User login
Virtual Respiratory Urgent Clinics for COVID-19 Symptoms
Virtual care (VC) has emerged as an effective mode of health care delivery especially in settings where significant barriers to traditional in-person visits exist; a large systematic review supports feasibility of telemedicine in primary care and suggests that telemedicine is at least as effective as traditional care.1 Nevertheless, broad adoption of VC into practice has lagged, impeded by government and private insurance reimbursement requirements as well as the persistent belief that care can best be delivered in person.2-4 Before the COVID-19 pandemic, states that enacted parity legislation that required private insurance companies to provide reimbursement coverage for telehealth services saw a significant increase in the number of outpatient telehealth visits (about ≥ 30% odds compared with nonparity states).3
With the onset of the COVID-19 pandemic, in-person medical appointments were converted to VC visits to reduce increased exposure risks to patients and health care workers.5 Prior government and private sector policies were suspended, and payment restrictions lifted, enabling adoption of VC modalities to rapidly accommodate the emergent need and Centers for Disease Control and Prevention (CDC) recommendations for virtual care.6-11
The CDC guidelines on managing operations during the COVID-19 pandemic highlighted the need to provide care in the safest way for patients and health care personnel and emphasized the importance of optimizing telehealth services. The federal government facilitated telehealth during the COVID-19 pandemic via temporary measures under the COVID-19 public health emergency declaration. This included Health Insurance Portability and Accountability Act flexibility to use everyday technology for VC visits, regulatory changes to deliver services to Medicare and Medicaid patients, permission of telehealth services across state lines, and prescribing of controlled substances via telehealth without an in-person medical evaluation.7
In response, health care providers (HCPs) and health care organizations created or expanded on existing telehealth infrastructure, developing virtual urgent care centers and telephone-based programs to evaluate patients remotely via screening questions that triaged them to a correct level of response, with possible subsequent virtual physician evaluation if indicated.12,13
The Veterans Health Administration (VHA) also shifted to a VC model in response to COVID-19 guided by a unique perspective from a well-developed prior VC experience.14-16 As a federally funded system, the VHA depends on workload documentation for budgeting. Since 2015, the VHA has provided workload credit and incentivized HCPs (via pay for performance) for the use of VC, including telephone visits, video visits, and secure messaging. These incentives resulted in higher rates of telehealth utilization before the COVID-19 pandemic compared with the private sector (with 4.2% and 0.7% of visits within the VHA being telephone and video visits, respectively, compared with telehealth utilization rates of 1.0% for Medicare recipients and 1.1% in an all-payer database).16
Historically, VHA care has successfully transitioned from in-person care models to exclusively virtual modalities to prevent suspension of medical services during natural disasters. Studies performed during these periods, specifically during the 2017 hurricane season (during which multiple VHA hospitals were closed or had limited in-person service available), supported telehealth as an efficient health care delivery method, and even recommended expanding telehealth services within non-VHA environments to accommodate needs of the general public during crises and postdisaster health care delivery.17
Armed with both a well-established telehealth infrastructure and prior knowledge gained from successful systemwide implementation of virtual care during times of disaster, US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACHS) primary care quickly transitioned to a VC model in response to COVID-19.16 Early in the pandemic, a rapid transition to virtual care (RTVC) model was developed, including implementation of virtual respiratory urgent clinics (VRUCs), defined as virtual respiratory symptom triage clinics, staffed by primary care providers (PCPs) aimed at minimizing patient and health care worker exposure risk.
Methods
VACHS consists of 8 primary care sites, including a major tertiary care center, a smaller medical center with full ambulatory services, and 6 community-based outpatient clinics with only primary care and mental health. There are 80 individual PCPs delivering care to 58,058 veterans. VRUCs were established during the COVID-19 pandemic to cover patients across the entire health care system, using a rotational schedule of VA PCPs.
COVID-19 Urgent Clinics Program
Within the first few weeks of the pandemic, VACHS primary care established VRUCS to provide expeditious virtual assessment of respiratory or flu-like symptoms. Using the established telehealth system, the intervention aimed to provide emergent screening, testing, and care to those with potential COVID-19 infections. The model also was designed to minimize exposures to the health care workforce and patients.
Retrospective analysis was performed using information obtained from the electronic health record (EHR) database to describe the characteristics of patients who received care through the VRUCs, such as demographics, era of military service, COVID-19 testing rates and results, as well as subsequent emergency department (ED) visits and hospital admissions. A secondary aim included collection of additional qualitative data via a random sample chart review.
Virtual clinics were established January 22, 2020, and data were analyzed over the next 3 months. Data were retrieved and analyzed from the EHR, and codes were used to categorize the VRUCs.
Results
A total of 445 unique patients used these clinics during this period. Unique patients were defined as individual patients (some may have used a clinic more than once but were counted only once). Of this group, 82% were male, and 48% served in the Gulf War era (1990 to present). A total of 51% of patients received a COVID-19 test (clinics began before wide testing availability), and 10% tested positive. Of all patients using the clinics, approximately 5% were admitted to the hospital, and 18% had at least 1 subsequent ED visit (Table).
A secondary aim included review of a random sample of 99 patient charts to gain additional information regarding whether the patient was given appropriate isolation precautions, was in a high-exposure occupation (eg, could expose a large number of people), and whether there was appropriate documentation of goals of care, health care proxy or referral to social work to discuss advance directives. In addition, we calculated the average length of time between patients’ initial contact with the health care system call center and the return call by the PCP (wait time).Of charts reviewed, the majority (71%) had documentation of appropriate isolation precautions. Although 25% of patients had documentation of a high-risk profession with potential to expose many people, more than half of the patients had no documentation of occupation. Most patients (86%) had no updated documentation regarding goals of care, health care proxy, or advance directives in their urgent care VC visit. The average time between the patient initiating contact with the health care system call center and a return call to the patient from a PCP was 104 minutes (excluding calls received after 3:30
Discussion
This analysis adds to the growing literature on use of VC during the COVID-19 pandemic. Specifically, we describe the population of patients who used VRUCs within a large health care system in a RTVC. This analysis was limited by lack of available testing during the initial phase of the pandemic, which contributed to the lower than expected rates of testing and test positivity in patients managed via VRUCs. In addition, chart review data are limited as the data includes only what was documented during the visit and not the entire discussion during the encounter.
Several important outcomes from this analysis can be applied to interventions in the future, which may have large public health implications: Several hundred patients who reported respiratory symptoms were expeditiously evaluated by a PCP using VC. The average wait time to full clinical assessment was about 1.5 hours. This short duration between contact and evaluation permitted early education about isolation precautions, which may have minimized spread. In addition, this innovation kept patients out of the medical center, eliminating chains of transmission to other vulnerable patients and health care workers.
Our retrospective chart review also revealed that more than half the patients were not queried about their occupation, but of those that were asked, a significant number were in high-risk professions potentially exposing large numbers of people. This would be an important aspect to add to future templated notes to minimize work-related exposures. Also, we identified that few HCPs discussed goals of care with patients. Given the nature of COVID-19 and potential for rapid decompensation especially in vulnerable patients, this also would be important to include in the future.
Conclusions
VC urgent care clinics to address possible COVID-19 symptoms facilitated expeditious PCP assessment while keeping potentially contagious patients outside of high-risk health care environments. Streamlining and optimizing clinical VC assessments will be imperative to future management of COVID-19 and potentially to other future infectious pandemics. This includes development of templated notes incorporating counseling regarding appropriate isolation, questions about high-contact occupations, and goals of care discussions.
Acknowledgment
The authors thank Robert F. Walsh, MHA.
1. Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
2. Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
3. Harvey JB, Valenta S, Simpson K, Lyles M, McElligott J. Utilization of outpatient telehealth services in parity and nonparity states 2010-2015. Telemed J E Health. 2019;25(2):132-136. doi:10.1089/tmj.2017.0265
4. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/NEJMra1601705
5. Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care. 2020;26(4):147-148. doi:10.37765/ajmc.2020.42784
6. Centers for Disease Control and Prevention. Healthcare facility guidance. Updated April 17, 2021. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html
7. US Department of Health and Human Services, Health Resources and Services Administration. Policy changes during COVID-19. Accessed August 20, 2021. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency
8. Coronavirus Preparedness and Response Supplemental Appropriation Act of 2020. 134 Stat. 146. Published February 2, 2021. Accessed August 20, 2021. https://www.govinfo.gov/content/pkg/CREC-2021-02-02/html/CREC-2021-02-02-pt1-PgS226.htm
9. US Department of Health and Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Updated January 20, 2021. Accessed August 20, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
10. Centers for Medicare and Medicaid Services. Coverage and payment related to COVID-19 Medicare. 2020. Published March 23, 2020. Accessed August 20, 2021. https://www.cms.gov/files/document/03052020-medicare-covid-19-fact-sheet.pdf
11. American Telemedicine Association. ATA commends 2020 Congress for giving HHS authority to waive restrictions on telehealth for Medicare beneficiaries in response to the COVID-19 outbreak [press release]. Published March 5, 2020. Accessed August 20, 2021. https://www.americantelemed.org/press-releases/ata-commends-congress-for-waiving-restrictions-on-telehealth-for-medicare-beneficiaries-in-response-to-the-covid-19-outbreak
12. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681. doi:10.1056/NEJMp2003539
13. Khairat S, Meng C, Xu Y, Edson B, Gianforcaro R. Interpreting COVID-19 and Virtual Care Trends: Cohort Study. JMIR Public Health Surveill. 2020;6(2):e18811. Published 2020 Apr 15. doi:10.2196/18811
14. Ferguson JM, Jacobs J, Yefimova M, Greene L, Heyworth L, Zulman DM. Virtual care expansion in the Veterans Health Administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2021;28(3):453-462. doi:10.1093/jamia/ocaa284
15. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
16. Spelman JF, Brienza R, Walsh RF, et al. A model for rapid transition to virtual care, VA Connecticut primary care response to COVID-19. J Gen Intern Med. 2020;35(10):3073-3076. doi:10.1007/s11606-020-06041-4
17. Der-Martirosian C, Chu K, Dobalian A. Use of telehealth to improve access to care at the United States Department of Veterans Affairs during the 2017 Atlantic hurricane season [published online ahead of print, 2020 Apr 13]. Disaster Med Public Health Prep. 2020;1-5. doi:10.1017/dmp.2020.88
Virtual care (VC) has emerged as an effective mode of health care delivery especially in settings where significant barriers to traditional in-person visits exist; a large systematic review supports feasibility of telemedicine in primary care and suggests that telemedicine is at least as effective as traditional care.1 Nevertheless, broad adoption of VC into practice has lagged, impeded by government and private insurance reimbursement requirements as well as the persistent belief that care can best be delivered in person.2-4 Before the COVID-19 pandemic, states that enacted parity legislation that required private insurance companies to provide reimbursement coverage for telehealth services saw a significant increase in the number of outpatient telehealth visits (about ≥ 30% odds compared with nonparity states).3
With the onset of the COVID-19 pandemic, in-person medical appointments were converted to VC visits to reduce increased exposure risks to patients and health care workers.5 Prior government and private sector policies were suspended, and payment restrictions lifted, enabling adoption of VC modalities to rapidly accommodate the emergent need and Centers for Disease Control and Prevention (CDC) recommendations for virtual care.6-11
The CDC guidelines on managing operations during the COVID-19 pandemic highlighted the need to provide care in the safest way for patients and health care personnel and emphasized the importance of optimizing telehealth services. The federal government facilitated telehealth during the COVID-19 pandemic via temporary measures under the COVID-19 public health emergency declaration. This included Health Insurance Portability and Accountability Act flexibility to use everyday technology for VC visits, regulatory changes to deliver services to Medicare and Medicaid patients, permission of telehealth services across state lines, and prescribing of controlled substances via telehealth without an in-person medical evaluation.7
In response, health care providers (HCPs) and health care organizations created or expanded on existing telehealth infrastructure, developing virtual urgent care centers and telephone-based programs to evaluate patients remotely via screening questions that triaged them to a correct level of response, with possible subsequent virtual physician evaluation if indicated.12,13
The Veterans Health Administration (VHA) also shifted to a VC model in response to COVID-19 guided by a unique perspective from a well-developed prior VC experience.14-16 As a federally funded system, the VHA depends on workload documentation for budgeting. Since 2015, the VHA has provided workload credit and incentivized HCPs (via pay for performance) for the use of VC, including telephone visits, video visits, and secure messaging. These incentives resulted in higher rates of telehealth utilization before the COVID-19 pandemic compared with the private sector (with 4.2% and 0.7% of visits within the VHA being telephone and video visits, respectively, compared with telehealth utilization rates of 1.0% for Medicare recipients and 1.1% in an all-payer database).16
Historically, VHA care has successfully transitioned from in-person care models to exclusively virtual modalities to prevent suspension of medical services during natural disasters. Studies performed during these periods, specifically during the 2017 hurricane season (during which multiple VHA hospitals were closed or had limited in-person service available), supported telehealth as an efficient health care delivery method, and even recommended expanding telehealth services within non-VHA environments to accommodate needs of the general public during crises and postdisaster health care delivery.17
Armed with both a well-established telehealth infrastructure and prior knowledge gained from successful systemwide implementation of virtual care during times of disaster, US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACHS) primary care quickly transitioned to a VC model in response to COVID-19.16 Early in the pandemic, a rapid transition to virtual care (RTVC) model was developed, including implementation of virtual respiratory urgent clinics (VRUCs), defined as virtual respiratory symptom triage clinics, staffed by primary care providers (PCPs) aimed at minimizing patient and health care worker exposure risk.
Methods
VACHS consists of 8 primary care sites, including a major tertiary care center, a smaller medical center with full ambulatory services, and 6 community-based outpatient clinics with only primary care and mental health. There are 80 individual PCPs delivering care to 58,058 veterans. VRUCs were established during the COVID-19 pandemic to cover patients across the entire health care system, using a rotational schedule of VA PCPs.
COVID-19 Urgent Clinics Program
Within the first few weeks of the pandemic, VACHS primary care established VRUCS to provide expeditious virtual assessment of respiratory or flu-like symptoms. Using the established telehealth system, the intervention aimed to provide emergent screening, testing, and care to those with potential COVID-19 infections. The model also was designed to minimize exposures to the health care workforce and patients.
Retrospective analysis was performed using information obtained from the electronic health record (EHR) database to describe the characteristics of patients who received care through the VRUCs, such as demographics, era of military service, COVID-19 testing rates and results, as well as subsequent emergency department (ED) visits and hospital admissions. A secondary aim included collection of additional qualitative data via a random sample chart review.
Virtual clinics were established January 22, 2020, and data were analyzed over the next 3 months. Data were retrieved and analyzed from the EHR, and codes were used to categorize the VRUCs.
Results
A total of 445 unique patients used these clinics during this period. Unique patients were defined as individual patients (some may have used a clinic more than once but were counted only once). Of this group, 82% were male, and 48% served in the Gulf War era (1990 to present). A total of 51% of patients received a COVID-19 test (clinics began before wide testing availability), and 10% tested positive. Of all patients using the clinics, approximately 5% were admitted to the hospital, and 18% had at least 1 subsequent ED visit (Table).
A secondary aim included review of a random sample of 99 patient charts to gain additional information regarding whether the patient was given appropriate isolation precautions, was in a high-exposure occupation (eg, could expose a large number of people), and whether there was appropriate documentation of goals of care, health care proxy or referral to social work to discuss advance directives. In addition, we calculated the average length of time between patients’ initial contact with the health care system call center and the return call by the PCP (wait time).Of charts reviewed, the majority (71%) had documentation of appropriate isolation precautions. Although 25% of patients had documentation of a high-risk profession with potential to expose many people, more than half of the patients had no documentation of occupation. Most patients (86%) had no updated documentation regarding goals of care, health care proxy, or advance directives in their urgent care VC visit. The average time between the patient initiating contact with the health care system call center and a return call to the patient from a PCP was 104 minutes (excluding calls received after 3:30
Discussion
This analysis adds to the growing literature on use of VC during the COVID-19 pandemic. Specifically, we describe the population of patients who used VRUCs within a large health care system in a RTVC. This analysis was limited by lack of available testing during the initial phase of the pandemic, which contributed to the lower than expected rates of testing and test positivity in patients managed via VRUCs. In addition, chart review data are limited as the data includes only what was documented during the visit and not the entire discussion during the encounter.
Several important outcomes from this analysis can be applied to interventions in the future, which may have large public health implications: Several hundred patients who reported respiratory symptoms were expeditiously evaluated by a PCP using VC. The average wait time to full clinical assessment was about 1.5 hours. This short duration between contact and evaluation permitted early education about isolation precautions, which may have minimized spread. In addition, this innovation kept patients out of the medical center, eliminating chains of transmission to other vulnerable patients and health care workers.
Our retrospective chart review also revealed that more than half the patients were not queried about their occupation, but of those that were asked, a significant number were in high-risk professions potentially exposing large numbers of people. This would be an important aspect to add to future templated notes to minimize work-related exposures. Also, we identified that few HCPs discussed goals of care with patients. Given the nature of COVID-19 and potential for rapid decompensation especially in vulnerable patients, this also would be important to include in the future.
Conclusions
VC urgent care clinics to address possible COVID-19 symptoms facilitated expeditious PCP assessment while keeping potentially contagious patients outside of high-risk health care environments. Streamlining and optimizing clinical VC assessments will be imperative to future management of COVID-19 and potentially to other future infectious pandemics. This includes development of templated notes incorporating counseling regarding appropriate isolation, questions about high-contact occupations, and goals of care discussions.
Acknowledgment
The authors thank Robert F. Walsh, MHA.
Virtual care (VC) has emerged as an effective mode of health care delivery especially in settings where significant barriers to traditional in-person visits exist; a large systematic review supports feasibility of telemedicine in primary care and suggests that telemedicine is at least as effective as traditional care.1 Nevertheless, broad adoption of VC into practice has lagged, impeded by government and private insurance reimbursement requirements as well as the persistent belief that care can best be delivered in person.2-4 Before the COVID-19 pandemic, states that enacted parity legislation that required private insurance companies to provide reimbursement coverage for telehealth services saw a significant increase in the number of outpatient telehealth visits (about ≥ 30% odds compared with nonparity states).3
With the onset of the COVID-19 pandemic, in-person medical appointments were converted to VC visits to reduce increased exposure risks to patients and health care workers.5 Prior government and private sector policies were suspended, and payment restrictions lifted, enabling adoption of VC modalities to rapidly accommodate the emergent need and Centers for Disease Control and Prevention (CDC) recommendations for virtual care.6-11
The CDC guidelines on managing operations during the COVID-19 pandemic highlighted the need to provide care in the safest way for patients and health care personnel and emphasized the importance of optimizing telehealth services. The federal government facilitated telehealth during the COVID-19 pandemic via temporary measures under the COVID-19 public health emergency declaration. This included Health Insurance Portability and Accountability Act flexibility to use everyday technology for VC visits, regulatory changes to deliver services to Medicare and Medicaid patients, permission of telehealth services across state lines, and prescribing of controlled substances via telehealth without an in-person medical evaluation.7
In response, health care providers (HCPs) and health care organizations created or expanded on existing telehealth infrastructure, developing virtual urgent care centers and telephone-based programs to evaluate patients remotely via screening questions that triaged them to a correct level of response, with possible subsequent virtual physician evaluation if indicated.12,13
The Veterans Health Administration (VHA) also shifted to a VC model in response to COVID-19 guided by a unique perspective from a well-developed prior VC experience.14-16 As a federally funded system, the VHA depends on workload documentation for budgeting. Since 2015, the VHA has provided workload credit and incentivized HCPs (via pay for performance) for the use of VC, including telephone visits, video visits, and secure messaging. These incentives resulted in higher rates of telehealth utilization before the COVID-19 pandemic compared with the private sector (with 4.2% and 0.7% of visits within the VHA being telephone and video visits, respectively, compared with telehealth utilization rates of 1.0% for Medicare recipients and 1.1% in an all-payer database).16
Historically, VHA care has successfully transitioned from in-person care models to exclusively virtual modalities to prevent suspension of medical services during natural disasters. Studies performed during these periods, specifically during the 2017 hurricane season (during which multiple VHA hospitals were closed or had limited in-person service available), supported telehealth as an efficient health care delivery method, and even recommended expanding telehealth services within non-VHA environments to accommodate needs of the general public during crises and postdisaster health care delivery.17
Armed with both a well-established telehealth infrastructure and prior knowledge gained from successful systemwide implementation of virtual care during times of disaster, US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACHS) primary care quickly transitioned to a VC model in response to COVID-19.16 Early in the pandemic, a rapid transition to virtual care (RTVC) model was developed, including implementation of virtual respiratory urgent clinics (VRUCs), defined as virtual respiratory symptom triage clinics, staffed by primary care providers (PCPs) aimed at minimizing patient and health care worker exposure risk.
Methods
VACHS consists of 8 primary care sites, including a major tertiary care center, a smaller medical center with full ambulatory services, and 6 community-based outpatient clinics with only primary care and mental health. There are 80 individual PCPs delivering care to 58,058 veterans. VRUCs were established during the COVID-19 pandemic to cover patients across the entire health care system, using a rotational schedule of VA PCPs.
COVID-19 Urgent Clinics Program
Within the first few weeks of the pandemic, VACHS primary care established VRUCS to provide expeditious virtual assessment of respiratory or flu-like symptoms. Using the established telehealth system, the intervention aimed to provide emergent screening, testing, and care to those with potential COVID-19 infections. The model also was designed to minimize exposures to the health care workforce and patients.
Retrospective analysis was performed using information obtained from the electronic health record (EHR) database to describe the characteristics of patients who received care through the VRUCs, such as demographics, era of military service, COVID-19 testing rates and results, as well as subsequent emergency department (ED) visits and hospital admissions. A secondary aim included collection of additional qualitative data via a random sample chart review.
Virtual clinics were established January 22, 2020, and data were analyzed over the next 3 months. Data were retrieved and analyzed from the EHR, and codes were used to categorize the VRUCs.
Results
A total of 445 unique patients used these clinics during this period. Unique patients were defined as individual patients (some may have used a clinic more than once but were counted only once). Of this group, 82% were male, and 48% served in the Gulf War era (1990 to present). A total of 51% of patients received a COVID-19 test (clinics began before wide testing availability), and 10% tested positive. Of all patients using the clinics, approximately 5% were admitted to the hospital, and 18% had at least 1 subsequent ED visit (Table).
A secondary aim included review of a random sample of 99 patient charts to gain additional information regarding whether the patient was given appropriate isolation precautions, was in a high-exposure occupation (eg, could expose a large number of people), and whether there was appropriate documentation of goals of care, health care proxy or referral to social work to discuss advance directives. In addition, we calculated the average length of time between patients’ initial contact with the health care system call center and the return call by the PCP (wait time).Of charts reviewed, the majority (71%) had documentation of appropriate isolation precautions. Although 25% of patients had documentation of a high-risk profession with potential to expose many people, more than half of the patients had no documentation of occupation. Most patients (86%) had no updated documentation regarding goals of care, health care proxy, or advance directives in their urgent care VC visit. The average time between the patient initiating contact with the health care system call center and a return call to the patient from a PCP was 104 minutes (excluding calls received after 3:30
Discussion
This analysis adds to the growing literature on use of VC during the COVID-19 pandemic. Specifically, we describe the population of patients who used VRUCs within a large health care system in a RTVC. This analysis was limited by lack of available testing during the initial phase of the pandemic, which contributed to the lower than expected rates of testing and test positivity in patients managed via VRUCs. In addition, chart review data are limited as the data includes only what was documented during the visit and not the entire discussion during the encounter.
Several important outcomes from this analysis can be applied to interventions in the future, which may have large public health implications: Several hundred patients who reported respiratory symptoms were expeditiously evaluated by a PCP using VC. The average wait time to full clinical assessment was about 1.5 hours. This short duration between contact and evaluation permitted early education about isolation precautions, which may have minimized spread. In addition, this innovation kept patients out of the medical center, eliminating chains of transmission to other vulnerable patients and health care workers.
Our retrospective chart review also revealed that more than half the patients were not queried about their occupation, but of those that were asked, a significant number were in high-risk professions potentially exposing large numbers of people. This would be an important aspect to add to future templated notes to minimize work-related exposures. Also, we identified that few HCPs discussed goals of care with patients. Given the nature of COVID-19 and potential for rapid decompensation especially in vulnerable patients, this also would be important to include in the future.
Conclusions
VC urgent care clinics to address possible COVID-19 symptoms facilitated expeditious PCP assessment while keeping potentially contagious patients outside of high-risk health care environments. Streamlining and optimizing clinical VC assessments will be imperative to future management of COVID-19 and potentially to other future infectious pandemics. This includes development of templated notes incorporating counseling regarding appropriate isolation, questions about high-contact occupations, and goals of care discussions.
Acknowledgment
The authors thank Robert F. Walsh, MHA.
1. Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
2. Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
3. Harvey JB, Valenta S, Simpson K, Lyles M, McElligott J. Utilization of outpatient telehealth services in parity and nonparity states 2010-2015. Telemed J E Health. 2019;25(2):132-136. doi:10.1089/tmj.2017.0265
4. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/NEJMra1601705
5. Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care. 2020;26(4):147-148. doi:10.37765/ajmc.2020.42784
6. Centers for Disease Control and Prevention. Healthcare facility guidance. Updated April 17, 2021. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html
7. US Department of Health and Human Services, Health Resources and Services Administration. Policy changes during COVID-19. Accessed August 20, 2021. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency
8. Coronavirus Preparedness and Response Supplemental Appropriation Act of 2020. 134 Stat. 146. Published February 2, 2021. Accessed August 20, 2021. https://www.govinfo.gov/content/pkg/CREC-2021-02-02/html/CREC-2021-02-02-pt1-PgS226.htm
9. US Department of Health and Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Updated January 20, 2021. Accessed August 20, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
10. Centers for Medicare and Medicaid Services. Coverage and payment related to COVID-19 Medicare. 2020. Published March 23, 2020. Accessed August 20, 2021. https://www.cms.gov/files/document/03052020-medicare-covid-19-fact-sheet.pdf
11. American Telemedicine Association. ATA commends 2020 Congress for giving HHS authority to waive restrictions on telehealth for Medicare beneficiaries in response to the COVID-19 outbreak [press release]. Published March 5, 2020. Accessed August 20, 2021. https://www.americantelemed.org/press-releases/ata-commends-congress-for-waiving-restrictions-on-telehealth-for-medicare-beneficiaries-in-response-to-the-covid-19-outbreak
12. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681. doi:10.1056/NEJMp2003539
13. Khairat S, Meng C, Xu Y, Edson B, Gianforcaro R. Interpreting COVID-19 and Virtual Care Trends: Cohort Study. JMIR Public Health Surveill. 2020;6(2):e18811. Published 2020 Apr 15. doi:10.2196/18811
14. Ferguson JM, Jacobs J, Yefimova M, Greene L, Heyworth L, Zulman DM. Virtual care expansion in the Veterans Health Administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2021;28(3):453-462. doi:10.1093/jamia/ocaa284
15. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
16. Spelman JF, Brienza R, Walsh RF, et al. A model for rapid transition to virtual care, VA Connecticut primary care response to COVID-19. J Gen Intern Med. 2020;35(10):3073-3076. doi:10.1007/s11606-020-06041-4
17. Der-Martirosian C, Chu K, Dobalian A. Use of telehealth to improve access to care at the United States Department of Veterans Affairs during the 2017 Atlantic hurricane season [published online ahead of print, 2020 Apr 13]. Disaster Med Public Health Prep. 2020;1-5. doi:10.1017/dmp.2020.88
1. Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
2. Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
3. Harvey JB, Valenta S, Simpson K, Lyles M, McElligott J. Utilization of outpatient telehealth services in parity and nonparity states 2010-2015. Telemed J E Health. 2019;25(2):132-136. doi:10.1089/tmj.2017.0265
4. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/NEJMra1601705
5. Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care. 2020;26(4):147-148. doi:10.37765/ajmc.2020.42784
6. Centers for Disease Control and Prevention. Healthcare facility guidance. Updated April 17, 2021. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html
7. US Department of Health and Human Services, Health Resources and Services Administration. Policy changes during COVID-19. Accessed August 20, 2021. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency
8. Coronavirus Preparedness and Response Supplemental Appropriation Act of 2020. 134 Stat. 146. Published February 2, 2021. Accessed August 20, 2021. https://www.govinfo.gov/content/pkg/CREC-2021-02-02/html/CREC-2021-02-02-pt1-PgS226.htm
9. US Department of Health and Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Updated January 20, 2021. Accessed August 20, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
10. Centers for Medicare and Medicaid Services. Coverage and payment related to COVID-19 Medicare. 2020. Published March 23, 2020. Accessed August 20, 2021. https://www.cms.gov/files/document/03052020-medicare-covid-19-fact-sheet.pdf
11. American Telemedicine Association. ATA commends 2020 Congress for giving HHS authority to waive restrictions on telehealth for Medicare beneficiaries in response to the COVID-19 outbreak [press release]. Published March 5, 2020. Accessed August 20, 2021. https://www.americantelemed.org/press-releases/ata-commends-congress-for-waiving-restrictions-on-telehealth-for-medicare-beneficiaries-in-response-to-the-covid-19-outbreak
12. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681. doi:10.1056/NEJMp2003539
13. Khairat S, Meng C, Xu Y, Edson B, Gianforcaro R. Interpreting COVID-19 and Virtual Care Trends: Cohort Study. JMIR Public Health Surveill. 2020;6(2):e18811. Published 2020 Apr 15. doi:10.2196/18811
14. Ferguson JM, Jacobs J, Yefimova M, Greene L, Heyworth L, Zulman DM. Virtual care expansion in the Veterans Health Administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2021;28(3):453-462. doi:10.1093/jamia/ocaa284
15. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
16. Spelman JF, Brienza R, Walsh RF, et al. A model for rapid transition to virtual care, VA Connecticut primary care response to COVID-19. J Gen Intern Med. 2020;35(10):3073-3076. doi:10.1007/s11606-020-06041-4
17. Der-Martirosian C, Chu K, Dobalian A. Use of telehealth to improve access to care at the United States Department of Veterans Affairs during the 2017 Atlantic hurricane season [published online ahead of print, 2020 Apr 13]. Disaster Med Public Health Prep. 2020;1-5. doi:10.1017/dmp.2020.88
The Delta Factor
Several weeks ago, I received a call from my brother who, though not a health care professional, wanted me to know he thought the public was being too critical of scientists and physicians who “are giving us the best advice they can about COVID. People think they should have all the answers. But this virus is complicated, and they don’t always know what is going to happen next.” What makes his charitable read of the public health situation remarkable is that he is a COVID-19 survivor of one of the first reported cases of Guillain-Barre syndrome, which several expert neurologists believe is the result of COVID-19. Like so many other COVID-19 long-haul patients, he is left with lingering symptoms and residual deficits.1
I use this personal story as the overture to this piece on why I am changing my opinion regarding a COVID-19 mandate for federal practitioners. In June I raised ethical concerns about compelling vaccination especially for service members of color based on a current and historical climate of mistrust and discrimination in health care that compulsory vaccination could exacerbate.2 Instead, I followed the lead of Secretary of Defense J. Lloyd Austin III and advocated continued education and encouragement for vaccine-hesitant troops.3 So in 2 months what has so radically changed to lead Secretary Austin and US Department of Veterans Affairs (VA) Secretary Denis R. McDonough to mandate vaccination for their workforce?4,5
I am calling the change the Delta Factor. This is not to be confused with the spy-thrillers that ironically involved rescuing a scientist! The Delta Factor is a catch-all phrase to cover the protean public health impacts of the devastating COVID-19 Delta variant now ravaging the country. Depending on the area of the country as of mid-August, the Centers for Disease Control and Prevention (CDC) estimated that 80% to > 90% of new cases were the Delta variant.6 An increasing number of these cases sadly are in children.7
According to the CDC, the Delta variant is more than twice as contagious as index or subsequent strains: making it about as contagious as chicken pox. The unvaccinated are the most susceptible to Delta and may develop more serious illness and risk of death than with other strains. Those who are fully vaccinated can still contract the virus although usually with milder cases. More worrisome is that individuals with these breakthrough infections have the same viral load as those without vaccinations, rendering them vectors of transmission, although for a shorter time than unvaccinated persons.8
The VA first mandated vaccination among its health care employees in July and then expanded it to all staff in August.9 The US Department of Defense (DoD) mandatory vaccination was announced prior to US Food and Drug Administration’s (FDA) full approval of the Pfizer-BioNTech vaccine.10 Secretary Austin asked President Biden to grant a waiver to permit mandatory vaccination even without full FDA approval, and Biden has indicated his support, but the full approval expedited the time line for implementation.11
Both agencies directly referenced Delta as a primary reason for their vaccination mandates. The VA argued that the mandate was necessary to protect the safety of veterans, while the DoD noted that vaccination was essential to ensure the health of the fighting force. In his initial announcement, Secretary McDonough explicitly mentioned the Delta variant as a primary reason for his decision. noting “it’s the best way to keep veterans safe, especially as the Delta variant spreads across the country.”4 Similarly, Secretary Austin declared, “We will also be keeping a close eye on infection rates, which are on the rise now due to the Delta variant and the impact these rates might have on our readiness.”5
VA and DoD leadership emphasized the safety and effectiveness of the vaccine and urged employees to voluntarily obtain the vaccine or obtain a religious or medical exemption. Those without such an exemption must adhere to masking, testing, and other restrictions.5 As anticipated in the earlier editorial, there has been opposition to the mandate from the workforce of the 2 agencies and their political supporters some of whom view vaccine mandates as violations of personal liberty and bodily integrity and for whom rampant disinformation has amplified entrenched distrust of the government.12
The decision to shift from voluntary to mandatory vaccination of federal employees responsible for the health care of veterans and the defense of citizens, which may seem
Finally and most important, for a vaccine or other public health intervention to be ethically mandated it must have a high probability of attaining a serious purpose: here preventing the harms of sickness and death especially in the most vulnerable. In July, the White House COVID-19 Response Team reported that “preliminary data from several states over the last few months suggest that 99.5% of deaths from COVID-19 in the United States were in unvaccinated people” and were preventable.15 Ethically, even as mandates are implemented across the federal workforce, efforts to educate, encourage, and empower vaccination especially among disenfranchised cohorts must continue. But as a recently leaked CDC internal document acknowledged about the Delta Factor, “the war has changed” and so has my opinion about mandating vaccination among those upon whose service depends the life and security of us all.16
1. CBS Good Morning. Christopher Cross on his near-fatal COVID illness. Published October 18, 2020. Accessed August 21, 2021. https://www.cbsnews.com/news/christopher-cross-on-his-near-fatal-covid-illness
2. Geppert CM. Mistrust and mandates: COVID-19 vaccination in the military. Fed Pract. 2021;38(6):254-255. doi:10.12788/fp.0143
3. Garmone J, US Department of Defense. Secretary of defense addresses vaccine hesitancy in the military. Published February 25, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military
4. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA mandates COVID-19 vaccines among its medical employees including VHA facilities staff [press release]. Published July 26, 2021. Accessed August 21, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5696
5. US Department of Defense, Secretary of Defense. Memorandum for all Department of Defense employees. Published August 9, 2021. Accessed August 23, 2021. https://media.defense.gov/2021/Aug/09/2002826254/-1/-1/0/MESSAGE-TO-THE-FORCE-MEMO-VACCINE.PDF
6. Centers for Disease Control and Prevention COVID data tracker. Variant proportions. Updated August 17, 2021. Accessed August 23, 2021. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
7. American Academy of Pediatrics. Children and COVID-19: state data level report. Updated August 23, 2021. Accessed August 23, 2021. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state|-level-data-report
8. Centers for Disease Control and Prevention. Delta variant: what we know about the science. Update August 19, 2021. Accessed August 23, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html
9. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands mandate for COVID-19 vaccines among VHA employees [press release]. Published August 12, 2021. Accessed August 23, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5703
10. US Food and Drug Administration. FDA approves first COVID-19 vaccine [press release]. Published August 23, 2021. Accessed August 23, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
11. Garamone J, US Department of Defense. Biden to approve Austin’s request to make COVID-19 vaccine mandatory for service members. Published August 9, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2724982/biden-to-approve-austins-request-to-make-covid-19-vaccine-mandatory-for-service
12. Watson J. Potential military vaccine mandate brings distrust, support. Associated Press. August 5, 2021. Accessed August 23, 2021. https://apnews.com/article/joe-biden-business-health-coronavirus-pandemic-6a0f94e11f5af1e0de740d44d7931d65
13. Giubilini A. Vaccination ethics. Br Med Bull. 2021;137(1):4-12. doi:10.1093/bmb/ldaa036
14. Steinhauer J. Military and V.A. struggle with vaccination rates in their ranks. The New York Times. July 1, 2021. Accessed August 23, 2021. https://www.nytimes.com/2021/07/01/us/politics/military-va-vaccines.html
15. The White House. Press briefing by White House COVID-19 Response Team and public health officials. Published July 8, 2021. Accessed August 23, 2021. https://www.whitehouse.gov/briefing-room/press-briefings/2021/07/08/press-briefing-by-white-house-covid-19-response-team-and-public-health-officials-44
16. Adutaleb Y, Johnson CY, Achenbach J. ‘The war has changed’: Internal CDC document urges new messaging, warns delta infections likely more severe. The Washington Post. July 29, 2021. Accessed August 21, 2021 https://www.washingtonpost.com/health/2021/07/29/cdc-mask-guidance
Several weeks ago, I received a call from my brother who, though not a health care professional, wanted me to know he thought the public was being too critical of scientists and physicians who “are giving us the best advice they can about COVID. People think they should have all the answers. But this virus is complicated, and they don’t always know what is going to happen next.” What makes his charitable read of the public health situation remarkable is that he is a COVID-19 survivor of one of the first reported cases of Guillain-Barre syndrome, which several expert neurologists believe is the result of COVID-19. Like so many other COVID-19 long-haul patients, he is left with lingering symptoms and residual deficits.1
I use this personal story as the overture to this piece on why I am changing my opinion regarding a COVID-19 mandate for federal practitioners. In June I raised ethical concerns about compelling vaccination especially for service members of color based on a current and historical climate of mistrust and discrimination in health care that compulsory vaccination could exacerbate.2 Instead, I followed the lead of Secretary of Defense J. Lloyd Austin III and advocated continued education and encouragement for vaccine-hesitant troops.3 So in 2 months what has so radically changed to lead Secretary Austin and US Department of Veterans Affairs (VA) Secretary Denis R. McDonough to mandate vaccination for their workforce?4,5
I am calling the change the Delta Factor. This is not to be confused with the spy-thrillers that ironically involved rescuing a scientist! The Delta Factor is a catch-all phrase to cover the protean public health impacts of the devastating COVID-19 Delta variant now ravaging the country. Depending on the area of the country as of mid-August, the Centers for Disease Control and Prevention (CDC) estimated that 80% to > 90% of new cases were the Delta variant.6 An increasing number of these cases sadly are in children.7
According to the CDC, the Delta variant is more than twice as contagious as index or subsequent strains: making it about as contagious as chicken pox. The unvaccinated are the most susceptible to Delta and may develop more serious illness and risk of death than with other strains. Those who are fully vaccinated can still contract the virus although usually with milder cases. More worrisome is that individuals with these breakthrough infections have the same viral load as those without vaccinations, rendering them vectors of transmission, although for a shorter time than unvaccinated persons.8
The VA first mandated vaccination among its health care employees in July and then expanded it to all staff in August.9 The US Department of Defense (DoD) mandatory vaccination was announced prior to US Food and Drug Administration’s (FDA) full approval of the Pfizer-BioNTech vaccine.10 Secretary Austin asked President Biden to grant a waiver to permit mandatory vaccination even without full FDA approval, and Biden has indicated his support, but the full approval expedited the time line for implementation.11
Both agencies directly referenced Delta as a primary reason for their vaccination mandates. The VA argued that the mandate was necessary to protect the safety of veterans, while the DoD noted that vaccination was essential to ensure the health of the fighting force. In his initial announcement, Secretary McDonough explicitly mentioned the Delta variant as a primary reason for his decision. noting “it’s the best way to keep veterans safe, especially as the Delta variant spreads across the country.”4 Similarly, Secretary Austin declared, “We will also be keeping a close eye on infection rates, which are on the rise now due to the Delta variant and the impact these rates might have on our readiness.”5
VA and DoD leadership emphasized the safety and effectiveness of the vaccine and urged employees to voluntarily obtain the vaccine or obtain a religious or medical exemption. Those without such an exemption must adhere to masking, testing, and other restrictions.5 As anticipated in the earlier editorial, there has been opposition to the mandate from the workforce of the 2 agencies and their political supporters some of whom view vaccine mandates as violations of personal liberty and bodily integrity and for whom rampant disinformation has amplified entrenched distrust of the government.12
The decision to shift from voluntary to mandatory vaccination of federal employees responsible for the health care of veterans and the defense of citizens, which may seem
Finally and most important, for a vaccine or other public health intervention to be ethically mandated it must have a high probability of attaining a serious purpose: here preventing the harms of sickness and death especially in the most vulnerable. In July, the White House COVID-19 Response Team reported that “preliminary data from several states over the last few months suggest that 99.5% of deaths from COVID-19 in the United States were in unvaccinated people” and were preventable.15 Ethically, even as mandates are implemented across the federal workforce, efforts to educate, encourage, and empower vaccination especially among disenfranchised cohorts must continue. But as a recently leaked CDC internal document acknowledged about the Delta Factor, “the war has changed” and so has my opinion about mandating vaccination among those upon whose service depends the life and security of us all.16
Several weeks ago, I received a call from my brother who, though not a health care professional, wanted me to know he thought the public was being too critical of scientists and physicians who “are giving us the best advice they can about COVID. People think they should have all the answers. But this virus is complicated, and they don’t always know what is going to happen next.” What makes his charitable read of the public health situation remarkable is that he is a COVID-19 survivor of one of the first reported cases of Guillain-Barre syndrome, which several expert neurologists believe is the result of COVID-19. Like so many other COVID-19 long-haul patients, he is left with lingering symptoms and residual deficits.1
I use this personal story as the overture to this piece on why I am changing my opinion regarding a COVID-19 mandate for federal practitioners. In June I raised ethical concerns about compelling vaccination especially for service members of color based on a current and historical climate of mistrust and discrimination in health care that compulsory vaccination could exacerbate.2 Instead, I followed the lead of Secretary of Defense J. Lloyd Austin III and advocated continued education and encouragement for vaccine-hesitant troops.3 So in 2 months what has so radically changed to lead Secretary Austin and US Department of Veterans Affairs (VA) Secretary Denis R. McDonough to mandate vaccination for their workforce?4,5
I am calling the change the Delta Factor. This is not to be confused with the spy-thrillers that ironically involved rescuing a scientist! The Delta Factor is a catch-all phrase to cover the protean public health impacts of the devastating COVID-19 Delta variant now ravaging the country. Depending on the area of the country as of mid-August, the Centers for Disease Control and Prevention (CDC) estimated that 80% to > 90% of new cases were the Delta variant.6 An increasing number of these cases sadly are in children.7
According to the CDC, the Delta variant is more than twice as contagious as index or subsequent strains: making it about as contagious as chicken pox. The unvaccinated are the most susceptible to Delta and may develop more serious illness and risk of death than with other strains. Those who are fully vaccinated can still contract the virus although usually with milder cases. More worrisome is that individuals with these breakthrough infections have the same viral load as those without vaccinations, rendering them vectors of transmission, although for a shorter time than unvaccinated persons.8
The VA first mandated vaccination among its health care employees in July and then expanded it to all staff in August.9 The US Department of Defense (DoD) mandatory vaccination was announced prior to US Food and Drug Administration’s (FDA) full approval of the Pfizer-BioNTech vaccine.10 Secretary Austin asked President Biden to grant a waiver to permit mandatory vaccination even without full FDA approval, and Biden has indicated his support, but the full approval expedited the time line for implementation.11
Both agencies directly referenced Delta as a primary reason for their vaccination mandates. The VA argued that the mandate was necessary to protect the safety of veterans, while the DoD noted that vaccination was essential to ensure the health of the fighting force. In his initial announcement, Secretary McDonough explicitly mentioned the Delta variant as a primary reason for his decision. noting “it’s the best way to keep veterans safe, especially as the Delta variant spreads across the country.”4 Similarly, Secretary Austin declared, “We will also be keeping a close eye on infection rates, which are on the rise now due to the Delta variant and the impact these rates might have on our readiness.”5
VA and DoD leadership emphasized the safety and effectiveness of the vaccine and urged employees to voluntarily obtain the vaccine or obtain a religious or medical exemption. Those without such an exemption must adhere to masking, testing, and other restrictions.5 As anticipated in the earlier editorial, there has been opposition to the mandate from the workforce of the 2 agencies and their political supporters some of whom view vaccine mandates as violations of personal liberty and bodily integrity and for whom rampant disinformation has amplified entrenched distrust of the government.12
The decision to shift from voluntary to mandatory vaccination of federal employees responsible for the health care of veterans and the defense of citizens, which may seem
Finally and most important, for a vaccine or other public health intervention to be ethically mandated it must have a high probability of attaining a serious purpose: here preventing the harms of sickness and death especially in the most vulnerable. In July, the White House COVID-19 Response Team reported that “preliminary data from several states over the last few months suggest that 99.5% of deaths from COVID-19 in the United States were in unvaccinated people” and were preventable.15 Ethically, even as mandates are implemented across the federal workforce, efforts to educate, encourage, and empower vaccination especially among disenfranchised cohorts must continue. But as a recently leaked CDC internal document acknowledged about the Delta Factor, “the war has changed” and so has my opinion about mandating vaccination among those upon whose service depends the life and security of us all.16
1. CBS Good Morning. Christopher Cross on his near-fatal COVID illness. Published October 18, 2020. Accessed August 21, 2021. https://www.cbsnews.com/news/christopher-cross-on-his-near-fatal-covid-illness
2. Geppert CM. Mistrust and mandates: COVID-19 vaccination in the military. Fed Pract. 2021;38(6):254-255. doi:10.12788/fp.0143
3. Garmone J, US Department of Defense. Secretary of defense addresses vaccine hesitancy in the military. Published February 25, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military
4. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA mandates COVID-19 vaccines among its medical employees including VHA facilities staff [press release]. Published July 26, 2021. Accessed August 21, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5696
5. US Department of Defense, Secretary of Defense. Memorandum for all Department of Defense employees. Published August 9, 2021. Accessed August 23, 2021. https://media.defense.gov/2021/Aug/09/2002826254/-1/-1/0/MESSAGE-TO-THE-FORCE-MEMO-VACCINE.PDF
6. Centers for Disease Control and Prevention COVID data tracker. Variant proportions. Updated August 17, 2021. Accessed August 23, 2021. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
7. American Academy of Pediatrics. Children and COVID-19: state data level report. Updated August 23, 2021. Accessed August 23, 2021. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state|-level-data-report
8. Centers for Disease Control and Prevention. Delta variant: what we know about the science. Update August 19, 2021. Accessed August 23, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html
9. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands mandate for COVID-19 vaccines among VHA employees [press release]. Published August 12, 2021. Accessed August 23, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5703
10. US Food and Drug Administration. FDA approves first COVID-19 vaccine [press release]. Published August 23, 2021. Accessed August 23, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
11. Garamone J, US Department of Defense. Biden to approve Austin’s request to make COVID-19 vaccine mandatory for service members. Published August 9, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2724982/biden-to-approve-austins-request-to-make-covid-19-vaccine-mandatory-for-service
12. Watson J. Potential military vaccine mandate brings distrust, support. Associated Press. August 5, 2021. Accessed August 23, 2021. https://apnews.com/article/joe-biden-business-health-coronavirus-pandemic-6a0f94e11f5af1e0de740d44d7931d65
13. Giubilini A. Vaccination ethics. Br Med Bull. 2021;137(1):4-12. doi:10.1093/bmb/ldaa036
14. Steinhauer J. Military and V.A. struggle with vaccination rates in their ranks. The New York Times. July 1, 2021. Accessed August 23, 2021. https://www.nytimes.com/2021/07/01/us/politics/military-va-vaccines.html
15. The White House. Press briefing by White House COVID-19 Response Team and public health officials. Published July 8, 2021. Accessed August 23, 2021. https://www.whitehouse.gov/briefing-room/press-briefings/2021/07/08/press-briefing-by-white-house-covid-19-response-team-and-public-health-officials-44
16. Adutaleb Y, Johnson CY, Achenbach J. ‘The war has changed’: Internal CDC document urges new messaging, warns delta infections likely more severe. The Washington Post. July 29, 2021. Accessed August 21, 2021 https://www.washingtonpost.com/health/2021/07/29/cdc-mask-guidance
1. CBS Good Morning. Christopher Cross on his near-fatal COVID illness. Published October 18, 2020. Accessed August 21, 2021. https://www.cbsnews.com/news/christopher-cross-on-his-near-fatal-covid-illness
2. Geppert CM. Mistrust and mandates: COVID-19 vaccination in the military. Fed Pract. 2021;38(6):254-255. doi:10.12788/fp.0143
3. Garmone J, US Department of Defense. Secretary of defense addresses vaccine hesitancy in the military. Published February 25, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military
4. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA mandates COVID-19 vaccines among its medical employees including VHA facilities staff [press release]. Published July 26, 2021. Accessed August 21, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5696
5. US Department of Defense, Secretary of Defense. Memorandum for all Department of Defense employees. Published August 9, 2021. Accessed August 23, 2021. https://media.defense.gov/2021/Aug/09/2002826254/-1/-1/0/MESSAGE-TO-THE-FORCE-MEMO-VACCINE.PDF
6. Centers for Disease Control and Prevention COVID data tracker. Variant proportions. Updated August 17, 2021. Accessed August 23, 2021. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
7. American Academy of Pediatrics. Children and COVID-19: state data level report. Updated August 23, 2021. Accessed August 23, 2021. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state|-level-data-report
8. Centers for Disease Control and Prevention. Delta variant: what we know about the science. Update August 19, 2021. Accessed August 23, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html
9. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands mandate for COVID-19 vaccines among VHA employees [press release]. Published August 12, 2021. Accessed August 23, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5703
10. US Food and Drug Administration. FDA approves first COVID-19 vaccine [press release]. Published August 23, 2021. Accessed August 23, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
11. Garamone J, US Department of Defense. Biden to approve Austin’s request to make COVID-19 vaccine mandatory for service members. Published August 9, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2724982/biden-to-approve-austins-request-to-make-covid-19-vaccine-mandatory-for-service
12. Watson J. Potential military vaccine mandate brings distrust, support. Associated Press. August 5, 2021. Accessed August 23, 2021. https://apnews.com/article/joe-biden-business-health-coronavirus-pandemic-6a0f94e11f5af1e0de740d44d7931d65
13. Giubilini A. Vaccination ethics. Br Med Bull. 2021;137(1):4-12. doi:10.1093/bmb/ldaa036
14. Steinhauer J. Military and V.A. struggle with vaccination rates in their ranks. The New York Times. July 1, 2021. Accessed August 23, 2021. https://www.nytimes.com/2021/07/01/us/politics/military-va-vaccines.html
15. The White House. Press briefing by White House COVID-19 Response Team and public health officials. Published July 8, 2021. Accessed August 23, 2021. https://www.whitehouse.gov/briefing-room/press-briefings/2021/07/08/press-briefing-by-white-house-covid-19-response-team-and-public-health-officials-44
16. Adutaleb Y, Johnson CY, Achenbach J. ‘The war has changed’: Internal CDC document urges new messaging, warns delta infections likely more severe. The Washington Post. July 29, 2021. Accessed August 21, 2021 https://www.washingtonpost.com/health/2021/07/29/cdc-mask-guidance
Right Ventricle Dilation Detected on Point-of-Care Ultrasound Is a Predictor of Poor Outcomes in Critically Ill Patients With COVID-19
Point-of-care ultrasound (POCUS) is increasingly being used by critical care physicians to augment the physical examination and guide clinical decision making, and several protocols have been established to standardize the POCUS evaluation.1 During the COVID-19 pandemic, POCUS has been a valuable tool as standard imaging techniques were used judiciously to minimize exposure of personnel and use of personal protective equipment (PPE).2
In the US Department of Veterans Affairs (VA) New York Harbor Healthcare System (VANYHHS) intensive care unit (ICU) on initial clinical examination included POCUS, which was helpful to examine deep vein thromboses, cardiac function, and the presence and extent of pneumonia. An international expert consensus on the use of POCUS for COVID-19 published in December 2020 called for further studies defining the role of lung and cardiac ultrasound in risk stratification, outcomes, and clinical management.3
The objective of this study was to review POCUS findings and correlate them with severity of illness and 30-day outcomes in critically ill patients with COVID-19.
Methods
The study was submitted to and reviewed by the VANYHHS Research and Development committee and study approval and informed consent waiver was granted. The study was a retrospective chart review of patients admitted to the VANYHHS ICU between March and April 2020, a tertiary health care center designated as a COVID-19 hospital.
Patients admitted to the ICU aged > 18 years with a diagnosis of acute hypoxemic respiratory failure, diagnosis of COVID-19, and documentation of POCUS findings in the chart were included in the study. A patient was considered to have a COVID-19 diagnosis following a positive SARS-CoV-2 polymerase chain reaction test documented in the electronic health record (EHR). Acute respiratory failure was defined as hypoxemia < 94% and the need for either supplemental oxygen by nasal cannula > 2 L/min, high flow nasal cannula, noninvasive ventilation, or mechanical ventilation.
To minimize personnel exposure, initial patient evaluations and POCUS examinations were performed by the most senior personnel (ie, fellowship trained, board-certified pulmonary critical care attending physicians or pulmonary and critical care fellowship trainees). Three members of the team had certification in advanced critical care echocardiography by the National Board of Echocardiography and oversaw POCUS imaging. POCUS examinations were performed with a GE Heathcare Venue POCUS or handheld unit. After use, ultrasound probes and ultrasound units were disinfected with wipes designated by the manufacturer and US Environmental Protection Agency for use during the COVID-19 pandemic.
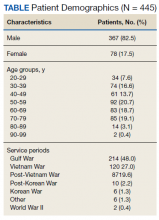
The POCUS protocol used by members of the team was as follows: POCUS lung—at least 2 anterior fields and 1 posterior/lateral field looking at the costophrenic angle on each hemithorax with a phased array or curvilinear probe. A linear probe was used to look for subpleural changes per physician discretion.4,5 Lung ultrasound findings in anterior lung fields were documented as A lines, B lines (as defined by the bedside lung ultrasound in emergency [BLUE] protocol)anterior pleural abnormalities or consolidations.4,5 The costophrenic point findings were documented as presence of consolidation or pleural effusion.
The POCUS cardiac examination consisted of parasternal long and short axis views, apical 4 chamber view, subcostal and inferior vena cava (IVC) view. Left ventricular (LV) ejection fraction was visually estimated as reduced or normal. Right ventricular (RV) dilation was considered present if RV size approached or exceeded LV size in the apical 4 chamber view. RV dysfunction was considered present if in addition there was flattening of interventricular septum, RV free wall hypokinesis or reduced tricuspid annular plane systolic excursion (TAPSE).6 IVC was documented as collapsible or plethoric by size and respirophasic variability (2 cm and 50%). Other POCUS examinations including venous compression were done at the discretion of the treating physician.7 POCUS was also used for the placement of central and arterial lines and to guide fluid management.8
The VA EHR and Venue image local archives were reviewed for patient demographics, laboratory findings, imaging studies and outcomes. All ICU attending physician and fellow notes were reviewed for POCUS lung, cardiac and vascular findings. The chart was also reviewed for management changes as a result of POCUS findings. Patients who had at minimum a POCUS lung or cardiac examination documented in the EHR were included in the study. For patients with serial POCUS the most severe findings were included.
Patients were divided into 2 groups based on 30-day outcome: discharge home vs mortality for comparison. POCUS findings were also compared by need for mechanical ventilation. Patients still hospitalized or transferred to other facilities were excluded from the analysis. A Student t test was used for comparison between the groups for continuous normally distributed variables. Linear and stepwise regression models were used to evaluate univariate and multivariate associations of baseline characteristics, biomarker, and ultrasound findings with patient outcomes. Analyses were performed using R 4.0.2 statistical software.
Results
Eighty-two patients were admitted to the VANYHHS ICU in March and April 2020, including 12 nonveterans. Sixty-four had COVID-19 and acute respiratory failure. POCUS findings were documented in 43 (67%) patients. Thirty-nine patients had documented lung examinations, and 25 patients had documented cardiac examinations. Patients were divided into 2 groups by 30-day outcome (discharge home vs mortality) for statistical analysis. Five patients who were either still hospitalized or had been transferred to another facility were excluded.
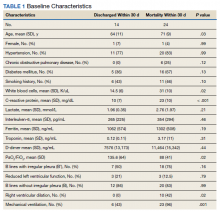
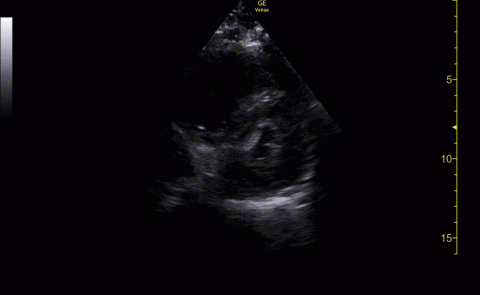
Baseline characteristics of patients included in the study stratified by 30-day outcomes are shown in Table 1. The study group was predominantly male (95%). Patients with poor 30-day outcomes were older, had higher white blood cell counts, more severe hypoxemia, higher rates of mechanical ventilation and RV dilation (Figures 1, 2, 3, 4, and 5). RV dilation was an independent predictor of mortality (odds ratio [OR], 12.0; P = .048).
Serial POCUS documented development or progression of RV dilation and dysfunction from the time of ICU admission in 4 of the patients. The presence of B lines with irregular pleura was predictive of a lower arterial pressure of oxygen to fraction of inspired oxygen ratio (PaO2/FiO2) by a value of 71 compared with those without B lines with irregular pleura (P = .005, adjusted R2 = 0.238). All patients with RV dilation had bilateral B lines with pleural irregularities on lung ultrasound. Vascular POCUS detected 4 deep vein thromboses (DVT).7 An arterial thrombus was also detected on focused examination. There was a higher mortality in patients who required mechanical ventilation; however, there was no difference in POCUS characteristics between the groups (Table 2).
Two severely hypoxemic patients received systemic tissue plasminogen activator (TPA) after findings of massive RV dilation with signs of volume and pressure overload and clinical suspicion of pulmonary embolism (PE). One of these patients also had a popliteal DVT. Both patients were too unstable to transport for additional imaging or therapies. Therapeutic anticoagulation was initiated on 4 patients with positive DVT examinations. In a fifth case an arterial thrombectomy and anticoagulation was required after diminished pulses led to the finding of an occlusive brachial artery thrombus on vascular POCUS.
Discussion
POCUS identified both lung and cardiac features that were associated with worse outcomes. While lung ultrasound abnormalities were very prevalent and associated with worse PaO2 to FiO2 ratios, the presence of RV dilation was associated most clearly with mortality and poor 30-day outcomes in the critical care setting.
Lung ultrasound abnormalities were pervasive in patients with acute respiratory failure and COVID-19. On linear regression we found that presence with bilateral B lines and pleural thickening was predictive of a lower PaO2/FiO2 (coefficient, -70; P = .005). Our study found that B lines with pleural irregularities, otherwise known as a B’ profile per the BLUE protocol, was seen in patients with severe COVID-19. Thus severe acute respiratory failure secondary to COVID-19 has similar lung ultrasound findings as non-COVID-19 acute respiratory distress syndrome (ARDS).4,5 Based on prior lung ultrasound studies in ARDS, lung ultrasound findings can be used as an alternate to chest radiography for the diagnosis of ARDS in COVID-19 and predict the severity of ARDS.9 This has particular implications in overwhelmed and resource poor health care settings.
We found no difference in 30-day mortality based on lung ultrasound findings or profile, probably because of small sample size or because the findings were tabulated as profiles and not differentiated further with lung ultrasound scores.10,11 However, there was a significant difference in RV dilation between the 2 groups by 30 days and its presence was found to be a predictor of mortality even when controlled for hypertension and diabetes mellitus (P = .048) with an OR of 12. RV dysfunction in patients with ARDS on mechanical ventilation ranges from 22 to 25% and is typically associated with high driving pressures.12-14 The mechanism is thought to be multifactorial including hypoxemic vasoconstriction in the pulmonary vasculature in addition to the increased transpulmonary pressure.15 While all of the above are at play in COVID-19 infection, there is reported damage to the pulmonary vascular endothelium and resultant hypercoagulability and thrombosis that further increases the RV afterload.16
While RV strain and dysfunction indices done by an echocardiographer would be ideal, given the surge in infections and hospitalizations and strain on health care resources, POCUS by the treating or examining clinician was considered the only feasible way to screen a large number of patients.17 Identification of RV dilation could influence clinical management including workup for venous thromboembolic disease and optimization of lung protective strategies. Further studies are needed to understand the particular etiology and pathophysiology of COVID-19 associated RV dilation. Given increased thrombosis events in COVID-19 infection we believe a POCUS vascular examination should be included as part of evaluation especially in the presence of increased D-dimers and has been discussed above for its important role in working up RV dilation.18
Limitations
Our study has several limitations. It was retrospective in nature and involved a small group of individuals. There was some variation in POCUS examinations done at the discretion of the examining physician. We did not have a blinded observer independently review all images. Since RV dilation was documented only when RV size approached or exceeded LV size in the apical 4 chamber view representing moderate or severe dilation, we may be underreporting the prevalence in critically ill patients.
Conclusions
POCUS is an invaluable adjunct to clinical evaluation and procedures in patients with severe COVID-19 with the ability to identity patients at risk for worse outcomes. B lines with pleural thickening is a sign of severe ARDS and RV dilatation is predictive of mortality. POCUS should be made available to the treating physician for monitoring and risk stratification and can be incorporated into management algorithms.
Additional point-of-care ultrasound videos.
Acknowledgments
We thank frontline healthcare workers and intensive care unit staff of the US Department of Veterans Affairs New York Harbor Healthcare System (NYHHS) for their dedication to the care of veterans and civilians during the COVID-19 pandemic in New York City. The authors acknowledge the NYHHS research and development committee and administration for their support.
1. Cardenas-Garcia J, Mayo PH. Bedside ultrasonography for the intensivist. Crit Care Clin. 2015;31(1):43-66. doi:10.1016/j.ccc.2014.08.003
2. Vetrugno L, Baciarello M, Bignami E, et al. The “pandemic” increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review. Ultrasound J. 2020;12(1):39. Published 2020 Aug 17. doi:10.1186/s13089-020-00185-4
3. Hussain A, Via G, Melniker L, et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care. 2020;24(1):702. Published 2020 Dec 24. doi:10.1186/s13054-020-03369-5
4. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol [published correction appears in Chest. 2013 Aug;144(2):721]. Chest. 2008;134(1):117-125. doi:10.1378/chest.07-2800
5. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-591. doi:10.1007/s00134-012-2513-4
6. Narasimhan M, Koenig SJ, Mayo PH. Advanced echocardiography for the critical care physician: part 1. Chest. 2014;145(1):129-134. doi:10.1378/chest.12-2441
7. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139(3):538-542. doi:10.1378/chest.10-1479
8. Bentzer P, Griesdale DE, Boyd J, MacLean K, Sirounis D, Ayas NT. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316(12):1298-1309. doi:10.1001/jama.2016.12310
9. See KC, Ong V, Tan YL, Sahagun J, Taculod J. Chest radiography versus lung ultrasound for identification of acute respiratory distress syndrome: a retrospective observational study. Crit Care. 2018;22(1):203. Published 2018 Aug 18. doi:10.1186/s13054-018-2105-y
10. Deng Q, Zhang Y, Wang H, et al. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single-center study. Acad Radiol. 2020;27(10):1363-1372. doi:10.1016/j.acra.2020.07.002
11. Brahier T, Meuwly JY, Pantet O, et al. Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study [published online ahead of print, 2020 Sep 17]. Clin Infect Dis. 2020;ciaa1408. doi:10.1093/cid/ciaa1408
12. Vieillard-Baron A, Schmitt JM, Augarde R, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis [published correction appears in Crit Care Med. 2002 Mar;30(3):726]. Crit Care Med. 2001;29(8):1551-1555. doi:10.1097/00003246-200108000-00009
13. Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1725-1733. doi:10.1007/s00134-013-2941-9
14. Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007;33(3):444-447. doi:10.1007/s00134-007-0552-z
15. Repessé X, Vieillard-Baron A. Right heart function during acute respiratory distress syndrome. Ann Transl Med 2017;5(14):295. doi:10.21037/atm.2017.06.66
16. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management [published correction appears in Thromb Res. 2020 Nov 26]. Thromb Res. 2020;194:101-115. doi:10.1016/j.thromres.2020.06.029
17. Kim J, Volodarskiy A, Sultana R, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 2020;76(17):1965-1977. doi:10.1016/j.jacc.2020.08.066
18. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. doi:10.1182/blood.2020006520
Point-of-care ultrasound (POCUS) is increasingly being used by critical care physicians to augment the physical examination and guide clinical decision making, and several protocols have been established to standardize the POCUS evaluation.1 During the COVID-19 pandemic, POCUS has been a valuable tool as standard imaging techniques were used judiciously to minimize exposure of personnel and use of personal protective equipment (PPE).2
In the US Department of Veterans Affairs (VA) New York Harbor Healthcare System (VANYHHS) intensive care unit (ICU) on initial clinical examination included POCUS, which was helpful to examine deep vein thromboses, cardiac function, and the presence and extent of pneumonia. An international expert consensus on the use of POCUS for COVID-19 published in December 2020 called for further studies defining the role of lung and cardiac ultrasound in risk stratification, outcomes, and clinical management.3
The objective of this study was to review POCUS findings and correlate them with severity of illness and 30-day outcomes in critically ill patients with COVID-19.
Methods
The study was submitted to and reviewed by the VANYHHS Research and Development committee and study approval and informed consent waiver was granted. The study was a retrospective chart review of patients admitted to the VANYHHS ICU between March and April 2020, a tertiary health care center designated as a COVID-19 hospital.
Patients admitted to the ICU aged > 18 years with a diagnosis of acute hypoxemic respiratory failure, diagnosis of COVID-19, and documentation of POCUS findings in the chart were included in the study. A patient was considered to have a COVID-19 diagnosis following a positive SARS-CoV-2 polymerase chain reaction test documented in the electronic health record (EHR). Acute respiratory failure was defined as hypoxemia < 94% and the need for either supplemental oxygen by nasal cannula > 2 L/min, high flow nasal cannula, noninvasive ventilation, or mechanical ventilation.
To minimize personnel exposure, initial patient evaluations and POCUS examinations were performed by the most senior personnel (ie, fellowship trained, board-certified pulmonary critical care attending physicians or pulmonary and critical care fellowship trainees). Three members of the team had certification in advanced critical care echocardiography by the National Board of Echocardiography and oversaw POCUS imaging. POCUS examinations were performed with a GE Heathcare Venue POCUS or handheld unit. After use, ultrasound probes and ultrasound units were disinfected with wipes designated by the manufacturer and US Environmental Protection Agency for use during the COVID-19 pandemic.
The POCUS protocol used by members of the team was as follows: POCUS lung—at least 2 anterior fields and 1 posterior/lateral field looking at the costophrenic angle on each hemithorax with a phased array or curvilinear probe. A linear probe was used to look for subpleural changes per physician discretion.4,5 Lung ultrasound findings in anterior lung fields were documented as A lines, B lines (as defined by the bedside lung ultrasound in emergency [BLUE] protocol)anterior pleural abnormalities or consolidations.4,5 The costophrenic point findings were documented as presence of consolidation or pleural effusion.
The POCUS cardiac examination consisted of parasternal long and short axis views, apical 4 chamber view, subcostal and inferior vena cava (IVC) view. Left ventricular (LV) ejection fraction was visually estimated as reduced or normal. Right ventricular (RV) dilation was considered present if RV size approached or exceeded LV size in the apical 4 chamber view. RV dysfunction was considered present if in addition there was flattening of interventricular septum, RV free wall hypokinesis or reduced tricuspid annular plane systolic excursion (TAPSE).6 IVC was documented as collapsible or plethoric by size and respirophasic variability (2 cm and 50%). Other POCUS examinations including venous compression were done at the discretion of the treating physician.7 POCUS was also used for the placement of central and arterial lines and to guide fluid management.8
The VA EHR and Venue image local archives were reviewed for patient demographics, laboratory findings, imaging studies and outcomes. All ICU attending physician and fellow notes were reviewed for POCUS lung, cardiac and vascular findings. The chart was also reviewed for management changes as a result of POCUS findings. Patients who had at minimum a POCUS lung or cardiac examination documented in the EHR were included in the study. For patients with serial POCUS the most severe findings were included.
Patients were divided into 2 groups based on 30-day outcome: discharge home vs mortality for comparison. POCUS findings were also compared by need for mechanical ventilation. Patients still hospitalized or transferred to other facilities were excluded from the analysis. A Student t test was used for comparison between the groups for continuous normally distributed variables. Linear and stepwise regression models were used to evaluate univariate and multivariate associations of baseline characteristics, biomarker, and ultrasound findings with patient outcomes. Analyses were performed using R 4.0.2 statistical software.
Results
Eighty-two patients were admitted to the VANYHHS ICU in March and April 2020, including 12 nonveterans. Sixty-four had COVID-19 and acute respiratory failure. POCUS findings were documented in 43 (67%) patients. Thirty-nine patients had documented lung examinations, and 25 patients had documented cardiac examinations. Patients were divided into 2 groups by 30-day outcome (discharge home vs mortality) for statistical analysis. Five patients who were either still hospitalized or had been transferred to another facility were excluded.
Baseline characteristics of patients included in the study stratified by 30-day outcomes are shown in Table 1. The study group was predominantly male (95%). Patients with poor 30-day outcomes were older, had higher white blood cell counts, more severe hypoxemia, higher rates of mechanical ventilation and RV dilation (Figures 1, 2, 3, 4, and 5). RV dilation was an independent predictor of mortality (odds ratio [OR], 12.0; P = .048).
Serial POCUS documented development or progression of RV dilation and dysfunction from the time of ICU admission in 4 of the patients. The presence of B lines with irregular pleura was predictive of a lower arterial pressure of oxygen to fraction of inspired oxygen ratio (PaO2/FiO2) by a value of 71 compared with those without B lines with irregular pleura (P = .005, adjusted R2 = 0.238). All patients with RV dilation had bilateral B lines with pleural irregularities on lung ultrasound. Vascular POCUS detected 4 deep vein thromboses (DVT).7 An arterial thrombus was also detected on focused examination. There was a higher mortality in patients who required mechanical ventilation; however, there was no difference in POCUS characteristics between the groups (Table 2).
Two severely hypoxemic patients received systemic tissue plasminogen activator (TPA) after findings of massive RV dilation with signs of volume and pressure overload and clinical suspicion of pulmonary embolism (PE). One of these patients also had a popliteal DVT. Both patients were too unstable to transport for additional imaging or therapies. Therapeutic anticoagulation was initiated on 4 patients with positive DVT examinations. In a fifth case an arterial thrombectomy and anticoagulation was required after diminished pulses led to the finding of an occlusive brachial artery thrombus on vascular POCUS.
Discussion
POCUS identified both lung and cardiac features that were associated with worse outcomes. While lung ultrasound abnormalities were very prevalent and associated with worse PaO2 to FiO2 ratios, the presence of RV dilation was associated most clearly with mortality and poor 30-day outcomes in the critical care setting.
Lung ultrasound abnormalities were pervasive in patients with acute respiratory failure and COVID-19. On linear regression we found that presence with bilateral B lines and pleural thickening was predictive of a lower PaO2/FiO2 (coefficient, -70; P = .005). Our study found that B lines with pleural irregularities, otherwise known as a B’ profile per the BLUE protocol, was seen in patients with severe COVID-19. Thus severe acute respiratory failure secondary to COVID-19 has similar lung ultrasound findings as non-COVID-19 acute respiratory distress syndrome (ARDS).4,5 Based on prior lung ultrasound studies in ARDS, lung ultrasound findings can be used as an alternate to chest radiography for the diagnosis of ARDS in COVID-19 and predict the severity of ARDS.9 This has particular implications in overwhelmed and resource poor health care settings.
We found no difference in 30-day mortality based on lung ultrasound findings or profile, probably because of small sample size or because the findings were tabulated as profiles and not differentiated further with lung ultrasound scores.10,11 However, there was a significant difference in RV dilation between the 2 groups by 30 days and its presence was found to be a predictor of mortality even when controlled for hypertension and diabetes mellitus (P = .048) with an OR of 12. RV dysfunction in patients with ARDS on mechanical ventilation ranges from 22 to 25% and is typically associated with high driving pressures.12-14 The mechanism is thought to be multifactorial including hypoxemic vasoconstriction in the pulmonary vasculature in addition to the increased transpulmonary pressure.15 While all of the above are at play in COVID-19 infection, there is reported damage to the pulmonary vascular endothelium and resultant hypercoagulability and thrombosis that further increases the RV afterload.16
While RV strain and dysfunction indices done by an echocardiographer would be ideal, given the surge in infections and hospitalizations and strain on health care resources, POCUS by the treating or examining clinician was considered the only feasible way to screen a large number of patients.17 Identification of RV dilation could influence clinical management including workup for venous thromboembolic disease and optimization of lung protective strategies. Further studies are needed to understand the particular etiology and pathophysiology of COVID-19 associated RV dilation. Given increased thrombosis events in COVID-19 infection we believe a POCUS vascular examination should be included as part of evaluation especially in the presence of increased D-dimers and has been discussed above for its important role in working up RV dilation.18
Limitations
Our study has several limitations. It was retrospective in nature and involved a small group of individuals. There was some variation in POCUS examinations done at the discretion of the examining physician. We did not have a blinded observer independently review all images. Since RV dilation was documented only when RV size approached or exceeded LV size in the apical 4 chamber view representing moderate or severe dilation, we may be underreporting the prevalence in critically ill patients.
Conclusions
POCUS is an invaluable adjunct to clinical evaluation and procedures in patients with severe COVID-19 with the ability to identity patients at risk for worse outcomes. B lines with pleural thickening is a sign of severe ARDS and RV dilatation is predictive of mortality. POCUS should be made available to the treating physician for monitoring and risk stratification and can be incorporated into management algorithms.
Additional point-of-care ultrasound videos.
Acknowledgments
We thank frontline healthcare workers and intensive care unit staff of the US Department of Veterans Affairs New York Harbor Healthcare System (NYHHS) for their dedication to the care of veterans and civilians during the COVID-19 pandemic in New York City. The authors acknowledge the NYHHS research and development committee and administration for their support.
Point-of-care ultrasound (POCUS) is increasingly being used by critical care physicians to augment the physical examination and guide clinical decision making, and several protocols have been established to standardize the POCUS evaluation.1 During the COVID-19 pandemic, POCUS has been a valuable tool as standard imaging techniques were used judiciously to minimize exposure of personnel and use of personal protective equipment (PPE).2
In the US Department of Veterans Affairs (VA) New York Harbor Healthcare System (VANYHHS) intensive care unit (ICU) on initial clinical examination included POCUS, which was helpful to examine deep vein thromboses, cardiac function, and the presence and extent of pneumonia. An international expert consensus on the use of POCUS for COVID-19 published in December 2020 called for further studies defining the role of lung and cardiac ultrasound in risk stratification, outcomes, and clinical management.3
The objective of this study was to review POCUS findings and correlate them with severity of illness and 30-day outcomes in critically ill patients with COVID-19.
Methods
The study was submitted to and reviewed by the VANYHHS Research and Development committee and study approval and informed consent waiver was granted. The study was a retrospective chart review of patients admitted to the VANYHHS ICU between March and April 2020, a tertiary health care center designated as a COVID-19 hospital.
Patients admitted to the ICU aged > 18 years with a diagnosis of acute hypoxemic respiratory failure, diagnosis of COVID-19, and documentation of POCUS findings in the chart were included in the study. A patient was considered to have a COVID-19 diagnosis following a positive SARS-CoV-2 polymerase chain reaction test documented in the electronic health record (EHR). Acute respiratory failure was defined as hypoxemia < 94% and the need for either supplemental oxygen by nasal cannula > 2 L/min, high flow nasal cannula, noninvasive ventilation, or mechanical ventilation.
To minimize personnel exposure, initial patient evaluations and POCUS examinations were performed by the most senior personnel (ie, fellowship trained, board-certified pulmonary critical care attending physicians or pulmonary and critical care fellowship trainees). Three members of the team had certification in advanced critical care echocardiography by the National Board of Echocardiography and oversaw POCUS imaging. POCUS examinations were performed with a GE Heathcare Venue POCUS or handheld unit. After use, ultrasound probes and ultrasound units were disinfected with wipes designated by the manufacturer and US Environmental Protection Agency for use during the COVID-19 pandemic.
The POCUS protocol used by members of the team was as follows: POCUS lung—at least 2 anterior fields and 1 posterior/lateral field looking at the costophrenic angle on each hemithorax with a phased array or curvilinear probe. A linear probe was used to look for subpleural changes per physician discretion.4,5 Lung ultrasound findings in anterior lung fields were documented as A lines, B lines (as defined by the bedside lung ultrasound in emergency [BLUE] protocol)anterior pleural abnormalities or consolidations.4,5 The costophrenic point findings were documented as presence of consolidation or pleural effusion.
The POCUS cardiac examination consisted of parasternal long and short axis views, apical 4 chamber view, subcostal and inferior vena cava (IVC) view. Left ventricular (LV) ejection fraction was visually estimated as reduced or normal. Right ventricular (RV) dilation was considered present if RV size approached or exceeded LV size in the apical 4 chamber view. RV dysfunction was considered present if in addition there was flattening of interventricular septum, RV free wall hypokinesis or reduced tricuspid annular plane systolic excursion (TAPSE).6 IVC was documented as collapsible or plethoric by size and respirophasic variability (2 cm and 50%). Other POCUS examinations including venous compression were done at the discretion of the treating physician.7 POCUS was also used for the placement of central and arterial lines and to guide fluid management.8
The VA EHR and Venue image local archives were reviewed for patient demographics, laboratory findings, imaging studies and outcomes. All ICU attending physician and fellow notes were reviewed for POCUS lung, cardiac and vascular findings. The chart was also reviewed for management changes as a result of POCUS findings. Patients who had at minimum a POCUS lung or cardiac examination documented in the EHR were included in the study. For patients with serial POCUS the most severe findings were included.
Patients were divided into 2 groups based on 30-day outcome: discharge home vs mortality for comparison. POCUS findings were also compared by need for mechanical ventilation. Patients still hospitalized or transferred to other facilities were excluded from the analysis. A Student t test was used for comparison between the groups for continuous normally distributed variables. Linear and stepwise regression models were used to evaluate univariate and multivariate associations of baseline characteristics, biomarker, and ultrasound findings with patient outcomes. Analyses were performed using R 4.0.2 statistical software.
Results
Eighty-two patients were admitted to the VANYHHS ICU in March and April 2020, including 12 nonveterans. Sixty-four had COVID-19 and acute respiratory failure. POCUS findings were documented in 43 (67%) patients. Thirty-nine patients had documented lung examinations, and 25 patients had documented cardiac examinations. Patients were divided into 2 groups by 30-day outcome (discharge home vs mortality) for statistical analysis. Five patients who were either still hospitalized or had been transferred to another facility were excluded.
Baseline characteristics of patients included in the study stratified by 30-day outcomes are shown in Table 1. The study group was predominantly male (95%). Patients with poor 30-day outcomes were older, had higher white blood cell counts, more severe hypoxemia, higher rates of mechanical ventilation and RV dilation (Figures 1, 2, 3, 4, and 5). RV dilation was an independent predictor of mortality (odds ratio [OR], 12.0; P = .048).
Serial POCUS documented development or progression of RV dilation and dysfunction from the time of ICU admission in 4 of the patients. The presence of B lines with irregular pleura was predictive of a lower arterial pressure of oxygen to fraction of inspired oxygen ratio (PaO2/FiO2) by a value of 71 compared with those without B lines with irregular pleura (P = .005, adjusted R2 = 0.238). All patients with RV dilation had bilateral B lines with pleural irregularities on lung ultrasound. Vascular POCUS detected 4 deep vein thromboses (DVT).7 An arterial thrombus was also detected on focused examination. There was a higher mortality in patients who required mechanical ventilation; however, there was no difference in POCUS characteristics between the groups (Table 2).
Two severely hypoxemic patients received systemic tissue plasminogen activator (TPA) after findings of massive RV dilation with signs of volume and pressure overload and clinical suspicion of pulmonary embolism (PE). One of these patients also had a popliteal DVT. Both patients were too unstable to transport for additional imaging or therapies. Therapeutic anticoagulation was initiated on 4 patients with positive DVT examinations. In a fifth case an arterial thrombectomy and anticoagulation was required after diminished pulses led to the finding of an occlusive brachial artery thrombus on vascular POCUS.
Discussion
POCUS identified both lung and cardiac features that were associated with worse outcomes. While lung ultrasound abnormalities were very prevalent and associated with worse PaO2 to FiO2 ratios, the presence of RV dilation was associated most clearly with mortality and poor 30-day outcomes in the critical care setting.
Lung ultrasound abnormalities were pervasive in patients with acute respiratory failure and COVID-19. On linear regression we found that presence with bilateral B lines and pleural thickening was predictive of a lower PaO2/FiO2 (coefficient, -70; P = .005). Our study found that B lines with pleural irregularities, otherwise known as a B’ profile per the BLUE protocol, was seen in patients with severe COVID-19. Thus severe acute respiratory failure secondary to COVID-19 has similar lung ultrasound findings as non-COVID-19 acute respiratory distress syndrome (ARDS).4,5 Based on prior lung ultrasound studies in ARDS, lung ultrasound findings can be used as an alternate to chest radiography for the diagnosis of ARDS in COVID-19 and predict the severity of ARDS.9 This has particular implications in overwhelmed and resource poor health care settings.
We found no difference in 30-day mortality based on lung ultrasound findings or profile, probably because of small sample size or because the findings were tabulated as profiles and not differentiated further with lung ultrasound scores.10,11 However, there was a significant difference in RV dilation between the 2 groups by 30 days and its presence was found to be a predictor of mortality even when controlled for hypertension and diabetes mellitus (P = .048) with an OR of 12. RV dysfunction in patients with ARDS on mechanical ventilation ranges from 22 to 25% and is typically associated with high driving pressures.12-14 The mechanism is thought to be multifactorial including hypoxemic vasoconstriction in the pulmonary vasculature in addition to the increased transpulmonary pressure.15 While all of the above are at play in COVID-19 infection, there is reported damage to the pulmonary vascular endothelium and resultant hypercoagulability and thrombosis that further increases the RV afterload.16
While RV strain and dysfunction indices done by an echocardiographer would be ideal, given the surge in infections and hospitalizations and strain on health care resources, POCUS by the treating or examining clinician was considered the only feasible way to screen a large number of patients.17 Identification of RV dilation could influence clinical management including workup for venous thromboembolic disease and optimization of lung protective strategies. Further studies are needed to understand the particular etiology and pathophysiology of COVID-19 associated RV dilation. Given increased thrombosis events in COVID-19 infection we believe a POCUS vascular examination should be included as part of evaluation especially in the presence of increased D-dimers and has been discussed above for its important role in working up RV dilation.18
Limitations
Our study has several limitations. It was retrospective in nature and involved a small group of individuals. There was some variation in POCUS examinations done at the discretion of the examining physician. We did not have a blinded observer independently review all images. Since RV dilation was documented only when RV size approached or exceeded LV size in the apical 4 chamber view representing moderate or severe dilation, we may be underreporting the prevalence in critically ill patients.
Conclusions
POCUS is an invaluable adjunct to clinical evaluation and procedures in patients with severe COVID-19 with the ability to identity patients at risk for worse outcomes. B lines with pleural thickening is a sign of severe ARDS and RV dilatation is predictive of mortality. POCUS should be made available to the treating physician for monitoring and risk stratification and can be incorporated into management algorithms.
Additional point-of-care ultrasound videos.
Acknowledgments
We thank frontline healthcare workers and intensive care unit staff of the US Department of Veterans Affairs New York Harbor Healthcare System (NYHHS) for their dedication to the care of veterans and civilians during the COVID-19 pandemic in New York City. The authors acknowledge the NYHHS research and development committee and administration for their support.
1. Cardenas-Garcia J, Mayo PH. Bedside ultrasonography for the intensivist. Crit Care Clin. 2015;31(1):43-66. doi:10.1016/j.ccc.2014.08.003
2. Vetrugno L, Baciarello M, Bignami E, et al. The “pandemic” increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review. Ultrasound J. 2020;12(1):39. Published 2020 Aug 17. doi:10.1186/s13089-020-00185-4
3. Hussain A, Via G, Melniker L, et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care. 2020;24(1):702. Published 2020 Dec 24. doi:10.1186/s13054-020-03369-5
4. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol [published correction appears in Chest. 2013 Aug;144(2):721]. Chest. 2008;134(1):117-125. doi:10.1378/chest.07-2800
5. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-591. doi:10.1007/s00134-012-2513-4
6. Narasimhan M, Koenig SJ, Mayo PH. Advanced echocardiography for the critical care physician: part 1. Chest. 2014;145(1):129-134. doi:10.1378/chest.12-2441
7. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139(3):538-542. doi:10.1378/chest.10-1479
8. Bentzer P, Griesdale DE, Boyd J, MacLean K, Sirounis D, Ayas NT. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316(12):1298-1309. doi:10.1001/jama.2016.12310
9. See KC, Ong V, Tan YL, Sahagun J, Taculod J. Chest radiography versus lung ultrasound for identification of acute respiratory distress syndrome: a retrospective observational study. Crit Care. 2018;22(1):203. Published 2018 Aug 18. doi:10.1186/s13054-018-2105-y
10. Deng Q, Zhang Y, Wang H, et al. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single-center study. Acad Radiol. 2020;27(10):1363-1372. doi:10.1016/j.acra.2020.07.002
11. Brahier T, Meuwly JY, Pantet O, et al. Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study [published online ahead of print, 2020 Sep 17]. Clin Infect Dis. 2020;ciaa1408. doi:10.1093/cid/ciaa1408
12. Vieillard-Baron A, Schmitt JM, Augarde R, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis [published correction appears in Crit Care Med. 2002 Mar;30(3):726]. Crit Care Med. 2001;29(8):1551-1555. doi:10.1097/00003246-200108000-00009
13. Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1725-1733. doi:10.1007/s00134-013-2941-9
14. Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007;33(3):444-447. doi:10.1007/s00134-007-0552-z
15. Repessé X, Vieillard-Baron A. Right heart function during acute respiratory distress syndrome. Ann Transl Med 2017;5(14):295. doi:10.21037/atm.2017.06.66
16. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management [published correction appears in Thromb Res. 2020 Nov 26]. Thromb Res. 2020;194:101-115. doi:10.1016/j.thromres.2020.06.029
17. Kim J, Volodarskiy A, Sultana R, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 2020;76(17):1965-1977. doi:10.1016/j.jacc.2020.08.066
18. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. doi:10.1182/blood.2020006520
1. Cardenas-Garcia J, Mayo PH. Bedside ultrasonography for the intensivist. Crit Care Clin. 2015;31(1):43-66. doi:10.1016/j.ccc.2014.08.003
2. Vetrugno L, Baciarello M, Bignami E, et al. The “pandemic” increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review. Ultrasound J. 2020;12(1):39. Published 2020 Aug 17. doi:10.1186/s13089-020-00185-4
3. Hussain A, Via G, Melniker L, et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care. 2020;24(1):702. Published 2020 Dec 24. doi:10.1186/s13054-020-03369-5
4. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol [published correction appears in Chest. 2013 Aug;144(2):721]. Chest. 2008;134(1):117-125. doi:10.1378/chest.07-2800
5. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-591. doi:10.1007/s00134-012-2513-4
6. Narasimhan M, Koenig SJ, Mayo PH. Advanced echocardiography for the critical care physician: part 1. Chest. 2014;145(1):129-134. doi:10.1378/chest.12-2441
7. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139(3):538-542. doi:10.1378/chest.10-1479
8. Bentzer P, Griesdale DE, Boyd J, MacLean K, Sirounis D, Ayas NT. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316(12):1298-1309. doi:10.1001/jama.2016.12310
9. See KC, Ong V, Tan YL, Sahagun J, Taculod J. Chest radiography versus lung ultrasound for identification of acute respiratory distress syndrome: a retrospective observational study. Crit Care. 2018;22(1):203. Published 2018 Aug 18. doi:10.1186/s13054-018-2105-y
10. Deng Q, Zhang Y, Wang H, et al. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single-center study. Acad Radiol. 2020;27(10):1363-1372. doi:10.1016/j.acra.2020.07.002
11. Brahier T, Meuwly JY, Pantet O, et al. Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study [published online ahead of print, 2020 Sep 17]. Clin Infect Dis. 2020;ciaa1408. doi:10.1093/cid/ciaa1408
12. Vieillard-Baron A, Schmitt JM, Augarde R, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis [published correction appears in Crit Care Med. 2002 Mar;30(3):726]. Crit Care Med. 2001;29(8):1551-1555. doi:10.1097/00003246-200108000-00009
13. Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1725-1733. doi:10.1007/s00134-013-2941-9
14. Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007;33(3):444-447. doi:10.1007/s00134-007-0552-z
15. Repessé X, Vieillard-Baron A. Right heart function during acute respiratory distress syndrome. Ann Transl Med 2017;5(14):295. doi:10.21037/atm.2017.06.66
16. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management [published correction appears in Thromb Res. 2020 Nov 26]. Thromb Res. 2020;194:101-115. doi:10.1016/j.thromres.2020.06.029
17. Kim J, Volodarskiy A, Sultana R, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 2020;76(17):1965-1977. doi:10.1016/j.jacc.2020.08.066
18. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. doi:10.1182/blood.2020006520
Flu and COVID-19 vaccines can be given on the same day: CDC and AAP
Previously, the CDC recommended that people receive their COVID-19 vaccinations alone and schedule any other vaccinations at least 2 weeks before or after their COVID-19 immunization. “This was out of an abundance of caution during a period when these vaccines were new and not due to any known safety or immunogenicity concerns,” the CDC guidance states. “However, substantial data have now been collected regarding the safety of COVID-19 vaccines currently approved or authorized by FDA.”
The guidance allowing for coadministration of COVID-19 vaccines with other immunizations, including the flu shot, was issued in mid-May 2021, and was restated in influenza vaccine recommendations released Aug. 27. The American Academy of Pediatrics soon followed suit, announcing that, for children eligible for the COVID-19 vaccine (age 12 and older), AAP recommendations allow for both the influenza and COVID-19 vaccines to be administered during the same visit.
Although there is limited data around giving COVID-19 vaccines with other vaccines, “extensive experience with non–COVID-19 vaccines has demonstrated that immunogenicity and adverse-event profiles are generally similar when vaccines are administered simultaneously as when they are administered alone,” the recommendations state. If administering other immunizations along with COVID-19 vaccines, providers should separate injection sites by at least 1 inch, the CDC recommends, and influenza vaccines that are more likely to cause a local reaction, like high-dose or the adjuvanted inactivated flu vaccine, should be administered in different limbs, if possible.
Whether someone should get their flu vaccine at the same time or separate from a COVID-19 vaccination or booster is a matter of personal preference as well as convenience, Susan Coffin, MD, MPH, an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, said in an interview. “It basically boils down to: Will you be able to get your flu shot without any difficulty in 2 weeks’ time?” she said. “We don’t want inconvenience or difficulties in access to get the way of people getting their flu shot this year.”
A version of this article first appeared on Medscape.com.
Previously, the CDC recommended that people receive their COVID-19 vaccinations alone and schedule any other vaccinations at least 2 weeks before or after their COVID-19 immunization. “This was out of an abundance of caution during a period when these vaccines were new and not due to any known safety or immunogenicity concerns,” the CDC guidance states. “However, substantial data have now been collected regarding the safety of COVID-19 vaccines currently approved or authorized by FDA.”
The guidance allowing for coadministration of COVID-19 vaccines with other immunizations, including the flu shot, was issued in mid-May 2021, and was restated in influenza vaccine recommendations released Aug. 27. The American Academy of Pediatrics soon followed suit, announcing that, for children eligible for the COVID-19 vaccine (age 12 and older), AAP recommendations allow for both the influenza and COVID-19 vaccines to be administered during the same visit.
Although there is limited data around giving COVID-19 vaccines with other vaccines, “extensive experience with non–COVID-19 vaccines has demonstrated that immunogenicity and adverse-event profiles are generally similar when vaccines are administered simultaneously as when they are administered alone,” the recommendations state. If administering other immunizations along with COVID-19 vaccines, providers should separate injection sites by at least 1 inch, the CDC recommends, and influenza vaccines that are more likely to cause a local reaction, like high-dose or the adjuvanted inactivated flu vaccine, should be administered in different limbs, if possible.
Whether someone should get their flu vaccine at the same time or separate from a COVID-19 vaccination or booster is a matter of personal preference as well as convenience, Susan Coffin, MD, MPH, an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, said in an interview. “It basically boils down to: Will you be able to get your flu shot without any difficulty in 2 weeks’ time?” she said. “We don’t want inconvenience or difficulties in access to get the way of people getting their flu shot this year.”
A version of this article first appeared on Medscape.com.
Previously, the CDC recommended that people receive their COVID-19 vaccinations alone and schedule any other vaccinations at least 2 weeks before or after their COVID-19 immunization. “This was out of an abundance of caution during a period when these vaccines were new and not due to any known safety or immunogenicity concerns,” the CDC guidance states. “However, substantial data have now been collected regarding the safety of COVID-19 vaccines currently approved or authorized by FDA.”
The guidance allowing for coadministration of COVID-19 vaccines with other immunizations, including the flu shot, was issued in mid-May 2021, and was restated in influenza vaccine recommendations released Aug. 27. The American Academy of Pediatrics soon followed suit, announcing that, for children eligible for the COVID-19 vaccine (age 12 and older), AAP recommendations allow for both the influenza and COVID-19 vaccines to be administered during the same visit.
Although there is limited data around giving COVID-19 vaccines with other vaccines, “extensive experience with non–COVID-19 vaccines has demonstrated that immunogenicity and adverse-event profiles are generally similar when vaccines are administered simultaneously as when they are administered alone,” the recommendations state. If administering other immunizations along with COVID-19 vaccines, providers should separate injection sites by at least 1 inch, the CDC recommends, and influenza vaccines that are more likely to cause a local reaction, like high-dose or the adjuvanted inactivated flu vaccine, should be administered in different limbs, if possible.
Whether someone should get their flu vaccine at the same time or separate from a COVID-19 vaccination or booster is a matter of personal preference as well as convenience, Susan Coffin, MD, MPH, an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, said in an interview. “It basically boils down to: Will you be able to get your flu shot without any difficulty in 2 weeks’ time?” she said. “We don’t want inconvenience or difficulties in access to get the way of people getting their flu shot this year.”
A version of this article first appeared on Medscape.com.
Medical boards: Docs who spread COVID misinformation put license at risk
Leaders of the American Board of Family Medicine, the American Board of Internal Medicine, and the American Board of Pediatrics said Sept. 9 that they support FSMB’s position.
“We also want all physicians certified by our boards to know that such unethical or unprofessional conduct may prompt their respective Board to take action that could put their certification at risk,” a statement read.
“Expertise matters, and board-certified physicians have demonstrated that they have stayed current in their field. Spreading misinformation or falsehoods to the public during a time of a public health emergency goes against everything our boards and our community of board-certified physicians stand for,” the leaders wrote.
“The evidence that we have safe, effective, and widely available vaccines against COVID-19 is overwhelming. We are particularly concerned about physicians who use their authority to denigrate vaccination at a time when vaccines continue to demonstrate excellent effectiveness against severe illness, hospitalization, and death.”
Small number spread false information
However, a small number of doctors continue to spread misinformation against the vaccines and communicate other false information surrounding COVID-19.
Some of the misinformation spreaders have had ultra-viral reach.
Among them is Daniel Stock, MD, a family physician in Indiana who has come out against COVID-19 vaccines. At a recent meeting of the Mt. Vernon Community School board in Indiana, he gave a speech urging the board to ignore the prevailing recommendations around COVID-19, such as test-and-trace measures.
Forbes reported in August that versions of the video of Stock›s speech on Facebook “have collected a total of 90 million engagements – a metric encompassing things such as comments, likes and shares – according to data collected by Media Matters for America, a liberal tech-watchdog group.”
This news organization published a story in August asking whether physicians who spread such information should lose their license and the question drew rapid-fire comments.
Commenters who argued with potential disciplinary actions raised questions about where the line will be drawn between misinformation and deeply held beliefs in terms of care.
Several comments centered on ivermectin, which is not approved by the Food and Drug Administration to treat COVID-19 but is enthusiastically supported as a COVID-19 treatment by a group of physicians called the Front Line COVID-19 Critical Care Alliance, whose website includes requests for donations.
Some cited free speech protections.
‘Not consistent with standards’
As for ivermectin, David G. Nichols, MD, president and CEO of the American Board of Pediatrics, gave this news organization an example: “Spreading the notion that one would not need to get vaccinated because if you get sick you could take ivermectin is a very dangerous statement. That is not consistent with the standards of professionalism required for certification or licensure.”
Ivermectin, he noted, is not an approved treatment for COVID-19.
“To say that it is or has any benefit is a false statement. We’re not willing to allow individuals who make false statements to devalue the terrific work of tens of thousands of physicians across the United States doing work under very difficult circumstances,” Dr. Nichols said.
He continued: “To suggest treatments that are known not to be effective in exchange for treatment that is known to be effective is dangerous – and ivermectin falls under that category.”
Asked whether such suggestions could result in suspension or revocation of a physician’s license, Dr. Nichols said, “It’s the kind of thing that would certainly trigger a review.”
He said the standard for separating misinformation from personal beliefs is based on whether there is scientific evidence to support the belief.
The boards are not, with this statement, attempting to referee legitimate scientific debate, he said.
The misinformation the boards are referring to, Dr. Nichols said, is “where the evidence is 100% on one side and zero on another. And the zero is not only that the opinions or beliefs are unsupported or unsubstantiated, they are indeed harmful if followed. That’s the distinction we’re trying to make here.”
As for free-speech arguments, he said, “Free speech is a constitutional right. You can say whatever you want. The issue here is you do not have the right to expect continued professional sanction of a board certificate if you are lying to the public.”
The board statement also said: “We all look to board-certified physicians to provide outstanding care and guidance; providing misinformation about a lethal disease is unethical, unprofessional, and dangerous. In times of medical emergency, the community of expert physicians committed to science and evidence collectively shares a responsibility for giving the public the most accurate and timely health information available, so they can make decisions that work best for themselves and their families.”
In addition to Dr. Nichols, the statement was signed by Warren Newton, MD, MPH, president and CEO of the American Board of Family Medicine, and Richard J. Baron, MD, president and CEO of the American Board of Internal Medicine.
A version of this article first appeared on Medscape.com.
Leaders of the American Board of Family Medicine, the American Board of Internal Medicine, and the American Board of Pediatrics said Sept. 9 that they support FSMB’s position.
“We also want all physicians certified by our boards to know that such unethical or unprofessional conduct may prompt their respective Board to take action that could put their certification at risk,” a statement read.
“Expertise matters, and board-certified physicians have demonstrated that they have stayed current in their field. Spreading misinformation or falsehoods to the public during a time of a public health emergency goes against everything our boards and our community of board-certified physicians stand for,” the leaders wrote.
“The evidence that we have safe, effective, and widely available vaccines against COVID-19 is overwhelming. We are particularly concerned about physicians who use their authority to denigrate vaccination at a time when vaccines continue to demonstrate excellent effectiveness against severe illness, hospitalization, and death.”
Small number spread false information
However, a small number of doctors continue to spread misinformation against the vaccines and communicate other false information surrounding COVID-19.
Some of the misinformation spreaders have had ultra-viral reach.
Among them is Daniel Stock, MD, a family physician in Indiana who has come out against COVID-19 vaccines. At a recent meeting of the Mt. Vernon Community School board in Indiana, he gave a speech urging the board to ignore the prevailing recommendations around COVID-19, such as test-and-trace measures.
Forbes reported in August that versions of the video of Stock›s speech on Facebook “have collected a total of 90 million engagements – a metric encompassing things such as comments, likes and shares – according to data collected by Media Matters for America, a liberal tech-watchdog group.”
This news organization published a story in August asking whether physicians who spread such information should lose their license and the question drew rapid-fire comments.
Commenters who argued with potential disciplinary actions raised questions about where the line will be drawn between misinformation and deeply held beliefs in terms of care.
Several comments centered on ivermectin, which is not approved by the Food and Drug Administration to treat COVID-19 but is enthusiastically supported as a COVID-19 treatment by a group of physicians called the Front Line COVID-19 Critical Care Alliance, whose website includes requests for donations.
Some cited free speech protections.
‘Not consistent with standards’
As for ivermectin, David G. Nichols, MD, president and CEO of the American Board of Pediatrics, gave this news organization an example: “Spreading the notion that one would not need to get vaccinated because if you get sick you could take ivermectin is a very dangerous statement. That is not consistent with the standards of professionalism required for certification or licensure.”
Ivermectin, he noted, is not an approved treatment for COVID-19.
“To say that it is or has any benefit is a false statement. We’re not willing to allow individuals who make false statements to devalue the terrific work of tens of thousands of physicians across the United States doing work under very difficult circumstances,” Dr. Nichols said.
He continued: “To suggest treatments that are known not to be effective in exchange for treatment that is known to be effective is dangerous – and ivermectin falls under that category.”
Asked whether such suggestions could result in suspension or revocation of a physician’s license, Dr. Nichols said, “It’s the kind of thing that would certainly trigger a review.”
He said the standard for separating misinformation from personal beliefs is based on whether there is scientific evidence to support the belief.
The boards are not, with this statement, attempting to referee legitimate scientific debate, he said.
The misinformation the boards are referring to, Dr. Nichols said, is “where the evidence is 100% on one side and zero on another. And the zero is not only that the opinions or beliefs are unsupported or unsubstantiated, they are indeed harmful if followed. That’s the distinction we’re trying to make here.”
As for free-speech arguments, he said, “Free speech is a constitutional right. You can say whatever you want. The issue here is you do not have the right to expect continued professional sanction of a board certificate if you are lying to the public.”
The board statement also said: “We all look to board-certified physicians to provide outstanding care and guidance; providing misinformation about a lethal disease is unethical, unprofessional, and dangerous. In times of medical emergency, the community of expert physicians committed to science and evidence collectively shares a responsibility for giving the public the most accurate and timely health information available, so they can make decisions that work best for themselves and their families.”
In addition to Dr. Nichols, the statement was signed by Warren Newton, MD, MPH, president and CEO of the American Board of Family Medicine, and Richard J. Baron, MD, president and CEO of the American Board of Internal Medicine.
A version of this article first appeared on Medscape.com.
Leaders of the American Board of Family Medicine, the American Board of Internal Medicine, and the American Board of Pediatrics said Sept. 9 that they support FSMB’s position.
“We also want all physicians certified by our boards to know that such unethical or unprofessional conduct may prompt their respective Board to take action that could put their certification at risk,” a statement read.
“Expertise matters, and board-certified physicians have demonstrated that they have stayed current in their field. Spreading misinformation or falsehoods to the public during a time of a public health emergency goes against everything our boards and our community of board-certified physicians stand for,” the leaders wrote.
“The evidence that we have safe, effective, and widely available vaccines against COVID-19 is overwhelming. We are particularly concerned about physicians who use their authority to denigrate vaccination at a time when vaccines continue to demonstrate excellent effectiveness against severe illness, hospitalization, and death.”
Small number spread false information
However, a small number of doctors continue to spread misinformation against the vaccines and communicate other false information surrounding COVID-19.
Some of the misinformation spreaders have had ultra-viral reach.
Among them is Daniel Stock, MD, a family physician in Indiana who has come out against COVID-19 vaccines. At a recent meeting of the Mt. Vernon Community School board in Indiana, he gave a speech urging the board to ignore the prevailing recommendations around COVID-19, such as test-and-trace measures.
Forbes reported in August that versions of the video of Stock›s speech on Facebook “have collected a total of 90 million engagements – a metric encompassing things such as comments, likes and shares – according to data collected by Media Matters for America, a liberal tech-watchdog group.”
This news organization published a story in August asking whether physicians who spread such information should lose their license and the question drew rapid-fire comments.
Commenters who argued with potential disciplinary actions raised questions about where the line will be drawn between misinformation and deeply held beliefs in terms of care.
Several comments centered on ivermectin, which is not approved by the Food and Drug Administration to treat COVID-19 but is enthusiastically supported as a COVID-19 treatment by a group of physicians called the Front Line COVID-19 Critical Care Alliance, whose website includes requests for donations.
Some cited free speech protections.
‘Not consistent with standards’
As for ivermectin, David G. Nichols, MD, president and CEO of the American Board of Pediatrics, gave this news organization an example: “Spreading the notion that one would not need to get vaccinated because if you get sick you could take ivermectin is a very dangerous statement. That is not consistent with the standards of professionalism required for certification or licensure.”
Ivermectin, he noted, is not an approved treatment for COVID-19.
“To say that it is or has any benefit is a false statement. We’re not willing to allow individuals who make false statements to devalue the terrific work of tens of thousands of physicians across the United States doing work under very difficult circumstances,” Dr. Nichols said.
He continued: “To suggest treatments that are known not to be effective in exchange for treatment that is known to be effective is dangerous – and ivermectin falls under that category.”
Asked whether such suggestions could result in suspension or revocation of a physician’s license, Dr. Nichols said, “It’s the kind of thing that would certainly trigger a review.”
He said the standard for separating misinformation from personal beliefs is based on whether there is scientific evidence to support the belief.
The boards are not, with this statement, attempting to referee legitimate scientific debate, he said.
The misinformation the boards are referring to, Dr. Nichols said, is “where the evidence is 100% on one side and zero on another. And the zero is not only that the opinions or beliefs are unsupported or unsubstantiated, they are indeed harmful if followed. That’s the distinction we’re trying to make here.”
As for free-speech arguments, he said, “Free speech is a constitutional right. You can say whatever you want. The issue here is you do not have the right to expect continued professional sanction of a board certificate if you are lying to the public.”
The board statement also said: “We all look to board-certified physicians to provide outstanding care and guidance; providing misinformation about a lethal disease is unethical, unprofessional, and dangerous. In times of medical emergency, the community of expert physicians committed to science and evidence collectively shares a responsibility for giving the public the most accurate and timely health information available, so they can make decisions that work best for themselves and their families.”
In addition to Dr. Nichols, the statement was signed by Warren Newton, MD, MPH, president and CEO of the American Board of Family Medicine, and Richard J. Baron, MD, president and CEO of the American Board of Internal Medicine.
A version of this article first appeared on Medscape.com.
Double Hit: Epstein-Barr Virus Causing Infectious Mononucleosis Followed by Hemolytic Uremic Syndrome
Introduction
Epstein-Barr virus (EBV) is a herpes virus that commonly causes infectious mononucleosis (IM) and linked to different hematological conditions. Here we present a case of EBV-triggered Hemolytic Uremic Syndrome (HUS) with pulmonary involvement.
Case Presentation
A 20-year-old male presented with fever, thrombocytopenia, and splenomegaly. Acute EBV serology was positive. Creatinine and hemoglobin were normal. He was diagnosed with IM. platelet count improved within 3 weeks. 4 weeks later, he returned with severe hemoptysis. Hgb 6.8g/dL, platelet 133,000/uL, lactate dehydrogenase 969u/L, creatinine 21mg/dL, and schistocytes on peripheral smear. Chest computed tomography showed bilateral opacities consistent with diffuse alveolar hemorrhage (DAH). Emergent hemodialysis and plasmapheresis were started. Infectious work up was negative. Autoimmune work up was also negative (anti-neutrophil cytoplasmic, anti-basement membrane antibodies, ANA). Aadamts13 activity was 62% (normal ~66%) ruling out thrombotic thrombocytopenic purpura (TTP). Kidney biopsy revealed thrombotic microangiopathic process. The patient was eventually diagnosed with HUS and treated with Eculizumab. 4 months later his renal function has partially recovered and no longer needs hemodialysis.
Discussion
HUS is a rare entity that is known to be triggered by different underlying pathologies. However, its link to EBV remains unclear. Literature review has revealed only two cases of EBV-triggered HUS, even though almost 90-95% of adults are EBV-seropositive. What unique about our case is the patient initially presented with documented IM, and HUS happened a month later. This raises the theory that HUS could be a sequela of the infection, rather than an effect of acute viral phase and this is the first case to report such correlation. The other unique thing is pulmonary involvement in HUS. With consultation with pulmonary service, we believe our patient had DAH based on clinical and radiographic findings. To our knowledge this is the first case to show this association.
Conclusion
EBV is a common virus with high seropositivity among world’s population. Its link to HUS remains unclear and needs more investigation. Providers should recognize HUS as a complication of EBV infection, either in the acute phase or as a sequela. Adolescents are at higher risk for such complication since IM is common in this population.
Introduction
Epstein-Barr virus (EBV) is a herpes virus that commonly causes infectious mononucleosis (IM) and linked to different hematological conditions. Here we present a case of EBV-triggered Hemolytic Uremic Syndrome (HUS) with pulmonary involvement.
Case Presentation
A 20-year-old male presented with fever, thrombocytopenia, and splenomegaly. Acute EBV serology was positive. Creatinine and hemoglobin were normal. He was diagnosed with IM. platelet count improved within 3 weeks. 4 weeks later, he returned with severe hemoptysis. Hgb 6.8g/dL, platelet 133,000/uL, lactate dehydrogenase 969u/L, creatinine 21mg/dL, and schistocytes on peripheral smear. Chest computed tomography showed bilateral opacities consistent with diffuse alveolar hemorrhage (DAH). Emergent hemodialysis and plasmapheresis were started. Infectious work up was negative. Autoimmune work up was also negative (anti-neutrophil cytoplasmic, anti-basement membrane antibodies, ANA). Aadamts13 activity was 62% (normal ~66%) ruling out thrombotic thrombocytopenic purpura (TTP). Kidney biopsy revealed thrombotic microangiopathic process. The patient was eventually diagnosed with HUS and treated with Eculizumab. 4 months later his renal function has partially recovered and no longer needs hemodialysis.
Discussion
HUS is a rare entity that is known to be triggered by different underlying pathologies. However, its link to EBV remains unclear. Literature review has revealed only two cases of EBV-triggered HUS, even though almost 90-95% of adults are EBV-seropositive. What unique about our case is the patient initially presented with documented IM, and HUS happened a month later. This raises the theory that HUS could be a sequela of the infection, rather than an effect of acute viral phase and this is the first case to report such correlation. The other unique thing is pulmonary involvement in HUS. With consultation with pulmonary service, we believe our patient had DAH based on clinical and radiographic findings. To our knowledge this is the first case to show this association.
Conclusion
EBV is a common virus with high seropositivity among world’s population. Its link to HUS remains unclear and needs more investigation. Providers should recognize HUS as a complication of EBV infection, either in the acute phase or as a sequela. Adolescents are at higher risk for such complication since IM is common in this population.
Introduction
Epstein-Barr virus (EBV) is a herpes virus that commonly causes infectious mononucleosis (IM) and linked to different hematological conditions. Here we present a case of EBV-triggered Hemolytic Uremic Syndrome (HUS) with pulmonary involvement.
Case Presentation
A 20-year-old male presented with fever, thrombocytopenia, and splenomegaly. Acute EBV serology was positive. Creatinine and hemoglobin were normal. He was diagnosed with IM. platelet count improved within 3 weeks. 4 weeks later, he returned with severe hemoptysis. Hgb 6.8g/dL, platelet 133,000/uL, lactate dehydrogenase 969u/L, creatinine 21mg/dL, and schistocytes on peripheral smear. Chest computed tomography showed bilateral opacities consistent with diffuse alveolar hemorrhage (DAH). Emergent hemodialysis and plasmapheresis were started. Infectious work up was negative. Autoimmune work up was also negative (anti-neutrophil cytoplasmic, anti-basement membrane antibodies, ANA). Aadamts13 activity was 62% (normal ~66%) ruling out thrombotic thrombocytopenic purpura (TTP). Kidney biopsy revealed thrombotic microangiopathic process. The patient was eventually diagnosed with HUS and treated with Eculizumab. 4 months later his renal function has partially recovered and no longer needs hemodialysis.
Discussion
HUS is a rare entity that is known to be triggered by different underlying pathologies. However, its link to EBV remains unclear. Literature review has revealed only two cases of EBV-triggered HUS, even though almost 90-95% of adults are EBV-seropositive. What unique about our case is the patient initially presented with documented IM, and HUS happened a month later. This raises the theory that HUS could be a sequela of the infection, rather than an effect of acute viral phase and this is the first case to report such correlation. The other unique thing is pulmonary involvement in HUS. With consultation with pulmonary service, we believe our patient had DAH based on clinical and radiographic findings. To our knowledge this is the first case to show this association.
Conclusion
EBV is a common virus with high seropositivity among world’s population. Its link to HUS remains unclear and needs more investigation. Providers should recognize HUS as a complication of EBV infection, either in the acute phase or as a sequela. Adolescents are at higher risk for such complication since IM is common in this population.
Choosing Wisely campaign targets waste and overuse in hospital pediatrics
“Health care spending and health care waste is a huge problem in the U.S., including for children,” Vivian Lee, MD, of Children’s Hospital, Los Angeles, said in a presentation at the 2021 virtual Pediatric Hospital Medicine conference.
Data from a 2019 study suggested that approximately 25% of health care spending in the United States qualifies as “wasteful spending,” in categories such as overtesting, and unnecessary hospitalization, Dr. Lee said. “It is essential for physicians in hospitals to be stewards of high-value care,” she emphasized.
To combat wasteful spending and control health care costs, the Choosing Wisely campaign was created in 2012 as an initiative from the American Board of Internal Medicine Foundation. An ongoing goal of the campaign is to raise awareness among physicians and patients about potential areas of low-value services and overuse. The overall campaign includes clinician-driven recommendations from multiple medical organizations.
The PHM produced its first set of five recommendations in 2012, Dr. Lee said. These recommendations, titled “Five Things Physicians and Patients Should Question,” have been updated for 2021. The updated recommendations were created as a partnership among the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine. A joint committee reviewed the latest evidence, and the updates were approved by the societies and published by the ABIM in January 2021.
“We think these recommendations truly reflect an exciting and evolving landscape for pediatric hospitalists,” Dr. Lee said. “There is a greater focus on opportunities to transition out of the hospital sooner, or avoid hospitalization altogether. There is an emphasis on antibiotic stewardship and a growing recognition of the impact that overuse may have on our vulnerable neonatal population,” she said. Several members of the Choosing Wisely panel presented the recommendations during the virtual presentation.
Revised recommendations
The new “Five Things Physicians and Patients Should Question” are as follows:
1. Do not prescribe IV antibiotics for predetermined durations for patients hospitalized with infections such as pyelonephritis, osteomyelitis, and complicated pneumonia. Consider early transition to oral antibiotics.
Many antibiotic doses used in clinical practice are preset durations that are not based on high-quality evidence, said Mike Tchou, MD, of Children’s Hospital of Colorado in Aurora. However, studies now show that earlier transition to enteral antibiotics can improve a range of outcomes including neonatal UTIs, osteomyelitis, and complicated pneumonia, he said. Considering early transition based on a patient’s response can decrease adverse events, pain, length of stay, and health care costs, he explained.
2. Do not continue hospitalization in well-appearing febrile infants once bacterial cultures (i.e., blood, cerebrospinal, and/or urine) have been confirmed negative for 24-36 hours, if adequate outpatient follow-up can be assured.
Recent data indicate that continuing hospitalization beyond 24-36 hours of confirmed negative bacterial cultures does not improve clinical outcomes for well-appearing infants admitted for concern of serious bacterial infection, said Paula Soung, MD, of Children’s Wisconsin in Milwaukee. In fact, “blood culture yield is highest in the first 12-36 hours after incubation with multiple studies demonstrating > 90% of pathogen cultures being positive by 24 hours,” Dr. Soung said. “If adequate outpatient follow-up can be assured, discharging well-appearing febrile infants at 24-36 hours after confirming cultures are negative has many positive outcomes,” she said.
3. Do not initiate phototherapy in term or late preterm well-appearing infants with neonatal hyperbilirubinemia if their bilirubin is below levels at which the AAP guidelines recommend treatment.
In making this recommendation, “we considered that the risk of kernicterus and cerebral palsy is extremely low in otherwise healthy term and late preterm newborns,” said Allison Holmes, MD, of Children’s Hospital at Dartmouth-Hitchcock, Manchester, N.H. “Subthreshold phototherapy leads to unnecessary hospitalization and its associated costs and harms,” and data show that kernicterus generally occurs close to 40 mg/dL and occurs most often in infants with hemolysis, she added.
The evidence for the recommendations included data showing that, among other factors, 8.6 of 100,000 babies have a bilirubin greater than 30 mg/dL, said Dr. Holmes. Risks of using subthreshold phototherapy include increased length of stay, increased readmissions, and increased costs, as well as decreased breastfeeding, bonding with parents, and increased parental anxiety. “Adding prolonged hospitalization for an intervention that might not be necessary can be stressful for parents,” she said.
4. Do not use broad-spectrum antibiotics such as ceftriaxone for children hospitalized with uncomplicated community-acquired pneumonia. Use narrow-spectrum antibiotics such as penicillin, ampicillin, or amoxicillin.
Michelle Lossius, MD, of the Shands Hospital for Children at the University of Florida, Gainesville, noted that the recommendations reflect IDSA guidelines from 2011 advising the use of ampicillin or penicillin for this population of children. More recent studies with large populations support the ability of narrow-spectrum antibiotics to limit the development of resistant organisms while achieving the same or better outcomes for children hospitalized with CAP, she said.
5. Do not start IV antibiotic therapy on well-appearing newborn infants with isolated risk factors for sepsis such as maternal chorioamnionitis, prolonged rupture of membranes, or untreated group-B streptococcal colonization. Use clinical tools such as an evidence-based sepsis risk calculator to guide management.
“This recommendation combines other recommendations,” said Prabi Rajbhandari, MD, of Akron (Ohio) Children’s Hospital. The evidence is ample, as the Centers for Disease Control and Prevention recommends the use of sepsis calculators to guide clinical management in sepsis patients, she said.
Data comparing periods before and after the adoption of a sepsis risk calculator showed a significant reduction in the use of blood cultures and antibiotics, she noted. Other risks of jumping to IV antibiotics include increased hospital stay, increased parental anxiety, and decreased parental bonding, Dr. Rajbhandari added.
Next steps include how to prioritize implementation, as well as deimplementation of outdated practices, said Francisco Alvarez, MD, of Lucile Packard Children’s Hospital, Palo Alto, Calif. “A lot of our practices were started without good evidence for why they should be done,” he said. Other steps include value improvement research; use of dashboards and benchmarking; involving other stakeholders including patients, families, and other health care providers; and addressing racial disparities, he concluded.
The presenters had no financial conflicts to disclose. The conference was sponsored by the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine.
“Health care spending and health care waste is a huge problem in the U.S., including for children,” Vivian Lee, MD, of Children’s Hospital, Los Angeles, said in a presentation at the 2021 virtual Pediatric Hospital Medicine conference.
Data from a 2019 study suggested that approximately 25% of health care spending in the United States qualifies as “wasteful spending,” in categories such as overtesting, and unnecessary hospitalization, Dr. Lee said. “It is essential for physicians in hospitals to be stewards of high-value care,” she emphasized.
To combat wasteful spending and control health care costs, the Choosing Wisely campaign was created in 2012 as an initiative from the American Board of Internal Medicine Foundation. An ongoing goal of the campaign is to raise awareness among physicians and patients about potential areas of low-value services and overuse. The overall campaign includes clinician-driven recommendations from multiple medical organizations.
The PHM produced its first set of five recommendations in 2012, Dr. Lee said. These recommendations, titled “Five Things Physicians and Patients Should Question,” have been updated for 2021. The updated recommendations were created as a partnership among the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine. A joint committee reviewed the latest evidence, and the updates were approved by the societies and published by the ABIM in January 2021.
“We think these recommendations truly reflect an exciting and evolving landscape for pediatric hospitalists,” Dr. Lee said. “There is a greater focus on opportunities to transition out of the hospital sooner, or avoid hospitalization altogether. There is an emphasis on antibiotic stewardship and a growing recognition of the impact that overuse may have on our vulnerable neonatal population,” she said. Several members of the Choosing Wisely panel presented the recommendations during the virtual presentation.
Revised recommendations
The new “Five Things Physicians and Patients Should Question” are as follows:
1. Do not prescribe IV antibiotics for predetermined durations for patients hospitalized with infections such as pyelonephritis, osteomyelitis, and complicated pneumonia. Consider early transition to oral antibiotics.
Many antibiotic doses used in clinical practice are preset durations that are not based on high-quality evidence, said Mike Tchou, MD, of Children’s Hospital of Colorado in Aurora. However, studies now show that earlier transition to enteral antibiotics can improve a range of outcomes including neonatal UTIs, osteomyelitis, and complicated pneumonia, he said. Considering early transition based on a patient’s response can decrease adverse events, pain, length of stay, and health care costs, he explained.
2. Do not continue hospitalization in well-appearing febrile infants once bacterial cultures (i.e., blood, cerebrospinal, and/or urine) have been confirmed negative for 24-36 hours, if adequate outpatient follow-up can be assured.
Recent data indicate that continuing hospitalization beyond 24-36 hours of confirmed negative bacterial cultures does not improve clinical outcomes for well-appearing infants admitted for concern of serious bacterial infection, said Paula Soung, MD, of Children’s Wisconsin in Milwaukee. In fact, “blood culture yield is highest in the first 12-36 hours after incubation with multiple studies demonstrating > 90% of pathogen cultures being positive by 24 hours,” Dr. Soung said. “If adequate outpatient follow-up can be assured, discharging well-appearing febrile infants at 24-36 hours after confirming cultures are negative has many positive outcomes,” she said.
3. Do not initiate phototherapy in term or late preterm well-appearing infants with neonatal hyperbilirubinemia if their bilirubin is below levels at which the AAP guidelines recommend treatment.
In making this recommendation, “we considered that the risk of kernicterus and cerebral palsy is extremely low in otherwise healthy term and late preterm newborns,” said Allison Holmes, MD, of Children’s Hospital at Dartmouth-Hitchcock, Manchester, N.H. “Subthreshold phototherapy leads to unnecessary hospitalization and its associated costs and harms,” and data show that kernicterus generally occurs close to 40 mg/dL and occurs most often in infants with hemolysis, she added.
The evidence for the recommendations included data showing that, among other factors, 8.6 of 100,000 babies have a bilirubin greater than 30 mg/dL, said Dr. Holmes. Risks of using subthreshold phototherapy include increased length of stay, increased readmissions, and increased costs, as well as decreased breastfeeding, bonding with parents, and increased parental anxiety. “Adding prolonged hospitalization for an intervention that might not be necessary can be stressful for parents,” she said.
4. Do not use broad-spectrum antibiotics such as ceftriaxone for children hospitalized with uncomplicated community-acquired pneumonia. Use narrow-spectrum antibiotics such as penicillin, ampicillin, or amoxicillin.
Michelle Lossius, MD, of the Shands Hospital for Children at the University of Florida, Gainesville, noted that the recommendations reflect IDSA guidelines from 2011 advising the use of ampicillin or penicillin for this population of children. More recent studies with large populations support the ability of narrow-spectrum antibiotics to limit the development of resistant organisms while achieving the same or better outcomes for children hospitalized with CAP, she said.
5. Do not start IV antibiotic therapy on well-appearing newborn infants with isolated risk factors for sepsis such as maternal chorioamnionitis, prolonged rupture of membranes, or untreated group-B streptococcal colonization. Use clinical tools such as an evidence-based sepsis risk calculator to guide management.
“This recommendation combines other recommendations,” said Prabi Rajbhandari, MD, of Akron (Ohio) Children’s Hospital. The evidence is ample, as the Centers for Disease Control and Prevention recommends the use of sepsis calculators to guide clinical management in sepsis patients, she said.
Data comparing periods before and after the adoption of a sepsis risk calculator showed a significant reduction in the use of blood cultures and antibiotics, she noted. Other risks of jumping to IV antibiotics include increased hospital stay, increased parental anxiety, and decreased parental bonding, Dr. Rajbhandari added.
Next steps include how to prioritize implementation, as well as deimplementation of outdated practices, said Francisco Alvarez, MD, of Lucile Packard Children’s Hospital, Palo Alto, Calif. “A lot of our practices were started without good evidence for why they should be done,” he said. Other steps include value improvement research; use of dashboards and benchmarking; involving other stakeholders including patients, families, and other health care providers; and addressing racial disparities, he concluded.
The presenters had no financial conflicts to disclose. The conference was sponsored by the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine.
“Health care spending and health care waste is a huge problem in the U.S., including for children,” Vivian Lee, MD, of Children’s Hospital, Los Angeles, said in a presentation at the 2021 virtual Pediatric Hospital Medicine conference.
Data from a 2019 study suggested that approximately 25% of health care spending in the United States qualifies as “wasteful spending,” in categories such as overtesting, and unnecessary hospitalization, Dr. Lee said. “It is essential for physicians in hospitals to be stewards of high-value care,” she emphasized.
To combat wasteful spending and control health care costs, the Choosing Wisely campaign was created in 2012 as an initiative from the American Board of Internal Medicine Foundation. An ongoing goal of the campaign is to raise awareness among physicians and patients about potential areas of low-value services and overuse. The overall campaign includes clinician-driven recommendations from multiple medical organizations.
The PHM produced its first set of five recommendations in 2012, Dr. Lee said. These recommendations, titled “Five Things Physicians and Patients Should Question,” have been updated for 2021. The updated recommendations were created as a partnership among the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine. A joint committee reviewed the latest evidence, and the updates were approved by the societies and published by the ABIM in January 2021.
“We think these recommendations truly reflect an exciting and evolving landscape for pediatric hospitalists,” Dr. Lee said. “There is a greater focus on opportunities to transition out of the hospital sooner, or avoid hospitalization altogether. There is an emphasis on antibiotic stewardship and a growing recognition of the impact that overuse may have on our vulnerable neonatal population,” she said. Several members of the Choosing Wisely panel presented the recommendations during the virtual presentation.
Revised recommendations
The new “Five Things Physicians and Patients Should Question” are as follows:
1. Do not prescribe IV antibiotics for predetermined durations for patients hospitalized with infections such as pyelonephritis, osteomyelitis, and complicated pneumonia. Consider early transition to oral antibiotics.
Many antibiotic doses used in clinical practice are preset durations that are not based on high-quality evidence, said Mike Tchou, MD, of Children’s Hospital of Colorado in Aurora. However, studies now show that earlier transition to enteral antibiotics can improve a range of outcomes including neonatal UTIs, osteomyelitis, and complicated pneumonia, he said. Considering early transition based on a patient’s response can decrease adverse events, pain, length of stay, and health care costs, he explained.
2. Do not continue hospitalization in well-appearing febrile infants once bacterial cultures (i.e., blood, cerebrospinal, and/or urine) have been confirmed negative for 24-36 hours, if adequate outpatient follow-up can be assured.
Recent data indicate that continuing hospitalization beyond 24-36 hours of confirmed negative bacterial cultures does not improve clinical outcomes for well-appearing infants admitted for concern of serious bacterial infection, said Paula Soung, MD, of Children’s Wisconsin in Milwaukee. In fact, “blood culture yield is highest in the first 12-36 hours after incubation with multiple studies demonstrating > 90% of pathogen cultures being positive by 24 hours,” Dr. Soung said. “If adequate outpatient follow-up can be assured, discharging well-appearing febrile infants at 24-36 hours after confirming cultures are negative has many positive outcomes,” she said.
3. Do not initiate phototherapy in term or late preterm well-appearing infants with neonatal hyperbilirubinemia if their bilirubin is below levels at which the AAP guidelines recommend treatment.
In making this recommendation, “we considered that the risk of kernicterus and cerebral palsy is extremely low in otherwise healthy term and late preterm newborns,” said Allison Holmes, MD, of Children’s Hospital at Dartmouth-Hitchcock, Manchester, N.H. “Subthreshold phototherapy leads to unnecessary hospitalization and its associated costs and harms,” and data show that kernicterus generally occurs close to 40 mg/dL and occurs most often in infants with hemolysis, she added.
The evidence for the recommendations included data showing that, among other factors, 8.6 of 100,000 babies have a bilirubin greater than 30 mg/dL, said Dr. Holmes. Risks of using subthreshold phototherapy include increased length of stay, increased readmissions, and increased costs, as well as decreased breastfeeding, bonding with parents, and increased parental anxiety. “Adding prolonged hospitalization for an intervention that might not be necessary can be stressful for parents,” she said.
4. Do not use broad-spectrum antibiotics such as ceftriaxone for children hospitalized with uncomplicated community-acquired pneumonia. Use narrow-spectrum antibiotics such as penicillin, ampicillin, or amoxicillin.
Michelle Lossius, MD, of the Shands Hospital for Children at the University of Florida, Gainesville, noted that the recommendations reflect IDSA guidelines from 2011 advising the use of ampicillin or penicillin for this population of children. More recent studies with large populations support the ability of narrow-spectrum antibiotics to limit the development of resistant organisms while achieving the same or better outcomes for children hospitalized with CAP, she said.
5. Do not start IV antibiotic therapy on well-appearing newborn infants with isolated risk factors for sepsis such as maternal chorioamnionitis, prolonged rupture of membranes, or untreated group-B streptococcal colonization. Use clinical tools such as an evidence-based sepsis risk calculator to guide management.
“This recommendation combines other recommendations,” said Prabi Rajbhandari, MD, of Akron (Ohio) Children’s Hospital. The evidence is ample, as the Centers for Disease Control and Prevention recommends the use of sepsis calculators to guide clinical management in sepsis patients, she said.
Data comparing periods before and after the adoption of a sepsis risk calculator showed a significant reduction in the use of blood cultures and antibiotics, she noted. Other risks of jumping to IV antibiotics include increased hospital stay, increased parental anxiety, and decreased parental bonding, Dr. Rajbhandari added.
Next steps include how to prioritize implementation, as well as deimplementation of outdated practices, said Francisco Alvarez, MD, of Lucile Packard Children’s Hospital, Palo Alto, Calif. “A lot of our practices were started without good evidence for why they should be done,” he said. Other steps include value improvement research; use of dashboards and benchmarking; involving other stakeholders including patients, families, and other health care providers; and addressing racial disparities, he concluded.
The presenters had no financial conflicts to disclose. The conference was sponsored by the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine.
FROM PHM 2021
United States reaches 5 million cases of child COVID
Cases of child COVID-19 set a new 1-week record and the total number of children infected during the pandemic passed 5 million, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The nearly 282,000 new cases reported in the United States during the week ending Sept. 2 broke the record of 211,000 set in mid-January and brought the cumulative count to 5,049,465 children with COVID-19 since the pandemic began, the AAP and the CHA said in their weekly COVID report.
Hospitalizations in children aged 0-17 years have also reached record levels in recent days. The highest daily admission rate since the pandemic began, 0.51 per 100,000 population, was recorded on Sept. 2, less than 2 months after the nation saw its lowest child COVID admission rate for 1 day: 0.07 per 100,000 on July 4. That’s an increase of 629%, according to data from the Centers for Disease Control and Prevention.
Vaccinations in children, however, did not follow suit. New vaccinations in children aged 12-17 years dropped by 4.5% for the week ending Sept. 6, compared with the week before. Initiations were actually up almost 12% for children aged 16-17, but that was not enough to overcome the continued decline among 12- to 15-year-olds, the CDC said on its COVID Data Tracker.
Despite the decline in new vaccinations, those younger children passed a noteworthy group milestone: 50.9% of all 12- to 15-year-olds now have received at least one dose, with 38.6% having completed the regimen. The 16- to 17-year-olds got an earlier start and have reached 58.9% coverage for one dose and 47.6% for two, the CDC said.
A total of 12.2 million children aged 12-17 years had received at least one dose of COVID vaccine as of Sept. 6, of whom almost 9.5 million are fully vaccinated, based on the CDC data.
At the state level, Vermont has the highest rates for vaccine initiation (75%) and full vaccination (65%), with Massachusetts (75%/62%) and Connecticut (73%/59%) just behind. The other end of the scale is occupied by Wyoming (28% initiation/19% full vaccination), Alabama (32%/19%), and North Dakota (32%/23%), the AAP said in a separate report.
In a recent letter to the Food and Drug Administration, AAP President Lee Savio Beers, MD, said that the “Delta variant is surging at extremely alarming rates in every region of America. This surge is seriously impacting all populations, including children.” Dr. Beers urged the FDA to work “aggressively toward authorizing safe and effective COVID-19 vaccines for children under age 12 as soon as possible.”
Cases of child COVID-19 set a new 1-week record and the total number of children infected during the pandemic passed 5 million, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The nearly 282,000 new cases reported in the United States during the week ending Sept. 2 broke the record of 211,000 set in mid-January and brought the cumulative count to 5,049,465 children with COVID-19 since the pandemic began, the AAP and the CHA said in their weekly COVID report.
Hospitalizations in children aged 0-17 years have also reached record levels in recent days. The highest daily admission rate since the pandemic began, 0.51 per 100,000 population, was recorded on Sept. 2, less than 2 months after the nation saw its lowest child COVID admission rate for 1 day: 0.07 per 100,000 on July 4. That’s an increase of 629%, according to data from the Centers for Disease Control and Prevention.
Vaccinations in children, however, did not follow suit. New vaccinations in children aged 12-17 years dropped by 4.5% for the week ending Sept. 6, compared with the week before. Initiations were actually up almost 12% for children aged 16-17, but that was not enough to overcome the continued decline among 12- to 15-year-olds, the CDC said on its COVID Data Tracker.
Despite the decline in new vaccinations, those younger children passed a noteworthy group milestone: 50.9% of all 12- to 15-year-olds now have received at least one dose, with 38.6% having completed the regimen. The 16- to 17-year-olds got an earlier start and have reached 58.9% coverage for one dose and 47.6% for two, the CDC said.
A total of 12.2 million children aged 12-17 years had received at least one dose of COVID vaccine as of Sept. 6, of whom almost 9.5 million are fully vaccinated, based on the CDC data.
At the state level, Vermont has the highest rates for vaccine initiation (75%) and full vaccination (65%), with Massachusetts (75%/62%) and Connecticut (73%/59%) just behind. The other end of the scale is occupied by Wyoming (28% initiation/19% full vaccination), Alabama (32%/19%), and North Dakota (32%/23%), the AAP said in a separate report.
In a recent letter to the Food and Drug Administration, AAP President Lee Savio Beers, MD, said that the “Delta variant is surging at extremely alarming rates in every region of America. This surge is seriously impacting all populations, including children.” Dr. Beers urged the FDA to work “aggressively toward authorizing safe and effective COVID-19 vaccines for children under age 12 as soon as possible.”
Cases of child COVID-19 set a new 1-week record and the total number of children infected during the pandemic passed 5 million, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

The nearly 282,000 new cases reported in the United States during the week ending Sept. 2 broke the record of 211,000 set in mid-January and brought the cumulative count to 5,049,465 children with COVID-19 since the pandemic began, the AAP and the CHA said in their weekly COVID report.
Hospitalizations in children aged 0-17 years have also reached record levels in recent days. The highest daily admission rate since the pandemic began, 0.51 per 100,000 population, was recorded on Sept. 2, less than 2 months after the nation saw its lowest child COVID admission rate for 1 day: 0.07 per 100,000 on July 4. That’s an increase of 629%, according to data from the Centers for Disease Control and Prevention.
Vaccinations in children, however, did not follow suit. New vaccinations in children aged 12-17 years dropped by 4.5% for the week ending Sept. 6, compared with the week before. Initiations were actually up almost 12% for children aged 16-17, but that was not enough to overcome the continued decline among 12- to 15-year-olds, the CDC said on its COVID Data Tracker.
Despite the decline in new vaccinations, those younger children passed a noteworthy group milestone: 50.9% of all 12- to 15-year-olds now have received at least one dose, with 38.6% having completed the regimen. The 16- to 17-year-olds got an earlier start and have reached 58.9% coverage for one dose and 47.6% for two, the CDC said.
A total of 12.2 million children aged 12-17 years had received at least one dose of COVID vaccine as of Sept. 6, of whom almost 9.5 million are fully vaccinated, based on the CDC data.
At the state level, Vermont has the highest rates for vaccine initiation (75%) and full vaccination (65%), with Massachusetts (75%/62%) and Connecticut (73%/59%) just behind. The other end of the scale is occupied by Wyoming (28% initiation/19% full vaccination), Alabama (32%/19%), and North Dakota (32%/23%), the AAP said in a separate report.
In a recent letter to the Food and Drug Administration, AAP President Lee Savio Beers, MD, said that the “Delta variant is surging at extremely alarming rates in every region of America. This surge is seriously impacting all populations, including children.” Dr. Beers urged the FDA to work “aggressively toward authorizing safe and effective COVID-19 vaccines for children under age 12 as soon as possible.”
Autoeczematization: A Strange Id Reaction of the Skin
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6

Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16
Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6

Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16

Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
Autoeczematization (AE), or id reaction, is a disseminated eczematous reaction that occurs days or weeks after exposure to a primary stimulus, resulting from a release of antigen(s). Whitfield1 first described AE in 1921, when he postulated that the id reaction was due to sensitization of the skin after a primary stimulus. He called it “a form of auto-intoxication derived from changes in the patient’s own tissues.”1 The exact prevalence of id reactions is unknown; one study showed that 17% of patients with dermatophyte infections developed an id reaction, typically tinea pedis linked with vesicles on the palms.2 Tinea capitis is one of the most common causes of AE in children, which is frequently misdiagnosed as a drug reaction. Approximately 37% of patients diagnosed with stasis dermatitis develop an id reaction (Figure 1). A history of contact dermatitis is common in patients presenting with AE.2-6

Pathophysiology of Id Reactions
An abnormal immune response against autologous skin antigens may be responsible for the development of AE. Shelley5 postulated that hair follicles play an important role in id reactions, as Sharquie et al6 recently emphasized for many skin disorders. The pathogenesis of AE is uncertain, but circulating T lymphocytes play a role in this reaction. Normally, T cells are activated by a release of antigens after a primary exposure to a stimulus. However, overactivation of these T cells induces autoimmune reactions such as AE.7 Activated T lymphocytes express HLA-DR and IL-2 receptor, markers elevated in the peripheral blood of patients undergoing id reactions. After treatment, the levels of activated T lymphocytes decline. An increase in the number of CD25+ T cells and a decrease in the number of suppressor T cells in the blood may occur during an id reaction.7-9 Keratinocytes produce proinflammatory cytokines, such as thymic stromal erythropoietin, IL-25, and IL-33, that activate T cells.10-12 Therefore, the most likely pathogenesis of an id reaction is that T lymphocytes are activated at the primary reaction site due to proinflammatory cytokines released by keratinocytes. These activated T cells then travel systemically via hematogenous dissemination. The spread of activated T lymphocytes produces an eczematous reaction at secondary locations distant to the primary site.9
Clinical and Histopathological Features of Id Reactions
Clinically, AE is first evident as a vesicular dissemination that groups to form papules or nummular patches and usually is present on the legs, feet, arms, and/or trunk (Figure 2). The primary dermatitis is localized to the area that was the site of contact to the offending stimuli. This localized eczematous eruption begins with an acute or subacute onset. It has the appearance of small crusted vesicles with erythema (Figure 1). The first sign of AE is vesicles presenting near the primary site on flexural surfaces or on the hands and feet. A classic example is tinea pedis linked with vesicles on the palms and sides of the fingers, resembling dyshidrotic eczema. Sites of prior cutaneous trauma, such as dermatoses, scars, and burns, are common locations for early AE. In later stages, vesicles disseminate to the legs, arms, and trunk, where they group to form papules and nummular patches in a symmetrical pattern.5,13-15 These lesions may be extremely pruritic. The pruritus may be so intense that it interrupts daily activities and disrupts the ability to fall or stay asleep.16

Histologically, biopsy specimens show psoriasiform spongiotic dermatitis with mononuclear cells contained in the vesicles. Interstitial edema and perivascular lymphohistiocytic infiltrates are evident. Eosinophils also may be present. This pattern is not unique toid reactions.17-19 Although AE is a reaction pattern that may be due to a fungal or bacterial infection, the etiologic agent is not evident microscopically within the eczema itself.
Etiology of Id Reactions
Id reactions most commonly occur from either stasis dermatitis or tinea pedis, although a wide variety of other causes should be considered. Evaluation of the primary site rather than the id reaction may identify an infectious or parasitic agent. Sometimes the AE reaction is specifically named: dermatophytid with dermatophytosis, bacterid with a bacterial infectious process, and tuberculid with tuberculosis. Similarly, there may be reactions to underlying candidiasis, sporotrichosis, histoplasmosis, and other fungal infections that can cause a cutaneous id reaction.18,20-22Mycobacterium species, Pseudomonas, Staphylococcus, and Streptococcus are bacterial causes of AE.15,23-26 Viral infections that can cause an id reaction are herpes simplex virus and molluscum contagiosum.27-29 Scabies, leishmaniasis, and pediculosis capitis are parasitic infections that may be etiologic.14,30,31 In addition, noninfectious stimuli besides stasis dermatitis that can produce id reactions include medications, topical creams, tattoo ink, sutures, radiotherapy, and dyshidrotic eczema. The primary reaction to these agents is a localized dermatitis followed by the immunological response that induces a secondary reaction distant from the primary site.17,18,32-38
Differential Diagnoses
Differential diagnoses include other types of eczema and some vesicular eruptions. Irritant contact dermatitis is another dermatosis that presents as a widespread vesicular eruption due to repetitive exposure to toxic irritants. The rash is erythematous with pustules, blisters, and crusts. It is only found in areas directly exposed to irritants, as opposed to AE, which spreads to areas distant to the primary reaction site. Irritant contact dermatitis presents with more of a burning sensation, whereas AE is more pruritic.39,40 Allergic contact dermatitis presents with erythematous vesicles and papules and sometimes with bullae. There is edema and crust formation, which often can spread past the point of contact in later stages. Similar to AE, there is intense pruritus. However, allergic contact dermatitis most commonly is caused by exposure to metals, cosmetics, and fragrances, whereas infectious agents and stasis dermatitis are the most common causes of AE.40,41 It may be challenging to distinguish AE from other causes of widespread eczematous dissemination. Vesicular eruptions sometimes require distinction from AE, including herpetic infections, insect bite reactions, and drug eruptions.18,42
Treatment
The underlying condition should be treated to mitigate the inflammatory response causing the id reaction. If not skillfully orchestrated, the id reaction can reoccur. For infectious causes of AE, an antifungal, antibacterial, antiviral, or antiparasitic should be given. If stasis dermatitis is responsible for the id reaction, compression stockings and leg elevation are indicated. The id reaction itself is treated with systemic or topical corticosteroids and wet compresses if acute. The goal of these treatments is to reduce patient discomfort caused by the inflammation and pruritus.18,43
Conclusion
Id reactions are an unusual phenomenon that commonly occurs after fungal skin infections and stasis dermatitis. T lymphocytes and keratinocytes may play a key role in this reaction, with newer research further delineating the process and possibly providing enhanced treatment options. Therapy focuses on treating the underlying condition, supplemented with corticosteroids for the autoeczema.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
- Whitfield A. Lumleian Lectures on Some Points in the Aetiology of Skin Diseases. Delivered before the Royal College of Physicians of London on March 10th, 15th, and 17th, 1921. Lecture II. Lancet. 1921;2:122-127.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:E453-E457.
- Schrom KP, Kobs A, Nedorost S. Clinical psoriasiform dermatitis following dupilumab use for autoeczematization secondary to chronic stasis dermatitis. Cureus. 2020;12:e7831. doi:10.7759/cureus.7831
- Templeton HJ, Lunsford CJ, Allington HV. Autosensitization dermatitis; report of five cases and protocol of an experiment. Arch Derm Syphilol. 1949;59:68-77.
- Shelley WB. Id reaction. In: Consultations in Dermatology. Saunders; 1972:262-267.
- Sharquie KE, Noaimi AA, Flayih RA. Clinical and histopathological findings in patients with follicular dermatoses: all skin diseases starts in the hair follicles as new hypothesis. Am J Clin Res Rev. 2020;4:17.
- Kasteler JS, Petersen MJ, Vance JE, et al. Circulating activated T lymphocytes in autoeczematization. Arch Dermatol. 1992;128:795-798.
- González-Amaro R, Baranda L, Abud-Mendoza C, et al. Autoeczematization is associated with abnormal immune recognition of autologous skin antigens. J Am Acad Dermatol. 1993;28:56-60.
- Cunningham MJ, Zone JJ, Petersen MJ, et al. Circulating activated (DR-positive) T lymphocytes in a patient with autoeczematization. J Am Acad Dermatol. 1986;14:1039-1041.
- Furue M, Ulzii D, Vu YH, et al. Pathogenesis of atopic dermatitis: current paradigm. Iran J Immunol. 2019;16:97-107.
- Uchi H, Terao H, Koga T, et al. Cytokines and chemokines in the epidermis. J Dermatol Sci. 2000;24(suppl 1):S29-S38.
- Bos JD, Kapsenberg ML. The skin immune system: progress in cutaneous biology. Immunol Today. 1993;14:75-78.
- Young AW Jr. Dynamics of autosensitization dermatitis; a clinical and microscopic concept of autoeczematization. AMA Arch Derm. 1958;77:495-502.
- Brenner S, Wolf R, Landau M. Scabid: an unusual id reaction to scabies. Int J Dermatol. 1993;32:128-129.
- Yamany T, Schwartz RA. Infectious eczematoid dermatitis: a comprehensive review. J Eur Acad Dermatol Venereol. 2015;29:203-208.
- Wang X, Li L, Shi X, et al. Itching and its related factors in subtypes of eczema: a cross-sectional multicenter study in tertiary hospitals of China. Sci Rep. 2018;8:10754.
- Price A, Tavazoie M, Meehan SA, et al. Id reaction associated with red tattoo ink. Cutis. 2018;102:E32-E34.
- Ilkit M, Durdu M, Karaks¸ M. Cutaneous id reactions: a comprehensive review of clinical manifestations, epidemiology, etiology, and management. Crit Rev Microbiol. 2012;38:191-202.
- Kaner SR. Dermatitis venenata of the feet with a generalized “id” reaction. J Am Podiatry Assoc. 1970;60:199-204.
- Jordan L, Jackson NA, Carter-Snell B, et al. Pustular tinea id reaction. Cutis. 2019;103:E3-E4.
- Crum N, Hardaway C, Graham B. Development of an idlike reaction during treatment for acute pulmonary histoplasmosis: a new cutaneous manifestation in histoplasmosis. J Am Acad Dermatol. 2003;48(2 suppl):S5-S6.
- Chirac A, Brzezinski P, Chiriac AE, et al. Autosensitisation (autoeczematisation) reactions in a case of diaper dermatitis candidiasis. Niger Med J. 2014;55:274-275.
- Singh PY, Sinha P, Baveja S, et al. Immune-mediated tuberculous uveitis—a rare association with papulonecrotic tuberculid. Indian J Ophthalmol. 2019;67:1207-1209.
- Urso B, Georgesen C, Harp J. Papulonecrotic tuberculid secondary to Mycobacterium avium complex. Cutis. 2019;104:E11-E13.
- Choudhri SH, Magro CM, Crowson AN, et al. An id reaction to Mycobacterium leprae: first documented case. Cutis. 1994;54:282-286.
- Park JW, Jeong GJ, Seo SJ, et al. Pseudomonas toe web infection and autosensitisation dermatitis: diagnostic and therapeutic challenge. Int Wound J. 2020;17:1543-1544. doi:10.1111/iwj.13386
- Netchiporouk E, Cohen BA. Recognizing and managing eczematous id reactions to molluscum contagiosum virus in children. Pediatrics. 2012;129:E1072-E1075.
- Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1.
- Rocamora V, Romaní J, Puig L, et al. Id reaction to molluscum contagiosum. Pediatr Dermatol. 1996;13:349-350.
- Yes¸ilova Y, Özbilgin A, Turan E, et al. Clinical exacerbation developing during treatment of cutaneous leishmaniasis: an id reaction? Turkiye Parazitol Derg. 2014;38:281-282.
- Connor CJ, Selby JC, Wanat KA. Severe pediculosis capitus: a case of “crusted lice” with autoeczematization. Dermatol Online J. 2016;22:13030/qt7c91z913.
- Shelley WB. The autoimmune mechanism in clinical dermatology. Arch Dermatol. 1962;86:27-34.
- Bosworth A, Hull PR. Disseminated eczema following radiotherapy: a case report. J Cutan Med Surg. 2018;22:353-355.
- Lowther C, Miedler JD, Cockerell CJ. Id-like reaction to BCG therapy for bladder cancer. Cutis. 2013;91:145-151.
- Huerth KA, Glick PL, Glick ZR. Cutaneous id reaction after using cyanoacrylate for wound closure. Cutis. 2020;105:E11-E13.
- Amini S, Burdick AE, Janniger CK. Dyshidrotic eczema (pompholyx). Updated April 22, 2020. Accessed August 23, 2021. https://emedicine.medscape.com/article/1122527-overview
- Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18:383-390.
- Hughes JDM, Pratt MD. Allergic contact dermatitis and autoeczematization to proctosedyl® cream and proctomyxin® cream. Case Rep Dermatol. 2018;10:238-246.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2019;56:99-109.
- Novak-Bilic´ G, Vucˇic´ M, Japundžic´ I, et al. Irritant and allergic contact dermatitis—skin lesion characteristics. Acta Clin Croat. 2018;57:713-720.
- Nassau S, Fonacier L. Allergic contact dermatitis. Med Clin North Am. 2020;104:61-76.
- Lewis DJ, Schlichte MJ, Dao H Jr. Atypical disseminated herpes zoster: management guidelines in immunocompromised patients. Cutis. 2017;100:321-330.
- Nedorost S, White S, Rowland DY, et al. Development and implementation of an order set to improve value of care for patients with severe stasis dermatitis. J Am Acad Dermatol. 2019;80:815-817.
Practice Points
- Autoeczematization, or id reaction, is a disseminated reaction of the skin occurring at a site distant to a primary cutaneous infection or stimulus.
- T lymphocytes and keratinocytes are postulated to be involved in the pathogenesis of id reactions.
- Therapy includes treating the underlying pathology while providing topical corticosteroids for the autoeczematous lesions.
Anakinra improved survival in hospitalized COVID-19 patients
Hospitalized COVID-19 patients at increased risk for respiratory failure showed significant improvement after treatment with anakinra, compared with placebo, based on data from a phase 3, randomized trial of nearly 600 patients who also received standard of care treatment.
Anakinra, a recombinant interleukin (IL)-1 receptor antagonist that blocks activity for both IL-1 alpha and beta, showed a 70% decrease in the risk of progression to severe respiratory failure in a prior open-label, phase 2, proof-of-concept study, wrote Evdoxia Kyriazopoulou, MD, PhD, of National and Kapodistrian University of Athens, and colleagues.
Previous research has shown that soluble urokinase plasminogen activator receptor (suPAR) serum levels can signal increased risk of progression to severe disease and respiratory failure in COVID-19 patients, they noted.
Supported by these early findings, “the SAVE-MORE study (suPAR-guided anakinra treatment for validation of the risk and early management of severe respiratory failure by COVID-19) is a pivotal, confirmatory, phase 3, double-blind, randomized controlled trial that evaluated the efficacy and safety of early initiation of anakinra treatment in hospitalized patients with moderate or severe COVID-19,” the researchers said.
In the SAVE-MORE study published Sept. 3 in Nature Medicine, the researchers identified 594 adults with COVID-19 who were hospitalized at 37 centers in Greece and Italy and at risk of progressing to respiratory failure based on plasma suPAR levels of at least 6 ng/mL.
The primary objective was to assess the impact of early anakinra treatment on the clinical status of COVID-19 patients at risk for severe disease according to the 11-point, ordinal World Health Organization Clinical Progression Scale (WHO-CPS) at 28 days after starting treatment. All patients received standard of care, which consisted of regular monitoring of physical signs, oximetry, and anticoagulation. Patients with severe disease by the WHO definition were also received 6 mg of dexamethasone intravenously daily for 10 days. A total of 405 were randomized to anakinra and 189 to placebo. Approximately 92% of the study participants had severe pneumonia according to the WHO classification for COVID-19. The average age of the patients was 62 years, 58% were male, and the average body mass index was 29.5 kg/m2.
At 28 days, 204 (50.4%) of the anakinra-treated patients had fully recovered, with no detectable viral RNA, compared with 50 (26.5%) of the placebo-treated patients (P < .0001). In addition, significantly fewer patients in the anakinra group had died by 28 days (13 patients, 3.2%), compared with patients in the placebo group (13 patients, 6.9%).
The median decrease in WHO-CPS scores from baseline to 28 days was 4 points in the anakinra group and 3 points in the placebo group, a statistically significant difference (P < .0001).
“Overall, the unadjusted proportional odds of having a worse score on the 11-point WHO-CPS at day 28 with anakinra was 0.36 versus placebo,” and this number remained the same in adjusted analysis, the researchers wrote.
All five secondary endpoints on the WHO-CPS showed significant benefits of anakinra, compared with placebo. These included an absolute decrease of WHO-CPS at day 28 and day 14 from baseline; an absolute decrease of Sequential Organ Failure Assessment scores at day 7 from baseline; and a significantly shorter mean time to both hospital and ICU discharge (1 day and 4 days, respectively) with anakinra versus placebo.
Follow-up laboratory data showed a significant increase in absolute lymphocyte count at 7 days, a significant decrease in circulating IL-6 levels at 4 and 7 days, and significantly decreased plasma C-reactive protein (CRP) levels at 7 days.
Serious treatment-emergent adverse events were reported in 16% with anakinra and in 21.7% with placebo; the most common of these events were infections (8.4% with anakinra and 15.9% with placebo). The next most common serious treatment-emergent adverse events were ventilator-associated pneumonia, septic shock and multiple organ dysfunction, bloodstream infections, and pulmonary embolism. The most common nonserious treatment-emergent adverse events were an increase of liver function tests and hyperglycemia (similar in anakinra and placebo groups) and nonserious anemia (lower in the anakinra group).
The study findings were limited by several factors, including the lack of patients with critical COVID-19 disease and the challenge of application of suPAR in all hospital settings, the researchers noted. However, “the results validate the findings of the previous SAVE open-label phase 2 trial,” they said. The results suggest “that suPAR should be measured upon admission of all patients with COVID-19 who do not need oxygen or who need nasal or mask oxygen, and that, if suPAR levels are 6 ng/mL or higher, anakinra treatment might be a suitable therapy,” they concluded.
Cytokine storm syndrome remains a treatment challenge
“Many who die from COVID-19 suffer hyperinflammation with features of cytokine storm syndrome (CSS) and associated acute respiratory distress syndrome,” wrote Randy Q. Cron, MD, and W. Winn Chatham, MD, of the University of Alabama at Birmingham, and Roberto Caricchio, MD, of Temple University, Philadelphia, in an accompanying editorial. They noted that the SAVE-MORE trial results contrast with another recent randomized trial of canakinumab, which failed to show notable benefits, compared with placebo, in treating hospitalized patients with COVID-19 pneumonia.
“There are some key differences between these trials, one being that anakinra blocks signaling of both IL-1 alpha and IL-1 beta, whereas canakinumab binds only IL-1 beta,” the editorialists explained. “SARS-CoV-2–infected endothelium may be a particularly important source of IL-1 alpha that is not targeted by canakinumab,” they noted.
Additional studies have examined IL-6 inhibition to treat COVID-19 patients, but data have been inconsistent, the editorialists said.
“One thing that is clearly emerging from this pandemic is that the CSS associated with COVID-19 is relatively unique, with only modestly elevated levels of IL-6, CRP, and ferritin, for example,” they noted. However, the SAVE-MORE study suggests that more targeted approaches, such as anakinra, “may allow earlier introduction of anticytokine treatment” and support the use of IL-1 blockade with anakinra for cases of severe COVID-19 pneumonia.
Predicting risk for severe disease
“One of the major challenges in the management of patients with COVID-19 is identifying patients at risk of severe disease who would warrant early intervention with anti-inflammatory therapy,” said Salim Hayek, MD, medical director of the University of Michigan’s Frankel Cardiovascular Center Clinics, in an interview. “We and others had found that soluble urokinase plasminogen activator receptor (suPAR) levels are the strongest predictor of severe disease amongst biomarkers of inflammation,” he said. “In this study, patients with high suPAR levels derived benefit from anakinra, compared to those with placebo. This study is a great example of how suPAR levels could be used to identify high-risk patients that would benefit from therapies targeting inflammation,” Dr. Hayek emphasized.
“The findings are in line with the hypothesis that patients with the highest degrees of inflammation would benefit the best from targeting the hyperinflammatory cascade using anakinra or other interleukin antagonists,” Dr. Hayek said. “Given suPAR levels are the best predictors of high-risk disease, it is not surprising to see that patients with high levels benefit from targeting inflammation,” he noted.
The take-home message for clinicians at this time is that anakinra effectively improves outcomes in COVID-19 patients with high suPAR levels, Dr. Hayek said. “SuPAR can be measured easily at the point of care. Thus, a targeted strategy using suPAR to identify patients who would benefit from anakinra appears to be viable,” he explained.
However, “Whether anakinra is effective in patients with lower suPAR levels (<6 ng/mL) is unclear and was not answered by this study,” he said. “We eagerly await results of other trials to make that determination. Whether suPAR levels can also help guide the use of other therapies for COVID-19 should be explored and would enhance the personalization of treatment for COVID-19 according to the underlying inflammatory state,” he added.
The SAVE-MORE study was funded by the Hellenic Institute for the Study of Sepsis and Sobi, which manufactures anakinra. Some of the study authors reported financial relationships with Sobi and other pharmaceutical companies.
Dr. Cron disclosed serving as a consultant to Sobi, Novartis, Pfizer, and Sironax. Dr. Cron and Dr. Chatham disclosed having received grant support from Sobi for investigator-initiated clinical trials, and Dr. Caricchio disclosed serving as a consultant to GlaxoSmithKline, Johnson & Johnson, Aurinia, and Bristol-Myers Squibb. Dr. Hayek had no relevant financial conflicts to disclose.
Hospitalized COVID-19 patients at increased risk for respiratory failure showed significant improvement after treatment with anakinra, compared with placebo, based on data from a phase 3, randomized trial of nearly 600 patients who also received standard of care treatment.
Anakinra, a recombinant interleukin (IL)-1 receptor antagonist that blocks activity for both IL-1 alpha and beta, showed a 70% decrease in the risk of progression to severe respiratory failure in a prior open-label, phase 2, proof-of-concept study, wrote Evdoxia Kyriazopoulou, MD, PhD, of National and Kapodistrian University of Athens, and colleagues.
Previous research has shown that soluble urokinase plasminogen activator receptor (suPAR) serum levels can signal increased risk of progression to severe disease and respiratory failure in COVID-19 patients, they noted.
Supported by these early findings, “the SAVE-MORE study (suPAR-guided anakinra treatment for validation of the risk and early management of severe respiratory failure by COVID-19) is a pivotal, confirmatory, phase 3, double-blind, randomized controlled trial that evaluated the efficacy and safety of early initiation of anakinra treatment in hospitalized patients with moderate or severe COVID-19,” the researchers said.
In the SAVE-MORE study published Sept. 3 in Nature Medicine, the researchers identified 594 adults with COVID-19 who were hospitalized at 37 centers in Greece and Italy and at risk of progressing to respiratory failure based on plasma suPAR levels of at least 6 ng/mL.
The primary objective was to assess the impact of early anakinra treatment on the clinical status of COVID-19 patients at risk for severe disease according to the 11-point, ordinal World Health Organization Clinical Progression Scale (WHO-CPS) at 28 days after starting treatment. All patients received standard of care, which consisted of regular monitoring of physical signs, oximetry, and anticoagulation. Patients with severe disease by the WHO definition were also received 6 mg of dexamethasone intravenously daily for 10 days. A total of 405 were randomized to anakinra and 189 to placebo. Approximately 92% of the study participants had severe pneumonia according to the WHO classification for COVID-19. The average age of the patients was 62 years, 58% were male, and the average body mass index was 29.5 kg/m2.
At 28 days, 204 (50.4%) of the anakinra-treated patients had fully recovered, with no detectable viral RNA, compared with 50 (26.5%) of the placebo-treated patients (P < .0001). In addition, significantly fewer patients in the anakinra group had died by 28 days (13 patients, 3.2%), compared with patients in the placebo group (13 patients, 6.9%).
The median decrease in WHO-CPS scores from baseline to 28 days was 4 points in the anakinra group and 3 points in the placebo group, a statistically significant difference (P < .0001).
“Overall, the unadjusted proportional odds of having a worse score on the 11-point WHO-CPS at day 28 with anakinra was 0.36 versus placebo,” and this number remained the same in adjusted analysis, the researchers wrote.
All five secondary endpoints on the WHO-CPS showed significant benefits of anakinra, compared with placebo. These included an absolute decrease of WHO-CPS at day 28 and day 14 from baseline; an absolute decrease of Sequential Organ Failure Assessment scores at day 7 from baseline; and a significantly shorter mean time to both hospital and ICU discharge (1 day and 4 days, respectively) with anakinra versus placebo.
Follow-up laboratory data showed a significant increase in absolute lymphocyte count at 7 days, a significant decrease in circulating IL-6 levels at 4 and 7 days, and significantly decreased plasma C-reactive protein (CRP) levels at 7 days.
Serious treatment-emergent adverse events were reported in 16% with anakinra and in 21.7% with placebo; the most common of these events were infections (8.4% with anakinra and 15.9% with placebo). The next most common serious treatment-emergent adverse events were ventilator-associated pneumonia, septic shock and multiple organ dysfunction, bloodstream infections, and pulmonary embolism. The most common nonserious treatment-emergent adverse events were an increase of liver function tests and hyperglycemia (similar in anakinra and placebo groups) and nonserious anemia (lower in the anakinra group).
The study findings were limited by several factors, including the lack of patients with critical COVID-19 disease and the challenge of application of suPAR in all hospital settings, the researchers noted. However, “the results validate the findings of the previous SAVE open-label phase 2 trial,” they said. The results suggest “that suPAR should be measured upon admission of all patients with COVID-19 who do not need oxygen or who need nasal or mask oxygen, and that, if suPAR levels are 6 ng/mL or higher, anakinra treatment might be a suitable therapy,” they concluded.
Cytokine storm syndrome remains a treatment challenge
“Many who die from COVID-19 suffer hyperinflammation with features of cytokine storm syndrome (CSS) and associated acute respiratory distress syndrome,” wrote Randy Q. Cron, MD, and W. Winn Chatham, MD, of the University of Alabama at Birmingham, and Roberto Caricchio, MD, of Temple University, Philadelphia, in an accompanying editorial. They noted that the SAVE-MORE trial results contrast with another recent randomized trial of canakinumab, which failed to show notable benefits, compared with placebo, in treating hospitalized patients with COVID-19 pneumonia.
“There are some key differences between these trials, one being that anakinra blocks signaling of both IL-1 alpha and IL-1 beta, whereas canakinumab binds only IL-1 beta,” the editorialists explained. “SARS-CoV-2–infected endothelium may be a particularly important source of IL-1 alpha that is not targeted by canakinumab,” they noted.
Additional studies have examined IL-6 inhibition to treat COVID-19 patients, but data have been inconsistent, the editorialists said.
“One thing that is clearly emerging from this pandemic is that the CSS associated with COVID-19 is relatively unique, with only modestly elevated levels of IL-6, CRP, and ferritin, for example,” they noted. However, the SAVE-MORE study suggests that more targeted approaches, such as anakinra, “may allow earlier introduction of anticytokine treatment” and support the use of IL-1 blockade with anakinra for cases of severe COVID-19 pneumonia.
Predicting risk for severe disease
“One of the major challenges in the management of patients with COVID-19 is identifying patients at risk of severe disease who would warrant early intervention with anti-inflammatory therapy,” said Salim Hayek, MD, medical director of the University of Michigan’s Frankel Cardiovascular Center Clinics, in an interview. “We and others had found that soluble urokinase plasminogen activator receptor (suPAR) levels are the strongest predictor of severe disease amongst biomarkers of inflammation,” he said. “In this study, patients with high suPAR levels derived benefit from anakinra, compared to those with placebo. This study is a great example of how suPAR levels could be used to identify high-risk patients that would benefit from therapies targeting inflammation,” Dr. Hayek emphasized.
“The findings are in line with the hypothesis that patients with the highest degrees of inflammation would benefit the best from targeting the hyperinflammatory cascade using anakinra or other interleukin antagonists,” Dr. Hayek said. “Given suPAR levels are the best predictors of high-risk disease, it is not surprising to see that patients with high levels benefit from targeting inflammation,” he noted.
The take-home message for clinicians at this time is that anakinra effectively improves outcomes in COVID-19 patients with high suPAR levels, Dr. Hayek said. “SuPAR can be measured easily at the point of care. Thus, a targeted strategy using suPAR to identify patients who would benefit from anakinra appears to be viable,” he explained.
However, “Whether anakinra is effective in patients with lower suPAR levels (<6 ng/mL) is unclear and was not answered by this study,” he said. “We eagerly await results of other trials to make that determination. Whether suPAR levels can also help guide the use of other therapies for COVID-19 should be explored and would enhance the personalization of treatment for COVID-19 according to the underlying inflammatory state,” he added.
The SAVE-MORE study was funded by the Hellenic Institute for the Study of Sepsis and Sobi, which manufactures anakinra. Some of the study authors reported financial relationships with Sobi and other pharmaceutical companies.
Dr. Cron disclosed serving as a consultant to Sobi, Novartis, Pfizer, and Sironax. Dr. Cron and Dr. Chatham disclosed having received grant support from Sobi for investigator-initiated clinical trials, and Dr. Caricchio disclosed serving as a consultant to GlaxoSmithKline, Johnson & Johnson, Aurinia, and Bristol-Myers Squibb. Dr. Hayek had no relevant financial conflicts to disclose.
Hospitalized COVID-19 patients at increased risk for respiratory failure showed significant improvement after treatment with anakinra, compared with placebo, based on data from a phase 3, randomized trial of nearly 600 patients who also received standard of care treatment.
Anakinra, a recombinant interleukin (IL)-1 receptor antagonist that blocks activity for both IL-1 alpha and beta, showed a 70% decrease in the risk of progression to severe respiratory failure in a prior open-label, phase 2, proof-of-concept study, wrote Evdoxia Kyriazopoulou, MD, PhD, of National and Kapodistrian University of Athens, and colleagues.
Previous research has shown that soluble urokinase plasminogen activator receptor (suPAR) serum levels can signal increased risk of progression to severe disease and respiratory failure in COVID-19 patients, they noted.
Supported by these early findings, “the SAVE-MORE study (suPAR-guided anakinra treatment for validation of the risk and early management of severe respiratory failure by COVID-19) is a pivotal, confirmatory, phase 3, double-blind, randomized controlled trial that evaluated the efficacy and safety of early initiation of anakinra treatment in hospitalized patients with moderate or severe COVID-19,” the researchers said.
In the SAVE-MORE study published Sept. 3 in Nature Medicine, the researchers identified 594 adults with COVID-19 who were hospitalized at 37 centers in Greece and Italy and at risk of progressing to respiratory failure based on plasma suPAR levels of at least 6 ng/mL.
The primary objective was to assess the impact of early anakinra treatment on the clinical status of COVID-19 patients at risk for severe disease according to the 11-point, ordinal World Health Organization Clinical Progression Scale (WHO-CPS) at 28 days after starting treatment. All patients received standard of care, which consisted of regular monitoring of physical signs, oximetry, and anticoagulation. Patients with severe disease by the WHO definition were also received 6 mg of dexamethasone intravenously daily for 10 days. A total of 405 were randomized to anakinra and 189 to placebo. Approximately 92% of the study participants had severe pneumonia according to the WHO classification for COVID-19. The average age of the patients was 62 years, 58% were male, and the average body mass index was 29.5 kg/m2.
At 28 days, 204 (50.4%) of the anakinra-treated patients had fully recovered, with no detectable viral RNA, compared with 50 (26.5%) of the placebo-treated patients (P < .0001). In addition, significantly fewer patients in the anakinra group had died by 28 days (13 patients, 3.2%), compared with patients in the placebo group (13 patients, 6.9%).
The median decrease in WHO-CPS scores from baseline to 28 days was 4 points in the anakinra group and 3 points in the placebo group, a statistically significant difference (P < .0001).
“Overall, the unadjusted proportional odds of having a worse score on the 11-point WHO-CPS at day 28 with anakinra was 0.36 versus placebo,” and this number remained the same in adjusted analysis, the researchers wrote.
All five secondary endpoints on the WHO-CPS showed significant benefits of anakinra, compared with placebo. These included an absolute decrease of WHO-CPS at day 28 and day 14 from baseline; an absolute decrease of Sequential Organ Failure Assessment scores at day 7 from baseline; and a significantly shorter mean time to both hospital and ICU discharge (1 day and 4 days, respectively) with anakinra versus placebo.
Follow-up laboratory data showed a significant increase in absolute lymphocyte count at 7 days, a significant decrease in circulating IL-6 levels at 4 and 7 days, and significantly decreased plasma C-reactive protein (CRP) levels at 7 days.
Serious treatment-emergent adverse events were reported in 16% with anakinra and in 21.7% with placebo; the most common of these events were infections (8.4% with anakinra and 15.9% with placebo). The next most common serious treatment-emergent adverse events were ventilator-associated pneumonia, septic shock and multiple organ dysfunction, bloodstream infections, and pulmonary embolism. The most common nonserious treatment-emergent adverse events were an increase of liver function tests and hyperglycemia (similar in anakinra and placebo groups) and nonserious anemia (lower in the anakinra group).
The study findings were limited by several factors, including the lack of patients with critical COVID-19 disease and the challenge of application of suPAR in all hospital settings, the researchers noted. However, “the results validate the findings of the previous SAVE open-label phase 2 trial,” they said. The results suggest “that suPAR should be measured upon admission of all patients with COVID-19 who do not need oxygen or who need nasal or mask oxygen, and that, if suPAR levels are 6 ng/mL or higher, anakinra treatment might be a suitable therapy,” they concluded.
Cytokine storm syndrome remains a treatment challenge
“Many who die from COVID-19 suffer hyperinflammation with features of cytokine storm syndrome (CSS) and associated acute respiratory distress syndrome,” wrote Randy Q. Cron, MD, and W. Winn Chatham, MD, of the University of Alabama at Birmingham, and Roberto Caricchio, MD, of Temple University, Philadelphia, in an accompanying editorial. They noted that the SAVE-MORE trial results contrast with another recent randomized trial of canakinumab, which failed to show notable benefits, compared with placebo, in treating hospitalized patients with COVID-19 pneumonia.
“There are some key differences between these trials, one being that anakinra blocks signaling of both IL-1 alpha and IL-1 beta, whereas canakinumab binds only IL-1 beta,” the editorialists explained. “SARS-CoV-2–infected endothelium may be a particularly important source of IL-1 alpha that is not targeted by canakinumab,” they noted.
Additional studies have examined IL-6 inhibition to treat COVID-19 patients, but data have been inconsistent, the editorialists said.
“One thing that is clearly emerging from this pandemic is that the CSS associated with COVID-19 is relatively unique, with only modestly elevated levels of IL-6, CRP, and ferritin, for example,” they noted. However, the SAVE-MORE study suggests that more targeted approaches, such as anakinra, “may allow earlier introduction of anticytokine treatment” and support the use of IL-1 blockade with anakinra for cases of severe COVID-19 pneumonia.
Predicting risk for severe disease
“One of the major challenges in the management of patients with COVID-19 is identifying patients at risk of severe disease who would warrant early intervention with anti-inflammatory therapy,” said Salim Hayek, MD, medical director of the University of Michigan’s Frankel Cardiovascular Center Clinics, in an interview. “We and others had found that soluble urokinase plasminogen activator receptor (suPAR) levels are the strongest predictor of severe disease amongst biomarkers of inflammation,” he said. “In this study, patients with high suPAR levels derived benefit from anakinra, compared to those with placebo. This study is a great example of how suPAR levels could be used to identify high-risk patients that would benefit from therapies targeting inflammation,” Dr. Hayek emphasized.
“The findings are in line with the hypothesis that patients with the highest degrees of inflammation would benefit the best from targeting the hyperinflammatory cascade using anakinra or other interleukin antagonists,” Dr. Hayek said. “Given suPAR levels are the best predictors of high-risk disease, it is not surprising to see that patients with high levels benefit from targeting inflammation,” he noted.
The take-home message for clinicians at this time is that anakinra effectively improves outcomes in COVID-19 patients with high suPAR levels, Dr. Hayek said. “SuPAR can be measured easily at the point of care. Thus, a targeted strategy using suPAR to identify patients who would benefit from anakinra appears to be viable,” he explained.
However, “Whether anakinra is effective in patients with lower suPAR levels (<6 ng/mL) is unclear and was not answered by this study,” he said. “We eagerly await results of other trials to make that determination. Whether suPAR levels can also help guide the use of other therapies for COVID-19 should be explored and would enhance the personalization of treatment for COVID-19 according to the underlying inflammatory state,” he added.
The SAVE-MORE study was funded by the Hellenic Institute for the Study of Sepsis and Sobi, which manufactures anakinra. Some of the study authors reported financial relationships with Sobi and other pharmaceutical companies.
Dr. Cron disclosed serving as a consultant to Sobi, Novartis, Pfizer, and Sironax. Dr. Cron and Dr. Chatham disclosed having received grant support from Sobi for investigator-initiated clinical trials, and Dr. Caricchio disclosed serving as a consultant to GlaxoSmithKline, Johnson & Johnson, Aurinia, and Bristol-Myers Squibb. Dr. Hayek had no relevant financial conflicts to disclose.
FROM NATURE MEDICINE