User login
Q&A: Meeting the challenge of giving COVID vaccines to younger kids
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.
This news organization spoke to several pediatric experts to get answers.
More than 6 million children and adolescents (up to age 18 years) in the United States have been infected with SARS-CoV-2. Children represent about 17% of all cases, and an estimated 0.1%-2% of infected children end up hospitalized, according to Oct. 28 data from the American Academy of Pediatrics.
Physicians and other health care practitioners are gearing up for what could be an influx of patients. “Pediatricians are standing by to talk with families about the vaccine and to administer the vaccine to children as soon as possible,” Lee Savio Beers, MD, FAAP, president of the AAP, said in a statement.
In this Q&A, this news organization asked for additional advice from Sara “Sally” Goza, MD, a pediatrician in Fayetteville, Georgia, and immediate past president of the AAP; Peter Hotez, MD, PhD, dean of the National School of Tropical Medicine at Baylor College of Medicine and codirector of the Texas Children’s Hospital Center for Vaccine Development, both in Houston; and Danielle M. Zerr, MD, professor and chief of the division of pediatric infectious disease at the University of Washington, Seattle, and medical director of infection prevention at Seattle Children’s Hospital.
Q: How are smaller pediatric practices and solo practitioners going to handle the additional vaccinations?
Dr. Goza: It’s a scheduling challenge with this rollout and all the people who want it and want it right now. They’re going to want it this week.
I’ve actually had some children asking their moms: “When can I get it? When can I get it?” It’s been very interesting – they are chomping at the bit.
If I give the vaccine to a patient this week, in 3 weeks the second dose will be right around Thanksgiving. No one in my office is going to want to be here to give the shot on Thanksgiving, and no patient is going to want to come in on Thanksgiving weekend. So I’m trying to delay those parents – saying, let’s do it next week. That way we’re not messing up a holiday.
Children are going to need two doses, and they won’t be fully protected until 2 weeks after their second dose. So they won’t get full protection for Thanksgiving, but they will have full protection for Christmas.
I know there are a lot of pediatricians who have preordered the vaccine. I know in our office they sent us an email ... to let us know our vaccines are being shipped. So I think a lot of pediatricians are going to have the vaccine.
Q: How should pediatricians counsel parents who are fearful or hesitant?
Dr. Hotez: It’s important to emphasize the severity of the 2021 summer Delta epidemic in children. We need to get beyond this false narrative that COVID only produces a mild disease in children. It’s caused thousands of pediatric hospitalizations, not to mention long COVID.
Dr. Zerr: It is key to find out what concerns parents have and then focus on answering their specific questions. It is helpful to emphasize the safety and efficacy of the vaccine and to explain the rigorous processes that the vaccine went through to receive Food and Drug Administration approval.
Q: How should pediatricians counter any misinformation/disinformation out there about the COVID-19 vaccines?
Dr. Goza: The most important thing is not to discount what they are saying. Don’t say: “That’s crazy” or “That’s not true.” Don’t roll your eyes and say: “Really, you’re going to believe all that?”
Instead, have a conversation with them about why we think that is not true, or why we know that’s not true. We really have to have that relationship and ask: “Well, what are your concerns?” And then really counter (any misinformation) with facts, with science, and based on your experience.
Q: Do the data presented to the FDA and the CDC about the safety and effectiveness of the COVID-19 vaccine for 5- to 11-year-olds seem robust to you?
Dr. Zerr: Yes, and data collection will be ongoing.
Dr. Hotez: I’ve only seen what’s publicly available so far, and it seems to support moving forward with emergency use authorization. The only shortfall is the size, roughly 2,200 children, which would not be of sufficient size to detect a rare safety signal.
Q: Do previous controversies around pediatric vaccines (for example, the MMR vaccine and autism) give pediatricians some background and experience so they can address any pushback on the COVID-19 vaccines?
Dr. Goza: Pediatricians have been dealing with vaccine hesitancy for a while now, ever since the MMR and autism controversy started. Even before then, there were certain groups of people who didn’t want vaccines.
We’ve really worked hard at helping teach pediatricians how to deal with the misinformation, how to counter it, and how to help parents understand the vaccines are safe and effective – and that they save lives.
That (experience) will help us in some ways. Unfortunately, there is more misinformation out there – there is almost a concerted effort on misinformation. It’s big.
Pediatricians will do everything we can, but we need help countering it. We need the misinformation to quit getting spread on social media. We can talk one on one with patients and families, but if all they are hearing on social media is the misinformation, it’s really hard.
Q: Are pediatricians, especially solo practitioners or pediatricians at smaller practices, going to face challenges with multidose vials and not wasting vaccine product?
Dr. Goza: I’m at a small practice. We have 3.5 FTEs (full-time equivalents) of MDs and three FTEs of nurse practitioners. So we’re not that big – about six providers.
You know, it is a challenge. We’re not going to buy the super-duper freezer, and we’re not going to be able to store these vaccines for a long period of time.
So when we order, we need smaller amounts. For the 12- to 18-year-olds, [maximum storage] was 45 days. Now for the 5- to 11-year-olds, we’re going to be able to store the vaccine in the refrigerator for 10 weeks, which gives us more leeway there.
We try to do all of vaccinations on 1 day, so we know how many people are coming in, and we are not going to waste too many doses.
Our Department of Public Health in Georgia has said: “We want these vaccines in the arms of kids, and if you have to waste some doses, don’t worry about it.” But it’s a 10-dose vial. It’s going to be hard for me to open it up for one child. I just don’t like wasting anything like this.
Our main goal is to get this vaccine in to the arms of children whose parents want it.
Q: What are some additional sources of information for pediatricians?
Dr. Zerr: There are a lot of great resources on vaccine hesitancy from reputable sources, including these from the CDC and from the National Academies of Sciences, Engineering, and Medicine:
- Building Confidence With OVID-19 Vaccines
- How to Talk With Parents About COVID-19 Vaccination
- Strategies for Building Confidence in the COVID-19 Vaccines
- Communication Strategies for Building Confidence in COVID-19 Vaccines: Addressing Variants and Childhood Vaccinations
A version of this article first appeared on Medscape.com.
Can adjunct corticosteroids help in childrens’ eye and throat infections?
Adding anti-inflammatory corticosteroids to antibiotics for certain pediatric throat and ocular infections may have some benefit, according to results from two recent database studies, but their benefit remains unclear.
Using steroids in this setting is a practice many pediatricians consider, although no clear guidance exists.
Drawing on data from a registry of 51 free-standing children’s hospitals in the Pediatric Health Information System (PHIS), and published online in Pediatrics, the analyses looked, respectively, at retro- and parapharyngeal abscesses (RPAs/PPAs) and acute orbital cellulitis.
Throat abscesses
In the first study, pediatrician Pratichi K. Goenka, MD, of Cohen Children’s Medical Center–Northwell Health and an assistant professor at Hofstra University, both in New Hyde Park, N.Y., and colleagues reported on the effect of systemic corticosteroids on several outcomes in RPAs/PPAs in 2,259 well-matched patients. The patients, aged 2 months to 8 years, were treated at 46 hospitals during the period from January 2016 to December 2019.
The data revealed that the 582 (25.8%) who received steroids had a significantly lower rate of surgical drainage, the study’s primary endpoint (odds ratio, 0.28; 95% confidence interval, 0.22-0.36). There was no difference, however, in length of hospital stay (rate ratio, 0.97; 95% CI, 0.92-1.02).
Those in the steroid group also had lower overall hospital costs and were less likely to be given opioid medications for pain. They were, however, more likely to undergo repeat CT imaging and also had a higher 7-day ED revisit rate but no increase in readmission 30 days after discharge: 4% versus 3% in the nonsteroid group (P = .29).
“As hospitalists, we share the care of young children with RPAs and PPAs with our otolaryngology colleagues. The primary therapy is antibiotics but there was no clear guidance on the next step, and the current literature had no answers as to how systemic corticosteroids might impact the care of these children,” Dr. Goenka said in an interview. “So we wanted to leverage the PHIS data to better understand the association with the need for surgery and length of stay. Surgery is painful and often involves IV administration of opioid painkillers. It’s something we may be able to avoid if we can optimize medical treatment.”
Pending results from randomized trials, what immediate impact could these registry findings have? “We hope that physicians will think about the best initial medical treatment plan for these children,” Dr. Goenka said. ”Given these data, I would be more likely to incorporate steroids early on in medical treatment.”
She emphasized, however, that before routine adoption prospective studies are needed to clearly identify which patients will have a strong benefit and which will not benefit. “That is the nuanced discussion that will happen with more prospective work.”
Dr. Goenka and associates explained that the rising incidence of RPAs and PPAs over the past 20 years has been attributed to more cases of tonsillitis because of a shift away from tonsillectomies, as well as the changing epidemiology of methicillin-resistant Staphylococcus aureus.
In an accompanying editorial, Ellen R. Wald, MD, and Jens C. Eickhoff, PhD, of the University of Wisconsin–Madison, stated that the use of corticosteroids in bacterial meningitis is often cited as an example of the benefits of steroids in infection. “The specific rationale for use of corticosteroids is [their] anti-inflammatory effects, which may result in decreases of swelling and/ or edema to facilitate drainage, perfusion, reduction in pain, and healing.”
They cautioned, however, that the pharmacologic effects of steroids are myriad and complicated, and include potential masking of the clinical course of disease, thereby delaying appropriate therapy for unrecognized deterioration, as well potential immunosuppression.
Acute orbital cellulitis
In the second retrospective analysis, a group led by pediatrician Maria Anna Leszczynska, MD, of Johns Hopkins All Children’s Hospital in Baltimore, analyzed a retrospective PHIS cohort of 5,645 children younger than 18 years with a primary diagnosis of orbital cellulitis treated at 51 hospitals from January 2007 to December 2018.
Of these, 1,347 (24%) received steroids, but, contrary to earlier reports, the data showed no reduction in length of stay associated with these drugs after adjustment for age, meningitis, abscess, or vision issues (ebeta, 1.01; 95% CI, 0.97-1.06). Corticosteroid exposure was, however, associated with operative episodes after 2 days’ hospitalization (OR, 2.05; 95% CI, 1.29-3.27) and 30-day readmission (OR, 2.40; 95% CI, 1.52-3.78).
“Among children hospitalized for orbital cellulitis, we did not observe the reduction in LOS [length of stay] for patients prescribed systemic corticosteroids as described previously in the literature,” the authors wrote.
In terms of surgical procedures, 52.0% of corticosteroid recipients versus 14.0% of nonrecipients underwent surgery (P < .001), and more were hospitalized in the pediatric ICU (4.4% vs 2.6%; P < .001).
According to the editorialists: “Both observations suggest that children who received steroids may have been a sicker group of patients.”
Dr. Wald and Dr. Eickhoff pointed out that the effect of steroids is ultimately unclear because of the retrospective study’s inherent potential for bias because of unobserved confounders. Were steroids prescribed more often when children were perceived to be sicker with more severe disease, or did these medications cause worse outcomes?
The authors agreed that the study could not determine causality. “Although we used all available markers of disease severity, there does not exist a validated disease severity clinical score for pediatric orbital cellulitis,” they wrote.
According to Ricardo A. Quinonez, MD, an associate professor of pediatrics and division and service chief of pediatric hospital medicine at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, “orbital cellulitis is a not very common thing in children so we don’t treat many patients with this. But having said that, there is usually some debate among providers about whether to use steroids.”
Some centers use them routinely for central nervous system and eye infections or extensions of sinusitis, he said, but there is variability in the prescribing of corticosteroids. “There’s ongoing discussion as to whether they‘re as helpful in orbital cellulitis as they are in similar conditions,” Dr. Quinonez said in an interview. “At our institution we don’t typically prescribe them – not never but not routinely. Children who are sicker tend to get steroids, as they do in other conditions.”
In the context of PPA as in the first study, he added, “I think the evidence favoring the use of steroids in infections that affect the airway is stronger, and their use is definitely more prevalent in those instances.”
While both PHIS analyses suggested some benefit from steroids, he continued, some children may not benefit and there may be harms. “The evidence is still mostly retrospective and observational with no multicenter randomized controlled data. Without those data the evidence is difficult to interpret and subject to all the biases that observational and retrospective data is subject to and the current evidence should not lead physicians to change their practice until controlled, randomized evidence is available.”
The editorialists concurred with the study authors and Dr. Quinonez that large, controlled, prospective clinical trials are needed to ascertain the effect of steroids and to standardize the approach to diagnosis and management. “Use of administrative databases are not optimal to answer questions related to outcome,” they wrote.
The study by Dr. Goenka and associates received no external funding; the study by Dr. Leszczynska and associates also received no external funding. None of the authors declared potential competing interests. Dr. Quinonez had no competing interests to declare. Dr. Wald and Dr. Eickhoff disclosed no competing interests with regard to their editorial.
Adding anti-inflammatory corticosteroids to antibiotics for certain pediatric throat and ocular infections may have some benefit, according to results from two recent database studies, but their benefit remains unclear.
Using steroids in this setting is a practice many pediatricians consider, although no clear guidance exists.
Drawing on data from a registry of 51 free-standing children’s hospitals in the Pediatric Health Information System (PHIS), and published online in Pediatrics, the analyses looked, respectively, at retro- and parapharyngeal abscesses (RPAs/PPAs) and acute orbital cellulitis.
Throat abscesses
In the first study, pediatrician Pratichi K. Goenka, MD, of Cohen Children’s Medical Center–Northwell Health and an assistant professor at Hofstra University, both in New Hyde Park, N.Y., and colleagues reported on the effect of systemic corticosteroids on several outcomes in RPAs/PPAs in 2,259 well-matched patients. The patients, aged 2 months to 8 years, were treated at 46 hospitals during the period from January 2016 to December 2019.
The data revealed that the 582 (25.8%) who received steroids had a significantly lower rate of surgical drainage, the study’s primary endpoint (odds ratio, 0.28; 95% confidence interval, 0.22-0.36). There was no difference, however, in length of hospital stay (rate ratio, 0.97; 95% CI, 0.92-1.02).
Those in the steroid group also had lower overall hospital costs and were less likely to be given opioid medications for pain. They were, however, more likely to undergo repeat CT imaging and also had a higher 7-day ED revisit rate but no increase in readmission 30 days after discharge: 4% versus 3% in the nonsteroid group (P = .29).
“As hospitalists, we share the care of young children with RPAs and PPAs with our otolaryngology colleagues. The primary therapy is antibiotics but there was no clear guidance on the next step, and the current literature had no answers as to how systemic corticosteroids might impact the care of these children,” Dr. Goenka said in an interview. “So we wanted to leverage the PHIS data to better understand the association with the need for surgery and length of stay. Surgery is painful and often involves IV administration of opioid painkillers. It’s something we may be able to avoid if we can optimize medical treatment.”
Pending results from randomized trials, what immediate impact could these registry findings have? “We hope that physicians will think about the best initial medical treatment plan for these children,” Dr. Goenka said. ”Given these data, I would be more likely to incorporate steroids early on in medical treatment.”
She emphasized, however, that before routine adoption prospective studies are needed to clearly identify which patients will have a strong benefit and which will not benefit. “That is the nuanced discussion that will happen with more prospective work.”
Dr. Goenka and associates explained that the rising incidence of RPAs and PPAs over the past 20 years has been attributed to more cases of tonsillitis because of a shift away from tonsillectomies, as well as the changing epidemiology of methicillin-resistant Staphylococcus aureus.
In an accompanying editorial, Ellen R. Wald, MD, and Jens C. Eickhoff, PhD, of the University of Wisconsin–Madison, stated that the use of corticosteroids in bacterial meningitis is often cited as an example of the benefits of steroids in infection. “The specific rationale for use of corticosteroids is [their] anti-inflammatory effects, which may result in decreases of swelling and/ or edema to facilitate drainage, perfusion, reduction in pain, and healing.”
They cautioned, however, that the pharmacologic effects of steroids are myriad and complicated, and include potential masking of the clinical course of disease, thereby delaying appropriate therapy for unrecognized deterioration, as well potential immunosuppression.
Acute orbital cellulitis
In the second retrospective analysis, a group led by pediatrician Maria Anna Leszczynska, MD, of Johns Hopkins All Children’s Hospital in Baltimore, analyzed a retrospective PHIS cohort of 5,645 children younger than 18 years with a primary diagnosis of orbital cellulitis treated at 51 hospitals from January 2007 to December 2018.
Of these, 1,347 (24%) received steroids, but, contrary to earlier reports, the data showed no reduction in length of stay associated with these drugs after adjustment for age, meningitis, abscess, or vision issues (ebeta, 1.01; 95% CI, 0.97-1.06). Corticosteroid exposure was, however, associated with operative episodes after 2 days’ hospitalization (OR, 2.05; 95% CI, 1.29-3.27) and 30-day readmission (OR, 2.40; 95% CI, 1.52-3.78).
“Among children hospitalized for orbital cellulitis, we did not observe the reduction in LOS [length of stay] for patients prescribed systemic corticosteroids as described previously in the literature,” the authors wrote.
In terms of surgical procedures, 52.0% of corticosteroid recipients versus 14.0% of nonrecipients underwent surgery (P < .001), and more were hospitalized in the pediatric ICU (4.4% vs 2.6%; P < .001).
According to the editorialists: “Both observations suggest that children who received steroids may have been a sicker group of patients.”
Dr. Wald and Dr. Eickhoff pointed out that the effect of steroids is ultimately unclear because of the retrospective study’s inherent potential for bias because of unobserved confounders. Were steroids prescribed more often when children were perceived to be sicker with more severe disease, or did these medications cause worse outcomes?
The authors agreed that the study could not determine causality. “Although we used all available markers of disease severity, there does not exist a validated disease severity clinical score for pediatric orbital cellulitis,” they wrote.
According to Ricardo A. Quinonez, MD, an associate professor of pediatrics and division and service chief of pediatric hospital medicine at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, “orbital cellulitis is a not very common thing in children so we don’t treat many patients with this. But having said that, there is usually some debate among providers about whether to use steroids.”
Some centers use them routinely for central nervous system and eye infections or extensions of sinusitis, he said, but there is variability in the prescribing of corticosteroids. “There’s ongoing discussion as to whether they‘re as helpful in orbital cellulitis as they are in similar conditions,” Dr. Quinonez said in an interview. “At our institution we don’t typically prescribe them – not never but not routinely. Children who are sicker tend to get steroids, as they do in other conditions.”
In the context of PPA as in the first study, he added, “I think the evidence favoring the use of steroids in infections that affect the airway is stronger, and their use is definitely more prevalent in those instances.”
While both PHIS analyses suggested some benefit from steroids, he continued, some children may not benefit and there may be harms. “The evidence is still mostly retrospective and observational with no multicenter randomized controlled data. Without those data the evidence is difficult to interpret and subject to all the biases that observational and retrospective data is subject to and the current evidence should not lead physicians to change their practice until controlled, randomized evidence is available.”
The editorialists concurred with the study authors and Dr. Quinonez that large, controlled, prospective clinical trials are needed to ascertain the effect of steroids and to standardize the approach to diagnosis and management. “Use of administrative databases are not optimal to answer questions related to outcome,” they wrote.
The study by Dr. Goenka and associates received no external funding; the study by Dr. Leszczynska and associates also received no external funding. None of the authors declared potential competing interests. Dr. Quinonez had no competing interests to declare. Dr. Wald and Dr. Eickhoff disclosed no competing interests with regard to their editorial.
Adding anti-inflammatory corticosteroids to antibiotics for certain pediatric throat and ocular infections may have some benefit, according to results from two recent database studies, but their benefit remains unclear.
Using steroids in this setting is a practice many pediatricians consider, although no clear guidance exists.
Drawing on data from a registry of 51 free-standing children’s hospitals in the Pediatric Health Information System (PHIS), and published online in Pediatrics, the analyses looked, respectively, at retro- and parapharyngeal abscesses (RPAs/PPAs) and acute orbital cellulitis.
Throat abscesses
In the first study, pediatrician Pratichi K. Goenka, MD, of Cohen Children’s Medical Center–Northwell Health and an assistant professor at Hofstra University, both in New Hyde Park, N.Y., and colleagues reported on the effect of systemic corticosteroids on several outcomes in RPAs/PPAs in 2,259 well-matched patients. The patients, aged 2 months to 8 years, were treated at 46 hospitals during the period from January 2016 to December 2019.
The data revealed that the 582 (25.8%) who received steroids had a significantly lower rate of surgical drainage, the study’s primary endpoint (odds ratio, 0.28; 95% confidence interval, 0.22-0.36). There was no difference, however, in length of hospital stay (rate ratio, 0.97; 95% CI, 0.92-1.02).
Those in the steroid group also had lower overall hospital costs and were less likely to be given opioid medications for pain. They were, however, more likely to undergo repeat CT imaging and also had a higher 7-day ED revisit rate but no increase in readmission 30 days after discharge: 4% versus 3% in the nonsteroid group (P = .29).
“As hospitalists, we share the care of young children with RPAs and PPAs with our otolaryngology colleagues. The primary therapy is antibiotics but there was no clear guidance on the next step, and the current literature had no answers as to how systemic corticosteroids might impact the care of these children,” Dr. Goenka said in an interview. “So we wanted to leverage the PHIS data to better understand the association with the need for surgery and length of stay. Surgery is painful and often involves IV administration of opioid painkillers. It’s something we may be able to avoid if we can optimize medical treatment.”
Pending results from randomized trials, what immediate impact could these registry findings have? “We hope that physicians will think about the best initial medical treatment plan for these children,” Dr. Goenka said. ”Given these data, I would be more likely to incorporate steroids early on in medical treatment.”
She emphasized, however, that before routine adoption prospective studies are needed to clearly identify which patients will have a strong benefit and which will not benefit. “That is the nuanced discussion that will happen with more prospective work.”
Dr. Goenka and associates explained that the rising incidence of RPAs and PPAs over the past 20 years has been attributed to more cases of tonsillitis because of a shift away from tonsillectomies, as well as the changing epidemiology of methicillin-resistant Staphylococcus aureus.
In an accompanying editorial, Ellen R. Wald, MD, and Jens C. Eickhoff, PhD, of the University of Wisconsin–Madison, stated that the use of corticosteroids in bacterial meningitis is often cited as an example of the benefits of steroids in infection. “The specific rationale for use of corticosteroids is [their] anti-inflammatory effects, which may result in decreases of swelling and/ or edema to facilitate drainage, perfusion, reduction in pain, and healing.”
They cautioned, however, that the pharmacologic effects of steroids are myriad and complicated, and include potential masking of the clinical course of disease, thereby delaying appropriate therapy for unrecognized deterioration, as well potential immunosuppression.
Acute orbital cellulitis
In the second retrospective analysis, a group led by pediatrician Maria Anna Leszczynska, MD, of Johns Hopkins All Children’s Hospital in Baltimore, analyzed a retrospective PHIS cohort of 5,645 children younger than 18 years with a primary diagnosis of orbital cellulitis treated at 51 hospitals from January 2007 to December 2018.
Of these, 1,347 (24%) received steroids, but, contrary to earlier reports, the data showed no reduction in length of stay associated with these drugs after adjustment for age, meningitis, abscess, or vision issues (ebeta, 1.01; 95% CI, 0.97-1.06). Corticosteroid exposure was, however, associated with operative episodes after 2 days’ hospitalization (OR, 2.05; 95% CI, 1.29-3.27) and 30-day readmission (OR, 2.40; 95% CI, 1.52-3.78).
“Among children hospitalized for orbital cellulitis, we did not observe the reduction in LOS [length of stay] for patients prescribed systemic corticosteroids as described previously in the literature,” the authors wrote.
In terms of surgical procedures, 52.0% of corticosteroid recipients versus 14.0% of nonrecipients underwent surgery (P < .001), and more were hospitalized in the pediatric ICU (4.4% vs 2.6%; P < .001).
According to the editorialists: “Both observations suggest that children who received steroids may have been a sicker group of patients.”
Dr. Wald and Dr. Eickhoff pointed out that the effect of steroids is ultimately unclear because of the retrospective study’s inherent potential for bias because of unobserved confounders. Were steroids prescribed more often when children were perceived to be sicker with more severe disease, or did these medications cause worse outcomes?
The authors agreed that the study could not determine causality. “Although we used all available markers of disease severity, there does not exist a validated disease severity clinical score for pediatric orbital cellulitis,” they wrote.
According to Ricardo A. Quinonez, MD, an associate professor of pediatrics and division and service chief of pediatric hospital medicine at Baylor College of Medicine and Texas Children’s Hospital, both in Houston, “orbital cellulitis is a not very common thing in children so we don’t treat many patients with this. But having said that, there is usually some debate among providers about whether to use steroids.”
Some centers use them routinely for central nervous system and eye infections or extensions of sinusitis, he said, but there is variability in the prescribing of corticosteroids. “There’s ongoing discussion as to whether they‘re as helpful in orbital cellulitis as they are in similar conditions,” Dr. Quinonez said in an interview. “At our institution we don’t typically prescribe them – not never but not routinely. Children who are sicker tend to get steroids, as they do in other conditions.”
In the context of PPA as in the first study, he added, “I think the evidence favoring the use of steroids in infections that affect the airway is stronger, and their use is definitely more prevalent in those instances.”
While both PHIS analyses suggested some benefit from steroids, he continued, some children may not benefit and there may be harms. “The evidence is still mostly retrospective and observational with no multicenter randomized controlled data. Without those data the evidence is difficult to interpret and subject to all the biases that observational and retrospective data is subject to and the current evidence should not lead physicians to change their practice until controlled, randomized evidence is available.”
The editorialists concurred with the study authors and Dr. Quinonez that large, controlled, prospective clinical trials are needed to ascertain the effect of steroids and to standardize the approach to diagnosis and management. “Use of administrative databases are not optimal to answer questions related to outcome,” they wrote.
The study by Dr. Goenka and associates received no external funding; the study by Dr. Leszczynska and associates also received no external funding. None of the authors declared potential competing interests. Dr. Quinonez had no competing interests to declare. Dr. Wald and Dr. Eickhoff disclosed no competing interests with regard to their editorial.
FROM PEDIATRICS
HCV in pregnancy: One piece of a bigger problem
Mirroring the opioid crisis, maternal and newborn hepatitis C infections (HCV) more than doubled in the United States between 2009 and 2019, with disproportionate increases in people of White, American Indian, and Alaska Native race, especially those with less education, according to a cross-sectional study published in JAMA Health Forum. However, the level of risk within these populations was mitigated in counties with higher employment, reported Stephen W. Patrick, MD, of Vanderbilt University, in Nashville, Tenn., and coauthors.
“As we develop public health approaches to prevent HCV infections, connect to treatment, and monitor exposed infants, understanding these factors can be of critical importance to tailoring interventions,” Dr. Patrick said in an interview. “HCV is one more complication of the opioid crisis,” he added. “These data also enable us to step back a bit from HCV and look at the landscape of how the opioid crisis continues to grow in complexity and scope. Throughout the opioid crisis we have often failed to recognize and address the unique needs of pregnant people and infants.”
The study authors used data from the National Center for Health Statistics at the Centers for Disease Control and Prevention, and from the Area Health Resource File to examine maternal-infant HCV infection among all U.S. births between 2009 and 2019. The researchers also examined community-level risk factors including rurality, employment, and access to medical care.
In counties reporting HCV, there were 39,380,122 people who had live births, of whom 138,343 (0.4%) were diagnosed with HCV. The overall rate of maternal HCV infection increased from 1.8 to 5.1 per 1,000 live births between 2009 and 2019.
Infection rates were highest in American Indian/Alaska Native (AI/AN) and White people (adjusted odds ratio [aOR] 7.94 and 7.37, respectively) compared with Black people. They were higher among individuals without a 4-year degree compared to those with higher education (aOR, 3.19).
Among these groups considered to be at higher risk for HCV infection, high employment rates somewhat mitigated the risk. Specifically, in counties in the 10th percentile of employment, the predicted probability of HCV increased from 0.16% to 1.37%, between 2009 and 2019, whereas in counties at the 90th percentile of employment, the predicted probability remained similar, at 0.36% in 2009 and 0.48% in 2019.
“With constrained national resources, understanding both individual and community-level factors associated with HCV infections in pregnant people could inform strategies to mitigate its spread, such as harm reduction efforts (e.g., syringe service programs), improving access to treatment for [opioid use disorder] or increasing the obstetrical workforce in high-risk communities, HCV testing strategies in pregnant people and people of childbearing age, and treatment with novel antiviral therapies,” wrote the authors.
In the time since the authors began the study, universal HCV screening for every pregnancy has been recommended by a number of groups, including the U.S. Preventive Services Task Force, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine (SMFM). However, Dr. Patrick says even though such recommendations are now adopted, it will be some time before they are fully operational, making knowledge of HCV risk factors important for obstetricians as well as pediatricians and family physicians. “We don’t know how if hospitals and clinicians have started universal screening for HCV and even when it is completely adopted, understanding individual and community-level factors associated with HCV in pregnant people is still of critical importance,” he explained. “In some of our previous work we have found that non-White HCV-exposed infants are less likely to be tested for HCV than are White infants, even after accounting for multiple individual and hospital-level factors. The pattern we are seeing in our research and in research in other groups is one of unequal treatment of pregnant people with substance use disorder in terms of being given evidence-based treatments, being tested for HCV, and even in child welfare outcomes like foster placement. It is important to know these issues are occurring, but we need specific equitable approaches to ensuring optimal outcomes for all families.
Jeffrey A. Kuller, MD, one of the authors of the SMFM’s new recommendations for universal HCV screening in pregnancy, agreed that until universal screening is widely adopted, awareness of maternal HCV risk factors is important, “to better determine who is at highest risk for hep C, barriers to care, and patients to better target.” This information also affects procedure at the time of delivery, added Dr. Kuller, professor of obstetrics and gynecology in the division of maternal-fetal medicine at Duke University, Durham, N.C. “We do not perform C-sections for the presence of hep C,” he told this publication. However, in labor, “we try to avoid internal fetal monitoring when possible, and early artificial rupture of membranes when possible, and avoid the use of routine episiotomy,” he said. “Hep C–positive patients should also be assessed for other sexually transmitted diseases including HIV, syphilis, gonorrhea, chlamydia, and hep B. “Although we do not typically treat hep C pharmacologically during pregnancy, we try to get the patient placed with a hepatologist for long-term management.”
The study has important implications for pediatric patients, added Audrey R. Lloyd, MD, a med-peds infectious disease fellow who is studying HCV in pregnancy at the University of Alabama at Birmingham. “In the setting of maternal HCV viremia, maternal-fetal transmission occurs in around 6% of exposed infants and around 10% if there is maternal HIV-HCV coinfection,” she said in an interview. “With the increasing rates of HCV in pregnant women described by Dr. Patrick et al., HCV infections among infants will also rise. Even when maternal HCV infection is documented, we often do not do a good job screening the infants for infection and linking them to treatment. This new data makes me worried we may see more complications of pediatric HCV infection in the future,” she added. She explained that safe and effective treatments for HCV infection are approved down to 3 years of age, but patients must first be diagnosed to receive treatment.
From whichever angle you approach it, tackling both the opioid epidemic and HCV infection in pregnancy will inevitably end up helping both parts of the mother-infant dyad, said Dr. Patrick. “Not too long ago I was caring for an opioid-exposed infant at the hospital where I practice who had transferred in from another center hours away. The mother had not been tested for HCV, so I tested the infant for HCV antibodies which were positive. Imagine that, determining a mother is HCV positive by testing the infant. There are so many layers of systems that should be fixed to make this not happen. And what are the chances the mother, after she found out, was able to access treatment for HCV? What about the infant being tested? The systems are just fragmented and we need to do better.”
The study was funded by the National Institute on Drug Abuse of the National Institutes of Health. Neither Dr. Patrick, Dr. Kuller, nor Dr. Lloyd reported any conflicts of interest.
Mirroring the opioid crisis, maternal and newborn hepatitis C infections (HCV) more than doubled in the United States between 2009 and 2019, with disproportionate increases in people of White, American Indian, and Alaska Native race, especially those with less education, according to a cross-sectional study published in JAMA Health Forum. However, the level of risk within these populations was mitigated in counties with higher employment, reported Stephen W. Patrick, MD, of Vanderbilt University, in Nashville, Tenn., and coauthors.
“As we develop public health approaches to prevent HCV infections, connect to treatment, and monitor exposed infants, understanding these factors can be of critical importance to tailoring interventions,” Dr. Patrick said in an interview. “HCV is one more complication of the opioid crisis,” he added. “These data also enable us to step back a bit from HCV and look at the landscape of how the opioid crisis continues to grow in complexity and scope. Throughout the opioid crisis we have often failed to recognize and address the unique needs of pregnant people and infants.”
The study authors used data from the National Center for Health Statistics at the Centers for Disease Control and Prevention, and from the Area Health Resource File to examine maternal-infant HCV infection among all U.S. births between 2009 and 2019. The researchers also examined community-level risk factors including rurality, employment, and access to medical care.
In counties reporting HCV, there were 39,380,122 people who had live births, of whom 138,343 (0.4%) were diagnosed with HCV. The overall rate of maternal HCV infection increased from 1.8 to 5.1 per 1,000 live births between 2009 and 2019.
Infection rates were highest in American Indian/Alaska Native (AI/AN) and White people (adjusted odds ratio [aOR] 7.94 and 7.37, respectively) compared with Black people. They were higher among individuals without a 4-year degree compared to those with higher education (aOR, 3.19).
Among these groups considered to be at higher risk for HCV infection, high employment rates somewhat mitigated the risk. Specifically, in counties in the 10th percentile of employment, the predicted probability of HCV increased from 0.16% to 1.37%, between 2009 and 2019, whereas in counties at the 90th percentile of employment, the predicted probability remained similar, at 0.36% in 2009 and 0.48% in 2019.
“With constrained national resources, understanding both individual and community-level factors associated with HCV infections in pregnant people could inform strategies to mitigate its spread, such as harm reduction efforts (e.g., syringe service programs), improving access to treatment for [opioid use disorder] or increasing the obstetrical workforce in high-risk communities, HCV testing strategies in pregnant people and people of childbearing age, and treatment with novel antiviral therapies,” wrote the authors.
In the time since the authors began the study, universal HCV screening for every pregnancy has been recommended by a number of groups, including the U.S. Preventive Services Task Force, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine (SMFM). However, Dr. Patrick says even though such recommendations are now adopted, it will be some time before they are fully operational, making knowledge of HCV risk factors important for obstetricians as well as pediatricians and family physicians. “We don’t know how if hospitals and clinicians have started universal screening for HCV and even when it is completely adopted, understanding individual and community-level factors associated with HCV in pregnant people is still of critical importance,” he explained. “In some of our previous work we have found that non-White HCV-exposed infants are less likely to be tested for HCV than are White infants, even after accounting for multiple individual and hospital-level factors. The pattern we are seeing in our research and in research in other groups is one of unequal treatment of pregnant people with substance use disorder in terms of being given evidence-based treatments, being tested for HCV, and even in child welfare outcomes like foster placement. It is important to know these issues are occurring, but we need specific equitable approaches to ensuring optimal outcomes for all families.
Jeffrey A. Kuller, MD, one of the authors of the SMFM’s new recommendations for universal HCV screening in pregnancy, agreed that until universal screening is widely adopted, awareness of maternal HCV risk factors is important, “to better determine who is at highest risk for hep C, barriers to care, and patients to better target.” This information also affects procedure at the time of delivery, added Dr. Kuller, professor of obstetrics and gynecology in the division of maternal-fetal medicine at Duke University, Durham, N.C. “We do not perform C-sections for the presence of hep C,” he told this publication. However, in labor, “we try to avoid internal fetal monitoring when possible, and early artificial rupture of membranes when possible, and avoid the use of routine episiotomy,” he said. “Hep C–positive patients should also be assessed for other sexually transmitted diseases including HIV, syphilis, gonorrhea, chlamydia, and hep B. “Although we do not typically treat hep C pharmacologically during pregnancy, we try to get the patient placed with a hepatologist for long-term management.”
The study has important implications for pediatric patients, added Audrey R. Lloyd, MD, a med-peds infectious disease fellow who is studying HCV in pregnancy at the University of Alabama at Birmingham. “In the setting of maternal HCV viremia, maternal-fetal transmission occurs in around 6% of exposed infants and around 10% if there is maternal HIV-HCV coinfection,” she said in an interview. “With the increasing rates of HCV in pregnant women described by Dr. Patrick et al., HCV infections among infants will also rise. Even when maternal HCV infection is documented, we often do not do a good job screening the infants for infection and linking them to treatment. This new data makes me worried we may see more complications of pediatric HCV infection in the future,” she added. She explained that safe and effective treatments for HCV infection are approved down to 3 years of age, but patients must first be diagnosed to receive treatment.
From whichever angle you approach it, tackling both the opioid epidemic and HCV infection in pregnancy will inevitably end up helping both parts of the mother-infant dyad, said Dr. Patrick. “Not too long ago I was caring for an opioid-exposed infant at the hospital where I practice who had transferred in from another center hours away. The mother had not been tested for HCV, so I tested the infant for HCV antibodies which were positive. Imagine that, determining a mother is HCV positive by testing the infant. There are so many layers of systems that should be fixed to make this not happen. And what are the chances the mother, after she found out, was able to access treatment for HCV? What about the infant being tested? The systems are just fragmented and we need to do better.”
The study was funded by the National Institute on Drug Abuse of the National Institutes of Health. Neither Dr. Patrick, Dr. Kuller, nor Dr. Lloyd reported any conflicts of interest.
Mirroring the opioid crisis, maternal and newborn hepatitis C infections (HCV) more than doubled in the United States between 2009 and 2019, with disproportionate increases in people of White, American Indian, and Alaska Native race, especially those with less education, according to a cross-sectional study published in JAMA Health Forum. However, the level of risk within these populations was mitigated in counties with higher employment, reported Stephen W. Patrick, MD, of Vanderbilt University, in Nashville, Tenn., and coauthors.
“As we develop public health approaches to prevent HCV infections, connect to treatment, and monitor exposed infants, understanding these factors can be of critical importance to tailoring interventions,” Dr. Patrick said in an interview. “HCV is one more complication of the opioid crisis,” he added. “These data also enable us to step back a bit from HCV and look at the landscape of how the opioid crisis continues to grow in complexity and scope. Throughout the opioid crisis we have often failed to recognize and address the unique needs of pregnant people and infants.”
The study authors used data from the National Center for Health Statistics at the Centers for Disease Control and Prevention, and from the Area Health Resource File to examine maternal-infant HCV infection among all U.S. births between 2009 and 2019. The researchers also examined community-level risk factors including rurality, employment, and access to medical care.
In counties reporting HCV, there were 39,380,122 people who had live births, of whom 138,343 (0.4%) were diagnosed with HCV. The overall rate of maternal HCV infection increased from 1.8 to 5.1 per 1,000 live births between 2009 and 2019.
Infection rates were highest in American Indian/Alaska Native (AI/AN) and White people (adjusted odds ratio [aOR] 7.94 and 7.37, respectively) compared with Black people. They were higher among individuals without a 4-year degree compared to those with higher education (aOR, 3.19).
Among these groups considered to be at higher risk for HCV infection, high employment rates somewhat mitigated the risk. Specifically, in counties in the 10th percentile of employment, the predicted probability of HCV increased from 0.16% to 1.37%, between 2009 and 2019, whereas in counties at the 90th percentile of employment, the predicted probability remained similar, at 0.36% in 2009 and 0.48% in 2019.
“With constrained national resources, understanding both individual and community-level factors associated with HCV infections in pregnant people could inform strategies to mitigate its spread, such as harm reduction efforts (e.g., syringe service programs), improving access to treatment for [opioid use disorder] or increasing the obstetrical workforce in high-risk communities, HCV testing strategies in pregnant people and people of childbearing age, and treatment with novel antiviral therapies,” wrote the authors.
In the time since the authors began the study, universal HCV screening for every pregnancy has been recommended by a number of groups, including the U.S. Preventive Services Task Force, the American College of Obstetricians and Gynecologists, and the Society for Maternal-Fetal Medicine (SMFM). However, Dr. Patrick says even though such recommendations are now adopted, it will be some time before they are fully operational, making knowledge of HCV risk factors important for obstetricians as well as pediatricians and family physicians. “We don’t know how if hospitals and clinicians have started universal screening for HCV and even when it is completely adopted, understanding individual and community-level factors associated with HCV in pregnant people is still of critical importance,” he explained. “In some of our previous work we have found that non-White HCV-exposed infants are less likely to be tested for HCV than are White infants, even after accounting for multiple individual and hospital-level factors. The pattern we are seeing in our research and in research in other groups is one of unequal treatment of pregnant people with substance use disorder in terms of being given evidence-based treatments, being tested for HCV, and even in child welfare outcomes like foster placement. It is important to know these issues are occurring, but we need specific equitable approaches to ensuring optimal outcomes for all families.
Jeffrey A. Kuller, MD, one of the authors of the SMFM’s new recommendations for universal HCV screening in pregnancy, agreed that until universal screening is widely adopted, awareness of maternal HCV risk factors is important, “to better determine who is at highest risk for hep C, barriers to care, and patients to better target.” This information also affects procedure at the time of delivery, added Dr. Kuller, professor of obstetrics and gynecology in the division of maternal-fetal medicine at Duke University, Durham, N.C. “We do not perform C-sections for the presence of hep C,” he told this publication. However, in labor, “we try to avoid internal fetal monitoring when possible, and early artificial rupture of membranes when possible, and avoid the use of routine episiotomy,” he said. “Hep C–positive patients should also be assessed for other sexually transmitted diseases including HIV, syphilis, gonorrhea, chlamydia, and hep B. “Although we do not typically treat hep C pharmacologically during pregnancy, we try to get the patient placed with a hepatologist for long-term management.”
The study has important implications for pediatric patients, added Audrey R. Lloyd, MD, a med-peds infectious disease fellow who is studying HCV in pregnancy at the University of Alabama at Birmingham. “In the setting of maternal HCV viremia, maternal-fetal transmission occurs in around 6% of exposed infants and around 10% if there is maternal HIV-HCV coinfection,” she said in an interview. “With the increasing rates of HCV in pregnant women described by Dr. Patrick et al., HCV infections among infants will also rise. Even when maternal HCV infection is documented, we often do not do a good job screening the infants for infection and linking them to treatment. This new data makes me worried we may see more complications of pediatric HCV infection in the future,” she added. She explained that safe and effective treatments for HCV infection are approved down to 3 years of age, but patients must first be diagnosed to receive treatment.
From whichever angle you approach it, tackling both the opioid epidemic and HCV infection in pregnancy will inevitably end up helping both parts of the mother-infant dyad, said Dr. Patrick. “Not too long ago I was caring for an opioid-exposed infant at the hospital where I practice who had transferred in from another center hours away. The mother had not been tested for HCV, so I tested the infant for HCV antibodies which were positive. Imagine that, determining a mother is HCV positive by testing the infant. There are so many layers of systems that should be fixed to make this not happen. And what are the chances the mother, after she found out, was able to access treatment for HCV? What about the infant being tested? The systems are just fragmented and we need to do better.”
The study was funded by the National Institute on Drug Abuse of the National Institutes of Health. Neither Dr. Patrick, Dr. Kuller, nor Dr. Lloyd reported any conflicts of interest.
FROM JAMA HEALTH FORUM
Children and COVID: A look at the pace of vaccination
With children aged 5-11 years about to enter the battle-of-the-COVID-vaccine phase of the war on COVID, there are many questions. MDedge takes a look at one: How long will it take to get 5- to 11-year-olds vaccinated?
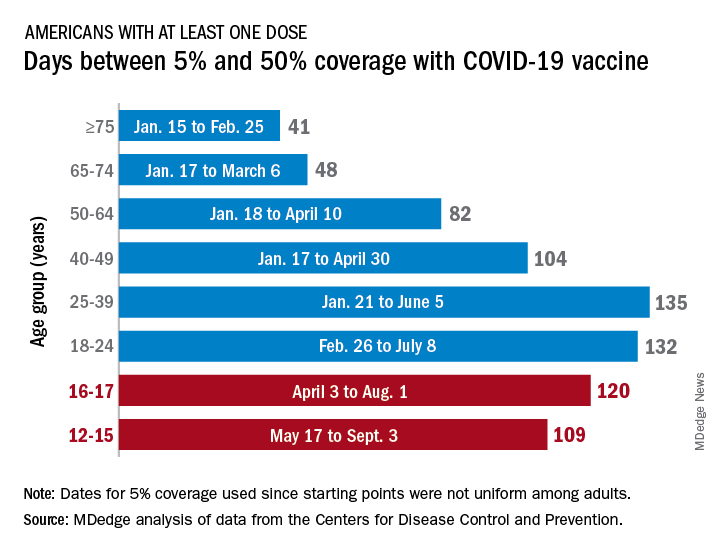
Previous experience may provide some guidance. The vaccine was approved by the Centers for Disease Control and Prevention for the closest group in age, 12- to 15-year-olds, on May 12, 2021, and according to data from the CDC.
(Use of the 5% figure acknowledges the uneven start after approval – the vaccine became available to different age groups at different times, even though it had been approved for all adults aged 18 years and older.)
The 16- to 17-year-olds, despite being a smaller group of less than 7.6 million individuals, took 120 days to go from 5% to 50% coverage. For those aged 18-24 years, the corresponding time was 132 days, while the 24- to 36-year-olds took longer than any other age group, 135 days, to reach the 50%-with-at-least-one-dose milestone. The time, in turn, decreased for each group as age increased, with those aged 75 and older taking just 41 days to get at least one dose in 50% of individuals, the CDC data show.
That trend also applies to full vaccination, for the most part. The oldest group, 75 and older, had the shortest time to 50% being fully vaccinated at 69 days, and the 25- to 39-year-olds had the longest time at 206 days, with the length rising as age decreased and dropping for groups younger than 25-39. Except for the 12- to 15-year-olds. It has been 160 days (as of Nov. 2) since the 5% mark was reached on May 17, but only 47.4% of the group is fully vaccinated, making it unlikely that the 50% mark will be reached earlier than the 169 days it took the 16- to 17-year-olds.
So where does that put the 5- to 11-year-olds?
The White House said on Nov. 1 that vaccinations could start the first week of November, pending approval from the CDC’s Advisory Committee on Immunization Practices, which meets on Nov. 2. “This is an important step forward in our nation’s fight against the virus,” Jeff Zients, the White House COVID-19 Response Coordinator, said in a briefing. “As we await the CDC decision, we are not waiting on the operations and logistics. In fact, we’ve been preparing for weeks.”
Availability, of course, is not the only factor involved. In a survey conducted Oct. 14-24, the Kaiser Family Foundation found that only 27% of parents of children aged 5-11 years are planning to have them vaccinated against COVID-19 “right away” once the vaccine is available, and that 33% would “wait and see” how the vaccine works.
“Parents of 5-11 year-olds cite a range of concerns when it comes to vaccinating their children for COVID-19, with safety issues topping off the list,” and “two-thirds say they are concerned the vaccine may negatively impact their child’s fertility in the future,” Kaiser said.
With children aged 5-11 years about to enter the battle-of-the-COVID-vaccine phase of the war on COVID, there are many questions. MDedge takes a look at one: How long will it take to get 5- to 11-year-olds vaccinated?

Previous experience may provide some guidance. The vaccine was approved by the Centers for Disease Control and Prevention for the closest group in age, 12- to 15-year-olds, on May 12, 2021, and according to data from the CDC.
(Use of the 5% figure acknowledges the uneven start after approval – the vaccine became available to different age groups at different times, even though it had been approved for all adults aged 18 years and older.)
The 16- to 17-year-olds, despite being a smaller group of less than 7.6 million individuals, took 120 days to go from 5% to 50% coverage. For those aged 18-24 years, the corresponding time was 132 days, while the 24- to 36-year-olds took longer than any other age group, 135 days, to reach the 50%-with-at-least-one-dose milestone. The time, in turn, decreased for each group as age increased, with those aged 75 and older taking just 41 days to get at least one dose in 50% of individuals, the CDC data show.
That trend also applies to full vaccination, for the most part. The oldest group, 75 and older, had the shortest time to 50% being fully vaccinated at 69 days, and the 25- to 39-year-olds had the longest time at 206 days, with the length rising as age decreased and dropping for groups younger than 25-39. Except for the 12- to 15-year-olds. It has been 160 days (as of Nov. 2) since the 5% mark was reached on May 17, but only 47.4% of the group is fully vaccinated, making it unlikely that the 50% mark will be reached earlier than the 169 days it took the 16- to 17-year-olds.
So where does that put the 5- to 11-year-olds?
The White House said on Nov. 1 that vaccinations could start the first week of November, pending approval from the CDC’s Advisory Committee on Immunization Practices, which meets on Nov. 2. “This is an important step forward in our nation’s fight against the virus,” Jeff Zients, the White House COVID-19 Response Coordinator, said in a briefing. “As we await the CDC decision, we are not waiting on the operations and logistics. In fact, we’ve been preparing for weeks.”
Availability, of course, is not the only factor involved. In a survey conducted Oct. 14-24, the Kaiser Family Foundation found that only 27% of parents of children aged 5-11 years are planning to have them vaccinated against COVID-19 “right away” once the vaccine is available, and that 33% would “wait and see” how the vaccine works.
“Parents of 5-11 year-olds cite a range of concerns when it comes to vaccinating their children for COVID-19, with safety issues topping off the list,” and “two-thirds say they are concerned the vaccine may negatively impact their child’s fertility in the future,” Kaiser said.
With children aged 5-11 years about to enter the battle-of-the-COVID-vaccine phase of the war on COVID, there are many questions. MDedge takes a look at one: How long will it take to get 5- to 11-year-olds vaccinated?

Previous experience may provide some guidance. The vaccine was approved by the Centers for Disease Control and Prevention for the closest group in age, 12- to 15-year-olds, on May 12, 2021, and according to data from the CDC.
(Use of the 5% figure acknowledges the uneven start after approval – the vaccine became available to different age groups at different times, even though it had been approved for all adults aged 18 years and older.)
The 16- to 17-year-olds, despite being a smaller group of less than 7.6 million individuals, took 120 days to go from 5% to 50% coverage. For those aged 18-24 years, the corresponding time was 132 days, while the 24- to 36-year-olds took longer than any other age group, 135 days, to reach the 50%-with-at-least-one-dose milestone. The time, in turn, decreased for each group as age increased, with those aged 75 and older taking just 41 days to get at least one dose in 50% of individuals, the CDC data show.
That trend also applies to full vaccination, for the most part. The oldest group, 75 and older, had the shortest time to 50% being fully vaccinated at 69 days, and the 25- to 39-year-olds had the longest time at 206 days, with the length rising as age decreased and dropping for groups younger than 25-39. Except for the 12- to 15-year-olds. It has been 160 days (as of Nov. 2) since the 5% mark was reached on May 17, but only 47.4% of the group is fully vaccinated, making it unlikely that the 50% mark will be reached earlier than the 169 days it took the 16- to 17-year-olds.
So where does that put the 5- to 11-year-olds?
The White House said on Nov. 1 that vaccinations could start the first week of November, pending approval from the CDC’s Advisory Committee on Immunization Practices, which meets on Nov. 2. “This is an important step forward in our nation’s fight against the virus,” Jeff Zients, the White House COVID-19 Response Coordinator, said in a briefing. “As we await the CDC decision, we are not waiting on the operations and logistics. In fact, we’ve been preparing for weeks.”
Availability, of course, is not the only factor involved. In a survey conducted Oct. 14-24, the Kaiser Family Foundation found that only 27% of parents of children aged 5-11 years are planning to have them vaccinated against COVID-19 “right away” once the vaccine is available, and that 33% would “wait and see” how the vaccine works.
“Parents of 5-11 year-olds cite a range of concerns when it comes to vaccinating their children for COVID-19, with safety issues topping off the list,” and “two-thirds say they are concerned the vaccine may negatively impact their child’s fertility in the future,” Kaiser said.
Influenza tied to long-term increased risk for Parkinson’s disease
Influenza infection is linked to a subsequent diagnosis of Parkinson’s disease (PD) more than 10 years later, resurfacing a long-held debate about whether infection increases the risk for movement disorders over the long term.
In a large case-control study, investigators found and by more than 70% for PD occurring more than 10 years after the flu.
“This study is not definitive by any means, but it certainly suggests there are potential long-term consequences from influenza,” study investigator Noelle M. Cocoros, DSc, research scientist at Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, said in an interview.
The study was published online Oct. 25 in JAMA Neurology.
Ongoing debate
The debate about whether influenza is associated with PD has been going on as far back as the 1918 influenza pandemic, when experts documented parkinsonism in affected individuals.
Using data from the Danish patient registry, researchers identified 10,271 subjects diagnosed with PD during a 17-year period (2000-2016). Of these, 38.7% were female, and the mean age was 71.4 years.
They matched these subjects for age and sex to 51,355 controls without PD. Compared with controls, slightly fewer individuals with PD had chronic obstructive pulmonary disease (COPD) or emphysema, but there was a similar distribution of cardiovascular disease and various other conditions.
Researchers collected data on influenza diagnoses from inpatient and outpatient hospital clinics from 1977 to 2016. They plotted these by month and year on a graph, calculated the median number of diagnoses per month, and identified peaks as those with more than threefold the median.
They categorized cases in groups related to the time between the infection and PD: More than 10 years, 10-15 years, and more than 15 years.
The time lapse accounts for a rather long “run-up” to PD, said Dr. Cocoros. There’s a sometimes decades-long preclinical phase before patients develop typical motor signs and a prodromal phase where they may present with nonmotor symptoms such as sleep disorders and constipation.
“We expected there would be at least 10 years between any infection and PD if there was an association present,” said Dr. Cocoros.
Investigators found an association between influenza exposure and PD diagnosis “that held up over time,” she said.
For more than 10 years before PD, the likelihood of a diagnosis for the infected compared with the unexposed was increased 73% (odds ratio [OR] 1.73; 95% confidence interval, 1.11-2.71; P = .02) after adjustment for cardiovascular disease, diabetes, chronic obstructive pulmonary disease, emphysema, lung cancer, Crohn’s disease, and ulcerative colitis.
The odds increased with more time from infection. For more than 15 years, the adjusted OR was 1.91 (95% CI, 1.14 - 3.19; P =.01).
However, for the 10- to 15-year time frame, the point estimate was reduced and the CI nonsignificant (OR, 1.33; 95% CI, 0.54-3.27; P = .53). This “is a little hard to interpret,” but could be a result of the small numbers, exposure misclassification, or because “the longer time interval is what’s meaningful,” said Dr. Cocoros.
Potential COVID-19–related PD surge?
In a sensitivity analysis, researchers looked at peak infection activity. “We wanted to increase the likelihood of these diagnoses representing actual infection,” Dr. Cocoros noted.
Here, the OR was still elevated at more than 10 years, but the CI was quite wide and included 1 (OR, 1.52; 95% CI, 0.80-2.89; P = .21). “So the association holds up, but the estimates are quite unstable,” said Dr. Cocoros.
Researchers examined associations with numerous other infection types, but did not see the same trend over time. Some infections – for example, gastrointestinal infections and septicemia – were associated with PD within 5 years, but most associations appeared to be null after more than 10 years.
“There seemed to be associations earlier between the infection and PD, which we interpret to suggest there’s actually not a meaningful association,” said Dr. Cocoros.
An exception might be urinary tract infections (UTIs), where after 10 years, the adjusted OR was 1.19 (95% CI, 1.01-1.40). Research suggests patients with PD often have UTIs and neurogenic bladder.
“It’s possible that UTIs could be an early symptom of PD rather than a causative factor,” said Dr. Cocoros.
It’s unclear how influenza might lead to PD but it could be that the virus gets into the central nervous system, resulting in neuroinflammation. Cytokines generated in response to the influenza infection might damage the brain.
“The infection could be a ‘primer’ or an initial ‘hit’ to the system, maybe setting people up for PD,” said Dr. Cocoros.
As for the current COVID-19 pandemic, some experts are concerned about a potential surge in PD cases in decades to come, and are calling for prospective monitoring of patients with this infection, said Dr. Cocoros.
However, she noted that infections don’t account for all PD cases and that genetic and environmental factors also influence risk.
Many individuals who contract influenza don’t seek medical care or get tested, so it’s possible the study counted those who had the infection as unexposed. Another potential study limitation was that small numbers for some infections, for example, Helicobacter pylori and hepatitis C, limited the ability to interpret results.
‘Exciting and important’ findings
Commenting on the research for this news organization, Aparna Wagle Shukla, MD, professor, Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the results amid the current pandemic are “exciting and important” and “have reinvigorated interest” in the role of infection in PD.
However, the study had some limitations, an important one being lack of accounting for confounding factors, including environmental factors, she said. Exposure to pesticides, living in a rural area, drinking well water, and having had a head injury may increase PD risk, whereas high intake of caffeine, nicotine, alcohol, and nonsteroidal anti-inflammatory drugs might lower the risk.
The researchers did not take into account exposure to multiple microbes or “infection burden,” said Dr. Wagle Shukla, who was not involved in the current study. In addition, as the data are from a single country with exposure to specific influenza strains, application of the findings elsewhere may be limited.
Dr. Wagle Shukla noted that a case-control design “isn’t ideal” from an epidemiological perspective. “Future studies should involve large cohorts followed longitudinally.”
The study was supported by grants from the Lundbeck Foundation and the Augustinus Foundation. Dr. Cocoros has disclosed no relevant financial relationships. Several coauthors have disclosed relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Influenza infection is linked to a subsequent diagnosis of Parkinson’s disease (PD) more than 10 years later, resurfacing a long-held debate about whether infection increases the risk for movement disorders over the long term.
In a large case-control study, investigators found and by more than 70% for PD occurring more than 10 years after the flu.
“This study is not definitive by any means, but it certainly suggests there are potential long-term consequences from influenza,” study investigator Noelle M. Cocoros, DSc, research scientist at Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, said in an interview.
The study was published online Oct. 25 in JAMA Neurology.
Ongoing debate
The debate about whether influenza is associated with PD has been going on as far back as the 1918 influenza pandemic, when experts documented parkinsonism in affected individuals.
Using data from the Danish patient registry, researchers identified 10,271 subjects diagnosed with PD during a 17-year period (2000-2016). Of these, 38.7% were female, and the mean age was 71.4 years.
They matched these subjects for age and sex to 51,355 controls without PD. Compared with controls, slightly fewer individuals with PD had chronic obstructive pulmonary disease (COPD) or emphysema, but there was a similar distribution of cardiovascular disease and various other conditions.
Researchers collected data on influenza diagnoses from inpatient and outpatient hospital clinics from 1977 to 2016. They plotted these by month and year on a graph, calculated the median number of diagnoses per month, and identified peaks as those with more than threefold the median.
They categorized cases in groups related to the time between the infection and PD: More than 10 years, 10-15 years, and more than 15 years.
The time lapse accounts for a rather long “run-up” to PD, said Dr. Cocoros. There’s a sometimes decades-long preclinical phase before patients develop typical motor signs and a prodromal phase where they may present with nonmotor symptoms such as sleep disorders and constipation.
“We expected there would be at least 10 years between any infection and PD if there was an association present,” said Dr. Cocoros.
Investigators found an association between influenza exposure and PD diagnosis “that held up over time,” she said.
For more than 10 years before PD, the likelihood of a diagnosis for the infected compared with the unexposed was increased 73% (odds ratio [OR] 1.73; 95% confidence interval, 1.11-2.71; P = .02) after adjustment for cardiovascular disease, diabetes, chronic obstructive pulmonary disease, emphysema, lung cancer, Crohn’s disease, and ulcerative colitis.
The odds increased with more time from infection. For more than 15 years, the adjusted OR was 1.91 (95% CI, 1.14 - 3.19; P =.01).
However, for the 10- to 15-year time frame, the point estimate was reduced and the CI nonsignificant (OR, 1.33; 95% CI, 0.54-3.27; P = .53). This “is a little hard to interpret,” but could be a result of the small numbers, exposure misclassification, or because “the longer time interval is what’s meaningful,” said Dr. Cocoros.
Potential COVID-19–related PD surge?
In a sensitivity analysis, researchers looked at peak infection activity. “We wanted to increase the likelihood of these diagnoses representing actual infection,” Dr. Cocoros noted.
Here, the OR was still elevated at more than 10 years, but the CI was quite wide and included 1 (OR, 1.52; 95% CI, 0.80-2.89; P = .21). “So the association holds up, but the estimates are quite unstable,” said Dr. Cocoros.
Researchers examined associations with numerous other infection types, but did not see the same trend over time. Some infections – for example, gastrointestinal infections and septicemia – were associated with PD within 5 years, but most associations appeared to be null after more than 10 years.
“There seemed to be associations earlier between the infection and PD, which we interpret to suggest there’s actually not a meaningful association,” said Dr. Cocoros.
An exception might be urinary tract infections (UTIs), where after 10 years, the adjusted OR was 1.19 (95% CI, 1.01-1.40). Research suggests patients with PD often have UTIs and neurogenic bladder.
“It’s possible that UTIs could be an early symptom of PD rather than a causative factor,” said Dr. Cocoros.
It’s unclear how influenza might lead to PD but it could be that the virus gets into the central nervous system, resulting in neuroinflammation. Cytokines generated in response to the influenza infection might damage the brain.
“The infection could be a ‘primer’ or an initial ‘hit’ to the system, maybe setting people up for PD,” said Dr. Cocoros.
As for the current COVID-19 pandemic, some experts are concerned about a potential surge in PD cases in decades to come, and are calling for prospective monitoring of patients with this infection, said Dr. Cocoros.
However, she noted that infections don’t account for all PD cases and that genetic and environmental factors also influence risk.
Many individuals who contract influenza don’t seek medical care or get tested, so it’s possible the study counted those who had the infection as unexposed. Another potential study limitation was that small numbers for some infections, for example, Helicobacter pylori and hepatitis C, limited the ability to interpret results.
‘Exciting and important’ findings
Commenting on the research for this news organization, Aparna Wagle Shukla, MD, professor, Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the results amid the current pandemic are “exciting and important” and “have reinvigorated interest” in the role of infection in PD.
However, the study had some limitations, an important one being lack of accounting for confounding factors, including environmental factors, she said. Exposure to pesticides, living in a rural area, drinking well water, and having had a head injury may increase PD risk, whereas high intake of caffeine, nicotine, alcohol, and nonsteroidal anti-inflammatory drugs might lower the risk.
The researchers did not take into account exposure to multiple microbes or “infection burden,” said Dr. Wagle Shukla, who was not involved in the current study. In addition, as the data are from a single country with exposure to specific influenza strains, application of the findings elsewhere may be limited.
Dr. Wagle Shukla noted that a case-control design “isn’t ideal” from an epidemiological perspective. “Future studies should involve large cohorts followed longitudinally.”
The study was supported by grants from the Lundbeck Foundation and the Augustinus Foundation. Dr. Cocoros has disclosed no relevant financial relationships. Several coauthors have disclosed relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Influenza infection is linked to a subsequent diagnosis of Parkinson’s disease (PD) more than 10 years later, resurfacing a long-held debate about whether infection increases the risk for movement disorders over the long term.
In a large case-control study, investigators found and by more than 70% for PD occurring more than 10 years after the flu.
“This study is not definitive by any means, but it certainly suggests there are potential long-term consequences from influenza,” study investigator Noelle M. Cocoros, DSc, research scientist at Harvard Pilgrim Health Care Institute and Harvard Medical School, Boston, said in an interview.
The study was published online Oct. 25 in JAMA Neurology.
Ongoing debate
The debate about whether influenza is associated with PD has been going on as far back as the 1918 influenza pandemic, when experts documented parkinsonism in affected individuals.
Using data from the Danish patient registry, researchers identified 10,271 subjects diagnosed with PD during a 17-year period (2000-2016). Of these, 38.7% were female, and the mean age was 71.4 years.
They matched these subjects for age and sex to 51,355 controls without PD. Compared with controls, slightly fewer individuals with PD had chronic obstructive pulmonary disease (COPD) or emphysema, but there was a similar distribution of cardiovascular disease and various other conditions.
Researchers collected data on influenza diagnoses from inpatient and outpatient hospital clinics from 1977 to 2016. They plotted these by month and year on a graph, calculated the median number of diagnoses per month, and identified peaks as those with more than threefold the median.
They categorized cases in groups related to the time between the infection and PD: More than 10 years, 10-15 years, and more than 15 years.
The time lapse accounts for a rather long “run-up” to PD, said Dr. Cocoros. There’s a sometimes decades-long preclinical phase before patients develop typical motor signs and a prodromal phase where they may present with nonmotor symptoms such as sleep disorders and constipation.
“We expected there would be at least 10 years between any infection and PD if there was an association present,” said Dr. Cocoros.
Investigators found an association between influenza exposure and PD diagnosis “that held up over time,” she said.
For more than 10 years before PD, the likelihood of a diagnosis for the infected compared with the unexposed was increased 73% (odds ratio [OR] 1.73; 95% confidence interval, 1.11-2.71; P = .02) after adjustment for cardiovascular disease, diabetes, chronic obstructive pulmonary disease, emphysema, lung cancer, Crohn’s disease, and ulcerative colitis.
The odds increased with more time from infection. For more than 15 years, the adjusted OR was 1.91 (95% CI, 1.14 - 3.19; P =.01).
However, for the 10- to 15-year time frame, the point estimate was reduced and the CI nonsignificant (OR, 1.33; 95% CI, 0.54-3.27; P = .53). This “is a little hard to interpret,” but could be a result of the small numbers, exposure misclassification, or because “the longer time interval is what’s meaningful,” said Dr. Cocoros.
Potential COVID-19–related PD surge?
In a sensitivity analysis, researchers looked at peak infection activity. “We wanted to increase the likelihood of these diagnoses representing actual infection,” Dr. Cocoros noted.
Here, the OR was still elevated at more than 10 years, but the CI was quite wide and included 1 (OR, 1.52; 95% CI, 0.80-2.89; P = .21). “So the association holds up, but the estimates are quite unstable,” said Dr. Cocoros.
Researchers examined associations with numerous other infection types, but did not see the same trend over time. Some infections – for example, gastrointestinal infections and septicemia – were associated with PD within 5 years, but most associations appeared to be null after more than 10 years.
“There seemed to be associations earlier between the infection and PD, which we interpret to suggest there’s actually not a meaningful association,” said Dr. Cocoros.
An exception might be urinary tract infections (UTIs), where after 10 years, the adjusted OR was 1.19 (95% CI, 1.01-1.40). Research suggests patients with PD often have UTIs and neurogenic bladder.
“It’s possible that UTIs could be an early symptom of PD rather than a causative factor,” said Dr. Cocoros.
It’s unclear how influenza might lead to PD but it could be that the virus gets into the central nervous system, resulting in neuroinflammation. Cytokines generated in response to the influenza infection might damage the brain.
“The infection could be a ‘primer’ or an initial ‘hit’ to the system, maybe setting people up for PD,” said Dr. Cocoros.
As for the current COVID-19 pandemic, some experts are concerned about a potential surge in PD cases in decades to come, and are calling for prospective monitoring of patients with this infection, said Dr. Cocoros.
However, she noted that infections don’t account for all PD cases and that genetic and environmental factors also influence risk.
Many individuals who contract influenza don’t seek medical care or get tested, so it’s possible the study counted those who had the infection as unexposed. Another potential study limitation was that small numbers for some infections, for example, Helicobacter pylori and hepatitis C, limited the ability to interpret results.
‘Exciting and important’ findings
Commenting on the research for this news organization, Aparna Wagle Shukla, MD, professor, Norman Fixel Institute for Neurological Diseases, University of Florida, Gainesville, said the results amid the current pandemic are “exciting and important” and “have reinvigorated interest” in the role of infection in PD.
However, the study had some limitations, an important one being lack of accounting for confounding factors, including environmental factors, she said. Exposure to pesticides, living in a rural area, drinking well water, and having had a head injury may increase PD risk, whereas high intake of caffeine, nicotine, alcohol, and nonsteroidal anti-inflammatory drugs might lower the risk.
The researchers did not take into account exposure to multiple microbes or “infection burden,” said Dr. Wagle Shukla, who was not involved in the current study. In addition, as the data are from a single country with exposure to specific influenza strains, application of the findings elsewhere may be limited.
Dr. Wagle Shukla noted that a case-control design “isn’t ideal” from an epidemiological perspective. “Future studies should involve large cohorts followed longitudinally.”
The study was supported by grants from the Lundbeck Foundation and the Augustinus Foundation. Dr. Cocoros has disclosed no relevant financial relationships. Several coauthors have disclosed relationships with industry. The full list can be found with the original article.
A version of this article first appeared on Medscape.com.
Without PrEP, a third of new HIV cases occur in MSM at low risk
Nearly one in three gay and bisexual men who were diagnosed with HIV at U.K. sexual health clinics didn’t meet the criteria for “high risk” that would signal to a clinician that they would be good candidates for pre-exposure prophylaxis (PrEP).
And that means that people who appear lower risk may still be good candidates for the HIV prevention pills, said Ann Sullivan, MD, consulting physician at Chelsea and Westminster Hospital, London.
“If people are coming forward for PrEP, they have self-identified that they need PrEP, [and] we should be allowing them to take PrEP,” said Dr. Sullivan at the 18th European AIDS Society Conference (EACS 2021). “We just need to trust patients. People know their risk, and we just have to accept that they know what they need best.”
And while this trial was made up of 95% gay and bisexual men, that ethos applies to every other group that could benefit from PrEP, including cisgender and transgender women and other gender-diverse people, Latinos, and Black Americans. In the United States, these groups make up nearly half of those who could benefit from PrEP under older guidelines but account for just 8% of people currently taking PrEP.
The finding also reinforces growing calls from health care providers to reduce gatekeeping around PrEP. For instance, there’s a move underway by the U.S. Centers for Disease Control and Prevention, where drafts of updated PrEP guidelines call for clinicians to talk to any sexually active teenager and adult about PrEP.
For the PrEP Impact trial, gay and bisexual men who received sexual health care at UK National Health Service sexual health clinics were invited to enroll in the study based on national PrEP guidelines. Those guidelines included being a cisgender man who had had sex with men not currently living with HIV and reporting condomless anal sex in the last 3 months; having a male partner whose HIV status they don’t know or who doesn’t have an undetectable viral load and with whom they’ve had condomless anal sex; or someone who doesn’t reach those criteria but whom the clinician thinks would be a good candidate.
Between Oct. 2017 and Feb. 2020, a total of 17,770 gay and bisexual men and 503 transgender or nonbinary people enrolled in the trial and were paired with 97,098 gay and bisexual men who didn’t use PrEP. (Data from the transgender participants were reported in a separate presentation.) The median age was 27 years, with 14.4% of the cisgender gay men between the ages of 16 and 24. Three out of four cis men were White, most lived in London, and more than half came from very-low-income neighborhoods.
Participants and controls were assessed for whether they were at particularly high risk for acquiring HIV, such as having used PrEP, having had two or more HIV tests, having had a rectal bacterial sexually transmitted infection (STI), or having had contact with someone with HIV or syphilis.
At the end of Feb. 2020, 24 cisgender men on PrEP had acquired HIV compared with 670 in the control group – an 87% reduction in HIV acquisition. Only one of those 24 cis men had lab-confirmed high adherence to PrEP. However, because the hair samples used to judge drug concentration weren’t long enough, Dr. Sullivan and colleagues were unable to assess whether the person really was fully adherent to treatment for the length of the trial.
But when they looked at the assessed behavior of people who acquired HIV, the two groups diverged. While a full 92% of people using PrEP had had STI diagnoses and other markers of increased risk, that was true for only 71% of people not taking PrEP. That meant, Dr. Sullivan said in an interview, that screening guidelines for PrEP were missing 29% of people with low assessed risk for HIV who nevertheless acquired the virus.
The findings led Antonio Urbina, MD, who both prescribes PrEP and manages Mount Sinai Medical Center’s PrEP program in New York, to the same conclusion that Dr. Sullivan and her team came to: that no screener is going to account for everything, and that there may be things that patients don’t want to tell their clinicians about their risk, either because of their own internalized stigma or their calculation that they aren’t comfortable enough with their providers to be honest.
“It reinforces to me that I need to ask more open-ended questions regarding risk and then just talk more about PrEP,” said Dr. Urbina, professor of medicine at Icahn School of Medicine. “Risk is dynamic and changes. And the great thing about PrEP is that if the risk goes up or down, if you have PrEP on board, you maintain this protection against HIV.”
An accompanying presentation on the transgender and nonbinary participants in the Impact Trial found that just one of 503 PrEP users acquired HIV. But here, too, there were people who could have benefited from PrEP but didn’t take it: Of the 477 trans and nonbinary participants who acted as controls, 97 were eligible by current guidelines but didn’t take PrEP. One in four of those declined the offer to take PrEP; the rest weren’t able to take it because they lived outside the treatment area. That, combined with a significantly lower likelihood that Black trans and nonbinary people took PrEP, indicated that work needs to be done to address the needs of people geographically and ethnically.
The data on gay men also raised the “who’s left out” issue for Gina Simoncini, MD, medical director for the Philadelphia AIDS Healthcare Foundation Healthcare Center. Dr. Simoncini previously taught attending physicians at Temple University how to prescribe PrEP and has done many grand rounds for primary care providers on how to manage PrEP.
“My biggest issue with this data is: What about the people who aren’t going to sexual health clinics?” she said. “What about the kid who’s 16 and maybe just barely putting his feet into the waters of sex and doesn’t feel quite comfortable going to a sexual health clinic? What about the trans Indian girl who can’t get to sexual health clinics because of family stigma and cultural stigma? The more we move toward primary care, the more people need to get on board with this.”
Dr. Sullivan reports no relevant financial relationships. Dr. Simoncini is an employee of AIDS Healthcare Foundation and has received advisory board fees from ViiV Healthcare. Dr. Urbina sits on the scientific advisory councils for Gilead Sciences, ViiV Healthcare, and Merck.
A version of this article first appeared on Medscape.com.
Nearly one in three gay and bisexual men who were diagnosed with HIV at U.K. sexual health clinics didn’t meet the criteria for “high risk” that would signal to a clinician that they would be good candidates for pre-exposure prophylaxis (PrEP).
And that means that people who appear lower risk may still be good candidates for the HIV prevention pills, said Ann Sullivan, MD, consulting physician at Chelsea and Westminster Hospital, London.
“If people are coming forward for PrEP, they have self-identified that they need PrEP, [and] we should be allowing them to take PrEP,” said Dr. Sullivan at the 18th European AIDS Society Conference (EACS 2021). “We just need to trust patients. People know their risk, and we just have to accept that they know what they need best.”
And while this trial was made up of 95% gay and bisexual men, that ethos applies to every other group that could benefit from PrEP, including cisgender and transgender women and other gender-diverse people, Latinos, and Black Americans. In the United States, these groups make up nearly half of those who could benefit from PrEP under older guidelines but account for just 8% of people currently taking PrEP.
The finding also reinforces growing calls from health care providers to reduce gatekeeping around PrEP. For instance, there’s a move underway by the U.S. Centers for Disease Control and Prevention, where drafts of updated PrEP guidelines call for clinicians to talk to any sexually active teenager and adult about PrEP.
For the PrEP Impact trial, gay and bisexual men who received sexual health care at UK National Health Service sexual health clinics were invited to enroll in the study based on national PrEP guidelines. Those guidelines included being a cisgender man who had had sex with men not currently living with HIV and reporting condomless anal sex in the last 3 months; having a male partner whose HIV status they don’t know or who doesn’t have an undetectable viral load and with whom they’ve had condomless anal sex; or someone who doesn’t reach those criteria but whom the clinician thinks would be a good candidate.
Between Oct. 2017 and Feb. 2020, a total of 17,770 gay and bisexual men and 503 transgender or nonbinary people enrolled in the trial and were paired with 97,098 gay and bisexual men who didn’t use PrEP. (Data from the transgender participants were reported in a separate presentation.) The median age was 27 years, with 14.4% of the cisgender gay men between the ages of 16 and 24. Three out of four cis men were White, most lived in London, and more than half came from very-low-income neighborhoods.
Participants and controls were assessed for whether they were at particularly high risk for acquiring HIV, such as having used PrEP, having had two or more HIV tests, having had a rectal bacterial sexually transmitted infection (STI), or having had contact with someone with HIV or syphilis.
At the end of Feb. 2020, 24 cisgender men on PrEP had acquired HIV compared with 670 in the control group – an 87% reduction in HIV acquisition. Only one of those 24 cis men had lab-confirmed high adherence to PrEP. However, because the hair samples used to judge drug concentration weren’t long enough, Dr. Sullivan and colleagues were unable to assess whether the person really was fully adherent to treatment for the length of the trial.
But when they looked at the assessed behavior of people who acquired HIV, the two groups diverged. While a full 92% of people using PrEP had had STI diagnoses and other markers of increased risk, that was true for only 71% of people not taking PrEP. That meant, Dr. Sullivan said in an interview, that screening guidelines for PrEP were missing 29% of people with low assessed risk for HIV who nevertheless acquired the virus.
The findings led Antonio Urbina, MD, who both prescribes PrEP and manages Mount Sinai Medical Center’s PrEP program in New York, to the same conclusion that Dr. Sullivan and her team came to: that no screener is going to account for everything, and that there may be things that patients don’t want to tell their clinicians about their risk, either because of their own internalized stigma or their calculation that they aren’t comfortable enough with their providers to be honest.
“It reinforces to me that I need to ask more open-ended questions regarding risk and then just talk more about PrEP,” said Dr. Urbina, professor of medicine at Icahn School of Medicine. “Risk is dynamic and changes. And the great thing about PrEP is that if the risk goes up or down, if you have PrEP on board, you maintain this protection against HIV.”
An accompanying presentation on the transgender and nonbinary participants in the Impact Trial found that just one of 503 PrEP users acquired HIV. But here, too, there were people who could have benefited from PrEP but didn’t take it: Of the 477 trans and nonbinary participants who acted as controls, 97 were eligible by current guidelines but didn’t take PrEP. One in four of those declined the offer to take PrEP; the rest weren’t able to take it because they lived outside the treatment area. That, combined with a significantly lower likelihood that Black trans and nonbinary people took PrEP, indicated that work needs to be done to address the needs of people geographically and ethnically.
The data on gay men also raised the “who’s left out” issue for Gina Simoncini, MD, medical director for the Philadelphia AIDS Healthcare Foundation Healthcare Center. Dr. Simoncini previously taught attending physicians at Temple University how to prescribe PrEP and has done many grand rounds for primary care providers on how to manage PrEP.
“My biggest issue with this data is: What about the people who aren’t going to sexual health clinics?” she said. “What about the kid who’s 16 and maybe just barely putting his feet into the waters of sex and doesn’t feel quite comfortable going to a sexual health clinic? What about the trans Indian girl who can’t get to sexual health clinics because of family stigma and cultural stigma? The more we move toward primary care, the more people need to get on board with this.”
Dr. Sullivan reports no relevant financial relationships. Dr. Simoncini is an employee of AIDS Healthcare Foundation and has received advisory board fees from ViiV Healthcare. Dr. Urbina sits on the scientific advisory councils for Gilead Sciences, ViiV Healthcare, and Merck.
A version of this article first appeared on Medscape.com.
Nearly one in three gay and bisexual men who were diagnosed with HIV at U.K. sexual health clinics didn’t meet the criteria for “high risk” that would signal to a clinician that they would be good candidates for pre-exposure prophylaxis (PrEP).
And that means that people who appear lower risk may still be good candidates for the HIV prevention pills, said Ann Sullivan, MD, consulting physician at Chelsea and Westminster Hospital, London.
“If people are coming forward for PrEP, they have self-identified that they need PrEP, [and] we should be allowing them to take PrEP,” said Dr. Sullivan at the 18th European AIDS Society Conference (EACS 2021). “We just need to trust patients. People know their risk, and we just have to accept that they know what they need best.”
And while this trial was made up of 95% gay and bisexual men, that ethos applies to every other group that could benefit from PrEP, including cisgender and transgender women and other gender-diverse people, Latinos, and Black Americans. In the United States, these groups make up nearly half of those who could benefit from PrEP under older guidelines but account for just 8% of people currently taking PrEP.
The finding also reinforces growing calls from health care providers to reduce gatekeeping around PrEP. For instance, there’s a move underway by the U.S. Centers for Disease Control and Prevention, where drafts of updated PrEP guidelines call for clinicians to talk to any sexually active teenager and adult about PrEP.
For the PrEP Impact trial, gay and bisexual men who received sexual health care at UK National Health Service sexual health clinics were invited to enroll in the study based on national PrEP guidelines. Those guidelines included being a cisgender man who had had sex with men not currently living with HIV and reporting condomless anal sex in the last 3 months; having a male partner whose HIV status they don’t know or who doesn’t have an undetectable viral load and with whom they’ve had condomless anal sex; or someone who doesn’t reach those criteria but whom the clinician thinks would be a good candidate.
Between Oct. 2017 and Feb. 2020, a total of 17,770 gay and bisexual men and 503 transgender or nonbinary people enrolled in the trial and were paired with 97,098 gay and bisexual men who didn’t use PrEP. (Data from the transgender participants were reported in a separate presentation.) The median age was 27 years, with 14.4% of the cisgender gay men between the ages of 16 and 24. Three out of four cis men were White, most lived in London, and more than half came from very-low-income neighborhoods.
Participants and controls were assessed for whether they were at particularly high risk for acquiring HIV, such as having used PrEP, having had two or more HIV tests, having had a rectal bacterial sexually transmitted infection (STI), or having had contact with someone with HIV or syphilis.
At the end of Feb. 2020, 24 cisgender men on PrEP had acquired HIV compared with 670 in the control group – an 87% reduction in HIV acquisition. Only one of those 24 cis men had lab-confirmed high adherence to PrEP. However, because the hair samples used to judge drug concentration weren’t long enough, Dr. Sullivan and colleagues were unable to assess whether the person really was fully adherent to treatment for the length of the trial.
But when they looked at the assessed behavior of people who acquired HIV, the two groups diverged. While a full 92% of people using PrEP had had STI diagnoses and other markers of increased risk, that was true for only 71% of people not taking PrEP. That meant, Dr. Sullivan said in an interview, that screening guidelines for PrEP were missing 29% of people with low assessed risk for HIV who nevertheless acquired the virus.
The findings led Antonio Urbina, MD, who both prescribes PrEP and manages Mount Sinai Medical Center’s PrEP program in New York, to the same conclusion that Dr. Sullivan and her team came to: that no screener is going to account for everything, and that there may be things that patients don’t want to tell their clinicians about their risk, either because of their own internalized stigma or their calculation that they aren’t comfortable enough with their providers to be honest.
“It reinforces to me that I need to ask more open-ended questions regarding risk and then just talk more about PrEP,” said Dr. Urbina, professor of medicine at Icahn School of Medicine. “Risk is dynamic and changes. And the great thing about PrEP is that if the risk goes up or down, if you have PrEP on board, you maintain this protection against HIV.”
An accompanying presentation on the transgender and nonbinary participants in the Impact Trial found that just one of 503 PrEP users acquired HIV. But here, too, there were people who could have benefited from PrEP but didn’t take it: Of the 477 trans and nonbinary participants who acted as controls, 97 were eligible by current guidelines but didn’t take PrEP. One in four of those declined the offer to take PrEP; the rest weren’t able to take it because they lived outside the treatment area. That, combined with a significantly lower likelihood that Black trans and nonbinary people took PrEP, indicated that work needs to be done to address the needs of people geographically and ethnically.
The data on gay men also raised the “who’s left out” issue for Gina Simoncini, MD, medical director for the Philadelphia AIDS Healthcare Foundation Healthcare Center. Dr. Simoncini previously taught attending physicians at Temple University how to prescribe PrEP and has done many grand rounds for primary care providers on how to manage PrEP.
“My biggest issue with this data is: What about the people who aren’t going to sexual health clinics?” she said. “What about the kid who’s 16 and maybe just barely putting his feet into the waters of sex and doesn’t feel quite comfortable going to a sexual health clinic? What about the trans Indian girl who can’t get to sexual health clinics because of family stigma and cultural stigma? The more we move toward primary care, the more people need to get on board with this.”
Dr. Sullivan reports no relevant financial relationships. Dr. Simoncini is an employee of AIDS Healthcare Foundation and has received advisory board fees from ViiV Healthcare. Dr. Urbina sits on the scientific advisory councils for Gilead Sciences, ViiV Healthcare, and Merck.
A version of this article first appeared on Medscape.com.
Long-acting HIV ART: Lessons from a year of Cabenuva
One year into offering the first long-acting injectable HIV treatment to his patients, Jonathan Angel, MD, head of the division of infectious diseases at the University of Ottawa, reported that 15 of the 21 of patients who started on the regimen are still taking it, all with viral suppression. Those who weren’t cited a combination of inconvenience, injection site pain, and “injection fatigue.”
These are just a few things HIV providers are learning as they begin what Chloe Orkin, MD, professor of HIV medicine at Queen Mary University of London, called a paradigm shift to long-acting treatment, which may soon include not just shots but rings, implants, and microarray patches.
“It’s a paradigm shift, and we are at the very beginning of this paradigm shift,” said Dr. Orkin, commenting during the discussion session of the European AIDS Clinical Society 2021 annual meeting. “We’re having to change our model, and it’s challenging.”
In the United States, the Food and Drug Administration approved the first long-acting injectable, a combination of cabotegravir and rilpivirine (CAB/RIL; Cabenuva, ViiV Healthcare) in January 2021. But it has been approved in Canada since March 2020 and available at Dr. Angel’s clinic since November 2020. It’s also available in Canada as an every-other-month shot. Injected into the buttocks, the shot was found to be noninferior to standard daily oral treatment in many studies, including the ATLAS, the ATLAS-2M – which tested the every-other-month approach – and FLAIR trials.
Dr. Angel’s clinic was part of all three of those trials, so his clinic has had 5 years’ experience preparing for the change in workflow and the new approach the shots require.
Of the 21 people Dr. Angel has treated, 11 were white Canadians, nine were Black African, and one was Indigenous Canadian, with women making up a third of the participants. Median age was 51 years, and all patients had had undetectable viral loads before beginning the regimen. (Studies of the drug’s effectiveness in people who struggle to take daily pills are still ongoing.)
Most of those 21 patients had had undetectable viral loads for more than 5 years, but a few had been undetectable for only 6 months before beginning the shots. Their immune systems were also healthy, with a median CD4 count of 618 cells/mcL. As in the clinical trials, none of the participants had experienced antiretroviral treatment failure. Because public health insurers in Canada have yet to approve the shots, Dr. Angel’s patients receiving Cabenuva also have private health insurance. Up to 90% of people in Canada receive pharmaceutical coverage through public insurance; therefore, the shot is not yet widely available.
Twenty patients switched from integrase-inhibitor regimens, and one had been receiving a nonnucleoside reverse transcriptase inhibitor–based regimen before starting Cabenuva.
And although the drug has not been approved for shot initiation this way, two patients requested – and Dr. Angel agreed – to start them on the shots without first doing a month of daily pills to check for safety.
“This is my conclusion from these data: the oral lead-in period is not necessary,” Dr. Angel said in his presentation at the meeting. “It can provide some comfort to either a physician or a patient, but it does not seem to be medically necessary.”
That approach is not without data to back it up. Research presented at HIV Glasgow 2020 showed that people who switched from daily oral dolutegravir/abacavir/lamivudine straight to the injections did so without problems.
At last clinic visit, 15 of those 21 were still receiving the shots. None have experienced treatment failure, and all were still virally suppressed. Four participants left the trials and one more person opted to return to daily pills, citing some level of what Dr. Angel called “injection fatigue.”
“Just as we use the term ‘pill fatigue’ for patients who are tired of taking pills, patients do get tired of coming in monthly for their visits and injections,” he said. They find the trip to the clinic for the intramuscular injections “inconvenient,” he said.
Unlike in the United States, where Cabenuva is approved for only monthly injections, Health Canada has already approved the shot for every-other-month injections, which Dr. Angel said may reduce the odds of injection fatigue.
Dr. Angel’s presentation drew comments, questions, and excitement from the crowd. Annemarie Wensing, MD, assistant professor of medicine at University Medical Center Utrecht (the Netherlands), asked whether dispensing with the oral lead-in period could mean that these shots could be useful for people going on longer trips, people having surgeries where they can’t swallow pills, or in other scenarios.
“These are not hypothetical conversations,” Dr. Angel said. “I’m having these conversations with patients now – temporary use, they travel for 3 months and come back, can they go from injectable to oral to injectable.”
For now, he said, the answer is, “We’ll figure it out.”
Meanwhile, there’s another big question when it comes to injectables, said Marta Vas ylyev, MD, from Lviv (Ukraine) Regional AIDS Center: When will they be available to the people who might benefit most from them – people in resource-limited settings, people who so far have struggled to remember to take their pills every day?
For now, Dr. Angel replied, injectables continue to be a treatment only for those who are already doing well while receiving HIV treatment: those with already suppressed viral load, who are good at taking daily pills, and who are being treated at well-resourced clinics.
“There are huge obstacles to overcome if this is ever to be available [in resource-limited settings], and way more obstacles than there are with any oral therapies,” he said. “There’s not been much discussion here about the necessity of cold-chain requirements of pharmacies either centrally or locally, [or] the requirements of additional nurses or health care staff to administer the medication. So you’re looking at a very resource-intensive therapy, which now is fairly restrictive [as to] who will have access to it.”
Dr. Angel reports serving on advisory boards for ViiV Healthcare and Gilead Sciences and has done contract research for ViiV Healthcare, Gilead, and Merck. Dr. Orkin has received research grants, fees as a consultant, travel sponsorship, and speaker fees from ViiV, Merck, and GlaxoSmithKline. Dr. Vasylyev reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One year into offering the first long-acting injectable HIV treatment to his patients, Jonathan Angel, MD, head of the division of infectious diseases at the University of Ottawa, reported that 15 of the 21 of patients who started on the regimen are still taking it, all with viral suppression. Those who weren’t cited a combination of inconvenience, injection site pain, and “injection fatigue.”
These are just a few things HIV providers are learning as they begin what Chloe Orkin, MD, professor of HIV medicine at Queen Mary University of London, called a paradigm shift to long-acting treatment, which may soon include not just shots but rings, implants, and microarray patches.
“It’s a paradigm shift, and we are at the very beginning of this paradigm shift,” said Dr. Orkin, commenting during the discussion session of the European AIDS Clinical Society 2021 annual meeting. “We’re having to change our model, and it’s challenging.”
In the United States, the Food and Drug Administration approved the first long-acting injectable, a combination of cabotegravir and rilpivirine (CAB/RIL; Cabenuva, ViiV Healthcare) in January 2021. But it has been approved in Canada since March 2020 and available at Dr. Angel’s clinic since November 2020. It’s also available in Canada as an every-other-month shot. Injected into the buttocks, the shot was found to be noninferior to standard daily oral treatment in many studies, including the ATLAS, the ATLAS-2M – which tested the every-other-month approach – and FLAIR trials.
Dr. Angel’s clinic was part of all three of those trials, so his clinic has had 5 years’ experience preparing for the change in workflow and the new approach the shots require.
Of the 21 people Dr. Angel has treated, 11 were white Canadians, nine were Black African, and one was Indigenous Canadian, with women making up a third of the participants. Median age was 51 years, and all patients had had undetectable viral loads before beginning the regimen. (Studies of the drug’s effectiveness in people who struggle to take daily pills are still ongoing.)
Most of those 21 patients had had undetectable viral loads for more than 5 years, but a few had been undetectable for only 6 months before beginning the shots. Their immune systems were also healthy, with a median CD4 count of 618 cells/mcL. As in the clinical trials, none of the participants had experienced antiretroviral treatment failure. Because public health insurers in Canada have yet to approve the shots, Dr. Angel’s patients receiving Cabenuva also have private health insurance. Up to 90% of people in Canada receive pharmaceutical coverage through public insurance; therefore, the shot is not yet widely available.
Twenty patients switched from integrase-inhibitor regimens, and one had been receiving a nonnucleoside reverse transcriptase inhibitor–based regimen before starting Cabenuva.
And although the drug has not been approved for shot initiation this way, two patients requested – and Dr. Angel agreed – to start them on the shots without first doing a month of daily pills to check for safety.
“This is my conclusion from these data: the oral lead-in period is not necessary,” Dr. Angel said in his presentation at the meeting. “It can provide some comfort to either a physician or a patient, but it does not seem to be medically necessary.”
That approach is not without data to back it up. Research presented at HIV Glasgow 2020 showed that people who switched from daily oral dolutegravir/abacavir/lamivudine straight to the injections did so without problems.
At last clinic visit, 15 of those 21 were still receiving the shots. None have experienced treatment failure, and all were still virally suppressed. Four participants left the trials and one more person opted to return to daily pills, citing some level of what Dr. Angel called “injection fatigue.”
“Just as we use the term ‘pill fatigue’ for patients who are tired of taking pills, patients do get tired of coming in monthly for their visits and injections,” he said. They find the trip to the clinic for the intramuscular injections “inconvenient,” he said.
Unlike in the United States, where Cabenuva is approved for only monthly injections, Health Canada has already approved the shot for every-other-month injections, which Dr. Angel said may reduce the odds of injection fatigue.
Dr. Angel’s presentation drew comments, questions, and excitement from the crowd. Annemarie Wensing, MD, assistant professor of medicine at University Medical Center Utrecht (the Netherlands), asked whether dispensing with the oral lead-in period could mean that these shots could be useful for people going on longer trips, people having surgeries where they can’t swallow pills, or in other scenarios.
“These are not hypothetical conversations,” Dr. Angel said. “I’m having these conversations with patients now – temporary use, they travel for 3 months and come back, can they go from injectable to oral to injectable.”
For now, he said, the answer is, “We’ll figure it out.”
Meanwhile, there’s another big question when it comes to injectables, said Marta Vas ylyev, MD, from Lviv (Ukraine) Regional AIDS Center: When will they be available to the people who might benefit most from them – people in resource-limited settings, people who so far have struggled to remember to take their pills every day?
For now, Dr. Angel replied, injectables continue to be a treatment only for those who are already doing well while receiving HIV treatment: those with already suppressed viral load, who are good at taking daily pills, and who are being treated at well-resourced clinics.
“There are huge obstacles to overcome if this is ever to be available [in resource-limited settings], and way more obstacles than there are with any oral therapies,” he said. “There’s not been much discussion here about the necessity of cold-chain requirements of pharmacies either centrally or locally, [or] the requirements of additional nurses or health care staff to administer the medication. So you’re looking at a very resource-intensive therapy, which now is fairly restrictive [as to] who will have access to it.”
Dr. Angel reports serving on advisory boards for ViiV Healthcare and Gilead Sciences and has done contract research for ViiV Healthcare, Gilead, and Merck. Dr. Orkin has received research grants, fees as a consultant, travel sponsorship, and speaker fees from ViiV, Merck, and GlaxoSmithKline. Dr. Vasylyev reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
One year into offering the first long-acting injectable HIV treatment to his patients, Jonathan Angel, MD, head of the division of infectious diseases at the University of Ottawa, reported that 15 of the 21 of patients who started on the regimen are still taking it, all with viral suppression. Those who weren’t cited a combination of inconvenience, injection site pain, and “injection fatigue.”
These are just a few things HIV providers are learning as they begin what Chloe Orkin, MD, professor of HIV medicine at Queen Mary University of London, called a paradigm shift to long-acting treatment, which may soon include not just shots but rings, implants, and microarray patches.
“It’s a paradigm shift, and we are at the very beginning of this paradigm shift,” said Dr. Orkin, commenting during the discussion session of the European AIDS Clinical Society 2021 annual meeting. “We’re having to change our model, and it’s challenging.”
In the United States, the Food and Drug Administration approved the first long-acting injectable, a combination of cabotegravir and rilpivirine (CAB/RIL; Cabenuva, ViiV Healthcare) in January 2021. But it has been approved in Canada since March 2020 and available at Dr. Angel’s clinic since November 2020. It’s also available in Canada as an every-other-month shot. Injected into the buttocks, the shot was found to be noninferior to standard daily oral treatment in many studies, including the ATLAS, the ATLAS-2M – which tested the every-other-month approach – and FLAIR trials.
Dr. Angel’s clinic was part of all three of those trials, so his clinic has had 5 years’ experience preparing for the change in workflow and the new approach the shots require.
Of the 21 people Dr. Angel has treated, 11 were white Canadians, nine were Black African, and one was Indigenous Canadian, with women making up a third of the participants. Median age was 51 years, and all patients had had undetectable viral loads before beginning the regimen. (Studies of the drug’s effectiveness in people who struggle to take daily pills are still ongoing.)
Most of those 21 patients had had undetectable viral loads for more than 5 years, but a few had been undetectable for only 6 months before beginning the shots. Their immune systems were also healthy, with a median CD4 count of 618 cells/mcL. As in the clinical trials, none of the participants had experienced antiretroviral treatment failure. Because public health insurers in Canada have yet to approve the shots, Dr. Angel’s patients receiving Cabenuva also have private health insurance. Up to 90% of people in Canada receive pharmaceutical coverage through public insurance; therefore, the shot is not yet widely available.
Twenty patients switched from integrase-inhibitor regimens, and one had been receiving a nonnucleoside reverse transcriptase inhibitor–based regimen before starting Cabenuva.
And although the drug has not been approved for shot initiation this way, two patients requested – and Dr. Angel agreed – to start them on the shots without first doing a month of daily pills to check for safety.
“This is my conclusion from these data: the oral lead-in period is not necessary,” Dr. Angel said in his presentation at the meeting. “It can provide some comfort to either a physician or a patient, but it does not seem to be medically necessary.”
That approach is not without data to back it up. Research presented at HIV Glasgow 2020 showed that people who switched from daily oral dolutegravir/abacavir/lamivudine straight to the injections did so without problems.
At last clinic visit, 15 of those 21 were still receiving the shots. None have experienced treatment failure, and all were still virally suppressed. Four participants left the trials and one more person opted to return to daily pills, citing some level of what Dr. Angel called “injection fatigue.”
“Just as we use the term ‘pill fatigue’ for patients who are tired of taking pills, patients do get tired of coming in monthly for their visits and injections,” he said. They find the trip to the clinic for the intramuscular injections “inconvenient,” he said.
Unlike in the United States, where Cabenuva is approved for only monthly injections, Health Canada has already approved the shot for every-other-month injections, which Dr. Angel said may reduce the odds of injection fatigue.
Dr. Angel’s presentation drew comments, questions, and excitement from the crowd. Annemarie Wensing, MD, assistant professor of medicine at University Medical Center Utrecht (the Netherlands), asked whether dispensing with the oral lead-in period could mean that these shots could be useful for people going on longer trips, people having surgeries where they can’t swallow pills, or in other scenarios.
“These are not hypothetical conversations,” Dr. Angel said. “I’m having these conversations with patients now – temporary use, they travel for 3 months and come back, can they go from injectable to oral to injectable.”
For now, he said, the answer is, “We’ll figure it out.”
Meanwhile, there’s another big question when it comes to injectables, said Marta Vas ylyev, MD, from Lviv (Ukraine) Regional AIDS Center: When will they be available to the people who might benefit most from them – people in resource-limited settings, people who so far have struggled to remember to take their pills every day?
For now, Dr. Angel replied, injectables continue to be a treatment only for those who are already doing well while receiving HIV treatment: those with already suppressed viral load, who are good at taking daily pills, and who are being treated at well-resourced clinics.
“There are huge obstacles to overcome if this is ever to be available [in resource-limited settings], and way more obstacles than there are with any oral therapies,” he said. “There’s not been much discussion here about the necessity of cold-chain requirements of pharmacies either centrally or locally, [or] the requirements of additional nurses or health care staff to administer the medication. So you’re looking at a very resource-intensive therapy, which now is fairly restrictive [as to] who will have access to it.”
Dr. Angel reports serving on advisory boards for ViiV Healthcare and Gilead Sciences and has done contract research for ViiV Healthcare, Gilead, and Merck. Dr. Orkin has received research grants, fees as a consultant, travel sponsorship, and speaker fees from ViiV, Merck, and GlaxoSmithKline. Dr. Vasylyev reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinicians may overprescribe clarithromycin for H. pylori
Clinicians are prescribing clarithromycin at high rates for Helicobacter pylori infections, despite increasing resistance to this antibiotic, researchers say.
In an analysis of 1 million U.S. prescriptions for H. pylori infections, 80% contained clarithromycin, said Carol Rockett, PharmD, associate vice president of RedHill Biopharma in Raleigh, N.C.
Dr. Rockett presented the findings at the annual meeting of the American College of Gastroenterology.
“Multiple talks [at the meeting] have suggested that the use of clarithromycin in H. pylori is obsolete,” she told this news organization. “Clarithromycin is particularly ineffective in people with a genetic variant that causes rapid metabolism.”
According to the 2017 ACG clinical guideline for treating H. pylori, patients diagnosed with this infection should be asked about their previous antibiotic exposure prior to treatment.
Additionally, clinicians should prescribe clarithromycin triple therapy with a proton pump inhibitor (PPI) and amoxicillin or metronidazole as a first-line treatment only in “regions where H. pylori clarithromycin resistance is known to be less than 15%” and in patients with no previous history of macrolide exposure.
The guideline puts bismuth quadruple therapy, consisting of a PPI, bismuth, tetracycline, and a nitroimidazole, at the top of its list of six alternative first-line therapies. However, three of the six alternatives include clarithromycin.
ERADICATE Hp and ERADICATE Hp2
To understand how U.S. physicians are treating patients with H. pylori, Dr. Rockett’s colleagues analyzed data from two phase 3 clinical trials of RedHill’s RHB-105 (Talicia): ERADICATE Hp and ERADICATE Hp2.
RHB-105 is an all-in‐one combination of omeprazole (40 mg), amoxicillin (1,000 mg), and rifabutin (50 mg) that the Food and Drug Administration approved for treatment of H pylori in 2019.
The researchers followed 38 subjects from ERADICATE Hp who remained positive for H. pylori after the study’s completion. A total of 33 had received a placebo in that trial, while the other 5 had received RHB-105.
The researchers obtained data on 31 of these patients. The overall cure rate was 61.3%. Of the 31 patients, 27 received a regimen including clarithromycin. Their cure rate was 59.3%.
Turning to ERADICATE Hp2, the researchers obtained data on 94 patients whose H. pylori infections persisted after the trial. Of those, 67 had received an active comparator (amoxicillin 250 mg and omeprazole 10 mg) and 27 had received RHB-105.
The overall cure rate was 56.2%. For the 48 subjects who received therapies including clarithromycin, the cure rate was 60.4%. For the 22 subjects who received a bismuth-based quadruple regimen, the cure rate was 45.4%.
In another analysis, the researchers crunched 12 months of numbers from IQVIA PharMetrics Plus medical and prescription claim database of over 1 million prescriptions for H. pylori. They found that 80% of the prescriptions made by gastroenterologists were for regimens containing clarithromycin. That proportion increased to 84% for physician assistants and internists, 85% for nurse practitioners, 86% for family practitioners, and 89% for general practitioners.
Finally, the researchers also analyzed patients for CYP2C19 gene status. They tested 65 subjects who received RHB-105 in ERADICATE Hp and all 445 subjects in ERADICATE Hp2. They found that 58.5% in ERADICATE Hp and 48.6% in ERADICATE Hp2 were normal metabolizers.
In 20 normal metabolizers who received clarithromycin, the drug eradicated the infection in 16 (80%). Out of 11 rapid metabolizers, clarithromycin eradicated the bacterium in 2 (18.2%). The difference was statistically significant (P = .0017).
“With clarithromycin, you can see that the efficacy is reduced in those patients who are rapid metabolizers,” Dr. Rockett said. “We didn’t see that with rifabutin [one of the drugs in RHB-105].”
Jared Magee, DO, MPH, a gastroenterology fellow at the Walter Reed National Military Medical Center in Bethesda, Md., said in treating H. pylori infections, he checks the patients’ medical records to see what antibiotics they have received in the past and generally begins treatment with the bismuth quadruple therapy.
“There is education needed to get the data out there that clarithromycin-based therapies may not be the right choice for patients,” he said. “There is a subset who will do well with it, but I think where we’re at now, with the frequency of macrolide prescriptions for other conditions, that clarithromycin is going to be a difficult therapy for a lot of people.”
Clinicians who are not gastroenterologists may not be aware of the guideline promulgated by the ACG, he pointed out.
Dr. Rockett is an employee of RedHill Biopharma. Dr. Magee has disclosed no relevant financial relationships. The study was funded by RedHill Biopharma.
A version of this article first appeared on Medscape.com.
Clinicians are prescribing clarithromycin at high rates for Helicobacter pylori infections, despite increasing resistance to this antibiotic, researchers say.
In an analysis of 1 million U.S. prescriptions for H. pylori infections, 80% contained clarithromycin, said Carol Rockett, PharmD, associate vice president of RedHill Biopharma in Raleigh, N.C.
Dr. Rockett presented the findings at the annual meeting of the American College of Gastroenterology.
“Multiple talks [at the meeting] have suggested that the use of clarithromycin in H. pylori is obsolete,” she told this news organization. “Clarithromycin is particularly ineffective in people with a genetic variant that causes rapid metabolism.”
According to the 2017 ACG clinical guideline for treating H. pylori, patients diagnosed with this infection should be asked about their previous antibiotic exposure prior to treatment.
Additionally, clinicians should prescribe clarithromycin triple therapy with a proton pump inhibitor (PPI) and amoxicillin or metronidazole as a first-line treatment only in “regions where H. pylori clarithromycin resistance is known to be less than 15%” and in patients with no previous history of macrolide exposure.
The guideline puts bismuth quadruple therapy, consisting of a PPI, bismuth, tetracycline, and a nitroimidazole, at the top of its list of six alternative first-line therapies. However, three of the six alternatives include clarithromycin.
ERADICATE Hp and ERADICATE Hp2
To understand how U.S. physicians are treating patients with H. pylori, Dr. Rockett’s colleagues analyzed data from two phase 3 clinical trials of RedHill’s RHB-105 (Talicia): ERADICATE Hp and ERADICATE Hp2.
RHB-105 is an all-in‐one combination of omeprazole (40 mg), amoxicillin (1,000 mg), and rifabutin (50 mg) that the Food and Drug Administration approved for treatment of H pylori in 2019.
The researchers followed 38 subjects from ERADICATE Hp who remained positive for H. pylori after the study’s completion. A total of 33 had received a placebo in that trial, while the other 5 had received RHB-105.
The researchers obtained data on 31 of these patients. The overall cure rate was 61.3%. Of the 31 patients, 27 received a regimen including clarithromycin. Their cure rate was 59.3%.
Turning to ERADICATE Hp2, the researchers obtained data on 94 patients whose H. pylori infections persisted after the trial. Of those, 67 had received an active comparator (amoxicillin 250 mg and omeprazole 10 mg) and 27 had received RHB-105.
The overall cure rate was 56.2%. For the 48 subjects who received therapies including clarithromycin, the cure rate was 60.4%. For the 22 subjects who received a bismuth-based quadruple regimen, the cure rate was 45.4%.
In another analysis, the researchers crunched 12 months of numbers from IQVIA PharMetrics Plus medical and prescription claim database of over 1 million prescriptions for H. pylori. They found that 80% of the prescriptions made by gastroenterologists were for regimens containing clarithromycin. That proportion increased to 84% for physician assistants and internists, 85% for nurse practitioners, 86% for family practitioners, and 89% for general practitioners.
Finally, the researchers also analyzed patients for CYP2C19 gene status. They tested 65 subjects who received RHB-105 in ERADICATE Hp and all 445 subjects in ERADICATE Hp2. They found that 58.5% in ERADICATE Hp and 48.6% in ERADICATE Hp2 were normal metabolizers.
In 20 normal metabolizers who received clarithromycin, the drug eradicated the infection in 16 (80%). Out of 11 rapid metabolizers, clarithromycin eradicated the bacterium in 2 (18.2%). The difference was statistically significant (P = .0017).
“With clarithromycin, you can see that the efficacy is reduced in those patients who are rapid metabolizers,” Dr. Rockett said. “We didn’t see that with rifabutin [one of the drugs in RHB-105].”
Jared Magee, DO, MPH, a gastroenterology fellow at the Walter Reed National Military Medical Center in Bethesda, Md., said in treating H. pylori infections, he checks the patients’ medical records to see what antibiotics they have received in the past and generally begins treatment with the bismuth quadruple therapy.
“There is education needed to get the data out there that clarithromycin-based therapies may not be the right choice for patients,” he said. “There is a subset who will do well with it, but I think where we’re at now, with the frequency of macrolide prescriptions for other conditions, that clarithromycin is going to be a difficult therapy for a lot of people.”
Clinicians who are not gastroenterologists may not be aware of the guideline promulgated by the ACG, he pointed out.
Dr. Rockett is an employee of RedHill Biopharma. Dr. Magee has disclosed no relevant financial relationships. The study was funded by RedHill Biopharma.
A version of this article first appeared on Medscape.com.
Clinicians are prescribing clarithromycin at high rates for Helicobacter pylori infections, despite increasing resistance to this antibiotic, researchers say.
In an analysis of 1 million U.S. prescriptions for H. pylori infections, 80% contained clarithromycin, said Carol Rockett, PharmD, associate vice president of RedHill Biopharma in Raleigh, N.C.
Dr. Rockett presented the findings at the annual meeting of the American College of Gastroenterology.
“Multiple talks [at the meeting] have suggested that the use of clarithromycin in H. pylori is obsolete,” she told this news organization. “Clarithromycin is particularly ineffective in people with a genetic variant that causes rapid metabolism.”
According to the 2017 ACG clinical guideline for treating H. pylori, patients diagnosed with this infection should be asked about their previous antibiotic exposure prior to treatment.
Additionally, clinicians should prescribe clarithromycin triple therapy with a proton pump inhibitor (PPI) and amoxicillin or metronidazole as a first-line treatment only in “regions where H. pylori clarithromycin resistance is known to be less than 15%” and in patients with no previous history of macrolide exposure.
The guideline puts bismuth quadruple therapy, consisting of a PPI, bismuth, tetracycline, and a nitroimidazole, at the top of its list of six alternative first-line therapies. However, three of the six alternatives include clarithromycin.
ERADICATE Hp and ERADICATE Hp2
To understand how U.S. physicians are treating patients with H. pylori, Dr. Rockett’s colleagues analyzed data from two phase 3 clinical trials of RedHill’s RHB-105 (Talicia): ERADICATE Hp and ERADICATE Hp2.
RHB-105 is an all-in‐one combination of omeprazole (40 mg), amoxicillin (1,000 mg), and rifabutin (50 mg) that the Food and Drug Administration approved for treatment of H pylori in 2019.
The researchers followed 38 subjects from ERADICATE Hp who remained positive for H. pylori after the study’s completion. A total of 33 had received a placebo in that trial, while the other 5 had received RHB-105.
The researchers obtained data on 31 of these patients. The overall cure rate was 61.3%. Of the 31 patients, 27 received a regimen including clarithromycin. Their cure rate was 59.3%.
Turning to ERADICATE Hp2, the researchers obtained data on 94 patients whose H. pylori infections persisted after the trial. Of those, 67 had received an active comparator (amoxicillin 250 mg and omeprazole 10 mg) and 27 had received RHB-105.
The overall cure rate was 56.2%. For the 48 subjects who received therapies including clarithromycin, the cure rate was 60.4%. For the 22 subjects who received a bismuth-based quadruple regimen, the cure rate was 45.4%.
In another analysis, the researchers crunched 12 months of numbers from IQVIA PharMetrics Plus medical and prescription claim database of over 1 million prescriptions for H. pylori. They found that 80% of the prescriptions made by gastroenterologists were for regimens containing clarithromycin. That proportion increased to 84% for physician assistants and internists, 85% for nurse practitioners, 86% for family practitioners, and 89% for general practitioners.
Finally, the researchers also analyzed patients for CYP2C19 gene status. They tested 65 subjects who received RHB-105 in ERADICATE Hp and all 445 subjects in ERADICATE Hp2. They found that 58.5% in ERADICATE Hp and 48.6% in ERADICATE Hp2 were normal metabolizers.
In 20 normal metabolizers who received clarithromycin, the drug eradicated the infection in 16 (80%). Out of 11 rapid metabolizers, clarithromycin eradicated the bacterium in 2 (18.2%). The difference was statistically significant (P = .0017).
“With clarithromycin, you can see that the efficacy is reduced in those patients who are rapid metabolizers,” Dr. Rockett said. “We didn’t see that with rifabutin [one of the drugs in RHB-105].”
Jared Magee, DO, MPH, a gastroenterology fellow at the Walter Reed National Military Medical Center in Bethesda, Md., said in treating H. pylori infections, he checks the patients’ medical records to see what antibiotics they have received in the past and generally begins treatment with the bismuth quadruple therapy.
“There is education needed to get the data out there that clarithromycin-based therapies may not be the right choice for patients,” he said. “There is a subset who will do well with it, but I think where we’re at now, with the frequency of macrolide prescriptions for other conditions, that clarithromycin is going to be a difficult therapy for a lot of people.”
Clinicians who are not gastroenterologists may not be aware of the guideline promulgated by the ACG, he pointed out.
Dr. Rockett is an employee of RedHill Biopharma. Dr. Magee has disclosed no relevant financial relationships. The study was funded by RedHill Biopharma.
A version of this article first appeared on Medscape.com.
Antidepressant may cut COVID-19–related hospitalization, mortality: TOGETHER
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
The antidepressant fluvoxamine (Luvox) may prevent hospitalization and death in outpatients with COVID-19, new research suggests.
Results from the placebo-controlled, multisite, phase 3 TOGETHER trial showed that in COVID-19 outpatients at high risk for complications, hospitalizations were cut by 66% and deaths were reduced by 91% in those who tolerated fluvoxamine.
“Our trial has found that fluvoxamine, an inexpensive existing drug, reduces the need for advanced disease care in this high-risk population,” wrote the investigators, led by Gilmar Reis, MD, PhD, research division, Cardresearch, Belo Horizonte, Brazil.
The findings were published online Oct. 27 in The Lancet Global Health.
Alternative mechanisms
Fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), is an antidepressant commonly prescribed for obsessive-compulsive disorder.
Besides its known effects on serotonin, the drug acts in other molecular pathways to dampen the production of inflammatory cytokines. Those alternative mechanisms are the ones believed to help patients with COVID-19, said coinvestigator Angela Reiersen, MD, child psychiatrist at Washington University, St. Louis.
Based on cell culture and mouse studies showing effects of the molecule’s binding to the sigma-1 receptor in the endoplasmic reticulum, Dr. Reiersen came up with the idea of testing if fluvoxamine could keep COVID-19 from progressing in newly infected patients.
Dr. Reiersen and psychiatrist Eric Lenze, MD, also from Washington University, led the phase 2 trial that initially suggested fluvoxamine’s promise as an outpatient medication. They are coinvestigators on the new phase 3 adaptive platform trial called TOGETHER, which was conducted by an international team of investigators in Brazil, Canada, and the United States.
For this latest study, researchers at McMaster University, Hamilton, Ont., partnered with the research clinic Cardresearch in Brazil to recruit unvaccinated, high-risk adults within 7 days of developing flu-like symptoms from COVID-19. They analyzed 1,497 newly symptomatic COVID-19 patients at 11 clinical sites in Brazil.
Patients entered the trial between January and August 2021 and were assigned to receive 100 mg fluvoxamine or placebo pills twice a day for 10 days. Investigators monitored participants through 28 days post treatment, noting whether complications developed requiring hospitalization or more than 6 hours of emergency care.
In the placebo group, 119 of 756 patients (15.7%) worsened to this extent. In comparison, 79 of 741 (10.7%) fluvoxamine-treated patients met these primary criteria. This represented a 32% reduction in hospitalizations and emergency visits.
Additional analysis requested
As Lancet Global Health reviewed these findings from the submitted manuscript, journal reviewers requested an additional “pre-protocol analysis” that was not specified in the trial’s original protocol. The request was to examine the subgroup of patients with good adherence (74% of treated group, 82% of placebo group).
Among these three quarters of patients who took at least 80% of their doses, benefits were better.
Fluvoxamine cut serious complications in this group by 66% and reduced mortality by 91%. In the placebo group, 12 people died compared with one who received the study drug.
from complications of the infection.
However, clinicians should note that the drug can cause side effects such as nausea, dizziness, and insomnia, she added. In addition, because it prevents the body from metabolizing caffeine, patients should limit their daily intake to half of a small cup of coffee or one can of soda or one tea while taking the drug.
Previous research has shown that fluvoxamine affects the metabolism of some drugs, such as theophylline, clozapine, olanzapine, and tizanidine.
Despite huge challenges with studying generic drugs as early COVID-19 treatment, the TOGETHER trial shows it is possible to produce quality evidence during a pandemic on a shoestring budget, noted co-principal investigator Edward Mills, PhD, professor in the department of health research methods, evidence, and impact at McMaster University.
To screen more than 12,000 patients and enroll 4,000 to test nine interventions, “our total budget was less than $8 million,” Dr. Mills said. The trial was funded by Fast Grants and the Rainwater Charitable Foundation.
‘A $10 medicine’
Commenting on the findings, David Boulware, MD, MPH, an infectious disease physician-researcher at the University of Minnesota in Minneapolis, noted fluvoxamine is “a $10 medicine that’s available and has a very good safety record.”
By comparison, a 5-day course of Merck’s antiviral molnupiravir, another oral drug that the company says can cut hospitalizations in COVID-19 outpatients, costs $700. However, the data have not been peer reviewed – and molnupiravir is not currently available and has unknown long-term safety implications, Dr. Boulware said.
Pharmaceutical companies typically spend tens of thousands of dollars on a trial evaluating a single drug, he noted.
In addition, the National Institutes of Health’s ACTIV-6 study, a nationwide trial on the effect of fluvoxamine and other repurposed generic drugs on thousands of COVID-19 outpatients, is a $110 million effort, according to Dr. Boulware, who cochairs its steering committee.
ACTIV-6 is currently enrolling outpatients with COVID-19 to test a lower dose of fluvoxamine, at 50 mg twice daily instead of the 100-mg dose used in the TOGETHER trial, as well as ivermectin and inhaled fluticasone. The COVID-OUT trial is also recruiting newly diagnosed COVID-19 patients to test various combinations of fluvoxamine, ivermectin, and the diabetes drug metformin.
Unanswered safety, efficacy questions
In an accompanying editorial in The Lancet Global Health, Otavio Berwanger, MD, cardiologist and clinical trialist, Academic Research Organization, Hospital Israelita Albert Einstein, São Paulo, Brazil, commends the investigators for rapidly generating evidence during the COVID-19 pandemic.
However, despite the important findings, “some questions related to efficacy and safety of fluvoxamine for patients with COVID-19 remain open,” Dr. Berwanger wrote.
The effects of the drug on reducing both mortality and hospitalizations also “still need addressing,” he noted.
“In addition, it remains to be established whether fluvoxamine has an additive effect to other therapies such as monoclonal antibodies and budesonide, and what is the optimal fluvoxamine therapeutic scheme,” wrote Dr. Berwanger.
In an interview, he noted that 74% of the Brazil population have currently received at least one dose of a COVID-19 vaccine and 52% have received two doses. In addition, deaths have gone down from 4,000 per day during the March-April second wave to about 400 per day. “That is still unfortunate and far from ideal,” he said. In total, they have had about 600,000 deaths because of COVID-19.
Asked whether public health authorities are now recommending fluvoxamine as an early treatment for COVID-19 based on the TOGETHER trial data, Dr. Berwanger answered, “Not yet.
“I believe medical and scientific societies will need to critically appraise the manuscript in order to inform their decisions and recommendations. This interesting trial adds another important piece of information in this regard,” he said.
Dr. Reiersen and Dr. Lenze are inventors on a patent application related to methods for treating COVID-19, which was filed by Washington University. Dr. Mills reports no relevant financial relationships, as does Dr. Boulware – except that the TOGETHER trial funders are also funding the University of Minnesota COVID-OUT trial. Dr. Berwanger reports having received research grants outside of the submitted work that were paid to his institution by AstraZeneca, Bayer, Amgen, Servier, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
CDC: Urgency remains to vaccinate children
The CDC is urging parents and guardians to vaccinate children ages 5-11 against COVID-19 once the shot is fully approved, despite questions from FDA advisers about the urgency given falling national case rates.
On Oct. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee voted to recommend a 10-microgram shot for children. Though 17 of the 18 panelists voted in favor of it, some members said it was a hard decision and questioned the need for it now that cases and hospitalizations are down.
“There’s urgency because we’re seeing disease in children, we’ve seen deaths in children, we’ve seen long COVID,” CDC Director Rochelle Walensky, MD, said at a White House briefing on Oct. 27. “Certainly we’ve seen cases come down before, and the way to prevent surges again is to get more and more people vaccinated.”
CDC data presented at an Oct. 26 advisory committee meeting show that among children 5-11, COVID-19 was one of top 10 causes of death over last year, Dr. Walensky said. There have been more than 8,300 hospitalizations and 745 deaths in children under 18.
As of yesterday, the 7-day average of daily COVID-19 cases was 65,900, a 16% decrease from the prior week. Hospitalizations are down 54% from the week of Aug. 28, Dr. Walensky said.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” James Hildreth, MD, president and CEO at Meharry Medical College in Nashville, said at the advisory committee meeting on Oct. 26.
But according to one CDC study, hospitalization rates for adolescents were 10 times higher in those who were unvaccinated. Another study found that COVID-related emergency room visits and hospital admissions among children were more than 3 times as high in states with the lowest vaccination rates.
“We are down from our peak in early September, and we are now heading in the right direction, but with cases still high, we must remain vigilant heading into the colder, drier winter months,” Dr. Walensky said, noting that the 7-day average of daily deaths still exceeds 1,000.
Meanwhile, the booster program is off to a “very strong start,” said White House COVID-19 Response Coordinator Jeff Zients.
In the 5 days since authorizations, about 15 million people have received an additional dose of the Pfizer, Moderna, and Johnson & Johnson vaccines.
A version of this article first appeared on WebMD.com.
The CDC is urging parents and guardians to vaccinate children ages 5-11 against COVID-19 once the shot is fully approved, despite questions from FDA advisers about the urgency given falling national case rates.
On Oct. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee voted to recommend a 10-microgram shot for children. Though 17 of the 18 panelists voted in favor of it, some members said it was a hard decision and questioned the need for it now that cases and hospitalizations are down.
“There’s urgency because we’re seeing disease in children, we’ve seen deaths in children, we’ve seen long COVID,” CDC Director Rochelle Walensky, MD, said at a White House briefing on Oct. 27. “Certainly we’ve seen cases come down before, and the way to prevent surges again is to get more and more people vaccinated.”
CDC data presented at an Oct. 26 advisory committee meeting show that among children 5-11, COVID-19 was one of top 10 causes of death over last year, Dr. Walensky said. There have been more than 8,300 hospitalizations and 745 deaths in children under 18.
As of yesterday, the 7-day average of daily COVID-19 cases was 65,900, a 16% decrease from the prior week. Hospitalizations are down 54% from the week of Aug. 28, Dr. Walensky said.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” James Hildreth, MD, president and CEO at Meharry Medical College in Nashville, said at the advisory committee meeting on Oct. 26.
But according to one CDC study, hospitalization rates for adolescents were 10 times higher in those who were unvaccinated. Another study found that COVID-related emergency room visits and hospital admissions among children were more than 3 times as high in states with the lowest vaccination rates.
“We are down from our peak in early September, and we are now heading in the right direction, but with cases still high, we must remain vigilant heading into the colder, drier winter months,” Dr. Walensky said, noting that the 7-day average of daily deaths still exceeds 1,000.
Meanwhile, the booster program is off to a “very strong start,” said White House COVID-19 Response Coordinator Jeff Zients.
In the 5 days since authorizations, about 15 million people have received an additional dose of the Pfizer, Moderna, and Johnson & Johnson vaccines.
A version of this article first appeared on WebMD.com.
The CDC is urging parents and guardians to vaccinate children ages 5-11 against COVID-19 once the shot is fully approved, despite questions from FDA advisers about the urgency given falling national case rates.
On Oct. 26, the FDA’s Vaccines and Related Biological Products Advisory Committee voted to recommend a 10-microgram shot for children. Though 17 of the 18 panelists voted in favor of it, some members said it was a hard decision and questioned the need for it now that cases and hospitalizations are down.
“There’s urgency because we’re seeing disease in children, we’ve seen deaths in children, we’ve seen long COVID,” CDC Director Rochelle Walensky, MD, said at a White House briefing on Oct. 27. “Certainly we’ve seen cases come down before, and the way to prevent surges again is to get more and more people vaccinated.”
CDC data presented at an Oct. 26 advisory committee meeting show that among children 5-11, COVID-19 was one of top 10 causes of death over last year, Dr. Walensky said. There have been more than 8,300 hospitalizations and 745 deaths in children under 18.
As of yesterday, the 7-day average of daily COVID-19 cases was 65,900, a 16% decrease from the prior week. Hospitalizations are down 54% from the week of Aug. 28, Dr. Walensky said.
“If the trends continue the way they are going, the emergency for children is not what we might think it would be. That was my concern,” James Hildreth, MD, president and CEO at Meharry Medical College in Nashville, said at the advisory committee meeting on Oct. 26.
But according to one CDC study, hospitalization rates for adolescents were 10 times higher in those who were unvaccinated. Another study found that COVID-related emergency room visits and hospital admissions among children were more than 3 times as high in states with the lowest vaccination rates.
“We are down from our peak in early September, and we are now heading in the right direction, but with cases still high, we must remain vigilant heading into the colder, drier winter months,” Dr. Walensky said, noting that the 7-day average of daily deaths still exceeds 1,000.
Meanwhile, the booster program is off to a “very strong start,” said White House COVID-19 Response Coordinator Jeff Zients.
In the 5 days since authorizations, about 15 million people have received an additional dose of the Pfizer, Moderna, and Johnson & Johnson vaccines.
A version of this article first appeared on WebMD.com.




