User login
Pilomatrical Carcinoma: Case Report and Review of the Literature
Cutaneous Manifestations of Diabetes Mellitus: A Case Series
Trichotillomania: An Important Psychocutaneous Disorder
Cultural Competence Key to Treating Skin of Color
NAPLES, Fla. — Vitiligo, keloids, acne keloidalis nuchae, and hair and scalp concerns are among the challenges dermatologists face when treating darker skin, Dr. George Cohen said.
Black skin is prone to adverse pigmentary or hyperproliferative responses to cryotherapy, lasers, and chemical skin treatments. Because of these and other concerns in a growing population of patients with skin types IV through VI, Dr. Cohen suggested dermatologists learn more about recognition and treatment of these important differences.
"We are a more diverse society. Become culturally competent and learn as much as you can," Dr. Cohen said at the annual meeting of the Florida Society of Dermatology & Dermatologic Surgeons. Respect, inquire, and do not make assumptions—those are the three pillars of cultural competence, he said.
Cultural competence is not only good for patients; it can be good for your practice as well. "Access and acceptance are good for us—this creates more demand for services," said Dr. Cohen, of the department of dermatology and cutaneous surgery at the University of South Florida in Tampa.
Vitiligo
Vitiligo is the prototypic pigmentary challenge for patients with skin of color, Dr. Cohen said. Although the etiology is not completely understood, it may be related to the immune system. The challenge for dermatologists is that "some people respond to some things some of the time, so we don't always know who is going to respond," Dr. Cohen said.
The myriad of treatments available for vitiligo include:
- Narrow-band UVB therapy.
- Targeted laser treatment with the XeCL Excimer (308 nm).
- Topical steroids.
- Calcineurin inhibitors.
- Surgery.
- Depigmentation (using medications or 694-nm Q-switched Ruby laser).
- Makeup.
Unfortunately, "none of these work perfectly," he said.
He cited the case of a patient with vitiligo who tried steroids, psoralen and UVA (PUVA), and other treatments to no avail. "He was desperate. He came to me with his family and asked: 'Doctor, can you make me one color?' I told him we can only make him one color—white—that is all we are able to do.
"Sometimes you cannot repigment the patient, and you have to know how to judiciously offer depigmentation. Some might say I robbed him of his culture. I say, no, I didn't, I robbed him of a disfiguring condition," said Dr. Cohen.
More research is clearly warranted to improve treatment options for vitiligo, such as studies to assess the biology of melanocytes, he said.
Keloids
Keloids are another challenge in skin of color patients. The therapeutic approach depends in part on the extent of the patient's condition. For example, a single keloid on the earlobe would be treated differently than more widespread presentation.
Again, more research is warranted on optimal treatments, Dr. Cohen said, because studies in the literature are contradictory and provide no consensus.
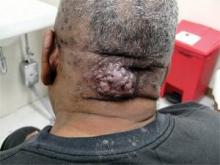
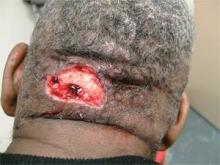
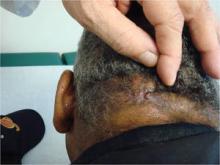
Acne keloidalis nuchae, "the keloids' cousin," most often occur in black men, he said. These nuchae can advance to plaque and form tumors, "and at the very least will need intralesional therapy." One clinical tip is to make an incision only within the keloid, he said.
If a patient presents with an acne keloidalis tumor, simply excise it. Once you get hemostasis, dress the wound with petroleum jelly only, and let it close by the magic of second intention
"I monitor these people. If I see any evidence of regrowth, I treat with triamcinolone early and often," he said.
Hair and Scalp Challenges
Hair and scalp concerns are common in patients with skin of color, Dr. Cohen said. A scalp biopsy is recommended to determine or confirm a diagnosis and to guide the course of clinical treatment.
These presentations can have a great psychosocial impact. "Do not underestimate the effect on patients," he said. Know your limitations and the limitations of therapy, and make sure you communicate those effectively to the patient. Otherwise, both the patient and provider can become frustrated.
Be honest with genetically-susceptible patients who present with scarring on the scalp from physical insult. In this population, scarring results when fibrous tissue replaces hair follicles. "Let them know up front that creams and other nonsense are not going to work," he said.
Contrary to popular belief, Dr. Cohen said, hair transplants are an option in patients with skin of color. "Hair transplants in black patients are not scary— I've been doing them for years and never had keloids," he said.
Black hair is heterogenous, so not everyone has curved follicles. If a skin of color patient has straight follicles, standard hair transplant procedures—for example, with 4-mm donor grafts—would be an option.
Dr. Cohen said that he did not have any relevant financial disclosures.
NAPLES, Fla. — Vitiligo, keloids, acne keloidalis nuchae, and hair and scalp concerns are among the challenges dermatologists face when treating darker skin, Dr. George Cohen said.
Black skin is prone to adverse pigmentary or hyperproliferative responses to cryotherapy, lasers, and chemical skin treatments. Because of these and other concerns in a growing population of patients with skin types IV through VI, Dr. Cohen suggested dermatologists learn more about recognition and treatment of these important differences.
"We are a more diverse society. Become culturally competent and learn as much as you can," Dr. Cohen said at the annual meeting of the Florida Society of Dermatology & Dermatologic Surgeons. Respect, inquire, and do not make assumptions—those are the three pillars of cultural competence, he said.
Cultural competence is not only good for patients; it can be good for your practice as well. "Access and acceptance are good for us—this creates more demand for services," said Dr. Cohen, of the department of dermatology and cutaneous surgery at the University of South Florida in Tampa.
Vitiligo
Vitiligo is the prototypic pigmentary challenge for patients with skin of color, Dr. Cohen said. Although the etiology is not completely understood, it may be related to the immune system. The challenge for dermatologists is that "some people respond to some things some of the time, so we don't always know who is going to respond," Dr. Cohen said.
The myriad of treatments available for vitiligo include:
- Narrow-band UVB therapy.
- Targeted laser treatment with the XeCL Excimer (308 nm).
- Topical steroids.
- Calcineurin inhibitors.
- Surgery.
- Depigmentation (using medications or 694-nm Q-switched Ruby laser).
- Makeup.
Unfortunately, "none of these work perfectly," he said.
He cited the case of a patient with vitiligo who tried steroids, psoralen and UVA (PUVA), and other treatments to no avail. "He was desperate. He came to me with his family and asked: 'Doctor, can you make me one color?' I told him we can only make him one color—white—that is all we are able to do.
"Sometimes you cannot repigment the patient, and you have to know how to judiciously offer depigmentation. Some might say I robbed him of his culture. I say, no, I didn't, I robbed him of a disfiguring condition," said Dr. Cohen.
More research is clearly warranted to improve treatment options for vitiligo, such as studies to assess the biology of melanocytes, he said.
Keloids
Keloids are another challenge in skin of color patients. The therapeutic approach depends in part on the extent of the patient's condition. For example, a single keloid on the earlobe would be treated differently than more widespread presentation.
Again, more research is warranted on optimal treatments, Dr. Cohen said, because studies in the literature are contradictory and provide no consensus.
Acne keloidalis nuchae, "the keloids' cousin," most often occur in black men, he said. These nuchae can advance to plaque and form tumors, "and at the very least will need intralesional therapy." One clinical tip is to make an incision only within the keloid, he said.
If a patient presents with an acne keloidalis tumor, simply excise it. Once you get hemostasis, dress the wound with petroleum jelly only, and let it close by the magic of second intention
"I monitor these people. If I see any evidence of regrowth, I treat with triamcinolone early and often," he said.
Hair and Scalp Challenges
Hair and scalp concerns are common in patients with skin of color, Dr. Cohen said. A scalp biopsy is recommended to determine or confirm a diagnosis and to guide the course of clinical treatment.
These presentations can have a great psychosocial impact. "Do not underestimate the effect on patients," he said. Know your limitations and the limitations of therapy, and make sure you communicate those effectively to the patient. Otherwise, both the patient and provider can become frustrated.
Be honest with genetically-susceptible patients who present with scarring on the scalp from physical insult. In this population, scarring results when fibrous tissue replaces hair follicles. "Let them know up front that creams and other nonsense are not going to work," he said.
Contrary to popular belief, Dr. Cohen said, hair transplants are an option in patients with skin of color. "Hair transplants in black patients are not scary— I've been doing them for years and never had keloids," he said.
Black hair is heterogenous, so not everyone has curved follicles. If a skin of color patient has straight follicles, standard hair transplant procedures—for example, with 4-mm donor grafts—would be an option.
Dr. Cohen said that he did not have any relevant financial disclosures.
NAPLES, Fla. — Vitiligo, keloids, acne keloidalis nuchae, and hair and scalp concerns are among the challenges dermatologists face when treating darker skin, Dr. George Cohen said.
Black skin is prone to adverse pigmentary or hyperproliferative responses to cryotherapy, lasers, and chemical skin treatments. Because of these and other concerns in a growing population of patients with skin types IV through VI, Dr. Cohen suggested dermatologists learn more about recognition and treatment of these important differences.
"We are a more diverse society. Become culturally competent and learn as much as you can," Dr. Cohen said at the annual meeting of the Florida Society of Dermatology & Dermatologic Surgeons. Respect, inquire, and do not make assumptions—those are the three pillars of cultural competence, he said.
Cultural competence is not only good for patients; it can be good for your practice as well. "Access and acceptance are good for us—this creates more demand for services," said Dr. Cohen, of the department of dermatology and cutaneous surgery at the University of South Florida in Tampa.
Vitiligo
Vitiligo is the prototypic pigmentary challenge for patients with skin of color, Dr. Cohen said. Although the etiology is not completely understood, it may be related to the immune system. The challenge for dermatologists is that "some people respond to some things some of the time, so we don't always know who is going to respond," Dr. Cohen said.
The myriad of treatments available for vitiligo include:
- Narrow-band UVB therapy.
- Targeted laser treatment with the XeCL Excimer (308 nm).
- Topical steroids.
- Calcineurin inhibitors.
- Surgery.
- Depigmentation (using medications or 694-nm Q-switched Ruby laser).
- Makeup.
Unfortunately, "none of these work perfectly," he said.
He cited the case of a patient with vitiligo who tried steroids, psoralen and UVA (PUVA), and other treatments to no avail. "He was desperate. He came to me with his family and asked: 'Doctor, can you make me one color?' I told him we can only make him one color—white—that is all we are able to do.
"Sometimes you cannot repigment the patient, and you have to know how to judiciously offer depigmentation. Some might say I robbed him of his culture. I say, no, I didn't, I robbed him of a disfiguring condition," said Dr. Cohen.
More research is clearly warranted to improve treatment options for vitiligo, such as studies to assess the biology of melanocytes, he said.
Keloids
Keloids are another challenge in skin of color patients. The therapeutic approach depends in part on the extent of the patient's condition. For example, a single keloid on the earlobe would be treated differently than more widespread presentation.
Again, more research is warranted on optimal treatments, Dr. Cohen said, because studies in the literature are contradictory and provide no consensus.
Acne keloidalis nuchae, "the keloids' cousin," most often occur in black men, he said. These nuchae can advance to plaque and form tumors, "and at the very least will need intralesional therapy." One clinical tip is to make an incision only within the keloid, he said.
If a patient presents with an acne keloidalis tumor, simply excise it. Once you get hemostasis, dress the wound with petroleum jelly only, and let it close by the magic of second intention
"I monitor these people. If I see any evidence of regrowth, I treat with triamcinolone early and often," he said.
Hair and Scalp Challenges
Hair and scalp concerns are common in patients with skin of color, Dr. Cohen said. A scalp biopsy is recommended to determine or confirm a diagnosis and to guide the course of clinical treatment.
These presentations can have a great psychosocial impact. "Do not underestimate the effect on patients," he said. Know your limitations and the limitations of therapy, and make sure you communicate those effectively to the patient. Otherwise, both the patient and provider can become frustrated.
Be honest with genetically-susceptible patients who present with scarring on the scalp from physical insult. In this population, scarring results when fibrous tissue replaces hair follicles. "Let them know up front that creams and other nonsense are not going to work," he said.
Contrary to popular belief, Dr. Cohen said, hair transplants are an option in patients with skin of color. "Hair transplants in black patients are not scary— I've been doing them for years and never had keloids," he said.
Black hair is heterogenous, so not everyone has curved follicles. If a skin of color patient has straight follicles, standard hair transplant procedures—for example, with 4-mm donor grafts—would be an option.
Dr. Cohen said that he did not have any relevant financial disclosures.
Are We Giving Nails Away? [editorial]
Two Lasers May Be Better Than One for Hair Removal
Recent advances in laser hair removal include using combination wavelengths, longer pulses, and larger spot sizes for all skin types, and using longer wavelengths for darker skin, according to Dr. E. Victor Ross.
Better pain control and cooling techniques also can make device-based hair removal a more comfortable option for patients, Dr. Ross said at a cosmetic dermatology seminar sponsored by Skin Disease Education Foundation in Santa Monica, Calif.
When using lasers for hair removal, cooling the skin before and after treatment can reduce pain and swelling, and cooling the skin during laser exposure "tends to minimize the dermal epidermal temperature," said Dr. Ross, director of the Scripps Clinic Laser and Cosmetic Dermatology Center in Carmel Valley, Calif.
Historically, fair-skinned patients have been treated with a 755-nm alexandrite laser for hair removal. For dark or tanned skin, or coarser hair, a 1064-nm Nd:YAG might be more effective, but it can be more painful for patients, Dr. Ross noted.
In his experience, a blended treatment including both the 755-nm and 1064-nm lasers can be more effective for removing fine hair on the legs than either laser alone, he said, adding that some patients still prefer the 755-nm alexandrite laser because the combination therapy is more painful than the 755 nm, although it is less painful than the 1064 nm alone.
New hair removal technologies include ultrasound and microwave radiation, as well as lower-fluence intense pulsed light and diode options with suction.
Approaches using high repetition with low fluence have been applied in some settings. The advantage is less pain, but more research is needed to determine whether lower fluences at high rates of repetition are effective, and what types of treatments are effective for white hair, said Dr. Ross.
Suction devices are an option to assist with permanent hair reduction over larger areas. A larger spot size allows more photons to remain in the target area, while vacuum-assisted suction concentrates more cumulative energy at any given depth and allows for effective treatment at a lower fluence.
Dr. Ross also addressed laser-diode hair removal devices being marketed for home use. The key issues to consider when evaluating at-home devices are safety for all skin types; safety with open or closed eyes; effectiveness in removing fine, gray, or white hair; and, of course, cost.
The TRIA hair removal device from TRIA Beauty Inc. is approved by the Food and Drug Administration for home use. The device packs an 800-nm wavelength and fluences of 7, 12, or 20 J/cm2, and efficacy data on this product are promising, Dr. Ross said.
Dr. Ross disclosed that he is a researcher for and receives funding from multiple laser companies, including Candela, Cutera, Lumenis, Sciton, and Syneron. SDEF and this news organization are both owned by Elsevier.
Recent advances in laser hair removal include using combination wavelengths, longer pulses, and larger spot sizes for all skin types, and using longer wavelengths for darker skin, according to Dr. E. Victor Ross.
Better pain control and cooling techniques also can make device-based hair removal a more comfortable option for patients, Dr. Ross said at a cosmetic dermatology seminar sponsored by Skin Disease Education Foundation in Santa Monica, Calif.
When using lasers for hair removal, cooling the skin before and after treatment can reduce pain and swelling, and cooling the skin during laser exposure "tends to minimize the dermal epidermal temperature," said Dr. Ross, director of the Scripps Clinic Laser and Cosmetic Dermatology Center in Carmel Valley, Calif.
Historically, fair-skinned patients have been treated with a 755-nm alexandrite laser for hair removal. For dark or tanned skin, or coarser hair, a 1064-nm Nd:YAG might be more effective, but it can be more painful for patients, Dr. Ross noted.
In his experience, a blended treatment including both the 755-nm and 1064-nm lasers can be more effective for removing fine hair on the legs than either laser alone, he said, adding that some patients still prefer the 755-nm alexandrite laser because the combination therapy is more painful than the 755 nm, although it is less painful than the 1064 nm alone.
New hair removal technologies include ultrasound and microwave radiation, as well as lower-fluence intense pulsed light and diode options with suction.
Approaches using high repetition with low fluence have been applied in some settings. The advantage is less pain, but more research is needed to determine whether lower fluences at high rates of repetition are effective, and what types of treatments are effective for white hair, said Dr. Ross.
Suction devices are an option to assist with permanent hair reduction over larger areas. A larger spot size allows more photons to remain in the target area, while vacuum-assisted suction concentrates more cumulative energy at any given depth and allows for effective treatment at a lower fluence.
Dr. Ross also addressed laser-diode hair removal devices being marketed for home use. The key issues to consider when evaluating at-home devices are safety for all skin types; safety with open or closed eyes; effectiveness in removing fine, gray, or white hair; and, of course, cost.
The TRIA hair removal device from TRIA Beauty Inc. is approved by the Food and Drug Administration for home use. The device packs an 800-nm wavelength and fluences of 7, 12, or 20 J/cm2, and efficacy data on this product are promising, Dr. Ross said.
Dr. Ross disclosed that he is a researcher for and receives funding from multiple laser companies, including Candela, Cutera, Lumenis, Sciton, and Syneron. SDEF and this news organization are both owned by Elsevier.
Recent advances in laser hair removal include using combination wavelengths, longer pulses, and larger spot sizes for all skin types, and using longer wavelengths for darker skin, according to Dr. E. Victor Ross.
Better pain control and cooling techniques also can make device-based hair removal a more comfortable option for patients, Dr. Ross said at a cosmetic dermatology seminar sponsored by Skin Disease Education Foundation in Santa Monica, Calif.
When using lasers for hair removal, cooling the skin before and after treatment can reduce pain and swelling, and cooling the skin during laser exposure "tends to minimize the dermal epidermal temperature," said Dr. Ross, director of the Scripps Clinic Laser and Cosmetic Dermatology Center in Carmel Valley, Calif.
Historically, fair-skinned patients have been treated with a 755-nm alexandrite laser for hair removal. For dark or tanned skin, or coarser hair, a 1064-nm Nd:YAG might be more effective, but it can be more painful for patients, Dr. Ross noted.
In his experience, a blended treatment including both the 755-nm and 1064-nm lasers can be more effective for removing fine hair on the legs than either laser alone, he said, adding that some patients still prefer the 755-nm alexandrite laser because the combination therapy is more painful than the 755 nm, although it is less painful than the 1064 nm alone.
New hair removal technologies include ultrasound and microwave radiation, as well as lower-fluence intense pulsed light and diode options with suction.
Approaches using high repetition with low fluence have been applied in some settings. The advantage is less pain, but more research is needed to determine whether lower fluences at high rates of repetition are effective, and what types of treatments are effective for white hair, said Dr. Ross.
Suction devices are an option to assist with permanent hair reduction over larger areas. A larger spot size allows more photons to remain in the target area, while vacuum-assisted suction concentrates more cumulative energy at any given depth and allows for effective treatment at a lower fluence.
Dr. Ross also addressed laser-diode hair removal devices being marketed for home use. The key issues to consider when evaluating at-home devices are safety for all skin types; safety with open or closed eyes; effectiveness in removing fine, gray, or white hair; and, of course, cost.
The TRIA hair removal device from TRIA Beauty Inc. is approved by the Food and Drug Administration for home use. The device packs an 800-nm wavelength and fluences of 7, 12, or 20 J/cm2, and efficacy data on this product are promising, Dr. Ross said.
Dr. Ross disclosed that he is a researcher for and receives funding from multiple laser companies, including Candela, Cutera, Lumenis, Sciton, and Syneron. SDEF and this news organization are both owned by Elsevier.
Hairpin-Induced Alopecia: Case Reports and a Review of the Literature
Transverse Melanonychia After Radiation Therapy [letter]
Cicatricial Alopecia
A great victory for our patients suffering from cicatricial alopecia—Dr. Pratima Karnik, assistant professor of dermatology at Cleveland’s Case Western Reserve University, received a $1.77 National Institutes of Health grant to fund a 5 year study on hair follicle, stem cell specific, PPAR-gamma deficiency in scarring alopecia.
Her research, published in the Journal of Investigative Dermatology, linked a defect in lipid processing and peroxisome biogenesis to cicatricial alopecia. As a result, it paved the way for a breakthrough finding in understanding the pathophysiology of the permanent hair loss disorder (J Invest Dermatol. 2009 May;129(5):1066-70).
Dr. Karnik and her colleagues found that unprocessed lipids are responsible for developing scarring hair loss. Their research suggests that processed lipids are necessary for hair growth and unprocessed lipids are toxic to hair. The bench-side research has led to clinical findings that treating patients with drugs that enhance lipid processing may relieve the clinical symptoms of the disorder.
Central centrifugal cicatricial alopecia, a scarring hair loss prevalent in African Americans, has no well-defined cause and has been difficult to and frustrating for patients.
Dr. Karnik’s research, and the work of the Cicatricial Alopecia Research Foundation (www.carfintl.org), is helping patients and physicians understand the biology, natural history, and treatment options for patients.
I personally attended a session with NIH Director Dr. Francis S. Collins, on behalf of CARF, where underrepresented and underfunded organizations had a chance to voice their opinions to the NIH and gain the well deserved attention they need.
Dr. Collins suggested that a new structure of communication was being established at the NIH, noting that any organization can send a brief summary of issues it would like to bring to the attention of the NIH. The e-mail address is [email protected]. He ensured us he would look at every e-mail and respond to each one.
Often, rare diseases are difficult to study given the lack of attention and funding. The work of Dr. Karnik and her collaborative team, and organizations like CARF, give hope to the thousands of people suffering from cicatricial alopecia.
A great victory for our patients suffering from cicatricial alopecia—Dr. Pratima Karnik, assistant professor of dermatology at Cleveland’s Case Western Reserve University, received a $1.77 National Institutes of Health grant to fund a 5 year study on hair follicle, stem cell specific, PPAR-gamma deficiency in scarring alopecia.
Her research, published in the Journal of Investigative Dermatology, linked a defect in lipid processing and peroxisome biogenesis to cicatricial alopecia. As a result, it paved the way for a breakthrough finding in understanding the pathophysiology of the permanent hair loss disorder (J Invest Dermatol. 2009 May;129(5):1066-70).
Dr. Karnik and her colleagues found that unprocessed lipids are responsible for developing scarring hair loss. Their research suggests that processed lipids are necessary for hair growth and unprocessed lipids are toxic to hair. The bench-side research has led to clinical findings that treating patients with drugs that enhance lipid processing may relieve the clinical symptoms of the disorder.
Central centrifugal cicatricial alopecia, a scarring hair loss prevalent in African Americans, has no well-defined cause and has been difficult to and frustrating for patients.
Dr. Karnik’s research, and the work of the Cicatricial Alopecia Research Foundation (www.carfintl.org), is helping patients and physicians understand the biology, natural history, and treatment options for patients.
I personally attended a session with NIH Director Dr. Francis S. Collins, on behalf of CARF, where underrepresented and underfunded organizations had a chance to voice their opinions to the NIH and gain the well deserved attention they need.
Dr. Collins suggested that a new structure of communication was being established at the NIH, noting that any organization can send a brief summary of issues it would like to bring to the attention of the NIH. The e-mail address is [email protected]. He ensured us he would look at every e-mail and respond to each one.
Often, rare diseases are difficult to study given the lack of attention and funding. The work of Dr. Karnik and her collaborative team, and organizations like CARF, give hope to the thousands of people suffering from cicatricial alopecia.
A great victory for our patients suffering from cicatricial alopecia—Dr. Pratima Karnik, assistant professor of dermatology at Cleveland’s Case Western Reserve University, received a $1.77 National Institutes of Health grant to fund a 5 year study on hair follicle, stem cell specific, PPAR-gamma deficiency in scarring alopecia.
Her research, published in the Journal of Investigative Dermatology, linked a defect in lipid processing and peroxisome biogenesis to cicatricial alopecia. As a result, it paved the way for a breakthrough finding in understanding the pathophysiology of the permanent hair loss disorder (J Invest Dermatol. 2009 May;129(5):1066-70).
Dr. Karnik and her colleagues found that unprocessed lipids are responsible for developing scarring hair loss. Their research suggests that processed lipids are necessary for hair growth and unprocessed lipids are toxic to hair. The bench-side research has led to clinical findings that treating patients with drugs that enhance lipid processing may relieve the clinical symptoms of the disorder.
Central centrifugal cicatricial alopecia, a scarring hair loss prevalent in African Americans, has no well-defined cause and has been difficult to and frustrating for patients.
Dr. Karnik’s research, and the work of the Cicatricial Alopecia Research Foundation (www.carfintl.org), is helping patients and physicians understand the biology, natural history, and treatment options for patients.
I personally attended a session with NIH Director Dr. Francis S. Collins, on behalf of CARF, where underrepresented and underfunded organizations had a chance to voice their opinions to the NIH and gain the well deserved attention they need.
Dr. Collins suggested that a new structure of communication was being established at the NIH, noting that any organization can send a brief summary of issues it would like to bring to the attention of the NIH. The e-mail address is [email protected]. He ensured us he would look at every e-mail and respond to each one.
Often, rare diseases are difficult to study given the lack of attention and funding. The work of Dr. Karnik and her collaborative team, and organizations like CARF, give hope to the thousands of people suffering from cicatricial alopecia.