User login
Achieving a ‘new sexual-health paradigm’ means expanding STI care
A vital aspect of expanding access and care for sexually transmitted infections (STIs) in the United States is broadening responsibility for this care across the health care system and other community resources, according to an article published online July 6 in Clinical Infectious Diseases. This expansion and decentralization of care are central to adopting the “new sexual health paradigm” recommended by a National Academies report that was published in March.
“STIs represent a sizable, longstanding, and growing public health challenge,” write Vincent Guilamo-Ramos, PhD, MPH, dean and professor at the Duke University School of Nursing and director of the Center for Latino Adolescent and Family Health (CLAFH) at Duke University, both in Durham, N.C., and his colleagues. Yet the limitations on the current STI workforce and limited federal funding and support for STI prevention and care mean it will take clinicians of all types from across the health care spectrum to meet the challenge, they explain.
“For too long, STI prevention and treatment has been perceived as the sole responsibility of a narrow workforce of specialized STI and HIV service providers,” Dr. Guilamo-Ramos and his coauthor, Marco Thimm-Kaiser, MPH, associate in research at Duke University and epidemiologist at CLAFH, wrote in an email.
“However, the resources allocated to this STI specialty workforce have diminished over time, along with decreasing investments in the broader U.S. public health infrastructure,” they continued. “At the same time – and in part due to this underinvestment – STI rates have soared, reaching a record high for the sixth year in a row in 2019.”
Those factors led to the National Academies report, which recommends moving “away from the traditional, disease-focused perspective on STIs in favor of a holistic perspective of sexual health as an integral component of overall health and well-being,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote to this news organization.
In their article, the authors review the limitations in the STI workforce, the implications of those limitations for the broader health care industry, and what it will take for STI and HIV specialists as well as regulators to ensure it’s possible to achieve the paradigm shift recommended by the National Academies.
Currently, the biggest limitation is access to care, said Laura Mercer, MD, MBA, of the department of obstetrics and gynecology and the ob.gyn. clerkship director at the University of Arizona, Phoenix. Dr. Mercer, who was not involved with the National Academies report or the analysis of it, said in an interview that it’s essential to emphasize “sexual health as a core element of routine primary and preventative care” to ensure it becomes more accessible to patients without the need to seek out specialty care.
Dr. Guilamo-Ramos and his colleagues drive home the importance of such a shift by noting that more than 200 million Americans live in counties with no practicing infectious disease physicians. The disparities are greatest in Southern states, which account for 40% of all reported STIs. The workforce shortage has continued to worsen alongside the deterioration of the clinical infrastructure supporting STI specialty services, the authors write.
Hence the need to expand accountability for care not only to primary-care physicians but also to nurses, pharmacists, physician assistants, nurse practitioners, and behavioral health practitioners. Doing so also requires normalizing sexual health services across health care professions.
“Prevention is a crucial first step” to this, Dr. Mercer said. “This is particularly important as we recall that almost half of new sexually transmitted infections occur in teenagers. Destigmatizing sexual health and sexual health education will also help encourage patients of all ages to request and accept testing.”
Further, with primary care practitioners managing most STI testing and treatment, subspecialists can focus primarily on complex or refractory cases, she added. Ways to help broaden care include developing point-of-care testing for STIs and improving the accuracy of existing testing, she said.
“The goal is to make routine sexual health services accessible in a wide range of settings, such as in primary care, at pharmacies, and in community-based settings, and to draw on a broader workforce for delivery of sexual health services,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser said in an interview.
Kevin Ault, MD, professor of obstetrics and gynecology and director of clinical and translational research at the University of Kansas Medical Center in Kansas City, said that many medical organizations, such as the American College of Obstetricians and Gynecologists, have long advocated incorporating sexual health into routine preventive care. He also noted that pharmacists have already become proactive in preventing STIs and could continue to do so.
“Vaccines for hepatitis and human papillomavirus are commonly available at pharmacies,” Dr. Ault said. He was not involved in the article by Dr. Guilamo-Ramos and colleagues or the original report. “Pharmacists could also fill a gap by administering injectable medications such as penicillin. States would have to approve changes in policy, but many states have already done this for expedited partner therapy.”
Dr. Guilamo-Ramos and Mr. Thimm-Kaiser noted similar barriers that must be removed to broaden delivery of STI services.
“Unfortunately, too many highly trained health care providers who are well-positioned for the delivery of sexual health services face regulatory or administrative barriers to practice to the full scope of their training,” they wrote. “These barriers can have a particularly negative impact in medically underserved communities, where physician shortages are common and where novel, decentralized health care service delivery models that draw on nonphysician providers may hold the greatest promise.”
As more diverse health care practitioners take on these roles, ID and HIV specialists can provide their expertise in developing training and technical assistance to support generalists, Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote. They can also aid in aligning “clinical training curricula, licensing criteria, and practice guidelines with routine delivery of sexual health services.”
Dr. Guilamo-Ramos and his coauthors offer specific recommendations for professional training, licensing, and practice guidelines to help overcome the “insufficient knowledge, inadequate training, and absence of explicit protocols” that currently impede delivery of STI services in general practice settings.
Although the paradigm shift recommended by the National Academies is ambitious, it’s also necessary, and “none of the recommendations are out of reach,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser said in an interview. They pointed out how the COVID-19 pandemic has highlighted how underresourced the health care workforce and infrastructure are and how great health care disparities are.
“There is momentum toward rebuilding the nation’s health and public health system in a more effective and efficient way,” they said, and many of the STI report’s recommendations “overlap with priorities for the broader health and public health system moving forward.”
Dr. Mercer also believes the recommendations are realistic, “but only the beginning,” she told this news organization. “Comprehensive sexual education to expand knowledge about STI prevention and public health campaigns to help destigmatize sexual health care in general will remain crucial,” she said.
Sexual education, expanded access, and destigmatizing sexual care are particularly important for reaching the populations most in need of care, such as adolescents and young adults, as well as ethnic, racial, sexual, and gender-minority youth.
“It cannot be overstated how important of a priority population adolescents and young adults are,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote. They noted that those aged 15-24 account for half of all STIs each year but represent only a quarter of the sexually active population. “Targeted efforts for STI prevention and treatment among adolescents and young adults are therefore essential for an overall successful strategy to address STIs and sexual health in the United States.”
The National Academies report was supported by the Centers for Disease Control and Prevention and the National Association of County and City Health Officials. Dr. Mercer, Dr. Ault, and Mr. Thimm-Kaiser have disclosed no relevant financial relationships. Dr. Guilamo-Ramos has received grants and personal fees from ViiV Health care.
A version of this article first appeared on Medscape.com.
A vital aspect of expanding access and care for sexually transmitted infections (STIs) in the United States is broadening responsibility for this care across the health care system and other community resources, according to an article published online July 6 in Clinical Infectious Diseases. This expansion and decentralization of care are central to adopting the “new sexual health paradigm” recommended by a National Academies report that was published in March.
“STIs represent a sizable, longstanding, and growing public health challenge,” write Vincent Guilamo-Ramos, PhD, MPH, dean and professor at the Duke University School of Nursing and director of the Center for Latino Adolescent and Family Health (CLAFH) at Duke University, both in Durham, N.C., and his colleagues. Yet the limitations on the current STI workforce and limited federal funding and support for STI prevention and care mean it will take clinicians of all types from across the health care spectrum to meet the challenge, they explain.
“For too long, STI prevention and treatment has been perceived as the sole responsibility of a narrow workforce of specialized STI and HIV service providers,” Dr. Guilamo-Ramos and his coauthor, Marco Thimm-Kaiser, MPH, associate in research at Duke University and epidemiologist at CLAFH, wrote in an email.
“However, the resources allocated to this STI specialty workforce have diminished over time, along with decreasing investments in the broader U.S. public health infrastructure,” they continued. “At the same time – and in part due to this underinvestment – STI rates have soared, reaching a record high for the sixth year in a row in 2019.”
Those factors led to the National Academies report, which recommends moving “away from the traditional, disease-focused perspective on STIs in favor of a holistic perspective of sexual health as an integral component of overall health and well-being,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote to this news organization.
In their article, the authors review the limitations in the STI workforce, the implications of those limitations for the broader health care industry, and what it will take for STI and HIV specialists as well as regulators to ensure it’s possible to achieve the paradigm shift recommended by the National Academies.
Currently, the biggest limitation is access to care, said Laura Mercer, MD, MBA, of the department of obstetrics and gynecology and the ob.gyn. clerkship director at the University of Arizona, Phoenix. Dr. Mercer, who was not involved with the National Academies report or the analysis of it, said in an interview that it’s essential to emphasize “sexual health as a core element of routine primary and preventative care” to ensure it becomes more accessible to patients without the need to seek out specialty care.
Dr. Guilamo-Ramos and his colleagues drive home the importance of such a shift by noting that more than 200 million Americans live in counties with no practicing infectious disease physicians. The disparities are greatest in Southern states, which account for 40% of all reported STIs. The workforce shortage has continued to worsen alongside the deterioration of the clinical infrastructure supporting STI specialty services, the authors write.
Hence the need to expand accountability for care not only to primary-care physicians but also to nurses, pharmacists, physician assistants, nurse practitioners, and behavioral health practitioners. Doing so also requires normalizing sexual health services across health care professions.
“Prevention is a crucial first step” to this, Dr. Mercer said. “This is particularly important as we recall that almost half of new sexually transmitted infections occur in teenagers. Destigmatizing sexual health and sexual health education will also help encourage patients of all ages to request and accept testing.”
Further, with primary care practitioners managing most STI testing and treatment, subspecialists can focus primarily on complex or refractory cases, she added. Ways to help broaden care include developing point-of-care testing for STIs and improving the accuracy of existing testing, she said.
“The goal is to make routine sexual health services accessible in a wide range of settings, such as in primary care, at pharmacies, and in community-based settings, and to draw on a broader workforce for delivery of sexual health services,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser said in an interview.
Kevin Ault, MD, professor of obstetrics and gynecology and director of clinical and translational research at the University of Kansas Medical Center in Kansas City, said that many medical organizations, such as the American College of Obstetricians and Gynecologists, have long advocated incorporating sexual health into routine preventive care. He also noted that pharmacists have already become proactive in preventing STIs and could continue to do so.
“Vaccines for hepatitis and human papillomavirus are commonly available at pharmacies,” Dr. Ault said. He was not involved in the article by Dr. Guilamo-Ramos and colleagues or the original report. “Pharmacists could also fill a gap by administering injectable medications such as penicillin. States would have to approve changes in policy, but many states have already done this for expedited partner therapy.”
Dr. Guilamo-Ramos and Mr. Thimm-Kaiser noted similar barriers that must be removed to broaden delivery of STI services.
“Unfortunately, too many highly trained health care providers who are well-positioned for the delivery of sexual health services face regulatory or administrative barriers to practice to the full scope of their training,” they wrote. “These barriers can have a particularly negative impact in medically underserved communities, where physician shortages are common and where novel, decentralized health care service delivery models that draw on nonphysician providers may hold the greatest promise.”
As more diverse health care practitioners take on these roles, ID and HIV specialists can provide their expertise in developing training and technical assistance to support generalists, Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote. They can also aid in aligning “clinical training curricula, licensing criteria, and practice guidelines with routine delivery of sexual health services.”
Dr. Guilamo-Ramos and his coauthors offer specific recommendations for professional training, licensing, and practice guidelines to help overcome the “insufficient knowledge, inadequate training, and absence of explicit protocols” that currently impede delivery of STI services in general practice settings.
Although the paradigm shift recommended by the National Academies is ambitious, it’s also necessary, and “none of the recommendations are out of reach,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser said in an interview. They pointed out how the COVID-19 pandemic has highlighted how underresourced the health care workforce and infrastructure are and how great health care disparities are.
“There is momentum toward rebuilding the nation’s health and public health system in a more effective and efficient way,” they said, and many of the STI report’s recommendations “overlap with priorities for the broader health and public health system moving forward.”
Dr. Mercer also believes the recommendations are realistic, “but only the beginning,” she told this news organization. “Comprehensive sexual education to expand knowledge about STI prevention and public health campaigns to help destigmatize sexual health care in general will remain crucial,” she said.
Sexual education, expanded access, and destigmatizing sexual care are particularly important for reaching the populations most in need of care, such as adolescents and young adults, as well as ethnic, racial, sexual, and gender-minority youth.
“It cannot be overstated how important of a priority population adolescents and young adults are,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote. They noted that those aged 15-24 account for half of all STIs each year but represent only a quarter of the sexually active population. “Targeted efforts for STI prevention and treatment among adolescents and young adults are therefore essential for an overall successful strategy to address STIs and sexual health in the United States.”
The National Academies report was supported by the Centers for Disease Control and Prevention and the National Association of County and City Health Officials. Dr. Mercer, Dr. Ault, and Mr. Thimm-Kaiser have disclosed no relevant financial relationships. Dr. Guilamo-Ramos has received grants and personal fees from ViiV Health care.
A version of this article first appeared on Medscape.com.
A vital aspect of expanding access and care for sexually transmitted infections (STIs) in the United States is broadening responsibility for this care across the health care system and other community resources, according to an article published online July 6 in Clinical Infectious Diseases. This expansion and decentralization of care are central to adopting the “new sexual health paradigm” recommended by a National Academies report that was published in March.
“STIs represent a sizable, longstanding, and growing public health challenge,” write Vincent Guilamo-Ramos, PhD, MPH, dean and professor at the Duke University School of Nursing and director of the Center for Latino Adolescent and Family Health (CLAFH) at Duke University, both in Durham, N.C., and his colleagues. Yet the limitations on the current STI workforce and limited federal funding and support for STI prevention and care mean it will take clinicians of all types from across the health care spectrum to meet the challenge, they explain.
“For too long, STI prevention and treatment has been perceived as the sole responsibility of a narrow workforce of specialized STI and HIV service providers,” Dr. Guilamo-Ramos and his coauthor, Marco Thimm-Kaiser, MPH, associate in research at Duke University and epidemiologist at CLAFH, wrote in an email.
“However, the resources allocated to this STI specialty workforce have diminished over time, along with decreasing investments in the broader U.S. public health infrastructure,” they continued. “At the same time – and in part due to this underinvestment – STI rates have soared, reaching a record high for the sixth year in a row in 2019.”
Those factors led to the National Academies report, which recommends moving “away from the traditional, disease-focused perspective on STIs in favor of a holistic perspective of sexual health as an integral component of overall health and well-being,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote to this news organization.
In their article, the authors review the limitations in the STI workforce, the implications of those limitations for the broader health care industry, and what it will take for STI and HIV specialists as well as regulators to ensure it’s possible to achieve the paradigm shift recommended by the National Academies.
Currently, the biggest limitation is access to care, said Laura Mercer, MD, MBA, of the department of obstetrics and gynecology and the ob.gyn. clerkship director at the University of Arizona, Phoenix. Dr. Mercer, who was not involved with the National Academies report or the analysis of it, said in an interview that it’s essential to emphasize “sexual health as a core element of routine primary and preventative care” to ensure it becomes more accessible to patients without the need to seek out specialty care.
Dr. Guilamo-Ramos and his colleagues drive home the importance of such a shift by noting that more than 200 million Americans live in counties with no practicing infectious disease physicians. The disparities are greatest in Southern states, which account for 40% of all reported STIs. The workforce shortage has continued to worsen alongside the deterioration of the clinical infrastructure supporting STI specialty services, the authors write.
Hence the need to expand accountability for care not only to primary-care physicians but also to nurses, pharmacists, physician assistants, nurse practitioners, and behavioral health practitioners. Doing so also requires normalizing sexual health services across health care professions.
“Prevention is a crucial first step” to this, Dr. Mercer said. “This is particularly important as we recall that almost half of new sexually transmitted infections occur in teenagers. Destigmatizing sexual health and sexual health education will also help encourage patients of all ages to request and accept testing.”
Further, with primary care practitioners managing most STI testing and treatment, subspecialists can focus primarily on complex or refractory cases, she added. Ways to help broaden care include developing point-of-care testing for STIs and improving the accuracy of existing testing, she said.
“The goal is to make routine sexual health services accessible in a wide range of settings, such as in primary care, at pharmacies, and in community-based settings, and to draw on a broader workforce for delivery of sexual health services,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser said in an interview.
Kevin Ault, MD, professor of obstetrics and gynecology and director of clinical and translational research at the University of Kansas Medical Center in Kansas City, said that many medical organizations, such as the American College of Obstetricians and Gynecologists, have long advocated incorporating sexual health into routine preventive care. He also noted that pharmacists have already become proactive in preventing STIs and could continue to do so.
“Vaccines for hepatitis and human papillomavirus are commonly available at pharmacies,” Dr. Ault said. He was not involved in the article by Dr. Guilamo-Ramos and colleagues or the original report. “Pharmacists could also fill a gap by administering injectable medications such as penicillin. States would have to approve changes in policy, but many states have already done this for expedited partner therapy.”
Dr. Guilamo-Ramos and Mr. Thimm-Kaiser noted similar barriers that must be removed to broaden delivery of STI services.
“Unfortunately, too many highly trained health care providers who are well-positioned for the delivery of sexual health services face regulatory or administrative barriers to practice to the full scope of their training,” they wrote. “These barriers can have a particularly negative impact in medically underserved communities, where physician shortages are common and where novel, decentralized health care service delivery models that draw on nonphysician providers may hold the greatest promise.”
As more diverse health care practitioners take on these roles, ID and HIV specialists can provide their expertise in developing training and technical assistance to support generalists, Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote. They can also aid in aligning “clinical training curricula, licensing criteria, and practice guidelines with routine delivery of sexual health services.”
Dr. Guilamo-Ramos and his coauthors offer specific recommendations for professional training, licensing, and practice guidelines to help overcome the “insufficient knowledge, inadequate training, and absence of explicit protocols” that currently impede delivery of STI services in general practice settings.
Although the paradigm shift recommended by the National Academies is ambitious, it’s also necessary, and “none of the recommendations are out of reach,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser said in an interview. They pointed out how the COVID-19 pandemic has highlighted how underresourced the health care workforce and infrastructure are and how great health care disparities are.
“There is momentum toward rebuilding the nation’s health and public health system in a more effective and efficient way,” they said, and many of the STI report’s recommendations “overlap with priorities for the broader health and public health system moving forward.”
Dr. Mercer also believes the recommendations are realistic, “but only the beginning,” she told this news organization. “Comprehensive sexual education to expand knowledge about STI prevention and public health campaigns to help destigmatize sexual health care in general will remain crucial,” she said.
Sexual education, expanded access, and destigmatizing sexual care are particularly important for reaching the populations most in need of care, such as adolescents and young adults, as well as ethnic, racial, sexual, and gender-minority youth.
“It cannot be overstated how important of a priority population adolescents and young adults are,” Dr. Guilamo-Ramos and Mr. Thimm-Kaiser wrote. They noted that those aged 15-24 account for half of all STIs each year but represent only a quarter of the sexually active population. “Targeted efforts for STI prevention and treatment among adolescents and young adults are therefore essential for an overall successful strategy to address STIs and sexual health in the United States.”
The National Academies report was supported by the Centers for Disease Control and Prevention and the National Association of County and City Health Officials. Dr. Mercer, Dr. Ault, and Mr. Thimm-Kaiser have disclosed no relevant financial relationships. Dr. Guilamo-Ramos has received grants and personal fees from ViiV Health care.
A version of this article first appeared on Medscape.com.
Exposure to marijuana smoke linked to increased risk of respiratory infections in children
Exposure to secondhand marijuana smoke is more strongly associated with viral respiratory infections in children, compared with children who were exposed to tobacco smoke and those with no smoke exposure, new research shows.
“The findings of this study are interesting and pleasantly raise further questions,” said Kristen Miller, MD, attending physician in the division of pulmonary and sleep medicine at Children’s Hospital of Philadelphia, who was not involved in the study. “Given the robust literature regarding secondhand smoke exposure and the current landscape surrounding marijuana, this is a timely study to evaluate the prevalence of marijuana use and the associated effects of marijuana exposure among children.”
Prior research has linked primary marijuana use with respiratory effects. A 2020 study associated cannabis use with an increased risk of severe bronchitis, lung hyperinflation, and increased central airway resistance. However, according to the Centers for Disease Control and Prevention, there are still a lot of unanswered questions surrounding secondhand marijuana smoke exposure and its effects.
“If kids are exposed to enough secondhand smoke, regardless of what the substance is, they’re going to have some negative health outcomes with it,” study author Adam Johnson, MD, of Wake Forest University, Winston-Salem, N.C., said in an interview.
The study, published in Pediatric Research, looked at rates of reported ED and urgent care visits and specific illnesses – such as otitis media, viral respiratory infections, and asthma exacerbations – among children with marijuana exposure and tobacco exposure.
For the study, Dr. Johnson and colleagues surveyed 1,500 parents and caregivers who went to an academic children’s hospital between Dec. 1, 2015, and July 30, 2017. Researchers found that children exposed to marijuana smoke had higher rates of ED visits at 2.21 within the past 12 months, compared with those exposed to tobacco smoke (2.14 within the past 12 months) and those with no smoke exposure (1.94 within the past 12 months). However, the difference in these visits were not statistically significant.
Researchers saw that children exposed to secondhand marijuana smoke saw a 30% increase in viral respiratory infections, compared with those who were not exposed to tobacco or marijuana smoke, Dr. Johnson said. Caregivers who smoked marijuana reported a rate of 1.31 viral infections in their children within the last year. Meanwhile those who smoked tobacco reported a rate of 1.00 infections within the last 12 months and caregivers who did not smoke reported 1.04 infections within the year.
“It suggests that components in marijuana smoke may depress the body’s immune responses to viral infections in children,” Dr. Miller said in an interview.
When it came to otitis media episodes, children exposed to marijuana had a rate of 0.96 episodes within the past 12 months. Children experiencing secondhand tobacco smoke had a rate of 0.83 episodes and those with no smoke exposure had 0.75 episodes within the past 12 months. Researchers did not note this difference as statistically significant.
When it came to asthma exacerbations, children exposed to marijuana smoke also had statistically insignificantly higher rates of exacerbations, compared with those exposed to tobacco smoke and those not exposed to smoke.
“I think it was surprising that the survey results found that marijuana seemed to be more strongly associated with the viral respiratory infections than tobacco,” Dr. Johnson said. “We know that secondhand tobacco smoke exposure in kids does lead to things like otitis media or ear infections, asthma attacks, and other processes, including colds. It was interesting that we didn’t find that association [in the new study], but we found that with marijuana.”
Dr. Johnson said the findings are especially concerning with increases in the acceptance and accessibility of marijuana as it becomes legalized in many states.
A 2015 study examined the effect of secondhand marijuana smoke exposure. Researchers found that exposure to secondhand marijuana smoke can increase heart rate, have mild to moderate sedative effects and can produce detectable cannabinoid levels in blood and urine. However, another study published in 2012 found that low to moderate primary marijuana use is less harmful to users’ lungs than tobacco exposure.
Dr. Miller added that little is known about how exposure to marijuana smoke can affect the innate responses to pathogens and there is a need to “study this in more detail” to figure out if secondhand marijuana smoke is a risk factor for either an increase in respiratory virus infections or their severity.
“These questions could have considerable implications for the health of our children and public health measures regarding marijuana use,” she explained. “As documented marijuana use increases, health care providers need to be aware of the effects of marijuana use and exposure.”
Neither Dr. Johnson nor Dr. Miller has any relevant financial disclosures.
Exposure to secondhand marijuana smoke is more strongly associated with viral respiratory infections in children, compared with children who were exposed to tobacco smoke and those with no smoke exposure, new research shows.
“The findings of this study are interesting and pleasantly raise further questions,” said Kristen Miller, MD, attending physician in the division of pulmonary and sleep medicine at Children’s Hospital of Philadelphia, who was not involved in the study. “Given the robust literature regarding secondhand smoke exposure and the current landscape surrounding marijuana, this is a timely study to evaluate the prevalence of marijuana use and the associated effects of marijuana exposure among children.”
Prior research has linked primary marijuana use with respiratory effects. A 2020 study associated cannabis use with an increased risk of severe bronchitis, lung hyperinflation, and increased central airway resistance. However, according to the Centers for Disease Control and Prevention, there are still a lot of unanswered questions surrounding secondhand marijuana smoke exposure and its effects.
“If kids are exposed to enough secondhand smoke, regardless of what the substance is, they’re going to have some negative health outcomes with it,” study author Adam Johnson, MD, of Wake Forest University, Winston-Salem, N.C., said in an interview.
The study, published in Pediatric Research, looked at rates of reported ED and urgent care visits and specific illnesses – such as otitis media, viral respiratory infections, and asthma exacerbations – among children with marijuana exposure and tobacco exposure.
For the study, Dr. Johnson and colleagues surveyed 1,500 parents and caregivers who went to an academic children’s hospital between Dec. 1, 2015, and July 30, 2017. Researchers found that children exposed to marijuana smoke had higher rates of ED visits at 2.21 within the past 12 months, compared with those exposed to tobacco smoke (2.14 within the past 12 months) and those with no smoke exposure (1.94 within the past 12 months). However, the difference in these visits were not statistically significant.
Researchers saw that children exposed to secondhand marijuana smoke saw a 30% increase in viral respiratory infections, compared with those who were not exposed to tobacco or marijuana smoke, Dr. Johnson said. Caregivers who smoked marijuana reported a rate of 1.31 viral infections in their children within the last year. Meanwhile those who smoked tobacco reported a rate of 1.00 infections within the last 12 months and caregivers who did not smoke reported 1.04 infections within the year.
“It suggests that components in marijuana smoke may depress the body’s immune responses to viral infections in children,” Dr. Miller said in an interview.
When it came to otitis media episodes, children exposed to marijuana had a rate of 0.96 episodes within the past 12 months. Children experiencing secondhand tobacco smoke had a rate of 0.83 episodes and those with no smoke exposure had 0.75 episodes within the past 12 months. Researchers did not note this difference as statistically significant.
When it came to asthma exacerbations, children exposed to marijuana smoke also had statistically insignificantly higher rates of exacerbations, compared with those exposed to tobacco smoke and those not exposed to smoke.
“I think it was surprising that the survey results found that marijuana seemed to be more strongly associated with the viral respiratory infections than tobacco,” Dr. Johnson said. “We know that secondhand tobacco smoke exposure in kids does lead to things like otitis media or ear infections, asthma attacks, and other processes, including colds. It was interesting that we didn’t find that association [in the new study], but we found that with marijuana.”
Dr. Johnson said the findings are especially concerning with increases in the acceptance and accessibility of marijuana as it becomes legalized in many states.
A 2015 study examined the effect of secondhand marijuana smoke exposure. Researchers found that exposure to secondhand marijuana smoke can increase heart rate, have mild to moderate sedative effects and can produce detectable cannabinoid levels in blood and urine. However, another study published in 2012 found that low to moderate primary marijuana use is less harmful to users’ lungs than tobacco exposure.
Dr. Miller added that little is known about how exposure to marijuana smoke can affect the innate responses to pathogens and there is a need to “study this in more detail” to figure out if secondhand marijuana smoke is a risk factor for either an increase in respiratory virus infections or their severity.
“These questions could have considerable implications for the health of our children and public health measures regarding marijuana use,” she explained. “As documented marijuana use increases, health care providers need to be aware of the effects of marijuana use and exposure.”
Neither Dr. Johnson nor Dr. Miller has any relevant financial disclosures.
Exposure to secondhand marijuana smoke is more strongly associated with viral respiratory infections in children, compared with children who were exposed to tobacco smoke and those with no smoke exposure, new research shows.
“The findings of this study are interesting and pleasantly raise further questions,” said Kristen Miller, MD, attending physician in the division of pulmonary and sleep medicine at Children’s Hospital of Philadelphia, who was not involved in the study. “Given the robust literature regarding secondhand smoke exposure and the current landscape surrounding marijuana, this is a timely study to evaluate the prevalence of marijuana use and the associated effects of marijuana exposure among children.”
Prior research has linked primary marijuana use with respiratory effects. A 2020 study associated cannabis use with an increased risk of severe bronchitis, lung hyperinflation, and increased central airway resistance. However, according to the Centers for Disease Control and Prevention, there are still a lot of unanswered questions surrounding secondhand marijuana smoke exposure and its effects.
“If kids are exposed to enough secondhand smoke, regardless of what the substance is, they’re going to have some negative health outcomes with it,” study author Adam Johnson, MD, of Wake Forest University, Winston-Salem, N.C., said in an interview.
The study, published in Pediatric Research, looked at rates of reported ED and urgent care visits and specific illnesses – such as otitis media, viral respiratory infections, and asthma exacerbations – among children with marijuana exposure and tobacco exposure.
For the study, Dr. Johnson and colleagues surveyed 1,500 parents and caregivers who went to an academic children’s hospital between Dec. 1, 2015, and July 30, 2017. Researchers found that children exposed to marijuana smoke had higher rates of ED visits at 2.21 within the past 12 months, compared with those exposed to tobacco smoke (2.14 within the past 12 months) and those with no smoke exposure (1.94 within the past 12 months). However, the difference in these visits were not statistically significant.
Researchers saw that children exposed to secondhand marijuana smoke saw a 30% increase in viral respiratory infections, compared with those who were not exposed to tobacco or marijuana smoke, Dr. Johnson said. Caregivers who smoked marijuana reported a rate of 1.31 viral infections in their children within the last year. Meanwhile those who smoked tobacco reported a rate of 1.00 infections within the last 12 months and caregivers who did not smoke reported 1.04 infections within the year.
“It suggests that components in marijuana smoke may depress the body’s immune responses to viral infections in children,” Dr. Miller said in an interview.
When it came to otitis media episodes, children exposed to marijuana had a rate of 0.96 episodes within the past 12 months. Children experiencing secondhand tobacco smoke had a rate of 0.83 episodes and those with no smoke exposure had 0.75 episodes within the past 12 months. Researchers did not note this difference as statistically significant.
When it came to asthma exacerbations, children exposed to marijuana smoke also had statistically insignificantly higher rates of exacerbations, compared with those exposed to tobacco smoke and those not exposed to smoke.
“I think it was surprising that the survey results found that marijuana seemed to be more strongly associated with the viral respiratory infections than tobacco,” Dr. Johnson said. “We know that secondhand tobacco smoke exposure in kids does lead to things like otitis media or ear infections, asthma attacks, and other processes, including colds. It was interesting that we didn’t find that association [in the new study], but we found that with marijuana.”
Dr. Johnson said the findings are especially concerning with increases in the acceptance and accessibility of marijuana as it becomes legalized in many states.
A 2015 study examined the effect of secondhand marijuana smoke exposure. Researchers found that exposure to secondhand marijuana smoke can increase heart rate, have mild to moderate sedative effects and can produce detectable cannabinoid levels in blood and urine. However, another study published in 2012 found that low to moderate primary marijuana use is less harmful to users’ lungs than tobacco exposure.
Dr. Miller added that little is known about how exposure to marijuana smoke can affect the innate responses to pathogens and there is a need to “study this in more detail” to figure out if secondhand marijuana smoke is a risk factor for either an increase in respiratory virus infections or their severity.
“These questions could have considerable implications for the health of our children and public health measures regarding marijuana use,” she explained. “As documented marijuana use increases, health care providers need to be aware of the effects of marijuana use and exposure.”
Neither Dr. Johnson nor Dr. Miller has any relevant financial disclosures.
FROM PEDIATRIC RESEARCH
Fauci says ‘unprecedented’ conditions could influence COVID vaccine approval for kids
“From a public health standpoint, I think we have an evolving situation,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, in a moderated session with Lee Beers, MD, president of the American Academy of Pediatrics, at the virtual Pediatric Hospital Medicine annual conference.
The reasons for this shift remain unclear, he said.
Dr. Beers emphasized the ability of pediatric hospitalists to be flexible in the face of uncertainty and the evolving virus, and asked Dr. Fauci to elaborate on the unique traits of the delta variant that make it especially challenging.
“There is no doubt that delta transmits much more efficiently than the alpha variant or any other variant,” Dr. Fauci said. The transmissibility is evident in comparisons of the level of virus in the nasopharynx of the delta variant, compared with the original alpha COVID-19 virus – delta is as much as 1,000 times higher, he explained.
In addition, the level of virus in the nasopharynx of vaccinated individuals who develop breakthrough infections with the delta variant is similar to the levels in unvaccinated individuals who are infected with the delta variant.
The delta variant is “the tough guy on the block” at the moment, Dr. Fauci said.
Dr. Fauci also responded to a question on the lack of winter viruses, such as RSV and the flu, last winter, but the surge in these viruses over the summer.
This winter’s activity remains uncertain, Dr. Fauci said. However, he speculated “with a strong dose of humility and modesty” that viruses tend to have niches, some are seasonal, and the winter viruses that were displaced by COVID-19 hit harder in the summer instead. “If I were a [non-COVID] virus looking for a niche, I would be really confused,” he said. “I don’t know what will happen this winter, but if we get good control over COVID-19 by winter, we could have a very vengeful influenza season,” he said. “This is speculation, I don’t have any data for this,” he cautioned.
Dr. Beers raised the issue of back-to-school safety, and the updated AAP guidance for universal masking for K-12 students. “Our guidance about return to school gets updated as the situation changes and we gain a better understanding of how kids can get to school safely,” she said. A combination of factors affect back-to-school guidance, including the ineligibility of children younger than 12 years to be vaccinated, the number of adolescents who are eligible but have not been vaccinated, and the challenge for educators to navigate which children should wear masks, Dr. Beers said.
“We want to get vaccines for our youngest kids as soon as safely possible,” Dr. Beers emphasized. She noted that the same urgency is needed to provide vaccines for children as for adults, although “we have to do it safely, and be sure and feel confident in the data.”
When asked to comment about the status of FDA authorization of COVID-19 vaccines for younger children, Dr. Fauci described the current situation as one that “might require some unprecedented and unique action” on the part of the FDA, which tends to move cautiously because of safety considerations. However, concerns about adverse events might get in the way of protecting children against what “you are really worried about,” in this case COVID-19 and its variants, he said. Despite the breakthrough infections, “vaccination continues to very adequately protect people from getting severe disease,” he emphasized.
Dr. Fauci also said that he believes the current data support boosters for the immune compromised; however “it is a different story about the general vaccinated population and the vaccinated elderly,” he said. Sooner or later most people will likely need boosters; “the question is who, when, and how soon,” he noted.
Dr. Fauci wrapped up the session with kudos and support for the pediatric health care community. “As a nonpediatrician, I have a great deal of respect for the job you are doing,” he said. “Keep up the great work.”
Dr. Beers echoed this sentiment, saying that she was “continually awed, impressed, and inspired” by how the pediatric hospitalists are navigating the ever-changing pandemic environment.
“From a public health standpoint, I think we have an evolving situation,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, in a moderated session with Lee Beers, MD, president of the American Academy of Pediatrics, at the virtual Pediatric Hospital Medicine annual conference.
The reasons for this shift remain unclear, he said.
Dr. Beers emphasized the ability of pediatric hospitalists to be flexible in the face of uncertainty and the evolving virus, and asked Dr. Fauci to elaborate on the unique traits of the delta variant that make it especially challenging.
“There is no doubt that delta transmits much more efficiently than the alpha variant or any other variant,” Dr. Fauci said. The transmissibility is evident in comparisons of the level of virus in the nasopharynx of the delta variant, compared with the original alpha COVID-19 virus – delta is as much as 1,000 times higher, he explained.
In addition, the level of virus in the nasopharynx of vaccinated individuals who develop breakthrough infections with the delta variant is similar to the levels in unvaccinated individuals who are infected with the delta variant.
The delta variant is “the tough guy on the block” at the moment, Dr. Fauci said.
Dr. Fauci also responded to a question on the lack of winter viruses, such as RSV and the flu, last winter, but the surge in these viruses over the summer.
This winter’s activity remains uncertain, Dr. Fauci said. However, he speculated “with a strong dose of humility and modesty” that viruses tend to have niches, some are seasonal, and the winter viruses that were displaced by COVID-19 hit harder in the summer instead. “If I were a [non-COVID] virus looking for a niche, I would be really confused,” he said. “I don’t know what will happen this winter, but if we get good control over COVID-19 by winter, we could have a very vengeful influenza season,” he said. “This is speculation, I don’t have any data for this,” he cautioned.
Dr. Beers raised the issue of back-to-school safety, and the updated AAP guidance for universal masking for K-12 students. “Our guidance about return to school gets updated as the situation changes and we gain a better understanding of how kids can get to school safely,” she said. A combination of factors affect back-to-school guidance, including the ineligibility of children younger than 12 years to be vaccinated, the number of adolescents who are eligible but have not been vaccinated, and the challenge for educators to navigate which children should wear masks, Dr. Beers said.
“We want to get vaccines for our youngest kids as soon as safely possible,” Dr. Beers emphasized. She noted that the same urgency is needed to provide vaccines for children as for adults, although “we have to do it safely, and be sure and feel confident in the data.”
When asked to comment about the status of FDA authorization of COVID-19 vaccines for younger children, Dr. Fauci described the current situation as one that “might require some unprecedented and unique action” on the part of the FDA, which tends to move cautiously because of safety considerations. However, concerns about adverse events might get in the way of protecting children against what “you are really worried about,” in this case COVID-19 and its variants, he said. Despite the breakthrough infections, “vaccination continues to very adequately protect people from getting severe disease,” he emphasized.
Dr. Fauci also said that he believes the current data support boosters for the immune compromised; however “it is a different story about the general vaccinated population and the vaccinated elderly,” he said. Sooner or later most people will likely need boosters; “the question is who, when, and how soon,” he noted.
Dr. Fauci wrapped up the session with kudos and support for the pediatric health care community. “As a nonpediatrician, I have a great deal of respect for the job you are doing,” he said. “Keep up the great work.”
Dr. Beers echoed this sentiment, saying that she was “continually awed, impressed, and inspired” by how the pediatric hospitalists are navigating the ever-changing pandemic environment.
“From a public health standpoint, I think we have an evolving situation,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, in a moderated session with Lee Beers, MD, president of the American Academy of Pediatrics, at the virtual Pediatric Hospital Medicine annual conference.
The reasons for this shift remain unclear, he said.
Dr. Beers emphasized the ability of pediatric hospitalists to be flexible in the face of uncertainty and the evolving virus, and asked Dr. Fauci to elaborate on the unique traits of the delta variant that make it especially challenging.
“There is no doubt that delta transmits much more efficiently than the alpha variant or any other variant,” Dr. Fauci said. The transmissibility is evident in comparisons of the level of virus in the nasopharynx of the delta variant, compared with the original alpha COVID-19 virus – delta is as much as 1,000 times higher, he explained.
In addition, the level of virus in the nasopharynx of vaccinated individuals who develop breakthrough infections with the delta variant is similar to the levels in unvaccinated individuals who are infected with the delta variant.
The delta variant is “the tough guy on the block” at the moment, Dr. Fauci said.
Dr. Fauci also responded to a question on the lack of winter viruses, such as RSV and the flu, last winter, but the surge in these viruses over the summer.
This winter’s activity remains uncertain, Dr. Fauci said. However, he speculated “with a strong dose of humility and modesty” that viruses tend to have niches, some are seasonal, and the winter viruses that were displaced by COVID-19 hit harder in the summer instead. “If I were a [non-COVID] virus looking for a niche, I would be really confused,” he said. “I don’t know what will happen this winter, but if we get good control over COVID-19 by winter, we could have a very vengeful influenza season,” he said. “This is speculation, I don’t have any data for this,” he cautioned.
Dr. Beers raised the issue of back-to-school safety, and the updated AAP guidance for universal masking for K-12 students. “Our guidance about return to school gets updated as the situation changes and we gain a better understanding of how kids can get to school safely,” she said. A combination of factors affect back-to-school guidance, including the ineligibility of children younger than 12 years to be vaccinated, the number of adolescents who are eligible but have not been vaccinated, and the challenge for educators to navigate which children should wear masks, Dr. Beers said.
“We want to get vaccines for our youngest kids as soon as safely possible,” Dr. Beers emphasized. She noted that the same urgency is needed to provide vaccines for children as for adults, although “we have to do it safely, and be sure and feel confident in the data.”
When asked to comment about the status of FDA authorization of COVID-19 vaccines for younger children, Dr. Fauci described the current situation as one that “might require some unprecedented and unique action” on the part of the FDA, which tends to move cautiously because of safety considerations. However, concerns about adverse events might get in the way of protecting children against what “you are really worried about,” in this case COVID-19 and its variants, he said. Despite the breakthrough infections, “vaccination continues to very adequately protect people from getting severe disease,” he emphasized.
Dr. Fauci also said that he believes the current data support boosters for the immune compromised; however “it is a different story about the general vaccinated population and the vaccinated elderly,” he said. Sooner or later most people will likely need boosters; “the question is who, when, and how soon,” he noted.
Dr. Fauci wrapped up the session with kudos and support for the pediatric health care community. “As a nonpediatrician, I have a great deal of respect for the job you are doing,” he said. “Keep up the great work.”
Dr. Beers echoed this sentiment, saying that she was “continually awed, impressed, and inspired” by how the pediatric hospitalists are navigating the ever-changing pandemic environment.
FROM PHM 2021
Injectable monoclonal antibodies prevent COVID-19 in trial
according to results of a randomized, double-blind, placebo-controlled clinical trial published online August 4, 2021, in the New England Journal of Medicine.
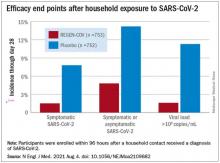
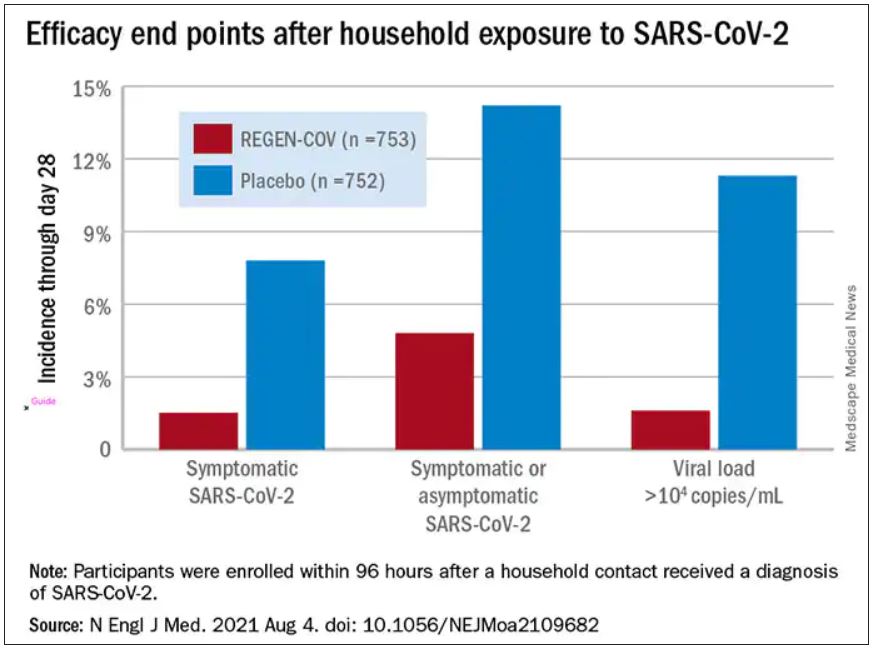
The cocktail of the monoclonal antibodies casirivimab and imdevimab (REGEN-COV, Regeneron Pharmaceuticals) reduced participants’ relative risk of infection by 72%, compared with placebo within the first week. After the first week, risk reduction increased to 93%.
“Long after you would be exposed by your household, there is an enduring effect that prevents you from community spread,” said David Wohl, MD, professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill, who was a site investigator for the trial but not a study author.
Participants were enrolled within 96 hours after someone in their household tested positive for SARS-CoV-2. Participants were randomly assigned to receive 1,200 mg of REGEN-COV subcutaneously or a placebo. Based on serologic testing, study participants showed no evidence of current or previous SARS-CoV-2 infection. The median age of participants was 42.9, but 45% were male teenagers (ages 12-17).
In the group that received REGEN-COV, 11 out of 753 participants developed symptomatic COVID-19, compared with 59 out of 752 participants who received placebo. The relative risk reduction for the study’s 4-week period was 81.4% (P < .001). Of the participants that did develop a SARS-CoV-2 infection, those that received REGEN-COV were less likely to be symptomatic. Asymptomatic infections developed in 25 participants who received REGEN-COV versus 48 in the placebo group. The relative risk of developing any SARS-CoV-2 infection, symptomatic or asymptomatic, was reduced by 66.4% with REGEN-COV (P < .001).
Among the patients who were symptomatic, symptoms subsided within a median of 1.2 weeks for the group that received REGEN-COV, 2 weeks earlier than the placebo group. These patients also had a shorter duration of a high viral load (>104 copies/mL). Few adverse events were reported in the treatment or placebo groups. Monoclonal antibodies “seem to be incredibly safe,” Dr. Wohl said.
“These monoclonal antibodies have proven they can reduce the viral replication in the nose,” said study author Myron Cohen, MD, an infectious disease specialist and professor of epidemiology at the University of North Carolina.
The Food and Drug Administration first granted REGEN-COV emergency use authorization (EUA) in November 2020 for use in patients with mild or moderate COVID-19 who were also at high risk for progressing to severe COVID-19. At that time, the cocktail of monoclonal antibodies was delivered by a single intravenous infusion.
In January, Regeneron first announced the success of this trial of the subcutaneous injection for exposed household contacts based on early results, and in June of 2021, the FDA expanded the EUA to include a subcutaneous delivery when IV is not feasible. On July 30, the EUA was expanded again to include prophylactic use in exposed patients based on these trial results.
The U.S. government has purchased approximately 1.5 million doses of REGEN-COV from Regeneron and has agreed to make the treatments free of charge to patients.
But despite being free, available, and backed by promising data, monoclonal antibodies as a therapeutic answer to COVID-19 still hasn’t really taken off. “The problem is, it first requires knowledge and awareness,” Dr. Wohl said. “A lot [of people] don’t know this exists. To be honest, vaccination has taken up all the oxygen in the room.”
Dr. Cohen agreed. One reason for the slow uptake may be because the drug supply is owned by the government and not a pharmaceutical company. There hasn’t been a typical marketing push to make physicians and consumers aware. Additionally, “the logistics are daunting,” Dr. Cohen said. The office spaces where many physicians care for patients “often aren’t appropriate for patients who think they have SARS-CoV-2.”
“Right now, there’s not a mechanism” to administer the drug to people who could benefit from it, Dr. Wohl said. Eligible patients are either immunocompromised and unlikely to mount a sufficient immune response with vaccination, or not fully vaccinated. They should have been exposed to an infected individual or have a high likelihood of exposure due to where they live, such as in a prison or nursing home. Local doctors are unlikely to be the primary administrators of the drug, Dr. Wohl added. “How do we operationalize this for people who fit the criteria?”
There’s also an issue of timing. REGEN-COV is most effective when given early, Dr. Cohen said. “[Monoclonal antibodies] really only work well in the replication phase.” Many patients who would be eligible delay care until they’ve had symptoms for several days, when REGEN-COV would no longer have the desired effect.
Eventually, Dr. Wohl suspects demand will increase when people realize REGEN-COV can help those with COVID-19 and those who have been exposed. But before then, “we do have to think about how to integrate this into a workflow people can access without being confused.”
The trial was done before there was widespread vaccination, so it’s unclear what the results mean for people who have been vaccinated. Dr. Cohen and Dr. Wohl said there are ongoing conversations about whether monoclonal antibodies could be complementary to vaccination and if there’s potential for continued monthly use of these therapies.
Cohen and Wohl reported no relevant financial relationships. The trial was supported by Regeneron Pharmaceuticals, F. Hoffmann–La Roche, the National Institute of Allergy and Infectious Diseases, NIH, and the COVID-19 Prevention Network.
A version of this article first appeared on Medscape.com.
according to results of a randomized, double-blind, placebo-controlled clinical trial published online August 4, 2021, in the New England Journal of Medicine.
The cocktail of the monoclonal antibodies casirivimab and imdevimab (REGEN-COV, Regeneron Pharmaceuticals) reduced participants’ relative risk of infection by 72%, compared with placebo within the first week. After the first week, risk reduction increased to 93%.
“Long after you would be exposed by your household, there is an enduring effect that prevents you from community spread,” said David Wohl, MD, professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill, who was a site investigator for the trial but not a study author.
Participants were enrolled within 96 hours after someone in their household tested positive for SARS-CoV-2. Participants were randomly assigned to receive 1,200 mg of REGEN-COV subcutaneously or a placebo. Based on serologic testing, study participants showed no evidence of current or previous SARS-CoV-2 infection. The median age of participants was 42.9, but 45% were male teenagers (ages 12-17).
In the group that received REGEN-COV, 11 out of 753 participants developed symptomatic COVID-19, compared with 59 out of 752 participants who received placebo. The relative risk reduction for the study’s 4-week period was 81.4% (P < .001). Of the participants that did develop a SARS-CoV-2 infection, those that received REGEN-COV were less likely to be symptomatic. Asymptomatic infections developed in 25 participants who received REGEN-COV versus 48 in the placebo group. The relative risk of developing any SARS-CoV-2 infection, symptomatic or asymptomatic, was reduced by 66.4% with REGEN-COV (P < .001).
Among the patients who were symptomatic, symptoms subsided within a median of 1.2 weeks for the group that received REGEN-COV, 2 weeks earlier than the placebo group. These patients also had a shorter duration of a high viral load (>104 copies/mL). Few adverse events were reported in the treatment or placebo groups. Monoclonal antibodies “seem to be incredibly safe,” Dr. Wohl said.
“These monoclonal antibodies have proven they can reduce the viral replication in the nose,” said study author Myron Cohen, MD, an infectious disease specialist and professor of epidemiology at the University of North Carolina.
The Food and Drug Administration first granted REGEN-COV emergency use authorization (EUA) in November 2020 for use in patients with mild or moderate COVID-19 who were also at high risk for progressing to severe COVID-19. At that time, the cocktail of monoclonal antibodies was delivered by a single intravenous infusion.
In January, Regeneron first announced the success of this trial of the subcutaneous injection for exposed household contacts based on early results, and in June of 2021, the FDA expanded the EUA to include a subcutaneous delivery when IV is not feasible. On July 30, the EUA was expanded again to include prophylactic use in exposed patients based on these trial results.
The U.S. government has purchased approximately 1.5 million doses of REGEN-COV from Regeneron and has agreed to make the treatments free of charge to patients.
But despite being free, available, and backed by promising data, monoclonal antibodies as a therapeutic answer to COVID-19 still hasn’t really taken off. “The problem is, it first requires knowledge and awareness,” Dr. Wohl said. “A lot [of people] don’t know this exists. To be honest, vaccination has taken up all the oxygen in the room.”
Dr. Cohen agreed. One reason for the slow uptake may be because the drug supply is owned by the government and not a pharmaceutical company. There hasn’t been a typical marketing push to make physicians and consumers aware. Additionally, “the logistics are daunting,” Dr. Cohen said. The office spaces where many physicians care for patients “often aren’t appropriate for patients who think they have SARS-CoV-2.”
“Right now, there’s not a mechanism” to administer the drug to people who could benefit from it, Dr. Wohl said. Eligible patients are either immunocompromised and unlikely to mount a sufficient immune response with vaccination, or not fully vaccinated. They should have been exposed to an infected individual or have a high likelihood of exposure due to where they live, such as in a prison or nursing home. Local doctors are unlikely to be the primary administrators of the drug, Dr. Wohl added. “How do we operationalize this for people who fit the criteria?”
There’s also an issue of timing. REGEN-COV is most effective when given early, Dr. Cohen said. “[Monoclonal antibodies] really only work well in the replication phase.” Many patients who would be eligible delay care until they’ve had symptoms for several days, when REGEN-COV would no longer have the desired effect.
Eventually, Dr. Wohl suspects demand will increase when people realize REGEN-COV can help those with COVID-19 and those who have been exposed. But before then, “we do have to think about how to integrate this into a workflow people can access without being confused.”
The trial was done before there was widespread vaccination, so it’s unclear what the results mean for people who have been vaccinated. Dr. Cohen and Dr. Wohl said there are ongoing conversations about whether monoclonal antibodies could be complementary to vaccination and if there’s potential for continued monthly use of these therapies.
Cohen and Wohl reported no relevant financial relationships. The trial was supported by Regeneron Pharmaceuticals, F. Hoffmann–La Roche, the National Institute of Allergy and Infectious Diseases, NIH, and the COVID-19 Prevention Network.
A version of this article first appeared on Medscape.com.
according to results of a randomized, double-blind, placebo-controlled clinical trial published online August 4, 2021, in the New England Journal of Medicine.
The cocktail of the monoclonal antibodies casirivimab and imdevimab (REGEN-COV, Regeneron Pharmaceuticals) reduced participants’ relative risk of infection by 72%, compared with placebo within the first week. After the first week, risk reduction increased to 93%.
“Long after you would be exposed by your household, there is an enduring effect that prevents you from community spread,” said David Wohl, MD, professor of medicine in the division of infectious diseases at the University of North Carolina at Chapel Hill, who was a site investigator for the trial but not a study author.
Participants were enrolled within 96 hours after someone in their household tested positive for SARS-CoV-2. Participants were randomly assigned to receive 1,200 mg of REGEN-COV subcutaneously or a placebo. Based on serologic testing, study participants showed no evidence of current or previous SARS-CoV-2 infection. The median age of participants was 42.9, but 45% were male teenagers (ages 12-17).
In the group that received REGEN-COV, 11 out of 753 participants developed symptomatic COVID-19, compared with 59 out of 752 participants who received placebo. The relative risk reduction for the study’s 4-week period was 81.4% (P < .001). Of the participants that did develop a SARS-CoV-2 infection, those that received REGEN-COV were less likely to be symptomatic. Asymptomatic infections developed in 25 participants who received REGEN-COV versus 48 in the placebo group. The relative risk of developing any SARS-CoV-2 infection, symptomatic or asymptomatic, was reduced by 66.4% with REGEN-COV (P < .001).
Among the patients who were symptomatic, symptoms subsided within a median of 1.2 weeks for the group that received REGEN-COV, 2 weeks earlier than the placebo group. These patients also had a shorter duration of a high viral load (>104 copies/mL). Few adverse events were reported in the treatment or placebo groups. Monoclonal antibodies “seem to be incredibly safe,” Dr. Wohl said.
“These monoclonal antibodies have proven they can reduce the viral replication in the nose,” said study author Myron Cohen, MD, an infectious disease specialist and professor of epidemiology at the University of North Carolina.
The Food and Drug Administration first granted REGEN-COV emergency use authorization (EUA) in November 2020 for use in patients with mild or moderate COVID-19 who were also at high risk for progressing to severe COVID-19. At that time, the cocktail of monoclonal antibodies was delivered by a single intravenous infusion.
In January, Regeneron first announced the success of this trial of the subcutaneous injection for exposed household contacts based on early results, and in June of 2021, the FDA expanded the EUA to include a subcutaneous delivery when IV is not feasible. On July 30, the EUA was expanded again to include prophylactic use in exposed patients based on these trial results.
The U.S. government has purchased approximately 1.5 million doses of REGEN-COV from Regeneron and has agreed to make the treatments free of charge to patients.
But despite being free, available, and backed by promising data, monoclonal antibodies as a therapeutic answer to COVID-19 still hasn’t really taken off. “The problem is, it first requires knowledge and awareness,” Dr. Wohl said. “A lot [of people] don’t know this exists. To be honest, vaccination has taken up all the oxygen in the room.”
Dr. Cohen agreed. One reason for the slow uptake may be because the drug supply is owned by the government and not a pharmaceutical company. There hasn’t been a typical marketing push to make physicians and consumers aware. Additionally, “the logistics are daunting,” Dr. Cohen said. The office spaces where many physicians care for patients “often aren’t appropriate for patients who think they have SARS-CoV-2.”
“Right now, there’s not a mechanism” to administer the drug to people who could benefit from it, Dr. Wohl said. Eligible patients are either immunocompromised and unlikely to mount a sufficient immune response with vaccination, or not fully vaccinated. They should have been exposed to an infected individual or have a high likelihood of exposure due to where they live, such as in a prison or nursing home. Local doctors are unlikely to be the primary administrators of the drug, Dr. Wohl added. “How do we operationalize this for people who fit the criteria?”
There’s also an issue of timing. REGEN-COV is most effective when given early, Dr. Cohen said. “[Monoclonal antibodies] really only work well in the replication phase.” Many patients who would be eligible delay care until they’ve had symptoms for several days, when REGEN-COV would no longer have the desired effect.
Eventually, Dr. Wohl suspects demand will increase when people realize REGEN-COV can help those with COVID-19 and those who have been exposed. But before then, “we do have to think about how to integrate this into a workflow people can access without being confused.”
The trial was done before there was widespread vaccination, so it’s unclear what the results mean for people who have been vaccinated. Dr. Cohen and Dr. Wohl said there are ongoing conversations about whether monoclonal antibodies could be complementary to vaccination and if there’s potential for continued monthly use of these therapies.
Cohen and Wohl reported no relevant financial relationships. The trial was supported by Regeneron Pharmaceuticals, F. Hoffmann–La Roche, the National Institute of Allergy and Infectious Diseases, NIH, and the COVID-19 Prevention Network.
A version of this article first appeared on Medscape.com.
Moderna says boosters may be needed after 6 months
Moderna says neutralizing antibodies generated by its COVID-19 vaccine against three variants of the virus that causes the disease waned substantially 6 months after the second dose.
Because of this, the company expects an increase in breakthrough infections with a need for boosters before winter.
In an experiment, a 50-mg dose of the vaccine, given as a third shot, boosted levels of antibodies in 20 previously vaccinated people by 32 times against the Beta variant, by 44 times against the Gamma variant, and by 42 times against Delta.
The new data was presented in an earnings call to investors and is based on a small study that hasn’t yet been published in medical literature.
The company also said its vaccine remained highly effective at preventing severe COVID outcomes through 6 months.
Last week, Pfizer released early data suggesting a similar drop in protection from its vaccine. The company also showed a third dose substantially boosted protection, including against the Delta variant.
The new results come just 1 day after the World Health Organization implored wealthy nations to hold off on third doses until more of the world’s population could get a first dose.
More than 80% of the 4 billion vaccine doses given around the world have been distributed to high-income countries.
A version of this article first appeared on WebMD.com.
Moderna says neutralizing antibodies generated by its COVID-19 vaccine against three variants of the virus that causes the disease waned substantially 6 months after the second dose.
Because of this, the company expects an increase in breakthrough infections with a need for boosters before winter.
In an experiment, a 50-mg dose of the vaccine, given as a third shot, boosted levels of antibodies in 20 previously vaccinated people by 32 times against the Beta variant, by 44 times against the Gamma variant, and by 42 times against Delta.
The new data was presented in an earnings call to investors and is based on a small study that hasn’t yet been published in medical literature.
The company also said its vaccine remained highly effective at preventing severe COVID outcomes through 6 months.
Last week, Pfizer released early data suggesting a similar drop in protection from its vaccine. The company also showed a third dose substantially boosted protection, including against the Delta variant.
The new results come just 1 day after the World Health Organization implored wealthy nations to hold off on third doses until more of the world’s population could get a first dose.
More than 80% of the 4 billion vaccine doses given around the world have been distributed to high-income countries.
A version of this article first appeared on WebMD.com.
Moderna says neutralizing antibodies generated by its COVID-19 vaccine against three variants of the virus that causes the disease waned substantially 6 months after the second dose.
Because of this, the company expects an increase in breakthrough infections with a need for boosters before winter.
In an experiment, a 50-mg dose of the vaccine, given as a third shot, boosted levels of antibodies in 20 previously vaccinated people by 32 times against the Beta variant, by 44 times against the Gamma variant, and by 42 times against Delta.
The new data was presented in an earnings call to investors and is based on a small study that hasn’t yet been published in medical literature.
The company also said its vaccine remained highly effective at preventing severe COVID outcomes through 6 months.
Last week, Pfizer released early data suggesting a similar drop in protection from its vaccine. The company also showed a third dose substantially boosted protection, including against the Delta variant.
The new results come just 1 day after the World Health Organization implored wealthy nations to hold off on third doses until more of the world’s population could get a first dose.
More than 80% of the 4 billion vaccine doses given around the world have been distributed to high-income countries.
A version of this article first appeared on WebMD.com.
New guideline for replacement ART: CAB/RPV LA not for everyone
“One of the most important considerations before switching HIV patients to injectable long-acting cabotegravir/rilpivirine [CAB/RPV LA; Cabenuva, ViiV Healthcare] is for the patient and the clinician to arrive at this decision together,” Elliot DeHaan, MD, told this news organization. “This therapy is not necessarily for everyone.”
Dr. DeHaan is lead author of the newly released clinical guideline from the New York State Department of Health AIDS Institute for use of CAB/RPV LA as replacement antiretroviral therapy (ART) in virally suppressed adults with HIV. He explained that the guidance expands upon Health & Human Services’ Feb. 24 CAB/RPV LA recommendations, highlighting some of the most important clinical and patient considerations necessary to implement injectable ART. “There are a lot of things that need to be laid out beforehand,” he said.
Gaining consensus
Approved by the FDA in late January 2021, CAB/RPV LA is considered an optimization strategy for individuals with HIV whose virus is suppressed by oral ART and who might prefer monthly injections to daily oral therapy. While there are various reasons why patients might wish to switch to a long-acting injectable, one of the primary concerns is adherence. Of note, the guidance points to phase 3 clinical study findings that suggest high levels (86%-91%) of patient satisfaction with CAB/RPV LA, which portends a promising future for this therapeutic approach.
With regard to patient preference, recommendations focus on the need to thoroughly discuss several critical requisites with potential candidates, including a 4-week lead-in daily oral ART course (CAB [Vocabria] 30 mg, RPV [Edurant] 25 mg) before initiating a loading dose. Patients should be advised of the potential for development of resistance should dosing be interrupted for any reason (CAB and RPV have extended half-lives ranging from mean 5.6 to 11.5 weeks for CAB and 13 to 28 weeks for RPV), as well as the need to return to oral bridging therapy if subsequent injections are not administered within the 7-day window period. If the maintenance dose is delayed beyond 2 months, a loading dose and restart is necessary.
CAB/RPV LA therapy is administered into opposing gluteal muscles (CAB into one gluteus medius and RPV into the contralateral gluteus medius), and injection-site pain beginning 1 day post-injection and lasting 3-4 days is common. In phase 3 clinical trials, as many as 83% of patients experienced adverse effects (AEs), which also include nodules, induration, and swelling at the injection sites. Fortunately, 99% of AEs were of mild to moderate severity. While pain tends to decline over several injections, Dr. DeHaan said that it’s an important part of the initial discussion about switching therapies.
Other considerations
Prior resistance testing, ART treatment history, and/or baseline genotypic resistance testing that includes both reverse transcriptase and integrase genes should be reviewed or conducted before initiating treatment. K103 mutations alone are not considered exclusionary. Virologic failures (defined as two consecutive plasma HIV-1 RNA measurements greater than 200 copies/mL), while rare, were reported in 13 clinical trial participants. Recent data suggest that patients who developed resistance despite adherence had at least two of three factors: a body mass index greater than 30 kg/m2, the HIV-1 subtype A6/A1, and the presence of proviral RPV RAMS.
CAB/RPV LA does not treat hepatitis B (HBV) coinfections, reinforcing the need for concurrent oral HBV therapy.
And there’s a paucity of data on the safety and efficacy of CAB/RPV in children and adolescents, or during pregnancy/lactation, precluding its use in those patient populations.
Clinical, institutional considerations
Adaptation of CAB/RPV LA as ART requires specific clinical institutional planning, especially in light of current pandemic-related resource and staffing limitations. Monthly dosing must be done within a 7-day window and requires preparations akin to initial loading doses. In addition to pharmacy resources and onsite storage requirements, the guidance points to patient scheduling and reminder systems, access (patient transportation, work constraints, parking), and most importantly, contingency plans for care (including oral bridging therapy) should a clinic be forced to shut down for any reason. Additional factors include billing protocols, insurance or third party authorizations, and provision of counseling and education training.
“Given that this is a completely new way of thinking among providers, we’re all learning together,” said David Koren, PharmD, MPH, a clinical pharmacy specialist in infectious diseases at Temple University, Philadelphia. “The guidelines provide a nice framework for taking the next step to operationalize these processes into practice, taking into account that barriers to implementation are still unknown.” Dr. Koren was not involved in the development of the guidelines.
Dr. DeHaan has disclosed no relevant financial relationships. Dr. Koren disclosed serving on a prior Advisory Panel for ViiV Healthcare US.
A version of this article first appeared on Medscape.com.
“One of the most important considerations before switching HIV patients to injectable long-acting cabotegravir/rilpivirine [CAB/RPV LA; Cabenuva, ViiV Healthcare] is for the patient and the clinician to arrive at this decision together,” Elliot DeHaan, MD, told this news organization. “This therapy is not necessarily for everyone.”
Dr. DeHaan is lead author of the newly released clinical guideline from the New York State Department of Health AIDS Institute for use of CAB/RPV LA as replacement antiretroviral therapy (ART) in virally suppressed adults with HIV. He explained that the guidance expands upon Health & Human Services’ Feb. 24 CAB/RPV LA recommendations, highlighting some of the most important clinical and patient considerations necessary to implement injectable ART. “There are a lot of things that need to be laid out beforehand,” he said.
Gaining consensus
Approved by the FDA in late January 2021, CAB/RPV LA is considered an optimization strategy for individuals with HIV whose virus is suppressed by oral ART and who might prefer monthly injections to daily oral therapy. While there are various reasons why patients might wish to switch to a long-acting injectable, one of the primary concerns is adherence. Of note, the guidance points to phase 3 clinical study findings that suggest high levels (86%-91%) of patient satisfaction with CAB/RPV LA, which portends a promising future for this therapeutic approach.
With regard to patient preference, recommendations focus on the need to thoroughly discuss several critical requisites with potential candidates, including a 4-week lead-in daily oral ART course (CAB [Vocabria] 30 mg, RPV [Edurant] 25 mg) before initiating a loading dose. Patients should be advised of the potential for development of resistance should dosing be interrupted for any reason (CAB and RPV have extended half-lives ranging from mean 5.6 to 11.5 weeks for CAB and 13 to 28 weeks for RPV), as well as the need to return to oral bridging therapy if subsequent injections are not administered within the 7-day window period. If the maintenance dose is delayed beyond 2 months, a loading dose and restart is necessary.
CAB/RPV LA therapy is administered into opposing gluteal muscles (CAB into one gluteus medius and RPV into the contralateral gluteus medius), and injection-site pain beginning 1 day post-injection and lasting 3-4 days is common. In phase 3 clinical trials, as many as 83% of patients experienced adverse effects (AEs), which also include nodules, induration, and swelling at the injection sites. Fortunately, 99% of AEs were of mild to moderate severity. While pain tends to decline over several injections, Dr. DeHaan said that it’s an important part of the initial discussion about switching therapies.
Other considerations
Prior resistance testing, ART treatment history, and/or baseline genotypic resistance testing that includes both reverse transcriptase and integrase genes should be reviewed or conducted before initiating treatment. K103 mutations alone are not considered exclusionary. Virologic failures (defined as two consecutive plasma HIV-1 RNA measurements greater than 200 copies/mL), while rare, were reported in 13 clinical trial participants. Recent data suggest that patients who developed resistance despite adherence had at least two of three factors: a body mass index greater than 30 kg/m2, the HIV-1 subtype A6/A1, and the presence of proviral RPV RAMS.
CAB/RPV LA does not treat hepatitis B (HBV) coinfections, reinforcing the need for concurrent oral HBV therapy.
And there’s a paucity of data on the safety and efficacy of CAB/RPV in children and adolescents, or during pregnancy/lactation, precluding its use in those patient populations.
Clinical, institutional considerations
Adaptation of CAB/RPV LA as ART requires specific clinical institutional planning, especially in light of current pandemic-related resource and staffing limitations. Monthly dosing must be done within a 7-day window and requires preparations akin to initial loading doses. In addition to pharmacy resources and onsite storage requirements, the guidance points to patient scheduling and reminder systems, access (patient transportation, work constraints, parking), and most importantly, contingency plans for care (including oral bridging therapy) should a clinic be forced to shut down for any reason. Additional factors include billing protocols, insurance or third party authorizations, and provision of counseling and education training.
“Given that this is a completely new way of thinking among providers, we’re all learning together,” said David Koren, PharmD, MPH, a clinical pharmacy specialist in infectious diseases at Temple University, Philadelphia. “The guidelines provide a nice framework for taking the next step to operationalize these processes into practice, taking into account that barriers to implementation are still unknown.” Dr. Koren was not involved in the development of the guidelines.
Dr. DeHaan has disclosed no relevant financial relationships. Dr. Koren disclosed serving on a prior Advisory Panel for ViiV Healthcare US.
A version of this article first appeared on Medscape.com.
“One of the most important considerations before switching HIV patients to injectable long-acting cabotegravir/rilpivirine [CAB/RPV LA; Cabenuva, ViiV Healthcare] is for the patient and the clinician to arrive at this decision together,” Elliot DeHaan, MD, told this news organization. “This therapy is not necessarily for everyone.”
Dr. DeHaan is lead author of the newly released clinical guideline from the New York State Department of Health AIDS Institute for use of CAB/RPV LA as replacement antiretroviral therapy (ART) in virally suppressed adults with HIV. He explained that the guidance expands upon Health & Human Services’ Feb. 24 CAB/RPV LA recommendations, highlighting some of the most important clinical and patient considerations necessary to implement injectable ART. “There are a lot of things that need to be laid out beforehand,” he said.
Gaining consensus
Approved by the FDA in late January 2021, CAB/RPV LA is considered an optimization strategy for individuals with HIV whose virus is suppressed by oral ART and who might prefer monthly injections to daily oral therapy. While there are various reasons why patients might wish to switch to a long-acting injectable, one of the primary concerns is adherence. Of note, the guidance points to phase 3 clinical study findings that suggest high levels (86%-91%) of patient satisfaction with CAB/RPV LA, which portends a promising future for this therapeutic approach.
With regard to patient preference, recommendations focus on the need to thoroughly discuss several critical requisites with potential candidates, including a 4-week lead-in daily oral ART course (CAB [Vocabria] 30 mg, RPV [Edurant] 25 mg) before initiating a loading dose. Patients should be advised of the potential for development of resistance should dosing be interrupted for any reason (CAB and RPV have extended half-lives ranging from mean 5.6 to 11.5 weeks for CAB and 13 to 28 weeks for RPV), as well as the need to return to oral bridging therapy if subsequent injections are not administered within the 7-day window period. If the maintenance dose is delayed beyond 2 months, a loading dose and restart is necessary.
CAB/RPV LA therapy is administered into opposing gluteal muscles (CAB into one gluteus medius and RPV into the contralateral gluteus medius), and injection-site pain beginning 1 day post-injection and lasting 3-4 days is common. In phase 3 clinical trials, as many as 83% of patients experienced adverse effects (AEs), which also include nodules, induration, and swelling at the injection sites. Fortunately, 99% of AEs were of mild to moderate severity. While pain tends to decline over several injections, Dr. DeHaan said that it’s an important part of the initial discussion about switching therapies.
Other considerations
Prior resistance testing, ART treatment history, and/or baseline genotypic resistance testing that includes both reverse transcriptase and integrase genes should be reviewed or conducted before initiating treatment. K103 mutations alone are not considered exclusionary. Virologic failures (defined as two consecutive plasma HIV-1 RNA measurements greater than 200 copies/mL), while rare, were reported in 13 clinical trial participants. Recent data suggest that patients who developed resistance despite adherence had at least two of three factors: a body mass index greater than 30 kg/m2, the HIV-1 subtype A6/A1, and the presence of proviral RPV RAMS.
CAB/RPV LA does not treat hepatitis B (HBV) coinfections, reinforcing the need for concurrent oral HBV therapy.
And there’s a paucity of data on the safety and efficacy of CAB/RPV in children and adolescents, or during pregnancy/lactation, precluding its use in those patient populations.
Clinical, institutional considerations
Adaptation of CAB/RPV LA as ART requires specific clinical institutional planning, especially in light of current pandemic-related resource and staffing limitations. Monthly dosing must be done within a 7-day window and requires preparations akin to initial loading doses. In addition to pharmacy resources and onsite storage requirements, the guidance points to patient scheduling and reminder systems, access (patient transportation, work constraints, parking), and most importantly, contingency plans for care (including oral bridging therapy) should a clinic be forced to shut down for any reason. Additional factors include billing protocols, insurance or third party authorizations, and provision of counseling and education training.
“Given that this is a completely new way of thinking among providers, we’re all learning together,” said David Koren, PharmD, MPH, a clinical pharmacy specialist in infectious diseases at Temple University, Philadelphia. “The guidelines provide a nice framework for taking the next step to operationalize these processes into practice, taking into account that barriers to implementation are still unknown.” Dr. Koren was not involved in the development of the guidelines.
Dr. DeHaan has disclosed no relevant financial relationships. Dr. Koren disclosed serving on a prior Advisory Panel for ViiV Healthcare US.
A version of this article first appeared on Medscape.com.
Treating bioterrorism-related plague: CDC issues new guidelines
The Centers for Disease Control has issued the first recommendations for the prevention and treatment of plague since 2000. The new guidelines focus on the possibility of bioterrorism with mass casualty events from an intentional release of Yersinia pestis.
Plague, a deadly infection caused by Y. pestis, has been feared throughout history because of large pandemics. The most well-known pandemic was the so-called Black Death in the fourteenth century, during which more than 50 million Europeans died. The biggest concern now is the spread of the bacteria by bioterrorism.
The CDC based their revised guidelines on an extensive systematic review of the literature and multiple sessions with about 90 experts in infectious disease, public health, emergency medicine, obgyn, maternal-fetal health, and pediatrics, in addition to representatives from a wide range of federal agencies.
Key changes
Christina Nelson, a medical officer with the CDC’s Division of Vector-Borne Diseases, told this news organization that now “we have been fortunate to have extended options for treatment.” Previously, “streptomycin and gentamicin were the first-line options for adults,” she said. Now, on the basis of additional evidence, “[we’re] able to … elevate the fluoroquinolones to first-line treatments.”
On the basis of the Animal Rule, which allows approval of antibiotics without human testing if such testing is not possible, the U.S. Food and Drug Administration has approved several quinolones for both treatment and prophylaxis of plague.
The guidelines offer same-class alternative antibiotics to meet surge capacity. Similarly, trimethoprim-sulfamethoxazole is now an alternative for prophylaxis.
There are additional oral options to conserve IV medications and supplies in a mass casualty event.
For the first time, the CDC added specific recommendations for pregnant women. Gigi Kwik Gronvall, PhD, senior scholar at the Johns Hopkins Center for Health Security, Baltimore, told this news organization that she was pleased to see this addition, because “effects on women and during pregnancy are not fully addressed, and it leads to problems down the road, like with COVID, [for which] they didn’t include pregnant people in their clinical trials for the vaccines [and] don’t have enough data to convince pregnant women to actually get the vaccine.”
Bubonic plague
Plague occurs globally, with natural sylvatic (wild animal) outbreaks occurring among rodents and small mammals. It is spread by fleas. When an infected flea bites a human, the person can become infected, most commonly as “bubonic” plague, with swollen lymph nodes, called buboes. Transmission can also occur between people by contact with infected fluids or inhalation of infectious droplets.
Gentamicin or streptomycin remain first-line agents for treating bubonic plague. When used as monotherapy, the survival rate is 91%. They have to be given parenterally and are associated with both nephroroxicity and ototoxicity; patients require monitoring.
Alternative first-line drugs now include high-dose ciprofloxicin, levofloxacin, moxifloxacin, and doxycycline. Each is administered either intravenously or orally.
Physicians should consider dual therapy and drainage for patients with large buboes. Treatment is for 10 to 14 days.
Pneumonic and septicemic plague
The pneumonic and septicemic forms of infection are deadlier than the bubonic. Pneumonic plague can be acquired from inhalation of infected bacteria from animals or people, from lab accidents, or from intentional aerosolization. Without treatment, these forms are almost always fatal. With treatment with aminoglycosides, fluoroquinolones, or tetracyclines, alone or in combination, survival is 82% to 83%. With naturally occurring pneumonic plague, the CDC now recommends levofloxacin or moxifloxacin to cover for community-acquired pneumonia if the source of the infection is uncertain.
Because plague is life threatening, doxycycline is not considered contraindicated in children. It has not been shown to cause tooth staining, unlike other tetracyclines, which should still be avoided if possible.
Meningitis
About 10% of people infected with bubonic plague develop plague meningitis. Symptoms are stiff neck, fever, headache, and coma. The current recommendation for treating plague meningitis is chloramphenicol and moxifloxacin or levofloxacin. However, quinolones can cause seizures, and clinicians should take that into account.
Infection control
Plague is transmitted between people by droplets, so caretakers should wear a mask in addition to taking standard precautions. They should add eye protection and a face shield if splashing is likely. Airborne precautions are not needed. Plague is not very transmissible from person to person; each infected person on average infects only 1.18 other people. In comparison, someone with chicken pox infects 9 to 10 people on average.
Bioterrorism
A deliberate attack would likely go undetected until a cluster or unusual pattern of disease became evident. With Y. pestis, the infectious dose is low. According to the guidelines, modeling suggests that a “release of 50 kg of Y. pestis into the air over a city of 5 million persons could result in 150,000 cases of pneumonic plague and 36,000 deaths.”
Because the former Union of the Soviet Socialist Republics (USSR) engineered antibiotic-resistant Y. pestis, antibiotics from two different classes should be used empirically until sensitivity tests become available.
Antibiotic prophylaxis would also have to be considered for exposed individuals. Recommendations would be developed at the time by federal and state experts, based in part on the magnitude of the event and the availability of masks and different classes of antibiotics.
Dr. Gronvall stressed the need for awareness, saying, “It’s important for people to remember that the first sign of the potential attack could be somebody coming into your hospital.”
Dr. Nelson added, “One of the main take-home messages ... is that plague still happens, it still happens in the western United States, it still happens around the world ... It’s not just a relic of history.” She emphasized that clinicians need to be thinking about it, because “it’s very important to get antibiotics on board early ... Then patients generally have a good prognosis.”
Dr. Nelson and Dr. Gronvall have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control has issued the first recommendations for the prevention and treatment of plague since 2000. The new guidelines focus on the possibility of bioterrorism with mass casualty events from an intentional release of Yersinia pestis.
Plague, a deadly infection caused by Y. pestis, has been feared throughout history because of large pandemics. The most well-known pandemic was the so-called Black Death in the fourteenth century, during which more than 50 million Europeans died. The biggest concern now is the spread of the bacteria by bioterrorism.
The CDC based their revised guidelines on an extensive systematic review of the literature and multiple sessions with about 90 experts in infectious disease, public health, emergency medicine, obgyn, maternal-fetal health, and pediatrics, in addition to representatives from a wide range of federal agencies.
Key changes
Christina Nelson, a medical officer with the CDC’s Division of Vector-Borne Diseases, told this news organization that now “we have been fortunate to have extended options for treatment.” Previously, “streptomycin and gentamicin were the first-line options for adults,” she said. Now, on the basis of additional evidence, “[we’re] able to … elevate the fluoroquinolones to first-line treatments.”
On the basis of the Animal Rule, which allows approval of antibiotics without human testing if such testing is not possible, the U.S. Food and Drug Administration has approved several quinolones for both treatment and prophylaxis of plague.
The guidelines offer same-class alternative antibiotics to meet surge capacity. Similarly, trimethoprim-sulfamethoxazole is now an alternative for prophylaxis.
There are additional oral options to conserve IV medications and supplies in a mass casualty event.
For the first time, the CDC added specific recommendations for pregnant women. Gigi Kwik Gronvall, PhD, senior scholar at the Johns Hopkins Center for Health Security, Baltimore, told this news organization that she was pleased to see this addition, because “effects on women and during pregnancy are not fully addressed, and it leads to problems down the road, like with COVID, [for which] they didn’t include pregnant people in their clinical trials for the vaccines [and] don’t have enough data to convince pregnant women to actually get the vaccine.”
Bubonic plague
Plague occurs globally, with natural sylvatic (wild animal) outbreaks occurring among rodents and small mammals. It is spread by fleas. When an infected flea bites a human, the person can become infected, most commonly as “bubonic” plague, with swollen lymph nodes, called buboes. Transmission can also occur between people by contact with infected fluids or inhalation of infectious droplets.
Gentamicin or streptomycin remain first-line agents for treating bubonic plague. When used as monotherapy, the survival rate is 91%. They have to be given parenterally and are associated with both nephroroxicity and ototoxicity; patients require monitoring.
Alternative first-line drugs now include high-dose ciprofloxicin, levofloxacin, moxifloxacin, and doxycycline. Each is administered either intravenously or orally.
Physicians should consider dual therapy and drainage for patients with large buboes. Treatment is for 10 to 14 days.
Pneumonic and septicemic plague
The pneumonic and septicemic forms of infection are deadlier than the bubonic. Pneumonic plague can be acquired from inhalation of infected bacteria from animals or people, from lab accidents, or from intentional aerosolization. Without treatment, these forms are almost always fatal. With treatment with aminoglycosides, fluoroquinolones, or tetracyclines, alone or in combination, survival is 82% to 83%. With naturally occurring pneumonic plague, the CDC now recommends levofloxacin or moxifloxacin to cover for community-acquired pneumonia if the source of the infection is uncertain.
Because plague is life threatening, doxycycline is not considered contraindicated in children. It has not been shown to cause tooth staining, unlike other tetracyclines, which should still be avoided if possible.
Meningitis
About 10% of people infected with bubonic plague develop plague meningitis. Symptoms are stiff neck, fever, headache, and coma. The current recommendation for treating plague meningitis is chloramphenicol and moxifloxacin or levofloxacin. However, quinolones can cause seizures, and clinicians should take that into account.
Infection control
Plague is transmitted between people by droplets, so caretakers should wear a mask in addition to taking standard precautions. They should add eye protection and a face shield if splashing is likely. Airborne precautions are not needed. Plague is not very transmissible from person to person; each infected person on average infects only 1.18 other people. In comparison, someone with chicken pox infects 9 to 10 people on average.
Bioterrorism
A deliberate attack would likely go undetected until a cluster or unusual pattern of disease became evident. With Y. pestis, the infectious dose is low. According to the guidelines, modeling suggests that a “release of 50 kg of Y. pestis into the air over a city of 5 million persons could result in 150,000 cases of pneumonic plague and 36,000 deaths.”
Because the former Union of the Soviet Socialist Republics (USSR) engineered antibiotic-resistant Y. pestis, antibiotics from two different classes should be used empirically until sensitivity tests become available.
Antibiotic prophylaxis would also have to be considered for exposed individuals. Recommendations would be developed at the time by federal and state experts, based in part on the magnitude of the event and the availability of masks and different classes of antibiotics.
Dr. Gronvall stressed the need for awareness, saying, “It’s important for people to remember that the first sign of the potential attack could be somebody coming into your hospital.”
Dr. Nelson added, “One of the main take-home messages ... is that plague still happens, it still happens in the western United States, it still happens around the world ... It’s not just a relic of history.” She emphasized that clinicians need to be thinking about it, because “it’s very important to get antibiotics on board early ... Then patients generally have a good prognosis.”
Dr. Nelson and Dr. Gronvall have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control has issued the first recommendations for the prevention and treatment of plague since 2000. The new guidelines focus on the possibility of bioterrorism with mass casualty events from an intentional release of Yersinia pestis.
Plague, a deadly infection caused by Y. pestis, has been feared throughout history because of large pandemics. The most well-known pandemic was the so-called Black Death in the fourteenth century, during which more than 50 million Europeans died. The biggest concern now is the spread of the bacteria by bioterrorism.
The CDC based their revised guidelines on an extensive systematic review of the literature and multiple sessions with about 90 experts in infectious disease, public health, emergency medicine, obgyn, maternal-fetal health, and pediatrics, in addition to representatives from a wide range of federal agencies.
Key changes
Christina Nelson, a medical officer with the CDC’s Division of Vector-Borne Diseases, told this news organization that now “we have been fortunate to have extended options for treatment.” Previously, “streptomycin and gentamicin were the first-line options for adults,” she said. Now, on the basis of additional evidence, “[we’re] able to … elevate the fluoroquinolones to first-line treatments.”
On the basis of the Animal Rule, which allows approval of antibiotics without human testing if such testing is not possible, the U.S. Food and Drug Administration has approved several quinolones for both treatment and prophylaxis of plague.
The guidelines offer same-class alternative antibiotics to meet surge capacity. Similarly, trimethoprim-sulfamethoxazole is now an alternative for prophylaxis.
There are additional oral options to conserve IV medications and supplies in a mass casualty event.
For the first time, the CDC added specific recommendations for pregnant women. Gigi Kwik Gronvall, PhD, senior scholar at the Johns Hopkins Center for Health Security, Baltimore, told this news organization that she was pleased to see this addition, because “effects on women and during pregnancy are not fully addressed, and it leads to problems down the road, like with COVID, [for which] they didn’t include pregnant people in their clinical trials for the vaccines [and] don’t have enough data to convince pregnant women to actually get the vaccine.”
Bubonic plague
Plague occurs globally, with natural sylvatic (wild animal) outbreaks occurring among rodents and small mammals. It is spread by fleas. When an infected flea bites a human, the person can become infected, most commonly as “bubonic” plague, with swollen lymph nodes, called buboes. Transmission can also occur between people by contact with infected fluids or inhalation of infectious droplets.
Gentamicin or streptomycin remain first-line agents for treating bubonic plague. When used as monotherapy, the survival rate is 91%. They have to be given parenterally and are associated with both nephroroxicity and ototoxicity; patients require monitoring.
Alternative first-line drugs now include high-dose ciprofloxicin, levofloxacin, moxifloxacin, and doxycycline. Each is administered either intravenously or orally.
Physicians should consider dual therapy and drainage for patients with large buboes. Treatment is for 10 to 14 days.
Pneumonic and septicemic plague
The pneumonic and septicemic forms of infection are deadlier than the bubonic. Pneumonic plague can be acquired from inhalation of infected bacteria from animals or people, from lab accidents, or from intentional aerosolization. Without treatment, these forms are almost always fatal. With treatment with aminoglycosides, fluoroquinolones, or tetracyclines, alone or in combination, survival is 82% to 83%. With naturally occurring pneumonic plague, the CDC now recommends levofloxacin or moxifloxacin to cover for community-acquired pneumonia if the source of the infection is uncertain.
Because plague is life threatening, doxycycline is not considered contraindicated in children. It has not been shown to cause tooth staining, unlike other tetracyclines, which should still be avoided if possible.
Meningitis
About 10% of people infected with bubonic plague develop plague meningitis. Symptoms are stiff neck, fever, headache, and coma. The current recommendation for treating plague meningitis is chloramphenicol and moxifloxacin or levofloxacin. However, quinolones can cause seizures, and clinicians should take that into account.
Infection control
Plague is transmitted between people by droplets, so caretakers should wear a mask in addition to taking standard precautions. They should add eye protection and a face shield if splashing is likely. Airborne precautions are not needed. Plague is not very transmissible from person to person; each infected person on average infects only 1.18 other people. In comparison, someone with chicken pox infects 9 to 10 people on average.
Bioterrorism
A deliberate attack would likely go undetected until a cluster or unusual pattern of disease became evident. With Y. pestis, the infectious dose is low. According to the guidelines, modeling suggests that a “release of 50 kg of Y. pestis into the air over a city of 5 million persons could result in 150,000 cases of pneumonic plague and 36,000 deaths.”
Because the former Union of the Soviet Socialist Republics (USSR) engineered antibiotic-resistant Y. pestis, antibiotics from two different classes should be used empirically until sensitivity tests become available.
Antibiotic prophylaxis would also have to be considered for exposed individuals. Recommendations would be developed at the time by federal and state experts, based in part on the magnitude of the event and the availability of masks and different classes of antibiotics.
Dr. Gronvall stressed the need for awareness, saying, “It’s important for people to remember that the first sign of the potential attack could be somebody coming into your hospital.”
Dr. Nelson added, “One of the main take-home messages ... is that plague still happens, it still happens in the western United States, it still happens around the world ... It’s not just a relic of history.” She emphasized that clinicians need to be thinking about it, because “it’s very important to get antibiotics on board early ... Then patients generally have a good prognosis.”
Dr. Nelson and Dr. Gronvall have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Summer campers spread COVID at home, follow-up finds
In a report published online in The New England Journal of Medicine, researchers found that campers spread COVID to household members after returning home – but transmission was more likely from some than others. Distancing and masking helped reduce the risk.
Victoria T. Chu, MD, MPH, with the Centers for Disease Control and Prevention, Atlanta, and colleagues with the agency and the Georgia Department of Health followed up with 224 camp attendees, aged 7 to 19 years, who had evidence of SARS-CoV-2 infection on laboratory testing.
These index patients – 88% of whom had symptoms – had 526 household contacts, mainly parents and siblings. Of 377 household contacts who underwent testing, 46 (12%) tested positive. Another two cases in household contacts were identified using clinical and epidemiologic criteria.
Family members hospitalized
Of the 41 adult household contacts who were infected, four (about 10%) were hospitalized. Their hospital stays ranged from 5 to 11 days. Of the seven infected household contacts who were younger than 18 years, none were hospitalized.
The four hospitalized adults were parents and grandparents aged 45 to 80 years, Dr. Chu said. Two of the four had underlying conditions. None of the household contacts died.
In an adjusted analysis, campers who had practiced physical distancing were less likely to transmit the virus at home, compared with those who had not practiced physical distancing (adjusted odds ratio, 0.4). Household members who had had close or direct contact with the index patients were more than 5 times more likely to become infected, compared with family members with minimal or no contact, analyses showed.
“This retrospective study showed that the efficient transmission of SARS-CoV-2 from school-age children and adolescents to household members led to the hospitalization of adults with secondary cases of COVID-19,” the researchers write. “In households in which transmission occurred, half the household contacts were infected.”
The secondary attack rates in this report may be an underestimate because testing was voluntary and participants reported the results themselves, the authors note. It is possible that infected household contacts spread the virus further, but this study did not address that question, Dr. Chu said.
For the study, investigators interviewed all camp attendees and their parents or guardians by phone between July 17, 2020 and Aug. 24, 2020, to collect information about demographic and clinical characteristics, SARS-CoV-2 testing, and preventive measures. The researchers’ analysis excluded households in which illness onset in a household contact occurred before or less than 2 days after a camper became sick.
About a third of the index patients began to have symptoms while still at camp. These campers may have been less infectious by the time they got home, compared with those whose symptoms started after they returned.
Two-thirds of the index patients adopted physical distancing at home, which “probably reduced the transmission of SARS-CoV-2 in the household,” Dr. Chu and colleagues wrote.
“Children who have had a known COVID-19 exposure should quarantine and obtain testing if they develop symptoms within the 14 days of returning home,” Dr. Chu advised. “If a child develops COVID-19, the child should be cared for and monitored using the proper combination of physical distancing, isolation when feasible, and mask use to prevent household transmission as much as possible. In addition, any person over the age of 12 is now eligible for vaccination in the United States. If eligible, children attending camp and their family members should get vaccinated to protect themselves and others, as vaccinations are our most effective public health prevention strategy.”
Mitigation can help
Another report regarding four overnight camps in Maine – in which three campers tested positive after they arrived last summer – shows that “aggressive mitigation strategies can be effective” in limiting transmission of the virus, William T. Basco Jr., MD, writes in a commentary for this news organization.
This summer, a range of factors, including vaccination rates at the camp, may influence transmission dynamics, Dr. Chu said in an interview. In July, the Associated Press reported outbreaks tied to summer camps in several states.
“Transmission dynamics will probably vary from summer camp to summer camp depending on many factors, such as vaccination rates of camp attendees, the mitigation measures in place, and the number of individual introductions during camp,” Dr. Chu said. “We would expect that a camp with a low vaccination rate among attendees and no enforcement of mitigation measures” still may experience a large outbreak.
“On the other hand, a large proportion of vaccinated individuals and appropriate implementation of multiple mitigation measures, such as wearing masks, may be quite effective at keeping their transmission rates low,” Dr. Chu added. “For camps with younger children who are not currently eligible for vaccination, implementing layered prevention strategies (e.g., mask use, physical distancing, and encouraging outdoor activities when feasible) is important to prevent transmission.”
Although COVID-19 transmission from children to adults, potentially leading to hospitalization, is not a new phenomenon, “data on the extent of transmission driven by children and adolescents in different settings are still quite sparse,” Dr. Chu said. “A better understanding of their impact on household and community transmission to help guide public health recommendations is particularly important, as most children are still not eligible for vaccination, and in-person schools will be reopening this fall.”
A version of this article first appeared on Medscape.com.
In a report published online in The New England Journal of Medicine, researchers found that campers spread COVID to household members after returning home – but transmission was more likely from some than others. Distancing and masking helped reduce the risk.
Victoria T. Chu, MD, MPH, with the Centers for Disease Control and Prevention, Atlanta, and colleagues with the agency and the Georgia Department of Health followed up with 224 camp attendees, aged 7 to 19 years, who had evidence of SARS-CoV-2 infection on laboratory testing.
These index patients – 88% of whom had symptoms – had 526 household contacts, mainly parents and siblings. Of 377 household contacts who underwent testing, 46 (12%) tested positive. Another two cases in household contacts were identified using clinical and epidemiologic criteria.
Family members hospitalized
Of the 41 adult household contacts who were infected, four (about 10%) were hospitalized. Their hospital stays ranged from 5 to 11 days. Of the seven infected household contacts who were younger than 18 years, none were hospitalized.
The four hospitalized adults were parents and grandparents aged 45 to 80 years, Dr. Chu said. Two of the four had underlying conditions. None of the household contacts died.
In an adjusted analysis, campers who had practiced physical distancing were less likely to transmit the virus at home, compared with those who had not practiced physical distancing (adjusted odds ratio, 0.4). Household members who had had close or direct contact with the index patients were more than 5 times more likely to become infected, compared with family members with minimal or no contact, analyses showed.
“This retrospective study showed that the efficient transmission of SARS-CoV-2 from school-age children and adolescents to household members led to the hospitalization of adults with secondary cases of COVID-19,” the researchers write. “In households in which transmission occurred, half the household contacts were infected.”
The secondary attack rates in this report may be an underestimate because testing was voluntary and participants reported the results themselves, the authors note. It is possible that infected household contacts spread the virus further, but this study did not address that question, Dr. Chu said.
For the study, investigators interviewed all camp attendees and their parents or guardians by phone between July 17, 2020 and Aug. 24, 2020, to collect information about demographic and clinical characteristics, SARS-CoV-2 testing, and preventive measures. The researchers’ analysis excluded households in which illness onset in a household contact occurred before or less than 2 days after a camper became sick.
About a third of the index patients began to have symptoms while still at camp. These campers may have been less infectious by the time they got home, compared with those whose symptoms started after they returned.
Two-thirds of the index patients adopted physical distancing at home, which “probably reduced the transmission of SARS-CoV-2 in the household,” Dr. Chu and colleagues wrote.
“Children who have had a known COVID-19 exposure should quarantine and obtain testing if they develop symptoms within the 14 days of returning home,” Dr. Chu advised. “If a child develops COVID-19, the child should be cared for and monitored using the proper combination of physical distancing, isolation when feasible, and mask use to prevent household transmission as much as possible. In addition, any person over the age of 12 is now eligible for vaccination in the United States. If eligible, children attending camp and their family members should get vaccinated to protect themselves and others, as vaccinations are our most effective public health prevention strategy.”
Mitigation can help
Another report regarding four overnight camps in Maine – in which three campers tested positive after they arrived last summer – shows that “aggressive mitigation strategies can be effective” in limiting transmission of the virus, William T. Basco Jr., MD, writes in a commentary for this news organization.
This summer, a range of factors, including vaccination rates at the camp, may influence transmission dynamics, Dr. Chu said in an interview. In July, the Associated Press reported outbreaks tied to summer camps in several states.
“Transmission dynamics will probably vary from summer camp to summer camp depending on many factors, such as vaccination rates of camp attendees, the mitigation measures in place, and the number of individual introductions during camp,” Dr. Chu said. “We would expect that a camp with a low vaccination rate among attendees and no enforcement of mitigation measures” still may experience a large outbreak.
“On the other hand, a large proportion of vaccinated individuals and appropriate implementation of multiple mitigation measures, such as wearing masks, may be quite effective at keeping their transmission rates low,” Dr. Chu added. “For camps with younger children who are not currently eligible for vaccination, implementing layered prevention strategies (e.g., mask use, physical distancing, and encouraging outdoor activities when feasible) is important to prevent transmission.”
Although COVID-19 transmission from children to adults, potentially leading to hospitalization, is not a new phenomenon, “data on the extent of transmission driven by children and adolescents in different settings are still quite sparse,” Dr. Chu said. “A better understanding of their impact on household and community transmission to help guide public health recommendations is particularly important, as most children are still not eligible for vaccination, and in-person schools will be reopening this fall.”
A version of this article first appeared on Medscape.com.
In a report published online in The New England Journal of Medicine, researchers found that campers spread COVID to household members after returning home – but transmission was more likely from some than others. Distancing and masking helped reduce the risk.
Victoria T. Chu, MD, MPH, with the Centers for Disease Control and Prevention, Atlanta, and colleagues with the agency and the Georgia Department of Health followed up with 224 camp attendees, aged 7 to 19 years, who had evidence of SARS-CoV-2 infection on laboratory testing.
These index patients – 88% of whom had symptoms – had 526 household contacts, mainly parents and siblings. Of 377 household contacts who underwent testing, 46 (12%) tested positive. Another two cases in household contacts were identified using clinical and epidemiologic criteria.
Family members hospitalized
Of the 41 adult household contacts who were infected, four (about 10%) were hospitalized. Their hospital stays ranged from 5 to 11 days. Of the seven infected household contacts who were younger than 18 years, none were hospitalized.
The four hospitalized adults were parents and grandparents aged 45 to 80 years, Dr. Chu said. Two of the four had underlying conditions. None of the household contacts died.
In an adjusted analysis, campers who had practiced physical distancing were less likely to transmit the virus at home, compared with those who had not practiced physical distancing (adjusted odds ratio, 0.4). Household members who had had close or direct contact with the index patients were more than 5 times more likely to become infected, compared with family members with minimal or no contact, analyses showed.
“This retrospective study showed that the efficient transmission of SARS-CoV-2 from school-age children and adolescents to household members led to the hospitalization of adults with secondary cases of COVID-19,” the researchers write. “In households in which transmission occurred, half the household contacts were infected.”
The secondary attack rates in this report may be an underestimate because testing was voluntary and participants reported the results themselves, the authors note. It is possible that infected household contacts spread the virus further, but this study did not address that question, Dr. Chu said.
For the study, investigators interviewed all camp attendees and their parents or guardians by phone between July 17, 2020 and Aug. 24, 2020, to collect information about demographic and clinical characteristics, SARS-CoV-2 testing, and preventive measures. The researchers’ analysis excluded households in which illness onset in a household contact occurred before or less than 2 days after a camper became sick.
About a third of the index patients began to have symptoms while still at camp. These campers may have been less infectious by the time they got home, compared with those whose symptoms started after they returned.
Two-thirds of the index patients adopted physical distancing at home, which “probably reduced the transmission of SARS-CoV-2 in the household,” Dr. Chu and colleagues wrote.
“Children who have had a known COVID-19 exposure should quarantine and obtain testing if they develop symptoms within the 14 days of returning home,” Dr. Chu advised. “If a child develops COVID-19, the child should be cared for and monitored using the proper combination of physical distancing, isolation when feasible, and mask use to prevent household transmission as much as possible. In addition, any person over the age of 12 is now eligible for vaccination in the United States. If eligible, children attending camp and their family members should get vaccinated to protect themselves and others, as vaccinations are our most effective public health prevention strategy.”
Mitigation can help
Another report regarding four overnight camps in Maine – in which three campers tested positive after they arrived last summer – shows that “aggressive mitigation strategies can be effective” in limiting transmission of the virus, William T. Basco Jr., MD, writes in a commentary for this news organization.
This summer, a range of factors, including vaccination rates at the camp, may influence transmission dynamics, Dr. Chu said in an interview. In July, the Associated Press reported outbreaks tied to summer camps in several states.
“Transmission dynamics will probably vary from summer camp to summer camp depending on many factors, such as vaccination rates of camp attendees, the mitigation measures in place, and the number of individual introductions during camp,” Dr. Chu said. “We would expect that a camp with a low vaccination rate among attendees and no enforcement of mitigation measures” still may experience a large outbreak.
“On the other hand, a large proportion of vaccinated individuals and appropriate implementation of multiple mitigation measures, such as wearing masks, may be quite effective at keeping their transmission rates low,” Dr. Chu added. “For camps with younger children who are not currently eligible for vaccination, implementing layered prevention strategies (e.g., mask use, physical distancing, and encouraging outdoor activities when feasible) is important to prevent transmission.”
Although COVID-19 transmission from children to adults, potentially leading to hospitalization, is not a new phenomenon, “data on the extent of transmission driven by children and adolescents in different settings are still quite sparse,” Dr. Chu said. “A better understanding of their impact on household and community transmission to help guide public health recommendations is particularly important, as most children are still not eligible for vaccination, and in-person schools will be reopening this fall.”
A version of this article first appeared on Medscape.com.
Long COVID symptoms rare but real in some kids
School-aged children with SARS-CoV-2 infection had only a few mild symptoms and typically recovered in 6 days, with more than 98% recovering in 8 weeks, a large U.K. study of smartphone data reassuringly reports.
In a small proportion (4.4%), however, COVID-19 symptoms such as fatigue, headache, or loss of smell persisted beyond a month, highlighting the need for ongoing pediatric care, according to Erika Molteni, PhD, a research fellow at King’s College, London, and colleagues.
The results, published online in The Lancet Child & Adolescent Health, also indicated that some children who had non-COVID infections were also susceptible to prolonged symptoms. “Our data highlight that other illnesses, such as colds and flu, can also have prolonged symptoms in children and it is important to consider this when planning for pediatric health services during the pandemic and beyond,” Michael Absoud, PhD, a senior coauthor and a King’s College consultant and senior lecturer, said in a news release. “All children who have persistent symptoms – from any illness – need timely multidisciplinary support linked with education, to enable them to find their individual pathway to recovery.”
Using a “citizen science” approach, the study extracted data from a smartphone app for tracking COVID symptoms in the ZOE COVID Study. The researchers looked at 258,790 children aged 5-17 years whose details were reported by adult proxies such as parents and carers from March 24, 2020, to Feb. 22, 2021. Of these, 75,529 had undergone a valid SARS-CoV-2 test.
The study also assessed symptoms in a randomly selected, age- and sex-matched cohort of 1,734 children in the app database who tested negative for COVID-19 but may have had other illnesses such as colds or flu.
In the 1,734 children testing positive for COVID-19 (approximately 50% each boys and girls), the most common symptoms were headache (62.2%) and fatigue (55.0%). More than 10% of the entire cohort had underlying asthma, but other comorbidities were very rare.
To assess the effect of age, the children were assessed in two groups: 5-11 years (n = 588) and 12-17 years (n = 1,146).
While unable to cross-check app reporting against actual medical records, the study suggested that illness lasted longer in COVID-positive than COVID-negative children, with a median of 6 days (interquartile range, 3-11) versus 3 days (IQR, 2-7). Furthermore, illness duration was positively associated with age: older children (median, 7 days; IQR, 3-12) versus younger children (median, 5 days; IQR, 2-9).
In 77 (4.4%) of the 1,734 COVID-positive children, illness persisted for at least 28 days, again more often in older than younger children: 5.1% of older children versus 3.1% of younger children (P = .046).
In addition, those with COVID-19 were more likely than children with non-COVID illness to be sick for more than 4 weeks: 4.4% versus 0.9%. At 4 weeks, however, the few children with other illnesses tended to have more symptoms, exhibiting a median of five symptoms versus two symptoms in the COVID-positive group.
“I tend to agree with the U.K. findings. COVID-19 in most school-age children is asymptomatic or a brief, self-limiting illness,” Sindhu Mohandas, MD, a pediatric infectious disease specialist at the Children’s Hospital Los Angeles, said in an interview. “The few children who need hospitalization have also mostly fully recovered by the time they are seen for their first outpatient clinic follow-up visit.”
Dr. Mohandas, who was not involved in the U.K. study, added that in her experience a small percentage, particularly adolescents, have some lingering symptoms after infection including fatigue, loss of appetite, and changes in smell and taste. “Identifying children with persistent illness and providing support and multidisciplinary care based on their symptomatology can make a positive impact on patients and their families.”
Recent research has suggested that long symptoms can persist for 3 months in 6% of children with COVID-19. And data from China have indicated that the prevalence of coinfection may be higher than in older patients.
In an accompanying comment, Dana Mahr, PhD, and Bruno J. Strasser, PhD, researchers in the faculty of science at the University of Geneva, said the app-based study “illustrates the potential and challenges of what has been called citizenship science,” in which projects rely on data input from nonscientists.
But while potentially democratizing participation in medical research, this subjective approach has the inherent bias of self-reporting (and in the case of the current study, proxy reporting), and can introduce potential conflicts of interest owing to the politicization of certain diseases.
In the case of the current study, Dr. Mahr and Dr. Strasser argued that, since the COVID-19 test result is known to participants, a pediatrician using objective criteria is better positioned to control for reporting biases than a parent asking a child about symptoms. “Entering data on a smartphone app is not equivalent to discussing with a pediatrician or health care worker who can answer further questions and concerns of participants, an especially important factor for underserved communities,” they wrote. “Citizen science will continue to require a close interaction with professional medical researchers to turn unique illness experiences into research data.”
This study was funded by Zoe Limited, the U.K. Government Department of Health and Social Care, Wellcome Trust, the U.K. Engineering and Physical Sciences Research Council, the U.K. Research and Innovation London Medical Imaging and Artificial Intelligence Centre for Value Based Healthcare, the U.K. National Institute for Health Research, the U.K. Medical Research Council, the British Heart Foundation, and the Alzheimer’s Society. Several study authors have disclosed support from various research-funding agencies and Zoe Limited supported all aspects of building and running the symptom-tracking application. Dr. Mahr and Dr. Strasser declared no competing interests. Dr. Mohandas disclosed no competing interests with regard to her comments.
School-aged children with SARS-CoV-2 infection had only a few mild symptoms and typically recovered in 6 days, with more than 98% recovering in 8 weeks, a large U.K. study of smartphone data reassuringly reports.
In a small proportion (4.4%), however, COVID-19 symptoms such as fatigue, headache, or loss of smell persisted beyond a month, highlighting the need for ongoing pediatric care, according to Erika Molteni, PhD, a research fellow at King’s College, London, and colleagues.
The results, published online in The Lancet Child & Adolescent Health, also indicated that some children who had non-COVID infections were also susceptible to prolonged symptoms. “Our data highlight that other illnesses, such as colds and flu, can also have prolonged symptoms in children and it is important to consider this when planning for pediatric health services during the pandemic and beyond,” Michael Absoud, PhD, a senior coauthor and a King’s College consultant and senior lecturer, said in a news release. “All children who have persistent symptoms – from any illness – need timely multidisciplinary support linked with education, to enable them to find their individual pathway to recovery.”
Using a “citizen science” approach, the study extracted data from a smartphone app for tracking COVID symptoms in the ZOE COVID Study. The researchers looked at 258,790 children aged 5-17 years whose details were reported by adult proxies such as parents and carers from March 24, 2020, to Feb. 22, 2021. Of these, 75,529 had undergone a valid SARS-CoV-2 test.
The study also assessed symptoms in a randomly selected, age- and sex-matched cohort of 1,734 children in the app database who tested negative for COVID-19 but may have had other illnesses such as colds or flu.
In the 1,734 children testing positive for COVID-19 (approximately 50% each boys and girls), the most common symptoms were headache (62.2%) and fatigue (55.0%). More than 10% of the entire cohort had underlying asthma, but other comorbidities were very rare.
To assess the effect of age, the children were assessed in two groups: 5-11 years (n = 588) and 12-17 years (n = 1,146).
While unable to cross-check app reporting against actual medical records, the study suggested that illness lasted longer in COVID-positive than COVID-negative children, with a median of 6 days (interquartile range, 3-11) versus 3 days (IQR, 2-7). Furthermore, illness duration was positively associated with age: older children (median, 7 days; IQR, 3-12) versus younger children (median, 5 days; IQR, 2-9).
In 77 (4.4%) of the 1,734 COVID-positive children, illness persisted for at least 28 days, again more often in older than younger children: 5.1% of older children versus 3.1% of younger children (P = .046).
In addition, those with COVID-19 were more likely than children with non-COVID illness to be sick for more than 4 weeks: 4.4% versus 0.9%. At 4 weeks, however, the few children with other illnesses tended to have more symptoms, exhibiting a median of five symptoms versus two symptoms in the COVID-positive group.
“I tend to agree with the U.K. findings. COVID-19 in most school-age children is asymptomatic or a brief, self-limiting illness,” Sindhu Mohandas, MD, a pediatric infectious disease specialist at the Children’s Hospital Los Angeles, said in an interview. “The few children who need hospitalization have also mostly fully recovered by the time they are seen for their first outpatient clinic follow-up visit.”
Dr. Mohandas, who was not involved in the U.K. study, added that in her experience a small percentage, particularly adolescents, have some lingering symptoms after infection including fatigue, loss of appetite, and changes in smell and taste. “Identifying children with persistent illness and providing support and multidisciplinary care based on their symptomatology can make a positive impact on patients and their families.”
Recent research has suggested that long symptoms can persist for 3 months in 6% of children with COVID-19. And data from China have indicated that the prevalence of coinfection may be higher than in older patients.
In an accompanying comment, Dana Mahr, PhD, and Bruno J. Strasser, PhD, researchers in the faculty of science at the University of Geneva, said the app-based study “illustrates the potential and challenges of what has been called citizenship science,” in which projects rely on data input from nonscientists.
But while potentially democratizing participation in medical research, this subjective approach has the inherent bias of self-reporting (and in the case of the current study, proxy reporting), and can introduce potential conflicts of interest owing to the politicization of certain diseases.
In the case of the current study, Dr. Mahr and Dr. Strasser argued that, since the COVID-19 test result is known to participants, a pediatrician using objective criteria is better positioned to control for reporting biases than a parent asking a child about symptoms. “Entering data on a smartphone app is not equivalent to discussing with a pediatrician or health care worker who can answer further questions and concerns of participants, an especially important factor for underserved communities,” they wrote. “Citizen science will continue to require a close interaction with professional medical researchers to turn unique illness experiences into research data.”
This study was funded by Zoe Limited, the U.K. Government Department of Health and Social Care, Wellcome Trust, the U.K. Engineering and Physical Sciences Research Council, the U.K. Research and Innovation London Medical Imaging and Artificial Intelligence Centre for Value Based Healthcare, the U.K. National Institute for Health Research, the U.K. Medical Research Council, the British Heart Foundation, and the Alzheimer’s Society. Several study authors have disclosed support from various research-funding agencies and Zoe Limited supported all aspects of building and running the symptom-tracking application. Dr. Mahr and Dr. Strasser declared no competing interests. Dr. Mohandas disclosed no competing interests with regard to her comments.
School-aged children with SARS-CoV-2 infection had only a few mild symptoms and typically recovered in 6 days, with more than 98% recovering in 8 weeks, a large U.K. study of smartphone data reassuringly reports.
In a small proportion (4.4%), however, COVID-19 symptoms such as fatigue, headache, or loss of smell persisted beyond a month, highlighting the need for ongoing pediatric care, according to Erika Molteni, PhD, a research fellow at King’s College, London, and colleagues.
The results, published online in The Lancet Child & Adolescent Health, also indicated that some children who had non-COVID infections were also susceptible to prolonged symptoms. “Our data highlight that other illnesses, such as colds and flu, can also have prolonged symptoms in children and it is important to consider this when planning for pediatric health services during the pandemic and beyond,” Michael Absoud, PhD, a senior coauthor and a King’s College consultant and senior lecturer, said in a news release. “All children who have persistent symptoms – from any illness – need timely multidisciplinary support linked with education, to enable them to find their individual pathway to recovery.”
Using a “citizen science” approach, the study extracted data from a smartphone app for tracking COVID symptoms in the ZOE COVID Study. The researchers looked at 258,790 children aged 5-17 years whose details were reported by adult proxies such as parents and carers from March 24, 2020, to Feb. 22, 2021. Of these, 75,529 had undergone a valid SARS-CoV-2 test.
The study also assessed symptoms in a randomly selected, age- and sex-matched cohort of 1,734 children in the app database who tested negative for COVID-19 but may have had other illnesses such as colds or flu.
In the 1,734 children testing positive for COVID-19 (approximately 50% each boys and girls), the most common symptoms were headache (62.2%) and fatigue (55.0%). More than 10% of the entire cohort had underlying asthma, but other comorbidities were very rare.
To assess the effect of age, the children were assessed in two groups: 5-11 years (n = 588) and 12-17 years (n = 1,146).
While unable to cross-check app reporting against actual medical records, the study suggested that illness lasted longer in COVID-positive than COVID-negative children, with a median of 6 days (interquartile range, 3-11) versus 3 days (IQR, 2-7). Furthermore, illness duration was positively associated with age: older children (median, 7 days; IQR, 3-12) versus younger children (median, 5 days; IQR, 2-9).
In 77 (4.4%) of the 1,734 COVID-positive children, illness persisted for at least 28 days, again more often in older than younger children: 5.1% of older children versus 3.1% of younger children (P = .046).
In addition, those with COVID-19 were more likely than children with non-COVID illness to be sick for more than 4 weeks: 4.4% versus 0.9%. At 4 weeks, however, the few children with other illnesses tended to have more symptoms, exhibiting a median of five symptoms versus two symptoms in the COVID-positive group.
“I tend to agree with the U.K. findings. COVID-19 in most school-age children is asymptomatic or a brief, self-limiting illness,” Sindhu Mohandas, MD, a pediatric infectious disease specialist at the Children’s Hospital Los Angeles, said in an interview. “The few children who need hospitalization have also mostly fully recovered by the time they are seen for their first outpatient clinic follow-up visit.”
Dr. Mohandas, who was not involved in the U.K. study, added that in her experience a small percentage, particularly adolescents, have some lingering symptoms after infection including fatigue, loss of appetite, and changes in smell and taste. “Identifying children with persistent illness and providing support and multidisciplinary care based on their symptomatology can make a positive impact on patients and their families.”
Recent research has suggested that long symptoms can persist for 3 months in 6% of children with COVID-19. And data from China have indicated that the prevalence of coinfection may be higher than in older patients.
In an accompanying comment, Dana Mahr, PhD, and Bruno J. Strasser, PhD, researchers in the faculty of science at the University of Geneva, said the app-based study “illustrates the potential and challenges of what has been called citizenship science,” in which projects rely on data input from nonscientists.
But while potentially democratizing participation in medical research, this subjective approach has the inherent bias of self-reporting (and in the case of the current study, proxy reporting), and can introduce potential conflicts of interest owing to the politicization of certain diseases.
In the case of the current study, Dr. Mahr and Dr. Strasser argued that, since the COVID-19 test result is known to participants, a pediatrician using objective criteria is better positioned to control for reporting biases than a parent asking a child about symptoms. “Entering data on a smartphone app is not equivalent to discussing with a pediatrician or health care worker who can answer further questions and concerns of participants, an especially important factor for underserved communities,” they wrote. “Citizen science will continue to require a close interaction with professional medical researchers to turn unique illness experiences into research data.”
This study was funded by Zoe Limited, the U.K. Government Department of Health and Social Care, Wellcome Trust, the U.K. Engineering and Physical Sciences Research Council, the U.K. Research and Innovation London Medical Imaging and Artificial Intelligence Centre for Value Based Healthcare, the U.K. National Institute for Health Research, the U.K. Medical Research Council, the British Heart Foundation, and the Alzheimer’s Society. Several study authors have disclosed support from various research-funding agencies and Zoe Limited supported all aspects of building and running the symptom-tracking application. Dr. Mahr and Dr. Strasser declared no competing interests. Dr. Mohandas disclosed no competing interests with regard to her comments.
FROM THE LANCET CHILD & ADOLESCENT HEALTH
Fulminant Hemorrhagic Bullae of the Upper Extremities Arising in the Setting of IV Placement During Severe COVID-19 Infection: Observations From a Major Consultative Practice
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.
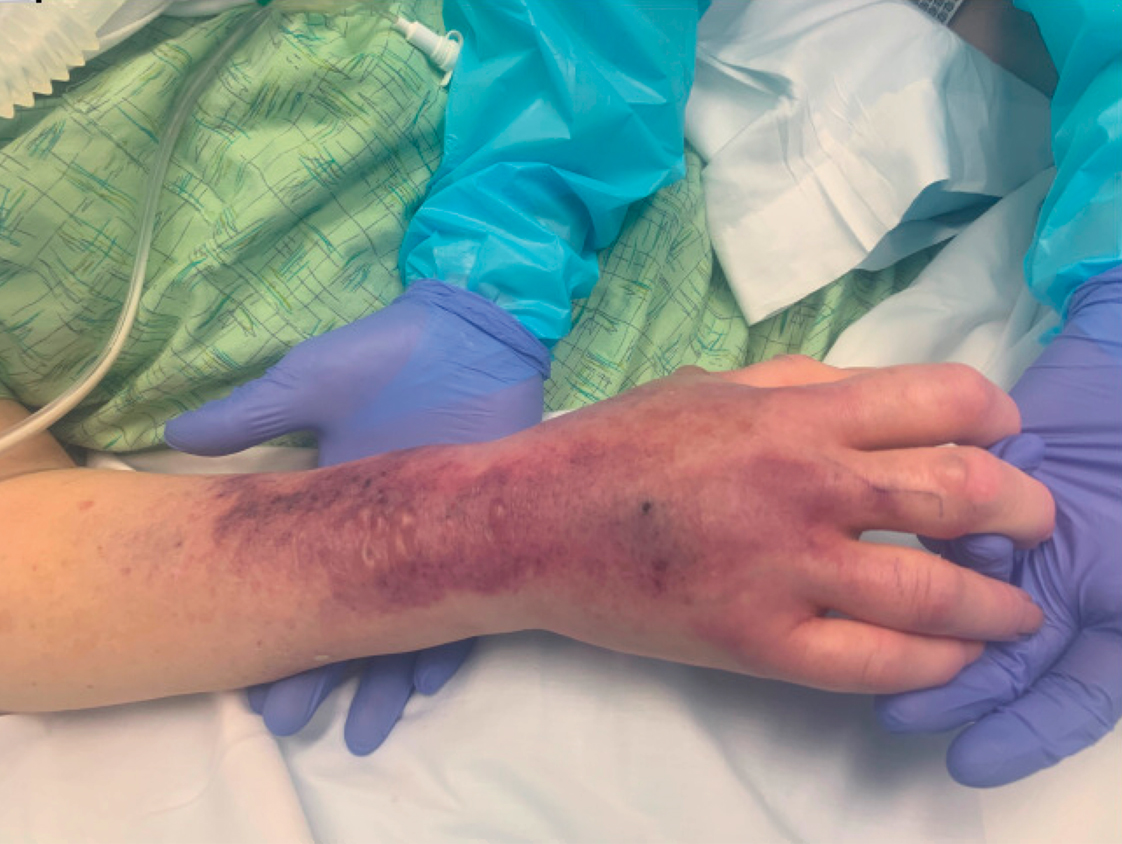
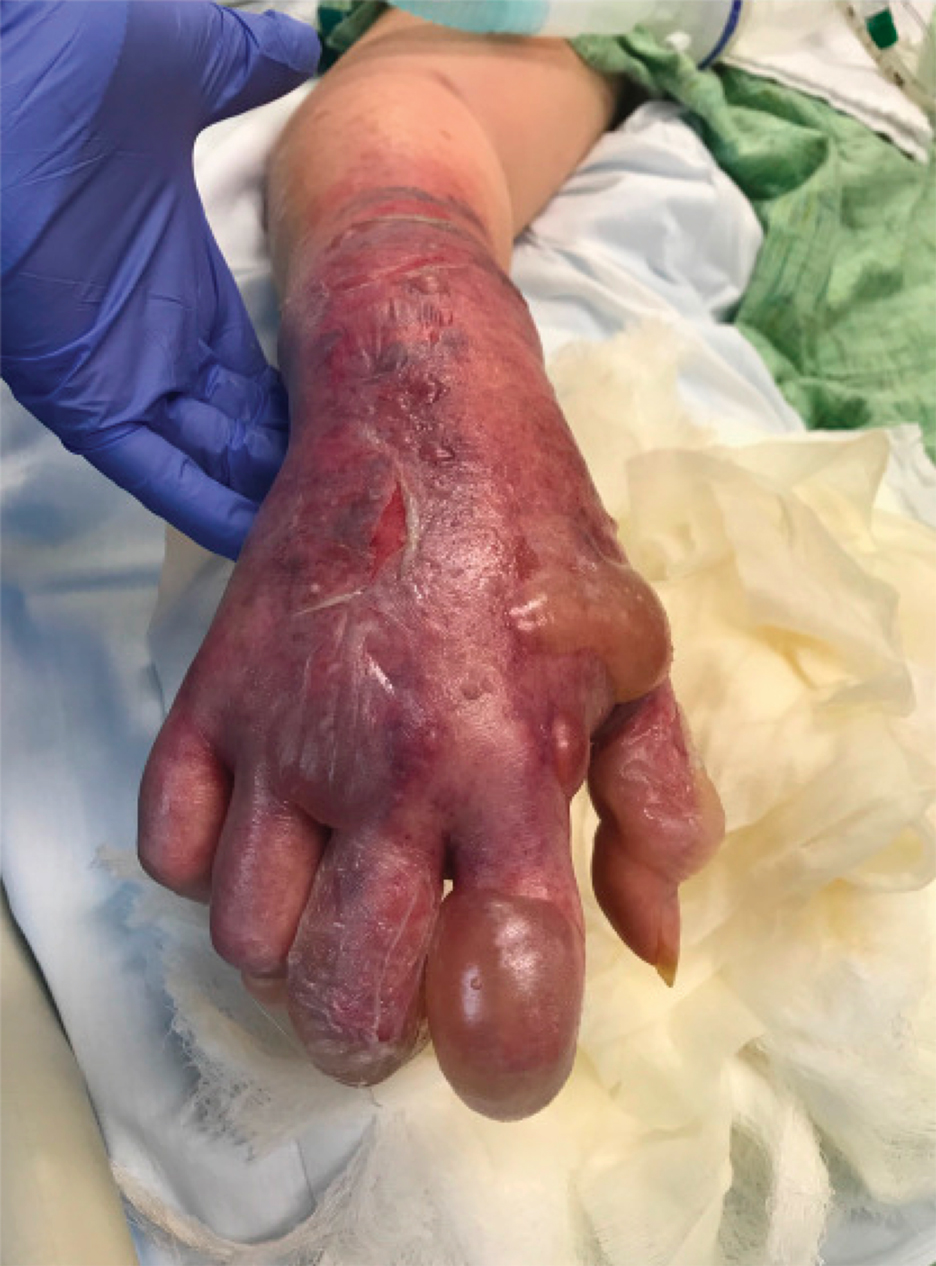
A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.

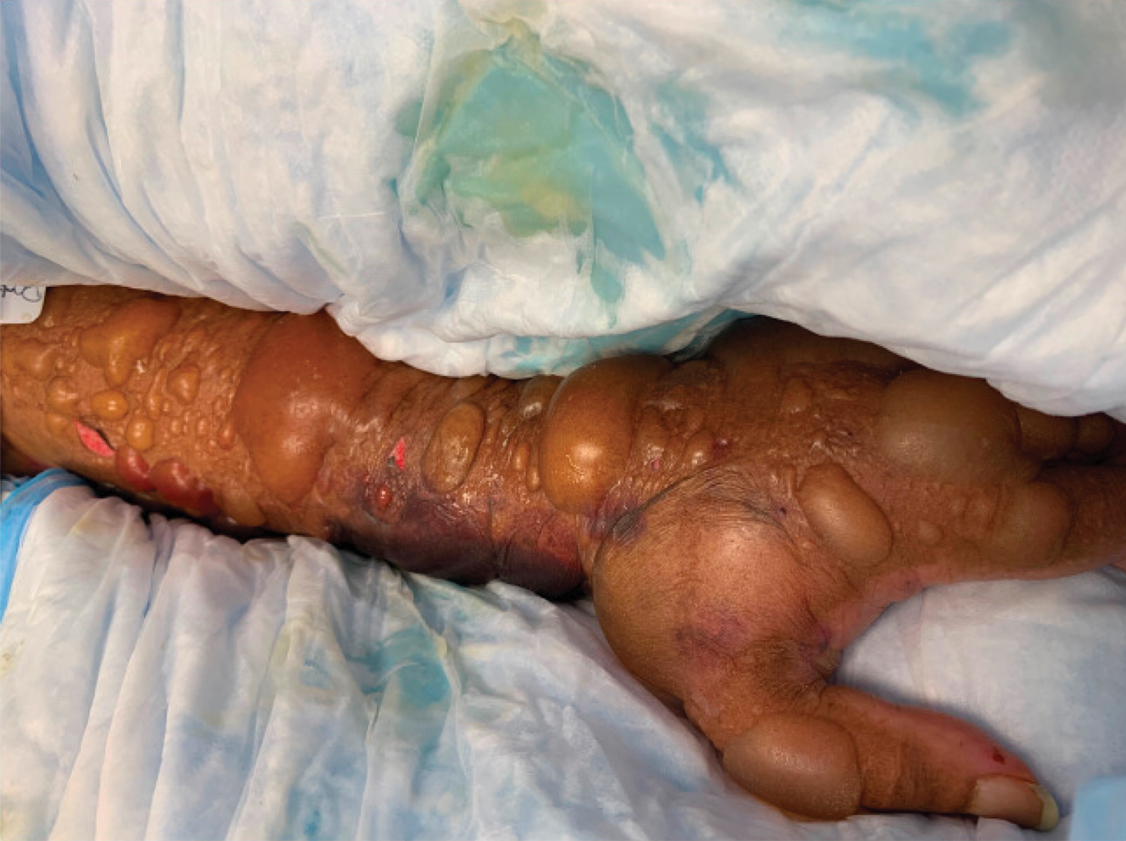
Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.


A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.


Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.


A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.


Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
Practice Points
- Hemorrhagic bullae are an uncommon cutaneous manifestation of COVID-19 infection in hospitalized individuals.
- Although there is no reported treatment for COVID-19–associated hemorrhagic bullae, we recommend supportive care and management of underlying etiology.