User login
Medical education programs tell how climate change affects health
Ms. Manivannan, copresident of Emory Medical Students for Climate Action, was in the first class of Emory’s medical students to experience the birth of a refined curriculum – lobbied for and partially created by students themselves. The new course of study addresses the myriad ways climate affects health: from air pollution and its effects on the lungs and cardiovascular system to heat-related kidney disease.
“We have known that climate has affected health for decades,” Ms. Manivannan said in a recent interview. “The narrative used to be that icebergs were melting and in 2050 polar bears would be extinct. The piece that’s different now is people are linking climate to increases in asthma and various diseases. We have a way to directly communicate that it’s not a far-off thing. It’s happening to your friends and family right now.”
Hospitals, medical schools, and public health programs are stepping up to educate the next generation of doctors as well as veteran medical workers on one of the most widespread, insidious health threats of our time – climate change – and specific ways it could affect their patients.
Although climate change may seem to many Americans like a distant threat, Marilyn Howarth, MD, a pediatrician in Philadelphia, is trying to make sure physicians are better prepared to treat a growing number of health problems associated with global warming.
“There isn’t a lot of education for pediatricians and internists on environmental health issues. It has not been a standard part of education in medical school or residency training,” Dr. Howarth, deputy director of the new Philadelphia Regional Center for Children’s Environmental Health, said. “With increasing attention on our climate, we really recognize there’s a real gap in physician knowledge, both in pediatric and adult care.”
Scientists have found that climate change can alter just about every system within the human body. Studies show that more extreme weather events, such as heat waves, thunderstorms, and floods, can worsen asthma and produce more pollen and mold, triggering debilitating respiratory problems.
According to the American Lung Association, ultrafine particles of air pollution can be inhaled and then travel throughout the bloodstream, wreaking havoc on organs and increasing risk of heart attack and stroke. Various types of air pollution also cause changes to the climate by trapping heat in the atmosphere, which leads to problems such as rising sea levels and extreme weather. Plus, in a new study published in Nature, scientists warn that warming climates are forcing animals to migrate to different areas, raising the risk that new infectious diseases will hop from animals – such as bats – to humans, a process called “zoonotic spillover” that many researchers believe is responsible for the COVID-19 pandemic.
The Philadelphia Regional Center for Children’s Environmental Health
One of the latest initiatives aimed at disseminating information about children’s health to health care providers is the Philadelphia Regional Center for Children’s Environmental Health, part of Children’s Hospital of Philadelphia and Penn Medicine. CHOP and Penn Medicine are jointly funding this center’s work, which will include educating health care providers on how to better screen for climate-caused health risks and treat related conditions, such as lead poisoning and asthma.
Outreach will focus on providers who treat patients with illnesses that researchers have linked to climate change, Dr. Howarth said. The center will offer clinicians access to seminars and webinars, along with online resources to help doctors treat environmental illnesses. For example, doctors at CHOP’s Poison Control Center are developing a toolkit for physicians to treat patients with elevated levels of lead in the blood. Scientists have linked extreme weather events related to climate change to flooding that pushes metals away from river banks where they were previously contained, allowing them to more easily contaminate homes, soils, and yards.
The initiative builds on CHOP’s Community Asthma Prevention Program (CAPP), which was launched in 1997 by Tyra Bryant-Stephens, MD, its current medical director. CAPP deploys community health workers into homes armed with supplies and tips for managing asthma. The new center will use similar tactics to provide education and resources to patients. The goal is to reach as many at-risk local children as possible.
Future generation of doctors fuel growth in climate change education
Lisa Doggett, MD, cofounder and president of the board of directors of Texas Physicians for Social Responsibility, announced in March that the University of Texas at Austin, Baylor College of Medicine, Houston, and the University of Texas Southwestern in Dallas have all decided to begin offering a course on environmental threats. Emory’s new curriculum has become more comprehensive every year since its start – thanks in part to the input of students like Ms. Manivannan. Faculty members tasked her with approving the new additions to the curriculum on how climate affects health, which in 2019 had consisted of a few slides about issues such as extreme heat exposure and air pollution and their effects on childbirth outcomes.
Material on climate change has now been woven into 13 courses. It is discussed at length in relation to pulmonology, cardiology, and gastropulmonology, for example, said Rebecca Philipsborn, MD, MPA, FAAP, faculty lead for the environmental and health curriculum at Emory.
The curriculum has only been incorporated into Emory’s program for the past 2 years. Dr. Philipsborn said the school plans to expand it to the clinical years to help trainees learn to treat conditions such as pediatric asthma.
“In the past few years, there has been so much momentum, and part of that is a testament to already seeing effects of climate change and how they affect delivery of health care,” she said.
At least one medical journal has recently ramped up its efforts to educate physicians on the links between health issues and climate change. Editors of Family Practice, from Oxford University Press, have announced that they plan to publish a special Climate Crisis and Primary Health Care issue in September.
Of course, not all climate initiatives in medicine are new. A select few have existed for decades.
But only now are physicians widely seeing the links between health and environment, according to Aaron Bernstein, MD, MPH, interim director of the Center for Climate, Health, and the Global Environment (C-CHANGE) at Harvard School of Public Health, Boston.
C-CHANGE, founded in 1996, was the first center in the world to focus on the health effects of environmental change.
“It’s taken 20 years, but what we’re seeing, I think, is the fruits of education,” Dr. Bernstein said. “There’s clearly a wave building here, and I think it really started with education and people younger than the people in charge calling them into account.”
Like the Philadelphia center, Harvard’s program conducts research on climate and health and educates people from high schoolers to health care veterans. Dr. Bernstein helps lead Climate MD, a program that aims to prepare health care workers for climate crises. The Climate MD team has published several articles in peer-reviewed journals on how to better treat patients struggling with environmental health problems. For example, an article on mapping patients in hurricane zones helped shed light on how systems can identify climate-vulnerable patients using public data.
They also developed a tool to help pediatricians provide “climate-informed primary care” – guidance on how to assess whether children are at risk of any harmful environmental exposures, a feature that is not part of standard pediatric visits.
Like the other programs, Climate MD uses community outreach to treat as many local patients as possible. Staff work with providers at more than 100 health clinics, particularly in areas where climate change disproportionately affects residents.
The next major step is to bring some of this into clinical practice, Dr. Bernstein said. In February 2020, C-CHANGE held its first symposium to address that issue.
“The key is to understand climate issues from a provider’s perspective,” he said. “Then those issues can really be brought to the bedside.”
A version of this article first appeared on Medscape.com.
Ms. Manivannan, copresident of Emory Medical Students for Climate Action, was in the first class of Emory’s medical students to experience the birth of a refined curriculum – lobbied for and partially created by students themselves. The new course of study addresses the myriad ways climate affects health: from air pollution and its effects on the lungs and cardiovascular system to heat-related kidney disease.
“We have known that climate has affected health for decades,” Ms. Manivannan said in a recent interview. “The narrative used to be that icebergs were melting and in 2050 polar bears would be extinct. The piece that’s different now is people are linking climate to increases in asthma and various diseases. We have a way to directly communicate that it’s not a far-off thing. It’s happening to your friends and family right now.”
Hospitals, medical schools, and public health programs are stepping up to educate the next generation of doctors as well as veteran medical workers on one of the most widespread, insidious health threats of our time – climate change – and specific ways it could affect their patients.
Although climate change may seem to many Americans like a distant threat, Marilyn Howarth, MD, a pediatrician in Philadelphia, is trying to make sure physicians are better prepared to treat a growing number of health problems associated with global warming.
“There isn’t a lot of education for pediatricians and internists on environmental health issues. It has not been a standard part of education in medical school or residency training,” Dr. Howarth, deputy director of the new Philadelphia Regional Center for Children’s Environmental Health, said. “With increasing attention on our climate, we really recognize there’s a real gap in physician knowledge, both in pediatric and adult care.”
Scientists have found that climate change can alter just about every system within the human body. Studies show that more extreme weather events, such as heat waves, thunderstorms, and floods, can worsen asthma and produce more pollen and mold, triggering debilitating respiratory problems.
According to the American Lung Association, ultrafine particles of air pollution can be inhaled and then travel throughout the bloodstream, wreaking havoc on organs and increasing risk of heart attack and stroke. Various types of air pollution also cause changes to the climate by trapping heat in the atmosphere, which leads to problems such as rising sea levels and extreme weather. Plus, in a new study published in Nature, scientists warn that warming climates are forcing animals to migrate to different areas, raising the risk that new infectious diseases will hop from animals – such as bats – to humans, a process called “zoonotic spillover” that many researchers believe is responsible for the COVID-19 pandemic.
The Philadelphia Regional Center for Children’s Environmental Health
One of the latest initiatives aimed at disseminating information about children’s health to health care providers is the Philadelphia Regional Center for Children’s Environmental Health, part of Children’s Hospital of Philadelphia and Penn Medicine. CHOP and Penn Medicine are jointly funding this center’s work, which will include educating health care providers on how to better screen for climate-caused health risks and treat related conditions, such as lead poisoning and asthma.
Outreach will focus on providers who treat patients with illnesses that researchers have linked to climate change, Dr. Howarth said. The center will offer clinicians access to seminars and webinars, along with online resources to help doctors treat environmental illnesses. For example, doctors at CHOP’s Poison Control Center are developing a toolkit for physicians to treat patients with elevated levels of lead in the blood. Scientists have linked extreme weather events related to climate change to flooding that pushes metals away from river banks where they were previously contained, allowing them to more easily contaminate homes, soils, and yards.
The initiative builds on CHOP’s Community Asthma Prevention Program (CAPP), which was launched in 1997 by Tyra Bryant-Stephens, MD, its current medical director. CAPP deploys community health workers into homes armed with supplies and tips for managing asthma. The new center will use similar tactics to provide education and resources to patients. The goal is to reach as many at-risk local children as possible.
Future generation of doctors fuel growth in climate change education
Lisa Doggett, MD, cofounder and president of the board of directors of Texas Physicians for Social Responsibility, announced in March that the University of Texas at Austin, Baylor College of Medicine, Houston, and the University of Texas Southwestern in Dallas have all decided to begin offering a course on environmental threats. Emory’s new curriculum has become more comprehensive every year since its start – thanks in part to the input of students like Ms. Manivannan. Faculty members tasked her with approving the new additions to the curriculum on how climate affects health, which in 2019 had consisted of a few slides about issues such as extreme heat exposure and air pollution and their effects on childbirth outcomes.
Material on climate change has now been woven into 13 courses. It is discussed at length in relation to pulmonology, cardiology, and gastropulmonology, for example, said Rebecca Philipsborn, MD, MPA, FAAP, faculty lead for the environmental and health curriculum at Emory.
The curriculum has only been incorporated into Emory’s program for the past 2 years. Dr. Philipsborn said the school plans to expand it to the clinical years to help trainees learn to treat conditions such as pediatric asthma.
“In the past few years, there has been so much momentum, and part of that is a testament to already seeing effects of climate change and how they affect delivery of health care,” she said.
At least one medical journal has recently ramped up its efforts to educate physicians on the links between health issues and climate change. Editors of Family Practice, from Oxford University Press, have announced that they plan to publish a special Climate Crisis and Primary Health Care issue in September.
Of course, not all climate initiatives in medicine are new. A select few have existed for decades.
But only now are physicians widely seeing the links between health and environment, according to Aaron Bernstein, MD, MPH, interim director of the Center for Climate, Health, and the Global Environment (C-CHANGE) at Harvard School of Public Health, Boston.
C-CHANGE, founded in 1996, was the first center in the world to focus on the health effects of environmental change.
“It’s taken 20 years, but what we’re seeing, I think, is the fruits of education,” Dr. Bernstein said. “There’s clearly a wave building here, and I think it really started with education and people younger than the people in charge calling them into account.”
Like the Philadelphia center, Harvard’s program conducts research on climate and health and educates people from high schoolers to health care veterans. Dr. Bernstein helps lead Climate MD, a program that aims to prepare health care workers for climate crises. The Climate MD team has published several articles in peer-reviewed journals on how to better treat patients struggling with environmental health problems. For example, an article on mapping patients in hurricane zones helped shed light on how systems can identify climate-vulnerable patients using public data.
They also developed a tool to help pediatricians provide “climate-informed primary care” – guidance on how to assess whether children are at risk of any harmful environmental exposures, a feature that is not part of standard pediatric visits.
Like the other programs, Climate MD uses community outreach to treat as many local patients as possible. Staff work with providers at more than 100 health clinics, particularly in areas where climate change disproportionately affects residents.
The next major step is to bring some of this into clinical practice, Dr. Bernstein said. In February 2020, C-CHANGE held its first symposium to address that issue.
“The key is to understand climate issues from a provider’s perspective,” he said. “Then those issues can really be brought to the bedside.”
A version of this article first appeared on Medscape.com.
Ms. Manivannan, copresident of Emory Medical Students for Climate Action, was in the first class of Emory’s medical students to experience the birth of a refined curriculum – lobbied for and partially created by students themselves. The new course of study addresses the myriad ways climate affects health: from air pollution and its effects on the lungs and cardiovascular system to heat-related kidney disease.
“We have known that climate has affected health for decades,” Ms. Manivannan said in a recent interview. “The narrative used to be that icebergs were melting and in 2050 polar bears would be extinct. The piece that’s different now is people are linking climate to increases in asthma and various diseases. We have a way to directly communicate that it’s not a far-off thing. It’s happening to your friends and family right now.”
Hospitals, medical schools, and public health programs are stepping up to educate the next generation of doctors as well as veteran medical workers on one of the most widespread, insidious health threats of our time – climate change – and specific ways it could affect their patients.
Although climate change may seem to many Americans like a distant threat, Marilyn Howarth, MD, a pediatrician in Philadelphia, is trying to make sure physicians are better prepared to treat a growing number of health problems associated with global warming.
“There isn’t a lot of education for pediatricians and internists on environmental health issues. It has not been a standard part of education in medical school or residency training,” Dr. Howarth, deputy director of the new Philadelphia Regional Center for Children’s Environmental Health, said. “With increasing attention on our climate, we really recognize there’s a real gap in physician knowledge, both in pediatric and adult care.”
Scientists have found that climate change can alter just about every system within the human body. Studies show that more extreme weather events, such as heat waves, thunderstorms, and floods, can worsen asthma and produce more pollen and mold, triggering debilitating respiratory problems.
According to the American Lung Association, ultrafine particles of air pollution can be inhaled and then travel throughout the bloodstream, wreaking havoc on organs and increasing risk of heart attack and stroke. Various types of air pollution also cause changes to the climate by trapping heat in the atmosphere, which leads to problems such as rising sea levels and extreme weather. Plus, in a new study published in Nature, scientists warn that warming climates are forcing animals to migrate to different areas, raising the risk that new infectious diseases will hop from animals – such as bats – to humans, a process called “zoonotic spillover” that many researchers believe is responsible for the COVID-19 pandemic.
The Philadelphia Regional Center for Children’s Environmental Health
One of the latest initiatives aimed at disseminating information about children’s health to health care providers is the Philadelphia Regional Center for Children’s Environmental Health, part of Children’s Hospital of Philadelphia and Penn Medicine. CHOP and Penn Medicine are jointly funding this center’s work, which will include educating health care providers on how to better screen for climate-caused health risks and treat related conditions, such as lead poisoning and asthma.
Outreach will focus on providers who treat patients with illnesses that researchers have linked to climate change, Dr. Howarth said. The center will offer clinicians access to seminars and webinars, along with online resources to help doctors treat environmental illnesses. For example, doctors at CHOP’s Poison Control Center are developing a toolkit for physicians to treat patients with elevated levels of lead in the blood. Scientists have linked extreme weather events related to climate change to flooding that pushes metals away from river banks where they were previously contained, allowing them to more easily contaminate homes, soils, and yards.
The initiative builds on CHOP’s Community Asthma Prevention Program (CAPP), which was launched in 1997 by Tyra Bryant-Stephens, MD, its current medical director. CAPP deploys community health workers into homes armed with supplies and tips for managing asthma. The new center will use similar tactics to provide education and resources to patients. The goal is to reach as many at-risk local children as possible.
Future generation of doctors fuel growth in climate change education
Lisa Doggett, MD, cofounder and president of the board of directors of Texas Physicians for Social Responsibility, announced in March that the University of Texas at Austin, Baylor College of Medicine, Houston, and the University of Texas Southwestern in Dallas have all decided to begin offering a course on environmental threats. Emory’s new curriculum has become more comprehensive every year since its start – thanks in part to the input of students like Ms. Manivannan. Faculty members tasked her with approving the new additions to the curriculum on how climate affects health, which in 2019 had consisted of a few slides about issues such as extreme heat exposure and air pollution and their effects on childbirth outcomes.
Material on climate change has now been woven into 13 courses. It is discussed at length in relation to pulmonology, cardiology, and gastropulmonology, for example, said Rebecca Philipsborn, MD, MPA, FAAP, faculty lead for the environmental and health curriculum at Emory.
The curriculum has only been incorporated into Emory’s program for the past 2 years. Dr. Philipsborn said the school plans to expand it to the clinical years to help trainees learn to treat conditions such as pediatric asthma.
“In the past few years, there has been so much momentum, and part of that is a testament to already seeing effects of climate change and how they affect delivery of health care,” she said.
At least one medical journal has recently ramped up its efforts to educate physicians on the links between health issues and climate change. Editors of Family Practice, from Oxford University Press, have announced that they plan to publish a special Climate Crisis and Primary Health Care issue in September.
Of course, not all climate initiatives in medicine are new. A select few have existed for decades.
But only now are physicians widely seeing the links between health and environment, according to Aaron Bernstein, MD, MPH, interim director of the Center for Climate, Health, and the Global Environment (C-CHANGE) at Harvard School of Public Health, Boston.
C-CHANGE, founded in 1996, was the first center in the world to focus on the health effects of environmental change.
“It’s taken 20 years, but what we’re seeing, I think, is the fruits of education,” Dr. Bernstein said. “There’s clearly a wave building here, and I think it really started with education and people younger than the people in charge calling them into account.”
Like the Philadelphia center, Harvard’s program conducts research on climate and health and educates people from high schoolers to health care veterans. Dr. Bernstein helps lead Climate MD, a program that aims to prepare health care workers for climate crises. The Climate MD team has published several articles in peer-reviewed journals on how to better treat patients struggling with environmental health problems. For example, an article on mapping patients in hurricane zones helped shed light on how systems can identify climate-vulnerable patients using public data.
They also developed a tool to help pediatricians provide “climate-informed primary care” – guidance on how to assess whether children are at risk of any harmful environmental exposures, a feature that is not part of standard pediatric visits.
Like the other programs, Climate MD uses community outreach to treat as many local patients as possible. Staff work with providers at more than 100 health clinics, particularly in areas where climate change disproportionately affects residents.
The next major step is to bring some of this into clinical practice, Dr. Bernstein said. In February 2020, C-CHANGE held its first symposium to address that issue.
“The key is to understand climate issues from a provider’s perspective,” he said. “Then those issues can really be brought to the bedside.”
A version of this article first appeared on Medscape.com.
How social determinants of health impact disparities in IBD care, outcomes
The incidence of inflammatory bowel disease (IBD) is on the rise among racial and ethnic minority groups in the United States, and social determinants of health (SDOH) contribute to disparities in IBD care and outcome, say the authors of a new paper on the topic.
It’s an “overdue priority to acknowledge the weight and influence of the SDOH on health disparities in IBD care,” write Adjoa Anyane-Yeboa, MD, PhD, with Massachusetts General Hospital, Boston, and co-authors.
“Only after this acknowledgement can we begin to develop alternative systems that work to rectify the deleterious effects of our current policies in a more longitudinal and effective manner,” they say.
Their paper was published online in Clinical Gastroenterology and Hepatology.
Upstream factors propagate downstream outcomes
The authors found multiple examples in the literature of how upstream SDOH (for example, racism, poverty, neighborhood violence, and under-insurance) lead to midstream SDOH (for example, lack of social support, lack of access to specialized IBD care, poor housing conditions, and food insecurity) that result in poor downstream outcomes in IBD (for example, delayed diagnosis, increased disease activity, IBD flares, and suboptimal medical management).
The IBD literature shows that Black/African American adults with IBD often have worse outcomes across the IBD care continuum than White peers, with higher hospitalization rates, longer stays, increased hospitalization costs, higher readmission rates, and more complications after IBD surgery.
Unequal access to specialized IBD care is a factor, with Black/African American patients less likely to undergo annual visits to a gastroenterologist or IBD specialist, twice as likely than White patients to visit the emergency department over a 12-month period, and less likely to receive treatment with infliximab.
As has been shown for other chronic digestive diseases and cancers, disparities in outcomes related to IBD exist across race, ethnicity, differential insurance status and coverage, and socioeconomic status, the authors note.
Yet, they point out that, interestingly, a 2021 study of patients with Medicaid insurance from four states revealed no disparities in the use of IBD-specific medications between Black/African American and White patients, suggesting that when access to care is equal, disparities diminish.
Target multiple stakeholders to achieve IBD health equity
Achieving health equity in IBD will require strategies targeting medical trainees, providers, practices, and health systems, as well as community and industry leaders and policymakers, Dr. Anyane-Yeboa and colleagues say.
At the medical trainee level, racism and bias should be addressed early in medical student, resident, and fellow training and education. Curricula should move away from race-based training, where race is considered an independent risk factor for disease and often used to guide differential diagnoses and treatment, they suggest.
At the provider level, they say self-reflection around one’s own beliefs, biases, perceptions, and interactions with diverse and vulnerable patient groups is “paramount.” Individual self-reflection should be coupled with mandatory and effective implicit bias and anti-racism training.
At the practice or hospital system level, screening for SDOH at the point of care, addressing barriers to needed treatment, and connecting patients to appropriate resources are all important, they write.
The researchers also call for policy-level changes to increase funding for health equity research, which is historically undervalued and underfunded.
“Focusing on SDOH as the root cause of health inequity in IBD is essential to improve outcomes for marginalized patients,” they write.
Given that research describing specific interventions to address SDOH in IBD is currently nonexistent, “our paper serves as a call to action for more work to be done in this area,” they say.
“As medical providers and health care organizations, we all have a responsibility to address the SDOH when caring for our patients in order to provide each patient with IBD the opportunity to achieve the best health possible,” they conclude.
This research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The incidence of inflammatory bowel disease (IBD) is on the rise among racial and ethnic minority groups in the United States, and social determinants of health (SDOH) contribute to disparities in IBD care and outcome, say the authors of a new paper on the topic.
It’s an “overdue priority to acknowledge the weight and influence of the SDOH on health disparities in IBD care,” write Adjoa Anyane-Yeboa, MD, PhD, with Massachusetts General Hospital, Boston, and co-authors.
“Only after this acknowledgement can we begin to develop alternative systems that work to rectify the deleterious effects of our current policies in a more longitudinal and effective manner,” they say.
Their paper was published online in Clinical Gastroenterology and Hepatology.
Upstream factors propagate downstream outcomes
The authors found multiple examples in the literature of how upstream SDOH (for example, racism, poverty, neighborhood violence, and under-insurance) lead to midstream SDOH (for example, lack of social support, lack of access to specialized IBD care, poor housing conditions, and food insecurity) that result in poor downstream outcomes in IBD (for example, delayed diagnosis, increased disease activity, IBD flares, and suboptimal medical management).
The IBD literature shows that Black/African American adults with IBD often have worse outcomes across the IBD care continuum than White peers, with higher hospitalization rates, longer stays, increased hospitalization costs, higher readmission rates, and more complications after IBD surgery.
Unequal access to specialized IBD care is a factor, with Black/African American patients less likely to undergo annual visits to a gastroenterologist or IBD specialist, twice as likely than White patients to visit the emergency department over a 12-month period, and less likely to receive treatment with infliximab.
As has been shown for other chronic digestive diseases and cancers, disparities in outcomes related to IBD exist across race, ethnicity, differential insurance status and coverage, and socioeconomic status, the authors note.
Yet, they point out that, interestingly, a 2021 study of patients with Medicaid insurance from four states revealed no disparities in the use of IBD-specific medications between Black/African American and White patients, suggesting that when access to care is equal, disparities diminish.
Target multiple stakeholders to achieve IBD health equity
Achieving health equity in IBD will require strategies targeting medical trainees, providers, practices, and health systems, as well as community and industry leaders and policymakers, Dr. Anyane-Yeboa and colleagues say.
At the medical trainee level, racism and bias should be addressed early in medical student, resident, and fellow training and education. Curricula should move away from race-based training, where race is considered an independent risk factor for disease and often used to guide differential diagnoses and treatment, they suggest.
At the provider level, they say self-reflection around one’s own beliefs, biases, perceptions, and interactions with diverse and vulnerable patient groups is “paramount.” Individual self-reflection should be coupled with mandatory and effective implicit bias and anti-racism training.
At the practice or hospital system level, screening for SDOH at the point of care, addressing barriers to needed treatment, and connecting patients to appropriate resources are all important, they write.
The researchers also call for policy-level changes to increase funding for health equity research, which is historically undervalued and underfunded.
“Focusing on SDOH as the root cause of health inequity in IBD is essential to improve outcomes for marginalized patients,” they write.
Given that research describing specific interventions to address SDOH in IBD is currently nonexistent, “our paper serves as a call to action for more work to be done in this area,” they say.
“As medical providers and health care organizations, we all have a responsibility to address the SDOH when caring for our patients in order to provide each patient with IBD the opportunity to achieve the best health possible,” they conclude.
This research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The incidence of inflammatory bowel disease (IBD) is on the rise among racial and ethnic minority groups in the United States, and social determinants of health (SDOH) contribute to disparities in IBD care and outcome, say the authors of a new paper on the topic.
It’s an “overdue priority to acknowledge the weight and influence of the SDOH on health disparities in IBD care,” write Adjoa Anyane-Yeboa, MD, PhD, with Massachusetts General Hospital, Boston, and co-authors.
“Only after this acknowledgement can we begin to develop alternative systems that work to rectify the deleterious effects of our current policies in a more longitudinal and effective manner,” they say.
Their paper was published online in Clinical Gastroenterology and Hepatology.
Upstream factors propagate downstream outcomes
The authors found multiple examples in the literature of how upstream SDOH (for example, racism, poverty, neighborhood violence, and under-insurance) lead to midstream SDOH (for example, lack of social support, lack of access to specialized IBD care, poor housing conditions, and food insecurity) that result in poor downstream outcomes in IBD (for example, delayed diagnosis, increased disease activity, IBD flares, and suboptimal medical management).
The IBD literature shows that Black/African American adults with IBD often have worse outcomes across the IBD care continuum than White peers, with higher hospitalization rates, longer stays, increased hospitalization costs, higher readmission rates, and more complications after IBD surgery.
Unequal access to specialized IBD care is a factor, with Black/African American patients less likely to undergo annual visits to a gastroenterologist or IBD specialist, twice as likely than White patients to visit the emergency department over a 12-month period, and less likely to receive treatment with infliximab.
As has been shown for other chronic digestive diseases and cancers, disparities in outcomes related to IBD exist across race, ethnicity, differential insurance status and coverage, and socioeconomic status, the authors note.
Yet, they point out that, interestingly, a 2021 study of patients with Medicaid insurance from four states revealed no disparities in the use of IBD-specific medications between Black/African American and White patients, suggesting that when access to care is equal, disparities diminish.
Target multiple stakeholders to achieve IBD health equity
Achieving health equity in IBD will require strategies targeting medical trainees, providers, practices, and health systems, as well as community and industry leaders and policymakers, Dr. Anyane-Yeboa and colleagues say.
At the medical trainee level, racism and bias should be addressed early in medical student, resident, and fellow training and education. Curricula should move away from race-based training, where race is considered an independent risk factor for disease and often used to guide differential diagnoses and treatment, they suggest.
At the provider level, they say self-reflection around one’s own beliefs, biases, perceptions, and interactions with diverse and vulnerable patient groups is “paramount.” Individual self-reflection should be coupled with mandatory and effective implicit bias and anti-racism training.
At the practice or hospital system level, screening for SDOH at the point of care, addressing barriers to needed treatment, and connecting patients to appropriate resources are all important, they write.
The researchers also call for policy-level changes to increase funding for health equity research, which is historically undervalued and underfunded.
“Focusing on SDOH as the root cause of health inequity in IBD is essential to improve outcomes for marginalized patients,” they write.
Given that research describing specific interventions to address SDOH in IBD is currently nonexistent, “our paper serves as a call to action for more work to be done in this area,” they say.
“As medical providers and health care organizations, we all have a responsibility to address the SDOH when caring for our patients in order to provide each patient with IBD the opportunity to achieve the best health possible,” they conclude.
This research had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
COVID fallout: ‘Alarming’ dip in routine vax for pregnant women
The percentage of low-income pregnant mothers who received influenza and Tdap vaccinations fell sharply during the COVID-19 pandemic, especially in Black and Hispanic patients, a new study finds.
The percentage of patients who received the influenza vaccines at two Medicaid clinics in Houston dropped from 78% before the pandemic to 61% during it (adjusted odds ratio, 0.38; 95% CI, 0.26-0.53; P < .01), researchers reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The percentage receiving the Tdap vaccine dipped from 85% to 76% (aOR, 0.56; 95% CI, 0.40-0.79; P < .01).
New York–Presbyterian/Weill Cornell Medical Center pediatrician Sallie Permar, MD, PhD, who’s familiar with the study findings, called them “alarming” and said in an interview that they should be “a call to action for providers.”
“Continuing the status quo in our routine preventative health care and clinic operations means that we are losing ground in reduction and elimination of vaccine-preventable diseases,” Dr. Permar said in an interview.
According to corresponding author Bani Ratan, MD, an ob.gyn. with the Baylor College of Medicine, Houston, there’s been little if any previous research into routine, non-COVID vaccination in pregnant women during the pandemic.
For the study, researchers retrospectively analyzed the records of 939 pregnant women who entered prenatal care before 20 weeks (462 from May–November 2019, and 477 from May–November 2020) and delivered at full term.
Among ethnic groups, non-Hispanic Blacks saw the largest decline in influenza vaccines. Among them, the percentage who got them fell from 64% (73/114) to 35% (35/101; aOR, 0.30; 95% CI, 0.17-0.52; P < .01). Only Hispanics had a statistically significant decline in Tdap vaccination (OR, 0.52, 95% CI, 0.34-0.80; P < .01, percentages not provided).
Another study presented at ACOG examined vaccination rates during the pandemic and found that Tdap vaccination rates dipped among pregnant women in a Philadelphia-area health care system.
Possible causes for the decline in routine vaccination include hesitancy linked to the COVID-19 vaccines and fewer office visits because of telemedicine, said Dr. Batan in an interview.
Dr. Permar blamed the role of vaccine misinformation during the pandemic and the mistrust caused by the exclusion of pregnant women from early vaccine trials. She added that “challenges in health care staffing and issues of health care provider burnout that worsened during the pandemic likely contributed to a fraying of the focus on preventive health maintenance simply due to bandwidth of health professionals.”
In a separate study presented at ACOG, researchers at the State University of New York, Syracuse, reported on a survey of 157 pregnant women of whom just 38.2% were vaccinated against COVID-19. Among the unvaccinated, who were more likely to have less education, 66% reported that lack of data about vaccination was their primary concern.
No funding or disclosures are reported by study authors. Dr. Permar reported consulting for Merck, Moderna, GlaxoSmithKline, Pfizer, Dynavax, and Hookipa on cytomegalovirus vaccine programs.
*This story was updated on 5/11/2022.
The percentage of low-income pregnant mothers who received influenza and Tdap vaccinations fell sharply during the COVID-19 pandemic, especially in Black and Hispanic patients, a new study finds.
The percentage of patients who received the influenza vaccines at two Medicaid clinics in Houston dropped from 78% before the pandemic to 61% during it (adjusted odds ratio, 0.38; 95% CI, 0.26-0.53; P < .01), researchers reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The percentage receiving the Tdap vaccine dipped from 85% to 76% (aOR, 0.56; 95% CI, 0.40-0.79; P < .01).
New York–Presbyterian/Weill Cornell Medical Center pediatrician Sallie Permar, MD, PhD, who’s familiar with the study findings, called them “alarming” and said in an interview that they should be “a call to action for providers.”
“Continuing the status quo in our routine preventative health care and clinic operations means that we are losing ground in reduction and elimination of vaccine-preventable diseases,” Dr. Permar said in an interview.
According to corresponding author Bani Ratan, MD, an ob.gyn. with the Baylor College of Medicine, Houston, there’s been little if any previous research into routine, non-COVID vaccination in pregnant women during the pandemic.
For the study, researchers retrospectively analyzed the records of 939 pregnant women who entered prenatal care before 20 weeks (462 from May–November 2019, and 477 from May–November 2020) and delivered at full term.
Among ethnic groups, non-Hispanic Blacks saw the largest decline in influenza vaccines. Among them, the percentage who got them fell from 64% (73/114) to 35% (35/101; aOR, 0.30; 95% CI, 0.17-0.52; P < .01). Only Hispanics had a statistically significant decline in Tdap vaccination (OR, 0.52, 95% CI, 0.34-0.80; P < .01, percentages not provided).
Another study presented at ACOG examined vaccination rates during the pandemic and found that Tdap vaccination rates dipped among pregnant women in a Philadelphia-area health care system.
Possible causes for the decline in routine vaccination include hesitancy linked to the COVID-19 vaccines and fewer office visits because of telemedicine, said Dr. Batan in an interview.
Dr. Permar blamed the role of vaccine misinformation during the pandemic and the mistrust caused by the exclusion of pregnant women from early vaccine trials. She added that “challenges in health care staffing and issues of health care provider burnout that worsened during the pandemic likely contributed to a fraying of the focus on preventive health maintenance simply due to bandwidth of health professionals.”
In a separate study presented at ACOG, researchers at the State University of New York, Syracuse, reported on a survey of 157 pregnant women of whom just 38.2% were vaccinated against COVID-19. Among the unvaccinated, who were more likely to have less education, 66% reported that lack of data about vaccination was their primary concern.
No funding or disclosures are reported by study authors. Dr. Permar reported consulting for Merck, Moderna, GlaxoSmithKline, Pfizer, Dynavax, and Hookipa on cytomegalovirus vaccine programs.
*This story was updated on 5/11/2022.
The percentage of low-income pregnant mothers who received influenza and Tdap vaccinations fell sharply during the COVID-19 pandemic, especially in Black and Hispanic patients, a new study finds.
The percentage of patients who received the influenza vaccines at two Medicaid clinics in Houston dropped from 78% before the pandemic to 61% during it (adjusted odds ratio, 0.38; 95% CI, 0.26-0.53; P < .01), researchers reported at the annual clinical and scientific meeting of the American College of Obstetricians and Gynecologists. The percentage receiving the Tdap vaccine dipped from 85% to 76% (aOR, 0.56; 95% CI, 0.40-0.79; P < .01).
New York–Presbyterian/Weill Cornell Medical Center pediatrician Sallie Permar, MD, PhD, who’s familiar with the study findings, called them “alarming” and said in an interview that they should be “a call to action for providers.”
“Continuing the status quo in our routine preventative health care and clinic operations means that we are losing ground in reduction and elimination of vaccine-preventable diseases,” Dr. Permar said in an interview.
According to corresponding author Bani Ratan, MD, an ob.gyn. with the Baylor College of Medicine, Houston, there’s been little if any previous research into routine, non-COVID vaccination in pregnant women during the pandemic.
For the study, researchers retrospectively analyzed the records of 939 pregnant women who entered prenatal care before 20 weeks (462 from May–November 2019, and 477 from May–November 2020) and delivered at full term.
Among ethnic groups, non-Hispanic Blacks saw the largest decline in influenza vaccines. Among them, the percentage who got them fell from 64% (73/114) to 35% (35/101; aOR, 0.30; 95% CI, 0.17-0.52; P < .01). Only Hispanics had a statistically significant decline in Tdap vaccination (OR, 0.52, 95% CI, 0.34-0.80; P < .01, percentages not provided).
Another study presented at ACOG examined vaccination rates during the pandemic and found that Tdap vaccination rates dipped among pregnant women in a Philadelphia-area health care system.
Possible causes for the decline in routine vaccination include hesitancy linked to the COVID-19 vaccines and fewer office visits because of telemedicine, said Dr. Batan in an interview.
Dr. Permar blamed the role of vaccine misinformation during the pandemic and the mistrust caused by the exclusion of pregnant women from early vaccine trials. She added that “challenges in health care staffing and issues of health care provider burnout that worsened during the pandemic likely contributed to a fraying of the focus on preventive health maintenance simply due to bandwidth of health professionals.”
In a separate study presented at ACOG, researchers at the State University of New York, Syracuse, reported on a survey of 157 pregnant women of whom just 38.2% were vaccinated against COVID-19. Among the unvaccinated, who were more likely to have less education, 66% reported that lack of data about vaccination was their primary concern.
No funding or disclosures are reported by study authors. Dr. Permar reported consulting for Merck, Moderna, GlaxoSmithKline, Pfizer, Dynavax, and Hookipa on cytomegalovirus vaccine programs.
*This story was updated on 5/11/2022.
FROM ACOG 2022
Screening for diabetes at normal BMIs could cut racial disparities
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
Use of race-based diabetes screening thresholds could reduce the disparity that arises from current screening guidelines in the United States, new research suggests.
In August 2021, the U.S. Preventive Services Task Force (USPSTF) lowered the recommended age for type 2 diabetes screening from 40 to 35 years among people with a body mass index of 25 kg/m2 or greater.
However, the diabetes rate among ethnic minorities aged 35-70 years in the United States is not just higher overall but, in certain populations, also occurs more frequently at a younger age and at lower BMIs, the new study indicates.
Among people with a BMI below 25 kg/m2, the diabetes prevalence is two to four times higher among Asian, Black, and Hispanic Americans than among the U.S. White population.
And the authors of the new study, led by Rahul Aggarwal, MD, predict that if screening begins at age 35 years, the BMI cut-off equivalent to 25 kg/m2 for White Americans would be 18.5 kg/m2 for Hispanic and Black Americans and 20 kg/m2 for Asian Americans.
“While diabetes has often been thought of as a disease that primarily affects adults with overweight or [obesity], our findings suggest that normal-weight adults in minority groups have surprisingly high rates of diabetes,” Dr. Aggarwal, senior resident physician in internal medicine at Harvard Medical School, Boston, told this news organization.
“Assessing diabetes risks in certain racial/ethnic groups will be necessary, even if these adults do not have overweight or [obesity],” he added.
Not screening in this way “is a missed opportunity for early intervention,” he noted.
And both the authors and an editorialist stress that the issue isn’t just theoretical.
“USPSTF recommendations influence what payers choose to cover, which in turn determines access to preventative services ... Addressing the staggering inequities in diabetes outcomes will require substantial investments in diabetes prevention and treatment, but making screening more equitable is a good place to start,” said senior author Dhruv S. Kazi, MD, of the Smith Center for Outcomes Research in Cardiology and director of the Cardiac Critical Care Unit at Beth Israel, Boston.
Screen minorities at a younger age if current BMI threshold kept
In their study, based on data from the National Health and Nutrition Examination Survey (NHANES) for 2011-2018, Dr. Aggarwal and colleagues also calculated that, if the BMI threshold is kept at 25 kg/m2, then the equivalent age cut-offs for Asian, Black, and Hispanic Americans would be 23, 21, and 25 years, respectively, compared with 35 years for White Americans.
The findings were published online in the Annals of Internal Medicine.
The prevalence of diabetes in those aged 35-70 years in the NHANES population was 17.3% for Asian Americans and 12.5% for those who were White (odds ratio, 1.51 vs. Whites). Among Black Americans and Mexican Americans, the prevalence was 20.7% and 20.6%, respectively, almost twice the prevalence in Whites (OR, 1.85 and 1.80). For other Hispanic Americans, the prevalence was 16.4% (OR, 1.37 vs. Whites). All of those differences were significant, compared with White Americans.
Undiagnosed diabetes was also significantly more common among minority populations, at 27.6%, 22.8%, 21.2%, and 23.5% for Asian, Black, Mexican, and other Hispanic Americans, respectively, versus 12.5% for White Americans.
‘The time has come for USPSTF to offer more concrete guidance’
“While there is more work to be done on carefully examining the long-term risk–benefit trade-off of various diabetes screening, I believe the time has come for USPSTF to offer more concrete guidance on the use of lower thresholds for screening higher-risk individuals,” Dr. Kazi told this news organization.
The author of an accompanying editorial agrees, noting that in a recent commentary the USPSTF, itself, “acknowledged the persistent inequalities across the screening-to-treatment continuum that result in racial/ethnic health disparities in the United States.”
And the USPSTF “emphasized the need to improve systems of care to ensure equitable and consistent delivery of high-quality preventive and treatment services, with special attention to racial/ethnic groups who may experience worse health outcomes,” continues Quyen Ngo-Metzger, MD, Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California.
For other conditions, including cancer, cardiovascular disease, and infectious disease, the USPSTF already recommends risk-based preventive services.
“To address the current inequity in diabetes screening, the USPSTF should apply the same consideration to its diabetes screening recommendation,” she notes.
‘Implementation will require an eye for pragmatism’
Asked about how this recommendation might be carried out in the real world, Dr. Aggarwal said in an interview that, because all three minority groups with normal weight had similar diabetes risk profiles to White adults with overweight, “one way for clinicians to easily implement these findings is by screening all Asian, Black, and Hispanic adults ages 35-70 years with normal weight for diabetes, similarly to how all White adults ages 35-70 years with overweight are currently recommended for screening.”
Dr. Kazi said: “I believe that implementation will require an eye for pragmatism,” noting that another option would be to have screening algorithms embedded in the electronic health record to flag individuals who qualify.
In any case, “the simplicity of the current one-size-fits-all approach is alluring, but it is profoundly inequitable. The more I look at the empiric evidence on diabetes burden in our communities, the more the status quo becomes untenable.”
However, Dr. Kazi also noted, “the benefit of any screening program relates to what we do with the information. The key is to ensure that folks identified as having diabetes – or better still prediabetes – receive timely lifestyle and pharmacological interventions to avert its long-term complications.”
This study was supported by institutional funds from the Richard A. and Susan F. Smith Center for Outcomes Research in Cardiology. Dr. Aggarwal, Dr. Kazi, and Dr. Ngo-Metzger have reported no relevant relationships.
A version of this article first appeared on Medscape.com.
Melanoma
THE COMPARISON
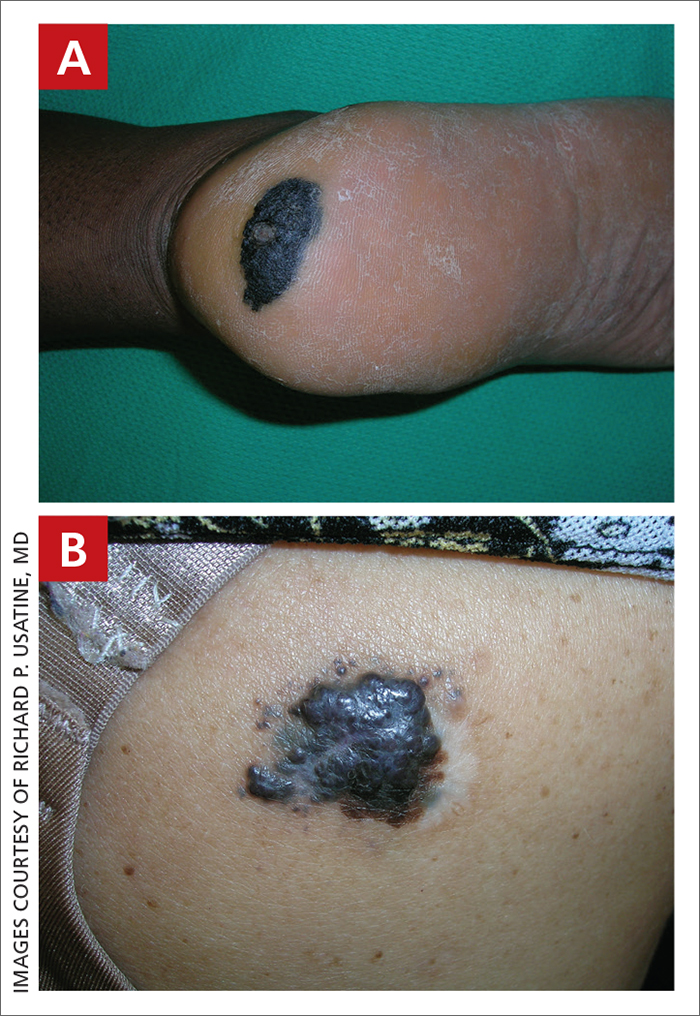
A Acral lentiginous melanoma on the sole of the foot of a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
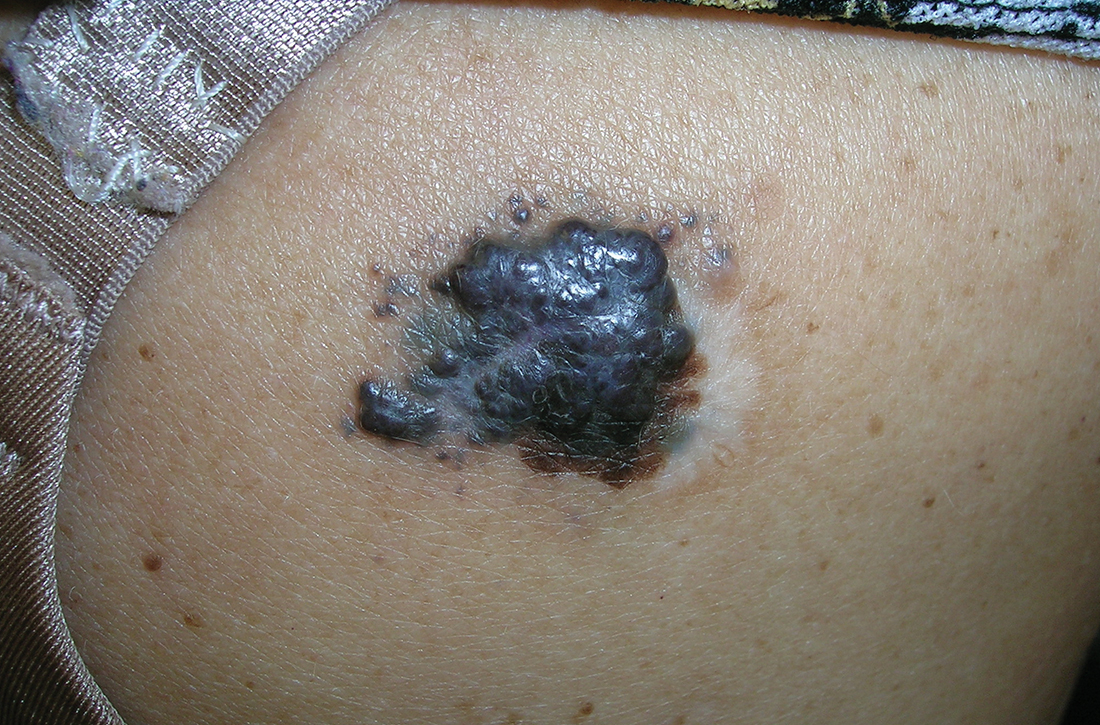
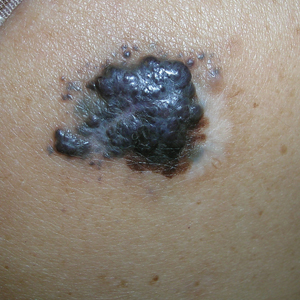
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in those with lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P <. 05), followed by Hispanic (P < .05), Asian American/Native American/Pacific Islander (P < .05), and Black (P < .05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P = .015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P < .001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P = .07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when researchers controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
1. Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi: 10.1016/j.jaad.2001.05.034
2. Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi: 10.1001/archinte.166.17.1907
3. Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi: 10.1023/a:1018432632528
4. Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi: 10.1016/ j.jaad.2004.05.005
5. Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142: 704-708. doi: 10.1001/archderm.142.6.704
6. Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi: 10.1097/DSS.0000000000001759
7. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi: 10.1016/j.jaad.2016.06.006
8. Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016) [published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi: 10.1016/ j.jaad.2020.08.097
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot of a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in those with lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.

Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P <. 05), followed by Hispanic (P < .05), Asian American/Native American/Pacific Islander (P < .05), and Black (P < .05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P = .015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P < .001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P = .07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when researchers controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot of a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in those with lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.

Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P <. 05), followed by Hispanic (P < .05), Asian American/Native American/Pacific Islander (P < .05), and Black (P < .05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P = .015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P < .001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P = .07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when researchers controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
1. Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi: 10.1016/j.jaad.2001.05.034
2. Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi: 10.1001/archinte.166.17.1907
3. Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi: 10.1023/a:1018432632528
4. Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi: 10.1016/ j.jaad.2004.05.005
5. Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142: 704-708. doi: 10.1001/archderm.142.6.704
6. Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi: 10.1097/DSS.0000000000001759
7. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi: 10.1016/j.jaad.2016.06.006
8. Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016) [published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi: 10.1016/ j.jaad.2020.08.097
1. Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi: 10.1016/j.jaad.2001.05.034
2. Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi: 10.1001/archinte.166.17.1907
3. Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi: 10.1023/a:1018432632528
4. Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi: 10.1016/ j.jaad.2004.05.005
5. Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142: 704-708. doi: 10.1001/archderm.142.6.704
6. Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi: 10.1097/DSS.0000000000001759
7. Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi: 10.1016/j.jaad.2016.06.006
8. Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016) [published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi: 10.1016/ j.jaad.2020.08.097
Dermatology attracts more than its share of physician assistants
Dermatology added PAs at a mean rate of 11.6% annually over that 6-year period, compared with a mean of 7.8% for all other specialties (P <.001), as the National Commission on Certification of Physician Assistants (NCCPA) tallied 2,324 working in dermatology and 64,490 in all other specialties in 2013 and 3,938/94,616, respectively, in 2018, Justin D. Arnold, MD, of the University of California, Irvine, and associates reported in JAMA Dermatology.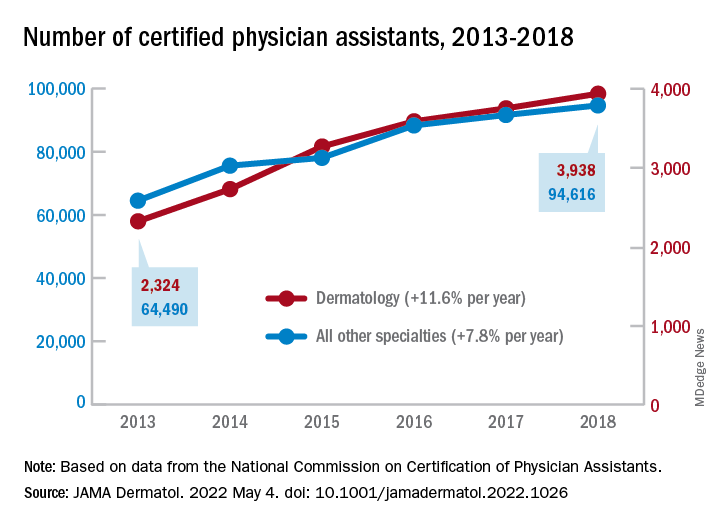
“There is, however, a lack of racial and ethnic diversity within the dermatology PA workforce,” they noted. A detailed comparison using the 2018 data showed that only 1.6% of dermatology PAs identified as Black, compared with 3.7% of those in all other specialties (P <.001), although “similar rates of Hispanic ethnicity were observed” in dermatology PAs (6.0%) and PAs in other fields (6.5%), the investigators added.
That was not the case for women in the profession, as 82% of PAs in dermatology were female in 2018, compared with 67% in the other specialties. Dermatology PAs also were significantly more likely to work in office-based practices than their nondermatology peers (93% vs. 37%, P < .001) and to reside in metropolitan areas (95% vs. 92%, P < .001), Dr. Arnold and associates said in the research letter.
The dermatology PAs also were more likely to work part time (30 or fewer hours per week) than those outside dermatology, 19.1% vs. 12.9% (P < .001). Despite that, the dermatology PAs reported seeing more patients per week (a mean of 119) than those in all of the other specialties (a mean of 71), the investigators said.
The total number of certified PAs was over 131,000 in 2018, but about 25% had not selected a principal specialty in their PA Professional Profiles and were not included in the study, they explained.
“Although this study did not assess the reasons for the substantial increase of dermatology PAs, numerous factors, such as a potential physician shortage or the expansion of private equity–owned practices, may contribute to the accelerating use of PAs within the field,” they wrote.
Dermatology added PAs at a mean rate of 11.6% annually over that 6-year period, compared with a mean of 7.8% for all other specialties (P <.001), as the National Commission on Certification of Physician Assistants (NCCPA) tallied 2,324 working in dermatology and 64,490 in all other specialties in 2013 and 3,938/94,616, respectively, in 2018, Justin D. Arnold, MD, of the University of California, Irvine, and associates reported in JAMA Dermatology.
“There is, however, a lack of racial and ethnic diversity within the dermatology PA workforce,” they noted. A detailed comparison using the 2018 data showed that only 1.6% of dermatology PAs identified as Black, compared with 3.7% of those in all other specialties (P <.001), although “similar rates of Hispanic ethnicity were observed” in dermatology PAs (6.0%) and PAs in other fields (6.5%), the investigators added.
That was not the case for women in the profession, as 82% of PAs in dermatology were female in 2018, compared with 67% in the other specialties. Dermatology PAs also were significantly more likely to work in office-based practices than their nondermatology peers (93% vs. 37%, P < .001) and to reside in metropolitan areas (95% vs. 92%, P < .001), Dr. Arnold and associates said in the research letter.
The dermatology PAs also were more likely to work part time (30 or fewer hours per week) than those outside dermatology, 19.1% vs. 12.9% (P < .001). Despite that, the dermatology PAs reported seeing more patients per week (a mean of 119) than those in all of the other specialties (a mean of 71), the investigators said.
The total number of certified PAs was over 131,000 in 2018, but about 25% had not selected a principal specialty in their PA Professional Profiles and were not included in the study, they explained.
“Although this study did not assess the reasons for the substantial increase of dermatology PAs, numerous factors, such as a potential physician shortage or the expansion of private equity–owned practices, may contribute to the accelerating use of PAs within the field,” they wrote.
Dermatology added PAs at a mean rate of 11.6% annually over that 6-year period, compared with a mean of 7.8% for all other specialties (P <.001), as the National Commission on Certification of Physician Assistants (NCCPA) tallied 2,324 working in dermatology and 64,490 in all other specialties in 2013 and 3,938/94,616, respectively, in 2018, Justin D. Arnold, MD, of the University of California, Irvine, and associates reported in JAMA Dermatology.
“There is, however, a lack of racial and ethnic diversity within the dermatology PA workforce,” they noted. A detailed comparison using the 2018 data showed that only 1.6% of dermatology PAs identified as Black, compared with 3.7% of those in all other specialties (P <.001), although “similar rates of Hispanic ethnicity were observed” in dermatology PAs (6.0%) and PAs in other fields (6.5%), the investigators added.
That was not the case for women in the profession, as 82% of PAs in dermatology were female in 2018, compared with 67% in the other specialties. Dermatology PAs also were significantly more likely to work in office-based practices than their nondermatology peers (93% vs. 37%, P < .001) and to reside in metropolitan areas (95% vs. 92%, P < .001), Dr. Arnold and associates said in the research letter.
The dermatology PAs also were more likely to work part time (30 or fewer hours per week) than those outside dermatology, 19.1% vs. 12.9% (P < .001). Despite that, the dermatology PAs reported seeing more patients per week (a mean of 119) than those in all of the other specialties (a mean of 71), the investigators said.
The total number of certified PAs was over 131,000 in 2018, but about 25% had not selected a principal specialty in their PA Professional Profiles and were not included in the study, they explained.
“Although this study did not assess the reasons for the substantial increase of dermatology PAs, numerous factors, such as a potential physician shortage or the expansion of private equity–owned practices, may contribute to the accelerating use of PAs within the field,” they wrote.
FROM JAMA DERMATOLOGY
Telehealth continues to loom large, say experts
This physician, Brian Hasselfeld, MD, said his university’s health system did 50-80 telemedicine visits a month before COVID, during a presentation at the annual meeting of the American College of Physicians. This soared to close to 100,000 a month in the pandemic, and now the health system does close to 40,000 a month, he continued.
“Life is definitely different in how we engage with our patients on a day-to-day basis,” said Dr. Hasselfeld, who oversees the telehealth for six hospitals and 50 ambulatory-care locations in Maryland and three other states.
Attitudes gauged in Johns Hopkins surveys suggest that a lot of medical care will continue to be provided by telemedicine. Nine out of 10 patients said they would likely recommend telemedicine to friends and family, and 88% said it would be either moderately, very, or extremely important to have video visit options in the future, he said.
A survey of the Hopkins system’s 3,600 physicians, which generated about 1,300 responses, found that physicians would like to have a considerable chunk of time set aside for telemedicine visits – the median response was 30%.
Virtual care is in ‘early-adopter phase’
But Dr. Hasselfeld said virtual care is still in the “early-adopter phase.” While many physicians said they would like more than half of their time devoted to telehealth, a larger proportion was more likely to say they wanted very little time devoted to it, Dr. Hasselfeld said. Among those wanting to do it are some who want to do all of their visits virtually, he said.
Those who are eager to do it will be those guiding the change, Dr. Hasselfeld said.
“As we move forward – and thinking about how to optimize virtual-care options for your patients – it’s not going to be a forced issue,” he said.
Providing better access to certain patient groups continues to be a challenge. A dashboard developed at Hopkins to identify groups who are at a technological disadvantage and don’t have ready access to telemedicine found that those living in low-income zip codes, African-Americans, and those on Medicaid and Medicare tend to have higher percentages of “audio-only” visits, mainly because of lack of connectivity allowing video visits, Dr. Hasselfeld said.
The lower share of video visits in the inner city suggests that access to telemedicine isn’t just a problem in remote rural areas, as the conventional wisdom has gone, he said.
“It doesn’t matter how many towers we have in downtown Baltimore, or how much fiber we have in the ground,” he said. “If you can’t have a data plan to access that high-speed Internet, or have a home with high-speed Internet, it doesn’t matter.”
Hopkins has developed a tool to assess how likely it is that someone will have trouble connecting for a telemedicine visit – if they’ve previously had an audio-only visit, for instance – and try to get in touch with those patients shortly before a visit so that it runs smoothly, Dr. Hasselfeld said.
The explosion of telemedicine has led to the rise of companies providing care through apps on phones and tablets, he said.
“This is real care being provided to our patients through nontraditional routes, and this is a new force, one our patients see out in the marketplace,” he said. “We have to acknowledge and wrestle with the fact that convenience is a new part of what it means to [provide] access [to] care for patients.”
Heather Hirsch, MD, an internist with Brigham and Women’s Hospital in Boston, said in an interview after the session that telemedicine is poised to improve care.
“I think the good is definitely going to outweigh the bad so long as the infrastructure and the legislation will allow it,” said Dr. Hirsch, who does about half of her visits in person and half through telemedicine, which she performs while at the office. “It does allow for a lot of flexibility for both patients and providers.”
But health care at academic medical centers, she said, needs to adjust to the times.
“We need [academic medicine] for so many reasons,” she said, “but the reality is that it moves very slowly, and the old infrastructure and the slowness to catch up with technology is the worry.”
Dr. Hasselfeld reported financial relationships with Humana and TRUE-See Systems.
This physician, Brian Hasselfeld, MD, said his university’s health system did 50-80 telemedicine visits a month before COVID, during a presentation at the annual meeting of the American College of Physicians. This soared to close to 100,000 a month in the pandemic, and now the health system does close to 40,000 a month, he continued.
“Life is definitely different in how we engage with our patients on a day-to-day basis,” said Dr. Hasselfeld, who oversees the telehealth for six hospitals and 50 ambulatory-care locations in Maryland and three other states.
Attitudes gauged in Johns Hopkins surveys suggest that a lot of medical care will continue to be provided by telemedicine. Nine out of 10 patients said they would likely recommend telemedicine to friends and family, and 88% said it would be either moderately, very, or extremely important to have video visit options in the future, he said.
A survey of the Hopkins system’s 3,600 physicians, which generated about 1,300 responses, found that physicians would like to have a considerable chunk of time set aside for telemedicine visits – the median response was 30%.
Virtual care is in ‘early-adopter phase’
But Dr. Hasselfeld said virtual care is still in the “early-adopter phase.” While many physicians said they would like more than half of their time devoted to telehealth, a larger proportion was more likely to say they wanted very little time devoted to it, Dr. Hasselfeld said. Among those wanting to do it are some who want to do all of their visits virtually, he said.
Those who are eager to do it will be those guiding the change, Dr. Hasselfeld said.
“As we move forward – and thinking about how to optimize virtual-care options for your patients – it’s not going to be a forced issue,” he said.
Providing better access to certain patient groups continues to be a challenge. A dashboard developed at Hopkins to identify groups who are at a technological disadvantage and don’t have ready access to telemedicine found that those living in low-income zip codes, African-Americans, and those on Medicaid and Medicare tend to have higher percentages of “audio-only” visits, mainly because of lack of connectivity allowing video visits, Dr. Hasselfeld said.
The lower share of video visits in the inner city suggests that access to telemedicine isn’t just a problem in remote rural areas, as the conventional wisdom has gone, he said.
“It doesn’t matter how many towers we have in downtown Baltimore, or how much fiber we have in the ground,” he said. “If you can’t have a data plan to access that high-speed Internet, or have a home with high-speed Internet, it doesn’t matter.”
Hopkins has developed a tool to assess how likely it is that someone will have trouble connecting for a telemedicine visit – if they’ve previously had an audio-only visit, for instance – and try to get in touch with those patients shortly before a visit so that it runs smoothly, Dr. Hasselfeld said.
The explosion of telemedicine has led to the rise of companies providing care through apps on phones and tablets, he said.
“This is real care being provided to our patients through nontraditional routes, and this is a new force, one our patients see out in the marketplace,” he said. “We have to acknowledge and wrestle with the fact that convenience is a new part of what it means to [provide] access [to] care for patients.”
Heather Hirsch, MD, an internist with Brigham and Women’s Hospital in Boston, said in an interview after the session that telemedicine is poised to improve care.
“I think the good is definitely going to outweigh the bad so long as the infrastructure and the legislation will allow it,” said Dr. Hirsch, who does about half of her visits in person and half through telemedicine, which she performs while at the office. “It does allow for a lot of flexibility for both patients and providers.”
But health care at academic medical centers, she said, needs to adjust to the times.
“We need [academic medicine] for so many reasons,” she said, “but the reality is that it moves very slowly, and the old infrastructure and the slowness to catch up with technology is the worry.”
Dr. Hasselfeld reported financial relationships with Humana and TRUE-See Systems.
This physician, Brian Hasselfeld, MD, said his university’s health system did 50-80 telemedicine visits a month before COVID, during a presentation at the annual meeting of the American College of Physicians. This soared to close to 100,000 a month in the pandemic, and now the health system does close to 40,000 a month, he continued.
“Life is definitely different in how we engage with our patients on a day-to-day basis,” said Dr. Hasselfeld, who oversees the telehealth for six hospitals and 50 ambulatory-care locations in Maryland and three other states.
Attitudes gauged in Johns Hopkins surveys suggest that a lot of medical care will continue to be provided by telemedicine. Nine out of 10 patients said they would likely recommend telemedicine to friends and family, and 88% said it would be either moderately, very, or extremely important to have video visit options in the future, he said.
A survey of the Hopkins system’s 3,600 physicians, which generated about 1,300 responses, found that physicians would like to have a considerable chunk of time set aside for telemedicine visits – the median response was 30%.
Virtual care is in ‘early-adopter phase’
But Dr. Hasselfeld said virtual care is still in the “early-adopter phase.” While many physicians said they would like more than half of their time devoted to telehealth, a larger proportion was more likely to say they wanted very little time devoted to it, Dr. Hasselfeld said. Among those wanting to do it are some who want to do all of their visits virtually, he said.
Those who are eager to do it will be those guiding the change, Dr. Hasselfeld said.
“As we move forward – and thinking about how to optimize virtual-care options for your patients – it’s not going to be a forced issue,” he said.
Providing better access to certain patient groups continues to be a challenge. A dashboard developed at Hopkins to identify groups who are at a technological disadvantage and don’t have ready access to telemedicine found that those living in low-income zip codes, African-Americans, and those on Medicaid and Medicare tend to have higher percentages of “audio-only” visits, mainly because of lack of connectivity allowing video visits, Dr. Hasselfeld said.
The lower share of video visits in the inner city suggests that access to telemedicine isn’t just a problem in remote rural areas, as the conventional wisdom has gone, he said.
“It doesn’t matter how many towers we have in downtown Baltimore, or how much fiber we have in the ground,” he said. “If you can’t have a data plan to access that high-speed Internet, or have a home with high-speed Internet, it doesn’t matter.”
Hopkins has developed a tool to assess how likely it is that someone will have trouble connecting for a telemedicine visit – if they’ve previously had an audio-only visit, for instance – and try to get in touch with those patients shortly before a visit so that it runs smoothly, Dr. Hasselfeld said.
The explosion of telemedicine has led to the rise of companies providing care through apps on phones and tablets, he said.
“This is real care being provided to our patients through nontraditional routes, and this is a new force, one our patients see out in the marketplace,” he said. “We have to acknowledge and wrestle with the fact that convenience is a new part of what it means to [provide] access [to] care for patients.”
Heather Hirsch, MD, an internist with Brigham and Women’s Hospital in Boston, said in an interview after the session that telemedicine is poised to improve care.
“I think the good is definitely going to outweigh the bad so long as the infrastructure and the legislation will allow it,” said Dr. Hirsch, who does about half of her visits in person and half through telemedicine, which she performs while at the office. “It does allow for a lot of flexibility for both patients and providers.”
But health care at academic medical centers, she said, needs to adjust to the times.
“We need [academic medicine] for so many reasons,” she said, “but the reality is that it moves very slowly, and the old infrastructure and the slowness to catch up with technology is the worry.”
Dr. Hasselfeld reported financial relationships with Humana and TRUE-See Systems.
AT INTERNAL MEDICINE 2022
How Dermatology Residents Can Best Serve the Needs of the LGBT Community
The chances are good that at least one patient you saw today could have been provided a better environment to foster your patient-physician relationship. A 2020 Gallup poll revealed that an estimated 5.6% of US adults identified as lesbian, gay, bisexual, and transgender (LGBT).1 Based on the estimated US population of 331.7 million individuals on December 3, 2020, this means that approximately 18.6 million identified as LGBT and could potentially require health care services.2 These numbers highlight the increasing need within the medical community to provide quality and accessible care to the LGBT community, and dermatologists have a role to play. They treat conditions that are apparent to the patient and others around them, attracting those that may not be motivated to see different physicians. They can not only help with skin diseases that affect all patients but also can train other physicians to screen for some dermatologic diseases that may have a higher prevalence within the LGBT community. Dermatologists have a unique opportunity to help patients better reflect themselves through both surgical and nonsurgical modalities.
Demographics and Definitions
To discuss this topic effectively, it is important to define LGBT terms (Table).3 As a disclaimer, language is fluid. Despite a word or term currently being used and accepted, it quickly can become obsolete. A clinician can always do research, follow the lead of the patient, and respectfully ask questions if there is ever confusion surrounding terminology. Patients do not expect every physician they encounter to be an expert in this subject. What is most important is that patients are approached with an open mind and humility with the goal of providing optimal care.
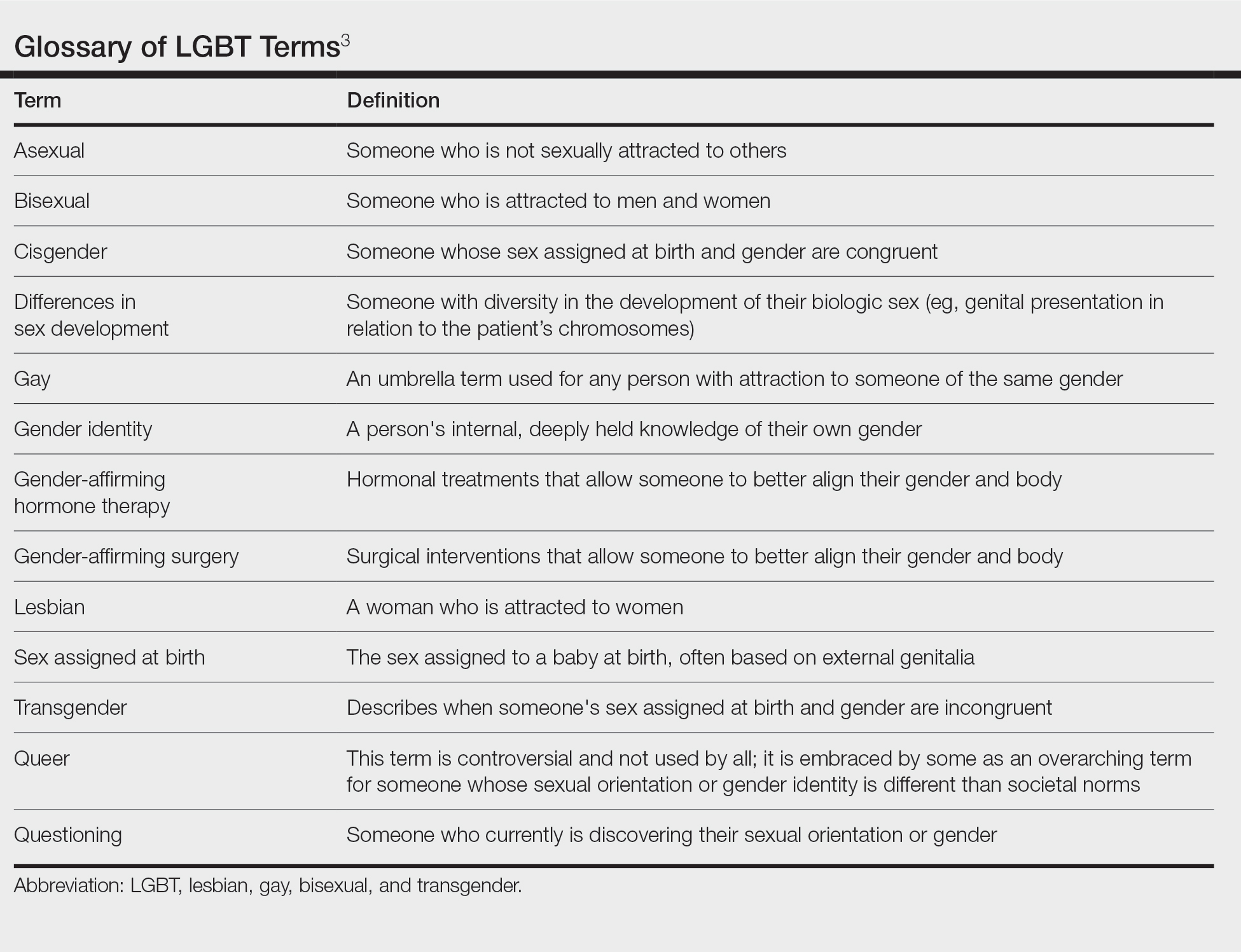
Although the federal government now uses the term sexual and gender minorities (SGM), the more specific terms lesbian, gay, bisexual, and transgender usually are preferred.3,4 Other letters are at times added to the acronym LGBT, including Q for questioning or queer, I for intersex, and A for asexual; all of these letters are under the larger SGM umbrella. Because LGBT is the most commonly used acronym in the daily vernacular, it will be the default for this article.
A term describing sexual orientation does not necessarily describe sexual practices. A woman who identifies as straight may have sex with both men and women, and a gay man may not have sex at all. To be more descriptive regarding sexual practices, one may use the terms men who have sex with men or women who have sex with women.3 Because of this nuance, it is important to elicit a sexual history when speaking to all patients in a forward nonjudgmental manner.
The term transgender is used to describe people whose gender identity differs from the sex they were assigned at birth. Two examples of transgender individuals would be transgender women who were assigned male at birth and transgender men who were assigned female at birth. The term transgender is used in opposition to the term cisgender, which is applied to a person whose gender and sex assigned at birth align.3 When a transgender patient presents to a physician, they may want to discuss methods of gender affirmation or transitioning. These terms encompass any action a person may take to align their body or gender expression with that of the gender they identify with. This could be in the form of gender-affirming hormone therapy (ie, estrogen or testosterone treatment) or gender-affirming surgery (ie, “top” and “bottom” surgeries, in which someone surgically treats their chest or genitals, respectively).3
Creating a Safe Space
The physician is responsible for providing a safe space for patients to disclose medically pertinent information. It is then the job of the dermatologist to be cognizant of health concerns that directly affect the LGBT population and to be prepared if one of these concerns should arise. A safe space consists of both the physical location in which the patient encounter will occur and the people that will be conducting and assisting in the patient encounter. Safe spaces provide a patient with reassurance that they will receive care in a judgement-free location. To create a safe space, both the physical and interpersonal aspects must be addressed to provide an environment that strengthens the patient-physician alliance.
Dermatology residents often spend more time with patients than their attending physicians, providing them the opportunity to foster robust relationships with those served. Although they may not be able to change the physical environment, residents can advocate for patients in their departments and show solidarity in subtle ways. One way to show support for the LGBT community is to publicly display a symbol of solidarity, which could be done by wearing a symbol of support on a white coat lapel. Although there are many designs and styles to choose from, one example is the American Medical Student Association pins that combine the caduceus (a common symbol for medicine) with a rainbow design.5 Whichever symbol is chosen, this small gesture allows patients to immediately know that their physician is an ally. Residents also can encourage their department to add a rainbow flag, a pink triangle, or another symbol somewhere prominent in the check-in area that conveys a message of support.6 Many institutions require residents to perform quality improvement projects. The resident can make a substantial difference in their patients’ experiences by revising their office’s intake forms as a quality improvement project, which can be done by including a section on assigned sex at birth separate from gender.7 When inquiring about gender, in addition to “male” and “female,” a space can be left for people that do not identify with the traditional binary. When asking about sexual orientation, inclusive language options can be provided with additional space for self-identification. Finally, residents can incorporate pronouns below their name in their email signature to normalize this disclosure of information.8 These small changes can have a substantial impact on the health care experience of SGM patients.
Medical Problems Encountered
The previously described changes can be implemented by residents to provide better care to SGM patients, a group usually considered to be more burdened by physical and psychological diseases.9 Furthermore, dermatologists can provide care for these patients in ways that other physicians cannot. There are special considerations for LGBT patients, as some dermatologic conditions may be more common in this patient population.
Prior studies have shown that men who have sex with men have a higher rate of HIV and other sexually transmitted infections, methicillin-resistant Staphylococcus aureus skin infections, and potentially nonmelanoma skin cancer.10-14 Transgender women also have been found to have higher rates of HIV, in addition to a higher incidence of anal human papillomavirus.15,16 Women who have sex with women have been shown to see physicians less frequently and to be less up to date on their pertinent cancer-related screenings.10,17 Although these associations should not dictate the patient encounter, awareness of them will lead to better patient care. Such awareness also can provide further motivation for dermatologists to discuss safe sexual practices, potential initiation of pre-exposure prophylactic antiretroviral therapy, sun-protective practices, and the importance of following up with a primary physician for examinations and age-specific cancer screening.
Transgender patients may present with unique dermatologic concerns. For transgender male patients, testosterone therapy can cause acne breakouts and androgenetic alopecia. Usually considered worse during the start of treatment, hormone-related acne can be managed with topical retinoids, topical and oral antibiotics, and isotretinoin (if severe).18,19 The iPLEDGE system necessary for prescribing isotretinoin to patients in the United States recently has changed its language to “patients who can get pregnant” and “patients who cannot get pregnant,” following urging by the medical community for inclusivity and progress.20,21 This change creates an inclusive space where registration is no longer centered around gender and instead focuses on the presence of anatomy. Although androgenetic alopecia is a side effect of hormone therapy, it may not be unwanted.18 Discussion about patient desires is important. If the alopecia is unwanted, the Endocrine Society recommends treating cisgender and transgender patients the same in terms of treatment modalities.22
Transgender female patients also can experience dermatologic manifestations of gender-affirming hormone therapy. Melasma may develop secondary to estrogen replacement and can be treated with topical bleaching creams, lasers, and phototherapy.23 Hair removal may be pursued for patients with refractory unwanted body hair, with laser hair removal being the most commonly pursued treatment. Patients also may desire cosmetic procedures, such as botulinum toxin or fillers, to augment their physical appearance.24 Providing these services to patients may allow them to better express themselves and live authentically.
Final Thoughts
There is no way to summarize the experience of everyone within a community. Each person has different thoughts, values, and goals. It also is impossible to encompass every topic that is important for SGM patients. The goal of this article is to empower clinicians to be comfortable discussing issues related to sexuality and gender while also offering resources to learn more, allowing optimal care to be provided to this population. Thus, this article is not comprehensive. There are articles to provide further resources and education, such as the continuing medical education series by Yeung et al10,25 in the Journal of the American Academy of Dermatology, as well as organizations within medicine, such as the GLMA: Health Professionals Advancing LGBTQ Equality (https://www.glma.org/), and in dermatology, such as GALDA, the Gay and Lesbian Dermatology Association (https://www.glderm.org/). By providing a safe space for our patients and learning about specific health-related risk factors, dermatologists can provide the best possible care to the LGBT community.
Acknowledgments—I thank Warren R. Heymann, MD (Camden, New Jersey), and Howa Yeung, MD, MSc (Atlanta, Georgia), for their guidance and mentorship in the creation of this article.
- Jones JM. LGBT identification rises to 5.6% in latest U.S. estimate. Gallup website. Published February 24, 2021. Accessed March 22, 2022. https://news.gallup.com/poll/329708/lgbt-identification-rises-latest-estimate.aspx
- U.S. and world population clock. US Census Bureau website. Accessed March 22, 2022. https://www.census.gov/popclock/
- National LGBTQIA+ Health Education Center. LGBTQIA+ glossary of terms for health care teams. Published February 2, 2022. Accessed April 11, 2022. https://www.lgbtqiahealtheducation.org/wp-content/uploads/2020/02/Glossary-2022.02.22-1.pdf
- National Institutes of Health Sexual and Gender Minority Research Coordinating Committee. NIH FY 2016-2020 strategic plan to advance research on the health and well-being of sexual and gender minorities. NIH website. Accessed March 23, 2022. https://www.edi.nih.gov/sites/default/files/EDI_Public_files/sgm-strategic-plan.pdf
- Caduceus pin—rainbow. American Medical Student Association website. Accessed March 23, 2022. https://www.amsa.org/member-center/store/Caduceus-Pin-Rainbow-p67375123
- 10 tips for caring for LGBTQIA+ patients. Nurse.org website. Accessed March 23, 2022. https://nurse.org/articles/culturally-competent-healthcare-for-LGBTQ-patients/
- Cartron AM, Raiciulescu S, Trinidad JC. Culturally competent care for LGBT patients in dermatology clinics. J Drugs Dermatol. 2020;19:786-787.
- Wareham J. Should you put pronouns in email signatures and social media bios? Forbes website. Published Dec 30, 2019. Accessed March 23, 2022. https://www.forbes.com/sites/jamiewareham/2020/12/30/should-you-put-pronouns-in-email-signatures-and-social-media-bios/?sh=5b74f1246320
- Hafeez H, Zeshan M, Tahir MA, et al. Healthcare disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons. part II. epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Centers for Disease Control and Prevention. CDC fact sheet: HIV among gay and bisexual men. CDC website. Accessed April 14, 2022. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2016. CDC website. Accessed April 14, 2022. https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017_1644.pdf
- Galindo GR, Casey AJ, Yeung A, et al. Community associated methicillin resistant Staphylococcus aureus among New York City men who have sex with men: qualitative research findings and implications for public health practice. J Community Health. 2012;37:458-467.
- Blashill AJ. Indoor tanning and skin cancer risk among diverse US youth: results from a national sample. JAMA Dermatol. 2017;153:344-345.
- Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1-17.
- Uaamnuichai S, Panyakhamlerd K, Suwan A, et al. Neovaginal and anal high-risk human papillomavirus DNA among Thai transgender women in gender health clinics. Sex Transm Dis. 2021;48:547-549.
- Valanis BG, Bowen DJ, Bassford T, et al. Sexual orientation and health: comparisons in the women’s health initiative sample. Arch Fam Med. 2000;9:843-853.
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Turrion-Merino L, Urech-Garcia-de-la-Vega M, Miguel-Gomez L, et al. Severe acne in female-to-male transgender patients. JAMA Dermatol. 2015;151:1260-1261.
- Questions and answers on the iPLEDGE REMS. US Food and Drug Administration website. Published October 12, 2021. Accessed March 23, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-ipledge-rems#:~:text=The%20modification%20will%20become%20effective,verify%20authorization%20to%20dispense%20isotretinoin
- Gao JL, Thoreson N, Dommasch ED. Navigating iPLEDGE enrollment for transgender and gender diverse patients: a guide for providing culturally competent care. J Am Acad Dermatol. 2021;85:790-791.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869-3903.
- Garcia-Rodriguez L, Spiegel JH. Melasma in a transgender woman. Am J Otolaryngol. 2018;39:788-790.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community.J Am Acad Dermatol. 2016;74:303-308.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian,gay, bisexual, and transgender persons. part I. terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
The chances are good that at least one patient you saw today could have been provided a better environment to foster your patient-physician relationship. A 2020 Gallup poll revealed that an estimated 5.6% of US adults identified as lesbian, gay, bisexual, and transgender (LGBT).1 Based on the estimated US population of 331.7 million individuals on December 3, 2020, this means that approximately 18.6 million identified as LGBT and could potentially require health care services.2 These numbers highlight the increasing need within the medical community to provide quality and accessible care to the LGBT community, and dermatologists have a role to play. They treat conditions that are apparent to the patient and others around them, attracting those that may not be motivated to see different physicians. They can not only help with skin diseases that affect all patients but also can train other physicians to screen for some dermatologic diseases that may have a higher prevalence within the LGBT community. Dermatologists have a unique opportunity to help patients better reflect themselves through both surgical and nonsurgical modalities.
Demographics and Definitions
To discuss this topic effectively, it is important to define LGBT terms (Table).3 As a disclaimer, language is fluid. Despite a word or term currently being used and accepted, it quickly can become obsolete. A clinician can always do research, follow the lead of the patient, and respectfully ask questions if there is ever confusion surrounding terminology. Patients do not expect every physician they encounter to be an expert in this subject. What is most important is that patients are approached with an open mind and humility with the goal of providing optimal care.

Although the federal government now uses the term sexual and gender minorities (SGM), the more specific terms lesbian, gay, bisexual, and transgender usually are preferred.3,4 Other letters are at times added to the acronym LGBT, including Q for questioning or queer, I for intersex, and A for asexual; all of these letters are under the larger SGM umbrella. Because LGBT is the most commonly used acronym in the daily vernacular, it will be the default for this article.
A term describing sexual orientation does not necessarily describe sexual practices. A woman who identifies as straight may have sex with both men and women, and a gay man may not have sex at all. To be more descriptive regarding sexual practices, one may use the terms men who have sex with men or women who have sex with women.3 Because of this nuance, it is important to elicit a sexual history when speaking to all patients in a forward nonjudgmental manner.
The term transgender is used to describe people whose gender identity differs from the sex they were assigned at birth. Two examples of transgender individuals would be transgender women who were assigned male at birth and transgender men who were assigned female at birth. The term transgender is used in opposition to the term cisgender, which is applied to a person whose gender and sex assigned at birth align.3 When a transgender patient presents to a physician, they may want to discuss methods of gender affirmation or transitioning. These terms encompass any action a person may take to align their body or gender expression with that of the gender they identify with. This could be in the form of gender-affirming hormone therapy (ie, estrogen or testosterone treatment) or gender-affirming surgery (ie, “top” and “bottom” surgeries, in which someone surgically treats their chest or genitals, respectively).3
Creating a Safe Space
The physician is responsible for providing a safe space for patients to disclose medically pertinent information. It is then the job of the dermatologist to be cognizant of health concerns that directly affect the LGBT population and to be prepared if one of these concerns should arise. A safe space consists of both the physical location in which the patient encounter will occur and the people that will be conducting and assisting in the patient encounter. Safe spaces provide a patient with reassurance that they will receive care in a judgement-free location. To create a safe space, both the physical and interpersonal aspects must be addressed to provide an environment that strengthens the patient-physician alliance.
Dermatology residents often spend more time with patients than their attending physicians, providing them the opportunity to foster robust relationships with those served. Although they may not be able to change the physical environment, residents can advocate for patients in their departments and show solidarity in subtle ways. One way to show support for the LGBT community is to publicly display a symbol of solidarity, which could be done by wearing a symbol of support on a white coat lapel. Although there are many designs and styles to choose from, one example is the American Medical Student Association pins that combine the caduceus (a common symbol for medicine) with a rainbow design.5 Whichever symbol is chosen, this small gesture allows patients to immediately know that their physician is an ally. Residents also can encourage their department to add a rainbow flag, a pink triangle, or another symbol somewhere prominent in the check-in area that conveys a message of support.6 Many institutions require residents to perform quality improvement projects. The resident can make a substantial difference in their patients’ experiences by revising their office’s intake forms as a quality improvement project, which can be done by including a section on assigned sex at birth separate from gender.7 When inquiring about gender, in addition to “male” and “female,” a space can be left for people that do not identify with the traditional binary. When asking about sexual orientation, inclusive language options can be provided with additional space for self-identification. Finally, residents can incorporate pronouns below their name in their email signature to normalize this disclosure of information.8 These small changes can have a substantial impact on the health care experience of SGM patients.
Medical Problems Encountered
The previously described changes can be implemented by residents to provide better care to SGM patients, a group usually considered to be more burdened by physical and psychological diseases.9 Furthermore, dermatologists can provide care for these patients in ways that other physicians cannot. There are special considerations for LGBT patients, as some dermatologic conditions may be more common in this patient population.
Prior studies have shown that men who have sex with men have a higher rate of HIV and other sexually transmitted infections, methicillin-resistant Staphylococcus aureus skin infections, and potentially nonmelanoma skin cancer.10-14 Transgender women also have been found to have higher rates of HIV, in addition to a higher incidence of anal human papillomavirus.15,16 Women who have sex with women have been shown to see physicians less frequently and to be less up to date on their pertinent cancer-related screenings.10,17 Although these associations should not dictate the patient encounter, awareness of them will lead to better patient care. Such awareness also can provide further motivation for dermatologists to discuss safe sexual practices, potential initiation of pre-exposure prophylactic antiretroviral therapy, sun-protective practices, and the importance of following up with a primary physician for examinations and age-specific cancer screening.
Transgender patients may present with unique dermatologic concerns. For transgender male patients, testosterone therapy can cause acne breakouts and androgenetic alopecia. Usually considered worse during the start of treatment, hormone-related acne can be managed with topical retinoids, topical and oral antibiotics, and isotretinoin (if severe).18,19 The iPLEDGE system necessary for prescribing isotretinoin to patients in the United States recently has changed its language to “patients who can get pregnant” and “patients who cannot get pregnant,” following urging by the medical community for inclusivity and progress.20,21 This change creates an inclusive space where registration is no longer centered around gender and instead focuses on the presence of anatomy. Although androgenetic alopecia is a side effect of hormone therapy, it may not be unwanted.18 Discussion about patient desires is important. If the alopecia is unwanted, the Endocrine Society recommends treating cisgender and transgender patients the same in terms of treatment modalities.22
Transgender female patients also can experience dermatologic manifestations of gender-affirming hormone therapy. Melasma may develop secondary to estrogen replacement and can be treated with topical bleaching creams, lasers, and phototherapy.23 Hair removal may be pursued for patients with refractory unwanted body hair, with laser hair removal being the most commonly pursued treatment. Patients also may desire cosmetic procedures, such as botulinum toxin or fillers, to augment their physical appearance.24 Providing these services to patients may allow them to better express themselves and live authentically.
Final Thoughts
There is no way to summarize the experience of everyone within a community. Each person has different thoughts, values, and goals. It also is impossible to encompass every topic that is important for SGM patients. The goal of this article is to empower clinicians to be comfortable discussing issues related to sexuality and gender while also offering resources to learn more, allowing optimal care to be provided to this population. Thus, this article is not comprehensive. There are articles to provide further resources and education, such as the continuing medical education series by Yeung et al10,25 in the Journal of the American Academy of Dermatology, as well as organizations within medicine, such as the GLMA: Health Professionals Advancing LGBTQ Equality (https://www.glma.org/), and in dermatology, such as GALDA, the Gay and Lesbian Dermatology Association (https://www.glderm.org/). By providing a safe space for our patients and learning about specific health-related risk factors, dermatologists can provide the best possible care to the LGBT community.
Acknowledgments—I thank Warren R. Heymann, MD (Camden, New Jersey), and Howa Yeung, MD, MSc (Atlanta, Georgia), for their guidance and mentorship in the creation of this article.
The chances are good that at least one patient you saw today could have been provided a better environment to foster your patient-physician relationship. A 2020 Gallup poll revealed that an estimated 5.6% of US adults identified as lesbian, gay, bisexual, and transgender (LGBT).1 Based on the estimated US population of 331.7 million individuals on December 3, 2020, this means that approximately 18.6 million identified as LGBT and could potentially require health care services.2 These numbers highlight the increasing need within the medical community to provide quality and accessible care to the LGBT community, and dermatologists have a role to play. They treat conditions that are apparent to the patient and others around them, attracting those that may not be motivated to see different physicians. They can not only help with skin diseases that affect all patients but also can train other physicians to screen for some dermatologic diseases that may have a higher prevalence within the LGBT community. Dermatologists have a unique opportunity to help patients better reflect themselves through both surgical and nonsurgical modalities.
Demographics and Definitions
To discuss this topic effectively, it is important to define LGBT terms (Table).3 As a disclaimer, language is fluid. Despite a word or term currently being used and accepted, it quickly can become obsolete. A clinician can always do research, follow the lead of the patient, and respectfully ask questions if there is ever confusion surrounding terminology. Patients do not expect every physician they encounter to be an expert in this subject. What is most important is that patients are approached with an open mind and humility with the goal of providing optimal care.

Although the federal government now uses the term sexual and gender minorities (SGM), the more specific terms lesbian, gay, bisexual, and transgender usually are preferred.3,4 Other letters are at times added to the acronym LGBT, including Q for questioning or queer, I for intersex, and A for asexual; all of these letters are under the larger SGM umbrella. Because LGBT is the most commonly used acronym in the daily vernacular, it will be the default for this article.
A term describing sexual orientation does not necessarily describe sexual practices. A woman who identifies as straight may have sex with both men and women, and a gay man may not have sex at all. To be more descriptive regarding sexual practices, one may use the terms men who have sex with men or women who have sex with women.3 Because of this nuance, it is important to elicit a sexual history when speaking to all patients in a forward nonjudgmental manner.
The term transgender is used to describe people whose gender identity differs from the sex they were assigned at birth. Two examples of transgender individuals would be transgender women who were assigned male at birth and transgender men who were assigned female at birth. The term transgender is used in opposition to the term cisgender, which is applied to a person whose gender and sex assigned at birth align.3 When a transgender patient presents to a physician, they may want to discuss methods of gender affirmation or transitioning. These terms encompass any action a person may take to align their body or gender expression with that of the gender they identify with. This could be in the form of gender-affirming hormone therapy (ie, estrogen or testosterone treatment) or gender-affirming surgery (ie, “top” and “bottom” surgeries, in which someone surgically treats their chest or genitals, respectively).3
Creating a Safe Space
The physician is responsible for providing a safe space for patients to disclose medically pertinent information. It is then the job of the dermatologist to be cognizant of health concerns that directly affect the LGBT population and to be prepared if one of these concerns should arise. A safe space consists of both the physical location in which the patient encounter will occur and the people that will be conducting and assisting in the patient encounter. Safe spaces provide a patient with reassurance that they will receive care in a judgement-free location. To create a safe space, both the physical and interpersonal aspects must be addressed to provide an environment that strengthens the patient-physician alliance.
Dermatology residents often spend more time with patients than their attending physicians, providing them the opportunity to foster robust relationships with those served. Although they may not be able to change the physical environment, residents can advocate for patients in their departments and show solidarity in subtle ways. One way to show support for the LGBT community is to publicly display a symbol of solidarity, which could be done by wearing a symbol of support on a white coat lapel. Although there are many designs and styles to choose from, one example is the American Medical Student Association pins that combine the caduceus (a common symbol for medicine) with a rainbow design.5 Whichever symbol is chosen, this small gesture allows patients to immediately know that their physician is an ally. Residents also can encourage their department to add a rainbow flag, a pink triangle, or another symbol somewhere prominent in the check-in area that conveys a message of support.6 Many institutions require residents to perform quality improvement projects. The resident can make a substantial difference in their patients’ experiences by revising their office’s intake forms as a quality improvement project, which can be done by including a section on assigned sex at birth separate from gender.7 When inquiring about gender, in addition to “male” and “female,” a space can be left for people that do not identify with the traditional binary. When asking about sexual orientation, inclusive language options can be provided with additional space for self-identification. Finally, residents can incorporate pronouns below their name in their email signature to normalize this disclosure of information.8 These small changes can have a substantial impact on the health care experience of SGM patients.
Medical Problems Encountered
The previously described changes can be implemented by residents to provide better care to SGM patients, a group usually considered to be more burdened by physical and psychological diseases.9 Furthermore, dermatologists can provide care for these patients in ways that other physicians cannot. There are special considerations for LGBT patients, as some dermatologic conditions may be more common in this patient population.
Prior studies have shown that men who have sex with men have a higher rate of HIV and other sexually transmitted infections, methicillin-resistant Staphylococcus aureus skin infections, and potentially nonmelanoma skin cancer.10-14 Transgender women also have been found to have higher rates of HIV, in addition to a higher incidence of anal human papillomavirus.15,16 Women who have sex with women have been shown to see physicians less frequently and to be less up to date on their pertinent cancer-related screenings.10,17 Although these associations should not dictate the patient encounter, awareness of them will lead to better patient care. Such awareness also can provide further motivation for dermatologists to discuss safe sexual practices, potential initiation of pre-exposure prophylactic antiretroviral therapy, sun-protective practices, and the importance of following up with a primary physician for examinations and age-specific cancer screening.
Transgender patients may present with unique dermatologic concerns. For transgender male patients, testosterone therapy can cause acne breakouts and androgenetic alopecia. Usually considered worse during the start of treatment, hormone-related acne can be managed with topical retinoids, topical and oral antibiotics, and isotretinoin (if severe).18,19 The iPLEDGE system necessary for prescribing isotretinoin to patients in the United States recently has changed its language to “patients who can get pregnant” and “patients who cannot get pregnant,” following urging by the medical community for inclusivity and progress.20,21 This change creates an inclusive space where registration is no longer centered around gender and instead focuses on the presence of anatomy. Although androgenetic alopecia is a side effect of hormone therapy, it may not be unwanted.18 Discussion about patient desires is important. If the alopecia is unwanted, the Endocrine Society recommends treating cisgender and transgender patients the same in terms of treatment modalities.22
Transgender female patients also can experience dermatologic manifestations of gender-affirming hormone therapy. Melasma may develop secondary to estrogen replacement and can be treated with topical bleaching creams, lasers, and phototherapy.23 Hair removal may be pursued for patients with refractory unwanted body hair, with laser hair removal being the most commonly pursued treatment. Patients also may desire cosmetic procedures, such as botulinum toxin or fillers, to augment their physical appearance.24 Providing these services to patients may allow them to better express themselves and live authentically.
Final Thoughts
There is no way to summarize the experience of everyone within a community. Each person has different thoughts, values, and goals. It also is impossible to encompass every topic that is important for SGM patients. The goal of this article is to empower clinicians to be comfortable discussing issues related to sexuality and gender while also offering resources to learn more, allowing optimal care to be provided to this population. Thus, this article is not comprehensive. There are articles to provide further resources and education, such as the continuing medical education series by Yeung et al10,25 in the Journal of the American Academy of Dermatology, as well as organizations within medicine, such as the GLMA: Health Professionals Advancing LGBTQ Equality (https://www.glma.org/), and in dermatology, such as GALDA, the Gay and Lesbian Dermatology Association (https://www.glderm.org/). By providing a safe space for our patients and learning about specific health-related risk factors, dermatologists can provide the best possible care to the LGBT community.
Acknowledgments—I thank Warren R. Heymann, MD (Camden, New Jersey), and Howa Yeung, MD, MSc (Atlanta, Georgia), for their guidance and mentorship in the creation of this article.
- Jones JM. LGBT identification rises to 5.6% in latest U.S. estimate. Gallup website. Published February 24, 2021. Accessed March 22, 2022. https://news.gallup.com/poll/329708/lgbt-identification-rises-latest-estimate.aspx
- U.S. and world population clock. US Census Bureau website. Accessed March 22, 2022. https://www.census.gov/popclock/
- National LGBTQIA+ Health Education Center. LGBTQIA+ glossary of terms for health care teams. Published February 2, 2022. Accessed April 11, 2022. https://www.lgbtqiahealtheducation.org/wp-content/uploads/2020/02/Glossary-2022.02.22-1.pdf
- National Institutes of Health Sexual and Gender Minority Research Coordinating Committee. NIH FY 2016-2020 strategic plan to advance research on the health and well-being of sexual and gender minorities. NIH website. Accessed March 23, 2022. https://www.edi.nih.gov/sites/default/files/EDI_Public_files/sgm-strategic-plan.pdf
- Caduceus pin—rainbow. American Medical Student Association website. Accessed March 23, 2022. https://www.amsa.org/member-center/store/Caduceus-Pin-Rainbow-p67375123
- 10 tips for caring for LGBTQIA+ patients. Nurse.org website. Accessed March 23, 2022. https://nurse.org/articles/culturally-competent-healthcare-for-LGBTQ-patients/
- Cartron AM, Raiciulescu S, Trinidad JC. Culturally competent care for LGBT patients in dermatology clinics. J Drugs Dermatol. 2020;19:786-787.
- Wareham J. Should you put pronouns in email signatures and social media bios? Forbes website. Published Dec 30, 2019. Accessed March 23, 2022. https://www.forbes.com/sites/jamiewareham/2020/12/30/should-you-put-pronouns-in-email-signatures-and-social-media-bios/?sh=5b74f1246320
- Hafeez H, Zeshan M, Tahir MA, et al. Healthcare disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons. part II. epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Centers for Disease Control and Prevention. CDC fact sheet: HIV among gay and bisexual men. CDC website. Accessed April 14, 2022. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2016. CDC website. Accessed April 14, 2022. https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017_1644.pdf
- Galindo GR, Casey AJ, Yeung A, et al. Community associated methicillin resistant Staphylococcus aureus among New York City men who have sex with men: qualitative research findings and implications for public health practice. J Community Health. 2012;37:458-467.
- Blashill AJ. Indoor tanning and skin cancer risk among diverse US youth: results from a national sample. JAMA Dermatol. 2017;153:344-345.
- Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1-17.
- Uaamnuichai S, Panyakhamlerd K, Suwan A, et al. Neovaginal and anal high-risk human papillomavirus DNA among Thai transgender women in gender health clinics. Sex Transm Dis. 2021;48:547-549.
- Valanis BG, Bowen DJ, Bassford T, et al. Sexual orientation and health: comparisons in the women’s health initiative sample. Arch Fam Med. 2000;9:843-853.
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Turrion-Merino L, Urech-Garcia-de-la-Vega M, Miguel-Gomez L, et al. Severe acne in female-to-male transgender patients. JAMA Dermatol. 2015;151:1260-1261.
- Questions and answers on the iPLEDGE REMS. US Food and Drug Administration website. Published October 12, 2021. Accessed March 23, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-ipledge-rems#:~:text=The%20modification%20will%20become%20effective,verify%20authorization%20to%20dispense%20isotretinoin
- Gao JL, Thoreson N, Dommasch ED. Navigating iPLEDGE enrollment for transgender and gender diverse patients: a guide for providing culturally competent care. J Am Acad Dermatol. 2021;85:790-791.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869-3903.
- Garcia-Rodriguez L, Spiegel JH. Melasma in a transgender woman. Am J Otolaryngol. 2018;39:788-790.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community.J Am Acad Dermatol. 2016;74:303-308.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian,gay, bisexual, and transgender persons. part I. terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
- Jones JM. LGBT identification rises to 5.6% in latest U.S. estimate. Gallup website. Published February 24, 2021. Accessed March 22, 2022. https://news.gallup.com/poll/329708/lgbt-identification-rises-latest-estimate.aspx
- U.S. and world population clock. US Census Bureau website. Accessed March 22, 2022. https://www.census.gov/popclock/
- National LGBTQIA+ Health Education Center. LGBTQIA+ glossary of terms for health care teams. Published February 2, 2022. Accessed April 11, 2022. https://www.lgbtqiahealtheducation.org/wp-content/uploads/2020/02/Glossary-2022.02.22-1.pdf
- National Institutes of Health Sexual and Gender Minority Research Coordinating Committee. NIH FY 2016-2020 strategic plan to advance research on the health and well-being of sexual and gender minorities. NIH website. Accessed March 23, 2022. https://www.edi.nih.gov/sites/default/files/EDI_Public_files/sgm-strategic-plan.pdf
- Caduceus pin—rainbow. American Medical Student Association website. Accessed March 23, 2022. https://www.amsa.org/member-center/store/Caduceus-Pin-Rainbow-p67375123
- 10 tips for caring for LGBTQIA+ patients. Nurse.org website. Accessed March 23, 2022. https://nurse.org/articles/culturally-competent-healthcare-for-LGBTQ-patients/
- Cartron AM, Raiciulescu S, Trinidad JC. Culturally competent care for LGBT patients in dermatology clinics. J Drugs Dermatol. 2020;19:786-787.
- Wareham J. Should you put pronouns in email signatures and social media bios? Forbes website. Published Dec 30, 2019. Accessed March 23, 2022. https://www.forbes.com/sites/jamiewareham/2020/12/30/should-you-put-pronouns-in-email-signatures-and-social-media-bios/?sh=5b74f1246320
- Hafeez H, Zeshan M, Tahir MA, et al. Healthcare disparities among lesbian, gay, bisexual, and transgender youth: a literature review. Cureus. 2017;9:E1184.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons. part II. epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Centers for Disease Control and Prevention. CDC fact sheet: HIV among gay and bisexual men. CDC website. Accessed April 14, 2022. https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/cdc-msm-508.pdf
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2016. CDC website. Accessed April 14, 2022. https://www.cdc.gov/std/stats16/CDC_2016_STDS_Report-for508WebSep21_2017_1644.pdf
- Galindo GR, Casey AJ, Yeung A, et al. Community associated methicillin resistant Staphylococcus aureus among New York City men who have sex with men: qualitative research findings and implications for public health practice. J Community Health. 2012;37:458-467.
- Blashill AJ. Indoor tanning and skin cancer risk among diverse US youth: results from a national sample. JAMA Dermatol. 2017;153:344-345.
- Herbst JH, Jacobs ED, Finlayson TJ, et al. Estimating HIV prevalence and risk behaviors of transgender persons in the United States: a systematic review. AIDS Behav. 2008;12:1-17.
- Uaamnuichai S, Panyakhamlerd K, Suwan A, et al. Neovaginal and anal high-risk human papillomavirus DNA among Thai transgender women in gender health clinics. Sex Transm Dis. 2021;48:547-549.
- Valanis BG, Bowen DJ, Bassford T, et al. Sexual orientation and health: comparisons in the women’s health initiative sample. Arch Fam Med. 2000;9:843-853.
- Wierckx K, Van de Peer F, Verhaeghe E, et al. Short- and long-term clinical skin effects of testosterone treatment in trans men. J Sex Med. 2014;11:222-229.
- Turrion-Merino L, Urech-Garcia-de-la-Vega M, Miguel-Gomez L, et al. Severe acne in female-to-male transgender patients. JAMA Dermatol. 2015;151:1260-1261.
- Questions and answers on the iPLEDGE REMS. US Food and Drug Administration website. Published October 12, 2021. Accessed March 23, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/questions-and-answers-ipledge-rems#:~:text=The%20modification%20will%20become%20effective,verify%20authorization%20to%20dispense%20isotretinoin
- Gao JL, Thoreson N, Dommasch ED. Navigating iPLEDGE enrollment for transgender and gender diverse patients: a guide for providing culturally competent care. J Am Acad Dermatol. 2021;85:790-791.
- Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102:3869-3903.
- Garcia-Rodriguez L, Spiegel JH. Melasma in a transgender woman. Am J Otolaryngol. 2018;39:788-790.
- Ginsberg BA, Calderon M, Seminara NM, et al. A potential role for the dermatologist in the physical transformation of transgender people: a survey of attitudes and practices within the transgender community.J Am Acad Dermatol. 2016;74:303-308.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian,gay, bisexual, and transgender persons. part I. terminology, demographics, health disparities, and approaches to care. J Am Acad Dermatol. 2019;80:581-589.
Resident Pearl
- Because of the longitudinal relationships dermatology residents make with their patients, they have a unique opportunity to provide a safe space and life-changing care to patients within the lesbian, gay, bisexual, and transgender community.
CDC flags uptick in hypertensive disorders in pregnancy
Hypertensive disorders in pregnancy affect nearly 16% of women who give birth in U.S. hospitals and appear to be increasing, according to an April 29 report from the Centers for Disease Control and Prevention.
Older patients and Black women are substantially more likely to experience hypertension in pregnancy, the analysis found.
“Addressing hypertensive disorders in pregnancy is a key strategy in reducing inequities in pregnancy-related mortality,” study coauthor Wanda Barfield, MD, MPH, director of CDC’s Division of Reproductive Health, said in a statement.
Age, obesity, diabetes
The overall prevalence of hypertensive disorders in pregnancy increased from 13.3% in 2017 to 15.9% in 2019, the researchers reported in the CDC’s Morbidity and Mortality Weekly Report.
The uptick in hypertension coincides with trends toward older maternal age and higher rates of obesity and diabetes, which may explain the increase, they said.
For the study, Dr. Barfield and her colleagues analyzed nationally representative data from the National Inpatient Sample. They identified patients with a diagnosis of chronic hypertension, pregnancy-associated hypertension, or unspecified maternal hypertension during their hospitalization.
Among women aged 45-55 years, the prevalence of hypertension was 31%. Among those aged 35-44 years, it was 18%.
Hypertension diagnoses were more common in women who were Black (20.9%) or American Indian or Alaska Native (16.4%), than in other groups.
Of patients who died during delivery hospitalization, 31.6% had a hypertensive disorder.
The study shows a marked increase in hypertensive disorders over a relatively short time, according to Jane van Dis, MD, of the department of obstetrics and gynecology at the University of Rochester (N.Y.), who was not involved in the research. The phenomenon is consistent with her own experience, she said.
“When I am admitting patients, I’m oftentimes surprised when someone does not have a hypertensive disorder because I feel like the majority of patients these days do,” Dr. van Dis told this news organization.
Dr. Van Dis speculated that factors related to the environment, including air pollution and endocrine disrupters, could contribute to elevated rates of hypertensive disorders.
Natalie Bello, MD, MPH, director of hypertension research at Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said rates of hypertension today could be even higher than in the study.
The CDC report relied on pre-COVID data, and the pandemic “increased disparities in health outcomes,” Dr. Bello said in an interview. “I’m worried that in actuality these numbers are an underestimation of the current state of hypertension in pregnancy.”
Dr. Bello, who has studied the need for better training in cardio-obstetrics, applauded Vice President Kamala Harris’ efforts to improve maternal health.
“The racial and geographic disparities that we continue to see in the field are disheartening but should be a call to action to redouble our work to improve maternal outcomes,” Dr. Bello told this news organization. “The good news is that a lot of morbidity related to hypertension can be avoided with timely diagnosis and treatment of blood pressure. However, we need to act to provide all pregnant persons with optimal care.”
Janet Wright, MD, director of CDC’s Division for Heart Disease and Stroke Prevention, said blood pressure home monitoring is a “great example” of a strategy clinicians can use to identify and manage patients with hypertension.
But one approach – self-monitoring blood pressure from home during pregnancy – did not significantly improve the health of pregnant women, according to new results from randomized trials in the United Kingdom.
Trial results published in JAMA show that blood pressure home-monitoring coupled to telemonitoring, as compared with usual care, did not significantly improve blood pressure control among patients with chronic or gestational hypertension.
A second trial published in JAMA that included patients at risk for preeclampsia found that self-monitoring with telemonitoring did not lead to significantly earlier diagnoses of hypertension.
“Individuals at risk for a hypertensive disorder of pregnancy, or with gestational or chronic hypertension, cannot be treated with a single approach,” Malavika Prabhu, MD, with Weill Cornell Medicine, New York, and coauthors write in an editorial accompanying the JAMA studies. Although the data suggest that self-monitoring of blood pressure is practical and tolerated, “More research is needed to determine optimal, high-value, equitable approaches to averting adverse perinatal outcomes associated with hypertensive disorders of pregnancy,” they write.
The CDC study authors and Dr. van Dis have disclosed no relevant financial relationships. Dr. Bello is funded by the National Institutes of Health to study blood pressure monitoring in pregnancy. The JAMA editorial authors disclosed university, government, and corporate grants and work with publishing companies.
A version of this article first appeared on Medscape.com.
Hypertensive disorders in pregnancy affect nearly 16% of women who give birth in U.S. hospitals and appear to be increasing, according to an April 29 report from the Centers for Disease Control and Prevention.
Older patients and Black women are substantially more likely to experience hypertension in pregnancy, the analysis found.
“Addressing hypertensive disorders in pregnancy is a key strategy in reducing inequities in pregnancy-related mortality,” study coauthor Wanda Barfield, MD, MPH, director of CDC’s Division of Reproductive Health, said in a statement.
Age, obesity, diabetes
The overall prevalence of hypertensive disorders in pregnancy increased from 13.3% in 2017 to 15.9% in 2019, the researchers reported in the CDC’s Morbidity and Mortality Weekly Report.
The uptick in hypertension coincides with trends toward older maternal age and higher rates of obesity and diabetes, which may explain the increase, they said.
For the study, Dr. Barfield and her colleagues analyzed nationally representative data from the National Inpatient Sample. They identified patients with a diagnosis of chronic hypertension, pregnancy-associated hypertension, or unspecified maternal hypertension during their hospitalization.
Among women aged 45-55 years, the prevalence of hypertension was 31%. Among those aged 35-44 years, it was 18%.
Hypertension diagnoses were more common in women who were Black (20.9%) or American Indian or Alaska Native (16.4%), than in other groups.
Of patients who died during delivery hospitalization, 31.6% had a hypertensive disorder.
The study shows a marked increase in hypertensive disorders over a relatively short time, according to Jane van Dis, MD, of the department of obstetrics and gynecology at the University of Rochester (N.Y.), who was not involved in the research. The phenomenon is consistent with her own experience, she said.
“When I am admitting patients, I’m oftentimes surprised when someone does not have a hypertensive disorder because I feel like the majority of patients these days do,” Dr. van Dis told this news organization.
Dr. Van Dis speculated that factors related to the environment, including air pollution and endocrine disrupters, could contribute to elevated rates of hypertensive disorders.
Natalie Bello, MD, MPH, director of hypertension research at Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said rates of hypertension today could be even higher than in the study.
The CDC report relied on pre-COVID data, and the pandemic “increased disparities in health outcomes,” Dr. Bello said in an interview. “I’m worried that in actuality these numbers are an underestimation of the current state of hypertension in pregnancy.”
Dr. Bello, who has studied the need for better training in cardio-obstetrics, applauded Vice President Kamala Harris’ efforts to improve maternal health.
“The racial and geographic disparities that we continue to see in the field are disheartening but should be a call to action to redouble our work to improve maternal outcomes,” Dr. Bello told this news organization. “The good news is that a lot of morbidity related to hypertension can be avoided with timely diagnosis and treatment of blood pressure. However, we need to act to provide all pregnant persons with optimal care.”
Janet Wright, MD, director of CDC’s Division for Heart Disease and Stroke Prevention, said blood pressure home monitoring is a “great example” of a strategy clinicians can use to identify and manage patients with hypertension.
But one approach – self-monitoring blood pressure from home during pregnancy – did not significantly improve the health of pregnant women, according to new results from randomized trials in the United Kingdom.
Trial results published in JAMA show that blood pressure home-monitoring coupled to telemonitoring, as compared with usual care, did not significantly improve blood pressure control among patients with chronic or gestational hypertension.
A second trial published in JAMA that included patients at risk for preeclampsia found that self-monitoring with telemonitoring did not lead to significantly earlier diagnoses of hypertension.
“Individuals at risk for a hypertensive disorder of pregnancy, or with gestational or chronic hypertension, cannot be treated with a single approach,” Malavika Prabhu, MD, with Weill Cornell Medicine, New York, and coauthors write in an editorial accompanying the JAMA studies. Although the data suggest that self-monitoring of blood pressure is practical and tolerated, “More research is needed to determine optimal, high-value, equitable approaches to averting adverse perinatal outcomes associated with hypertensive disorders of pregnancy,” they write.
The CDC study authors and Dr. van Dis have disclosed no relevant financial relationships. Dr. Bello is funded by the National Institutes of Health to study blood pressure monitoring in pregnancy. The JAMA editorial authors disclosed university, government, and corporate grants and work with publishing companies.
A version of this article first appeared on Medscape.com.
Hypertensive disorders in pregnancy affect nearly 16% of women who give birth in U.S. hospitals and appear to be increasing, according to an April 29 report from the Centers for Disease Control and Prevention.
Older patients and Black women are substantially more likely to experience hypertension in pregnancy, the analysis found.
“Addressing hypertensive disorders in pregnancy is a key strategy in reducing inequities in pregnancy-related mortality,” study coauthor Wanda Barfield, MD, MPH, director of CDC’s Division of Reproductive Health, said in a statement.
Age, obesity, diabetes
The overall prevalence of hypertensive disorders in pregnancy increased from 13.3% in 2017 to 15.9% in 2019, the researchers reported in the CDC’s Morbidity and Mortality Weekly Report.
The uptick in hypertension coincides with trends toward older maternal age and higher rates of obesity and diabetes, which may explain the increase, they said.
For the study, Dr. Barfield and her colleagues analyzed nationally representative data from the National Inpatient Sample. They identified patients with a diagnosis of chronic hypertension, pregnancy-associated hypertension, or unspecified maternal hypertension during their hospitalization.
Among women aged 45-55 years, the prevalence of hypertension was 31%. Among those aged 35-44 years, it was 18%.
Hypertension diagnoses were more common in women who were Black (20.9%) or American Indian or Alaska Native (16.4%), than in other groups.
Of patients who died during delivery hospitalization, 31.6% had a hypertensive disorder.
The study shows a marked increase in hypertensive disorders over a relatively short time, according to Jane van Dis, MD, of the department of obstetrics and gynecology at the University of Rochester (N.Y.), who was not involved in the research. The phenomenon is consistent with her own experience, she said.
“When I am admitting patients, I’m oftentimes surprised when someone does not have a hypertensive disorder because I feel like the majority of patients these days do,” Dr. van Dis told this news organization.
Dr. Van Dis speculated that factors related to the environment, including air pollution and endocrine disrupters, could contribute to elevated rates of hypertensive disorders.
Natalie Bello, MD, MPH, director of hypertension research at Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, said rates of hypertension today could be even higher than in the study.
The CDC report relied on pre-COVID data, and the pandemic “increased disparities in health outcomes,” Dr. Bello said in an interview. “I’m worried that in actuality these numbers are an underestimation of the current state of hypertension in pregnancy.”
Dr. Bello, who has studied the need for better training in cardio-obstetrics, applauded Vice President Kamala Harris’ efforts to improve maternal health.
“The racial and geographic disparities that we continue to see in the field are disheartening but should be a call to action to redouble our work to improve maternal outcomes,” Dr. Bello told this news organization. “The good news is that a lot of morbidity related to hypertension can be avoided with timely diagnosis and treatment of blood pressure. However, we need to act to provide all pregnant persons with optimal care.”
Janet Wright, MD, director of CDC’s Division for Heart Disease and Stroke Prevention, said blood pressure home monitoring is a “great example” of a strategy clinicians can use to identify and manage patients with hypertension.
But one approach – self-monitoring blood pressure from home during pregnancy – did not significantly improve the health of pregnant women, according to new results from randomized trials in the United Kingdom.
Trial results published in JAMA show that blood pressure home-monitoring coupled to telemonitoring, as compared with usual care, did not significantly improve blood pressure control among patients with chronic or gestational hypertension.
A second trial published in JAMA that included patients at risk for preeclampsia found that self-monitoring with telemonitoring did not lead to significantly earlier diagnoses of hypertension.
“Individuals at risk for a hypertensive disorder of pregnancy, or with gestational or chronic hypertension, cannot be treated with a single approach,” Malavika Prabhu, MD, with Weill Cornell Medicine, New York, and coauthors write in an editorial accompanying the JAMA studies. Although the data suggest that self-monitoring of blood pressure is practical and tolerated, “More research is needed to determine optimal, high-value, equitable approaches to averting adverse perinatal outcomes associated with hypertensive disorders of pregnancy,” they write.
The CDC study authors and Dr. van Dis have disclosed no relevant financial relationships. Dr. Bello is funded by the National Institutes of Health to study blood pressure monitoring in pregnancy. The JAMA editorial authors disclosed university, government, and corporate grants and work with publishing companies.
A version of this article first appeared on Medscape.com.
Melanoma
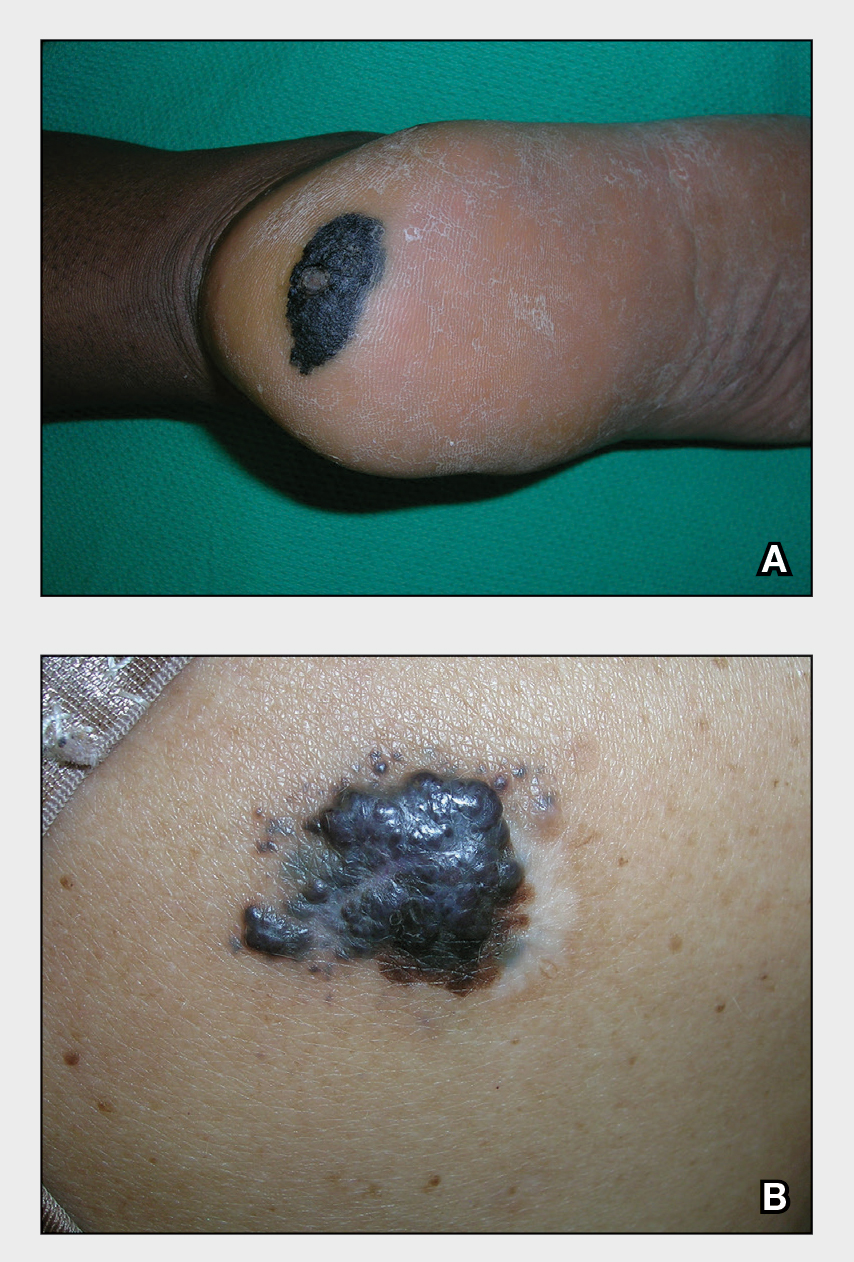
THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097

THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.

THE COMPARISON
A Acral lentiginous melanoma on the sole of the foot in a 30-year-old Black woman. The depth of the lesion was 2 mm with a positive sentinel lymph node biopsy.
B Nodular melanoma on the shoulder of a 63-year-old Hispanic woman. The depth of the lesion was 5.5 mm with a positive sentinel lymph node biopsy.
Melanoma occurs less frequently in individuals with darker skin types than in lighter skin types but is associated with higher rates of morbidity and mortality in this patient population.1-7 In the cases shown here (A and B), both patients had advanced melanomas with large primary lesions and lymph node metastases.
Epidemiology
A systematic review by Higgins et al6 reported the following on the epidemiology of melanomas in patients with skin of color:
- African Americans have deeper tumors at the time of diagnosis, in addition to increased rates of regionally advanced and distant disease. Lesions generally are located on the lower extremities and have an increased propensity for ulceration. Acral lentiginous melanoma is the most common melanoma subtype found in African American patients.6
- In Hispanic individuals, superficial spreading melanoma is the most common melanoma subtype. Lower extremity lesions are more common relative to White individuals. Hispanic individuals have the highest rate of oral cavity melanomas across all ethnic groups.6
- In Asian individuals, acral and subungual sites are most common. Specifically, Pacific Islanders have the highest proportion of mucosal melanomas across all ethnic groups.6
Key clinical features in people with darker skin tones
Melanomas are found more often on the palms, soles, nail units, oral cavity, and mucosae.6 The melanomas have the same clinical and dermoscopic features found in individuals with lighter skin tones.
Worth noting
Factors that may contribute to the diagnosis of more advanced melanomas in racial/ethnic minorities in the United States include:
- decreased access to health care based on lack of health insurance and low socioeconomic status,
- less awareness of the risk of melanoma among patients and health care providers because melanoma is less common in persons of color, and
- lesions found in areas less likely to be seen in screening examinations, such as the soles of the feet and the oral and genital mucosae.
Health disparity highlight
- In a large US study of 96,953 patients with a diagnosis of cutaneous melanoma from 1992 to 2009, the proportion of later-stage melanoma—stages II to IV—was greater in Black patients compared to White patients.7
- Based on this same data set, White patients had the longest survival time (P<.05), followed by Hispanic (P<.05), Asian American/Native American/Pacific Islander (P<.05), and Black (P<.05) patients, respectively.7
- In Miami-Dade County, one study of 1690 melanoma cases found that 48% of Black patients had regional or distant disease at presentation compared to 22% of White patients (P=.015).5 Analysis of multiple factors found that only race was a significant predictor for late-stage melanoma (P<.001). Black patients in this study were 3 times more likely than others to be diagnosed with melanoma at a late stage (P=.07).5
- Black patients in the United States are more likely to have a delayed time from diagnosis to definitive surgery even when controlled for type of health insurance and stage of diagnosis.8
Final thoughts
Efforts are needed to overcome these disparities by:
- educating patients with skin of color and their health care providers about the risks of advanced melanoma with the goal of prevention and earlier diagnosis;
- breaking down barriers to care caused by poverty, lack of health insurance, and systemic racism; and
- eliminating factors that lead to delays from diagnosis to definitive surgery.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37. doi:10.1016/j.jaad.2001.05.034
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914. doi:10.1001/archinte.166.17.1907
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California cancer registry data, 1988-93. Cancer Causes Control. 1997;8:246-252. doi:10.1023/a:1018432632528
- Hu S, Parker DF, Thomas AG, et al. Advanced presentation of melanoma in African Americans: the Miami-Dade County experience. J Am Acad Dermatol. 2004;51:1031-1032. doi:10.1016/j. jaad.2004.05.005
- Hu S, Soza-Vento RM, Parker DF, et al. Comparison of stage at diagnosis of melanoma among Hispanic, black, and white patients in Miami-Dade County, Florida. Arch Dermatol. 2006;142:704-708. doi:10.1001/archderm.142.6.704
- Higgins S, Nazemi A, Feinstein S, et al. Clinical presentations of melanoma in African Americans, Hispanics, and Asians. Dermatol Surg. 2019;45:791-801. doi:10.1097/DSS.0000000000001759
- Dawes SM, Tsai S, Gittleman H, et al. Racial disparities in melanoma survival [published online July 28, 2016]. J Am Acad Dermatol. 2016;75:983-991. doi:10.1016/j.jaad.2016.06.006
- Qian Y, Johannet P, Sawyers A, et al. The ongoing racial disparities in melanoma: an analysis of the Surveillance, Epidemiology, and End Results database (1975-2016)[published online August 27, 2020]. J Am Acad Dermatol. 2021;84:1585-1593. doi:10.1016/j. jaad.2020.08.097