User login
Vetiver: More than a pleasant aroma?
An important ingredient in the contemporary perfume and cosmetics industries, vetiver, is the only grass cultivated throughout the world to retain its essential oil, which contains sesquiterpene alcohols and hydrocarbons.1-3 Field and glasshouse studies have revealed that vetiver grass can tolerate extreme variations in climate well, including protracted drought, floods, submergence, temperature, and soils high in acidity, alkalinity, and various heavy metals. Its heartiness may explain its continuing or even increasing use in fragrances and other products pertinent to skin health as humanity strives to adapt to climate change.4 In a 2017 review of various commercial essential oils as antimicrobial therapy for cutaneous disorders, Orchard and van Vuuren identified vetiver as warranting particular attention for its capacity to confer broad benefits to the skin in addressing acne, cuts, eczema, oiliness, sores, wounds, and aging skin.5 The focus of this column will be the dermatologic potential of vetiver.
Chemical constituents
Vetiver is thought to be one of the most complex of the essential oils owing to the hundreds of sesquiterpene derivatives with large structural diversity that contribute to its composition. 3
In a 2012 analysis of the components of South Indian vetiver oils, Mallavarapu et al. found an abundance of sesquiterpenes and oxygenated sesquiterpenes with cedrane, bisabolane, eudesmane, eremophilane, and zizaane skeletons. The primary constituents identified in the four oils evaluated included eudesma-4,6-diene (delta-selinene) + beta-vetispirene (3.9%-6.1%), beta-vetivenene (0.9%-9.4%), 13-nor-trans-eudesma-4(15),7-dien-11-one + amorph-4-en-10-ol (5.0%-6.4%), trans-eudesma-4(15),7-dien-12-ol (vetiselinenol) + (E)-opposita-4(15),7(11)-dien-12-ol (3.7%-5.9%), eremophila-1 (10),11-dien-2alpha-ol (nootkatol) + ziza-6(13)-en-12-ol (khusimol) (16.1%-19.2%), and eremophila-1(10),7(11)-dien-2alpha-ol (isonootkatol) + (E)-eremophila-1(10),7(11)-12-ol (isovalencenol) (5.6%-6.9%).6
Antimicrobial activity
In 2012, Saikia et al. assessed the antimycobacterial activity of Vetiveria zizanioides against Mycobacterium tuberculosis H(37)Rv and H(37)Ra strains. Their results showed that ethanolic extracts and hexane fractions displayed robust antimycobacterial properties, buttressing the traditional medical uses of the plant, as well as consideration of this agent as a modern antituberculosis agent.7
Two years later, Dos Santos et al. showed that Vetiveria zizanioides roots grown in Brazil exhibited notable antimicrobial effects against various pathogenic organisms.8In 2017, Burger et al. showed that vetiver essential oil primarily contributes its scent to cosmetic formulations but also displayed antimicrobial activity against Gram-positive bacterial strains, as well as one strain of Candida glabrata. They suggest that vetiver should be considered for its antimicrobial capacity as an added bonus to cosmetic formulations.2
In a 2018 study to ascertain the antimicrobial activity of 247 essential oil combinations against five reference strains of wound pathogens, Orchard et al. found that 26 combinations exhibited extensive antimicrobial activity. Sandalwood and vetiver were found to contribute most to antimicrobial function when used in combination. The investigators concluded that such combinations warrant consideration for wound therapy.9
Antiacne activity
In 2018, Orchard et al. conducted another study of the efficacy of commercial essential oil combinations against the two pathogens responsible for acne, Propionibacterium acnes and Staphlyococcus epidermidis. They investigated 408 combinations, of which 167 exhibited notable antimicrobial activity. They observed that the combination with the lowest minimum inhibitory concentration value against P. acnes and S. epidermidis was vetiver and cinnamon bark.10 This usage points to the potential of vetiver use as an antiacne ingredient.
Safety
The Scientific Committee on Consumer Safety (SCCS) offered a final opinion on the safety of the fragrance ingredient acetylated vetiver oil in 2019, declaring its use with 1% alpha-tocopherol in cosmetic leave-on and rinse-off products safe at proposed concentration levels. They noted that acetylated vetiver oil has been used for several years without provoking contact allergies.11
Conclusion
Much more research is necessary to determine just what kind of a role this perfumery powerhouse can play in dermatology.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Del Giudice L et al. Environ Microbiol. 2008 Oct;10(10):2824-41.
2. Burger P et al. Medicines (Basel). 2017 Jun 16;4(2):41.
3. Belhassen E et al. Chem Biodivers. 2014 Nov;11(11):1821–42.
4. Danh LT et al. Int J Phytoremediation. 2009 Oct-Dec;11(8):664–91.
5. Orchard A and van Vuuren S. Evid Based Complement Alternat Med. 2017;2017:4517971.
6. Mallavarapu GR et al. Nat Prod Commun. 2012 Feb;7(2):223–5.
7. Saikia D et al. Complement Ther Med. 2012 Dec;20(6):434–6.
8. Dos Santos DS et al. Acta Pharm. 2014 Dec;64(4):495-501.
9. Orchard A et al. Chem Biodivers. 2018 Dec;15(12):e1800405.
10. Orchard A et al. Int J Cosmet Sci. 2018 Mar 24. [Epub ahead of print].
11. SCCS members & External experts. Regul Toxicol Pharmacol. 2019 Oct;107:104389.
An important ingredient in the contemporary perfume and cosmetics industries, vetiver, is the only grass cultivated throughout the world to retain its essential oil, which contains sesquiterpene alcohols and hydrocarbons.1-3 Field and glasshouse studies have revealed that vetiver grass can tolerate extreme variations in climate well, including protracted drought, floods, submergence, temperature, and soils high in acidity, alkalinity, and various heavy metals. Its heartiness may explain its continuing or even increasing use in fragrances and other products pertinent to skin health as humanity strives to adapt to climate change.4 In a 2017 review of various commercial essential oils as antimicrobial therapy for cutaneous disorders, Orchard and van Vuuren identified vetiver as warranting particular attention for its capacity to confer broad benefits to the skin in addressing acne, cuts, eczema, oiliness, sores, wounds, and aging skin.5 The focus of this column will be the dermatologic potential of vetiver.
Chemical constituents
Vetiver is thought to be one of the most complex of the essential oils owing to the hundreds of sesquiterpene derivatives with large structural diversity that contribute to its composition. 3
In a 2012 analysis of the components of South Indian vetiver oils, Mallavarapu et al. found an abundance of sesquiterpenes and oxygenated sesquiterpenes with cedrane, bisabolane, eudesmane, eremophilane, and zizaane skeletons. The primary constituents identified in the four oils evaluated included eudesma-4,6-diene (delta-selinene) + beta-vetispirene (3.9%-6.1%), beta-vetivenene (0.9%-9.4%), 13-nor-trans-eudesma-4(15),7-dien-11-one + amorph-4-en-10-ol (5.0%-6.4%), trans-eudesma-4(15),7-dien-12-ol (vetiselinenol) + (E)-opposita-4(15),7(11)-dien-12-ol (3.7%-5.9%), eremophila-1 (10),11-dien-2alpha-ol (nootkatol) + ziza-6(13)-en-12-ol (khusimol) (16.1%-19.2%), and eremophila-1(10),7(11)-dien-2alpha-ol (isonootkatol) + (E)-eremophila-1(10),7(11)-12-ol (isovalencenol) (5.6%-6.9%).6
Antimicrobial activity
In 2012, Saikia et al. assessed the antimycobacterial activity of Vetiveria zizanioides against Mycobacterium tuberculosis H(37)Rv and H(37)Ra strains. Their results showed that ethanolic extracts and hexane fractions displayed robust antimycobacterial properties, buttressing the traditional medical uses of the plant, as well as consideration of this agent as a modern antituberculosis agent.7
Two years later, Dos Santos et al. showed that Vetiveria zizanioides roots grown in Brazil exhibited notable antimicrobial effects against various pathogenic organisms.8In 2017, Burger et al. showed that vetiver essential oil primarily contributes its scent to cosmetic formulations but also displayed antimicrobial activity against Gram-positive bacterial strains, as well as one strain of Candida glabrata. They suggest that vetiver should be considered for its antimicrobial capacity as an added bonus to cosmetic formulations.2
In a 2018 study to ascertain the antimicrobial activity of 247 essential oil combinations against five reference strains of wound pathogens, Orchard et al. found that 26 combinations exhibited extensive antimicrobial activity. Sandalwood and vetiver were found to contribute most to antimicrobial function when used in combination. The investigators concluded that such combinations warrant consideration for wound therapy.9
Antiacne activity
In 2018, Orchard et al. conducted another study of the efficacy of commercial essential oil combinations against the two pathogens responsible for acne, Propionibacterium acnes and Staphlyococcus epidermidis. They investigated 408 combinations, of which 167 exhibited notable antimicrobial activity. They observed that the combination with the lowest minimum inhibitory concentration value against P. acnes and S. epidermidis was vetiver and cinnamon bark.10 This usage points to the potential of vetiver use as an antiacne ingredient.
Safety
The Scientific Committee on Consumer Safety (SCCS) offered a final opinion on the safety of the fragrance ingredient acetylated vetiver oil in 2019, declaring its use with 1% alpha-tocopherol in cosmetic leave-on and rinse-off products safe at proposed concentration levels. They noted that acetylated vetiver oil has been used for several years without provoking contact allergies.11
Conclusion
Much more research is necessary to determine just what kind of a role this perfumery powerhouse can play in dermatology.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Del Giudice L et al. Environ Microbiol. 2008 Oct;10(10):2824-41.
2. Burger P et al. Medicines (Basel). 2017 Jun 16;4(2):41.
3. Belhassen E et al. Chem Biodivers. 2014 Nov;11(11):1821–42.
4. Danh LT et al. Int J Phytoremediation. 2009 Oct-Dec;11(8):664–91.
5. Orchard A and van Vuuren S. Evid Based Complement Alternat Med. 2017;2017:4517971.
6. Mallavarapu GR et al. Nat Prod Commun. 2012 Feb;7(2):223–5.
7. Saikia D et al. Complement Ther Med. 2012 Dec;20(6):434–6.
8. Dos Santos DS et al. Acta Pharm. 2014 Dec;64(4):495-501.
9. Orchard A et al. Chem Biodivers. 2018 Dec;15(12):e1800405.
10. Orchard A et al. Int J Cosmet Sci. 2018 Mar 24. [Epub ahead of print].
11. SCCS members & External experts. Regul Toxicol Pharmacol. 2019 Oct;107:104389.
An important ingredient in the contemporary perfume and cosmetics industries, vetiver, is the only grass cultivated throughout the world to retain its essential oil, which contains sesquiterpene alcohols and hydrocarbons.1-3 Field and glasshouse studies have revealed that vetiver grass can tolerate extreme variations in climate well, including protracted drought, floods, submergence, temperature, and soils high in acidity, alkalinity, and various heavy metals. Its heartiness may explain its continuing or even increasing use in fragrances and other products pertinent to skin health as humanity strives to adapt to climate change.4 In a 2017 review of various commercial essential oils as antimicrobial therapy for cutaneous disorders, Orchard and van Vuuren identified vetiver as warranting particular attention for its capacity to confer broad benefits to the skin in addressing acne, cuts, eczema, oiliness, sores, wounds, and aging skin.5 The focus of this column will be the dermatologic potential of vetiver.
Chemical constituents
Vetiver is thought to be one of the most complex of the essential oils owing to the hundreds of sesquiterpene derivatives with large structural diversity that contribute to its composition. 3
In a 2012 analysis of the components of South Indian vetiver oils, Mallavarapu et al. found an abundance of sesquiterpenes and oxygenated sesquiterpenes with cedrane, bisabolane, eudesmane, eremophilane, and zizaane skeletons. The primary constituents identified in the four oils evaluated included eudesma-4,6-diene (delta-selinene) + beta-vetispirene (3.9%-6.1%), beta-vetivenene (0.9%-9.4%), 13-nor-trans-eudesma-4(15),7-dien-11-one + amorph-4-en-10-ol (5.0%-6.4%), trans-eudesma-4(15),7-dien-12-ol (vetiselinenol) + (E)-opposita-4(15),7(11)-dien-12-ol (3.7%-5.9%), eremophila-1 (10),11-dien-2alpha-ol (nootkatol) + ziza-6(13)-en-12-ol (khusimol) (16.1%-19.2%), and eremophila-1(10),7(11)-dien-2alpha-ol (isonootkatol) + (E)-eremophila-1(10),7(11)-12-ol (isovalencenol) (5.6%-6.9%).6
Antimicrobial activity
In 2012, Saikia et al. assessed the antimycobacterial activity of Vetiveria zizanioides against Mycobacterium tuberculosis H(37)Rv and H(37)Ra strains. Their results showed that ethanolic extracts and hexane fractions displayed robust antimycobacterial properties, buttressing the traditional medical uses of the plant, as well as consideration of this agent as a modern antituberculosis agent.7
Two years later, Dos Santos et al. showed that Vetiveria zizanioides roots grown in Brazil exhibited notable antimicrobial effects against various pathogenic organisms.8In 2017, Burger et al. showed that vetiver essential oil primarily contributes its scent to cosmetic formulations but also displayed antimicrobial activity against Gram-positive bacterial strains, as well as one strain of Candida glabrata. They suggest that vetiver should be considered for its antimicrobial capacity as an added bonus to cosmetic formulations.2
In a 2018 study to ascertain the antimicrobial activity of 247 essential oil combinations against five reference strains of wound pathogens, Orchard et al. found that 26 combinations exhibited extensive antimicrobial activity. Sandalwood and vetiver were found to contribute most to antimicrobial function when used in combination. The investigators concluded that such combinations warrant consideration for wound therapy.9
Antiacne activity
In 2018, Orchard et al. conducted another study of the efficacy of commercial essential oil combinations against the two pathogens responsible for acne, Propionibacterium acnes and Staphlyococcus epidermidis. They investigated 408 combinations, of which 167 exhibited notable antimicrobial activity. They observed that the combination with the lowest minimum inhibitory concentration value against P. acnes and S. epidermidis was vetiver and cinnamon bark.10 This usage points to the potential of vetiver use as an antiacne ingredient.
Safety
The Scientific Committee on Consumer Safety (SCCS) offered a final opinion on the safety of the fragrance ingredient acetylated vetiver oil in 2019, declaring its use with 1% alpha-tocopherol in cosmetic leave-on and rinse-off products safe at proposed concentration levels. They noted that acetylated vetiver oil has been used for several years without provoking contact allergies.11
Conclusion
Much more research is necessary to determine just what kind of a role this perfumery powerhouse can play in dermatology.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Del Giudice L et al. Environ Microbiol. 2008 Oct;10(10):2824-41.
2. Burger P et al. Medicines (Basel). 2017 Jun 16;4(2):41.
3. Belhassen E et al. Chem Biodivers. 2014 Nov;11(11):1821–42.
4. Danh LT et al. Int J Phytoremediation. 2009 Oct-Dec;11(8):664–91.
5. Orchard A and van Vuuren S. Evid Based Complement Alternat Med. 2017;2017:4517971.
6. Mallavarapu GR et al. Nat Prod Commun. 2012 Feb;7(2):223–5.
7. Saikia D et al. Complement Ther Med. 2012 Dec;20(6):434–6.
8. Dos Santos DS et al. Acta Pharm. 2014 Dec;64(4):495-501.
9. Orchard A et al. Chem Biodivers. 2018 Dec;15(12):e1800405.
10. Orchard A et al. Int J Cosmet Sci. 2018 Mar 24. [Epub ahead of print].
11. SCCS members & External experts. Regul Toxicol Pharmacol. 2019 Oct;107:104389.
Pandemic goal deficiency disorder
In August I shared with you my observations on two opposing op-ed pieces from two major newspapers, one was in favor of masking mandates for public schools, the other against. (Masking in school: A battle of the op-eds. MDedge Pediatrics. Letters from Maine, 2021 Aug 12). Neither group of authors could offer us evidence from controlled studies to support their views. However, both agreed that returning children to school deserves a high priority. But neither the authors nor I treaded into the uncharted waters of exactly how masking fits into our national goals for managing the pandemic because ... no one in this country has articulated what these goals should be. A third op-ed appearing 3 weeks later suggests why we are floundering in this goal-deficient limbo.
Writing in the New York Times, two epidemiologists in Boston ask the simple question: “What are we actually trying to achieve in the United States?” when it comes to the pandemic. (Allen AG and Jenkins H. The Hard Covid-19 Questions We’re Not Asking. 2021 Aug 30). Is our goal zero infections? Is it hammering on the virus until we can treat it like the seasonal flu? We do seem to agree that not having kids in school has been a disaster economically, educationally, and psychologically. But, where does the goal of getting them back in school fit into a larger and as yet undefined national goal? Without that target we have little idea of what compromises and risks we should be willing to accept.
How much serious pediatric disease is acceptable? It appears that the number of fatal complications in the pediatric population is very small in comparison with other demographic groups. Although few in number, there have been and there will continue to be pediatric deaths because of COVID. Is our goal zero pediatric deaths? If it is then this dictates a level of response that ripples back upstream to every child in every classroom and could threaten our overarching goal of returning children to school. Because none of us likes the thought of a child dying, some of us may be hesitant to even consider a strategy that doesn’t include zero pediatric deaths as a goal.
Are we looking to have zero serious pediatric infections? Achieving this goal is unlikely. Even if we develop a pediatric vaccine in the near future it probably won’t be in the arms of enough children by the end of this school year to make a significant dent in the number of serious pediatric infections. Waiting until an optimal number of children are immunized doesn’t feel like it will achieve a primary goal of getting kids back in school if we continue to focus on driving the level of serious pediatric infections to zero. We have already endured a year in which many communities made decisions that seemed to have prioritized an unstated goal of no school exposure–related educator deaths. Again, a goal based on little if any evidence.
The problem we face in this country is that our response to the pandemic has been nonuniform. Here in Brunswick, Maine, 99% of the eligible adults have been vaccinated. Even with the recent surge, we may be ready for a strategy that avoids wholesale quarantining. A targeted and robust antibody testing system might work for us and make an unproven and unpopular masking mandate unnecessary. Britain seems to be moving in a similar direction to meet its goal of keeping children in school.
However, there are large population groups in regions of this country that have stumbled at taking the initial steps to get the pandemic under control. Articulating a national goal that covers both communities where the response to the pandemic has been less thoughtful and robust along with states that have been more successful is going to be difficult. But it must be done.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In August I shared with you my observations on two opposing op-ed pieces from two major newspapers, one was in favor of masking mandates for public schools, the other against. (Masking in school: A battle of the op-eds. MDedge Pediatrics. Letters from Maine, 2021 Aug 12). Neither group of authors could offer us evidence from controlled studies to support their views. However, both agreed that returning children to school deserves a high priority. But neither the authors nor I treaded into the uncharted waters of exactly how masking fits into our national goals for managing the pandemic because ... no one in this country has articulated what these goals should be. A third op-ed appearing 3 weeks later suggests why we are floundering in this goal-deficient limbo.
Writing in the New York Times, two epidemiologists in Boston ask the simple question: “What are we actually trying to achieve in the United States?” when it comes to the pandemic. (Allen AG and Jenkins H. The Hard Covid-19 Questions We’re Not Asking. 2021 Aug 30). Is our goal zero infections? Is it hammering on the virus until we can treat it like the seasonal flu? We do seem to agree that not having kids in school has been a disaster economically, educationally, and psychologically. But, where does the goal of getting them back in school fit into a larger and as yet undefined national goal? Without that target we have little idea of what compromises and risks we should be willing to accept.
How much serious pediatric disease is acceptable? It appears that the number of fatal complications in the pediatric population is very small in comparison with other demographic groups. Although few in number, there have been and there will continue to be pediatric deaths because of COVID. Is our goal zero pediatric deaths? If it is then this dictates a level of response that ripples back upstream to every child in every classroom and could threaten our overarching goal of returning children to school. Because none of us likes the thought of a child dying, some of us may be hesitant to even consider a strategy that doesn’t include zero pediatric deaths as a goal.
Are we looking to have zero serious pediatric infections? Achieving this goal is unlikely. Even if we develop a pediatric vaccine in the near future it probably won’t be in the arms of enough children by the end of this school year to make a significant dent in the number of serious pediatric infections. Waiting until an optimal number of children are immunized doesn’t feel like it will achieve a primary goal of getting kids back in school if we continue to focus on driving the level of serious pediatric infections to zero. We have already endured a year in which many communities made decisions that seemed to have prioritized an unstated goal of no school exposure–related educator deaths. Again, a goal based on little if any evidence.
The problem we face in this country is that our response to the pandemic has been nonuniform. Here in Brunswick, Maine, 99% of the eligible adults have been vaccinated. Even with the recent surge, we may be ready for a strategy that avoids wholesale quarantining. A targeted and robust antibody testing system might work for us and make an unproven and unpopular masking mandate unnecessary. Britain seems to be moving in a similar direction to meet its goal of keeping children in school.
However, there are large population groups in regions of this country that have stumbled at taking the initial steps to get the pandemic under control. Articulating a national goal that covers both communities where the response to the pandemic has been less thoughtful and robust along with states that have been more successful is going to be difficult. But it must be done.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
In August I shared with you my observations on two opposing op-ed pieces from two major newspapers, one was in favor of masking mandates for public schools, the other against. (Masking in school: A battle of the op-eds. MDedge Pediatrics. Letters from Maine, 2021 Aug 12). Neither group of authors could offer us evidence from controlled studies to support their views. However, both agreed that returning children to school deserves a high priority. But neither the authors nor I treaded into the uncharted waters of exactly how masking fits into our national goals for managing the pandemic because ... no one in this country has articulated what these goals should be. A third op-ed appearing 3 weeks later suggests why we are floundering in this goal-deficient limbo.
Writing in the New York Times, two epidemiologists in Boston ask the simple question: “What are we actually trying to achieve in the United States?” when it comes to the pandemic. (Allen AG and Jenkins H. The Hard Covid-19 Questions We’re Not Asking. 2021 Aug 30). Is our goal zero infections? Is it hammering on the virus until we can treat it like the seasonal flu? We do seem to agree that not having kids in school has been a disaster economically, educationally, and psychologically. But, where does the goal of getting them back in school fit into a larger and as yet undefined national goal? Without that target we have little idea of what compromises and risks we should be willing to accept.
How much serious pediatric disease is acceptable? It appears that the number of fatal complications in the pediatric population is very small in comparison with other demographic groups. Although few in number, there have been and there will continue to be pediatric deaths because of COVID. Is our goal zero pediatric deaths? If it is then this dictates a level of response that ripples back upstream to every child in every classroom and could threaten our overarching goal of returning children to school. Because none of us likes the thought of a child dying, some of us may be hesitant to even consider a strategy that doesn’t include zero pediatric deaths as a goal.
Are we looking to have zero serious pediatric infections? Achieving this goal is unlikely. Even if we develop a pediatric vaccine in the near future it probably won’t be in the arms of enough children by the end of this school year to make a significant dent in the number of serious pediatric infections. Waiting until an optimal number of children are immunized doesn’t feel like it will achieve a primary goal of getting kids back in school if we continue to focus on driving the level of serious pediatric infections to zero. We have already endured a year in which many communities made decisions that seemed to have prioritized an unstated goal of no school exposure–related educator deaths. Again, a goal based on little if any evidence.
The problem we face in this country is that our response to the pandemic has been nonuniform. Here in Brunswick, Maine, 99% of the eligible adults have been vaccinated. Even with the recent surge, we may be ready for a strategy that avoids wholesale quarantining. A targeted and robust antibody testing system might work for us and make an unproven and unpopular masking mandate unnecessary. Britain seems to be moving in a similar direction to meet its goal of keeping children in school.
However, there are large population groups in regions of this country that have stumbled at taking the initial steps to get the pandemic under control. Articulating a national goal that covers both communities where the response to the pandemic has been less thoughtful and robust along with states that have been more successful is going to be difficult. But it must be done.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
HPV infection during pregnancy ups risk of premature birth
Persistent human papillomavirus (HPV) 16 and HPV 18 during a pregnancy may be associated with an increased risk of premature birth.
Findings published online in JAMA Network Open found that 15.9% of individuals who had a persistent HPV 16 or 18 infection during the first and third trimesters of their pregnancy gave birth prematurely, compared with 5.6% of those who did not have an HPV infection at all.
The findings prompted the question of “the pathophysiology of HPV in pregnancy and how the virus is affecting the placenta,” said Lisette Davidson Tanner, MD, MPH, FACOG, who was not involved in the study.
Researchers said the findings are the first to show the association between preterm birth and HPV, which is an incurable virus that most sexually active individuals will get at some point in their lives, according to the Centers for Disease Control and Prevention.
“The results of this study are very important in helping us understand the burden caused by HPV in pregnancy,” study author Helen Trottier, MSc, PhD, researcher at the Centre Hospitalier Universitaire Sainte-Justine, said in an interview. “We may have just pinpointed an important cause of preterm birth that has so far been unexplained.”
Dr. Trottier and colleagues examined data from 1,052 pregnant women from three university-affiliated health care centers in Montreal between Nov. 8, 2010, and Oct. 16, 2016.
Only 5.6% of those who did not have an HPV infection had a premature birth, compared with 6.9% of those who tested positive for any HPV infection in the first trimester.
When looking at the first trimester, researchers found 12% of those diagnosed with HPV 16 and 18 had a preterm birth, compared to 4.9% of those who had a high-risk HPV infection other than HPV 16/18. When looking at the third trimester, researchers found that 15.9% of those with HPV 16/18 had an increased risk of giving birth prematurely, compared to those who had other high-risk HPV infections.
When researchers looked at the persistence of these HPV infections, they found that most HPV infections detected in the first trimester persist to the third trimester. The findings also revealed that persistent vaginal HPV 16/18 detection was significantly associated with all preterm births and spontaneous preterm births. This association was also found among those who had HPV infections detected in their placentas.
Meanwhile, 5.8% of those who had an HPV infection only during the first trimester experienced a preterm birth.
The researchers also found that HPV infections were frequent in pregnancy even among populations “considered to be at low risk based on sociodemographic and sexual history characteristics,” they wrote. Dr. Trottier said she hopes the findings will strengthen support for HPV vaccination.
Dr. Trottier’s study adds to a growing body of research regarding the adverse effects of HPV, according to Dr. Tanner, assistant professor of gynecology and obstetrics at Emory University, Atlanta. “It is already well known that HPV is associated with a number of anogenital and oropharyngeal cancers,” Dr. Tanner said in an interview. “The potential association with preterm birth only adds weight to the recommendations to screen for and prevent HPV infection.”
HPV 16 and 18 are high-risk types that cause about 70% of cervical cancers and precancerous cervical lesions, according to the World Health Organization. However, there are three HPV vaccines – 9-valent HPV vaccine (Gardasil), quadrivalent HPV vaccine (Gardasil®, 4vHPV), and bivalent HPV vaccine (Cervarix) – that help protect against HPV 16/18.
The findings strengthen the benefits of HPV vaccination, Dr. Trottier explained. “There is no cure when the HPV infection is present,” Dr. Trottier said. “If the link [between preterm birth and HPV infections] is indeed causal, we can expect a greater risk of preterm delivery in these women. The effective tool we have is the HPV vaccination, but it should ideally be received before the start of sexual activity in order to prevent future infections that could occur in women.”
The American College of Obstetricians and Gynecologists recommends HPV vaccination for girls and women between the ages of 11 and 26 years old. However, Dr. Tanner said, women aged 27-45 who were previously unvaccinated may still receive benefit from the vaccine.
“Despite the known efficacy of the vaccine, only 50% of patients are up to date with their HPV vaccination,” Dr. Tanner explained. “This study further highlights the need to educate and encourage patients to be vaccinated.”
The researchers said future studies should investigate the association of HPV vaccination and vaccination programs with the risk of preterm birth.
The experts disclosed no conflicts of interest.
Persistent human papillomavirus (HPV) 16 and HPV 18 during a pregnancy may be associated with an increased risk of premature birth.
Findings published online in JAMA Network Open found that 15.9% of individuals who had a persistent HPV 16 or 18 infection during the first and third trimesters of their pregnancy gave birth prematurely, compared with 5.6% of those who did not have an HPV infection at all.
The findings prompted the question of “the pathophysiology of HPV in pregnancy and how the virus is affecting the placenta,” said Lisette Davidson Tanner, MD, MPH, FACOG, who was not involved in the study.
Researchers said the findings are the first to show the association between preterm birth and HPV, which is an incurable virus that most sexually active individuals will get at some point in their lives, according to the Centers for Disease Control and Prevention.
“The results of this study are very important in helping us understand the burden caused by HPV in pregnancy,” study author Helen Trottier, MSc, PhD, researcher at the Centre Hospitalier Universitaire Sainte-Justine, said in an interview. “We may have just pinpointed an important cause of preterm birth that has so far been unexplained.”
Dr. Trottier and colleagues examined data from 1,052 pregnant women from three university-affiliated health care centers in Montreal between Nov. 8, 2010, and Oct. 16, 2016.
Only 5.6% of those who did not have an HPV infection had a premature birth, compared with 6.9% of those who tested positive for any HPV infection in the first trimester.
When looking at the first trimester, researchers found 12% of those diagnosed with HPV 16 and 18 had a preterm birth, compared to 4.9% of those who had a high-risk HPV infection other than HPV 16/18. When looking at the third trimester, researchers found that 15.9% of those with HPV 16/18 had an increased risk of giving birth prematurely, compared to those who had other high-risk HPV infections.
When researchers looked at the persistence of these HPV infections, they found that most HPV infections detected in the first trimester persist to the third trimester. The findings also revealed that persistent vaginal HPV 16/18 detection was significantly associated with all preterm births and spontaneous preterm births. This association was also found among those who had HPV infections detected in their placentas.
Meanwhile, 5.8% of those who had an HPV infection only during the first trimester experienced a preterm birth.
The researchers also found that HPV infections were frequent in pregnancy even among populations “considered to be at low risk based on sociodemographic and sexual history characteristics,” they wrote. Dr. Trottier said she hopes the findings will strengthen support for HPV vaccination.
Dr. Trottier’s study adds to a growing body of research regarding the adverse effects of HPV, according to Dr. Tanner, assistant professor of gynecology and obstetrics at Emory University, Atlanta. “It is already well known that HPV is associated with a number of anogenital and oropharyngeal cancers,” Dr. Tanner said in an interview. “The potential association with preterm birth only adds weight to the recommendations to screen for and prevent HPV infection.”
HPV 16 and 18 are high-risk types that cause about 70% of cervical cancers and precancerous cervical lesions, according to the World Health Organization. However, there are three HPV vaccines – 9-valent HPV vaccine (Gardasil), quadrivalent HPV vaccine (Gardasil®, 4vHPV), and bivalent HPV vaccine (Cervarix) – that help protect against HPV 16/18.
The findings strengthen the benefits of HPV vaccination, Dr. Trottier explained. “There is no cure when the HPV infection is present,” Dr. Trottier said. “If the link [between preterm birth and HPV infections] is indeed causal, we can expect a greater risk of preterm delivery in these women. The effective tool we have is the HPV vaccination, but it should ideally be received before the start of sexual activity in order to prevent future infections that could occur in women.”
The American College of Obstetricians and Gynecologists recommends HPV vaccination for girls and women between the ages of 11 and 26 years old. However, Dr. Tanner said, women aged 27-45 who were previously unvaccinated may still receive benefit from the vaccine.
“Despite the known efficacy of the vaccine, only 50% of patients are up to date with their HPV vaccination,” Dr. Tanner explained. “This study further highlights the need to educate and encourage patients to be vaccinated.”
The researchers said future studies should investigate the association of HPV vaccination and vaccination programs with the risk of preterm birth.
The experts disclosed no conflicts of interest.
Persistent human papillomavirus (HPV) 16 and HPV 18 during a pregnancy may be associated with an increased risk of premature birth.
Findings published online in JAMA Network Open found that 15.9% of individuals who had a persistent HPV 16 or 18 infection during the first and third trimesters of their pregnancy gave birth prematurely, compared with 5.6% of those who did not have an HPV infection at all.
The findings prompted the question of “the pathophysiology of HPV in pregnancy and how the virus is affecting the placenta,” said Lisette Davidson Tanner, MD, MPH, FACOG, who was not involved in the study.
Researchers said the findings are the first to show the association between preterm birth and HPV, which is an incurable virus that most sexually active individuals will get at some point in their lives, according to the Centers for Disease Control and Prevention.
“The results of this study are very important in helping us understand the burden caused by HPV in pregnancy,” study author Helen Trottier, MSc, PhD, researcher at the Centre Hospitalier Universitaire Sainte-Justine, said in an interview. “We may have just pinpointed an important cause of preterm birth that has so far been unexplained.”
Dr. Trottier and colleagues examined data from 1,052 pregnant women from three university-affiliated health care centers in Montreal between Nov. 8, 2010, and Oct. 16, 2016.
Only 5.6% of those who did not have an HPV infection had a premature birth, compared with 6.9% of those who tested positive for any HPV infection in the first trimester.
When looking at the first trimester, researchers found 12% of those diagnosed with HPV 16 and 18 had a preterm birth, compared to 4.9% of those who had a high-risk HPV infection other than HPV 16/18. When looking at the third trimester, researchers found that 15.9% of those with HPV 16/18 had an increased risk of giving birth prematurely, compared to those who had other high-risk HPV infections.
When researchers looked at the persistence of these HPV infections, they found that most HPV infections detected in the first trimester persist to the third trimester. The findings also revealed that persistent vaginal HPV 16/18 detection was significantly associated with all preterm births and spontaneous preterm births. This association was also found among those who had HPV infections detected in their placentas.
Meanwhile, 5.8% of those who had an HPV infection only during the first trimester experienced a preterm birth.
The researchers also found that HPV infections were frequent in pregnancy even among populations “considered to be at low risk based on sociodemographic and sexual history characteristics,” they wrote. Dr. Trottier said she hopes the findings will strengthen support for HPV vaccination.
Dr. Trottier’s study adds to a growing body of research regarding the adverse effects of HPV, according to Dr. Tanner, assistant professor of gynecology and obstetrics at Emory University, Atlanta. “It is already well known that HPV is associated with a number of anogenital and oropharyngeal cancers,” Dr. Tanner said in an interview. “The potential association with preterm birth only adds weight to the recommendations to screen for and prevent HPV infection.”
HPV 16 and 18 are high-risk types that cause about 70% of cervical cancers and precancerous cervical lesions, according to the World Health Organization. However, there are three HPV vaccines – 9-valent HPV vaccine (Gardasil), quadrivalent HPV vaccine (Gardasil®, 4vHPV), and bivalent HPV vaccine (Cervarix) – that help protect against HPV 16/18.
The findings strengthen the benefits of HPV vaccination, Dr. Trottier explained. “There is no cure when the HPV infection is present,” Dr. Trottier said. “If the link [between preterm birth and HPV infections] is indeed causal, we can expect a greater risk of preterm delivery in these women. The effective tool we have is the HPV vaccination, but it should ideally be received before the start of sexual activity in order to prevent future infections that could occur in women.”
The American College of Obstetricians and Gynecologists recommends HPV vaccination for girls and women between the ages of 11 and 26 years old. However, Dr. Tanner said, women aged 27-45 who were previously unvaccinated may still receive benefit from the vaccine.
“Despite the known efficacy of the vaccine, only 50% of patients are up to date with their HPV vaccination,” Dr. Tanner explained. “This study further highlights the need to educate and encourage patients to be vaccinated.”
The researchers said future studies should investigate the association of HPV vaccination and vaccination programs with the risk of preterm birth.
The experts disclosed no conflicts of interest.
FROM JAMA NETWORK OPEN
How could this happen? Judge forces doctors to give ivermectin
The judge’s order was a major affront to many clinical ethicists. A county judge in Ohio ordered a hospital to give ivermectin to a COVID-19 patient on a ventilator. This order occurred against the advice and judgment of the local physicians. It occurred in spite of the hospital’s lawyers fighting the order. How could such a situation occur?
This column is not the appropriate forum to debate the use of ivermectin. The Food and Drug Administration has not approved the drug for treating COVID-19. Indeed, the FDA has specifically recommended against its use.1 So has the Centers for Disease Control and Prevention.2 Poison control centers report a large uptick in exposures this summer because of self-medication, sometimes from veterinary sources.3
Fortunately for this case, the judge who overruled the order, Judge Michael A. Oster, wrote in his decision a summary of facts presented by both sides. The topic here is how a judge could order a medical institution and its staff to provide care against medical judgment. A key tenet of clinical ethics consultation is that the consultant needs to do their own investigation. Most veteran consultants have a litany of anecdotes wherein the initial story changed markedly as new facts were uncovered. The more outrageous the initial story, the more likely a major distortion is found. Therefore, most clinical ethics consultants are reluctant to discuss case studies based solely on publicly available information. Often, it is nearly impossible to obtain further information. One side of the story may be gagged by privacy laws. However, cases must sometimes be discussed based on the limited information available because, without that discussion, egregious violations of medical ethics would not be brought to light.
Fortunately for this case, Judge Osler’s decision contains a summary of facts presented by both sides. In August 2021, a 51-year-old patient with severe COVID-19 is in an Ohio intensive care unit on a ventilator. His wife seeks and obtains a prescription for ivermectin from a physician who has an Ohio state medical license but lives elsewhere, has no clinical privileges at the involved hospital, and has never examined the patient. The wife, as a surrogate decision maker, demands her husband receive the medication. The medical staff involved do not consider it a valid treatment. The wife seeks an injunction. A county judge orders the hospital to administer a specified dose of ivermectin daily for 21 days.4 That judge further grants an emergency preliminary injunction for 14 days that orders administration of the medication while legal appeals are made. Two weeks later, a second county judge hearing the case rules that the wife has not presented convincing evidence that she is likely to ultimately win the case on the merits.5 Therefore, the second judge reverses the preliminary injunction. The hospital need not continue to give the medication while further legal proceedings take place.
Cases like this are uncommon. Judges generally defer the authority for medical decisions to physicians. Various attitudes combine to make such an event happen. The judge may view the hospital as a local monopoly of health care and the patient may be too unstable to transport elsewhere. A judge in that situation, combined with a “the consumer is always right” mentality, and a sympathetic plaintiff, may seek to make miracles happen.
Judges overriding science are more likely to manifest when they see the science as ambiguous. Scientists have lost some of the gravitas they had when men walked on the moon. The spectacular success of the mRNA vaccines has surprisingly not reversed that loss. Science has been tainted by mercenary scientists, biased researchers seeking publications, and the large volume of published medical research that is false.
But there is more going on here. In the United States there has been a significant rebellion against any form of expertise and any form of authority. The echo chambers of misinformation on social media have led to polarization, conspiracy theories, and loyalty to political tribe rather than truth; hence the battle over masks and vaccines. This breakdown in authority is accompanied by losses in virtues such as civic duty and loving one’s neighbor. This is a failure of modern moral institutions. When major medical journals print opinion pieces portraying physicians as interchangeable automatons,6 it should not be surprising to see judges tempted by similar imagery.
One part of the solution is accountability in peer review. With 30,000 county judges scattered in 50 states, there will always be a few rogue and maverick attitudes among judges. The judiciary has a means of reassigning rebels to less impactful tasks. Similarly, if the physician who counseled the wife to use ivermectin had privileges at the admitting hospital, then peer review and credential committees could discipline behaviors that were too far outside accepted norms. Even when a consensus on best practice is hard to establish, damage can be mitigated by creating consequences for promoting aberrant care.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Why you should not use ivermectin to treat or prevent COVID-19,” FDA Consumer Updates, Sept. 3, 2021.
2. “Rapid increase in ivermectin prescriptions and reports of severe illness associated with use of products containing ivermectin to prevent or treat COVID-19,” CDC Health Advisory, Aug. 26, 2021.
3. National Poison Data System Bulletin: COVID-19 (Ivermectin), American Association of Poison Control Centers, 2021.
4. Smith v West Chester Hosptial, LLC, DBA West Chester Hospital, Butler County Clerk of Courts, Aug. 23, 2021.
5. Smith v West Chester Hosptial, LLC, Decision denying plaintiff’s action for a preliminary injunction, Butler County Clerk of Courts, Sept. 6, 2021.
6. “Conscientious objection in medicine,” BMJ 2006 Feb 2. doi: 10.1136/bmj.332.7536.294.
The judge’s order was a major affront to many clinical ethicists. A county judge in Ohio ordered a hospital to give ivermectin to a COVID-19 patient on a ventilator. This order occurred against the advice and judgment of the local physicians. It occurred in spite of the hospital’s lawyers fighting the order. How could such a situation occur?
This column is not the appropriate forum to debate the use of ivermectin. The Food and Drug Administration has not approved the drug for treating COVID-19. Indeed, the FDA has specifically recommended against its use.1 So has the Centers for Disease Control and Prevention.2 Poison control centers report a large uptick in exposures this summer because of self-medication, sometimes from veterinary sources.3
Fortunately for this case, the judge who overruled the order, Judge Michael A. Oster, wrote in his decision a summary of facts presented by both sides. The topic here is how a judge could order a medical institution and its staff to provide care against medical judgment. A key tenet of clinical ethics consultation is that the consultant needs to do their own investigation. Most veteran consultants have a litany of anecdotes wherein the initial story changed markedly as new facts were uncovered. The more outrageous the initial story, the more likely a major distortion is found. Therefore, most clinical ethics consultants are reluctant to discuss case studies based solely on publicly available information. Often, it is nearly impossible to obtain further information. One side of the story may be gagged by privacy laws. However, cases must sometimes be discussed based on the limited information available because, without that discussion, egregious violations of medical ethics would not be brought to light.
Fortunately for this case, Judge Osler’s decision contains a summary of facts presented by both sides. In August 2021, a 51-year-old patient with severe COVID-19 is in an Ohio intensive care unit on a ventilator. His wife seeks and obtains a prescription for ivermectin from a physician who has an Ohio state medical license but lives elsewhere, has no clinical privileges at the involved hospital, and has never examined the patient. The wife, as a surrogate decision maker, demands her husband receive the medication. The medical staff involved do not consider it a valid treatment. The wife seeks an injunction. A county judge orders the hospital to administer a specified dose of ivermectin daily for 21 days.4 That judge further grants an emergency preliminary injunction for 14 days that orders administration of the medication while legal appeals are made. Two weeks later, a second county judge hearing the case rules that the wife has not presented convincing evidence that she is likely to ultimately win the case on the merits.5 Therefore, the second judge reverses the preliminary injunction. The hospital need not continue to give the medication while further legal proceedings take place.
Cases like this are uncommon. Judges generally defer the authority for medical decisions to physicians. Various attitudes combine to make such an event happen. The judge may view the hospital as a local monopoly of health care and the patient may be too unstable to transport elsewhere. A judge in that situation, combined with a “the consumer is always right” mentality, and a sympathetic plaintiff, may seek to make miracles happen.
Judges overriding science are more likely to manifest when they see the science as ambiguous. Scientists have lost some of the gravitas they had when men walked on the moon. The spectacular success of the mRNA vaccines has surprisingly not reversed that loss. Science has been tainted by mercenary scientists, biased researchers seeking publications, and the large volume of published medical research that is false.
But there is more going on here. In the United States there has been a significant rebellion against any form of expertise and any form of authority. The echo chambers of misinformation on social media have led to polarization, conspiracy theories, and loyalty to political tribe rather than truth; hence the battle over masks and vaccines. This breakdown in authority is accompanied by losses in virtues such as civic duty and loving one’s neighbor. This is a failure of modern moral institutions. When major medical journals print opinion pieces portraying physicians as interchangeable automatons,6 it should not be surprising to see judges tempted by similar imagery.
One part of the solution is accountability in peer review. With 30,000 county judges scattered in 50 states, there will always be a few rogue and maverick attitudes among judges. The judiciary has a means of reassigning rebels to less impactful tasks. Similarly, if the physician who counseled the wife to use ivermectin had privileges at the admitting hospital, then peer review and credential committees could discipline behaviors that were too far outside accepted norms. Even when a consensus on best practice is hard to establish, damage can be mitigated by creating consequences for promoting aberrant care.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Why you should not use ivermectin to treat or prevent COVID-19,” FDA Consumer Updates, Sept. 3, 2021.
2. “Rapid increase in ivermectin prescriptions and reports of severe illness associated with use of products containing ivermectin to prevent or treat COVID-19,” CDC Health Advisory, Aug. 26, 2021.
3. National Poison Data System Bulletin: COVID-19 (Ivermectin), American Association of Poison Control Centers, 2021.
4. Smith v West Chester Hosptial, LLC, DBA West Chester Hospital, Butler County Clerk of Courts, Aug. 23, 2021.
5. Smith v West Chester Hosptial, LLC, Decision denying plaintiff’s action for a preliminary injunction, Butler County Clerk of Courts, Sept. 6, 2021.
6. “Conscientious objection in medicine,” BMJ 2006 Feb 2. doi: 10.1136/bmj.332.7536.294.
The judge’s order was a major affront to many clinical ethicists. A county judge in Ohio ordered a hospital to give ivermectin to a COVID-19 patient on a ventilator. This order occurred against the advice and judgment of the local physicians. It occurred in spite of the hospital’s lawyers fighting the order. How could such a situation occur?
This column is not the appropriate forum to debate the use of ivermectin. The Food and Drug Administration has not approved the drug for treating COVID-19. Indeed, the FDA has specifically recommended against its use.1 So has the Centers for Disease Control and Prevention.2 Poison control centers report a large uptick in exposures this summer because of self-medication, sometimes from veterinary sources.3
Fortunately for this case, the judge who overruled the order, Judge Michael A. Oster, wrote in his decision a summary of facts presented by both sides. The topic here is how a judge could order a medical institution and its staff to provide care against medical judgment. A key tenet of clinical ethics consultation is that the consultant needs to do their own investigation. Most veteran consultants have a litany of anecdotes wherein the initial story changed markedly as new facts were uncovered. The more outrageous the initial story, the more likely a major distortion is found. Therefore, most clinical ethics consultants are reluctant to discuss case studies based solely on publicly available information. Often, it is nearly impossible to obtain further information. One side of the story may be gagged by privacy laws. However, cases must sometimes be discussed based on the limited information available because, without that discussion, egregious violations of medical ethics would not be brought to light.
Fortunately for this case, Judge Osler’s decision contains a summary of facts presented by both sides. In August 2021, a 51-year-old patient with severe COVID-19 is in an Ohio intensive care unit on a ventilator. His wife seeks and obtains a prescription for ivermectin from a physician who has an Ohio state medical license but lives elsewhere, has no clinical privileges at the involved hospital, and has never examined the patient. The wife, as a surrogate decision maker, demands her husband receive the medication. The medical staff involved do not consider it a valid treatment. The wife seeks an injunction. A county judge orders the hospital to administer a specified dose of ivermectin daily for 21 days.4 That judge further grants an emergency preliminary injunction for 14 days that orders administration of the medication while legal appeals are made. Two weeks later, a second county judge hearing the case rules that the wife has not presented convincing evidence that she is likely to ultimately win the case on the merits.5 Therefore, the second judge reverses the preliminary injunction. The hospital need not continue to give the medication while further legal proceedings take place.
Cases like this are uncommon. Judges generally defer the authority for medical decisions to physicians. Various attitudes combine to make such an event happen. The judge may view the hospital as a local monopoly of health care and the patient may be too unstable to transport elsewhere. A judge in that situation, combined with a “the consumer is always right” mentality, and a sympathetic plaintiff, may seek to make miracles happen.
Judges overriding science are more likely to manifest when they see the science as ambiguous. Scientists have lost some of the gravitas they had when men walked on the moon. The spectacular success of the mRNA vaccines has surprisingly not reversed that loss. Science has been tainted by mercenary scientists, biased researchers seeking publications, and the large volume of published medical research that is false.
But there is more going on here. In the United States there has been a significant rebellion against any form of expertise and any form of authority. The echo chambers of misinformation on social media have led to polarization, conspiracy theories, and loyalty to political tribe rather than truth; hence the battle over masks and vaccines. This breakdown in authority is accompanied by losses in virtues such as civic duty and loving one’s neighbor. This is a failure of modern moral institutions. When major medical journals print opinion pieces portraying physicians as interchangeable automatons,6 it should not be surprising to see judges tempted by similar imagery.
One part of the solution is accountability in peer review. With 30,000 county judges scattered in 50 states, there will always be a few rogue and maverick attitudes among judges. The judiciary has a means of reassigning rebels to less impactful tasks. Similarly, if the physician who counseled the wife to use ivermectin had privileges at the admitting hospital, then peer review and credential committees could discipline behaviors that were too far outside accepted norms. Even when a consensus on best practice is hard to establish, damage can be mitigated by creating consequences for promoting aberrant care.
Dr. Powell is a retired pediatric hospitalist and clinical ethics consultant living in St. Louis. Email him at [email protected].
References
1. “Why you should not use ivermectin to treat or prevent COVID-19,” FDA Consumer Updates, Sept. 3, 2021.
2. “Rapid increase in ivermectin prescriptions and reports of severe illness associated with use of products containing ivermectin to prevent or treat COVID-19,” CDC Health Advisory, Aug. 26, 2021.
3. National Poison Data System Bulletin: COVID-19 (Ivermectin), American Association of Poison Control Centers, 2021.
4. Smith v West Chester Hosptial, LLC, DBA West Chester Hospital, Butler County Clerk of Courts, Aug. 23, 2021.
5. Smith v West Chester Hosptial, LLC, Decision denying plaintiff’s action for a preliminary injunction, Butler County Clerk of Courts, Sept. 6, 2021.
6. “Conscientious objection in medicine,” BMJ 2006 Feb 2. doi: 10.1136/bmj.332.7536.294.
USPSTF: Continue gonorrhea, chlamydia screening in sexually active young women, teens
The U.S. Preventive Services Task Force (USPSTF) announced on Tuesday that it is standing by its 2014 recommendations that sexually active girls and young women be screened for chlamydia and gonorrhea. But the panel is not ready to provide guidance about screening males even amid an outbreak of gonorrhea infections among men who have sex with men (MSM).
“For men in general, there’s not enough evidence to determine whether screening will reduce the risk of complications or spreading infections to others,” said Marti Kubik, PhD, RN, in an interview. Dr. Kubik is a professor at the George Mason University School of Nursing, Fairfax, Va., and is a member of the task force. “We need further research so we will know how to make those recommendations,” she said.
The screening recommendations for chlamydia and gonorrhea were published Sept. 14 in the Journal of the American Medical Association. The guidance is identical to the panel’s 2014 recommendations. The task force recommends screening for chlamydia and gonorrhea in all sexually active females aged 24 years or younger and in sexually active women aged 25 and older if they are at higher risk because of factors such as new or multiple sex partners.
“We continue to see rising rates of these infections in spite of consistent screening recommendations,” Dr. Kubik said. “In 2019, the CDC recorded nearly 2 million cases of chlamydia and a half million cases of gonorrhea. The big clincher is that chlamydia and gonorrhea can occur without symptoms. It’s critical to screen if we’re going to prevent serious health complications.”
The report notes that chlamydia and gonorrhea may lead to pelvic inflammatory disease in women and to multiple complications in infants born to infected mothers. Men can develop urethritis and epididymitis. Both diseases can boost the risk for HIV infection and transmission.
“We want clinicians to review the new recommendation and feel confident about the evidence base that supports a need for us to be screening young women and older women who are at increased risk,” Dr. Kubik said. She noted that almost two-thirds of chlamydia cases and more than half of gonorrhea cases occur in men and women aged 15-24.
Unlike the CDC, which recommends annual chlamydia and gonorrhea screening in appropriate female patients, the task force provides no guidance on screening frequency. “We didn’t have the evidence base to make a recommendation about how often to screen,” Dr. Kubik said. “But recognizing that these often occur without symptoms, it’s reasonable for clinicians to screen patients whose sexual history reveals new or consistent risk factors.”
Philip A. Chan, MD, an associate professor at Brown University, Providence, R.I., who directs a sexually transmitted disease clinic, told this news organization that he found it frustrating that the task force didn’t make recommendations about screening of MSM. According to a commentary accompanying the new recommendations, the rate of gonorrhea in MSM – 5,166 cases per 100,000, or more than 5% – is at a historic high.
In contrast to the task force, the CDC recommends annual or more frequent testing for gonorrhea and chlamydia plus HIV and syphilis in sexually active MSM.
Dr. Chan noted that the task force’s guidance “tends to be the most evidence-based recommendations that exist. If the evidence isn’t there, they usually don’t make a recommendation.” Still, he said, “I would argue that there’s good evidence that in MSM, the risk for HIV acquisition warrants routine screening.”
Jeanne Marrazzo, MD, MPH, director of the division of infectious diseases at the University of Alabama at Birmingham, also noted the limits of the task force’s insistence on certain kinds of evidence. Dr. Marrazzo, who coauthored a commentary that accompanies the recommendations, said in an interview that the panel’s “reliance on randomized-controlled-trial-level evidence tends to limit its ability to evolve their recommendations in a way that could account for evolving epidemiology or advances in our understanding of pathophysiology of these infections.”
Dr. Chan noted that obstacles exist for patients even when screening recommendations are in place. Although insurers typically cover costs of chlamydia and gonorrhea screening tests, he said, the uninsured may have to pay $100 or more each.
The USPSTF is supported by the U.S. Agency for Healthcare Research and Quality. Dr. Kubik, Dr. Chan, and Dr. Marrazzo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force (USPSTF) announced on Tuesday that it is standing by its 2014 recommendations that sexually active girls and young women be screened for chlamydia and gonorrhea. But the panel is not ready to provide guidance about screening males even amid an outbreak of gonorrhea infections among men who have sex with men (MSM).
“For men in general, there’s not enough evidence to determine whether screening will reduce the risk of complications or spreading infections to others,” said Marti Kubik, PhD, RN, in an interview. Dr. Kubik is a professor at the George Mason University School of Nursing, Fairfax, Va., and is a member of the task force. “We need further research so we will know how to make those recommendations,” she said.
The screening recommendations for chlamydia and gonorrhea were published Sept. 14 in the Journal of the American Medical Association. The guidance is identical to the panel’s 2014 recommendations. The task force recommends screening for chlamydia and gonorrhea in all sexually active females aged 24 years or younger and in sexually active women aged 25 and older if they are at higher risk because of factors such as new or multiple sex partners.
“We continue to see rising rates of these infections in spite of consistent screening recommendations,” Dr. Kubik said. “In 2019, the CDC recorded nearly 2 million cases of chlamydia and a half million cases of gonorrhea. The big clincher is that chlamydia and gonorrhea can occur without symptoms. It’s critical to screen if we’re going to prevent serious health complications.”
The report notes that chlamydia and gonorrhea may lead to pelvic inflammatory disease in women and to multiple complications in infants born to infected mothers. Men can develop urethritis and epididymitis. Both diseases can boost the risk for HIV infection and transmission.
“We want clinicians to review the new recommendation and feel confident about the evidence base that supports a need for us to be screening young women and older women who are at increased risk,” Dr. Kubik said. She noted that almost two-thirds of chlamydia cases and more than half of gonorrhea cases occur in men and women aged 15-24.
Unlike the CDC, which recommends annual chlamydia and gonorrhea screening in appropriate female patients, the task force provides no guidance on screening frequency. “We didn’t have the evidence base to make a recommendation about how often to screen,” Dr. Kubik said. “But recognizing that these often occur without symptoms, it’s reasonable for clinicians to screen patients whose sexual history reveals new or consistent risk factors.”
Philip A. Chan, MD, an associate professor at Brown University, Providence, R.I., who directs a sexually transmitted disease clinic, told this news organization that he found it frustrating that the task force didn’t make recommendations about screening of MSM. According to a commentary accompanying the new recommendations, the rate of gonorrhea in MSM – 5,166 cases per 100,000, or more than 5% – is at a historic high.
In contrast to the task force, the CDC recommends annual or more frequent testing for gonorrhea and chlamydia plus HIV and syphilis in sexually active MSM.
Dr. Chan noted that the task force’s guidance “tends to be the most evidence-based recommendations that exist. If the evidence isn’t there, they usually don’t make a recommendation.” Still, he said, “I would argue that there’s good evidence that in MSM, the risk for HIV acquisition warrants routine screening.”
Jeanne Marrazzo, MD, MPH, director of the division of infectious diseases at the University of Alabama at Birmingham, also noted the limits of the task force’s insistence on certain kinds of evidence. Dr. Marrazzo, who coauthored a commentary that accompanies the recommendations, said in an interview that the panel’s “reliance on randomized-controlled-trial-level evidence tends to limit its ability to evolve their recommendations in a way that could account for evolving epidemiology or advances in our understanding of pathophysiology of these infections.”
Dr. Chan noted that obstacles exist for patients even when screening recommendations are in place. Although insurers typically cover costs of chlamydia and gonorrhea screening tests, he said, the uninsured may have to pay $100 or more each.
The USPSTF is supported by the U.S. Agency for Healthcare Research and Quality. Dr. Kubik, Dr. Chan, and Dr. Marrazzo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The U.S. Preventive Services Task Force (USPSTF) announced on Tuesday that it is standing by its 2014 recommendations that sexually active girls and young women be screened for chlamydia and gonorrhea. But the panel is not ready to provide guidance about screening males even amid an outbreak of gonorrhea infections among men who have sex with men (MSM).
“For men in general, there’s not enough evidence to determine whether screening will reduce the risk of complications or spreading infections to others,” said Marti Kubik, PhD, RN, in an interview. Dr. Kubik is a professor at the George Mason University School of Nursing, Fairfax, Va., and is a member of the task force. “We need further research so we will know how to make those recommendations,” she said.
The screening recommendations for chlamydia and gonorrhea were published Sept. 14 in the Journal of the American Medical Association. The guidance is identical to the panel’s 2014 recommendations. The task force recommends screening for chlamydia and gonorrhea in all sexually active females aged 24 years or younger and in sexually active women aged 25 and older if they are at higher risk because of factors such as new or multiple sex partners.
“We continue to see rising rates of these infections in spite of consistent screening recommendations,” Dr. Kubik said. “In 2019, the CDC recorded nearly 2 million cases of chlamydia and a half million cases of gonorrhea. The big clincher is that chlamydia and gonorrhea can occur without symptoms. It’s critical to screen if we’re going to prevent serious health complications.”
The report notes that chlamydia and gonorrhea may lead to pelvic inflammatory disease in women and to multiple complications in infants born to infected mothers. Men can develop urethritis and epididymitis. Both diseases can boost the risk for HIV infection and transmission.
“We want clinicians to review the new recommendation and feel confident about the evidence base that supports a need for us to be screening young women and older women who are at increased risk,” Dr. Kubik said. She noted that almost two-thirds of chlamydia cases and more than half of gonorrhea cases occur in men and women aged 15-24.
Unlike the CDC, which recommends annual chlamydia and gonorrhea screening in appropriate female patients, the task force provides no guidance on screening frequency. “We didn’t have the evidence base to make a recommendation about how often to screen,” Dr. Kubik said. “But recognizing that these often occur without symptoms, it’s reasonable for clinicians to screen patients whose sexual history reveals new or consistent risk factors.”
Philip A. Chan, MD, an associate professor at Brown University, Providence, R.I., who directs a sexually transmitted disease clinic, told this news organization that he found it frustrating that the task force didn’t make recommendations about screening of MSM. According to a commentary accompanying the new recommendations, the rate of gonorrhea in MSM – 5,166 cases per 100,000, or more than 5% – is at a historic high.
In contrast to the task force, the CDC recommends annual or more frequent testing for gonorrhea and chlamydia plus HIV and syphilis in sexually active MSM.
Dr. Chan noted that the task force’s guidance “tends to be the most evidence-based recommendations that exist. If the evidence isn’t there, they usually don’t make a recommendation.” Still, he said, “I would argue that there’s good evidence that in MSM, the risk for HIV acquisition warrants routine screening.”
Jeanne Marrazzo, MD, MPH, director of the division of infectious diseases at the University of Alabama at Birmingham, also noted the limits of the task force’s insistence on certain kinds of evidence. Dr. Marrazzo, who coauthored a commentary that accompanies the recommendations, said in an interview that the panel’s “reliance on randomized-controlled-trial-level evidence tends to limit its ability to evolve their recommendations in a way that could account for evolving epidemiology or advances in our understanding of pathophysiology of these infections.”
Dr. Chan noted that obstacles exist for patients even when screening recommendations are in place. Although insurers typically cover costs of chlamydia and gonorrhea screening tests, he said, the uninsured may have to pay $100 or more each.
The USPSTF is supported by the U.S. Agency for Healthcare Research and Quality. Dr. Kubik, Dr. Chan, and Dr. Marrazzo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Candida auris transmission can be contained in postacute care settings
A new study from Orange County, California, shows how Candida auris, an emerging pathogen, was successfully identified and contained in long-term acute care hospitals (LTACHs) and ventilator-capable skilled-nursing facilities (vSNFs).
Lead author Ellora Karmarkar, MD, MSc, formerly an epidemic intelligence service officer with the Centers for Disease Control and Prevention and currently with the California Department of Public Health, said in an interview that the prospective surveillance of urine cultures for C. auris was prompted by “seeing what was happening in New York, New Jersey, and Illinois [being] pretty alarming for a lot of the health officials in California, [who] know that LTACHs are high-risk facilities because they take care of really sick people. Some of those people are there for a very long time.”
Therefore, the study authors decided to focus their investigations there, rather than in acute care hospitals, which were believed to be at lower risk for C. auris outbreaks.
The Orange County Health Department, working with the California Department of Health and the CDC, asked labs to prospectively identify all Candida isolates in urines from LTACHs between September 2018 and February 2019. Normally, labs do not speciate Candida from nonsterile body sites.
Dan Diekema, MD, an epidemiologist and clinical microbiologist at the University of Iowa, Iowa City, who was not involved in the study, told this news organization, “Acute care hospitals really ought to be moving toward doing species identification of Candida from nonsterile sites if they really want to have a better chance of detecting this early.”
The OCHD also screened LTACH and vSNF patients with composite cultures from the axilla-groin or nasal swabs. Screening was undertaken because 5%-10% of colonized patients later develop invasive infections, and 30%-60% die.
The first bloodstream infection was detected in May 2019. Per the report, published online Sept. 7 in Annals of Internal Medicine, “As of 1 January 2020, of 182 patients, 22 (12%) died within 30 days of C. auris identification; 47 (26%) died within 90 days. One of 47 deaths was attributed to C. auris.” Whole-genome sequencing showed that the isolates were all closely related in clade III.
Experts conducted extensive education in infection control at the LTACHs, and communication among the LTACHs and between the long-term facilities and acute care hospitals was improved. As a result, receiving facilities accepting transfers began culturing their newly admitted patients and quickly identified 4 of 99 patients with C. auris who had no known history of colonization. By October 2019, the outbreak was contained in two facilities, down from the nine where C. auris was initially found.
Dr. Diekema noted, “The challenge, of course, for a new emerging MDRO [multidrug-resistant organism] like Candida auris, is that the initial approach, in general, has to be almost passive, when you have not seen the organism. ... Passive surveillance means that you just carefully monitor your clinical cultures, and the first time you detect the MDRO of concern, then you begin doing the point prevalence surveys. ... This [prospective] kind of approach is really good for how we should move forward with both initial detection and containment of MDRO spread.”
Many outbreak studies are confined to a particular institution. Authors of an accompanying editorial commented that this study “underlines the importance of proactive protocols for outbreak investigations and containment measures across the entirety of the health care network serving at-risk patients.”
In her research, Dr. Karmarkar observed that, “some of these facilities don’t have the same infrastructure and infection prevention and control that an acute care hospital might.”
She said in an interview that, “one of the challenges was that people were so focused on COVID that they forgot about the MDROs. ... Some of the things that we recommend to help control Candida auris are also excellent practices for every other organism including COVID care. ... What I appreciated about this investigation is that every facility that we went to was so open to learning, so happy to have us there. They’re very interested in learning about Candida auris and understanding what they could do to control it.”
While recent attention has been on the frightening levels of multidrug resistance in C. auris, Dr. Karmarkar concluded that the “central message in our investigation is that with the right effort, the right approach, and the right team this is an intervenable issue. It’s not inevitable if the attention is focused on it to pick it up early and then try to contain it.”
Dr. Karmarkar reports no relevant financial relationships. Dr. Diekema reports research funding from bioMerieux and consulting fees from Opgen.
A version of this article first appeared on Medscape.com.
A new study from Orange County, California, shows how Candida auris, an emerging pathogen, was successfully identified and contained in long-term acute care hospitals (LTACHs) and ventilator-capable skilled-nursing facilities (vSNFs).
Lead author Ellora Karmarkar, MD, MSc, formerly an epidemic intelligence service officer with the Centers for Disease Control and Prevention and currently with the California Department of Public Health, said in an interview that the prospective surveillance of urine cultures for C. auris was prompted by “seeing what was happening in New York, New Jersey, and Illinois [being] pretty alarming for a lot of the health officials in California, [who] know that LTACHs are high-risk facilities because they take care of really sick people. Some of those people are there for a very long time.”
Therefore, the study authors decided to focus their investigations there, rather than in acute care hospitals, which were believed to be at lower risk for C. auris outbreaks.
The Orange County Health Department, working with the California Department of Health and the CDC, asked labs to prospectively identify all Candida isolates in urines from LTACHs between September 2018 and February 2019. Normally, labs do not speciate Candida from nonsterile body sites.
Dan Diekema, MD, an epidemiologist and clinical microbiologist at the University of Iowa, Iowa City, who was not involved in the study, told this news organization, “Acute care hospitals really ought to be moving toward doing species identification of Candida from nonsterile sites if they really want to have a better chance of detecting this early.”
The OCHD also screened LTACH and vSNF patients with composite cultures from the axilla-groin or nasal swabs. Screening was undertaken because 5%-10% of colonized patients later develop invasive infections, and 30%-60% die.
The first bloodstream infection was detected in May 2019. Per the report, published online Sept. 7 in Annals of Internal Medicine, “As of 1 January 2020, of 182 patients, 22 (12%) died within 30 days of C. auris identification; 47 (26%) died within 90 days. One of 47 deaths was attributed to C. auris.” Whole-genome sequencing showed that the isolates were all closely related in clade III.
Experts conducted extensive education in infection control at the LTACHs, and communication among the LTACHs and between the long-term facilities and acute care hospitals was improved. As a result, receiving facilities accepting transfers began culturing their newly admitted patients and quickly identified 4 of 99 patients with C. auris who had no known history of colonization. By October 2019, the outbreak was contained in two facilities, down from the nine where C. auris was initially found.
Dr. Diekema noted, “The challenge, of course, for a new emerging MDRO [multidrug-resistant organism] like Candida auris, is that the initial approach, in general, has to be almost passive, when you have not seen the organism. ... Passive surveillance means that you just carefully monitor your clinical cultures, and the first time you detect the MDRO of concern, then you begin doing the point prevalence surveys. ... This [prospective] kind of approach is really good for how we should move forward with both initial detection and containment of MDRO spread.”
Many outbreak studies are confined to a particular institution. Authors of an accompanying editorial commented that this study “underlines the importance of proactive protocols for outbreak investigations and containment measures across the entirety of the health care network serving at-risk patients.”
In her research, Dr. Karmarkar observed that, “some of these facilities don’t have the same infrastructure and infection prevention and control that an acute care hospital might.”
She said in an interview that, “one of the challenges was that people were so focused on COVID that they forgot about the MDROs. ... Some of the things that we recommend to help control Candida auris are also excellent practices for every other organism including COVID care. ... What I appreciated about this investigation is that every facility that we went to was so open to learning, so happy to have us there. They’re very interested in learning about Candida auris and understanding what they could do to control it.”
While recent attention has been on the frightening levels of multidrug resistance in C. auris, Dr. Karmarkar concluded that the “central message in our investigation is that with the right effort, the right approach, and the right team this is an intervenable issue. It’s not inevitable if the attention is focused on it to pick it up early and then try to contain it.”
Dr. Karmarkar reports no relevant financial relationships. Dr. Diekema reports research funding from bioMerieux and consulting fees from Opgen.
A version of this article first appeared on Medscape.com.
A new study from Orange County, California, shows how Candida auris, an emerging pathogen, was successfully identified and contained in long-term acute care hospitals (LTACHs) and ventilator-capable skilled-nursing facilities (vSNFs).
Lead author Ellora Karmarkar, MD, MSc, formerly an epidemic intelligence service officer with the Centers for Disease Control and Prevention and currently with the California Department of Public Health, said in an interview that the prospective surveillance of urine cultures for C. auris was prompted by “seeing what was happening in New York, New Jersey, and Illinois [being] pretty alarming for a lot of the health officials in California, [who] know that LTACHs are high-risk facilities because they take care of really sick people. Some of those people are there for a very long time.”
Therefore, the study authors decided to focus their investigations there, rather than in acute care hospitals, which were believed to be at lower risk for C. auris outbreaks.
The Orange County Health Department, working with the California Department of Health and the CDC, asked labs to prospectively identify all Candida isolates in urines from LTACHs between September 2018 and February 2019. Normally, labs do not speciate Candida from nonsterile body sites.
Dan Diekema, MD, an epidemiologist and clinical microbiologist at the University of Iowa, Iowa City, who was not involved in the study, told this news organization, “Acute care hospitals really ought to be moving toward doing species identification of Candida from nonsterile sites if they really want to have a better chance of detecting this early.”
The OCHD also screened LTACH and vSNF patients with composite cultures from the axilla-groin or nasal swabs. Screening was undertaken because 5%-10% of colonized patients later develop invasive infections, and 30%-60% die.
The first bloodstream infection was detected in May 2019. Per the report, published online Sept. 7 in Annals of Internal Medicine, “As of 1 January 2020, of 182 patients, 22 (12%) died within 30 days of C. auris identification; 47 (26%) died within 90 days. One of 47 deaths was attributed to C. auris.” Whole-genome sequencing showed that the isolates were all closely related in clade III.
Experts conducted extensive education in infection control at the LTACHs, and communication among the LTACHs and between the long-term facilities and acute care hospitals was improved. As a result, receiving facilities accepting transfers began culturing their newly admitted patients and quickly identified 4 of 99 patients with C. auris who had no known history of colonization. By October 2019, the outbreak was contained in two facilities, down from the nine where C. auris was initially found.
Dr. Diekema noted, “The challenge, of course, for a new emerging MDRO [multidrug-resistant organism] like Candida auris, is that the initial approach, in general, has to be almost passive, when you have not seen the organism. ... Passive surveillance means that you just carefully monitor your clinical cultures, and the first time you detect the MDRO of concern, then you begin doing the point prevalence surveys. ... This [prospective] kind of approach is really good for how we should move forward with both initial detection and containment of MDRO spread.”
Many outbreak studies are confined to a particular institution. Authors of an accompanying editorial commented that this study “underlines the importance of proactive protocols for outbreak investigations and containment measures across the entirety of the health care network serving at-risk patients.”
In her research, Dr. Karmarkar observed that, “some of these facilities don’t have the same infrastructure and infection prevention and control that an acute care hospital might.”
She said in an interview that, “one of the challenges was that people were so focused on COVID that they forgot about the MDROs. ... Some of the things that we recommend to help control Candida auris are also excellent practices for every other organism including COVID care. ... What I appreciated about this investigation is that every facility that we went to was so open to learning, so happy to have us there. They’re very interested in learning about Candida auris and understanding what they could do to control it.”
While recent attention has been on the frightening levels of multidrug resistance in C. auris, Dr. Karmarkar concluded that the “central message in our investigation is that with the right effort, the right approach, and the right team this is an intervenable issue. It’s not inevitable if the attention is focused on it to pick it up early and then try to contain it.”
Dr. Karmarkar reports no relevant financial relationships. Dr. Diekema reports research funding from bioMerieux and consulting fees from Opgen.
A version of this article first appeared on Medscape.com.
Children and COVID: New cases down slightly from record high
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.
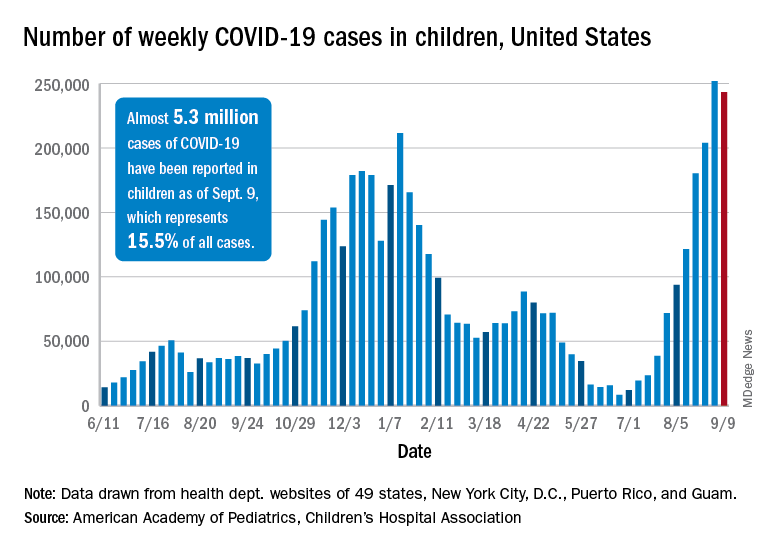
Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).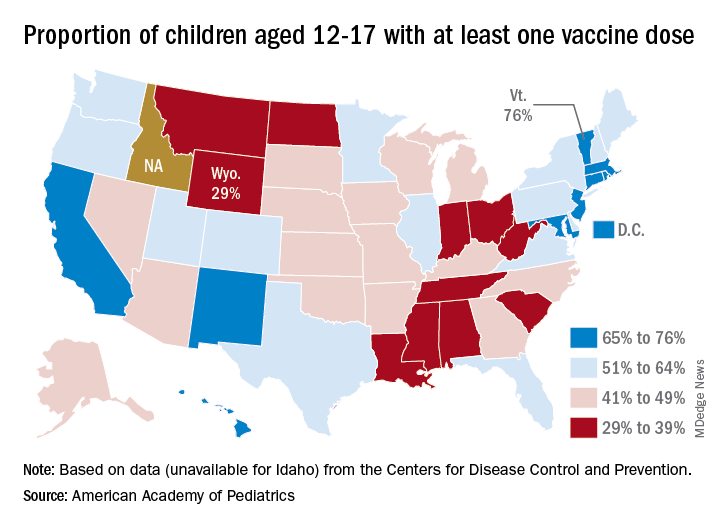
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.

Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.

Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
Antibiotic use and colon cancer: More evidence of link
The latest data come from a Swedish population study. Investigators analyzed data from more than 40,000 colorectal cancer patients and 200,000 cancer-free control persons.
They found that moderate use of antibiotics increased the risk for proximal colon cancer by 9% and that very high antibiotic use increased the risk by 17%.
In contrast, the risk for rectal cancer was reduced by 4% with moderate use and 9% with very high use, but this association was confined to women.
Antibiotic use was categorized as no use (no reported use of antibiotics during the study period), low (use during a period of 1-10 days), moderate (11-60 days), high (61-180 days), and very high (>180 days).
The study, led by Sophia Harlid, PhD, department of radiation sciences, oncology, Umeå University, Sweden, was published online on Sept. 1 in the Journal of the National Cancer Institute.
The results complement findings from a recent study from Scotland, which found that a history of antibiotic use among individuals younger than 50 appeared to increase the risk of developing colon cancer but not rectal cancer by 49%.
The new data from Sweden “strengthen prior evidence and provide new insights into site-specific carcinogenesis as well as indirect support for the role of gut microbiota,” lead author Dr. Dr. Harlid commented in an interview.
“The positive associations between antibiotics use and proximal colon cancer began at the lowest level of antibiotics use, providing a potential justification for reducing antibiotics prescriptions in clinical practice,” she added.
In their article, the team suggests that the increased risk could be a result of antibiotics having a “disruptive effect” on the gut microbiome.
The finding of an increased risk for cancer in the proximal colon but not further along the alimentary tract “is consistent with a high microbial impact in the proximal colon and a decreasing concentration of short-chain fatty acids along the colon,” the authors comment.
This results “in higher bacterial activity, biofilm formation, and fermentation in the proximal compared with the distal colon and rectum.”
A further analysis showed that the use of quinolones and sulfonamides and/or trimethoprims was associated with an increased risk for proximal colon cancer, whereas use of nitrofurantoins, macrolides and/or lincosamides, and metronidazoles and/or tinidazoles was inversely associated with rectal cancer.
Details of the study findings
For their study, the team analyzed complete-population data from Swedish national registers for the period July 1, 2005 to Dec. 31, 2016.
They matched case patients who were diagnosed with colorectal cancer from Jan. 1, 2010 to Dec. 31, 2016 with cancer-free control persons in a 1:5 ratio. Data on antibiotic use were extracted from the Swedish Prescribed Drug Register.
Other variables, such as socioeconomic factors and health care utilization, were obtained from the Swedish Inpatient Register and the Longitudinal Integration Database for Health Insurance and Labor Market Studies.
The team identified 40,545 patients with colorectal cancer cases; there were 202,720 control persons. Just over half (52.9%) of the participants were men; the mean age at cancer diagnosis was 72 years. Among the cases, 36.4% were proximal colon cancers, 29.3% were distal colon cancers, and 33.0% rectal cancers.
Control patients were more likely to have been prescribed no antibiotics, at 22.4% versus 18.7% for case patients. Case patients were more likely than control persons to have used antibiotics for more than 2 months, at 20.8% versus 19.3% (P < .001).
Overall, antibiotic use was positively associated with colorectal cancer. In comparison with no use, the odds ratio for moderate use was 1.15; for very high use, it was 1.17 (P < .001 for trend).
Excluding all antibiotic use during the 2 years prior to a colorectal cancer diagnosis attenuated the association, such that it was no longer significant for very high use versus no antibiotic use.
Applying this cutoff to the remaining analyses, the team found that the dose-response relationship between antibiotic use and colorectal cancer was largely confined to proximal colon cancer, at an odds ratio of 1.09 for moderate use and 1.17 for very high use in comparison with no use (P < .001 for trend).
For distal colon cancer, the relationship was “close to null.”
There was a slight inverse relationship between rectal cancer and antibiotic use, at an odds rate of 0.96 for moderate use and 0.91 for very high use versus no use (P < .001 for trend). This association was found in women only, whereas the other associations were seen in both men and women.
The study was supported by the Lion’s Cancer Research Foundation, Umeå University, and Region Västerbotten. Dr. Harlid has disclosed no relevant financial relationships. Three coauthors report various relationships with industry, as noted in the original article.
A version of this article first appeared on Medscape.com.
The latest data come from a Swedish population study. Investigators analyzed data from more than 40,000 colorectal cancer patients and 200,000 cancer-free control persons.
They found that moderate use of antibiotics increased the risk for proximal colon cancer by 9% and that very high antibiotic use increased the risk by 17%.
In contrast, the risk for rectal cancer was reduced by 4% with moderate use and 9% with very high use, but this association was confined to women.
Antibiotic use was categorized as no use (no reported use of antibiotics during the study period), low (use during a period of 1-10 days), moderate (11-60 days), high (61-180 days), and very high (>180 days).
The study, led by Sophia Harlid, PhD, department of radiation sciences, oncology, Umeå University, Sweden, was published online on Sept. 1 in the Journal of the National Cancer Institute.
The results complement findings from a recent study from Scotland, which found that a history of antibiotic use among individuals younger than 50 appeared to increase the risk of developing colon cancer but not rectal cancer by 49%.
The new data from Sweden “strengthen prior evidence and provide new insights into site-specific carcinogenesis as well as indirect support for the role of gut microbiota,” lead author Dr. Dr. Harlid commented in an interview.
“The positive associations between antibiotics use and proximal colon cancer began at the lowest level of antibiotics use, providing a potential justification for reducing antibiotics prescriptions in clinical practice,” she added.
In their article, the team suggests that the increased risk could be a result of antibiotics having a “disruptive effect” on the gut microbiome.
The finding of an increased risk for cancer in the proximal colon but not further along the alimentary tract “is consistent with a high microbial impact in the proximal colon and a decreasing concentration of short-chain fatty acids along the colon,” the authors comment.
This results “in higher bacterial activity, biofilm formation, and fermentation in the proximal compared with the distal colon and rectum.”
A further analysis showed that the use of quinolones and sulfonamides and/or trimethoprims was associated with an increased risk for proximal colon cancer, whereas use of nitrofurantoins, macrolides and/or lincosamides, and metronidazoles and/or tinidazoles was inversely associated with rectal cancer.
Details of the study findings
For their study, the team analyzed complete-population data from Swedish national registers for the period July 1, 2005 to Dec. 31, 2016.
They matched case patients who were diagnosed with colorectal cancer from Jan. 1, 2010 to Dec. 31, 2016 with cancer-free control persons in a 1:5 ratio. Data on antibiotic use were extracted from the Swedish Prescribed Drug Register.
Other variables, such as socioeconomic factors and health care utilization, were obtained from the Swedish Inpatient Register and the Longitudinal Integration Database for Health Insurance and Labor Market Studies.
The team identified 40,545 patients with colorectal cancer cases; there were 202,720 control persons. Just over half (52.9%) of the participants were men; the mean age at cancer diagnosis was 72 years. Among the cases, 36.4% were proximal colon cancers, 29.3% were distal colon cancers, and 33.0% rectal cancers.
Control patients were more likely to have been prescribed no antibiotics, at 22.4% versus 18.7% for case patients. Case patients were more likely than control persons to have used antibiotics for more than 2 months, at 20.8% versus 19.3% (P < .001).
Overall, antibiotic use was positively associated with colorectal cancer. In comparison with no use, the odds ratio for moderate use was 1.15; for very high use, it was 1.17 (P < .001 for trend).
Excluding all antibiotic use during the 2 years prior to a colorectal cancer diagnosis attenuated the association, such that it was no longer significant for very high use versus no antibiotic use.
Applying this cutoff to the remaining analyses, the team found that the dose-response relationship between antibiotic use and colorectal cancer was largely confined to proximal colon cancer, at an odds ratio of 1.09 for moderate use and 1.17 for very high use in comparison with no use (P < .001 for trend).
For distal colon cancer, the relationship was “close to null.”
There was a slight inverse relationship between rectal cancer and antibiotic use, at an odds rate of 0.96 for moderate use and 0.91 for very high use versus no use (P < .001 for trend). This association was found in women only, whereas the other associations were seen in both men and women.
The study was supported by the Lion’s Cancer Research Foundation, Umeå University, and Region Västerbotten. Dr. Harlid has disclosed no relevant financial relationships. Three coauthors report various relationships with industry, as noted in the original article.
A version of this article first appeared on Medscape.com.
The latest data come from a Swedish population study. Investigators analyzed data from more than 40,000 colorectal cancer patients and 200,000 cancer-free control persons.
They found that moderate use of antibiotics increased the risk for proximal colon cancer by 9% and that very high antibiotic use increased the risk by 17%.
In contrast, the risk for rectal cancer was reduced by 4% with moderate use and 9% with very high use, but this association was confined to women.
Antibiotic use was categorized as no use (no reported use of antibiotics during the study period), low (use during a period of 1-10 days), moderate (11-60 days), high (61-180 days), and very high (>180 days).
The study, led by Sophia Harlid, PhD, department of radiation sciences, oncology, Umeå University, Sweden, was published online on Sept. 1 in the Journal of the National Cancer Institute.
The results complement findings from a recent study from Scotland, which found that a history of antibiotic use among individuals younger than 50 appeared to increase the risk of developing colon cancer but not rectal cancer by 49%.
The new data from Sweden “strengthen prior evidence and provide new insights into site-specific carcinogenesis as well as indirect support for the role of gut microbiota,” lead author Dr. Dr. Harlid commented in an interview.
“The positive associations between antibiotics use and proximal colon cancer began at the lowest level of antibiotics use, providing a potential justification for reducing antibiotics prescriptions in clinical practice,” she added.
In their article, the team suggests that the increased risk could be a result of antibiotics having a “disruptive effect” on the gut microbiome.
The finding of an increased risk for cancer in the proximal colon but not further along the alimentary tract “is consistent with a high microbial impact in the proximal colon and a decreasing concentration of short-chain fatty acids along the colon,” the authors comment.
This results “in higher bacterial activity, biofilm formation, and fermentation in the proximal compared with the distal colon and rectum.”
A further analysis showed that the use of quinolones and sulfonamides and/or trimethoprims was associated with an increased risk for proximal colon cancer, whereas use of nitrofurantoins, macrolides and/or lincosamides, and metronidazoles and/or tinidazoles was inversely associated with rectal cancer.
Details of the study findings
For their study, the team analyzed complete-population data from Swedish national registers for the period July 1, 2005 to Dec. 31, 2016.
They matched case patients who were diagnosed with colorectal cancer from Jan. 1, 2010 to Dec. 31, 2016 with cancer-free control persons in a 1:5 ratio. Data on antibiotic use were extracted from the Swedish Prescribed Drug Register.
Other variables, such as socioeconomic factors and health care utilization, were obtained from the Swedish Inpatient Register and the Longitudinal Integration Database for Health Insurance and Labor Market Studies.
The team identified 40,545 patients with colorectal cancer cases; there were 202,720 control persons. Just over half (52.9%) of the participants were men; the mean age at cancer diagnosis was 72 years. Among the cases, 36.4% were proximal colon cancers, 29.3% were distal colon cancers, and 33.0% rectal cancers.
Control patients were more likely to have been prescribed no antibiotics, at 22.4% versus 18.7% for case patients. Case patients were more likely than control persons to have used antibiotics for more than 2 months, at 20.8% versus 19.3% (P < .001).
Overall, antibiotic use was positively associated with colorectal cancer. In comparison with no use, the odds ratio for moderate use was 1.15; for very high use, it was 1.17 (P < .001 for trend).
Excluding all antibiotic use during the 2 years prior to a colorectal cancer diagnosis attenuated the association, such that it was no longer significant for very high use versus no antibiotic use.
Applying this cutoff to the remaining analyses, the team found that the dose-response relationship between antibiotic use and colorectal cancer was largely confined to proximal colon cancer, at an odds ratio of 1.09 for moderate use and 1.17 for very high use in comparison with no use (P < .001 for trend).
For distal colon cancer, the relationship was “close to null.”
There was a slight inverse relationship between rectal cancer and antibiotic use, at an odds rate of 0.96 for moderate use and 0.91 for very high use versus no use (P < .001 for trend). This association was found in women only, whereas the other associations were seen in both men and women.
The study was supported by the Lion’s Cancer Research Foundation, Umeå University, and Region Västerbotten. Dr. Harlid has disclosed no relevant financial relationships. Three coauthors report various relationships with industry, as noted in the original article.
A version of this article first appeared on Medscape.com.
USPSTF update: Screen young asymptomatic women for chlamydia and gonorrhea
But evidence for screening men remains insufficient, task force says
The U.S. Preventive Services Task Force has updated its 2014 statement on screening asymptomatic individuals for chlamydia and gonorrhea infection.
Published online in JAMA, the 2021 version recommends that all sexually active women aged 24 years or younger and at-risk women 25 years or older should be screened for chlamydia and gonorrhea.
As in 2014, the task force made no screening recommendation for men owing to inconclusive evidence of benefit.
With cases of sexually transmitted infections reaching all-time highs, Amy G. Cantor, MD, MPH, of the Pacific Northwest Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues noted that chlamydia and gonorrhea are among the most common STIs in this country. According to the Centers for Disease Control and Prevention, 2019 saw approximately 1.8 million reported cases of chlamydia and more than 600,000 of gonorrhea.
In the current analysis of 27 observational and randomized studies comprising 179,515 patients, the USPSTF panel found that, compared with no screening, chlamydia screening was significantly associated with a reduced risk of pelvic inflammatory disease (PID) in young women in 2 out of 4 trials.
The authors cautioned, however, that the magnitude of benefit was relatively small. No studies reported on screening effectiveness in men, except for one reporting rates of epididymitis, and no studies were done on pregnant women for any outcome.
The largest and newest study, the Australian Chlamydia Control Effectiveness Pilot trial of 2018, assessed chlamydia screening against usual care in 180,355 men and women aged 16-29 years in 130 rural Australian primary care clinics. Screening was associated with a reduced risk of hospital-diagnosed PID: the absolute risk was 0.24% for screening versus 0.38% for usual care (unadjusted risk ratio, 0.6; 95% confidence interval, 0.4-1.0). It was not, however, significantly associated with a reduced risk of clinic-diagnosed PID, with an absolute risk of 0.45% versus 0.39% (RR, 1.1; 95% CI, 0.7-18). Nor did it correlate with a risk reduction for clinic-diagnosed epididymitis: 0.26% vs. 0.27% (RR, 0.9; 95% CI, 0.6-1.4).
While risk prediction criteria apart from age were only minimally accurate, testing for asymptomatic chlamydial and gonococcal infections was highly accurate at most anatomical sites, including urine and self-collected specimens, the investigators observed. Age 22 years or younger alone versus multi-item risk criteria demonstrated similar discrimination in a study that included symptomatic and asymptomatic women.
Sensitivity of chlamydial testing was similar at endocervical (89%-100%) and self- and clinician-collected vaginal (90%-100%) sites for women and at meatal (100%), urethral (99%), and rectal (92%) sites for men. It was lower, however, at pharyngeal sites (69.2%) for men who have sex with men (MSM).
Sensitivity of gonococcal testing was 89% or greater for all anatomical samples. False-positive and false-negative testing rates were low across anatomical sites and collection methods.
“Effectiveness of screening in men and during pregnancy, optimal screening intervals, and adverse effects of screening require further evaluation, Dr. Cantor and associates concluded.
In an accompanying editorial, Jeanne Marrazzo, MD, MPH, and Jodie Dionne-Odom, MD, MSPH, of the division of infectious diseases at the University of Alabama at Birmingham, called the guidelines “timely” and “powerful agents of change” that “influence a wide spectrum of health-based metrics, from quality assurance measures to criteria for financial reimbursement.”
They pointed out that men who have sex with men are experiencing historically high rates of gonorrhea, with most infections occurring extragenitally at the pharynx or rectum. In 2019 CDC data, MSM had substantially higher rates of gonorrhea than men who had sex only with women. They recommended that guidelines for men consider STI risk because of sexual relations with men, women, or both.
“Comprehensive screening guidelines for common STIs like chlamydia and gonorrhea could incorporate the limited evidence base for MSM, whether it is regular practice or not,” they wrote, with the same approach for women who have sex with women but may be at risk for chlamydia, particularly if they also have sex with men.
In their view, these latest guidelines appropriately prioritize high-level clinically based data. They pointed, however, to recent progress in understanding the pathogenesis of upper reproductive tract infection in women and the sexual networks behind the current resurgence of STIs in the United States in the failure to manage exposed sex partners.
“Considering these critical advances in the evolution of clinic-based screening guidelines is a work in progress,” they wrote, “the dialogue among basic scientists, clinical trial investigators, and public health professionals to inform the next version of updated USPSTF chlamydia and gonorrhea screening guidelines should start now.”
In the opinion of Jennifer L. Reed, MD, MS, a professor of pediatrics and an emergency medicine physician at Cincinnati Children’s Hospital Medical Center and not involved in the updated statement, the recommendations are very reasonable. “The highest rates of infection occur in females 15-24 years of age, and therefore asymptomatic screening for chlamydia and gonorrhea is imperative at least annually or more often if they are high risk,” she said in an interview.
“I would hope that providers increase their asymptomatic screening as a result of these recommendations and highly consider it in the younger men,” Dr. Reed added. “I see a very high rate of gonorrhea and chlamydia infections.” Her center is studying the implementation of gonorrhea and chlamydia asymptomatic screening for adolescents in the pediatric emergency department, a high-risk patient population that will benefit from STI screening opportunities in nontraditional settings.
This research was funded by the Agency for Healthcare Research and Quality and the Department of Health & Human Services under a contract to support the USPSTF. One statement coauthor reported personal fees from Insmed, Paratek, RedHill, and Spero, as well as grants from Insmed. No other disclosures were reported. Dr. Dionne-Odom reported grants from the National Institutes of Health/National Institute of Child Health and Development. Dr. Reed reported a grant from NIH/NICHD for a pragmatic trial of improving STI detection in the pediatric ED.
But evidence for screening men remains insufficient, task force says
But evidence for screening men remains insufficient, task force says
The U.S. Preventive Services Task Force has updated its 2014 statement on screening asymptomatic individuals for chlamydia and gonorrhea infection.
Published online in JAMA, the 2021 version recommends that all sexually active women aged 24 years or younger and at-risk women 25 years or older should be screened for chlamydia and gonorrhea.
As in 2014, the task force made no screening recommendation for men owing to inconclusive evidence of benefit.
With cases of sexually transmitted infections reaching all-time highs, Amy G. Cantor, MD, MPH, of the Pacific Northwest Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues noted that chlamydia and gonorrhea are among the most common STIs in this country. According to the Centers for Disease Control and Prevention, 2019 saw approximately 1.8 million reported cases of chlamydia and more than 600,000 of gonorrhea.
In the current analysis of 27 observational and randomized studies comprising 179,515 patients, the USPSTF panel found that, compared with no screening, chlamydia screening was significantly associated with a reduced risk of pelvic inflammatory disease (PID) in young women in 2 out of 4 trials.
The authors cautioned, however, that the magnitude of benefit was relatively small. No studies reported on screening effectiveness in men, except for one reporting rates of epididymitis, and no studies were done on pregnant women for any outcome.
The largest and newest study, the Australian Chlamydia Control Effectiveness Pilot trial of 2018, assessed chlamydia screening against usual care in 180,355 men and women aged 16-29 years in 130 rural Australian primary care clinics. Screening was associated with a reduced risk of hospital-diagnosed PID: the absolute risk was 0.24% for screening versus 0.38% for usual care (unadjusted risk ratio, 0.6; 95% confidence interval, 0.4-1.0). It was not, however, significantly associated with a reduced risk of clinic-diagnosed PID, with an absolute risk of 0.45% versus 0.39% (RR, 1.1; 95% CI, 0.7-18). Nor did it correlate with a risk reduction for clinic-diagnosed epididymitis: 0.26% vs. 0.27% (RR, 0.9; 95% CI, 0.6-1.4).
While risk prediction criteria apart from age were only minimally accurate, testing for asymptomatic chlamydial and gonococcal infections was highly accurate at most anatomical sites, including urine and self-collected specimens, the investigators observed. Age 22 years or younger alone versus multi-item risk criteria demonstrated similar discrimination in a study that included symptomatic and asymptomatic women.
Sensitivity of chlamydial testing was similar at endocervical (89%-100%) and self- and clinician-collected vaginal (90%-100%) sites for women and at meatal (100%), urethral (99%), and rectal (92%) sites for men. It was lower, however, at pharyngeal sites (69.2%) for men who have sex with men (MSM).
Sensitivity of gonococcal testing was 89% or greater for all anatomical samples. False-positive and false-negative testing rates were low across anatomical sites and collection methods.
“Effectiveness of screening in men and during pregnancy, optimal screening intervals, and adverse effects of screening require further evaluation, Dr. Cantor and associates concluded.
In an accompanying editorial, Jeanne Marrazzo, MD, MPH, and Jodie Dionne-Odom, MD, MSPH, of the division of infectious diseases at the University of Alabama at Birmingham, called the guidelines “timely” and “powerful agents of change” that “influence a wide spectrum of health-based metrics, from quality assurance measures to criteria for financial reimbursement.”
They pointed out that men who have sex with men are experiencing historically high rates of gonorrhea, with most infections occurring extragenitally at the pharynx or rectum. In 2019 CDC data, MSM had substantially higher rates of gonorrhea than men who had sex only with women. They recommended that guidelines for men consider STI risk because of sexual relations with men, women, or both.
“Comprehensive screening guidelines for common STIs like chlamydia and gonorrhea could incorporate the limited evidence base for MSM, whether it is regular practice or not,” they wrote, with the same approach for women who have sex with women but may be at risk for chlamydia, particularly if they also have sex with men.
In their view, these latest guidelines appropriately prioritize high-level clinically based data. They pointed, however, to recent progress in understanding the pathogenesis of upper reproductive tract infection in women and the sexual networks behind the current resurgence of STIs in the United States in the failure to manage exposed sex partners.
“Considering these critical advances in the evolution of clinic-based screening guidelines is a work in progress,” they wrote, “the dialogue among basic scientists, clinical trial investigators, and public health professionals to inform the next version of updated USPSTF chlamydia and gonorrhea screening guidelines should start now.”
In the opinion of Jennifer L. Reed, MD, MS, a professor of pediatrics and an emergency medicine physician at Cincinnati Children’s Hospital Medical Center and not involved in the updated statement, the recommendations are very reasonable. “The highest rates of infection occur in females 15-24 years of age, and therefore asymptomatic screening for chlamydia and gonorrhea is imperative at least annually or more often if they are high risk,” she said in an interview.
“I would hope that providers increase their asymptomatic screening as a result of these recommendations and highly consider it in the younger men,” Dr. Reed added. “I see a very high rate of gonorrhea and chlamydia infections.” Her center is studying the implementation of gonorrhea and chlamydia asymptomatic screening for adolescents in the pediatric emergency department, a high-risk patient population that will benefit from STI screening opportunities in nontraditional settings.
This research was funded by the Agency for Healthcare Research and Quality and the Department of Health & Human Services under a contract to support the USPSTF. One statement coauthor reported personal fees from Insmed, Paratek, RedHill, and Spero, as well as grants from Insmed. No other disclosures were reported. Dr. Dionne-Odom reported grants from the National Institutes of Health/National Institute of Child Health and Development. Dr. Reed reported a grant from NIH/NICHD for a pragmatic trial of improving STI detection in the pediatric ED.
The U.S. Preventive Services Task Force has updated its 2014 statement on screening asymptomatic individuals for chlamydia and gonorrhea infection.
Published online in JAMA, the 2021 version recommends that all sexually active women aged 24 years or younger and at-risk women 25 years or older should be screened for chlamydia and gonorrhea.
As in 2014, the task force made no screening recommendation for men owing to inconclusive evidence of benefit.
With cases of sexually transmitted infections reaching all-time highs, Amy G. Cantor, MD, MPH, of the Pacific Northwest Evidence-based Practice Center at Oregon Health & Science University, Portland, and colleagues noted that chlamydia and gonorrhea are among the most common STIs in this country. According to the Centers for Disease Control and Prevention, 2019 saw approximately 1.8 million reported cases of chlamydia and more than 600,000 of gonorrhea.
In the current analysis of 27 observational and randomized studies comprising 179,515 patients, the USPSTF panel found that, compared with no screening, chlamydia screening was significantly associated with a reduced risk of pelvic inflammatory disease (PID) in young women in 2 out of 4 trials.
The authors cautioned, however, that the magnitude of benefit was relatively small. No studies reported on screening effectiveness in men, except for one reporting rates of epididymitis, and no studies were done on pregnant women for any outcome.
The largest and newest study, the Australian Chlamydia Control Effectiveness Pilot trial of 2018, assessed chlamydia screening against usual care in 180,355 men and women aged 16-29 years in 130 rural Australian primary care clinics. Screening was associated with a reduced risk of hospital-diagnosed PID: the absolute risk was 0.24% for screening versus 0.38% for usual care (unadjusted risk ratio, 0.6; 95% confidence interval, 0.4-1.0). It was not, however, significantly associated with a reduced risk of clinic-diagnosed PID, with an absolute risk of 0.45% versus 0.39% (RR, 1.1; 95% CI, 0.7-18). Nor did it correlate with a risk reduction for clinic-diagnosed epididymitis: 0.26% vs. 0.27% (RR, 0.9; 95% CI, 0.6-1.4).
While risk prediction criteria apart from age were only minimally accurate, testing for asymptomatic chlamydial and gonococcal infections was highly accurate at most anatomical sites, including urine and self-collected specimens, the investigators observed. Age 22 years or younger alone versus multi-item risk criteria demonstrated similar discrimination in a study that included symptomatic and asymptomatic women.
Sensitivity of chlamydial testing was similar at endocervical (89%-100%) and self- and clinician-collected vaginal (90%-100%) sites for women and at meatal (100%), urethral (99%), and rectal (92%) sites for men. It was lower, however, at pharyngeal sites (69.2%) for men who have sex with men (MSM).
Sensitivity of gonococcal testing was 89% or greater for all anatomical samples. False-positive and false-negative testing rates were low across anatomical sites and collection methods.
“Effectiveness of screening in men and during pregnancy, optimal screening intervals, and adverse effects of screening require further evaluation, Dr. Cantor and associates concluded.
In an accompanying editorial, Jeanne Marrazzo, MD, MPH, and Jodie Dionne-Odom, MD, MSPH, of the division of infectious diseases at the University of Alabama at Birmingham, called the guidelines “timely” and “powerful agents of change” that “influence a wide spectrum of health-based metrics, from quality assurance measures to criteria for financial reimbursement.”
They pointed out that men who have sex with men are experiencing historically high rates of gonorrhea, with most infections occurring extragenitally at the pharynx or rectum. In 2019 CDC data, MSM had substantially higher rates of gonorrhea than men who had sex only with women. They recommended that guidelines for men consider STI risk because of sexual relations with men, women, or both.
“Comprehensive screening guidelines for common STIs like chlamydia and gonorrhea could incorporate the limited evidence base for MSM, whether it is regular practice or not,” they wrote, with the same approach for women who have sex with women but may be at risk for chlamydia, particularly if they also have sex with men.
In their view, these latest guidelines appropriately prioritize high-level clinically based data. They pointed, however, to recent progress in understanding the pathogenesis of upper reproductive tract infection in women and the sexual networks behind the current resurgence of STIs in the United States in the failure to manage exposed sex partners.
“Considering these critical advances in the evolution of clinic-based screening guidelines is a work in progress,” they wrote, “the dialogue among basic scientists, clinical trial investigators, and public health professionals to inform the next version of updated USPSTF chlamydia and gonorrhea screening guidelines should start now.”
In the opinion of Jennifer L. Reed, MD, MS, a professor of pediatrics and an emergency medicine physician at Cincinnati Children’s Hospital Medical Center and not involved in the updated statement, the recommendations are very reasonable. “The highest rates of infection occur in females 15-24 years of age, and therefore asymptomatic screening for chlamydia and gonorrhea is imperative at least annually or more often if they are high risk,” she said in an interview.
“I would hope that providers increase their asymptomatic screening as a result of these recommendations and highly consider it in the younger men,” Dr. Reed added. “I see a very high rate of gonorrhea and chlamydia infections.” Her center is studying the implementation of gonorrhea and chlamydia asymptomatic screening for adolescents in the pediatric emergency department, a high-risk patient population that will benefit from STI screening opportunities in nontraditional settings.
This research was funded by the Agency for Healthcare Research and Quality and the Department of Health & Human Services under a contract to support the USPSTF. One statement coauthor reported personal fees from Insmed, Paratek, RedHill, and Spero, as well as grants from Insmed. No other disclosures were reported. Dr. Dionne-Odom reported grants from the National Institutes of Health/National Institute of Child Health and Development. Dr. Reed reported a grant from NIH/NICHD for a pragmatic trial of improving STI detection in the pediatric ED.
FROM JAMA
Study: Use urine sampling more broadly to rule out pediatric UTI
of diagnostic test accuracy studies in ambulatory care (Ann Fam Med 2021;19:437-46).
“Urine sampling is often restricted to children with clinical features such as pain while urinating, frequent urination or children presenting with fever without any abnormalities found on clinical examination,” said lead author Jan Y. Verbakel, MD, PhD, from the University of Leuven (Belgium) in an interview. “Our study findings suggest that, in children, pain while urinating or frequent urination are less accurate than in adults and increase the probability of UTI only moderately.”
Urine sampling “should be applied more broadly in ambulatory care, given that appropriate sampling techniques are available,” he and his coauthors advised in the paper.
Methods and results
The analysis included 35 studies, involving a total of 78,427 patients, which provided information on 58 clinical features and 6 prediction rules of UTI, compared with urine culture. For urine sampling, most studies used catheterization (n = 23), suprapubic aspiration (n = 17), or midstream catch (n = 14), and fewer studies used clean catch (n = 7), bag specimens (n = 5), or diaper pads (n = 2).
The study showed that only three features substantially decreased the likelihood of UTI: being circumcised, the presence of stridor, and the presence of diaper rash. “In febrile children, finding an apparent source of infection decreased the probability of UTI; however, this was not useful for ruling out UTI by itself,” the authors noted.
Additionally, they found that red flags for UTI were cloudy or malodorous urine, hematuria, no fluid intake, suprapubic tenderness, and loin tenderness.
Study implications
“We recommend to sample urine in children that have one or more features that increase the probability of UTI … and less so pain while urinating, frequent urination, urgency, bed wetting, or previous UTI history,” said Dr. Verbakel, who is also a researcher at the University of Oxford (England).
In terms of prediction rules, the analysis showed the Diagnosis of Urinary Tract Infection in Young Children (DUTY) score, Gorelick Scale score, and UTIcalc might be useful to identify which children should have urine sampling, the authors stated in the paper.
Specifically, a DUTY clean-catch score of less than one point was useful for ruling out UTI in children aged less than 5 years, and in girls aged less than 3 years with unexplained fever. The Gorelick Scale score was useful for ruling out UTI when less than two of five variables were present.
“The present meta-analyses confirm that few clinical features are useful for diagnosing or ruling out UTI without further urine analysis. Signs and symptoms combined in a clinical prediction rule, such as with the DUTY or UTIcalc score, might increase accuracy for ruling out UTI; however, these should be validated externally,” Dr. Verbakel said in an interview.
Is urine sampling guideline too broad?
Commenting on the new paper, Martin Koyle, MD, former division chief of urology at the Hospital for Sick Children and professor of surgery at the University of Toronto, expressed concern that unexplained fever is not included as a “differentiating” red flag.
“Many contemporary guidelines define fever as an important diagnostic symptom, as the goal truly is to differentiate lower urinary tract from actual kidney infection, the latter thought to be more important for severity of illness, and potential for developing kidney damage,” he said in an interview. “It begs the question as to which nonfebrile patients who don’t have symptoms related to the respiratory tract for instance [for example, stridor], should be under suspicion for an afebrile urinary tract infection, and have their urine sampled. This paper does not answer that question.”
Dr. Koyle added that an overly broad guideline for urine sampling could come at a cost, and he raised the following questions.
“Will there be an overdiagnosis based on urines alone? Will this lead to overtreatment, often unnecessary, just because there is a positive urine specimen or asymptomatic bacteriuria? Will overtreatment lead to resistant bacteria and side effects related to antibiotics? Will such treatment actually prevent clinical illness and/or renal damage?”
The study authors and Dr. Koyle reported no conflicts of interest.
of diagnostic test accuracy studies in ambulatory care (Ann Fam Med 2021;19:437-46).
“Urine sampling is often restricted to children with clinical features such as pain while urinating, frequent urination or children presenting with fever without any abnormalities found on clinical examination,” said lead author Jan Y. Verbakel, MD, PhD, from the University of Leuven (Belgium) in an interview. “Our study findings suggest that, in children, pain while urinating or frequent urination are less accurate than in adults and increase the probability of UTI only moderately.”
Urine sampling “should be applied more broadly in ambulatory care, given that appropriate sampling techniques are available,” he and his coauthors advised in the paper.
Methods and results
The analysis included 35 studies, involving a total of 78,427 patients, which provided information on 58 clinical features and 6 prediction rules of UTI, compared with urine culture. For urine sampling, most studies used catheterization (n = 23), suprapubic aspiration (n = 17), or midstream catch (n = 14), and fewer studies used clean catch (n = 7), bag specimens (n = 5), or diaper pads (n = 2).
The study showed that only three features substantially decreased the likelihood of UTI: being circumcised, the presence of stridor, and the presence of diaper rash. “In febrile children, finding an apparent source of infection decreased the probability of UTI; however, this was not useful for ruling out UTI by itself,” the authors noted.
Additionally, they found that red flags for UTI were cloudy or malodorous urine, hematuria, no fluid intake, suprapubic tenderness, and loin tenderness.
Study implications
“We recommend to sample urine in children that have one or more features that increase the probability of UTI … and less so pain while urinating, frequent urination, urgency, bed wetting, or previous UTI history,” said Dr. Verbakel, who is also a researcher at the University of Oxford (England).
In terms of prediction rules, the analysis showed the Diagnosis of Urinary Tract Infection in Young Children (DUTY) score, Gorelick Scale score, and UTIcalc might be useful to identify which children should have urine sampling, the authors stated in the paper.
Specifically, a DUTY clean-catch score of less than one point was useful for ruling out UTI in children aged less than 5 years, and in girls aged less than 3 years with unexplained fever. The Gorelick Scale score was useful for ruling out UTI when less than two of five variables were present.
“The present meta-analyses confirm that few clinical features are useful for diagnosing or ruling out UTI without further urine analysis. Signs and symptoms combined in a clinical prediction rule, such as with the DUTY or UTIcalc score, might increase accuracy for ruling out UTI; however, these should be validated externally,” Dr. Verbakel said in an interview.
Is urine sampling guideline too broad?
Commenting on the new paper, Martin Koyle, MD, former division chief of urology at the Hospital for Sick Children and professor of surgery at the University of Toronto, expressed concern that unexplained fever is not included as a “differentiating” red flag.
“Many contemporary guidelines define fever as an important diagnostic symptom, as the goal truly is to differentiate lower urinary tract from actual kidney infection, the latter thought to be more important for severity of illness, and potential for developing kidney damage,” he said in an interview. “It begs the question as to which nonfebrile patients who don’t have symptoms related to the respiratory tract for instance [for example, stridor], should be under suspicion for an afebrile urinary tract infection, and have their urine sampled. This paper does not answer that question.”
Dr. Koyle added that an overly broad guideline for urine sampling could come at a cost, and he raised the following questions.
“Will there be an overdiagnosis based on urines alone? Will this lead to overtreatment, often unnecessary, just because there is a positive urine specimen or asymptomatic bacteriuria? Will overtreatment lead to resistant bacteria and side effects related to antibiotics? Will such treatment actually prevent clinical illness and/or renal damage?”
The study authors and Dr. Koyle reported no conflicts of interest.
of diagnostic test accuracy studies in ambulatory care (Ann Fam Med 2021;19:437-46).
“Urine sampling is often restricted to children with clinical features such as pain while urinating, frequent urination or children presenting with fever without any abnormalities found on clinical examination,” said lead author Jan Y. Verbakel, MD, PhD, from the University of Leuven (Belgium) in an interview. “Our study findings suggest that, in children, pain while urinating or frequent urination are less accurate than in adults and increase the probability of UTI only moderately.”
Urine sampling “should be applied more broadly in ambulatory care, given that appropriate sampling techniques are available,” he and his coauthors advised in the paper.
Methods and results
The analysis included 35 studies, involving a total of 78,427 patients, which provided information on 58 clinical features and 6 prediction rules of UTI, compared with urine culture. For urine sampling, most studies used catheterization (n = 23), suprapubic aspiration (n = 17), or midstream catch (n = 14), and fewer studies used clean catch (n = 7), bag specimens (n = 5), or diaper pads (n = 2).
The study showed that only three features substantially decreased the likelihood of UTI: being circumcised, the presence of stridor, and the presence of diaper rash. “In febrile children, finding an apparent source of infection decreased the probability of UTI; however, this was not useful for ruling out UTI by itself,” the authors noted.
Additionally, they found that red flags for UTI were cloudy or malodorous urine, hematuria, no fluid intake, suprapubic tenderness, and loin tenderness.
Study implications
“We recommend to sample urine in children that have one or more features that increase the probability of UTI … and less so pain while urinating, frequent urination, urgency, bed wetting, or previous UTI history,” said Dr. Verbakel, who is also a researcher at the University of Oxford (England).
In terms of prediction rules, the analysis showed the Diagnosis of Urinary Tract Infection in Young Children (DUTY) score, Gorelick Scale score, and UTIcalc might be useful to identify which children should have urine sampling, the authors stated in the paper.
Specifically, a DUTY clean-catch score of less than one point was useful for ruling out UTI in children aged less than 5 years, and in girls aged less than 3 years with unexplained fever. The Gorelick Scale score was useful for ruling out UTI when less than two of five variables were present.
“The present meta-analyses confirm that few clinical features are useful for diagnosing or ruling out UTI without further urine analysis. Signs and symptoms combined in a clinical prediction rule, such as with the DUTY or UTIcalc score, might increase accuracy for ruling out UTI; however, these should be validated externally,” Dr. Verbakel said in an interview.
Is urine sampling guideline too broad?
Commenting on the new paper, Martin Koyle, MD, former division chief of urology at the Hospital for Sick Children and professor of surgery at the University of Toronto, expressed concern that unexplained fever is not included as a “differentiating” red flag.
“Many contemporary guidelines define fever as an important diagnostic symptom, as the goal truly is to differentiate lower urinary tract from actual kidney infection, the latter thought to be more important for severity of illness, and potential for developing kidney damage,” he said in an interview. “It begs the question as to which nonfebrile patients who don’t have symptoms related to the respiratory tract for instance [for example, stridor], should be under suspicion for an afebrile urinary tract infection, and have their urine sampled. This paper does not answer that question.”
Dr. Koyle added that an overly broad guideline for urine sampling could come at a cost, and he raised the following questions.
“Will there be an overdiagnosis based on urines alone? Will this lead to overtreatment, often unnecessary, just because there is a positive urine specimen or asymptomatic bacteriuria? Will overtreatment lead to resistant bacteria and side effects related to antibiotics? Will such treatment actually prevent clinical illness and/or renal damage?”
The study authors and Dr. Koyle reported no conflicts of interest.