User login
Children and COVID: Weekly cases top 200,000, vaccinations down
Weekly pediatric cases of COVID-19 exceeded 200,000 for just the second time during the pandemic, while new vaccinations in children continued to decline.
The weekly count has now increased for 9 consecutive weeks, during which time it has risen by over 2,300%, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report. Total cases in children number almost 4.8 million since the pandemic started.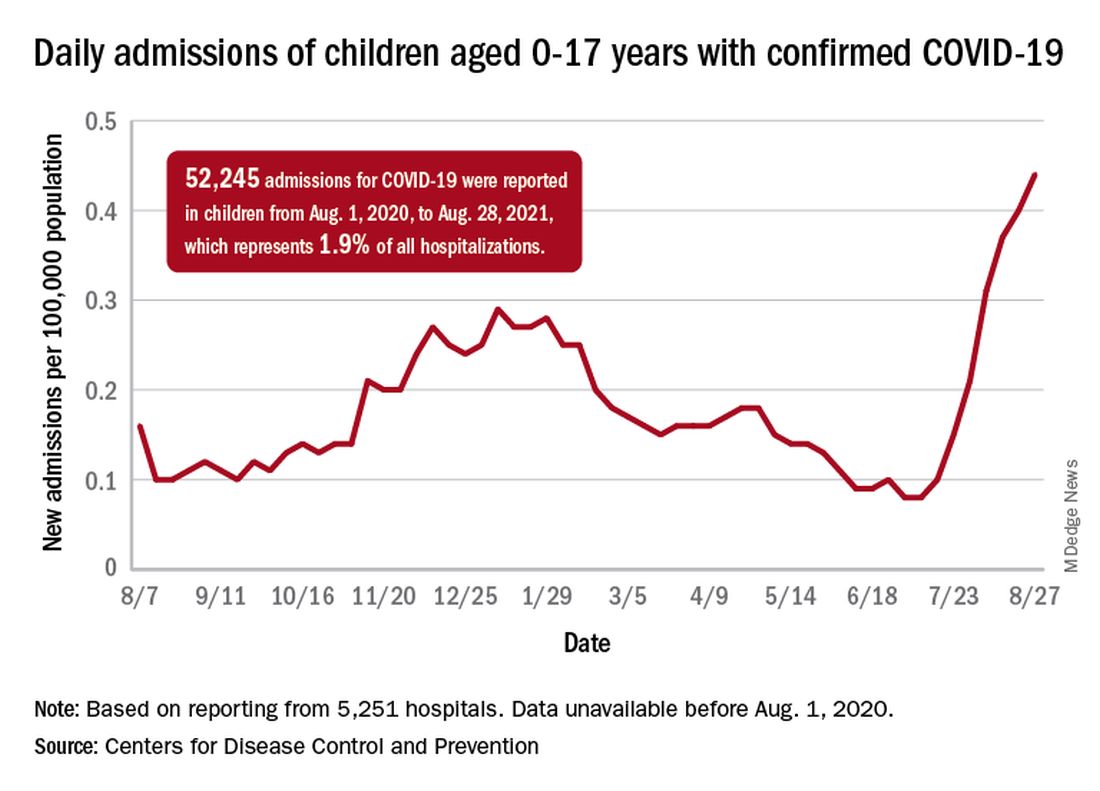
Vaccinations in children are following a different trend. Vaccine initiation has dropped 3 weeks in a row for both of the eligible age groups: First doses administered were down by 29% among 12- to 15-year-olds over that span and by 32% in 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
Since vaccination for children aged 12-15 years started in May, 49% had received at least one dose, and just over 36% were fully vaccinated as of Aug. 30. Among children aged 16-17 years, who have been eligible since December, 57.5% had gotten at least one dose of the vaccine and 46% have completed the two-dose regimen. The total number of children with at least one dose, including those under age 12 who are involved in clinical trials, was about 12 million, the CDC said on its COVID Data Tracker.
Hospitalizations are higher than ever
The recent rise in new child cases has been accompanied by an unprecedented increase in hospitalizations. The daily rate in children aged 0-17 years, which did not surpass 0.30 new admissions per 100,000 population during the worst of the winter surge, had risen to 0.45 per 100,000 by Aug. 26. Since July 4, when the new-admission rate was at its low point of 0.07 per 100,000, hospitalizations in children have jumped by 543%, based on data reported to the CDC by 5,251 hospitals.
A total of 52,245 children were admitted with confirmed COVID-19 from Aug. 1, 2020, when the CDC dataset begins, to Aug. 28, 2021. Those children represent 1.9% of all COVID admissions (2.7 million) in the United States over that period, the CDC said.
Total COVID-related deaths in children are up to 425 in the 48 jurisdictions (45 states, New York City, Puerto Rico, and Guam) that provide mortality data by age, the AAP and the CHA said.
Record-high numbers for the previous 2 reporting weeks – 23 deaths during Aug. 20-26 and 24 deaths during Aug. 13-19, when the previous weekly high was 16 – at least partially reflect the recent addition of South Carolina and New Mexico to the AAP/CHA database, as the two states just started reporting age-related data.
Weekly pediatric cases of COVID-19 exceeded 200,000 for just the second time during the pandemic, while new vaccinations in children continued to decline.
The weekly count has now increased for 9 consecutive weeks, during which time it has risen by over 2,300%, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report. Total cases in children number almost 4.8 million since the pandemic started.
Vaccinations in children are following a different trend. Vaccine initiation has dropped 3 weeks in a row for both of the eligible age groups: First doses administered were down by 29% among 12- to 15-year-olds over that span and by 32% in 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
Since vaccination for children aged 12-15 years started in May, 49% had received at least one dose, and just over 36% were fully vaccinated as of Aug. 30. Among children aged 16-17 years, who have been eligible since December, 57.5% had gotten at least one dose of the vaccine and 46% have completed the two-dose regimen. The total number of children with at least one dose, including those under age 12 who are involved in clinical trials, was about 12 million, the CDC said on its COVID Data Tracker.
Hospitalizations are higher than ever
The recent rise in new child cases has been accompanied by an unprecedented increase in hospitalizations. The daily rate in children aged 0-17 years, which did not surpass 0.30 new admissions per 100,000 population during the worst of the winter surge, had risen to 0.45 per 100,000 by Aug. 26. Since July 4, when the new-admission rate was at its low point of 0.07 per 100,000, hospitalizations in children have jumped by 543%, based on data reported to the CDC by 5,251 hospitals.
A total of 52,245 children were admitted with confirmed COVID-19 from Aug. 1, 2020, when the CDC dataset begins, to Aug. 28, 2021. Those children represent 1.9% of all COVID admissions (2.7 million) in the United States over that period, the CDC said.
Total COVID-related deaths in children are up to 425 in the 48 jurisdictions (45 states, New York City, Puerto Rico, and Guam) that provide mortality data by age, the AAP and the CHA said.
Record-high numbers for the previous 2 reporting weeks – 23 deaths during Aug. 20-26 and 24 deaths during Aug. 13-19, when the previous weekly high was 16 – at least partially reflect the recent addition of South Carolina and New Mexico to the AAP/CHA database, as the two states just started reporting age-related data.
Weekly pediatric cases of COVID-19 exceeded 200,000 for just the second time during the pandemic, while new vaccinations in children continued to decline.
The weekly count has now increased for 9 consecutive weeks, during which time it has risen by over 2,300%, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report. Total cases in children number almost 4.8 million since the pandemic started.
Vaccinations in children are following a different trend. Vaccine initiation has dropped 3 weeks in a row for both of the eligible age groups: First doses administered were down by 29% among 12- to 15-year-olds over that span and by 32% in 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
Since vaccination for children aged 12-15 years started in May, 49% had received at least one dose, and just over 36% were fully vaccinated as of Aug. 30. Among children aged 16-17 years, who have been eligible since December, 57.5% had gotten at least one dose of the vaccine and 46% have completed the two-dose regimen. The total number of children with at least one dose, including those under age 12 who are involved in clinical trials, was about 12 million, the CDC said on its COVID Data Tracker.
Hospitalizations are higher than ever
The recent rise in new child cases has been accompanied by an unprecedented increase in hospitalizations. The daily rate in children aged 0-17 years, which did not surpass 0.30 new admissions per 100,000 population during the worst of the winter surge, had risen to 0.45 per 100,000 by Aug. 26. Since July 4, when the new-admission rate was at its low point of 0.07 per 100,000, hospitalizations in children have jumped by 543%, based on data reported to the CDC by 5,251 hospitals.
A total of 52,245 children were admitted with confirmed COVID-19 from Aug. 1, 2020, when the CDC dataset begins, to Aug. 28, 2021. Those children represent 1.9% of all COVID admissions (2.7 million) in the United States over that period, the CDC said.
Total COVID-related deaths in children are up to 425 in the 48 jurisdictions (45 states, New York City, Puerto Rico, and Guam) that provide mortality data by age, the AAP and the CHA said.
Record-high numbers for the previous 2 reporting weeks – 23 deaths during Aug. 20-26 and 24 deaths during Aug. 13-19, when the previous weekly high was 16 – at least partially reflect the recent addition of South Carolina and New Mexico to the AAP/CHA database, as the two states just started reporting age-related data.
ICU infections and all-cause hospital mortality rate
Background: Many articles have been published on sepsis and mortality in ICUs, but there are not many analyzing outcomes in patients with infections, nor types of infections. More information on the infection rate, types of infection, and possible impact on mortality should heighten awareness of infection effects, as well as guide resource allocation and help direct policy development for diagnosis and treatment.
Study design: 24-hour point-prevalence study with longitudinal follow-up.
Setting: ICUs in 1,150 centers in 88 countries.
Synopsis: The study included 15,202 patients who were aged 18 or older (mean, 61.6) within a 24-hour time period on Sept. 13, 2017, who were admitted to the ICU in participating centers and had documented, confirmed, or suspected infection. The investigators looked at prevalence of infection and antibiotic exposure on the study day and the main outcome measure was all cause in-hospital mortality, which was compiled 60 days later. The prevalence of suspected or proven infection in ICUs was 54% (8,135) and that of ICU-acquired infection was 22%. Of confirmed or suspected infection, 65% (5,259) had at least one positive microbiology culture. Of those cultures, 67% were gram-negative and 37% gram-positive bacteria, and 16% were fungal. 70% of ICU patients received at least one antibiotic. The in-hospital mortality rate with proven or suspected infection was 30% (2,404 of 7,936). Multilevel analysis disclosed two independent risk factors for mortality, which were ICU-acquired infections and antibiotic-resistant organisms, specifically, vancomycin-resistant Enterococcus, Klebsiella resistant to beta-lactam antibiotics, and carbapenem-resistant Acinetobacter.
Despite limitations related to being an observational study, 24-hour point evaluation, a centrally controlled database, and different geographic locations, this study elucidated the world-wide prevalence of ICU infection and high hospital-in mortality in those patients.
Bottom line: There is a high prevalence of infection in ICUs: 43%-60% depending on location. This is associated with 30% in-hospital mortality.
Citation: Vincent J-L et al. Prevalance and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020 Mar 24;323(15):1478-87.
Dr. Rogozinska is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Many articles have been published on sepsis and mortality in ICUs, but there are not many analyzing outcomes in patients with infections, nor types of infections. More information on the infection rate, types of infection, and possible impact on mortality should heighten awareness of infection effects, as well as guide resource allocation and help direct policy development for diagnosis and treatment.
Study design: 24-hour point-prevalence study with longitudinal follow-up.
Setting: ICUs in 1,150 centers in 88 countries.
Synopsis: The study included 15,202 patients who were aged 18 or older (mean, 61.6) within a 24-hour time period on Sept. 13, 2017, who were admitted to the ICU in participating centers and had documented, confirmed, or suspected infection. The investigators looked at prevalence of infection and antibiotic exposure on the study day and the main outcome measure was all cause in-hospital mortality, which was compiled 60 days later. The prevalence of suspected or proven infection in ICUs was 54% (8,135) and that of ICU-acquired infection was 22%. Of confirmed or suspected infection, 65% (5,259) had at least one positive microbiology culture. Of those cultures, 67% were gram-negative and 37% gram-positive bacteria, and 16% were fungal. 70% of ICU patients received at least one antibiotic. The in-hospital mortality rate with proven or suspected infection was 30% (2,404 of 7,936). Multilevel analysis disclosed two independent risk factors for mortality, which were ICU-acquired infections and antibiotic-resistant organisms, specifically, vancomycin-resistant Enterococcus, Klebsiella resistant to beta-lactam antibiotics, and carbapenem-resistant Acinetobacter.
Despite limitations related to being an observational study, 24-hour point evaluation, a centrally controlled database, and different geographic locations, this study elucidated the world-wide prevalence of ICU infection and high hospital-in mortality in those patients.
Bottom line: There is a high prevalence of infection in ICUs: 43%-60% depending on location. This is associated with 30% in-hospital mortality.
Citation: Vincent J-L et al. Prevalance and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020 Mar 24;323(15):1478-87.
Dr. Rogozinska is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Many articles have been published on sepsis and mortality in ICUs, but there are not many analyzing outcomes in patients with infections, nor types of infections. More information on the infection rate, types of infection, and possible impact on mortality should heighten awareness of infection effects, as well as guide resource allocation and help direct policy development for diagnosis and treatment.
Study design: 24-hour point-prevalence study with longitudinal follow-up.
Setting: ICUs in 1,150 centers in 88 countries.
Synopsis: The study included 15,202 patients who were aged 18 or older (mean, 61.6) within a 24-hour time period on Sept. 13, 2017, who were admitted to the ICU in participating centers and had documented, confirmed, or suspected infection. The investigators looked at prevalence of infection and antibiotic exposure on the study day and the main outcome measure was all cause in-hospital mortality, which was compiled 60 days later. The prevalence of suspected or proven infection in ICUs was 54% (8,135) and that of ICU-acquired infection was 22%. Of confirmed or suspected infection, 65% (5,259) had at least one positive microbiology culture. Of those cultures, 67% were gram-negative and 37% gram-positive bacteria, and 16% were fungal. 70% of ICU patients received at least one antibiotic. The in-hospital mortality rate with proven or suspected infection was 30% (2,404 of 7,936). Multilevel analysis disclosed two independent risk factors for mortality, which were ICU-acquired infections and antibiotic-resistant organisms, specifically, vancomycin-resistant Enterococcus, Klebsiella resistant to beta-lactam antibiotics, and carbapenem-resistant Acinetobacter.
Despite limitations related to being an observational study, 24-hour point evaluation, a centrally controlled database, and different geographic locations, this study elucidated the world-wide prevalence of ICU infection and high hospital-in mortality in those patients.
Bottom line: There is a high prevalence of infection in ICUs: 43%-60% depending on location. This is associated with 30% in-hospital mortality.
Citation: Vincent J-L et al. Prevalance and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020 Mar 24;323(15):1478-87.
Dr. Rogozinska is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Study evaluates OTC treatments for molluscum contagiosum
“It’s important for clinicians who see children with molluscum to be aware of the many products marketed to patients and to be able to provide objective information about them,” senior author Elaine Siegfried, MD, said in an interview following the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session.
In the text of their abstract, Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and coauthors Isaac Hoft, of Open Mind Holistics in Ft. Collins, Colo., and Samantha K. Ong, BA, a student at SLU, noted that MC primarily infects children, with an annual incidence of 8%. “Although the disease is self-limited, associated symptoms, contagion and an average 1-year duration prompt concern and frequent medical visits,” they wrote.
The optimal treatment for MC has not been defined and there is currently no approved medication approved for the condition, although three products are in development: VP-102 (cantharidin) by Verrica Pharmaceuticals; SB206, a topical antiviral by Novan; and 10%-15% KOH formulation by the Gurina Foundation.
But many OTC products have been marketed to treat the condition. To identify the OTC products and to assess accompanying information related to safety, efficacy, and cost, the researchers performed an internet search using the terms “molluscum” plus “treatment,” “treatment at home,” “relief,” and “medication.” Eight products were identified for analysis: Conzerol (Elroselabs), Molleave (Innovative Med), Mollenol (Jeva Laboratories), MolluscumBLAST (Revitalize Life Organics), Molluscum Away Patches (Molluscum Away), Naturasil (Nature’s Innovation), Terrasil (Advanced Skincare % Topical Solutions), and Zymaderm (Naturopathix). Package sizes ranged from 0.78 to 1.5 ounces, and prices ranged from about $19 to almost $55.
Dr. Siegfried and colleagues found that all products provided instructions on application and use but most package labels did not include sufficient information about their plant-based ingredients or appropriate dosing. Six of the eight products contained Thuja occidentalis (Arbor vitae), a coniferous cedar whose essential oil has been used in homeopathic products for its anti-inflammatory and antiviral properties. Lemon extract, tea tree oil, and other botanicals were present in no more than three products each. Only two of the products provided information about the number of lesions that could be treated per package.
“The lack of national oversight as well as robust methods for high-level data analysis make safety and efficacy unclear for a Thuja extract marketed to treat MC,” the researchers wrote. “Numerous adverse drug events and positive intradermal skin tests related to Thuja have been reported.”
Dr. Siegfried added that many OTC products offer a money-back guarantee, “so when seeing a patient who failed to respond to one of these products, encourage them, at least, to request a refund, but to also submit a comment about lack of efficacy, in order to provide more balanced Internet information.”
Dr. Siegfried disclosed that she has served as an investigator and consultant for Verrica Pharmaceuticals, and as a consultant and Data Safety Monitoring board member for Novan, two of the companies currently developing drugs to treat molluscum. Her coauthors had no conflicts of interest to disclose.
“It’s important for clinicians who see children with molluscum to be aware of the many products marketed to patients and to be able to provide objective information about them,” senior author Elaine Siegfried, MD, said in an interview following the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session.
In the text of their abstract, Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and coauthors Isaac Hoft, of Open Mind Holistics in Ft. Collins, Colo., and Samantha K. Ong, BA, a student at SLU, noted that MC primarily infects children, with an annual incidence of 8%. “Although the disease is self-limited, associated symptoms, contagion and an average 1-year duration prompt concern and frequent medical visits,” they wrote.
The optimal treatment for MC has not been defined and there is currently no approved medication approved for the condition, although three products are in development: VP-102 (cantharidin) by Verrica Pharmaceuticals; SB206, a topical antiviral by Novan; and 10%-15% KOH formulation by the Gurina Foundation.
But many OTC products have been marketed to treat the condition. To identify the OTC products and to assess accompanying information related to safety, efficacy, and cost, the researchers performed an internet search using the terms “molluscum” plus “treatment,” “treatment at home,” “relief,” and “medication.” Eight products were identified for analysis: Conzerol (Elroselabs), Molleave (Innovative Med), Mollenol (Jeva Laboratories), MolluscumBLAST (Revitalize Life Organics), Molluscum Away Patches (Molluscum Away), Naturasil (Nature’s Innovation), Terrasil (Advanced Skincare % Topical Solutions), and Zymaderm (Naturopathix). Package sizes ranged from 0.78 to 1.5 ounces, and prices ranged from about $19 to almost $55.
Dr. Siegfried and colleagues found that all products provided instructions on application and use but most package labels did not include sufficient information about their plant-based ingredients or appropriate dosing. Six of the eight products contained Thuja occidentalis (Arbor vitae), a coniferous cedar whose essential oil has been used in homeopathic products for its anti-inflammatory and antiviral properties. Lemon extract, tea tree oil, and other botanicals were present in no more than three products each. Only two of the products provided information about the number of lesions that could be treated per package.
“The lack of national oversight as well as robust methods for high-level data analysis make safety and efficacy unclear for a Thuja extract marketed to treat MC,” the researchers wrote. “Numerous adverse drug events and positive intradermal skin tests related to Thuja have been reported.”
Dr. Siegfried added that many OTC products offer a money-back guarantee, “so when seeing a patient who failed to respond to one of these products, encourage them, at least, to request a refund, but to also submit a comment about lack of efficacy, in order to provide more balanced Internet information.”
Dr. Siegfried disclosed that she has served as an investigator and consultant for Verrica Pharmaceuticals, and as a consultant and Data Safety Monitoring board member for Novan, two of the companies currently developing drugs to treat molluscum. Her coauthors had no conflicts of interest to disclose.
“It’s important for clinicians who see children with molluscum to be aware of the many products marketed to patients and to be able to provide objective information about them,” senior author Elaine Siegfried, MD, said in an interview following the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session.
In the text of their abstract, Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and coauthors Isaac Hoft, of Open Mind Holistics in Ft. Collins, Colo., and Samantha K. Ong, BA, a student at SLU, noted that MC primarily infects children, with an annual incidence of 8%. “Although the disease is self-limited, associated symptoms, contagion and an average 1-year duration prompt concern and frequent medical visits,” they wrote.
The optimal treatment for MC has not been defined and there is currently no approved medication approved for the condition, although three products are in development: VP-102 (cantharidin) by Verrica Pharmaceuticals; SB206, a topical antiviral by Novan; and 10%-15% KOH formulation by the Gurina Foundation.
But many OTC products have been marketed to treat the condition. To identify the OTC products and to assess accompanying information related to safety, efficacy, and cost, the researchers performed an internet search using the terms “molluscum” plus “treatment,” “treatment at home,” “relief,” and “medication.” Eight products were identified for analysis: Conzerol (Elroselabs), Molleave (Innovative Med), Mollenol (Jeva Laboratories), MolluscumBLAST (Revitalize Life Organics), Molluscum Away Patches (Molluscum Away), Naturasil (Nature’s Innovation), Terrasil (Advanced Skincare % Topical Solutions), and Zymaderm (Naturopathix). Package sizes ranged from 0.78 to 1.5 ounces, and prices ranged from about $19 to almost $55.
Dr. Siegfried and colleagues found that all products provided instructions on application and use but most package labels did not include sufficient information about their plant-based ingredients or appropriate dosing. Six of the eight products contained Thuja occidentalis (Arbor vitae), a coniferous cedar whose essential oil has been used in homeopathic products for its anti-inflammatory and antiviral properties. Lemon extract, tea tree oil, and other botanicals were present in no more than three products each. Only two of the products provided information about the number of lesions that could be treated per package.
“The lack of national oversight as well as robust methods for high-level data analysis make safety and efficacy unclear for a Thuja extract marketed to treat MC,” the researchers wrote. “Numerous adverse drug events and positive intradermal skin tests related to Thuja have been reported.”
Dr. Siegfried added that many OTC products offer a money-back guarantee, “so when seeing a patient who failed to respond to one of these products, encourage them, at least, to request a refund, but to also submit a comment about lack of efficacy, in order to provide more balanced Internet information.”
Dr. Siegfried disclosed that she has served as an investigator and consultant for Verrica Pharmaceuticals, and as a consultant and Data Safety Monitoring board member for Novan, two of the companies currently developing drugs to treat molluscum. Her coauthors had no conflicts of interest to disclose.
FROM SPD 2021
TB prevention in people with HIV: How short can we go?
A 3-month, 12-dose regimen of rifapentine and isoniazid (INH) was less toxic, had better compliance, and showed similar efficacy as 6 months of INH alone in preventing tuberculosis (TB) in people with HIV, according to the results of a clinical trial reported in Annals of Internal Medicine.
The study, a randomized pragmatic trial in South Africa, Ethiopia, and Mozambique, was called WHIP3TB (Weekly High Dose Isoniazid and Rifapentine [P] Periodic Prophylaxis for TB).
Investigators randomized patients to three groups, comparing a 3-month course of weekly rifapentine-INH, given either once or repeated in a year, with daily isoniazid for 6 months. At 1 year, 90% of the rifapentine-INH group (3HP) were still on therapy, compared with only 50.5% in the INH group.
In the study, patients were initially assessed for TB using the World Health Organization four-symptom screen, but the sensitivity in HIV patients on antiretrovirals (ARVs) was only 53%. In addition to symptoms, screening at 12 months included a chest x-ray and sputum culture.
Of the 30 patients at month 12 with confirmed TB, 26 were asymptomatic, suggesting physicians should do further evaluation prior to initiating preventive TB treatment (which was not part of the WHO recommendation when the study was initiated).
Another unexpected finding was that 10.2% of the TB cases detected in the combined 3HP groups in South Africa, along with 18% of the cases in Mozambique, had rifampin resistance.
Investigator Gavin Churchyard, MBBCh, PhD, CEO of the Aurum Institute in Johannesburg, South Africa, said in an interview: “It appeared that taking this potent short course regimen – they’re just taking a single course – provided the same level of protection as taking repeat courses of the antibiotics. So that’s good news.” He noted, too, that TB transmission rates have been declining in sub-Saharan Africa because of ARV, and “so it may just be that a single course is now adequate because the risk of exposure and reinfection” is decreasing.
But Madhu Pai, MD, PhD, associate director, McGill International TB Centre, Montreal, who was not involved in the study, shared a more cautious interpretation. He said in an interview that the 2020 WHO Consolidated Guidelines on Tuberculosis state: “In settings with high TB transmission, adults and adolescents living with HIV ... should receive at least 36 months of daily isoniazid preventive therapy (IPT) ... whether or not the person is on ART.” The problem is that almost no one can tolerate prolonged therapy with INH because of side effects, as has been shown in numerous studies.
For successful TB treatment, Dr. Pai said, “Even 3HP is not going to cut it; they’re going to get reinfected again. So that shortening of that 36 months is what this trial is really all about, in terms of new information ... and they were not successful.” But because this is still the most practical course, Dr. Pai suggests that follow-up monitoring for reinfection will be the most likely path forward.
Dr. Churchyard concluded: “If we wanted to end the global TB epidemic, we need to continue to find ways to further reduce the risk of TB overall at a population level, and then amongst high-risk groups such as people with HIV, including those on ARVs, and who have had a course of preventive therapy. ... We need to look for other strategies to further reduce that risk. Part of those strategies may be doing a more intensive screen. But also, it may be adding another intervention, particularly TB vaccines. ... No single intervention by itself will adequately address the risk of TB in people with HIV in these high TB transmission settings.”
Dr. Pai reported no relevant financial relationships. Dr. Churchyard has reported participation in a Sanofi advisory committee on the prevention of TB. Judy Stone, MD, is an infectious disease specialist and author of “Resilience: One Family’s Story of Hope and Triumph Over Evil” and of “Conducting Clinical Research.”
A version of this article first appeared on Medscape.com.
A 3-month, 12-dose regimen of rifapentine and isoniazid (INH) was less toxic, had better compliance, and showed similar efficacy as 6 months of INH alone in preventing tuberculosis (TB) in people with HIV, according to the results of a clinical trial reported in Annals of Internal Medicine.
The study, a randomized pragmatic trial in South Africa, Ethiopia, and Mozambique, was called WHIP3TB (Weekly High Dose Isoniazid and Rifapentine [P] Periodic Prophylaxis for TB).
Investigators randomized patients to three groups, comparing a 3-month course of weekly rifapentine-INH, given either once or repeated in a year, with daily isoniazid for 6 months. At 1 year, 90% of the rifapentine-INH group (3HP) were still on therapy, compared with only 50.5% in the INH group.
In the study, patients were initially assessed for TB using the World Health Organization four-symptom screen, but the sensitivity in HIV patients on antiretrovirals (ARVs) was only 53%. In addition to symptoms, screening at 12 months included a chest x-ray and sputum culture.
Of the 30 patients at month 12 with confirmed TB, 26 were asymptomatic, suggesting physicians should do further evaluation prior to initiating preventive TB treatment (which was not part of the WHO recommendation when the study was initiated).
Another unexpected finding was that 10.2% of the TB cases detected in the combined 3HP groups in South Africa, along with 18% of the cases in Mozambique, had rifampin resistance.
Investigator Gavin Churchyard, MBBCh, PhD, CEO of the Aurum Institute in Johannesburg, South Africa, said in an interview: “It appeared that taking this potent short course regimen – they’re just taking a single course – provided the same level of protection as taking repeat courses of the antibiotics. So that’s good news.” He noted, too, that TB transmission rates have been declining in sub-Saharan Africa because of ARV, and “so it may just be that a single course is now adequate because the risk of exposure and reinfection” is decreasing.
But Madhu Pai, MD, PhD, associate director, McGill International TB Centre, Montreal, who was not involved in the study, shared a more cautious interpretation. He said in an interview that the 2020 WHO Consolidated Guidelines on Tuberculosis state: “In settings with high TB transmission, adults and adolescents living with HIV ... should receive at least 36 months of daily isoniazid preventive therapy (IPT) ... whether or not the person is on ART.” The problem is that almost no one can tolerate prolonged therapy with INH because of side effects, as has been shown in numerous studies.
For successful TB treatment, Dr. Pai said, “Even 3HP is not going to cut it; they’re going to get reinfected again. So that shortening of that 36 months is what this trial is really all about, in terms of new information ... and they were not successful.” But because this is still the most practical course, Dr. Pai suggests that follow-up monitoring for reinfection will be the most likely path forward.
Dr. Churchyard concluded: “If we wanted to end the global TB epidemic, we need to continue to find ways to further reduce the risk of TB overall at a population level, and then amongst high-risk groups such as people with HIV, including those on ARVs, and who have had a course of preventive therapy. ... We need to look for other strategies to further reduce that risk. Part of those strategies may be doing a more intensive screen. But also, it may be adding another intervention, particularly TB vaccines. ... No single intervention by itself will adequately address the risk of TB in people with HIV in these high TB transmission settings.”
Dr. Pai reported no relevant financial relationships. Dr. Churchyard has reported participation in a Sanofi advisory committee on the prevention of TB. Judy Stone, MD, is an infectious disease specialist and author of “Resilience: One Family’s Story of Hope and Triumph Over Evil” and of “Conducting Clinical Research.”
A version of this article first appeared on Medscape.com.
A 3-month, 12-dose regimen of rifapentine and isoniazid (INH) was less toxic, had better compliance, and showed similar efficacy as 6 months of INH alone in preventing tuberculosis (TB) in people with HIV, according to the results of a clinical trial reported in Annals of Internal Medicine.
The study, a randomized pragmatic trial in South Africa, Ethiopia, and Mozambique, was called WHIP3TB (Weekly High Dose Isoniazid and Rifapentine [P] Periodic Prophylaxis for TB).
Investigators randomized patients to three groups, comparing a 3-month course of weekly rifapentine-INH, given either once or repeated in a year, with daily isoniazid for 6 months. At 1 year, 90% of the rifapentine-INH group (3HP) were still on therapy, compared with only 50.5% in the INH group.
In the study, patients were initially assessed for TB using the World Health Organization four-symptom screen, but the sensitivity in HIV patients on antiretrovirals (ARVs) was only 53%. In addition to symptoms, screening at 12 months included a chest x-ray and sputum culture.
Of the 30 patients at month 12 with confirmed TB, 26 were asymptomatic, suggesting physicians should do further evaluation prior to initiating preventive TB treatment (which was not part of the WHO recommendation when the study was initiated).
Another unexpected finding was that 10.2% of the TB cases detected in the combined 3HP groups in South Africa, along with 18% of the cases in Mozambique, had rifampin resistance.
Investigator Gavin Churchyard, MBBCh, PhD, CEO of the Aurum Institute in Johannesburg, South Africa, said in an interview: “It appeared that taking this potent short course regimen – they’re just taking a single course – provided the same level of protection as taking repeat courses of the antibiotics. So that’s good news.” He noted, too, that TB transmission rates have been declining in sub-Saharan Africa because of ARV, and “so it may just be that a single course is now adequate because the risk of exposure and reinfection” is decreasing.
But Madhu Pai, MD, PhD, associate director, McGill International TB Centre, Montreal, who was not involved in the study, shared a more cautious interpretation. He said in an interview that the 2020 WHO Consolidated Guidelines on Tuberculosis state: “In settings with high TB transmission, adults and adolescents living with HIV ... should receive at least 36 months of daily isoniazid preventive therapy (IPT) ... whether or not the person is on ART.” The problem is that almost no one can tolerate prolonged therapy with INH because of side effects, as has been shown in numerous studies.
For successful TB treatment, Dr. Pai said, “Even 3HP is not going to cut it; they’re going to get reinfected again. So that shortening of that 36 months is what this trial is really all about, in terms of new information ... and they were not successful.” But because this is still the most practical course, Dr. Pai suggests that follow-up monitoring for reinfection will be the most likely path forward.
Dr. Churchyard concluded: “If we wanted to end the global TB epidemic, we need to continue to find ways to further reduce the risk of TB overall at a population level, and then amongst high-risk groups such as people with HIV, including those on ARVs, and who have had a course of preventive therapy. ... We need to look for other strategies to further reduce that risk. Part of those strategies may be doing a more intensive screen. But also, it may be adding another intervention, particularly TB vaccines. ... No single intervention by itself will adequately address the risk of TB in people with HIV in these high TB transmission settings.”
Dr. Pai reported no relevant financial relationships. Dr. Churchyard has reported participation in a Sanofi advisory committee on the prevention of TB. Judy Stone, MD, is an infectious disease specialist and author of “Resilience: One Family’s Story of Hope and Triumph Over Evil” and of “Conducting Clinical Research.”
A version of this article first appeared on Medscape.com.
Genetic link may tie cannabis use disorder to severe COVID-19
The same genetic variations may boost susceptibility to both severe COVID-19 and cannabis use disorder (CUD), a new study suggests. The research does not confirm a genetic link, but the lead author said the signs of an association are still “troubling.”
“Reducing cannabis use among heavy users may potentially provide protection against severe COVID-19 presentations,” Alexander S. Hatoum, PhD, a postdoctoral scholar at Washington University, St. Louis, said in an interview. “Outside of individual risk, these data also have important implications for policy regarding vaccination as well as treatment prioritization in an overly taxed medical system.”
The study was published in the journal Biological Psychiatry Global Open Science.
Dr. Hatoum and colleagues launched the study to gain insight into whether CUD might be a risk factor for severe COVID-19 presentations.
As defined by the DSM-5, people with CUD suffer from impairment or distress because of their cannabis use and meet at least 2 of 11 criteria over a 12-month period, such as cravings, cannabis tolerance, and withdrawal symptoms. According to a 2020 study that examined 2008-2016 data, 2.72% of children aged 12-17 showed signs of CUD, as did 1.23% of those aged over 26.
The primary reasons for hospitalization and death related to COVID-19 are respiratory symptoms. “And we have observed that genetic vulnerability to CUD is shared with respiratory disease, even after tobacco use is considered,” Dr. Hatoum said.
He and his colleagues examined data from genomewide association studies and searched for genetic correlations between CUD (14,080 cases, 343,726 controls) and COVID-19 hospitalization (9,373 cases, 1,197,256 controls). “Genetic vulnerability to COVID-19 was correlated with genetic liability to CUD (P = 1.33e–6),” the researchers wrote. “This association remained when accounting for genetic liability to related risk factors and covariates (P = .012-.049).”
According to Dr. Hatoum, the researchers found inconclusive evidence that CUD might worsen COVID-19 cases. “We applied statistical causal models, which found an effect consistent with causality, but it was nonsignificant,” he said.
Despite the absence of causality, the study findings could prove useful for clinicians and policy makers.
“Those struggling with CUD may be prioritized for vaccination and vaccination boosters to mitigate their higher likelihood of a severe COVID-19 presentation,” Dr. Hatoum said. “When testing positive for COVID-19, they may also be prioritized for earlier treatment.”
The study authors also added that the findings “urge caution” in regard to the wave of U.S. states legalizing cannabis. “Our data suggest that heavy cannabis use, but not lifetime cannabis use, represents a risk factor for severe COVID-19 presentations,” Dr. Hatoum said.
In an interview, Danielle Dick, PhD, who was not involved with the study, said it applies “cutting-edge methods to an important research question” and offers a “hint” of a genetic risk factor that makes some people more likely to be hospitalized for COVID-19. However, “the study does not tell us what those underlying genetically influenced processes might be,” added Dr. Dick, professor of psychology, and human and molecular genetics at Virginia Commonwealth University, Richmond. “And it’s an important caveat to point out that the results from this study are limited in that they are based on data from people from European descent – so they can’t necessarily be applied to address the harm experienced by so many people of color from the COVID pandemic. That’s an unfortunate limitation.”
As for the idea that the study findings should prompt caution about marijuana legalization, Dr. Dick said it’s true that increased acceptability of drug use “increases the likelihood that individuals who are genetically vulnerable will develop problems. There is robust evidence of this.”
However, Dr. Dick said, “the legalization of marijuana is a complex topic because the health consequences aren’t the only consideration when it comes to legalization. The other side of the coin is the huge harm that has been caused to communities of color through marijuana criminalization. Legalization will hopefully lead to decreased harm on that front. So it’s a double-edged sword.”
Dr. Hatoum, his colleagues, and Dr. Dick reported no relevant disclosures.
The same genetic variations may boost susceptibility to both severe COVID-19 and cannabis use disorder (CUD), a new study suggests. The research does not confirm a genetic link, but the lead author said the signs of an association are still “troubling.”
“Reducing cannabis use among heavy users may potentially provide protection against severe COVID-19 presentations,” Alexander S. Hatoum, PhD, a postdoctoral scholar at Washington University, St. Louis, said in an interview. “Outside of individual risk, these data also have important implications for policy regarding vaccination as well as treatment prioritization in an overly taxed medical system.”
The study was published in the journal Biological Psychiatry Global Open Science.
Dr. Hatoum and colleagues launched the study to gain insight into whether CUD might be a risk factor for severe COVID-19 presentations.
As defined by the DSM-5, people with CUD suffer from impairment or distress because of their cannabis use and meet at least 2 of 11 criteria over a 12-month period, such as cravings, cannabis tolerance, and withdrawal symptoms. According to a 2020 study that examined 2008-2016 data, 2.72% of children aged 12-17 showed signs of CUD, as did 1.23% of those aged over 26.
The primary reasons for hospitalization and death related to COVID-19 are respiratory symptoms. “And we have observed that genetic vulnerability to CUD is shared with respiratory disease, even after tobacco use is considered,” Dr. Hatoum said.
He and his colleagues examined data from genomewide association studies and searched for genetic correlations between CUD (14,080 cases, 343,726 controls) and COVID-19 hospitalization (9,373 cases, 1,197,256 controls). “Genetic vulnerability to COVID-19 was correlated with genetic liability to CUD (P = 1.33e–6),” the researchers wrote. “This association remained when accounting for genetic liability to related risk factors and covariates (P = .012-.049).”
According to Dr. Hatoum, the researchers found inconclusive evidence that CUD might worsen COVID-19 cases. “We applied statistical causal models, which found an effect consistent with causality, but it was nonsignificant,” he said.
Despite the absence of causality, the study findings could prove useful for clinicians and policy makers.
“Those struggling with CUD may be prioritized for vaccination and vaccination boosters to mitigate their higher likelihood of a severe COVID-19 presentation,” Dr. Hatoum said. “When testing positive for COVID-19, they may also be prioritized for earlier treatment.”
The study authors also added that the findings “urge caution” in regard to the wave of U.S. states legalizing cannabis. “Our data suggest that heavy cannabis use, but not lifetime cannabis use, represents a risk factor for severe COVID-19 presentations,” Dr. Hatoum said.
In an interview, Danielle Dick, PhD, who was not involved with the study, said it applies “cutting-edge methods to an important research question” and offers a “hint” of a genetic risk factor that makes some people more likely to be hospitalized for COVID-19. However, “the study does not tell us what those underlying genetically influenced processes might be,” added Dr. Dick, professor of psychology, and human and molecular genetics at Virginia Commonwealth University, Richmond. “And it’s an important caveat to point out that the results from this study are limited in that they are based on data from people from European descent – so they can’t necessarily be applied to address the harm experienced by so many people of color from the COVID pandemic. That’s an unfortunate limitation.”
As for the idea that the study findings should prompt caution about marijuana legalization, Dr. Dick said it’s true that increased acceptability of drug use “increases the likelihood that individuals who are genetically vulnerable will develop problems. There is robust evidence of this.”
However, Dr. Dick said, “the legalization of marijuana is a complex topic because the health consequences aren’t the only consideration when it comes to legalization. The other side of the coin is the huge harm that has been caused to communities of color through marijuana criminalization. Legalization will hopefully lead to decreased harm on that front. So it’s a double-edged sword.”
Dr. Hatoum, his colleagues, and Dr. Dick reported no relevant disclosures.
The same genetic variations may boost susceptibility to both severe COVID-19 and cannabis use disorder (CUD), a new study suggests. The research does not confirm a genetic link, but the lead author said the signs of an association are still “troubling.”
“Reducing cannabis use among heavy users may potentially provide protection against severe COVID-19 presentations,” Alexander S. Hatoum, PhD, a postdoctoral scholar at Washington University, St. Louis, said in an interview. “Outside of individual risk, these data also have important implications for policy regarding vaccination as well as treatment prioritization in an overly taxed medical system.”
The study was published in the journal Biological Psychiatry Global Open Science.
Dr. Hatoum and colleagues launched the study to gain insight into whether CUD might be a risk factor for severe COVID-19 presentations.
As defined by the DSM-5, people with CUD suffer from impairment or distress because of their cannabis use and meet at least 2 of 11 criteria over a 12-month period, such as cravings, cannabis tolerance, and withdrawal symptoms. According to a 2020 study that examined 2008-2016 data, 2.72% of children aged 12-17 showed signs of CUD, as did 1.23% of those aged over 26.
The primary reasons for hospitalization and death related to COVID-19 are respiratory symptoms. “And we have observed that genetic vulnerability to CUD is shared with respiratory disease, even after tobacco use is considered,” Dr. Hatoum said.
He and his colleagues examined data from genomewide association studies and searched for genetic correlations between CUD (14,080 cases, 343,726 controls) and COVID-19 hospitalization (9,373 cases, 1,197,256 controls). “Genetic vulnerability to COVID-19 was correlated with genetic liability to CUD (P = 1.33e–6),” the researchers wrote. “This association remained when accounting for genetic liability to related risk factors and covariates (P = .012-.049).”
According to Dr. Hatoum, the researchers found inconclusive evidence that CUD might worsen COVID-19 cases. “We applied statistical causal models, which found an effect consistent with causality, but it was nonsignificant,” he said.
Despite the absence of causality, the study findings could prove useful for clinicians and policy makers.
“Those struggling with CUD may be prioritized for vaccination and vaccination boosters to mitigate their higher likelihood of a severe COVID-19 presentation,” Dr. Hatoum said. “When testing positive for COVID-19, they may also be prioritized for earlier treatment.”
The study authors also added that the findings “urge caution” in regard to the wave of U.S. states legalizing cannabis. “Our data suggest that heavy cannabis use, but not lifetime cannabis use, represents a risk factor for severe COVID-19 presentations,” Dr. Hatoum said.
In an interview, Danielle Dick, PhD, who was not involved with the study, said it applies “cutting-edge methods to an important research question” and offers a “hint” of a genetic risk factor that makes some people more likely to be hospitalized for COVID-19. However, “the study does not tell us what those underlying genetically influenced processes might be,” added Dr. Dick, professor of psychology, and human and molecular genetics at Virginia Commonwealth University, Richmond. “And it’s an important caveat to point out that the results from this study are limited in that they are based on data from people from European descent – so they can’t necessarily be applied to address the harm experienced by so many people of color from the COVID pandemic. That’s an unfortunate limitation.”
As for the idea that the study findings should prompt caution about marijuana legalization, Dr. Dick said it’s true that increased acceptability of drug use “increases the likelihood that individuals who are genetically vulnerable will develop problems. There is robust evidence of this.”
However, Dr. Dick said, “the legalization of marijuana is a complex topic because the health consequences aren’t the only consideration when it comes to legalization. The other side of the coin is the huge harm that has been caused to communities of color through marijuana criminalization. Legalization will hopefully lead to decreased harm on that front. So it’s a double-edged sword.”
Dr. Hatoum, his colleagues, and Dr. Dick reported no relevant disclosures.
FROM BIOLOGICAL PSYCHIATRY GLOBAL OPEN SCIENCE
Study: More than half of people taking HIV PrEP discontinue use
More than half of individuals who started HIV preexposure prophylaxis (PrEP) in a large Northern California care management organization discontinued PrEP in a 6.5-year study period, researchers report. African American and Latinx individuals, women, and participants with substance use disorder were more likely to experience gaps in the PrEP care continuum, from initial contact with a provider to adherence over time.
While PrEP is highly effective at preventing HIV when taken as prescribed, research suggests that access to and usage of the medication is lower in the communities that need it most. Even if someone in these groups gets access to PrEP, he or she is less likely to start taking the medication and more likely to discontinue treatment, Carlo Hojilla, PhD, RN, lead author of the study and research fellow with the Kaiser Permanente Northern California Division of Research in Oakland, Calif., said in an interview.
By identifying and tracking these at-risk individuals and subgroups, “we can better characterize at what points in the PrEP continuum people are falling off so we can then better develop interventions to address those gaps,” he said. The results of the analysis were published Aug. 26.
The investigators looked at the electronic health records (EHR) from 13,906 adults (18 years or older) linked to PrEP services at Kaiser Permanente Northern California (KPNC) from July 16, 2012 – when PrEP received regulatory approval in the United States – through March 31, 2019. The total follow-up in the study was 26,210 person-years.
Individuals were included if they had a PrEP referral or a PrEP-coded clinical encounter in the EHR and were KPNC health members for at least 6 months during the study period. The analysis also included age, sex, self-reported race and ethnicity, and socioeconomic status, approximated by participants’ zip codes. Individuals were followed from the initiation of PrEP services to the end of the study period or until HIV diagnosis, discontinuation of KPNC health plan membership, or death.
Nearly all of the study cohort (95.1%) were male, and the median age of participants was 33. Nearly half (48.7%) of the cohort was White, 21.6% were Latinx, 14.8% were Asian, and 7% were African American.
Of all individuals linked to PrEP care in the study, 88.1% received a PrEP prescription. Of those, 98.2% filled their prescription and were assumed to have initiated the medication. More than half (52.2%) of participants discontinued PrEP at least once during the study period, and 60.2% of those participants eventually restarted their regimen.
Participants were most likely to discontinue PrEP within the first 2 years of treatment, the authors found. “With earlier data that we’ve gotten from PrEP trials and studies, we’ve been under the impression that the first few months were the most critical to keeping people engaged in care and maintaining a high degree of adherence,” Dr. Hojilla said. “But I think our findings suggest that it may be more than just a few months.”
Compared with White participants, both African American and Latinx participants had lower rates of PrEP prescriptions, were less likely to initiate PrEP, and more frequently discontinued PrEP. Compared with men, women had lower rates of PrEP prescription and initiation, and were nearly twice as likely (hazard ratio, 1.99) to discontinue their regimen during the study period. Young adults (18-25 years of age), individuals with lower socioeconomic status, and people with substance use disorder also experienced disparities throughout the PrEP continuum of care.
Over the study period, 136 individuals were diagnosed with HIV, with one-third (33.1%) diagnosed during their initial PrEP assessment. Excluding this group, the overall HIV incidence was 0.35 new infections per 100 person-years, with the highest incidence among those who had discontinued and did not reinitiate PrEP (1.28 new infections per 100 person-years.) No individuals who consistently took PrEP were diagnosed with HIV during the study period.
Although the findings are not surprising, the study “corroborates what a lot of us have looked at on the clinic level, which is basically that a lot of people discontinue PrEP who probably need it,” said Amy Nunn, ScD, a professor of behavioral and social sciences at the Brown University School of Public Health in Providence, R.I. She was not involved with the study. As “one of the largest studies to date” to look at HIV PrEP adherence, the study also gives a better picture of what is going on at a population level, she said.
Because the authors retrospectively looked at EHR data, a limitation they acknowledged, it was not clear what was driving these patients to discontinue care or neglect adherence, Dr. Nunn noted.
Dr. Nunn’s previous research found that unexpected out-of-pocket costs can be one reason people discontinue PrEP, and there are many structural barriers such as medical mistrust and community stigma that can contribute to disrupted care, added Jessica Jaiswal, PhD, MPH, a public health scientist at the University of Alabama in Tuscaloosa. And since all the study’s participants had health insurance, the findings do not reflect additional struggles of accessing care while uninsured. “If this is what they found among folks who are insured, then it’s very likely that the barriers are more intense or formidable for folks without insurance,” said Dr. Jaiswal, who was not associated with the study.
Dr. Hojilla reported receiving grants from the National Institute on Drug Abuse and Kaiser Permanente Northern California during the conduct of the study and salary for clinical work from the San Francisco Department of Public Health outside the submitted work. Dr. Jaiswal and Dr. Nunn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than half of individuals who started HIV preexposure prophylaxis (PrEP) in a large Northern California care management organization discontinued PrEP in a 6.5-year study period, researchers report. African American and Latinx individuals, women, and participants with substance use disorder were more likely to experience gaps in the PrEP care continuum, from initial contact with a provider to adherence over time.
While PrEP is highly effective at preventing HIV when taken as prescribed, research suggests that access to and usage of the medication is lower in the communities that need it most. Even if someone in these groups gets access to PrEP, he or she is less likely to start taking the medication and more likely to discontinue treatment, Carlo Hojilla, PhD, RN, lead author of the study and research fellow with the Kaiser Permanente Northern California Division of Research in Oakland, Calif., said in an interview.
By identifying and tracking these at-risk individuals and subgroups, “we can better characterize at what points in the PrEP continuum people are falling off so we can then better develop interventions to address those gaps,” he said. The results of the analysis were published Aug. 26.
The investigators looked at the electronic health records (EHR) from 13,906 adults (18 years or older) linked to PrEP services at Kaiser Permanente Northern California (KPNC) from July 16, 2012 – when PrEP received regulatory approval in the United States – through March 31, 2019. The total follow-up in the study was 26,210 person-years.
Individuals were included if they had a PrEP referral or a PrEP-coded clinical encounter in the EHR and were KPNC health members for at least 6 months during the study period. The analysis also included age, sex, self-reported race and ethnicity, and socioeconomic status, approximated by participants’ zip codes. Individuals were followed from the initiation of PrEP services to the end of the study period or until HIV diagnosis, discontinuation of KPNC health plan membership, or death.
Nearly all of the study cohort (95.1%) were male, and the median age of participants was 33. Nearly half (48.7%) of the cohort was White, 21.6% were Latinx, 14.8% were Asian, and 7% were African American.
Of all individuals linked to PrEP care in the study, 88.1% received a PrEP prescription. Of those, 98.2% filled their prescription and were assumed to have initiated the medication. More than half (52.2%) of participants discontinued PrEP at least once during the study period, and 60.2% of those participants eventually restarted their regimen.
Participants were most likely to discontinue PrEP within the first 2 years of treatment, the authors found. “With earlier data that we’ve gotten from PrEP trials and studies, we’ve been under the impression that the first few months were the most critical to keeping people engaged in care and maintaining a high degree of adherence,” Dr. Hojilla said. “But I think our findings suggest that it may be more than just a few months.”
Compared with White participants, both African American and Latinx participants had lower rates of PrEP prescriptions, were less likely to initiate PrEP, and more frequently discontinued PrEP. Compared with men, women had lower rates of PrEP prescription and initiation, and were nearly twice as likely (hazard ratio, 1.99) to discontinue their regimen during the study period. Young adults (18-25 years of age), individuals with lower socioeconomic status, and people with substance use disorder also experienced disparities throughout the PrEP continuum of care.
Over the study period, 136 individuals were diagnosed with HIV, with one-third (33.1%) diagnosed during their initial PrEP assessment. Excluding this group, the overall HIV incidence was 0.35 new infections per 100 person-years, with the highest incidence among those who had discontinued and did not reinitiate PrEP (1.28 new infections per 100 person-years.) No individuals who consistently took PrEP were diagnosed with HIV during the study period.
Although the findings are not surprising, the study “corroborates what a lot of us have looked at on the clinic level, which is basically that a lot of people discontinue PrEP who probably need it,” said Amy Nunn, ScD, a professor of behavioral and social sciences at the Brown University School of Public Health in Providence, R.I. She was not involved with the study. As “one of the largest studies to date” to look at HIV PrEP adherence, the study also gives a better picture of what is going on at a population level, she said.
Because the authors retrospectively looked at EHR data, a limitation they acknowledged, it was not clear what was driving these patients to discontinue care or neglect adherence, Dr. Nunn noted.
Dr. Nunn’s previous research found that unexpected out-of-pocket costs can be one reason people discontinue PrEP, and there are many structural barriers such as medical mistrust and community stigma that can contribute to disrupted care, added Jessica Jaiswal, PhD, MPH, a public health scientist at the University of Alabama in Tuscaloosa. And since all the study’s participants had health insurance, the findings do not reflect additional struggles of accessing care while uninsured. “If this is what they found among folks who are insured, then it’s very likely that the barriers are more intense or formidable for folks without insurance,” said Dr. Jaiswal, who was not associated with the study.
Dr. Hojilla reported receiving grants from the National Institute on Drug Abuse and Kaiser Permanente Northern California during the conduct of the study and salary for clinical work from the San Francisco Department of Public Health outside the submitted work. Dr. Jaiswal and Dr. Nunn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More than half of individuals who started HIV preexposure prophylaxis (PrEP) in a large Northern California care management organization discontinued PrEP in a 6.5-year study period, researchers report. African American and Latinx individuals, women, and participants with substance use disorder were more likely to experience gaps in the PrEP care continuum, from initial contact with a provider to adherence over time.
While PrEP is highly effective at preventing HIV when taken as prescribed, research suggests that access to and usage of the medication is lower in the communities that need it most. Even if someone in these groups gets access to PrEP, he or she is less likely to start taking the medication and more likely to discontinue treatment, Carlo Hojilla, PhD, RN, lead author of the study and research fellow with the Kaiser Permanente Northern California Division of Research in Oakland, Calif., said in an interview.
By identifying and tracking these at-risk individuals and subgroups, “we can better characterize at what points in the PrEP continuum people are falling off so we can then better develop interventions to address those gaps,” he said. The results of the analysis were published Aug. 26.
The investigators looked at the electronic health records (EHR) from 13,906 adults (18 years or older) linked to PrEP services at Kaiser Permanente Northern California (KPNC) from July 16, 2012 – when PrEP received regulatory approval in the United States – through March 31, 2019. The total follow-up in the study was 26,210 person-years.
Individuals were included if they had a PrEP referral or a PrEP-coded clinical encounter in the EHR and were KPNC health members for at least 6 months during the study period. The analysis also included age, sex, self-reported race and ethnicity, and socioeconomic status, approximated by participants’ zip codes. Individuals were followed from the initiation of PrEP services to the end of the study period or until HIV diagnosis, discontinuation of KPNC health plan membership, or death.
Nearly all of the study cohort (95.1%) were male, and the median age of participants was 33. Nearly half (48.7%) of the cohort was White, 21.6% were Latinx, 14.8% were Asian, and 7% were African American.
Of all individuals linked to PrEP care in the study, 88.1% received a PrEP prescription. Of those, 98.2% filled their prescription and were assumed to have initiated the medication. More than half (52.2%) of participants discontinued PrEP at least once during the study period, and 60.2% of those participants eventually restarted their regimen.
Participants were most likely to discontinue PrEP within the first 2 years of treatment, the authors found. “With earlier data that we’ve gotten from PrEP trials and studies, we’ve been under the impression that the first few months were the most critical to keeping people engaged in care and maintaining a high degree of adherence,” Dr. Hojilla said. “But I think our findings suggest that it may be more than just a few months.”
Compared with White participants, both African American and Latinx participants had lower rates of PrEP prescriptions, were less likely to initiate PrEP, and more frequently discontinued PrEP. Compared with men, women had lower rates of PrEP prescription and initiation, and were nearly twice as likely (hazard ratio, 1.99) to discontinue their regimen during the study period. Young adults (18-25 years of age), individuals with lower socioeconomic status, and people with substance use disorder also experienced disparities throughout the PrEP continuum of care.
Over the study period, 136 individuals were diagnosed with HIV, with one-third (33.1%) diagnosed during their initial PrEP assessment. Excluding this group, the overall HIV incidence was 0.35 new infections per 100 person-years, with the highest incidence among those who had discontinued and did not reinitiate PrEP (1.28 new infections per 100 person-years.) No individuals who consistently took PrEP were diagnosed with HIV during the study period.
Although the findings are not surprising, the study “corroborates what a lot of us have looked at on the clinic level, which is basically that a lot of people discontinue PrEP who probably need it,” said Amy Nunn, ScD, a professor of behavioral and social sciences at the Brown University School of Public Health in Providence, R.I. She was not involved with the study. As “one of the largest studies to date” to look at HIV PrEP adherence, the study also gives a better picture of what is going on at a population level, she said.
Because the authors retrospectively looked at EHR data, a limitation they acknowledged, it was not clear what was driving these patients to discontinue care or neglect adherence, Dr. Nunn noted.
Dr. Nunn’s previous research found that unexpected out-of-pocket costs can be one reason people discontinue PrEP, and there are many structural barriers such as medical mistrust and community stigma that can contribute to disrupted care, added Jessica Jaiswal, PhD, MPH, a public health scientist at the University of Alabama in Tuscaloosa. And since all the study’s participants had health insurance, the findings do not reflect additional struggles of accessing care while uninsured. “If this is what they found among folks who are insured, then it’s very likely that the barriers are more intense or formidable for folks without insurance,” said Dr. Jaiswal, who was not associated with the study.
Dr. Hojilla reported receiving grants from the National Institute on Drug Abuse and Kaiser Permanente Northern California during the conduct of the study and salary for clinical work from the San Francisco Department of Public Health outside the submitted work. Dr. Jaiswal and Dr. Nunn have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Time to positivity doesn’t predict mortality in bloodstream infections with enterococci
A short time to positivity (TTP), the period from incubation to blood culture positivity, may help predict mortality rates for patients with Enterococcus faecalis and vancomycin-sensitive E faecium (VSEfm) bloodstream infections (BSIs), but it is not an independent predictor of risk for death from bloodstream infections caused by enterococci, new research indicates.
Katharina Michelson, of the Institute of Microbiology, Jena University Hospital, Germany, and colleagues conducted a single-site study at Jena University Hospital that included 244 patients with monomicrobial BSIs to assess the value of TTP as a prognostic or diagnostic tool.
Death in the hospital was the primary endpoint considered in the study, which was conducted from January 2014 through December 2016. The shortest TTP of blood cultures was compared among groups.
Findings were published online in April in Diagnostic Microbiology and Infectious Disease.
Among the 244 patients with monomicrobial BSIs, 22.1% of cases were caused by E faecalis, 55.3% were caused by VSEfm, and 22.5% were caused by vancomycin-resistant E faecium (VREfm).
Average TTP of Enterococcus BSI (E-BSI) was 11.6 hours. The researchers found no significant association between risk for death and time to positivity with bloodstream infections with E faecalis, VSEfm, or VREfm, or its cutoffs.
The mortality rate of patients with bloodstream infections with E faecalis was 16.7%; for VSEfm, 26.7%; and for vancomycin-resistant E faecium, 38.2%. Cutoffs showed a significantly higher death rate when TTP was longer but were not risk factors in survival analysis.
The authors explain that “in literature, TTP has not always been proven to be a reliable parameter.”
Sam Aitken, PharmD, MPH, who is a pharmacy specialist for infectious diseases at Michigan Medicine, Ann Arbor, said in an interview that the main message from the article is that the TTP of E faecalis is quite different from that of E faecium and that “that’s in line with what we know about generally with how these organisms come about in patients.”
“This paper reinforces the differences that are sometimes underappreciated between these organisms because they are both enterococci,” he said.
The authors say appropriate antimicrobial therapy can lead to misinterpretation of TTP, so only patients who received inappropriate antimicrobial therapy on the day of positive blood culture were included in the study.
However, Dr. Aitken said that methodology doesn’t account for “immortal time bias.”
“They didn’t account for the fact that patients who tend to get active antibiotics are the ones who live longer. So unless you account for it, you’re not necessarily going to find that patients who get active antibiotics have improved survival,” he said.
The authors point out that finding new methods for quickly identifying patients with E-BSI is a high priority.
The mortality rates of E-BSI vary between 20% for E faecalis and 50% for E faecium.
Resistance to vancomycin is common in E faecium infections and is associated with high mortality, longer hospital stays, and increased costs. Vancomycin-resistant E faecium is part of a group of bacteria that is associated with multidrug resistance and nosocomial infections.
Dr. Aitken said that rather than TTP, “the best risk predictors are going to be in the microbiome studies we’re seeing. If there is a future for figuring out who’s going to get significant E faecium infections, at least, it’s going to be in the microbiome.”
Limitations of the study include its small size; the possibility of missing data, owing to the fact that the study was retrospective; potential delays to incubation; and the possibility of contamination of blood cultures.
The authors and Dr. Aitken have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A short time to positivity (TTP), the period from incubation to blood culture positivity, may help predict mortality rates for patients with Enterococcus faecalis and vancomycin-sensitive E faecium (VSEfm) bloodstream infections (BSIs), but it is not an independent predictor of risk for death from bloodstream infections caused by enterococci, new research indicates.
Katharina Michelson, of the Institute of Microbiology, Jena University Hospital, Germany, and colleagues conducted a single-site study at Jena University Hospital that included 244 patients with monomicrobial BSIs to assess the value of TTP as a prognostic or diagnostic tool.
Death in the hospital was the primary endpoint considered in the study, which was conducted from January 2014 through December 2016. The shortest TTP of blood cultures was compared among groups.
Findings were published online in April in Diagnostic Microbiology and Infectious Disease.
Among the 244 patients with monomicrobial BSIs, 22.1% of cases were caused by E faecalis, 55.3% were caused by VSEfm, and 22.5% were caused by vancomycin-resistant E faecium (VREfm).
Average TTP of Enterococcus BSI (E-BSI) was 11.6 hours. The researchers found no significant association between risk for death and time to positivity with bloodstream infections with E faecalis, VSEfm, or VREfm, or its cutoffs.
The mortality rate of patients with bloodstream infections with E faecalis was 16.7%; for VSEfm, 26.7%; and for vancomycin-resistant E faecium, 38.2%. Cutoffs showed a significantly higher death rate when TTP was longer but were not risk factors in survival analysis.
The authors explain that “in literature, TTP has not always been proven to be a reliable parameter.”
Sam Aitken, PharmD, MPH, who is a pharmacy specialist for infectious diseases at Michigan Medicine, Ann Arbor, said in an interview that the main message from the article is that the TTP of E faecalis is quite different from that of E faecium and that “that’s in line with what we know about generally with how these organisms come about in patients.”
“This paper reinforces the differences that are sometimes underappreciated between these organisms because they are both enterococci,” he said.
The authors say appropriate antimicrobial therapy can lead to misinterpretation of TTP, so only patients who received inappropriate antimicrobial therapy on the day of positive blood culture were included in the study.
However, Dr. Aitken said that methodology doesn’t account for “immortal time bias.”
“They didn’t account for the fact that patients who tend to get active antibiotics are the ones who live longer. So unless you account for it, you’re not necessarily going to find that patients who get active antibiotics have improved survival,” he said.
The authors point out that finding new methods for quickly identifying patients with E-BSI is a high priority.
The mortality rates of E-BSI vary between 20% for E faecalis and 50% for E faecium.
Resistance to vancomycin is common in E faecium infections and is associated with high mortality, longer hospital stays, and increased costs. Vancomycin-resistant E faecium is part of a group of bacteria that is associated with multidrug resistance and nosocomial infections.
Dr. Aitken said that rather than TTP, “the best risk predictors are going to be in the microbiome studies we’re seeing. If there is a future for figuring out who’s going to get significant E faecium infections, at least, it’s going to be in the microbiome.”
Limitations of the study include its small size; the possibility of missing data, owing to the fact that the study was retrospective; potential delays to incubation; and the possibility of contamination of blood cultures.
The authors and Dr. Aitken have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A short time to positivity (TTP), the period from incubation to blood culture positivity, may help predict mortality rates for patients with Enterococcus faecalis and vancomycin-sensitive E faecium (VSEfm) bloodstream infections (BSIs), but it is not an independent predictor of risk for death from bloodstream infections caused by enterococci, new research indicates.
Katharina Michelson, of the Institute of Microbiology, Jena University Hospital, Germany, and colleagues conducted a single-site study at Jena University Hospital that included 244 patients with monomicrobial BSIs to assess the value of TTP as a prognostic or diagnostic tool.
Death in the hospital was the primary endpoint considered in the study, which was conducted from January 2014 through December 2016. The shortest TTP of blood cultures was compared among groups.
Findings were published online in April in Diagnostic Microbiology and Infectious Disease.
Among the 244 patients with monomicrobial BSIs, 22.1% of cases were caused by E faecalis, 55.3% were caused by VSEfm, and 22.5% were caused by vancomycin-resistant E faecium (VREfm).
Average TTP of Enterococcus BSI (E-BSI) was 11.6 hours. The researchers found no significant association between risk for death and time to positivity with bloodstream infections with E faecalis, VSEfm, or VREfm, or its cutoffs.
The mortality rate of patients with bloodstream infections with E faecalis was 16.7%; for VSEfm, 26.7%; and for vancomycin-resistant E faecium, 38.2%. Cutoffs showed a significantly higher death rate when TTP was longer but were not risk factors in survival analysis.
The authors explain that “in literature, TTP has not always been proven to be a reliable parameter.”
Sam Aitken, PharmD, MPH, who is a pharmacy specialist for infectious diseases at Michigan Medicine, Ann Arbor, said in an interview that the main message from the article is that the TTP of E faecalis is quite different from that of E faecium and that “that’s in line with what we know about generally with how these organisms come about in patients.”
“This paper reinforces the differences that are sometimes underappreciated between these organisms because they are both enterococci,” he said.
The authors say appropriate antimicrobial therapy can lead to misinterpretation of TTP, so only patients who received inappropriate antimicrobial therapy on the day of positive blood culture were included in the study.
However, Dr. Aitken said that methodology doesn’t account for “immortal time bias.”
“They didn’t account for the fact that patients who tend to get active antibiotics are the ones who live longer. So unless you account for it, you’re not necessarily going to find that patients who get active antibiotics have improved survival,” he said.
The authors point out that finding new methods for quickly identifying patients with E-BSI is a high priority.
The mortality rates of E-BSI vary between 20% for E faecalis and 50% for E faecium.
Resistance to vancomycin is common in E faecium infections and is associated with high mortality, longer hospital stays, and increased costs. Vancomycin-resistant E faecium is part of a group of bacteria that is associated with multidrug resistance and nosocomial infections.
Dr. Aitken said that rather than TTP, “the best risk predictors are going to be in the microbiome studies we’re seeing. If there is a future for figuring out who’s going to get significant E faecium infections, at least, it’s going to be in the microbiome.”
Limitations of the study include its small size; the possibility of missing data, owing to the fact that the study was retrospective; potential delays to incubation; and the possibility of contamination of blood cultures.
The authors and Dr. Aitken have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cutaneous Protothecosis
To the Editor:
Protothecosis infections are caused by an achlorophyllic algae of the species Prototheca. Prototheca organisms are found mostly in soil and water.1 Human infections are rare and involve 2 species, Prototheca wickerhamii and Prototheca zopfii. The former most commonly is responsible for human infections, though P zopfii results in more serious systemic infections with a poor prognosis. There are various types of Prototheca infection presentations, with a 2007 review of 117 cases reporting that cutaneous infections are most common (66%), followed by systemic infections (19%), and olecranon bursitis (15%).2 Skin lesions most commonly occur on the extremities and face, and they present as vesiculobullous and ulcerative lesions with purulent drainage. The skin lesions also may appear as erythematous plaques or nodules, subcutaneous papules, verrucous or herpetiformis lesions, or pyogenic granuloma–like lesions.3 Protothecosis typically affects immunocompromised individuals, especially those with a history of chronic corticosteroid use, malignancy, diabetes mellitus, AIDS, and/or organ transplant.1 We present a case of cutaneous protothecosis on the dorsal distal extremity of a 94-year-old woman. History of exposure to soil while gardening was elicited from the patient, and no immunosuppressive history was present aside from the patient’s age. This case may prompt workup for malignancy or immunosuppression in this patient subset.
A 94-year-old woman with a medical history of cutaneous squamous cell carcinoma (SCC) presented with a growing lesion on the dorsal surface of the left fourth digit of 2 months’ duration. The patient reported the lesion was painful, and she noted preceding trauma to the area that was suspected to have occurred while gardening. Physical examination revealed an ulcerated, hypertrophic, erythematous nodule on the dorsal surface of the left fourth metacarpophalangeal joint. The differential diagnosis included SCC, inflamed cyst, verruca vulgaris, and orf virus due to the clinical presentation. A shave biopsy was performed, and the lesion subsequently was treated with electrodesiccation and curettage.
Histopathologic evaluation revealed pseudoepitheliomatous hyperplasia with a mixed inflammatory infiltrate including lymphocytes and histiocytes. A morula within the dermis was characteristic of a protothecosis infection (Figure 1). On follow-up visit 6 weeks later, the lesion had grown back to its original size and morphology (Figure 2). At this time, the lesion was again treated with shave removal, followed by electrodesiccation and curettage, and the patient was placed on oral fluconazole 200 mg daily for 1 month. When the lesion did not resolve with fluconazole, she was referred to infectious disease as well as general surgery for surgical removal and debridement of the lesion. Unfortunately, the patient was lost to follow-up.
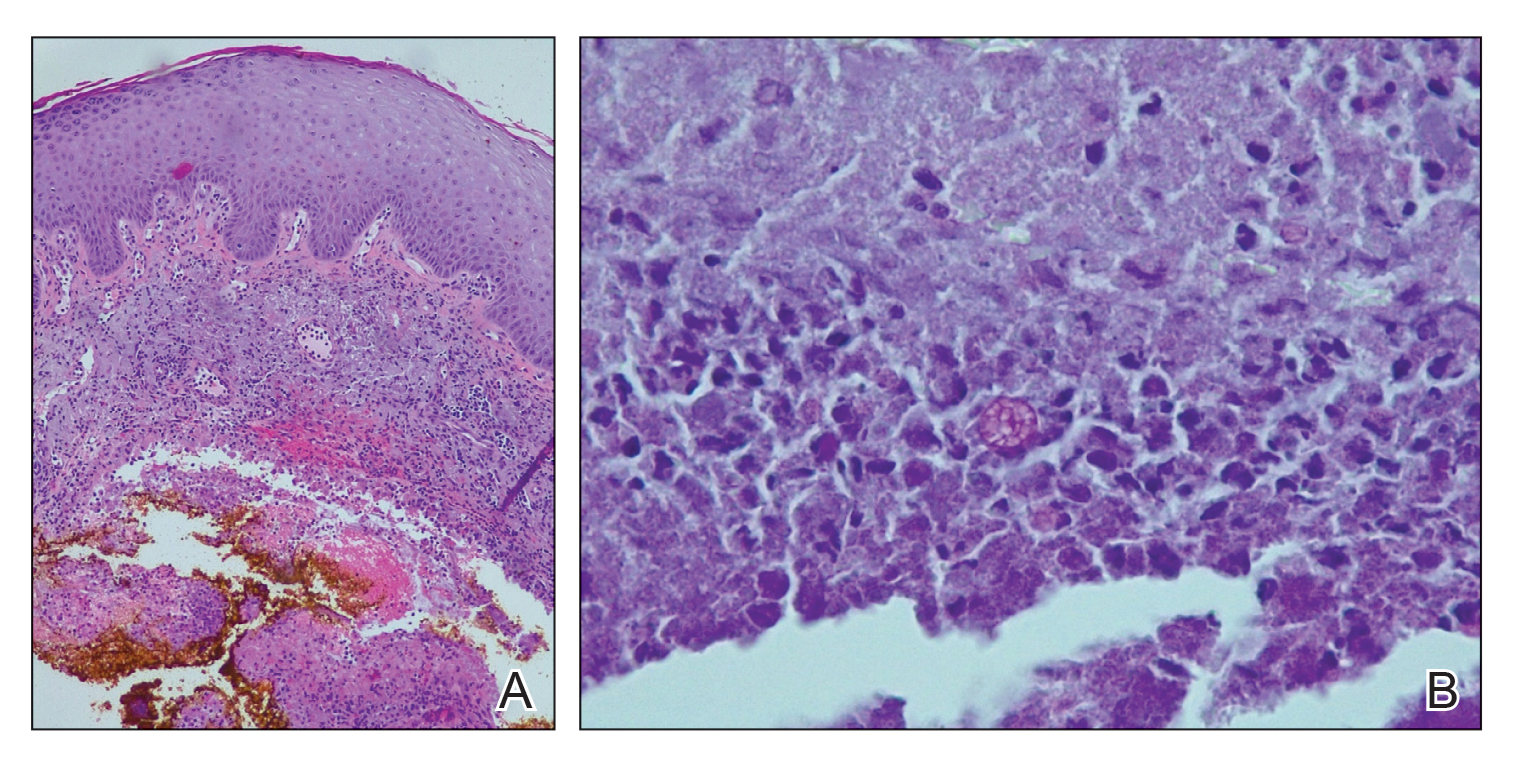
Protothecosis is an infectious disease comprised of achlorophyllic algae found in soil and water that rarely affects humans. When it does affect humans, cutaneous infections are most common. All human cases in which organisms were identified to species level have been caused by P wickerhamii or P zopfii species.2 Inoculation is suspected to occur through trauma to affected skin, especially when in the context of contaminated water. Our patient reported history of trauma to the hand, with soil from gardening as the potential aquagenic source of the infection.

The clinical presentation of protothecosis ranges from localized cutaneous to disseminated systemic infections, with most reported cases of systemic disease occurring in immunocompromised individuals. The cutaneous lesions of protothecosis vary greatly in clinical appearance including ulcerative nodules (as in our case), papules, plaques, pustules, and vesicles with erosion or crusting.4
Cutaneous protothecosis has the potential to mimic many other skin diseases and lesions, and, given its rarity, it may not be on the radar of dermatologists. Our patient’s lesion was presumed to be a skin cancer and was treated as such because of the history of SCC and clinical presentation. Although excision of individual lesions of protothecosis can be curative, electrodesiccation and curettage does not appear to be an adequate treatment, as the lesion subsequently recurred. It also is possible that this case represents P zopfii infection, as it did not respond to treatment with oral fluconazole, though in vitro studies with fluconazole to both P zopfii and P wickerhamii had variable treatment success.2 Also, the histopathologic findings were most consistent with P wickerhamii, revealing small, round, symmetrical morula, compared to P zopfii, which typically will display oval or cylindrical, asymmetrical, random internal segmentation.5 This case may warrant determination of species, which can be accomplished by a culture on Sabouraud dextrose agar, carbohydrate and alcohol assimilation test, yeast biochemical card, serological typing by immunoblotting, immunofluorescence study using species-specific antibodies, or amplification by polymerase chain reaction for small subunit ribosomal DNA sequences.2,6-8
The natural history of isolated skin disease is an indolent progressive course; however, reports do exist noting spontaneous resolution.4,9 Treatment options for Prototheca infections can be disappointing and consist of both surgical and medical management, or a combination of the 2 approaches. Reports in the literature support the use of antifungals including ketoconazole, voriconazole, itraconazole, fluconazole, and amphotericin B, with the latter displaying the best activity against Prototheca species.2 Tetracycline has been used in combination with oral or topical amphotericin B and was found to be synergistic in vitro and in case reports at successfully treating cutaneous protothecosis infections. It is possible that our patient was not treated with fluconazole long enough for it to become therapeutic, as most reported treatment regimens are weeks to months in length. Conversely, it may have been of benefit to transition the patient to topical amphotericin B and tetracycline, as fluconazole failed in this patient. However, treatment successes and failures are limited to case reports/case series and in vitro studies, with prospective studies lacking. Due to the variability with in vitro susceptibility profiles for Prototheca species, it generally is not recommended to pursue in vitro susceptibility testing in the management of Prototheca skin infections due to the inconsistency demonstrated between in vitro activity and clinical response to therapy.2
- Silva PC, Costa e Silva SB, Lima RB, et al. Cutaneous protothecosis—case report. An Bras Dermatol. 2013;88:183-185.
- Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20:230-242.
- Seok JY, Lee Y, Lee H, et al. Human cutaneous protothecosis: report of a case and literature review. Korean J Pathol. 2013;47:575-578.
- Mayorga J, Barba-Gómez JF, Verduzco-Martínez AP, et al. Protothecosis. Clin Dermatol. 2012;30:432-436.
- Walsh SV, Johnson RA, Tahan SR. Protothecosis: an unusual cause of chronic subcutaneous and soft tissue infection. Am J Dermatopathol. 1998;20:379-382.
- Casal MJ, Gutierrez J. Simple new test for rapid differentiation of Prototheca wickerhamii from Prototheca zopfii. J Clin Microbiol. 1983;18:992-993.
- Arnold, P, Ahearn, DG. The systematics of the genus Prototheca with a description of a new species P. filamenta. Mycologia 1972;64:265-275.
- Roesler U, Scholz H, Hensel H. Emended phenotypic characterization of Prototheca zopfii: a proposal for three biotypes and standards for their identification. Int J Syst Evol Microbiol. 2003;53:1195-1199.
- Todd JR, King JW, Oberle A, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol. 2012;50:673-689.
To the Editor:
Protothecosis infections are caused by an achlorophyllic algae of the species Prototheca. Prototheca organisms are found mostly in soil and water.1 Human infections are rare and involve 2 species, Prototheca wickerhamii and Prototheca zopfii. The former most commonly is responsible for human infections, though P zopfii results in more serious systemic infections with a poor prognosis. There are various types of Prototheca infection presentations, with a 2007 review of 117 cases reporting that cutaneous infections are most common (66%), followed by systemic infections (19%), and olecranon bursitis (15%).2 Skin lesions most commonly occur on the extremities and face, and they present as vesiculobullous and ulcerative lesions with purulent drainage. The skin lesions also may appear as erythematous plaques or nodules, subcutaneous papules, verrucous or herpetiformis lesions, or pyogenic granuloma–like lesions.3 Protothecosis typically affects immunocompromised individuals, especially those with a history of chronic corticosteroid use, malignancy, diabetes mellitus, AIDS, and/or organ transplant.1 We present a case of cutaneous protothecosis on the dorsal distal extremity of a 94-year-old woman. History of exposure to soil while gardening was elicited from the patient, and no immunosuppressive history was present aside from the patient’s age. This case may prompt workup for malignancy or immunosuppression in this patient subset.
A 94-year-old woman with a medical history of cutaneous squamous cell carcinoma (SCC) presented with a growing lesion on the dorsal surface of the left fourth digit of 2 months’ duration. The patient reported the lesion was painful, and she noted preceding trauma to the area that was suspected to have occurred while gardening. Physical examination revealed an ulcerated, hypertrophic, erythematous nodule on the dorsal surface of the left fourth metacarpophalangeal joint. The differential diagnosis included SCC, inflamed cyst, verruca vulgaris, and orf virus due to the clinical presentation. A shave biopsy was performed, and the lesion subsequently was treated with electrodesiccation and curettage.
Histopathologic evaluation revealed pseudoepitheliomatous hyperplasia with a mixed inflammatory infiltrate including lymphocytes and histiocytes. A morula within the dermis was characteristic of a protothecosis infection (Figure 1). On follow-up visit 6 weeks later, the lesion had grown back to its original size and morphology (Figure 2). At this time, the lesion was again treated with shave removal, followed by electrodesiccation and curettage, and the patient was placed on oral fluconazole 200 mg daily for 1 month. When the lesion did not resolve with fluconazole, she was referred to infectious disease as well as general surgery for surgical removal and debridement of the lesion. Unfortunately, the patient was lost to follow-up.

Protothecosis is an infectious disease comprised of achlorophyllic algae found in soil and water that rarely affects humans. When it does affect humans, cutaneous infections are most common. All human cases in which organisms were identified to species level have been caused by P wickerhamii or P zopfii species.2 Inoculation is suspected to occur through trauma to affected skin, especially when in the context of contaminated water. Our patient reported history of trauma to the hand, with soil from gardening as the potential aquagenic source of the infection.

The clinical presentation of protothecosis ranges from localized cutaneous to disseminated systemic infections, with most reported cases of systemic disease occurring in immunocompromised individuals. The cutaneous lesions of protothecosis vary greatly in clinical appearance including ulcerative nodules (as in our case), papules, plaques, pustules, and vesicles with erosion or crusting.4
Cutaneous protothecosis has the potential to mimic many other skin diseases and lesions, and, given its rarity, it may not be on the radar of dermatologists. Our patient’s lesion was presumed to be a skin cancer and was treated as such because of the history of SCC and clinical presentation. Although excision of individual lesions of protothecosis can be curative, electrodesiccation and curettage does not appear to be an adequate treatment, as the lesion subsequently recurred. It also is possible that this case represents P zopfii infection, as it did not respond to treatment with oral fluconazole, though in vitro studies with fluconazole to both P zopfii and P wickerhamii had variable treatment success.2 Also, the histopathologic findings were most consistent with P wickerhamii, revealing small, round, symmetrical morula, compared to P zopfii, which typically will display oval or cylindrical, asymmetrical, random internal segmentation.5 This case may warrant determination of species, which can be accomplished by a culture on Sabouraud dextrose agar, carbohydrate and alcohol assimilation test, yeast biochemical card, serological typing by immunoblotting, immunofluorescence study using species-specific antibodies, or amplification by polymerase chain reaction for small subunit ribosomal DNA sequences.2,6-8
The natural history of isolated skin disease is an indolent progressive course; however, reports do exist noting spontaneous resolution.4,9 Treatment options for Prototheca infections can be disappointing and consist of both surgical and medical management, or a combination of the 2 approaches. Reports in the literature support the use of antifungals including ketoconazole, voriconazole, itraconazole, fluconazole, and amphotericin B, with the latter displaying the best activity against Prototheca species.2 Tetracycline has been used in combination with oral or topical amphotericin B and was found to be synergistic in vitro and in case reports at successfully treating cutaneous protothecosis infections. It is possible that our patient was not treated with fluconazole long enough for it to become therapeutic, as most reported treatment regimens are weeks to months in length. Conversely, it may have been of benefit to transition the patient to topical amphotericin B and tetracycline, as fluconazole failed in this patient. However, treatment successes and failures are limited to case reports/case series and in vitro studies, with prospective studies lacking. Due to the variability with in vitro susceptibility profiles for Prototheca species, it generally is not recommended to pursue in vitro susceptibility testing in the management of Prototheca skin infections due to the inconsistency demonstrated between in vitro activity and clinical response to therapy.2
To the Editor:
Protothecosis infections are caused by an achlorophyllic algae of the species Prototheca. Prototheca organisms are found mostly in soil and water.1 Human infections are rare and involve 2 species, Prototheca wickerhamii and Prototheca zopfii. The former most commonly is responsible for human infections, though P zopfii results in more serious systemic infections with a poor prognosis. There are various types of Prototheca infection presentations, with a 2007 review of 117 cases reporting that cutaneous infections are most common (66%), followed by systemic infections (19%), and olecranon bursitis (15%).2 Skin lesions most commonly occur on the extremities and face, and they present as vesiculobullous and ulcerative lesions with purulent drainage. The skin lesions also may appear as erythematous plaques or nodules, subcutaneous papules, verrucous or herpetiformis lesions, or pyogenic granuloma–like lesions.3 Protothecosis typically affects immunocompromised individuals, especially those with a history of chronic corticosteroid use, malignancy, diabetes mellitus, AIDS, and/or organ transplant.1 We present a case of cutaneous protothecosis on the dorsal distal extremity of a 94-year-old woman. History of exposure to soil while gardening was elicited from the patient, and no immunosuppressive history was present aside from the patient’s age. This case may prompt workup for malignancy or immunosuppression in this patient subset.
A 94-year-old woman with a medical history of cutaneous squamous cell carcinoma (SCC) presented with a growing lesion on the dorsal surface of the left fourth digit of 2 months’ duration. The patient reported the lesion was painful, and she noted preceding trauma to the area that was suspected to have occurred while gardening. Physical examination revealed an ulcerated, hypertrophic, erythematous nodule on the dorsal surface of the left fourth metacarpophalangeal joint. The differential diagnosis included SCC, inflamed cyst, verruca vulgaris, and orf virus due to the clinical presentation. A shave biopsy was performed, and the lesion subsequently was treated with electrodesiccation and curettage.
Histopathologic evaluation revealed pseudoepitheliomatous hyperplasia with a mixed inflammatory infiltrate including lymphocytes and histiocytes. A morula within the dermis was characteristic of a protothecosis infection (Figure 1). On follow-up visit 6 weeks later, the lesion had grown back to its original size and morphology (Figure 2). At this time, the lesion was again treated with shave removal, followed by electrodesiccation and curettage, and the patient was placed on oral fluconazole 200 mg daily for 1 month. When the lesion did not resolve with fluconazole, she was referred to infectious disease as well as general surgery for surgical removal and debridement of the lesion. Unfortunately, the patient was lost to follow-up.

Protothecosis is an infectious disease comprised of achlorophyllic algae found in soil and water that rarely affects humans. When it does affect humans, cutaneous infections are most common. All human cases in which organisms were identified to species level have been caused by P wickerhamii or P zopfii species.2 Inoculation is suspected to occur through trauma to affected skin, especially when in the context of contaminated water. Our patient reported history of trauma to the hand, with soil from gardening as the potential aquagenic source of the infection.

The clinical presentation of protothecosis ranges from localized cutaneous to disseminated systemic infections, with most reported cases of systemic disease occurring in immunocompromised individuals. The cutaneous lesions of protothecosis vary greatly in clinical appearance including ulcerative nodules (as in our case), papules, plaques, pustules, and vesicles with erosion or crusting.4
Cutaneous protothecosis has the potential to mimic many other skin diseases and lesions, and, given its rarity, it may not be on the radar of dermatologists. Our patient’s lesion was presumed to be a skin cancer and was treated as such because of the history of SCC and clinical presentation. Although excision of individual lesions of protothecosis can be curative, electrodesiccation and curettage does not appear to be an adequate treatment, as the lesion subsequently recurred. It also is possible that this case represents P zopfii infection, as it did not respond to treatment with oral fluconazole, though in vitro studies with fluconazole to both P zopfii and P wickerhamii had variable treatment success.2 Also, the histopathologic findings were most consistent with P wickerhamii, revealing small, round, symmetrical morula, compared to P zopfii, which typically will display oval or cylindrical, asymmetrical, random internal segmentation.5 This case may warrant determination of species, which can be accomplished by a culture on Sabouraud dextrose agar, carbohydrate and alcohol assimilation test, yeast biochemical card, serological typing by immunoblotting, immunofluorescence study using species-specific antibodies, or amplification by polymerase chain reaction for small subunit ribosomal DNA sequences.2,6-8
The natural history of isolated skin disease is an indolent progressive course; however, reports do exist noting spontaneous resolution.4,9 Treatment options for Prototheca infections can be disappointing and consist of both surgical and medical management, or a combination of the 2 approaches. Reports in the literature support the use of antifungals including ketoconazole, voriconazole, itraconazole, fluconazole, and amphotericin B, with the latter displaying the best activity against Prototheca species.2 Tetracycline has been used in combination with oral or topical amphotericin B and was found to be synergistic in vitro and in case reports at successfully treating cutaneous protothecosis infections. It is possible that our patient was not treated with fluconazole long enough for it to become therapeutic, as most reported treatment regimens are weeks to months in length. Conversely, it may have been of benefit to transition the patient to topical amphotericin B and tetracycline, as fluconazole failed in this patient. However, treatment successes and failures are limited to case reports/case series and in vitro studies, with prospective studies lacking. Due to the variability with in vitro susceptibility profiles for Prototheca species, it generally is not recommended to pursue in vitro susceptibility testing in the management of Prototheca skin infections due to the inconsistency demonstrated between in vitro activity and clinical response to therapy.2
- Silva PC, Costa e Silva SB, Lima RB, et al. Cutaneous protothecosis—case report. An Bras Dermatol. 2013;88:183-185.
- Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20:230-242.
- Seok JY, Lee Y, Lee H, et al. Human cutaneous protothecosis: report of a case and literature review. Korean J Pathol. 2013;47:575-578.
- Mayorga J, Barba-Gómez JF, Verduzco-Martínez AP, et al. Protothecosis. Clin Dermatol. 2012;30:432-436.
- Walsh SV, Johnson RA, Tahan SR. Protothecosis: an unusual cause of chronic subcutaneous and soft tissue infection. Am J Dermatopathol. 1998;20:379-382.
- Casal MJ, Gutierrez J. Simple new test for rapid differentiation of Prototheca wickerhamii from Prototheca zopfii. J Clin Microbiol. 1983;18:992-993.
- Arnold, P, Ahearn, DG. The systematics of the genus Prototheca with a description of a new species P. filamenta. Mycologia 1972;64:265-275.
- Roesler U, Scholz H, Hensel H. Emended phenotypic characterization of Prototheca zopfii: a proposal for three biotypes and standards for their identification. Int J Syst Evol Microbiol. 2003;53:1195-1199.
- Todd JR, King JW, Oberle A, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol. 2012;50:673-689.
- Silva PC, Costa e Silva SB, Lima RB, et al. Cutaneous protothecosis—case report. An Bras Dermatol. 2013;88:183-185.
- Lass-Flörl C, Mayr A. Human protothecosis. Clin Microbiol Rev. 2007;20:230-242.
- Seok JY, Lee Y, Lee H, et al. Human cutaneous protothecosis: report of a case and literature review. Korean J Pathol. 2013;47:575-578.
- Mayorga J, Barba-Gómez JF, Verduzco-Martínez AP, et al. Protothecosis. Clin Dermatol. 2012;30:432-436.
- Walsh SV, Johnson RA, Tahan SR. Protothecosis: an unusual cause of chronic subcutaneous and soft tissue infection. Am J Dermatopathol. 1998;20:379-382.
- Casal MJ, Gutierrez J. Simple new test for rapid differentiation of Prototheca wickerhamii from Prototheca zopfii. J Clin Microbiol. 1983;18:992-993.
- Arnold, P, Ahearn, DG. The systematics of the genus Prototheca with a description of a new species P. filamenta. Mycologia 1972;64:265-275.
- Roesler U, Scholz H, Hensel H. Emended phenotypic characterization of Prototheca zopfii: a proposal for three biotypes and standards for their identification. Int J Syst Evol Microbiol. 2003;53:1195-1199.
- Todd JR, King JW, Oberle A, et al. Protothecosis: report of a case with 20-year follow-up, and review of previously published cases. Med Mycol. 2012;50:673-689.
Practice Points
- Cutaneous protothecosis is a rare skin infection most commonly reported in immunocompromised individuals with recent exposure to contaminated soil or water. Cutaneous protothecosis has the potential to mimic many other skin diseases and lesions, including eczema; nonmelanoma skin cancer; or bacterial, viral, and fungal skin infections.
- A skin biopsy is essential for diagnosis, and histopathology is characteristic with soccer ball–appearing morula noted in a mixed inflammatory infiltrate.
Children and COVID: New cases soar to near-record level
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.
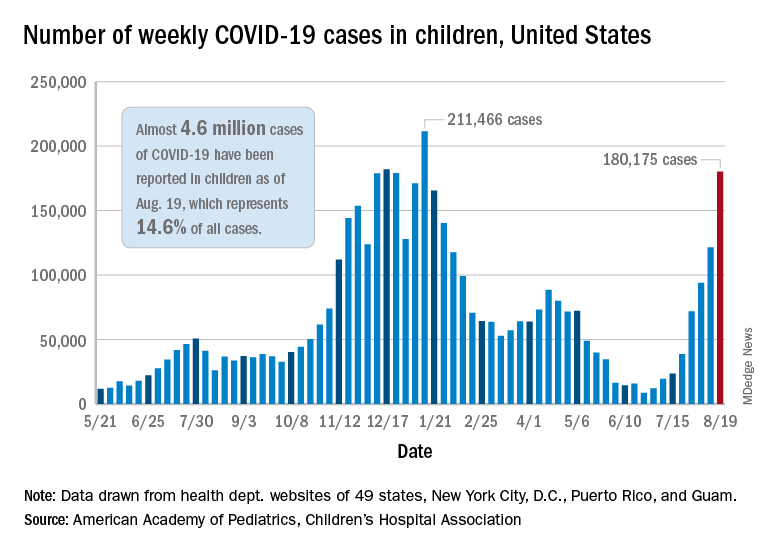
The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.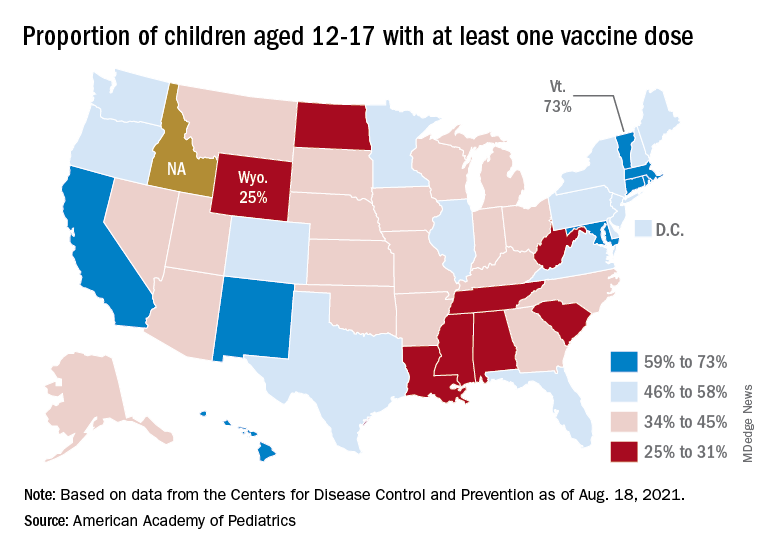
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.

The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Weekly cases of COVID-19 in children jumped by nearly 50% in the United States, posting the highest count since hitting a pandemic high back in mid-January, a new report shows.

The latest weekly figure represents a 48% increase over the previous week and an increase of over 2,000% in the 8 weeks since the national count dropped to a low of 8,500 cases for the week of June 18-24, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID report.
Vaccinations, in the meantime, appear to be headed in the opposite direction. Vaccine initiations were down for the second consecutive week, falling by 18% among 12- to 15-year-olds and by 15% in those aged 16-17 years, according to data from the Centers for Disease Control and Prevention.
Nationally, about 47% of children aged 12-15 and 56% of those aged 16-17 have received at least one dose of COVID vaccine as of Aug. 23, with 34% and 44%, respectively, reaching full vaccination. The total number of children with at least one dose is 11.6 million, including a relatively small number (about 200,000) of children under age 12 years, the CDC said on its COVID Data Tracker.
At the state level, vaccination is a source of considerable disparity. In Vermont, 73% of children aged 12-17 had received at least one dose by Aug. 18, and 63% were fully vaccinated. In Wyoming, however, just 25% of children had received at least one dose (17% are fully vaccinated), while Alabama has a lowest-in-the-nation full vaccination rate of 14%, based on a separate AAP analysis of CDC data.
There are seven states in which over 60% of 12- to 17-year-olds have at least started the vaccine regimen and five states where less than 30% have received at least one dose, the AAP noted.
Back on the incidence side of the pandemic, Mississippi and Hawaii had the largest increases in new cases over the past 2 weeks, followed by Florida and West Virginia. Cumulative figures show that California has had the most cases overall in children (550,337), Vermont has the highest proportion of all cases in children (22.9%), and Rhode Island has the highest rate of cases per 100,000 (10,636), the AAP and CHA said in the joint report based on data from 49 states, the District of Columbia, New York City, Puerto Rico, and Guam.
Add up all those jurisdictions, and it works out to 4.6 million children infected with SARS-CoV-2 as of Aug. 19, with children representing 14.6% of all cases since the start of the pandemic. There have been over 18,000 hospitalizations so far, which is just 2.3% of the total for all ages in the 23 states (and New York City) that are reporting such data on their health department websites, the AAP and CHA said.
The number of COVID-related deaths in children is now 402 after the largest 1-week increase (24) since late May of 2020, when the AAP/CHA coverage began. Mortality data by age are available from 44 states, New York City, Puerto Rico, and Guam.
Prevalence of high-risk HPV types dwindled since vaccine approval
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Young women who received the quadrivalent human papillomavirus (HPV) vaccine had fewer and fewer infections with high-risk HPV strains covered by the vaccine year after year, but the incidence of high-risk strains that were not covered by the vaccine increased over the same 12-year period, researchers report in a study published August 23 in JAMA Open Network.
“One of the unique contributions that this study provides is the evaluation of a real-world example of the HPV infection rates following immunization in a population of adolescent girls and young adult women at a single health center in a large U.S. city, reflecting strong evidence of vaccine effectiveness,” write Nicolas F. Schlecht, PhD, a professor of oncology at Roswell Park Comprehensive Cancer Center, Buffalo, and his colleagues. “Previous surveillance studies from the U.S. have involved older women and populations with relatively low vaccine coverage.”
In addition to supporting the value of continuing to vaccinate teens against HPV, the findings underscore the importance of continuing to screen women for cervical cancer, Dr. Schlecht said in an interview.
“HPV has not and is not going away,” he said. “We need to keep on our toes with screening and other measures to continue to prevent the development of cervix cancer,” including monitoring different high-risk HPV types and keeping a close eye on cervical precancer rates, particularly CIN3 and cervix cancer, he said. “The vaccines are definitely a good thing. Just getting rid of HPV16 is an amazing accomplishment.”
Kevin Ault, MD, a professor of ob/gyn and academic specialist director of clinical and translational research at the University of Kansas, Kansas City, told this news organization that other studies have had similar findings, but this one is larger with longer follow-up.
“The take-home message is that vaccines work, and this is especially true for the HPV vaccine,” said Dr. Ault, who was not involved in the research. “The vaccine prevents HPV infections and the consequences of these infections, such as cervical cancer. The results are consistent with other studies in different settings, so they are likely generalizable.”
The researchers collected data from October 2007, shortly after the vaccine was approved, through September 2019 on sexually active adolescent and young women aged 13 to 21 years who had received the HPV vaccine and had agreed to follow-up assessments every 6 months until they turned 26. Each follow-up included the collecting of samples of cervical and anal cells for polymerase chain reaction testing for the presence of HPV types.
More than half of the 1,453 participants were Hispanic (58.8%), and half were Black (50.4%), including 15% Hispanic and Black patients. The average age of the participants was 18 years. They were tracked for a median 2.4 years. Nearly half the participants (48%) received the HPV vaccine prior to sexual debut.
For the longitudinal study, the researchers adjusted for participants’ age, the year they received the vaccine, and the years since they were vaccinated. They also tracked breakthrough infections for the four types of HPV covered by the vaccine in participants who received the vaccine before sexual debut.
“We evaluated whether infection rates for HPV have changed since the administration of the vaccine by assessing longitudinally the probability of HPV detection over time among vaccinated participants while adjusting for changes in cohort characteristics over time,” the researchers write. In their statistical analysis, they made adjustments for the number of vaccine doses participants received before their first study visit, age at sexual debut, age at first vaccine dose, number of sexual partners in the preceding 6 months, consistency of condom use during sex, history of a positive chlamydia test, and, for anal HPV analyses, whether the participants had had anal sex in the previous 6 months.
The average age at first intercourse remained steady at 15 years throughout the study, but the average age of vaccination dropped from 18 years in 2008 to 12 years in 2019 (P < .001). More than half the participants (64%) had had at least three lifetime sexual partners at baseline.
After adjustment for age, the researchers found that the incidence of the four HPV types covered by the vaccine – HPV-6, HPV-11, HPV-16, and HPV-18 – dropped more each year, shifting from 9.1% from 2008-2010 to 4.7% from 2017-2019. The effect was even greater among those vaccinated prior to sexual debut; for those patients, the incidence of the four vaccine types dropped from 8.8% to 1.7% over the course of the study. Declines over time also occurred for anal types HPV-31 (adjusted odds ratio [aOR] = 0.76) and HPV-45 (aOR = 0.77). Those vaccinated prior to any sexual intercourse had 19% lower odds of infection per year with a vaccine-covered HPV type.
“We were really excited to see that the types targeted by the vaccines were considerably lower over time in our population,” Dr. Schlecht told this news organization. “This is an important observation, since most of these types are the most worrisome for cervical cancer.”
They were surprised, however, to see overall HPV prevalence increase over time, particularly with the high-risk HPV types that were not covered by the quadrivalent vaccine.
Prevalence of cervical high-risk types not in the vaccine increased from 25.1% from 2008-2010 to 30.5% from 2017-2019. Odds of detection of high-risk HPV types not covered by the vaccine increased 8% each year, particularly for HPV-56 and HPV-68; anal HPV types increased 11% each year. Neither age nor recent number of sexual partners affected the findings.
“The underlying mechanisms for the observed increased detection of specific non-vaccine HPV types over time are not yet clear.”
“We hope this doesn’t translate into some increase in cervical neoplasia that is unanticipated,” Dr. Schlecht said. He noted that the attributable risks for cancer associated with nonvaccine high-risk HPV types remain low. “Theoretical concerns are one thing; actual data is what drives the show,” he said.
The research was funded by the National Institutes of Health and the Icahn School of Medicine at Mount Sinai, New York. Dr. Schlecht has served on advisory boards for Merck, GlaxoSmithKline (GSK), and PDS Biotechnology. One author previously served on a GSK advisory board, and another worked with Merck on an early vaccine trial. Dr. Ault has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.