User login
Updated Moderna booster shows greater activity against COVID in adults
WASHINGTON –
The bivalent booster was superior regardless of age and whether a person had previously been infected with SARS-CoV-2.
Additionally, no new safety concerns emerged.
Spyros Chalkias, MD, senior medical director of clinical development at Moderna, presented the data during an annual scientific meeting on infectious diseases.
In the phase 2/3 trial, participants received either 50 mcg of the bivalent vaccine mRNA-1273.214 (25 mcg each of the original Wuhan-Hu-1 and Omicron BA.1 spike mRNAs) or 50 mcg of the standard authorized mRNA-1273. The doses were given as second boosters in adults who had previously received a two-dose primary series and a first booster at least 3 months before.
The model-based geometric mean titers (GMTs) ratio of the enhanced booster compared with the standard booster was 1.74 (1.49-2.04), meeting the prespecified bar for superiority against Omicron BA.1.
In participants without prior SARS-CoV-2 infection who received updated booster doses and those who received standard boosters, the neutralizing antibody GMTs against Omicron BA.1 were 2372.4 and 1473.5, respectively.
Additionally, the updated booster elicited higher GMTs (727.4) than the standard booster (492.1) against Omicron subvariants BA.4/BA.5. Safety and reactogenicity were similar for both vaccine groups.
“By the end of this year, we expect to also have clinical trial data from our BA.4/BA.5 bivalent booster,” Dr. Chalkias said.
In the interim, the U.S. Food and Drug Administration recently granted emergency use authorization for Moderna’s BA.4/BA.5 Omicron-targeting bivalent COVID-19 booster vaccine in children and adolescents aged 6-17 years.
Pfizer/BioNTech also has recently issued an announcement that their COVID-19 booster, adapted for the BA.4 and the BA.5 Omicron subvariants, generated a strong immune response and was well tolerated in human tests.
Pfizer/BioNTech said data from roughly 80 adult patients showed that the booster led to a substantial increase in neutralizing antibody levels against the BA.4/BA.5 variants after 1 week.
Separate study of causes of severe breakthrough infections in early vaccine formulations
Though COVID vaccines reduce the incidence of severe outcomes, there are reports of breakthrough infections in persons who received the original vaccines, and some of these have been serious.
In a separate study, also presented at the meeting, researchers led by first author Austin D. Vo, BS, with the VA Boston Healthcare System, used data collected from Dec. 15, 2020, through Feb. 28, 2022, in a U.S. veteran population to assess those at highest risk for severe disease despite vaccination.
Results of the large, nationwide retrospective study were simultaneously published in JAMA Network Open.
The primary outcome was development of severe COVID, defined as a hospitalization within 14 days of a confirmed positive SARS-CoV-2 test, receipt of supplemental oxygen, mechanical ventilation, or death within 28 days.
Among 110,760 participants with severe disease after primary vaccination, 13% (14,690) were hospitalized with severe COVID-19 or died.
The strongest risk factor for severe disease despite vaccination was age, the researchers found.
Presenting author Westyn Branch-Elliman, MD, associate professor of medicine with VA Boston Healthcare System, said, “We found that age greater than 50 was associated with an adjusted odds ratio of 1.42 for every 5-year increase.”
To put that in perspective, she said, “compared to patients who are 45 to 50, those over 80 had an adjusted odds ratio of 16 for hospitalization or death following breakthrough infection.”
Priya Nori, MD, an infectious disease specialist at Montefiore Medical Center in New York, said in an interview that the evidence that age is a strong risk factor for severe disease – even after vaccination – confirms that attention should be focused on those in the highest age groups, particularly those 80 years and older.
Other top risk factors included having immunocompromising conditions; having received cytotoxic chemotherapy within 6 months (adjusted odds ratio, 2.69; 95% confidence interval, 2.25-3.21); having leukemias/lymphomas (aOR, 1.84; 95% CI, 1.59-2.14); and having chronic conditions associated with end-organ disease.
“We also found that receipt of an additional booster dose of vaccine was associated with a 50% reduction in adjusted odds of severe disease,” noted Dr. Branch-Elliman.
Dr. Nori emphasized that, given these data, emphatic messaging is needed to encourage uptake of the updated Omicron-targeted vaccines for these high-risk age groups.
The study by Dr. Chalkias and colleagues was funded by Moderna. Dr. Chalkias and several coauthors are employed by Moderna. One coauthor has relationships with DLA Piper/Medtronic, and Gilead Pharmaceuticals, and one has relationships with Celgene/Bristol-Myers Squibb, ChemoCentryx, Gilead, and Kiniksa. Dr. Nori has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON –
The bivalent booster was superior regardless of age and whether a person had previously been infected with SARS-CoV-2.
Additionally, no new safety concerns emerged.
Spyros Chalkias, MD, senior medical director of clinical development at Moderna, presented the data during an annual scientific meeting on infectious diseases.
In the phase 2/3 trial, participants received either 50 mcg of the bivalent vaccine mRNA-1273.214 (25 mcg each of the original Wuhan-Hu-1 and Omicron BA.1 spike mRNAs) or 50 mcg of the standard authorized mRNA-1273. The doses were given as second boosters in adults who had previously received a two-dose primary series and a first booster at least 3 months before.
The model-based geometric mean titers (GMTs) ratio of the enhanced booster compared with the standard booster was 1.74 (1.49-2.04), meeting the prespecified bar for superiority against Omicron BA.1.
In participants without prior SARS-CoV-2 infection who received updated booster doses and those who received standard boosters, the neutralizing antibody GMTs against Omicron BA.1 were 2372.4 and 1473.5, respectively.
Additionally, the updated booster elicited higher GMTs (727.4) than the standard booster (492.1) against Omicron subvariants BA.4/BA.5. Safety and reactogenicity were similar for both vaccine groups.
“By the end of this year, we expect to also have clinical trial data from our BA.4/BA.5 bivalent booster,” Dr. Chalkias said.
In the interim, the U.S. Food and Drug Administration recently granted emergency use authorization for Moderna’s BA.4/BA.5 Omicron-targeting bivalent COVID-19 booster vaccine in children and adolescents aged 6-17 years.
Pfizer/BioNTech also has recently issued an announcement that their COVID-19 booster, adapted for the BA.4 and the BA.5 Omicron subvariants, generated a strong immune response and was well tolerated in human tests.
Pfizer/BioNTech said data from roughly 80 adult patients showed that the booster led to a substantial increase in neutralizing antibody levels against the BA.4/BA.5 variants after 1 week.
Separate study of causes of severe breakthrough infections in early vaccine formulations
Though COVID vaccines reduce the incidence of severe outcomes, there are reports of breakthrough infections in persons who received the original vaccines, and some of these have been serious.
In a separate study, also presented at the meeting, researchers led by first author Austin D. Vo, BS, with the VA Boston Healthcare System, used data collected from Dec. 15, 2020, through Feb. 28, 2022, in a U.S. veteran population to assess those at highest risk for severe disease despite vaccination.
Results of the large, nationwide retrospective study were simultaneously published in JAMA Network Open.
The primary outcome was development of severe COVID, defined as a hospitalization within 14 days of a confirmed positive SARS-CoV-2 test, receipt of supplemental oxygen, mechanical ventilation, or death within 28 days.
Among 110,760 participants with severe disease after primary vaccination, 13% (14,690) were hospitalized with severe COVID-19 or died.
The strongest risk factor for severe disease despite vaccination was age, the researchers found.
Presenting author Westyn Branch-Elliman, MD, associate professor of medicine with VA Boston Healthcare System, said, “We found that age greater than 50 was associated with an adjusted odds ratio of 1.42 for every 5-year increase.”
To put that in perspective, she said, “compared to patients who are 45 to 50, those over 80 had an adjusted odds ratio of 16 for hospitalization or death following breakthrough infection.”
Priya Nori, MD, an infectious disease specialist at Montefiore Medical Center in New York, said in an interview that the evidence that age is a strong risk factor for severe disease – even after vaccination – confirms that attention should be focused on those in the highest age groups, particularly those 80 years and older.
Other top risk factors included having immunocompromising conditions; having received cytotoxic chemotherapy within 6 months (adjusted odds ratio, 2.69; 95% confidence interval, 2.25-3.21); having leukemias/lymphomas (aOR, 1.84; 95% CI, 1.59-2.14); and having chronic conditions associated with end-organ disease.
“We also found that receipt of an additional booster dose of vaccine was associated with a 50% reduction in adjusted odds of severe disease,” noted Dr. Branch-Elliman.
Dr. Nori emphasized that, given these data, emphatic messaging is needed to encourage uptake of the updated Omicron-targeted vaccines for these high-risk age groups.
The study by Dr. Chalkias and colleagues was funded by Moderna. Dr. Chalkias and several coauthors are employed by Moderna. One coauthor has relationships with DLA Piper/Medtronic, and Gilead Pharmaceuticals, and one has relationships with Celgene/Bristol-Myers Squibb, ChemoCentryx, Gilead, and Kiniksa. Dr. Nori has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON –
The bivalent booster was superior regardless of age and whether a person had previously been infected with SARS-CoV-2.
Additionally, no new safety concerns emerged.
Spyros Chalkias, MD, senior medical director of clinical development at Moderna, presented the data during an annual scientific meeting on infectious diseases.
In the phase 2/3 trial, participants received either 50 mcg of the bivalent vaccine mRNA-1273.214 (25 mcg each of the original Wuhan-Hu-1 and Omicron BA.1 spike mRNAs) or 50 mcg of the standard authorized mRNA-1273. The doses were given as second boosters in adults who had previously received a two-dose primary series and a first booster at least 3 months before.
The model-based geometric mean titers (GMTs) ratio of the enhanced booster compared with the standard booster was 1.74 (1.49-2.04), meeting the prespecified bar for superiority against Omicron BA.1.
In participants without prior SARS-CoV-2 infection who received updated booster doses and those who received standard boosters, the neutralizing antibody GMTs against Omicron BA.1 were 2372.4 and 1473.5, respectively.
Additionally, the updated booster elicited higher GMTs (727.4) than the standard booster (492.1) against Omicron subvariants BA.4/BA.5. Safety and reactogenicity were similar for both vaccine groups.
“By the end of this year, we expect to also have clinical trial data from our BA.4/BA.5 bivalent booster,” Dr. Chalkias said.
In the interim, the U.S. Food and Drug Administration recently granted emergency use authorization for Moderna’s BA.4/BA.5 Omicron-targeting bivalent COVID-19 booster vaccine in children and adolescents aged 6-17 years.
Pfizer/BioNTech also has recently issued an announcement that their COVID-19 booster, adapted for the BA.4 and the BA.5 Omicron subvariants, generated a strong immune response and was well tolerated in human tests.
Pfizer/BioNTech said data from roughly 80 adult patients showed that the booster led to a substantial increase in neutralizing antibody levels against the BA.4/BA.5 variants after 1 week.
Separate study of causes of severe breakthrough infections in early vaccine formulations
Though COVID vaccines reduce the incidence of severe outcomes, there are reports of breakthrough infections in persons who received the original vaccines, and some of these have been serious.
In a separate study, also presented at the meeting, researchers led by first author Austin D. Vo, BS, with the VA Boston Healthcare System, used data collected from Dec. 15, 2020, through Feb. 28, 2022, in a U.S. veteran population to assess those at highest risk for severe disease despite vaccination.
Results of the large, nationwide retrospective study were simultaneously published in JAMA Network Open.
The primary outcome was development of severe COVID, defined as a hospitalization within 14 days of a confirmed positive SARS-CoV-2 test, receipt of supplemental oxygen, mechanical ventilation, or death within 28 days.
Among 110,760 participants with severe disease after primary vaccination, 13% (14,690) were hospitalized with severe COVID-19 or died.
The strongest risk factor for severe disease despite vaccination was age, the researchers found.
Presenting author Westyn Branch-Elliman, MD, associate professor of medicine with VA Boston Healthcare System, said, “We found that age greater than 50 was associated with an adjusted odds ratio of 1.42 for every 5-year increase.”
To put that in perspective, she said, “compared to patients who are 45 to 50, those over 80 had an adjusted odds ratio of 16 for hospitalization or death following breakthrough infection.”
Priya Nori, MD, an infectious disease specialist at Montefiore Medical Center in New York, said in an interview that the evidence that age is a strong risk factor for severe disease – even after vaccination – confirms that attention should be focused on those in the highest age groups, particularly those 80 years and older.
Other top risk factors included having immunocompromising conditions; having received cytotoxic chemotherapy within 6 months (adjusted odds ratio, 2.69; 95% confidence interval, 2.25-3.21); having leukemias/lymphomas (aOR, 1.84; 95% CI, 1.59-2.14); and having chronic conditions associated with end-organ disease.
“We also found that receipt of an additional booster dose of vaccine was associated with a 50% reduction in adjusted odds of severe disease,” noted Dr. Branch-Elliman.
Dr. Nori emphasized that, given these data, emphatic messaging is needed to encourage uptake of the updated Omicron-targeted vaccines for these high-risk age groups.
The study by Dr. Chalkias and colleagues was funded by Moderna. Dr. Chalkias and several coauthors are employed by Moderna. One coauthor has relationships with DLA Piper/Medtronic, and Gilead Pharmaceuticals, and one has relationships with Celgene/Bristol-Myers Squibb, ChemoCentryx, Gilead, and Kiniksa. Dr. Nori has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT IDWEEK 2022
More data suggest preexisting statin use improves COVID outcomes
Compared with patients who didn’t take statins, statin users had better health outcomes. For those who used these medications, the researchers saw lower mortality, lower clinical severity, and shorter hospital stays, aligning with previous observational studies, said lead author Ettore Crimi, MD, of the University of Central Florida, Orlando, and colleagues in their abstract, which was part of the agenda for the Anesthesiology annual meeting.
They attributed these clinical improvements to the pleiotropic – non–cholesterol lowering – effects of statins.
“[These] benefits of statins have been reported since the 1990s,” Dr. Crimi said in an interview. “Statin treatment has been associated with a marked reduction of markers of inflammation, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), interleukin-6 (IL-6), ferritin, and white blood cell count, among others.”
He noted that these effects have been studied in an array of conditions, including cancer, autoimmune diseases, chronic inflammatory disease, and in the perioperative setting, and with infectious diseases, including COVID-19.
In those previous studies, “preexisting statin use was protective among hospitalized COVID-19 patients, but a large, multicenter cohort study has not been reported in the United States,” Dr. Crimi and his colleagues wrote in their abstract.
To address this knowledge gap, they turned to electronic medical records from 38,875 patients hospitalized with COVID-19 from January to September 2020. Almost one-third of the population (n = 11,533) were using statins prior to hospitalization, while the remainder (n = 27,342) were nonusers.
The primary outcome was all-cause mortality. Secondary outcomes included death from COVID-19, along with a variety of severe complications. While the analysis did account for a range of potentially confounding variables, the effects of different SARS-CoV-2 variants and new therapeutics were not considered. Vaccines were not yet available at the time the data were collected.
Statin users had a 31% lower rate of all-cause mortality (odds ratio, 0.69; 95% confidence interval, 0.64-0.75; P = .001) and a 37% reduced rate of death from COVID-19 (OR, 0.63; 95% CI, 0.58-0.69; P = .001).
A litany of other secondary variables also favored statin users, including reduced rates of discharge to hospice (OR, 0.79), ICU admission (OR, 0.69), severe acute respiratory distress syndrome (ARDs; OR, 0.72), critical ARDs (OR, 0.57), mechanical ventilation (OR, 0.60), severe sepsis with septic shock (OR, 0.66), and thrombosis (OR, 0.46). Statin users also had, on average, shorter hospital stays and briefer mechanical ventilation.
“Our study showed a strong association between preexisting statin use and reduced mortality and morbidity rates in hospitalized COVID-19 patients,” the investigators concluded. “Pleiotropic benefits of statins could be repurposed for COVID-19 illness.”
Prospective studies needed before practice changes
How to best use statins against COVID-19, if at all, remains unclear, Dr. Crimi said, as initiation upon infection has generated mixed results in other studies, possibly because of statin pharmacodynamics. Cholesterol normalization can take about 6 weeks, so other benefits may track a similar timeline.
“The delayed onset of statins’ pleiotropic effects may likely fail to keep pace with the rapidly progressive, devastating COVID-19 disease,” Dr. Crimi said. “Therefore, initiating statins for an acute disease may not be an ideal first-line treatment.”
Stronger data are on the horizon, he added, noting that 19 federally funded prospective trials are underway to better understand the relationship between statins and COVID-19.
Daniel Rader, MD, of the University of Pennsylvania, Philadelphia, said the present findings are “not especially notable” because they “mostly confirm previous studies, but in a large U.S. cohort.”
Dr. Rader, who wrote about the potential repurposing of statins for COVID-19 back in the first year of the pandemic (Cell Metab. 2020 Aug 4;32[2]:145-7), agreed with the investigators that recommending changes to clinical practice would be imprudent until randomized controlled data confirm the benefits of initiating statins in patients with active COVID-19.
“More research on the impact of cellular cholesterol metabolism on SARS-CoV-2 infection of cells and generation of inflammation would also be of interest,” he added.
The investigators disclosed no competing interests. Dr. Rader disclosed relationships with Novartis, Pfizer, Verve, and others.
Compared with patients who didn’t take statins, statin users had better health outcomes. For those who used these medications, the researchers saw lower mortality, lower clinical severity, and shorter hospital stays, aligning with previous observational studies, said lead author Ettore Crimi, MD, of the University of Central Florida, Orlando, and colleagues in their abstract, which was part of the agenda for the Anesthesiology annual meeting.
They attributed these clinical improvements to the pleiotropic – non–cholesterol lowering – effects of statins.
“[These] benefits of statins have been reported since the 1990s,” Dr. Crimi said in an interview. “Statin treatment has been associated with a marked reduction of markers of inflammation, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), interleukin-6 (IL-6), ferritin, and white blood cell count, among others.”
He noted that these effects have been studied in an array of conditions, including cancer, autoimmune diseases, chronic inflammatory disease, and in the perioperative setting, and with infectious diseases, including COVID-19.
In those previous studies, “preexisting statin use was protective among hospitalized COVID-19 patients, but a large, multicenter cohort study has not been reported in the United States,” Dr. Crimi and his colleagues wrote in their abstract.
To address this knowledge gap, they turned to electronic medical records from 38,875 patients hospitalized with COVID-19 from January to September 2020. Almost one-third of the population (n = 11,533) were using statins prior to hospitalization, while the remainder (n = 27,342) were nonusers.
The primary outcome was all-cause mortality. Secondary outcomes included death from COVID-19, along with a variety of severe complications. While the analysis did account for a range of potentially confounding variables, the effects of different SARS-CoV-2 variants and new therapeutics were not considered. Vaccines were not yet available at the time the data were collected.
Statin users had a 31% lower rate of all-cause mortality (odds ratio, 0.69; 95% confidence interval, 0.64-0.75; P = .001) and a 37% reduced rate of death from COVID-19 (OR, 0.63; 95% CI, 0.58-0.69; P = .001).
A litany of other secondary variables also favored statin users, including reduced rates of discharge to hospice (OR, 0.79), ICU admission (OR, 0.69), severe acute respiratory distress syndrome (ARDs; OR, 0.72), critical ARDs (OR, 0.57), mechanical ventilation (OR, 0.60), severe sepsis with septic shock (OR, 0.66), and thrombosis (OR, 0.46). Statin users also had, on average, shorter hospital stays and briefer mechanical ventilation.
“Our study showed a strong association between preexisting statin use and reduced mortality and morbidity rates in hospitalized COVID-19 patients,” the investigators concluded. “Pleiotropic benefits of statins could be repurposed for COVID-19 illness.”
Prospective studies needed before practice changes
How to best use statins against COVID-19, if at all, remains unclear, Dr. Crimi said, as initiation upon infection has generated mixed results in other studies, possibly because of statin pharmacodynamics. Cholesterol normalization can take about 6 weeks, so other benefits may track a similar timeline.
“The delayed onset of statins’ pleiotropic effects may likely fail to keep pace with the rapidly progressive, devastating COVID-19 disease,” Dr. Crimi said. “Therefore, initiating statins for an acute disease may not be an ideal first-line treatment.”
Stronger data are on the horizon, he added, noting that 19 federally funded prospective trials are underway to better understand the relationship between statins and COVID-19.
Daniel Rader, MD, of the University of Pennsylvania, Philadelphia, said the present findings are “not especially notable” because they “mostly confirm previous studies, but in a large U.S. cohort.”
Dr. Rader, who wrote about the potential repurposing of statins for COVID-19 back in the first year of the pandemic (Cell Metab. 2020 Aug 4;32[2]:145-7), agreed with the investigators that recommending changes to clinical practice would be imprudent until randomized controlled data confirm the benefits of initiating statins in patients with active COVID-19.
“More research on the impact of cellular cholesterol metabolism on SARS-CoV-2 infection of cells and generation of inflammation would also be of interest,” he added.
The investigators disclosed no competing interests. Dr. Rader disclosed relationships with Novartis, Pfizer, Verve, and others.
Compared with patients who didn’t take statins, statin users had better health outcomes. For those who used these medications, the researchers saw lower mortality, lower clinical severity, and shorter hospital stays, aligning with previous observational studies, said lead author Ettore Crimi, MD, of the University of Central Florida, Orlando, and colleagues in their abstract, which was part of the agenda for the Anesthesiology annual meeting.
They attributed these clinical improvements to the pleiotropic – non–cholesterol lowering – effects of statins.
“[These] benefits of statins have been reported since the 1990s,” Dr. Crimi said in an interview. “Statin treatment has been associated with a marked reduction of markers of inflammation, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), interleukin-6 (IL-6), ferritin, and white blood cell count, among others.”
He noted that these effects have been studied in an array of conditions, including cancer, autoimmune diseases, chronic inflammatory disease, and in the perioperative setting, and with infectious diseases, including COVID-19.
In those previous studies, “preexisting statin use was protective among hospitalized COVID-19 patients, but a large, multicenter cohort study has not been reported in the United States,” Dr. Crimi and his colleagues wrote in their abstract.
To address this knowledge gap, they turned to electronic medical records from 38,875 patients hospitalized with COVID-19 from January to September 2020. Almost one-third of the population (n = 11,533) were using statins prior to hospitalization, while the remainder (n = 27,342) were nonusers.
The primary outcome was all-cause mortality. Secondary outcomes included death from COVID-19, along with a variety of severe complications. While the analysis did account for a range of potentially confounding variables, the effects of different SARS-CoV-2 variants and new therapeutics were not considered. Vaccines were not yet available at the time the data were collected.
Statin users had a 31% lower rate of all-cause mortality (odds ratio, 0.69; 95% confidence interval, 0.64-0.75; P = .001) and a 37% reduced rate of death from COVID-19 (OR, 0.63; 95% CI, 0.58-0.69; P = .001).
A litany of other secondary variables also favored statin users, including reduced rates of discharge to hospice (OR, 0.79), ICU admission (OR, 0.69), severe acute respiratory distress syndrome (ARDs; OR, 0.72), critical ARDs (OR, 0.57), mechanical ventilation (OR, 0.60), severe sepsis with septic shock (OR, 0.66), and thrombosis (OR, 0.46). Statin users also had, on average, shorter hospital stays and briefer mechanical ventilation.
“Our study showed a strong association between preexisting statin use and reduced mortality and morbidity rates in hospitalized COVID-19 patients,” the investigators concluded. “Pleiotropic benefits of statins could be repurposed for COVID-19 illness.”
Prospective studies needed before practice changes
How to best use statins against COVID-19, if at all, remains unclear, Dr. Crimi said, as initiation upon infection has generated mixed results in other studies, possibly because of statin pharmacodynamics. Cholesterol normalization can take about 6 weeks, so other benefits may track a similar timeline.
“The delayed onset of statins’ pleiotropic effects may likely fail to keep pace with the rapidly progressive, devastating COVID-19 disease,” Dr. Crimi said. “Therefore, initiating statins for an acute disease may not be an ideal first-line treatment.”
Stronger data are on the horizon, he added, noting that 19 federally funded prospective trials are underway to better understand the relationship between statins and COVID-19.
Daniel Rader, MD, of the University of Pennsylvania, Philadelphia, said the present findings are “not especially notable” because they “mostly confirm previous studies, but in a large U.S. cohort.”
Dr. Rader, who wrote about the potential repurposing of statins for COVID-19 back in the first year of the pandemic (Cell Metab. 2020 Aug 4;32[2]:145-7), agreed with the investigators that recommending changes to clinical practice would be imprudent until randomized controlled data confirm the benefits of initiating statins in patients with active COVID-19.
“More research on the impact of cellular cholesterol metabolism on SARS-CoV-2 infection of cells and generation of inflammation would also be of interest,” he added.
The investigators disclosed no competing interests. Dr. Rader disclosed relationships with Novartis, Pfizer, Verve, and others.
FROM ANESTHESIOLOGY 2022
COVID lawsuits have arrived: Which doctors are at risk?
A pregnant patient who had COVID-19 showed up at a hospital with respiratory difficulty caused by her illness. Physicians had to perform an emergency delivery of her near-term baby.
The infant survived, but the woman lost oxygen during the ordeal and suffered hypoxic brain damage. She is now suing an obstetrician, a pulmonologist, and an intensive care unit physician for medical malpractice.
The plaintiff contends there was a failure “to adequately recognize and treat her condition,” said Peter Kolbert, senior vice president for claim and litigation services for Healthcare Risk Advisors, part of TDC Group, which includes national medical liability insurer The Doctors Company.
“The physicians involved vehemently disagree and believe they treated her appropriately,” Mr. Kolbert said. “In fact, we believe their actions were heroic.”
In another case, a patient with COVID-19 and multiple comorbidities was admitted to a hospital. Physicians sedated and intubated the patient to maintain her airway. She recovered, but the patient now alleges doctors were negligent because she developed ulcers during her hospital stay. The case occurred during the height of the pandemic. In addition to the hospital, a pulmonologist, an ICU physician, and an acute care physician are named in the suit.
Both of these lawsuits are being defined as COVID claims because at the time, the plaintiffs either had COVID and needed care because of COVID, or because the care that physicians provided was affected by COVID in some way.
In the second case, the patient had COVID and needed treatment. During her recovery, ulcers developed. A significant aspect of this case is that it occurred during the height of the pandemic. Hospitals were overcrowded, the staff was swamped, and resources were limited. One factor may be that physicians were doing the best they could at the time but that the pandemic affected the extent of care they could provide.
Now, new data reflect the grim news: COVID claims have arrived. These cases from the claims database of The Doctors Company are just two examples of many COVID-related claims that have been levied since the pandemic started.
Currently, there are 162 open COVID-related claims in The Doctors Company database, according to Mr. Kolbert. A September 2022 benchmark report from Aon and the American Society for Health Care Risk Management indicates that 245 claims that pertain to patients with confirmed or suspected COVID-19 have been filed since the pandemic began. The findings in this report stem from an analysis of 95,600 hospital and physician liability claims that occurred between 2012 and 2021.
Of the 245 cases, 89 claims have been closed. The average cost was $43,000 per claim, said Kanika Vats, a director and actuary for Aon, a global firm that provides risk, reinsurance, and health solutions. Six of the claims cost $300,000 or more; the highest settlement was for $700,000.
“Most of the allegations in these claims revolve around delay in treatment or delay in diagnosis,” Ms. Vats said.
Which specialties are involved in legal actions?
Physicians working in acute care settings such as emergency departments and urgent care centers are the primary targets in COVID-related lawsuits involving doctors, say legal analysts. However, other specialties are also being affected. Physicians being sued include some who practiced telemedicine during the pandemic.
In one case, a primary care physician saw a patient via telemedicine because the physical medical office was closed. The patient was evaluated virtually and was sent for bloodwork and an x-ray.
The patient is now suing the primary care physician, alleging that failure to immediately send her to a hospital resulted in tuberculosis going untreated and that the failure led to a bad outcome. The allegation is that the physician underevaluated the case during the telemedicine visit, Mr. Kolbert said.
Drew Graham, an attorney at Hall Booth Smith PC, which is based in New York, said that most of the COVID-related liability claims he has seen involve facilities that provide postacute care, such as nursing homes and assisted living facilities. His firm has also seen a small number of COVID-related claims against physicians.
At least two of the claims involved allegations of improper treatment of COVID during hospitalizations, he said. Another involved a telehealth visit in which the patient claimed the virtual care that was provided was improper and that their condition required an in-person examination. Mr. Graham declined to specify the specialties of the physicians sued.
The Medical Professional Liability Association reports similar trends in COVID-related claims. Long-term facilities and hospitals are the most common focus of COVID-19 claims, followed by emergency medicine, primary care, and ob/gyn medical specialties, according to Kwon Miller, manager of data and analytics for MPL Association, a national trade association for medical liability insurers that operates a large claims database.
Between January 2020 and June 2022, the MPL Association Data Sharing Project recorded 280 COVID-19 events. “Events” refers to notifications, licensing board inquiries, and claims involving COVID. Of these events, 180 were closed with no indemnity payment, and 13 were closed with an average indemnity payment of $3,816, Mr. Miller said.
Complaints of delayed care associated with the pandemic are also on the rise. For example, one patient is suing a gastroenterologist for delaying his colonoscopy, alleging the postponement led to a delayed colon cancer diagnosis and worse prognosis, Mr. Kolbert said.
“It was delayed because all elective procedures at the time were being put off,” he said. “The patient claims that had they received the scheduled screening, the cancer would have been diagnosed at stage I as opposed to stage III.”
Why isn’t federal immunity shielding physicians?
A pressing question about the growing number of COVID claims is why state and federal immunity isn’t preventing such lawsuits.
In 2020, the U.S. Department of Health & Human Services published a declaration under the Public Readiness and Emergency Preparedness Act (PREP Act) that provided liability immunity to health care professionals for any activity related to medical countermeasures against COVID-19. The act allows an exception for negligence claims associated with death or serious injury caused by willful misconduct.
At the same time, most states implemented laws or executive orders shielding physicians from liability claims related to the prevention and treatment of COVID-19, unless gross negligence or willful misconduct is proven.
Mr. Graham said some COVID-related claims against physicians have included allegations of gross negligence to avoid the application of state immunity, while others combine allegations of deviations from standard of care unrelated to the pandemic.
Some plaintiffs are attempting to skirt the protections by making complaints sound as if they’re not related to COVID-19, Mr. Kolbert said. That way, they don’t have to prove gross negligence or willful misconduct at all.
“The filings at first blush may not tell you it’s a COVID case, but it may be a COVID case,” he said. “Plaintiffs’ attorneys are trying to assert that COVID defenses do not apply and that these cases are ‘traditional physician negligence’ claims. They’re trying to plead around the protections.”
The federal and state immunities are likely keeping the volume of COVID claims down overall and are discouraging some complaints from moving forward, attorneys say.
But because some plaintiffs are downplaying or ignoring the COVID association, it’s likely that more COVID lawsuits exist than anyone realizes, according to Mr. Kolbert.
“I expect there’s an underestimation of how many COVID claims are really out there,” he said.
What does the future hold for COVID claims?
Currently, the frequency and the severity of COVID claims are low, Ms. Vats said. She believes the cost of such claims will continue to remain at low levels.
“But again, there is a lot of uncertainty,” she said. “This year, states have started to roll back their immunity protections, and in a lot of states, there is no cap in awarding [noneconomic] damages. There could well be a scenario where they allege wrongful death, and in a state with no cap on the pain and suffering component, if juries continue to behave the way they have been behaving, we could see aberration verdicts.”
Another lingering issue concerns which court systems have jurisdiction in cases involving COVID-related claims. Because of the nationwide response to the pandemic, Mr. Graham thinks it makes sense that federal courts handle the cases, but the plaintiffs’ bar has generally been opposed to federal jurisdiction.
“A second issue is the long-term impact of COVID litigation on our providers,” he said. “If the protections in place to limit liability are determined to be ineffective, our state and federal leaders must act aggressively and in a bipartisan way to make sure our health care providers are protected when we face the next crisis.”
A version of this article first appeared on Medscape.com.
A pregnant patient who had COVID-19 showed up at a hospital with respiratory difficulty caused by her illness. Physicians had to perform an emergency delivery of her near-term baby.
The infant survived, but the woman lost oxygen during the ordeal and suffered hypoxic brain damage. She is now suing an obstetrician, a pulmonologist, and an intensive care unit physician for medical malpractice.
The plaintiff contends there was a failure “to adequately recognize and treat her condition,” said Peter Kolbert, senior vice president for claim and litigation services for Healthcare Risk Advisors, part of TDC Group, which includes national medical liability insurer The Doctors Company.
“The physicians involved vehemently disagree and believe they treated her appropriately,” Mr. Kolbert said. “In fact, we believe their actions were heroic.”
In another case, a patient with COVID-19 and multiple comorbidities was admitted to a hospital. Physicians sedated and intubated the patient to maintain her airway. She recovered, but the patient now alleges doctors were negligent because she developed ulcers during her hospital stay. The case occurred during the height of the pandemic. In addition to the hospital, a pulmonologist, an ICU physician, and an acute care physician are named in the suit.
Both of these lawsuits are being defined as COVID claims because at the time, the plaintiffs either had COVID and needed care because of COVID, or because the care that physicians provided was affected by COVID in some way.
In the second case, the patient had COVID and needed treatment. During her recovery, ulcers developed. A significant aspect of this case is that it occurred during the height of the pandemic. Hospitals were overcrowded, the staff was swamped, and resources were limited. One factor may be that physicians were doing the best they could at the time but that the pandemic affected the extent of care they could provide.
Now, new data reflect the grim news: COVID claims have arrived. These cases from the claims database of The Doctors Company are just two examples of many COVID-related claims that have been levied since the pandemic started.
Currently, there are 162 open COVID-related claims in The Doctors Company database, according to Mr. Kolbert. A September 2022 benchmark report from Aon and the American Society for Health Care Risk Management indicates that 245 claims that pertain to patients with confirmed or suspected COVID-19 have been filed since the pandemic began. The findings in this report stem from an analysis of 95,600 hospital and physician liability claims that occurred between 2012 and 2021.
Of the 245 cases, 89 claims have been closed. The average cost was $43,000 per claim, said Kanika Vats, a director and actuary for Aon, a global firm that provides risk, reinsurance, and health solutions. Six of the claims cost $300,000 or more; the highest settlement was for $700,000.
“Most of the allegations in these claims revolve around delay in treatment or delay in diagnosis,” Ms. Vats said.
Which specialties are involved in legal actions?
Physicians working in acute care settings such as emergency departments and urgent care centers are the primary targets in COVID-related lawsuits involving doctors, say legal analysts. However, other specialties are also being affected. Physicians being sued include some who practiced telemedicine during the pandemic.
In one case, a primary care physician saw a patient via telemedicine because the physical medical office was closed. The patient was evaluated virtually and was sent for bloodwork and an x-ray.
The patient is now suing the primary care physician, alleging that failure to immediately send her to a hospital resulted in tuberculosis going untreated and that the failure led to a bad outcome. The allegation is that the physician underevaluated the case during the telemedicine visit, Mr. Kolbert said.
Drew Graham, an attorney at Hall Booth Smith PC, which is based in New York, said that most of the COVID-related liability claims he has seen involve facilities that provide postacute care, such as nursing homes and assisted living facilities. His firm has also seen a small number of COVID-related claims against physicians.
At least two of the claims involved allegations of improper treatment of COVID during hospitalizations, he said. Another involved a telehealth visit in which the patient claimed the virtual care that was provided was improper and that their condition required an in-person examination. Mr. Graham declined to specify the specialties of the physicians sued.
The Medical Professional Liability Association reports similar trends in COVID-related claims. Long-term facilities and hospitals are the most common focus of COVID-19 claims, followed by emergency medicine, primary care, and ob/gyn medical specialties, according to Kwon Miller, manager of data and analytics for MPL Association, a national trade association for medical liability insurers that operates a large claims database.
Between January 2020 and June 2022, the MPL Association Data Sharing Project recorded 280 COVID-19 events. “Events” refers to notifications, licensing board inquiries, and claims involving COVID. Of these events, 180 were closed with no indemnity payment, and 13 were closed with an average indemnity payment of $3,816, Mr. Miller said.
Complaints of delayed care associated with the pandemic are also on the rise. For example, one patient is suing a gastroenterologist for delaying his colonoscopy, alleging the postponement led to a delayed colon cancer diagnosis and worse prognosis, Mr. Kolbert said.
“It was delayed because all elective procedures at the time were being put off,” he said. “The patient claims that had they received the scheduled screening, the cancer would have been diagnosed at stage I as opposed to stage III.”
Why isn’t federal immunity shielding physicians?
A pressing question about the growing number of COVID claims is why state and federal immunity isn’t preventing such lawsuits.
In 2020, the U.S. Department of Health & Human Services published a declaration under the Public Readiness and Emergency Preparedness Act (PREP Act) that provided liability immunity to health care professionals for any activity related to medical countermeasures against COVID-19. The act allows an exception for negligence claims associated with death or serious injury caused by willful misconduct.
At the same time, most states implemented laws or executive orders shielding physicians from liability claims related to the prevention and treatment of COVID-19, unless gross negligence or willful misconduct is proven.
Mr. Graham said some COVID-related claims against physicians have included allegations of gross negligence to avoid the application of state immunity, while others combine allegations of deviations from standard of care unrelated to the pandemic.
Some plaintiffs are attempting to skirt the protections by making complaints sound as if they’re not related to COVID-19, Mr. Kolbert said. That way, they don’t have to prove gross negligence or willful misconduct at all.
“The filings at first blush may not tell you it’s a COVID case, but it may be a COVID case,” he said. “Plaintiffs’ attorneys are trying to assert that COVID defenses do not apply and that these cases are ‘traditional physician negligence’ claims. They’re trying to plead around the protections.”
The federal and state immunities are likely keeping the volume of COVID claims down overall and are discouraging some complaints from moving forward, attorneys say.
But because some plaintiffs are downplaying or ignoring the COVID association, it’s likely that more COVID lawsuits exist than anyone realizes, according to Mr. Kolbert.
“I expect there’s an underestimation of how many COVID claims are really out there,” he said.
What does the future hold for COVID claims?
Currently, the frequency and the severity of COVID claims are low, Ms. Vats said. She believes the cost of such claims will continue to remain at low levels.
“But again, there is a lot of uncertainty,” she said. “This year, states have started to roll back their immunity protections, and in a lot of states, there is no cap in awarding [noneconomic] damages. There could well be a scenario where they allege wrongful death, and in a state with no cap on the pain and suffering component, if juries continue to behave the way they have been behaving, we could see aberration verdicts.”
Another lingering issue concerns which court systems have jurisdiction in cases involving COVID-related claims. Because of the nationwide response to the pandemic, Mr. Graham thinks it makes sense that federal courts handle the cases, but the plaintiffs’ bar has generally been opposed to federal jurisdiction.
“A second issue is the long-term impact of COVID litigation on our providers,” he said. “If the protections in place to limit liability are determined to be ineffective, our state and federal leaders must act aggressively and in a bipartisan way to make sure our health care providers are protected when we face the next crisis.”
A version of this article first appeared on Medscape.com.
A pregnant patient who had COVID-19 showed up at a hospital with respiratory difficulty caused by her illness. Physicians had to perform an emergency delivery of her near-term baby.
The infant survived, but the woman lost oxygen during the ordeal and suffered hypoxic brain damage. She is now suing an obstetrician, a pulmonologist, and an intensive care unit physician for medical malpractice.
The plaintiff contends there was a failure “to adequately recognize and treat her condition,” said Peter Kolbert, senior vice president for claim and litigation services for Healthcare Risk Advisors, part of TDC Group, which includes national medical liability insurer The Doctors Company.
“The physicians involved vehemently disagree and believe they treated her appropriately,” Mr. Kolbert said. “In fact, we believe their actions were heroic.”
In another case, a patient with COVID-19 and multiple comorbidities was admitted to a hospital. Physicians sedated and intubated the patient to maintain her airway. She recovered, but the patient now alleges doctors were negligent because she developed ulcers during her hospital stay. The case occurred during the height of the pandemic. In addition to the hospital, a pulmonologist, an ICU physician, and an acute care physician are named in the suit.
Both of these lawsuits are being defined as COVID claims because at the time, the plaintiffs either had COVID and needed care because of COVID, or because the care that physicians provided was affected by COVID in some way.
In the second case, the patient had COVID and needed treatment. During her recovery, ulcers developed. A significant aspect of this case is that it occurred during the height of the pandemic. Hospitals were overcrowded, the staff was swamped, and resources were limited. One factor may be that physicians were doing the best they could at the time but that the pandemic affected the extent of care they could provide.
Now, new data reflect the grim news: COVID claims have arrived. These cases from the claims database of The Doctors Company are just two examples of many COVID-related claims that have been levied since the pandemic started.
Currently, there are 162 open COVID-related claims in The Doctors Company database, according to Mr. Kolbert. A September 2022 benchmark report from Aon and the American Society for Health Care Risk Management indicates that 245 claims that pertain to patients with confirmed or suspected COVID-19 have been filed since the pandemic began. The findings in this report stem from an analysis of 95,600 hospital and physician liability claims that occurred between 2012 and 2021.
Of the 245 cases, 89 claims have been closed. The average cost was $43,000 per claim, said Kanika Vats, a director and actuary for Aon, a global firm that provides risk, reinsurance, and health solutions. Six of the claims cost $300,000 or more; the highest settlement was for $700,000.
“Most of the allegations in these claims revolve around delay in treatment or delay in diagnosis,” Ms. Vats said.
Which specialties are involved in legal actions?
Physicians working in acute care settings such as emergency departments and urgent care centers are the primary targets in COVID-related lawsuits involving doctors, say legal analysts. However, other specialties are also being affected. Physicians being sued include some who practiced telemedicine during the pandemic.
In one case, a primary care physician saw a patient via telemedicine because the physical medical office was closed. The patient was evaluated virtually and was sent for bloodwork and an x-ray.
The patient is now suing the primary care physician, alleging that failure to immediately send her to a hospital resulted in tuberculosis going untreated and that the failure led to a bad outcome. The allegation is that the physician underevaluated the case during the telemedicine visit, Mr. Kolbert said.
Drew Graham, an attorney at Hall Booth Smith PC, which is based in New York, said that most of the COVID-related liability claims he has seen involve facilities that provide postacute care, such as nursing homes and assisted living facilities. His firm has also seen a small number of COVID-related claims against physicians.
At least two of the claims involved allegations of improper treatment of COVID during hospitalizations, he said. Another involved a telehealth visit in which the patient claimed the virtual care that was provided was improper and that their condition required an in-person examination. Mr. Graham declined to specify the specialties of the physicians sued.
The Medical Professional Liability Association reports similar trends in COVID-related claims. Long-term facilities and hospitals are the most common focus of COVID-19 claims, followed by emergency medicine, primary care, and ob/gyn medical specialties, according to Kwon Miller, manager of data and analytics for MPL Association, a national trade association for medical liability insurers that operates a large claims database.
Between January 2020 and June 2022, the MPL Association Data Sharing Project recorded 280 COVID-19 events. “Events” refers to notifications, licensing board inquiries, and claims involving COVID. Of these events, 180 were closed with no indemnity payment, and 13 were closed with an average indemnity payment of $3,816, Mr. Miller said.
Complaints of delayed care associated with the pandemic are also on the rise. For example, one patient is suing a gastroenterologist for delaying his colonoscopy, alleging the postponement led to a delayed colon cancer diagnosis and worse prognosis, Mr. Kolbert said.
“It was delayed because all elective procedures at the time were being put off,” he said. “The patient claims that had they received the scheduled screening, the cancer would have been diagnosed at stage I as opposed to stage III.”
Why isn’t federal immunity shielding physicians?
A pressing question about the growing number of COVID claims is why state and federal immunity isn’t preventing such lawsuits.
In 2020, the U.S. Department of Health & Human Services published a declaration under the Public Readiness and Emergency Preparedness Act (PREP Act) that provided liability immunity to health care professionals for any activity related to medical countermeasures against COVID-19. The act allows an exception for negligence claims associated with death or serious injury caused by willful misconduct.
At the same time, most states implemented laws or executive orders shielding physicians from liability claims related to the prevention and treatment of COVID-19, unless gross negligence or willful misconduct is proven.
Mr. Graham said some COVID-related claims against physicians have included allegations of gross negligence to avoid the application of state immunity, while others combine allegations of deviations from standard of care unrelated to the pandemic.
Some plaintiffs are attempting to skirt the protections by making complaints sound as if they’re not related to COVID-19, Mr. Kolbert said. That way, they don’t have to prove gross negligence or willful misconduct at all.
“The filings at first blush may not tell you it’s a COVID case, but it may be a COVID case,” he said. “Plaintiffs’ attorneys are trying to assert that COVID defenses do not apply and that these cases are ‘traditional physician negligence’ claims. They’re trying to plead around the protections.”
The federal and state immunities are likely keeping the volume of COVID claims down overall and are discouraging some complaints from moving forward, attorneys say.
But because some plaintiffs are downplaying or ignoring the COVID association, it’s likely that more COVID lawsuits exist than anyone realizes, according to Mr. Kolbert.
“I expect there’s an underestimation of how many COVID claims are really out there,” he said.
What does the future hold for COVID claims?
Currently, the frequency and the severity of COVID claims are low, Ms. Vats said. She believes the cost of such claims will continue to remain at low levels.
“But again, there is a lot of uncertainty,” she said. “This year, states have started to roll back their immunity protections, and in a lot of states, there is no cap in awarding [noneconomic] damages. There could well be a scenario where they allege wrongful death, and in a state with no cap on the pain and suffering component, if juries continue to behave the way they have been behaving, we could see aberration verdicts.”
Another lingering issue concerns which court systems have jurisdiction in cases involving COVID-related claims. Because of the nationwide response to the pandemic, Mr. Graham thinks it makes sense that federal courts handle the cases, but the plaintiffs’ bar has generally been opposed to federal jurisdiction.
“A second issue is the long-term impact of COVID litigation on our providers,” he said. “If the protections in place to limit liability are determined to be ineffective, our state and federal leaders must act aggressively and in a bipartisan way to make sure our health care providers are protected when we face the next crisis.”
A version of this article first appeared on Medscape.com.
Ten-day methotrexate pause after COVID vaccine booster enhances immunity against Omicron variant
People taking methotrexate for immunomodulatory diseases can skip one or two scheduled doses after they get an mRNA-based vaccine booster for COVID-19 and achieve a level of immunity against Omicron variants that’s comparable to people who aren’t immunosuppressed, a small observational cohort study from Germany reported.
“In general, the data suggest that pausing methotrexate is feasible, and it’s sufficient if the last dose occurs 1-3 days before the vaccination,” study coauthor Gerd Burmester, MD, a senior professor of rheumatology and immunology at the University of Medicine Berlin, told this news organization. “In pragmatic terms: pausing the methotrexate injection just twice after the vaccine is finished and, interestingly, not prior to the vaccination.”
The study, published online in RMD Open, included a statistical analysis that determined that a 10-day pause after the vaccination would be optimal, Dr. Burmester said.
Dr. Burmester and coauthors claimed this is the first study to evaluate the antibody response in patients on methotrexate against Omicron variants – in this study, variants BA.1 and BA.2 – after getting a COVID-19 mRNA booster. The study compared neutralizing serum activity of 50 patients taking methotrexate – 24 of whom continued treatments uninterrupted and 26 of whom paused treatments after getting a second booster – with 25 nonimmunosuppressed patients who served as controls. A total of 24% of the patients taking methotrexate received the mRNA-1273 vaccine while the entire control group received the Pfizer/BioNTech BNT162b2 vaccine.
The researchers used SARS-CoV-2 pseudovirus neutralization assays to evaluate post-vaccination antibody levels.
The U.S. Centers for Disease Control and Prevention and other government health agencies have recommended that immunocompromised patients get a fourth COVID-19 vaccination. But these vaccines can be problematic in patients taking methotrexate, which was linked to a reduced response after the second and third doses of the COVID-19 vaccine.
Previous studies reported that pausing methotrexate for 10 or 14 days after the first two vaccinations improved the production of neutralizing antibodies. A 2022 study found that a 2-week pause after a booster increased antibody response against S1 RBD (receptor binding domain) of the SARS-CoV-2 spike protein about twofold. Another recently published study of mRNA vaccines found that taking methotrexate with either a biologic or targeted synthetic disease-modifying antirheumatic drug reduces the efficacy of a third (booster) shot of SARS-CoV-2 mRNA vaccine in older adults but not younger patients with RA.
“Our study and also the other studies suggested that you can pause methotrexate treatment safely from a point of view of disease activity of rheumatoid arthritis,” Dr. Burmester said. “If you do the pause just twice or once only, it doesn’t lead to significant flares.”
Study results
The study found that serum neutralizing activity against the Omicron BA.1 variant, measured as geometric mean 50% inhibitory serum dilution (ID50s), wasn’t significantly different between the methotrexate and the nonimmunosuppressed groups before getting their mRNA booster (P = .657). However, 4 weeks after getting the booster, the nonimmunosuppressed group had a 68-fold increase in antibody activity versus a 20-fold increase in the methotrexate patients. After 12 weeks, ID50s in both groups decreased by about half (P = .001).
The methotrexate patients who continued therapy after the booster had significantly lower neutralization against Omicron BA.1 at both 4 weeks and 12 weeks than did their counterparts who paused therapy, as well as control patients.
The results were very similar in the same group comparisons of the serum neutralizing activity against the Omicron BA.2 variant at 4 and 12 weeks after booster vaccination.
Expert commentary
This study is noteworthy because it used SARS-CoV-2 pseudovirus neutralization assays to evaluate antibody levels, Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study, said. “A lot of studies don’t look at neutralizing antibody titers, and that’s really what we care about,” Dr. Winthrop said. “What we want are functional antibodies that are doing something, and the only way to do that is to test them.”
The study is “confirmatory” of other studies that call for pausing methotrexate after vaccination, Dr. Winthrop said, including a study he coauthored, and which the German researchers cited, that found pausing methotrexate for a week or so after the influenza vaccination in RA patients improved vaccine immunogenicity. He added that the findings with the early Omicron variants are important because the newest boosters target the later Omicron variants, BA.4 and BA.5.
“The bottom line is that when someone comes in for a COVID-19 vaccination, tell them to be off of methotrexate for 7-10 days,” Dr. Winthrop said. “This is for the booster, but it raises the question: If you go out to three, four, or five vaccinations, does this matter anymore? With the flu vaccine, most people are out to 10 or 15 boosters, and we haven’t seen any significant increase in disease flares.”
The study received funding from Medac, Gilead/Galapagos, and Friends and Sponsors of Berlin Charity. Dr. Burmester reported no relevant disclosures. Dr. Winthrop is a research consultant to Pfizer.
A version of this article first appeared on Medscape.com.
People taking methotrexate for immunomodulatory diseases can skip one or two scheduled doses after they get an mRNA-based vaccine booster for COVID-19 and achieve a level of immunity against Omicron variants that’s comparable to people who aren’t immunosuppressed, a small observational cohort study from Germany reported.
“In general, the data suggest that pausing methotrexate is feasible, and it’s sufficient if the last dose occurs 1-3 days before the vaccination,” study coauthor Gerd Burmester, MD, a senior professor of rheumatology and immunology at the University of Medicine Berlin, told this news organization. “In pragmatic terms: pausing the methotrexate injection just twice after the vaccine is finished and, interestingly, not prior to the vaccination.”
The study, published online in RMD Open, included a statistical analysis that determined that a 10-day pause after the vaccination would be optimal, Dr. Burmester said.
Dr. Burmester and coauthors claimed this is the first study to evaluate the antibody response in patients on methotrexate against Omicron variants – in this study, variants BA.1 and BA.2 – after getting a COVID-19 mRNA booster. The study compared neutralizing serum activity of 50 patients taking methotrexate – 24 of whom continued treatments uninterrupted and 26 of whom paused treatments after getting a second booster – with 25 nonimmunosuppressed patients who served as controls. A total of 24% of the patients taking methotrexate received the mRNA-1273 vaccine while the entire control group received the Pfizer/BioNTech BNT162b2 vaccine.
The researchers used SARS-CoV-2 pseudovirus neutralization assays to evaluate post-vaccination antibody levels.
The U.S. Centers for Disease Control and Prevention and other government health agencies have recommended that immunocompromised patients get a fourth COVID-19 vaccination. But these vaccines can be problematic in patients taking methotrexate, which was linked to a reduced response after the second and third doses of the COVID-19 vaccine.
Previous studies reported that pausing methotrexate for 10 or 14 days after the first two vaccinations improved the production of neutralizing antibodies. A 2022 study found that a 2-week pause after a booster increased antibody response against S1 RBD (receptor binding domain) of the SARS-CoV-2 spike protein about twofold. Another recently published study of mRNA vaccines found that taking methotrexate with either a biologic or targeted synthetic disease-modifying antirheumatic drug reduces the efficacy of a third (booster) shot of SARS-CoV-2 mRNA vaccine in older adults but not younger patients with RA.
“Our study and also the other studies suggested that you can pause methotrexate treatment safely from a point of view of disease activity of rheumatoid arthritis,” Dr. Burmester said. “If you do the pause just twice or once only, it doesn’t lead to significant flares.”
Study results
The study found that serum neutralizing activity against the Omicron BA.1 variant, measured as geometric mean 50% inhibitory serum dilution (ID50s), wasn’t significantly different between the methotrexate and the nonimmunosuppressed groups before getting their mRNA booster (P = .657). However, 4 weeks after getting the booster, the nonimmunosuppressed group had a 68-fold increase in antibody activity versus a 20-fold increase in the methotrexate patients. After 12 weeks, ID50s in both groups decreased by about half (P = .001).
The methotrexate patients who continued therapy after the booster had significantly lower neutralization against Omicron BA.1 at both 4 weeks and 12 weeks than did their counterparts who paused therapy, as well as control patients.
The results were very similar in the same group comparisons of the serum neutralizing activity against the Omicron BA.2 variant at 4 and 12 weeks after booster vaccination.
Expert commentary
This study is noteworthy because it used SARS-CoV-2 pseudovirus neutralization assays to evaluate antibody levels, Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study, said. “A lot of studies don’t look at neutralizing antibody titers, and that’s really what we care about,” Dr. Winthrop said. “What we want are functional antibodies that are doing something, and the only way to do that is to test them.”
The study is “confirmatory” of other studies that call for pausing methotrexate after vaccination, Dr. Winthrop said, including a study he coauthored, and which the German researchers cited, that found pausing methotrexate for a week or so after the influenza vaccination in RA patients improved vaccine immunogenicity. He added that the findings with the early Omicron variants are important because the newest boosters target the later Omicron variants, BA.4 and BA.5.
“The bottom line is that when someone comes in for a COVID-19 vaccination, tell them to be off of methotrexate for 7-10 days,” Dr. Winthrop said. “This is for the booster, but it raises the question: If you go out to three, four, or five vaccinations, does this matter anymore? With the flu vaccine, most people are out to 10 or 15 boosters, and we haven’t seen any significant increase in disease flares.”
The study received funding from Medac, Gilead/Galapagos, and Friends and Sponsors of Berlin Charity. Dr. Burmester reported no relevant disclosures. Dr. Winthrop is a research consultant to Pfizer.
A version of this article first appeared on Medscape.com.
People taking methotrexate for immunomodulatory diseases can skip one or two scheduled doses after they get an mRNA-based vaccine booster for COVID-19 and achieve a level of immunity against Omicron variants that’s comparable to people who aren’t immunosuppressed, a small observational cohort study from Germany reported.
“In general, the data suggest that pausing methotrexate is feasible, and it’s sufficient if the last dose occurs 1-3 days before the vaccination,” study coauthor Gerd Burmester, MD, a senior professor of rheumatology and immunology at the University of Medicine Berlin, told this news organization. “In pragmatic terms: pausing the methotrexate injection just twice after the vaccine is finished and, interestingly, not prior to the vaccination.”
The study, published online in RMD Open, included a statistical analysis that determined that a 10-day pause after the vaccination would be optimal, Dr. Burmester said.
Dr. Burmester and coauthors claimed this is the first study to evaluate the antibody response in patients on methotrexate against Omicron variants – in this study, variants BA.1 and BA.2 – after getting a COVID-19 mRNA booster. The study compared neutralizing serum activity of 50 patients taking methotrexate – 24 of whom continued treatments uninterrupted and 26 of whom paused treatments after getting a second booster – with 25 nonimmunosuppressed patients who served as controls. A total of 24% of the patients taking methotrexate received the mRNA-1273 vaccine while the entire control group received the Pfizer/BioNTech BNT162b2 vaccine.
The researchers used SARS-CoV-2 pseudovirus neutralization assays to evaluate post-vaccination antibody levels.
The U.S. Centers for Disease Control and Prevention and other government health agencies have recommended that immunocompromised patients get a fourth COVID-19 vaccination. But these vaccines can be problematic in patients taking methotrexate, which was linked to a reduced response after the second and third doses of the COVID-19 vaccine.
Previous studies reported that pausing methotrexate for 10 or 14 days after the first two vaccinations improved the production of neutralizing antibodies. A 2022 study found that a 2-week pause after a booster increased antibody response against S1 RBD (receptor binding domain) of the SARS-CoV-2 spike protein about twofold. Another recently published study of mRNA vaccines found that taking methotrexate with either a biologic or targeted synthetic disease-modifying antirheumatic drug reduces the efficacy of a third (booster) shot of SARS-CoV-2 mRNA vaccine in older adults but not younger patients with RA.
“Our study and also the other studies suggested that you can pause methotrexate treatment safely from a point of view of disease activity of rheumatoid arthritis,” Dr. Burmester said. “If you do the pause just twice or once only, it doesn’t lead to significant flares.”
Study results
The study found that serum neutralizing activity against the Omicron BA.1 variant, measured as geometric mean 50% inhibitory serum dilution (ID50s), wasn’t significantly different between the methotrexate and the nonimmunosuppressed groups before getting their mRNA booster (P = .657). However, 4 weeks after getting the booster, the nonimmunosuppressed group had a 68-fold increase in antibody activity versus a 20-fold increase in the methotrexate patients. After 12 weeks, ID50s in both groups decreased by about half (P = .001).
The methotrexate patients who continued therapy after the booster had significantly lower neutralization against Omicron BA.1 at both 4 weeks and 12 weeks than did their counterparts who paused therapy, as well as control patients.
The results were very similar in the same group comparisons of the serum neutralizing activity against the Omicron BA.2 variant at 4 and 12 weeks after booster vaccination.
Expert commentary
This study is noteworthy because it used SARS-CoV-2 pseudovirus neutralization assays to evaluate antibody levels, Kevin Winthrop, MD, MPH, professor of infectious disease and public health at Oregon Health & Science University, Portland, who was not involved in the study, said. “A lot of studies don’t look at neutralizing antibody titers, and that’s really what we care about,” Dr. Winthrop said. “What we want are functional antibodies that are doing something, and the only way to do that is to test them.”
The study is “confirmatory” of other studies that call for pausing methotrexate after vaccination, Dr. Winthrop said, including a study he coauthored, and which the German researchers cited, that found pausing methotrexate for a week or so after the influenza vaccination in RA patients improved vaccine immunogenicity. He added that the findings with the early Omicron variants are important because the newest boosters target the later Omicron variants, BA.4 and BA.5.
“The bottom line is that when someone comes in for a COVID-19 vaccination, tell them to be off of methotrexate for 7-10 days,” Dr. Winthrop said. “This is for the booster, but it raises the question: If you go out to three, four, or five vaccinations, does this matter anymore? With the flu vaccine, most people are out to 10 or 15 boosters, and we haven’t seen any significant increase in disease flares.”
The study received funding from Medac, Gilead/Galapagos, and Friends and Sponsors of Berlin Charity. Dr. Burmester reported no relevant disclosures. Dr. Winthrop is a research consultant to Pfizer.
A version of this article first appeared on Medscape.com.
FROM RMD OPEN
Why the 5-day isolation period for COVID makes no sense
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
One of the more baffling decisions the CDC made during this pandemic was when they reduced the duration of isolation after a positive COVID test from 10 days to 5 days and did not require a negative antigen test to end isolation.
Multiple studies had suggested, after all, that positive antigen tests, while not perfect, were a decent proxy for infectivity. And if the purpose of isolation is to keep other community members safe, why not use a readily available test to know when it might be safe to go out in public again?
Also, 5 days just wasn’t that much time. Many individuals are symptomatic long after that point. Many people test positive long after that point. What exactly is the point of the 5-day isolation period?
We got some hard numbers this week to show just how good (or bad) an arbitrary-seeming 5-day isolation period is, thanks to this study from JAMA Network Open, which gives us a low-end estimate for the proportion of people who remain positive on antigen tests, which is to say infectious, after an isolation period.
This study estimates the low end of postisolation infectivity because of the study population: student athletes at an NCAA Division I school, which may or may not be Stanford. These athletes tested positive for COVID after having at least one dose of vaccine from January to May 2022. School protocol was to put the students in isolation for 7 days, at which time they could “test out” with a negative antigen test.
Put simply, these were healthy people. They were young. They were athletes. They were vaccinated. If anyone is going to have a brief, easy COVID course, it would be them. And they are doing at least a week of isolation, not 5 days.
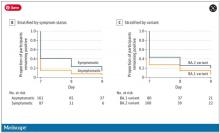
So – isolation for 7 days. Antigen testing on day 7. How many still tested positive? Of 248 individuals tested, 67 (27%) tested positive. One in four.
More than half of those positive on day 7 tested positive on day 8, and more than half of those tested positive again on day 9. By day 10, they were released from isolation without further testing.
So, right there .
There were some predictors of prolonged positivity.
Symptomatic athletes were much more likely to test positive than asymptomatic athletes.
And the particular variant seemed to matter as well. In this time period, BA.1 and BA.2 were dominant, and it was pretty clear that BA.2 persisted longer than BA.1.
This brings me back to my original question: What is the point of the 5-day isolation period? On the basis of this study, you could imagine a guideline based on symptoms: Stay home until you feel better. You could imagine a guideline based on testing: Stay home until you test negative. A guideline based on time alone just doesn’t comport with the data. The benefit of policies based on symptoms or testing are obvious; some people would be out of isolation even before 5 days. But the downside, of course, is that some people would be stuck in isolation for much longer.
Maybe we should just say it. At this point, you could even imagine there being no recommendation at all – no isolation period. Like, you just stay home if you feel like you should stay home. I’m not entirely sure that such a policy would necessarily result in a greater number of infectious people out in the community.
In any case, as the arbitrariness of this particular 5-day isolation policy becomes more clear, the policy itself may be living on borrowed time.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com. He disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
One of the more baffling decisions the CDC made during this pandemic was when they reduced the duration of isolation after a positive COVID test from 10 days to 5 days and did not require a negative antigen test to end isolation.
Multiple studies had suggested, after all, that positive antigen tests, while not perfect, were a decent proxy for infectivity. And if the purpose of isolation is to keep other community members safe, why not use a readily available test to know when it might be safe to go out in public again?
Also, 5 days just wasn’t that much time. Many individuals are symptomatic long after that point. Many people test positive long after that point. What exactly is the point of the 5-day isolation period?
We got some hard numbers this week to show just how good (or bad) an arbitrary-seeming 5-day isolation period is, thanks to this study from JAMA Network Open, which gives us a low-end estimate for the proportion of people who remain positive on antigen tests, which is to say infectious, after an isolation period.
This study estimates the low end of postisolation infectivity because of the study population: student athletes at an NCAA Division I school, which may or may not be Stanford. These athletes tested positive for COVID after having at least one dose of vaccine from January to May 2022. School protocol was to put the students in isolation for 7 days, at which time they could “test out” with a negative antigen test.
Put simply, these were healthy people. They were young. They were athletes. They were vaccinated. If anyone is going to have a brief, easy COVID course, it would be them. And they are doing at least a week of isolation, not 5 days.
So – isolation for 7 days. Antigen testing on day 7. How many still tested positive? Of 248 individuals tested, 67 (27%) tested positive. One in four.
More than half of those positive on day 7 tested positive on day 8, and more than half of those tested positive again on day 9. By day 10, they were released from isolation without further testing.
So, right there .
There were some predictors of prolonged positivity.
Symptomatic athletes were much more likely to test positive than asymptomatic athletes.
And the particular variant seemed to matter as well. In this time period, BA.1 and BA.2 were dominant, and it was pretty clear that BA.2 persisted longer than BA.1.
This brings me back to my original question: What is the point of the 5-day isolation period? On the basis of this study, you could imagine a guideline based on symptoms: Stay home until you feel better. You could imagine a guideline based on testing: Stay home until you test negative. A guideline based on time alone just doesn’t comport with the data. The benefit of policies based on symptoms or testing are obvious; some people would be out of isolation even before 5 days. But the downside, of course, is that some people would be stuck in isolation for much longer.
Maybe we should just say it. At this point, you could even imagine there being no recommendation at all – no isolation period. Like, you just stay home if you feel like you should stay home. I’m not entirely sure that such a policy would necessarily result in a greater number of infectious people out in the community.
In any case, as the arbitrariness of this particular 5-day isolation policy becomes more clear, the policy itself may be living on borrowed time.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com. He disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study. I’m Dr. F. Perry Wilson of the Yale School of Medicine.
One of the more baffling decisions the CDC made during this pandemic was when they reduced the duration of isolation after a positive COVID test from 10 days to 5 days and did not require a negative antigen test to end isolation.
Multiple studies had suggested, after all, that positive antigen tests, while not perfect, were a decent proxy for infectivity. And if the purpose of isolation is to keep other community members safe, why not use a readily available test to know when it might be safe to go out in public again?
Also, 5 days just wasn’t that much time. Many individuals are symptomatic long after that point. Many people test positive long after that point. What exactly is the point of the 5-day isolation period?
We got some hard numbers this week to show just how good (or bad) an arbitrary-seeming 5-day isolation period is, thanks to this study from JAMA Network Open, which gives us a low-end estimate for the proportion of people who remain positive on antigen tests, which is to say infectious, after an isolation period.
This study estimates the low end of postisolation infectivity because of the study population: student athletes at an NCAA Division I school, which may or may not be Stanford. These athletes tested positive for COVID after having at least one dose of vaccine from January to May 2022. School protocol was to put the students in isolation for 7 days, at which time they could “test out” with a negative antigen test.
Put simply, these were healthy people. They were young. They were athletes. They were vaccinated. If anyone is going to have a brief, easy COVID course, it would be them. And they are doing at least a week of isolation, not 5 days.
So – isolation for 7 days. Antigen testing on day 7. How many still tested positive? Of 248 individuals tested, 67 (27%) tested positive. One in four.
More than half of those positive on day 7 tested positive on day 8, and more than half of those tested positive again on day 9. By day 10, they were released from isolation without further testing.
So, right there .
There were some predictors of prolonged positivity.
Symptomatic athletes were much more likely to test positive than asymptomatic athletes.
And the particular variant seemed to matter as well. In this time period, BA.1 and BA.2 were dominant, and it was pretty clear that BA.2 persisted longer than BA.1.
This brings me back to my original question: What is the point of the 5-day isolation period? On the basis of this study, you could imagine a guideline based on symptoms: Stay home until you feel better. You could imagine a guideline based on testing: Stay home until you test negative. A guideline based on time alone just doesn’t comport with the data. The benefit of policies based on symptoms or testing are obvious; some people would be out of isolation even before 5 days. But the downside, of course, is that some people would be stuck in isolation for much longer.
Maybe we should just say it. At this point, you could even imagine there being no recommendation at all – no isolation period. Like, you just stay home if you feel like you should stay home. I’m not entirely sure that such a policy would necessarily result in a greater number of infectious people out in the community.
In any case, as the arbitrariness of this particular 5-day isolation policy becomes more clear, the policy itself may be living on borrowed time.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale’s Clinical and Translational Research Accelerator. His science communication work can be found in the Huffington Post, on NPR, and on Medscape. He tweets @fperrywilson and hosts a repository of his communication work at www.methodsman.com. He disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Vaccine adherence hinges on improving science communication
“I’m not getting the vaccine. Nobody knows the long-term effects, and I heard that people are getting clots.”
We were screening patients at a low-cost clinic in Philadelphia for concerns surrounding social determinants of health. During one patient visit, in addition to concerns including housing, medication affordability, and transportation, we found that she had not received the COVID-19 vaccine, and we asked if she was interested in being immunized.
News reports have endlessly covered antivaccine sentiment, but this personal encounter hit home. From simple face masks to groundbreaking vaccines, we failed as physicians to encourage widespread uptake of health-protective measures despite strong scientific backing.
Large swaths of the public deny these tools’ importance or question their safety. This is ultimately rooted in the inability of community leaders and health care professionals to communicate with the public.
Science communication is inherently difficult. Scientists use complex language, and it is hard to evaluate the lay public’s baseline knowledge. Moreover, we are trained to speak with qualifications, encourage doubt, and accept change and evolution of fact. These qualities contrast the definitive messaging necessary in public settings. COVID-19 highlighted these gaps, where regardless of novel scientific solutions, poor communication led to a resistance to accept the tested scientific solution, which ultimately was the rate-limiting factor for overcoming the virus.
As directors of Physician Executive Leadership, an organization that trains future physicians at Thomas Jefferson University to tackle emerging health care issues, we hosted Paul Offit, MD, a national media figure and vaccine advocate. Dr. Offit shared his personal growth during the pandemic, from being abruptly thrown into the spotlight to eventually honing his communication skills. Dr. Offit discussed the challenges of sharing medical knowledge with laypeople and adaptations that are necessary. We found this transformative, realizing the importance of science communication training early in medical education.
Emphasizing the humanities and building soft skills will improve outcomes and benefit broader society by producing physician-leaders in public health and policy. We hope to improve our own communication skills and work in medical education to incorporate similar training into education paradigms for future students.
As seen in our patient interaction, strong science alone will not drive patient adherence; instead, we must work at personal and system levels to induce change. Physicians have a unique opportunity to generate trust and guide evidence-based policy. We must communicate, whether one-on-one with patients, or to millions of viewers via media or policymaker settings. We hope to not only be doctors, but to be advocates, leaders, and trusted advisers for the public.
Mr. Kieran and Mr. Shah are second-year medical students at Sidney Kimmel Medical College, Philadelphia. Neither disclosed any relevant conflicts of interest. A version of this article first appeared on Medscape.com.
“I’m not getting the vaccine. Nobody knows the long-term effects, and I heard that people are getting clots.”
We were screening patients at a low-cost clinic in Philadelphia for concerns surrounding social determinants of health. During one patient visit, in addition to concerns including housing, medication affordability, and transportation, we found that she had not received the COVID-19 vaccine, and we asked if she was interested in being immunized.
News reports have endlessly covered antivaccine sentiment, but this personal encounter hit home. From simple face masks to groundbreaking vaccines, we failed as physicians to encourage widespread uptake of health-protective measures despite strong scientific backing.
Large swaths of the public deny these tools’ importance or question their safety. This is ultimately rooted in the inability of community leaders and health care professionals to communicate with the public.
Science communication is inherently difficult. Scientists use complex language, and it is hard to evaluate the lay public’s baseline knowledge. Moreover, we are trained to speak with qualifications, encourage doubt, and accept change and evolution of fact. These qualities contrast the definitive messaging necessary in public settings. COVID-19 highlighted these gaps, where regardless of novel scientific solutions, poor communication led to a resistance to accept the tested scientific solution, which ultimately was the rate-limiting factor for overcoming the virus.
As directors of Physician Executive Leadership, an organization that trains future physicians at Thomas Jefferson University to tackle emerging health care issues, we hosted Paul Offit, MD, a national media figure and vaccine advocate. Dr. Offit shared his personal growth during the pandemic, from being abruptly thrown into the spotlight to eventually honing his communication skills. Dr. Offit discussed the challenges of sharing medical knowledge with laypeople and adaptations that are necessary. We found this transformative, realizing the importance of science communication training early in medical education.
Emphasizing the humanities and building soft skills will improve outcomes and benefit broader society by producing physician-leaders in public health and policy. We hope to improve our own communication skills and work in medical education to incorporate similar training into education paradigms for future students.
As seen in our patient interaction, strong science alone will not drive patient adherence; instead, we must work at personal and system levels to induce change. Physicians have a unique opportunity to generate trust and guide evidence-based policy. We must communicate, whether one-on-one with patients, or to millions of viewers via media or policymaker settings. We hope to not only be doctors, but to be advocates, leaders, and trusted advisers for the public.
Mr. Kieran and Mr. Shah are second-year medical students at Sidney Kimmel Medical College, Philadelphia. Neither disclosed any relevant conflicts of interest. A version of this article first appeared on Medscape.com.
“I’m not getting the vaccine. Nobody knows the long-term effects, and I heard that people are getting clots.”
We were screening patients at a low-cost clinic in Philadelphia for concerns surrounding social determinants of health. During one patient visit, in addition to concerns including housing, medication affordability, and transportation, we found that she had not received the COVID-19 vaccine, and we asked if she was interested in being immunized.
News reports have endlessly covered antivaccine sentiment, but this personal encounter hit home. From simple face masks to groundbreaking vaccines, we failed as physicians to encourage widespread uptake of health-protective measures despite strong scientific backing.
Large swaths of the public deny these tools’ importance or question their safety. This is ultimately rooted in the inability of community leaders and health care professionals to communicate with the public.
Science communication is inherently difficult. Scientists use complex language, and it is hard to evaluate the lay public’s baseline knowledge. Moreover, we are trained to speak with qualifications, encourage doubt, and accept change and evolution of fact. These qualities contrast the definitive messaging necessary in public settings. COVID-19 highlighted these gaps, where regardless of novel scientific solutions, poor communication led to a resistance to accept the tested scientific solution, which ultimately was the rate-limiting factor for overcoming the virus.
As directors of Physician Executive Leadership, an organization that trains future physicians at Thomas Jefferson University to tackle emerging health care issues, we hosted Paul Offit, MD, a national media figure and vaccine advocate. Dr. Offit shared his personal growth during the pandemic, from being abruptly thrown into the spotlight to eventually honing his communication skills. Dr. Offit discussed the challenges of sharing medical knowledge with laypeople and adaptations that are necessary. We found this transformative, realizing the importance of science communication training early in medical education.
Emphasizing the humanities and building soft skills will improve outcomes and benefit broader society by producing physician-leaders in public health and policy. We hope to improve our own communication skills and work in medical education to incorporate similar training into education paradigms for future students.
As seen in our patient interaction, strong science alone will not drive patient adherence; instead, we must work at personal and system levels to induce change. Physicians have a unique opportunity to generate trust and guide evidence-based policy. We must communicate, whether one-on-one with patients, or to millions of viewers via media or policymaker settings. We hope to not only be doctors, but to be advocates, leaders, and trusted advisers for the public.
Mr. Kieran and Mr. Shah are second-year medical students at Sidney Kimmel Medical College, Philadelphia. Neither disclosed any relevant conflicts of interest. A version of this article first appeared on Medscape.com.
Research fails to justify post-COVID-19 wave of new-onset parkinsonism
, a multinational team of researchers reported at the International Congress of Parkinson’s Disease and Movement Disorders.
SARS-CoV-2 led to numerous discussions about a potential post–COVID-19 emergence of new-onset parkinsonism in susceptible individuals, often referred to in the literature as a “perfect storm” or a “wave” of parkinsonism, according to lead study author Iro Boura, MD.
Postviral precedence
“Although pathogens have been associated both with parkinsonism cases and Parkinson’s disease pathogenesis, the main concern of a potential connection between COVID-19 and new-onset parkinsonism arose from the historically documented parkinsonism cases appearing with encephalitis lethargica,” said Dr. Boura, a PhD candidate with the University of Crete in Greece and ex-fellow at King’s College London.
Encephalitis lethargica appeared between 1916 and 1930 and has been epidemiologically related to the Spanish influenza pandemic, “although this link has been strongly debated by other researchers,” she added.
Because the connection of COVID-19 and parkinsonism seemed highly speculative, Dr. Boura and movement disorder specialist Kallol Ray Chaudhuri DSc, FRCP, MD, decided to search for any data supporting this notion. “Such a possibility would have a significant impact on everyday practice, including long follow-up neurological assessments of COVID-19 patients, along with greater vigilance in recognizing potential symptoms,” said Dr. Boura.
They found no organized research exploring this link, aside from published case reports.
Scant evidence of a parkinsonism wave
The investigators conducted a review of the literature up to February 2022 to identify and analyze published cases of new-onset parkinsonism following a confirmed SARS-CoV-2 infection in otherwise healthy individuals. They ended up with 20 such cases.
Although some cases presented during or shortly after a COVID-19 infection, “the numbers are currently quite low to draw safe conclusions and generalize these findings as a risk of parkinsonism for the general population,” said Dr. Boura. Overall, parkinsonism appeared in the context of encephalopathy in 11 patients. Four patients developed postinfectious parkinsonism without encephalopathy. Another four had phenotypic similarities to idiopathic Parkinson’s disease.
Nine patients were responsive to levodopa, while four required immunomodulatory treatment.
Although cases have already been reported, current data do not yet justify the concept of a post–COVID-19 parkinsonism wave. However, long-term surveillance is crucial to ensure that reports of further cases are carefully documented and analyzed.
Dr. Chaudhuri’s research team recently wrote a book exploring the numerous aspects of COVID-19 and parkinsonism, including Parkinson’s disease, said Dr. Boura.
“Moreover, the COVID-19 Clinical Neuroscience Study (COVID-CNS), with serial follow-up visits for COVID-19 patients, including imaging, is currently running in the United Kingdom with the active participation of Prof Chaudhuri’s team, aiming at revealing any potential parkinsonism cases after a COVID-19 infection,” she said.
, a multinational team of researchers reported at the International Congress of Parkinson’s Disease and Movement Disorders.
SARS-CoV-2 led to numerous discussions about a potential post–COVID-19 emergence of new-onset parkinsonism in susceptible individuals, often referred to in the literature as a “perfect storm” or a “wave” of parkinsonism, according to lead study author Iro Boura, MD.
Postviral precedence
“Although pathogens have been associated both with parkinsonism cases and Parkinson’s disease pathogenesis, the main concern of a potential connection between COVID-19 and new-onset parkinsonism arose from the historically documented parkinsonism cases appearing with encephalitis lethargica,” said Dr. Boura, a PhD candidate with the University of Crete in Greece and ex-fellow at King’s College London.
Encephalitis lethargica appeared between 1916 and 1930 and has been epidemiologically related to the Spanish influenza pandemic, “although this link has been strongly debated by other researchers,” she added.
Because the connection of COVID-19 and parkinsonism seemed highly speculative, Dr. Boura and movement disorder specialist Kallol Ray Chaudhuri DSc, FRCP, MD, decided to search for any data supporting this notion. “Such a possibility would have a significant impact on everyday practice, including long follow-up neurological assessments of COVID-19 patients, along with greater vigilance in recognizing potential symptoms,” said Dr. Boura.
They found no organized research exploring this link, aside from published case reports.
Scant evidence of a parkinsonism wave
The investigators conducted a review of the literature up to February 2022 to identify and analyze published cases of new-onset parkinsonism following a confirmed SARS-CoV-2 infection in otherwise healthy individuals. They ended up with 20 such cases.
Although some cases presented during or shortly after a COVID-19 infection, “the numbers are currently quite low to draw safe conclusions and generalize these findings as a risk of parkinsonism for the general population,” said Dr. Boura. Overall, parkinsonism appeared in the context of encephalopathy in 11 patients. Four patients developed postinfectious parkinsonism without encephalopathy. Another four had phenotypic similarities to idiopathic Parkinson’s disease.
Nine patients were responsive to levodopa, while four required immunomodulatory treatment.
Although cases have already been reported, current data do not yet justify the concept of a post–COVID-19 parkinsonism wave. However, long-term surveillance is crucial to ensure that reports of further cases are carefully documented and analyzed.
Dr. Chaudhuri’s research team recently wrote a book exploring the numerous aspects of COVID-19 and parkinsonism, including Parkinson’s disease, said Dr. Boura.
“Moreover, the COVID-19 Clinical Neuroscience Study (COVID-CNS), with serial follow-up visits for COVID-19 patients, including imaging, is currently running in the United Kingdom with the active participation of Prof Chaudhuri’s team, aiming at revealing any potential parkinsonism cases after a COVID-19 infection,” she said.
, a multinational team of researchers reported at the International Congress of Parkinson’s Disease and Movement Disorders.
SARS-CoV-2 led to numerous discussions about a potential post–COVID-19 emergence of new-onset parkinsonism in susceptible individuals, often referred to in the literature as a “perfect storm” or a “wave” of parkinsonism, according to lead study author Iro Boura, MD.
Postviral precedence
“Although pathogens have been associated both with parkinsonism cases and Parkinson’s disease pathogenesis, the main concern of a potential connection between COVID-19 and new-onset parkinsonism arose from the historically documented parkinsonism cases appearing with encephalitis lethargica,” said Dr. Boura, a PhD candidate with the University of Crete in Greece and ex-fellow at King’s College London.
Encephalitis lethargica appeared between 1916 and 1930 and has been epidemiologically related to the Spanish influenza pandemic, “although this link has been strongly debated by other researchers,” she added.
Because the connection of COVID-19 and parkinsonism seemed highly speculative, Dr. Boura and movement disorder specialist Kallol Ray Chaudhuri DSc, FRCP, MD, decided to search for any data supporting this notion. “Such a possibility would have a significant impact on everyday practice, including long follow-up neurological assessments of COVID-19 patients, along with greater vigilance in recognizing potential symptoms,” said Dr. Boura.
They found no organized research exploring this link, aside from published case reports.
Scant evidence of a parkinsonism wave
The investigators conducted a review of the literature up to February 2022 to identify and analyze published cases of new-onset parkinsonism following a confirmed SARS-CoV-2 infection in otherwise healthy individuals. They ended up with 20 such cases.
Although some cases presented during or shortly after a COVID-19 infection, “the numbers are currently quite low to draw safe conclusions and generalize these findings as a risk of parkinsonism for the general population,” said Dr. Boura. Overall, parkinsonism appeared in the context of encephalopathy in 11 patients. Four patients developed postinfectious parkinsonism without encephalopathy. Another four had phenotypic similarities to idiopathic Parkinson’s disease.
Nine patients were responsive to levodopa, while four required immunomodulatory treatment.
Although cases have already been reported, current data do not yet justify the concept of a post–COVID-19 parkinsonism wave. However, long-term surveillance is crucial to ensure that reports of further cases are carefully documented and analyzed.
Dr. Chaudhuri’s research team recently wrote a book exploring the numerous aspects of COVID-19 and parkinsonism, including Parkinson’s disease, said Dr. Boura.
“Moreover, the COVID-19 Clinical Neuroscience Study (COVID-CNS), with serial follow-up visits for COVID-19 patients, including imaging, is currently running in the United Kingdom with the active participation of Prof Chaudhuri’s team, aiming at revealing any potential parkinsonism cases after a COVID-19 infection,” she said.
FROM MDS 2022
Reminder that COVID-19 and cancer can be a deadly combo
A new study underscores the importance of COVID-19 and regular COVID-19 testing among adults with a recent cancer diagnosis.
The Indiana statewide study, conducted at the beginning of the pandemic, found that
“This analysis provides additional empirical evidence on the magnitude of risk to patients with cancer whose immune systems are often weakened either by the disease or treatment,” the study team wrote.
The study was published online in JMIR Cancer.
Although evidence has consistently revealed similar findings, the risk of death among unvaccinated people with cancer and COVID-19 has not been nearly as high in previous studies, lead author Brian E. Dixon, PhD, MBA, with Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, said in a statement. Previous studies from China, for instance, reported a two- to threefold greater risk of all-cause mortality among unvaccinated adults with cancer and COVID-19.
A potential reason for this discrepancy, Dr. Dixon noted, is that earlier studies were “generally smaller and made calculations based on data from a single cancer center or health system.”
Another reason is testing for COVID-19 early in the pandemic was limited to symptomatic individuals who may have had more severe infections, possibly leading to an overestimate of the association between SARS-CoV-2 infection, cancer, and all-cause mortality.
In the current analysis, researchers used electronic health records linked to Indiana’s statewide SARS-CoV-2 testing database and state vital records to evaluate the association between SARS-CoV-2 infection and all-cause mortality among 41,924 adults newly diagnosed with cancer between Jan. 1, 2019, and Dec. 31, 2020.
Most people with cancer were White (78.4%) and about half were male. At the time of diagnosis, 17% had one comorbid condition and about 10% had two or more. Most patients had breast cancer (14%), prostate cancer (13%), or melanoma (13%).
During the study period, 2,894 patients (7%) tested positive for SARS-CoV-2.
In multivariate adjusted analysis, the risk of death among those newly diagnosed with cancer increased by 91% (adjusted hazard ratio, 1.91) during the first year of the pandemic before vaccines were available, compared with the year before (January 2019 to Jan. 14, 2020).
During the pandemic period, the risk of death was roughly threefold higher among adults 65 years old and older, compared with adults 18-44 years old (aHR, 3.35).
When looking at the time from a cancer diagnosis to SARS-CoV-2 infection, infection was associated with an almost sevenfold increase in all-cause mortality (aHR, 6.91). Adults 65 years old and older had an almost threefold increased risk of dying, compared with their younger peers (aHR, 2.74).
Dr. Dixon and colleagues also observed an increased risk of death in men with cancer and COVID, compared with women (aHR, 1.23) and those with at least two comorbid conditions versus none (aHR, 2.12). In addition, the risk of dying was 9% higher among Indiana’s rural population than urban dwellers.
Compared with other cancer types, individuals with lung cancer and other digestive cancers had the highest risk of death after SARS-CoV-2 infection (aHR, 1.45 and 1.80, respectively).
“Our findings highlight the increased risk of death for adult cancer patients who test positive for COVID and underscore the importance to cancer patients – including those in remission – of vaccinations, boosters, and regular COVID testing,” Dr. Dixon commented.
“Our results should encourage individuals diagnosed with cancer not only to take preventive action, but also to expeditiously seek out treatments available in the marketplace should they test positive for COVID,” he added.
Support for the study was provided by Indiana University Simon Cancer Center and the Centers for Disease Control and Prevention. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study underscores the importance of COVID-19 and regular COVID-19 testing among adults with a recent cancer diagnosis.
The Indiana statewide study, conducted at the beginning of the pandemic, found that
“This analysis provides additional empirical evidence on the magnitude of risk to patients with cancer whose immune systems are often weakened either by the disease or treatment,” the study team wrote.
The study was published online in JMIR Cancer.
Although evidence has consistently revealed similar findings, the risk of death among unvaccinated people with cancer and COVID-19 has not been nearly as high in previous studies, lead author Brian E. Dixon, PhD, MBA, with Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, said in a statement. Previous studies from China, for instance, reported a two- to threefold greater risk of all-cause mortality among unvaccinated adults with cancer and COVID-19.
A potential reason for this discrepancy, Dr. Dixon noted, is that earlier studies were “generally smaller and made calculations based on data from a single cancer center or health system.”
Another reason is testing for COVID-19 early in the pandemic was limited to symptomatic individuals who may have had more severe infections, possibly leading to an overestimate of the association between SARS-CoV-2 infection, cancer, and all-cause mortality.
In the current analysis, researchers used electronic health records linked to Indiana’s statewide SARS-CoV-2 testing database and state vital records to evaluate the association between SARS-CoV-2 infection and all-cause mortality among 41,924 adults newly diagnosed with cancer between Jan. 1, 2019, and Dec. 31, 2020.
Most people with cancer were White (78.4%) and about half were male. At the time of diagnosis, 17% had one comorbid condition and about 10% had two or more. Most patients had breast cancer (14%), prostate cancer (13%), or melanoma (13%).
During the study period, 2,894 patients (7%) tested positive for SARS-CoV-2.
In multivariate adjusted analysis, the risk of death among those newly diagnosed with cancer increased by 91% (adjusted hazard ratio, 1.91) during the first year of the pandemic before vaccines were available, compared with the year before (January 2019 to Jan. 14, 2020).
During the pandemic period, the risk of death was roughly threefold higher among adults 65 years old and older, compared with adults 18-44 years old (aHR, 3.35).
When looking at the time from a cancer diagnosis to SARS-CoV-2 infection, infection was associated with an almost sevenfold increase in all-cause mortality (aHR, 6.91). Adults 65 years old and older had an almost threefold increased risk of dying, compared with their younger peers (aHR, 2.74).
Dr. Dixon and colleagues also observed an increased risk of death in men with cancer and COVID, compared with women (aHR, 1.23) and those with at least two comorbid conditions versus none (aHR, 2.12). In addition, the risk of dying was 9% higher among Indiana’s rural population than urban dwellers.
Compared with other cancer types, individuals with lung cancer and other digestive cancers had the highest risk of death after SARS-CoV-2 infection (aHR, 1.45 and 1.80, respectively).
“Our findings highlight the increased risk of death for adult cancer patients who test positive for COVID and underscore the importance to cancer patients – including those in remission – of vaccinations, boosters, and regular COVID testing,” Dr. Dixon commented.
“Our results should encourage individuals diagnosed with cancer not only to take preventive action, but also to expeditiously seek out treatments available in the marketplace should they test positive for COVID,” he added.
Support for the study was provided by Indiana University Simon Cancer Center and the Centers for Disease Control and Prevention. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study underscores the importance of COVID-19 and regular COVID-19 testing among adults with a recent cancer diagnosis.
The Indiana statewide study, conducted at the beginning of the pandemic, found that
“This analysis provides additional empirical evidence on the magnitude of risk to patients with cancer whose immune systems are often weakened either by the disease or treatment,” the study team wrote.
The study was published online in JMIR Cancer.
Although evidence has consistently revealed similar findings, the risk of death among unvaccinated people with cancer and COVID-19 has not been nearly as high in previous studies, lead author Brian E. Dixon, PhD, MBA, with Indiana University Richard M. Fairbanks School of Public Health, Indianapolis, said in a statement. Previous studies from China, for instance, reported a two- to threefold greater risk of all-cause mortality among unvaccinated adults with cancer and COVID-19.
A potential reason for this discrepancy, Dr. Dixon noted, is that earlier studies were “generally smaller and made calculations based on data from a single cancer center or health system.”
Another reason is testing for COVID-19 early in the pandemic was limited to symptomatic individuals who may have had more severe infections, possibly leading to an overestimate of the association between SARS-CoV-2 infection, cancer, and all-cause mortality.
In the current analysis, researchers used electronic health records linked to Indiana’s statewide SARS-CoV-2 testing database and state vital records to evaluate the association between SARS-CoV-2 infection and all-cause mortality among 41,924 adults newly diagnosed with cancer between Jan. 1, 2019, and Dec. 31, 2020.
Most people with cancer were White (78.4%) and about half were male. At the time of diagnosis, 17% had one comorbid condition and about 10% had two or more. Most patients had breast cancer (14%), prostate cancer (13%), or melanoma (13%).
During the study period, 2,894 patients (7%) tested positive for SARS-CoV-2.
In multivariate adjusted analysis, the risk of death among those newly diagnosed with cancer increased by 91% (adjusted hazard ratio, 1.91) during the first year of the pandemic before vaccines were available, compared with the year before (January 2019 to Jan. 14, 2020).
During the pandemic period, the risk of death was roughly threefold higher among adults 65 years old and older, compared with adults 18-44 years old (aHR, 3.35).
When looking at the time from a cancer diagnosis to SARS-CoV-2 infection, infection was associated with an almost sevenfold increase in all-cause mortality (aHR, 6.91). Adults 65 years old and older had an almost threefold increased risk of dying, compared with their younger peers (aHR, 2.74).
Dr. Dixon and colleagues also observed an increased risk of death in men with cancer and COVID, compared with women (aHR, 1.23) and those with at least two comorbid conditions versus none (aHR, 2.12). In addition, the risk of dying was 9% higher among Indiana’s rural population than urban dwellers.
Compared with other cancer types, individuals with lung cancer and other digestive cancers had the highest risk of death after SARS-CoV-2 infection (aHR, 1.45 and 1.80, respectively).
“Our findings highlight the increased risk of death for adult cancer patients who test positive for COVID and underscore the importance to cancer patients – including those in remission – of vaccinations, boosters, and regular COVID testing,” Dr. Dixon commented.
“Our results should encourage individuals diagnosed with cancer not only to take preventive action, but also to expeditiously seek out treatments available in the marketplace should they test positive for COVID,” he added.
Support for the study was provided by Indiana University Simon Cancer Center and the Centers for Disease Control and Prevention. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JMIR CANCER
New COVID variant gaining traction in U.S.
, according to the CDC’s latest data.
Just 1 month ago, the variant accounted for less than 1% of cases.
“When you get variants like that, you look at what their rate of increase is as a relative proportion of the variants, and this has a pretty troublesome doubling time,” Anthony Fauci, MD, said in an interview with CBS News. Dr. Fauci is the director of the National Institute of Allergy and Infectious Diseases and also the chief medical adviser to President Joe Biden.
There are also concerning features of the BQ.1 variant, which include mutations that could potentially escape vaccines and treatments for COVID-19.
Currently, the most widespread variant in the U.S. is the Omicron subvariant known as BA.5, which accounts for 68% of all infections. One of the go-to treatments for BA.5 infections is monoclonal antibodies, which may not be as effective when fighting the up-and-coming strains of BQ.1 and its descendant BQ.1.1, according to experts.
“That’s the reason why people are concerned about BQ.1.1, for the double reason of its doubling time and the fact that it seems to elude important monoclonal antibodies,” Dr. Fauci told CBS News.
Currently, BQ.1 and BQ.1.1 appear most widespread in the New York and New Jersey region, accounting for nearly 20% of infections there, according to the CDC.
But because the new variant is a descendant of Omicron, Dr. Fauci said the currently available booster shots are still the best first line of protection against this up-and-coming threat.
“The bad news is that there’s a new variant that’s emerging and that has qualities or characteristics that could evade some of the interventions we have. But, the somewhat encouraging news is that it’s a BA.5 sub-lineage, so there is almost certainly going to be some cross-protection that you can boost up,” he said.
A version of this article first appeared on WebMD.com.
, according to the CDC’s latest data.
Just 1 month ago, the variant accounted for less than 1% of cases.
“When you get variants like that, you look at what their rate of increase is as a relative proportion of the variants, and this has a pretty troublesome doubling time,” Anthony Fauci, MD, said in an interview with CBS News. Dr. Fauci is the director of the National Institute of Allergy and Infectious Diseases and also the chief medical adviser to President Joe Biden.
There are also concerning features of the BQ.1 variant, which include mutations that could potentially escape vaccines and treatments for COVID-19.
Currently, the most widespread variant in the U.S. is the Omicron subvariant known as BA.5, which accounts for 68% of all infections. One of the go-to treatments for BA.5 infections is monoclonal antibodies, which may not be as effective when fighting the up-and-coming strains of BQ.1 and its descendant BQ.1.1, according to experts.
“That’s the reason why people are concerned about BQ.1.1, for the double reason of its doubling time and the fact that it seems to elude important monoclonal antibodies,” Dr. Fauci told CBS News.
Currently, BQ.1 and BQ.1.1 appear most widespread in the New York and New Jersey region, accounting for nearly 20% of infections there, according to the CDC.
But because the new variant is a descendant of Omicron, Dr. Fauci said the currently available booster shots are still the best first line of protection against this up-and-coming threat.
“The bad news is that there’s a new variant that’s emerging and that has qualities or characteristics that could evade some of the interventions we have. But, the somewhat encouraging news is that it’s a BA.5 sub-lineage, so there is almost certainly going to be some cross-protection that you can boost up,” he said.
A version of this article first appeared on WebMD.com.
, according to the CDC’s latest data.
Just 1 month ago, the variant accounted for less than 1% of cases.
“When you get variants like that, you look at what their rate of increase is as a relative proportion of the variants, and this has a pretty troublesome doubling time,” Anthony Fauci, MD, said in an interview with CBS News. Dr. Fauci is the director of the National Institute of Allergy and Infectious Diseases and also the chief medical adviser to President Joe Biden.
There are also concerning features of the BQ.1 variant, which include mutations that could potentially escape vaccines and treatments for COVID-19.
Currently, the most widespread variant in the U.S. is the Omicron subvariant known as BA.5, which accounts for 68% of all infections. One of the go-to treatments for BA.5 infections is monoclonal antibodies, which may not be as effective when fighting the up-and-coming strains of BQ.1 and its descendant BQ.1.1, according to experts.
“That’s the reason why people are concerned about BQ.1.1, for the double reason of its doubling time and the fact that it seems to elude important monoclonal antibodies,” Dr. Fauci told CBS News.
Currently, BQ.1 and BQ.1.1 appear most widespread in the New York and New Jersey region, accounting for nearly 20% of infections there, according to the CDC.
But because the new variant is a descendant of Omicron, Dr. Fauci said the currently available booster shots are still the best first line of protection against this up-and-coming threat.
“The bad news is that there’s a new variant that’s emerging and that has qualities or characteristics that could evade some of the interventions we have. But, the somewhat encouraging news is that it’s a BA.5 sub-lineage, so there is almost certainly going to be some cross-protection that you can boost up,” he said.
A version of this article first appeared on WebMD.com.
You and the skeptical patient: Who’s the doctor here?
“I spoke to him on many occasions about the dangers of COVID, but he just didn’t believe me,” said Dr. Hood, an internist in Lexington, Ky. “He just didn’t give me enough time to help him. He waited to let me know he was ill with COVID and took days to pick up the medicine. Unfortunately, he then passed away.”
The rise of the skeptical patient
It can be extremely frustrating for doctors when patients question or disbelieve their physician’s medical advice and explanations. And many physicians resent the amount of time they spend trying to explain or make their case, especially during a busy day. But patients’ skepticism about the validity of some treatments seems to be increasing.
“Patients are now more likely to have their own medical explanation for their complaint than they used to, and that can be bad for their health,” Dr. Hood said.
Dr. Hood sees medical cynicism as part of Americans’ growing distrust of experts, leveraged by easy access to the internet. “When people Google, they tend to look for support of their opinions, rather than arrive at a fully educated decision.”
Only about half of patients believe their physicians “provide fair and accurate treatment information all or most of the time,” according to a 2019 survey by the Pew Research Center.
Patients’ distrust has become more obvious during the COVID-19 pandemic, said John Schumann, MD, an internist with Oak Street Health, a practice with more than 500 physicians and other providers in 20 states, treating almost exclusively Medicare patients.
“The skeptics became more entrenched during the pandemic,” said Dr. Schumann, who is based in Tulsa, Okla. “They may think the COVID vaccines were approved too quickly, or believe the pandemic itself is a hoax.”
“There’s a lot of antiscience rhetoric now,” Dr. Schumann added. “I’d say about half of my patients are comfortable with science-based decisions and the other half are not.”
What are patients mistrustful about?
Patients’ suspicions of certain therapies began long before the pandemic. In dermatology, for example, some patients refuse to take topical steroids, said Steven R. Feldman, MD, a dermatologist in Winston-Salem, N.C.
“Their distrust is usually based on anecdotal stories they read about,” he noted. “Patients in other specialties are dead set against vaccinations.”
In addition to refusing treatments and inoculations, some patients ask for questionable regimens mentioned in the news. “Some patients have demanded hydroxychloroquine or Noromectin, drugs that are unproven in the treatment of COVID,” Dr. Schumann said. “We refuse to prescribe them.”
Dr. Hood said patients’ reluctance to follow medical advice can often be based on cost. “I have a patient who was more willing to save $20 than to save his life. But when the progression of his test results fit my predictions, he became more willing to take treatments. I had to wait for the opportune moment to convince him.”
Many naysayer patients keep their views to themselves, and physicians may be unaware that the patients are stonewalling. A 2006 study estimated that about 10%-16% of primary care patients actively resist medical authority.
Dr. Schumann cited patients who don’t want to hear an upsetting diagnosis. “Some patients might refuse to take a biopsy to see if they have cancer because they don’t want to know,” he said. “In many cases, they simply won’t get the biopsy and won’t tell the doctor that they didn’t.”
Sometimes skeptics’ arguments have merit
Some patients’ concerns can be valid, such as when they refuse to go on statins, said Zain Hakeem, DO, a physician in Austin, Tex.
“In some cases, I feel that statins are not necessary,” he said. “The science on statins for primary prevention is not strong, although they should be used for exceedingly high-risk patients.”
Certain patients, especially those with chronic conditions, do a great deal of research, using legitimate sources on the Web, and their research is well supported.
However, these patients can be overconfident in their conclusions. Several studies have shown that with just a little experience, people can replace beginners’ caution with a false sense of competence.
For example, “Patients may not weigh the risks correctly,” Dr. Hakeem said. “They can be more concerned about the risk of having their colon perforated during a colonoscopy, while the risk of cancer if they don’t have a colonoscopy is much higher.”
Some highly successful people may be more likely to trust their own medical instincts. When Steve Jobs, the founder of Apple, was diagnosed with pancreatic cancer in 2003, he put off surgery for 9 months while he tried to cure his disease with a vegan diet, acupuncture, herbs, bowel cleansings, and other remedies he read about. He died in 2011. Some experts believe that delay hastened his death.
Of course, not all physicians’ diagnoses or treatments are correct. One study indicated doctors’ diagnostic error rate could be as high as 15%. And just as patients can be overconfident in their conclusions, so can doctors. Another study found that physicians’ stated confidence in their diagnosis was only slightly affected by the inaccuracy of that diagnosis or the difficulty of the case.
Best ways to deal with cynical patients
Patients’ skepticism can frustrate doctors, reduce the efficiency of care delivery, and interfere with recovery. What can doctors do to deal with these problems?
1. Build the patient’s trust in you. “Getting patients to adhere to your advice involves making sure they feel they have a caring doctor whom they trust,” Dr. Feldman said.
“I want to show patients that I am entirely focused on them,” he added. “For example, I may rush to the door of the exam room from my last appointment, but I open the door very slowly and deliberately, because I want the patient to see that I won’t hurry with them.”
2. Spend time with the patient. Familiarity builds trust. Dr. Schumann said doctors at Oak Street Health see their patients an average of six to eight times a year, an unusually high number. “The more patients see their physicians, the more likely they are to trust them.”
3. Keep up to date. “I make sure I’m up to date with the literature, and I try to present a truthful message,” Dr. Hood said. “For instance, my research showed that inflammation played a strong role in developing complications from COVID, so I wrote a detailed treatment protocol aimed at the inflammation and the immune response, which has been very effective.”
4. Confront patients tactfully. Patients who do research on the Web don’t want to be scolded, Dr. Feldman said. In fact, he praises them, even if he doesn’t agree with their findings. “I might say: ‘What a relief to finally find patients who’ve taken the time to educate themselves before coming here.’ ”
Dr. Feldman is careful not to dispute patients’ conclusions. “Debating the issues is not an effective approach to get patients to trust you. The last thing you want to tell a patient is: ‘Listen to me! I’m an expert.’ People just dig in.”
However, it does help to give patients feedback. “I’m a big fan of patients arguing with me,” Dr. Hakeem said. “It means you can straighten out misunderstandings and improve decision-making.”
5. Explain your reasoning. “You need to communicate clearly and show them your thinking,” Dr. Hood said. “For instance, I’ll explain why a patient has a strong risk for heart attack.”
6. Acknowledge uncertainties. “The doctor may present the science as far more certain than it is,” Dr. Hakeem said. “If you don’t acknowledge the uncertainties, you could break the patient’s trust in you.”
7. Don’t use a lot of numbers. “Data is not a good tool to convince patients,” Dr. Feldman said. “The human brain isn’t designed to work that way.”
If you want to use numbers to show clinical risk, Dr. Hakeem advisd using natural frequencies, such as 10 out of 10,000, which is less confusing to the patient than the equivalent percentage of 0.1%.
It can be helpful to refer to familiar concepts. One way to understand a risk is to compare it with risks in daily life, such as the dangers of driving or falling in the shower, Dr. Hakeem added.
Dr. Feldman often refers to another person’s experience when presenting his medical advice. “I might say to the patient: ‘You remind me of another patient I had. They were sitting in the same chair you’re sitting in. They did really well on this drug, and I think it’s probably the best choice for you, too.’ ”
8. Adopt shared decision-making. This approach involves empowering the patient to become an equal partner in medical decisions. The patient is given information through portals and is encouraged to do research. Critics, however, say that most patients don’t want this degree of empowerment and would rather depend on the doctor’s advice.
Conclusion
It’s often impossible to get through to a skeptical patient, which can be disheartening for doctors. “Physicians want to do what is best for the patient, so when the patient doesn’t listen, they may take it personally,” Dr. Hood said. “But you always have to remember, the patient is the one with disease, and it’s up to the patient to open the door.”
Still, some skeptical patients ultimately change their minds. Dr. Schumann said patients who initially declined the COVID vaccine eventually decided to get it. “It often took them more than a year. but it’s never too late.”
A version of this article first appeared on Medscape.com.
“I spoke to him on many occasions about the dangers of COVID, but he just didn’t believe me,” said Dr. Hood, an internist in Lexington, Ky. “He just didn’t give me enough time to help him. He waited to let me know he was ill with COVID and took days to pick up the medicine. Unfortunately, he then passed away.”
The rise of the skeptical patient
It can be extremely frustrating for doctors when patients question or disbelieve their physician’s medical advice and explanations. And many physicians resent the amount of time they spend trying to explain or make their case, especially during a busy day. But patients’ skepticism about the validity of some treatments seems to be increasing.
“Patients are now more likely to have their own medical explanation for their complaint than they used to, and that can be bad for their health,” Dr. Hood said.
Dr. Hood sees medical cynicism as part of Americans’ growing distrust of experts, leveraged by easy access to the internet. “When people Google, they tend to look for support of their opinions, rather than arrive at a fully educated decision.”
Only about half of patients believe their physicians “provide fair and accurate treatment information all or most of the time,” according to a 2019 survey by the Pew Research Center.
Patients’ distrust has become more obvious during the COVID-19 pandemic, said John Schumann, MD, an internist with Oak Street Health, a practice with more than 500 physicians and other providers in 20 states, treating almost exclusively Medicare patients.
“The skeptics became more entrenched during the pandemic,” said Dr. Schumann, who is based in Tulsa, Okla. “They may think the COVID vaccines were approved too quickly, or believe the pandemic itself is a hoax.”
“There’s a lot of antiscience rhetoric now,” Dr. Schumann added. “I’d say about half of my patients are comfortable with science-based decisions and the other half are not.”
What are patients mistrustful about?
Patients’ suspicions of certain therapies began long before the pandemic. In dermatology, for example, some patients refuse to take topical steroids, said Steven R. Feldman, MD, a dermatologist in Winston-Salem, N.C.
“Their distrust is usually based on anecdotal stories they read about,” he noted. “Patients in other specialties are dead set against vaccinations.”
In addition to refusing treatments and inoculations, some patients ask for questionable regimens mentioned in the news. “Some patients have demanded hydroxychloroquine or Noromectin, drugs that are unproven in the treatment of COVID,” Dr. Schumann said. “We refuse to prescribe them.”
Dr. Hood said patients’ reluctance to follow medical advice can often be based on cost. “I have a patient who was more willing to save $20 than to save his life. But when the progression of his test results fit my predictions, he became more willing to take treatments. I had to wait for the opportune moment to convince him.”
Many naysayer patients keep their views to themselves, and physicians may be unaware that the patients are stonewalling. A 2006 study estimated that about 10%-16% of primary care patients actively resist medical authority.
Dr. Schumann cited patients who don’t want to hear an upsetting diagnosis. “Some patients might refuse to take a biopsy to see if they have cancer because they don’t want to know,” he said. “In many cases, they simply won’t get the biopsy and won’t tell the doctor that they didn’t.”
Sometimes skeptics’ arguments have merit
Some patients’ concerns can be valid, such as when they refuse to go on statins, said Zain Hakeem, DO, a physician in Austin, Tex.
“In some cases, I feel that statins are not necessary,” he said. “The science on statins for primary prevention is not strong, although they should be used for exceedingly high-risk patients.”
Certain patients, especially those with chronic conditions, do a great deal of research, using legitimate sources on the Web, and their research is well supported.
However, these patients can be overconfident in their conclusions. Several studies have shown that with just a little experience, people can replace beginners’ caution with a false sense of competence.
For example, “Patients may not weigh the risks correctly,” Dr. Hakeem said. “They can be more concerned about the risk of having their colon perforated during a colonoscopy, while the risk of cancer if they don’t have a colonoscopy is much higher.”
Some highly successful people may be more likely to trust their own medical instincts. When Steve Jobs, the founder of Apple, was diagnosed with pancreatic cancer in 2003, he put off surgery for 9 months while he tried to cure his disease with a vegan diet, acupuncture, herbs, bowel cleansings, and other remedies he read about. He died in 2011. Some experts believe that delay hastened his death.
Of course, not all physicians’ diagnoses or treatments are correct. One study indicated doctors’ diagnostic error rate could be as high as 15%. And just as patients can be overconfident in their conclusions, so can doctors. Another study found that physicians’ stated confidence in their diagnosis was only slightly affected by the inaccuracy of that diagnosis or the difficulty of the case.
Best ways to deal with cynical patients
Patients’ skepticism can frustrate doctors, reduce the efficiency of care delivery, and interfere with recovery. What can doctors do to deal with these problems?
1. Build the patient’s trust in you. “Getting patients to adhere to your advice involves making sure they feel they have a caring doctor whom they trust,” Dr. Feldman said.
“I want to show patients that I am entirely focused on them,” he added. “For example, I may rush to the door of the exam room from my last appointment, but I open the door very slowly and deliberately, because I want the patient to see that I won’t hurry with them.”
2. Spend time with the patient. Familiarity builds trust. Dr. Schumann said doctors at Oak Street Health see their patients an average of six to eight times a year, an unusually high number. “The more patients see their physicians, the more likely they are to trust them.”
3. Keep up to date. “I make sure I’m up to date with the literature, and I try to present a truthful message,” Dr. Hood said. “For instance, my research showed that inflammation played a strong role in developing complications from COVID, so I wrote a detailed treatment protocol aimed at the inflammation and the immune response, which has been very effective.”
4. Confront patients tactfully. Patients who do research on the Web don’t want to be scolded, Dr. Feldman said. In fact, he praises them, even if he doesn’t agree with their findings. “I might say: ‘What a relief to finally find patients who’ve taken the time to educate themselves before coming here.’ ”
Dr. Feldman is careful not to dispute patients’ conclusions. “Debating the issues is not an effective approach to get patients to trust you. The last thing you want to tell a patient is: ‘Listen to me! I’m an expert.’ People just dig in.”
However, it does help to give patients feedback. “I’m a big fan of patients arguing with me,” Dr. Hakeem said. “It means you can straighten out misunderstandings and improve decision-making.”
5. Explain your reasoning. “You need to communicate clearly and show them your thinking,” Dr. Hood said. “For instance, I’ll explain why a patient has a strong risk for heart attack.”
6. Acknowledge uncertainties. “The doctor may present the science as far more certain than it is,” Dr. Hakeem said. “If you don’t acknowledge the uncertainties, you could break the patient’s trust in you.”
7. Don’t use a lot of numbers. “Data is not a good tool to convince patients,” Dr. Feldman said. “The human brain isn’t designed to work that way.”
If you want to use numbers to show clinical risk, Dr. Hakeem advisd using natural frequencies, such as 10 out of 10,000, which is less confusing to the patient than the equivalent percentage of 0.1%.
It can be helpful to refer to familiar concepts. One way to understand a risk is to compare it with risks in daily life, such as the dangers of driving or falling in the shower, Dr. Hakeem added.
Dr. Feldman often refers to another person’s experience when presenting his medical advice. “I might say to the patient: ‘You remind me of another patient I had. They were sitting in the same chair you’re sitting in. They did really well on this drug, and I think it’s probably the best choice for you, too.’ ”
8. Adopt shared decision-making. This approach involves empowering the patient to become an equal partner in medical decisions. The patient is given information through portals and is encouraged to do research. Critics, however, say that most patients don’t want this degree of empowerment and would rather depend on the doctor’s advice.
Conclusion
It’s often impossible to get through to a skeptical patient, which can be disheartening for doctors. “Physicians want to do what is best for the patient, so when the patient doesn’t listen, they may take it personally,” Dr. Hood said. “But you always have to remember, the patient is the one with disease, and it’s up to the patient to open the door.”
Still, some skeptical patients ultimately change their minds. Dr. Schumann said patients who initially declined the COVID vaccine eventually decided to get it. “It often took them more than a year. but it’s never too late.”
A version of this article first appeared on Medscape.com.
“I spoke to him on many occasions about the dangers of COVID, but he just didn’t believe me,” said Dr. Hood, an internist in Lexington, Ky. “He just didn’t give me enough time to help him. He waited to let me know he was ill with COVID and took days to pick up the medicine. Unfortunately, he then passed away.”
The rise of the skeptical patient
It can be extremely frustrating for doctors when patients question or disbelieve their physician’s medical advice and explanations. And many physicians resent the amount of time they spend trying to explain or make their case, especially during a busy day. But patients’ skepticism about the validity of some treatments seems to be increasing.
“Patients are now more likely to have their own medical explanation for their complaint than they used to, and that can be bad for their health,” Dr. Hood said.
Dr. Hood sees medical cynicism as part of Americans’ growing distrust of experts, leveraged by easy access to the internet. “When people Google, they tend to look for support of their opinions, rather than arrive at a fully educated decision.”
Only about half of patients believe their physicians “provide fair and accurate treatment information all or most of the time,” according to a 2019 survey by the Pew Research Center.
Patients’ distrust has become more obvious during the COVID-19 pandemic, said John Schumann, MD, an internist with Oak Street Health, a practice with more than 500 physicians and other providers in 20 states, treating almost exclusively Medicare patients.
“The skeptics became more entrenched during the pandemic,” said Dr. Schumann, who is based in Tulsa, Okla. “They may think the COVID vaccines were approved too quickly, or believe the pandemic itself is a hoax.”
“There’s a lot of antiscience rhetoric now,” Dr. Schumann added. “I’d say about half of my patients are comfortable with science-based decisions and the other half are not.”
What are patients mistrustful about?
Patients’ suspicions of certain therapies began long before the pandemic. In dermatology, for example, some patients refuse to take topical steroids, said Steven R. Feldman, MD, a dermatologist in Winston-Salem, N.C.
“Their distrust is usually based on anecdotal stories they read about,” he noted. “Patients in other specialties are dead set against vaccinations.”
In addition to refusing treatments and inoculations, some patients ask for questionable regimens mentioned in the news. “Some patients have demanded hydroxychloroquine or Noromectin, drugs that are unproven in the treatment of COVID,” Dr. Schumann said. “We refuse to prescribe them.”
Dr. Hood said patients’ reluctance to follow medical advice can often be based on cost. “I have a patient who was more willing to save $20 than to save his life. But when the progression of his test results fit my predictions, he became more willing to take treatments. I had to wait for the opportune moment to convince him.”
Many naysayer patients keep their views to themselves, and physicians may be unaware that the patients are stonewalling. A 2006 study estimated that about 10%-16% of primary care patients actively resist medical authority.
Dr. Schumann cited patients who don’t want to hear an upsetting diagnosis. “Some patients might refuse to take a biopsy to see if they have cancer because they don’t want to know,” he said. “In many cases, they simply won’t get the biopsy and won’t tell the doctor that they didn’t.”
Sometimes skeptics’ arguments have merit
Some patients’ concerns can be valid, such as when they refuse to go on statins, said Zain Hakeem, DO, a physician in Austin, Tex.
“In some cases, I feel that statins are not necessary,” he said. “The science on statins for primary prevention is not strong, although they should be used for exceedingly high-risk patients.”
Certain patients, especially those with chronic conditions, do a great deal of research, using legitimate sources on the Web, and their research is well supported.
However, these patients can be overconfident in their conclusions. Several studies have shown that with just a little experience, people can replace beginners’ caution with a false sense of competence.
For example, “Patients may not weigh the risks correctly,” Dr. Hakeem said. “They can be more concerned about the risk of having their colon perforated during a colonoscopy, while the risk of cancer if they don’t have a colonoscopy is much higher.”
Some highly successful people may be more likely to trust their own medical instincts. When Steve Jobs, the founder of Apple, was diagnosed with pancreatic cancer in 2003, he put off surgery for 9 months while he tried to cure his disease with a vegan diet, acupuncture, herbs, bowel cleansings, and other remedies he read about. He died in 2011. Some experts believe that delay hastened his death.
Of course, not all physicians’ diagnoses or treatments are correct. One study indicated doctors’ diagnostic error rate could be as high as 15%. And just as patients can be overconfident in their conclusions, so can doctors. Another study found that physicians’ stated confidence in their diagnosis was only slightly affected by the inaccuracy of that diagnosis or the difficulty of the case.
Best ways to deal with cynical patients
Patients’ skepticism can frustrate doctors, reduce the efficiency of care delivery, and interfere with recovery. What can doctors do to deal with these problems?
1. Build the patient’s trust in you. “Getting patients to adhere to your advice involves making sure they feel they have a caring doctor whom they trust,” Dr. Feldman said.
“I want to show patients that I am entirely focused on them,” he added. “For example, I may rush to the door of the exam room from my last appointment, but I open the door very slowly and deliberately, because I want the patient to see that I won’t hurry with them.”
2. Spend time with the patient. Familiarity builds trust. Dr. Schumann said doctors at Oak Street Health see their patients an average of six to eight times a year, an unusually high number. “The more patients see their physicians, the more likely they are to trust them.”
3. Keep up to date. “I make sure I’m up to date with the literature, and I try to present a truthful message,” Dr. Hood said. “For instance, my research showed that inflammation played a strong role in developing complications from COVID, so I wrote a detailed treatment protocol aimed at the inflammation and the immune response, which has been very effective.”
4. Confront patients tactfully. Patients who do research on the Web don’t want to be scolded, Dr. Feldman said. In fact, he praises them, even if he doesn’t agree with their findings. “I might say: ‘What a relief to finally find patients who’ve taken the time to educate themselves before coming here.’ ”
Dr. Feldman is careful not to dispute patients’ conclusions. “Debating the issues is not an effective approach to get patients to trust you. The last thing you want to tell a patient is: ‘Listen to me! I’m an expert.’ People just dig in.”
However, it does help to give patients feedback. “I’m a big fan of patients arguing with me,” Dr. Hakeem said. “It means you can straighten out misunderstandings and improve decision-making.”
5. Explain your reasoning. “You need to communicate clearly and show them your thinking,” Dr. Hood said. “For instance, I’ll explain why a patient has a strong risk for heart attack.”
6. Acknowledge uncertainties. “The doctor may present the science as far more certain than it is,” Dr. Hakeem said. “If you don’t acknowledge the uncertainties, you could break the patient’s trust in you.”
7. Don’t use a lot of numbers. “Data is not a good tool to convince patients,” Dr. Feldman said. “The human brain isn’t designed to work that way.”
If you want to use numbers to show clinical risk, Dr. Hakeem advisd using natural frequencies, such as 10 out of 10,000, which is less confusing to the patient than the equivalent percentage of 0.1%.
It can be helpful to refer to familiar concepts. One way to understand a risk is to compare it with risks in daily life, such as the dangers of driving or falling in the shower, Dr. Hakeem added.
Dr. Feldman often refers to another person’s experience when presenting his medical advice. “I might say to the patient: ‘You remind me of another patient I had. They were sitting in the same chair you’re sitting in. They did really well on this drug, and I think it’s probably the best choice for you, too.’ ”
8. Adopt shared decision-making. This approach involves empowering the patient to become an equal partner in medical decisions. The patient is given information through portals and is encouraged to do research. Critics, however, say that most patients don’t want this degree of empowerment and would rather depend on the doctor’s advice.
Conclusion
It’s often impossible to get through to a skeptical patient, which can be disheartening for doctors. “Physicians want to do what is best for the patient, so when the patient doesn’t listen, they may take it personally,” Dr. Hood said. “But you always have to remember, the patient is the one with disease, and it’s up to the patient to open the door.”
Still, some skeptical patients ultimately change their minds. Dr. Schumann said patients who initially declined the COVID vaccine eventually decided to get it. “It often took them more than a year. but it’s never too late.”
A version of this article first appeared on Medscape.com.