User login
Granulomatous Dermatitis in a Patient With Cholangiocarcinoma Treated With BRAF and MEK Inhibitors
To the Editor:
Granulomatous dermatitis (GD) has been described as a rare side effect of MEK and BRAF inhibitor use in the treatment of BRAF V600E mutation–positive metastatic melanoma. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation. We present the case of a 52-year-old woman who developed GD while being treated with vemurafenib and cobimetinib for BRAF V600E mutation–positive metastatic cholangiocarcinoma.
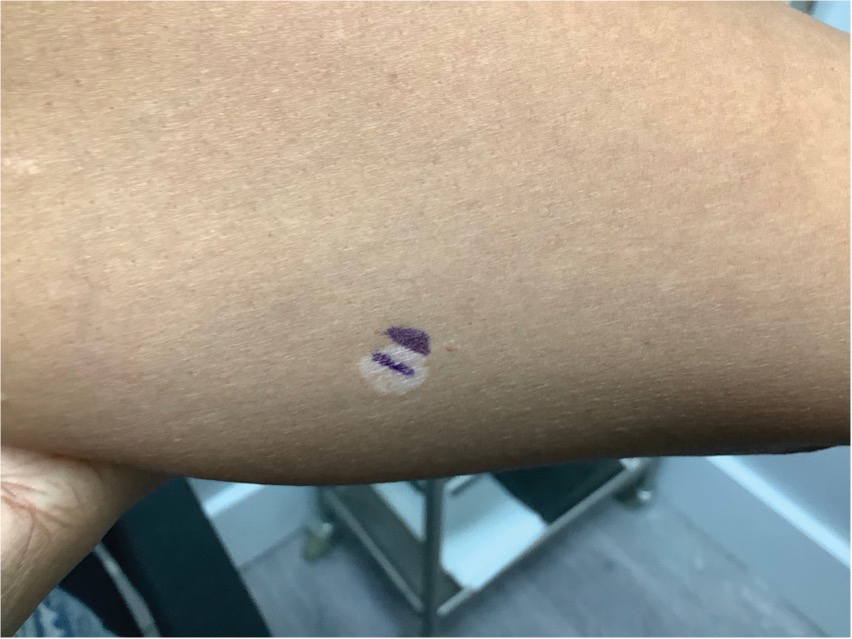
A 52-year-old White woman presented with faint patches of nonpalpable violaceous mottling that extended distally to proximally from the ankles to the thighs on the medial aspects of both legs. She was diagnosed with cholangiocarcinoma 10 months prior, with metastases to the lung, liver, and sternum. She underwent treatment with gemcitabine and cisplatin therapy. Computed tomography after several treatment cycles revealed progressive disease with multiple pulmonary nodules as well as metastatic intrathoracic and abdominal adenopathy. Treatment with gemcitabine and cisplatin failed to produce a favorable response and was discontinued after 6 treatment cycles.
Genomic testing performed at the time of diagnosis revealed a positive mutation for BRAF V600E. The patient subsequently enrolled in a clinical trial and started treatment with the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib. She developed sun sensitivity and multiple sunburns after starting these therapies. The patient tolerated the next few cycles of therapy well with only moderate concerns of dry sensitive skin.
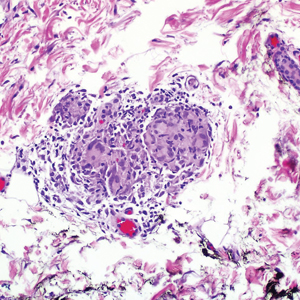
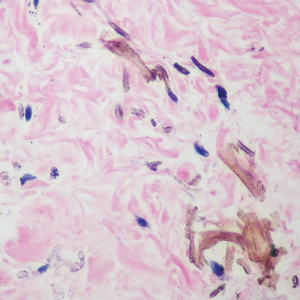
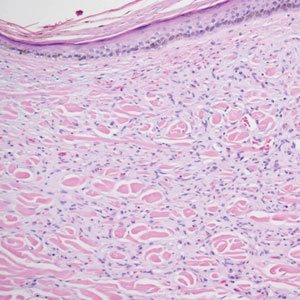
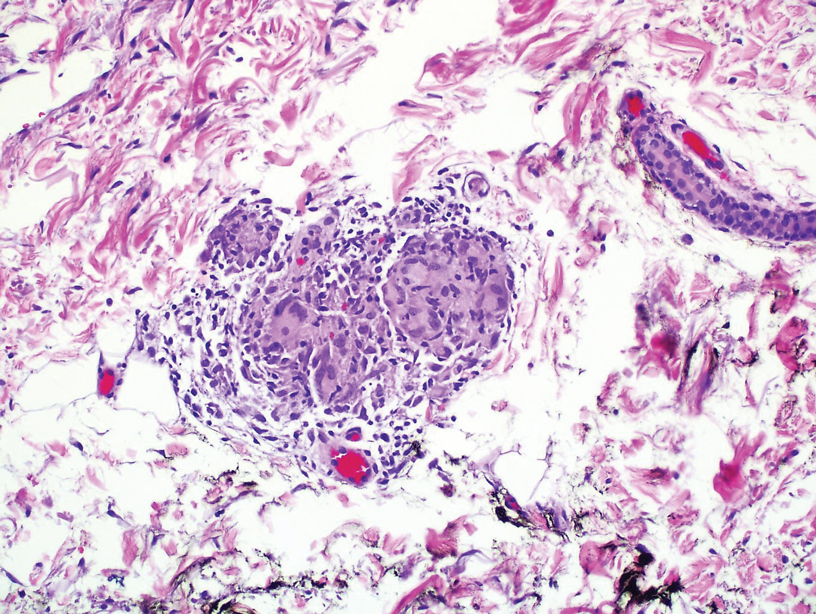
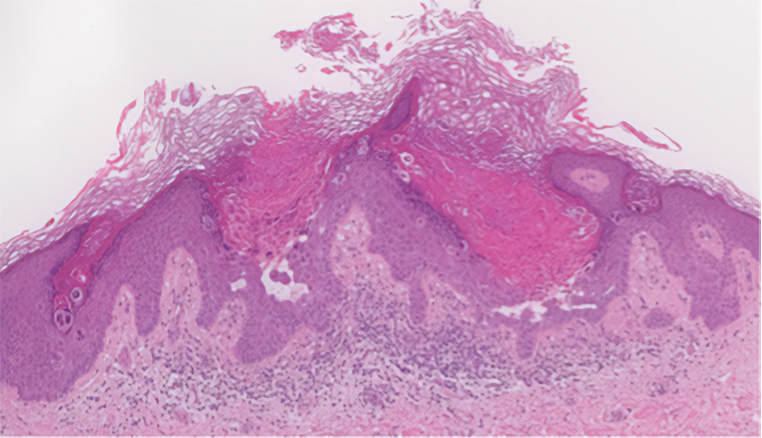
During the sixth cycle of therapy, she presented to dermatology after developing a rash. Over the next 2 weeks, similar lesions appeared on the arms. The patient denied the use of any new lotions, soaps, or other medications. Punch biopsies of the right forearm and right medial thigh revealed nonnecrotizing granulomas in the superficial dermis that extended into the subcutaneous adipose tissue (Figure 1). Surrounding chronic inflammation was scant, and the presence of rare eosinophils was noted (Figure 2). The histiocytes were highlighted by a CD68 immunohistochemical stain. An auramine-O special stain test was negative for acid-fast bacilli, and a Grocott methenamine-silver special stain test for fungal organisms was negative. These findings were consistent with GD. Computed tomography of the chest performed 2 months prior and 1 month after biopsy of the skin lesions revealed no axillary, mediastinal, or hilar lymphadenopathy. The calcium level at the time of skin biopsy was within reference range.
A topical steroid was prescribed; however, it was not utilized by the patient. Within 2 months of onset, the GD lesions resolved with no treatment. The GD lesions did not affect the patient’s enrollment in the clinical trial, and no dose reductions were made. Due to progressive disease with metastases to the brain, the patient eventually discontinued the clinical trial.
BRAF inhibitors are US Food and Drug Administration approved for the treatment of metastatic melanoma to deactivate the serine-threonine kinase BRAF gene mutation, which leads to decreased generation and survival of melanoma cells.1,2 Vemurafenib, dabrafenib, and encorafenib are the only BRAF inhibitors approved in the United States.3 The most common side effects of vemurafenib include arthralgia, fatigue, rash, and photosensitivity.1,4 There are 4 MEK inhibitors currently available in the United States: cobimetinib, trametinib, selumetinib and binimetinib. The addition of a MEK inhibitor to BRAF inhibitor therapy has shown increased patient response rates and prolonged survival in 3 phase 3 studies.5-10
Response rates remain low in the treatment of advanced cholangiocarcinoma with standard chemotherapy. Recent research has explored if targeted therapies at the molecular level would be of benefit.11 Our patient was enrolled in the American Society of Clinical Oncology Targeted Agent and Profiling Utilization Registry (TAPUR) trial, a phase 2, prospective, nonrandomized trial that matches eligible participants to US Food and Drug Administration–approved study medications based on specific data from their molecular testing results.12 Some of the most common mutations in intrahepatic cholangiocarcinoma include HER2, KRAS, MET, and BRAF.13-17 Our patient’s molecular test results were positive for a BRAF V600E–positive mutation, and she subsequently started therapy with vemurafenib and cobimetinib. The use of personalized genomic treatment approaches for BRAF V600E mutation–positive cholangiocarcinoma has produced a dramatic patient response to BRAF and MEK inhibitor combination therapies.11,18-20
Drug-induced GD most likely is caused by vascular insults that lead to deposition of immune complexes in vessels causing inflammation and a consequent granulomatous infiltrate.21,22 Although cordlike lesions in the subcutaneous tissue on the trunk commonly are reported, the presentation of GD can vary considerably. Other presentations include areas of violaceous or erythematous patches or plaques on the limbs, intertriginous areas, and upper trunk. Diffuse macular erythema or small flesh-colored papules also can be observed.23
Granulomatous dermatitis secondary to drug reactions can have varying morphologies. The infiltrate often can have an interstitial appearance with the presence of lymphocytes, plasma cells, histiocytes, eosinophils, and multinucleated giant cells.24 These findings can be confused with interstitial granuloma annulare. Other cases, such as in our patient, can have discrete granulomata formation with a sarcoidlike appearance. These naked granulomas lack surrounding inflammation and suggest a differential diagnosis of sarcoidosis and infection. Use of immune checkpoint inhibitors (CIs) and kinase inhibitors has been proven to cause sarcoidosislike reactions.25 The development of granulomatous/sarcoidlike lesions associated with the use of BRAF and MEK inhibitors may clinically and radiographically mimic disease recurrence. An awareness of this type of reaction by clinicians and pathologists is important to ensure appropriate management in patients who develop GD.26
Checkpoint inhibitor–induced GD that remains asymptomatic does not necessarily warrant treatment; however, corticosteroid use and elimination of CI therapies have resolved GD in prior cases. Responsiveness of the cancer to CI therapy and severity of GD symptoms should be considered before discontinuation of a CI trial.25
One case report described complete resolution of a GD eruption without interruption of the scheduled BRAF and MEK inhibitor therapies for the treatment of metastatic melanoma. There was no reported use of a steroidal cream or other topical medication to aid in controlling the eruption.27 The exact mechanism of how GD resolves while continuing therapy is unknown; however, it has been suggested that a GD eruption may be the consequence of a BRAF and MEK inhibitor–mediated immune response against a subclinical area of metastatic melanoma.28 If the immune response successfully eliminates the subclinical tumor, one could postulate that the inflammatory response and granulomatous eruption would resolve. Future studies are necessary to further elucidate the exact mechanisms involved.
There have been several case reports of GD with vemurafenib treatment,29,30 1 report of GD and erythema induratum with vemurafenib and cobimetinib treatment,31 2 reports of GD with dabrafenib treatment,27,30 and a few reports of GD with the BRAF inhibitor dabrafenib combined with the MEK inhibitor trametinib,28,32,33 all for the treatment of metastatic melanoma. Additionally, a report described a 3-year-old boy who developed GD secondary to vemurafenib for the treatment of Langerhans cell histiocytosis.34 We present a unique case of BRAF and MEK inhibitor therapy–induced GD in the treatment of metastatic cholangiocarcinoma with vemurafenib and cobimetinib.
BRAF and MEK inhibitor therapy is used in patients with metastatic melanomas with a positive BRAF V600E mutation. Due to advancements in next-generation DNA sequencing, these therapies also are being tested in clinical trials for use in the treatment of other cancers with the same checkpoint mutation, such as metastatic cholangiocarcinoma. Cutaneous reactions frequently are documented side effects that occur during treatment with BRAF and MEK inhibitors; GD is an uncommon finding. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of other cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation.
- Mackiewicz J, Mackiewicz A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Comtemp Oncol (Pozn). 2018;22:68-72.
- Jovanovic B, Krockel D, Linden D, et al. Lack of cytoplasmic ERK activation is an independent adverse prognostic factor in primary cutaneous melanoma. J Invest Dermatol. 2008;128:2696-2704.
- Alqathama A. BRAF in malignant melanoma progression and metastasis: potentials and challenges. Am J Cancer Res. 2020;10:1103-1114.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Casey D, Demko S, Sinha A, et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin Cancer Res. 2021;27;4142-4146
- Flaherty K, Davies MA, Grob JJ, et al. Genomic analysis and 3-y efficacy and safety update of COMBI-d: a phase 3 study of dabrafenib (D) fl trametinib (T) vs D monotherapy in patients (pts) with unresectable or metastatic BRAF V600E/K-mutant cutaneous melanoma. Abstract presented at: American Society of Clinical Oncology Annual Meeting; June 3-7, 2016; Chicago, IL. P9502.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Robert C, Karaszewska B, Schachter J, et al. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/K–mutant cutaneous melanoma. Ann Oncol. 2016;27(suppl 6):vi552-vi587.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876.
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advance BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomized, double-blind, phase 3 trial. Lancet Once. 2016;17:1248-1260.
- Kocsis J, Árokszállási A, András C, et al. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion. J Gastrointest Oncol. 2017;8:E32-E38.
- Mangat PK, Halabi S, Bruinooge SS, et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study [published online July 11, 2018]. JCO Precis Oncol. doi:10.1200/PO.18.00122
- Terada T, Ashida K, Endo K, et al. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325-331.
- Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16INK4A alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721-727.
- Momoi H, Itoh T, Nozaki Y, et al. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol. 2001;35:235-244.
- Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180.
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706-712.
- Bunyatov T, Zhao A, Kovalenko J, et al. Personalised approach in combined treatment of cholangiocarcinoma: a case report of healing from cholangiocellular carcinoma at stage IV. J Gastrointest Oncol. 2019;10:815-820.
- Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7:E98-E102.
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience. 2014;8:479.
- Rosenbach M, English JC. Reactive granulomatous dermatitis. Dermatol Clin. 2015;33:373-387.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol 2012;166:775-783.
- Calonje JE, Brenn T, Lazar A, Billings S. Lichenoid and interface dermatitis. In: McKee’s Pathology of the Skin. 5th ed. China: Elsevier Limited: 2018;7:241-282.
- Gkiozos I, Kopitopoulou A, Kalkanis A, et al. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 2018;13:1076-1082.
- Tetzlaff MT, Nelson KC, Diab A, et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6:14.
- Garrido MC, Gutiérrez C, Riveiro-Falkenbach E, et al. BRAF inhibitor-induced antitumoral granulomatous dermatitis eruption in advanced melanoma. Am J Dermatopathol. 2015;37:795-798.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307‐311.
- Ong ELH, Sinha R, Jmor S, et al. BRAF inhibitor-associated granulomatous dermatitis: a report of 3 cases. Am J of Dermatopathol. 2019;41:214-217.
- Wali GN, Stonard C, Espinosa O, et al. Persistent granulomatous cutaneous drug eruption to a BRAF inhibitor. J Am Acad Dermatol. 2017;76(suppl 1):AB195.
- Aj lafolla M, Ramsay J, Wismer J, et al. Cobimetinib- and vemurafenib-induced granulomatous dermatitis and erythema induratum: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847358
- Jansen YJ, Janssens P, Hoorens A, et al. Granulomatous nephritis and dermatitis in a patient with BRAF V600E mutant metastatic melanoma treated with dabrafenib and trametinib. Melanoma Res. 2015;25:550‐554.
- Green JS, Norris DA, Wisell J. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Chen L, His A, Kothari A, et al. Granulomatous dermatitis secondary to vemurafenib in a child with Langerhans cell histiocytosis. Pediatr Dermatol. 2018;35:E402-E403.
To the Editor:
Granulomatous dermatitis (GD) has been described as a rare side effect of MEK and BRAF inhibitor use in the treatment of BRAF V600E mutation–positive metastatic melanoma. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation. We present the case of a 52-year-old woman who developed GD while being treated with vemurafenib and cobimetinib for BRAF V600E mutation–positive metastatic cholangiocarcinoma.
A 52-year-old White woman presented with faint patches of nonpalpable violaceous mottling that extended distally to proximally from the ankles to the thighs on the medial aspects of both legs. She was diagnosed with cholangiocarcinoma 10 months prior, with metastases to the lung, liver, and sternum. She underwent treatment with gemcitabine and cisplatin therapy. Computed tomography after several treatment cycles revealed progressive disease with multiple pulmonary nodules as well as metastatic intrathoracic and abdominal adenopathy. Treatment with gemcitabine and cisplatin failed to produce a favorable response and was discontinued after 6 treatment cycles.
Genomic testing performed at the time of diagnosis revealed a positive mutation for BRAF V600E. The patient subsequently enrolled in a clinical trial and started treatment with the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib. She developed sun sensitivity and multiple sunburns after starting these therapies. The patient tolerated the next few cycles of therapy well with only moderate concerns of dry sensitive skin.
During the sixth cycle of therapy, she presented to dermatology after developing a rash. Over the next 2 weeks, similar lesions appeared on the arms. The patient denied the use of any new lotions, soaps, or other medications. Punch biopsies of the right forearm and right medial thigh revealed nonnecrotizing granulomas in the superficial dermis that extended into the subcutaneous adipose tissue (Figure 1). Surrounding chronic inflammation was scant, and the presence of rare eosinophils was noted (Figure 2). The histiocytes were highlighted by a CD68 immunohistochemical stain. An auramine-O special stain test was negative for acid-fast bacilli, and a Grocott methenamine-silver special stain test for fungal organisms was negative. These findings were consistent with GD. Computed tomography of the chest performed 2 months prior and 1 month after biopsy of the skin lesions revealed no axillary, mediastinal, or hilar lymphadenopathy. The calcium level at the time of skin biopsy was within reference range.

A topical steroid was prescribed; however, it was not utilized by the patient. Within 2 months of onset, the GD lesions resolved with no treatment. The GD lesions did not affect the patient’s enrollment in the clinical trial, and no dose reductions were made. Due to progressive disease with metastases to the brain, the patient eventually discontinued the clinical trial.

BRAF inhibitors are US Food and Drug Administration approved for the treatment of metastatic melanoma to deactivate the serine-threonine kinase BRAF gene mutation, which leads to decreased generation and survival of melanoma cells.1,2 Vemurafenib, dabrafenib, and encorafenib are the only BRAF inhibitors approved in the United States.3 The most common side effects of vemurafenib include arthralgia, fatigue, rash, and photosensitivity.1,4 There are 4 MEK inhibitors currently available in the United States: cobimetinib, trametinib, selumetinib and binimetinib. The addition of a MEK inhibitor to BRAF inhibitor therapy has shown increased patient response rates and prolonged survival in 3 phase 3 studies.5-10
Response rates remain low in the treatment of advanced cholangiocarcinoma with standard chemotherapy. Recent research has explored if targeted therapies at the molecular level would be of benefit.11 Our patient was enrolled in the American Society of Clinical Oncology Targeted Agent and Profiling Utilization Registry (TAPUR) trial, a phase 2, prospective, nonrandomized trial that matches eligible participants to US Food and Drug Administration–approved study medications based on specific data from their molecular testing results.12 Some of the most common mutations in intrahepatic cholangiocarcinoma include HER2, KRAS, MET, and BRAF.13-17 Our patient’s molecular test results were positive for a BRAF V600E–positive mutation, and she subsequently started therapy with vemurafenib and cobimetinib. The use of personalized genomic treatment approaches for BRAF V600E mutation–positive cholangiocarcinoma has produced a dramatic patient response to BRAF and MEK inhibitor combination therapies.11,18-20
Drug-induced GD most likely is caused by vascular insults that lead to deposition of immune complexes in vessels causing inflammation and a consequent granulomatous infiltrate.21,22 Although cordlike lesions in the subcutaneous tissue on the trunk commonly are reported, the presentation of GD can vary considerably. Other presentations include areas of violaceous or erythematous patches or plaques on the limbs, intertriginous areas, and upper trunk. Diffuse macular erythema or small flesh-colored papules also can be observed.23
Granulomatous dermatitis secondary to drug reactions can have varying morphologies. The infiltrate often can have an interstitial appearance with the presence of lymphocytes, plasma cells, histiocytes, eosinophils, and multinucleated giant cells.24 These findings can be confused with interstitial granuloma annulare. Other cases, such as in our patient, can have discrete granulomata formation with a sarcoidlike appearance. These naked granulomas lack surrounding inflammation and suggest a differential diagnosis of sarcoidosis and infection. Use of immune checkpoint inhibitors (CIs) and kinase inhibitors has been proven to cause sarcoidosislike reactions.25 The development of granulomatous/sarcoidlike lesions associated with the use of BRAF and MEK inhibitors may clinically and radiographically mimic disease recurrence. An awareness of this type of reaction by clinicians and pathologists is important to ensure appropriate management in patients who develop GD.26
Checkpoint inhibitor–induced GD that remains asymptomatic does not necessarily warrant treatment; however, corticosteroid use and elimination of CI therapies have resolved GD in prior cases. Responsiveness of the cancer to CI therapy and severity of GD symptoms should be considered before discontinuation of a CI trial.25
One case report described complete resolution of a GD eruption without interruption of the scheduled BRAF and MEK inhibitor therapies for the treatment of metastatic melanoma. There was no reported use of a steroidal cream or other topical medication to aid in controlling the eruption.27 The exact mechanism of how GD resolves while continuing therapy is unknown; however, it has been suggested that a GD eruption may be the consequence of a BRAF and MEK inhibitor–mediated immune response against a subclinical area of metastatic melanoma.28 If the immune response successfully eliminates the subclinical tumor, one could postulate that the inflammatory response and granulomatous eruption would resolve. Future studies are necessary to further elucidate the exact mechanisms involved.
There have been several case reports of GD with vemurafenib treatment,29,30 1 report of GD and erythema induratum with vemurafenib and cobimetinib treatment,31 2 reports of GD with dabrafenib treatment,27,30 and a few reports of GD with the BRAF inhibitor dabrafenib combined with the MEK inhibitor trametinib,28,32,33 all for the treatment of metastatic melanoma. Additionally, a report described a 3-year-old boy who developed GD secondary to vemurafenib for the treatment of Langerhans cell histiocytosis.34 We present a unique case of BRAF and MEK inhibitor therapy–induced GD in the treatment of metastatic cholangiocarcinoma with vemurafenib and cobimetinib.
BRAF and MEK inhibitor therapy is used in patients with metastatic melanomas with a positive BRAF V600E mutation. Due to advancements in next-generation DNA sequencing, these therapies also are being tested in clinical trials for use in the treatment of other cancers with the same checkpoint mutation, such as metastatic cholangiocarcinoma. Cutaneous reactions frequently are documented side effects that occur during treatment with BRAF and MEK inhibitors; GD is an uncommon finding. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of other cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation.
To the Editor:
Granulomatous dermatitis (GD) has been described as a rare side effect of MEK and BRAF inhibitor use in the treatment of BRAF V600E mutation–positive metastatic melanoma. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation. We present the case of a 52-year-old woman who developed GD while being treated with vemurafenib and cobimetinib for BRAF V600E mutation–positive metastatic cholangiocarcinoma.
A 52-year-old White woman presented with faint patches of nonpalpable violaceous mottling that extended distally to proximally from the ankles to the thighs on the medial aspects of both legs. She was diagnosed with cholangiocarcinoma 10 months prior, with metastases to the lung, liver, and sternum. She underwent treatment with gemcitabine and cisplatin therapy. Computed tomography after several treatment cycles revealed progressive disease with multiple pulmonary nodules as well as metastatic intrathoracic and abdominal adenopathy. Treatment with gemcitabine and cisplatin failed to produce a favorable response and was discontinued after 6 treatment cycles.
Genomic testing performed at the time of diagnosis revealed a positive mutation for BRAF V600E. The patient subsequently enrolled in a clinical trial and started treatment with the BRAF inhibitor vemurafenib and the MEK inhibitor cobimetinib. She developed sun sensitivity and multiple sunburns after starting these therapies. The patient tolerated the next few cycles of therapy well with only moderate concerns of dry sensitive skin.
During the sixth cycle of therapy, she presented to dermatology after developing a rash. Over the next 2 weeks, similar lesions appeared on the arms. The patient denied the use of any new lotions, soaps, or other medications. Punch biopsies of the right forearm and right medial thigh revealed nonnecrotizing granulomas in the superficial dermis that extended into the subcutaneous adipose tissue (Figure 1). Surrounding chronic inflammation was scant, and the presence of rare eosinophils was noted (Figure 2). The histiocytes were highlighted by a CD68 immunohistochemical stain. An auramine-O special stain test was negative for acid-fast bacilli, and a Grocott methenamine-silver special stain test for fungal organisms was negative. These findings were consistent with GD. Computed tomography of the chest performed 2 months prior and 1 month after biopsy of the skin lesions revealed no axillary, mediastinal, or hilar lymphadenopathy. The calcium level at the time of skin biopsy was within reference range.

A topical steroid was prescribed; however, it was not utilized by the patient. Within 2 months of onset, the GD lesions resolved with no treatment. The GD lesions did not affect the patient’s enrollment in the clinical trial, and no dose reductions were made. Due to progressive disease with metastases to the brain, the patient eventually discontinued the clinical trial.

BRAF inhibitors are US Food and Drug Administration approved for the treatment of metastatic melanoma to deactivate the serine-threonine kinase BRAF gene mutation, which leads to decreased generation and survival of melanoma cells.1,2 Vemurafenib, dabrafenib, and encorafenib are the only BRAF inhibitors approved in the United States.3 The most common side effects of vemurafenib include arthralgia, fatigue, rash, and photosensitivity.1,4 There are 4 MEK inhibitors currently available in the United States: cobimetinib, trametinib, selumetinib and binimetinib. The addition of a MEK inhibitor to BRAF inhibitor therapy has shown increased patient response rates and prolonged survival in 3 phase 3 studies.5-10
Response rates remain low in the treatment of advanced cholangiocarcinoma with standard chemotherapy. Recent research has explored if targeted therapies at the molecular level would be of benefit.11 Our patient was enrolled in the American Society of Clinical Oncology Targeted Agent and Profiling Utilization Registry (TAPUR) trial, a phase 2, prospective, nonrandomized trial that matches eligible participants to US Food and Drug Administration–approved study medications based on specific data from their molecular testing results.12 Some of the most common mutations in intrahepatic cholangiocarcinoma include HER2, KRAS, MET, and BRAF.13-17 Our patient’s molecular test results were positive for a BRAF V600E–positive mutation, and she subsequently started therapy with vemurafenib and cobimetinib. The use of personalized genomic treatment approaches for BRAF V600E mutation–positive cholangiocarcinoma has produced a dramatic patient response to BRAF and MEK inhibitor combination therapies.11,18-20
Drug-induced GD most likely is caused by vascular insults that lead to deposition of immune complexes in vessels causing inflammation and a consequent granulomatous infiltrate.21,22 Although cordlike lesions in the subcutaneous tissue on the trunk commonly are reported, the presentation of GD can vary considerably. Other presentations include areas of violaceous or erythematous patches or plaques on the limbs, intertriginous areas, and upper trunk. Diffuse macular erythema or small flesh-colored papules also can be observed.23
Granulomatous dermatitis secondary to drug reactions can have varying morphologies. The infiltrate often can have an interstitial appearance with the presence of lymphocytes, plasma cells, histiocytes, eosinophils, and multinucleated giant cells.24 These findings can be confused with interstitial granuloma annulare. Other cases, such as in our patient, can have discrete granulomata formation with a sarcoidlike appearance. These naked granulomas lack surrounding inflammation and suggest a differential diagnosis of sarcoidosis and infection. Use of immune checkpoint inhibitors (CIs) and kinase inhibitors has been proven to cause sarcoidosislike reactions.25 The development of granulomatous/sarcoidlike lesions associated with the use of BRAF and MEK inhibitors may clinically and radiographically mimic disease recurrence. An awareness of this type of reaction by clinicians and pathologists is important to ensure appropriate management in patients who develop GD.26
Checkpoint inhibitor–induced GD that remains asymptomatic does not necessarily warrant treatment; however, corticosteroid use and elimination of CI therapies have resolved GD in prior cases. Responsiveness of the cancer to CI therapy and severity of GD symptoms should be considered before discontinuation of a CI trial.25
One case report described complete resolution of a GD eruption without interruption of the scheduled BRAF and MEK inhibitor therapies for the treatment of metastatic melanoma. There was no reported use of a steroidal cream or other topical medication to aid in controlling the eruption.27 The exact mechanism of how GD resolves while continuing therapy is unknown; however, it has been suggested that a GD eruption may be the consequence of a BRAF and MEK inhibitor–mediated immune response against a subclinical area of metastatic melanoma.28 If the immune response successfully eliminates the subclinical tumor, one could postulate that the inflammatory response and granulomatous eruption would resolve. Future studies are necessary to further elucidate the exact mechanisms involved.
There have been several case reports of GD with vemurafenib treatment,29,30 1 report of GD and erythema induratum with vemurafenib and cobimetinib treatment,31 2 reports of GD with dabrafenib treatment,27,30 and a few reports of GD with the BRAF inhibitor dabrafenib combined with the MEK inhibitor trametinib,28,32,33 all for the treatment of metastatic melanoma. Additionally, a report described a 3-year-old boy who developed GD secondary to vemurafenib for the treatment of Langerhans cell histiocytosis.34 We present a unique case of BRAF and MEK inhibitor therapy–induced GD in the treatment of metastatic cholangiocarcinoma with vemurafenib and cobimetinib.
BRAF and MEK inhibitor therapy is used in patients with metastatic melanomas with a positive BRAF V600E mutation. Due to advancements in next-generation DNA sequencing, these therapies also are being tested in clinical trials for use in the treatment of other cancers with the same checkpoint mutation, such as metastatic cholangiocarcinoma. Cutaneous reactions frequently are documented side effects that occur during treatment with BRAF and MEK inhibitors; GD is an uncommon finding. As the utilization of BRAF and MEK inhibitors increases for the treatment of a variety of other cancers, it is essential that clinicians and pathologists recognize GD as a potential cutaneous manifestation.
- Mackiewicz J, Mackiewicz A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Comtemp Oncol (Pozn). 2018;22:68-72.
- Jovanovic B, Krockel D, Linden D, et al. Lack of cytoplasmic ERK activation is an independent adverse prognostic factor in primary cutaneous melanoma. J Invest Dermatol. 2008;128:2696-2704.
- Alqathama A. BRAF in malignant melanoma progression and metastasis: potentials and challenges. Am J Cancer Res. 2020;10:1103-1114.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Casey D, Demko S, Sinha A, et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin Cancer Res. 2021;27;4142-4146
- Flaherty K, Davies MA, Grob JJ, et al. Genomic analysis and 3-y efficacy and safety update of COMBI-d: a phase 3 study of dabrafenib (D) fl trametinib (T) vs D monotherapy in patients (pts) with unresectable or metastatic BRAF V600E/K-mutant cutaneous melanoma. Abstract presented at: American Society of Clinical Oncology Annual Meeting; June 3-7, 2016; Chicago, IL. P9502.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Robert C, Karaszewska B, Schachter J, et al. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/K–mutant cutaneous melanoma. Ann Oncol. 2016;27(suppl 6):vi552-vi587.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876.
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advance BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomized, double-blind, phase 3 trial. Lancet Once. 2016;17:1248-1260.
- Kocsis J, Árokszállási A, András C, et al. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion. J Gastrointest Oncol. 2017;8:E32-E38.
- Mangat PK, Halabi S, Bruinooge SS, et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study [published online July 11, 2018]. JCO Precis Oncol. doi:10.1200/PO.18.00122
- Terada T, Ashida K, Endo K, et al. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325-331.
- Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16INK4A alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721-727.
- Momoi H, Itoh T, Nozaki Y, et al. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol. 2001;35:235-244.
- Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180.
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706-712.
- Bunyatov T, Zhao A, Kovalenko J, et al. Personalised approach in combined treatment of cholangiocarcinoma: a case report of healing from cholangiocellular carcinoma at stage IV. J Gastrointest Oncol. 2019;10:815-820.
- Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7:E98-E102.
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience. 2014;8:479.
- Rosenbach M, English JC. Reactive granulomatous dermatitis. Dermatol Clin. 2015;33:373-387.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol 2012;166:775-783.
- Calonje JE, Brenn T, Lazar A, Billings S. Lichenoid and interface dermatitis. In: McKee’s Pathology of the Skin. 5th ed. China: Elsevier Limited: 2018;7:241-282.
- Gkiozos I, Kopitopoulou A, Kalkanis A, et al. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 2018;13:1076-1082.
- Tetzlaff MT, Nelson KC, Diab A, et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6:14.
- Garrido MC, Gutiérrez C, Riveiro-Falkenbach E, et al. BRAF inhibitor-induced antitumoral granulomatous dermatitis eruption in advanced melanoma. Am J Dermatopathol. 2015;37:795-798.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307‐311.
- Ong ELH, Sinha R, Jmor S, et al. BRAF inhibitor-associated granulomatous dermatitis: a report of 3 cases. Am J of Dermatopathol. 2019;41:214-217.
- Wali GN, Stonard C, Espinosa O, et al. Persistent granulomatous cutaneous drug eruption to a BRAF inhibitor. J Am Acad Dermatol. 2017;76(suppl 1):AB195.
- Aj lafolla M, Ramsay J, Wismer J, et al. Cobimetinib- and vemurafenib-induced granulomatous dermatitis and erythema induratum: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847358
- Jansen YJ, Janssens P, Hoorens A, et al. Granulomatous nephritis and dermatitis in a patient with BRAF V600E mutant metastatic melanoma treated with dabrafenib and trametinib. Melanoma Res. 2015;25:550‐554.
- Green JS, Norris DA, Wisell J. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Chen L, His A, Kothari A, et al. Granulomatous dermatitis secondary to vemurafenib in a child with Langerhans cell histiocytosis. Pediatr Dermatol. 2018;35:E402-E403.
- Mackiewicz J, Mackiewicz A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Comtemp Oncol (Pozn). 2018;22:68-72.
- Jovanovic B, Krockel D, Linden D, et al. Lack of cytoplasmic ERK activation is an independent adverse prognostic factor in primary cutaneous melanoma. J Invest Dermatol. 2008;128:2696-2704.
- Alqathama A. BRAF in malignant melanoma progression and metastasis: potentials and challenges. Am J Cancer Res. 2020;10:1103-1114.
- Zimmer L, Hillen U, Livingstone E, et al. Atypical melanocytic proliferations and new primary melanomas in patients with advanced melanoma undergoing selective BRAF inhibition. J Clin Oncol. 2012;30:2375-2383.
- Casey D, Demko S, Sinha A, et al. FDA approval summary: selumetinib for plexiform neurofibroma. Clin Cancer Res. 2021;27;4142-4146
- Flaherty K, Davies MA, Grob JJ, et al. Genomic analysis and 3-y efficacy and safety update of COMBI-d: a phase 3 study of dabrafenib (D) fl trametinib (T) vs D monotherapy in patients (pts) with unresectable or metastatic BRAF V600E/K-mutant cutaneous melanoma. Abstract presented at: American Society of Clinical Oncology Annual Meeting; June 3-7, 2016; Chicago, IL. P9502.
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30-39.
- Robert C, Karaszewska B, Schachter J, et al. Three-year estimate of overall survival in COMBI-v, a randomized phase 3 study evaluating first-line dabrafenib (D) + trametinib (T) in patients (pts) with unresectable or metastatic BRAF V600E/K–mutant cutaneous melanoma. Ann Oncol. 2016;27(suppl 6):vi552-vi587.
- Larkin J, Ascierto PA, Dreno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med. 2014;371:1867-1876.
- Ascierto PA, McArthur GA, Dréno B, et al. Cobimetinib combined with vemurafenib in advance BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomized, double-blind, phase 3 trial. Lancet Once. 2016;17:1248-1260.
- Kocsis J, Árokszállási A, András C, et al. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: dramatic clinical and radiological response with a confusing synchronic new liver lesion. J Gastrointest Oncol. 2017;8:E32-E38.
- Mangat PK, Halabi S, Bruinooge SS, et al. Rationale and design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study [published online July 11, 2018]. JCO Precis Oncol. doi:10.1200/PO.18.00122
- Terada T, Ashida K, Endo K, et al. c-erbB-2 protein is expressed in hepatolithiasis and cholangiocarcinoma. Histopathology. 1998;33:325-331.
- Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16INK4A alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721-727.
- Momoi H, Itoh T, Nozaki Y, et al. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol. 2001;35:235-244.
- Terada T, Nakanuma Y, Sirica AE. Immunohistochemical demonstration of MET overexpression in human intrahepatic cholangiocarcinoma and in hepatolithiasis. Hum Pathol. 1998;29:175-180.
- Tannapfel A, Sommerer F, Benicke M, et al. Mutations of the BRAF gene in cholangiocarcinoma but not in hepatocellular carcinoma. Gut. 2003;52:706-712.
- Bunyatov T, Zhao A, Kovalenko J, et al. Personalised approach in combined treatment of cholangiocarcinoma: a case report of healing from cholangiocellular carcinoma at stage IV. J Gastrointest Oncol. 2019;10:815-820.
- Lavingia V, Fakih M. Impressive response to dual BRAF and MEK inhibition in patients with BRAF mutant intrahepatic cholangiocarcinoma-2 case reports and a brief review. J Gastrointest Oncol. 2016;7:E98-E102.
- Loaiza-Bonilla A, Clayton E, Furth E, et al. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience. 2014;8:479.
- Rosenbach M, English JC. Reactive granulomatous dermatitis. Dermatol Clin. 2015;33:373-387.
- Tomasini C, Pippione M. Interstitial granulomatous dermatitis with plaques. J Am Acad Dermatol. 2002;46:892-899.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol 2012;166:775-783.
- Calonje JE, Brenn T, Lazar A, Billings S. Lichenoid and interface dermatitis. In: McKee’s Pathology of the Skin. 5th ed. China: Elsevier Limited: 2018;7:241-282.
- Gkiozos I, Kopitopoulou A, Kalkanis A, et al. Sarcoidosis-like reactions induced by checkpoint inhibitors. J Thorac Oncol. 2018;13:1076-1082.
- Tetzlaff MT, Nelson KC, Diab A, et al. Granulomatous/sarcoid-like lesions associated with checkpoint inhibitors: a marker of therapy response in a subset of melanoma patients. J Immunother Cancer. 2018;6:14.
- Garrido MC, Gutiérrez C, Riveiro-Falkenbach E, et al. BRAF inhibitor-induced antitumoral granulomatous dermatitis eruption in advanced melanoma. Am J Dermatopathol. 2015;37:795-798.
- Park JJ, Hawryluk EB, Tahan SR, et al. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. JAMA Dermatol. 2014;150:307‐311.
- Ong ELH, Sinha R, Jmor S, et al. BRAF inhibitor-associated granulomatous dermatitis: a report of 3 cases. Am J of Dermatopathol. 2019;41:214-217.
- Wali GN, Stonard C, Espinosa O, et al. Persistent granulomatous cutaneous drug eruption to a BRAF inhibitor. J Am Acad Dermatol. 2017;76(suppl 1):AB195.
- Aj lafolla M, Ramsay J, Wismer J, et al. Cobimetinib- and vemurafenib-induced granulomatous dermatitis and erythema induratum: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19847358
- Jansen YJ, Janssens P, Hoorens A, et al. Granulomatous nephritis and dermatitis in a patient with BRAF V600E mutant metastatic melanoma treated with dabrafenib and trametinib. Melanoma Res. 2015;25:550‐554.
- Green JS, Norris DA, Wisell J. Novel cutaneous effects of combination chemotherapy with BRAF and MEK inhibitors: a report of two cases. Br J Dermatol. 2013;169:172-176.
- Chen L, His A, Kothari A, et al. Granulomatous dermatitis secondary to vemurafenib in a child with Langerhans cell histiocytosis. Pediatr Dermatol. 2018;35:E402-E403.
Practice Points
- Granulomatous dermatitis (GD) is a potential rare side effect of the use of BRAF and MEK inhibitors for the treatment of BRAF V600 mutation–positive cancers, including metastatic cholangiocarcinoma.
- Granulomatous dermatitis can resolve despite continuation of BRAF and MEK inhibitor therapies.
- Histologically, GD can appear similar to disease recurrence. It is imperative that clinicians and pathologists recognize the cutaneous manifestations of BRAF and MEK inhibitors.
Pruritic Papules in the Perianal and Gluteal Cleft Regions
The Diagnosis: Papular Acantholytic Dyskeratosis
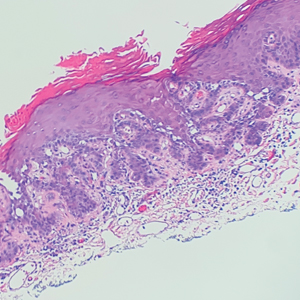
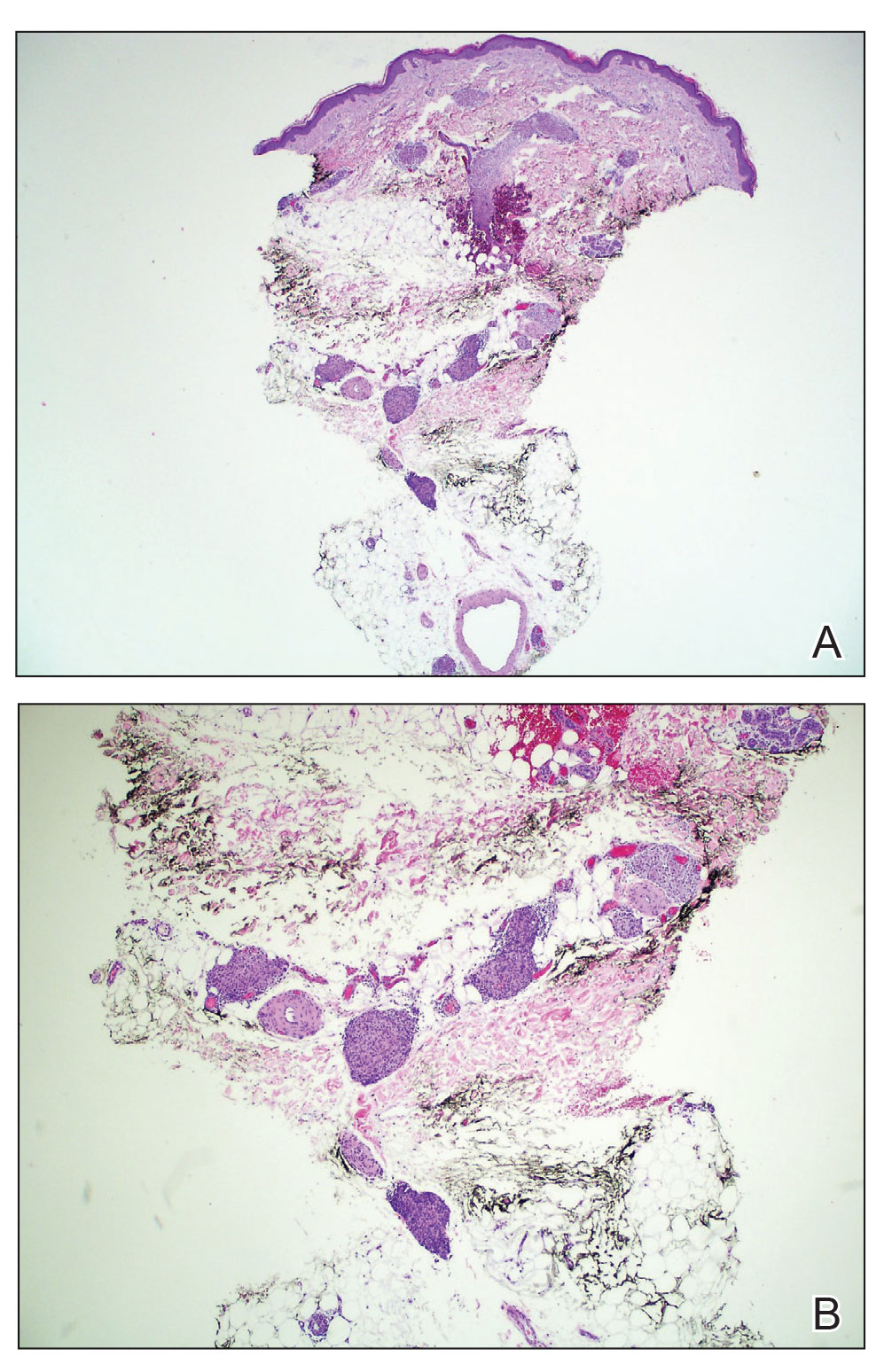
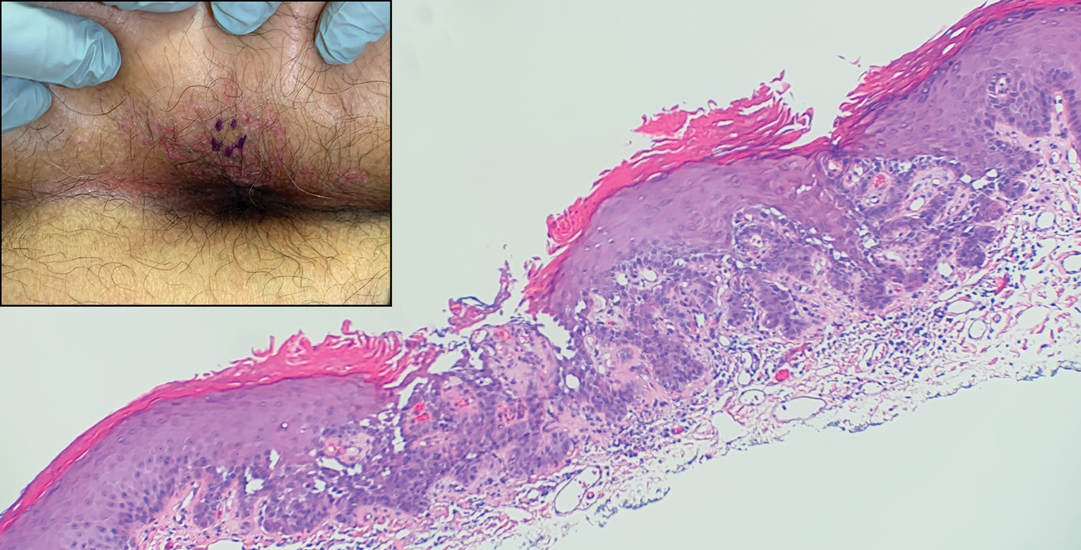
The shave biopsy revealed suprabasal clefts associated with acantholytic and dyskeratotic cells as well as overlying hyperkeratosis. Direct immunofluorescence (DIF) was negative. Based on the combined clinical and histological findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD), a rare disease that clinically presents as small whitishgreyish papules with the potential to coalesce into larger plaques.1,2 The condition predominantly manifests without symptoms, though pruritus and burning have been reported in affected sites. Most cases of PAD have been reported in older adults rather than in children or adolescents; it is more prevalent in women than in men. Lesions generally are localized to the penis, vulva, scrotum, inguinal folds, and perianal region.3 More specific terms have been used to describe this presentation such as papular acantholytic dyskeratosis of the anogenital region and papular acantholytic dyskeratosis of the genital-crural region. Histologic findings of PAD include epidermal acantholysis and dyskeratosis with hyperkeratosis and parakeratosis (quiz image).
The histologic differential diagnosis of PAD is broad due to its overlapping features with other diseases such as pemphigus vulgaris, Hailey-Hailey disease (HHD), Darier disease, and Grover disease. The acantholytic pathophysiology of these conditions involves dysfunction in cell adhesion markers. The correct diagnosis can be made by considering both the clinical location of involvement and histopathologic clues.
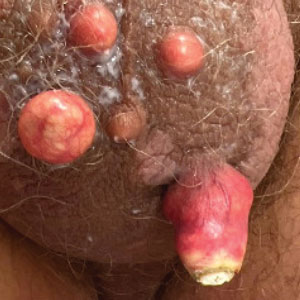
Pemphigus is a family of disorders involving mucocutaneous blistering of an autoimmune nature (Figure 1). Pemphigus vulgaris is the most prevalent variant of the pemphigus family, with symptomatically painful involvement of mucosal and cutaneous tissue. Autoantibodies to desmoglein 3 alone or both desmoglein 1 and 3 are present. Pemphigus vulgaris displays positive DIF findings with intercellular IgG and C3.
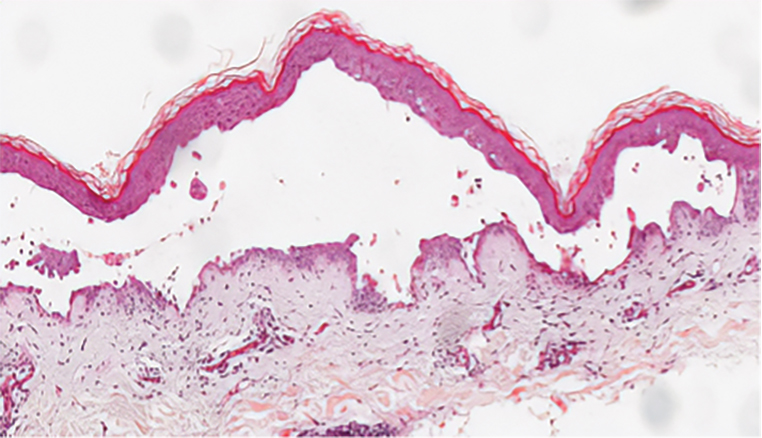
Hailey-Hailey disease (also known as benign familial pemphigus) is an autosomal-dominant disease that shares the acantholytic feature that is common in this class of diseases and caused by a defect in cell-cell adhesion as well as a loss of function in the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1. Blistering lesions typically appear in the neck, axillary, inguinal, or genital regions, and they can develop into crusted, exudate-filled lesions. No autoimmunity has been associated with this disease, unlike other diseases in the pemphigus family, and mutations in the ATP2C1 gene have been linked with dysregulation of cell-cell adhesion, particularly in cadherins and calcium-dependent cell adhesion processes. Histologically, HHD will show diffuse keratinocyte acantholysis with suprabasal clefting (Figure 2).4 Dyskeratosis is mild, if present at all, and dyskeratotic keratinocytes show a well-defined nucleus with cytoplasmic preservation. In contrast to HHD, PAD typically shows more dyskeratosis.
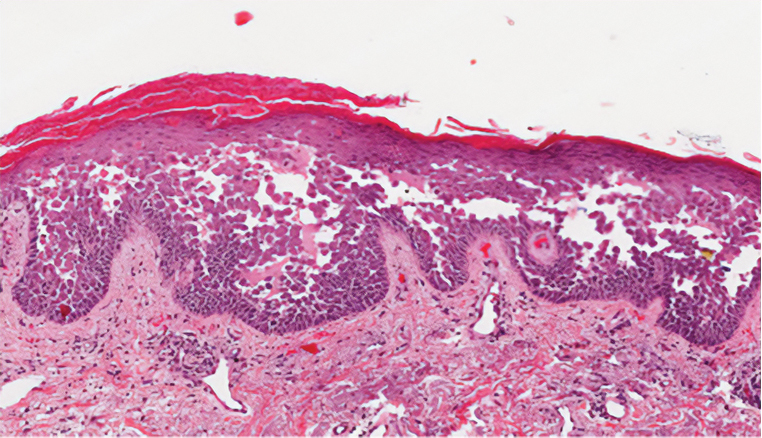
Darier disease (also known as keratosis follicularis) is an autosomal-dominant condition that normally presents with seborrheic eruptions in intertriginous areas, usually with onset during adolescence. Darier disease is caused by a loss-of-function mutation in the ATP2A2 gene found on chromosome 12q23-24.1 that encodes for the sarco(endo)plasmic reticulum calcium ATPase2 (SERCA2) enzymes involved in calcium-dependent transport of the endoplasmic reticulum within the cell. Due to calcium dysregulation, desmosomes are unable to carry out their function in cell-cell adhesion, resulting in keratinocyte acantholysis. Histopathology of Darier disease is identical to HHD but displays more dyskeratosis than HHD (Figure 3), possibly due to the endoplasmic reticulum calcium stores that are affected in Darier disease compared to the Golgi apparatus calcium stores that are implicated in HHD.5 The lowered endoplasmic reticulum calcium stores in Darier-White disease are associated with more pronounced dyskeratosis, which is seen histologically as corps ronds. Suprabasal hyperkeratosis also is found in Darier disease. The histopathologic findings of Darier disease and PAD can be identical, but the clinical presentations are distinct, with Darier disease typically manifesting as seborrheic eruptions appearing in adolescence and PAD presenting as small white papules in the anogenital or crural regions.
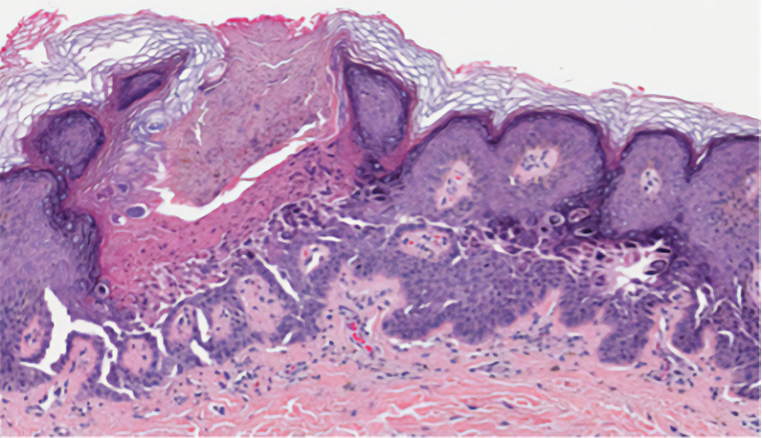
Grover disease (also referred to as transient acantholytic dermatosis) has an idiopathic pathophysiology. It clinically manifests with eruptions of erythematous, pruritic, truncal papules on the chest or back. Grover disease has a predilection for White men older than 50 years, and symptoms may be exacerbated in heat and diaphoretic conditions. Histologically, Grover disease may show acantholytic features seen in pemphigus vulgaris, HHD, and Darier disease; the pattern can only follow a specific disease or consist of a combination of all disease features (Figure 4). The acantholytic pattern of Grover disease was found to be similar to pemphigus vulgaris, Darier disease, pemphigus foliaceus, and HHD 47%, 18%, 9%, and 8% of the time, respectively. In 9% of cases, Grover disease will exhibit a mixed histopathology in which its acantholytic pattern will consist of a combination of features seen in the pemphigus family of diseases.6 Biopsy results showing mixed histologic patterns or a combination of different acantholytic features are suggestive of Grover disease over PAD. Moreover, the clinical distribution helps to differentiate Grover disease from PAD.
Because the histologic characteristics of these diseases overlap, certain nuances in clinical correlations and histology allow for distinction. In our patient, the diagnosis was most consistent with PAD based on the clinical manifestation of the disease and the biopsy results. Considering solely the clinical location of the lesions, Grover disease was a less likely diagnosis because our patient’s lesions were observed in the perianal region, not the truncal region as typically seen in Grover disease. Taking into account the DIF assay results in our patient, the pemphigus family of diseases also moved lower on the differential diagnosis. Finally, because the biopsy showed more dyskeratosis than would be present in HHD and also was inconsistent with the location and onset that would be expected to be seen in Darier disease, PAD was the most probable diagnosis. Interestingly, studies have shown mosaic mutations in ATP2A2 and ATP2C1 as possible causes of PAD, suggesting that this may be an allelic variant of Darier disease and HHD.7-9 No genetic testing was performed in our patient.
- Dowd ML, Ansell LH, Husain S, et al. Papular acantholytic dyskeratosis of the genitocrural area: a rare unilateral asymptomatic intertrigo. JAAD Case Rep. 2016;2:132-134. doi:10.1016/j.jdcr.2015.11.003
- Konstantinou MP, Krasagakis K. Benign familial pemphigus (Hailey Hailey disease). StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK585136/
- Montis-Palos MC, Acebo-Mariñas E, Catón-Santarén B, et al. Papular acantholytic dermatosis in the genito-crural region: a localized form of Darier disease or Hailey-Hailey disease? Actas Dermosifiliogr (Engl Ed). 2013;104:170-172. https://doi.org/10.1016/j.adengl.2012.02.008
- Verma SB. Papular acantholytic dyskeratosis localized to the perineal and perianal area in a young male. Indian J Dermatol. 2013;58:393-395.
- Schmieder SJ, Rosario-Collazo JA. Keratosis follicularis. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm .nih.gov/books/NBK519557/
- Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133:1490-1494.
- Knopp EA, Saraceni C, Moss J, et al. Somatic ATP2A2 mutation in a case of papular acantholytic dyskeratosis: mosaic Darier disease [published online August 12, 2015]. J Cutan Pathol. 2015;42:853-857. doi:10.1111/cup.12551
- Lipoff JB, Mudgil AV, Young S, et al. Acantholytic dermatosis of the crural folds with ATP2C1 mutation is a possible variant of Hailey-Hailey Disease. J Cutan Med Surg. 2009;13:151.
- Vodo D, Malchin N, Furman M, et al. Identification of a recurrent mutation in ATP2C1 demonstrates that papular acantholytic dyskeratosis and Hailey-Hailey disease are allelic disorders. Br J Dermatol. 2018;179:1001-1002.
The Diagnosis: Papular Acantholytic Dyskeratosis
The shave biopsy revealed suprabasal clefts associated with acantholytic and dyskeratotic cells as well as overlying hyperkeratosis. Direct immunofluorescence (DIF) was negative. Based on the combined clinical and histological findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD), a rare disease that clinically presents as small whitishgreyish papules with the potential to coalesce into larger plaques.1,2 The condition predominantly manifests without symptoms, though pruritus and burning have been reported in affected sites. Most cases of PAD have been reported in older adults rather than in children or adolescents; it is more prevalent in women than in men. Lesions generally are localized to the penis, vulva, scrotum, inguinal folds, and perianal region.3 More specific terms have been used to describe this presentation such as papular acantholytic dyskeratosis of the anogenital region and papular acantholytic dyskeratosis of the genital-crural region. Histologic findings of PAD include epidermal acantholysis and dyskeratosis with hyperkeratosis and parakeratosis (quiz image).
The histologic differential diagnosis of PAD is broad due to its overlapping features with other diseases such as pemphigus vulgaris, Hailey-Hailey disease (HHD), Darier disease, and Grover disease. The acantholytic pathophysiology of these conditions involves dysfunction in cell adhesion markers. The correct diagnosis can be made by considering both the clinical location of involvement and histopathologic clues.
Pemphigus is a family of disorders involving mucocutaneous blistering of an autoimmune nature (Figure 1). Pemphigus vulgaris is the most prevalent variant of the pemphigus family, with symptomatically painful involvement of mucosal and cutaneous tissue. Autoantibodies to desmoglein 3 alone or both desmoglein 1 and 3 are present. Pemphigus vulgaris displays positive DIF findings with intercellular IgG and C3.

Hailey-Hailey disease (also known as benign familial pemphigus) is an autosomal-dominant disease that shares the acantholytic feature that is common in this class of diseases and caused by a defect in cell-cell adhesion as well as a loss of function in the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1. Blistering lesions typically appear in the neck, axillary, inguinal, or genital regions, and they can develop into crusted, exudate-filled lesions. No autoimmunity has been associated with this disease, unlike other diseases in the pemphigus family, and mutations in the ATP2C1 gene have been linked with dysregulation of cell-cell adhesion, particularly in cadherins and calcium-dependent cell adhesion processes. Histologically, HHD will show diffuse keratinocyte acantholysis with suprabasal clefting (Figure 2).4 Dyskeratosis is mild, if present at all, and dyskeratotic keratinocytes show a well-defined nucleus with cytoplasmic preservation. In contrast to HHD, PAD typically shows more dyskeratosis.

Darier disease (also known as keratosis follicularis) is an autosomal-dominant condition that normally presents with seborrheic eruptions in intertriginous areas, usually with onset during adolescence. Darier disease is caused by a loss-of-function mutation in the ATP2A2 gene found on chromosome 12q23-24.1 that encodes for the sarco(endo)plasmic reticulum calcium ATPase2 (SERCA2) enzymes involved in calcium-dependent transport of the endoplasmic reticulum within the cell. Due to calcium dysregulation, desmosomes are unable to carry out their function in cell-cell adhesion, resulting in keratinocyte acantholysis. Histopathology of Darier disease is identical to HHD but displays more dyskeratosis than HHD (Figure 3), possibly due to the endoplasmic reticulum calcium stores that are affected in Darier disease compared to the Golgi apparatus calcium stores that are implicated in HHD.5 The lowered endoplasmic reticulum calcium stores in Darier-White disease are associated with more pronounced dyskeratosis, which is seen histologically as corps ronds. Suprabasal hyperkeratosis also is found in Darier disease. The histopathologic findings of Darier disease and PAD can be identical, but the clinical presentations are distinct, with Darier disease typically manifesting as seborrheic eruptions appearing in adolescence and PAD presenting as small white papules in the anogenital or crural regions.

Grover disease (also referred to as transient acantholytic dermatosis) has an idiopathic pathophysiology. It clinically manifests with eruptions of erythematous, pruritic, truncal papules on the chest or back. Grover disease has a predilection for White men older than 50 years, and symptoms may be exacerbated in heat and diaphoretic conditions. Histologically, Grover disease may show acantholytic features seen in pemphigus vulgaris, HHD, and Darier disease; the pattern can only follow a specific disease or consist of a combination of all disease features (Figure 4). The acantholytic pattern of Grover disease was found to be similar to pemphigus vulgaris, Darier disease, pemphigus foliaceus, and HHD 47%, 18%, 9%, and 8% of the time, respectively. In 9% of cases, Grover disease will exhibit a mixed histopathology in which its acantholytic pattern will consist of a combination of features seen in the pemphigus family of diseases.6 Biopsy results showing mixed histologic patterns or a combination of different acantholytic features are suggestive of Grover disease over PAD. Moreover, the clinical distribution helps to differentiate Grover disease from PAD.

Because the histologic characteristics of these diseases overlap, certain nuances in clinical correlations and histology allow for distinction. In our patient, the diagnosis was most consistent with PAD based on the clinical manifestation of the disease and the biopsy results. Considering solely the clinical location of the lesions, Grover disease was a less likely diagnosis because our patient’s lesions were observed in the perianal region, not the truncal region as typically seen in Grover disease. Taking into account the DIF assay results in our patient, the pemphigus family of diseases also moved lower on the differential diagnosis. Finally, because the biopsy showed more dyskeratosis than would be present in HHD and also was inconsistent with the location and onset that would be expected to be seen in Darier disease, PAD was the most probable diagnosis. Interestingly, studies have shown mosaic mutations in ATP2A2 and ATP2C1 as possible causes of PAD, suggesting that this may be an allelic variant of Darier disease and HHD.7-9 No genetic testing was performed in our patient.
The Diagnosis: Papular Acantholytic Dyskeratosis
The shave biopsy revealed suprabasal clefts associated with acantholytic and dyskeratotic cells as well as overlying hyperkeratosis. Direct immunofluorescence (DIF) was negative. Based on the combined clinical and histological findings, the patient was diagnosed with papular acantholytic dyskeratosis (PAD), a rare disease that clinically presents as small whitishgreyish papules with the potential to coalesce into larger plaques.1,2 The condition predominantly manifests without symptoms, though pruritus and burning have been reported in affected sites. Most cases of PAD have been reported in older adults rather than in children or adolescents; it is more prevalent in women than in men. Lesions generally are localized to the penis, vulva, scrotum, inguinal folds, and perianal region.3 More specific terms have been used to describe this presentation such as papular acantholytic dyskeratosis of the anogenital region and papular acantholytic dyskeratosis of the genital-crural region. Histologic findings of PAD include epidermal acantholysis and dyskeratosis with hyperkeratosis and parakeratosis (quiz image).
The histologic differential diagnosis of PAD is broad due to its overlapping features with other diseases such as pemphigus vulgaris, Hailey-Hailey disease (HHD), Darier disease, and Grover disease. The acantholytic pathophysiology of these conditions involves dysfunction in cell adhesion markers. The correct diagnosis can be made by considering both the clinical location of involvement and histopathologic clues.
Pemphigus is a family of disorders involving mucocutaneous blistering of an autoimmune nature (Figure 1). Pemphigus vulgaris is the most prevalent variant of the pemphigus family, with symptomatically painful involvement of mucosal and cutaneous tissue. Autoantibodies to desmoglein 3 alone or both desmoglein 1 and 3 are present. Pemphigus vulgaris displays positive DIF findings with intercellular IgG and C3.

Hailey-Hailey disease (also known as benign familial pemphigus) is an autosomal-dominant disease that shares the acantholytic feature that is common in this class of diseases and caused by a defect in cell-cell adhesion as well as a loss of function in the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1. Blistering lesions typically appear in the neck, axillary, inguinal, or genital regions, and they can develop into crusted, exudate-filled lesions. No autoimmunity has been associated with this disease, unlike other diseases in the pemphigus family, and mutations in the ATP2C1 gene have been linked with dysregulation of cell-cell adhesion, particularly in cadherins and calcium-dependent cell adhesion processes. Histologically, HHD will show diffuse keratinocyte acantholysis with suprabasal clefting (Figure 2).4 Dyskeratosis is mild, if present at all, and dyskeratotic keratinocytes show a well-defined nucleus with cytoplasmic preservation. In contrast to HHD, PAD typically shows more dyskeratosis.

Darier disease (also known as keratosis follicularis) is an autosomal-dominant condition that normally presents with seborrheic eruptions in intertriginous areas, usually with onset during adolescence. Darier disease is caused by a loss-of-function mutation in the ATP2A2 gene found on chromosome 12q23-24.1 that encodes for the sarco(endo)plasmic reticulum calcium ATPase2 (SERCA2) enzymes involved in calcium-dependent transport of the endoplasmic reticulum within the cell. Due to calcium dysregulation, desmosomes are unable to carry out their function in cell-cell adhesion, resulting in keratinocyte acantholysis. Histopathology of Darier disease is identical to HHD but displays more dyskeratosis than HHD (Figure 3), possibly due to the endoplasmic reticulum calcium stores that are affected in Darier disease compared to the Golgi apparatus calcium stores that are implicated in HHD.5 The lowered endoplasmic reticulum calcium stores in Darier-White disease are associated with more pronounced dyskeratosis, which is seen histologically as corps ronds. Suprabasal hyperkeratosis also is found in Darier disease. The histopathologic findings of Darier disease and PAD can be identical, but the clinical presentations are distinct, with Darier disease typically manifesting as seborrheic eruptions appearing in adolescence and PAD presenting as small white papules in the anogenital or crural regions.

Grover disease (also referred to as transient acantholytic dermatosis) has an idiopathic pathophysiology. It clinically manifests with eruptions of erythematous, pruritic, truncal papules on the chest or back. Grover disease has a predilection for White men older than 50 years, and symptoms may be exacerbated in heat and diaphoretic conditions. Histologically, Grover disease may show acantholytic features seen in pemphigus vulgaris, HHD, and Darier disease; the pattern can only follow a specific disease or consist of a combination of all disease features (Figure 4). The acantholytic pattern of Grover disease was found to be similar to pemphigus vulgaris, Darier disease, pemphigus foliaceus, and HHD 47%, 18%, 9%, and 8% of the time, respectively. In 9% of cases, Grover disease will exhibit a mixed histopathology in which its acantholytic pattern will consist of a combination of features seen in the pemphigus family of diseases.6 Biopsy results showing mixed histologic patterns or a combination of different acantholytic features are suggestive of Grover disease over PAD. Moreover, the clinical distribution helps to differentiate Grover disease from PAD.

Because the histologic characteristics of these diseases overlap, certain nuances in clinical correlations and histology allow for distinction. In our patient, the diagnosis was most consistent with PAD based on the clinical manifestation of the disease and the biopsy results. Considering solely the clinical location of the lesions, Grover disease was a less likely diagnosis because our patient’s lesions were observed in the perianal region, not the truncal region as typically seen in Grover disease. Taking into account the DIF assay results in our patient, the pemphigus family of diseases also moved lower on the differential diagnosis. Finally, because the biopsy showed more dyskeratosis than would be present in HHD and also was inconsistent with the location and onset that would be expected to be seen in Darier disease, PAD was the most probable diagnosis. Interestingly, studies have shown mosaic mutations in ATP2A2 and ATP2C1 as possible causes of PAD, suggesting that this may be an allelic variant of Darier disease and HHD.7-9 No genetic testing was performed in our patient.
- Dowd ML, Ansell LH, Husain S, et al. Papular acantholytic dyskeratosis of the genitocrural area: a rare unilateral asymptomatic intertrigo. JAAD Case Rep. 2016;2:132-134. doi:10.1016/j.jdcr.2015.11.003
- Konstantinou MP, Krasagakis K. Benign familial pemphigus (Hailey Hailey disease). StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK585136/
- Montis-Palos MC, Acebo-Mariñas E, Catón-Santarén B, et al. Papular acantholytic dermatosis in the genito-crural region: a localized form of Darier disease or Hailey-Hailey disease? Actas Dermosifiliogr (Engl Ed). 2013;104:170-172. https://doi.org/10.1016/j.adengl.2012.02.008
- Verma SB. Papular acantholytic dyskeratosis localized to the perineal and perianal area in a young male. Indian J Dermatol. 2013;58:393-395.
- Schmieder SJ, Rosario-Collazo JA. Keratosis follicularis. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm .nih.gov/books/NBK519557/
- Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133:1490-1494.
- Knopp EA, Saraceni C, Moss J, et al. Somatic ATP2A2 mutation in a case of papular acantholytic dyskeratosis: mosaic Darier disease [published online August 12, 2015]. J Cutan Pathol. 2015;42:853-857. doi:10.1111/cup.12551
- Lipoff JB, Mudgil AV, Young S, et al. Acantholytic dermatosis of the crural folds with ATP2C1 mutation is a possible variant of Hailey-Hailey Disease. J Cutan Med Surg. 2009;13:151.
- Vodo D, Malchin N, Furman M, et al. Identification of a recurrent mutation in ATP2C1 demonstrates that papular acantholytic dyskeratosis and Hailey-Hailey disease are allelic disorders. Br J Dermatol. 2018;179:1001-1002.
- Dowd ML, Ansell LH, Husain S, et al. Papular acantholytic dyskeratosis of the genitocrural area: a rare unilateral asymptomatic intertrigo. JAAD Case Rep. 2016;2:132-134. doi:10.1016/j.jdcr.2015.11.003
- Konstantinou MP, Krasagakis K. Benign familial pemphigus (Hailey Hailey disease). StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK585136/
- Montis-Palos MC, Acebo-Mariñas E, Catón-Santarén B, et al. Papular acantholytic dermatosis in the genito-crural region: a localized form of Darier disease or Hailey-Hailey disease? Actas Dermosifiliogr (Engl Ed). 2013;104:170-172. https://doi.org/10.1016/j.adengl.2012.02.008
- Verma SB. Papular acantholytic dyskeratosis localized to the perineal and perianal area in a young male. Indian J Dermatol. 2013;58:393-395.
- Schmieder SJ, Rosario-Collazo JA. Keratosis follicularis. StatPearls [Internet]. StatPearls Publishing; 2023. https://www.ncbi.nlm .nih.gov/books/NBK519557/
- Weaver J, Bergfeld WF. Grover disease (transient acantholytic dermatosis). Arch Pathol Lab Med. 2009;133:1490-1494.
- Knopp EA, Saraceni C, Moss J, et al. Somatic ATP2A2 mutation in a case of papular acantholytic dyskeratosis: mosaic Darier disease [published online August 12, 2015]. J Cutan Pathol. 2015;42:853-857. doi:10.1111/cup.12551
- Lipoff JB, Mudgil AV, Young S, et al. Acantholytic dermatosis of the crural folds with ATP2C1 mutation is a possible variant of Hailey-Hailey Disease. J Cutan Med Surg. 2009;13:151.
- Vodo D, Malchin N, Furman M, et al. Identification of a recurrent mutation in ATP2C1 demonstrates that papular acantholytic dyskeratosis and Hailey-Hailey disease are allelic disorders. Br J Dermatol. 2018;179:1001-1002.
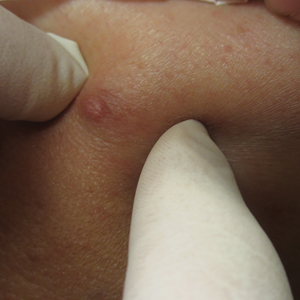
A 66-year-old man presented to the dermatology clinic with pruritus of the gluteal cleft and perianal region of several months’ duration. He had been prescribed permethrin by an outside physician, as well as oral acyclovir, triamcinolone-nystatin combination ointment, and topical zinc oxide prescribed by dermatology, without improvement. Physical examination showed several papules and erosions (<1 mm) in the perianal and gluteal cleft regions (inset). Hyperpigmented macules also were noted in the inguinal folds. A shave biopsy of a lesion from the perianal region was performed.
Disseminated Papules and Nodules on the Skin and Oral Mucosa in an Infant
The Diagnosis: Congenital Cutaneous Langerhans Cell Histiocytosis
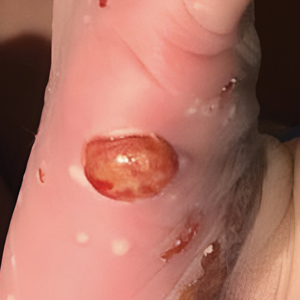
Although the infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies, serologies for the rest of the TORCH (toxoplasmosis, other agents [syphilis, hepatitis B virus], rubella, cytomegalovirus) group of infections, as well as other bacterial, fungal, and viral infections, were negative. A skin biopsy from the right fifth toe showed a dense infiltrate of CD1a+ histiocytic cells with folded or kidney-shaped nuclei mixed with eosinophils, which was consistent with Langerhans cell histiocytosis (LCH) (Figure 1). Skin lesions were treated with hydrocortisone cream 2.5% and progressively faded over a few weeks.

Langerhans cell histiocytosis is a rare disorder with a variable clinical presentation depending on the sites affected and the extent of involvement. It can involve multiple organ systems, most commonly the skeletal system and the skin. Organ involvement is characterized by histiocyte infiltration. Acute disseminated multisystem disease most commonly is seen in children younger than 3 years.1
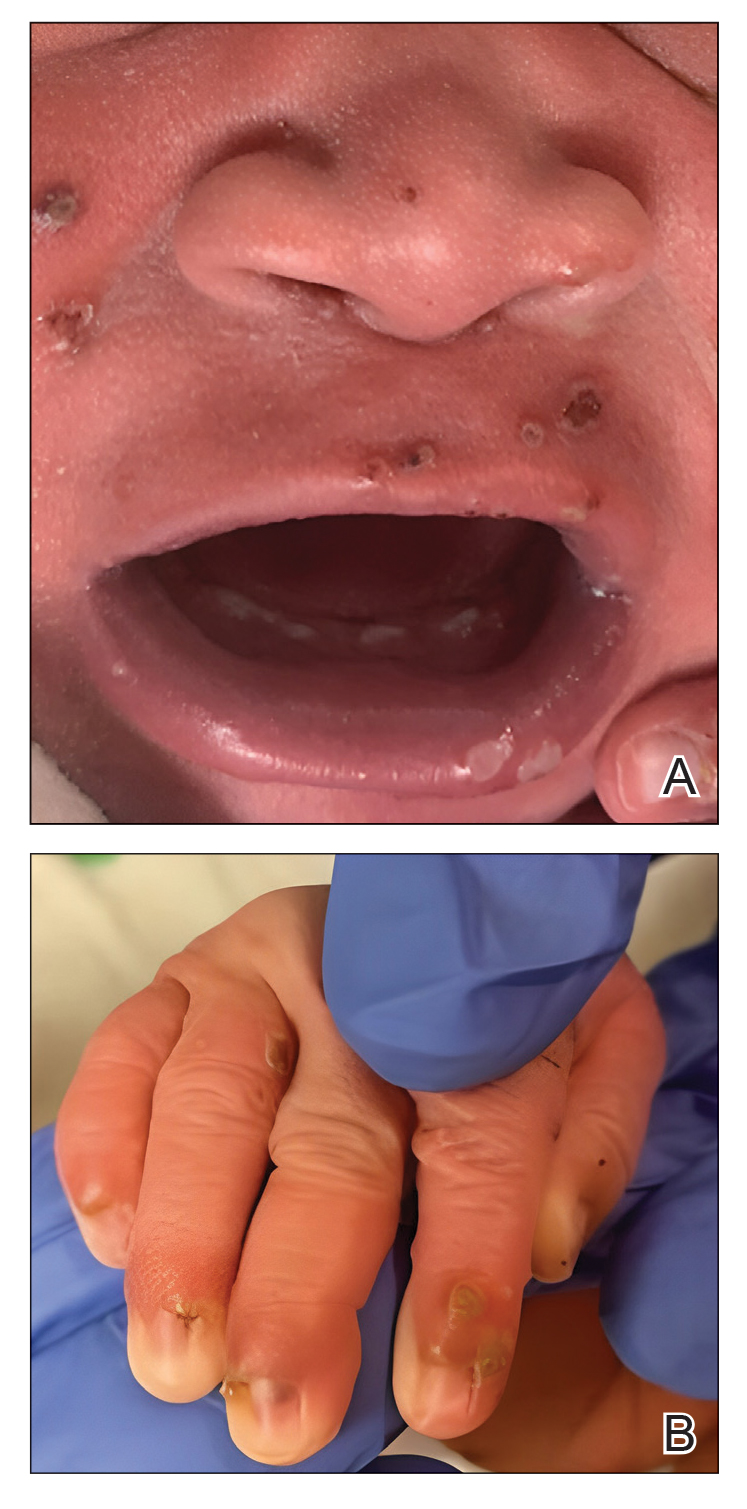
Congenital cutaneous LCH presents with variable skin lesions ranging from papules to vesicles, pustules, and ulcers, with onset at birth or in the neonatal period. Various morphologic traits of skin lesions have been described; the most common presentation is multiple red to yellow-brown, crusted papules with accompanying hemorrhage or erosion.1 Other cases have described an eczematous, seborrheic, diffuse eruption or erosive intertrigo. One case of a child with a solitary necrotic nodule on the scalp has been reported.2
Our patient presented with disseminated, nonblanching, purple to dark red papules and nodules of the skin and oral mucosa, as well as nail dystrophy (Figure 2). However, LCH in a neonate can mimic other causes of congenital papulonodular eruptions. Red-brown papules and nodules with or without crusting in a newborn can be mistaken for erythema toxicum neonatorum, transient neonatal pustular melanosis, congenital leukemia cutis, neonatal erythropoiesis, disseminated neonatal hemangiomatosis, infantile acropustulosis, or congenital TORCH infections such as rubella or syphilis. When LCH presents as vesicles or eroded papules or nodules in a newborn, the differential diagnosis includes incontinentia pigmenti and hereditary epidermolysis bullosa.
Langerhans cell histiocytosis may even present with a classic blueberry muffin rash that can lead clinicians to consider cutaneous metastasis from various hematologic malignancies or the more common TORCH infections. Several diagnostic tests can be performed to clarify the diagnosis, including bacterial and viral cultures and stains, serology, immunohistochemistry, flow cytometry, bone marrow aspiration, or skin biopsy.3 Langerhans cell histiocytosis is diagnosed with a combination of histology, immunohistochemistry, and clinical presentation; however, a skin biopsy is crucial. Tissue should be taken from the most easily accessible yet representative lesion. The characteristic appearance of LCH lesions is described as a dense infiltrate of histiocytic cells mixed with numerous eosinophils in the dermis.1 Histiocytes usually have folded nuclei and eosinophilic cytoplasm or kidney-shaped nuclei with prominent nucleoli. Positive CD1a and/or CD207 (Langerin) staining of the cells is required for definitive diagnosis.4 After diagnosis, it is important to obtain baseline laboratory and radiographic studies to determine the extent of systemic involvement.
Treatment of congenital LCH is tailored to the extent of organ involvement. The dermatologic manifestations resolve without medications in many cases. However, true self-resolving LCH can only be diagnosed retrospectively after a full evaluation for other sites of disease. Disseminated disease can be life-threatening and requires more active management. In cases of skin-limited disease, therapies include topical steroids, nitrogen mustard, or imiquimod; surgical resection of isolated lesions; phototherapy; or systemic therapies such as methotrexate, 6-mercaptopurine, vinblastine/vincristine, cladribine, and/or cytarabine. Symptomatic patients initially are treated with methotrexate and 6-mercaptopurine.5 Asymptomatic infants with skin-limited involvement can be managed with topical treatments.
Our patient had skin-limited disease. Abdominal ultrasonography, skeletal survey, and magnetic resonance imaging of the brain revealed no abnormalities. The patient’s family was advised to monitor him for reoccurrence of the skin lesions and to continue close follow-up with hematology and dermatology. Although congenital LCH often is self-resolving, extensive skin involvement increases the risk for internal organ involvement for several years.6 These patients require long-term follow-up for potential musculoskeletal, ophthalmologic, endocrine, hepatic, and/or pulmonary disease.
- Pan Y, Zeng X, Ge J, et al. Congenital self-healing Langerhans cell histiocytosis: clinical and pathological characteristics. Int J Clin Exp Pathol. 2019;12:2275-2278.
- Morren MA, Vanden Broecke K, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492. doi:10.1002/pbc.25834
- Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: diagnosis, differential diagnosis, treatment, sequelae, and standardized follow-up. J Am Acad Dermatol. 2018;78:1047-1056. doi:10.1016/j.jaad.2017.05.060
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
- Allen CE, Ladisch S, McClain KL. How I treat Langerhans cell histiocytosis. Blood. 2015;126:26-35. doi:10.1182/blood-2014-12-569301
- Jezierska M, Stefanowicz J, Romanowicz G, et al. Langerhans cell histiocytosis in children—a disease with many faces. recent advances in pathogenesis, diagnostic examinations and treatment. Postepy Dermatol Alergol. 2018;35:6-17. doi:10.5114/pdia.2017.67095
The Diagnosis: Congenital Cutaneous Langerhans Cell Histiocytosis
Although the infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies, serologies for the rest of the TORCH (toxoplasmosis, other agents [syphilis, hepatitis B virus], rubella, cytomegalovirus) group of infections, as well as other bacterial, fungal, and viral infections, were negative. A skin biopsy from the right fifth toe showed a dense infiltrate of CD1a+ histiocytic cells with folded or kidney-shaped nuclei mixed with eosinophils, which was consistent with Langerhans cell histiocytosis (LCH) (Figure 1). Skin lesions were treated with hydrocortisone cream 2.5% and progressively faded over a few weeks.

Langerhans cell histiocytosis is a rare disorder with a variable clinical presentation depending on the sites affected and the extent of involvement. It can involve multiple organ systems, most commonly the skeletal system and the skin. Organ involvement is characterized by histiocyte infiltration. Acute disseminated multisystem disease most commonly is seen in children younger than 3 years.1
Congenital cutaneous LCH presents with variable skin lesions ranging from papules to vesicles, pustules, and ulcers, with onset at birth or in the neonatal period. Various morphologic traits of skin lesions have been described; the most common presentation is multiple red to yellow-brown, crusted papules with accompanying hemorrhage or erosion.1 Other cases have described an eczematous, seborrheic, diffuse eruption or erosive intertrigo. One case of a child with a solitary necrotic nodule on the scalp has been reported.2
Our patient presented with disseminated, nonblanching, purple to dark red papules and nodules of the skin and oral mucosa, as well as nail dystrophy (Figure 2). However, LCH in a neonate can mimic other causes of congenital papulonodular eruptions. Red-brown papules and nodules with or without crusting in a newborn can be mistaken for erythema toxicum neonatorum, transient neonatal pustular melanosis, congenital leukemia cutis, neonatal erythropoiesis, disseminated neonatal hemangiomatosis, infantile acropustulosis, or congenital TORCH infections such as rubella or syphilis. When LCH presents as vesicles or eroded papules or nodules in a newborn, the differential diagnosis includes incontinentia pigmenti and hereditary epidermolysis bullosa.

Langerhans cell histiocytosis may even present with a classic blueberry muffin rash that can lead clinicians to consider cutaneous metastasis from various hematologic malignancies or the more common TORCH infections. Several diagnostic tests can be performed to clarify the diagnosis, including bacterial and viral cultures and stains, serology, immunohistochemistry, flow cytometry, bone marrow aspiration, or skin biopsy.3 Langerhans cell histiocytosis is diagnosed with a combination of histology, immunohistochemistry, and clinical presentation; however, a skin biopsy is crucial. Tissue should be taken from the most easily accessible yet representative lesion. The characteristic appearance of LCH lesions is described as a dense infiltrate of histiocytic cells mixed with numerous eosinophils in the dermis.1 Histiocytes usually have folded nuclei and eosinophilic cytoplasm or kidney-shaped nuclei with prominent nucleoli. Positive CD1a and/or CD207 (Langerin) staining of the cells is required for definitive diagnosis.4 After diagnosis, it is important to obtain baseline laboratory and radiographic studies to determine the extent of systemic involvement.
Treatment of congenital LCH is tailored to the extent of organ involvement. The dermatologic manifestations resolve without medications in many cases. However, true self-resolving LCH can only be diagnosed retrospectively after a full evaluation for other sites of disease. Disseminated disease can be life-threatening and requires more active management. In cases of skin-limited disease, therapies include topical steroids, nitrogen mustard, or imiquimod; surgical resection of isolated lesions; phototherapy; or systemic therapies such as methotrexate, 6-mercaptopurine, vinblastine/vincristine, cladribine, and/or cytarabine. Symptomatic patients initially are treated with methotrexate and 6-mercaptopurine.5 Asymptomatic infants with skin-limited involvement can be managed with topical treatments.
Our patient had skin-limited disease. Abdominal ultrasonography, skeletal survey, and magnetic resonance imaging of the brain revealed no abnormalities. The patient’s family was advised to monitor him for reoccurrence of the skin lesions and to continue close follow-up with hematology and dermatology. Although congenital LCH often is self-resolving, extensive skin involvement increases the risk for internal organ involvement for several years.6 These patients require long-term follow-up for potential musculoskeletal, ophthalmologic, endocrine, hepatic, and/or pulmonary disease.
The Diagnosis: Congenital Cutaneous Langerhans Cell Histiocytosis
Although the infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies, serologies for the rest of the TORCH (toxoplasmosis, other agents [syphilis, hepatitis B virus], rubella, cytomegalovirus) group of infections, as well as other bacterial, fungal, and viral infections, were negative. A skin biopsy from the right fifth toe showed a dense infiltrate of CD1a+ histiocytic cells with folded or kidney-shaped nuclei mixed with eosinophils, which was consistent with Langerhans cell histiocytosis (LCH) (Figure 1). Skin lesions were treated with hydrocortisone cream 2.5% and progressively faded over a few weeks.

Langerhans cell histiocytosis is a rare disorder with a variable clinical presentation depending on the sites affected and the extent of involvement. It can involve multiple organ systems, most commonly the skeletal system and the skin. Organ involvement is characterized by histiocyte infiltration. Acute disseminated multisystem disease most commonly is seen in children younger than 3 years.1
Congenital cutaneous LCH presents with variable skin lesions ranging from papules to vesicles, pustules, and ulcers, with onset at birth or in the neonatal period. Various morphologic traits of skin lesions have been described; the most common presentation is multiple red to yellow-brown, crusted papules with accompanying hemorrhage or erosion.1 Other cases have described an eczematous, seborrheic, diffuse eruption or erosive intertrigo. One case of a child with a solitary necrotic nodule on the scalp has been reported.2
Our patient presented with disseminated, nonblanching, purple to dark red papules and nodules of the skin and oral mucosa, as well as nail dystrophy (Figure 2). However, LCH in a neonate can mimic other causes of congenital papulonodular eruptions. Red-brown papules and nodules with or without crusting in a newborn can be mistaken for erythema toxicum neonatorum, transient neonatal pustular melanosis, congenital leukemia cutis, neonatal erythropoiesis, disseminated neonatal hemangiomatosis, infantile acropustulosis, or congenital TORCH infections such as rubella or syphilis. When LCH presents as vesicles or eroded papules or nodules in a newborn, the differential diagnosis includes incontinentia pigmenti and hereditary epidermolysis bullosa.

Langerhans cell histiocytosis may even present with a classic blueberry muffin rash that can lead clinicians to consider cutaneous metastasis from various hematologic malignancies or the more common TORCH infections. Several diagnostic tests can be performed to clarify the diagnosis, including bacterial and viral cultures and stains, serology, immunohistochemistry, flow cytometry, bone marrow aspiration, or skin biopsy.3 Langerhans cell histiocytosis is diagnosed with a combination of histology, immunohistochemistry, and clinical presentation; however, a skin biopsy is crucial. Tissue should be taken from the most easily accessible yet representative lesion. The characteristic appearance of LCH lesions is described as a dense infiltrate of histiocytic cells mixed with numerous eosinophils in the dermis.1 Histiocytes usually have folded nuclei and eosinophilic cytoplasm or kidney-shaped nuclei with prominent nucleoli. Positive CD1a and/or CD207 (Langerin) staining of the cells is required for definitive diagnosis.4 After diagnosis, it is important to obtain baseline laboratory and radiographic studies to determine the extent of systemic involvement.
Treatment of congenital LCH is tailored to the extent of organ involvement. The dermatologic manifestations resolve without medications in many cases. However, true self-resolving LCH can only be diagnosed retrospectively after a full evaluation for other sites of disease. Disseminated disease can be life-threatening and requires more active management. In cases of skin-limited disease, therapies include topical steroids, nitrogen mustard, or imiquimod; surgical resection of isolated lesions; phototherapy; or systemic therapies such as methotrexate, 6-mercaptopurine, vinblastine/vincristine, cladribine, and/or cytarabine. Symptomatic patients initially are treated with methotrexate and 6-mercaptopurine.5 Asymptomatic infants with skin-limited involvement can be managed with topical treatments.
Our patient had skin-limited disease. Abdominal ultrasonography, skeletal survey, and magnetic resonance imaging of the brain revealed no abnormalities. The patient’s family was advised to monitor him for reoccurrence of the skin lesions and to continue close follow-up with hematology and dermatology. Although congenital LCH often is self-resolving, extensive skin involvement increases the risk for internal organ involvement for several years.6 These patients require long-term follow-up for potential musculoskeletal, ophthalmologic, endocrine, hepatic, and/or pulmonary disease.
- Pan Y, Zeng X, Ge J, et al. Congenital self-healing Langerhans cell histiocytosis: clinical and pathological characteristics. Int J Clin Exp Pathol. 2019;12:2275-2278.
- Morren MA, Vanden Broecke K, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492. doi:10.1002/pbc.25834
- Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: diagnosis, differential diagnosis, treatment, sequelae, and standardized follow-up. J Am Acad Dermatol. 2018;78:1047-1056. doi:10.1016/j.jaad.2017.05.060
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
- Allen CE, Ladisch S, McClain KL. How I treat Langerhans cell histiocytosis. Blood. 2015;126:26-35. doi:10.1182/blood-2014-12-569301
- Jezierska M, Stefanowicz J, Romanowicz G, et al. Langerhans cell histiocytosis in children—a disease with many faces. recent advances in pathogenesis, diagnostic examinations and treatment. Postepy Dermatol Alergol. 2018;35:6-17. doi:10.5114/pdia.2017.67095
- Pan Y, Zeng X, Ge J, et al. Congenital self-healing Langerhans cell histiocytosis: clinical and pathological characteristics. Int J Clin Exp Pathol. 2019;12:2275-2278.
- Morren MA, Vanden Broecke K, Vangeebergen L, et al. Diverse cutaneous presentations of Langerhans cell histiocytosis in children: a retrospective cohort study. Pediatr Blood Cancer. 2016;63:486-492. doi:10.1002/pbc.25834
- Krooks J, Minkov M, Weatherall AG. Langerhans cell histiocytosis in children: diagnosis, differential diagnosis, treatment, sequelae, and standardized follow-up. J Am Acad Dermatol. 2018;78:1047-1056. doi:10.1016/j.jaad.2017.05.060
- Haupt R, Minkov M, Astigarraga I, et al. Langerhans cell histiocytosis (LCH): guidelines for diagnosis, clinical work-up, and treatment for patients till the age of 18 years. Pediatr Blood Cancer. 2013;60:175-184. doi:10.1002/pbc.24367
- Allen CE, Ladisch S, McClain KL. How I treat Langerhans cell histiocytosis. Blood. 2015;126:26-35. doi:10.1182/blood-2014-12-569301
- Jezierska M, Stefanowicz J, Romanowicz G, et al. Langerhans cell histiocytosis in children—a disease with many faces. recent advances in pathogenesis, diagnostic examinations and treatment. Postepy Dermatol Alergol. 2018;35:6-17. doi:10.5114/pdia.2017.67095
A 38-week-old infant boy presented at birth with disseminated, nonblanching, purple to dark red papules and nodules on the skin and oral mucosa. He was born spontaneously after an uncomplicated pregnancy. The mother experienced an episode of oral herpes simplex virus during pregnancy. The infant was otherwise healthy. Laboratory tests including a complete blood cell count and routine serum biochemical analyses were within reference range; however, an infectious workup was positive for herpes simplex virus type 1 and cytomegalovirus antibodies. Ophthalmologic and auditory screenings were normal.
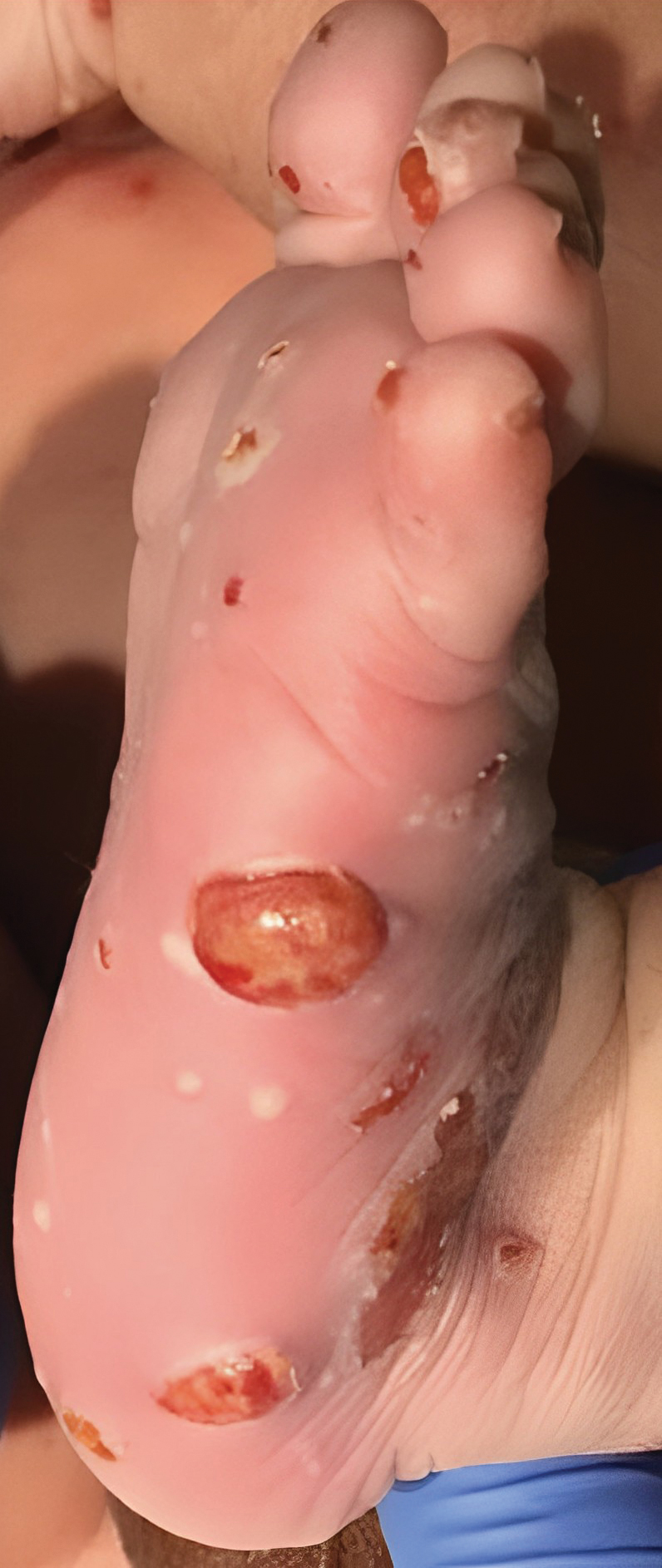
Cystic Presentation of High-Grade Ductal Carcinoma In Situ in an Inframammary Accessory Nipple
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.
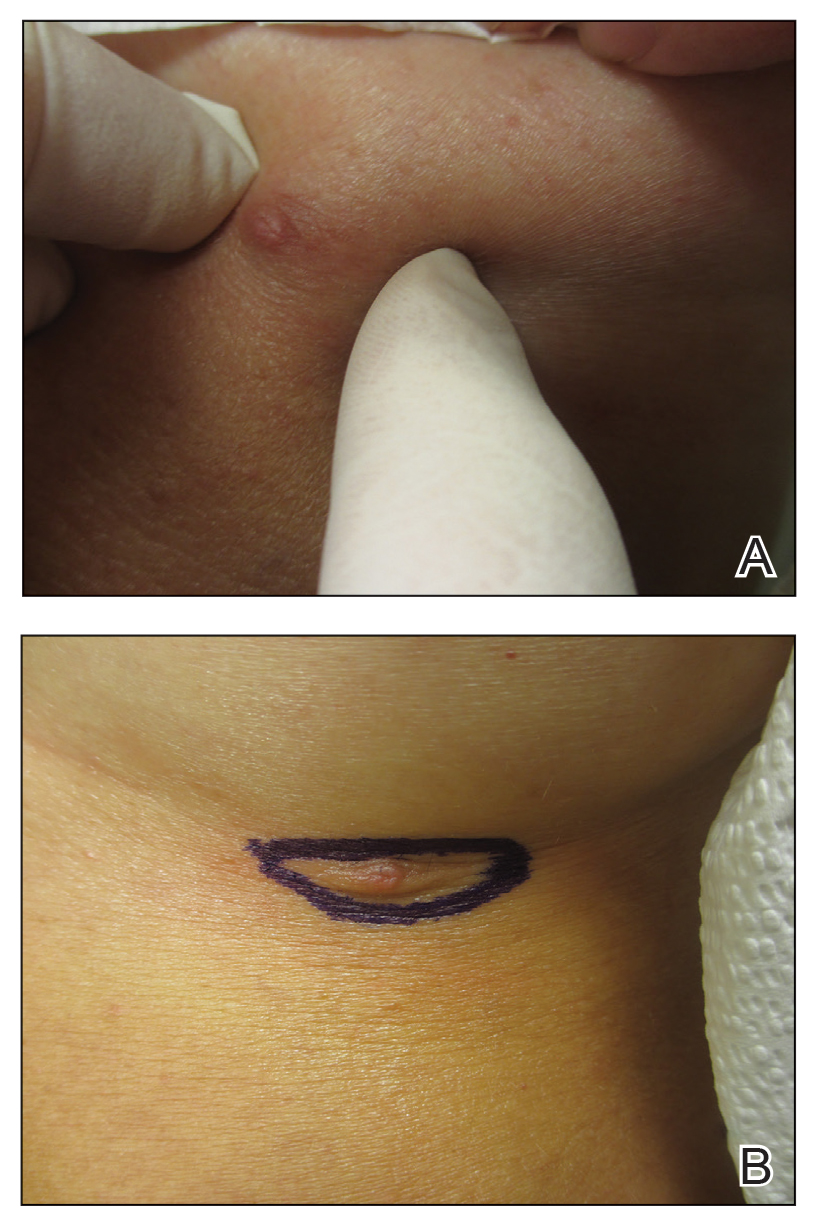
A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).
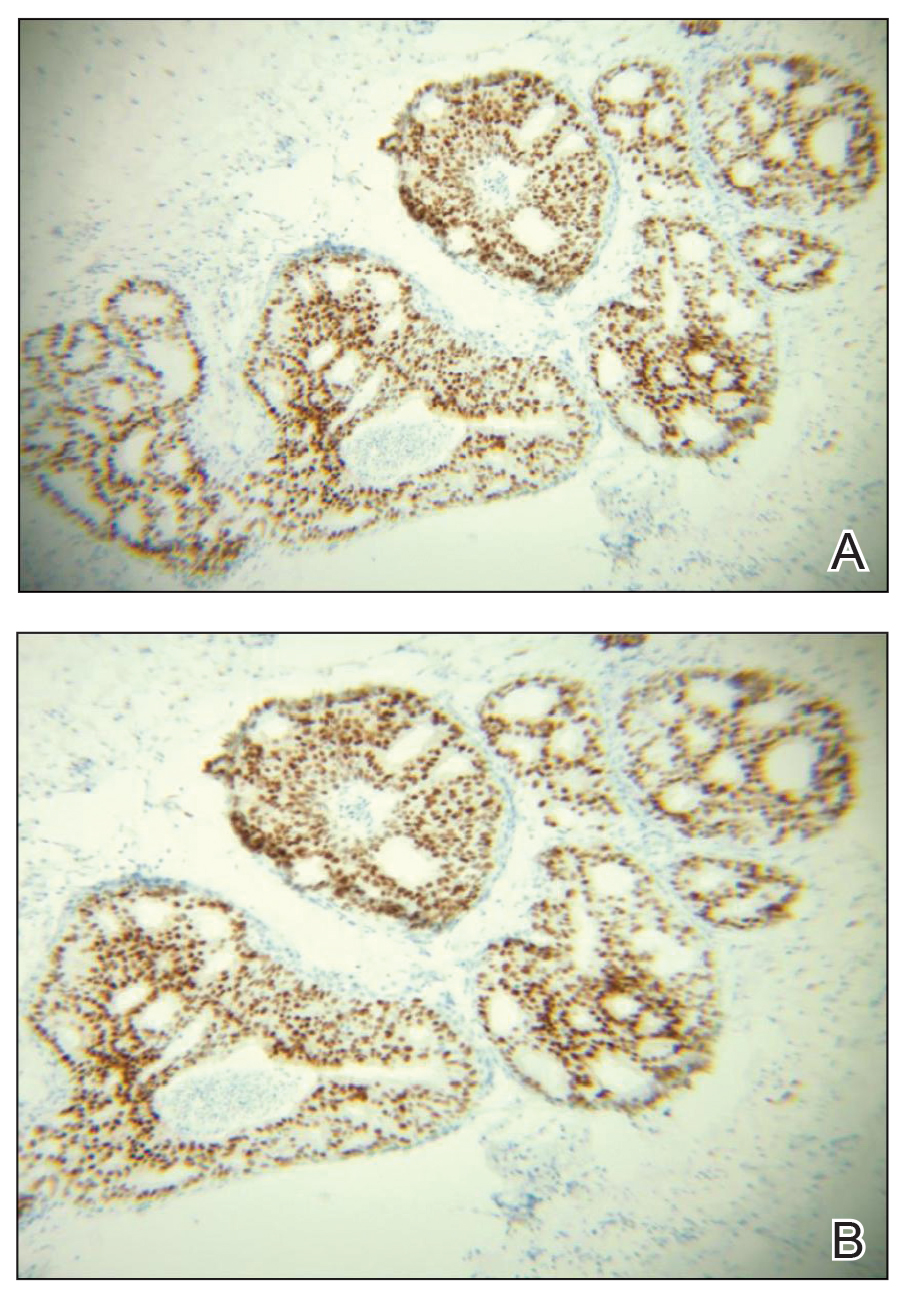
Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11
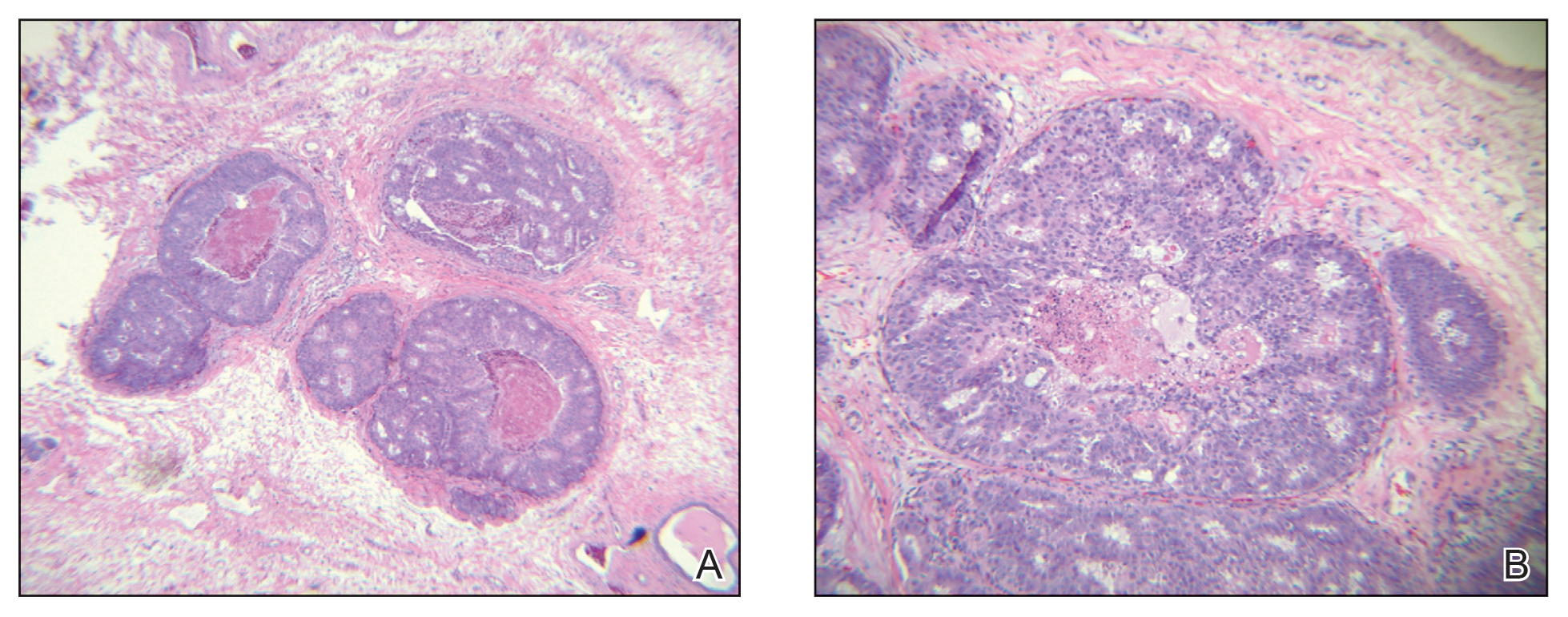
There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.

A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).

Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11

There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
To the Editor:
The term ectopic breast tissue serves as an umbrella term that encompasses breast tissue positioned in anatomically incorrect locations, including the subtypes of supernumerary and aberrant breasts.1 However, the more frequently used term is accessory breast tissue (ABT).1 Supernumerary breasts have diverse variations of a nipple, areola, and/or ductal tissue and can span in size from a small mole to a fully functioning breast. This breast type maintains structured ductal systems connected to the overlying skin and experiences regular changes during the reproductive cycle. In contrast, an aberrant breast is isolated breast tissue that does not contain organized ductal systems.1 Accessory breast tissue is prevalent in up to 6.0% of the world population, with Japanese individuals being the most affected and White individuals being the least affected.1
Accessory breasts typically are located along the milk line—the embryologic precursor to mammary glands and nipples, which extend from the axillae to the groin and regress from the caudal end spanning to the groin.2 For this reason, incomplete regression of the mammary ridge results in ABT, most commonly in the axillary region.3 Accessory breast tissue usually is benign and is considered an anatomical variant; however, because the histomorphology is similar to mammary gland tissue, accessory breasts have the same proliferative potential as anatomically correct breasts and therefore can form fibroadenomas, cysts, abscesses, mastitis, or breast cancer.4 Accessory breast carcinomas comprise 0.3% to 0.6% of all breast malignancies.5 Certain genodermatoses (ie, Cowden syndrome) also may predispose patients to benign or malignant pathology in ABT.6 We present a rare case of accessory breast cancer in the inframammary region masquerading as a cyst. These findings were further supported by ultrasonography and mammography.

A 45-year-old White woman presented to our clinic for removal of a dermal mass underlying a supernumerary nipple at the left inframammary fold. Her medical history was noncontributory and was only remarkable for uterine fibroids. She developed pain and swelling in the left breast 1 year prior, which prompted her to seek medical attention from her primary care physician. Diagnostic mammography was negative for any concerning malignant nodules, and subsequent BRCA genetic testing also was negative. Six months after the diagnostic mammography, she continued to experience pain and swelling in the left breast and was then referred for diagnostic ultrasonography; 2 masses in the left breast suspected as infected cysts with rupture were identified (Figure 1). She was then referred to our dermatology clinic for evaluation and surgical extirpation of the suspected cyst underlying the accessory breast. The area subsequently was excised under local anesthesia, and a second similar but smaller mass also was identified adjacent to the initial growth. Dermatopathologic examination revealed an estrogen receptor– (Figure 2A) and progesterone receptor–positive (Figure 2B), ERBB2 (HER2/neu)–negative, nuclear grade III ductal carcinoma in situ (Figure 3).

Various ABT classification methods have been proposed with Brightmore7 categorizing polymastia into 8 subtypes: (1) complete breast; (2) glandular tissue and nipple; (3) glandular tissue and areola; (4) glandular tissue only; (5) nipple, areola, and fat; (6) nipple only; (7) areola only; and (8) patch of hair only. De Cholnokey8 focused on axillary polymastia, dividing it into 4 classes: (1) axillary tumor in milk line without nipple or areola; (2) axillary tumor with areola with or without pigmentation; (3) nipple or areola without underlying breast tissue; and (4) complete breast with nipple, areola, and glandular tissue. Fenench’s9 method is preferred and simply describes ABT as 2 subtypes: supernumerary and aberrant.1,2,10 One study observed 6% of ABT cancers were the supernumerary type and 94% were the aberrant type.1 Ductal lumen stagnation increases the risk for accessory breast carcinoma development.10 Men have a higher prevalence of cancer in ABT compared to anatomically correct breast tissue.11

There currently is no standardized guideline for ABT cancer treatment. The initial clinical impression of cancer of ABT may be misdiagnosed as lymphadenopathy, abscesses, or lipomas.12 The risk for misdiagnosis is higher for cancer of ABT compared to normal breast tissue and is associated with a poorer prognosis.1 Despite multiple screening modalities, our patient’s initial breast cancer screenings proved unreliable. A mammogram failed to detect malignancy, likely secondary to the area of concern being out of the standard imaging field. Ultrasonography also was unreliable and led to misdiagnosis as an infected sebaceous cyst with rupture in our patient. Upon review of the ultrasound, concerns were raised by dermatology that the mass was more likely an epidermal inclusion cyst with rupture given the more superficial and sac-free nature of sebaceous cysts, which commonly are associated with steatocystoma multiplex.13 Definitive diagnosis of ductal carcinoma in situ was made with dermatopathologic examination.
Prophylactic surgical excision of ABT has been recommended, suggesting that excisional biopsy and histopathologic examination is the more appropriate method to rule out malignancy. Surgical treatment of ABT may omit any risk for malignant transformation and may provide psychological relief to patients for aesthetic reasons.10,12,14 The risk and benefits of prophylactic excision of ABT has been compared to prophylactic mastectomy of anatomically correct breasts,15 with some clinicians considering this definitive procedure unnecessary except in high-risk patients with a strong genetic predisposition.16,17
Accessory breast tissue should be viewed as an anatomical variant with the option of surgical removal for symptomatic concerns, such as firm nodules, discharge, and pain. Although ABT is rare and cancer in ABT is even more uncommon (<1% of all breast cancers),5,11 clinicians should be suspicious of benign diagnostic reports when the clinical situation does not fit the proposed narrative.
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
- Marshall MB, Moynihan JJ, Frost A, et al. Ectopic breast cancer: case report and literature review. Surg Oncol. 1994;3:295-304. doi:10.1016/0960-7404(94)90032-9
- DeFilippis EM, Arleo EK. The ABCs of accessory breast tissue: basic information every radiologist should know. Am J Roentgenol. 2014;202:1157-1162. doi:10.2214/AJR.13.10930
- Famá F, Cicciú M, Sindoni A, et al. Prevalence of ectopic breast tissue and tumor: a 20-year single center experience. Clin Breast Cancer. 2016;16:E107-E112. doi:10.1016/j.clbc.2016.03.004
- Brown J, Schwartz RA. Supernumerary nipples: an overview. Cutis. 2003;71:344-346.
- Nihon-Yanagi Y, Ueda T, Kameda N, et al. A case of ectopic breast cancer with a literature review. Surg Oncol. 2011;20:35-42. doi:10.1016/j.suronc.2009.09.005
- Hedayat AA, Pettus JR, Marotti JD, et al. Proliferative lesion of anogenital mammary-like glands in the setting of Cowden syndrome: case report and review of the literature. J Cutan Pathol. 2016;43:707-710. doi:10.1111/cup.12721
- Brightmore T. Bilateral double nipples. Br J Surg. 1972;59:55-57. https://doi.org/10.1002/bjs.1800590114
- De Cholnoky T. Accessory breast tissue in the axilla. N Y State J Med. 1951;51:2245-2248.
- Fenech HB. Aberrant breast tissue; case report. Harper Hosp Bull. 1949;7:268-271.
- Francone E, Nathan MJ, Murelli F, et al. Ectopic breast cancer: case report and review of the literature. Aesthetic Plast Surg. 2013;37:746-749. doi:10.1007/s00266-013-0125-1
- Yamamura J, Masuda N, Kodama Y, et al. Male breast cancer originating in an accessory mammary gland in the axilla: a case report. Case Rep Med. 2012;2012:286210. doi:10.1155/2012/286210.
- Ghosn SH, Khatri KA, Bhawan J. Bilateral aberrant axillary breast tissue mimicking lipomas: report of a case and review of the literature. J Cutan Pathol. 2007;34(suppl 1):9-13. doi:10.1111/j.1600-0560.2006.00713.x
- Arceu M, Martinez G, Alfaro D, et al. Ultrasound morphologic features of steatocystoma multiplex with clinical correlation. J Ultrasound Med. 2020;39:2255-2260. doi:10.1002/jum.15320
- Lesavoy MA, Gomez-Garcia A, Nejdl R, et al. Axillary breast tissue: clinical presentation and surgical treatment. Ann Plast Surg. 1995;35:356-360. doi:10.1097/00000637-199510000-00004
- Bank J. Management of ectopic breast tissue. Aesthetic Plast Surg. 2013;37:750-751. doi:10.1007/s00266-013-0143-z
- Morrow M. Prophylactic mastectomy of the contralateral breast. Breast. 2011;20(suppl 3):S108-S110. doi:10.1016/S0960-9776(11)70306-X
- Teoh V, Tasoulis M-K, Gui G. Contralateral prophylactic mastectomy in women with unilateral breast cancer who are genetic carriers, have a strong family history or are just young at presentation. Cancers (Basel). 2020;12:140. doi:10.3390/cancers12010140
Practice Points
- Accessory breasts (also referred to as ectopic breast tissue) develop when breast tissue is retained along the mammary ridge outside of the usual pectoral regions.
- Because accessory breasts may contain the same structures as anatomically correct breasts, they can be subject to the same benign or malignant changes.
- Clinical and pathologic correlation is prudent when interpreting ectopic mammary tissue, as various benign or malignant neoplasms may arise in this setting, especially if there are underlying genetic aberrancies or genodermatoses.
Enlarging Pigmented Lesion on the Thigh
The Diagnosis: Localized Cutaneous Argyria
The differential diagnosis of an enlarging pigmented lesion is broad, including various neoplasms, pigmented deep fungal infections, and cutaneous deposits secondary to systemic or topical medications or other exogenous substances. In our patient, identification of black particulate material on biopsy prompted further questioning. After the sinus tract persisted for 6 months, our patient’s infectious disease physician started applying silver nitrate at 3-week intervals to minimize drainage, exudate, and granulation tissue formation. After 3 months, marked pigmentation of the skin around the sinus tract was noted.
Argyria is a rare skin disorder that results from deposition of silver via localized exposure or systemic ingestion. Discoloration can either be reversible or irreversible, usually dependent on the length of silver exposure.1 Affected individuals exhibit blue-gray pigmentation of the skin that may be localized or diffuse. Photoactivated reduction of silver salts leads to conversion to elemental silver in the skin.2 Although argyria is most common on sun-exposed areas, the mucosae and nails may be involved in systemic cases. The etiology of argyria includes occupational exposure by ingestion of dust or traumatic cutaneous exposure in jewelry manufacturing, mining, or photographic or radiograph manufacturing. Other sources of localized argyria include prolonged contact with topical silver nitrate or silver sulfadiazine for wound care, silver-coated jewelry or piercings, acupuncture, tooth restoration procedures using dental amalgam, silver-containing surgical implants, or other silver-containing medications or wound dressings. Discontinuing contact with the source of silver minimizes further pigmentation, and excision of deposits may be helpful in some instances.3
Histopathologic findings in argyria may be subtle and diverse. Small particulate material may be apparent on careful examination at high magnification only, and the depth of deposition can depend on the etiology of absorption or implantation as well as the length of exposure. Short-term exposure may be associated with deposition of dark, brown-black, coarse granules confined to the stratum corneum.1 Frequently, cases of argyria reveal small, extracellular, brown-black, pigmented granules in a bandlike distribution primarily around vasculature, eccrine glands, perineural tissue, hair follicles, or arrector pili muscles or free in the dermis around collagen bundles. The granules can be highlighted by dark-field microscopy that will display scattered, refractile, white particles, described as a “stars in heaven” pattern.3 Rare ochre-colored collagen bundles have been reported in some cases, described as a pseudo-ochronosis pattern of argyria.4
Given the clinical history in our patient, a melanocytic lesion was considered but was excluded based on the histopathologic findings. Regressed melanoma clinically may resemble cutaneous silver deposition, as tumoral melanosis can be associated with an intense blue-black presentation. Histopathology will reveal an absence of melanocytes with residual coarse melanin in melanophages (Figure 1) rather than the particulate material associated with silver deposition. Although argyria can be associated with increased melanin in the basal epidermal keratinocytes and melanophages in the papillary dermis, silver granules can be distinguished by their uniform appearance and location throughout the skin (dermis, around vasculature/adnexal structures vs melanin in melanophages and basal epidermal keratinocytes).3,5,6
Blue nevi typically present as well-circumscribed, blue to gray or even dark brown lesions most often located on the arms, legs, head, and neck. Histopathology reveals spindle-shaped dendritic melanocytes dissecting through collagen bundles in the dermis with melanophages (Figure 2). Pigmentation may vary from extensive to little or even none. Blue nevi are demarcated and may be associated with dermal sclerosis.7
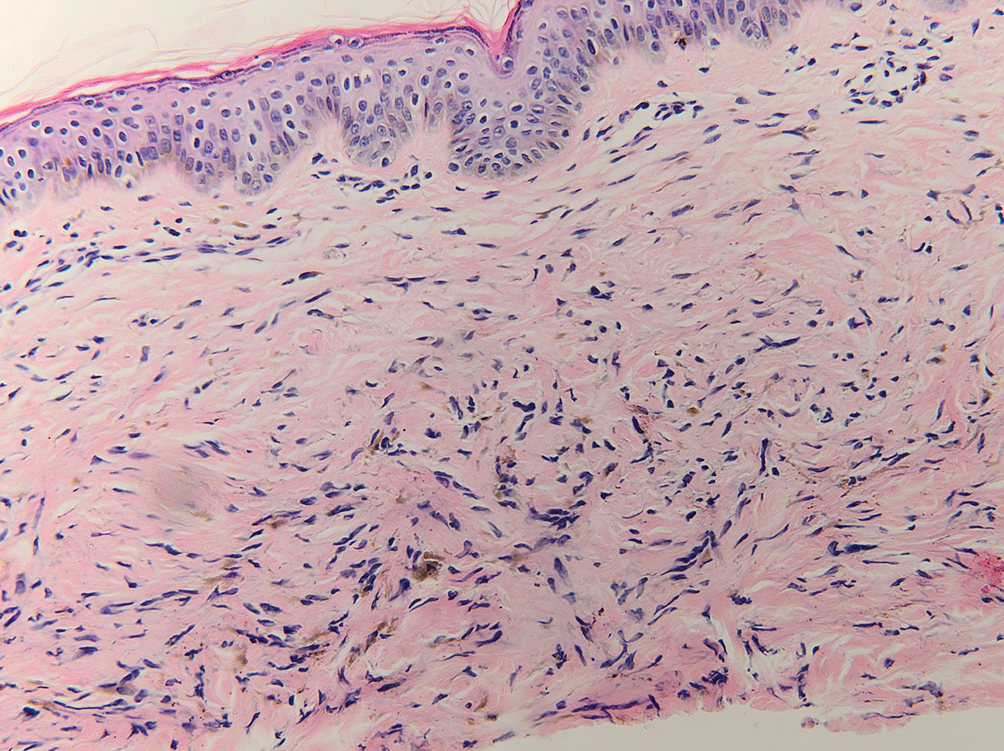
Drug-induced hyperpigmentation has a variable presentation both clinically and histologically depending on the type of drug implicated. Tetracyclines, particularly minocycline, are known culprits of drug-induced pigmentation, which can present as blue-gray to brown discoloration in at least 3 classically described patterns: (1) blue-black pigmentation around scars or prior inflammatory sites, (2) blue-black pigmentation on the shins or upper extremities, or (3) brown pigmentation in photosensitive areas. Histopathology reveals brown-black granules intracellularly in macrophages or fibroblasts or localized around vessels or eccrine glands (Figure 3). Special stains such as Perls Prussian blue or Fontana-Masson may highlight the pigmented granules. Widespread pigmentation in other organs, such as the thyroid, and history of long-standing tetracycline use are helpful clues to distinguish drug-induced pigmentation from other entities.8
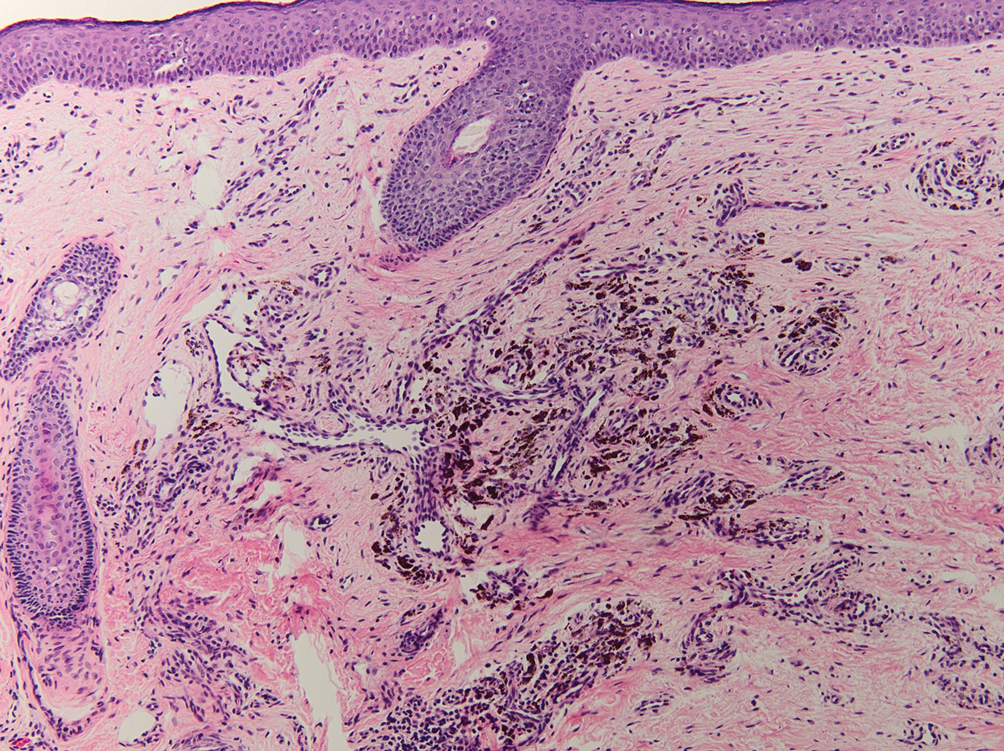
Tattoo ink reaction frequently presents as an irregular pigmented lesion that can have associated features of inflammation including rash, erythema, and swelling. Histopathology reveals small clumped pigment in the dermis localized either extracellularly preferentially around vascular structures and collagen fibers or intracellularly in macrophages or fibroblasts (Figure 4). Considering the pigment is foreign material, a mixed inflammatory infiltrate can be present or more rarely the presence of pigment may induce pseudoepitheliomatous hyperplasia. The inflammatory reaction pattern on histology can vary, but granulomatous and lichenoid patterns frequently have been described. Other helpful clues to suggest tattoo pigment include refractile granules under polarized light and multiple pigmented colors.3
Dermal melanocytosis also may be considered, which consists of blue-gray irregular macules to patches on the skin that are frequently present at birth but may develop later in life. Histopathology reveals pigmented dendritic to spindle-shaped dermal melanocytes and melanophages dissecting between collagen fibers localized to the deep dermis. In addition, some hematologic or vascular disorders, including resolving hemorrhage or cyanosis, may be considered in the clinical differential. Deposition disorders such as chrysiasis and ochronosis could exhibit clinical or histopathologic similarities.3,8
Occasionally, prolonged use of topical silver nitrate may result in a pigmented lesion that mimics a melanocytic neoplasm or other pigmented lesions. However, these conditions can be readily differentiated by their characteristic histopathologic findings along with detailed clinical history.
- Ondrasik RM, Jordan P, Sriharan A. A clinical mimicker of melanoma with distinctive histopathology: topical silver nitrate exposure. J Cutan Pathol. 2020;47:1205-1210.
- Gill P, Richards K, Cho WC, et al. Localized cutaneous argyria: review of a rare clinical mimicker of melanocytic lesions. Ann Diagn Pathol. 2021;54:151776.
- Molina-Ruiz AM, Cerroni L, Kutzner H, et al. Cutaneous deposits. Am J Dermatopathol. 2014;36:1-48.
- Lee J, Korgavkar K, DiMarco C, et al. Localized argyria with pseudoochronosis. J Cutan Pathol. 2020;47:671-674.
- El Sharouni MA, Aivazian K, Witkamp AJ, et al. Association of histologic regression with a favorable outcome in patients with stage 1 and stage 2 cutaneous melanoma. JAMA Dermatol. 2021;157:166-173.
- Staser K, Chen D, Solus J, et al. Extensive tumoral melanosis associated with ipilimumab-treated melanoma. Br J Dermatol. 2016;175:391-393.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and “malignant blue nevus”: a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Wang RF, Ko D, Friedman BJ, et al. Disorders of hyperpigmentation. part I. pathogenesis and clinical features of common pigmentary disorders. J Am Acad Dermatol. 2023;88:271-288.
The Diagnosis: Localized Cutaneous Argyria
The differential diagnosis of an enlarging pigmented lesion is broad, including various neoplasms, pigmented deep fungal infections, and cutaneous deposits secondary to systemic or topical medications or other exogenous substances. In our patient, identification of black particulate material on biopsy prompted further questioning. After the sinus tract persisted for 6 months, our patient’s infectious disease physician started applying silver nitrate at 3-week intervals to minimize drainage, exudate, and granulation tissue formation. After 3 months, marked pigmentation of the skin around the sinus tract was noted.
Argyria is a rare skin disorder that results from deposition of silver via localized exposure or systemic ingestion. Discoloration can either be reversible or irreversible, usually dependent on the length of silver exposure.1 Affected individuals exhibit blue-gray pigmentation of the skin that may be localized or diffuse. Photoactivated reduction of silver salts leads to conversion to elemental silver in the skin.2 Although argyria is most common on sun-exposed areas, the mucosae and nails may be involved in systemic cases. The etiology of argyria includes occupational exposure by ingestion of dust or traumatic cutaneous exposure in jewelry manufacturing, mining, or photographic or radiograph manufacturing. Other sources of localized argyria include prolonged contact with topical silver nitrate or silver sulfadiazine for wound care, silver-coated jewelry or piercings, acupuncture, tooth restoration procedures using dental amalgam, silver-containing surgical implants, or other silver-containing medications or wound dressings. Discontinuing contact with the source of silver minimizes further pigmentation, and excision of deposits may be helpful in some instances.3
Histopathologic findings in argyria may be subtle and diverse. Small particulate material may be apparent on careful examination at high magnification only, and the depth of deposition can depend on the etiology of absorption or implantation as well as the length of exposure. Short-term exposure may be associated with deposition of dark, brown-black, coarse granules confined to the stratum corneum.1 Frequently, cases of argyria reveal small, extracellular, brown-black, pigmented granules in a bandlike distribution primarily around vasculature, eccrine glands, perineural tissue, hair follicles, or arrector pili muscles or free in the dermis around collagen bundles. The granules can be highlighted by dark-field microscopy that will display scattered, refractile, white particles, described as a “stars in heaven” pattern.3 Rare ochre-colored collagen bundles have been reported in some cases, described as a pseudo-ochronosis pattern of argyria.4
Given the clinical history in our patient, a melanocytic lesion was considered but was excluded based on the histopathologic findings. Regressed melanoma clinically may resemble cutaneous silver deposition, as tumoral melanosis can be associated with an intense blue-black presentation. Histopathology will reveal an absence of melanocytes with residual coarse melanin in melanophages (Figure 1) rather than the particulate material associated with silver deposition. Although argyria can be associated with increased melanin in the basal epidermal keratinocytes and melanophages in the papillary dermis, silver granules can be distinguished by their uniform appearance and location throughout the skin (dermis, around vasculature/adnexal structures vs melanin in melanophages and basal epidermal keratinocytes).3,5,6

Blue nevi typically present as well-circumscribed, blue to gray or even dark brown lesions most often located on the arms, legs, head, and neck. Histopathology reveals spindle-shaped dendritic melanocytes dissecting through collagen bundles in the dermis with melanophages (Figure 2). Pigmentation may vary from extensive to little or even none. Blue nevi are demarcated and may be associated with dermal sclerosis.7

Drug-induced hyperpigmentation has a variable presentation both clinically and histologically depending on the type of drug implicated. Tetracyclines, particularly minocycline, are known culprits of drug-induced pigmentation, which can present as blue-gray to brown discoloration in at least 3 classically described patterns: (1) blue-black pigmentation around scars or prior inflammatory sites, (2) blue-black pigmentation on the shins or upper extremities, or (3) brown pigmentation in photosensitive areas. Histopathology reveals brown-black granules intracellularly in macrophages or fibroblasts or localized around vessels or eccrine glands (Figure 3). Special stains such as Perls Prussian blue or Fontana-Masson may highlight the pigmented granules. Widespread pigmentation in other organs, such as the thyroid, and history of long-standing tetracycline use are helpful clues to distinguish drug-induced pigmentation from other entities.8

Tattoo ink reaction frequently presents as an irregular pigmented lesion that can have associated features of inflammation including rash, erythema, and swelling. Histopathology reveals small clumped pigment in the dermis localized either extracellularly preferentially around vascular structures and collagen fibers or intracellularly in macrophages or fibroblasts (Figure 4). Considering the pigment is foreign material, a mixed inflammatory infiltrate can be present or more rarely the presence of pigment may induce pseudoepitheliomatous hyperplasia. The inflammatory reaction pattern on histology can vary, but granulomatous and lichenoid patterns frequently have been described. Other helpful clues to suggest tattoo pigment include refractile granules under polarized light and multiple pigmented colors.3

Dermal melanocytosis also may be considered, which consists of blue-gray irregular macules to patches on the skin that are frequently present at birth but may develop later in life. Histopathology reveals pigmented dendritic to spindle-shaped dermal melanocytes and melanophages dissecting between collagen fibers localized to the deep dermis. In addition, some hematologic or vascular disorders, including resolving hemorrhage or cyanosis, may be considered in the clinical differential. Deposition disorders such as chrysiasis and ochronosis could exhibit clinical or histopathologic similarities.3,8
Occasionally, prolonged use of topical silver nitrate may result in a pigmented lesion that mimics a melanocytic neoplasm or other pigmented lesions. However, these conditions can be readily differentiated by their characteristic histopathologic findings along with detailed clinical history.
The Diagnosis: Localized Cutaneous Argyria
The differential diagnosis of an enlarging pigmented lesion is broad, including various neoplasms, pigmented deep fungal infections, and cutaneous deposits secondary to systemic or topical medications or other exogenous substances. In our patient, identification of black particulate material on biopsy prompted further questioning. After the sinus tract persisted for 6 months, our patient’s infectious disease physician started applying silver nitrate at 3-week intervals to minimize drainage, exudate, and granulation tissue formation. After 3 months, marked pigmentation of the skin around the sinus tract was noted.
Argyria is a rare skin disorder that results from deposition of silver via localized exposure or systemic ingestion. Discoloration can either be reversible or irreversible, usually dependent on the length of silver exposure.1 Affected individuals exhibit blue-gray pigmentation of the skin that may be localized or diffuse. Photoactivated reduction of silver salts leads to conversion to elemental silver in the skin.2 Although argyria is most common on sun-exposed areas, the mucosae and nails may be involved in systemic cases. The etiology of argyria includes occupational exposure by ingestion of dust or traumatic cutaneous exposure in jewelry manufacturing, mining, or photographic or radiograph manufacturing. Other sources of localized argyria include prolonged contact with topical silver nitrate or silver sulfadiazine for wound care, silver-coated jewelry or piercings, acupuncture, tooth restoration procedures using dental amalgam, silver-containing surgical implants, or other silver-containing medications or wound dressings. Discontinuing contact with the source of silver minimizes further pigmentation, and excision of deposits may be helpful in some instances.3
Histopathologic findings in argyria may be subtle and diverse. Small particulate material may be apparent on careful examination at high magnification only, and the depth of deposition can depend on the etiology of absorption or implantation as well as the length of exposure. Short-term exposure may be associated with deposition of dark, brown-black, coarse granules confined to the stratum corneum.1 Frequently, cases of argyria reveal small, extracellular, brown-black, pigmented granules in a bandlike distribution primarily around vasculature, eccrine glands, perineural tissue, hair follicles, or arrector pili muscles or free in the dermis around collagen bundles. The granules can be highlighted by dark-field microscopy that will display scattered, refractile, white particles, described as a “stars in heaven” pattern.3 Rare ochre-colored collagen bundles have been reported in some cases, described as a pseudo-ochronosis pattern of argyria.4
Given the clinical history in our patient, a melanocytic lesion was considered but was excluded based on the histopathologic findings. Regressed melanoma clinically may resemble cutaneous silver deposition, as tumoral melanosis can be associated with an intense blue-black presentation. Histopathology will reveal an absence of melanocytes with residual coarse melanin in melanophages (Figure 1) rather than the particulate material associated with silver deposition. Although argyria can be associated with increased melanin in the basal epidermal keratinocytes and melanophages in the papillary dermis, silver granules can be distinguished by their uniform appearance and location throughout the skin (dermis, around vasculature/adnexal structures vs melanin in melanophages and basal epidermal keratinocytes).3,5,6

Blue nevi typically present as well-circumscribed, blue to gray or even dark brown lesions most often located on the arms, legs, head, and neck. Histopathology reveals spindle-shaped dendritic melanocytes dissecting through collagen bundles in the dermis with melanophages (Figure 2). Pigmentation may vary from extensive to little or even none. Blue nevi are demarcated and may be associated with dermal sclerosis.7

Drug-induced hyperpigmentation has a variable presentation both clinically and histologically depending on the type of drug implicated. Tetracyclines, particularly minocycline, are known culprits of drug-induced pigmentation, which can present as blue-gray to brown discoloration in at least 3 classically described patterns: (1) blue-black pigmentation around scars or prior inflammatory sites, (2) blue-black pigmentation on the shins or upper extremities, or (3) brown pigmentation in photosensitive areas. Histopathology reveals brown-black granules intracellularly in macrophages or fibroblasts or localized around vessels or eccrine glands (Figure 3). Special stains such as Perls Prussian blue or Fontana-Masson may highlight the pigmented granules. Widespread pigmentation in other organs, such as the thyroid, and history of long-standing tetracycline use are helpful clues to distinguish drug-induced pigmentation from other entities.8

Tattoo ink reaction frequently presents as an irregular pigmented lesion that can have associated features of inflammation including rash, erythema, and swelling. Histopathology reveals small clumped pigment in the dermis localized either extracellularly preferentially around vascular structures and collagen fibers or intracellularly in macrophages or fibroblasts (Figure 4). Considering the pigment is foreign material, a mixed inflammatory infiltrate can be present or more rarely the presence of pigment may induce pseudoepitheliomatous hyperplasia. The inflammatory reaction pattern on histology can vary, but granulomatous and lichenoid patterns frequently have been described. Other helpful clues to suggest tattoo pigment include refractile granules under polarized light and multiple pigmented colors.3

Dermal melanocytosis also may be considered, which consists of blue-gray irregular macules to patches on the skin that are frequently present at birth but may develop later in life. Histopathology reveals pigmented dendritic to spindle-shaped dermal melanocytes and melanophages dissecting between collagen fibers localized to the deep dermis. In addition, some hematologic or vascular disorders, including resolving hemorrhage or cyanosis, may be considered in the clinical differential. Deposition disorders such as chrysiasis and ochronosis could exhibit clinical or histopathologic similarities.3,8
Occasionally, prolonged use of topical silver nitrate may result in a pigmented lesion that mimics a melanocytic neoplasm or other pigmented lesions. However, these conditions can be readily differentiated by their characteristic histopathologic findings along with detailed clinical history.
- Ondrasik RM, Jordan P, Sriharan A. A clinical mimicker of melanoma with distinctive histopathology: topical silver nitrate exposure. J Cutan Pathol. 2020;47:1205-1210.
- Gill P, Richards K, Cho WC, et al. Localized cutaneous argyria: review of a rare clinical mimicker of melanocytic lesions. Ann Diagn Pathol. 2021;54:151776.
- Molina-Ruiz AM, Cerroni L, Kutzner H, et al. Cutaneous deposits. Am J Dermatopathol. 2014;36:1-48.
- Lee J, Korgavkar K, DiMarco C, et al. Localized argyria with pseudoochronosis. J Cutan Pathol. 2020;47:671-674.
- El Sharouni MA, Aivazian K, Witkamp AJ, et al. Association of histologic regression with a favorable outcome in patients with stage 1 and stage 2 cutaneous melanoma. JAMA Dermatol. 2021;157:166-173.
- Staser K, Chen D, Solus J, et al. Extensive tumoral melanosis associated with ipilimumab-treated melanoma. Br J Dermatol. 2016;175:391-393.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and “malignant blue nevus”: a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Wang RF, Ko D, Friedman BJ, et al. Disorders of hyperpigmentation. part I. pathogenesis and clinical features of common pigmentary disorders. J Am Acad Dermatol. 2023;88:271-288.
- Ondrasik RM, Jordan P, Sriharan A. A clinical mimicker of melanoma with distinctive histopathology: topical silver nitrate exposure. J Cutan Pathol. 2020;47:1205-1210.
- Gill P, Richards K, Cho WC, et al. Localized cutaneous argyria: review of a rare clinical mimicker of melanocytic lesions. Ann Diagn Pathol. 2021;54:151776.
- Molina-Ruiz AM, Cerroni L, Kutzner H, et al. Cutaneous deposits. Am J Dermatopathol. 2014;36:1-48.
- Lee J, Korgavkar K, DiMarco C, et al. Localized argyria with pseudoochronosis. J Cutan Pathol. 2020;47:671-674.
- El Sharouni MA, Aivazian K, Witkamp AJ, et al. Association of histologic regression with a favorable outcome in patients with stage 1 and stage 2 cutaneous melanoma. JAMA Dermatol. 2021;157:166-173.
- Staser K, Chen D, Solus J, et al. Extensive tumoral melanosis associated with ipilimumab-treated melanoma. Br J Dermatol. 2016;175:391-393.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and “malignant blue nevus”: a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Wang RF, Ko D, Friedman BJ, et al. Disorders of hyperpigmentation. part I. pathogenesis and clinical features of common pigmentary disorders. J Am Acad Dermatol. 2023;88:271-288.
An 80-year-old man presented with a pigmented lesion on the left lateral thigh near the knee that had been gradually enlarging over several weeks (top [inset]). He underwent a left knee replacement surgery for advanced osteoarthritis many months prior that was complicated by postoperative Staphylococcus aureus infection with sinus tract formation that was persistent for 6 months and treated with a topical medication. A pigmented lesion developed near the opening of the sinus tract. His medical history was remarkable for extensive actinic damage as well as multiple actinic keratoses treated with cryotherapy but no history of melanoma. An excisional biopsy was performed (top and bottom).
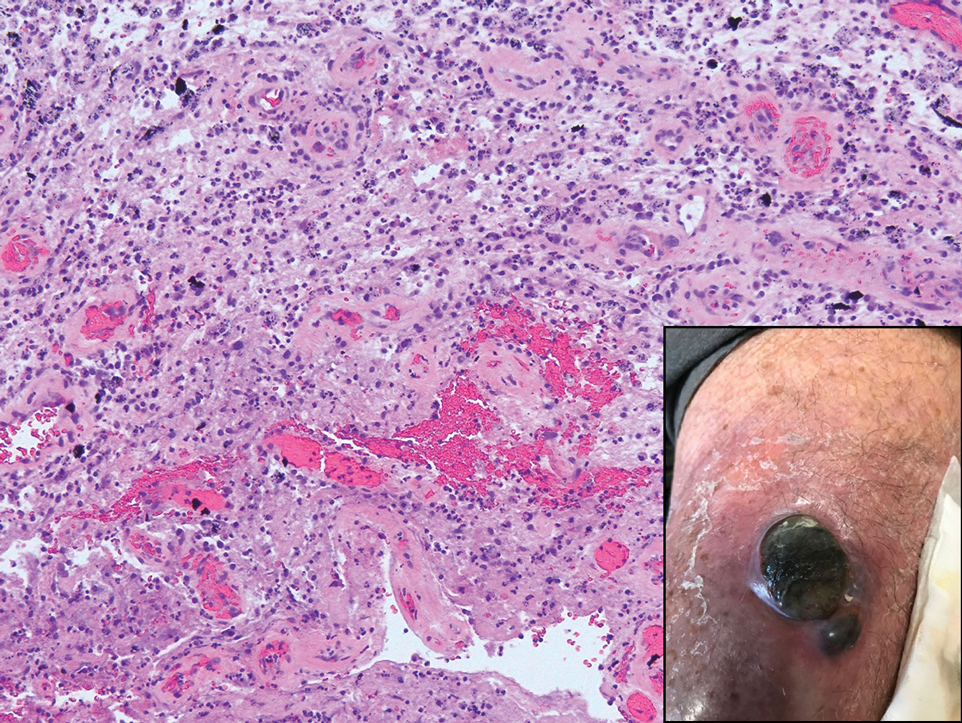
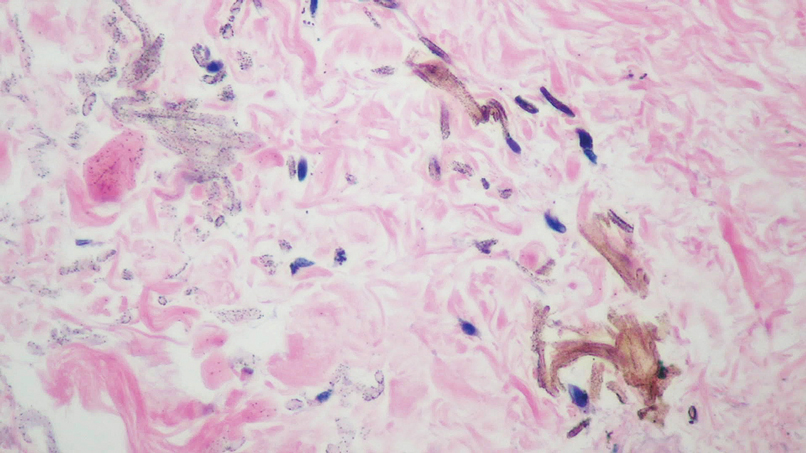
Multiple Nodules on the Scrotum
The Diagnosis: Scrotal Calcinosis
Scrotal calcinosis is a rare benign disease that results from the deposition of calcium, magnesium, phosphate, and carbonate within the dermis and subcutaneous layer of the skin in the absence of underlying systemic disease or serum calcium and phosphorus abnormalities.1,2 Lesions usually are asymptomatic but can be mildly painful or pruritic. They usually present in childhood or early adulthood as yellow-white firm nodules ranging in size from a few millimeters to a few centimeters that increase in size and number over time. Additionally, lesions can ulcerate and discharge a chalklike exudative material. Although benign in nature, the quality-of-life impact in patients with this condition can be substantial, specifically regarding cosmesis, which may cause patients to feel embarrassed and even avoid sexual activity. This condition rarely has been associated with infection.1
Our patient elected to undergo surgical excision under local anesthesia, and the lesions were sent for histopathologic examination. His postoperative course was unremarkable, and he was pleased with the cosmetic result of the surgery (Figure 1). Histopathology revealed calcified deposits that appeared as intradermal basophilic nodules lacking an epithelial lining (Figure 2), consistent with the diagnosis of scrotal calcinosis.2 No recurrence of the lesions was documented over the course of 18 months.
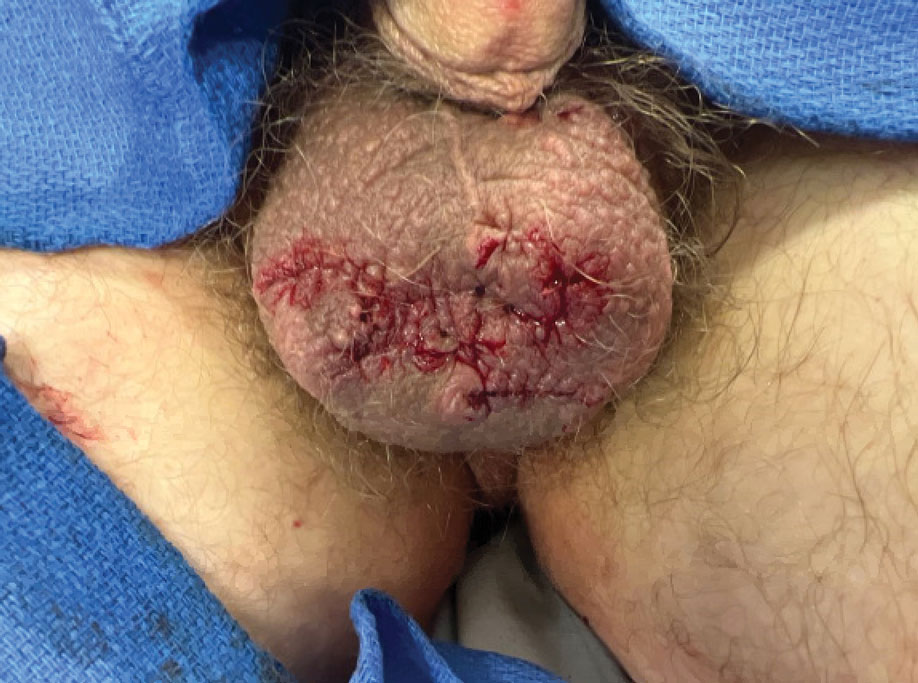
The pathogenesis of this condition is not clear. Most research supports scrotal calcinosis resulting from dystrophic calcification of epidermal inclusion cysts.3 There have been cases of scrotal calcinosis coinciding with epidermal inclusion cysts of the scrotum in varying stages of inflammation (some intact and some ruptured).2 Some research also suggests dystrophic calcification of eccrine epithelial cysts and degenerated dartos muscle as the origin of scrotal calcinosis.3
The differential diagnosis for this case included calcified steatocystoma multiplex, eruptive xanthomas, nodular scabies, and epidermal inclusion cysts. Steatocystoma multiplex can be inherited in an autosomal-dominant fashion or can develop sporadically with mutations in the KRT17 gene.4 It is characterized by multiple sebum-filled, cystic lesions of the pilosebaceous unit that may become calcified. Calcified lesions appear as yellow, firm, irregularly shaped papules or nodules ranging from a few millimeters to centimeters in size. Cysts can develop anywhere on the body with a predilection for the chest, upper extremities, axillae, trunk, groin, and scrotum.4 Histologically, our patient’s lesions were not associated with the pilosebaceous unit. Additionally, our patient denied a family history of similar skin lesions, which made calcified steatocystoma multiplex an unlikely diagnosis.
Eruptive xanthomas result from localized deposition of lipids within the dermis, typically in the setting of dyslipidemia or poorly controlled diabetes mellitus. They commonly appear on the extremities or buttocks as pruritic crops of yellow-red papules or nodules that are a few millimeters in size. Although our patient has a history of hyperlipidemia, his lesions differed substantially from eruptive xanthomas in clinical presentation.
Nodular scabies is a manifestation of classic scabies that presents with intensely pruritic erythematous papules and nodules that are a few millimeters in size and commonly occur on the axillae, groin, and genitalia. Our patient’s skin lesions were not pruritic and differed in appearance from nodular scabies.
Although research indicates scrotal calcinosis may result from dystrophic calcification of epidermal inclusion cysts,2 the latter present as dome-shaped, flesh-colored nodules with central pores representing the opening of hair follicles. Our patient lacked characteristic findings of epidermal inclusion cysts on histology.
The preferred treatment for scrotal calcinosis is surgical excision, which improves the aesthetic appearance, relieves itch, and removes ulcerative lesions.5 Additionally, surgical excision provides histological diagnostic confirmation. Recurrence with incomplete excision is possible; therefore, all lesions should be completely excised to reduce the risk for recurrence.3
- Pompeo A, Molina WR, Pohlman GD, et al. Idiopathic scrotal calcinosis: a rare entity and a review of the literature. Can Urol Assoc J. 2013;7:E439-E441. doi:10.5489/cuaj.1387
- Swinehart JM, Golitz LE. Scrotal calcinosis: dystrophic calcification of epidermoid cysts. Arch Dermatol. 1982;118:985-988. doi:10.1001 /archderm.1982.01650240029016
- Khallouk A, Yazami OE, Mellas S, et al. Idiopathic scrotal calcinosis: a nonelucidated pathogenesis and its surgical treatment. Rev Urol. 2011;13:95-97.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480. doi:10.1046/j.1365-2133.1998.02413.x
- Solanki A, Narang S, Kathpalia R, et al. Scrotal calcinosis: pathogenetic link with epidermal cyst. BMJ Case Rep. 2015;2015:bcr2015211163. doi:10.1136/bcr-2015-211163
The Diagnosis: Scrotal Calcinosis
Scrotal calcinosis is a rare benign disease that results from the deposition of calcium, magnesium, phosphate, and carbonate within the dermis and subcutaneous layer of the skin in the absence of underlying systemic disease or serum calcium and phosphorus abnormalities.1,2 Lesions usually are asymptomatic but can be mildly painful or pruritic. They usually present in childhood or early adulthood as yellow-white firm nodules ranging in size from a few millimeters to a few centimeters that increase in size and number over time. Additionally, lesions can ulcerate and discharge a chalklike exudative material. Although benign in nature, the quality-of-life impact in patients with this condition can be substantial, specifically regarding cosmesis, which may cause patients to feel embarrassed and even avoid sexual activity. This condition rarely has been associated with infection.1
Our patient elected to undergo surgical excision under local anesthesia, and the lesions were sent for histopathologic examination. His postoperative course was unremarkable, and he was pleased with the cosmetic result of the surgery (Figure 1). Histopathology revealed calcified deposits that appeared as intradermal basophilic nodules lacking an epithelial lining (Figure 2), consistent with the diagnosis of scrotal calcinosis.2 No recurrence of the lesions was documented over the course of 18 months.

The pathogenesis of this condition is not clear. Most research supports scrotal calcinosis resulting from dystrophic calcification of epidermal inclusion cysts.3 There have been cases of scrotal calcinosis coinciding with epidermal inclusion cysts of the scrotum in varying stages of inflammation (some intact and some ruptured).2 Some research also suggests dystrophic calcification of eccrine epithelial cysts and degenerated dartos muscle as the origin of scrotal calcinosis.3

The differential diagnosis for this case included calcified steatocystoma multiplex, eruptive xanthomas, nodular scabies, and epidermal inclusion cysts. Steatocystoma multiplex can be inherited in an autosomal-dominant fashion or can develop sporadically with mutations in the KRT17 gene.4 It is characterized by multiple sebum-filled, cystic lesions of the pilosebaceous unit that may become calcified. Calcified lesions appear as yellow, firm, irregularly shaped papules or nodules ranging from a few millimeters to centimeters in size. Cysts can develop anywhere on the body with a predilection for the chest, upper extremities, axillae, trunk, groin, and scrotum.4 Histologically, our patient’s lesions were not associated with the pilosebaceous unit. Additionally, our patient denied a family history of similar skin lesions, which made calcified steatocystoma multiplex an unlikely diagnosis.
Eruptive xanthomas result from localized deposition of lipids within the dermis, typically in the setting of dyslipidemia or poorly controlled diabetes mellitus. They commonly appear on the extremities or buttocks as pruritic crops of yellow-red papules or nodules that are a few millimeters in size. Although our patient has a history of hyperlipidemia, his lesions differed substantially from eruptive xanthomas in clinical presentation.
Nodular scabies is a manifestation of classic scabies that presents with intensely pruritic erythematous papules and nodules that are a few millimeters in size and commonly occur on the axillae, groin, and genitalia. Our patient’s skin lesions were not pruritic and differed in appearance from nodular scabies.
Although research indicates scrotal calcinosis may result from dystrophic calcification of epidermal inclusion cysts,2 the latter present as dome-shaped, flesh-colored nodules with central pores representing the opening of hair follicles. Our patient lacked characteristic findings of epidermal inclusion cysts on histology.
The preferred treatment for scrotal calcinosis is surgical excision, which improves the aesthetic appearance, relieves itch, and removes ulcerative lesions.5 Additionally, surgical excision provides histological diagnostic confirmation. Recurrence with incomplete excision is possible; therefore, all lesions should be completely excised to reduce the risk for recurrence.3
The Diagnosis: Scrotal Calcinosis
Scrotal calcinosis is a rare benign disease that results from the deposition of calcium, magnesium, phosphate, and carbonate within the dermis and subcutaneous layer of the skin in the absence of underlying systemic disease or serum calcium and phosphorus abnormalities.1,2 Lesions usually are asymptomatic but can be mildly painful or pruritic. They usually present in childhood or early adulthood as yellow-white firm nodules ranging in size from a few millimeters to a few centimeters that increase in size and number over time. Additionally, lesions can ulcerate and discharge a chalklike exudative material. Although benign in nature, the quality-of-life impact in patients with this condition can be substantial, specifically regarding cosmesis, which may cause patients to feel embarrassed and even avoid sexual activity. This condition rarely has been associated with infection.1
Our patient elected to undergo surgical excision under local anesthesia, and the lesions were sent for histopathologic examination. His postoperative course was unremarkable, and he was pleased with the cosmetic result of the surgery (Figure 1). Histopathology revealed calcified deposits that appeared as intradermal basophilic nodules lacking an epithelial lining (Figure 2), consistent with the diagnosis of scrotal calcinosis.2 No recurrence of the lesions was documented over the course of 18 months.

The pathogenesis of this condition is not clear. Most research supports scrotal calcinosis resulting from dystrophic calcification of epidermal inclusion cysts.3 There have been cases of scrotal calcinosis coinciding with epidermal inclusion cysts of the scrotum in varying stages of inflammation (some intact and some ruptured).2 Some research also suggests dystrophic calcification of eccrine epithelial cysts and degenerated dartos muscle as the origin of scrotal calcinosis.3

The differential diagnosis for this case included calcified steatocystoma multiplex, eruptive xanthomas, nodular scabies, and epidermal inclusion cysts. Steatocystoma multiplex can be inherited in an autosomal-dominant fashion or can develop sporadically with mutations in the KRT17 gene.4 It is characterized by multiple sebum-filled, cystic lesions of the pilosebaceous unit that may become calcified. Calcified lesions appear as yellow, firm, irregularly shaped papules or nodules ranging from a few millimeters to centimeters in size. Cysts can develop anywhere on the body with a predilection for the chest, upper extremities, axillae, trunk, groin, and scrotum.4 Histologically, our patient’s lesions were not associated with the pilosebaceous unit. Additionally, our patient denied a family history of similar skin lesions, which made calcified steatocystoma multiplex an unlikely diagnosis.
Eruptive xanthomas result from localized deposition of lipids within the dermis, typically in the setting of dyslipidemia or poorly controlled diabetes mellitus. They commonly appear on the extremities or buttocks as pruritic crops of yellow-red papules or nodules that are a few millimeters in size. Although our patient has a history of hyperlipidemia, his lesions differed substantially from eruptive xanthomas in clinical presentation.
Nodular scabies is a manifestation of classic scabies that presents with intensely pruritic erythematous papules and nodules that are a few millimeters in size and commonly occur on the axillae, groin, and genitalia. Our patient’s skin lesions were not pruritic and differed in appearance from nodular scabies.
Although research indicates scrotal calcinosis may result from dystrophic calcification of epidermal inclusion cysts,2 the latter present as dome-shaped, flesh-colored nodules with central pores representing the opening of hair follicles. Our patient lacked characteristic findings of epidermal inclusion cysts on histology.
The preferred treatment for scrotal calcinosis is surgical excision, which improves the aesthetic appearance, relieves itch, and removes ulcerative lesions.5 Additionally, surgical excision provides histological diagnostic confirmation. Recurrence with incomplete excision is possible; therefore, all lesions should be completely excised to reduce the risk for recurrence.3
- Pompeo A, Molina WR, Pohlman GD, et al. Idiopathic scrotal calcinosis: a rare entity and a review of the literature. Can Urol Assoc J. 2013;7:E439-E441. doi:10.5489/cuaj.1387
- Swinehart JM, Golitz LE. Scrotal calcinosis: dystrophic calcification of epidermoid cysts. Arch Dermatol. 1982;118:985-988. doi:10.1001 /archderm.1982.01650240029016
- Khallouk A, Yazami OE, Mellas S, et al. Idiopathic scrotal calcinosis: a nonelucidated pathogenesis and its surgical treatment. Rev Urol. 2011;13:95-97.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480. doi:10.1046/j.1365-2133.1998.02413.x
- Solanki A, Narang S, Kathpalia R, et al. Scrotal calcinosis: pathogenetic link with epidermal cyst. BMJ Case Rep. 2015;2015:bcr2015211163. doi:10.1136/bcr-2015-211163
- Pompeo A, Molina WR, Pohlman GD, et al. Idiopathic scrotal calcinosis: a rare entity and a review of the literature. Can Urol Assoc J. 2013;7:E439-E441. doi:10.5489/cuaj.1387
- Swinehart JM, Golitz LE. Scrotal calcinosis: dystrophic calcification of epidermoid cysts. Arch Dermatol. 1982;118:985-988. doi:10.1001 /archderm.1982.01650240029016
- Khallouk A, Yazami OE, Mellas S, et al. Idiopathic scrotal calcinosis: a nonelucidated pathogenesis and its surgical treatment. Rev Urol. 2011;13:95-97.
- Covello SP, Smith FJ, Sillevis Smitt JH, et al. Keratin 17 mutations cause either steatocystoma multiplex or pachyonychia congenita type 2. Br J Dermatol. 1998;139:475-480. doi:10.1046/j.1365-2133.1998.02413.x
- Solanki A, Narang S, Kathpalia R, et al. Scrotal calcinosis: pathogenetic link with epidermal cyst. BMJ Case Rep. 2015;2015:bcr2015211163. doi:10.1136/bcr-2015-211163
A 33-year-old man presented with progressively enlarging bumps on the scrotum that were present since adolescence. He had a history of hyperlipidemia but no history of systemic or autoimmune disease. The lesions were asymptomatic without associated pruritus, pain, or discharge. No treatments had been administered, and he had no known personal or family history of similar skin conditions or skin cancer. He endorsed a monogamous relationship with his wife. Physical examination revealed 15 firm, yellow-white, subcutaneous nodules on the scrotum that varied in size.
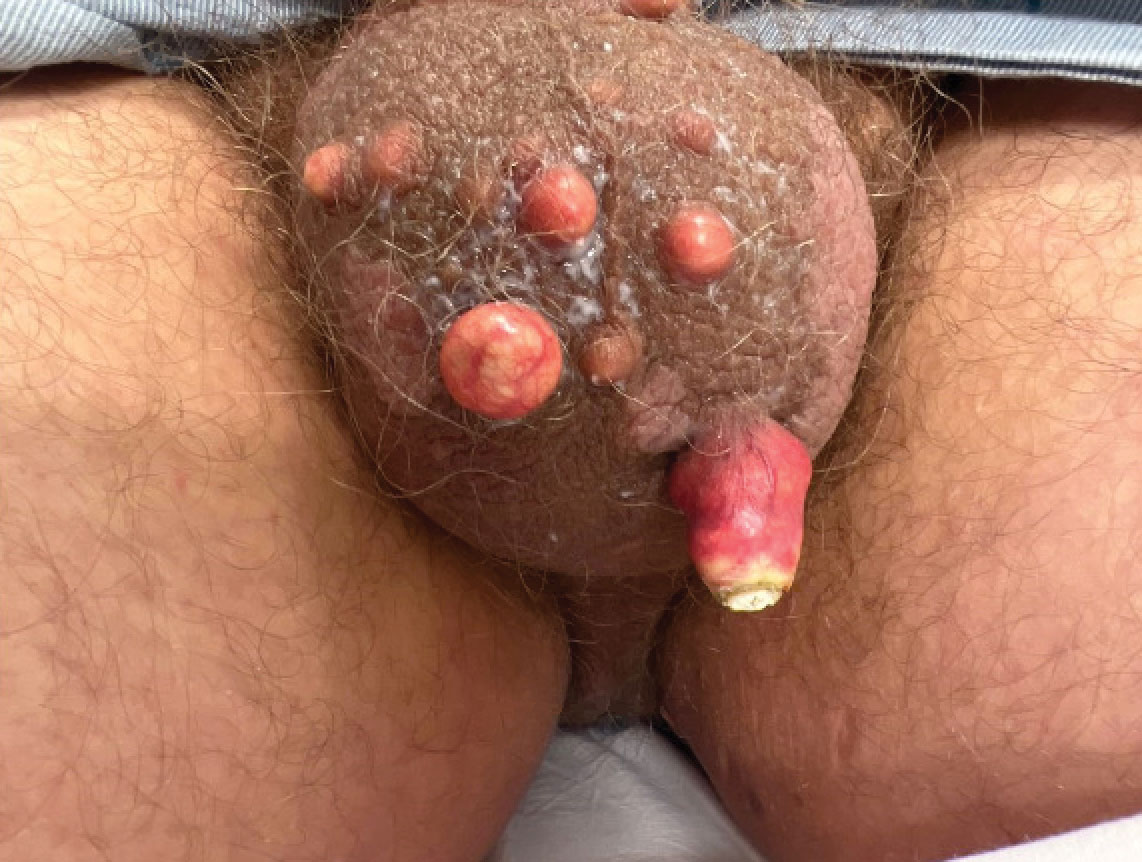
Porcelain White, Crinkled, Violaceous Patches on the Inner Thighs
The Diagnosis: Extragenital Lichen Sclerosus et Atrophicus
A punch biopsy of the lesion revealed epidermal hyperkeratosis, atrophy, follicular plugs with basal vacuolar degeneration, and homogenous dense fibrosis in the papillary dermis with a dense lymphocytic infiltrate beneath the fibrosis (Figure 1). Dermoscopic examination was remarkable for a distinctive rainbow pattern. Clinical, histopathologic, and dermoscopic findings led to the diagnosis of extragenital lichen sclerosus et atrophicus (LSEA). A potent corticosteroid cream was prescribed twice daily for 2 months, after which the lesions completely resolved. At 2-year follow-up, a relapse was not observed (Figure 2).
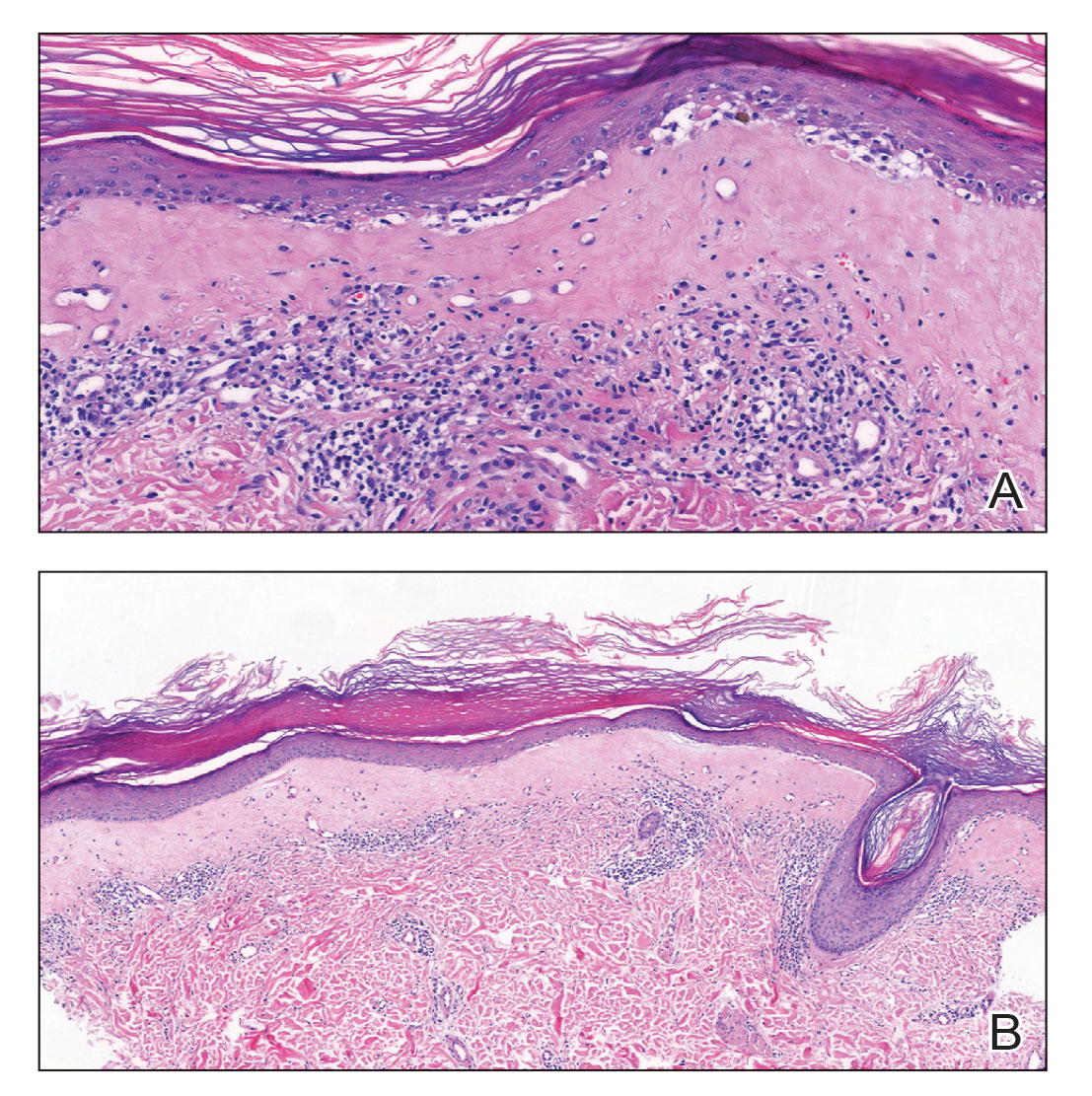
Lichen sclerosus et atrophicus is an inflammatory dermatosis that clinically presents as atrophic or hypertrophic plaques that may show pigmentation changes with anogenital and extragenital involvement. It is common among females and predominantly occurs in prepubescent girls and postmenopausal women. The exact etiology is unclear; however, it is hypothesized to occur secondary to autoimmunity with an underlying genetic predisposition. Local trauma, hormonal influences, and infections are other suspected etiologic factors. Genital lesions often lead to itching, pain, and dyspareunia, whereas extragenital lesions predominantly are asymptomatic. When symptomatic, itching usually is the main concern. Unlike genital LSEA, extragenital lesions are not associated with squamous cell carcinoma development. Reported dermoscopic features of LSEA are white structureless areas with scaling, comedolike openings, follicular plugs, white shiny streaks, blue-gray peppering, pigment network, and red-purple globules.1 In our case, the dermoscopic finding of a rainbow pattern in LSEA is rare.2 Although the mechanism behind this appearance unclear, it can be the result of the birefringence effect—local variations in refractive index—influenced by the direction of structures within the dermis such as collagen. In this case, there was diffuse and dense homogenous fibrosis in the superficial dermis that corresponded to dermoscopic white polygonal clods.

Extragenital LSEA commonly is located on the neck, shoulders, wrists, and upper trunk and manifests clinically as whitish papules coalescing into scarlike plaques. Of all patients who have LSEA, 20% have extragenital lesions, and most of these lesions are seen in patients who also have genital LSEA. Approximately 6% of all LSEA patients have extragenital LSEA without genital involvement.3
For experienced dermatologists, clinical symptoms and lesion characteristics usually are sufficient for diagnosis; however, a differential diagnosis of atypical lesions and isolated extragenital presentations such as morphea, lichen simplex chronicus, lichen planus, and vitiligo requires the correlation of clinical findings with histopathology and dermoscopy. Morphea, known as localized scleroderma, is an idiopathic inflammatory skin disease with sclerotic changes. It manifests as inflammatory plaques that vary in color from red to purple. If there is moderate sclerosis in the center of this plaque, the color progressively fades to white, leaving a purplish ring around the edges. Dermoscopic features of morphea are reported as areas of erythema; red-focused vessels of linear, irregular, or dotted morphology; white fibrotic beams; and pigmentary structures.2 Lichen simplex chronicus is characterized by single or multiple dry and patchy skin lesions that are intensely pruritic. It commonly occurs on the neck, scalp, extremities, genital areas, and buttocks. Scratching the lesions leads to scarring, thickening of the skin, and increased frequency of itching. Histopathology of lichen simplex chronicus most frequently demonstrates a thickening of the epidermis and papillary dermis, irregularly elongated rete ridges, and fibroplasia with stellate or multinucleated fibroblasts completed by perivascular lymphocytic inflammation.4 Lichen planus presents with pruritic, polygonal, purple papules and/or plaques that can present in a variety of clinical forms, including atrophic and hypertrophic lichen planus.5 Lichen planus was an unlikely diagnosis for our patient due to the presence of patchy scarlike lesions and dermoscopic features that are well described in patients with LSEA. Lichen sclerosus et atrophicus presents with hypopigmented and/or hyperpigmented patches and plaques, distinguishing itself from vitiligo, which has flat lesions.
Topical steroids are the first-line therapeutic agents in the treatment of LSEA.6 Despite frequent use in this setting, common side effects such as localized scarring and atrophic degenerations have led to debate about their use. In our patient, the lesions resolved almost completely in 2 months, and no relapse was observed in the following 2 years. In the setting of topical steroid resistance, topical calcineurin inhibitors, UVA/UVB phototherapy, and topical tacrolimus can be used for treatment.6
The diagnosis of isolated extragenital LSEA may be a clinical challenge and generally requires further workup. When evaluating extragenital lesions, dermatologists should keep in mind extragenital LSEA as a differential diagnosis in the presence of a dermoscopic rainbow pattern arranged over white polygonal clods.
- Wang Y-K, Hao J-C, Liu J, et al. Dermoscopic features of morphea and extragenital lichen sclerosus in Chinese patients. Chin Med J (Engl). 2020;133:2109-2111.
- Errichetti E, Lallas A, Apalla Z, et al. Dermoscopy of morphea and cutaneous lichen sclerosus: clinicopathological correlation study and comparative analysis. Dermatology. 2017;233:462-470.
- Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9-30.
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149.
- Kirtschig G, Becker K, Günthert A, et al. Evidence-based (S3) guideline on (anogenital) lichen sclerosus. J Eur Acad Dermatol Venereol. 2015;29:E1-E43.
The Diagnosis: Extragenital Lichen Sclerosus et Atrophicus
A punch biopsy of the lesion revealed epidermal hyperkeratosis, atrophy, follicular plugs with basal vacuolar degeneration, and homogenous dense fibrosis in the papillary dermis with a dense lymphocytic infiltrate beneath the fibrosis (Figure 1). Dermoscopic examination was remarkable for a distinctive rainbow pattern. Clinical, histopathologic, and dermoscopic findings led to the diagnosis of extragenital lichen sclerosus et atrophicus (LSEA). A potent corticosteroid cream was prescribed twice daily for 2 months, after which the lesions completely resolved. At 2-year follow-up, a relapse was not observed (Figure 2).

Lichen sclerosus et atrophicus is an inflammatory dermatosis that clinically presents as atrophic or hypertrophic plaques that may show pigmentation changes with anogenital and extragenital involvement. It is common among females and predominantly occurs in prepubescent girls and postmenopausal women. The exact etiology is unclear; however, it is hypothesized to occur secondary to autoimmunity with an underlying genetic predisposition. Local trauma, hormonal influences, and infections are other suspected etiologic factors. Genital lesions often lead to itching, pain, and dyspareunia, whereas extragenital lesions predominantly are asymptomatic. When symptomatic, itching usually is the main concern. Unlike genital LSEA, extragenital lesions are not associated with squamous cell carcinoma development. Reported dermoscopic features of LSEA are white structureless areas with scaling, comedolike openings, follicular plugs, white shiny streaks, blue-gray peppering, pigment network, and red-purple globules.1 In our case, the dermoscopic finding of a rainbow pattern in LSEA is rare.2 Although the mechanism behind this appearance unclear, it can be the result of the birefringence effect—local variations in refractive index—influenced by the direction of structures within the dermis such as collagen. In this case, there was diffuse and dense homogenous fibrosis in the superficial dermis that corresponded to dermoscopic white polygonal clods.

Extragenital LSEA commonly is located on the neck, shoulders, wrists, and upper trunk and manifests clinically as whitish papules coalescing into scarlike plaques. Of all patients who have LSEA, 20% have extragenital lesions, and most of these lesions are seen in patients who also have genital LSEA. Approximately 6% of all LSEA patients have extragenital LSEA without genital involvement.3
For experienced dermatologists, clinical symptoms and lesion characteristics usually are sufficient for diagnosis; however, a differential diagnosis of atypical lesions and isolated extragenital presentations such as morphea, lichen simplex chronicus, lichen planus, and vitiligo requires the correlation of clinical findings with histopathology and dermoscopy. Morphea, known as localized scleroderma, is an idiopathic inflammatory skin disease with sclerotic changes. It manifests as inflammatory plaques that vary in color from red to purple. If there is moderate sclerosis in the center of this plaque, the color progressively fades to white, leaving a purplish ring around the edges. Dermoscopic features of morphea are reported as areas of erythema; red-focused vessels of linear, irregular, or dotted morphology; white fibrotic beams; and pigmentary structures.2 Lichen simplex chronicus is characterized by single or multiple dry and patchy skin lesions that are intensely pruritic. It commonly occurs on the neck, scalp, extremities, genital areas, and buttocks. Scratching the lesions leads to scarring, thickening of the skin, and increased frequency of itching. Histopathology of lichen simplex chronicus most frequently demonstrates a thickening of the epidermis and papillary dermis, irregularly elongated rete ridges, and fibroplasia with stellate or multinucleated fibroblasts completed by perivascular lymphocytic inflammation.4 Lichen planus presents with pruritic, polygonal, purple papules and/or plaques that can present in a variety of clinical forms, including atrophic and hypertrophic lichen planus.5 Lichen planus was an unlikely diagnosis for our patient due to the presence of patchy scarlike lesions and dermoscopic features that are well described in patients with LSEA. Lichen sclerosus et atrophicus presents with hypopigmented and/or hyperpigmented patches and plaques, distinguishing itself from vitiligo, which has flat lesions.
Topical steroids are the first-line therapeutic agents in the treatment of LSEA.6 Despite frequent use in this setting, common side effects such as localized scarring and atrophic degenerations have led to debate about their use. In our patient, the lesions resolved almost completely in 2 months, and no relapse was observed in the following 2 years. In the setting of topical steroid resistance, topical calcineurin inhibitors, UVA/UVB phototherapy, and topical tacrolimus can be used for treatment.6
The diagnosis of isolated extragenital LSEA may be a clinical challenge and generally requires further workup. When evaluating extragenital lesions, dermatologists should keep in mind extragenital LSEA as a differential diagnosis in the presence of a dermoscopic rainbow pattern arranged over white polygonal clods.
The Diagnosis: Extragenital Lichen Sclerosus et Atrophicus
A punch biopsy of the lesion revealed epidermal hyperkeratosis, atrophy, follicular plugs with basal vacuolar degeneration, and homogenous dense fibrosis in the papillary dermis with a dense lymphocytic infiltrate beneath the fibrosis (Figure 1). Dermoscopic examination was remarkable for a distinctive rainbow pattern. Clinical, histopathologic, and dermoscopic findings led to the diagnosis of extragenital lichen sclerosus et atrophicus (LSEA). A potent corticosteroid cream was prescribed twice daily for 2 months, after which the lesions completely resolved. At 2-year follow-up, a relapse was not observed (Figure 2).

Lichen sclerosus et atrophicus is an inflammatory dermatosis that clinically presents as atrophic or hypertrophic plaques that may show pigmentation changes with anogenital and extragenital involvement. It is common among females and predominantly occurs in prepubescent girls and postmenopausal women. The exact etiology is unclear; however, it is hypothesized to occur secondary to autoimmunity with an underlying genetic predisposition. Local trauma, hormonal influences, and infections are other suspected etiologic factors. Genital lesions often lead to itching, pain, and dyspareunia, whereas extragenital lesions predominantly are asymptomatic. When symptomatic, itching usually is the main concern. Unlike genital LSEA, extragenital lesions are not associated with squamous cell carcinoma development. Reported dermoscopic features of LSEA are white structureless areas with scaling, comedolike openings, follicular plugs, white shiny streaks, blue-gray peppering, pigment network, and red-purple globules.1 In our case, the dermoscopic finding of a rainbow pattern in LSEA is rare.2 Although the mechanism behind this appearance unclear, it can be the result of the birefringence effect—local variations in refractive index—influenced by the direction of structures within the dermis such as collagen. In this case, there was diffuse and dense homogenous fibrosis in the superficial dermis that corresponded to dermoscopic white polygonal clods.

Extragenital LSEA commonly is located on the neck, shoulders, wrists, and upper trunk and manifests clinically as whitish papules coalescing into scarlike plaques. Of all patients who have LSEA, 20% have extragenital lesions, and most of these lesions are seen in patients who also have genital LSEA. Approximately 6% of all LSEA patients have extragenital LSEA without genital involvement.3
For experienced dermatologists, clinical symptoms and lesion characteristics usually are sufficient for diagnosis; however, a differential diagnosis of atypical lesions and isolated extragenital presentations such as morphea, lichen simplex chronicus, lichen planus, and vitiligo requires the correlation of clinical findings with histopathology and dermoscopy. Morphea, known as localized scleroderma, is an idiopathic inflammatory skin disease with sclerotic changes. It manifests as inflammatory plaques that vary in color from red to purple. If there is moderate sclerosis in the center of this plaque, the color progressively fades to white, leaving a purplish ring around the edges. Dermoscopic features of morphea are reported as areas of erythema; red-focused vessels of linear, irregular, or dotted morphology; white fibrotic beams; and pigmentary structures.2 Lichen simplex chronicus is characterized by single or multiple dry and patchy skin lesions that are intensely pruritic. It commonly occurs on the neck, scalp, extremities, genital areas, and buttocks. Scratching the lesions leads to scarring, thickening of the skin, and increased frequency of itching. Histopathology of lichen simplex chronicus most frequently demonstrates a thickening of the epidermis and papillary dermis, irregularly elongated rete ridges, and fibroplasia with stellate or multinucleated fibroblasts completed by perivascular lymphocytic inflammation.4 Lichen planus presents with pruritic, polygonal, purple papules and/or plaques that can present in a variety of clinical forms, including atrophic and hypertrophic lichen planus.5 Lichen planus was an unlikely diagnosis for our patient due to the presence of patchy scarlike lesions and dermoscopic features that are well described in patients with LSEA. Lichen sclerosus et atrophicus presents with hypopigmented and/or hyperpigmented patches and plaques, distinguishing itself from vitiligo, which has flat lesions.
Topical steroids are the first-line therapeutic agents in the treatment of LSEA.6 Despite frequent use in this setting, common side effects such as localized scarring and atrophic degenerations have led to debate about their use. In our patient, the lesions resolved almost completely in 2 months, and no relapse was observed in the following 2 years. In the setting of topical steroid resistance, topical calcineurin inhibitors, UVA/UVB phototherapy, and topical tacrolimus can be used for treatment.6
The diagnosis of isolated extragenital LSEA may be a clinical challenge and generally requires further workup. When evaluating extragenital lesions, dermatologists should keep in mind extragenital LSEA as a differential diagnosis in the presence of a dermoscopic rainbow pattern arranged over white polygonal clods.
- Wang Y-K, Hao J-C, Liu J, et al. Dermoscopic features of morphea and extragenital lichen sclerosus in Chinese patients. Chin Med J (Engl). 2020;133:2109-2111.
- Errichetti E, Lallas A, Apalla Z, et al. Dermoscopy of morphea and cutaneous lichen sclerosus: clinicopathological correlation study and comparative analysis. Dermatology. 2017;233:462-470.
- Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9-30.
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149.
- Kirtschig G, Becker K, Günthert A, et al. Evidence-based (S3) guideline on (anogenital) lichen sclerosus. J Eur Acad Dermatol Venereol. 2015;29:E1-E43.
- Wang Y-K, Hao J-C, Liu J, et al. Dermoscopic features of morphea and extragenital lichen sclerosus in Chinese patients. Chin Med J (Engl). 2020;133:2109-2111.
- Errichetti E, Lallas A, Apalla Z, et al. Dermoscopy of morphea and cutaneous lichen sclerosus: clinicopathological correlation study and comparative analysis. Dermatology. 2017;233:462-470.
- Wallace HJ. Lichen sclerosus et atrophicus. Trans St Johns Hosp Dermatol Soc. 1971;57:9-30.
- Balan R, Grigoras¸ A, Popovici D, et al. The histopathological landscape of the major psoriasiform dermatoses. Arch Clin Cases. 2021;6:59-68.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149.
- Kirtschig G, Becker K, Günthert A, et al. Evidence-based (S3) guideline on (anogenital) lichen sclerosus. J Eur Acad Dermatol Venereol. 2015;29:E1-E43.
A 50-year-old woman presented with multiple pruritic lesions on the right inner thigh of 2 years’ duration. Physical examination revealed porcelain white, crinkled, violaceous patches extending from the right inner thigh to the inguinal fold (top). Dermoscopic examination revealed follicular plugs, white structureless areas, white lines, and a rainbow pattern arranged over white polygonal clods on polarized mode (bottom).
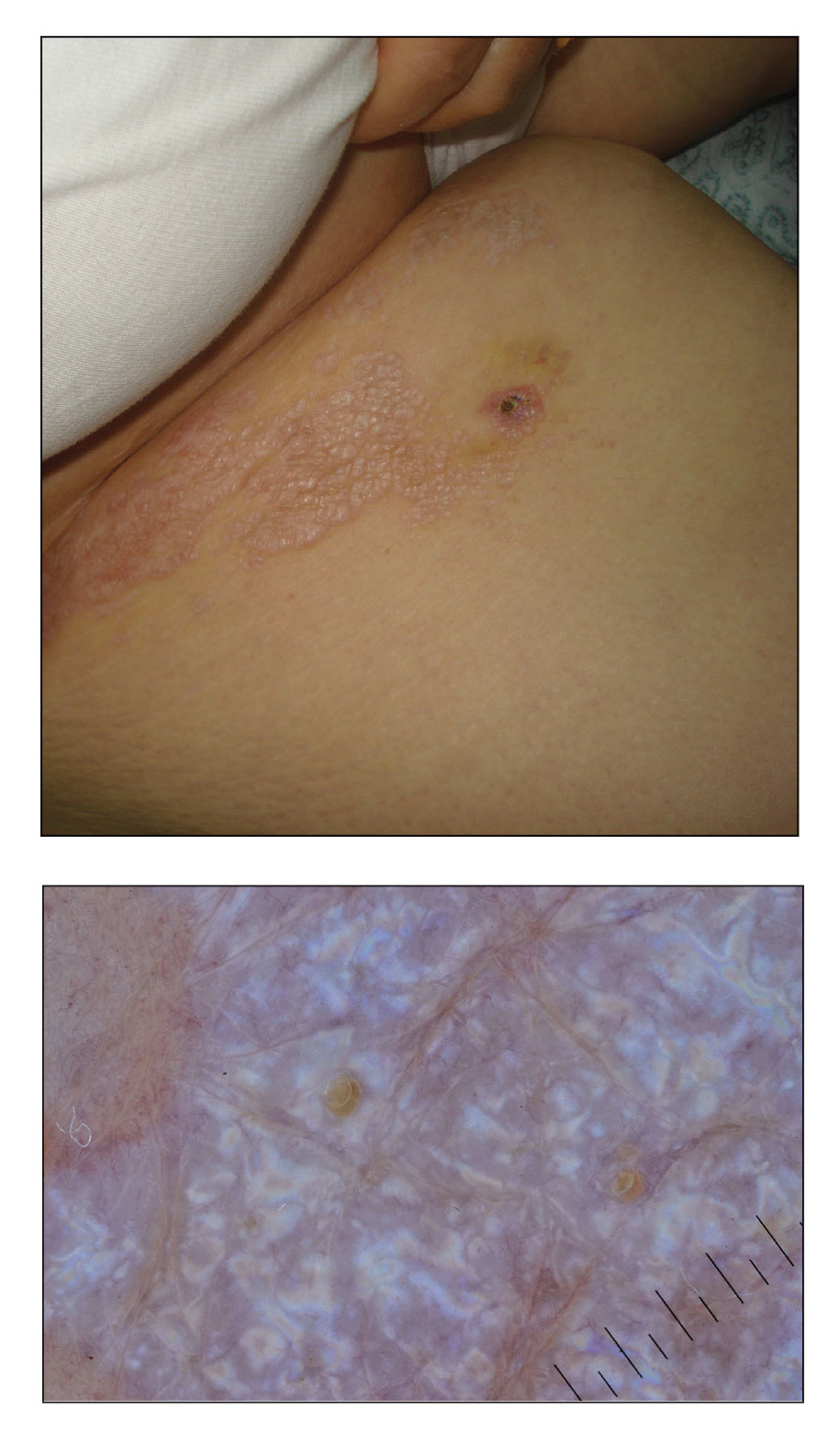
Painful, Nonhealing, Violaceus Plaque on the Right Breast
The Diagnosis: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is an acquired reactive vascular proliferation in the spectrum of cutaneous reactive angioendotheliomatoses. Clinically, DDA presents as violaceous reticulated plaques, often with secondary ulceration and sometimes necrosis.1-3 Diffuse dermal angiomatosis more commonly presents in patients with a history of severe peripheral vascular disease, coagulopathies, or infection, and it frequently arises on the extremities. Diffuse dermal angiomatosis also has been shown to develop on the breasts, particularly in patients with pendulous breast tissue. Vascular proliferation in DDA is hypothesized to be from ischemia and hypoxia, leading to angiogenesis.1-3 Diffuse dermal angiomatosis is characterized histologically by the presence of a diffuse proliferation of spindled endothelial cells distributed between the collagen bundles throughout the dermis (quiz image and Figure 1). Spindle-shaped endothelial cells exhibit a vacuolated cytoplasm. On immunohistochemistry, these dermal spindle cells classically stain positive for CD31, CD34, and erythroblast transformation specific–related gene (Erg) and stain negative for both human herpesvirus 8 (HHV-8) and factor XIIIa.
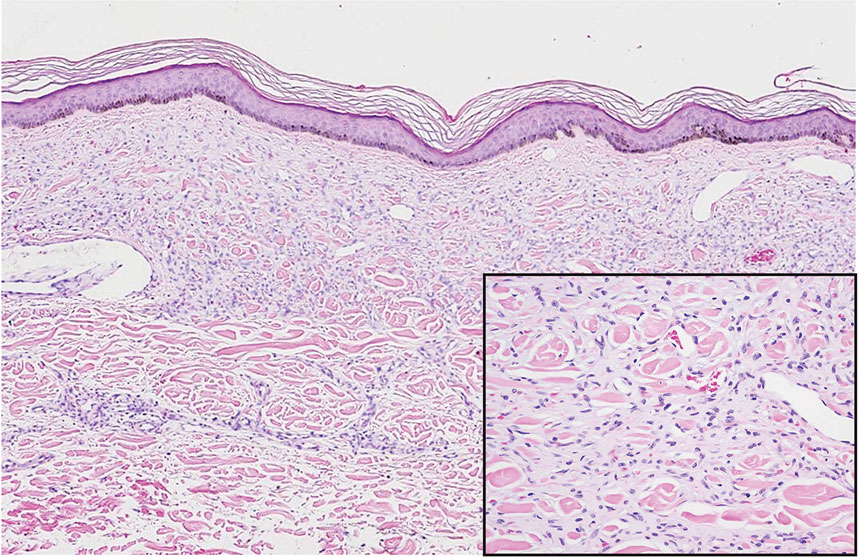
Cutaneous fibrous histiocytoma, more commonly referred to as dermatofibroma, is a common benign lesion that presents clinically as a solitary firm nodule most commonly on the extremities in areas of repetitive trauma or pressure. It classically exhibits dimpling of the overlaying skin with lateral pressure on the lesion, known as the dimple sign.4 Histologically, dermatofibromas share similar features to DDA and demonstrate the presence of bland-appearing spindle cells within the dermis between the collagen bundles, resulting in collagen trapping. However, a distinguishing histologic feature of a dermatofibroma in comparison to DDA is the presence of epidermal hyperplasia overlying the dermatofibroma, leading to tabled rete ridges (Figure 2). Spindle cells in dermatofibromas are fibroblasts and have a distinct immunophenotype that includes factor XIIIa positivity and negative staining for CD31, CD34, and Erg.4,5
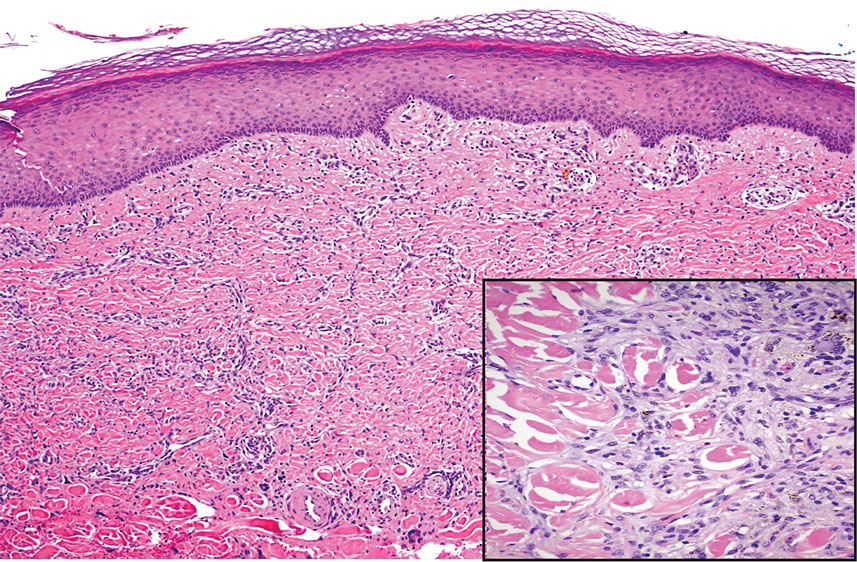
Dermatofibrosarcoma protuberans (DFSP) is a rare malignant soft-tissue sarcoma that clinically presents as a firm, flesh-colored, dermal plaque on the trunk, proximal extremities, head, or neck.5 Histologically, DFSP can be distinguished from DDA by the high density of spindle cells that are arranged in a storiform pattern, extending and infiltrating the underlying subcutaneous fat in a honeycomblike pattern (Figure 3). Spindle cells in DFSP typically show expression of CD34 but are negative for CD31, Erg, and factor XIIIa.5

Kaposi sarcoma (KS) is an endothelial cell–driven angioproliferative neoplasm that is associated with HHV-8 infection.6 The clinical presentation of KS can range from isolated pink or purple papules and patches to more extensive ulcerated plaques or nodules. Histopathology exhibits proliferation of monomorphic spindled endothelial cells within the dermis staining positive for HHV-8, Erg, CD31, and CD34, in conjunction with extravasated erythrocytes arranged within slitlike vascular spaces (Figure 4). Additionally, KS classically exhibits aberrant endothelial cell proliferation and vessel formation around preexisting vessels, which is referred to as the promontory sign (Figure 4).
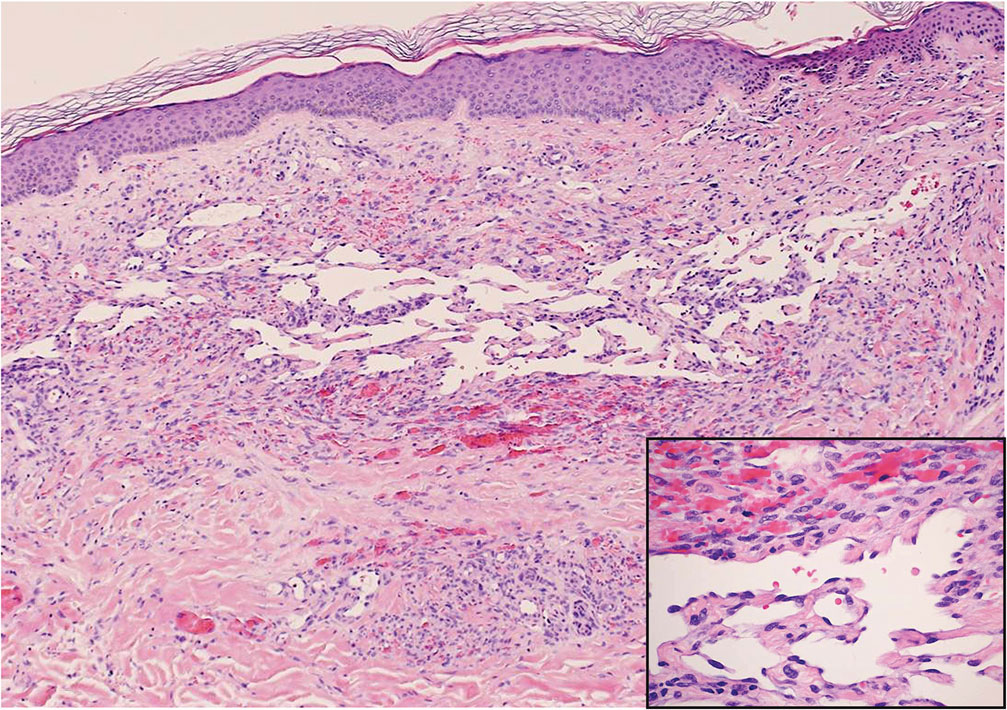
Angiosarcoma is a rare and highly aggressive vascular tumor arising from endothelial cells lining the blood vessels and lymphatics.7,8 Clinically, angiosarcoma presents as ulcerated violaceous nodules or plaques on the head, neck, or trunk. Histologic evaluation of angiosarcoma reveals a complex and poorly demarcated vascular network dissecting between collagen bundles in the dermis (Figure 5). Multilayering of endothelial cells, papillary projections extending into the vessel lumina, and mitoses frequently are seen. On immunohistochemistry, endothelial cells demonstrate prominent cellular atypia and stain positive with CD31, CD34, and Erg.
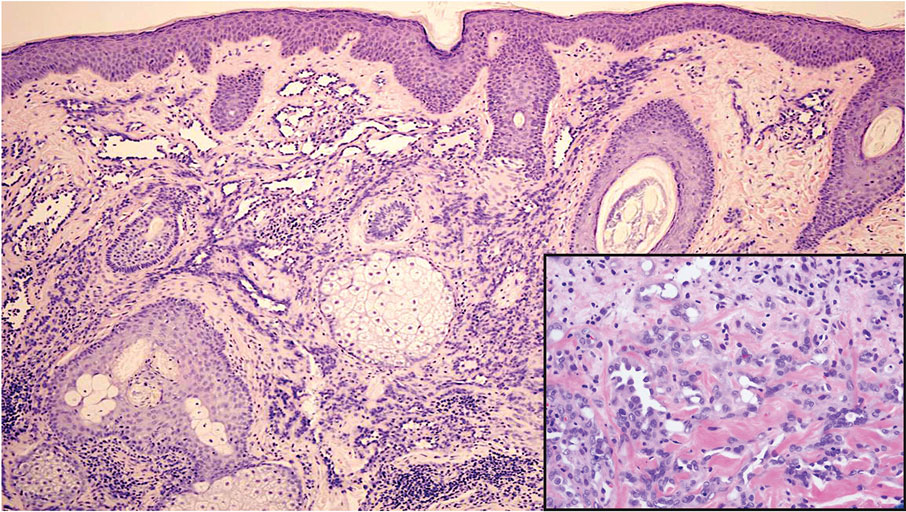
- Touloei K, Tongdee E, Smirnov B, et al. Diffuse dermal angiomatosis. Cutis. 2019;103:181-184.
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Baylor Univ Med Cent Proc. 2020;33:273-275.
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Etemad SA, Dewan AK. Kaposi sarcoma updates. Dermatol Clin. 2019;37:505-517.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
The Diagnosis: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is an acquired reactive vascular proliferation in the spectrum of cutaneous reactive angioendotheliomatoses. Clinically, DDA presents as violaceous reticulated plaques, often with secondary ulceration and sometimes necrosis.1-3 Diffuse dermal angiomatosis more commonly presents in patients with a history of severe peripheral vascular disease, coagulopathies, or infection, and it frequently arises on the extremities. Diffuse dermal angiomatosis also has been shown to develop on the breasts, particularly in patients with pendulous breast tissue. Vascular proliferation in DDA is hypothesized to be from ischemia and hypoxia, leading to angiogenesis.1-3 Diffuse dermal angiomatosis is characterized histologically by the presence of a diffuse proliferation of spindled endothelial cells distributed between the collagen bundles throughout the dermis (quiz image and Figure 1). Spindle-shaped endothelial cells exhibit a vacuolated cytoplasm. On immunohistochemistry, these dermal spindle cells classically stain positive for CD31, CD34, and erythroblast transformation specific–related gene (Erg) and stain negative for both human herpesvirus 8 (HHV-8) and factor XIIIa.

Cutaneous fibrous histiocytoma, more commonly referred to as dermatofibroma, is a common benign lesion that presents clinically as a solitary firm nodule most commonly on the extremities in areas of repetitive trauma or pressure. It classically exhibits dimpling of the overlaying skin with lateral pressure on the lesion, known as the dimple sign.4 Histologically, dermatofibromas share similar features to DDA and demonstrate the presence of bland-appearing spindle cells within the dermis between the collagen bundles, resulting in collagen trapping. However, a distinguishing histologic feature of a dermatofibroma in comparison to DDA is the presence of epidermal hyperplasia overlying the dermatofibroma, leading to tabled rete ridges (Figure 2). Spindle cells in dermatofibromas are fibroblasts and have a distinct immunophenotype that includes factor XIIIa positivity and negative staining for CD31, CD34, and Erg.4,5

Dermatofibrosarcoma protuberans (DFSP) is a rare malignant soft-tissue sarcoma that clinically presents as a firm, flesh-colored, dermal plaque on the trunk, proximal extremities, head, or neck.5 Histologically, DFSP can be distinguished from DDA by the high density of spindle cells that are arranged in a storiform pattern, extending and infiltrating the underlying subcutaneous fat in a honeycomblike pattern (Figure 3). Spindle cells in DFSP typically show expression of CD34 but are negative for CD31, Erg, and factor XIIIa.5

Kaposi sarcoma (KS) is an endothelial cell–driven angioproliferative neoplasm that is associated with HHV-8 infection.6 The clinical presentation of KS can range from isolated pink or purple papules and patches to more extensive ulcerated plaques or nodules. Histopathology exhibits proliferation of monomorphic spindled endothelial cells within the dermis staining positive for HHV-8, Erg, CD31, and CD34, in conjunction with extravasated erythrocytes arranged within slitlike vascular spaces (Figure 4). Additionally, KS classically exhibits aberrant endothelial cell proliferation and vessel formation around preexisting vessels, which is referred to as the promontory sign (Figure 4).

Angiosarcoma is a rare and highly aggressive vascular tumor arising from endothelial cells lining the blood vessels and lymphatics.7,8 Clinically, angiosarcoma presents as ulcerated violaceous nodules or plaques on the head, neck, or trunk. Histologic evaluation of angiosarcoma reveals a complex and poorly demarcated vascular network dissecting between collagen bundles in the dermis (Figure 5). Multilayering of endothelial cells, papillary projections extending into the vessel lumina, and mitoses frequently are seen. On immunohistochemistry, endothelial cells demonstrate prominent cellular atypia and stain positive with CD31, CD34, and Erg.

The Diagnosis: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is an acquired reactive vascular proliferation in the spectrum of cutaneous reactive angioendotheliomatoses. Clinically, DDA presents as violaceous reticulated plaques, often with secondary ulceration and sometimes necrosis.1-3 Diffuse dermal angiomatosis more commonly presents in patients with a history of severe peripheral vascular disease, coagulopathies, or infection, and it frequently arises on the extremities. Diffuse dermal angiomatosis also has been shown to develop on the breasts, particularly in patients with pendulous breast tissue. Vascular proliferation in DDA is hypothesized to be from ischemia and hypoxia, leading to angiogenesis.1-3 Diffuse dermal angiomatosis is characterized histologically by the presence of a diffuse proliferation of spindled endothelial cells distributed between the collagen bundles throughout the dermis (quiz image and Figure 1). Spindle-shaped endothelial cells exhibit a vacuolated cytoplasm. On immunohistochemistry, these dermal spindle cells classically stain positive for CD31, CD34, and erythroblast transformation specific–related gene (Erg) and stain negative for both human herpesvirus 8 (HHV-8) and factor XIIIa.

Cutaneous fibrous histiocytoma, more commonly referred to as dermatofibroma, is a common benign lesion that presents clinically as a solitary firm nodule most commonly on the extremities in areas of repetitive trauma or pressure. It classically exhibits dimpling of the overlaying skin with lateral pressure on the lesion, known as the dimple sign.4 Histologically, dermatofibromas share similar features to DDA and demonstrate the presence of bland-appearing spindle cells within the dermis between the collagen bundles, resulting in collagen trapping. However, a distinguishing histologic feature of a dermatofibroma in comparison to DDA is the presence of epidermal hyperplasia overlying the dermatofibroma, leading to tabled rete ridges (Figure 2). Spindle cells in dermatofibromas are fibroblasts and have a distinct immunophenotype that includes factor XIIIa positivity and negative staining for CD31, CD34, and Erg.4,5

Dermatofibrosarcoma protuberans (DFSP) is a rare malignant soft-tissue sarcoma that clinically presents as a firm, flesh-colored, dermal plaque on the trunk, proximal extremities, head, or neck.5 Histologically, DFSP can be distinguished from DDA by the high density of spindle cells that are arranged in a storiform pattern, extending and infiltrating the underlying subcutaneous fat in a honeycomblike pattern (Figure 3). Spindle cells in DFSP typically show expression of CD34 but are negative for CD31, Erg, and factor XIIIa.5

Kaposi sarcoma (KS) is an endothelial cell–driven angioproliferative neoplasm that is associated with HHV-8 infection.6 The clinical presentation of KS can range from isolated pink or purple papules and patches to more extensive ulcerated plaques or nodules. Histopathology exhibits proliferation of monomorphic spindled endothelial cells within the dermis staining positive for HHV-8, Erg, CD31, and CD34, in conjunction with extravasated erythrocytes arranged within slitlike vascular spaces (Figure 4). Additionally, KS classically exhibits aberrant endothelial cell proliferation and vessel formation around preexisting vessels, which is referred to as the promontory sign (Figure 4).

Angiosarcoma is a rare and highly aggressive vascular tumor arising from endothelial cells lining the blood vessels and lymphatics.7,8 Clinically, angiosarcoma presents as ulcerated violaceous nodules or plaques on the head, neck, or trunk. Histologic evaluation of angiosarcoma reveals a complex and poorly demarcated vascular network dissecting between collagen bundles in the dermis (Figure 5). Multilayering of endothelial cells, papillary projections extending into the vessel lumina, and mitoses frequently are seen. On immunohistochemistry, endothelial cells demonstrate prominent cellular atypia and stain positive with CD31, CD34, and Erg.

- Touloei K, Tongdee E, Smirnov B, et al. Diffuse dermal angiomatosis. Cutis. 2019;103:181-184.
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Baylor Univ Med Cent Proc. 2020;33:273-275.
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Etemad SA, Dewan AK. Kaposi sarcoma updates. Dermatol Clin. 2019;37:505-517.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
- Touloei K, Tongdee E, Smirnov B, et al. Diffuse dermal angiomatosis. Cutis. 2019;103:181-184.
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Baylor Univ Med Cent Proc. 2020;33:273-275.
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Etemad SA, Dewan AK. Kaposi sarcoma updates. Dermatol Clin. 2019;37:505-517.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
A 42-year-old woman with a medical history of hypertension and smoking tobacco (5 pack years) presented with a painful, nonhealing, violaceous, reticulated plaque with ulceration on the right breast of 3 months’ duration. Histopathology revealed diffuse, interstitial, bland-appearing spindle cells throughout the papillary and reticular dermis that were distributed between the collagen bundles. Dermal interstitial spindle cells were positive for CD31, CD34, and erythroblast transformation specific–related gene immunostains. Factor XIIIa and human herpesvirus 8 immunostaining was negative.
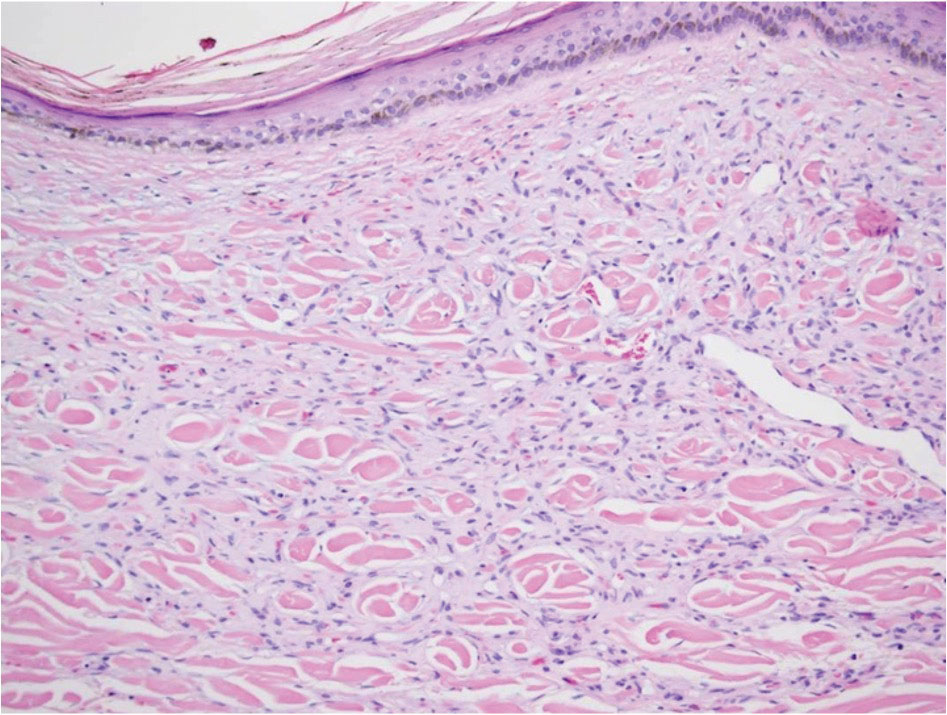
Ulcerated Nodule on the Lip
The Diagnosis: Cutaneous Metastasis
A shave biopsy of the lip revealed a diffuse cellular infiltrate filling the superficial and deep dermis (Figure 1A). Morphologically, the cells had abundant clear cytoplasm with eccentrically located, pleomorphic, hyperchromatic nuclei with occasional prominent nucleoli (Figure 1B). The cells stained positive for AE1/ AE3 on immunohistochemistry (Figure 2). A punch biopsy of the nodule in the right axillary vault revealed a morphologically similar proliferation of cells. A colonoscopy revealed a completely obstructing circumferential mass in the distal ascending colon. A biopsy of the mass confirmed invasive adenocarcinoma, supporting a diagnosis of cutaneous metastases from adenocarcinoma of the colon. The patient underwent resection of the lip tumor and started multiagent chemotherapy for his newly diagnosed stage IV adenocarcinoma of the colon. The patient died, despite therapy.

Cutaneous metastasis from solid malignancies is uncommon, as only 1.3% of them exhibit cutaneous manifestations at presentation.1 Cutaneous metastasis from signet ring cell adenocarcinoma (SRCA) of the colon is uncommon, and cutaneous metastasis of colorectal SRCA rarely precedes the diagnosis of the primary lesion.2 Among the colorectal cancers that metastasize to the skin, metastasis to the face occurs in only 0.5% of patients.3

Signet ring cell adenocarcinomas are poorly differentiated adenocarcinomas histologically characterized by the neoplastic cells’ circular to ovoid appearance with a flattened top.4,5 This distinctive shape is from the displacement of the nucleus to the periphery of the cell due to the accumulation of intracytoplasmic mucin.4 Classically, malignancies are characterized as an SRCA if more than 50% of the cells have a signet ring cell morphology; if the signet ring cells comprise less than 50% of the neoplasm, the tumor is designated as an adenocarcinoma with signet ring morphology.4 The most common cause of cutaneous metastasis with signet ring morphology is gastric cancer, while colorectal carcinoma is less common.1 Colorectal SRCAs usually are found in the right colon or the rectum in comparison to other colonic sites.6
Clinically, cutaneous metastasis can present in a variety of ways. The most common presentation is nodular lesions that may coalesce to become zosteriform in configuration or lesions that mimic inflammatory dermatoses.7 Cutaneous metastasis is more common in breast and lung cancer, and when it occurs secondary to colorectal cancer, cutaneous metastasis rarely predates the detection of the primary neoplasm.2
The clinical appearance of metastasis is not specific and can mimic many entities8; therefore, a high index of suspicion must be employed when managing patients, even those without a history of internal malignancy. In our patient, the smooth nodular lesion appeared similar to a basal cell carcinoma; however, basal cell carcinomas appear more pearly, and arborizing telangiectasia often is seen.9 Merkel cell carcinoma is common on sundamaged skin of the head and neck but clinically appears more violaceous than the lesion seen in our patient.10 Paracoccidioidomycosis may form ulcerated papulonodules or plaques, especially around the nose and mouth. In many of these cases, lesions develop from contiguous lesions of the oral mucosa; therefore, the presence of oral lesions will help distinguish this infectious entity from cutaneous metastasis. Multiple lesions usually are identified when there is hematogenous dissemination.11 Mycosis fungoides is a subtype of cutaneous T-cell lymphoma and is characterized by multiple patches, plaques, and nodules on sun-protected areas. Involvement of the head and neck is not common, except in the folliculotropic subtype, which has a separate and distinct clinical morphology.12
The development of signet ring morphology from an adenocarcinoma can be attributed to the activation of phosphatidylinositol 3-kinase (PI3K), which leads to downstream activation of mitogen-activated protein kinase (MAPK) and the subsequent loss of intercellular tight junctions. The mucin 4 gene, MUC4, also is upregulated by PI3K activation and possesses antiapoptotic and mitogenic effects in addition to its mucin secretory function.13
The neoplastic cells in SRCAs stain positive for mucicarmine, Alcian blue, and periodic acid–Schiff, which highlights the mucinous component of the cells.7 Immunohistochemical stains with CK7, CK20, AE1/AE3, and epithelial membrane antigen can be implemented to confirm an epithelial origin of the primary cancer.7,13 CK20 is a low-molecular-weight cytokeratin normally expressed by Merkel cells and by the epithelium of the gastrointestinal tract and urothelium, whereas CK7 expression typically is expressed in the lungs, ovaries, endometrium, and breasts, but not in the lower gastrointestinal tract.14 Differentiating primary cutaneous adenocarcinoma from cutaneous metastasis can be accomplished with a thorough clinical history; however, p63 positivity supports a primary cutaneous lesion and may be useful in certain situations.7 CDX2 stains can be utilized to aid in localizing the primary neoplasm when it is unknown, and when positive, it suggests a lower gastrointestinal tract origin. However, special AT-rich sequence-binding protein 2 (SATB2) recently has been proposed as a replacement immunohistochemical marker for CDX2, as it has greater specificity for SRCA of the lower gastrointestinal tract.15 Benign entities with signet ring cell morphology are difficult to distinguish from SRCA; however, malignant lesions are more likely to demonstrate an infiltrative growth pattern, frequent mitotic figures, and apoptosis. Immunohistochemistry also can be utilized to support the diagnosis of benign proliferation with signet ring morphology, as benign lesions often will demonstrate E-cadherin positivity and negativity for p53 and Ki-67.13
Cutaneous metastasis usually correlates to advanced disease and generally indicates a worse prognosis.13 Signet ring cell morphology in both gastric and colorectal cancer portends a poor prognosis, and there is a lower overall survival in patients with these malignancies compared to cancers of the same organ with non–signet ring cell morphology.4,8
- Mandzhieva B, Jalil A, Nadeem M, et al. Most common pathway of metastasis of rectal signet ring cell carcinoma to the skin: hematogenous. Cureus. 2020;12:E6890.
- Parente P, Ciardiello D, Reggiani Bonetti L, et al. Cutaneous metastasis from colorectal cancer: making light on an unusual and misdiagnosed event. Life. 2021;11:954.
- Picciariello A, Tomasicchio G, Lantone G, et al. Synchronous “skip” facial metastases from colorectal adenocarcinoma: a case report and review of literature. BMC Gastroenterol. 2022;22:68.
- Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers. 2020;12:1544.
- Xu Q, Karouji Y, Kobayashi M, et al. The PI 3-kinase-Rac-p38 MAP kinase pathway is involved in the formation of signet-ring cell carcinoma. Oncogene. 2003;22:5537-5544.
- Morales-Cruz M, Salgado-Nesme N, Trolle-Silva AM, et al. Signet ring cell carcinoma of the rectum: atypical metastatic presentation. BMJ Case Rep CP. 2019;12:E229135.
- Demirciog˘lu D, Öztürk Durmaz E, Demirkesen C, et al. Livedoid cutaneous metastasis of signet‐ring cell gastric carcinoma. J Cutan Pathol. 2021;48:785-788.
- Dong X, Sun G, Qu H, et al. Prognostic significance of signet-ring cell components in patients with gastric carcinoma of different stages. Front Surg. 2021;8:642468.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Nguyen AH, Tahseen AI, Vaudreuil AM, et al. Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review. Gynecol Oncol Res Pract. 2017;4:2.
- Marques, SA. Paracoccidioidomycosis. Clin Dermatol. 2012;30:610-615.
- Larocca C, Kupper T. Mycosis fungoides and Sézary syndrome. Hematol Oncol Clin. 2019;33:103-120.
- Gündüz Ö, Emeksiz MC, Atasoy P, et al. Signet-ring cells in the skin: a case of late-onset cutaneous metastasis of gastric carcinoma and a brief review of histological approach. Dermatol Rep. 2017;8:6819.
- Al-Taee A, Almukhtar R, Lai J, et al. Metastatic signet ring cell carcinoma of unknown primary origin: a case report and review of the literature. Ann Transl Med. 2016;4:283.
- Ma C, Lowenthal BM, Pai RK. SATB2 is superior to CDX2 in distinguishing signet ring cell carcinoma of the upper gastrointestinal tract and lower gastrointestinal tract. Am J Surg Pathol. 2018; 42:1715-1722.
The Diagnosis: Cutaneous Metastasis
A shave biopsy of the lip revealed a diffuse cellular infiltrate filling the superficial and deep dermis (Figure 1A). Morphologically, the cells had abundant clear cytoplasm with eccentrically located, pleomorphic, hyperchromatic nuclei with occasional prominent nucleoli (Figure 1B). The cells stained positive for AE1/ AE3 on immunohistochemistry (Figure 2). A punch biopsy of the nodule in the right axillary vault revealed a morphologically similar proliferation of cells. A colonoscopy revealed a completely obstructing circumferential mass in the distal ascending colon. A biopsy of the mass confirmed invasive adenocarcinoma, supporting a diagnosis of cutaneous metastases from adenocarcinoma of the colon. The patient underwent resection of the lip tumor and started multiagent chemotherapy for his newly diagnosed stage IV adenocarcinoma of the colon. The patient died, despite therapy.

Cutaneous metastasis from solid malignancies is uncommon, as only 1.3% of them exhibit cutaneous manifestations at presentation.1 Cutaneous metastasis from signet ring cell adenocarcinoma (SRCA) of the colon is uncommon, and cutaneous metastasis of colorectal SRCA rarely precedes the diagnosis of the primary lesion.2 Among the colorectal cancers that metastasize to the skin, metastasis to the face occurs in only 0.5% of patients.3

Signet ring cell adenocarcinomas are poorly differentiated adenocarcinomas histologically characterized by the neoplastic cells’ circular to ovoid appearance with a flattened top.4,5 This distinctive shape is from the displacement of the nucleus to the periphery of the cell due to the accumulation of intracytoplasmic mucin.4 Classically, malignancies are characterized as an SRCA if more than 50% of the cells have a signet ring cell morphology; if the signet ring cells comprise less than 50% of the neoplasm, the tumor is designated as an adenocarcinoma with signet ring morphology.4 The most common cause of cutaneous metastasis with signet ring morphology is gastric cancer, while colorectal carcinoma is less common.1 Colorectal SRCAs usually are found in the right colon or the rectum in comparison to other colonic sites.6
Clinically, cutaneous metastasis can present in a variety of ways. The most common presentation is nodular lesions that may coalesce to become zosteriform in configuration or lesions that mimic inflammatory dermatoses.7 Cutaneous metastasis is more common in breast and lung cancer, and when it occurs secondary to colorectal cancer, cutaneous metastasis rarely predates the detection of the primary neoplasm.2
The clinical appearance of metastasis is not specific and can mimic many entities8; therefore, a high index of suspicion must be employed when managing patients, even those without a history of internal malignancy. In our patient, the smooth nodular lesion appeared similar to a basal cell carcinoma; however, basal cell carcinomas appear more pearly, and arborizing telangiectasia often is seen.9 Merkel cell carcinoma is common on sundamaged skin of the head and neck but clinically appears more violaceous than the lesion seen in our patient.10 Paracoccidioidomycosis may form ulcerated papulonodules or plaques, especially around the nose and mouth. In many of these cases, lesions develop from contiguous lesions of the oral mucosa; therefore, the presence of oral lesions will help distinguish this infectious entity from cutaneous metastasis. Multiple lesions usually are identified when there is hematogenous dissemination.11 Mycosis fungoides is a subtype of cutaneous T-cell lymphoma and is characterized by multiple patches, plaques, and nodules on sun-protected areas. Involvement of the head and neck is not common, except in the folliculotropic subtype, which has a separate and distinct clinical morphology.12
The development of signet ring morphology from an adenocarcinoma can be attributed to the activation of phosphatidylinositol 3-kinase (PI3K), which leads to downstream activation of mitogen-activated protein kinase (MAPK) and the subsequent loss of intercellular tight junctions. The mucin 4 gene, MUC4, also is upregulated by PI3K activation and possesses antiapoptotic and mitogenic effects in addition to its mucin secretory function.13
The neoplastic cells in SRCAs stain positive for mucicarmine, Alcian blue, and periodic acid–Schiff, which highlights the mucinous component of the cells.7 Immunohistochemical stains with CK7, CK20, AE1/AE3, and epithelial membrane antigen can be implemented to confirm an epithelial origin of the primary cancer.7,13 CK20 is a low-molecular-weight cytokeratin normally expressed by Merkel cells and by the epithelium of the gastrointestinal tract and urothelium, whereas CK7 expression typically is expressed in the lungs, ovaries, endometrium, and breasts, but not in the lower gastrointestinal tract.14 Differentiating primary cutaneous adenocarcinoma from cutaneous metastasis can be accomplished with a thorough clinical history; however, p63 positivity supports a primary cutaneous lesion and may be useful in certain situations.7 CDX2 stains can be utilized to aid in localizing the primary neoplasm when it is unknown, and when positive, it suggests a lower gastrointestinal tract origin. However, special AT-rich sequence-binding protein 2 (SATB2) recently has been proposed as a replacement immunohistochemical marker for CDX2, as it has greater specificity for SRCA of the lower gastrointestinal tract.15 Benign entities with signet ring cell morphology are difficult to distinguish from SRCA; however, malignant lesions are more likely to demonstrate an infiltrative growth pattern, frequent mitotic figures, and apoptosis. Immunohistochemistry also can be utilized to support the diagnosis of benign proliferation with signet ring morphology, as benign lesions often will demonstrate E-cadherin positivity and negativity for p53 and Ki-67.13
Cutaneous metastasis usually correlates to advanced disease and generally indicates a worse prognosis.13 Signet ring cell morphology in both gastric and colorectal cancer portends a poor prognosis, and there is a lower overall survival in patients with these malignancies compared to cancers of the same organ with non–signet ring cell morphology.4,8
The Diagnosis: Cutaneous Metastasis
A shave biopsy of the lip revealed a diffuse cellular infiltrate filling the superficial and deep dermis (Figure 1A). Morphologically, the cells had abundant clear cytoplasm with eccentrically located, pleomorphic, hyperchromatic nuclei with occasional prominent nucleoli (Figure 1B). The cells stained positive for AE1/ AE3 on immunohistochemistry (Figure 2). A punch biopsy of the nodule in the right axillary vault revealed a morphologically similar proliferation of cells. A colonoscopy revealed a completely obstructing circumferential mass in the distal ascending colon. A biopsy of the mass confirmed invasive adenocarcinoma, supporting a diagnosis of cutaneous metastases from adenocarcinoma of the colon. The patient underwent resection of the lip tumor and started multiagent chemotherapy for his newly diagnosed stage IV adenocarcinoma of the colon. The patient died, despite therapy.

Cutaneous metastasis from solid malignancies is uncommon, as only 1.3% of them exhibit cutaneous manifestations at presentation.1 Cutaneous metastasis from signet ring cell adenocarcinoma (SRCA) of the colon is uncommon, and cutaneous metastasis of colorectal SRCA rarely precedes the diagnosis of the primary lesion.2 Among the colorectal cancers that metastasize to the skin, metastasis to the face occurs in only 0.5% of patients.3

Signet ring cell adenocarcinomas are poorly differentiated adenocarcinomas histologically characterized by the neoplastic cells’ circular to ovoid appearance with a flattened top.4,5 This distinctive shape is from the displacement of the nucleus to the periphery of the cell due to the accumulation of intracytoplasmic mucin.4 Classically, malignancies are characterized as an SRCA if more than 50% of the cells have a signet ring cell morphology; if the signet ring cells comprise less than 50% of the neoplasm, the tumor is designated as an adenocarcinoma with signet ring morphology.4 The most common cause of cutaneous metastasis with signet ring morphology is gastric cancer, while colorectal carcinoma is less common.1 Colorectal SRCAs usually are found in the right colon or the rectum in comparison to other colonic sites.6
Clinically, cutaneous metastasis can present in a variety of ways. The most common presentation is nodular lesions that may coalesce to become zosteriform in configuration or lesions that mimic inflammatory dermatoses.7 Cutaneous metastasis is more common in breast and lung cancer, and when it occurs secondary to colorectal cancer, cutaneous metastasis rarely predates the detection of the primary neoplasm.2
The clinical appearance of metastasis is not specific and can mimic many entities8; therefore, a high index of suspicion must be employed when managing patients, even those without a history of internal malignancy. In our patient, the smooth nodular lesion appeared similar to a basal cell carcinoma; however, basal cell carcinomas appear more pearly, and arborizing telangiectasia often is seen.9 Merkel cell carcinoma is common on sundamaged skin of the head and neck but clinically appears more violaceous than the lesion seen in our patient.10 Paracoccidioidomycosis may form ulcerated papulonodules or plaques, especially around the nose and mouth. In many of these cases, lesions develop from contiguous lesions of the oral mucosa; therefore, the presence of oral lesions will help distinguish this infectious entity from cutaneous metastasis. Multiple lesions usually are identified when there is hematogenous dissemination.11 Mycosis fungoides is a subtype of cutaneous T-cell lymphoma and is characterized by multiple patches, plaques, and nodules on sun-protected areas. Involvement of the head and neck is not common, except in the folliculotropic subtype, which has a separate and distinct clinical morphology.12
The development of signet ring morphology from an adenocarcinoma can be attributed to the activation of phosphatidylinositol 3-kinase (PI3K), which leads to downstream activation of mitogen-activated protein kinase (MAPK) and the subsequent loss of intercellular tight junctions. The mucin 4 gene, MUC4, also is upregulated by PI3K activation and possesses antiapoptotic and mitogenic effects in addition to its mucin secretory function.13
The neoplastic cells in SRCAs stain positive for mucicarmine, Alcian blue, and periodic acid–Schiff, which highlights the mucinous component of the cells.7 Immunohistochemical stains with CK7, CK20, AE1/AE3, and epithelial membrane antigen can be implemented to confirm an epithelial origin of the primary cancer.7,13 CK20 is a low-molecular-weight cytokeratin normally expressed by Merkel cells and by the epithelium of the gastrointestinal tract and urothelium, whereas CK7 expression typically is expressed in the lungs, ovaries, endometrium, and breasts, but not in the lower gastrointestinal tract.14 Differentiating primary cutaneous adenocarcinoma from cutaneous metastasis can be accomplished with a thorough clinical history; however, p63 positivity supports a primary cutaneous lesion and may be useful in certain situations.7 CDX2 stains can be utilized to aid in localizing the primary neoplasm when it is unknown, and when positive, it suggests a lower gastrointestinal tract origin. However, special AT-rich sequence-binding protein 2 (SATB2) recently has been proposed as a replacement immunohistochemical marker for CDX2, as it has greater specificity for SRCA of the lower gastrointestinal tract.15 Benign entities with signet ring cell morphology are difficult to distinguish from SRCA; however, malignant lesions are more likely to demonstrate an infiltrative growth pattern, frequent mitotic figures, and apoptosis. Immunohistochemistry also can be utilized to support the diagnosis of benign proliferation with signet ring morphology, as benign lesions often will demonstrate E-cadherin positivity and negativity for p53 and Ki-67.13
Cutaneous metastasis usually correlates to advanced disease and generally indicates a worse prognosis.13 Signet ring cell morphology in both gastric and colorectal cancer portends a poor prognosis, and there is a lower overall survival in patients with these malignancies compared to cancers of the same organ with non–signet ring cell morphology.4,8
- Mandzhieva B, Jalil A, Nadeem M, et al. Most common pathway of metastasis of rectal signet ring cell carcinoma to the skin: hematogenous. Cureus. 2020;12:E6890.
- Parente P, Ciardiello D, Reggiani Bonetti L, et al. Cutaneous metastasis from colorectal cancer: making light on an unusual and misdiagnosed event. Life. 2021;11:954.
- Picciariello A, Tomasicchio G, Lantone G, et al. Synchronous “skip” facial metastases from colorectal adenocarcinoma: a case report and review of literature. BMC Gastroenterol. 2022;22:68.
- Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers. 2020;12:1544.
- Xu Q, Karouji Y, Kobayashi M, et al. The PI 3-kinase-Rac-p38 MAP kinase pathway is involved in the formation of signet-ring cell carcinoma. Oncogene. 2003;22:5537-5544.
- Morales-Cruz M, Salgado-Nesme N, Trolle-Silva AM, et al. Signet ring cell carcinoma of the rectum: atypical metastatic presentation. BMJ Case Rep CP. 2019;12:E229135.
- Demirciog˘lu D, Öztürk Durmaz E, Demirkesen C, et al. Livedoid cutaneous metastasis of signet‐ring cell gastric carcinoma. J Cutan Pathol. 2021;48:785-788.
- Dong X, Sun G, Qu H, et al. Prognostic significance of signet-ring cell components in patients with gastric carcinoma of different stages. Front Surg. 2021;8:642468.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Nguyen AH, Tahseen AI, Vaudreuil AM, et al. Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review. Gynecol Oncol Res Pract. 2017;4:2.
- Marques, SA. Paracoccidioidomycosis. Clin Dermatol. 2012;30:610-615.
- Larocca C, Kupper T. Mycosis fungoides and Sézary syndrome. Hematol Oncol Clin. 2019;33:103-120.
- Gündüz Ö, Emeksiz MC, Atasoy P, et al. Signet-ring cells in the skin: a case of late-onset cutaneous metastasis of gastric carcinoma and a brief review of histological approach. Dermatol Rep. 2017;8:6819.
- Al-Taee A, Almukhtar R, Lai J, et al. Metastatic signet ring cell carcinoma of unknown primary origin: a case report and review of the literature. Ann Transl Med. 2016;4:283.
- Ma C, Lowenthal BM, Pai RK. SATB2 is superior to CDX2 in distinguishing signet ring cell carcinoma of the upper gastrointestinal tract and lower gastrointestinal tract. Am J Surg Pathol. 2018; 42:1715-1722.
- Mandzhieva B, Jalil A, Nadeem M, et al. Most common pathway of metastasis of rectal signet ring cell carcinoma to the skin: hematogenous. Cureus. 2020;12:E6890.
- Parente P, Ciardiello D, Reggiani Bonetti L, et al. Cutaneous metastasis from colorectal cancer: making light on an unusual and misdiagnosed event. Life. 2021;11:954.
- Picciariello A, Tomasicchio G, Lantone G, et al. Synchronous “skip” facial metastases from colorectal adenocarcinoma: a case report and review of literature. BMC Gastroenterol. 2022;22:68.
- Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers. 2020;12:1544.
- Xu Q, Karouji Y, Kobayashi M, et al. The PI 3-kinase-Rac-p38 MAP kinase pathway is involved in the formation of signet-ring cell carcinoma. Oncogene. 2003;22:5537-5544.
- Morales-Cruz M, Salgado-Nesme N, Trolle-Silva AM, et al. Signet ring cell carcinoma of the rectum: atypical metastatic presentation. BMJ Case Rep CP. 2019;12:E229135.
- Demirciog˘lu D, Öztürk Durmaz E, Demirkesen C, et al. Livedoid cutaneous metastasis of signet‐ring cell gastric carcinoma. J Cutan Pathol. 2021;48:785-788.
- Dong X, Sun G, Qu H, et al. Prognostic significance of signet-ring cell components in patients with gastric carcinoma of different stages. Front Surg. 2021;8:642468.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Nguyen AH, Tahseen AI, Vaudreuil AM, et al. Clinical features and treatment of vulvar Merkel cell carcinoma: a systematic review. Gynecol Oncol Res Pract. 2017;4:2.
- Marques, SA. Paracoccidioidomycosis. Clin Dermatol. 2012;30:610-615.
- Larocca C, Kupper T. Mycosis fungoides and Sézary syndrome. Hematol Oncol Clin. 2019;33:103-120.
- Gündüz Ö, Emeksiz MC, Atasoy P, et al. Signet-ring cells in the skin: a case of late-onset cutaneous metastasis of gastric carcinoma and a brief review of histological approach. Dermatol Rep. 2017;8:6819.
- Al-Taee A, Almukhtar R, Lai J, et al. Metastatic signet ring cell carcinoma of unknown primary origin: a case report and review of the literature. Ann Transl Med. 2016;4:283.
- Ma C, Lowenthal BM, Pai RK. SATB2 is superior to CDX2 in distinguishing signet ring cell carcinoma of the upper gastrointestinal tract and lower gastrointestinal tract. Am J Surg Pathol. 2018; 42:1715-1722.
A 79-year-old man with a medical history of type 2 diabetes mellitus, hypothyroidism, and atrial fibrillation presented with an enlarging lesion on the right side of the upper cutaneous lip of 5 weeks’ duration. He had no personal history of skin cancer or other malignancy and was up to date on all routine cancer screenings. He reported associated lip and oral cavity tenderness, weakness, and a 13.6-kg (30-lb) unintentional weight loss over the last 6 months. He had used over-the-counter bacitracin ointment on the lesion without relief. A full-body skin examination revealed a firm, mobile, flesh-colored, nondraining nodule in the right axillary vault.
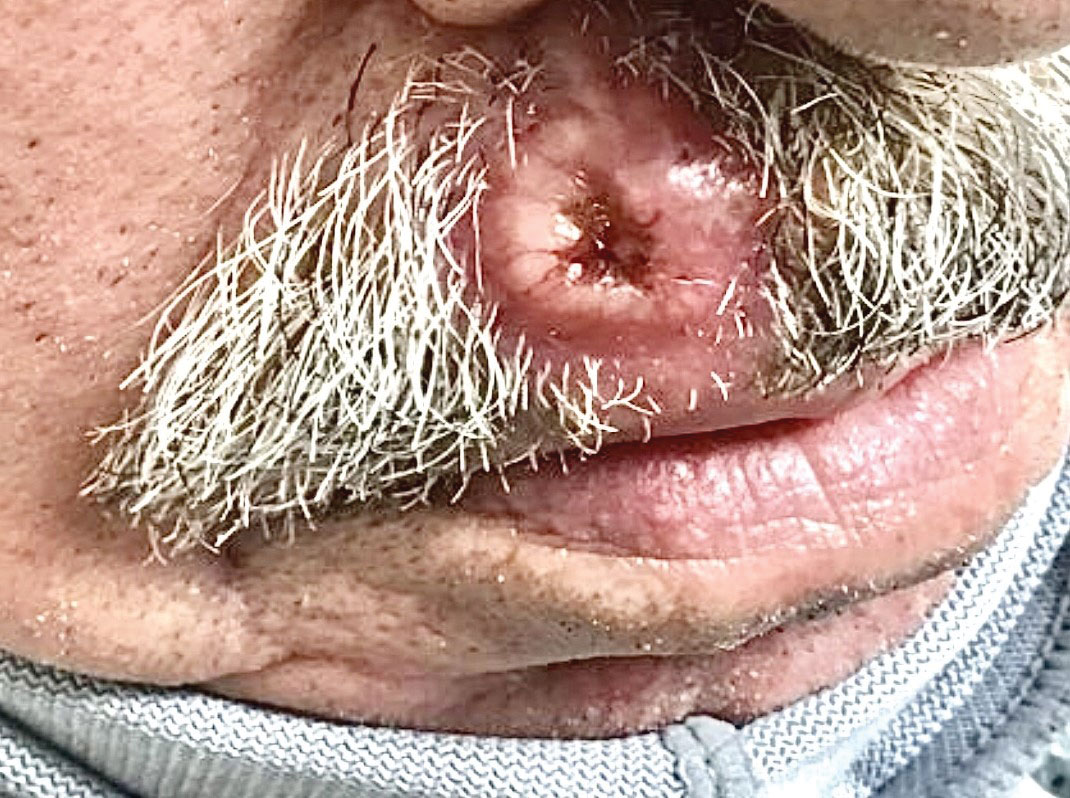
White Spots on the Extremities
The Diagnosis: Hypopigmented Mycosis Fungoides
Histopathology showed an atypical lymphoid infiltrate with expanded cytoplasm and hyperchromatic nuclei of irregular contours in the dermoepidermal junction (Figure 1). Immunohistochemical stains of atypical lymphocytes demonstrated the presence of CD3, CD8, and CD5, as well as the absence of CD7 and CD4 lymphocytes (Figure 2). The T-cell γ rearrangement showed polyclonal lymphocytes with 5% tumor cells. The histologic and clinical findings along with our patient’s medical history led to a diagnosis of stage IA (<10% body surface area involvement) hypopigmented mycosis fungoides (hMF).1 Our patient was treated with triamcinolone cream 0.1%; she noted an improvement in her symptoms at 2-month follow-up.

Hypopigmented MF is an uncommon manifestation of MF with unknown prevalence and incidence rates. Mycosis fungoides is considered the most common subtype of cutaneous T-cell lymphoma that classically presents as a chronic, indolent, hypopigmented or depigmented macule or patch, commonly with scaling, in sunprotected areas such as the trunk and proximal arms and legs. It predominantly affects younger adults with darker skin tones and may be present in the pediatric population within the first decade of life.1 Classically, MF affects White patients aged 55 to 60 years. Disease progression is slow, with an incidence rate of 10% of tumor or extracutaneous involvement in the early stages of disease. A lack of specificity on the clinical and histopathologic findings in the initial stage often contributes to the diagnostic delay of hMF. As seen in our patient, this disease can be misdiagnosed as tinea versicolor, postinflammatory hypopigmentation, vitiligo, pityriasis alba, subcutaneous lupus erythematosus, or Hansen disease due to prolonged hypopigmented lesions.2 The clinical findings and histopathologic results including immunohistochemistry confirmed the diagnosis of hMF and ruled out pityriasis alba, postinflammatory hypopigmentation, subcutaneous lupus erythematosus, and vitiligo.

The etiology and pathophysiology of hMF are not fully understood; however, it is hypothesized that melanocyte degeneration, abnormal melanogenesis, and disturbance of melanosome transfer result from the clonal expansion of T helper memory cells. T-cell dyscrasia has been reported to evolve into hMF during etanercept therapy.3 Clinically, hMF presents as hypopigmented papulosquamous, eczematous, or erythrodermic patches, plaques, and tumors with poorly defined atrophied borders. Multiple biopsies of steroid-naive lesions are needed for the diagnosis, as the initial hMF histologic finding cannot be specific for diagnostic confirmation. Common histopathologic findings include a bandlike lymphocytic infiltrate with epidermotropism, intraepidermal nests of atypical cells, or cerebriform nuclei lymphocytes on hematoxylin and eosin staining. In comparison to classical MF epidermotropism, CD4− and CD8+ atypical cells aid in the diagnosis of hMF. Although hMF carries a good prognosis and a benign clinical course,4 full-body computed tomography or positron emission tomography/computed tomography as well as laboratory analysis for lactate dehydrogenase should be pursued if lymphadenopathy, systemic symptoms, or advancedstage hMF are present.
Treatment of hMF depends on the disease stage. Psoralen plus UVA and narrowband UVB can be utilized for the initial stages with a relatively fast response and remission of lesions as early as the first 2 months of treatment. In addition to phototherapy, stage IA to IIA mycosis fungoides with localized skin lesions can benefit from topical steroids, topical retinoids, imiquimod, nitrogen mustard, and carmustine. For advanced stages of mycosis fungoides, combination therapy consisting of psoralen plus UVA with an oral retinoid, interferon alfa, and systemic chemotherapy commonly are prescribed. Maintenance therapy is used for prolonging remission; however, long-term phototherapy is not recommended due to the risk for skin cancer. Unfortunately, hMF requires long-term treatment due to its waxing and waning course, and recurrence may occur after complete resolution.5
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Lambroza E, Cohen SR, Lebwohl M, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- Chuang GS, Wasserman DI, Byers HR, et al. Hypopigmented T-cell dyscrasia evolving to hypopigmented mycosis fungoides during etanercept therapy. J Am Acad Dermatol. 2008;59(5 suppl):S121-S122.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/ European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014; 70:223.e1-17; quiz 240-242.
The Diagnosis: Hypopigmented Mycosis Fungoides
Histopathology showed an atypical lymphoid infiltrate with expanded cytoplasm and hyperchromatic nuclei of irregular contours in the dermoepidermal junction (Figure 1). Immunohistochemical stains of atypical lymphocytes demonstrated the presence of CD3, CD8, and CD5, as well as the absence of CD7 and CD4 lymphocytes (Figure 2). The T-cell γ rearrangement showed polyclonal lymphocytes with 5% tumor cells. The histologic and clinical findings along with our patient’s medical history led to a diagnosis of stage IA (<10% body surface area involvement) hypopigmented mycosis fungoides (hMF).1 Our patient was treated with triamcinolone cream 0.1%; she noted an improvement in her symptoms at 2-month follow-up.

Hypopigmented MF is an uncommon manifestation of MF with unknown prevalence and incidence rates. Mycosis fungoides is considered the most common subtype of cutaneous T-cell lymphoma that classically presents as a chronic, indolent, hypopigmented or depigmented macule or patch, commonly with scaling, in sunprotected areas such as the trunk and proximal arms and legs. It predominantly affects younger adults with darker skin tones and may be present in the pediatric population within the first decade of life.1 Classically, MF affects White patients aged 55 to 60 years. Disease progression is slow, with an incidence rate of 10% of tumor or extracutaneous involvement in the early stages of disease. A lack of specificity on the clinical and histopathologic findings in the initial stage often contributes to the diagnostic delay of hMF. As seen in our patient, this disease can be misdiagnosed as tinea versicolor, postinflammatory hypopigmentation, vitiligo, pityriasis alba, subcutaneous lupus erythematosus, or Hansen disease due to prolonged hypopigmented lesions.2 The clinical findings and histopathologic results including immunohistochemistry confirmed the diagnosis of hMF and ruled out pityriasis alba, postinflammatory hypopigmentation, subcutaneous lupus erythematosus, and vitiligo.

The etiology and pathophysiology of hMF are not fully understood; however, it is hypothesized that melanocyte degeneration, abnormal melanogenesis, and disturbance of melanosome transfer result from the clonal expansion of T helper memory cells. T-cell dyscrasia has been reported to evolve into hMF during etanercept therapy.3 Clinically, hMF presents as hypopigmented papulosquamous, eczematous, or erythrodermic patches, plaques, and tumors with poorly defined atrophied borders. Multiple biopsies of steroid-naive lesions are needed for the diagnosis, as the initial hMF histologic finding cannot be specific for diagnostic confirmation. Common histopathologic findings include a bandlike lymphocytic infiltrate with epidermotropism, intraepidermal nests of atypical cells, or cerebriform nuclei lymphocytes on hematoxylin and eosin staining. In comparison to classical MF epidermotropism, CD4− and CD8+ atypical cells aid in the diagnosis of hMF. Although hMF carries a good prognosis and a benign clinical course,4 full-body computed tomography or positron emission tomography/computed tomography as well as laboratory analysis for lactate dehydrogenase should be pursued if lymphadenopathy, systemic symptoms, or advancedstage hMF are present.
Treatment of hMF depends on the disease stage. Psoralen plus UVA and narrowband UVB can be utilized for the initial stages with a relatively fast response and remission of lesions as early as the first 2 months of treatment. In addition to phototherapy, stage IA to IIA mycosis fungoides with localized skin lesions can benefit from topical steroids, topical retinoids, imiquimod, nitrogen mustard, and carmustine. For advanced stages of mycosis fungoides, combination therapy consisting of psoralen plus UVA with an oral retinoid, interferon alfa, and systemic chemotherapy commonly are prescribed. Maintenance therapy is used for prolonging remission; however, long-term phototherapy is not recommended due to the risk for skin cancer. Unfortunately, hMF requires long-term treatment due to its waxing and waning course, and recurrence may occur after complete resolution.5
The Diagnosis: Hypopigmented Mycosis Fungoides
Histopathology showed an atypical lymphoid infiltrate with expanded cytoplasm and hyperchromatic nuclei of irregular contours in the dermoepidermal junction (Figure 1). Immunohistochemical stains of atypical lymphocytes demonstrated the presence of CD3, CD8, and CD5, as well as the absence of CD7 and CD4 lymphocytes (Figure 2). The T-cell γ rearrangement showed polyclonal lymphocytes with 5% tumor cells. The histologic and clinical findings along with our patient’s medical history led to a diagnosis of stage IA (<10% body surface area involvement) hypopigmented mycosis fungoides (hMF).1 Our patient was treated with triamcinolone cream 0.1%; she noted an improvement in her symptoms at 2-month follow-up.

Hypopigmented MF is an uncommon manifestation of MF with unknown prevalence and incidence rates. Mycosis fungoides is considered the most common subtype of cutaneous T-cell lymphoma that classically presents as a chronic, indolent, hypopigmented or depigmented macule or patch, commonly with scaling, in sunprotected areas such as the trunk and proximal arms and legs. It predominantly affects younger adults with darker skin tones and may be present in the pediatric population within the first decade of life.1 Classically, MF affects White patients aged 55 to 60 years. Disease progression is slow, with an incidence rate of 10% of tumor or extracutaneous involvement in the early stages of disease. A lack of specificity on the clinical and histopathologic findings in the initial stage often contributes to the diagnostic delay of hMF. As seen in our patient, this disease can be misdiagnosed as tinea versicolor, postinflammatory hypopigmentation, vitiligo, pityriasis alba, subcutaneous lupus erythematosus, or Hansen disease due to prolonged hypopigmented lesions.2 The clinical findings and histopathologic results including immunohistochemistry confirmed the diagnosis of hMF and ruled out pityriasis alba, postinflammatory hypopigmentation, subcutaneous lupus erythematosus, and vitiligo.

The etiology and pathophysiology of hMF are not fully understood; however, it is hypothesized that melanocyte degeneration, abnormal melanogenesis, and disturbance of melanosome transfer result from the clonal expansion of T helper memory cells. T-cell dyscrasia has been reported to evolve into hMF during etanercept therapy.3 Clinically, hMF presents as hypopigmented papulosquamous, eczematous, or erythrodermic patches, plaques, and tumors with poorly defined atrophied borders. Multiple biopsies of steroid-naive lesions are needed for the diagnosis, as the initial hMF histologic finding cannot be specific for diagnostic confirmation. Common histopathologic findings include a bandlike lymphocytic infiltrate with epidermotropism, intraepidermal nests of atypical cells, or cerebriform nuclei lymphocytes on hematoxylin and eosin staining. In comparison to classical MF epidermotropism, CD4− and CD8+ atypical cells aid in the diagnosis of hMF. Although hMF carries a good prognosis and a benign clinical course,4 full-body computed tomography or positron emission tomography/computed tomography as well as laboratory analysis for lactate dehydrogenase should be pursued if lymphadenopathy, systemic symptoms, or advancedstage hMF are present.
Treatment of hMF depends on the disease stage. Psoralen plus UVA and narrowband UVB can be utilized for the initial stages with a relatively fast response and remission of lesions as early as the first 2 months of treatment. In addition to phototherapy, stage IA to IIA mycosis fungoides with localized skin lesions can benefit from topical steroids, topical retinoids, imiquimod, nitrogen mustard, and carmustine. For advanced stages of mycosis fungoides, combination therapy consisting of psoralen plus UVA with an oral retinoid, interferon alfa, and systemic chemotherapy commonly are prescribed. Maintenance therapy is used for prolonging remission; however, long-term phototherapy is not recommended due to the risk for skin cancer. Unfortunately, hMF requires long-term treatment due to its waxing and waning course, and recurrence may occur after complete resolution.5
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Lambroza E, Cohen SR, Lebwohl M, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- Chuang GS, Wasserman DI, Byers HR, et al. Hypopigmented T-cell dyscrasia evolving to hypopigmented mycosis fungoides during etanercept therapy. J Am Acad Dermatol. 2008;59(5 suppl):S121-S122.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/ European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014; 70:223.e1-17; quiz 240-242.
- Furlan FC, Sanches JA. Hypopigmented mycosis fungoides: a review of its clinical features and pathophysiology. An Bras Dermatol. 2013;88:954-960.
- Lambroza E, Cohen SR, Lebwohl M, et al. Hypopigmented variant of mycosis fungoides: demography, histopathology, and treatment of seven cases. J Am Acad Dermatol. 1995;32:987-993.
- Chuang GS, Wasserman DI, Byers HR, et al. Hypopigmented T-cell dyscrasia evolving to hypopigmented mycosis fungoides during etanercept therapy. J Am Acad Dermatol. 2008;59(5 suppl):S121-S122.
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/ European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014; 70:223.e1-17; quiz 240-242.
A 52-year-old Black woman presented with self-described whitened spots on the arms and legs of 2 years’ duration. She experienced no improvement with ketoconazole cream and topical calcineurin inhibitors prescribed during a prior dermatology visit at an outside institution. She denied pain or pruritus. A review of systems as well as the patient’s medical history were noncontributory. A prior biopsy at an outside institution revealed an interface dermatitis suggestive of cutaneous lupus erythematosus. The patient noted social drinking and denied tobacco use. She had no known allergies to medications and currently was on tamoxifen for breast cancer following a right mastectomy. Physical examination showed hypopigmented macules and patches on the left upper arm and right proximal leg. The center of the lesions was not erythematous or scaly. Palpation did not reveal enlarged lymph nodes, and laboratory analyses ruled out low levels of red blood cells, white blood cells, or platelets. Punch biopsies from the left arm and right thigh were performed.