User login
CRP as a biomarker for community-acquired pneumonia
Background: In the United States, CAP was responsible for nearly 50,000 deaths in 2017. Prompt and accurate diagnosis promotes early treatment and avoids unnecessary antibiotic treatment for nonpneumonia lower respiratory tract infection patients. Diagnosis is based on signs and symptoms, as well as available imaging. Inflammatory markers such as CRP, white blood cell count, and procalcitonin are readily available in the ED and outpatient settings.
Study design: Bivariate meta-analysis.
Setting: A systematic review of literature was done via PubMed search to identify prospective studies evaluating the accuracy of biomarkers in patients with cough or suspected CAP.
Synopsis: Fourteen studies met the criteria to be included in the meta-analysis. Summary receiver operating characteristic (ROC) curves generated reported area under the curve of 0.802 for CRP (95% confidence interval, 0.78-0.85), 0.777 for leukocytosis (95% CI, 0.74-0.81), and 0.771 for procalcitonin (95% CI, 0.74-0.81). The combination of CRP greater than 49.5 mg/L and procalcitonin greater than 0.1 mcg/L had a positive likelihood ratio of 2.24 and a negative likelihood ratio of 0.44.
The study had a some of limitations. The blinding of the person performing the index test to the reference standard and vice versa was not clear. Further, it was unclear if the person interpreting the reference standard was blinded to the index test in five studies and absent in one. Other limitations were inconsistent reporting of abnormal post hoc cutoffs and only two biomarkers being reported in a single study.
Combining a biomarker with signs and symptoms has the potential to improve diagnostic accuracy in the outpatient setting further. CRP was found to be most accurate regardless of the cutoff used; however, further studies without threshold effect will prove beneficial.
Bottom line: CRP is a more accurate and useful biomarker for outpatient CAP diagnosis than procalcitonin or leukocytosis.
Citation: Ebell MH et al. Accuracy of biomarkers for the diagnosis of adult community-acquired pneumonia: A meta-analysis. Acad Emerg Med. 2020;27(3):195-206.
Dr. Castellanos is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: In the United States, CAP was responsible for nearly 50,000 deaths in 2017. Prompt and accurate diagnosis promotes early treatment and avoids unnecessary antibiotic treatment for nonpneumonia lower respiratory tract infection patients. Diagnosis is based on signs and symptoms, as well as available imaging. Inflammatory markers such as CRP, white blood cell count, and procalcitonin are readily available in the ED and outpatient settings.
Study design: Bivariate meta-analysis.
Setting: A systematic review of literature was done via PubMed search to identify prospective studies evaluating the accuracy of biomarkers in patients with cough or suspected CAP.
Synopsis: Fourteen studies met the criteria to be included in the meta-analysis. Summary receiver operating characteristic (ROC) curves generated reported area under the curve of 0.802 for CRP (95% confidence interval, 0.78-0.85), 0.777 for leukocytosis (95% CI, 0.74-0.81), and 0.771 for procalcitonin (95% CI, 0.74-0.81). The combination of CRP greater than 49.5 mg/L and procalcitonin greater than 0.1 mcg/L had a positive likelihood ratio of 2.24 and a negative likelihood ratio of 0.44.
The study had a some of limitations. The blinding of the person performing the index test to the reference standard and vice versa was not clear. Further, it was unclear if the person interpreting the reference standard was blinded to the index test in five studies and absent in one. Other limitations were inconsistent reporting of abnormal post hoc cutoffs and only two biomarkers being reported in a single study.
Combining a biomarker with signs and symptoms has the potential to improve diagnostic accuracy in the outpatient setting further. CRP was found to be most accurate regardless of the cutoff used; however, further studies without threshold effect will prove beneficial.
Bottom line: CRP is a more accurate and useful biomarker for outpatient CAP diagnosis than procalcitonin or leukocytosis.
Citation: Ebell MH et al. Accuracy of biomarkers for the diagnosis of adult community-acquired pneumonia: A meta-analysis. Acad Emerg Med. 2020;27(3):195-206.
Dr. Castellanos is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: In the United States, CAP was responsible for nearly 50,000 deaths in 2017. Prompt and accurate diagnosis promotes early treatment and avoids unnecessary antibiotic treatment for nonpneumonia lower respiratory tract infection patients. Diagnosis is based on signs and symptoms, as well as available imaging. Inflammatory markers such as CRP, white blood cell count, and procalcitonin are readily available in the ED and outpatient settings.
Study design: Bivariate meta-analysis.
Setting: A systematic review of literature was done via PubMed search to identify prospective studies evaluating the accuracy of biomarkers in patients with cough or suspected CAP.
Synopsis: Fourteen studies met the criteria to be included in the meta-analysis. Summary receiver operating characteristic (ROC) curves generated reported area under the curve of 0.802 for CRP (95% confidence interval, 0.78-0.85), 0.777 for leukocytosis (95% CI, 0.74-0.81), and 0.771 for procalcitonin (95% CI, 0.74-0.81). The combination of CRP greater than 49.5 mg/L and procalcitonin greater than 0.1 mcg/L had a positive likelihood ratio of 2.24 and a negative likelihood ratio of 0.44.
The study had a some of limitations. The blinding of the person performing the index test to the reference standard and vice versa was not clear. Further, it was unclear if the person interpreting the reference standard was blinded to the index test in five studies and absent in one. Other limitations were inconsistent reporting of abnormal post hoc cutoffs and only two biomarkers being reported in a single study.
Combining a biomarker with signs and symptoms has the potential to improve diagnostic accuracy in the outpatient setting further. CRP was found to be most accurate regardless of the cutoff used; however, further studies without threshold effect will prove beneficial.
Bottom line: CRP is a more accurate and useful biomarker for outpatient CAP diagnosis than procalcitonin or leukocytosis.
Citation: Ebell MH et al. Accuracy of biomarkers for the diagnosis of adult community-acquired pneumonia: A meta-analysis. Acad Emerg Med. 2020;27(3):195-206.
Dr. Castellanos is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Q&A: Get flu shot early this year? Same time as COVID vaccine?
With first-time COVID-19 immunizations continuing and the plan to offer booster vaccines to most Americans starting next month, what are the considerations for getting COVID-19 and flu shots at the same time?
This news organization asked Andrew T. Pavia, MD, for his advice. He is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, and a fellow of the Infectious Diseases Society of America.
Q: With COVID-19 cases surging, is it a good idea to get the flu shot early this season?
Dr. Pavia: I don’t think there is a rush to do it in August, but it is a good idea to get a flu shot this season. The consequences of getting the flu while COVID is circulating are serious.
Q: What are the implications?
There are some we know and some we don’t know. If you develop flu-like symptoms, you’re going to have to get tested. You’re going to have to stay home quite a bit longer if you get a definitive (positive COVID-19) test than you would simply with flu symptoms. Also, you’re probably going to miss work when your workplace is very stressed or your children are stressed by having COVID circulating in schools.
The part we know less about are the implications of getting the flu and COVID together. There is some reason to believe if you get them together, the illness will be more severe. We are seeing that with RSV (respiratory syncytial virus) and parainfluenza and COVID coinfections in children. They appear to be quite severe.
But for flu, we just don’t have the data yet. That’s because there really was no cocirculation of COVID and influenza with the exception of parts of China for a brief part of February and March.
Q: Will the planned administration of booster COVID-19 shots this fall affect the number of people who get the flu vaccine or how it’s distributed?
It creates a lot of logistical challenges, particularly for hospitals and other places that need to vaccinate a large number of their employees for flu and that will need to give COVID boosters at about the same time period. It also creates logistical challenges for doctors’ offices.
But we don’t know of any reason why you can’t give the two shots together.
Q: Is it possible flu season will be more severe because we isolated and wore masks, etc., last winter? Any science behind that?
The more you study flu, the less you can predict, and I’ve been studying flu for a long time. There are reasons that might suggest a severe flu season – there has been limited immunity, and some people are not wearing masks effectively and they are gathering again. Those are things we believe protected us from influenza last season.
But we have not seen flu emerge yet. Normally we look to Australia, New Zealand, and South Africa during their winter – which is our summer – to get some idea of what is over the horizon for the Northern Hemisphere. Flu activity in Australia has been very modest this year.
That might mean flu may not show up for a while, but I would be loathe to make a prediction.
Q: What are the chances we’ll see a flu outbreak like we’re seeing with RSV, which is normally a winter illness?
The fact that we had a summer RSV surge just gives you an idea of how the normal epidemiology of viral infections has been disrupted. It means anything could happen with influenza. It could show up late summer or fall or wait until next spring.
We really don’t understand how those interactions work. When a new flu strain emerges, it often ignores the traditional behavior and shows up in the spring or fall. It happened in the 2009 pandemic, it happened in 1918.
The one thing I would safely predict about the next flu wave is that it will surprise us.
Q: Are you hopeful that combination vaccines in development from a number of companies, such as Moderna, Novavax, and Vivaldi, will be effective?
It is beginning to look like COVID will be with us for the foreseeable future – maybe as a seasonal virus or maybe as an ongoing pandemic. We are going to need to protect (ourselves) simultaneously against the flu and COVID. A single shot is a great way to do that – nobody wants two needles; nobody wants two trips to get vaccinated.
An effective combination vaccine would be a really great tool.
We have to wait to see what the science shows us, because they are quite different viruses. We won’t know if a combination vaccine works well and has acceptable side effects until we do those studies.
Q. Do you know at this point whether the side effects from two vaccines would be additive? Is there any way to predict that?
There is no way to predict. There are so many things that go into whether someone has side effects that we don’t understand. With fairly reactogenic vaccines like the mRNA vaccines, lots of people have no side effects whatsoever and others are really uncomfortable for 24 hours.
Flu is generally a better tolerated vaccine. There are still people who get muscle aches and very sore arms. I don’t think we can predict if getting two will be additive or just the same as getting one vaccine.
Q: Other than convenience and the benefit for people who are needle-phobic, are there any other advantages of combining them into one shot?
The logistics alone are enough to justify having one effective product if we can make one. It should reduce the overall cost of administration and reduce time off from work.
The combination vaccines given by pediatricians have been very successful. They reduce the number of needles for kids and make it much easier for parents and the pediatricians administering them. The same principle should apply to adults, who sometimes are less brave about needles than kids are.
Historically, combined vaccines in general have worked as well as vaccines given alone, but there have been exceptions. We just have to see what the products look like.
Q: For now, the flu vaccine and COVID-19 vaccine are single products. If you get them separately, is it better to put some time between the two?
We don’t know. There are studies that probably won’t be out in time to decide in September. They are looking at whether you get an equivalent immune response if you give them together or apart.
For now, I would say the advantage of getting them together is if you do get side effects, you’ll only get them once – one day to suffer through them. Also, it’s one trip to the doctor.
The potential advantage of separating them is that is how we developed and tested the vaccines. If you do react to them, side effects could be milder, but it will be on two separate days.
I would recommend doing whatever works so that you get both vaccines in a timely manner.
I’m going to get my flu shot as soon as it’s available. If I’m due for a COVID booster at that time, I would probably do them together.
Q: Do you foresee a point in the future when the predominant strain of SARS-CoV-2 will be one of the components of a flu vaccine, like we did in the past with H1N1, etc?
It really remains to be seen, but it is very conceivable it could happen. The same companies that developed COVID-19 vaccines are working on flu vaccines.
Q: Any other advice for people concerned about getting immunized against both COVID-19 and influenza in the coming months?
There is no side effect of the vaccine that begins to approach the risk you face from either disease. It’s really one of the best things you can do to protect yourself is to get vaccinated.
In the case of flu, the vaccine is only modestly effective, but it still saves tens of thousands of lives each year. The SARS-CoV-2 vaccine is a much better vaccine and a deadlier disease.
Dr. Pavia consulted for GlaxoSmithKline on influenza testing.
A version of this article first appeared on Medscape.com.
With first-time COVID-19 immunizations continuing and the plan to offer booster vaccines to most Americans starting next month, what are the considerations for getting COVID-19 and flu shots at the same time?
This news organization asked Andrew T. Pavia, MD, for his advice. He is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, and a fellow of the Infectious Diseases Society of America.
Q: With COVID-19 cases surging, is it a good idea to get the flu shot early this season?
Dr. Pavia: I don’t think there is a rush to do it in August, but it is a good idea to get a flu shot this season. The consequences of getting the flu while COVID is circulating are serious.
Q: What are the implications?
There are some we know and some we don’t know. If you develop flu-like symptoms, you’re going to have to get tested. You’re going to have to stay home quite a bit longer if you get a definitive (positive COVID-19) test than you would simply with flu symptoms. Also, you’re probably going to miss work when your workplace is very stressed or your children are stressed by having COVID circulating in schools.
The part we know less about are the implications of getting the flu and COVID together. There is some reason to believe if you get them together, the illness will be more severe. We are seeing that with RSV (respiratory syncytial virus) and parainfluenza and COVID coinfections in children. They appear to be quite severe.
But for flu, we just don’t have the data yet. That’s because there really was no cocirculation of COVID and influenza with the exception of parts of China for a brief part of February and March.
Q: Will the planned administration of booster COVID-19 shots this fall affect the number of people who get the flu vaccine or how it’s distributed?
It creates a lot of logistical challenges, particularly for hospitals and other places that need to vaccinate a large number of their employees for flu and that will need to give COVID boosters at about the same time period. It also creates logistical challenges for doctors’ offices.
But we don’t know of any reason why you can’t give the two shots together.
Q: Is it possible flu season will be more severe because we isolated and wore masks, etc., last winter? Any science behind that?
The more you study flu, the less you can predict, and I’ve been studying flu for a long time. There are reasons that might suggest a severe flu season – there has been limited immunity, and some people are not wearing masks effectively and they are gathering again. Those are things we believe protected us from influenza last season.
But we have not seen flu emerge yet. Normally we look to Australia, New Zealand, and South Africa during their winter – which is our summer – to get some idea of what is over the horizon for the Northern Hemisphere. Flu activity in Australia has been very modest this year.
That might mean flu may not show up for a while, but I would be loathe to make a prediction.
Q: What are the chances we’ll see a flu outbreak like we’re seeing with RSV, which is normally a winter illness?
The fact that we had a summer RSV surge just gives you an idea of how the normal epidemiology of viral infections has been disrupted. It means anything could happen with influenza. It could show up late summer or fall or wait until next spring.
We really don’t understand how those interactions work. When a new flu strain emerges, it often ignores the traditional behavior and shows up in the spring or fall. It happened in the 2009 pandemic, it happened in 1918.
The one thing I would safely predict about the next flu wave is that it will surprise us.
Q: Are you hopeful that combination vaccines in development from a number of companies, such as Moderna, Novavax, and Vivaldi, will be effective?
It is beginning to look like COVID will be with us for the foreseeable future – maybe as a seasonal virus or maybe as an ongoing pandemic. We are going to need to protect (ourselves) simultaneously against the flu and COVID. A single shot is a great way to do that – nobody wants two needles; nobody wants two trips to get vaccinated.
An effective combination vaccine would be a really great tool.
We have to wait to see what the science shows us, because they are quite different viruses. We won’t know if a combination vaccine works well and has acceptable side effects until we do those studies.
Q. Do you know at this point whether the side effects from two vaccines would be additive? Is there any way to predict that?
There is no way to predict. There are so many things that go into whether someone has side effects that we don’t understand. With fairly reactogenic vaccines like the mRNA vaccines, lots of people have no side effects whatsoever and others are really uncomfortable for 24 hours.
Flu is generally a better tolerated vaccine. There are still people who get muscle aches and very sore arms. I don’t think we can predict if getting two will be additive or just the same as getting one vaccine.
Q: Other than convenience and the benefit for people who are needle-phobic, are there any other advantages of combining them into one shot?
The logistics alone are enough to justify having one effective product if we can make one. It should reduce the overall cost of administration and reduce time off from work.
The combination vaccines given by pediatricians have been very successful. They reduce the number of needles for kids and make it much easier for parents and the pediatricians administering them. The same principle should apply to adults, who sometimes are less brave about needles than kids are.
Historically, combined vaccines in general have worked as well as vaccines given alone, but there have been exceptions. We just have to see what the products look like.
Q: For now, the flu vaccine and COVID-19 vaccine are single products. If you get them separately, is it better to put some time between the two?
We don’t know. There are studies that probably won’t be out in time to decide in September. They are looking at whether you get an equivalent immune response if you give them together or apart.
For now, I would say the advantage of getting them together is if you do get side effects, you’ll only get them once – one day to suffer through them. Also, it’s one trip to the doctor.
The potential advantage of separating them is that is how we developed and tested the vaccines. If you do react to them, side effects could be milder, but it will be on two separate days.
I would recommend doing whatever works so that you get both vaccines in a timely manner.
I’m going to get my flu shot as soon as it’s available. If I’m due for a COVID booster at that time, I would probably do them together.
Q: Do you foresee a point in the future when the predominant strain of SARS-CoV-2 will be one of the components of a flu vaccine, like we did in the past with H1N1, etc?
It really remains to be seen, but it is very conceivable it could happen. The same companies that developed COVID-19 vaccines are working on flu vaccines.
Q: Any other advice for people concerned about getting immunized against both COVID-19 and influenza in the coming months?
There is no side effect of the vaccine that begins to approach the risk you face from either disease. It’s really one of the best things you can do to protect yourself is to get vaccinated.
In the case of flu, the vaccine is only modestly effective, but it still saves tens of thousands of lives each year. The SARS-CoV-2 vaccine is a much better vaccine and a deadlier disease.
Dr. Pavia consulted for GlaxoSmithKline on influenza testing.
A version of this article first appeared on Medscape.com.
With first-time COVID-19 immunizations continuing and the plan to offer booster vaccines to most Americans starting next month, what are the considerations for getting COVID-19 and flu shots at the same time?
This news organization asked Andrew T. Pavia, MD, for his advice. He is the George and Esther Gross Presidential Professor and chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, and a fellow of the Infectious Diseases Society of America.
Q: With COVID-19 cases surging, is it a good idea to get the flu shot early this season?
Dr. Pavia: I don’t think there is a rush to do it in August, but it is a good idea to get a flu shot this season. The consequences of getting the flu while COVID is circulating are serious.
Q: What are the implications?
There are some we know and some we don’t know. If you develop flu-like symptoms, you’re going to have to get tested. You’re going to have to stay home quite a bit longer if you get a definitive (positive COVID-19) test than you would simply with flu symptoms. Also, you’re probably going to miss work when your workplace is very stressed or your children are stressed by having COVID circulating in schools.
The part we know less about are the implications of getting the flu and COVID together. There is some reason to believe if you get them together, the illness will be more severe. We are seeing that with RSV (respiratory syncytial virus) and parainfluenza and COVID coinfections in children. They appear to be quite severe.
But for flu, we just don’t have the data yet. That’s because there really was no cocirculation of COVID and influenza with the exception of parts of China for a brief part of February and March.
Q: Will the planned administration of booster COVID-19 shots this fall affect the number of people who get the flu vaccine or how it’s distributed?
It creates a lot of logistical challenges, particularly for hospitals and other places that need to vaccinate a large number of their employees for flu and that will need to give COVID boosters at about the same time period. It also creates logistical challenges for doctors’ offices.
But we don’t know of any reason why you can’t give the two shots together.
Q: Is it possible flu season will be more severe because we isolated and wore masks, etc., last winter? Any science behind that?
The more you study flu, the less you can predict, and I’ve been studying flu for a long time. There are reasons that might suggest a severe flu season – there has been limited immunity, and some people are not wearing masks effectively and they are gathering again. Those are things we believe protected us from influenza last season.
But we have not seen flu emerge yet. Normally we look to Australia, New Zealand, and South Africa during their winter – which is our summer – to get some idea of what is over the horizon for the Northern Hemisphere. Flu activity in Australia has been very modest this year.
That might mean flu may not show up for a while, but I would be loathe to make a prediction.
Q: What are the chances we’ll see a flu outbreak like we’re seeing with RSV, which is normally a winter illness?
The fact that we had a summer RSV surge just gives you an idea of how the normal epidemiology of viral infections has been disrupted. It means anything could happen with influenza. It could show up late summer or fall or wait until next spring.
We really don’t understand how those interactions work. When a new flu strain emerges, it often ignores the traditional behavior and shows up in the spring or fall. It happened in the 2009 pandemic, it happened in 1918.
The one thing I would safely predict about the next flu wave is that it will surprise us.
Q: Are you hopeful that combination vaccines in development from a number of companies, such as Moderna, Novavax, and Vivaldi, will be effective?
It is beginning to look like COVID will be with us for the foreseeable future – maybe as a seasonal virus or maybe as an ongoing pandemic. We are going to need to protect (ourselves) simultaneously against the flu and COVID. A single shot is a great way to do that – nobody wants two needles; nobody wants two trips to get vaccinated.
An effective combination vaccine would be a really great tool.
We have to wait to see what the science shows us, because they are quite different viruses. We won’t know if a combination vaccine works well and has acceptable side effects until we do those studies.
Q. Do you know at this point whether the side effects from two vaccines would be additive? Is there any way to predict that?
There is no way to predict. There are so many things that go into whether someone has side effects that we don’t understand. With fairly reactogenic vaccines like the mRNA vaccines, lots of people have no side effects whatsoever and others are really uncomfortable for 24 hours.
Flu is generally a better tolerated vaccine. There are still people who get muscle aches and very sore arms. I don’t think we can predict if getting two will be additive or just the same as getting one vaccine.
Q: Other than convenience and the benefit for people who are needle-phobic, are there any other advantages of combining them into one shot?
The logistics alone are enough to justify having one effective product if we can make one. It should reduce the overall cost of administration and reduce time off from work.
The combination vaccines given by pediatricians have been very successful. They reduce the number of needles for kids and make it much easier for parents and the pediatricians administering them. The same principle should apply to adults, who sometimes are less brave about needles than kids are.
Historically, combined vaccines in general have worked as well as vaccines given alone, but there have been exceptions. We just have to see what the products look like.
Q: For now, the flu vaccine and COVID-19 vaccine are single products. If you get them separately, is it better to put some time between the two?
We don’t know. There are studies that probably won’t be out in time to decide in September. They are looking at whether you get an equivalent immune response if you give them together or apart.
For now, I would say the advantage of getting them together is if you do get side effects, you’ll only get them once – one day to suffer through them. Also, it’s one trip to the doctor.
The potential advantage of separating them is that is how we developed and tested the vaccines. If you do react to them, side effects could be milder, but it will be on two separate days.
I would recommend doing whatever works so that you get both vaccines in a timely manner.
I’m going to get my flu shot as soon as it’s available. If I’m due for a COVID booster at that time, I would probably do them together.
Q: Do you foresee a point in the future when the predominant strain of SARS-CoV-2 will be one of the components of a flu vaccine, like we did in the past with H1N1, etc?
It really remains to be seen, but it is very conceivable it could happen. The same companies that developed COVID-19 vaccines are working on flu vaccines.
Q: Any other advice for people concerned about getting immunized against both COVID-19 and influenza in the coming months?
There is no side effect of the vaccine that begins to approach the risk you face from either disease. It’s really one of the best things you can do to protect yourself is to get vaccinated.
In the case of flu, the vaccine is only modestly effective, but it still saves tens of thousands of lives each year. The SARS-CoV-2 vaccine is a much better vaccine and a deadlier disease.
Dr. Pavia consulted for GlaxoSmithKline on influenza testing.
A version of this article first appeared on Medscape.com.
CDC launches new center to watch for future outbreaks
The Centers for Disease Control and Prevention is setting up a new hub to watch for early warning signs of future infectious outbreaks, the agency announced on Aug. 18.
Epidemiologists learn about emerging outbreaks by tracking information, and the quality of their analysis depends on their access to high-quality data. Gaps in existing systems became obvious during the COVID-19 pandemic as experts were challenged by the crisis.
The new Center for Forecasting and Outbreak Analytics will, in part, work like a meteorological office that tracks weather-related changes, only the center will track possible flareups in infectious disease.
The day after he took office, President Joe Biden pledged to modernize the country’s system for public health data. First funding for the initiative will come from the American Rescue Plan.
“We are excited to have the expertise and ability to model and forecast public health concerns and share information in real-time to activate governmental, private sector, and public actions in anticipation of threats both domestically and abroad,” CDC Director Rochelle Walensky, MD, said in a statement.
Devastating toll of COVID-19
Many world leaders are now responding to the destruction of the health crisis and are investing in new infrastructure. A July report from a G-20 panel calls for $75 billion in international financing for pandemic prevention and preparedness –twice as much as current spending levels.
Testifying in a congressional hearing, epidemiologist Caitlin Rivers, PhD, from the Johns Hopkins Center for Health Security, Baltimore, voiced the importance of never being caught unprepared again.
“We were unprepared to manage the emergence and swift global spread of the novel coronavirus, and we were late to recognize when it reached our shores. Those delays set us on a worse trajectory than we might have otherwise faced,” she said.
Dr. Rivers will join the new center’s leadership team as associate director working alongside Marc Lipsitch, PhD, director for science.
“The new center will meet a longstanding need for a national focal point to analyze data and forecast the trajectory of pandemics with the express goal of informing and improving decisions with the best available evidence,” Dr. Lipsitch said in the CDC’s news release announcing the new center.
Experts will map what data sources are needed to assist disease modelers and public health emergency responders tracking emerging problems that they can share with decision-makers. They will expand tracking capability and data sharing using open-source software and application programming with existing and new data streams from the public health ecosystem and elsewhere.
Dylan George, PhD, who will be the center’s director for operations, said in the CDC news release that the center will provide critical information to communities so they can respond.
“Pandemics threaten our families and communities at speed and scale – our response needs to move at speed and scale, too,” he said.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention is setting up a new hub to watch for early warning signs of future infectious outbreaks, the agency announced on Aug. 18.
Epidemiologists learn about emerging outbreaks by tracking information, and the quality of their analysis depends on their access to high-quality data. Gaps in existing systems became obvious during the COVID-19 pandemic as experts were challenged by the crisis.
The new Center for Forecasting and Outbreak Analytics will, in part, work like a meteorological office that tracks weather-related changes, only the center will track possible flareups in infectious disease.
The day after he took office, President Joe Biden pledged to modernize the country’s system for public health data. First funding for the initiative will come from the American Rescue Plan.
“We are excited to have the expertise and ability to model and forecast public health concerns and share information in real-time to activate governmental, private sector, and public actions in anticipation of threats both domestically and abroad,” CDC Director Rochelle Walensky, MD, said in a statement.
Devastating toll of COVID-19
Many world leaders are now responding to the destruction of the health crisis and are investing in new infrastructure. A July report from a G-20 panel calls for $75 billion in international financing for pandemic prevention and preparedness –twice as much as current spending levels.
Testifying in a congressional hearing, epidemiologist Caitlin Rivers, PhD, from the Johns Hopkins Center for Health Security, Baltimore, voiced the importance of never being caught unprepared again.
“We were unprepared to manage the emergence and swift global spread of the novel coronavirus, and we were late to recognize when it reached our shores. Those delays set us on a worse trajectory than we might have otherwise faced,” she said.
Dr. Rivers will join the new center’s leadership team as associate director working alongside Marc Lipsitch, PhD, director for science.
“The new center will meet a longstanding need for a national focal point to analyze data and forecast the trajectory of pandemics with the express goal of informing and improving decisions with the best available evidence,” Dr. Lipsitch said in the CDC’s news release announcing the new center.
Experts will map what data sources are needed to assist disease modelers and public health emergency responders tracking emerging problems that they can share with decision-makers. They will expand tracking capability and data sharing using open-source software and application programming with existing and new data streams from the public health ecosystem and elsewhere.
Dylan George, PhD, who will be the center’s director for operations, said in the CDC news release that the center will provide critical information to communities so they can respond.
“Pandemics threaten our families and communities at speed and scale – our response needs to move at speed and scale, too,” he said.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention is setting up a new hub to watch for early warning signs of future infectious outbreaks, the agency announced on Aug. 18.
Epidemiologists learn about emerging outbreaks by tracking information, and the quality of their analysis depends on their access to high-quality data. Gaps in existing systems became obvious during the COVID-19 pandemic as experts were challenged by the crisis.
The new Center for Forecasting and Outbreak Analytics will, in part, work like a meteorological office that tracks weather-related changes, only the center will track possible flareups in infectious disease.
The day after he took office, President Joe Biden pledged to modernize the country’s system for public health data. First funding for the initiative will come from the American Rescue Plan.
“We are excited to have the expertise and ability to model and forecast public health concerns and share information in real-time to activate governmental, private sector, and public actions in anticipation of threats both domestically and abroad,” CDC Director Rochelle Walensky, MD, said in a statement.
Devastating toll of COVID-19
Many world leaders are now responding to the destruction of the health crisis and are investing in new infrastructure. A July report from a G-20 panel calls for $75 billion in international financing for pandemic prevention and preparedness –twice as much as current spending levels.
Testifying in a congressional hearing, epidemiologist Caitlin Rivers, PhD, from the Johns Hopkins Center for Health Security, Baltimore, voiced the importance of never being caught unprepared again.
“We were unprepared to manage the emergence and swift global spread of the novel coronavirus, and we were late to recognize when it reached our shores. Those delays set us on a worse trajectory than we might have otherwise faced,” she said.
Dr. Rivers will join the new center’s leadership team as associate director working alongside Marc Lipsitch, PhD, director for science.
“The new center will meet a longstanding need for a national focal point to analyze data and forecast the trajectory of pandemics with the express goal of informing and improving decisions with the best available evidence,” Dr. Lipsitch said in the CDC’s news release announcing the new center.
Experts will map what data sources are needed to assist disease modelers and public health emergency responders tracking emerging problems that they can share with decision-makers. They will expand tracking capability and data sharing using open-source software and application programming with existing and new data streams from the public health ecosystem and elsewhere.
Dylan George, PhD, who will be the center’s director for operations, said in the CDC news release that the center will provide critical information to communities so they can respond.
“Pandemics threaten our families and communities at speed and scale – our response needs to move at speed and scale, too,” he said.
A version of this article first appeared on WebMD.com.
Cutaneous Chaetomium globosum Infection in a Vedolizumab-Treated Patient
To the Editor:
Broader availability and utilization of novel biologic treatments has heralded the emergence of unusual infections, including skin and soft tissue infections. These unusual infections may not be seen in clinical trials due to their overall rare incidence. In modern society, exposure to unusual pathogens can occur in locations far from their natural habitat.1 Tissue culture remains the gold standard, as histopathology and smears may not identify the organisms. Tissue culture of these less-common pathogens is challenging and may require multiple samples and specialized laboratory evaluations.2 In some cases, a skin biopsy with histopathologic examination is an efficient means to confirm or exclude a dermatologic manifestation of an inflammatory disease. This information can quickly change the course of treatment, especially for those on immunosuppressive medications.3 We report a case of unusual cutaneous infection with Chaetomium globosum in a patient concomitantly treated with vedolizumab, a gut-specific integrin inhibitor, alongside traditional immunosuppressive therapy.
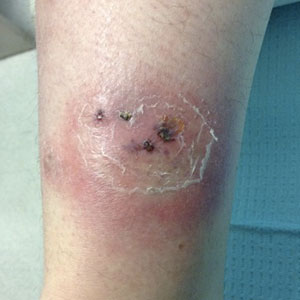
A 33-year-old woman with Crohn disease on vedolizumab and mercaptopurine was referred to the dermatology clinic with firm, tender, erythematous lesions on the legs of 1 month’s duration (Figure, A). She had a history of inflammatory bowel disease with perianal fistula, sacroiliitis, uveitis, guttate psoriasis, and erythema nodosum. She denied recent medication changes, foreign travel, swimming in freshwater or a hot tub, chills, fever, malaise, night sweats, and weight loss. Physical examination revealed several tender, indurated, erythematous plaques across the legs, ranging in size from 4 to 12 cm. The plaques had central hyperpigmentation, atrophy, and scant scale without ulceration, drainage, or pustules. The largest plaque demonstrated a well-defined area of central fluctuance. Prednisone (60 mg) with taper was initiated for presumed recurrence of erythema nodosum with close follow-up.
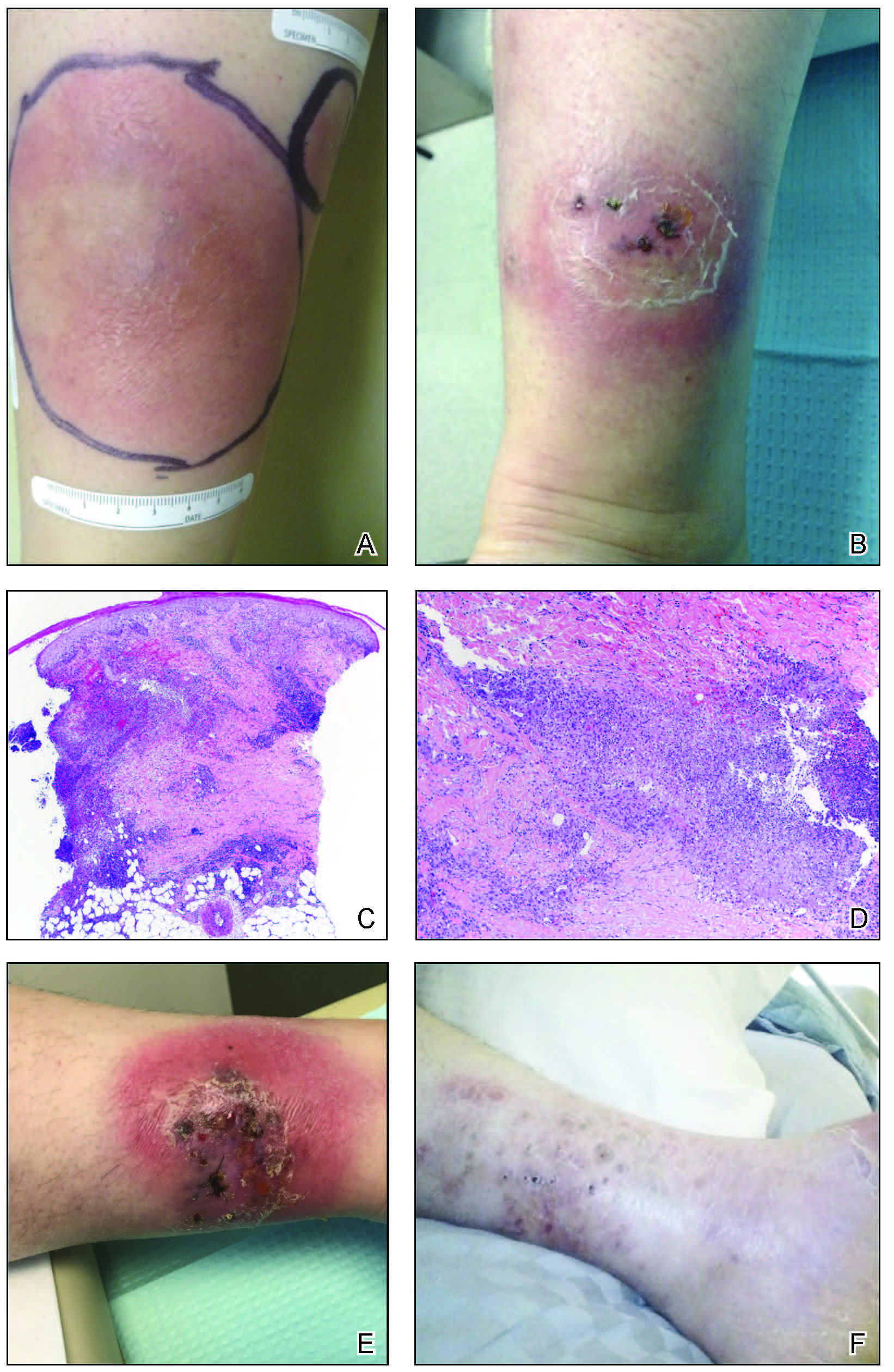
Five weeks later, most indurated plaques healed, leaving depressed scars; however, at 10 mg of prednisone she developed 2 additional nodules on the shin that, unlike earlier plaques, developed a central pustule and drained. The prednisone dose was increased to control the new areas and tapered thereafter to 20 mg daily. Despite the overall improvement, 2 plaques remained on the left side of the shin. Initially, erythema nodosum recurrence was considered, given the setting of inflammatory bowel disease and recent more classic presentation4; however, the disease progression and lack of response to standard treatment suggested an alternate pathology. Further history revealed that the patient had a pedicure 3 weeks prior to initial symptom onset. A swab was sent for routine bacterial culture at an outside clinic; no infectious agents were identified.
Three weeks later, the patient's condition had worsened again with increased edema, pain with standing, and more drainage (Figure, B). She did not report fevers or joint swelling. A punch biopsy was performed for tissue culture and histopathologic evaluation, which revealed granulomatous and suppurative inflammation and excluded erythema nodosum. Special stains for organisms were negative (Figure, C and D). Two weeks later, tissue culture began growing an unspecified mold. Mercaptopurine and prednisone were immediately discontinued. The patient remained on vedolizumab, started itraconazole (200 mg), and was referred to an infectious disease (ID) specialist. The sample was eventually identified as C globosum (Figure, E) at a specialized facility (University of Texas, San Antonio). Despite several weeks of itraconazole therapy, the patient developed edema surrounding the knee. Upon evaluation by orthopedics, the patient was diagnosed with reactive arthritis in the left knee and ankle. The knee fluid was drained, and cultures were negative. At recommendation of the ID physician, the itraconazole dosage was doubled given the limited clinical response. After several weeks at the increased dosage, she began to experience slow improvement (Figure, F). Because Chaetomium species infections are rare and have limited response to many antifungal agents,5 no standard treatment protocol was available. Initial recommendations for treatment were for 1 year, based on the experience and expertise of the ID physician. Treatment with itraconazole was continued for 10 months, at which point the patient chose to discontinue therapy prior to her follow-up appointments. The patient had no evidence of infection recurrence 2 months after discontinuing therapy.
In the expanding landscape of targeted biologic therapies for chronic inflammatory disease, physicians of various specialties are increasingly encountering unanticipated cutaneous eruptions and infections. Chaetomium is a dematiaceous mold found primarily in soil, water, decaying plants, paper, or dung. Based on its habitat, populations at risk for infection with Chaetomium species include farmers (plant and animal husbandry), children who play on the ground, and people with inadequate foot protection.1,2Chaetomium globosum has been identified in indoor environments, such as moldy rugs and mattresses. In one report, it was cultured from the environmental air in a bone marrow transplant patient’s room after the patient presented with delayed infection.6 Although human infection is uncommon, clinical isolation of Chaetomium species has occurred mainly in superficial samples from the skin, hair, nails, eyes, and respiratory tract.1 It been reported as a causative agent of onychomycosis in several immunocompetent patients7,8 but rarely is a cause of deep-skin infection. Chaetomium is thought to cause superficial infections, as it uses extracellular keratinases1 to degrade protective keratin structures, such as human nails. Infections in the brain, blood, and lymph nodes also have been noted but are quite rare. Deep skin infections present as painful papules and nodules to nonhealing ulcers that develop into inflammatory granulomas on the extremities.3 Local edema and yellow-brown crust often is present and fevers have been reported. Hyphae may be identified in skin biopsy.8 We posit that our patient may have been exposed to Chaetomium during her pedicure, as recirculating baths in nail salons have been a reported site of other infectious organisms, such as atypical mycobacteria.9
Vedolizumab is a humanized IgG1 monoclonal antibody used in the treatment of ulcerative colitis and Crohn disease. It targets the α4β7 integrin, a specific modulator of gut-trafficking lymphocytes. In vedolizumab’s clinical trial for Crohn disease, there was no increased incidence of life-threatening, severe infection.10,11 Often, new biologic treatments are used with known immunosuppressive medications. Mercaptopurine and prednisone are implicated in infections; however, recovery from the immune suppression usually is seen at 1 month after discontinuation.12 Our patient continued to worsen for several weeks and required increased dosing of itraconazole, despite stopping both prednisone and mercaptopurine. It opens the question as to whether vedolizumab played a role in the recalcitrant disease.
This case illustrates the importance of a high index of suspicion for unusual infections in the setting of biologic therapy. An infectious etiology of a cutaneous eruption in an immunosuppressed patient should always be included in the differential diagnosis and actively pursued early on; tissue culture may shorten the treatment course and decrease severity of the disease. Although a direct link between the mechanism of action of vedolizumab and cutaneous infection is not clear, given the rare incidence of this infection, a report of such a case is important to the practicing clinician.
- de Hoog GS, Ahmed SA, Najafzadeh MJ, et al. Phylogenetic findings suggest possible new habitat and routes of infection of human eumycetoma. PLoS Negl Trop Dis. 2013;7:e2229. doi:10.1371/journal.pntd.0002229
- Zhang H, Ran Y, Li D, et al. Clavispora lusitaniae and Chaetomium atrobrunneum as rare agents of cutaneous infection. Mycopathologia. 2010;169:373-380. doi:10.1007/s11046-009-9266-9
- Schieffelin JS, Garcia-Diaz JB, Loss GE, et al. Phaeohyphomycosis fungal infections in solid organ transplant recipients: clinical presentation, pathology, and treatment. Transpl Infect Dis Off J Transplant Soc. 2014;16:270-278. doi:10.1111/tid.12197
- Farhi D, Cosnes J, Zizi N, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore). 2008;87:281-293. doi:10.1097/MD.0b013e318187cc9c
- Guarro J, Soler L, Rinaldi MG. Pathogenicity and antifungal susceptibility of Chaetomium species. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 1995;14:613-618.
- Teixeira ABA, Trabasso P, Moretti-Branchini ML, et al. Phaeohyphomycosis caused by Chaetomium globosum in an allogeneic bone marrow transplant recipient. Mycopathologia. 2003;156:309-312.
- Falcón CS, Falcón MDMS, Ceballos JD, et al. Onychomycosis by Chaetomium spp. Mycoses. 2009;52:77-79. doi:10.1111/j.14390507.2008.01519.x
- Kim DM, Lee MH, Suh MK, et al. Onychomycosis caused by Chaetomium globosum. Ann Dermatol. 2013;25:232-236. doi:10.5021/ad.2013.25.2.232
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
- Luthra P, Peyrin-Biroulet L, Ford AC. Systematic review and meta-analysis: opportunistic infections and malignancies during treatment with anti-integrin antibodies in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:1227-1236. doi:10.1111/apt.13215
- Colombel J-F, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839-851. doi:10.1136/gutjnl-2015-311079
- Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081-1085.
To the Editor:
Broader availability and utilization of novel biologic treatments has heralded the emergence of unusual infections, including skin and soft tissue infections. These unusual infections may not be seen in clinical trials due to their overall rare incidence. In modern society, exposure to unusual pathogens can occur in locations far from their natural habitat.1 Tissue culture remains the gold standard, as histopathology and smears may not identify the organisms. Tissue culture of these less-common pathogens is challenging and may require multiple samples and specialized laboratory evaluations.2 In some cases, a skin biopsy with histopathologic examination is an efficient means to confirm or exclude a dermatologic manifestation of an inflammatory disease. This information can quickly change the course of treatment, especially for those on immunosuppressive medications.3 We report a case of unusual cutaneous infection with Chaetomium globosum in a patient concomitantly treated with vedolizumab, a gut-specific integrin inhibitor, alongside traditional immunosuppressive therapy.
A 33-year-old woman with Crohn disease on vedolizumab and mercaptopurine was referred to the dermatology clinic with firm, tender, erythematous lesions on the legs of 1 month’s duration (Figure, A). She had a history of inflammatory bowel disease with perianal fistula, sacroiliitis, uveitis, guttate psoriasis, and erythema nodosum. She denied recent medication changes, foreign travel, swimming in freshwater or a hot tub, chills, fever, malaise, night sweats, and weight loss. Physical examination revealed several tender, indurated, erythematous plaques across the legs, ranging in size from 4 to 12 cm. The plaques had central hyperpigmentation, atrophy, and scant scale without ulceration, drainage, or pustules. The largest plaque demonstrated a well-defined area of central fluctuance. Prednisone (60 mg) with taper was initiated for presumed recurrence of erythema nodosum with close follow-up.

Five weeks later, most indurated plaques healed, leaving depressed scars; however, at 10 mg of prednisone she developed 2 additional nodules on the shin that, unlike earlier plaques, developed a central pustule and drained. The prednisone dose was increased to control the new areas and tapered thereafter to 20 mg daily. Despite the overall improvement, 2 plaques remained on the left side of the shin. Initially, erythema nodosum recurrence was considered, given the setting of inflammatory bowel disease and recent more classic presentation4; however, the disease progression and lack of response to standard treatment suggested an alternate pathology. Further history revealed that the patient had a pedicure 3 weeks prior to initial symptom onset. A swab was sent for routine bacterial culture at an outside clinic; no infectious agents were identified.
Three weeks later, the patient's condition had worsened again with increased edema, pain with standing, and more drainage (Figure, B). She did not report fevers or joint swelling. A punch biopsy was performed for tissue culture and histopathologic evaluation, which revealed granulomatous and suppurative inflammation and excluded erythema nodosum. Special stains for organisms were negative (Figure, C and D). Two weeks later, tissue culture began growing an unspecified mold. Mercaptopurine and prednisone were immediately discontinued. The patient remained on vedolizumab, started itraconazole (200 mg), and was referred to an infectious disease (ID) specialist. The sample was eventually identified as C globosum (Figure, E) at a specialized facility (University of Texas, San Antonio). Despite several weeks of itraconazole therapy, the patient developed edema surrounding the knee. Upon evaluation by orthopedics, the patient was diagnosed with reactive arthritis in the left knee and ankle. The knee fluid was drained, and cultures were negative. At recommendation of the ID physician, the itraconazole dosage was doubled given the limited clinical response. After several weeks at the increased dosage, she began to experience slow improvement (Figure, F). Because Chaetomium species infections are rare and have limited response to many antifungal agents,5 no standard treatment protocol was available. Initial recommendations for treatment were for 1 year, based on the experience and expertise of the ID physician. Treatment with itraconazole was continued for 10 months, at which point the patient chose to discontinue therapy prior to her follow-up appointments. The patient had no evidence of infection recurrence 2 months after discontinuing therapy.
In the expanding landscape of targeted biologic therapies for chronic inflammatory disease, physicians of various specialties are increasingly encountering unanticipated cutaneous eruptions and infections. Chaetomium is a dematiaceous mold found primarily in soil, water, decaying plants, paper, or dung. Based on its habitat, populations at risk for infection with Chaetomium species include farmers (plant and animal husbandry), children who play on the ground, and people with inadequate foot protection.1,2Chaetomium globosum has been identified in indoor environments, such as moldy rugs and mattresses. In one report, it was cultured from the environmental air in a bone marrow transplant patient’s room after the patient presented with delayed infection.6 Although human infection is uncommon, clinical isolation of Chaetomium species has occurred mainly in superficial samples from the skin, hair, nails, eyes, and respiratory tract.1 It been reported as a causative agent of onychomycosis in several immunocompetent patients7,8 but rarely is a cause of deep-skin infection. Chaetomium is thought to cause superficial infections, as it uses extracellular keratinases1 to degrade protective keratin structures, such as human nails. Infections in the brain, blood, and lymph nodes also have been noted but are quite rare. Deep skin infections present as painful papules and nodules to nonhealing ulcers that develop into inflammatory granulomas on the extremities.3 Local edema and yellow-brown crust often is present and fevers have been reported. Hyphae may be identified in skin biopsy.8 We posit that our patient may have been exposed to Chaetomium during her pedicure, as recirculating baths in nail salons have been a reported site of other infectious organisms, such as atypical mycobacteria.9
Vedolizumab is a humanized IgG1 monoclonal antibody used in the treatment of ulcerative colitis and Crohn disease. It targets the α4β7 integrin, a specific modulator of gut-trafficking lymphocytes. In vedolizumab’s clinical trial for Crohn disease, there was no increased incidence of life-threatening, severe infection.10,11 Often, new biologic treatments are used with known immunosuppressive medications. Mercaptopurine and prednisone are implicated in infections; however, recovery from the immune suppression usually is seen at 1 month after discontinuation.12 Our patient continued to worsen for several weeks and required increased dosing of itraconazole, despite stopping both prednisone and mercaptopurine. It opens the question as to whether vedolizumab played a role in the recalcitrant disease.
This case illustrates the importance of a high index of suspicion for unusual infections in the setting of biologic therapy. An infectious etiology of a cutaneous eruption in an immunosuppressed patient should always be included in the differential diagnosis and actively pursued early on; tissue culture may shorten the treatment course and decrease severity of the disease. Although a direct link between the mechanism of action of vedolizumab and cutaneous infection is not clear, given the rare incidence of this infection, a report of such a case is important to the practicing clinician.
To the Editor:
Broader availability and utilization of novel biologic treatments has heralded the emergence of unusual infections, including skin and soft tissue infections. These unusual infections may not be seen in clinical trials due to their overall rare incidence. In modern society, exposure to unusual pathogens can occur in locations far from their natural habitat.1 Tissue culture remains the gold standard, as histopathology and smears may not identify the organisms. Tissue culture of these less-common pathogens is challenging and may require multiple samples and specialized laboratory evaluations.2 In some cases, a skin biopsy with histopathologic examination is an efficient means to confirm or exclude a dermatologic manifestation of an inflammatory disease. This information can quickly change the course of treatment, especially for those on immunosuppressive medications.3 We report a case of unusual cutaneous infection with Chaetomium globosum in a patient concomitantly treated with vedolizumab, a gut-specific integrin inhibitor, alongside traditional immunosuppressive therapy.
A 33-year-old woman with Crohn disease on vedolizumab and mercaptopurine was referred to the dermatology clinic with firm, tender, erythematous lesions on the legs of 1 month’s duration (Figure, A). She had a history of inflammatory bowel disease with perianal fistula, sacroiliitis, uveitis, guttate psoriasis, and erythema nodosum. She denied recent medication changes, foreign travel, swimming in freshwater or a hot tub, chills, fever, malaise, night sweats, and weight loss. Physical examination revealed several tender, indurated, erythematous plaques across the legs, ranging in size from 4 to 12 cm. The plaques had central hyperpigmentation, atrophy, and scant scale without ulceration, drainage, or pustules. The largest plaque demonstrated a well-defined area of central fluctuance. Prednisone (60 mg) with taper was initiated for presumed recurrence of erythema nodosum with close follow-up.

Five weeks later, most indurated plaques healed, leaving depressed scars; however, at 10 mg of prednisone she developed 2 additional nodules on the shin that, unlike earlier plaques, developed a central pustule and drained. The prednisone dose was increased to control the new areas and tapered thereafter to 20 mg daily. Despite the overall improvement, 2 plaques remained on the left side of the shin. Initially, erythema nodosum recurrence was considered, given the setting of inflammatory bowel disease and recent more classic presentation4; however, the disease progression and lack of response to standard treatment suggested an alternate pathology. Further history revealed that the patient had a pedicure 3 weeks prior to initial symptom onset. A swab was sent for routine bacterial culture at an outside clinic; no infectious agents were identified.
Three weeks later, the patient's condition had worsened again with increased edema, pain with standing, and more drainage (Figure, B). She did not report fevers or joint swelling. A punch biopsy was performed for tissue culture and histopathologic evaluation, which revealed granulomatous and suppurative inflammation and excluded erythema nodosum. Special stains for organisms were negative (Figure, C and D). Two weeks later, tissue culture began growing an unspecified mold. Mercaptopurine and prednisone were immediately discontinued. The patient remained on vedolizumab, started itraconazole (200 mg), and was referred to an infectious disease (ID) specialist. The sample was eventually identified as C globosum (Figure, E) at a specialized facility (University of Texas, San Antonio). Despite several weeks of itraconazole therapy, the patient developed edema surrounding the knee. Upon evaluation by orthopedics, the patient was diagnosed with reactive arthritis in the left knee and ankle. The knee fluid was drained, and cultures were negative. At recommendation of the ID physician, the itraconazole dosage was doubled given the limited clinical response. After several weeks at the increased dosage, she began to experience slow improvement (Figure, F). Because Chaetomium species infections are rare and have limited response to many antifungal agents,5 no standard treatment protocol was available. Initial recommendations for treatment were for 1 year, based on the experience and expertise of the ID physician. Treatment with itraconazole was continued for 10 months, at which point the patient chose to discontinue therapy prior to her follow-up appointments. The patient had no evidence of infection recurrence 2 months after discontinuing therapy.
In the expanding landscape of targeted biologic therapies for chronic inflammatory disease, physicians of various specialties are increasingly encountering unanticipated cutaneous eruptions and infections. Chaetomium is a dematiaceous mold found primarily in soil, water, decaying plants, paper, or dung. Based on its habitat, populations at risk for infection with Chaetomium species include farmers (plant and animal husbandry), children who play on the ground, and people with inadequate foot protection.1,2Chaetomium globosum has been identified in indoor environments, such as moldy rugs and mattresses. In one report, it was cultured from the environmental air in a bone marrow transplant patient’s room after the patient presented with delayed infection.6 Although human infection is uncommon, clinical isolation of Chaetomium species has occurred mainly in superficial samples from the skin, hair, nails, eyes, and respiratory tract.1 It been reported as a causative agent of onychomycosis in several immunocompetent patients7,8 but rarely is a cause of deep-skin infection. Chaetomium is thought to cause superficial infections, as it uses extracellular keratinases1 to degrade protective keratin structures, such as human nails. Infections in the brain, blood, and lymph nodes also have been noted but are quite rare. Deep skin infections present as painful papules and nodules to nonhealing ulcers that develop into inflammatory granulomas on the extremities.3 Local edema and yellow-brown crust often is present and fevers have been reported. Hyphae may be identified in skin biopsy.8 We posit that our patient may have been exposed to Chaetomium during her pedicure, as recirculating baths in nail salons have been a reported site of other infectious organisms, such as atypical mycobacteria.9
Vedolizumab is a humanized IgG1 monoclonal antibody used in the treatment of ulcerative colitis and Crohn disease. It targets the α4β7 integrin, a specific modulator of gut-trafficking lymphocytes. In vedolizumab’s clinical trial for Crohn disease, there was no increased incidence of life-threatening, severe infection.10,11 Often, new biologic treatments are used with known immunosuppressive medications. Mercaptopurine and prednisone are implicated in infections; however, recovery from the immune suppression usually is seen at 1 month after discontinuation.12 Our patient continued to worsen for several weeks and required increased dosing of itraconazole, despite stopping both prednisone and mercaptopurine. It opens the question as to whether vedolizumab played a role in the recalcitrant disease.
This case illustrates the importance of a high index of suspicion for unusual infections in the setting of biologic therapy. An infectious etiology of a cutaneous eruption in an immunosuppressed patient should always be included in the differential diagnosis and actively pursued early on; tissue culture may shorten the treatment course and decrease severity of the disease. Although a direct link between the mechanism of action of vedolizumab and cutaneous infection is not clear, given the rare incidence of this infection, a report of such a case is important to the practicing clinician.
- de Hoog GS, Ahmed SA, Najafzadeh MJ, et al. Phylogenetic findings suggest possible new habitat and routes of infection of human eumycetoma. PLoS Negl Trop Dis. 2013;7:e2229. doi:10.1371/journal.pntd.0002229
- Zhang H, Ran Y, Li D, et al. Clavispora lusitaniae and Chaetomium atrobrunneum as rare agents of cutaneous infection. Mycopathologia. 2010;169:373-380. doi:10.1007/s11046-009-9266-9
- Schieffelin JS, Garcia-Diaz JB, Loss GE, et al. Phaeohyphomycosis fungal infections in solid organ transplant recipients: clinical presentation, pathology, and treatment. Transpl Infect Dis Off J Transplant Soc. 2014;16:270-278. doi:10.1111/tid.12197
- Farhi D, Cosnes J, Zizi N, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore). 2008;87:281-293. doi:10.1097/MD.0b013e318187cc9c
- Guarro J, Soler L, Rinaldi MG. Pathogenicity and antifungal susceptibility of Chaetomium species. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 1995;14:613-618.
- Teixeira ABA, Trabasso P, Moretti-Branchini ML, et al. Phaeohyphomycosis caused by Chaetomium globosum in an allogeneic bone marrow transplant recipient. Mycopathologia. 2003;156:309-312.
- Falcón CS, Falcón MDMS, Ceballos JD, et al. Onychomycosis by Chaetomium spp. Mycoses. 2009;52:77-79. doi:10.1111/j.14390507.2008.01519.x
- Kim DM, Lee MH, Suh MK, et al. Onychomycosis caused by Chaetomium globosum. Ann Dermatol. 2013;25:232-236. doi:10.5021/ad.2013.25.2.232
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
- Luthra P, Peyrin-Biroulet L, Ford AC. Systematic review and meta-analysis: opportunistic infections and malignancies during treatment with anti-integrin antibodies in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:1227-1236. doi:10.1111/apt.13215
- Colombel J-F, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839-851. doi:10.1136/gutjnl-2015-311079
- Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081-1085.
- de Hoog GS, Ahmed SA, Najafzadeh MJ, et al. Phylogenetic findings suggest possible new habitat and routes of infection of human eumycetoma. PLoS Negl Trop Dis. 2013;7:e2229. doi:10.1371/journal.pntd.0002229
- Zhang H, Ran Y, Li D, et al. Clavispora lusitaniae and Chaetomium atrobrunneum as rare agents of cutaneous infection. Mycopathologia. 2010;169:373-380. doi:10.1007/s11046-009-9266-9
- Schieffelin JS, Garcia-Diaz JB, Loss GE, et al. Phaeohyphomycosis fungal infections in solid organ transplant recipients: clinical presentation, pathology, and treatment. Transpl Infect Dis Off J Transplant Soc. 2014;16:270-278. doi:10.1111/tid.12197
- Farhi D, Cosnes J, Zizi N, et al. Significance of erythema nodosum and pyoderma gangrenosum in inflammatory bowel diseases: a cohort study of 2402 patients. Medicine (Baltimore). 2008;87:281-293. doi:10.1097/MD.0b013e318187cc9c
- Guarro J, Soler L, Rinaldi MG. Pathogenicity and antifungal susceptibility of Chaetomium species. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 1995;14:613-618.
- Teixeira ABA, Trabasso P, Moretti-Branchini ML, et al. Phaeohyphomycosis caused by Chaetomium globosum in an allogeneic bone marrow transplant recipient. Mycopathologia. 2003;156:309-312.
- Falcón CS, Falcón MDMS, Ceballos JD, et al. Onychomycosis by Chaetomium spp. Mycoses. 2009;52:77-79. doi:10.1111/j.14390507.2008.01519.x
- Kim DM, Lee MH, Suh MK, et al. Onychomycosis caused by Chaetomium globosum. Ann Dermatol. 2013;25:232-236. doi:10.5021/ad.2013.25.2.232
- Vugia DJ, Jang Y, Zizek C, et al. Mycobacteria in nail salon whirlpool footbaths, California. Emerg Infect Dis. 2005;11:616-618. doi:10.3201/eid1104.040936
- Luthra P, Peyrin-Biroulet L, Ford AC. Systematic review and meta-analysis: opportunistic infections and malignancies during treatment with anti-integrin antibodies in inflammatory bowel disease. Aliment Pharmacol Ther. 2015;41:1227-1236. doi:10.1111/apt.13215
- Colombel J-F, Sands BE, Rutgeerts P, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017;66:839-851. doi:10.1136/gutjnl-2015-311079
- Connell WR, Kamm MA, Ritchie JK, et al. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081-1085.
Practice Points
- Tissue culture remains the gold standard for deep fungal infections.
- Physicians must maintain a high index of suspicion for alternate diagnoses when a disease progresses along an unexpected course.
- Biologic medications may have low-incidence side effects that emerge in postmarket use.
COVID-19 booster shots to start in September: Officials
at a press briefing August 18.
Those who received the Pfizer-BioNTech and Moderna vaccines would be eligible to get a booster shot 8 months after they received the second dose of those vaccines, officials said. Information on boosters for those who got the one-dose Johnson & Johnson vaccine will be forthcoming.
“We anticipate a booster will [also] likely be needed,” said U.S. Surgeon General Vivek Murthy, MD. The J&J vaccine was not available in the U.S. until March, he said, and ‘’we expect more data on J&J in the coming weeks, so that plan is coming.”
The plan for boosters for the two mRNA vaccines is pending the FDA’s conducting of an independent review and authorizing the third dose of the Moderna and Pfizer-BioNTech vaccines, as well as an advisory committee of the CDC making the recommendation.
“We know that even highly effective vaccines become less effective over time,” Dr. Murthy said. “Having reviewed the most current data, it is now our clinical judgment that the time to lay out a plan for the COVID-19 boosters is now.”
Research released Aug. 18 shows waning effectiveness of the two mRNA vaccines.
At the briefing, Dr. Murthy and others continually reassured listeners that while effectiveness against infection declines, the vaccines continue to protect against severe infections, hospitalizations, and death.
“If you are fully vaccinated, you still have a high degree of protection against the worst outcomes,” Dr. Murthy said.
Data driving the plan
CDC Director Rochelle Walensky, MD, cited three research studies published Aug. 18 in the CDC’s Morbidity and Mortality Weekly Report that helped to drive the decision to recommend boosters.
Analysis of nursing home COVID-19 data from the CDC’s National Healthcare Safety Network showed a significant decline in the effectiveness of the full mRNA vaccine against lab-confirmed COVID-19 infection, from 74.7% before the Delta variant (March 1-May 9, 2021) to 53% when the Delta variant became predominant in the United States. The analysis during the Delta dominant period included 85,000 weekly reports from nearly 15,000 facilities.
Another study looked at more than 10 million New York adults who had been fully vaccinated with either the Moderna, Pfizer, or J&J vaccine by July 25. During the period from May 3 to July 25, overall, the age-adjusted vaccine effectiveness against infection decreased from 91.7% to 79.8%.
Vaccine effectiveness against hospitalization remains high, another study found. An analysis of 1,129 patients who had gotten two doses of an mRNA vaccine showed vaccine effectiveness against hospitalization after 24 weeks. It was 86% at weeks 2-12 and 84% at weeks 13-24.
Immunologic facts
Immunologic information also points to the need for a booster, said Anthony Fauci, MD, the chief medical advisor to the president and director of the National Institute of Allergy and Infectious Diseases.
“Antibody levels decline over time,” he said, “and higher antibody levels are associated with higher efficacy of the vaccine. Higher levels of antibody may be needed to protect against Delta.”
A booster increased antibody levels by ‘’at least tenfold and possibly more,” he said. And higher levels of antibody may be required to protect against Delta. Taken together, he said, the data support the use of a booster to increase the overall level of protection.
Booster details
“We will make sure it is convenient and easy to get the booster shot,” said Jeff Zients, the White House COVID-19 response coordinator. As with the previous immunization, he said, the booster will be free, and no one will be asked about immigration status.
The plan for booster shots is an attempt to stay ahead of the virus, officials stressed
Big picture
Not everyone agrees with the booster dose idea. At a World Health Organization briefing Aug. 18, WHO’s Chief Scientist Soumya Swaminathan, MD, an Indian pediatrician, said that the right thing to do right now ‘’is to wait for the science to tell us when boosters, which groups of people, and which vaccines need boosters.”
Like others, she also broached the ‘’moral and ethical argument of giving people third doses, when they’re already well protected and while the rest of the world is waiting for their primary immunization.”
Dr. Swaminathan does see a role for boosters to protect immunocompromised people but noted that ‘’that’s a small number of people.” Widespread boosters ‘’will only lead to more variants, to more escape variants, and perhaps we’re heading into more dire situations.”
A version of this article first appeared on WebMD.com.
at a press briefing August 18.
Those who received the Pfizer-BioNTech and Moderna vaccines would be eligible to get a booster shot 8 months after they received the second dose of those vaccines, officials said. Information on boosters for those who got the one-dose Johnson & Johnson vaccine will be forthcoming.
“We anticipate a booster will [also] likely be needed,” said U.S. Surgeon General Vivek Murthy, MD. The J&J vaccine was not available in the U.S. until March, he said, and ‘’we expect more data on J&J in the coming weeks, so that plan is coming.”
The plan for boosters for the two mRNA vaccines is pending the FDA’s conducting of an independent review and authorizing the third dose of the Moderna and Pfizer-BioNTech vaccines, as well as an advisory committee of the CDC making the recommendation.
“We know that even highly effective vaccines become less effective over time,” Dr. Murthy said. “Having reviewed the most current data, it is now our clinical judgment that the time to lay out a plan for the COVID-19 boosters is now.”
Research released Aug. 18 shows waning effectiveness of the two mRNA vaccines.
At the briefing, Dr. Murthy and others continually reassured listeners that while effectiveness against infection declines, the vaccines continue to protect against severe infections, hospitalizations, and death.
“If you are fully vaccinated, you still have a high degree of protection against the worst outcomes,” Dr. Murthy said.
Data driving the plan
CDC Director Rochelle Walensky, MD, cited three research studies published Aug. 18 in the CDC’s Morbidity and Mortality Weekly Report that helped to drive the decision to recommend boosters.
Analysis of nursing home COVID-19 data from the CDC’s National Healthcare Safety Network showed a significant decline in the effectiveness of the full mRNA vaccine against lab-confirmed COVID-19 infection, from 74.7% before the Delta variant (March 1-May 9, 2021) to 53% when the Delta variant became predominant in the United States. The analysis during the Delta dominant period included 85,000 weekly reports from nearly 15,000 facilities.
Another study looked at more than 10 million New York adults who had been fully vaccinated with either the Moderna, Pfizer, or J&J vaccine by July 25. During the period from May 3 to July 25, overall, the age-adjusted vaccine effectiveness against infection decreased from 91.7% to 79.8%.
Vaccine effectiveness against hospitalization remains high, another study found. An analysis of 1,129 patients who had gotten two doses of an mRNA vaccine showed vaccine effectiveness against hospitalization after 24 weeks. It was 86% at weeks 2-12 and 84% at weeks 13-24.
Immunologic facts
Immunologic information also points to the need for a booster, said Anthony Fauci, MD, the chief medical advisor to the president and director of the National Institute of Allergy and Infectious Diseases.
“Antibody levels decline over time,” he said, “and higher antibody levels are associated with higher efficacy of the vaccine. Higher levels of antibody may be needed to protect against Delta.”
A booster increased antibody levels by ‘’at least tenfold and possibly more,” he said. And higher levels of antibody may be required to protect against Delta. Taken together, he said, the data support the use of a booster to increase the overall level of protection.
Booster details
“We will make sure it is convenient and easy to get the booster shot,” said Jeff Zients, the White House COVID-19 response coordinator. As with the previous immunization, he said, the booster will be free, and no one will be asked about immigration status.
The plan for booster shots is an attempt to stay ahead of the virus, officials stressed
Big picture
Not everyone agrees with the booster dose idea. At a World Health Organization briefing Aug. 18, WHO’s Chief Scientist Soumya Swaminathan, MD, an Indian pediatrician, said that the right thing to do right now ‘’is to wait for the science to tell us when boosters, which groups of people, and which vaccines need boosters.”
Like others, she also broached the ‘’moral and ethical argument of giving people third doses, when they’re already well protected and while the rest of the world is waiting for their primary immunization.”
Dr. Swaminathan does see a role for boosters to protect immunocompromised people but noted that ‘’that’s a small number of people.” Widespread boosters ‘’will only lead to more variants, to more escape variants, and perhaps we’re heading into more dire situations.”
A version of this article first appeared on WebMD.com.
at a press briefing August 18.
Those who received the Pfizer-BioNTech and Moderna vaccines would be eligible to get a booster shot 8 months after they received the second dose of those vaccines, officials said. Information on boosters for those who got the one-dose Johnson & Johnson vaccine will be forthcoming.
“We anticipate a booster will [also] likely be needed,” said U.S. Surgeon General Vivek Murthy, MD. The J&J vaccine was not available in the U.S. until March, he said, and ‘’we expect more data on J&J in the coming weeks, so that plan is coming.”
The plan for boosters for the two mRNA vaccines is pending the FDA’s conducting of an independent review and authorizing the third dose of the Moderna and Pfizer-BioNTech vaccines, as well as an advisory committee of the CDC making the recommendation.
“We know that even highly effective vaccines become less effective over time,” Dr. Murthy said. “Having reviewed the most current data, it is now our clinical judgment that the time to lay out a plan for the COVID-19 boosters is now.”
Research released Aug. 18 shows waning effectiveness of the two mRNA vaccines.
At the briefing, Dr. Murthy and others continually reassured listeners that while effectiveness against infection declines, the vaccines continue to protect against severe infections, hospitalizations, and death.
“If you are fully vaccinated, you still have a high degree of protection against the worst outcomes,” Dr. Murthy said.
Data driving the plan
CDC Director Rochelle Walensky, MD, cited three research studies published Aug. 18 in the CDC’s Morbidity and Mortality Weekly Report that helped to drive the decision to recommend boosters.
Analysis of nursing home COVID-19 data from the CDC’s National Healthcare Safety Network showed a significant decline in the effectiveness of the full mRNA vaccine against lab-confirmed COVID-19 infection, from 74.7% before the Delta variant (March 1-May 9, 2021) to 53% when the Delta variant became predominant in the United States. The analysis during the Delta dominant period included 85,000 weekly reports from nearly 15,000 facilities.
Another study looked at more than 10 million New York adults who had been fully vaccinated with either the Moderna, Pfizer, or J&J vaccine by July 25. During the period from May 3 to July 25, overall, the age-adjusted vaccine effectiveness against infection decreased from 91.7% to 79.8%.
Vaccine effectiveness against hospitalization remains high, another study found. An analysis of 1,129 patients who had gotten two doses of an mRNA vaccine showed vaccine effectiveness against hospitalization after 24 weeks. It was 86% at weeks 2-12 and 84% at weeks 13-24.
Immunologic facts
Immunologic information also points to the need for a booster, said Anthony Fauci, MD, the chief medical advisor to the president and director of the National Institute of Allergy and Infectious Diseases.
“Antibody levels decline over time,” he said, “and higher antibody levels are associated with higher efficacy of the vaccine. Higher levels of antibody may be needed to protect against Delta.”
A booster increased antibody levels by ‘’at least tenfold and possibly more,” he said. And higher levels of antibody may be required to protect against Delta. Taken together, he said, the data support the use of a booster to increase the overall level of protection.
Booster details
“We will make sure it is convenient and easy to get the booster shot,” said Jeff Zients, the White House COVID-19 response coordinator. As with the previous immunization, he said, the booster will be free, and no one will be asked about immigration status.
The plan for booster shots is an attempt to stay ahead of the virus, officials stressed
Big picture
Not everyone agrees with the booster dose idea. At a World Health Organization briefing Aug. 18, WHO’s Chief Scientist Soumya Swaminathan, MD, an Indian pediatrician, said that the right thing to do right now ‘’is to wait for the science to tell us when boosters, which groups of people, and which vaccines need boosters.”
Like others, she also broached the ‘’moral and ethical argument of giving people third doses, when they’re already well protected and while the rest of the world is waiting for their primary immunization.”
Dr. Swaminathan does see a role for boosters to protect immunocompromised people but noted that ‘’that’s a small number of people.” Widespread boosters ‘’will only lead to more variants, to more escape variants, and perhaps we’re heading into more dire situations.”
A version of this article first appeared on WebMD.com.
Latest data show increase in breakthrough COVID-19 cases
Breakthrough cases accounted for about one in five newly diagnosed cases in six of the states, according to the New York Times. Hospitalizations and deaths among vaccinated people may be higher than previously thought as well.
“Remember when the early vaccine studies came out, it was like nobody gets hospitalized, nobody dies,” Robert Wachter, MD, chairman of the department of medicine at the University of California, San Francisco, said in an interview. “That clearly is not true.”
The New York Times analyzed data in seven states – California, Colorado, Massachusetts, Oregon, Utah, Vermont, and Virginia – that are tracking the most detailed information. The trends in these states may not reflect the numbers throughout the country, the newspaper reported.
Even still, the numbers back up the idea that vaccinated people may need booster shots this fall to support their earlier vaccine doses. Federal health officials are scheduled to approve the extra shots in coming weeks, potentially in September. The first people to receive booster shots will likely be health care workers and nursing home residents who took the first vaccines in December and January.
“If the chances of a breakthrough infection have gone up considerably, and I think the evidence is clear that they have, and the level of protection against severe illness is no longer as robust as it was, I think the case for boosters goes up pretty quickly,” Dr. Wachter said.
Previous analyses of breakthrough cases included data from June and earlier, the newspaper reported. But since July, COVID-19 cases have soared again because of the Delta variant, and the most recent numbers show an uptick among vaccinated people. In Los Angeles County, for instance, fully vaccinated people account for 20% of new COVID-19 cases, which is up from 11% in May, 5% in April, and 2% in March, according to a late July report from the Los Angeles County Department of Public Health.
What’s more, breakthrough infections in the seven states accounted for 12%-24% of COVID-19 hospitalizations in those states. About 8,000 breakthrough hospitalizations have been reported to the CDC. Still, the overall numbers remain low – in California, for instance, about 1,615 people have been hospitalized with breakthrough infections, which accounts for 0.007% of the state’s 22 million vaccinated people, the Times reported.
The breakthrough infections appear to be more severe among vaccinated people who are older or have weakened immune systems. About 74% of breakthrough cases are among adults 65 or older, the CDC reported.
The increase may shift how vaccinated people see their risks for infection and interact with loved ones. Public health officials have suggested that people follow some COVID-19 safety protocols again, such as wearing masks in public indoor spaces regardless of vaccination status.
As the Delta variant continues to circulate this fall, public health researchers will be researching more about breakthrough cases among vaccinated people, including whether they have prolonged symptoms and how easily they may pass the virus to others.
“I think some of us have been challenged by the numbers of clusters that we’ve seen,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, told this news organization.
“I think that really needs to be examined more,” he said.
A version of this article first appeared on WebMD.com.
Breakthrough cases accounted for about one in five newly diagnosed cases in six of the states, according to the New York Times. Hospitalizations and deaths among vaccinated people may be higher than previously thought as well.
“Remember when the early vaccine studies came out, it was like nobody gets hospitalized, nobody dies,” Robert Wachter, MD, chairman of the department of medicine at the University of California, San Francisco, said in an interview. “That clearly is not true.”
The New York Times analyzed data in seven states – California, Colorado, Massachusetts, Oregon, Utah, Vermont, and Virginia – that are tracking the most detailed information. The trends in these states may not reflect the numbers throughout the country, the newspaper reported.
Even still, the numbers back up the idea that vaccinated people may need booster shots this fall to support their earlier vaccine doses. Federal health officials are scheduled to approve the extra shots in coming weeks, potentially in September. The first people to receive booster shots will likely be health care workers and nursing home residents who took the first vaccines in December and January.
“If the chances of a breakthrough infection have gone up considerably, and I think the evidence is clear that they have, and the level of protection against severe illness is no longer as robust as it was, I think the case for boosters goes up pretty quickly,” Dr. Wachter said.
Previous analyses of breakthrough cases included data from June and earlier, the newspaper reported. But since July, COVID-19 cases have soared again because of the Delta variant, and the most recent numbers show an uptick among vaccinated people. In Los Angeles County, for instance, fully vaccinated people account for 20% of new COVID-19 cases, which is up from 11% in May, 5% in April, and 2% in March, according to a late July report from the Los Angeles County Department of Public Health.
What’s more, breakthrough infections in the seven states accounted for 12%-24% of COVID-19 hospitalizations in those states. About 8,000 breakthrough hospitalizations have been reported to the CDC. Still, the overall numbers remain low – in California, for instance, about 1,615 people have been hospitalized with breakthrough infections, which accounts for 0.007% of the state’s 22 million vaccinated people, the Times reported.
The breakthrough infections appear to be more severe among vaccinated people who are older or have weakened immune systems. About 74% of breakthrough cases are among adults 65 or older, the CDC reported.
The increase may shift how vaccinated people see their risks for infection and interact with loved ones. Public health officials have suggested that people follow some COVID-19 safety protocols again, such as wearing masks in public indoor spaces regardless of vaccination status.
As the Delta variant continues to circulate this fall, public health researchers will be researching more about breakthrough cases among vaccinated people, including whether they have prolonged symptoms and how easily they may pass the virus to others.
“I think some of us have been challenged by the numbers of clusters that we’ve seen,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, told this news organization.
“I think that really needs to be examined more,” he said.
A version of this article first appeared on WebMD.com.
Breakthrough cases accounted for about one in five newly diagnosed cases in six of the states, according to the New York Times. Hospitalizations and deaths among vaccinated people may be higher than previously thought as well.
“Remember when the early vaccine studies came out, it was like nobody gets hospitalized, nobody dies,” Robert Wachter, MD, chairman of the department of medicine at the University of California, San Francisco, said in an interview. “That clearly is not true.”
The New York Times analyzed data in seven states – California, Colorado, Massachusetts, Oregon, Utah, Vermont, and Virginia – that are tracking the most detailed information. The trends in these states may not reflect the numbers throughout the country, the newspaper reported.
Even still, the numbers back up the idea that vaccinated people may need booster shots this fall to support their earlier vaccine doses. Federal health officials are scheduled to approve the extra shots in coming weeks, potentially in September. The first people to receive booster shots will likely be health care workers and nursing home residents who took the first vaccines in December and January.
“If the chances of a breakthrough infection have gone up considerably, and I think the evidence is clear that they have, and the level of protection against severe illness is no longer as robust as it was, I think the case for boosters goes up pretty quickly,” Dr. Wachter said.
Previous analyses of breakthrough cases included data from June and earlier, the newspaper reported. But since July, COVID-19 cases have soared again because of the Delta variant, and the most recent numbers show an uptick among vaccinated people. In Los Angeles County, for instance, fully vaccinated people account for 20% of new COVID-19 cases, which is up from 11% in May, 5% in April, and 2% in March, according to a late July report from the Los Angeles County Department of Public Health.
What’s more, breakthrough infections in the seven states accounted for 12%-24% of COVID-19 hospitalizations in those states. About 8,000 breakthrough hospitalizations have been reported to the CDC. Still, the overall numbers remain low – in California, for instance, about 1,615 people have been hospitalized with breakthrough infections, which accounts for 0.007% of the state’s 22 million vaccinated people, the Times reported.
The breakthrough infections appear to be more severe among vaccinated people who are older or have weakened immune systems. About 74% of breakthrough cases are among adults 65 or older, the CDC reported.
The increase may shift how vaccinated people see their risks for infection and interact with loved ones. Public health officials have suggested that people follow some COVID-19 safety protocols again, such as wearing masks in public indoor spaces regardless of vaccination status.
As the Delta variant continues to circulate this fall, public health researchers will be researching more about breakthrough cases among vaccinated people, including whether they have prolonged symptoms and how easily they may pass the virus to others.
“I think some of us have been challenged by the numbers of clusters that we’ve seen,” Michael Osterholm, PhD, director of the Center for Infectious Disease Research and Policy at the University of Minnesota, told this news organization.
“I think that really needs to be examined more,” he said.
A version of this article first appeared on WebMD.com.
FDA approves Pfizer’s tick-borne encephalitis vaccine
The U.S. Food and Drug Administration has approved Pfizer’s TicoVac vaccine for the treatment of tick-borne encephalitis (TBE). The vaccine is approved outside of the United States, and more than 170 million doses have been administered since 1976. The World Health Organization recommends vaccination for everyone in areas where the annual incidence of clinical disease is highly endemic, defined as more than five cases per 100,000 population, which is primarily the Baltic countries of Europe but includes some regions of Central and East Asia.
GlaxoSmithKline’s Encepur is also approved outside the United States, as is a vaccine from China and two from Russia. The efficacy of all the vaccines is greater than 95%. Pfizer’s protection is 98.7% to 100.0% after the three-dose course. With the new approval, American travelers will be able to get immunized before their departure instead of waiting until they are overseas to start the series.
TicoVac can cause injection-site pain, headache, myalgia, and fever, as is typical with many vaccines.
Tick-borne encephalitis
TBE is caused by a flavivirus and is transmitted by the bite of an infected Ixodes scapularis, or deer tick. Like the Powassan virus, another flavivirus, infection can be transmitted in minutes through the tick’s saliva, so early removal of the tick might not prevent illness. This is different than Lyme disease, where vigilance and early removal of the tick can prevent transmission.
Reservoirs for the virus include mice, voles, and shrews. Large mammals (deer, sheep, cattle, goats) also serve to support tick multiplication. In addition to tick bites, ingestion of unpasteurized milk from infected mammals can transmit TBE.
TBE symptoms can range from none to severe encephalitis (brain inflammation). One-quarter of infected people develop encephalitis. Most recover fully, but one-third of those infected can develop lifelong damage and paralysis or cognitive deficits. Death is rare, except in those infected with the Russian strain.
The first phase of a TBE infection is typical of viral infections, with nonspecific fever, headache, nausea, and myalgia. The next phase involves an asymptomatic interval of about a week (range, 1 to 33 days), followed by symptoms of a central nervous system infection.
There is no treatment for TBE and no antivirals with proven benefit. However, a recent case report describes the successful treatment of TBE with favipiravir.
For now, if you are unvaccinated, prevention is the only viable option. If you plan to travel to an endemic region and anticipate participating in outdoor activities (such as hunting or hiking), wear permethrin-treated clothes, use an insecticide, and don’t eat or drink unpasteurized dairy products.
Judy Stone, MD, is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. You can find her at drjudystone.com or on Twitter @drjudystone.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved Pfizer’s TicoVac vaccine for the treatment of tick-borne encephalitis (TBE). The vaccine is approved outside of the United States, and more than 170 million doses have been administered since 1976. The World Health Organization recommends vaccination for everyone in areas where the annual incidence of clinical disease is highly endemic, defined as more than five cases per 100,000 population, which is primarily the Baltic countries of Europe but includes some regions of Central and East Asia.
GlaxoSmithKline’s Encepur is also approved outside the United States, as is a vaccine from China and two from Russia. The efficacy of all the vaccines is greater than 95%. Pfizer’s protection is 98.7% to 100.0% after the three-dose course. With the new approval, American travelers will be able to get immunized before their departure instead of waiting until they are overseas to start the series.
TicoVac can cause injection-site pain, headache, myalgia, and fever, as is typical with many vaccines.
Tick-borne encephalitis
TBE is caused by a flavivirus and is transmitted by the bite of an infected Ixodes scapularis, or deer tick. Like the Powassan virus, another flavivirus, infection can be transmitted in minutes through the tick’s saliva, so early removal of the tick might not prevent illness. This is different than Lyme disease, where vigilance and early removal of the tick can prevent transmission.
Reservoirs for the virus include mice, voles, and shrews. Large mammals (deer, sheep, cattle, goats) also serve to support tick multiplication. In addition to tick bites, ingestion of unpasteurized milk from infected mammals can transmit TBE.
TBE symptoms can range from none to severe encephalitis (brain inflammation). One-quarter of infected people develop encephalitis. Most recover fully, but one-third of those infected can develop lifelong damage and paralysis or cognitive deficits. Death is rare, except in those infected with the Russian strain.
The first phase of a TBE infection is typical of viral infections, with nonspecific fever, headache, nausea, and myalgia. The next phase involves an asymptomatic interval of about a week (range, 1 to 33 days), followed by symptoms of a central nervous system infection.
There is no treatment for TBE and no antivirals with proven benefit. However, a recent case report describes the successful treatment of TBE with favipiravir.
For now, if you are unvaccinated, prevention is the only viable option. If you plan to travel to an endemic region and anticipate participating in outdoor activities (such as hunting or hiking), wear permethrin-treated clothes, use an insecticide, and don’t eat or drink unpasteurized dairy products.
Judy Stone, MD, is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. You can find her at drjudystone.com or on Twitter @drjudystone.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has approved Pfizer’s TicoVac vaccine for the treatment of tick-borne encephalitis (TBE). The vaccine is approved outside of the United States, and more than 170 million doses have been administered since 1976. The World Health Organization recommends vaccination for everyone in areas where the annual incidence of clinical disease is highly endemic, defined as more than five cases per 100,000 population, which is primarily the Baltic countries of Europe but includes some regions of Central and East Asia.
GlaxoSmithKline’s Encepur is also approved outside the United States, as is a vaccine from China and two from Russia. The efficacy of all the vaccines is greater than 95%. Pfizer’s protection is 98.7% to 100.0% after the three-dose course. With the new approval, American travelers will be able to get immunized before their departure instead of waiting until they are overseas to start the series.
TicoVac can cause injection-site pain, headache, myalgia, and fever, as is typical with many vaccines.
Tick-borne encephalitis
TBE is caused by a flavivirus and is transmitted by the bite of an infected Ixodes scapularis, or deer tick. Like the Powassan virus, another flavivirus, infection can be transmitted in minutes through the tick’s saliva, so early removal of the tick might not prevent illness. This is different than Lyme disease, where vigilance and early removal of the tick can prevent transmission.
Reservoirs for the virus include mice, voles, and shrews. Large mammals (deer, sheep, cattle, goats) also serve to support tick multiplication. In addition to tick bites, ingestion of unpasteurized milk from infected mammals can transmit TBE.
TBE symptoms can range from none to severe encephalitis (brain inflammation). One-quarter of infected people develop encephalitis. Most recover fully, but one-third of those infected can develop lifelong damage and paralysis or cognitive deficits. Death is rare, except in those infected with the Russian strain.
The first phase of a TBE infection is typical of viral infections, with nonspecific fever, headache, nausea, and myalgia. The next phase involves an asymptomatic interval of about a week (range, 1 to 33 days), followed by symptoms of a central nervous system infection.
There is no treatment for TBE and no antivirals with proven benefit. However, a recent case report describes the successful treatment of TBE with favipiravir.
For now, if you are unvaccinated, prevention is the only viable option. If you plan to travel to an endemic region and anticipate participating in outdoor activities (such as hunting or hiking), wear permethrin-treated clothes, use an insecticide, and don’t eat or drink unpasteurized dairy products.
Judy Stone, MD, is an infectious disease specialist and author of Resilience: One Family’s Story of Hope and Triumph Over Evil and of Conducting Clinical Research, the essential guide to the topic. You can find her at drjudystone.com or on Twitter @drjudystone.
A version of this article first appeared on Medscape.com.
Children and COVID: New cases rise to winter levels
Weekly cases of COVID-19 in children topped 100,000 for the first time since early February, according to the American Academy of Pediatrics and the Children’s Hospital Association.
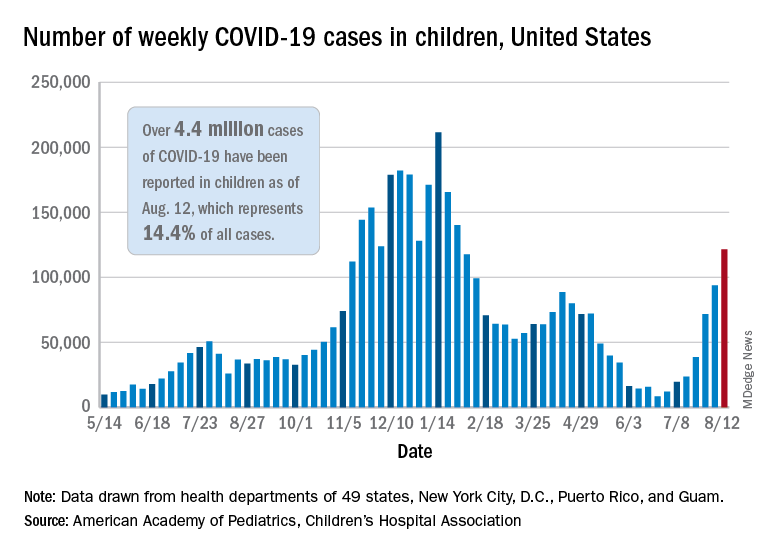
the AAP and CHA said in their weekly COVD-19 report. The recent surge in child COVID has also brought a record high in hospitalizations and shortages of pediatric ICU beds in some areas.
The 121,000 new cases represent an increase of almost 1,400% since June 18-24, when the weekly tally was just 8,447 and at its lowest point in over a year, the AAP/CHA data show.
On the vaccination front in the last week (Aug. 10-16), vaccine initiation for 12- to 17-year-olds was fairly robust but still down slightly, compared with the previous week. Just over 402,000 children aged 12-15 years received a first vaccination, which was down slightly from 411,000 the week before but still higher than any of the 6 weeks from June 22 to Aug. 2, based on data from the Centers for Disease Control and Prevention. Vaccinations were down by a similar margin for 15- to-17-year-olds.
Over 10.9 million children aged 12-17 have had at least one dose of COVID-19 vaccine administered, of whom 8.1 million are fully vaccinated. Among those aged 12-15 years, 44.5% have gotten at least one dose and 31.8% are fully vaccinated, with corresponding figures of 53.9% and 42.5% for 16- and 17-year-olds, according to the CDC’s COVID Data Tracker.
The number of COVID-19 cases reported in children since the start of the pandemic is up to 4.4 million, which makes up 14.4% of all cases in the United States, the AAP and CHA said. Other cumulative figures through Aug. 12 include almost 18,000 hospitalizations – reported by 23 states and New York City – and 378 deaths – reported by 43 states, New York City, Puerto Rico, and Guam.
In the latest edition of their ongoing report, compiled using state data since the summer of 2020, the two groups noted that, “in the summer of 2021, some states have revised cases counts previously reported, begun reporting less frequently, or dropped metrics previously reported.” Among those states are Nebraska, which shut down its online COVID dashboard in late June, and Alabama, which stopped reporting cumulative cases and deaths after July 29.
Weekly cases of COVID-19 in children topped 100,000 for the first time since early February, according to the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVD-19 report. The recent surge in child COVID has also brought a record high in hospitalizations and shortages of pediatric ICU beds in some areas.
The 121,000 new cases represent an increase of almost 1,400% since June 18-24, when the weekly tally was just 8,447 and at its lowest point in over a year, the AAP/CHA data show.
On the vaccination front in the last week (Aug. 10-16), vaccine initiation for 12- to 17-year-olds was fairly robust but still down slightly, compared with the previous week. Just over 402,000 children aged 12-15 years received a first vaccination, which was down slightly from 411,000 the week before but still higher than any of the 6 weeks from June 22 to Aug. 2, based on data from the Centers for Disease Control and Prevention. Vaccinations were down by a similar margin for 15- to-17-year-olds.
Over 10.9 million children aged 12-17 have had at least one dose of COVID-19 vaccine administered, of whom 8.1 million are fully vaccinated. Among those aged 12-15 years, 44.5% have gotten at least one dose and 31.8% are fully vaccinated, with corresponding figures of 53.9% and 42.5% for 16- and 17-year-olds, according to the CDC’s COVID Data Tracker.
The number of COVID-19 cases reported in children since the start of the pandemic is up to 4.4 million, which makes up 14.4% of all cases in the United States, the AAP and CHA said. Other cumulative figures through Aug. 12 include almost 18,000 hospitalizations – reported by 23 states and New York City – and 378 deaths – reported by 43 states, New York City, Puerto Rico, and Guam.
In the latest edition of their ongoing report, compiled using state data since the summer of 2020, the two groups noted that, “in the summer of 2021, some states have revised cases counts previously reported, begun reporting less frequently, or dropped metrics previously reported.” Among those states are Nebraska, which shut down its online COVID dashboard in late June, and Alabama, which stopped reporting cumulative cases and deaths after July 29.
Weekly cases of COVID-19 in children topped 100,000 for the first time since early February, according to the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVD-19 report. The recent surge in child COVID has also brought a record high in hospitalizations and shortages of pediatric ICU beds in some areas.
The 121,000 new cases represent an increase of almost 1,400% since June 18-24, when the weekly tally was just 8,447 and at its lowest point in over a year, the AAP/CHA data show.
On the vaccination front in the last week (Aug. 10-16), vaccine initiation for 12- to 17-year-olds was fairly robust but still down slightly, compared with the previous week. Just over 402,000 children aged 12-15 years received a first vaccination, which was down slightly from 411,000 the week before but still higher than any of the 6 weeks from June 22 to Aug. 2, based on data from the Centers for Disease Control and Prevention. Vaccinations were down by a similar margin for 15- to-17-year-olds.
Over 10.9 million children aged 12-17 have had at least one dose of COVID-19 vaccine administered, of whom 8.1 million are fully vaccinated. Among those aged 12-15 years, 44.5% have gotten at least one dose and 31.8% are fully vaccinated, with corresponding figures of 53.9% and 42.5% for 16- and 17-year-olds, according to the CDC’s COVID Data Tracker.
The number of COVID-19 cases reported in children since the start of the pandemic is up to 4.4 million, which makes up 14.4% of all cases in the United States, the AAP and CHA said. Other cumulative figures through Aug. 12 include almost 18,000 hospitalizations – reported by 23 states and New York City – and 378 deaths – reported by 43 states, New York City, Puerto Rico, and Guam.
In the latest edition of their ongoing report, compiled using state data since the summer of 2020, the two groups noted that, “in the summer of 2021, some states have revised cases counts previously reported, begun reporting less frequently, or dropped metrics previously reported.” Among those states are Nebraska, which shut down its online COVID dashboard in late June, and Alabama, which stopped reporting cumulative cases and deaths after July 29.
Ulcerated and Verrucous Plaque on the Chest
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
The Diagnosis: Disseminated Coccidioidomycosis
A6-mm punch biopsy was performed at the periphery of the ulcerated cutaneous lesion on the chest revealing extensive spherules. Serum antibody immunodiffusion for histoplasmosis and blastomycoses both were negative; however, B-D-glucan assay was positive at 364 pg/mL (reference range: <60 pg/mL, negative). Initial HIV-1 and HIV-2 antibody and antigen testing was negative as well as repeat testing at 3 weeks. Immunodiffusion for Coccidioides IgM and IgG was positive, and cocci antibody IgG complement fixation assays were positive at titers of 1:64 (reference range: <1:2, negative). A computed tomography needle-guided biopsy of the paravertebral soft tissue was performed. Gram stains and bacterial cultures of the biopsies were negative; however, fungal cultures were notable for growth of Coccidioides. Given the pertinent testing, a diagnosis of disseminated coccidioidomycosis was made.
Cutaneous coccidioidomycosis can occur in 3 situations: direct inoculation (primary cutaneous coccidioidomycosis), disseminated infection (disseminated cutaneous coccidioidomycosis), or as a reactive component of pulmonary infection.1,2 Of them, primary and disseminated cutaneous coccidioidomycosis are organism specific and display characteristic spherules and fungus on histopathology and cultures, respectively. Reactive coccidioidomycosis differs from organism-specific disease, as it does not contain spherules in histopathologic sections of tissue biopsies.1 Reactive skin manifestations occur in 12% to 50% of patients with primary pulmonary infection and include erythema nodosum, erythema multiforme, acute generalized exanthema, reactive interstitial granulomatous dermatitis, and Sweet syndrome.3
Organism-specific cutaneous coccidioidomycosis most often is correlated with hematogenous dissemination of primary pulmonary disease rather than direct inoculation of skin.1 The skin is the most common site of extrapulmonary involvement in disseminated coccidioidomycosis, and cutaneous lesions have been reported in 15% to 67% of cases of disseminated disease.1,4 In cutaneous disseminated disease, nodules, papules, macules, and verrucous plaques have been described. In a case series of disseminated cutaneous coccidioidomycosis, nodules were the most common cutaneous presentation and occurred in 39% (7/18) of patients, while verrucous plaques were the rarest and occurred in only 6% (1/18) of patients.5
The rate of coccidioidomycosis dissemination varies based on exposure and patient characteristics. Increased rates of dissemination have been reported in patients of African and Filipino descent, along with individuals that are immunosuppressed due to disease or medical therapy. Dissemination is clinically significant, as patients with multifocal dissemination have a greater than 50% risk for mortality.6
Disseminated coccidioidomycosis is a relatively rare manifestation of Coccidioides infection; approximately 1.6% of patients exposed to and infected with Coccidioides ultimately will develop systemic or disseminated disease.7,8 Although the rates of primary pulmonary infection are similar between patients of varying ethnicities, the rates of dissemination are higher in patients of African and Filipino ethnicity.8 In population studies of coccidioidomycosis (N=332), Black patients represented 33.3% (4/12) of disseminated cases but only 8.7% of Coccidioides cases overall.7
Population studies of Black patients with coccidioidomycosis have shown a 4-fold higher predisposition for severe disease compared to mild disease.9 Spondylitis and meningitis also are disproportionately more common in Black patients.8 Black patients comprised 75% of all spondylitis cases in a cohort where only 25% of patients were Black. Additionally, 33% of all meningitis cases occurred in Black patients in a cohort where 8% of total cases were Black patients.8 Within the United States, the highest rates of coccidioidomycosis meningitis are seen in Black patients.10
The pathophysiology underlying the increased susceptibility of individuals of African or Filipino descent to disseminated and severe coccidioidomycosis remains unknown.8 The level of vulnerability within this patient population has no association with increased environmental exposure or poor immunologic response to Coccidioides, as demonstrated by the ability of these populations to respond to experimental vaccination and skin testing (spherulin, coccidioidin) to a similar extent as other ethnicities.8 Class II HLA-DRB1*1301 alleles have been associated with an increased risk for severe disseminated Coccidioides infection regardless of ethnicity; however, these alleles are not overrepresented in these patient populations.8
In patients with primary pulmonary coccidioidomycosis with no evidence of dissemination, guidelines generally recommend offering treatment to groups at high risk of dissemination, such as pregnant women and patients with diabetes mellitus. Given the high incidence of disseminated and severe disease in Black and Filipino patients, some guidelines endorse treatment of all cases of coccidioidomycosis in this patient population.8 No current data are available to help determine whether this broad treatment approach reduces the development of disseminated infection in these populations. Frequent monitoring for disease progression and/or dissemination involving clinical and laboratory reevaluation every 3 months for 2 years is highly recommended.8
Treatment generally is based on location and severity of infection, with disseminated nonmeningeal infection being treated with oral azole therapy (ketoconazole, itraconazole, or fluconazole).11 If there is involvement of the central nervous system structures or rapidly worsening disease despite azole therapy, amphotericin B is recommended at 0.5 to 0.7 mg/kg daily. In patients with disseminated meningeal infection, oral fluconazole (800–1000 mg/d) or a combination of an azole with intrathecal amphotericin B (0.01–1.5 mg/dose, interval ranging from daily to 1 week) is recommended to improve response.11
The differential diagnosis of cutaneous disseminated coccidioidomycosis is broad and includes other systemic endemic mycoses (histoplasmosis, blastomycosis) and infections (mycobacteria, leishmania). Lupus vulgaris, a form of cutaneous tuberculosis, presents as a palpable tubercular lesion that may coalesce into erythematous plaques, which may mimic endemic mycoses, especially in patients with risk factors for both infectious etiologies such as our patient.12 Disseminated histoplasmosis may present as polymorphic plaques, pustules, nodules, and ulcerated skin lesions, whereas disseminated blastomycosis characteristically presents as a crusted verrucous lesion with raised borders and painful ulcers, both of which may mimic coccidioidomycosis.13 Biopsy would reveal the characteristic intracellular yeast in Histoplasma capsulatum and broad-based budding yeast form of Blastomyces dermatitidis in histoplasmosis and blastomycosis, respectively, in contrast to the spherules seen in our patient’s biopsy.13 Localized cutaneous leishmaniasis initially develops as a nodular or papular lesion and can progress to open ulcerations with raised borders. Biopsy and histopathology would reveal round protozoal amastigotes.14 Other diagnoses that should be considered include mycetoma, nocardiosis, and sporotrichosis.15 As the cutaneous manifestations of Coccidioides infections are varied, a broad differential diagnosis should be maintained, and probable environmental and infectious exposures should be considered prior to ordering diagnostic studies.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
- Garcia Garcia SC, Salas Alanis JC, Flores MG, et al. Coccidioidomycosis and the skin: a comprehensive review. An Bras Dermatol. 2015; 90:610-619.
- DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-925.
- DiCaudo DJ, Yiannias JA, Laman SD, et al. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of 3 cases and review of the literature. Arch Dermatol. 2006;142:744-746.
- Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections. Ann N Y Acad Sci. 2007;1111:411-421.
- Crum NF, Lederman ER, Stafford CM, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. clinical characteristics and current controversies. Medicine (Baltimore). 2004;83:149-175.
- Borchers AT, Gershwin ME. The immune response in coccidioidomycosis. Autoimmun Rev. 2010;10:94-102.
- Smith CE, Beard RR. Varieties of coccidioidal infection in relation to the epidemiology and control of the diseases. Am J Public Health Nations Health. 1946;36:1394-1402.
- Ruddy BE, Mayer AP, Ko MG, et al. Coccidioidomycosis in African Americans. Mayo Clin Proc. 2011;86:63-69.
- Louie L, Ng S, Hajjeh R, et al. Influence of host genetics on the severity of coccidioidomycosis. Emerg Infect Dis. 1999;5:672-680.
- McCotter OZ, Benedict K, Engelthaler DM, et al. Update on the epidemiology of coccidioidomycosis in the United States. Med Mycol. 2019;57(suppl 1):S30-S40.
- Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:658-661.
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973.
- Smith JA, Riddell JT, Kauffman CA. Cutaneous manifestations of endemic mycoses. Curr Infect Dis Rep. 2013;15:440-449.
- Scorza BM, Carvalho EM, Wilson ME. Cutaneous manifestations of human and murine leishmaniasis. Int J Mol Sci. 2017;18:1296.
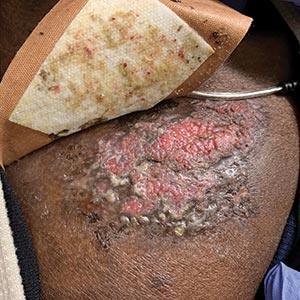
A 36-year-old man presented to an emergency department in the southwestern United States with a cough, fatigue, and worsening back pain associated with night sweats of 1 month’s duration. He experienced a 9.07-kg weight loss, as well as development of a rough, nontender, nonpruritic rash along the left upper chest over the prior month. The patient was born in West Africa and reported that he had moved to the southwestern United States from the eastern United States approximately 6 years prior to presentation. Physical examination on admission revealed a 5×3-cm, purple-brown, verrucous plaque with a central pink cobblestone appearance and ulceration. Chest radiography was notable for perihilar adenopathy with no focal infiltrates or cavitary lesions. Computed tomography and magnetic resonance imaging of the chest were notable for miliary nodules throughout the lungs; extensive lytic spine lesions of cervical, thoracic, and lumbar vertebral bodies and left twelfth rib; and a left paraspinal thoracic epidural soft tissue phlegmon. Initial laboratory investigations revealed peripheral eosinophilia without absolute leukocytosis and a microcytic anemia.
Universal masking is the key to safe school attendance
“I want my child to go back to school,” the mother said to me. “I just want you to tell me it will be safe.”
As the summer break winds down for children across the United States, pediatric COVID-19 cases are rising. According to the American Academy of Pediatrics, nearly 94,000 cases were reported for the week ending Aug. 5, more than double the case count from 2 weeks earlier.1
Anecdotally, some children’s hospitals are reporting an increase in pediatric COVID-19 admissions. In the hospital in which I practice, we are seeing numbers similar to those we saw in December and January: a typical daily census of 10 kids admitted with COVID-19, with 4 of them in the intensive care unit. It is a stark contrast to June when, most days, we had no patients with COVID-19 in the hospital. About half of our hospitalized patients are too young to be vaccinated against COVID-19, while the rest are unvaccinated children 12 years and older.
Vaccination of eligible children and teachers is an essential strategy for preventing the spread of COVID-19 in schools, but as children head back to school, immunization rates of educators are largely unknown and are suboptimal among students in most states. As of Aug. 11, 10.7 million U.S. children had received at least one dose of COVID-19 vaccine, representing 43% of 12- to 15-year-olds and 53% of 16- to 17-year-olds.2 Rates vary substantially by state, with more than 70% of kids in Vermont receiving at least one dose of vaccine, compared with less than 25% in Wyoming and Alabama.
Still, in the absence of robust immunization rates, we have data that schools can still reopen successfully. We need to follow the science and implement universal masking, a safe, effective, and practical mitigation strategy.
It worked in Wisconsin. Seventeen K-12 schools in rural Wisconsin opened last fall for in-person instruction.3 Reported compliance with masking was high, ranging from 92.1% to 97.4%, and in-school transmission of COVID-19 was low, with seven cases among 4,876 students.
It worked in Salt Lake City.4 In 20 elementary schools open for in-person instruction Dec. 3, 2020, to Jan. 31, 2021, compliance with mask-wearing was high and in-school transmission was very low, despite a high community incidence of COVID-19. Notably, students’ classroom seats were less than 6 feet apart, suggesting that consistent mask-wearing works even when physical distancing is challenging.
One of the best examples of successful school reopening happened in North Carolina, where pediatricians, pediatric infectious disease specialists, and other experts affiliated with Duke University formed the ABC Science Collaborative to support school districts that requested scientific input to help guide return-to-school policies during the COVID-19 pandemic. From Oct. 26, 2020, to Feb. 28, 2021, the ABC Science Collaborative worked with 13 school districts that were open for in-person instruction using basic mitigation strategies, including universal masking.5 During this time period, there were 4,969 community-acquired SARS-CoV-2 infections in the more than 100,000 students and staff present in schools. Transmission to school contacts was identified in only 209 individuals for a secondary attack rate of less than 1%.
Duke investigator Kanecia Zimmerman, MD, told Duke Today, “We know that, if our goal is to reduce transmission of COVID-19 in schools, there are two effective ways to do that: 1. vaccination, 2. masking. In the setting of schools ... the science suggests masking can be extremely effective, particularly for those who can’t get vaccinated while COVID-19 is still circulating.”
Both the AAP6 and the Pediatric Infectious Diseases Society7 have emphasized the importance of in-person instruction and endorsed universal masking in school. Mask-optional policies or “mask-if-you-are-unvaccinated” policies don’t work, as we have seen in society at large. They are likely to be especially challenging in school settings. Given an option, many, if not most kids, will take off their masks. Kids who leave them on run the risk of stigmatization or bullying.
On Aug. 4, the Centers for Disease Control and Prevention updated its guidance to recommend universal indoor masking for all students, staff, teachers, and visitors to K-12 schools, regardless of vaccination status. Now we’ll have to wait and see if school districts, elected officials, and parents will get on board with masks. ... and we’ll be left to count the number of rising COVID-19 cases that occur until they do.
Case in point: Kids in Greater Clark County, Ind., headed back to school on July 28. Masks were not required on school property, although unvaccinated students and teachers were “strongly encouraged” to wear them.8
Over the first 8 days of in-person instruction, schools in Greater Clark County identified 70 cases of COVID-19 in students and quarantined more than 1,100 of the district’s 10,300 students. Only the unvaccinated were required to quarantine. The district began requiring masks in all school buildings on Aug. 9.9
The worried mother had one last question for me. “What’s the best mask for a child to wear?” For most kids, a simple, well-fitting cloth mask is fine. The best mask is ultimately the mask a child will wear. A toolkit with practical tips for helping children successfully wear a mask is available on the ABC Science Collaborative website.
Dr. Bryant, president of the Pediatric Infectious Diseases Society, is a pediatrician at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. American Academy of Pediatrics. “Children and COVID-19: State-level data report.”
2. American Academy of Pediatrics. “Children and COVID-19 vaccination trends.”
3. Falk A et al. MMWR Morb Mortal Wkly Rep. 2021;70:136-40.
4. Hershow RB et al. MMWR Morb Mortal Wkly Rep 2021;70:442-8.
5. Zimmerman KO et al. Pediatrics. 2021 Jul;e2021052686. doi: 10.1542/peds.2021-052686.
6. American Academy of Pediatrics. “American Academy of Pediatrics updates recommendations for opening schools in fall 2021.”
7. Pediatric Infectious Diseases Society. “PIDS supports universal masking for students, school staff.”
8. Courtney Hayden. WHAS11. “Greater Clark County Schools return to class July 28.”
9. Dustin Vogt. WAVE3 News. “Greater Clark Country Schools to require masks amid 70 positive cases.”
“I want my child to go back to school,” the mother said to me. “I just want you to tell me it will be safe.”
As the summer break winds down for children across the United States, pediatric COVID-19 cases are rising. According to the American Academy of Pediatrics, nearly 94,000 cases were reported for the week ending Aug. 5, more than double the case count from 2 weeks earlier.1
Anecdotally, some children’s hospitals are reporting an increase in pediatric COVID-19 admissions. In the hospital in which I practice, we are seeing numbers similar to those we saw in December and January: a typical daily census of 10 kids admitted with COVID-19, with 4 of them in the intensive care unit. It is a stark contrast to June when, most days, we had no patients with COVID-19 in the hospital. About half of our hospitalized patients are too young to be vaccinated against COVID-19, while the rest are unvaccinated children 12 years and older.
Vaccination of eligible children and teachers is an essential strategy for preventing the spread of COVID-19 in schools, but as children head back to school, immunization rates of educators are largely unknown and are suboptimal among students in most states. As of Aug. 11, 10.7 million U.S. children had received at least one dose of COVID-19 vaccine, representing 43% of 12- to 15-year-olds and 53% of 16- to 17-year-olds.2 Rates vary substantially by state, with more than 70% of kids in Vermont receiving at least one dose of vaccine, compared with less than 25% in Wyoming and Alabama.
Still, in the absence of robust immunization rates, we have data that schools can still reopen successfully. We need to follow the science and implement universal masking, a safe, effective, and practical mitigation strategy.
It worked in Wisconsin. Seventeen K-12 schools in rural Wisconsin opened last fall for in-person instruction.3 Reported compliance with masking was high, ranging from 92.1% to 97.4%, and in-school transmission of COVID-19 was low, with seven cases among 4,876 students.
It worked in Salt Lake City.4 In 20 elementary schools open for in-person instruction Dec. 3, 2020, to Jan. 31, 2021, compliance with mask-wearing was high and in-school transmission was very low, despite a high community incidence of COVID-19. Notably, students’ classroom seats were less than 6 feet apart, suggesting that consistent mask-wearing works even when physical distancing is challenging.
One of the best examples of successful school reopening happened in North Carolina, where pediatricians, pediatric infectious disease specialists, and other experts affiliated with Duke University formed the ABC Science Collaborative to support school districts that requested scientific input to help guide return-to-school policies during the COVID-19 pandemic. From Oct. 26, 2020, to Feb. 28, 2021, the ABC Science Collaborative worked with 13 school districts that were open for in-person instruction using basic mitigation strategies, including universal masking.5 During this time period, there were 4,969 community-acquired SARS-CoV-2 infections in the more than 100,000 students and staff present in schools. Transmission to school contacts was identified in only 209 individuals for a secondary attack rate of less than 1%.
Duke investigator Kanecia Zimmerman, MD, told Duke Today, “We know that, if our goal is to reduce transmission of COVID-19 in schools, there are two effective ways to do that: 1. vaccination, 2. masking. In the setting of schools ... the science suggests masking can be extremely effective, particularly for those who can’t get vaccinated while COVID-19 is still circulating.”
Both the AAP6 and the Pediatric Infectious Diseases Society7 have emphasized the importance of in-person instruction and endorsed universal masking in school. Mask-optional policies or “mask-if-you-are-unvaccinated” policies don’t work, as we have seen in society at large. They are likely to be especially challenging in school settings. Given an option, many, if not most kids, will take off their masks. Kids who leave them on run the risk of stigmatization or bullying.
On Aug. 4, the Centers for Disease Control and Prevention updated its guidance to recommend universal indoor masking for all students, staff, teachers, and visitors to K-12 schools, regardless of vaccination status. Now we’ll have to wait and see if school districts, elected officials, and parents will get on board with masks. ... and we’ll be left to count the number of rising COVID-19 cases that occur until they do.
Case in point: Kids in Greater Clark County, Ind., headed back to school on July 28. Masks were not required on school property, although unvaccinated students and teachers were “strongly encouraged” to wear them.8
Over the first 8 days of in-person instruction, schools in Greater Clark County identified 70 cases of COVID-19 in students and quarantined more than 1,100 of the district’s 10,300 students. Only the unvaccinated were required to quarantine. The district began requiring masks in all school buildings on Aug. 9.9
The worried mother had one last question for me. “What’s the best mask for a child to wear?” For most kids, a simple, well-fitting cloth mask is fine. The best mask is ultimately the mask a child will wear. A toolkit with practical tips for helping children successfully wear a mask is available on the ABC Science Collaborative website.
Dr. Bryant, president of the Pediatric Infectious Diseases Society, is a pediatrician at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. American Academy of Pediatrics. “Children and COVID-19: State-level data report.”
2. American Academy of Pediatrics. “Children and COVID-19 vaccination trends.”
3. Falk A et al. MMWR Morb Mortal Wkly Rep. 2021;70:136-40.
4. Hershow RB et al. MMWR Morb Mortal Wkly Rep 2021;70:442-8.
5. Zimmerman KO et al. Pediatrics. 2021 Jul;e2021052686. doi: 10.1542/peds.2021-052686.
6. American Academy of Pediatrics. “American Academy of Pediatrics updates recommendations for opening schools in fall 2021.”
7. Pediatric Infectious Diseases Society. “PIDS supports universal masking for students, school staff.”
8. Courtney Hayden. WHAS11. “Greater Clark County Schools return to class July 28.”
9. Dustin Vogt. WAVE3 News. “Greater Clark Country Schools to require masks amid 70 positive cases.”
“I want my child to go back to school,” the mother said to me. “I just want you to tell me it will be safe.”
As the summer break winds down for children across the United States, pediatric COVID-19 cases are rising. According to the American Academy of Pediatrics, nearly 94,000 cases were reported for the week ending Aug. 5, more than double the case count from 2 weeks earlier.1
Anecdotally, some children’s hospitals are reporting an increase in pediatric COVID-19 admissions. In the hospital in which I practice, we are seeing numbers similar to those we saw in December and January: a typical daily census of 10 kids admitted with COVID-19, with 4 of them in the intensive care unit. It is a stark contrast to June when, most days, we had no patients with COVID-19 in the hospital. About half of our hospitalized patients are too young to be vaccinated against COVID-19, while the rest are unvaccinated children 12 years and older.
Vaccination of eligible children and teachers is an essential strategy for preventing the spread of COVID-19 in schools, but as children head back to school, immunization rates of educators are largely unknown and are suboptimal among students in most states. As of Aug. 11, 10.7 million U.S. children had received at least one dose of COVID-19 vaccine, representing 43% of 12- to 15-year-olds and 53% of 16- to 17-year-olds.2 Rates vary substantially by state, with more than 70% of kids in Vermont receiving at least one dose of vaccine, compared with less than 25% in Wyoming and Alabama.
Still, in the absence of robust immunization rates, we have data that schools can still reopen successfully. We need to follow the science and implement universal masking, a safe, effective, and practical mitigation strategy.
It worked in Wisconsin. Seventeen K-12 schools in rural Wisconsin opened last fall for in-person instruction.3 Reported compliance with masking was high, ranging from 92.1% to 97.4%, and in-school transmission of COVID-19 was low, with seven cases among 4,876 students.
It worked in Salt Lake City.4 In 20 elementary schools open for in-person instruction Dec. 3, 2020, to Jan. 31, 2021, compliance with mask-wearing was high and in-school transmission was very low, despite a high community incidence of COVID-19. Notably, students’ classroom seats were less than 6 feet apart, suggesting that consistent mask-wearing works even when physical distancing is challenging.
One of the best examples of successful school reopening happened in North Carolina, where pediatricians, pediatric infectious disease specialists, and other experts affiliated with Duke University formed the ABC Science Collaborative to support school districts that requested scientific input to help guide return-to-school policies during the COVID-19 pandemic. From Oct. 26, 2020, to Feb. 28, 2021, the ABC Science Collaborative worked with 13 school districts that were open for in-person instruction using basic mitigation strategies, including universal masking.5 During this time period, there were 4,969 community-acquired SARS-CoV-2 infections in the more than 100,000 students and staff present in schools. Transmission to school contacts was identified in only 209 individuals for a secondary attack rate of less than 1%.
Duke investigator Kanecia Zimmerman, MD, told Duke Today, “We know that, if our goal is to reduce transmission of COVID-19 in schools, there are two effective ways to do that: 1. vaccination, 2. masking. In the setting of schools ... the science suggests masking can be extremely effective, particularly for those who can’t get vaccinated while COVID-19 is still circulating.”
Both the AAP6 and the Pediatric Infectious Diseases Society7 have emphasized the importance of in-person instruction and endorsed universal masking in school. Mask-optional policies or “mask-if-you-are-unvaccinated” policies don’t work, as we have seen in society at large. They are likely to be especially challenging in school settings. Given an option, many, if not most kids, will take off their masks. Kids who leave them on run the risk of stigmatization or bullying.
On Aug. 4, the Centers for Disease Control and Prevention updated its guidance to recommend universal indoor masking for all students, staff, teachers, and visitors to K-12 schools, regardless of vaccination status. Now we’ll have to wait and see if school districts, elected officials, and parents will get on board with masks. ... and we’ll be left to count the number of rising COVID-19 cases that occur until they do.
Case in point: Kids in Greater Clark County, Ind., headed back to school on July 28. Masks were not required on school property, although unvaccinated students and teachers were “strongly encouraged” to wear them.8
Over the first 8 days of in-person instruction, schools in Greater Clark County identified 70 cases of COVID-19 in students and quarantined more than 1,100 of the district’s 10,300 students. Only the unvaccinated were required to quarantine. The district began requiring masks in all school buildings on Aug. 9.9
The worried mother had one last question for me. “What’s the best mask for a child to wear?” For most kids, a simple, well-fitting cloth mask is fine. The best mask is ultimately the mask a child will wear. A toolkit with practical tips for helping children successfully wear a mask is available on the ABC Science Collaborative website.
Dr. Bryant, president of the Pediatric Infectious Diseases Society, is a pediatrician at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at [email protected].
References
1. American Academy of Pediatrics. “Children and COVID-19: State-level data report.”
2. American Academy of Pediatrics. “Children and COVID-19 vaccination trends.”
3. Falk A et al. MMWR Morb Mortal Wkly Rep. 2021;70:136-40.
4. Hershow RB et al. MMWR Morb Mortal Wkly Rep 2021;70:442-8.
5. Zimmerman KO et al. Pediatrics. 2021 Jul;e2021052686. doi: 10.1542/peds.2021-052686.
6. American Academy of Pediatrics. “American Academy of Pediatrics updates recommendations for opening schools in fall 2021.”
7. Pediatric Infectious Diseases Society. “PIDS supports universal masking for students, school staff.”
8. Courtney Hayden. WHAS11. “Greater Clark County Schools return to class July 28.”
9. Dustin Vogt. WAVE3 News. “Greater Clark Country Schools to require masks amid 70 positive cases.”