User login
Practice Question Answers: Allergic Contact Dermatitis, Part 3
1. Which of the following is an amide-type anesthetic?
a. benzocaine
b. cocaine
c. lidocaine
d. procaine
e. tetracaine
2. A patient is referred for patch testing for suspected allergic contact dermatitis and is found to have positivity to hydrocortisone butyrate. The patient should try to avoid all of the following, except:
a. desonide
b. desoximetasone
c. fluocinolone
d. fluocinonide
e. triamcinolone
3. A patient with a documented contact allergy to neomycin sulfate should avoid all of the following medications, except:
a. bacitracin
b. gentamicin
c. kanamycin
d. mupirocin
e. streptomycin
4. Imidazolidinyl urea can cross-react with all of the following, except:
a. diazolidinyl urea
b. DMDM hydantoin
c. para-aminobenzoic acid
d. quaternium-15
e. tris(hydroxymethyl)nitromethane
5. Mercaptobenzothiazole can coreact with all of the following, except:
a. carbamates
b. dibenzothiazyl disulfide
c. mercapto mix
d. methyldibromo glutaronitrile
e. thiurams
1. Which of the following is an amide-type anesthetic?
a. benzocaine
b. cocaine
c. lidocaine
d. procaine
e. tetracaine
2. A patient is referred for patch testing for suspected allergic contact dermatitis and is found to have positivity to hydrocortisone butyrate. The patient should try to avoid all of the following, except:
a. desonide
b. desoximetasone
c. fluocinolone
d. fluocinonide
e. triamcinolone
3. A patient with a documented contact allergy to neomycin sulfate should avoid all of the following medications, except:
a. bacitracin
b. gentamicin
c. kanamycin
d. mupirocin
e. streptomycin
4. Imidazolidinyl urea can cross-react with all of the following, except:
a. diazolidinyl urea
b. DMDM hydantoin
c. para-aminobenzoic acid
d. quaternium-15
e. tris(hydroxymethyl)nitromethane
5. Mercaptobenzothiazole can coreact with all of the following, except:
a. carbamates
b. dibenzothiazyl disulfide
c. mercapto mix
d. methyldibromo glutaronitrile
e. thiurams
1. Which of the following is an amide-type anesthetic?
a. benzocaine
b. cocaine
c. lidocaine
d. procaine
e. tetracaine
2. A patient is referred for patch testing for suspected allergic contact dermatitis and is found to have positivity to hydrocortisone butyrate. The patient should try to avoid all of the following, except:
a. desonide
b. desoximetasone
c. fluocinolone
d. fluocinonide
e. triamcinolone
3. A patient with a documented contact allergy to neomycin sulfate should avoid all of the following medications, except:
a. bacitracin
b. gentamicin
c. kanamycin
d. mupirocin
e. streptomycin
4. Imidazolidinyl urea can cross-react with all of the following, except:
a. diazolidinyl urea
b. DMDM hydantoin
c. para-aminobenzoic acid
d. quaternium-15
e. tris(hydroxymethyl)nitromethane
5. Mercaptobenzothiazole can coreact with all of the following, except:
a. carbamates
b. dibenzothiazyl disulfide
c. mercapto mix
d. methyldibromo glutaronitrile
e. thiurams
Allergic Contact Dermatitis, Part 3
What’s Eating You? Cutaneous Larva Migrans
Cutaneous larva migrans (CLM), also known as creeping eruption, is a pruritic serpiginous eruption caused by the migration of animal hookworm larvae through the epidermis.1,2 The most common parasites are Ancylostoma braziliense (common in dogs and cats) and Ancylostoma caninum (common in dogs).1
Disease Transmission
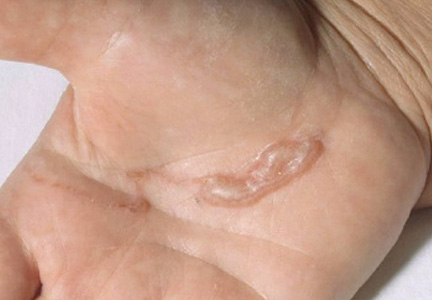
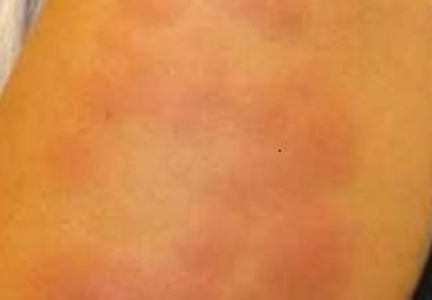
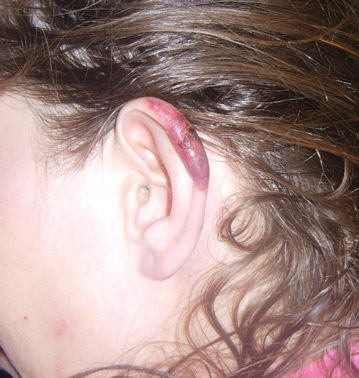
The infection is typically acquired in warm climates and tropical areas after coming in direct contact with sand or soil that is contaminated with animal feces. Therefore, the eruption most commonly occurs as a single or unilateral erythematous, pruritic, serpiginous tract on the feet, hands, or buttocks (Figure).2 The larval tract typically migrates at a rate of 1 to 2 cm per day,3 which is in contrast to the serpiginous urticarial rash of larva currens of strongyloidiasis that can travel up to 10 cm per hour.4
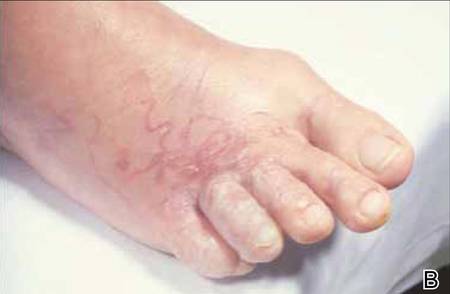
|
Clinical Presentation
Rarely, CLM can present with bilateral lesions5; in severe cases a single patient can have hundreds of lesions. It also may present as folliculitis and urticarial papules.6 Shih et al7 reported a patient with CLM that presented as a diffuse papular urticarialike eruption following a trip to Thailand. This case may represent an underdiagnosed presentation of CLM. Patients with a history of exposure to contaminated sand or soil diffusely on the body may exhibit lesions in less classic locations, such as the trunk and upper proximal extremities.3
Cutaneous larva migrans is a self-limited eruption, as the larvae cannot complete their lifecycles in the human body and typically die within 2 to 8 weeks.2 However, rare cases lasting up to a year have been reported.3 Sarasombath and Young2 reported a case of CLM that persisted for 4 months with intermittent symptoms characterized by several weeklong intervals with no symptoms or visible rash.
Cutaneous larva migrans typically presents with isolated dermatologic symptoms. Rare cases associated with Löffler syndrome characterized by migratory pulmonary infiltrates and peripheral eosinophilia have been reported.8 Two proposed mechanisms for pulmonary involvement include direct invasion of the lungs by the helminths and a systemic immunologic process triggered by the helminths, resulting in eosinophilic pulmonary infiltration.9
Diagnosis
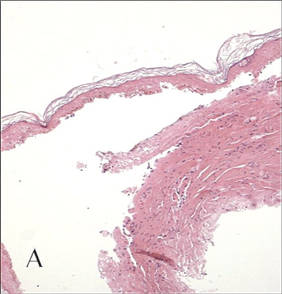
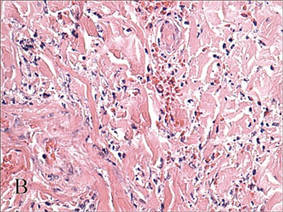
Cutaneous larva migrans is a clinical diagnosis and skin biopsy usually is not obtained because the larvae often are located 1 to 2 cm beyond the visible erythematous border.3,5 Rarely, the parasites are found on biopsy, revealing larvae that are 0.5-mm thick and up to 10-mm long.10 The larvae typically are confined to the deep epidermis because the parasite lacks the collagenase required to penetrate the basement membrane.2
Langley et al11 showed that confocal scanning laser microscopy can be an effective method for identifying the highly refractile oval larva that disrupt the normal honeycomb pattern of the epidermis. Performing a 4-mm punch biopsy over the identified site can allow for precise excision and treatment of the intact hookworm larvae of CLM. There also are limited reports of dermoscopy being used to facilitate diagnosis of CLM.12 Dermoscopic features of CLM include translucent, brown, structureless areas in a segmental arrangement corresponding to the larval bodies and red-dotted vessels corresponding to an empty burrow.13 However, Zalaudek et al13 concluded that the efficacy of dermoscopy in aiding in the diagnosis of CLM has not been fully established.
Treatment
Cutaneous larva migrans is a self-limited condition that often resolves within 2 to 8 weeks; however, pruritus can be intense and patients therefore are seldom willing to forego treatment. Treatment options include a single oral dose of albendazole 400 mg in adults, with increased efficacy if administered daily for 3 to 5 days (or 10–15 mg/kg, with a maximum dose of 800 mg daily in children), a single oral dose of ivermectin 12 mg in adults (or 150 µg/kg in children), or topical application of thiabendazole 10% to 15% three times daily for at least 15 days.14 Cases of CLM complicated by Löffler syndrome may require a longer treatment course, such as a 7-day course of albendazole 400 mg daily. Tan and Liu9 reported a case of CLM complicated by Löffler syndrome that was successfully treated with albendazole. In this patient, initial treatment with 2 courses of mebendazole (3 days each for a total of 6 days) resulted in improvement of cutaneous lesions but not the pulmonary infiltrate. A subsequent prolonged course of albendazole and intravenous hydrocortisone for 5 days resulted in complete resolution of the pulmonary infiltrate and peripheral eosinophilia. The authors concluded that inadequacy of treatment with mebendazole may be related to differences in the rate of absorption and efficacy when compared to albendazole.9
Conclusion
Cutaneous larva migrans is a self-limited and pruritic skin eruption that is acquired after direct inoculation with sand or soil that is contaminated with feces containing A braziliense or A caninum. Although the classic presentation is readily identifiable, there are a variety of atypical presentations that may go undiagnosed. Symptomatic relief usually can be achieved with short courses of oral or topical antihelminth medications.
1. Berlin JM, Goldberg SJ, McDonough RD, et al. JAAD grand rounds quiz. serpiginous eruption on the leg. J Am Acad Dermatol. 2010;63:921-922.
2. Sarasombath PA, Young PK. An unusual presentation of cutaneous larva migrans. Arch Dermatol. 2007;143:955.
3. Patel S, Aboutalebi S, Vindhya PL, et al. What’s eating you? extensive cutaneous larva migrans (Ancylostoma braziliense). Cutis. 2008;82:239-240.
4. Elston DM, Czarnik K, Brockett R, et al. What’s eating you? Strongyloides stercoralis. Cutis. 2003;71:22-24.
5. Duarte De Sousa ICV, De La Pascua L. Bilateral cutaneous larva migrans [poster reference number 4677]. J Am Acad Dermatol. 2012;66(4, suppl 1):AB106.
6. Caumes E, Ly F, Bricaire F. Cutaneous larva migrans with folliculitis: report of seven cases and review of the literature. Br J Dermatol. 2002;146:314-316.
7. Shih PY, Hsieh MY, Huang YH, et al. Multiple pruritic erythematous papules on the trunk after a trip to Thailand–quiz case. Arch Dermatol. 2010;146:557-562.
8. Wright DO, Gold ED. Löffler’s syndrome associated with creeping eruption (cutaneous helminthiasis): report of twenty-six cases. Arch Intern Med. 1946;78:303-312.
9. Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler’s syndrome. Arch Dermatol. 2010;146:210-212.
10. Rapini RP, ed. Practical Dermatopathology. Philadelphia, PA: Elsevier; 2005.
11. Langley R, Webb A, Haldane D, et al. Confocal microscopy of cutaneous larva migrans. J Am Acad Dermatol. 2011;64(2, suppl 1):AB100.
12. Aljasser MI, Lui H, Zeng H, et al. Dermoscopy and near-infrared fluorescence imaging of cutaneous larva migrans. Photodermatol Photoimmunol Photomed. 2013;29:337-338.
13. Zalaudek I, Giacomel J, Cabo H, et al. Entodermoscopy: a new tool for diagnosing skin infections and infestations. Dermatology. 2008;216:14-23.
14. Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814.
Cutaneous larva migrans (CLM), also known as creeping eruption, is a pruritic serpiginous eruption caused by the migration of animal hookworm larvae through the epidermis.1,2 The most common parasites are Ancylostoma braziliense (common in dogs and cats) and Ancylostoma caninum (common in dogs).1
Disease Transmission
The infection is typically acquired in warm climates and tropical areas after coming in direct contact with sand or soil that is contaminated with animal feces. Therefore, the eruption most commonly occurs as a single or unilateral erythematous, pruritic, serpiginous tract on the feet, hands, or buttocks (Figure).2 The larval tract typically migrates at a rate of 1 to 2 cm per day,3 which is in contrast to the serpiginous urticarial rash of larva currens of strongyloidiasis that can travel up to 10 cm per hour.4


|
Clinical Presentation
Rarely, CLM can present with bilateral lesions5; in severe cases a single patient can have hundreds of lesions. It also may present as folliculitis and urticarial papules.6 Shih et al7 reported a patient with CLM that presented as a diffuse papular urticarialike eruption following a trip to Thailand. This case may represent an underdiagnosed presentation of CLM. Patients with a history of exposure to contaminated sand or soil diffusely on the body may exhibit lesions in less classic locations, such as the trunk and upper proximal extremities.3
Cutaneous larva migrans is a self-limited eruption, as the larvae cannot complete their lifecycles in the human body and typically die within 2 to 8 weeks.2 However, rare cases lasting up to a year have been reported.3 Sarasombath and Young2 reported a case of CLM that persisted for 4 months with intermittent symptoms characterized by several weeklong intervals with no symptoms or visible rash.
Cutaneous larva migrans typically presents with isolated dermatologic symptoms. Rare cases associated with Löffler syndrome characterized by migratory pulmonary infiltrates and peripheral eosinophilia have been reported.8 Two proposed mechanisms for pulmonary involvement include direct invasion of the lungs by the helminths and a systemic immunologic process triggered by the helminths, resulting in eosinophilic pulmonary infiltration.9
Diagnosis
Cutaneous larva migrans is a clinical diagnosis and skin biopsy usually is not obtained because the larvae often are located 1 to 2 cm beyond the visible erythematous border.3,5 Rarely, the parasites are found on biopsy, revealing larvae that are 0.5-mm thick and up to 10-mm long.10 The larvae typically are confined to the deep epidermis because the parasite lacks the collagenase required to penetrate the basement membrane.2
Langley et al11 showed that confocal scanning laser microscopy can be an effective method for identifying the highly refractile oval larva that disrupt the normal honeycomb pattern of the epidermis. Performing a 4-mm punch biopsy over the identified site can allow for precise excision and treatment of the intact hookworm larvae of CLM. There also are limited reports of dermoscopy being used to facilitate diagnosis of CLM.12 Dermoscopic features of CLM include translucent, brown, structureless areas in a segmental arrangement corresponding to the larval bodies and red-dotted vessels corresponding to an empty burrow.13 However, Zalaudek et al13 concluded that the efficacy of dermoscopy in aiding in the diagnosis of CLM has not been fully established.
Treatment
Cutaneous larva migrans is a self-limited condition that often resolves within 2 to 8 weeks; however, pruritus can be intense and patients therefore are seldom willing to forego treatment. Treatment options include a single oral dose of albendazole 400 mg in adults, with increased efficacy if administered daily for 3 to 5 days (or 10–15 mg/kg, with a maximum dose of 800 mg daily in children), a single oral dose of ivermectin 12 mg in adults (or 150 µg/kg in children), or topical application of thiabendazole 10% to 15% three times daily for at least 15 days.14 Cases of CLM complicated by Löffler syndrome may require a longer treatment course, such as a 7-day course of albendazole 400 mg daily. Tan and Liu9 reported a case of CLM complicated by Löffler syndrome that was successfully treated with albendazole. In this patient, initial treatment with 2 courses of mebendazole (3 days each for a total of 6 days) resulted in improvement of cutaneous lesions but not the pulmonary infiltrate. A subsequent prolonged course of albendazole and intravenous hydrocortisone for 5 days resulted in complete resolution of the pulmonary infiltrate and peripheral eosinophilia. The authors concluded that inadequacy of treatment with mebendazole may be related to differences in the rate of absorption and efficacy when compared to albendazole.9
Conclusion
Cutaneous larva migrans is a self-limited and pruritic skin eruption that is acquired after direct inoculation with sand or soil that is contaminated with feces containing A braziliense or A caninum. Although the classic presentation is readily identifiable, there are a variety of atypical presentations that may go undiagnosed. Symptomatic relief usually can be achieved with short courses of oral or topical antihelminth medications.
Cutaneous larva migrans (CLM), also known as creeping eruption, is a pruritic serpiginous eruption caused by the migration of animal hookworm larvae through the epidermis.1,2 The most common parasites are Ancylostoma braziliense (common in dogs and cats) and Ancylostoma caninum (common in dogs).1
Disease Transmission
The infection is typically acquired in warm climates and tropical areas after coming in direct contact with sand or soil that is contaminated with animal feces. Therefore, the eruption most commonly occurs as a single or unilateral erythematous, pruritic, serpiginous tract on the feet, hands, or buttocks (Figure).2 The larval tract typically migrates at a rate of 1 to 2 cm per day,3 which is in contrast to the serpiginous urticarial rash of larva currens of strongyloidiasis that can travel up to 10 cm per hour.4


|
Clinical Presentation
Rarely, CLM can present with bilateral lesions5; in severe cases a single patient can have hundreds of lesions. It also may present as folliculitis and urticarial papules.6 Shih et al7 reported a patient with CLM that presented as a diffuse papular urticarialike eruption following a trip to Thailand. This case may represent an underdiagnosed presentation of CLM. Patients with a history of exposure to contaminated sand or soil diffusely on the body may exhibit lesions in less classic locations, such as the trunk and upper proximal extremities.3
Cutaneous larva migrans is a self-limited eruption, as the larvae cannot complete their lifecycles in the human body and typically die within 2 to 8 weeks.2 However, rare cases lasting up to a year have been reported.3 Sarasombath and Young2 reported a case of CLM that persisted for 4 months with intermittent symptoms characterized by several weeklong intervals with no symptoms or visible rash.
Cutaneous larva migrans typically presents with isolated dermatologic symptoms. Rare cases associated with Löffler syndrome characterized by migratory pulmonary infiltrates and peripheral eosinophilia have been reported.8 Two proposed mechanisms for pulmonary involvement include direct invasion of the lungs by the helminths and a systemic immunologic process triggered by the helminths, resulting in eosinophilic pulmonary infiltration.9
Diagnosis
Cutaneous larva migrans is a clinical diagnosis and skin biopsy usually is not obtained because the larvae often are located 1 to 2 cm beyond the visible erythematous border.3,5 Rarely, the parasites are found on biopsy, revealing larvae that are 0.5-mm thick and up to 10-mm long.10 The larvae typically are confined to the deep epidermis because the parasite lacks the collagenase required to penetrate the basement membrane.2
Langley et al11 showed that confocal scanning laser microscopy can be an effective method for identifying the highly refractile oval larva that disrupt the normal honeycomb pattern of the epidermis. Performing a 4-mm punch biopsy over the identified site can allow for precise excision and treatment of the intact hookworm larvae of CLM. There also are limited reports of dermoscopy being used to facilitate diagnosis of CLM.12 Dermoscopic features of CLM include translucent, brown, structureless areas in a segmental arrangement corresponding to the larval bodies and red-dotted vessels corresponding to an empty burrow.13 However, Zalaudek et al13 concluded that the efficacy of dermoscopy in aiding in the diagnosis of CLM has not been fully established.
Treatment
Cutaneous larva migrans is a self-limited condition that often resolves within 2 to 8 weeks; however, pruritus can be intense and patients therefore are seldom willing to forego treatment. Treatment options include a single oral dose of albendazole 400 mg in adults, with increased efficacy if administered daily for 3 to 5 days (or 10–15 mg/kg, with a maximum dose of 800 mg daily in children), a single oral dose of ivermectin 12 mg in adults (or 150 µg/kg in children), or topical application of thiabendazole 10% to 15% three times daily for at least 15 days.14 Cases of CLM complicated by Löffler syndrome may require a longer treatment course, such as a 7-day course of albendazole 400 mg daily. Tan and Liu9 reported a case of CLM complicated by Löffler syndrome that was successfully treated with albendazole. In this patient, initial treatment with 2 courses of mebendazole (3 days each for a total of 6 days) resulted in improvement of cutaneous lesions but not the pulmonary infiltrate. A subsequent prolonged course of albendazole and intravenous hydrocortisone for 5 days resulted in complete resolution of the pulmonary infiltrate and peripheral eosinophilia. The authors concluded that inadequacy of treatment with mebendazole may be related to differences in the rate of absorption and efficacy when compared to albendazole.9
Conclusion
Cutaneous larva migrans is a self-limited and pruritic skin eruption that is acquired after direct inoculation with sand or soil that is contaminated with feces containing A braziliense or A caninum. Although the classic presentation is readily identifiable, there are a variety of atypical presentations that may go undiagnosed. Symptomatic relief usually can be achieved with short courses of oral or topical antihelminth medications.
1. Berlin JM, Goldberg SJ, McDonough RD, et al. JAAD grand rounds quiz. serpiginous eruption on the leg. J Am Acad Dermatol. 2010;63:921-922.
2. Sarasombath PA, Young PK. An unusual presentation of cutaneous larva migrans. Arch Dermatol. 2007;143:955.
3. Patel S, Aboutalebi S, Vindhya PL, et al. What’s eating you? extensive cutaneous larva migrans (Ancylostoma braziliense). Cutis. 2008;82:239-240.
4. Elston DM, Czarnik K, Brockett R, et al. What’s eating you? Strongyloides stercoralis. Cutis. 2003;71:22-24.
5. Duarte De Sousa ICV, De La Pascua L. Bilateral cutaneous larva migrans [poster reference number 4677]. J Am Acad Dermatol. 2012;66(4, suppl 1):AB106.
6. Caumes E, Ly F, Bricaire F. Cutaneous larva migrans with folliculitis: report of seven cases and review of the literature. Br J Dermatol. 2002;146:314-316.
7. Shih PY, Hsieh MY, Huang YH, et al. Multiple pruritic erythematous papules on the trunk after a trip to Thailand–quiz case. Arch Dermatol. 2010;146:557-562.
8. Wright DO, Gold ED. Löffler’s syndrome associated with creeping eruption (cutaneous helminthiasis): report of twenty-six cases. Arch Intern Med. 1946;78:303-312.
9. Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler’s syndrome. Arch Dermatol. 2010;146:210-212.
10. Rapini RP, ed. Practical Dermatopathology. Philadelphia, PA: Elsevier; 2005.
11. Langley R, Webb A, Haldane D, et al. Confocal microscopy of cutaneous larva migrans. J Am Acad Dermatol. 2011;64(2, suppl 1):AB100.
12. Aljasser MI, Lui H, Zeng H, et al. Dermoscopy and near-infrared fluorescence imaging of cutaneous larva migrans. Photodermatol Photoimmunol Photomed. 2013;29:337-338.
13. Zalaudek I, Giacomel J, Cabo H, et al. Entodermoscopy: a new tool for diagnosing skin infections and infestations. Dermatology. 2008;216:14-23.
14. Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814.
1. Berlin JM, Goldberg SJ, McDonough RD, et al. JAAD grand rounds quiz. serpiginous eruption on the leg. J Am Acad Dermatol. 2010;63:921-922.
2. Sarasombath PA, Young PK. An unusual presentation of cutaneous larva migrans. Arch Dermatol. 2007;143:955.
3. Patel S, Aboutalebi S, Vindhya PL, et al. What’s eating you? extensive cutaneous larva migrans (Ancylostoma braziliense). Cutis. 2008;82:239-240.
4. Elston DM, Czarnik K, Brockett R, et al. What’s eating you? Strongyloides stercoralis. Cutis. 2003;71:22-24.
5. Duarte De Sousa ICV, De La Pascua L. Bilateral cutaneous larva migrans [poster reference number 4677]. J Am Acad Dermatol. 2012;66(4, suppl 1):AB106.
6. Caumes E, Ly F, Bricaire F. Cutaneous larva migrans with folliculitis: report of seven cases and review of the literature. Br J Dermatol. 2002;146:314-316.
7. Shih PY, Hsieh MY, Huang YH, et al. Multiple pruritic erythematous papules on the trunk after a trip to Thailand–quiz case. Arch Dermatol. 2010;146:557-562.
8. Wright DO, Gold ED. Löffler’s syndrome associated with creeping eruption (cutaneous helminthiasis): report of twenty-six cases. Arch Intern Med. 1946;78:303-312.
9. Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler’s syndrome. Arch Dermatol. 2010;146:210-212.
10. Rapini RP, ed. Practical Dermatopathology. Philadelphia, PA: Elsevier; 2005.
11. Langley R, Webb A, Haldane D, et al. Confocal microscopy of cutaneous larva migrans. J Am Acad Dermatol. 2011;64(2, suppl 1):AB100.
12. Aljasser MI, Lui H, Zeng H, et al. Dermoscopy and near-infrared fluorescence imaging of cutaneous larva migrans. Photodermatol Photoimmunol Photomed. 2013;29:337-338.
13. Zalaudek I, Giacomel J, Cabo H, et al. Entodermoscopy: a new tool for diagnosing skin infections and infestations. Dermatology. 2008;216:14-23.
14. Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814.
Practice Points
- Classic cutaneous larva migrans (CLM) presents with a unilateral, serpiginous, pruritic eruption on the hands, feet, or buttocks following direct contact with sand or soil that is contaminated with Ancylostoma braziliense or Ancylostoma caninum.
- Atypical presentations of CLM include bilateral distribution; folliculitis and urticarial plaques; prolonged cases lasting up to 1 year; and Löffler syndrome characterized by migratory pulmonary infiltrates and peripheral eosinophilia.
- Cutaneous larva migrans is self-limited, but treatment often is necessary due to intense pruritus. Treatment options include a single oral dose of albendazole or ivermectin, topical thiabendazole, and prolonged courses of oral albendazole in cases complicated by Löffler syndrome.
Inability to Grow Long Hair: A Presentation of Trichorrhexis Nodosa
To the Editor:
First identified by Samuel Wilks in 1852, trichorrhexis nodosa (TN) is a congenital or acquired hair shaft disorder that is characterized by fragile and easily broken hair.1 Congenital TN is rare and can occur in syndromes such as pseudomonilethrix, Netherton syndrome, pili annulati,2 argininosuccinic aciduria,3 trichothiodystrophy,4 Menkes syndrome,5 and trichohepatoenteric syndrome.6 The primary congenital form of TN is inherited as an autosomal-dominant trait in some families. Acquired TN is the most common hair shaft abnormality and often is overlooked. It is provoked by hair injury, usually mechanical or physical, or chemical trauma.7,8
Chemical trauma is caused by the use of permanent hair liquids or dyes. Mechanical injuries are the result of frequent brushing, scalp massage, or lengthy backcombing, and physical damage includes excessive UV exposure or repeated application of heat. Habit tics, trichotillomania, and the scratching and pulling associated with pruritic dermatoses also can result in sufficient damage to provoke TN. Furthermore, this acquired disorder may develop from malnutrition, particularly iron deficiency, or endocrinopathy such as hypothyroidism.9 Seasonal recurrence of TN has been reported from the cumulative effect of repeated soaking in salt water and exposure to UV light. Macroscopically, hair shafts affected by TN contain small white nodes at irregular intervals throughout the length of the hair shaft. These nodes represent areas of cuticular cell disruption, which allows the underlying cortical fibers to separate and fray and gives the node the microscopic appearance of 2 brooms or paintbrushes thrusting together end-to-end by the bristles. The classic description is known as paintbrush fracture.10 Generally, complete breakage occurs at these nodes.
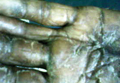
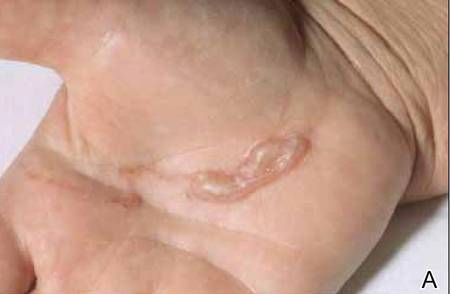
A 21-year-old white woman presented to our clinic with hair fragility and inability to grow long hair of 2 years’ duration. The hair was lusterless and dry. Dermoscopic examination revealed broken blunt-ended hair of uneven length with minute pinpoint grayish white nodules (Figure 1). Small fragments could be easily broken off with gentle tugging on the distal ends. She reported a history of severe sunlight and seawater exposure during the last 2 summers and the continuous use of a flat iron in the last year. Microscopic examination of hair samples with a scanning electron microscope showed the characteristic paintbrush fracture (Figure 2). She had no history of diseases, and blood examinations including complete blood cell count, thyroid function test, and iron levels were within reference range.

|
We hypothesize that the seasonal damage caused by exposure to UV light and salt water with repeated trauma from the heat of the flat iron caused distal TN. The patient was given an explanation about the diagnosis of TN and was instructed to avoid the practices that were suspected causes of the condition. Use of a gentle shampoo and conditioner also was recommended. At 6-month follow-up, we noticed an improvement of the quality of hair with a reduction in the whitish nodules and a revival of hair growth.
Acquired TN has been classified into 3 clinical forms: proximal, distal, and localized.1 Proximal TN is common in black individuals who use caustic chemicals when styling the hair. The involved hairs develop the characteristic nodes that break within a few centimeters from the scalp, especially in areas subject to friction from combing or sleeping. Distal TN primarily occurs in white or Asian individuals. In this disorder, nodes and breakage occur near the ends of the hairs that appear dull, dry, and uneven. Breakage commonly is associated with trichoptilosis, or longitudinal splitting, commonly referred to as split ends. This breakage may reflect frequent use of shampoo or heat treatments. The distal acquired form may simulate dandruff or pediculosis and the detection of this hair defect often is casual.
Localized TN, described by Raymond Sabouraud in 1921, is a rare disorder. It occurs in a patch that is usually a few centimeters long. It generally is accompanied by a pruritic dermatosis, such as circumscribed neurodermatitis, contact dermatitis, or atopic dermatitis. Scratching and rubbing most likely are the ultimate causes.
Trichorrhexis nodosa can spontaneously resolve. In all cases, diagnosis depends on careful microscopy examination and, if possible, scanning electron microscopy. Treatment is aimed at minimizing mechanical and physical injury, and chemical trauma. Excessive brushing, hot-combing, permanent waving, and other harsh hair treatments should be avoided. If the hair is long and the damage is distal, it may be sufficient to cut the distal fraction and to change cosmetic practices to prevent relapse.
Dermatologists who see patients with hair fragility and inability to grow long hair should consider the diagnosis of TN. Acquired TN often is reversible. Complete resolution may take 2 to 4 years depending on the growth of new anagen hairs. All patients with a history of white flecking on the scalp, abnormal fragility of the hair, and failure to attain normal hair length should be questioned about their routine hair care habits as well as environmental or chemical exposures to determine and remove the source of physical or chemical trauma.
1. Whiting DA. Structural abnormalities of hair shaft. J Am Acad Dermatol. 1987;16(1, pt 1):1-25.
2. Leider M. Multiple simultaneous anomalies of the hair; report of a case exhibiting trichorrhexis nodosa, pili annulati and trichostasis spinulosa. AMA Arch Derm Syphilol. 1950;62:510-514.
3. Allan JD, Cusworth DC, Dent CE, et al. A disease, probably hereditary characterised by severe mental deficiency and a constant gross abnormality of aminoacid metabolism. Lancet. 1958;1:182-187.
4. Liang C, Morris A, Schlücker S, et al. Structural and molecular hair abnormalities in trichothiodystrophy [published online ahead of print May 25, 2006]. J Invest Dermatol. 2006;126:2210-2216.
5. Taylor CJ, Green SH. Menkes’ syndrome (trichopoliodystrophy): use of scanning electron-microscope in diagnosis and carrier identification. Dev Med Child Neurol. 1981;23:361-368.
6. Hartley JL, Zachos NC, Dawood B, et al. Mutations in TTC37 cause trichohepatoenteric syndrome (phenotypic diarrhea of infancy)[published online ahead of print February 20, 2010]. Gastroenterology. 2010;138:2388-2398.
7. Chernosky ME, Owens DW. Trichorrhexis nodosa. clinical and investigative studies. Arch Dermatol. 1966;94:577-585.
8. Owens DW, Chernosky ME. Trichorrhexis nodosa; in vitro reproduction. Arch Dermatol. 1966;94:586-588.
9. Lurie R, Hodak E, Ginzburg A, et al. Trichorrhexis nodosa: a manifestation of hypothyroidism. Cutis. 1996;57:358-359.
10. Miyamoto M, Tsuboi R, Oh-I T. Case of acquired trichorrhexis nodosa: scanning electron microscopic observation. J Dermatol. 2009;36:109-110.
To the Editor:
First identified by Samuel Wilks in 1852, trichorrhexis nodosa (TN) is a congenital or acquired hair shaft disorder that is characterized by fragile and easily broken hair.1 Congenital TN is rare and can occur in syndromes such as pseudomonilethrix, Netherton syndrome, pili annulati,2 argininosuccinic aciduria,3 trichothiodystrophy,4 Menkes syndrome,5 and trichohepatoenteric syndrome.6 The primary congenital form of TN is inherited as an autosomal-dominant trait in some families. Acquired TN is the most common hair shaft abnormality and often is overlooked. It is provoked by hair injury, usually mechanical or physical, or chemical trauma.7,8
Chemical trauma is caused by the use of permanent hair liquids or dyes. Mechanical injuries are the result of frequent brushing, scalp massage, or lengthy backcombing, and physical damage includes excessive UV exposure or repeated application of heat. Habit tics, trichotillomania, and the scratching and pulling associated with pruritic dermatoses also can result in sufficient damage to provoke TN. Furthermore, this acquired disorder may develop from malnutrition, particularly iron deficiency, or endocrinopathy such as hypothyroidism.9 Seasonal recurrence of TN has been reported from the cumulative effect of repeated soaking in salt water and exposure to UV light. Macroscopically, hair shafts affected by TN contain small white nodes at irregular intervals throughout the length of the hair shaft. These nodes represent areas of cuticular cell disruption, which allows the underlying cortical fibers to separate and fray and gives the node the microscopic appearance of 2 brooms or paintbrushes thrusting together end-to-end by the bristles. The classic description is known as paintbrush fracture.10 Generally, complete breakage occurs at these nodes.
A 21-year-old white woman presented to our clinic with hair fragility and inability to grow long hair of 2 years’ duration. The hair was lusterless and dry. Dermoscopic examination revealed broken blunt-ended hair of uneven length with minute pinpoint grayish white nodules (Figure 1). Small fragments could be easily broken off with gentle tugging on the distal ends. She reported a history of severe sunlight and seawater exposure during the last 2 summers and the continuous use of a flat iron in the last year. Microscopic examination of hair samples with a scanning electron microscope showed the characteristic paintbrush fracture (Figure 2). She had no history of diseases, and blood examinations including complete blood cell count, thyroid function test, and iron levels were within reference range.

|
We hypothesize that the seasonal damage caused by exposure to UV light and salt water with repeated trauma from the heat of the flat iron caused distal TN. The patient was given an explanation about the diagnosis of TN and was instructed to avoid the practices that were suspected causes of the condition. Use of a gentle shampoo and conditioner also was recommended. At 6-month follow-up, we noticed an improvement of the quality of hair with a reduction in the whitish nodules and a revival of hair growth.
Acquired TN has been classified into 3 clinical forms: proximal, distal, and localized.1 Proximal TN is common in black individuals who use caustic chemicals when styling the hair. The involved hairs develop the characteristic nodes that break within a few centimeters from the scalp, especially in areas subject to friction from combing or sleeping. Distal TN primarily occurs in white or Asian individuals. In this disorder, nodes and breakage occur near the ends of the hairs that appear dull, dry, and uneven. Breakage commonly is associated with trichoptilosis, or longitudinal splitting, commonly referred to as split ends. This breakage may reflect frequent use of shampoo or heat treatments. The distal acquired form may simulate dandruff or pediculosis and the detection of this hair defect often is casual.
Localized TN, described by Raymond Sabouraud in 1921, is a rare disorder. It occurs in a patch that is usually a few centimeters long. It generally is accompanied by a pruritic dermatosis, such as circumscribed neurodermatitis, contact dermatitis, or atopic dermatitis. Scratching and rubbing most likely are the ultimate causes.
Trichorrhexis nodosa can spontaneously resolve. In all cases, diagnosis depends on careful microscopy examination and, if possible, scanning electron microscopy. Treatment is aimed at minimizing mechanical and physical injury, and chemical trauma. Excessive brushing, hot-combing, permanent waving, and other harsh hair treatments should be avoided. If the hair is long and the damage is distal, it may be sufficient to cut the distal fraction and to change cosmetic practices to prevent relapse.
Dermatologists who see patients with hair fragility and inability to grow long hair should consider the diagnosis of TN. Acquired TN often is reversible. Complete resolution may take 2 to 4 years depending on the growth of new anagen hairs. All patients with a history of white flecking on the scalp, abnormal fragility of the hair, and failure to attain normal hair length should be questioned about their routine hair care habits as well as environmental or chemical exposures to determine and remove the source of physical or chemical trauma.
To the Editor:
First identified by Samuel Wilks in 1852, trichorrhexis nodosa (TN) is a congenital or acquired hair shaft disorder that is characterized by fragile and easily broken hair.1 Congenital TN is rare and can occur in syndromes such as pseudomonilethrix, Netherton syndrome, pili annulati,2 argininosuccinic aciduria,3 trichothiodystrophy,4 Menkes syndrome,5 and trichohepatoenteric syndrome.6 The primary congenital form of TN is inherited as an autosomal-dominant trait in some families. Acquired TN is the most common hair shaft abnormality and often is overlooked. It is provoked by hair injury, usually mechanical or physical, or chemical trauma.7,8
Chemical trauma is caused by the use of permanent hair liquids or dyes. Mechanical injuries are the result of frequent brushing, scalp massage, or lengthy backcombing, and physical damage includes excessive UV exposure or repeated application of heat. Habit tics, trichotillomania, and the scratching and pulling associated with pruritic dermatoses also can result in sufficient damage to provoke TN. Furthermore, this acquired disorder may develop from malnutrition, particularly iron deficiency, or endocrinopathy such as hypothyroidism.9 Seasonal recurrence of TN has been reported from the cumulative effect of repeated soaking in salt water and exposure to UV light. Macroscopically, hair shafts affected by TN contain small white nodes at irregular intervals throughout the length of the hair shaft. These nodes represent areas of cuticular cell disruption, which allows the underlying cortical fibers to separate and fray and gives the node the microscopic appearance of 2 brooms or paintbrushes thrusting together end-to-end by the bristles. The classic description is known as paintbrush fracture.10 Generally, complete breakage occurs at these nodes.
A 21-year-old white woman presented to our clinic with hair fragility and inability to grow long hair of 2 years’ duration. The hair was lusterless and dry. Dermoscopic examination revealed broken blunt-ended hair of uneven length with minute pinpoint grayish white nodules (Figure 1). Small fragments could be easily broken off with gentle tugging on the distal ends. She reported a history of severe sunlight and seawater exposure during the last 2 summers and the continuous use of a flat iron in the last year. Microscopic examination of hair samples with a scanning electron microscope showed the characteristic paintbrush fracture (Figure 2). She had no history of diseases, and blood examinations including complete blood cell count, thyroid function test, and iron levels were within reference range.

|
We hypothesize that the seasonal damage caused by exposure to UV light and salt water with repeated trauma from the heat of the flat iron caused distal TN. The patient was given an explanation about the diagnosis of TN and was instructed to avoid the practices that were suspected causes of the condition. Use of a gentle shampoo and conditioner also was recommended. At 6-month follow-up, we noticed an improvement of the quality of hair with a reduction in the whitish nodules and a revival of hair growth.
Acquired TN has been classified into 3 clinical forms: proximal, distal, and localized.1 Proximal TN is common in black individuals who use caustic chemicals when styling the hair. The involved hairs develop the characteristic nodes that break within a few centimeters from the scalp, especially in areas subject to friction from combing or sleeping. Distal TN primarily occurs in white or Asian individuals. In this disorder, nodes and breakage occur near the ends of the hairs that appear dull, dry, and uneven. Breakage commonly is associated with trichoptilosis, or longitudinal splitting, commonly referred to as split ends. This breakage may reflect frequent use of shampoo or heat treatments. The distal acquired form may simulate dandruff or pediculosis and the detection of this hair defect often is casual.
Localized TN, described by Raymond Sabouraud in 1921, is a rare disorder. It occurs in a patch that is usually a few centimeters long. It generally is accompanied by a pruritic dermatosis, such as circumscribed neurodermatitis, contact dermatitis, or atopic dermatitis. Scratching and rubbing most likely are the ultimate causes.
Trichorrhexis nodosa can spontaneously resolve. In all cases, diagnosis depends on careful microscopy examination and, if possible, scanning electron microscopy. Treatment is aimed at minimizing mechanical and physical injury, and chemical trauma. Excessive brushing, hot-combing, permanent waving, and other harsh hair treatments should be avoided. If the hair is long and the damage is distal, it may be sufficient to cut the distal fraction and to change cosmetic practices to prevent relapse.
Dermatologists who see patients with hair fragility and inability to grow long hair should consider the diagnosis of TN. Acquired TN often is reversible. Complete resolution may take 2 to 4 years depending on the growth of new anagen hairs. All patients with a history of white flecking on the scalp, abnormal fragility of the hair, and failure to attain normal hair length should be questioned about their routine hair care habits as well as environmental or chemical exposures to determine and remove the source of physical or chemical trauma.
1. Whiting DA. Structural abnormalities of hair shaft. J Am Acad Dermatol. 1987;16(1, pt 1):1-25.
2. Leider M. Multiple simultaneous anomalies of the hair; report of a case exhibiting trichorrhexis nodosa, pili annulati and trichostasis spinulosa. AMA Arch Derm Syphilol. 1950;62:510-514.
3. Allan JD, Cusworth DC, Dent CE, et al. A disease, probably hereditary characterised by severe mental deficiency and a constant gross abnormality of aminoacid metabolism. Lancet. 1958;1:182-187.
4. Liang C, Morris A, Schlücker S, et al. Structural and molecular hair abnormalities in trichothiodystrophy [published online ahead of print May 25, 2006]. J Invest Dermatol. 2006;126:2210-2216.
5. Taylor CJ, Green SH. Menkes’ syndrome (trichopoliodystrophy): use of scanning electron-microscope in diagnosis and carrier identification. Dev Med Child Neurol. 1981;23:361-368.
6. Hartley JL, Zachos NC, Dawood B, et al. Mutations in TTC37 cause trichohepatoenteric syndrome (phenotypic diarrhea of infancy)[published online ahead of print February 20, 2010]. Gastroenterology. 2010;138:2388-2398.
7. Chernosky ME, Owens DW. Trichorrhexis nodosa. clinical and investigative studies. Arch Dermatol. 1966;94:577-585.
8. Owens DW, Chernosky ME. Trichorrhexis nodosa; in vitro reproduction. Arch Dermatol. 1966;94:586-588.
9. Lurie R, Hodak E, Ginzburg A, et al. Trichorrhexis nodosa: a manifestation of hypothyroidism. Cutis. 1996;57:358-359.
10. Miyamoto M, Tsuboi R, Oh-I T. Case of acquired trichorrhexis nodosa: scanning electron microscopic observation. J Dermatol. 2009;36:109-110.
1. Whiting DA. Structural abnormalities of hair shaft. J Am Acad Dermatol. 1987;16(1, pt 1):1-25.
2. Leider M. Multiple simultaneous anomalies of the hair; report of a case exhibiting trichorrhexis nodosa, pili annulati and trichostasis spinulosa. AMA Arch Derm Syphilol. 1950;62:510-514.
3. Allan JD, Cusworth DC, Dent CE, et al. A disease, probably hereditary characterised by severe mental deficiency and a constant gross abnormality of aminoacid metabolism. Lancet. 1958;1:182-187.
4. Liang C, Morris A, Schlücker S, et al. Structural and molecular hair abnormalities in trichothiodystrophy [published online ahead of print May 25, 2006]. J Invest Dermatol. 2006;126:2210-2216.
5. Taylor CJ, Green SH. Menkes’ syndrome (trichopoliodystrophy): use of scanning electron-microscope in diagnosis and carrier identification. Dev Med Child Neurol. 1981;23:361-368.
6. Hartley JL, Zachos NC, Dawood B, et al. Mutations in TTC37 cause trichohepatoenteric syndrome (phenotypic diarrhea of infancy)[published online ahead of print February 20, 2010]. Gastroenterology. 2010;138:2388-2398.
7. Chernosky ME, Owens DW. Trichorrhexis nodosa. clinical and investigative studies. Arch Dermatol. 1966;94:577-585.
8. Owens DW, Chernosky ME. Trichorrhexis nodosa; in vitro reproduction. Arch Dermatol. 1966;94:586-588.
9. Lurie R, Hodak E, Ginzburg A, et al. Trichorrhexis nodosa: a manifestation of hypothyroidism. Cutis. 1996;57:358-359.
10. Miyamoto M, Tsuboi R, Oh-I T. Case of acquired trichorrhexis nodosa: scanning electron microscopic observation. J Dermatol. 2009;36:109-110.
Dermatologic Toxicity in a Patient Receiving Liposomal Doxorubicin
To the Editor:
Liposomal doxorubicin hydrochloride is an anthracycline topoisomerase inhibitor indicated for ovarian cancer, AIDS-related Kaposi sarcoma, and multiple myeloma.1 It also has been used with limited success in a clinical trial of previously treated patients with endometrial cancer.2 The most common adverse reactions include asthenia, fatigue, fever, anorexia, nausea, vomiting, stomatitis, diarrhea, constipation, hand-and-foot syndrome, rash, neutropenia, thrombocytopenia, and anemia.1
A 58-year-old woman with a history of stage IIIA endometrial cancer underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy soon after diagnosis. She then completed 5 high-dose-rate brachytherapy treatments and 6 cycles of paclitaxel and carboplatin. Follow-up imaging revealed pulmonary metastasis. The patient was then enrolled in a clinical trial but was switched to 40 mg/m2 liposomal doxorubicin given once every 28 days for 5 cycles after progression of disease.
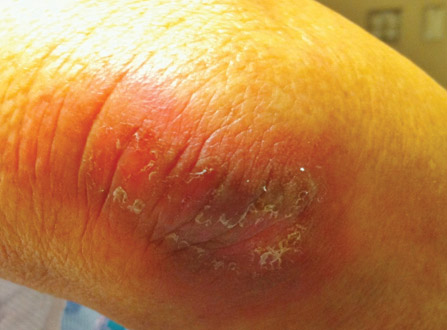
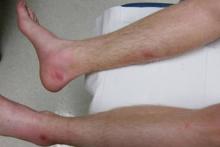
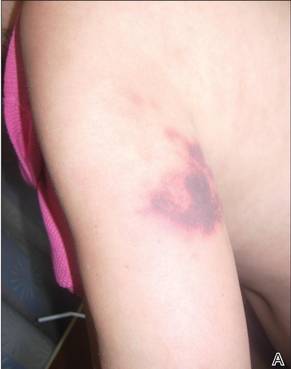
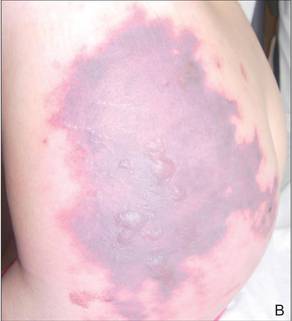
After each dose of doxorubicin, she developed redness of the palms and soles. Following the third cycle of doxorubicin, a painful rash involving the thighs and axilla appeared with some desquamation in the left axilla. Three weeks after the fourth dose of doxorubicin, she presented with severe worsening of the rash to involve the extensor elbows (Figure 1), back, and lower legs with bilateral axillary desquamation. The bilateral medial thighs were erythematous with maceration that was tender and blanchable (Figure 2). The total affected body surface area was 10% to 15%. There was no involvement of the mucosa. She was treated with hydrogel sheet dressings and silver sulfadiazine cream 1%.
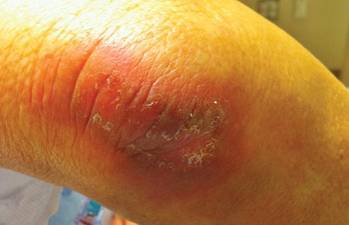
|
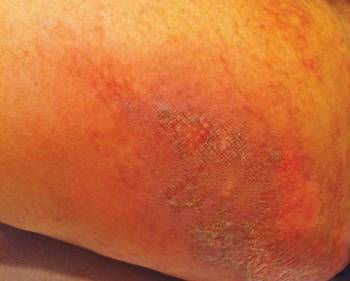
|
The patient’s rash was thought to be due to doxorubicin toxicity; however, a 4-mm punch biopsy specimen from the left thigh was taken for culture and hemotoxylin and eosin stain to rule out other possibilities. Biopsy was consistent with a drug reaction, revealing superficial perivascular dermatitis with keratinocyte atypia of the epidermis. Doxorubicin was discontinued and the rash resolved completely within 2 weeks, except for some thickening of the skin on the palms, soles, and thighs. After a delay of approximately 1 week, doxorubicin was resumed at a lower dose of 30 mg/m2. No dermatologic symptoms followed treatment at this dose.
Four clinical patterns of doxorubicin toxicity are recognized. The most common pattern is acral erythema, also known as hand-and-foot syndrome, which is followed by desquamation of the palms and soles, occurring in approximately 50% of patients. Ten percent of patients experience a diffuse follicular rash with mild, diffuse, scaly erythema and follicular accentuation that often occurs over the lateral limbs but also may occur over the trunk. New melanotic macules may appear on the trunk or extremities including palms and soles.3 Finally, an intertrigolike eruption exacerbated by friction with erythematous patches over skin folds or in areas of friction also has been described.3-5 Our patient presented with a combination of dermatologic toxicities including acral erythema and intertrigolike eruption. Acral erythema occurred in 24 of 60 patients and intertrigolike eruption occurred in 5 of 60 patients in one study.3 Another report documented both occurring together.5
Treatment of doxorubicin skin toxicity consists of reduction of the dose of doxorubicin, supportive care, and patient education. Specific treatments include topical wound care, emollient creams, and pain management with analgesics. Other interventions include wearing loose clothing, avoiding vigorous exercise, and sitting on padded surfaces.6
Doxorubicin skin toxicity presents in several clinical patterns. Although acral erythema is the most common pattern, severe intertrigolike eruptions similar to our case may occur. Physicians caring for patients receiving doxorubicin should be aware of the variety of presentations of skin toxicity and the possible need for dose reduction to decrease symptoms.
1. Doxil [package insert]. Horsham, PA: Janssen Products, LP; 2014.
2. Muggia FM, Blessing JA, Sorosky J, et al. Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2002;20:2360-2364.
3. Lotem M, Hubert A, Lyass O, et al. Skin toxic effects of polyethylene glycol-coated liposomal doxorubicin. Arch Dermatol. 2000;136:1475-1480.
4. Korver GE, Ronald H, Petersen MJ. An intertrigo-like eruption from pegylated liposomal doxorubicin. J Drugs Dermatol. 2006;5:901-902.
5. Sánchez Henarejos P, Ros Martinez S, Marín Zafra GR,
et al. Intertrigo-like eruption caused by pegylated liposomal doxorubicin (PLD). Clin Transl Oncol. 2009;11:486-487.
6. von Moos R, Thuerlimann BJ, Aapro M, et al. Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts [published online ahead of print March 10, 2008]. Eur J Cancer. 2008;44:781-790.
To the Editor:
Liposomal doxorubicin hydrochloride is an anthracycline topoisomerase inhibitor indicated for ovarian cancer, AIDS-related Kaposi sarcoma, and multiple myeloma.1 It also has been used with limited success in a clinical trial of previously treated patients with endometrial cancer.2 The most common adverse reactions include asthenia, fatigue, fever, anorexia, nausea, vomiting, stomatitis, diarrhea, constipation, hand-and-foot syndrome, rash, neutropenia, thrombocytopenia, and anemia.1
A 58-year-old woman with a history of stage IIIA endometrial cancer underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy soon after diagnosis. She then completed 5 high-dose-rate brachytherapy treatments and 6 cycles of paclitaxel and carboplatin. Follow-up imaging revealed pulmonary metastasis. The patient was then enrolled in a clinical trial but was switched to 40 mg/m2 liposomal doxorubicin given once every 28 days for 5 cycles after progression of disease.
After each dose of doxorubicin, she developed redness of the palms and soles. Following the third cycle of doxorubicin, a painful rash involving the thighs and axilla appeared with some desquamation in the left axilla. Three weeks after the fourth dose of doxorubicin, she presented with severe worsening of the rash to involve the extensor elbows (Figure 1), back, and lower legs with bilateral axillary desquamation. The bilateral medial thighs were erythematous with maceration that was tender and blanchable (Figure 2). The total affected body surface area was 10% to 15%. There was no involvement of the mucosa. She was treated with hydrogel sheet dressings and silver sulfadiazine cream 1%.

|

|
The patient’s rash was thought to be due to doxorubicin toxicity; however, a 4-mm punch biopsy specimen from the left thigh was taken for culture and hemotoxylin and eosin stain to rule out other possibilities. Biopsy was consistent with a drug reaction, revealing superficial perivascular dermatitis with keratinocyte atypia of the epidermis. Doxorubicin was discontinued and the rash resolved completely within 2 weeks, except for some thickening of the skin on the palms, soles, and thighs. After a delay of approximately 1 week, doxorubicin was resumed at a lower dose of 30 mg/m2. No dermatologic symptoms followed treatment at this dose.
Four clinical patterns of doxorubicin toxicity are recognized. The most common pattern is acral erythema, also known as hand-and-foot syndrome, which is followed by desquamation of the palms and soles, occurring in approximately 50% of patients. Ten percent of patients experience a diffuse follicular rash with mild, diffuse, scaly erythema and follicular accentuation that often occurs over the lateral limbs but also may occur over the trunk. New melanotic macules may appear on the trunk or extremities including palms and soles.3 Finally, an intertrigolike eruption exacerbated by friction with erythematous patches over skin folds or in areas of friction also has been described.3-5 Our patient presented with a combination of dermatologic toxicities including acral erythema and intertrigolike eruption. Acral erythema occurred in 24 of 60 patients and intertrigolike eruption occurred in 5 of 60 patients in one study.3 Another report documented both occurring together.5
Treatment of doxorubicin skin toxicity consists of reduction of the dose of doxorubicin, supportive care, and patient education. Specific treatments include topical wound care, emollient creams, and pain management with analgesics. Other interventions include wearing loose clothing, avoiding vigorous exercise, and sitting on padded surfaces.6
Doxorubicin skin toxicity presents in several clinical patterns. Although acral erythema is the most common pattern, severe intertrigolike eruptions similar to our case may occur. Physicians caring for patients receiving doxorubicin should be aware of the variety of presentations of skin toxicity and the possible need for dose reduction to decrease symptoms.
To the Editor:
Liposomal doxorubicin hydrochloride is an anthracycline topoisomerase inhibitor indicated for ovarian cancer, AIDS-related Kaposi sarcoma, and multiple myeloma.1 It also has been used with limited success in a clinical trial of previously treated patients with endometrial cancer.2 The most common adverse reactions include asthenia, fatigue, fever, anorexia, nausea, vomiting, stomatitis, diarrhea, constipation, hand-and-foot syndrome, rash, neutropenia, thrombocytopenia, and anemia.1
A 58-year-old woman with a history of stage IIIA endometrial cancer underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy soon after diagnosis. She then completed 5 high-dose-rate brachytherapy treatments and 6 cycles of paclitaxel and carboplatin. Follow-up imaging revealed pulmonary metastasis. The patient was then enrolled in a clinical trial but was switched to 40 mg/m2 liposomal doxorubicin given once every 28 days for 5 cycles after progression of disease.
After each dose of doxorubicin, she developed redness of the palms and soles. Following the third cycle of doxorubicin, a painful rash involving the thighs and axilla appeared with some desquamation in the left axilla. Three weeks after the fourth dose of doxorubicin, she presented with severe worsening of the rash to involve the extensor elbows (Figure 1), back, and lower legs with bilateral axillary desquamation. The bilateral medial thighs were erythematous with maceration that was tender and blanchable (Figure 2). The total affected body surface area was 10% to 15%. There was no involvement of the mucosa. She was treated with hydrogel sheet dressings and silver sulfadiazine cream 1%.

|

|
The patient’s rash was thought to be due to doxorubicin toxicity; however, a 4-mm punch biopsy specimen from the left thigh was taken for culture and hemotoxylin and eosin stain to rule out other possibilities. Biopsy was consistent with a drug reaction, revealing superficial perivascular dermatitis with keratinocyte atypia of the epidermis. Doxorubicin was discontinued and the rash resolved completely within 2 weeks, except for some thickening of the skin on the palms, soles, and thighs. After a delay of approximately 1 week, doxorubicin was resumed at a lower dose of 30 mg/m2. No dermatologic symptoms followed treatment at this dose.
Four clinical patterns of doxorubicin toxicity are recognized. The most common pattern is acral erythema, also known as hand-and-foot syndrome, which is followed by desquamation of the palms and soles, occurring in approximately 50% of patients. Ten percent of patients experience a diffuse follicular rash with mild, diffuse, scaly erythema and follicular accentuation that often occurs over the lateral limbs but also may occur over the trunk. New melanotic macules may appear on the trunk or extremities including palms and soles.3 Finally, an intertrigolike eruption exacerbated by friction with erythematous patches over skin folds or in areas of friction also has been described.3-5 Our patient presented with a combination of dermatologic toxicities including acral erythema and intertrigolike eruption. Acral erythema occurred in 24 of 60 patients and intertrigolike eruption occurred in 5 of 60 patients in one study.3 Another report documented both occurring together.5
Treatment of doxorubicin skin toxicity consists of reduction of the dose of doxorubicin, supportive care, and patient education. Specific treatments include topical wound care, emollient creams, and pain management with analgesics. Other interventions include wearing loose clothing, avoiding vigorous exercise, and sitting on padded surfaces.6
Doxorubicin skin toxicity presents in several clinical patterns. Although acral erythema is the most common pattern, severe intertrigolike eruptions similar to our case may occur. Physicians caring for patients receiving doxorubicin should be aware of the variety of presentations of skin toxicity and the possible need for dose reduction to decrease symptoms.
1. Doxil [package insert]. Horsham, PA: Janssen Products, LP; 2014.
2. Muggia FM, Blessing JA, Sorosky J, et al. Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2002;20:2360-2364.
3. Lotem M, Hubert A, Lyass O, et al. Skin toxic effects of polyethylene glycol-coated liposomal doxorubicin. Arch Dermatol. 2000;136:1475-1480.
4. Korver GE, Ronald H, Petersen MJ. An intertrigo-like eruption from pegylated liposomal doxorubicin. J Drugs Dermatol. 2006;5:901-902.
5. Sánchez Henarejos P, Ros Martinez S, Marín Zafra GR,
et al. Intertrigo-like eruption caused by pegylated liposomal doxorubicin (PLD). Clin Transl Oncol. 2009;11:486-487.
6. von Moos R, Thuerlimann BJ, Aapro M, et al. Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts [published online ahead of print March 10, 2008]. Eur J Cancer. 2008;44:781-790.
1. Doxil [package insert]. Horsham, PA: Janssen Products, LP; 2014.
2. Muggia FM, Blessing JA, Sorosky J, et al. Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2002;20:2360-2364.
3. Lotem M, Hubert A, Lyass O, et al. Skin toxic effects of polyethylene glycol-coated liposomal doxorubicin. Arch Dermatol. 2000;136:1475-1480.
4. Korver GE, Ronald H, Petersen MJ. An intertrigo-like eruption from pegylated liposomal doxorubicin. J Drugs Dermatol. 2006;5:901-902.
5. Sánchez Henarejos P, Ros Martinez S, Marín Zafra GR,
et al. Intertrigo-like eruption caused by pegylated liposomal doxorubicin (PLD). Clin Transl Oncol. 2009;11:486-487.
6. von Moos R, Thuerlimann BJ, Aapro M, et al. Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts [published online ahead of print March 10, 2008]. Eur J Cancer. 2008;44:781-790.
Cold Panniculitis: Delayed Onset in an Adult
The panniculitides can be a complex dermatologic entity for both dermatologists and dermatopathologists. The history, clinical examination, and histology need to be correlated to arrive at a differential diagnosis that will ultimately provide a diagnosis for the subcutaneous lesions. Panniculitis is an inflammation of the subcutaneous adipose tissue and can be associated with systemic diseases. According to Peters and Su,1 “Anatomic location of lesions, presence or absence of ulceration, occurrence of lipoatrophy, history of trauma, association with immunologic or metabolic disorders, and age of the patient are important clinical data to consider in conjunction with the microscopic features.” The panniculitides histologic differences may be subtle because they all include septal and lobular components, but one is usually more dominant in leading to a diagnosis along with the clinical findings.2
Cold panniculitis is a form of traumatic panniculitis. We present a unique case of this condition that was caused by use of a cold therapy unit following surgery to relieve pain.
Case Report
A 37-year-old woman presented for a routine postoperative visit 15 days following arthroscopic repair of a superior labrum anterior posterior tear in the left shoulder with a single suture anchor. The patient reported a rash that had developed 10 days postoperatively on the left upper arm. The rash started as red dots that progressively became larger, painful, and warm to the touch. The rash did not spread anywhere else on the patient’s body, and she denied fever, chills, and pruritus. She had tried using diphenhydramine without relief. The only new medication the patient had started prior to the eruption was oxycodone, which was initiated immediately following surgery. Prior to surgery, the entire left upper extremity including the shoulder had been prepared with a preoperative surgical skin antiseptic. There were no visible signs of the antiseptic on the skin at the time of presentation. The patient reported that she had applied a cold therapy unit to the left upper arm over her clothing for 1 hour every night since surgery. The cold therapy unit frequently is used to help decrease postoperative pain, swelling, inflammation, and narcotic use following surgical procedures.
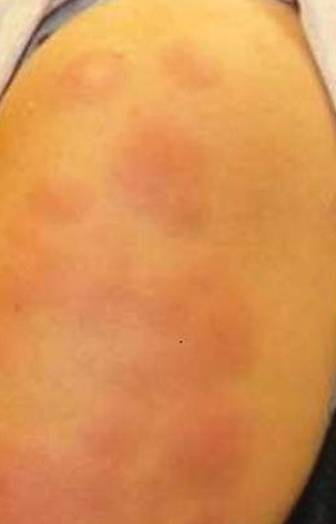
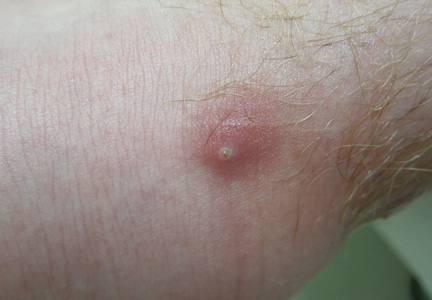
Physical examination revealed multiple well-defined, erythematous, tender, indurated, warm nodules on the lateral aspect of the left upper arm (Figure 1). No other areas of eruption were noted on the body, and there was no swelling of the left elbow, forearm, wrist, or hand. The left upper extremity demonstrated intact sensation, rapid capillary refill, and a palpable radial pulse. Her weight was 230.1 lb with a body mass index of 35.
|
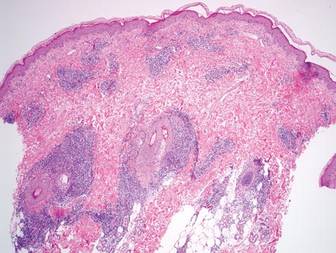
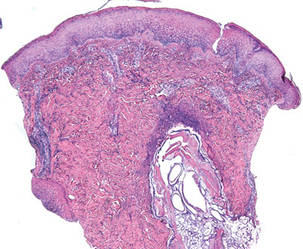
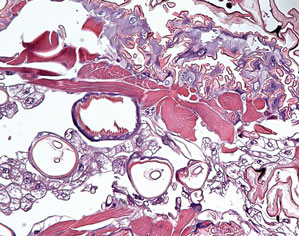
A 5-mm punch biopsy from a nodule on the left upper arm was performed, and pathology demonstrated vacuolar interface changes with patchy parakeratosis, spongiosis, and dyskeratosis on staining with hematoxylin and eosin. Pandermal and subcutaneous perivascular, periadnexal, and mild interstitial lymphohistiocytic infiltrate with occasional neutrophils and eosinophils were noted (Figure 2). The inflammation extended to the subcutaneous fat involving both septae and lobules with a primarily lobular distribution.
Clinical and pathologic correlation was required to arrive at a definitive diagnosis of cold panniculitis. The epidermal and dermal changes were consistent with a pernio or chilblains type of insult, and the septal and lobular panniculitis was indicative of cold panniculitis. The patient was advised to discontinue use of the cold therapy device as well as any other form of icing of the left shoulder or arm. She continued the oxycodone for pain control. Four weeks postoperatively, only desquamation remained where the nodules had previously appeared, which also eventually resolved.
Comment
Infants and small children are more predisposed to cold panniculitis than adults. In their 2008 review, Quesada-Cortés et al3 found the first report of cold panniculitis by Hochsinger in 1902 in a German pediatric journal, followed by reports from Lemez in 1928 and Haxthausen in 1941, which subsequently described similar cases in infants. Adult cases were not reported until 1963 by Solomon and Beerman4 and then in 1980 by Beacham et al.5
Etiologies for children have included popsicles, ice packs applied to the face to control supraventricular tachycardia or to the lower extremities after vaccinations, and cold weather exposure.6 The chemical composition of fat tissue plays a role in pediatric patients. According to Quesada-Cortés et al,3 subcutaneous fat in newborns is rich in saturated oils such as palmitic and stearic acids that have a higher solidification point. A small decrease in an infant’s temperature may result in crystallization of fat. The subcutaneous fat tends to become more unsaturated with aging with more oleic acid, and the solidification temperature diminishes.7
Cryoglobulins and cold agglutinins have not been demonstrated to be a cause of cold panniculitis in infants.7 Severe cold exposure or predisposition to certain conditions such as cryofibrinogenemia may occur in some adult patients. Gender does not seem to be a factor in children; however, in adults, women tend to be more predisposed to cold panniculitis secondary to obesity and participation in activities such as cycling, motorcycling, or horseback riding in cold conditions.3
On clinical examination, cold panniculitis features erythematous, firm, tender nodules on the cheeks and chin in infants and small children.2 These areas often are exposed to cold weather or wind because they typically are not covered with protective clothing.3 Nodules generally occur 1 to 3 days following exposure to cold and usually resolve spontaneously within 2 weeks.8 Popsicle panniculitis is characterized by a reddish discoloration on both cheeks 1 or 2 days after sucking on popsicles or ice cubes. This reaction can be reproduced in a half day by applying an ice cube to the volar forearm for 2 minutes, which can help diagnose and differentiate this subset of cold panniuculits.3 The red area in cold panniculitis eventually turns purple, becomes less indurated, and fades in approximately 3 months, but occasionally residual hyperpigmentation will last for a few months. Ice packs used as treatment of congenital cardiac arrhythmias in some cardiac surgeries and as surface cooling for management of birth asphyxia can produce a similar physical presentation.3
Equestrian panniculitis is characterized by erythematous, violaceous, tender plaques on the upper lateral thighs of young females who participate in horseback riding in the winter while wearing tight-fitting pants.2,5 These plaques typically occur within several hours and over the next week become painful, violaceous, and indurated or develop red nodules or plaques that can ulcerate or become crusted.3 These lesions usually will spontaneously resolve within 3 weeks, but new areas may occur again during the winter on further exposure with occasional persistent hyperpigmentation. These areas usually disappear at the end of winter with warmer weather or when horseback riding is discontinued. Perniosis also needs to be considered in the differential diagnosis due to the location and appearance of the lesions.3
It is important to obtain the correct specimen for biopsy. According to Peters and Su,1 a deep excisional biopsy that includes multiple fat lobules in addition to dermis and epidermis is critical. On histology, cold panniculitis usually demonstrates a primarily lobular inflammation. There typically is a superficial and deep perivascular lymphocytic infiltrate in the papillary dermis with edema noted in the connective tissue around the eccrine glands that can appear similar to perniosis on histopathology.9 Deposition of mucin, focal panniculitis surrounded by fatty tissue without inflammatory changes within the same field, and fat necrosis with pseudocysts and numerous lipophages also are characteristic features of cold panniculitis.10 Needlelike clefts are not present in cold panniculitis but appear in subcutaneous fat necrosis of the newborn.1
Different treatments have been tried, but no substantial impact on the rate of dissipation of the lesions has been noted. The plaques slowly resolve without scarring over 2 to 3 weeks if the cold source is removed.2 Application of a heating pad to the affected area has been used with limited success. Vasodilators such as nifedipine have been used but have not been found to be effective.3 Antihistamines also have failed to control the lesions.11
Treatment of cold panniculitis is based on the prevention of further insult versus trying to cure the condition. Avoidance of cold and wind exposure as well as direct contact with ice are key methods in preventing cold panniculitis.
Our patient’s presentation of this condition was unique. Although cold panniculitis lesions usually develop 1 to 3 days after cold exposure, our patient did not develop lesions until 10 days following surgery. The cold therapy unit used by our patient was evaluated in our office and also by the manufacturer and was found to be functioning normally with no defects. The late onset of the lesions was attributed to limited application of the cold therapy unit; our patient used it for only 1 hour every night, whereas application for 6 to 8 hours continuously is normally recommended. The lesions may have occurred sooner had the patient been using a solid ice pack versus the continuous cold circulating water of the cold therapy unit. Pathology was consistent with the patient’s history and physical examination indicating a diagnosis of cold panniculitis. The challenge of treatment was to alleviate the pain of the lesions as well as the postoperative shoulder pain without the aid of any form of cold therapy. The patient only needed a tincture of time, as the lesions resolved after 4 weeks. Patient education was provided on future prevention of this condition by avoiding exposure to cold or applying cold packs directly to the skin.
Acknowledgment
The authors thank the staff at the Office of Scientific Writing and Publication at the Marshfield Clinic Research Foundation, Wisconsin, for their editorial assistance in the preparation of this manuscript.
1. Peters MS, Su WP. Panniculitis. Dermatol Clin. 1992;10:37-57.
2. Patterson JW. Panniculitis. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 2nd ed. St. Louis, MO: Mosby Elsevier; 2008:1515-1530.
3. Quesada-Cortés A, Campos-Muñoz L, Díaz-Díaz RM, et al. Cold panniculitis. Dermatol Clin. 2008;26:485-489.
4. Solomon LM, Beerman H. Cold panniculitis. Arch Dermatol. 1963;88:897-900.
5. Beacham BE, Cooper PH, Buchanan CS, et al. Equestrian cold panniculitis in women. Arch Dermatol. 1980;116:1025-1027.
6. Ter Poorten MC, Thiers BH. Panniculitis. Dermatol Clin. 2002;20:421-433.
7. Ter Poorten JC, Hebert AA, Ilkiw R. Cold panniculitis in a neonate. J Am Acad Dermatol. 1995;33(2, pt 2):383-385.
8. Page EH, Shear NH. Temperature-dependent skin disorders. J Am Acad Dermatol. 1988;18(5, pt 1):1003-1019.
9. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
10. Diaz Cascajo C, Borghi S, Weyers W. Panniculitis: definition of terms and diagnostic strategy. Am J Dermatopathol. 2000;22:530-549.
11. Duncan WC, Freeman RG, Heaton CL. Cold panniculitis. Arch Dermatol. 1966;94:722-724.
The panniculitides can be a complex dermatologic entity for both dermatologists and dermatopathologists. The history, clinical examination, and histology need to be correlated to arrive at a differential diagnosis that will ultimately provide a diagnosis for the subcutaneous lesions. Panniculitis is an inflammation of the subcutaneous adipose tissue and can be associated with systemic diseases. According to Peters and Su,1 “Anatomic location of lesions, presence or absence of ulceration, occurrence of lipoatrophy, history of trauma, association with immunologic or metabolic disorders, and age of the patient are important clinical data to consider in conjunction with the microscopic features.” The panniculitides histologic differences may be subtle because they all include septal and lobular components, but one is usually more dominant in leading to a diagnosis along with the clinical findings.2
Cold panniculitis is a form of traumatic panniculitis. We present a unique case of this condition that was caused by use of a cold therapy unit following surgery to relieve pain.
Case Report
A 37-year-old woman presented for a routine postoperative visit 15 days following arthroscopic repair of a superior labrum anterior posterior tear in the left shoulder with a single suture anchor. The patient reported a rash that had developed 10 days postoperatively on the left upper arm. The rash started as red dots that progressively became larger, painful, and warm to the touch. The rash did not spread anywhere else on the patient’s body, and she denied fever, chills, and pruritus. She had tried using diphenhydramine without relief. The only new medication the patient had started prior to the eruption was oxycodone, which was initiated immediately following surgery. Prior to surgery, the entire left upper extremity including the shoulder had been prepared with a preoperative surgical skin antiseptic. There were no visible signs of the antiseptic on the skin at the time of presentation. The patient reported that she had applied a cold therapy unit to the left upper arm over her clothing for 1 hour every night since surgery. The cold therapy unit frequently is used to help decrease postoperative pain, swelling, inflammation, and narcotic use following surgical procedures.
Physical examination revealed multiple well-defined, erythematous, tender, indurated, warm nodules on the lateral aspect of the left upper arm (Figure 1). No other areas of eruption were noted on the body, and there was no swelling of the left elbow, forearm, wrist, or hand. The left upper extremity demonstrated intact sensation, rapid capillary refill, and a palpable radial pulse. Her weight was 230.1 lb with a body mass index of 35.
|
A 5-mm punch biopsy from a nodule on the left upper arm was performed, and pathology demonstrated vacuolar interface changes with patchy parakeratosis, spongiosis, and dyskeratosis on staining with hematoxylin and eosin. Pandermal and subcutaneous perivascular, periadnexal, and mild interstitial lymphohistiocytic infiltrate with occasional neutrophils and eosinophils were noted (Figure 2). The inflammation extended to the subcutaneous fat involving both septae and lobules with a primarily lobular distribution.
Clinical and pathologic correlation was required to arrive at a definitive diagnosis of cold panniculitis. The epidermal and dermal changes were consistent with a pernio or chilblains type of insult, and the septal and lobular panniculitis was indicative of cold panniculitis. The patient was advised to discontinue use of the cold therapy device as well as any other form of icing of the left shoulder or arm. She continued the oxycodone for pain control. Four weeks postoperatively, only desquamation remained where the nodules had previously appeared, which also eventually resolved.
Comment
Infants and small children are more predisposed to cold panniculitis than adults. In their 2008 review, Quesada-Cortés et al3 found the first report of cold panniculitis by Hochsinger in 1902 in a German pediatric journal, followed by reports from Lemez in 1928 and Haxthausen in 1941, which subsequently described similar cases in infants. Adult cases were not reported until 1963 by Solomon and Beerman4 and then in 1980 by Beacham et al.5
Etiologies for children have included popsicles, ice packs applied to the face to control supraventricular tachycardia or to the lower extremities after vaccinations, and cold weather exposure.6 The chemical composition of fat tissue plays a role in pediatric patients. According to Quesada-Cortés et al,3 subcutaneous fat in newborns is rich in saturated oils such as palmitic and stearic acids that have a higher solidification point. A small decrease in an infant’s temperature may result in crystallization of fat. The subcutaneous fat tends to become more unsaturated with aging with more oleic acid, and the solidification temperature diminishes.7
Cryoglobulins and cold agglutinins have not been demonstrated to be a cause of cold panniculitis in infants.7 Severe cold exposure or predisposition to certain conditions such as cryofibrinogenemia may occur in some adult patients. Gender does not seem to be a factor in children; however, in adults, women tend to be more predisposed to cold panniculitis secondary to obesity and participation in activities such as cycling, motorcycling, or horseback riding in cold conditions.3
On clinical examination, cold panniculitis features erythematous, firm, tender nodules on the cheeks and chin in infants and small children.2 These areas often are exposed to cold weather or wind because they typically are not covered with protective clothing.3 Nodules generally occur 1 to 3 days following exposure to cold and usually resolve spontaneously within 2 weeks.8 Popsicle panniculitis is characterized by a reddish discoloration on both cheeks 1 or 2 days after sucking on popsicles or ice cubes. This reaction can be reproduced in a half day by applying an ice cube to the volar forearm for 2 minutes, which can help diagnose and differentiate this subset of cold panniuculits.3 The red area in cold panniculitis eventually turns purple, becomes less indurated, and fades in approximately 3 months, but occasionally residual hyperpigmentation will last for a few months. Ice packs used as treatment of congenital cardiac arrhythmias in some cardiac surgeries and as surface cooling for management of birth asphyxia can produce a similar physical presentation.3
Equestrian panniculitis is characterized by erythematous, violaceous, tender plaques on the upper lateral thighs of young females who participate in horseback riding in the winter while wearing tight-fitting pants.2,5 These plaques typically occur within several hours and over the next week become painful, violaceous, and indurated or develop red nodules or plaques that can ulcerate or become crusted.3 These lesions usually will spontaneously resolve within 3 weeks, but new areas may occur again during the winter on further exposure with occasional persistent hyperpigmentation. These areas usually disappear at the end of winter with warmer weather or when horseback riding is discontinued. Perniosis also needs to be considered in the differential diagnosis due to the location and appearance of the lesions.3
It is important to obtain the correct specimen for biopsy. According to Peters and Su,1 a deep excisional biopsy that includes multiple fat lobules in addition to dermis and epidermis is critical. On histology, cold panniculitis usually demonstrates a primarily lobular inflammation. There typically is a superficial and deep perivascular lymphocytic infiltrate in the papillary dermis with edema noted in the connective tissue around the eccrine glands that can appear similar to perniosis on histopathology.9 Deposition of mucin, focal panniculitis surrounded by fatty tissue without inflammatory changes within the same field, and fat necrosis with pseudocysts and numerous lipophages also are characteristic features of cold panniculitis.10 Needlelike clefts are not present in cold panniculitis but appear in subcutaneous fat necrosis of the newborn.1
Different treatments have been tried, but no substantial impact on the rate of dissipation of the lesions has been noted. The plaques slowly resolve without scarring over 2 to 3 weeks if the cold source is removed.2 Application of a heating pad to the affected area has been used with limited success. Vasodilators such as nifedipine have been used but have not been found to be effective.3 Antihistamines also have failed to control the lesions.11
Treatment of cold panniculitis is based on the prevention of further insult versus trying to cure the condition. Avoidance of cold and wind exposure as well as direct contact with ice are key methods in preventing cold panniculitis.
Our patient’s presentation of this condition was unique. Although cold panniculitis lesions usually develop 1 to 3 days after cold exposure, our patient did not develop lesions until 10 days following surgery. The cold therapy unit used by our patient was evaluated in our office and also by the manufacturer and was found to be functioning normally with no defects. The late onset of the lesions was attributed to limited application of the cold therapy unit; our patient used it for only 1 hour every night, whereas application for 6 to 8 hours continuously is normally recommended. The lesions may have occurred sooner had the patient been using a solid ice pack versus the continuous cold circulating water of the cold therapy unit. Pathology was consistent with the patient’s history and physical examination indicating a diagnosis of cold panniculitis. The challenge of treatment was to alleviate the pain of the lesions as well as the postoperative shoulder pain without the aid of any form of cold therapy. The patient only needed a tincture of time, as the lesions resolved after 4 weeks. Patient education was provided on future prevention of this condition by avoiding exposure to cold or applying cold packs directly to the skin.
Acknowledgment
The authors thank the staff at the Office of Scientific Writing and Publication at the Marshfield Clinic Research Foundation, Wisconsin, for their editorial assistance in the preparation of this manuscript.
The panniculitides can be a complex dermatologic entity for both dermatologists and dermatopathologists. The history, clinical examination, and histology need to be correlated to arrive at a differential diagnosis that will ultimately provide a diagnosis for the subcutaneous lesions. Panniculitis is an inflammation of the subcutaneous adipose tissue and can be associated with systemic diseases. According to Peters and Su,1 “Anatomic location of lesions, presence or absence of ulceration, occurrence of lipoatrophy, history of trauma, association with immunologic or metabolic disorders, and age of the patient are important clinical data to consider in conjunction with the microscopic features.” The panniculitides histologic differences may be subtle because they all include septal and lobular components, but one is usually more dominant in leading to a diagnosis along with the clinical findings.2
Cold panniculitis is a form of traumatic panniculitis. We present a unique case of this condition that was caused by use of a cold therapy unit following surgery to relieve pain.
Case Report
A 37-year-old woman presented for a routine postoperative visit 15 days following arthroscopic repair of a superior labrum anterior posterior tear in the left shoulder with a single suture anchor. The patient reported a rash that had developed 10 days postoperatively on the left upper arm. The rash started as red dots that progressively became larger, painful, and warm to the touch. The rash did not spread anywhere else on the patient’s body, and she denied fever, chills, and pruritus. She had tried using diphenhydramine without relief. The only new medication the patient had started prior to the eruption was oxycodone, which was initiated immediately following surgery. Prior to surgery, the entire left upper extremity including the shoulder had been prepared with a preoperative surgical skin antiseptic. There were no visible signs of the antiseptic on the skin at the time of presentation. The patient reported that she had applied a cold therapy unit to the left upper arm over her clothing for 1 hour every night since surgery. The cold therapy unit frequently is used to help decrease postoperative pain, swelling, inflammation, and narcotic use following surgical procedures.
Physical examination revealed multiple well-defined, erythematous, tender, indurated, warm nodules on the lateral aspect of the left upper arm (Figure 1). No other areas of eruption were noted on the body, and there was no swelling of the left elbow, forearm, wrist, or hand. The left upper extremity demonstrated intact sensation, rapid capillary refill, and a palpable radial pulse. Her weight was 230.1 lb with a body mass index of 35.
|
A 5-mm punch biopsy from a nodule on the left upper arm was performed, and pathology demonstrated vacuolar interface changes with patchy parakeratosis, spongiosis, and dyskeratosis on staining with hematoxylin and eosin. Pandermal and subcutaneous perivascular, periadnexal, and mild interstitial lymphohistiocytic infiltrate with occasional neutrophils and eosinophils were noted (Figure 2). The inflammation extended to the subcutaneous fat involving both septae and lobules with a primarily lobular distribution.
Clinical and pathologic correlation was required to arrive at a definitive diagnosis of cold panniculitis. The epidermal and dermal changes were consistent with a pernio or chilblains type of insult, and the septal and lobular panniculitis was indicative of cold panniculitis. The patient was advised to discontinue use of the cold therapy device as well as any other form of icing of the left shoulder or arm. She continued the oxycodone for pain control. Four weeks postoperatively, only desquamation remained where the nodules had previously appeared, which also eventually resolved.
Comment
Infants and small children are more predisposed to cold panniculitis than adults. In their 2008 review, Quesada-Cortés et al3 found the first report of cold panniculitis by Hochsinger in 1902 in a German pediatric journal, followed by reports from Lemez in 1928 and Haxthausen in 1941, which subsequently described similar cases in infants. Adult cases were not reported until 1963 by Solomon and Beerman4 and then in 1980 by Beacham et al.5
Etiologies for children have included popsicles, ice packs applied to the face to control supraventricular tachycardia or to the lower extremities after vaccinations, and cold weather exposure.6 The chemical composition of fat tissue plays a role in pediatric patients. According to Quesada-Cortés et al,3 subcutaneous fat in newborns is rich in saturated oils such as palmitic and stearic acids that have a higher solidification point. A small decrease in an infant’s temperature may result in crystallization of fat. The subcutaneous fat tends to become more unsaturated with aging with more oleic acid, and the solidification temperature diminishes.7
Cryoglobulins and cold agglutinins have not been demonstrated to be a cause of cold panniculitis in infants.7 Severe cold exposure or predisposition to certain conditions such as cryofibrinogenemia may occur in some adult patients. Gender does not seem to be a factor in children; however, in adults, women tend to be more predisposed to cold panniculitis secondary to obesity and participation in activities such as cycling, motorcycling, or horseback riding in cold conditions.3
On clinical examination, cold panniculitis features erythematous, firm, tender nodules on the cheeks and chin in infants and small children.2 These areas often are exposed to cold weather or wind because they typically are not covered with protective clothing.3 Nodules generally occur 1 to 3 days following exposure to cold and usually resolve spontaneously within 2 weeks.8 Popsicle panniculitis is characterized by a reddish discoloration on both cheeks 1 or 2 days after sucking on popsicles or ice cubes. This reaction can be reproduced in a half day by applying an ice cube to the volar forearm for 2 minutes, which can help diagnose and differentiate this subset of cold panniuculits.3 The red area in cold panniculitis eventually turns purple, becomes less indurated, and fades in approximately 3 months, but occasionally residual hyperpigmentation will last for a few months. Ice packs used as treatment of congenital cardiac arrhythmias in some cardiac surgeries and as surface cooling for management of birth asphyxia can produce a similar physical presentation.3
Equestrian panniculitis is characterized by erythematous, violaceous, tender plaques on the upper lateral thighs of young females who participate in horseback riding in the winter while wearing tight-fitting pants.2,5 These plaques typically occur within several hours and over the next week become painful, violaceous, and indurated or develop red nodules or plaques that can ulcerate or become crusted.3 These lesions usually will spontaneously resolve within 3 weeks, but new areas may occur again during the winter on further exposure with occasional persistent hyperpigmentation. These areas usually disappear at the end of winter with warmer weather or when horseback riding is discontinued. Perniosis also needs to be considered in the differential diagnosis due to the location and appearance of the lesions.3
It is important to obtain the correct specimen for biopsy. According to Peters and Su,1 a deep excisional biopsy that includes multiple fat lobules in addition to dermis and epidermis is critical. On histology, cold panniculitis usually demonstrates a primarily lobular inflammation. There typically is a superficial and deep perivascular lymphocytic infiltrate in the papillary dermis with edema noted in the connective tissue around the eccrine glands that can appear similar to perniosis on histopathology.9 Deposition of mucin, focal panniculitis surrounded by fatty tissue without inflammatory changes within the same field, and fat necrosis with pseudocysts and numerous lipophages also are characteristic features of cold panniculitis.10 Needlelike clefts are not present in cold panniculitis but appear in subcutaneous fat necrosis of the newborn.1
Different treatments have been tried, but no substantial impact on the rate of dissipation of the lesions has been noted. The plaques slowly resolve without scarring over 2 to 3 weeks if the cold source is removed.2 Application of a heating pad to the affected area has been used with limited success. Vasodilators such as nifedipine have been used but have not been found to be effective.3 Antihistamines also have failed to control the lesions.11
Treatment of cold panniculitis is based on the prevention of further insult versus trying to cure the condition. Avoidance of cold and wind exposure as well as direct contact with ice are key methods in preventing cold panniculitis.
Our patient’s presentation of this condition was unique. Although cold panniculitis lesions usually develop 1 to 3 days after cold exposure, our patient did not develop lesions until 10 days following surgery. The cold therapy unit used by our patient was evaluated in our office and also by the manufacturer and was found to be functioning normally with no defects. The late onset of the lesions was attributed to limited application of the cold therapy unit; our patient used it for only 1 hour every night, whereas application for 6 to 8 hours continuously is normally recommended. The lesions may have occurred sooner had the patient been using a solid ice pack versus the continuous cold circulating water of the cold therapy unit. Pathology was consistent with the patient’s history and physical examination indicating a diagnosis of cold panniculitis. The challenge of treatment was to alleviate the pain of the lesions as well as the postoperative shoulder pain without the aid of any form of cold therapy. The patient only needed a tincture of time, as the lesions resolved after 4 weeks. Patient education was provided on future prevention of this condition by avoiding exposure to cold or applying cold packs directly to the skin.
Acknowledgment
The authors thank the staff at the Office of Scientific Writing and Publication at the Marshfield Clinic Research Foundation, Wisconsin, for their editorial assistance in the preparation of this manuscript.
1. Peters MS, Su WP. Panniculitis. Dermatol Clin. 1992;10:37-57.
2. Patterson JW. Panniculitis. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 2nd ed. St. Louis, MO: Mosby Elsevier; 2008:1515-1530.
3. Quesada-Cortés A, Campos-Muñoz L, Díaz-Díaz RM, et al. Cold panniculitis. Dermatol Clin. 2008;26:485-489.
4. Solomon LM, Beerman H. Cold panniculitis. Arch Dermatol. 1963;88:897-900.
5. Beacham BE, Cooper PH, Buchanan CS, et al. Equestrian cold panniculitis in women. Arch Dermatol. 1980;116:1025-1027.
6. Ter Poorten MC, Thiers BH. Panniculitis. Dermatol Clin. 2002;20:421-433.
7. Ter Poorten JC, Hebert AA, Ilkiw R. Cold panniculitis in a neonate. J Am Acad Dermatol. 1995;33(2, pt 2):383-385.
8. Page EH, Shear NH. Temperature-dependent skin disorders. J Am Acad Dermatol. 1988;18(5, pt 1):1003-1019.
9. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
10. Diaz Cascajo C, Borghi S, Weyers W. Panniculitis: definition of terms and diagnostic strategy. Am J Dermatopathol. 2000;22:530-549.
11. Duncan WC, Freeman RG, Heaton CL. Cold panniculitis. Arch Dermatol. 1966;94:722-724.
1. Peters MS, Su WP. Panniculitis. Dermatol Clin. 1992;10:37-57.
2. Patterson JW. Panniculitis. In: Bolognia J, Jorizzo J, Rapini R, eds. Dermatology. 2nd ed. St. Louis, MO: Mosby Elsevier; 2008:1515-1530.
3. Quesada-Cortés A, Campos-Muñoz L, Díaz-Díaz RM, et al. Cold panniculitis. Dermatol Clin. 2008;26:485-489.
4. Solomon LM, Beerman H. Cold panniculitis. Arch Dermatol. 1963;88:897-900.
5. Beacham BE, Cooper PH, Buchanan CS, et al. Equestrian cold panniculitis in women. Arch Dermatol. 1980;116:1025-1027.
6. Ter Poorten MC, Thiers BH. Panniculitis. Dermatol Clin. 2002;20:421-433.
7. Ter Poorten JC, Hebert AA, Ilkiw R. Cold panniculitis in a neonate. J Am Acad Dermatol. 1995;33(2, pt 2):383-385.
8. Page EH, Shear NH. Temperature-dependent skin disorders. J Am Acad Dermatol. 1988;18(5, pt 1):1003-1019.
9. Requena L, Sánchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
10. Diaz Cascajo C, Borghi S, Weyers W. Panniculitis: definition of terms and diagnostic strategy. Am J Dermatopathol. 2000;22:530-549.
11. Duncan WC, Freeman RG, Heaton CL. Cold panniculitis. Arch Dermatol. 1966;94:722-724.
Practice Points
- Cold panniculitis is a form of traumatic panniculitis.
- Cold panniculitis often appears on the cheeks and chin, areas that are exposed to cold weather or wind because they are not covered with protective clothing, in infants and small children.
- Treatment of cold panniculitis is based on the prevention of further insult.
Aspergillus nidulans Causing Primary Cutaneous Aspergillosis in an Immunocompetent Patient
To the Editor:
Cutaneous aspergillosis mostly has been reported in immunosuppressed hosts and usually is caused by Aspergillus flavus or Aspergillus fumigatus. We report the occurrence of primary cutaneous aspergillosis (PCA) caused by a relatively rare species, Aspergillus nidulans, in a middle-aged patient without overt immunosuppression or history of trauma.
A 57-year-old woman was referred to the dermatology outpatient department for evaluation of a lesion on the right hand of 1 month's duration. On examination the lesion measured approximately 4×3 cm with central necrosis (Figure 1). Her medical history was unremarkable and routine laboratory test results were within reference range.

The patient was an agricultural worker with no history of trauma. Her history was unremarkable. A 20% potassium hydroxide mount of the tissue revealed septate, branched, hyaline hyphae. A soft, wooly, greenish brown growth was observed after 3 days of incubation on Sabouraud dextrose agar (Figure 2). No growth was observed on dermatophyte test medium. A lactophenol cotton blue mount revealed columnar conidial heads with brown, short, smooth-walled conidiophores (Figures 3–6). Vesicles were hemispheric and small (8–12 µm in diameter), with metulae and phialides occurring in the upper portion. Conidia were globose (3–4 µm) and rough. Based on these findings the fungus was identified as A nidulans. The patient did not respond to daily oral ketoconazole, and after 1 month of therapy the lesion did not regress. She was eventually treated with oral itraconazole and the lesion completely healed within 15 weeks.
An overwhelming majority of the cases of cutaneous aspergillosis have been reported either in immunocompromised hosts (ie, leukemia, cutaneous T-cell lymphoma, Hodgkin disease, human immunodeficiency virus/AIDS, solid-organ or hematopoietic stem cell transplant recipients) or in patients with contributing risk factors (ie, severe burns, diabetes mellitus, preterm or underweight neonates, elderly patients). Two outbreaks of this condition have been reported in neonatal intensive care units, with the source of contamination being linked to nonsterile disposable gloves, incubators, and humidity chambers.1,2 However, PCA is a relatively rare condition and often is associated with disruption of dermal integrity by trauma or maceration, followed by colonization of the wound by Aspergillus spores that are ubiquitously present in soil and decomposed vegetation.3-5 Our case was remarkable, as the patient was not immunosuppressed and did not have a history of trauma. However, we surmise that fungal inoculation might have inadvertently occurred through some trivial trauma sustained through her professional work.
The 2 species that have most commonly been associated with PCA are A flavus and A fumigatus.6,7 There have been isolated reports of PCA caused by other organisms such as Aspergillus niger,8,9 Aspergillus terreus,10Aspergillus ustus,11 or Aspergillus calidoustus.12 In a report of a neutropenic 56-year-old patient suffering from acute myeloblastic leukemia, PCA developed in association with a double-lumen Hickman catheter after a period of prolonged hospitalization.13 A study by the National Institutes of Health (1976-1997) revealed 6 life-threatening cases of A nidulans infection in patients with chronic granulomatous disease.14
We did not perform antifungal susceptibility testing on the isolate in our patient. However, we observed disease that was refractory to ketoconazole therapy but successfully resolved with oral itraconazole. Antifungal susceptibility was noted in a large number of reported cases of Aspergillus infections that were resistant to conventional treatment, such as voriconazole, itraconazole, and amphotericin B.15 Thus antifungal susceptibility testing is necessary before starting treatment. There also have been reports of recurrence of cutaneous aspergillosis following incomplete and irregular treatment.16 Our case of PCA also failed to respond to ketoconazole therapy, thus stressing the need for thorough mycological characterization, including the determination of an antifungal susceptibility profile, for successful and complete management of this condition.
Acknowledgment
The authors would like to thank Arunaloke Chakraborti, MD, Chandigarh, India, for the help extended for identification of the fungus.
- Stock C, Veyrier M, Raberin H, et al. Severe cutaneous aspergillosis in a premature neonate linked to nonsterile disposable glove contamination [published online ahead of print August 31, 2011]? Am J Infect Control. 2012;40:465-467.
- Etienne KA, Subudhi CP, Chadwick PR, et al. Investigation of a cluster of cutaneous aspergillosis in a neonatal intensive care unit [published online ahead of print August 12, 2011]. J Hosp Infect. 2011;79:344-348.
- Isaac M. Cutaneous aspergillosis. Dermatol Clin. 1996;14:137-140.
- Cahill KM, Mofty AM, Kawaguchi TP. Primary cutaneous aspergillosis. Arch Dermatol. 1967;96:545-547.
- Carlile JR, Millet RE, Cho CT, et al. Primary cutaneous aspergillosis in a leukemic child. Arch Dermatol. 1978;114:78-80.
- John PU, Shadomy HJ. Deep fungal infections. In: Fitzpatrick TB, Eisen AZ, Wolff K, et al, eds. Dermatology in General Medicine. New York, NY: McGraw Hill; 1987:2266-2268.
- Chakrabarti A, Gupta V, Biswas G, et al. Primary cutaneous aspergillosis: our experience in 10 years. J Infect. 1998;37:24-27.
- Robinson A, Fien S, Grassi MA. Nonhealing scalp wound infected with Aspergillus niger in an elderly patient. Cutis. 2011;87:197-200.
- Thomas LM, Rand HK, Miller JL, et al. Primary cutaneous aspergillosis in a patient with a solid organ transplant: case report and review of the literature. Cutis. 2008;81:127-130.
- Yuanjie Z, Jingxia D, Hai W, et al. Primary cutaneous aspergillosis in a patient with cutaneous T-cell lymphoma [published online ahead of print October 22, 2008]. Mycoses. 2009;52:462-464.
- Krishnan-Natesan S, Chandrasekar PH, Manavathu EK, et al. Successful treatment of primary cutaneous Aspergillus ustus infection with surgical debridement and a combination of voriconazole and terbinafine [published online ahead of print October 7, 2008]. Diagn Microbiol Infect Dis. 2008;62:443-446.
- Sato Y, Suzino K, Suzuki A, et al. Case of primary cutaneous Aspergillus calidoustus infection caused by nerve block therapy [in Japanese]. Med Mycol J. 2011;52:239-244.
- Lucas GM, Tucker P, Merz WG. Primary cutaneous Aspergillus nidulans infection associated with a Hickman catheter in a patient with neutropenia. Clin Infect Dis. 1999;29:1594-1596.
- Segal BH, DeCarlo ES, Kwon-Chung KJ, et al. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore). 1998;77:345-354.
- Woodruff CA, Hebert AA. Neonatal primary cutaneous aspergillosis: case report and review of the literature. Pediatr Dermatol. 2002;19:439-444.
- Mohapatra S, Xess I, Swetha JV, et al. Primary cutaneous aspergillosis due to Aspergillus niger in an immunocompetent patient. Indian J Med Microbiol. 2009;27:367-370.
To the Editor:
Cutaneous aspergillosis mostly has been reported in immunosuppressed hosts and usually is caused by Aspergillus flavus or Aspergillus fumigatus. We report the occurrence of primary cutaneous aspergillosis (PCA) caused by a relatively rare species, Aspergillus nidulans, in a middle-aged patient without overt immunosuppression or history of trauma.
A 57-year-old woman was referred to the dermatology outpatient department for evaluation of a lesion on the right hand of 1 month's duration. On examination the lesion measured approximately 4×3 cm with central necrosis (Figure 1). Her medical history was unremarkable and routine laboratory test results were within reference range.


The patient was an agricultural worker with no history of trauma. Her history was unremarkable. A 20% potassium hydroxide mount of the tissue revealed septate, branched, hyaline hyphae. A soft, wooly, greenish brown growth was observed after 3 days of incubation on Sabouraud dextrose agar (Figure 2). No growth was observed on dermatophyte test medium. A lactophenol cotton blue mount revealed columnar conidial heads with brown, short, smooth-walled conidiophores (Figures 3–6). Vesicles were hemispheric and small (8–12 µm in diameter), with metulae and phialides occurring in the upper portion. Conidia were globose (3–4 µm) and rough. Based on these findings the fungus was identified as A nidulans. The patient did not respond to daily oral ketoconazole, and after 1 month of therapy the lesion did not regress. She was eventually treated with oral itraconazole and the lesion completely healed within 15 weeks.




An overwhelming majority of the cases of cutaneous aspergillosis have been reported either in immunocompromised hosts (ie, leukemia, cutaneous T-cell lymphoma, Hodgkin disease, human immunodeficiency virus/AIDS, solid-organ or hematopoietic stem cell transplant recipients) or in patients with contributing risk factors (ie, severe burns, diabetes mellitus, preterm or underweight neonates, elderly patients). Two outbreaks of this condition have been reported in neonatal intensive care units, with the source of contamination being linked to nonsterile disposable gloves, incubators, and humidity chambers.1,2 However, PCA is a relatively rare condition and often is associated with disruption of dermal integrity by trauma or maceration, followed by colonization of the wound by Aspergillus spores that are ubiquitously present in soil and decomposed vegetation.3-5 Our case was remarkable, as the patient was not immunosuppressed and did not have a history of trauma. However, we surmise that fungal inoculation might have inadvertently occurred through some trivial trauma sustained through her professional work.
The 2 species that have most commonly been associated with PCA are A flavus and A fumigatus.6,7 There have been isolated reports of PCA caused by other organisms such as Aspergillus niger,8,9 Aspergillus terreus,10Aspergillus ustus,11 or Aspergillus calidoustus.12 In a report of a neutropenic 56-year-old patient suffering from acute myeloblastic leukemia, PCA developed in association with a double-lumen Hickman catheter after a period of prolonged hospitalization.13 A study by the National Institutes of Health (1976-1997) revealed 6 life-threatening cases of A nidulans infection in patients with chronic granulomatous disease.14
We did not perform antifungal susceptibility testing on the isolate in our patient. However, we observed disease that was refractory to ketoconazole therapy but successfully resolved with oral itraconazole. Antifungal susceptibility was noted in a large number of reported cases of Aspergillus infections that were resistant to conventional treatment, such as voriconazole, itraconazole, and amphotericin B.15 Thus antifungal susceptibility testing is necessary before starting treatment. There also have been reports of recurrence of cutaneous aspergillosis following incomplete and irregular treatment.16 Our case of PCA also failed to respond to ketoconazole therapy, thus stressing the need for thorough mycological characterization, including the determination of an antifungal susceptibility profile, for successful and complete management of this condition.
Acknowledgment
The authors would like to thank Arunaloke Chakraborti, MD, Chandigarh, India, for the help extended for identification of the fungus.
To the Editor:
Cutaneous aspergillosis mostly has been reported in immunosuppressed hosts and usually is caused by Aspergillus flavus or Aspergillus fumigatus. We report the occurrence of primary cutaneous aspergillosis (PCA) caused by a relatively rare species, Aspergillus nidulans, in a middle-aged patient without overt immunosuppression or history of trauma.
A 57-year-old woman was referred to the dermatology outpatient department for evaluation of a lesion on the right hand of 1 month's duration. On examination the lesion measured approximately 4×3 cm with central necrosis (Figure 1). Her medical history was unremarkable and routine laboratory test results were within reference range.


The patient was an agricultural worker with no history of trauma. Her history was unremarkable. A 20% potassium hydroxide mount of the tissue revealed septate, branched, hyaline hyphae. A soft, wooly, greenish brown growth was observed after 3 days of incubation on Sabouraud dextrose agar (Figure 2). No growth was observed on dermatophyte test medium. A lactophenol cotton blue mount revealed columnar conidial heads with brown, short, smooth-walled conidiophores (Figures 3–6). Vesicles were hemispheric and small (8–12 µm in diameter), with metulae and phialides occurring in the upper portion. Conidia were globose (3–4 µm) and rough. Based on these findings the fungus was identified as A nidulans. The patient did not respond to daily oral ketoconazole, and after 1 month of therapy the lesion did not regress. She was eventually treated with oral itraconazole and the lesion completely healed within 15 weeks.




An overwhelming majority of the cases of cutaneous aspergillosis have been reported either in immunocompromised hosts (ie, leukemia, cutaneous T-cell lymphoma, Hodgkin disease, human immunodeficiency virus/AIDS, solid-organ or hematopoietic stem cell transplant recipients) or in patients with contributing risk factors (ie, severe burns, diabetes mellitus, preterm or underweight neonates, elderly patients). Two outbreaks of this condition have been reported in neonatal intensive care units, with the source of contamination being linked to nonsterile disposable gloves, incubators, and humidity chambers.1,2 However, PCA is a relatively rare condition and often is associated with disruption of dermal integrity by trauma or maceration, followed by colonization of the wound by Aspergillus spores that are ubiquitously present in soil and decomposed vegetation.3-5 Our case was remarkable, as the patient was not immunosuppressed and did not have a history of trauma. However, we surmise that fungal inoculation might have inadvertently occurred through some trivial trauma sustained through her professional work.
The 2 species that have most commonly been associated with PCA are A flavus and A fumigatus.6,7 There have been isolated reports of PCA caused by other organisms such as Aspergillus niger,8,9 Aspergillus terreus,10Aspergillus ustus,11 or Aspergillus calidoustus.12 In a report of a neutropenic 56-year-old patient suffering from acute myeloblastic leukemia, PCA developed in association with a double-lumen Hickman catheter after a period of prolonged hospitalization.13 A study by the National Institutes of Health (1976-1997) revealed 6 life-threatening cases of A nidulans infection in patients with chronic granulomatous disease.14
We did not perform antifungal susceptibility testing on the isolate in our patient. However, we observed disease that was refractory to ketoconazole therapy but successfully resolved with oral itraconazole. Antifungal susceptibility was noted in a large number of reported cases of Aspergillus infections that were resistant to conventional treatment, such as voriconazole, itraconazole, and amphotericin B.15 Thus antifungal susceptibility testing is necessary before starting treatment. There also have been reports of recurrence of cutaneous aspergillosis following incomplete and irregular treatment.16 Our case of PCA also failed to respond to ketoconazole therapy, thus stressing the need for thorough mycological characterization, including the determination of an antifungal susceptibility profile, for successful and complete management of this condition.
Acknowledgment
The authors would like to thank Arunaloke Chakraborti, MD, Chandigarh, India, for the help extended for identification of the fungus.
- Stock C, Veyrier M, Raberin H, et al. Severe cutaneous aspergillosis in a premature neonate linked to nonsterile disposable glove contamination [published online ahead of print August 31, 2011]? Am J Infect Control. 2012;40:465-467.
- Etienne KA, Subudhi CP, Chadwick PR, et al. Investigation of a cluster of cutaneous aspergillosis in a neonatal intensive care unit [published online ahead of print August 12, 2011]. J Hosp Infect. 2011;79:344-348.
- Isaac M. Cutaneous aspergillosis. Dermatol Clin. 1996;14:137-140.
- Cahill KM, Mofty AM, Kawaguchi TP. Primary cutaneous aspergillosis. Arch Dermatol. 1967;96:545-547.
- Carlile JR, Millet RE, Cho CT, et al. Primary cutaneous aspergillosis in a leukemic child. Arch Dermatol. 1978;114:78-80.
- John PU, Shadomy HJ. Deep fungal infections. In: Fitzpatrick TB, Eisen AZ, Wolff K, et al, eds. Dermatology in General Medicine. New York, NY: McGraw Hill; 1987:2266-2268.
- Chakrabarti A, Gupta V, Biswas G, et al. Primary cutaneous aspergillosis: our experience in 10 years. J Infect. 1998;37:24-27.
- Robinson A, Fien S, Grassi MA. Nonhealing scalp wound infected with Aspergillus niger in an elderly patient. Cutis. 2011;87:197-200.
- Thomas LM, Rand HK, Miller JL, et al. Primary cutaneous aspergillosis in a patient with a solid organ transplant: case report and review of the literature. Cutis. 2008;81:127-130.
- Yuanjie Z, Jingxia D, Hai W, et al. Primary cutaneous aspergillosis in a patient with cutaneous T-cell lymphoma [published online ahead of print October 22, 2008]. Mycoses. 2009;52:462-464.
- Krishnan-Natesan S, Chandrasekar PH, Manavathu EK, et al. Successful treatment of primary cutaneous Aspergillus ustus infection with surgical debridement and a combination of voriconazole and terbinafine [published online ahead of print October 7, 2008]. Diagn Microbiol Infect Dis. 2008;62:443-446.
- Sato Y, Suzino K, Suzuki A, et al. Case of primary cutaneous Aspergillus calidoustus infection caused by nerve block therapy [in Japanese]. Med Mycol J. 2011;52:239-244.
- Lucas GM, Tucker P, Merz WG. Primary cutaneous Aspergillus nidulans infection associated with a Hickman catheter in a patient with neutropenia. Clin Infect Dis. 1999;29:1594-1596.
- Segal BH, DeCarlo ES, Kwon-Chung KJ, et al. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore). 1998;77:345-354.
- Woodruff CA, Hebert AA. Neonatal primary cutaneous aspergillosis: case report and review of the literature. Pediatr Dermatol. 2002;19:439-444.
- Mohapatra S, Xess I, Swetha JV, et al. Primary cutaneous aspergillosis due to Aspergillus niger in an immunocompetent patient. Indian J Med Microbiol. 2009;27:367-370.
- Stock C, Veyrier M, Raberin H, et al. Severe cutaneous aspergillosis in a premature neonate linked to nonsterile disposable glove contamination [published online ahead of print August 31, 2011]? Am J Infect Control. 2012;40:465-467.
- Etienne KA, Subudhi CP, Chadwick PR, et al. Investigation of a cluster of cutaneous aspergillosis in a neonatal intensive care unit [published online ahead of print August 12, 2011]. J Hosp Infect. 2011;79:344-348.
- Isaac M. Cutaneous aspergillosis. Dermatol Clin. 1996;14:137-140.
- Cahill KM, Mofty AM, Kawaguchi TP. Primary cutaneous aspergillosis. Arch Dermatol. 1967;96:545-547.
- Carlile JR, Millet RE, Cho CT, et al. Primary cutaneous aspergillosis in a leukemic child. Arch Dermatol. 1978;114:78-80.
- John PU, Shadomy HJ. Deep fungal infections. In: Fitzpatrick TB, Eisen AZ, Wolff K, et al, eds. Dermatology in General Medicine. New York, NY: McGraw Hill; 1987:2266-2268.
- Chakrabarti A, Gupta V, Biswas G, et al. Primary cutaneous aspergillosis: our experience in 10 years. J Infect. 1998;37:24-27.
- Robinson A, Fien S, Grassi MA. Nonhealing scalp wound infected with Aspergillus niger in an elderly patient. Cutis. 2011;87:197-200.
- Thomas LM, Rand HK, Miller JL, et al. Primary cutaneous aspergillosis in a patient with a solid organ transplant: case report and review of the literature. Cutis. 2008;81:127-130.
- Yuanjie Z, Jingxia D, Hai W, et al. Primary cutaneous aspergillosis in a patient with cutaneous T-cell lymphoma [published online ahead of print October 22, 2008]. Mycoses. 2009;52:462-464.
- Krishnan-Natesan S, Chandrasekar PH, Manavathu EK, et al. Successful treatment of primary cutaneous Aspergillus ustus infection with surgical debridement and a combination of voriconazole and terbinafine [published online ahead of print October 7, 2008]. Diagn Microbiol Infect Dis. 2008;62:443-446.
- Sato Y, Suzino K, Suzuki A, et al. Case of primary cutaneous Aspergillus calidoustus infection caused by nerve block therapy [in Japanese]. Med Mycol J. 2011;52:239-244.
- Lucas GM, Tucker P, Merz WG. Primary cutaneous Aspergillus nidulans infection associated with a Hickman catheter in a patient with neutropenia. Clin Infect Dis. 1999;29:1594-1596.
- Segal BH, DeCarlo ES, Kwon-Chung KJ, et al. Aspergillus nidulans infection in chronic granulomatous disease. Medicine (Baltimore). 1998;77:345-354.
- Woodruff CA, Hebert AA. Neonatal primary cutaneous aspergillosis: case report and review of the literature. Pediatr Dermatol. 2002;19:439-444.
- Mohapatra S, Xess I, Swetha JV, et al. Primary cutaneous aspergillosis due to Aspergillus niger in an immunocompetent patient. Indian J Med Microbiol. 2009;27:367-370.
Most Common Dermatologic Conditions Encountered by Dermatologists and Nondermatologists
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).
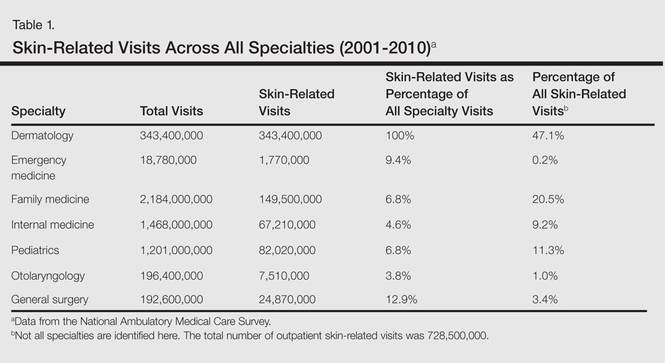
Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).
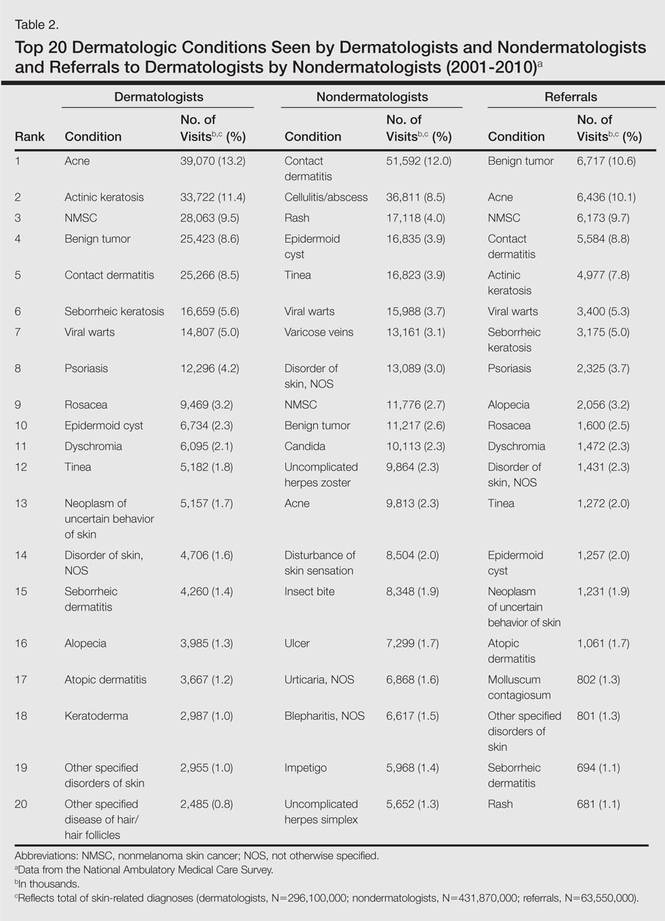
The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.
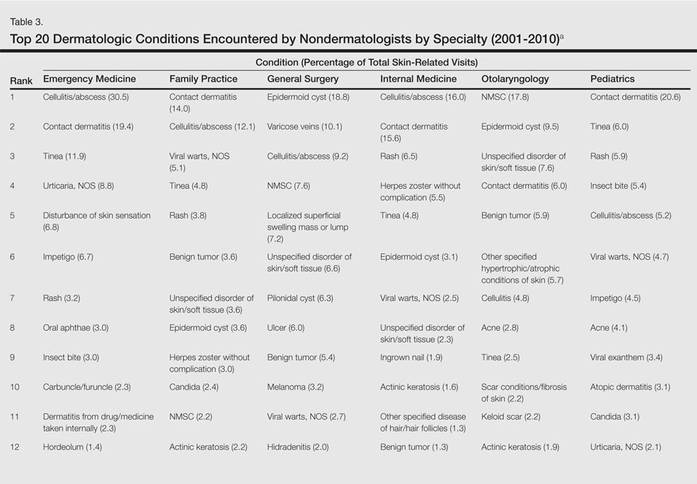
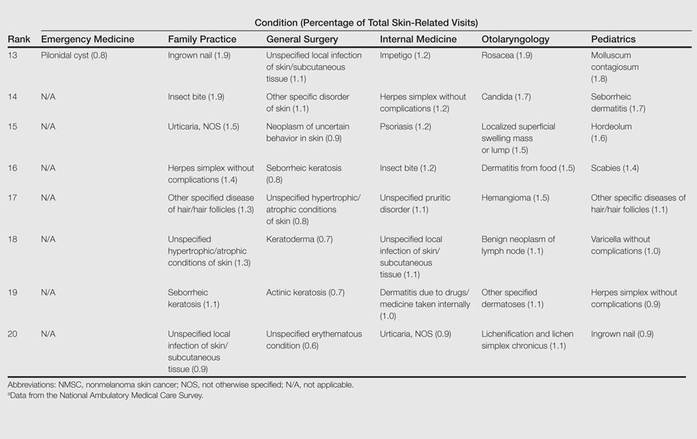
Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).

Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).

The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.


Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
Skin diseases are highly prevalent in the United States, affecting an estimated 1 in 3 Americans at any given time.1,2 In 2009 the direct medical costs associated with skin-related diseases, including health services and prescriptions, was approximately $22 billion; the annual total economic burden was estimated to be closer to $96 billion when factoring in the cost of lost productivity and pay for symptom relief.3,4 Effective and efficient management of skin disease is essential to minimizing cost and morbidity. Nondermatologists traditionally have diagnosed the majority of skin diseases.5,6 In particular, primary care physicians commonly manage dermatologic conditions and often are the first health care providers to encounter patients presenting with skin problems. A predicted shortage of dermatologists will likely contribute to an increase in this trend.7,8 Therefore, it is important to adequately prepare nondermatologists to evaluate and treat the skin conditions that they are most likely to encounter in their scope of practice.
Residents, particularly in primary care specialties, often have opportunities to spend 2 to 4 weeks with a dermatologist to learn about skin diseases; however, the skin conditions most often encountered by dermatologists may differ from those most often encountered by physicians in other specialties. For instance, one study demonstrated a disparity between the most common skin problems seen by dermatologists and internists.9 These dissimilarities should be recognized and addressed in curriculum content. The purpose of this study was to identify and compare the 20 most common dermatologic conditions reported by dermatologists versus those reported by nondermatologists (ie, internists, pediatricians, family physicians, emergency medicine physicians, general surgeons, otolaryngologists) from 2001 to 2010. Data also were analyzed to determine the top 20 conditions referred to dermatologists by nondermatologists as a potential indicator for areas of further improvement within medical education. With this knowledge, we hope educational curricula and self-study can be modified to reflect the current epidemiology of cutaneous diseases, thereby improving patient care.
Methods
Data from 2001 to 2010 were extracted from the National Ambulatory Medical Care Survey (NAMCS), which is an ongoing survey conducted by the National Center for Health Statistics. The NAMCS collects descriptive data regarding ambulatory visits to nonfederal office-based physicians in the United States. Participating physicians are instructed to record information about patient visits for a 1-week period, including patient demographics, insurance status, reason for visit, diagnoses, procedures, therapeutics, and referrals made at that time. Data collected for the NAMCS are entered into a multistage probability sample to produce national estimates. Within dermatology, an average of 118 dermatologists are sampled each year, and over the last 10 years, participation rates have ranged from 47% to 77%.
International Classification of Diseases, Ninth Revision, Clinical Modification codes were identified to determine the diagnoses that could be classified as dermatologic conditions. Select infectious and neoplastic disorders of the skin and mucous membrane conditions were included as well as the codes for skin diseases. Nondermatologic diagnoses and V codes were not included in the study. Data for all providers were studied to identify outpatient visits associated with the primary diagnosis of a dermatologic condition. Minor diagnoses that were considered to be subsets of major diagnoses were combined to allow better analysis of the data. For example, all tinea infections (ie, dermatophytosis of various sites, dermatomycosis unspecified) were combined into 1 diagnosis referred to as tinea because the recognition and treatment of this disease does not vary tremendously by anatomic location. Visits to dermatologists that listed nonspecific diagnoses and codes (eg, other postsurgical status [V45.89], neoplasm of uncertain behavior site unspecified [238.9]) were assumed to be for dermatologic problems.
Sampling weights were applied to obtain estimates for the number of each diagnosis made nationally. All data analyses were performed using SAS software and linear regression models were generated using SAS PROC SURVEYREG.
Data were analyzed to determine the dermatologic conditions most commonly encountered by dermatologists and nondermatologists in emergency medicine, family medicine, general surgery, internal medicine, otolaryngology, and pediatrics; these specialties include physicians who are known to commonly diagnose and treat skin diseases.10 Data also were analyzed to determine the most common conditions referred to dermatologists for treatment by nondermatologists from the selected specialties. Permission to conduct this study was obtained from the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Results
From 2001 to 2010, more than 700 million outpatient visits for skin-related problems were identified, with 676.3 million visits to dermatologists, emergency medicine physicians, family practitioners, general surgeons, internists, otolaryngologists, and pediatricians. More than half (52.9%) of all skin-related visits were addressed by nondermatologists during this time. Among nondermatologists, family practitioners encountered the greatest number of skin diseases (20.5%), followed by pediatricians (11.3%), internists (9.2%), general surgeons (3.4%), otolaryngologists (1.0%), and emergency medicine physicians (0.2%)(Table 1).

Benign tumors and acne were the most common cutaneous conditions referred to dermatologists by nondermatologists (10.6% and 10.1% of all dermatology referrals, respectively), followed by nonmelanoma skin cancers (9.7%), contact dermatitis (8.8%), and actinic keratosis (7.8%)(Table 2). The top 20 conditions referred to dermatologists accounted for 83.7% of all outpatient referrals to dermatologists.
Among the diseases most frequently reported by nondermatologists, contact dermatitis was the most common (12.0%), with twice the number of visits to nondermatologists for contact dermatitis than to dermatologists (51.6 million vs 25.3 million). In terms of disease categories, infectious skin diseases (ie, bacterial [cellulitis/abscess], viral [warts, herpesvirus], fungal [tinea] and yeast [candida] etiologies) were the most common dermatologic conditions reported by nondermatologists (Table 2).

The top 20 dermatologic conditions reported by dermatologists accounted for 85.4% of all diagnoses made by dermatologists. Diseases that were among the top 20 conditions encountered by dermatologists but were not among the top 20 for nondermatologists included actinic keratosis, seborrheic keratosis, atopic dermatitis, psoriasis, alopecia, rosacea, dyschromia, seborrheic dermatitis, follicular disease, and neoplasm of uncertain behavior of skin. Additionally, 5 of the top 20 conditions encountered by dermatologists also were among the top 20 for only 1 individual nondermatologic specialty; these included atopic dermatitis (pediatrics), seborrheic dermatitis (pediatrics), psoriasis (internal medicine), rosacea (otolaryngology), and keratoderma (general surgery). Seborrheic dermatitis, psoriasis, and rosacea also were among the top 20 conditions most commonly referred to dermatologists for treatment by nondermatologists. Table 3 shows the top 20 dermatologic conditions encountered by nondermatologists by comparison.


Comment
According to NAMCS data from 2001 to 2010, visits to nondermatologists accounted for more than half of total outpatient visits for cutaneous diseases in the United States, whereas visits to dermatologists accounted for 47.1%. These findings are consistent with historical data indicating that 30% to 40% of skin-related visits are to dermatologists, and the majority of patients with skin disease are diagnosed by nondermatologists.5,6
Past data indicate that most visits to dermatologists were for evaluation of acne, infections, psoriasis, and neoplasms, whereas most visits to nondermatologists were for evaluation of epidermoid cysts, impetigo, plant dermatitis, cellulitis, and diaper rash.9 Over the last 10 years, acne has been more commonly encountered by nondermatologists, especially pediatricians. Additionally, infectious etiologies have been seen in larger volume by nondermatologists.9 Together, infectious cutaneous conditions make up nearly one-fourth of dermatologic encounters by emergency medicine physicians, internists, and family practitioners but are not within the top 20 diagnoses referred to dermatologists, which suggests that uncomplicated cases of cellulitis, herpes zoster, and other skin-related infections are largely managed by nondermatologists.5,6 Contact dermatitis, often caused by specific allergens such as detergents, solvents, and topical products, was one of the most common reported dermatologic encounters among dermatologists and nondermatologists and also was the fourth most common condition referred to dermatologists by nondermatologists for treatment; however, there may be an element of overuse of the International Classification of Diseases, Ninth Revision code, as any presumed contact dermatitis of unspecified cause can be reported under 692.9 defined as contact dermatitis and other eczema, unspecified cause. The high rate of referrals to dermatologists by nondermatologists may be for patch testing and further management. Additionally, there are no specific codes for allergic or irritant dermatitis, thus these diseases may be lumped together.
Although nearly half of all dermatologic encounters were seen by nondermatologists, dermatologists see a much larger proportion of patients with skin disease than nondermatologists and nondermatologists often have limited exposure to the field of dermatology during residency training. Studies have demonstrated differences in the abilities of dermatologists and nondermatologists to correctly diagnose common cutaneous diseases, which unsurprisingly revealed greater diagnostic accuracy demonstrated by dermatologists.11-16 The increase in acne and skin-related infections reported by nondermatologists is consistent with possible efforts to increase formal training in frequently encountered skin diseases. In one study evaluating the impact of a formal 3-week dermatology curriculum on an internal medicine department, internists demonstrated 100% accuracy in the diagnosis of acne and herpes zoster in contrast to 29% for tinea and 12% for lichen planus.5,6
The current Accreditation Council for Graduate Medical Education guidelines place little emphasis on exposure to dermatology training during residency for internists and pediatricians, as this training is not a required component of these programs.17 Two core problems with current training regarding the evaluation and management of cutaneous disease are minimal exposure to dermatologic conditions in medical school and residency and lack of consensus on the core topics that should be taught to nondermatologists.18 Exposure to dermatologic conditions through rotations in medical school has been shown to increase residents’ self-reported confidence in diagnosing and treating alopecia, cutaneous drug eruptions, warts, acne, rosacea, nonmelanoma skin cancers, sun damage, psoriasis, seborrhea, atopic dermatitis, and contact dermatitis; however, the majority of primary care residents surveyed still felt that this exposure in medical school was inadequate.19
In creating a core curriculum for dermatology training for nondermatologists, it is important to consider the dermatologic conditions that are most frequently encountered by these specialties. Our study revealed that the most commonly encountered dermatologic conditions differ among dermatologists and nondermatologists, with a fair degree of variation even among individual specialties. Failure to recognize these discrepancies has likely contributed to the challenges faced by nondermatologists in the diagnosis and management of dermatologic disease. In this study, contact dermatitis, epidermoid cysts, and skin infections were the most common dermatologic conditions encountered by nondermatologists and also were among the top skin diseases referred to dermatologists by nondermatologists. This finding suggests that nondermatologists are able to identify these conditions but have a tendency to refer approximately 10% of these patients to dermatology for further management. Clinical evaluation and medical management of these cutaneous diseases may be an important area of focus for medical school curricula, as the treatment of these diseases is within the capabilities of the nondermatologist. For example, initial management of dermatitis requires determination of the type of dermatitis (ie, essential, contact, atopic, seborrheic, stasis) and selection of an appropriate topical steroid, with referral to a dermatologist needed for questionable or refractory cases. Although a curriculum cannot be built solely on a list of the top 20 diagnoses provided here, these data may serve as a preliminary platform for medical school dermatology curriculum design. The curriculum also should include serious skin diseases, such as melanoma and severe drug eruptions. Although these conditions are less commonly encountered by nondermatologists, missed diagnosis and/or improper management can be life threatening.
The use of NAMCS data presents a few limitations. For instance, these data only represent outpatient management of skin disease. There is the potential for misdiagnosis and coding errors by the reporting physicians. The volume of data (ie, billions of office visits) prevents verification of diagnostic accuracy. The coding system requires physicians to give a diagnosis but does not provide any means by which to determine the physician’s confidence in that diagnosis. There is no code for “uncertain” or “diagnosis not determined.” Additionally, an “unspecified” diagnosis may reflect uncertainty or may simply imply that no other code accurately described the condition. Despite these limitations, the NAMCS database is a large, nationally representative survey of actual patient visits and represents some of the best data available for a study such as ours.
Conclusion
This study provides an important analysis of the most common outpatient dermatologic conditions encountered by dermatologists and nondermatologists of various specialties and offers a foundation from which to construct curricula for dermatology training tailored to individual specialties based on their needs. In the future, identification of the most common inpatient dermatologic conditions managed by each specialty also may benefit curriculum design.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
- Thorpe KE, Florence CS, Joski P. Which medical conditions account for the rise in health care spending? Health Aff (Millwood). 2004;(suppl web exclusives):W4-437-445.
- Johnson ML. Defining the burden of skin disease in the United States—a historical perspective. J Investig Dermatol Symp Proc. 2004;9:108-110.
- Agency for Healthcare Research and Quality. Medical expenditure panel survey. US Department of Health & Human Services Web site. http://meps.ahrq.gov. Accessed November 17, 2014.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Johnson ML. On teaching dermatology to nondermatologists. Arch Dermatol. 1994;130:850-852.
- Ramsay DL, Weary PE. Primary care in dermatology: whose role should it be? J Am Acad Dermatol. 1996;35:1005-1008.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Resneck JS Jr, Kimball AB. Who else is providing care in dermatology practices? trends in the use of nonphysician clinicians. J Am Acad Dermatol. 2008;58:211-216.
- Feldman SR, Fleischer AB Jr, McConnell RC. Most common dermatologic problems identified by internists, 1990-1994. Arch Intern Med. 1998;158:726-730.
- Ahn CS, Davis SA, Debade TS, et al. Noncosmetic skin-related procedures performed in the United States: an analysis of national ambulatory medical care survey data from 1995 to 2010. Dermatol Surg. 2013;39:1912-1921.
- Antic M, Conen D, Itin PH. Teaching effects of dermatological consultations on nondermatologists in the field of internal medicine. a study of 1290 inpatients. Dermatology. 2004;208:32-37.
- Federman DG, Concato J, Kirsner RS. Comparison of dermatologic diagnoses by primary care practitioners and dermatologists. a review of the literature. Arch Fam Med. 1999;8:170-172.
- Fleischer AB Jr, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by nondermatologists. Am J Manag Care. 2000;6:1149-1156.
- Kirsner RS, Federman DG. Lack of correlation between internists’ ability in dermatology and their patterns of treating patients with skin disease. Arch Dermatol. 1996;132:1043-1046.
- McCarthy GM, Lamb GC, Russell TJ, et al. Primary care-based dermatology practice: internists need more training. J Gen Intern Med. 1991;6:52-56.
- Sellheyer K, Bergfeld WF. A retrospective biopsy study of the clinical diagnostic accuracy of common skin diseases by different specialties compared with dermatology. J Am Acad Dermatol. 2005;52:823-830.
- Medical specialties. Accreditation Council for Graduate Medical Education Web site. http://www.acgme.org/acgmeweb/tabid/368ProgramandInstitutionalGuidelines/MedicalAccreditation.aspx. Accessed November 17, 2014.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
- Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
Practice Points
- Approximately half of skin-related visits are to nondermatologists, such as family medicine physicians, pediatricians, and internists.
- Skin conditions that most frequently present to nondermatologists are different from those seen by dermatologists.
- Education efforts in nondermatology specialties should be targeted toward the common skin diseases that present to these specialties to maximize the yield of medical education and improve diagnostic accuracy and patient outcomes.
Furuncular Myiasis in 2 American Travelers Returning From Senegal
Case Reports
Patient 1
A 16-year-old adolescent boy presented to the emergency department with painful, pruritic, erythematous nodules on the bilateral legs of 1 week’s duration. The lesions had developed 1 week after returning from a monthlong trip to Senegal with a volunteer youth group. He did not recall sustaining any painful insect bites or illnesses while traveling in Africa and only noticed the erythematous papules on the legs when he returned home to the United States. After consulting with his primary care physician and a local dermatologist, the patient began taking oral cephalexin for suspected bacterial furunculosis with no considerable improvement. Over the course of 1 week, the lesions became increasingly painful and pruritic, prompting a visit to the emergency department. Prior to his arrival, the patient reported squeezing a live worm from one of the lesions on the right ankle.
On presentation, the patient was afebrile (temperature, 36.7°C) and his vital signs revealed no abnormalities. Physical examination revealed tender erythematous nodules on the bilateral heels, ankles, and shins with pinpoint puncta noted at the center of many of the lesions (Figure 1). The nodules were warm and indurated and no pulsatile movement was appreciated. The legs appeared to be well perfused with intact sensation and motor function. The patient brought in the live mobile larva that he extruded from the lesion on the right ankle. Both the departments of infectious diseases and dermatology were consulted and a preliminary diagnosis of furuncular myiasis was made.
The lesions were occluded with petroleum jelly and the patient was instructed to follow-up with the dermatology department later that same day. On follow-up in the dermatology clinic, the tips of intact larvae were appreciated at the central puncta of some of the lesions (Figure 2). Lidocaine adrenaline tetracaine gel was applied to lesions on the legs for 40 minutes, then lidocaine gel 1% was injected into each lesion. On injection, immobile larvae were ejected from the central puncta of most of the lesions; the remaining lesions were treated via 3-mm punch biopsy as a means of extraction. Each nodule contained only a single larva, all of which were dead at the time of removal (Figure 3). The wounds were left open and the patient was instructed to continue treatment with cephalexin with leg elevation and rest. Pathologic examination of deep dermal skin sections revealed larval fragments encased by a thick chitinous cuticle with spines that were consistent with furuncular myiasis (Figures 4 and 5). Given the patient’s recent history of travel to Africa along with the morphology of the extracted specimens, the larvae were identified as Cordylobia anthropophaga, a common cause of furuncular myiasis in that region.
Patient 2
The next week, a 17-year-old adolescent girl who had been on the same trip to Senegal as patient 1 presented with 2 similar erythematous nodules with central crusts on the left inner thigh and buttock. On noticing the lesions approximately 3 days prior to presentation, the patient applied topical antibiotic ointment to each nodule, which incited the evacuation of white tube-shaped structures that were presented for examination. On presentation, the nodules were healing well. Given the patient’s travel history and physical examination, a presumptive diagnosis of furuncular myiasis from C anthropophaga also was made.
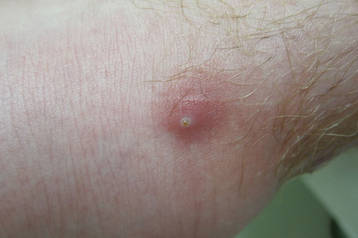

|
Comment
The term myiasis stems from the Greek term for fly and is used to describe the infestation of fly larvae in living vertebrates.1 Myiasis has many classifications, the 3 most common being furuncular, migratory, and wound myiasis, which are differentiated by the different fly species found in distinct regions of the world. Furuncular myiasis is the most benign form, usually affecting only a localized region of the skin; migratory myiasis is characterized by larvae traveling substantial distances from one anatomic site to another within the lower layers of the epidermis; and wound myiasis involves rapid reproduction of larvae in necrotic tissue with subsequent tissue destruction.2
The clinical presentation of the lesions noted in our patients suggested a diagnosis of furuncular myiasis, which commonly is caused by Dermatobia hominis, C anthropophaga, Cuterebra species, Wohlfahrtia vigil, and Wohlfahrtia opaca larvae.3Dermatobia hominis is the most common cause of furuncular myiasis and usually is found in Central and South America. Our patients likely developed an infestation of C anthropophaga (also known as the tumbu fly), a yellow-brown, 7- to 12-mm blowfly commonly found throughout tropical Africa.3 Although C anthropophaga is historically limited to sub-Saharan Africa, there has been a report of a case acquired in Portugal.4
In a review of the literature, C anthropophaga myiasis was documented in Italian travelers returning from Senegal5-7; our cases are unique because they represent North American travelers returning from Senegal with furuncular myiasis. Furuncular myiasis from C anthropophaga has been reported in travelers returning to North America from other African countries, including Angola,8 Tanzania,9-11 Kenya,9 Sierra Leone,12 and Ivory Coast.13 Several cases of ocular myiasis from D hominis and Oestrus ovis have been reported in European travelers returning from Tunisia.14,15
Tumbu fly infestations typically affect dogs and rodents but can arise in human hosts.3 Children may be affected by C anthropophaga furuncular myiasis more often than adults because they have thinner skin and less immunity to the larvae.2
|
There are 2 mechanisms by which infestation of human hosts by C anthropophaga can occur. Most commonly, female flies lay eggs in shady areas in soil that is contaminated by feces or urine. The hatched larvae can survive in the ground for up to 2 weeks and later attach to a host when prompted by heat or movement.3 Therefore, clothing set out to dry may be contaminated by this soil. Alternatively, female flies can lay eggs directly onto clothing that is contaminated by feces or urine and the larvae subsequently hatch outside the soil with easy access to human skin once the clothing is worn.2
Common penetration sites are the head, neck, and back, as well as areas covered by contaminated or infested clothing.2,3 Penetration of the human skin occurs instantly and is a painless process that is rarely noticed by the human host.3 The larvae burrow into the skin for 8 to 12 days, resulting in a furuncle that occasionally secretes a serous fluid.2 Within the first 2 days of infestation, the host may experience symptoms ranging from local pruritus to severe pain. Six days following initial onset, an intense inflammatory response may result in local lymphadenopathy along with fever and fatigue.2 The larvae use their posterior spiracles to create openings in the skin to create air holes that allow them to breathe.3 On physical examination, the spiracles generally appear as 1- to 3-mm dark linear streaks within furuncles, which is important in the diagnosis of C anthropophaga furuncular myiasis.1,3 If spiracles are not appreciated on initial examination, diagnosis can be made by submerging the affected areas in water or saliva to look for air bubbles arising from the central puncta of the lesions.1
All causes of furuncular myiasis are characterized by a ratio of 1 larva to 1 furuncle.16 Although most of these types of larvae that can cause furuncular myiasis result in single lesions, C anthropophaga infestation often produces several furuncles that may coalesce into plaques.1,2 The differential diagnosis for C anthropophaga furuncular myiasis includes pyoderma, impetigo, staphylococcal furunculosis, cutaneous leishmaniasis, infected cyst, retained foreign body, and facticial disease.2,3 Dracunculiasis also may be considered, which occurs after ingestion of contaminated water.2 Ultrasonography may be helpful for the diagnosis of furuncular myiasis, as it can facilitate identification of foreign bodies, abscesses, and even larvae in some cases.17 Definitive diagnosis of any type of myiasis involves extraction of the larva and identification of the family, genus, and species by a parasitologist.1 Some experts suggest rearing preserved live larvae with raw meat after extraction because adult specimens are more reliable than larvae for species diagnosis.1
Treatment of furuncular myiasis involves occlusion and extraction of the larvae from the skin. Suffocation of the larvae by occlusion of air holes with petroleum jelly, paraffin oil, bacon fat, glue, and other obstructing substances forces the larvae to emerge in search of oxygen, though immature larvae may be more reluctant than mature ones.2,3 Definitive treatment involves the direct removal of the larvae by surgery or expulsion by pressure, though it is recommended that lesions are pretreated with occlusive techniques.1,3 Other reported methods of extraction include injection of lidocaine and the use of a commercial venom extractor.1 It should be noted that rupture and incomplete extraction of larvae can lead to secondary infections and allergic reactions. Lesions can be pretreated with lidocaine gel prior to extraction, and antibiotics should be used in cases of secondary bacterial infection. Ivermectin also has been reported as a treatment of furuncular myiasis and other types of myiasis.1 Prevention of infestation by C anthropophaga includes avoidance of endemic areas, maintaining good hygiene, and ironing clothing or drying it in sunny locations.1,2 Overall, furuncular myiasis has a good prognosis with rapid recovery and a low incidence of complications.1
Conclusion
We present 2 cases of travelers returning to North America from Senegal with C anthropophaga furuncular myiasis. Careful review of travel history, physical examination, and identification of fly larvae are important for diagnosis. Individuals traveling to sub-Saharan Africa should avoid drying clothes in shady places and lying on the ground. They also are urged to iron their clothing before wearing it.
1. Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
2. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926.
3. Robbins K, Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49:1092-1098.
4. Curtis SJ, Edwards C, Athulathmuda C, et al. Case of the month: cutaneous myiasis in a returning traveller from the Algarve: first report of tumbu maggots, Cordylobia anthropophaga, acquired in Portugal. Emerg Med J. 2006;23:236-237.
5. Veraldi S, Brusasco A, Süss L. Cutaneous myiasis caused by larvae of Cordylobia anthropophaga (Blanchard). Int J Dermatol. 1993;32:184-187.
6. Cultrera R, Dettori G, Calderaro A, et al. Cutaneous myiasis caused by Cordylobia anthropophaga (Blanchard 1872): description of 5 cases from costal regions of Senegal [in Italian]. Parassitologia. 1993;35:47-49.
7. Fusco FM, Nardiello S, Brancaccio G, et al. Cutaneous myiasis from Cordylobia anthropophaga in a traveller returning from Senegal: a case study [in Italian]. Infez Med. 2005;13:109-111.
8. Lee EJ, Robinson F. Furuncular myiasis of the face caused by larva of the tumbu fly (Cordylobia anthropophaga)[published online ahead of print July 21, 2006]. Eye (Lond). 2007;21:268-269.
9. Rice PL, Gleason N. Two cases of myiasis in the United States by the African tumbu fly, Cordylobia anthropophaga (Diptera, Calliphoridae). Am J Trop Med Hyg. 1972;21:62-65.
10. March CH. A case of “ver du Cayor” in Manhattan. Arch Dermatol. 1964;90:32-33.
11. Schorr WF. Tumbu-fly myiasis in Marshfield, Wis. Arch Dermatol. 1967;95:61-62.
12. Potter TS, Dorman MA, Ghaemi M, et al. Inflammatory papules on the back of a traveling businessman. tumbu
fly myiasis. Arch Dermatol. 1995;131:951, 954.
13. Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
14. Kaouech E, Kallel K, Belhadj S, et al. Dermatobia hominis furuncular myiasis in a man returning from Latin America: first imported case in Tunisia [in French]. Med Trop (Mars). 2010;70:135-136.
15. Zayani A, Chaabouni M, Gouiaa R, et al. Conjuctival myiasis. 23 cases in the Tunisian Sahel [in French]. Arch Inst Pasteur Tunis. 1989;66:289-292.
16. Latorre M, Ullate JV, Sanchez J, et al. A case of myiasis due to Dermatobia hominis. Eur J Clin Microbiol Infect Dis. 1993;12:968-969.
17. Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominis: a case of human botfly infestation [published online ahead of print February 1, 2010]. J Emerg Med. 2012;43:618-621.
Case Reports
Patient 1
A 16-year-old adolescent boy presented to the emergency department with painful, pruritic, erythematous nodules on the bilateral legs of 1 week’s duration. The lesions had developed 1 week after returning from a monthlong trip to Senegal with a volunteer youth group. He did not recall sustaining any painful insect bites or illnesses while traveling in Africa and only noticed the erythematous papules on the legs when he returned home to the United States. After consulting with his primary care physician and a local dermatologist, the patient began taking oral cephalexin for suspected bacterial furunculosis with no considerable improvement. Over the course of 1 week, the lesions became increasingly painful and pruritic, prompting a visit to the emergency department. Prior to his arrival, the patient reported squeezing a live worm from one of the lesions on the right ankle.
On presentation, the patient was afebrile (temperature, 36.7°C) and his vital signs revealed no abnormalities. Physical examination revealed tender erythematous nodules on the bilateral heels, ankles, and shins with pinpoint puncta noted at the center of many of the lesions (Figure 1). The nodules were warm and indurated and no pulsatile movement was appreciated. The legs appeared to be well perfused with intact sensation and motor function. The patient brought in the live mobile larva that he extruded from the lesion on the right ankle. Both the departments of infectious diseases and dermatology were consulted and a preliminary diagnosis of furuncular myiasis was made.
The lesions were occluded with petroleum jelly and the patient was instructed to follow-up with the dermatology department later that same day. On follow-up in the dermatology clinic, the tips of intact larvae were appreciated at the central puncta of some of the lesions (Figure 2). Lidocaine adrenaline tetracaine gel was applied to lesions on the legs for 40 minutes, then lidocaine gel 1% was injected into each lesion. On injection, immobile larvae were ejected from the central puncta of most of the lesions; the remaining lesions were treated via 3-mm punch biopsy as a means of extraction. Each nodule contained only a single larva, all of which were dead at the time of removal (Figure 3). The wounds were left open and the patient was instructed to continue treatment with cephalexin with leg elevation and rest. Pathologic examination of deep dermal skin sections revealed larval fragments encased by a thick chitinous cuticle with spines that were consistent with furuncular myiasis (Figures 4 and 5). Given the patient’s recent history of travel to Africa along with the morphology of the extracted specimens, the larvae were identified as Cordylobia anthropophaga, a common cause of furuncular myiasis in that region.
Patient 2
The next week, a 17-year-old adolescent girl who had been on the same trip to Senegal as patient 1 presented with 2 similar erythematous nodules with central crusts on the left inner thigh and buttock. On noticing the lesions approximately 3 days prior to presentation, the patient applied topical antibiotic ointment to each nodule, which incited the evacuation of white tube-shaped structures that were presented for examination. On presentation, the nodules were healing well. Given the patient’s travel history and physical examination, a presumptive diagnosis of furuncular myiasis from C anthropophaga also was made.


|
Comment
The term myiasis stems from the Greek term for fly and is used to describe the infestation of fly larvae in living vertebrates.1 Myiasis has many classifications, the 3 most common being furuncular, migratory, and wound myiasis, which are differentiated by the different fly species found in distinct regions of the world. Furuncular myiasis is the most benign form, usually affecting only a localized region of the skin; migratory myiasis is characterized by larvae traveling substantial distances from one anatomic site to another within the lower layers of the epidermis; and wound myiasis involves rapid reproduction of larvae in necrotic tissue with subsequent tissue destruction.2
The clinical presentation of the lesions noted in our patients suggested a diagnosis of furuncular myiasis, which commonly is caused by Dermatobia hominis, C anthropophaga, Cuterebra species, Wohlfahrtia vigil, and Wohlfahrtia opaca larvae.3Dermatobia hominis is the most common cause of furuncular myiasis and usually is found in Central and South America. Our patients likely developed an infestation of C anthropophaga (also known as the tumbu fly), a yellow-brown, 7- to 12-mm blowfly commonly found throughout tropical Africa.3 Although C anthropophaga is historically limited to sub-Saharan Africa, there has been a report of a case acquired in Portugal.4
In a review of the literature, C anthropophaga myiasis was documented in Italian travelers returning from Senegal5-7; our cases are unique because they represent North American travelers returning from Senegal with furuncular myiasis. Furuncular myiasis from C anthropophaga has been reported in travelers returning to North America from other African countries, including Angola,8 Tanzania,9-11 Kenya,9 Sierra Leone,12 and Ivory Coast.13 Several cases of ocular myiasis from D hominis and Oestrus ovis have been reported in European travelers returning from Tunisia.14,15
Tumbu fly infestations typically affect dogs and rodents but can arise in human hosts.3 Children may be affected by C anthropophaga furuncular myiasis more often than adults because they have thinner skin and less immunity to the larvae.2
|
There are 2 mechanisms by which infestation of human hosts by C anthropophaga can occur. Most commonly, female flies lay eggs in shady areas in soil that is contaminated by feces or urine. The hatched larvae can survive in the ground for up to 2 weeks and later attach to a host when prompted by heat or movement.3 Therefore, clothing set out to dry may be contaminated by this soil. Alternatively, female flies can lay eggs directly onto clothing that is contaminated by feces or urine and the larvae subsequently hatch outside the soil with easy access to human skin once the clothing is worn.2
Common penetration sites are the head, neck, and back, as well as areas covered by contaminated or infested clothing.2,3 Penetration of the human skin occurs instantly and is a painless process that is rarely noticed by the human host.3 The larvae burrow into the skin for 8 to 12 days, resulting in a furuncle that occasionally secretes a serous fluid.2 Within the first 2 days of infestation, the host may experience symptoms ranging from local pruritus to severe pain. Six days following initial onset, an intense inflammatory response may result in local lymphadenopathy along with fever and fatigue.2 The larvae use their posterior spiracles to create openings in the skin to create air holes that allow them to breathe.3 On physical examination, the spiracles generally appear as 1- to 3-mm dark linear streaks within furuncles, which is important in the diagnosis of C anthropophaga furuncular myiasis.1,3 If spiracles are not appreciated on initial examination, diagnosis can be made by submerging the affected areas in water or saliva to look for air bubbles arising from the central puncta of the lesions.1
All causes of furuncular myiasis are characterized by a ratio of 1 larva to 1 furuncle.16 Although most of these types of larvae that can cause furuncular myiasis result in single lesions, C anthropophaga infestation often produces several furuncles that may coalesce into plaques.1,2 The differential diagnosis for C anthropophaga furuncular myiasis includes pyoderma, impetigo, staphylococcal furunculosis, cutaneous leishmaniasis, infected cyst, retained foreign body, and facticial disease.2,3 Dracunculiasis also may be considered, which occurs after ingestion of contaminated water.2 Ultrasonography may be helpful for the diagnosis of furuncular myiasis, as it can facilitate identification of foreign bodies, abscesses, and even larvae in some cases.17 Definitive diagnosis of any type of myiasis involves extraction of the larva and identification of the family, genus, and species by a parasitologist.1 Some experts suggest rearing preserved live larvae with raw meat after extraction because adult specimens are more reliable than larvae for species diagnosis.1
Treatment of furuncular myiasis involves occlusion and extraction of the larvae from the skin. Suffocation of the larvae by occlusion of air holes with petroleum jelly, paraffin oil, bacon fat, glue, and other obstructing substances forces the larvae to emerge in search of oxygen, though immature larvae may be more reluctant than mature ones.2,3 Definitive treatment involves the direct removal of the larvae by surgery or expulsion by pressure, though it is recommended that lesions are pretreated with occlusive techniques.1,3 Other reported methods of extraction include injection of lidocaine and the use of a commercial venom extractor.1 It should be noted that rupture and incomplete extraction of larvae can lead to secondary infections and allergic reactions. Lesions can be pretreated with lidocaine gel prior to extraction, and antibiotics should be used in cases of secondary bacterial infection. Ivermectin also has been reported as a treatment of furuncular myiasis and other types of myiasis.1 Prevention of infestation by C anthropophaga includes avoidance of endemic areas, maintaining good hygiene, and ironing clothing or drying it in sunny locations.1,2 Overall, furuncular myiasis has a good prognosis with rapid recovery and a low incidence of complications.1
Conclusion
We present 2 cases of travelers returning to North America from Senegal with C anthropophaga furuncular myiasis. Careful review of travel history, physical examination, and identification of fly larvae are important for diagnosis. Individuals traveling to sub-Saharan Africa should avoid drying clothes in shady places and lying on the ground. They also are urged to iron their clothing before wearing it.
Case Reports
Patient 1
A 16-year-old adolescent boy presented to the emergency department with painful, pruritic, erythematous nodules on the bilateral legs of 1 week’s duration. The lesions had developed 1 week after returning from a monthlong trip to Senegal with a volunteer youth group. He did not recall sustaining any painful insect bites or illnesses while traveling in Africa and only noticed the erythematous papules on the legs when he returned home to the United States. After consulting with his primary care physician and a local dermatologist, the patient began taking oral cephalexin for suspected bacterial furunculosis with no considerable improvement. Over the course of 1 week, the lesions became increasingly painful and pruritic, prompting a visit to the emergency department. Prior to his arrival, the patient reported squeezing a live worm from one of the lesions on the right ankle.
On presentation, the patient was afebrile (temperature, 36.7°C) and his vital signs revealed no abnormalities. Physical examination revealed tender erythematous nodules on the bilateral heels, ankles, and shins with pinpoint puncta noted at the center of many of the lesions (Figure 1). The nodules were warm and indurated and no pulsatile movement was appreciated. The legs appeared to be well perfused with intact sensation and motor function. The patient brought in the live mobile larva that he extruded from the lesion on the right ankle. Both the departments of infectious diseases and dermatology were consulted and a preliminary diagnosis of furuncular myiasis was made.
The lesions were occluded with petroleum jelly and the patient was instructed to follow-up with the dermatology department later that same day. On follow-up in the dermatology clinic, the tips of intact larvae were appreciated at the central puncta of some of the lesions (Figure 2). Lidocaine adrenaline tetracaine gel was applied to lesions on the legs for 40 minutes, then lidocaine gel 1% was injected into each lesion. On injection, immobile larvae were ejected from the central puncta of most of the lesions; the remaining lesions were treated via 3-mm punch biopsy as a means of extraction. Each nodule contained only a single larva, all of which were dead at the time of removal (Figure 3). The wounds were left open and the patient was instructed to continue treatment with cephalexin with leg elevation and rest. Pathologic examination of deep dermal skin sections revealed larval fragments encased by a thick chitinous cuticle with spines that were consistent with furuncular myiasis (Figures 4 and 5). Given the patient’s recent history of travel to Africa along with the morphology of the extracted specimens, the larvae were identified as Cordylobia anthropophaga, a common cause of furuncular myiasis in that region.
Patient 2
The next week, a 17-year-old adolescent girl who had been on the same trip to Senegal as patient 1 presented with 2 similar erythematous nodules with central crusts on the left inner thigh and buttock. On noticing the lesions approximately 3 days prior to presentation, the patient applied topical antibiotic ointment to each nodule, which incited the evacuation of white tube-shaped structures that were presented for examination. On presentation, the nodules were healing well. Given the patient’s travel history and physical examination, a presumptive diagnosis of furuncular myiasis from C anthropophaga also was made.


|
Comment
The term myiasis stems from the Greek term for fly and is used to describe the infestation of fly larvae in living vertebrates.1 Myiasis has many classifications, the 3 most common being furuncular, migratory, and wound myiasis, which are differentiated by the different fly species found in distinct regions of the world. Furuncular myiasis is the most benign form, usually affecting only a localized region of the skin; migratory myiasis is characterized by larvae traveling substantial distances from one anatomic site to another within the lower layers of the epidermis; and wound myiasis involves rapid reproduction of larvae in necrotic tissue with subsequent tissue destruction.2
The clinical presentation of the lesions noted in our patients suggested a diagnosis of furuncular myiasis, which commonly is caused by Dermatobia hominis, C anthropophaga, Cuterebra species, Wohlfahrtia vigil, and Wohlfahrtia opaca larvae.3Dermatobia hominis is the most common cause of furuncular myiasis and usually is found in Central and South America. Our patients likely developed an infestation of C anthropophaga (also known as the tumbu fly), a yellow-brown, 7- to 12-mm blowfly commonly found throughout tropical Africa.3 Although C anthropophaga is historically limited to sub-Saharan Africa, there has been a report of a case acquired in Portugal.4
In a review of the literature, C anthropophaga myiasis was documented in Italian travelers returning from Senegal5-7; our cases are unique because they represent North American travelers returning from Senegal with furuncular myiasis. Furuncular myiasis from C anthropophaga has been reported in travelers returning to North America from other African countries, including Angola,8 Tanzania,9-11 Kenya,9 Sierra Leone,12 and Ivory Coast.13 Several cases of ocular myiasis from D hominis and Oestrus ovis have been reported in European travelers returning from Tunisia.14,15
Tumbu fly infestations typically affect dogs and rodents but can arise in human hosts.3 Children may be affected by C anthropophaga furuncular myiasis more often than adults because they have thinner skin and less immunity to the larvae.2
|
There are 2 mechanisms by which infestation of human hosts by C anthropophaga can occur. Most commonly, female flies lay eggs in shady areas in soil that is contaminated by feces or urine. The hatched larvae can survive in the ground for up to 2 weeks and later attach to a host when prompted by heat or movement.3 Therefore, clothing set out to dry may be contaminated by this soil. Alternatively, female flies can lay eggs directly onto clothing that is contaminated by feces or urine and the larvae subsequently hatch outside the soil with easy access to human skin once the clothing is worn.2
Common penetration sites are the head, neck, and back, as well as areas covered by contaminated or infested clothing.2,3 Penetration of the human skin occurs instantly and is a painless process that is rarely noticed by the human host.3 The larvae burrow into the skin for 8 to 12 days, resulting in a furuncle that occasionally secretes a serous fluid.2 Within the first 2 days of infestation, the host may experience symptoms ranging from local pruritus to severe pain. Six days following initial onset, an intense inflammatory response may result in local lymphadenopathy along with fever and fatigue.2 The larvae use their posterior spiracles to create openings in the skin to create air holes that allow them to breathe.3 On physical examination, the spiracles generally appear as 1- to 3-mm dark linear streaks within furuncles, which is important in the diagnosis of C anthropophaga furuncular myiasis.1,3 If spiracles are not appreciated on initial examination, diagnosis can be made by submerging the affected areas in water or saliva to look for air bubbles arising from the central puncta of the lesions.1
All causes of furuncular myiasis are characterized by a ratio of 1 larva to 1 furuncle.16 Although most of these types of larvae that can cause furuncular myiasis result in single lesions, C anthropophaga infestation often produces several furuncles that may coalesce into plaques.1,2 The differential diagnosis for C anthropophaga furuncular myiasis includes pyoderma, impetigo, staphylococcal furunculosis, cutaneous leishmaniasis, infected cyst, retained foreign body, and facticial disease.2,3 Dracunculiasis also may be considered, which occurs after ingestion of contaminated water.2 Ultrasonography may be helpful for the diagnosis of furuncular myiasis, as it can facilitate identification of foreign bodies, abscesses, and even larvae in some cases.17 Definitive diagnosis of any type of myiasis involves extraction of the larva and identification of the family, genus, and species by a parasitologist.1 Some experts suggest rearing preserved live larvae with raw meat after extraction because adult specimens are more reliable than larvae for species diagnosis.1
Treatment of furuncular myiasis involves occlusion and extraction of the larvae from the skin. Suffocation of the larvae by occlusion of air holes with petroleum jelly, paraffin oil, bacon fat, glue, and other obstructing substances forces the larvae to emerge in search of oxygen, though immature larvae may be more reluctant than mature ones.2,3 Definitive treatment involves the direct removal of the larvae by surgery or expulsion by pressure, though it is recommended that lesions are pretreated with occlusive techniques.1,3 Other reported methods of extraction include injection of lidocaine and the use of a commercial venom extractor.1 It should be noted that rupture and incomplete extraction of larvae can lead to secondary infections and allergic reactions. Lesions can be pretreated with lidocaine gel prior to extraction, and antibiotics should be used in cases of secondary bacterial infection. Ivermectin also has been reported as a treatment of furuncular myiasis and other types of myiasis.1 Prevention of infestation by C anthropophaga includes avoidance of endemic areas, maintaining good hygiene, and ironing clothing or drying it in sunny locations.1,2 Overall, furuncular myiasis has a good prognosis with rapid recovery and a low incidence of complications.1
Conclusion
We present 2 cases of travelers returning to North America from Senegal with C anthropophaga furuncular myiasis. Careful review of travel history, physical examination, and identification of fly larvae are important for diagnosis. Individuals traveling to sub-Saharan Africa should avoid drying clothes in shady places and lying on the ground. They also are urged to iron their clothing before wearing it.
1. Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
2. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926.
3. Robbins K, Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49:1092-1098.
4. Curtis SJ, Edwards C, Athulathmuda C, et al. Case of the month: cutaneous myiasis in a returning traveller from the Algarve: first report of tumbu maggots, Cordylobia anthropophaga, acquired in Portugal. Emerg Med J. 2006;23:236-237.
5. Veraldi S, Brusasco A, Süss L. Cutaneous myiasis caused by larvae of Cordylobia anthropophaga (Blanchard). Int J Dermatol. 1993;32:184-187.
6. Cultrera R, Dettori G, Calderaro A, et al. Cutaneous myiasis caused by Cordylobia anthropophaga (Blanchard 1872): description of 5 cases from costal regions of Senegal [in Italian]. Parassitologia. 1993;35:47-49.
7. Fusco FM, Nardiello S, Brancaccio G, et al. Cutaneous myiasis from Cordylobia anthropophaga in a traveller returning from Senegal: a case study [in Italian]. Infez Med. 2005;13:109-111.
8. Lee EJ, Robinson F. Furuncular myiasis of the face caused by larva of the tumbu fly (Cordylobia anthropophaga)[published online ahead of print July 21, 2006]. Eye (Lond). 2007;21:268-269.
9. Rice PL, Gleason N. Two cases of myiasis in the United States by the African tumbu fly, Cordylobia anthropophaga (Diptera, Calliphoridae). Am J Trop Med Hyg. 1972;21:62-65.
10. March CH. A case of “ver du Cayor” in Manhattan. Arch Dermatol. 1964;90:32-33.
11. Schorr WF. Tumbu-fly myiasis in Marshfield, Wis. Arch Dermatol. 1967;95:61-62.
12. Potter TS, Dorman MA, Ghaemi M, et al. Inflammatory papules on the back of a traveling businessman. tumbu
fly myiasis. Arch Dermatol. 1995;131:951, 954.
13. Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
14. Kaouech E, Kallel K, Belhadj S, et al. Dermatobia hominis furuncular myiasis in a man returning from Latin America: first imported case in Tunisia [in French]. Med Trop (Mars). 2010;70:135-136.
15. Zayani A, Chaabouni M, Gouiaa R, et al. Conjuctival myiasis. 23 cases in the Tunisian Sahel [in French]. Arch Inst Pasteur Tunis. 1989;66:289-292.
16. Latorre M, Ullate JV, Sanchez J, et al. A case of myiasis due to Dermatobia hominis. Eur J Clin Microbiol Infect Dis. 1993;12:968-969.
17. Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominis: a case of human botfly infestation [published online ahead of print February 1, 2010]. J Emerg Med. 2012;43:618-621.
1. Caissie R, Beaulieu F, Giroux M, et al. Cutaneous myiasis: diagnosis, treatment, and prevention. J Oral Maxillofac Surg. 2008;66:560-568.
2. McGraw TA, Turiansky GW. Cutaneous myiasis. J Am Acad Dermatol. 2008;58:907-926.
3. Robbins K, Khachemoune A. Cutaneous myiasis: a review of the common types of myiasis. Int J Dermatol. 2010;49:1092-1098.
4. Curtis SJ, Edwards C, Athulathmuda C, et al. Case of the month: cutaneous myiasis in a returning traveller from the Algarve: first report of tumbu maggots, Cordylobia anthropophaga, acquired in Portugal. Emerg Med J. 2006;23:236-237.
5. Veraldi S, Brusasco A, Süss L. Cutaneous myiasis caused by larvae of Cordylobia anthropophaga (Blanchard). Int J Dermatol. 1993;32:184-187.
6. Cultrera R, Dettori G, Calderaro A, et al. Cutaneous myiasis caused by Cordylobia anthropophaga (Blanchard 1872): description of 5 cases from costal regions of Senegal [in Italian]. Parassitologia. 1993;35:47-49.
7. Fusco FM, Nardiello S, Brancaccio G, et al. Cutaneous myiasis from Cordylobia anthropophaga in a traveller returning from Senegal: a case study [in Italian]. Infez Med. 2005;13:109-111.
8. Lee EJ, Robinson F. Furuncular myiasis of the face caused by larva of the tumbu fly (Cordylobia anthropophaga)[published online ahead of print July 21, 2006]. Eye (Lond). 2007;21:268-269.
9. Rice PL, Gleason N. Two cases of myiasis in the United States by the African tumbu fly, Cordylobia anthropophaga (Diptera, Calliphoridae). Am J Trop Med Hyg. 1972;21:62-65.
10. March CH. A case of “ver du Cayor” in Manhattan. Arch Dermatol. 1964;90:32-33.
11. Schorr WF. Tumbu-fly myiasis in Marshfield, Wis. Arch Dermatol. 1967;95:61-62.
12. Potter TS, Dorman MA, Ghaemi M, et al. Inflammatory papules on the back of a traveling businessman. tumbu
fly myiasis. Arch Dermatol. 1995;131:951, 954.
13. Ockenhouse CF, Samlaska CP, Benson PM, et al. Cutaneous myiasis caused by the African tumbu fly (Cordylobia anthropophaga). Arch Dermatol. 1990;126:199-202.
14. Kaouech E, Kallel K, Belhadj S, et al. Dermatobia hominis furuncular myiasis in a man returning from Latin America: first imported case in Tunisia [in French]. Med Trop (Mars). 2010;70:135-136.
15. Zayani A, Chaabouni M, Gouiaa R, et al. Conjuctival myiasis. 23 cases in the Tunisian Sahel [in French]. Arch Inst Pasteur Tunis. 1989;66:289-292.
16. Latorre M, Ullate JV, Sanchez J, et al. A case of myiasis due to Dermatobia hominis. Eur J Clin Microbiol Infect Dis. 1993;12:968-969.
17. Mahal JJ, Sperling JD. Furuncular myiasis from Dermatobia hominis: a case of human botfly infestation [published online ahead of print February 1, 2010]. J Emerg Med. 2012;43:618-621.
Practice Points
- Cutaneous myiasis is caused by an infestation of fly larvae and can present as furuncles (furuncular myiasis), migratory inflammatory linear plaques (migratory myiasis), and worsening tissue destruction in existing wounds (wound myiasis).
- Furuncular myiasis should be included in the differential diagnosis in patients with furuncular skin lesions who have recently traveled to Central America, South America, or sub-Saharan Africa.
- Furuncular myiasis may be treated by both occlusive and extraction techniques.
Cutaneous Manifestations of Cocaine Use
The Diagnosis: Levamisole-Induced Cutaneous Vasculopathy
In our patient, tender stellate purpura and occasional bullae were present on the ears, arms and legs, groin, and buttocks (Figure 1). Histopathologic examination revealed subepidermal detachment, perivascular neutrophilic infiltrate, and red blood cell extravasation, consistent with early leukocyctoclastic vasculitis (Figure 2).
|
Levamisole-induced vasculopathy is a condition related primarily to cocaine use. Levamisole is an immunomodulatory agent, historically used as a disease-modifying antirheumatic drug for rheumatoid arthritis and as adjuvant chemotherapy for various types of cancer. However, levamisole for human use was banned from US and Canadian markets in 1999 and 2003, respectively, due to increased risk for agranulocytosis, retiform purpura, and epilepsy.1 Currently, veterinarians use levamisole as an anthelminthic agent to deworm house and farm animals. In Europe, pediatric nephrologists use it as a steroid-sparing agent in children with steroid-dependent nephritic syndrome.
Over the last decade, levamisole has increasingly been used as a cocaine adulterant or bulking agent. This contaminant closely resembles cocaine physically and is theorized to prolong or attenuate cocaine’s “high.” Approximately 69% of cocaine sampled by the US Drug Enforcement Administration is adulterated with levamisole.2 Similarly, levamisole-contaminated cocaine also has been found in Europe, Australia, and other parts of the world. Potential complications include vasculitis, thromboembolism, neutropenia, and agranulocytosis.3
Levamisole-induced vasculopathy appears to affect cocaine users of all ages, ethnicities, and genders. Cocaine can be smoked, snorted, or injected. In nearly all reported cases, patients characteristically present with hemorrhagic bullae of the bilateral ear helix, cheeks, or nasal tip. Any body site can be affected with retiform purpura or necrotic bullae. Along with skin lesions, arthralgia is commonly reported, as are constitutional symptoms (eg, fever, night sweats, weight loss, malaise)4; oral mucosal involvement also has been reported.5 Laboratory investigation can reveal neutropenia, positive antineutrophil cytoplasmic antibodies (ANCAs) in the perinuclear or cytoplasmic pattern, positive proteinase 3, and negative or mildly elevated antimyeloperoxidase.3-5 Acute renal injury and pulmonary hemorrhage are other potentially serious copmlications.4 Antihuman neutrophil elastase antibody testing can help distinguish levamisole-induced vasculopathy from other forms of immune-mediated vasculitis and will be negative in immune-mediated vasculitides such as Churg-Strauss syndrome (allergic granulomatosis), Wegener granulomatosis (granulomatosis with polyangiitis), and polyarteritis nodosa.6 On histology, microvascular thrombosis or leukocytoclastic vasculitis can both, or individually, be seen. Epidermal necrosis, dermal hemorrhage, and endothelial hyperplasia have all been noted in skin biopsied from necrotic bullae.
|
Levamisole’s short half-life (approximately 5–6 hours) makes it difficult to detect on routine blood draws. An astute physician suspecting this diagnosis on initial presentation can ask for levamisole detection on urine toxicology screening.7 Urine samples also can be sent for testing with gas chromatography–mass spectrometry, though this test may only be available at major research centers.8
Differential diagnosis of levamisole toxicity includes different types of vasculitides such as cryoglobulinemia (positive serum IgM and IgG cryoglobulins; possible hepatitis C infection), Wegener granulomatosis (cytoplasmic ANCA positive; associated with upper and lower respiratory tract inflammation, glomerulonephritis), Churg-Strauss syndrome (perinuclear ANCA positive; associated with asthma and eosinophilia), and polyarteritis nodosa (medium vessel involvement only; associated with livedo reticularis, subcutaneous nodules, ulcers).9 Necrotic lesions also may raise the possibility of warfarin necrosis, heparin necrosis, or cholesterol emboli. Cholesterol embolism most frequently presents with small vessel vasculitis and necrosis of distal extremities such as the toes. With large areas of skin involvement and bullae, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis also should be considered.9
Definitive treatment of this condition requires complete and immediate cessation of cocaine use. Levamisole also has been found as a contaminant in heroin.1 Thus, it may be prudent to recommend heroin avoidance to the patient to prevent recurrences. Management of acute levamisole-induced vasculopathy is primarily symptomatic. Some patients with severe neutropenia at risk for infection have been treated with granulocyte colony-stimulating factor, while others have only required pain control, usually with nonsteroidal anti-inflammatory drugs.10 Oral prednisone and colchicine also have been used with reported success.5
Given the increasing incidence of levamisole toxicity and public health implications, clinicians should be aware of this association and the classic clinical and laboratory findings.
1. Aberastury MN, Silva WH, Vaccarezza MM, et al. Epilepsia partialis continua associated with levamisole. Pediatr Neurol. 2011;44:385-388.
2. Nationwide public health alert issued concerning life-threatening risk posed by cocaine laced with veterinary anti-parasite drug [press release]. Rockville, MD: Substance Abuse and Mental Health Services Administration; September 21, 2009. http://beta.samhsa.gov/newsroom/press-announcements/200909211245. Accessed October 9, 2014.
3. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
4. McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasm antibody-associated disease. Clin J Am Soc Nephrol. 2011;6:2799-2805.
5. Poon SH, Baliog CR Jr, Sams RN, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leucopenia. Semin Arthritis Rheum. 2011;41:434-444.
6. Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol. 2010;37:1212-1219.
7. Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
8. Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2001;35:545-550.
9. Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
10. Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
The Diagnosis: Levamisole-Induced Cutaneous Vasculopathy
In our patient, tender stellate purpura and occasional bullae were present on the ears, arms and legs, groin, and buttocks (Figure 1). Histopathologic examination revealed subepidermal detachment, perivascular neutrophilic infiltrate, and red blood cell extravasation, consistent with early leukocyctoclastic vasculitis (Figure 2).
|
Levamisole-induced vasculopathy is a condition related primarily to cocaine use. Levamisole is an immunomodulatory agent, historically used as a disease-modifying antirheumatic drug for rheumatoid arthritis and as adjuvant chemotherapy for various types of cancer. However, levamisole for human use was banned from US and Canadian markets in 1999 and 2003, respectively, due to increased risk for agranulocytosis, retiform purpura, and epilepsy.1 Currently, veterinarians use levamisole as an anthelminthic agent to deworm house and farm animals. In Europe, pediatric nephrologists use it as a steroid-sparing agent in children with steroid-dependent nephritic syndrome.
Over the last decade, levamisole has increasingly been used as a cocaine adulterant or bulking agent. This contaminant closely resembles cocaine physically and is theorized to prolong or attenuate cocaine’s “high.” Approximately 69% of cocaine sampled by the US Drug Enforcement Administration is adulterated with levamisole.2 Similarly, levamisole-contaminated cocaine also has been found in Europe, Australia, and other parts of the world. Potential complications include vasculitis, thromboembolism, neutropenia, and agranulocytosis.3
Levamisole-induced vasculopathy appears to affect cocaine users of all ages, ethnicities, and genders. Cocaine can be smoked, snorted, or injected. In nearly all reported cases, patients characteristically present with hemorrhagic bullae of the bilateral ear helix, cheeks, or nasal tip. Any body site can be affected with retiform purpura or necrotic bullae. Along with skin lesions, arthralgia is commonly reported, as are constitutional symptoms (eg, fever, night sweats, weight loss, malaise)4; oral mucosal involvement also has been reported.5 Laboratory investigation can reveal neutropenia, positive antineutrophil cytoplasmic antibodies (ANCAs) in the perinuclear or cytoplasmic pattern, positive proteinase 3, and negative or mildly elevated antimyeloperoxidase.3-5 Acute renal injury and pulmonary hemorrhage are other potentially serious copmlications.4 Antihuman neutrophil elastase antibody testing can help distinguish levamisole-induced vasculopathy from other forms of immune-mediated vasculitis and will be negative in immune-mediated vasculitides such as Churg-Strauss syndrome (allergic granulomatosis), Wegener granulomatosis (granulomatosis with polyangiitis), and polyarteritis nodosa.6 On histology, microvascular thrombosis or leukocytoclastic vasculitis can both, or individually, be seen. Epidermal necrosis, dermal hemorrhage, and endothelial hyperplasia have all been noted in skin biopsied from necrotic bullae.
|
Levamisole’s short half-life (approximately 5–6 hours) makes it difficult to detect on routine blood draws. An astute physician suspecting this diagnosis on initial presentation can ask for levamisole detection on urine toxicology screening.7 Urine samples also can be sent for testing with gas chromatography–mass spectrometry, though this test may only be available at major research centers.8
Differential diagnosis of levamisole toxicity includes different types of vasculitides such as cryoglobulinemia (positive serum IgM and IgG cryoglobulins; possible hepatitis C infection), Wegener granulomatosis (cytoplasmic ANCA positive; associated with upper and lower respiratory tract inflammation, glomerulonephritis), Churg-Strauss syndrome (perinuclear ANCA positive; associated with asthma and eosinophilia), and polyarteritis nodosa (medium vessel involvement only; associated with livedo reticularis, subcutaneous nodules, ulcers).9 Necrotic lesions also may raise the possibility of warfarin necrosis, heparin necrosis, or cholesterol emboli. Cholesterol embolism most frequently presents with small vessel vasculitis and necrosis of distal extremities such as the toes. With large areas of skin involvement and bullae, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis also should be considered.9
Definitive treatment of this condition requires complete and immediate cessation of cocaine use. Levamisole also has been found as a contaminant in heroin.1 Thus, it may be prudent to recommend heroin avoidance to the patient to prevent recurrences. Management of acute levamisole-induced vasculopathy is primarily symptomatic. Some patients with severe neutropenia at risk for infection have been treated with granulocyte colony-stimulating factor, while others have only required pain control, usually with nonsteroidal anti-inflammatory drugs.10 Oral prednisone and colchicine also have been used with reported success.5
Given the increasing incidence of levamisole toxicity and public health implications, clinicians should be aware of this association and the classic clinical and laboratory findings.
The Diagnosis: Levamisole-Induced Cutaneous Vasculopathy
In our patient, tender stellate purpura and occasional bullae were present on the ears, arms and legs, groin, and buttocks (Figure 1). Histopathologic examination revealed subepidermal detachment, perivascular neutrophilic infiltrate, and red blood cell extravasation, consistent with early leukocyctoclastic vasculitis (Figure 2).
|
Levamisole-induced vasculopathy is a condition related primarily to cocaine use. Levamisole is an immunomodulatory agent, historically used as a disease-modifying antirheumatic drug for rheumatoid arthritis and as adjuvant chemotherapy for various types of cancer. However, levamisole for human use was banned from US and Canadian markets in 1999 and 2003, respectively, due to increased risk for agranulocytosis, retiform purpura, and epilepsy.1 Currently, veterinarians use levamisole as an anthelminthic agent to deworm house and farm animals. In Europe, pediatric nephrologists use it as a steroid-sparing agent in children with steroid-dependent nephritic syndrome.
Over the last decade, levamisole has increasingly been used as a cocaine adulterant or bulking agent. This contaminant closely resembles cocaine physically and is theorized to prolong or attenuate cocaine’s “high.” Approximately 69% of cocaine sampled by the US Drug Enforcement Administration is adulterated with levamisole.2 Similarly, levamisole-contaminated cocaine also has been found in Europe, Australia, and other parts of the world. Potential complications include vasculitis, thromboembolism, neutropenia, and agranulocytosis.3
Levamisole-induced vasculopathy appears to affect cocaine users of all ages, ethnicities, and genders. Cocaine can be smoked, snorted, or injected. In nearly all reported cases, patients characteristically present with hemorrhagic bullae of the bilateral ear helix, cheeks, or nasal tip. Any body site can be affected with retiform purpura or necrotic bullae. Along with skin lesions, arthralgia is commonly reported, as are constitutional symptoms (eg, fever, night sweats, weight loss, malaise)4; oral mucosal involvement also has been reported.5 Laboratory investigation can reveal neutropenia, positive antineutrophil cytoplasmic antibodies (ANCAs) in the perinuclear or cytoplasmic pattern, positive proteinase 3, and negative or mildly elevated antimyeloperoxidase.3-5 Acute renal injury and pulmonary hemorrhage are other potentially serious copmlications.4 Antihuman neutrophil elastase antibody testing can help distinguish levamisole-induced vasculopathy from other forms of immune-mediated vasculitis and will be negative in immune-mediated vasculitides such as Churg-Strauss syndrome (allergic granulomatosis), Wegener granulomatosis (granulomatosis with polyangiitis), and polyarteritis nodosa.6 On histology, microvascular thrombosis or leukocytoclastic vasculitis can both, or individually, be seen. Epidermal necrosis, dermal hemorrhage, and endothelial hyperplasia have all been noted in skin biopsied from necrotic bullae.
|
Levamisole’s short half-life (approximately 5–6 hours) makes it difficult to detect on routine blood draws. An astute physician suspecting this diagnosis on initial presentation can ask for levamisole detection on urine toxicology screening.7 Urine samples also can be sent for testing with gas chromatography–mass spectrometry, though this test may only be available at major research centers.8
Differential diagnosis of levamisole toxicity includes different types of vasculitides such as cryoglobulinemia (positive serum IgM and IgG cryoglobulins; possible hepatitis C infection), Wegener granulomatosis (cytoplasmic ANCA positive; associated with upper and lower respiratory tract inflammation, glomerulonephritis), Churg-Strauss syndrome (perinuclear ANCA positive; associated with asthma and eosinophilia), and polyarteritis nodosa (medium vessel involvement only; associated with livedo reticularis, subcutaneous nodules, ulcers).9 Necrotic lesions also may raise the possibility of warfarin necrosis, heparin necrosis, or cholesterol emboli. Cholesterol embolism most frequently presents with small vessel vasculitis and necrosis of distal extremities such as the toes. With large areas of skin involvement and bullae, erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis also should be considered.9
Definitive treatment of this condition requires complete and immediate cessation of cocaine use. Levamisole also has been found as a contaminant in heroin.1 Thus, it may be prudent to recommend heroin avoidance to the patient to prevent recurrences. Management of acute levamisole-induced vasculopathy is primarily symptomatic. Some patients with severe neutropenia at risk for infection have been treated with granulocyte colony-stimulating factor, while others have only required pain control, usually with nonsteroidal anti-inflammatory drugs.10 Oral prednisone and colchicine also have been used with reported success.5
Given the increasing incidence of levamisole toxicity and public health implications, clinicians should be aware of this association and the classic clinical and laboratory findings.
1. Aberastury MN, Silva WH, Vaccarezza MM, et al. Epilepsia partialis continua associated with levamisole. Pediatr Neurol. 2011;44:385-388.
2. Nationwide public health alert issued concerning life-threatening risk posed by cocaine laced with veterinary anti-parasite drug [press release]. Rockville, MD: Substance Abuse and Mental Health Services Administration; September 21, 2009. http://beta.samhsa.gov/newsroom/press-announcements/200909211245. Accessed October 9, 2014.
3. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
4. McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasm antibody-associated disease. Clin J Am Soc Nephrol. 2011;6:2799-2805.
5. Poon SH, Baliog CR Jr, Sams RN, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leucopenia. Semin Arthritis Rheum. 2011;41:434-444.
6. Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol. 2010;37:1212-1219.
7. Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
8. Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2001;35:545-550.
9. Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
10. Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
1. Aberastury MN, Silva WH, Vaccarezza MM, et al. Epilepsia partialis continua associated with levamisole. Pediatr Neurol. 2011;44:385-388.
2. Nationwide public health alert issued concerning life-threatening risk posed by cocaine laced with veterinary anti-parasite drug [press release]. Rockville, MD: Substance Abuse and Mental Health Services Administration; September 21, 2009. http://beta.samhsa.gov/newsroom/press-announcements/200909211245. Accessed October 9, 2014.
3. Lee KC, Culpepper K, Kessler M. Levamisole-induced thrombosis: literature review and pertinent laboratory findings. J Am Acad Dermatol. 2011;65:e128-e129.
4. McGrath MM, Isakova T, Rennke HG, et al. Contaminated cocaine and antineutrophil cytoplasm antibody-associated disease. Clin J Am Soc Nephrol. 2011;6:2799-2805.
5. Poon SH, Baliog CR Jr, Sams RN, et al. Syndrome of cocaine-levamisole-induced cutaneous vasculitis and immune-mediated leucopenia. Semin Arthritis Rheum. 2011;41:434-444.
6. Walsh NM, Green PJ, Burlingame RW, et al. Cocaine-related retiform purpura: evidence to incriminate the adulterant, levamisole. J Cutan Pathol. 2010;37:1212-1219.
7. Buchanan JA, Heard K, Burbach C, et al. Prevalence of levamisole in urine toxicology screens positive for cocaine in an inner-city hospital. JAMA. 2011;305:1657-1658.
8. Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2001;35:545-550.
9. Lee KC, Ladizinski B, Federman DG. Complications associated with use of levamisole-contaminated cocaine: an emerging public health challenge. Mayo Clin Proc. 2012;87:581-586.
10. Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
A 43-year-old woman presented to the emergency department with painful skin lesions of 1 day’s duration. Physical examination revealed tender stellate purpura and occasional bullae on the ears, arms and legs, groin, and buttocks. Laboratory results revealed neutropenia and positive lupus anticoagulant; antineutrophil cytoplasmic antibody, antinuclear antibody, and double-stranded DNA antibodies were all negative. Urine toxicology was positive for cocaine and opioids. An incisional biopsy of the left arm was performed.