User login
Biden vaccine mandate rule could be ready within weeks
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
The emergency rule ordering large employers to require COVID-19 vaccines or weekly tests for their workers could be ready “within weeks,” officials said in a news briefing Sept. 10.
Labor Secretary Martin Walsh will oversee the Occupational Safety and Health Administration as the agency drafts what’s known as an emergency temporary standard, similar to the one that was issued a few months ago to protect health care workers during the pandemic.
The rule should be ready within weeks, said Jeff Zients, coordinator of the White House COVID-19 response team.
He said the ultimate goal of the president’s plan is to increase vaccinations as quickly as possible to keep schools open, the economy recovering, and to decrease hospitalizations and deaths from COVID.
Mr. Zients declined to set hard numbers around those goals, but other experts did.
“What we need to get to is 85% to 90% population immunity, and that’s going to be immunity both from vaccines and infections, before that really begins to have a substantial dampening effect on viral spread,” Ashish Jha, MD, dean of the Brown University School of Public Health, Providence, R.I., said on a call with reporters Sept. 9.
He said immunity needs to be that high because the Delta variant is so contagious.
Mandates are seen as the most effective way to increase immunity and do it quickly.
David Michaels, PhD, an epidemiologist and professor at George Washington University, Washington, says OSHA will have to work through a number of steps to develop the rule.
“OSHA will have to write a preamble explaining the standard, its justifications, its costs, and how it will be enforced,” says Dr. Michaels, who led OSHA for the Obama administration. After that, the rule will be reviewed by the White House. Then employers will have some time – typically 30 days – to comply.
In addition to drafting the standard, OSHA will oversee its enforcement.
Companies that refuse to follow the standard could be fined $13,600 per violation, Mr. Zients said.
Dr. Michaels said he doesn’t expect enforcement to be a big issue, and he said we’re likely to see the rule well before it is final.
“Most employers are law-abiding. When OSHA issues a standard, they try to meet whatever those requirements are, and generally that starts to happen when the rule is announced, even before it goes into effect,” he said.
The rule may face legal challenges as well. Several governors and state attorneys general, as well as the Republican National Committee, have promised lawsuits to stop the vaccine mandates.
Critics of the new mandates say they impinge on personal freedom and impose burdens on businesses.
But the president hit back at that notion Sept. 10.
“Look, I am so disappointed that, particularly some of the Republican governors, have been so cavalier with the health of these kids, so cavalier of the health of their communities,” President Biden told reporters.
“I don’t know of any scientist out there in this field who doesn’t think it makes considerable sense to do the six things I’ve suggested.”
Yet, others feel the new requirements didn’t go far enough.
“These are good steps in the right direction, but they’re not enough to get the job done,” said Leana Wen, MD, in an op-ed for The Washington Post.
Dr. Wen, an expert in public health, wondered why President Biden didn’t mandate vaccinations for plane and train travel. She was disappointed that children 12 and older weren’t required to be vaccinated, too.
“There are mandates for childhood immunizations in every state. The coronavirus vaccine should be no different,” she wrote.
Vaccines remain the cornerstone of U.S. plans to control the pandemic.
On Sept. 10, there was new research from the CDC and state health departments showing that the COVID-19 vaccines continue to be highly effective at preventing severe illness and death.
But the study also found that the vaccines became less effective in the United States after Delta became the dominant cause of infections here.
The study, which included more than 600,000 COVID-19 cases, analyzed breakthrough infections – cases where people got sick despite being fully vaccinated – in 13 jurisdictions in the United States between April 4 and July 17, 2021.
Epidemiologists compared breakthrough infections between two distinct points in time: Before and after the period when the Delta variant began causing most infections.
From April 4 to June 19, fully vaccinated people made up just 5% of cases, 7% of hospitalizations, and 8% of deaths. From June 20 to July 17, 18% of cases, 14% of hospitalizations, and 16% of deaths occurred in fully vaccinated people.
“After the week of June 20, 2021, when the SARS-CoV-2 Delta variant became predominant, the percentage of fully vaccinated persons among cases increased more than expected,” the study authors wrote.
Even after Delta swept the United States, fully vaccinated people were 5 times less likely to get a COVID-19 infection and more than 10 times less likely to be hospitalized or die from one.
“As we have shown in study after study, vaccination works,” CDC Director Rochelle Walensky, MD, said during the White House news briefing.
“We have the scientific tools we need to turn the corner on this pandemic. Vaccination works and will protect us from the severe complications of COVID-19,” she said.
A version of this article first appeared on WebMD.com.
The Delta Factor
Several weeks ago, I received a call from my brother who, though not a health care professional, wanted me to know he thought the public was being too critical of scientists and physicians who “are giving us the best advice they can about COVID. People think they should have all the answers. But this virus is complicated, and they don’t always know what is going to happen next.” What makes his charitable read of the public health situation remarkable is that he is a COVID-19 survivor of one of the first reported cases of Guillain-Barre syndrome, which several expert neurologists believe is the result of COVID-19. Like so many other COVID-19 long-haul patients, he is left with lingering symptoms and residual deficits.1
I use this personal story as the overture to this piece on why I am changing my opinion regarding a COVID-19 mandate for federal practitioners. In June I raised ethical concerns about compelling vaccination especially for service members of color based on a current and historical climate of mistrust and discrimination in health care that compulsory vaccination could exacerbate.2 Instead, I followed the lead of Secretary of Defense J. Lloyd Austin III and advocated continued education and encouragement for vaccine-hesitant troops.3 So in 2 months what has so radically changed to lead Secretary Austin and US Department of Veterans Affairs (VA) Secretary Denis R. McDonough to mandate vaccination for their workforce?4,5
I am calling the change the Delta Factor. This is not to be confused with the spy-thrillers that ironically involved rescuing a scientist! The Delta Factor is a catch-all phrase to cover the protean public health impacts of the devastating COVID-19 Delta variant now ravaging the country. Depending on the area of the country as of mid-August, the Centers for Disease Control and Prevention (CDC) estimated that 80% to > 90% of new cases were the Delta variant.6 An increasing number of these cases sadly are in children.7
According to the CDC, the Delta variant is more than twice as contagious as index or subsequent strains: making it about as contagious as chicken pox. The unvaccinated are the most susceptible to Delta and may develop more serious illness and risk of death than with other strains. Those who are fully vaccinated can still contract the virus although usually with milder cases. More worrisome is that individuals with these breakthrough infections have the same viral load as those without vaccinations, rendering them vectors of transmission, although for a shorter time than unvaccinated persons.8
The VA first mandated vaccination among its health care employees in July and then expanded it to all staff in August.9 The US Department of Defense (DoD) mandatory vaccination was announced prior to US Food and Drug Administration’s (FDA) full approval of the Pfizer-BioNTech vaccine.10 Secretary Austin asked President Biden to grant a waiver to permit mandatory vaccination even without full FDA approval, and Biden has indicated his support, but the full approval expedited the time line for implementation.11
Both agencies directly referenced Delta as a primary reason for their vaccination mandates. The VA argued that the mandate was necessary to protect the safety of veterans, while the DoD noted that vaccination was essential to ensure the health of the fighting force. In his initial announcement, Secretary McDonough explicitly mentioned the Delta variant as a primary reason for his decision. noting “it’s the best way to keep veterans safe, especially as the Delta variant spreads across the country.”4 Similarly, Secretary Austin declared, “We will also be keeping a close eye on infection rates, which are on the rise now due to the Delta variant and the impact these rates might have on our readiness.”5
VA and DoD leadership emphasized the safety and effectiveness of the vaccine and urged employees to voluntarily obtain the vaccine or obtain a religious or medical exemption. Those without such an exemption must adhere to masking, testing, and other restrictions.5 As anticipated in the earlier editorial, there has been opposition to the mandate from the workforce of the 2 agencies and their political supporters some of whom view vaccine mandates as violations of personal liberty and bodily integrity and for whom rampant disinformation has amplified entrenched distrust of the government.12
The decision to shift from voluntary to mandatory vaccination of federal employees responsible for the health care of veterans and the defense of citizens, which may seem
Finally and most important, for a vaccine or other public health intervention to be ethically mandated it must have a high probability of attaining a serious purpose: here preventing the harms of sickness and death especially in the most vulnerable. In July, the White House COVID-19 Response Team reported that “preliminary data from several states over the last few months suggest that 99.5% of deaths from COVID-19 in the United States were in unvaccinated people” and were preventable.15 Ethically, even as mandates are implemented across the federal workforce, efforts to educate, encourage, and empower vaccination especially among disenfranchised cohorts must continue. But as a recently leaked CDC internal document acknowledged about the Delta Factor, “the war has changed” and so has my opinion about mandating vaccination among those upon whose service depends the life and security of us all.16
1. CBS Good Morning. Christopher Cross on his near-fatal COVID illness. Published October 18, 2020. Accessed August 21, 2021. https://www.cbsnews.com/news/christopher-cross-on-his-near-fatal-covid-illness
2. Geppert CM. Mistrust and mandates: COVID-19 vaccination in the military. Fed Pract. 2021;38(6):254-255. doi:10.12788/fp.0143
3. Garmone J, US Department of Defense. Secretary of defense addresses vaccine hesitancy in the military. Published February 25, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military
4. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA mandates COVID-19 vaccines among its medical employees including VHA facilities staff [press release]. Published July 26, 2021. Accessed August 21, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5696
5. US Department of Defense, Secretary of Defense. Memorandum for all Department of Defense employees. Published August 9, 2021. Accessed August 23, 2021. https://media.defense.gov/2021/Aug/09/2002826254/-1/-1/0/MESSAGE-TO-THE-FORCE-MEMO-VACCINE.PDF
6. Centers for Disease Control and Prevention COVID data tracker. Variant proportions. Updated August 17, 2021. Accessed August 23, 2021. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
7. American Academy of Pediatrics. Children and COVID-19: state data level report. Updated August 23, 2021. Accessed August 23, 2021. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state|-level-data-report
8. Centers for Disease Control and Prevention. Delta variant: what we know about the science. Update August 19, 2021. Accessed August 23, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html
9. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands mandate for COVID-19 vaccines among VHA employees [press release]. Published August 12, 2021. Accessed August 23, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5703
10. US Food and Drug Administration. FDA approves first COVID-19 vaccine [press release]. Published August 23, 2021. Accessed August 23, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
11. Garamone J, US Department of Defense. Biden to approve Austin’s request to make COVID-19 vaccine mandatory for service members. Published August 9, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2724982/biden-to-approve-austins-request-to-make-covid-19-vaccine-mandatory-for-service
12. Watson J. Potential military vaccine mandate brings distrust, support. Associated Press. August 5, 2021. Accessed August 23, 2021. https://apnews.com/article/joe-biden-business-health-coronavirus-pandemic-6a0f94e11f5af1e0de740d44d7931d65
13. Giubilini A. Vaccination ethics. Br Med Bull. 2021;137(1):4-12. doi:10.1093/bmb/ldaa036
14. Steinhauer J. Military and V.A. struggle with vaccination rates in their ranks. The New York Times. July 1, 2021. Accessed August 23, 2021. https://www.nytimes.com/2021/07/01/us/politics/military-va-vaccines.html
15. The White House. Press briefing by White House COVID-19 Response Team and public health officials. Published July 8, 2021. Accessed August 23, 2021. https://www.whitehouse.gov/briefing-room/press-briefings/2021/07/08/press-briefing-by-white-house-covid-19-response-team-and-public-health-officials-44
16. Adutaleb Y, Johnson CY, Achenbach J. ‘The war has changed’: Internal CDC document urges new messaging, warns delta infections likely more severe. The Washington Post. July 29, 2021. Accessed August 21, 2021 https://www.washingtonpost.com/health/2021/07/29/cdc-mask-guidance
Several weeks ago, I received a call from my brother who, though not a health care professional, wanted me to know he thought the public was being too critical of scientists and physicians who “are giving us the best advice they can about COVID. People think they should have all the answers. But this virus is complicated, and they don’t always know what is going to happen next.” What makes his charitable read of the public health situation remarkable is that he is a COVID-19 survivor of one of the first reported cases of Guillain-Barre syndrome, which several expert neurologists believe is the result of COVID-19. Like so many other COVID-19 long-haul patients, he is left with lingering symptoms and residual deficits.1
I use this personal story as the overture to this piece on why I am changing my opinion regarding a COVID-19 mandate for federal practitioners. In June I raised ethical concerns about compelling vaccination especially for service members of color based on a current and historical climate of mistrust and discrimination in health care that compulsory vaccination could exacerbate.2 Instead, I followed the lead of Secretary of Defense J. Lloyd Austin III and advocated continued education and encouragement for vaccine-hesitant troops.3 So in 2 months what has so radically changed to lead Secretary Austin and US Department of Veterans Affairs (VA) Secretary Denis R. McDonough to mandate vaccination for their workforce?4,5
I am calling the change the Delta Factor. This is not to be confused with the spy-thrillers that ironically involved rescuing a scientist! The Delta Factor is a catch-all phrase to cover the protean public health impacts of the devastating COVID-19 Delta variant now ravaging the country. Depending on the area of the country as of mid-August, the Centers for Disease Control and Prevention (CDC) estimated that 80% to > 90% of new cases were the Delta variant.6 An increasing number of these cases sadly are in children.7
According to the CDC, the Delta variant is more than twice as contagious as index or subsequent strains: making it about as contagious as chicken pox. The unvaccinated are the most susceptible to Delta and may develop more serious illness and risk of death than with other strains. Those who are fully vaccinated can still contract the virus although usually with milder cases. More worrisome is that individuals with these breakthrough infections have the same viral load as those without vaccinations, rendering them vectors of transmission, although for a shorter time than unvaccinated persons.8
The VA first mandated vaccination among its health care employees in July and then expanded it to all staff in August.9 The US Department of Defense (DoD) mandatory vaccination was announced prior to US Food and Drug Administration’s (FDA) full approval of the Pfizer-BioNTech vaccine.10 Secretary Austin asked President Biden to grant a waiver to permit mandatory vaccination even without full FDA approval, and Biden has indicated his support, but the full approval expedited the time line for implementation.11
Both agencies directly referenced Delta as a primary reason for their vaccination mandates. The VA argued that the mandate was necessary to protect the safety of veterans, while the DoD noted that vaccination was essential to ensure the health of the fighting force. In his initial announcement, Secretary McDonough explicitly mentioned the Delta variant as a primary reason for his decision. noting “it’s the best way to keep veterans safe, especially as the Delta variant spreads across the country.”4 Similarly, Secretary Austin declared, “We will also be keeping a close eye on infection rates, which are on the rise now due to the Delta variant and the impact these rates might have on our readiness.”5
VA and DoD leadership emphasized the safety and effectiveness of the vaccine and urged employees to voluntarily obtain the vaccine or obtain a religious or medical exemption. Those without such an exemption must adhere to masking, testing, and other restrictions.5 As anticipated in the earlier editorial, there has been opposition to the mandate from the workforce of the 2 agencies and their political supporters some of whom view vaccine mandates as violations of personal liberty and bodily integrity and for whom rampant disinformation has amplified entrenched distrust of the government.12
The decision to shift from voluntary to mandatory vaccination of federal employees responsible for the health care of veterans and the defense of citizens, which may seem
Finally and most important, for a vaccine or other public health intervention to be ethically mandated it must have a high probability of attaining a serious purpose: here preventing the harms of sickness and death especially in the most vulnerable. In July, the White House COVID-19 Response Team reported that “preliminary data from several states over the last few months suggest that 99.5% of deaths from COVID-19 in the United States were in unvaccinated people” and were preventable.15 Ethically, even as mandates are implemented across the federal workforce, efforts to educate, encourage, and empower vaccination especially among disenfranchised cohorts must continue. But as a recently leaked CDC internal document acknowledged about the Delta Factor, “the war has changed” and so has my opinion about mandating vaccination among those upon whose service depends the life and security of us all.16
Several weeks ago, I received a call from my brother who, though not a health care professional, wanted me to know he thought the public was being too critical of scientists and physicians who “are giving us the best advice they can about COVID. People think they should have all the answers. But this virus is complicated, and they don’t always know what is going to happen next.” What makes his charitable read of the public health situation remarkable is that he is a COVID-19 survivor of one of the first reported cases of Guillain-Barre syndrome, which several expert neurologists believe is the result of COVID-19. Like so many other COVID-19 long-haul patients, he is left with lingering symptoms and residual deficits.1
I use this personal story as the overture to this piece on why I am changing my opinion regarding a COVID-19 mandate for federal practitioners. In June I raised ethical concerns about compelling vaccination especially for service members of color based on a current and historical climate of mistrust and discrimination in health care that compulsory vaccination could exacerbate.2 Instead, I followed the lead of Secretary of Defense J. Lloyd Austin III and advocated continued education and encouragement for vaccine-hesitant troops.3 So in 2 months what has so radically changed to lead Secretary Austin and US Department of Veterans Affairs (VA) Secretary Denis R. McDonough to mandate vaccination for their workforce?4,5
I am calling the change the Delta Factor. This is not to be confused with the spy-thrillers that ironically involved rescuing a scientist! The Delta Factor is a catch-all phrase to cover the protean public health impacts of the devastating COVID-19 Delta variant now ravaging the country. Depending on the area of the country as of mid-August, the Centers for Disease Control and Prevention (CDC) estimated that 80% to > 90% of new cases were the Delta variant.6 An increasing number of these cases sadly are in children.7
According to the CDC, the Delta variant is more than twice as contagious as index or subsequent strains: making it about as contagious as chicken pox. The unvaccinated are the most susceptible to Delta and may develop more serious illness and risk of death than with other strains. Those who are fully vaccinated can still contract the virus although usually with milder cases. More worrisome is that individuals with these breakthrough infections have the same viral load as those without vaccinations, rendering them vectors of transmission, although for a shorter time than unvaccinated persons.8
The VA first mandated vaccination among its health care employees in July and then expanded it to all staff in August.9 The US Department of Defense (DoD) mandatory vaccination was announced prior to US Food and Drug Administration’s (FDA) full approval of the Pfizer-BioNTech vaccine.10 Secretary Austin asked President Biden to grant a waiver to permit mandatory vaccination even without full FDA approval, and Biden has indicated his support, but the full approval expedited the time line for implementation.11
Both agencies directly referenced Delta as a primary reason for their vaccination mandates. The VA argued that the mandate was necessary to protect the safety of veterans, while the DoD noted that vaccination was essential to ensure the health of the fighting force. In his initial announcement, Secretary McDonough explicitly mentioned the Delta variant as a primary reason for his decision. noting “it’s the best way to keep veterans safe, especially as the Delta variant spreads across the country.”4 Similarly, Secretary Austin declared, “We will also be keeping a close eye on infection rates, which are on the rise now due to the Delta variant and the impact these rates might have on our readiness.”5
VA and DoD leadership emphasized the safety and effectiveness of the vaccine and urged employees to voluntarily obtain the vaccine or obtain a religious or medical exemption. Those without such an exemption must adhere to masking, testing, and other restrictions.5 As anticipated in the earlier editorial, there has been opposition to the mandate from the workforce of the 2 agencies and their political supporters some of whom view vaccine mandates as violations of personal liberty and bodily integrity and for whom rampant disinformation has amplified entrenched distrust of the government.12
The decision to shift from voluntary to mandatory vaccination of federal employees responsible for the health care of veterans and the defense of citizens, which may seem
Finally and most important, for a vaccine or other public health intervention to be ethically mandated it must have a high probability of attaining a serious purpose: here preventing the harms of sickness and death especially in the most vulnerable. In July, the White House COVID-19 Response Team reported that “preliminary data from several states over the last few months suggest that 99.5% of deaths from COVID-19 in the United States were in unvaccinated people” and were preventable.15 Ethically, even as mandates are implemented across the federal workforce, efforts to educate, encourage, and empower vaccination especially among disenfranchised cohorts must continue. But as a recently leaked CDC internal document acknowledged about the Delta Factor, “the war has changed” and so has my opinion about mandating vaccination among those upon whose service depends the life and security of us all.16
1. CBS Good Morning. Christopher Cross on his near-fatal COVID illness. Published October 18, 2020. Accessed August 21, 2021. https://www.cbsnews.com/news/christopher-cross-on-his-near-fatal-covid-illness
2. Geppert CM. Mistrust and mandates: COVID-19 vaccination in the military. Fed Pract. 2021;38(6):254-255. doi:10.12788/fp.0143
3. Garmone J, US Department of Defense. Secretary of defense addresses vaccine hesitancy in the military. Published February 25, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military
4. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA mandates COVID-19 vaccines among its medical employees including VHA facilities staff [press release]. Published July 26, 2021. Accessed August 21, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5696
5. US Department of Defense, Secretary of Defense. Memorandum for all Department of Defense employees. Published August 9, 2021. Accessed August 23, 2021. https://media.defense.gov/2021/Aug/09/2002826254/-1/-1/0/MESSAGE-TO-THE-FORCE-MEMO-VACCINE.PDF
6. Centers for Disease Control and Prevention COVID data tracker. Variant proportions. Updated August 17, 2021. Accessed August 23, 2021. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
7. American Academy of Pediatrics. Children and COVID-19: state data level report. Updated August 23, 2021. Accessed August 23, 2021. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state|-level-data-report
8. Centers for Disease Control and Prevention. Delta variant: what we know about the science. Update August 19, 2021. Accessed August 23, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html
9. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands mandate for COVID-19 vaccines among VHA employees [press release]. Published August 12, 2021. Accessed August 23, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5703
10. US Food and Drug Administration. FDA approves first COVID-19 vaccine [press release]. Published August 23, 2021. Accessed August 23, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
11. Garamone J, US Department of Defense. Biden to approve Austin’s request to make COVID-19 vaccine mandatory for service members. Published August 9, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2724982/biden-to-approve-austins-request-to-make-covid-19-vaccine-mandatory-for-service
12. Watson J. Potential military vaccine mandate brings distrust, support. Associated Press. August 5, 2021. Accessed August 23, 2021. https://apnews.com/article/joe-biden-business-health-coronavirus-pandemic-6a0f94e11f5af1e0de740d44d7931d65
13. Giubilini A. Vaccination ethics. Br Med Bull. 2021;137(1):4-12. doi:10.1093/bmb/ldaa036
14. Steinhauer J. Military and V.A. struggle with vaccination rates in their ranks. The New York Times. July 1, 2021. Accessed August 23, 2021. https://www.nytimes.com/2021/07/01/us/politics/military-va-vaccines.html
15. The White House. Press briefing by White House COVID-19 Response Team and public health officials. Published July 8, 2021. Accessed August 23, 2021. https://www.whitehouse.gov/briefing-room/press-briefings/2021/07/08/press-briefing-by-white-house-covid-19-response-team-and-public-health-officials-44
16. Adutaleb Y, Johnson CY, Achenbach J. ‘The war has changed’: Internal CDC document urges new messaging, warns delta infections likely more severe. The Washington Post. July 29, 2021. Accessed August 21, 2021 https://www.washingtonpost.com/health/2021/07/29/cdc-mask-guidance
1. CBS Good Morning. Christopher Cross on his near-fatal COVID illness. Published October 18, 2020. Accessed August 21, 2021. https://www.cbsnews.com/news/christopher-cross-on-his-near-fatal-covid-illness
2. Geppert CM. Mistrust and mandates: COVID-19 vaccination in the military. Fed Pract. 2021;38(6):254-255. doi:10.12788/fp.0143
3. Garmone J, US Department of Defense. Secretary of defense addresses vaccine hesitancy in the military. Published February 25, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2516511/secretary-of-defense-addresses-vaccine-hesitancy-in-military
4. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA mandates COVID-19 vaccines among its medical employees including VHA facilities staff [press release]. Published July 26, 2021. Accessed August 21, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5696
5. US Department of Defense, Secretary of Defense. Memorandum for all Department of Defense employees. Published August 9, 2021. Accessed August 23, 2021. https://media.defense.gov/2021/Aug/09/2002826254/-1/-1/0/MESSAGE-TO-THE-FORCE-MEMO-VACCINE.PDF
6. Centers for Disease Control and Prevention COVID data tracker. Variant proportions. Updated August 17, 2021. Accessed August 23, 2021. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
7. American Academy of Pediatrics. Children and COVID-19: state data level report. Updated August 23, 2021. Accessed August 23, 2021. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state|-level-data-report
8. Centers for Disease Control and Prevention. Delta variant: what we know about the science. Update August 19, 2021. Accessed August 23, 2021. https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html
9. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands mandate for COVID-19 vaccines among VHA employees [press release]. Published August 12, 2021. Accessed August 23, 2021. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5703
10. US Food and Drug Administration. FDA approves first COVID-19 vaccine [press release]. Published August 23, 2021. Accessed August 23, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
11. Garamone J, US Department of Defense. Biden to approve Austin’s request to make COVID-19 vaccine mandatory for service members. Published August 9, 2021. Accessed August 23, 2021. https://www.defense.gov/Explore/News/Article/Article/2724982/biden-to-approve-austins-request-to-make-covid-19-vaccine-mandatory-for-service
12. Watson J. Potential military vaccine mandate brings distrust, support. Associated Press. August 5, 2021. Accessed August 23, 2021. https://apnews.com/article/joe-biden-business-health-coronavirus-pandemic-6a0f94e11f5af1e0de740d44d7931d65
13. Giubilini A. Vaccination ethics. Br Med Bull. 2021;137(1):4-12. doi:10.1093/bmb/ldaa036
14. Steinhauer J. Military and V.A. struggle with vaccination rates in their ranks. The New York Times. July 1, 2021. Accessed August 23, 2021. https://www.nytimes.com/2021/07/01/us/politics/military-va-vaccines.html
15. The White House. Press briefing by White House COVID-19 Response Team and public health officials. Published July 8, 2021. Accessed August 23, 2021. https://www.whitehouse.gov/briefing-room/press-briefings/2021/07/08/press-briefing-by-white-house-covid-19-response-team-and-public-health-officials-44
16. Adutaleb Y, Johnson CY, Achenbach J. ‘The war has changed’: Internal CDC document urges new messaging, warns delta infections likely more severe. The Washington Post. July 29, 2021. Accessed August 21, 2021 https://www.washingtonpost.com/health/2021/07/29/cdc-mask-guidance
Right Ventricle Dilation Detected on Point-of-Care Ultrasound Is a Predictor of Poor Outcomes in Critically Ill Patients With COVID-19
Point-of-care ultrasound (POCUS) is increasingly being used by critical care physicians to augment the physical examination and guide clinical decision making, and several protocols have been established to standardize the POCUS evaluation.1 During the COVID-19 pandemic, POCUS has been a valuable tool as standard imaging techniques were used judiciously to minimize exposure of personnel and use of personal protective equipment (PPE).2
In the US Department of Veterans Affairs (VA) New York Harbor Healthcare System (VANYHHS) intensive care unit (ICU) on initial clinical examination included POCUS, which was helpful to examine deep vein thromboses, cardiac function, and the presence and extent of pneumonia. An international expert consensus on the use of POCUS for COVID-19 published in December 2020 called for further studies defining the role of lung and cardiac ultrasound in risk stratification, outcomes, and clinical management.3
The objective of this study was to review POCUS findings and correlate them with severity of illness and 30-day outcomes in critically ill patients with COVID-19.
Methods
The study was submitted to and reviewed by the VANYHHS Research and Development committee and study approval and informed consent waiver was granted. The study was a retrospective chart review of patients admitted to the VANYHHS ICU between March and April 2020, a tertiary health care center designated as a COVID-19 hospital.
Patients admitted to the ICU aged > 18 years with a diagnosis of acute hypoxemic respiratory failure, diagnosis of COVID-19, and documentation of POCUS findings in the chart were included in the study. A patient was considered to have a COVID-19 diagnosis following a positive SARS-CoV-2 polymerase chain reaction test documented in the electronic health record (EHR). Acute respiratory failure was defined as hypoxemia < 94% and the need for either supplemental oxygen by nasal cannula > 2 L/min, high flow nasal cannula, noninvasive ventilation, or mechanical ventilation.
To minimize personnel exposure, initial patient evaluations and POCUS examinations were performed by the most senior personnel (ie, fellowship trained, board-certified pulmonary critical care attending physicians or pulmonary and critical care fellowship trainees). Three members of the team had certification in advanced critical care echocardiography by the National Board of Echocardiography and oversaw POCUS imaging. POCUS examinations were performed with a GE Heathcare Venue POCUS or handheld unit. After use, ultrasound probes and ultrasound units were disinfected with wipes designated by the manufacturer and US Environmental Protection Agency for use during the COVID-19 pandemic.
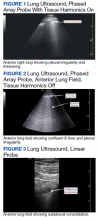
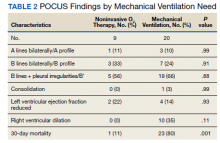
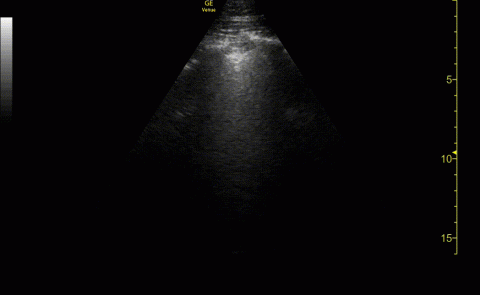
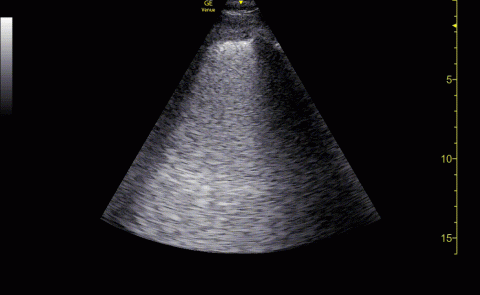
The POCUS protocol used by members of the team was as follows: POCUS lung—at least 2 anterior fields and 1 posterior/lateral field looking at the costophrenic angle on each hemithorax with a phased array or curvilinear probe. A linear probe was used to look for subpleural changes per physician discretion.4,5 Lung ultrasound findings in anterior lung fields were documented as A lines, B lines (as defined by the bedside lung ultrasound in emergency [BLUE] protocol)anterior pleural abnormalities or consolidations.4,5 The costophrenic point findings were documented as presence of consolidation or pleural effusion.
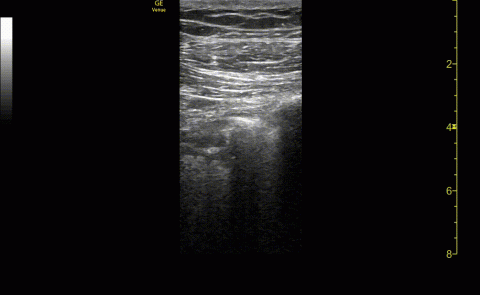
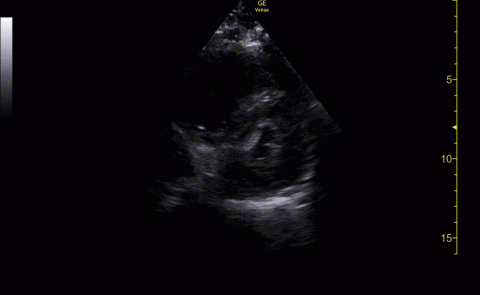
The POCUS cardiac examination consisted of parasternal long and short axis views, apical 4 chamber view, subcostal and inferior vena cava (IVC) view. Left ventricular (LV) ejection fraction was visually estimated as reduced or normal. Right ventricular (RV) dilation was considered present if RV size approached or exceeded LV size in the apical 4 chamber view. RV dysfunction was considered present if in addition there was flattening of interventricular septum, RV free wall hypokinesis or reduced tricuspid annular plane systolic excursion (TAPSE).6 IVC was documented as collapsible or plethoric by size and respirophasic variability (2 cm and 50%). Other POCUS examinations including venous compression were done at the discretion of the treating physician.7 POCUS was also used for the placement of central and arterial lines and to guide fluid management.8
The VA EHR and Venue image local archives were reviewed for patient demographics, laboratory findings, imaging studies and outcomes. All ICU attending physician and fellow notes were reviewed for POCUS lung, cardiac and vascular findings. The chart was also reviewed for management changes as a result of POCUS findings. Patients who had at minimum a POCUS lung or cardiac examination documented in the EHR were included in the study. For patients with serial POCUS the most severe findings were included.
Patients were divided into 2 groups based on 30-day outcome: discharge home vs mortality for comparison. POCUS findings were also compared by need for mechanical ventilation. Patients still hospitalized or transferred to other facilities were excluded from the analysis. A Student t test was used for comparison between the groups for continuous normally distributed variables. Linear and stepwise regression models were used to evaluate univariate and multivariate associations of baseline characteristics, biomarker, and ultrasound findings with patient outcomes. Analyses were performed using R 4.0.2 statistical software.
Results
Eighty-two patients were admitted to the VANYHHS ICU in March and April 2020, including 12 nonveterans. Sixty-four had COVID-19 and acute respiratory failure. POCUS findings were documented in 43 (67%) patients. Thirty-nine patients had documented lung examinations, and 25 patients had documented cardiac examinations. Patients were divided into 2 groups by 30-day outcome (discharge home vs mortality) for statistical analysis. Five patients who were either still hospitalized or had been transferred to another facility were excluded.
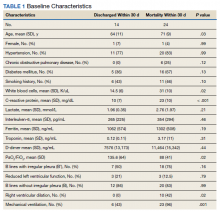
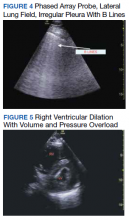
Baseline characteristics of patients included in the study stratified by 30-day outcomes are shown in Table 1. The study group was predominantly male (95%). Patients with poor 30-day outcomes were older, had higher white blood cell counts, more severe hypoxemia, higher rates of mechanical ventilation and RV dilation (Figures 1, 2, 3, 4, and 5). RV dilation was an independent predictor of mortality (odds ratio [OR], 12.0; P = .048).
Serial POCUS documented development or progression of RV dilation and dysfunction from the time of ICU admission in 4 of the patients. The presence of B lines with irregular pleura was predictive of a lower arterial pressure of oxygen to fraction of inspired oxygen ratio (PaO2/FiO2) by a value of 71 compared with those without B lines with irregular pleura (P = .005, adjusted R2 = 0.238). All patients with RV dilation had bilateral B lines with pleural irregularities on lung ultrasound. Vascular POCUS detected 4 deep vein thromboses (DVT).7 An arterial thrombus was also detected on focused examination. There was a higher mortality in patients who required mechanical ventilation; however, there was no difference in POCUS characteristics between the groups (Table 2).
Two severely hypoxemic patients received systemic tissue plasminogen activator (TPA) after findings of massive RV dilation with signs of volume and pressure overload and clinical suspicion of pulmonary embolism (PE). One of these patients also had a popliteal DVT. Both patients were too unstable to transport for additional imaging or therapies. Therapeutic anticoagulation was initiated on 4 patients with positive DVT examinations. In a fifth case an arterial thrombectomy and anticoagulation was required after diminished pulses led to the finding of an occlusive brachial artery thrombus on vascular POCUS.
Discussion
POCUS identified both lung and cardiac features that were associated with worse outcomes. While lung ultrasound abnormalities were very prevalent and associated with worse PaO2 to FiO2 ratios, the presence of RV dilation was associated most clearly with mortality and poor 30-day outcomes in the critical care setting.
Lung ultrasound abnormalities were pervasive in patients with acute respiratory failure and COVID-19. On linear regression we found that presence with bilateral B lines and pleural thickening was predictive of a lower PaO2/FiO2 (coefficient, -70; P = .005). Our study found that B lines with pleural irregularities, otherwise known as a B’ profile per the BLUE protocol, was seen in patients with severe COVID-19. Thus severe acute respiratory failure secondary to COVID-19 has similar lung ultrasound findings as non-COVID-19 acute respiratory distress syndrome (ARDS).4,5 Based on prior lung ultrasound studies in ARDS, lung ultrasound findings can be used as an alternate to chest radiography for the diagnosis of ARDS in COVID-19 and predict the severity of ARDS.9 This has particular implications in overwhelmed and resource poor health care settings.
We found no difference in 30-day mortality based on lung ultrasound findings or profile, probably because of small sample size or because the findings were tabulated as profiles and not differentiated further with lung ultrasound scores.10,11 However, there was a significant difference in RV dilation between the 2 groups by 30 days and its presence was found to be a predictor of mortality even when controlled for hypertension and diabetes mellitus (P = .048) with an OR of 12. RV dysfunction in patients with ARDS on mechanical ventilation ranges from 22 to 25% and is typically associated with high driving pressures.12-14 The mechanism is thought to be multifactorial including hypoxemic vasoconstriction in the pulmonary vasculature in addition to the increased transpulmonary pressure.15 While all of the above are at play in COVID-19 infection, there is reported damage to the pulmonary vascular endothelium and resultant hypercoagulability and thrombosis that further increases the RV afterload.16
While RV strain and dysfunction indices done by an echocardiographer would be ideal, given the surge in infections and hospitalizations and strain on health care resources, POCUS by the treating or examining clinician was considered the only feasible way to screen a large number of patients.17 Identification of RV dilation could influence clinical management including workup for venous thromboembolic disease and optimization of lung protective strategies. Further studies are needed to understand the particular etiology and pathophysiology of COVID-19 associated RV dilation. Given increased thrombosis events in COVID-19 infection we believe a POCUS vascular examination should be included as part of evaluation especially in the presence of increased D-dimers and has been discussed above for its important role in working up RV dilation.18
Limitations
Our study has several limitations. It was retrospective in nature and involved a small group of individuals. There was some variation in POCUS examinations done at the discretion of the examining physician. We did not have a blinded observer independently review all images. Since RV dilation was documented only when RV size approached or exceeded LV size in the apical 4 chamber view representing moderate or severe dilation, we may be underreporting the prevalence in critically ill patients.
Conclusions
POCUS is an invaluable adjunct to clinical evaluation and procedures in patients with severe COVID-19 with the ability to identity patients at risk for worse outcomes. B lines with pleural thickening is a sign of severe ARDS and RV dilatation is predictive of mortality. POCUS should be made available to the treating physician for monitoring and risk stratification and can be incorporated into management algorithms.
Additional point-of-care ultrasound videos.
Acknowledgments
We thank frontline healthcare workers and intensive care unit staff of the US Department of Veterans Affairs New York Harbor Healthcare System (NYHHS) for their dedication to the care of veterans and civilians during the COVID-19 pandemic in New York City. The authors acknowledge the NYHHS research and development committee and administration for their support.
1. Cardenas-Garcia J, Mayo PH. Bedside ultrasonography for the intensivist. Crit Care Clin. 2015;31(1):43-66. doi:10.1016/j.ccc.2014.08.003
2. Vetrugno L, Baciarello M, Bignami E, et al. The “pandemic” increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review. Ultrasound J. 2020;12(1):39. Published 2020 Aug 17. doi:10.1186/s13089-020-00185-4
3. Hussain A, Via G, Melniker L, et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care. 2020;24(1):702. Published 2020 Dec 24. doi:10.1186/s13054-020-03369-5
4. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol [published correction appears in Chest. 2013 Aug;144(2):721]. Chest. 2008;134(1):117-125. doi:10.1378/chest.07-2800
5. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-591. doi:10.1007/s00134-012-2513-4
6. Narasimhan M, Koenig SJ, Mayo PH. Advanced echocardiography for the critical care physician: part 1. Chest. 2014;145(1):129-134. doi:10.1378/chest.12-2441
7. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139(3):538-542. doi:10.1378/chest.10-1479
8. Bentzer P, Griesdale DE, Boyd J, MacLean K, Sirounis D, Ayas NT. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316(12):1298-1309. doi:10.1001/jama.2016.12310
9. See KC, Ong V, Tan YL, Sahagun J, Taculod J. Chest radiography versus lung ultrasound for identification of acute respiratory distress syndrome: a retrospective observational study. Crit Care. 2018;22(1):203. Published 2018 Aug 18. doi:10.1186/s13054-018-2105-y
10. Deng Q, Zhang Y, Wang H, et al. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single-center study. Acad Radiol. 2020;27(10):1363-1372. doi:10.1016/j.acra.2020.07.002
11. Brahier T, Meuwly JY, Pantet O, et al. Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study [published online ahead of print, 2020 Sep 17]. Clin Infect Dis. 2020;ciaa1408. doi:10.1093/cid/ciaa1408
12. Vieillard-Baron A, Schmitt JM, Augarde R, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis [published correction appears in Crit Care Med. 2002 Mar;30(3):726]. Crit Care Med. 2001;29(8):1551-1555. doi:10.1097/00003246-200108000-00009
13. Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1725-1733. doi:10.1007/s00134-013-2941-9
14. Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007;33(3):444-447. doi:10.1007/s00134-007-0552-z
15. Repessé X, Vieillard-Baron A. Right heart function during acute respiratory distress syndrome. Ann Transl Med 2017;5(14):295. doi:10.21037/atm.2017.06.66
16. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management [published correction appears in Thromb Res. 2020 Nov 26]. Thromb Res. 2020;194:101-115. doi:10.1016/j.thromres.2020.06.029
17. Kim J, Volodarskiy A, Sultana R, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 2020;76(17):1965-1977. doi:10.1016/j.jacc.2020.08.066
18. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. doi:10.1182/blood.2020006520
Point-of-care ultrasound (POCUS) is increasingly being used by critical care physicians to augment the physical examination and guide clinical decision making, and several protocols have been established to standardize the POCUS evaluation.1 During the COVID-19 pandemic, POCUS has been a valuable tool as standard imaging techniques were used judiciously to minimize exposure of personnel and use of personal protective equipment (PPE).2
In the US Department of Veterans Affairs (VA) New York Harbor Healthcare System (VANYHHS) intensive care unit (ICU) on initial clinical examination included POCUS, which was helpful to examine deep vein thromboses, cardiac function, and the presence and extent of pneumonia. An international expert consensus on the use of POCUS for COVID-19 published in December 2020 called for further studies defining the role of lung and cardiac ultrasound in risk stratification, outcomes, and clinical management.3
The objective of this study was to review POCUS findings and correlate them with severity of illness and 30-day outcomes in critically ill patients with COVID-19.
Methods
The study was submitted to and reviewed by the VANYHHS Research and Development committee and study approval and informed consent waiver was granted. The study was a retrospective chart review of patients admitted to the VANYHHS ICU between March and April 2020, a tertiary health care center designated as a COVID-19 hospital.
Patients admitted to the ICU aged > 18 years with a diagnosis of acute hypoxemic respiratory failure, diagnosis of COVID-19, and documentation of POCUS findings in the chart were included in the study. A patient was considered to have a COVID-19 diagnosis following a positive SARS-CoV-2 polymerase chain reaction test documented in the electronic health record (EHR). Acute respiratory failure was defined as hypoxemia < 94% and the need for either supplemental oxygen by nasal cannula > 2 L/min, high flow nasal cannula, noninvasive ventilation, or mechanical ventilation.
To minimize personnel exposure, initial patient evaluations and POCUS examinations were performed by the most senior personnel (ie, fellowship trained, board-certified pulmonary critical care attending physicians or pulmonary and critical care fellowship trainees). Three members of the team had certification in advanced critical care echocardiography by the National Board of Echocardiography and oversaw POCUS imaging. POCUS examinations were performed with a GE Heathcare Venue POCUS or handheld unit. After use, ultrasound probes and ultrasound units were disinfected with wipes designated by the manufacturer and US Environmental Protection Agency for use during the COVID-19 pandemic.
The POCUS protocol used by members of the team was as follows: POCUS lung—at least 2 anterior fields and 1 posterior/lateral field looking at the costophrenic angle on each hemithorax with a phased array or curvilinear probe. A linear probe was used to look for subpleural changes per physician discretion.4,5 Lung ultrasound findings in anterior lung fields were documented as A lines, B lines (as defined by the bedside lung ultrasound in emergency [BLUE] protocol)anterior pleural abnormalities or consolidations.4,5 The costophrenic point findings were documented as presence of consolidation or pleural effusion.
The POCUS cardiac examination consisted of parasternal long and short axis views, apical 4 chamber view, subcostal and inferior vena cava (IVC) view. Left ventricular (LV) ejection fraction was visually estimated as reduced or normal. Right ventricular (RV) dilation was considered present if RV size approached or exceeded LV size in the apical 4 chamber view. RV dysfunction was considered present if in addition there was flattening of interventricular septum, RV free wall hypokinesis or reduced tricuspid annular plane systolic excursion (TAPSE).6 IVC was documented as collapsible or plethoric by size and respirophasic variability (2 cm and 50%). Other POCUS examinations including venous compression were done at the discretion of the treating physician.7 POCUS was also used for the placement of central and arterial lines and to guide fluid management.8
The VA EHR and Venue image local archives were reviewed for patient demographics, laboratory findings, imaging studies and outcomes. All ICU attending physician and fellow notes were reviewed for POCUS lung, cardiac and vascular findings. The chart was also reviewed for management changes as a result of POCUS findings. Patients who had at minimum a POCUS lung or cardiac examination documented in the EHR were included in the study. For patients with serial POCUS the most severe findings were included.
Patients were divided into 2 groups based on 30-day outcome: discharge home vs mortality for comparison. POCUS findings were also compared by need for mechanical ventilation. Patients still hospitalized or transferred to other facilities were excluded from the analysis. A Student t test was used for comparison between the groups for continuous normally distributed variables. Linear and stepwise regression models were used to evaluate univariate and multivariate associations of baseline characteristics, biomarker, and ultrasound findings with patient outcomes. Analyses were performed using R 4.0.2 statistical software.
Results
Eighty-two patients were admitted to the VANYHHS ICU in March and April 2020, including 12 nonveterans. Sixty-four had COVID-19 and acute respiratory failure. POCUS findings were documented in 43 (67%) patients. Thirty-nine patients had documented lung examinations, and 25 patients had documented cardiac examinations. Patients were divided into 2 groups by 30-day outcome (discharge home vs mortality) for statistical analysis. Five patients who were either still hospitalized or had been transferred to another facility were excluded.
Baseline characteristics of patients included in the study stratified by 30-day outcomes are shown in Table 1. The study group was predominantly male (95%). Patients with poor 30-day outcomes were older, had higher white blood cell counts, more severe hypoxemia, higher rates of mechanical ventilation and RV dilation (Figures 1, 2, 3, 4, and 5). RV dilation was an independent predictor of mortality (odds ratio [OR], 12.0; P = .048).
Serial POCUS documented development or progression of RV dilation and dysfunction from the time of ICU admission in 4 of the patients. The presence of B lines with irregular pleura was predictive of a lower arterial pressure of oxygen to fraction of inspired oxygen ratio (PaO2/FiO2) by a value of 71 compared with those without B lines with irregular pleura (P = .005, adjusted R2 = 0.238). All patients with RV dilation had bilateral B lines with pleural irregularities on lung ultrasound. Vascular POCUS detected 4 deep vein thromboses (DVT).7 An arterial thrombus was also detected on focused examination. There was a higher mortality in patients who required mechanical ventilation; however, there was no difference in POCUS characteristics between the groups (Table 2).
Two severely hypoxemic patients received systemic tissue plasminogen activator (TPA) after findings of massive RV dilation with signs of volume and pressure overload and clinical suspicion of pulmonary embolism (PE). One of these patients also had a popliteal DVT. Both patients were too unstable to transport for additional imaging or therapies. Therapeutic anticoagulation was initiated on 4 patients with positive DVT examinations. In a fifth case an arterial thrombectomy and anticoagulation was required after diminished pulses led to the finding of an occlusive brachial artery thrombus on vascular POCUS.
Discussion
POCUS identified both lung and cardiac features that were associated with worse outcomes. While lung ultrasound abnormalities were very prevalent and associated with worse PaO2 to FiO2 ratios, the presence of RV dilation was associated most clearly with mortality and poor 30-day outcomes in the critical care setting.
Lung ultrasound abnormalities were pervasive in patients with acute respiratory failure and COVID-19. On linear regression we found that presence with bilateral B lines and pleural thickening was predictive of a lower PaO2/FiO2 (coefficient, -70; P = .005). Our study found that B lines with pleural irregularities, otherwise known as a B’ profile per the BLUE protocol, was seen in patients with severe COVID-19. Thus severe acute respiratory failure secondary to COVID-19 has similar lung ultrasound findings as non-COVID-19 acute respiratory distress syndrome (ARDS).4,5 Based on prior lung ultrasound studies in ARDS, lung ultrasound findings can be used as an alternate to chest radiography for the diagnosis of ARDS in COVID-19 and predict the severity of ARDS.9 This has particular implications in overwhelmed and resource poor health care settings.
We found no difference in 30-day mortality based on lung ultrasound findings or profile, probably because of small sample size or because the findings were tabulated as profiles and not differentiated further with lung ultrasound scores.10,11 However, there was a significant difference in RV dilation between the 2 groups by 30 days and its presence was found to be a predictor of mortality even when controlled for hypertension and diabetes mellitus (P = .048) with an OR of 12. RV dysfunction in patients with ARDS on mechanical ventilation ranges from 22 to 25% and is typically associated with high driving pressures.12-14 The mechanism is thought to be multifactorial including hypoxemic vasoconstriction in the pulmonary vasculature in addition to the increased transpulmonary pressure.15 While all of the above are at play in COVID-19 infection, there is reported damage to the pulmonary vascular endothelium and resultant hypercoagulability and thrombosis that further increases the RV afterload.16
While RV strain and dysfunction indices done by an echocardiographer would be ideal, given the surge in infections and hospitalizations and strain on health care resources, POCUS by the treating or examining clinician was considered the only feasible way to screen a large number of patients.17 Identification of RV dilation could influence clinical management including workup for venous thromboembolic disease and optimization of lung protective strategies. Further studies are needed to understand the particular etiology and pathophysiology of COVID-19 associated RV dilation. Given increased thrombosis events in COVID-19 infection we believe a POCUS vascular examination should be included as part of evaluation especially in the presence of increased D-dimers and has been discussed above for its important role in working up RV dilation.18
Limitations
Our study has several limitations. It was retrospective in nature and involved a small group of individuals. There was some variation in POCUS examinations done at the discretion of the examining physician. We did not have a blinded observer independently review all images. Since RV dilation was documented only when RV size approached or exceeded LV size in the apical 4 chamber view representing moderate or severe dilation, we may be underreporting the prevalence in critically ill patients.
Conclusions
POCUS is an invaluable adjunct to clinical evaluation and procedures in patients with severe COVID-19 with the ability to identity patients at risk for worse outcomes. B lines with pleural thickening is a sign of severe ARDS and RV dilatation is predictive of mortality. POCUS should be made available to the treating physician for monitoring and risk stratification and can be incorporated into management algorithms.
Additional point-of-care ultrasound videos.
Acknowledgments
We thank frontline healthcare workers and intensive care unit staff of the US Department of Veterans Affairs New York Harbor Healthcare System (NYHHS) for their dedication to the care of veterans and civilians during the COVID-19 pandemic in New York City. The authors acknowledge the NYHHS research and development committee and administration for their support.
Point-of-care ultrasound (POCUS) is increasingly being used by critical care physicians to augment the physical examination and guide clinical decision making, and several protocols have been established to standardize the POCUS evaluation.1 During the COVID-19 pandemic, POCUS has been a valuable tool as standard imaging techniques were used judiciously to minimize exposure of personnel and use of personal protective equipment (PPE).2
In the US Department of Veterans Affairs (VA) New York Harbor Healthcare System (VANYHHS) intensive care unit (ICU) on initial clinical examination included POCUS, which was helpful to examine deep vein thromboses, cardiac function, and the presence and extent of pneumonia. An international expert consensus on the use of POCUS for COVID-19 published in December 2020 called for further studies defining the role of lung and cardiac ultrasound in risk stratification, outcomes, and clinical management.3
The objective of this study was to review POCUS findings and correlate them with severity of illness and 30-day outcomes in critically ill patients with COVID-19.
Methods
The study was submitted to and reviewed by the VANYHHS Research and Development committee and study approval and informed consent waiver was granted. The study was a retrospective chart review of patients admitted to the VANYHHS ICU between March and April 2020, a tertiary health care center designated as a COVID-19 hospital.
Patients admitted to the ICU aged > 18 years with a diagnosis of acute hypoxemic respiratory failure, diagnosis of COVID-19, and documentation of POCUS findings in the chart were included in the study. A patient was considered to have a COVID-19 diagnosis following a positive SARS-CoV-2 polymerase chain reaction test documented in the electronic health record (EHR). Acute respiratory failure was defined as hypoxemia < 94% and the need for either supplemental oxygen by nasal cannula > 2 L/min, high flow nasal cannula, noninvasive ventilation, or mechanical ventilation.
To minimize personnel exposure, initial patient evaluations and POCUS examinations were performed by the most senior personnel (ie, fellowship trained, board-certified pulmonary critical care attending physicians or pulmonary and critical care fellowship trainees). Three members of the team had certification in advanced critical care echocardiography by the National Board of Echocardiography and oversaw POCUS imaging. POCUS examinations were performed with a GE Heathcare Venue POCUS or handheld unit. After use, ultrasound probes and ultrasound units were disinfected with wipes designated by the manufacturer and US Environmental Protection Agency for use during the COVID-19 pandemic.
The POCUS protocol used by members of the team was as follows: POCUS lung—at least 2 anterior fields and 1 posterior/lateral field looking at the costophrenic angle on each hemithorax with a phased array or curvilinear probe. A linear probe was used to look for subpleural changes per physician discretion.4,5 Lung ultrasound findings in anterior lung fields were documented as A lines, B lines (as defined by the bedside lung ultrasound in emergency [BLUE] protocol)anterior pleural abnormalities or consolidations.4,5 The costophrenic point findings were documented as presence of consolidation or pleural effusion.
The POCUS cardiac examination consisted of parasternal long and short axis views, apical 4 chamber view, subcostal and inferior vena cava (IVC) view. Left ventricular (LV) ejection fraction was visually estimated as reduced or normal. Right ventricular (RV) dilation was considered present if RV size approached or exceeded LV size in the apical 4 chamber view. RV dysfunction was considered present if in addition there was flattening of interventricular septum, RV free wall hypokinesis or reduced tricuspid annular plane systolic excursion (TAPSE).6 IVC was documented as collapsible or plethoric by size and respirophasic variability (2 cm and 50%). Other POCUS examinations including venous compression were done at the discretion of the treating physician.7 POCUS was also used for the placement of central and arterial lines and to guide fluid management.8
The VA EHR and Venue image local archives were reviewed for patient demographics, laboratory findings, imaging studies and outcomes. All ICU attending physician and fellow notes were reviewed for POCUS lung, cardiac and vascular findings. The chart was also reviewed for management changes as a result of POCUS findings. Patients who had at minimum a POCUS lung or cardiac examination documented in the EHR were included in the study. For patients with serial POCUS the most severe findings were included.
Patients were divided into 2 groups based on 30-day outcome: discharge home vs mortality for comparison. POCUS findings were also compared by need for mechanical ventilation. Patients still hospitalized or transferred to other facilities were excluded from the analysis. A Student t test was used for comparison between the groups for continuous normally distributed variables. Linear and stepwise regression models were used to evaluate univariate and multivariate associations of baseline characteristics, biomarker, and ultrasound findings with patient outcomes. Analyses were performed using R 4.0.2 statistical software.
Results
Eighty-two patients were admitted to the VANYHHS ICU in March and April 2020, including 12 nonveterans. Sixty-four had COVID-19 and acute respiratory failure. POCUS findings were documented in 43 (67%) patients. Thirty-nine patients had documented lung examinations, and 25 patients had documented cardiac examinations. Patients were divided into 2 groups by 30-day outcome (discharge home vs mortality) for statistical analysis. Five patients who were either still hospitalized or had been transferred to another facility were excluded.
Baseline characteristics of patients included in the study stratified by 30-day outcomes are shown in Table 1. The study group was predominantly male (95%). Patients with poor 30-day outcomes were older, had higher white blood cell counts, more severe hypoxemia, higher rates of mechanical ventilation and RV dilation (Figures 1, 2, 3, 4, and 5). RV dilation was an independent predictor of mortality (odds ratio [OR], 12.0; P = .048).
Serial POCUS documented development or progression of RV dilation and dysfunction from the time of ICU admission in 4 of the patients. The presence of B lines with irregular pleura was predictive of a lower arterial pressure of oxygen to fraction of inspired oxygen ratio (PaO2/FiO2) by a value of 71 compared with those without B lines with irregular pleura (P = .005, adjusted R2 = 0.238). All patients with RV dilation had bilateral B lines with pleural irregularities on lung ultrasound. Vascular POCUS detected 4 deep vein thromboses (DVT).7 An arterial thrombus was also detected on focused examination. There was a higher mortality in patients who required mechanical ventilation; however, there was no difference in POCUS characteristics between the groups (Table 2).
Two severely hypoxemic patients received systemic tissue plasminogen activator (TPA) after findings of massive RV dilation with signs of volume and pressure overload and clinical suspicion of pulmonary embolism (PE). One of these patients also had a popliteal DVT. Both patients were too unstable to transport for additional imaging or therapies. Therapeutic anticoagulation was initiated on 4 patients with positive DVT examinations. In a fifth case an arterial thrombectomy and anticoagulation was required after diminished pulses led to the finding of an occlusive brachial artery thrombus on vascular POCUS.
Discussion
POCUS identified both lung and cardiac features that were associated with worse outcomes. While lung ultrasound abnormalities were very prevalent and associated with worse PaO2 to FiO2 ratios, the presence of RV dilation was associated most clearly with mortality and poor 30-day outcomes in the critical care setting.
Lung ultrasound abnormalities were pervasive in patients with acute respiratory failure and COVID-19. On linear regression we found that presence with bilateral B lines and pleural thickening was predictive of a lower PaO2/FiO2 (coefficient, -70; P = .005). Our study found that B lines with pleural irregularities, otherwise known as a B’ profile per the BLUE protocol, was seen in patients with severe COVID-19. Thus severe acute respiratory failure secondary to COVID-19 has similar lung ultrasound findings as non-COVID-19 acute respiratory distress syndrome (ARDS).4,5 Based on prior lung ultrasound studies in ARDS, lung ultrasound findings can be used as an alternate to chest radiography for the diagnosis of ARDS in COVID-19 and predict the severity of ARDS.9 This has particular implications in overwhelmed and resource poor health care settings.
We found no difference in 30-day mortality based on lung ultrasound findings or profile, probably because of small sample size or because the findings were tabulated as profiles and not differentiated further with lung ultrasound scores.10,11 However, there was a significant difference in RV dilation between the 2 groups by 30 days and its presence was found to be a predictor of mortality even when controlled for hypertension and diabetes mellitus (P = .048) with an OR of 12. RV dysfunction in patients with ARDS on mechanical ventilation ranges from 22 to 25% and is typically associated with high driving pressures.12-14 The mechanism is thought to be multifactorial including hypoxemic vasoconstriction in the pulmonary vasculature in addition to the increased transpulmonary pressure.15 While all of the above are at play in COVID-19 infection, there is reported damage to the pulmonary vascular endothelium and resultant hypercoagulability and thrombosis that further increases the RV afterload.16
While RV strain and dysfunction indices done by an echocardiographer would be ideal, given the surge in infections and hospitalizations and strain on health care resources, POCUS by the treating or examining clinician was considered the only feasible way to screen a large number of patients.17 Identification of RV dilation could influence clinical management including workup for venous thromboembolic disease and optimization of lung protective strategies. Further studies are needed to understand the particular etiology and pathophysiology of COVID-19 associated RV dilation. Given increased thrombosis events in COVID-19 infection we believe a POCUS vascular examination should be included as part of evaluation especially in the presence of increased D-dimers and has been discussed above for its important role in working up RV dilation.18
Limitations
Our study has several limitations. It was retrospective in nature and involved a small group of individuals. There was some variation in POCUS examinations done at the discretion of the examining physician. We did not have a blinded observer independently review all images. Since RV dilation was documented only when RV size approached or exceeded LV size in the apical 4 chamber view representing moderate or severe dilation, we may be underreporting the prevalence in critically ill patients.
Conclusions
POCUS is an invaluable adjunct to clinical evaluation and procedures in patients with severe COVID-19 with the ability to identity patients at risk for worse outcomes. B lines with pleural thickening is a sign of severe ARDS and RV dilatation is predictive of mortality. POCUS should be made available to the treating physician for monitoring and risk stratification and can be incorporated into management algorithms.
Additional point-of-care ultrasound videos.
Acknowledgments
We thank frontline healthcare workers and intensive care unit staff of the US Department of Veterans Affairs New York Harbor Healthcare System (NYHHS) for their dedication to the care of veterans and civilians during the COVID-19 pandemic in New York City. The authors acknowledge the NYHHS research and development committee and administration for their support.
1. Cardenas-Garcia J, Mayo PH. Bedside ultrasonography for the intensivist. Crit Care Clin. 2015;31(1):43-66. doi:10.1016/j.ccc.2014.08.003
2. Vetrugno L, Baciarello M, Bignami E, et al. The “pandemic” increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review. Ultrasound J. 2020;12(1):39. Published 2020 Aug 17. doi:10.1186/s13089-020-00185-4
3. Hussain A, Via G, Melniker L, et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care. 2020;24(1):702. Published 2020 Dec 24. doi:10.1186/s13054-020-03369-5
4. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol [published correction appears in Chest. 2013 Aug;144(2):721]. Chest. 2008;134(1):117-125. doi:10.1378/chest.07-2800
5. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-591. doi:10.1007/s00134-012-2513-4
6. Narasimhan M, Koenig SJ, Mayo PH. Advanced echocardiography for the critical care physician: part 1. Chest. 2014;145(1):129-134. doi:10.1378/chest.12-2441
7. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139(3):538-542. doi:10.1378/chest.10-1479
8. Bentzer P, Griesdale DE, Boyd J, MacLean K, Sirounis D, Ayas NT. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316(12):1298-1309. doi:10.1001/jama.2016.12310
9. See KC, Ong V, Tan YL, Sahagun J, Taculod J. Chest radiography versus lung ultrasound for identification of acute respiratory distress syndrome: a retrospective observational study. Crit Care. 2018;22(1):203. Published 2018 Aug 18. doi:10.1186/s13054-018-2105-y
10. Deng Q, Zhang Y, Wang H, et al. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single-center study. Acad Radiol. 2020;27(10):1363-1372. doi:10.1016/j.acra.2020.07.002
11. Brahier T, Meuwly JY, Pantet O, et al. Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study [published online ahead of print, 2020 Sep 17]. Clin Infect Dis. 2020;ciaa1408. doi:10.1093/cid/ciaa1408
12. Vieillard-Baron A, Schmitt JM, Augarde R, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis [published correction appears in Crit Care Med. 2002 Mar;30(3):726]. Crit Care Med. 2001;29(8):1551-1555. doi:10.1097/00003246-200108000-00009
13. Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1725-1733. doi:10.1007/s00134-013-2941-9
14. Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007;33(3):444-447. doi:10.1007/s00134-007-0552-z
15. Repessé X, Vieillard-Baron A. Right heart function during acute respiratory distress syndrome. Ann Transl Med 2017;5(14):295. doi:10.21037/atm.2017.06.66
16. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management [published correction appears in Thromb Res. 2020 Nov 26]. Thromb Res. 2020;194:101-115. doi:10.1016/j.thromres.2020.06.029
17. Kim J, Volodarskiy A, Sultana R, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 2020;76(17):1965-1977. doi:10.1016/j.jacc.2020.08.066
18. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. doi:10.1182/blood.2020006520
1. Cardenas-Garcia J, Mayo PH. Bedside ultrasonography for the intensivist. Crit Care Clin. 2015;31(1):43-66. doi:10.1016/j.ccc.2014.08.003
2. Vetrugno L, Baciarello M, Bignami E, et al. The “pandemic” increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review. Ultrasound J. 2020;12(1):39. Published 2020 Aug 17. doi:10.1186/s13089-020-00185-4
3. Hussain A, Via G, Melniker L, et al. Multi-organ point-of-care ultrasound for COVID-19 (PoCUS4COVID): international expert consensus. Crit Care. 2020;24(1):702. Published 2020 Dec 24. doi:10.1186/s13054-020-03369-5
4. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol [published correction appears in Chest. 2013 Aug;144(2):721]. Chest. 2008;134(1):117-125. doi:10.1378/chest.07-2800
5. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577-591. doi:10.1007/s00134-012-2513-4
6. Narasimhan M, Koenig SJ, Mayo PH. Advanced echocardiography for the critical care physician: part 1. Chest. 2014;145(1):129-134. doi:10.1378/chest.12-2441
7. Kory PD, Pellecchia CM, Shiloh AL, Mayo PH, DiBello C, Koenig S. Accuracy of ultrasonography performed by critical care physicians for the diagnosis of DVT. Chest. 2011;139(3):538-542. doi:10.1378/chest.10-1479
8. Bentzer P, Griesdale DE, Boyd J, MacLean K, Sirounis D, Ayas NT. Will this hemodynamically unstable patient respond to a bolus of intravenous fluids? JAMA. 2016;316(12):1298-1309. doi:10.1001/jama.2016.12310
9. See KC, Ong V, Tan YL, Sahagun J, Taculod J. Chest radiography versus lung ultrasound for identification of acute respiratory distress syndrome: a retrospective observational study. Crit Care. 2018;22(1):203. Published 2018 Aug 18. doi:10.1186/s13054-018-2105-y
10. Deng Q, Zhang Y, Wang H, et al. Semiquantitative lung ultrasound scores in the evaluation and follow-up of critically ill patients with COVID-19: a single-center study. Acad Radiol. 2020;27(10):1363-1372. doi:10.1016/j.acra.2020.07.002
11. Brahier T, Meuwly JY, Pantet O, et al. Lung ultrasonography for risk stratification in patients with COVID-19: a prospective observational cohort study [published online ahead of print, 2020 Sep 17]. Clin Infect Dis. 2020;ciaa1408. doi:10.1093/cid/ciaa1408
12. Vieillard-Baron A, Schmitt JM, Augarde R, et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis [published correction appears in Crit Care Med. 2002 Mar;30(3):726]. Crit Care Med. 2001;29(8):1551-1555. doi:10.1097/00003246-200108000-00009
13. Boissier F, Katsahian S, Razazi K, et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013;39(10):1725-1733. doi:10.1007/s00134-013-2941-9
14. Jardin F, Vieillard-Baron A. Is there a safe plateau pressure in ARDS? The right heart only knows. Intensive Care Med. 2007;33(3):444-447. doi:10.1007/s00134-007-0552-z
15. Repessé X, Vieillard-Baron A. Right heart function during acute respiratory distress syndrome. Ann Transl Med 2017;5(14):295. doi:10.21037/atm.2017.06.66
16. Abou-Ismail MY, Diamond A, Kapoor S, Arafah Y, Nayak L. The hypercoagulable state in COVID-19: Incidence, pathophysiology, and management [published correction appears in Thromb Res. 2020 Nov 26]. Thromb Res. 2020;194:101-115. doi:10.1016/j.thromres.2020.06.029
17. Kim J, Volodarskiy A, Sultana R, et al. Prognostic utility of right ventricular remodeling over conventional risk stratification in patients with COVID-19. J Am Coll Cardiol. 2020;76(17):1965-1977. doi:10.1016/j.jacc.2020.08.066
18. Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500. doi:10.1182/blood.2020006520
Flu and COVID-19 vaccines can be given on the same day: CDC and AAP
Previously, the CDC recommended that people receive their COVID-19 vaccinations alone and schedule any other vaccinations at least 2 weeks before or after their COVID-19 immunization. “This was out of an abundance of caution during a period when these vaccines were new and not due to any known safety or immunogenicity concerns,” the CDC guidance states. “However, substantial data have now been collected regarding the safety of COVID-19 vaccines currently approved or authorized by FDA.”
The guidance allowing for coadministration of COVID-19 vaccines with other immunizations, including the flu shot, was issued in mid-May 2021, and was restated in influenza vaccine recommendations released Aug. 27. The American Academy of Pediatrics soon followed suit, announcing that, for children eligible for the COVID-19 vaccine (age 12 and older), AAP recommendations allow for both the influenza and COVID-19 vaccines to be administered during the same visit.
Although there is limited data around giving COVID-19 vaccines with other vaccines, “extensive experience with non–COVID-19 vaccines has demonstrated that immunogenicity and adverse-event profiles are generally similar when vaccines are administered simultaneously as when they are administered alone,” the recommendations state. If administering other immunizations along with COVID-19 vaccines, providers should separate injection sites by at least 1 inch, the CDC recommends, and influenza vaccines that are more likely to cause a local reaction, like high-dose or the adjuvanted inactivated flu vaccine, should be administered in different limbs, if possible.
Whether someone should get their flu vaccine at the same time or separate from a COVID-19 vaccination or booster is a matter of personal preference as well as convenience, Susan Coffin, MD, MPH, an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, said in an interview. “It basically boils down to: Will you be able to get your flu shot without any difficulty in 2 weeks’ time?” she said. “We don’t want inconvenience or difficulties in access to get the way of people getting their flu shot this year.”
A version of this article first appeared on Medscape.com.
Previously, the CDC recommended that people receive their COVID-19 vaccinations alone and schedule any other vaccinations at least 2 weeks before or after their COVID-19 immunization. “This was out of an abundance of caution during a period when these vaccines were new and not due to any known safety or immunogenicity concerns,” the CDC guidance states. “However, substantial data have now been collected regarding the safety of COVID-19 vaccines currently approved or authorized by FDA.”
The guidance allowing for coadministration of COVID-19 vaccines with other immunizations, including the flu shot, was issued in mid-May 2021, and was restated in influenza vaccine recommendations released Aug. 27. The American Academy of Pediatrics soon followed suit, announcing that, for children eligible for the COVID-19 vaccine (age 12 and older), AAP recommendations allow for both the influenza and COVID-19 vaccines to be administered during the same visit.
Although there is limited data around giving COVID-19 vaccines with other vaccines, “extensive experience with non–COVID-19 vaccines has demonstrated that immunogenicity and adverse-event profiles are generally similar when vaccines are administered simultaneously as when they are administered alone,” the recommendations state. If administering other immunizations along with COVID-19 vaccines, providers should separate injection sites by at least 1 inch, the CDC recommends, and influenza vaccines that are more likely to cause a local reaction, like high-dose or the adjuvanted inactivated flu vaccine, should be administered in different limbs, if possible.
Whether someone should get their flu vaccine at the same time or separate from a COVID-19 vaccination or booster is a matter of personal preference as well as convenience, Susan Coffin, MD, MPH, an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, said in an interview. “It basically boils down to: Will you be able to get your flu shot without any difficulty in 2 weeks’ time?” she said. “We don’t want inconvenience or difficulties in access to get the way of people getting their flu shot this year.”
A version of this article first appeared on Medscape.com.
Previously, the CDC recommended that people receive their COVID-19 vaccinations alone and schedule any other vaccinations at least 2 weeks before or after their COVID-19 immunization. “This was out of an abundance of caution during a period when these vaccines were new and not due to any known safety or immunogenicity concerns,” the CDC guidance states. “However, substantial data have now been collected regarding the safety of COVID-19 vaccines currently approved or authorized by FDA.”
The guidance allowing for coadministration of COVID-19 vaccines with other immunizations, including the flu shot, was issued in mid-May 2021, and was restated in influenza vaccine recommendations released Aug. 27. The American Academy of Pediatrics soon followed suit, announcing that, for children eligible for the COVID-19 vaccine (age 12 and older), AAP recommendations allow for both the influenza and COVID-19 vaccines to be administered during the same visit.
Although there is limited data around giving COVID-19 vaccines with other vaccines, “extensive experience with non–COVID-19 vaccines has demonstrated that immunogenicity and adverse-event profiles are generally similar when vaccines are administered simultaneously as when they are administered alone,” the recommendations state. If administering other immunizations along with COVID-19 vaccines, providers should separate injection sites by at least 1 inch, the CDC recommends, and influenza vaccines that are more likely to cause a local reaction, like high-dose or the adjuvanted inactivated flu vaccine, should be administered in different limbs, if possible.
Whether someone should get their flu vaccine at the same time or separate from a COVID-19 vaccination or booster is a matter of personal preference as well as convenience, Susan Coffin, MD, MPH, an attending physician in the division of infectious diseases at Children’s Hospital of Philadelphia, said in an interview. “It basically boils down to: Will you be able to get your flu shot without any difficulty in 2 weeks’ time?” she said. “We don’t want inconvenience or difficulties in access to get the way of people getting their flu shot this year.”
A version of this article first appeared on Medscape.com.
My experience of a COVID-19 vaccine breakthrough infection
Friday, July 16, 2021, marked the end of a week on duty in the hospital, and it was time to celebrate with a nice dinner out with my wife, since COVID-19 masking requirements had been lifted in our part of California for people like us who were fully vaccinated.
We always loved a nice dinner out and missed it so much during the pandemic. Unlike 6 months earlier, when I was administering dexamethasone, remdesivir, and high-flow oxygen to half of the patients on my panel, not a single patient was diagnosed with COVID-19, much less treated for it, during the previous week. We were doing so well in Sacramento that the hospital visitation rules had been relaxed and vaccinated patients were not required to have a negative COVID-19 test prior to hospital admission.
Saturday was game 5 of the NBA finals, so we had two couples join us for the game at our house; no masks because we were all vaccinated. On Sunday, we visited our neighbors who had just had a new baby boy and made them the gift of some baby books. The new mom had struggled with the decision of whether to get vaccinated during her pregnancy, but eventually decided to complete the vaccination cycle prior to delivery. She was fully immune at the time of the baby’s birth, wisely wanting the baby to have passive immunity through her. We kept an appropriate distance, and never touched baby or mom, but since masking guidelines had been lifted for the vaccinated,we didn’t bother with them.
On Monday, I felt a little something in my nose but still pursued my usual workout. Interestingly, my performance wasn’t up to my usual standards. There was a meeting that evening that I had to prepare for, when all of a sudden I felt very fatigued. I lay down and slept for a good hour, which disrupted my preparation. I warned the participants that I was feeling a little under the weather, but they wanted to proceed. At this point, I decided it was time to start wearing a mask again.
More meetings on Tuesday morning, but I made sure that I was fully masked. That little thing in my nose had blown up into a full-scale rhinitis, requiring Kleenex and decongestants. Plus, the fatigue was hitting me very hard. “Dang!” I thought. “I haven’t had a cold since 2019. All those COVID-19 precautions not only worked against COVID-19 (which I never got) but also worked against the common cold, which I had now.”
I finished up my meetings and laid down for a good hour and a half. As the father of two, I had plenty of experience with the common cold, and I knew that plenty of rest and hydration was the key to kicking this thing. Besides, my 55th birthday was coming up, and I wanted to make sure I was fully recovered for the festivities my wife was planning for me. Nonetheless, I scheduled myself for a COVID-19 test. I knew this couldn’t be COVID-19 because I was fully vaccinated, but it was hitting me so hard. It had to be a virus that my body had never seen before; maybe the human metapneumovirus. That was my line of reasoning, anyway.
Wednesday was another day on the couch because of continued severe fatigue and myalgias. I figured another good day of rest would help me kick this cold in time for my birthday celebration. Then the COVID-19 results came back positive. “How could this be? I was vaccinated?!” Admittedly I had been more relaxed with masking, per the CDC and county guidelines, but I always wore a mask when I was seeing patients in the hospital. Yeah, I wasn’t wearing an N95 anymore, and I had given up my goggles months ago, but we just weren’t seeing much COVID-19 anymore, so a plain surgical mask was all that was required and seemed sufficient. I had been reading articles about the new Delta variant that was becoming dominant across the country, and reports were that the vaccine was still effective against the Delta variant. However, I was experiencing the COVID-19 vaccine breakthrough infection because of the remarkable talent the Delta variant has for replicating and producing high levels of viremia.
My first thoughts were for my family, of course. As my illness unfolded, I had kept checking in with them to see if they had any of these “cold” symptoms I had; none of them did. When my test came back positive, we all went into quarantine immediately and they went to get tested; all of them were negative. Next, I contacted the people I had been meeting with that week and warned them that I had tested positive. Despite my mask, and their fully vaccinated status, they needed to get tested. They did, and they were negative. I realized that I was probably contagious, though asymptomatic, on Saturday night when we had friends over to watch the NBA finals. Yeah, everyone was vaccinated, but if I could get sick from this new Delta variant, they could too. The public health department sent me a survey when they found out about my positive test, and they pinpointed Saturday as the day I started to be contagious. I told my friends that I was probably contagious when they were over for the game, and that they should get tested. They did, and everyone came back negative for COVID-19.
Wait a minute; what about Sunday night? The newborn baby and the sleep-deprived mom. Oh no! I was contagious then as well. We kept our distance, and were only there for about 10 minutes, but if I felt bad from COVID-19, I felt worse for exposing them to the virus.
I am no Anthony Fauci, and I am grateful that we have had levelheaded scientists like him to lead us through this terrible experience. I am sure there will be many papers written about COVID-19 breakthrough infections in the future, but I have many thoughts from this experience. First, my practice of wearing an N95 and goggles for all patients, not just COVID-19 patients, during the height of the pandemic was effective. Prior to getting vaccinated, my antibody tests were negative, so I never contracted the illness when I stuck to this regimen. Second, we all want to get back to something that looks like “normal,” but because there are large unvaccinated populations in the community the virus will continue to propagate and evolve, and hence everyone is at risk. While the guidelines said it was okay to ease up on our restrictions, because so many people are not vaccinated, we all must continue to keep our guard up. Third, would a booster shot have saved me from this fate? Because I was on the front lines of the pandemic as a hospitalist, I was also among the first members of my community to get vaccinated, receiving my second shot on Jan. 14, 2021. My wife was not in any risk group, was not on any vaccine priority list, and didn’t complete the series until early April. If I was going to give the infection to anyone, it would have been her. Not only did she never develop symptoms, but she also repeatedly tested negative, as did everyone else that I was in contact with when I was most contagious. The thing that was different about me from everyone else was that I had gotten the vaccine well ahead of them. Had my immunity waned over the months?
The good news is that, while I wouldn’t characterize what I had as “mild,” it certainly wasn’t protracted. Yes, I was a good boy, and did the basics: stay hydrated and get plenty of sleep. I was really bad off for about 3 days, and I hate to think what it would have been like if I had coexisting conditions such as asthma or diabetes. We all know what a bad case of COVID-19 looks like in the unvaccinated, with months in the hospital, intravenous infusions, and high-flow oxygen for the lucky ones. I had nothing remotely like that. The dominant symptom I had was incapacitating fatigue and significant body aches. The second night I had some major chills, sweats, and wild dreams. From a respiratory standpoint, I had bad rhinitis and a wicked cough for a while that tapered off. My oxygen saturations dropped into the mid 90’s, but never below 94%. But if I had been ten times sicker, I doubt I would have survived. I was on quarantine for 10 days but I highly doubt I was at all contagious by day 5, based on my symptoms and the fact that nobody around me turned COVID positive with repeat testing.
I was so relieved that none of my contacts when I was most contagious turned positive for COVID-19. Though not scientific, I find that illustrative. While I should have canceled my meetings on Monday and Tuesday, everybody knew I had a “cold” and nobody wanted to cancel. Nobody thought it possible that I had COVID-19, especially me. The Delta variant is notorious for generating high levels of viremia, yet I didn’t get anybody sick, not even my wife. That suggests to me that, while the vaccine doesn’t eliminate the risk of infection – which we already knew – it probably significantly reduced my infectivity. For that I am very grateful. Now that I can say that I had the COVID-19 experience, I can tell you it feels terrible. But I would have felt much worse if I had gotten others ill. My personal belief is that while the vaccine didn’t save me from disease, it dramatically truncated my illness, and significantly reduced my risk of passing the virus on to my friends and family.
So where did I contract the virus? We were unmasked at dinner on Friday night, which was acceptable in Yolo County at that time. By the way, I actually live in Yolo County, not YOLO (you only live once) county. You can imagine the latter would be a bit more loosey-goosey with the masking requirements. That notwithstanding, I don’t think the dinner was where I picked it up because it was too short of an incubation period. My wife and I obviously reacted differently, as I discussed, but we were both at the restaurant. She didn’t get COVID-19 and I did. I think that I probably picked it up at the hospital, because, while I was wearing a mask there, I was only wearing a surgical mask, not an N95. And I wasn’t wearing goggles anymore. While none of my patients were officially diagnosed with COVID-19, I was encountering a lot of people, getting in relatively close contact, and guidelines were being relaxed, including preadmission COVID-19 testing.
I was an outlier, as I have pointed out; none of my other close contacts contracted COVID-19. A lot of politics and public opinion is driven by outlier cases, and even pure fabrications these days; we certainly can’t create public health policy based on an outlier. I am not suggesting that my experience is any basis for rewriting the rules of COVID-19. The experience has given me pause to think through many facets of this horrible illness we have had to deal with in so many ways, however. And I have also reexamined my own practice for protecting myself in the hospital. Clearly what I was doing in the height of the pandemic was effective, and my more relaxed recent practices were not. Now that I am fully recovered after a relatively unique encounter with the condition, I look forward to seeing what the scientists and public policy makers do with COVID-19 vaccine breakthrough cases. So, between us hospitalist friends and colleagues, regardless of the policy guidelines, I say we keep on masking.
Dr. McIlraith is the founding chairman of the hospital medicine department at Mercy Medical Group in Sacramento. He received the SHM Award for Outstanding Service in Hospital Medicine in 2016.
Friday, July 16, 2021, marked the end of a week on duty in the hospital, and it was time to celebrate with a nice dinner out with my wife, since COVID-19 masking requirements had been lifted in our part of California for people like us who were fully vaccinated.
We always loved a nice dinner out and missed it so much during the pandemic. Unlike 6 months earlier, when I was administering dexamethasone, remdesivir, and high-flow oxygen to half of the patients on my panel, not a single patient was diagnosed with COVID-19, much less treated for it, during the previous week. We were doing so well in Sacramento that the hospital visitation rules had been relaxed and vaccinated patients were not required to have a negative COVID-19 test prior to hospital admission.
Saturday was game 5 of the NBA finals, so we had two couples join us for the game at our house; no masks because we were all vaccinated. On Sunday, we visited our neighbors who had just had a new baby boy and made them the gift of some baby books. The new mom had struggled with the decision of whether to get vaccinated during her pregnancy, but eventually decided to complete the vaccination cycle prior to delivery. She was fully immune at the time of the baby’s birth, wisely wanting the baby to have passive immunity through her. We kept an appropriate distance, and never touched baby or mom, but since masking guidelines had been lifted for the vaccinated,we didn’t bother with them.
On Monday, I felt a little something in my nose but still pursued my usual workout. Interestingly, my performance wasn’t up to my usual standards. There was a meeting that evening that I had to prepare for, when all of a sudden I felt very fatigued. I lay down and slept for a good hour, which disrupted my preparation. I warned the participants that I was feeling a little under the weather, but they wanted to proceed. At this point, I decided it was time to start wearing a mask again.
More meetings on Tuesday morning, but I made sure that I was fully masked. That little thing in my nose had blown up into a full-scale rhinitis, requiring Kleenex and decongestants. Plus, the fatigue was hitting me very hard. “Dang!” I thought. “I haven’t had a cold since 2019. All those COVID-19 precautions not only worked against COVID-19 (which I never got) but also worked against the common cold, which I had now.”
I finished up my meetings and laid down for a good hour and a half. As the father of two, I had plenty of experience with the common cold, and I knew that plenty of rest and hydration was the key to kicking this thing. Besides, my 55th birthday was coming up, and I wanted to make sure I was fully recovered for the festivities my wife was planning for me. Nonetheless, I scheduled myself for a COVID-19 test. I knew this couldn’t be COVID-19 because I was fully vaccinated, but it was hitting me so hard. It had to be a virus that my body had never seen before; maybe the human metapneumovirus. That was my line of reasoning, anyway.
Wednesday was another day on the couch because of continued severe fatigue and myalgias. I figured another good day of rest would help me kick this cold in time for my birthday celebration. Then the COVID-19 results came back positive. “How could this be? I was vaccinated?!” Admittedly I had been more relaxed with masking, per the CDC and county guidelines, but I always wore a mask when I was seeing patients in the hospital. Yeah, I wasn’t wearing an N95 anymore, and I had given up my goggles months ago, but we just weren’t seeing much COVID-19 anymore, so a plain surgical mask was all that was required and seemed sufficient. I had been reading articles about the new Delta variant that was becoming dominant across the country, and reports were that the vaccine was still effective against the Delta variant. However, I was experiencing the COVID-19 vaccine breakthrough infection because of the remarkable talent the Delta variant has for replicating and producing high levels of viremia.
My first thoughts were for my family, of course. As my illness unfolded, I had kept checking in with them to see if they had any of these “cold” symptoms I had; none of them did. When my test came back positive, we all went into quarantine immediately and they went to get tested; all of them were negative. Next, I contacted the people I had been meeting with that week and warned them that I had tested positive. Despite my mask, and their fully vaccinated status, they needed to get tested. They did, and they were negative. I realized that I was probably contagious, though asymptomatic, on Saturday night when we had friends over to watch the NBA finals. Yeah, everyone was vaccinated, but if I could get sick from this new Delta variant, they could too. The public health department sent me a survey when they found out about my positive test, and they pinpointed Saturday as the day I started to be contagious. I told my friends that I was probably contagious when they were over for the game, and that they should get tested. They did, and everyone came back negative for COVID-19.
Wait a minute; what about Sunday night? The newborn baby and the sleep-deprived mom. Oh no! I was contagious then as well. We kept our distance, and were only there for about 10 minutes, but if I felt bad from COVID-19, I felt worse for exposing them to the virus.
I am no Anthony Fauci, and I am grateful that we have had levelheaded scientists like him to lead us through this terrible experience. I am sure there will be many papers written about COVID-19 breakthrough infections in the future, but I have many thoughts from this experience. First, my practice of wearing an N95 and goggles for all patients, not just COVID-19 patients, during the height of the pandemic was effective. Prior to getting vaccinated, my antibody tests were negative, so I never contracted the illness when I stuck to this regimen. Second, we all want to get back to something that looks like “normal,” but because there are large unvaccinated populations in the community the virus will continue to propagate and evolve, and hence everyone is at risk. While the guidelines said it was okay to ease up on our restrictions, because so many people are not vaccinated, we all must continue to keep our guard up. Third, would a booster shot have saved me from this fate? Because I was on the front lines of the pandemic as a hospitalist, I was also among the first members of my community to get vaccinated, receiving my second shot on Jan. 14, 2021. My wife was not in any risk group, was not on any vaccine priority list, and didn’t complete the series until early April. If I was going to give the infection to anyone, it would have been her. Not only did she never develop symptoms, but she also repeatedly tested negative, as did everyone else that I was in contact with when I was most contagious. The thing that was different about me from everyone else was that I had gotten the vaccine well ahead of them. Had my immunity waned over the months?
The good news is that, while I wouldn’t characterize what I had as “mild,” it certainly wasn’t protracted. Yes, I was a good boy, and did the basics: stay hydrated and get plenty of sleep. I was really bad off for about 3 days, and I hate to think what it would have been like if I had coexisting conditions such as asthma or diabetes. We all know what a bad case of COVID-19 looks like in the unvaccinated, with months in the hospital, intravenous infusions, and high-flow oxygen for the lucky ones. I had nothing remotely like that. The dominant symptom I had was incapacitating fatigue and significant body aches. The second night I had some major chills, sweats, and wild dreams. From a respiratory standpoint, I had bad rhinitis and a wicked cough for a while that tapered off. My oxygen saturations dropped into the mid 90’s, but never below 94%. But if I had been ten times sicker, I doubt I would have survived. I was on quarantine for 10 days but I highly doubt I was at all contagious by day 5, based on my symptoms and the fact that nobody around me turned COVID positive with repeat testing.
I was so relieved that none of my contacts when I was most contagious turned positive for COVID-19. Though not scientific, I find that illustrative. While I should have canceled my meetings on Monday and Tuesday, everybody knew I had a “cold” and nobody wanted to cancel. Nobody thought it possible that I had COVID-19, especially me. The Delta variant is notorious for generating high levels of viremia, yet I didn’t get anybody sick, not even my wife. That suggests to me that, while the vaccine doesn’t eliminate the risk of infection – which we already knew – it probably significantly reduced my infectivity. For that I am very grateful. Now that I can say that I had the COVID-19 experience, I can tell you it feels terrible. But I would have felt much worse if I had gotten others ill. My personal belief is that while the vaccine didn’t save me from disease, it dramatically truncated my illness, and significantly reduced my risk of passing the virus on to my friends and family.
So where did I contract the virus? We were unmasked at dinner on Friday night, which was acceptable in Yolo County at that time. By the way, I actually live in Yolo County, not YOLO (you only live once) county. You can imagine the latter would be a bit more loosey-goosey with the masking requirements. That notwithstanding, I don’t think the dinner was where I picked it up because it was too short of an incubation period. My wife and I obviously reacted differently, as I discussed, but we were both at the restaurant. She didn’t get COVID-19 and I did. I think that I probably picked it up at the hospital, because, while I was wearing a mask there, I was only wearing a surgical mask, not an N95. And I wasn’t wearing goggles anymore. While none of my patients were officially diagnosed with COVID-19, I was encountering a lot of people, getting in relatively close contact, and guidelines were being relaxed, including preadmission COVID-19 testing.
I was an outlier, as I have pointed out; none of my other close contacts contracted COVID-19. A lot of politics and public opinion is driven by outlier cases, and even pure fabrications these days; we certainly can’t create public health policy based on an outlier. I am not suggesting that my experience is any basis for rewriting the rules of COVID-19. The experience has given me pause to think through many facets of this horrible illness we have had to deal with in so many ways, however. And I have also reexamined my own practice for protecting myself in the hospital. Clearly what I was doing in the height of the pandemic was effective, and my more relaxed recent practices were not. Now that I am fully recovered after a relatively unique encounter with the condition, I look forward to seeing what the scientists and public policy makers do with COVID-19 vaccine breakthrough cases. So, between us hospitalist friends and colleagues, regardless of the policy guidelines, I say we keep on masking.
Dr. McIlraith is the founding chairman of the hospital medicine department at Mercy Medical Group in Sacramento. He received the SHM Award for Outstanding Service in Hospital Medicine in 2016.
Friday, July 16, 2021, marked the end of a week on duty in the hospital, and it was time to celebrate with a nice dinner out with my wife, since COVID-19 masking requirements had been lifted in our part of California for people like us who were fully vaccinated.
We always loved a nice dinner out and missed it so much during the pandemic. Unlike 6 months earlier, when I was administering dexamethasone, remdesivir, and high-flow oxygen to half of the patients on my panel, not a single patient was diagnosed with COVID-19, much less treated for it, during the previous week. We were doing so well in Sacramento that the hospital visitation rules had been relaxed and vaccinated patients were not required to have a negative COVID-19 test prior to hospital admission.
Saturday was game 5 of the NBA finals, so we had two couples join us for the game at our house; no masks because we were all vaccinated. On Sunday, we visited our neighbors who had just had a new baby boy and made them the gift of some baby books. The new mom had struggled with the decision of whether to get vaccinated during her pregnancy, but eventually decided to complete the vaccination cycle prior to delivery. She was fully immune at the time of the baby’s birth, wisely wanting the baby to have passive immunity through her. We kept an appropriate distance, and never touched baby or mom, but since masking guidelines had been lifted for the vaccinated,we didn’t bother with them.
On Monday, I felt a little something in my nose but still pursued my usual workout. Interestingly, my performance wasn’t up to my usual standards. There was a meeting that evening that I had to prepare for, when all of a sudden I felt very fatigued. I lay down and slept for a good hour, which disrupted my preparation. I warned the participants that I was feeling a little under the weather, but they wanted to proceed. At this point, I decided it was time to start wearing a mask again.
More meetings on Tuesday morning, but I made sure that I was fully masked. That little thing in my nose had blown up into a full-scale rhinitis, requiring Kleenex and decongestants. Plus, the fatigue was hitting me very hard. “Dang!” I thought. “I haven’t had a cold since 2019. All those COVID-19 precautions not only worked against COVID-19 (which I never got) but also worked against the common cold, which I had now.”
I finished up my meetings and laid down for a good hour and a half. As the father of two, I had plenty of experience with the common cold, and I knew that plenty of rest and hydration was the key to kicking this thing. Besides, my 55th birthday was coming up, and I wanted to make sure I was fully recovered for the festivities my wife was planning for me. Nonetheless, I scheduled myself for a COVID-19 test. I knew this couldn’t be COVID-19 because I was fully vaccinated, but it was hitting me so hard. It had to be a virus that my body had never seen before; maybe the human metapneumovirus. That was my line of reasoning, anyway.
Wednesday was another day on the couch because of continued severe fatigue and myalgias. I figured another good day of rest would help me kick this cold in time for my birthday celebration. Then the COVID-19 results came back positive. “How could this be? I was vaccinated?!” Admittedly I had been more relaxed with masking, per the CDC and county guidelines, but I always wore a mask when I was seeing patients in the hospital. Yeah, I wasn’t wearing an N95 anymore, and I had given up my goggles months ago, but we just weren’t seeing much COVID-19 anymore, so a plain surgical mask was all that was required and seemed sufficient. I had been reading articles about the new Delta variant that was becoming dominant across the country, and reports were that the vaccine was still effective against the Delta variant. However, I was experiencing the COVID-19 vaccine breakthrough infection because of the remarkable talent the Delta variant has for replicating and producing high levels of viremia.
My first thoughts were for my family, of course. As my illness unfolded, I had kept checking in with them to see if they had any of these “cold” symptoms I had; none of them did. When my test came back positive, we all went into quarantine immediately and they went to get tested; all of them were negative. Next, I contacted the people I had been meeting with that week and warned them that I had tested positive. Despite my mask, and their fully vaccinated status, they needed to get tested. They did, and they were negative. I realized that I was probably contagious, though asymptomatic, on Saturday night when we had friends over to watch the NBA finals. Yeah, everyone was vaccinated, but if I could get sick from this new Delta variant, they could too. The public health department sent me a survey when they found out about my positive test, and they pinpointed Saturday as the day I started to be contagious. I told my friends that I was probably contagious when they were over for the game, and that they should get tested. They did, and everyone came back negative for COVID-19.
Wait a minute; what about Sunday night? The newborn baby and the sleep-deprived mom. Oh no! I was contagious then as well. We kept our distance, and were only there for about 10 minutes, but if I felt bad from COVID-19, I felt worse for exposing them to the virus.
I am no Anthony Fauci, and I am grateful that we have had levelheaded scientists like him to lead us through this terrible experience. I am sure there will be many papers written about COVID-19 breakthrough infections in the future, but I have many thoughts from this experience. First, my practice of wearing an N95 and goggles for all patients, not just COVID-19 patients, during the height of the pandemic was effective. Prior to getting vaccinated, my antibody tests were negative, so I never contracted the illness when I stuck to this regimen. Second, we all want to get back to something that looks like “normal,” but because there are large unvaccinated populations in the community the virus will continue to propagate and evolve, and hence everyone is at risk. While the guidelines said it was okay to ease up on our restrictions, because so many people are not vaccinated, we all must continue to keep our guard up. Third, would a booster shot have saved me from this fate? Because I was on the front lines of the pandemic as a hospitalist, I was also among the first members of my community to get vaccinated, receiving my second shot on Jan. 14, 2021. My wife was not in any risk group, was not on any vaccine priority list, and didn’t complete the series until early April. If I was going to give the infection to anyone, it would have been her. Not only did she never develop symptoms, but she also repeatedly tested negative, as did everyone else that I was in contact with when I was most contagious. The thing that was different about me from everyone else was that I had gotten the vaccine well ahead of them. Had my immunity waned over the months?
The good news is that, while I wouldn’t characterize what I had as “mild,” it certainly wasn’t protracted. Yes, I was a good boy, and did the basics: stay hydrated and get plenty of sleep. I was really bad off for about 3 days, and I hate to think what it would have been like if I had coexisting conditions such as asthma or diabetes. We all know what a bad case of COVID-19 looks like in the unvaccinated, with months in the hospital, intravenous infusions, and high-flow oxygen for the lucky ones. I had nothing remotely like that. The dominant symptom I had was incapacitating fatigue and significant body aches. The second night I had some major chills, sweats, and wild dreams. From a respiratory standpoint, I had bad rhinitis and a wicked cough for a while that tapered off. My oxygen saturations dropped into the mid 90’s, but never below 94%. But if I had been ten times sicker, I doubt I would have survived. I was on quarantine for 10 days but I highly doubt I was at all contagious by day 5, based on my symptoms and the fact that nobody around me turned COVID positive with repeat testing.
I was so relieved that none of my contacts when I was most contagious turned positive for COVID-19. Though not scientific, I find that illustrative. While I should have canceled my meetings on Monday and Tuesday, everybody knew I had a “cold” and nobody wanted to cancel. Nobody thought it possible that I had COVID-19, especially me. The Delta variant is notorious for generating high levels of viremia, yet I didn’t get anybody sick, not even my wife. That suggests to me that, while the vaccine doesn’t eliminate the risk of infection – which we already knew – it probably significantly reduced my infectivity. For that I am very grateful. Now that I can say that I had the COVID-19 experience, I can tell you it feels terrible. But I would have felt much worse if I had gotten others ill. My personal belief is that while the vaccine didn’t save me from disease, it dramatically truncated my illness, and significantly reduced my risk of passing the virus on to my friends and family.
So where did I contract the virus? We were unmasked at dinner on Friday night, which was acceptable in Yolo County at that time. By the way, I actually live in Yolo County, not YOLO (you only live once) county. You can imagine the latter would be a bit more loosey-goosey with the masking requirements. That notwithstanding, I don’t think the dinner was where I picked it up because it was too short of an incubation period. My wife and I obviously reacted differently, as I discussed, but we were both at the restaurant. She didn’t get COVID-19 and I did. I think that I probably picked it up at the hospital, because, while I was wearing a mask there, I was only wearing a surgical mask, not an N95. And I wasn’t wearing goggles anymore. While none of my patients were officially diagnosed with COVID-19, I was encountering a lot of people, getting in relatively close contact, and guidelines were being relaxed, including preadmission COVID-19 testing.
I was an outlier, as I have pointed out; none of my other close contacts contracted COVID-19. A lot of politics and public opinion is driven by outlier cases, and even pure fabrications these days; we certainly can’t create public health policy based on an outlier. I am not suggesting that my experience is any basis for rewriting the rules of COVID-19. The experience has given me pause to think through many facets of this horrible illness we have had to deal with in so many ways, however. And I have also reexamined my own practice for protecting myself in the hospital. Clearly what I was doing in the height of the pandemic was effective, and my more relaxed recent practices were not. Now that I am fully recovered after a relatively unique encounter with the condition, I look forward to seeing what the scientists and public policy makers do with COVID-19 vaccine breakthrough cases. So, between us hospitalist friends and colleagues, regardless of the policy guidelines, I say we keep on masking.
Dr. McIlraith is the founding chairman of the hospital medicine department at Mercy Medical Group in Sacramento. He received the SHM Award for Outstanding Service in Hospital Medicine in 2016.
Researchers describe first reports of breakthrough COVID infections, booster shots in rheumatology patients
Although breakthrough COVID-19 infections appear to be infrequent in people with inflammatory rheumatic and musculoskeletal diseases (iRMDs), these patients’ comparatively low antibodies after their initial vaccine series validate the recommendation that booster doses could reinforce their immune responses. These findings were highlighted in three letters recently published in Annals of the Rheumatic Diseases.
In the first letter, the researchers assessed breakthrough COVID-19 infections among vaccinated patients with iRMDs who were treated within the Mass General Brigham health care system in the Boston area. Of the 340 COVID-19 infections in patients with iRMDs after vaccinations were approved by the Food and Drug Administration for emergency use, 16 (4.7%) were breakthrough infections. All but one of the breakthrough infections were symptomatic, and six of the patients were hospitalized.
Patients who had breakthrough infections took disease-modifying antirheumatic drugs (DMARDs) that included rituximab and glucocorticoids (five patients each), mycophenolate mofetil or mycophenolic acid (four patients), and methotrexate (three patients). Two of the patients died, both of whom were on rituximab and had interstitial lung disease.
“Some DMARD users may require alternative risk-mitigation strategies, including passive immunity or booster vaccines, and may need to continue shielding practices,” the authors wrote.
“Honestly, it’s hard to know what to make of that rate of breakthrough infections,” Camille Kotton, MD, clinical director of transplant and immunocompromised host infectious diseases in the infectious diseases division at Massachusetts General Hospital in Boston, said in an interview. “People who are immunocompromised were strongly advised to change behavior so as to avoid infection, which probably greatly alters their risk of breakthrough infection. It’s thus hard to evaluate vaccine efficacy.
“Also, 93% were symptomatic, which is fairly high,” she added. “I’m not sure if these patients were more likely to be symptomatic or if there was some bias in testing based on symptoms.”
In the second letter, the researchers assessed postvaccination COVID-19 infections in European patients with iRMDs. Two COVID-19 registries with thousands of patients were reviewed, with less than 1% of patients in each deemed eligible for this study. Of the 34 patients who were ultimately analyzed with available COVID-19 outcomes – 10 were fully vaccinated and 24 were partially vaccinated – 28 fully recovered, 3 recovered with ongoing sequelae, and 3 patients died. The three patients who died were all over 70 years old and had been treated with glucocorticoids and mycophenolate mofetil, glucocorticoids, and rituximab, respectively.
The medications most frequently used by the iRMDs patients with breakthrough cases included glucocorticoids (32%), methotrexate (26%), and tumor necrosis factor inhibitors (26%).
“Overall, the low numbers of SARS-CoV-2 infection post vaccination in both registries are encouraging,” the authors wrote, adding that “all three deceased patients were treated with medications that are potential negative influences on postvaccination SARS-CoV-2 immunogenicity in the RMD population.”
Patients with RMDs: Consider COVID-19 booster shots
In the third letter, the researchers investigated booster doses of COVID-19 vaccine in patients with autoimmune diseases. Of the 18 participants who received a booster dose, 14 were on antimetabolite therapy and 8 of those were on mycophenolate. At a median of 29 days after completion of their initial vaccine series, antispike antibodies were negative in 10 of the participants and low positive in 6 others, with a median antispike antibody level of less than 0.4 U/mL (interquartile range, <0.4-222 U/mL).
Booster doses were administered at a median of 77 days after completion of the initial series. At a median of 30 days after booster dose, 89% of the participants had an augmented humoral response, with a median antispike antibody level of 2,500 (IQR, 885-2,500 U/mL). Of the 10 participants who had negative anti-spike antibodies after the initial series, 80% were positive after the booster.
“I think this study supports the wealth of evidence that contributed to the [Centers for Disease Control and Prevention]’s and the FDA’s recommendation to get the third dose of the COVID vaccination,” coauthor Julie J. Paik, MD, of Johns Hopkins University, Baltimore, said in an interview. “Our patients are a very vulnerable group, including lupus patients or myositis patients, both of whom can get severe COVID if they were to contract it. They think they’re protected after a two-dose series, but in reality they’re not.
“We were just happy that they had a response,” she added. “Most of them had absolutely no response whatsoever after the first series.”
One other recently published case report in Arthritis & Rheumatology describes booster vaccination with the viral vector Johnson & Johnson vaccine in a man with seropositive RA who had previously received both doses of the Moderna mRNA-1273 vaccine. The 74-year-old man, who had low disease activity over the past 5 years on hydroxychloroquine, etanercept, and leflunomide, received the booster dose of his own accord after undergoing testing that showed a semiquantitative spike protein receptor binding domain (RBD) antibody level of 53.9 U/mL (reference range, 0-2,500 U/mL) and a negative SARS-CoV-2 antispike (S1/RBD) IgG test, as well as less than 10% blocking activity on an assay designed to detect blocking of the interaction between the SARS-CoV-2 spike protein RBD and the human ACE2 receptor and a negative interferon-gamma release assay detecting SARS-CoV-2–specific T cells. Several weeks after the booster dose, a repeat semiquantitative spike protein RBD antibody level was 2,455.0 U/mL and the S1/RBD IgG level was positive. An ACE2 blocking assay demonstrated 90%-100% blocking activity, but the interferon-gamma release assay remained negative.
“I would recommend abiding by the CDC guidelines regarding boosters for immunocompromised patients,” Dr. Kotton stated. “Patients with rheumatologic disease generally fit into the last category on that list. We don’t have an antibody titer that ensures protection, and as per CDC guidance, we don’t recommend checking antibody titers. Furthermore, boosters were given for this study before the CDC recommendation came out.”
Dr. Paik and coauthors acknowledged their study’s limitations, including a small, inhomogeneous sample and a lack of data on memory B-cell and T-cell response. They also echoed Dr. Kotton’s thoughts by noting that, although this subset of patients had notably limited antibody responses, “no antibody titer has been defined to correlate with protection.”
“Of course, the humoral response isn’t the whole story,” Dr. Paik said. “Some studies are showing that some vaccine recipients may not have the antibodies but their T-cell response may still be intact; it just takes time, and we’re not picking it up. Even if the antibody test is coming up negative, there may be some immunogenicity to the vaccine that we’re not detecting.
“Hopefully at some point, we’ll have more T-cell immunophenotyping to provide better insight into the full vaccine response.”
The Boston-area breakthrough study and the booster shot study were both funded primarily by grants from various institutes within the National Institutes of Health. The European study was financially supported by the European Alliance of Associations for Rheumatology.
Although breakthrough COVID-19 infections appear to be infrequent in people with inflammatory rheumatic and musculoskeletal diseases (iRMDs), these patients’ comparatively low antibodies after their initial vaccine series validate the recommendation that booster doses could reinforce their immune responses. These findings were highlighted in three letters recently published in Annals of the Rheumatic Diseases.
In the first letter, the researchers assessed breakthrough COVID-19 infections among vaccinated patients with iRMDs who were treated within the Mass General Brigham health care system in the Boston area. Of the 340 COVID-19 infections in patients with iRMDs after vaccinations were approved by the Food and Drug Administration for emergency use, 16 (4.7%) were breakthrough infections. All but one of the breakthrough infections were symptomatic, and six of the patients were hospitalized.
Patients who had breakthrough infections took disease-modifying antirheumatic drugs (DMARDs) that included rituximab and glucocorticoids (five patients each), mycophenolate mofetil or mycophenolic acid (four patients), and methotrexate (three patients). Two of the patients died, both of whom were on rituximab and had interstitial lung disease.
“Some DMARD users may require alternative risk-mitigation strategies, including passive immunity or booster vaccines, and may need to continue shielding practices,” the authors wrote.
“Honestly, it’s hard to know what to make of that rate of breakthrough infections,” Camille Kotton, MD, clinical director of transplant and immunocompromised host infectious diseases in the infectious diseases division at Massachusetts General Hospital in Boston, said in an interview. “People who are immunocompromised were strongly advised to change behavior so as to avoid infection, which probably greatly alters their risk of breakthrough infection. It’s thus hard to evaluate vaccine efficacy.
“Also, 93% were symptomatic, which is fairly high,” she added. “I’m not sure if these patients were more likely to be symptomatic or if there was some bias in testing based on symptoms.”
In the second letter, the researchers assessed postvaccination COVID-19 infections in European patients with iRMDs. Two COVID-19 registries with thousands of patients were reviewed, with less than 1% of patients in each deemed eligible for this study. Of the 34 patients who were ultimately analyzed with available COVID-19 outcomes – 10 were fully vaccinated and 24 were partially vaccinated – 28 fully recovered, 3 recovered with ongoing sequelae, and 3 patients died. The three patients who died were all over 70 years old and had been treated with glucocorticoids and mycophenolate mofetil, glucocorticoids, and rituximab, respectively.
The medications most frequently used by the iRMDs patients with breakthrough cases included glucocorticoids (32%), methotrexate (26%), and tumor necrosis factor inhibitors (26%).
“Overall, the low numbers of SARS-CoV-2 infection post vaccination in both registries are encouraging,” the authors wrote, adding that “all three deceased patients were treated with medications that are potential negative influences on postvaccination SARS-CoV-2 immunogenicity in the RMD population.”
Patients with RMDs: Consider COVID-19 booster shots
In the third letter, the researchers investigated booster doses of COVID-19 vaccine in patients with autoimmune diseases. Of the 18 participants who received a booster dose, 14 were on antimetabolite therapy and 8 of those were on mycophenolate. At a median of 29 days after completion of their initial vaccine series, antispike antibodies were negative in 10 of the participants and low positive in 6 others, with a median antispike antibody level of less than 0.4 U/mL (interquartile range, <0.4-222 U/mL).
Booster doses were administered at a median of 77 days after completion of the initial series. At a median of 30 days after booster dose, 89% of the participants had an augmented humoral response, with a median antispike antibody level of 2,500 (IQR, 885-2,500 U/mL). Of the 10 participants who had negative anti-spike antibodies after the initial series, 80% were positive after the booster.
“I think this study supports the wealth of evidence that contributed to the [Centers for Disease Control and Prevention]’s and the FDA’s recommendation to get the third dose of the COVID vaccination,” coauthor Julie J. Paik, MD, of Johns Hopkins University, Baltimore, said in an interview. “Our patients are a very vulnerable group, including lupus patients or myositis patients, both of whom can get severe COVID if they were to contract it. They think they’re protected after a two-dose series, but in reality they’re not.
“We were just happy that they had a response,” she added. “Most of them had absolutely no response whatsoever after the first series.”
One other recently published case report in Arthritis & Rheumatology describes booster vaccination with the viral vector Johnson & Johnson vaccine in a man with seropositive RA who had previously received both doses of the Moderna mRNA-1273 vaccine. The 74-year-old man, who had low disease activity over the past 5 years on hydroxychloroquine, etanercept, and leflunomide, received the booster dose of his own accord after undergoing testing that showed a semiquantitative spike protein receptor binding domain (RBD) antibody level of 53.9 U/mL (reference range, 0-2,500 U/mL) and a negative SARS-CoV-2 antispike (S1/RBD) IgG test, as well as less than 10% blocking activity on an assay designed to detect blocking of the interaction between the SARS-CoV-2 spike protein RBD and the human ACE2 receptor and a negative interferon-gamma release assay detecting SARS-CoV-2–specific T cells. Several weeks after the booster dose, a repeat semiquantitative spike protein RBD antibody level was 2,455.0 U/mL and the S1/RBD IgG level was positive. An ACE2 blocking assay demonstrated 90%-100% blocking activity, but the interferon-gamma release assay remained negative.
“I would recommend abiding by the CDC guidelines regarding boosters for immunocompromised patients,” Dr. Kotton stated. “Patients with rheumatologic disease generally fit into the last category on that list. We don’t have an antibody titer that ensures protection, and as per CDC guidance, we don’t recommend checking antibody titers. Furthermore, boosters were given for this study before the CDC recommendation came out.”
Dr. Paik and coauthors acknowledged their study’s limitations, including a small, inhomogeneous sample and a lack of data on memory B-cell and T-cell response. They also echoed Dr. Kotton’s thoughts by noting that, although this subset of patients had notably limited antibody responses, “no antibody titer has been defined to correlate with protection.”
“Of course, the humoral response isn’t the whole story,” Dr. Paik said. “Some studies are showing that some vaccine recipients may not have the antibodies but their T-cell response may still be intact; it just takes time, and we’re not picking it up. Even if the antibody test is coming up negative, there may be some immunogenicity to the vaccine that we’re not detecting.
“Hopefully at some point, we’ll have more T-cell immunophenotyping to provide better insight into the full vaccine response.”
The Boston-area breakthrough study and the booster shot study were both funded primarily by grants from various institutes within the National Institutes of Health. The European study was financially supported by the European Alliance of Associations for Rheumatology.
Although breakthrough COVID-19 infections appear to be infrequent in people with inflammatory rheumatic and musculoskeletal diseases (iRMDs), these patients’ comparatively low antibodies after their initial vaccine series validate the recommendation that booster doses could reinforce their immune responses. These findings were highlighted in three letters recently published in Annals of the Rheumatic Diseases.
In the first letter, the researchers assessed breakthrough COVID-19 infections among vaccinated patients with iRMDs who were treated within the Mass General Brigham health care system in the Boston area. Of the 340 COVID-19 infections in patients with iRMDs after vaccinations were approved by the Food and Drug Administration for emergency use, 16 (4.7%) were breakthrough infections. All but one of the breakthrough infections were symptomatic, and six of the patients were hospitalized.
Patients who had breakthrough infections took disease-modifying antirheumatic drugs (DMARDs) that included rituximab and glucocorticoids (five patients each), mycophenolate mofetil or mycophenolic acid (four patients), and methotrexate (three patients). Two of the patients died, both of whom were on rituximab and had interstitial lung disease.
“Some DMARD users may require alternative risk-mitigation strategies, including passive immunity or booster vaccines, and may need to continue shielding practices,” the authors wrote.
“Honestly, it’s hard to know what to make of that rate of breakthrough infections,” Camille Kotton, MD, clinical director of transplant and immunocompromised host infectious diseases in the infectious diseases division at Massachusetts General Hospital in Boston, said in an interview. “People who are immunocompromised were strongly advised to change behavior so as to avoid infection, which probably greatly alters their risk of breakthrough infection. It’s thus hard to evaluate vaccine efficacy.
“Also, 93% were symptomatic, which is fairly high,” she added. “I’m not sure if these patients were more likely to be symptomatic or if there was some bias in testing based on symptoms.”
In the second letter, the researchers assessed postvaccination COVID-19 infections in European patients with iRMDs. Two COVID-19 registries with thousands of patients were reviewed, with less than 1% of patients in each deemed eligible for this study. Of the 34 patients who were ultimately analyzed with available COVID-19 outcomes – 10 were fully vaccinated and 24 were partially vaccinated – 28 fully recovered, 3 recovered with ongoing sequelae, and 3 patients died. The three patients who died were all over 70 years old and had been treated with glucocorticoids and mycophenolate mofetil, glucocorticoids, and rituximab, respectively.
The medications most frequently used by the iRMDs patients with breakthrough cases included glucocorticoids (32%), methotrexate (26%), and tumor necrosis factor inhibitors (26%).
“Overall, the low numbers of SARS-CoV-2 infection post vaccination in both registries are encouraging,” the authors wrote, adding that “all three deceased patients were treated with medications that are potential negative influences on postvaccination SARS-CoV-2 immunogenicity in the RMD population.”
Patients with RMDs: Consider COVID-19 booster shots
In the third letter, the researchers investigated booster doses of COVID-19 vaccine in patients with autoimmune diseases. Of the 18 participants who received a booster dose, 14 were on antimetabolite therapy and 8 of those were on mycophenolate. At a median of 29 days after completion of their initial vaccine series, antispike antibodies were negative in 10 of the participants and low positive in 6 others, with a median antispike antibody level of less than 0.4 U/mL (interquartile range, <0.4-222 U/mL).
Booster doses were administered at a median of 77 days after completion of the initial series. At a median of 30 days after booster dose, 89% of the participants had an augmented humoral response, with a median antispike antibody level of 2,500 (IQR, 885-2,500 U/mL). Of the 10 participants who had negative anti-spike antibodies after the initial series, 80% were positive after the booster.
“I think this study supports the wealth of evidence that contributed to the [Centers for Disease Control and Prevention]’s and the FDA’s recommendation to get the third dose of the COVID vaccination,” coauthor Julie J. Paik, MD, of Johns Hopkins University, Baltimore, said in an interview. “Our patients are a very vulnerable group, including lupus patients or myositis patients, both of whom can get severe COVID if they were to contract it. They think they’re protected after a two-dose series, but in reality they’re not.
“We were just happy that they had a response,” she added. “Most of them had absolutely no response whatsoever after the first series.”
One other recently published case report in Arthritis & Rheumatology describes booster vaccination with the viral vector Johnson & Johnson vaccine in a man with seropositive RA who had previously received both doses of the Moderna mRNA-1273 vaccine. The 74-year-old man, who had low disease activity over the past 5 years on hydroxychloroquine, etanercept, and leflunomide, received the booster dose of his own accord after undergoing testing that showed a semiquantitative spike protein receptor binding domain (RBD) antibody level of 53.9 U/mL (reference range, 0-2,500 U/mL) and a negative SARS-CoV-2 antispike (S1/RBD) IgG test, as well as less than 10% blocking activity on an assay designed to detect blocking of the interaction between the SARS-CoV-2 spike protein RBD and the human ACE2 receptor and a negative interferon-gamma release assay detecting SARS-CoV-2–specific T cells. Several weeks after the booster dose, a repeat semiquantitative spike protein RBD antibody level was 2,455.0 U/mL and the S1/RBD IgG level was positive. An ACE2 blocking assay demonstrated 90%-100% blocking activity, but the interferon-gamma release assay remained negative.
“I would recommend abiding by the CDC guidelines regarding boosters for immunocompromised patients,” Dr. Kotton stated. “Patients with rheumatologic disease generally fit into the last category on that list. We don’t have an antibody titer that ensures protection, and as per CDC guidance, we don’t recommend checking antibody titers. Furthermore, boosters were given for this study before the CDC recommendation came out.”
Dr. Paik and coauthors acknowledged their study’s limitations, including a small, inhomogeneous sample and a lack of data on memory B-cell and T-cell response. They also echoed Dr. Kotton’s thoughts by noting that, although this subset of patients had notably limited antibody responses, “no antibody titer has been defined to correlate with protection.”
“Of course, the humoral response isn’t the whole story,” Dr. Paik said. “Some studies are showing that some vaccine recipients may not have the antibodies but their T-cell response may still be intact; it just takes time, and we’re not picking it up. Even if the antibody test is coming up negative, there may be some immunogenicity to the vaccine that we’re not detecting.
“Hopefully at some point, we’ll have more T-cell immunophenotyping to provide better insight into the full vaccine response.”
The Boston-area breakthrough study and the booster shot study were both funded primarily by grants from various institutes within the National Institutes of Health. The European study was financially supported by the European Alliance of Associations for Rheumatology.
FROM ANNALS OF THE RHEUMATIC DISEASES
Even those who just test positive at more risk for long COVID: CDC
Long-term symptoms, like those linked with COVID-19, were common in people who had even just a single positive test, new Centers for Disease Control and Prevention data show.
The data show that symptoms in this group – including fatigue, cough, and headache – tended to last for more than a month.
Frequency of symptoms in people with a positive test was 1.5 times higher, compared with people whose tests had always been negative, according to the research published in the CDC’s latest Morbidity and Mortality Weekly Report.
Lead author Valentine Wanga, PhD, with the CDC’s COVID-19 response team, and colleagues conducted a non–probability-based internet panel survey of about 6,000 U.S. adults to assess long-term symptoms often associated with COVID-19 among those who had ever tested positive or always tested negative for COVID-19 between January 2020 and April 2021.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., said in an interview that this research “establishes more securely than before that you don’t have to be hospitalized with COVID in order to develop long COVID symptoms.”
That’s better known among infectious disease experts, he said, but added that “this survey really gives a firm database for that.”
Study results
The study’s results showed that, compared with respondents who had a negative test result, those who received a positive result reported a significantly higher prevalence of any long-term symptom (65.9% vs. 42.9%), fatigue (22.5% vs. 12.0%), change in sense of smell or taste (17.3% vs. 1.7%), shortness of breath (15.5% vs. 5.2%), cough (14.5% vs. 4.9%), and headache (13.8% vs. 9.9%).
More people who had a positive test result (76.2%) reported persistence for more than a month of at least one initially occurring symptom, compared with those whose test results were always negative (69.6%).
The numbers are further proof, Dr. Schaffner said, that COVID not only will be an acute stressor on the health care system but patients with long COVID will need help with managing care for the long term.
“We still don’t know what the COVID virus does that results in these long COVID symptoms,” he said. Vanderbilt and many other institutions have developed “long COVID” centers as a testament to how important the problem is.
Long COVID symptoms are not well understood and most studies have looked at the effects from patients who had been hospitalized with COVID-19.
In this survey, respondents self-reported whether they had ever had a positive SARS-CoV-2 test result (698), always received a negative test result (2,437), or never were tested for SARS-CoV-2 (2,750).
Compared with those who always tested negative, a larger proportion of those who tested positive (28.7% vs. 15.7%) reported believing that receiving a COVID-19 vaccine made their long-term symptoms better. No difference was found in reported beliefs that a vaccine made long-term symptoms worse.
Dr. Schaffner said he found that survey result interesting, but said that is not backed up by current data and would need further study.
“I would treat that with great caution,” he said. “I’m not dismissing it, but you can’t take that at face value. All of us who get sick and those of us who care for people who are sick – if there’s an intervention, we all hope for the best. We’re being optimistic. It’s when you do a randomized, double-blind, placebo-controlled study that you can find out whether your instincts or hopes were correct.”
The authors said that findings can inform public health preparedness, help guide care for people with post-COVID conditions, and help make the case for vaccines.
The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term symptoms, like those linked with COVID-19, were common in people who had even just a single positive test, new Centers for Disease Control and Prevention data show.
The data show that symptoms in this group – including fatigue, cough, and headache – tended to last for more than a month.
Frequency of symptoms in people with a positive test was 1.5 times higher, compared with people whose tests had always been negative, according to the research published in the CDC’s latest Morbidity and Mortality Weekly Report.
Lead author Valentine Wanga, PhD, with the CDC’s COVID-19 response team, and colleagues conducted a non–probability-based internet panel survey of about 6,000 U.S. adults to assess long-term symptoms often associated with COVID-19 among those who had ever tested positive or always tested negative for COVID-19 between January 2020 and April 2021.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., said in an interview that this research “establishes more securely than before that you don’t have to be hospitalized with COVID in order to develop long COVID symptoms.”
That’s better known among infectious disease experts, he said, but added that “this survey really gives a firm database for that.”
Study results
The study’s results showed that, compared with respondents who had a negative test result, those who received a positive result reported a significantly higher prevalence of any long-term symptom (65.9% vs. 42.9%), fatigue (22.5% vs. 12.0%), change in sense of smell or taste (17.3% vs. 1.7%), shortness of breath (15.5% vs. 5.2%), cough (14.5% vs. 4.9%), and headache (13.8% vs. 9.9%).
More people who had a positive test result (76.2%) reported persistence for more than a month of at least one initially occurring symptom, compared with those whose test results were always negative (69.6%).
The numbers are further proof, Dr. Schaffner said, that COVID not only will be an acute stressor on the health care system but patients with long COVID will need help with managing care for the long term.
“We still don’t know what the COVID virus does that results in these long COVID symptoms,” he said. Vanderbilt and many other institutions have developed “long COVID” centers as a testament to how important the problem is.
Long COVID symptoms are not well understood and most studies have looked at the effects from patients who had been hospitalized with COVID-19.
In this survey, respondents self-reported whether they had ever had a positive SARS-CoV-2 test result (698), always received a negative test result (2,437), or never were tested for SARS-CoV-2 (2,750).
Compared with those who always tested negative, a larger proportion of those who tested positive (28.7% vs. 15.7%) reported believing that receiving a COVID-19 vaccine made their long-term symptoms better. No difference was found in reported beliefs that a vaccine made long-term symptoms worse.
Dr. Schaffner said he found that survey result interesting, but said that is not backed up by current data and would need further study.
“I would treat that with great caution,” he said. “I’m not dismissing it, but you can’t take that at face value. All of us who get sick and those of us who care for people who are sick – if there’s an intervention, we all hope for the best. We’re being optimistic. It’s when you do a randomized, double-blind, placebo-controlled study that you can find out whether your instincts or hopes were correct.”
The authors said that findings can inform public health preparedness, help guide care for people with post-COVID conditions, and help make the case for vaccines.
The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term symptoms, like those linked with COVID-19, were common in people who had even just a single positive test, new Centers for Disease Control and Prevention data show.
The data show that symptoms in this group – including fatigue, cough, and headache – tended to last for more than a month.
Frequency of symptoms in people with a positive test was 1.5 times higher, compared with people whose tests had always been negative, according to the research published in the CDC’s latest Morbidity and Mortality Weekly Report.
Lead author Valentine Wanga, PhD, with the CDC’s COVID-19 response team, and colleagues conducted a non–probability-based internet panel survey of about 6,000 U.S. adults to assess long-term symptoms often associated with COVID-19 among those who had ever tested positive or always tested negative for COVID-19 between January 2020 and April 2021.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., said in an interview that this research “establishes more securely than before that you don’t have to be hospitalized with COVID in order to develop long COVID symptoms.”
That’s better known among infectious disease experts, he said, but added that “this survey really gives a firm database for that.”
Study results
The study’s results showed that, compared with respondents who had a negative test result, those who received a positive result reported a significantly higher prevalence of any long-term symptom (65.9% vs. 42.9%), fatigue (22.5% vs. 12.0%), change in sense of smell or taste (17.3% vs. 1.7%), shortness of breath (15.5% vs. 5.2%), cough (14.5% vs. 4.9%), and headache (13.8% vs. 9.9%).
More people who had a positive test result (76.2%) reported persistence for more than a month of at least one initially occurring symptom, compared with those whose test results were always negative (69.6%).
The numbers are further proof, Dr. Schaffner said, that COVID not only will be an acute stressor on the health care system but patients with long COVID will need help with managing care for the long term.
“We still don’t know what the COVID virus does that results in these long COVID symptoms,” he said. Vanderbilt and many other institutions have developed “long COVID” centers as a testament to how important the problem is.
Long COVID symptoms are not well understood and most studies have looked at the effects from patients who had been hospitalized with COVID-19.
In this survey, respondents self-reported whether they had ever had a positive SARS-CoV-2 test result (698), always received a negative test result (2,437), or never were tested for SARS-CoV-2 (2,750).
Compared with those who always tested negative, a larger proportion of those who tested positive (28.7% vs. 15.7%) reported believing that receiving a COVID-19 vaccine made their long-term symptoms better. No difference was found in reported beliefs that a vaccine made long-term symptoms worse.
Dr. Schaffner said he found that survey result interesting, but said that is not backed up by current data and would need further study.
“I would treat that with great caution,” he said. “I’m not dismissing it, but you can’t take that at face value. All of us who get sick and those of us who care for people who are sick – if there’s an intervention, we all hope for the best. We’re being optimistic. It’s when you do a randomized, double-blind, placebo-controlled study that you can find out whether your instincts or hopes were correct.”
The authors said that findings can inform public health preparedness, help guide care for people with post-COVID conditions, and help make the case for vaccines.
The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Seizure a first sign of COVID in kids?
Unlike in adults, seizures, including status epilepticus (SE), may be the first and main manifestation of COVID-19 in children, new research suggests.
Seizures may occur even in children with no history of epilepsy and in the absence of fever or severe COVID-19 illness, necessitating a “high index” of suspicion for the virus to make an early diagnosis and allow for appropriate infection control measures, say the researchers.
“We hope to increase physicians’ awareness of noninfluenza-like presentations of COVID in children. In areas with a high prevalence of COVID, we suggest that children with seizures be tested for COVID,” Tal Gilboa, MD, director of the child neurology unit and codirector of epilepsy, Hadassah Medical Center, Jerusalem, told this news organization.
The study was published online August 27 in the journal Seizure.
Presenting symptom
Among 175 children diagnosed with acute SARS-CoV-2 infection in the emergency department over 10 months in 2020, 11 (6%) presented with seizures. Studies in adults with COVID-19 have reported seizures in 0% to 2% of cases, the investigators note.
The 11 children with seizures (seven boys) ranged in age from 6 months to 17 years (median age, 11.5 years). All of them had seizures as the presenting sign of infection and none had severe COVID-19 requiring ventilatory or hemodynamic support. Six of the 11 children presented with fever.
Seven of the children had a prior history of neurological disorder: Five had epilepsy, one had a single unprovoked seizure 3 years before admission, and one had an intellectual disability. Three of the children had uncontrolled seizures despite appropriate treatment with antiseizure medication.
Nine of the 11 children presented with generalized tonic-clonic seizures. One child with a prior history of uncontrolled epilepsy with multiple seizure types had a focal tonic seizure. The youngest patient, a 5-month-old infant, presented with bilateral asymmetrical tonic-clonic seizure.
Of note, say the investigators, five of the 11 children presented with convulsive SE; none had a history of prior SE, and one had no history of seizures.
Although young age, especially under 12 months, is a known risk factor for SE, four of the five patients with SE were between 5 and 17 years old. All five children with SE responded to treatment with antiseizure medications.
All 11 children made a full recovery while in hospital, although further follow-up is essential to determine long-term outcomes, the researchers report.
“Children with no prior history of epilepsy and those with well-controlled epilepsy who present with breakthrough seizures, regardless of their body temperature, should be considered as potentially infected by SARS-CoV-2,” said Dr. Gilboa.
“It is possible, however unlikely, that a child, especially with prior epilepsy, may have an unprovoked seizure while being asymptomatically infected by SARS-CoV-2; in any case, infection control measures should be taken,” Dr. Gilboa added.
Need for replication
Weighing in on the study, Carl E. Stafstrom, MD, PhD, professor of neurology and pediatrics, Johns Hopkins University, Baltimore, said it’s important to note that “about half of the children had had epilepsy already, and for whatever reason, had a seizure, which required an ED visit, and then they found COVID.”
“Nevertheless, this article is interesting and surprising in what they found because nobody else has found nearly as frequent a seizure presentation,” said Dr. Stafstrom, director of the John M. Freeman Pediatric Epilepsy Center, Johns Hopkins Medicine.
“We would want to see some replication from other institutions and other populations,” he added.
The study had no specific funding. Dr. Gilboa and Dr. Stafstrom have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unlike in adults, seizures, including status epilepticus (SE), may be the first and main manifestation of COVID-19 in children, new research suggests.
Seizures may occur even in children with no history of epilepsy and in the absence of fever or severe COVID-19 illness, necessitating a “high index” of suspicion for the virus to make an early diagnosis and allow for appropriate infection control measures, say the researchers.
“We hope to increase physicians’ awareness of noninfluenza-like presentations of COVID in children. In areas with a high prevalence of COVID, we suggest that children with seizures be tested for COVID,” Tal Gilboa, MD, director of the child neurology unit and codirector of epilepsy, Hadassah Medical Center, Jerusalem, told this news organization.
The study was published online August 27 in the journal Seizure.
Presenting symptom
Among 175 children diagnosed with acute SARS-CoV-2 infection in the emergency department over 10 months in 2020, 11 (6%) presented with seizures. Studies in adults with COVID-19 have reported seizures in 0% to 2% of cases, the investigators note.
The 11 children with seizures (seven boys) ranged in age from 6 months to 17 years (median age, 11.5 years). All of them had seizures as the presenting sign of infection and none had severe COVID-19 requiring ventilatory or hemodynamic support. Six of the 11 children presented with fever.
Seven of the children had a prior history of neurological disorder: Five had epilepsy, one had a single unprovoked seizure 3 years before admission, and one had an intellectual disability. Three of the children had uncontrolled seizures despite appropriate treatment with antiseizure medication.
Nine of the 11 children presented with generalized tonic-clonic seizures. One child with a prior history of uncontrolled epilepsy with multiple seizure types had a focal tonic seizure. The youngest patient, a 5-month-old infant, presented with bilateral asymmetrical tonic-clonic seizure.
Of note, say the investigators, five of the 11 children presented with convulsive SE; none had a history of prior SE, and one had no history of seizures.
Although young age, especially under 12 months, is a known risk factor for SE, four of the five patients with SE were between 5 and 17 years old. All five children with SE responded to treatment with antiseizure medications.
All 11 children made a full recovery while in hospital, although further follow-up is essential to determine long-term outcomes, the researchers report.
“Children with no prior history of epilepsy and those with well-controlled epilepsy who present with breakthrough seizures, regardless of their body temperature, should be considered as potentially infected by SARS-CoV-2,” said Dr. Gilboa.
“It is possible, however unlikely, that a child, especially with prior epilepsy, may have an unprovoked seizure while being asymptomatically infected by SARS-CoV-2; in any case, infection control measures should be taken,” Dr. Gilboa added.
Need for replication
Weighing in on the study, Carl E. Stafstrom, MD, PhD, professor of neurology and pediatrics, Johns Hopkins University, Baltimore, said it’s important to note that “about half of the children had had epilepsy already, and for whatever reason, had a seizure, which required an ED visit, and then they found COVID.”
“Nevertheless, this article is interesting and surprising in what they found because nobody else has found nearly as frequent a seizure presentation,” said Dr. Stafstrom, director of the John M. Freeman Pediatric Epilepsy Center, Johns Hopkins Medicine.
“We would want to see some replication from other institutions and other populations,” he added.
The study had no specific funding. Dr. Gilboa and Dr. Stafstrom have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unlike in adults, seizures, including status epilepticus (SE), may be the first and main manifestation of COVID-19 in children, new research suggests.
Seizures may occur even in children with no history of epilepsy and in the absence of fever or severe COVID-19 illness, necessitating a “high index” of suspicion for the virus to make an early diagnosis and allow for appropriate infection control measures, say the researchers.
“We hope to increase physicians’ awareness of noninfluenza-like presentations of COVID in children. In areas with a high prevalence of COVID, we suggest that children with seizures be tested for COVID,” Tal Gilboa, MD, director of the child neurology unit and codirector of epilepsy, Hadassah Medical Center, Jerusalem, told this news organization.
The study was published online August 27 in the journal Seizure.
Presenting symptom
Among 175 children diagnosed with acute SARS-CoV-2 infection in the emergency department over 10 months in 2020, 11 (6%) presented with seizures. Studies in adults with COVID-19 have reported seizures in 0% to 2% of cases, the investigators note.
The 11 children with seizures (seven boys) ranged in age from 6 months to 17 years (median age, 11.5 years). All of them had seizures as the presenting sign of infection and none had severe COVID-19 requiring ventilatory or hemodynamic support. Six of the 11 children presented with fever.
Seven of the children had a prior history of neurological disorder: Five had epilepsy, one had a single unprovoked seizure 3 years before admission, and one had an intellectual disability. Three of the children had uncontrolled seizures despite appropriate treatment with antiseizure medication.
Nine of the 11 children presented with generalized tonic-clonic seizures. One child with a prior history of uncontrolled epilepsy with multiple seizure types had a focal tonic seizure. The youngest patient, a 5-month-old infant, presented with bilateral asymmetrical tonic-clonic seizure.
Of note, say the investigators, five of the 11 children presented with convulsive SE; none had a history of prior SE, and one had no history of seizures.
Although young age, especially under 12 months, is a known risk factor for SE, four of the five patients with SE were between 5 and 17 years old. All five children with SE responded to treatment with antiseizure medications.
All 11 children made a full recovery while in hospital, although further follow-up is essential to determine long-term outcomes, the researchers report.
“Children with no prior history of epilepsy and those with well-controlled epilepsy who present with breakthrough seizures, regardless of their body temperature, should be considered as potentially infected by SARS-CoV-2,” said Dr. Gilboa.
“It is possible, however unlikely, that a child, especially with prior epilepsy, may have an unprovoked seizure while being asymptomatically infected by SARS-CoV-2; in any case, infection control measures should be taken,” Dr. Gilboa added.
Need for replication
Weighing in on the study, Carl E. Stafstrom, MD, PhD, professor of neurology and pediatrics, Johns Hopkins University, Baltimore, said it’s important to note that “about half of the children had had epilepsy already, and for whatever reason, had a seizure, which required an ED visit, and then they found COVID.”
“Nevertheless, this article is interesting and surprising in what they found because nobody else has found nearly as frequent a seizure presentation,” said Dr. Stafstrom, director of the John M. Freeman Pediatric Epilepsy Center, Johns Hopkins Medicine.
“We would want to see some replication from other institutions and other populations,” he added.
The study had no specific funding. Dr. Gilboa and Dr. Stafstrom have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Medical boards: Docs who spread COVID misinformation put license at risk
Leaders of the American Board of Family Medicine, the American Board of Internal Medicine, and the American Board of Pediatrics said Sept. 9 that they support FSMB’s position.
“We also want all physicians certified by our boards to know that such unethical or unprofessional conduct may prompt their respective Board to take action that could put their certification at risk,” a statement read.
“Expertise matters, and board-certified physicians have demonstrated that they have stayed current in their field. Spreading misinformation or falsehoods to the public during a time of a public health emergency goes against everything our boards and our community of board-certified physicians stand for,” the leaders wrote.
“The evidence that we have safe, effective, and widely available vaccines against COVID-19 is overwhelming. We are particularly concerned about physicians who use their authority to denigrate vaccination at a time when vaccines continue to demonstrate excellent effectiveness against severe illness, hospitalization, and death.”
Small number spread false information
However, a small number of doctors continue to spread misinformation against the vaccines and communicate other false information surrounding COVID-19.
Some of the misinformation spreaders have had ultra-viral reach.
Among them is Daniel Stock, MD, a family physician in Indiana who has come out against COVID-19 vaccines. At a recent meeting of the Mt. Vernon Community School board in Indiana, he gave a speech urging the board to ignore the prevailing recommendations around COVID-19, such as test-and-trace measures.
Forbes reported in August that versions of the video of Stock›s speech on Facebook “have collected a total of 90 million engagements – a metric encompassing things such as comments, likes and shares – according to data collected by Media Matters for America, a liberal tech-watchdog group.”
This news organization published a story in August asking whether physicians who spread such information should lose their license and the question drew rapid-fire comments.
Commenters who argued with potential disciplinary actions raised questions about where the line will be drawn between misinformation and deeply held beliefs in terms of care.
Several comments centered on ivermectin, which is not approved by the Food and Drug Administration to treat COVID-19 but is enthusiastically supported as a COVID-19 treatment by a group of physicians called the Front Line COVID-19 Critical Care Alliance, whose website includes requests for donations.
Some cited free speech protections.
‘Not consistent with standards’
As for ivermectin, David G. Nichols, MD, president and CEO of the American Board of Pediatrics, gave this news organization an example: “Spreading the notion that one would not need to get vaccinated because if you get sick you could take ivermectin is a very dangerous statement. That is not consistent with the standards of professionalism required for certification or licensure.”
Ivermectin, he noted, is not an approved treatment for COVID-19.
“To say that it is or has any benefit is a false statement. We’re not willing to allow individuals who make false statements to devalue the terrific work of tens of thousands of physicians across the United States doing work under very difficult circumstances,” Dr. Nichols said.
He continued: “To suggest treatments that are known not to be effective in exchange for treatment that is known to be effective is dangerous – and ivermectin falls under that category.”
Asked whether such suggestions could result in suspension or revocation of a physician’s license, Dr. Nichols said, “It’s the kind of thing that would certainly trigger a review.”
He said the standard for separating misinformation from personal beliefs is based on whether there is scientific evidence to support the belief.
The boards are not, with this statement, attempting to referee legitimate scientific debate, he said.
The misinformation the boards are referring to, Dr. Nichols said, is “where the evidence is 100% on one side and zero on another. And the zero is not only that the opinions or beliefs are unsupported or unsubstantiated, they are indeed harmful if followed. That’s the distinction we’re trying to make here.”
As for free-speech arguments, he said, “Free speech is a constitutional right. You can say whatever you want. The issue here is you do not have the right to expect continued professional sanction of a board certificate if you are lying to the public.”
The board statement also said: “We all look to board-certified physicians to provide outstanding care and guidance; providing misinformation about a lethal disease is unethical, unprofessional, and dangerous. In times of medical emergency, the community of expert physicians committed to science and evidence collectively shares a responsibility for giving the public the most accurate and timely health information available, so they can make decisions that work best for themselves and their families.”
In addition to Dr. Nichols, the statement was signed by Warren Newton, MD, MPH, president and CEO of the American Board of Family Medicine, and Richard J. Baron, MD, president and CEO of the American Board of Internal Medicine.
A version of this article first appeared on Medscape.com.
Leaders of the American Board of Family Medicine, the American Board of Internal Medicine, and the American Board of Pediatrics said Sept. 9 that they support FSMB’s position.
“We also want all physicians certified by our boards to know that such unethical or unprofessional conduct may prompt their respective Board to take action that could put their certification at risk,” a statement read.
“Expertise matters, and board-certified physicians have demonstrated that they have stayed current in their field. Spreading misinformation or falsehoods to the public during a time of a public health emergency goes against everything our boards and our community of board-certified physicians stand for,” the leaders wrote.
“The evidence that we have safe, effective, and widely available vaccines against COVID-19 is overwhelming. We are particularly concerned about physicians who use their authority to denigrate vaccination at a time when vaccines continue to demonstrate excellent effectiveness against severe illness, hospitalization, and death.”
Small number spread false information
However, a small number of doctors continue to spread misinformation against the vaccines and communicate other false information surrounding COVID-19.
Some of the misinformation spreaders have had ultra-viral reach.
Among them is Daniel Stock, MD, a family physician in Indiana who has come out against COVID-19 vaccines. At a recent meeting of the Mt. Vernon Community School board in Indiana, he gave a speech urging the board to ignore the prevailing recommendations around COVID-19, such as test-and-trace measures.
Forbes reported in August that versions of the video of Stock›s speech on Facebook “have collected a total of 90 million engagements – a metric encompassing things such as comments, likes and shares – according to data collected by Media Matters for America, a liberal tech-watchdog group.”
This news organization published a story in August asking whether physicians who spread such information should lose their license and the question drew rapid-fire comments.
Commenters who argued with potential disciplinary actions raised questions about where the line will be drawn between misinformation and deeply held beliefs in terms of care.
Several comments centered on ivermectin, which is not approved by the Food and Drug Administration to treat COVID-19 but is enthusiastically supported as a COVID-19 treatment by a group of physicians called the Front Line COVID-19 Critical Care Alliance, whose website includes requests for donations.
Some cited free speech protections.
‘Not consistent with standards’
As for ivermectin, David G. Nichols, MD, president and CEO of the American Board of Pediatrics, gave this news organization an example: “Spreading the notion that one would not need to get vaccinated because if you get sick you could take ivermectin is a very dangerous statement. That is not consistent with the standards of professionalism required for certification or licensure.”
Ivermectin, he noted, is not an approved treatment for COVID-19.
“To say that it is or has any benefit is a false statement. We’re not willing to allow individuals who make false statements to devalue the terrific work of tens of thousands of physicians across the United States doing work under very difficult circumstances,” Dr. Nichols said.
He continued: “To suggest treatments that are known not to be effective in exchange for treatment that is known to be effective is dangerous – and ivermectin falls under that category.”
Asked whether such suggestions could result in suspension or revocation of a physician’s license, Dr. Nichols said, “It’s the kind of thing that would certainly trigger a review.”
He said the standard for separating misinformation from personal beliefs is based on whether there is scientific evidence to support the belief.
The boards are not, with this statement, attempting to referee legitimate scientific debate, he said.
The misinformation the boards are referring to, Dr. Nichols said, is “where the evidence is 100% on one side and zero on another. And the zero is not only that the opinions or beliefs are unsupported or unsubstantiated, they are indeed harmful if followed. That’s the distinction we’re trying to make here.”
As for free-speech arguments, he said, “Free speech is a constitutional right. You can say whatever you want. The issue here is you do not have the right to expect continued professional sanction of a board certificate if you are lying to the public.”
The board statement also said: “We all look to board-certified physicians to provide outstanding care and guidance; providing misinformation about a lethal disease is unethical, unprofessional, and dangerous. In times of medical emergency, the community of expert physicians committed to science and evidence collectively shares a responsibility for giving the public the most accurate and timely health information available, so they can make decisions that work best for themselves and their families.”
In addition to Dr. Nichols, the statement was signed by Warren Newton, MD, MPH, president and CEO of the American Board of Family Medicine, and Richard J. Baron, MD, president and CEO of the American Board of Internal Medicine.
A version of this article first appeared on Medscape.com.
Leaders of the American Board of Family Medicine, the American Board of Internal Medicine, and the American Board of Pediatrics said Sept. 9 that they support FSMB’s position.
“We also want all physicians certified by our boards to know that such unethical or unprofessional conduct may prompt their respective Board to take action that could put their certification at risk,” a statement read.
“Expertise matters, and board-certified physicians have demonstrated that they have stayed current in their field. Spreading misinformation or falsehoods to the public during a time of a public health emergency goes against everything our boards and our community of board-certified physicians stand for,” the leaders wrote.
“The evidence that we have safe, effective, and widely available vaccines against COVID-19 is overwhelming. We are particularly concerned about physicians who use their authority to denigrate vaccination at a time when vaccines continue to demonstrate excellent effectiveness against severe illness, hospitalization, and death.”
Small number spread false information
However, a small number of doctors continue to spread misinformation against the vaccines and communicate other false information surrounding COVID-19.
Some of the misinformation spreaders have had ultra-viral reach.
Among them is Daniel Stock, MD, a family physician in Indiana who has come out against COVID-19 vaccines. At a recent meeting of the Mt. Vernon Community School board in Indiana, he gave a speech urging the board to ignore the prevailing recommendations around COVID-19, such as test-and-trace measures.
Forbes reported in August that versions of the video of Stock›s speech on Facebook “have collected a total of 90 million engagements – a metric encompassing things such as comments, likes and shares – according to data collected by Media Matters for America, a liberal tech-watchdog group.”
This news organization published a story in August asking whether physicians who spread such information should lose their license and the question drew rapid-fire comments.
Commenters who argued with potential disciplinary actions raised questions about where the line will be drawn between misinformation and deeply held beliefs in terms of care.
Several comments centered on ivermectin, which is not approved by the Food and Drug Administration to treat COVID-19 but is enthusiastically supported as a COVID-19 treatment by a group of physicians called the Front Line COVID-19 Critical Care Alliance, whose website includes requests for donations.
Some cited free speech protections.
‘Not consistent with standards’
As for ivermectin, David G. Nichols, MD, president and CEO of the American Board of Pediatrics, gave this news organization an example: “Spreading the notion that one would not need to get vaccinated because if you get sick you could take ivermectin is a very dangerous statement. That is not consistent with the standards of professionalism required for certification or licensure.”
Ivermectin, he noted, is not an approved treatment for COVID-19.
“To say that it is or has any benefit is a false statement. We’re not willing to allow individuals who make false statements to devalue the terrific work of tens of thousands of physicians across the United States doing work under very difficult circumstances,” Dr. Nichols said.
He continued: “To suggest treatments that are known not to be effective in exchange for treatment that is known to be effective is dangerous – and ivermectin falls under that category.”
Asked whether such suggestions could result in suspension or revocation of a physician’s license, Dr. Nichols said, “It’s the kind of thing that would certainly trigger a review.”
He said the standard for separating misinformation from personal beliefs is based on whether there is scientific evidence to support the belief.
The boards are not, with this statement, attempting to referee legitimate scientific debate, he said.
The misinformation the boards are referring to, Dr. Nichols said, is “where the evidence is 100% on one side and zero on another. And the zero is not only that the opinions or beliefs are unsupported or unsubstantiated, they are indeed harmful if followed. That’s the distinction we’re trying to make here.”
As for free-speech arguments, he said, “Free speech is a constitutional right. You can say whatever you want. The issue here is you do not have the right to expect continued professional sanction of a board certificate if you are lying to the public.”
The board statement also said: “We all look to board-certified physicians to provide outstanding care and guidance; providing misinformation about a lethal disease is unethical, unprofessional, and dangerous. In times of medical emergency, the community of expert physicians committed to science and evidence collectively shares a responsibility for giving the public the most accurate and timely health information available, so they can make decisions that work best for themselves and their families.”
In addition to Dr. Nichols, the statement was signed by Warren Newton, MD, MPH, president and CEO of the American Board of Family Medicine, and Richard J. Baron, MD, president and CEO of the American Board of Internal Medicine.
A version of this article first appeared on Medscape.com.
More reassuring data on COVID-19 vaccines and pregnancy
Receiving a COVID-19 vaccine early in pregnancy is not associated with an increased risk for spontaneous abortion, new research suggests.
The study, published online in JAMA, evaluated the proportion of women who received the vaccine and had ongoing pregnancies in comparison with those who experienced a miscarriage or spontaneous abortion. The researchers analyzed data from 105,446 unique pregnancies over seven 4-week surveillance periods between December 2020 and June 2021. Ongoing pregnancies between 6 and 19 weeks’ gestation were identified on the last day of each 4-week surveillance period (index date). Spontaneous abortions were assigned to a 4-week surveillance period on the basis of their outcome date. There were 13,160 spontaneous abortions and 92,286 ongoing pregnancies.
Overall, a COVID-19 vaccine was received within 28 days prior to an index date among 8.0% of ongoing pregnancy surveillance periods versus 8.6% of spontaneous abortions.
“We’re hoping that this data can inform the ongoing conversations between providers and pregnant women [about the COVID-19 vaccines],” study author Elyse O. Kharbanda, MD, MPH, senior research investigator at HealthPartners Institute, told this news organization. “It should be considered in the context of all the data that’s coming out both on the risks of COVID infection and pregnancy and data on outcomes among women who are vaccinated and pregnant.”
Among the women whose pregnancies were followed, 7.8% received at least one dose of the Pfizer COVID-19 vaccine, 6% received at least one dose of the Moderna COVID-19 vaccine, and 0.5% received the Janssen vaccine.
In August, the American College of Obstetricians and Gynecologists (ACOG), the Centers for Disease Control and Prevention, and the Society for Maternal-Fetal Medicine strongly recommended that all pregnant women be vaccinated against COVID-19.
The new findings provide reassuring evidence about the safety of COVID vaccines, particularly mRNA vaccines, during pregnancy, said Denise J. Jamieson, MD, MPH, chair of the department of gynecology and obstetrics at Emory University, Atlanta, who was not involved in the study.
“The study design was a carefully conducted case-control study. Although ideally the best design for studying vaccine safety and efficacy is a randomized clinical trial, data are rapidly accumulating from a variety of sources that COVID vaccines are safe in pregnancy,” said Dr. Jamieson, who serves on several ACOG committees.
A version of this article first appeared on Medscape.com.
Receiving a COVID-19 vaccine early in pregnancy is not associated with an increased risk for spontaneous abortion, new research suggests.
The study, published online in JAMA, evaluated the proportion of women who received the vaccine and had ongoing pregnancies in comparison with those who experienced a miscarriage or spontaneous abortion. The researchers analyzed data from 105,446 unique pregnancies over seven 4-week surveillance periods between December 2020 and June 2021. Ongoing pregnancies between 6 and 19 weeks’ gestation were identified on the last day of each 4-week surveillance period (index date). Spontaneous abortions were assigned to a 4-week surveillance period on the basis of their outcome date. There were 13,160 spontaneous abortions and 92,286 ongoing pregnancies.
Overall, a COVID-19 vaccine was received within 28 days prior to an index date among 8.0% of ongoing pregnancy surveillance periods versus 8.6% of spontaneous abortions.
“We’re hoping that this data can inform the ongoing conversations between providers and pregnant women [about the COVID-19 vaccines],” study author Elyse O. Kharbanda, MD, MPH, senior research investigator at HealthPartners Institute, told this news organization. “It should be considered in the context of all the data that’s coming out both on the risks of COVID infection and pregnancy and data on outcomes among women who are vaccinated and pregnant.”
Among the women whose pregnancies were followed, 7.8% received at least one dose of the Pfizer COVID-19 vaccine, 6% received at least one dose of the Moderna COVID-19 vaccine, and 0.5% received the Janssen vaccine.
In August, the American College of Obstetricians and Gynecologists (ACOG), the Centers for Disease Control and Prevention, and the Society for Maternal-Fetal Medicine strongly recommended that all pregnant women be vaccinated against COVID-19.
The new findings provide reassuring evidence about the safety of COVID vaccines, particularly mRNA vaccines, during pregnancy, said Denise J. Jamieson, MD, MPH, chair of the department of gynecology and obstetrics at Emory University, Atlanta, who was not involved in the study.
“The study design was a carefully conducted case-control study. Although ideally the best design for studying vaccine safety and efficacy is a randomized clinical trial, data are rapidly accumulating from a variety of sources that COVID vaccines are safe in pregnancy,” said Dr. Jamieson, who serves on several ACOG committees.
A version of this article first appeared on Medscape.com.
Receiving a COVID-19 vaccine early in pregnancy is not associated with an increased risk for spontaneous abortion, new research suggests.
The study, published online in JAMA, evaluated the proportion of women who received the vaccine and had ongoing pregnancies in comparison with those who experienced a miscarriage or spontaneous abortion. The researchers analyzed data from 105,446 unique pregnancies over seven 4-week surveillance periods between December 2020 and June 2021. Ongoing pregnancies between 6 and 19 weeks’ gestation were identified on the last day of each 4-week surveillance period (index date). Spontaneous abortions were assigned to a 4-week surveillance period on the basis of their outcome date. There were 13,160 spontaneous abortions and 92,286 ongoing pregnancies.
Overall, a COVID-19 vaccine was received within 28 days prior to an index date among 8.0% of ongoing pregnancy surveillance periods versus 8.6% of spontaneous abortions.
“We’re hoping that this data can inform the ongoing conversations between providers and pregnant women [about the COVID-19 vaccines],” study author Elyse O. Kharbanda, MD, MPH, senior research investigator at HealthPartners Institute, told this news organization. “It should be considered in the context of all the data that’s coming out both on the risks of COVID infection and pregnancy and data on outcomes among women who are vaccinated and pregnant.”
Among the women whose pregnancies were followed, 7.8% received at least one dose of the Pfizer COVID-19 vaccine, 6% received at least one dose of the Moderna COVID-19 vaccine, and 0.5% received the Janssen vaccine.
In August, the American College of Obstetricians and Gynecologists (ACOG), the Centers for Disease Control and Prevention, and the Society for Maternal-Fetal Medicine strongly recommended that all pregnant women be vaccinated against COVID-19.
The new findings provide reassuring evidence about the safety of COVID vaccines, particularly mRNA vaccines, during pregnancy, said Denise J. Jamieson, MD, MPH, chair of the department of gynecology and obstetrics at Emory University, Atlanta, who was not involved in the study.
“The study design was a carefully conducted case-control study. Although ideally the best design for studying vaccine safety and efficacy is a randomized clinical trial, data are rapidly accumulating from a variety of sources that COVID vaccines are safe in pregnancy,” said Dr. Jamieson, who serves on several ACOG committees.
A version of this article first appeared on Medscape.com.