User login
ACIP issues adult vaccination schedule 2022
by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC).
The Clinical Guideline on the “Recommended Adult Immunization Schedule, United States, 2022” appears online Feb. 17 in Annals of Internal Medicine and in the CDC’s Morbidity and Mortality Weekly Report.
The document features changes to the zoster, pneumococcal, and hepatitis B vaccines. COVID-19 vaccinations are now included in the notes section of the schedule and can be co-administered with other vaccines, according to ACIP.
The 2022 schedule is particularly important because the pandemic has caused many adults to fall behind in routine vaccinations, according to lead author Neil Murthy, MD, MPH, MSJ, of the CDC’s immunization services division, National Center for Immunization and Respiratory Diseases, and colleagues.
“Providers should administer all due and overdue vaccines according to the routine immunization schedule during the same visit,” the group wrote. “In addition, providers should implement strategies to catch up all patients on any overdue vaccines.”
Among other changes appearing in the 2022 recommendations:
- A new step 4 in the form of an appendix lists all the contraindications and precautions for each vaccine.
- The zoster vaccine now is recommended for use in everyone aged 19 years and older who are or will be immunodeficient or immunosuppressed through disease or therapy. The new purple color bar reflects ACIP’s new two-dose series regimen for immunocompromised adults aged 19 to 49.
- The simplified pneumococcal recommendation includes guidance on using the new PCV15 and PCV20 vaccines.
- The hepatitis B recommendation has been made more inclusive, with vaccination recommended for all adults aged 19 to 59. The Special Situations section in the Notes outlines the risk-based recommendations for the hepatitis B vaccine in adults aged 60 and older. The schedule has been harmonized with the 2022 Child and Adolescent Immunization Schedule.
A welcome change
Sandra A. Fryhofer, MD, a member of the ACIP Combined Immunization Work Group, said the new pneumococcal recommendation is a particularly welcome change.
“The old recommendation was complicated and confusing. The new one is much more straightforward,” Dr. Fryhofer, an internist in Atlanta, said in an interview. Now there are only two options: a two-vaccine series of PCV15 (Vaxneuvance), in combination with the already familiar PPSV23 polysaccharide vaccine (Pneumovax 23), and a single dose of the new PCV20, Prevnar 20.
“Some work group members favored a universal age-based recommendation starting at 50 instead of 65,” Fryhofer said. “This would provide more opportunities to vaccinate adults but could lead to waning immunity later in life when risk of disease is higher.”
Although none of the updates is likely to stir controversy, discussion among ACIP members was particularly lively around hepatitis B vaccination, Dr. Fryhofer said. This vaccine has historically been recommended based on risk and has had poor uptake, while age-based vaccine recommendations generally have greater uptake.
“ACIP approved hepatitis B vaccine universally for those up to age 60, but for those 60 and older, the recommendation remains risk-based with a loophole: Anyone 60 and older who wants it can get it,” she told this news organization. “Some of the risk indications for hepatitis B vaccination may be uncomfortable or embarrassing to disclose, especially for older patients. The loophole takes care of that, but patients may have to ask for the vaccine.”
As usual, the graphics have been fine-tuned for greater accuracy and readability. “You can print a color copy to have in the exam room or at your workspace or give it a bookmark and check it online,” Dr. Fryhofer said. “It’s a great resource to have at your fingertips.”
Dr. Fryhofer has made a series of videos explaining ACIP’s approval process, the use of the schedule, and changes to vaccines including influenza. These can be accessed on the American College of Physicians website.
The authors of the recommendations stress that physicians should pay careful attention to the notes section for each vaccine, as these details clarify who needs what vaccine, when, and at what dose.
Co-author Henry Bernstein, DO, reported that he is the editor of Current Opinion in Pediatrics Office Pediatrics Series and received a presentation honorarium from the Florida chapter of the American Academy of Pediatrics. Co-author Kevin Ault, MD, reported having received a grant from the National Cancer Institute, consulting fees from PathoVax, and payments supporting attending meetings and/or travel from the American College of Obstetricians and Gynecologists.
A version of this article first appeared on Medscape.com.
by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC).
The Clinical Guideline on the “Recommended Adult Immunization Schedule, United States, 2022” appears online Feb. 17 in Annals of Internal Medicine and in the CDC’s Morbidity and Mortality Weekly Report.
The document features changes to the zoster, pneumococcal, and hepatitis B vaccines. COVID-19 vaccinations are now included in the notes section of the schedule and can be co-administered with other vaccines, according to ACIP.
The 2022 schedule is particularly important because the pandemic has caused many adults to fall behind in routine vaccinations, according to lead author Neil Murthy, MD, MPH, MSJ, of the CDC’s immunization services division, National Center for Immunization and Respiratory Diseases, and colleagues.
“Providers should administer all due and overdue vaccines according to the routine immunization schedule during the same visit,” the group wrote. “In addition, providers should implement strategies to catch up all patients on any overdue vaccines.”
Among other changes appearing in the 2022 recommendations:
- A new step 4 in the form of an appendix lists all the contraindications and precautions for each vaccine.
- The zoster vaccine now is recommended for use in everyone aged 19 years and older who are or will be immunodeficient or immunosuppressed through disease or therapy. The new purple color bar reflects ACIP’s new two-dose series regimen for immunocompromised adults aged 19 to 49.
- The simplified pneumococcal recommendation includes guidance on using the new PCV15 and PCV20 vaccines.
- The hepatitis B recommendation has been made more inclusive, with vaccination recommended for all adults aged 19 to 59. The Special Situations section in the Notes outlines the risk-based recommendations for the hepatitis B vaccine in adults aged 60 and older. The schedule has been harmonized with the 2022 Child and Adolescent Immunization Schedule.
A welcome change
Sandra A. Fryhofer, MD, a member of the ACIP Combined Immunization Work Group, said the new pneumococcal recommendation is a particularly welcome change.
“The old recommendation was complicated and confusing. The new one is much more straightforward,” Dr. Fryhofer, an internist in Atlanta, said in an interview. Now there are only two options: a two-vaccine series of PCV15 (Vaxneuvance), in combination with the already familiar PPSV23 polysaccharide vaccine (Pneumovax 23), and a single dose of the new PCV20, Prevnar 20.
“Some work group members favored a universal age-based recommendation starting at 50 instead of 65,” Fryhofer said. “This would provide more opportunities to vaccinate adults but could lead to waning immunity later in life when risk of disease is higher.”
Although none of the updates is likely to stir controversy, discussion among ACIP members was particularly lively around hepatitis B vaccination, Dr. Fryhofer said. This vaccine has historically been recommended based on risk and has had poor uptake, while age-based vaccine recommendations generally have greater uptake.
“ACIP approved hepatitis B vaccine universally for those up to age 60, but for those 60 and older, the recommendation remains risk-based with a loophole: Anyone 60 and older who wants it can get it,” she told this news organization. “Some of the risk indications for hepatitis B vaccination may be uncomfortable or embarrassing to disclose, especially for older patients. The loophole takes care of that, but patients may have to ask for the vaccine.”
As usual, the graphics have been fine-tuned for greater accuracy and readability. “You can print a color copy to have in the exam room or at your workspace or give it a bookmark and check it online,” Dr. Fryhofer said. “It’s a great resource to have at your fingertips.”
Dr. Fryhofer has made a series of videos explaining ACIP’s approval process, the use of the schedule, and changes to vaccines including influenza. These can be accessed on the American College of Physicians website.
The authors of the recommendations stress that physicians should pay careful attention to the notes section for each vaccine, as these details clarify who needs what vaccine, when, and at what dose.
Co-author Henry Bernstein, DO, reported that he is the editor of Current Opinion in Pediatrics Office Pediatrics Series and received a presentation honorarium from the Florida chapter of the American Academy of Pediatrics. Co-author Kevin Ault, MD, reported having received a grant from the National Cancer Institute, consulting fees from PathoVax, and payments supporting attending meetings and/or travel from the American College of Obstetricians and Gynecologists.
A version of this article first appeared on Medscape.com.
by the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC).
The Clinical Guideline on the “Recommended Adult Immunization Schedule, United States, 2022” appears online Feb. 17 in Annals of Internal Medicine and in the CDC’s Morbidity and Mortality Weekly Report.
The document features changes to the zoster, pneumococcal, and hepatitis B vaccines. COVID-19 vaccinations are now included in the notes section of the schedule and can be co-administered with other vaccines, according to ACIP.
The 2022 schedule is particularly important because the pandemic has caused many adults to fall behind in routine vaccinations, according to lead author Neil Murthy, MD, MPH, MSJ, of the CDC’s immunization services division, National Center for Immunization and Respiratory Diseases, and colleagues.
“Providers should administer all due and overdue vaccines according to the routine immunization schedule during the same visit,” the group wrote. “In addition, providers should implement strategies to catch up all patients on any overdue vaccines.”
Among other changes appearing in the 2022 recommendations:
- A new step 4 in the form of an appendix lists all the contraindications and precautions for each vaccine.
- The zoster vaccine now is recommended for use in everyone aged 19 years and older who are or will be immunodeficient or immunosuppressed through disease or therapy. The new purple color bar reflects ACIP’s new two-dose series regimen for immunocompromised adults aged 19 to 49.
- The simplified pneumococcal recommendation includes guidance on using the new PCV15 and PCV20 vaccines.
- The hepatitis B recommendation has been made more inclusive, with vaccination recommended for all adults aged 19 to 59. The Special Situations section in the Notes outlines the risk-based recommendations for the hepatitis B vaccine in adults aged 60 and older. The schedule has been harmonized with the 2022 Child and Adolescent Immunization Schedule.
A welcome change
Sandra A. Fryhofer, MD, a member of the ACIP Combined Immunization Work Group, said the new pneumococcal recommendation is a particularly welcome change.
“The old recommendation was complicated and confusing. The new one is much more straightforward,” Dr. Fryhofer, an internist in Atlanta, said in an interview. Now there are only two options: a two-vaccine series of PCV15 (Vaxneuvance), in combination with the already familiar PPSV23 polysaccharide vaccine (Pneumovax 23), and a single dose of the new PCV20, Prevnar 20.
“Some work group members favored a universal age-based recommendation starting at 50 instead of 65,” Fryhofer said. “This would provide more opportunities to vaccinate adults but could lead to waning immunity later in life when risk of disease is higher.”
Although none of the updates is likely to stir controversy, discussion among ACIP members was particularly lively around hepatitis B vaccination, Dr. Fryhofer said. This vaccine has historically been recommended based on risk and has had poor uptake, while age-based vaccine recommendations generally have greater uptake.
“ACIP approved hepatitis B vaccine universally for those up to age 60, but for those 60 and older, the recommendation remains risk-based with a loophole: Anyone 60 and older who wants it can get it,” she told this news organization. “Some of the risk indications for hepatitis B vaccination may be uncomfortable or embarrassing to disclose, especially for older patients. The loophole takes care of that, but patients may have to ask for the vaccine.”
As usual, the graphics have been fine-tuned for greater accuracy and readability. “You can print a color copy to have in the exam room or at your workspace or give it a bookmark and check it online,” Dr. Fryhofer said. “It’s a great resource to have at your fingertips.”
Dr. Fryhofer has made a series of videos explaining ACIP’s approval process, the use of the schedule, and changes to vaccines including influenza. These can be accessed on the American College of Physicians website.
The authors of the recommendations stress that physicians should pay careful attention to the notes section for each vaccine, as these details clarify who needs what vaccine, when, and at what dose.
Co-author Henry Bernstein, DO, reported that he is the editor of Current Opinion in Pediatrics Office Pediatrics Series and received a presentation honorarium from the Florida chapter of the American Academy of Pediatrics. Co-author Kevin Ault, MD, reported having received a grant from the National Cancer Institute, consulting fees from PathoVax, and payments supporting attending meetings and/or travel from the American College of Obstetricians and Gynecologists.
A version of this article first appeared on Medscape.com.
16 toddlers with HIV at birth had no detectable virus 2 years later
Hours after their births, 34 infants began a three-drug combination HIV treatment. Now, 2 years later, a third of those toddlers have tested negative for HIV antibodies and have no detectable HIV DNA in their blood. The children aren’t cured of HIV, but as many as 16 of them may be candidates to stop treatment and see if they are in fact in HIV remission.
If one or more are,
At the Conference on Retroviruses and Opportunistic Infections, Deborah Persaud, MD, interim director of pediatric infectious diseases and professor of pediatrics at Johns Hopkins University School of Medicine, Baltimore, Md., told this news organization that the evidence suggests that more U.S. clinicians should start infants at high risk for HIV on presumptive treatment – not only to potentially prevent transmission but also to set the child up for the lowest possible viral reservoir, the first step to HIV remission.
The three-drug preemptive treatment is “not uniformly practiced,” Dr. Persaud said in an interview. “We’re at a point now where we don’t have to wait to see if we have remission” to act on these findings, she said. “The question is, should this now become standard of care for in-utero infected infants?”
Every year, about 150 infants are born with HIV in the United States, according to the Elizabeth Glaser Pediatric AIDS Foundation. Current U.S. perinatal treatment guidelines already suggest either treatment with one or more HIV drugs at birth to attempt preventing transmission or initiating three-drug regimens for infants at high risk for perinatally acquired HIV. In this case “high risk” is defined as infants born to:
- people who haven’t received any HIV treatment before delivery or during delivery,
- people who did receive treatment but failed to achieve undetectable viral loads, or
- people who acquire HIV during pregnancy, or who otherwise weren’t diagnosed until after birth.
Trying to replicate the Mississippi baby
The Mississippi baby did eventually relapse. But ever since Dr. Persaud reported the case of that 2-year-old who went into treatment-free remission in 2013, she has been trying to figure out how to duplicate that initial success. There were several factors in that remission, but one piece researchers could control was starting treatment very early – before HIV blood tests even come back positive. So, in this trial, researchers enrolled 440 infants in Africa and Asia at high risk for in utero HIV transmission.
All 440 of those infants received their first doses of the three-drug preemptive treatment within 24 hours of birth. Of those 440 infants, 34 tested positive for HIV and remained in the trial.*
Meanwhile, in North America, South America, and African countries, another 20 infants enrolled in the trial – not as part of the protocol but because their clinicians had been influenced by the news of the Mississippi baby, Dr. Persaud said, and decided on their own to start high-risk infants on three-drug regimens preemptively.
“We wanted to take advantage of those real-world situations of infants being treated outside the clinical trials,” Dr. Persaud said.
Now there were 54 infants trying this very early treatment. In Cohort One, they started their first drug cocktail 7 hours after delivery. In Cohort Two, their first antiretroviral combination treatment was at 32.8 hours of life, and they enrolled in the trial at 8 days. Then researchers followed the infants closely, adding on lopinavir and ritonavir when age-appropriate.
Meeting milestones
To continue in the trial and be considered for treatment interruption, infants had to meet certain milestones. At 24 weeks, HIV RNA needed to be below 200 copies per milliliter. Then their HIV RNA needed to stay below 200 copies consistently until week 48. At week 48, they had to have an HIV RNA that was even lower – below 20 or 40 copies – with “target not detected” in the test in HIV RNA. That’s a sign that there weren’t even any trace levels of viral nucleic acid RNA in the blood to indicate HIV. Then, from week 48 on, they had to maintain that level of viral suppression until age 2.
At that point, not only did they need to maintain that level of viral suppression, they also needed to have a negative HIV antibody test and a PCR test for total HIV DNA, which had to be undetectable down to the limit of 4 copies per 106 – that is, there were fewer than 4 copies of the virus out of 1 million cells tested. Only then would they be considered for treatment interruption.
“After week 28 there was no leeway,” Dr. Persaud said. Then “they had to have nothing detectable from the first year of age. We thought the best shot at remission were cases that achieved very good and strict virologic control.”
Criteria for consideration
Of the 34 infants in Cohort One, 24 infants made it past the first hurdle at 24 weeks and 6 had PCR tests that found no cell-associated HIV DNA. In Cohort Two, 15 made it past the week-24 hurdle and 4 had no detectable HIV DNA via PCR test.
Now, more than 2 years out from study initiation, Dr. Persaud and colleagues are evaluating each child to see if any still meet the requirements for treatment interruption. The COVID-19 pandemic has delayed their evaluations, and it’s possible that fewer children now meet the requirements. But Dr. Persaud said there are still candidates left. An analysis suggests that up to 30% of the children, or 16, were candidates at 2 years.
“We have kids who are eligible for [antiretroviral therapy] cessation years out from this, which I think is really important,” she said in an interview. “It’s not game over.”
And although 30% is not an overwhelming victory, Dr. Persaud said the team’s goal was “to identify an N of 1 to replicate the Mississippi baby.” The study team, led by Ellen Chadwick, MD, of Northwestern University’s Feinberg School of Medicine, Chicago, and a member of the board that creates HIV perinatal treatment guidelines, is starting a new trial, using more modern, integrase inhibitor-based, three-drug regimens for infants and pairing them with broadly neutralizing antibodies. The combination used in this trial included zidovudine, or AZT.
If one of the children is able to go off treatment, it would be the first step toward creating a functional cure for HIV, starting with the youngest people affected by the virus.
“This trial convinces me that very early treatment was the key strategy that led to remission in the Mississippi baby,” Dr. Persaud said in an interview. “We’re confirming here that the first step toward remission and cure is reducing reservoirs. We’ve got that here. Whether we need more on top of that – therapeutic vaccines, immunotherapies, or a better regimen to start out with – needs to be determined.”
The presentation was met with excitement and questions. For instance, if very early treatment works, why does it work for just 30% of the children?
Were some of the children able to control HIV on their own because they were rare post-treatment controllers? And was 30% really a victory? Others were convinced of it.
“Amazing outcome to have 30% so well suppressed after 2 years with CA-DNA not detected,” commented Hermione Lyall, MBChB, a pediatric infectious disease doctor at Imperial College Healthcare NHS Trust in the United Kingdom, in the virtual chat.
As for whether the study should change practice, Elaine Abrams, MD, professor of epidemiology and pediatrics at Columbia University Medical Center, New York, and CROI cochair, said that this study proves that the three-drug regimen is at the very least safe to start immediately.
Whether it should become standard of care everywhere is still up for discussion, she told this news organization.
“It very much depends on what you’re trying to achieve,” she said. “Postnatal prophylaxis is provided to reduce the risk of acquiring infection. That’s a different objective than early treatment. If you have 1,000 high-risk babies, how many are likely to turn out to have HIV infection? And how many of those will you be treating with three drugs and actually making this impact by doing so? And how many babies are going to be getting possibly extra treatment that they don’t need?”
Regardless, what’s clear is that treatment is essential – for mother and infant, said Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases. It needs to start, he said, by making sure all mothers know their HIV status and have access early in pregnancy to the treatment that can prevent transmission.
“So much of what’s wrong in the world is about implementation of health care,” he said in an interview. Still, “if you could demonstrate that early treatment to the mother plus early treatment to the babies [is efficacious], we could really talk about an HIV-free generation of kids.”
The study was funded by the National Institutes of Health. Dr. Persaud, Dr. Dieffenbach, Dr. Abrams, and Dr. Lyall all report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Correction, 2/16/22: An earlier version of this article misstated the number that tested positive for HIV and remained in the trial.
This article was updated 2/16/22.
Hours after their births, 34 infants began a three-drug combination HIV treatment. Now, 2 years later, a third of those toddlers have tested negative for HIV antibodies and have no detectable HIV DNA in their blood. The children aren’t cured of HIV, but as many as 16 of them may be candidates to stop treatment and see if they are in fact in HIV remission.
If one or more are,
At the Conference on Retroviruses and Opportunistic Infections, Deborah Persaud, MD, interim director of pediatric infectious diseases and professor of pediatrics at Johns Hopkins University School of Medicine, Baltimore, Md., told this news organization that the evidence suggests that more U.S. clinicians should start infants at high risk for HIV on presumptive treatment – not only to potentially prevent transmission but also to set the child up for the lowest possible viral reservoir, the first step to HIV remission.
The three-drug preemptive treatment is “not uniformly practiced,” Dr. Persaud said in an interview. “We’re at a point now where we don’t have to wait to see if we have remission” to act on these findings, she said. “The question is, should this now become standard of care for in-utero infected infants?”
Every year, about 150 infants are born with HIV in the United States, according to the Elizabeth Glaser Pediatric AIDS Foundation. Current U.S. perinatal treatment guidelines already suggest either treatment with one or more HIV drugs at birth to attempt preventing transmission or initiating three-drug regimens for infants at high risk for perinatally acquired HIV. In this case “high risk” is defined as infants born to:
- people who haven’t received any HIV treatment before delivery or during delivery,
- people who did receive treatment but failed to achieve undetectable viral loads, or
- people who acquire HIV during pregnancy, or who otherwise weren’t diagnosed until after birth.
Trying to replicate the Mississippi baby
The Mississippi baby did eventually relapse. But ever since Dr. Persaud reported the case of that 2-year-old who went into treatment-free remission in 2013, she has been trying to figure out how to duplicate that initial success. There were several factors in that remission, but one piece researchers could control was starting treatment very early – before HIV blood tests even come back positive. So, in this trial, researchers enrolled 440 infants in Africa and Asia at high risk for in utero HIV transmission.
All 440 of those infants received their first doses of the three-drug preemptive treatment within 24 hours of birth. Of those 440 infants, 34 tested positive for HIV and remained in the trial.*
Meanwhile, in North America, South America, and African countries, another 20 infants enrolled in the trial – not as part of the protocol but because their clinicians had been influenced by the news of the Mississippi baby, Dr. Persaud said, and decided on their own to start high-risk infants on three-drug regimens preemptively.
“We wanted to take advantage of those real-world situations of infants being treated outside the clinical trials,” Dr. Persaud said.
Now there were 54 infants trying this very early treatment. In Cohort One, they started their first drug cocktail 7 hours after delivery. In Cohort Two, their first antiretroviral combination treatment was at 32.8 hours of life, and they enrolled in the trial at 8 days. Then researchers followed the infants closely, adding on lopinavir and ritonavir when age-appropriate.
Meeting milestones
To continue in the trial and be considered for treatment interruption, infants had to meet certain milestones. At 24 weeks, HIV RNA needed to be below 200 copies per milliliter. Then their HIV RNA needed to stay below 200 copies consistently until week 48. At week 48, they had to have an HIV RNA that was even lower – below 20 or 40 copies – with “target not detected” in the test in HIV RNA. That’s a sign that there weren’t even any trace levels of viral nucleic acid RNA in the blood to indicate HIV. Then, from week 48 on, they had to maintain that level of viral suppression until age 2.
At that point, not only did they need to maintain that level of viral suppression, they also needed to have a negative HIV antibody test and a PCR test for total HIV DNA, which had to be undetectable down to the limit of 4 copies per 106 – that is, there were fewer than 4 copies of the virus out of 1 million cells tested. Only then would they be considered for treatment interruption.
“After week 28 there was no leeway,” Dr. Persaud said. Then “they had to have nothing detectable from the first year of age. We thought the best shot at remission were cases that achieved very good and strict virologic control.”
Criteria for consideration
Of the 34 infants in Cohort One, 24 infants made it past the first hurdle at 24 weeks and 6 had PCR tests that found no cell-associated HIV DNA. In Cohort Two, 15 made it past the week-24 hurdle and 4 had no detectable HIV DNA via PCR test.
Now, more than 2 years out from study initiation, Dr. Persaud and colleagues are evaluating each child to see if any still meet the requirements for treatment interruption. The COVID-19 pandemic has delayed their evaluations, and it’s possible that fewer children now meet the requirements. But Dr. Persaud said there are still candidates left. An analysis suggests that up to 30% of the children, or 16, were candidates at 2 years.
“We have kids who are eligible for [antiretroviral therapy] cessation years out from this, which I think is really important,” she said in an interview. “It’s not game over.”
And although 30% is not an overwhelming victory, Dr. Persaud said the team’s goal was “to identify an N of 1 to replicate the Mississippi baby.” The study team, led by Ellen Chadwick, MD, of Northwestern University’s Feinberg School of Medicine, Chicago, and a member of the board that creates HIV perinatal treatment guidelines, is starting a new trial, using more modern, integrase inhibitor-based, three-drug regimens for infants and pairing them with broadly neutralizing antibodies. The combination used in this trial included zidovudine, or AZT.
If one of the children is able to go off treatment, it would be the first step toward creating a functional cure for HIV, starting with the youngest people affected by the virus.
“This trial convinces me that very early treatment was the key strategy that led to remission in the Mississippi baby,” Dr. Persaud said in an interview. “We’re confirming here that the first step toward remission and cure is reducing reservoirs. We’ve got that here. Whether we need more on top of that – therapeutic vaccines, immunotherapies, or a better regimen to start out with – needs to be determined.”
The presentation was met with excitement and questions. For instance, if very early treatment works, why does it work for just 30% of the children?
Were some of the children able to control HIV on their own because they were rare post-treatment controllers? And was 30% really a victory? Others were convinced of it.
“Amazing outcome to have 30% so well suppressed after 2 years with CA-DNA not detected,” commented Hermione Lyall, MBChB, a pediatric infectious disease doctor at Imperial College Healthcare NHS Trust in the United Kingdom, in the virtual chat.
As for whether the study should change practice, Elaine Abrams, MD, professor of epidemiology and pediatrics at Columbia University Medical Center, New York, and CROI cochair, said that this study proves that the three-drug regimen is at the very least safe to start immediately.
Whether it should become standard of care everywhere is still up for discussion, she told this news organization.
“It very much depends on what you’re trying to achieve,” she said. “Postnatal prophylaxis is provided to reduce the risk of acquiring infection. That’s a different objective than early treatment. If you have 1,000 high-risk babies, how many are likely to turn out to have HIV infection? And how many of those will you be treating with three drugs and actually making this impact by doing so? And how many babies are going to be getting possibly extra treatment that they don’t need?”
Regardless, what’s clear is that treatment is essential – for mother and infant, said Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases. It needs to start, he said, by making sure all mothers know their HIV status and have access early in pregnancy to the treatment that can prevent transmission.
“So much of what’s wrong in the world is about implementation of health care,” he said in an interview. Still, “if you could demonstrate that early treatment to the mother plus early treatment to the babies [is efficacious], we could really talk about an HIV-free generation of kids.”
The study was funded by the National Institutes of Health. Dr. Persaud, Dr. Dieffenbach, Dr. Abrams, and Dr. Lyall all report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Correction, 2/16/22: An earlier version of this article misstated the number that tested positive for HIV and remained in the trial.
This article was updated 2/16/22.
Hours after their births, 34 infants began a three-drug combination HIV treatment. Now, 2 years later, a third of those toddlers have tested negative for HIV antibodies and have no detectable HIV DNA in their blood. The children aren’t cured of HIV, but as many as 16 of them may be candidates to stop treatment and see if they are in fact in HIV remission.
If one or more are,
At the Conference on Retroviruses and Opportunistic Infections, Deborah Persaud, MD, interim director of pediatric infectious diseases and professor of pediatrics at Johns Hopkins University School of Medicine, Baltimore, Md., told this news organization that the evidence suggests that more U.S. clinicians should start infants at high risk for HIV on presumptive treatment – not only to potentially prevent transmission but also to set the child up for the lowest possible viral reservoir, the first step to HIV remission.
The three-drug preemptive treatment is “not uniformly practiced,” Dr. Persaud said in an interview. “We’re at a point now where we don’t have to wait to see if we have remission” to act on these findings, she said. “The question is, should this now become standard of care for in-utero infected infants?”
Every year, about 150 infants are born with HIV in the United States, according to the Elizabeth Glaser Pediatric AIDS Foundation. Current U.S. perinatal treatment guidelines already suggest either treatment with one or more HIV drugs at birth to attempt preventing transmission or initiating three-drug regimens for infants at high risk for perinatally acquired HIV. In this case “high risk” is defined as infants born to:
- people who haven’t received any HIV treatment before delivery or during delivery,
- people who did receive treatment but failed to achieve undetectable viral loads, or
- people who acquire HIV during pregnancy, or who otherwise weren’t diagnosed until after birth.
Trying to replicate the Mississippi baby
The Mississippi baby did eventually relapse. But ever since Dr. Persaud reported the case of that 2-year-old who went into treatment-free remission in 2013, she has been trying to figure out how to duplicate that initial success. There were several factors in that remission, but one piece researchers could control was starting treatment very early – before HIV blood tests even come back positive. So, in this trial, researchers enrolled 440 infants in Africa and Asia at high risk for in utero HIV transmission.
All 440 of those infants received their first doses of the three-drug preemptive treatment within 24 hours of birth. Of those 440 infants, 34 tested positive for HIV and remained in the trial.*
Meanwhile, in North America, South America, and African countries, another 20 infants enrolled in the trial – not as part of the protocol but because their clinicians had been influenced by the news of the Mississippi baby, Dr. Persaud said, and decided on their own to start high-risk infants on three-drug regimens preemptively.
“We wanted to take advantage of those real-world situations of infants being treated outside the clinical trials,” Dr. Persaud said.
Now there were 54 infants trying this very early treatment. In Cohort One, they started their first drug cocktail 7 hours after delivery. In Cohort Two, their first antiretroviral combination treatment was at 32.8 hours of life, and they enrolled in the trial at 8 days. Then researchers followed the infants closely, adding on lopinavir and ritonavir when age-appropriate.
Meeting milestones
To continue in the trial and be considered for treatment interruption, infants had to meet certain milestones. At 24 weeks, HIV RNA needed to be below 200 copies per milliliter. Then their HIV RNA needed to stay below 200 copies consistently until week 48. At week 48, they had to have an HIV RNA that was even lower – below 20 or 40 copies – with “target not detected” in the test in HIV RNA. That’s a sign that there weren’t even any trace levels of viral nucleic acid RNA in the blood to indicate HIV. Then, from week 48 on, they had to maintain that level of viral suppression until age 2.
At that point, not only did they need to maintain that level of viral suppression, they also needed to have a negative HIV antibody test and a PCR test for total HIV DNA, which had to be undetectable down to the limit of 4 copies per 106 – that is, there were fewer than 4 copies of the virus out of 1 million cells tested. Only then would they be considered for treatment interruption.
“After week 28 there was no leeway,” Dr. Persaud said. Then “they had to have nothing detectable from the first year of age. We thought the best shot at remission were cases that achieved very good and strict virologic control.”
Criteria for consideration
Of the 34 infants in Cohort One, 24 infants made it past the first hurdle at 24 weeks and 6 had PCR tests that found no cell-associated HIV DNA. In Cohort Two, 15 made it past the week-24 hurdle and 4 had no detectable HIV DNA via PCR test.
Now, more than 2 years out from study initiation, Dr. Persaud and colleagues are evaluating each child to see if any still meet the requirements for treatment interruption. The COVID-19 pandemic has delayed their evaluations, and it’s possible that fewer children now meet the requirements. But Dr. Persaud said there are still candidates left. An analysis suggests that up to 30% of the children, or 16, were candidates at 2 years.
“We have kids who are eligible for [antiretroviral therapy] cessation years out from this, which I think is really important,” she said in an interview. “It’s not game over.”
And although 30% is not an overwhelming victory, Dr. Persaud said the team’s goal was “to identify an N of 1 to replicate the Mississippi baby.” The study team, led by Ellen Chadwick, MD, of Northwestern University’s Feinberg School of Medicine, Chicago, and a member of the board that creates HIV perinatal treatment guidelines, is starting a new trial, using more modern, integrase inhibitor-based, three-drug regimens for infants and pairing them with broadly neutralizing antibodies. The combination used in this trial included zidovudine, or AZT.
If one of the children is able to go off treatment, it would be the first step toward creating a functional cure for HIV, starting with the youngest people affected by the virus.
“This trial convinces me that very early treatment was the key strategy that led to remission in the Mississippi baby,” Dr. Persaud said in an interview. “We’re confirming here that the first step toward remission and cure is reducing reservoirs. We’ve got that here. Whether we need more on top of that – therapeutic vaccines, immunotherapies, or a better regimen to start out with – needs to be determined.”
The presentation was met with excitement and questions. For instance, if very early treatment works, why does it work for just 30% of the children?
Were some of the children able to control HIV on their own because they were rare post-treatment controllers? And was 30% really a victory? Others were convinced of it.
“Amazing outcome to have 30% so well suppressed after 2 years with CA-DNA not detected,” commented Hermione Lyall, MBChB, a pediatric infectious disease doctor at Imperial College Healthcare NHS Trust in the United Kingdom, in the virtual chat.
As for whether the study should change practice, Elaine Abrams, MD, professor of epidemiology and pediatrics at Columbia University Medical Center, New York, and CROI cochair, said that this study proves that the three-drug regimen is at the very least safe to start immediately.
Whether it should become standard of care everywhere is still up for discussion, she told this news organization.
“It very much depends on what you’re trying to achieve,” she said. “Postnatal prophylaxis is provided to reduce the risk of acquiring infection. That’s a different objective than early treatment. If you have 1,000 high-risk babies, how many are likely to turn out to have HIV infection? And how many of those will you be treating with three drugs and actually making this impact by doing so? And how many babies are going to be getting possibly extra treatment that they don’t need?”
Regardless, what’s clear is that treatment is essential – for mother and infant, said Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases. It needs to start, he said, by making sure all mothers know their HIV status and have access early in pregnancy to the treatment that can prevent transmission.
“So much of what’s wrong in the world is about implementation of health care,” he said in an interview. Still, “if you could demonstrate that early treatment to the mother plus early treatment to the babies [is efficacious], we could really talk about an HIV-free generation of kids.”
The study was funded by the National Institutes of Health. Dr. Persaud, Dr. Dieffenbach, Dr. Abrams, and Dr. Lyall all report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Correction, 2/16/22: An earlier version of this article misstated the number that tested positive for HIV and remained in the trial.
This article was updated 2/16/22.
FROM CROI 22
A third person living with HIV has been cured by transplant
In a first, If she remains off treatment without any hint of HIV, she would be only the third person in the world – after the Berlin Patient and the London Patient – to be cured through a transplant.
“Her own virus could not infect her cells,” said Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, who presented the study at the Conference on Retroviruses and Opportunistic Infections, which both presenters and the audience attended remotely.
The middle-aged New York woman of mixed race, who has asked that her specific race and age not be shared to protect her privacy, was diagnosed with HIV in 2013 when she was still in the very early stages of infection. She started treatment immediately and quickly achieved an undetectable viral load. An undetectable viral load not only prevents someone from transmitting HIV to others but also reduces or eliminates HIV replication, which means fewer variants and less time for the virus to infiltrate cells where it can hide.
But in 2017, she was diagnosed with leukemia. As a last resort to cure her of the cancer, she received a combination of adult stem cells from a relative’s blood that closely matched her own and umbilical cord blood obtained from a cord blood bank. That particular sample of cord blood was selected for its genetic mutation against the CCR5 receptor on immune cells, CD4 T cells. That mutation makes the immune system resistant to HIV.
The two previous HIV cures, of Berlin Patient Timothy Ray Brown and London Patient Adam Castillejo, also used stem cell transplantation with a CCR5 mutation, but theirs were bone marrow transplants. Bone marrow transplants are more arduous than cord blood transplants, which are commonly used in pediatric cancer treatment.
In this case, the physicians treating her used both.
“This allows the adult cells to accelerate and grow up until the cord blood takes over,” said Dr. Bryson. During her presentation, Dr. Bryson pointed to two types of data: First, she presented data showing the level of HIV in the patient’s blood. Soon after HIV diagnosis and treatment, her viral load dropped to undetectable levels. She had a spike of virus when she received the transplant, but then it went back to undetectable and has stayed that way ever since.
Meanwhile, following the transplant, her immune system started rebuilding itself using the new, HIV-resistant cells provided in the transplant. As her care team watched, no graft-versus-host (GVH) disease, a common side effect of stem cell transplants, emerged. In fact, the transplant went so well that she was discharged early from the hospital.
One hundred days after the transplant, the immune system contained within the cord blood had taken over. Her CD4 immune cells returned to normal levels a little more than a year after the transplant. By 27 months, she decided to stop all HIV treatment to see if the transplant had worked.
This was the real test. But as Dr. Bryson and colleagues continued to watch her HIV viral load and her CD4 counts and search for infectious virus, they didn’t find any. She tested negative for HIV by antibody test. Dr. Bryson grew 75 million of her cells in a lab to look for any HIV. None. Aside from one blip in detectable HIV DNA at 14 weeks, researchers never found HIV in the patient again.
“Her cells are resistant to HIV now – both her own strains and laboratory strains,” Dr. Bryson told this news organization. “It’s been 14 months since then. She has no rebound and no detectable virus.”
The presentation drew as raucous as praise gets in a virtual environment. The comments began pouring in.
“Impressive results,” wrote Jim Hoxie, MD, professor emeritus at the University of Pennsylvania, Philadelphia.
“Exciting case,” wrote Allison Agwu, MD, a professor of pediatrics at Johns Hopkins University, Baltimore.
And Dennis Copertino, a research specialist at Weill Cornell Medicine, New York, wrote: “Thank you so much for translating this important cure strategy to people of color.”
Most donors with CCR5 mutations are White, Dr. Bryson said, suggesting that this approach, in a mixed-race woman, could expand the pool of people living with HIV and cancer who are good candidates for the approach.
But other observers had questions, ones that may require more research to answer. Some asked why this woman’s virus, after transplantation, wasn’t just immune to viruses with CCR5 but also another variant, called CXCR4, that one wouldn’t expect. Luis Montaner, DVM, director of the Immunopathogenesis Laboratory at the Wistar Institute in Philadelphia, wondered whether it was more than the blood that had cleared HIV. Did it get into the tissue, too? That question has not yet been answered.
For Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases, the lack of GVH disease was a powerful and hopeful finding.
“There’s been this ongoing hypothesis that maybe graft-versus-host disease was needed at some level to help clear out every last single CD4+ T cell that may or may not have been harboring replication-competent virus,” Dr. Dieffenbach said in an interview. “But there was no GVH disease. That’s incredible. It’s a wonderful thing.”
Now the challenge is to move from a single case to making cure available to other people living with HIV.
The case also got cure researchers thinking.
Dr. Montaner called the case “an encouraging roadmap supporting anti-CCR5 strategies by CRISPR Cas9,” studies that are now underway.
Steven Deeks, MD, called the case “perhaps a model for how we might do this using a person’s own cells. Because we were never really going to be transplanting cells from another person as a scalable cure.”
For people living with HIV, particularly women of color, the results raise hopes and questions. Nina Martinez knows something about being a “first.” In 2019, she was the first American woman of color living with HIV to donate a kidney to another person living with the virus. To her, the excitement over the first woman of color being cured of HIV just shines a light on how very White and male HIV cure studies have been until now.
“For me, I’m not looking for a cure in which the successful step forward is me getting cancer,” she said in an interview. “I’m looking at, what’s going to be sustainable? I want to know what’s going to work for a group of people.”
Gina Marie Brown, a social worker living with HIV in New Orleans, is also thinking of groups of people.
“Every time we get a breakthrough, it’s like the sun is taken from behind the clouds a little more,” said Ms. Brown. “I think about people in the South, who bear a huge burden of HIV. I think about trans women. I think about Black women, and gay, bisexual, and same-gender-loving men. This could really impact HIV – in the same way that PrEP [pre-exposure prophylaxis] has, the same way that one pill once a day has.”
When Ms. Brown was diagnosed with HIV 22 years ago, she started to plan her funeral.
“That’s how much I thought HIV was a death sentence,” she told this news organization. “Oh my goodness! Glad you stuck around, Gina.”
The study was funded by the National Institutes of Health. Dr. Bryson, Dr. Dieffenbach, Dr. Deeks, and Dr. Montaner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a first, If she remains off treatment without any hint of HIV, she would be only the third person in the world – after the Berlin Patient and the London Patient – to be cured through a transplant.
“Her own virus could not infect her cells,” said Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, who presented the study at the Conference on Retroviruses and Opportunistic Infections, which both presenters and the audience attended remotely.
The middle-aged New York woman of mixed race, who has asked that her specific race and age not be shared to protect her privacy, was diagnosed with HIV in 2013 when she was still in the very early stages of infection. She started treatment immediately and quickly achieved an undetectable viral load. An undetectable viral load not only prevents someone from transmitting HIV to others but also reduces or eliminates HIV replication, which means fewer variants and less time for the virus to infiltrate cells where it can hide.
But in 2017, she was diagnosed with leukemia. As a last resort to cure her of the cancer, she received a combination of adult stem cells from a relative’s blood that closely matched her own and umbilical cord blood obtained from a cord blood bank. That particular sample of cord blood was selected for its genetic mutation against the CCR5 receptor on immune cells, CD4 T cells. That mutation makes the immune system resistant to HIV.
The two previous HIV cures, of Berlin Patient Timothy Ray Brown and London Patient Adam Castillejo, also used stem cell transplantation with a CCR5 mutation, but theirs were bone marrow transplants. Bone marrow transplants are more arduous than cord blood transplants, which are commonly used in pediatric cancer treatment.
In this case, the physicians treating her used both.
“This allows the adult cells to accelerate and grow up until the cord blood takes over,” said Dr. Bryson. During her presentation, Dr. Bryson pointed to two types of data: First, she presented data showing the level of HIV in the patient’s blood. Soon after HIV diagnosis and treatment, her viral load dropped to undetectable levels. She had a spike of virus when she received the transplant, but then it went back to undetectable and has stayed that way ever since.
Meanwhile, following the transplant, her immune system started rebuilding itself using the new, HIV-resistant cells provided in the transplant. As her care team watched, no graft-versus-host (GVH) disease, a common side effect of stem cell transplants, emerged. In fact, the transplant went so well that she was discharged early from the hospital.
One hundred days after the transplant, the immune system contained within the cord blood had taken over. Her CD4 immune cells returned to normal levels a little more than a year after the transplant. By 27 months, she decided to stop all HIV treatment to see if the transplant had worked.
This was the real test. But as Dr. Bryson and colleagues continued to watch her HIV viral load and her CD4 counts and search for infectious virus, they didn’t find any. She tested negative for HIV by antibody test. Dr. Bryson grew 75 million of her cells in a lab to look for any HIV. None. Aside from one blip in detectable HIV DNA at 14 weeks, researchers never found HIV in the patient again.
“Her cells are resistant to HIV now – both her own strains and laboratory strains,” Dr. Bryson told this news organization. “It’s been 14 months since then. She has no rebound and no detectable virus.”
The presentation drew as raucous as praise gets in a virtual environment. The comments began pouring in.
“Impressive results,” wrote Jim Hoxie, MD, professor emeritus at the University of Pennsylvania, Philadelphia.
“Exciting case,” wrote Allison Agwu, MD, a professor of pediatrics at Johns Hopkins University, Baltimore.
And Dennis Copertino, a research specialist at Weill Cornell Medicine, New York, wrote: “Thank you so much for translating this important cure strategy to people of color.”
Most donors with CCR5 mutations are White, Dr. Bryson said, suggesting that this approach, in a mixed-race woman, could expand the pool of people living with HIV and cancer who are good candidates for the approach.
But other observers had questions, ones that may require more research to answer. Some asked why this woman’s virus, after transplantation, wasn’t just immune to viruses with CCR5 but also another variant, called CXCR4, that one wouldn’t expect. Luis Montaner, DVM, director of the Immunopathogenesis Laboratory at the Wistar Institute in Philadelphia, wondered whether it was more than the blood that had cleared HIV. Did it get into the tissue, too? That question has not yet been answered.
For Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases, the lack of GVH disease was a powerful and hopeful finding.
“There’s been this ongoing hypothesis that maybe graft-versus-host disease was needed at some level to help clear out every last single CD4+ T cell that may or may not have been harboring replication-competent virus,” Dr. Dieffenbach said in an interview. “But there was no GVH disease. That’s incredible. It’s a wonderful thing.”
Now the challenge is to move from a single case to making cure available to other people living with HIV.
The case also got cure researchers thinking.
Dr. Montaner called the case “an encouraging roadmap supporting anti-CCR5 strategies by CRISPR Cas9,” studies that are now underway.
Steven Deeks, MD, called the case “perhaps a model for how we might do this using a person’s own cells. Because we were never really going to be transplanting cells from another person as a scalable cure.”
For people living with HIV, particularly women of color, the results raise hopes and questions. Nina Martinez knows something about being a “first.” In 2019, she was the first American woman of color living with HIV to donate a kidney to another person living with the virus. To her, the excitement over the first woman of color being cured of HIV just shines a light on how very White and male HIV cure studies have been until now.
“For me, I’m not looking for a cure in which the successful step forward is me getting cancer,” she said in an interview. “I’m looking at, what’s going to be sustainable? I want to know what’s going to work for a group of people.”
Gina Marie Brown, a social worker living with HIV in New Orleans, is also thinking of groups of people.
“Every time we get a breakthrough, it’s like the sun is taken from behind the clouds a little more,” said Ms. Brown. “I think about people in the South, who bear a huge burden of HIV. I think about trans women. I think about Black women, and gay, bisexual, and same-gender-loving men. This could really impact HIV – in the same way that PrEP [pre-exposure prophylaxis] has, the same way that one pill once a day has.”
When Ms. Brown was diagnosed with HIV 22 years ago, she started to plan her funeral.
“That’s how much I thought HIV was a death sentence,” she told this news organization. “Oh my goodness! Glad you stuck around, Gina.”
The study was funded by the National Institutes of Health. Dr. Bryson, Dr. Dieffenbach, Dr. Deeks, and Dr. Montaner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a first, If she remains off treatment without any hint of HIV, she would be only the third person in the world – after the Berlin Patient and the London Patient – to be cured through a transplant.
“Her own virus could not infect her cells,” said Yvonne Bryson, MD, chief of pediatric infectious diseases at the University of California, Los Angeles, who presented the study at the Conference on Retroviruses and Opportunistic Infections, which both presenters and the audience attended remotely.
The middle-aged New York woman of mixed race, who has asked that her specific race and age not be shared to protect her privacy, was diagnosed with HIV in 2013 when she was still in the very early stages of infection. She started treatment immediately and quickly achieved an undetectable viral load. An undetectable viral load not only prevents someone from transmitting HIV to others but also reduces or eliminates HIV replication, which means fewer variants and less time for the virus to infiltrate cells where it can hide.
But in 2017, she was diagnosed with leukemia. As a last resort to cure her of the cancer, she received a combination of adult stem cells from a relative’s blood that closely matched her own and umbilical cord blood obtained from a cord blood bank. That particular sample of cord blood was selected for its genetic mutation against the CCR5 receptor on immune cells, CD4 T cells. That mutation makes the immune system resistant to HIV.
The two previous HIV cures, of Berlin Patient Timothy Ray Brown and London Patient Adam Castillejo, also used stem cell transplantation with a CCR5 mutation, but theirs were bone marrow transplants. Bone marrow transplants are more arduous than cord blood transplants, which are commonly used in pediatric cancer treatment.
In this case, the physicians treating her used both.
“This allows the adult cells to accelerate and grow up until the cord blood takes over,” said Dr. Bryson. During her presentation, Dr. Bryson pointed to two types of data: First, she presented data showing the level of HIV in the patient’s blood. Soon after HIV diagnosis and treatment, her viral load dropped to undetectable levels. She had a spike of virus when she received the transplant, but then it went back to undetectable and has stayed that way ever since.
Meanwhile, following the transplant, her immune system started rebuilding itself using the new, HIV-resistant cells provided in the transplant. As her care team watched, no graft-versus-host (GVH) disease, a common side effect of stem cell transplants, emerged. In fact, the transplant went so well that she was discharged early from the hospital.
One hundred days after the transplant, the immune system contained within the cord blood had taken over. Her CD4 immune cells returned to normal levels a little more than a year after the transplant. By 27 months, she decided to stop all HIV treatment to see if the transplant had worked.
This was the real test. But as Dr. Bryson and colleagues continued to watch her HIV viral load and her CD4 counts and search for infectious virus, they didn’t find any. She tested negative for HIV by antibody test. Dr. Bryson grew 75 million of her cells in a lab to look for any HIV. None. Aside from one blip in detectable HIV DNA at 14 weeks, researchers never found HIV in the patient again.
“Her cells are resistant to HIV now – both her own strains and laboratory strains,” Dr. Bryson told this news organization. “It’s been 14 months since then. She has no rebound and no detectable virus.”
The presentation drew as raucous as praise gets in a virtual environment. The comments began pouring in.
“Impressive results,” wrote Jim Hoxie, MD, professor emeritus at the University of Pennsylvania, Philadelphia.
“Exciting case,” wrote Allison Agwu, MD, a professor of pediatrics at Johns Hopkins University, Baltimore.
And Dennis Copertino, a research specialist at Weill Cornell Medicine, New York, wrote: “Thank you so much for translating this important cure strategy to people of color.”
Most donors with CCR5 mutations are White, Dr. Bryson said, suggesting that this approach, in a mixed-race woman, could expand the pool of people living with HIV and cancer who are good candidates for the approach.
But other observers had questions, ones that may require more research to answer. Some asked why this woman’s virus, after transplantation, wasn’t just immune to viruses with CCR5 but also another variant, called CXCR4, that one wouldn’t expect. Luis Montaner, DVM, director of the Immunopathogenesis Laboratory at the Wistar Institute in Philadelphia, wondered whether it was more than the blood that had cleared HIV. Did it get into the tissue, too? That question has not yet been answered.
For Carl Dieffenbach, PhD, director of the division of AIDS at the National Institute of Allergy and Infectious Diseases, the lack of GVH disease was a powerful and hopeful finding.
“There’s been this ongoing hypothesis that maybe graft-versus-host disease was needed at some level to help clear out every last single CD4+ T cell that may or may not have been harboring replication-competent virus,” Dr. Dieffenbach said in an interview. “But there was no GVH disease. That’s incredible. It’s a wonderful thing.”
Now the challenge is to move from a single case to making cure available to other people living with HIV.
The case also got cure researchers thinking.
Dr. Montaner called the case “an encouraging roadmap supporting anti-CCR5 strategies by CRISPR Cas9,” studies that are now underway.
Steven Deeks, MD, called the case “perhaps a model for how we might do this using a person’s own cells. Because we were never really going to be transplanting cells from another person as a scalable cure.”
For people living with HIV, particularly women of color, the results raise hopes and questions. Nina Martinez knows something about being a “first.” In 2019, she was the first American woman of color living with HIV to donate a kidney to another person living with the virus. To her, the excitement over the first woman of color being cured of HIV just shines a light on how very White and male HIV cure studies have been until now.
“For me, I’m not looking for a cure in which the successful step forward is me getting cancer,” she said in an interview. “I’m looking at, what’s going to be sustainable? I want to know what’s going to work for a group of people.”
Gina Marie Brown, a social worker living with HIV in New Orleans, is also thinking of groups of people.
“Every time we get a breakthrough, it’s like the sun is taken from behind the clouds a little more,” said Ms. Brown. “I think about people in the South, who bear a huge burden of HIV. I think about trans women. I think about Black women, and gay, bisexual, and same-gender-loving men. This could really impact HIV – in the same way that PrEP [pre-exposure prophylaxis] has, the same way that one pill once a day has.”
When Ms. Brown was diagnosed with HIV 22 years ago, she started to plan her funeral.
“That’s how much I thought HIV was a death sentence,” she told this news organization. “Oh my goodness! Glad you stuck around, Gina.”
The study was funded by the National Institutes of Health. Dr. Bryson, Dr. Dieffenbach, Dr. Deeks, and Dr. Montaner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CROI 2022
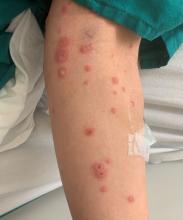
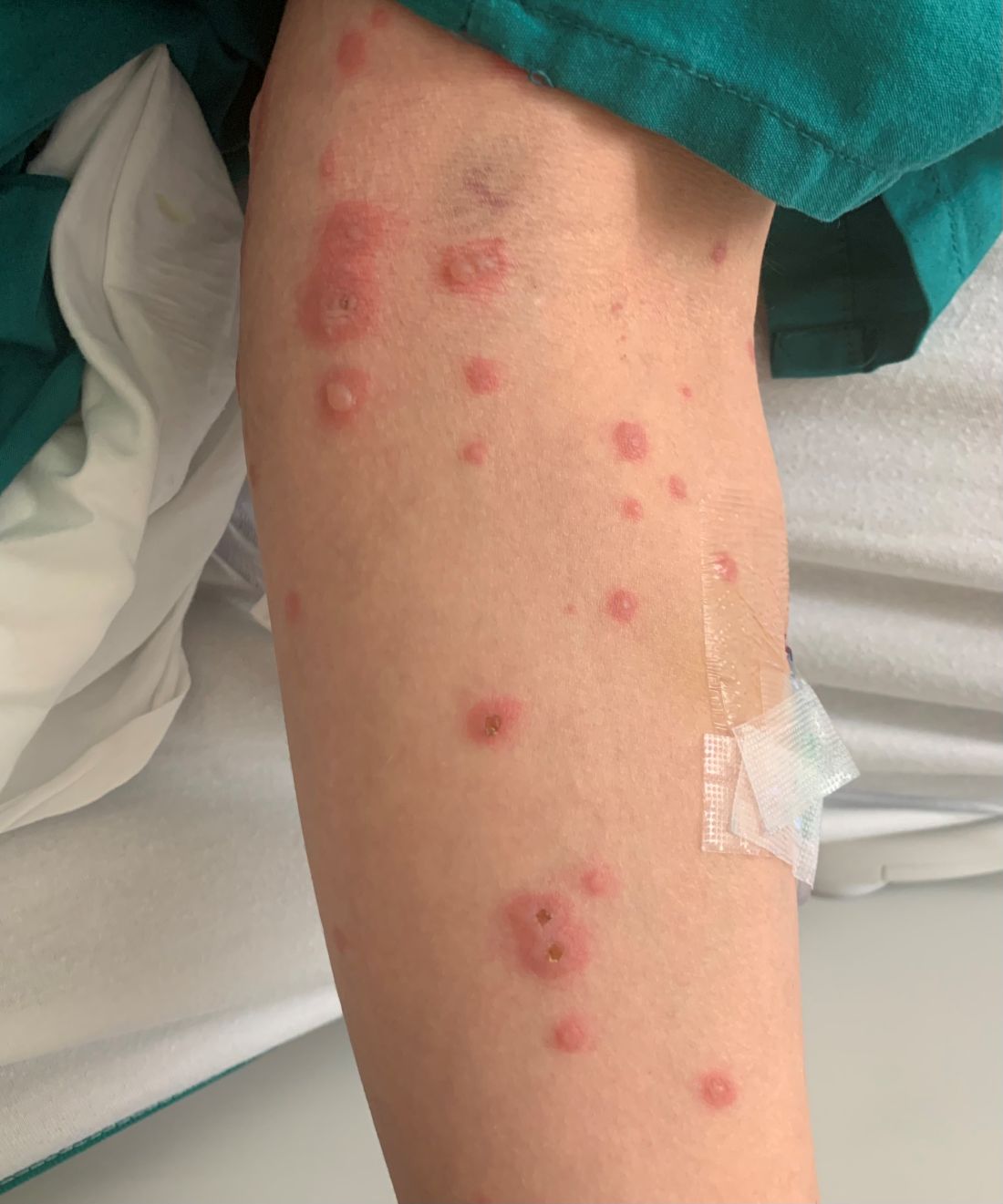
A 34-year-old male presented with 10 days of a pruritic rash
Less frequently observable infectious agents associated with EM are Mycoplasma pneumoniae, Histoplasma capsulatum, and parapoxvirus (orf). Rarely, EM is triggered by drug eruption or systemic disease. Individuals of all age groups and races can be affected by EM. However, it is predominantly observed in young adult patients (20-40 years of age), and there is a male predominance.
Patients typically present with the abrupt onset of symmetrical red papules that evolve into typical and atypical targetoid lesions. Lesions evolve in 48-72 hours, favoring acrofacial sites that then spread down towards the trunk. Systemic symptoms such as fever and arthralgia may accompany the skin lesions.1-3
Erythema multiforme is recognized in two forms: EM minor and EM major. Both forms share the same characteristic of target lesions. However, the presence of mucosal involvement distinguishes the two. Mucosal involvement is absent or mild in EM minor, while mucosal involvement in EM major is often severe.2,3 Painful bullous lesions are commonly present in the mouth, genital, and ocular mucous membranes. Severe symptoms can often result in difficulty eating and drinking.
Diagnosis is largely clinical. Further histologic study may accompany diagnoses to exclude differential diagnosis. In EM, direct immunofluorescence (DIF) is negative. Histopathology reveals apoptosis of individual keratinocytes.1,2
Therapeutic treatment for painful bullous lesions in the mouth involve antiseptic rinses and anesthetic solutions. Preventive treatment for patients with HSV-associated EM recurrence includes oral acyclovir or valacyclovir.2
In this patient, a punch biopsy was performed, confirming the diagnosis. A DIF was negative, and a chest x-ray was negative. Treatment was initiated with oral acyclovir, doxycycline, and a prednisone taper. In addition, topical clobetasol propionate and magic mouthwash (Maalox/lidocaine/nystatin) was prescribed. The patient was placed on daily suppressive valacyclovir to prevent frequent recurrence of EM.
This case and photo were submitted by Ms. Pham, the University of California, Los Angeles, and Dr. Sateesh, San Diego Family Dermatology. Dr. Bilu-Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Hafsi W and Badri T. Erythema Multiforme, in “StatPearls [Internet].” Treasure Island, Fla.: StatPearls Publishing, 2022 Jan.
2. Bolognia J et al. Dermatology. St. Louis: Mosby/Elsevier, 2008.
3. Oakley A. Erythema Multiforme. DermNet NZ. 2015 Oct.
Less frequently observable infectious agents associated with EM are Mycoplasma pneumoniae, Histoplasma capsulatum, and parapoxvirus (orf). Rarely, EM is triggered by drug eruption or systemic disease. Individuals of all age groups and races can be affected by EM. However, it is predominantly observed in young adult patients (20-40 years of age), and there is a male predominance.
Patients typically present with the abrupt onset of symmetrical red papules that evolve into typical and atypical targetoid lesions. Lesions evolve in 48-72 hours, favoring acrofacial sites that then spread down towards the trunk. Systemic symptoms such as fever and arthralgia may accompany the skin lesions.1-3
Erythema multiforme is recognized in two forms: EM minor and EM major. Both forms share the same characteristic of target lesions. However, the presence of mucosal involvement distinguishes the two. Mucosal involvement is absent or mild in EM minor, while mucosal involvement in EM major is often severe.2,3 Painful bullous lesions are commonly present in the mouth, genital, and ocular mucous membranes. Severe symptoms can often result in difficulty eating and drinking.
Diagnosis is largely clinical. Further histologic study may accompany diagnoses to exclude differential diagnosis. In EM, direct immunofluorescence (DIF) is negative. Histopathology reveals apoptosis of individual keratinocytes.1,2
Therapeutic treatment for painful bullous lesions in the mouth involve antiseptic rinses and anesthetic solutions. Preventive treatment for patients with HSV-associated EM recurrence includes oral acyclovir or valacyclovir.2
In this patient, a punch biopsy was performed, confirming the diagnosis. A DIF was negative, and a chest x-ray was negative. Treatment was initiated with oral acyclovir, doxycycline, and a prednisone taper. In addition, topical clobetasol propionate and magic mouthwash (Maalox/lidocaine/nystatin) was prescribed. The patient was placed on daily suppressive valacyclovir to prevent frequent recurrence of EM.
This case and photo were submitted by Ms. Pham, the University of California, Los Angeles, and Dr. Sateesh, San Diego Family Dermatology. Dr. Bilu-Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Hafsi W and Badri T. Erythema Multiforme, in “StatPearls [Internet].” Treasure Island, Fla.: StatPearls Publishing, 2022 Jan.
2. Bolognia J et al. Dermatology. St. Louis: Mosby/Elsevier, 2008.
3. Oakley A. Erythema Multiforme. DermNet NZ. 2015 Oct.
Less frequently observable infectious agents associated with EM are Mycoplasma pneumoniae, Histoplasma capsulatum, and parapoxvirus (orf). Rarely, EM is triggered by drug eruption or systemic disease. Individuals of all age groups and races can be affected by EM. However, it is predominantly observed in young adult patients (20-40 years of age), and there is a male predominance.
Patients typically present with the abrupt onset of symmetrical red papules that evolve into typical and atypical targetoid lesions. Lesions evolve in 48-72 hours, favoring acrofacial sites that then spread down towards the trunk. Systemic symptoms such as fever and arthralgia may accompany the skin lesions.1-3
Erythema multiforme is recognized in two forms: EM minor and EM major. Both forms share the same characteristic of target lesions. However, the presence of mucosal involvement distinguishes the two. Mucosal involvement is absent or mild in EM minor, while mucosal involvement in EM major is often severe.2,3 Painful bullous lesions are commonly present in the mouth, genital, and ocular mucous membranes. Severe symptoms can often result in difficulty eating and drinking.
Diagnosis is largely clinical. Further histologic study may accompany diagnoses to exclude differential diagnosis. In EM, direct immunofluorescence (DIF) is negative. Histopathology reveals apoptosis of individual keratinocytes.1,2
Therapeutic treatment for painful bullous lesions in the mouth involve antiseptic rinses and anesthetic solutions. Preventive treatment for patients with HSV-associated EM recurrence includes oral acyclovir or valacyclovir.2
In this patient, a punch biopsy was performed, confirming the diagnosis. A DIF was negative, and a chest x-ray was negative. Treatment was initiated with oral acyclovir, doxycycline, and a prednisone taper. In addition, topical clobetasol propionate and magic mouthwash (Maalox/lidocaine/nystatin) was prescribed. The patient was placed on daily suppressive valacyclovir to prevent frequent recurrence of EM.
This case and photo were submitted by Ms. Pham, the University of California, Los Angeles, and Dr. Sateesh, San Diego Family Dermatology. Dr. Bilu-Martin edited the column.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Hafsi W and Badri T. Erythema Multiforme, in “StatPearls [Internet].” Treasure Island, Fla.: StatPearls Publishing, 2022 Jan.
2. Bolognia J et al. Dermatology. St. Louis: Mosby/Elsevier, 2008.
3. Oakley A. Erythema Multiforme. DermNet NZ. 2015 Oct.
Children and COVID: Weekly cases down by more than half
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.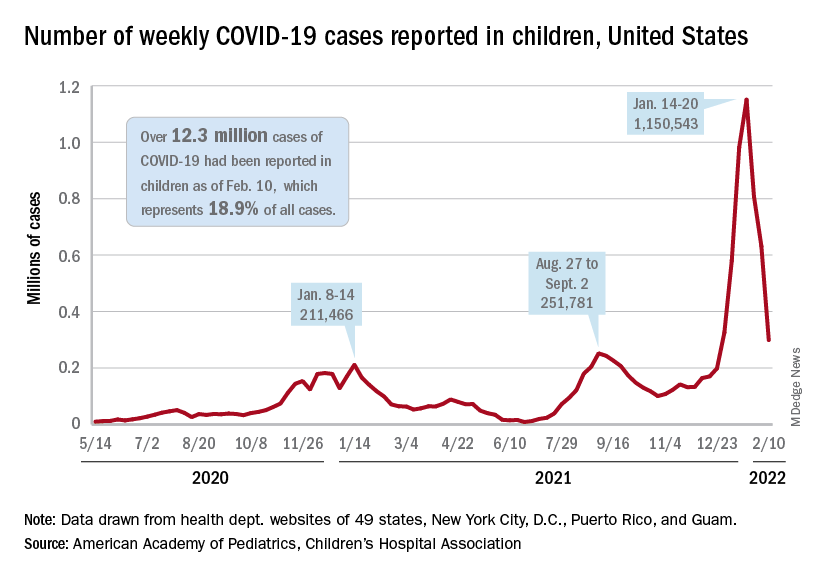
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
A third consecutive week of declines in new COVID-19 cases among children has brought the weekly count down by 74% since the Omicron surge peaked in mid-January, based on data from the American Academy of Pediatrics and the Children’s Hospital Association.
and by 74% from the peak of 1.15 million cases recorded for the week of Jan. 14-20, the AAP and CHA said in their weekly COVID report. They also noted that the weekly tally was still higher than anything seen during the Delta surge.
The total number of pediatric cases was over 12.3 million as of Feb. 10, with children representing 18.9% of cases in all ages, according to the AAP/CHA report. The Centers for Disease Control and Prevention puts the two measures at 10.4 million and 17.3% on its COVID Data Tracker, based on availability of age data for 59.6 million total cases as of Feb. 14. The CDC also reported that 1,282 children have died from COVID-19 so far, which is about 0.17% of all deaths with age data available.
The AAP and CHA have been collecting data from state and territorial health departments, which have not always been consistently available over the course of the pandemic. Also, the CDC defines children as those under age 18 years, but that upper boundary varies from 14 to 20 among the states.
The decline of the Omicron variant also can be seen in new admissions of children with confirmed COVID-19, which continued to drop. The 7-day average of 435 admissions per day for the week of Feb. 6-12 was less than half of the peak seen in mid-January, when it reached 914 per day. The daily admission rate on Feb. 12 was 0.60 per 100,000 children aged 0-17 years – again, less than half the peak rate of 1.25 reported on Jan. 16, CDC data show.
The fading threat of Omicron also seems to be reflected in recent vaccination trends. Both initial doses and completions declined for the fourth consecutive week (Feb. 3-9) among children aged 5-11 years, while initiations held steady for 12- to 17-year-olds but completions declined for the third straight week, the AAP said in its separate vaccination report, which is based on data from the CDC.
As of Feb. 14, almost 32% of children aged 5-11 – that’s almost 9.2 million individuals – had received at least one dose of the COVID-19 vaccine and just over 24% (6.9 million) were fully vaccinated, the CDC reported. For children aged 12-17, the corresponding figures are 67% (16.9 million) and 57% (14.4 million). Newly available data from the CDC also indicate that 19.5% (2.8 million) of children aged 12-17 have received a booster dose.
Ear tubes not recommended for recurrent AOM without effusion, ENTs maintain
A practice guideline update from the ENT community on tympanostomy tubes in children reaffirms that tube insertion should not be considered in cases of otitis media with effusion (OME) lasting less than 3 months, or in children with recurrent acute otitis media (AOM) without middle ear effusion at the time of assessment for the procedure.
New in the update from the American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) is a strong recommendation for timely follow-up after surgery and recommendations against both routine use of prophylactic antibiotic ear drops after surgery and the initial use of long-term tubes except when there are specific reasons for doing so.
The update also expands the list of risk factors that place children with OME at increased risk of developmental difficulties – and often in need of timely ear tube placement – to include intellectual disability, learning disorder, and attention-deficit/hyperactivity disorder.
“Most of what we said in the 2013 [original] guideline was good and still valid ... and [important for] pediatricians, who are the key players” in managing otitis media, Jesse Hackell, MD, one of two general pediatricians who served on the Academy’s guideline update committee, said in an interview.
OME spontaneously clears up to 90% of the time within 3 months, said Dr. Hackell, of Pomona (New York) Pediatrics, and chair of the American Academy of Pediatrics (AAP) Committee on Practice and Ambulatory Medicine.
The updated guideline, for children 6 months to 12 years, reaffirms a recommendation that tube insertion be offered to children with “bilateral OME for 3 months or longer AND documented hearing difficulties.”
It also reaffirms “options” (a lesser quality of evidence) that in the absence of hearing difficulties, surgery may be performed for children with chronic OME (3 months or longer) in one or both ears if 1) they are at increased risk of developmental difficulties from OME or 2) effusion is likely contributing to balance problems, poor school performance, behavioral problems, ear discomfort, or reduced quality of life.
Children with chronic OME who do not undergo surgery should be reevaluated at 3- to 6-month intervals and monitored until effusion is no longer present, significant hearing loss is detected, or structural abnormalities of the tympanic membrane or middle ear are detected, the update again recommends.
Tympanostomy tube placement is the most common ambulatory surgery performed on children in the United States, the guideline authors say. In 2014, about 9% of children had undergone the surgery, they wrote, noting also that “tubes were placed in 25%-30% of children with frequent ear infections.”
Recurrent AOM
The AAO-HNSF guidance regarding tympanostomy tubes for OME is similar overall to management guidance issued by the AAP in its clinical practice guideline on OME.
The organizations differ, however, on their guidance for tube insertion for recurrent AOM. In its 2013 clinical practice guideline on AOM, the AAP recommends that clinicians may offer tube insertion for recurrent AOM, with no mention of the presence or absence of persistent fluid as a consideration.
According to the AAO-HNSF update, grade A evidence, including some research published since its original 2013 guideline, has shown little benefit to tube insertion in reducing the incidence of AOM in otherwise healthy children who don’t have middle ear effusion.
One study published in 2019 assessed outcomes after watchful waiting and found that only one-third of 123 children eventually went on to tympanostomy tube placement, noted Richard M. Rosenfeld, MD, distinguished professor and chairman of otolaryngology at SUNY Downstate Health Sciences University in Brooklyn, N.Y., and lead author of the original and updated guidelines.
In practice, “the real question [for the ENT] is the future. If the ears are perfectly clear, will tubes really reduce the frequency of infections going forward?” Dr. Rosenfeld said in an interview. “All the evidence seems to say no, it doesn’t make much of a difference.”
Dr. Hackell said he’s confident that the question “is settled enough.” While there “could be stronger research and higher quality studies, the evidence is still pretty good to suggest you gain little to no benefit with tubes when you’re dealing with recurrent AOM without effusion,” he said.
Asked to comment on the ENT update and its guidance on tympanostomy tubes for children with recurrent AOM, an AAP spokesperson said the “issue is under review” and that the AAP did not currently have a statement.
At-risk children
The AAO-HNSF update renews a recommendation to evaluate children with either recurrent AOM or OME of any duration for increased risk for speech, language, or learning problems from OME because of baseline factors (sensory, physical, cognitive, or behavioral).
When OME becomes chronic – or when a tympanogram gives a flat-line reading – OME is likely to persist, and families of at-risk children especially should be encouraged to pursue tube placement, Dr. Rosenfeld said.
Despite prior guidance to this effect, he said, ear tubes are being underutilized in at-risk children, with effusion being missed in primary care and with ENTs not expediting tube placement upon referral.
“These children have learning issues, cognitive issues, developmental issues,” he said in the interview. “It’s a population that does very poorly with ears full of fluid ... and despite guidance suggesting these children should be prioritized with tubes, it doesn’t seem to be happening enough.”
Formulating guidelines for at-risk children is challenging because they are often excluded from trials, Dr. Rosenfeld said, which limits evidence about the benefits of tubes and limits the strength of recommendations.
The addition of attention-deficit/hyperactivity disorder, intellectual disability, and learning disorder to the list of risk factors is notable, Dr. Hackell said. (The list includes autism spectrum disorder, developmental delay, and suspected or confirmed speech and language delay or disorder.)
“We know that kids with ADHD take in and process information a little differently ... it may be harder to get their attention with auditory stimulation,” he said. “So anything that would impact the taking in of information even for a short period of time increases their risk.”
Surgical practice
ENTs are advised in the new guidance to use long-term tubes and perioperative antibiotic ear drops more judiciously. “Long-term tubes have a role, but there are some doctors who routinely use them, even for a first-time surgery,” said Dr. Rosenfeld.
Overuse of long-term tubes results in a higher incidence of tympanic membrane perforation, chronic drainage, and other complications, as well as greater need for long-term follow-up. “There needs to be a reason – something to justify the need for prolonged ventilation,” he said.
Perioperative antibiotic ear drops are often administered during surgery and then prescribed routinely for all children afterward, but research has shown that saline irrigation during surgery and a single application of antibiotic/steroid drops is similarly efficacious in preventing otorrhea, the guideline says. Antibiotic ear drops are also “expensive,” noted Dr. Hackell. “There’s not enough benefit to justify it.”
The update also more explicitly advises selective use of adenoidectomy. A new option says that clinicians may perform the procedure as an adjunct to tube insertion for children 4 years or older to potentially reduce the future incidence of recurrent OME or the need for repeat surgery.
However, in younger children, it should not be offered unless there are symptoms directly related to adenoid infection or nasal obstruction. “Under 4 years, there’s no primary benefit for the ears,” said Dr. Rosenfeld.
Follow-up with the surgeon after tympanostomy tube insertion should occur within 3 months to assess outcomes and educate the family, the update strongly recommends.
And pediatricians should know, Dr. Hackell notes, that clinical evidence continues to show that earplugs and other water precautions are not routinely needed for children who have tubes in place. A good approach, the guideline says, is to “first avoid water precautions and instead reserve them for children with recurrent or persistent tympanostomy tube otorrhea.”
Asked to comment on the guideline update, Tim Joos, MD, MPH, who practices combined internal medicine/pediatrics in Seattle and is an editorial advisory board member of Pediatric News, noted the inclusion of patient information sheets with frequently asked questions – resources that can be useful for guiding parents through what’s often a shared decision-making process.
Neither Dr. Rosenfeld nor Dr. Hackell reported any disclosures. Other members of the guideline update committee reported various book royalties, consulting fees, and other disclosures. Dr. Joos reported he has no connections to the guideline authors.
A practice guideline update from the ENT community on tympanostomy tubes in children reaffirms that tube insertion should not be considered in cases of otitis media with effusion (OME) lasting less than 3 months, or in children with recurrent acute otitis media (AOM) without middle ear effusion at the time of assessment for the procedure.
New in the update from the American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) is a strong recommendation for timely follow-up after surgery and recommendations against both routine use of prophylactic antibiotic ear drops after surgery and the initial use of long-term tubes except when there are specific reasons for doing so.
The update also expands the list of risk factors that place children with OME at increased risk of developmental difficulties – and often in need of timely ear tube placement – to include intellectual disability, learning disorder, and attention-deficit/hyperactivity disorder.
“Most of what we said in the 2013 [original] guideline was good and still valid ... and [important for] pediatricians, who are the key players” in managing otitis media, Jesse Hackell, MD, one of two general pediatricians who served on the Academy’s guideline update committee, said in an interview.
OME spontaneously clears up to 90% of the time within 3 months, said Dr. Hackell, of Pomona (New York) Pediatrics, and chair of the American Academy of Pediatrics (AAP) Committee on Practice and Ambulatory Medicine.
The updated guideline, for children 6 months to 12 years, reaffirms a recommendation that tube insertion be offered to children with “bilateral OME for 3 months or longer AND documented hearing difficulties.”
It also reaffirms “options” (a lesser quality of evidence) that in the absence of hearing difficulties, surgery may be performed for children with chronic OME (3 months or longer) in one or both ears if 1) they are at increased risk of developmental difficulties from OME or 2) effusion is likely contributing to balance problems, poor school performance, behavioral problems, ear discomfort, or reduced quality of life.
Children with chronic OME who do not undergo surgery should be reevaluated at 3- to 6-month intervals and monitored until effusion is no longer present, significant hearing loss is detected, or structural abnormalities of the tympanic membrane or middle ear are detected, the update again recommends.
Tympanostomy tube placement is the most common ambulatory surgery performed on children in the United States, the guideline authors say. In 2014, about 9% of children had undergone the surgery, they wrote, noting also that “tubes were placed in 25%-30% of children with frequent ear infections.”
Recurrent AOM
The AAO-HNSF guidance regarding tympanostomy tubes for OME is similar overall to management guidance issued by the AAP in its clinical practice guideline on OME.
The organizations differ, however, on their guidance for tube insertion for recurrent AOM. In its 2013 clinical practice guideline on AOM, the AAP recommends that clinicians may offer tube insertion for recurrent AOM, with no mention of the presence or absence of persistent fluid as a consideration.
According to the AAO-HNSF update, grade A evidence, including some research published since its original 2013 guideline, has shown little benefit to tube insertion in reducing the incidence of AOM in otherwise healthy children who don’t have middle ear effusion.
One study published in 2019 assessed outcomes after watchful waiting and found that only one-third of 123 children eventually went on to tympanostomy tube placement, noted Richard M. Rosenfeld, MD, distinguished professor and chairman of otolaryngology at SUNY Downstate Health Sciences University in Brooklyn, N.Y., and lead author of the original and updated guidelines.
In practice, “the real question [for the ENT] is the future. If the ears are perfectly clear, will tubes really reduce the frequency of infections going forward?” Dr. Rosenfeld said in an interview. “All the evidence seems to say no, it doesn’t make much of a difference.”
Dr. Hackell said he’s confident that the question “is settled enough.” While there “could be stronger research and higher quality studies, the evidence is still pretty good to suggest you gain little to no benefit with tubes when you’re dealing with recurrent AOM without effusion,” he said.
Asked to comment on the ENT update and its guidance on tympanostomy tubes for children with recurrent AOM, an AAP spokesperson said the “issue is under review” and that the AAP did not currently have a statement.
At-risk children
The AAO-HNSF update renews a recommendation to evaluate children with either recurrent AOM or OME of any duration for increased risk for speech, language, or learning problems from OME because of baseline factors (sensory, physical, cognitive, or behavioral).
When OME becomes chronic – or when a tympanogram gives a flat-line reading – OME is likely to persist, and families of at-risk children especially should be encouraged to pursue tube placement, Dr. Rosenfeld said.
Despite prior guidance to this effect, he said, ear tubes are being underutilized in at-risk children, with effusion being missed in primary care and with ENTs not expediting tube placement upon referral.
“These children have learning issues, cognitive issues, developmental issues,” he said in the interview. “It’s a population that does very poorly with ears full of fluid ... and despite guidance suggesting these children should be prioritized with tubes, it doesn’t seem to be happening enough.”
Formulating guidelines for at-risk children is challenging because they are often excluded from trials, Dr. Rosenfeld said, which limits evidence about the benefits of tubes and limits the strength of recommendations.
The addition of attention-deficit/hyperactivity disorder, intellectual disability, and learning disorder to the list of risk factors is notable, Dr. Hackell said. (The list includes autism spectrum disorder, developmental delay, and suspected or confirmed speech and language delay or disorder.)
“We know that kids with ADHD take in and process information a little differently ... it may be harder to get their attention with auditory stimulation,” he said. “So anything that would impact the taking in of information even for a short period of time increases their risk.”
Surgical practice
ENTs are advised in the new guidance to use long-term tubes and perioperative antibiotic ear drops more judiciously. “Long-term tubes have a role, but there are some doctors who routinely use them, even for a first-time surgery,” said Dr. Rosenfeld.
Overuse of long-term tubes results in a higher incidence of tympanic membrane perforation, chronic drainage, and other complications, as well as greater need for long-term follow-up. “There needs to be a reason – something to justify the need for prolonged ventilation,” he said.
Perioperative antibiotic ear drops are often administered during surgery and then prescribed routinely for all children afterward, but research has shown that saline irrigation during surgery and a single application of antibiotic/steroid drops is similarly efficacious in preventing otorrhea, the guideline says. Antibiotic ear drops are also “expensive,” noted Dr. Hackell. “There’s not enough benefit to justify it.”
The update also more explicitly advises selective use of adenoidectomy. A new option says that clinicians may perform the procedure as an adjunct to tube insertion for children 4 years or older to potentially reduce the future incidence of recurrent OME or the need for repeat surgery.
However, in younger children, it should not be offered unless there are symptoms directly related to adenoid infection or nasal obstruction. “Under 4 years, there’s no primary benefit for the ears,” said Dr. Rosenfeld.
Follow-up with the surgeon after tympanostomy tube insertion should occur within 3 months to assess outcomes and educate the family, the update strongly recommends.
And pediatricians should know, Dr. Hackell notes, that clinical evidence continues to show that earplugs and other water precautions are not routinely needed for children who have tubes in place. A good approach, the guideline says, is to “first avoid water precautions and instead reserve them for children with recurrent or persistent tympanostomy tube otorrhea.”
Asked to comment on the guideline update, Tim Joos, MD, MPH, who practices combined internal medicine/pediatrics in Seattle and is an editorial advisory board member of Pediatric News, noted the inclusion of patient information sheets with frequently asked questions – resources that can be useful for guiding parents through what’s often a shared decision-making process.
Neither Dr. Rosenfeld nor Dr. Hackell reported any disclosures. Other members of the guideline update committee reported various book royalties, consulting fees, and other disclosures. Dr. Joos reported he has no connections to the guideline authors.
A practice guideline update from the ENT community on tympanostomy tubes in children reaffirms that tube insertion should not be considered in cases of otitis media with effusion (OME) lasting less than 3 months, or in children with recurrent acute otitis media (AOM) without middle ear effusion at the time of assessment for the procedure.
New in the update from the American Academy of Otolaryngology–Head and Neck Surgery Foundation (AAO-HNSF) is a strong recommendation for timely follow-up after surgery and recommendations against both routine use of prophylactic antibiotic ear drops after surgery and the initial use of long-term tubes except when there are specific reasons for doing so.
The update also expands the list of risk factors that place children with OME at increased risk of developmental difficulties – and often in need of timely ear tube placement – to include intellectual disability, learning disorder, and attention-deficit/hyperactivity disorder.
“Most of what we said in the 2013 [original] guideline was good and still valid ... and [important for] pediatricians, who are the key players” in managing otitis media, Jesse Hackell, MD, one of two general pediatricians who served on the Academy’s guideline update committee, said in an interview.
OME spontaneously clears up to 90% of the time within 3 months, said Dr. Hackell, of Pomona (New York) Pediatrics, and chair of the American Academy of Pediatrics (AAP) Committee on Practice and Ambulatory Medicine.
The updated guideline, for children 6 months to 12 years, reaffirms a recommendation that tube insertion be offered to children with “bilateral OME for 3 months or longer AND documented hearing difficulties.”
It also reaffirms “options” (a lesser quality of evidence) that in the absence of hearing difficulties, surgery may be performed for children with chronic OME (3 months or longer) in one or both ears if 1) they are at increased risk of developmental difficulties from OME or 2) effusion is likely contributing to balance problems, poor school performance, behavioral problems, ear discomfort, or reduced quality of life.
Children with chronic OME who do not undergo surgery should be reevaluated at 3- to 6-month intervals and monitored until effusion is no longer present, significant hearing loss is detected, or structural abnormalities of the tympanic membrane or middle ear are detected, the update again recommends.
Tympanostomy tube placement is the most common ambulatory surgery performed on children in the United States, the guideline authors say. In 2014, about 9% of children had undergone the surgery, they wrote, noting also that “tubes were placed in 25%-30% of children with frequent ear infections.”
Recurrent AOM
The AAO-HNSF guidance regarding tympanostomy tubes for OME is similar overall to management guidance issued by the AAP in its clinical practice guideline on OME.
The organizations differ, however, on their guidance for tube insertion for recurrent AOM. In its 2013 clinical practice guideline on AOM, the AAP recommends that clinicians may offer tube insertion for recurrent AOM, with no mention of the presence or absence of persistent fluid as a consideration.
According to the AAO-HNSF update, grade A evidence, including some research published since its original 2013 guideline, has shown little benefit to tube insertion in reducing the incidence of AOM in otherwise healthy children who don’t have middle ear effusion.
One study published in 2019 assessed outcomes after watchful waiting and found that only one-third of 123 children eventually went on to tympanostomy tube placement, noted Richard M. Rosenfeld, MD, distinguished professor and chairman of otolaryngology at SUNY Downstate Health Sciences University in Brooklyn, N.Y., and lead author of the original and updated guidelines.
In practice, “the real question [for the ENT] is the future. If the ears are perfectly clear, will tubes really reduce the frequency of infections going forward?” Dr. Rosenfeld said in an interview. “All the evidence seems to say no, it doesn’t make much of a difference.”
Dr. Hackell said he’s confident that the question “is settled enough.” While there “could be stronger research and higher quality studies, the evidence is still pretty good to suggest you gain little to no benefit with tubes when you’re dealing with recurrent AOM without effusion,” he said.
Asked to comment on the ENT update and its guidance on tympanostomy tubes for children with recurrent AOM, an AAP spokesperson said the “issue is under review” and that the AAP did not currently have a statement.
At-risk children
The AAO-HNSF update renews a recommendation to evaluate children with either recurrent AOM or OME of any duration for increased risk for speech, language, or learning problems from OME because of baseline factors (sensory, physical, cognitive, or behavioral).
When OME becomes chronic – or when a tympanogram gives a flat-line reading – OME is likely to persist, and families of at-risk children especially should be encouraged to pursue tube placement, Dr. Rosenfeld said.
Despite prior guidance to this effect, he said, ear tubes are being underutilized in at-risk children, with effusion being missed in primary care and with ENTs not expediting tube placement upon referral.
“These children have learning issues, cognitive issues, developmental issues,” he said in the interview. “It’s a population that does very poorly with ears full of fluid ... and despite guidance suggesting these children should be prioritized with tubes, it doesn’t seem to be happening enough.”
Formulating guidelines for at-risk children is challenging because they are often excluded from trials, Dr. Rosenfeld said, which limits evidence about the benefits of tubes and limits the strength of recommendations.
The addition of attention-deficit/hyperactivity disorder, intellectual disability, and learning disorder to the list of risk factors is notable, Dr. Hackell said. (The list includes autism spectrum disorder, developmental delay, and suspected or confirmed speech and language delay or disorder.)
“We know that kids with ADHD take in and process information a little differently ... it may be harder to get their attention with auditory stimulation,” he said. “So anything that would impact the taking in of information even for a short period of time increases their risk.”
Surgical practice
ENTs are advised in the new guidance to use long-term tubes and perioperative antibiotic ear drops more judiciously. “Long-term tubes have a role, but there are some doctors who routinely use them, even for a first-time surgery,” said Dr. Rosenfeld.
Overuse of long-term tubes results in a higher incidence of tympanic membrane perforation, chronic drainage, and other complications, as well as greater need for long-term follow-up. “There needs to be a reason – something to justify the need for prolonged ventilation,” he said.
Perioperative antibiotic ear drops are often administered during surgery and then prescribed routinely for all children afterward, but research has shown that saline irrigation during surgery and a single application of antibiotic/steroid drops is similarly efficacious in preventing otorrhea, the guideline says. Antibiotic ear drops are also “expensive,” noted Dr. Hackell. “There’s not enough benefit to justify it.”
The update also more explicitly advises selective use of adenoidectomy. A new option says that clinicians may perform the procedure as an adjunct to tube insertion for children 4 years or older to potentially reduce the future incidence of recurrent OME or the need for repeat surgery.
However, in younger children, it should not be offered unless there are symptoms directly related to adenoid infection or nasal obstruction. “Under 4 years, there’s no primary benefit for the ears,” said Dr. Rosenfeld.
Follow-up with the surgeon after tympanostomy tube insertion should occur within 3 months to assess outcomes and educate the family, the update strongly recommends.
And pediatricians should know, Dr. Hackell notes, that clinical evidence continues to show that earplugs and other water precautions are not routinely needed for children who have tubes in place. A good approach, the guideline says, is to “first avoid water precautions and instead reserve them for children with recurrent or persistent tympanostomy tube otorrhea.”
Asked to comment on the guideline update, Tim Joos, MD, MPH, who practices combined internal medicine/pediatrics in Seattle and is an editorial advisory board member of Pediatric News, noted the inclusion of patient information sheets with frequently asked questions – resources that can be useful for guiding parents through what’s often a shared decision-making process.
Neither Dr. Rosenfeld nor Dr. Hackell reported any disclosures. Other members of the guideline update committee reported various book royalties, consulting fees, and other disclosures. Dr. Joos reported he has no connections to the guideline authors.
FROM OTOLARYNGOLOGY HEAD AND NECK SURGERY
Treatment duration for acute otitis media – so many choices
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Derms in survey say climate change is impacting their patients
in which the majority of participants said their patients are already being impacted.
Almost 80% of the 148 participants who responded to an electronic survey reported this belief.
The survey was designed and distributed to the membership of various dermatological organizations by Misha Rosenbach, MD, and coauthors. The results were published in the British Journal of Dermatology.
Asked also about specific types of climate-driven phenomena with a current – or future – impact on their patients, 80.1% reported that they believed that increased exposure to ultraviolet radiation (UVR) is impactful, or will be. Changes in temporal or geographic patterns of vector-borne illnesses were affirmed by 78.7%, and an increase in social displacement caused by extreme weather or other events was affirmed by 67.1% as having an impact on their patients currently or in the future.
Other phenomena affirmed by respondents as already having an impact or impacting patients in the future were an increased incidence of heat exposure or heat-related illness (58.2%); an increase in rates of inflammatory skin disease flares (43.2%); increased incidence of waterborne infections (42.5%); and increased rates of allergic contact dermatitis (29.5%).
The survey was sent to the membership of the American Society of Dermatologic Surgery, the Society for Pediatric Dermatology, the Society for Investigative Dermatology, and the American Academy of Dermatology’s Climate Change Expert Resource Group (ERG), among other organizations.
The study design and membership overlap made it impossible to calculate a response rate, the authors said, but they estimated it to be about 10%.
Almost all respondents were from the United States, and most (86.3%) practiced in an academic setting. The findings are similar to those of an online survey of members of the International Society of Dermatology (ISD), published in 2020, which found that 89% of 158 respondents believed climate change will impact the incidence of skin diseases in their area.
“Physicians, including dermatologists, are starting to understand the impact of the climate crisis on both their patients and themselves ... both through lived experiences and [issues raised] more in the scientific literature and in meetings,” Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
A majority of participants in the U.S. survey agreed they have a responsibility to bring awareness of the health effects of climate change to patients (77.2%) and to policymakers (88.6%). (In the ISD survey, 88% said they believed that dermatologists should play an advocacy role in climate change-related issues).
Only a minority of respondents in the U.S. survey said that they would feel comfortable discussing climate change with their patients (37.2%). Almost one-third of the respondents said they would like to be better informed about climate change before doing so. And 81.8% said they would like to read more about the dermatological effects of climate change in scientific journals.
“There continues to be unfilled interest in education and advocacy regarding climate change, suggesting a ‘practice gap’ even among dermatologists,” Dr. Rosenbach and his colleagues wrote, noting opportunities for professional organizations and journals to provide more resources and “actionable items” regarding climate change.
Some dermatologists have been taking action, in the meantime, to reduce the carbon footprint of their practices and institutions. Reductions in facility energy consumption, and reductions in medical waste/optimization of recycling, were each reported by more than one-third of survey respondents.
And almost half indicated that their practice or institution had increased capacity for telemedicine or telecommuting in response to climate change. Only 8% said their practice or institution had divested from fossil fuel stocks and/or bonds.
“There are a lot of sustainability-in-medicine solutions that are actually cost-neutral or cost-saving for practices,” said Dr. Rosenbach, who is a founder and co-chair of the AAD’s ERG on Climate Change and Environmental Issues.
Research in dermatology is starting to quantify the environmental impact of some of these changes. In a research letter also published in the British Journal of Dermatology, researchers from Cardiff University and the department of dermatology at University Hospital of Wales, described how they determined that reusable surgical packs used for skin surgery are more sustainable than single-use packs because of their reduced cost and reduced greenhouse gas emissions.
Such single-site reports are “early feeders” into what will become a stream of larger studies quantifying the impact of measures taken in dermatology, Dr. Rosenbach said.
Across medicine, there is evidence that health care professionals are now seeing climate change as a threat to their patients. In a multinational survey published last year in The Lancet Planetary Health, 77% of 3,977 participants said that climate change will cause a moderate or great deal of harm for their patients.
Climate change will be discussed at the AAD’s annual meeting in late March in a session devoted to the topic, and as part of a broader session on controversies in dermatology.
Dr. Rosenbach and two of the five authors of the dermatology research letter are members of the AAD’s ERG on climate change, but in the publication they noted that they were not writing on behalf of the AAD. None of the authors reported any disclosures, and there was no funding source for the survey.
in which the majority of participants said their patients are already being impacted.
Almost 80% of the 148 participants who responded to an electronic survey reported this belief.
The survey was designed and distributed to the membership of various dermatological organizations by Misha Rosenbach, MD, and coauthors. The results were published in the British Journal of Dermatology.
Asked also about specific types of climate-driven phenomena with a current – or future – impact on their patients, 80.1% reported that they believed that increased exposure to ultraviolet radiation (UVR) is impactful, or will be. Changes in temporal or geographic patterns of vector-borne illnesses were affirmed by 78.7%, and an increase in social displacement caused by extreme weather or other events was affirmed by 67.1% as having an impact on their patients currently or in the future.
Other phenomena affirmed by respondents as already having an impact or impacting patients in the future were an increased incidence of heat exposure or heat-related illness (58.2%); an increase in rates of inflammatory skin disease flares (43.2%); increased incidence of waterborne infections (42.5%); and increased rates of allergic contact dermatitis (29.5%).
The survey was sent to the membership of the American Society of Dermatologic Surgery, the Society for Pediatric Dermatology, the Society for Investigative Dermatology, and the American Academy of Dermatology’s Climate Change Expert Resource Group (ERG), among other organizations.
The study design and membership overlap made it impossible to calculate a response rate, the authors said, but they estimated it to be about 10%.
Almost all respondents were from the United States, and most (86.3%) practiced in an academic setting. The findings are similar to those of an online survey of members of the International Society of Dermatology (ISD), published in 2020, which found that 89% of 158 respondents believed climate change will impact the incidence of skin diseases in their area.
“Physicians, including dermatologists, are starting to understand the impact of the climate crisis on both their patients and themselves ... both through lived experiences and [issues raised] more in the scientific literature and in meetings,” Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
A majority of participants in the U.S. survey agreed they have a responsibility to bring awareness of the health effects of climate change to patients (77.2%) and to policymakers (88.6%). (In the ISD survey, 88% said they believed that dermatologists should play an advocacy role in climate change-related issues).
Only a minority of respondents in the U.S. survey said that they would feel comfortable discussing climate change with their patients (37.2%). Almost one-third of the respondents said they would like to be better informed about climate change before doing so. And 81.8% said they would like to read more about the dermatological effects of climate change in scientific journals.
“There continues to be unfilled interest in education and advocacy regarding climate change, suggesting a ‘practice gap’ even among dermatologists,” Dr. Rosenbach and his colleagues wrote, noting opportunities for professional organizations and journals to provide more resources and “actionable items” regarding climate change.
Some dermatologists have been taking action, in the meantime, to reduce the carbon footprint of their practices and institutions. Reductions in facility energy consumption, and reductions in medical waste/optimization of recycling, were each reported by more than one-third of survey respondents.
And almost half indicated that their practice or institution had increased capacity for telemedicine or telecommuting in response to climate change. Only 8% said their practice or institution had divested from fossil fuel stocks and/or bonds.
“There are a lot of sustainability-in-medicine solutions that are actually cost-neutral or cost-saving for practices,” said Dr. Rosenbach, who is a founder and co-chair of the AAD’s ERG on Climate Change and Environmental Issues.
Research in dermatology is starting to quantify the environmental impact of some of these changes. In a research letter also published in the British Journal of Dermatology, researchers from Cardiff University and the department of dermatology at University Hospital of Wales, described how they determined that reusable surgical packs used for skin surgery are more sustainable than single-use packs because of their reduced cost and reduced greenhouse gas emissions.
Such single-site reports are “early feeders” into what will become a stream of larger studies quantifying the impact of measures taken in dermatology, Dr. Rosenbach said.
Across medicine, there is evidence that health care professionals are now seeing climate change as a threat to their patients. In a multinational survey published last year in The Lancet Planetary Health, 77% of 3,977 participants said that climate change will cause a moderate or great deal of harm for their patients.
Climate change will be discussed at the AAD’s annual meeting in late March in a session devoted to the topic, and as part of a broader session on controversies in dermatology.
Dr. Rosenbach and two of the five authors of the dermatology research letter are members of the AAD’s ERG on climate change, but in the publication they noted that they were not writing on behalf of the AAD. None of the authors reported any disclosures, and there was no funding source for the survey.
in which the majority of participants said their patients are already being impacted.
Almost 80% of the 148 participants who responded to an electronic survey reported this belief.
The survey was designed and distributed to the membership of various dermatological organizations by Misha Rosenbach, MD, and coauthors. The results were published in the British Journal of Dermatology.
Asked also about specific types of climate-driven phenomena with a current – or future – impact on their patients, 80.1% reported that they believed that increased exposure to ultraviolet radiation (UVR) is impactful, or will be. Changes in temporal or geographic patterns of vector-borne illnesses were affirmed by 78.7%, and an increase in social displacement caused by extreme weather or other events was affirmed by 67.1% as having an impact on their patients currently or in the future.
Other phenomena affirmed by respondents as already having an impact or impacting patients in the future were an increased incidence of heat exposure or heat-related illness (58.2%); an increase in rates of inflammatory skin disease flares (43.2%); increased incidence of waterborne infections (42.5%); and increased rates of allergic contact dermatitis (29.5%).
The survey was sent to the membership of the American Society of Dermatologic Surgery, the Society for Pediatric Dermatology, the Society for Investigative Dermatology, and the American Academy of Dermatology’s Climate Change Expert Resource Group (ERG), among other organizations.
The study design and membership overlap made it impossible to calculate a response rate, the authors said, but they estimated it to be about 10%.
Almost all respondents were from the United States, and most (86.3%) practiced in an academic setting. The findings are similar to those of an online survey of members of the International Society of Dermatology (ISD), published in 2020, which found that 89% of 158 respondents believed climate change will impact the incidence of skin diseases in their area.
“Physicians, including dermatologists, are starting to understand the impact of the climate crisis on both their patients and themselves ... both through lived experiences and [issues raised] more in the scientific literature and in meetings,” Dr. Rosenbach, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said in an interview.
A majority of participants in the U.S. survey agreed they have a responsibility to bring awareness of the health effects of climate change to patients (77.2%) and to policymakers (88.6%). (In the ISD survey, 88% said they believed that dermatologists should play an advocacy role in climate change-related issues).
Only a minority of respondents in the U.S. survey said that they would feel comfortable discussing climate change with their patients (37.2%). Almost one-third of the respondents said they would like to be better informed about climate change before doing so. And 81.8% said they would like to read more about the dermatological effects of climate change in scientific journals.
“There continues to be unfilled interest in education and advocacy regarding climate change, suggesting a ‘practice gap’ even among dermatologists,” Dr. Rosenbach and his colleagues wrote, noting opportunities for professional organizations and journals to provide more resources and “actionable items” regarding climate change.
Some dermatologists have been taking action, in the meantime, to reduce the carbon footprint of their practices and institutions. Reductions in facility energy consumption, and reductions in medical waste/optimization of recycling, were each reported by more than one-third of survey respondents.
And almost half indicated that their practice or institution had increased capacity for telemedicine or telecommuting in response to climate change. Only 8% said their practice or institution had divested from fossil fuel stocks and/or bonds.
“There are a lot of sustainability-in-medicine solutions that are actually cost-neutral or cost-saving for practices,” said Dr. Rosenbach, who is a founder and co-chair of the AAD’s ERG on Climate Change and Environmental Issues.
Research in dermatology is starting to quantify the environmental impact of some of these changes. In a research letter also published in the British Journal of Dermatology, researchers from Cardiff University and the department of dermatology at University Hospital of Wales, described how they determined that reusable surgical packs used for skin surgery are more sustainable than single-use packs because of their reduced cost and reduced greenhouse gas emissions.
Such single-site reports are “early feeders” into what will become a stream of larger studies quantifying the impact of measures taken in dermatology, Dr. Rosenbach said.
Across medicine, there is evidence that health care professionals are now seeing climate change as a threat to their patients. In a multinational survey published last year in The Lancet Planetary Health, 77% of 3,977 participants said that climate change will cause a moderate or great deal of harm for their patients.
Climate change will be discussed at the AAD’s annual meeting in late March in a session devoted to the topic, and as part of a broader session on controversies in dermatology.
Dr. Rosenbach and two of the five authors of the dermatology research letter are members of the AAD’s ERG on climate change, but in the publication they noted that they were not writing on behalf of the AAD. None of the authors reported any disclosures, and there was no funding source for the survey.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Small group of higher-volume antibiotic prescribers identified
“Higher-volume prescribers prescribed antibiotics to a larger share of their patient panel and their prescribing rate was 60% higher than that of lower-volume prescribers, indicating that their prescribing practices might be independent of the number of beneficiaries under their care,” Katryna A. Gouin, MPH, and associates wrote in the Morbidity and Mortality Weekly Report.
In 2019, 41% of all Part D antibiotics – that’s 24.4 million prescriptions – were prescribed by 69,835 higher-volume prescribers. The other 59% of all antibiotics were prescribed by the 627,000 lower-volume health care providers included in the analysis (those who prescribed fewer than 11 antibiotics were excluded), Ms. Gouin of Chenega in Anchorage, Alaska, and associates noted.
The analysis involved the Medicare Part D Prescribers by Provider data set and defined the highest-volume prescribers “as those in the highest 10th percentile of prescriber-level antibiotic volume (number of antibiotic prescriptions filled) across all Medicare providers nationwide,” the investigators explained.
The antibiotic-prescribing rate for the higher-volume prescribers was 680 prescriptions per 1,000 beneficiaries, which was 60% higher than the 426 prescriptions per 1,000 among the lower 90% of prescribers. Another way to look at it: The top 10% of health care providers “wrote a median of 284 antibiotic prescriptions, compared with a median of 41 among lower-volume prescribers,” the investigators said.
Physicians in internal medicine and family practice, the two largest medical specialties, were the most likely to be 10-percenters, accounting for 24.6% and 27.5%, respectively, of the higher-volume group. They were followed by nurse practitioners (14.1%) and physician assistants (7.4%), who were classified as specialists for the purposes of the study, Ms. Gouin and associates said.
The only other group of physicians among the top six specialties were urologists, who represented 6.8% of high-volume prescribers but only 1% of all prescribers, they noted.
The highest antibiotic prescription rate in the six largest groups of providers occurred among dentists, whose highest-prescribing practitioners wrote 1,271 prescriptions per 1,000 beneficiaries. Even the lower-prescribing 90% of dentists prescribed more antibiotics (1,068 per 1,000) than did the higher-prescribing family physicians (611 per 1,000) and internists (590 per 1,000), the researchers said.
The prescribing rates for all the other specialties that were not included separately also were higher than the family physicians’ and internists’. These rates were 850 per 1,000 beneficiaries for the higher-prescribers and 360 per 1,000 for the lower-prescribers, the researchers wrote.
The considerable differences in prescribing practices between specialties and even among those of the same specialty present “opportunities for improved prescribing through antibiotic stewardship activities focusing on these higher-volume prescribers, independent of specialty,” Ms. Gouin and associates wrote.
“Higher-volume prescribers prescribed antibiotics to a larger share of their patient panel and their prescribing rate was 60% higher than that of lower-volume prescribers, indicating that their prescribing practices might be independent of the number of beneficiaries under their care,” Katryna A. Gouin, MPH, and associates wrote in the Morbidity and Mortality Weekly Report.
In 2019, 41% of all Part D antibiotics – that’s 24.4 million prescriptions – were prescribed by 69,835 higher-volume prescribers. The other 59% of all antibiotics were prescribed by the 627,000 lower-volume health care providers included in the analysis (those who prescribed fewer than 11 antibiotics were excluded), Ms. Gouin of Chenega in Anchorage, Alaska, and associates noted.
The analysis involved the Medicare Part D Prescribers by Provider data set and defined the highest-volume prescribers “as those in the highest 10th percentile of prescriber-level antibiotic volume (number of antibiotic prescriptions filled) across all Medicare providers nationwide,” the investigators explained.
The antibiotic-prescribing rate for the higher-volume prescribers was 680 prescriptions per 1,000 beneficiaries, which was 60% higher than the 426 prescriptions per 1,000 among the lower 90% of prescribers. Another way to look at it: The top 10% of health care providers “wrote a median of 284 antibiotic prescriptions, compared with a median of 41 among lower-volume prescribers,” the investigators said.
Physicians in internal medicine and family practice, the two largest medical specialties, were the most likely to be 10-percenters, accounting for 24.6% and 27.5%, respectively, of the higher-volume group. They were followed by nurse practitioners (14.1%) and physician assistants (7.4%), who were classified as specialists for the purposes of the study, Ms. Gouin and associates said.
The only other group of physicians among the top six specialties were urologists, who represented 6.8% of high-volume prescribers but only 1% of all prescribers, they noted.
The highest antibiotic prescription rate in the six largest groups of providers occurred among dentists, whose highest-prescribing practitioners wrote 1,271 prescriptions per 1,000 beneficiaries. Even the lower-prescribing 90% of dentists prescribed more antibiotics (1,068 per 1,000) than did the higher-prescribing family physicians (611 per 1,000) and internists (590 per 1,000), the researchers said.
The prescribing rates for all the other specialties that were not included separately also were higher than the family physicians’ and internists’. These rates were 850 per 1,000 beneficiaries for the higher-prescribers and 360 per 1,000 for the lower-prescribers, the researchers wrote.
The considerable differences in prescribing practices between specialties and even among those of the same specialty present “opportunities for improved prescribing through antibiotic stewardship activities focusing on these higher-volume prescribers, independent of specialty,” Ms. Gouin and associates wrote.
“Higher-volume prescribers prescribed antibiotics to a larger share of their patient panel and their prescribing rate was 60% higher than that of lower-volume prescribers, indicating that their prescribing practices might be independent of the number of beneficiaries under their care,” Katryna A. Gouin, MPH, and associates wrote in the Morbidity and Mortality Weekly Report.
In 2019, 41% of all Part D antibiotics – that’s 24.4 million prescriptions – were prescribed by 69,835 higher-volume prescribers. The other 59% of all antibiotics were prescribed by the 627,000 lower-volume health care providers included in the analysis (those who prescribed fewer than 11 antibiotics were excluded), Ms. Gouin of Chenega in Anchorage, Alaska, and associates noted.
The analysis involved the Medicare Part D Prescribers by Provider data set and defined the highest-volume prescribers “as those in the highest 10th percentile of prescriber-level antibiotic volume (number of antibiotic prescriptions filled) across all Medicare providers nationwide,” the investigators explained.
The antibiotic-prescribing rate for the higher-volume prescribers was 680 prescriptions per 1,000 beneficiaries, which was 60% higher than the 426 prescriptions per 1,000 among the lower 90% of prescribers. Another way to look at it: The top 10% of health care providers “wrote a median of 284 antibiotic prescriptions, compared with a median of 41 among lower-volume prescribers,” the investigators said.
Physicians in internal medicine and family practice, the two largest medical specialties, were the most likely to be 10-percenters, accounting for 24.6% and 27.5%, respectively, of the higher-volume group. They were followed by nurse practitioners (14.1%) and physician assistants (7.4%), who were classified as specialists for the purposes of the study, Ms. Gouin and associates said.
The only other group of physicians among the top six specialties were urologists, who represented 6.8% of high-volume prescribers but only 1% of all prescribers, they noted.
The highest antibiotic prescription rate in the six largest groups of providers occurred among dentists, whose highest-prescribing practitioners wrote 1,271 prescriptions per 1,000 beneficiaries. Even the lower-prescribing 90% of dentists prescribed more antibiotics (1,068 per 1,000) than did the higher-prescribing family physicians (611 per 1,000) and internists (590 per 1,000), the researchers said.
The prescribing rates for all the other specialties that were not included separately also were higher than the family physicians’ and internists’. These rates were 850 per 1,000 beneficiaries for the higher-prescribers and 360 per 1,000 for the lower-prescribers, the researchers wrote.
The considerable differences in prescribing practices between specialties and even among those of the same specialty present “opportunities for improved prescribing through antibiotic stewardship activities focusing on these higher-volume prescribers, independent of specialty,” Ms. Gouin and associates wrote.
FROM THE MMWR
Strep infection and tics in children: new data
Group A streptococcus (GAS) infection is not associated with new-onset tic disorders in at-risk children, findings from a large prospective study show.
The results mean that if preteens present with a new-onset tic condition, “they’re unlikely to have it as a result of a group A streptococcal throat infection,” study author Anette Eleonore Schrag, MD, PhD, professor, department of clinical neuroscience, Institute of Neurology, University College London, told this news organization.
Therefore, clinicians should not automatically prescribe antibiotics for children with tics, which sometimes occurs, said Dr. Schrag.
The study was published online Feb. 2 in Neurology.
Ongoing controversy
Research shows that genetic and environmental factors contribute to chronic tic disorders (CTDs) and Tourette syndrome (TS). Prenatal exposure to maternal smoking and central nervous system (CNS) stimulants, as well as psychosocial stress, may play a role.
There has been an ongoing controversy regarding the possible role of GAS in tics, with some studies showing an association and others not showing a link. However, previous studies have been retrospective, registry based, or had limited sample size.
This new prospective study is the first in children without a tic disorder but who were at relatively high risk of developing one. The children were followed to assess development of streptococcal infections and tics, said Dr. Schrag.
The study included 259 children aged 3-10 years (mean baseline age, 6.8 years; over half female) who had a first-degree relative such as a parent or sibling with TS or CTD.
The average age at TS onset is 7 years, peaking in prevalence and severity at about 9-12 years. GAS throat infections are common in this age group.
Although study participants did not have tics themselves, they represented “an enriched group,” said Dr. Schrag. “Because they had family history, we knew they were at increased risk for developing tics.”
Participants were evaluated every 2 months, alternating between scheduled hospital visits and telephone interviews. Parents kept a weekly diary and were instructed to bring their child in for assessment if they showed any signs of tics.
The average follow-up period was 1.6 years, but some of the children were followed for up to 48 months. During the study, there were a total of 1,944 assessments, including 939 telephone interviews and 1,005 clinical visits.
More common in boys
Investigators defined tic onset as the first occurrence of any sudden, rapid, recurrent, nonrhythmic involuntary movement and/or vocalization on at least three separate days within a period of 3 weeks.
The investigators assessed GAS exposure using parameters from throat swabs, serum anti-streptolysin O titers, and anti-DNAse B titers.
They used multiple definitions and combinations of GAS exposures “to make sure we weren’t missing any association because we didn’t use the right definition,” said Dr. Schrag. She explained a definitive strep infection is not always clear-cut.
At baseline, 17.0% participants tested positive for GAS, and 78.8% tested negative. No throat swab was available from 4.2% of participants.
During follow-up, the number of confirmed positive GAS exposures was 59, 102, 125, and 138, depending on the definition.
Researchers identified 61 tic cases during the study period. There was no evidence of an association of tic onset with GAS exposure after adjusting for age, sex, and parental education level.
However, there was a strong association between tic onset and sex, with girls being 60% less likely to develop tics than boys (hazard ratio, 0.4; 95% CI, 0.2-0.7; P < .01).
This result wasn’t particularly surprising, as it’s known that more boys develop tics than girls. “We just confirmed that in a prospective way,” said Dr. Schrag.
Results from sensitivity analyses confirmed the results. This was also the case with analyses that excluded visits with missing data on GAS exposure and that further adjusted for clinical site and psychotropic medication use.
Other pathogens?
Although the results showed no association between strep and tics in this population, it does not “close the door completely” on a potential relationship, said Dr. Schrag.
“By and large, the development of tics in children is not associated with group A strep, but differences in small subgroups can never be excluded by a study like this.”
Participants in this study were part of the European Multicentre Tics in Children Studies (EMTICS), a prospective cohort study exploring the role of environmental and genetic factors in pediatric CTD. That project is also looking at immune system factors, “which might play a role in the development of chronic tic disorder and associated conditions,” said Dr. Schrag.
It’s still possible, she added, that other pathogens could play a role in tic development. “That’s going to be the subject of further analysis and future studies,” she said.
Tamara Pringsheim, MD, professor of clinical neurosciences, psychiatry, pediatrics, and community health sciences, University of Calgary (Alta.), praised the research.
“This was a well-designed study, with a large sample of 260 children followed for up to 4 years, using a standardized protocol to assess for group A streptococcal infection and new onset of tics.”
The study, which did not uncover an association between GAS exposure and tic onset, “provides high level evidence that group A streptococcal exposure is not an important risk factor for the new onset of tics in children with a family history of tic disorders.”
The study received funding from the European Union Seventh Framework Program for research technological development and demonstration. Dr. Schrag reports receiving consultancy or advisory board honoraria from Biogen, Abbvie, Bial, and Neurotechnology; research support from the National Institute of Health Research, Parkinsons UK, and the Economic and Social Research Council and the European Commission; and Royalties from Oxford University Press. Dr. Pringsheim reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Group A streptococcus (GAS) infection is not associated with new-onset tic disorders in at-risk children, findings from a large prospective study show.
The results mean that if preteens present with a new-onset tic condition, “they’re unlikely to have it as a result of a group A streptococcal throat infection,” study author Anette Eleonore Schrag, MD, PhD, professor, department of clinical neuroscience, Institute of Neurology, University College London, told this news organization.
Therefore, clinicians should not automatically prescribe antibiotics for children with tics, which sometimes occurs, said Dr. Schrag.
The study was published online Feb. 2 in Neurology.
Ongoing controversy
Research shows that genetic and environmental factors contribute to chronic tic disorders (CTDs) and Tourette syndrome (TS). Prenatal exposure to maternal smoking and central nervous system (CNS) stimulants, as well as psychosocial stress, may play a role.
There has been an ongoing controversy regarding the possible role of GAS in tics, with some studies showing an association and others not showing a link. However, previous studies have been retrospective, registry based, or had limited sample size.
This new prospective study is the first in children without a tic disorder but who were at relatively high risk of developing one. The children were followed to assess development of streptococcal infections and tics, said Dr. Schrag.
The study included 259 children aged 3-10 years (mean baseline age, 6.8 years; over half female) who had a first-degree relative such as a parent or sibling with TS or CTD.
The average age at TS onset is 7 years, peaking in prevalence and severity at about 9-12 years. GAS throat infections are common in this age group.
Although study participants did not have tics themselves, they represented “an enriched group,” said Dr. Schrag. “Because they had family history, we knew they were at increased risk for developing tics.”
Participants were evaluated every 2 months, alternating between scheduled hospital visits and telephone interviews. Parents kept a weekly diary and were instructed to bring their child in for assessment if they showed any signs of tics.
The average follow-up period was 1.6 years, but some of the children were followed for up to 48 months. During the study, there were a total of 1,944 assessments, including 939 telephone interviews and 1,005 clinical visits.
More common in boys
Investigators defined tic onset as the first occurrence of any sudden, rapid, recurrent, nonrhythmic involuntary movement and/or vocalization on at least three separate days within a period of 3 weeks.
The investigators assessed GAS exposure using parameters from throat swabs, serum anti-streptolysin O titers, and anti-DNAse B titers.
They used multiple definitions and combinations of GAS exposures “to make sure we weren’t missing any association because we didn’t use the right definition,” said Dr. Schrag. She explained a definitive strep infection is not always clear-cut.
At baseline, 17.0% participants tested positive for GAS, and 78.8% tested negative. No throat swab was available from 4.2% of participants.
During follow-up, the number of confirmed positive GAS exposures was 59, 102, 125, and 138, depending on the definition.
Researchers identified 61 tic cases during the study period. There was no evidence of an association of tic onset with GAS exposure after adjusting for age, sex, and parental education level.
However, there was a strong association between tic onset and sex, with girls being 60% less likely to develop tics than boys (hazard ratio, 0.4; 95% CI, 0.2-0.7; P < .01).
This result wasn’t particularly surprising, as it’s known that more boys develop tics than girls. “We just confirmed that in a prospective way,” said Dr. Schrag.
Results from sensitivity analyses confirmed the results. This was also the case with analyses that excluded visits with missing data on GAS exposure and that further adjusted for clinical site and psychotropic medication use.
Other pathogens?
Although the results showed no association between strep and tics in this population, it does not “close the door completely” on a potential relationship, said Dr. Schrag.
“By and large, the development of tics in children is not associated with group A strep, but differences in small subgroups can never be excluded by a study like this.”
Participants in this study were part of the European Multicentre Tics in Children Studies (EMTICS), a prospective cohort study exploring the role of environmental and genetic factors in pediatric CTD. That project is also looking at immune system factors, “which might play a role in the development of chronic tic disorder and associated conditions,” said Dr. Schrag.
It’s still possible, she added, that other pathogens could play a role in tic development. “That’s going to be the subject of further analysis and future studies,” she said.
Tamara Pringsheim, MD, professor of clinical neurosciences, psychiatry, pediatrics, and community health sciences, University of Calgary (Alta.), praised the research.
“This was a well-designed study, with a large sample of 260 children followed for up to 4 years, using a standardized protocol to assess for group A streptococcal infection and new onset of tics.”
The study, which did not uncover an association between GAS exposure and tic onset, “provides high level evidence that group A streptococcal exposure is not an important risk factor for the new onset of tics in children with a family history of tic disorders.”
The study received funding from the European Union Seventh Framework Program for research technological development and demonstration. Dr. Schrag reports receiving consultancy or advisory board honoraria from Biogen, Abbvie, Bial, and Neurotechnology; research support from the National Institute of Health Research, Parkinsons UK, and the Economic and Social Research Council and the European Commission; and Royalties from Oxford University Press. Dr. Pringsheim reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Group A streptococcus (GAS) infection is not associated with new-onset tic disorders in at-risk children, findings from a large prospective study show.
The results mean that if preteens present with a new-onset tic condition, “they’re unlikely to have it as a result of a group A streptococcal throat infection,” study author Anette Eleonore Schrag, MD, PhD, professor, department of clinical neuroscience, Institute of Neurology, University College London, told this news organization.
Therefore, clinicians should not automatically prescribe antibiotics for children with tics, which sometimes occurs, said Dr. Schrag.
The study was published online Feb. 2 in Neurology.
Ongoing controversy
Research shows that genetic and environmental factors contribute to chronic tic disorders (CTDs) and Tourette syndrome (TS). Prenatal exposure to maternal smoking and central nervous system (CNS) stimulants, as well as psychosocial stress, may play a role.
There has been an ongoing controversy regarding the possible role of GAS in tics, with some studies showing an association and others not showing a link. However, previous studies have been retrospective, registry based, or had limited sample size.
This new prospective study is the first in children without a tic disorder but who were at relatively high risk of developing one. The children were followed to assess development of streptococcal infections and tics, said Dr. Schrag.
The study included 259 children aged 3-10 years (mean baseline age, 6.8 years; over half female) who had a first-degree relative such as a parent or sibling with TS or CTD.
The average age at TS onset is 7 years, peaking in prevalence and severity at about 9-12 years. GAS throat infections are common in this age group.
Although study participants did not have tics themselves, they represented “an enriched group,” said Dr. Schrag. “Because they had family history, we knew they were at increased risk for developing tics.”
Participants were evaluated every 2 months, alternating between scheduled hospital visits and telephone interviews. Parents kept a weekly diary and were instructed to bring their child in for assessment if they showed any signs of tics.
The average follow-up period was 1.6 years, but some of the children were followed for up to 48 months. During the study, there were a total of 1,944 assessments, including 939 telephone interviews and 1,005 clinical visits.
More common in boys
Investigators defined tic onset as the first occurrence of any sudden, rapid, recurrent, nonrhythmic involuntary movement and/or vocalization on at least three separate days within a period of 3 weeks.
The investigators assessed GAS exposure using parameters from throat swabs, serum anti-streptolysin O titers, and anti-DNAse B titers.
They used multiple definitions and combinations of GAS exposures “to make sure we weren’t missing any association because we didn’t use the right definition,” said Dr. Schrag. She explained a definitive strep infection is not always clear-cut.
At baseline, 17.0% participants tested positive for GAS, and 78.8% tested negative. No throat swab was available from 4.2% of participants.
During follow-up, the number of confirmed positive GAS exposures was 59, 102, 125, and 138, depending on the definition.
Researchers identified 61 tic cases during the study period. There was no evidence of an association of tic onset with GAS exposure after adjusting for age, sex, and parental education level.
However, there was a strong association between tic onset and sex, with girls being 60% less likely to develop tics than boys (hazard ratio, 0.4; 95% CI, 0.2-0.7; P < .01).
This result wasn’t particularly surprising, as it’s known that more boys develop tics than girls. “We just confirmed that in a prospective way,” said Dr. Schrag.
Results from sensitivity analyses confirmed the results. This was also the case with analyses that excluded visits with missing data on GAS exposure and that further adjusted for clinical site and psychotropic medication use.
Other pathogens?
Although the results showed no association between strep and tics in this population, it does not “close the door completely” on a potential relationship, said Dr. Schrag.
“By and large, the development of tics in children is not associated with group A strep, but differences in small subgroups can never be excluded by a study like this.”
Participants in this study were part of the European Multicentre Tics in Children Studies (EMTICS), a prospective cohort study exploring the role of environmental and genetic factors in pediatric CTD. That project is also looking at immune system factors, “which might play a role in the development of chronic tic disorder and associated conditions,” said Dr. Schrag.
It’s still possible, she added, that other pathogens could play a role in tic development. “That’s going to be the subject of further analysis and future studies,” she said.
Tamara Pringsheim, MD, professor of clinical neurosciences, psychiatry, pediatrics, and community health sciences, University of Calgary (Alta.), praised the research.
“This was a well-designed study, with a large sample of 260 children followed for up to 4 years, using a standardized protocol to assess for group A streptococcal infection and new onset of tics.”
The study, which did not uncover an association between GAS exposure and tic onset, “provides high level evidence that group A streptococcal exposure is not an important risk factor for the new onset of tics in children with a family history of tic disorders.”
The study received funding from the European Union Seventh Framework Program for research technological development and demonstration. Dr. Schrag reports receiving consultancy or advisory board honoraria from Biogen, Abbvie, Bial, and Neurotechnology; research support from the National Institute of Health Research, Parkinsons UK, and the Economic and Social Research Council and the European Commission; and Royalties from Oxford University Press. Dr. Pringsheim reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.