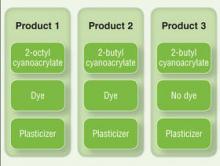
User login
Practice Question Answers: Allergic Contact Dermatitis, Part 2
1. Which of the following is not a component of fragrance mix?
a. abietic acid
b. α-amylcinnamaldehyde
c. geraniol
d. hydroxycitronellal
e. oakmoss
2. A patient is referred for patch testing for suspected allergic contact dermatitis and is found to have positivity to disperse blue dye 106. The patient should avoid all of the following except:
a. black-colored clothing
b. pure acetate clothing
c. pure polyester clothing
d. purple-colored clothing
e. red-colored clothing
3. A patient with a documented contact allergy to ethylenediamine dihydrochloride should avoid all of the following systemic medications except:
a. aminophylline
b. disulfiram
c. hydroxyzine
d. meclizine
e. promethazine
4. Formaldehyde can cross-react with all of the following except:
a. diazolidinyl urea
b. DMDM hydantoin
c. imidazolidinyl urea
d. para-aminobenzoic acid
e. quaternium-15
5. Colophony can be found in all of the following trees except:
a. cedars
b. firs
c. junipers
d. maples
e. pines
1. Which of the following is not a component of fragrance mix?
a. abietic acid
b. α-amylcinnamaldehyde
c. geraniol
d. hydroxycitronellal
e. oakmoss
2. A patient is referred for patch testing for suspected allergic contact dermatitis and is found to have positivity to disperse blue dye 106. The patient should avoid all of the following except:
a. black-colored clothing
b. pure acetate clothing
c. pure polyester clothing
d. purple-colored clothing
e. red-colored clothing
3. A patient with a documented contact allergy to ethylenediamine dihydrochloride should avoid all of the following systemic medications except:
a. aminophylline
b. disulfiram
c. hydroxyzine
d. meclizine
e. promethazine
4. Formaldehyde can cross-react with all of the following except:
a. diazolidinyl urea
b. DMDM hydantoin
c. imidazolidinyl urea
d. para-aminobenzoic acid
e. quaternium-15
5. Colophony can be found in all of the following trees except:
a. cedars
b. firs
c. junipers
d. maples
e. pines
1. Which of the following is not a component of fragrance mix?
a. abietic acid
b. α-amylcinnamaldehyde
c. geraniol
d. hydroxycitronellal
e. oakmoss
2. A patient is referred for patch testing for suspected allergic contact dermatitis and is found to have positivity to disperse blue dye 106. The patient should avoid all of the following except:
a. black-colored clothing
b. pure acetate clothing
c. pure polyester clothing
d. purple-colored clothing
e. red-colored clothing
3. A patient with a documented contact allergy to ethylenediamine dihydrochloride should avoid all of the following systemic medications except:
a. aminophylline
b. disulfiram
c. hydroxyzine
d. meclizine
e. promethazine
4. Formaldehyde can cross-react with all of the following except:
a. diazolidinyl urea
b. DMDM hydantoin
c. imidazolidinyl urea
d. para-aminobenzoic acid
e. quaternium-15
5. Colophony can be found in all of the following trees except:
a. cedars
b. firs
c. junipers
d. maples
e. pines
Allergic Contact Dermatitis, Part 2
Applications of Lasers in Medical Dermatology
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
The use of lasers in dermatology has had a major impact on the treatment of many dermatologic conditions. In this column practical applications of lasers in medical dermatology will be discussed to give dermatology residents a broad overview of both established indications and the reasoning behind the usage of lasers in treating these skin conditions. The applications for lasers in aesthetic dermatology are numerous and are constantly being refined and developed; they have been discussed extensively in the literature. Given the vast variety of uses of lasers in dermatology today, a comprehensive review of this topic would likely span several volumes. This article will focus on recent evidence regarding the use of lasers in medical dermatology, specifically laser treatment of selected common dermatoses and cutaneous malignancies.
Laser Treatment of Skin Diseases
Many common dermatoses seen in the dermatologist’s office (eg, discoid lupus erythematosus [DLE], morphea, alopecia) already have an established therapeutic ladder, with most patients responding to either first- or second-line therapies; however, a number of patients present with refractory disease that can be difficult to treat due to either treatment resistance or other contraindications to therapy. With the advent and development of modern lasers, we are now able to target many of these conditions and provide a viable safe treatment option for these patients. Although many physicians may be familiar with the use of the excimer laser in the treatment of psoriasis,1 a long-standing and well-accepted treatment modality for this condition, many novel applications for different types of lasers have been developed.
First, it is important to consider what a laser is able to accomplish to modulate the skin. With ablative lasers such as the CO2 laser, it is possible to destroy superficial layers of the skin (ie, the epidermis). It would stand to reason that this approach would be ideal for treating epidermal processes such as viral warts; in fact, this modality has been used for this indication for more than 3 decades, with the earliest references coming from the podiatric and urologic literature.2,3 Despite conflicting reports of the risk for human papillomavirus aerosolization and subsequent contamination of the treatment area,4,5 CO2 laser therapy has been advocated as a nonsurgical approach to difficult-to-treat cases of viral warts.
On the other hand, the pulsed dye laser (PDL) can target blood vessels because the wavelength corresponds to the absorption spectrum of hemoglobin and penetrates to the level of the dermis, while the pulse duration can be set to be shorter than the thermal relaxation time of a small cutaneous blood vessel.6 In clinical practice, the PDL has been used for the treatment of vascular lesions including hemangiomas, nevus flammeus, and other vascular proliferations.7-9 However, the PDL also can be used to target the vessels in cutaneous inflammatory diseases that feature vascular dilation and/or perivascular inflammation as a prominent feature.
Discoid lupus erythematosus is a form of chronic cutaneous lupus erythematosus that may be difficult to treat, with recalcitrant lesions displaying continued inflammation leading to chronic scarring and dyspigmentation. A small study (N=12) presented the efficacy of the PDL in the treatment of DLE lesions, suggesting that it has good efficacy in treating recalcitrant lesions with significant reduction in the cutaneous lupus erythematosus disease area and severity index after 6 weeks of treatment and 6 weeks of follow-up (P<.0001) with decreased erythema and scaling.10 It is important to note, however, that scarring, dyspigmentation, and atrophy were not affected, which suggests that early intervention may be optimal to prevent development of these sequelae. More interestingly, a more recent study expounded on this idea and attempted to examine pathophysiologic mechanisms behind this observed improvement. Evaluation of biopsy specimens before and after treatment and immunohistochemistry revealed that PDL treatment of cutaneous DLE lesions led to a decrease in vascular endothelial proteins—intercellular adhesion molecule 1 and vascular cell adhesion molecule 1—with a coincident reduction in the dermal lymphocytic infiltrate in treated lesions.11 These results offer a somewhat satisfying view on the correlation between the theory and basic science of laser therapy and the subsequent clinical benefits afforded by laser treatment. A case series provided further evidence that PDL or intense pulsed light can ameliorate the cutaneous lesions of DLE in 16 patients in whom all other treatments had failed.12
Several other inflammatory dermatoses can be treated with PDL, though the evidence for most of these conditions is sporadic at best, consisting mostly of case reports and a few case series. Granuloma faciale is one such condition, with evidence of efficacy of the PDL dating back as far as 1999,13 though a more recent case series of 4 patients only showed response in 2 patients.14 Because granuloma faciale features vasculitis as a prominent feature in its pathology, targeting the blood vessels may be helpful, but it is important to remember that there is a complex interplay between multiple factors. For example, treatment with typical fluences used in dermatology can be proinflammatory, leading to tissue damage, necrosis, and posttreatment erythema. However, low-level laser therapy (LLLT) has been shown to downregulate proinflammatory mediators.15 Additionally, the presence of a large burden of inflammatory cells also may alter the effectiveness of the laser. Several case reports also the show effectiveness of both PDL and the CO2 laser in treating lesions of cutaneous sarcoidosis, especially lupus pernio.16-19 Of these 2 modalities, the use of the CO2 laser for effective remodeling of lupus pernio may be more intuitive; however, it is still important to note that the mechanism of action of several of these laser modalities is unclear with regard to the clinical benefit shown. Morphea and scleroderma also have been treated with laser therapy. It is essential to understand that in many cases, laser therapy may be targeted to treat the precise cutaneous manifestations of disease in each individual patient (eg, CO2 laser to treat disabling contractures and calcinosis cutis,20,21 PDL to treat telangiectases related to morphea22). Again, the most critical consideration is that the treatment modality should align with the cutaneous lesion being targeted.
A relatively recent development in the use of lasers has been LLLT, which refers to the use of lasers below levels where they would cause any thermal effects, thereby limiting tissue damage. Although the technology has existed for decades, there has been a recent flurry of reports extolling the many benefits of LLLT; however, the true physiologic effects of LLLT have yet to be determined, with many studies trying to elucidate its numerous effects on various signaling pathways, cell proliferation, and cellular respiration.23-26 Upon reviewing the literature, the list of cutaneous conditions that are being treated with LLLT is vast, spanning acne, vitiligo, wounds, burns, psoriasis, and alopecia, among others.15 It is important to consider that the definition of LLLT in the literature is rather broad with a wide range of wavelengths, fluences, and power densities. As such, the specific laser settings and protocols may vary considerably among different practitioners and therefore the treatment results also may vary. Nevertheless, many studies have hinted at promising results in the use of LLLT in conditions that may have previously been extremely difficult to treat (eg, alopecia). Earlier trials had demonstrated a faster resolution time in patients with alopecia areata when LLLT was added to a topical regimen27; however, the improvement was modest and lesions tended to improve with or without LLLT. Perhaps more compelling is the use of LLLT in treating androgenetic alopecia, a condition for which a satisfying facile treatment would truly carry great impact. Although physicians should be cautious of studies regarding LLLT and hair regrowth that are conducted by groups who may stand to benefit from producing such a device, the results are nonetheless notable, if only for the relative paucity of other therapeutic approaches toward this condition.28,29 A randomized, double-blind, controlled, multicenter trial showed significant improvements in median hair thickness and density with LLLT (P=.01 and P=.003, respectively), though global appearance did not change significantly.30
Laser Treatment of Skin Cancer
Lasers also have been used to treat cutaneous malignancies. Although they may be powerful in the treatment of these conditions, this treatment approach must be used with caution. As with any superficial treatment modality for skin cancer, it is difficult to ascertain if a lesion has been completely treated without any residual cancer cells, and therein lies the main caveat of laser treatment. With the use of a modality that causes a cutaneous response that may mask any underlying process, it is important to ensure that there is a reasonable degree of certainty that this treatment can effectively remove a cancerous lesion in its entirety while avoiding the theoretical risk that disturbing underlying vasculature and/or lymphatics may be modulating the ability of a cancer to metastasize. Thankfully, current evidence does not suggest that there are any downsides to laser treatment for malignancies. Clinically, we know that basal cell carcinomas (BCCs) often feature prominent vasculature, with telangiectases being used as a clinical marker to suggest the diagnosis of a BCC. Capitalizing on this aspect of the clinical lesion, PDL has been used to treat BCCs in 2 small studies with a response rate of approximately 75% for small BCCs in both studies.31,32 A recent randomized controlled trial showed significant superiority of PDL as compared to the control (P<.0001) in treatment of BCC, with nearly 80% (44/56) of cases showing histologically proven complete remission at 6-month follow-up.33 Thus, we have some promising data that suggest PDL may be a viable treatment option in BCC, especially in areas that are difficult to treat surgically.
Additionally, a newer treatment approach for BCC capitalizes on the ability of confocal microscopy to provide a feasible, bedside imaging modality to identify tumor margins. Confocal microscopy has been used as a road map to identify where and how to apply the laser treatment, thus allowing for a higher likelihood of complete destruction of the tumor, at least in theory.34 Although the concept of using confocal microscopy to guide laser treatment of skin cancer has been shown in smaller proof-of-concept case series, it remains to be seen if it is not only an efficacious approach that may be widely adopted but also whether it is pragmatic to do so, as the equipment and expertise involved in using confocal microscopy is not trivial.
Finally, lasers also have been used in the treatment of mycosis fungoides (MF), or cutaneous T-cell lymphoma. It has been suggested that this modality is an excellent treatment option as a skin-directed therapy for stage IA or IB MFs limited to the acral surfaces or MF palmaris et plantaris.35 The reasoning behind this approach was the effectiveness of narrowband UVB for early-stage MF, with an excimer laser operating at a similar wavelength (308 nm) and offering similar therapeutic benefits while limiting adverse effects to surrounding skin.36 More recently, the excimer laser was applied to a small population of 6 patients, with 3 achieving complete response, 1 with partial response, 1 with stable disease, and 1 with progressive disease. The authors were careful to point out that the excimer laser should not be thought of as a replacement for narrowband UVB in early-stage MF but rather as an adjunctive treatment of specific targeted lesional areas.36
Conclusion
Lasers are an important part of the dermatologist’s treatment arsenal. Although much attention has been focused on laser treatment for aesthetic indications, it is important not to overlook the fact that lasers also can be useful in the treatment of refractory skin diseases, as a first-line treatment in some conditions such as vascular lesions, or as an adjunctive treatment modality. There is a great deal of exciting research that may lead to new indications and a better understanding of how to best use these powerful tools, and the outlook is bright for the use of lasers in dermatology.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.
1. Bonis B, Kemeny L, Dobozy A, et al. 308 nm UVB excimer laser for psoriasis. Lancet. 1997;350:1522.
2. Fuselier HA Jr, McBurney EI, Brannan W, et al. Treatment of condylomata acuminata with carbon dioxide laser. Urology. 1980;15:265-266.
3. Mueller TJ, Carlson BA, Lindy MP. The use of the carbon dioxide surgical laser for the treatment of verrucae. J Am Podiatry Assoc. 1980;70:136-141.
4. Weyandt GH, Tollmann F, Kristen P, et al. Low risk of contamination with human papilloma virus during treatment of condylomata acuminata with multilayer argon plasma coagulation and CO2 laser ablation. Arch Dermatol Res. 2011;303:141-144.
5. Ferenczy A, Bergeron C, Richart RM. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75:114-118.
6. Anderson RR, Parrish JA. Microvasculature can be selectively damaged using dye lasers: a basic theory and experimental evidence in human skin. Lasers Surg Med. 1981:263-276.
7. Morelli JG, Tan OT, Garden J, et al. Tunable dye laser (577 nm) treatment of port wine stains. Lasers Surg Med. 1986;6:94-99.
8. Reyes BA, Geronemus R. Treatment of port-wine stains during childhood with the flashlamp-pumped pulsed dye laser. J Am Acad Dermatol. 1990;23:1142-1148.
9. Ashinoff R, Geronemus RG. Capillary hemangiomas and treatment with the flash lamp-pumped pulsed dye laser. Arch Dermatol. 1991;127:202-205.
10. Erceg A, Bovenschen HJ, van de Kerkhof PC, et al. Efficacy and safety of pulsed dye laser treatment for cutaneous discoid lupus erythematosus. J Am Acad Dermatol. 2009;60:626-632.
11. Diez MT, Boixeda P, Moreno C, et al. Histopathology and immunohistochemistry of cutaneous lupus erythematosus after pulsed dye laser treatment. Dermatol Surg. 2011;37:971-981.
12. Ekback MP, Troilius A. Laser therapy for refractory discoid lupus erythematosus when everything else has failed. J Cosmet Laser Ther. 2013;15:260-265.
13. Welsh JH, Schroeder TL, Levy ML. Granuloma faciale in a child successfully treated with the pulsed dye laser. J Am Acad Dermatol. 1999;41:351-353.
14. Cheung ST, Lanigan SW. Granuloma faciale treated with the pulsed-dye laser: a case series. Clin Exp Dermatol. 2005;30:373-375.
15. Avci P, Gupta A, Sadasivam M, et al. Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. 2013;32:41-52.
16. Roos S, Raulin C, Ockenfels HM, et al. Successful treatment of cutaneous sarcoidosis lesions with the flashlamp pumped pulsed dye laser: a case report. Dermatol Surg. 2009;35:1139-1140.
17. Cliff S, Felix RH, Singh L, et al. The successful treatment of lupus pernio with the flashlamp pulsed dye laser. J Cutan Laser Ther. 1999;1:49-52.
18. O’Donoghue NB, Barlow RJ. Laser remodelling of nodular nasal lupus pernio. Clin Exp Dermatol. 2006;31:27-29.
19. Young HS, Chalmers RJ, Griffiths CE, et al. CO2 laser vaporization for disfiguring lupus pernio. J Cosmet Laser Ther. 2002;4:87-90.
20. Kineston D, Kwan JM, Uebelhoer NS, et al. Use of a fractional ablative 10.6-mum carbon dioxide laser in the treatment of a morphea-related contracture. Arch Dermatol. 2011;147:1148-1150.
21. Chamberlain AJ, Walker NP. Successful palliation and significant remission of cutaneous calcinosis in CREST syndrome with carbon dioxide laser. Dermatol Surg. 2003;29:968-970.
22. Ciatti S, Varga J, Greenbaum SS. The 585 nm flashlamp-pumped pulsed dye laser for the treatment of telangiectases in patients with scleroderma. J Am Acad Dermatol. 1996;35:487-488.
23. Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg. 2005;23:355-361.
24. Greco M, Guida G, Perlino E, et al. Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser. Biochem Biophys Res Commun. 1989;163:1428-1434.
25. Karu TI, Pyatibrat LV, Kalendo GS. Photobiological modulation of cell attachment via cytochrome c oxidase. Photochem Photobiol Sci. 2004;3:211-216.
26. Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem. 2005;280:4761-4771.
27. Yamazaki M, Miura Y, Tsuboi R, et al. Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol. 2003;42:738-740.
28. Leavitt M, Charles G, Heyman E, et al. HairMax LaserComb laser phototherapy device in the treatment of male androgenetic alopecia: a randomized, double-blind, sham device-controlled, multicentre trial. Clin Drug Investig. 2009;29:283-292.
29. Munck A, Gavazzoni MF, Trueb RM. Use of low-level laser therapy as monotherapy or concomitant therapy for male and female androgenetic alopecia. Int J Trichology. 2014;6:45-49.
30. Kim H, Choi JW, Kim JY, et al. Low-level light therapy for androgenetic alopecia: a 24-week, randomized, double-blind, sham device-controlled multicenter trial. Dermatol Surg. 2013;39:1177-1183.
31. Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series [published online ahead of print December 13, 2012]. J Skin Cancer. 2012;2012:286480.
32. Jalian HR, Avram MM, Stankiewicz KJ, et al. Combined 585 nm pulsed-dye and 1,064 nm Nd:YAG lasers for the treatment of basal cell carcinoma. Lasers Surg Med. 2014;46:1-7.
33. Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial [published online ahead of print July 12, 2014]. Br J Dermatol. doi:10.1111/bjd.13266.
34. Chen CS, Sierra H, Cordova M, et al. Confocal microscopy-guided laser ablation for superficial and early nodular Basal cell carcinoma: a promising surgical alternative for superficial skin cancers. JAMA Dermatol. 2014;150:994-998.
35. Jin SP, Jeon YK, Cho KH, et al. Excimer laser therapy (308 nm) for mycosis fungoides palmaris et plantaris: a skin-directed and anatomically feasible treatment. Br J Dermatol. 2010;163:651-653.
36. Deaver D, Cauthen A, Cohen G, et al. Excimer laser in the treatment of mycosis fungoides. J Am Acad Dermatol. 2014;70:1058-1060.
Sulfur Spring Dermatitis
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
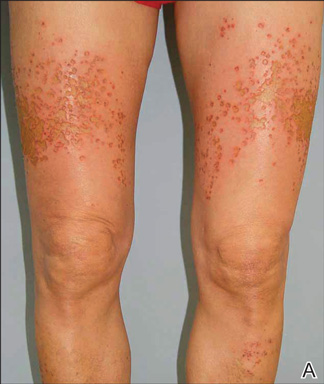
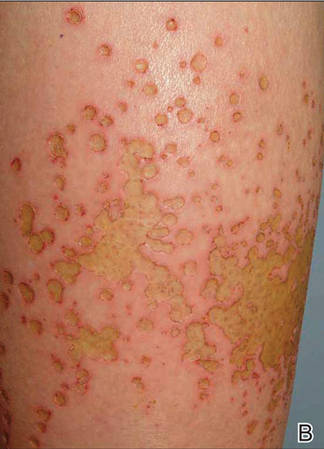
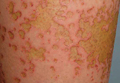
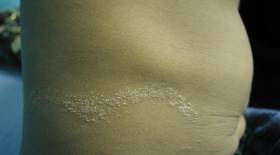
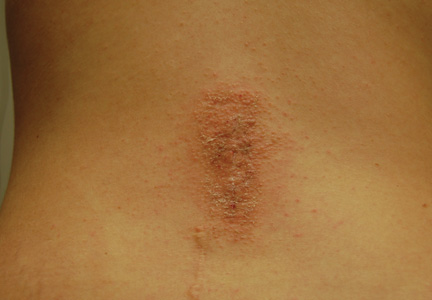
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
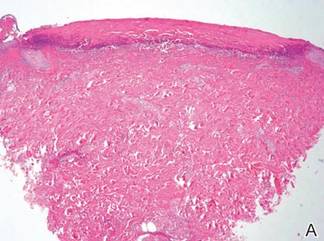
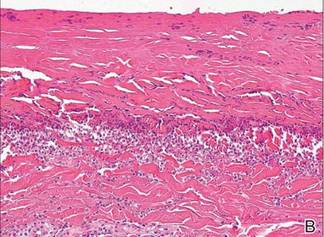
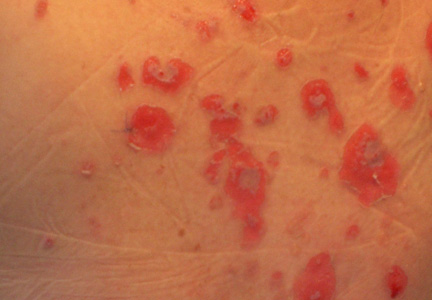
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
Sulfur spring dermatitis is characterized by multiple punched-out erosions and pits. In prior case reports, patients often presented with painful swollen lesions that developed within 24 hours of bathing in hot sulfur springs.1 Because spa therapy and thermal spring baths are common in modern society, dermatologists should be aware of sulfur spring dermatitis as a potential adverse effect.
Case Report
A healthy 65-year-old man presented with painful skin lesions on the legs that developed after bathing for 25 minutes in a hot sulfur spring 1 day prior. The patient had no history of dermatologic disease. He reported a 10-year history of bathing in a hot sulfur spring for 20 minutes every 3 days in the winter. This time, he bathed 5 minutes longer than usual. No skin condition was noted prior to bathing, but he reported feeling a tickling sensation and scratching the legs while he was immersed in the water. One hour after bathing, he noted confluent, punched-out, round ulcers with peripheral erythema on the thighs and shins (Figure 1).
|
|
A skin biopsy revealed sharply demarcated, homogeneous coagulation necrosis of the epidermis. Many neutrophils were present under the necrosis (Figure 2). Periodic acid–Schiff and acid-fast stains were negative for infectious organisms, and a skin tissue culture yielded negative results. Intensive wound care was started with nitrofurazone ointment 0.2%. The ulcers healed gradually in the following months with scar formation and hyperpigmentation.
Comment
Thermal sulfur baths are a form of balneotherapy promoted in many cultures for improvement of skin conditions; however, certain uncommon skin problems may occur after bathing in hot sulfur springs.2 In particular, sulfur spring dermatitis is a potential adverse effect.
Thermal sulfur water is known to exert anti-inflammatory, keratoplastic, and antipruriginous effects. As a result, it often is used in many cultures as an alternative treatment of various skin conditions.2-4 Moreover, thermal sulfur baths are popular in northeastern Asian countries for their effects on mental health.5 Hot springs in northern Taiwan, which contain large amounts of hydrogen sulfide, sulfate, and sulfur differ from other thermal springs in that they are rather acidic in nature and release geothermal energy from volcanic activity.6 In addition to hot sulfur springs, there are neutral salt and CO2 springs in Taiwan.5 However, spring dermatitis has only been associated with bathing in hot sulfur springs due to high concentrations of hydrogen sulfide that break down keratin and cause dissolution of the stratum corneum.7
The incidence of sulfur spring dermatitis is unknown. Although the largest known case series reported 44 cases occurring within a decade in Taiwan,1 it is rarely seen in our daily practice. Previously reported cases of sulfur spring dermatitis noted clinical findings of swelling of the affected area followed by punched-out erosions with surrounding erythema. Most lesions gradually healed with dry brownish crusts. A patch test with sulfur spring water and sulfur compounds showed negative results; therefore, the mechanism is unlikely to be allergic reaction.1 The clinical differential diagnosis includes factitious ulcers as well as viral and fungal infections. A tissue culture should be performed to exclude infectious conditions.
This characteristic skin disease does not present in all individuals after bathing in hot sulfur springs. Lesions may present anywhere on the body with a predilection for skin folds, including the penis and scrotum. Preexisting skin conditions such as pruritus and xerosis are considered to be contributing factors. The possible etiology of sulfur spring dermatitis may be acid irritation from the unstable amount of soluble sulfur in the water, which is enhanced by the heat.1 In our patient, no prior skin disease was noted, but he scratched the skin on the thighs while bathing, which may have contributed to the development of lesions in this area rather than in the skin folds.
The skin biopsy specimen demonstrated epidermal coagulation necrosis, mild superficial dermal damage, and preservation of the pilosebaceous appendages. The ulcers were painful during healing and resolved with scarring and hyperpigmentation. The histopathologic findings and clinical course in our patient were similar to cases of superficial second-degree burns.8 It is possible that the keratoplastic effect of sulfur at high concentrations along with thermal water caused the skin condition.
Conclusion
Individuals who engage in thermal sulfur baths should be aware of potential adverse effects such as sulfur spring dermatitis, especially those with preexisting skin disorders.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
1. Sun CC, Sue MS. Sulfur spring dermatitis. Contact Dermatitis. 1995;32:31-34.
2. Matz H, Orion E, Wolf R. Balneotherapy in dermatology. Dermatol Ther. 2003;16:132-140.
3. Leslie KS, Millington GW, Levell NJ. Sulphur and skin: from Satan to Saddam! J Cosmet Dermatol. 2004;3:94-98.
4. Millikan LE. Unapproved treatments or indications in dermatology: physical therapy including balneotherapy. Clin Dermatol. 2000;18:125-129.
5. Nirei H, Furuno K, Kusuda T. Medical geology in Japan. In: Selinus O, Finkelman RB, Centeno JA, eds. Medical Geology: A Regional Synthesis. New York, NY: Springer; 2010:329-354.
6. Liu CM, Song SR, Chen YL, et al. Characteristics and origins of hot springs in the Tatun Volcano Group in northern Taiwan. Terr Atmos Ocean Sci. 2011;22:475-489.
7. Lin AN, Reimer RJ, Carter DM. Sulfur revisited. J Am Acad Dermatol. 1988;18:553-558.
8. Weedon D. Reaction to physical agents. In: Weedon D. Weedon’s Skin Pathology. 3rd ed. London, England: Churchill Livingstone, Elsevier Health; 2010:525-540.
Practice Points
- The clinical findings of sulfur spring dermatitis are similar to those of a superficial second-degree burn.
- Careful evaluation of the patient’s clinical history and recognition of characteristic findings are important for correct diagnosis.
- Patients with preexisting skin disorders who engage in thermal sulfur baths should be aware of the potential adverse effect of sulfur spring dermatitis.
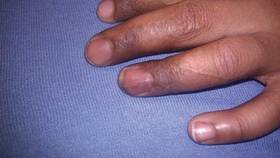
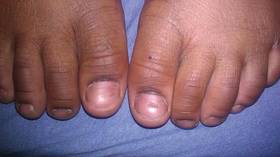
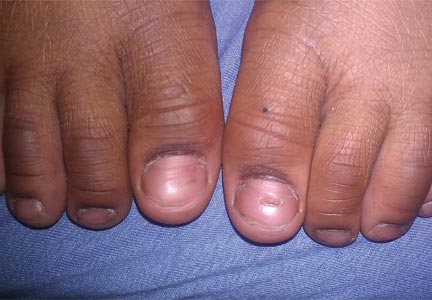
Bilateral Onychodystrophy in a Boy With a History of Isolated Lichen Striatus
Lichen striatus (LS) is a relatively rare and self-limited linear dermatosis of unknown etiology. Lichen striatus primarily affects children, with more than 50% of cases occurring in patients aged 5 to 15 years.1,2 It presents clinically as a single unilateral linear band consisting of scaly, 1- to 3-mm papules that coalesce to form long streaks.3,4 The diagnosis usually is made clinically based on the characteristic appearance of skin lesions and a pattern of distribution that follows the lines of Blaschko.5,6 The papules usually are asymptomatic; however, if the patient is symptomatic, pruritus is the most common concern. Lichen striatus may resolve with postinflammatory hyperpigmentation or hypopigmentation that may last for several months to years.
Nail involvement is uncommon in LS; a review of the literature has shown that 30 cases have been reported in the world literature since 1941.7 Nail changes may present before, after, or concurrently with the skin lesions.4,8 On rare occasions, nail involvement may be the only area of involvement without the presence of typical skin lesions.8 The involved nails may show longitudinal ridging, splitting, hyperkeratosis of the nail beds, thinning or thickening of the nail plate, nail pitting, and overcurvature of the nail plate, and rarely the nails may fall off completely.8-10
We report the case of a boy who was diagnosed with isolated LS at 2 years of age. The lesions spontaneously resolved within 6 months. Three years later the patient presented with a rare manifestation of LS in the form of bilateral onychodystrophy.
Case Report
An otherwise healthy 2-year-old boy presented for evaluation of a nonpruritic linear rash on the right lower side of the abdomen of 3 weeks’ duration. A review of systems was negative for any other constitutional signs or symptoms. No sick contacts were reported at the patient’s home, and his immunizations were up-to-date. His medical history was remarkable for a burn on the left hand from contact with a hot object at 11 months of age that required skin grafting.
Dermatologic examination revealed a linear band of small, 1- to 3-mm, flesh-colored lichenoid papules. Many of the papules had a scaly appearance and some had a vesicular component or were flat topped. The band ranged from 2- to 3-cm wide and was 25 cm in length, extending from the right anterolateral part of the lower abdomen to the right upper lateral part of the buttocks (Figure 1). No abnormalities were noted on the rest of the skin. A diagnosis of LS was made.
|
At 5 years of age, the patient returned for evaluation of bluish discoloration and thinning of the nails of the left middle and ring fingers of several months duration. The patient was afebrile and appeared to be healthy. There was no lymphadenopathy or hepatomegaly and the rest of the physical examination by a pediatrician was unremarkable. The nails of the 2 affected fingers had fallen off 2 months prior to presentation and had started to regrow. On dermatologic examination, it was noted that the regrown nails showed some residual longitudinal ridging, thinning, and dark discoloration of the proximal nail folds (Figure 2). On examination of the other toenails and fingernails there was evidence of bilateral pitting, ridging, and discoloration (Figure 3). The left great toenail was predominantly affected. The patient’s guardians were not aware of the toenail changes and denied any history of trauma to the fingers. When asked about the course of the prior abdominal linear rash, they reported that the lesions had completely resolved within 6 months. The rare diagnosis of isolated onychodystrophy as a late manifestation of the prior LS was made.
Comment
The etiology of LS remains unknown, but there have been several hypotheses suggesting environmental triggers such as trauma11 or infection.12 Others have suggested a possible autoimmune response13 or genetic components.6 Reports of simultaneous occurrences of LS in siblings as well as in a mother and her son14,15; outbreaks of LS among children who are not biologically related but in a shared living environment; and a possible seasonal variation suggest an environmental infectious agent (eg, a virus) as the possible triggering factor. However, laboratory testing for viral etiology in LS has not been helpful.
Many of the reported cases of LS have described a pattern of distribution along the lines of Blaschko.5,6,16,17 Lines of Blaschko are thought to be embryologic in origin and caused by the segmental growth of clones of cutaneous cells or the mutation-induced mosaicism of cutaneous cells, which led to the theory that mosaicism is involved in LS. Lichen striatus needs to be differentiated from other conditions with similar cutaneous appearances (eg, lichen nitidus, linear lichen planus of the digits, linear psoriasis, linear keratosis follicularis, linear epidermal nevus).
Skin biopsy to confirm the diagnosis rarely is necessary, as LS is a self-limited disorder and generally no treatment is recommended. Topical and intralesional steroids do not routinely impact the resolution of LS; however, emollients and topical steroids may be used to treat associated dryness and pruritus, if present.18 Immunomodulators such as tacrolimus and pimecrolimus have been successfully used in treating persistent and pruritic LS lesions on the face and extremities.19,20 Tacrolimus also has been successfully used to treat nail abnormalities in LS.21
Guardians and family members should be reassured that LS is a benign condition that generally resolves spontaneously within 3 to 12 months. Also, guardians should be counseled regarding the possibility of postinflammatory hyperpigmentation or hypopigmentation, which may last for several months to years, particularly in children with darker skin types. Lichen striatus of the nails may have a more protracted course, lasting from 6 months to 5 years,22 but usually resolves spontaneously and without deformity.
Our patient developed a rare case of isolated LS at 2 years of age. Reports have suggested later onset of the condition, with more than 50% of all LS cases occurring in children aged 5 to 15 years.1,2 Despite the earlier onset in our case, the patient still presented with the classic nonpruritic single linear band of papules that is characteristic of LS.
The nail involvement in our case is quite intriguing because of its rarity, timing, and extent of involvement. Nail involvement is generally uncommon in LS, with approximately 30 cases reported worldwide since 1941.7 The nail changes in our patient were unique in their timing, with the isolated onychodystrophy developing 3 years after the initial skin lesion. This subtle timing may pose a diagnostic challenge in patients with LS if treating physicians are unable to link the presenting onychodystrophy to the earlier cutaneous component of the condition. Two reports have shown that nail changes in association with LS may occur at any time before, after, or concurrently with the skin lesions,4,8 suggesting that on rare occasions, as in our case, nail involvement may be the only area of involvement without the presence of typical LS skin lesions.8
The nail involvement in our patient also showed a greater severity than prior reports,8,9 as he lost 2 fingernails completely before regrowth. Also, the bilateral distribution of onychodystrophy in our patient involving both the fingernails and toenails appeared to be consistent with a report by Al-Niaimi and Cox.22
Nail involvement in cases of LS may be underreported when, as in our case, nail dystrophy presents as the only area of involvement without the presence of the typical skin lesions characteristic of LS. It is reasonable to recommend that clinicians facing similar presentations of isolated onychodystrophy should include the possibility of LS in the differential diagnosis before committing patients to a more common diagnosis (eg, onychomycosis). Clinicians should inquire about any history of cutaneous LS and counsel patients to return for treatment should skin lesions develop that are suggestive of LS.
1. Hofer T. Lichen striatus in adults or ‘adult blaschkitis’? there is no need for a new naming. Dermatology. 2003;207:89-92.
2. Taniguchi Abagge K, Parolin Marinoni L, Giraldi S, et al. Lichen striatus: description of 89 cases in children. Pediatr Dermatol. 2004;21:440-443.
3. Hauber K, Rose C, Brocker EB, et al. Lichen striatus: clinical features and follow-up in 12 patients. Eur J Dermatol. 2000;10:536-539.
4. Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361.
5. Arias-Santiago SA, Sierra Girón-Prieto M, Fernández-Pugnarie MA, et al. Lichen striatus following Blaschko lines [published online ahead of print May 8, 2009]. An Pediatr (Barc). 2009;71:76-77.
6. Racette AJ, Adams AD, Kessler SE. Simultaneous lichen striatus in siblings along the same Blaschko line [published online ahead of print February 16, 2009]. Pediatr Dermatol. 2009;26:50-54.
7. Markouch I, Clérici T, Saiag P, et al. Lichen striatus with nail dystrophy in an infant. Ann Dermatol Venereol. 2009;136:883-886.
8. Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36:908-913.
9. Leposavic R, Belsito DV. Onychodystrophy and subungual hyperkeratosis due to lichen striatus. Arch Dermatol. 2002;138:1099-1100.
10. Baran R, Dupré A, Lauret P, et al. Lichen striatus with nail involvement. report of 4 cases and review of the 4 cases in the literature. Ann Dermatol Venereol. 1979;106:885-891.
11. Shepherd V, Lun K, Strutton G. Lichen striatus in an adult following trauma. Australas J Dermatol. 2005;46:25-28.
12. Hafner C, Landthaler M, Vogt T. Lichen striatus (blaschkitis) following varicella infection. J Eur Acad Dermatol Venereol. 2006;20:1345-1347.
13. Brennand S, Khan S, Chong AH. Lichen striatus in a pregnant woman. Australas J Dermatol. 2005;46:184-186.
14. Patrizi A, Neri I, Fiorentini C, et al. Simultaneous occurrence of lichen striatus in siblings. Pediatr Dermatol. 1997;14:293-295.
15. Yaosaka M, Sawamura D, Iitoyo M, et al. Lichen striatus affecting a mother and her son. J Am Acad Dermatol. 2005;53:352-353.
16. Keegan BR, Kamino H, Fangman W, et al. “Pediatric blaschkitis”: expanding the spectrum of childhood acquired Blaschko-linear dermatoses. Pediatr Dermatol. 2007;24:621-627.
17. Taieb A, el Youbi A, Grosshans E, et al. Lichen striatus: a Blaschko linear acquired inflammatory skin eruption. J Am Acad Dermatol. 1991;25:637-642.
18. Tilly JJ, Drolet BA, Esterly NB. Lichenoid eruptions in children. J Am Acad Dermatol. 2004;51:606-624.
19. Vukićević J, Milobratović D, Vesić S, et al. Unilateral multiple lichen striatus treated with tacrolimus ointment: a case report. Acta Dermatovenerol Alp Panonica Adriat. 2009;18:35-38.
20. Fujimoto N, Tajima S, Ishibashi A. Facial lichen striatus: successful treatment with tacrolimus ointment. Br J Dermatol. 2003;148:587-590.
21. Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617.
22. Al-Niaimi FA, Cox NH. Unilateral lichen striatus with bilateral onychodystrophy [published online ahead of print June 5, 2009]. Eur J Dermatol. 2009;19:511.
Lichen striatus (LS) is a relatively rare and self-limited linear dermatosis of unknown etiology. Lichen striatus primarily affects children, with more than 50% of cases occurring in patients aged 5 to 15 years.1,2 It presents clinically as a single unilateral linear band consisting of scaly, 1- to 3-mm papules that coalesce to form long streaks.3,4 The diagnosis usually is made clinically based on the characteristic appearance of skin lesions and a pattern of distribution that follows the lines of Blaschko.5,6 The papules usually are asymptomatic; however, if the patient is symptomatic, pruritus is the most common concern. Lichen striatus may resolve with postinflammatory hyperpigmentation or hypopigmentation that may last for several months to years.
Nail involvement is uncommon in LS; a review of the literature has shown that 30 cases have been reported in the world literature since 1941.7 Nail changes may present before, after, or concurrently with the skin lesions.4,8 On rare occasions, nail involvement may be the only area of involvement without the presence of typical skin lesions.8 The involved nails may show longitudinal ridging, splitting, hyperkeratosis of the nail beds, thinning or thickening of the nail plate, nail pitting, and overcurvature of the nail plate, and rarely the nails may fall off completely.8-10
We report the case of a boy who was diagnosed with isolated LS at 2 years of age. The lesions spontaneously resolved within 6 months. Three years later the patient presented with a rare manifestation of LS in the form of bilateral onychodystrophy.
Case Report
An otherwise healthy 2-year-old boy presented for evaluation of a nonpruritic linear rash on the right lower side of the abdomen of 3 weeks’ duration. A review of systems was negative for any other constitutional signs or symptoms. No sick contacts were reported at the patient’s home, and his immunizations were up-to-date. His medical history was remarkable for a burn on the left hand from contact with a hot object at 11 months of age that required skin grafting.
Dermatologic examination revealed a linear band of small, 1- to 3-mm, flesh-colored lichenoid papules. Many of the papules had a scaly appearance and some had a vesicular component or were flat topped. The band ranged from 2- to 3-cm wide and was 25 cm in length, extending from the right anterolateral part of the lower abdomen to the right upper lateral part of the buttocks (Figure 1). No abnormalities were noted on the rest of the skin. A diagnosis of LS was made.
|
At 5 years of age, the patient returned for evaluation of bluish discoloration and thinning of the nails of the left middle and ring fingers of several months duration. The patient was afebrile and appeared to be healthy. There was no lymphadenopathy or hepatomegaly and the rest of the physical examination by a pediatrician was unremarkable. The nails of the 2 affected fingers had fallen off 2 months prior to presentation and had started to regrow. On dermatologic examination, it was noted that the regrown nails showed some residual longitudinal ridging, thinning, and dark discoloration of the proximal nail folds (Figure 2). On examination of the other toenails and fingernails there was evidence of bilateral pitting, ridging, and discoloration (Figure 3). The left great toenail was predominantly affected. The patient’s guardians were not aware of the toenail changes and denied any history of trauma to the fingers. When asked about the course of the prior abdominal linear rash, they reported that the lesions had completely resolved within 6 months. The rare diagnosis of isolated onychodystrophy as a late manifestation of the prior LS was made.
Comment
The etiology of LS remains unknown, but there have been several hypotheses suggesting environmental triggers such as trauma11 or infection.12 Others have suggested a possible autoimmune response13 or genetic components.6 Reports of simultaneous occurrences of LS in siblings as well as in a mother and her son14,15; outbreaks of LS among children who are not biologically related but in a shared living environment; and a possible seasonal variation suggest an environmental infectious agent (eg, a virus) as the possible triggering factor. However, laboratory testing for viral etiology in LS has not been helpful.
Many of the reported cases of LS have described a pattern of distribution along the lines of Blaschko.5,6,16,17 Lines of Blaschko are thought to be embryologic in origin and caused by the segmental growth of clones of cutaneous cells or the mutation-induced mosaicism of cutaneous cells, which led to the theory that mosaicism is involved in LS. Lichen striatus needs to be differentiated from other conditions with similar cutaneous appearances (eg, lichen nitidus, linear lichen planus of the digits, linear psoriasis, linear keratosis follicularis, linear epidermal nevus).
Skin biopsy to confirm the diagnosis rarely is necessary, as LS is a self-limited disorder and generally no treatment is recommended. Topical and intralesional steroids do not routinely impact the resolution of LS; however, emollients and topical steroids may be used to treat associated dryness and pruritus, if present.18 Immunomodulators such as tacrolimus and pimecrolimus have been successfully used in treating persistent and pruritic LS lesions on the face and extremities.19,20 Tacrolimus also has been successfully used to treat nail abnormalities in LS.21
Guardians and family members should be reassured that LS is a benign condition that generally resolves spontaneously within 3 to 12 months. Also, guardians should be counseled regarding the possibility of postinflammatory hyperpigmentation or hypopigmentation, which may last for several months to years, particularly in children with darker skin types. Lichen striatus of the nails may have a more protracted course, lasting from 6 months to 5 years,22 but usually resolves spontaneously and without deformity.
Our patient developed a rare case of isolated LS at 2 years of age. Reports have suggested later onset of the condition, with more than 50% of all LS cases occurring in children aged 5 to 15 years.1,2 Despite the earlier onset in our case, the patient still presented with the classic nonpruritic single linear band of papules that is characteristic of LS.
The nail involvement in our case is quite intriguing because of its rarity, timing, and extent of involvement. Nail involvement is generally uncommon in LS, with approximately 30 cases reported worldwide since 1941.7 The nail changes in our patient were unique in their timing, with the isolated onychodystrophy developing 3 years after the initial skin lesion. This subtle timing may pose a diagnostic challenge in patients with LS if treating physicians are unable to link the presenting onychodystrophy to the earlier cutaneous component of the condition. Two reports have shown that nail changes in association with LS may occur at any time before, after, or concurrently with the skin lesions,4,8 suggesting that on rare occasions, as in our case, nail involvement may be the only area of involvement without the presence of typical LS skin lesions.8
The nail involvement in our patient also showed a greater severity than prior reports,8,9 as he lost 2 fingernails completely before regrowth. Also, the bilateral distribution of onychodystrophy in our patient involving both the fingernails and toenails appeared to be consistent with a report by Al-Niaimi and Cox.22
Nail involvement in cases of LS may be underreported when, as in our case, nail dystrophy presents as the only area of involvement without the presence of the typical skin lesions characteristic of LS. It is reasonable to recommend that clinicians facing similar presentations of isolated onychodystrophy should include the possibility of LS in the differential diagnosis before committing patients to a more common diagnosis (eg, onychomycosis). Clinicians should inquire about any history of cutaneous LS and counsel patients to return for treatment should skin lesions develop that are suggestive of LS.
Lichen striatus (LS) is a relatively rare and self-limited linear dermatosis of unknown etiology. Lichen striatus primarily affects children, with more than 50% of cases occurring in patients aged 5 to 15 years.1,2 It presents clinically as a single unilateral linear band consisting of scaly, 1- to 3-mm papules that coalesce to form long streaks.3,4 The diagnosis usually is made clinically based on the characteristic appearance of skin lesions and a pattern of distribution that follows the lines of Blaschko.5,6 The papules usually are asymptomatic; however, if the patient is symptomatic, pruritus is the most common concern. Lichen striatus may resolve with postinflammatory hyperpigmentation or hypopigmentation that may last for several months to years.
Nail involvement is uncommon in LS; a review of the literature has shown that 30 cases have been reported in the world literature since 1941.7 Nail changes may present before, after, or concurrently with the skin lesions.4,8 On rare occasions, nail involvement may be the only area of involvement without the presence of typical skin lesions.8 The involved nails may show longitudinal ridging, splitting, hyperkeratosis of the nail beds, thinning or thickening of the nail plate, nail pitting, and overcurvature of the nail plate, and rarely the nails may fall off completely.8-10
We report the case of a boy who was diagnosed with isolated LS at 2 years of age. The lesions spontaneously resolved within 6 months. Three years later the patient presented with a rare manifestation of LS in the form of bilateral onychodystrophy.
Case Report
An otherwise healthy 2-year-old boy presented for evaluation of a nonpruritic linear rash on the right lower side of the abdomen of 3 weeks’ duration. A review of systems was negative for any other constitutional signs or symptoms. No sick contacts were reported at the patient’s home, and his immunizations were up-to-date. His medical history was remarkable for a burn on the left hand from contact with a hot object at 11 months of age that required skin grafting.
Dermatologic examination revealed a linear band of small, 1- to 3-mm, flesh-colored lichenoid papules. Many of the papules had a scaly appearance and some had a vesicular component or were flat topped. The band ranged from 2- to 3-cm wide and was 25 cm in length, extending from the right anterolateral part of the lower abdomen to the right upper lateral part of the buttocks (Figure 1). No abnormalities were noted on the rest of the skin. A diagnosis of LS was made.
|
At 5 years of age, the patient returned for evaluation of bluish discoloration and thinning of the nails of the left middle and ring fingers of several months duration. The patient was afebrile and appeared to be healthy. There was no lymphadenopathy or hepatomegaly and the rest of the physical examination by a pediatrician was unremarkable. The nails of the 2 affected fingers had fallen off 2 months prior to presentation and had started to regrow. On dermatologic examination, it was noted that the regrown nails showed some residual longitudinal ridging, thinning, and dark discoloration of the proximal nail folds (Figure 2). On examination of the other toenails and fingernails there was evidence of bilateral pitting, ridging, and discoloration (Figure 3). The left great toenail was predominantly affected. The patient’s guardians were not aware of the toenail changes and denied any history of trauma to the fingers. When asked about the course of the prior abdominal linear rash, they reported that the lesions had completely resolved within 6 months. The rare diagnosis of isolated onychodystrophy as a late manifestation of the prior LS was made.
Comment
The etiology of LS remains unknown, but there have been several hypotheses suggesting environmental triggers such as trauma11 or infection.12 Others have suggested a possible autoimmune response13 or genetic components.6 Reports of simultaneous occurrences of LS in siblings as well as in a mother and her son14,15; outbreaks of LS among children who are not biologically related but in a shared living environment; and a possible seasonal variation suggest an environmental infectious agent (eg, a virus) as the possible triggering factor. However, laboratory testing for viral etiology in LS has not been helpful.
Many of the reported cases of LS have described a pattern of distribution along the lines of Blaschko.5,6,16,17 Lines of Blaschko are thought to be embryologic in origin and caused by the segmental growth of clones of cutaneous cells or the mutation-induced mosaicism of cutaneous cells, which led to the theory that mosaicism is involved in LS. Lichen striatus needs to be differentiated from other conditions with similar cutaneous appearances (eg, lichen nitidus, linear lichen planus of the digits, linear psoriasis, linear keratosis follicularis, linear epidermal nevus).
Skin biopsy to confirm the diagnosis rarely is necessary, as LS is a self-limited disorder and generally no treatment is recommended. Topical and intralesional steroids do not routinely impact the resolution of LS; however, emollients and topical steroids may be used to treat associated dryness and pruritus, if present.18 Immunomodulators such as tacrolimus and pimecrolimus have been successfully used in treating persistent and pruritic LS lesions on the face and extremities.19,20 Tacrolimus also has been successfully used to treat nail abnormalities in LS.21
Guardians and family members should be reassured that LS is a benign condition that generally resolves spontaneously within 3 to 12 months. Also, guardians should be counseled regarding the possibility of postinflammatory hyperpigmentation or hypopigmentation, which may last for several months to years, particularly in children with darker skin types. Lichen striatus of the nails may have a more protracted course, lasting from 6 months to 5 years,22 but usually resolves spontaneously and without deformity.
Our patient developed a rare case of isolated LS at 2 years of age. Reports have suggested later onset of the condition, with more than 50% of all LS cases occurring in children aged 5 to 15 years.1,2 Despite the earlier onset in our case, the patient still presented with the classic nonpruritic single linear band of papules that is characteristic of LS.
The nail involvement in our case is quite intriguing because of its rarity, timing, and extent of involvement. Nail involvement is generally uncommon in LS, with approximately 30 cases reported worldwide since 1941.7 The nail changes in our patient were unique in their timing, with the isolated onychodystrophy developing 3 years after the initial skin lesion. This subtle timing may pose a diagnostic challenge in patients with LS if treating physicians are unable to link the presenting onychodystrophy to the earlier cutaneous component of the condition. Two reports have shown that nail changes in association with LS may occur at any time before, after, or concurrently with the skin lesions,4,8 suggesting that on rare occasions, as in our case, nail involvement may be the only area of involvement without the presence of typical LS skin lesions.8
The nail involvement in our patient also showed a greater severity than prior reports,8,9 as he lost 2 fingernails completely before regrowth. Also, the bilateral distribution of onychodystrophy in our patient involving both the fingernails and toenails appeared to be consistent with a report by Al-Niaimi and Cox.22
Nail involvement in cases of LS may be underreported when, as in our case, nail dystrophy presents as the only area of involvement without the presence of the typical skin lesions characteristic of LS. It is reasonable to recommend that clinicians facing similar presentations of isolated onychodystrophy should include the possibility of LS in the differential diagnosis before committing patients to a more common diagnosis (eg, onychomycosis). Clinicians should inquire about any history of cutaneous LS and counsel patients to return for treatment should skin lesions develop that are suggestive of LS.
1. Hofer T. Lichen striatus in adults or ‘adult blaschkitis’? there is no need for a new naming. Dermatology. 2003;207:89-92.
2. Taniguchi Abagge K, Parolin Marinoni L, Giraldi S, et al. Lichen striatus: description of 89 cases in children. Pediatr Dermatol. 2004;21:440-443.
3. Hauber K, Rose C, Brocker EB, et al. Lichen striatus: clinical features and follow-up in 12 patients. Eur J Dermatol. 2000;10:536-539.
4. Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361.
5. Arias-Santiago SA, Sierra Girón-Prieto M, Fernández-Pugnarie MA, et al. Lichen striatus following Blaschko lines [published online ahead of print May 8, 2009]. An Pediatr (Barc). 2009;71:76-77.
6. Racette AJ, Adams AD, Kessler SE. Simultaneous lichen striatus in siblings along the same Blaschko line [published online ahead of print February 16, 2009]. Pediatr Dermatol. 2009;26:50-54.
7. Markouch I, Clérici T, Saiag P, et al. Lichen striatus with nail dystrophy in an infant. Ann Dermatol Venereol. 2009;136:883-886.
8. Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36:908-913.
9. Leposavic R, Belsito DV. Onychodystrophy and subungual hyperkeratosis due to lichen striatus. Arch Dermatol. 2002;138:1099-1100.
10. Baran R, Dupré A, Lauret P, et al. Lichen striatus with nail involvement. report of 4 cases and review of the 4 cases in the literature. Ann Dermatol Venereol. 1979;106:885-891.
11. Shepherd V, Lun K, Strutton G. Lichen striatus in an adult following trauma. Australas J Dermatol. 2005;46:25-28.
12. Hafner C, Landthaler M, Vogt T. Lichen striatus (blaschkitis) following varicella infection. J Eur Acad Dermatol Venereol. 2006;20:1345-1347.
13. Brennand S, Khan S, Chong AH. Lichen striatus in a pregnant woman. Australas J Dermatol. 2005;46:184-186.
14. Patrizi A, Neri I, Fiorentini C, et al. Simultaneous occurrence of lichen striatus in siblings. Pediatr Dermatol. 1997;14:293-295.
15. Yaosaka M, Sawamura D, Iitoyo M, et al. Lichen striatus affecting a mother and her son. J Am Acad Dermatol. 2005;53:352-353.
16. Keegan BR, Kamino H, Fangman W, et al. “Pediatric blaschkitis”: expanding the spectrum of childhood acquired Blaschko-linear dermatoses. Pediatr Dermatol. 2007;24:621-627.
17. Taieb A, el Youbi A, Grosshans E, et al. Lichen striatus: a Blaschko linear acquired inflammatory skin eruption. J Am Acad Dermatol. 1991;25:637-642.
18. Tilly JJ, Drolet BA, Esterly NB. Lichenoid eruptions in children. J Am Acad Dermatol. 2004;51:606-624.
19. Vukićević J, Milobratović D, Vesić S, et al. Unilateral multiple lichen striatus treated with tacrolimus ointment: a case report. Acta Dermatovenerol Alp Panonica Adriat. 2009;18:35-38.
20. Fujimoto N, Tajima S, Ishibashi A. Facial lichen striatus: successful treatment with tacrolimus ointment. Br J Dermatol. 2003;148:587-590.
21. Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617.
22. Al-Niaimi FA, Cox NH. Unilateral lichen striatus with bilateral onychodystrophy [published online ahead of print June 5, 2009]. Eur J Dermatol. 2009;19:511.
1. Hofer T. Lichen striatus in adults or ‘adult blaschkitis’? there is no need for a new naming. Dermatology. 2003;207:89-92.
2. Taniguchi Abagge K, Parolin Marinoni L, Giraldi S, et al. Lichen striatus: description of 89 cases in children. Pediatr Dermatol. 2004;21:440-443.
3. Hauber K, Rose C, Brocker EB, et al. Lichen striatus: clinical features and follow-up in 12 patients. Eur J Dermatol. 2000;10:536-539.
4. Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361.
5. Arias-Santiago SA, Sierra Girón-Prieto M, Fernández-Pugnarie MA, et al. Lichen striatus following Blaschko lines [published online ahead of print May 8, 2009]. An Pediatr (Barc). 2009;71:76-77.
6. Racette AJ, Adams AD, Kessler SE. Simultaneous lichen striatus in siblings along the same Blaschko line [published online ahead of print February 16, 2009]. Pediatr Dermatol. 2009;26:50-54.
7. Markouch I, Clérici T, Saiag P, et al. Lichen striatus with nail dystrophy in an infant. Ann Dermatol Venereol. 2009;136:883-886.
8. Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36:908-913.
9. Leposavic R, Belsito DV. Onychodystrophy and subungual hyperkeratosis due to lichen striatus. Arch Dermatol. 2002;138:1099-1100.
10. Baran R, Dupré A, Lauret P, et al. Lichen striatus with nail involvement. report of 4 cases and review of the 4 cases in the literature. Ann Dermatol Venereol. 1979;106:885-891.
11. Shepherd V, Lun K, Strutton G. Lichen striatus in an adult following trauma. Australas J Dermatol. 2005;46:25-28.
12. Hafner C, Landthaler M, Vogt T. Lichen striatus (blaschkitis) following varicella infection. J Eur Acad Dermatol Venereol. 2006;20:1345-1347.
13. Brennand S, Khan S, Chong AH. Lichen striatus in a pregnant woman. Australas J Dermatol. 2005;46:184-186.
14. Patrizi A, Neri I, Fiorentini C, et al. Simultaneous occurrence of lichen striatus in siblings. Pediatr Dermatol. 1997;14:293-295.
15. Yaosaka M, Sawamura D, Iitoyo M, et al. Lichen striatus affecting a mother and her son. J Am Acad Dermatol. 2005;53:352-353.
16. Keegan BR, Kamino H, Fangman W, et al. “Pediatric blaschkitis”: expanding the spectrum of childhood acquired Blaschko-linear dermatoses. Pediatr Dermatol. 2007;24:621-627.
17. Taieb A, el Youbi A, Grosshans E, et al. Lichen striatus: a Blaschko linear acquired inflammatory skin eruption. J Am Acad Dermatol. 1991;25:637-642.
18. Tilly JJ, Drolet BA, Esterly NB. Lichenoid eruptions in children. J Am Acad Dermatol. 2004;51:606-624.
19. Vukićević J, Milobratović D, Vesić S, et al. Unilateral multiple lichen striatus treated with tacrolimus ointment: a case report. Acta Dermatovenerol Alp Panonica Adriat. 2009;18:35-38.
20. Fujimoto N, Tajima S, Ishibashi A. Facial lichen striatus: successful treatment with tacrolimus ointment. Br J Dermatol. 2003;148:587-590.
21. Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617.
22. Al-Niaimi FA, Cox NH. Unilateral lichen striatus with bilateral onychodystrophy [published online ahead of print June 5, 2009]. Eur J Dermatol. 2009;19:511.
Practice Points
- Lichen striatus (LS) is a relatively rare and self-limited linear dermatosis of unknown etiology and diagnosis usually is made clinically.
- Nail involvement is uncommon in LS but also may be underreported. When present, nail changes may appear before, after, or concurrently with skin lesions.
- If a patient presents with a similar case of isolated onychodystrophy, the clinician should inquire about history of cutaneous LS and should consider the possibility of LS in the differential diagnosis.
Photosensitivity Reaction From Dronedarone for Atrial Fibrillation
To the Editor:
A 61-year-old woman with a history of atrial fibrillation, type 2 diabetes mellitus, and hyperlipidemia presented with an erythematous, edematous, pruritic eruption on the chest, neck, and arms of 2 weeks’ duration. The patient had no history of considerable sun exposure or reports of photosensitivity. One month prior to presentation she had started taking dronedarone for improved control of atrial fibrillation. She had no known history of drug allergies. Other medications included valsartan, digoxin, pioglitazone, simvastatin, aspirin, hydrocodone, and zolpidem, all of which were unchanged for years. There were no changes in topical products used.
Physical examination revealed confluent, well-demarcated, erythematous and edematous papules and plaques over the anterior aspect of the neck, bilateral forearms, and dorsal aspect of the hands, with a v-shaped distribution on the chest (Figure). There was notable sparing of the submental region, upper arms, abdomen, back, and legs. Dronedarone was discontinued and she was started on fluocinonide ointment 0.05% and oral hydroxyzine for pruritus. Her rash resolved within the following few weeks.
|
|
| Confluent, well-demarcated, erythematous and edematous papules and plaques in a v-shaped distribution on the chest (A) and dorsal aspect of the hands (B). |
Dronedarone is a noniodinated benzofuran derivative. It is structurally similar to and shares the antiarrhythmic properties of amiodarone,1 and thus it is used in the treatment of atrial fibrillation and atrial flutter. However, the pulmonary and thyroid toxicities sometimes associated with amiodarone have not been observed with dronedarone. The primary side effect of dronedarone is gastrointestinal distress, specifically nausea, vomiting, and diarrhea. Dronedarone has been associated with severe liver injury and hepatic failure.2 Cutaneous reactions appear to be an uncommon side effect of dronedarone therapy. Across 5 clinical studies (N=6285), adverse events involving skin and subcutaneous tissue including eczema, allergic dermatitis, pruritus, and nonspecific rash occurred in 5% of dronedarone and 3% of placebo patients. Photosensitivity reactions occurred in less than 1% of dronedarone recipients.3
Although the lack of a biopsy leaves the possibility of a contact or photocontact dermatitis, our patient demonstrated the potential for dronedarone to cause a photodistributed drug eruption that resolved after cessation of the medication.
1. Hoy SM, Keam SJ. Dronedarone. Drugs. 2009;69:1647-1663.
2. In brief: FDA warning on dronedarone (Multaq). Med Lett Drugs Ther. 2011;53:17.
3. Multaq [package insert]. Bridgewater, NJ: sanofi-aventis; 2009.
To the Editor:
A 61-year-old woman with a history of atrial fibrillation, type 2 diabetes mellitus, and hyperlipidemia presented with an erythematous, edematous, pruritic eruption on the chest, neck, and arms of 2 weeks’ duration. The patient had no history of considerable sun exposure or reports of photosensitivity. One month prior to presentation she had started taking dronedarone for improved control of atrial fibrillation. She had no known history of drug allergies. Other medications included valsartan, digoxin, pioglitazone, simvastatin, aspirin, hydrocodone, and zolpidem, all of which were unchanged for years. There were no changes in topical products used.
Physical examination revealed confluent, well-demarcated, erythematous and edematous papules and plaques over the anterior aspect of the neck, bilateral forearms, and dorsal aspect of the hands, with a v-shaped distribution on the chest (Figure). There was notable sparing of the submental region, upper arms, abdomen, back, and legs. Dronedarone was discontinued and she was started on fluocinonide ointment 0.05% and oral hydroxyzine for pruritus. Her rash resolved within the following few weeks.
|
|
| Confluent, well-demarcated, erythematous and edematous papules and plaques in a v-shaped distribution on the chest (A) and dorsal aspect of the hands (B). |
Dronedarone is a noniodinated benzofuran derivative. It is structurally similar to and shares the antiarrhythmic properties of amiodarone,1 and thus it is used in the treatment of atrial fibrillation and atrial flutter. However, the pulmonary and thyroid toxicities sometimes associated with amiodarone have not been observed with dronedarone. The primary side effect of dronedarone is gastrointestinal distress, specifically nausea, vomiting, and diarrhea. Dronedarone has been associated with severe liver injury and hepatic failure.2 Cutaneous reactions appear to be an uncommon side effect of dronedarone therapy. Across 5 clinical studies (N=6285), adverse events involving skin and subcutaneous tissue including eczema, allergic dermatitis, pruritus, and nonspecific rash occurred in 5% of dronedarone and 3% of placebo patients. Photosensitivity reactions occurred in less than 1% of dronedarone recipients.3
Although the lack of a biopsy leaves the possibility of a contact or photocontact dermatitis, our patient demonstrated the potential for dronedarone to cause a photodistributed drug eruption that resolved after cessation of the medication.
To the Editor:
A 61-year-old woman with a history of atrial fibrillation, type 2 diabetes mellitus, and hyperlipidemia presented with an erythematous, edematous, pruritic eruption on the chest, neck, and arms of 2 weeks’ duration. The patient had no history of considerable sun exposure or reports of photosensitivity. One month prior to presentation she had started taking dronedarone for improved control of atrial fibrillation. She had no known history of drug allergies. Other medications included valsartan, digoxin, pioglitazone, simvastatin, aspirin, hydrocodone, and zolpidem, all of which were unchanged for years. There were no changes in topical products used.
Physical examination revealed confluent, well-demarcated, erythematous and edematous papules and plaques over the anterior aspect of the neck, bilateral forearms, and dorsal aspect of the hands, with a v-shaped distribution on the chest (Figure). There was notable sparing of the submental region, upper arms, abdomen, back, and legs. Dronedarone was discontinued and she was started on fluocinonide ointment 0.05% and oral hydroxyzine for pruritus. Her rash resolved within the following few weeks.
|
|
| Confluent, well-demarcated, erythematous and edematous papules and plaques in a v-shaped distribution on the chest (A) and dorsal aspect of the hands (B). |
Dronedarone is a noniodinated benzofuran derivative. It is structurally similar to and shares the antiarrhythmic properties of amiodarone,1 and thus it is used in the treatment of atrial fibrillation and atrial flutter. However, the pulmonary and thyroid toxicities sometimes associated with amiodarone have not been observed with dronedarone. The primary side effect of dronedarone is gastrointestinal distress, specifically nausea, vomiting, and diarrhea. Dronedarone has been associated with severe liver injury and hepatic failure.2 Cutaneous reactions appear to be an uncommon side effect of dronedarone therapy. Across 5 clinical studies (N=6285), adverse events involving skin and subcutaneous tissue including eczema, allergic dermatitis, pruritus, and nonspecific rash occurred in 5% of dronedarone and 3% of placebo patients. Photosensitivity reactions occurred in less than 1% of dronedarone recipients.3
Although the lack of a biopsy leaves the possibility of a contact or photocontact dermatitis, our patient demonstrated the potential for dronedarone to cause a photodistributed drug eruption that resolved after cessation of the medication.
1. Hoy SM, Keam SJ. Dronedarone. Drugs. 2009;69:1647-1663.
2. In brief: FDA warning on dronedarone (Multaq). Med Lett Drugs Ther. 2011;53:17.
3. Multaq [package insert]. Bridgewater, NJ: sanofi-aventis; 2009.
1. Hoy SM, Keam SJ. Dronedarone. Drugs. 2009;69:1647-1663.
2. In brief: FDA warning on dronedarone (Multaq). Med Lett Drugs Ther. 2011;53:17.
3. Multaq [package insert]. Bridgewater, NJ: sanofi-aventis; 2009.
Practice Question Answers: Vulvar Diseases, Part 1
1. The risk for subsequently developing squamous cell carcinoma in situ of the vulva is most strongly associated with:
a. candidiasis
b. cicatricial pemphigoid
c. lichen planus
d. lichen sclerosus
e. recurrent Trichomonas infections
2. Vitamin D supplements and topical antibiotics commonly are used to treat:
a. desquamative inflammatory vaginitis
b. dysesthetic vulvodynia
c. human papillomavirus–related severe squamous dysplasia of the vulva and vagina
d. lichen sclerosus
e. psoriasis
3. A 28-year-old diabetic woman presented to your clinic with well-developed vulvar pruritus. She was known to have an implanted copper intrauterine device. A Papanicolaou test would most likely reveal:
a. bacteria
b. herpetic virocytes
c. high-grade dysplastic squamous cells
d. koilocytic squamous cells
e. pseudohyphae
4. A 54-year-old woman with Sjögren syndrome and atrophic gastritis presented to your clinic with vulvar pruritus. Atrophy of the skin and mucosa with fissures was clinically suggestive of:
a. candidiasis
b. dysesthetic vulvodynia
c. lichen sclerosus
d. lichen simplex chronicus
e. psoriasis
5. A 48-year-old woman was referred to your clinic for evaluation of persistent burning vulvar pain of 3 months’ duration. She said she felt tired most of the time. On physical examination the vulva looked normal. Commonly this condition is associated with:
a. diabetes mellitus
b. fibromyalgia
c. hypothyroidism
d. iron deficiency anemia
e. psoriasis
1. The risk for subsequently developing squamous cell carcinoma in situ of the vulva is most strongly associated with:
a. candidiasis
b. cicatricial pemphigoid
c. lichen planus
d. lichen sclerosus
e. recurrent Trichomonas infections
2. Vitamin D supplements and topical antibiotics commonly are used to treat:
a. desquamative inflammatory vaginitis
b. dysesthetic vulvodynia
c. human papillomavirus–related severe squamous dysplasia of the vulva and vagina
d. lichen sclerosus
e. psoriasis
3. A 28-year-old diabetic woman presented to your clinic with well-developed vulvar pruritus. She was known to have an implanted copper intrauterine device. A Papanicolaou test would most likely reveal:
a. bacteria
b. herpetic virocytes
c. high-grade dysplastic squamous cells
d. koilocytic squamous cells
e. pseudohyphae
4. A 54-year-old woman with Sjögren syndrome and atrophic gastritis presented to your clinic with vulvar pruritus. Atrophy of the skin and mucosa with fissures was clinically suggestive of:
a. candidiasis
b. dysesthetic vulvodynia
c. lichen sclerosus
d. lichen simplex chronicus
e. psoriasis
5. A 48-year-old woman was referred to your clinic for evaluation of persistent burning vulvar pain of 3 months’ duration. She said she felt tired most of the time. On physical examination the vulva looked normal. Commonly this condition is associated with:
a. diabetes mellitus
b. fibromyalgia
c. hypothyroidism
d. iron deficiency anemia
e. psoriasis
1. The risk for subsequently developing squamous cell carcinoma in situ of the vulva is most strongly associated with:
a. candidiasis
b. cicatricial pemphigoid
c. lichen planus
d. lichen sclerosus
e. recurrent Trichomonas infections
2. Vitamin D supplements and topical antibiotics commonly are used to treat:
a. desquamative inflammatory vaginitis
b. dysesthetic vulvodynia
c. human papillomavirus–related severe squamous dysplasia of the vulva and vagina
d. lichen sclerosus
e. psoriasis
3. A 28-year-old diabetic woman presented to your clinic with well-developed vulvar pruritus. She was known to have an implanted copper intrauterine device. A Papanicolaou test would most likely reveal:
a. bacteria
b. herpetic virocytes
c. high-grade dysplastic squamous cells
d. koilocytic squamous cells
e. pseudohyphae
4. A 54-year-old woman with Sjögren syndrome and atrophic gastritis presented to your clinic with vulvar pruritus. Atrophy of the skin and mucosa with fissures was clinically suggestive of:
a. candidiasis
b. dysesthetic vulvodynia
c. lichen sclerosus
d. lichen simplex chronicus
e. psoriasis
5. A 48-year-old woman was referred to your clinic for evaluation of persistent burning vulvar pain of 3 months’ duration. She said she felt tired most of the time. On physical examination the vulva looked normal. Commonly this condition is associated with:
a. diabetes mellitus
b. fibromyalgia
c. hypothyroidism
d. iron deficiency anemia
e. psoriasis
Vulvar Diseases, Part 1
Allergic Contact Dermatitis to 2-Octyl Cyanoacrylate
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.

| 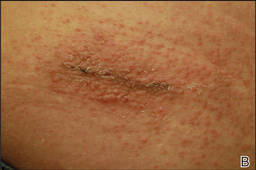
|
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.
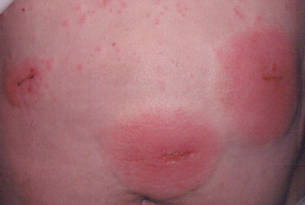
|
| Figure 2. Acute eczematous plaques at wound closures. |
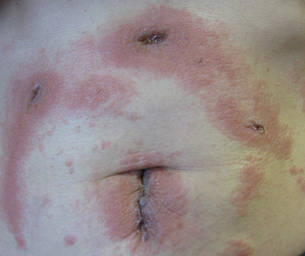
|
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.

| 
|
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.

|
| Figure 2. Acute eczematous plaques at wound closures. |

|
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.

| 
|
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.

|
| Figure 2. Acute eczematous plaques at wound closures. |

|
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
Practice Points
- It is important for physicians to recognize that skin adhesives are a potential source of allergic contact dermatitis (ACD) in a postsurgical setting.
- There are 3 primary components of skin adhesives that are potential contactants, including a cyanoacrylate, a plasticizer, and a dye.
- Treatment of ACD to skin adhesives is straightforward, including removal of any remaining adhesive and applying topical steroids.
Pemphigus Vulgaris in Pregnancy
Pemphigus vulgaris (PV) is a rare autoimmune bullous dermatosis that has not shown a predilection toward a particular race or sex.1 Autoantibodies for desmoglein 1 and desmoglein 3, members of the cadherin family that are involved in cellular adhesion, have been linked to the pathogenesis of PV.2 These autoantibodies play a role in the loss of cell-to-cell adhesion in the basal and suprabasal layers of the deep epidermis while cellular adhesion in the superficial epidermis remains intact, leading to the clinical presentation of epidermal blistering and ulcerations most commonly found on the scalp, face, groin, and axillae. Diagnosis typically is made based on skin biopsy and confirmed by direct immunofluorescence. Histologically, PV displays acantholysis and suprabasal cleft formation. Immunofluorescence may show IgG antibodies against the PV antigen in the epidermis.3 Once a diagnosis has been made, treatment typically consists of systemic steroids, as the use of steroids has had great effect in preventing infections, sepsis, and fatality that were once associated with PV.4 Mortality rates associated with PV have decreased to 10% to 15% with systemic steroids from a mortality rate as high as 70% in the presteroid era.1,5 Treatment of PV during pregnancy, as in our patient, requires obstetric and pediatric consultations before therapy is initiated. Use of corticosteroids during pregnancy can be potentially dangerous to the fetus, particularly if high doses are necessary to control maternal disease.6,7
Case Report
A 34-year-old pregnant woman at 6 weeks’ gestation presented with widespread blistering dermatitis and associated burning and pruritus. Her obstetrical history was gravida 3, para 2. The patient reported a “rash” on the scalp that had developed 9 months prior. She had been treated as an outpatient at an outside institution with topical antibiotics and antifungal medications, yet the dermatitis progressed. Three weeks prior to hospitalization, the rash was present on the skin and mucosal surfaces, including the groin, chest, face, hard palate, buccal mucosa, lips (Figure 1), and back (Figure 2). Nontender bullae ruptured after 3 days, releasing clear, yellow, serous fluid with associated burning and pruritus. The bullae were hemorrhagic and erythematous at the base.
|
| Figure 1. Facial involvement with bullae, crusted hemorrhagic lesions, and eschar in a 34-year-old pregnant woman. |
|
| Figure 2. Involvement of the back with bullae in various stages. Some bullae were intact while others newly erupted. |
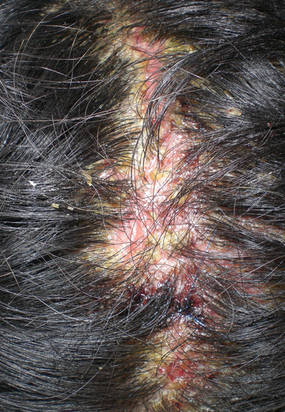
|
| Figure 3. Superinfected and flaking scalp. |
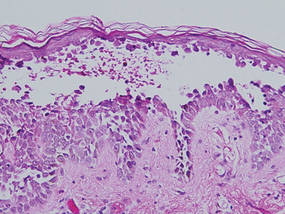
|
| Figure 4. Biopsy revealed suprabasal acantholysis with a tombstone effect of residual basal cells (H&E, original magnification ×200). |
At the current presentation, the patient had several excoriated 1- to 2-cm oval denudations; some were crusted with eschar. Nikolsky sign was negative. Multiple confluent bullous lesions had erupted on the entire scalp with a thick, impetiginous, yellow crust. She had a wet, boggy, foul-smelling, superinfected scalp that was mildly tender to touch with flaking tissue debris (Figure 3). A white blood cell count was 13.2×109/L (reference range, 4.5–11.0×109/L) with 5% eosinophils (reference range, 2.7%). The differential diagnosis included bullous impetigo, pemphigoid, Stevens-Johnson syndrome, dermatitis herpetiformis, and pemphigus vulgaris.
Biopsies of the scalp and back were taken and showed suprabasal acantholysis with a tombstone effect of residual basal cells standing up on the basement membrane without the characteristic acantholysis into skin appendages (Figure 4). The acantholytic cells in the bullous chamber did not round up as in Hailey-Hailey disease nor was there the dyskeratosis of Grover disease. Direct immunofluorescence on an elbow punch biopsy found diffuse 1+ intercellular IgG in the epidermis and diffuse 1+ basal intercellular C3, and was negative for IgA, IgM, and C1q, thus confirming a diagnosis of PV.
The patient was started on prednisone 20 mg once daily. An increase to prednisone 60 mg led to initial improvement of symptoms, but there was a relapse after several days, which is typical of PV in pregnancy,7 prompting the dose to be increased to 120 mg. Following alleviation of symptoms, the dose was later tapered back to 60 mg. No lesions were present at discharge or for 2.5 months thereafter, as the prednisone was tapered from 60 to 45 mg daily after discharge.
On follow-up, the patient’s PV was well controlled, but the prednisone dose was back up to 60 mg daily because of 2 new skin lesions that had developed since her last visit 2.5 months prior. Ultrasonography showed no fetal abnormalities as the pregnancy progressed to 28 weeks’ gestation. The patient developed hypertension and went into premature labor due to placenta previa. The neonate showed no skin lesions or anomalies while in the neonatal intensive care unit. The mother’s prednisone dose was tapered from 60 to 20 mg daily while the white blood cell count was 7.1×109/L with 2% eosinophils and a new scalp lesion appeared. Seven months after her initial discharge from the hospital for the dermatologic condition, she was no longer nursing and azathioprine was added to prednisone 60 mg daily.
Comment
Pemphigus vulgaris is associated with infertility in its active phase; therefore, PV during pregnancy is rare.8 Pregnancy may exacerbate PV, which has been a similar finding in other well-documented autoimmune diseases.7 One review of PV in pregnancy reported that 11 of 49 patients (22%) experienced an exacerbation of the disease.8 This finding pre-sents 2 problems: (1) severe active disease during pregnancy with high antibody titers has been shown to heighten risk for morbidity and mortality for the fetus, and (2) a patient with active PV during pregnancy may require systemic therapy with doses high enough to subdue the disease. The presence of PV was a challenge throughout our patient’s pregnancy. Transient skin lesions may occasionally appear in the neonate and seem to have an increased association with severe active PV in the mother; however, neonatal PV also has been present in mild cases in the mother.7 These lesions are secondary to passive transplacental transfer of PV antibodies but do not have long-lasting clinical implications because of an antibody’s brief half-life.9 The lesions either spontaneously resolve or can be treated with a topical corticosteroid.
Treatment with high-dose systemic corticosteroids or immunosuppressants can be problematic because of the risks posed to the fetus, especially if the mother must be treated when the embryo is particularly susceptible (eg, during organogenesis).10 If a woman with known PV is planning to become pregnant, it is recommended to first control and suppress the disease so that therapy can be minimal during the pregnancy. It also is recommended to use aggressive topical therapy if possible to control PV in a pregnant woman.8 This option would not have been efficacious in our patient because of her severe widespread disease.
Prednisone is considered one of the first-line treatments of PV and has been historically successful as a treatment for pregnant patients with PV if maintained at a low dosage. Prednisone, similar to other corticosteroids, can cross the placental barrier and can increase the chance of premature birth, infection, and mortality in high doses.7 Similar to prednisone, azathioprine is not recommended during pregnancy, but if use is necessary, it is suggested to keep the dose low to prevent fetal harm.11 Inadequate treatment and control of PV can be life threatening to the patient because of the severe infection that may ensue; thus it is necessary for the health of the patient and fetus to suppress the PV. One alternative to treatment with steroids and immunosuppressants is plasma exchange, which has been successful in the clinical context of pregnancy.12 The cons of plasma exchange are repeat procedures, the need to give the patient more immunosuppressants to prevent a rejection, and the return of the autoantibody.7
Several studies have evaluated the safety and efficacy of rituximab in the treatment of refractory PV. Multiple case reports state that both 1 and 2 courses of intravenous rituximab therapy at a dosage of 375 mg per square meter of body surface area affected once weekly for 4 weeks proved to be useful in clinical improvement for patients with refractory disease.13,14 Studies are currently underway to look at the effects of rituximab on pregnancy and the fetus. Preliminary findings show neonates may have B-cell abnormalities initially yet recover fully without infectious complications or sequelae.15 Rituximab currently is a pregnancy category C drug, and women are counseled to avoid pregnancy for at least 12 months after rituximab exposure and use contraception while actively taking the drug.16
Conclusion
Contrary to traditional thinking, PV itself may be associated with poor neonatal outcome, including prematurity and fetal death. These complications seem to be restricted to pregnancies with clinically severe PV.7 Our patient decided to progress with her pregnancy despite the potential risk to the fetus from the disease and treatment. Ultimately, the infant was delivered prematurely but was free of disease.
1. Fainaru O, Mashiach R, Kupferminc M, et al. Pemphigus vulgaris in pregnancy: a case report and review of literature. Hum Reprod. 2000;15:1195-1197.
2. Joly P, Gilbert D, Thomine E, et al. Identification of a new antibody population directed against a desmosomal plaque antigen in pemphigus vulgaris and pemphigus foliaceus. J Invest Dermatol. 1997;108:469-475.
3. Daniel Y, Shenhav M, Botchan A, et al. Pregnancy associated with pemphigus. Br J Obstet Gynecol. 1995;102:667-669.
4. Ruach M, Ohel G, Rahav D, et al. Pemphigus vulgaris and pregnancy. Obstet Gynecol Surv. 1995;50:755-760.
5. Carson PJ, Hameed A, Ahmed AR. Influence of treatment on clinical course of pemphigus vulgaris. J Am Acad Dermatol. 1996;34:645-652.
6. Goldberg NS, DeFeo C, Kirshenbaum N. Pemphigus and pregnancy: risk factors and recommendations. J Am Acad Dermatol. 1993;28(5, pt 2):877-879.
7. Lehman JS, Mueller KK, Schraith DF. Do safe and effective treatment options exist for patients with active pemphigus vulgaris who plan conception and pregnancy? Arch Dermatol. 2008;144:783-785.
8. Kardos M, Levine D, Gurcan H, et al. Pemphigus vulgaris in pregnancy: analysis of current data on the management and outcomes. Obstet Gynecol Surv. 2009;64:739-749.
9. Fenniche S, Benmously R, Marrak H, et al. Neonatal pemphigus vulgaris in an infant born to a mother with pemphigus vulgaris in remission. Pediatr Dermatol. 2006;23:124-127.
10. Kalayciyan A, Engin B, Serdaroglu S, et al. A retrospective analysis of patients with pemphigus vulgaris associated with pregnancy. Br J Dermatol. 2002;147:396-397.
11. Hup JM, Bruinsma RA, Boersma ER, et al. Neonatal pemphigus vulgaris: transplacental transmission of antibodies. Pediatr Dermatol. 1986;3:468-472.
12. Piontek JO, Borberg H, Sollberg S, et al. Severe exacerbation of pemphigus vulgaris in pregnancy: successful treatment with plasma exchange. Br J Dermatol. 2000;143:455-456.
13. Faurschou A, Gniadecki R. Two courses of rituximab (anti-CD20 monoclonal antibody) for recalcitrant pemphigus vulgaris. Int J Dermatol. 2008;47:292-294.
14. Marzano AV, Fanoni D, Venegoni L, et al. Treatment of refractory pemphigus with the anti-CD20 monoclonal antibody (rituximab). Dermatology. 2007;214:310-318.
15. Braunstein I, Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther. 2013;26:354-363.
16. Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506.
Pemphigus vulgaris (PV) is a rare autoimmune bullous dermatosis that has not shown a predilection toward a particular race or sex.1 Autoantibodies for desmoglein 1 and desmoglein 3, members of the cadherin family that are involved in cellular adhesion, have been linked to the pathogenesis of PV.2 These autoantibodies play a role in the loss of cell-to-cell adhesion in the basal and suprabasal layers of the deep epidermis while cellular adhesion in the superficial epidermis remains intact, leading to the clinical presentation of epidermal blistering and ulcerations most commonly found on the scalp, face, groin, and axillae. Diagnosis typically is made based on skin biopsy and confirmed by direct immunofluorescence. Histologically, PV displays acantholysis and suprabasal cleft formation. Immunofluorescence may show IgG antibodies against the PV antigen in the epidermis.3 Once a diagnosis has been made, treatment typically consists of systemic steroids, as the use of steroids has had great effect in preventing infections, sepsis, and fatality that were once associated with PV.4 Mortality rates associated with PV have decreased to 10% to 15% with systemic steroids from a mortality rate as high as 70% in the presteroid era.1,5 Treatment of PV during pregnancy, as in our patient, requires obstetric and pediatric consultations before therapy is initiated. Use of corticosteroids during pregnancy can be potentially dangerous to the fetus, particularly if high doses are necessary to control maternal disease.6,7
Case Report
A 34-year-old pregnant woman at 6 weeks’ gestation presented with widespread blistering dermatitis and associated burning and pruritus. Her obstetrical history was gravida 3, para 2. The patient reported a “rash” on the scalp that had developed 9 months prior. She had been treated as an outpatient at an outside institution with topical antibiotics and antifungal medications, yet the dermatitis progressed. Three weeks prior to hospitalization, the rash was present on the skin and mucosal surfaces, including the groin, chest, face, hard palate, buccal mucosa, lips (Figure 1), and back (Figure 2). Nontender bullae ruptured after 3 days, releasing clear, yellow, serous fluid with associated burning and pruritus. The bullae were hemorrhagic and erythematous at the base.

|
| Figure 1. Facial involvement with bullae, crusted hemorrhagic lesions, and eschar in a 34-year-old pregnant woman. |

|
| Figure 2. Involvement of the back with bullae in various stages. Some bullae were intact while others newly erupted. |

|
| Figure 3. Superinfected and flaking scalp. |

|
| Figure 4. Biopsy revealed suprabasal acantholysis with a tombstone effect of residual basal cells (H&E, original magnification ×200). |
At the current presentation, the patient had several excoriated 1- to 2-cm oval denudations; some were crusted with eschar. Nikolsky sign was negative. Multiple confluent bullous lesions had erupted on the entire scalp with a thick, impetiginous, yellow crust. She had a wet, boggy, foul-smelling, superinfected scalp that was mildly tender to touch with flaking tissue debris (Figure 3). A white blood cell count was 13.2×109/L (reference range, 4.5–11.0×109/L) with 5% eosinophils (reference range, 2.7%). The differential diagnosis included bullous impetigo, pemphigoid, Stevens-Johnson syndrome, dermatitis herpetiformis, and pemphigus vulgaris.
Biopsies of the scalp and back were taken and showed suprabasal acantholysis with a tombstone effect of residual basal cells standing up on the basement membrane without the characteristic acantholysis into skin appendages (Figure 4). The acantholytic cells in the bullous chamber did not round up as in Hailey-Hailey disease nor was there the dyskeratosis of Grover disease. Direct immunofluorescence on an elbow punch biopsy found diffuse 1+ intercellular IgG in the epidermis and diffuse 1+ basal intercellular C3, and was negative for IgA, IgM, and C1q, thus confirming a diagnosis of PV.
The patient was started on prednisone 20 mg once daily. An increase to prednisone 60 mg led to initial improvement of symptoms, but there was a relapse after several days, which is typical of PV in pregnancy,7 prompting the dose to be increased to 120 mg. Following alleviation of symptoms, the dose was later tapered back to 60 mg. No lesions were present at discharge or for 2.5 months thereafter, as the prednisone was tapered from 60 to 45 mg daily after discharge.
On follow-up, the patient’s PV was well controlled, but the prednisone dose was back up to 60 mg daily because of 2 new skin lesions that had developed since her last visit 2.5 months prior. Ultrasonography showed no fetal abnormalities as the pregnancy progressed to 28 weeks’ gestation. The patient developed hypertension and went into premature labor due to placenta previa. The neonate showed no skin lesions or anomalies while in the neonatal intensive care unit. The mother’s prednisone dose was tapered from 60 to 20 mg daily while the white blood cell count was 7.1×109/L with 2% eosinophils and a new scalp lesion appeared. Seven months after her initial discharge from the hospital for the dermatologic condition, she was no longer nursing and azathioprine was added to prednisone 60 mg daily.
Comment
Pemphigus vulgaris is associated with infertility in its active phase; therefore, PV during pregnancy is rare.8 Pregnancy may exacerbate PV, which has been a similar finding in other well-documented autoimmune diseases.7 One review of PV in pregnancy reported that 11 of 49 patients (22%) experienced an exacerbation of the disease.8 This finding pre-sents 2 problems: (1) severe active disease during pregnancy with high antibody titers has been shown to heighten risk for morbidity and mortality for the fetus, and (2) a patient with active PV during pregnancy may require systemic therapy with doses high enough to subdue the disease. The presence of PV was a challenge throughout our patient’s pregnancy. Transient skin lesions may occasionally appear in the neonate and seem to have an increased association with severe active PV in the mother; however, neonatal PV also has been present in mild cases in the mother.7 These lesions are secondary to passive transplacental transfer of PV antibodies but do not have long-lasting clinical implications because of an antibody’s brief half-life.9 The lesions either spontaneously resolve or can be treated with a topical corticosteroid.
Treatment with high-dose systemic corticosteroids or immunosuppressants can be problematic because of the risks posed to the fetus, especially if the mother must be treated when the embryo is particularly susceptible (eg, during organogenesis).10 If a woman with known PV is planning to become pregnant, it is recommended to first control and suppress the disease so that therapy can be minimal during the pregnancy. It also is recommended to use aggressive topical therapy if possible to control PV in a pregnant woman.8 This option would not have been efficacious in our patient because of her severe widespread disease.
Prednisone is considered one of the first-line treatments of PV and has been historically successful as a treatment for pregnant patients with PV if maintained at a low dosage. Prednisone, similar to other corticosteroids, can cross the placental barrier and can increase the chance of premature birth, infection, and mortality in high doses.7 Similar to prednisone, azathioprine is not recommended during pregnancy, but if use is necessary, it is suggested to keep the dose low to prevent fetal harm.11 Inadequate treatment and control of PV can be life threatening to the patient because of the severe infection that may ensue; thus it is necessary for the health of the patient and fetus to suppress the PV. One alternative to treatment with steroids and immunosuppressants is plasma exchange, which has been successful in the clinical context of pregnancy.12 The cons of plasma exchange are repeat procedures, the need to give the patient more immunosuppressants to prevent a rejection, and the return of the autoantibody.7
Several studies have evaluated the safety and efficacy of rituximab in the treatment of refractory PV. Multiple case reports state that both 1 and 2 courses of intravenous rituximab therapy at a dosage of 375 mg per square meter of body surface area affected once weekly for 4 weeks proved to be useful in clinical improvement for patients with refractory disease.13,14 Studies are currently underway to look at the effects of rituximab on pregnancy and the fetus. Preliminary findings show neonates may have B-cell abnormalities initially yet recover fully without infectious complications or sequelae.15 Rituximab currently is a pregnancy category C drug, and women are counseled to avoid pregnancy for at least 12 months after rituximab exposure and use contraception while actively taking the drug.16
Conclusion
Contrary to traditional thinking, PV itself may be associated with poor neonatal outcome, including prematurity and fetal death. These complications seem to be restricted to pregnancies with clinically severe PV.7 Our patient decided to progress with her pregnancy despite the potential risk to the fetus from the disease and treatment. Ultimately, the infant was delivered prematurely but was free of disease.
Pemphigus vulgaris (PV) is a rare autoimmune bullous dermatosis that has not shown a predilection toward a particular race or sex.1 Autoantibodies for desmoglein 1 and desmoglein 3, members of the cadherin family that are involved in cellular adhesion, have been linked to the pathogenesis of PV.2 These autoantibodies play a role in the loss of cell-to-cell adhesion in the basal and suprabasal layers of the deep epidermis while cellular adhesion in the superficial epidermis remains intact, leading to the clinical presentation of epidermal blistering and ulcerations most commonly found on the scalp, face, groin, and axillae. Diagnosis typically is made based on skin biopsy and confirmed by direct immunofluorescence. Histologically, PV displays acantholysis and suprabasal cleft formation. Immunofluorescence may show IgG antibodies against the PV antigen in the epidermis.3 Once a diagnosis has been made, treatment typically consists of systemic steroids, as the use of steroids has had great effect in preventing infections, sepsis, and fatality that were once associated with PV.4 Mortality rates associated with PV have decreased to 10% to 15% with systemic steroids from a mortality rate as high as 70% in the presteroid era.1,5 Treatment of PV during pregnancy, as in our patient, requires obstetric and pediatric consultations before therapy is initiated. Use of corticosteroids during pregnancy can be potentially dangerous to the fetus, particularly if high doses are necessary to control maternal disease.6,7
Case Report
A 34-year-old pregnant woman at 6 weeks’ gestation presented with widespread blistering dermatitis and associated burning and pruritus. Her obstetrical history was gravida 3, para 2. The patient reported a “rash” on the scalp that had developed 9 months prior. She had been treated as an outpatient at an outside institution with topical antibiotics and antifungal medications, yet the dermatitis progressed. Three weeks prior to hospitalization, the rash was present on the skin and mucosal surfaces, including the groin, chest, face, hard palate, buccal mucosa, lips (Figure 1), and back (Figure 2). Nontender bullae ruptured after 3 days, releasing clear, yellow, serous fluid with associated burning and pruritus. The bullae were hemorrhagic and erythematous at the base.

|
| Figure 1. Facial involvement with bullae, crusted hemorrhagic lesions, and eschar in a 34-year-old pregnant woman. |

|
| Figure 2. Involvement of the back with bullae in various stages. Some bullae were intact while others newly erupted. |

|
| Figure 3. Superinfected and flaking scalp. |

|
| Figure 4. Biopsy revealed suprabasal acantholysis with a tombstone effect of residual basal cells (H&E, original magnification ×200). |
At the current presentation, the patient had several excoriated 1- to 2-cm oval denudations; some were crusted with eschar. Nikolsky sign was negative. Multiple confluent bullous lesions had erupted on the entire scalp with a thick, impetiginous, yellow crust. She had a wet, boggy, foul-smelling, superinfected scalp that was mildly tender to touch with flaking tissue debris (Figure 3). A white blood cell count was 13.2×109/L (reference range, 4.5–11.0×109/L) with 5% eosinophils (reference range, 2.7%). The differential diagnosis included bullous impetigo, pemphigoid, Stevens-Johnson syndrome, dermatitis herpetiformis, and pemphigus vulgaris.
Biopsies of the scalp and back were taken and showed suprabasal acantholysis with a tombstone effect of residual basal cells standing up on the basement membrane without the characteristic acantholysis into skin appendages (Figure 4). The acantholytic cells in the bullous chamber did not round up as in Hailey-Hailey disease nor was there the dyskeratosis of Grover disease. Direct immunofluorescence on an elbow punch biopsy found diffuse 1+ intercellular IgG in the epidermis and diffuse 1+ basal intercellular C3, and was negative for IgA, IgM, and C1q, thus confirming a diagnosis of PV.
The patient was started on prednisone 20 mg once daily. An increase to prednisone 60 mg led to initial improvement of symptoms, but there was a relapse after several days, which is typical of PV in pregnancy,7 prompting the dose to be increased to 120 mg. Following alleviation of symptoms, the dose was later tapered back to 60 mg. No lesions were present at discharge or for 2.5 months thereafter, as the prednisone was tapered from 60 to 45 mg daily after discharge.
On follow-up, the patient’s PV was well controlled, but the prednisone dose was back up to 60 mg daily because of 2 new skin lesions that had developed since her last visit 2.5 months prior. Ultrasonography showed no fetal abnormalities as the pregnancy progressed to 28 weeks’ gestation. The patient developed hypertension and went into premature labor due to placenta previa. The neonate showed no skin lesions or anomalies while in the neonatal intensive care unit. The mother’s prednisone dose was tapered from 60 to 20 mg daily while the white blood cell count was 7.1×109/L with 2% eosinophils and a new scalp lesion appeared. Seven months after her initial discharge from the hospital for the dermatologic condition, she was no longer nursing and azathioprine was added to prednisone 60 mg daily.
Comment
Pemphigus vulgaris is associated with infertility in its active phase; therefore, PV during pregnancy is rare.8 Pregnancy may exacerbate PV, which has been a similar finding in other well-documented autoimmune diseases.7 One review of PV in pregnancy reported that 11 of 49 patients (22%) experienced an exacerbation of the disease.8 This finding pre-sents 2 problems: (1) severe active disease during pregnancy with high antibody titers has been shown to heighten risk for morbidity and mortality for the fetus, and (2) a patient with active PV during pregnancy may require systemic therapy with doses high enough to subdue the disease. The presence of PV was a challenge throughout our patient’s pregnancy. Transient skin lesions may occasionally appear in the neonate and seem to have an increased association with severe active PV in the mother; however, neonatal PV also has been present in mild cases in the mother.7 These lesions are secondary to passive transplacental transfer of PV antibodies but do not have long-lasting clinical implications because of an antibody’s brief half-life.9 The lesions either spontaneously resolve or can be treated with a topical corticosteroid.
Treatment with high-dose systemic corticosteroids or immunosuppressants can be problematic because of the risks posed to the fetus, especially if the mother must be treated when the embryo is particularly susceptible (eg, during organogenesis).10 If a woman with known PV is planning to become pregnant, it is recommended to first control and suppress the disease so that therapy can be minimal during the pregnancy. It also is recommended to use aggressive topical therapy if possible to control PV in a pregnant woman.8 This option would not have been efficacious in our patient because of her severe widespread disease.
Prednisone is considered one of the first-line treatments of PV and has been historically successful as a treatment for pregnant patients with PV if maintained at a low dosage. Prednisone, similar to other corticosteroids, can cross the placental barrier and can increase the chance of premature birth, infection, and mortality in high doses.7 Similar to prednisone, azathioprine is not recommended during pregnancy, but if use is necessary, it is suggested to keep the dose low to prevent fetal harm.11 Inadequate treatment and control of PV can be life threatening to the patient because of the severe infection that may ensue; thus it is necessary for the health of the patient and fetus to suppress the PV. One alternative to treatment with steroids and immunosuppressants is plasma exchange, which has been successful in the clinical context of pregnancy.12 The cons of plasma exchange are repeat procedures, the need to give the patient more immunosuppressants to prevent a rejection, and the return of the autoantibody.7
Several studies have evaluated the safety and efficacy of rituximab in the treatment of refractory PV. Multiple case reports state that both 1 and 2 courses of intravenous rituximab therapy at a dosage of 375 mg per square meter of body surface area affected once weekly for 4 weeks proved to be useful in clinical improvement for patients with refractory disease.13,14 Studies are currently underway to look at the effects of rituximab on pregnancy and the fetus. Preliminary findings show neonates may have B-cell abnormalities initially yet recover fully without infectious complications or sequelae.15 Rituximab currently is a pregnancy category C drug, and women are counseled to avoid pregnancy for at least 12 months after rituximab exposure and use contraception while actively taking the drug.16
Conclusion
Contrary to traditional thinking, PV itself may be associated with poor neonatal outcome, including prematurity and fetal death. These complications seem to be restricted to pregnancies with clinically severe PV.7 Our patient decided to progress with her pregnancy despite the potential risk to the fetus from the disease and treatment. Ultimately, the infant was delivered prematurely but was free of disease.
1. Fainaru O, Mashiach R, Kupferminc M, et al. Pemphigus vulgaris in pregnancy: a case report and review of literature. Hum Reprod. 2000;15:1195-1197.
2. Joly P, Gilbert D, Thomine E, et al. Identification of a new antibody population directed against a desmosomal plaque antigen in pemphigus vulgaris and pemphigus foliaceus. J Invest Dermatol. 1997;108:469-475.
3. Daniel Y, Shenhav M, Botchan A, et al. Pregnancy associated with pemphigus. Br J Obstet Gynecol. 1995;102:667-669.
4. Ruach M, Ohel G, Rahav D, et al. Pemphigus vulgaris and pregnancy. Obstet Gynecol Surv. 1995;50:755-760.
5. Carson PJ, Hameed A, Ahmed AR. Influence of treatment on clinical course of pemphigus vulgaris. J Am Acad Dermatol. 1996;34:645-652.
6. Goldberg NS, DeFeo C, Kirshenbaum N. Pemphigus and pregnancy: risk factors and recommendations. J Am Acad Dermatol. 1993;28(5, pt 2):877-879.
7. Lehman JS, Mueller KK, Schraith DF. Do safe and effective treatment options exist for patients with active pemphigus vulgaris who plan conception and pregnancy? Arch Dermatol. 2008;144:783-785.
8. Kardos M, Levine D, Gurcan H, et al. Pemphigus vulgaris in pregnancy: analysis of current data on the management and outcomes. Obstet Gynecol Surv. 2009;64:739-749.
9. Fenniche S, Benmously R, Marrak H, et al. Neonatal pemphigus vulgaris in an infant born to a mother with pemphigus vulgaris in remission. Pediatr Dermatol. 2006;23:124-127.
10. Kalayciyan A, Engin B, Serdaroglu S, et al. A retrospective analysis of patients with pemphigus vulgaris associated with pregnancy. Br J Dermatol. 2002;147:396-397.
11. Hup JM, Bruinsma RA, Boersma ER, et al. Neonatal pemphigus vulgaris: transplacental transmission of antibodies. Pediatr Dermatol. 1986;3:468-472.
12. Piontek JO, Borberg H, Sollberg S, et al. Severe exacerbation of pemphigus vulgaris in pregnancy: successful treatment with plasma exchange. Br J Dermatol. 2000;143:455-456.
13. Faurschou A, Gniadecki R. Two courses of rituximab (anti-CD20 monoclonal antibody) for recalcitrant pemphigus vulgaris. Int J Dermatol. 2008;47:292-294.
14. Marzano AV, Fanoni D, Venegoni L, et al. Treatment of refractory pemphigus with the anti-CD20 monoclonal antibody (rituximab). Dermatology. 2007;214:310-318.
15. Braunstein I, Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther. 2013;26:354-363.
16. Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506.
1. Fainaru O, Mashiach R, Kupferminc M, et al. Pemphigus vulgaris in pregnancy: a case report and review of literature. Hum Reprod. 2000;15:1195-1197.
2. Joly P, Gilbert D, Thomine E, et al. Identification of a new antibody population directed against a desmosomal plaque antigen in pemphigus vulgaris and pemphigus foliaceus. J Invest Dermatol. 1997;108:469-475.
3. Daniel Y, Shenhav M, Botchan A, et al. Pregnancy associated with pemphigus. Br J Obstet Gynecol. 1995;102:667-669.
4. Ruach M, Ohel G, Rahav D, et al. Pemphigus vulgaris and pregnancy. Obstet Gynecol Surv. 1995;50:755-760.
5. Carson PJ, Hameed A, Ahmed AR. Influence of treatment on clinical course of pemphigus vulgaris. J Am Acad Dermatol. 1996;34:645-652.
6. Goldberg NS, DeFeo C, Kirshenbaum N. Pemphigus and pregnancy: risk factors and recommendations. J Am Acad Dermatol. 1993;28(5, pt 2):877-879.
7. Lehman JS, Mueller KK, Schraith DF. Do safe and effective treatment options exist for patients with active pemphigus vulgaris who plan conception and pregnancy? Arch Dermatol. 2008;144:783-785.
8. Kardos M, Levine D, Gurcan H, et al. Pemphigus vulgaris in pregnancy: analysis of current data on the management and outcomes. Obstet Gynecol Surv. 2009;64:739-749.
9. Fenniche S, Benmously R, Marrak H, et al. Neonatal pemphigus vulgaris in an infant born to a mother with pemphigus vulgaris in remission. Pediatr Dermatol. 2006;23:124-127.
10. Kalayciyan A, Engin B, Serdaroglu S, et al. A retrospective analysis of patients with pemphigus vulgaris associated with pregnancy. Br J Dermatol. 2002;147:396-397.
11. Hup JM, Bruinsma RA, Boersma ER, et al. Neonatal pemphigus vulgaris: transplacental transmission of antibodies. Pediatr Dermatol. 1986;3:468-472.
12. Piontek JO, Borberg H, Sollberg S, et al. Severe exacerbation of pemphigus vulgaris in pregnancy: successful treatment with plasma exchange. Br J Dermatol. 2000;143:455-456.
13. Faurschou A, Gniadecki R. Two courses of rituximab (anti-CD20 monoclonal antibody) for recalcitrant pemphigus vulgaris. Int J Dermatol. 2008;47:292-294.
14. Marzano AV, Fanoni D, Venegoni L, et al. Treatment of refractory pemphigus with the anti-CD20 monoclonal antibody (rituximab). Dermatology. 2007;214:310-318.
15. Braunstein I, Werth V. Treatment of dermatologic connective tissue disease and autoimmune blistering disorders in pregnancy. Dermatol Ther. 2013;26:354-363.
16. Chakravarty EF, Murray ER, Kelman A, et al. Pregnancy outcomes after maternal exposure to rituximab. Blood. 2011;117:1499-1506.
Practice Points
- Early diagnosis and appropriate treatment of pemphigus vulgaris in pregnancy is paramount in protecting the health of the mother and fetus.
- Management of autoimmune diseases during pregnancy continues to present numerous challenges for physicians due to the pathology of the diseases as well as the sensitive nature of pregnancy and lack of robust data in this patient population.