User login
Why misinformation spreads
Over the past 16 months, the COVID-19 pandemic has highlighted not only our vulnerability to disease outbreaks but also our susceptibility to misinformation and the dangers of “fake news.”
In fact, COVID-19 is not a pandemic but rather a syndemic of viral disease and misinformation. In the current digital age, there is an abundance of information at our fingertips. This has resulted in a surplus of accurate as well as inaccurate information – information that is subject to the various biases we humans are subject to.
Our decision making and cognition are colored by our internal and external environmental biases, whether through our emotions, societal influences, or cues from the “machines” that are now such an omnipresent part of our lives.
Let’s break them down:
- Emotional bias: We’re only human, and our emotions often overwhelm objective judgment. Even when the evidence is of low quality, emotional attachments can deter us from rational thinking. This kind of bias can be rooted in personal experiences.
- Societal bias: Thoughts, opinions, or perspectives of peers are powerful forces that may influence our decisions and viewpoints. We can conceptualize our social networks as partisan circles and “echo chambers.” This bias is perhaps most evident in various online social media platforms.
- Machine bias: Our online platforms are laced with algorithms that tailor the content we see. Accordingly, the curated content we see (and, by extension, the less diverse content we view) may reinforce existing biases, such as confirmation bias.
- Although bias plays a significant role in decision making, we should also consider intuition versus deliberation – and whether the “gut” is a reliable source of information.
Intuition versus deliberation: The power of reasoning
The dual process theory suggests that thought may be categorized in two ways: System 1, referred to as rapid, intuitive, or automatic thinking (which may be a result of personal experience); and system 2, referred to as deliberate or controlled thinking (for example, reasoned thinking). System 1 versus system 2 may be conceptualized as fast versus slow thinking.
Let’s use the Cognitive Reflection Test to illustrate the dual process theory. This test measures the ability to reflect and deliberate on a question and to forgo an intuitive, rapid response. One of the questions asks: “A bat and a ball cost $1.10 in total. The bat costs $1.00 more than the ball. How much does the ball cost?” A common answer is that the ball costs $0.10. However, the ball actually costs $0.05. The common response is a “gut” response, rather than an analytic or deliberate response.
This example can be extrapolated to social media behavior, such as when individuals endorse beliefs and behaviors that may be far from the truth (for example, conspiracy ideation). It is not uncommon for individuals to rely on intuition, which may be incorrect, as a driving source of truth. Although one’s intuition can be correct, it’s important to be careful and to deliberate.
But would deliberate engagement lead to more politically valenced perspectives? One hypothesis posits that system 2 can lead to false claims and worsening discernment of truth. Another, and more popular, account of classical reasoning says that more thoughtful engagement (regardless of one’s political beliefs) is less susceptible to false news (for example, hyperpartisan news).
Additionally, having good literacy (political, scientific, or general) is important for discerning the truth, especially regarding events in which the information and/or claims of knowledge have been heavily manipulated.
Are believing and sharing the same?
Interestingly, believing in a headline and sharing it are not the same. A study that investigated the difference between the two found that although individuals were able to discern the validity of headlines, the veracity of those headlines was not a determining factor in sharing the story on social media.
It has been suggested that social media context may distract individuals from engaging in deliberate thinking that would enhance their ability to determine the accuracy of the content. The dissociation between truthfulness and sharing may be a result of the “attention economy,” which refers to user engagement of likes, comments, shares, and so forth. As such, social media behavior and content consumption may not necessarily reflect one’s beliefs and may be influenced by what others value.
To combat the spread of misinformation, it has been suggested that proactive interventions – “prebunking” or “inoculation” – are necessary. This idea is in accordance with the inoculation theory, which suggests that pre-exposure can confer resistance to challenge. This line of thinking is aligned with the use of vaccines to counter medical illnesses. Increasing awareness of individual vulnerability to manipulation and misinformation has also been proposed as a strategy to resist persuasion.
The age old tale of what others think of us versus what we believe to be true has existed long before the viral overtake of social media. The main difference today is that social media acts as a catalyst for pockets of misinformation. Although social media outlets are cracking down on “false news,” we must consider what criteria should be employed to identify false information. Should external bodies regulate our content consumption? We are certainly entering a gray zone of “wrong” versus “right.” With the overabundance of information available online, it may be the case of “them” versus “us” – that is, those who do not believe in the existence of misinformation versus those who do.
Leanna M. W. Lui, HBSc, completed an HBSc global health specialist degree at the University of Toronto, where she is now an MSc candidate.
A version of this article first appeared on Medscape.com.
Over the past 16 months, the COVID-19 pandemic has highlighted not only our vulnerability to disease outbreaks but also our susceptibility to misinformation and the dangers of “fake news.”
In fact, COVID-19 is not a pandemic but rather a syndemic of viral disease and misinformation. In the current digital age, there is an abundance of information at our fingertips. This has resulted in a surplus of accurate as well as inaccurate information – information that is subject to the various biases we humans are subject to.
Our decision making and cognition are colored by our internal and external environmental biases, whether through our emotions, societal influences, or cues from the “machines” that are now such an omnipresent part of our lives.
Let’s break them down:
- Emotional bias: We’re only human, and our emotions often overwhelm objective judgment. Even when the evidence is of low quality, emotional attachments can deter us from rational thinking. This kind of bias can be rooted in personal experiences.
- Societal bias: Thoughts, opinions, or perspectives of peers are powerful forces that may influence our decisions and viewpoints. We can conceptualize our social networks as partisan circles and “echo chambers.” This bias is perhaps most evident in various online social media platforms.
- Machine bias: Our online platforms are laced with algorithms that tailor the content we see. Accordingly, the curated content we see (and, by extension, the less diverse content we view) may reinforce existing biases, such as confirmation bias.
- Although bias plays a significant role in decision making, we should also consider intuition versus deliberation – and whether the “gut” is a reliable source of information.
Intuition versus deliberation: The power of reasoning
The dual process theory suggests that thought may be categorized in two ways: System 1, referred to as rapid, intuitive, or automatic thinking (which may be a result of personal experience); and system 2, referred to as deliberate or controlled thinking (for example, reasoned thinking). System 1 versus system 2 may be conceptualized as fast versus slow thinking.
Let’s use the Cognitive Reflection Test to illustrate the dual process theory. This test measures the ability to reflect and deliberate on a question and to forgo an intuitive, rapid response. One of the questions asks: “A bat and a ball cost $1.10 in total. The bat costs $1.00 more than the ball. How much does the ball cost?” A common answer is that the ball costs $0.10. However, the ball actually costs $0.05. The common response is a “gut” response, rather than an analytic or deliberate response.
This example can be extrapolated to social media behavior, such as when individuals endorse beliefs and behaviors that may be far from the truth (for example, conspiracy ideation). It is not uncommon for individuals to rely on intuition, which may be incorrect, as a driving source of truth. Although one’s intuition can be correct, it’s important to be careful and to deliberate.
But would deliberate engagement lead to more politically valenced perspectives? One hypothesis posits that system 2 can lead to false claims and worsening discernment of truth. Another, and more popular, account of classical reasoning says that more thoughtful engagement (regardless of one’s political beliefs) is less susceptible to false news (for example, hyperpartisan news).
Additionally, having good literacy (political, scientific, or general) is important for discerning the truth, especially regarding events in which the information and/or claims of knowledge have been heavily manipulated.
Are believing and sharing the same?
Interestingly, believing in a headline and sharing it are not the same. A study that investigated the difference between the two found that although individuals were able to discern the validity of headlines, the veracity of those headlines was not a determining factor in sharing the story on social media.
It has been suggested that social media context may distract individuals from engaging in deliberate thinking that would enhance their ability to determine the accuracy of the content. The dissociation between truthfulness and sharing may be a result of the “attention economy,” which refers to user engagement of likes, comments, shares, and so forth. As such, social media behavior and content consumption may not necessarily reflect one’s beliefs and may be influenced by what others value.
To combat the spread of misinformation, it has been suggested that proactive interventions – “prebunking” or “inoculation” – are necessary. This idea is in accordance with the inoculation theory, which suggests that pre-exposure can confer resistance to challenge. This line of thinking is aligned with the use of vaccines to counter medical illnesses. Increasing awareness of individual vulnerability to manipulation and misinformation has also been proposed as a strategy to resist persuasion.
The age old tale of what others think of us versus what we believe to be true has existed long before the viral overtake of social media. The main difference today is that social media acts as a catalyst for pockets of misinformation. Although social media outlets are cracking down on “false news,” we must consider what criteria should be employed to identify false information. Should external bodies regulate our content consumption? We are certainly entering a gray zone of “wrong” versus “right.” With the overabundance of information available online, it may be the case of “them” versus “us” – that is, those who do not believe in the existence of misinformation versus those who do.
Leanna M. W. Lui, HBSc, completed an HBSc global health specialist degree at the University of Toronto, where she is now an MSc candidate.
A version of this article first appeared on Medscape.com.
Over the past 16 months, the COVID-19 pandemic has highlighted not only our vulnerability to disease outbreaks but also our susceptibility to misinformation and the dangers of “fake news.”
In fact, COVID-19 is not a pandemic but rather a syndemic of viral disease and misinformation. In the current digital age, there is an abundance of information at our fingertips. This has resulted in a surplus of accurate as well as inaccurate information – information that is subject to the various biases we humans are subject to.
Our decision making and cognition are colored by our internal and external environmental biases, whether through our emotions, societal influences, or cues from the “machines” that are now such an omnipresent part of our lives.
Let’s break them down:
- Emotional bias: We’re only human, and our emotions often overwhelm objective judgment. Even when the evidence is of low quality, emotional attachments can deter us from rational thinking. This kind of bias can be rooted in personal experiences.
- Societal bias: Thoughts, opinions, or perspectives of peers are powerful forces that may influence our decisions and viewpoints. We can conceptualize our social networks as partisan circles and “echo chambers.” This bias is perhaps most evident in various online social media platforms.
- Machine bias: Our online platforms are laced with algorithms that tailor the content we see. Accordingly, the curated content we see (and, by extension, the less diverse content we view) may reinforce existing biases, such as confirmation bias.
- Although bias plays a significant role in decision making, we should also consider intuition versus deliberation – and whether the “gut” is a reliable source of information.
Intuition versus deliberation: The power of reasoning
The dual process theory suggests that thought may be categorized in two ways: System 1, referred to as rapid, intuitive, or automatic thinking (which may be a result of personal experience); and system 2, referred to as deliberate or controlled thinking (for example, reasoned thinking). System 1 versus system 2 may be conceptualized as fast versus slow thinking.
Let’s use the Cognitive Reflection Test to illustrate the dual process theory. This test measures the ability to reflect and deliberate on a question and to forgo an intuitive, rapid response. One of the questions asks: “A bat and a ball cost $1.10 in total. The bat costs $1.00 more than the ball. How much does the ball cost?” A common answer is that the ball costs $0.10. However, the ball actually costs $0.05. The common response is a “gut” response, rather than an analytic or deliberate response.
This example can be extrapolated to social media behavior, such as when individuals endorse beliefs and behaviors that may be far from the truth (for example, conspiracy ideation). It is not uncommon for individuals to rely on intuition, which may be incorrect, as a driving source of truth. Although one’s intuition can be correct, it’s important to be careful and to deliberate.
But would deliberate engagement lead to more politically valenced perspectives? One hypothesis posits that system 2 can lead to false claims and worsening discernment of truth. Another, and more popular, account of classical reasoning says that more thoughtful engagement (regardless of one’s political beliefs) is less susceptible to false news (for example, hyperpartisan news).
Additionally, having good literacy (political, scientific, or general) is important for discerning the truth, especially regarding events in which the information and/or claims of knowledge have been heavily manipulated.
Are believing and sharing the same?
Interestingly, believing in a headline and sharing it are not the same. A study that investigated the difference between the two found that although individuals were able to discern the validity of headlines, the veracity of those headlines was not a determining factor in sharing the story on social media.
It has been suggested that social media context may distract individuals from engaging in deliberate thinking that would enhance their ability to determine the accuracy of the content. The dissociation between truthfulness and sharing may be a result of the “attention economy,” which refers to user engagement of likes, comments, shares, and so forth. As such, social media behavior and content consumption may not necessarily reflect one’s beliefs and may be influenced by what others value.
To combat the spread of misinformation, it has been suggested that proactive interventions – “prebunking” or “inoculation” – are necessary. This idea is in accordance with the inoculation theory, which suggests that pre-exposure can confer resistance to challenge. This line of thinking is aligned with the use of vaccines to counter medical illnesses. Increasing awareness of individual vulnerability to manipulation and misinformation has also been proposed as a strategy to resist persuasion.
The age old tale of what others think of us versus what we believe to be true has existed long before the viral overtake of social media. The main difference today is that social media acts as a catalyst for pockets of misinformation. Although social media outlets are cracking down on “false news,” we must consider what criteria should be employed to identify false information. Should external bodies regulate our content consumption? We are certainly entering a gray zone of “wrong” versus “right.” With the overabundance of information available online, it may be the case of “them” versus “us” – that is, those who do not believe in the existence of misinformation versus those who do.
Leanna M. W. Lui, HBSc, completed an HBSc global health specialist degree at the University of Toronto, where she is now an MSc candidate.
A version of this article first appeared on Medscape.com.
A boy went to a COVID-swamped ER. He waited for hours. Then his appendix burst.
Seth was finally diagnosed with appendicitis more than six hours after arriving at Cleveland Clinic Martin Health North Hospital in late July. Around midnight, he was taken by ambulance to a sister hospital about a half-hour away that was better equipped to perform pediatric emergency surgery, his father said.
But by the time the doctor operated in the early morning hours, Seth’s appendix had burst – a potentially fatal complication.
They, too, need emergency care, but the sheer number of COVID-19 cases is crowding them out. Treatment has often been delayed as ERs scramble to find a bed that may be hundreds of miles away.
Some health officials now worry about looming ethical decisions. Last week, Idaho activated a “crisis standard of care,” which one official described as a “last resort.” It allows overwhelmed hospitals to ration care, including “in rare cases, ventilator (breathing machines) or intensive care unit (ICU) beds may need to be used for those who are most likely to survive, while patients who are not likely to survive may not be able to receive one,” the state’s website said.
The federal government’s latest data shows Alabama is at 100% of its intensive care unit capacity, with Texas, Georgia, Mississippi and Arkansas at more than 90% ICU capacity. Florida is just under 90%.
It’s the COVID-19 cases that are dominating. In Georgia, 62% of the ICU beds are now filled with just COVID-19 patients. In Texas, the percentage is nearly half.
To have so many ICU beds pressed into service for a single diagnosis is “unheard of,” said Dr. Hasan Kakli, an emergency room physician at Bellville Medical Center in Bellville, Texas, about an hour from Houston. “It’s approaching apocalyptic.”
In Texas, state data released Monday showed there were only 319 adult and 104 pediatric staffed ICU beds available across a state of 29 million people.
Hospitals need to hold some ICU beds for other patients, such as those recovering from major surgery or other critical conditions such as stroke, trauma or heart failure.
“This is not just a COVID issue,” said Dr. Normaliz Rodriguez, pediatric emergency physician at Johns Hopkins All Children’s Hospital in St. Petersburg, Florida. “This is an everyone issue.”
While the latest hospital crisis echoes previous pandemic spikes, there are troubling differences this time around.
Before, localized COVID-19 hot spots led to bed shortages, but there were usually hospitals in the region not as affected that could accept a transfer.
Now, as the highly contagious delta variant envelops swaths of low-vaccination states all at once, it becomes harder to find nearby hospitals that are not slammed.
“Wait times can now be measured in days,” said Darrell Pile, CEO of the SouthEast Texas Regional Advisory Council, which helps coordinate patient transfers across a 25-county region.
Recently, Dr. Cedric Dark, a Houston emergency physician and assistant professor of emergency medicine at Baylor College of Medicine, said he saw a critically ill COVID-19 patient waiting in the emergency room for an ICU bed to open. The doctor worked eight hours, went home and came in the next day. The patient was still waiting.
Holding a seriously ill patient in an emergency room while waiting for an in-patient bed to open is known as boarding. The longer the wait, the more dangerous it can be for the patient, studies have found.
Not only do patients ultimately end up staying in the hospital or the ICU longer, some research suggests that long waits for a bed will worsen their condition and may increase the risk of in-hospital death.
That’s what happened last month in Texas.
On Aug. 21, around 11:30 a.m., Michelle Puget took her adult son, Daniel Wilkinson, to the Bellville Medical Center’s emergency room as a pain in his abdomen became unbearable. “Mama,” he said, “take me to the hospital.”
Wilkinson, a 46-year-old decorated Army veteran who did two tours of duty in Afghanistan, was ushered into an exam room about half an hour later. Kakli, the emergency room physician there, diagnosed gallstone pancreatitis, a serious but treatable condition that required a specialist to perform a surgical procedure and an ICU bed.
In other times, the transfer to a larger facility would be easy. But soon Kakli found himself on a frantic, six-hour quest to find a bed for his patient. Not only did he call hospitals across Texas, but he also tried Kansas, Missouri, Oklahoma and Colorado. It was like throwing darts at a map and hoping to get lucky, he told ProPublica. But no one could or would take the transfer.
By 2:30 p.m., Wilkinson’s condition was deteriorating. Kakli told Puget to come back to the hospital. “I have to tell you,” she said he told her, “Your son is a very, very sick man. If he doesn’t get this procedure he will die.” She began to weep.
Two hours later, Wilkinson’s blood pressure was dropping, signaling his organs were failing, she said.
Kakli went on Facebook and posted an all-caps plea to physician groups around the nation: “GETTING REJECTED BY ALL HOSPITALS IN TEXAS DUE TO NO ICU BEDS. PLEASE HELP. MESSAGE ME IF YOU HAVE A BED. PATIENT IS IN ER NOW. I AM THE ER DOC. WILL FLY ANYWHERE.”
The doctor tried Michael E. DeBakey VA Medical Center in Houston for a second time. This time he found a bed.
Around 7 p.m., Wilkinson, still conscious but in grave condition, was flown by helicopter to the hospital. He was put in a medically induced coma. Through the night and into the next morning, medical teams worked to stabilize him enough to perform the procedure. They could not.
Doctors told his family the internal damage was catastrophic. “We made the decision we had to let him go,” Puget said.
Time of death: 1:37 p.m. Aug. 22 – 26 hours after he first arrived in the emergency room.
The story was first reported by CBS News. Kakli told ProPublica last week he still sometimes does the math in his head: It should have been 40 minutes from diagnosis in Bellville to transfer to the ICU in Houston. “If he had 40 minutes to wait instead of six hours, I strongly believe he would have had a different outcome.”
Another difference with the latest surge is how it’s affecting children.
Last year, schools were closed, and children were more protected because they were mostly isolated at home. In fact, children’s hospitals were often so empty during previous spikes they opened beds to adult patients.
Now, families are out more. Schools have reopened, some with mask mandates, some without. Vaccines are not yet available to those under 12. Suddenly the numbers of hospitalized children are on the rise, setting up the same type of competition for resources between young COVID-19 patients and those with other illnesses such as new onset diabetes, trauma, pneumonia or appendicitis.
Dr. Rafael Santiago, a pediatric emergency physician in Central Florida, said at Lakeland Regional Health Medical Center, the average number of children coming into the emergency room is around 130 per day. During the lockdown last spring, that number dropped to 33. Last month – “the busiest month ever” – the average daily number of children in the emergency room was 160.
Pediatric transfers are not yet as fraught as adult ones, Santiago said, but it does take more calls than it once did to secure a bed.
Seth Osborn, the 12-year-old whose appendix burst after a long wait, spent five days and four nights in the hospital as doctors pumped his body full of antibiotics to stave off infection from the rupture. The typical hospitalization for a routine appendectomy is about 24 hours.
The initial hospital bill for the stay came to more than $48,000, Nathaniel Osborn said. Although insurance paid for most of it, he said the family still borrowed against its house to cover the more than $5,000 in out-of-pocket costs so far.
While the hospital system where Seth was treated declined to comment about his case because of patient privacy laws, it did email a statement about the strain the pandemic is creating.
“Since July 2021, we have seen a tremendous spike in COVID-19 patients needing care and hospitalization. In mid-August, we saw the highest number of patients hospitalized with COVID-19 across the Cleveland Clinic Florida region, a total of 395 COVID-19 patients in four hospitals. Those hospitals have approximately 1,000 total beds,” the email to ProPublica said. “We strongly encourage vaccination. Approximately 90% of our patients hospitalized due to COVID-19 are unvaccinated.”
On Sunday, The Washington Post reported that a hospital in Alabama called 43 others across three states before finding a bed for Ray DeMonia, a critically ill heart patient who later died. In his obituary his family wrote: “In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies. ... He would not want any other family to go through what his did.”
Today, Seth is mostly recovered. “Twelve-year-old boys bounce back,” his father said. Still, the experience has left Nathaniel Osborn shaken.
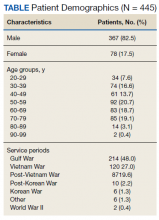
The high school history teacher said he likes to stay upbeat and apolitical in his social media musings, posting about Florida wildlife preservation and favorite books. But on Sept. 7, he tweeted: “My 12-year-old had appendicitis. The ER was overwhelmed with unvaccinated Covid patients and we had to wait 6+ hours. While waiting, his appendix ruptured and had to spend 5 days in hospital. ... So yeah, your decision to not vaccinate does affect others.”
It was retweeted 34,700 times, with 143,000 likes. Most comments were sympathetic and wished his child a speedy recovery. Some, though, went straight to hate, apparently triggered by his last line. He was attacked personally and accused of making up the story: “Good try with the guilt, jerk.”
Osborn, who is vaccinated, as are his wife and son, told ProPublica he only shared Seth’s story on Twitter to encourage vaccinations.
“I have no ill will towards the hospitals or the care received at either hospital,” he said this week, “but had these hospitals not been so crowded with COVID patients, we wouldn’t have had to wait so long and perhaps my son’s appendix would not have burst.”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Seth was finally diagnosed with appendicitis more than six hours after arriving at Cleveland Clinic Martin Health North Hospital in late July. Around midnight, he was taken by ambulance to a sister hospital about a half-hour away that was better equipped to perform pediatric emergency surgery, his father said.
But by the time the doctor operated in the early morning hours, Seth’s appendix had burst – a potentially fatal complication.
They, too, need emergency care, but the sheer number of COVID-19 cases is crowding them out. Treatment has often been delayed as ERs scramble to find a bed that may be hundreds of miles away.
Some health officials now worry about looming ethical decisions. Last week, Idaho activated a “crisis standard of care,” which one official described as a “last resort.” It allows overwhelmed hospitals to ration care, including “in rare cases, ventilator (breathing machines) or intensive care unit (ICU) beds may need to be used for those who are most likely to survive, while patients who are not likely to survive may not be able to receive one,” the state’s website said.
The federal government’s latest data shows Alabama is at 100% of its intensive care unit capacity, with Texas, Georgia, Mississippi and Arkansas at more than 90% ICU capacity. Florida is just under 90%.
It’s the COVID-19 cases that are dominating. In Georgia, 62% of the ICU beds are now filled with just COVID-19 patients. In Texas, the percentage is nearly half.
To have so many ICU beds pressed into service for a single diagnosis is “unheard of,” said Dr. Hasan Kakli, an emergency room physician at Bellville Medical Center in Bellville, Texas, about an hour from Houston. “It’s approaching apocalyptic.”
In Texas, state data released Monday showed there were only 319 adult and 104 pediatric staffed ICU beds available across a state of 29 million people.
Hospitals need to hold some ICU beds for other patients, such as those recovering from major surgery or other critical conditions such as stroke, trauma or heart failure.
“This is not just a COVID issue,” said Dr. Normaliz Rodriguez, pediatric emergency physician at Johns Hopkins All Children’s Hospital in St. Petersburg, Florida. “This is an everyone issue.”
While the latest hospital crisis echoes previous pandemic spikes, there are troubling differences this time around.
Before, localized COVID-19 hot spots led to bed shortages, but there were usually hospitals in the region not as affected that could accept a transfer.
Now, as the highly contagious delta variant envelops swaths of low-vaccination states all at once, it becomes harder to find nearby hospitals that are not slammed.
“Wait times can now be measured in days,” said Darrell Pile, CEO of the SouthEast Texas Regional Advisory Council, which helps coordinate patient transfers across a 25-county region.
Recently, Dr. Cedric Dark, a Houston emergency physician and assistant professor of emergency medicine at Baylor College of Medicine, said he saw a critically ill COVID-19 patient waiting in the emergency room for an ICU bed to open. The doctor worked eight hours, went home and came in the next day. The patient was still waiting.
Holding a seriously ill patient in an emergency room while waiting for an in-patient bed to open is known as boarding. The longer the wait, the more dangerous it can be for the patient, studies have found.
Not only do patients ultimately end up staying in the hospital or the ICU longer, some research suggests that long waits for a bed will worsen their condition and may increase the risk of in-hospital death.
That’s what happened last month in Texas.
On Aug. 21, around 11:30 a.m., Michelle Puget took her adult son, Daniel Wilkinson, to the Bellville Medical Center’s emergency room as a pain in his abdomen became unbearable. “Mama,” he said, “take me to the hospital.”
Wilkinson, a 46-year-old decorated Army veteran who did two tours of duty in Afghanistan, was ushered into an exam room about half an hour later. Kakli, the emergency room physician there, diagnosed gallstone pancreatitis, a serious but treatable condition that required a specialist to perform a surgical procedure and an ICU bed.
In other times, the transfer to a larger facility would be easy. But soon Kakli found himself on a frantic, six-hour quest to find a bed for his patient. Not only did he call hospitals across Texas, but he also tried Kansas, Missouri, Oklahoma and Colorado. It was like throwing darts at a map and hoping to get lucky, he told ProPublica. But no one could or would take the transfer.
By 2:30 p.m., Wilkinson’s condition was deteriorating. Kakli told Puget to come back to the hospital. “I have to tell you,” she said he told her, “Your son is a very, very sick man. If he doesn’t get this procedure he will die.” She began to weep.
Two hours later, Wilkinson’s blood pressure was dropping, signaling his organs were failing, she said.
Kakli went on Facebook and posted an all-caps plea to physician groups around the nation: “GETTING REJECTED BY ALL HOSPITALS IN TEXAS DUE TO NO ICU BEDS. PLEASE HELP. MESSAGE ME IF YOU HAVE A BED. PATIENT IS IN ER NOW. I AM THE ER DOC. WILL FLY ANYWHERE.”
The doctor tried Michael E. DeBakey VA Medical Center in Houston for a second time. This time he found a bed.
Around 7 p.m., Wilkinson, still conscious but in grave condition, was flown by helicopter to the hospital. He was put in a medically induced coma. Through the night and into the next morning, medical teams worked to stabilize him enough to perform the procedure. They could not.
Doctors told his family the internal damage was catastrophic. “We made the decision we had to let him go,” Puget said.
Time of death: 1:37 p.m. Aug. 22 – 26 hours after he first arrived in the emergency room.
The story was first reported by CBS News. Kakli told ProPublica last week he still sometimes does the math in his head: It should have been 40 minutes from diagnosis in Bellville to transfer to the ICU in Houston. “If he had 40 minutes to wait instead of six hours, I strongly believe he would have had a different outcome.”
Another difference with the latest surge is how it’s affecting children.
Last year, schools were closed, and children were more protected because they were mostly isolated at home. In fact, children’s hospitals were often so empty during previous spikes they opened beds to adult patients.
Now, families are out more. Schools have reopened, some with mask mandates, some without. Vaccines are not yet available to those under 12. Suddenly the numbers of hospitalized children are on the rise, setting up the same type of competition for resources between young COVID-19 patients and those with other illnesses such as new onset diabetes, trauma, pneumonia or appendicitis.
Dr. Rafael Santiago, a pediatric emergency physician in Central Florida, said at Lakeland Regional Health Medical Center, the average number of children coming into the emergency room is around 130 per day. During the lockdown last spring, that number dropped to 33. Last month – “the busiest month ever” – the average daily number of children in the emergency room was 160.
Pediatric transfers are not yet as fraught as adult ones, Santiago said, but it does take more calls than it once did to secure a bed.
Seth Osborn, the 12-year-old whose appendix burst after a long wait, spent five days and four nights in the hospital as doctors pumped his body full of antibiotics to stave off infection from the rupture. The typical hospitalization for a routine appendectomy is about 24 hours.
The initial hospital bill for the stay came to more than $48,000, Nathaniel Osborn said. Although insurance paid for most of it, he said the family still borrowed against its house to cover the more than $5,000 in out-of-pocket costs so far.
While the hospital system where Seth was treated declined to comment about his case because of patient privacy laws, it did email a statement about the strain the pandemic is creating.
“Since July 2021, we have seen a tremendous spike in COVID-19 patients needing care and hospitalization. In mid-August, we saw the highest number of patients hospitalized with COVID-19 across the Cleveland Clinic Florida region, a total of 395 COVID-19 patients in four hospitals. Those hospitals have approximately 1,000 total beds,” the email to ProPublica said. “We strongly encourage vaccination. Approximately 90% of our patients hospitalized due to COVID-19 are unvaccinated.”
On Sunday, The Washington Post reported that a hospital in Alabama called 43 others across three states before finding a bed for Ray DeMonia, a critically ill heart patient who later died. In his obituary his family wrote: “In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies. ... He would not want any other family to go through what his did.”
Today, Seth is mostly recovered. “Twelve-year-old boys bounce back,” his father said. Still, the experience has left Nathaniel Osborn shaken.
The high school history teacher said he likes to stay upbeat and apolitical in his social media musings, posting about Florida wildlife preservation and favorite books. But on Sept. 7, he tweeted: “My 12-year-old had appendicitis. The ER was overwhelmed with unvaccinated Covid patients and we had to wait 6+ hours. While waiting, his appendix ruptured and had to spend 5 days in hospital. ... So yeah, your decision to not vaccinate does affect others.”
It was retweeted 34,700 times, with 143,000 likes. Most comments were sympathetic and wished his child a speedy recovery. Some, though, went straight to hate, apparently triggered by his last line. He was attacked personally and accused of making up the story: “Good try with the guilt, jerk.”
Osborn, who is vaccinated, as are his wife and son, told ProPublica he only shared Seth’s story on Twitter to encourage vaccinations.
“I have no ill will towards the hospitals or the care received at either hospital,” he said this week, “but had these hospitals not been so crowded with COVID patients, we wouldn’t have had to wait so long and perhaps my son’s appendix would not have burst.”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Seth was finally diagnosed with appendicitis more than six hours after arriving at Cleveland Clinic Martin Health North Hospital in late July. Around midnight, he was taken by ambulance to a sister hospital about a half-hour away that was better equipped to perform pediatric emergency surgery, his father said.
But by the time the doctor operated in the early morning hours, Seth’s appendix had burst – a potentially fatal complication.
They, too, need emergency care, but the sheer number of COVID-19 cases is crowding them out. Treatment has often been delayed as ERs scramble to find a bed that may be hundreds of miles away.
Some health officials now worry about looming ethical decisions. Last week, Idaho activated a “crisis standard of care,” which one official described as a “last resort.” It allows overwhelmed hospitals to ration care, including “in rare cases, ventilator (breathing machines) or intensive care unit (ICU) beds may need to be used for those who are most likely to survive, while patients who are not likely to survive may not be able to receive one,” the state’s website said.
The federal government’s latest data shows Alabama is at 100% of its intensive care unit capacity, with Texas, Georgia, Mississippi and Arkansas at more than 90% ICU capacity. Florida is just under 90%.
It’s the COVID-19 cases that are dominating. In Georgia, 62% of the ICU beds are now filled with just COVID-19 patients. In Texas, the percentage is nearly half.
To have so many ICU beds pressed into service for a single diagnosis is “unheard of,” said Dr. Hasan Kakli, an emergency room physician at Bellville Medical Center in Bellville, Texas, about an hour from Houston. “It’s approaching apocalyptic.”
In Texas, state data released Monday showed there were only 319 adult and 104 pediatric staffed ICU beds available across a state of 29 million people.
Hospitals need to hold some ICU beds for other patients, such as those recovering from major surgery or other critical conditions such as stroke, trauma or heart failure.
“This is not just a COVID issue,” said Dr. Normaliz Rodriguez, pediatric emergency physician at Johns Hopkins All Children’s Hospital in St. Petersburg, Florida. “This is an everyone issue.”
While the latest hospital crisis echoes previous pandemic spikes, there are troubling differences this time around.
Before, localized COVID-19 hot spots led to bed shortages, but there were usually hospitals in the region not as affected that could accept a transfer.
Now, as the highly contagious delta variant envelops swaths of low-vaccination states all at once, it becomes harder to find nearby hospitals that are not slammed.
“Wait times can now be measured in days,” said Darrell Pile, CEO of the SouthEast Texas Regional Advisory Council, which helps coordinate patient transfers across a 25-county region.
Recently, Dr. Cedric Dark, a Houston emergency physician and assistant professor of emergency medicine at Baylor College of Medicine, said he saw a critically ill COVID-19 patient waiting in the emergency room for an ICU bed to open. The doctor worked eight hours, went home and came in the next day. The patient was still waiting.
Holding a seriously ill patient in an emergency room while waiting for an in-patient bed to open is known as boarding. The longer the wait, the more dangerous it can be for the patient, studies have found.
Not only do patients ultimately end up staying in the hospital or the ICU longer, some research suggests that long waits for a bed will worsen their condition and may increase the risk of in-hospital death.
That’s what happened last month in Texas.
On Aug. 21, around 11:30 a.m., Michelle Puget took her adult son, Daniel Wilkinson, to the Bellville Medical Center’s emergency room as a pain in his abdomen became unbearable. “Mama,” he said, “take me to the hospital.”
Wilkinson, a 46-year-old decorated Army veteran who did two tours of duty in Afghanistan, was ushered into an exam room about half an hour later. Kakli, the emergency room physician there, diagnosed gallstone pancreatitis, a serious but treatable condition that required a specialist to perform a surgical procedure and an ICU bed.
In other times, the transfer to a larger facility would be easy. But soon Kakli found himself on a frantic, six-hour quest to find a bed for his patient. Not only did he call hospitals across Texas, but he also tried Kansas, Missouri, Oklahoma and Colorado. It was like throwing darts at a map and hoping to get lucky, he told ProPublica. But no one could or would take the transfer.
By 2:30 p.m., Wilkinson’s condition was deteriorating. Kakli told Puget to come back to the hospital. “I have to tell you,” she said he told her, “Your son is a very, very sick man. If he doesn’t get this procedure he will die.” She began to weep.
Two hours later, Wilkinson’s blood pressure was dropping, signaling his organs were failing, she said.
Kakli went on Facebook and posted an all-caps plea to physician groups around the nation: “GETTING REJECTED BY ALL HOSPITALS IN TEXAS DUE TO NO ICU BEDS. PLEASE HELP. MESSAGE ME IF YOU HAVE A BED. PATIENT IS IN ER NOW. I AM THE ER DOC. WILL FLY ANYWHERE.”
The doctor tried Michael E. DeBakey VA Medical Center in Houston for a second time. This time he found a bed.
Around 7 p.m., Wilkinson, still conscious but in grave condition, was flown by helicopter to the hospital. He was put in a medically induced coma. Through the night and into the next morning, medical teams worked to stabilize him enough to perform the procedure. They could not.
Doctors told his family the internal damage was catastrophic. “We made the decision we had to let him go,” Puget said.
Time of death: 1:37 p.m. Aug. 22 – 26 hours after he first arrived in the emergency room.
The story was first reported by CBS News. Kakli told ProPublica last week he still sometimes does the math in his head: It should have been 40 minutes from diagnosis in Bellville to transfer to the ICU in Houston. “If he had 40 minutes to wait instead of six hours, I strongly believe he would have had a different outcome.”
Another difference with the latest surge is how it’s affecting children.
Last year, schools were closed, and children were more protected because they were mostly isolated at home. In fact, children’s hospitals were often so empty during previous spikes they opened beds to adult patients.
Now, families are out more. Schools have reopened, some with mask mandates, some without. Vaccines are not yet available to those under 12. Suddenly the numbers of hospitalized children are on the rise, setting up the same type of competition for resources between young COVID-19 patients and those with other illnesses such as new onset diabetes, trauma, pneumonia or appendicitis.
Dr. Rafael Santiago, a pediatric emergency physician in Central Florida, said at Lakeland Regional Health Medical Center, the average number of children coming into the emergency room is around 130 per day. During the lockdown last spring, that number dropped to 33. Last month – “the busiest month ever” – the average daily number of children in the emergency room was 160.
Pediatric transfers are not yet as fraught as adult ones, Santiago said, but it does take more calls than it once did to secure a bed.
Seth Osborn, the 12-year-old whose appendix burst after a long wait, spent five days and four nights in the hospital as doctors pumped his body full of antibiotics to stave off infection from the rupture. The typical hospitalization for a routine appendectomy is about 24 hours.
The initial hospital bill for the stay came to more than $48,000, Nathaniel Osborn said. Although insurance paid for most of it, he said the family still borrowed against its house to cover the more than $5,000 in out-of-pocket costs so far.
While the hospital system where Seth was treated declined to comment about his case because of patient privacy laws, it did email a statement about the strain the pandemic is creating.
“Since July 2021, we have seen a tremendous spike in COVID-19 patients needing care and hospitalization. In mid-August, we saw the highest number of patients hospitalized with COVID-19 across the Cleveland Clinic Florida region, a total of 395 COVID-19 patients in four hospitals. Those hospitals have approximately 1,000 total beds,” the email to ProPublica said. “We strongly encourage vaccination. Approximately 90% of our patients hospitalized due to COVID-19 are unvaccinated.”
On Sunday, The Washington Post reported that a hospital in Alabama called 43 others across three states before finding a bed for Ray DeMonia, a critically ill heart patient who later died. In his obituary his family wrote: “In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies. ... He would not want any other family to go through what his did.”
Today, Seth is mostly recovered. “Twelve-year-old boys bounce back,” his father said. Still, the experience has left Nathaniel Osborn shaken.
The high school history teacher said he likes to stay upbeat and apolitical in his social media musings, posting about Florida wildlife preservation and favorite books. But on Sept. 7, he tweeted: “My 12-year-old had appendicitis. The ER was overwhelmed with unvaccinated Covid patients and we had to wait 6+ hours. While waiting, his appendix ruptured and had to spend 5 days in hospital. ... So yeah, your decision to not vaccinate does affect others.”
It was retweeted 34,700 times, with 143,000 likes. Most comments were sympathetic and wished his child a speedy recovery. Some, though, went straight to hate, apparently triggered by his last line. He was attacked personally and accused of making up the story: “Good try with the guilt, jerk.”
Osborn, who is vaccinated, as are his wife and son, told ProPublica he only shared Seth’s story on Twitter to encourage vaccinations.
“I have no ill will towards the hospitals or the care received at either hospital,” he said this week, “but had these hospitals not been so crowded with COVID patients, we wouldn’t have had to wait so long and perhaps my son’s appendix would not have burst.”
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive their biggest stories as soon as they’re published.
Want to see what COVID strain you have? The government says no
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.
The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.

The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Every day, more than 140,000 people in the United States are diagnosed with COVID-19. But no matter how curious they are about which variant they are fighting, none of them will find out.

The country is dotted with labs that sequence the genomes of COVID-19 cases, and the Centers for Disease Control and Prevention tracks those results. But federal rules say those results are not allowed to make their way back to patients or doctors.
According to public health and infectious disease experts, this is unlikely to change any time soon.
“I know people want to know – I’ve had a lot of friends or family who’ve asked me how they can find out,” says Aubree Gordon, PhD, an epidemiology specialist at the University of Michigan, Ann Arbor. “I think it’s an interesting thing to find out, for sure. And it would certainly be nice to know. But because it probably isn’t necessary, there is little motivation to change the rules.”
Because the tests that are used have not been approved as diagnostic tools under the Clinical Laboratory Improvement Amendments program, which is overseen by the Centers for Medicare & Medicaid Services, they can only be used for research purposes.
In fact, the scientists doing the sequencing rarely have any patient information, Dr. Gordon says. For example, the Lauring Lab at University of Michigan – run by Adam Lauring, MD – focuses on viral evolution and currently tests for variants. But this is not done for the sake of the patient or the doctors treating the patient.
“The samples come in ... and they’ve been de-identified,”Dr. Gordon says. “This is just for research purposes. Not much patient information is shared with the researchers.”
But as of now, aside from sheer curiosity, there is not a reason to change this, says Timothy Brewer, MD, a professor of medicine and epidemiology at University of California, Los Angeles.
Although there are emerging variants – including the new Mu variant, also known as B.1.621 and recently classified as a “variant of interest” – the Delta variant accounts for about 99% of U.S. cases.
In addition, Dr. Brewer says, treatments are the same for all COVID-19 patients, regardless of the variant.
“There would have to be some clinical significance for there to be a good reason to give this information,” he says. “That would mean we would be doing something different treatment-wise depending on the variant. As of now, that is not the case.”
There is a loophole that allows labs to release variant information: They can develop their own tests. But they then must go through a lengthy validation process that proves their tests are as effective as the gold standard, says Mark Pandori, PhD, director of the Nevada State Public Health Laboratory.
But even with validation, it is too time-consuming and costly to sequence large numbers of cases, he says.
“The reason we’re not doing it routinely is there’s no way to do the genomic analysis on all the positives,” Dr. Pandori says. “It is about $110 dollars to do a sequence. It’s not like a standard PCR test.”
There is a hypothetical situation that may warrant the release of these results, Dr. Brewer says: If a variant emerges that evades vaccines.
“That would be a real public health issue,” he says. “You want to make sure there aren’t variants emerging somewhere that are escaping immunity.”
A version of this article first appeared on WebMD.com.
Low RA flare rate reported after Pfizer COVID vaccination
Patients with rheumatoid arthritis in remission had a rate of flare following vaccination with the Pfizer/BioNtech COVID-19 vaccine that appears to be on par with rates seen with other vaccines in patients with RA, according to results from a small Italian cohort study.
“Our data show a very low flare rate [7.8% (6 of 77)] after the BNT162b2 COVID-19 vaccine in patients with RA in remission and are consistent with previous findings about varicella-zoster virus (6.7%) and hepatitis B virus (2.2%) vaccinations,” Riccardo Bixio, MD, and colleagues from University of Verona (Italy) Hospital Trust wrote in ACR Open Rheumatology. “Because remission is not commonly obtained in the real world, we are aware that our findings may not be generalizable to all patients with RA receiving COVID-19 vaccination.”
Other studies of flare rate after COVID-19 vaccination in patients with a variety of rheumatic and musculoskeletal diseases have reported rates ranging from 5% to 17%, they said.
The 77 consecutive patients from the University of Verona center that conducted the study were all in clinical remission in the 3 months before vaccination based on a 28-joint Disease Activity Score based on C-reactive protein (DAS28-CRP) of less than 2.6, and all had discontinued antirheumatic therapies according to American College of Rheumatology COVID-19 recommendations. The researchers defined flares as agreement between patient and rheumatologist assessments and a DAS28-CRP increase of more than 1.2.
Five of the six people with a flare had it occur after the second dose at a mean of 2.6 days later, and all flares were resolved within 2 weeks using glucocorticoids with or without anti-inflammatory drugs. One flare was called severe. The overall disease activity of the cohort after 3 months was not significantly changed after vaccination.
In noting that five out of the six patients with flares had withdrawn or delayed antirheumatic therapies around the time of vaccination according to ACR recommendations, the authors wrote that “Even if there is no direct evidence that holding therapies could occur in a higher proportion of disease flares, we suggest that clinicians consider this possibility when counseling patients about COVID-19 vaccination.”
The authors had no outside funding for the study and had no potential conflicts of interest to disclose.
Patients with rheumatoid arthritis in remission had a rate of flare following vaccination with the Pfizer/BioNtech COVID-19 vaccine that appears to be on par with rates seen with other vaccines in patients with RA, according to results from a small Italian cohort study.
“Our data show a very low flare rate [7.8% (6 of 77)] after the BNT162b2 COVID-19 vaccine in patients with RA in remission and are consistent with previous findings about varicella-zoster virus (6.7%) and hepatitis B virus (2.2%) vaccinations,” Riccardo Bixio, MD, and colleagues from University of Verona (Italy) Hospital Trust wrote in ACR Open Rheumatology. “Because remission is not commonly obtained in the real world, we are aware that our findings may not be generalizable to all patients with RA receiving COVID-19 vaccination.”
Other studies of flare rate after COVID-19 vaccination in patients with a variety of rheumatic and musculoskeletal diseases have reported rates ranging from 5% to 17%, they said.
The 77 consecutive patients from the University of Verona center that conducted the study were all in clinical remission in the 3 months before vaccination based on a 28-joint Disease Activity Score based on C-reactive protein (DAS28-CRP) of less than 2.6, and all had discontinued antirheumatic therapies according to American College of Rheumatology COVID-19 recommendations. The researchers defined flares as agreement between patient and rheumatologist assessments and a DAS28-CRP increase of more than 1.2.
Five of the six people with a flare had it occur after the second dose at a mean of 2.6 days later, and all flares were resolved within 2 weeks using glucocorticoids with or without anti-inflammatory drugs. One flare was called severe. The overall disease activity of the cohort after 3 months was not significantly changed after vaccination.
In noting that five out of the six patients with flares had withdrawn or delayed antirheumatic therapies around the time of vaccination according to ACR recommendations, the authors wrote that “Even if there is no direct evidence that holding therapies could occur in a higher proportion of disease flares, we suggest that clinicians consider this possibility when counseling patients about COVID-19 vaccination.”
The authors had no outside funding for the study and had no potential conflicts of interest to disclose.
Patients with rheumatoid arthritis in remission had a rate of flare following vaccination with the Pfizer/BioNtech COVID-19 vaccine that appears to be on par with rates seen with other vaccines in patients with RA, according to results from a small Italian cohort study.
“Our data show a very low flare rate [7.8% (6 of 77)] after the BNT162b2 COVID-19 vaccine in patients with RA in remission and are consistent with previous findings about varicella-zoster virus (6.7%) and hepatitis B virus (2.2%) vaccinations,” Riccardo Bixio, MD, and colleagues from University of Verona (Italy) Hospital Trust wrote in ACR Open Rheumatology. “Because remission is not commonly obtained in the real world, we are aware that our findings may not be generalizable to all patients with RA receiving COVID-19 vaccination.”
Other studies of flare rate after COVID-19 vaccination in patients with a variety of rheumatic and musculoskeletal diseases have reported rates ranging from 5% to 17%, they said.
The 77 consecutive patients from the University of Verona center that conducted the study were all in clinical remission in the 3 months before vaccination based on a 28-joint Disease Activity Score based on C-reactive protein (DAS28-CRP) of less than 2.6, and all had discontinued antirheumatic therapies according to American College of Rheumatology COVID-19 recommendations. The researchers defined flares as agreement between patient and rheumatologist assessments and a DAS28-CRP increase of more than 1.2.
Five of the six people with a flare had it occur after the second dose at a mean of 2.6 days later, and all flares were resolved within 2 weeks using glucocorticoids with or without anti-inflammatory drugs. One flare was called severe. The overall disease activity of the cohort after 3 months was not significantly changed after vaccination.
In noting that five out of the six patients with flares had withdrawn or delayed antirheumatic therapies around the time of vaccination according to ACR recommendations, the authors wrote that “Even if there is no direct evidence that holding therapies could occur in a higher proportion of disease flares, we suggest that clinicians consider this possibility when counseling patients about COVID-19 vaccination.”
The authors had no outside funding for the study and had no potential conflicts of interest to disclose.
FROM ACR OPEN RHEUMATOLOGY
Children and COVID: New cases down slightly from record high
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.
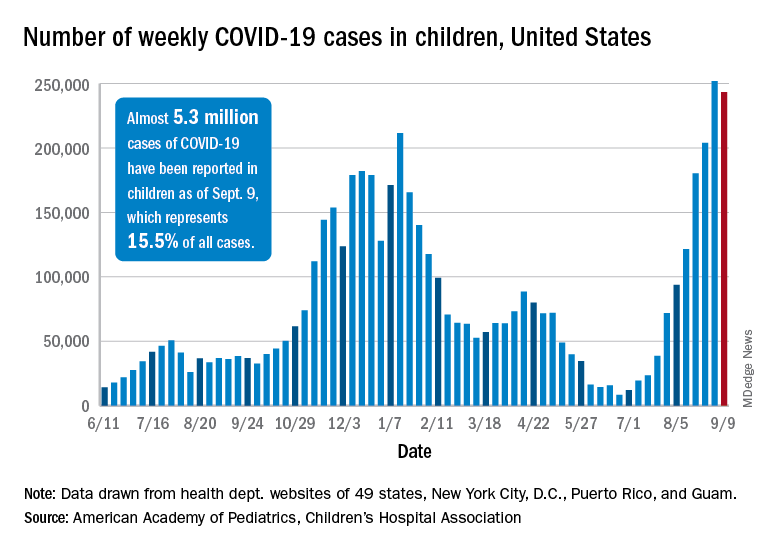
Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).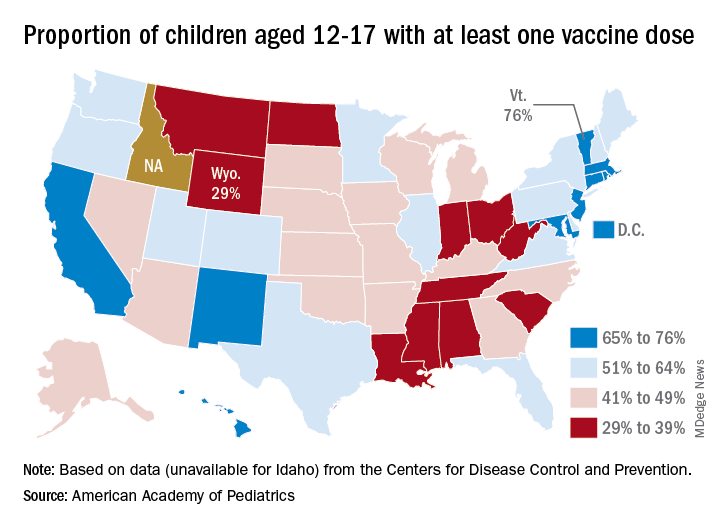
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.

Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
Weekly cases of COVID-19 in children dropped for the first time since June, and daily hospitalizations appear to be falling, even as the pace of vaccinations continues to slow among the youngest eligible recipients, according to new data.

Despite the 3.3% decline from the previous week’s record high, the new-case count still topped 243,000 for the week of Sept. 3-9, putting the total number of cases in children at almost 5.3 million since the pandemic began.
Hospitalizations seem to have peaked on Sept. 4, when the rate for children aged 0-17 years reached 0.51 per 100,000 population. The admission rate for confirmed COVID-19 has dropped steadily since then and was down to 0.45 per 100,000 on Sept. 11, the last day for which preliminary data from the Centers for Disease Control and Prevention were available.
On the prevention side, fully vaccinated children aged 12-17 years represented 5.5% of all Americans who had completed the vaccine regimen as of Sept. 13. Vaccine initiation, however, has dropped for 5 consecutive weeks in 12- to 15-year-olds and in 4 of the last 5 weeks among 16- and 17-year-olds, the CDC said on its COVID Data Tracker.
Just under 199,000 children aged 12-15 received their first dose of the COVID-19 vaccine during the week of Sept. 7-13. That’s down by 18.5% from the week before and by 51.6% since Aug. 9, the last week that vaccine initiation increased for the age group. Among 16- and 17-year-olds, the 83,000 new recipients that week was a decrease of 25.7% from the previous week and a decline of 47% since the summer peak of Aug. 9, the CDC data show.
Those newest recipients bring at-least-one-dose status to 52.0% of those aged 12-15 and 59.9% of the 16- and 17-year-olds, while 40.3% and 48.9% were fully vaccinated as of Sept. 13. Corresponding figures for some of the older groups are 61.6%/49.7% (age 18-24 years), 73.8%/63.1% (40-49 years), and 95.1%/84.5% (65-74 years), the CDC said.
Vaccine coverage for children at the state level deviates considerably from the national averages. The highest rates for children aged 12-17 are to be found in Vermont, where 76% have received at least one dose, the AAP reported in a separate analysis. Massachusetts is just below that but also comes in at 76% by virtue of a rounding error. The other states in the top five are Connecticut (74%), Hawaii (73%), and Rhode Island (71%).
The lowest vaccination rate for children comes from Wyoming (29%), which is preceded by North Dakota (33%), West Virginia (33%), Alabama (33%), and Mississippi (34%). the AAP said based on data from the CDC, which does not include Idaho.
In a bit of a side note, West Virginia’s Republican governor, Jim Justice, recently said this about vaccine reluctance in his state: “For God’s sakes a livin’, how difficult is this to understand? Why in the world do we have to come up with these crazy ideas – and they’re crazy ideas – that the vaccine’s got something in it and it’s tracing people wherever they go? And the same very people that are saying that are carrying their cellphones around. I mean, come on. Come on.”
Over the last 3 weeks, the District of Columbia has had the largest increase in children having received at least one dose: 10 percentage points, as it went from 58% to 68%. The next-largest improvement – 7 percentage points – occurred in Georgia (34% to 41%), New Mexico (61% to 68%), New York (55% to 62%), and Washington (57% to 64%), the AAP said in its weekly vaccination trends report.
Man dies after 43 full ICUs turn him away
Ray Martin DeMonia, 73, of Cullman, Alabama, ran an antiques business for 40 years and served as an auctioneer at charity events, the obituary said.
He had a stroke in 2020 during the first months of the COVID pandemic and made sure to get vaccinated, his daughter, Raven DeMonia, told The Washington Post.
“He knew what the vaccine meant for his health and what it meant to staying alive,” she said. “He said, ‘I just want to get back to shaking hands with people, selling stuff, and talking antiques.’”
His daughter told the Post that her father went to Cullman Regional Medical Center on Aug. 23 with heart problems.
About 12 hours after he was admitted, her mother got a call from the hospital saying they’d called 43 hospitals and were unable to find a “specialized cardiac ICU bed” for him, Ms. DeMonia told the Post.
He was finally airlifted to Rush Foundation Hospital in Meridian, Mississippi, almost 200 miles from his home, but died there Sept. 1. His family decided to make a plea for increased vaccinations in his obituary.
“In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies,” the obit said. “Due to COVID 19, CRMC emergency staff contacted 43 hospitals in 3 states in search of a Cardiac ICU bed and finally located one in Meridian, MS. He would not want any other family to go through what his did.”
Mr. DeMonia is survived by his wife, daughter, grandson, and other family members.
The Alabama Hospital Association says state hospitals are still short of ICU beds. On Sept. 12, the AHA website said the state had 1,530 staffed ICU beds to accommodate 1,541 ICU patients.
The AHA said 83% of COVID patients in ICU had not been vaccinated against COVID, 4% were partially vaccinated, and 13% were fully vaccinated. Alabama trails other states in vaccination rates. Newsweek, citing CDC data, said 53.7% of people in Alabama were fully vaccinated. In comparison, 53.8% of all Americans nationally are fully vaccinated.
A version of this article first appeared on WebMD.com.
Ray Martin DeMonia, 73, of Cullman, Alabama, ran an antiques business for 40 years and served as an auctioneer at charity events, the obituary said.
He had a stroke in 2020 during the first months of the COVID pandemic and made sure to get vaccinated, his daughter, Raven DeMonia, told The Washington Post.
“He knew what the vaccine meant for his health and what it meant to staying alive,” she said. “He said, ‘I just want to get back to shaking hands with people, selling stuff, and talking antiques.’”
His daughter told the Post that her father went to Cullman Regional Medical Center on Aug. 23 with heart problems.
About 12 hours after he was admitted, her mother got a call from the hospital saying they’d called 43 hospitals and were unable to find a “specialized cardiac ICU bed” for him, Ms. DeMonia told the Post.
He was finally airlifted to Rush Foundation Hospital in Meridian, Mississippi, almost 200 miles from his home, but died there Sept. 1. His family decided to make a plea for increased vaccinations in his obituary.
“In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies,” the obit said. “Due to COVID 19, CRMC emergency staff contacted 43 hospitals in 3 states in search of a Cardiac ICU bed and finally located one in Meridian, MS. He would not want any other family to go through what his did.”
Mr. DeMonia is survived by his wife, daughter, grandson, and other family members.
The Alabama Hospital Association says state hospitals are still short of ICU beds. On Sept. 12, the AHA website said the state had 1,530 staffed ICU beds to accommodate 1,541 ICU patients.
The AHA said 83% of COVID patients in ICU had not been vaccinated against COVID, 4% were partially vaccinated, and 13% were fully vaccinated. Alabama trails other states in vaccination rates. Newsweek, citing CDC data, said 53.7% of people in Alabama were fully vaccinated. In comparison, 53.8% of all Americans nationally are fully vaccinated.
A version of this article first appeared on WebMD.com.
Ray Martin DeMonia, 73, of Cullman, Alabama, ran an antiques business for 40 years and served as an auctioneer at charity events, the obituary said.
He had a stroke in 2020 during the first months of the COVID pandemic and made sure to get vaccinated, his daughter, Raven DeMonia, told The Washington Post.
“He knew what the vaccine meant for his health and what it meant to staying alive,” she said. “He said, ‘I just want to get back to shaking hands with people, selling stuff, and talking antiques.’”
His daughter told the Post that her father went to Cullman Regional Medical Center on Aug. 23 with heart problems.
About 12 hours after he was admitted, her mother got a call from the hospital saying they’d called 43 hospitals and were unable to find a “specialized cardiac ICU bed” for him, Ms. DeMonia told the Post.
He was finally airlifted to Rush Foundation Hospital in Meridian, Mississippi, almost 200 miles from his home, but died there Sept. 1. His family decided to make a plea for increased vaccinations in his obituary.
“In honor of Ray, please get vaccinated if you have not, in an effort to free up resources for non COVID related emergencies,” the obit said. “Due to COVID 19, CRMC emergency staff contacted 43 hospitals in 3 states in search of a Cardiac ICU bed and finally located one in Meridian, MS. He would not want any other family to go through what his did.”
Mr. DeMonia is survived by his wife, daughter, grandson, and other family members.
The Alabama Hospital Association says state hospitals are still short of ICU beds. On Sept. 12, the AHA website said the state had 1,530 staffed ICU beds to accommodate 1,541 ICU patients.
The AHA said 83% of COVID patients in ICU had not been vaccinated against COVID, 4% were partially vaccinated, and 13% were fully vaccinated. Alabama trails other states in vaccination rates. Newsweek, citing CDC data, said 53.7% of people in Alabama were fully vaccinated. In comparison, 53.8% of all Americans nationally are fully vaccinated.
A version of this article first appeared on WebMD.com.
Pandemic strategies to boost trial enrollment should stay
Although enrollment into lung cancer clinical trials fell during the early months of the COVID-19 pandemic, it increased after a number of mitigation strategies were introduced.
These strategies should now be maintained, say experts, in order to improve enrollment and access to trials and to ensure that trials are more pragmatic and streamlined.
These were the findings from a survey sent to 173 sites of clinical trials in 45 countries around the world. The findings were presented recently at the World Conference on Lung Cancer (WCLC) 2021. The meeting and the survey were organized by the International Association for the Study of Lung Cancer (IASLC).
Responses to the survey revealed that enrollment into lung cancer trials fell by 43% during the early months of the pandemic. Patients stopped attending clinics, and some trials were suspended.
Patients were less willing to visit clinical trial sites, and lockdown restrictions made travel difficult.
Organizers of clinical trials responded by implementing mitigation strategies, such as changing monitoring requirements, increasing use of telehealth, and using local non-study facilities for laboratory and radiology services.
These measures led to an increase in trial enrollment toward the end of 2020, the survey results show.
“The COVID-19 pandemic created many challenges [that led to] reductions in lung cancer clinical trial enrollment,” commented study presenter Matthew P. Smeltzer, PhD, from the Division of Epidemiology, Biostatistics, and Environmental Health, University of Memphis.
The employment of mitigation strategies allowed the removal of “barriers,” and although the pandemic “worsened, trial enrollment began to improve due in part to these strategies,” Dr. Smeltzer said.
Many of these measures were successful and should be maintained, he suggested. Strategies include allowing telehealth visits, performing testing at local laboratories, using local radiology services, mailing experimental agents “where possible,” and allowing flexibility in trial schedules.
This is a “very important” study, commented Marina Garassino, MD, professor of medicine, hematology, and oncology, the University of Chicago Medicine, in her discussion of the abstract.
Irrespective of the pandemic, the regulation and the bureaucracy of clinical trials hinder participation by patients and physicians, she said.
Many of the mitigation strategies highlighted by the survey were similar to recommendations on the conduct of clinical trials published by the American Society of Clinical Oncology during the pandemic. Those recommendations emphasize the use of telehealth and offsite strategies to help with patient monitoring, she noted.
The findings from the survey show that it is possible to conduct more “streamlined and pragmatic trials,” she said.
“More flexible approaches should be approved by the sponsors of clinical trials and global regulatory bodies,” she added.
However, she expressed concern that “with the telehealth visits, we can create some disparities.”
“We have to remember that lung cancer patients are sometimes a very old population, and they are not digitally evolved,” she commented.
Commenting on Twitter, Jennifer C. King, PhD, chief scientific officer at the GO2 Foundation for Lung Cancer, in Washington, D.C., agreed that many of the mitigation strategies identified in the study “are good for patients all of the time, not just during a pandemic.”
Impact on lung cancer clinical trials
The survey, which included 64 questions, was intended to assess the impact of the COVID pandemic on lung cancer clinical trials.
Most of the survey responses came from sites in Europe (37.6%); 21.4% came from Asia, 13.3% came from the United States, and 7.5% came from Canada.
The team found that enrollment into lung cancer trials declined by 43% in 2020 compared to 2019, at an incidence rate ratio of 0.57 (P = .0115).
The largest decreases in enrollment were between April and August 2020, Dr. Smeltzer noted. However, in the last quarter of 2020 (October to December), the differences in enrollment were significantly smaller (P = .0160), despite a marked increase in global COVID-19 cases per month, he added.
The most common challenges faced by clinical trial sites during the pandemic were the following: There were fewer eligible patients (cited by 67% of respondents); compliance protocol was worse (61%); trials were suspended (60%); there was a lack of research staff (48%); and there were institutional closures (39%).
Regarding patient-related challenges, 67% of sites cited less willingness to visit the site. Other challenges included less ability to travel (cited by 60%), reduced access to the trial site (52%), quarantining because of exposure to COVID-19 (40%), and SARS-CoV-2 infection (26%).
Concerns of patients included the following: Fear of SARS-CoV-2 infection, which was cited by 83%; travel restrictions (47%); securing transportation (38%); and access to the laboratory/radiology services (14%).
“Patient willingness to visit the site was a consistent barrier reported across Europe, the U.S., and Canada,” said Dr. Smeltzer, although the effect was smaller in North America, he added.
Regarding mitigation strategies that were employed during the pandemic to combat the challenges and concerns, the team found that the most common measure was the modification of monitoring requirements, used by 44% of sites.
This was followed by the use of telehealth visits (43% sites), the use of laboratories at non-study facilities ( 27%), and alterations to the number of required visits (25%).
Other mitigation strategies included use of mail-order medications, (24%), using radiology services at a non-study site (20%), and altering the trial schedules (19%).
The most effective mitigation strategies were felt to be those that allowed flexibility with respect to location. These measures included use of remote monitoring, remote diagnostics, telehealth visits, and modified symptom monitoring.
Effective strategies that increased flexibility in time were delayed visits, delayed assessments, and changes to the Institutional Review Board.
The study was funded by the IASLC, which received industry support to conduct the project. Dr. Smeltzer reported no relevant financial relationships. Dr. Garassino has relationships with AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Ignyta, Incyte, MedImmune, Mirati, MSD International, Novartis, Pfizer, Regeneron, Roche, Takeda, and Seattle Genetics.
A version of this article first appeared on Medscape.com.
Although enrollment into lung cancer clinical trials fell during the early months of the COVID-19 pandemic, it increased after a number of mitigation strategies were introduced.
These strategies should now be maintained, say experts, in order to improve enrollment and access to trials and to ensure that trials are more pragmatic and streamlined.
These were the findings from a survey sent to 173 sites of clinical trials in 45 countries around the world. The findings were presented recently at the World Conference on Lung Cancer (WCLC) 2021. The meeting and the survey were organized by the International Association for the Study of Lung Cancer (IASLC).
Responses to the survey revealed that enrollment into lung cancer trials fell by 43% during the early months of the pandemic. Patients stopped attending clinics, and some trials were suspended.
Patients were less willing to visit clinical trial sites, and lockdown restrictions made travel difficult.
Organizers of clinical trials responded by implementing mitigation strategies, such as changing monitoring requirements, increasing use of telehealth, and using local non-study facilities for laboratory and radiology services.
These measures led to an increase in trial enrollment toward the end of 2020, the survey results show.
“The COVID-19 pandemic created many challenges [that led to] reductions in lung cancer clinical trial enrollment,” commented study presenter Matthew P. Smeltzer, PhD, from the Division of Epidemiology, Biostatistics, and Environmental Health, University of Memphis.
The employment of mitigation strategies allowed the removal of “barriers,” and although the pandemic “worsened, trial enrollment began to improve due in part to these strategies,” Dr. Smeltzer said.
Many of these measures were successful and should be maintained, he suggested. Strategies include allowing telehealth visits, performing testing at local laboratories, using local radiology services, mailing experimental agents “where possible,” and allowing flexibility in trial schedules.
This is a “very important” study, commented Marina Garassino, MD, professor of medicine, hematology, and oncology, the University of Chicago Medicine, in her discussion of the abstract.
Irrespective of the pandemic, the regulation and the bureaucracy of clinical trials hinder participation by patients and physicians, she said.
Many of the mitigation strategies highlighted by the survey were similar to recommendations on the conduct of clinical trials published by the American Society of Clinical Oncology during the pandemic. Those recommendations emphasize the use of telehealth and offsite strategies to help with patient monitoring, she noted.
The findings from the survey show that it is possible to conduct more “streamlined and pragmatic trials,” she said.
“More flexible approaches should be approved by the sponsors of clinical trials and global regulatory bodies,” she added.
However, she expressed concern that “with the telehealth visits, we can create some disparities.”
“We have to remember that lung cancer patients are sometimes a very old population, and they are not digitally evolved,” she commented.
Commenting on Twitter, Jennifer C. King, PhD, chief scientific officer at the GO2 Foundation for Lung Cancer, in Washington, D.C., agreed that many of the mitigation strategies identified in the study “are good for patients all of the time, not just during a pandemic.”
Impact on lung cancer clinical trials
The survey, which included 64 questions, was intended to assess the impact of the COVID pandemic on lung cancer clinical trials.
Most of the survey responses came from sites in Europe (37.6%); 21.4% came from Asia, 13.3% came from the United States, and 7.5% came from Canada.
The team found that enrollment into lung cancer trials declined by 43% in 2020 compared to 2019, at an incidence rate ratio of 0.57 (P = .0115).
The largest decreases in enrollment were between April and August 2020, Dr. Smeltzer noted. However, in the last quarter of 2020 (October to December), the differences in enrollment were significantly smaller (P = .0160), despite a marked increase in global COVID-19 cases per month, he added.
The most common challenges faced by clinical trial sites during the pandemic were the following: There were fewer eligible patients (cited by 67% of respondents); compliance protocol was worse (61%); trials were suspended (60%); there was a lack of research staff (48%); and there were institutional closures (39%).
Regarding patient-related challenges, 67% of sites cited less willingness to visit the site. Other challenges included less ability to travel (cited by 60%), reduced access to the trial site (52%), quarantining because of exposure to COVID-19 (40%), and SARS-CoV-2 infection (26%).
Concerns of patients included the following: Fear of SARS-CoV-2 infection, which was cited by 83%; travel restrictions (47%); securing transportation (38%); and access to the laboratory/radiology services (14%).
“Patient willingness to visit the site was a consistent barrier reported across Europe, the U.S., and Canada,” said Dr. Smeltzer, although the effect was smaller in North America, he added.
Regarding mitigation strategies that were employed during the pandemic to combat the challenges and concerns, the team found that the most common measure was the modification of monitoring requirements, used by 44% of sites.
This was followed by the use of telehealth visits (43% sites), the use of laboratories at non-study facilities ( 27%), and alterations to the number of required visits (25%).
Other mitigation strategies included use of mail-order medications, (24%), using radiology services at a non-study site (20%), and altering the trial schedules (19%).
The most effective mitigation strategies were felt to be those that allowed flexibility with respect to location. These measures included use of remote monitoring, remote diagnostics, telehealth visits, and modified symptom monitoring.
Effective strategies that increased flexibility in time were delayed visits, delayed assessments, and changes to the Institutional Review Board.
The study was funded by the IASLC, which received industry support to conduct the project. Dr. Smeltzer reported no relevant financial relationships. Dr. Garassino has relationships with AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Ignyta, Incyte, MedImmune, Mirati, MSD International, Novartis, Pfizer, Regeneron, Roche, Takeda, and Seattle Genetics.
A version of this article first appeared on Medscape.com.
Although enrollment into lung cancer clinical trials fell during the early months of the COVID-19 pandemic, it increased after a number of mitigation strategies were introduced.
These strategies should now be maintained, say experts, in order to improve enrollment and access to trials and to ensure that trials are more pragmatic and streamlined.
These were the findings from a survey sent to 173 sites of clinical trials in 45 countries around the world. The findings were presented recently at the World Conference on Lung Cancer (WCLC) 2021. The meeting and the survey were organized by the International Association for the Study of Lung Cancer (IASLC).
Responses to the survey revealed that enrollment into lung cancer trials fell by 43% during the early months of the pandemic. Patients stopped attending clinics, and some trials were suspended.
Patients were less willing to visit clinical trial sites, and lockdown restrictions made travel difficult.
Organizers of clinical trials responded by implementing mitigation strategies, such as changing monitoring requirements, increasing use of telehealth, and using local non-study facilities for laboratory and radiology services.
These measures led to an increase in trial enrollment toward the end of 2020, the survey results show.
“The COVID-19 pandemic created many challenges [that led to] reductions in lung cancer clinical trial enrollment,” commented study presenter Matthew P. Smeltzer, PhD, from the Division of Epidemiology, Biostatistics, and Environmental Health, University of Memphis.
The employment of mitigation strategies allowed the removal of “barriers,” and although the pandemic “worsened, trial enrollment began to improve due in part to these strategies,” Dr. Smeltzer said.
Many of these measures were successful and should be maintained, he suggested. Strategies include allowing telehealth visits, performing testing at local laboratories, using local radiology services, mailing experimental agents “where possible,” and allowing flexibility in trial schedules.
This is a “very important” study, commented Marina Garassino, MD, professor of medicine, hematology, and oncology, the University of Chicago Medicine, in her discussion of the abstract.
Irrespective of the pandemic, the regulation and the bureaucracy of clinical trials hinder participation by patients and physicians, she said.
Many of the mitigation strategies highlighted by the survey were similar to recommendations on the conduct of clinical trials published by the American Society of Clinical Oncology during the pandemic. Those recommendations emphasize the use of telehealth and offsite strategies to help with patient monitoring, she noted.
The findings from the survey show that it is possible to conduct more “streamlined and pragmatic trials,” she said.
“More flexible approaches should be approved by the sponsors of clinical trials and global regulatory bodies,” she added.
However, she expressed concern that “with the telehealth visits, we can create some disparities.”
“We have to remember that lung cancer patients are sometimes a very old population, and they are not digitally evolved,” she commented.
Commenting on Twitter, Jennifer C. King, PhD, chief scientific officer at the GO2 Foundation for Lung Cancer, in Washington, D.C., agreed that many of the mitigation strategies identified in the study “are good for patients all of the time, not just during a pandemic.”
Impact on lung cancer clinical trials
The survey, which included 64 questions, was intended to assess the impact of the COVID pandemic on lung cancer clinical trials.
Most of the survey responses came from sites in Europe (37.6%); 21.4% came from Asia, 13.3% came from the United States, and 7.5% came from Canada.
The team found that enrollment into lung cancer trials declined by 43% in 2020 compared to 2019, at an incidence rate ratio of 0.57 (P = .0115).
The largest decreases in enrollment were between April and August 2020, Dr. Smeltzer noted. However, in the last quarter of 2020 (October to December), the differences in enrollment were significantly smaller (P = .0160), despite a marked increase in global COVID-19 cases per month, he added.
The most common challenges faced by clinical trial sites during the pandemic were the following: There were fewer eligible patients (cited by 67% of respondents); compliance protocol was worse (61%); trials were suspended (60%); there was a lack of research staff (48%); and there were institutional closures (39%).
Regarding patient-related challenges, 67% of sites cited less willingness to visit the site. Other challenges included less ability to travel (cited by 60%), reduced access to the trial site (52%), quarantining because of exposure to COVID-19 (40%), and SARS-CoV-2 infection (26%).
Concerns of patients included the following: Fear of SARS-CoV-2 infection, which was cited by 83%; travel restrictions (47%); securing transportation (38%); and access to the laboratory/radiology services (14%).
“Patient willingness to visit the site was a consistent barrier reported across Europe, the U.S., and Canada,” said Dr. Smeltzer, although the effect was smaller in North America, he added.
Regarding mitigation strategies that were employed during the pandemic to combat the challenges and concerns, the team found that the most common measure was the modification of monitoring requirements, used by 44% of sites.
This was followed by the use of telehealth visits (43% sites), the use of laboratories at non-study facilities ( 27%), and alterations to the number of required visits (25%).
Other mitigation strategies included use of mail-order medications, (24%), using radiology services at a non-study site (20%), and altering the trial schedules (19%).
The most effective mitigation strategies were felt to be those that allowed flexibility with respect to location. These measures included use of remote monitoring, remote diagnostics, telehealth visits, and modified symptom monitoring.
Effective strategies that increased flexibility in time were delayed visits, delayed assessments, and changes to the Institutional Review Board.
The study was funded by the IASLC, which received industry support to conduct the project. Dr. Smeltzer reported no relevant financial relationships. Dr. Garassino has relationships with AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Daiichi Sankyo, Eli Lilly, Ignyta, Incyte, MedImmune, Mirati, MSD International, Novartis, Pfizer, Regeneron, Roche, Takeda, and Seattle Genetics.
A version of this article first appeared on Medscape.com.
At 18 months, much still unknown about diabetes and COVID-19
At 18 months into the COVID-19 pandemic, many of the direct and indirect effects of SARS-CoV-2 on people with diabetes have become clearer, but knowledge gaps remain, say epidemiologists.
“COVID-19 has had a devastating effect on the population with diabetes, and conversely, the high prevalence of diabetes and uncontrolled diabetes has exacerbated the problem,” Edward W. Gregg, PhD, Imperial College London, lead author of a new literature review, told this news organization.
“As it becomes clear that the COVID-19 pandemic will be with us in different forms for the foreseeable future, the emphasis for people with diabetes needs to be continued primary care, glycemic management, and vaccination to reduce the long-term impact of COVID-19 in this population,” he added.
In data, mostly from case series, the review shows that more than one-third of people hospitalized with COVID-19 have diabetes. It is published in the September issue of Diabetes Care.
People with diabetes are more than three times as likely to be hospitalized for COVID-19 than those without diabetes, even after adjustment for age, sex, and other underlying conditions. Diabetes also accounts for 30%-40% of severe COVID-19 cases and deaths. Among those with diabetes hospitalized for COVID-19, 21%-43% require intensive care, and the case fatality rate is about 25%.
In one of the few multivariate analyses that examined type 1 and type 2 diabetes separately, conducted in the U.K., the odds of in-hospital COVID-19–related deaths, compared with people without diabetes, were almost three times higher (odds ratio, 2.9) for individuals with type 1 diabetes and almost twice as high (OR, 1.8) for those with type 2, after adjustment for comorbidities.
The causes of death appear to be a combination of factors specific to the SARS-CoV-2 infection and to diabetes-related factors, Dr. Gregg said in an interview.
“Much of the increased risk is due to the fact that people with diabetes have more comorbid factors, but there are many other mechanisms that appear to further increase risk, including the inflammatory and immune responses of people with diabetes, and hyperglycemia appears to have an exacerbating effect by itself.”
Elevated glucose is clear risk factor for COVID-19 severity
Elevated A1c was identified among several other overall predictors of poor COVID-19 outcomes, including obesity as well as comorbid kidney and cardiovascular disease.
High blood glucose levels at the time of admission in people with previously diagnosed or undiagnosed diabetes emerged as a clear predictor of worse outcomes. For example, among 605 people hospitalized with COVID-19 in China, those with fasting plasma glucose 6.1-6.9 mmol/L (110-125 mg/dL) and ≥7 mmol/L (126 mg/dL) had odds ratios of poor outcomes within 28 days of 2.6 and 4.0 compared with FPG <6.1 mmol/L (110 mg/dL).
Population-based studies in the U.K. found that A1c levels measured months before COVID-19 hospitalization were associated with risk for intensive care unit admission and/or death, particularly among those with type 1 diabetes. Overall, the death rate was 36% higher for those with A1c of 9%-9.9% versus 6.5%-7%.
Despite the link between high A1c and death, there is as yet no clear evidence that normalizing blood glucose levels minimizes COVID-19 severity, Dr. Gregg said.
“There are data that suggest poor glycemic control is associated with higher risk of poor outcomes. This is indirect evidence that managing blood sugar will help, but more direct evidence is needed.”
Evidence gaps identified
Dr. Gregg and co-authors Marisa Sophiea, PhD, MSc, and Misghina Weldegiorgis, PhD, BSc, also from Imperial College London, identify three areas in which more data are needed.
First, more information is needed to determine whether exposure, infection, and hospitalization risks differ by diabetes status and how those factors affect outcomes. The same studies would also be important to identify how factors such as behavior, masking, and lockdown policies, risk factor control, and household/community environments affect risk in people with diabetes.
Second, studies are needed to better understand indirect effects of the pandemic, such as care and management factors. Some of these, such as the advent of telehealth, may turn out to be beneficial in the long run, they note.
Finally, the pandemic has “brought a wealth of natural experiments,” such as how vaccination programs and other interventions are affecting people with diabetes specifically. Finally, population studies are needed in many parts of the world beyond the U.S. and the U.K., where most of that work has been done thus far.
“Many of the most important unanswered questions lie in the potential indirect and long-term impact of the pandemic that require population-based studies,” Dr. Gregg said. “Most of our knowledge so far is from case series, which only assess patients from the time of hospitalization.”
Indeed, very little data are available for people with diabetes who get COVID-19 but are not hospitalized, so it’s not known whether they have a longer duration of illness or are at greater risk for “long COVID” than those without diabetes who experience COVID-19 at home.
“I have not seen published data on this yet, and it’s an important unanswered question,” Dr. Gregg said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At 18 months into the COVID-19 pandemic, many of the direct and indirect effects of SARS-CoV-2 on people with diabetes have become clearer, but knowledge gaps remain, say epidemiologists.
“COVID-19 has had a devastating effect on the population with diabetes, and conversely, the high prevalence of diabetes and uncontrolled diabetes has exacerbated the problem,” Edward W. Gregg, PhD, Imperial College London, lead author of a new literature review, told this news organization.
“As it becomes clear that the COVID-19 pandemic will be with us in different forms for the foreseeable future, the emphasis for people with diabetes needs to be continued primary care, glycemic management, and vaccination to reduce the long-term impact of COVID-19 in this population,” he added.
In data, mostly from case series, the review shows that more than one-third of people hospitalized with COVID-19 have diabetes. It is published in the September issue of Diabetes Care.
People with diabetes are more than three times as likely to be hospitalized for COVID-19 than those without diabetes, even after adjustment for age, sex, and other underlying conditions. Diabetes also accounts for 30%-40% of severe COVID-19 cases and deaths. Among those with diabetes hospitalized for COVID-19, 21%-43% require intensive care, and the case fatality rate is about 25%.
In one of the few multivariate analyses that examined type 1 and type 2 diabetes separately, conducted in the U.K., the odds of in-hospital COVID-19–related deaths, compared with people without diabetes, were almost three times higher (odds ratio, 2.9) for individuals with type 1 diabetes and almost twice as high (OR, 1.8) for those with type 2, after adjustment for comorbidities.
The causes of death appear to be a combination of factors specific to the SARS-CoV-2 infection and to diabetes-related factors, Dr. Gregg said in an interview.
“Much of the increased risk is due to the fact that people with diabetes have more comorbid factors, but there are many other mechanisms that appear to further increase risk, including the inflammatory and immune responses of people with diabetes, and hyperglycemia appears to have an exacerbating effect by itself.”
Elevated glucose is clear risk factor for COVID-19 severity
Elevated A1c was identified among several other overall predictors of poor COVID-19 outcomes, including obesity as well as comorbid kidney and cardiovascular disease.
High blood glucose levels at the time of admission in people with previously diagnosed or undiagnosed diabetes emerged as a clear predictor of worse outcomes. For example, among 605 people hospitalized with COVID-19 in China, those with fasting plasma glucose 6.1-6.9 mmol/L (110-125 mg/dL) and ≥7 mmol/L (126 mg/dL) had odds ratios of poor outcomes within 28 days of 2.6 and 4.0 compared with FPG <6.1 mmol/L (110 mg/dL).
Population-based studies in the U.K. found that A1c levels measured months before COVID-19 hospitalization were associated with risk for intensive care unit admission and/or death, particularly among those with type 1 diabetes. Overall, the death rate was 36% higher for those with A1c of 9%-9.9% versus 6.5%-7%.
Despite the link between high A1c and death, there is as yet no clear evidence that normalizing blood glucose levels minimizes COVID-19 severity, Dr. Gregg said.
“There are data that suggest poor glycemic control is associated with higher risk of poor outcomes. This is indirect evidence that managing blood sugar will help, but more direct evidence is needed.”
Evidence gaps identified
Dr. Gregg and co-authors Marisa Sophiea, PhD, MSc, and Misghina Weldegiorgis, PhD, BSc, also from Imperial College London, identify three areas in which more data are needed.
First, more information is needed to determine whether exposure, infection, and hospitalization risks differ by diabetes status and how those factors affect outcomes. The same studies would also be important to identify how factors such as behavior, masking, and lockdown policies, risk factor control, and household/community environments affect risk in people with diabetes.
Second, studies are needed to better understand indirect effects of the pandemic, such as care and management factors. Some of these, such as the advent of telehealth, may turn out to be beneficial in the long run, they note.
Finally, the pandemic has “brought a wealth of natural experiments,” such as how vaccination programs and other interventions are affecting people with diabetes specifically. Finally, population studies are needed in many parts of the world beyond the U.S. and the U.K., where most of that work has been done thus far.
“Many of the most important unanswered questions lie in the potential indirect and long-term impact of the pandemic that require population-based studies,” Dr. Gregg said. “Most of our knowledge so far is from case series, which only assess patients from the time of hospitalization.”
Indeed, very little data are available for people with diabetes who get COVID-19 but are not hospitalized, so it’s not known whether they have a longer duration of illness or are at greater risk for “long COVID” than those without diabetes who experience COVID-19 at home.
“I have not seen published data on this yet, and it’s an important unanswered question,” Dr. Gregg said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
At 18 months into the COVID-19 pandemic, many of the direct and indirect effects of SARS-CoV-2 on people with diabetes have become clearer, but knowledge gaps remain, say epidemiologists.
“COVID-19 has had a devastating effect on the population with diabetes, and conversely, the high prevalence of diabetes and uncontrolled diabetes has exacerbated the problem,” Edward W. Gregg, PhD, Imperial College London, lead author of a new literature review, told this news organization.
“As it becomes clear that the COVID-19 pandemic will be with us in different forms for the foreseeable future, the emphasis for people with diabetes needs to be continued primary care, glycemic management, and vaccination to reduce the long-term impact of COVID-19 in this population,” he added.
In data, mostly from case series, the review shows that more than one-third of people hospitalized with COVID-19 have diabetes. It is published in the September issue of Diabetes Care.
People with diabetes are more than three times as likely to be hospitalized for COVID-19 than those without diabetes, even after adjustment for age, sex, and other underlying conditions. Diabetes also accounts for 30%-40% of severe COVID-19 cases and deaths. Among those with diabetes hospitalized for COVID-19, 21%-43% require intensive care, and the case fatality rate is about 25%.
In one of the few multivariate analyses that examined type 1 and type 2 diabetes separately, conducted in the U.K., the odds of in-hospital COVID-19–related deaths, compared with people without diabetes, were almost three times higher (odds ratio, 2.9) for individuals with type 1 diabetes and almost twice as high (OR, 1.8) for those with type 2, after adjustment for comorbidities.
The causes of death appear to be a combination of factors specific to the SARS-CoV-2 infection and to diabetes-related factors, Dr. Gregg said in an interview.
“Much of the increased risk is due to the fact that people with diabetes have more comorbid factors, but there are many other mechanisms that appear to further increase risk, including the inflammatory and immune responses of people with diabetes, and hyperglycemia appears to have an exacerbating effect by itself.”
Elevated glucose is clear risk factor for COVID-19 severity
Elevated A1c was identified among several other overall predictors of poor COVID-19 outcomes, including obesity as well as comorbid kidney and cardiovascular disease.
High blood glucose levels at the time of admission in people with previously diagnosed or undiagnosed diabetes emerged as a clear predictor of worse outcomes. For example, among 605 people hospitalized with COVID-19 in China, those with fasting plasma glucose 6.1-6.9 mmol/L (110-125 mg/dL) and ≥7 mmol/L (126 mg/dL) had odds ratios of poor outcomes within 28 days of 2.6 and 4.0 compared with FPG <6.1 mmol/L (110 mg/dL).
Population-based studies in the U.K. found that A1c levels measured months before COVID-19 hospitalization were associated with risk for intensive care unit admission and/or death, particularly among those with type 1 diabetes. Overall, the death rate was 36% higher for those with A1c of 9%-9.9% versus 6.5%-7%.
Despite the link between high A1c and death, there is as yet no clear evidence that normalizing blood glucose levels minimizes COVID-19 severity, Dr. Gregg said.
“There are data that suggest poor glycemic control is associated with higher risk of poor outcomes. This is indirect evidence that managing blood sugar will help, but more direct evidence is needed.”
Evidence gaps identified
Dr. Gregg and co-authors Marisa Sophiea, PhD, MSc, and Misghina Weldegiorgis, PhD, BSc, also from Imperial College London, identify three areas in which more data are needed.
First, more information is needed to determine whether exposure, infection, and hospitalization risks differ by diabetes status and how those factors affect outcomes. The same studies would also be important to identify how factors such as behavior, masking, and lockdown policies, risk factor control, and household/community environments affect risk in people with diabetes.
Second, studies are needed to better understand indirect effects of the pandemic, such as care and management factors. Some of these, such as the advent of telehealth, may turn out to be beneficial in the long run, they note.
Finally, the pandemic has “brought a wealth of natural experiments,” such as how vaccination programs and other interventions are affecting people with diabetes specifically. Finally, population studies are needed in many parts of the world beyond the U.S. and the U.K., where most of that work has been done thus far.
“Many of the most important unanswered questions lie in the potential indirect and long-term impact of the pandemic that require population-based studies,” Dr. Gregg said. “Most of our knowledge so far is from case series, which only assess patients from the time of hospitalization.”
Indeed, very little data are available for people with diabetes who get COVID-19 but are not hospitalized, so it’s not known whether they have a longer duration of illness or are at greater risk for “long COVID” than those without diabetes who experience COVID-19 at home.
“I have not seen published data on this yet, and it’s an important unanswered question,” Dr. Gregg said.
The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA could authorize COVID-19 vaccine for ages 5-11 in October
The timeline is based on the expectation that Pfizer will have enough data from clinical trials to request Food and Drug Administration emergency use authorization for the age group near the end of September. Then the FDA would likely make a decision about the vaccine’s safety and effectiveness in children within about 3 weeks, two sources told Reuters.
Anthony Fauci, MD, chief medical adviser to President Joe Biden and director of the National Institute of Allergy and Infectious Diseases, spoke about the timeline during an online town hall meeting Friday, Reuters reported. The meeting was attended by thousands of staff members at the National Institutes of Health.
If Pfizer submits paperwork to the FDA by the end of September, the vaccine could be available for kids around mid-October, Dr. Fauci said, and approval for the Moderna vaccine could come in November. Moderna will take about 3 weeks longer to collect and analyze data for ages 5-11.
Pfizer has said it would have enough data for ages 5-11 in September and would submit its documentation for FDA authorization soon after. Moderna told investors on Sept. 9 that data for ages 6-11 would be available by the end of the year.
On Sept. 10, the FDA said it would work to approve COVID-19 vaccines for children quickly once companies submit their data, according to Reuters. The agency said it would consider applications for emergency use, which would allow for faster approval.
Pfizer’s vaccine is the only one to receive full FDA approval, but only for people ages 16 and older. Adolescents ages 12-15 can receive the Pfizer vaccine under the FDA’s emergency use authorization.
For emergency use authorization, companies must submit 2 months of safety data versus 6 months for full approval. The FDA said on Sept. 10 that children in clinical trials should be monitored for at least 2 months to observe side effects.
BioNTech, Pfizer’s vaccine manufacturing partner, told a news outlet in Germany that it plans to request authorization globally for ages 5-11 in coming weeks, according to Reuters.
“Already over the next few weeks, we will file the results of our trial in 5- to 11-year-olds with regulators across the world and will request approval of the vaccine in this age group, also here in Europe,” Oezlem Tuereci, MD, the chief medical officer for BioNTech, told Der Spiegel.
The company is completing the final production steps to make the vaccine at lower doses for the younger age group, she said. Pfizer and BioNTech will also seek vaccine approval for ages 6 months to 2 years later this year.
“Things are looking good, everything is going according to plan,” Ugur Sahin, MD, the CEO of BioNTech, told Der Spiegel.
A version of this article first appeared on WebMD.com.
The timeline is based on the expectation that Pfizer will have enough data from clinical trials to request Food and Drug Administration emergency use authorization for the age group near the end of September. Then the FDA would likely make a decision about the vaccine’s safety and effectiveness in children within about 3 weeks, two sources told Reuters.
Anthony Fauci, MD, chief medical adviser to President Joe Biden and director of the National Institute of Allergy and Infectious Diseases, spoke about the timeline during an online town hall meeting Friday, Reuters reported. The meeting was attended by thousands of staff members at the National Institutes of Health.
If Pfizer submits paperwork to the FDA by the end of September, the vaccine could be available for kids around mid-October, Dr. Fauci said, and approval for the Moderna vaccine could come in November. Moderna will take about 3 weeks longer to collect and analyze data for ages 5-11.
Pfizer has said it would have enough data for ages 5-11 in September and would submit its documentation for FDA authorization soon after. Moderna told investors on Sept. 9 that data for ages 6-11 would be available by the end of the year.
On Sept. 10, the FDA said it would work to approve COVID-19 vaccines for children quickly once companies submit their data, according to Reuters. The agency said it would consider applications for emergency use, which would allow for faster approval.
Pfizer’s vaccine is the only one to receive full FDA approval, but only for people ages 16 and older. Adolescents ages 12-15 can receive the Pfizer vaccine under the FDA’s emergency use authorization.
For emergency use authorization, companies must submit 2 months of safety data versus 6 months for full approval. The FDA said on Sept. 10 that children in clinical trials should be monitored for at least 2 months to observe side effects.
BioNTech, Pfizer’s vaccine manufacturing partner, told a news outlet in Germany that it plans to request authorization globally for ages 5-11 in coming weeks, according to Reuters.
“Already over the next few weeks, we will file the results of our trial in 5- to 11-year-olds with regulators across the world and will request approval of the vaccine in this age group, also here in Europe,” Oezlem Tuereci, MD, the chief medical officer for BioNTech, told Der Spiegel.
The company is completing the final production steps to make the vaccine at lower doses for the younger age group, she said. Pfizer and BioNTech will also seek vaccine approval for ages 6 months to 2 years later this year.
“Things are looking good, everything is going according to plan,” Ugur Sahin, MD, the CEO of BioNTech, told Der Spiegel.
A version of this article first appeared on WebMD.com.
The timeline is based on the expectation that Pfizer will have enough data from clinical trials to request Food and Drug Administration emergency use authorization for the age group near the end of September. Then the FDA would likely make a decision about the vaccine’s safety and effectiveness in children within about 3 weeks, two sources told Reuters.
Anthony Fauci, MD, chief medical adviser to President Joe Biden and director of the National Institute of Allergy and Infectious Diseases, spoke about the timeline during an online town hall meeting Friday, Reuters reported. The meeting was attended by thousands of staff members at the National Institutes of Health.
If Pfizer submits paperwork to the FDA by the end of September, the vaccine could be available for kids around mid-October, Dr. Fauci said, and approval for the Moderna vaccine could come in November. Moderna will take about 3 weeks longer to collect and analyze data for ages 5-11.
Pfizer has said it would have enough data for ages 5-11 in September and would submit its documentation for FDA authorization soon after. Moderna told investors on Sept. 9 that data for ages 6-11 would be available by the end of the year.
On Sept. 10, the FDA said it would work to approve COVID-19 vaccines for children quickly once companies submit their data, according to Reuters. The agency said it would consider applications for emergency use, which would allow for faster approval.
Pfizer’s vaccine is the only one to receive full FDA approval, but only for people ages 16 and older. Adolescents ages 12-15 can receive the Pfizer vaccine under the FDA’s emergency use authorization.
For emergency use authorization, companies must submit 2 months of safety data versus 6 months for full approval. The FDA said on Sept. 10 that children in clinical trials should be monitored for at least 2 months to observe side effects.
BioNTech, Pfizer’s vaccine manufacturing partner, told a news outlet in Germany that it plans to request authorization globally for ages 5-11 in coming weeks, according to Reuters.
“Already over the next few weeks, we will file the results of our trial in 5- to 11-year-olds with regulators across the world and will request approval of the vaccine in this age group, also here in Europe,” Oezlem Tuereci, MD, the chief medical officer for BioNTech, told Der Spiegel.
The company is completing the final production steps to make the vaccine at lower doses for the younger age group, she said. Pfizer and BioNTech will also seek vaccine approval for ages 6 months to 2 years later this year.
“Things are looking good, everything is going according to plan,” Ugur Sahin, MD, the CEO of BioNTech, told Der Spiegel.
A version of this article first appeared on WebMD.com.
Virtual Respiratory Urgent Clinics for COVID-19 Symptoms
Virtual care (VC) has emerged as an effective mode of health care delivery especially in settings where significant barriers to traditional in-person visits exist; a large systematic review supports feasibility of telemedicine in primary care and suggests that telemedicine is at least as effective as traditional care.1 Nevertheless, broad adoption of VC into practice has lagged, impeded by government and private insurance reimbursement requirements as well as the persistent belief that care can best be delivered in person.2-4 Before the COVID-19 pandemic, states that enacted parity legislation that required private insurance companies to provide reimbursement coverage for telehealth services saw a significant increase in the number of outpatient telehealth visits (about ≥ 30% odds compared with nonparity states).3
With the onset of the COVID-19 pandemic, in-person medical appointments were converted to VC visits to reduce increased exposure risks to patients and health care workers.5 Prior government and private sector policies were suspended, and payment restrictions lifted, enabling adoption of VC modalities to rapidly accommodate the emergent need and Centers for Disease Control and Prevention (CDC) recommendations for virtual care.6-11
The CDC guidelines on managing operations during the COVID-19 pandemic highlighted the need to provide care in the safest way for patients and health care personnel and emphasized the importance of optimizing telehealth services. The federal government facilitated telehealth during the COVID-19 pandemic via temporary measures under the COVID-19 public health emergency declaration. This included Health Insurance Portability and Accountability Act flexibility to use everyday technology for VC visits, regulatory changes to deliver services to Medicare and Medicaid patients, permission of telehealth services across state lines, and prescribing of controlled substances via telehealth without an in-person medical evaluation.7
In response, health care providers (HCPs) and health care organizations created or expanded on existing telehealth infrastructure, developing virtual urgent care centers and telephone-based programs to evaluate patients remotely via screening questions that triaged them to a correct level of response, with possible subsequent virtual physician evaluation if indicated.12,13
The Veterans Health Administration (VHA) also shifted to a VC model in response to COVID-19 guided by a unique perspective from a well-developed prior VC experience.14-16 As a federally funded system, the VHA depends on workload documentation for budgeting. Since 2015, the VHA has provided workload credit and incentivized HCPs (via pay for performance) for the use of VC, including telephone visits, video visits, and secure messaging. These incentives resulted in higher rates of telehealth utilization before the COVID-19 pandemic compared with the private sector (with 4.2% and 0.7% of visits within the VHA being telephone and video visits, respectively, compared with telehealth utilization rates of 1.0% for Medicare recipients and 1.1% in an all-payer database).16
Historically, VHA care has successfully transitioned from in-person care models to exclusively virtual modalities to prevent suspension of medical services during natural disasters. Studies performed during these periods, specifically during the 2017 hurricane season (during which multiple VHA hospitals were closed or had limited in-person service available), supported telehealth as an efficient health care delivery method, and even recommended expanding telehealth services within non-VHA environments to accommodate needs of the general public during crises and postdisaster health care delivery.17
Armed with both a well-established telehealth infrastructure and prior knowledge gained from successful systemwide implementation of virtual care during times of disaster, US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACHS) primary care quickly transitioned to a VC model in response to COVID-19.16 Early in the pandemic, a rapid transition to virtual care (RTVC) model was developed, including implementation of virtual respiratory urgent clinics (VRUCs), defined as virtual respiratory symptom triage clinics, staffed by primary care providers (PCPs) aimed at minimizing patient and health care worker exposure risk.
Methods
VACHS consists of 8 primary care sites, including a major tertiary care center, a smaller medical center with full ambulatory services, and 6 community-based outpatient clinics with only primary care and mental health. There are 80 individual PCPs delivering care to 58,058 veterans. VRUCs were established during the COVID-19 pandemic to cover patients across the entire health care system, using a rotational schedule of VA PCPs.
COVID-19 Urgent Clinics Program
Within the first few weeks of the pandemic, VACHS primary care established VRUCS to provide expeditious virtual assessment of respiratory or flu-like symptoms. Using the established telehealth system, the intervention aimed to provide emergent screening, testing, and care to those with potential COVID-19 infections. The model also was designed to minimize exposures to the health care workforce and patients.
Retrospective analysis was performed using information obtained from the electronic health record (EHR) database to describe the characteristics of patients who received care through the VRUCs, such as demographics, era of military service, COVID-19 testing rates and results, as well as subsequent emergency department (ED) visits and hospital admissions. A secondary aim included collection of additional qualitative data via a random sample chart review.
Virtual clinics were established January 22, 2020, and data were analyzed over the next 3 months. Data were retrieved and analyzed from the EHR, and codes were used to categorize the VRUCs.
Results
A total of 445 unique patients used these clinics during this period. Unique patients were defined as individual patients (some may have used a clinic more than once but were counted only once). Of this group, 82% were male, and 48% served in the Gulf War era (1990 to present). A total of 51% of patients received a COVID-19 test (clinics began before wide testing availability), and 10% tested positive. Of all patients using the clinics, approximately 5% were admitted to the hospital, and 18% had at least 1 subsequent ED visit (Table).
A secondary aim included review of a random sample of 99 patient charts to gain additional information regarding whether the patient was given appropriate isolation precautions, was in a high-exposure occupation (eg, could expose a large number of people), and whether there was appropriate documentation of goals of care, health care proxy or referral to social work to discuss advance directives. In addition, we calculated the average length of time between patients’ initial contact with the health care system call center and the return call by the PCP (wait time).Of charts reviewed, the majority (71%) had documentation of appropriate isolation precautions. Although 25% of patients had documentation of a high-risk profession with potential to expose many people, more than half of the patients had no documentation of occupation. Most patients (86%) had no updated documentation regarding goals of care, health care proxy, or advance directives in their urgent care VC visit. The average time between the patient initiating contact with the health care system call center and a return call to the patient from a PCP was 104 minutes (excluding calls received after 3:30
Discussion
This analysis adds to the growing literature on use of VC during the COVID-19 pandemic. Specifically, we describe the population of patients who used VRUCs within a large health care system in a RTVC. This analysis was limited by lack of available testing during the initial phase of the pandemic, which contributed to the lower than expected rates of testing and test positivity in patients managed via VRUCs. In addition, chart review data are limited as the data includes only what was documented during the visit and not the entire discussion during the encounter.
Several important outcomes from this analysis can be applied to interventions in the future, which may have large public health implications: Several hundred patients who reported respiratory symptoms were expeditiously evaluated by a PCP using VC. The average wait time to full clinical assessment was about 1.5 hours. This short duration between contact and evaluation permitted early education about isolation precautions, which may have minimized spread. In addition, this innovation kept patients out of the medical center, eliminating chains of transmission to other vulnerable patients and health care workers.
Our retrospective chart review also revealed that more than half the patients were not queried about their occupation, but of those that were asked, a significant number were in high-risk professions potentially exposing large numbers of people. This would be an important aspect to add to future templated notes to minimize work-related exposures. Also, we identified that few HCPs discussed goals of care with patients. Given the nature of COVID-19 and potential for rapid decompensation especially in vulnerable patients, this also would be important to include in the future.
Conclusions
VC urgent care clinics to address possible COVID-19 symptoms facilitated expeditious PCP assessment while keeping potentially contagious patients outside of high-risk health care environments. Streamlining and optimizing clinical VC assessments will be imperative to future management of COVID-19 and potentially to other future infectious pandemics. This includes development of templated notes incorporating counseling regarding appropriate isolation, questions about high-contact occupations, and goals of care discussions.
Acknowledgment
The authors thank Robert F. Walsh, MHA.
1. Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
2. Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
3. Harvey JB, Valenta S, Simpson K, Lyles M, McElligott J. Utilization of outpatient telehealth services in parity and nonparity states 2010-2015. Telemed J E Health. 2019;25(2):132-136. doi:10.1089/tmj.2017.0265
4. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/NEJMra1601705
5. Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care. 2020;26(4):147-148. doi:10.37765/ajmc.2020.42784
6. Centers for Disease Control and Prevention. Healthcare facility guidance. Updated April 17, 2021. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html
7. US Department of Health and Human Services, Health Resources and Services Administration. Policy changes during COVID-19. Accessed August 20, 2021. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency
8. Coronavirus Preparedness and Response Supplemental Appropriation Act of 2020. 134 Stat. 146. Published February 2, 2021. Accessed August 20, 2021. https://www.govinfo.gov/content/pkg/CREC-2021-02-02/html/CREC-2021-02-02-pt1-PgS226.htm
9. US Department of Health and Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Updated January 20, 2021. Accessed August 20, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
10. Centers for Medicare and Medicaid Services. Coverage and payment related to COVID-19 Medicare. 2020. Published March 23, 2020. Accessed August 20, 2021. https://www.cms.gov/files/document/03052020-medicare-covid-19-fact-sheet.pdf
11. American Telemedicine Association. ATA commends 2020 Congress for giving HHS authority to waive restrictions on telehealth for Medicare beneficiaries in response to the COVID-19 outbreak [press release]. Published March 5, 2020. Accessed August 20, 2021. https://www.americantelemed.org/press-releases/ata-commends-congress-for-waiving-restrictions-on-telehealth-for-medicare-beneficiaries-in-response-to-the-covid-19-outbreak
12. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681. doi:10.1056/NEJMp2003539
13. Khairat S, Meng C, Xu Y, Edson B, Gianforcaro R. Interpreting COVID-19 and Virtual Care Trends: Cohort Study. JMIR Public Health Surveill. 2020;6(2):e18811. Published 2020 Apr 15. doi:10.2196/18811
14. Ferguson JM, Jacobs J, Yefimova M, Greene L, Heyworth L, Zulman DM. Virtual care expansion in the Veterans Health Administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2021;28(3):453-462. doi:10.1093/jamia/ocaa284
15. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
16. Spelman JF, Brienza R, Walsh RF, et al. A model for rapid transition to virtual care, VA Connecticut primary care response to COVID-19. J Gen Intern Med. 2020;35(10):3073-3076. doi:10.1007/s11606-020-06041-4
17. Der-Martirosian C, Chu K, Dobalian A. Use of telehealth to improve access to care at the United States Department of Veterans Affairs during the 2017 Atlantic hurricane season [published online ahead of print, 2020 Apr 13]. Disaster Med Public Health Prep. 2020;1-5. doi:10.1017/dmp.2020.88
Virtual care (VC) has emerged as an effective mode of health care delivery especially in settings where significant barriers to traditional in-person visits exist; a large systematic review supports feasibility of telemedicine in primary care and suggests that telemedicine is at least as effective as traditional care.1 Nevertheless, broad adoption of VC into practice has lagged, impeded by government and private insurance reimbursement requirements as well as the persistent belief that care can best be delivered in person.2-4 Before the COVID-19 pandemic, states that enacted parity legislation that required private insurance companies to provide reimbursement coverage for telehealth services saw a significant increase in the number of outpatient telehealth visits (about ≥ 30% odds compared with nonparity states).3
With the onset of the COVID-19 pandemic, in-person medical appointments were converted to VC visits to reduce increased exposure risks to patients and health care workers.5 Prior government and private sector policies were suspended, and payment restrictions lifted, enabling adoption of VC modalities to rapidly accommodate the emergent need and Centers for Disease Control and Prevention (CDC) recommendations for virtual care.6-11
The CDC guidelines on managing operations during the COVID-19 pandemic highlighted the need to provide care in the safest way for patients and health care personnel and emphasized the importance of optimizing telehealth services. The federal government facilitated telehealth during the COVID-19 pandemic via temporary measures under the COVID-19 public health emergency declaration. This included Health Insurance Portability and Accountability Act flexibility to use everyday technology for VC visits, regulatory changes to deliver services to Medicare and Medicaid patients, permission of telehealth services across state lines, and prescribing of controlled substances via telehealth without an in-person medical evaluation.7
In response, health care providers (HCPs) and health care organizations created or expanded on existing telehealth infrastructure, developing virtual urgent care centers and telephone-based programs to evaluate patients remotely via screening questions that triaged them to a correct level of response, with possible subsequent virtual physician evaluation if indicated.12,13
The Veterans Health Administration (VHA) also shifted to a VC model in response to COVID-19 guided by a unique perspective from a well-developed prior VC experience.14-16 As a federally funded system, the VHA depends on workload documentation for budgeting. Since 2015, the VHA has provided workload credit and incentivized HCPs (via pay for performance) for the use of VC, including telephone visits, video visits, and secure messaging. These incentives resulted in higher rates of telehealth utilization before the COVID-19 pandemic compared with the private sector (with 4.2% and 0.7% of visits within the VHA being telephone and video visits, respectively, compared with telehealth utilization rates of 1.0% for Medicare recipients and 1.1% in an all-payer database).16
Historically, VHA care has successfully transitioned from in-person care models to exclusively virtual modalities to prevent suspension of medical services during natural disasters. Studies performed during these periods, specifically during the 2017 hurricane season (during which multiple VHA hospitals were closed or had limited in-person service available), supported telehealth as an efficient health care delivery method, and even recommended expanding telehealth services within non-VHA environments to accommodate needs of the general public during crises and postdisaster health care delivery.17
Armed with both a well-established telehealth infrastructure and prior knowledge gained from successful systemwide implementation of virtual care during times of disaster, US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACHS) primary care quickly transitioned to a VC model in response to COVID-19.16 Early in the pandemic, a rapid transition to virtual care (RTVC) model was developed, including implementation of virtual respiratory urgent clinics (VRUCs), defined as virtual respiratory symptom triage clinics, staffed by primary care providers (PCPs) aimed at minimizing patient and health care worker exposure risk.
Methods
VACHS consists of 8 primary care sites, including a major tertiary care center, a smaller medical center with full ambulatory services, and 6 community-based outpatient clinics with only primary care and mental health. There are 80 individual PCPs delivering care to 58,058 veterans. VRUCs were established during the COVID-19 pandemic to cover patients across the entire health care system, using a rotational schedule of VA PCPs.
COVID-19 Urgent Clinics Program
Within the first few weeks of the pandemic, VACHS primary care established VRUCS to provide expeditious virtual assessment of respiratory or flu-like symptoms. Using the established telehealth system, the intervention aimed to provide emergent screening, testing, and care to those with potential COVID-19 infections. The model also was designed to minimize exposures to the health care workforce and patients.
Retrospective analysis was performed using information obtained from the electronic health record (EHR) database to describe the characteristics of patients who received care through the VRUCs, such as demographics, era of military service, COVID-19 testing rates and results, as well as subsequent emergency department (ED) visits and hospital admissions. A secondary aim included collection of additional qualitative data via a random sample chart review.
Virtual clinics were established January 22, 2020, and data were analyzed over the next 3 months. Data were retrieved and analyzed from the EHR, and codes were used to categorize the VRUCs.
Results
A total of 445 unique patients used these clinics during this period. Unique patients were defined as individual patients (some may have used a clinic more than once but were counted only once). Of this group, 82% were male, and 48% served in the Gulf War era (1990 to present). A total of 51% of patients received a COVID-19 test (clinics began before wide testing availability), and 10% tested positive. Of all patients using the clinics, approximately 5% were admitted to the hospital, and 18% had at least 1 subsequent ED visit (Table).
A secondary aim included review of a random sample of 99 patient charts to gain additional information regarding whether the patient was given appropriate isolation precautions, was in a high-exposure occupation (eg, could expose a large number of people), and whether there was appropriate documentation of goals of care, health care proxy or referral to social work to discuss advance directives. In addition, we calculated the average length of time between patients’ initial contact with the health care system call center and the return call by the PCP (wait time).Of charts reviewed, the majority (71%) had documentation of appropriate isolation precautions. Although 25% of patients had documentation of a high-risk profession with potential to expose many people, more than half of the patients had no documentation of occupation. Most patients (86%) had no updated documentation regarding goals of care, health care proxy, or advance directives in their urgent care VC visit. The average time between the patient initiating contact with the health care system call center and a return call to the patient from a PCP was 104 minutes (excluding calls received after 3:30
Discussion
This analysis adds to the growing literature on use of VC during the COVID-19 pandemic. Specifically, we describe the population of patients who used VRUCs within a large health care system in a RTVC. This analysis was limited by lack of available testing during the initial phase of the pandemic, which contributed to the lower than expected rates of testing and test positivity in patients managed via VRUCs. In addition, chart review data are limited as the data includes only what was documented during the visit and not the entire discussion during the encounter.
Several important outcomes from this analysis can be applied to interventions in the future, which may have large public health implications: Several hundred patients who reported respiratory symptoms were expeditiously evaluated by a PCP using VC. The average wait time to full clinical assessment was about 1.5 hours. This short duration between contact and evaluation permitted early education about isolation precautions, which may have minimized spread. In addition, this innovation kept patients out of the medical center, eliminating chains of transmission to other vulnerable patients and health care workers.
Our retrospective chart review also revealed that more than half the patients were not queried about their occupation, but of those that were asked, a significant number were in high-risk professions potentially exposing large numbers of people. This would be an important aspect to add to future templated notes to minimize work-related exposures. Also, we identified that few HCPs discussed goals of care with patients. Given the nature of COVID-19 and potential for rapid decompensation especially in vulnerable patients, this also would be important to include in the future.
Conclusions
VC urgent care clinics to address possible COVID-19 symptoms facilitated expeditious PCP assessment while keeping potentially contagious patients outside of high-risk health care environments. Streamlining and optimizing clinical VC assessments will be imperative to future management of COVID-19 and potentially to other future infectious pandemics. This includes development of templated notes incorporating counseling regarding appropriate isolation, questions about high-contact occupations, and goals of care discussions.
Acknowledgment
The authors thank Robert F. Walsh, MHA.
Virtual care (VC) has emerged as an effective mode of health care delivery especially in settings where significant barriers to traditional in-person visits exist; a large systematic review supports feasibility of telemedicine in primary care and suggests that telemedicine is at least as effective as traditional care.1 Nevertheless, broad adoption of VC into practice has lagged, impeded by government and private insurance reimbursement requirements as well as the persistent belief that care can best be delivered in person.2-4 Before the COVID-19 pandemic, states that enacted parity legislation that required private insurance companies to provide reimbursement coverage for telehealth services saw a significant increase in the number of outpatient telehealth visits (about ≥ 30% odds compared with nonparity states).3
With the onset of the COVID-19 pandemic, in-person medical appointments were converted to VC visits to reduce increased exposure risks to patients and health care workers.5 Prior government and private sector policies were suspended, and payment restrictions lifted, enabling adoption of VC modalities to rapidly accommodate the emergent need and Centers for Disease Control and Prevention (CDC) recommendations for virtual care.6-11
The CDC guidelines on managing operations during the COVID-19 pandemic highlighted the need to provide care in the safest way for patients and health care personnel and emphasized the importance of optimizing telehealth services. The federal government facilitated telehealth during the COVID-19 pandemic via temporary measures under the COVID-19 public health emergency declaration. This included Health Insurance Portability and Accountability Act flexibility to use everyday technology for VC visits, regulatory changes to deliver services to Medicare and Medicaid patients, permission of telehealth services across state lines, and prescribing of controlled substances via telehealth without an in-person medical evaluation.7
In response, health care providers (HCPs) and health care organizations created or expanded on existing telehealth infrastructure, developing virtual urgent care centers and telephone-based programs to evaluate patients remotely via screening questions that triaged them to a correct level of response, with possible subsequent virtual physician evaluation if indicated.12,13
The Veterans Health Administration (VHA) also shifted to a VC model in response to COVID-19 guided by a unique perspective from a well-developed prior VC experience.14-16 As a federally funded system, the VHA depends on workload documentation for budgeting. Since 2015, the VHA has provided workload credit and incentivized HCPs (via pay for performance) for the use of VC, including telephone visits, video visits, and secure messaging. These incentives resulted in higher rates of telehealth utilization before the COVID-19 pandemic compared with the private sector (with 4.2% and 0.7% of visits within the VHA being telephone and video visits, respectively, compared with telehealth utilization rates of 1.0% for Medicare recipients and 1.1% in an all-payer database).16
Historically, VHA care has successfully transitioned from in-person care models to exclusively virtual modalities to prevent suspension of medical services during natural disasters. Studies performed during these periods, specifically during the 2017 hurricane season (during which multiple VHA hospitals were closed or had limited in-person service available), supported telehealth as an efficient health care delivery method, and even recommended expanding telehealth services within non-VHA environments to accommodate needs of the general public during crises and postdisaster health care delivery.17
Armed with both a well-established telehealth infrastructure and prior knowledge gained from successful systemwide implementation of virtual care during times of disaster, US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACHS) primary care quickly transitioned to a VC model in response to COVID-19.16 Early in the pandemic, a rapid transition to virtual care (RTVC) model was developed, including implementation of virtual respiratory urgent clinics (VRUCs), defined as virtual respiratory symptom triage clinics, staffed by primary care providers (PCPs) aimed at minimizing patient and health care worker exposure risk.
Methods
VACHS consists of 8 primary care sites, including a major tertiary care center, a smaller medical center with full ambulatory services, and 6 community-based outpatient clinics with only primary care and mental health. There are 80 individual PCPs delivering care to 58,058 veterans. VRUCs were established during the COVID-19 pandemic to cover patients across the entire health care system, using a rotational schedule of VA PCPs.
COVID-19 Urgent Clinics Program
Within the first few weeks of the pandemic, VACHS primary care established VRUCS to provide expeditious virtual assessment of respiratory or flu-like symptoms. Using the established telehealth system, the intervention aimed to provide emergent screening, testing, and care to those with potential COVID-19 infections. The model also was designed to minimize exposures to the health care workforce and patients.
Retrospective analysis was performed using information obtained from the electronic health record (EHR) database to describe the characteristics of patients who received care through the VRUCs, such as demographics, era of military service, COVID-19 testing rates and results, as well as subsequent emergency department (ED) visits and hospital admissions. A secondary aim included collection of additional qualitative data via a random sample chart review.
Virtual clinics were established January 22, 2020, and data were analyzed over the next 3 months. Data were retrieved and analyzed from the EHR, and codes were used to categorize the VRUCs.
Results
A total of 445 unique patients used these clinics during this period. Unique patients were defined as individual patients (some may have used a clinic more than once but were counted only once). Of this group, 82% were male, and 48% served in the Gulf War era (1990 to present). A total of 51% of patients received a COVID-19 test (clinics began before wide testing availability), and 10% tested positive. Of all patients using the clinics, approximately 5% were admitted to the hospital, and 18% had at least 1 subsequent ED visit (Table).
A secondary aim included review of a random sample of 99 patient charts to gain additional information regarding whether the patient was given appropriate isolation precautions, was in a high-exposure occupation (eg, could expose a large number of people), and whether there was appropriate documentation of goals of care, health care proxy or referral to social work to discuss advance directives. In addition, we calculated the average length of time between patients’ initial contact with the health care system call center and the return call by the PCP (wait time).Of charts reviewed, the majority (71%) had documentation of appropriate isolation precautions. Although 25% of patients had documentation of a high-risk profession with potential to expose many people, more than half of the patients had no documentation of occupation. Most patients (86%) had no updated documentation regarding goals of care, health care proxy, or advance directives in their urgent care VC visit. The average time between the patient initiating contact with the health care system call center and a return call to the patient from a PCP was 104 minutes (excluding calls received after 3:30
Discussion
This analysis adds to the growing literature on use of VC during the COVID-19 pandemic. Specifically, we describe the population of patients who used VRUCs within a large health care system in a RTVC. This analysis was limited by lack of available testing during the initial phase of the pandemic, which contributed to the lower than expected rates of testing and test positivity in patients managed via VRUCs. In addition, chart review data are limited as the data includes only what was documented during the visit and not the entire discussion during the encounter.
Several important outcomes from this analysis can be applied to interventions in the future, which may have large public health implications: Several hundred patients who reported respiratory symptoms were expeditiously evaluated by a PCP using VC. The average wait time to full clinical assessment was about 1.5 hours. This short duration between contact and evaluation permitted early education about isolation precautions, which may have minimized spread. In addition, this innovation kept patients out of the medical center, eliminating chains of transmission to other vulnerable patients and health care workers.
Our retrospective chart review also revealed that more than half the patients were not queried about their occupation, but of those that were asked, a significant number were in high-risk professions potentially exposing large numbers of people. This would be an important aspect to add to future templated notes to minimize work-related exposures. Also, we identified that few HCPs discussed goals of care with patients. Given the nature of COVID-19 and potential for rapid decompensation especially in vulnerable patients, this also would be important to include in the future.
Conclusions
VC urgent care clinics to address possible COVID-19 symptoms facilitated expeditious PCP assessment while keeping potentially contagious patients outside of high-risk health care environments. Streamlining and optimizing clinical VC assessments will be imperative to future management of COVID-19 and potentially to other future infectious pandemics. This includes development of templated notes incorporating counseling regarding appropriate isolation, questions about high-contact occupations, and goals of care discussions.
Acknowledgment
The authors thank Robert F. Walsh, MHA.
1. Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
2. Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
3. Harvey JB, Valenta S, Simpson K, Lyles M, McElligott J. Utilization of outpatient telehealth services in parity and nonparity states 2010-2015. Telemed J E Health. 2019;25(2):132-136. doi:10.1089/tmj.2017.0265
4. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/NEJMra1601705
5. Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care. 2020;26(4):147-148. doi:10.37765/ajmc.2020.42784
6. Centers for Disease Control and Prevention. Healthcare facility guidance. Updated April 17, 2021. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html
7. US Department of Health and Human Services, Health Resources and Services Administration. Policy changes during COVID-19. Accessed August 20, 2021. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency
8. Coronavirus Preparedness and Response Supplemental Appropriation Act of 2020. 134 Stat. 146. Published February 2, 2021. Accessed August 20, 2021. https://www.govinfo.gov/content/pkg/CREC-2021-02-02/html/CREC-2021-02-02-pt1-PgS226.htm
9. US Department of Health and Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Updated January 20, 2021. Accessed August 20, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
10. Centers for Medicare and Medicaid Services. Coverage and payment related to COVID-19 Medicare. 2020. Published March 23, 2020. Accessed August 20, 2021. https://www.cms.gov/files/document/03052020-medicare-covid-19-fact-sheet.pdf
11. American Telemedicine Association. ATA commends 2020 Congress for giving HHS authority to waive restrictions on telehealth for Medicare beneficiaries in response to the COVID-19 outbreak [press release]. Published March 5, 2020. Accessed August 20, 2021. https://www.americantelemed.org/press-releases/ata-commends-congress-for-waiving-restrictions-on-telehealth-for-medicare-beneficiaries-in-response-to-the-covid-19-outbreak
12. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681. doi:10.1056/NEJMp2003539
13. Khairat S, Meng C, Xu Y, Edson B, Gianforcaro R. Interpreting COVID-19 and Virtual Care Trends: Cohort Study. JMIR Public Health Surveill. 2020;6(2):e18811. Published 2020 Apr 15. doi:10.2196/18811
14. Ferguson JM, Jacobs J, Yefimova M, Greene L, Heyworth L, Zulman DM. Virtual care expansion in the Veterans Health Administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2021;28(3):453-462. doi:10.1093/jamia/ocaa284
15. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
16. Spelman JF, Brienza R, Walsh RF, et al. A model for rapid transition to virtual care, VA Connecticut primary care response to COVID-19. J Gen Intern Med. 2020;35(10):3073-3076. doi:10.1007/s11606-020-06041-4
17. Der-Martirosian C, Chu K, Dobalian A. Use of telehealth to improve access to care at the United States Department of Veterans Affairs during the 2017 Atlantic hurricane season [published online ahead of print, 2020 Apr 13]. Disaster Med Public Health Prep. 2020;1-5. doi:10.1017/dmp.2020.88
1. Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
2. Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
3. Harvey JB, Valenta S, Simpson K, Lyles M, McElligott J. Utilization of outpatient telehealth services in parity and nonparity states 2010-2015. Telemed J E Health. 2019;25(2):132-136. doi:10.1089/tmj.2017.0265
4. Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/NEJMra1601705
5. Rockwell KL, Gilroy AS. Incorporating telemedicine as part of COVID-19 outbreak response systems. Am J Manag Care. 2020;26(4):147-148. doi:10.37765/ajmc.2020.42784
6. Centers for Disease Control and Prevention. Healthcare facility guidance. Updated April 17, 2021. Accessed August 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care.html
7. US Department of Health and Human Services, Health Resources and Services Administration. Policy changes during COVID-19. Accessed August 20, 2021. https://telehealth.hhs.gov/providers/policy-changes-during-the-covid-19-public-health-emergency
8. Coronavirus Preparedness and Response Supplemental Appropriation Act of 2020. 134 Stat. 146. Published February 2, 2021. Accessed August 20, 2021. https://www.govinfo.gov/content/pkg/CREC-2021-02-02/html/CREC-2021-02-02-pt1-PgS226.htm
9. US Department of Health and Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. Updated January 20, 2021. Accessed August 20, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
10. Centers for Medicare and Medicaid Services. Coverage and payment related to COVID-19 Medicare. 2020. Published March 23, 2020. Accessed August 20, 2021. https://www.cms.gov/files/document/03052020-medicare-covid-19-fact-sheet.pdf
11. American Telemedicine Association. ATA commends 2020 Congress for giving HHS authority to waive restrictions on telehealth for Medicare beneficiaries in response to the COVID-19 outbreak [press release]. Published March 5, 2020. Accessed August 20, 2021. https://www.americantelemed.org/press-releases/ata-commends-congress-for-waiving-restrictions-on-telehealth-for-medicare-beneficiaries-in-response-to-the-covid-19-outbreak
12. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681. doi:10.1056/NEJMp2003539
13. Khairat S, Meng C, Xu Y, Edson B, Gianforcaro R. Interpreting COVID-19 and Virtual Care Trends: Cohort Study. JMIR Public Health Surveill. 2020;6(2):e18811. Published 2020 Apr 15. doi:10.2196/18811
14. Ferguson JM, Jacobs J, Yefimova M, Greene L, Heyworth L, Zulman DM. Virtual care expansion in the Veterans Health Administration during the COVID-19 pandemic: clinical services and patient characteristics associated with utilization. J Am Med Inform Assoc. 2021;28(3):453-462. doi:10.1093/jamia/ocaa284
15. Baum A, Kaboli PJ, Schwartz MD. Reduced in-person and increased telehealth outpatient visits during the COVID-19 Pandemic. Ann Intern Med. 2021;174(1):129-131. doi:10.7326/M20-3026
16. Spelman JF, Brienza R, Walsh RF, et al. A model for rapid transition to virtual care, VA Connecticut primary care response to COVID-19. J Gen Intern Med. 2020;35(10):3073-3076. doi:10.1007/s11606-020-06041-4
17. Der-Martirosian C, Chu K, Dobalian A. Use of telehealth to improve access to care at the United States Department of Veterans Affairs during the 2017 Atlantic hurricane season [published online ahead of print, 2020 Apr 13]. Disaster Med Public Health Prep. 2020;1-5. doi:10.1017/dmp.2020.88