User login
Large study affirms what we already know: Masks work to prevent COVID-19
It also shows that surgical masks are more effective than cloth face coverings.
The study, which was published ahead of peer review, demonstrates the power of careful investigation and offers a host of lessons about mask wearing that will be important worldwide. One key finding of the study, for example, is that wearing a mask doesn’t lead people to abandon social distancing, something public health officials had feared might happen if masks gave people a false sense of security.
“What we really were able to achieve is to demonstrate that masks are effective against COVID-19, even under a rigorous and systematic evaluation that was done in the throes of the pandemic,” said Ashley Styczynski, MD, who was an infectious disease fellow at Stanford (Calif.) University when she collaborated on the study with other colleagues at Stanford, Yale, and Innovations for Poverty Action, a large research and policy nonprofit organization that currently works in 22 countries.
“And so, I think people who have been holding out on wearing masks because [they] felt like there wasn’t enough evidence for it, we’re hoping this will really help bridge that gap for them,” she said.
It included more than 600 unions – or local governmental districts in Bangladesh – and roughly 340,000 people.
Half of the districts were given cloth or surgical face masks along with continual reminders to wear them properly; the other half were tracked with no intervention. Blood tests of people who developed symptoms during the study verified their infections.
Compared to villages that didn’t mask, those in which masks of any type were worn had about 9% fewer symptomatic cases of COVID-19. The finding was statistically significant and was unlikely to have occurred by chance alone.
“Somebody could read this study and say, ‘OK, you reduced COVID-19 by 9%. Big deal.’ And what I would respond to that would be that, if anything, we think that that is a substantial underestimate,” Dr. Styczynski said.
One reason they think they underestimated the effectiveness of masks is that they tested only people who were having symptoms, so people who had only very mild or asymptomatic infections were missed.
Another reason is that, among people who had symptoms, only one-third agreed to undergo a blood test. The effect may have been bigger had participation been universal.
Local transmission may have played a role, too. Rates of COVID-19 in Bangladesh were relatively low during the study. Most infections were caused by the B.1.1.7, or Alpha, variant.
Since then, Delta has taken over. Delta is thought to be more transmissible, and some studies have suggested that people infected with Delta shed more viral particles. Masks may be more effective when more virus is circulating.
The investigators also found important differences by age and by the type of mask. Villages in which surgical masks were worn had 11% fewer COVID-19 cases than villages in which masks were not worn. In villages in which cloth masks were worn, on the other hand, infections were reduced by only 5%.
The cloth masks were substantial. Each had three layers – two layers of fabric with an outer layer of polypropylene. On testing, the filtration efficiency of the cloth masks was only about 37%, compared with 95% for the three-layer surgical masks, which were also made of polypropylene.
Masks were most effective for older individuals. People aged 50-60 years who wore surgical masks were 23% less likely to test positive for COVID, compared with their peers who didn’t wear masks. For people older than 60, the reduction in risk was greater – 35%.
Rigorous research
The study took place over a period of 8 weeks in each district. The interventions were rolled out in waves, with the first starting in November 2020 and the last in January 2021.
Investigators gave each household free cloth or surgical face masks and showed families a video about proper mask wearing with promotional messages from the prime minister, a head imam, and a national cricket star. They also handed out free masks.
Previous studies have shown that people aren’t always truthful about wearing masks in public. In Kenya, for example, 88% of people answering a phone survey said that they wore masks regularly, but researchers determined that only 10% of them actually did so.
Investigators in the Bangladesh study didn’t just ask people if they’d worn masks, they stationed themselves in public markets, mosques, tea stalls, and on roads that were the main entrances to the villages and took notes.
They also tested various ways to educate people and to remind them to wear masks. They found that four factors were effective at promoting the wearing of masks, and they gave them an acronym – NORM.
- N for no-cost masks.
- O for offering information through the video and local leaders.
- R for regular reminders to people by investigators who stand in public markets and offer masks or encourage anyone who wasn’t wearing one or wasn’t wearing it correctly.
- M for modeling, in which local leaders, such as imams, wear masks and remind their followers to wear them.
These four measures tripled the wearing of masks in the intervention communities, from a baseline level of 13% to 42%. People continued to wear their masks properly for about 2 weeks after the study ended, indicating that they’d gotten used to wearing them.
Dr. Styczynski said that nothing else – not text message reminders, or signs posted in public places, or local incentives – moved the needle on mask wearing.
Saved lives and money
The study found that the strategy was cost effective, too. Giving masks to a large population and getting people to use them costs about $10,000 per life saved from COVID, on par with the cost of deploying mosquito nets to save people from malaria, Dr. Styczynski said.
“I think that what we’ve been able to show is that this is a really important tool to be used globally, especially as countries have delays in getting access to vaccines and rolling them out,” she said.
Dr. Styczynski said masks will continue to be important even in countries such as the United States, where vaccines aren’t stopping transmission 100% and there are still large portions of the population who are unvaccinated, such as children.
“If we want to reduce COVID-19 here, it’s really important that we consider the ongoing utility of masks, in addition to vaccines, and not really thinking of them as one or the other,” she said.
The study was funded by a grant from GiveWell.org. The funder had no role in the study design, interpretation, or the decision to publish.
A version of this article first appeared on Medscape.com.
It also shows that surgical masks are more effective than cloth face coverings.
The study, which was published ahead of peer review, demonstrates the power of careful investigation and offers a host of lessons about mask wearing that will be important worldwide. One key finding of the study, for example, is that wearing a mask doesn’t lead people to abandon social distancing, something public health officials had feared might happen if masks gave people a false sense of security.
“What we really were able to achieve is to demonstrate that masks are effective against COVID-19, even under a rigorous and systematic evaluation that was done in the throes of the pandemic,” said Ashley Styczynski, MD, who was an infectious disease fellow at Stanford (Calif.) University when she collaborated on the study with other colleagues at Stanford, Yale, and Innovations for Poverty Action, a large research and policy nonprofit organization that currently works in 22 countries.
“And so, I think people who have been holding out on wearing masks because [they] felt like there wasn’t enough evidence for it, we’re hoping this will really help bridge that gap for them,” she said.
It included more than 600 unions – or local governmental districts in Bangladesh – and roughly 340,000 people.
Half of the districts were given cloth or surgical face masks along with continual reminders to wear them properly; the other half were tracked with no intervention. Blood tests of people who developed symptoms during the study verified their infections.
Compared to villages that didn’t mask, those in which masks of any type were worn had about 9% fewer symptomatic cases of COVID-19. The finding was statistically significant and was unlikely to have occurred by chance alone.
“Somebody could read this study and say, ‘OK, you reduced COVID-19 by 9%. Big deal.’ And what I would respond to that would be that, if anything, we think that that is a substantial underestimate,” Dr. Styczynski said.
One reason they think they underestimated the effectiveness of masks is that they tested only people who were having symptoms, so people who had only very mild or asymptomatic infections were missed.
Another reason is that, among people who had symptoms, only one-third agreed to undergo a blood test. The effect may have been bigger had participation been universal.
Local transmission may have played a role, too. Rates of COVID-19 in Bangladesh were relatively low during the study. Most infections were caused by the B.1.1.7, or Alpha, variant.
Since then, Delta has taken over. Delta is thought to be more transmissible, and some studies have suggested that people infected with Delta shed more viral particles. Masks may be more effective when more virus is circulating.
The investigators also found important differences by age and by the type of mask. Villages in which surgical masks were worn had 11% fewer COVID-19 cases than villages in which masks were not worn. In villages in which cloth masks were worn, on the other hand, infections were reduced by only 5%.
The cloth masks were substantial. Each had three layers – two layers of fabric with an outer layer of polypropylene. On testing, the filtration efficiency of the cloth masks was only about 37%, compared with 95% for the three-layer surgical masks, which were also made of polypropylene.
Masks were most effective for older individuals. People aged 50-60 years who wore surgical masks were 23% less likely to test positive for COVID, compared with their peers who didn’t wear masks. For people older than 60, the reduction in risk was greater – 35%.
Rigorous research
The study took place over a period of 8 weeks in each district. The interventions were rolled out in waves, with the first starting in November 2020 and the last in January 2021.
Investigators gave each household free cloth or surgical face masks and showed families a video about proper mask wearing with promotional messages from the prime minister, a head imam, and a national cricket star. They also handed out free masks.
Previous studies have shown that people aren’t always truthful about wearing masks in public. In Kenya, for example, 88% of people answering a phone survey said that they wore masks regularly, but researchers determined that only 10% of them actually did so.
Investigators in the Bangladesh study didn’t just ask people if they’d worn masks, they stationed themselves in public markets, mosques, tea stalls, and on roads that were the main entrances to the villages and took notes.
They also tested various ways to educate people and to remind them to wear masks. They found that four factors were effective at promoting the wearing of masks, and they gave them an acronym – NORM.
- N for no-cost masks.
- O for offering information through the video and local leaders.
- R for regular reminders to people by investigators who stand in public markets and offer masks or encourage anyone who wasn’t wearing one or wasn’t wearing it correctly.
- M for modeling, in which local leaders, such as imams, wear masks and remind their followers to wear them.
These four measures tripled the wearing of masks in the intervention communities, from a baseline level of 13% to 42%. People continued to wear their masks properly for about 2 weeks after the study ended, indicating that they’d gotten used to wearing them.
Dr. Styczynski said that nothing else – not text message reminders, or signs posted in public places, or local incentives – moved the needle on mask wearing.
Saved lives and money
The study found that the strategy was cost effective, too. Giving masks to a large population and getting people to use them costs about $10,000 per life saved from COVID, on par with the cost of deploying mosquito nets to save people from malaria, Dr. Styczynski said.
“I think that what we’ve been able to show is that this is a really important tool to be used globally, especially as countries have delays in getting access to vaccines and rolling them out,” she said.
Dr. Styczynski said masks will continue to be important even in countries such as the United States, where vaccines aren’t stopping transmission 100% and there are still large portions of the population who are unvaccinated, such as children.
“If we want to reduce COVID-19 here, it’s really important that we consider the ongoing utility of masks, in addition to vaccines, and not really thinking of them as one or the other,” she said.
The study was funded by a grant from GiveWell.org. The funder had no role in the study design, interpretation, or the decision to publish.
A version of this article first appeared on Medscape.com.
It also shows that surgical masks are more effective than cloth face coverings.
The study, which was published ahead of peer review, demonstrates the power of careful investigation and offers a host of lessons about mask wearing that will be important worldwide. One key finding of the study, for example, is that wearing a mask doesn’t lead people to abandon social distancing, something public health officials had feared might happen if masks gave people a false sense of security.
“What we really were able to achieve is to demonstrate that masks are effective against COVID-19, even under a rigorous and systematic evaluation that was done in the throes of the pandemic,” said Ashley Styczynski, MD, who was an infectious disease fellow at Stanford (Calif.) University when she collaborated on the study with other colleagues at Stanford, Yale, and Innovations for Poverty Action, a large research and policy nonprofit organization that currently works in 22 countries.
“And so, I think people who have been holding out on wearing masks because [they] felt like there wasn’t enough evidence for it, we’re hoping this will really help bridge that gap for them,” she said.
It included more than 600 unions – or local governmental districts in Bangladesh – and roughly 340,000 people.
Half of the districts were given cloth or surgical face masks along with continual reminders to wear them properly; the other half were tracked with no intervention. Blood tests of people who developed symptoms during the study verified their infections.
Compared to villages that didn’t mask, those in which masks of any type were worn had about 9% fewer symptomatic cases of COVID-19. The finding was statistically significant and was unlikely to have occurred by chance alone.
“Somebody could read this study and say, ‘OK, you reduced COVID-19 by 9%. Big deal.’ And what I would respond to that would be that, if anything, we think that that is a substantial underestimate,” Dr. Styczynski said.
One reason they think they underestimated the effectiveness of masks is that they tested only people who were having symptoms, so people who had only very mild or asymptomatic infections were missed.
Another reason is that, among people who had symptoms, only one-third agreed to undergo a blood test. The effect may have been bigger had participation been universal.
Local transmission may have played a role, too. Rates of COVID-19 in Bangladesh were relatively low during the study. Most infections were caused by the B.1.1.7, or Alpha, variant.
Since then, Delta has taken over. Delta is thought to be more transmissible, and some studies have suggested that people infected with Delta shed more viral particles. Masks may be more effective when more virus is circulating.
The investigators also found important differences by age and by the type of mask. Villages in which surgical masks were worn had 11% fewer COVID-19 cases than villages in which masks were not worn. In villages in which cloth masks were worn, on the other hand, infections were reduced by only 5%.
The cloth masks were substantial. Each had three layers – two layers of fabric with an outer layer of polypropylene. On testing, the filtration efficiency of the cloth masks was only about 37%, compared with 95% for the three-layer surgical masks, which were also made of polypropylene.
Masks were most effective for older individuals. People aged 50-60 years who wore surgical masks were 23% less likely to test positive for COVID, compared with their peers who didn’t wear masks. For people older than 60, the reduction in risk was greater – 35%.
Rigorous research
The study took place over a period of 8 weeks in each district. The interventions were rolled out in waves, with the first starting in November 2020 and the last in January 2021.
Investigators gave each household free cloth or surgical face masks and showed families a video about proper mask wearing with promotional messages from the prime minister, a head imam, and a national cricket star. They also handed out free masks.
Previous studies have shown that people aren’t always truthful about wearing masks in public. In Kenya, for example, 88% of people answering a phone survey said that they wore masks regularly, but researchers determined that only 10% of them actually did so.
Investigators in the Bangladesh study didn’t just ask people if they’d worn masks, they stationed themselves in public markets, mosques, tea stalls, and on roads that were the main entrances to the villages and took notes.
They also tested various ways to educate people and to remind them to wear masks. They found that four factors were effective at promoting the wearing of masks, and they gave them an acronym – NORM.
- N for no-cost masks.
- O for offering information through the video and local leaders.
- R for regular reminders to people by investigators who stand in public markets and offer masks or encourage anyone who wasn’t wearing one or wasn’t wearing it correctly.
- M for modeling, in which local leaders, such as imams, wear masks and remind their followers to wear them.
These four measures tripled the wearing of masks in the intervention communities, from a baseline level of 13% to 42%. People continued to wear their masks properly for about 2 weeks after the study ended, indicating that they’d gotten used to wearing them.
Dr. Styczynski said that nothing else – not text message reminders, or signs posted in public places, or local incentives – moved the needle on mask wearing.
Saved lives and money
The study found that the strategy was cost effective, too. Giving masks to a large population and getting people to use them costs about $10,000 per life saved from COVID, on par with the cost of deploying mosquito nets to save people from malaria, Dr. Styczynski said.
“I think that what we’ve been able to show is that this is a really important tool to be used globally, especially as countries have delays in getting access to vaccines and rolling them out,” she said.
Dr. Styczynski said masks will continue to be important even in countries such as the United States, where vaccines aren’t stopping transmission 100% and there are still large portions of the population who are unvaccinated, such as children.
“If we want to reduce COVID-19 here, it’s really important that we consider the ongoing utility of masks, in addition to vaccines, and not really thinking of them as one or the other,” she said.
The study was funded by a grant from GiveWell.org. The funder had no role in the study design, interpretation, or the decision to publish.
A version of this article first appeared on Medscape.com.
Health care–associated infections spiked in 2020 in U.S. hospitals
Several health care-associated infections in U.S. hospitals spiked in 2020 compared to the previous year, according to a Centers for Disease Control and Prevention analysis published Sept. 2 in Infection Control and Hospital Epidemiology. Soaring hospitalization rates, sicker patients who required more frequent and intense care, and staffing and supply shortages caused by the COVID-19 pandemic are thought to have contributed to this increase.
This is the first increase in health care–associated infections since 2015.
These findings “are a reflection of the enormous stress that COVID has placed on our health care system,” Arjun Srinivasan, MD (Capt, USPHS), the associate director of the CDC’s Health care-Associated Infection Prevention Programs, Atlanta, told this news organization. He was not an author of the article, but he supervised the research. “We don’t want anyone to read this report and think that it represents a failure of the individual provider or a failure of health care providers in this country in their care of COVID patients,” he said. He noted that health care professionals have provided “tremendously good care to patients under extremely difficult circumstances.”
“People don’t fail – systems fail – and that’s what happened here,” he said. “Our systems that we need to have in place to prevent health care–associated infection simply were not as strong as they needed to be to survive this challenge.”
In the study, researchers used data reported to the National Healthcare Safety Network, the CDC’s tracking system for health care–associated infections. The team compared national standard infection ratios – calculated by dividing the number of reported infections by the number of predicted infections – between 2019 and 2020 for six routinely tracked events:
- Central line–associated bloodstream infections.
- Catheter-associated urinary tract infections (CAUTIs).
- Ventilator-associated events (VAEs).
- Infections associated with colon surgery and abdominal hysterectomy.
- Clostridioides difficile infections.
- Methicillin-resistant Staphylococcus aureus (MRSA) infections.
Infections were estimated using regression models created with baseline data from 2015.
“The new report highlights the need for health care facilities to strengthen their infection prevention programs and support them with adequate resources so that they can handle emerging threats to public health, while at the same time ensuring that gains made in combating HAIs [health care–associated infections] are not lost,” said the Association for Professionals in Infection Control and Epidemiology in a statement.
The analysis revealed significant national increases in central line–associated bloodstream infections, CAUTIs, VAEs, and MRSA infections in 2020 compared to 2019. Among all infection types, the greatest increase was in central-line infections, which were 46% to 47% higher in the third quarter and fourth quarter (Q4) of 2020 relative to the same periods the previous year. VAEs rose by 45%, MRSA infections increased by 34%, and CAUTIs increased by 19% in Q4 of 2020 compared to 2019.
The influx of sicker patients in hospitals throughout 2020 led to more frequent and longer use of medical devices such as catheters and ventilators. The use of these devices increases risk for infection, David P. Calfee, MD, chief medical epidemiologist at the New York–Presbyterian/Weill Cornell Medical Center, said in an interview. He is an editor of Infection Control and Hospital Epidemiology and was not involved with the study. Shortages in personal protective equipment and crowded intensive care units could also have affected how care was delivered, he said. These factors could have led to “reductions in the ability to provide some of the types of care that are needed to optimally reduce the risk of infection.”
There was either no change or decreases in infections associated with colon surgery or abdominal hysterectomy, likely because there were fewer elective surgeries performed, said Dr. Srinivasan. C. difficile–associated infections also decreased throughout 2020 compared to the previous year. Common practices to prevent the spread of COVID-19 in hospitals, such as environmental cleaning, use of personal protective equipment, and patient isolation, likely helped to curb the spread of C. difficile. Although these mitigating procedures do help protect against MRSA infection, many other factors, notably, the use of medical devices such as ventilators and catheters, can increase the risk for MRSA infection, Dr. Srinivasan added.
Although more research is needed to identify the reasons for these spikes in infection, the findings help quantify the scope of these increases across the United States, Dr. Calfee said. The data allow hospitals and health care professionals to “look back at what we did and then think forward in terms of what we can do different in the future,” he added, “so that these stresses to the system have less of an impact on how we are able to provide care.”
Dr. Srinivasan and Dr. Calfee report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Several health care-associated infections in U.S. hospitals spiked in 2020 compared to the previous year, according to a Centers for Disease Control and Prevention analysis published Sept. 2 in Infection Control and Hospital Epidemiology. Soaring hospitalization rates, sicker patients who required more frequent and intense care, and staffing and supply shortages caused by the COVID-19 pandemic are thought to have contributed to this increase.
This is the first increase in health care–associated infections since 2015.
These findings “are a reflection of the enormous stress that COVID has placed on our health care system,” Arjun Srinivasan, MD (Capt, USPHS), the associate director of the CDC’s Health care-Associated Infection Prevention Programs, Atlanta, told this news organization. He was not an author of the article, but he supervised the research. “We don’t want anyone to read this report and think that it represents a failure of the individual provider or a failure of health care providers in this country in their care of COVID patients,” he said. He noted that health care professionals have provided “tremendously good care to patients under extremely difficult circumstances.”
“People don’t fail – systems fail – and that’s what happened here,” he said. “Our systems that we need to have in place to prevent health care–associated infection simply were not as strong as they needed to be to survive this challenge.”
In the study, researchers used data reported to the National Healthcare Safety Network, the CDC’s tracking system for health care–associated infections. The team compared national standard infection ratios – calculated by dividing the number of reported infections by the number of predicted infections – between 2019 and 2020 for six routinely tracked events:
- Central line–associated bloodstream infections.
- Catheter-associated urinary tract infections (CAUTIs).
- Ventilator-associated events (VAEs).
- Infections associated with colon surgery and abdominal hysterectomy.
- Clostridioides difficile infections.
- Methicillin-resistant Staphylococcus aureus (MRSA) infections.
Infections were estimated using regression models created with baseline data from 2015.
“The new report highlights the need for health care facilities to strengthen their infection prevention programs and support them with adequate resources so that they can handle emerging threats to public health, while at the same time ensuring that gains made in combating HAIs [health care–associated infections] are not lost,” said the Association for Professionals in Infection Control and Epidemiology in a statement.
The analysis revealed significant national increases in central line–associated bloodstream infections, CAUTIs, VAEs, and MRSA infections in 2020 compared to 2019. Among all infection types, the greatest increase was in central-line infections, which were 46% to 47% higher in the third quarter and fourth quarter (Q4) of 2020 relative to the same periods the previous year. VAEs rose by 45%, MRSA infections increased by 34%, and CAUTIs increased by 19% in Q4 of 2020 compared to 2019.
The influx of sicker patients in hospitals throughout 2020 led to more frequent and longer use of medical devices such as catheters and ventilators. The use of these devices increases risk for infection, David P. Calfee, MD, chief medical epidemiologist at the New York–Presbyterian/Weill Cornell Medical Center, said in an interview. He is an editor of Infection Control and Hospital Epidemiology and was not involved with the study. Shortages in personal protective equipment and crowded intensive care units could also have affected how care was delivered, he said. These factors could have led to “reductions in the ability to provide some of the types of care that are needed to optimally reduce the risk of infection.”
There was either no change or decreases in infections associated with colon surgery or abdominal hysterectomy, likely because there were fewer elective surgeries performed, said Dr. Srinivasan. C. difficile–associated infections also decreased throughout 2020 compared to the previous year. Common practices to prevent the spread of COVID-19 in hospitals, such as environmental cleaning, use of personal protective equipment, and patient isolation, likely helped to curb the spread of C. difficile. Although these mitigating procedures do help protect against MRSA infection, many other factors, notably, the use of medical devices such as ventilators and catheters, can increase the risk for MRSA infection, Dr. Srinivasan added.
Although more research is needed to identify the reasons for these spikes in infection, the findings help quantify the scope of these increases across the United States, Dr. Calfee said. The data allow hospitals and health care professionals to “look back at what we did and then think forward in terms of what we can do different in the future,” he added, “so that these stresses to the system have less of an impact on how we are able to provide care.”
Dr. Srinivasan and Dr. Calfee report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Several health care-associated infections in U.S. hospitals spiked in 2020 compared to the previous year, according to a Centers for Disease Control and Prevention analysis published Sept. 2 in Infection Control and Hospital Epidemiology. Soaring hospitalization rates, sicker patients who required more frequent and intense care, and staffing and supply shortages caused by the COVID-19 pandemic are thought to have contributed to this increase.
This is the first increase in health care–associated infections since 2015.
These findings “are a reflection of the enormous stress that COVID has placed on our health care system,” Arjun Srinivasan, MD (Capt, USPHS), the associate director of the CDC’s Health care-Associated Infection Prevention Programs, Atlanta, told this news organization. He was not an author of the article, but he supervised the research. “We don’t want anyone to read this report and think that it represents a failure of the individual provider or a failure of health care providers in this country in their care of COVID patients,” he said. He noted that health care professionals have provided “tremendously good care to patients under extremely difficult circumstances.”
“People don’t fail – systems fail – and that’s what happened here,” he said. “Our systems that we need to have in place to prevent health care–associated infection simply were not as strong as they needed to be to survive this challenge.”
In the study, researchers used data reported to the National Healthcare Safety Network, the CDC’s tracking system for health care–associated infections. The team compared national standard infection ratios – calculated by dividing the number of reported infections by the number of predicted infections – between 2019 and 2020 for six routinely tracked events:
- Central line–associated bloodstream infections.
- Catheter-associated urinary tract infections (CAUTIs).
- Ventilator-associated events (VAEs).
- Infections associated with colon surgery and abdominal hysterectomy.
- Clostridioides difficile infections.
- Methicillin-resistant Staphylococcus aureus (MRSA) infections.
Infections were estimated using regression models created with baseline data from 2015.
“The new report highlights the need for health care facilities to strengthen their infection prevention programs and support them with adequate resources so that they can handle emerging threats to public health, while at the same time ensuring that gains made in combating HAIs [health care–associated infections] are not lost,” said the Association for Professionals in Infection Control and Epidemiology in a statement.
The analysis revealed significant national increases in central line–associated bloodstream infections, CAUTIs, VAEs, and MRSA infections in 2020 compared to 2019. Among all infection types, the greatest increase was in central-line infections, which were 46% to 47% higher in the third quarter and fourth quarter (Q4) of 2020 relative to the same periods the previous year. VAEs rose by 45%, MRSA infections increased by 34%, and CAUTIs increased by 19% in Q4 of 2020 compared to 2019.
The influx of sicker patients in hospitals throughout 2020 led to more frequent and longer use of medical devices such as catheters and ventilators. The use of these devices increases risk for infection, David P. Calfee, MD, chief medical epidemiologist at the New York–Presbyterian/Weill Cornell Medical Center, said in an interview. He is an editor of Infection Control and Hospital Epidemiology and was not involved with the study. Shortages in personal protective equipment and crowded intensive care units could also have affected how care was delivered, he said. These factors could have led to “reductions in the ability to provide some of the types of care that are needed to optimally reduce the risk of infection.”
There was either no change or decreases in infections associated with colon surgery or abdominal hysterectomy, likely because there were fewer elective surgeries performed, said Dr. Srinivasan. C. difficile–associated infections also decreased throughout 2020 compared to the previous year. Common practices to prevent the spread of COVID-19 in hospitals, such as environmental cleaning, use of personal protective equipment, and patient isolation, likely helped to curb the spread of C. difficile. Although these mitigating procedures do help protect against MRSA infection, many other factors, notably, the use of medical devices such as ventilators and catheters, can increase the risk for MRSA infection, Dr. Srinivasan added.
Although more research is needed to identify the reasons for these spikes in infection, the findings help quantify the scope of these increases across the United States, Dr. Calfee said. The data allow hospitals and health care professionals to “look back at what we did and then think forward in terms of what we can do different in the future,” he added, “so that these stresses to the system have less of an impact on how we are able to provide care.”
Dr. Srinivasan and Dr. Calfee report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NIH on HIV vaccine failure: ‘Get your HIV-negative, at-risk patients on PrEP tomorrow’
Last year, Katherine Gill, MBChB, an HIV prevention researcher in Cape Town, South Africa, realized how jaded she’d become to vaccine research when the Pfizer COVID-19 vaccine came back as 95% effective. In her career conducting HIV clinical trials, she had never seen anything like it. Even HIV prevention methods she had studied that had worked, such as the dapivirine ring, had an overall efficacy of 30%.
The COVID-19 success story started to soften her views toward another vaccine trial she was helping to conduct, a trial in HIV that used the same platform as Johnson & Johnson’s successful COVID-19 vaccine.
“When the COVID vaccine was cracked so quickly and seemingly quite easily, I did start to think, ‘Well, maybe … maybe this will work for HIV,’ “ she said in an interview.
That turned out to be false hope. The National Institutes of Health (NIH) announced that the trial Dr. Gill was helping to conduct, HVTN 705, was stopping early because it hadn’t generated enough of an immune response in participants to justify continuing. It was the second HIV vaccine to fail in the last year. It’s also the latest in what has been a litany of disappointments in the attempt to boost the human immune system to fight HIV without the need for ongoing HIV treatment.
In HVTN 705, known as the Imbokodo study (imbokodo is a Zulu word that’s part of a saying about women being strong as rocks), researchers used the platform made up of a common cold virus, adenovirus 26, to deliver a computer-generated mosaic of HIV antigens to participants’ immune systems. That mosaic of antigens is meant to goose the immune system into recognizing HIV if it were exposed to it.
When HIV enters the body, it infiltrates immune cells and replicates within them. To the rest of the immune system, those cells still register as just typical T-cells. The rest of the immune system can’t see that the virus is spreading through the very cells meant to protect the body from illness. That, plus the armor of sugary glycoproteins encasing the virus, has made HIV nearly impervious to vaccination.
Then, those so-called “prime” shots were followed by a second shot that targets glycoprotein 140, on the most common HIV subtype (or clade) in Africa, clade C. In the Imbokodo trial, a total of 2,637 women from five sub-Saharan African countries received shots at baseline, 3 months, 6 months, and 1 year. Then researchers followed the women from month 7 to 2 years after their third dose, testing their blood to see if their immune systems had generated the immune response the vaccine was meant to induce – and whether such immune response was associated with lower rates of HIV.
When researchers looked at the first 2 years of data, they learned that the vaccine was safe. And they found a total of 114 new cases of HIV – 51 among women who received the vaccine and 63 among those who received a placebo. That’s a 25.2% efficacy rate – but it wasn’t statistically significant.
The results are frustrating, said Carl Dieffenbach, PhD, director of the AIDS division at the National Institute of Allergy and Infectious Diseases (NIAID). NIAID is one of the funders of the study.
“This [trial] is a little more confounding, in that there is this low level of statistically insignificant difference between vaccine and placebo that starts somewhere around month 9 and then just kind of indolently is maintained over the next 15 months,” he said in an interview. “That’s kind of frustrating. Does it mean there’s a signal or is this just chance? Because that’s what statistics tell us, not to believe your last data point.”
What this means for the future direction of vaccine research is unclear. A sister study to Imbokodo, called Mosaico, recently finished enrolling participants. Mosaico uses the same adenovirus 26 platform, but it’s loaded with different antigens and targets a different glycoprotein for a different HIV subtype. If that trial shows success, it could mean that the platform is right, but the targets in the Imbokodo vaccine were wrong.
Dr. Dieffenbach said that before NIAID decides what to do with Mosaico they’ve asked researchers to analyze the data on the people who did respond, to see if those people have some specific variant of HIV or some other biomarker that could be used to form the next iteration of an HIV vaccine candidate. Only after that will they make a decision about Mosaico.
But he added that it does make him wonder if vaccine approaches that rely on nonneutralizing antibodies like this one have a ceiling of effectiveness that’s just too low to alter the course of the epidemic.
“I think we’ve discovered that there’s not a floor to [these nonneutralizing approaches], but there probably is a ceiling,” he said. “I don’t know if we’re going to get better than” a 25%-29% efficacy rate with those approaches.
The Imbokodo findings reminded Mitchell Warren, executive director of the global HIV prevention nonprofit, AVAC, of the data released in January from the Antibody Mediated Prevention (AMP) trial. That trial pitted the broadly neutralizing antibody (bNAb) VCR01 against HIV – and mostly, it lost.
VCR01 worked only on HIV variants that 30% of participants had. But in those 30%, it was 75% effective at preventing HIV. Now you have Imbokodo, with its potential 25% activity against HIV, something that may have been a fluke. This, to Mr. Warren, requires a rethinking of the whole HIV vaccine enterprise while “doubling, tripling, quadrupling down” on the HIV prevention methods we know work, such as preexposure prophylaxis (PrEP).
Dr. Dieffenbach agreed. To clinicians, Dr. Dieffenbach said the message of this HIV vaccine trial is flush with urgency: “Get your HIV-negative, at-risk people on PrEP tomorrow.”
There are now two pills approved for HIV prevention, both of which have been found to be up to 99% effective when taken consistently. A third option, injectable cabotegravir (Vocabria), has been submitted to the Food and Drug Administration for approval. The federal Ready, Set, PrEP program makes the pill available for free for those who qualify, and recently the Biden administration reaffirmed that, under the Affordable Care Act, insurance companies should cover all costs associated with PrEP, including lab work and exam visits.
But for the 157 women who participated in the trial at Dr. Gill’s site in Masiphumelele, on the southwestern tip of South Africa, the trial was personal, said Jason Naidoo, community liaison officer at the Desmond Tutu HIV Foundation, which conducted a portion of the study. These were women whose parents, siblings, or children were living with HIV or had died from AIDS-defining illnesses, he said. Their lives were chaotic, traveling at a moment’s notice to hometowns on the Eastern Cape, an 11-hour car ride away – longer by bus – for traditional prayers, funerals, and other important events.
Mr. Naidoo remembers arranging buses for the women to return for scheduled clinic visits, leaving the Eastern Cape in the afternoon and arriving in Masiphumelele in the early morning hours, just to keep the clinic appointment. Then, they’d turn around and return east.
They did this for 3 years, he said.
“The fact that these participants have stuck to this and been dedicated amidst all of the chaos talks about their commitment to actually having a vaccine for HIV,” he said. “They know their own risk profile as young Black women in South Africa, and they understand the need for an intervention for the future generations.
“So you can understand the emotion and the sense of sadness, the disappointment – the incredible [dis]belief that this [the failure of the vaccine] could have happened, because the expectations are so, so high.”
For Dr. Gill, who is lead investigator for Imbokodo in Masiphumelele, the weariness toward vaccines is back. Another trial is underway for an HIV vaccine with a platform that was successful in COVID-19 – using messenger RNA (mRNA), like the Pfizer and Moderna COVID-19 vaccines did.
“I think we need to be careful,” she said, “thinking that the mRNA vaccines are going to crack it.”
Dr. Dieffenbach, Dr. Gill, and Mr. Naidoo have disclosed no relevant financial relationships. The study was funded by Janssen, a Johnson & Johnson company, with NIAID and the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape.com.
Last year, Katherine Gill, MBChB, an HIV prevention researcher in Cape Town, South Africa, realized how jaded she’d become to vaccine research when the Pfizer COVID-19 vaccine came back as 95% effective. In her career conducting HIV clinical trials, she had never seen anything like it. Even HIV prevention methods she had studied that had worked, such as the dapivirine ring, had an overall efficacy of 30%.
The COVID-19 success story started to soften her views toward another vaccine trial she was helping to conduct, a trial in HIV that used the same platform as Johnson & Johnson’s successful COVID-19 vaccine.
“When the COVID vaccine was cracked so quickly and seemingly quite easily, I did start to think, ‘Well, maybe … maybe this will work for HIV,’ “ she said in an interview.
That turned out to be false hope. The National Institutes of Health (NIH) announced that the trial Dr. Gill was helping to conduct, HVTN 705, was stopping early because it hadn’t generated enough of an immune response in participants to justify continuing. It was the second HIV vaccine to fail in the last year. It’s also the latest in what has been a litany of disappointments in the attempt to boost the human immune system to fight HIV without the need for ongoing HIV treatment.
In HVTN 705, known as the Imbokodo study (imbokodo is a Zulu word that’s part of a saying about women being strong as rocks), researchers used the platform made up of a common cold virus, adenovirus 26, to deliver a computer-generated mosaic of HIV antigens to participants’ immune systems. That mosaic of antigens is meant to goose the immune system into recognizing HIV if it were exposed to it.
When HIV enters the body, it infiltrates immune cells and replicates within them. To the rest of the immune system, those cells still register as just typical T-cells. The rest of the immune system can’t see that the virus is spreading through the very cells meant to protect the body from illness. That, plus the armor of sugary glycoproteins encasing the virus, has made HIV nearly impervious to vaccination.
Then, those so-called “prime” shots were followed by a second shot that targets glycoprotein 140, on the most common HIV subtype (or clade) in Africa, clade C. In the Imbokodo trial, a total of 2,637 women from five sub-Saharan African countries received shots at baseline, 3 months, 6 months, and 1 year. Then researchers followed the women from month 7 to 2 years after their third dose, testing their blood to see if their immune systems had generated the immune response the vaccine was meant to induce – and whether such immune response was associated with lower rates of HIV.
When researchers looked at the first 2 years of data, they learned that the vaccine was safe. And they found a total of 114 new cases of HIV – 51 among women who received the vaccine and 63 among those who received a placebo. That’s a 25.2% efficacy rate – but it wasn’t statistically significant.
The results are frustrating, said Carl Dieffenbach, PhD, director of the AIDS division at the National Institute of Allergy and Infectious Diseases (NIAID). NIAID is one of the funders of the study.
“This [trial] is a little more confounding, in that there is this low level of statistically insignificant difference between vaccine and placebo that starts somewhere around month 9 and then just kind of indolently is maintained over the next 15 months,” he said in an interview. “That’s kind of frustrating. Does it mean there’s a signal or is this just chance? Because that’s what statistics tell us, not to believe your last data point.”
What this means for the future direction of vaccine research is unclear. A sister study to Imbokodo, called Mosaico, recently finished enrolling participants. Mosaico uses the same adenovirus 26 platform, but it’s loaded with different antigens and targets a different glycoprotein for a different HIV subtype. If that trial shows success, it could mean that the platform is right, but the targets in the Imbokodo vaccine were wrong.
Dr. Dieffenbach said that before NIAID decides what to do with Mosaico they’ve asked researchers to analyze the data on the people who did respond, to see if those people have some specific variant of HIV or some other biomarker that could be used to form the next iteration of an HIV vaccine candidate. Only after that will they make a decision about Mosaico.
But he added that it does make him wonder if vaccine approaches that rely on nonneutralizing antibodies like this one have a ceiling of effectiveness that’s just too low to alter the course of the epidemic.
“I think we’ve discovered that there’s not a floor to [these nonneutralizing approaches], but there probably is a ceiling,” he said. “I don’t know if we’re going to get better than” a 25%-29% efficacy rate with those approaches.
The Imbokodo findings reminded Mitchell Warren, executive director of the global HIV prevention nonprofit, AVAC, of the data released in January from the Antibody Mediated Prevention (AMP) trial. That trial pitted the broadly neutralizing antibody (bNAb) VCR01 against HIV – and mostly, it lost.
VCR01 worked only on HIV variants that 30% of participants had. But in those 30%, it was 75% effective at preventing HIV. Now you have Imbokodo, with its potential 25% activity against HIV, something that may have been a fluke. This, to Mr. Warren, requires a rethinking of the whole HIV vaccine enterprise while “doubling, tripling, quadrupling down” on the HIV prevention methods we know work, such as preexposure prophylaxis (PrEP).
Dr. Dieffenbach agreed. To clinicians, Dr. Dieffenbach said the message of this HIV vaccine trial is flush with urgency: “Get your HIV-negative, at-risk people on PrEP tomorrow.”
There are now two pills approved for HIV prevention, both of which have been found to be up to 99% effective when taken consistently. A third option, injectable cabotegravir (Vocabria), has been submitted to the Food and Drug Administration for approval. The federal Ready, Set, PrEP program makes the pill available for free for those who qualify, and recently the Biden administration reaffirmed that, under the Affordable Care Act, insurance companies should cover all costs associated with PrEP, including lab work and exam visits.
But for the 157 women who participated in the trial at Dr. Gill’s site in Masiphumelele, on the southwestern tip of South Africa, the trial was personal, said Jason Naidoo, community liaison officer at the Desmond Tutu HIV Foundation, which conducted a portion of the study. These were women whose parents, siblings, or children were living with HIV or had died from AIDS-defining illnesses, he said. Their lives were chaotic, traveling at a moment’s notice to hometowns on the Eastern Cape, an 11-hour car ride away – longer by bus – for traditional prayers, funerals, and other important events.
Mr. Naidoo remembers arranging buses for the women to return for scheduled clinic visits, leaving the Eastern Cape in the afternoon and arriving in Masiphumelele in the early morning hours, just to keep the clinic appointment. Then, they’d turn around and return east.
They did this for 3 years, he said.
“The fact that these participants have stuck to this and been dedicated amidst all of the chaos talks about their commitment to actually having a vaccine for HIV,” he said. “They know their own risk profile as young Black women in South Africa, and they understand the need for an intervention for the future generations.
“So you can understand the emotion and the sense of sadness, the disappointment – the incredible [dis]belief that this [the failure of the vaccine] could have happened, because the expectations are so, so high.”
For Dr. Gill, who is lead investigator for Imbokodo in Masiphumelele, the weariness toward vaccines is back. Another trial is underway for an HIV vaccine with a platform that was successful in COVID-19 – using messenger RNA (mRNA), like the Pfizer and Moderna COVID-19 vaccines did.
“I think we need to be careful,” she said, “thinking that the mRNA vaccines are going to crack it.”
Dr. Dieffenbach, Dr. Gill, and Mr. Naidoo have disclosed no relevant financial relationships. The study was funded by Janssen, a Johnson & Johnson company, with NIAID and the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape.com.
Last year, Katherine Gill, MBChB, an HIV prevention researcher in Cape Town, South Africa, realized how jaded she’d become to vaccine research when the Pfizer COVID-19 vaccine came back as 95% effective. In her career conducting HIV clinical trials, she had never seen anything like it. Even HIV prevention methods she had studied that had worked, such as the dapivirine ring, had an overall efficacy of 30%.
The COVID-19 success story started to soften her views toward another vaccine trial she was helping to conduct, a trial in HIV that used the same platform as Johnson & Johnson’s successful COVID-19 vaccine.
“When the COVID vaccine was cracked so quickly and seemingly quite easily, I did start to think, ‘Well, maybe … maybe this will work for HIV,’ “ she said in an interview.
That turned out to be false hope. The National Institutes of Health (NIH) announced that the trial Dr. Gill was helping to conduct, HVTN 705, was stopping early because it hadn’t generated enough of an immune response in participants to justify continuing. It was the second HIV vaccine to fail in the last year. It’s also the latest in what has been a litany of disappointments in the attempt to boost the human immune system to fight HIV without the need for ongoing HIV treatment.
In HVTN 705, known as the Imbokodo study (imbokodo is a Zulu word that’s part of a saying about women being strong as rocks), researchers used the platform made up of a common cold virus, adenovirus 26, to deliver a computer-generated mosaic of HIV antigens to participants’ immune systems. That mosaic of antigens is meant to goose the immune system into recognizing HIV if it were exposed to it.
When HIV enters the body, it infiltrates immune cells and replicates within them. To the rest of the immune system, those cells still register as just typical T-cells. The rest of the immune system can’t see that the virus is spreading through the very cells meant to protect the body from illness. That, plus the armor of sugary glycoproteins encasing the virus, has made HIV nearly impervious to vaccination.
Then, those so-called “prime” shots were followed by a second shot that targets glycoprotein 140, on the most common HIV subtype (or clade) in Africa, clade C. In the Imbokodo trial, a total of 2,637 women from five sub-Saharan African countries received shots at baseline, 3 months, 6 months, and 1 year. Then researchers followed the women from month 7 to 2 years after their third dose, testing their blood to see if their immune systems had generated the immune response the vaccine was meant to induce – and whether such immune response was associated with lower rates of HIV.
When researchers looked at the first 2 years of data, they learned that the vaccine was safe. And they found a total of 114 new cases of HIV – 51 among women who received the vaccine and 63 among those who received a placebo. That’s a 25.2% efficacy rate – but it wasn’t statistically significant.
The results are frustrating, said Carl Dieffenbach, PhD, director of the AIDS division at the National Institute of Allergy and Infectious Diseases (NIAID). NIAID is one of the funders of the study.
“This [trial] is a little more confounding, in that there is this low level of statistically insignificant difference between vaccine and placebo that starts somewhere around month 9 and then just kind of indolently is maintained over the next 15 months,” he said in an interview. “That’s kind of frustrating. Does it mean there’s a signal or is this just chance? Because that’s what statistics tell us, not to believe your last data point.”
What this means for the future direction of vaccine research is unclear. A sister study to Imbokodo, called Mosaico, recently finished enrolling participants. Mosaico uses the same adenovirus 26 platform, but it’s loaded with different antigens and targets a different glycoprotein for a different HIV subtype. If that trial shows success, it could mean that the platform is right, but the targets in the Imbokodo vaccine were wrong.
Dr. Dieffenbach said that before NIAID decides what to do with Mosaico they’ve asked researchers to analyze the data on the people who did respond, to see if those people have some specific variant of HIV or some other biomarker that could be used to form the next iteration of an HIV vaccine candidate. Only after that will they make a decision about Mosaico.
But he added that it does make him wonder if vaccine approaches that rely on nonneutralizing antibodies like this one have a ceiling of effectiveness that’s just too low to alter the course of the epidemic.
“I think we’ve discovered that there’s not a floor to [these nonneutralizing approaches], but there probably is a ceiling,” he said. “I don’t know if we’re going to get better than” a 25%-29% efficacy rate with those approaches.
The Imbokodo findings reminded Mitchell Warren, executive director of the global HIV prevention nonprofit, AVAC, of the data released in January from the Antibody Mediated Prevention (AMP) trial. That trial pitted the broadly neutralizing antibody (bNAb) VCR01 against HIV – and mostly, it lost.
VCR01 worked only on HIV variants that 30% of participants had. But in those 30%, it was 75% effective at preventing HIV. Now you have Imbokodo, with its potential 25% activity against HIV, something that may have been a fluke. This, to Mr. Warren, requires a rethinking of the whole HIV vaccine enterprise while “doubling, tripling, quadrupling down” on the HIV prevention methods we know work, such as preexposure prophylaxis (PrEP).
Dr. Dieffenbach agreed. To clinicians, Dr. Dieffenbach said the message of this HIV vaccine trial is flush with urgency: “Get your HIV-negative, at-risk people on PrEP tomorrow.”
There are now two pills approved for HIV prevention, both of which have been found to be up to 99% effective when taken consistently. A third option, injectable cabotegravir (Vocabria), has been submitted to the Food and Drug Administration for approval. The federal Ready, Set, PrEP program makes the pill available for free for those who qualify, and recently the Biden administration reaffirmed that, under the Affordable Care Act, insurance companies should cover all costs associated with PrEP, including lab work and exam visits.
But for the 157 women who participated in the trial at Dr. Gill’s site in Masiphumelele, on the southwestern tip of South Africa, the trial was personal, said Jason Naidoo, community liaison officer at the Desmond Tutu HIV Foundation, which conducted a portion of the study. These were women whose parents, siblings, or children were living with HIV or had died from AIDS-defining illnesses, he said. Their lives were chaotic, traveling at a moment’s notice to hometowns on the Eastern Cape, an 11-hour car ride away – longer by bus – for traditional prayers, funerals, and other important events.
Mr. Naidoo remembers arranging buses for the women to return for scheduled clinic visits, leaving the Eastern Cape in the afternoon and arriving in Masiphumelele in the early morning hours, just to keep the clinic appointment. Then, they’d turn around and return east.
They did this for 3 years, he said.
“The fact that these participants have stuck to this and been dedicated amidst all of the chaos talks about their commitment to actually having a vaccine for HIV,” he said. “They know their own risk profile as young Black women in South Africa, and they understand the need for an intervention for the future generations.
“So you can understand the emotion and the sense of sadness, the disappointment – the incredible [dis]belief that this [the failure of the vaccine] could have happened, because the expectations are so, so high.”
For Dr. Gill, who is lead investigator for Imbokodo in Masiphumelele, the weariness toward vaccines is back. Another trial is underway for an HIV vaccine with a platform that was successful in COVID-19 – using messenger RNA (mRNA), like the Pfizer and Moderna COVID-19 vaccines did.
“I think we need to be careful,” she said, “thinking that the mRNA vaccines are going to crack it.”
Dr. Dieffenbach, Dr. Gill, and Mr. Naidoo have disclosed no relevant financial relationships. The study was funded by Janssen, a Johnson & Johnson company, with NIAID and the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape.com.
Antiviral Therapy Improves Hepatocellular Cancer Survival
Hepatocellular cancer (HCC) is the most common type of hepatic cancers, accounting for 65% of all hepatic cancers.1 Among all cancers, HCC is one of the fastest growing causes of death in the United States, and the rate of new HCC cases are on the rise over several decades.2 There are many risk factors leading to HCC, including alcohol use, obesity, and smoking. Infection with hepatitis C virus (HCV) poses a significant risk.1
The pathogenesis of HCV-induced carcinogenesis is mediated by a unique host-induced immunologic response. Viral replication induces production of inflammatory factors, such as tumor necrosis factor (TNF-α), interferon (IFN), and oxidative stress on hepatocytes, resulting in cell injury, death, and regeneration. Repetitive cycles of cellular death and regeneration induce fibrosis, which may lead to cirrhosis.3 Hence, early treatment of HCV infection and achieving sustained virologic response (SVR) may lead to decreased incidence and mortality associated with HCC.
Treatment of HCV infection has become more effective with the development of direct-acting antivirals (DAAs) leading to SVR in > 90% of patients compared with 40 to 50% with IFN-based treatment.4,5 DAAs have been proved safe and highly effective in eradicating HCV infection even in patients with advanced liver disease with decompensated cirrhosis.6 Although achieving SVR indicates a complete cure from chronic HCV infection, several studies have shown subsequent risk of developing HCC persists even after successful HCV treatment.7-9 Some studies show that using DAAs to achieve SVR in patients with HCV infection leads to a decreased relative risk of HCC development compared with patients who do not receive treatment.10-12 But data on HCC risk following DAA-induced SVR vs IFN-induced SVR are somewhat conflicting.
Much of the information regarding the association between SVR and HCC has been gleaned from large data banks without accounting for individual patient characteristics that can be obtained through full chart review. Due to small sample sizes in many chart review studies, the impact that SVR from DAA therapy has on the progression and severity of HCC is not entirely clear. The aim of our study is to evaluate the effect of HCV treatment and SVR status on overall survival (OS) in patients with HCC. Second, we aim to compare survival benefits, if any exist, among the 2 major HCV treatment modalities (IFN vs DAA).
Methods
We performed a retrospective review of patients at Memphis Veterans Affairs Medical Center (VAMC) in Tennessee to determine whether treatment for HCV infection in general, and achieving SVR in particular, makes a difference in progression, recurrence, or OS among patients with HCV infection who develop HCC. We identified 111 patients with a diagnosis of both HCV and new or recurrent HCC lesions from November 2008 to March 2019 (Table 1). We divided these patients based on their HCV treatment status, SVR status, and treatment types (IFN vs DAA).
The inclusion criteria were patients aged > 18 years treated at the Memphis VAMC who have HCV infection and developed HCC. Exclusion criteria were patients who developed HCC from other causes such as alcoholic steatohepatitis, hepatitis B virus infection, hemochromatosis, patients without HCV infection, and patients who were not established at the Memphis VAMC. This protocol was approved by the Memphis VAMC Institutional Review Board.
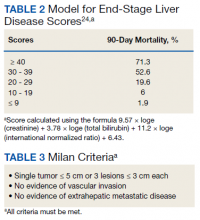
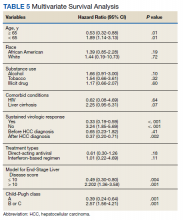
HCC diagnosis was determined using International Classification of Diseases codes (9th revision: 155 and 155.2; 10th revision: CD 22 and 22.9). We also used records of multidisciplinary gastrointestinal malignancy tumor conferences to identify patient who had been diagnosed and treated for HCV infection. We identified patients who were treated with DAA vs IFN as well as patients who had achieved SVR (classified as having negative HCV RNA tests at the end of DAA treatment). We were unable to evaluate Barcelona Clinic Liver Cancer staging since this required documented performance status that was not available in many patient records. We selected cases consistent with both treatment for HCV infection and subsequent development of HCC. Patient data included age; OS time; HIV status HCV genotype; time and status of progression to HCC; type and duration of treatment; and alcohol, tobacco, and drug use. Disease status was measured using the Model for End-Stage Liver Disease (MELD) score (Table 2), Milan criteria (Table 3), and Child-Pugh score (Table 4).
Statistical Analysis
OS was measured from the date of HCC diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was defined from the date of HCC treatment initiation to the date of first HCC recurrence. We compared survival data for the SVR and non-SVR subgroups, the HCV treatment vs non-HCV treatment subgroups, and the IFN therapy vs DAA therapy subgroups, using the Kaplan-Meier method. The differences between subgroups were assessed using a log-rank test. Multivariate analysis using Cox proportional hazards regression model was used to identify factors that had significant impact on OS. Those factors included age; race; alcohol, tobacco, and illicit drug use; SVR status; HCV treatment status; IFN-based regimen vs DAA; MELD, and Child-Pugh scores. The results were expressed as hazard ratios (HRs) and 95% CI. Calculations were made using Statistical Analysis SAS and IBM SPSS software.
Results
The study included 111 patients. The mean age was 65.7 years; all were male and half of were Black patients. The gender imbalance was due to the predominantly male patient population at Memphis VAMC. Among 111 patients with HCV infection and HCC, 68 patients were treated for HCV infection and had significantly improved OS and PFS compared with the nontreatment group. The median 5-year OS was 44.6 months (95% CI, 966-3202) in the treated HCV infection group compared with 15.1 months in the untreated HCV infection group with a Wilcoxon P = .0005 (Figure 1). Similarly, patients treated for HCV infection had a significantly better 5-year PFS of 15.3 months (95% CI, 294-726) compared with the nontreatment group 9.5 months (95% CI, 205-405) with a Wilcoxon P = .04 (Figure 2).
Among 68 patients treated for HCV infection, 51 achieved SVR, and 34 achieved SVR after the diagnosis of HCC. Patients who achieved SVR had an improved 5-year OS when compared with patients who did not achieve SVR (median 65.8 months [95% CI, 1222-NA] vs 15.7 months [95% CI, 242-853], Wilcoxon P < .001) (Figure 3). Similarly, patients with SVR had improved 5-year PFS when compared with the non-SVR group (median 20.5 months [95% CI, 431-914] vs 8.9 months [95% CI, 191-340], Wilcoxon P = .007 (Figure 4). Achievement of SVR after HCC diagnosis suggests a significantly improved OS (HR 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI, 0.23-1.82, P = .41)
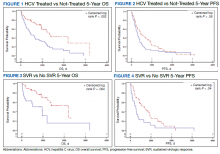
Multivariate Cox regression was used to determine factors with significant survival impact. Advanced age at diagnosis (aged ≥ 65 years) (HR, 0.53; 95% CI, 0.320-0.880; P = .01), SVR status (HR, 0.33; 95% CI, 0.190-0.587; P < .001), achieving SVR after HCC diagnosis (HR, 0.37; 95% CI, 0.20-0.71; P = .002), low MELD score (< 10) (HR, 0.49; 95% CI, 0.30-0.80; P = .004) and low Child-Pugh score (class A) (HR, 0.39; 95% CI, 0.24-0.64; P = .001) have a significant positive impact on OS. Survival was not significantly influenced by race, tobacco, drug use, HIV or cirrhosis status, or HCV treatment type. In addition, higher Child-Pugh class (B or C), higher MELD score (> 10), and younger age at diagnosis (< 65 years) have a negative impact on survival outcome (Table 5).
Discussion
The survival benefit of HCV eradication and achieving SVR status has been well established in patients with HCC.13 In a retrospective cohort study of 250 patients with HCV infection who had received curative treatment for HCC, multivariate analysis demonstrated that achieving SVR is an independent predictor of OS.14 The 3-year and 5-year OS rates were 97% and 94% for the SVR group, and 91% and 60% for the non‐SVR group, respectively (P < .001). Similarly, according to Sou and colleagues, of 122 patients with HCV-related HCC, patients with SVR had longer OS than patients with no SVR (P = .04).15 One of the hypotheses that could explain the survival benefit in patients who achieved SVR is the effect of achieving SVR in reducing persistent liver inflammation and associated liver mortality, and therefore lowering risks of complication in patients with HCC.16 In our study, multivariate analysis shows that achieving SVR is associated with significant improved OS (HR, 0.33). In contrast, patients with HCC who have not achieved SVR are associated with worse survival (HR, 3.24). This finding supports early treatment of HCV to obtain SVR in HCV-related patients with HCC, even after development of HCC.
Among 68 patients treated for HCV infection, 45 patients were treated after HCC diagnosis, and 34 patients achieved SVR after HCC diagnosis. The average time between HCV infection treatment after HCC diagnosis was 6 months. Our data suggested that achievement of SVR after HCC diagnosis suggests an improved OS (HR, 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI,0.23-1.82; P = .41). This lack of statistical significance is likely due to small sample size of patients achieving SVR prior to HCC diagnosis. Our results are consistent with the findings regarding the efficacy and timing of DAA treatment in patients with active HCC. According to Singal and colleagues, achieving SVR after DAA therapy may result in improved liver function and facilitate additional HCC-directed therapy, which potentially improves survival.17-19
Nagaoki and colleagues found that there was no significant difference in OS in patients with HCC between the DAA and IFN groups. According to the study, the 3-year and 5-year OS rates were 96% and 96% for DAA patients and 93% and 73% for IFN patients, respectively (P = .16).14 This finding is consistent with the results of our study. HCV treatment type (IFN vs DAA) was not found to be associated with either OS or PFS time, regardless of time period.
A higher MELD score (> 10) and a higher Child-Pugh class (B or C) score are associated with worse survival outcome regardless of SVR status. While patients with a low MELD score (≤ 10) have a better survival rate (HR 0.49), a higher MELD score has a significantly higher HR and therefore worse survival outcomes (HR, 2.20). Similarly, patients with Child-Pugh A (HR, 0.39) have a better survival outcome compared with those patients with Child-Pugh class B or C (HR, 2.57). This finding is consistent with results of multiple studies indicating that advanced liver disease, as measured by a high MELD score and Child-Pugh class score, can be used to predict the survival outcome in patients with HCV-related HCC.20-22
Unlike other studies that look at a single prognostic variable, our study evaluated prognostic impacts of multiple variables (age, SVR status, the order of SVR in relation to HCC development, HCV treatment type, MELD score and Child-Pugh class) in patients with HCC. The study included patients treated for HCV after development of HCC along with other multiple variables leading to OS benefit. It is one of the only studies in the United States that compared 5-year OS and PFS among patients with HCC treated for HCV and achieved SVR. The studies by Nagaoki and colleagues and Sou and colleagues were conducted in Japan, and some of their subset analyses were univariate. Among our study population of veterans, 50% were African American patients, suggesting that they may have similar OS benefit when compared to White patients with HCC and HCV treatment.
Limitations
Our findings were limited in that our study population is too small to conduct further subset analysis that would allow statistical significance of those subsets, such as the suggested benefit of SVR in patients who presented with HCC after antiviral therapy. Another limitation is the all-male population, likely a result of the older veteran population at the Memphis VAMC. The mean age at diagnosis was 65 years, which is slightly higher than the general population. Compared to the SEER database, HCC is most frequently diagnosed among people aged 55 to 64 years.23 The age difference was likely due to our aging veteran population.
Further studies are needed to determine the significance of SVR on HCC recurrence and treatment. Immunotherapy is now first-line treatment for patients with local advanced HCC. All the immunotherapy studies excluded patients with active HCV infection. Hence, we need more data on HCV treatment timing among patients scheduled to start treatment with immunotherapy.
Conclusions
In a population of older veterans, treatment of HCV infection leads to OS benefit among patients with HCC. In addition, patients with HCV infection who achieve SVR have an OS benefit over patients unable to achieve SVR. The type of treatment, DAA vs IFN-based regimen, did not show significant survival benefit.
1. Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. Published 2017 May 29. doi:10.4103/jcar.JCar_9_16
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
3. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674-687. doi:10.1038/nrc1934
4. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis c virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
5. Kouris G, Hydery T, Greenwood BC, et al. Effectiveness of Ledipasvir/Sofosbuvir and predictors of treatment failure in members with hepatitis C genotype 1 infection: a retrospective cohort study in a medicaid population. J Manag Care Spec Pharm. 2018;24(7):591-597. doi:10.18553/jmcp.2018.24.7.591
6. Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis c virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology. 2017;152(6):1372-1382.e2. doi:10.1053/j.gastro.2017.01.050
7. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155(5):1436-1450.e6. doi:10.1053/j.gastro.2018.07.01510.
8. Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2018;68(4):646-654. doi:10.1016/j.jhep.2017.10.033
9. Romano A, Angeli P, Piovesan S, et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J Hepatol. 2018;69(2):345-352. doi:10.1016/j.jhep.2018.03.009
10. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996-1005.e1. doi:10.1053/j.gastro.2017.06.0122
11. Singh S, Nautiyal A, Loke YK. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. Frontline Gastroenterol. 2018;9(4):262-270. doi:10.1136/flgastro-2018-101017
12. Kuftinec G, Loehfelm T, Corwin M, et al. De novo hepatocellular carcinoma occurrence in hepatitis C cirrhotics treated with direct-acting antiviral agents. Hepat Oncol. 2018;5(1):HEP06. Published 2018 Jul 25. doi:10.2217/hep-2018-00033
13. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
14. Nagaoki Y, Imamura M, Nishida Y, et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: comparison with interferon-based therapy. J Med Virol. 2019;91(4):650-658. doi:10.1002/jmv.25352
15. Sou FM, Wu CK, Chang KC, et al. Clinical characteristics and prognosis of HCC occurrence after antiviral therapy for HCV patients between sustained and non-sustained responders. J Formos Med Assoc. 2019;118(1 Pt 3):504-513. doi:10.1016/j.jfma.2018.10.017
16. Roche B, Coilly A, Duclos-Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38(suppl 1):139-145. doi:10.1111/liv.13659
17. Singal AG, Lim JK, Kanwal F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology. 2019;156(8):2149-2157. doi:10.1053/j.gastro.2019.02.046
18. Toyoda H, Kumada T, Hayashi K, et al. Characteristics and prognosis of hepatocellular carcinoma detected in sustained responders to interferon therapy for chronic hepatitis C. Cancer Detect Prev. 2003;27(6):498-502. doi:10.1016/j.cdp.2003.09.00719. Okamura Y, Sugiura T, Ito T, et al. The achievement of a sustained virological response either before or after hepatectomy improves the prognosis of patients with primary hepatitis C virus-related hepatocellular carcinoma. Ann Surg Oncol. 2019; 26(13):4566-4575. doi:10.1245/s10434-019-07911-w
20. Wray CJ, Harvin JA, Silberfein EJ, Ko TC, Kao LS. Pilot prognostic model of extremely poor survival among high-risk hepatocellular carcinoma patients. Cancer. 2012;118(24):6118-6125. doi:10.1002/cncr.27649
21. Kim JH, Kim JH, Choi JH, et al. Value of the model for end-stage liver disease for predicting survival in hepatocellular carcinoma patients treated with transarterial chemoembolization. Scand J Gastroenterol. 2009;44(3):346-357. doi:10.1080/00365520802530838
22. Vogeler M, Mohr I, Pfeiffenberger J, et al. Applicability of scoring systems predicting outcome of transarterial chemoembolization for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(4):1033-1050. doi:10.1007/s00432-020-03135-8
23. National Institutes of Health, Surveillance, Epidemiology, and End Results. Cancer stat facts: cancer of the liver and intrahepatic bile duct. Accessed July 15, 2021. https://seer.cancer.gov/statfacts/html/livibd.html
24. Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
Hepatocellular cancer (HCC) is the most common type of hepatic cancers, accounting for 65% of all hepatic cancers.1 Among all cancers, HCC is one of the fastest growing causes of death in the United States, and the rate of new HCC cases are on the rise over several decades.2 There are many risk factors leading to HCC, including alcohol use, obesity, and smoking. Infection with hepatitis C virus (HCV) poses a significant risk.1
The pathogenesis of HCV-induced carcinogenesis is mediated by a unique host-induced immunologic response. Viral replication induces production of inflammatory factors, such as tumor necrosis factor (TNF-α), interferon (IFN), and oxidative stress on hepatocytes, resulting in cell injury, death, and regeneration. Repetitive cycles of cellular death and regeneration induce fibrosis, which may lead to cirrhosis.3 Hence, early treatment of HCV infection and achieving sustained virologic response (SVR) may lead to decreased incidence and mortality associated with HCC.
Treatment of HCV infection has become more effective with the development of direct-acting antivirals (DAAs) leading to SVR in > 90% of patients compared with 40 to 50% with IFN-based treatment.4,5 DAAs have been proved safe and highly effective in eradicating HCV infection even in patients with advanced liver disease with decompensated cirrhosis.6 Although achieving SVR indicates a complete cure from chronic HCV infection, several studies have shown subsequent risk of developing HCC persists even after successful HCV treatment.7-9 Some studies show that using DAAs to achieve SVR in patients with HCV infection leads to a decreased relative risk of HCC development compared with patients who do not receive treatment.10-12 But data on HCC risk following DAA-induced SVR vs IFN-induced SVR are somewhat conflicting.
Much of the information regarding the association between SVR and HCC has been gleaned from large data banks without accounting for individual patient characteristics that can be obtained through full chart review. Due to small sample sizes in many chart review studies, the impact that SVR from DAA therapy has on the progression and severity of HCC is not entirely clear. The aim of our study is to evaluate the effect of HCV treatment and SVR status on overall survival (OS) in patients with HCC. Second, we aim to compare survival benefits, if any exist, among the 2 major HCV treatment modalities (IFN vs DAA).
Methods
We performed a retrospective review of patients at Memphis Veterans Affairs Medical Center (VAMC) in Tennessee to determine whether treatment for HCV infection in general, and achieving SVR in particular, makes a difference in progression, recurrence, or OS among patients with HCV infection who develop HCC. We identified 111 patients with a diagnosis of both HCV and new or recurrent HCC lesions from November 2008 to March 2019 (Table 1). We divided these patients based on their HCV treatment status, SVR status, and treatment types (IFN vs DAA).
The inclusion criteria were patients aged > 18 years treated at the Memphis VAMC who have HCV infection and developed HCC. Exclusion criteria were patients who developed HCC from other causes such as alcoholic steatohepatitis, hepatitis B virus infection, hemochromatosis, patients without HCV infection, and patients who were not established at the Memphis VAMC. This protocol was approved by the Memphis VAMC Institutional Review Board.
HCC diagnosis was determined using International Classification of Diseases codes (9th revision: 155 and 155.2; 10th revision: CD 22 and 22.9). We also used records of multidisciplinary gastrointestinal malignancy tumor conferences to identify patient who had been diagnosed and treated for HCV infection. We identified patients who were treated with DAA vs IFN as well as patients who had achieved SVR (classified as having negative HCV RNA tests at the end of DAA treatment). We were unable to evaluate Barcelona Clinic Liver Cancer staging since this required documented performance status that was not available in many patient records. We selected cases consistent with both treatment for HCV infection and subsequent development of HCC. Patient data included age; OS time; HIV status HCV genotype; time and status of progression to HCC; type and duration of treatment; and alcohol, tobacco, and drug use. Disease status was measured using the Model for End-Stage Liver Disease (MELD) score (Table 2), Milan criteria (Table 3), and Child-Pugh score (Table 4).
Statistical Analysis
OS was measured from the date of HCC diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was defined from the date of HCC treatment initiation to the date of first HCC recurrence. We compared survival data for the SVR and non-SVR subgroups, the HCV treatment vs non-HCV treatment subgroups, and the IFN therapy vs DAA therapy subgroups, using the Kaplan-Meier method. The differences between subgroups were assessed using a log-rank test. Multivariate analysis using Cox proportional hazards regression model was used to identify factors that had significant impact on OS. Those factors included age; race; alcohol, tobacco, and illicit drug use; SVR status; HCV treatment status; IFN-based regimen vs DAA; MELD, and Child-Pugh scores. The results were expressed as hazard ratios (HRs) and 95% CI. Calculations were made using Statistical Analysis SAS and IBM SPSS software.
Results
The study included 111 patients. The mean age was 65.7 years; all were male and half of were Black patients. The gender imbalance was due to the predominantly male patient population at Memphis VAMC. Among 111 patients with HCV infection and HCC, 68 patients were treated for HCV infection and had significantly improved OS and PFS compared with the nontreatment group. The median 5-year OS was 44.6 months (95% CI, 966-3202) in the treated HCV infection group compared with 15.1 months in the untreated HCV infection group with a Wilcoxon P = .0005 (Figure 1). Similarly, patients treated for HCV infection had a significantly better 5-year PFS of 15.3 months (95% CI, 294-726) compared with the nontreatment group 9.5 months (95% CI, 205-405) with a Wilcoxon P = .04 (Figure 2).
Among 68 patients treated for HCV infection, 51 achieved SVR, and 34 achieved SVR after the diagnosis of HCC. Patients who achieved SVR had an improved 5-year OS when compared with patients who did not achieve SVR (median 65.8 months [95% CI, 1222-NA] vs 15.7 months [95% CI, 242-853], Wilcoxon P < .001) (Figure 3). Similarly, patients with SVR had improved 5-year PFS when compared with the non-SVR group (median 20.5 months [95% CI, 431-914] vs 8.9 months [95% CI, 191-340], Wilcoxon P = .007 (Figure 4). Achievement of SVR after HCC diagnosis suggests a significantly improved OS (HR 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI, 0.23-1.82, P = .41)
Multivariate Cox regression was used to determine factors with significant survival impact. Advanced age at diagnosis (aged ≥ 65 years) (HR, 0.53; 95% CI, 0.320-0.880; P = .01), SVR status (HR, 0.33; 95% CI, 0.190-0.587; P < .001), achieving SVR after HCC diagnosis (HR, 0.37; 95% CI, 0.20-0.71; P = .002), low MELD score (< 10) (HR, 0.49; 95% CI, 0.30-0.80; P = .004) and low Child-Pugh score (class A) (HR, 0.39; 95% CI, 0.24-0.64; P = .001) have a significant positive impact on OS. Survival was not significantly influenced by race, tobacco, drug use, HIV or cirrhosis status, or HCV treatment type. In addition, higher Child-Pugh class (B or C), higher MELD score (> 10), and younger age at diagnosis (< 65 years) have a negative impact on survival outcome (Table 5).
Discussion
The survival benefit of HCV eradication and achieving SVR status has been well established in patients with HCC.13 In a retrospective cohort study of 250 patients with HCV infection who had received curative treatment for HCC, multivariate analysis demonstrated that achieving SVR is an independent predictor of OS.14 The 3-year and 5-year OS rates were 97% and 94% for the SVR group, and 91% and 60% for the non‐SVR group, respectively (P < .001). Similarly, according to Sou and colleagues, of 122 patients with HCV-related HCC, patients with SVR had longer OS than patients with no SVR (P = .04).15 One of the hypotheses that could explain the survival benefit in patients who achieved SVR is the effect of achieving SVR in reducing persistent liver inflammation and associated liver mortality, and therefore lowering risks of complication in patients with HCC.16 In our study, multivariate analysis shows that achieving SVR is associated with significant improved OS (HR, 0.33). In contrast, patients with HCC who have not achieved SVR are associated with worse survival (HR, 3.24). This finding supports early treatment of HCV to obtain SVR in HCV-related patients with HCC, even after development of HCC.
Among 68 patients treated for HCV infection, 45 patients were treated after HCC diagnosis, and 34 patients achieved SVR after HCC diagnosis. The average time between HCV infection treatment after HCC diagnosis was 6 months. Our data suggested that achievement of SVR after HCC diagnosis suggests an improved OS (HR, 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI,0.23-1.82; P = .41). This lack of statistical significance is likely due to small sample size of patients achieving SVR prior to HCC diagnosis. Our results are consistent with the findings regarding the efficacy and timing of DAA treatment in patients with active HCC. According to Singal and colleagues, achieving SVR after DAA therapy may result in improved liver function and facilitate additional HCC-directed therapy, which potentially improves survival.17-19
Nagaoki and colleagues found that there was no significant difference in OS in patients with HCC between the DAA and IFN groups. According to the study, the 3-year and 5-year OS rates were 96% and 96% for DAA patients and 93% and 73% for IFN patients, respectively (P = .16).14 This finding is consistent with the results of our study. HCV treatment type (IFN vs DAA) was not found to be associated with either OS or PFS time, regardless of time period.
A higher MELD score (> 10) and a higher Child-Pugh class (B or C) score are associated with worse survival outcome regardless of SVR status. While patients with a low MELD score (≤ 10) have a better survival rate (HR 0.49), a higher MELD score has a significantly higher HR and therefore worse survival outcomes (HR, 2.20). Similarly, patients with Child-Pugh A (HR, 0.39) have a better survival outcome compared with those patients with Child-Pugh class B or C (HR, 2.57). This finding is consistent with results of multiple studies indicating that advanced liver disease, as measured by a high MELD score and Child-Pugh class score, can be used to predict the survival outcome in patients with HCV-related HCC.20-22
Unlike other studies that look at a single prognostic variable, our study evaluated prognostic impacts of multiple variables (age, SVR status, the order of SVR in relation to HCC development, HCV treatment type, MELD score and Child-Pugh class) in patients with HCC. The study included patients treated for HCV after development of HCC along with other multiple variables leading to OS benefit. It is one of the only studies in the United States that compared 5-year OS and PFS among patients with HCC treated for HCV and achieved SVR. The studies by Nagaoki and colleagues and Sou and colleagues were conducted in Japan, and some of their subset analyses were univariate. Among our study population of veterans, 50% were African American patients, suggesting that they may have similar OS benefit when compared to White patients with HCC and HCV treatment.
Limitations
Our findings were limited in that our study population is too small to conduct further subset analysis that would allow statistical significance of those subsets, such as the suggested benefit of SVR in patients who presented with HCC after antiviral therapy. Another limitation is the all-male population, likely a result of the older veteran population at the Memphis VAMC. The mean age at diagnosis was 65 years, which is slightly higher than the general population. Compared to the SEER database, HCC is most frequently diagnosed among people aged 55 to 64 years.23 The age difference was likely due to our aging veteran population.
Further studies are needed to determine the significance of SVR on HCC recurrence and treatment. Immunotherapy is now first-line treatment for patients with local advanced HCC. All the immunotherapy studies excluded patients with active HCV infection. Hence, we need more data on HCV treatment timing among patients scheduled to start treatment with immunotherapy.
Conclusions
In a population of older veterans, treatment of HCV infection leads to OS benefit among patients with HCC. In addition, patients with HCV infection who achieve SVR have an OS benefit over patients unable to achieve SVR. The type of treatment, DAA vs IFN-based regimen, did not show significant survival benefit.
Hepatocellular cancer (HCC) is the most common type of hepatic cancers, accounting for 65% of all hepatic cancers.1 Among all cancers, HCC is one of the fastest growing causes of death in the United States, and the rate of new HCC cases are on the rise over several decades.2 There are many risk factors leading to HCC, including alcohol use, obesity, and smoking. Infection with hepatitis C virus (HCV) poses a significant risk.1
The pathogenesis of HCV-induced carcinogenesis is mediated by a unique host-induced immunologic response. Viral replication induces production of inflammatory factors, such as tumor necrosis factor (TNF-α), interferon (IFN), and oxidative stress on hepatocytes, resulting in cell injury, death, and regeneration. Repetitive cycles of cellular death and regeneration induce fibrosis, which may lead to cirrhosis.3 Hence, early treatment of HCV infection and achieving sustained virologic response (SVR) may lead to decreased incidence and mortality associated with HCC.
Treatment of HCV infection has become more effective with the development of direct-acting antivirals (DAAs) leading to SVR in > 90% of patients compared with 40 to 50% with IFN-based treatment.4,5 DAAs have been proved safe and highly effective in eradicating HCV infection even in patients with advanced liver disease with decompensated cirrhosis.6 Although achieving SVR indicates a complete cure from chronic HCV infection, several studies have shown subsequent risk of developing HCC persists even after successful HCV treatment.7-9 Some studies show that using DAAs to achieve SVR in patients with HCV infection leads to a decreased relative risk of HCC development compared with patients who do not receive treatment.10-12 But data on HCC risk following DAA-induced SVR vs IFN-induced SVR are somewhat conflicting.
Much of the information regarding the association between SVR and HCC has been gleaned from large data banks without accounting for individual patient characteristics that can be obtained through full chart review. Due to small sample sizes in many chart review studies, the impact that SVR from DAA therapy has on the progression and severity of HCC is not entirely clear. The aim of our study is to evaluate the effect of HCV treatment and SVR status on overall survival (OS) in patients with HCC. Second, we aim to compare survival benefits, if any exist, among the 2 major HCV treatment modalities (IFN vs DAA).
Methods
We performed a retrospective review of patients at Memphis Veterans Affairs Medical Center (VAMC) in Tennessee to determine whether treatment for HCV infection in general, and achieving SVR in particular, makes a difference in progression, recurrence, or OS among patients with HCV infection who develop HCC. We identified 111 patients with a diagnosis of both HCV and new or recurrent HCC lesions from November 2008 to March 2019 (Table 1). We divided these patients based on their HCV treatment status, SVR status, and treatment types (IFN vs DAA).
The inclusion criteria were patients aged > 18 years treated at the Memphis VAMC who have HCV infection and developed HCC. Exclusion criteria were patients who developed HCC from other causes such as alcoholic steatohepatitis, hepatitis B virus infection, hemochromatosis, patients without HCV infection, and patients who were not established at the Memphis VAMC. This protocol was approved by the Memphis VAMC Institutional Review Board.
HCC diagnosis was determined using International Classification of Diseases codes (9th revision: 155 and 155.2; 10th revision: CD 22 and 22.9). We also used records of multidisciplinary gastrointestinal malignancy tumor conferences to identify patient who had been diagnosed and treated for HCV infection. We identified patients who were treated with DAA vs IFN as well as patients who had achieved SVR (classified as having negative HCV RNA tests at the end of DAA treatment). We were unable to evaluate Barcelona Clinic Liver Cancer staging since this required documented performance status that was not available in many patient records. We selected cases consistent with both treatment for HCV infection and subsequent development of HCC. Patient data included age; OS time; HIV status HCV genotype; time and status of progression to HCC; type and duration of treatment; and alcohol, tobacco, and drug use. Disease status was measured using the Model for End-Stage Liver Disease (MELD) score (Table 2), Milan criteria (Table 3), and Child-Pugh score (Table 4).
Statistical Analysis
OS was measured from the date of HCC diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was defined from the date of HCC treatment initiation to the date of first HCC recurrence. We compared survival data for the SVR and non-SVR subgroups, the HCV treatment vs non-HCV treatment subgroups, and the IFN therapy vs DAA therapy subgroups, using the Kaplan-Meier method. The differences between subgroups were assessed using a log-rank test. Multivariate analysis using Cox proportional hazards regression model was used to identify factors that had significant impact on OS. Those factors included age; race; alcohol, tobacco, and illicit drug use; SVR status; HCV treatment status; IFN-based regimen vs DAA; MELD, and Child-Pugh scores. The results were expressed as hazard ratios (HRs) and 95% CI. Calculations were made using Statistical Analysis SAS and IBM SPSS software.
Results
The study included 111 patients. The mean age was 65.7 years; all were male and half of were Black patients. The gender imbalance was due to the predominantly male patient population at Memphis VAMC. Among 111 patients with HCV infection and HCC, 68 patients were treated for HCV infection and had significantly improved OS and PFS compared with the nontreatment group. The median 5-year OS was 44.6 months (95% CI, 966-3202) in the treated HCV infection group compared with 15.1 months in the untreated HCV infection group with a Wilcoxon P = .0005 (Figure 1). Similarly, patients treated for HCV infection had a significantly better 5-year PFS of 15.3 months (95% CI, 294-726) compared with the nontreatment group 9.5 months (95% CI, 205-405) with a Wilcoxon P = .04 (Figure 2).
Among 68 patients treated for HCV infection, 51 achieved SVR, and 34 achieved SVR after the diagnosis of HCC. Patients who achieved SVR had an improved 5-year OS when compared with patients who did not achieve SVR (median 65.8 months [95% CI, 1222-NA] vs 15.7 months [95% CI, 242-853], Wilcoxon P < .001) (Figure 3). Similarly, patients with SVR had improved 5-year PFS when compared with the non-SVR group (median 20.5 months [95% CI, 431-914] vs 8.9 months [95% CI, 191-340], Wilcoxon P = .007 (Figure 4). Achievement of SVR after HCC diagnosis suggests a significantly improved OS (HR 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI, 0.23-1.82, P = .41)
Multivariate Cox regression was used to determine factors with significant survival impact. Advanced age at diagnosis (aged ≥ 65 years) (HR, 0.53; 95% CI, 0.320-0.880; P = .01), SVR status (HR, 0.33; 95% CI, 0.190-0.587; P < .001), achieving SVR after HCC diagnosis (HR, 0.37; 95% CI, 0.20-0.71; P = .002), low MELD score (< 10) (HR, 0.49; 95% CI, 0.30-0.80; P = .004) and low Child-Pugh score (class A) (HR, 0.39; 95% CI, 0.24-0.64; P = .001) have a significant positive impact on OS. Survival was not significantly influenced by race, tobacco, drug use, HIV or cirrhosis status, or HCV treatment type. In addition, higher Child-Pugh class (B or C), higher MELD score (> 10), and younger age at diagnosis (< 65 years) have a negative impact on survival outcome (Table 5).
Discussion
The survival benefit of HCV eradication and achieving SVR status has been well established in patients with HCC.13 In a retrospective cohort study of 250 patients with HCV infection who had received curative treatment for HCC, multivariate analysis demonstrated that achieving SVR is an independent predictor of OS.14 The 3-year and 5-year OS rates were 97% and 94% for the SVR group, and 91% and 60% for the non‐SVR group, respectively (P < .001). Similarly, according to Sou and colleagues, of 122 patients with HCV-related HCC, patients with SVR had longer OS than patients with no SVR (P = .04).15 One of the hypotheses that could explain the survival benefit in patients who achieved SVR is the effect of achieving SVR in reducing persistent liver inflammation and associated liver mortality, and therefore lowering risks of complication in patients with HCC.16 In our study, multivariate analysis shows that achieving SVR is associated with significant improved OS (HR, 0.33). In contrast, patients with HCC who have not achieved SVR are associated with worse survival (HR, 3.24). This finding supports early treatment of HCV to obtain SVR in HCV-related patients with HCC, even after development of HCC.
Among 68 patients treated for HCV infection, 45 patients were treated after HCC diagnosis, and 34 patients achieved SVR after HCC diagnosis. The average time between HCV infection treatment after HCC diagnosis was 6 months. Our data suggested that achievement of SVR after HCC diagnosis suggests an improved OS (HR, 0.37) compared with achievement prior to HCC diagnosis (HR, 0.65; 95% CI,0.23-1.82; P = .41). This lack of statistical significance is likely due to small sample size of patients achieving SVR prior to HCC diagnosis. Our results are consistent with the findings regarding the efficacy and timing of DAA treatment in patients with active HCC. According to Singal and colleagues, achieving SVR after DAA therapy may result in improved liver function and facilitate additional HCC-directed therapy, which potentially improves survival.17-19
Nagaoki and colleagues found that there was no significant difference in OS in patients with HCC between the DAA and IFN groups. According to the study, the 3-year and 5-year OS rates were 96% and 96% for DAA patients and 93% and 73% for IFN patients, respectively (P = .16).14 This finding is consistent with the results of our study. HCV treatment type (IFN vs DAA) was not found to be associated with either OS or PFS time, regardless of time period.
A higher MELD score (> 10) and a higher Child-Pugh class (B or C) score are associated with worse survival outcome regardless of SVR status. While patients with a low MELD score (≤ 10) have a better survival rate (HR 0.49), a higher MELD score has a significantly higher HR and therefore worse survival outcomes (HR, 2.20). Similarly, patients with Child-Pugh A (HR, 0.39) have a better survival outcome compared with those patients with Child-Pugh class B or C (HR, 2.57). This finding is consistent with results of multiple studies indicating that advanced liver disease, as measured by a high MELD score and Child-Pugh class score, can be used to predict the survival outcome in patients with HCV-related HCC.20-22
Unlike other studies that look at a single prognostic variable, our study evaluated prognostic impacts of multiple variables (age, SVR status, the order of SVR in relation to HCC development, HCV treatment type, MELD score and Child-Pugh class) in patients with HCC. The study included patients treated for HCV after development of HCC along with other multiple variables leading to OS benefit. It is one of the only studies in the United States that compared 5-year OS and PFS among patients with HCC treated for HCV and achieved SVR. The studies by Nagaoki and colleagues and Sou and colleagues were conducted in Japan, and some of their subset analyses were univariate. Among our study population of veterans, 50% were African American patients, suggesting that they may have similar OS benefit when compared to White patients with HCC and HCV treatment.
Limitations
Our findings were limited in that our study population is too small to conduct further subset analysis that would allow statistical significance of those subsets, such as the suggested benefit of SVR in patients who presented with HCC after antiviral therapy. Another limitation is the all-male population, likely a result of the older veteran population at the Memphis VAMC. The mean age at diagnosis was 65 years, which is slightly higher than the general population. Compared to the SEER database, HCC is most frequently diagnosed among people aged 55 to 64 years.23 The age difference was likely due to our aging veteran population.
Further studies are needed to determine the significance of SVR on HCC recurrence and treatment. Immunotherapy is now first-line treatment for patients with local advanced HCC. All the immunotherapy studies excluded patients with active HCV infection. Hence, we need more data on HCV treatment timing among patients scheduled to start treatment with immunotherapy.
Conclusions
In a population of older veterans, treatment of HCV infection leads to OS benefit among patients with HCC. In addition, patients with HCV infection who achieve SVR have an OS benefit over patients unable to achieve SVR. The type of treatment, DAA vs IFN-based regimen, did not show significant survival benefit.
1. Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. Published 2017 May 29. doi:10.4103/jcar.JCar_9_16
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
3. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674-687. doi:10.1038/nrc1934
4. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis c virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
5. Kouris G, Hydery T, Greenwood BC, et al. Effectiveness of Ledipasvir/Sofosbuvir and predictors of treatment failure in members with hepatitis C genotype 1 infection: a retrospective cohort study in a medicaid population. J Manag Care Spec Pharm. 2018;24(7):591-597. doi:10.18553/jmcp.2018.24.7.591
6. Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis c virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology. 2017;152(6):1372-1382.e2. doi:10.1053/j.gastro.2017.01.050
7. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155(5):1436-1450.e6. doi:10.1053/j.gastro.2018.07.01510.
8. Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2018;68(4):646-654. doi:10.1016/j.jhep.2017.10.033
9. Romano A, Angeli P, Piovesan S, et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J Hepatol. 2018;69(2):345-352. doi:10.1016/j.jhep.2018.03.009
10. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996-1005.e1. doi:10.1053/j.gastro.2017.06.0122
11. Singh S, Nautiyal A, Loke YK. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. Frontline Gastroenterol. 2018;9(4):262-270. doi:10.1136/flgastro-2018-101017
12. Kuftinec G, Loehfelm T, Corwin M, et al. De novo hepatocellular carcinoma occurrence in hepatitis C cirrhotics treated with direct-acting antiviral agents. Hepat Oncol. 2018;5(1):HEP06. Published 2018 Jul 25. doi:10.2217/hep-2018-00033
13. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
14. Nagaoki Y, Imamura M, Nishida Y, et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: comparison with interferon-based therapy. J Med Virol. 2019;91(4):650-658. doi:10.1002/jmv.25352
15. Sou FM, Wu CK, Chang KC, et al. Clinical characteristics and prognosis of HCC occurrence after antiviral therapy for HCV patients between sustained and non-sustained responders. J Formos Med Assoc. 2019;118(1 Pt 3):504-513. doi:10.1016/j.jfma.2018.10.017
16. Roche B, Coilly A, Duclos-Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38(suppl 1):139-145. doi:10.1111/liv.13659
17. Singal AG, Lim JK, Kanwal F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology. 2019;156(8):2149-2157. doi:10.1053/j.gastro.2019.02.046
18. Toyoda H, Kumada T, Hayashi K, et al. Characteristics and prognosis of hepatocellular carcinoma detected in sustained responders to interferon therapy for chronic hepatitis C. Cancer Detect Prev. 2003;27(6):498-502. doi:10.1016/j.cdp.2003.09.00719. Okamura Y, Sugiura T, Ito T, et al. The achievement of a sustained virological response either before or after hepatectomy improves the prognosis of patients with primary hepatitis C virus-related hepatocellular carcinoma. Ann Surg Oncol. 2019; 26(13):4566-4575. doi:10.1245/s10434-019-07911-w
20. Wray CJ, Harvin JA, Silberfein EJ, Ko TC, Kao LS. Pilot prognostic model of extremely poor survival among high-risk hepatocellular carcinoma patients. Cancer. 2012;118(24):6118-6125. doi:10.1002/cncr.27649
21. Kim JH, Kim JH, Choi JH, et al. Value of the model for end-stage liver disease for predicting survival in hepatocellular carcinoma patients treated with transarterial chemoembolization. Scand J Gastroenterol. 2009;44(3):346-357. doi:10.1080/00365520802530838
22. Vogeler M, Mohr I, Pfeiffenberger J, et al. Applicability of scoring systems predicting outcome of transarterial chemoembolization for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(4):1033-1050. doi:10.1007/s00432-020-03135-8
23. National Institutes of Health, Surveillance, Epidemiology, and End Results. Cancer stat facts: cancer of the liver and intrahepatic bile duct. Accessed July 15, 2021. https://seer.cancer.gov/statfacts/html/livibd.html
24. Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
1. Ghouri YA, Mian I, Rowe JH. Review of hepatocellular carcinoma: epidemiology, etiology, and carcinogenesis. J Carcinog. 2017;16:1. Published 2017 May 29. doi:10.4103/jcar.JCar_9_16
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
3. Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674-687. doi:10.1038/nrc1934
4. Falade-Nwulia O, Suarez-Cuervo C, Nelson DR, Fried MW, Segal JB, Sulkowski MS. Oral direct-acting agent therapy for hepatitis c virus infection: a systematic review. Ann Intern Med. 2017;166(9):637-648. doi:10.7326/M16-2575
5. Kouris G, Hydery T, Greenwood BC, et al. Effectiveness of Ledipasvir/Sofosbuvir and predictors of treatment failure in members with hepatitis C genotype 1 infection: a retrospective cohort study in a medicaid population. J Manag Care Spec Pharm. 2018;24(7):591-597. doi:10.18553/jmcp.2018.24.7.591
6. Jacobson IM, Lawitz E, Kwo PY, et al. Safety and efficacy of elbasvir/grazoprevir in patients with hepatitis c virus infection and compensated cirrhosis: an integrated analysis. Gastroenterology. 2017;152(6):1372-1382.e2. doi:10.1053/j.gastro.2017.01.050
7. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology. 2018;155(5):1436-1450.e6. doi:10.1053/j.gastro.2018.07.01510.
8. Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: role of the treatment regimen. J Hepatol. 2018;68(4):646-654. doi:10.1016/j.jhep.2017.10.033
9. Romano A, Angeli P, Piovesan S, et al. Newly diagnosed hepatocellular carcinoma in patients with advanced hepatitis C treated with DAAs: a prospective population study. J Hepatol. 2018;69(2):345-352. doi:10.1016/j.jhep.2018.03.009
10. Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153(4):996-1005.e1. doi:10.1053/j.gastro.2017.06.0122
11. Singh S, Nautiyal A, Loke YK. Oral direct-acting antivirals and the incidence or recurrence of hepatocellular carcinoma: a systematic review and meta-analysis. Frontline Gastroenterol. 2018;9(4):262-270. doi:10.1136/flgastro-2018-101017
12. Kuftinec G, Loehfelm T, Corwin M, et al. De novo hepatocellular carcinoma occurrence in hepatitis C cirrhotics treated with direct-acting antiviral agents. Hepat Oncol. 2018;5(1):HEP06. Published 2018 Jul 25. doi:10.2217/hep-2018-00033
13. Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158(5 Pt 1):329-337. doi:10.7326/0003-4819-158-5-201303050-00005
14. Nagaoki Y, Imamura M, Nishida Y, et al. The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: comparison with interferon-based therapy. J Med Virol. 2019;91(4):650-658. doi:10.1002/jmv.25352
15. Sou FM, Wu CK, Chang KC, et al. Clinical characteristics and prognosis of HCC occurrence after antiviral therapy for HCV patients between sustained and non-sustained responders. J Formos Med Assoc. 2019;118(1 Pt 3):504-513. doi:10.1016/j.jfma.2018.10.017
16. Roche B, Coilly A, Duclos-Vallee JC, Samuel D. The impact of treatment of hepatitis C with DAAs on the occurrence of HCC. Liver Int. 2018;38(suppl 1):139-145. doi:10.1111/liv.13659
17. Singal AG, Lim JK, Kanwal F. AGA clinical practice update on interaction between oral direct-acting antivirals for chronic hepatitis C infection and hepatocellular carcinoma: expert review. Gastroenterology. 2019;156(8):2149-2157. doi:10.1053/j.gastro.2019.02.046
18. Toyoda H, Kumada T, Hayashi K, et al. Characteristics and prognosis of hepatocellular carcinoma detected in sustained responders to interferon therapy for chronic hepatitis C. Cancer Detect Prev. 2003;27(6):498-502. doi:10.1016/j.cdp.2003.09.00719. Okamura Y, Sugiura T, Ito T, et al. The achievement of a sustained virological response either before or after hepatectomy improves the prognosis of patients with primary hepatitis C virus-related hepatocellular carcinoma. Ann Surg Oncol. 2019; 26(13):4566-4575. doi:10.1245/s10434-019-07911-w
20. Wray CJ, Harvin JA, Silberfein EJ, Ko TC, Kao LS. Pilot prognostic model of extremely poor survival among high-risk hepatocellular carcinoma patients. Cancer. 2012;118(24):6118-6125. doi:10.1002/cncr.27649
21. Kim JH, Kim JH, Choi JH, et al. Value of the model for end-stage liver disease for predicting survival in hepatocellular carcinoma patients treated with transarterial chemoembolization. Scand J Gastroenterol. 2009;44(3):346-357. doi:10.1080/00365520802530838
22. Vogeler M, Mohr I, Pfeiffenberger J, et al. Applicability of scoring systems predicting outcome of transarterial chemoembolization for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2020;146(4):1033-1050. doi:10.1007/s00432-020-03135-8
23. National Institutes of Health, Surveillance, Epidemiology, and End Results. Cancer stat facts: cancer of the liver and intrahepatic bile duct. Accessed July 15, 2021. https://seer.cancer.gov/statfacts/html/livibd.html
24. Singal AK, Kamath PS. Model for End-stage Liver Disease. J Clin Exp Hepatol. 2013;3(1):50-60. doi:10.1016/j.jceh.2012.11.002
Politics or protection? What’s behind the push for boosters?
That plan, which was first announced on Aug. 18, has raised eyebrows because it comes in advance of regulatory reviews by the Food and Drug Administration and recommendations from the Centers for Disease Control and Prevention. Those reviews are needed to determine whether third doses of these vaccines are effective or even safe. The move could have important legal ramifications for doctors and patients, too.
On Aug. 31, two high-level officials in the FDA’s Office of Vaccines Research and Review abruptly resigned amid reports that they were angry that the Biden administration was making decisions that should be left up to that agency.
So far, data show that the vaccines are highly effective at preventing the most severe consequences of COVID-19 – hospitalization and death – even regarding the Delta variant. The World Health Organization has urged wealthy nations such as the United States not to offer boosters so that the limited supply of vaccines can be directed to countries with fewer resources.
White House supports boosters
In a recent press briefing, Jeff Zients, the White House COVID-19 response coordinator, defended the move.
“You know, the booster decision, which you referenced ... was made by and announced by the nation’s leading public health officials, including Dr. Walensky; Dr. Fauci; Surgeon General Vivek Murthy; Dr. Janet Woodcock; the FDA acting commissioner, Dr. Francis Collins; Dr. Kessler; and others,” Mr. Zients said.
“And as our medical experts laid out, having reviewed all of the available data, it is in their clinical judgment that it is time to prepare Americans for a booster shot.”
He said a target date of Sept. 20 was announced so as to give states and practitioners time to prepare. He also said the move to give boosters was meant to help the United States stay ahead of a rapidly changing virus. Mr. Zients added that whether boosters will be administered starting on Sept. 20 depends on the FDA’s and CDC’s giving the go-ahead.
“Booster doses are going to be handled the same way all vaccines are handled,” said Kristen Nordlund, a CDC spokesperson. “Companies will have to provide data to FDA. FDA will have to make a decision and authorize the use of those, and ACIP [the Advisory Committee on Immunization Practices] will have to look at the evidence as well and make recommendations on top of FDA’s regulatory action,” she said.
Ms. Nordlund agreed that the planned Sept. 20 start date for boosters was something to which they aspired and was not necessarily set.
Historically, the FDA has needed at least 4 months to review a change to a vaccine’s approval, even on an accelerated schedule. Reviewers use that time to assess data regarding individual patients in a study, to review raw data, and essentially to check a drug company’s math and conclusions. The Biden administration’s timeline would shorten that review period from months to just a few weeks.
‘FDA in a very difficult position’
After the FDA approves, the ACIP of the CDC must meet to review the evidence and make recommendations on the use of the boosters in the United States.
Pfizer says it completed its submission for a supplemental biologics license application to the FDA on Aug. 27. To meet a Sept. 20 timeline, the entire process would have to be completed within 3 weeks.
“I don’t think that was handled, you know, ideally,” said Peter Lurie, MD, president of the Center for Science in the Public Interest and former associate commissioner of public health strategy and analysis at the FDA.
“It puts FDA in a very difficult position,” Dr. Lurie said. “It’s almost as if the decision has been made and they’re just checking a box, and that is, you know, contrary to the what FDA – at least the internal people at FDA – have been trying to do for ages.”
He said the agency took great pains with the emergency use authorizations and the full approvals of the vaccines to work as rapidly but thoroughly as possible. They did not skip steps.
“I think all of that reflected very well on the agency,” Dr. Lurie said. “And I think it worked out well in terms of trust in the vaccines.”
Although additional doses of vaccine are expected to be safe, little is known about side effects or adverse events after a third dose.
“It’s critical to wait for additional data and regulatory allowance for booster doses,” Sara Oliver, MD, a member of the CDC’s epidemic intelligence service, said in an Aug. 30 presentation to the ACIP, which is charged with making recommendations for use of all vaccines in the United States.
Boosters already being given
But after the White House announced that boosters were on the way, many people are not waiting.
Many health care practitioners and pharmacies have already been giving people third doses of vaccines, even if they are not among the immunocompromised – the group for which the shots are currently approved.
“You can walk into a pharmacy and ask for a third dose. Depending on which pharmacy you go to, you may get it,” said Helen Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., and a member of the ACIP.
She says she has a friend who recently went for a checkup and was offered a third dose. His physician is already giving extra doses to everyone who is older than 65.
Dr. Talbot said that in fairness, pharmacies in the United States are throwing away doses of vaccine because they are expiring before they get used.
“Many of us may or may not be ready to give a third dose but would rather give someone a third dose than throw a vaccine away,” she said.
Consequences of a third shot
But giving or getting a third dose before approval by the FDA may have legal consequences.
In the ACIP meeting on Aug. 30, Demetre Daskalakis, MD, who leads vaccine equity efforts at the CDC, cautioned that physicians who give extra doses of the vaccine before the FDA and CDC have signed off may be in violation of practitioner agreements with the federal government and might not be covered by the federal PREP Act. The PREP Act provides immunity from lawsuits for people who administer COVID-19 vaccines and compensates patients in the event of injury. Patients who get a vaccine and suffer a rare but serious side effect may lose the ability to claim compensation offered by the act.
“Many of us gasped when he said that,” Dr. Talbot said, “because that’s a big deal.”
The ACIP signaled that it is considering recommending boosters for a much narrower slice of the American population than the Biden administration has suggested.
They said that so far, the data point only to the need for boosters for seniors, who are the patients most likely to experience breakthrough infections that require hospitalization, and health care workers, who are needed now more than ever and cannot work if they’re sick.
In a White House news briefing Aug. 31, CDC Director Rochelle Walensky, MD, was asked about the ACIP’s conclusions and whether she believed there were enough data to recommend booster shots for most Americans 8 months after their last dose.
“The ACIP did not review international data that actually has led us to be even more concerned about increased risk of vaccine effectiveness waning against hospitalization, severe disease, and death. They will be reviewing that as well,” she said.
A version of this article first appeared on Medscape.com.
That plan, which was first announced on Aug. 18, has raised eyebrows because it comes in advance of regulatory reviews by the Food and Drug Administration and recommendations from the Centers for Disease Control and Prevention. Those reviews are needed to determine whether third doses of these vaccines are effective or even safe. The move could have important legal ramifications for doctors and patients, too.
On Aug. 31, two high-level officials in the FDA’s Office of Vaccines Research and Review abruptly resigned amid reports that they were angry that the Biden administration was making decisions that should be left up to that agency.
So far, data show that the vaccines are highly effective at preventing the most severe consequences of COVID-19 – hospitalization and death – even regarding the Delta variant. The World Health Organization has urged wealthy nations such as the United States not to offer boosters so that the limited supply of vaccines can be directed to countries with fewer resources.
White House supports boosters
In a recent press briefing, Jeff Zients, the White House COVID-19 response coordinator, defended the move.
“You know, the booster decision, which you referenced ... was made by and announced by the nation’s leading public health officials, including Dr. Walensky; Dr. Fauci; Surgeon General Vivek Murthy; Dr. Janet Woodcock; the FDA acting commissioner, Dr. Francis Collins; Dr. Kessler; and others,” Mr. Zients said.
“And as our medical experts laid out, having reviewed all of the available data, it is in their clinical judgment that it is time to prepare Americans for a booster shot.”
He said a target date of Sept. 20 was announced so as to give states and practitioners time to prepare. He also said the move to give boosters was meant to help the United States stay ahead of a rapidly changing virus. Mr. Zients added that whether boosters will be administered starting on Sept. 20 depends on the FDA’s and CDC’s giving the go-ahead.
“Booster doses are going to be handled the same way all vaccines are handled,” said Kristen Nordlund, a CDC spokesperson. “Companies will have to provide data to FDA. FDA will have to make a decision and authorize the use of those, and ACIP [the Advisory Committee on Immunization Practices] will have to look at the evidence as well and make recommendations on top of FDA’s regulatory action,” she said.
Ms. Nordlund agreed that the planned Sept. 20 start date for boosters was something to which they aspired and was not necessarily set.
Historically, the FDA has needed at least 4 months to review a change to a vaccine’s approval, even on an accelerated schedule. Reviewers use that time to assess data regarding individual patients in a study, to review raw data, and essentially to check a drug company’s math and conclusions. The Biden administration’s timeline would shorten that review period from months to just a few weeks.
‘FDA in a very difficult position’
After the FDA approves, the ACIP of the CDC must meet to review the evidence and make recommendations on the use of the boosters in the United States.
Pfizer says it completed its submission for a supplemental biologics license application to the FDA on Aug. 27. To meet a Sept. 20 timeline, the entire process would have to be completed within 3 weeks.
“I don’t think that was handled, you know, ideally,” said Peter Lurie, MD, president of the Center for Science in the Public Interest and former associate commissioner of public health strategy and analysis at the FDA.
“It puts FDA in a very difficult position,” Dr. Lurie said. “It’s almost as if the decision has been made and they’re just checking a box, and that is, you know, contrary to the what FDA – at least the internal people at FDA – have been trying to do for ages.”
He said the agency took great pains with the emergency use authorizations and the full approvals of the vaccines to work as rapidly but thoroughly as possible. They did not skip steps.
“I think all of that reflected very well on the agency,” Dr. Lurie said. “And I think it worked out well in terms of trust in the vaccines.”
Although additional doses of vaccine are expected to be safe, little is known about side effects or adverse events after a third dose.
“It’s critical to wait for additional data and regulatory allowance for booster doses,” Sara Oliver, MD, a member of the CDC’s epidemic intelligence service, said in an Aug. 30 presentation to the ACIP, which is charged with making recommendations for use of all vaccines in the United States.
Boosters already being given
But after the White House announced that boosters were on the way, many people are not waiting.
Many health care practitioners and pharmacies have already been giving people third doses of vaccines, even if they are not among the immunocompromised – the group for which the shots are currently approved.
“You can walk into a pharmacy and ask for a third dose. Depending on which pharmacy you go to, you may get it,” said Helen Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., and a member of the ACIP.
She says she has a friend who recently went for a checkup and was offered a third dose. His physician is already giving extra doses to everyone who is older than 65.
Dr. Talbot said that in fairness, pharmacies in the United States are throwing away doses of vaccine because they are expiring before they get used.
“Many of us may or may not be ready to give a third dose but would rather give someone a third dose than throw a vaccine away,” she said.
Consequences of a third shot
But giving or getting a third dose before approval by the FDA may have legal consequences.
In the ACIP meeting on Aug. 30, Demetre Daskalakis, MD, who leads vaccine equity efforts at the CDC, cautioned that physicians who give extra doses of the vaccine before the FDA and CDC have signed off may be in violation of practitioner agreements with the federal government and might not be covered by the federal PREP Act. The PREP Act provides immunity from lawsuits for people who administer COVID-19 vaccines and compensates patients in the event of injury. Patients who get a vaccine and suffer a rare but serious side effect may lose the ability to claim compensation offered by the act.
“Many of us gasped when he said that,” Dr. Talbot said, “because that’s a big deal.”
The ACIP signaled that it is considering recommending boosters for a much narrower slice of the American population than the Biden administration has suggested.
They said that so far, the data point only to the need for boosters for seniors, who are the patients most likely to experience breakthrough infections that require hospitalization, and health care workers, who are needed now more than ever and cannot work if they’re sick.
In a White House news briefing Aug. 31, CDC Director Rochelle Walensky, MD, was asked about the ACIP’s conclusions and whether she believed there were enough data to recommend booster shots for most Americans 8 months after their last dose.
“The ACIP did not review international data that actually has led us to be even more concerned about increased risk of vaccine effectiveness waning against hospitalization, severe disease, and death. They will be reviewing that as well,” she said.
A version of this article first appeared on Medscape.com.
That plan, which was first announced on Aug. 18, has raised eyebrows because it comes in advance of regulatory reviews by the Food and Drug Administration and recommendations from the Centers for Disease Control and Prevention. Those reviews are needed to determine whether third doses of these vaccines are effective or even safe. The move could have important legal ramifications for doctors and patients, too.
On Aug. 31, two high-level officials in the FDA’s Office of Vaccines Research and Review abruptly resigned amid reports that they were angry that the Biden administration was making decisions that should be left up to that agency.
So far, data show that the vaccines are highly effective at preventing the most severe consequences of COVID-19 – hospitalization and death – even regarding the Delta variant. The World Health Organization has urged wealthy nations such as the United States not to offer boosters so that the limited supply of vaccines can be directed to countries with fewer resources.
White House supports boosters
In a recent press briefing, Jeff Zients, the White House COVID-19 response coordinator, defended the move.
“You know, the booster decision, which you referenced ... was made by and announced by the nation’s leading public health officials, including Dr. Walensky; Dr. Fauci; Surgeon General Vivek Murthy; Dr. Janet Woodcock; the FDA acting commissioner, Dr. Francis Collins; Dr. Kessler; and others,” Mr. Zients said.
“And as our medical experts laid out, having reviewed all of the available data, it is in their clinical judgment that it is time to prepare Americans for a booster shot.”
He said a target date of Sept. 20 was announced so as to give states and practitioners time to prepare. He also said the move to give boosters was meant to help the United States stay ahead of a rapidly changing virus. Mr. Zients added that whether boosters will be administered starting on Sept. 20 depends on the FDA’s and CDC’s giving the go-ahead.
“Booster doses are going to be handled the same way all vaccines are handled,” said Kristen Nordlund, a CDC spokesperson. “Companies will have to provide data to FDA. FDA will have to make a decision and authorize the use of those, and ACIP [the Advisory Committee on Immunization Practices] will have to look at the evidence as well and make recommendations on top of FDA’s regulatory action,” she said.
Ms. Nordlund agreed that the planned Sept. 20 start date for boosters was something to which they aspired and was not necessarily set.
Historically, the FDA has needed at least 4 months to review a change to a vaccine’s approval, even on an accelerated schedule. Reviewers use that time to assess data regarding individual patients in a study, to review raw data, and essentially to check a drug company’s math and conclusions. The Biden administration’s timeline would shorten that review period from months to just a few weeks.
‘FDA in a very difficult position’
After the FDA approves, the ACIP of the CDC must meet to review the evidence and make recommendations on the use of the boosters in the United States.
Pfizer says it completed its submission for a supplemental biologics license application to the FDA on Aug. 27. To meet a Sept. 20 timeline, the entire process would have to be completed within 3 weeks.
“I don’t think that was handled, you know, ideally,” said Peter Lurie, MD, president of the Center for Science in the Public Interest and former associate commissioner of public health strategy and analysis at the FDA.
“It puts FDA in a very difficult position,” Dr. Lurie said. “It’s almost as if the decision has been made and they’re just checking a box, and that is, you know, contrary to the what FDA – at least the internal people at FDA – have been trying to do for ages.”
He said the agency took great pains with the emergency use authorizations and the full approvals of the vaccines to work as rapidly but thoroughly as possible. They did not skip steps.
“I think all of that reflected very well on the agency,” Dr. Lurie said. “And I think it worked out well in terms of trust in the vaccines.”
Although additional doses of vaccine are expected to be safe, little is known about side effects or adverse events after a third dose.
“It’s critical to wait for additional data and regulatory allowance for booster doses,” Sara Oliver, MD, a member of the CDC’s epidemic intelligence service, said in an Aug. 30 presentation to the ACIP, which is charged with making recommendations for use of all vaccines in the United States.
Boosters already being given
But after the White House announced that boosters were on the way, many people are not waiting.
Many health care practitioners and pharmacies have already been giving people third doses of vaccines, even if they are not among the immunocompromised – the group for which the shots are currently approved.
“You can walk into a pharmacy and ask for a third dose. Depending on which pharmacy you go to, you may get it,” said Helen Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., and a member of the ACIP.
She says she has a friend who recently went for a checkup and was offered a third dose. His physician is already giving extra doses to everyone who is older than 65.
Dr. Talbot said that in fairness, pharmacies in the United States are throwing away doses of vaccine because they are expiring before they get used.
“Many of us may or may not be ready to give a third dose but would rather give someone a third dose than throw a vaccine away,” she said.
Consequences of a third shot
But giving or getting a third dose before approval by the FDA may have legal consequences.
In the ACIP meeting on Aug. 30, Demetre Daskalakis, MD, who leads vaccine equity efforts at the CDC, cautioned that physicians who give extra doses of the vaccine before the FDA and CDC have signed off may be in violation of practitioner agreements with the federal government and might not be covered by the federal PREP Act. The PREP Act provides immunity from lawsuits for people who administer COVID-19 vaccines and compensates patients in the event of injury. Patients who get a vaccine and suffer a rare but serious side effect may lose the ability to claim compensation offered by the act.
“Many of us gasped when he said that,” Dr. Talbot said, “because that’s a big deal.”
The ACIP signaled that it is considering recommending boosters for a much narrower slice of the American population than the Biden administration has suggested.
They said that so far, the data point only to the need for boosters for seniors, who are the patients most likely to experience breakthrough infections that require hospitalization, and health care workers, who are needed now more than ever and cannot work if they’re sick.
In a White House news briefing Aug. 31, CDC Director Rochelle Walensky, MD, was asked about the ACIP’s conclusions and whether she believed there were enough data to recommend booster shots for most Americans 8 months after their last dose.
“The ACIP did not review international data that actually has led us to be even more concerned about increased risk of vaccine effectiveness waning against hospitalization, severe disease, and death. They will be reviewing that as well,” she said.
A version of this article first appeared on Medscape.com.
Expert shares vulvovaginal candidiasis treatment pearls
that was approved in June 2021, Aruna Venkatesan, MD, recommends.
“Ibrexafungerp, an inhibitor of beta (1-3)–glucan synthase, is important for many reasons,” Dr. Venkatesan, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center, San Jose, Calif., said during the annual meeting of the Pacific Dermatologic Association. “It’s one of the few drugs that can be used to treat Candida glabrata when C. glabrata is resistant to azoles and echinocandins. As the second-most common Candida species after C. albicans, C. glabrata is more common in immunosuppressed patients and it can cause mucosal and invasive disease, so ibrexafungerp is a welcome addition to our treatment armamentarium,” said Dr. Venkatesan, clinical professor of dermatology (affiliated) at Stanford (Calif.) Hospital and Clinics, adding that that vulvovaginal candidiasis can be tricky to diagnose. “In medical school, we learned that yeast infection in a woman presents as white, curd-like discharge, but that’s actually a minority of patients.”
For a patient who is being treated with topical steroids or estrogen for a genital condition, but is experiencing worsening itch, redness, or thick white discharge, she recommends performing a KOH exam.
“Instead of using a 15-blade scalpel, as we are used to performing on the skin for tinea, take a sterile [cotton swab], and swab the affected area. You can then apply it to a slide and perform a KOH exam as you normally would. Then look for yeast elements under the microscope. I also find it helpful to send for fungal culture to get speciation, especially in someone who’s not responding to therapy. This is because non-albicans yeast can be more resistant to azoles and require a different treatment plan.”
Often, patients with vulvovaginal candidiasis who present to her clinic are referred from an ob.gyn. and other general practitioners because they have failed a topical or oral azole. “I tend to avoid the topicals,” said Dr. Venkatesan, who is also president-elect of the North American chapter of the International Society for the Study of Vulvovaginal Disease. “If the culture shows C. albicans, I usually treat with oral fluconazole, 150 mg or 200 mg once, and consider repeat weekly dosing. Many patients come to me because they have recurrent refractory disease, so giving it once weekly for 6-8 weeks while they work on their potential risk factors such as diabetic blood sugar control is sensible.”
Non-albicans yeast can be resistant to azoles. If the fungal culture shows C. glabrata in such patients, “consider a course of intravaginal boric acid suppositories,” she advised. “These used to be difficult to give patients, because you would either have to send the prescription to a compounding pharmacy, or have the patients buy the capsules and boric acid crystals separately and make them themselves. That always made me nervous because of the chance of errors. The safety and the concern of taking it by mouth is an issue.” But now, intravaginal boric acid suppositories are available on Amazon and other web sites, and are relatively affordable, she said, adding, “just make sure the patient doesn’t take it by mouth as this is very toxic.”
Dr. Venkatesan reported having no financial disclosures.
that was approved in June 2021, Aruna Venkatesan, MD, recommends.
“Ibrexafungerp, an inhibitor of beta (1-3)–glucan synthase, is important for many reasons,” Dr. Venkatesan, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center, San Jose, Calif., said during the annual meeting of the Pacific Dermatologic Association. “It’s one of the few drugs that can be used to treat Candida glabrata when C. glabrata is resistant to azoles and echinocandins. As the second-most common Candida species after C. albicans, C. glabrata is more common in immunosuppressed patients and it can cause mucosal and invasive disease, so ibrexafungerp is a welcome addition to our treatment armamentarium,” said Dr. Venkatesan, clinical professor of dermatology (affiliated) at Stanford (Calif.) Hospital and Clinics, adding that that vulvovaginal candidiasis can be tricky to diagnose. “In medical school, we learned that yeast infection in a woman presents as white, curd-like discharge, but that’s actually a minority of patients.”
For a patient who is being treated with topical steroids or estrogen for a genital condition, but is experiencing worsening itch, redness, or thick white discharge, she recommends performing a KOH exam.
“Instead of using a 15-blade scalpel, as we are used to performing on the skin for tinea, take a sterile [cotton swab], and swab the affected area. You can then apply it to a slide and perform a KOH exam as you normally would. Then look for yeast elements under the microscope. I also find it helpful to send for fungal culture to get speciation, especially in someone who’s not responding to therapy. This is because non-albicans yeast can be more resistant to azoles and require a different treatment plan.”
Often, patients with vulvovaginal candidiasis who present to her clinic are referred from an ob.gyn. and other general practitioners because they have failed a topical or oral azole. “I tend to avoid the topicals,” said Dr. Venkatesan, who is also president-elect of the North American chapter of the International Society for the Study of Vulvovaginal Disease. “If the culture shows C. albicans, I usually treat with oral fluconazole, 150 mg or 200 mg once, and consider repeat weekly dosing. Many patients come to me because they have recurrent refractory disease, so giving it once weekly for 6-8 weeks while they work on their potential risk factors such as diabetic blood sugar control is sensible.”
Non-albicans yeast can be resistant to azoles. If the fungal culture shows C. glabrata in such patients, “consider a course of intravaginal boric acid suppositories,” she advised. “These used to be difficult to give patients, because you would either have to send the prescription to a compounding pharmacy, or have the patients buy the capsules and boric acid crystals separately and make them themselves. That always made me nervous because of the chance of errors. The safety and the concern of taking it by mouth is an issue.” But now, intravaginal boric acid suppositories are available on Amazon and other web sites, and are relatively affordable, she said, adding, “just make sure the patient doesn’t take it by mouth as this is very toxic.”
Dr. Venkatesan reported having no financial disclosures.
that was approved in June 2021, Aruna Venkatesan, MD, recommends.
“Ibrexafungerp, an inhibitor of beta (1-3)–glucan synthase, is important for many reasons,” Dr. Venkatesan, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center, San Jose, Calif., said during the annual meeting of the Pacific Dermatologic Association. “It’s one of the few drugs that can be used to treat Candida glabrata when C. glabrata is resistant to azoles and echinocandins. As the second-most common Candida species after C. albicans, C. glabrata is more common in immunosuppressed patients and it can cause mucosal and invasive disease, so ibrexafungerp is a welcome addition to our treatment armamentarium,” said Dr. Venkatesan, clinical professor of dermatology (affiliated) at Stanford (Calif.) Hospital and Clinics, adding that that vulvovaginal candidiasis can be tricky to diagnose. “In medical school, we learned that yeast infection in a woman presents as white, curd-like discharge, but that’s actually a minority of patients.”
For a patient who is being treated with topical steroids or estrogen for a genital condition, but is experiencing worsening itch, redness, or thick white discharge, she recommends performing a KOH exam.
“Instead of using a 15-blade scalpel, as we are used to performing on the skin for tinea, take a sterile [cotton swab], and swab the affected area. You can then apply it to a slide and perform a KOH exam as you normally would. Then look for yeast elements under the microscope. I also find it helpful to send for fungal culture to get speciation, especially in someone who’s not responding to therapy. This is because non-albicans yeast can be more resistant to azoles and require a different treatment plan.”
Often, patients with vulvovaginal candidiasis who present to her clinic are referred from an ob.gyn. and other general practitioners because they have failed a topical or oral azole. “I tend to avoid the topicals,” said Dr. Venkatesan, who is also president-elect of the North American chapter of the International Society for the Study of Vulvovaginal Disease. “If the culture shows C. albicans, I usually treat with oral fluconazole, 150 mg or 200 mg once, and consider repeat weekly dosing. Many patients come to me because they have recurrent refractory disease, so giving it once weekly for 6-8 weeks while they work on their potential risk factors such as diabetic blood sugar control is sensible.”
Non-albicans yeast can be resistant to azoles. If the fungal culture shows C. glabrata in such patients, “consider a course of intravaginal boric acid suppositories,” she advised. “These used to be difficult to give patients, because you would either have to send the prescription to a compounding pharmacy, or have the patients buy the capsules and boric acid crystals separately and make them themselves. That always made me nervous because of the chance of errors. The safety and the concern of taking it by mouth is an issue.” But now, intravaginal boric acid suppositories are available on Amazon and other web sites, and are relatively affordable, she said, adding, “just make sure the patient doesn’t take it by mouth as this is very toxic.”
Dr. Venkatesan reported having no financial disclosures.
FROM PDA 2021
COVID-19 linked to baby bust in high-income countries
In an assessment of the pandemic’s early effects, Arnstein Aassve, PhD, and colleagues found a significant COVID-19–related decline in crude birth rates (CBRs) in 7 of 22 high-income countries, particularly in Southwestern Europe.
Dr. Aassve, an economist at the Carlo F. Dondena Center for Research on Social Dynamics and Public Policy at the Università Commerciale Luigi Bocconi, Milan, and colleagues report the results in an article published online August 30 in the Proceedings of the National Academy of Sciences.
Defining the start of the COVID-19 pandemic as February 2020, the study identifies strong declines in Italy (-9.1%), Hungary (-8.5%), Spain (-8.4%), and Portugal (-6.6%) beyond those predicted by past trends. In the United States, CBRs fell by 7.1% relative to 2019 for births occurring in Nov. and Dec. 2020 following conceptions in February and March of that year.
Significant declines in CBR also occurred in Belgium, Austria, and Singapore.
A year-to-year comparison of the mean for monthly CBRs per 1,000 population before and during the pandemic suggests a negative difference for all countries studied except for Denmark, Finland, Germany, and the Netherlands, Dr. Aassve and colleagues write. These findings may have policy implications for childcare, housing, and the labor market.
The Milan researchers compared monthly vital statistics data on live births from the international Human Fertility Database for the period of Jan. 2016 to March 2021. These figures reflect conceptions carried to term between April 2015 and June 2020. The 22 countries in the analysis represent 37% of the total reported COVID-19 cases and 34% of deaths worldwide.
The study findings align with surveys on “fertility intentions” collected early in the first COVID-19 wave in Germany, France, Spain, and the United Kingdom. These surveys indicated that 73% of people who were planning pregnancies in 2020 either decided to delay the pregnancy or they abandoned their plans.
“The popular media speculated that the lockdown would lead to a baby boom, as couples spent more time together,” Dr. Aassve told this news organization. “There’s very little evidence of this when you look to previous disasters and shocks, and the first data suggest more of an immediate collapse than a boom. But as you also see from the paper, the collapse is not seen everywhere.” Other current studies suggest the fertility drop is immediate but temporary, says Dr. Aassve, who is also a professor of demography.
Interestingly, Dr. Aassve and colleagues found that CBRs were relatively stable in Northern Europe. The authors point to supportive social and family policies in that region that might have reduced the effect of the pandemic on births. “These factors are likely to affect CBRs in the subsequent pandemic waves,” they write. They call for future studies to assess the full population implications of the pandemic, the moderating impact of policy interventions, and the nexus between short- and long-run effects in relation to the various waves of the COVID-19 pandemic.
Rebounds
Some regions have already reported a rebound from the COVID-19 fertility trough. Molly J. Stout, MD, director of maternal fetal medicine at the University of Michigan, Ann Arbor, and colleagues used electronic medical records to predict a surge in births after the initial decline.
“The surge we’ve seen at the end of this summer is exceeding the usual annual birth rate, as predicted,” she said in an interview. “But I think there’ll be a return to normal after this transient escalation. I don’t think birth rates will stay elevated above the normal because the birth surge is a temporary response to an event, although there will likely be regional differences.”
Looking ahead, Dr. Stout, who was not involved in Dr. Aassve’s analysis, is not certain how a fourth pandemic wave might ultimately modify a couple’s overall family size. But the toll the health crisis has taken on working women who have been forced to withdraw from the economy because of a lack of childcare points to a societal need that should be addressed.
According to Philip N. Cohen, PhD, a professor of sociology at the University of Maryland, College Park, who’s been tracking fertility trends since the onset of the COVID-19 emergency, the pandemic has combined a health crisis with an economic crisis, along with “the additional factor of social distancing and isolation, which all contributed to the decline in birth rates. Some people changed their plans to hold off on having children, while others didn’t get pregnant because they weren’t socializing and meeting people as much.”
Dr. Cohen, who was not involved in the study by Dr. Aassve and associates, said his provisional data show that although in many places, birth rates have rebounded more or less to prepandemic levels after a nadir around Jan. 2021, some areas of the United States still show substantially lower rates, including California, Hawaii, and Oregon.
As to the duration of the pandemic effect, Dr. Aassve cautions that his group’s estimates refer to the first wave only. “We then have the second, third, and currently the fourth wave. We can’t be sure about the impact of these waves on fertility since the data are not there yet, but I’d be surprised if they didn’t continue to have an impact on fertility rates,” he said.
Dr. Cohen agreed: “Some people who delayed childbearing will make up the delay. However, whenever there’s a delay, there’s inevitably some portion of the decline that’s not recouped.”
As for the wider effect across the world, Dr. Aassve said his team’s figures derive from high-income countries where data are readily available. For middle- and low-income countries, fewer data exist, and the quality of those data is not as good.
The lessons from this and other upheavals teach us that unforeseen shocks almost always have a negative impact on fertility, says Dr. Aassve. “[B]ut these effects may be separate from existing declining trends. The issue here is that those overall declining trends may be driven by other factors. In contrast, the shock of the pandemic is short-lived, and we may return to normal rather quickly. But if the pandemic also impacts other societal structures, such as the occupational and industrial sectors, then the pandemic might exacerbate the negative trend.”
The study was supported by funding from the European Research Council for funding under the European Union’s Horizon 2020 Research and Innovation Programme. The study authors, Dr. Stout, and Dr. Cohen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an assessment of the pandemic’s early effects, Arnstein Aassve, PhD, and colleagues found a significant COVID-19–related decline in crude birth rates (CBRs) in 7 of 22 high-income countries, particularly in Southwestern Europe.
Dr. Aassve, an economist at the Carlo F. Dondena Center for Research on Social Dynamics and Public Policy at the Università Commerciale Luigi Bocconi, Milan, and colleagues report the results in an article published online August 30 in the Proceedings of the National Academy of Sciences.
Defining the start of the COVID-19 pandemic as February 2020, the study identifies strong declines in Italy (-9.1%), Hungary (-8.5%), Spain (-8.4%), and Portugal (-6.6%) beyond those predicted by past trends. In the United States, CBRs fell by 7.1% relative to 2019 for births occurring in Nov. and Dec. 2020 following conceptions in February and March of that year.
Significant declines in CBR also occurred in Belgium, Austria, and Singapore.
A year-to-year comparison of the mean for monthly CBRs per 1,000 population before and during the pandemic suggests a negative difference for all countries studied except for Denmark, Finland, Germany, and the Netherlands, Dr. Aassve and colleagues write. These findings may have policy implications for childcare, housing, and the labor market.
The Milan researchers compared monthly vital statistics data on live births from the international Human Fertility Database for the period of Jan. 2016 to March 2021. These figures reflect conceptions carried to term between April 2015 and June 2020. The 22 countries in the analysis represent 37% of the total reported COVID-19 cases and 34% of deaths worldwide.
The study findings align with surveys on “fertility intentions” collected early in the first COVID-19 wave in Germany, France, Spain, and the United Kingdom. These surveys indicated that 73% of people who were planning pregnancies in 2020 either decided to delay the pregnancy or they abandoned their plans.
“The popular media speculated that the lockdown would lead to a baby boom, as couples spent more time together,” Dr. Aassve told this news organization. “There’s very little evidence of this when you look to previous disasters and shocks, and the first data suggest more of an immediate collapse than a boom. But as you also see from the paper, the collapse is not seen everywhere.” Other current studies suggest the fertility drop is immediate but temporary, says Dr. Aassve, who is also a professor of demography.
Interestingly, Dr. Aassve and colleagues found that CBRs were relatively stable in Northern Europe. The authors point to supportive social and family policies in that region that might have reduced the effect of the pandemic on births. “These factors are likely to affect CBRs in the subsequent pandemic waves,” they write. They call for future studies to assess the full population implications of the pandemic, the moderating impact of policy interventions, and the nexus between short- and long-run effects in relation to the various waves of the COVID-19 pandemic.
Rebounds
Some regions have already reported a rebound from the COVID-19 fertility trough. Molly J. Stout, MD, director of maternal fetal medicine at the University of Michigan, Ann Arbor, and colleagues used electronic medical records to predict a surge in births after the initial decline.
“The surge we’ve seen at the end of this summer is exceeding the usual annual birth rate, as predicted,” she said in an interview. “But I think there’ll be a return to normal after this transient escalation. I don’t think birth rates will stay elevated above the normal because the birth surge is a temporary response to an event, although there will likely be regional differences.”
Looking ahead, Dr. Stout, who was not involved in Dr. Aassve’s analysis, is not certain how a fourth pandemic wave might ultimately modify a couple’s overall family size. But the toll the health crisis has taken on working women who have been forced to withdraw from the economy because of a lack of childcare points to a societal need that should be addressed.
According to Philip N. Cohen, PhD, a professor of sociology at the University of Maryland, College Park, who’s been tracking fertility trends since the onset of the COVID-19 emergency, the pandemic has combined a health crisis with an economic crisis, along with “the additional factor of social distancing and isolation, which all contributed to the decline in birth rates. Some people changed their plans to hold off on having children, while others didn’t get pregnant because they weren’t socializing and meeting people as much.”
Dr. Cohen, who was not involved in the study by Dr. Aassve and associates, said his provisional data show that although in many places, birth rates have rebounded more or less to prepandemic levels after a nadir around Jan. 2021, some areas of the United States still show substantially lower rates, including California, Hawaii, and Oregon.
As to the duration of the pandemic effect, Dr. Aassve cautions that his group’s estimates refer to the first wave only. “We then have the second, third, and currently the fourth wave. We can’t be sure about the impact of these waves on fertility since the data are not there yet, but I’d be surprised if they didn’t continue to have an impact on fertility rates,” he said.
Dr. Cohen agreed: “Some people who delayed childbearing will make up the delay. However, whenever there’s a delay, there’s inevitably some portion of the decline that’s not recouped.”
As for the wider effect across the world, Dr. Aassve said his team’s figures derive from high-income countries where data are readily available. For middle- and low-income countries, fewer data exist, and the quality of those data is not as good.
The lessons from this and other upheavals teach us that unforeseen shocks almost always have a negative impact on fertility, says Dr. Aassve. “[B]ut these effects may be separate from existing declining trends. The issue here is that those overall declining trends may be driven by other factors. In contrast, the shock of the pandemic is short-lived, and we may return to normal rather quickly. But if the pandemic also impacts other societal structures, such as the occupational and industrial sectors, then the pandemic might exacerbate the negative trend.”
The study was supported by funding from the European Research Council for funding under the European Union’s Horizon 2020 Research and Innovation Programme. The study authors, Dr. Stout, and Dr. Cohen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In an assessment of the pandemic’s early effects, Arnstein Aassve, PhD, and colleagues found a significant COVID-19–related decline in crude birth rates (CBRs) in 7 of 22 high-income countries, particularly in Southwestern Europe.
Dr. Aassve, an economist at the Carlo F. Dondena Center for Research on Social Dynamics and Public Policy at the Università Commerciale Luigi Bocconi, Milan, and colleagues report the results in an article published online August 30 in the Proceedings of the National Academy of Sciences.
Defining the start of the COVID-19 pandemic as February 2020, the study identifies strong declines in Italy (-9.1%), Hungary (-8.5%), Spain (-8.4%), and Portugal (-6.6%) beyond those predicted by past trends. In the United States, CBRs fell by 7.1% relative to 2019 for births occurring in Nov. and Dec. 2020 following conceptions in February and March of that year.
Significant declines in CBR also occurred in Belgium, Austria, and Singapore.
A year-to-year comparison of the mean for monthly CBRs per 1,000 population before and during the pandemic suggests a negative difference for all countries studied except for Denmark, Finland, Germany, and the Netherlands, Dr. Aassve and colleagues write. These findings may have policy implications for childcare, housing, and the labor market.
The Milan researchers compared monthly vital statistics data on live births from the international Human Fertility Database for the period of Jan. 2016 to March 2021. These figures reflect conceptions carried to term between April 2015 and June 2020. The 22 countries in the analysis represent 37% of the total reported COVID-19 cases and 34% of deaths worldwide.
The study findings align with surveys on “fertility intentions” collected early in the first COVID-19 wave in Germany, France, Spain, and the United Kingdom. These surveys indicated that 73% of people who were planning pregnancies in 2020 either decided to delay the pregnancy or they abandoned their plans.
“The popular media speculated that the lockdown would lead to a baby boom, as couples spent more time together,” Dr. Aassve told this news organization. “There’s very little evidence of this when you look to previous disasters and shocks, and the first data suggest more of an immediate collapse than a boom. But as you also see from the paper, the collapse is not seen everywhere.” Other current studies suggest the fertility drop is immediate but temporary, says Dr. Aassve, who is also a professor of demography.
Interestingly, Dr. Aassve and colleagues found that CBRs were relatively stable in Northern Europe. The authors point to supportive social and family policies in that region that might have reduced the effect of the pandemic on births. “These factors are likely to affect CBRs in the subsequent pandemic waves,” they write. They call for future studies to assess the full population implications of the pandemic, the moderating impact of policy interventions, and the nexus between short- and long-run effects in relation to the various waves of the COVID-19 pandemic.
Rebounds
Some regions have already reported a rebound from the COVID-19 fertility trough. Molly J. Stout, MD, director of maternal fetal medicine at the University of Michigan, Ann Arbor, and colleagues used electronic medical records to predict a surge in births after the initial decline.
“The surge we’ve seen at the end of this summer is exceeding the usual annual birth rate, as predicted,” she said in an interview. “But I think there’ll be a return to normal after this transient escalation. I don’t think birth rates will stay elevated above the normal because the birth surge is a temporary response to an event, although there will likely be regional differences.”
Looking ahead, Dr. Stout, who was not involved in Dr. Aassve’s analysis, is not certain how a fourth pandemic wave might ultimately modify a couple’s overall family size. But the toll the health crisis has taken on working women who have been forced to withdraw from the economy because of a lack of childcare points to a societal need that should be addressed.
According to Philip N. Cohen, PhD, a professor of sociology at the University of Maryland, College Park, who’s been tracking fertility trends since the onset of the COVID-19 emergency, the pandemic has combined a health crisis with an economic crisis, along with “the additional factor of social distancing and isolation, which all contributed to the decline in birth rates. Some people changed their plans to hold off on having children, while others didn’t get pregnant because they weren’t socializing and meeting people as much.”
Dr. Cohen, who was not involved in the study by Dr. Aassve and associates, said his provisional data show that although in many places, birth rates have rebounded more or less to prepandemic levels after a nadir around Jan. 2021, some areas of the United States still show substantially lower rates, including California, Hawaii, and Oregon.
As to the duration of the pandemic effect, Dr. Aassve cautions that his group’s estimates refer to the first wave only. “We then have the second, third, and currently the fourth wave. We can’t be sure about the impact of these waves on fertility since the data are not there yet, but I’d be surprised if they didn’t continue to have an impact on fertility rates,” he said.
Dr. Cohen agreed: “Some people who delayed childbearing will make up the delay. However, whenever there’s a delay, there’s inevitably some portion of the decline that’s not recouped.”
As for the wider effect across the world, Dr. Aassve said his team’s figures derive from high-income countries where data are readily available. For middle- and low-income countries, fewer data exist, and the quality of those data is not as good.
The lessons from this and other upheavals teach us that unforeseen shocks almost always have a negative impact on fertility, says Dr. Aassve. “[B]ut these effects may be separate from existing declining trends. The issue here is that those overall declining trends may be driven by other factors. In contrast, the shock of the pandemic is short-lived, and we may return to normal rather quickly. But if the pandemic also impacts other societal structures, such as the occupational and industrial sectors, then the pandemic might exacerbate the negative trend.”
The study was supported by funding from the European Research Council for funding under the European Union’s Horizon 2020 Research and Innovation Programme. The study authors, Dr. Stout, and Dr. Cohen have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Malaria study shows remarkable 70% reduction in severe disease and death
A new study from Africa shows a remarkable 70% reduction in malaria if two treatments — a vaccine and an antimalarial medication — are combined instead of giving them individually.
Malaria is endemic in the tropics. The World Health Organization (WHO) reports that in 2019, there were 229 million cases and 409,000 deaths from this parasitic infection. Most of the burden (94%) occurs in Africa, and children younger than age 5 account for 67% of the deaths.
In the Sahel region of Africa, a broad, sub-Saharan band that stretches across the continent, high malaria transmission is seasonal. Children in some countries there are treated with monthly courses of sulfadoxine-pyrimethamine and amodiaquine chemoprophylaxis during the four higher-risk months. Such seasonal malaria chemoprophylaxis (SMC) has been shown to reduce infections by up to 88% and costs an average of $3.43 per child per year.
This double-blind, randomized controlled trial enrolled young children (5-17 months old) in Burkina Faso and Mali, where SMC is the current treatment regimen. Nearly 6,000 children received either chemoprophylaxis, the RTS,S/AS01E malaria vaccine (RTS,S), or both treatments. The study, led by investigators at the London School of Hygiene and Tropical Medicine (LSHTM), was reported in the New England Journal of Medicine.
Co-lead investigator Daniel Chandramohan, MBBS, PhD, MSc, professor of public health at LSHTM, said in an interview that SMC administration is quite labor-intensive and that “we thought we can replace these four cycles of seasonal cure prevention with one seasonal vaccination like the flu vaccine ... and that there might be some additive benefit.”
Instead, the study found the combination reduces the incidence of malaria by 62% against clinical malaria infection, 70% against severe malaria, and 73% against death from malaria compared with SMC alone. “Not in our wildest dreams would I have hypothesized that this is a possibility,” Dr. Chandramohan said. He continued that this was unlikely a “freak result” because the findings are “consistent between both countries. Two, it is consistent across the years. Three, all the malaria outcomes ... are consistently showing the protective effect at the same level.”
To maintain the blinded study design, children received injections of rabies vaccine and hepatitis A vaccine instead of a placebo for RTS,S. Both were chosen to provide additional benefits by protecting children against those infections.
With so many children followed over years, accuracy in providing the correct treatment for each study arm can be difficult. Each child was given a QR code and picture identification to facilitate drug distribution each year in this study.
Miriam K. Laufer, MD, professor and associate director for malaria research at the University of Maryland, Baltimore, who was not involved in the study, said in an interview, “This is a spectacular result, you know, decreasing disease by 60%-70% using interventions that we already have.”
RTS,S is not a new vaccine; it was developed in 2001 by GlaxoSmithKline with Path’s Malaria Vaccine Initiative, then manufactured by GSK. The Gates Foundation has supported production. Dr. Chandramohan said GSK has transferred the technology to Bharat, in India, and that it will take 2-3 years to ramp up production. Until then, enough vaccine is available to supply Kenya, Malawi, and Ghana, where the pilot studies are being done.
Dr. Laufer stressed that the “group that got RTS,S did as well as the group that received SMC.” She noted that the use of SMC is limited to specific areas of the Sahel sub-region of Africa, with a brief transmission period. In other areas of Africa where malaria has a longer transmission period, SMC isn’t as effective. “RTS,S vaccine could really have an impact” there, she added.
Asked if RTS,S might be substituted for SMC to reduce the likelihood of resistance emerging, Dr. Laufer said, “Giving RTS,S vaccine is as good as using repeated treatment of malaria drugs during the malaria season. And that’s important for two reasons. One is that the advantage of a vaccine is that you’re not producing pressure of drugs that would enable drug resistance to emerge and spread. So maybe your vaccine efficacy could last longer than drug efficacy. We don’t know the answer to that.”
Hypothesizing about the unexpectedly good trial results, Dr. Laufer explained, “We know that RTS,S decreases the number of parasites that make it into the blood when a child is bitten by an infected mosquito. When drugs like sulfadoxine-pyrimethamine and amodiaquine that have moderate efficacy only have to kill off a small number of parasites, they can work better. Maybe that explains why the combination of RTS,S and SMC created such a positive outcome.”
Dr. Laufer echoed Chandramohan, saying, “Results were much more dramatic than anybody – certainly than I anticipated.” Both physicians anticipate that WHO will give full approval for this combination this fall.
Dr. Chandramohan and Dr. Laufer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study from Africa shows a remarkable 70% reduction in malaria if two treatments — a vaccine and an antimalarial medication — are combined instead of giving them individually.
Malaria is endemic in the tropics. The World Health Organization (WHO) reports that in 2019, there were 229 million cases and 409,000 deaths from this parasitic infection. Most of the burden (94%) occurs in Africa, and children younger than age 5 account for 67% of the deaths.
In the Sahel region of Africa, a broad, sub-Saharan band that stretches across the continent, high malaria transmission is seasonal. Children in some countries there are treated with monthly courses of sulfadoxine-pyrimethamine and amodiaquine chemoprophylaxis during the four higher-risk months. Such seasonal malaria chemoprophylaxis (SMC) has been shown to reduce infections by up to 88% and costs an average of $3.43 per child per year.
This double-blind, randomized controlled trial enrolled young children (5-17 months old) in Burkina Faso and Mali, where SMC is the current treatment regimen. Nearly 6,000 children received either chemoprophylaxis, the RTS,S/AS01E malaria vaccine (RTS,S), or both treatments. The study, led by investigators at the London School of Hygiene and Tropical Medicine (LSHTM), was reported in the New England Journal of Medicine.
Co-lead investigator Daniel Chandramohan, MBBS, PhD, MSc, professor of public health at LSHTM, said in an interview that SMC administration is quite labor-intensive and that “we thought we can replace these four cycles of seasonal cure prevention with one seasonal vaccination like the flu vaccine ... and that there might be some additive benefit.”
Instead, the study found the combination reduces the incidence of malaria by 62% against clinical malaria infection, 70% against severe malaria, and 73% against death from malaria compared with SMC alone. “Not in our wildest dreams would I have hypothesized that this is a possibility,” Dr. Chandramohan said. He continued that this was unlikely a “freak result” because the findings are “consistent between both countries. Two, it is consistent across the years. Three, all the malaria outcomes ... are consistently showing the protective effect at the same level.”
To maintain the blinded study design, children received injections of rabies vaccine and hepatitis A vaccine instead of a placebo for RTS,S. Both were chosen to provide additional benefits by protecting children against those infections.
With so many children followed over years, accuracy in providing the correct treatment for each study arm can be difficult. Each child was given a QR code and picture identification to facilitate drug distribution each year in this study.
Miriam K. Laufer, MD, professor and associate director for malaria research at the University of Maryland, Baltimore, who was not involved in the study, said in an interview, “This is a spectacular result, you know, decreasing disease by 60%-70% using interventions that we already have.”
RTS,S is not a new vaccine; it was developed in 2001 by GlaxoSmithKline with Path’s Malaria Vaccine Initiative, then manufactured by GSK. The Gates Foundation has supported production. Dr. Chandramohan said GSK has transferred the technology to Bharat, in India, and that it will take 2-3 years to ramp up production. Until then, enough vaccine is available to supply Kenya, Malawi, and Ghana, where the pilot studies are being done.
Dr. Laufer stressed that the “group that got RTS,S did as well as the group that received SMC.” She noted that the use of SMC is limited to specific areas of the Sahel sub-region of Africa, with a brief transmission period. In other areas of Africa where malaria has a longer transmission period, SMC isn’t as effective. “RTS,S vaccine could really have an impact” there, she added.
Asked if RTS,S might be substituted for SMC to reduce the likelihood of resistance emerging, Dr. Laufer said, “Giving RTS,S vaccine is as good as using repeated treatment of malaria drugs during the malaria season. And that’s important for two reasons. One is that the advantage of a vaccine is that you’re not producing pressure of drugs that would enable drug resistance to emerge and spread. So maybe your vaccine efficacy could last longer than drug efficacy. We don’t know the answer to that.”
Hypothesizing about the unexpectedly good trial results, Dr. Laufer explained, “We know that RTS,S decreases the number of parasites that make it into the blood when a child is bitten by an infected mosquito. When drugs like sulfadoxine-pyrimethamine and amodiaquine that have moderate efficacy only have to kill off a small number of parasites, they can work better. Maybe that explains why the combination of RTS,S and SMC created such a positive outcome.”
Dr. Laufer echoed Chandramohan, saying, “Results were much more dramatic than anybody – certainly than I anticipated.” Both physicians anticipate that WHO will give full approval for this combination this fall.
Dr. Chandramohan and Dr. Laufer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study from Africa shows a remarkable 70% reduction in malaria if two treatments — a vaccine and an antimalarial medication — are combined instead of giving them individually.
Malaria is endemic in the tropics. The World Health Organization (WHO) reports that in 2019, there were 229 million cases and 409,000 deaths from this parasitic infection. Most of the burden (94%) occurs in Africa, and children younger than age 5 account for 67% of the deaths.
In the Sahel region of Africa, a broad, sub-Saharan band that stretches across the continent, high malaria transmission is seasonal. Children in some countries there are treated with monthly courses of sulfadoxine-pyrimethamine and amodiaquine chemoprophylaxis during the four higher-risk months. Such seasonal malaria chemoprophylaxis (SMC) has been shown to reduce infections by up to 88% and costs an average of $3.43 per child per year.
This double-blind, randomized controlled trial enrolled young children (5-17 months old) in Burkina Faso and Mali, where SMC is the current treatment regimen. Nearly 6,000 children received either chemoprophylaxis, the RTS,S/AS01E malaria vaccine (RTS,S), or both treatments. The study, led by investigators at the London School of Hygiene and Tropical Medicine (LSHTM), was reported in the New England Journal of Medicine.
Co-lead investigator Daniel Chandramohan, MBBS, PhD, MSc, professor of public health at LSHTM, said in an interview that SMC administration is quite labor-intensive and that “we thought we can replace these four cycles of seasonal cure prevention with one seasonal vaccination like the flu vaccine ... and that there might be some additive benefit.”
Instead, the study found the combination reduces the incidence of malaria by 62% against clinical malaria infection, 70% against severe malaria, and 73% against death from malaria compared with SMC alone. “Not in our wildest dreams would I have hypothesized that this is a possibility,” Dr. Chandramohan said. He continued that this was unlikely a “freak result” because the findings are “consistent between both countries. Two, it is consistent across the years. Three, all the malaria outcomes ... are consistently showing the protective effect at the same level.”
To maintain the blinded study design, children received injections of rabies vaccine and hepatitis A vaccine instead of a placebo for RTS,S. Both were chosen to provide additional benefits by protecting children against those infections.
With so many children followed over years, accuracy in providing the correct treatment for each study arm can be difficult. Each child was given a QR code and picture identification to facilitate drug distribution each year in this study.
Miriam K. Laufer, MD, professor and associate director for malaria research at the University of Maryland, Baltimore, who was not involved in the study, said in an interview, “This is a spectacular result, you know, decreasing disease by 60%-70% using interventions that we already have.”
RTS,S is not a new vaccine; it was developed in 2001 by GlaxoSmithKline with Path’s Malaria Vaccine Initiative, then manufactured by GSK. The Gates Foundation has supported production. Dr. Chandramohan said GSK has transferred the technology to Bharat, in India, and that it will take 2-3 years to ramp up production. Until then, enough vaccine is available to supply Kenya, Malawi, and Ghana, where the pilot studies are being done.
Dr. Laufer stressed that the “group that got RTS,S did as well as the group that received SMC.” She noted that the use of SMC is limited to specific areas of the Sahel sub-region of Africa, with a brief transmission period. In other areas of Africa where malaria has a longer transmission period, SMC isn’t as effective. “RTS,S vaccine could really have an impact” there, she added.
Asked if RTS,S might be substituted for SMC to reduce the likelihood of resistance emerging, Dr. Laufer said, “Giving RTS,S vaccine is as good as using repeated treatment of malaria drugs during the malaria season. And that’s important for two reasons. One is that the advantage of a vaccine is that you’re not producing pressure of drugs that would enable drug resistance to emerge and spread. So maybe your vaccine efficacy could last longer than drug efficacy. We don’t know the answer to that.”
Hypothesizing about the unexpectedly good trial results, Dr. Laufer explained, “We know that RTS,S decreases the number of parasites that make it into the blood when a child is bitten by an infected mosquito. When drugs like sulfadoxine-pyrimethamine and amodiaquine that have moderate efficacy only have to kill off a small number of parasites, they can work better. Maybe that explains why the combination of RTS,S and SMC created such a positive outcome.”
Dr. Laufer echoed Chandramohan, saying, “Results were much more dramatic than anybody – certainly than I anticipated.” Both physicians anticipate that WHO will give full approval for this combination this fall.
Dr. Chandramohan and Dr. Laufer have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Verruca Vulgaris Arising Within the Red Portion of a Multicolored Tattoo
To the Editor:
The art of tattooing continues to gain popularity in the 21st century, albeit with accompanying hazards.1 Reported adverse reactions to tattoos include infections, tumors, and hypersensitivity and granulomatous reactions.2 Various infectious agents may involve tattoos, including human papillomavirus (HPV), molluscum contagiosum, herpes simplex virus, hepatitis C virus, tuberculoid and nontuberculoid mycobacteria, and Staphylococcus aureus.2 Verruca vulgaris infrequently has been reported to develop in tattoos.3,4 Previously reported cases of verruca in tattoos suggest a predilection for blue or black pigment.1-5 We report a case of verruca vulgaris occurring within the red-inked areas of a tattoo that first appeared approximately 18 years after the initial tattoo placement.
A 44-year-old woman presented with erythema, induration, and irritation of a tattoo on the left leg of 2 years’ duration. The tattoo initially was inscribed more than 20 years prior. The patient had a history of type 2 diabetes mellitus and chronic obstructive pulmonary disease. She reported no prior trauma to the area, prior rash or irritation, or similar changes to her other tattoos, including those with red ink. The affected tattoo was inscribed at a separate time from the other tattoos. Physical examination of the irritated tattoo revealed hyperkeratotic papules with firm scaling in the zone of dermal red pigment (Figure 1). Notable nodularity or deep induration was not present. The clinical differential diagnosis included a hypersensitivity reaction to red tattoo ink, sarcoidosis, and an infectious process, such as an atypical mycobacterial infection. A punch biopsy demonstrated papillomatous epidermal hyperplasia with hyperkeratosis, focal parakeratosis, and frequent vacuolization of keratinocytes with enlarged keratohyalin granules, diagnostic of verruca vulgaris (Figure 2). Of note, the patient did not have clinically apparent viral warts elsewhere on physical examination. The patient was successfully managedwith a combination of 2 treatments of intralesional Candida antigen and 3 treatments of cryotherapy with resolution of most lesions over the course of 8 months. Over the following several months, the patient applied topical salicylic acid, which led to the resolution of the remaining lesions. The verrucae had not recurred 19 months after the initial presentation.
The development of verruca vulgaris within a tattoo may occur secondary to various mechanisms of HPV inoculation, including introduction of the virus through contaminated ink, the tattoo artist’s saliva, autoinoculation, or koebnerization of a pre-existing verruca vulgaris.4 Local immune system dysregulation secondary to tattoo ink also has been proposed as a mechanism for HPV infection in this setting.1,5 The contents of darker tattoo pigments may promote formation of reactive oxygen species inducing local immunocompromise.5
The pathogenic mechanism was elusive in our patient. Although the localization of verruca vulgaris to the zones of red pigment may be merely coincidental, this phenomenon raised suspicion for direct inoculation via contaminated red ink. The patient’s other red ink–containing tattoos that were inscribed separately were spared, compatible with contamination of the red ink used for the affected tattoo. However, the delayed onset of nearly 2 decades was exceptional, given the shorter previously reported latencies ranging from months to 10 years.4 Autoinoculation or koebnerization is plausible, though greater involvement of nonred pigments would be expected as well as a briefer latency. Finally, the possibility of local immune dysregulation seemed feasible, given the slow evolution of the lesions largely restricted to one pigment type.
We report a case of verruca vulgaris within the red area of a multicolored tattoo that occurred approximately 18 years after tattoo placement. This case highlights a rare presentation of an infectious agent that may complicate tattoos. Both predilection for red pigment rather than black or blue pigment and the long latency period raised interesting questions regarding pathogenesis. Confirmatory biopsy enables effective management of this tattoo complication.
- Huynh TN, Jackson JD, Brodell RT. Tattoo and vaccination sites: possible nest for opportunistic infections, tumors, and dysimmune reactions. Clin Dermatol. 2014;32:678-684.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Trefzer U, Schmollack K, Stockfleth E, et al. Verrucae in a multicolored decorative tattoo. J Am Acad Dermatol. 2004;50:478-479.
- Wanat KA, Tyring S, Rady P, et al. Human papillomavirus type 27 associated with multiple verruca within a tattoo: report of a case and review of the literature. Int J Dermatol. 2014;53:882-884.
- Ramey K, Ibrahim J, Brodell RT. Verruca localization predominately in black tattoo ink: a retrospective case series. J Eur Acad Dermatol Venereol. 2016;30:E34-E36.
To the Editor:
The art of tattooing continues to gain popularity in the 21st century, albeit with accompanying hazards.1 Reported adverse reactions to tattoos include infections, tumors, and hypersensitivity and granulomatous reactions.2 Various infectious agents may involve tattoos, including human papillomavirus (HPV), molluscum contagiosum, herpes simplex virus, hepatitis C virus, tuberculoid and nontuberculoid mycobacteria, and Staphylococcus aureus.2 Verruca vulgaris infrequently has been reported to develop in tattoos.3,4 Previously reported cases of verruca in tattoos suggest a predilection for blue or black pigment.1-5 We report a case of verruca vulgaris occurring within the red-inked areas of a tattoo that first appeared approximately 18 years after the initial tattoo placement.
A 44-year-old woman presented with erythema, induration, and irritation of a tattoo on the left leg of 2 years’ duration. The tattoo initially was inscribed more than 20 years prior. The patient had a history of type 2 diabetes mellitus and chronic obstructive pulmonary disease. She reported no prior trauma to the area, prior rash or irritation, or similar changes to her other tattoos, including those with red ink. The affected tattoo was inscribed at a separate time from the other tattoos. Physical examination of the irritated tattoo revealed hyperkeratotic papules with firm scaling in the zone of dermal red pigment (Figure 1). Notable nodularity or deep induration was not present. The clinical differential diagnosis included a hypersensitivity reaction to red tattoo ink, sarcoidosis, and an infectious process, such as an atypical mycobacterial infection. A punch biopsy demonstrated papillomatous epidermal hyperplasia with hyperkeratosis, focal parakeratosis, and frequent vacuolization of keratinocytes with enlarged keratohyalin granules, diagnostic of verruca vulgaris (Figure 2). Of note, the patient did not have clinically apparent viral warts elsewhere on physical examination. The patient was successfully managedwith a combination of 2 treatments of intralesional Candida antigen and 3 treatments of cryotherapy with resolution of most lesions over the course of 8 months. Over the following several months, the patient applied topical salicylic acid, which led to the resolution of the remaining lesions. The verrucae had not recurred 19 months after the initial presentation.


The development of verruca vulgaris within a tattoo may occur secondary to various mechanisms of HPV inoculation, including introduction of the virus through contaminated ink, the tattoo artist’s saliva, autoinoculation, or koebnerization of a pre-existing verruca vulgaris.4 Local immune system dysregulation secondary to tattoo ink also has been proposed as a mechanism for HPV infection in this setting.1,5 The contents of darker tattoo pigments may promote formation of reactive oxygen species inducing local immunocompromise.5
The pathogenic mechanism was elusive in our patient. Although the localization of verruca vulgaris to the zones of red pigment may be merely coincidental, this phenomenon raised suspicion for direct inoculation via contaminated red ink. The patient’s other red ink–containing tattoos that were inscribed separately were spared, compatible with contamination of the red ink used for the affected tattoo. However, the delayed onset of nearly 2 decades was exceptional, given the shorter previously reported latencies ranging from months to 10 years.4 Autoinoculation or koebnerization is plausible, though greater involvement of nonred pigments would be expected as well as a briefer latency. Finally, the possibility of local immune dysregulation seemed feasible, given the slow evolution of the lesions largely restricted to one pigment type.
We report a case of verruca vulgaris within the red area of a multicolored tattoo that occurred approximately 18 years after tattoo placement. This case highlights a rare presentation of an infectious agent that may complicate tattoos. Both predilection for red pigment rather than black or blue pigment and the long latency period raised interesting questions regarding pathogenesis. Confirmatory biopsy enables effective management of this tattoo complication.
To the Editor:
The art of tattooing continues to gain popularity in the 21st century, albeit with accompanying hazards.1 Reported adverse reactions to tattoos include infections, tumors, and hypersensitivity and granulomatous reactions.2 Various infectious agents may involve tattoos, including human papillomavirus (HPV), molluscum contagiosum, herpes simplex virus, hepatitis C virus, tuberculoid and nontuberculoid mycobacteria, and Staphylococcus aureus.2 Verruca vulgaris infrequently has been reported to develop in tattoos.3,4 Previously reported cases of verruca in tattoos suggest a predilection for blue or black pigment.1-5 We report a case of verruca vulgaris occurring within the red-inked areas of a tattoo that first appeared approximately 18 years after the initial tattoo placement.
A 44-year-old woman presented with erythema, induration, and irritation of a tattoo on the left leg of 2 years’ duration. The tattoo initially was inscribed more than 20 years prior. The patient had a history of type 2 diabetes mellitus and chronic obstructive pulmonary disease. She reported no prior trauma to the area, prior rash or irritation, or similar changes to her other tattoos, including those with red ink. The affected tattoo was inscribed at a separate time from the other tattoos. Physical examination of the irritated tattoo revealed hyperkeratotic papules with firm scaling in the zone of dermal red pigment (Figure 1). Notable nodularity or deep induration was not present. The clinical differential diagnosis included a hypersensitivity reaction to red tattoo ink, sarcoidosis, and an infectious process, such as an atypical mycobacterial infection. A punch biopsy demonstrated papillomatous epidermal hyperplasia with hyperkeratosis, focal parakeratosis, and frequent vacuolization of keratinocytes with enlarged keratohyalin granules, diagnostic of verruca vulgaris (Figure 2). Of note, the patient did not have clinically apparent viral warts elsewhere on physical examination. The patient was successfully managedwith a combination of 2 treatments of intralesional Candida antigen and 3 treatments of cryotherapy with resolution of most lesions over the course of 8 months. Over the following several months, the patient applied topical salicylic acid, which led to the resolution of the remaining lesions. The verrucae had not recurred 19 months after the initial presentation.


The development of verruca vulgaris within a tattoo may occur secondary to various mechanisms of HPV inoculation, including introduction of the virus through contaminated ink, the tattoo artist’s saliva, autoinoculation, or koebnerization of a pre-existing verruca vulgaris.4 Local immune system dysregulation secondary to tattoo ink also has been proposed as a mechanism for HPV infection in this setting.1,5 The contents of darker tattoo pigments may promote formation of reactive oxygen species inducing local immunocompromise.5
The pathogenic mechanism was elusive in our patient. Although the localization of verruca vulgaris to the zones of red pigment may be merely coincidental, this phenomenon raised suspicion for direct inoculation via contaminated red ink. The patient’s other red ink–containing tattoos that were inscribed separately were spared, compatible with contamination of the red ink used for the affected tattoo. However, the delayed onset of nearly 2 decades was exceptional, given the shorter previously reported latencies ranging from months to 10 years.4 Autoinoculation or koebnerization is plausible, though greater involvement of nonred pigments would be expected as well as a briefer latency. Finally, the possibility of local immune dysregulation seemed feasible, given the slow evolution of the lesions largely restricted to one pigment type.
We report a case of verruca vulgaris within the red area of a multicolored tattoo that occurred approximately 18 years after tattoo placement. This case highlights a rare presentation of an infectious agent that may complicate tattoos. Both predilection for red pigment rather than black or blue pigment and the long latency period raised interesting questions regarding pathogenesis. Confirmatory biopsy enables effective management of this tattoo complication.
- Huynh TN, Jackson JD, Brodell RT. Tattoo and vaccination sites: possible nest for opportunistic infections, tumors, and dysimmune reactions. Clin Dermatol. 2014;32:678-684.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Trefzer U, Schmollack K, Stockfleth E, et al. Verrucae in a multicolored decorative tattoo. J Am Acad Dermatol. 2004;50:478-479.
- Wanat KA, Tyring S, Rady P, et al. Human papillomavirus type 27 associated with multiple verruca within a tattoo: report of a case and review of the literature. Int J Dermatol. 2014;53:882-884.
- Ramey K, Ibrahim J, Brodell RT. Verruca localization predominately in black tattoo ink: a retrospective case series. J Eur Acad Dermatol Venereol. 2016;30:E34-E36.
- Huynh TN, Jackson JD, Brodell RT. Tattoo and vaccination sites: possible nest for opportunistic infections, tumors, and dysimmune reactions. Clin Dermatol. 2014;32:678-684.
- Wenzel SM, Rittmann I, Landthaler M, et al. Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology. 2013;226:138-147.
- Trefzer U, Schmollack K, Stockfleth E, et al. Verrucae in a multicolored decorative tattoo. J Am Acad Dermatol. 2004;50:478-479.
- Wanat KA, Tyring S, Rady P, et al. Human papillomavirus type 27 associated with multiple verruca within a tattoo: report of a case and review of the literature. Int J Dermatol. 2014;53:882-884.
- Ramey K, Ibrahim J, Brodell RT. Verruca localization predominately in black tattoo ink: a retrospective case series. J Eur Acad Dermatol Venereol. 2016;30:E34-E36.
Practice Points
- Various adverse reactions and infectious agents may involve tattoos.
- Verruca vulgaris may affect tattoos in a color-restricted manner and demonstrate latency of many years after tattoo placement.
- Timely diagnosis of the tattoo-involving process, confirmed by biopsy, allows for appropriate management.
‘Deeper dive’ into opioid overdose deaths during COVID pandemic
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.
Opioid overdose deaths were significantly higher during 2020, but occurrences were not homogeneous across nine states. Male deaths were higher than in the 2 previous years in two states, according to a new, granular examination of data collected by researchers at the Massachusetts General Hospital (Mass General), Boston.
The analysis also showed that synthetic opioids such as fentanyl played an outsized role in most of the states that were reviewed. Additional drugs of abuse found in decedents, such as cocaine and psychostimulants, were more prevalent in some states than in others.
The Centers for Disease Control and Prevention used provisional death data in its recent report. It found that opioid-related deaths substantially rose in 2020 and that synthetic opioids were a primary driver.
The current Mass General analysis provides a more timely and detailed dive, senior author Mohammad Jalali, PhD, who is a senior scientist at Mass General’s Institute for Technology Assessment, told this news organization.
The findings, which have not yet been peer reviewed, were published in MedRxiv.
Shifting sands of opioid use disorder
to analyze and project trends and also to be better prepared to address the shifting sands of opioid use disorder in the United States.
They attempted to collect data on confirmed opioid overdose deaths from all 50 states and Washington, D.C. to assess what might have changed during the COVID-19 pandemic. Only nine states provided enough data for the analysis, which has been submitted to a peer reviewed publication.
These states were Alaska, Connecticut, Indiana, Massachusetts, North Carolina, Rhode Island, Colorado, Utah, and Wyoming.
“Drug overdose data are collected and reported more slowly than COVID-19 data,” Dr. Jalali said in a press release. The data reflected a lag time of about 4 to 8 months in Massachusetts and North Carolina to more than a year in Maryland and Ohio, he noted.
The reporting lag “has clouded the understanding of the effects of the COVID-19 pandemic on opioid-related overdose deaths,” said Dr. Jalali.
Commenting on the findings, Brandon Marshall, PhD, associate professor of epidemiology at Brown University, Providence, R.I, said that “the overall pattern of what’s being reported here is not surprising,” given the national trends seen in the CDC data.
“This paper adds a deeper dive into some of the sociodemographic trends that we’re starting to observe in specific states,” Dr. Marshall said.
Also commenting for this news organization, Brian Fuehrlein, MD, PhD, director of the psychiatric emergency department at the VA Connecticut Healthcare System in West Haven, Connecticut, noted that the current study “highlights things that we are currently seeing at VA Connecticut.”
Decrease in heroin, rise in fentanyl
The investigators found a significant reduction in overdose deaths that involved heroin in Alaska, Connecticut, Indiana, Massachusetts, North Carolina, and Rhode Island. That was a new trend for Alaska, Indiana, and Rhode Island, although with only 3 years of data, it’s hard to say whether it will continue, Dr. Jalali noted.
The decrease in heroin involvement seemed to continue a trend previously observed in Colorado, Connecticut, Massachusetts, and North Carolina.
In Connecticut, heroin was involved in 36% of deaths in 2018, 30% in 2019, and 16% in 2020, according to the study.
“We have begun seeing more and more heroin-negative, fentanyl-positive drug screens,” said Dr. Fuehrlein, who is also associate professor of psychiatry at Yale University, New Haven, Conn.
“There is a shift from fentanyl being an adulterant to fentanyl being what is sold and used exclusively,” he added.
In 2020, 92% (n = 887) of deaths in Connecticut involved synthetic opioids, continuing a trend. In Alaska, however, synthetic opioids were involved in 60% (44) of deaths, which is a big jump from 23% (9) in 2018.
Synthetic opioids were involved in the largest percentage of overdoses in all of the states studied. The fewest deaths, 17 (49%), occurred in Wyoming.
Cocaine is also increasingly found in addition to other substances in decedents. In Alaska, about 14% of individuals who overdosed in 2020 also had cocaine in their system, which was a jump from 2% in the prior year.
In Colorado, 19% (94) of those who died also had taken cocaine, up from 13% in 2019. Cocaine was also frequently found in those who died in the northeast: 39% (467) of those who died in Massachusetts, 29% (280) in Connecticut, and 47% (109) in Rhode Island.
There was also an increase in psychostimulants found in those who had died in Massachusetts in 2020.
More male overdoses in 2020
Results also showed that, compared to 2019, significantly more men died from overdoses in 2020 in Colorado (61% vs. 70%, P = .017) and Indiana (62% vs. 70%, P = .026).
This finding was unexpected, said Dr. Marshall, who has observed the same phenomenon in Rhode Island. He is the scientific director of PreventOverdoseRI, Rhode Island’s drug overdose surveillance and information dashboard.
Dr. Marshall and his colleagues conducted a study that also found disproportionate increases in overdoses among men. The findings of that study will be published in September.
“We’re still trying to wrap our head around why that is,” he said. He added that a deeper dive into the Rhode Island data showed that the deaths were increased especially among middle-aged men who had been diagnosed with depression and anxiety.
The same patterns were not seen among women in either Dr. Jalali’s study or his own analysis of the Rhode Island data, said Dr. Marshall.
“That suggests the COVID-19 pandemic impacted men who are at risk for overdose in some particularly severe way,” he noted.
Dr. Fuehrlein said he believes a variety of factors have led to an increase in overdose deaths during the pandemic, including the fact that many patients who would normally seek help avoided care or dropped out of treatment because of COVID fears. In addition, other support systems, such as group therapy and Narcotics Anonymous, were unavailable.
The pandemic increased stress, which can lead to worsening substance use, said Dr. Fuehrlein. He also noted that regular opioid suppliers were often not available, which led some to buy from different dealers, “which can lead to overdose if the fentanyl content is different.”
Identifying at-risk individuals
Dr. Jalali and colleagues note that clinicians and policymakers could use the new study to help identify and treat at-risk individuals.
“Practitioners and policy makers can use our findings to help them anticipate which groups of people might be most affected by opioid overdose and which types of policy interventions might be most effective given each state’s unique situation,” said lead study author Gian-Gabriel P. Garcia, PhD, in a press release. At the time of the study, Dr. Garcia was a postdoctoral fellow at Mass General and Harvard Medical School. He is currently an assistant professor at Georgia Tech, Atlanta.
Dr. Marshall pointed out that Dr. Jalali’s study is also relevant for emergency departments.
ED clinicians “are and will be seeing patients coming in who have no idea they were exposed to an opioid, nevermind fentanyl,” he said. ED clinicians can discuss with patients various harm reduction techniques, including the use of naloxone as well as test strips that can detect fentanyl in the drug supply, he added.
“Given the increasing use of fentanyl, which is very dangerous in overdose, clinicians need to be well versed in a harm reduction/overdose prevention approach to patient care,” Dr. Fuehrlein agreed.
A version of this article first appeared on Medscape.com.