User login
Aberrant Expression of CD56 in Metastatic Malignant Melanoma
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
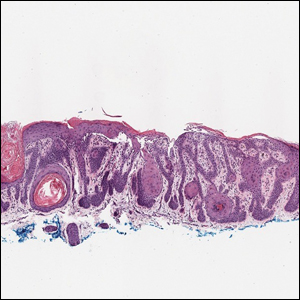
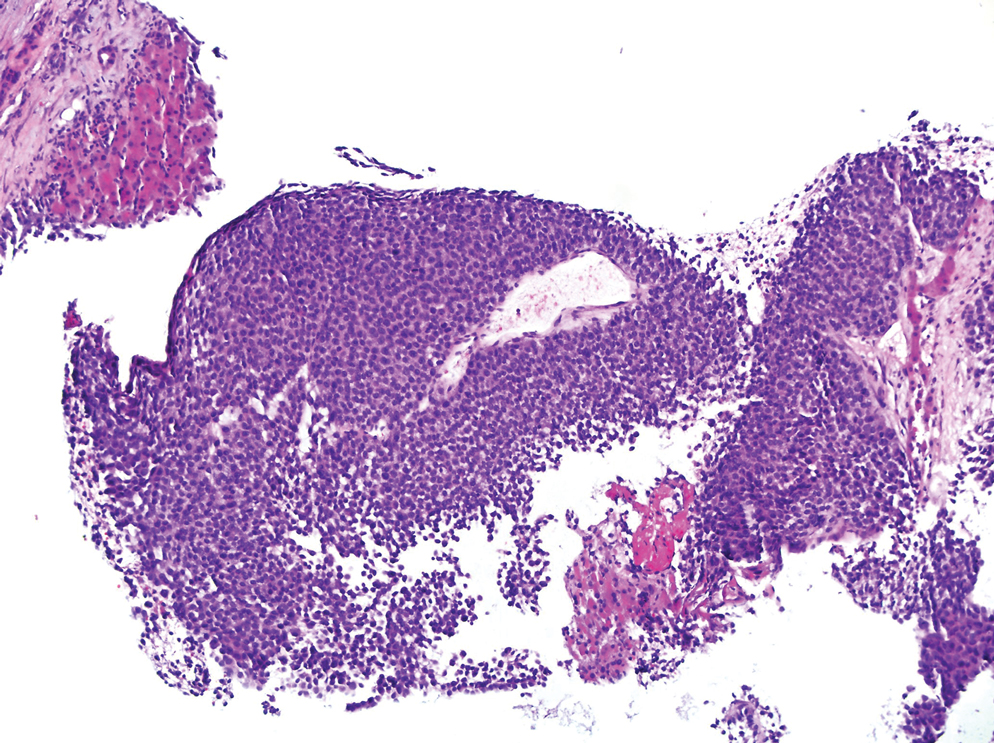
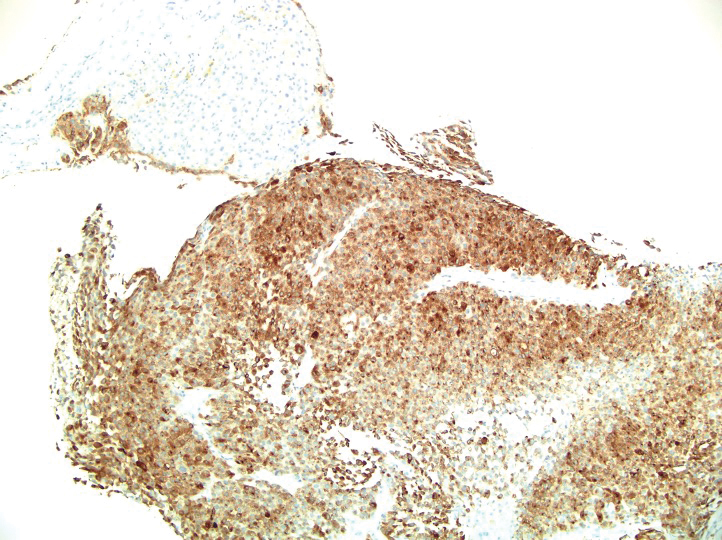
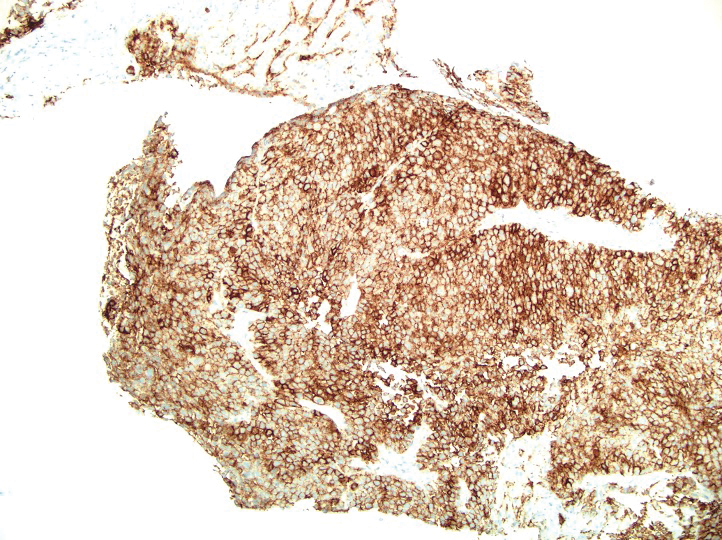
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.
BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.
More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5
Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.

BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.

More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5

Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
To the Editor:
Many types of neoplasms can show aberrant immunoreactivity or unexpected expression of markers.1 Malignant melanoma is a tumor that can show not only aberrant immunohistochemical staining patterns but also notable histologic diversity,1,2 which often makes the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.2
The incidence of malignant melanoma continues to grow.3 Maintaining a high degree of suspicion for this disease, recognizing its heterogeneity and divergent differentiation, and knowing potential aberrant immunohistochemical staining patterns are imperative for accurate diagnosis.
A 36-year-old man presented to a primary care physician with right-sided chest pain, upper and lower back aches, bilateral hip pain, neck pain, headache, night sweats, chills, and nausea. After infectious causes were ruled out, he was placed on a steroid taper without improvement. He presented to the emergency department a few days later with muscle spasms and was found to also have diffuse abdominal tenderness and guarding. The patient’s medical history was noncontributory; he was a lifelong nonsmoker. Laboratory studies revealed elevated levels of alanine aminotransferase and C-reactive protein. Computed tomography of the chest and abdomen revealed innumerable liver and lung lesions that were suspicious for metastatic malignancy. A liver biopsy revealed nests and sheets of metastatic tumor with pleomorphic nuclei, inconspicuous nucleoli, and areas of intranuclear clearing (Figures 1 and 2). Immunohistochemical staining was performed to further characterize the tumor. Neoplastic cells were positive for MART-1 (also known as Melan-A and melanoma-associated antigen recognized by T cells)(Figure 3), SOX10, S-100, HMB-45, and vimentin. Nonspecific staining with CD56 (Figure 4), a neuroendocrine marker, also was noted; however, the neoplasm was negative for synaptophysin, another neuroendocrine marker. Other markers for which staining was negative included pan-keratin, CD138 (syndecan-1), desmin, placental alkaline phosphatase (PLAP), inhibin, OCT-4, cytokeratin 7, and cytokeratin 20. This staining pattern was compatible with metastatic melanoma with aberrant CD56 expression.

BRAF V600E immunohistochemical staining also was performed and showed strong and diffuse positivity within neoplastic cells. A subsequent positron emission tomography scan revealed widespread metastatic disease involving the lungs, liver, spleen, and bones. The patient did not have a history of an excised skin lesion; no primary cutaneous or mucosal lesions were identified.

The patient was started on targeted therapy with trametinib, a mitogen-activated extracellular signal-related kinase kinase (MEK) inhibitor, and dabrafenib, a BRAF inhibitor. The disease continued to progress; he developed extensive leptomeningeal metastatic disease for which palliative radiation therapy was administered. The patient died 4 months after the initial diagnosis.

More than 90% of melanoma cases are of cutaneous origin; however, 4% to 8% of cases present as a metastatic lesion in the absence of an identified primary lesion,4 similar to our patient. The diagnosis of melanoma often is challenging; the tumor can show notable histologic diversity and has the potential to express aberrant immunophenotypes.1,2 The histologic diversity of melanoma includes a variety of architectural patterns (eg, nests, trabeculae, fascicular, pseudoglandular, pseudopapillary, or pseudorosette patterns), cytomorphologic features, and stromal changes. Cytomorphologic features of melanoma can be large pleomorphic cells; small cells; spindle cells; clear cells; signet-ring cells; and rhabdoid, plasmacytoid, and balloon cells.5

Melanoma can mimic carcinoma, sarcoma, lymphoma, benign stromal tumors, plasmacytoma, and germ-cell tumors.5 Nuclei can binucleated, multinucleated, or lobated and may contain inclusions or grooves. Stroma may become myxoid or desmoplastic in appearance or rarely show granulomatous inflammation or osteoclastic giant cells.5 These variations render the diagnosis of melanoma challenging and ultimately can lead to diagnostic uncertainty.
Melanomas typically express MART-1, HMB-45, S-100, tyrosinase, NK1C3, vimentin, and neuron-specific enolase. However, melanoma is among the many neoplasms that sometimes exhibit aberrant immunoreactivity and differentiation toward nonmelanocytic elements.6 The most commonly expressed immunophenotypic aberration is cytokeratin, especially the low-molecular-weight keratin marker CAM5.2.5 CAM5.2 positivity also is seen more often in metastatic melanoma. Melanomas rarely express other intermediate filaments, including desmin, neurofilament protein, and glial fibrillary acidic protein; expression of smooth-muscle actin is rare.5
Only a few cases of melanoma showing expression of neuroendocrine markers have been reported. However, one study reported synaptophysin positivity in 29% (10/34) of cases of primary and metastatic melanoma, making the stain a relatively common finding.1
In contrast, expression of CD56 (also known as neural-cell adhesion molecule 1) in melanoma has been reported only rarely. CD56 is a nonspecific neuroendocrine marker that normally is expressed on neurons, glial tissue, skeletal muscle, and natural killer cells. Riddle and Bui7 reported a case of metastatic malignant melanoma with focal CD56 positivity and no expression of other neuroendocrine markers, similar to our patient. Suzuki and colleagues4 also reported a case of melanoma metastatic to bone marrow that showed CD56 expression in true nonhematologic tumor cells and negative immunoreactivity with synaptophysin and chromogranin A.
It is important to document cases of melanoma that express neuroendocrine markers to prevent an incorrect diagnosis of a neuroendocrine tumor.1 In some cases, distinguishing amelanotic melanoma from poorly differentiated squamous cell carcinoma, neuroendocrine tumor, and lymphoma can be difficult.5
The term neuroendocrine differentiation is reserved for cases of melanoma that show areas of ultrastructural change consistent with a neuroendocrine tumor.2 Neuroendocrine differentiation in melanoma is not common; its prognostic significance is unknown.8 We do not consider our case to be true neuroendocrine differentiation, as the tumor lacked the morphologic changes of a neuroendocrine tumor. Furthermore, CD56 is a nonspecific neuroendocrine marker, and the tumor was negative for synaptophysin.
Melanoma has the potential to show notable histologic diversity as well as aberrant immunohistochemical staining patterns.1,2 Our patient had metastatic melanoma with aberrant neuroendocrine expression of CD56, which could have been a potential diagnostic pitfall. Because expression of CD56 in melanoma is rare, it is imperative to recognize this potential aberrant staining pattern to ensure the accurate diagnosis of melanoma and appropriate provision of care.
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
1. Romano RC, Carter JM, Folpe AL. Aberrant intermediate filament and synaptophysin expression is a frequent event in malignant melanoma: an immunohistochemical study of 73 cases. Mod Pathol. 2015;28:1033-1042. doi:10.1038/modpathol.2015.62
2. Eyden B, Pandit D, Banerjee SS. Malignant melanoma with neuroendocrine differentiation: clinical, histological, immunohistochemical and ultrastructural features of three cases. Histopathology. 2005;47:402-409. doi:10.1111/j.1365-2559.2005.02240.x
3. Katerji H, Childs JM, Bratton LE, et al. Primary esophageal melanoma with aberrant CD56 expression: a potential diagnostic pitfall. Case Rep Pathol. 2017;2017:9052637. doi:10.1155/2017/9052637
4. Suzuki T, Kusumoto S, Iida S, et al. Amelanotic malignant melanoma of unknown primary origin metastasizing to the bone marrow: a case report and review of the literature. Intern Med. 2014;53:325-328. doi:10.2169/internalmedicine.53.1412
5. Banerjee SS, Harris M. Morphological and immunophenotypic variations in malignant melanoma. Histopathology. 2000;36:387-402. doi:10.1046/j.1365-2559.2000.00894.x
6. Banerjee SS, Eyden B. Divergent differentiation in malignant melanomas: a review. Histopathology. 2008;52:119-129. doi:10.1111/j.1365-2559.2007.02823.x
7. Riddle ND, Bui MM. When melanoma is negative for S100: diagnostic pitfalls. Arch Pathol Lab Med. 2012;136:237-239. doi:10.5858/arpa.2011-0405-LE
8. Ilardi G, Caroppo D, Varricchio S, et al. Anal melanoma with neuroendocrine differentiation: report of a case. Int J Surg Pathol. 2015;23:329-332. doi:10.1177/1066896915573568
Practice Points
- The diagnosis of melanoma often is challenging as tumors can show notable histologic diversity and have the potential to express aberrant immunophenotypes including CD56 expression.
- Because expression of CD56 in melanoma is rare, it is important to be aware of this potential aberrant staining pattern.
- Recognizing this heterogeneity and divergent differentiation as well as knowing potential aberrant immunohistochemical staining patterns are imperative for accurate and timely diagnosis.
Specialty-trained pathologists more likely to make higher-grade diagnoses for melanocytic lesions
, results from an exploratory study showed.
The findings “could in part play a role in the rising incidence of early-stage melanoma with low risk of progression or patient morbidity, thereby contributing to increasing rates of overdiagnosis,” researchers led by co–senior authors Joann G. Elmore, MD, MPH, of the University of California, Los Angeles, and Raymond L. Barnhill, MD, MBA, of the Institut Curie, Paris, wrote in their study, published online in JAMA Dermatology.
To investigate the characteristics associated with rendering higher-grade diagnoses, including invasive melanoma, the researchers drew from two national data sets: the Melanoma Pathology (M-Path) study, conducted from July 2013 to May 2016, and the Reducing Errors in Melanocytic Interpretations (REMI) study, conducted from August 2018 to March 2021. In both studies, pathologists who interpreted melanocytic lesions in their clinical practices interpreted study cases in glass slide format. For the current study, researchers used logistic regression to examine the association of pathologist characteristics with diagnosis of a study case as higher grade (including severely dysplastic and melanoma in situ) vs. lower grade (including mild to moderately dysplastic nevi) and diagnosis of invasive melanoma vs. any less severe diagnosis.
A total of 338 pathologists were included in the analysis. Of these, 113 were general pathologists and 225 were dermatopathologists (those who were board certified and/or fellowship trained in dermatopathology).
The researchers found that, compared with general pathologists, dermatopathologists were 2.63 times more likely to render higher-grade diagnoses and 1.95 times more likely to diagnose invasive melanoma (P < .001 for both associations). Diagnoses of stage pT1a melanomas with no mitotic activity completely accounted for the difference between dermatopathologists and general pathologists in diagnosing invasive melanoma.
For the analysis limited to the 225 dermatopathologists, those with a higher practice caseload of melanocytic lesions were more likely to assign higher-grade diagnoses (odds ratio for trend, 1.27; P = .02), while those affiliated with an academic center had lower odds of diagnosing invasive melanoma (OR, 0.61; P = .049).
The researchers acknowledged limitations of their analysis, including the lack of data on patient outcomes, “so we could not make conclusions about the clinical outcome of any particular diagnosis by a study participant,” they wrote. “While our analyses revealed pathologist characteristics associated with assigning more vs. less severe diagnoses of melanocytic lesions, we could not conclude that any particular diagnosis by a study participant was overcalling or undercalling. However, the epidemiologic evidence that melanoma is overdiagnosed suggests that overcalling by some pathologists may be contributing to increasing rates of low-risk melanoma diagnoses.”
In an accompanying editorial, authors Klaus J. Busam, MD, of the department of pathology and laboratory medicine at Memorial Sloan Kettering Cancer Center, New York, Pedram Gerami, MD, of the department of dermatology at Northwestern University, Chicago, and Richard A. Scolyer, MD, of the Melanoma Institute, Wollstonecraft, Australia, wrote that the study findings “raise the question of whether subspecialization in dermatopathology may be a factor contributing to the epidemiologic phenomenon of overdiagnosis – that is, the discordance in the rise of melanoma incidence and relatively constant annual mortality rates over many decades. The findings also invite a discussion about strategies to minimize harm from overdiagnosis for both patients and the health care system.”
To minimize misdiagnoses, they continued, efforts to facilitate diagnostic accuracy should be encouraged. “Excisional (rather than partial) biopsies and provision of relevant clinical information would facilitate rendering of the correct histopathologic diagnosis,” they wrote. “When the diagnosis is uncertain, this is best acknowledged. If felt necessary, a reexcision of a lesion with an uncertain diagnosis can be recommended without upgrading the diagnosis.”
In addition, “improvements in prognosis are needed beyond American Joint Committee on Cancer staging,” they noted. “This will likely require a multimodal approach with novel methods, including artificial intelligence and biomarkers that help distinguish low-risk melanomas, for which a conservative approach may be appropriate, from those that require surgical intervention.”
The study was supported by the National Center for Advancing Translational Sciences and by the National Institutes of Health. One author disclosed receiving grants from the National Cancer Institute during the conduct of the study, and another disclosed serving as editor in chief of Primary Care topics at UpToDate; other authors had no disclosures. Dr. Busam reported receiving nonfinancial support from the American Society of Dermatopathology. Dr. Gerami reported receiving consulting fees from Castle Biosciences. Dr. Scolyer reported receiving an investigator grant from the National Health and Medical Research Council of Australia during the conduct of the study and personal fees from several pharmaceutical companies outside the submitted work.
, results from an exploratory study showed.
The findings “could in part play a role in the rising incidence of early-stage melanoma with low risk of progression or patient morbidity, thereby contributing to increasing rates of overdiagnosis,” researchers led by co–senior authors Joann G. Elmore, MD, MPH, of the University of California, Los Angeles, and Raymond L. Barnhill, MD, MBA, of the Institut Curie, Paris, wrote in their study, published online in JAMA Dermatology.
To investigate the characteristics associated with rendering higher-grade diagnoses, including invasive melanoma, the researchers drew from two national data sets: the Melanoma Pathology (M-Path) study, conducted from July 2013 to May 2016, and the Reducing Errors in Melanocytic Interpretations (REMI) study, conducted from August 2018 to March 2021. In both studies, pathologists who interpreted melanocytic lesions in their clinical practices interpreted study cases in glass slide format. For the current study, researchers used logistic regression to examine the association of pathologist characteristics with diagnosis of a study case as higher grade (including severely dysplastic and melanoma in situ) vs. lower grade (including mild to moderately dysplastic nevi) and diagnosis of invasive melanoma vs. any less severe diagnosis.
A total of 338 pathologists were included in the analysis. Of these, 113 were general pathologists and 225 were dermatopathologists (those who were board certified and/or fellowship trained in dermatopathology).
The researchers found that, compared with general pathologists, dermatopathologists were 2.63 times more likely to render higher-grade diagnoses and 1.95 times more likely to diagnose invasive melanoma (P < .001 for both associations). Diagnoses of stage pT1a melanomas with no mitotic activity completely accounted for the difference between dermatopathologists and general pathologists in diagnosing invasive melanoma.
For the analysis limited to the 225 dermatopathologists, those with a higher practice caseload of melanocytic lesions were more likely to assign higher-grade diagnoses (odds ratio for trend, 1.27; P = .02), while those affiliated with an academic center had lower odds of diagnosing invasive melanoma (OR, 0.61; P = .049).
The researchers acknowledged limitations of their analysis, including the lack of data on patient outcomes, “so we could not make conclusions about the clinical outcome of any particular diagnosis by a study participant,” they wrote. “While our analyses revealed pathologist characteristics associated with assigning more vs. less severe diagnoses of melanocytic lesions, we could not conclude that any particular diagnosis by a study participant was overcalling or undercalling. However, the epidemiologic evidence that melanoma is overdiagnosed suggests that overcalling by some pathologists may be contributing to increasing rates of low-risk melanoma diagnoses.”
In an accompanying editorial, authors Klaus J. Busam, MD, of the department of pathology and laboratory medicine at Memorial Sloan Kettering Cancer Center, New York, Pedram Gerami, MD, of the department of dermatology at Northwestern University, Chicago, and Richard A. Scolyer, MD, of the Melanoma Institute, Wollstonecraft, Australia, wrote that the study findings “raise the question of whether subspecialization in dermatopathology may be a factor contributing to the epidemiologic phenomenon of overdiagnosis – that is, the discordance in the rise of melanoma incidence and relatively constant annual mortality rates over many decades. The findings also invite a discussion about strategies to minimize harm from overdiagnosis for both patients and the health care system.”
To minimize misdiagnoses, they continued, efforts to facilitate diagnostic accuracy should be encouraged. “Excisional (rather than partial) biopsies and provision of relevant clinical information would facilitate rendering of the correct histopathologic diagnosis,” they wrote. “When the diagnosis is uncertain, this is best acknowledged. If felt necessary, a reexcision of a lesion with an uncertain diagnosis can be recommended without upgrading the diagnosis.”
In addition, “improvements in prognosis are needed beyond American Joint Committee on Cancer staging,” they noted. “This will likely require a multimodal approach with novel methods, including artificial intelligence and biomarkers that help distinguish low-risk melanomas, for which a conservative approach may be appropriate, from those that require surgical intervention.”
The study was supported by the National Center for Advancing Translational Sciences and by the National Institutes of Health. One author disclosed receiving grants from the National Cancer Institute during the conduct of the study, and another disclosed serving as editor in chief of Primary Care topics at UpToDate; other authors had no disclosures. Dr. Busam reported receiving nonfinancial support from the American Society of Dermatopathology. Dr. Gerami reported receiving consulting fees from Castle Biosciences. Dr. Scolyer reported receiving an investigator grant from the National Health and Medical Research Council of Australia during the conduct of the study and personal fees from several pharmaceutical companies outside the submitted work.
, results from an exploratory study showed.
The findings “could in part play a role in the rising incidence of early-stage melanoma with low risk of progression or patient morbidity, thereby contributing to increasing rates of overdiagnosis,” researchers led by co–senior authors Joann G. Elmore, MD, MPH, of the University of California, Los Angeles, and Raymond L. Barnhill, MD, MBA, of the Institut Curie, Paris, wrote in their study, published online in JAMA Dermatology.
To investigate the characteristics associated with rendering higher-grade diagnoses, including invasive melanoma, the researchers drew from two national data sets: the Melanoma Pathology (M-Path) study, conducted from July 2013 to May 2016, and the Reducing Errors in Melanocytic Interpretations (REMI) study, conducted from August 2018 to March 2021. In both studies, pathologists who interpreted melanocytic lesions in their clinical practices interpreted study cases in glass slide format. For the current study, researchers used logistic regression to examine the association of pathologist characteristics with diagnosis of a study case as higher grade (including severely dysplastic and melanoma in situ) vs. lower grade (including mild to moderately dysplastic nevi) and diagnosis of invasive melanoma vs. any less severe diagnosis.
A total of 338 pathologists were included in the analysis. Of these, 113 were general pathologists and 225 were dermatopathologists (those who were board certified and/or fellowship trained in dermatopathology).
The researchers found that, compared with general pathologists, dermatopathologists were 2.63 times more likely to render higher-grade diagnoses and 1.95 times more likely to diagnose invasive melanoma (P < .001 for both associations). Diagnoses of stage pT1a melanomas with no mitotic activity completely accounted for the difference between dermatopathologists and general pathologists in diagnosing invasive melanoma.
For the analysis limited to the 225 dermatopathologists, those with a higher practice caseload of melanocytic lesions were more likely to assign higher-grade diagnoses (odds ratio for trend, 1.27; P = .02), while those affiliated with an academic center had lower odds of diagnosing invasive melanoma (OR, 0.61; P = .049).
The researchers acknowledged limitations of their analysis, including the lack of data on patient outcomes, “so we could not make conclusions about the clinical outcome of any particular diagnosis by a study participant,” they wrote. “While our analyses revealed pathologist characteristics associated with assigning more vs. less severe diagnoses of melanocytic lesions, we could not conclude that any particular diagnosis by a study participant was overcalling or undercalling. However, the epidemiologic evidence that melanoma is overdiagnosed suggests that overcalling by some pathologists may be contributing to increasing rates of low-risk melanoma diagnoses.”
In an accompanying editorial, authors Klaus J. Busam, MD, of the department of pathology and laboratory medicine at Memorial Sloan Kettering Cancer Center, New York, Pedram Gerami, MD, of the department of dermatology at Northwestern University, Chicago, and Richard A. Scolyer, MD, of the Melanoma Institute, Wollstonecraft, Australia, wrote that the study findings “raise the question of whether subspecialization in dermatopathology may be a factor contributing to the epidemiologic phenomenon of overdiagnosis – that is, the discordance in the rise of melanoma incidence and relatively constant annual mortality rates over many decades. The findings also invite a discussion about strategies to minimize harm from overdiagnosis for both patients and the health care system.”
To minimize misdiagnoses, they continued, efforts to facilitate diagnostic accuracy should be encouraged. “Excisional (rather than partial) biopsies and provision of relevant clinical information would facilitate rendering of the correct histopathologic diagnosis,” they wrote. “When the diagnosis is uncertain, this is best acknowledged. If felt necessary, a reexcision of a lesion with an uncertain diagnosis can be recommended without upgrading the diagnosis.”
In addition, “improvements in prognosis are needed beyond American Joint Committee on Cancer staging,” they noted. “This will likely require a multimodal approach with novel methods, including artificial intelligence and biomarkers that help distinguish low-risk melanomas, for which a conservative approach may be appropriate, from those that require surgical intervention.”
The study was supported by the National Center for Advancing Translational Sciences and by the National Institutes of Health. One author disclosed receiving grants from the National Cancer Institute during the conduct of the study, and another disclosed serving as editor in chief of Primary Care topics at UpToDate; other authors had no disclosures. Dr. Busam reported receiving nonfinancial support from the American Society of Dermatopathology. Dr. Gerami reported receiving consulting fees from Castle Biosciences. Dr. Scolyer reported receiving an investigator grant from the National Health and Medical Research Council of Australia during the conduct of the study and personal fees from several pharmaceutical companies outside the submitted work.
FROM JAMA DERMATOLOGY
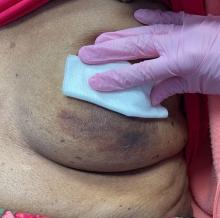
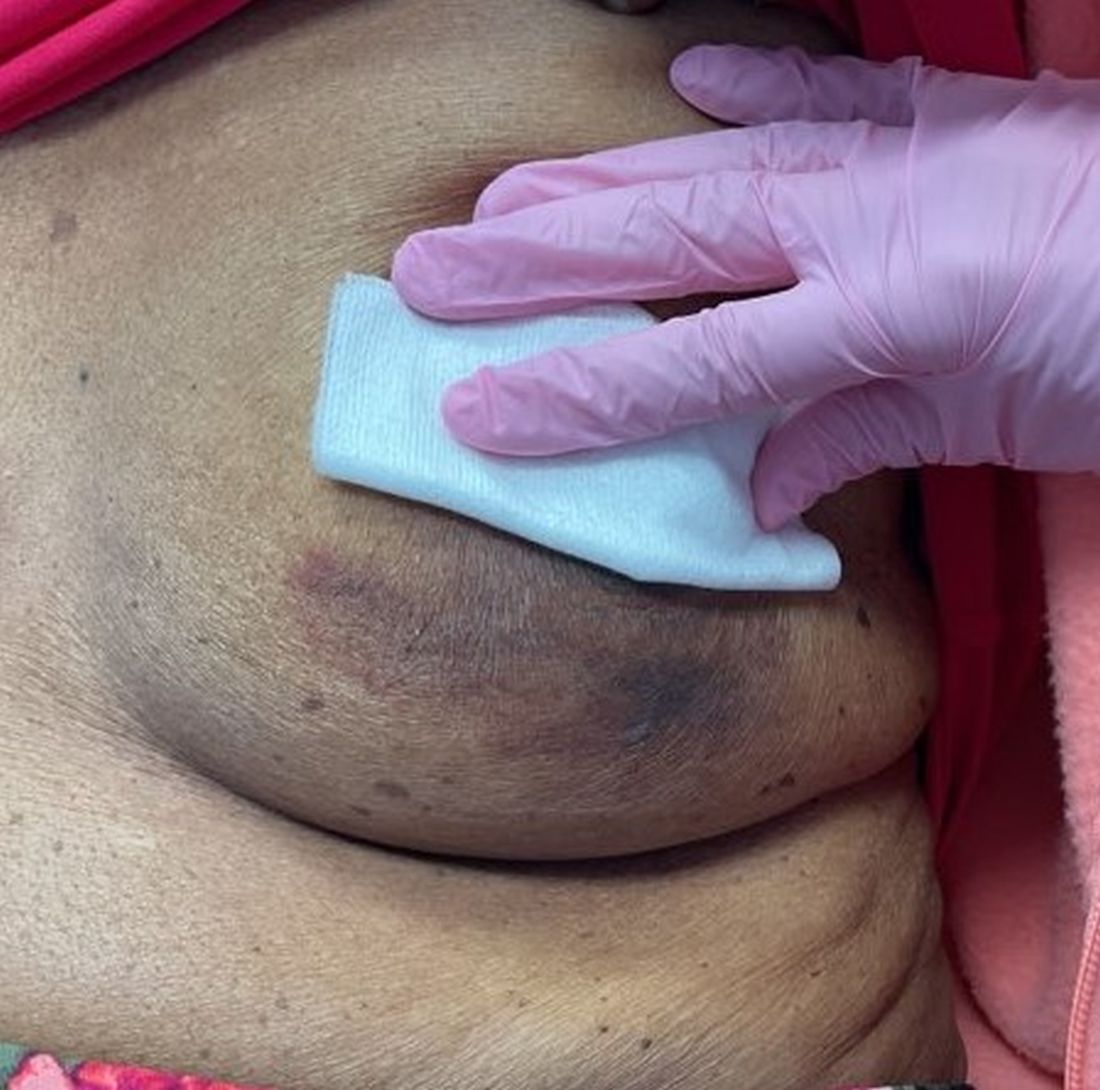
An 88-year-old Black woman presented with 3 months duration of asymptomatic, violaceous patches on the left breast
Angiosarcomas are uncommon, high-grade malignant tumors of endothelial cell origin that can arise via the lymphatics or vasculature. They typically occur spontaneously; however, there have been cases reported of benign vascular transformation. These tumors are more commonly found in elderly men on the head and neck in sun-damaged skin. . This is a late complication, typically occurring about 5-10 years after radiation. Stewart-Treves syndrome, chronic lymphedema occurring after breast cancer treatment with axillary node dissection, increases the risk of angiosarcoma. As a vascular tumor, angiosarcoma spreads hematogenously and carries a poor prognosis if not caught early. Differential diagnoses include other vascular tumors such as retiform hemangioendothelioma. In this specific patient, the differential diagnosis includes Paget’s disease, chronic radiation skin changes, and eczema.
Histopathologically, angiosarcomas exhibit abnormal, pleomorphic, malignant endothelial cells. As the tumor progresses, the cell architecture becomes more distorted and cells form layers with papillary projections into the vascular lumen. Malignant cells may stain positive for CD31, CD34, the oncogene ERG and the proto-oncogene FLI-1. Histology in this patient revealed radiation changes in the dermis, as well as few vascular channels lined by large endothelial cells with marked nuclear atypia, in the form of large nucleoli and variably coarse chromatin. The cells were positive for MYC.
Treatment of angiosarcoma involves a multidisciplinary approach. Resection with wide margins is generally the treatment of choice. However, recurrence is relatively common, which may be a result of microsatellite deposits of the tumor. Perioperative radiation is recommended, and adjuvant chemotherapy often is recommended for metastatic disease. Specifically, paclitaxel has been found to promote survival in some cases of cutaneous angiosarcoma. Metastatic disease may be treated with cytotoxic drugs such as anthracyclines and taxanes. Additionally, targeted therapy including anti-VEGF drugs and tyrosine kinase inhibitors have been tested.
The case and photo were submitted by Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Dr. Bilu Martin. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cohen-Hallaleh RB et al. Clin Sarcoma Res. 2017 Aug 7:7:15.
Cozzi S et al. Rep Pract Oncol Radiother. 2021 Sep 30;26(5):827-32.
Spiker AM, Mangla A, Ramsey ML. Angiosarcoma. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK441983/
Angiosarcomas are uncommon, high-grade malignant tumors of endothelial cell origin that can arise via the lymphatics or vasculature. They typically occur spontaneously; however, there have been cases reported of benign vascular transformation. These tumors are more commonly found in elderly men on the head and neck in sun-damaged skin. . This is a late complication, typically occurring about 5-10 years after radiation. Stewart-Treves syndrome, chronic lymphedema occurring after breast cancer treatment with axillary node dissection, increases the risk of angiosarcoma. As a vascular tumor, angiosarcoma spreads hematogenously and carries a poor prognosis if not caught early. Differential diagnoses include other vascular tumors such as retiform hemangioendothelioma. In this specific patient, the differential diagnosis includes Paget’s disease, chronic radiation skin changes, and eczema.
Histopathologically, angiosarcomas exhibit abnormal, pleomorphic, malignant endothelial cells. As the tumor progresses, the cell architecture becomes more distorted and cells form layers with papillary projections into the vascular lumen. Malignant cells may stain positive for CD31, CD34, the oncogene ERG and the proto-oncogene FLI-1. Histology in this patient revealed radiation changes in the dermis, as well as few vascular channels lined by large endothelial cells with marked nuclear atypia, in the form of large nucleoli and variably coarse chromatin. The cells were positive for MYC.
Treatment of angiosarcoma involves a multidisciplinary approach. Resection with wide margins is generally the treatment of choice. However, recurrence is relatively common, which may be a result of microsatellite deposits of the tumor. Perioperative radiation is recommended, and adjuvant chemotherapy often is recommended for metastatic disease. Specifically, paclitaxel has been found to promote survival in some cases of cutaneous angiosarcoma. Metastatic disease may be treated with cytotoxic drugs such as anthracyclines and taxanes. Additionally, targeted therapy including anti-VEGF drugs and tyrosine kinase inhibitors have been tested.
The case and photo were submitted by Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Dr. Bilu Martin. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cohen-Hallaleh RB et al. Clin Sarcoma Res. 2017 Aug 7:7:15.
Cozzi S et al. Rep Pract Oncol Radiother. 2021 Sep 30;26(5):827-32.
Spiker AM, Mangla A, Ramsey ML. Angiosarcoma. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK441983/
Angiosarcomas are uncommon, high-grade malignant tumors of endothelial cell origin that can arise via the lymphatics or vasculature. They typically occur spontaneously; however, there have been cases reported of benign vascular transformation. These tumors are more commonly found in elderly men on the head and neck in sun-damaged skin. . This is a late complication, typically occurring about 5-10 years after radiation. Stewart-Treves syndrome, chronic lymphedema occurring after breast cancer treatment with axillary node dissection, increases the risk of angiosarcoma. As a vascular tumor, angiosarcoma spreads hematogenously and carries a poor prognosis if not caught early. Differential diagnoses include other vascular tumors such as retiform hemangioendothelioma. In this specific patient, the differential diagnosis includes Paget’s disease, chronic radiation skin changes, and eczema.
Histopathologically, angiosarcomas exhibit abnormal, pleomorphic, malignant endothelial cells. As the tumor progresses, the cell architecture becomes more distorted and cells form layers with papillary projections into the vascular lumen. Malignant cells may stain positive for CD31, CD34, the oncogene ERG and the proto-oncogene FLI-1. Histology in this patient revealed radiation changes in the dermis, as well as few vascular channels lined by large endothelial cells with marked nuclear atypia, in the form of large nucleoli and variably coarse chromatin. The cells were positive for MYC.
Treatment of angiosarcoma involves a multidisciplinary approach. Resection with wide margins is generally the treatment of choice. However, recurrence is relatively common, which may be a result of microsatellite deposits of the tumor. Perioperative radiation is recommended, and adjuvant chemotherapy often is recommended for metastatic disease. Specifically, paclitaxel has been found to promote survival in some cases of cutaneous angiosarcoma. Metastatic disease may be treated with cytotoxic drugs such as anthracyclines and taxanes. Additionally, targeted therapy including anti-VEGF drugs and tyrosine kinase inhibitors have been tested.
The case and photo were submitted by Mr. Shapiro of Nova Southeastern University College of Osteopathic Medicine, Fort Lauderdale, Fla., and Dr. Bilu Martin. The column was edited by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cohen-Hallaleh RB et al. Clin Sarcoma Res. 2017 Aug 7:7:15.
Cozzi S et al. Rep Pract Oncol Radiother. 2021 Sep 30;26(5):827-32.
Spiker AM, Mangla A, Ramsey ML. Angiosarcoma. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing; 2023 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK441983/
Keratotic Nodules in a Patient With End-Stage Renal Disease
The Diagnosis: Reactive Perforating Collagenosis
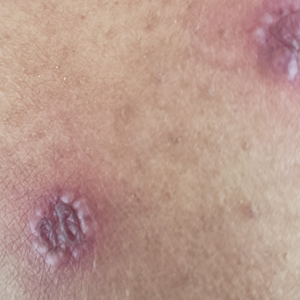
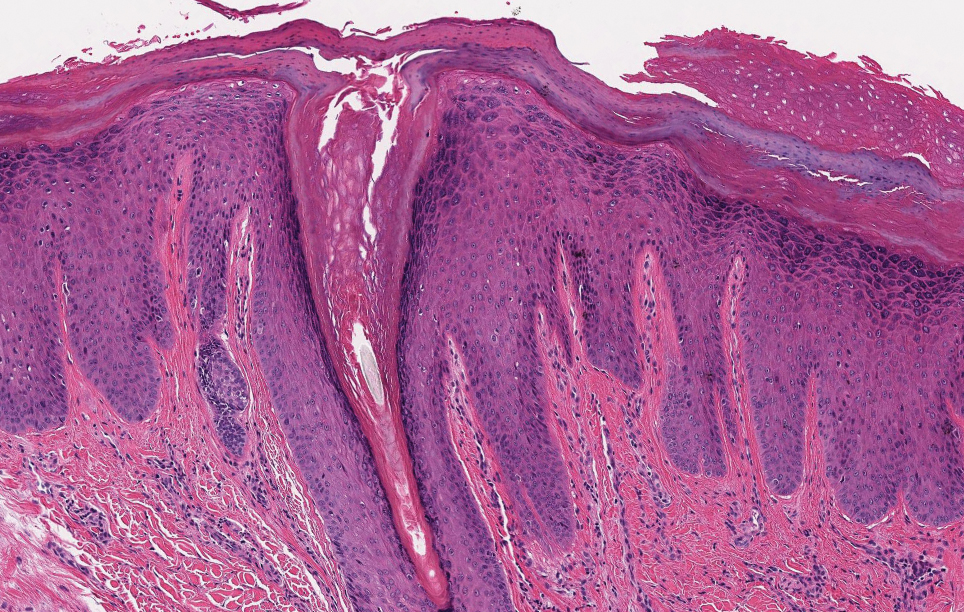
Reactive perforating collagenosis (RPC) is the most common type of primary perforating dermatosis and is characterized by the transepithelial elimination of collagen from the dermis. Although familial RPC usually presents in infancy or early childhood, the acquired form has a strong association with type 2 diabetes mellitus and chronic renal disease. Up to 10% of hemodialysis patients develop RPC.1 Patients with RPC develop red-brown, umbilicated, papulonodular lesions, often with a central keratotic crust and erythematous halo. The lesions are variable in shape and size (typically up to 10 mm in diameter) and commonly are located on the trunk or extensor aspects of the limbs. Pruritus is the primary concern, and the Koebner phenomenon commonly is seen.2
Although the histopathology can vary depending on the stage of the lesion, an invaginating epidermal process with prominent epidermal hyperplasia surrounding a central plug of keratin, basophilic inflammatory debris, and degenerated collagen are findings indicative of RPC. At the base of the invagination, the altered collagen perforates through the epidermis by the process of transepidermal elimination.3 Trichrome stains can highlight the collagen, while Verhoeff–van Gieson staining is negative (no elastic fiber elimination). Anecdotal reports have described a variety of successful therapies including retinoids, allopurinol, doxycycline, dupilumab, and phototherapy, with phototherapy being especially effective in patients with coexistent renal disease.4-8 Our patient was started on dupilumab 300 mg every other week and triamcinolone cream 0.1% twice daily (Monday through Friday) for itchy areas. The efficacy of the treatment was to be assessed at the next visit.
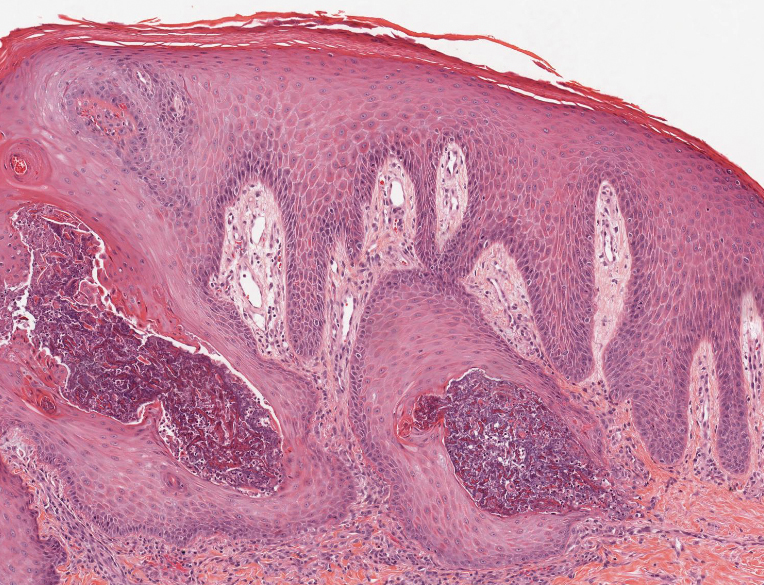
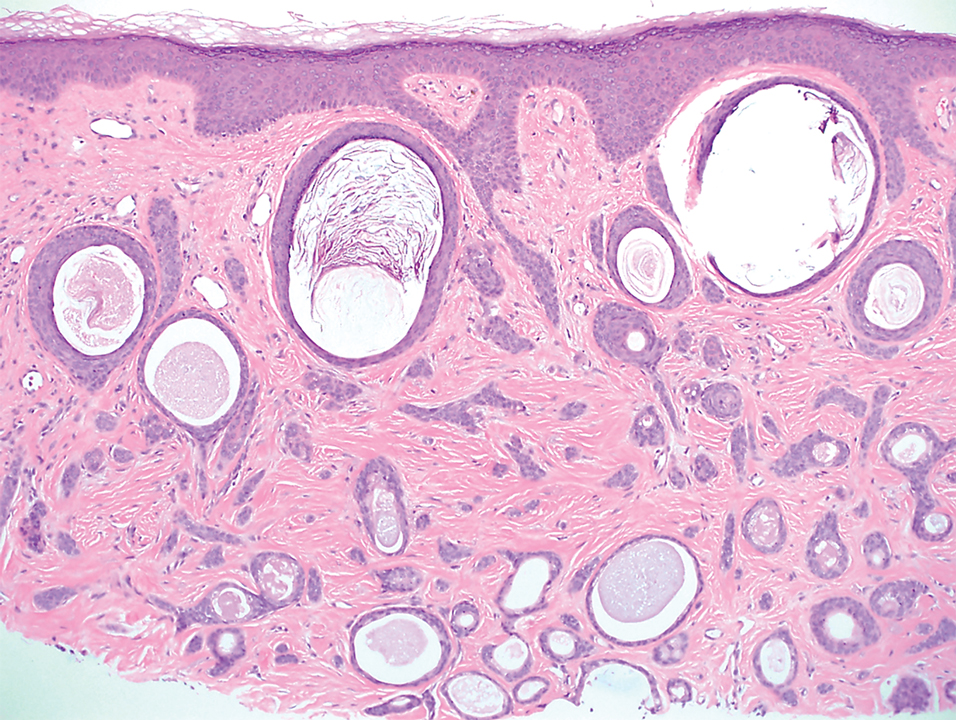
Elastosis perforans serpiginosa (EPS) is a rare skin disease that presents as small papules arranged in serpiginous or annular patterns on the neck, face, arms, or other flexural areas in early adulthood. It more commonly is seen in males and can be associated with other inherited disorders such as Down syndrome, Ehlers-Danlos syndrome, and Marfan syndrome. In rare instances, EPS has been linked to D-penicillamine.9 Elastosis perforans serpiginosa is characterized by focal dermal elastosis and transepithelial elimination of abnormal elastic fibers instead of collagen. The formation of narrow channels extending upward from the dermis in straight or corkscrew patterns commonly is seen (Figure 1). The dermis also may contain a chronic inflammatory infiltrate consisting of lymphocytes, macrophages, or multinucleated giant cells.10 Verhoeff– van Gieson stain highlights the altered elastic fibers in the papillary dermis.
Prurigo nodularis involves chronic, intensely pruritic, lichenified, excoriated nodules that often present as grouped symmetric lesions predominantly on the extensor aspects of the distal extremities and occasionally the trunk. Histologically, prurigo nodularis appears similar to lichen simplex chronicus but in a nodular form with pronounced hyperkeratosis and acanthosis, sometimes to the degree of pseudoepitheliomatous hyperplasia (Figure 2).11 Its features may resemble chronic eczema with mild spongiosis and focal parakeratosis. In the dermis, there is vascular hyperplasia surrounded by perivascular inflammatory infiltrates. Immunohistochemical staining for calcitonin gene-related peptide and substance P may show a large increase of immunoreactive nerves in the lesional skin of nodular prurigo patients compared to the lichenified skin of eczema patients.12 However, neural hyperplasia is not a diagnostic prerequisite in prurigo nodularis.13 Rarely, hyperplasic nerve trunks associated with Schwann cell proliferation may give rise to small neuromata that can be detected on electron microscopy.14 Screening for underlying systemic disease is recommended to rule out cancer, liver disease, chronic kidney disease, thyroid disorders, or HIV.
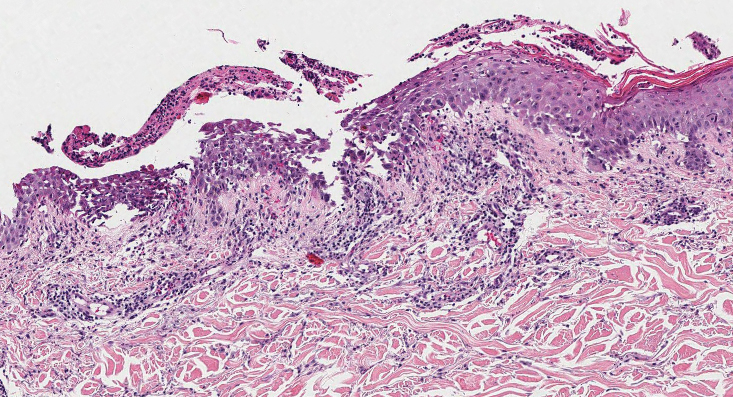
Ecthyma can affect children, adults, and especially immunocompromised patients at sites of trauma that allow entry of Streptococcus pyogenes or Staphylococcus aureus. Histologically, there is ulceration of the epidermis with a thick overlying inflammatory crust (Figure 3). The heavy infiltrate of neutrophils in the reticular dermis forms the base of the ulcer, and gram-positive cocci may be detected within the inflammatory crust. Ecthyma lesions may resemble the excoriations and shallow ulcers that are seen in a variety of other pruritic conditions.15
Pityriasis lichenoides et varioliformis acuta is a T-cell–mediated disease that is characterized by crops of lesions in varying sizes and stages including vesicular, hemorrhagic, ulcerated, and necrotic. It often results in varioliform scarring. Histologic findings can include parakeratosis, lichenoid inflammation, extravasation of red blood cells, vasculitis, and apoptotic keratinocytes (Figure 4).16
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288. doi:10.3346/jkms.2004.19.2.283
- Mullins TB, Sickinger M, Zito PM. Reactive perforating collagenosis. StatPearls [Internet]. StatPearls Publishing; 2022.
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritus! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Cullen SI. Successful treatment of reactive perforating collagenosis with tretinoin. Cutis. 1979;23:187-193.
- Tilz H, Becker JC, Legat F, et al. Allopurinol in the treatment of acquired reactive perforating collagenosis. An Bras Dermatol. 2013;88:94-97. doi:10.1590/s0365-05962013000100012
- Brinkmeier T, Schaller J, Herbst RA, et al. Successful treatment of acquired reactive perforating collagenosis with doxycycline. Acta Derm Venereol. 2002;82:393-395. doi:10.1080/000155502320624249
- Gil-Lianes J, Riquelme-McLoughlin C, Mascaró JM Jr. Reactive perforating collagenosis successfully treated with dupilumab. Australas J Dermatol. 2022;63:398-400. doi:10.1111/ajd.13874
- Gambichler T, Altmeyer P, Kreuter A. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2005;52:363-364. doi:10.1016/j.jaad.2004.08.018
- Na SY, Choi M, Kim MJ, et al. Penicillamine-induced elastosis perforans serpiginosa and cutis laxa in a patient with Wilson’s disease. Ann Dermatol. 2010;22:468-471. doi:10.5021/ad.2010.22.4.468
- Lee SH, Choi Y, Kim SC. Elastosis perforans serpiginosa. Ann Dermatol. 2014;26:103-106. doi:10.5021/ad.2014.26.1.103
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Abadía Molina F, Burrows NP, Jones RR, et al. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127:344-351. doi:10.1111/j.1365-2133.1992.tb00452.x
- Lindley RP, Payne CM. Neural hyperplasia is not a diagnostic prerequisite in nodular prurigo. a controlled morphometric microscopic study of 26 biopsy specimens. J Cutan Pathol. 1989;16:14-18. doi:10.1111/j.1600-0560.1989.tb00003.x
- Feuerman EJ, Sandbank M. Prurigo nodularis. histological and electron microscopical study. Arch Dermatol. 1975;111:1472-1477. doi:10.1001/archderm.111.11.1472
- Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone; 2010. 16. Clarey DD, Lauer SR, Trowbridge RM. Clinical, dermatoscopic, and histological findings in a diagnosis of pityriasis lichenoides [published online June 20, 2020]. Cureus. 2020;12:E8725. doi:10.7759 /cureus.8725
The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) is the most common type of primary perforating dermatosis and is characterized by the transepithelial elimination of collagen from the dermis. Although familial RPC usually presents in infancy or early childhood, the acquired form has a strong association with type 2 diabetes mellitus and chronic renal disease. Up to 10% of hemodialysis patients develop RPC.1 Patients with RPC develop red-brown, umbilicated, papulonodular lesions, often with a central keratotic crust and erythematous halo. The lesions are variable in shape and size (typically up to 10 mm in diameter) and commonly are located on the trunk or extensor aspects of the limbs. Pruritus is the primary concern, and the Koebner phenomenon commonly is seen.2
Although the histopathology can vary depending on the stage of the lesion, an invaginating epidermal process with prominent epidermal hyperplasia surrounding a central plug of keratin, basophilic inflammatory debris, and degenerated collagen are findings indicative of RPC. At the base of the invagination, the altered collagen perforates through the epidermis by the process of transepidermal elimination.3 Trichrome stains can highlight the collagen, while Verhoeff–van Gieson staining is negative (no elastic fiber elimination). Anecdotal reports have described a variety of successful therapies including retinoids, allopurinol, doxycycline, dupilumab, and phototherapy, with phototherapy being especially effective in patients with coexistent renal disease.4-8 Our patient was started on dupilumab 300 mg every other week and triamcinolone cream 0.1% twice daily (Monday through Friday) for itchy areas. The efficacy of the treatment was to be assessed at the next visit.
Elastosis perforans serpiginosa (EPS) is a rare skin disease that presents as small papules arranged in serpiginous or annular patterns on the neck, face, arms, or other flexural areas in early adulthood. It more commonly is seen in males and can be associated with other inherited disorders such as Down syndrome, Ehlers-Danlos syndrome, and Marfan syndrome. In rare instances, EPS has been linked to D-penicillamine.9 Elastosis perforans serpiginosa is characterized by focal dermal elastosis and transepithelial elimination of abnormal elastic fibers instead of collagen. The formation of narrow channels extending upward from the dermis in straight or corkscrew patterns commonly is seen (Figure 1). The dermis also may contain a chronic inflammatory infiltrate consisting of lymphocytes, macrophages, or multinucleated giant cells.10 Verhoeff– van Gieson stain highlights the altered elastic fibers in the papillary dermis.

Prurigo nodularis involves chronic, intensely pruritic, lichenified, excoriated nodules that often present as grouped symmetric lesions predominantly on the extensor aspects of the distal extremities and occasionally the trunk. Histologically, prurigo nodularis appears similar to lichen simplex chronicus but in a nodular form with pronounced hyperkeratosis and acanthosis, sometimes to the degree of pseudoepitheliomatous hyperplasia (Figure 2).11 Its features may resemble chronic eczema with mild spongiosis and focal parakeratosis. In the dermis, there is vascular hyperplasia surrounded by perivascular inflammatory infiltrates. Immunohistochemical staining for calcitonin gene-related peptide and substance P may show a large increase of immunoreactive nerves in the lesional skin of nodular prurigo patients compared to the lichenified skin of eczema patients.12 However, neural hyperplasia is not a diagnostic prerequisite in prurigo nodularis.13 Rarely, hyperplasic nerve trunks associated with Schwann cell proliferation may give rise to small neuromata that can be detected on electron microscopy.14 Screening for underlying systemic disease is recommended to rule out cancer, liver disease, chronic kidney disease, thyroid disorders, or HIV.

Ecthyma can affect children, adults, and especially immunocompromised patients at sites of trauma that allow entry of Streptococcus pyogenes or Staphylococcus aureus. Histologically, there is ulceration of the epidermis with a thick overlying inflammatory crust (Figure 3). The heavy infiltrate of neutrophils in the reticular dermis forms the base of the ulcer, and gram-positive cocci may be detected within the inflammatory crust. Ecthyma lesions may resemble the excoriations and shallow ulcers that are seen in a variety of other pruritic conditions.15

Pityriasis lichenoides et varioliformis acuta is a T-cell–mediated disease that is characterized by crops of lesions in varying sizes and stages including vesicular, hemorrhagic, ulcerated, and necrotic. It often results in varioliform scarring. Histologic findings can include parakeratosis, lichenoid inflammation, extravasation of red blood cells, vasculitis, and apoptotic keratinocytes (Figure 4).16

The Diagnosis: Reactive Perforating Collagenosis
Reactive perforating collagenosis (RPC) is the most common type of primary perforating dermatosis and is characterized by the transepithelial elimination of collagen from the dermis. Although familial RPC usually presents in infancy or early childhood, the acquired form has a strong association with type 2 diabetes mellitus and chronic renal disease. Up to 10% of hemodialysis patients develop RPC.1 Patients with RPC develop red-brown, umbilicated, papulonodular lesions, often with a central keratotic crust and erythematous halo. The lesions are variable in shape and size (typically up to 10 mm in diameter) and commonly are located on the trunk or extensor aspects of the limbs. Pruritus is the primary concern, and the Koebner phenomenon commonly is seen.2
Although the histopathology can vary depending on the stage of the lesion, an invaginating epidermal process with prominent epidermal hyperplasia surrounding a central plug of keratin, basophilic inflammatory debris, and degenerated collagen are findings indicative of RPC. At the base of the invagination, the altered collagen perforates through the epidermis by the process of transepidermal elimination.3 Trichrome stains can highlight the collagen, while Verhoeff–van Gieson staining is negative (no elastic fiber elimination). Anecdotal reports have described a variety of successful therapies including retinoids, allopurinol, doxycycline, dupilumab, and phototherapy, with phototherapy being especially effective in patients with coexistent renal disease.4-8 Our patient was started on dupilumab 300 mg every other week and triamcinolone cream 0.1% twice daily (Monday through Friday) for itchy areas. The efficacy of the treatment was to be assessed at the next visit.
Elastosis perforans serpiginosa (EPS) is a rare skin disease that presents as small papules arranged in serpiginous or annular patterns on the neck, face, arms, or other flexural areas in early adulthood. It more commonly is seen in males and can be associated with other inherited disorders such as Down syndrome, Ehlers-Danlos syndrome, and Marfan syndrome. In rare instances, EPS has been linked to D-penicillamine.9 Elastosis perforans serpiginosa is characterized by focal dermal elastosis and transepithelial elimination of abnormal elastic fibers instead of collagen. The formation of narrow channels extending upward from the dermis in straight or corkscrew patterns commonly is seen (Figure 1). The dermis also may contain a chronic inflammatory infiltrate consisting of lymphocytes, macrophages, or multinucleated giant cells.10 Verhoeff– van Gieson stain highlights the altered elastic fibers in the papillary dermis.

Prurigo nodularis involves chronic, intensely pruritic, lichenified, excoriated nodules that often present as grouped symmetric lesions predominantly on the extensor aspects of the distal extremities and occasionally the trunk. Histologically, prurigo nodularis appears similar to lichen simplex chronicus but in a nodular form with pronounced hyperkeratosis and acanthosis, sometimes to the degree of pseudoepitheliomatous hyperplasia (Figure 2).11 Its features may resemble chronic eczema with mild spongiosis and focal parakeratosis. In the dermis, there is vascular hyperplasia surrounded by perivascular inflammatory infiltrates. Immunohistochemical staining for calcitonin gene-related peptide and substance P may show a large increase of immunoreactive nerves in the lesional skin of nodular prurigo patients compared to the lichenified skin of eczema patients.12 However, neural hyperplasia is not a diagnostic prerequisite in prurigo nodularis.13 Rarely, hyperplasic nerve trunks associated with Schwann cell proliferation may give rise to small neuromata that can be detected on electron microscopy.14 Screening for underlying systemic disease is recommended to rule out cancer, liver disease, chronic kidney disease, thyroid disorders, or HIV.

Ecthyma can affect children, adults, and especially immunocompromised patients at sites of trauma that allow entry of Streptococcus pyogenes or Staphylococcus aureus. Histologically, there is ulceration of the epidermis with a thick overlying inflammatory crust (Figure 3). The heavy infiltrate of neutrophils in the reticular dermis forms the base of the ulcer, and gram-positive cocci may be detected within the inflammatory crust. Ecthyma lesions may resemble the excoriations and shallow ulcers that are seen in a variety of other pruritic conditions.15

Pityriasis lichenoides et varioliformis acuta is a T-cell–mediated disease that is characterized by crops of lesions in varying sizes and stages including vesicular, hemorrhagic, ulcerated, and necrotic. It often results in varioliform scarring. Histologic findings can include parakeratosis, lichenoid inflammation, extravasation of red blood cells, vasculitis, and apoptotic keratinocytes (Figure 4).16

- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288. doi:10.3346/jkms.2004.19.2.283
- Mullins TB, Sickinger M, Zito PM. Reactive perforating collagenosis. StatPearls [Internet]. StatPearls Publishing; 2022.
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritus! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Cullen SI. Successful treatment of reactive perforating collagenosis with tretinoin. Cutis. 1979;23:187-193.
- Tilz H, Becker JC, Legat F, et al. Allopurinol in the treatment of acquired reactive perforating collagenosis. An Bras Dermatol. 2013;88:94-97. doi:10.1590/s0365-05962013000100012
- Brinkmeier T, Schaller J, Herbst RA, et al. Successful treatment of acquired reactive perforating collagenosis with doxycycline. Acta Derm Venereol. 2002;82:393-395. doi:10.1080/000155502320624249
- Gil-Lianes J, Riquelme-McLoughlin C, Mascaró JM Jr. Reactive perforating collagenosis successfully treated with dupilumab. Australas J Dermatol. 2022;63:398-400. doi:10.1111/ajd.13874
- Gambichler T, Altmeyer P, Kreuter A. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2005;52:363-364. doi:10.1016/j.jaad.2004.08.018
- Na SY, Choi M, Kim MJ, et al. Penicillamine-induced elastosis perforans serpiginosa and cutis laxa in a patient with Wilson’s disease. Ann Dermatol. 2010;22:468-471. doi:10.5021/ad.2010.22.4.468
- Lee SH, Choi Y, Kim SC. Elastosis perforans serpiginosa. Ann Dermatol. 2014;26:103-106. doi:10.5021/ad.2014.26.1.103
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Abadía Molina F, Burrows NP, Jones RR, et al. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127:344-351. doi:10.1111/j.1365-2133.1992.tb00452.x
- Lindley RP, Payne CM. Neural hyperplasia is not a diagnostic prerequisite in nodular prurigo. a controlled morphometric microscopic study of 26 biopsy specimens. J Cutan Pathol. 1989;16:14-18. doi:10.1111/j.1600-0560.1989.tb00003.x
- Feuerman EJ, Sandbank M. Prurigo nodularis. histological and electron microscopical study. Arch Dermatol. 1975;111:1472-1477. doi:10.1001/archderm.111.11.1472
- Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone; 2010. 16. Clarey DD, Lauer SR, Trowbridge RM. Clinical, dermatoscopic, and histological findings in a diagnosis of pityriasis lichenoides [published online June 20, 2020]. Cureus. 2020;12:E8725. doi:10.7759 /cureus.8725
- Hong SB, Park JH, Ihm CG, et al. Acquired perforating dermatosis in patients with chronic renal failure and diabetes mellitus. J Korean Med Sci. 2004;19:283-288. doi:10.3346/jkms.2004.19.2.283
- Mullins TB, Sickinger M, Zito PM. Reactive perforating collagenosis. StatPearls [Internet]. StatPearls Publishing; 2022.
- Bejjanki H, Siroy AE, Koratala A. Reactive perforating collagenosis in end-stage renal disease: not all that itches is uremic pruritus! Am J Med. 2019;132:E658-E660. doi:10.1016/j.amjmed.2019.03.015
- Cullen SI. Successful treatment of reactive perforating collagenosis with tretinoin. Cutis. 1979;23:187-193.
- Tilz H, Becker JC, Legat F, et al. Allopurinol in the treatment of acquired reactive perforating collagenosis. An Bras Dermatol. 2013;88:94-97. doi:10.1590/s0365-05962013000100012
- Brinkmeier T, Schaller J, Herbst RA, et al. Successful treatment of acquired reactive perforating collagenosis with doxycycline. Acta Derm Venereol. 2002;82:393-395. doi:10.1080/000155502320624249
- Gil-Lianes J, Riquelme-McLoughlin C, Mascaró JM Jr. Reactive perforating collagenosis successfully treated with dupilumab. Australas J Dermatol. 2022;63:398-400. doi:10.1111/ajd.13874
- Gambichler T, Altmeyer P, Kreuter A. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol. 2005;52:363-364. doi:10.1016/j.jaad.2004.08.018
- Na SY, Choi M, Kim MJ, et al. Penicillamine-induced elastosis perforans serpiginosa and cutis laxa in a patient with Wilson’s disease. Ann Dermatol. 2010;22:468-471. doi:10.5021/ad.2010.22.4.468
- Lee SH, Choi Y, Kim SC. Elastosis perforans serpiginosa. Ann Dermatol. 2014;26:103-106. doi:10.5021/ad.2014.26.1.103
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Abadía Molina F, Burrows NP, Jones RR, et al. Increased sensory neuropeptides in nodular prurigo: a quantitative immunohistochemical analysis. Br J Dermatol. 1992;127:344-351. doi:10.1111/j.1365-2133.1992.tb00452.x
- Lindley RP, Payne CM. Neural hyperplasia is not a diagnostic prerequisite in nodular prurigo. a controlled morphometric microscopic study of 26 biopsy specimens. J Cutan Pathol. 1989;16:14-18. doi:10.1111/j.1600-0560.1989.tb00003.x
- Feuerman EJ, Sandbank M. Prurigo nodularis. histological and electron microscopical study. Arch Dermatol. 1975;111:1472-1477. doi:10.1001/archderm.111.11.1472
- Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Churchill Livingstone; 2010. 16. Clarey DD, Lauer SR, Trowbridge RM. Clinical, dermatoscopic, and histological findings in a diagnosis of pityriasis lichenoides [published online June 20, 2020]. Cureus. 2020;12:E8725. doi:10.7759 /cureus.8725
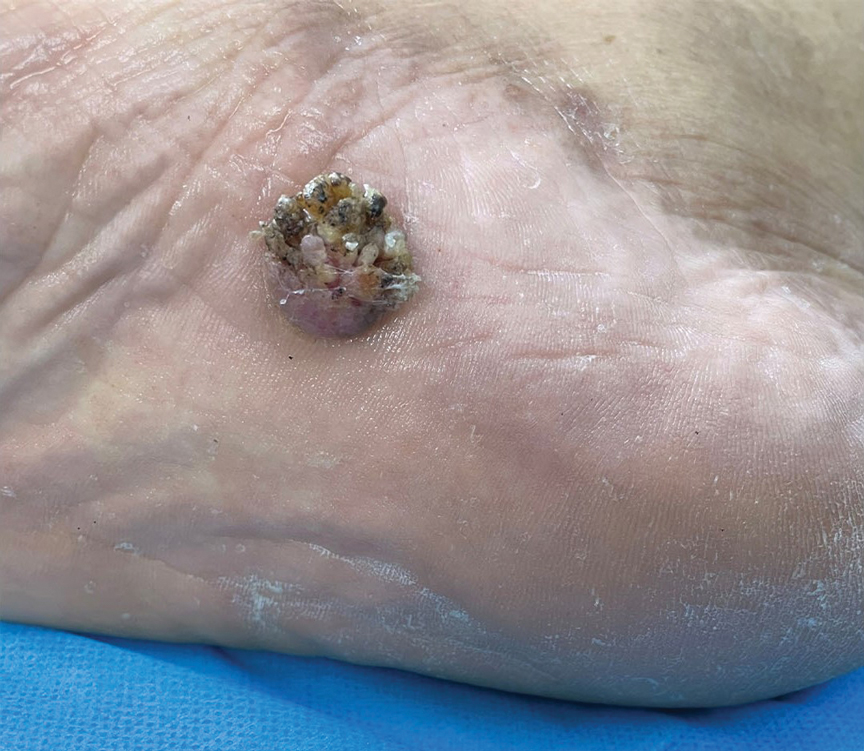
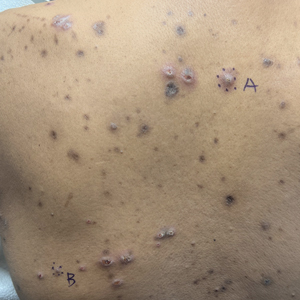
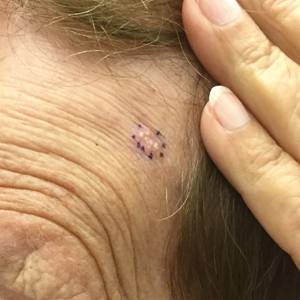
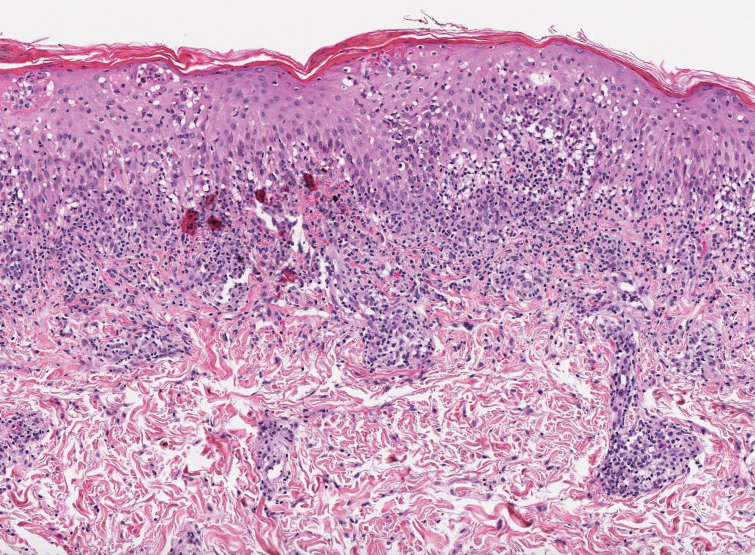
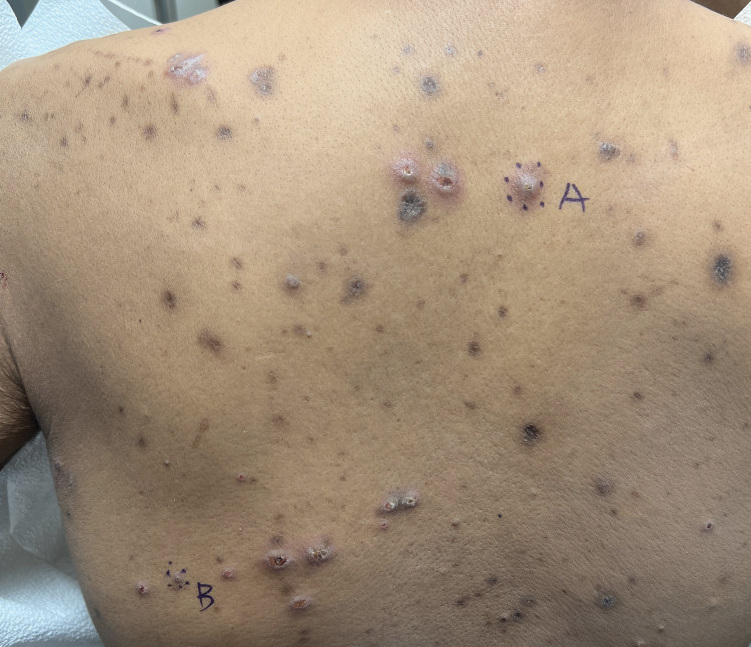
A 42-year-old man with end-stage renal disease on hemodialysis presented with generalized body itching and nodules on the scalp and back of 1 year’s duration. Physical examination revealed diffuse, hyperpigmented, pruritic, keratotic nodules and macules on the scalp and back (top). A punch biopsy was performed (bottom).
Plaquelike Syringoma Mimicking Microcystic Adnexal Carcinoma: A Potential Histologic Pitfall
To the Editor:
Plaquelike or plaque-type syringoma is a lesser-known variant of syringoma that can appear histologically indistinguishable from the superficial portion of microcystic adnexal carcinoma (MAC). The plaquelike variant of syringoma holds a benign clinical course, and no treatment is necessary. Microcystic adnexal carcinoma is distinguished from plaquelike syringoma by an aggressive growth pattern with a high risk for local invasion and recurrence if inadequately treated. Thus, treatment with Mohs micrographic surgery (MMS) has been recommended as the mainstay for MAC. If superficial biopsy specimens reveal suspicion for MAC and patients are referred for MMS, careful consideration should be made to differentiate MAC and plaquelike syringoma early to prevent unnecessary morbidity.
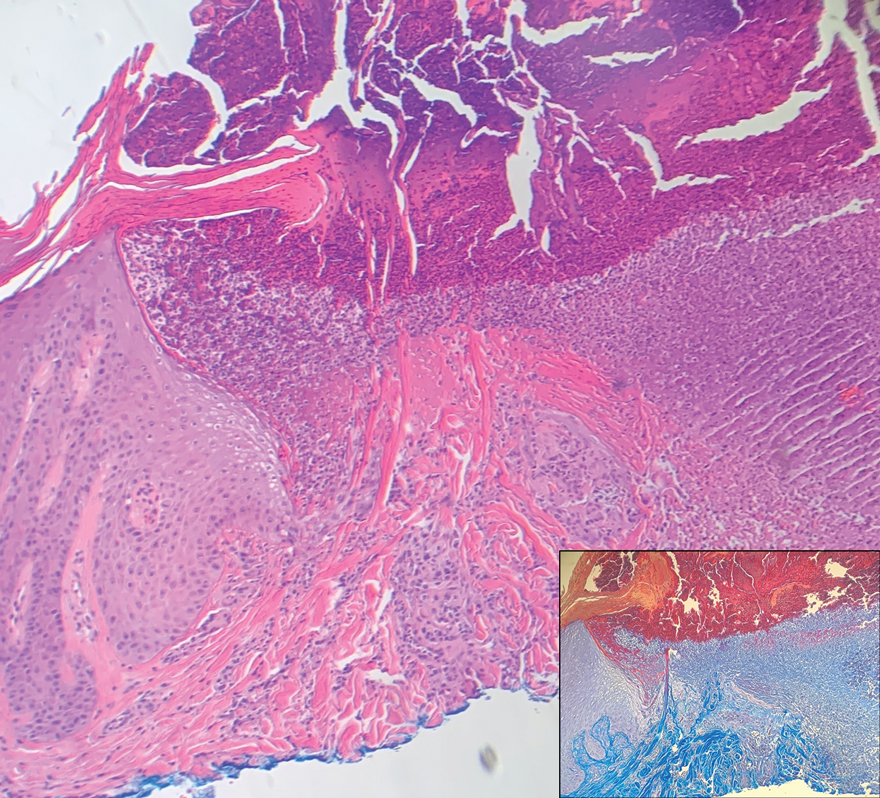
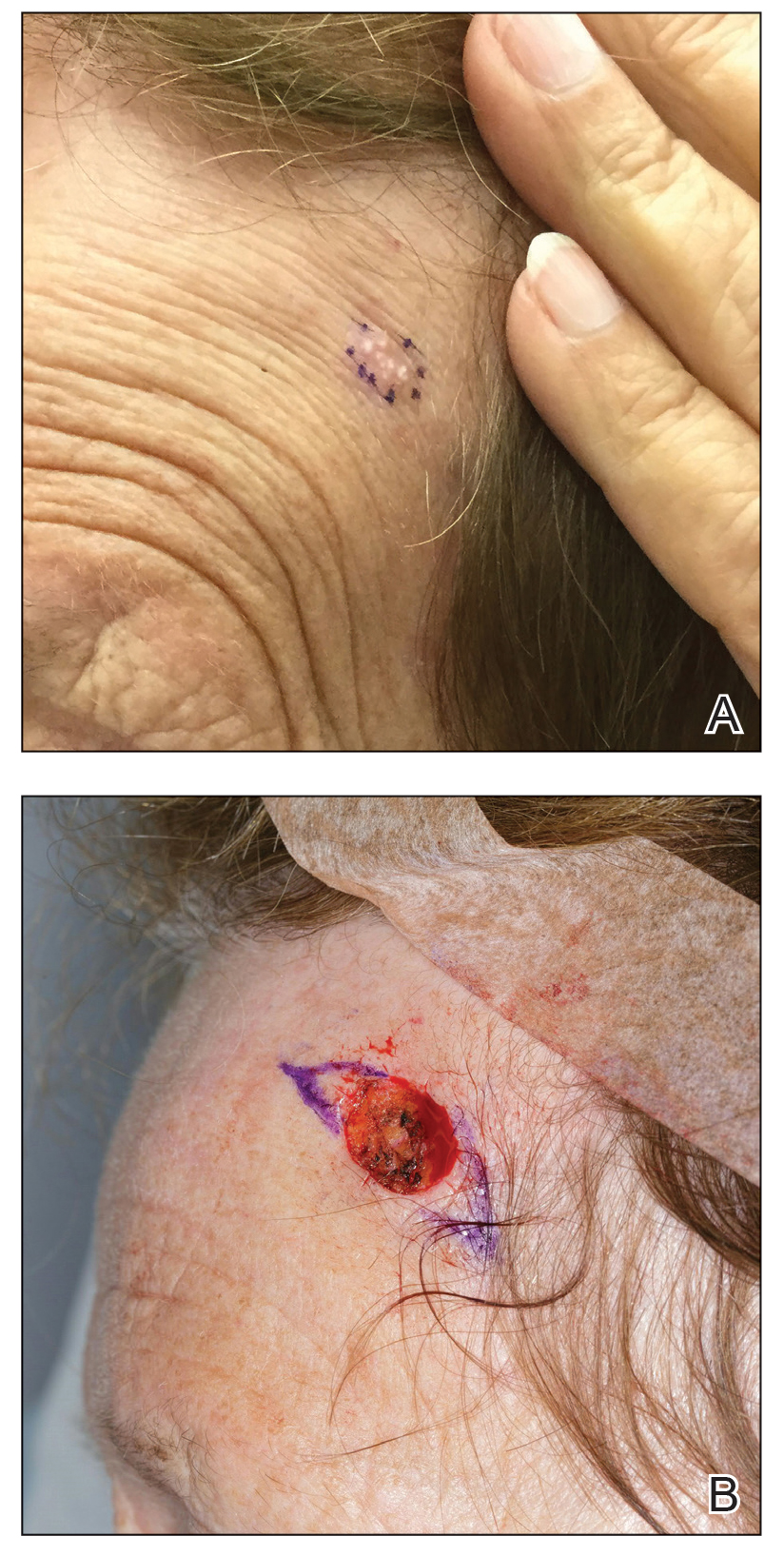
A 78-year-old woman was referred for MMS for a left forehead lesion that was diagnosed via shave biopsy as a desmoplastic and cystic adnexal neoplasm with suspicion for desmoplastic trichoepithelioma or MAC (Figure 1). Upon presentation for MMS, a well-healed, 1.0×0.9-cm scar at the biopsy site on the left forehead was observed (Figure 2A). One stage was obtained by standard MMS technique and sent for intraoperative processing (Figure 2B). Frozen section examination of the first stage demonstrated peripheral margin involvement with syringomatous change confined to the superficial and mid dermis (Figure 3). Before proceeding further, these findings were reviewed with an in-house dermatopathologist, and it was determined that no infiltrative tumor, perineural involvement, or other features to indicate malignancy were noted. A decision was made to refrain from obtaining any additional layers and to send excised Burow triangles for permanent section analysis. A primary linear closure was performed without complication, and the patient was discharged from the ambulatory surgery suite. Histopathologic examination of the Burow triangles later confirmed findings consistent with plaquelike syringoma with no evidence of malignancy (Figure 4).
Syringomas present as small flesh-colored papules in the periorbital areas. These benign neoplasms previously have been classified into 4 major clinical variants: localized, generalized, Down syndrome associated, and familial.1 The lesser-known plaquelike variant of syringoma was first described by Kikuchi et al2 in 1979. Aside from our report, a PubMed search of articles indexed for MEDLINE using the terms plaquelike or plaque-type syringoma yielded 16 cases in the literature.2-14 Of these, 6 were referred to or encountered in the MMS setting.8,9,11,12,14 Plaquelike syringoma can be solitary or multiple in presentation.6 It most commonly involves the head and neck but also can present on the trunk, arms, legs, and groin areas. The clinical size of plaquelike syringoma is variable, with the largest reported cases extending several centimeters in diameter.2,6 Similar to reported associations with conventional syringoma, the plaquelike subtype of syringoma has been reported in association with Down syndrome.13
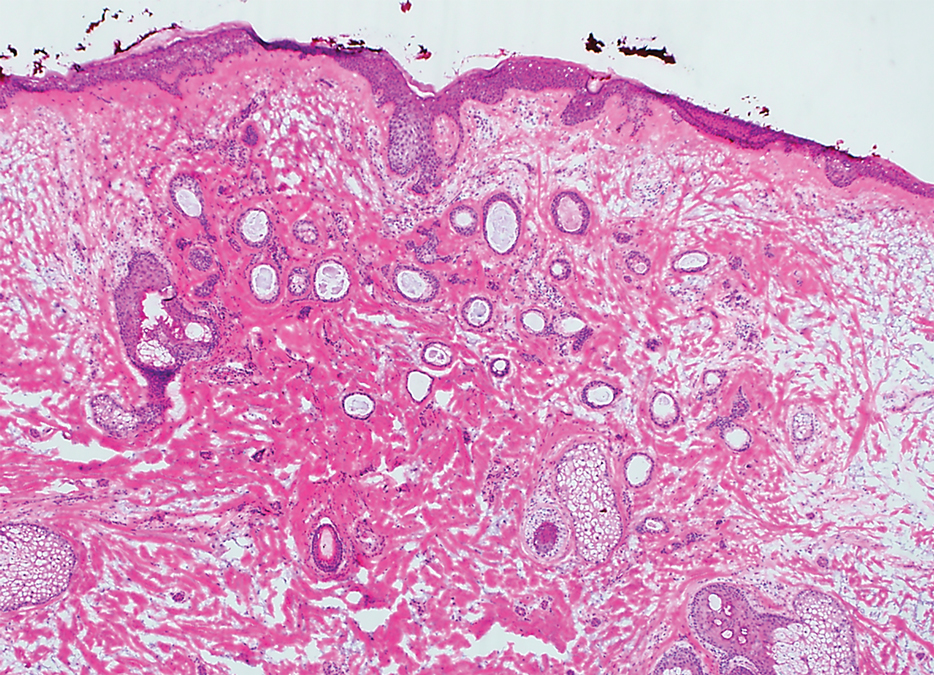
Histopathologically, plaquelike syringoma shares features with MAC as well as desmoplastic trichoepithelioma and desmoplastic basal cell carcinoma. Plaquelike syringoma demonstrates broad proliferations of small tubules morphologically reminiscent of tadpoles confined within the dermis. Ducts typically are lined with 2 or 3 layers of small cuboidal cells. Microcystic adnexal carcinoma typically features asymmetric ductal structures lined with single cells extending from the dermis into the subcutis and even underlying muscle, cartilage, or bone.8 There are no reliable immunohistochemical stains to differentiate between these 2 entities; thus, the primary distinction lies in the depth of involvement. Desmoplastic trichoepithelioma is composed of narrow cords and nests of basaloid cells of follicular origin commonly admixed with small cornifying cysts appearing in the dermis.8 Colonizing Merkel cells positive for cytokeratin 20 often are present in desmoplastic trichoepithelioma and not in syringoma or MAC.15 Desmoplastic basal cell carcinoma demonstrates narrow strands of basaloid cells of follicular origin appearing in the dermis. Desmoplastic trichoepithelioma and desmoplastic basal cell carcinoma are each fundamentally differentiated from plaquelike syringoma in that proliferations of cords and nests are not of eccrine or apocrine origin.
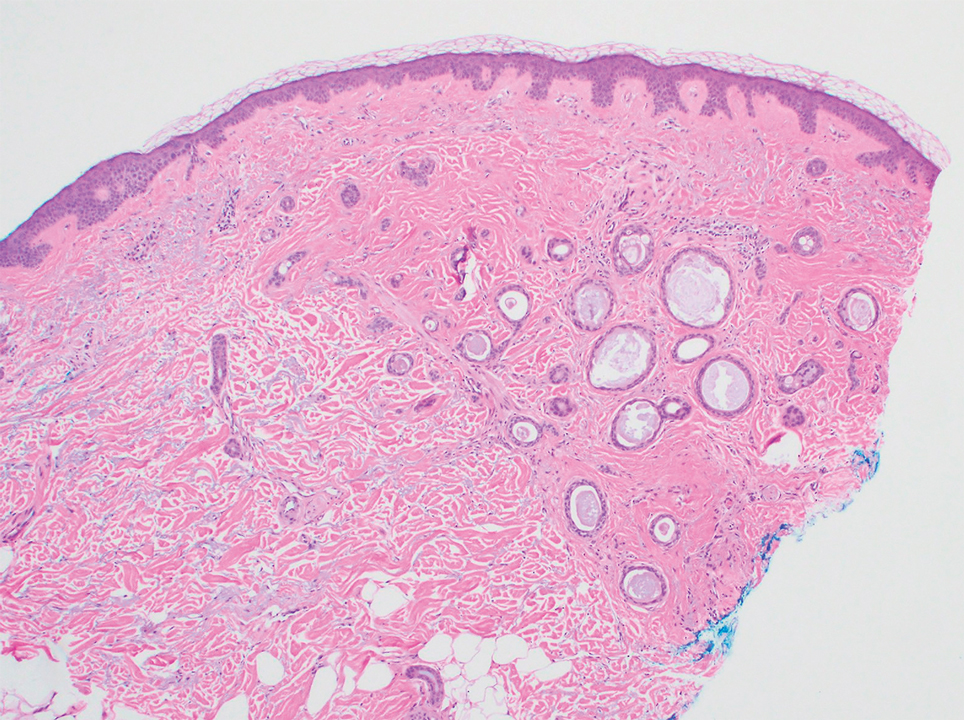
Several cases of plaquelike syringoma have been challenging to distinguish from MAC in performing MMS.8,9,11 Underlying extension of this syringoma variant can be far-reaching, extending to several centimeters in size and involving multiple cosmetic subunits.6,11,14 Inadvertent overtreatment with multiple MMS stages can be avoided with careful recognition of the differentiating histopathologic features. Syringomatous lesions commonly are encountered in MMS and may even be present at the edge of other tumor types. Plaquelike syringoma has been reported as a coexistent entity with nodular basal cell carcinoma.12 Boos et al16 similarly reported the presence of deceptive ductal proliferations along the immediate peripheral margin of MAC, which prompted multiple re-excisions. Pursuit of permanent section analysis in these cases revealed the appearance of small syringomas, and a diagnosis of benign subclinical syringomatous proliferations was made, averting further intervention.16
Our case sheds light on the threat of commission bias in dermatologic surgery, which is the tendency for action rather than inaction.17 In this context, it is important to avoid the perspective that harm to the patient can only be prevented by active intervention. Cognitive bias has been increasingly recognized as a source of medical error, and methods to mitigate bias in medical practice have been well described.17 Microcystic adnexal carcinoma and plaquelike syringoma can be hard to differentiate especially initially, as demonstrated in our case, which particularly illustrates the importance of slowing down a surgical case at the appropriate time, considering and revisiting alternative diagnoses, implementing checklists, and seeking histopathologic collaboration with colleagues when necessary. Our attempted implementation of these principles, especially early collaboration with colleagues, led to intraoperative recognition of plaquelike syringoma within the first stage of MMS.
We seek to raise the index of suspicion for plaquelike syringoma among dermatologists and dermatologic surgeons, especially when syringomatous structures are limited to the superficial dermis. We encourage familiarity with the plaquelike syringoma entity as well as careful consideration of further investigation via scouting biopsies or permanent section analysis when other characteristic features of MAC are unclear or lacking. Adequate sampling as well as collaboration with a dermatopathologist in cases of suspected syringoma can help to reduce the susceptibility to commission bias and prevent histopathologic pitfalls and unwarranted surgical morbidity.
- Friedman SJ, Butler DF. Syringoma presenting as milia. J Am Acad Dermatol. 1987;16:310-314.
- Kikuchi I, Idemori M, Okazaki M. Plaque type syringoma. J Dermatol. 1979;6:329-331.
- Dekio S, Jidoi J. Submammary syringoma—report of a case. J Dermatol. 1988;15:351-352.
- Patrizi A, Neri I, Marzaduri S, et al. Syringoma: a review of twenty-nine cases. Acta Derm Venereol. 1998;78:460-462.
- Nguyen DB, Patterson JW, Wilson BB. Syringoma of the moustache area. J Am Acad Dermatol. 2003;49:337-339.
- Rongioletti F, Semino MT, Rebora A. Unilateral multiple plaque-like syringomas. Br J Dermatol. 1996;135:623-625.
- Chi HI. A case of unusual syringoma: unilateral linear distribution and plaque formation. J Dermatol. 1996;23:505-506.
- Suwatee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Wallace JS, Bond JS, Seidel GD, et al. An important mimicker: plaque-type syringoma mistakenly diagnosed as microcystic adnexal carcinoma. Dermatol Surg. 2014;40:810-812.
- Mitkov M, Balagula Y, Taube JM, et al. Plaque-like syringoma with involvement of deep reticular dermis. J Am Acad Dermatol. 2014;71:E206-E207.
- Schleich C, Ferringer T, Petrick M. Plaque type syringoma mimicking a microcystic adnexal carcinoma. J Am Acad Dermatol. 2016;74(suppl 1):AB287.
- Yang Y, Srivastava D. Plaque-type syringoma coexisting with basal cell carcinoma. Dermatol Surg. 2018;44:1464-1466.
- Motegi SI, Sekiguchi A, Fujiwara C, et al. Milia-like idiopathic calcinosis cutis and plaque-type syringoma in a girl with Down syndrome. J Dermatol. 2019;46:E136-E137.
- Clark M, Duprey C, Sutton A, et al. Plaque-type syringoma masquerading as microcystic adnexal carcinoma: review of the literature and description of a novel technique that emphasizes lesion architecture to help make the diagnosis. Am J Dermatopathol. 2019;41:E98-E101.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Boos MD, Elenitsas R, Seykora J, et al. Benign subclinical syringomatous proliferations adjacent to a microcystic adnexal carcinoma: a tumor mimic with significant patient implications. Am J Dermatopathol. 2014;36:174-178.
- O’Sullivan ED, Schofield SJ. Cognitive bias in clinical medicine. J R Coll Physicians Edinb. 2018;48:225-232.
To the Editor:
Plaquelike or plaque-type syringoma is a lesser-known variant of syringoma that can appear histologically indistinguishable from the superficial portion of microcystic adnexal carcinoma (MAC). The plaquelike variant of syringoma holds a benign clinical course, and no treatment is necessary. Microcystic adnexal carcinoma is distinguished from plaquelike syringoma by an aggressive growth pattern with a high risk for local invasion and recurrence if inadequately treated. Thus, treatment with Mohs micrographic surgery (MMS) has been recommended as the mainstay for MAC. If superficial biopsy specimens reveal suspicion for MAC and patients are referred for MMS, careful consideration should be made to differentiate MAC and plaquelike syringoma early to prevent unnecessary morbidity.

A 78-year-old woman was referred for MMS for a left forehead lesion that was diagnosed via shave biopsy as a desmoplastic and cystic adnexal neoplasm with suspicion for desmoplastic trichoepithelioma or MAC (Figure 1). Upon presentation for MMS, a well-healed, 1.0×0.9-cm scar at the biopsy site on the left forehead was observed (Figure 2A). One stage was obtained by standard MMS technique and sent for intraoperative processing (Figure 2B). Frozen section examination of the first stage demonstrated peripheral margin involvement with syringomatous change confined to the superficial and mid dermis (Figure 3). Before proceeding further, these findings were reviewed with an in-house dermatopathologist, and it was determined that no infiltrative tumor, perineural involvement, or other features to indicate malignancy were noted. A decision was made to refrain from obtaining any additional layers and to send excised Burow triangles for permanent section analysis. A primary linear closure was performed without complication, and the patient was discharged from the ambulatory surgery suite. Histopathologic examination of the Burow triangles later confirmed findings consistent with plaquelike syringoma with no evidence of malignancy (Figure 4).

Syringomas present as small flesh-colored papules in the periorbital areas. These benign neoplasms previously have been classified into 4 major clinical variants: localized, generalized, Down syndrome associated, and familial.1 The lesser-known plaquelike variant of syringoma was first described by Kikuchi et al2 in 1979. Aside from our report, a PubMed search of articles indexed for MEDLINE using the terms plaquelike or plaque-type syringoma yielded 16 cases in the literature.2-14 Of these, 6 were referred to or encountered in the MMS setting.8,9,11,12,14 Plaquelike syringoma can be solitary or multiple in presentation.6 It most commonly involves the head and neck but also can present on the trunk, arms, legs, and groin areas. The clinical size of plaquelike syringoma is variable, with the largest reported cases extending several centimeters in diameter.2,6 Similar to reported associations with conventional syringoma, the plaquelike subtype of syringoma has been reported in association with Down syndrome.13

Histopathologically, plaquelike syringoma shares features with MAC as well as desmoplastic trichoepithelioma and desmoplastic basal cell carcinoma. Plaquelike syringoma demonstrates broad proliferations of small tubules morphologically reminiscent of tadpoles confined within the dermis. Ducts typically are lined with 2 or 3 layers of small cuboidal cells. Microcystic adnexal carcinoma typically features asymmetric ductal structures lined with single cells extending from the dermis into the subcutis and even underlying muscle, cartilage, or bone.8 There are no reliable immunohistochemical stains to differentiate between these 2 entities; thus, the primary distinction lies in the depth of involvement. Desmoplastic trichoepithelioma is composed of narrow cords and nests of basaloid cells of follicular origin commonly admixed with small cornifying cysts appearing in the dermis.8 Colonizing Merkel cells positive for cytokeratin 20 often are present in desmoplastic trichoepithelioma and not in syringoma or MAC.15 Desmoplastic basal cell carcinoma demonstrates narrow strands of basaloid cells of follicular origin appearing in the dermis. Desmoplastic trichoepithelioma and desmoplastic basal cell carcinoma are each fundamentally differentiated from plaquelike syringoma in that proliferations of cords and nests are not of eccrine or apocrine origin.

Several cases of plaquelike syringoma have been challenging to distinguish from MAC in performing MMS.8,9,11 Underlying extension of this syringoma variant can be far-reaching, extending to several centimeters in size and involving multiple cosmetic subunits.6,11,14 Inadvertent overtreatment with multiple MMS stages can be avoided with careful recognition of the differentiating histopathologic features. Syringomatous lesions commonly are encountered in MMS and may even be present at the edge of other tumor types. Plaquelike syringoma has been reported as a coexistent entity with nodular basal cell carcinoma.12 Boos et al16 similarly reported the presence of deceptive ductal proliferations along the immediate peripheral margin of MAC, which prompted multiple re-excisions. Pursuit of permanent section analysis in these cases revealed the appearance of small syringomas, and a diagnosis of benign subclinical syringomatous proliferations was made, averting further intervention.16
Our case sheds light on the threat of commission bias in dermatologic surgery, which is the tendency for action rather than inaction.17 In this context, it is important to avoid the perspective that harm to the patient can only be prevented by active intervention. Cognitive bias has been increasingly recognized as a source of medical error, and methods to mitigate bias in medical practice have been well described.17 Microcystic adnexal carcinoma and plaquelike syringoma can be hard to differentiate especially initially, as demonstrated in our case, which particularly illustrates the importance of slowing down a surgical case at the appropriate time, considering and revisiting alternative diagnoses, implementing checklists, and seeking histopathologic collaboration with colleagues when necessary. Our attempted implementation of these principles, especially early collaboration with colleagues, led to intraoperative recognition of plaquelike syringoma within the first stage of MMS.
We seek to raise the index of suspicion for plaquelike syringoma among dermatologists and dermatologic surgeons, especially when syringomatous structures are limited to the superficial dermis. We encourage familiarity with the plaquelike syringoma entity as well as careful consideration of further investigation via scouting biopsies or permanent section analysis when other characteristic features of MAC are unclear or lacking. Adequate sampling as well as collaboration with a dermatopathologist in cases of suspected syringoma can help to reduce the susceptibility to commission bias and prevent histopathologic pitfalls and unwarranted surgical morbidity.
To the Editor:
Plaquelike or plaque-type syringoma is a lesser-known variant of syringoma that can appear histologically indistinguishable from the superficial portion of microcystic adnexal carcinoma (MAC). The plaquelike variant of syringoma holds a benign clinical course, and no treatment is necessary. Microcystic adnexal carcinoma is distinguished from plaquelike syringoma by an aggressive growth pattern with a high risk for local invasion and recurrence if inadequately treated. Thus, treatment with Mohs micrographic surgery (MMS) has been recommended as the mainstay for MAC. If superficial biopsy specimens reveal suspicion for MAC and patients are referred for MMS, careful consideration should be made to differentiate MAC and plaquelike syringoma early to prevent unnecessary morbidity.

A 78-year-old woman was referred for MMS for a left forehead lesion that was diagnosed via shave biopsy as a desmoplastic and cystic adnexal neoplasm with suspicion for desmoplastic trichoepithelioma or MAC (Figure 1). Upon presentation for MMS, a well-healed, 1.0×0.9-cm scar at the biopsy site on the left forehead was observed (Figure 2A). One stage was obtained by standard MMS technique and sent for intraoperative processing (Figure 2B). Frozen section examination of the first stage demonstrated peripheral margin involvement with syringomatous change confined to the superficial and mid dermis (Figure 3). Before proceeding further, these findings were reviewed with an in-house dermatopathologist, and it was determined that no infiltrative tumor, perineural involvement, or other features to indicate malignancy were noted. A decision was made to refrain from obtaining any additional layers and to send excised Burow triangles for permanent section analysis. A primary linear closure was performed without complication, and the patient was discharged from the ambulatory surgery suite. Histopathologic examination of the Burow triangles later confirmed findings consistent with plaquelike syringoma with no evidence of malignancy (Figure 4).

Syringomas present as small flesh-colored papules in the periorbital areas. These benign neoplasms previously have been classified into 4 major clinical variants: localized, generalized, Down syndrome associated, and familial.1 The lesser-known plaquelike variant of syringoma was first described by Kikuchi et al2 in 1979. Aside from our report, a PubMed search of articles indexed for MEDLINE using the terms plaquelike or plaque-type syringoma yielded 16 cases in the literature.2-14 Of these, 6 were referred to or encountered in the MMS setting.8,9,11,12,14 Plaquelike syringoma can be solitary or multiple in presentation.6 It most commonly involves the head and neck but also can present on the trunk, arms, legs, and groin areas. The clinical size of plaquelike syringoma is variable, with the largest reported cases extending several centimeters in diameter.2,6 Similar to reported associations with conventional syringoma, the plaquelike subtype of syringoma has been reported in association with Down syndrome.13

Histopathologically, plaquelike syringoma shares features with MAC as well as desmoplastic trichoepithelioma and desmoplastic basal cell carcinoma. Plaquelike syringoma demonstrates broad proliferations of small tubules morphologically reminiscent of tadpoles confined within the dermis. Ducts typically are lined with 2 or 3 layers of small cuboidal cells. Microcystic adnexal carcinoma typically features asymmetric ductal structures lined with single cells extending from the dermis into the subcutis and even underlying muscle, cartilage, or bone.8 There are no reliable immunohistochemical stains to differentiate between these 2 entities; thus, the primary distinction lies in the depth of involvement. Desmoplastic trichoepithelioma is composed of narrow cords and nests of basaloid cells of follicular origin commonly admixed with small cornifying cysts appearing in the dermis.8 Colonizing Merkel cells positive for cytokeratin 20 often are present in desmoplastic trichoepithelioma and not in syringoma or MAC.15 Desmoplastic basal cell carcinoma demonstrates narrow strands of basaloid cells of follicular origin appearing in the dermis. Desmoplastic trichoepithelioma and desmoplastic basal cell carcinoma are each fundamentally differentiated from plaquelike syringoma in that proliferations of cords and nests are not of eccrine or apocrine origin.

Several cases of plaquelike syringoma have been challenging to distinguish from MAC in performing MMS.8,9,11 Underlying extension of this syringoma variant can be far-reaching, extending to several centimeters in size and involving multiple cosmetic subunits.6,11,14 Inadvertent overtreatment with multiple MMS stages can be avoided with careful recognition of the differentiating histopathologic features. Syringomatous lesions commonly are encountered in MMS and may even be present at the edge of other tumor types. Plaquelike syringoma has been reported as a coexistent entity with nodular basal cell carcinoma.12 Boos et al16 similarly reported the presence of deceptive ductal proliferations along the immediate peripheral margin of MAC, which prompted multiple re-excisions. Pursuit of permanent section analysis in these cases revealed the appearance of small syringomas, and a diagnosis of benign subclinical syringomatous proliferations was made, averting further intervention.16
Our case sheds light on the threat of commission bias in dermatologic surgery, which is the tendency for action rather than inaction.17 In this context, it is important to avoid the perspective that harm to the patient can only be prevented by active intervention. Cognitive bias has been increasingly recognized as a source of medical error, and methods to mitigate bias in medical practice have been well described.17 Microcystic adnexal carcinoma and plaquelike syringoma can be hard to differentiate especially initially, as demonstrated in our case, which particularly illustrates the importance of slowing down a surgical case at the appropriate time, considering and revisiting alternative diagnoses, implementing checklists, and seeking histopathologic collaboration with colleagues when necessary. Our attempted implementation of these principles, especially early collaboration with colleagues, led to intraoperative recognition of plaquelike syringoma within the first stage of MMS.
We seek to raise the index of suspicion for plaquelike syringoma among dermatologists and dermatologic surgeons, especially when syringomatous structures are limited to the superficial dermis. We encourage familiarity with the plaquelike syringoma entity as well as careful consideration of further investigation via scouting biopsies or permanent section analysis when other characteristic features of MAC are unclear or lacking. Adequate sampling as well as collaboration with a dermatopathologist in cases of suspected syringoma can help to reduce the susceptibility to commission bias and prevent histopathologic pitfalls and unwarranted surgical morbidity.
- Friedman SJ, Butler DF. Syringoma presenting as milia. J Am Acad Dermatol. 1987;16:310-314.
- Kikuchi I, Idemori M, Okazaki M. Plaque type syringoma. J Dermatol. 1979;6:329-331.
- Dekio S, Jidoi J. Submammary syringoma—report of a case. J Dermatol. 1988;15:351-352.
- Patrizi A, Neri I, Marzaduri S, et al. Syringoma: a review of twenty-nine cases. Acta Derm Venereol. 1998;78:460-462.
- Nguyen DB, Patterson JW, Wilson BB. Syringoma of the moustache area. J Am Acad Dermatol. 2003;49:337-339.
- Rongioletti F, Semino MT, Rebora A. Unilateral multiple plaque-like syringomas. Br J Dermatol. 1996;135:623-625.
- Chi HI. A case of unusual syringoma: unilateral linear distribution and plaque formation. J Dermatol. 1996;23:505-506.
- Suwatee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Wallace JS, Bond JS, Seidel GD, et al. An important mimicker: plaque-type syringoma mistakenly diagnosed as microcystic adnexal carcinoma. Dermatol Surg. 2014;40:810-812.
- Mitkov M, Balagula Y, Taube JM, et al. Plaque-like syringoma with involvement of deep reticular dermis. J Am Acad Dermatol. 2014;71:E206-E207.
- Schleich C, Ferringer T, Petrick M. Plaque type syringoma mimicking a microcystic adnexal carcinoma. J Am Acad Dermatol. 2016;74(suppl 1):AB287.
- Yang Y, Srivastava D. Plaque-type syringoma coexisting with basal cell carcinoma. Dermatol Surg. 2018;44:1464-1466.
- Motegi SI, Sekiguchi A, Fujiwara C, et al. Milia-like idiopathic calcinosis cutis and plaque-type syringoma in a girl with Down syndrome. J Dermatol. 2019;46:E136-E137.
- Clark M, Duprey C, Sutton A, et al. Plaque-type syringoma masquerading as microcystic adnexal carcinoma: review of the literature and description of a novel technique that emphasizes lesion architecture to help make the diagnosis. Am J Dermatopathol. 2019;41:E98-E101.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Boos MD, Elenitsas R, Seykora J, et al. Benign subclinical syringomatous proliferations adjacent to a microcystic adnexal carcinoma: a tumor mimic with significant patient implications. Am J Dermatopathol. 2014;36:174-178.
- O’Sullivan ED, Schofield SJ. Cognitive bias in clinical medicine. J R Coll Physicians Edinb. 2018;48:225-232.
- Friedman SJ, Butler DF. Syringoma presenting as milia. J Am Acad Dermatol. 1987;16:310-314.
- Kikuchi I, Idemori M, Okazaki M. Plaque type syringoma. J Dermatol. 1979;6:329-331.
- Dekio S, Jidoi J. Submammary syringoma—report of a case. J Dermatol. 1988;15:351-352.
- Patrizi A, Neri I, Marzaduri S, et al. Syringoma: a review of twenty-nine cases. Acta Derm Venereol. 1998;78:460-462.
- Nguyen DB, Patterson JW, Wilson BB. Syringoma of the moustache area. J Am Acad Dermatol. 2003;49:337-339.
- Rongioletti F, Semino MT, Rebora A. Unilateral multiple plaque-like syringomas. Br J Dermatol. 1996;135:623-625.
- Chi HI. A case of unusual syringoma: unilateral linear distribution and plaque formation. J Dermatol. 1996;23:505-506.
- Suwatee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Wallace JS, Bond JS, Seidel GD, et al. An important mimicker: plaque-type syringoma mistakenly diagnosed as microcystic adnexal carcinoma. Dermatol Surg. 2014;40:810-812.
- Mitkov M, Balagula Y, Taube JM, et al. Plaque-like syringoma with involvement of deep reticular dermis. J Am Acad Dermatol. 2014;71:E206-E207.
- Schleich C, Ferringer T, Petrick M. Plaque type syringoma mimicking a microcystic adnexal carcinoma. J Am Acad Dermatol. 2016;74(suppl 1):AB287.
- Yang Y, Srivastava D. Plaque-type syringoma coexisting with basal cell carcinoma. Dermatol Surg. 2018;44:1464-1466.
- Motegi SI, Sekiguchi A, Fujiwara C, et al. Milia-like idiopathic calcinosis cutis and plaque-type syringoma in a girl with Down syndrome. J Dermatol. 2019;46:E136-E137.
- Clark M, Duprey C, Sutton A, et al. Plaque-type syringoma masquerading as microcystic adnexal carcinoma: review of the literature and description of a novel technique that emphasizes lesion architecture to help make the diagnosis. Am J Dermatopathol. 2019;41:E98-E101.
- Abesamis-Cubillan E, El-Shabrawi-Caelen L, LeBoit PE. Merkel cells and sclerosing epithelial neoplasms. Am J Dermatopathol. 2000;22:311-315.
- Boos MD, Elenitsas R, Seykora J, et al. Benign subclinical syringomatous proliferations adjacent to a microcystic adnexal carcinoma: a tumor mimic with significant patient implications. Am J Dermatopathol. 2014;36:174-178.
- O’Sullivan ED, Schofield SJ. Cognitive bias in clinical medicine. J R Coll Physicians Edinb. 2018;48:225-232.
Practice Points
- Dermatologists should familiarize themselves with the plaquelike subtype of syringoma, which can histologically mimic the superficial portion of microcystic adnexal carcinoma (MAC).
- Careful recognition of plaquelike syringoma in the Mohs micrographic surgery setting may prevent unnecessary surgical morbidity.
- Further diagnostic investigation is warranted for superficial biopsies suggestive of MAC or when other characteristic features are lacking.
Blue Nodules on the Forearms in an Active-Duty Military Servicemember
The Diagnosis: Glomangiomyoma
A punch biopsy of the right forearm revealed a collection of vascular and smooth muscle components with small and spindled bland cells containing minimal eosinophilic cytoplasm (Figure 1), confirming the diagnosis of glomangiomyoma. Immunohistochemical stains also supported the diagnosis and were positive for smooth muscle actin, desmin, and CD34 (Figure 2). Magnetic resonance imaging from a prior attempt at treatment with sclerotherapy demonstrated scattered vascular malformations with no notable internal derangement. There was no improvement with sclerotherapy. Given the number and vascular nature of the lesions, a trial of pulsed dye laser (PDL) therapy was administered and tolerated by the patient. He subsequently moved to a new military duty station. On follow-up, he reported no noticeable clinical improvement in the lesions after PDL and opted not to continue with laser treatment.

Glomangiomyoma is a rare and benign glomus tumor variant that demonstrates differentiation into the smooth muscle and potentially can result in substantial complications.1 Glomus tumors generally are benign neoplasms of the glomus apparatus, and glomus cells function as thermoregulators in the reticular dermis.2 Glomus tumors comprise less than 2% of soft tissue neoplasms and generally are solitary nodules; only 10% of glomus tumors occur with multiple lesions, and among them, glomangiomyoma is the rarest subtype, presenting in only 15% of cases.2,3 The 3 main subtypes of glomus tumors are solid, glomangioma, and glomangiomyoma.4 Clinically, the lesions may present as small blue nodules with associated pain and cold or pressure sensitivity. Although there appears to be variation of the nomenclature depending on the source in the literature, glomangiomas are characterized by their predominant vascular malformations on biopsy. Glomangiomyomas are a subset of glomus tumors with distinct smooth muscle differentiation.4 Given their pathologic presentation, our patient’s lesions were most consistent with the diagnosis of glomangiomyoma.
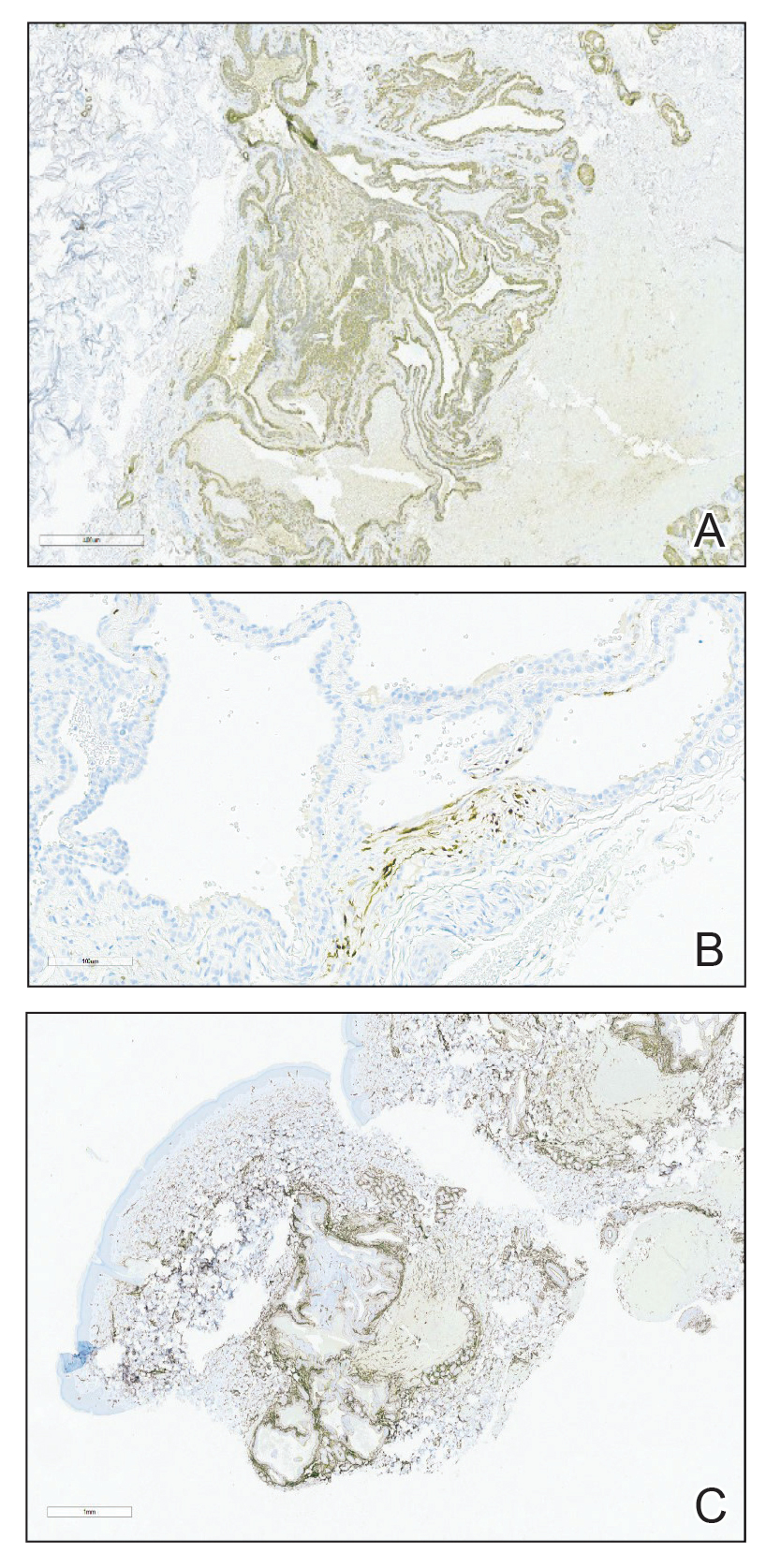
The small size of the lesions may result in difficulty establishing a clinical diagnosis, particularly if there is no hand involvement, where lesions most commonly occur.2 Therefore, histopathologic evaluation is essential and is the best initial step in evaluating glomangiomyomas.4 Biopsy is the most reliable means of confirming a diagnosis2,4,5; however, diagnostic imaging such as a computed tomography also should be performed if considering blue rubber bleb nevus syndrome due to the primary site of involvement. Surgical excision is the treatment of choice after confirming the diagnosis in most cases of symptomatic glomangiomyomas, particularly with painful lesions.6
Neurilemmomas (also known as schwannomas) are benign lesions that generally present as asymptomatic, soft, smooth nodules most often on the neck; however, they also may present on the flexor extremities or in internal organs. Although primarily asymptomatic, the tumors may be associated with pain and paresthesia as they enlarge and affect surrounding structures. Neurilemmomas may occur spontaneously or as part of a syndrome, such as neurofibromatosis type 2 or Carney complex.7
Hereditary hemorrhagic telangiectasia (formerly known as Osler-Weber-Rendu syndrome) is an autosomal-dominant disease that presents with arteriovenous malformations and telangiectases. Patients generally present in the third decade of life, with the main concern generally being epistaxis.8
Kaposi sarcoma is a viral infection secondary to human herpesvirus 8 that results in red-purple lesions commonly on mucocutaneous sites. Kaposi sarcoma can be AIDS associated and non-HIV associated. Although clinically indistinguishable, a few subtle histologic features can assist in differentiating the 2 etiologies. In addition to a potential history of immunodeficiency, evaluating for involvement of the lymphatic system, respiratory tract, or gastrointestinal tract can aid in differentiating this entity from glomus tumors.9
Leiomyomas are smooth muscle lesions divided into 3 subcategories: angioleiomyoma, piloleiomyoma, and genital leiomyoma. The clinical presentation and histopathology will vary depending on the subcategory. Although cutaneous leiomyomas are benign, further workup for piloleiomyoma may be required given the reported association with hereditary leiomyomatosis and renal cell cancer (Reed syndrome).10
Imaging can be helpful when the clinical diagnosis of a glomus tumor vs other painful neoplasms of the skin is unclear, such as in blue rubber bleb nevus syndrome, angioleiomyomas, neuromas, glomus tumors, leiomyomas, eccrine spiradenomas, congenital vascular malformations, schwannomas, or hemangiomas.4 Radiologic findings for glomus tumors may demonstrate cortical or cystic osseous defects. Magnetic resonance imaging and ultrasonography can help provide additional information on the lesion size and depth of involvement.1 Additionally, deeper glomangiomyomas have been associated with malignancy,2 potentially highlighting the benefit of early incorporation of imaging in the workup for this condition. Malignant transformation is rare and has been reported in less than 1% of cases.6
Treatment of glomus tumors predominantly is directed to the patient’s symptoms; asymptomatic lesions may be monitored.4 For symptomatic lesions, therapeutic options include wide local excision; sclerotherapy; and incorporation of various lasers, including Nd:YAG, CO2, and flashlamp tunable dye laser.4,5 One case report documented use of a PDL that successfully eliminated the pain associated with glomangiomyoma; however, the lesion in that report was not biopsy proven.11
Our case highlights the need to consider glomus tumors in patients presenting with multiple small nodules given the potential for misdiagnosis, impact on quality of life with associated psychological distress, and potential utility of incorporating PDL in treatment. Although our patient did not report clinical improvement in the appearance of the lesions with PDL therapy, additional treatment sessions may have helped,11 but he opted to discontinue. Follow-up for persistently symptomatic or changing lesions is necessary, given the minimal risk for malignant transformation.6
- Lee DY, Hwang SC, Jeong ST, et al. The value of diagnostic ultrasonography in the assessment of a glomus tumor of the subcutaneous layer of the forearm mimicking a hemangioma: a case report. J Med Case Rep. 2015;9:191. doi:10.1186/s13256-015-0672-y
- Li L, Bardsley V, Grainger A, et al. Extradigital glomangiomyoma of the forearm mimicking peripheral nerve sheath tumour and thrombosed varicose vein. BMJ Case Rep. 2021;14:E241221. doi: 10.1136 /bcr-2020-241221
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mohammadi O, Suarez M. Glomus cancer. StatPearls [Internet]. StatPearls Publishing; 2021.
- Maxey ML, Houghton CC, Mastriani KS, et al. Large prepatellar glomangioma: a case report [published online July 10, 2015]. Int J Surg Case Rep. 2015;14:80-84. doi:10.1016/j.ijscr.2015.07.002
- Brathwaite CD, Poppiti RJ Jr. Malignant glomus tumor. a case report of widespread metastases in a patient with multiple glomus body hamartomas. Am J Surg Pathol. 1996;20:233-238. doi:10.1097/00000478-199602000-00012
- Davis DD, Kane SM. Neurilemmoma. StatPearls [Internet]. StatPearls Publishing; 2022.
- Kühnel T, Wirsching K, Wohlgemuth W, et al. Hereditary hemorrhagic telangiectasia. Otolaryngol Clin North Am. 2018;51:237-254. doi:10.1016/j.otc.2017.09.017
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294. doi:10.5858/arpa.2012-0101-RS
- Bernett CN, Mammino JJ. Cutaneous leiomyomas. StatPearls [Internet]. StatPearls Publishing; 2022.
- Antony FC, Cliff S, Cowley N. Complete pain relief following treatment of a glomangiomyoma with the pulsed dye laser. Clin Exp Dermatol. 2003;28:617-619. doi:10.1046/j.1365-2230.2003.01403.x
The Diagnosis: Glomangiomyoma
A punch biopsy of the right forearm revealed a collection of vascular and smooth muscle components with small and spindled bland cells containing minimal eosinophilic cytoplasm (Figure 1), confirming the diagnosis of glomangiomyoma. Immunohistochemical stains also supported the diagnosis and were positive for smooth muscle actin, desmin, and CD34 (Figure 2). Magnetic resonance imaging from a prior attempt at treatment with sclerotherapy demonstrated scattered vascular malformations with no notable internal derangement. There was no improvement with sclerotherapy. Given the number and vascular nature of the lesions, a trial of pulsed dye laser (PDL) therapy was administered and tolerated by the patient. He subsequently moved to a new military duty station. On follow-up, he reported no noticeable clinical improvement in the lesions after PDL and opted not to continue with laser treatment.

Glomangiomyoma is a rare and benign glomus tumor variant that demonstrates differentiation into the smooth muscle and potentially can result in substantial complications.1 Glomus tumors generally are benign neoplasms of the glomus apparatus, and glomus cells function as thermoregulators in the reticular dermis.2 Glomus tumors comprise less than 2% of soft tissue neoplasms and generally are solitary nodules; only 10% of glomus tumors occur with multiple lesions, and among them, glomangiomyoma is the rarest subtype, presenting in only 15% of cases.2,3 The 3 main subtypes of glomus tumors are solid, glomangioma, and glomangiomyoma.4 Clinically, the lesions may present as small blue nodules with associated pain and cold or pressure sensitivity. Although there appears to be variation of the nomenclature depending on the source in the literature, glomangiomas are characterized by their predominant vascular malformations on biopsy. Glomangiomyomas are a subset of glomus tumors with distinct smooth muscle differentiation.4 Given their pathologic presentation, our patient’s lesions were most consistent with the diagnosis of glomangiomyoma.

The small size of the lesions may result in difficulty establishing a clinical diagnosis, particularly if there is no hand involvement, where lesions most commonly occur.2 Therefore, histopathologic evaluation is essential and is the best initial step in evaluating glomangiomyomas.4 Biopsy is the most reliable means of confirming a diagnosis2,4,5; however, diagnostic imaging such as a computed tomography also should be performed if considering blue rubber bleb nevus syndrome due to the primary site of involvement. Surgical excision is the treatment of choice after confirming the diagnosis in most cases of symptomatic glomangiomyomas, particularly with painful lesions.6
Neurilemmomas (also known as schwannomas) are benign lesions that generally present as asymptomatic, soft, smooth nodules most often on the neck; however, they also may present on the flexor extremities or in internal organs. Although primarily asymptomatic, the tumors may be associated with pain and paresthesia as they enlarge and affect surrounding structures. Neurilemmomas may occur spontaneously or as part of a syndrome, such as neurofibromatosis type 2 or Carney complex.7
Hereditary hemorrhagic telangiectasia (formerly known as Osler-Weber-Rendu syndrome) is an autosomal-dominant disease that presents with arteriovenous malformations and telangiectases. Patients generally present in the third decade of life, with the main concern generally being epistaxis.8
Kaposi sarcoma is a viral infection secondary to human herpesvirus 8 that results in red-purple lesions commonly on mucocutaneous sites. Kaposi sarcoma can be AIDS associated and non-HIV associated. Although clinically indistinguishable, a few subtle histologic features can assist in differentiating the 2 etiologies. In addition to a potential history of immunodeficiency, evaluating for involvement of the lymphatic system, respiratory tract, or gastrointestinal tract can aid in differentiating this entity from glomus tumors.9
Leiomyomas are smooth muscle lesions divided into 3 subcategories: angioleiomyoma, piloleiomyoma, and genital leiomyoma. The clinical presentation and histopathology will vary depending on the subcategory. Although cutaneous leiomyomas are benign, further workup for piloleiomyoma may be required given the reported association with hereditary leiomyomatosis and renal cell cancer (Reed syndrome).10
Imaging can be helpful when the clinical diagnosis of a glomus tumor vs other painful neoplasms of the skin is unclear, such as in blue rubber bleb nevus syndrome, angioleiomyomas, neuromas, glomus tumors, leiomyomas, eccrine spiradenomas, congenital vascular malformations, schwannomas, or hemangiomas.4 Radiologic findings for glomus tumors may demonstrate cortical or cystic osseous defects. Magnetic resonance imaging and ultrasonography can help provide additional information on the lesion size and depth of involvement.1 Additionally, deeper glomangiomyomas have been associated with malignancy,2 potentially highlighting the benefit of early incorporation of imaging in the workup for this condition. Malignant transformation is rare and has been reported in less than 1% of cases.6
Treatment of glomus tumors predominantly is directed to the patient’s symptoms; asymptomatic lesions may be monitored.4 For symptomatic lesions, therapeutic options include wide local excision; sclerotherapy; and incorporation of various lasers, including Nd:YAG, CO2, and flashlamp tunable dye laser.4,5 One case report documented use of a PDL that successfully eliminated the pain associated with glomangiomyoma; however, the lesion in that report was not biopsy proven.11
Our case highlights the need to consider glomus tumors in patients presenting with multiple small nodules given the potential for misdiagnosis, impact on quality of life with associated psychological distress, and potential utility of incorporating PDL in treatment. Although our patient did not report clinical improvement in the appearance of the lesions with PDL therapy, additional treatment sessions may have helped,11 but he opted to discontinue. Follow-up for persistently symptomatic or changing lesions is necessary, given the minimal risk for malignant transformation.6
The Diagnosis: Glomangiomyoma
A punch biopsy of the right forearm revealed a collection of vascular and smooth muscle components with small and spindled bland cells containing minimal eosinophilic cytoplasm (Figure 1), confirming the diagnosis of glomangiomyoma. Immunohistochemical stains also supported the diagnosis and were positive for smooth muscle actin, desmin, and CD34 (Figure 2). Magnetic resonance imaging from a prior attempt at treatment with sclerotherapy demonstrated scattered vascular malformations with no notable internal derangement. There was no improvement with sclerotherapy. Given the number and vascular nature of the lesions, a trial of pulsed dye laser (PDL) therapy was administered and tolerated by the patient. He subsequently moved to a new military duty station. On follow-up, he reported no noticeable clinical improvement in the lesions after PDL and opted not to continue with laser treatment.

Glomangiomyoma is a rare and benign glomus tumor variant that demonstrates differentiation into the smooth muscle and potentially can result in substantial complications.1 Glomus tumors generally are benign neoplasms of the glomus apparatus, and glomus cells function as thermoregulators in the reticular dermis.2 Glomus tumors comprise less than 2% of soft tissue neoplasms and generally are solitary nodules; only 10% of glomus tumors occur with multiple lesions, and among them, glomangiomyoma is the rarest subtype, presenting in only 15% of cases.2,3 The 3 main subtypes of glomus tumors are solid, glomangioma, and glomangiomyoma.4 Clinically, the lesions may present as small blue nodules with associated pain and cold or pressure sensitivity. Although there appears to be variation of the nomenclature depending on the source in the literature, glomangiomas are characterized by their predominant vascular malformations on biopsy. Glomangiomyomas are a subset of glomus tumors with distinct smooth muscle differentiation.4 Given their pathologic presentation, our patient’s lesions were most consistent with the diagnosis of glomangiomyoma.

The small size of the lesions may result in difficulty establishing a clinical diagnosis, particularly if there is no hand involvement, where lesions most commonly occur.2 Therefore, histopathologic evaluation is essential and is the best initial step in evaluating glomangiomyomas.4 Biopsy is the most reliable means of confirming a diagnosis2,4,5; however, diagnostic imaging such as a computed tomography also should be performed if considering blue rubber bleb nevus syndrome due to the primary site of involvement. Surgical excision is the treatment of choice after confirming the diagnosis in most cases of symptomatic glomangiomyomas, particularly with painful lesions.6
Neurilemmomas (also known as schwannomas) are benign lesions that generally present as asymptomatic, soft, smooth nodules most often on the neck; however, they also may present on the flexor extremities or in internal organs. Although primarily asymptomatic, the tumors may be associated with pain and paresthesia as they enlarge and affect surrounding structures. Neurilemmomas may occur spontaneously or as part of a syndrome, such as neurofibromatosis type 2 or Carney complex.7
Hereditary hemorrhagic telangiectasia (formerly known as Osler-Weber-Rendu syndrome) is an autosomal-dominant disease that presents with arteriovenous malformations and telangiectases. Patients generally present in the third decade of life, with the main concern generally being epistaxis.8
Kaposi sarcoma is a viral infection secondary to human herpesvirus 8 that results in red-purple lesions commonly on mucocutaneous sites. Kaposi sarcoma can be AIDS associated and non-HIV associated. Although clinically indistinguishable, a few subtle histologic features can assist in differentiating the 2 etiologies. In addition to a potential history of immunodeficiency, evaluating for involvement of the lymphatic system, respiratory tract, or gastrointestinal tract can aid in differentiating this entity from glomus tumors.9
Leiomyomas are smooth muscle lesions divided into 3 subcategories: angioleiomyoma, piloleiomyoma, and genital leiomyoma. The clinical presentation and histopathology will vary depending on the subcategory. Although cutaneous leiomyomas are benign, further workup for piloleiomyoma may be required given the reported association with hereditary leiomyomatosis and renal cell cancer (Reed syndrome).10
Imaging can be helpful when the clinical diagnosis of a glomus tumor vs other painful neoplasms of the skin is unclear, such as in blue rubber bleb nevus syndrome, angioleiomyomas, neuromas, glomus tumors, leiomyomas, eccrine spiradenomas, congenital vascular malformations, schwannomas, or hemangiomas.4 Radiologic findings for glomus tumors may demonstrate cortical or cystic osseous defects. Magnetic resonance imaging and ultrasonography can help provide additional information on the lesion size and depth of involvement.1 Additionally, deeper glomangiomyomas have been associated with malignancy,2 potentially highlighting the benefit of early incorporation of imaging in the workup for this condition. Malignant transformation is rare and has been reported in less than 1% of cases.6
Treatment of glomus tumors predominantly is directed to the patient’s symptoms; asymptomatic lesions may be monitored.4 For symptomatic lesions, therapeutic options include wide local excision; sclerotherapy; and incorporation of various lasers, including Nd:YAG, CO2, and flashlamp tunable dye laser.4,5 One case report documented use of a PDL that successfully eliminated the pain associated with glomangiomyoma; however, the lesion in that report was not biopsy proven.11
Our case highlights the need to consider glomus tumors in patients presenting with multiple small nodules given the potential for misdiagnosis, impact on quality of life with associated psychological distress, and potential utility of incorporating PDL in treatment. Although our patient did not report clinical improvement in the appearance of the lesions with PDL therapy, additional treatment sessions may have helped,11 but he opted to discontinue. Follow-up for persistently symptomatic or changing lesions is necessary, given the minimal risk for malignant transformation.6
- Lee DY, Hwang SC, Jeong ST, et al. The value of diagnostic ultrasonography in the assessment of a glomus tumor of the subcutaneous layer of the forearm mimicking a hemangioma: a case report. J Med Case Rep. 2015;9:191. doi:10.1186/s13256-015-0672-y
- Li L, Bardsley V, Grainger A, et al. Extradigital glomangiomyoma of the forearm mimicking peripheral nerve sheath tumour and thrombosed varicose vein. BMJ Case Rep. 2021;14:E241221. doi: 10.1136 /bcr-2020-241221
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mohammadi O, Suarez M. Glomus cancer. StatPearls [Internet]. StatPearls Publishing; 2021.
- Maxey ML, Houghton CC, Mastriani KS, et al. Large prepatellar glomangioma: a case report [published online July 10, 2015]. Int J Surg Case Rep. 2015;14:80-84. doi:10.1016/j.ijscr.2015.07.002
- Brathwaite CD, Poppiti RJ Jr. Malignant glomus tumor. a case report of widespread metastases in a patient with multiple glomus body hamartomas. Am J Surg Pathol. 1996;20:233-238. doi:10.1097/00000478-199602000-00012
- Davis DD, Kane SM. Neurilemmoma. StatPearls [Internet]. StatPearls Publishing; 2022.
- Kühnel T, Wirsching K, Wohlgemuth W, et al. Hereditary hemorrhagic telangiectasia. Otolaryngol Clin North Am. 2018;51:237-254. doi:10.1016/j.otc.2017.09.017
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294. doi:10.5858/arpa.2012-0101-RS
- Bernett CN, Mammino JJ. Cutaneous leiomyomas. StatPearls [Internet]. StatPearls Publishing; 2022.
- Antony FC, Cliff S, Cowley N. Complete pain relief following treatment of a glomangiomyoma with the pulsed dye laser. Clin Exp Dermatol. 2003;28:617-619. doi:10.1046/j.1365-2230.2003.01403.x
- Lee DY, Hwang SC, Jeong ST, et al. The value of diagnostic ultrasonography in the assessment of a glomus tumor of the subcutaneous layer of the forearm mimicking a hemangioma: a case report. J Med Case Rep. 2015;9:191. doi:10.1186/s13256-015-0672-y
- Li L, Bardsley V, Grainger A, et al. Extradigital glomangiomyoma of the forearm mimicking peripheral nerve sheath tumour and thrombosed varicose vein. BMJ Case Rep. 2021;14:E241221. doi: 10.1136 /bcr-2020-241221
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mohammadi O, Suarez M. Glomus cancer. StatPearls [Internet]. StatPearls Publishing; 2021.
- Maxey ML, Houghton CC, Mastriani KS, et al. Large prepatellar glomangioma: a case report [published online July 10, 2015]. Int J Surg Case Rep. 2015;14:80-84. doi:10.1016/j.ijscr.2015.07.002
- Brathwaite CD, Poppiti RJ Jr. Malignant glomus tumor. a case report of widespread metastases in a patient with multiple glomus body hamartomas. Am J Surg Pathol. 1996;20:233-238. doi:10.1097/00000478-199602000-00012
- Davis DD, Kane SM. Neurilemmoma. StatPearls [Internet]. StatPearls Publishing; 2022.
- Kühnel T, Wirsching K, Wohlgemuth W, et al. Hereditary hemorrhagic telangiectasia. Otolaryngol Clin North Am. 2018;51:237-254. doi:10.1016/j.otc.2017.09.017
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294. doi:10.5858/arpa.2012-0101-RS
- Bernett CN, Mammino JJ. Cutaneous leiomyomas. StatPearls [Internet]. StatPearls Publishing; 2022.
- Antony FC, Cliff S, Cowley N. Complete pain relief following treatment of a glomangiomyoma with the pulsed dye laser. Clin Exp Dermatol. 2003;28:617-619. doi:10.1046/j.1365-2230.2003.01403.x
A 31-year-old active-duty military servicemember presented to the dermatology clinic for evaluation of 0.3- to 2-cm, tender, blue nodules on the wrists and forearms. The lesions first appeared on the right volar wrist secondary to a presumed injury sustained approximately 10 years prior to presentation and spread to the proximal forearm as well as the left wrist and forearm. He denied fevers, chills, chest pain, hematochezia, hematuria, or other skin findings. Physical examination revealed blue-violaceous, firm nodules on the right volar wrist and forearm that were tender to palpation. Blue-violaceous, papulonodular lesions on the left volar wrist and dorsal hand were not tender to palpation. A punch biopsy was performed.
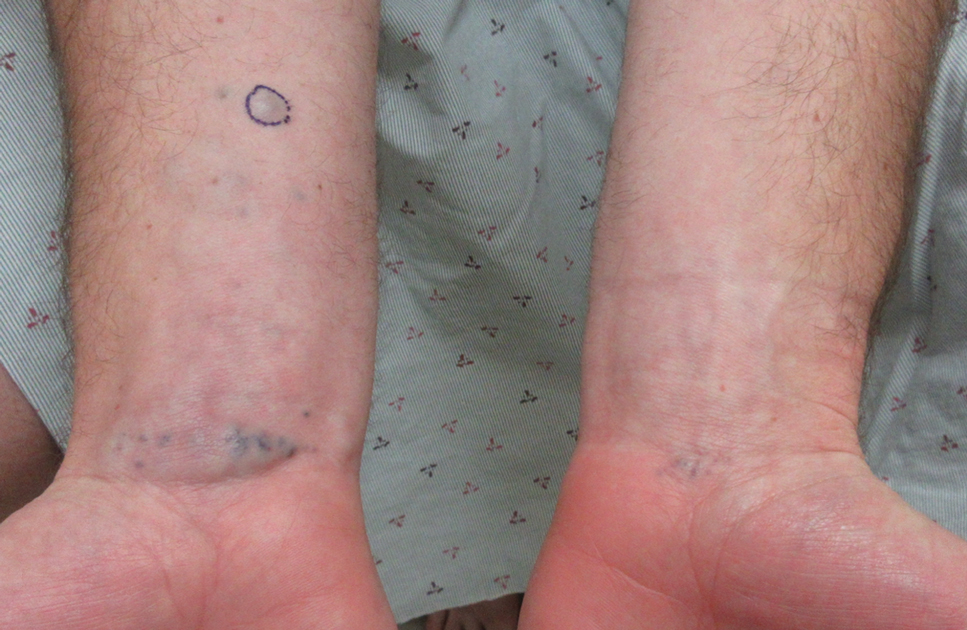
Iododerma Simulating Cryptococcal Infection
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-

The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.

Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-

The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.

Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
To the Editor:
A woman in her 40s presented with acute onset of rapidly spreading lesions on the face, trunk, and extremities. She reported high fever and endorsed malaise. She had a history of end-stage renal disease and was on renal dialysis. She recently underwent revision of an arteriovenous fistula.
Physical examination revealed diffuse, erythematous, firm papules and plaques with central hemorrhage and umbilication on the dorsal aspect of the nose, forehead, temples, and cheeks. There also were purpuric papules and plaques with a peripheral rim of vesiculation (Figure 1) on the medial and posterior thighs and buttocks. Histopathology of a biopsy specimen revealed an interstitial neutrophilic infiltrate in the superficial dermis and mid dermis with scattered, haloed, acellular structures simulating cryptococcal organisms (Figure 2). Periodic acid–Schiff (PAS), Grocott methenamine-silver, and mucicarmine staining was negative. Repeat biopsy showed similar findings. A (1-3)-β-

The findings compatible with a diagnosis of iododerma included umbilicated hemorrhagic papules and plaques, cryptococcal-like structures with negative staining on histopathology, and elevated iodine levels with a negative infectious workup. The patient was treated with topical corticosteroids. At 1-month follow-up, the lesions had resolved.

Iododerma is a halogenoderma, a skin eruption that occurs after ingestion of or exposure to a halogen-containing substance (eg, iodine, bromine, fluorine) or medication (eg, lithium).1 Common sources of iodine include iodinated contrast media, potassium iodide ingestion, topical application of povidone–iodine, radioactive iodine administration, and the antiarrhythmic amiodarone. Excess exposure to iodine-containing compounds typically occurs in the setting of kidney disease or failure as well as due to reduced iodine clearance.1 Although the pathogenesis of iododerma is unknown, the most common hypothesis is that lesions are delayed hypersensitivity reactions secondary to formation of a protein-halogen complex.2
The presentation of iododerma is polymorphous and includes acneform, vegetative, or pustular eruptions; umbilicated papules and plaques can be present.2,3 Lesions can be either asymptomatic or painful and pruritic. Timing between iodine exposure and onset of lesions varies from hours to days to years.2,4
Systemic symptoms of iododerma can occur, including salivary gland swelling, hypotension and bradycardia, kidney injury, or thyroid and liver abnormalities. Histopathologic analysis demonstrates a dense neutrophilic dermatitis with negative staining for infectious causes.4,5 Cryptococcal-like structures have been described in iododerma3; neutrophilic dermatoses of various causes that mimic cryptococcal infection have been reported.6 Ultimately, iododerma remains a diagnosis of exclusion.
Withdrawal of an offending compound is remedial. Dialysis is beneficial in end-stage renal disease. Topical, intralesional, and systemic corticosteroids, as well as antibiotics, provide variable benefit.4,7 Lesions can take 4 to 6 weeks to clear after withdrawal of the offending agent. It is unclear whether recurrences happen; iodine-containing compounds need to be avoided after a patient has been affected.
Iododerma has a broad differential diagnosis due to the polymorphous presentation of the disorder, including acute febrile neutrophilic dermatosis (also known as Sweet syndrome), cutaneous cryptococcosis, and cutaneous histoplasmosis. Sweet syndrome presents as abrupt onset of edematous erythematous plaques with fever and leukocytosis. It is associated with infection, inflammatory disorders, medication, and malignancy.8 Histopathologic analysis reveals papillary dermal edema and a neutrophilic dermatosis. Cytoplasmic vacuolization resembling C neoformans has been reported.9 The diagnosis is less favored in the presence of renal disease, temporal association of the eruption with iodine exposure, and elevated blood and urine iodine levels, as in our patient.
Cutaneous cryptococcosis, an infection caused by C neoformans, typically occurs secondary to dissemination from the lungs; rarely, the disease is primary. Acneform plaques, vegetative plaques, and umbilicated lesions are seen.10 Histopathologic analysis shows characteristic yeast forms of cryptococcosis surrounded by gelatinous edema, which create a haloed effect, typically throughout the dermis. Capsules are positive for PAS or mucicarmine staining. Although C neoformans can closely mimic iododerma both clinically and histopathologically, negative infectious staining, localization of haloed structures to the upper dermis, a negative test for cryptococcal antigen, and elevated blood and urine iodine levels in this case all favored iododerma.
Cutaneous histoplasmosis is an infection caused by Histoplasma capsulatum, most commonly as secondary dissemination from pulmonary infection but rarely from direct inoculation of the skin.11 Presentation includes erythematous to hemorrhagic, umbilicated papules and plaques. Histopathologic findings are round to oval, narrow-based, budding yeasts that stain positive for PAS or mucicarmine. Although histoplasmosis can clinically mimic iododerma, the disease is distinguished histologically by the presence of fungal microorganisms that lack the gelatinous edema and haloed effect of iododerma.
We presented a unique case of iododerma simulating cryptococcal infection both clinically and histopathologically. Prompt recognition of histologic mimickers of true infectious microorganisms is essential to prevent unnecessary delay of withdrawal of the offending substance and to initiate appropriate therapy.
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
- Alagheband M, Engineer L. Lithium and halogenoderma. Arch Dermatol. 2000;136:126-127. doi:10.1001/archderm.136.1.126
- Young AL, Grossman ME. Acute iododerma secondary to iodinated contrast media. Br J Dermatol. 2014;170:1377-1379. doi:10.1111/bjd.12852
- Runge M, Williams K, Scharnitz T, et al. Iodine toxicity after iodinated contrast: new observations in iododerma. JAAD Case Rep. 2020;6:319-322. doi:10.1016/j.jdcr.2020.02.006
- Chalela JG, Aguilar L. Iododerma from contrast material. N Engl J Med. 2016;374:2477. doi:10.1056/NEJMicm1512512
- Chang MW, Miner JE, Moiin A, et al. Iododerma after computed tomographic scan with intravenous radiopaque contrast media. J Am Acad Dermatol. 1997;36:1014-1016. doi:10.1016/s0190-9622(97)80291-5
- Ko JS, Fernandez AP, Anderson KA, et al. Morphologic mimickers of Cryptococcus occurring within inflammatory infiltrates in the setting of neutrophilic dermatitis: a series of three cases highlighting clinical dilemmas associated with a novel histopathologic pitfall. J Cutan Pathol. 2013;40:38-45. doi:10.1111/cup.12019
- Pranteda G, Grimaldi M, Salzetta M, et al. Vegetating iododerma and pulmonary eosinophilic infiltration. a simple co-occurrence? Acta Derm Venereol. 2004;84:480-481.
- Nelson CA, Stephen S, Ashchyan HJ, et al. M. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/j.jaad.2017.11.064
- Wilson J, Gleghorn K, Kelly B. Cryptococcoid Sweet’s syndrome: two reports of Sweet’s syndrome mimicking cutaneous cryptococcosis. J Cutan Pathol. 2017;44:413-419. doi:10.1111/cup.12921
- Beatson M, Harwood M, Reese V, et al. Primary cutaneous cryptococcosis in an elderly pigeon breeder. JAAD Case Rep. 2019;5:433-435. doi:10.1016/j.jdcr.2019.03.006
- Raggio B. Primary cutaneous histoplasmosis. Ear Nose Throat J. 2018;97:346-348. doi:10.1177/0145561318097010-1108
Practice Points
- Halogenodermas are rare cutaneous reactions to excess exposure to or ingestion of halogen-containing drugs or substances such as bromine, iodine (iododerma), fluorine, and rarely lithium.
- The clinical presentation of a halogenoderma varies; the most characteristic manifestation is a vegetative or exudative plaque with a peripheral rim of pustules.
- Histologically, lesions of a halogenoderma are characterized by pseudoepitheliomatous hyperplasia associated with numerous intraepidermal microabscesses overlying a dense mixed inflammatory infiltrate of neutrophils, plasma cells, eosinophils, histiocytes, and scattered multinucleated giant cells.
- Rarely, the dermal infiltrate of a halogenoderma contains abundant acellular bodies surrounded by capsulelike vacuolated spaces mimicking Cryptococcus neoformans.
Reticular Hyperpigmentation With Keratotic Papules in the Axillae and Groin
The Diagnosis: Galli-Galli Disease
Several cutaneous conditions can present as reticulated hyperpigmentation or keratotic papules. Although genetic testing can help identify some of these dermatoses, biopsy typically is sufficient for diagnosis, and genetic testing can be considered for more clinically challenging cases. In our case, the clinical evidence and histopathologic findings were diagnostic of Galli-Galli disease (GGD), an autosomal-dominant genodermatosis with incomplete penetrance. Our patient was unaware of any family members with a diagnosis of GGD; however, she reported a great uncle with similar clinical findings.
Galli-Galli disease is a rare allelic variant of Dowling- Degos disease (DDD), both caused by a loss-of-function mutation in the keratin 5 gene, KRT5. Both conditions present as reticulated papules distributed symmetrically in the flexural regions, most commonly the axillae and groin, but also as comedolike papules, typically in patients aged 30 to 50 years.1 Cutaneous lesions primarily are of cosmetic concern but can be extremely pruritic, especially for patients with GGD. Gene mutations in protein O-fucosyltransferase 1, POFUT1; protein O-glucosyltransferase 1, POGLUT1; and presenilin enhancer 2, PSENEN, also have been discovered in cases of DDD and GGD.2,3
Galli-Galli disease and DDD are distinguishable by their histologic appearance. Both diseases show elongated fingerlike rete ridges and a thin suprapapillary epidermis. The basal projections often are described as bulbous or resembling antler horns.4 Galli-Galli disease can be differentiated from DDD by focal suprabasal acantholysis with minimal dyskeratosis (quiz images).5 Due to the genetic and clinical similarities, many consider GGD an acantholytic variant of DDD rather than its own entity. Indeed, some patients have shown acantholysis in one area of biopsy but not others.6
Hailey-Hailey disease (HHD)(also known as benign familial or benign chronic pemphigus) is an autosomaldominant disorder caused by mutation of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1. Clinically, patients tend to present at a wide age range with fragile flaccid vesicles that commonly develop on the neck, axillae, and groin. Histologically, the epidermis is acanthotic with a dilapidated brick wall– like appearance from a few persistent intercellular connections amid widespread acantholysis (Figure 1).7 Unlike in autoimmune pemphigus, direct immunofluorescence is negative, and acantholysis spares the adnexal structures. Hailey-Hailey disease does not involve reticulated hyperpigmentation or the elongated bulbous rete seen in GGD. Confluent and reticulated papillomatosis is a rare, typically asymptomatic, hyperpigmented dermatosis. It presents as a conglomeration of scaly hyperpigmented macules or papillomatous papules that coalesce centrally and are reticulated toward the periphery.
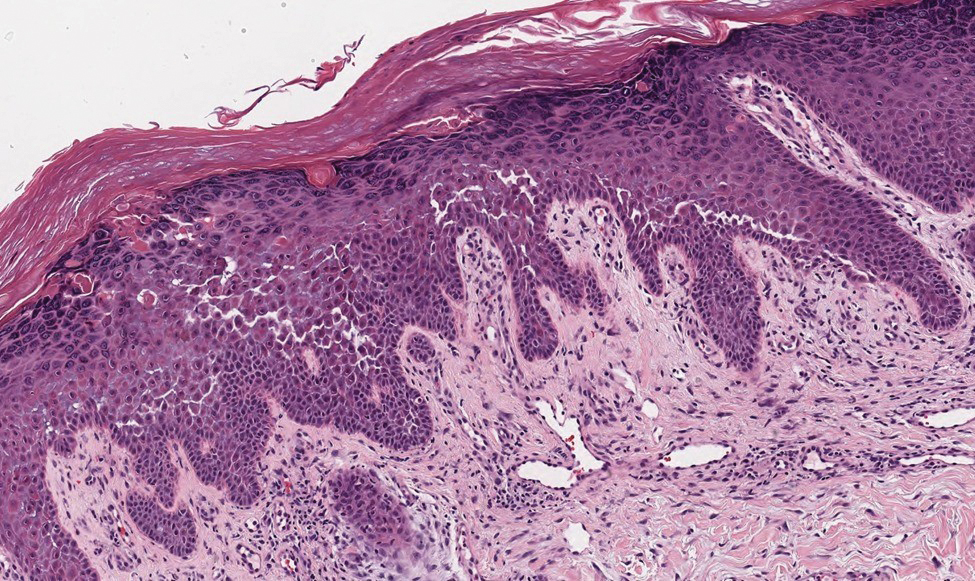
Confluent and reticulated papillomatosis most commonly is seen on the trunk, initially presenting in adolescents and young adults. Confluent and reticulated papillomatosis is histologically similar to acanthosis nigricans. Histopathology will show hyperkeratosis, papillomatosis, and minimal to no inflammatory infiltrate, with no elongated rete ridges or acantholysis (Figure 2).8
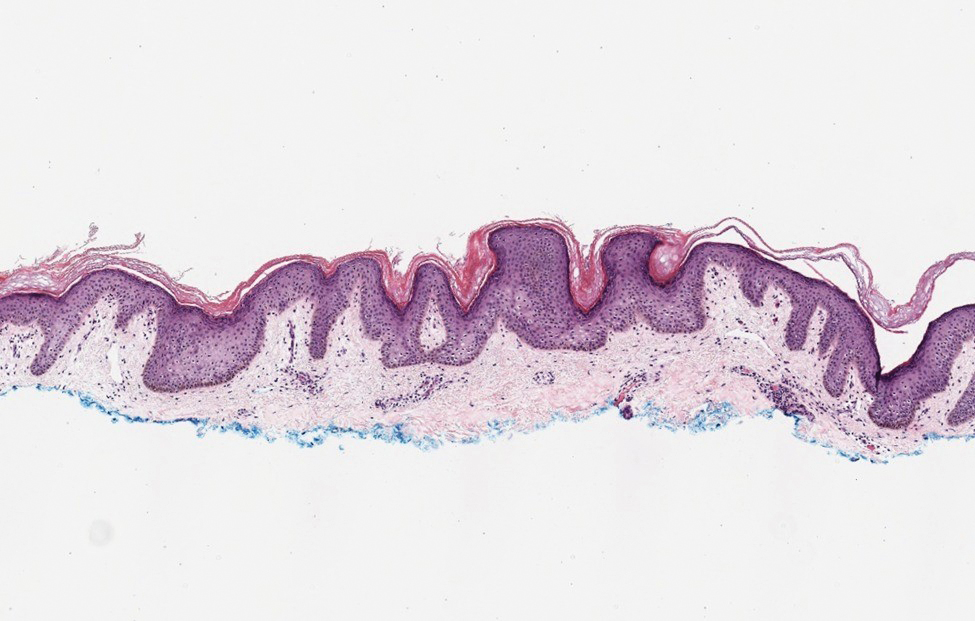
Pemphigus vulgaris is a blistering disease resulting from the development of autoantibodies against desmogleins 1 and 3. Similar to GGD, there is suprabasal acantholysis, which often results in a tombstonelike appearance consisting of separation between the basal layer cells of the epidermis but with maintained attachment to the underlying basement membrane zone. Unlike HHD, the acantholysis tends to involve the follicular epithelium in pemphigus vulgaris (Figure 3). Clinically, the blisters are positive for Nikolsky sign and can be both cutaneous or mucosal, commonly arising initially in the mouth during the fourth or fifth decades of life. Ruptured blisters can result in painful and hemorrhagic erosions.9 Direct immunofluorescence exhibits a classic chicken wire–like deposition of IgG and C3 between keratinocytes of the epidermis. Although sometimes difficult to appreciate, the deposition can be more prominent in the lower epidermis, in contrast to pemphigus foliaceus, which can have more prominent deposition in the upper epidermis.

Darier disease (or dyskeratosis follicularis) is an autosomal-dominant genodermatosis caused by mutation of the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene, ATP2A2. Clinically, this disorder arises in adolescents as red-brown, greasy, crusted papules in seborrheic areas that may coalesce into papillomatous clusters. Palmar punctate keratoses and pits also are common. Histologically, Darier disease can appear similar to GGD, as both can show acantholysis and dyskeratosis. Darier disease will tend to show more prominent dyskeratosis with corps ronds and grains, as well as thicker villilike projections of keratinocytes into the papillary dermis, in contrast to the thinner, fingerlike or bulbous projections that hang down from the epidermis in GGD (Figure 4).10
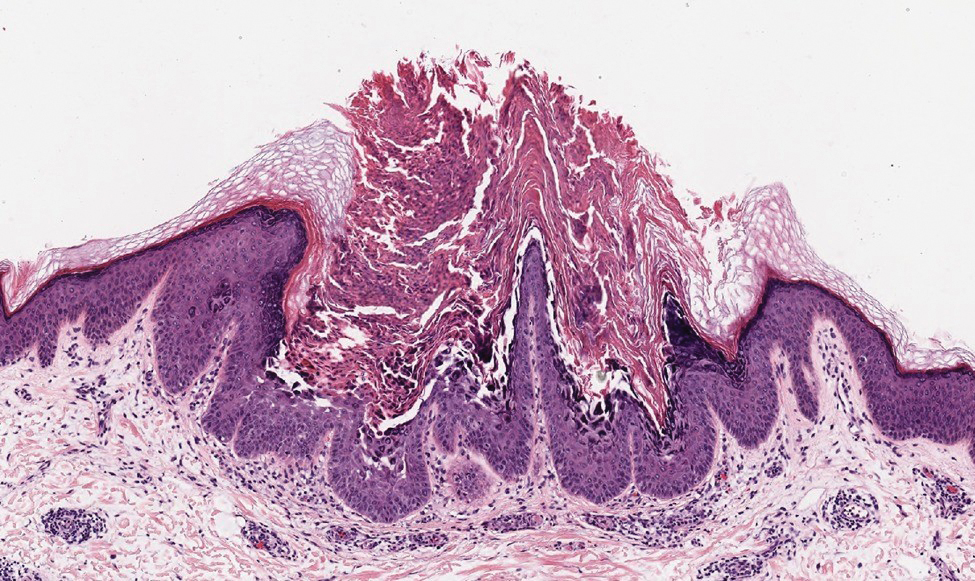
- Hanneken S, Rütten A, Eigelshoven S, et al. Morbus Galli-Galli. Hautarzt. 2013;64:282.
- Wilson NJ, Cole C, Kroboth K, et al. Mutations in POGLUT1 in Galli- Galli/Dowling-Degos disease. Br J Dermatol. 2017;176:270-274.
- Ralser DJ, Basmanav FB, Tafazzoli A, et al. Mutations in γ-secretase subunit–encoding PSENEN underlie Dowling-Degos disease associated with acne inversa. J Clin Invest. 2017;127:1485-1490.
- Desai CA, Virmani N, Sakhiya J, et al. An uncommon presentation of Galli-Galli disease. Indian J Dermatol Venereol Leprol. 2016; 82:720-723.
- Joshi TP, Shaver S, Tschen J. Exacerbation of Galli-Galli disease following dialysis treatment: a case report and review of aggravating factors. Cureus. 2021;13:E15401.
- Muller CS, Pfohler C, Tilgen W. Changing a concept—controversy on the confusion spectrum of the reticulate pigmented disorders of the skin. J Cutan Pathol. 2008;36:44-48.
- Dai Y, Yu L, Wang Y, et al. Case report: a case of Hailey-Hailey disease mimicking condyloma acuminatum and a novel splice-site mutation of ATP2C1 gene. Front Genet. 2021;12:777630.
- Banjar TA, Abdulwahab RA, Al Hawsawi KA. Confluent and reticulated papillomatosis of Gougerot and Carteaud: a case report and review of the literature. Cureus. 2022;14:E24557.
- Porro AM, Seque CA, Ferreira MCC, et al. Pemphigus vulgaris. An Bras Dermatol. 2019;94:264-278.
- Bachar-Wikström E, Wikström JD. Darier disease—a multi-organ condition? Acta Derm Venereol. 2021;101:adv00430.
The Diagnosis: Galli-Galli Disease
Several cutaneous conditions can present as reticulated hyperpigmentation or keratotic papules. Although genetic testing can help identify some of these dermatoses, biopsy typically is sufficient for diagnosis, and genetic testing can be considered for more clinically challenging cases. In our case, the clinical evidence and histopathologic findings were diagnostic of Galli-Galli disease (GGD), an autosomal-dominant genodermatosis with incomplete penetrance. Our patient was unaware of any family members with a diagnosis of GGD; however, she reported a great uncle with similar clinical findings.
Galli-Galli disease is a rare allelic variant of Dowling- Degos disease (DDD), both caused by a loss-of-function mutation in the keratin 5 gene, KRT5. Both conditions present as reticulated papules distributed symmetrically in the flexural regions, most commonly the axillae and groin, but also as comedolike papules, typically in patients aged 30 to 50 years.1 Cutaneous lesions primarily are of cosmetic concern but can be extremely pruritic, especially for patients with GGD. Gene mutations in protein O-fucosyltransferase 1, POFUT1; protein O-glucosyltransferase 1, POGLUT1; and presenilin enhancer 2, PSENEN, also have been discovered in cases of DDD and GGD.2,3
Galli-Galli disease and DDD are distinguishable by their histologic appearance. Both diseases show elongated fingerlike rete ridges and a thin suprapapillary epidermis. The basal projections often are described as bulbous or resembling antler horns.4 Galli-Galli disease can be differentiated from DDD by focal suprabasal acantholysis with minimal dyskeratosis (quiz images).5 Due to the genetic and clinical similarities, many consider GGD an acantholytic variant of DDD rather than its own entity. Indeed, some patients have shown acantholysis in one area of biopsy but not others.6
Hailey-Hailey disease (HHD)(also known as benign familial or benign chronic pemphigus) is an autosomaldominant disorder caused by mutation of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1. Clinically, patients tend to present at a wide age range with fragile flaccid vesicles that commonly develop on the neck, axillae, and groin. Histologically, the epidermis is acanthotic with a dilapidated brick wall– like appearance from a few persistent intercellular connections amid widespread acantholysis (Figure 1).7 Unlike in autoimmune pemphigus, direct immunofluorescence is negative, and acantholysis spares the adnexal structures. Hailey-Hailey disease does not involve reticulated hyperpigmentation or the elongated bulbous rete seen in GGD. Confluent and reticulated papillomatosis is a rare, typically asymptomatic, hyperpigmented dermatosis. It presents as a conglomeration of scaly hyperpigmented macules or papillomatous papules that coalesce centrally and are reticulated toward the periphery.

Confluent and reticulated papillomatosis most commonly is seen on the trunk, initially presenting in adolescents and young adults. Confluent and reticulated papillomatosis is histologically similar to acanthosis nigricans. Histopathology will show hyperkeratosis, papillomatosis, and minimal to no inflammatory infiltrate, with no elongated rete ridges or acantholysis (Figure 2).8

Pemphigus vulgaris is a blistering disease resulting from the development of autoantibodies against desmogleins 1 and 3. Similar to GGD, there is suprabasal acantholysis, which often results in a tombstonelike appearance consisting of separation between the basal layer cells of the epidermis but with maintained attachment to the underlying basement membrane zone. Unlike HHD, the acantholysis tends to involve the follicular epithelium in pemphigus vulgaris (Figure 3). Clinically, the blisters are positive for Nikolsky sign and can be both cutaneous or mucosal, commonly arising initially in the mouth during the fourth or fifth decades of life. Ruptured blisters can result in painful and hemorrhagic erosions.9 Direct immunofluorescence exhibits a classic chicken wire–like deposition of IgG and C3 between keratinocytes of the epidermis. Although sometimes difficult to appreciate, the deposition can be more prominent in the lower epidermis, in contrast to pemphigus foliaceus, which can have more prominent deposition in the upper epidermis.

Darier disease (or dyskeratosis follicularis) is an autosomal-dominant genodermatosis caused by mutation of the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene, ATP2A2. Clinically, this disorder arises in adolescents as red-brown, greasy, crusted papules in seborrheic areas that may coalesce into papillomatous clusters. Palmar punctate keratoses and pits also are common. Histologically, Darier disease can appear similar to GGD, as both can show acantholysis and dyskeratosis. Darier disease will tend to show more prominent dyskeratosis with corps ronds and grains, as well as thicker villilike projections of keratinocytes into the papillary dermis, in contrast to the thinner, fingerlike or bulbous projections that hang down from the epidermis in GGD (Figure 4).10

The Diagnosis: Galli-Galli Disease
Several cutaneous conditions can present as reticulated hyperpigmentation or keratotic papules. Although genetic testing can help identify some of these dermatoses, biopsy typically is sufficient for diagnosis, and genetic testing can be considered for more clinically challenging cases. In our case, the clinical evidence and histopathologic findings were diagnostic of Galli-Galli disease (GGD), an autosomal-dominant genodermatosis with incomplete penetrance. Our patient was unaware of any family members with a diagnosis of GGD; however, she reported a great uncle with similar clinical findings.
Galli-Galli disease is a rare allelic variant of Dowling- Degos disease (DDD), both caused by a loss-of-function mutation in the keratin 5 gene, KRT5. Both conditions present as reticulated papules distributed symmetrically in the flexural regions, most commonly the axillae and groin, but also as comedolike papules, typically in patients aged 30 to 50 years.1 Cutaneous lesions primarily are of cosmetic concern but can be extremely pruritic, especially for patients with GGD. Gene mutations in protein O-fucosyltransferase 1, POFUT1; protein O-glucosyltransferase 1, POGLUT1; and presenilin enhancer 2, PSENEN, also have been discovered in cases of DDD and GGD.2,3
Galli-Galli disease and DDD are distinguishable by their histologic appearance. Both diseases show elongated fingerlike rete ridges and a thin suprapapillary epidermis. The basal projections often are described as bulbous or resembling antler horns.4 Galli-Galli disease can be differentiated from DDD by focal suprabasal acantholysis with minimal dyskeratosis (quiz images).5 Due to the genetic and clinical similarities, many consider GGD an acantholytic variant of DDD rather than its own entity. Indeed, some patients have shown acantholysis in one area of biopsy but not others.6
Hailey-Hailey disease (HHD)(also known as benign familial or benign chronic pemphigus) is an autosomaldominant disorder caused by mutation of the ATPase secretory pathway Ca2+ transporting 1 gene, ATP2C1. Clinically, patients tend to present at a wide age range with fragile flaccid vesicles that commonly develop on the neck, axillae, and groin. Histologically, the epidermis is acanthotic with a dilapidated brick wall– like appearance from a few persistent intercellular connections amid widespread acantholysis (Figure 1).7 Unlike in autoimmune pemphigus, direct immunofluorescence is negative, and acantholysis spares the adnexal structures. Hailey-Hailey disease does not involve reticulated hyperpigmentation or the elongated bulbous rete seen in GGD. Confluent and reticulated papillomatosis is a rare, typically asymptomatic, hyperpigmented dermatosis. It presents as a conglomeration of scaly hyperpigmented macules or papillomatous papules that coalesce centrally and are reticulated toward the periphery.

Confluent and reticulated papillomatosis most commonly is seen on the trunk, initially presenting in adolescents and young adults. Confluent and reticulated papillomatosis is histologically similar to acanthosis nigricans. Histopathology will show hyperkeratosis, papillomatosis, and minimal to no inflammatory infiltrate, with no elongated rete ridges or acantholysis (Figure 2).8

Pemphigus vulgaris is a blistering disease resulting from the development of autoantibodies against desmogleins 1 and 3. Similar to GGD, there is suprabasal acantholysis, which often results in a tombstonelike appearance consisting of separation between the basal layer cells of the epidermis but with maintained attachment to the underlying basement membrane zone. Unlike HHD, the acantholysis tends to involve the follicular epithelium in pemphigus vulgaris (Figure 3). Clinically, the blisters are positive for Nikolsky sign and can be both cutaneous or mucosal, commonly arising initially in the mouth during the fourth or fifth decades of life. Ruptured blisters can result in painful and hemorrhagic erosions.9 Direct immunofluorescence exhibits a classic chicken wire–like deposition of IgG and C3 between keratinocytes of the epidermis. Although sometimes difficult to appreciate, the deposition can be more prominent in the lower epidermis, in contrast to pemphigus foliaceus, which can have more prominent deposition in the upper epidermis.

Darier disease (or dyskeratosis follicularis) is an autosomal-dominant genodermatosis caused by mutation of the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene, ATP2A2. Clinically, this disorder arises in adolescents as red-brown, greasy, crusted papules in seborrheic areas that may coalesce into papillomatous clusters. Palmar punctate keratoses and pits also are common. Histologically, Darier disease can appear similar to GGD, as both can show acantholysis and dyskeratosis. Darier disease will tend to show more prominent dyskeratosis with corps ronds and grains, as well as thicker villilike projections of keratinocytes into the papillary dermis, in contrast to the thinner, fingerlike or bulbous projections that hang down from the epidermis in GGD (Figure 4).10

- Hanneken S, Rütten A, Eigelshoven S, et al. Morbus Galli-Galli. Hautarzt. 2013;64:282.
- Wilson NJ, Cole C, Kroboth K, et al. Mutations in POGLUT1 in Galli- Galli/Dowling-Degos disease. Br J Dermatol. 2017;176:270-274.
- Ralser DJ, Basmanav FB, Tafazzoli A, et al. Mutations in γ-secretase subunit–encoding PSENEN underlie Dowling-Degos disease associated with acne inversa. J Clin Invest. 2017;127:1485-1490.
- Desai CA, Virmani N, Sakhiya J, et al. An uncommon presentation of Galli-Galli disease. Indian J Dermatol Venereol Leprol. 2016; 82:720-723.
- Joshi TP, Shaver S, Tschen J. Exacerbation of Galli-Galli disease following dialysis treatment: a case report and review of aggravating factors. Cureus. 2021;13:E15401.
- Muller CS, Pfohler C, Tilgen W. Changing a concept—controversy on the confusion spectrum of the reticulate pigmented disorders of the skin. J Cutan Pathol. 2008;36:44-48.
- Dai Y, Yu L, Wang Y, et al. Case report: a case of Hailey-Hailey disease mimicking condyloma acuminatum and a novel splice-site mutation of ATP2C1 gene. Front Genet. 2021;12:777630.
- Banjar TA, Abdulwahab RA, Al Hawsawi KA. Confluent and reticulated papillomatosis of Gougerot and Carteaud: a case report and review of the literature. Cureus. 2022;14:E24557.
- Porro AM, Seque CA, Ferreira MCC, et al. Pemphigus vulgaris. An Bras Dermatol. 2019;94:264-278.
- Bachar-Wikström E, Wikström JD. Darier disease—a multi-organ condition? Acta Derm Venereol. 2021;101:adv00430.
- Hanneken S, Rütten A, Eigelshoven S, et al. Morbus Galli-Galli. Hautarzt. 2013;64:282.
- Wilson NJ, Cole C, Kroboth K, et al. Mutations in POGLUT1 in Galli- Galli/Dowling-Degos disease. Br J Dermatol. 2017;176:270-274.
- Ralser DJ, Basmanav FB, Tafazzoli A, et al. Mutations in γ-secretase subunit–encoding PSENEN underlie Dowling-Degos disease associated with acne inversa. J Clin Invest. 2017;127:1485-1490.
- Desai CA, Virmani N, Sakhiya J, et al. An uncommon presentation of Galli-Galli disease. Indian J Dermatol Venereol Leprol. 2016; 82:720-723.
- Joshi TP, Shaver S, Tschen J. Exacerbation of Galli-Galli disease following dialysis treatment: a case report and review of aggravating factors. Cureus. 2021;13:E15401.
- Muller CS, Pfohler C, Tilgen W. Changing a concept—controversy on the confusion spectrum of the reticulate pigmented disorders of the skin. J Cutan Pathol. 2008;36:44-48.
- Dai Y, Yu L, Wang Y, et al. Case report: a case of Hailey-Hailey disease mimicking condyloma acuminatum and a novel splice-site mutation of ATP2C1 gene. Front Genet. 2021;12:777630.
- Banjar TA, Abdulwahab RA, Al Hawsawi KA. Confluent and reticulated papillomatosis of Gougerot and Carteaud: a case report and review of the literature. Cureus. 2022;14:E24557.
- Porro AM, Seque CA, Ferreira MCC, et al. Pemphigus vulgaris. An Bras Dermatol. 2019;94:264-278.
- Bachar-Wikström E, Wikström JD. Darier disease—a multi-organ condition? Acta Derm Venereol. 2021;101:adv00430.
A 37-year-old woman presented with multiple hyperkeratotic small papules in the axillae and groin of 1 year’s duration. She reported pruritus and occasional sleep disruption. Subtle background reticulated hyperpigmentation was present. The patient reported that she had a great uncle with similar findings.


Update on Dermatology Reimbursement in 2024
Health care spending in the United States remained relatively flat from 2019 to 2021 and only increased 2.7% in 2021, reaching $4.3 billion or $12,914 per person. Physician services account for 15% of health care spending (Figure). Relative value units (RVUs) signify the time it took a physician to complete a task multiplied by a conversion factor (CF). When RVUs initially were created in 1992 by what is now the Centers for Medicare &Medicaid Services (CMS), the CF was $32.00. Thirty-one years later, the CF is $33.89 in 2023; however, it would be $66.00 if the CF had increased with inflation.1 If the proposed 2024 Medicare physician fee schedule (MPFS) is adopted, the payment formula would decrease by 3.4% ($32.75) relative to the 2023 fee schedule ($33.89), which would be a 9% decrease relative to 2019 ($36.04).2,3 This reduction is due to the budget neutrality adjustment required by changes in RVUs, implementation of the evaluation and management (E/M) add-on code G2211, and proposed increases in primary are services.2,3 Since 2001, Medicare physician payment has declined by 26%.4 Adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index (MEI); (2) an expenditure target performance adjustment; and (3) miscellaneous adjustments, including those for budget neutrality required by law. Despite continued substantial increases in practice expenses, physicians’ reimbursement has remained flat while other service providers, such as those in skilled nursing facilities and hospitals, have received favorable payment increases compared to practice cost inflation and the Consumer Price Index.4
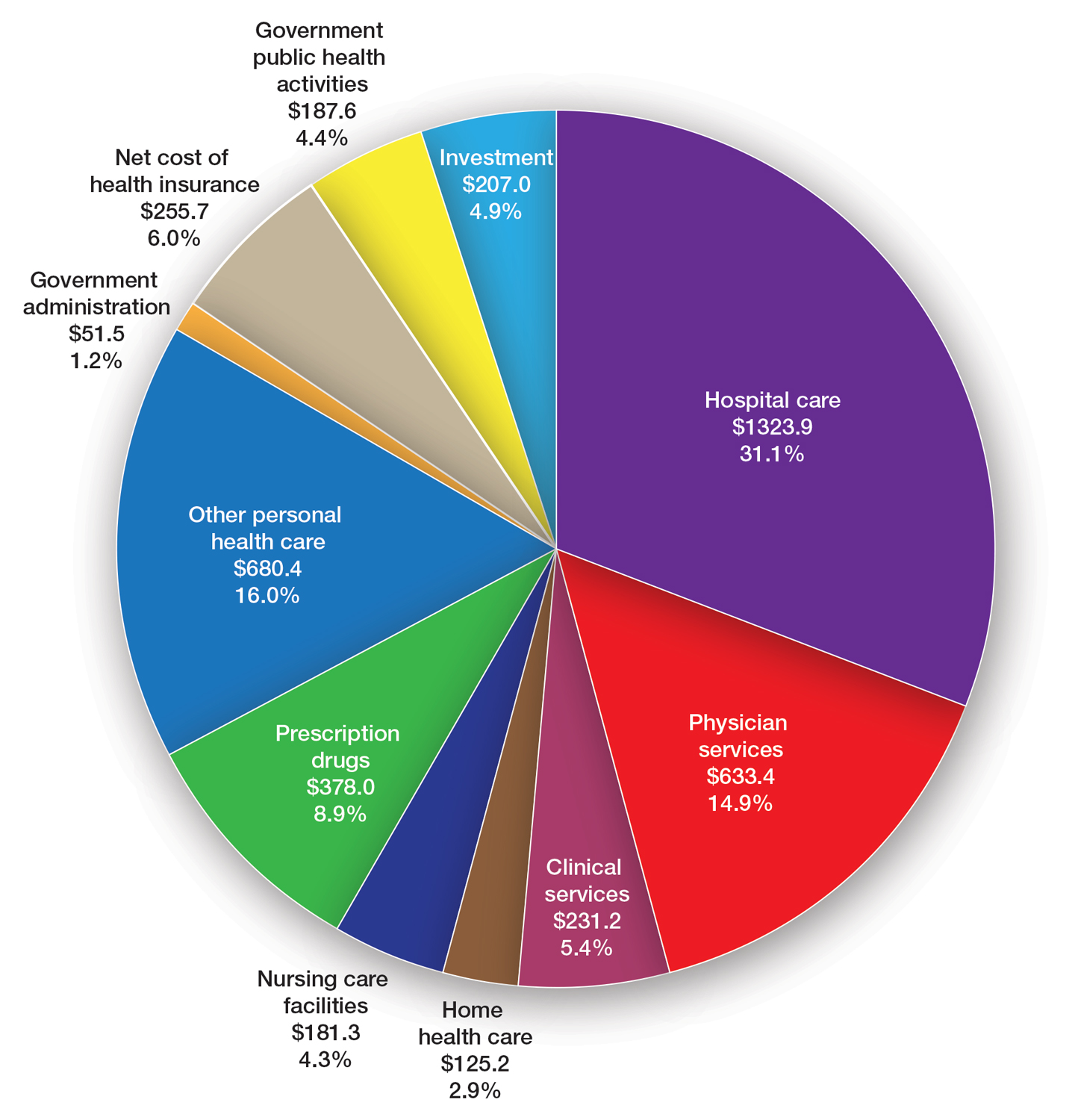
The CMS will not incorporate 2017 MEI cost weights for the RVUs in the MPFS rate setting for 2024 because all key measures of practice expenses in the MEI accelerated in 2022. Instead, the CMS is updating data on practice expense per hour to calculate payment for physician services with a survey for physician practices that launched on July 31, 2023.5 The American Medical Association contracted with Mathematica, an independent research company, to conduct a physician practice information survey that will be used to determine indirect practice expenses. Physicians should be on the lookout for emails regarding completion of these surveys and the appropriate financial expert in their practice should be contacted so the responses are accurate, as these data are key to future updates in the Medicare pay formula used to reimburse physicians.
Impact of Medicare Cuts
The recent congressional debt limit deal set spending caps for the next 2 fiscal years. Dermatology is facing an overall payment reduction of 1.87% (range, 1%–4%).2,3 The impact will depend on the services offered in an individual practice; for example, payment for a punch biopsy (Current Procedural Terminology [CPT] code 11104) would decrease by 3.9%. Payment for benign destruction (CPT code 17110) would decrease by 2.8%, and payment for even simple E/M of an established patient (CPT code 99213) would decrease by 1.6%. Overall, there would be a reduction of 2.75% for dermatopathology services, with a decrease of 2% for CPT code 88305 global and decreases for the technical component of 1% and professional component of 3%.2,3
Medicare cuts have reached a critical level, and physicians cannot continue to absorb the costs to own and operate their practices.4 This has led to health market consolidation, which in turn limits competition and patient access while driving up health care costs and driving down the quality of care. Small independent rural practices as well as those caring for historically marginalized patients will be disproportionately affected.
Proposed Addition of E/M Code G2211
In the calendar year (CY) 2021 final rule, the CMS tried to adopt a new add-on code—G2211—patients with a serious or complex condition that typically require referral and coordination of multispecialty care. Per the CMS, the primary policy goal of G2211 is to increase payments to primary care physicians and to reimburse them more appropriately for the care provided to patients with a serious or complex condition.2,3 It can be reported in conjunction with all office and outpatient E/M visits to better account for additional resources associated with primary care, or similarly ongoing medical care related to a patient’s single, serious condition, or complex condition.3 Typically, G2211 would not be used by dermatologists, as this add-on code requires visit complexity inherent to E/M associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single serious condition or a complex condition.2,3
Initially, the CMS assumed that G2211 would be reported with 90% of all office and outpatient E/M visit claims, which would account for a considerable portion of total MPFS schedule spending; however, the House of Medicine disagreed and believed it would be 75%.2,3 Given the extremely high utilization estimate, G2211 would have had a substantial effect on budget neutrality, accounting for an estimated increase of $3.3 billion and a corresponding 3.0% cut to the CY 2021 MPFS. Because of the potential payment reductions to physicians and a successful advocacy effort by organized medicine, including the American Academy of Dermatology Association (AADA), Congress delayed implementation of G2211 until CY 2024. Modifier -25 cannot be reported with G2211. The CMS revised its utilization assumptions from 90% of all E/M services to an initial utilization of 38% and then 54% when fully adopted. The proposed 2024 payment for G2211 is an additional $16.05.2,3
Advancing Health Equity With Healthcare Common Procedure Coding System G Codes
The CMS is proposing coding and payment for several new services to help underserved populations, including addressing unmet health-related social needs that can potentially interfere with the diagnosis and treatment of medical conditions, which includes paying for certain caregiver training services as well as payment for community health integration services.2,3 These are the first MPFS services designed to include care involving community health workers, who link underserved communities with critical health care and social services in the community. Additionally, the rule also proposes coding and payment for evaluating the risks related to social factors that affect a patient’s health, such as access to affordable quality health care, that can take place during an annual wellness visit or in combination with an E/M visit.2,3 As dermatologists, we should be familiar with this set of G codes, as we will likely use them in practice for patients with transportation needs.
Advocacy Efforts on Medicare Payment Reform
Medicare physician payment reform needs to happen at a national level. Advocacy efforts by the AADA and other groups have been underway to mitigate the proposed 2024 cuts. The Strengthening Medicare for Patients and Providers Act (HR 2474) is a bill that was introduced by a bipartisan coalition of physicians to provide an inflation-based increase in Medicare payments in 2024 and beyond.6
Other Legislative Updates Affecting Dermatology
Modifier -25—Cigna’s policy requiring dermatologists to submit documentation to use modifier -25 when billing with E/M CPT codes 99212 through 99215 has been delayed indefinitely.7 If a payer denies a dermatologist payment, contact the AADA Patient Access and Payer Relations committee ([email protected]) for assistance.
Telehealth and Digital Pathology—Recent legislation authorized extension of many of the Medicare telehealth and digital pathology flexibilities that were put in place during the COVID-19 public health emergency through December 31, 2024.8,9 Seventeen newly approved CPT telemedicine codes for new and established patient audio-visual and audio-only visits recently were surveyed.2,3 The data from the survey will be used as a key element in assigning a specific RVU to the CMS and will be included in the MPFS.
Thirty additional new digital pathology add-on CPT category III codes for 2024 were added to the ones from 2023.2,3 These codes can be used to report additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis. They cannot be used for archival or educational purposes, clinical conferences, training, or validating artificial intelligence algorithms. Category III codes used for emerging technologies have no assigned RVUs or reimbursement.2,3
The Cures Act—The Cures Act aims to ensure that patients have timely access to their health information.10 It requires all physicians to make their office notes, laboratory results, and other diagnostic reports available to patients as soon as the office receives them. The rules went into effect on April 5, 2021, with a limited definition of electronic health information; on October 6, 2022, the Cures Act rule expanded to include all electronic health information. The AADA has urged the Office of the National Coordinator for Health Information Technology to collaborate with stakeholder organizations to re-evaluate federal policies concerning the immediate release of electronic health information and information blocking, particularly in cases with life-altering diagnoses.10 They stressed the importance of prioritizing the well-being and emotional stability of patients and enhancing care by providing patients adequate time and support to process, comprehend, and discuss findings with their physician.
Proposed 2024 Medicare Quality Payment Program Requirements
The CMS proposed to increase the performance threshold in the quality payment program from 75 to 82 points for the 2024 Merit-based Incentive Payment System (MIPS) performance period, impacting the 2026 payment year.2,3,11 As a result of this increase, there could be more MIPS-eligible clinicians receiving penalties, which could be a reduction of up to 9%. The AADA will firmly oppose any increase in the threshold and strongly urge CMS to maintain the 75-point threshold. The performance category weights for the 2024 performance year will remain unchanged from the 2023 performance year.2,3,11
2024 Proposed Quality MIPS Measures Set—The CMS proposed to remove the topped-out MIPS measure 138 (coordination of care for melanoma).2,3,11 Additionally, it proposed to remove MIPS measure 402 (tobacco use and help with quitting among adolescents) as a quality measure from MIPS because the agency believes it is duplicative of measure 226 (preventive care and screening: tobacco use: screening and cessation intervention).2,3,11
MIPS Value Pathways—The CMS consolidated 2 previously established MIPS value pathways (MVPs): the Promoting Wellness MVP and the Optimizing Chronic Disease Management MVP.2,3,11 Proposed new MVPs for 2024 include Focusing on Women’s Health; Quality Care for the Treatment of Ear, Nose, and Throat Disorders; Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV; Quality Care in Mental Health and Substance Use Disorders; and Rehabilitative Support for Musculoskeletal Care. Dermatology is not impacted; however, the CMS plans to sunset traditional MIPS and replace it with MVPs—the future of MIPS.2,3,11 The AADA maintains that traditional MIPS should continue to be an option because MVPs have a limited number of measures for dermatologists.
Update on Reporting Suture Removal
There are 2 new CPT add-on codes—15853 and 15854—for the removal of sutures or staples not requiring anesthesia to be listed separately in addition to an appropriate E/M service. These add-on codes went into effect on January 1, 2023.12 These codes were created with the intent to capture and ensure remuneration for practice expenses that are not included in a stand-alone E/M encounter that occur after a 0-day procedure (eg, services reported with CPT codes 11102–11107 and 11300–11313) for wound check and suture removal where appropriate. These new add-on codes do not have physician work RVUs assigned to them because they are only for practice expenses (eg, clinical staff time, disposable supplies, use of equipment); CPT code 15853 is reported for the removal of sutures or staples, and CPT code 15854 is reported when both sutures and staples are removed. These codes can only be reported if an E/M service also is reported for the patient encounter.12
Final Thoughts
The AADA is working with the House of Medicine and the medical specialty community to develop specific proposals to reform the Medicare payment system.4 The proposed 2024 MPFS was released on July 13, 2023, and final regulations are expected in the late fall of 2023. The AADA will continue to engage with the CMS, but it is important for physicians to learn about and support advocacy priorities and efforts as well as join forces to protect their practices. As health care professionals, we have unique insights into the challenges and needs of our patients and the health care system. Advocacy can take various forms, such as supporting or opposing specific legislations, participating in grassroots campaigns, engaging with policymakers, and/or joining professional organizations that advocate for health care–related issues. Get involved, stay informed, and stay engaged through dermatology medical societies; together we can make a difference.
- Centers for Medicare & Medicaid Services. NHE fact sheet. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Medicare and Medicaid Programs; CY 2024 payment policies under the physician fee schedule and other changes to part B payment and coverage policies; Medicare shared savings program requirements; Medicare advantage; Medicare and Medicaid provider and supplier enrollment policies; and basic health program. Fed Regist. 2023;88:52262-53197. To be codified at 42 CFR §405, §410, §411, §414, §415, §418, §422, §423, §424, §425, §455, §489, §491, §495, §498, and §600. https://www.federalregister.gov/documents/2023/08/07/2023-14624/medicare-and-medicaid-programs-cy-2024-payment-policies-under-the-physician-fee-schedule-and-other
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2024 Medicare physician fee schedule proposed rule. Published July 13, 2023. Accessed September 18, 2023. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-proposed-rule
- American Medical Association. Payment reform. Accessed September 18, 2023. https://www.ama-assn.org/health-care-advocacypayment-reform
- American Medical Association. Physician answers on this survey will shape future Medicare pay. Published July 31, 2023. Accessed September 18, 2023. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future -medicare-pay
- Strengthening Medicare for Patients and Providers Act, HR 2474, 118 Congress (2023-2024). https://www.congress.gov/bill/118th-congress/house-bill/2474
- American Academy of Dermatology Association. Academy advocacy priorities. Accessed September 18, 2023. https://www.aad.org/member/advocacy/priorities
- College of American Pathologists. Remote sign-out of cases with digital pathology FAQs. Accessed September 18, 2023. https://www.cap.org/covid-19/remote-sign-out-faqs
- Centers for Medicare & Medicaid Services. Telehealth. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/medicare/coverage/telehealth
- The Office of the National Coordinator for Health Information Technology. ONC’s Cures Act final rule. Accessed September 18, 2023. https://www.healthit.gov/topic/oncs-cures-act-final-rule
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Notice of Proposed Rule Making Quality Payment Program Policy Overview: Proposals and Requests for Information. Accessed September 12, 2023. https://email.aadresources.org/e3t/Ctc/I6+113/cVKqx04/VVWzj43dDbctW8c23GW1ZLnJHW1xTZ7Q50Y DYN89Qzy5nCVhV3Zsc37CgFV9W5Ck4-D42qs9BW38PtXn4LSlNLW1QKpPL4xT8BMW6Mcwww3FdwCHN3vfGTMXbtF-W2-Zzfy5WHDg6W88tx1F1KgsgxW7zDzT46C2sFXW800vQJ3lLsS_W5D6f1d30-f3cN1njgZ_dX7xkW447ldH2-kgc5VCs7Xg1GY6dsN87pLVJqJG5XW8VWwD-7VxVkJN777f5fJL7jBW8RxkQM1lcSDjVV746T3C-stpN52V_S5xj7q6W3_vldf3p1Yk2Vbd4ZD3cPrHqW5Pwv9m567fkzW1vfDm51H-T7rW1jVrxl8gstXyW5RVTn8863CVFW8g6LgK2YdhpkW34HC4z3_pGYgW8V_qWH3g-tTlW4S3RD-1dKry7W4_rW8d1ssZ1fVwXQjQ9krVMW8Y0bTt8Nr5CNW6vbG0h3wyx59W8WCrNW50p5n6W1r-VBC2rKh93N4W2RyYr7vvm3kxG1
- Centers for Medicare & Medicaid Services. Chapter III surgery: integumentary system CPT codes 10000-19999 for Medicare national correct coding initiative policy manual. Updated January 1, 2023. Accessed September 26, 2023. https://www.cms.gov/files/document/medicare-ncci-policy-manual-2023-chapter-3.pdf
Health care spending in the United States remained relatively flat from 2019 to 2021 and only increased 2.7% in 2021, reaching $4.3 billion or $12,914 per person. Physician services account for 15% of health care spending (Figure). Relative value units (RVUs) signify the time it took a physician to complete a task multiplied by a conversion factor (CF). When RVUs initially were created in 1992 by what is now the Centers for Medicare &Medicaid Services (CMS), the CF was $32.00. Thirty-one years later, the CF is $33.89 in 2023; however, it would be $66.00 if the CF had increased with inflation.1 If the proposed 2024 Medicare physician fee schedule (MPFS) is adopted, the payment formula would decrease by 3.4% ($32.75) relative to the 2023 fee schedule ($33.89), which would be a 9% decrease relative to 2019 ($36.04).2,3 This reduction is due to the budget neutrality adjustment required by changes in RVUs, implementation of the evaluation and management (E/M) add-on code G2211, and proposed increases in primary are services.2,3 Since 2001, Medicare physician payment has declined by 26%.4 Adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index (MEI); (2) an expenditure target performance adjustment; and (3) miscellaneous adjustments, including those for budget neutrality required by law. Despite continued substantial increases in practice expenses, physicians’ reimbursement has remained flat while other service providers, such as those in skilled nursing facilities and hospitals, have received favorable payment increases compared to practice cost inflation and the Consumer Price Index.4

The CMS will not incorporate 2017 MEI cost weights for the RVUs in the MPFS rate setting for 2024 because all key measures of practice expenses in the MEI accelerated in 2022. Instead, the CMS is updating data on practice expense per hour to calculate payment for physician services with a survey for physician practices that launched on July 31, 2023.5 The American Medical Association contracted with Mathematica, an independent research company, to conduct a physician practice information survey that will be used to determine indirect practice expenses. Physicians should be on the lookout for emails regarding completion of these surveys and the appropriate financial expert in their practice should be contacted so the responses are accurate, as these data are key to future updates in the Medicare pay formula used to reimburse physicians.
Impact of Medicare Cuts
The recent congressional debt limit deal set spending caps for the next 2 fiscal years. Dermatology is facing an overall payment reduction of 1.87% (range, 1%–4%).2,3 The impact will depend on the services offered in an individual practice; for example, payment for a punch biopsy (Current Procedural Terminology [CPT] code 11104) would decrease by 3.9%. Payment for benign destruction (CPT code 17110) would decrease by 2.8%, and payment for even simple E/M of an established patient (CPT code 99213) would decrease by 1.6%. Overall, there would be a reduction of 2.75% for dermatopathology services, with a decrease of 2% for CPT code 88305 global and decreases for the technical component of 1% and professional component of 3%.2,3
Medicare cuts have reached a critical level, and physicians cannot continue to absorb the costs to own and operate their practices.4 This has led to health market consolidation, which in turn limits competition and patient access while driving up health care costs and driving down the quality of care. Small independent rural practices as well as those caring for historically marginalized patients will be disproportionately affected.
Proposed Addition of E/M Code G2211
In the calendar year (CY) 2021 final rule, the CMS tried to adopt a new add-on code—G2211—patients with a serious or complex condition that typically require referral and coordination of multispecialty care. Per the CMS, the primary policy goal of G2211 is to increase payments to primary care physicians and to reimburse them more appropriately for the care provided to patients with a serious or complex condition.2,3 It can be reported in conjunction with all office and outpatient E/M visits to better account for additional resources associated with primary care, or similarly ongoing medical care related to a patient’s single, serious condition, or complex condition.3 Typically, G2211 would not be used by dermatologists, as this add-on code requires visit complexity inherent to E/M associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single serious condition or a complex condition.2,3
Initially, the CMS assumed that G2211 would be reported with 90% of all office and outpatient E/M visit claims, which would account for a considerable portion of total MPFS schedule spending; however, the House of Medicine disagreed and believed it would be 75%.2,3 Given the extremely high utilization estimate, G2211 would have had a substantial effect on budget neutrality, accounting for an estimated increase of $3.3 billion and a corresponding 3.0% cut to the CY 2021 MPFS. Because of the potential payment reductions to physicians and a successful advocacy effort by organized medicine, including the American Academy of Dermatology Association (AADA), Congress delayed implementation of G2211 until CY 2024. Modifier -25 cannot be reported with G2211. The CMS revised its utilization assumptions from 90% of all E/M services to an initial utilization of 38% and then 54% when fully adopted. The proposed 2024 payment for G2211 is an additional $16.05.2,3
Advancing Health Equity With Healthcare Common Procedure Coding System G Codes
The CMS is proposing coding and payment for several new services to help underserved populations, including addressing unmet health-related social needs that can potentially interfere with the diagnosis and treatment of medical conditions, which includes paying for certain caregiver training services as well as payment for community health integration services.2,3 These are the first MPFS services designed to include care involving community health workers, who link underserved communities with critical health care and social services in the community. Additionally, the rule also proposes coding and payment for evaluating the risks related to social factors that affect a patient’s health, such as access to affordable quality health care, that can take place during an annual wellness visit or in combination with an E/M visit.2,3 As dermatologists, we should be familiar with this set of G codes, as we will likely use them in practice for patients with transportation needs.
Advocacy Efforts on Medicare Payment Reform
Medicare physician payment reform needs to happen at a national level. Advocacy efforts by the AADA and other groups have been underway to mitigate the proposed 2024 cuts. The Strengthening Medicare for Patients and Providers Act (HR 2474) is a bill that was introduced by a bipartisan coalition of physicians to provide an inflation-based increase in Medicare payments in 2024 and beyond.6
Other Legislative Updates Affecting Dermatology
Modifier -25—Cigna’s policy requiring dermatologists to submit documentation to use modifier -25 when billing with E/M CPT codes 99212 through 99215 has been delayed indefinitely.7 If a payer denies a dermatologist payment, contact the AADA Patient Access and Payer Relations committee ([email protected]) for assistance.
Telehealth and Digital Pathology—Recent legislation authorized extension of many of the Medicare telehealth and digital pathology flexibilities that were put in place during the COVID-19 public health emergency through December 31, 2024.8,9 Seventeen newly approved CPT telemedicine codes for new and established patient audio-visual and audio-only visits recently were surveyed.2,3 The data from the survey will be used as a key element in assigning a specific RVU to the CMS and will be included in the MPFS.
Thirty additional new digital pathology add-on CPT category III codes for 2024 were added to the ones from 2023.2,3 These codes can be used to report additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis. They cannot be used for archival or educational purposes, clinical conferences, training, or validating artificial intelligence algorithms. Category III codes used for emerging technologies have no assigned RVUs or reimbursement.2,3
The Cures Act—The Cures Act aims to ensure that patients have timely access to their health information.10 It requires all physicians to make their office notes, laboratory results, and other diagnostic reports available to patients as soon as the office receives them. The rules went into effect on April 5, 2021, with a limited definition of electronic health information; on October 6, 2022, the Cures Act rule expanded to include all electronic health information. The AADA has urged the Office of the National Coordinator for Health Information Technology to collaborate with stakeholder organizations to re-evaluate federal policies concerning the immediate release of electronic health information and information blocking, particularly in cases with life-altering diagnoses.10 They stressed the importance of prioritizing the well-being and emotional stability of patients and enhancing care by providing patients adequate time and support to process, comprehend, and discuss findings with their physician.
Proposed 2024 Medicare Quality Payment Program Requirements
The CMS proposed to increase the performance threshold in the quality payment program from 75 to 82 points for the 2024 Merit-based Incentive Payment System (MIPS) performance period, impacting the 2026 payment year.2,3,11 As a result of this increase, there could be more MIPS-eligible clinicians receiving penalties, which could be a reduction of up to 9%. The AADA will firmly oppose any increase in the threshold and strongly urge CMS to maintain the 75-point threshold. The performance category weights for the 2024 performance year will remain unchanged from the 2023 performance year.2,3,11
2024 Proposed Quality MIPS Measures Set—The CMS proposed to remove the topped-out MIPS measure 138 (coordination of care for melanoma).2,3,11 Additionally, it proposed to remove MIPS measure 402 (tobacco use and help with quitting among adolescents) as a quality measure from MIPS because the agency believes it is duplicative of measure 226 (preventive care and screening: tobacco use: screening and cessation intervention).2,3,11
MIPS Value Pathways—The CMS consolidated 2 previously established MIPS value pathways (MVPs): the Promoting Wellness MVP and the Optimizing Chronic Disease Management MVP.2,3,11 Proposed new MVPs for 2024 include Focusing on Women’s Health; Quality Care for the Treatment of Ear, Nose, and Throat Disorders; Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV; Quality Care in Mental Health and Substance Use Disorders; and Rehabilitative Support for Musculoskeletal Care. Dermatology is not impacted; however, the CMS plans to sunset traditional MIPS and replace it with MVPs—the future of MIPS.2,3,11 The AADA maintains that traditional MIPS should continue to be an option because MVPs have a limited number of measures for dermatologists.
Update on Reporting Suture Removal
There are 2 new CPT add-on codes—15853 and 15854—for the removal of sutures or staples not requiring anesthesia to be listed separately in addition to an appropriate E/M service. These add-on codes went into effect on January 1, 2023.12 These codes were created with the intent to capture and ensure remuneration for practice expenses that are not included in a stand-alone E/M encounter that occur after a 0-day procedure (eg, services reported with CPT codes 11102–11107 and 11300–11313) for wound check and suture removal where appropriate. These new add-on codes do not have physician work RVUs assigned to them because they are only for practice expenses (eg, clinical staff time, disposable supplies, use of equipment); CPT code 15853 is reported for the removal of sutures or staples, and CPT code 15854 is reported when both sutures and staples are removed. These codes can only be reported if an E/M service also is reported for the patient encounter.12
Final Thoughts
The AADA is working with the House of Medicine and the medical specialty community to develop specific proposals to reform the Medicare payment system.4 The proposed 2024 MPFS was released on July 13, 2023, and final regulations are expected in the late fall of 2023. The AADA will continue to engage with the CMS, but it is important for physicians to learn about and support advocacy priorities and efforts as well as join forces to protect their practices. As health care professionals, we have unique insights into the challenges and needs of our patients and the health care system. Advocacy can take various forms, such as supporting or opposing specific legislations, participating in grassroots campaigns, engaging with policymakers, and/or joining professional organizations that advocate for health care–related issues. Get involved, stay informed, and stay engaged through dermatology medical societies; together we can make a difference.
Health care spending in the United States remained relatively flat from 2019 to 2021 and only increased 2.7% in 2021, reaching $4.3 billion or $12,914 per person. Physician services account for 15% of health care spending (Figure). Relative value units (RVUs) signify the time it took a physician to complete a task multiplied by a conversion factor (CF). When RVUs initially were created in 1992 by what is now the Centers for Medicare &Medicaid Services (CMS), the CF was $32.00. Thirty-one years later, the CF is $33.89 in 2023; however, it would be $66.00 if the CF had increased with inflation.1 If the proposed 2024 Medicare physician fee schedule (MPFS) is adopted, the payment formula would decrease by 3.4% ($32.75) relative to the 2023 fee schedule ($33.89), which would be a 9% decrease relative to 2019 ($36.04).2,3 This reduction is due to the budget neutrality adjustment required by changes in RVUs, implementation of the evaluation and management (E/M) add-on code G2211, and proposed increases in primary are services.2,3 Since 2001, Medicare physician payment has declined by 26%.4 Adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index (MEI); (2) an expenditure target performance adjustment; and (3) miscellaneous adjustments, including those for budget neutrality required by law. Despite continued substantial increases in practice expenses, physicians’ reimbursement has remained flat while other service providers, such as those in skilled nursing facilities and hospitals, have received favorable payment increases compared to practice cost inflation and the Consumer Price Index.4

The CMS will not incorporate 2017 MEI cost weights for the RVUs in the MPFS rate setting for 2024 because all key measures of practice expenses in the MEI accelerated in 2022. Instead, the CMS is updating data on practice expense per hour to calculate payment for physician services with a survey for physician practices that launched on July 31, 2023.5 The American Medical Association contracted with Mathematica, an independent research company, to conduct a physician practice information survey that will be used to determine indirect practice expenses. Physicians should be on the lookout for emails regarding completion of these surveys and the appropriate financial expert in their practice should be contacted so the responses are accurate, as these data are key to future updates in the Medicare pay formula used to reimburse physicians.
Impact of Medicare Cuts
The recent congressional debt limit deal set spending caps for the next 2 fiscal years. Dermatology is facing an overall payment reduction of 1.87% (range, 1%–4%).2,3 The impact will depend on the services offered in an individual practice; for example, payment for a punch biopsy (Current Procedural Terminology [CPT] code 11104) would decrease by 3.9%. Payment for benign destruction (CPT code 17110) would decrease by 2.8%, and payment for even simple E/M of an established patient (CPT code 99213) would decrease by 1.6%. Overall, there would be a reduction of 2.75% for dermatopathology services, with a decrease of 2% for CPT code 88305 global and decreases for the technical component of 1% and professional component of 3%.2,3
Medicare cuts have reached a critical level, and physicians cannot continue to absorb the costs to own and operate their practices.4 This has led to health market consolidation, which in turn limits competition and patient access while driving up health care costs and driving down the quality of care. Small independent rural practices as well as those caring for historically marginalized patients will be disproportionately affected.
Proposed Addition of E/M Code G2211
In the calendar year (CY) 2021 final rule, the CMS tried to adopt a new add-on code—G2211—patients with a serious or complex condition that typically require referral and coordination of multispecialty care. Per the CMS, the primary policy goal of G2211 is to increase payments to primary care physicians and to reimburse them more appropriately for the care provided to patients with a serious or complex condition.2,3 It can be reported in conjunction with all office and outpatient E/M visits to better account for additional resources associated with primary care, or similarly ongoing medical care related to a patient’s single, serious condition, or complex condition.3 Typically, G2211 would not be used by dermatologists, as this add-on code requires visit complexity inherent to E/M associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single serious condition or a complex condition.2,3
Initially, the CMS assumed that G2211 would be reported with 90% of all office and outpatient E/M visit claims, which would account for a considerable portion of total MPFS schedule spending; however, the House of Medicine disagreed and believed it would be 75%.2,3 Given the extremely high utilization estimate, G2211 would have had a substantial effect on budget neutrality, accounting for an estimated increase of $3.3 billion and a corresponding 3.0% cut to the CY 2021 MPFS. Because of the potential payment reductions to physicians and a successful advocacy effort by organized medicine, including the American Academy of Dermatology Association (AADA), Congress delayed implementation of G2211 until CY 2024. Modifier -25 cannot be reported with G2211. The CMS revised its utilization assumptions from 90% of all E/M services to an initial utilization of 38% and then 54% when fully adopted. The proposed 2024 payment for G2211 is an additional $16.05.2,3
Advancing Health Equity With Healthcare Common Procedure Coding System G Codes
The CMS is proposing coding and payment for several new services to help underserved populations, including addressing unmet health-related social needs that can potentially interfere with the diagnosis and treatment of medical conditions, which includes paying for certain caregiver training services as well as payment for community health integration services.2,3 These are the first MPFS services designed to include care involving community health workers, who link underserved communities with critical health care and social services in the community. Additionally, the rule also proposes coding and payment for evaluating the risks related to social factors that affect a patient’s health, such as access to affordable quality health care, that can take place during an annual wellness visit or in combination with an E/M visit.2,3 As dermatologists, we should be familiar with this set of G codes, as we will likely use them in practice for patients with transportation needs.
Advocacy Efforts on Medicare Payment Reform
Medicare physician payment reform needs to happen at a national level. Advocacy efforts by the AADA and other groups have been underway to mitigate the proposed 2024 cuts. The Strengthening Medicare for Patients and Providers Act (HR 2474) is a bill that was introduced by a bipartisan coalition of physicians to provide an inflation-based increase in Medicare payments in 2024 and beyond.6
Other Legislative Updates Affecting Dermatology
Modifier -25—Cigna’s policy requiring dermatologists to submit documentation to use modifier -25 when billing with E/M CPT codes 99212 through 99215 has been delayed indefinitely.7 If a payer denies a dermatologist payment, contact the AADA Patient Access and Payer Relations committee ([email protected]) for assistance.
Telehealth and Digital Pathology—Recent legislation authorized extension of many of the Medicare telehealth and digital pathology flexibilities that were put in place during the COVID-19 public health emergency through December 31, 2024.8,9 Seventeen newly approved CPT telemedicine codes for new and established patient audio-visual and audio-only visits recently were surveyed.2,3 The data from the survey will be used as a key element in assigning a specific RVU to the CMS and will be included in the MPFS.
Thirty additional new digital pathology add-on CPT category III codes for 2024 were added to the ones from 2023.2,3 These codes can be used to report additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis. They cannot be used for archival or educational purposes, clinical conferences, training, or validating artificial intelligence algorithms. Category III codes used for emerging technologies have no assigned RVUs or reimbursement.2,3
The Cures Act—The Cures Act aims to ensure that patients have timely access to their health information.10 It requires all physicians to make their office notes, laboratory results, and other diagnostic reports available to patients as soon as the office receives them. The rules went into effect on April 5, 2021, with a limited definition of electronic health information; on October 6, 2022, the Cures Act rule expanded to include all electronic health information. The AADA has urged the Office of the National Coordinator for Health Information Technology to collaborate with stakeholder organizations to re-evaluate federal policies concerning the immediate release of electronic health information and information blocking, particularly in cases with life-altering diagnoses.10 They stressed the importance of prioritizing the well-being and emotional stability of patients and enhancing care by providing patients adequate time and support to process, comprehend, and discuss findings with their physician.
Proposed 2024 Medicare Quality Payment Program Requirements
The CMS proposed to increase the performance threshold in the quality payment program from 75 to 82 points for the 2024 Merit-based Incentive Payment System (MIPS) performance period, impacting the 2026 payment year.2,3,11 As a result of this increase, there could be more MIPS-eligible clinicians receiving penalties, which could be a reduction of up to 9%. The AADA will firmly oppose any increase in the threshold and strongly urge CMS to maintain the 75-point threshold. The performance category weights for the 2024 performance year will remain unchanged from the 2023 performance year.2,3,11
2024 Proposed Quality MIPS Measures Set—The CMS proposed to remove the topped-out MIPS measure 138 (coordination of care for melanoma).2,3,11 Additionally, it proposed to remove MIPS measure 402 (tobacco use and help with quitting among adolescents) as a quality measure from MIPS because the agency believes it is duplicative of measure 226 (preventive care and screening: tobacco use: screening and cessation intervention).2,3,11
MIPS Value Pathways—The CMS consolidated 2 previously established MIPS value pathways (MVPs): the Promoting Wellness MVP and the Optimizing Chronic Disease Management MVP.2,3,11 Proposed new MVPs for 2024 include Focusing on Women’s Health; Quality Care for the Treatment of Ear, Nose, and Throat Disorders; Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV; Quality Care in Mental Health and Substance Use Disorders; and Rehabilitative Support for Musculoskeletal Care. Dermatology is not impacted; however, the CMS plans to sunset traditional MIPS and replace it with MVPs—the future of MIPS.2,3,11 The AADA maintains that traditional MIPS should continue to be an option because MVPs have a limited number of measures for dermatologists.
Update on Reporting Suture Removal
There are 2 new CPT add-on codes—15853 and 15854—for the removal of sutures or staples not requiring anesthesia to be listed separately in addition to an appropriate E/M service. These add-on codes went into effect on January 1, 2023.12 These codes were created with the intent to capture and ensure remuneration for practice expenses that are not included in a stand-alone E/M encounter that occur after a 0-day procedure (eg, services reported with CPT codes 11102–11107 and 11300–11313) for wound check and suture removal where appropriate. These new add-on codes do not have physician work RVUs assigned to them because they are only for practice expenses (eg, clinical staff time, disposable supplies, use of equipment); CPT code 15853 is reported for the removal of sutures or staples, and CPT code 15854 is reported when both sutures and staples are removed. These codes can only be reported if an E/M service also is reported for the patient encounter.12
Final Thoughts
The AADA is working with the House of Medicine and the medical specialty community to develop specific proposals to reform the Medicare payment system.4 The proposed 2024 MPFS was released on July 13, 2023, and final regulations are expected in the late fall of 2023. The AADA will continue to engage with the CMS, but it is important for physicians to learn about and support advocacy priorities and efforts as well as join forces to protect their practices. As health care professionals, we have unique insights into the challenges and needs of our patients and the health care system. Advocacy can take various forms, such as supporting or opposing specific legislations, participating in grassroots campaigns, engaging with policymakers, and/or joining professional organizations that advocate for health care–related issues. Get involved, stay informed, and stay engaged through dermatology medical societies; together we can make a difference.
- Centers for Medicare & Medicaid Services. NHE fact sheet. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Medicare and Medicaid Programs; CY 2024 payment policies under the physician fee schedule and other changes to part B payment and coverage policies; Medicare shared savings program requirements; Medicare advantage; Medicare and Medicaid provider and supplier enrollment policies; and basic health program. Fed Regist. 2023;88:52262-53197. To be codified at 42 CFR §405, §410, §411, §414, §415, §418, §422, §423, §424, §425, §455, §489, §491, §495, §498, and §600. https://www.federalregister.gov/documents/2023/08/07/2023-14624/medicare-and-medicaid-programs-cy-2024-payment-policies-under-the-physician-fee-schedule-and-other
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2024 Medicare physician fee schedule proposed rule. Published July 13, 2023. Accessed September 18, 2023. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-proposed-rule
- American Medical Association. Payment reform. Accessed September 18, 2023. https://www.ama-assn.org/health-care-advocacypayment-reform
- American Medical Association. Physician answers on this survey will shape future Medicare pay. Published July 31, 2023. Accessed September 18, 2023. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future -medicare-pay
- Strengthening Medicare for Patients and Providers Act, HR 2474, 118 Congress (2023-2024). https://www.congress.gov/bill/118th-congress/house-bill/2474
- American Academy of Dermatology Association. Academy advocacy priorities. Accessed September 18, 2023. https://www.aad.org/member/advocacy/priorities
- College of American Pathologists. Remote sign-out of cases with digital pathology FAQs. Accessed September 18, 2023. https://www.cap.org/covid-19/remote-sign-out-faqs
- Centers for Medicare & Medicaid Services. Telehealth. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/medicare/coverage/telehealth
- The Office of the National Coordinator for Health Information Technology. ONC’s Cures Act final rule. Accessed September 18, 2023. https://www.healthit.gov/topic/oncs-cures-act-final-rule
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Notice of Proposed Rule Making Quality Payment Program Policy Overview: Proposals and Requests for Information. Accessed September 12, 2023. https://email.aadresources.org/e3t/Ctc/I6+113/cVKqx04/VVWzj43dDbctW8c23GW1ZLnJHW1xTZ7Q50Y DYN89Qzy5nCVhV3Zsc37CgFV9W5Ck4-D42qs9BW38PtXn4LSlNLW1QKpPL4xT8BMW6Mcwww3FdwCHN3vfGTMXbtF-W2-Zzfy5WHDg6W88tx1F1KgsgxW7zDzT46C2sFXW800vQJ3lLsS_W5D6f1d30-f3cN1njgZ_dX7xkW447ldH2-kgc5VCs7Xg1GY6dsN87pLVJqJG5XW8VWwD-7VxVkJN777f5fJL7jBW8RxkQM1lcSDjVV746T3C-stpN52V_S5xj7q6W3_vldf3p1Yk2Vbd4ZD3cPrHqW5Pwv9m567fkzW1vfDm51H-T7rW1jVrxl8gstXyW5RVTn8863CVFW8g6LgK2YdhpkW34HC4z3_pGYgW8V_qWH3g-tTlW4S3RD-1dKry7W4_rW8d1ssZ1fVwXQjQ9krVMW8Y0bTt8Nr5CNW6vbG0h3wyx59W8WCrNW50p5n6W1r-VBC2rKh93N4W2RyYr7vvm3kxG1
- Centers for Medicare & Medicaid Services. Chapter III surgery: integumentary system CPT codes 10000-19999 for Medicare national correct coding initiative policy manual. Updated January 1, 2023. Accessed September 26, 2023. https://www.cms.gov/files/document/medicare-ncci-policy-manual-2023-chapter-3.pdf
- Centers for Medicare & Medicaid Services. NHE fact sheet. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Medicare and Medicaid Programs; CY 2024 payment policies under the physician fee schedule and other changes to part B payment and coverage policies; Medicare shared savings program requirements; Medicare advantage; Medicare and Medicaid provider and supplier enrollment policies; and basic health program. Fed Regist. 2023;88:52262-53197. To be codified at 42 CFR §405, §410, §411, §414, §415, §418, §422, §423, §424, §425, §455, §489, §491, §495, §498, and §600. https://www.federalregister.gov/documents/2023/08/07/2023-14624/medicare-and-medicaid-programs-cy-2024-payment-policies-under-the-physician-fee-schedule-and-other
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2024 Medicare physician fee schedule proposed rule. Published July 13, 2023. Accessed September 18, 2023. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-proposed-rule
- American Medical Association. Payment reform. Accessed September 18, 2023. https://www.ama-assn.org/health-care-advocacypayment-reform
- American Medical Association. Physician answers on this survey will shape future Medicare pay. Published July 31, 2023. Accessed September 18, 2023. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future -medicare-pay
- Strengthening Medicare for Patients and Providers Act, HR 2474, 118 Congress (2023-2024). https://www.congress.gov/bill/118th-congress/house-bill/2474
- American Academy of Dermatology Association. Academy advocacy priorities. Accessed September 18, 2023. https://www.aad.org/member/advocacy/priorities
- College of American Pathologists. Remote sign-out of cases with digital pathology FAQs. Accessed September 18, 2023. https://www.cap.org/covid-19/remote-sign-out-faqs
- Centers for Medicare & Medicaid Services. Telehealth. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/medicare/coverage/telehealth
- The Office of the National Coordinator for Health Information Technology. ONC’s Cures Act final rule. Accessed September 18, 2023. https://www.healthit.gov/topic/oncs-cures-act-final-rule
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Notice of Proposed Rule Making Quality Payment Program Policy Overview: Proposals and Requests for Information. Accessed September 12, 2023. https://email.aadresources.org/e3t/Ctc/I6+113/cVKqx04/VVWzj43dDbctW8c23GW1ZLnJHW1xTZ7Q50Y DYN89Qzy5nCVhV3Zsc37CgFV9W5Ck4-D42qs9BW38PtXn4LSlNLW1QKpPL4xT8BMW6Mcwww3FdwCHN3vfGTMXbtF-W2-Zzfy5WHDg6W88tx1F1KgsgxW7zDzT46C2sFXW800vQJ3lLsS_W5D6f1d30-f3cN1njgZ_dX7xkW447ldH2-kgc5VCs7Xg1GY6dsN87pLVJqJG5XW8VWwD-7VxVkJN777f5fJL7jBW8RxkQM1lcSDjVV746T3C-stpN52V_S5xj7q6W3_vldf3p1Yk2Vbd4ZD3cPrHqW5Pwv9m567fkzW1vfDm51H-T7rW1jVrxl8gstXyW5RVTn8863CVFW8g6LgK2YdhpkW34HC4z3_pGYgW8V_qWH3g-tTlW4S3RD-1dKry7W4_rW8d1ssZ1fVwXQjQ9krVMW8Y0bTt8Nr5CNW6vbG0h3wyx59W8WCrNW50p5n6W1r-VBC2rKh93N4W2RyYr7vvm3kxG1
- Centers for Medicare & Medicaid Services. Chapter III surgery: integumentary system CPT codes 10000-19999 for Medicare national correct coding initiative policy manual. Updated January 1, 2023. Accessed September 26, 2023. https://www.cms.gov/files/document/medicare-ncci-policy-manual-2023-chapter-3.pdf
PRACTICE POINTS
- The proposed 2024 Medicare physician fee schedule published by the Centers for Medicare & Medicaid Services in July 2023 will negatively impact dermatology practices.
- The final regulations are expected in November 2023.
Verrucous Plaque on the Foot
The Diagnosis: Eccrine Poroma
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.
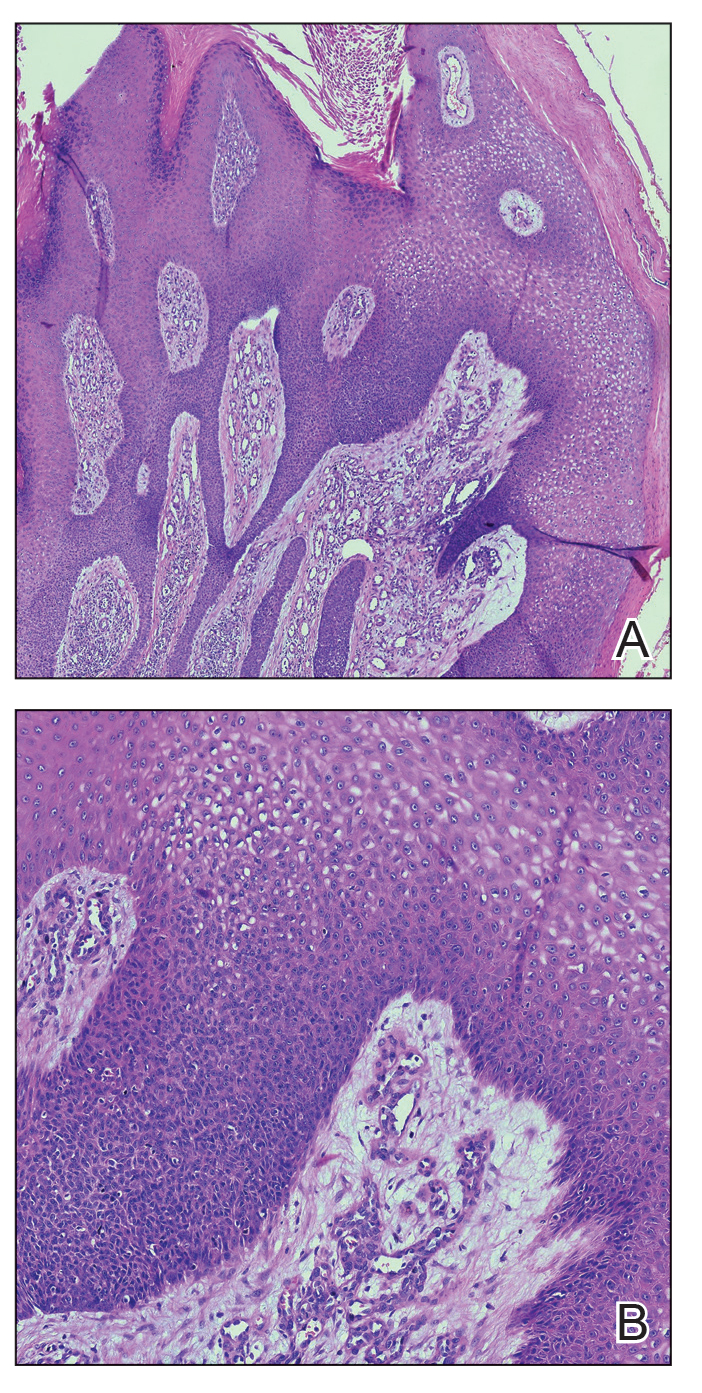
Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.
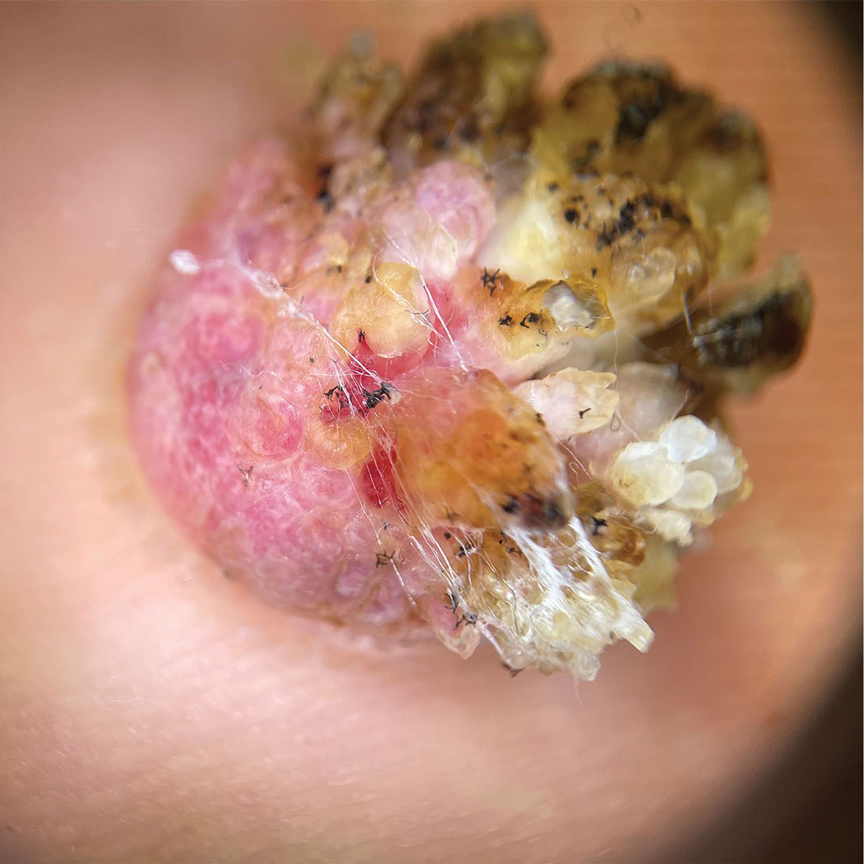
Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9
There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
The Diagnosis: Eccrine Poroma
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.

Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.

Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9

There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
The Diagnosis: Eccrine Poroma
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.

Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.

Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9

There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
A 62-year-old man presented with an enlarging plaque on the foot of 3 years’ duration. He experienced minor pain while walking but reported no other symptoms. His family history was negative for similar anomalies, and his medical history was negative for the presence of malignant tumors. Physical examination revealed a 2-mm erythematous plaque on the plantar aspect of the right foot with prominent overlying verrucous changes and no ulceration or regional lymphadenopathy. Dermoscopy and reflectance confocal microscopy of the lesion were performed along with a histopathologic examination after complete surgical excision.