User login
Erythematous Friable Papule Under the Great Toenail
The Diagnosis: Subungual Eccrine Poroma
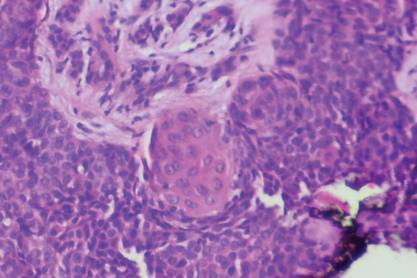
Histologic examination revealed a solitary papule (Figure 1). The epidermis was replaced with a well-defined proliferation of cuboidal and poroid cells. These cells demonstrated a downgrowth into the dermis in broad anastomosing bands that were surrounded by a fibrovascular stroma. Notably, there were few scattered foci of maturation into the ductal lumina of eccrine origin, which confirmed the diagnosis (Figure 2).
|
First described in 1956 by Goldman et al,1 eccrine poromas are benign, slow-growing tumors that account for approximately 10% of sweat gland neoplasms.2 Onset is typically in mid to late adulthood, and there is no ethnic or gender predilection. Classically, eccrine poromas present as soft, sessile, reddish papules or nodules measuring less than 2 cm that protrude from a well-circumscribed depression.
Although eccrine poromas can develop on hair-bearing regions, they most commonly arise on acral skin. In acral locations, bleeding, discharge, rapid growth, and localized pain can occur. These symptoms are even more common in this lesion’s malignant counterpart, eccrine porocarcinoma.3
Solar damage, radiation exposure, trauma, and human papillomavirus have been indicated in the pathogenesis of eccrine poroma; however, the exact etiology has yet to be defined.2,4 The differential diagnosis includes nevus, pyogenic granu-loma, acrochordon, basal cell carcinoma, and verruca vulgaris.5
Histologically, eccrine poromas consist of a combination of 5 distinct features: poroid cells, cuticular cells, intracytoplasmic or intercellular vacuolization en route to duct formation, massive necrosis or necrosis en masse, and nuclear monomorphism of the poroid and cuticular cells.6 However, all 5 histologic features do not have to be present for the diagnosis. Classically, there is a sharp demarcation of the lesion from the surrounding epidermis.7
Treatment of choice is complete excision to prevent recurrence and risk for malignant transformation in long-standing lesions. One study of eccrine porocarcinomas found that 18% (11/62) arose from a benign preexistent poroma.8 These malignant lesions are found more commonly on the extremities and tend to show a slight female predominance.9
Although there have been 2 reported cases of subungual eccrine porocarcinomas9,10 and 1 case of periungual eccrine porocarcinoma,11 according to an Ovid search using the terms porocarcinoma and nail, the benign subungual eccrine poroma is more rare.
1. Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956;74:511-521.
2. Orlandi C, Arcangeli F, Patrizi A, et al. Eccrine poroma in a child. Pediatr Dermatol. 2005;22:279-280.
3. Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011;88:227-229.
4. Kang MC, Kim SA, Lee KS, et al. A case of an unusual eccrine poroma on the left forearm area. Ann Dermatol. 2011;23:250-253.
5. Moore TO, Orman HL, Orman SK, et al. Poromas of the head and neck. J Am Acad Dermatol. 2001;44:48-52.
6. Chen CC, Chang YT, Liu HN. Clinical and histological characteristics of poroid neoplasms: a study of 25 cases in Taiwan. Int J Dermatol. 2006;45:722-727.
7. Smith EV, Madan V, Joshi A, et al. A pigmented lesion on the foot. Clin Exp Dermatol. 2012;37:84-86.
8. Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
9. Moussallem CD, Abi Hatem NE, El-Khoury ZN.Malignant porocarcinoma of the nail fold: a tricky diagnosis. Dermatol Online J. 2008;14:10.
10. Requena L, Sánchez M, Aguilar A, et al. Periungual porocarcinoma. Dermatologica. 1990;180:177-180.
11. van Gorp J, van der Putte SC. Periungual eccrine porocarcinoma. Dermatology. 1993;187:67-70.
The Diagnosis: Subungual Eccrine Poroma
Histologic examination revealed a solitary papule (Figure 1). The epidermis was replaced with a well-defined proliferation of cuboidal and poroid cells. These cells demonstrated a downgrowth into the dermis in broad anastomosing bands that were surrounded by a fibrovascular stroma. Notably, there were few scattered foci of maturation into the ductal lumina of eccrine origin, which confirmed the diagnosis (Figure 2).
|
First described in 1956 by Goldman et al,1 eccrine poromas are benign, slow-growing tumors that account for approximately 10% of sweat gland neoplasms.2 Onset is typically in mid to late adulthood, and there is no ethnic or gender predilection. Classically, eccrine poromas present as soft, sessile, reddish papules or nodules measuring less than 2 cm that protrude from a well-circumscribed depression.
Although eccrine poromas can develop on hair-bearing regions, they most commonly arise on acral skin. In acral locations, bleeding, discharge, rapid growth, and localized pain can occur. These symptoms are even more common in this lesion’s malignant counterpart, eccrine porocarcinoma.3
Solar damage, radiation exposure, trauma, and human papillomavirus have been indicated in the pathogenesis of eccrine poroma; however, the exact etiology has yet to be defined.2,4 The differential diagnosis includes nevus, pyogenic granu-loma, acrochordon, basal cell carcinoma, and verruca vulgaris.5
Histologically, eccrine poromas consist of a combination of 5 distinct features: poroid cells, cuticular cells, intracytoplasmic or intercellular vacuolization en route to duct formation, massive necrosis or necrosis en masse, and nuclear monomorphism of the poroid and cuticular cells.6 However, all 5 histologic features do not have to be present for the diagnosis. Classically, there is a sharp demarcation of the lesion from the surrounding epidermis.7
Treatment of choice is complete excision to prevent recurrence and risk for malignant transformation in long-standing lesions. One study of eccrine porocarcinomas found that 18% (11/62) arose from a benign preexistent poroma.8 These malignant lesions are found more commonly on the extremities and tend to show a slight female predominance.9
Although there have been 2 reported cases of subungual eccrine porocarcinomas9,10 and 1 case of periungual eccrine porocarcinoma,11 according to an Ovid search using the terms porocarcinoma and nail, the benign subungual eccrine poroma is more rare.
The Diagnosis: Subungual Eccrine Poroma
Histologic examination revealed a solitary papule (Figure 1). The epidermis was replaced with a well-defined proliferation of cuboidal and poroid cells. These cells demonstrated a downgrowth into the dermis in broad anastomosing bands that were surrounded by a fibrovascular stroma. Notably, there were few scattered foci of maturation into the ductal lumina of eccrine origin, which confirmed the diagnosis (Figure 2).
|
First described in 1956 by Goldman et al,1 eccrine poromas are benign, slow-growing tumors that account for approximately 10% of sweat gland neoplasms.2 Onset is typically in mid to late adulthood, and there is no ethnic or gender predilection. Classically, eccrine poromas present as soft, sessile, reddish papules or nodules measuring less than 2 cm that protrude from a well-circumscribed depression.
Although eccrine poromas can develop on hair-bearing regions, they most commonly arise on acral skin. In acral locations, bleeding, discharge, rapid growth, and localized pain can occur. These symptoms are even more common in this lesion’s malignant counterpart, eccrine porocarcinoma.3
Solar damage, radiation exposure, trauma, and human papillomavirus have been indicated in the pathogenesis of eccrine poroma; however, the exact etiology has yet to be defined.2,4 The differential diagnosis includes nevus, pyogenic granu-loma, acrochordon, basal cell carcinoma, and verruca vulgaris.5
Histologically, eccrine poromas consist of a combination of 5 distinct features: poroid cells, cuticular cells, intracytoplasmic or intercellular vacuolization en route to duct formation, massive necrosis or necrosis en masse, and nuclear monomorphism of the poroid and cuticular cells.6 However, all 5 histologic features do not have to be present for the diagnosis. Classically, there is a sharp demarcation of the lesion from the surrounding epidermis.7
Treatment of choice is complete excision to prevent recurrence and risk for malignant transformation in long-standing lesions. One study of eccrine porocarcinomas found that 18% (11/62) arose from a benign preexistent poroma.8 These malignant lesions are found more commonly on the extremities and tend to show a slight female predominance.9
Although there have been 2 reported cases of subungual eccrine porocarcinomas9,10 and 1 case of periungual eccrine porocarcinoma,11 according to an Ovid search using the terms porocarcinoma and nail, the benign subungual eccrine poroma is more rare.
1. Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956;74:511-521.
2. Orlandi C, Arcangeli F, Patrizi A, et al. Eccrine poroma in a child. Pediatr Dermatol. 2005;22:279-280.
3. Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011;88:227-229.
4. Kang MC, Kim SA, Lee KS, et al. A case of an unusual eccrine poroma on the left forearm area. Ann Dermatol. 2011;23:250-253.
5. Moore TO, Orman HL, Orman SK, et al. Poromas of the head and neck. J Am Acad Dermatol. 2001;44:48-52.
6. Chen CC, Chang YT, Liu HN. Clinical and histological characteristics of poroid neoplasms: a study of 25 cases in Taiwan. Int J Dermatol. 2006;45:722-727.
7. Smith EV, Madan V, Joshi A, et al. A pigmented lesion on the foot. Clin Exp Dermatol. 2012;37:84-86.
8. Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
9. Moussallem CD, Abi Hatem NE, El-Khoury ZN.Malignant porocarcinoma of the nail fold: a tricky diagnosis. Dermatol Online J. 2008;14:10.
10. Requena L, Sánchez M, Aguilar A, et al. Periungual porocarcinoma. Dermatologica. 1990;180:177-180.
11. van Gorp J, van der Putte SC. Periungual eccrine porocarcinoma. Dermatology. 1993;187:67-70.
1. Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956;74:511-521.
2. Orlandi C, Arcangeli F, Patrizi A, et al. Eccrine poroma in a child. Pediatr Dermatol. 2005;22:279-280.
3. Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011;88:227-229.
4. Kang MC, Kim SA, Lee KS, et al. A case of an unusual eccrine poroma on the left forearm area. Ann Dermatol. 2011;23:250-253.
5. Moore TO, Orman HL, Orman SK, et al. Poromas of the head and neck. J Am Acad Dermatol. 2001;44:48-52.
6. Chen CC, Chang YT, Liu HN. Clinical and histological characteristics of poroid neoplasms: a study of 25 cases in Taiwan. Int J Dermatol. 2006;45:722-727.
7. Smith EV, Madan V, Joshi A, et al. A pigmented lesion on the foot. Clin Exp Dermatol. 2012;37:84-86.
8. Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720.
9. Moussallem CD, Abi Hatem NE, El-Khoury ZN.Malignant porocarcinoma of the nail fold: a tricky diagnosis. Dermatol Online J. 2008;14:10.
10. Requena L, Sánchez M, Aguilar A, et al. Periungual porocarcinoma. Dermatologica. 1990;180:177-180.
11. van Gorp J, van der Putte SC. Periungual eccrine porocarcinoma. Dermatology. 1993;187:67-70.
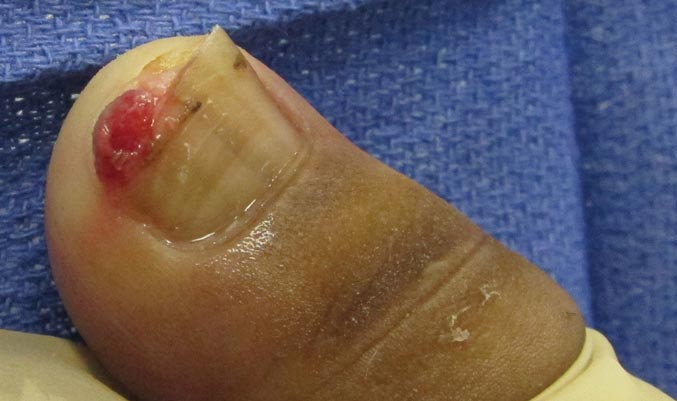
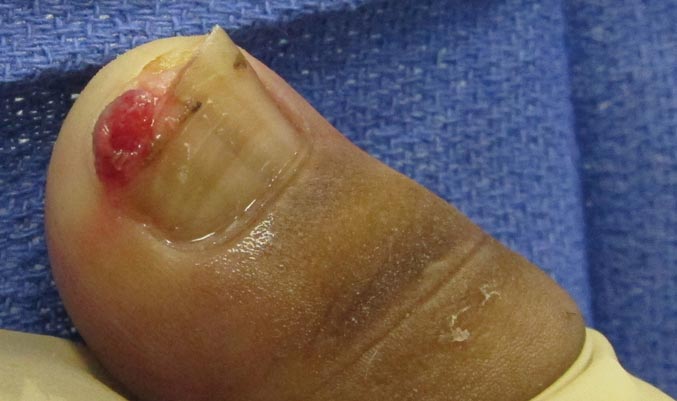
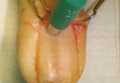
A 20-year-old woman presented with a subungual growth of 1 year’s duration that would intermittently bleed. Despite treatment with silver nitrate in 2 sequential treatments, the lesion continued to increase in size. Physical examination revealed a 6×7-mm erythematous, friable, well-defined papule under the medial aspect of the distal great toenail. Complete surgical excision of the lesion was performed.
Dreadlocks
The Diagnosis: “Pseudonits”
Dreadlocks are matted hairs formed into thick ropelike strands (Figure 1). As a chosen hairstyle dreadlocks are worn by individuals of many different ethnic groups but are most commonly associated with members of the Rastafarian movement, or Rastas. Various techniques are used to form dreadlocks including backcombing (also known as teasing) in which the hair is combed toward the scalp to facilitate tangles and knotting or the neglect method in which the hair is not combed, brushed, or cut, becoming tangled and twisted as it grows long. Manicuring and perming techniques may be used to create the starting point for dreadlocks.
Telogen hairs are the hairs shed as part of normal hair cycling. The average person is estimated to lose 50 telogen hairs per day.1 With dreadlocks, the hairs are entangled distally, so when telogen hairs are released from scalp follicles, the shed hairs remain part of the locks. These “club” hairs have a bulbous white tip situated at the proximal end of the hair shaft (Figure 2) and should not be mistaken for the eggs of Pediculus humanus var capitis, hence the designation pseudonits.2 Hair casts, keratinous material surrounding the hair shafts when there is infundibular or perifollicular hyperkeratosis, also may resemble nits.3 Hair cast pseudonits can be distinguished from true nits by one’s ability to slide the hair casts freely along the hair shaft, whereas lice ova are cemented to the hair shaft and fixed in place.
Figure 2. “Club” hairs with a bulbous white tip situated at the
proximal end of the hair shaft (“pseudonits”).
1. Sperling LC. An Atlas of Hair Pathology with Clinical Correlations. New York, NY: The Parthenon Publishing Group; 2003.
2. Salih S, Bowling JC. Pseudonits in dreadlocked hair: A louse-y case of nits. Dermatology. 2006;213:245.
3. Lam M, Crutchfield CE 3rd, Lewis EJ. Hair casts: a case of pseudonits. Cutis. 1997;60:251-252.
The Diagnosis: “Pseudonits”
Dreadlocks are matted hairs formed into thick ropelike strands (Figure 1). As a chosen hairstyle dreadlocks are worn by individuals of many different ethnic groups but are most commonly associated with members of the Rastafarian movement, or Rastas. Various techniques are used to form dreadlocks including backcombing (also known as teasing) in which the hair is combed toward the scalp to facilitate tangles and knotting or the neglect method in which the hair is not combed, brushed, or cut, becoming tangled and twisted as it grows long. Manicuring and perming techniques may be used to create the starting point for dreadlocks.
Telogen hairs are the hairs shed as part of normal hair cycling. The average person is estimated to lose 50 telogen hairs per day.1 With dreadlocks, the hairs are entangled distally, so when telogen hairs are released from scalp follicles, the shed hairs remain part of the locks. These “club” hairs have a bulbous white tip situated at the proximal end of the hair shaft (Figure 2) and should not be mistaken for the eggs of Pediculus humanus var capitis, hence the designation pseudonits.2 Hair casts, keratinous material surrounding the hair shafts when there is infundibular or perifollicular hyperkeratosis, also may resemble nits.3 Hair cast pseudonits can be distinguished from true nits by one’s ability to slide the hair casts freely along the hair shaft, whereas lice ova are cemented to the hair shaft and fixed in place.
Figure 2. “Club” hairs with a bulbous white tip situated at the
proximal end of the hair shaft (“pseudonits”).
The Diagnosis: “Pseudonits”
Dreadlocks are matted hairs formed into thick ropelike strands (Figure 1). As a chosen hairstyle dreadlocks are worn by individuals of many different ethnic groups but are most commonly associated with members of the Rastafarian movement, or Rastas. Various techniques are used to form dreadlocks including backcombing (also known as teasing) in which the hair is combed toward the scalp to facilitate tangles and knotting or the neglect method in which the hair is not combed, brushed, or cut, becoming tangled and twisted as it grows long. Manicuring and perming techniques may be used to create the starting point for dreadlocks.
Telogen hairs are the hairs shed as part of normal hair cycling. The average person is estimated to lose 50 telogen hairs per day.1 With dreadlocks, the hairs are entangled distally, so when telogen hairs are released from scalp follicles, the shed hairs remain part of the locks. These “club” hairs have a bulbous white tip situated at the proximal end of the hair shaft (Figure 2) and should not be mistaken for the eggs of Pediculus humanus var capitis, hence the designation pseudonits.2 Hair casts, keratinous material surrounding the hair shafts when there is infundibular or perifollicular hyperkeratosis, also may resemble nits.3 Hair cast pseudonits can be distinguished from true nits by one’s ability to slide the hair casts freely along the hair shaft, whereas lice ova are cemented to the hair shaft and fixed in place.
Figure 2. “Club” hairs with a bulbous white tip situated at the
proximal end of the hair shaft (“pseudonits”).
1. Sperling LC. An Atlas of Hair Pathology with Clinical Correlations. New York, NY: The Parthenon Publishing Group; 2003.
2. Salih S, Bowling JC. Pseudonits in dreadlocked hair: A louse-y case of nits. Dermatology. 2006;213:245.
3. Lam M, Crutchfield CE 3rd, Lewis EJ. Hair casts: a case of pseudonits. Cutis. 1997;60:251-252.
1. Sperling LC. An Atlas of Hair Pathology with Clinical Correlations. New York, NY: The Parthenon Publishing Group; 2003.
2. Salih S, Bowling JC. Pseudonits in dreadlocked hair: A louse-y case of nits. Dermatology. 2006;213:245.
3. Lam M, Crutchfield CE 3rd, Lewis EJ. Hair casts: a case of pseudonits. Cutis. 1997;60:251-252.

A 17-year-old adolescent girl presented to our dermatology office with dreadlocks that were unrelated to the reason for her visit. She had mild scalp pruritus. Close inspection of the hair and scalp was performed.
Onychomycosis Treatment in the United States
Onychomycosis is a common progressive infection of the nails caused by dermatophytes, nondermatophyte molds, and yeasts, with Trichophyton rubrum being the most common causative organism.1-3 Onychomycosis affects approximately 2% to 26% of different populations worldwide. It represents 20% to 50% of onychopathies and approximately 30% of fungal cutaneous infections.4-9 Less than 30% of infected persons seek medical advice or treatment even in developed areas of the world.10 Onychomycosis may be a source of more widespread fungal skin infections or give rise to complications such as cellulitis. Chronic, long-lasting infection may result in nail dystrophy and can lead to pain, absence from work, and decreased quality of life.1,11 Because the dermatophyte can contaminate communal bathing facilities and spread to others,12 it is important to effectively target and treat patients with onychomycosis, thus reducing the rate of related morbidities.1,9
The primary aim of onychomycosis treatment is to cure the infection and prevent relapse. Both topical and oral agents are available for the treatment of fungal nail infections. Generally, systemic therapy for onychomycosis is more successful than topical treatment, likely due to poor penetration of topical medications into the nail plate.1,2,9 However, newer topical drugs have shown promising results in treating some types of onychomycosis.13 In its guidelines for treatment of onychomycosis, the British Association of Dermatologists recommends use of topical treatment under the following conditions: (1) when there is not extensive involvement of the nail plate (eg, candidal paronychia, superficial white onychomycosis, early stages of distal and lateral subungual onychomycosis), (2) when systemic therapy is contraindicated, or (3) in combination with systemic therapy.1 Although there are multiple treatments for fungal nail infections, there are limited reports on the ways in which physicians actually use these treatments or the frequency with which they prescribe them.
This study provides a representative portrayal of onychomycosis visits in the US outpatient setting using a large nationally sampled survey. In particular, we aimed to assess the number of visits related to onychomycosis, the demographics of patients, and the treatments being prescribed for onychomycosis.
Methods
Study Design
Data from January 1, 1993, to December 31, 2010, were collected from the National Ambulatory Medical Care Survey (NAMCS), an ongoing survey of nonfederal employed US office-based physicians who are primarily engaged in direct patient care. The NAMCS has been conducted by the National Center for Health Statistics every year since 1989 to estimate the utilization of ambulatory care services in the United States. Since 1989 including 1993 to 2010, the NAMCS sampled approximately 30,000 visits per year. For each visit sampled, a 1-page patient log including demographic data, physicians’ diagnoses, services provided, and medications was completed. In the NAMCS survey, visits were divided into 2 groups: (1) visits from established patients that have been seen in that office before for any reason, and (2) visits for new (ie, first-time) patients. The current study included all visits in which fungal nail infection (code 110.1 according to the International Classification of Diseases, Ninth Revision [ICD-9]) was listed as 1 of 3 possible diagnoses.
Statistical Analysis
Sampling weights were applied to data to produce estimates for the total US outpatient setting.14 Data were analyzed using SAS version 9.2, and SAS survey analysis procedures were used to account for the clustered sampling of the survey. The total numbers of visits for which onychomycosis was 1 of 3 possible diagnoses and for which it was the sole diagnosis were reported. Visit rates per population by demographic characteristics (ie, patient sex, age, race, and ethnicity) were calculated. Population estimates were based on the 2001 NAMCS Public Micro-Data File Documentation records of the US census estimates for noninstitutionalized civilian persons.15 Trends in proportion of visits linked with an onychomycosis diagnosis over time were evaluated using the SAS SURVEYREG procedure. Types of physicians who attended to these visits as well as leading comorbidities that had been diagnosed and documented in the medical record were characterized. Onychomycosis-related medications prescribed at these visits were reported and prescribing trends over time were evaluated. Differences in the treatment prescribed according to the type of visit (ie, first-time or return visit); physician specialty; and patients’ gender, race, and health conditions (eg, obesity, diabetes mellitus) were examined. To exclude the possibility that fluconazole and other broad-spectrum antifungals were being used for secondary diagnoses, we determined the number of visits that had an additional diagnosis of either candidiasis (ICD-9 codes 112.0–112.9) or “other specified erythematous conditions” (ICD-9 code 695.89).
Results
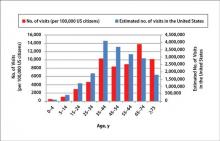
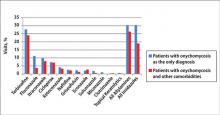
During the 18-year study period, 636 visits with a diagnosis of onychomycosis were recorded in the NAMCS database. This unweighted number of visits corresponded with approximately 19,350,000 visits (an average of 1,075,000 visits per year) to physicians’ offices with a diagnosis of onychomycosis in the United States during this period. Among these visits, there were an estimated 4,250,000 visits with fungal nail infection as the only diagnosis (no other comorbidities recorded). The recorded visits included more female (57.6%) than male (42.4%) patients, and 85% of patients were white (Table). Patients aged 35 to 44 years accounted for the largest number of visits; however, the estimated rate of onychomycosis visits per 100,000 US citizens was highest among those aged 65 to 74 years (Figure 1).
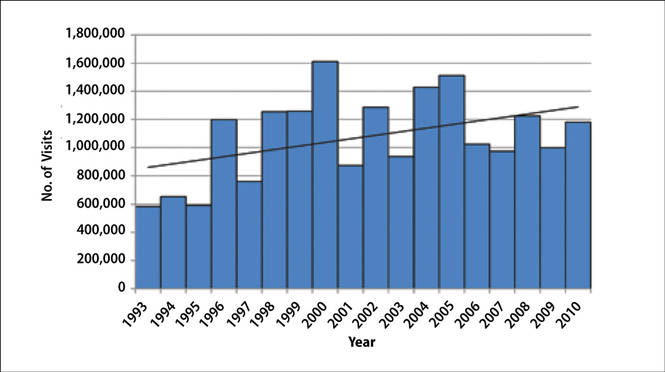
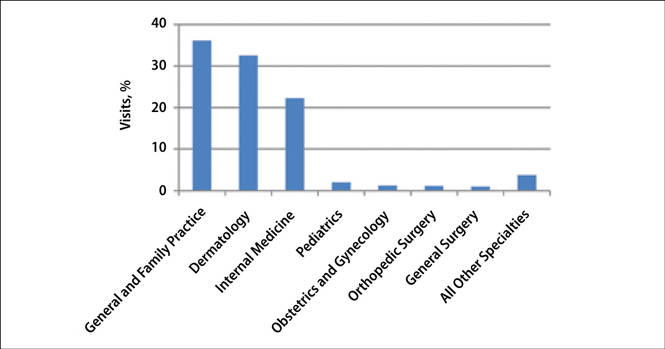
The number of US outpatient visits with a recorded diagnosis of onychomycosis increased from 1993 to 2010 (Figure 2); however, there was no change in the ratio of onychomycosis visits to the total number of recorded visits in NAMCS database during the study period (P=.9). A combined total of 91% of onychomycosis visits were to general and family practitioners, dermatologists, or internal medicine practitioners (Figure 3). Although cardiovascular diseases and diabetes mellitus accounted for a large proportion of comorbidities, conditions affecting the feet (eg, tinea pedis, ingrown nails) also were among the most common comorbidities (Figure 4).
|
In both topical and systemic form, terbinafine was the most commonly prescribed antifungal agent, followed by systemic fluconazole, systemic itraconazole, and topical ciclopirox (Figure 5). Over the 18-year study period, there was an increasing trend in the frequency of terbinafine prescription (regression coefficient [r]=0.01319; P=.004); a decreasing trend for fluconazole (r=-0.0053851; P=.04), itraconazole (r=-0.0113988; P<.001), griseofulvin (r=-0.0073942; P<.001), and econazole prescription (r=-0.0032405; P=.01); and no significant trend for ketoconazole (r=-0.0034553; P=.1), naftifine (r=-0.0029067; P=.06), sulconazole (r=-0.0001619; P=.8), ciclopirox (r=0.0032684; P=.1), and miconazole prescription (r=0.0002074; P=.5).
Eighty-six percent of visits were for established patients who had been seen in the related office with any diagnosis before the recorded visit and 14% of visits were for new (first-time) patients. Fluconazole was the most frequently used antifungal drug for new patients, while terbinafine was the most frequently used in other visits. Terbinafine was the most frequently prescribed antifungal drug by general and family practitioners, dermatologists, internal medicine practitioners, and all other specialties not listed.
Terbinafine was the most frequently prescribed antifungal drug in both genders and in white and black patients. Itraconazole was the most frequently prescribed antifungal drug for Hispanic patients and those of other ethnicities not listed. Terbinafine was the most frequently prescribed antifungal drug for patients with diabetes and obesity (ie, body mass index ≥30). In 19,330,000 of 19,350,000 total estimated visits included in this study, onychomycosis was the only diagnosis with a potential indication for an antifungal drug therapy, ruling out the possibility that fluconazole or other drugs were used for patients who also had candidiasis or “other specified erythematous conditions.”
Discussion
Onychomycosis is a common progressive infection of the nails that is more prevalent in older age groups, with equal prevalence in both genders and a higher prevalence in males. The NAMCS data showed higher rates of onychomycosis visits among older age groups, which is in agreement with results from prior studies.16,17 In the current study, we observed a higher prevalence of onychomycosis visits among females as well as white and Hispanic patients. These results may be due to a higher prevalence of onychomycosis in these populations or simply a result of difference in socioeconomic level or importance of aesthetics. Although there are limited data regarding the prevalence of onychomycosis among different races and ethnicities in the United States, a high incidence of onychomycosis has been reported in Mexico.18
Repeated trauma to the great toenail from ill-fitting shoes is a predisposing factor for onychomycosis.16 In the current study, ingrown nails were among the most common comorbidities found in onychomycosis patients. Although nail dystrophy caused by onychomycosis may lead to ingrown nails, it also is possible that both conditions may be caused by trauma.
Patients with immunodeficiencies (eg, diabetes) may be predisposed to onychomycosis as well as its associated complications and morbidities (eg, cellulitis).16,19 Diabetes affects 4% to 22% of patients with onychomycosis in different populations, including Denmark, Mexico, and India.18,20,21 In our study, diabetes was among the most common recorded comorbidities reported during onychomycosis visits, with a prevalence of 3.4%. It is likely that many more visits involved patients with diabetes that had not been diagnosed or reported. With the increased risk for complications with diabetes, it is important for physicians to treat these patients when they have a nail infection.
The available systemic therapies for treatment of onychomycosis include griseofulvin, allylamines, and imidazoles. Comparison of griseofulvin with newer systemic antifungal agents such as terbinafine and itraconazole suggests that griseofulvin has lower efficacy and therefore is not a first-line treatment of onychomycosis.1 Terbinafine is the most active of the currently available antidermatophyte drugs both in vitro and in vivo, with synergistic effects with imidazoles and ciclopirox.1,22-27 A combination of topical and systemic therapies may improve cure rates of onychomycosis or possibly shorten the duration of therapy with the systemic agent.1,2 Treatment strategies can vary according to the specialty of the treating physician, with general practitioners often preferring monotherapies and dermatologists preferring combination therapies.28 In Europe, the most commonly prescribed medication for onychomycosis was topical amorolfine followed by systemic terbinafine and itraconazole.28 In the current study, we could not separate data for topical versus systemic terbinafine because the NAMCS uses similar names for reporting the drug; however, the rates of prescription for allylamines and imidazoles were nearly equal (Figure 5), with terbinafine showing an increased use over time as opposed to a decreased use of imidazoles. Although fluconazole is not approved by the US Food and Drug Administration for treatment of onychomycosis, oral fluconazole was the second most common treatment prescribed in our study. Griseofulvin, which is not considered as a drug of choice in onychomycosis,1 was prescribed in a small fraction of the visits, with a decreasing trend of usage over time.
Conclusion
Our analysis of the NAMCS data revealed that the treatment of onychomycosis in the United States is in accordance with recommendations in current guidelines. An encouraging finding was the notable downward trend in use of griseofulvin, suggesting that health care providers are changing practice to meet standard of care. Increased efforts must be made to uniformly modify practices in compliance with evidence-based recommendations and to minimize unnecessary risk and cost associated with use of drugs with lower efficacy.
1. Roberts DT, Taylor WD, Boyle J; British Association of Dermatologists. Guidelines for treatment of onychomycosis. Br J Dermatol. 2003;148:402-410.
2. Seebacher C, Brasch J, Abeck D, et al. Onychomycosis. Mycoses. 2007;50:321-327.
3. Summerbell RC, Kane J, Krajden S. Onychomycosis, tinea pedis and tinea manuum caused by non-dermatophytic filamentous fungi. Mycoses. 1989;32:609-619.
4. Murray SC, Dawber RP. Onychomycosis of toenails: orthopaedic and podiatric considerations. Australas J Dermatol. 2002;43:105-112.
5. Achten G, Wanet-Rouard J. Onychomycoses in the laboratory. Mykosen Suppl. 1978;1:125-127.
6. Haneke E, Roseeuw D. The scope of onychomycosis: epidemiology and clinical features. Int J Dermatol. 1999;38(suppl 2):7-12.
7. Haneke E. Fungal infections of the nail. Semin Dermatol. 1991;10:41-53.
8. Karmakar S, Kalla G, Joshi KR, et al. Dermatophytoses in a desert district of Western Rajasthan. Indian J Dermatol Venereol Leprol. 1995;61:280-283.
9. Drake LA. Guidelines of care for superficial mycotic infections of the skin: onychomycosis. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:116-121.
10. Roberts DT. Prevalence of dermatophyte onychomycosis in the United Kingdom: results of an omnibus survey. Br J Dermatol. 1992;126(suppl 39):23-27.
11. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5 pt 1):702-704.
12. Detandt M, Nolard N. Fungal contamination of the floors of swimming pools, particularly subtropical swimming paradises. Mycoses. 1995;38:509-513.
13. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
14. Fleischer AB Jr, Feldman SR, Bradham DD. Office-based physician services provided by dermatologists in the United States in 1990. J Invest Dermatol. 1994;102:93-97.
15. 2001 NAMCS Micro-Data File Documentation. http://www.nber.org/namcs/docs/namcs2001.pdf. National Bureau of Economic Research Web site. Accessed April 27, 2015.
16. Williams HC. The epidemiology of onychomycosis in Britain. Br J Dermatol. 1993;129:101-109.
17. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
18. Arenas R, Bonifaz A, Padilla MC, et al. Onychomycosis. a Mexican survey. Eur J Dermatol. 2010;20:611-614.
19. Faergemann J, Baran R. Epidemiology, clinical presentation and diagnosis of onychomycosis. Br J Dermatol. 2003;149(suppl 65):1-4.
20. Sarma S, Capoor MR, Deb M, et al. Epidemiologic and clinicomycologic profile of onychomycosis from north India. Int J Dermatol. 2008;47:584-587.
21. Svejgaard EL, Nilsson J. Onychomycosis in Denmark: prevalence of fungal nail infection in general practice. Mycoses. 2004;47:131-135.
22. Santos DA, Hamdan JS. In vitro antifungal oral drug and drug-combination activity against onychomycosis causative dermatophytes. Med Mycol. 2006;44:357-362.
23. Gupta AK, Kohli Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br J Dermatol. 2003;149:296-305.
24. Gupta AK, Lynch LE. Management of onychomycosis: examining the role of monotherapy and dual, triple, or quadruple therapies. Cutis. 2004;74(suppl 1):5-9.
25. Harman S, Ashbee HR, Evans EG. Testing of antifungal combinations against yeasts and dermatophytes. J Dermatolog Treat. 2004;15:104-107.
26. Spader TB, Venturini TP, Rossato L, et al. Synergisms of voriconazole or itraconazole combined with other antifungal agents against Fusarium spp. Rev Iberoam Micol. 2013;30:200-204.
27. Biancalana FS, Lyra L, Moretti ML, et al. Susceptibility testing of terbinafine alone and in combination with amphotericin B, itraconazole, or voriconazole against conidia and hyphae of dematiaceous molds. Diagn Microbiol Infect Dis. 2011;71:378-385.
28. Effendy I, Lecha M, Feuilhade de CM, et al. Epidemiology and clinical classification of onychomycosis. J Eur Acad Dermatol Venereol. 2005;19(suppl 1):8-12.
Onychomycosis is a common progressive infection of the nails caused by dermatophytes, nondermatophyte molds, and yeasts, with Trichophyton rubrum being the most common causative organism.1-3 Onychomycosis affects approximately 2% to 26% of different populations worldwide. It represents 20% to 50% of onychopathies and approximately 30% of fungal cutaneous infections.4-9 Less than 30% of infected persons seek medical advice or treatment even in developed areas of the world.10 Onychomycosis may be a source of more widespread fungal skin infections or give rise to complications such as cellulitis. Chronic, long-lasting infection may result in nail dystrophy and can lead to pain, absence from work, and decreased quality of life.1,11 Because the dermatophyte can contaminate communal bathing facilities and spread to others,12 it is important to effectively target and treat patients with onychomycosis, thus reducing the rate of related morbidities.1,9
The primary aim of onychomycosis treatment is to cure the infection and prevent relapse. Both topical and oral agents are available for the treatment of fungal nail infections. Generally, systemic therapy for onychomycosis is more successful than topical treatment, likely due to poor penetration of topical medications into the nail plate.1,2,9 However, newer topical drugs have shown promising results in treating some types of onychomycosis.13 In its guidelines for treatment of onychomycosis, the British Association of Dermatologists recommends use of topical treatment under the following conditions: (1) when there is not extensive involvement of the nail plate (eg, candidal paronychia, superficial white onychomycosis, early stages of distal and lateral subungual onychomycosis), (2) when systemic therapy is contraindicated, or (3) in combination with systemic therapy.1 Although there are multiple treatments for fungal nail infections, there are limited reports on the ways in which physicians actually use these treatments or the frequency with which they prescribe them.
This study provides a representative portrayal of onychomycosis visits in the US outpatient setting using a large nationally sampled survey. In particular, we aimed to assess the number of visits related to onychomycosis, the demographics of patients, and the treatments being prescribed for onychomycosis.
Methods
Study Design
Data from January 1, 1993, to December 31, 2010, were collected from the National Ambulatory Medical Care Survey (NAMCS), an ongoing survey of nonfederal employed US office-based physicians who are primarily engaged in direct patient care. The NAMCS has been conducted by the National Center for Health Statistics every year since 1989 to estimate the utilization of ambulatory care services in the United States. Since 1989 including 1993 to 2010, the NAMCS sampled approximately 30,000 visits per year. For each visit sampled, a 1-page patient log including demographic data, physicians’ diagnoses, services provided, and medications was completed. In the NAMCS survey, visits were divided into 2 groups: (1) visits from established patients that have been seen in that office before for any reason, and (2) visits for new (ie, first-time) patients. The current study included all visits in which fungal nail infection (code 110.1 according to the International Classification of Diseases, Ninth Revision [ICD-9]) was listed as 1 of 3 possible diagnoses.
Statistical Analysis
Sampling weights were applied to data to produce estimates for the total US outpatient setting.14 Data were analyzed using SAS version 9.2, and SAS survey analysis procedures were used to account for the clustered sampling of the survey. The total numbers of visits for which onychomycosis was 1 of 3 possible diagnoses and for which it was the sole diagnosis were reported. Visit rates per population by demographic characteristics (ie, patient sex, age, race, and ethnicity) were calculated. Population estimates were based on the 2001 NAMCS Public Micro-Data File Documentation records of the US census estimates for noninstitutionalized civilian persons.15 Trends in proportion of visits linked with an onychomycosis diagnosis over time were evaluated using the SAS SURVEYREG procedure. Types of physicians who attended to these visits as well as leading comorbidities that had been diagnosed and documented in the medical record were characterized. Onychomycosis-related medications prescribed at these visits were reported and prescribing trends over time were evaluated. Differences in the treatment prescribed according to the type of visit (ie, first-time or return visit); physician specialty; and patients’ gender, race, and health conditions (eg, obesity, diabetes mellitus) were examined. To exclude the possibility that fluconazole and other broad-spectrum antifungals were being used for secondary diagnoses, we determined the number of visits that had an additional diagnosis of either candidiasis (ICD-9 codes 112.0–112.9) or “other specified erythematous conditions” (ICD-9 code 695.89).
Results
During the 18-year study period, 636 visits with a diagnosis of onychomycosis were recorded in the NAMCS database. This unweighted number of visits corresponded with approximately 19,350,000 visits (an average of 1,075,000 visits per year) to physicians’ offices with a diagnosis of onychomycosis in the United States during this period. Among these visits, there were an estimated 4,250,000 visits with fungal nail infection as the only diagnosis (no other comorbidities recorded). The recorded visits included more female (57.6%) than male (42.4%) patients, and 85% of patients were white (Table). Patients aged 35 to 44 years accounted for the largest number of visits; however, the estimated rate of onychomycosis visits per 100,000 US citizens was highest among those aged 65 to 74 years (Figure 1).
The number of US outpatient visits with a recorded diagnosis of onychomycosis increased from 1993 to 2010 (Figure 2); however, there was no change in the ratio of onychomycosis visits to the total number of recorded visits in NAMCS database during the study period (P=.9). A combined total of 91% of onychomycosis visits were to general and family practitioners, dermatologists, or internal medicine practitioners (Figure 3). Although cardiovascular diseases and diabetes mellitus accounted for a large proportion of comorbidities, conditions affecting the feet (eg, tinea pedis, ingrown nails) also were among the most common comorbidities (Figure 4).

|
In both topical and systemic form, terbinafine was the most commonly prescribed antifungal agent, followed by systemic fluconazole, systemic itraconazole, and topical ciclopirox (Figure 5). Over the 18-year study period, there was an increasing trend in the frequency of terbinafine prescription (regression coefficient [r]=0.01319; P=.004); a decreasing trend for fluconazole (r=-0.0053851; P=.04), itraconazole (r=-0.0113988; P<.001), griseofulvin (r=-0.0073942; P<.001), and econazole prescription (r=-0.0032405; P=.01); and no significant trend for ketoconazole (r=-0.0034553; P=.1), naftifine (r=-0.0029067; P=.06), sulconazole (r=-0.0001619; P=.8), ciclopirox (r=0.0032684; P=.1), and miconazole prescription (r=0.0002074; P=.5).
Eighty-six percent of visits were for established patients who had been seen in the related office with any diagnosis before the recorded visit and 14% of visits were for new (first-time) patients. Fluconazole was the most frequently used antifungal drug for new patients, while terbinafine was the most frequently used in other visits. Terbinafine was the most frequently prescribed antifungal drug by general and family practitioners, dermatologists, internal medicine practitioners, and all other specialties not listed.
Terbinafine was the most frequently prescribed antifungal drug in both genders and in white and black patients. Itraconazole was the most frequently prescribed antifungal drug for Hispanic patients and those of other ethnicities not listed. Terbinafine was the most frequently prescribed antifungal drug for patients with diabetes and obesity (ie, body mass index ≥30). In 19,330,000 of 19,350,000 total estimated visits included in this study, onychomycosis was the only diagnosis with a potential indication for an antifungal drug therapy, ruling out the possibility that fluconazole or other drugs were used for patients who also had candidiasis or “other specified erythematous conditions.”
Discussion
Onychomycosis is a common progressive infection of the nails that is more prevalent in older age groups, with equal prevalence in both genders and a higher prevalence in males. The NAMCS data showed higher rates of onychomycosis visits among older age groups, which is in agreement with results from prior studies.16,17 In the current study, we observed a higher prevalence of onychomycosis visits among females as well as white and Hispanic patients. These results may be due to a higher prevalence of onychomycosis in these populations or simply a result of difference in socioeconomic level or importance of aesthetics. Although there are limited data regarding the prevalence of onychomycosis among different races and ethnicities in the United States, a high incidence of onychomycosis has been reported in Mexico.18
Repeated trauma to the great toenail from ill-fitting shoes is a predisposing factor for onychomycosis.16 In the current study, ingrown nails were among the most common comorbidities found in onychomycosis patients. Although nail dystrophy caused by onychomycosis may lead to ingrown nails, it also is possible that both conditions may be caused by trauma.
Patients with immunodeficiencies (eg, diabetes) may be predisposed to onychomycosis as well as its associated complications and morbidities (eg, cellulitis).16,19 Diabetes affects 4% to 22% of patients with onychomycosis in different populations, including Denmark, Mexico, and India.18,20,21 In our study, diabetes was among the most common recorded comorbidities reported during onychomycosis visits, with a prevalence of 3.4%. It is likely that many more visits involved patients with diabetes that had not been diagnosed or reported. With the increased risk for complications with diabetes, it is important for physicians to treat these patients when they have a nail infection.
The available systemic therapies for treatment of onychomycosis include griseofulvin, allylamines, and imidazoles. Comparison of griseofulvin with newer systemic antifungal agents such as terbinafine and itraconazole suggests that griseofulvin has lower efficacy and therefore is not a first-line treatment of onychomycosis.1 Terbinafine is the most active of the currently available antidermatophyte drugs both in vitro and in vivo, with synergistic effects with imidazoles and ciclopirox.1,22-27 A combination of topical and systemic therapies may improve cure rates of onychomycosis or possibly shorten the duration of therapy with the systemic agent.1,2 Treatment strategies can vary according to the specialty of the treating physician, with general practitioners often preferring monotherapies and dermatologists preferring combination therapies.28 In Europe, the most commonly prescribed medication for onychomycosis was topical amorolfine followed by systemic terbinafine and itraconazole.28 In the current study, we could not separate data for topical versus systemic terbinafine because the NAMCS uses similar names for reporting the drug; however, the rates of prescription for allylamines and imidazoles were nearly equal (Figure 5), with terbinafine showing an increased use over time as opposed to a decreased use of imidazoles. Although fluconazole is not approved by the US Food and Drug Administration for treatment of onychomycosis, oral fluconazole was the second most common treatment prescribed in our study. Griseofulvin, which is not considered as a drug of choice in onychomycosis,1 was prescribed in a small fraction of the visits, with a decreasing trend of usage over time.
Conclusion
Our analysis of the NAMCS data revealed that the treatment of onychomycosis in the United States is in accordance with recommendations in current guidelines. An encouraging finding was the notable downward trend in use of griseofulvin, suggesting that health care providers are changing practice to meet standard of care. Increased efforts must be made to uniformly modify practices in compliance with evidence-based recommendations and to minimize unnecessary risk and cost associated with use of drugs with lower efficacy.
Onychomycosis is a common progressive infection of the nails caused by dermatophytes, nondermatophyte molds, and yeasts, with Trichophyton rubrum being the most common causative organism.1-3 Onychomycosis affects approximately 2% to 26% of different populations worldwide. It represents 20% to 50% of onychopathies and approximately 30% of fungal cutaneous infections.4-9 Less than 30% of infected persons seek medical advice or treatment even in developed areas of the world.10 Onychomycosis may be a source of more widespread fungal skin infections or give rise to complications such as cellulitis. Chronic, long-lasting infection may result in nail dystrophy and can lead to pain, absence from work, and decreased quality of life.1,11 Because the dermatophyte can contaminate communal bathing facilities and spread to others,12 it is important to effectively target and treat patients with onychomycosis, thus reducing the rate of related morbidities.1,9
The primary aim of onychomycosis treatment is to cure the infection and prevent relapse. Both topical and oral agents are available for the treatment of fungal nail infections. Generally, systemic therapy for onychomycosis is more successful than topical treatment, likely due to poor penetration of topical medications into the nail plate.1,2,9 However, newer topical drugs have shown promising results in treating some types of onychomycosis.13 In its guidelines for treatment of onychomycosis, the British Association of Dermatologists recommends use of topical treatment under the following conditions: (1) when there is not extensive involvement of the nail plate (eg, candidal paronychia, superficial white onychomycosis, early stages of distal and lateral subungual onychomycosis), (2) when systemic therapy is contraindicated, or (3) in combination with systemic therapy.1 Although there are multiple treatments for fungal nail infections, there are limited reports on the ways in which physicians actually use these treatments or the frequency with which they prescribe them.
This study provides a representative portrayal of onychomycosis visits in the US outpatient setting using a large nationally sampled survey. In particular, we aimed to assess the number of visits related to onychomycosis, the demographics of patients, and the treatments being prescribed for onychomycosis.
Methods
Study Design
Data from January 1, 1993, to December 31, 2010, were collected from the National Ambulatory Medical Care Survey (NAMCS), an ongoing survey of nonfederal employed US office-based physicians who are primarily engaged in direct patient care. The NAMCS has been conducted by the National Center for Health Statistics every year since 1989 to estimate the utilization of ambulatory care services in the United States. Since 1989 including 1993 to 2010, the NAMCS sampled approximately 30,000 visits per year. For each visit sampled, a 1-page patient log including demographic data, physicians’ diagnoses, services provided, and medications was completed. In the NAMCS survey, visits were divided into 2 groups: (1) visits from established patients that have been seen in that office before for any reason, and (2) visits for new (ie, first-time) patients. The current study included all visits in which fungal nail infection (code 110.1 according to the International Classification of Diseases, Ninth Revision [ICD-9]) was listed as 1 of 3 possible diagnoses.
Statistical Analysis
Sampling weights were applied to data to produce estimates for the total US outpatient setting.14 Data were analyzed using SAS version 9.2, and SAS survey analysis procedures were used to account for the clustered sampling of the survey. The total numbers of visits for which onychomycosis was 1 of 3 possible diagnoses and for which it was the sole diagnosis were reported. Visit rates per population by demographic characteristics (ie, patient sex, age, race, and ethnicity) were calculated. Population estimates were based on the 2001 NAMCS Public Micro-Data File Documentation records of the US census estimates for noninstitutionalized civilian persons.15 Trends in proportion of visits linked with an onychomycosis diagnosis over time were evaluated using the SAS SURVEYREG procedure. Types of physicians who attended to these visits as well as leading comorbidities that had been diagnosed and documented in the medical record were characterized. Onychomycosis-related medications prescribed at these visits were reported and prescribing trends over time were evaluated. Differences in the treatment prescribed according to the type of visit (ie, first-time or return visit); physician specialty; and patients’ gender, race, and health conditions (eg, obesity, diabetes mellitus) were examined. To exclude the possibility that fluconazole and other broad-spectrum antifungals were being used for secondary diagnoses, we determined the number of visits that had an additional diagnosis of either candidiasis (ICD-9 codes 112.0–112.9) or “other specified erythematous conditions” (ICD-9 code 695.89).
Results
During the 18-year study period, 636 visits with a diagnosis of onychomycosis were recorded in the NAMCS database. This unweighted number of visits corresponded with approximately 19,350,000 visits (an average of 1,075,000 visits per year) to physicians’ offices with a diagnosis of onychomycosis in the United States during this period. Among these visits, there were an estimated 4,250,000 visits with fungal nail infection as the only diagnosis (no other comorbidities recorded). The recorded visits included more female (57.6%) than male (42.4%) patients, and 85% of patients were white (Table). Patients aged 35 to 44 years accounted for the largest number of visits; however, the estimated rate of onychomycosis visits per 100,000 US citizens was highest among those aged 65 to 74 years (Figure 1).
The number of US outpatient visits with a recorded diagnosis of onychomycosis increased from 1993 to 2010 (Figure 2); however, there was no change in the ratio of onychomycosis visits to the total number of recorded visits in NAMCS database during the study period (P=.9). A combined total of 91% of onychomycosis visits were to general and family practitioners, dermatologists, or internal medicine practitioners (Figure 3). Although cardiovascular diseases and diabetes mellitus accounted for a large proportion of comorbidities, conditions affecting the feet (eg, tinea pedis, ingrown nails) also were among the most common comorbidities (Figure 4).

|
In both topical and systemic form, terbinafine was the most commonly prescribed antifungal agent, followed by systemic fluconazole, systemic itraconazole, and topical ciclopirox (Figure 5). Over the 18-year study period, there was an increasing trend in the frequency of terbinafine prescription (regression coefficient [r]=0.01319; P=.004); a decreasing trend for fluconazole (r=-0.0053851; P=.04), itraconazole (r=-0.0113988; P<.001), griseofulvin (r=-0.0073942; P<.001), and econazole prescription (r=-0.0032405; P=.01); and no significant trend for ketoconazole (r=-0.0034553; P=.1), naftifine (r=-0.0029067; P=.06), sulconazole (r=-0.0001619; P=.8), ciclopirox (r=0.0032684; P=.1), and miconazole prescription (r=0.0002074; P=.5).
Eighty-six percent of visits were for established patients who had been seen in the related office with any diagnosis before the recorded visit and 14% of visits were for new (first-time) patients. Fluconazole was the most frequently used antifungal drug for new patients, while terbinafine was the most frequently used in other visits. Terbinafine was the most frequently prescribed antifungal drug by general and family practitioners, dermatologists, internal medicine practitioners, and all other specialties not listed.
Terbinafine was the most frequently prescribed antifungal drug in both genders and in white and black patients. Itraconazole was the most frequently prescribed antifungal drug for Hispanic patients and those of other ethnicities not listed. Terbinafine was the most frequently prescribed antifungal drug for patients with diabetes and obesity (ie, body mass index ≥30). In 19,330,000 of 19,350,000 total estimated visits included in this study, onychomycosis was the only diagnosis with a potential indication for an antifungal drug therapy, ruling out the possibility that fluconazole or other drugs were used for patients who also had candidiasis or “other specified erythematous conditions.”
Discussion
Onychomycosis is a common progressive infection of the nails that is more prevalent in older age groups, with equal prevalence in both genders and a higher prevalence in males. The NAMCS data showed higher rates of onychomycosis visits among older age groups, which is in agreement with results from prior studies.16,17 In the current study, we observed a higher prevalence of onychomycosis visits among females as well as white and Hispanic patients. These results may be due to a higher prevalence of onychomycosis in these populations or simply a result of difference in socioeconomic level or importance of aesthetics. Although there are limited data regarding the prevalence of onychomycosis among different races and ethnicities in the United States, a high incidence of onychomycosis has been reported in Mexico.18
Repeated trauma to the great toenail from ill-fitting shoes is a predisposing factor for onychomycosis.16 In the current study, ingrown nails were among the most common comorbidities found in onychomycosis patients. Although nail dystrophy caused by onychomycosis may lead to ingrown nails, it also is possible that both conditions may be caused by trauma.
Patients with immunodeficiencies (eg, diabetes) may be predisposed to onychomycosis as well as its associated complications and morbidities (eg, cellulitis).16,19 Diabetes affects 4% to 22% of patients with onychomycosis in different populations, including Denmark, Mexico, and India.18,20,21 In our study, diabetes was among the most common recorded comorbidities reported during onychomycosis visits, with a prevalence of 3.4%. It is likely that many more visits involved patients with diabetes that had not been diagnosed or reported. With the increased risk for complications with diabetes, it is important for physicians to treat these patients when they have a nail infection.
The available systemic therapies for treatment of onychomycosis include griseofulvin, allylamines, and imidazoles. Comparison of griseofulvin with newer systemic antifungal agents such as terbinafine and itraconazole suggests that griseofulvin has lower efficacy and therefore is not a first-line treatment of onychomycosis.1 Terbinafine is the most active of the currently available antidermatophyte drugs both in vitro and in vivo, with synergistic effects with imidazoles and ciclopirox.1,22-27 A combination of topical and systemic therapies may improve cure rates of onychomycosis or possibly shorten the duration of therapy with the systemic agent.1,2 Treatment strategies can vary according to the specialty of the treating physician, with general practitioners often preferring monotherapies and dermatologists preferring combination therapies.28 In Europe, the most commonly prescribed medication for onychomycosis was topical amorolfine followed by systemic terbinafine and itraconazole.28 In the current study, we could not separate data for topical versus systemic terbinafine because the NAMCS uses similar names for reporting the drug; however, the rates of prescription for allylamines and imidazoles were nearly equal (Figure 5), with terbinafine showing an increased use over time as opposed to a decreased use of imidazoles. Although fluconazole is not approved by the US Food and Drug Administration for treatment of onychomycosis, oral fluconazole was the second most common treatment prescribed in our study. Griseofulvin, which is not considered as a drug of choice in onychomycosis,1 was prescribed in a small fraction of the visits, with a decreasing trend of usage over time.
Conclusion
Our analysis of the NAMCS data revealed that the treatment of onychomycosis in the United States is in accordance with recommendations in current guidelines. An encouraging finding was the notable downward trend in use of griseofulvin, suggesting that health care providers are changing practice to meet standard of care. Increased efforts must be made to uniformly modify practices in compliance with evidence-based recommendations and to minimize unnecessary risk and cost associated with use of drugs with lower efficacy.
1. Roberts DT, Taylor WD, Boyle J; British Association of Dermatologists. Guidelines for treatment of onychomycosis. Br J Dermatol. 2003;148:402-410.
2. Seebacher C, Brasch J, Abeck D, et al. Onychomycosis. Mycoses. 2007;50:321-327.
3. Summerbell RC, Kane J, Krajden S. Onychomycosis, tinea pedis and tinea manuum caused by non-dermatophytic filamentous fungi. Mycoses. 1989;32:609-619.
4. Murray SC, Dawber RP. Onychomycosis of toenails: orthopaedic and podiatric considerations. Australas J Dermatol. 2002;43:105-112.
5. Achten G, Wanet-Rouard J. Onychomycoses in the laboratory. Mykosen Suppl. 1978;1:125-127.
6. Haneke E, Roseeuw D. The scope of onychomycosis: epidemiology and clinical features. Int J Dermatol. 1999;38(suppl 2):7-12.
7. Haneke E. Fungal infections of the nail. Semin Dermatol. 1991;10:41-53.
8. Karmakar S, Kalla G, Joshi KR, et al. Dermatophytoses in a desert district of Western Rajasthan. Indian J Dermatol Venereol Leprol. 1995;61:280-283.
9. Drake LA. Guidelines of care for superficial mycotic infections of the skin: onychomycosis. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:116-121.
10. Roberts DT. Prevalence of dermatophyte onychomycosis in the United Kingdom: results of an omnibus survey. Br J Dermatol. 1992;126(suppl 39):23-27.
11. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5 pt 1):702-704.
12. Detandt M, Nolard N. Fungal contamination of the floors of swimming pools, particularly subtropical swimming paradises. Mycoses. 1995;38:509-513.
13. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
14. Fleischer AB Jr, Feldman SR, Bradham DD. Office-based physician services provided by dermatologists in the United States in 1990. J Invest Dermatol. 1994;102:93-97.
15. 2001 NAMCS Micro-Data File Documentation. http://www.nber.org/namcs/docs/namcs2001.pdf. National Bureau of Economic Research Web site. Accessed April 27, 2015.
16. Williams HC. The epidemiology of onychomycosis in Britain. Br J Dermatol. 1993;129:101-109.
17. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
18. Arenas R, Bonifaz A, Padilla MC, et al. Onychomycosis. a Mexican survey. Eur J Dermatol. 2010;20:611-614.
19. Faergemann J, Baran R. Epidemiology, clinical presentation and diagnosis of onychomycosis. Br J Dermatol. 2003;149(suppl 65):1-4.
20. Sarma S, Capoor MR, Deb M, et al. Epidemiologic and clinicomycologic profile of onychomycosis from north India. Int J Dermatol. 2008;47:584-587.
21. Svejgaard EL, Nilsson J. Onychomycosis in Denmark: prevalence of fungal nail infection in general practice. Mycoses. 2004;47:131-135.
22. Santos DA, Hamdan JS. In vitro antifungal oral drug and drug-combination activity against onychomycosis causative dermatophytes. Med Mycol. 2006;44:357-362.
23. Gupta AK, Kohli Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br J Dermatol. 2003;149:296-305.
24. Gupta AK, Lynch LE. Management of onychomycosis: examining the role of monotherapy and dual, triple, or quadruple therapies. Cutis. 2004;74(suppl 1):5-9.
25. Harman S, Ashbee HR, Evans EG. Testing of antifungal combinations against yeasts and dermatophytes. J Dermatolog Treat. 2004;15:104-107.
26. Spader TB, Venturini TP, Rossato L, et al. Synergisms of voriconazole or itraconazole combined with other antifungal agents against Fusarium spp. Rev Iberoam Micol. 2013;30:200-204.
27. Biancalana FS, Lyra L, Moretti ML, et al. Susceptibility testing of terbinafine alone and in combination with amphotericin B, itraconazole, or voriconazole against conidia and hyphae of dematiaceous molds. Diagn Microbiol Infect Dis. 2011;71:378-385.
28. Effendy I, Lecha M, Feuilhade de CM, et al. Epidemiology and clinical classification of onychomycosis. J Eur Acad Dermatol Venereol. 2005;19(suppl 1):8-12.
1. Roberts DT, Taylor WD, Boyle J; British Association of Dermatologists. Guidelines for treatment of onychomycosis. Br J Dermatol. 2003;148:402-410.
2. Seebacher C, Brasch J, Abeck D, et al. Onychomycosis. Mycoses. 2007;50:321-327.
3. Summerbell RC, Kane J, Krajden S. Onychomycosis, tinea pedis and tinea manuum caused by non-dermatophytic filamentous fungi. Mycoses. 1989;32:609-619.
4. Murray SC, Dawber RP. Onychomycosis of toenails: orthopaedic and podiatric considerations. Australas J Dermatol. 2002;43:105-112.
5. Achten G, Wanet-Rouard J. Onychomycoses in the laboratory. Mykosen Suppl. 1978;1:125-127.
6. Haneke E, Roseeuw D. The scope of onychomycosis: epidemiology and clinical features. Int J Dermatol. 1999;38(suppl 2):7-12.
7. Haneke E. Fungal infections of the nail. Semin Dermatol. 1991;10:41-53.
8. Karmakar S, Kalla G, Joshi KR, et al. Dermatophytoses in a desert district of Western Rajasthan. Indian J Dermatol Venereol Leprol. 1995;61:280-283.
9. Drake LA. Guidelines of care for superficial mycotic infections of the skin: onychomycosis. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol. 1996;34:116-121.
10. Roberts DT. Prevalence of dermatophyte onychomycosis in the United Kingdom: results of an omnibus survey. Br J Dermatol. 1992;126(suppl 39):23-27.
11. Drake LA, Scher RK, Smith EB, et al. Effect of onychomycosis on quality of life. J Am Acad Dermatol. 1998;38(5 pt 1):702-704.
12. Detandt M, Nolard N. Fungal contamination of the floors of swimming pools, particularly subtropical swimming paradises. Mycoses. 1995;38:509-513.
13. Elewski BE, Rich P, Pollak R, et al. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J Am Acad Dermatol. 2013;68:600-608.
14. Fleischer AB Jr, Feldman SR, Bradham DD. Office-based physician services provided by dermatologists in the United States in 1990. J Invest Dermatol. 1994;102:93-97.
15. 2001 NAMCS Micro-Data File Documentation. http://www.nber.org/namcs/docs/namcs2001.pdf. National Bureau of Economic Research Web site. Accessed April 27, 2015.
16. Williams HC. The epidemiology of onychomycosis in Britain. Br J Dermatol. 1993;129:101-109.
17. Elewski BE, Charif MA. Prevalence of onychomycosis in patients attending a dermatology clinic in northeastern Ohio for other conditions. Arch Dermatol. 1997;133:1172-1173.
18. Arenas R, Bonifaz A, Padilla MC, et al. Onychomycosis. a Mexican survey. Eur J Dermatol. 2010;20:611-614.
19. Faergemann J, Baran R. Epidemiology, clinical presentation and diagnosis of onychomycosis. Br J Dermatol. 2003;149(suppl 65):1-4.
20. Sarma S, Capoor MR, Deb M, et al. Epidemiologic and clinicomycologic profile of onychomycosis from north India. Int J Dermatol. 2008;47:584-587.
21. Svejgaard EL, Nilsson J. Onychomycosis in Denmark: prevalence of fungal nail infection in general practice. Mycoses. 2004;47:131-135.
22. Santos DA, Hamdan JS. In vitro antifungal oral drug and drug-combination activity against onychomycosis causative dermatophytes. Med Mycol. 2006;44:357-362.
23. Gupta AK, Kohli Y. In vitro susceptibility testing of ciclopirox, terbinafine, ketoconazole and itraconazole against dermatophytes and nondermatophytes, and in vitro evaluation of combination antifungal activity. Br J Dermatol. 2003;149:296-305.
24. Gupta AK, Lynch LE. Management of onychomycosis: examining the role of monotherapy and dual, triple, or quadruple therapies. Cutis. 2004;74(suppl 1):5-9.
25. Harman S, Ashbee HR, Evans EG. Testing of antifungal combinations against yeasts and dermatophytes. J Dermatolog Treat. 2004;15:104-107.
26. Spader TB, Venturini TP, Rossato L, et al. Synergisms of voriconazole or itraconazole combined with other antifungal agents against Fusarium spp. Rev Iberoam Micol. 2013;30:200-204.
27. Biancalana FS, Lyra L, Moretti ML, et al. Susceptibility testing of terbinafine alone and in combination with amphotericin B, itraconazole, or voriconazole against conidia and hyphae of dematiaceous molds. Diagn Microbiol Infect Dis. 2011;71:378-385.
28. Effendy I, Lecha M, Feuilhade de CM, et al. Epidemiology and clinical classification of onychomycosis. J Eur Acad Dermatol Venereol. 2005;19(suppl 1):8-12.
Practice Points
- Onychomycosis is a common progressive infection of the nails that may result in remarkable morbidity. Effective treatment may reduce the rate of transmission and related morbidities.
- Onychomycosis is most commonly found in patients older than 35 years.
- Terbinafine has been the most commonly prescribed antifungal agent for onychomycosis in the United States between 1993 and 2010, followed by systemic fluconazole, systemic itraconazole, and topical ciclopirox.
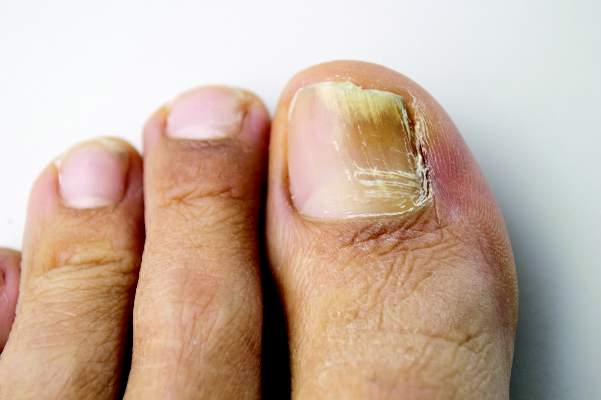
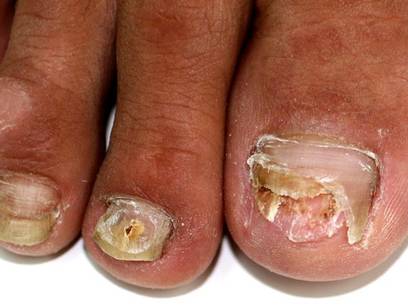
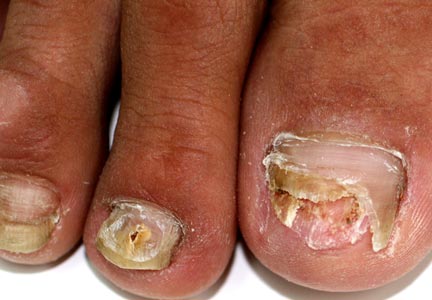
Combine topicals, orals for onychomycosis
MIAMI BEACH – Two new topical solutions approved in 2014 for the treatment of distal subungual onychomycosis don’t eliminate the need for oral treatment, but they do represent improvement in the options available to patients, according to Dr. Boni E. Elewski.
Oral treatments, including terbinafine, itraconazole, and fluconazole are still of value – either alone or in combination with the new solutions or other agents – for this type of onychomycosis, which is “essentially a nail bed dermatophytosis,” she said at the South Beach Symposium.
Terbinafine has been used for 2 decades, and is probably the most commonly used treatment for onychomycosis. It is approved as a once-daily pill given for 90 days, and reportedly has a cure rate just under 40%, she said.
Itraconazole is approved as a 200-mg daily dose for 12 weeks, although most physicians use pulse dosing, prescribing 400 mg daily for a week, then for 1 week each month.
“I like this drug for my nondermatophyte patients … if you have something that you don’t think is a dermatophyte, or if they’ve failed terbinafine, this is an excellent option,” she said.
Fluconazole, her “personal favorite,” is used off label for onychomycosis, but has been shown to have good cure rates with once-weekly treatment, said Dr. Elewski of the University of Alabama at Birmingham.
“I’ll confess, I like this drug because I feel comfortable with it,” she said adding that she tells her patients to think about “Fungal Fridays” or “Toesdays” as a way to remember to take their weekly treatments.
The cure rates are quite good, she said, noting that because the nails grow so slowly, the pace of the treatment matches patient expectations. They don’t finish treatment and still have a “cruddy-looking” nail, she explained.
If any of these oral drugs are used, laboratory monitoring and periodic assessments are necessary, so treatment is a bit complicated. Adverse events remain a concern – particularly drug-drug interactions, drug eruption, cardiac issues (with itraconazole), and loss of taste (with terbinafine).
“So we do have to worry about some of these conditions, which is why having other treatments is so nice,” Dr. Elewski said.
Another reason it’s good to have more options is that no matter which drug you choose, it won’t cure everyone, she noted. Sometimes that’s because the condition is severe; patients with a dermatophyte abscess, those with very thick nails, and those with complete nail involvement associated with a nondermatophyte mold, for example, will have a poorer prognosis, regardless of which oral treatment given. Also, the condition is often complicated by concomitant disorders such as psoriasis. About 5% of patients with psoriasis have nail-only disease, and about a third of them also have onychomycosis. Others are misdiagnosed as having onychomycosis.
Alternatives that can be used alone or in combination with the oral therapies, include the two new topical solutions: efinaconazole and tavaborole, Dr. Elewski said.
Solutions are good, because you can apply them on, under, and around the nail. Both of the drugs have demonstrated effective penetration of the nail plate, allowing penetration to the nail bed where the infection exists, she noted.
The mycological cure rate with efinaconazole yields outcomes comparable to those with oral drugs.
“I think [the mycological cure] is actually the most important endpoint. Because when you want to get rid of the fungus, what do you do? You want to kill the fungus,” said Dr. Elewski, adding that mycological cure is the first sign a patient will go on to experience complete cure.
The complete cure rate is lower, but that appears to be a time-related factor. The nail takes a long time to grow, so the complete cure rate will lag behind the mycological cure rate, Dr. Elewski explained.
The other topical solution – tavaborole – is a new molecule that contains boron. Mycological cure rates in studies of the drug were in the mid-30% range, and it appears able to be used with nail polish without issues to the polish or the outcomes, she said.
In Dr. Elewski’s experience, these topicals work better than expected in clinical practice, based on the clinical trials. One patient who wasn’t eligible for the clinical trials because of a dermatophytosis, for example, was nearly clear within 5 months, and is now totally cured, she said.
“So these treatments are effective as monotherapy, but could be used as an adjunct with systemic therapy, and perhaps also in nondermatophyte cases of onychomycosis,” Dr. Elewski noted. “I think it would be a better option to pick one of these topical drugs than putting someone on a prolonged course of itraconazole if at all possible.” The safety issue would be more favorable with the topical antifungal solution, she said.
A treatment that should never be used is ketoconazole, she noted, explaining that although the drug was never approved specifically for onychomycosis, it was often prescribed for the treatment of tinea versicolor. Because of safety concerns, the FDA removed its indication for all cutaneous fungal infections in 2013.
Dr. Elewski is a consultant for Valeant Pharmaceuticals and a contracted researcher for Anacor Pharmaceuticals.
MIAMI BEACH – Two new topical solutions approved in 2014 for the treatment of distal subungual onychomycosis don’t eliminate the need for oral treatment, but they do represent improvement in the options available to patients, according to Dr. Boni E. Elewski.
Oral treatments, including terbinafine, itraconazole, and fluconazole are still of value – either alone or in combination with the new solutions or other agents – for this type of onychomycosis, which is “essentially a nail bed dermatophytosis,” she said at the South Beach Symposium.
Terbinafine has been used for 2 decades, and is probably the most commonly used treatment for onychomycosis. It is approved as a once-daily pill given for 90 days, and reportedly has a cure rate just under 40%, she said.
Itraconazole is approved as a 200-mg daily dose for 12 weeks, although most physicians use pulse dosing, prescribing 400 mg daily for a week, then for 1 week each month.
“I like this drug for my nondermatophyte patients … if you have something that you don’t think is a dermatophyte, or if they’ve failed terbinafine, this is an excellent option,” she said.
Fluconazole, her “personal favorite,” is used off label for onychomycosis, but has been shown to have good cure rates with once-weekly treatment, said Dr. Elewski of the University of Alabama at Birmingham.
“I’ll confess, I like this drug because I feel comfortable with it,” she said adding that she tells her patients to think about “Fungal Fridays” or “Toesdays” as a way to remember to take their weekly treatments.
The cure rates are quite good, she said, noting that because the nails grow so slowly, the pace of the treatment matches patient expectations. They don’t finish treatment and still have a “cruddy-looking” nail, she explained.
If any of these oral drugs are used, laboratory monitoring and periodic assessments are necessary, so treatment is a bit complicated. Adverse events remain a concern – particularly drug-drug interactions, drug eruption, cardiac issues (with itraconazole), and loss of taste (with terbinafine).
“So we do have to worry about some of these conditions, which is why having other treatments is so nice,” Dr. Elewski said.
Another reason it’s good to have more options is that no matter which drug you choose, it won’t cure everyone, she noted. Sometimes that’s because the condition is severe; patients with a dermatophyte abscess, those with very thick nails, and those with complete nail involvement associated with a nondermatophyte mold, for example, will have a poorer prognosis, regardless of which oral treatment given. Also, the condition is often complicated by concomitant disorders such as psoriasis. About 5% of patients with psoriasis have nail-only disease, and about a third of them also have onychomycosis. Others are misdiagnosed as having onychomycosis.
Alternatives that can be used alone or in combination with the oral therapies, include the two new topical solutions: efinaconazole and tavaborole, Dr. Elewski said.
Solutions are good, because you can apply them on, under, and around the nail. Both of the drugs have demonstrated effective penetration of the nail plate, allowing penetration to the nail bed where the infection exists, she noted.
The mycological cure rate with efinaconazole yields outcomes comparable to those with oral drugs.
“I think [the mycological cure] is actually the most important endpoint. Because when you want to get rid of the fungus, what do you do? You want to kill the fungus,” said Dr. Elewski, adding that mycological cure is the first sign a patient will go on to experience complete cure.
The complete cure rate is lower, but that appears to be a time-related factor. The nail takes a long time to grow, so the complete cure rate will lag behind the mycological cure rate, Dr. Elewski explained.
The other topical solution – tavaborole – is a new molecule that contains boron. Mycological cure rates in studies of the drug were in the mid-30% range, and it appears able to be used with nail polish without issues to the polish or the outcomes, she said.
In Dr. Elewski’s experience, these topicals work better than expected in clinical practice, based on the clinical trials. One patient who wasn’t eligible for the clinical trials because of a dermatophytosis, for example, was nearly clear within 5 months, and is now totally cured, she said.
“So these treatments are effective as monotherapy, but could be used as an adjunct with systemic therapy, and perhaps also in nondermatophyte cases of onychomycosis,” Dr. Elewski noted. “I think it would be a better option to pick one of these topical drugs than putting someone on a prolonged course of itraconazole if at all possible.” The safety issue would be more favorable with the topical antifungal solution, she said.
A treatment that should never be used is ketoconazole, she noted, explaining that although the drug was never approved specifically for onychomycosis, it was often prescribed for the treatment of tinea versicolor. Because of safety concerns, the FDA removed its indication for all cutaneous fungal infections in 2013.
Dr. Elewski is a consultant for Valeant Pharmaceuticals and a contracted researcher for Anacor Pharmaceuticals.
MIAMI BEACH – Two new topical solutions approved in 2014 for the treatment of distal subungual onychomycosis don’t eliminate the need for oral treatment, but they do represent improvement in the options available to patients, according to Dr. Boni E. Elewski.
Oral treatments, including terbinafine, itraconazole, and fluconazole are still of value – either alone or in combination with the new solutions or other agents – for this type of onychomycosis, which is “essentially a nail bed dermatophytosis,” she said at the South Beach Symposium.
Terbinafine has been used for 2 decades, and is probably the most commonly used treatment for onychomycosis. It is approved as a once-daily pill given for 90 days, and reportedly has a cure rate just under 40%, she said.
Itraconazole is approved as a 200-mg daily dose for 12 weeks, although most physicians use pulse dosing, prescribing 400 mg daily for a week, then for 1 week each month.
“I like this drug for my nondermatophyte patients … if you have something that you don’t think is a dermatophyte, or if they’ve failed terbinafine, this is an excellent option,” she said.
Fluconazole, her “personal favorite,” is used off label for onychomycosis, but has been shown to have good cure rates with once-weekly treatment, said Dr. Elewski of the University of Alabama at Birmingham.
“I’ll confess, I like this drug because I feel comfortable with it,” she said adding that she tells her patients to think about “Fungal Fridays” or “Toesdays” as a way to remember to take their weekly treatments.
The cure rates are quite good, she said, noting that because the nails grow so slowly, the pace of the treatment matches patient expectations. They don’t finish treatment and still have a “cruddy-looking” nail, she explained.
If any of these oral drugs are used, laboratory monitoring and periodic assessments are necessary, so treatment is a bit complicated. Adverse events remain a concern – particularly drug-drug interactions, drug eruption, cardiac issues (with itraconazole), and loss of taste (with terbinafine).
“So we do have to worry about some of these conditions, which is why having other treatments is so nice,” Dr. Elewski said.
Another reason it’s good to have more options is that no matter which drug you choose, it won’t cure everyone, she noted. Sometimes that’s because the condition is severe; patients with a dermatophyte abscess, those with very thick nails, and those with complete nail involvement associated with a nondermatophyte mold, for example, will have a poorer prognosis, regardless of which oral treatment given. Also, the condition is often complicated by concomitant disorders such as psoriasis. About 5% of patients with psoriasis have nail-only disease, and about a third of them also have onychomycosis. Others are misdiagnosed as having onychomycosis.
Alternatives that can be used alone or in combination with the oral therapies, include the two new topical solutions: efinaconazole and tavaborole, Dr. Elewski said.
Solutions are good, because you can apply them on, under, and around the nail. Both of the drugs have demonstrated effective penetration of the nail plate, allowing penetration to the nail bed where the infection exists, she noted.
The mycological cure rate with efinaconazole yields outcomes comparable to those with oral drugs.
“I think [the mycological cure] is actually the most important endpoint. Because when you want to get rid of the fungus, what do you do? You want to kill the fungus,” said Dr. Elewski, adding that mycological cure is the first sign a patient will go on to experience complete cure.
The complete cure rate is lower, but that appears to be a time-related factor. The nail takes a long time to grow, so the complete cure rate will lag behind the mycological cure rate, Dr. Elewski explained.
The other topical solution – tavaborole – is a new molecule that contains boron. Mycological cure rates in studies of the drug were in the mid-30% range, and it appears able to be used with nail polish without issues to the polish or the outcomes, she said.
In Dr. Elewski’s experience, these topicals work better than expected in clinical practice, based on the clinical trials. One patient who wasn’t eligible for the clinical trials because of a dermatophytosis, for example, was nearly clear within 5 months, and is now totally cured, she said.
“So these treatments are effective as monotherapy, but could be used as an adjunct with systemic therapy, and perhaps also in nondermatophyte cases of onychomycosis,” Dr. Elewski noted. “I think it would be a better option to pick one of these topical drugs than putting someone on a prolonged course of itraconazole if at all possible.” The safety issue would be more favorable with the topical antifungal solution, she said.
A treatment that should never be used is ketoconazole, she noted, explaining that although the drug was never approved specifically for onychomycosis, it was often prescribed for the treatment of tinea versicolor. Because of safety concerns, the FDA removed its indication for all cutaneous fungal infections in 2013.
Dr. Elewski is a consultant for Valeant Pharmaceuticals and a contracted researcher for Anacor Pharmaceuticals.
AT THE SOUTH BEACH SYMPOSIUM
Scalp Hyperkeratosis in Children With Skin of Color: Diagnostic and Therapeutic Considerations
Scalp hyperkeratosis (scaling or flaking) is a common symptom in childhood and is typified by fine to thick hyperkeratosis of the scalp with or without underlying erythema. The causes of scalp hyperkeratosis in childhood vary based on the demographics of the population. In a population where approximately half of the pediatric patients were white, scaling of the scalp was more common in patients with seborrheic dermatitis and/or atopic dermatitis (AD) who were aged 0 to 2 years, and tinea capitis was only noted in children who were black.1 In children with skin of color, scalp hyperkeratosis has been noted as a marker of tinea capitis, especially in patients aged 3 to 11 years,2,3 and the level of suspicion should consistently remain high for this age group. In another study of an all-black population of schoolchildren aged 5 to 13 years (N=224), 3% demonstrated signs and symptoms of tinea capitis and 14% were found to be asymptomatic carriers.4 Although generally benign in nature, scalp hyperkeratosis can be associated with systemic illnesses such as juvenile dermatomyositis and Langerhans cell histiocytosis.5 This article addresses the diagnosis and treatment of scalp hyperkeratosis in children with skin of color, focusing on differences in exposure to contagious cases, hairstyling practices, and biological factors that may impact the disease process.
CAUSES OF SCALP HYPERKERATOSIS IN CHILDHOOD
Scalp hyperkeratosis in childhood usually is caused by common benign conditions, but some level of suspicion should be maintained for more severe etiologic conditions such as Langerhans cell histiocytosis and collagen vascular diseases (eg, juvenile dermatomyositis).6 Langerhans cell histiocytosis of the scalp might be obscured by background pigmentation in black children.
Scalp scaling can be a minor criterion in the diagnosis of AD. Atopic dermatitis should be suspected in Asian children with scalp scaling. Although one study in Bangladesh revealed scalp involvement in only 5.2% of pediatric patients with AD,7 a study in China reported an incidence rate as high as 49.7% (with a similarly high incidence of eyelid dermatitis).8 Children with AD also may have dry hair.9 Atopic dermatitis of the scalp is typified by itching, fine hyperkeratosis, and notably eczematous scalp lesions ranging from excoriated or oozing erythematous plaques to lichenification with hair miniaturization, primarily from scratch-induced breakage.10 The latter finding often is noted in black adolescent girls with long-term moderate to severe AD (personal observation).
Seborrheic dermatitis is a hypersensitivity response to yeast colonization of the scalp with Malassezia species. The infantile form is extremely common (also known as cradle cap). Characteristically, greasy yellow hyperkeratosis in fine to thick sheets is noted on the scalp in children younger than 2 years, especially infants, often with involvement of skin folds. One study noted that seborrheic dermatitis occurs in 6% of school-aged children as opposed to 19% of children younger than 2 years.1 Severe seborrheic dermatitis in infancy may be a prelude to AD, with the incidence being 3 times higher in children with prior seborrheic dermatitis.11 In teenagers, seborrheic dermatitis often accompanies acne onset in the early pubertal years.12
Psoriasis is an autoimmune inflammatory dermatosis that most commonly affects white children. In childhood, pityriasis amiantacea, psoriasiform scalp hyperkeratosis, is more common than in adulthood, with thick, stuck-on scales bound to the hairs. This variant is uncommon in Hispanic and Asian children and is almost never seen in black children but has been reported in cohorts of Turkish children.13 In a series of 85 Egyptian children with pityriasis amiantacea, diagnosis of scalp psoriasis was made in 35.3%, eczematous dermatitis in 34.2%, and tinea capitis in 12.9%.14 Consequently, a high degree of suspicion for tinea capitis should be held if pityriasis amiantacea is found in children with skin of color.15,16
Tinea capitis is a dermatophyte infection of the scalp, hair, and surrounding skin. The presence of tinea capitis on the scalp is associated with environmental exposure to dermatophytes (eg, school, household).4,17 The infection is largely caused by Trichophyton tonsurans in the United States, which causes a seborrheic appearance and less commonly alopecia (black dot or thinning), plaques with scale, or kerion. The presence of cervical lymph nodes and/or alopecia increases the chances of tinea being the diagnosis. Potassium hydroxide preparation and fungal culture can be performed to corroborate the diagnosis.1-3 Other etiologies of scalp hyperkeratosis such as juvenile pityriasis rubra pilaris and lice are extremely uncommon in black children, but lice may be seen in Hispanic and Asian girls with long straight hair who attend school. Discoid lupus is more common in children with skin of color but is rare overall. When noted, accompanying mottled dyspigmentation and scarring alopecia are noted in addition to a high risk for developing systemic lupus erythematosus. Biopsy and screening for systemic lupus are necessary, as the risk for progression from discoid lupus to systemic disease is 26% over 3 years.18
THE BIOLOGY OF HAIR IN CHILDREN WITH SKIN OF COLOR
To some extent, the biology of hair impacts the occurrence, appearance, and treatment of scalp hyperkeratosis in children with skin of color. First, it is important to remember that follicular density is lower in black patients as compared to Asian patients with a consequently lower hair count overall, which results in the easy appearance of hair loss, particularly at the margins of the scalp.19,20 Second, the shape of the hair follicle differs among races and ethnicities. Asian patients have round hair shafts coming from straight follicles, which allows for greater natural hair hydration, resulting in somewhat less aggressive scalp disease. Hispanic patients may have similarly straight hair or may have elliptical or curled shafts, the latter being noted in black patients. Furthermore, a curled hair shaft results in poor flow of sebum across the hair, resulting in greater scalp xerosis, more susceptibility to traction alopecia, and ultimately a greater risk for infections.20-23 Finally, the scalp is continuous with the face and neck, and Asian patients have greater sensitivity to skin care products in these areas, resulting in difficulty of treatment in this patient population and the need for use of gentle products.
HAIR CARE PRACTICES IN CHILDREN WITH SKIN OF COLOR
Hair care in patients with skin of color can be costly, difficult, and potentially damaging, with 99% of black girls reporting pomade or oil usage. Costly and complex hair care practices begin in childhood for patients with skin of color. In a series of 201 surveyed black girls with a mean age of 9.8 years, 80% had used hot combs and 42% used relaxers.24 Traction styles were common with 81% using ponytails, 67% braids, and 49% cornrows in the last 12 months. These styles are thought to affect hair health, particularly through induction of traction-related damage, folliculitis, and alopecia. Furthermore, chemical relaxers, hot combs, blowouts, and hair setting may be introduced during childhood.24 These practices appear to disturb the integrity of the hair follicle, leaving it more susceptible to irritation and infection.
Hair care in the pediatric population often is complicated by the fact that multiple children are being styled in tandem, either at home or in a salon, resulting in shared equipment and fomite spread. Even just proximity to a case of tinea capitis in the household will increase risk for tinea capitis. Furthermore, it is quite commonplace for black patients to use pomades and shampoos that contain antifungals, especially selenium sulfide, which makes it difficult to obtain accurate culture results. In India, use of mustard oil also has been linked to increased risk for tinea capitis.25
Other issues related to hair care include frequent dry scalp in patients with skin of color due to poor sebum distribution along the hair shaft. As a result, frequent washing may exacerbate scalp xerosis and further irritate seborrheic dermatitis and/or AD.
DIAGNOSTIC CONSIDERATIONS FOR SCALP HYPERKERATOSIS IN CHILDHOOD
Dermatologists should have a greater level of suspicion for tinea capitis in black and Hispanic children compared to white children. The index of suspicion should be high given that antifungal shampoos and pomades may minimize the clinical appearance. Although trends in overall incidence in the United States suggest tinea capitis is becoming less common, there still is a stronger representation of the disease in black patients.26 A study of positive fungal cultures from one clinic in Mississippi (N=1220) showed that two-thirds of patients were children younger than 13 years; 87% of patients with positive cultures for dermatophytes were black.27 The endothrix type of tinea capitis caused by T tonsurans often presents with a seborrheic appearance, and fungal culture is warranted in all pediatric patients with skin of color who have scalp hyperkeratosis. Asian children can be regarded with a lower level of suspicion for tinea capitis, similar to white patients in the United States. Variation in incidence of tinea capitis does exist worldwide and the practitioner may need to address these issues in patients who travel or are recent immigrants.
When identifying tinea capitis infections in children with skin of color, physicians should consider the patient’s personal and family history, comorbid skin disorders, dermoscopy, microscopy and fungal staining, and fungal culture (Figure).
A paradigm for the diagnosis of scalp hyperkeratosis in children with skin of color.
Personal and Family History
The first diagnostic consideration is the patient’s personal and family history. A history of AD, asthma, or allergies will support but not confirm the diagnosis of AD. Prior tinea capitis infections and household contacts with tinea infections support the presence of tinea capitis.17 Recent implementation of anti–tumor necrosis factor a inhibitor therapy in a psoriatic child can flare scalp disease, mimicking tinea capitis.28 The patient’s guardians should be queried about potential infectious contacts, whether they themselves have signs of scalp disease or tinea corporis (ringworm) or whether they have a pet with problematic fur. Physicians also should query patients and their guardians about recent use of topical antifungal shampoos, pomades, creams (both over-the-counter [OTC] and prescription), and/or oral antifungals. When these agents are used, there is a possibility that fungal examinations may be negative in the presence of true infection with tinea capitis. Traction alopecia, often preceded by fine scale, is more likely to present in patients who wear their hair in cornrows, while seborrheic dermatitis may be associated with hair extensions, reduced frequency of washing (61% of black girls surveyed wash every 2 weeks), and/or reduced usage of hair oils in black girls.24 Knowledge of the patient’s personal hair care history, such as use of pomades; frequency and method of washing/drying hair; types of hair care products used daily to wash and style hair; use of chemical relaxers; or recent hairstyling with cornrows, braids, or hair extensions, also is essential to the diagnosis of tinea capitis. Usage of traction-related styling practices in patients with chemically relaxed hair can enhance the risk for traction alopecia.29
Comorbid Skin Disorders
The patient also should be examined for comorbid skin disorders, including tinea corporis, alopecia (particularly in the areas of hyperkeratosis), and the presence of nuchal lymphadenopathy. For each extra clinical finding, the chances of a final diagnosis of tinea capitis rises, allowing for empiric diagnosis to be made that can be confirmed by a variety of tests.1-3
Dermoscopy
Next, the patient should undergo dermoscopic evaluation. On dermoscopy, tinea capitis typically presents with broken hairs, black dots on the scalp, comma-shaped hairs, and short corkscrew hairs, all of which should clear with therapy.30-33 Dermoscopic findings of AD would reveal underlying xerosis and prominent vasculature due to inflammation, and alopecia areata would present with yellow dots at the orifices of the hair follicles, exclamation point hairs, and vellus hairs.34,35 Traction alopecia may be noted by retained hairs along the hairline, which is known as the fringe sign.36
Microscopy and Fungal Staining
Microscopic preparations can be performed to identify tinea capitis using fungal stains of slide-based specimens. Breakage of short hairs onto the slide and/or cotton swab is a soft sign corroborating endothrix infection of the hairs. Potassium hydroxide can enhance visualization of the hyperkeratotic scalp, but for most black patients, use of antifungal agents reduces fungal hyphae and spores in the areas of hyperkeratosis and may limit the utility of examining the skin microscopically. Assessment of the broken hairs obtained by gentle friction with one glass slide and catching the scales onto another glass slide may yield the best results in the evaluation of tinea capitis (a technique taught to me by Robin Hornung, MD, Everett, Washington). Hairs obtained in this manner often are fragile and break due to endothrix infection replacing and weakening the shaft of the hairs. In the United States, fungal samples usually are obtained with cotton swabs, but a recent study suggested that brushing is superior to scraping to obtain samples; the combination of sampling techniques may improve the yield of a culture.37 Because topical agents are unable to enter the hair cortex, the hair shaft is the most likely to show fungal spores under the microscope when antifungal shampoos or pomades are used. Other testing methods such as Swartz-Lamkins or calcofluor white staining can be used on similar scrapings. Biopsy and periodic acid–Schiff staining of thick scales or crust can help differentiate tinea capitis from pityriasis amiantacea when the crust is too thick to be softened via potassium hydroxide preparation.38
Fungal Culture
Fungal culture onto media that contains nutrients for dermatophyte growth can be used for 4 purposes in tinea capitis: (1) to confirm infection, (2) to identify species of infection, (3) to confirm mycological cure when difficulty in clearance of disease has been noted, and (4) to obtain a specimen for sensitivity screening regarding antifungals when necessary, an uncommon but occasionally useful test in individuals with disease that has failed treatment with 1 or more antifungals.27
THERAPY FOR SCALP HYPERKERATOSIS IN CHILDREN WITH SKIN OF COLOR
In patients with scalp hyperkeratosis, it is important to address the specific cause of the disease. Therapy for scalp hyperkeratosis in children with skin of color includes altered hair care practices, use of OTC and prescription agents, and containment of fomites in the case of infections. Biopsy of atypical scalp hyperkeratosis cases is needed to diagnose rare etiologies such as discoid lupus or Langerhans cell histiocytosis. For individuals with systemic disease including Langerhans cell histiocytosis, which is generally accompanied by nodes and plaques in the inguinal region or other intertriginous sites, immediate hematology and oncology workup is required.39 For collagen vascular diseases such as lupus or dermatomyositis, appropriate referral to rheumatology and systemic therapy is warranted.
Altered Hair Care Practices
The use of prophylactic ketoconazole 1% shampoo may not reduce the risk for recurrence of tinea capitis over standard good hygiene, removal of fomites, and adherence to prescribed therapy.40 Use of selenium sulfide has been shown to effectively reduce contagion risk.41
Fragrance- and dye-free shampoos can be helpful in providing gentle cleansing of the scalp, which is especially important in Asian patients who have greater facial and eyelid sensitivity. Free-and-clear shampoos can be used alternatively with shampoos containing selenium sulfide or sulfur to eliminate comorbid seborrhea. Black patients should be advised to shampoo and condition their hair once weekly, and Asian and Hispanic patients should shampoo and condition 2 to 3 times weekly to remove scale and potentially reduce risk for tinea acquisition.42 Children with straight hair should shampoo with increased frequency in the summer to manually remove sweat-induced macerated hyperkeratosis. Conditioners also should be used consistently after shampooing to enhance hair health.
Use of OTC and Prescription Agents
Atopic Dermatitis
Topical corticosteroid agents can be used in increasing strengths to treat AD of the scalp in children with skin of color, from OTC scalp products containing hydrocortisone 1% to prescription-based agents. Hydration of the hair also is needed to counteract reduced water content.43 Due to the innate xerosis of the scalp in black patients and atopic patients, the use of oil-based or lotion products may provide the most hydration for patients with scalp disease.44 Alcohol-based agents, either drops or foams, may enhance xerosis and should be used sparingly.
Seborrheic Dermatitis
Alternating treatment with medicated shampoos containing selenium sulfide and ketoconazole can aid in the removal of seborrhea. Pomades including borage seed oil–based agents can be massaged into the scalp,45 particularly for treatment of infantile seborrhea, and should not necessarily be washed off daily in dark-skinned patients. Additional focused application of topical corticosteroids to the scalp also is helpful. Due to innate scalp xerosis in black children, therapy should be similar to AD.
Psoriasis
In the setting of pityriasis amiantacea, albeit rare in children with skin of color, oil-based agents can soften hyperkeratosis for removal. Sterile mineral oil or commercially available scalp preparations of peanut oil with fluocinolone or mineral oil with glycerin can aid in the removal of scales without harming the hair, but usage must be age appropriate. The addition of focused application of age-appropriate topical corticosteroids for areas of severe hyperkeratosis can aid in clearance of the lesions.44 Recently, a stable combination of calcipo-triene 0.005%–betamethasone dipropionate 0.064% has been approved in the United States for the therapy of scalp psoriasis in adolescents.46
Tinea Capitis
Antifungal shampoos including selenium sulfide will reduce contagion risk when used by both the patient and his/her family members. Frequency of shampooing is similar to that described for AD. Between shampooing, pomades with selenium sulfide can be applied to the scalp to enhance overall clearance.
Oral antifungals are the basis of treatment and use of griseofulvin is the gold standard. Terbinafine has been approved by the US Food and Drug Administration for treatment of tinea capitis; for children weighing less than 25 kg the dosage is 125 mg daily, for 25 to 35 kg the dosage is 187.5 mg daily, and for more than 35 kg the dosage is 250 mg daily. Shorter therapeutic courses may be required, making it a good second-line agent. Laboratory screening in children prior to therapy is not always performed but should be done in cases where fatty liver might be suspected.47 Monitoring liver function tests is best when exceeding 3 months of usage or shifting from one antifungal to another.3
Containment of Fomites
There are several procedures that should be followed to contain scalp infection in children with skin of color. First, all objects that come into contact with the scalp (eg, hats, hoods, brushes, pillowcases) should be washed with hot water or replaced weekly. Sharing these objects with friends or family should be strongly discouraged. Patients and their family members also should be instructed to use medicated (eg, selenium sulfide) shampoos and conditioners. Finally, patients are advised to avoid use of shared classroom garments or mats for sleeping.
LONG-TERM SEQUELAE OF SCALP HYPERKERATOSIS
Long-term sequelae of scalp hyperkeratosis often are discounted in children, but the disease can have lasting and damaging effects on the scalp. Sequelae include discomfort from chronicity and psychological distress. In particular, years of scalp pruritus can promote lichenification of the scalp and miniaturization of the hair follicles. Furthermore, itching due to sweating can limit participation in sports. Finally, tinea capitis is thought to be a risk factor for central centrifugal cicatricial alopecia (or can occur comorbidly with central centrifugal cicatricial alopecia causing severe pruritus), a chronic scarring hair loss that is seen primarily in black adult females.48 Erythema nodosum also has been reported as an associated finding in the case of kerion.49 One study reported associated findings that included thyroid cancer in individuals irradiated for tinea capitis in the 1950s.50
Conclusion
Scalp hyperkeratosis in children with skin of color, especially black patients, is more likely to be associated with tinea capitis and is more challenging to treat due to innate scalp xerosis in black patients and increased sensitivity of facial skin in Asian children. Ultimately, institution of therapy when needed and good scalp and hair care may prevent long-term sequelae.
1. Williams JV, Eichenfield LF, Burke BL, et al. Prevalence of scalp scaling in prepubertal children. Pediatrics. 2005;115:e1-e6.
2. Coley MK, Bhanusali DG, Silverberg JI, et al. Scalp hyperkeratosis and alopecia in children of color. J Drugs Dermatol. 2011;10:511-516.
3. Bhanusali D, Coley M, Silverberg JI, et al. Treatment outcomes for tinea capitis in a skin of color population. J Drugs Dermatol. 2012;11:852-856.
4. Williams JV, Honig PJ, McGinley KJ, et al. Semiquantitative study of tinea capitis and the asymptomatic carrier state in inner-city school children. Pediatrics. 1995;96:265-267.
5. McDonald LL, Smith ML. Diagnostic dilemmas in pediatric/adolescent dermatology: scaly scalp. J Pediatr Health Care. 1998;12:80-84.
6. Peloro TM, Miller OF 3rd, Hahn TF, et al. Juvenile dermatomyositis: a retrospective review of a 30-year experience. J Am Acad Dermatol. 2001;45:28-34.
7. Wahab MA, Rahman MH, Khondker L, et al. Minor criteria for atopic dermatitis in children. Mymensingh Med J. 2011;20:419-424.
8. Shi M, Zhang H, Chen X, et al. Clinical features of atopic dermatitis in a hospital-based setting in China. J Eur Acad Dermatol Venereol [published online ahead of print January 9, 2011]. 2011;25:1206-1212.
9. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair. Microsc Res Tech. 2012;75:620-625.
10. Sabin BR, Peters N, Peters AT. Chapter 20: atopic dermatitis. Allergy Asthma Proc. 2012;33:S67-S69.
11. Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis [published online ahead of print November 13, 2013]. Pediatr Dermatol. 2014;31:125-130.
12. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
13. Sarifakioglu E, Yilmaz AE, Gorpelioglu C, et al. Prevalence of scalp disorders and hair loss in children. Cutis. 2012;90:225-229.
14. Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
15. Oostveen AM, Jong EM, Evers AW, et al. Reliability, responsiveness and validity of Scalpdex in children with scalp psoriasis: the Dutch study. Acta Derm Venereol. 2014;94:198-202.
16. Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity: Comparative Dermatologic Atlas of Pediatric Skin of All Colors. New York, NY: Springer; 2012.
17. Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
18. Moises-Alfaro C, Berrón-Pérez R, Carrasco-Daza D, et al. Discoid lupus erythematosus in children: clinical, histopathologic, and follow-up features in 27 cases. Pediatr Dermatol. 2003;20:103-107.
19. Ramos-e-Silva M. Ethnic hair and skin: what is the state of the science? Chicago, Illinois—September 29-30, 2001. Clin Dermatol. 2002;20:321-324.
20. Heath CR, McMichael AJ. Biology of hair follicle. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. New York, NY: McGraw Hill; 2009:105-109.
21. Khumalo NP. African hair morphology: macrostructure to ultrastructure. Int J Dermatol. 2005;44(suppl 1):10-12.
22. Thibaut S, Bernard BA. The biology of hair shape. Int J Dermatol. 2005;44(suppl 1):2-3.
23. Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
24. Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
25. Kumar V, Sharma RC, Chander R. Clinicomycological study of tinea capitis. Indian J Dermatol Venereol Leprol. 1996;62:207-209.
26. Mirmirani P, Tucker LY. Epidemiologic trends in pediatric tinea capitis: a population-based study from Kaiser Permanente Northern California [published online ahead of print October 2, 2013]. J Am Acad Dermatol. 2013;69:916-921.
27. Chapman JC, Daniel CR 3rd, Daniel JG, et al. Tinea capitis caused by dermatophytes: a 15-year retrospective study from a Mississippi Dermatology Clinic. Cutis. 2011;88:230-233.
28. Perman MJ, Lovell DJ, Denson LA, et al. Five cases of anti-tumor necrosis factor alpha-induced psoriasis presenting with severe scalp involvement in children. Pediatr Dermatol. 2012;29:454-459.
29. Khumalo NP, Jessop S, Gumedze F, et al. Determinants of marginal traction alopecia in African girls and women. J Am Acad Dermatol. 2008;59:432-438.
30. Vazquez-Lopez F, Palacios-Garcia L, Argenziano G. Dermoscopic corkscrew hairs dissolve after successful therapy of Trichophyton violaceum tinea capitis: a case report. Australas J Dermatol. 2012;53:118-119.
31. Pinheiro AM, Lobato LA, Varella TC. Dermoscopy findings in tinea capitis: case report and literature review. An Bras Dermatol. 2012;87:313-314.
32. Mapelli ET, Gualandri L, Cerri A, et al. Comma hairs in tinea capitis: a useful dermatoscopic sign for diagnosis of tinea capitis. Pediatr Dermatol. 2012;29:223-224.
33. Hughes R, Chiaverini C, Bahadoran P, et al. Corkscrew hair: a new dermoscopic sign for diagnosis of tinea capitis in black children. Arch Dermatol. 2011;147:355-356.
34. Ekiz O, Sen BB, Rifaiog˘lu EN, et al. Trichoscopy in paediatric patients with tinea capitis: a useful method to differentiate from alopecia areata [published online ahead of print August 24, 2013]. J Eur Acad Dermatol Venereol. 2014;28:1255-1258.
35. Lencastre A, Tosti A. Role of trichoscopy in children’s scalp and hair disorders [published online ahead of print Aug 13, 2013]. Pediatr Dermatol. 2013;30:674-682.
36. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
37. Nasir S, Ralph N, O’Neill C, et al. Trends in tinea capitis in an Irish pediatric population and a comparison of scalp brushings versus scalp scrapings as methods of investigation [published online ahead of print February 22, 2013]. Pediatr Dermatol. 2014;31:622-623.
38. Alvarez MS, Silverberg NB. Tinea capitis. Cutis. 2006;78:189-196.
39. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996.
40. Bookstaver PB, Watson HJ, Winters SD, et al. Prophylactic ketoconazole shampoo for tinea capitis in a high-risk pediatric population. J Pediatr Pharmacol Ther. 2011;16:199-203.
41. Allen HB, Honig PJ, Leyden JJ, et al. Selenium sulfide: adjunctive therapy for tinea capitis. Pediatrics. 1982;69:81-83.
42. Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
43. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair [published online ahead of print November 7, 2011]. Microsc Res Tech. 2012;75:620-625.
44. Kapila S, Hong E, Fischer G. A comparative study of childhood psoriasis and atopic dermatitis and greater understanding of the overlapping condition, psoriasis-dermatitis. Australas J Dermatol. 2012;53:98-105.
45. Tollesson A, Frithz A. Borage oil, an effective new treatment for infantile seborrhoeic dermatitis. Br J Dermatol. 1993;129:95.
46. Gooderham M, Debarre JM, Keddy-Grant J, et al. Safety and efficacy of calcipotriol plus betamethasone dipropionate gel in the treatment of scalp psoriasis in adolescents 12-17 years of age [published online ahead of print October 22, 2014]. Br J Dermatol. 2014;171:1470-1477.
47. Singer C, Stancu P, Coşoveanu S, et al. Non-alcoholic fatty liver disease in children. Curr Health Sci J. 2014;40:170-176.
48. Chiang C, Price V, Mirmirani P. Central centrifugal cicatricial alopecia: superimposed tinea capitis as the etiology of chronic scalp pruritus. Dermatol Online J. 2008;14:3.
49. Morrone A, Calcaterra R, Valenzano M, et al. Erythema nodosum induced by kerion celsi of the scalp in a woman. Mycoses. 2011;54:e237-e239.
50. Boaventura P, Pereira D, Celestino R, et al. Genetic alterations in thyroid tumors from patients irradiated in childhood for tinea capitis treatment. Eur J Endocrinol. 2013;169:673-679.
Scalp hyperkeratosis (scaling or flaking) is a common symptom in childhood and is typified by fine to thick hyperkeratosis of the scalp with or without underlying erythema. The causes of scalp hyperkeratosis in childhood vary based on the demographics of the population. In a population where approximately half of the pediatric patients were white, scaling of the scalp was more common in patients with seborrheic dermatitis and/or atopic dermatitis (AD) who were aged 0 to 2 years, and tinea capitis was only noted in children who were black.1 In children with skin of color, scalp hyperkeratosis has been noted as a marker of tinea capitis, especially in patients aged 3 to 11 years,2,3 and the level of suspicion should consistently remain high for this age group. In another study of an all-black population of schoolchildren aged 5 to 13 years (N=224), 3% demonstrated signs and symptoms of tinea capitis and 14% were found to be asymptomatic carriers.4 Although generally benign in nature, scalp hyperkeratosis can be associated with systemic illnesses such as juvenile dermatomyositis and Langerhans cell histiocytosis.5 This article addresses the diagnosis and treatment of scalp hyperkeratosis in children with skin of color, focusing on differences in exposure to contagious cases, hairstyling practices, and biological factors that may impact the disease process.
CAUSES OF SCALP HYPERKERATOSIS IN CHILDHOOD
Scalp hyperkeratosis in childhood usually is caused by common benign conditions, but some level of suspicion should be maintained for more severe etiologic conditions such as Langerhans cell histiocytosis and collagen vascular diseases (eg, juvenile dermatomyositis).6 Langerhans cell histiocytosis of the scalp might be obscured by background pigmentation in black children.
Scalp scaling can be a minor criterion in the diagnosis of AD. Atopic dermatitis should be suspected in Asian children with scalp scaling. Although one study in Bangladesh revealed scalp involvement in only 5.2% of pediatric patients with AD,7 a study in China reported an incidence rate as high as 49.7% (with a similarly high incidence of eyelid dermatitis).8 Children with AD also may have dry hair.9 Atopic dermatitis of the scalp is typified by itching, fine hyperkeratosis, and notably eczematous scalp lesions ranging from excoriated or oozing erythematous plaques to lichenification with hair miniaturization, primarily from scratch-induced breakage.10 The latter finding often is noted in black adolescent girls with long-term moderate to severe AD (personal observation).
Seborrheic dermatitis is a hypersensitivity response to yeast colonization of the scalp with Malassezia species. The infantile form is extremely common (also known as cradle cap). Characteristically, greasy yellow hyperkeratosis in fine to thick sheets is noted on the scalp in children younger than 2 years, especially infants, often with involvement of skin folds. One study noted that seborrheic dermatitis occurs in 6% of school-aged children as opposed to 19% of children younger than 2 years.1 Severe seborrheic dermatitis in infancy may be a prelude to AD, with the incidence being 3 times higher in children with prior seborrheic dermatitis.11 In teenagers, seborrheic dermatitis often accompanies acne onset in the early pubertal years.12
Psoriasis is an autoimmune inflammatory dermatosis that most commonly affects white children. In childhood, pityriasis amiantacea, psoriasiform scalp hyperkeratosis, is more common than in adulthood, with thick, stuck-on scales bound to the hairs. This variant is uncommon in Hispanic and Asian children and is almost never seen in black children but has been reported in cohorts of Turkish children.13 In a series of 85 Egyptian children with pityriasis amiantacea, diagnosis of scalp psoriasis was made in 35.3%, eczematous dermatitis in 34.2%, and tinea capitis in 12.9%.14 Consequently, a high degree of suspicion for tinea capitis should be held if pityriasis amiantacea is found in children with skin of color.15,16
Tinea capitis is a dermatophyte infection of the scalp, hair, and surrounding skin. The presence of tinea capitis on the scalp is associated with environmental exposure to dermatophytes (eg, school, household).4,17 The infection is largely caused by Trichophyton tonsurans in the United States, which causes a seborrheic appearance and less commonly alopecia (black dot or thinning), plaques with scale, or kerion. The presence of cervical lymph nodes and/or alopecia increases the chances of tinea being the diagnosis. Potassium hydroxide preparation and fungal culture can be performed to corroborate the diagnosis.1-3 Other etiologies of scalp hyperkeratosis such as juvenile pityriasis rubra pilaris and lice are extremely uncommon in black children, but lice may be seen in Hispanic and Asian girls with long straight hair who attend school. Discoid lupus is more common in children with skin of color but is rare overall. When noted, accompanying mottled dyspigmentation and scarring alopecia are noted in addition to a high risk for developing systemic lupus erythematosus. Biopsy and screening for systemic lupus are necessary, as the risk for progression from discoid lupus to systemic disease is 26% over 3 years.18
THE BIOLOGY OF HAIR IN CHILDREN WITH SKIN OF COLOR
To some extent, the biology of hair impacts the occurrence, appearance, and treatment of scalp hyperkeratosis in children with skin of color. First, it is important to remember that follicular density is lower in black patients as compared to Asian patients with a consequently lower hair count overall, which results in the easy appearance of hair loss, particularly at the margins of the scalp.19,20 Second, the shape of the hair follicle differs among races and ethnicities. Asian patients have round hair shafts coming from straight follicles, which allows for greater natural hair hydration, resulting in somewhat less aggressive scalp disease. Hispanic patients may have similarly straight hair or may have elliptical or curled shafts, the latter being noted in black patients. Furthermore, a curled hair shaft results in poor flow of sebum across the hair, resulting in greater scalp xerosis, more susceptibility to traction alopecia, and ultimately a greater risk for infections.20-23 Finally, the scalp is continuous with the face and neck, and Asian patients have greater sensitivity to skin care products in these areas, resulting in difficulty of treatment in this patient population and the need for use of gentle products.
HAIR CARE PRACTICES IN CHILDREN WITH SKIN OF COLOR
Hair care in patients with skin of color can be costly, difficult, and potentially damaging, with 99% of black girls reporting pomade or oil usage. Costly and complex hair care practices begin in childhood for patients with skin of color. In a series of 201 surveyed black girls with a mean age of 9.8 years, 80% had used hot combs and 42% used relaxers.24 Traction styles were common with 81% using ponytails, 67% braids, and 49% cornrows in the last 12 months. These styles are thought to affect hair health, particularly through induction of traction-related damage, folliculitis, and alopecia. Furthermore, chemical relaxers, hot combs, blowouts, and hair setting may be introduced during childhood.24 These practices appear to disturb the integrity of the hair follicle, leaving it more susceptible to irritation and infection.
Hair care in the pediatric population often is complicated by the fact that multiple children are being styled in tandem, either at home or in a salon, resulting in shared equipment and fomite spread. Even just proximity to a case of tinea capitis in the household will increase risk for tinea capitis. Furthermore, it is quite commonplace for black patients to use pomades and shampoos that contain antifungals, especially selenium sulfide, which makes it difficult to obtain accurate culture results. In India, use of mustard oil also has been linked to increased risk for tinea capitis.25
Other issues related to hair care include frequent dry scalp in patients with skin of color due to poor sebum distribution along the hair shaft. As a result, frequent washing may exacerbate scalp xerosis and further irritate seborrheic dermatitis and/or AD.
DIAGNOSTIC CONSIDERATIONS FOR SCALP HYPERKERATOSIS IN CHILDHOOD
Dermatologists should have a greater level of suspicion for tinea capitis in black and Hispanic children compared to white children. The index of suspicion should be high given that antifungal shampoos and pomades may minimize the clinical appearance. Although trends in overall incidence in the United States suggest tinea capitis is becoming less common, there still is a stronger representation of the disease in black patients.26 A study of positive fungal cultures from one clinic in Mississippi (N=1220) showed that two-thirds of patients were children younger than 13 years; 87% of patients with positive cultures for dermatophytes were black.27 The endothrix type of tinea capitis caused by T tonsurans often presents with a seborrheic appearance, and fungal culture is warranted in all pediatric patients with skin of color who have scalp hyperkeratosis. Asian children can be regarded with a lower level of suspicion for tinea capitis, similar to white patients in the United States. Variation in incidence of tinea capitis does exist worldwide and the practitioner may need to address these issues in patients who travel or are recent immigrants.
When identifying tinea capitis infections in children with skin of color, physicians should consider the patient’s personal and family history, comorbid skin disorders, dermoscopy, microscopy and fungal staining, and fungal culture (Figure).
A paradigm for the diagnosis of scalp hyperkeratosis in children with skin of color.
Personal and Family History
The first diagnostic consideration is the patient’s personal and family history. A history of AD, asthma, or allergies will support but not confirm the diagnosis of AD. Prior tinea capitis infections and household contacts with tinea infections support the presence of tinea capitis.17 Recent implementation of anti–tumor necrosis factor a inhibitor therapy in a psoriatic child can flare scalp disease, mimicking tinea capitis.28 The patient’s guardians should be queried about potential infectious contacts, whether they themselves have signs of scalp disease or tinea corporis (ringworm) or whether they have a pet with problematic fur. Physicians also should query patients and their guardians about recent use of topical antifungal shampoos, pomades, creams (both over-the-counter [OTC] and prescription), and/or oral antifungals. When these agents are used, there is a possibility that fungal examinations may be negative in the presence of true infection with tinea capitis. Traction alopecia, often preceded by fine scale, is more likely to present in patients who wear their hair in cornrows, while seborrheic dermatitis may be associated with hair extensions, reduced frequency of washing (61% of black girls surveyed wash every 2 weeks), and/or reduced usage of hair oils in black girls.24 Knowledge of the patient’s personal hair care history, such as use of pomades; frequency and method of washing/drying hair; types of hair care products used daily to wash and style hair; use of chemical relaxers; or recent hairstyling with cornrows, braids, or hair extensions, also is essential to the diagnosis of tinea capitis. Usage of traction-related styling practices in patients with chemically relaxed hair can enhance the risk for traction alopecia.29
Comorbid Skin Disorders
The patient also should be examined for comorbid skin disorders, including tinea corporis, alopecia (particularly in the areas of hyperkeratosis), and the presence of nuchal lymphadenopathy. For each extra clinical finding, the chances of a final diagnosis of tinea capitis rises, allowing for empiric diagnosis to be made that can be confirmed by a variety of tests.1-3
Dermoscopy
Next, the patient should undergo dermoscopic evaluation. On dermoscopy, tinea capitis typically presents with broken hairs, black dots on the scalp, comma-shaped hairs, and short corkscrew hairs, all of which should clear with therapy.30-33 Dermoscopic findings of AD would reveal underlying xerosis and prominent vasculature due to inflammation, and alopecia areata would present with yellow dots at the orifices of the hair follicles, exclamation point hairs, and vellus hairs.34,35 Traction alopecia may be noted by retained hairs along the hairline, which is known as the fringe sign.36
Microscopy and Fungal Staining
Microscopic preparations can be performed to identify tinea capitis using fungal stains of slide-based specimens. Breakage of short hairs onto the slide and/or cotton swab is a soft sign corroborating endothrix infection of the hairs. Potassium hydroxide can enhance visualization of the hyperkeratotic scalp, but for most black patients, use of antifungal agents reduces fungal hyphae and spores in the areas of hyperkeratosis and may limit the utility of examining the skin microscopically. Assessment of the broken hairs obtained by gentle friction with one glass slide and catching the scales onto another glass slide may yield the best results in the evaluation of tinea capitis (a technique taught to me by Robin Hornung, MD, Everett, Washington). Hairs obtained in this manner often are fragile and break due to endothrix infection replacing and weakening the shaft of the hairs. In the United States, fungal samples usually are obtained with cotton swabs, but a recent study suggested that brushing is superior to scraping to obtain samples; the combination of sampling techniques may improve the yield of a culture.37 Because topical agents are unable to enter the hair cortex, the hair shaft is the most likely to show fungal spores under the microscope when antifungal shampoos or pomades are used. Other testing methods such as Swartz-Lamkins or calcofluor white staining can be used on similar scrapings. Biopsy and periodic acid–Schiff staining of thick scales or crust can help differentiate tinea capitis from pityriasis amiantacea when the crust is too thick to be softened via potassium hydroxide preparation.38
Fungal Culture
Fungal culture onto media that contains nutrients for dermatophyte growth can be used for 4 purposes in tinea capitis: (1) to confirm infection, (2) to identify species of infection, (3) to confirm mycological cure when difficulty in clearance of disease has been noted, and (4) to obtain a specimen for sensitivity screening regarding antifungals when necessary, an uncommon but occasionally useful test in individuals with disease that has failed treatment with 1 or more antifungals.27
THERAPY FOR SCALP HYPERKERATOSIS IN CHILDREN WITH SKIN OF COLOR
In patients with scalp hyperkeratosis, it is important to address the specific cause of the disease. Therapy for scalp hyperkeratosis in children with skin of color includes altered hair care practices, use of OTC and prescription agents, and containment of fomites in the case of infections. Biopsy of atypical scalp hyperkeratosis cases is needed to diagnose rare etiologies such as discoid lupus or Langerhans cell histiocytosis. For individuals with systemic disease including Langerhans cell histiocytosis, which is generally accompanied by nodes and plaques in the inguinal region or other intertriginous sites, immediate hematology and oncology workup is required.39 For collagen vascular diseases such as lupus or dermatomyositis, appropriate referral to rheumatology and systemic therapy is warranted.
Altered Hair Care Practices
The use of prophylactic ketoconazole 1% shampoo may not reduce the risk for recurrence of tinea capitis over standard good hygiene, removal of fomites, and adherence to prescribed therapy.40 Use of selenium sulfide has been shown to effectively reduce contagion risk.41
Fragrance- and dye-free shampoos can be helpful in providing gentle cleansing of the scalp, which is especially important in Asian patients who have greater facial and eyelid sensitivity. Free-and-clear shampoos can be used alternatively with shampoos containing selenium sulfide or sulfur to eliminate comorbid seborrhea. Black patients should be advised to shampoo and condition their hair once weekly, and Asian and Hispanic patients should shampoo and condition 2 to 3 times weekly to remove scale and potentially reduce risk for tinea acquisition.42 Children with straight hair should shampoo with increased frequency in the summer to manually remove sweat-induced macerated hyperkeratosis. Conditioners also should be used consistently after shampooing to enhance hair health.
Use of OTC and Prescription Agents
Atopic Dermatitis
Topical corticosteroid agents can be used in increasing strengths to treat AD of the scalp in children with skin of color, from OTC scalp products containing hydrocortisone 1% to prescription-based agents. Hydration of the hair also is needed to counteract reduced water content.43 Due to the innate xerosis of the scalp in black patients and atopic patients, the use of oil-based or lotion products may provide the most hydration for patients with scalp disease.44 Alcohol-based agents, either drops or foams, may enhance xerosis and should be used sparingly.
Seborrheic Dermatitis
Alternating treatment with medicated shampoos containing selenium sulfide and ketoconazole can aid in the removal of seborrhea. Pomades including borage seed oil–based agents can be massaged into the scalp,45 particularly for treatment of infantile seborrhea, and should not necessarily be washed off daily in dark-skinned patients. Additional focused application of topical corticosteroids to the scalp also is helpful. Due to innate scalp xerosis in black children, therapy should be similar to AD.
Psoriasis
In the setting of pityriasis amiantacea, albeit rare in children with skin of color, oil-based agents can soften hyperkeratosis for removal. Sterile mineral oil or commercially available scalp preparations of peanut oil with fluocinolone or mineral oil with glycerin can aid in the removal of scales without harming the hair, but usage must be age appropriate. The addition of focused application of age-appropriate topical corticosteroids for areas of severe hyperkeratosis can aid in clearance of the lesions.44 Recently, a stable combination of calcipo-triene 0.005%–betamethasone dipropionate 0.064% has been approved in the United States for the therapy of scalp psoriasis in adolescents.46
Tinea Capitis
Antifungal shampoos including selenium sulfide will reduce contagion risk when used by both the patient and his/her family members. Frequency of shampooing is similar to that described for AD. Between shampooing, pomades with selenium sulfide can be applied to the scalp to enhance overall clearance.
Oral antifungals are the basis of treatment and use of griseofulvin is the gold standard. Terbinafine has been approved by the US Food and Drug Administration for treatment of tinea capitis; for children weighing less than 25 kg the dosage is 125 mg daily, for 25 to 35 kg the dosage is 187.5 mg daily, and for more than 35 kg the dosage is 250 mg daily. Shorter therapeutic courses may be required, making it a good second-line agent. Laboratory screening in children prior to therapy is not always performed but should be done in cases where fatty liver might be suspected.47 Monitoring liver function tests is best when exceeding 3 months of usage or shifting from one antifungal to another.3
Containment of Fomites
There are several procedures that should be followed to contain scalp infection in children with skin of color. First, all objects that come into contact with the scalp (eg, hats, hoods, brushes, pillowcases) should be washed with hot water or replaced weekly. Sharing these objects with friends or family should be strongly discouraged. Patients and their family members also should be instructed to use medicated (eg, selenium sulfide) shampoos and conditioners. Finally, patients are advised to avoid use of shared classroom garments or mats for sleeping.
LONG-TERM SEQUELAE OF SCALP HYPERKERATOSIS
Long-term sequelae of scalp hyperkeratosis often are discounted in children, but the disease can have lasting and damaging effects on the scalp. Sequelae include discomfort from chronicity and psychological distress. In particular, years of scalp pruritus can promote lichenification of the scalp and miniaturization of the hair follicles. Furthermore, itching due to sweating can limit participation in sports. Finally, tinea capitis is thought to be a risk factor for central centrifugal cicatricial alopecia (or can occur comorbidly with central centrifugal cicatricial alopecia causing severe pruritus), a chronic scarring hair loss that is seen primarily in black adult females.48 Erythema nodosum also has been reported as an associated finding in the case of kerion.49 One study reported associated findings that included thyroid cancer in individuals irradiated for tinea capitis in the 1950s.50
Conclusion
Scalp hyperkeratosis in children with skin of color, especially black patients, is more likely to be associated with tinea capitis and is more challenging to treat due to innate scalp xerosis in black patients and increased sensitivity of facial skin in Asian children. Ultimately, institution of therapy when needed and good scalp and hair care may prevent long-term sequelae.
Scalp hyperkeratosis (scaling or flaking) is a common symptom in childhood and is typified by fine to thick hyperkeratosis of the scalp with or without underlying erythema. The causes of scalp hyperkeratosis in childhood vary based on the demographics of the population. In a population where approximately half of the pediatric patients were white, scaling of the scalp was more common in patients with seborrheic dermatitis and/or atopic dermatitis (AD) who were aged 0 to 2 years, and tinea capitis was only noted in children who were black.1 In children with skin of color, scalp hyperkeratosis has been noted as a marker of tinea capitis, especially in patients aged 3 to 11 years,2,3 and the level of suspicion should consistently remain high for this age group. In another study of an all-black population of schoolchildren aged 5 to 13 years (N=224), 3% demonstrated signs and symptoms of tinea capitis and 14% were found to be asymptomatic carriers.4 Although generally benign in nature, scalp hyperkeratosis can be associated with systemic illnesses such as juvenile dermatomyositis and Langerhans cell histiocytosis.5 This article addresses the diagnosis and treatment of scalp hyperkeratosis in children with skin of color, focusing on differences in exposure to contagious cases, hairstyling practices, and biological factors that may impact the disease process.
CAUSES OF SCALP HYPERKERATOSIS IN CHILDHOOD
Scalp hyperkeratosis in childhood usually is caused by common benign conditions, but some level of suspicion should be maintained for more severe etiologic conditions such as Langerhans cell histiocytosis and collagen vascular diseases (eg, juvenile dermatomyositis).6 Langerhans cell histiocytosis of the scalp might be obscured by background pigmentation in black children.
Scalp scaling can be a minor criterion in the diagnosis of AD. Atopic dermatitis should be suspected in Asian children with scalp scaling. Although one study in Bangladesh revealed scalp involvement in only 5.2% of pediatric patients with AD,7 a study in China reported an incidence rate as high as 49.7% (with a similarly high incidence of eyelid dermatitis).8 Children with AD also may have dry hair.9 Atopic dermatitis of the scalp is typified by itching, fine hyperkeratosis, and notably eczematous scalp lesions ranging from excoriated or oozing erythematous plaques to lichenification with hair miniaturization, primarily from scratch-induced breakage.10 The latter finding often is noted in black adolescent girls with long-term moderate to severe AD (personal observation).
Seborrheic dermatitis is a hypersensitivity response to yeast colonization of the scalp with Malassezia species. The infantile form is extremely common (also known as cradle cap). Characteristically, greasy yellow hyperkeratosis in fine to thick sheets is noted on the scalp in children younger than 2 years, especially infants, often with involvement of skin folds. One study noted that seborrheic dermatitis occurs in 6% of school-aged children as opposed to 19% of children younger than 2 years.1 Severe seborrheic dermatitis in infancy may be a prelude to AD, with the incidence being 3 times higher in children with prior seborrheic dermatitis.11 In teenagers, seborrheic dermatitis often accompanies acne onset in the early pubertal years.12
Psoriasis is an autoimmune inflammatory dermatosis that most commonly affects white children. In childhood, pityriasis amiantacea, psoriasiform scalp hyperkeratosis, is more common than in adulthood, with thick, stuck-on scales bound to the hairs. This variant is uncommon in Hispanic and Asian children and is almost never seen in black children but has been reported in cohorts of Turkish children.13 In a series of 85 Egyptian children with pityriasis amiantacea, diagnosis of scalp psoriasis was made in 35.3%, eczematous dermatitis in 34.2%, and tinea capitis in 12.9%.14 Consequently, a high degree of suspicion for tinea capitis should be held if pityriasis amiantacea is found in children with skin of color.15,16
Tinea capitis is a dermatophyte infection of the scalp, hair, and surrounding skin. The presence of tinea capitis on the scalp is associated with environmental exposure to dermatophytes (eg, school, household).4,17 The infection is largely caused by Trichophyton tonsurans in the United States, which causes a seborrheic appearance and less commonly alopecia (black dot or thinning), plaques with scale, or kerion. The presence of cervical lymph nodes and/or alopecia increases the chances of tinea being the diagnosis. Potassium hydroxide preparation and fungal culture can be performed to corroborate the diagnosis.1-3 Other etiologies of scalp hyperkeratosis such as juvenile pityriasis rubra pilaris and lice are extremely uncommon in black children, but lice may be seen in Hispanic and Asian girls with long straight hair who attend school. Discoid lupus is more common in children with skin of color but is rare overall. When noted, accompanying mottled dyspigmentation and scarring alopecia are noted in addition to a high risk for developing systemic lupus erythematosus. Biopsy and screening for systemic lupus are necessary, as the risk for progression from discoid lupus to systemic disease is 26% over 3 years.18
THE BIOLOGY OF HAIR IN CHILDREN WITH SKIN OF COLOR
To some extent, the biology of hair impacts the occurrence, appearance, and treatment of scalp hyperkeratosis in children with skin of color. First, it is important to remember that follicular density is lower in black patients as compared to Asian patients with a consequently lower hair count overall, which results in the easy appearance of hair loss, particularly at the margins of the scalp.19,20 Second, the shape of the hair follicle differs among races and ethnicities. Asian patients have round hair shafts coming from straight follicles, which allows for greater natural hair hydration, resulting in somewhat less aggressive scalp disease. Hispanic patients may have similarly straight hair or may have elliptical or curled shafts, the latter being noted in black patients. Furthermore, a curled hair shaft results in poor flow of sebum across the hair, resulting in greater scalp xerosis, more susceptibility to traction alopecia, and ultimately a greater risk for infections.20-23 Finally, the scalp is continuous with the face and neck, and Asian patients have greater sensitivity to skin care products in these areas, resulting in difficulty of treatment in this patient population and the need for use of gentle products.
HAIR CARE PRACTICES IN CHILDREN WITH SKIN OF COLOR
Hair care in patients with skin of color can be costly, difficult, and potentially damaging, with 99% of black girls reporting pomade or oil usage. Costly and complex hair care practices begin in childhood for patients with skin of color. In a series of 201 surveyed black girls with a mean age of 9.8 years, 80% had used hot combs and 42% used relaxers.24 Traction styles were common with 81% using ponytails, 67% braids, and 49% cornrows in the last 12 months. These styles are thought to affect hair health, particularly through induction of traction-related damage, folliculitis, and alopecia. Furthermore, chemical relaxers, hot combs, blowouts, and hair setting may be introduced during childhood.24 These practices appear to disturb the integrity of the hair follicle, leaving it more susceptible to irritation and infection.
Hair care in the pediatric population often is complicated by the fact that multiple children are being styled in tandem, either at home or in a salon, resulting in shared equipment and fomite spread. Even just proximity to a case of tinea capitis in the household will increase risk for tinea capitis. Furthermore, it is quite commonplace for black patients to use pomades and shampoos that contain antifungals, especially selenium sulfide, which makes it difficult to obtain accurate culture results. In India, use of mustard oil also has been linked to increased risk for tinea capitis.25
Other issues related to hair care include frequent dry scalp in patients with skin of color due to poor sebum distribution along the hair shaft. As a result, frequent washing may exacerbate scalp xerosis and further irritate seborrheic dermatitis and/or AD.
DIAGNOSTIC CONSIDERATIONS FOR SCALP HYPERKERATOSIS IN CHILDHOOD
Dermatologists should have a greater level of suspicion for tinea capitis in black and Hispanic children compared to white children. The index of suspicion should be high given that antifungal shampoos and pomades may minimize the clinical appearance. Although trends in overall incidence in the United States suggest tinea capitis is becoming less common, there still is a stronger representation of the disease in black patients.26 A study of positive fungal cultures from one clinic in Mississippi (N=1220) showed that two-thirds of patients were children younger than 13 years; 87% of patients with positive cultures for dermatophytes were black.27 The endothrix type of tinea capitis caused by T tonsurans often presents with a seborrheic appearance, and fungal culture is warranted in all pediatric patients with skin of color who have scalp hyperkeratosis. Asian children can be regarded with a lower level of suspicion for tinea capitis, similar to white patients in the United States. Variation in incidence of tinea capitis does exist worldwide and the practitioner may need to address these issues in patients who travel or are recent immigrants.
When identifying tinea capitis infections in children with skin of color, physicians should consider the patient’s personal and family history, comorbid skin disorders, dermoscopy, microscopy and fungal staining, and fungal culture (Figure).
A paradigm for the diagnosis of scalp hyperkeratosis in children with skin of color.
Personal and Family History
The first diagnostic consideration is the patient’s personal and family history. A history of AD, asthma, or allergies will support but not confirm the diagnosis of AD. Prior tinea capitis infections and household contacts with tinea infections support the presence of tinea capitis.17 Recent implementation of anti–tumor necrosis factor a inhibitor therapy in a psoriatic child can flare scalp disease, mimicking tinea capitis.28 The patient’s guardians should be queried about potential infectious contacts, whether they themselves have signs of scalp disease or tinea corporis (ringworm) or whether they have a pet with problematic fur. Physicians also should query patients and their guardians about recent use of topical antifungal shampoos, pomades, creams (both over-the-counter [OTC] and prescription), and/or oral antifungals. When these agents are used, there is a possibility that fungal examinations may be negative in the presence of true infection with tinea capitis. Traction alopecia, often preceded by fine scale, is more likely to present in patients who wear their hair in cornrows, while seborrheic dermatitis may be associated with hair extensions, reduced frequency of washing (61% of black girls surveyed wash every 2 weeks), and/or reduced usage of hair oils in black girls.24 Knowledge of the patient’s personal hair care history, such as use of pomades; frequency and method of washing/drying hair; types of hair care products used daily to wash and style hair; use of chemical relaxers; or recent hairstyling with cornrows, braids, or hair extensions, also is essential to the diagnosis of tinea capitis. Usage of traction-related styling practices in patients with chemically relaxed hair can enhance the risk for traction alopecia.29
Comorbid Skin Disorders
The patient also should be examined for comorbid skin disorders, including tinea corporis, alopecia (particularly in the areas of hyperkeratosis), and the presence of nuchal lymphadenopathy. For each extra clinical finding, the chances of a final diagnosis of tinea capitis rises, allowing for empiric diagnosis to be made that can be confirmed by a variety of tests.1-3
Dermoscopy
Next, the patient should undergo dermoscopic evaluation. On dermoscopy, tinea capitis typically presents with broken hairs, black dots on the scalp, comma-shaped hairs, and short corkscrew hairs, all of which should clear with therapy.30-33 Dermoscopic findings of AD would reveal underlying xerosis and prominent vasculature due to inflammation, and alopecia areata would present with yellow dots at the orifices of the hair follicles, exclamation point hairs, and vellus hairs.34,35 Traction alopecia may be noted by retained hairs along the hairline, which is known as the fringe sign.36
Microscopy and Fungal Staining
Microscopic preparations can be performed to identify tinea capitis using fungal stains of slide-based specimens. Breakage of short hairs onto the slide and/or cotton swab is a soft sign corroborating endothrix infection of the hairs. Potassium hydroxide can enhance visualization of the hyperkeratotic scalp, but for most black patients, use of antifungal agents reduces fungal hyphae and spores in the areas of hyperkeratosis and may limit the utility of examining the skin microscopically. Assessment of the broken hairs obtained by gentle friction with one glass slide and catching the scales onto another glass slide may yield the best results in the evaluation of tinea capitis (a technique taught to me by Robin Hornung, MD, Everett, Washington). Hairs obtained in this manner often are fragile and break due to endothrix infection replacing and weakening the shaft of the hairs. In the United States, fungal samples usually are obtained with cotton swabs, but a recent study suggested that brushing is superior to scraping to obtain samples; the combination of sampling techniques may improve the yield of a culture.37 Because topical agents are unable to enter the hair cortex, the hair shaft is the most likely to show fungal spores under the microscope when antifungal shampoos or pomades are used. Other testing methods such as Swartz-Lamkins or calcofluor white staining can be used on similar scrapings. Biopsy and periodic acid–Schiff staining of thick scales or crust can help differentiate tinea capitis from pityriasis amiantacea when the crust is too thick to be softened via potassium hydroxide preparation.38
Fungal Culture
Fungal culture onto media that contains nutrients for dermatophyte growth can be used for 4 purposes in tinea capitis: (1) to confirm infection, (2) to identify species of infection, (3) to confirm mycological cure when difficulty in clearance of disease has been noted, and (4) to obtain a specimen for sensitivity screening regarding antifungals when necessary, an uncommon but occasionally useful test in individuals with disease that has failed treatment with 1 or more antifungals.27
THERAPY FOR SCALP HYPERKERATOSIS IN CHILDREN WITH SKIN OF COLOR
In patients with scalp hyperkeratosis, it is important to address the specific cause of the disease. Therapy for scalp hyperkeratosis in children with skin of color includes altered hair care practices, use of OTC and prescription agents, and containment of fomites in the case of infections. Biopsy of atypical scalp hyperkeratosis cases is needed to diagnose rare etiologies such as discoid lupus or Langerhans cell histiocytosis. For individuals with systemic disease including Langerhans cell histiocytosis, which is generally accompanied by nodes and plaques in the inguinal region or other intertriginous sites, immediate hematology and oncology workup is required.39 For collagen vascular diseases such as lupus or dermatomyositis, appropriate referral to rheumatology and systemic therapy is warranted.
Altered Hair Care Practices
The use of prophylactic ketoconazole 1% shampoo may not reduce the risk for recurrence of tinea capitis over standard good hygiene, removal of fomites, and adherence to prescribed therapy.40 Use of selenium sulfide has been shown to effectively reduce contagion risk.41
Fragrance- and dye-free shampoos can be helpful in providing gentle cleansing of the scalp, which is especially important in Asian patients who have greater facial and eyelid sensitivity. Free-and-clear shampoos can be used alternatively with shampoos containing selenium sulfide or sulfur to eliminate comorbid seborrhea. Black patients should be advised to shampoo and condition their hair once weekly, and Asian and Hispanic patients should shampoo and condition 2 to 3 times weekly to remove scale and potentially reduce risk for tinea acquisition.42 Children with straight hair should shampoo with increased frequency in the summer to manually remove sweat-induced macerated hyperkeratosis. Conditioners also should be used consistently after shampooing to enhance hair health.
Use of OTC and Prescription Agents
Atopic Dermatitis
Topical corticosteroid agents can be used in increasing strengths to treat AD of the scalp in children with skin of color, from OTC scalp products containing hydrocortisone 1% to prescription-based agents. Hydration of the hair also is needed to counteract reduced water content.43 Due to the innate xerosis of the scalp in black patients and atopic patients, the use of oil-based or lotion products may provide the most hydration for patients with scalp disease.44 Alcohol-based agents, either drops or foams, may enhance xerosis and should be used sparingly.
Seborrheic Dermatitis
Alternating treatment with medicated shampoos containing selenium sulfide and ketoconazole can aid in the removal of seborrhea. Pomades including borage seed oil–based agents can be massaged into the scalp,45 particularly for treatment of infantile seborrhea, and should not necessarily be washed off daily in dark-skinned patients. Additional focused application of topical corticosteroids to the scalp also is helpful. Due to innate scalp xerosis in black children, therapy should be similar to AD.
Psoriasis
In the setting of pityriasis amiantacea, albeit rare in children with skin of color, oil-based agents can soften hyperkeratosis for removal. Sterile mineral oil or commercially available scalp preparations of peanut oil with fluocinolone or mineral oil with glycerin can aid in the removal of scales without harming the hair, but usage must be age appropriate. The addition of focused application of age-appropriate topical corticosteroids for areas of severe hyperkeratosis can aid in clearance of the lesions.44 Recently, a stable combination of calcipo-triene 0.005%–betamethasone dipropionate 0.064% has been approved in the United States for the therapy of scalp psoriasis in adolescents.46
Tinea Capitis
Antifungal shampoos including selenium sulfide will reduce contagion risk when used by both the patient and his/her family members. Frequency of shampooing is similar to that described for AD. Between shampooing, pomades with selenium sulfide can be applied to the scalp to enhance overall clearance.
Oral antifungals are the basis of treatment and use of griseofulvin is the gold standard. Terbinafine has been approved by the US Food and Drug Administration for treatment of tinea capitis; for children weighing less than 25 kg the dosage is 125 mg daily, for 25 to 35 kg the dosage is 187.5 mg daily, and for more than 35 kg the dosage is 250 mg daily. Shorter therapeutic courses may be required, making it a good second-line agent. Laboratory screening in children prior to therapy is not always performed but should be done in cases where fatty liver might be suspected.47 Monitoring liver function tests is best when exceeding 3 months of usage or shifting from one antifungal to another.3
Containment of Fomites
There are several procedures that should be followed to contain scalp infection in children with skin of color. First, all objects that come into contact with the scalp (eg, hats, hoods, brushes, pillowcases) should be washed with hot water or replaced weekly. Sharing these objects with friends or family should be strongly discouraged. Patients and their family members also should be instructed to use medicated (eg, selenium sulfide) shampoos and conditioners. Finally, patients are advised to avoid use of shared classroom garments or mats for sleeping.
LONG-TERM SEQUELAE OF SCALP HYPERKERATOSIS
Long-term sequelae of scalp hyperkeratosis often are discounted in children, but the disease can have lasting and damaging effects on the scalp. Sequelae include discomfort from chronicity and psychological distress. In particular, years of scalp pruritus can promote lichenification of the scalp and miniaturization of the hair follicles. Furthermore, itching due to sweating can limit participation in sports. Finally, tinea capitis is thought to be a risk factor for central centrifugal cicatricial alopecia (or can occur comorbidly with central centrifugal cicatricial alopecia causing severe pruritus), a chronic scarring hair loss that is seen primarily in black adult females.48 Erythema nodosum also has been reported as an associated finding in the case of kerion.49 One study reported associated findings that included thyroid cancer in individuals irradiated for tinea capitis in the 1950s.50
Conclusion
Scalp hyperkeratosis in children with skin of color, especially black patients, is more likely to be associated with tinea capitis and is more challenging to treat due to innate scalp xerosis in black patients and increased sensitivity of facial skin in Asian children. Ultimately, institution of therapy when needed and good scalp and hair care may prevent long-term sequelae.
1. Williams JV, Eichenfield LF, Burke BL, et al. Prevalence of scalp scaling in prepubertal children. Pediatrics. 2005;115:e1-e6.
2. Coley MK, Bhanusali DG, Silverberg JI, et al. Scalp hyperkeratosis and alopecia in children of color. J Drugs Dermatol. 2011;10:511-516.
3. Bhanusali D, Coley M, Silverberg JI, et al. Treatment outcomes for tinea capitis in a skin of color population. J Drugs Dermatol. 2012;11:852-856.
4. Williams JV, Honig PJ, McGinley KJ, et al. Semiquantitative study of tinea capitis and the asymptomatic carrier state in inner-city school children. Pediatrics. 1995;96:265-267.
5. McDonald LL, Smith ML. Diagnostic dilemmas in pediatric/adolescent dermatology: scaly scalp. J Pediatr Health Care. 1998;12:80-84.
6. Peloro TM, Miller OF 3rd, Hahn TF, et al. Juvenile dermatomyositis: a retrospective review of a 30-year experience. J Am Acad Dermatol. 2001;45:28-34.
7. Wahab MA, Rahman MH, Khondker L, et al. Minor criteria for atopic dermatitis in children. Mymensingh Med J. 2011;20:419-424.
8. Shi M, Zhang H, Chen X, et al. Clinical features of atopic dermatitis in a hospital-based setting in China. J Eur Acad Dermatol Venereol [published online ahead of print January 9, 2011]. 2011;25:1206-1212.
9. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair. Microsc Res Tech. 2012;75:620-625.
10. Sabin BR, Peters N, Peters AT. Chapter 20: atopic dermatitis. Allergy Asthma Proc. 2012;33:S67-S69.
11. Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis [published online ahead of print November 13, 2013]. Pediatr Dermatol. 2014;31:125-130.
12. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
13. Sarifakioglu E, Yilmaz AE, Gorpelioglu C, et al. Prevalence of scalp disorders and hair loss in children. Cutis. 2012;90:225-229.
14. Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
15. Oostveen AM, Jong EM, Evers AW, et al. Reliability, responsiveness and validity of Scalpdex in children with scalp psoriasis: the Dutch study. Acta Derm Venereol. 2014;94:198-202.
16. Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity: Comparative Dermatologic Atlas of Pediatric Skin of All Colors. New York, NY: Springer; 2012.
17. Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
18. Moises-Alfaro C, Berrón-Pérez R, Carrasco-Daza D, et al. Discoid lupus erythematosus in children: clinical, histopathologic, and follow-up features in 27 cases. Pediatr Dermatol. 2003;20:103-107.
19. Ramos-e-Silva M. Ethnic hair and skin: what is the state of the science? Chicago, Illinois—September 29-30, 2001. Clin Dermatol. 2002;20:321-324.
20. Heath CR, McMichael AJ. Biology of hair follicle. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. New York, NY: McGraw Hill; 2009:105-109.
21. Khumalo NP. African hair morphology: macrostructure to ultrastructure. Int J Dermatol. 2005;44(suppl 1):10-12.
22. Thibaut S, Bernard BA. The biology of hair shape. Int J Dermatol. 2005;44(suppl 1):2-3.
23. Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
24. Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
25. Kumar V, Sharma RC, Chander R. Clinicomycological study of tinea capitis. Indian J Dermatol Venereol Leprol. 1996;62:207-209.
26. Mirmirani P, Tucker LY. Epidemiologic trends in pediatric tinea capitis: a population-based study from Kaiser Permanente Northern California [published online ahead of print October 2, 2013]. J Am Acad Dermatol. 2013;69:916-921.
27. Chapman JC, Daniel CR 3rd, Daniel JG, et al. Tinea capitis caused by dermatophytes: a 15-year retrospective study from a Mississippi Dermatology Clinic. Cutis. 2011;88:230-233.
28. Perman MJ, Lovell DJ, Denson LA, et al. Five cases of anti-tumor necrosis factor alpha-induced psoriasis presenting with severe scalp involvement in children. Pediatr Dermatol. 2012;29:454-459.
29. Khumalo NP, Jessop S, Gumedze F, et al. Determinants of marginal traction alopecia in African girls and women. J Am Acad Dermatol. 2008;59:432-438.
30. Vazquez-Lopez F, Palacios-Garcia L, Argenziano G. Dermoscopic corkscrew hairs dissolve after successful therapy of Trichophyton violaceum tinea capitis: a case report. Australas J Dermatol. 2012;53:118-119.
31. Pinheiro AM, Lobato LA, Varella TC. Dermoscopy findings in tinea capitis: case report and literature review. An Bras Dermatol. 2012;87:313-314.
32. Mapelli ET, Gualandri L, Cerri A, et al. Comma hairs in tinea capitis: a useful dermatoscopic sign for diagnosis of tinea capitis. Pediatr Dermatol. 2012;29:223-224.
33. Hughes R, Chiaverini C, Bahadoran P, et al. Corkscrew hair: a new dermoscopic sign for diagnosis of tinea capitis in black children. Arch Dermatol. 2011;147:355-356.
34. Ekiz O, Sen BB, Rifaiog˘lu EN, et al. Trichoscopy in paediatric patients with tinea capitis: a useful method to differentiate from alopecia areata [published online ahead of print August 24, 2013]. J Eur Acad Dermatol Venereol. 2014;28:1255-1258.
35. Lencastre A, Tosti A. Role of trichoscopy in children’s scalp and hair disorders [published online ahead of print Aug 13, 2013]. Pediatr Dermatol. 2013;30:674-682.
36. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
37. Nasir S, Ralph N, O’Neill C, et al. Trends in tinea capitis in an Irish pediatric population and a comparison of scalp brushings versus scalp scrapings as methods of investigation [published online ahead of print February 22, 2013]. Pediatr Dermatol. 2014;31:622-623.
38. Alvarez MS, Silverberg NB. Tinea capitis. Cutis. 2006;78:189-196.
39. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996.
40. Bookstaver PB, Watson HJ, Winters SD, et al. Prophylactic ketoconazole shampoo for tinea capitis in a high-risk pediatric population. J Pediatr Pharmacol Ther. 2011;16:199-203.
41. Allen HB, Honig PJ, Leyden JJ, et al. Selenium sulfide: adjunctive therapy for tinea capitis. Pediatrics. 1982;69:81-83.
42. Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
43. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair [published online ahead of print November 7, 2011]. Microsc Res Tech. 2012;75:620-625.
44. Kapila S, Hong E, Fischer G. A comparative study of childhood psoriasis and atopic dermatitis and greater understanding of the overlapping condition, psoriasis-dermatitis. Australas J Dermatol. 2012;53:98-105.
45. Tollesson A, Frithz A. Borage oil, an effective new treatment for infantile seborrhoeic dermatitis. Br J Dermatol. 1993;129:95.
46. Gooderham M, Debarre JM, Keddy-Grant J, et al. Safety and efficacy of calcipotriol plus betamethasone dipropionate gel in the treatment of scalp psoriasis in adolescents 12-17 years of age [published online ahead of print October 22, 2014]. Br J Dermatol. 2014;171:1470-1477.
47. Singer C, Stancu P, Coşoveanu S, et al. Non-alcoholic fatty liver disease in children. Curr Health Sci J. 2014;40:170-176.
48. Chiang C, Price V, Mirmirani P. Central centrifugal cicatricial alopecia: superimposed tinea capitis as the etiology of chronic scalp pruritus. Dermatol Online J. 2008;14:3.
49. Morrone A, Calcaterra R, Valenzano M, et al. Erythema nodosum induced by kerion celsi of the scalp in a woman. Mycoses. 2011;54:e237-e239.
50. Boaventura P, Pereira D, Celestino R, et al. Genetic alterations in thyroid tumors from patients irradiated in childhood for tinea capitis treatment. Eur J Endocrinol. 2013;169:673-679.
1. Williams JV, Eichenfield LF, Burke BL, et al. Prevalence of scalp scaling in prepubertal children. Pediatrics. 2005;115:e1-e6.
2. Coley MK, Bhanusali DG, Silverberg JI, et al. Scalp hyperkeratosis and alopecia in children of color. J Drugs Dermatol. 2011;10:511-516.
3. Bhanusali D, Coley M, Silverberg JI, et al. Treatment outcomes for tinea capitis in a skin of color population. J Drugs Dermatol. 2012;11:852-856.
4. Williams JV, Honig PJ, McGinley KJ, et al. Semiquantitative study of tinea capitis and the asymptomatic carrier state in inner-city school children. Pediatrics. 1995;96:265-267.
5. McDonald LL, Smith ML. Diagnostic dilemmas in pediatric/adolescent dermatology: scaly scalp. J Pediatr Health Care. 1998;12:80-84.
6. Peloro TM, Miller OF 3rd, Hahn TF, et al. Juvenile dermatomyositis: a retrospective review of a 30-year experience. J Am Acad Dermatol. 2001;45:28-34.
7. Wahab MA, Rahman MH, Khondker L, et al. Minor criteria for atopic dermatitis in children. Mymensingh Med J. 2011;20:419-424.
8. Shi M, Zhang H, Chen X, et al. Clinical features of atopic dermatitis in a hospital-based setting in China. J Eur Acad Dermatol Venereol [published online ahead of print January 9, 2011]. 2011;25:1206-1212.
9. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair. Microsc Res Tech. 2012;75:620-625.
10. Sabin BR, Peters N, Peters AT. Chapter 20: atopic dermatitis. Allergy Asthma Proc. 2012;33:S67-S69.
11. Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis [published online ahead of print November 13, 2013]. Pediatr Dermatol. 2014;31:125-130.
12. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
13. Sarifakioglu E, Yilmaz AE, Gorpelioglu C, et al. Prevalence of scalp disorders and hair loss in children. Cutis. 2012;90:225-229.
14. Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
15. Oostveen AM, Jong EM, Evers AW, et al. Reliability, responsiveness and validity of Scalpdex in children with scalp psoriasis: the Dutch study. Acta Derm Venereol. 2014;94:198-202.
16. Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity: Comparative Dermatologic Atlas of Pediatric Skin of All Colors. New York, NY: Springer; 2012.
17. Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
18. Moises-Alfaro C, Berrón-Pérez R, Carrasco-Daza D, et al. Discoid lupus erythematosus in children: clinical, histopathologic, and follow-up features in 27 cases. Pediatr Dermatol. 2003;20:103-107.
19. Ramos-e-Silva M. Ethnic hair and skin: what is the state of the science? Chicago, Illinois—September 29-30, 2001. Clin Dermatol. 2002;20:321-324.
20. Heath CR, McMichael AJ. Biology of hair follicle. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. New York, NY: McGraw Hill; 2009:105-109.
21. Khumalo NP. African hair morphology: macrostructure to ultrastructure. Int J Dermatol. 2005;44(suppl 1):10-12.
22. Thibaut S, Bernard BA. The biology of hair shape. Int J Dermatol. 2005;44(suppl 1):2-3.
23. Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
24. Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
25. Kumar V, Sharma RC, Chander R. Clinicomycological study of tinea capitis. Indian J Dermatol Venereol Leprol. 1996;62:207-209.
26. Mirmirani P, Tucker LY. Epidemiologic trends in pediatric tinea capitis: a population-based study from Kaiser Permanente Northern California [published online ahead of print October 2, 2013]. J Am Acad Dermatol. 2013;69:916-921.
27. Chapman JC, Daniel CR 3rd, Daniel JG, et al. Tinea capitis caused by dermatophytes: a 15-year retrospective study from a Mississippi Dermatology Clinic. Cutis. 2011;88:230-233.
28. Perman MJ, Lovell DJ, Denson LA, et al. Five cases of anti-tumor necrosis factor alpha-induced psoriasis presenting with severe scalp involvement in children. Pediatr Dermatol. 2012;29:454-459.
29. Khumalo NP, Jessop S, Gumedze F, et al. Determinants of marginal traction alopecia in African girls and women. J Am Acad Dermatol. 2008;59:432-438.
30. Vazquez-Lopez F, Palacios-Garcia L, Argenziano G. Dermoscopic corkscrew hairs dissolve after successful therapy of Trichophyton violaceum tinea capitis: a case report. Australas J Dermatol. 2012;53:118-119.
31. Pinheiro AM, Lobato LA, Varella TC. Dermoscopy findings in tinea capitis: case report and literature review. An Bras Dermatol. 2012;87:313-314.
32. Mapelli ET, Gualandri L, Cerri A, et al. Comma hairs in tinea capitis: a useful dermatoscopic sign for diagnosis of tinea capitis. Pediatr Dermatol. 2012;29:223-224.
33. Hughes R, Chiaverini C, Bahadoran P, et al. Corkscrew hair: a new dermoscopic sign for diagnosis of tinea capitis in black children. Arch Dermatol. 2011;147:355-356.
34. Ekiz O, Sen BB, Rifaiog˘lu EN, et al. Trichoscopy in paediatric patients with tinea capitis: a useful method to differentiate from alopecia areata [published online ahead of print August 24, 2013]. J Eur Acad Dermatol Venereol. 2014;28:1255-1258.
35. Lencastre A, Tosti A. Role of trichoscopy in children’s scalp and hair disorders [published online ahead of print Aug 13, 2013]. Pediatr Dermatol. 2013;30:674-682.
36. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
37. Nasir S, Ralph N, O’Neill C, et al. Trends in tinea capitis in an Irish pediatric population and a comparison of scalp brushings versus scalp scrapings as methods of investigation [published online ahead of print February 22, 2013]. Pediatr Dermatol. 2014;31:622-623.
38. Alvarez MS, Silverberg NB. Tinea capitis. Cutis. 2006;78:189-196.
39. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996.
40. Bookstaver PB, Watson HJ, Winters SD, et al. Prophylactic ketoconazole shampoo for tinea capitis in a high-risk pediatric population. J Pediatr Pharmacol Ther. 2011;16:199-203.
41. Allen HB, Honig PJ, Leyden JJ, et al. Selenium sulfide: adjunctive therapy for tinea capitis. Pediatrics. 1982;69:81-83.
42. Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
43. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair [published online ahead of print November 7, 2011]. Microsc Res Tech. 2012;75:620-625.
44. Kapila S, Hong E, Fischer G. A comparative study of childhood psoriasis and atopic dermatitis and greater understanding of the overlapping condition, psoriasis-dermatitis. Australas J Dermatol. 2012;53:98-105.
45. Tollesson A, Frithz A. Borage oil, an effective new treatment for infantile seborrhoeic dermatitis. Br J Dermatol. 1993;129:95.
46. Gooderham M, Debarre JM, Keddy-Grant J, et al. Safety and efficacy of calcipotriol plus betamethasone dipropionate gel in the treatment of scalp psoriasis in adolescents 12-17 years of age [published online ahead of print October 22, 2014]. Br J Dermatol. 2014;171:1470-1477.
47. Singer C, Stancu P, Coşoveanu S, et al. Non-alcoholic fatty liver disease in children. Curr Health Sci J. 2014;40:170-176.
48. Chiang C, Price V, Mirmirani P. Central centrifugal cicatricial alopecia: superimposed tinea capitis as the etiology of chronic scalp pruritus. Dermatol Online J. 2008;14:3.
49. Morrone A, Calcaterra R, Valenzano M, et al. Erythema nodosum induced by kerion celsi of the scalp in a woman. Mycoses. 2011;54:e237-e239.
50. Boaventura P, Pereira D, Celestino R, et al. Genetic alterations in thyroid tumors from patients irradiated in childhood for tinea capitis treatment. Eur J Endocrinol. 2013;169:673-679.
Practice Points
- Scalp hyperkeratosis is a common finding in children, especially those with skin of color.
- Fungal culture may be helpful in the diagnosis of scalp hyperkeratosis in children of any age but should be performed in patients aged 3 to 11 years with skin of color.
- Therapy of scalp disease in children with skin of color should be adjusted based on hair type and disease features.
In Vivo Confocal Microscopy in the Diagnosis of Onychomycosis
To the Editor:
Onychomycosis is a common nail disease that frequently is caused by dermatophytes and is diagnosed by direct microscopy. Conventional diagnostic methods are often time consuming and can produce false-positive or false-negative results. We report a case of onychomycosis diagnosed by confocal microscopy and confirmed with routine potassium hydroxide (KOH) examination and fungal culture. Confocal microscopy is a reliable, practical, and noninvasive technique in the diagnosis of onychomycosis.
A 46-year-old woman presented with yellow-brown discoloration and dystrophy of the toenails (Figure 1) that had become worse over a 5-year period. She was otherwise healthy and had no other dermatologic problems. Examination revealed yellow-brown discoloration, subungual hyperkeratosis, and onycholysis of the toenails. Clinically, a diagnosis of onychomycosis was made. Potassium hydroxide examination of a scraping from the subungual region showed fungal elements. Trichophyton rubrum on Sabouraud dextrose agar was determined.
|
We performed both in vivo and in vitro confocal laser scanning microscopic examination of the nail of the right great toe (Figure 2). For the diagnosis of onychomycosis in our case, we used a multilaser reflectance confocal microscope (RCM) with a wavelength of 786 nm. In vivo confocal microscopy of the nail revealed branching hyphae just below the surface of the nail plate. Hyphae were seen as refractile, bright, linear structures along the laminates of the nail.
Onychomycosis is a common condition affecting 5.5% of the population worldwide and representing 20% to 40% of all onychopathies and approximately 30% of cutaneous mycotic infections.1,2 There are many methods available to confirm the clinical diagnosis of onychomycosis by detecting the causative organisms. Direct microscopic examination of the scraping with a KOH culture, histopathologic assessment with periodic acid–Schiff staining, immunofluorescence analysis with calcofluor white staining, enzyme analysis, and polymerase chain reaction can be used for diagnosis of fungal infections. The most frequently used diagnostic method for onychomycosis is KOH examination of the scraping; however, fungal culture and histopathologic examination also can be used in cases having diagnostic difficulties.1,3,4 There are many studies comparing the efficacies of these methods in the literature.5-9
The causative fungal agent should be determined with at least 1 laboratory method due to the high cost, long duration, and serious potential adverse effects of systemic antifungal treatment. Direct microscopic examination with KOH in the diagnosis of onychomycosis is simple, fast, and inexpensive. However, inadequate material, using crystallized KOH for hydrolysis, insufficient or too much hydrolysis of scrapings, inappropriate staining, and not scanning all areas in the microscopy produce false-negative results. Similarly, secondary contamination of hair, cotton, yarn, or air bubbles mimicking fungal structures can cause false-positive results.9,10
Fungal culture is another diagnostic method that is accepted as the gold standard for diagnosis of onychomycosis.9 However, fungal cultures were positive in only 43% to 50% of all cases of onychomycosis that were diagnosed with other methods,11,12 which may be due to the loss of viability and ability of the fungi to grow in culture media during the transport. A major advantage of fungal culture is that the fungal agent can be classified as dermatophyte, nondermatophyte, mold, or yeast. However, culture does determine if the growing fungi is contamination or the real pathogen. Moreover, it is necessary to wait 3 to 4 weeks for culture results. For nondermatophyte fungi this time may be much longer.12
In vivo RCM is a noninvasive imaging method that allows optical en face sectioning of the living tissue with high resolution. Currently, RCM has a wide range of applications, such as the evaluation of both benign and malignant skin lesions in clinical dermatology.13
In vivo RCM was used first by Hongcharu et al.14 The diagnoses of onychomycosis and fungal hyphae were shown both in vivo and in vitro.14 The sensitivity and specificity of confocal examination in the diagnosis of onychomycosis is not known yet. Large clinical trials are needed to assess the sensitivity and specificity of this method in diagnosing fungal infections.
Onychomycosis is a contagious infectious disease characterized by hyphae proliferation in the nail plate. Definitive diagnosis is necessary before treatment because onychomycosis can be mistaken for many infectious or noninfectious skin diseases with nail involvement. Conventional methods are time consuming, laborious, and less reliable. Instead of high-cost procedures, in vivo confocal microscopic examination can be a rapid and reliable diagnostic method for onychomycosis in the near future.
1. Singal A, Khanna D. Onychomycosis: diagnosis and management. Indian J Dermatol Venereol Leprol. 2011;77:659-672.
2. Kaur R, Kashyap B, Bhalla P. Onychomycosis—epidemiology, diagnosis and management. Indian J Med Microbiol. 2008;26:108-116.
3. Richardson MD. Diagnosis and pathogenesis of dermatophyte infections. Br J Clin Pract Suppl. 1990;71:98-102.
4. Jensen RH, Arendrup MC. Molecular diagnosis of dermato-phyte infections. Curr Opin Infect Dis. 2012;25:126-134.
5. Weinberg JM, Koestenblatt EK, Tutrone WD, et al. Comparison of diagnostic methods in the evaluation of onychomycosis. J Am Acad Dermatol. 2003;49:193-197.
6. Gianni C, Morelli V, Cerri A, et al. Usefulness of histological examination for the diagnosis of onychomycosis. Dermatology. 2001;202:283-288.
7. Machler BC, Kirsner RS, Elgart GW. Routine histologic examination for the diagnosis of onychomycosis: an evaluation of sensitivity and specificity. Cutis. 1998;61:217-219.
8. Wilsmann-Theis D, Sareika F, Bieber T, et al. New reasons for histopathological nail-clipping examination in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2011;25:235-237.
9. Reisberger EM, Abels C, Landthaler M, et al. Histopathological diagnosis of onychomycosis by periodic acid-Schiff-stained nail clippings. Br J Dermatol. 2003;148:749-754.
10. Shemer A, Trau H, Davidovici B, et al. Collection of fungi samples from nails: comparative study of curettage and drilling techniques. J Eur Acad Dermatol Venereol. 2008;22:182-185.
11. Daniel CR 3rd, Elewski BE. The diagnosis of nail fungus infection revisited. Arch Dermatol. 2000;136:1162-1164.
12. Borkowski P, Williams M, Holewinski J, et al. Onychomycosis: an analysis of 50 cases and a comparison of diagnostic techniques. J Am Podiatr Med Assoc. 2001;91:351-355.
13. Rajadhyaksha M, Gonzalez S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113:293-303.
14. Hongcharu W, Dwyer P, Gonzalez S, et al. Confirmation of onychomycosis by in vivo confocal microscopy. J Am Acad Dermatol. 2000;42(2, pt 1):214-216.
To the Editor:
Onychomycosis is a common nail disease that frequently is caused by dermatophytes and is diagnosed by direct microscopy. Conventional diagnostic methods are often time consuming and can produce false-positive or false-negative results. We report a case of onychomycosis diagnosed by confocal microscopy and confirmed with routine potassium hydroxide (KOH) examination and fungal culture. Confocal microscopy is a reliable, practical, and noninvasive technique in the diagnosis of onychomycosis.
A 46-year-old woman presented with yellow-brown discoloration and dystrophy of the toenails (Figure 1) that had become worse over a 5-year period. She was otherwise healthy and had no other dermatologic problems. Examination revealed yellow-brown discoloration, subungual hyperkeratosis, and onycholysis of the toenails. Clinically, a diagnosis of onychomycosis was made. Potassium hydroxide examination of a scraping from the subungual region showed fungal elements. Trichophyton rubrum on Sabouraud dextrose agar was determined.
|
We performed both in vivo and in vitro confocal laser scanning microscopic examination of the nail of the right great toe (Figure 2). For the diagnosis of onychomycosis in our case, we used a multilaser reflectance confocal microscope (RCM) with a wavelength of 786 nm. In vivo confocal microscopy of the nail revealed branching hyphae just below the surface of the nail plate. Hyphae were seen as refractile, bright, linear structures along the laminates of the nail.
Onychomycosis is a common condition affecting 5.5% of the population worldwide and representing 20% to 40% of all onychopathies and approximately 30% of cutaneous mycotic infections.1,2 There are many methods available to confirm the clinical diagnosis of onychomycosis by detecting the causative organisms. Direct microscopic examination of the scraping with a KOH culture, histopathologic assessment with periodic acid–Schiff staining, immunofluorescence analysis with calcofluor white staining, enzyme analysis, and polymerase chain reaction can be used for diagnosis of fungal infections. The most frequently used diagnostic method for onychomycosis is KOH examination of the scraping; however, fungal culture and histopathologic examination also can be used in cases having diagnostic difficulties.1,3,4 There are many studies comparing the efficacies of these methods in the literature.5-9
The causative fungal agent should be determined with at least 1 laboratory method due to the high cost, long duration, and serious potential adverse effects of systemic antifungal treatment. Direct microscopic examination with KOH in the diagnosis of onychomycosis is simple, fast, and inexpensive. However, inadequate material, using crystallized KOH for hydrolysis, insufficient or too much hydrolysis of scrapings, inappropriate staining, and not scanning all areas in the microscopy produce false-negative results. Similarly, secondary contamination of hair, cotton, yarn, or air bubbles mimicking fungal structures can cause false-positive results.9,10
Fungal culture is another diagnostic method that is accepted as the gold standard for diagnosis of onychomycosis.9 However, fungal cultures were positive in only 43% to 50% of all cases of onychomycosis that were diagnosed with other methods,11,12 which may be due to the loss of viability and ability of the fungi to grow in culture media during the transport. A major advantage of fungal culture is that the fungal agent can be classified as dermatophyte, nondermatophyte, mold, or yeast. However, culture does determine if the growing fungi is contamination or the real pathogen. Moreover, it is necessary to wait 3 to 4 weeks for culture results. For nondermatophyte fungi this time may be much longer.12
In vivo RCM is a noninvasive imaging method that allows optical en face sectioning of the living tissue with high resolution. Currently, RCM has a wide range of applications, such as the evaluation of both benign and malignant skin lesions in clinical dermatology.13
In vivo RCM was used first by Hongcharu et al.14 The diagnoses of onychomycosis and fungal hyphae were shown both in vivo and in vitro.14 The sensitivity and specificity of confocal examination in the diagnosis of onychomycosis is not known yet. Large clinical trials are needed to assess the sensitivity and specificity of this method in diagnosing fungal infections.
Onychomycosis is a contagious infectious disease characterized by hyphae proliferation in the nail plate. Definitive diagnosis is necessary before treatment because onychomycosis can be mistaken for many infectious or noninfectious skin diseases with nail involvement. Conventional methods are time consuming, laborious, and less reliable. Instead of high-cost procedures, in vivo confocal microscopic examination can be a rapid and reliable diagnostic method for onychomycosis in the near future.
To the Editor:
Onychomycosis is a common nail disease that frequently is caused by dermatophytes and is diagnosed by direct microscopy. Conventional diagnostic methods are often time consuming and can produce false-positive or false-negative results. We report a case of onychomycosis diagnosed by confocal microscopy and confirmed with routine potassium hydroxide (KOH) examination and fungal culture. Confocal microscopy is a reliable, practical, and noninvasive technique in the diagnosis of onychomycosis.
A 46-year-old woman presented with yellow-brown discoloration and dystrophy of the toenails (Figure 1) that had become worse over a 5-year period. She was otherwise healthy and had no other dermatologic problems. Examination revealed yellow-brown discoloration, subungual hyperkeratosis, and onycholysis of the toenails. Clinically, a diagnosis of onychomycosis was made. Potassium hydroxide examination of a scraping from the subungual region showed fungal elements. Trichophyton rubrum on Sabouraud dextrose agar was determined.
|
We performed both in vivo and in vitro confocal laser scanning microscopic examination of the nail of the right great toe (Figure 2). For the diagnosis of onychomycosis in our case, we used a multilaser reflectance confocal microscope (RCM) with a wavelength of 786 nm. In vivo confocal microscopy of the nail revealed branching hyphae just below the surface of the nail plate. Hyphae were seen as refractile, bright, linear structures along the laminates of the nail.
Onychomycosis is a common condition affecting 5.5% of the population worldwide and representing 20% to 40% of all onychopathies and approximately 30% of cutaneous mycotic infections.1,2 There are many methods available to confirm the clinical diagnosis of onychomycosis by detecting the causative organisms. Direct microscopic examination of the scraping with a KOH culture, histopathologic assessment with periodic acid–Schiff staining, immunofluorescence analysis with calcofluor white staining, enzyme analysis, and polymerase chain reaction can be used for diagnosis of fungal infections. The most frequently used diagnostic method for onychomycosis is KOH examination of the scraping; however, fungal culture and histopathologic examination also can be used in cases having diagnostic difficulties.1,3,4 There are many studies comparing the efficacies of these methods in the literature.5-9
The causative fungal agent should be determined with at least 1 laboratory method due to the high cost, long duration, and serious potential adverse effects of systemic antifungal treatment. Direct microscopic examination with KOH in the diagnosis of onychomycosis is simple, fast, and inexpensive. However, inadequate material, using crystallized KOH for hydrolysis, insufficient or too much hydrolysis of scrapings, inappropriate staining, and not scanning all areas in the microscopy produce false-negative results. Similarly, secondary contamination of hair, cotton, yarn, or air bubbles mimicking fungal structures can cause false-positive results.9,10
Fungal culture is another diagnostic method that is accepted as the gold standard for diagnosis of onychomycosis.9 However, fungal cultures were positive in only 43% to 50% of all cases of onychomycosis that were diagnosed with other methods,11,12 which may be due to the loss of viability and ability of the fungi to grow in culture media during the transport. A major advantage of fungal culture is that the fungal agent can be classified as dermatophyte, nondermatophyte, mold, or yeast. However, culture does determine if the growing fungi is contamination or the real pathogen. Moreover, it is necessary to wait 3 to 4 weeks for culture results. For nondermatophyte fungi this time may be much longer.12
In vivo RCM is a noninvasive imaging method that allows optical en face sectioning of the living tissue with high resolution. Currently, RCM has a wide range of applications, such as the evaluation of both benign and malignant skin lesions in clinical dermatology.13
In vivo RCM was used first by Hongcharu et al.14 The diagnoses of onychomycosis and fungal hyphae were shown both in vivo and in vitro.14 The sensitivity and specificity of confocal examination in the diagnosis of onychomycosis is not known yet. Large clinical trials are needed to assess the sensitivity and specificity of this method in diagnosing fungal infections.
Onychomycosis is a contagious infectious disease characterized by hyphae proliferation in the nail plate. Definitive diagnosis is necessary before treatment because onychomycosis can be mistaken for many infectious or noninfectious skin diseases with nail involvement. Conventional methods are time consuming, laborious, and less reliable. Instead of high-cost procedures, in vivo confocal microscopic examination can be a rapid and reliable diagnostic method for onychomycosis in the near future.
1. Singal A, Khanna D. Onychomycosis: diagnosis and management. Indian J Dermatol Venereol Leprol. 2011;77:659-672.
2. Kaur R, Kashyap B, Bhalla P. Onychomycosis—epidemiology, diagnosis and management. Indian J Med Microbiol. 2008;26:108-116.
3. Richardson MD. Diagnosis and pathogenesis of dermatophyte infections. Br J Clin Pract Suppl. 1990;71:98-102.
4. Jensen RH, Arendrup MC. Molecular diagnosis of dermato-phyte infections. Curr Opin Infect Dis. 2012;25:126-134.
5. Weinberg JM, Koestenblatt EK, Tutrone WD, et al. Comparison of diagnostic methods in the evaluation of onychomycosis. J Am Acad Dermatol. 2003;49:193-197.
6. Gianni C, Morelli V, Cerri A, et al. Usefulness of histological examination for the diagnosis of onychomycosis. Dermatology. 2001;202:283-288.
7. Machler BC, Kirsner RS, Elgart GW. Routine histologic examination for the diagnosis of onychomycosis: an evaluation of sensitivity and specificity. Cutis. 1998;61:217-219.
8. Wilsmann-Theis D, Sareika F, Bieber T, et al. New reasons for histopathological nail-clipping examination in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2011;25:235-237.
9. Reisberger EM, Abels C, Landthaler M, et al. Histopathological diagnosis of onychomycosis by periodic acid-Schiff-stained nail clippings. Br J Dermatol. 2003;148:749-754.
10. Shemer A, Trau H, Davidovici B, et al. Collection of fungi samples from nails: comparative study of curettage and drilling techniques. J Eur Acad Dermatol Venereol. 2008;22:182-185.
11. Daniel CR 3rd, Elewski BE. The diagnosis of nail fungus infection revisited. Arch Dermatol. 2000;136:1162-1164.
12. Borkowski P, Williams M, Holewinski J, et al. Onychomycosis: an analysis of 50 cases and a comparison of diagnostic techniques. J Am Podiatr Med Assoc. 2001;91:351-355.
13. Rajadhyaksha M, Gonzalez S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113:293-303.
14. Hongcharu W, Dwyer P, Gonzalez S, et al. Confirmation of onychomycosis by in vivo confocal microscopy. J Am Acad Dermatol. 2000;42(2, pt 1):214-216.
1. Singal A, Khanna D. Onychomycosis: diagnosis and management. Indian J Dermatol Venereol Leprol. 2011;77:659-672.
2. Kaur R, Kashyap B, Bhalla P. Onychomycosis—epidemiology, diagnosis and management. Indian J Med Microbiol. 2008;26:108-116.
3. Richardson MD. Diagnosis and pathogenesis of dermatophyte infections. Br J Clin Pract Suppl. 1990;71:98-102.
4. Jensen RH, Arendrup MC. Molecular diagnosis of dermato-phyte infections. Curr Opin Infect Dis. 2012;25:126-134.
5. Weinberg JM, Koestenblatt EK, Tutrone WD, et al. Comparison of diagnostic methods in the evaluation of onychomycosis. J Am Acad Dermatol. 2003;49:193-197.
6. Gianni C, Morelli V, Cerri A, et al. Usefulness of histological examination for the diagnosis of onychomycosis. Dermatology. 2001;202:283-288.
7. Machler BC, Kirsner RS, Elgart GW. Routine histologic examination for the diagnosis of onychomycosis: an evaluation of sensitivity and specificity. Cutis. 1998;61:217-219.
8. Wilsmann-Theis D, Sareika F, Bieber T, et al. New reasons for histopathological nail-clipping examination in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2011;25:235-237.
9. Reisberger EM, Abels C, Landthaler M, et al. Histopathological diagnosis of onychomycosis by periodic acid-Schiff-stained nail clippings. Br J Dermatol. 2003;148:749-754.
10. Shemer A, Trau H, Davidovici B, et al. Collection of fungi samples from nails: comparative study of curettage and drilling techniques. J Eur Acad Dermatol Venereol. 2008;22:182-185.
11. Daniel CR 3rd, Elewski BE. The diagnosis of nail fungus infection revisited. Arch Dermatol. 2000;136:1162-1164.
12. Borkowski P, Williams M, Holewinski J, et al. Onychomycosis: an analysis of 50 cases and a comparison of diagnostic techniques. J Am Podiatr Med Assoc. 2001;91:351-355.
13. Rajadhyaksha M, Gonzalez S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113:293-303.
14. Hongcharu W, Dwyer P, Gonzalez S, et al. Confirmation of onychomycosis by in vivo confocal microscopy. J Am Acad Dermatol. 2000;42(2, pt 1):214-216.
Nail Biopsy: 6 Techniques to Biopsy the Nail Matrix
Nail matrix biopsies are performed to confirm a diagnosis or surgically remove a skin lesion that is affecting the growth of the nail plate. The procedure may be used to identify:
- Inflammatory conditions such as nail psoriasis and lichen planus
- Benign tumors
- Solitary melanonychia
- Squamous cell carcinoma (SCC)
- Other nail disorders
Nail biopsy can lead to complications such as bleeding, infection, or scarring. Postoperative scarring can cause permanent nail splitting, dystrophy, or both.
In a Cosmetic Dermatology article, “Matrix Biopsy of Longitudinal Melanonychia and Longitudinal Erythronychia: A Step-by-Step Approach,” Drs. Siobhan C. Collins and Nathaniel J. Jellinek review 6 techniques used to biopsy the nail matrix.
- Punch excision
- Matrix shave
- Lateral longitudinal excision
- Midline/paramedian longitudinal excision
- Transverse excision
- Longitudinal excision of erythronychia
In the setting of longitudinal melanonychia (to diagnose nail melanoma or SCC) and longitudinal erythronychia (to diagnose SCC and rarely amelanotic melanoma or basal cell carcinoma), the techniques they describe accomplish 3 fundamental goals of nail surgery:
- Obtain adequate tissue via an excisional biopsy to make an accurate diagnosis and avoid sampling error
- Avoid unnecessary trauma to surrounding nail tissues by the judicious use of partial plate avulsions whenever feasible
- Avoid unnecessary postoperative nail scarring whenever possible
Dermatologists must be confident when performing nail biopsies and the techniques discussed by the authors will help approach nail surgery with more certainty.
At the 73rd Annual Meeting of the American Academy of Dermatology, Dr. Jellinek provides a hands-on approach to nail surgery. On Saturday, March 21, he will provide tips for nail surgeries at the “Medical and Surgical Management of Nail Disorders” lecture.
For more information, read the Collins and Jellinek article from Cosmetic Dermatology.
Nail matrix biopsies are performed to confirm a diagnosis or surgically remove a skin lesion that is affecting the growth of the nail plate. The procedure may be used to identify:
- Inflammatory conditions such as nail psoriasis and lichen planus
- Benign tumors
- Solitary melanonychia
- Squamous cell carcinoma (SCC)
- Other nail disorders
Nail biopsy can lead to complications such as bleeding, infection, or scarring. Postoperative scarring can cause permanent nail splitting, dystrophy, or both.
In a Cosmetic Dermatology article, “Matrix Biopsy of Longitudinal Melanonychia and Longitudinal Erythronychia: A Step-by-Step Approach,” Drs. Siobhan C. Collins and Nathaniel J. Jellinek review 6 techniques used to biopsy the nail matrix.
- Punch excision
- Matrix shave
- Lateral longitudinal excision
- Midline/paramedian longitudinal excision
- Transverse excision
- Longitudinal excision of erythronychia
In the setting of longitudinal melanonychia (to diagnose nail melanoma or SCC) and longitudinal erythronychia (to diagnose SCC and rarely amelanotic melanoma or basal cell carcinoma), the techniques they describe accomplish 3 fundamental goals of nail surgery:
- Obtain adequate tissue via an excisional biopsy to make an accurate diagnosis and avoid sampling error
- Avoid unnecessary trauma to surrounding nail tissues by the judicious use of partial plate avulsions whenever feasible
- Avoid unnecessary postoperative nail scarring whenever possible
Dermatologists must be confident when performing nail biopsies and the techniques discussed by the authors will help approach nail surgery with more certainty.
At the 73rd Annual Meeting of the American Academy of Dermatology, Dr. Jellinek provides a hands-on approach to nail surgery. On Saturday, March 21, he will provide tips for nail surgeries at the “Medical and Surgical Management of Nail Disorders” lecture.
For more information, read the Collins and Jellinek article from Cosmetic Dermatology.
Nail matrix biopsies are performed to confirm a diagnosis or surgically remove a skin lesion that is affecting the growth of the nail plate. The procedure may be used to identify:
- Inflammatory conditions such as nail psoriasis and lichen planus
- Benign tumors
- Solitary melanonychia
- Squamous cell carcinoma (SCC)
- Other nail disorders
Nail biopsy can lead to complications such as bleeding, infection, or scarring. Postoperative scarring can cause permanent nail splitting, dystrophy, or both.
In a Cosmetic Dermatology article, “Matrix Biopsy of Longitudinal Melanonychia and Longitudinal Erythronychia: A Step-by-Step Approach,” Drs. Siobhan C. Collins and Nathaniel J. Jellinek review 6 techniques used to biopsy the nail matrix.
- Punch excision
- Matrix shave
- Lateral longitudinal excision
- Midline/paramedian longitudinal excision
- Transverse excision
- Longitudinal excision of erythronychia
In the setting of longitudinal melanonychia (to diagnose nail melanoma or SCC) and longitudinal erythronychia (to diagnose SCC and rarely amelanotic melanoma or basal cell carcinoma), the techniques they describe accomplish 3 fundamental goals of nail surgery:
- Obtain adequate tissue via an excisional biopsy to make an accurate diagnosis and avoid sampling error
- Avoid unnecessary trauma to surrounding nail tissues by the judicious use of partial plate avulsions whenever feasible
- Avoid unnecessary postoperative nail scarring whenever possible
Dermatologists must be confident when performing nail biopsies and the techniques discussed by the authors will help approach nail surgery with more certainty.
At the 73rd Annual Meeting of the American Academy of Dermatology, Dr. Jellinek provides a hands-on approach to nail surgery. On Saturday, March 21, he will provide tips for nail surgeries at the “Medical and Surgical Management of Nail Disorders” lecture.
For more information, read the Collins and Jellinek article from Cosmetic Dermatology.
Inability to Grow Long Hair: A Presentation of Trichorrhexis Nodosa
To the Editor:
First identified by Samuel Wilks in 1852, trichorrhexis nodosa (TN) is a congenital or acquired hair shaft disorder that is characterized by fragile and easily broken hair.1 Congenital TN is rare and can occur in syndromes such as pseudomonilethrix, Netherton syndrome, pili annulati,2 argininosuccinic aciduria,3 trichothiodystrophy,4 Menkes syndrome,5 and trichohepatoenteric syndrome.6 The primary congenital form of TN is inherited as an autosomal-dominant trait in some families. Acquired TN is the most common hair shaft abnormality and often is overlooked. It is provoked by hair injury, usually mechanical or physical, or chemical trauma.7,8
Chemical trauma is caused by the use of permanent hair liquids or dyes. Mechanical injuries are the result of frequent brushing, scalp massage, or lengthy backcombing, and physical damage includes excessive UV exposure or repeated application of heat. Habit tics, trichotillomania, and the scratching and pulling associated with pruritic dermatoses also can result in sufficient damage to provoke TN. Furthermore, this acquired disorder may develop from malnutrition, particularly iron deficiency, or endocrinopathy such as hypothyroidism.9 Seasonal recurrence of TN has been reported from the cumulative effect of repeated soaking in salt water and exposure to UV light. Macroscopically, hair shafts affected by TN contain small white nodes at irregular intervals throughout the length of the hair shaft. These nodes represent areas of cuticular cell disruption, which allows the underlying cortical fibers to separate and fray and gives the node the microscopic appearance of 2 brooms or paintbrushes thrusting together end-to-end by the bristles. The classic description is known as paintbrush fracture.10 Generally, complete breakage occurs at these nodes.
A 21-year-old white woman presented to our clinic with hair fragility and inability to grow long hair of 2 years’ duration. The hair was lusterless and dry. Dermoscopic examination revealed broken blunt-ended hair of uneven length with minute pinpoint grayish white nodules (Figure 1). Small fragments could be easily broken off with gentle tugging on the distal ends. She reported a history of severe sunlight and seawater exposure during the last 2 summers and the continuous use of a flat iron in the last year. Microscopic examination of hair samples with a scanning electron microscope showed the characteristic paintbrush fracture (Figure 2). She had no history of diseases, and blood examinations including complete blood cell count, thyroid function test, and iron levels were within reference range.

|
We hypothesize that the seasonal damage caused by exposure to UV light and salt water with repeated trauma from the heat of the flat iron caused distal TN. The patient was given an explanation about the diagnosis of TN and was instructed to avoid the practices that were suspected causes of the condition. Use of a gentle shampoo and conditioner also was recommended. At 6-month follow-up, we noticed an improvement of the quality of hair with a reduction in the whitish nodules and a revival of hair growth.
Acquired TN has been classified into 3 clinical forms: proximal, distal, and localized.1 Proximal TN is common in black individuals who use caustic chemicals when styling the hair. The involved hairs develop the characteristic nodes that break within a few centimeters from the scalp, especially in areas subject to friction from combing or sleeping. Distal TN primarily occurs in white or Asian individuals. In this disorder, nodes and breakage occur near the ends of the hairs that appear dull, dry, and uneven. Breakage commonly is associated with trichoptilosis, or longitudinal splitting, commonly referred to as split ends. This breakage may reflect frequent use of shampoo or heat treatments. The distal acquired form may simulate dandruff or pediculosis and the detection of this hair defect often is casual.
Localized TN, described by Raymond Sabouraud in 1921, is a rare disorder. It occurs in a patch that is usually a few centimeters long. It generally is accompanied by a pruritic dermatosis, such as circumscribed neurodermatitis, contact dermatitis, or atopic dermatitis. Scratching and rubbing most likely are the ultimate causes.
Trichorrhexis nodosa can spontaneously resolve. In all cases, diagnosis depends on careful microscopy examination and, if possible, scanning electron microscopy. Treatment is aimed at minimizing mechanical and physical injury, and chemical trauma. Excessive brushing, hot-combing, permanent waving, and other harsh hair treatments should be avoided. If the hair is long and the damage is distal, it may be sufficient to cut the distal fraction and to change cosmetic practices to prevent relapse.
Dermatologists who see patients with hair fragility and inability to grow long hair should consider the diagnosis of TN. Acquired TN often is reversible. Complete resolution may take 2 to 4 years depending on the growth of new anagen hairs. All patients with a history of white flecking on the scalp, abnormal fragility of the hair, and failure to attain normal hair length should be questioned about their routine hair care habits as well as environmental or chemical exposures to determine and remove the source of physical or chemical trauma.
1. Whiting DA. Structural abnormalities of hair shaft. J Am Acad Dermatol. 1987;16(1, pt 1):1-25.
2. Leider M. Multiple simultaneous anomalies of the hair; report of a case exhibiting trichorrhexis nodosa, pili annulati and trichostasis spinulosa. AMA Arch Derm Syphilol. 1950;62:510-514.
3. Allan JD, Cusworth DC, Dent CE, et al. A disease, probably hereditary characterised by severe mental deficiency and a constant gross abnormality of aminoacid metabolism. Lancet. 1958;1:182-187.
4. Liang C, Morris A, Schlücker S, et al. Structural and molecular hair abnormalities in trichothiodystrophy [published online ahead of print May 25, 2006]. J Invest Dermatol. 2006;126:2210-2216.
5. Taylor CJ, Green SH. Menkes’ syndrome (trichopoliodystrophy): use of scanning electron-microscope in diagnosis and carrier identification. Dev Med Child Neurol. 1981;23:361-368.
6. Hartley JL, Zachos NC, Dawood B, et al. Mutations in TTC37 cause trichohepatoenteric syndrome (phenotypic diarrhea of infancy)[published online ahead of print February 20, 2010]. Gastroenterology. 2010;138:2388-2398.
7. Chernosky ME, Owens DW. Trichorrhexis nodosa. clinical and investigative studies. Arch Dermatol. 1966;94:577-585.
8. Owens DW, Chernosky ME. Trichorrhexis nodosa; in vitro reproduction. Arch Dermatol. 1966;94:586-588.
9. Lurie R, Hodak E, Ginzburg A, et al. Trichorrhexis nodosa: a manifestation of hypothyroidism. Cutis. 1996;57:358-359.
10. Miyamoto M, Tsuboi R, Oh-I T. Case of acquired trichorrhexis nodosa: scanning electron microscopic observation. J Dermatol. 2009;36:109-110.
To the Editor:
First identified by Samuel Wilks in 1852, trichorrhexis nodosa (TN) is a congenital or acquired hair shaft disorder that is characterized by fragile and easily broken hair.1 Congenital TN is rare and can occur in syndromes such as pseudomonilethrix, Netherton syndrome, pili annulati,2 argininosuccinic aciduria,3 trichothiodystrophy,4 Menkes syndrome,5 and trichohepatoenteric syndrome.6 The primary congenital form of TN is inherited as an autosomal-dominant trait in some families. Acquired TN is the most common hair shaft abnormality and often is overlooked. It is provoked by hair injury, usually mechanical or physical, or chemical trauma.7,8
Chemical trauma is caused by the use of permanent hair liquids or dyes. Mechanical injuries are the result of frequent brushing, scalp massage, or lengthy backcombing, and physical damage includes excessive UV exposure or repeated application of heat. Habit tics, trichotillomania, and the scratching and pulling associated with pruritic dermatoses also can result in sufficient damage to provoke TN. Furthermore, this acquired disorder may develop from malnutrition, particularly iron deficiency, or endocrinopathy such as hypothyroidism.9 Seasonal recurrence of TN has been reported from the cumulative effect of repeated soaking in salt water and exposure to UV light. Macroscopically, hair shafts affected by TN contain small white nodes at irregular intervals throughout the length of the hair shaft. These nodes represent areas of cuticular cell disruption, which allows the underlying cortical fibers to separate and fray and gives the node the microscopic appearance of 2 brooms or paintbrushes thrusting together end-to-end by the bristles. The classic description is known as paintbrush fracture.10 Generally, complete breakage occurs at these nodes.
A 21-year-old white woman presented to our clinic with hair fragility and inability to grow long hair of 2 years’ duration. The hair was lusterless and dry. Dermoscopic examination revealed broken blunt-ended hair of uneven length with minute pinpoint grayish white nodules (Figure 1). Small fragments could be easily broken off with gentle tugging on the distal ends. She reported a history of severe sunlight and seawater exposure during the last 2 summers and the continuous use of a flat iron in the last year. Microscopic examination of hair samples with a scanning electron microscope showed the characteristic paintbrush fracture (Figure 2). She had no history of diseases, and blood examinations including complete blood cell count, thyroid function test, and iron levels were within reference range.

|
We hypothesize that the seasonal damage caused by exposure to UV light and salt water with repeated trauma from the heat of the flat iron caused distal TN. The patient was given an explanation about the diagnosis of TN and was instructed to avoid the practices that were suspected causes of the condition. Use of a gentle shampoo and conditioner also was recommended. At 6-month follow-up, we noticed an improvement of the quality of hair with a reduction in the whitish nodules and a revival of hair growth.
Acquired TN has been classified into 3 clinical forms: proximal, distal, and localized.1 Proximal TN is common in black individuals who use caustic chemicals when styling the hair. The involved hairs develop the characteristic nodes that break within a few centimeters from the scalp, especially in areas subject to friction from combing or sleeping. Distal TN primarily occurs in white or Asian individuals. In this disorder, nodes and breakage occur near the ends of the hairs that appear dull, dry, and uneven. Breakage commonly is associated with trichoptilosis, or longitudinal splitting, commonly referred to as split ends. This breakage may reflect frequent use of shampoo or heat treatments. The distal acquired form may simulate dandruff or pediculosis and the detection of this hair defect often is casual.
Localized TN, described by Raymond Sabouraud in 1921, is a rare disorder. It occurs in a patch that is usually a few centimeters long. It generally is accompanied by a pruritic dermatosis, such as circumscribed neurodermatitis, contact dermatitis, or atopic dermatitis. Scratching and rubbing most likely are the ultimate causes.
Trichorrhexis nodosa can spontaneously resolve. In all cases, diagnosis depends on careful microscopy examination and, if possible, scanning electron microscopy. Treatment is aimed at minimizing mechanical and physical injury, and chemical trauma. Excessive brushing, hot-combing, permanent waving, and other harsh hair treatments should be avoided. If the hair is long and the damage is distal, it may be sufficient to cut the distal fraction and to change cosmetic practices to prevent relapse.
Dermatologists who see patients with hair fragility and inability to grow long hair should consider the diagnosis of TN. Acquired TN often is reversible. Complete resolution may take 2 to 4 years depending on the growth of new anagen hairs. All patients with a history of white flecking on the scalp, abnormal fragility of the hair, and failure to attain normal hair length should be questioned about their routine hair care habits as well as environmental or chemical exposures to determine and remove the source of physical or chemical trauma.
To the Editor:
First identified by Samuel Wilks in 1852, trichorrhexis nodosa (TN) is a congenital or acquired hair shaft disorder that is characterized by fragile and easily broken hair.1 Congenital TN is rare and can occur in syndromes such as pseudomonilethrix, Netherton syndrome, pili annulati,2 argininosuccinic aciduria,3 trichothiodystrophy,4 Menkes syndrome,5 and trichohepatoenteric syndrome.6 The primary congenital form of TN is inherited as an autosomal-dominant trait in some families. Acquired TN is the most common hair shaft abnormality and often is overlooked. It is provoked by hair injury, usually mechanical or physical, or chemical trauma.7,8
Chemical trauma is caused by the use of permanent hair liquids or dyes. Mechanical injuries are the result of frequent brushing, scalp massage, or lengthy backcombing, and physical damage includes excessive UV exposure or repeated application of heat. Habit tics, trichotillomania, and the scratching and pulling associated with pruritic dermatoses also can result in sufficient damage to provoke TN. Furthermore, this acquired disorder may develop from malnutrition, particularly iron deficiency, or endocrinopathy such as hypothyroidism.9 Seasonal recurrence of TN has been reported from the cumulative effect of repeated soaking in salt water and exposure to UV light. Macroscopically, hair shafts affected by TN contain small white nodes at irregular intervals throughout the length of the hair shaft. These nodes represent areas of cuticular cell disruption, which allows the underlying cortical fibers to separate and fray and gives the node the microscopic appearance of 2 brooms or paintbrushes thrusting together end-to-end by the bristles. The classic description is known as paintbrush fracture.10 Generally, complete breakage occurs at these nodes.
A 21-year-old white woman presented to our clinic with hair fragility and inability to grow long hair of 2 years’ duration. The hair was lusterless and dry. Dermoscopic examination revealed broken blunt-ended hair of uneven length with minute pinpoint grayish white nodules (Figure 1). Small fragments could be easily broken off with gentle tugging on the distal ends. She reported a history of severe sunlight and seawater exposure during the last 2 summers and the continuous use of a flat iron in the last year. Microscopic examination of hair samples with a scanning electron microscope showed the characteristic paintbrush fracture (Figure 2). She had no history of diseases, and blood examinations including complete blood cell count, thyroid function test, and iron levels were within reference range.

|
We hypothesize that the seasonal damage caused by exposure to UV light and salt water with repeated trauma from the heat of the flat iron caused distal TN. The patient was given an explanation about the diagnosis of TN and was instructed to avoid the practices that were suspected causes of the condition. Use of a gentle shampoo and conditioner also was recommended. At 6-month follow-up, we noticed an improvement of the quality of hair with a reduction in the whitish nodules and a revival of hair growth.
Acquired TN has been classified into 3 clinical forms: proximal, distal, and localized.1 Proximal TN is common in black individuals who use caustic chemicals when styling the hair. The involved hairs develop the characteristic nodes that break within a few centimeters from the scalp, especially in areas subject to friction from combing or sleeping. Distal TN primarily occurs in white or Asian individuals. In this disorder, nodes and breakage occur near the ends of the hairs that appear dull, dry, and uneven. Breakage commonly is associated with trichoptilosis, or longitudinal splitting, commonly referred to as split ends. This breakage may reflect frequent use of shampoo or heat treatments. The distal acquired form may simulate dandruff or pediculosis and the detection of this hair defect often is casual.
Localized TN, described by Raymond Sabouraud in 1921, is a rare disorder. It occurs in a patch that is usually a few centimeters long. It generally is accompanied by a pruritic dermatosis, such as circumscribed neurodermatitis, contact dermatitis, or atopic dermatitis. Scratching and rubbing most likely are the ultimate causes.
Trichorrhexis nodosa can spontaneously resolve. In all cases, diagnosis depends on careful microscopy examination and, if possible, scanning electron microscopy. Treatment is aimed at minimizing mechanical and physical injury, and chemical trauma. Excessive brushing, hot-combing, permanent waving, and other harsh hair treatments should be avoided. If the hair is long and the damage is distal, it may be sufficient to cut the distal fraction and to change cosmetic practices to prevent relapse.
Dermatologists who see patients with hair fragility and inability to grow long hair should consider the diagnosis of TN. Acquired TN often is reversible. Complete resolution may take 2 to 4 years depending on the growth of new anagen hairs. All patients with a history of white flecking on the scalp, abnormal fragility of the hair, and failure to attain normal hair length should be questioned about their routine hair care habits as well as environmental or chemical exposures to determine and remove the source of physical or chemical trauma.
1. Whiting DA. Structural abnormalities of hair shaft. J Am Acad Dermatol. 1987;16(1, pt 1):1-25.
2. Leider M. Multiple simultaneous anomalies of the hair; report of a case exhibiting trichorrhexis nodosa, pili annulati and trichostasis spinulosa. AMA Arch Derm Syphilol. 1950;62:510-514.
3. Allan JD, Cusworth DC, Dent CE, et al. A disease, probably hereditary characterised by severe mental deficiency and a constant gross abnormality of aminoacid metabolism. Lancet. 1958;1:182-187.
4. Liang C, Morris A, Schlücker S, et al. Structural and molecular hair abnormalities in trichothiodystrophy [published online ahead of print May 25, 2006]. J Invest Dermatol. 2006;126:2210-2216.
5. Taylor CJ, Green SH. Menkes’ syndrome (trichopoliodystrophy): use of scanning electron-microscope in diagnosis and carrier identification. Dev Med Child Neurol. 1981;23:361-368.
6. Hartley JL, Zachos NC, Dawood B, et al. Mutations in TTC37 cause trichohepatoenteric syndrome (phenotypic diarrhea of infancy)[published online ahead of print February 20, 2010]. Gastroenterology. 2010;138:2388-2398.
7. Chernosky ME, Owens DW. Trichorrhexis nodosa. clinical and investigative studies. Arch Dermatol. 1966;94:577-585.
8. Owens DW, Chernosky ME. Trichorrhexis nodosa; in vitro reproduction. Arch Dermatol. 1966;94:586-588.
9. Lurie R, Hodak E, Ginzburg A, et al. Trichorrhexis nodosa: a manifestation of hypothyroidism. Cutis. 1996;57:358-359.
10. Miyamoto M, Tsuboi R, Oh-I T. Case of acquired trichorrhexis nodosa: scanning electron microscopic observation. J Dermatol. 2009;36:109-110.
1. Whiting DA. Structural abnormalities of hair shaft. J Am Acad Dermatol. 1987;16(1, pt 1):1-25.
2. Leider M. Multiple simultaneous anomalies of the hair; report of a case exhibiting trichorrhexis nodosa, pili annulati and trichostasis spinulosa. AMA Arch Derm Syphilol. 1950;62:510-514.
3. Allan JD, Cusworth DC, Dent CE, et al. A disease, probably hereditary characterised by severe mental deficiency and a constant gross abnormality of aminoacid metabolism. Lancet. 1958;1:182-187.
4. Liang C, Morris A, Schlücker S, et al. Structural and molecular hair abnormalities in trichothiodystrophy [published online ahead of print May 25, 2006]. J Invest Dermatol. 2006;126:2210-2216.
5. Taylor CJ, Green SH. Menkes’ syndrome (trichopoliodystrophy): use of scanning electron-microscope in diagnosis and carrier identification. Dev Med Child Neurol. 1981;23:361-368.
6. Hartley JL, Zachos NC, Dawood B, et al. Mutations in TTC37 cause trichohepatoenteric syndrome (phenotypic diarrhea of infancy)[published online ahead of print February 20, 2010]. Gastroenterology. 2010;138:2388-2398.
7. Chernosky ME, Owens DW. Trichorrhexis nodosa. clinical and investigative studies. Arch Dermatol. 1966;94:577-585.
8. Owens DW, Chernosky ME. Trichorrhexis nodosa; in vitro reproduction. Arch Dermatol. 1966;94:586-588.
9. Lurie R, Hodak E, Ginzburg A, et al. Trichorrhexis nodosa: a manifestation of hypothyroidism. Cutis. 1996;57:358-359.
10. Miyamoto M, Tsuboi R, Oh-I T. Case of acquired trichorrhexis nodosa: scanning electron microscopic observation. J Dermatol. 2009;36:109-110.
Never inject epinephrine in the fingers or toes?
A 30-year-old woman cuts her finger on a glass jar. She goes to the clinic and needs to have sutures on her right ring finger. What would you recommend for anesthesia to prepare the patient for repair?
A. 1% lidocaine.
B. 1% lidocaine with epinephrine.
C. Bupivacaine.
Myth: You should not use lidocaine with epinephrine on a digit.
Many of us were taught to avoid the use of epinephrine on digits because of the concern of precipitating digital ischemia. This was a common warning in emergency and surgical textbooks (J.C. Vance. Anesthesia. R.K. Roenigk, H.H. Roenigk [Eds.], Dermatologic Surgery, Principles and Practice [2nd ed.], Marcel Decker, New York, N.Y. [1996], pp. 31-52.).
Over the past 20 years, there has been a growing body of evidence that the concern is unwarranted and that there may be benefit to the addition of epinephrine.
Dr. Bradon J. Wilhelmi and his colleagues performed a randomized, double-blind trial comparing lidocaine with epinephrine (31 patients) and lidocaine (29 patients) in patients with traumatic injuries or elective procedures (Plast. Reconstr. Surg. 2001;107:393-7). The need for control of bleeding required digital tourniquet use in 20 of 29 block procedures with plain lidocaine and in 9 of 31 procedures using lidocaine with epinephrine (P < .002). There were no complications in the patients who received lidocaine with epinephrine.
A retrospective study was done by Dr. Saeed Chowdhry and his colleagues of 1,111 patients who had hand surgery and received digital blocks (Plast. Reconstr. Surg. 2010;126:2031-4). A total of 611 patients received lidocaine with epinephrine, and 500 patients received lidocaine alone. The concentration of lidocaine with epinephrine was 1:100,000, with an average dose of 4.33 cc.
There were no cases of digital gangrene or other complications because of the use of epinephrine in this retrospective study.
In a large, retrospective study of nine hand surgeons’ practices, looking at 3,110 cases of elective injection of low-dose epinephrine in hands and fingers, there were no cases of digital tissue loss or need for phentolamine rescue (J. Hand Surg. Am. 2005;30:1061-7).
Several studies have been done using epinephrine digital injections of the toes. In a prospective, randomized, controlled trial, 44 patients undergoing phenolization matricectomy involving digital block injection of 70 toes received either anesthetic and epinephrine or anesthetic and digital tourniquet (J. Eur. Acad. Dermatol. Venereol. 2014 [doi:10.1111/jdv.12746]). The outcome measures were rate of recurrence, bleeding, pain, and duration of anesthetic effect.
There was no difference in recurrence rates, but postoperative bleeding was higher in the procedures done with digital tourniquet and no epinephrine (P = .001). Anesthetic effect as measured by less pain and duration of effect was superior in the patients receiving digital block with epinephrine (P = .001).
In another study looking at chemical matricectomy, Dr. Cevdet Altinyazar and his colleagues randomized patients to receive either 2% lidocaine or lidocaine with epinephrine for anesthesia for chemical matricectomy of ingrown toenails of the great toe (Dermatol. Surg. 2010;36:1568-71). There was less anesthetic needed in the patients who received lidocaine with epinephrine, and a statistically significant reduction in days of drainage following procedure in the lidocaine with epinephrine group (11.1 days +/- 2.5 days), compared with the lidocaine-only group (19.0 days +/- 3.8 days). There were no complications because of the use of epinephrine.
The belief in this myth is still quite common, despite the evidence from randomized, controlled trials and the experience of more than 3,500 patients who have received epinephrine in the fingers without any complications. The evidence from the podiatry literature on safety in use in the toes mirrors the evidence of safety in the fingers.
Dr. Paauw is a professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington Medical School. He is the Rathmann Family Foundation Chair in Patient-Centered Clinical Education. Contact Dr. Paauw at [email protected].
A 30-year-old woman cuts her finger on a glass jar. She goes to the clinic and needs to have sutures on her right ring finger. What would you recommend for anesthesia to prepare the patient for repair?
A. 1% lidocaine.
B. 1% lidocaine with epinephrine.
C. Bupivacaine.
Myth: You should not use lidocaine with epinephrine on a digit.
Many of us were taught to avoid the use of epinephrine on digits because of the concern of precipitating digital ischemia. This was a common warning in emergency and surgical textbooks (J.C. Vance. Anesthesia. R.K. Roenigk, H.H. Roenigk [Eds.], Dermatologic Surgery, Principles and Practice [2nd ed.], Marcel Decker, New York, N.Y. [1996], pp. 31-52.).
Over the past 20 years, there has been a growing body of evidence that the concern is unwarranted and that there may be benefit to the addition of epinephrine.
Dr. Bradon J. Wilhelmi and his colleagues performed a randomized, double-blind trial comparing lidocaine with epinephrine (31 patients) and lidocaine (29 patients) in patients with traumatic injuries or elective procedures (Plast. Reconstr. Surg. 2001;107:393-7). The need for control of bleeding required digital tourniquet use in 20 of 29 block procedures with plain lidocaine and in 9 of 31 procedures using lidocaine with epinephrine (P < .002). There were no complications in the patients who received lidocaine with epinephrine.
A retrospective study was done by Dr. Saeed Chowdhry and his colleagues of 1,111 patients who had hand surgery and received digital blocks (Plast. Reconstr. Surg. 2010;126:2031-4). A total of 611 patients received lidocaine with epinephrine, and 500 patients received lidocaine alone. The concentration of lidocaine with epinephrine was 1:100,000, with an average dose of 4.33 cc.
There were no cases of digital gangrene or other complications because of the use of epinephrine in this retrospective study.
In a large, retrospective study of nine hand surgeons’ practices, looking at 3,110 cases of elective injection of low-dose epinephrine in hands and fingers, there were no cases of digital tissue loss or need for phentolamine rescue (J. Hand Surg. Am. 2005;30:1061-7).
Several studies have been done using epinephrine digital injections of the toes. In a prospective, randomized, controlled trial, 44 patients undergoing phenolization matricectomy involving digital block injection of 70 toes received either anesthetic and epinephrine or anesthetic and digital tourniquet (J. Eur. Acad. Dermatol. Venereol. 2014 [doi:10.1111/jdv.12746]). The outcome measures were rate of recurrence, bleeding, pain, and duration of anesthetic effect.
There was no difference in recurrence rates, but postoperative bleeding was higher in the procedures done with digital tourniquet and no epinephrine (P = .001). Anesthetic effect as measured by less pain and duration of effect was superior in the patients receiving digital block with epinephrine (P = .001).
In another study looking at chemical matricectomy, Dr. Cevdet Altinyazar and his colleagues randomized patients to receive either 2% lidocaine or lidocaine with epinephrine for anesthesia for chemical matricectomy of ingrown toenails of the great toe (Dermatol. Surg. 2010;36:1568-71). There was less anesthetic needed in the patients who received lidocaine with epinephrine, and a statistically significant reduction in days of drainage following procedure in the lidocaine with epinephrine group (11.1 days +/- 2.5 days), compared with the lidocaine-only group (19.0 days +/- 3.8 days). There were no complications because of the use of epinephrine.
The belief in this myth is still quite common, despite the evidence from randomized, controlled trials and the experience of more than 3,500 patients who have received epinephrine in the fingers without any complications. The evidence from the podiatry literature on safety in use in the toes mirrors the evidence of safety in the fingers.
Dr. Paauw is a professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington Medical School. He is the Rathmann Family Foundation Chair in Patient-Centered Clinical Education. Contact Dr. Paauw at [email protected].
A 30-year-old woman cuts her finger on a glass jar. She goes to the clinic and needs to have sutures on her right ring finger. What would you recommend for anesthesia to prepare the patient for repair?
A. 1% lidocaine.
B. 1% lidocaine with epinephrine.
C. Bupivacaine.
Myth: You should not use lidocaine with epinephrine on a digit.
Many of us were taught to avoid the use of epinephrine on digits because of the concern of precipitating digital ischemia. This was a common warning in emergency and surgical textbooks (J.C. Vance. Anesthesia. R.K. Roenigk, H.H. Roenigk [Eds.], Dermatologic Surgery, Principles and Practice [2nd ed.], Marcel Decker, New York, N.Y. [1996], pp. 31-52.).
Over the past 20 years, there has been a growing body of evidence that the concern is unwarranted and that there may be benefit to the addition of epinephrine.
Dr. Bradon J. Wilhelmi and his colleagues performed a randomized, double-blind trial comparing lidocaine with epinephrine (31 patients) and lidocaine (29 patients) in patients with traumatic injuries or elective procedures (Plast. Reconstr. Surg. 2001;107:393-7). The need for control of bleeding required digital tourniquet use in 20 of 29 block procedures with plain lidocaine and in 9 of 31 procedures using lidocaine with epinephrine (P < .002). There were no complications in the patients who received lidocaine with epinephrine.
A retrospective study was done by Dr. Saeed Chowdhry and his colleagues of 1,111 patients who had hand surgery and received digital blocks (Plast. Reconstr. Surg. 2010;126:2031-4). A total of 611 patients received lidocaine with epinephrine, and 500 patients received lidocaine alone. The concentration of lidocaine with epinephrine was 1:100,000, with an average dose of 4.33 cc.
There were no cases of digital gangrene or other complications because of the use of epinephrine in this retrospective study.
In a large, retrospective study of nine hand surgeons’ practices, looking at 3,110 cases of elective injection of low-dose epinephrine in hands and fingers, there were no cases of digital tissue loss or need for phentolamine rescue (J. Hand Surg. Am. 2005;30:1061-7).
Several studies have been done using epinephrine digital injections of the toes. In a prospective, randomized, controlled trial, 44 patients undergoing phenolization matricectomy involving digital block injection of 70 toes received either anesthetic and epinephrine or anesthetic and digital tourniquet (J. Eur. Acad. Dermatol. Venereol. 2014 [doi:10.1111/jdv.12746]). The outcome measures were rate of recurrence, bleeding, pain, and duration of anesthetic effect.
There was no difference in recurrence rates, but postoperative bleeding was higher in the procedures done with digital tourniquet and no epinephrine (P = .001). Anesthetic effect as measured by less pain and duration of effect was superior in the patients receiving digital block with epinephrine (P = .001).
In another study looking at chemical matricectomy, Dr. Cevdet Altinyazar and his colleagues randomized patients to receive either 2% lidocaine or lidocaine with epinephrine for anesthesia for chemical matricectomy of ingrown toenails of the great toe (Dermatol. Surg. 2010;36:1568-71). There was less anesthetic needed in the patients who received lidocaine with epinephrine, and a statistically significant reduction in days of drainage following procedure in the lidocaine with epinephrine group (11.1 days +/- 2.5 days), compared with the lidocaine-only group (19.0 days +/- 3.8 days). There were no complications because of the use of epinephrine.
The belief in this myth is still quite common, despite the evidence from randomized, controlled trials and the experience of more than 3,500 patients who have received epinephrine in the fingers without any complications. The evidence from the podiatry literature on safety in use in the toes mirrors the evidence of safety in the fingers.
Dr. Paauw is a professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington Medical School. He is the Rathmann Family Foundation Chair in Patient-Centered Clinical Education. Contact Dr. Paauw at [email protected].
A Primer to Natural Hair Care Practices in Black Patients
The phenomenon of natural (nonchemically treated) hair in individuals of African and Afro-Caribbean descent is sweeping across the United States. The ideals of beauty among this patient population have shifted from a relaxed, straightened, noncurly look to a more natural curly and/or kinky appearance. The discussion on natural hair versus straight hair has been brought to the mainstream by films such as Good Hair (2009). Furthermore, major hair care companies have increased their marketing of natural hair products to address the needs of these patients.
Popular traumatic hair care practices such as chemical relaxation and thermal straightening may lead to hair damage. Although the role of hair care practices in various scalp and hair disorders is ambiguous, traumatic practices commonly are performed by patients who are diagnosed with dermatologic conditions such as scarring alopecia.1 Alopecia is the fourth most common dermatologic diagnosis in black patients.2 Central centrifugal cicatricial alopecia is the most common form of scarring alopecia in this patient population3 and has been associated with traumatic hair care practices. As a result, many patients have switched to natural hairstyles that are less traumatic and damaging, often due to recommendations by dermatologists.
As the US population continues to become more diverse, dermatologists will be faced with many questions regarding hair disease and natural hair care in patients with skin of color. A basic understanding of hair care practices among black individuals is important to aid in the diagnosis and treatment of hair shaft and scalp disorders.4 When patients switch to natural hairstyles, are dermatologists prepared to answer questions that may arise during this process? This article will familiarize dermatologists with basic hair care terminology and general recommendations they can make to black patients who are transitioning to natural hairstyles.
Characteristics of Hair in the Skin of Color Population
A basic understanding of the structural properties of hair is fundamental. Human hair is categorized into 3 groups: Asian, Caucasian, and African.5 African hair typically is curly and, depending on the degree of the curl, is more susceptible to damage due to increased mechanical fragility. It also has a tendency to form knots and fissures along the hair shaft, which causes additional fracturing with simple manipulation. African hair grows more slowly than Asian and Caucasian hair, which can be discouraging to patients. It also has a lower water concentration and does not become coated with sebum as naturally as straightened hair.5 A simplified explanation of these characteristics can help patients understand how to proceed in managing and styling their natural hair.
As physicians, it is important for us to treat any underlying conditions related to the hair and scalp in black patients. Common dermatologic conditions such as seborrheic dermatitis, lupus, folliculitis, and alopecia can affect patients’ hair health. In addition to traumatic hair care practices, inflammation secondary to bacterial infections can contribute to the onset of central centrifugal cicatricial alopecia.6 Therefore, a detailed history and physical examination are needed to evaluate the etiology of associated symptoms. Treatment of these associated symptoms will aid in the overall care of patients.
Transitioning to Natural Hairstyles
Following evaluation and treatment of any hair or scalp conditions, how can dermatologists help black patients transition to natural hairstyles? The term transition refers to the process of switching from a chemically relaxed or thermally straightened hairstyle to a natural hairstyle. Dermatologists must understand the common terminology used to describe natural hair practices in this patient population.
There are several methods patients can use to transition from chemically treated hairstyles to natural hairstyles. Patients may consider the option of the “big chop,” or cutting off all chemically treated hair. This option typically leaves women with very short hairstyles down to the new growth, or hair that has grown since the last chemical relaxer. Other commonly used methods during the transition phase include protective styling (eg, braids, weaves, extensions) or simply growing out the chemically treated hair.
Protective styling methods such as braids, weaves, and extensions allow hair to be easily styled while the chemically treated hair grows out over time.7 Typically, protective styles may be worn for weeks to months, allowing hair growth without hair breakage and shedding. Hair weaving is a practice that incorporates artificial (synthetic) or human hair into one’s natural scalp hair.8 There are various techniques to extend hair including clip-in extensions, hair bonding and fusion with adhesives, sewing hair into braided hair, or the application of single strands of hair into a cap made of nylon mesh known as a lace front. Braided styles, weaves, and hair extensions cannot be washed as often as natural hair, but it is important to remind patients to replenish moisture as often as possible. Moisturizing or greasing the exposed scalp and proximal hair shafts can assist with water retention. It is imperative to inform patients that overuse of tight braids and glues for weaves and extensions may further damage the hair and scalp. Some of the natural ingredients commonly used in moisturizers include olive oil, jojoba oil, coconut oil, castor oil, and glycerin. These products can commonly cause pomade acne, which should be recognized and treated by dermatologists. Furthermore, long weaves and extensions can put excess weight on natural hair causing breakage. To prevent breakage, wearing an updo (a hairstyle in which the hair is pulled upward) can reduce the heavy strain on the hair.
Dermatologists should remind patients who wish to grow out chemically treated hair to frequently moisturize the hair and scalp as well as to avoid trauma to prevent hair breakage. As the natural hair grows out, the patient will experience varying hair textures from the natural curly hair to the previously processed straightened hair; as a result, the hair may tangle and become damaged. Manual detangling and detangling conditioners can help prevent damage. Patients should be advised to detangle the hair in sections first with the fingers, then with a wide-tooth comb working retrograde from the hair end to the roots.
Frequent hair trimming, ranging from every 4 to 6 weeks to every 2 to 4 months, should be recommended to patients who are experiencing breakage or wish to prevent damage. Trimming damaged hair can relieve excess weight on the natural hair and remove split ends, which promotes hair growth. Braiding and other lengthening techniques can prevent the hair from curling upon itself or tangling, causing less kinking and thereby decreasing the need for trimming.7 Wearing bonnets, using satin pillowcases, and wearing protective hairstyles while sleeping also can decrease hair breakage and hair loss. A commonly used hairstyle to protect the hair while sleeping is called “pineappling,” which is used to preserve and protect curls. This technique is described as gathering the hair in a high but loose ponytail at the top of the head. For patients with straightened hair, wrapping the hair underneath a bonnet or satin scarf while sleeping can prevent damage.
Managing Natural Hairstyles
An important factor in the management of natural hairstyles is the retention of hair moisture, as there is less water content in African hair compared to other hair types.5 Overuse of heat and harsh shampoos can strip moisture from the hair. Similar to patients with atopic dermatitis who should restore and maintain the skin barrier to prevent transepidermal water loss, it is important to remind patients with natural hairstyles to avoid using products and styling practices that may further deplete water content in the hair. Moisture is crucial to healthy hair.
A common culprit in shampoos that leads to hair dryness is sodium lauryl sulfate/sodium laureth sulfate, a detergent/surfactant used as a foaming agent. Sodium lauryl sulfate is a potent degreaser that binds dirt and excess product on the hair and scalp. It also dissolves oil in the hair, causing additional dryness and breakage.
Patients with natural hairstyles commonly use sulfate-free shampoos to prevent stripping the hair of its moisture and natural oils. Another method used to prevent hair dryness is co-washing, or washing the hair with a conditioner. Co-washing can effectively cleanse the hair while maintaining moisture. The use of cationic ingredients in conditioners aids in sealing moisture within the hair shaft. Hair consists of the negatively charged protein keratin, which binds to cationic surfactants in conditioners.9 The hydrophobic ends of the surfactant prevent the substance from being rinsed out and act to restore the hair barrier.
Silicone is another important ingredient in hair care products. In patients with natural hair, there are varying views on the use of products containing silicone. Silicones are added to products designed to coat the hair, adding shine, retaining moisture, and providing thermal protection. Silicones are used to provide “slip.” Slip is a term that is commonly used among patients with natural hair to describe how slippery a product is and how easily the product will help comb or detangle the hair. There are 2 basic types of silicones: water insoluble and water soluble. Water-insoluble silicones traditionally build up on the hair and require surfactant-containing shampoos to becompletely removed. Residue buildup on the hair weighs the hair down and causes damage. In contrast, water-soluble silicones do not build up and typically do not cause damage.
Silicones with the prefixes PEG- or PPG- typically are water soluble and will not build up on the hair. Dimethicone copolyol and lauryl methicone copolyol are other water-soluble silicones. In general, water-soluble silicones provide moisturizing properties without leaving residue. Other silicones such as amodimethicone and cyclomethicone are not water soluble but have properties that prevent buildup.
It is common practice for patients with natural hairstyles to avoid using water-insoluble silicones. As dermatologists, we can recommend silicone-free conditioners or conditioners containing water-soluble silicones to prevent hair dehydration and subsequent breakage. It may be advantageous to have patients try various products to determine which ones work best for their hair.
More Resources for Patients
Dermatologists have extensive knowledge of the pathophysiology of skin, hair, and nail diseases; however, despite our vast knowledge, we also need to recognize our limits. In addition to increasing your own knowledge of natural hair care practices to help your patients, it is important to recommend that your patients search for additional resources to aid in their transition to natural hairstyles. Natural hairstylists can be great resources for patients to help with hair management. In the current digital age, there also are thousands of blogs and social media forums dedicated to the topic of natural hair care. Advising patients to consult natural hair care resources can be beneficial, but as hair specialists, it also is important for us to dispel any false information that our patients may receive. As physicians, it is essential not only to manage patients who present to our offices with conditions resulting from damaging hair practices but also to help prevent such conditions from occurring. Although there may not be an overwhelming amount of evidence-based medical research to guide our decisions, we also can learn from the thousands of patients who have articulated their stories and experiences. Through observing and listening to our patients, we can incorporate this new knowledge in the management of our patients.
1. Shah SK, Alexis AF. Central centrifugal cicatricial alopecia: retrospective chart review. J Cutan Med Surg. 2010;14:212-222.
2. Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
3. Uhlenhake EE, Mehregan DM. Prospective histologic examinations in patients who practice traumatic hairstyling [published online ahead of print March 3, 2013]. Int J Dermatol. 2013;52:1506-1512.
4. Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108.
5. Kelly AP, Taylor S, eds. Dermatology for Skin of Color. New York: McGraw-Hill; 2009.
6. Kyei A, Bergfeld WF, Piliang M, et al. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: a population study [published online ahead of print April 11, 2011]. Arch Dermatol. 2011;147:909-914.
7. Walton N, Carter ET. Better Than Good Hair: The Curly Girl Guide to Healthy, Gorgeous Natural Hair! New York, NY: Amistad; 2013.
8. Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
9. Cruz CF, Fernandes MM, Gomes AC, et al. Keratins and lipids in ethnic hair [published online ahead of print January 24, 2013]. Int J Cosmet Sci. 2013;35:244-249.
The phenomenon of natural (nonchemically treated) hair in individuals of African and Afro-Caribbean descent is sweeping across the United States. The ideals of beauty among this patient population have shifted from a relaxed, straightened, noncurly look to a more natural curly and/or kinky appearance. The discussion on natural hair versus straight hair has been brought to the mainstream by films such as Good Hair (2009). Furthermore, major hair care companies have increased their marketing of natural hair products to address the needs of these patients.
Popular traumatic hair care practices such as chemical relaxation and thermal straightening may lead to hair damage. Although the role of hair care practices in various scalp and hair disorders is ambiguous, traumatic practices commonly are performed by patients who are diagnosed with dermatologic conditions such as scarring alopecia.1 Alopecia is the fourth most common dermatologic diagnosis in black patients.2 Central centrifugal cicatricial alopecia is the most common form of scarring alopecia in this patient population3 and has been associated with traumatic hair care practices. As a result, many patients have switched to natural hairstyles that are less traumatic and damaging, often due to recommendations by dermatologists.
As the US population continues to become more diverse, dermatologists will be faced with many questions regarding hair disease and natural hair care in patients with skin of color. A basic understanding of hair care practices among black individuals is important to aid in the diagnosis and treatment of hair shaft and scalp disorders.4 When patients switch to natural hairstyles, are dermatologists prepared to answer questions that may arise during this process? This article will familiarize dermatologists with basic hair care terminology and general recommendations they can make to black patients who are transitioning to natural hairstyles.
Characteristics of Hair in the Skin of Color Population
A basic understanding of the structural properties of hair is fundamental. Human hair is categorized into 3 groups: Asian, Caucasian, and African.5 African hair typically is curly and, depending on the degree of the curl, is more susceptible to damage due to increased mechanical fragility. It also has a tendency to form knots and fissures along the hair shaft, which causes additional fracturing with simple manipulation. African hair grows more slowly than Asian and Caucasian hair, which can be discouraging to patients. It also has a lower water concentration and does not become coated with sebum as naturally as straightened hair.5 A simplified explanation of these characteristics can help patients understand how to proceed in managing and styling their natural hair.
As physicians, it is important for us to treat any underlying conditions related to the hair and scalp in black patients. Common dermatologic conditions such as seborrheic dermatitis, lupus, folliculitis, and alopecia can affect patients’ hair health. In addition to traumatic hair care practices, inflammation secondary to bacterial infections can contribute to the onset of central centrifugal cicatricial alopecia.6 Therefore, a detailed history and physical examination are needed to evaluate the etiology of associated symptoms. Treatment of these associated symptoms will aid in the overall care of patients.
Transitioning to Natural Hairstyles
Following evaluation and treatment of any hair or scalp conditions, how can dermatologists help black patients transition to natural hairstyles? The term transition refers to the process of switching from a chemically relaxed or thermally straightened hairstyle to a natural hairstyle. Dermatologists must understand the common terminology used to describe natural hair practices in this patient population.
There are several methods patients can use to transition from chemically treated hairstyles to natural hairstyles. Patients may consider the option of the “big chop,” or cutting off all chemically treated hair. This option typically leaves women with very short hairstyles down to the new growth, or hair that has grown since the last chemical relaxer. Other commonly used methods during the transition phase include protective styling (eg, braids, weaves, extensions) or simply growing out the chemically treated hair.
Protective styling methods such as braids, weaves, and extensions allow hair to be easily styled while the chemically treated hair grows out over time.7 Typically, protective styles may be worn for weeks to months, allowing hair growth without hair breakage and shedding. Hair weaving is a practice that incorporates artificial (synthetic) or human hair into one’s natural scalp hair.8 There are various techniques to extend hair including clip-in extensions, hair bonding and fusion with adhesives, sewing hair into braided hair, or the application of single strands of hair into a cap made of nylon mesh known as a lace front. Braided styles, weaves, and hair extensions cannot be washed as often as natural hair, but it is important to remind patients to replenish moisture as often as possible. Moisturizing or greasing the exposed scalp and proximal hair shafts can assist with water retention. It is imperative to inform patients that overuse of tight braids and glues for weaves and extensions may further damage the hair and scalp. Some of the natural ingredients commonly used in moisturizers include olive oil, jojoba oil, coconut oil, castor oil, and glycerin. These products can commonly cause pomade acne, which should be recognized and treated by dermatologists. Furthermore, long weaves and extensions can put excess weight on natural hair causing breakage. To prevent breakage, wearing an updo (a hairstyle in which the hair is pulled upward) can reduce the heavy strain on the hair.
Dermatologists should remind patients who wish to grow out chemically treated hair to frequently moisturize the hair and scalp as well as to avoid trauma to prevent hair breakage. As the natural hair grows out, the patient will experience varying hair textures from the natural curly hair to the previously processed straightened hair; as a result, the hair may tangle and become damaged. Manual detangling and detangling conditioners can help prevent damage. Patients should be advised to detangle the hair in sections first with the fingers, then with a wide-tooth comb working retrograde from the hair end to the roots.
Frequent hair trimming, ranging from every 4 to 6 weeks to every 2 to 4 months, should be recommended to patients who are experiencing breakage or wish to prevent damage. Trimming damaged hair can relieve excess weight on the natural hair and remove split ends, which promotes hair growth. Braiding and other lengthening techniques can prevent the hair from curling upon itself or tangling, causing less kinking and thereby decreasing the need for trimming.7 Wearing bonnets, using satin pillowcases, and wearing protective hairstyles while sleeping also can decrease hair breakage and hair loss. A commonly used hairstyle to protect the hair while sleeping is called “pineappling,” which is used to preserve and protect curls. This technique is described as gathering the hair in a high but loose ponytail at the top of the head. For patients with straightened hair, wrapping the hair underneath a bonnet or satin scarf while sleeping can prevent damage.
Managing Natural Hairstyles
An important factor in the management of natural hairstyles is the retention of hair moisture, as there is less water content in African hair compared to other hair types.5 Overuse of heat and harsh shampoos can strip moisture from the hair. Similar to patients with atopic dermatitis who should restore and maintain the skin barrier to prevent transepidermal water loss, it is important to remind patients with natural hairstyles to avoid using products and styling practices that may further deplete water content in the hair. Moisture is crucial to healthy hair.
A common culprit in shampoos that leads to hair dryness is sodium lauryl sulfate/sodium laureth sulfate, a detergent/surfactant used as a foaming agent. Sodium lauryl sulfate is a potent degreaser that binds dirt and excess product on the hair and scalp. It also dissolves oil in the hair, causing additional dryness and breakage.
Patients with natural hairstyles commonly use sulfate-free shampoos to prevent stripping the hair of its moisture and natural oils. Another method used to prevent hair dryness is co-washing, or washing the hair with a conditioner. Co-washing can effectively cleanse the hair while maintaining moisture. The use of cationic ingredients in conditioners aids in sealing moisture within the hair shaft. Hair consists of the negatively charged protein keratin, which binds to cationic surfactants in conditioners.9 The hydrophobic ends of the surfactant prevent the substance from being rinsed out and act to restore the hair barrier.
Silicone is another important ingredient in hair care products. In patients with natural hair, there are varying views on the use of products containing silicone. Silicones are added to products designed to coat the hair, adding shine, retaining moisture, and providing thermal protection. Silicones are used to provide “slip.” Slip is a term that is commonly used among patients with natural hair to describe how slippery a product is and how easily the product will help comb or detangle the hair. There are 2 basic types of silicones: water insoluble and water soluble. Water-insoluble silicones traditionally build up on the hair and require surfactant-containing shampoos to becompletely removed. Residue buildup on the hair weighs the hair down and causes damage. In contrast, water-soluble silicones do not build up and typically do not cause damage.
Silicones with the prefixes PEG- or PPG- typically are water soluble and will not build up on the hair. Dimethicone copolyol and lauryl methicone copolyol are other water-soluble silicones. In general, water-soluble silicones provide moisturizing properties without leaving residue. Other silicones such as amodimethicone and cyclomethicone are not water soluble but have properties that prevent buildup.
It is common practice for patients with natural hairstyles to avoid using water-insoluble silicones. As dermatologists, we can recommend silicone-free conditioners or conditioners containing water-soluble silicones to prevent hair dehydration and subsequent breakage. It may be advantageous to have patients try various products to determine which ones work best for their hair.
More Resources for Patients
Dermatologists have extensive knowledge of the pathophysiology of skin, hair, and nail diseases; however, despite our vast knowledge, we also need to recognize our limits. In addition to increasing your own knowledge of natural hair care practices to help your patients, it is important to recommend that your patients search for additional resources to aid in their transition to natural hairstyles. Natural hairstylists can be great resources for patients to help with hair management. In the current digital age, there also are thousands of blogs and social media forums dedicated to the topic of natural hair care. Advising patients to consult natural hair care resources can be beneficial, but as hair specialists, it also is important for us to dispel any false information that our patients may receive. As physicians, it is essential not only to manage patients who present to our offices with conditions resulting from damaging hair practices but also to help prevent such conditions from occurring. Although there may not be an overwhelming amount of evidence-based medical research to guide our decisions, we also can learn from the thousands of patients who have articulated their stories and experiences. Through observing and listening to our patients, we can incorporate this new knowledge in the management of our patients.
The phenomenon of natural (nonchemically treated) hair in individuals of African and Afro-Caribbean descent is sweeping across the United States. The ideals of beauty among this patient population have shifted from a relaxed, straightened, noncurly look to a more natural curly and/or kinky appearance. The discussion on natural hair versus straight hair has been brought to the mainstream by films such as Good Hair (2009). Furthermore, major hair care companies have increased their marketing of natural hair products to address the needs of these patients.
Popular traumatic hair care practices such as chemical relaxation and thermal straightening may lead to hair damage. Although the role of hair care practices in various scalp and hair disorders is ambiguous, traumatic practices commonly are performed by patients who are diagnosed with dermatologic conditions such as scarring alopecia.1 Alopecia is the fourth most common dermatologic diagnosis in black patients.2 Central centrifugal cicatricial alopecia is the most common form of scarring alopecia in this patient population3 and has been associated with traumatic hair care practices. As a result, many patients have switched to natural hairstyles that are less traumatic and damaging, often due to recommendations by dermatologists.
As the US population continues to become more diverse, dermatologists will be faced with many questions regarding hair disease and natural hair care in patients with skin of color. A basic understanding of hair care practices among black individuals is important to aid in the diagnosis and treatment of hair shaft and scalp disorders.4 When patients switch to natural hairstyles, are dermatologists prepared to answer questions that may arise during this process? This article will familiarize dermatologists with basic hair care terminology and general recommendations they can make to black patients who are transitioning to natural hairstyles.
Characteristics of Hair in the Skin of Color Population
A basic understanding of the structural properties of hair is fundamental. Human hair is categorized into 3 groups: Asian, Caucasian, and African.5 African hair typically is curly and, depending on the degree of the curl, is more susceptible to damage due to increased mechanical fragility. It also has a tendency to form knots and fissures along the hair shaft, which causes additional fracturing with simple manipulation. African hair grows more slowly than Asian and Caucasian hair, which can be discouraging to patients. It also has a lower water concentration and does not become coated with sebum as naturally as straightened hair.5 A simplified explanation of these characteristics can help patients understand how to proceed in managing and styling their natural hair.
As physicians, it is important for us to treat any underlying conditions related to the hair and scalp in black patients. Common dermatologic conditions such as seborrheic dermatitis, lupus, folliculitis, and alopecia can affect patients’ hair health. In addition to traumatic hair care practices, inflammation secondary to bacterial infections can contribute to the onset of central centrifugal cicatricial alopecia.6 Therefore, a detailed history and physical examination are needed to evaluate the etiology of associated symptoms. Treatment of these associated symptoms will aid in the overall care of patients.
Transitioning to Natural Hairstyles
Following evaluation and treatment of any hair or scalp conditions, how can dermatologists help black patients transition to natural hairstyles? The term transition refers to the process of switching from a chemically relaxed or thermally straightened hairstyle to a natural hairstyle. Dermatologists must understand the common terminology used to describe natural hair practices in this patient population.
There are several methods patients can use to transition from chemically treated hairstyles to natural hairstyles. Patients may consider the option of the “big chop,” or cutting off all chemically treated hair. This option typically leaves women with very short hairstyles down to the new growth, or hair that has grown since the last chemical relaxer. Other commonly used methods during the transition phase include protective styling (eg, braids, weaves, extensions) or simply growing out the chemically treated hair.
Protective styling methods such as braids, weaves, and extensions allow hair to be easily styled while the chemically treated hair grows out over time.7 Typically, protective styles may be worn for weeks to months, allowing hair growth without hair breakage and shedding. Hair weaving is a practice that incorporates artificial (synthetic) or human hair into one’s natural scalp hair.8 There are various techniques to extend hair including clip-in extensions, hair bonding and fusion with adhesives, sewing hair into braided hair, or the application of single strands of hair into a cap made of nylon mesh known as a lace front. Braided styles, weaves, and hair extensions cannot be washed as often as natural hair, but it is important to remind patients to replenish moisture as often as possible. Moisturizing or greasing the exposed scalp and proximal hair shafts can assist with water retention. It is imperative to inform patients that overuse of tight braids and glues for weaves and extensions may further damage the hair and scalp. Some of the natural ingredients commonly used in moisturizers include olive oil, jojoba oil, coconut oil, castor oil, and glycerin. These products can commonly cause pomade acne, which should be recognized and treated by dermatologists. Furthermore, long weaves and extensions can put excess weight on natural hair causing breakage. To prevent breakage, wearing an updo (a hairstyle in which the hair is pulled upward) can reduce the heavy strain on the hair.
Dermatologists should remind patients who wish to grow out chemically treated hair to frequently moisturize the hair and scalp as well as to avoid trauma to prevent hair breakage. As the natural hair grows out, the patient will experience varying hair textures from the natural curly hair to the previously processed straightened hair; as a result, the hair may tangle and become damaged. Manual detangling and detangling conditioners can help prevent damage. Patients should be advised to detangle the hair in sections first with the fingers, then with a wide-tooth comb working retrograde from the hair end to the roots.
Frequent hair trimming, ranging from every 4 to 6 weeks to every 2 to 4 months, should be recommended to patients who are experiencing breakage or wish to prevent damage. Trimming damaged hair can relieve excess weight on the natural hair and remove split ends, which promotes hair growth. Braiding and other lengthening techniques can prevent the hair from curling upon itself or tangling, causing less kinking and thereby decreasing the need for trimming.7 Wearing bonnets, using satin pillowcases, and wearing protective hairstyles while sleeping also can decrease hair breakage and hair loss. A commonly used hairstyle to protect the hair while sleeping is called “pineappling,” which is used to preserve and protect curls. This technique is described as gathering the hair in a high but loose ponytail at the top of the head. For patients with straightened hair, wrapping the hair underneath a bonnet or satin scarf while sleeping can prevent damage.
Managing Natural Hairstyles
An important factor in the management of natural hairstyles is the retention of hair moisture, as there is less water content in African hair compared to other hair types.5 Overuse of heat and harsh shampoos can strip moisture from the hair. Similar to patients with atopic dermatitis who should restore and maintain the skin barrier to prevent transepidermal water loss, it is important to remind patients with natural hairstyles to avoid using products and styling practices that may further deplete water content in the hair. Moisture is crucial to healthy hair.
A common culprit in shampoos that leads to hair dryness is sodium lauryl sulfate/sodium laureth sulfate, a detergent/surfactant used as a foaming agent. Sodium lauryl sulfate is a potent degreaser that binds dirt and excess product on the hair and scalp. It also dissolves oil in the hair, causing additional dryness and breakage.
Patients with natural hairstyles commonly use sulfate-free shampoos to prevent stripping the hair of its moisture and natural oils. Another method used to prevent hair dryness is co-washing, or washing the hair with a conditioner. Co-washing can effectively cleanse the hair while maintaining moisture. The use of cationic ingredients in conditioners aids in sealing moisture within the hair shaft. Hair consists of the negatively charged protein keratin, which binds to cationic surfactants in conditioners.9 The hydrophobic ends of the surfactant prevent the substance from being rinsed out and act to restore the hair barrier.
Silicone is another important ingredient in hair care products. In patients with natural hair, there are varying views on the use of products containing silicone. Silicones are added to products designed to coat the hair, adding shine, retaining moisture, and providing thermal protection. Silicones are used to provide “slip.” Slip is a term that is commonly used among patients with natural hair to describe how slippery a product is and how easily the product will help comb or detangle the hair. There are 2 basic types of silicones: water insoluble and water soluble. Water-insoluble silicones traditionally build up on the hair and require surfactant-containing shampoos to becompletely removed. Residue buildup on the hair weighs the hair down and causes damage. In contrast, water-soluble silicones do not build up and typically do not cause damage.
Silicones with the prefixes PEG- or PPG- typically are water soluble and will not build up on the hair. Dimethicone copolyol and lauryl methicone copolyol are other water-soluble silicones. In general, water-soluble silicones provide moisturizing properties without leaving residue. Other silicones such as amodimethicone and cyclomethicone are not water soluble but have properties that prevent buildup.
It is common practice for patients with natural hairstyles to avoid using water-insoluble silicones. As dermatologists, we can recommend silicone-free conditioners or conditioners containing water-soluble silicones to prevent hair dehydration and subsequent breakage. It may be advantageous to have patients try various products to determine which ones work best for their hair.
More Resources for Patients
Dermatologists have extensive knowledge of the pathophysiology of skin, hair, and nail diseases; however, despite our vast knowledge, we also need to recognize our limits. In addition to increasing your own knowledge of natural hair care practices to help your patients, it is important to recommend that your patients search for additional resources to aid in their transition to natural hairstyles. Natural hairstylists can be great resources for patients to help with hair management. In the current digital age, there also are thousands of blogs and social media forums dedicated to the topic of natural hair care. Advising patients to consult natural hair care resources can be beneficial, but as hair specialists, it also is important for us to dispel any false information that our patients may receive. As physicians, it is essential not only to manage patients who present to our offices with conditions resulting from damaging hair practices but also to help prevent such conditions from occurring. Although there may not be an overwhelming amount of evidence-based medical research to guide our decisions, we also can learn from the thousands of patients who have articulated their stories and experiences. Through observing and listening to our patients, we can incorporate this new knowledge in the management of our patients.
1. Shah SK, Alexis AF. Central centrifugal cicatricial alopecia: retrospective chart review. J Cutan Med Surg. 2010;14:212-222.
2. Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
3. Uhlenhake EE, Mehregan DM. Prospective histologic examinations in patients who practice traumatic hairstyling [published online ahead of print March 3, 2013]. Int J Dermatol. 2013;52:1506-1512.
4. Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108.
5. Kelly AP, Taylor S, eds. Dermatology for Skin of Color. New York: McGraw-Hill; 2009.
6. Kyei A, Bergfeld WF, Piliang M, et al. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: a population study [published online ahead of print April 11, 2011]. Arch Dermatol. 2011;147:909-914.
7. Walton N, Carter ET. Better Than Good Hair: The Curly Girl Guide to Healthy, Gorgeous Natural Hair! New York, NY: Amistad; 2013.
8. Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
9. Cruz CF, Fernandes MM, Gomes AC, et al. Keratins and lipids in ethnic hair [published online ahead of print January 24, 2013]. Int J Cosmet Sci. 2013;35:244-249.
1. Shah SK, Alexis AF. Central centrifugal cicatricial alopecia: retrospective chart review. J Cutan Med Surg. 2010;14:212-222.
2. Alexis AF, Sergay AB, Taylor SC. Common dermatologic disorders in skin of color: a comparative practice survey. Cutis. 2007;80:387-394.
3. Uhlenhake EE, Mehregan DM. Prospective histologic examinations in patients who practice traumatic hairstyling [published online ahead of print March 3, 2013]. Int J Dermatol. 2013;52:1506-1512.
4. Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108.
5. Kelly AP, Taylor S, eds. Dermatology for Skin of Color. New York: McGraw-Hill; 2009.
6. Kyei A, Bergfeld WF, Piliang M, et al. Medical and environmental risk factors for the development of central centrifugal cicatricial alopecia: a population study [published online ahead of print April 11, 2011]. Arch Dermatol. 2011;147:909-914.
7. Walton N, Carter ET. Better Than Good Hair: The Curly Girl Guide to Healthy, Gorgeous Natural Hair! New York, NY: Amistad; 2013.
8. Quinn CR, Quinn TM, Kelly AP. Hair care practices in African American women. Cutis. 2003;72:280-282, 285-289.
9. Cruz CF, Fernandes MM, Gomes AC, et al. Keratins and lipids in ethnic hair [published online ahead of print January 24, 2013]. Int J Cosmet Sci. 2013;35:244-249.
Practice Points
- Many scalp and hair diseases in patients of African and Afro-Caribbean descent result from traumatic hairstyling practices and poor management. Proper care of these patients requires an understanding of hair variances and styling techniques across ethnicities.
- The use of protective hairstyles and adequate trimming can aid black patients in the transition to healthier natural hair.
- The use of natural oils for scalp health and the avoidance of products containing chemicals that remove moisture from the hair are helpful in maintaining healthy natural hair.