User login
25% of patients with cancer lack immunity against measles
Before the onslaught of COVID-19, researchers at the Fred Hutchinson Cancer Research Center in Seattle had another infectious disease worry: an “unprecedented” outbreak of measles.
“In 2019, we saw the most measles cases in any year since the 1990s,” said Sara Marquis, MPH, a clinical research coordinator at the center. The worry, she says, was that various oncology treatments, such as bone marrow transplantations and assorted biologics, “may leave cancer patients severely immunosuppressed” and thus vulnerable to infectious diseases.
Measles-related illness is typically not severe but can lead to pneumonia, deafness, and death, even in immunocompetent people, Ms. Marquis added.
So in 2019, a team at Fred Hutchinson initiated a study to get a sense of immunity to measles among patients with cancer.
They now report that of a group of 900-plus patients, 25% lacked protective antibodies for measles. That’s “significantly more” than the general population, in which about 8% of people lack these antibodies, Ms. Marquis said.
The study, published online in JAMA Network Open, also found that 38% lacked protection against the less-worrisome infectious disease of mumps, which is more than the 13% found in the general population.
“The scary thing about measles is that it is one of the most contagious diseases known,” Ms. Marquis told this news organization, adding that it is about twice as contagious as the COVID-19 Delta variant.
And it’s not just in the state of Washington. “We’re seeing it more and more in the community,” as various outbreaks continue to happen, she said.
“Deficits in protective antibodies underscore patients’ increased risk during outbreaks and emphasize the need for community-based efforts to increase herd immunity to protect this population,” the study authors conclude.
In short, administration of the measles-mumps-rubella (MMR) vaccine, introduced in 1963, must continue universally, they said
“We’ve had so many incredible advances in cancer treatment in recent years. … it would be devastating to see something like measles, which is a vaccine-preventable disease, come through and negate those efforts,” said study coauthor Elizabeth Krantz, MS, a biostatistician at Fred Hutchinson.
The health care teams and family caregivers of patients with cancer should also make sure they are vaccinated, said Ms. Marquis. However, some patients may not be able to get a measles booster vaccine because it is a live vaccine or because they cannot generate enough antibodies for it to be protective, she explained.
Three subgroups more likely to have deficits
The new study, which is one of the first to measure measles and mumps seroprevalence among patients with cancer in the modern era of cancer treatment, also identified three subgroups that more commonly had immunity deficits: those aged 30-59 years; those with hematologic malignant neoplasms, and those who had received a hematopoietic cell transplant.
In the study, residual clinical plasma samples were obtained from 959 consecutive patients with cancer at Seattle Cancer Care Alliance and Fred Hutchinson in August 2019. These samples were tested for measles and mumps IgG by using a commercial enzyme-linked immunosorbent assay. In all, 60% of patients had a solid tumor and 40% had a blood cancer.
As noted above, the seroprevalence of measles antibodies was 0.75 and the seroprevalence of mumps antibodies was 0.62.
A study author explained why the study included mumps, a less threatening infection.
“We assessed mumps in this study out of interest to compare response in the MMR vaccine component – particularly as we could assess a potent vaccine (measles) versus one that has a weaker immunologic response (mumps). We remain worried about outbreaks of mumps as MMR vaccination rates drop across the U.S.,” wrote Steven Pergam, MD, MPH, infectious disease specialist at Fred Hutchinson, in an email.
Vaccination vigilance is one of the study’s messages. “We all need to do our part to make sure we are up to date with our vaccinations so we can make sure we protect those who are vulnerable,” said Ms. Krantz.
The study was funded by the National Cancer Institute and Seattle Cancer Care Alliance. Multiple study authors have ties to pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Before the onslaught of COVID-19, researchers at the Fred Hutchinson Cancer Research Center in Seattle had another infectious disease worry: an “unprecedented” outbreak of measles.
“In 2019, we saw the most measles cases in any year since the 1990s,” said Sara Marquis, MPH, a clinical research coordinator at the center. The worry, she says, was that various oncology treatments, such as bone marrow transplantations and assorted biologics, “may leave cancer patients severely immunosuppressed” and thus vulnerable to infectious diseases.
Measles-related illness is typically not severe but can lead to pneumonia, deafness, and death, even in immunocompetent people, Ms. Marquis added.
So in 2019, a team at Fred Hutchinson initiated a study to get a sense of immunity to measles among patients with cancer.
They now report that of a group of 900-plus patients, 25% lacked protective antibodies for measles. That’s “significantly more” than the general population, in which about 8% of people lack these antibodies, Ms. Marquis said.
The study, published online in JAMA Network Open, also found that 38% lacked protection against the less-worrisome infectious disease of mumps, which is more than the 13% found in the general population.
“The scary thing about measles is that it is one of the most contagious diseases known,” Ms. Marquis told this news organization, adding that it is about twice as contagious as the COVID-19 Delta variant.
And it’s not just in the state of Washington. “We’re seeing it more and more in the community,” as various outbreaks continue to happen, she said.
“Deficits in protective antibodies underscore patients’ increased risk during outbreaks and emphasize the need for community-based efforts to increase herd immunity to protect this population,” the study authors conclude.
In short, administration of the measles-mumps-rubella (MMR) vaccine, introduced in 1963, must continue universally, they said
“We’ve had so many incredible advances in cancer treatment in recent years. … it would be devastating to see something like measles, which is a vaccine-preventable disease, come through and negate those efforts,” said study coauthor Elizabeth Krantz, MS, a biostatistician at Fred Hutchinson.
The health care teams and family caregivers of patients with cancer should also make sure they are vaccinated, said Ms. Marquis. However, some patients may not be able to get a measles booster vaccine because it is a live vaccine or because they cannot generate enough antibodies for it to be protective, she explained.
Three subgroups more likely to have deficits
The new study, which is one of the first to measure measles and mumps seroprevalence among patients with cancer in the modern era of cancer treatment, also identified three subgroups that more commonly had immunity deficits: those aged 30-59 years; those with hematologic malignant neoplasms, and those who had received a hematopoietic cell transplant.
In the study, residual clinical plasma samples were obtained from 959 consecutive patients with cancer at Seattle Cancer Care Alliance and Fred Hutchinson in August 2019. These samples were tested for measles and mumps IgG by using a commercial enzyme-linked immunosorbent assay. In all, 60% of patients had a solid tumor and 40% had a blood cancer.
As noted above, the seroprevalence of measles antibodies was 0.75 and the seroprevalence of mumps antibodies was 0.62.
A study author explained why the study included mumps, a less threatening infection.
“We assessed mumps in this study out of interest to compare response in the MMR vaccine component – particularly as we could assess a potent vaccine (measles) versus one that has a weaker immunologic response (mumps). We remain worried about outbreaks of mumps as MMR vaccination rates drop across the U.S.,” wrote Steven Pergam, MD, MPH, infectious disease specialist at Fred Hutchinson, in an email.
Vaccination vigilance is one of the study’s messages. “We all need to do our part to make sure we are up to date with our vaccinations so we can make sure we protect those who are vulnerable,” said Ms. Krantz.
The study was funded by the National Cancer Institute and Seattle Cancer Care Alliance. Multiple study authors have ties to pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Before the onslaught of COVID-19, researchers at the Fred Hutchinson Cancer Research Center in Seattle had another infectious disease worry: an “unprecedented” outbreak of measles.
“In 2019, we saw the most measles cases in any year since the 1990s,” said Sara Marquis, MPH, a clinical research coordinator at the center. The worry, she says, was that various oncology treatments, such as bone marrow transplantations and assorted biologics, “may leave cancer patients severely immunosuppressed” and thus vulnerable to infectious diseases.
Measles-related illness is typically not severe but can lead to pneumonia, deafness, and death, even in immunocompetent people, Ms. Marquis added.
So in 2019, a team at Fred Hutchinson initiated a study to get a sense of immunity to measles among patients with cancer.
They now report that of a group of 900-plus patients, 25% lacked protective antibodies for measles. That’s “significantly more” than the general population, in which about 8% of people lack these antibodies, Ms. Marquis said.
The study, published online in JAMA Network Open, also found that 38% lacked protection against the less-worrisome infectious disease of mumps, which is more than the 13% found in the general population.
“The scary thing about measles is that it is one of the most contagious diseases known,” Ms. Marquis told this news organization, adding that it is about twice as contagious as the COVID-19 Delta variant.
And it’s not just in the state of Washington. “We’re seeing it more and more in the community,” as various outbreaks continue to happen, she said.
“Deficits in protective antibodies underscore patients’ increased risk during outbreaks and emphasize the need for community-based efforts to increase herd immunity to protect this population,” the study authors conclude.
In short, administration of the measles-mumps-rubella (MMR) vaccine, introduced in 1963, must continue universally, they said
“We’ve had so many incredible advances in cancer treatment in recent years. … it would be devastating to see something like measles, which is a vaccine-preventable disease, come through and negate those efforts,” said study coauthor Elizabeth Krantz, MS, a biostatistician at Fred Hutchinson.
The health care teams and family caregivers of patients with cancer should also make sure they are vaccinated, said Ms. Marquis. However, some patients may not be able to get a measles booster vaccine because it is a live vaccine or because they cannot generate enough antibodies for it to be protective, she explained.
Three subgroups more likely to have deficits
The new study, which is one of the first to measure measles and mumps seroprevalence among patients with cancer in the modern era of cancer treatment, also identified three subgroups that more commonly had immunity deficits: those aged 30-59 years; those with hematologic malignant neoplasms, and those who had received a hematopoietic cell transplant.
In the study, residual clinical plasma samples were obtained from 959 consecutive patients with cancer at Seattle Cancer Care Alliance and Fred Hutchinson in August 2019. These samples were tested for measles and mumps IgG by using a commercial enzyme-linked immunosorbent assay. In all, 60% of patients had a solid tumor and 40% had a blood cancer.
As noted above, the seroprevalence of measles antibodies was 0.75 and the seroprevalence of mumps antibodies was 0.62.
A study author explained why the study included mumps, a less threatening infection.
“We assessed mumps in this study out of interest to compare response in the MMR vaccine component – particularly as we could assess a potent vaccine (measles) versus one that has a weaker immunologic response (mumps). We remain worried about outbreaks of mumps as MMR vaccination rates drop across the U.S.,” wrote Steven Pergam, MD, MPH, infectious disease specialist at Fred Hutchinson, in an email.
Vaccination vigilance is one of the study’s messages. “We all need to do our part to make sure we are up to date with our vaccinations so we can make sure we protect those who are vulnerable,” said Ms. Krantz.
The study was funded by the National Cancer Institute and Seattle Cancer Care Alliance. Multiple study authors have ties to pharmaceutical companies.
A version of this article first appeared on Medscape.com.
CDC reports Burkholderia cepacia and B. pseudomallei outbreaks
The Centers for Disease Control and Prevention and Food and Drug Administration have announced an outbreak of at least 15 Burkholderia cepacia infections associated with contaminated ultrasound gel used to guide invasive procedures as well as an unrelated outbreak of Burkholderia pseudomallei that caused two deaths.
The procedures involved in the B. cepacia outbreak included placement of both central and peripheral intravenous catheters and paracentesis (removal of peritoneal fluid from the abdominal cavity). Cases have occurred in several states.
Further testing has shown the presence of Burkholderia stabilis, a member of B. cepacia complex (Bcc), in four lots of unopened bottles of MediChoice M500812 ultrasound gel. Eco-Med Pharmaceuticals of Etobicoke, Ont., the parent manufacturer, has issued a recall of MediChoice M500812 or Eco-Gel 200 with the following lot numbers: B029, B030, B031, B032, B040, B041, B048, B055. A similar outbreak occurred in Canada.
Some of these cases resulted in bloodstream infections. Further details are not yet available. Bcc infections have ranged from asymptomatic to life-threatening pneumonias, particularly in patients with cystic fibrosis. Other risk factors include immunosuppression, mechanical ventilation, and the use of other invasive venous or urinary catheters.
Kiran M. Perkins, MD, MPH, outbreak lead with the CDC’s Prevention Research Branch, said in an interview via email that automated systems such as Vitek might have trouble identifying the organism as “the system may only reveal the microbial species at the genus level but not at the species level, and/or it may have difficulty distinguishing between members of closely related group members.”
In the CDC’s experience, “most facilities do not conduct further species identification.” The agency added that it cannot tell if there has been any increase in cases associated with COVID-19, as they are not notifiable diseases and the “CDC does not systematically collect information on B. cepacia complex infections.”
Rodney Rohde, PhD, professor of clinical laboratory science and chair of the clinical laboratory science program, Texas State University, San Marcos, told this news organization via email that Burkholderia’s “detection in the manufacturing process is difficult, and product recalls are frequent.” He added, “A recent review by the Food and Drug Administration in the U.S. found that almost 40% of contamination reports in both sterile and nonsterile pharmaceutical products were caused by Bcc bacteria.” Another problem is that they often create biofilms, so “they are tenacious environmental colonizers of medical equipment and surfaces in general.”
There have been many other outbreaks as a result to B. cepacia complex. Because it is often in the water supply used in pharmaceutical manufacturing and is resistant to preservatives, the FDA cautions that it poses a risk of contamination in all nonsterile, water-based drug products.
Recalls have included contaminated antiseptics, such as povidone iodine, benzalkonium chloride, and chlorhexidine gluconate. Contamination in manufacturing may not be uniform, and only some samples may be affected. Antiseptic mouthwashes have also been affected. So have nonbacterial soaps and docusate (a stool softener) solutions, and various personal care products, including nasal sprays, lotions, simethicone gas relief drops (Mylicon), and baby wipes.
Although Bcc are considered “objectionable organisms,” there have been no strong or consistent standards for their detection from the U.S. Pharmacopeia, and some manufacturers reportedly underestimate the consequences of contamination. The FDA issued a guidance to manufacturers in 2017 on quality assurance and cleaning procedures. This is particularly important since preservatives are ineffective against Bcc, and sterility has to be insured at each step of production.
Burkholderia isolates are generally resistant to commonly used antibiotics. Treatment might therefore include a combination of two drugs (to try to limit the emergence of more resistance) such as ceftazidime, piperacillin, meropenem with trimethoprim-sulfamethoxazole, or a beta-lactam plus aminoglycoside.
Interestingly, an outbreak of Burkholderia pseudomallei was just reported by the CDC as well. This is a related gram-negative bacillus which is quite uncommon in the United States. It causes melioidosis, usually a tropical infection, which presents with nonspecific symptoms or serious pneumonia, abscesses, or bloodstream infections.
Four cases have been identified this year in Georgia, Kansas, Minnesota, and Texas, two of them fatal. It is usually acquired from soil or water. By genomic analysis, the four cases are felt to be related, but no common source of exposure has been identified. They also appear to be closely related to South Asian strains, although none of the patients had traveled internationally. Prolonged antibiotic therapy with ceftazidime or meropenem, followed by 3-6 months of trimethoprim-sulfamethoxazole, is often required.
In his email, Dr. Rohde stated, “Melioidosis causes cough, chest pain, high fever, headache or unexplained weight loss, but it may take 2-3 weeks for symptoms of melioidosis to appear after a person’s initial exposure to the bacteria. So, one could see how this might be overlooked as COVID per symptoms and per the limitations of laboratory identification.”
It’s essential for clinicians to recognize that automated microbiology identification systems can misidentify B. pseudomallei as B. cepacia and to ask the lab for more specialized molecular diagnostics, particularly when relatively unusual organisms are isolated.
Candice Hoffmann, a public affairs specialist at the CDC, told this news organization that “clinicians should consider melioidosis as a differential diagnosis in both adult and pediatric patients who are suspected to have a bacterial infection (pneumonia, sepsis, meningitis, wound) and are not responding to antibacterial treatment, even if they have not traveled outside of the continental United States.”
Dr. Rohde has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and Food and Drug Administration have announced an outbreak of at least 15 Burkholderia cepacia infections associated with contaminated ultrasound gel used to guide invasive procedures as well as an unrelated outbreak of Burkholderia pseudomallei that caused two deaths.
The procedures involved in the B. cepacia outbreak included placement of both central and peripheral intravenous catheters and paracentesis (removal of peritoneal fluid from the abdominal cavity). Cases have occurred in several states.
Further testing has shown the presence of Burkholderia stabilis, a member of B. cepacia complex (Bcc), in four lots of unopened bottles of MediChoice M500812 ultrasound gel. Eco-Med Pharmaceuticals of Etobicoke, Ont., the parent manufacturer, has issued a recall of MediChoice M500812 or Eco-Gel 200 with the following lot numbers: B029, B030, B031, B032, B040, B041, B048, B055. A similar outbreak occurred in Canada.
Some of these cases resulted in bloodstream infections. Further details are not yet available. Bcc infections have ranged from asymptomatic to life-threatening pneumonias, particularly in patients with cystic fibrosis. Other risk factors include immunosuppression, mechanical ventilation, and the use of other invasive venous or urinary catheters.
Kiran M. Perkins, MD, MPH, outbreak lead with the CDC’s Prevention Research Branch, said in an interview via email that automated systems such as Vitek might have trouble identifying the organism as “the system may only reveal the microbial species at the genus level but not at the species level, and/or it may have difficulty distinguishing between members of closely related group members.”
In the CDC’s experience, “most facilities do not conduct further species identification.” The agency added that it cannot tell if there has been any increase in cases associated with COVID-19, as they are not notifiable diseases and the “CDC does not systematically collect information on B. cepacia complex infections.”
Rodney Rohde, PhD, professor of clinical laboratory science and chair of the clinical laboratory science program, Texas State University, San Marcos, told this news organization via email that Burkholderia’s “detection in the manufacturing process is difficult, and product recalls are frequent.” He added, “A recent review by the Food and Drug Administration in the U.S. found that almost 40% of contamination reports in both sterile and nonsterile pharmaceutical products were caused by Bcc bacteria.” Another problem is that they often create biofilms, so “they are tenacious environmental colonizers of medical equipment and surfaces in general.”
There have been many other outbreaks as a result to B. cepacia complex. Because it is often in the water supply used in pharmaceutical manufacturing and is resistant to preservatives, the FDA cautions that it poses a risk of contamination in all nonsterile, water-based drug products.
Recalls have included contaminated antiseptics, such as povidone iodine, benzalkonium chloride, and chlorhexidine gluconate. Contamination in manufacturing may not be uniform, and only some samples may be affected. Antiseptic mouthwashes have also been affected. So have nonbacterial soaps and docusate (a stool softener) solutions, and various personal care products, including nasal sprays, lotions, simethicone gas relief drops (Mylicon), and baby wipes.
Although Bcc are considered “objectionable organisms,” there have been no strong or consistent standards for their detection from the U.S. Pharmacopeia, and some manufacturers reportedly underestimate the consequences of contamination. The FDA issued a guidance to manufacturers in 2017 on quality assurance and cleaning procedures. This is particularly important since preservatives are ineffective against Bcc, and sterility has to be insured at each step of production.
Burkholderia isolates are generally resistant to commonly used antibiotics. Treatment might therefore include a combination of two drugs (to try to limit the emergence of more resistance) such as ceftazidime, piperacillin, meropenem with trimethoprim-sulfamethoxazole, or a beta-lactam plus aminoglycoside.
Interestingly, an outbreak of Burkholderia pseudomallei was just reported by the CDC as well. This is a related gram-negative bacillus which is quite uncommon in the United States. It causes melioidosis, usually a tropical infection, which presents with nonspecific symptoms or serious pneumonia, abscesses, or bloodstream infections.
Four cases have been identified this year in Georgia, Kansas, Minnesota, and Texas, two of them fatal. It is usually acquired from soil or water. By genomic analysis, the four cases are felt to be related, but no common source of exposure has been identified. They also appear to be closely related to South Asian strains, although none of the patients had traveled internationally. Prolonged antibiotic therapy with ceftazidime or meropenem, followed by 3-6 months of trimethoprim-sulfamethoxazole, is often required.
In his email, Dr. Rohde stated, “Melioidosis causes cough, chest pain, high fever, headache or unexplained weight loss, but it may take 2-3 weeks for symptoms of melioidosis to appear after a person’s initial exposure to the bacteria. So, one could see how this might be overlooked as COVID per symptoms and per the limitations of laboratory identification.”
It’s essential for clinicians to recognize that automated microbiology identification systems can misidentify B. pseudomallei as B. cepacia and to ask the lab for more specialized molecular diagnostics, particularly when relatively unusual organisms are isolated.
Candice Hoffmann, a public affairs specialist at the CDC, told this news organization that “clinicians should consider melioidosis as a differential diagnosis in both adult and pediatric patients who are suspected to have a bacterial infection (pneumonia, sepsis, meningitis, wound) and are not responding to antibacterial treatment, even if they have not traveled outside of the continental United States.”
Dr. Rohde has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention and Food and Drug Administration have announced an outbreak of at least 15 Burkholderia cepacia infections associated with contaminated ultrasound gel used to guide invasive procedures as well as an unrelated outbreak of Burkholderia pseudomallei that caused two deaths.
The procedures involved in the B. cepacia outbreak included placement of both central and peripheral intravenous catheters and paracentesis (removal of peritoneal fluid from the abdominal cavity). Cases have occurred in several states.
Further testing has shown the presence of Burkholderia stabilis, a member of B. cepacia complex (Bcc), in four lots of unopened bottles of MediChoice M500812 ultrasound gel. Eco-Med Pharmaceuticals of Etobicoke, Ont., the parent manufacturer, has issued a recall of MediChoice M500812 or Eco-Gel 200 with the following lot numbers: B029, B030, B031, B032, B040, B041, B048, B055. A similar outbreak occurred in Canada.
Some of these cases resulted in bloodstream infections. Further details are not yet available. Bcc infections have ranged from asymptomatic to life-threatening pneumonias, particularly in patients with cystic fibrosis. Other risk factors include immunosuppression, mechanical ventilation, and the use of other invasive venous or urinary catheters.
Kiran M. Perkins, MD, MPH, outbreak lead with the CDC’s Prevention Research Branch, said in an interview via email that automated systems such as Vitek might have trouble identifying the organism as “the system may only reveal the microbial species at the genus level but not at the species level, and/or it may have difficulty distinguishing between members of closely related group members.”
In the CDC’s experience, “most facilities do not conduct further species identification.” The agency added that it cannot tell if there has been any increase in cases associated with COVID-19, as they are not notifiable diseases and the “CDC does not systematically collect information on B. cepacia complex infections.”
Rodney Rohde, PhD, professor of clinical laboratory science and chair of the clinical laboratory science program, Texas State University, San Marcos, told this news organization via email that Burkholderia’s “detection in the manufacturing process is difficult, and product recalls are frequent.” He added, “A recent review by the Food and Drug Administration in the U.S. found that almost 40% of contamination reports in both sterile and nonsterile pharmaceutical products were caused by Bcc bacteria.” Another problem is that they often create biofilms, so “they are tenacious environmental colonizers of medical equipment and surfaces in general.”
There have been many other outbreaks as a result to B. cepacia complex. Because it is often in the water supply used in pharmaceutical manufacturing and is resistant to preservatives, the FDA cautions that it poses a risk of contamination in all nonsterile, water-based drug products.
Recalls have included contaminated antiseptics, such as povidone iodine, benzalkonium chloride, and chlorhexidine gluconate. Contamination in manufacturing may not be uniform, and only some samples may be affected. Antiseptic mouthwashes have also been affected. So have nonbacterial soaps and docusate (a stool softener) solutions, and various personal care products, including nasal sprays, lotions, simethicone gas relief drops (Mylicon), and baby wipes.
Although Bcc are considered “objectionable organisms,” there have been no strong or consistent standards for their detection from the U.S. Pharmacopeia, and some manufacturers reportedly underestimate the consequences of contamination. The FDA issued a guidance to manufacturers in 2017 on quality assurance and cleaning procedures. This is particularly important since preservatives are ineffective against Bcc, and sterility has to be insured at each step of production.
Burkholderia isolates are generally resistant to commonly used antibiotics. Treatment might therefore include a combination of two drugs (to try to limit the emergence of more resistance) such as ceftazidime, piperacillin, meropenem with trimethoprim-sulfamethoxazole, or a beta-lactam plus aminoglycoside.
Interestingly, an outbreak of Burkholderia pseudomallei was just reported by the CDC as well. This is a related gram-negative bacillus which is quite uncommon in the United States. It causes melioidosis, usually a tropical infection, which presents with nonspecific symptoms or serious pneumonia, abscesses, or bloodstream infections.
Four cases have been identified this year in Georgia, Kansas, Minnesota, and Texas, two of them fatal. It is usually acquired from soil or water. By genomic analysis, the four cases are felt to be related, but no common source of exposure has been identified. They also appear to be closely related to South Asian strains, although none of the patients had traveled internationally. Prolonged antibiotic therapy with ceftazidime or meropenem, followed by 3-6 months of trimethoprim-sulfamethoxazole, is often required.
In his email, Dr. Rohde stated, “Melioidosis causes cough, chest pain, high fever, headache or unexplained weight loss, but it may take 2-3 weeks for symptoms of melioidosis to appear after a person’s initial exposure to the bacteria. So, one could see how this might be overlooked as COVID per symptoms and per the limitations of laboratory identification.”
It’s essential for clinicians to recognize that automated microbiology identification systems can misidentify B. pseudomallei as B. cepacia and to ask the lab for more specialized molecular diagnostics, particularly when relatively unusual organisms are isolated.
Candice Hoffmann, a public affairs specialist at the CDC, told this news organization that “clinicians should consider melioidosis as a differential diagnosis in both adult and pediatric patients who are suspected to have a bacterial infection (pneumonia, sepsis, meningitis, wound) and are not responding to antibacterial treatment, even if they have not traveled outside of the continental United States.”
Dr. Rohde has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Specific COVID-19 antibodies found in breast milk of vaccinated women
The breast milk of women who had received Pfizer’s COVID-19 vaccine contained specific antibodies against the infectious disease, new research found.
“The COVID-19 pandemic has raised questions among individuals who are breastfeeding, both because of the possibility of viral transmission to infants during breastfeeding and, more recently, of the potential risks and benefits of vaccination in this specific population,” researchers wrote.
In August, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and most recently, the Centers for Disease Control and Prevention, recommended that pregnant people receive the COVID-19 vaccine.
The study, published Aug. 11 in JAMA Network Open, adds to a growing collection of research that has found COVID-19 antibodies in the breast milk of women who were vaccinated against or have been infected with the illness.
Study author Erika Esteve-Palau, MD, PhD, and her colleagues collected blood and milk samples from 33 people who were on average 37 years old and who were on average 17.5 months post partum to examine the correlation of the levels of immunoglobulin G antibodies against the spike protein (S1 subunit) and against the nucleocapsid (NC) of SARS-CoV-2.
Blood and milk samples were taken from each study participant at three time points – 2 weeks after receiving the first dose of the vaccine, 2 weeks after receiving the second dose, and 4 weeks after the second dose. No participants had confirmed SARS-CoV-2 infection prior to vaccination or during the study period.
Researchers found that, after the second dose of the vaccine, IgG(S1) levels in breast milk increased and were positively associated with corresponding levels in the blood samples. The median range of IgG(S1) levels for serum-milk pairs at each time point were 519 to 1 arbitrary units (AU) per mL 2 weeks after receiving the first dose of the vaccine, 8,644 to 78 AU/mL 2 weeks after receiving the second dose, and 12,478 to 50.4 AU/mL 4 weeks after receiving the second dose.
Lisette D. Tanner, MD, MPH, FACOG, who was not involved in the study, said she was not surprised by the findings as previous studies have shown the passage of antibodies in breast milk in vaccinated women. One 2021 study published in JAMA found SARS-CoV-2–specific IgA and IgG antibodies in breast milk for 6 weeks after vaccination. IgA secretion was evident as early as 2 weeks after vaccination followed by a spike in IgG after 4 weeks (a week after the second vaccine). Meanwhile, another 2021 study published in mBio found that breast milk produced by parents with COVID-19 is a source of SARS-CoV-2 IgA and IgG antibodies and can neutralize COVID-19 activity.
“While the data from this and other studies is promising in regards to the passage of antibodies, it is currently unclear what the long-term effects for children will be,” said Dr. Tanner of the department of gynecology and obstetrics at Emory University, Atlanta. “It is not yet known what level of antibodies is necessary to convey protection to either neonates or children. This is an active area of investigation at multiple institutions.”
Dr. Tanner said she wished the study “evaluated neonatal cord blood or serum levels to better understand the immune response mounted by the children of women who received vaccination.”
Researchers of the current study said larger prospective studies are needed to confirm the safety of SARS-CoV-2 vaccination in individuals who are breastfeeding and further assess the association of vaccination with infants’ health and SARS-CoV-2–specific immunity.
Dr. Palau and Dr. Tanner had no relevant financial disclosures.
The breast milk of women who had received Pfizer’s COVID-19 vaccine contained specific antibodies against the infectious disease, new research found.
“The COVID-19 pandemic has raised questions among individuals who are breastfeeding, both because of the possibility of viral transmission to infants during breastfeeding and, more recently, of the potential risks and benefits of vaccination in this specific population,” researchers wrote.
In August, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and most recently, the Centers for Disease Control and Prevention, recommended that pregnant people receive the COVID-19 vaccine.
The study, published Aug. 11 in JAMA Network Open, adds to a growing collection of research that has found COVID-19 antibodies in the breast milk of women who were vaccinated against or have been infected with the illness.
Study author Erika Esteve-Palau, MD, PhD, and her colleagues collected blood and milk samples from 33 people who were on average 37 years old and who were on average 17.5 months post partum to examine the correlation of the levels of immunoglobulin G antibodies against the spike protein (S1 subunit) and against the nucleocapsid (NC) of SARS-CoV-2.
Blood and milk samples were taken from each study participant at three time points – 2 weeks after receiving the first dose of the vaccine, 2 weeks after receiving the second dose, and 4 weeks after the second dose. No participants had confirmed SARS-CoV-2 infection prior to vaccination or during the study period.
Researchers found that, after the second dose of the vaccine, IgG(S1) levels in breast milk increased and were positively associated with corresponding levels in the blood samples. The median range of IgG(S1) levels for serum-milk pairs at each time point were 519 to 1 arbitrary units (AU) per mL 2 weeks after receiving the first dose of the vaccine, 8,644 to 78 AU/mL 2 weeks after receiving the second dose, and 12,478 to 50.4 AU/mL 4 weeks after receiving the second dose.
Lisette D. Tanner, MD, MPH, FACOG, who was not involved in the study, said she was not surprised by the findings as previous studies have shown the passage of antibodies in breast milk in vaccinated women. One 2021 study published in JAMA found SARS-CoV-2–specific IgA and IgG antibodies in breast milk for 6 weeks after vaccination. IgA secretion was evident as early as 2 weeks after vaccination followed by a spike in IgG after 4 weeks (a week after the second vaccine). Meanwhile, another 2021 study published in mBio found that breast milk produced by parents with COVID-19 is a source of SARS-CoV-2 IgA and IgG antibodies and can neutralize COVID-19 activity.
“While the data from this and other studies is promising in regards to the passage of antibodies, it is currently unclear what the long-term effects for children will be,” said Dr. Tanner of the department of gynecology and obstetrics at Emory University, Atlanta. “It is not yet known what level of antibodies is necessary to convey protection to either neonates or children. This is an active area of investigation at multiple institutions.”
Dr. Tanner said she wished the study “evaluated neonatal cord blood or serum levels to better understand the immune response mounted by the children of women who received vaccination.”
Researchers of the current study said larger prospective studies are needed to confirm the safety of SARS-CoV-2 vaccination in individuals who are breastfeeding and further assess the association of vaccination with infants’ health and SARS-CoV-2–specific immunity.
Dr. Palau and Dr. Tanner had no relevant financial disclosures.
The breast milk of women who had received Pfizer’s COVID-19 vaccine contained specific antibodies against the infectious disease, new research found.
“The COVID-19 pandemic has raised questions among individuals who are breastfeeding, both because of the possibility of viral transmission to infants during breastfeeding and, more recently, of the potential risks and benefits of vaccination in this specific population,” researchers wrote.
In August, the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine, and most recently, the Centers for Disease Control and Prevention, recommended that pregnant people receive the COVID-19 vaccine.
The study, published Aug. 11 in JAMA Network Open, adds to a growing collection of research that has found COVID-19 antibodies in the breast milk of women who were vaccinated against or have been infected with the illness.
Study author Erika Esteve-Palau, MD, PhD, and her colleagues collected blood and milk samples from 33 people who were on average 37 years old and who were on average 17.5 months post partum to examine the correlation of the levels of immunoglobulin G antibodies against the spike protein (S1 subunit) and against the nucleocapsid (NC) of SARS-CoV-2.
Blood and milk samples were taken from each study participant at three time points – 2 weeks after receiving the first dose of the vaccine, 2 weeks after receiving the second dose, and 4 weeks after the second dose. No participants had confirmed SARS-CoV-2 infection prior to vaccination or during the study period.
Researchers found that, after the second dose of the vaccine, IgG(S1) levels in breast milk increased and were positively associated with corresponding levels in the blood samples. The median range of IgG(S1) levels for serum-milk pairs at each time point were 519 to 1 arbitrary units (AU) per mL 2 weeks after receiving the first dose of the vaccine, 8,644 to 78 AU/mL 2 weeks after receiving the second dose, and 12,478 to 50.4 AU/mL 4 weeks after receiving the second dose.
Lisette D. Tanner, MD, MPH, FACOG, who was not involved in the study, said she was not surprised by the findings as previous studies have shown the passage of antibodies in breast milk in vaccinated women. One 2021 study published in JAMA found SARS-CoV-2–specific IgA and IgG antibodies in breast milk for 6 weeks after vaccination. IgA secretion was evident as early as 2 weeks after vaccination followed by a spike in IgG after 4 weeks (a week after the second vaccine). Meanwhile, another 2021 study published in mBio found that breast milk produced by parents with COVID-19 is a source of SARS-CoV-2 IgA and IgG antibodies and can neutralize COVID-19 activity.
“While the data from this and other studies is promising in regards to the passage of antibodies, it is currently unclear what the long-term effects for children will be,” said Dr. Tanner of the department of gynecology and obstetrics at Emory University, Atlanta. “It is not yet known what level of antibodies is necessary to convey protection to either neonates or children. This is an active area of investigation at multiple institutions.”
Dr. Tanner said she wished the study “evaluated neonatal cord blood or serum levels to better understand the immune response mounted by the children of women who received vaccination.”
Researchers of the current study said larger prospective studies are needed to confirm the safety of SARS-CoV-2 vaccination in individuals who are breastfeeding and further assess the association of vaccination with infants’ health and SARS-CoV-2–specific immunity.
Dr. Palau and Dr. Tanner had no relevant financial disclosures.
JAMA NETWORK OPEN
Hep B vaccine response varied among youth with inflammatory, autoimmune disorders
“Hepatitis B is a common viral infection with 2 billion people worldwide having evidence of prior or current infection, and it can present as an acute or chronic infection,” or with chronic sequelae, including cirrhosis and hepatocellular carcinoma, Alexandra Ritter said during the annual meeting of the Society for Pediatric Dermatology. A three-dose vaccination series is recommended beginning at birth, and in 2016, the Centers for Disease Control and Prevention reported that 90.5% of U.S. children aged 19-35 months had completed the series.
While the vaccine series provides protection in healthy individuals more than 95% of the time, a decreased response has been noted in specific pediatric populations, including those with inflammatory and autoimmune diseases. “This is important to note and investigate further because a decreased vaccine response increases the risk for this high-risk population, and the use of boosters is currently debated,” said Ms. Ritter, who is a fourth-year student at the Medical University of South Carolina, Charleston.
To determine the percent of pediatric patients with inflammatory or autoimmune disease who lack evidence of immunity following the hepatitis B vaccine series, Ms. Ritter and colleagues Abigail Truitt and pediatric dermatologist Lara Wine Lee, MD, PhD, of MUSC, retrospectively reviewed the charts of 160 patients between the ages of 6 months and 21 years, who were diagnosed with an autoimmune or autoinflammatory disease, or inflammatory bowel disease (IBD), and had documented evidence of vaccination and serologic testing prior to the start of immunosuppressive therapy.
Of the 160 patients, 100 (63%) had IBD, 34 (21%) had an autoimmune disease, 26 (16%) had an autoinflammatory disease, 89 (56%) were female, and their mean age was 15 years.
The researchers observed variation in the testing ordered between the three patient groups. Specifically, 88.2% of autoimmune patients had hepatitis B surface antigen (HBsAg) testing, compared with 96.15% of patients with an autoinflammatory disease and 67% of patients with IBD, while 76.47% of patients with an autoimmune disease had hepatitis B core antibody (anti-HBc) testing, compared with 88.46% of patients with an autoinflammatory disease and 31% of patients with IBD.
In addition, 82.35% of patients with an autoimmune disease had HBsAg testing, compared with 100% of patients with an autoinflammatory disease and 94% of patients with IBD.
Of the 148 patients who had HBsAg testing ordered and completed prior to starting an immunosuppressive drug, there was no statistically significant difference in the percent of patients showing evidence of an immune response to the hepatitis B vaccine (32.14% among patients with an autoimmune disease, 34.62% among patients with an autoinflammatory disease, and 31.91% among patients with IBD). Combined, 67.57% of tested negative for the hepatitis B surface antibody.
“Our study showed that the majority of these patients did not show serologic evidence of immunity despite being fully vaccinated,” Ms. Ritter said. “There was also variation in the testing ordered and a more standardized approach is needed in this high-risk population.” She acknowledged certain limitations of the study, including its retrospective design and lack of a control group.
“This brings us to our next question of whether this indicates a failure of the vaccine, or the way immunity is tested,” she continued. “The CDC and the European Consensus Group on Hepatitis B Immunity recommend a cutoff of greater than 10 mIU/mL. Those that achieve immunity are protected for up to 20 years due to immune memory, even if their antibody levels later drop. There have been rare cases of immunocompetent individuals having evidence of transient asymptomatic infections when antibody levels drop. The chronic disease has only been documented in infants born to positive mothers. In hemodialysis patients, however, clinically significant infections have been documented when antibody levels drop.”
The CDC only recommends postvaccination testing to infants born to positive mothers, health care workers at high risk, hemodialysis patients, people with HIV and other immunocompromised people, and needle-sharing partners of chronically infected people. This is completed 1-2 months following the third vaccine dose, and those with antibody levels less than 10 mIU/mL should be revaccinated. “As some groups do not respond to the vaccine series, alternative dosing and the intradermal vaccine have been studied and shown to be effective in certain groups,” she said.
When it comes to monitoring immunocompromised individuals and giving booster shots, however, there are conflicting recommendations. The CDC recommends yearly testing and booster shots when levels drop below 10 mIU/mL only in hemodialysis patients, while the European Consensus Group recommends testing every 6-12 months for immunocompromised individuals and boosters when their levels drop below 10 mIU/mL.
“The CDC has not yet determined if other immunocompromised individuals should receive a booster, with more research required, but studies have shown it to be effective,” Ms. Ritter said. In a similar study looking at evidence of immunity in children with connective tissue disease who were on immunosuppressive treatment, 50% had no evidence of protective antibodies, compared with 96% in the control group. “In that study, a booster shot was given, and protective antibody concentrations were found at follow-up,” she said.
The researchers reported having no financial disclosures.
[email protected]
“Hepatitis B is a common viral infection with 2 billion people worldwide having evidence of prior or current infection, and it can present as an acute or chronic infection,” or with chronic sequelae, including cirrhosis and hepatocellular carcinoma, Alexandra Ritter said during the annual meeting of the Society for Pediatric Dermatology. A three-dose vaccination series is recommended beginning at birth, and in 2016, the Centers for Disease Control and Prevention reported that 90.5% of U.S. children aged 19-35 months had completed the series.
While the vaccine series provides protection in healthy individuals more than 95% of the time, a decreased response has been noted in specific pediatric populations, including those with inflammatory and autoimmune diseases. “This is important to note and investigate further because a decreased vaccine response increases the risk for this high-risk population, and the use of boosters is currently debated,” said Ms. Ritter, who is a fourth-year student at the Medical University of South Carolina, Charleston.
To determine the percent of pediatric patients with inflammatory or autoimmune disease who lack evidence of immunity following the hepatitis B vaccine series, Ms. Ritter and colleagues Abigail Truitt and pediatric dermatologist Lara Wine Lee, MD, PhD, of MUSC, retrospectively reviewed the charts of 160 patients between the ages of 6 months and 21 years, who were diagnosed with an autoimmune or autoinflammatory disease, or inflammatory bowel disease (IBD), and had documented evidence of vaccination and serologic testing prior to the start of immunosuppressive therapy.
Of the 160 patients, 100 (63%) had IBD, 34 (21%) had an autoimmune disease, 26 (16%) had an autoinflammatory disease, 89 (56%) were female, and their mean age was 15 years.
The researchers observed variation in the testing ordered between the three patient groups. Specifically, 88.2% of autoimmune patients had hepatitis B surface antigen (HBsAg) testing, compared with 96.15% of patients with an autoinflammatory disease and 67% of patients with IBD, while 76.47% of patients with an autoimmune disease had hepatitis B core antibody (anti-HBc) testing, compared with 88.46% of patients with an autoinflammatory disease and 31% of patients with IBD.
In addition, 82.35% of patients with an autoimmune disease had HBsAg testing, compared with 100% of patients with an autoinflammatory disease and 94% of patients with IBD.
Of the 148 patients who had HBsAg testing ordered and completed prior to starting an immunosuppressive drug, there was no statistically significant difference in the percent of patients showing evidence of an immune response to the hepatitis B vaccine (32.14% among patients with an autoimmune disease, 34.62% among patients with an autoinflammatory disease, and 31.91% among patients with IBD). Combined, 67.57% of tested negative for the hepatitis B surface antibody.
“Our study showed that the majority of these patients did not show serologic evidence of immunity despite being fully vaccinated,” Ms. Ritter said. “There was also variation in the testing ordered and a more standardized approach is needed in this high-risk population.” She acknowledged certain limitations of the study, including its retrospective design and lack of a control group.
“This brings us to our next question of whether this indicates a failure of the vaccine, or the way immunity is tested,” she continued. “The CDC and the European Consensus Group on Hepatitis B Immunity recommend a cutoff of greater than 10 mIU/mL. Those that achieve immunity are protected for up to 20 years due to immune memory, even if their antibody levels later drop. There have been rare cases of immunocompetent individuals having evidence of transient asymptomatic infections when antibody levels drop. The chronic disease has only been documented in infants born to positive mothers. In hemodialysis patients, however, clinically significant infections have been documented when antibody levels drop.”
The CDC only recommends postvaccination testing to infants born to positive mothers, health care workers at high risk, hemodialysis patients, people with HIV and other immunocompromised people, and needle-sharing partners of chronically infected people. This is completed 1-2 months following the third vaccine dose, and those with antibody levels less than 10 mIU/mL should be revaccinated. “As some groups do not respond to the vaccine series, alternative dosing and the intradermal vaccine have been studied and shown to be effective in certain groups,” she said.
When it comes to monitoring immunocompromised individuals and giving booster shots, however, there are conflicting recommendations. The CDC recommends yearly testing and booster shots when levels drop below 10 mIU/mL only in hemodialysis patients, while the European Consensus Group recommends testing every 6-12 months for immunocompromised individuals and boosters when their levels drop below 10 mIU/mL.
“The CDC has not yet determined if other immunocompromised individuals should receive a booster, with more research required, but studies have shown it to be effective,” Ms. Ritter said. In a similar study looking at evidence of immunity in children with connective tissue disease who were on immunosuppressive treatment, 50% had no evidence of protective antibodies, compared with 96% in the control group. “In that study, a booster shot was given, and protective antibody concentrations were found at follow-up,” she said.
The researchers reported having no financial disclosures.
[email protected]
“Hepatitis B is a common viral infection with 2 billion people worldwide having evidence of prior or current infection, and it can present as an acute or chronic infection,” or with chronic sequelae, including cirrhosis and hepatocellular carcinoma, Alexandra Ritter said during the annual meeting of the Society for Pediatric Dermatology. A three-dose vaccination series is recommended beginning at birth, and in 2016, the Centers for Disease Control and Prevention reported that 90.5% of U.S. children aged 19-35 months had completed the series.
While the vaccine series provides protection in healthy individuals more than 95% of the time, a decreased response has been noted in specific pediatric populations, including those with inflammatory and autoimmune diseases. “This is important to note and investigate further because a decreased vaccine response increases the risk for this high-risk population, and the use of boosters is currently debated,” said Ms. Ritter, who is a fourth-year student at the Medical University of South Carolina, Charleston.
To determine the percent of pediatric patients with inflammatory or autoimmune disease who lack evidence of immunity following the hepatitis B vaccine series, Ms. Ritter and colleagues Abigail Truitt and pediatric dermatologist Lara Wine Lee, MD, PhD, of MUSC, retrospectively reviewed the charts of 160 patients between the ages of 6 months and 21 years, who were diagnosed with an autoimmune or autoinflammatory disease, or inflammatory bowel disease (IBD), and had documented evidence of vaccination and serologic testing prior to the start of immunosuppressive therapy.
Of the 160 patients, 100 (63%) had IBD, 34 (21%) had an autoimmune disease, 26 (16%) had an autoinflammatory disease, 89 (56%) were female, and their mean age was 15 years.
The researchers observed variation in the testing ordered between the three patient groups. Specifically, 88.2% of autoimmune patients had hepatitis B surface antigen (HBsAg) testing, compared with 96.15% of patients with an autoinflammatory disease and 67% of patients with IBD, while 76.47% of patients with an autoimmune disease had hepatitis B core antibody (anti-HBc) testing, compared with 88.46% of patients with an autoinflammatory disease and 31% of patients with IBD.
In addition, 82.35% of patients with an autoimmune disease had HBsAg testing, compared with 100% of patients with an autoinflammatory disease and 94% of patients with IBD.
Of the 148 patients who had HBsAg testing ordered and completed prior to starting an immunosuppressive drug, there was no statistically significant difference in the percent of patients showing evidence of an immune response to the hepatitis B vaccine (32.14% among patients with an autoimmune disease, 34.62% among patients with an autoinflammatory disease, and 31.91% among patients with IBD). Combined, 67.57% of tested negative for the hepatitis B surface antibody.
“Our study showed that the majority of these patients did not show serologic evidence of immunity despite being fully vaccinated,” Ms. Ritter said. “There was also variation in the testing ordered and a more standardized approach is needed in this high-risk population.” She acknowledged certain limitations of the study, including its retrospective design and lack of a control group.
“This brings us to our next question of whether this indicates a failure of the vaccine, or the way immunity is tested,” she continued. “The CDC and the European Consensus Group on Hepatitis B Immunity recommend a cutoff of greater than 10 mIU/mL. Those that achieve immunity are protected for up to 20 years due to immune memory, even if their antibody levels later drop. There have been rare cases of immunocompetent individuals having evidence of transient asymptomatic infections when antibody levels drop. The chronic disease has only been documented in infants born to positive mothers. In hemodialysis patients, however, clinically significant infections have been documented when antibody levels drop.”
The CDC only recommends postvaccination testing to infants born to positive mothers, health care workers at high risk, hemodialysis patients, people with HIV and other immunocompromised people, and needle-sharing partners of chronically infected people. This is completed 1-2 months following the third vaccine dose, and those with antibody levels less than 10 mIU/mL should be revaccinated. “As some groups do not respond to the vaccine series, alternative dosing and the intradermal vaccine have been studied and shown to be effective in certain groups,” she said.
When it comes to monitoring immunocompromised individuals and giving booster shots, however, there are conflicting recommendations. The CDC recommends yearly testing and booster shots when levels drop below 10 mIU/mL only in hemodialysis patients, while the European Consensus Group recommends testing every 6-12 months for immunocompromised individuals and boosters when their levels drop below 10 mIU/mL.
“The CDC has not yet determined if other immunocompromised individuals should receive a booster, with more research required, but studies have shown it to be effective,” Ms. Ritter said. In a similar study looking at evidence of immunity in children with connective tissue disease who were on immunosuppressive treatment, 50% had no evidence of protective antibodies, compared with 96% in the control group. “In that study, a booster shot was given, and protective antibody concentrations were found at follow-up,” she said.
The researchers reported having no financial disclosures.
[email protected]
FROM SPD 2021
Febrile infant guideline allows wiggle room on hospital admission, testing
The long-anticipated American Academy of Pediatrics guidelines for the treatment of well-appearing febrile infants have arrived, and key points include updated guidance for cerebrospinal fluid testing and urine cultures, according to Robert Pantell, MD, and Kenneth Roberts, MD, who presented the guidelines at the virtual Pediatric Hospital Medicine annual conference.
The AAP guideline was published in the August 2021 issue of Pediatrics. The guideline includes 21 key action statements and 40 total recommendations, and describes separate management algorithms for three age groups: infants aged 8-21 days, 22-28 days, and 29-60 days.
Dr. Roberts, of the University of North Carolina at Chapel Hill, and Dr. Pantell, of the University of California, San Francisco, emphasized that all pediatricians should read the full guideline, but they offered an overview of some of the notable points.
Some changes that drove the development of evidence-based guideline included changes in technology, such as the increased use of procalcitonin, the development of large research networks for studies of sufficient size, and a need to reduce the costs of unnecessary care and unnecessary trauma for infants, Dr. Roberts said. Use of data from large networks such as the Pediatric Emergency Care Applied Research Network provided enough evidence to support dividing the aged 8- to 60-day population into three groups.
The guideline applies to well-appearing term infants aged 8-60 days and at least 37 weeks’ gestation, with fever of 38° C (100.4° F) or higher in the past 24 hours in the home or clinical setting. The decision to exclude infants in the first week of life from the guideline was because at this age, infants “are sufficiently different in rates and types of illness, including early-onset bacterial infection,” according to the authors.
Dr. Roberts emphasized that the guidelines apply to “well-appearing infants,” which is not always obvious. “If a clinician is not confident an infant is well appearing, the clinical practice guideline should not be applied,” he said.
The guideline also includes a visual algorithm for each age group.
Dr. Pantell summarized the key action statements for the three age groups, and encouraged pediatricians to review the visual algorithms and footnotes available in the full text of the guideline.
The guideline includes seven key action statements for each of the three age groups. Four of these address evaluations, using urine, blood culture, inflammatory markers (IM), and cerebrospinal fluid (CSF). One action statement focuses on initial treatment, and two on management: hospital admission versus monitoring at home, and treatment cessation.
Infants aged 8-21 days
The key action statements for well-appearing infants aged 8-21 days are similar to what clinicians likely would do for ill-appearing infants, the authors noted, based in part on the challenge of assessing an infant this age as “well appearing,” because they don’t yet have the ability to interact with the clinician.
For the 8- to 21-day group, the first two key actions are to obtain a urine specimen and blood culture, Dr. Pantell said. Also, clinicians “should” obtain a CSF for analysis and culture. “We recognize that the ability to get CSF quickly is a challenge,” he added. However, for the 8- to 21-day age group, a new feature is that these infants may be discharged if the CSF is negative. Evaluation in this youngest group states that clinicians “may assess inflammatory markers” including height of fever, absolute neutrophil count, C-reactive protein, and procalcitonin.
Treatment of infants in the 8- to 21-day group “should” include parenteral antimicrobial therapy, according to the guideline, and these infants “should” be actively monitored in the hospital by nurses and staff experienced in neonatal care, Dr. Pantell said. The guideline also includes a key action statement to stop antimicrobials at 24-36 hours if cultures are negative, but to treat identified organisms.
Infants aged 22-28 days
In both the 22- to 28-day-old and 29- to 60-day-old groups, the guideline offers opportunities for less testing and treatment, such as avoiding a lumbar puncture, and fewer hospitalizations. The development of a separate guideline for the 22- to 28-day group is something new, said Dr. Pantell. The guideline states that clinicians should obtain urine specimens and blood culture, and should assess IM in this group. Further key action statements note that clinicians “should obtain a CSF if any IM is positive,” but “may” obtain CSF if the infant is hospitalized, if blood and urine cultures have been obtained, and if none of the IMs are abnormal.
As with younger patients, those with a negative CSF can go home, he said. As for treatment, clinicians “should” administer parenteral antimicrobial therapy to infants managed at home even if they have a negative CSF and urinalysis (UA), and no abnormal inflammatory markers Other points for management of infants in this age group at home include verbal teaching and written instructions for caregivers, plans for a re-evaluation at home in 24 hours, and a plan for communication and access to emergency care in case of a change in clinical status, Dr. Pantell explained. The guideline states that infants “should” be hospitalized if CSF is either not obtained or not interpretable, which leaves room for clinical judgment and individual circumstances. Antimicrobials “should” be discontinued in this group once all cultures are negative after 24-36 hours and no other infection requires treatment.
Infants aged 29-60 days
For the 29- to 60-day group, there are some differences, the main one is the recommendation of blood cultures in this group, said Dr. Pantell. “We are seeing a lot of UTIs [urinary tract infections], and we would like those treated.” However, clinicians need not obtain a CSF if other IMs are normal, but may do so if any IM is abnormal. Antimicrobial therapy may include ceftriaxone or cephalexin for UTIs, or vancomycin for bacteremia.
Although antimicrobial therapy is an option for UTIs and bacterial meningitis, clinicians “need not” use antimicrobials if CSF is normal, if UA is negative, and if no IMs are abnormal, Dr. Pantell added. Overall, further management of infants in this oldest age group should focus on discharge to home in the absence of abnormal findings, but hospitalization in the presence of abnormal CSF, IMs, or other concerns.
During a question-and-answer session, Dr. Roberts said that, while rectal temperature is preferable, any method is acceptable as a starting point for applying the guideline. Importantly, the guideline still leaves room for clinical judgment. “We hope this will change some thinking as far as whether one model fits all,” he noted. The authors tried to temper the word “should” with the word “may” when possible, so clinicians can say: “I’m going to individualize my decision to the infant in front of me.”
Ultimately, the guideline is meant as a guide, and not an absolute standard of care, the authors said. The language of the key action statements includes the words “should, may, need not” in place of “must, must not.” The guideline recommends factoring family values and preferences into any treatment decisions. “Variations, taking into account individual circumstances, may be appropriate.”
The guideline received no outside funding. The authors had no financial conflicts to disclose.
The long-anticipated American Academy of Pediatrics guidelines for the treatment of well-appearing febrile infants have arrived, and key points include updated guidance for cerebrospinal fluid testing and urine cultures, according to Robert Pantell, MD, and Kenneth Roberts, MD, who presented the guidelines at the virtual Pediatric Hospital Medicine annual conference.
The AAP guideline was published in the August 2021 issue of Pediatrics. The guideline includes 21 key action statements and 40 total recommendations, and describes separate management algorithms for three age groups: infants aged 8-21 days, 22-28 days, and 29-60 days.
Dr. Roberts, of the University of North Carolina at Chapel Hill, and Dr. Pantell, of the University of California, San Francisco, emphasized that all pediatricians should read the full guideline, but they offered an overview of some of the notable points.
Some changes that drove the development of evidence-based guideline included changes in technology, such as the increased use of procalcitonin, the development of large research networks for studies of sufficient size, and a need to reduce the costs of unnecessary care and unnecessary trauma for infants, Dr. Roberts said. Use of data from large networks such as the Pediatric Emergency Care Applied Research Network provided enough evidence to support dividing the aged 8- to 60-day population into three groups.
The guideline applies to well-appearing term infants aged 8-60 days and at least 37 weeks’ gestation, with fever of 38° C (100.4° F) or higher in the past 24 hours in the home or clinical setting. The decision to exclude infants in the first week of life from the guideline was because at this age, infants “are sufficiently different in rates and types of illness, including early-onset bacterial infection,” according to the authors.
Dr. Roberts emphasized that the guidelines apply to “well-appearing infants,” which is not always obvious. “If a clinician is not confident an infant is well appearing, the clinical practice guideline should not be applied,” he said.
The guideline also includes a visual algorithm for each age group.
Dr. Pantell summarized the key action statements for the three age groups, and encouraged pediatricians to review the visual algorithms and footnotes available in the full text of the guideline.
The guideline includes seven key action statements for each of the three age groups. Four of these address evaluations, using urine, blood culture, inflammatory markers (IM), and cerebrospinal fluid (CSF). One action statement focuses on initial treatment, and two on management: hospital admission versus monitoring at home, and treatment cessation.
Infants aged 8-21 days
The key action statements for well-appearing infants aged 8-21 days are similar to what clinicians likely would do for ill-appearing infants, the authors noted, based in part on the challenge of assessing an infant this age as “well appearing,” because they don’t yet have the ability to interact with the clinician.
For the 8- to 21-day group, the first two key actions are to obtain a urine specimen and blood culture, Dr. Pantell said. Also, clinicians “should” obtain a CSF for analysis and culture. “We recognize that the ability to get CSF quickly is a challenge,” he added. However, for the 8- to 21-day age group, a new feature is that these infants may be discharged if the CSF is negative. Evaluation in this youngest group states that clinicians “may assess inflammatory markers” including height of fever, absolute neutrophil count, C-reactive protein, and procalcitonin.
Treatment of infants in the 8- to 21-day group “should” include parenteral antimicrobial therapy, according to the guideline, and these infants “should” be actively monitored in the hospital by nurses and staff experienced in neonatal care, Dr. Pantell said. The guideline also includes a key action statement to stop antimicrobials at 24-36 hours if cultures are negative, but to treat identified organisms.
Infants aged 22-28 days
In both the 22- to 28-day-old and 29- to 60-day-old groups, the guideline offers opportunities for less testing and treatment, such as avoiding a lumbar puncture, and fewer hospitalizations. The development of a separate guideline for the 22- to 28-day group is something new, said Dr. Pantell. The guideline states that clinicians should obtain urine specimens and blood culture, and should assess IM in this group. Further key action statements note that clinicians “should obtain a CSF if any IM is positive,” but “may” obtain CSF if the infant is hospitalized, if blood and urine cultures have been obtained, and if none of the IMs are abnormal.
As with younger patients, those with a negative CSF can go home, he said. As for treatment, clinicians “should” administer parenteral antimicrobial therapy to infants managed at home even if they have a negative CSF and urinalysis (UA), and no abnormal inflammatory markers Other points for management of infants in this age group at home include verbal teaching and written instructions for caregivers, plans for a re-evaluation at home in 24 hours, and a plan for communication and access to emergency care in case of a change in clinical status, Dr. Pantell explained. The guideline states that infants “should” be hospitalized if CSF is either not obtained or not interpretable, which leaves room for clinical judgment and individual circumstances. Antimicrobials “should” be discontinued in this group once all cultures are negative after 24-36 hours and no other infection requires treatment.
Infants aged 29-60 days
For the 29- to 60-day group, there are some differences, the main one is the recommendation of blood cultures in this group, said Dr. Pantell. “We are seeing a lot of UTIs [urinary tract infections], and we would like those treated.” However, clinicians need not obtain a CSF if other IMs are normal, but may do so if any IM is abnormal. Antimicrobial therapy may include ceftriaxone or cephalexin for UTIs, or vancomycin for bacteremia.
Although antimicrobial therapy is an option for UTIs and bacterial meningitis, clinicians “need not” use antimicrobials if CSF is normal, if UA is negative, and if no IMs are abnormal, Dr. Pantell added. Overall, further management of infants in this oldest age group should focus on discharge to home in the absence of abnormal findings, but hospitalization in the presence of abnormal CSF, IMs, or other concerns.
During a question-and-answer session, Dr. Roberts said that, while rectal temperature is preferable, any method is acceptable as a starting point for applying the guideline. Importantly, the guideline still leaves room for clinical judgment. “We hope this will change some thinking as far as whether one model fits all,” he noted. The authors tried to temper the word “should” with the word “may” when possible, so clinicians can say: “I’m going to individualize my decision to the infant in front of me.”
Ultimately, the guideline is meant as a guide, and not an absolute standard of care, the authors said. The language of the key action statements includes the words “should, may, need not” in place of “must, must not.” The guideline recommends factoring family values and preferences into any treatment decisions. “Variations, taking into account individual circumstances, may be appropriate.”
The guideline received no outside funding. The authors had no financial conflicts to disclose.
The long-anticipated American Academy of Pediatrics guidelines for the treatment of well-appearing febrile infants have arrived, and key points include updated guidance for cerebrospinal fluid testing and urine cultures, according to Robert Pantell, MD, and Kenneth Roberts, MD, who presented the guidelines at the virtual Pediatric Hospital Medicine annual conference.
The AAP guideline was published in the August 2021 issue of Pediatrics. The guideline includes 21 key action statements and 40 total recommendations, and describes separate management algorithms for three age groups: infants aged 8-21 days, 22-28 days, and 29-60 days.
Dr. Roberts, of the University of North Carolina at Chapel Hill, and Dr. Pantell, of the University of California, San Francisco, emphasized that all pediatricians should read the full guideline, but they offered an overview of some of the notable points.
Some changes that drove the development of evidence-based guideline included changes in technology, such as the increased use of procalcitonin, the development of large research networks for studies of sufficient size, and a need to reduce the costs of unnecessary care and unnecessary trauma for infants, Dr. Roberts said. Use of data from large networks such as the Pediatric Emergency Care Applied Research Network provided enough evidence to support dividing the aged 8- to 60-day population into three groups.
The guideline applies to well-appearing term infants aged 8-60 days and at least 37 weeks’ gestation, with fever of 38° C (100.4° F) or higher in the past 24 hours in the home or clinical setting. The decision to exclude infants in the first week of life from the guideline was because at this age, infants “are sufficiently different in rates and types of illness, including early-onset bacterial infection,” according to the authors.
Dr. Roberts emphasized that the guidelines apply to “well-appearing infants,” which is not always obvious. “If a clinician is not confident an infant is well appearing, the clinical practice guideline should not be applied,” he said.
The guideline also includes a visual algorithm for each age group.
Dr. Pantell summarized the key action statements for the three age groups, and encouraged pediatricians to review the visual algorithms and footnotes available in the full text of the guideline.
The guideline includes seven key action statements for each of the three age groups. Four of these address evaluations, using urine, blood culture, inflammatory markers (IM), and cerebrospinal fluid (CSF). One action statement focuses on initial treatment, and two on management: hospital admission versus monitoring at home, and treatment cessation.
Infants aged 8-21 days
The key action statements for well-appearing infants aged 8-21 days are similar to what clinicians likely would do for ill-appearing infants, the authors noted, based in part on the challenge of assessing an infant this age as “well appearing,” because they don’t yet have the ability to interact with the clinician.
For the 8- to 21-day group, the first two key actions are to obtain a urine specimen and blood culture, Dr. Pantell said. Also, clinicians “should” obtain a CSF for analysis and culture. “We recognize that the ability to get CSF quickly is a challenge,” he added. However, for the 8- to 21-day age group, a new feature is that these infants may be discharged if the CSF is negative. Evaluation in this youngest group states that clinicians “may assess inflammatory markers” including height of fever, absolute neutrophil count, C-reactive protein, and procalcitonin.
Treatment of infants in the 8- to 21-day group “should” include parenteral antimicrobial therapy, according to the guideline, and these infants “should” be actively monitored in the hospital by nurses and staff experienced in neonatal care, Dr. Pantell said. The guideline also includes a key action statement to stop antimicrobials at 24-36 hours if cultures are negative, but to treat identified organisms.
Infants aged 22-28 days
In both the 22- to 28-day-old and 29- to 60-day-old groups, the guideline offers opportunities for less testing and treatment, such as avoiding a lumbar puncture, and fewer hospitalizations. The development of a separate guideline for the 22- to 28-day group is something new, said Dr. Pantell. The guideline states that clinicians should obtain urine specimens and blood culture, and should assess IM in this group. Further key action statements note that clinicians “should obtain a CSF if any IM is positive,” but “may” obtain CSF if the infant is hospitalized, if blood and urine cultures have been obtained, and if none of the IMs are abnormal.
As with younger patients, those with a negative CSF can go home, he said. As for treatment, clinicians “should” administer parenteral antimicrobial therapy to infants managed at home even if they have a negative CSF and urinalysis (UA), and no abnormal inflammatory markers Other points for management of infants in this age group at home include verbal teaching and written instructions for caregivers, plans for a re-evaluation at home in 24 hours, and a plan for communication and access to emergency care in case of a change in clinical status, Dr. Pantell explained. The guideline states that infants “should” be hospitalized if CSF is either not obtained or not interpretable, which leaves room for clinical judgment and individual circumstances. Antimicrobials “should” be discontinued in this group once all cultures are negative after 24-36 hours and no other infection requires treatment.
Infants aged 29-60 days
For the 29- to 60-day group, there are some differences, the main one is the recommendation of blood cultures in this group, said Dr. Pantell. “We are seeing a lot of UTIs [urinary tract infections], and we would like those treated.” However, clinicians need not obtain a CSF if other IMs are normal, but may do so if any IM is abnormal. Antimicrobial therapy may include ceftriaxone or cephalexin for UTIs, or vancomycin for bacteremia.
Although antimicrobial therapy is an option for UTIs and bacterial meningitis, clinicians “need not” use antimicrobials if CSF is normal, if UA is negative, and if no IMs are abnormal, Dr. Pantell added. Overall, further management of infants in this oldest age group should focus on discharge to home in the absence of abnormal findings, but hospitalization in the presence of abnormal CSF, IMs, or other concerns.
During a question-and-answer session, Dr. Roberts said that, while rectal temperature is preferable, any method is acceptable as a starting point for applying the guideline. Importantly, the guideline still leaves room for clinical judgment. “We hope this will change some thinking as far as whether one model fits all,” he noted. The authors tried to temper the word “should” with the word “may” when possible, so clinicians can say: “I’m going to individualize my decision to the infant in front of me.”
Ultimately, the guideline is meant as a guide, and not an absolute standard of care, the authors said. The language of the key action statements includes the words “should, may, need not” in place of “must, must not.” The guideline recommends factoring family values and preferences into any treatment decisions. “Variations, taking into account individual circumstances, may be appropriate.”
The guideline received no outside funding. The authors had no financial conflicts to disclose.
FROM PHM 2021
Injectable cabotegravir PrEP superior to oral TDF-FTC; trial halted early
The future of preexposure prophylaxis (PrEP) is here, according to interim study results demonstrating the superiority of long-acting, injectable cabotegravir (CAB-LA) over the current workhorse, daily oral tenofovir disoproxil fumarate-emtricitabine (TDF-FTC).
In a prospective, phase 2b-3 randomized, double-blind, double-dummy, active-controlled trial among 4,566 cisgender MSM (men who have sex with men) and transgender women, The study was terminated early, owing to strong evidence of efficacy in the first preplanned interim endpoint analysis. The study findings were published Aug. 11 in the New England Journal of Medicine.
“The lesson is not that TDF-FTC doesn’t work or has major problems; it is a very safe, very well-tolerated agent and astonishingly effective if taken as prescribed,” Raphael J. Landovitz, MD, lead author and codirector of the Center for HIV Identification, Prevention, and Treatment Services at the University of California, Los Angeles, said in an interview.
“The reason that we were able to show that cabotegravir was superior is because we enrolled a very young, very highly at-risk, very underresourced, underrepresented, highly sexually active group who weren’t able to take PrEP the way it was prescribed,” he said.
Study participants were assigned to receive either active CAB 600 mg intramuscularly with TDF-FTC placebo or active TDF-FTC (300 mg/200 mg) with a CAB procedure in three phases:
- An oral-tablet 5-week lead-in phase, a blinded injection phase beginning at week 5.
- An injection at week 9 and every 8 weeks thereafter through week 153.
- An open-label “tail” phase consisting of oral TDF/FTC to provide ongoing for participants discontinuing injections.
The median age of study participants was 26 years (interquartile range [IQR], 22-32 years); 12.5% (570) identified as transgender women; 49.8% (845/1,698) of U.S. participants were Black patients.
During follow-up, HIV infections were identified in 57 participants, including 52 who acquired HIV infections after enrollment (13 in the CAB group, incidence 0.41 per 100 person-years, vs. 39 in the TDF-FTC group, incidence 1.22 per 100 person-years). The hazard ratio for incident HIV infection was 0.34 (95% confidence interval, 0.18-0.62) CAB vs. TDF-FTC (P < .001). Consistent effects were observed across prespecified subgroups and populations.
Among participants in the CAB group, integrase strand-transfer inhibitor resistance mutations were detected in one of four of the baseline HIV infection cases. Among participants in the TDF-FTC group, 2 of 39 incident infections occurred despite drug concentration measurements that indicated good PrEP adherence.
Although injection site reactions were reported in 81.4% (1,724) of the CAB group, only 2.4% of patients (50) discontinued treatment. Most reactions began a median of 1 day (IQR, 0-2 days) post injection. They were of mild to moderate severity (60.8% pain, 23.7% tenderness) and lasted a median of 3 days (IQR, 2-6 days). Injection site reactions were reported in 31.3% of the participants in the TDF-FTC group who received at least one placebo injection.
Rates of severe adverse effects (grade 3 or higher) were similar between participants in the CAB and TDF-FTC groups. They consisted mostly of an increase in creatine kinase level (14.2% with CAB vs. 13.5% with TDF-FTC) and a decrease in creatine clearance (7.0% with CAB vs. 8.3% with TDF-FTC).
In a post hoc analysis, the mean annualized weight increase was 1.23 kg/y (95% CI, 1.05-1.42) in the CAB group, compared with 0.37 kg/y (95% CI, 0.18-0.55) in the TDF-FTC group. Most of these differences were observed during the first 40 weeks and were driven by weight loss among TDF-FTC participants; weight changes between groups were similar thereafter (~1 kg/y for both groups).
New modality, new challenges
“We’re constantly searching for new modalities to expand our repertoire of what we can provide patients, especially those folks with the highest need for PrEP,” Lina Rosengren-Hovee, MD, assistant professor of medicine and infectious disease specialist at UNC-Health, Chapel Hill, N.C., said in an interview. “Being able to offer an injectable option is going to be a game changer, but it will be critical to pinpoint structural factors that affect adherence,” she added.
Dr. Rosengren-Hovee also pointed to cases of integrase inhibitor resistance (both in the study and the larger clinical arena), which she believes are concerning. “It’s still a conversation that you’ll want to have with a patient; I wonder if we need more discussion about how we handle that in the clinical setting, even if it’s fairly uncommon,” she said.
When asked, Dr. Landovitz emphasized the rarity of breakthrough cases but acknowledged that there appears to be a pattern whereby the first breakthrough occurs with a trickle of virus and then bursts out with higher levels of virus at some point.
“CDC is actually thinking very hard about whether these long-acting PrEP agents obligate a change to the HIV screening process [e.g., a viral load or RNA-based test] rather than a conventional HIV test,” Dr. Landovitz said. He went on to say that in the ongoing, open-label portion of the study, investigators hope to learn whether one can avoid resistance by catching the first breakthrough earlier. That would help inform clinical implementation, he explained. He said that he challenges practitioners and health care communities to avoid some of the mistakes made with the oral PrEP rollout, namely, universal access without proper implementation of planning and testing protocols.
“By default, PrEP is much more decentralized and demedicalized, especially in primary care,” said Dr. Rosengren-Hovee. “We need more studies looking at real-world scenarios.”
Dr. Rosengren-Hovee reports no relevant financial relationships. Dr. Landovitz has consulting relationships with Gilead, Janssen, Roche, and Cepheus.
A version of this article first appeared on Medscape.com.
The future of preexposure prophylaxis (PrEP) is here, according to interim study results demonstrating the superiority of long-acting, injectable cabotegravir (CAB-LA) over the current workhorse, daily oral tenofovir disoproxil fumarate-emtricitabine (TDF-FTC).
In a prospective, phase 2b-3 randomized, double-blind, double-dummy, active-controlled trial among 4,566 cisgender MSM (men who have sex with men) and transgender women, The study was terminated early, owing to strong evidence of efficacy in the first preplanned interim endpoint analysis. The study findings were published Aug. 11 in the New England Journal of Medicine.
“The lesson is not that TDF-FTC doesn’t work or has major problems; it is a very safe, very well-tolerated agent and astonishingly effective if taken as prescribed,” Raphael J. Landovitz, MD, lead author and codirector of the Center for HIV Identification, Prevention, and Treatment Services at the University of California, Los Angeles, said in an interview.
“The reason that we were able to show that cabotegravir was superior is because we enrolled a very young, very highly at-risk, very underresourced, underrepresented, highly sexually active group who weren’t able to take PrEP the way it was prescribed,” he said.
Study participants were assigned to receive either active CAB 600 mg intramuscularly with TDF-FTC placebo or active TDF-FTC (300 mg/200 mg) with a CAB procedure in three phases:
- An oral-tablet 5-week lead-in phase, a blinded injection phase beginning at week 5.
- An injection at week 9 and every 8 weeks thereafter through week 153.
- An open-label “tail” phase consisting of oral TDF/FTC to provide ongoing for participants discontinuing injections.
The median age of study participants was 26 years (interquartile range [IQR], 22-32 years); 12.5% (570) identified as transgender women; 49.8% (845/1,698) of U.S. participants were Black patients.
During follow-up, HIV infections were identified in 57 participants, including 52 who acquired HIV infections after enrollment (13 in the CAB group, incidence 0.41 per 100 person-years, vs. 39 in the TDF-FTC group, incidence 1.22 per 100 person-years). The hazard ratio for incident HIV infection was 0.34 (95% confidence interval, 0.18-0.62) CAB vs. TDF-FTC (P < .001). Consistent effects were observed across prespecified subgroups and populations.
Among participants in the CAB group, integrase strand-transfer inhibitor resistance mutations were detected in one of four of the baseline HIV infection cases. Among participants in the TDF-FTC group, 2 of 39 incident infections occurred despite drug concentration measurements that indicated good PrEP adherence.
Although injection site reactions were reported in 81.4% (1,724) of the CAB group, only 2.4% of patients (50) discontinued treatment. Most reactions began a median of 1 day (IQR, 0-2 days) post injection. They were of mild to moderate severity (60.8% pain, 23.7% tenderness) and lasted a median of 3 days (IQR, 2-6 days). Injection site reactions were reported in 31.3% of the participants in the TDF-FTC group who received at least one placebo injection.
Rates of severe adverse effects (grade 3 or higher) were similar between participants in the CAB and TDF-FTC groups. They consisted mostly of an increase in creatine kinase level (14.2% with CAB vs. 13.5% with TDF-FTC) and a decrease in creatine clearance (7.0% with CAB vs. 8.3% with TDF-FTC).
In a post hoc analysis, the mean annualized weight increase was 1.23 kg/y (95% CI, 1.05-1.42) in the CAB group, compared with 0.37 kg/y (95% CI, 0.18-0.55) in the TDF-FTC group. Most of these differences were observed during the first 40 weeks and were driven by weight loss among TDF-FTC participants; weight changes between groups were similar thereafter (~1 kg/y for both groups).
New modality, new challenges
“We’re constantly searching for new modalities to expand our repertoire of what we can provide patients, especially those folks with the highest need for PrEP,” Lina Rosengren-Hovee, MD, assistant professor of medicine and infectious disease specialist at UNC-Health, Chapel Hill, N.C., said in an interview. “Being able to offer an injectable option is going to be a game changer, but it will be critical to pinpoint structural factors that affect adherence,” she added.
Dr. Rosengren-Hovee also pointed to cases of integrase inhibitor resistance (both in the study and the larger clinical arena), which she believes are concerning. “It’s still a conversation that you’ll want to have with a patient; I wonder if we need more discussion about how we handle that in the clinical setting, even if it’s fairly uncommon,” she said.
When asked, Dr. Landovitz emphasized the rarity of breakthrough cases but acknowledged that there appears to be a pattern whereby the first breakthrough occurs with a trickle of virus and then bursts out with higher levels of virus at some point.
“CDC is actually thinking very hard about whether these long-acting PrEP agents obligate a change to the HIV screening process [e.g., a viral load or RNA-based test] rather than a conventional HIV test,” Dr. Landovitz said. He went on to say that in the ongoing, open-label portion of the study, investigators hope to learn whether one can avoid resistance by catching the first breakthrough earlier. That would help inform clinical implementation, he explained. He said that he challenges practitioners and health care communities to avoid some of the mistakes made with the oral PrEP rollout, namely, universal access without proper implementation of planning and testing protocols.
“By default, PrEP is much more decentralized and demedicalized, especially in primary care,” said Dr. Rosengren-Hovee. “We need more studies looking at real-world scenarios.”
Dr. Rosengren-Hovee reports no relevant financial relationships. Dr. Landovitz has consulting relationships with Gilead, Janssen, Roche, and Cepheus.
A version of this article first appeared on Medscape.com.
The future of preexposure prophylaxis (PrEP) is here, according to interim study results demonstrating the superiority of long-acting, injectable cabotegravir (CAB-LA) over the current workhorse, daily oral tenofovir disoproxil fumarate-emtricitabine (TDF-FTC).
In a prospective, phase 2b-3 randomized, double-blind, double-dummy, active-controlled trial among 4,566 cisgender MSM (men who have sex with men) and transgender women, The study was terminated early, owing to strong evidence of efficacy in the first preplanned interim endpoint analysis. The study findings were published Aug. 11 in the New England Journal of Medicine.
“The lesson is not that TDF-FTC doesn’t work or has major problems; it is a very safe, very well-tolerated agent and astonishingly effective if taken as prescribed,” Raphael J. Landovitz, MD, lead author and codirector of the Center for HIV Identification, Prevention, and Treatment Services at the University of California, Los Angeles, said in an interview.
“The reason that we were able to show that cabotegravir was superior is because we enrolled a very young, very highly at-risk, very underresourced, underrepresented, highly sexually active group who weren’t able to take PrEP the way it was prescribed,” he said.
Study participants were assigned to receive either active CAB 600 mg intramuscularly with TDF-FTC placebo or active TDF-FTC (300 mg/200 mg) with a CAB procedure in three phases:
- An oral-tablet 5-week lead-in phase, a blinded injection phase beginning at week 5.
- An injection at week 9 and every 8 weeks thereafter through week 153.
- An open-label “tail” phase consisting of oral TDF/FTC to provide ongoing for participants discontinuing injections.
The median age of study participants was 26 years (interquartile range [IQR], 22-32 years); 12.5% (570) identified as transgender women; 49.8% (845/1,698) of U.S. participants were Black patients.
During follow-up, HIV infections were identified in 57 participants, including 52 who acquired HIV infections after enrollment (13 in the CAB group, incidence 0.41 per 100 person-years, vs. 39 in the TDF-FTC group, incidence 1.22 per 100 person-years). The hazard ratio for incident HIV infection was 0.34 (95% confidence interval, 0.18-0.62) CAB vs. TDF-FTC (P < .001). Consistent effects were observed across prespecified subgroups and populations.
Among participants in the CAB group, integrase strand-transfer inhibitor resistance mutations were detected in one of four of the baseline HIV infection cases. Among participants in the TDF-FTC group, 2 of 39 incident infections occurred despite drug concentration measurements that indicated good PrEP adherence.
Although injection site reactions were reported in 81.4% (1,724) of the CAB group, only 2.4% of patients (50) discontinued treatment. Most reactions began a median of 1 day (IQR, 0-2 days) post injection. They were of mild to moderate severity (60.8% pain, 23.7% tenderness) and lasted a median of 3 days (IQR, 2-6 days). Injection site reactions were reported in 31.3% of the participants in the TDF-FTC group who received at least one placebo injection.
Rates of severe adverse effects (grade 3 or higher) were similar between participants in the CAB and TDF-FTC groups. They consisted mostly of an increase in creatine kinase level (14.2% with CAB vs. 13.5% with TDF-FTC) and a decrease in creatine clearance (7.0% with CAB vs. 8.3% with TDF-FTC).
In a post hoc analysis, the mean annualized weight increase was 1.23 kg/y (95% CI, 1.05-1.42) in the CAB group, compared with 0.37 kg/y (95% CI, 0.18-0.55) in the TDF-FTC group. Most of these differences were observed during the first 40 weeks and were driven by weight loss among TDF-FTC participants; weight changes between groups were similar thereafter (~1 kg/y for both groups).
New modality, new challenges
“We’re constantly searching for new modalities to expand our repertoire of what we can provide patients, especially those folks with the highest need for PrEP,” Lina Rosengren-Hovee, MD, assistant professor of medicine and infectious disease specialist at UNC-Health, Chapel Hill, N.C., said in an interview. “Being able to offer an injectable option is going to be a game changer, but it will be critical to pinpoint structural factors that affect adherence,” she added.
Dr. Rosengren-Hovee also pointed to cases of integrase inhibitor resistance (both in the study and the larger clinical arena), which she believes are concerning. “It’s still a conversation that you’ll want to have with a patient; I wonder if we need more discussion about how we handle that in the clinical setting, even if it’s fairly uncommon,” she said.
When asked, Dr. Landovitz emphasized the rarity of breakthrough cases but acknowledged that there appears to be a pattern whereby the first breakthrough occurs with a trickle of virus and then bursts out with higher levels of virus at some point.
“CDC is actually thinking very hard about whether these long-acting PrEP agents obligate a change to the HIV screening process [e.g., a viral load or RNA-based test] rather than a conventional HIV test,” Dr. Landovitz said. He went on to say that in the ongoing, open-label portion of the study, investigators hope to learn whether one can avoid resistance by catching the first breakthrough earlier. That would help inform clinical implementation, he explained. He said that he challenges practitioners and health care communities to avoid some of the mistakes made with the oral PrEP rollout, namely, universal access without proper implementation of planning and testing protocols.
“By default, PrEP is much more decentralized and demedicalized, especially in primary care,” said Dr. Rosengren-Hovee. “We need more studies looking at real-world scenarios.”
Dr. Rosengren-Hovee reports no relevant financial relationships. Dr. Landovitz has consulting relationships with Gilead, Janssen, Roche, and Cepheus.
A version of this article first appeared on Medscape.com.
Masking in school: A battle of the op-eds
Traditionally, as the ides of August descend upon us we expect to be bombarded with advertisements encouraging parents and students to finish up their back-to-school shopping. But, this year the question on every parent and school administrator’s mind is not which color back pack will be the most popular this year but whether a mask should be a required part of the back-to-school ensemble.
The American Academy of Pediatrics has recommended that “All students older than 2 years and all school staff should wear a mask at school” (“American Academy of Pediatrics Updates Recommendations for Opening Schools in Fall 2021.” 2021 Jul 19). The academy’s statement includes a generous list of common sense caveats but it does not include a statement that masks have been shown to be protective for children in school environments. The Centers for Disease Control and Prevention “recommends” universal indoor masking along with keeping a 3-foot separation but again fails to include any references to support the effectiveness of masks (“Guidance for COVID-19 Prevention in K-12 Schools.” 2021 Aug 5).
Not surprisingly, into this void have stepped two pairs of experts – one group purporting to have evidence that masking is effective in school environments and the other warning that masks may not only be ineffective but that they also carry some significant downsides. And, where can you find these opposing positions? Not in The Lancet. Not in the New England Journal of Medicine. We don’t have time for any of that peer-reviewed monkey business. No, this is pandemic-era science where we have an abundance of opinions and paucity of facts. You will find these opposing articles on the op-ed pages of two of this country’s major newspapers.
In the Aug. 10, 2021, edition of the New York Times you will find an article (“We Studied One Million Students. This Is What We Learned About Masking”) by two pediatricians, Kanecia Zimmerman, MD, and Danny Benjamin Jr., MD, who have “studied” a million students in North Carolina school systems and tell us universal masking is “one of the most effective and efficient strategies for preventing SARS-CoV-2 transmission in schools. These investigators write that they “believe” the low rate of in school transmission they observed in North Carolina was “because of the mask-on-mask school environment.”
However, in the next paragraph the authors admit, “Because North Carolina had a mask mandate for all K-12 schools, we could not compare masked schools with unmasked schools.” They lean instead on studies from three other states with mask mandates that also had low transmission rates and a single report of an outbreak in Israel that employed neither masking nor safe distancing.
On the other side of the divide is an article in the Wall Street Journal titled “The Case Against Masks for Children” by Marty Makary, MD, and H. Cody Meissner, MD, (2021 Aug 9). The authors, one a pediatric infectious disease specialist, argue that there is “no science behind mask mandates for children.” And, observe that, of the $46 billion spent on research grants by the National Institutes of Health, “not a single grant was dedicated to studying masking in children.”
Dr. Makary and Dr. Meissner present a variety of concerns about the effects of masking including those on the development and communication skills of young children. None of their theoretical concerns of course are supported by controlled studies. They also observe that in previous studies children seem to be less likely to transmit COVID-19 than adults. Although we all know the landscape is changing with the emergence of the delta strain. In their strongest statement the authors claim, “It is abusive to force kids who struggle with them [masks] to sacrifice for the sake of unvaccinated adults.”
So there you have it. It is a situation we have come to expect over the last 2 years – plenty of opinions and too few facts supported by controlled studies. Both pairs of authors, however, agree on two things: Vaccination should continue to be considered our primary tool in prevention and control of COVID-19. and children need to be in school. Based on nothing more than a hunch and 7 decades of hunching, I tend to side with Dr. Makary and Dr. Meissner. Depending on the situation, I suggest masking but wouldn’t mandate it for children in school.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Traditionally, as the ides of August descend upon us we expect to be bombarded with advertisements encouraging parents and students to finish up their back-to-school shopping. But, this year the question on every parent and school administrator’s mind is not which color back pack will be the most popular this year but whether a mask should be a required part of the back-to-school ensemble.
The American Academy of Pediatrics has recommended that “All students older than 2 years and all school staff should wear a mask at school” (“American Academy of Pediatrics Updates Recommendations for Opening Schools in Fall 2021.” 2021 Jul 19). The academy’s statement includes a generous list of common sense caveats but it does not include a statement that masks have been shown to be protective for children in school environments. The Centers for Disease Control and Prevention “recommends” universal indoor masking along with keeping a 3-foot separation but again fails to include any references to support the effectiveness of masks (“Guidance for COVID-19 Prevention in K-12 Schools.” 2021 Aug 5).
Not surprisingly, into this void have stepped two pairs of experts – one group purporting to have evidence that masking is effective in school environments and the other warning that masks may not only be ineffective but that they also carry some significant downsides. And, where can you find these opposing positions? Not in The Lancet. Not in the New England Journal of Medicine. We don’t have time for any of that peer-reviewed monkey business. No, this is pandemic-era science where we have an abundance of opinions and paucity of facts. You will find these opposing articles on the op-ed pages of two of this country’s major newspapers.
In the Aug. 10, 2021, edition of the New York Times you will find an article (“We Studied One Million Students. This Is What We Learned About Masking”) by two pediatricians, Kanecia Zimmerman, MD, and Danny Benjamin Jr., MD, who have “studied” a million students in North Carolina school systems and tell us universal masking is “one of the most effective and efficient strategies for preventing SARS-CoV-2 transmission in schools. These investigators write that they “believe” the low rate of in school transmission they observed in North Carolina was “because of the mask-on-mask school environment.”
However, in the next paragraph the authors admit, “Because North Carolina had a mask mandate for all K-12 schools, we could not compare masked schools with unmasked schools.” They lean instead on studies from three other states with mask mandates that also had low transmission rates and a single report of an outbreak in Israel that employed neither masking nor safe distancing.
On the other side of the divide is an article in the Wall Street Journal titled “The Case Against Masks for Children” by Marty Makary, MD, and H. Cody Meissner, MD, (2021 Aug 9). The authors, one a pediatric infectious disease specialist, argue that there is “no science behind mask mandates for children.” And, observe that, of the $46 billion spent on research grants by the National Institutes of Health, “not a single grant was dedicated to studying masking in children.”
Dr. Makary and Dr. Meissner present a variety of concerns about the effects of masking including those on the development and communication skills of young children. None of their theoretical concerns of course are supported by controlled studies. They also observe that in previous studies children seem to be less likely to transmit COVID-19 than adults. Although we all know the landscape is changing with the emergence of the delta strain. In their strongest statement the authors claim, “It is abusive to force kids who struggle with them [masks] to sacrifice for the sake of unvaccinated adults.”
So there you have it. It is a situation we have come to expect over the last 2 years – plenty of opinions and too few facts supported by controlled studies. Both pairs of authors, however, agree on two things: Vaccination should continue to be considered our primary tool in prevention and control of COVID-19. and children need to be in school. Based on nothing more than a hunch and 7 decades of hunching, I tend to side with Dr. Makary and Dr. Meissner. Depending on the situation, I suggest masking but wouldn’t mandate it for children in school.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Traditionally, as the ides of August descend upon us we expect to be bombarded with advertisements encouraging parents and students to finish up their back-to-school shopping. But, this year the question on every parent and school administrator’s mind is not which color back pack will be the most popular this year but whether a mask should be a required part of the back-to-school ensemble.
The American Academy of Pediatrics has recommended that “All students older than 2 years and all school staff should wear a mask at school” (“American Academy of Pediatrics Updates Recommendations for Opening Schools in Fall 2021.” 2021 Jul 19). The academy’s statement includes a generous list of common sense caveats but it does not include a statement that masks have been shown to be protective for children in school environments. The Centers for Disease Control and Prevention “recommends” universal indoor masking along with keeping a 3-foot separation but again fails to include any references to support the effectiveness of masks (“Guidance for COVID-19 Prevention in K-12 Schools.” 2021 Aug 5).
Not surprisingly, into this void have stepped two pairs of experts – one group purporting to have evidence that masking is effective in school environments and the other warning that masks may not only be ineffective but that they also carry some significant downsides. And, where can you find these opposing positions? Not in The Lancet. Not in the New England Journal of Medicine. We don’t have time for any of that peer-reviewed monkey business. No, this is pandemic-era science where we have an abundance of opinions and paucity of facts. You will find these opposing articles on the op-ed pages of two of this country’s major newspapers.
In the Aug. 10, 2021, edition of the New York Times you will find an article (“We Studied One Million Students. This Is What We Learned About Masking”) by two pediatricians, Kanecia Zimmerman, MD, and Danny Benjamin Jr., MD, who have “studied” a million students in North Carolina school systems and tell us universal masking is “one of the most effective and efficient strategies for preventing SARS-CoV-2 transmission in schools. These investigators write that they “believe” the low rate of in school transmission they observed in North Carolina was “because of the mask-on-mask school environment.”
However, in the next paragraph the authors admit, “Because North Carolina had a mask mandate for all K-12 schools, we could not compare masked schools with unmasked schools.” They lean instead on studies from three other states with mask mandates that also had low transmission rates and a single report of an outbreak in Israel that employed neither masking nor safe distancing.
On the other side of the divide is an article in the Wall Street Journal titled “The Case Against Masks for Children” by Marty Makary, MD, and H. Cody Meissner, MD, (2021 Aug 9). The authors, one a pediatric infectious disease specialist, argue that there is “no science behind mask mandates for children.” And, observe that, of the $46 billion spent on research grants by the National Institutes of Health, “not a single grant was dedicated to studying masking in children.”
Dr. Makary and Dr. Meissner present a variety of concerns about the effects of masking including those on the development and communication skills of young children. None of their theoretical concerns of course are supported by controlled studies. They also observe that in previous studies children seem to be less likely to transmit COVID-19 than adults. Although we all know the landscape is changing with the emergence of the delta strain. In their strongest statement the authors claim, “It is abusive to force kids who struggle with them [masks] to sacrifice for the sake of unvaccinated adults.”
So there you have it. It is a situation we have come to expect over the last 2 years – plenty of opinions and too few facts supported by controlled studies. Both pairs of authors, however, agree on two things: Vaccination should continue to be considered our primary tool in prevention and control of COVID-19. and children need to be in school. Based on nothing more than a hunch and 7 decades of hunching, I tend to side with Dr. Makary and Dr. Meissner. Depending on the situation, I suggest masking but wouldn’t mandate it for children in school.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
COVID-19 mitigation measures led to shifts in typical annual respiratory virus patterns
Nonpharmaceutical interventions, such as masking, staying home, limiting travel, and social distancing, have been doing more than reducing the risk for COVID-19. They’re also having an impact on infection rates and the timing of seasonal surges of other common respiratory diseases, according to an article published July 23 in Morbidity and Mortality Weekly Report.
Typically, respiratory pathogens such as respiratory syncytial virus (RSV), common cold coronaviruses, parainfluenza viruses, and respiratory adenoviruses increase in the fall and remain high throughout winter, following the same basic patterns as influenza. Although the historically low rates of influenza remained low into spring 2021, that’s not the case for several other common respiratory viruses.
“Clinicians should be aware of increases in some respiratory virus activity and remain vigilant for off-season increases,” wrote Sonja J. Olsen, PhD, and her colleagues at the Centers for Disease Control and Prevention. She told this news organization that clinicians should use multipathogen testing to help guide treatment.
The authors also underscore the importance of fall influenza vaccination campaigns for anyone aged 6 months or older.
Timothy Brewer, MD, MPH, a professor of medicine in the Division of Infectious Diseases at the University of California, Los Angeles (UCLA), and of epidemiology at the UCLA Fielding School of Public Health, agreed that it’s important for health care professionals to consider off-season illnesses in their patients.
“Practitioners should be aware that if they see a sick child in the summer, outside of what normally might be influenza season, but they look like they have influenza, consider potentially influenza and test for it, because it might be possible that we may have disrupted that natural pattern,” Dr. Brewer told this news organization. Dr. Brewer, who was not involved in the CDC research, said it’s also “critically important” to encourage influenza vaccination as the season approaches.
The CDC researchers used the U.S. World Health Organization Collaborating Laboratories System and the CDC’s National Respiratory and Enteric Virus Surveillance System to analyze virologic data from Oct. 3, 2020, to May 22, 2021, for influenza and Jan. 4, 2020, to May 22, 2021, for other respiratory viruses. The authors compared virus circulation during these periods to circulation during the same dates from four previous years.
Data to calculate influenza and RSV hospitalization rates came from the Influenza Hospitalization Surveillance Network and RSV Hospitalization Surveillance Network.
The authors report that flu activity dropped dramatically in March 2020 to its lowest levels since 1997, the earliest season for which data are available. Only 0.2% of more than 1 million specimens tested positive for influenza; the rate of hospitalizations for lab-confirmed flu was 0.8 per 100,000 people. Flu levels remained low through the summer, fall, and on to May 2021.
A potential drawback to this low activity, however, is a more prevalent and severe upcoming flu season, the authors write. The repeated exposure to flu viruses every year often “does not lead to illness, but it does serve to boost our immune response to influenza viruses,” Dr. Olsen said in an interview. “The absence of influenza viruses in the community over the last year means that we are not getting these regular boosts to our immune system. When we finally get exposed, our body may mount a weak response, and this could mean we develop a more clinically severe illness.”
Children are most susceptible to that phenomenon because they haven’t had a lifetime of exposure to flu viruses, Dr. Olsen said.
“An immunologically naive child may be more likely to develop a severe illness than someone who has lived through several influenza seasons,” she said. “This is why it is especially important for everyone 6 months and older to get vaccinated against influenza this season.”
Rhinovirus and enterovirus infections rebounded fairly quickly after their decline in March 2020 and started increasing in May 2020 until they reached “near prepandemic seasonal levels,” the authors write.
RSV infections dropped from 15.3% of weekly positive results in January 2020 to 1.4% by April and then stayed below 1% through the end of 2020. In past years, weekly positive results climbed to 3% in October and peaked at 12.5% to 16.7% in late December. Instead, RSV weekly positive results began increasing in April 2021, rising from 1.1% to 2.8% in May.
The “unusually timed” late spring increase in RSV “is probably associated with various nonpharmaceutical measures that have been in place but are now relaxing,” Dr. Olsen stated.
The RSV hospitalization rate was 0.3 per 100,000 people from October 2020 to April 2021, compared to 27.1 and 33.4 per 100,000 people in the previous 2 years. Of all RSV hospitalizations in the past year, 76.5% occurred in April-May 2021.
Rates of illness caused by the four common human coronaviruses (OC43, NL63, 229E, and HKU1) dropped from 7.5% of weekly positive results in January 2020 to 1.3% in April 2020 and stayed below 1% through February 2021. Then they climbed to 6.6% by May 2021. Infection rates of parainfluenza viruses types 1-4 similarly dropped from 2.6% in January 2020 to 1% in March 2020 and stayed below 1% until April 2021. Since then, rates of the common coronaviruses increased to 6.6% and parainfluenza viruses to 10.9% in May 2021.
Normally, parainfluenza viruses peak in October-November and May-June, so “the current increase could represent a return to prepandemic seasonality,” the authors write.
Human pneumoviruses’ weekly positive results initially increased from 4.2% in January 2020 to 7% in March and then fell to 1.9% the second week of April and remained below 1% through May 2021. In typical years, these viruses peak from 6.2% to 7.7% in March-April. Respiratory adenovirus activity similarly dropped to historically low levels in April 2021 and then began increasing to reach 3% by May 2021, the usual level for that month.
“The different circulation patterns observed across respiratory viruses probably also reflect differences in the virus transmission routes and how effective various nonpharmaceutical measures are at stopping transmission,” Dr. Olsen said in an interview. “As pandemic mitigation measures continue to be adjusted, we expect to see more changes in the circulation of these viruses, including a return to prepandemic circulation, as seen for rhinoviruses and enteroviruses.”
Rhinovirus and enterovirus rates dropped from 14.9% in March 2020 to 3.2% in May – lower than typical – and then climbed to a peak in October 2020. The peak (21.7% weekly positive results) was, however, still lower than the usual median of 32.8%. After dropping to 9.9% in January 2021, it then rose 19.1% in May, potentially reflecting “the usual spring peak that has occurred in previous years,” the authors write.
The authors note that it’s not yet clear how the COVID-19 pandemic and related mitigation measures will continue to affect respiratory virus circulation.
The authors hypothesize that the reasons for a seeming return to seasonal activity of respiratory adenoviruses, rhinoviruses, and enteroviruses could involve “different transmission mechanisms, the role of asymptomatic transmission, and prolonged survival of these nonenveloped viruses on surfaces, all of which might make these viruses less susceptible to nonpharmaceutical interventions.”
Dr. Brewer, of UCLA, agreed.
All the viruses basically “flatline except for adenoviruses and enteroviruses, and they behave a little differently in terms of how they spread,” he said. “Enteroviruses are much more likely to be fecal-oral spread than the other viruses [in the study].”
The delayed circulation of parainfluenza and human coronaviruses may have resulted from suspension of in-person classes through late winter 2020, they write, but that doesn’t explain the relative absence of pneumovirus activity, which usually affects the same young pediatric populations as RSV.
Dr. Brewer said California is seeing a surge of RSV right now, as are many states, especially throughout in the South. He’s not surprised by RSV’s deferred season, because those most affected – children younger than 2 years – are less likely to wear masks now and were “not going to daycare, not being out in public” in 2020. “As people are doing more activities, that’s probably why RSV has been starting to go up since April,” he said.
Despite the fact that, unlike many East Asian cultures, the United States has not traditionally been a mask-wearing culture, Dr. Brewer wouldn’t be surprised if more Americans begin wearing masks during flu season. “Hopefully another thing that will come out of this is better hand hygiene, with people just getting used to washing their hands more, particularly after they come home from being out,” he added.
Dr. Brewer similarly emphasized the importance of flu vaccination for the upcoming season, especially for younger children who may have poorer natural immunity to influenza, owing to its low circulation rates in 2020-2021.
The study was funded by the CDC. Dr. Brewer and Dr. Olsen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nonpharmaceutical interventions, such as masking, staying home, limiting travel, and social distancing, have been doing more than reducing the risk for COVID-19. They’re also having an impact on infection rates and the timing of seasonal surges of other common respiratory diseases, according to an article published July 23 in Morbidity and Mortality Weekly Report.
Typically, respiratory pathogens such as respiratory syncytial virus (RSV), common cold coronaviruses, parainfluenza viruses, and respiratory adenoviruses increase in the fall and remain high throughout winter, following the same basic patterns as influenza. Although the historically low rates of influenza remained low into spring 2021, that’s not the case for several other common respiratory viruses.
“Clinicians should be aware of increases in some respiratory virus activity and remain vigilant for off-season increases,” wrote Sonja J. Olsen, PhD, and her colleagues at the Centers for Disease Control and Prevention. She told this news organization that clinicians should use multipathogen testing to help guide treatment.
The authors also underscore the importance of fall influenza vaccination campaigns for anyone aged 6 months or older.
Timothy Brewer, MD, MPH, a professor of medicine in the Division of Infectious Diseases at the University of California, Los Angeles (UCLA), and of epidemiology at the UCLA Fielding School of Public Health, agreed that it’s important for health care professionals to consider off-season illnesses in their patients.
“Practitioners should be aware that if they see a sick child in the summer, outside of what normally might be influenza season, but they look like they have influenza, consider potentially influenza and test for it, because it might be possible that we may have disrupted that natural pattern,” Dr. Brewer told this news organization. Dr. Brewer, who was not involved in the CDC research, said it’s also “critically important” to encourage influenza vaccination as the season approaches.
The CDC researchers used the U.S. World Health Organization Collaborating Laboratories System and the CDC’s National Respiratory and Enteric Virus Surveillance System to analyze virologic data from Oct. 3, 2020, to May 22, 2021, for influenza and Jan. 4, 2020, to May 22, 2021, for other respiratory viruses. The authors compared virus circulation during these periods to circulation during the same dates from four previous years.
Data to calculate influenza and RSV hospitalization rates came from the Influenza Hospitalization Surveillance Network and RSV Hospitalization Surveillance Network.
The authors report that flu activity dropped dramatically in March 2020 to its lowest levels since 1997, the earliest season for which data are available. Only 0.2% of more than 1 million specimens tested positive for influenza; the rate of hospitalizations for lab-confirmed flu was 0.8 per 100,000 people. Flu levels remained low through the summer, fall, and on to May 2021.
A potential drawback to this low activity, however, is a more prevalent and severe upcoming flu season, the authors write. The repeated exposure to flu viruses every year often “does not lead to illness, but it does serve to boost our immune response to influenza viruses,” Dr. Olsen said in an interview. “The absence of influenza viruses in the community over the last year means that we are not getting these regular boosts to our immune system. When we finally get exposed, our body may mount a weak response, and this could mean we develop a more clinically severe illness.”
Children are most susceptible to that phenomenon because they haven’t had a lifetime of exposure to flu viruses, Dr. Olsen said.
“An immunologically naive child may be more likely to develop a severe illness than someone who has lived through several influenza seasons,” she said. “This is why it is especially important for everyone 6 months and older to get vaccinated against influenza this season.”
Rhinovirus and enterovirus infections rebounded fairly quickly after their decline in March 2020 and started increasing in May 2020 until they reached “near prepandemic seasonal levels,” the authors write.
RSV infections dropped from 15.3% of weekly positive results in January 2020 to 1.4% by April and then stayed below 1% through the end of 2020. In past years, weekly positive results climbed to 3% in October and peaked at 12.5% to 16.7% in late December. Instead, RSV weekly positive results began increasing in April 2021, rising from 1.1% to 2.8% in May.
The “unusually timed” late spring increase in RSV “is probably associated with various nonpharmaceutical measures that have been in place but are now relaxing,” Dr. Olsen stated.
The RSV hospitalization rate was 0.3 per 100,000 people from October 2020 to April 2021, compared to 27.1 and 33.4 per 100,000 people in the previous 2 years. Of all RSV hospitalizations in the past year, 76.5% occurred in April-May 2021.
Rates of illness caused by the four common human coronaviruses (OC43, NL63, 229E, and HKU1) dropped from 7.5% of weekly positive results in January 2020 to 1.3% in April 2020 and stayed below 1% through February 2021. Then they climbed to 6.6% by May 2021. Infection rates of parainfluenza viruses types 1-4 similarly dropped from 2.6% in January 2020 to 1% in March 2020 and stayed below 1% until April 2021. Since then, rates of the common coronaviruses increased to 6.6% and parainfluenza viruses to 10.9% in May 2021.
Normally, parainfluenza viruses peak in October-November and May-June, so “the current increase could represent a return to prepandemic seasonality,” the authors write.
Human pneumoviruses’ weekly positive results initially increased from 4.2% in January 2020 to 7% in March and then fell to 1.9% the second week of April and remained below 1% through May 2021. In typical years, these viruses peak from 6.2% to 7.7% in March-April. Respiratory adenovirus activity similarly dropped to historically low levels in April 2021 and then began increasing to reach 3% by May 2021, the usual level for that month.
“The different circulation patterns observed across respiratory viruses probably also reflect differences in the virus transmission routes and how effective various nonpharmaceutical measures are at stopping transmission,” Dr. Olsen said in an interview. “As pandemic mitigation measures continue to be adjusted, we expect to see more changes in the circulation of these viruses, including a return to prepandemic circulation, as seen for rhinoviruses and enteroviruses.”
Rhinovirus and enterovirus rates dropped from 14.9% in March 2020 to 3.2% in May – lower than typical – and then climbed to a peak in October 2020. The peak (21.7% weekly positive results) was, however, still lower than the usual median of 32.8%. After dropping to 9.9% in January 2021, it then rose 19.1% in May, potentially reflecting “the usual spring peak that has occurred in previous years,” the authors write.
The authors note that it’s not yet clear how the COVID-19 pandemic and related mitigation measures will continue to affect respiratory virus circulation.
The authors hypothesize that the reasons for a seeming return to seasonal activity of respiratory adenoviruses, rhinoviruses, and enteroviruses could involve “different transmission mechanisms, the role of asymptomatic transmission, and prolonged survival of these nonenveloped viruses on surfaces, all of which might make these viruses less susceptible to nonpharmaceutical interventions.”
Dr. Brewer, of UCLA, agreed.
All the viruses basically “flatline except for adenoviruses and enteroviruses, and they behave a little differently in terms of how they spread,” he said. “Enteroviruses are much more likely to be fecal-oral spread than the other viruses [in the study].”
The delayed circulation of parainfluenza and human coronaviruses may have resulted from suspension of in-person classes through late winter 2020, they write, but that doesn’t explain the relative absence of pneumovirus activity, which usually affects the same young pediatric populations as RSV.
Dr. Brewer said California is seeing a surge of RSV right now, as are many states, especially throughout in the South. He’s not surprised by RSV’s deferred season, because those most affected – children younger than 2 years – are less likely to wear masks now and were “not going to daycare, not being out in public” in 2020. “As people are doing more activities, that’s probably why RSV has been starting to go up since April,” he said.
Despite the fact that, unlike many East Asian cultures, the United States has not traditionally been a mask-wearing culture, Dr. Brewer wouldn’t be surprised if more Americans begin wearing masks during flu season. “Hopefully another thing that will come out of this is better hand hygiene, with people just getting used to washing their hands more, particularly after they come home from being out,” he added.
Dr. Brewer similarly emphasized the importance of flu vaccination for the upcoming season, especially for younger children who may have poorer natural immunity to influenza, owing to its low circulation rates in 2020-2021.
The study was funded by the CDC. Dr. Brewer and Dr. Olsen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Nonpharmaceutical interventions, such as masking, staying home, limiting travel, and social distancing, have been doing more than reducing the risk for COVID-19. They’re also having an impact on infection rates and the timing of seasonal surges of other common respiratory diseases, according to an article published July 23 in Morbidity and Mortality Weekly Report.
Typically, respiratory pathogens such as respiratory syncytial virus (RSV), common cold coronaviruses, parainfluenza viruses, and respiratory adenoviruses increase in the fall and remain high throughout winter, following the same basic patterns as influenza. Although the historically low rates of influenza remained low into spring 2021, that’s not the case for several other common respiratory viruses.
“Clinicians should be aware of increases in some respiratory virus activity and remain vigilant for off-season increases,” wrote Sonja J. Olsen, PhD, and her colleagues at the Centers for Disease Control and Prevention. She told this news organization that clinicians should use multipathogen testing to help guide treatment.
The authors also underscore the importance of fall influenza vaccination campaigns for anyone aged 6 months or older.
Timothy Brewer, MD, MPH, a professor of medicine in the Division of Infectious Diseases at the University of California, Los Angeles (UCLA), and of epidemiology at the UCLA Fielding School of Public Health, agreed that it’s important for health care professionals to consider off-season illnesses in their patients.
“Practitioners should be aware that if they see a sick child in the summer, outside of what normally might be influenza season, but they look like they have influenza, consider potentially influenza and test for it, because it might be possible that we may have disrupted that natural pattern,” Dr. Brewer told this news organization. Dr. Brewer, who was not involved in the CDC research, said it’s also “critically important” to encourage influenza vaccination as the season approaches.
The CDC researchers used the U.S. World Health Organization Collaborating Laboratories System and the CDC’s National Respiratory and Enteric Virus Surveillance System to analyze virologic data from Oct. 3, 2020, to May 22, 2021, for influenza and Jan. 4, 2020, to May 22, 2021, for other respiratory viruses. The authors compared virus circulation during these periods to circulation during the same dates from four previous years.
Data to calculate influenza and RSV hospitalization rates came from the Influenza Hospitalization Surveillance Network and RSV Hospitalization Surveillance Network.
The authors report that flu activity dropped dramatically in March 2020 to its lowest levels since 1997, the earliest season for which data are available. Only 0.2% of more than 1 million specimens tested positive for influenza; the rate of hospitalizations for lab-confirmed flu was 0.8 per 100,000 people. Flu levels remained low through the summer, fall, and on to May 2021.
A potential drawback to this low activity, however, is a more prevalent and severe upcoming flu season, the authors write. The repeated exposure to flu viruses every year often “does not lead to illness, but it does serve to boost our immune response to influenza viruses,” Dr. Olsen said in an interview. “The absence of influenza viruses in the community over the last year means that we are not getting these regular boosts to our immune system. When we finally get exposed, our body may mount a weak response, and this could mean we develop a more clinically severe illness.”
Children are most susceptible to that phenomenon because they haven’t had a lifetime of exposure to flu viruses, Dr. Olsen said.
“An immunologically naive child may be more likely to develop a severe illness than someone who has lived through several influenza seasons,” she said. “This is why it is especially important for everyone 6 months and older to get vaccinated against influenza this season.”
Rhinovirus and enterovirus infections rebounded fairly quickly after their decline in March 2020 and started increasing in May 2020 until they reached “near prepandemic seasonal levels,” the authors write.
RSV infections dropped from 15.3% of weekly positive results in January 2020 to 1.4% by April and then stayed below 1% through the end of 2020. In past years, weekly positive results climbed to 3% in October and peaked at 12.5% to 16.7% in late December. Instead, RSV weekly positive results began increasing in April 2021, rising from 1.1% to 2.8% in May.
The “unusually timed” late spring increase in RSV “is probably associated with various nonpharmaceutical measures that have been in place but are now relaxing,” Dr. Olsen stated.
The RSV hospitalization rate was 0.3 per 100,000 people from October 2020 to April 2021, compared to 27.1 and 33.4 per 100,000 people in the previous 2 years. Of all RSV hospitalizations in the past year, 76.5% occurred in April-May 2021.
Rates of illness caused by the four common human coronaviruses (OC43, NL63, 229E, and HKU1) dropped from 7.5% of weekly positive results in January 2020 to 1.3% in April 2020 and stayed below 1% through February 2021. Then they climbed to 6.6% by May 2021. Infection rates of parainfluenza viruses types 1-4 similarly dropped from 2.6% in January 2020 to 1% in March 2020 and stayed below 1% until April 2021. Since then, rates of the common coronaviruses increased to 6.6% and parainfluenza viruses to 10.9% in May 2021.
Normally, parainfluenza viruses peak in October-November and May-June, so “the current increase could represent a return to prepandemic seasonality,” the authors write.
Human pneumoviruses’ weekly positive results initially increased from 4.2% in January 2020 to 7% in March and then fell to 1.9% the second week of April and remained below 1% through May 2021. In typical years, these viruses peak from 6.2% to 7.7% in March-April. Respiratory adenovirus activity similarly dropped to historically low levels in April 2021 and then began increasing to reach 3% by May 2021, the usual level for that month.
“The different circulation patterns observed across respiratory viruses probably also reflect differences in the virus transmission routes and how effective various nonpharmaceutical measures are at stopping transmission,” Dr. Olsen said in an interview. “As pandemic mitigation measures continue to be adjusted, we expect to see more changes in the circulation of these viruses, including a return to prepandemic circulation, as seen for rhinoviruses and enteroviruses.”
Rhinovirus and enterovirus rates dropped from 14.9% in March 2020 to 3.2% in May – lower than typical – and then climbed to a peak in October 2020. The peak (21.7% weekly positive results) was, however, still lower than the usual median of 32.8%. After dropping to 9.9% in January 2021, it then rose 19.1% in May, potentially reflecting “the usual spring peak that has occurred in previous years,” the authors write.
The authors note that it’s not yet clear how the COVID-19 pandemic and related mitigation measures will continue to affect respiratory virus circulation.
The authors hypothesize that the reasons for a seeming return to seasonal activity of respiratory adenoviruses, rhinoviruses, and enteroviruses could involve “different transmission mechanisms, the role of asymptomatic transmission, and prolonged survival of these nonenveloped viruses on surfaces, all of which might make these viruses less susceptible to nonpharmaceutical interventions.”
Dr. Brewer, of UCLA, agreed.
All the viruses basically “flatline except for adenoviruses and enteroviruses, and they behave a little differently in terms of how they spread,” he said. “Enteroviruses are much more likely to be fecal-oral spread than the other viruses [in the study].”
The delayed circulation of parainfluenza and human coronaviruses may have resulted from suspension of in-person classes through late winter 2020, they write, but that doesn’t explain the relative absence of pneumovirus activity, which usually affects the same young pediatric populations as RSV.
Dr. Brewer said California is seeing a surge of RSV right now, as are many states, especially throughout in the South. He’s not surprised by RSV’s deferred season, because those most affected – children younger than 2 years – are less likely to wear masks now and were “not going to daycare, not being out in public” in 2020. “As people are doing more activities, that’s probably why RSV has been starting to go up since April,” he said.
Despite the fact that, unlike many East Asian cultures, the United States has not traditionally been a mask-wearing culture, Dr. Brewer wouldn’t be surprised if more Americans begin wearing masks during flu season. “Hopefully another thing that will come out of this is better hand hygiene, with people just getting used to washing their hands more, particularly after they come home from being out,” he added.
Dr. Brewer similarly emphasized the importance of flu vaccination for the upcoming season, especially for younger children who may have poorer natural immunity to influenza, owing to its low circulation rates in 2020-2021.
The study was funded by the CDC. Dr. Brewer and Dr. Olsen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anaplasmosis quadruples in New York state
Anaplasmosis prevalence in New York state nearly quadrupled statewide from 2010 to 2018, new research suggests, increasing by more than eightfold in the region surrounding Albany, the state capital. The proportion of ticks carrying Anaplasma phagocytophilum, the bacterium that causes the tick-borne disease, also increased during the study period.
Although not as well-recognized as Lyme disease, anaplasmosis is one of the most common tickborne diseases in the United States. The bacterial disease is primarily transmitted to humans by the bites of blacklegged ticks infected with A. phagocytophilum, and often causes fever, headache, muscle aches, and chills. If treatment is delayed – or if a patient has underlying medical conditions – anaplasmosis can lead to difficulty breathing, bleeding problems, organ failure, and even death.
Since anaplasmosis become a nationally notifiable disease in 1999, cases have increased 16-fold in the United States, from 351 cases in 2000 to a high of 5,762 cases in 2017, according to data from the Centers for Disease Control and Prevention. Just eight states – Vermont, Maine, Rhode Island, Minnesota, Massachusetts, Wisconsin, New Hampshire, and New York – make up 90% of reported cases.
“While Lyme disease remains the most common tick-borne illness reported in New York state, anaplasmosis continues to account for a growing proportion of our tick-borne disease cases each year,” Melissa Prusinski, a research scientist at the New York State Department of Health and author of the study, told this news organization in an email. “It is critically important to investigate the environmental and epidemiological drivers facilitating this increase to better understand why and how risk for this serious illness is increasing.” The results were published in Emerging Infectious Diseases.
For the study, investigators analyzed human anaplasmosis cases reported to the New York State Department of Health from 2010-2018. They also included data from tick collection and pathogen testing in order to determine whether the prevalence of A. phagocytophilum in ticks increased along with cases. All New York State counties were included in the study, apart from the five boroughs of New York City: Manhattan, Brooklyn, the Bronx, Queens, and Staten Island.
There were 5,146 reported anaplasmosis cases in New York, with annual case numbers peaking at 1,112 in 2017. Researchers reported a dip in cases in 2018, a trend that was also seen nationally. Anaplasmosis incidence surged in the area surrounding Albany, increasing 8.4-fold from 4.3 cases per 100,000 people in 2010 to 36.3 cases per 100,000 persons in 2018.
Ms. Prusinski noted that the rapid increase in and around this inland hot spot is unlike the gradual spread of Lyme disease and other tick-borne illnesses like babesiosis, which spreads from coastal areas both northward and westward across New York. The research team also found that the incidence of ticks infected with A. phagocytophilum nearly doubled statewide and increased fourfold – from 2.9% to 12% – between 2010 and 2018 in the Albany area.
This increase in cases could be the result, at least in part, of more robust testing efforts over time, said Susan Elias, PhD, of the Vector-Borne Disease Laboratory at the Maine Medical Center Research Institute in Scarborough. She was not involved with the recently published study. “The more you look for something, the more you find,” she said. For example, she added, a 602% surge in anaplasmosis cases in Maine from 2013-2017 occurred alongside a 10-fold increase in use of tick-borne disease panels that test for multiple pathogens.
Ms. Prusinski agreed that increased testing at least partially explains the surge of cases in New York, but she did not have data on how many tick-borne disease panels were used to diagnose cases in the state.
Proliferation of A. phagocytophilum in tick populations could also partially explain this dramatic increase in cases. With the suburbanization of America, “we have basically laid out a buffet” for ticks, Dr. Elias said. Patches of forest and yards create edge habitats where ticks, and the small mammals they feed on, thrive. “Then, once you have a large expanding blacklegged tick population, it makes it easier for the pathogens and carriers to amplify,” she added.
While the study did not differentiate between a variant of A. phagocytophilum associated with small mammals that causes illness and another found in white-tailed deer that is nonpathogenic, Ms. Prusinski suspects that the infectious variant is likely more prevalent and is circulating in animals and ticks in and around Albany. Research is ongoing to see if this could help explain the spread of disease in this anaplasmosis hotspot.
“The unique geographic pattern of anaplasmosis spread in New York state and elsewhere leads to many further questions about the vector ecology and epidemiology of this emerging tick-borne illness,” Ms. Prusinski added. “Learning all we can about this dynamic disease system will help us better identify at-risk populations and may lead to novel ways to prevent anaplasmosis.”
Dr. Elias and Ms. Prusinski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anaplasmosis prevalence in New York state nearly quadrupled statewide from 2010 to 2018, new research suggests, increasing by more than eightfold in the region surrounding Albany, the state capital. The proportion of ticks carrying Anaplasma phagocytophilum, the bacterium that causes the tick-borne disease, also increased during the study period.
Although not as well-recognized as Lyme disease, anaplasmosis is one of the most common tickborne diseases in the United States. The bacterial disease is primarily transmitted to humans by the bites of blacklegged ticks infected with A. phagocytophilum, and often causes fever, headache, muscle aches, and chills. If treatment is delayed – or if a patient has underlying medical conditions – anaplasmosis can lead to difficulty breathing, bleeding problems, organ failure, and even death.
Since anaplasmosis become a nationally notifiable disease in 1999, cases have increased 16-fold in the United States, from 351 cases in 2000 to a high of 5,762 cases in 2017, according to data from the Centers for Disease Control and Prevention. Just eight states – Vermont, Maine, Rhode Island, Minnesota, Massachusetts, Wisconsin, New Hampshire, and New York – make up 90% of reported cases.
“While Lyme disease remains the most common tick-borne illness reported in New York state, anaplasmosis continues to account for a growing proportion of our tick-borne disease cases each year,” Melissa Prusinski, a research scientist at the New York State Department of Health and author of the study, told this news organization in an email. “It is critically important to investigate the environmental and epidemiological drivers facilitating this increase to better understand why and how risk for this serious illness is increasing.” The results were published in Emerging Infectious Diseases.
For the study, investigators analyzed human anaplasmosis cases reported to the New York State Department of Health from 2010-2018. They also included data from tick collection and pathogen testing in order to determine whether the prevalence of A. phagocytophilum in ticks increased along with cases. All New York State counties were included in the study, apart from the five boroughs of New York City: Manhattan, Brooklyn, the Bronx, Queens, and Staten Island.
There were 5,146 reported anaplasmosis cases in New York, with annual case numbers peaking at 1,112 in 2017. Researchers reported a dip in cases in 2018, a trend that was also seen nationally. Anaplasmosis incidence surged in the area surrounding Albany, increasing 8.4-fold from 4.3 cases per 100,000 people in 2010 to 36.3 cases per 100,000 persons in 2018.
Ms. Prusinski noted that the rapid increase in and around this inland hot spot is unlike the gradual spread of Lyme disease and other tick-borne illnesses like babesiosis, which spreads from coastal areas both northward and westward across New York. The research team also found that the incidence of ticks infected with A. phagocytophilum nearly doubled statewide and increased fourfold – from 2.9% to 12% – between 2010 and 2018 in the Albany area.
This increase in cases could be the result, at least in part, of more robust testing efforts over time, said Susan Elias, PhD, of the Vector-Borne Disease Laboratory at the Maine Medical Center Research Institute in Scarborough. She was not involved with the recently published study. “The more you look for something, the more you find,” she said. For example, she added, a 602% surge in anaplasmosis cases in Maine from 2013-2017 occurred alongside a 10-fold increase in use of tick-borne disease panels that test for multiple pathogens.
Ms. Prusinski agreed that increased testing at least partially explains the surge of cases in New York, but she did not have data on how many tick-borne disease panels were used to diagnose cases in the state.
Proliferation of A. phagocytophilum in tick populations could also partially explain this dramatic increase in cases. With the suburbanization of America, “we have basically laid out a buffet” for ticks, Dr. Elias said. Patches of forest and yards create edge habitats where ticks, and the small mammals they feed on, thrive. “Then, once you have a large expanding blacklegged tick population, it makes it easier for the pathogens and carriers to amplify,” she added.
While the study did not differentiate between a variant of A. phagocytophilum associated with small mammals that causes illness and another found in white-tailed deer that is nonpathogenic, Ms. Prusinski suspects that the infectious variant is likely more prevalent and is circulating in animals and ticks in and around Albany. Research is ongoing to see if this could help explain the spread of disease in this anaplasmosis hotspot.
“The unique geographic pattern of anaplasmosis spread in New York state and elsewhere leads to many further questions about the vector ecology and epidemiology of this emerging tick-borne illness,” Ms. Prusinski added. “Learning all we can about this dynamic disease system will help us better identify at-risk populations and may lead to novel ways to prevent anaplasmosis.”
Dr. Elias and Ms. Prusinski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Anaplasmosis prevalence in New York state nearly quadrupled statewide from 2010 to 2018, new research suggests, increasing by more than eightfold in the region surrounding Albany, the state capital. The proportion of ticks carrying Anaplasma phagocytophilum, the bacterium that causes the tick-borne disease, also increased during the study period.
Although not as well-recognized as Lyme disease, anaplasmosis is one of the most common tickborne diseases in the United States. The bacterial disease is primarily transmitted to humans by the bites of blacklegged ticks infected with A. phagocytophilum, and often causes fever, headache, muscle aches, and chills. If treatment is delayed – or if a patient has underlying medical conditions – anaplasmosis can lead to difficulty breathing, bleeding problems, organ failure, and even death.
Since anaplasmosis become a nationally notifiable disease in 1999, cases have increased 16-fold in the United States, from 351 cases in 2000 to a high of 5,762 cases in 2017, according to data from the Centers for Disease Control and Prevention. Just eight states – Vermont, Maine, Rhode Island, Minnesota, Massachusetts, Wisconsin, New Hampshire, and New York – make up 90% of reported cases.
“While Lyme disease remains the most common tick-borne illness reported in New York state, anaplasmosis continues to account for a growing proportion of our tick-borne disease cases each year,” Melissa Prusinski, a research scientist at the New York State Department of Health and author of the study, told this news organization in an email. “It is critically important to investigate the environmental and epidemiological drivers facilitating this increase to better understand why and how risk for this serious illness is increasing.” The results were published in Emerging Infectious Diseases.
For the study, investigators analyzed human anaplasmosis cases reported to the New York State Department of Health from 2010-2018. They also included data from tick collection and pathogen testing in order to determine whether the prevalence of A. phagocytophilum in ticks increased along with cases. All New York State counties were included in the study, apart from the five boroughs of New York City: Manhattan, Brooklyn, the Bronx, Queens, and Staten Island.
There were 5,146 reported anaplasmosis cases in New York, with annual case numbers peaking at 1,112 in 2017. Researchers reported a dip in cases in 2018, a trend that was also seen nationally. Anaplasmosis incidence surged in the area surrounding Albany, increasing 8.4-fold from 4.3 cases per 100,000 people in 2010 to 36.3 cases per 100,000 persons in 2018.
Ms. Prusinski noted that the rapid increase in and around this inland hot spot is unlike the gradual spread of Lyme disease and other tick-borne illnesses like babesiosis, which spreads from coastal areas both northward and westward across New York. The research team also found that the incidence of ticks infected with A. phagocytophilum nearly doubled statewide and increased fourfold – from 2.9% to 12% – between 2010 and 2018 in the Albany area.
This increase in cases could be the result, at least in part, of more robust testing efforts over time, said Susan Elias, PhD, of the Vector-Borne Disease Laboratory at the Maine Medical Center Research Institute in Scarborough. She was not involved with the recently published study. “The more you look for something, the more you find,” she said. For example, she added, a 602% surge in anaplasmosis cases in Maine from 2013-2017 occurred alongside a 10-fold increase in use of tick-borne disease panels that test for multiple pathogens.
Ms. Prusinski agreed that increased testing at least partially explains the surge of cases in New York, but she did not have data on how many tick-borne disease panels were used to diagnose cases in the state.
Proliferation of A. phagocytophilum in tick populations could also partially explain this dramatic increase in cases. With the suburbanization of America, “we have basically laid out a buffet” for ticks, Dr. Elias said. Patches of forest and yards create edge habitats where ticks, and the small mammals they feed on, thrive. “Then, once you have a large expanding blacklegged tick population, it makes it easier for the pathogens and carriers to amplify,” she added.
While the study did not differentiate between a variant of A. phagocytophilum associated with small mammals that causes illness and another found in white-tailed deer that is nonpathogenic, Ms. Prusinski suspects that the infectious variant is likely more prevalent and is circulating in animals and ticks in and around Albany. Research is ongoing to see if this could help explain the spread of disease in this anaplasmosis hotspot.
“The unique geographic pattern of anaplasmosis spread in New York state and elsewhere leads to many further questions about the vector ecology and epidemiology of this emerging tick-borne illness,” Ms. Prusinski added. “Learning all we can about this dynamic disease system will help us better identify at-risk populations and may lead to novel ways to prevent anaplasmosis.”
Dr. Elias and Ms. Prusinski disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Surge of new child COVID cases continues for 6th consecutive week
The current COVID-19 surge has brought new cases in children to their highest level since February, according to a new report.
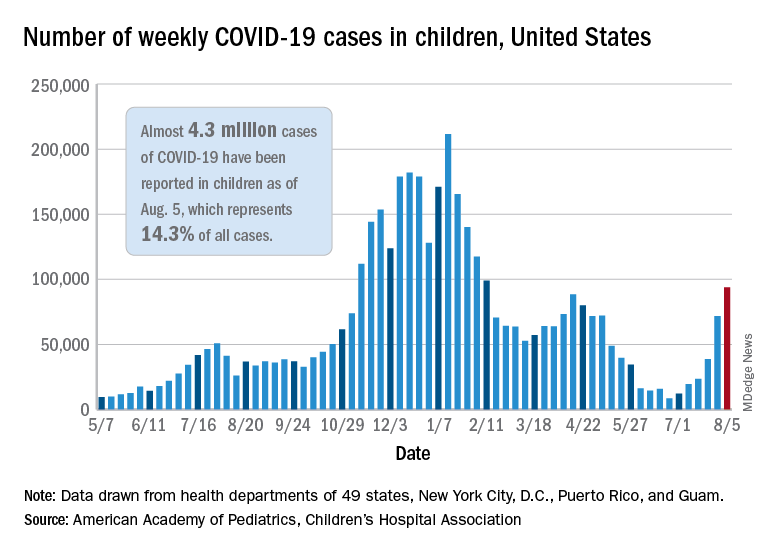
New pediatric cases rose for the 6th straight week, with almost 94,000 reported for the week ending Aug. 5.
That weekly total was up by 31% over the previous week and by over 1,000% since late June, when the new-case figure was at its lowest point (8,447) since early in the pandemic, the American Academy of Pediatrics and the Children’s Hospital Association said. COVID-related deaths – 13 for the week – were also higher than at any time since March 2021.
Almost 4.3 million children have been infected with SARS-CoV-2, which is 14.3% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. Children represented 15.0% of the new cases reported in those jurisdictions during the week ending Aug. 5, the AAP and CHA said in their weekly report.
Another measure that has been trending upward recently is vaccine initiation among 12- to 15-year-olds, although the latest weekly total is still well below the high of 1.4 million seen in May. First-time vaccinations reached almost 411,000 for the week of Aug. 3-9, marking the fourth consecutive increase in that age group, the Centers for Disease Control and Prevention said on its COVID Data Tracker. Vaccinations also increased, although more modestly, for 16- and 17-year-olds in the most recent week.
Cumulative figures for children aged 12-17 show that almost 10.4 million have received at least one dose and that 7.7 million are fully vaccinated as of Aug. 9. By age group, 42.2% of those aged 12-15 have received at least one dose, and 30.4% have completed the vaccine regimen. Among those aged 16-17 years, 52.2% have gotten their first dose, and 41.4% are fully vaccinated, according to the COVID Data Tracker.
Looking at vaccination rates on the state level shows that only 20% of children aged 12-17 in Wyoming and 21% in Mississippi have gotten at least one dose as of Aug. 4, while Massachusetts is up to 68% and Vermont reports 70%. Rates for full vaccination range from 11% in Mississippi and Alabama to 61% in Vermont, based on an AAP analysis of CDC data, which is not available for Idaho.
The current COVID-19 surge has brought new cases in children to their highest level since February, according to a new report.

New pediatric cases rose for the 6th straight week, with almost 94,000 reported for the week ending Aug. 5.
That weekly total was up by 31% over the previous week and by over 1,000% since late June, when the new-case figure was at its lowest point (8,447) since early in the pandemic, the American Academy of Pediatrics and the Children’s Hospital Association said. COVID-related deaths – 13 for the week – were also higher than at any time since March 2021.
Almost 4.3 million children have been infected with SARS-CoV-2, which is 14.3% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. Children represented 15.0% of the new cases reported in those jurisdictions during the week ending Aug. 5, the AAP and CHA said in their weekly report.
Another measure that has been trending upward recently is vaccine initiation among 12- to 15-year-olds, although the latest weekly total is still well below the high of 1.4 million seen in May. First-time vaccinations reached almost 411,000 for the week of Aug. 3-9, marking the fourth consecutive increase in that age group, the Centers for Disease Control and Prevention said on its COVID Data Tracker. Vaccinations also increased, although more modestly, for 16- and 17-year-olds in the most recent week.
Cumulative figures for children aged 12-17 show that almost 10.4 million have received at least one dose and that 7.7 million are fully vaccinated as of Aug. 9. By age group, 42.2% of those aged 12-15 have received at least one dose, and 30.4% have completed the vaccine regimen. Among those aged 16-17 years, 52.2% have gotten their first dose, and 41.4% are fully vaccinated, according to the COVID Data Tracker.
Looking at vaccination rates on the state level shows that only 20% of children aged 12-17 in Wyoming and 21% in Mississippi have gotten at least one dose as of Aug. 4, while Massachusetts is up to 68% and Vermont reports 70%. Rates for full vaccination range from 11% in Mississippi and Alabama to 61% in Vermont, based on an AAP analysis of CDC data, which is not available for Idaho.
The current COVID-19 surge has brought new cases in children to their highest level since February, according to a new report.

New pediatric cases rose for the 6th straight week, with almost 94,000 reported for the week ending Aug. 5.
That weekly total was up by 31% over the previous week and by over 1,000% since late June, when the new-case figure was at its lowest point (8,447) since early in the pandemic, the American Academy of Pediatrics and the Children’s Hospital Association said. COVID-related deaths – 13 for the week – were also higher than at any time since March 2021.
Almost 4.3 million children have been infected with SARS-CoV-2, which is 14.3% of all cases reported in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. Children represented 15.0% of the new cases reported in those jurisdictions during the week ending Aug. 5, the AAP and CHA said in their weekly report.
Another measure that has been trending upward recently is vaccine initiation among 12- to 15-year-olds, although the latest weekly total is still well below the high of 1.4 million seen in May. First-time vaccinations reached almost 411,000 for the week of Aug. 3-9, marking the fourth consecutive increase in that age group, the Centers for Disease Control and Prevention said on its COVID Data Tracker. Vaccinations also increased, although more modestly, for 16- and 17-year-olds in the most recent week.
Cumulative figures for children aged 12-17 show that almost 10.4 million have received at least one dose and that 7.7 million are fully vaccinated as of Aug. 9. By age group, 42.2% of those aged 12-15 have received at least one dose, and 30.4% have completed the vaccine regimen. Among those aged 16-17 years, 52.2% have gotten their first dose, and 41.4% are fully vaccinated, according to the COVID Data Tracker.
Looking at vaccination rates on the state level shows that only 20% of children aged 12-17 in Wyoming and 21% in Mississippi have gotten at least one dose as of Aug. 4, while Massachusetts is up to 68% and Vermont reports 70%. Rates for full vaccination range from 11% in Mississippi and Alabama to 61% in Vermont, based on an AAP analysis of CDC data, which is not available for Idaho.



