User login
An Unusual Presentation of Congenital Dermal Melanocytosis Fitting the Rare Diagnosis of Dermal Melanocyte Hamartoma
To the Editor:
Dermal melanocytosis is thought to be the result of a defect in melanoblast migration during embryogenesis and is characterized by the presence of functional fusiform and dendritic melanocytes in the dermis. Congenital dermal melanocytosis is classified into various subtypes based on the distribution, morphology, natural history of lesions, and distinctive histologic findings. We present an unusual case of congenital dermal melanocytosis that might fit the rare entity of dermal melanocyte hamartoma (DMH).
|
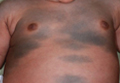
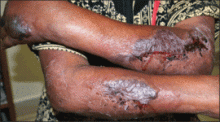
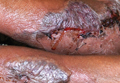
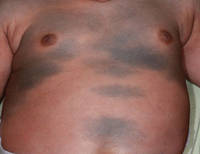
| Figure 1. Uniform grayish blue patches over the torso with sharp demarcation lines that followed a dermatomal distribution. |
|
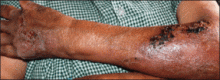
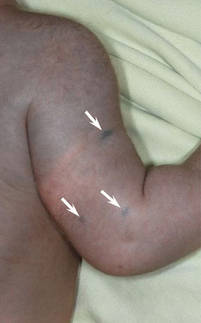
| Figure 2. Three conspicuous darker blue macules (arrows) within a bluish patch. |
|
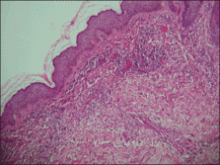
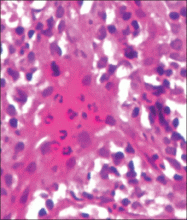
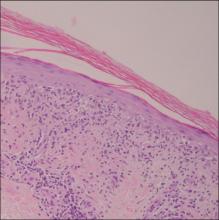
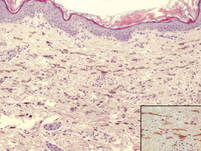
| Figure 3. Intradermal dendritic pigmented melanocytes localized in the upper dermis and arranged parallel to the skin surface (H&E, original magnification ×100 [inset, original magnification ×400]). |
A 4-month-old girl presented with bilateral bluish patches over the trunk and upper extremities. The lesions were present since birth, and remained entirely unchanged during a follow-up period of 18 months. Mental and physical development was normal. There was no family history of pigmentary disorders. On physical examination uniform grayish blue patches were seen over the torso and upper extremities. The patches seemed to follow a dermatomal distribution along the trunk and extremities (Figure 1). Several well-circumscribed, much darker blue macules were scattered within the bluish patches (Figure 2). The rest of the physical examination was unremarkable. Complete blood cell count and blood chemistry values were within reference range. Skin biopsy specimens from both the grayish blue patches and the conspicuous darker blue macules showed a fair amount of dermal bipolar dendritic pigmented melanocytes arranged parallel to the skin surface with no apparent disturbance of collagen bundles (Figure 3). No melanophages were seen. The clinical, histologic, and laboratory findings were consistent with a diagnosis of congenital dermal melanocytosis. An underlying lysosomal storage disease was ruled out through metabolic screening that included liver function tests, abdominal sonography, and neurologic and ophthalmologic examinations.
The clinical spectrum of congenital dermal melanocytosis includes several clinical entities such as mongolian spots, Ota nevus, Ito nevus, blue nevi, and DMH.1 The differentiation between these different types of dermal melanocytosis can be challenging, especially when the process of melanocytosis is extensive. Among the several types of congenital dermal melanocytosis, Ota or Ito nevi can be ruled out in our patient based on the clinical presentation.
The term dermal melanocyte hamartoma was introduced by Burkhart and Gohara.2 It is characterized by congenital dermal melanocytosis that follows a dermatomal pattern. The original case report included speckled, darker blue macules with a background of grayish blue patches similar to our patient.2 Melanocytes in DMH typically are located in the upper half of the reticular dermis, as opposed to extensive mongolian spots in which ectopic melanocytes usually are found in the lower half of the dermis. The pigmentation in our patient did not change during 22 months of follow-up, which is more consistent with the diagnosis of DMH versus extensive mongolian spots. Our patient did not present with any systemic anomalies. However, a case of congenital melanocytosis and neuroectodermal malformation has been described in the literature.3
Little is known about the etiology of dermal melanocytosis. Mutations in the guanine nucleotide binding protein q polypeptide gene, GNAQ, or guanine nucleotide binding protein alpha 11 gene, GNA11, were found to cause dermal melanocytosis in mice.4 Also, GNAQ mutations have been demonstrated in humans with dermal melanocytosis as well as uveal melanoma.5 Progress in our understanding of the pathogenesis of dermal melanocytosis is expected to lead to a more accurate classification of dermal pigmentation disorders.
1. Stanford DG, Georgouras KE. Dermal melanocytosis: a clinical spectrum. Australas J Dermatol. 1996;37:19-25.
2. Burkhart CG, Gohara A. Dermal melanocyte hamartoma: a distinctive new form of dermal melanocytosis.
Arch Dermatol. 1981;117:102-104.
3. Schwartz RA, Cohen-Addad N, Lambert MW, et al. Congenital melanocytosis with myelomeningocele and hydrocephalus. Cutis. 1986;37:37-39.
4. Van Raamsdonk CD, Fitch KR, Fuchs H, et al. Effects of G-protein mutations on skin color. Nat Genet. 2004;36:961-968.
5. Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue nevi. Nature. 2009;457:599-602.
To the Editor:
Dermal melanocytosis is thought to be the result of a defect in melanoblast migration during embryogenesis and is characterized by the presence of functional fusiform and dendritic melanocytes in the dermis. Congenital dermal melanocytosis is classified into various subtypes based on the distribution, morphology, natural history of lesions, and distinctive histologic findings. We present an unusual case of congenital dermal melanocytosis that might fit the rare entity of dermal melanocyte hamartoma (DMH).

|
| Figure 1. Uniform grayish blue patches over the torso with sharp demarcation lines that followed a dermatomal distribution. |

|
| Figure 2. Three conspicuous darker blue macules (arrows) within a bluish patch. |

|
| Figure 3. Intradermal dendritic pigmented melanocytes localized in the upper dermis and arranged parallel to the skin surface (H&E, original magnification ×100 [inset, original magnification ×400]). |
A 4-month-old girl presented with bilateral bluish patches over the trunk and upper extremities. The lesions were present since birth, and remained entirely unchanged during a follow-up period of 18 months. Mental and physical development was normal. There was no family history of pigmentary disorders. On physical examination uniform grayish blue patches were seen over the torso and upper extremities. The patches seemed to follow a dermatomal distribution along the trunk and extremities (Figure 1). Several well-circumscribed, much darker blue macules were scattered within the bluish patches (Figure 2). The rest of the physical examination was unremarkable. Complete blood cell count and blood chemistry values were within reference range. Skin biopsy specimens from both the grayish blue patches and the conspicuous darker blue macules showed a fair amount of dermal bipolar dendritic pigmented melanocytes arranged parallel to the skin surface with no apparent disturbance of collagen bundles (Figure 3). No melanophages were seen. The clinical, histologic, and laboratory findings were consistent with a diagnosis of congenital dermal melanocytosis. An underlying lysosomal storage disease was ruled out through metabolic screening that included liver function tests, abdominal sonography, and neurologic and ophthalmologic examinations.
The clinical spectrum of congenital dermal melanocytosis includes several clinical entities such as mongolian spots, Ota nevus, Ito nevus, blue nevi, and DMH.1 The differentiation between these different types of dermal melanocytosis can be challenging, especially when the process of melanocytosis is extensive. Among the several types of congenital dermal melanocytosis, Ota or Ito nevi can be ruled out in our patient based on the clinical presentation.
The term dermal melanocyte hamartoma was introduced by Burkhart and Gohara.2 It is characterized by congenital dermal melanocytosis that follows a dermatomal pattern. The original case report included speckled, darker blue macules with a background of grayish blue patches similar to our patient.2 Melanocytes in DMH typically are located in the upper half of the reticular dermis, as opposed to extensive mongolian spots in which ectopic melanocytes usually are found in the lower half of the dermis. The pigmentation in our patient did not change during 22 months of follow-up, which is more consistent with the diagnosis of DMH versus extensive mongolian spots. Our patient did not present with any systemic anomalies. However, a case of congenital melanocytosis and neuroectodermal malformation has been described in the literature.3
Little is known about the etiology of dermal melanocytosis. Mutations in the guanine nucleotide binding protein q polypeptide gene, GNAQ, or guanine nucleotide binding protein alpha 11 gene, GNA11, were found to cause dermal melanocytosis in mice.4 Also, GNAQ mutations have been demonstrated in humans with dermal melanocytosis as well as uveal melanoma.5 Progress in our understanding of the pathogenesis of dermal melanocytosis is expected to lead to a more accurate classification of dermal pigmentation disorders.
To the Editor:
Dermal melanocytosis is thought to be the result of a defect in melanoblast migration during embryogenesis and is characterized by the presence of functional fusiform and dendritic melanocytes in the dermis. Congenital dermal melanocytosis is classified into various subtypes based on the distribution, morphology, natural history of lesions, and distinctive histologic findings. We present an unusual case of congenital dermal melanocytosis that might fit the rare entity of dermal melanocyte hamartoma (DMH).

|
| Figure 1. Uniform grayish blue patches over the torso with sharp demarcation lines that followed a dermatomal distribution. |

|
| Figure 2. Three conspicuous darker blue macules (arrows) within a bluish patch. |

|
| Figure 3. Intradermal dendritic pigmented melanocytes localized in the upper dermis and arranged parallel to the skin surface (H&E, original magnification ×100 [inset, original magnification ×400]). |
A 4-month-old girl presented with bilateral bluish patches over the trunk and upper extremities. The lesions were present since birth, and remained entirely unchanged during a follow-up period of 18 months. Mental and physical development was normal. There was no family history of pigmentary disorders. On physical examination uniform grayish blue patches were seen over the torso and upper extremities. The patches seemed to follow a dermatomal distribution along the trunk and extremities (Figure 1). Several well-circumscribed, much darker blue macules were scattered within the bluish patches (Figure 2). The rest of the physical examination was unremarkable. Complete blood cell count and blood chemistry values were within reference range. Skin biopsy specimens from both the grayish blue patches and the conspicuous darker blue macules showed a fair amount of dermal bipolar dendritic pigmented melanocytes arranged parallel to the skin surface with no apparent disturbance of collagen bundles (Figure 3). No melanophages were seen. The clinical, histologic, and laboratory findings were consistent with a diagnosis of congenital dermal melanocytosis. An underlying lysosomal storage disease was ruled out through metabolic screening that included liver function tests, abdominal sonography, and neurologic and ophthalmologic examinations.
The clinical spectrum of congenital dermal melanocytosis includes several clinical entities such as mongolian spots, Ota nevus, Ito nevus, blue nevi, and DMH.1 The differentiation between these different types of dermal melanocytosis can be challenging, especially when the process of melanocytosis is extensive. Among the several types of congenital dermal melanocytosis, Ota or Ito nevi can be ruled out in our patient based on the clinical presentation.
The term dermal melanocyte hamartoma was introduced by Burkhart and Gohara.2 It is characterized by congenital dermal melanocytosis that follows a dermatomal pattern. The original case report included speckled, darker blue macules with a background of grayish blue patches similar to our patient.2 Melanocytes in DMH typically are located in the upper half of the reticular dermis, as opposed to extensive mongolian spots in which ectopic melanocytes usually are found in the lower half of the dermis. The pigmentation in our patient did not change during 22 months of follow-up, which is more consistent with the diagnosis of DMH versus extensive mongolian spots. Our patient did not present with any systemic anomalies. However, a case of congenital melanocytosis and neuroectodermal malformation has been described in the literature.3
Little is known about the etiology of dermal melanocytosis. Mutations in the guanine nucleotide binding protein q polypeptide gene, GNAQ, or guanine nucleotide binding protein alpha 11 gene, GNA11, were found to cause dermal melanocytosis in mice.4 Also, GNAQ mutations have been demonstrated in humans with dermal melanocytosis as well as uveal melanoma.5 Progress in our understanding of the pathogenesis of dermal melanocytosis is expected to lead to a more accurate classification of dermal pigmentation disorders.
1. Stanford DG, Georgouras KE. Dermal melanocytosis: a clinical spectrum. Australas J Dermatol. 1996;37:19-25.
2. Burkhart CG, Gohara A. Dermal melanocyte hamartoma: a distinctive new form of dermal melanocytosis.
Arch Dermatol. 1981;117:102-104.
3. Schwartz RA, Cohen-Addad N, Lambert MW, et al. Congenital melanocytosis with myelomeningocele and hydrocephalus. Cutis. 1986;37:37-39.
4. Van Raamsdonk CD, Fitch KR, Fuchs H, et al. Effects of G-protein mutations on skin color. Nat Genet. 2004;36:961-968.
5. Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue nevi. Nature. 2009;457:599-602.
1. Stanford DG, Georgouras KE. Dermal melanocytosis: a clinical spectrum. Australas J Dermatol. 1996;37:19-25.
2. Burkhart CG, Gohara A. Dermal melanocyte hamartoma: a distinctive new form of dermal melanocytosis.
Arch Dermatol. 1981;117:102-104.
3. Schwartz RA, Cohen-Addad N, Lambert MW, et al. Congenital melanocytosis with myelomeningocele and hydrocephalus. Cutis. 1986;37:37-39.
4. Van Raamsdonk CD, Fitch KR, Fuchs H, et al. Effects of G-protein mutations on skin color. Nat Genet. 2004;36:961-968.
5. Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue nevi. Nature. 2009;457:599-602.
Allergic Contact Dermatitis From Ketoconazole
Case Report
A 65-year-old man presented to the dermatology department for treatment of a scaly rash on the face and scalp. A diagnosis of seborrheic dermatitis was made, and he was prescribed ketoconazole cream 2% and shampoo 2%. Two days later, the patient presented to the emergency department for facial swelling and pruritus, which began 1 day after he began using the ketoconazole cream and shampoo. He reported itching and burning on the face that began within several hours of application followed by progressive facial edema. The patient denied shortness of breath or swelling of the tongue. Physical examination revealed mild facial induration with erythematous plaques on the bilateral cheeks, forehead, and eyelids. The patient was instructed to stop using the ketoconazole cream and shampoo. Within several days of discontinuing use of the ketoconazole products, the dermatitis resolved following treatment with oral diphenhydramine and topical desonide.
Review of the patient’s medical record revealed several likely relevant incidences of undiagnosed recurrent dermatitis. Approximately 2 years earlier, the patient had called his primary care provider to report pain, burning, redness, and itching in the right buttock area following use of ketoconazole cream that the physician had prescribed. Allergic contact dermatitis also had been documented in the patient’s dermatology problem list approximately 1.5 years prior to the current presentation, though a likely causative agent was not listed. Approximately 3 months prior to the current presentation, the patient presented with lower leg rash and edema with documentation of possible allergic reaction to ketoconazole cream.
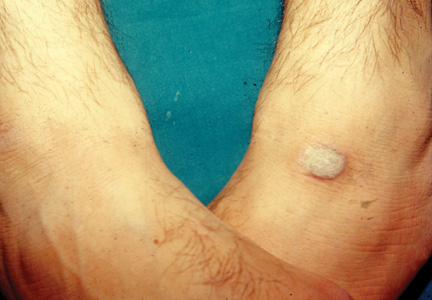
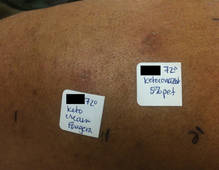
The patient was patch tested several weeks after discontinuation of the ketoconazole products using the 2012 North American Contact Dermatitis Group series (70 allergens), a supplemental series (36 allergens), an antifungal series (10 allergens), and personal products including ketoconazole cream and shampoo (diluted 1:100). Clinically relevant reactions at 72 hours included an extreme reaction (+++) to the patient’s personal ketoconazole cream 2% (E. Fougera & Co)(Figure 1), and strong reactions (++) to purified ketoconazole 5% in petrolatum and ketoconazole cream 2% (E. Fougera & Co) in an antifungal series (Figure 2). A doubtful reaction to methyl methacrylate was not deemed clinically relevant. No reactions were noted to terbinafine cream 1%, clotrimazole cream 1%, nystatin cream, nystatin ointment, econazole nitrate cream 1%, miconazole nitrate cream 2%, tolnaftate cream 1%, or purified clotrimazole 1% in petrolatum.

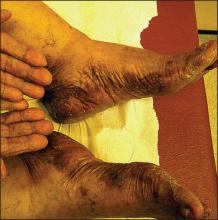
|
Figure 1. Reading at 72 hours of patient’s personal products (ketoconazole cream 2% and ketoconazole shampoo 2%). |
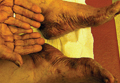
|
Figure 2. Reading at 72 hours of an antifungal series (ketoconazole cream 2% and purified ketocona-zole 5% in petrolatum). |
Comment
Ketoconazole is a widely used antifungal but rarely is reported as a cause of allergic contact dermatitis. Allergies to inactive ingredients, especially vehicles and preservatives, are more common than allergies to ketoconazole itself. In our patient, allergy to inactive ingredients was ruled out by negative reactions to individual constituents and/or negative reactions to other products containing those ingredients. A literature review via Ovid using the search terms ketoconazole, allergic contact dermatitis, and allergy found 4 reports involving 9 documented patients with type IV hypersensitivity to ketoconazole,1-4 and 1 report of 2 patients who developed anaphylaxis from oral ketoconazole.1 Of the 9 dermatitis cases, 3 patients had positive patch tests to only ketoconazole with no reactions to other imidazoles.2,3 Monoallergy to clotrimazole also has been reported.5 A study by Dooms-Goossens et al4 showed that ketoconazole ranked seventh of 11 imidazole derivatives in its frequency to cause allergic contact dermatitis and did not demonstrate statistically significant cross-reactivity with other imidazoles; cross-reactivity usually occurred with miconazole and sulconazole.
Conclusion
This case of contact dermatitis to ketoconazole demonstrates the importance of patch testing with personal products as well as the unpredictability of cross-reactions within the imidazole class of antifungals.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Health Care System.
1. Garcia-Bravo B, Mazuecos J, Rodriguez-Pichardo A, et al. Hypersensitivity to ketoconazole preparations: study of 4 cases. Contact Dermatitis. 1989;21:346-348.
2. Valsecchi R, Pansera B, di Landro A, et al. Contact dermatitis from ketoconazole. Contact Dermatitis. 1993;29:162.
3. Santucci B, Cannistraci C, Cristaudo A, et al. Contact dermatitis from ketoconazole cream. Contact Dermatitis. 1992;27:274-275.
4. Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77.
5. Pullen SK, Warshaw EM. Vulvar allergic contact dermatitis from clotrimazole. Dermatitis. 2010;21:59-60.
Case Report
A 65-year-old man presented to the dermatology department for treatment of a scaly rash on the face and scalp. A diagnosis of seborrheic dermatitis was made, and he was prescribed ketoconazole cream 2% and shampoo 2%. Two days later, the patient presented to the emergency department for facial swelling and pruritus, which began 1 day after he began using the ketoconazole cream and shampoo. He reported itching and burning on the face that began within several hours of application followed by progressive facial edema. The patient denied shortness of breath or swelling of the tongue. Physical examination revealed mild facial induration with erythematous plaques on the bilateral cheeks, forehead, and eyelids. The patient was instructed to stop using the ketoconazole cream and shampoo. Within several days of discontinuing use of the ketoconazole products, the dermatitis resolved following treatment with oral diphenhydramine and topical desonide.
Review of the patient’s medical record revealed several likely relevant incidences of undiagnosed recurrent dermatitis. Approximately 2 years earlier, the patient had called his primary care provider to report pain, burning, redness, and itching in the right buttock area following use of ketoconazole cream that the physician had prescribed. Allergic contact dermatitis also had been documented in the patient’s dermatology problem list approximately 1.5 years prior to the current presentation, though a likely causative agent was not listed. Approximately 3 months prior to the current presentation, the patient presented with lower leg rash and edema with documentation of possible allergic reaction to ketoconazole cream.
The patient was patch tested several weeks after discontinuation of the ketoconazole products using the 2012 North American Contact Dermatitis Group series (70 allergens), a supplemental series (36 allergens), an antifungal series (10 allergens), and personal products including ketoconazole cream and shampoo (diluted 1:100). Clinically relevant reactions at 72 hours included an extreme reaction (+++) to the patient’s personal ketoconazole cream 2% (E. Fougera & Co)(Figure 1), and strong reactions (++) to purified ketoconazole 5% in petrolatum and ketoconazole cream 2% (E. Fougera & Co) in an antifungal series (Figure 2). A doubtful reaction to methyl methacrylate was not deemed clinically relevant. No reactions were noted to terbinafine cream 1%, clotrimazole cream 1%, nystatin cream, nystatin ointment, econazole nitrate cream 1%, miconazole nitrate cream 2%, tolnaftate cream 1%, or purified clotrimazole 1% in petrolatum.

|
Figure 1. Reading at 72 hours of patient’s personal products (ketoconazole cream 2% and ketoconazole shampoo 2%). |

|
Figure 2. Reading at 72 hours of an antifungal series (ketoconazole cream 2% and purified ketocona-zole 5% in petrolatum). |
Comment
Ketoconazole is a widely used antifungal but rarely is reported as a cause of allergic contact dermatitis. Allergies to inactive ingredients, especially vehicles and preservatives, are more common than allergies to ketoconazole itself. In our patient, allergy to inactive ingredients was ruled out by negative reactions to individual constituents and/or negative reactions to other products containing those ingredients. A literature review via Ovid using the search terms ketoconazole, allergic contact dermatitis, and allergy found 4 reports involving 9 documented patients with type IV hypersensitivity to ketoconazole,1-4 and 1 report of 2 patients who developed anaphylaxis from oral ketoconazole.1 Of the 9 dermatitis cases, 3 patients had positive patch tests to only ketoconazole with no reactions to other imidazoles.2,3 Monoallergy to clotrimazole also has been reported.5 A study by Dooms-Goossens et al4 showed that ketoconazole ranked seventh of 11 imidazole derivatives in its frequency to cause allergic contact dermatitis and did not demonstrate statistically significant cross-reactivity with other imidazoles; cross-reactivity usually occurred with miconazole and sulconazole.
Conclusion
This case of contact dermatitis to ketoconazole demonstrates the importance of patch testing with personal products as well as the unpredictability of cross-reactions within the imidazole class of antifungals.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Health Care System.
Case Report
A 65-year-old man presented to the dermatology department for treatment of a scaly rash on the face and scalp. A diagnosis of seborrheic dermatitis was made, and he was prescribed ketoconazole cream 2% and shampoo 2%. Two days later, the patient presented to the emergency department for facial swelling and pruritus, which began 1 day after he began using the ketoconazole cream and shampoo. He reported itching and burning on the face that began within several hours of application followed by progressive facial edema. The patient denied shortness of breath or swelling of the tongue. Physical examination revealed mild facial induration with erythematous plaques on the bilateral cheeks, forehead, and eyelids. The patient was instructed to stop using the ketoconazole cream and shampoo. Within several days of discontinuing use of the ketoconazole products, the dermatitis resolved following treatment with oral diphenhydramine and topical desonide.
Review of the patient’s medical record revealed several likely relevant incidences of undiagnosed recurrent dermatitis. Approximately 2 years earlier, the patient had called his primary care provider to report pain, burning, redness, and itching in the right buttock area following use of ketoconazole cream that the physician had prescribed. Allergic contact dermatitis also had been documented in the patient’s dermatology problem list approximately 1.5 years prior to the current presentation, though a likely causative agent was not listed. Approximately 3 months prior to the current presentation, the patient presented with lower leg rash and edema with documentation of possible allergic reaction to ketoconazole cream.
The patient was patch tested several weeks after discontinuation of the ketoconazole products using the 2012 North American Contact Dermatitis Group series (70 allergens), a supplemental series (36 allergens), an antifungal series (10 allergens), and personal products including ketoconazole cream and shampoo (diluted 1:100). Clinically relevant reactions at 72 hours included an extreme reaction (+++) to the patient’s personal ketoconazole cream 2% (E. Fougera & Co)(Figure 1), and strong reactions (++) to purified ketoconazole 5% in petrolatum and ketoconazole cream 2% (E. Fougera & Co) in an antifungal series (Figure 2). A doubtful reaction to methyl methacrylate was not deemed clinically relevant. No reactions were noted to terbinafine cream 1%, clotrimazole cream 1%, nystatin cream, nystatin ointment, econazole nitrate cream 1%, miconazole nitrate cream 2%, tolnaftate cream 1%, or purified clotrimazole 1% in petrolatum.

|
Figure 1. Reading at 72 hours of patient’s personal products (ketoconazole cream 2% and ketoconazole shampoo 2%). |

|
Figure 2. Reading at 72 hours of an antifungal series (ketoconazole cream 2% and purified ketocona-zole 5% in petrolatum). |
Comment
Ketoconazole is a widely used antifungal but rarely is reported as a cause of allergic contact dermatitis. Allergies to inactive ingredients, especially vehicles and preservatives, are more common than allergies to ketoconazole itself. In our patient, allergy to inactive ingredients was ruled out by negative reactions to individual constituents and/or negative reactions to other products containing those ingredients. A literature review via Ovid using the search terms ketoconazole, allergic contact dermatitis, and allergy found 4 reports involving 9 documented patients with type IV hypersensitivity to ketoconazole,1-4 and 1 report of 2 patients who developed anaphylaxis from oral ketoconazole.1 Of the 9 dermatitis cases, 3 patients had positive patch tests to only ketoconazole with no reactions to other imidazoles.2,3 Monoallergy to clotrimazole also has been reported.5 A study by Dooms-Goossens et al4 showed that ketoconazole ranked seventh of 11 imidazole derivatives in its frequency to cause allergic contact dermatitis and did not demonstrate statistically significant cross-reactivity with other imidazoles; cross-reactivity usually occurred with miconazole and sulconazole.
Conclusion
This case of contact dermatitis to ketoconazole demonstrates the importance of patch testing with personal products as well as the unpredictability of cross-reactions within the imidazole class of antifungals.
Acknowledgment
This material is the result of work supported with resources and the use of facilities at the Minneapolis Veterans Affairs Health Care System.
1. Garcia-Bravo B, Mazuecos J, Rodriguez-Pichardo A, et al. Hypersensitivity to ketoconazole preparations: study of 4 cases. Contact Dermatitis. 1989;21:346-348.
2. Valsecchi R, Pansera B, di Landro A, et al. Contact dermatitis from ketoconazole. Contact Dermatitis. 1993;29:162.
3. Santucci B, Cannistraci C, Cristaudo A, et al. Contact dermatitis from ketoconazole cream. Contact Dermatitis. 1992;27:274-275.
4. Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77.
5. Pullen SK, Warshaw EM. Vulvar allergic contact dermatitis from clotrimazole. Dermatitis. 2010;21:59-60.
1. Garcia-Bravo B, Mazuecos J, Rodriguez-Pichardo A, et al. Hypersensitivity to ketoconazole preparations: study of 4 cases. Contact Dermatitis. 1989;21:346-348.
2. Valsecchi R, Pansera B, di Landro A, et al. Contact dermatitis from ketoconazole. Contact Dermatitis. 1993;29:162.
3. Santucci B, Cannistraci C, Cristaudo A, et al. Contact dermatitis from ketoconazole cream. Contact Dermatitis. 1992;27:274-275.
4. Dooms-Goossens A, Matura M, Drieghe J, et al. Contact allergy to imidazoles used as antimycotic agents. Contact Dermatitis. 1995;33:73-77.
5. Pullen SK, Warshaw EM. Vulvar allergic contact dermatitis from clotrimazole. Dermatitis. 2010;21:59-60.
- Contact allergy to topical ketoconazole is rare and its cross-reactivity with other imidazole antifungals is unpredictable.
- Patch testing to personal products often is important for detecting rare allergies.
Patching Psoriasis
Patch testing is one of the major diagnostic tools in the evaluation of allergic contact dermatitis. One limitation of patch testing is the use of steroids prior to testing. Because steroids may suppress a positive test reaction, the use of topical steroids on the test site or oral steroids should be discontinued for at least 2 weeks prior to testing. Therefore, it is interesting to consider the effect of biologics on the reliability of patch testing.
Kim et al (Dermatitis. 2014;25:182-190) evaluated the prevalence of positive patch tests in psoriasis patients receiving biologics and whether these results differed from those of psoriasis patients who were not receiving biologics. An institutional review board–approved retrospective chart review was performed for individuals with psoriasis who were patch tested from January 2002 to 2012 at Tufts Medical Center in Boston, Massachusetts. Patients were selected if they had a history of psoriasis, psoriatic arthritis, and patch testing as identified by International Classification of Diseases, Ninth Revision, codes 696.1, 696.0, and 95044, respectively, in their medical records. Patients were patch tested using a modified North American Contact Dermatitis Group standard and cosmetics series. Readings were performed at 48 hours and 72 to 96 hours. The North American Contact Dermatitis Group grading system was used to grade reactions.
The chart review included 15 psoriasis patients who were on biologics (cases) and 16 psoriasis patients who were not on biologics (control subjects). The biologics used were ustekinumab (n=7), etanercept (n=4), adalimumab (n=3), and infliximab (n=1). The authors determined that 80% (12/15) of cases had at least 1 positive reaction compared with 81% (13/16) of control subjects, 67% (10/15) of cases had 2+ positive reactions compared with 63% (10/16) of control subjects, and 27% (4/15) of cases had 3+ positive reactions compared with 38% (6/16) of control subjects. These differences were not statistically significant.
Given the limitation of the small number of patients evaluated, the authors concluded that biologics do not appear to influence the abilities of patients with psoriasis to mount a positive patch test.
What’s the issue?
This study is small, but the findings do give an indication that the biologic agents utilized for psoriasis do not suppress patch test reactions. These data are not typically what we collect in this population, but it is nice to know. What has been your experience in patch testing patients on biologic therapy?
Patch testing is one of the major diagnostic tools in the evaluation of allergic contact dermatitis. One limitation of patch testing is the use of steroids prior to testing. Because steroids may suppress a positive test reaction, the use of topical steroids on the test site or oral steroids should be discontinued for at least 2 weeks prior to testing. Therefore, it is interesting to consider the effect of biologics on the reliability of patch testing.
Kim et al (Dermatitis. 2014;25:182-190) evaluated the prevalence of positive patch tests in psoriasis patients receiving biologics and whether these results differed from those of psoriasis patients who were not receiving biologics. An institutional review board–approved retrospective chart review was performed for individuals with psoriasis who were patch tested from January 2002 to 2012 at Tufts Medical Center in Boston, Massachusetts. Patients were selected if they had a history of psoriasis, psoriatic arthritis, and patch testing as identified by International Classification of Diseases, Ninth Revision, codes 696.1, 696.0, and 95044, respectively, in their medical records. Patients were patch tested using a modified North American Contact Dermatitis Group standard and cosmetics series. Readings were performed at 48 hours and 72 to 96 hours. The North American Contact Dermatitis Group grading system was used to grade reactions.
The chart review included 15 psoriasis patients who were on biologics (cases) and 16 psoriasis patients who were not on biologics (control subjects). The biologics used were ustekinumab (n=7), etanercept (n=4), adalimumab (n=3), and infliximab (n=1). The authors determined that 80% (12/15) of cases had at least 1 positive reaction compared with 81% (13/16) of control subjects, 67% (10/15) of cases had 2+ positive reactions compared with 63% (10/16) of control subjects, and 27% (4/15) of cases had 3+ positive reactions compared with 38% (6/16) of control subjects. These differences were not statistically significant.
Given the limitation of the small number of patients evaluated, the authors concluded that biologics do not appear to influence the abilities of patients with psoriasis to mount a positive patch test.
What’s the issue?
This study is small, but the findings do give an indication that the biologic agents utilized for psoriasis do not suppress patch test reactions. These data are not typically what we collect in this population, but it is nice to know. What has been your experience in patch testing patients on biologic therapy?
Patch testing is one of the major diagnostic tools in the evaluation of allergic contact dermatitis. One limitation of patch testing is the use of steroids prior to testing. Because steroids may suppress a positive test reaction, the use of topical steroids on the test site or oral steroids should be discontinued for at least 2 weeks prior to testing. Therefore, it is interesting to consider the effect of biologics on the reliability of patch testing.
Kim et al (Dermatitis. 2014;25:182-190) evaluated the prevalence of positive patch tests in psoriasis patients receiving biologics and whether these results differed from those of psoriasis patients who were not receiving biologics. An institutional review board–approved retrospective chart review was performed for individuals with psoriasis who were patch tested from January 2002 to 2012 at Tufts Medical Center in Boston, Massachusetts. Patients were selected if they had a history of psoriasis, psoriatic arthritis, and patch testing as identified by International Classification of Diseases, Ninth Revision, codes 696.1, 696.0, and 95044, respectively, in their medical records. Patients were patch tested using a modified North American Contact Dermatitis Group standard and cosmetics series. Readings were performed at 48 hours and 72 to 96 hours. The North American Contact Dermatitis Group grading system was used to grade reactions.
The chart review included 15 psoriasis patients who were on biologics (cases) and 16 psoriasis patients who were not on biologics (control subjects). The biologics used were ustekinumab (n=7), etanercept (n=4), adalimumab (n=3), and infliximab (n=1). The authors determined that 80% (12/15) of cases had at least 1 positive reaction compared with 81% (13/16) of control subjects, 67% (10/15) of cases had 2+ positive reactions compared with 63% (10/16) of control subjects, and 27% (4/15) of cases had 3+ positive reactions compared with 38% (6/16) of control subjects. These differences were not statistically significant.
Given the limitation of the small number of patients evaluated, the authors concluded that biologics do not appear to influence the abilities of patients with psoriasis to mount a positive patch test.
What’s the issue?
This study is small, but the findings do give an indication that the biologic agents utilized for psoriasis do not suppress patch test reactions. These data are not typically what we collect in this population, but it is nice to know. What has been your experience in patch testing patients on biologic therapy?
A New Appraisal of Dermatologic Manifestations of Diabetes Mellitus
Diabetes mellitus is a morbid and costly condition that carries a high burden of disease for patients (both with and without a diagnosis) and for society as a whole. The economic burden of diabetes in the United States recently was estimated at nearly $250 billion annually,1 and this number continues to rise. The cutaneous manifestations of diabetes are diverse and far-reaching, ranging from benign cosmetic concerns to severe dermatologic conditions. Given the wide range of etiology for diabetes mellitus and its existence on a spectrum of severity, it is perhaps not surprising that some of these entities are the subject of debate (vis-à-vis the strength of association between these skin conditions and diabetes) and can manifest in different forms. However, it is clear that the cutaneous manifestations of diabetes are equally as important to consider and manage as the systemic complications of the disease. In analyzing associations with diabetes, it is important to note that given such a high incidence of diabetes among the general population and its close association with other disease states, such as the metabolic syndrome, studies aimed at determining direct relationships to this entity must be well controlled for confounding factors, which may not even always be possible. Regardless, it is important for dermatologists and dermatology residents to recognize and understand the protean cutaneous manifestations of diabetes mellitus, and this column will explore skin findings that are characteristic of diabetes (Table 1) as well as other dermatoses with a reported but less clear association with diabetes (Table 2).
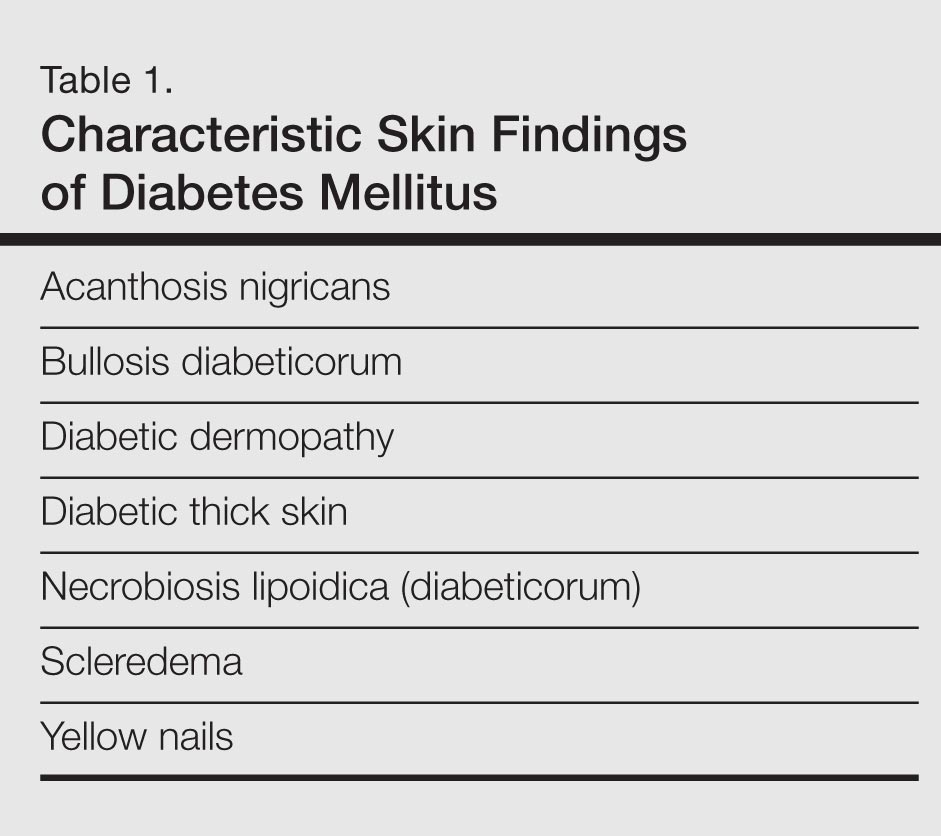

Skin Findings Characteristic of Diabetes
Diabetic Thick Skin
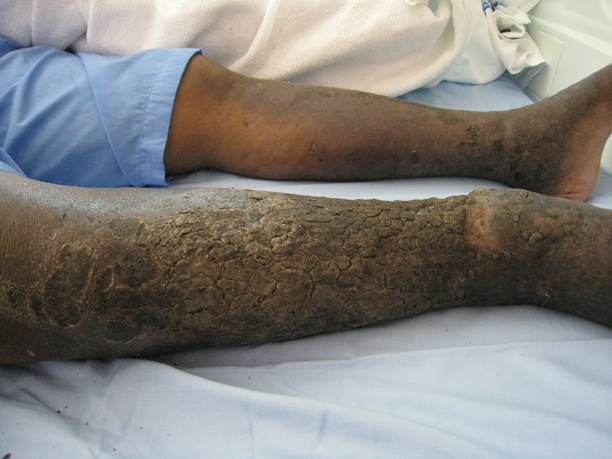
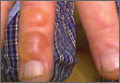
The association between diabetes and thick skin is well described as either a mobility-limiting affliction of the joints of the hands (cheiroarthropathy) or as an asymptomatic thickening of the skin. It has been estimated that 8% to 36% of patients with insulin-dependent diabetes develop some form of skin thickening2; one series also found this association to be true for patients with non–insulin-dependent diabetes mellitus (NIDDM).3 Skin thickening is readily observable on clinical presentation or ultrasonography, with increasing thickness in many cases associated with long-term disease progression. This increasing thickness was shown histopathologically to be a direct result of activated fibroblasts and increased collagen polymerization, with some similar features to progressive systemic sclerosis.4 Interestingly, even clinically normal skin showed some degree of fibroblast activation in diabetic patients, but collagen fibers in each case were smaller in diameter than those found in progressive systemic sclerosis. This finding clearly has implications on quality of life, as a lack of hand mobility due to the cheiroarthropathy can be severely disabling. Underlining the need for strict glycemic control, it has been suggested that tight control of blood sugar levels can lead to improvement in diabetic thick skin; however, reports of improvement are based on a small sample population.5 Huntley papules are localized to areas on the dorsum of the hands overlying the joints, demonstrating hyperkeratosis and enlarged dermal papillae.6 They also can be found in a minority of patients without diabetes. Interestingly, diabetic thick skin also has been associated with neurologic disorders in diabetes.7 Diabetic thick skin was found to be significantly (P<.05) correlated with diabetic neuropathy, independent of duration of diabetes, age, or glycosylated hemoglobin levels, though no causal or etiologic link between these entities has been proven.
Yellow Nails
Nail changes are well described in diabetes, ranging from periungual telangiectases to complications from infections, such as paronychia; however, a well-recognized finding, especially in elderly diabetic patients, is a characteristic yellowing of the nails, reported to affect up to 40% of patients with diabetes.8 The mechanism behind it likely includes accumulation of glycation end products, which also has been thought to lead to yellowing of the skin, and vascular impairment.9 These nails tend to exhibit slow growth, likely resulting from a nail matrix that is poorly supplied with blood, and also can be more curved than normal with longitudinal ridges (onychorrhexis).10 It is important, however, not to attribute yellow nails to diabetes without considering other causes of yellow nails, such as onychomycosis, yellow nail syndrome, and yellow nails associated with lymphedema or respiratory tract disease (eg, pleural effusion, bronchiectasis).11
Diabetic Dermopathy
Colloquially known as shin spots, diabetic dermopathy is perhaps the most common skin finding in this patient population, though it also can occur in up to 1 in 5 individuals without diabetes.12 Although it is very common, it is not a condition that should be overlooked, as numerous studies have shown an increase in microangiopathic complications such as retinopathy in patients with diabetic dermopathy.13,14 Although follow-up studies may be necessary to fully characterize the relationship between shin spots and diabetes, it certainly is reasonable to be more wary of diabetic patients presenting with many shin spots, as the general consensus is that these areas represent postinflammatory hyperpigmentation and cutaneous atrophy in the setting of poor vascular supply, which should prompt analysis of other areas that might be affected by poor vasculature, such as an ophthalmologic examination. Antecedent and perhaps unnoticed trauma has been implicated given a possible underlying neuropathy, but this theory has not been supported by studies.
Bullosis Diabeticorum
Bullosis diabeticorum is a rare but well-described occurrence of self-resolving, nonscarring blisters that arise on the extremities of diabetic patients. This entity should be distinguished from other primary autoimmune blistering disorders and from simple mechanobullous lesions. Several types of bullosis diabeticorum have been described, with the most classic form showing an intraepidermal cleavage plane.15 These lesions tend to resolve in weeks but can be recurrent. The location of the pathology underlines its nonscarring nature, though similar lesions have been reported showing a cleavage plane in the lamina lucida of the dermoepidermal junction, which underlines the confusion in the literature surrounding diabetic bullae.16 Some may even use this term interchangeably with trauma or friction-induced blisters, which diabetics may be prone to develop due to peripheral neuropathy. Confounding reports have stated there is a correlation between bullosis diabeticorum and neuropathy as well as the acral location of these blisters. Although many authors cite the incidence of bullosis diabeticorum being 0.5%,17 no population-based studies have confirmed this figure and some have speculated that the actual incidence is higher.18 In the end, the term bullosis diabeticorum is probably best reserved for a rapidly appearing blister on the extremities of diabetic patients with at most minimal trauma, with a lesion containing sterile fluid and negative immunofluorescence. The mechanism for these blisters is thought to be microangiopathy, with scant blood supply to the skin causing it to be more prone to acantholysis and blister formation.19 This theory was reinforced in a study showing a reduced threshold for suction blister formation in diabetic patients.20 Care should be taken to prevent secondary infections at these sites.
Acanthosis Nigricans
Acanthosis nigricans, which consists of dark brown plaques in the flexural areas, especially the posterior neck and axillae, is a common finding in diabetic patients and is no doubt familiar to clinicians. The pathophysiology of these lesions has been well studied and is a prototype for the effects of insulin resistance in the skin. In this model, high concentrations of insulin binding to insulinlike growth factor receptor in the skin stimulate keratinocyte proliferation,21 leading to the clinical appearance and the histologic finding of hyperkeratosis and papillomatosis, which in turn is responsible for the observed hyperpigmentation. It is an important finding, especially in those without a known history of diabetes, as it can also signal an underlying endocrinopathy (eg, Cushing syndrome, acromegaly, polycystic ovary syndrome) or malignancy (ie, adenocarcinoma of the gastrointestinal tract). Several distinct mechanisms of insulin resistance have been described, including insulin resistance due to receptor defects, such as those seen with insulin resistance in NIDDM; autoimmune processes; and postreceptor defects in insulin action.22 Keratolytics and topical retinoids have been used to ameliorate the appearance of these lesions.
Necrobiosis Lipoidica (Diabeticorum)
Necrobiosis lipoidica diabeticorum was first described by Urback23 in 1932, but reports of similar lesions were described in nondiabetic patients soon after. The dermatologic community has since come to realize that perhaps a more accurate nomenclature is necrobiosis lipoidica to fully encompass this entity. Clinically, lesions appear as erythematous papules and plaques that expand into a larger well-circumscribed plaque with a waxy yellowish atrophic center, often with telangiectases, and usually presenting in the pretibial area. Lesions can become ulcerated in up to one-third of cases. Necrobiosis lipoidica also is defined by characteristic histologic findings, including important features such as palisaded granulomas arranged in a tierlike fashion, necrotizing vasculitis, collagen degradation, and panniculitis. Necrobiosis lipoidica is still relatively rare, developing in approximately 0.3% of patients with diabetes,24 though its relationship with insulin resistance and diabetes is strong. Approximately two-thirds of patients with necrobiosis lipoidica have diabetes and an even higher number go on to develop diabetes or have a positive family history of diabetes.
Although these figures are interesting, the data are nearly a half-century-old, and it is unclear if these findings still hold true today. The etiology of necrobiosis lipoidica also remains elusive, with theories focusing on the role of microangiopathy, immunoglobulin deposition leading to vasculitis, structural abnormalities in collagen or fibroblasts, and trauma; however, the true nature of this condition is likely some combination of these factors.25 These lesions are difficult to treat, especially at an advanced stage. Management with topical steroids to limit the inflammatory progression of the lesions is the mainstay of therapy.
Scleredema
Scleredema adultorum (Buschke disease) refers to indurated plaques over the posterior neck and upper back. It is usually thought of as 3 distinct forms. The form that is known to occur in diabetic patients is sometimes referred to as scleredema diabeticorum; the other 2 occur as postinfectious, usually Streptococcus, or malignancy-related forms. The prevalence of scleredema diabeticorum among diabetic individuals most frequently is reported as 2.5%26; however, it is worth noting that other estimates have been as high as 14%.27 Although there has been some correlation between poorly controlled NIDDM, treatment and tight glucose control does not seem to readily resolve these lesions with only few conflicting case studies serving as evidence for and against this premise.28-30 The lesions often are recalcitrant toward a wide variety of treatment approaches. Histopathologic analysis generally reveals a thickened dermis with large collagen bundles, with clear spaces between the collagen representing mucin and increased numbers of mast cells. Proposed mechanisms include stimulation of collagen synthesis by fibroblasts and retarded collagen degradation, likely due to excess glucose.31
Dermatoses Demonstrating an Association With Diabetes
Granuloma Annulare
Granuloma annulare (GA) is a dermatologic condition existing in numerous forms. The generalized form has been suggested to have some association with diabetes. The lesions of GA are classically round, flesh-colored to erythematous papules arising in the dermis that may start on the dorsal extremities where the localized form typically presents, though larger annular plaques or patches may exist in the generalized form. Histologically, GA has a characteristic granulomatous infiltrate and palisaded granulomatous dermatitis, depending on the stage of the evolution. Many studies dating back to the mid-20th century have attempted to elucidate a link between GA and diabetes, with numerous reports showing conflicting results across their study populations.32-36 This issue is further muddled by links between generalized GA and a host of other diseases, such as malignancy, thyroid disease, hepatitis, and human immunodeficiency virus infection. The usual course of GA is spontaneous resolution, including a peculiar phenomenon noted in the literature whereby biopsy of one of the lesions led to clearance of other lesions on the body.37 However, the generalized form may be more difficult to treat, with therapeutic approaches including topical steroids, light therapy, and systemic immunomodulators.
Lichen Planus
A recent small population study in Turkey demonstrated a strong relationship between lichen planus and abnormal glucose tolerance. In this study of 30 patients with lichen planus, approximately half (14/30) had abnormal glucose metabolism and a quarter (8/30) had known diabetes, but larger studies are needed to clarify this relationship.38 Prior to this report, a link between oral lichen planus and diabetes had been shown in larger case series.39,40 Clinically, one may see white plaques with a characteristic lacy reticular pattern in the mouth. At other cutaneous sites, lichen planus generally appears as pruritic, purple, flat-topped polygonal papules. The clinical finding of lichen planus also is linked with many other disease states, most notably hepatitis C virus, but also thymoma, liver disease, and inflammatory bowel disease, among other associations.41
Vitiligo
As an autoimmune entity, it stands to reason that vitiligo may be seen more commonly associated with insulin-dependent diabetes, which has been shown to hold true in one study, while no association was found between later-onset NIDDM and vitiligo.42 Given the nonspecific nature of this association and the relatively common presentation of vitiligo, no special consideration is likely needed when examining a patient with vitiligo, but its presence should remind the clinician that these autoimmune entities tend to travel together.
Acquired Perforating Dermatosis
Although the classic presentation of acquired perforating dermatosis (Kyrle disease) is linked to renal failure, diabetes also has been connected to its presentation. Extremely rare outside of the setting of chronic renal failure, acquired perforating dermatosis occurs in up to 10% of dialysis patients.43,44 It is characterized by papules with a central keratin plug, representing transepidermal elimination of keratin, collagen, and other cellular material; its etiology has not been elucidated. The connection between acquired perforating dermatosis and diabetes is not completely clear; it would seem that renal failure is a prerequisite for itspresentation. A large proportion of renal failure necessitating hemodialysis occurs in patients with diabetic nephropathy, which may explain the coincidence of diabetes, renal failure, and acquired perforating dermatosis.45 The presentation of this cutaneous finding should not, however, affect treatment of the underlying conditions. Symptom relief in the form of topical steroids can be used as a first-line treatment of these often pruritic lesions.
Eruptive Xanthomas
The link between diabetes and eruptive xanthomas seems to be a rather tenuous one, hinging on the fact that many diabetic patients have abnormalities in carbohydrate and lipid metabolism. A central feature of eruptive xanthomas is an elevation in triglycerides, which can occur in diabetes. Indeed, it has been estimated that only 0.1% of diabetics will develop eruptive xanthomas,46 and its main importance may be to prompt the physician to treat the hypertriglyceridemia and consider other concerning possibilities such as acute pancreatitis.
Psoriasis
Psoriasis is a common dermatologic condition that has been shown to have a far-reaching impact both on patients’ quality of life and cardiovascular risk profiles. Data have emerged linking psoriasis with diabetes as an independent risk factor47; although this retrospective study had its limitations, it certainly is interesting to note that patients with psoriasis may have an increased risk for developing diabetes. Perhaps more importantly, though, this study also implied that patients with severe psoriasis may present with diabetes that is more difficult to control, evidenced by increased treatment with systemic therapies as opposed to milder forms of intervention such as diet control.47 There almost certainly are other confounding factors and further studies would serve to reveal the strength of this association, but it is certainly an intriguing concept. Echoing these findings, a more recent nationwide study from Denmark demonstrated that psoriasis is associated with increased incidence of new-onset diabetes, adjusting for numerous confounding factors.48 The relationship between psoriasis and diabetes is worth noting as evidence continues to emerge.
Conclusion
Given the diverse cutaneous manifestations of diabetes, it is important to distinguish those that are directly related to diabetes from those that suggest there may be another underlying process. For example, a new patient presenting to a primary care physician with acanthosis nigricans and yellow nails should immediately trigger a test for a hemoglobin A1c (glycated hemoglobin) level to investigate for diabetes; however, clinicians also should be wary of patients with acanthosis nigricans who report early satiety, as this asso-ciation may be a sign of underlying malignancy. Conversely, the presence of yellow nails in a patient with chronic diabetes should not be ignored. The physician should consider onychomycosis and query the patient about possible respiratory symptoms. In the case of a multisystem disease such as diabetes, it may be challenging to reconcile seemingly disparate skin findings, but having a framework to approach the cutaneous manifestations of diabetes can help to properly identify and treat an individual patient’s afflictions.
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012 [published online instead of print March 16, 2013]. Diabetes Care. 2013;36:1033-1046.
- Collier A, Matthews DM, Kellett HA, et al. Change in skin thickness associated with cheiroarthropathy in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed). 1986;292:936.
- Fitzcharles MA, Duby S, Waddell RW, et al. Limitation of joint mobility (cheiroarthropathy) in adult noninsulin-dependent diabetic patients. Ann Rheum Dis. 1984;43:251-254.
- Hanna W, Friesen D, Bombardier C, et al. Pathologic features of diabetic thick skin. J Am Acad Dermatol. 1987;16:546-553.
- Lieberman LS, Rosenbloom AL, Riley WJ, et al. Reduced skin thickness with pump administration of insulin. N Engl J Med. 1980;303:940-941.
- Guarneri C, Guarneri F, Borgia F, et al. Finger pebbles in a diabetic patient: Huntley's papules. Int J Dermatol. 2005;44:755-756.
- Forst T, Kann P, Pfützner A, et al. Association between "diabetic thick skin syndrome" and neurological disorders in diabetes mellitus. Acta Diabetol. 1994;31:73-77.
- Nikoleishvili LR, Kurashvili RB, Virsaladze DK, et al. Characteristic changes of skin and its accessories in type 2 diabetes mellitus [in Russian]. Georgian Med News. 2006:43-46.
- Lithner F. Purpura, pigmentation and yellow nails of the lower extremities in diabetics. Acta Med Scand. 1976;199:203-208.
- Greene RA, Scher RK. Nail changes associated with diabetes mellitus. J Am Acad Dermatol. 1987;16:1015-1021.
- Hiller E, Rosenow EC 3rd, Olsen AM. Pulmonary manifestations of the yellow nail syndrome. Chest. 1972;61:452-458.
- Feingold KR, Elias PM. Endocrine-skin interactions. cutaneous manifestations of pituitary disease, thyroid disease, calcium disorders, and diabetes. J Am Acad Dermatol. 1987;17:921-940.
- Abdollahi A, Daneshpazhooh M, Amirchaghmaghi E, et al. Dermopathy and retinopathy in diabetes: is there an association? Dermatology, 2007;214:133-136.
- Morgan AJ, Schwartz RA. Diabetic dermopathy: A subtle sign with grave implications. J Am Acad Dermatol. 2008;58:447-451.
- Perez MI, Kohn SR. Cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol. 1994;30:519-531.
- Cantwell AR, Martz W. Idiopathic bullae in diabetics. Bullosis diabeticorum. Arch Dermatol. 1967;96:42-44.
- Larsen K, Jensen T, Karlsmark T, Holstein PE. Incidence of bullosis diabeticorum – a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596.
- Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200.
- Basarab T, Munn SE, McGrath J, et al. Bullosis diabeticorum. a case report and literature review. Clin Exp Dermatol. 1995;20:218-220.
- Bernstein JE, Levine LE, Medenica MM, et al. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8:790-791.
- Cruz PD Jr, Hud JA Jr. Excess insulin binding to insulin-like growth factor receptors: proposed mechanism for acanthosis nigricans. J Invest Dermatol. 1992;98(suppl 6):S82-S85.
- Romano G, Moretti G, Di Benedetto A, et al. Skin lesions in diabetes mellitus: prevalence and clinical correlations. Diabetes Res Clin Pract. 1998;39:101-106.
- Urback E. Eine neue diabetische Stoffwechseldermatose: Nekrobiosis lipoidica diabeticorum. Arch. f. Dermat. u Syph. 1932;166:273.
- Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. a clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93:272-281.
- Engel MF, Smith JG Jr. The pathogenesis of necrobiosis lipoidica. necrobiosis lipoidica, a form fruste of diabetes mellitus. Arch Dermatol. 1960;82:791-797.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care. 1983;6:189-192.
- Sattar MA, Diab S, Sugathan TN, et al. Scleroedema diabeticorum: a minor but often unrecognized complication of diabetes mellitus. Diabet Med. 1988;5:465-468.
- Rho YW, Suhr KB, Lee JH, et al. A clinical observation of scleredema adultorum and its relationship to diabetes. J Dermatol. 1998;25:103-107.
- Baillot-Rudoni S, Apostol D, Vaillant G, et al. Implantable pump therapy restores metabolic control and quality of life in type 1 diabetic patients with Buschke's nonsystemic scleroderma. Diabetes Care. 2006;29:1710.
- Meguerditchian C, Jacquet P, Béliard S, et al. Scleredema adultorum of Buschke: an under recognized skin complication of diabetes. Diabetes Metab. 2006;32:481-484.
- Behm B, Schreml S, Landthaler M, et al. Skin signs in diabetes mellitus. J Eur Acad Dermatol Venereol. 2012;26:1203-1211.
- Nebesio CL, Lewis C, Chuang TY. Lack of an association between granuloma annulare and type 2 diabetes mellitus. Br J Dermatol. 2002;146:122-124.
- Stankler L, Leslie G. Generalized granuloma annulare. a report of a case and review of the literature. Arch Dermatol. 1976;95:509-513.
- Williamson DM, Dykes JR. Carbohydrate metabolism in granuloma annulare. J Invest Dermatol. 1972;58:400-404.
- Dabski K, Winkelmann RK. Generalized granuloma annulare: clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989;20:39-47.
- Veraldi S, Bencini PL, Drudi E, et al. Laboratory abnormalities in granuloma annulare: a case-control study. Br J Dermatol. 1997;136:652-653.
- Levin NA, Patterson JW, Yao LL, et al. Resolution of patch-type granuloma annulare lesions after biopsy. J Am Acad Dermatol. 2002;46:426-429.
- Seyhan M, Ozcan H, Sahin I, et al. High prevalence of glucose metabolism disturbance in patients with lichen planus. Diabetes Res Clin Pract. 2007;77:198-202.
- Romero MA, Seoane J, Varela-Centelles P, et al. Prevalence of diabetes mellitus amongst oral lichen planus patients. clinical and pathological characteristics. Med Oral. 2002;7:121-129.
- Albrecht M, Banoczy J, Dinya E, et al. Occurrence of oral leukoplakia and lichen planus in diabetes mellitus. J Oral Pathol Med. 1992;21:364-366.
- Lehman JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Gould IM, Gray RS, Urbaniak SJ, et al. Vitiligo in diabetes mellitus. Br J Dermatol. 1985;113:153-155.
- White CR Jr, Heskel NS, Pokorny DJ. Perforating folliculitis of hemodialysis. Am J Dermatopathol. 1982;4:109-116.
- Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Muller SA. Dermatologic disorders associated with diabetes mellitus. Mayo Clin Proc. 1966;41:689-703.
- Azfar RS, Seminara NM, Shin DB, et al. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol. 2012;148:995-1000.
- Khalid U, Hansen PR, Gislason GH, et al. Psoriasis and new-onset diabetes: a Danish nationwide cohort study. Diabetes Care. 2013;36:2402-2407.
Diabetes mellitus is a morbid and costly condition that carries a high burden of disease for patients (both with and without a diagnosis) and for society as a whole. The economic burden of diabetes in the United States recently was estimated at nearly $250 billion annually,1 and this number continues to rise. The cutaneous manifestations of diabetes are diverse and far-reaching, ranging from benign cosmetic concerns to severe dermatologic conditions. Given the wide range of etiology for diabetes mellitus and its existence on a spectrum of severity, it is perhaps not surprising that some of these entities are the subject of debate (vis-à-vis the strength of association between these skin conditions and diabetes) and can manifest in different forms. However, it is clear that the cutaneous manifestations of diabetes are equally as important to consider and manage as the systemic complications of the disease. In analyzing associations with diabetes, it is important to note that given such a high incidence of diabetes among the general population and its close association with other disease states, such as the metabolic syndrome, studies aimed at determining direct relationships to this entity must be well controlled for confounding factors, which may not even always be possible. Regardless, it is important for dermatologists and dermatology residents to recognize and understand the protean cutaneous manifestations of diabetes mellitus, and this column will explore skin findings that are characteristic of diabetes (Table 1) as well as other dermatoses with a reported but less clear association with diabetes (Table 2).


Skin Findings Characteristic of Diabetes
Diabetic Thick Skin
The association between diabetes and thick skin is well described as either a mobility-limiting affliction of the joints of the hands (cheiroarthropathy) or as an asymptomatic thickening of the skin. It has been estimated that 8% to 36% of patients with insulin-dependent diabetes develop some form of skin thickening2; one series also found this association to be true for patients with non–insulin-dependent diabetes mellitus (NIDDM).3 Skin thickening is readily observable on clinical presentation or ultrasonography, with increasing thickness in many cases associated with long-term disease progression. This increasing thickness was shown histopathologically to be a direct result of activated fibroblasts and increased collagen polymerization, with some similar features to progressive systemic sclerosis.4 Interestingly, even clinically normal skin showed some degree of fibroblast activation in diabetic patients, but collagen fibers in each case were smaller in diameter than those found in progressive systemic sclerosis. This finding clearly has implications on quality of life, as a lack of hand mobility due to the cheiroarthropathy can be severely disabling. Underlining the need for strict glycemic control, it has been suggested that tight control of blood sugar levels can lead to improvement in diabetic thick skin; however, reports of improvement are based on a small sample population.5 Huntley papules are localized to areas on the dorsum of the hands overlying the joints, demonstrating hyperkeratosis and enlarged dermal papillae.6 They also can be found in a minority of patients without diabetes. Interestingly, diabetic thick skin also has been associated with neurologic disorders in diabetes.7 Diabetic thick skin was found to be significantly (P<.05) correlated with diabetic neuropathy, independent of duration of diabetes, age, or glycosylated hemoglobin levels, though no causal or etiologic link between these entities has been proven.
Yellow Nails
Nail changes are well described in diabetes, ranging from periungual telangiectases to complications from infections, such as paronychia; however, a well-recognized finding, especially in elderly diabetic patients, is a characteristic yellowing of the nails, reported to affect up to 40% of patients with diabetes.8 The mechanism behind it likely includes accumulation of glycation end products, which also has been thought to lead to yellowing of the skin, and vascular impairment.9 These nails tend to exhibit slow growth, likely resulting from a nail matrix that is poorly supplied with blood, and also can be more curved than normal with longitudinal ridges (onychorrhexis).10 It is important, however, not to attribute yellow nails to diabetes without considering other causes of yellow nails, such as onychomycosis, yellow nail syndrome, and yellow nails associated with lymphedema or respiratory tract disease (eg, pleural effusion, bronchiectasis).11
Diabetic Dermopathy
Colloquially known as shin spots, diabetic dermopathy is perhaps the most common skin finding in this patient population, though it also can occur in up to 1 in 5 individuals without diabetes.12 Although it is very common, it is not a condition that should be overlooked, as numerous studies have shown an increase in microangiopathic complications such as retinopathy in patients with diabetic dermopathy.13,14 Although follow-up studies may be necessary to fully characterize the relationship between shin spots and diabetes, it certainly is reasonable to be more wary of diabetic patients presenting with many shin spots, as the general consensus is that these areas represent postinflammatory hyperpigmentation and cutaneous atrophy in the setting of poor vascular supply, which should prompt analysis of other areas that might be affected by poor vasculature, such as an ophthalmologic examination. Antecedent and perhaps unnoticed trauma has been implicated given a possible underlying neuropathy, but this theory has not been supported by studies.
Bullosis Diabeticorum
Bullosis diabeticorum is a rare but well-described occurrence of self-resolving, nonscarring blisters that arise on the extremities of diabetic patients. This entity should be distinguished from other primary autoimmune blistering disorders and from simple mechanobullous lesions. Several types of bullosis diabeticorum have been described, with the most classic form showing an intraepidermal cleavage plane.15 These lesions tend to resolve in weeks but can be recurrent. The location of the pathology underlines its nonscarring nature, though similar lesions have been reported showing a cleavage plane in the lamina lucida of the dermoepidermal junction, which underlines the confusion in the literature surrounding diabetic bullae.16 Some may even use this term interchangeably with trauma or friction-induced blisters, which diabetics may be prone to develop due to peripheral neuropathy. Confounding reports have stated there is a correlation between bullosis diabeticorum and neuropathy as well as the acral location of these blisters. Although many authors cite the incidence of bullosis diabeticorum being 0.5%,17 no population-based studies have confirmed this figure and some have speculated that the actual incidence is higher.18 In the end, the term bullosis diabeticorum is probably best reserved for a rapidly appearing blister on the extremities of diabetic patients with at most minimal trauma, with a lesion containing sterile fluid and negative immunofluorescence. The mechanism for these blisters is thought to be microangiopathy, with scant blood supply to the skin causing it to be more prone to acantholysis and blister formation.19 This theory was reinforced in a study showing a reduced threshold for suction blister formation in diabetic patients.20 Care should be taken to prevent secondary infections at these sites.
Acanthosis Nigricans
Acanthosis nigricans, which consists of dark brown plaques in the flexural areas, especially the posterior neck and axillae, is a common finding in diabetic patients and is no doubt familiar to clinicians. The pathophysiology of these lesions has been well studied and is a prototype for the effects of insulin resistance in the skin. In this model, high concentrations of insulin binding to insulinlike growth factor receptor in the skin stimulate keratinocyte proliferation,21 leading to the clinical appearance and the histologic finding of hyperkeratosis and papillomatosis, which in turn is responsible for the observed hyperpigmentation. It is an important finding, especially in those without a known history of diabetes, as it can also signal an underlying endocrinopathy (eg, Cushing syndrome, acromegaly, polycystic ovary syndrome) or malignancy (ie, adenocarcinoma of the gastrointestinal tract). Several distinct mechanisms of insulin resistance have been described, including insulin resistance due to receptor defects, such as those seen with insulin resistance in NIDDM; autoimmune processes; and postreceptor defects in insulin action.22 Keratolytics and topical retinoids have been used to ameliorate the appearance of these lesions.
Necrobiosis Lipoidica (Diabeticorum)
Necrobiosis lipoidica diabeticorum was first described by Urback23 in 1932, but reports of similar lesions were described in nondiabetic patients soon after. The dermatologic community has since come to realize that perhaps a more accurate nomenclature is necrobiosis lipoidica to fully encompass this entity. Clinically, lesions appear as erythematous papules and plaques that expand into a larger well-circumscribed plaque with a waxy yellowish atrophic center, often with telangiectases, and usually presenting in the pretibial area. Lesions can become ulcerated in up to one-third of cases. Necrobiosis lipoidica also is defined by characteristic histologic findings, including important features such as palisaded granulomas arranged in a tierlike fashion, necrotizing vasculitis, collagen degradation, and panniculitis. Necrobiosis lipoidica is still relatively rare, developing in approximately 0.3% of patients with diabetes,24 though its relationship with insulin resistance and diabetes is strong. Approximately two-thirds of patients with necrobiosis lipoidica have diabetes and an even higher number go on to develop diabetes or have a positive family history of diabetes.
Although these figures are interesting, the data are nearly a half-century-old, and it is unclear if these findings still hold true today. The etiology of necrobiosis lipoidica also remains elusive, with theories focusing on the role of microangiopathy, immunoglobulin deposition leading to vasculitis, structural abnormalities in collagen or fibroblasts, and trauma; however, the true nature of this condition is likely some combination of these factors.25 These lesions are difficult to treat, especially at an advanced stage. Management with topical steroids to limit the inflammatory progression of the lesions is the mainstay of therapy.
Scleredema
Scleredema adultorum (Buschke disease) refers to indurated plaques over the posterior neck and upper back. It is usually thought of as 3 distinct forms. The form that is known to occur in diabetic patients is sometimes referred to as scleredema diabeticorum; the other 2 occur as postinfectious, usually Streptococcus, or malignancy-related forms. The prevalence of scleredema diabeticorum among diabetic individuals most frequently is reported as 2.5%26; however, it is worth noting that other estimates have been as high as 14%.27 Although there has been some correlation between poorly controlled NIDDM, treatment and tight glucose control does not seem to readily resolve these lesions with only few conflicting case studies serving as evidence for and against this premise.28-30 The lesions often are recalcitrant toward a wide variety of treatment approaches. Histopathologic analysis generally reveals a thickened dermis with large collagen bundles, with clear spaces between the collagen representing mucin and increased numbers of mast cells. Proposed mechanisms include stimulation of collagen synthesis by fibroblasts and retarded collagen degradation, likely due to excess glucose.31
Dermatoses Demonstrating an Association With Diabetes
Granuloma Annulare
Granuloma annulare (GA) is a dermatologic condition existing in numerous forms. The generalized form has been suggested to have some association with diabetes. The lesions of GA are classically round, flesh-colored to erythematous papules arising in the dermis that may start on the dorsal extremities where the localized form typically presents, though larger annular plaques or patches may exist in the generalized form. Histologically, GA has a characteristic granulomatous infiltrate and palisaded granulomatous dermatitis, depending on the stage of the evolution. Many studies dating back to the mid-20th century have attempted to elucidate a link between GA and diabetes, with numerous reports showing conflicting results across their study populations.32-36 This issue is further muddled by links between generalized GA and a host of other diseases, such as malignancy, thyroid disease, hepatitis, and human immunodeficiency virus infection. The usual course of GA is spontaneous resolution, including a peculiar phenomenon noted in the literature whereby biopsy of one of the lesions led to clearance of other lesions on the body.37 However, the generalized form may be more difficult to treat, with therapeutic approaches including topical steroids, light therapy, and systemic immunomodulators.
Lichen Planus
A recent small population study in Turkey demonstrated a strong relationship between lichen planus and abnormal glucose tolerance. In this study of 30 patients with lichen planus, approximately half (14/30) had abnormal glucose metabolism and a quarter (8/30) had known diabetes, but larger studies are needed to clarify this relationship.38 Prior to this report, a link between oral lichen planus and diabetes had been shown in larger case series.39,40 Clinically, one may see white plaques with a characteristic lacy reticular pattern in the mouth. At other cutaneous sites, lichen planus generally appears as pruritic, purple, flat-topped polygonal papules. The clinical finding of lichen planus also is linked with many other disease states, most notably hepatitis C virus, but also thymoma, liver disease, and inflammatory bowel disease, among other associations.41
Vitiligo
As an autoimmune entity, it stands to reason that vitiligo may be seen more commonly associated with insulin-dependent diabetes, which has been shown to hold true in one study, while no association was found between later-onset NIDDM and vitiligo.42 Given the nonspecific nature of this association and the relatively common presentation of vitiligo, no special consideration is likely needed when examining a patient with vitiligo, but its presence should remind the clinician that these autoimmune entities tend to travel together.
Acquired Perforating Dermatosis
Although the classic presentation of acquired perforating dermatosis (Kyrle disease) is linked to renal failure, diabetes also has been connected to its presentation. Extremely rare outside of the setting of chronic renal failure, acquired perforating dermatosis occurs in up to 10% of dialysis patients.43,44 It is characterized by papules with a central keratin plug, representing transepidermal elimination of keratin, collagen, and other cellular material; its etiology has not been elucidated. The connection between acquired perforating dermatosis and diabetes is not completely clear; it would seem that renal failure is a prerequisite for itspresentation. A large proportion of renal failure necessitating hemodialysis occurs in patients with diabetic nephropathy, which may explain the coincidence of diabetes, renal failure, and acquired perforating dermatosis.45 The presentation of this cutaneous finding should not, however, affect treatment of the underlying conditions. Symptom relief in the form of topical steroids can be used as a first-line treatment of these often pruritic lesions.
Eruptive Xanthomas
The link between diabetes and eruptive xanthomas seems to be a rather tenuous one, hinging on the fact that many diabetic patients have abnormalities in carbohydrate and lipid metabolism. A central feature of eruptive xanthomas is an elevation in triglycerides, which can occur in diabetes. Indeed, it has been estimated that only 0.1% of diabetics will develop eruptive xanthomas,46 and its main importance may be to prompt the physician to treat the hypertriglyceridemia and consider other concerning possibilities such as acute pancreatitis.
Psoriasis
Psoriasis is a common dermatologic condition that has been shown to have a far-reaching impact both on patients’ quality of life and cardiovascular risk profiles. Data have emerged linking psoriasis with diabetes as an independent risk factor47; although this retrospective study had its limitations, it certainly is interesting to note that patients with psoriasis may have an increased risk for developing diabetes. Perhaps more importantly, though, this study also implied that patients with severe psoriasis may present with diabetes that is more difficult to control, evidenced by increased treatment with systemic therapies as opposed to milder forms of intervention such as diet control.47 There almost certainly are other confounding factors and further studies would serve to reveal the strength of this association, but it is certainly an intriguing concept. Echoing these findings, a more recent nationwide study from Denmark demonstrated that psoriasis is associated with increased incidence of new-onset diabetes, adjusting for numerous confounding factors.48 The relationship between psoriasis and diabetes is worth noting as evidence continues to emerge.
Conclusion
Given the diverse cutaneous manifestations of diabetes, it is important to distinguish those that are directly related to diabetes from those that suggest there may be another underlying process. For example, a new patient presenting to a primary care physician with acanthosis nigricans and yellow nails should immediately trigger a test for a hemoglobin A1c (glycated hemoglobin) level to investigate for diabetes; however, clinicians also should be wary of patients with acanthosis nigricans who report early satiety, as this asso-ciation may be a sign of underlying malignancy. Conversely, the presence of yellow nails in a patient with chronic diabetes should not be ignored. The physician should consider onychomycosis and query the patient about possible respiratory symptoms. In the case of a multisystem disease such as diabetes, it may be challenging to reconcile seemingly disparate skin findings, but having a framework to approach the cutaneous manifestations of diabetes can help to properly identify and treat an individual patient’s afflictions.
Diabetes mellitus is a morbid and costly condition that carries a high burden of disease for patients (both with and without a diagnosis) and for society as a whole. The economic burden of diabetes in the United States recently was estimated at nearly $250 billion annually,1 and this number continues to rise. The cutaneous manifestations of diabetes are diverse and far-reaching, ranging from benign cosmetic concerns to severe dermatologic conditions. Given the wide range of etiology for diabetes mellitus and its existence on a spectrum of severity, it is perhaps not surprising that some of these entities are the subject of debate (vis-à-vis the strength of association between these skin conditions and diabetes) and can manifest in different forms. However, it is clear that the cutaneous manifestations of diabetes are equally as important to consider and manage as the systemic complications of the disease. In analyzing associations with diabetes, it is important to note that given such a high incidence of diabetes among the general population and its close association with other disease states, such as the metabolic syndrome, studies aimed at determining direct relationships to this entity must be well controlled for confounding factors, which may not even always be possible. Regardless, it is important for dermatologists and dermatology residents to recognize and understand the protean cutaneous manifestations of diabetes mellitus, and this column will explore skin findings that are characteristic of diabetes (Table 1) as well as other dermatoses with a reported but less clear association with diabetes (Table 2).


Skin Findings Characteristic of Diabetes
Diabetic Thick Skin
The association between diabetes and thick skin is well described as either a mobility-limiting affliction of the joints of the hands (cheiroarthropathy) or as an asymptomatic thickening of the skin. It has been estimated that 8% to 36% of patients with insulin-dependent diabetes develop some form of skin thickening2; one series also found this association to be true for patients with non–insulin-dependent diabetes mellitus (NIDDM).3 Skin thickening is readily observable on clinical presentation or ultrasonography, with increasing thickness in many cases associated with long-term disease progression. This increasing thickness was shown histopathologically to be a direct result of activated fibroblasts and increased collagen polymerization, with some similar features to progressive systemic sclerosis.4 Interestingly, even clinically normal skin showed some degree of fibroblast activation in diabetic patients, but collagen fibers in each case were smaller in diameter than those found in progressive systemic sclerosis. This finding clearly has implications on quality of life, as a lack of hand mobility due to the cheiroarthropathy can be severely disabling. Underlining the need for strict glycemic control, it has been suggested that tight control of blood sugar levels can lead to improvement in diabetic thick skin; however, reports of improvement are based on a small sample population.5 Huntley papules are localized to areas on the dorsum of the hands overlying the joints, demonstrating hyperkeratosis and enlarged dermal papillae.6 They also can be found in a minority of patients without diabetes. Interestingly, diabetic thick skin also has been associated with neurologic disorders in diabetes.7 Diabetic thick skin was found to be significantly (P<.05) correlated with diabetic neuropathy, independent of duration of diabetes, age, or glycosylated hemoglobin levels, though no causal or etiologic link between these entities has been proven.
Yellow Nails
Nail changes are well described in diabetes, ranging from periungual telangiectases to complications from infections, such as paronychia; however, a well-recognized finding, especially in elderly diabetic patients, is a characteristic yellowing of the nails, reported to affect up to 40% of patients with diabetes.8 The mechanism behind it likely includes accumulation of glycation end products, which also has been thought to lead to yellowing of the skin, and vascular impairment.9 These nails tend to exhibit slow growth, likely resulting from a nail matrix that is poorly supplied with blood, and also can be more curved than normal with longitudinal ridges (onychorrhexis).10 It is important, however, not to attribute yellow nails to diabetes without considering other causes of yellow nails, such as onychomycosis, yellow nail syndrome, and yellow nails associated with lymphedema or respiratory tract disease (eg, pleural effusion, bronchiectasis).11
Diabetic Dermopathy
Colloquially known as shin spots, diabetic dermopathy is perhaps the most common skin finding in this patient population, though it also can occur in up to 1 in 5 individuals without diabetes.12 Although it is very common, it is not a condition that should be overlooked, as numerous studies have shown an increase in microangiopathic complications such as retinopathy in patients with diabetic dermopathy.13,14 Although follow-up studies may be necessary to fully characterize the relationship between shin spots and diabetes, it certainly is reasonable to be more wary of diabetic patients presenting with many shin spots, as the general consensus is that these areas represent postinflammatory hyperpigmentation and cutaneous atrophy in the setting of poor vascular supply, which should prompt analysis of other areas that might be affected by poor vasculature, such as an ophthalmologic examination. Antecedent and perhaps unnoticed trauma has been implicated given a possible underlying neuropathy, but this theory has not been supported by studies.
Bullosis Diabeticorum
Bullosis diabeticorum is a rare but well-described occurrence of self-resolving, nonscarring blisters that arise on the extremities of diabetic patients. This entity should be distinguished from other primary autoimmune blistering disorders and from simple mechanobullous lesions. Several types of bullosis diabeticorum have been described, with the most classic form showing an intraepidermal cleavage plane.15 These lesions tend to resolve in weeks but can be recurrent. The location of the pathology underlines its nonscarring nature, though similar lesions have been reported showing a cleavage plane in the lamina lucida of the dermoepidermal junction, which underlines the confusion in the literature surrounding diabetic bullae.16 Some may even use this term interchangeably with trauma or friction-induced blisters, which diabetics may be prone to develop due to peripheral neuropathy. Confounding reports have stated there is a correlation between bullosis diabeticorum and neuropathy as well as the acral location of these blisters. Although many authors cite the incidence of bullosis diabeticorum being 0.5%,17 no population-based studies have confirmed this figure and some have speculated that the actual incidence is higher.18 In the end, the term bullosis diabeticorum is probably best reserved for a rapidly appearing blister on the extremities of diabetic patients with at most minimal trauma, with a lesion containing sterile fluid and negative immunofluorescence. The mechanism for these blisters is thought to be microangiopathy, with scant blood supply to the skin causing it to be more prone to acantholysis and blister formation.19 This theory was reinforced in a study showing a reduced threshold for suction blister formation in diabetic patients.20 Care should be taken to prevent secondary infections at these sites.
Acanthosis Nigricans
Acanthosis nigricans, which consists of dark brown plaques in the flexural areas, especially the posterior neck and axillae, is a common finding in diabetic patients and is no doubt familiar to clinicians. The pathophysiology of these lesions has been well studied and is a prototype for the effects of insulin resistance in the skin. In this model, high concentrations of insulin binding to insulinlike growth factor receptor in the skin stimulate keratinocyte proliferation,21 leading to the clinical appearance and the histologic finding of hyperkeratosis and papillomatosis, which in turn is responsible for the observed hyperpigmentation. It is an important finding, especially in those without a known history of diabetes, as it can also signal an underlying endocrinopathy (eg, Cushing syndrome, acromegaly, polycystic ovary syndrome) or malignancy (ie, adenocarcinoma of the gastrointestinal tract). Several distinct mechanisms of insulin resistance have been described, including insulin resistance due to receptor defects, such as those seen with insulin resistance in NIDDM; autoimmune processes; and postreceptor defects in insulin action.22 Keratolytics and topical retinoids have been used to ameliorate the appearance of these lesions.
Necrobiosis Lipoidica (Diabeticorum)
Necrobiosis lipoidica diabeticorum was first described by Urback23 in 1932, but reports of similar lesions were described in nondiabetic patients soon after. The dermatologic community has since come to realize that perhaps a more accurate nomenclature is necrobiosis lipoidica to fully encompass this entity. Clinically, lesions appear as erythematous papules and plaques that expand into a larger well-circumscribed plaque with a waxy yellowish atrophic center, often with telangiectases, and usually presenting in the pretibial area. Lesions can become ulcerated in up to one-third of cases. Necrobiosis lipoidica also is defined by characteristic histologic findings, including important features such as palisaded granulomas arranged in a tierlike fashion, necrotizing vasculitis, collagen degradation, and panniculitis. Necrobiosis lipoidica is still relatively rare, developing in approximately 0.3% of patients with diabetes,24 though its relationship with insulin resistance and diabetes is strong. Approximately two-thirds of patients with necrobiosis lipoidica have diabetes and an even higher number go on to develop diabetes or have a positive family history of diabetes.
Although these figures are interesting, the data are nearly a half-century-old, and it is unclear if these findings still hold true today. The etiology of necrobiosis lipoidica also remains elusive, with theories focusing on the role of microangiopathy, immunoglobulin deposition leading to vasculitis, structural abnormalities in collagen or fibroblasts, and trauma; however, the true nature of this condition is likely some combination of these factors.25 These lesions are difficult to treat, especially at an advanced stage. Management with topical steroids to limit the inflammatory progression of the lesions is the mainstay of therapy.
Scleredema
Scleredema adultorum (Buschke disease) refers to indurated plaques over the posterior neck and upper back. It is usually thought of as 3 distinct forms. The form that is known to occur in diabetic patients is sometimes referred to as scleredema diabeticorum; the other 2 occur as postinfectious, usually Streptococcus, or malignancy-related forms. The prevalence of scleredema diabeticorum among diabetic individuals most frequently is reported as 2.5%26; however, it is worth noting that other estimates have been as high as 14%.27 Although there has been some correlation between poorly controlled NIDDM, treatment and tight glucose control does not seem to readily resolve these lesions with only few conflicting case studies serving as evidence for and against this premise.28-30 The lesions often are recalcitrant toward a wide variety of treatment approaches. Histopathologic analysis generally reveals a thickened dermis with large collagen bundles, with clear spaces between the collagen representing mucin and increased numbers of mast cells. Proposed mechanisms include stimulation of collagen synthesis by fibroblasts and retarded collagen degradation, likely due to excess glucose.31
Dermatoses Demonstrating an Association With Diabetes
Granuloma Annulare
Granuloma annulare (GA) is a dermatologic condition existing in numerous forms. The generalized form has been suggested to have some association with diabetes. The lesions of GA are classically round, flesh-colored to erythematous papules arising in the dermis that may start on the dorsal extremities where the localized form typically presents, though larger annular plaques or patches may exist in the generalized form. Histologically, GA has a characteristic granulomatous infiltrate and palisaded granulomatous dermatitis, depending on the stage of the evolution. Many studies dating back to the mid-20th century have attempted to elucidate a link between GA and diabetes, with numerous reports showing conflicting results across their study populations.32-36 This issue is further muddled by links between generalized GA and a host of other diseases, such as malignancy, thyroid disease, hepatitis, and human immunodeficiency virus infection. The usual course of GA is spontaneous resolution, including a peculiar phenomenon noted in the literature whereby biopsy of one of the lesions led to clearance of other lesions on the body.37 However, the generalized form may be more difficult to treat, with therapeutic approaches including topical steroids, light therapy, and systemic immunomodulators.
Lichen Planus
A recent small population study in Turkey demonstrated a strong relationship between lichen planus and abnormal glucose tolerance. In this study of 30 patients with lichen planus, approximately half (14/30) had abnormal glucose metabolism and a quarter (8/30) had known diabetes, but larger studies are needed to clarify this relationship.38 Prior to this report, a link between oral lichen planus and diabetes had been shown in larger case series.39,40 Clinically, one may see white plaques with a characteristic lacy reticular pattern in the mouth. At other cutaneous sites, lichen planus generally appears as pruritic, purple, flat-topped polygonal papules. The clinical finding of lichen planus also is linked with many other disease states, most notably hepatitis C virus, but also thymoma, liver disease, and inflammatory bowel disease, among other associations.41
Vitiligo
As an autoimmune entity, it stands to reason that vitiligo may be seen more commonly associated with insulin-dependent diabetes, which has been shown to hold true in one study, while no association was found between later-onset NIDDM and vitiligo.42 Given the nonspecific nature of this association and the relatively common presentation of vitiligo, no special consideration is likely needed when examining a patient with vitiligo, but its presence should remind the clinician that these autoimmune entities tend to travel together.
Acquired Perforating Dermatosis
Although the classic presentation of acquired perforating dermatosis (Kyrle disease) is linked to renal failure, diabetes also has been connected to its presentation. Extremely rare outside of the setting of chronic renal failure, acquired perforating dermatosis occurs in up to 10% of dialysis patients.43,44 It is characterized by papules with a central keratin plug, representing transepidermal elimination of keratin, collagen, and other cellular material; its etiology has not been elucidated. The connection between acquired perforating dermatosis and diabetes is not completely clear; it would seem that renal failure is a prerequisite for itspresentation. A large proportion of renal failure necessitating hemodialysis occurs in patients with diabetic nephropathy, which may explain the coincidence of diabetes, renal failure, and acquired perforating dermatosis.45 The presentation of this cutaneous finding should not, however, affect treatment of the underlying conditions. Symptom relief in the form of topical steroids can be used as a first-line treatment of these often pruritic lesions.
Eruptive Xanthomas
The link between diabetes and eruptive xanthomas seems to be a rather tenuous one, hinging on the fact that many diabetic patients have abnormalities in carbohydrate and lipid metabolism. A central feature of eruptive xanthomas is an elevation in triglycerides, which can occur in diabetes. Indeed, it has been estimated that only 0.1% of diabetics will develop eruptive xanthomas,46 and its main importance may be to prompt the physician to treat the hypertriglyceridemia and consider other concerning possibilities such as acute pancreatitis.
Psoriasis
Psoriasis is a common dermatologic condition that has been shown to have a far-reaching impact both on patients’ quality of life and cardiovascular risk profiles. Data have emerged linking psoriasis with diabetes as an independent risk factor47; although this retrospective study had its limitations, it certainly is interesting to note that patients with psoriasis may have an increased risk for developing diabetes. Perhaps more importantly, though, this study also implied that patients with severe psoriasis may present with diabetes that is more difficult to control, evidenced by increased treatment with systemic therapies as opposed to milder forms of intervention such as diet control.47 There almost certainly are other confounding factors and further studies would serve to reveal the strength of this association, but it is certainly an intriguing concept. Echoing these findings, a more recent nationwide study from Denmark demonstrated that psoriasis is associated with increased incidence of new-onset diabetes, adjusting for numerous confounding factors.48 The relationship between psoriasis and diabetes is worth noting as evidence continues to emerge.
Conclusion
Given the diverse cutaneous manifestations of diabetes, it is important to distinguish those that are directly related to diabetes from those that suggest there may be another underlying process. For example, a new patient presenting to a primary care physician with acanthosis nigricans and yellow nails should immediately trigger a test for a hemoglobin A1c (glycated hemoglobin) level to investigate for diabetes; however, clinicians also should be wary of patients with acanthosis nigricans who report early satiety, as this asso-ciation may be a sign of underlying malignancy. Conversely, the presence of yellow nails in a patient with chronic diabetes should not be ignored. The physician should consider onychomycosis and query the patient about possible respiratory symptoms. In the case of a multisystem disease such as diabetes, it may be challenging to reconcile seemingly disparate skin findings, but having a framework to approach the cutaneous manifestations of diabetes can help to properly identify and treat an individual patient’s afflictions.
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012 [published online instead of print March 16, 2013]. Diabetes Care. 2013;36:1033-1046.
- Collier A, Matthews DM, Kellett HA, et al. Change in skin thickness associated with cheiroarthropathy in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed). 1986;292:936.
- Fitzcharles MA, Duby S, Waddell RW, et al. Limitation of joint mobility (cheiroarthropathy) in adult noninsulin-dependent diabetic patients. Ann Rheum Dis. 1984;43:251-254.
- Hanna W, Friesen D, Bombardier C, et al. Pathologic features of diabetic thick skin. J Am Acad Dermatol. 1987;16:546-553.
- Lieberman LS, Rosenbloom AL, Riley WJ, et al. Reduced skin thickness with pump administration of insulin. N Engl J Med. 1980;303:940-941.
- Guarneri C, Guarneri F, Borgia F, et al. Finger pebbles in a diabetic patient: Huntley's papules. Int J Dermatol. 2005;44:755-756.
- Forst T, Kann P, Pfützner A, et al. Association between "diabetic thick skin syndrome" and neurological disorders in diabetes mellitus. Acta Diabetol. 1994;31:73-77.
- Nikoleishvili LR, Kurashvili RB, Virsaladze DK, et al. Characteristic changes of skin and its accessories in type 2 diabetes mellitus [in Russian]. Georgian Med News. 2006:43-46.
- Lithner F. Purpura, pigmentation and yellow nails of the lower extremities in diabetics. Acta Med Scand. 1976;199:203-208.
- Greene RA, Scher RK. Nail changes associated with diabetes mellitus. J Am Acad Dermatol. 1987;16:1015-1021.
- Hiller E, Rosenow EC 3rd, Olsen AM. Pulmonary manifestations of the yellow nail syndrome. Chest. 1972;61:452-458.
- Feingold KR, Elias PM. Endocrine-skin interactions. cutaneous manifestations of pituitary disease, thyroid disease, calcium disorders, and diabetes. J Am Acad Dermatol. 1987;17:921-940.
- Abdollahi A, Daneshpazhooh M, Amirchaghmaghi E, et al. Dermopathy and retinopathy in diabetes: is there an association? Dermatology, 2007;214:133-136.
- Morgan AJ, Schwartz RA. Diabetic dermopathy: A subtle sign with grave implications. J Am Acad Dermatol. 2008;58:447-451.
- Perez MI, Kohn SR. Cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol. 1994;30:519-531.
- Cantwell AR, Martz W. Idiopathic bullae in diabetics. Bullosis diabeticorum. Arch Dermatol. 1967;96:42-44.
- Larsen K, Jensen T, Karlsmark T, Holstein PE. Incidence of bullosis diabeticorum – a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596.
- Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200.
- Basarab T, Munn SE, McGrath J, et al. Bullosis diabeticorum. a case report and literature review. Clin Exp Dermatol. 1995;20:218-220.
- Bernstein JE, Levine LE, Medenica MM, et al. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8:790-791.
- Cruz PD Jr, Hud JA Jr. Excess insulin binding to insulin-like growth factor receptors: proposed mechanism for acanthosis nigricans. J Invest Dermatol. 1992;98(suppl 6):S82-S85.
- Romano G, Moretti G, Di Benedetto A, et al. Skin lesions in diabetes mellitus: prevalence and clinical correlations. Diabetes Res Clin Pract. 1998;39:101-106.
- Urback E. Eine neue diabetische Stoffwechseldermatose: Nekrobiosis lipoidica diabeticorum. Arch. f. Dermat. u Syph. 1932;166:273.
- Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. a clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93:272-281.
- Engel MF, Smith JG Jr. The pathogenesis of necrobiosis lipoidica. necrobiosis lipoidica, a form fruste of diabetes mellitus. Arch Dermatol. 1960;82:791-797.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care. 1983;6:189-192.
- Sattar MA, Diab S, Sugathan TN, et al. Scleroedema diabeticorum: a minor but often unrecognized complication of diabetes mellitus. Diabet Med. 1988;5:465-468.
- Rho YW, Suhr KB, Lee JH, et al. A clinical observation of scleredema adultorum and its relationship to diabetes. J Dermatol. 1998;25:103-107.
- Baillot-Rudoni S, Apostol D, Vaillant G, et al. Implantable pump therapy restores metabolic control and quality of life in type 1 diabetic patients with Buschke's nonsystemic scleroderma. Diabetes Care. 2006;29:1710.
- Meguerditchian C, Jacquet P, Béliard S, et al. Scleredema adultorum of Buschke: an under recognized skin complication of diabetes. Diabetes Metab. 2006;32:481-484.
- Behm B, Schreml S, Landthaler M, et al. Skin signs in diabetes mellitus. J Eur Acad Dermatol Venereol. 2012;26:1203-1211.
- Nebesio CL, Lewis C, Chuang TY. Lack of an association between granuloma annulare and type 2 diabetes mellitus. Br J Dermatol. 2002;146:122-124.
- Stankler L, Leslie G. Generalized granuloma annulare. a report of a case and review of the literature. Arch Dermatol. 1976;95:509-513.
- Williamson DM, Dykes JR. Carbohydrate metabolism in granuloma annulare. J Invest Dermatol. 1972;58:400-404.
- Dabski K, Winkelmann RK. Generalized granuloma annulare: clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989;20:39-47.
- Veraldi S, Bencini PL, Drudi E, et al. Laboratory abnormalities in granuloma annulare: a case-control study. Br J Dermatol. 1997;136:652-653.
- Levin NA, Patterson JW, Yao LL, et al. Resolution of patch-type granuloma annulare lesions after biopsy. J Am Acad Dermatol. 2002;46:426-429.
- Seyhan M, Ozcan H, Sahin I, et al. High prevalence of glucose metabolism disturbance in patients with lichen planus. Diabetes Res Clin Pract. 2007;77:198-202.
- Romero MA, Seoane J, Varela-Centelles P, et al. Prevalence of diabetes mellitus amongst oral lichen planus patients. clinical and pathological characteristics. Med Oral. 2002;7:121-129.
- Albrecht M, Banoczy J, Dinya E, et al. Occurrence of oral leukoplakia and lichen planus in diabetes mellitus. J Oral Pathol Med. 1992;21:364-366.
- Lehman JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Gould IM, Gray RS, Urbaniak SJ, et al. Vitiligo in diabetes mellitus. Br J Dermatol. 1985;113:153-155.
- White CR Jr, Heskel NS, Pokorny DJ. Perforating folliculitis of hemodialysis. Am J Dermatopathol. 1982;4:109-116.
- Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Muller SA. Dermatologic disorders associated with diabetes mellitus. Mayo Clin Proc. 1966;41:689-703.
- Azfar RS, Seminara NM, Shin DB, et al. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol. 2012;148:995-1000.
- Khalid U, Hansen PR, Gislason GH, et al. Psoriasis and new-onset diabetes: a Danish nationwide cohort study. Diabetes Care. 2013;36:2402-2407.
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012 [published online instead of print March 16, 2013]. Diabetes Care. 2013;36:1033-1046.
- Collier A, Matthews DM, Kellett HA, et al. Change in skin thickness associated with cheiroarthropathy in insulin dependent diabetes mellitus. Br Med J (Clin Res Ed). 1986;292:936.
- Fitzcharles MA, Duby S, Waddell RW, et al. Limitation of joint mobility (cheiroarthropathy) in adult noninsulin-dependent diabetic patients. Ann Rheum Dis. 1984;43:251-254.
- Hanna W, Friesen D, Bombardier C, et al. Pathologic features of diabetic thick skin. J Am Acad Dermatol. 1987;16:546-553.
- Lieberman LS, Rosenbloom AL, Riley WJ, et al. Reduced skin thickness with pump administration of insulin. N Engl J Med. 1980;303:940-941.
- Guarneri C, Guarneri F, Borgia F, et al. Finger pebbles in a diabetic patient: Huntley's papules. Int J Dermatol. 2005;44:755-756.
- Forst T, Kann P, Pfützner A, et al. Association between "diabetic thick skin syndrome" and neurological disorders in diabetes mellitus. Acta Diabetol. 1994;31:73-77.
- Nikoleishvili LR, Kurashvili RB, Virsaladze DK, et al. Characteristic changes of skin and its accessories in type 2 diabetes mellitus [in Russian]. Georgian Med News. 2006:43-46.
- Lithner F. Purpura, pigmentation and yellow nails of the lower extremities in diabetics. Acta Med Scand. 1976;199:203-208.
- Greene RA, Scher RK. Nail changes associated with diabetes mellitus. J Am Acad Dermatol. 1987;16:1015-1021.
- Hiller E, Rosenow EC 3rd, Olsen AM. Pulmonary manifestations of the yellow nail syndrome. Chest. 1972;61:452-458.
- Feingold KR, Elias PM. Endocrine-skin interactions. cutaneous manifestations of pituitary disease, thyroid disease, calcium disorders, and diabetes. J Am Acad Dermatol. 1987;17:921-940.
- Abdollahi A, Daneshpazhooh M, Amirchaghmaghi E, et al. Dermopathy and retinopathy in diabetes: is there an association? Dermatology, 2007;214:133-136.
- Morgan AJ, Schwartz RA. Diabetic dermopathy: A subtle sign with grave implications. J Am Acad Dermatol. 2008;58:447-451.
- Perez MI, Kohn SR. Cutaneous manifestations of diabetes mellitus. J Am Acad Dermatol. 1994;30:519-531.
- Cantwell AR, Martz W. Idiopathic bullae in diabetics. Bullosis diabeticorum. Arch Dermatol. 1967;96:42-44.
- Larsen K, Jensen T, Karlsmark T, Holstein PE. Incidence of bullosis diabeticorum – a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596.
- Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200.
- Basarab T, Munn SE, McGrath J, et al. Bullosis diabeticorum. a case report and literature review. Clin Exp Dermatol. 1995;20:218-220.
- Bernstein JE, Levine LE, Medenica MM, et al. Reduced threshold to suction-induced blister formation in insulin-dependent diabetics. J Am Acad Dermatol. 1983;8:790-791.
- Cruz PD Jr, Hud JA Jr. Excess insulin binding to insulin-like growth factor receptors: proposed mechanism for acanthosis nigricans. J Invest Dermatol. 1992;98(suppl 6):S82-S85.
- Romano G, Moretti G, Di Benedetto A, et al. Skin lesions in diabetes mellitus: prevalence and clinical correlations. Diabetes Res Clin Pract. 1998;39:101-106.
- Urback E. Eine neue diabetische Stoffwechseldermatose: Nekrobiosis lipoidica diabeticorum. Arch. f. Dermat. u Syph. 1932;166:273.
- Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. a clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93:272-281.
- Engel MF, Smith JG Jr. The pathogenesis of necrobiosis lipoidica. necrobiosis lipoidica, a form fruste of diabetes mellitus. Arch Dermatol. 1960;82:791-797.
- Cole GW, Headley J, Skowsky R. Scleredema diabeticorum: a common and distinct cutaneous manifestation of diabetes mellitus. Diabetes Care. 1983;6:189-192.
- Sattar MA, Diab S, Sugathan TN, et al. Scleroedema diabeticorum: a minor but often unrecognized complication of diabetes mellitus. Diabet Med. 1988;5:465-468.
- Rho YW, Suhr KB, Lee JH, et al. A clinical observation of scleredema adultorum and its relationship to diabetes. J Dermatol. 1998;25:103-107.
- Baillot-Rudoni S, Apostol D, Vaillant G, et al. Implantable pump therapy restores metabolic control and quality of life in type 1 diabetic patients with Buschke's nonsystemic scleroderma. Diabetes Care. 2006;29:1710.
- Meguerditchian C, Jacquet P, Béliard S, et al. Scleredema adultorum of Buschke: an under recognized skin complication of diabetes. Diabetes Metab. 2006;32:481-484.
- Behm B, Schreml S, Landthaler M, et al. Skin signs in diabetes mellitus. J Eur Acad Dermatol Venereol. 2012;26:1203-1211.
- Nebesio CL, Lewis C, Chuang TY. Lack of an association between granuloma annulare and type 2 diabetes mellitus. Br J Dermatol. 2002;146:122-124.
- Stankler L, Leslie G. Generalized granuloma annulare. a report of a case and review of the literature. Arch Dermatol. 1976;95:509-513.
- Williamson DM, Dykes JR. Carbohydrate metabolism in granuloma annulare. J Invest Dermatol. 1972;58:400-404.
- Dabski K, Winkelmann RK. Generalized granuloma annulare: clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989;20:39-47.
- Veraldi S, Bencini PL, Drudi E, et al. Laboratory abnormalities in granuloma annulare: a case-control study. Br J Dermatol. 1997;136:652-653.
- Levin NA, Patterson JW, Yao LL, et al. Resolution of patch-type granuloma annulare lesions after biopsy. J Am Acad Dermatol. 2002;46:426-429.
- Seyhan M, Ozcan H, Sahin I, et al. High prevalence of glucose metabolism disturbance in patients with lichen planus. Diabetes Res Clin Pract. 2007;77:198-202.
- Romero MA, Seoane J, Varela-Centelles P, et al. Prevalence of diabetes mellitus amongst oral lichen planus patients. clinical and pathological characteristics. Med Oral. 2002;7:121-129.
- Albrecht M, Banoczy J, Dinya E, et al. Occurrence of oral leukoplakia and lichen planus in diabetes mellitus. J Oral Pathol Med. 1992;21:364-366.
- Lehman JS, Tollefson MM, Gibson LE. Lichen planus. Int J Dermatol. 2009;48:682-694.
- Gould IM, Gray RS, Urbaniak SJ, et al. Vitiligo in diabetes mellitus. Br J Dermatol. 1985;113:153-155.
- White CR Jr, Heskel NS, Pokorny DJ. Perforating folliculitis of hemodialysis. Am J Dermatopathol. 1982;4:109-116.
- Hurwitz RM, Melton ME, Creech FT 3rd, et al. Perforating folliculitis in association with hemodialysis. Am J Dermatopathol. 1982;4:101-108.
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol. 1989;125:1074-1078.
- Muller SA. Dermatologic disorders associated with diabetes mellitus. Mayo Clin Proc. 1966;41:689-703.
- Azfar RS, Seminara NM, Shin DB, et al. Increased risk of diabetes mellitus and likelihood of receiving diabetes mellitus treatment in patients with psoriasis. Arch Dermatol. 2012;148:995-1000.
- Khalid U, Hansen PR, Gislason GH, et al. Psoriasis and new-onset diabetes: a Danish nationwide cohort study. Diabetes Care. 2013;36:2402-2407.
Verrucous Cobblestonelike Papules and Nodules of the Right Lower Limb
The Diagnosis: Elephantiasis Nostras Verrucosa
Elephantiasis nostras verrucosa (ENV) is a chronic deforming disorder characterized by hyperkeratosis and papillomatosis of the epidermis with underlying woody fibrosis of the dermis and the subcutaneous tissue in the setting of chronic nonfilarial lymphedema. Mossy papules, plaques, and cobblestonelike nodules are classic clinical features of ENV (Figure). The cobblestone lesion often is foul smelling and may be colonized with multiple bacteria and fungi.1
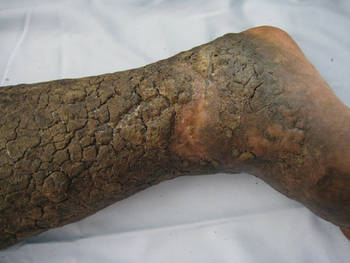
The differential diagnosis includes filariasis, chromoblastomycosis, and venous stasis dermatitis. Filariasis is infection with the filarial worms Wuchereria bancrofti, Brugia malayi, or Brugia timori. These parasites are transmitted to humans through the bite of an infected mosquito and develop into adult worms in the lymphatic vessels, causing lymphedema. Elephantiasis, the classic sign of late-stage disease, also causes swelling of the legs and genital organs that can be disfiguring. Thus travel history to Southeast Asia and Africa regions is particularly important.
Chromoblastomycosis is a chronic fungal infection of the skin and subcutaneous tissue caused by traumatic inoculation of a specific group of dematiaceous fungi, usually Fonsecaea pedrosoi, Phialophora verrucosa, Cladosporium carrionii, or Fonsecaea compacta. Chromoblastomycosis-induced lymphedema may mimic elephantiasis. Thus it is important to perform fungal culture and fungal scraping, which will show typical thick-walled, cigar-colored, sclerotic cells (known as Medlar bodies).
The skin changes of venous stasis dermatitis include edema, varicosities, hyperpigmentation, atrophie blanche, diffuse red-brown discoloration representing deep dermal deposits of hemosiderin, and lipodermatosclerosis. Venous stasis dermatitis is caused by chronic venous insufficiency. It is one of the predispositions to ENV, as it can cause repeated infections and cellulitis leading to blockage of lymphatic drainage and secondary lymphedema. A study of 21 cases highlighted that chronic venous insufficiency may be an underappreciated risk factor in the pathogenesis of ENV.2
The development of verrucous cobblestonelike plaques in ENV occurs with chronic lymphedema. Chronic lymphedema can be caused by a variety of etiologies including infection, chronic venous stasis, congestive heart failure, obesity, trauma, tumor obstruction, and radiation. Our patient was obese with chronic venous stasis, congestive heart failure, and recurrent cellulitis that can lead to secondary lymphatic obstruction and edema, all contributing to the picture of ENV. Because of the unilateral presentation in our patient, we believe that recurrent infections and inflammation are the important risk factors that lead to fibrosis of the dermis and lymph channels, ultimately resulting in ENV.
The diagnosis of ENV is based on the patient’s history and characteristic skin changes. However, a biopsy should be performed if the diagnosis is not clinically apparent, if the lesion looks suspicious for malignancy, or if areas of chronic ulceration exist. Histologic findings include hyperkeratosis as well as a collarette of acanthotic epidermis extending around dilated lymph vessels in the upper dermis. Sometimes a few dilated lymph vessels present in the deeper or subcutaneous tissue. Bacterial and fungal culture should be done to exclude concomitant infection. Ultrasonography can be used to evaluate the lymphatic and venous systems.
Treatment of ENV aims at improving lymphedema and preventing infection. Physical therapy to improve lymphedema includes manual lymphatic drainage, massage, compression stockings, elastic bandages, and leg elevation. In cases associated with obesity, weight loss is recommended. In cases of recurrent cellulitis or lymphangitis, long-term antibiotic therapy with agents such as penicillins or cephalosporins is sometimes used.3 Success has been reported with the use of oral and topical retinoids, such as acitretin and tazarotene, which are thought to decrease epidermal proliferation, fibrogenesis, and inflammation.4,5 Our patient was started on tretinoin cream. Surgery is reserved for cases of lymphedema resistant to medical therapies, including debridement,6 lymphatic and lymphovenous anastomosis,7 lymphatic transplantation, and amputation for severe cases.
Malignancies can develop in areas of chronic lymphedema such as lymphangiosarcoma, squamous cell carcinoma, Kaposi sarcoma, B-cell lymphoma, and malignant fibrous histiocytoma. The morbidity and mortality usually are from the underlying medical problems rather than ENV itself. Our patient died 1 month after the diagnosis of ENV from his poorly controlled heart failure.
- Schissel DJ, Hivnor C, Elston DM. Elephantiasis nostras verrucosa. Cutis. 1998;62:77-80.
- Dean SM, Zirwas MJ, Horst AV. Elephantiasis nostras verrucosa: an institutional analysis of 21 cases. J Am Acad Dermatol. 2011;64:1104-1110.
- Olszewski WL, Jamal S, Manokaran G, et al. The effectiveness of long-acting penicillin (penidur) in preventing recurrences of dermatolymphangioadenitis (DLA) and controlling skin, deep tissues, and lymph bacterial flora in patients with “filarial” lymphedema. Lymphology. 2005;38:66-80.
- Feind-Koopmans A, van de Kerkhof PC. Successful treatment of papillomatosis cutis lymphostatica with acitretin. Acta Derm Venereol. 1995;75:411.
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004;3:446-448.
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-41.
- Motegi S, Tamura A, Okada E, et al. Successful treatment with lymphaticovenular anastomosis for secondary skin lesions of chronic lymphedema. Dermatology. 2007;215:147-151.
The Diagnosis: Elephantiasis Nostras Verrucosa
Elephantiasis nostras verrucosa (ENV) is a chronic deforming disorder characterized by hyperkeratosis and papillomatosis of the epidermis with underlying woody fibrosis of the dermis and the subcutaneous tissue in the setting of chronic nonfilarial lymphedema. Mossy papules, plaques, and cobblestonelike nodules are classic clinical features of ENV (Figure). The cobblestone lesion often is foul smelling and may be colonized with multiple bacteria and fungi.1

The differential diagnosis includes filariasis, chromoblastomycosis, and venous stasis dermatitis. Filariasis is infection with the filarial worms Wuchereria bancrofti, Brugia malayi, or Brugia timori. These parasites are transmitted to humans through the bite of an infected mosquito and develop into adult worms in the lymphatic vessels, causing lymphedema. Elephantiasis, the classic sign of late-stage disease, also causes swelling of the legs and genital organs that can be disfiguring. Thus travel history to Southeast Asia and Africa regions is particularly important.
Chromoblastomycosis is a chronic fungal infection of the skin and subcutaneous tissue caused by traumatic inoculation of a specific group of dematiaceous fungi, usually Fonsecaea pedrosoi, Phialophora verrucosa, Cladosporium carrionii, or Fonsecaea compacta. Chromoblastomycosis-induced lymphedema may mimic elephantiasis. Thus it is important to perform fungal culture and fungal scraping, which will show typical thick-walled, cigar-colored, sclerotic cells (known as Medlar bodies).
The skin changes of venous stasis dermatitis include edema, varicosities, hyperpigmentation, atrophie blanche, diffuse red-brown discoloration representing deep dermal deposits of hemosiderin, and lipodermatosclerosis. Venous stasis dermatitis is caused by chronic venous insufficiency. It is one of the predispositions to ENV, as it can cause repeated infections and cellulitis leading to blockage of lymphatic drainage and secondary lymphedema. A study of 21 cases highlighted that chronic venous insufficiency may be an underappreciated risk factor in the pathogenesis of ENV.2
The development of verrucous cobblestonelike plaques in ENV occurs with chronic lymphedema. Chronic lymphedema can be caused by a variety of etiologies including infection, chronic venous stasis, congestive heart failure, obesity, trauma, tumor obstruction, and radiation. Our patient was obese with chronic venous stasis, congestive heart failure, and recurrent cellulitis that can lead to secondary lymphatic obstruction and edema, all contributing to the picture of ENV. Because of the unilateral presentation in our patient, we believe that recurrent infections and inflammation are the important risk factors that lead to fibrosis of the dermis and lymph channels, ultimately resulting in ENV.
The diagnosis of ENV is based on the patient’s history and characteristic skin changes. However, a biopsy should be performed if the diagnosis is not clinically apparent, if the lesion looks suspicious for malignancy, or if areas of chronic ulceration exist. Histologic findings include hyperkeratosis as well as a collarette of acanthotic epidermis extending around dilated lymph vessels in the upper dermis. Sometimes a few dilated lymph vessels present in the deeper or subcutaneous tissue. Bacterial and fungal culture should be done to exclude concomitant infection. Ultrasonography can be used to evaluate the lymphatic and venous systems.
Treatment of ENV aims at improving lymphedema and preventing infection. Physical therapy to improve lymphedema includes manual lymphatic drainage, massage, compression stockings, elastic bandages, and leg elevation. In cases associated with obesity, weight loss is recommended. In cases of recurrent cellulitis or lymphangitis, long-term antibiotic therapy with agents such as penicillins or cephalosporins is sometimes used.3 Success has been reported with the use of oral and topical retinoids, such as acitretin and tazarotene, which are thought to decrease epidermal proliferation, fibrogenesis, and inflammation.4,5 Our patient was started on tretinoin cream. Surgery is reserved for cases of lymphedema resistant to medical therapies, including debridement,6 lymphatic and lymphovenous anastomosis,7 lymphatic transplantation, and amputation for severe cases.
Malignancies can develop in areas of chronic lymphedema such as lymphangiosarcoma, squamous cell carcinoma, Kaposi sarcoma, B-cell lymphoma, and malignant fibrous histiocytoma. The morbidity and mortality usually are from the underlying medical problems rather than ENV itself. Our patient died 1 month after the diagnosis of ENV from his poorly controlled heart failure.
The Diagnosis: Elephantiasis Nostras Verrucosa
Elephantiasis nostras verrucosa (ENV) is a chronic deforming disorder characterized by hyperkeratosis and papillomatosis of the epidermis with underlying woody fibrosis of the dermis and the subcutaneous tissue in the setting of chronic nonfilarial lymphedema. Mossy papules, plaques, and cobblestonelike nodules are classic clinical features of ENV (Figure). The cobblestone lesion often is foul smelling and may be colonized with multiple bacteria and fungi.1

The differential diagnosis includes filariasis, chromoblastomycosis, and venous stasis dermatitis. Filariasis is infection with the filarial worms Wuchereria bancrofti, Brugia malayi, or Brugia timori. These parasites are transmitted to humans through the bite of an infected mosquito and develop into adult worms in the lymphatic vessels, causing lymphedema. Elephantiasis, the classic sign of late-stage disease, also causes swelling of the legs and genital organs that can be disfiguring. Thus travel history to Southeast Asia and Africa regions is particularly important.
Chromoblastomycosis is a chronic fungal infection of the skin and subcutaneous tissue caused by traumatic inoculation of a specific group of dematiaceous fungi, usually Fonsecaea pedrosoi, Phialophora verrucosa, Cladosporium carrionii, or Fonsecaea compacta. Chromoblastomycosis-induced lymphedema may mimic elephantiasis. Thus it is important to perform fungal culture and fungal scraping, which will show typical thick-walled, cigar-colored, sclerotic cells (known as Medlar bodies).
The skin changes of venous stasis dermatitis include edema, varicosities, hyperpigmentation, atrophie blanche, diffuse red-brown discoloration representing deep dermal deposits of hemosiderin, and lipodermatosclerosis. Venous stasis dermatitis is caused by chronic venous insufficiency. It is one of the predispositions to ENV, as it can cause repeated infections and cellulitis leading to blockage of lymphatic drainage and secondary lymphedema. A study of 21 cases highlighted that chronic venous insufficiency may be an underappreciated risk factor in the pathogenesis of ENV.2
The development of verrucous cobblestonelike plaques in ENV occurs with chronic lymphedema. Chronic lymphedema can be caused by a variety of etiologies including infection, chronic venous stasis, congestive heart failure, obesity, trauma, tumor obstruction, and radiation. Our patient was obese with chronic venous stasis, congestive heart failure, and recurrent cellulitis that can lead to secondary lymphatic obstruction and edema, all contributing to the picture of ENV. Because of the unilateral presentation in our patient, we believe that recurrent infections and inflammation are the important risk factors that lead to fibrosis of the dermis and lymph channels, ultimately resulting in ENV.
The diagnosis of ENV is based on the patient’s history and characteristic skin changes. However, a biopsy should be performed if the diagnosis is not clinically apparent, if the lesion looks suspicious for malignancy, or if areas of chronic ulceration exist. Histologic findings include hyperkeratosis as well as a collarette of acanthotic epidermis extending around dilated lymph vessels in the upper dermis. Sometimes a few dilated lymph vessels present in the deeper or subcutaneous tissue. Bacterial and fungal culture should be done to exclude concomitant infection. Ultrasonography can be used to evaluate the lymphatic and venous systems.
Treatment of ENV aims at improving lymphedema and preventing infection. Physical therapy to improve lymphedema includes manual lymphatic drainage, massage, compression stockings, elastic bandages, and leg elevation. In cases associated with obesity, weight loss is recommended. In cases of recurrent cellulitis or lymphangitis, long-term antibiotic therapy with agents such as penicillins or cephalosporins is sometimes used.3 Success has been reported with the use of oral and topical retinoids, such as acitretin and tazarotene, which are thought to decrease epidermal proliferation, fibrogenesis, and inflammation.4,5 Our patient was started on tretinoin cream. Surgery is reserved for cases of lymphedema resistant to medical therapies, including debridement,6 lymphatic and lymphovenous anastomosis,7 lymphatic transplantation, and amputation for severe cases.
Malignancies can develop in areas of chronic lymphedema such as lymphangiosarcoma, squamous cell carcinoma, Kaposi sarcoma, B-cell lymphoma, and malignant fibrous histiocytoma. The morbidity and mortality usually are from the underlying medical problems rather than ENV itself. Our patient died 1 month after the diagnosis of ENV from his poorly controlled heart failure.
- Schissel DJ, Hivnor C, Elston DM. Elephantiasis nostras verrucosa. Cutis. 1998;62:77-80.
- Dean SM, Zirwas MJ, Horst AV. Elephantiasis nostras verrucosa: an institutional analysis of 21 cases. J Am Acad Dermatol. 2011;64:1104-1110.
- Olszewski WL, Jamal S, Manokaran G, et al. The effectiveness of long-acting penicillin (penidur) in preventing recurrences of dermatolymphangioadenitis (DLA) and controlling skin, deep tissues, and lymph bacterial flora in patients with “filarial” lymphedema. Lymphology. 2005;38:66-80.
- Feind-Koopmans A, van de Kerkhof PC. Successful treatment of papillomatosis cutis lymphostatica with acitretin. Acta Derm Venereol. 1995;75:411.
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004;3:446-448.
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-41.
- Motegi S, Tamura A, Okada E, et al. Successful treatment with lymphaticovenular anastomosis for secondary skin lesions of chronic lymphedema. Dermatology. 2007;215:147-151.
- Schissel DJ, Hivnor C, Elston DM. Elephantiasis nostras verrucosa. Cutis. 1998;62:77-80.
- Dean SM, Zirwas MJ, Horst AV. Elephantiasis nostras verrucosa: an institutional analysis of 21 cases. J Am Acad Dermatol. 2011;64:1104-1110.
- Olszewski WL, Jamal S, Manokaran G, et al. The effectiveness of long-acting penicillin (penidur) in preventing recurrences of dermatolymphangioadenitis (DLA) and controlling skin, deep tissues, and lymph bacterial flora in patients with “filarial” lymphedema. Lymphology. 2005;38:66-80.
- Feind-Koopmans A, van de Kerkhof PC. Successful treatment of papillomatosis cutis lymphostatica with acitretin. Acta Derm Venereol. 1995;75:411.
- Boyd J, Sloan S, Meffert J. Elephantiasis nostrum verrucosa of the abdomen: clinical results with tazarotene. J Drugs Dermatol. 2004;3:446-448.
- Iwao F, Sato-Matsumura KC, Sawamura D, et al. Elephantiasis nostras verrucosa successfully treated by surgical debridement. Dermatol Surg. 2004;30:939-41.
- Motegi S, Tamura A, Okada E, et al. Successful treatment with lymphaticovenular anastomosis for secondary skin lesions of chronic lymphedema. Dermatology. 2007;215:147-151.
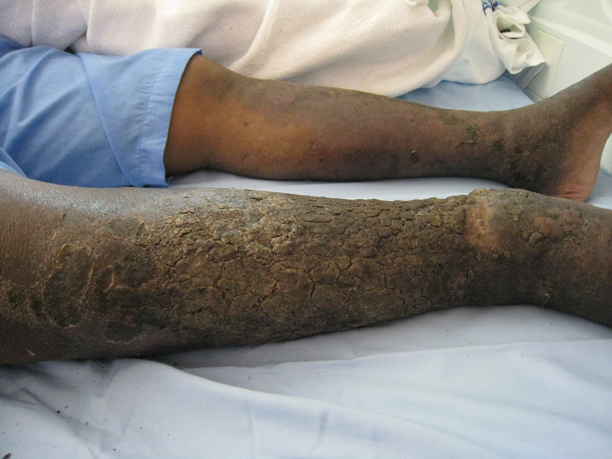
An obese 58-year-old man was admitted to the cardiology department for poorly controlled congestive heart failure. He was referred to the dermatology department with progressive painless swelling of the right lower limb of a year’s duration. He had chronic right lower limb insufficiency of 3 years’ duration with a history of a recurrent right medial malleolus ulcer and cellulitis. There was no notable travel or family history. Physical examination revealed woody edema of the right lower limb with verrucous cobblestonelike papules and nodules, foul-smelling odor, and thick crusts that were easily removed.
Assessing Attributes of Topical Vehicles for the Treatment of Acne, Atopic Dermatitis, and Plaque Psoriasis
The skin care market includes topical product formulations (eg, foams, lotions, ointments, creams) for a number of dermatologic therapeutic targets; however, there is limited information available regarding patient preference for product vehicles by specific dermatologic disease. There are few studies in the literature examining patient adherence to topical medications.1 In the current study, 6 focus groups comprised of patients with 3 dermatologic conditions of interest—acne, atopic dermatitis (AD), or plaque psoriasis (PP)—were surveyed to gain a better understanding of treatment preferences among patients and attributes of various formulations that are most desirable and important to this patient population.
Patient preference for a vehicle is relevant to treatment adherence in patients with conditions such as acne, AD, and PP because noncompliance is a major factor in the high rates of failure that have been associated with topical dermatologic treatments.2 The aesthetic attributes of a given vehicle formulation depend on the disease state being treated, the site of application, and the length of treatment.3 A limited number of studies have linked patient preferences to the attributes of topical medications. In one study, participants preferred an aqueous gel formulation compared with previously used topical treatments for AD.3 In another study, participants ranked the following properties of a hypothetical topical medication as most important: gel formulation, room temperature storage, product life of up to 18 months once opened, application with fingers, and a once-daily regimen.2 A third study found that foams were preferred among other formulations (ie, creams, gels, and ointments) for a variety of disease states, including AD, PP, and seborrheic dermatitis.4 The current study uses a qualitative analysis to further explore patient preferences of vehicle attributes for the topical treatment of acne, AD, and PP.
Methods
Study Participants
Six focus groups were conducted with patients who were using topical prescription medications for the treatment of acne, AD, or PP from March to April 2012. Participants were recruited from Raleigh, North Carolina, and New York, New York, with 3 focus groups (1 for each condition) from each city.
Following institutional review board approval of the study processes and recruitment materials, participants meeting the following criteria were included in the study: 18 years or older; diagnosed with acne, AD, or PP by a physician (participants who currently reported more than 1 of these conditions were not eligible); diagnosed with the respective skin condition at least 6 months prior to screening; current or prior use of topical prescription medications in at least 2 different vehicle formulations (eg, cream and foam, ointment and gel); use of at least 1 topical prescription treatment 5 or more times per month; and English speaking and able to provide written consent.
Study Design
A semistructured discussion guide was developed to ensure consistency in the topics surveyed among all 6 focus groups, and the same 2 moderators conducted the discussion for all 6 groups. At the beginning of the study, after providing written informed consent, participants were given an overview of the study and were asked general questions intended to get the participants talking about their experiences with their respective conditions. To avoid or minimize bias, participants were only asked open-ended questions designed to ascertain what symptoms they experienced in relation to their respective conditions. Finally, the discussions were focused on the topical prescription treatments that participants had tried and the properties of each treatment they liked and disliked.
The focus groups were recorded (audio) and transcribed; transcriptions were then verified through an iterative process of technical and editorial review. Analysis of the results was conducted by evaluation and review of the field notes as well as the transcripts from the focus groups.
Results
A total of 54 participants were surveyed (average age, 40.9 years). Although the average age of participants in the acne and AD groups was generally the same (35.2 and 35.4 years, respectively), the average age of participants in the PP group was higher (52.2 years). The majority of participants were white females who had at least a college degree. On average, participants had been diagnosed with their respective condition approximately 15.5 years prior to screening. Participant demographics and clinical characteristics are presented in more detail in Table 1.
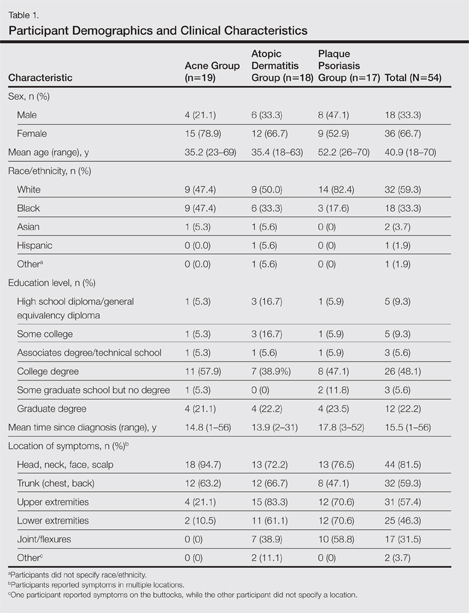
At the time of screening, participants reported prior or present use of topical prescription medications in various formulations for treatment of their respective conditions. The most commonly reported vehicles across all 3 conditions were creams and ointments, followed by lotions, gels, and foams.
Symptoms Across Conditions
At the beginning of the study, participants reported symptoms they experienced in association with their respective skin conditions. Itching and redness were the only symptoms reported across all 3 conditions in all 6 focus groups. Dry skin/dryness, flaking/scaling/peeling, and pain were reported by at least 1 participant in 5 of 6 focus groups. Other common symptoms reported in at least 3 focus groups included pimples/bumps/boils, sensitivity, bleeding, discoloration of skin, cracking/cuts/skin breaking, and buildup of dead or thickened skin/chunks of skin/plaque. As shown in Table 2, there was more variability in the symptoms reported across the 2 acne groups compared with those reported by the AD and PP groups. Table 2 displays all symptoms spontaneously reported across each of the 6 focus groups. Symptoms are listed according to the terms/descriptions provided by focus group participants.
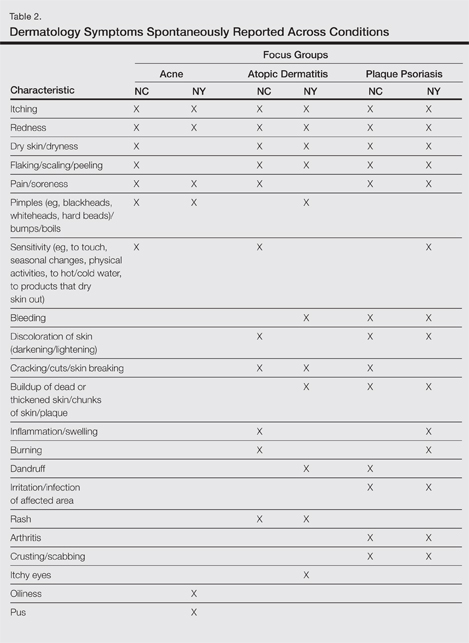
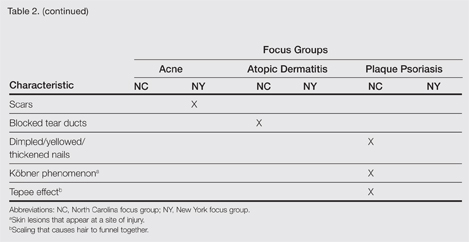
Results by Condition
Unless otherwise noted, participant responses generally were consistent across the North Carolina and New York focus groups. Results from the study, which included a total of 54 participants, suggested a high degree of similarity among preferences for topical treatment attributes across the 3 conditions. Moisturizing was the single attribute that was mentioned across all 3 conditions in all 6 focus groups as an important characteristic in a topical dermatologic treatment. Other attributes mentioned by at least 1 participant in 5 of 6 focus groups included the following: absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy/oily, is not sticky/tacky, is long lasting/long acting/stays on/lasts through sweating or hand washing, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
A few condition-specific attributes also were noted. An attribute was considered condition specific if it was mentioned by at least 1 participant in both groups for any condition. Preferred properties for topical medications that were specific to acne patients included the following: easy to dispense/dispenses right amount, nondrying, product goes on/spreads smoothly, container (eg, tube, pump) is not easily broken/does not leak, and creamy. Preferred properties that were specific to AD patients included the following: is not noticeable to others/conceals area, good consistency, and cooling. Preferred properties that were specific to 2 conditions included no residue among acne and AD patients and soothing among AD and PP patients. Preferred properties for topical medications across all focus groups are described in further detail in Table 3.
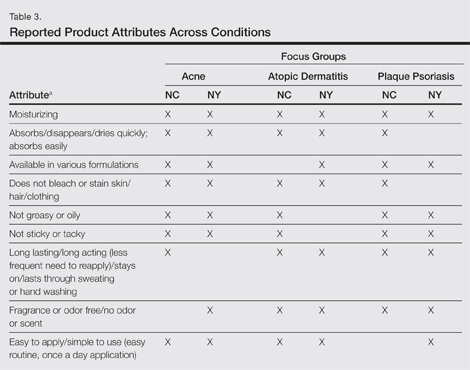
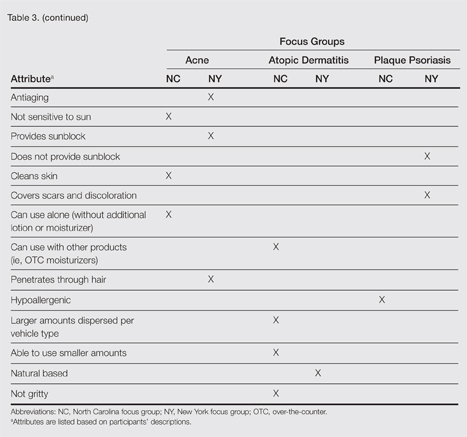
Regarding vehicle-type preferences, acne patients tended to prefer washes, creams, and lotions; AD patients preferred creams; and psoriasis patients preferred creams, ointments, and foams (particularly for the scalp).
Participants across all 3 conditions reported that during daytime hours (versus at night), they would be less likely to use products that are oily, shiny, thick (eg, ointments, oils); bleach or stain the skin/clothing; interfere with makeup, work, or other activities; or are visible to others. Rather, a majority of participants noted that they were more likely to use thinner, less oily products (eg, creams, lotions) during daytime hours, in social situations, or during certain activities (eg, exercise). Participants across all 3 conditions noted that they only used prescription shampoos when they had enough time to repeatedly rinse their hair to eliminate the smell. Participants across all 6 groups noted that they tended to choose creams or lotions over ointments (when available) during the summer because they considered these products to be lighter and less greasy.
Overall, participants indicated that condition-specific symptoms did not influence their preference for topical formulations; rather, they used whatever prescriptions they currently had. However, in some instances, participants noted that the location of the affected area might influence the type of product selected. For example, some participants tended not to use ointments on the scalp or in locations where their clothes might come into contact with the medicated area to avoid clothes sticking to medicated areas. Other participants used lighter, less stringent products on the face versus other locations. Participants noted that foams were preferred for more discrete localized areas.
Comment
Despite the variability in symptoms and conditions across the study population as well as participants’ varying experience with and access to topical treatments, the attributes participants valued most in topical prescription treatments were relatively consistent across the 3 conditions: moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time. Preferred vehicle attributes were generally consistent across the 3 conditions, but preferences for vehicle types tended to vary by condition. Although some vehicles were more closely associated with specific attributes (eg, the majority considered lotions to be moisturizing), no one vehicle was associated with the complete list of attributes for the ideal topical medication. Regardless of the symptoms experienced or particular situations/activities, participants noted that they tended to use the topical medications that were available to them, even if intended for different locations of the body, and nearly all reported using more than 1 type of topical treatment.
Caution should be used in interpreting these findings, as participant preferences are largely dependent on prior experience with different vehicle types. The small number of participants across the 3 conditions in this analysis also limits conclusions that can be drawn from the data sets, as each condition has different though somewhat overlapping treatment algorithms. A focused inquiry into each of these conditions through separate evaluations may provide for a more robust analysis. Future research might employ questionnaire methodology to develop items assessing the attributes of interest (eg, moisturizing ability, rate of absorption, greasiness/oiliness, stickiness/tackiness, fragrance/odor). The final set of desirable attributes may vary depending on the condition (eg, a moisturizing product may be more important to PP and AD patients than acne patients) as well as comparator products.
Acknowledgment—The authors would like to thank Jennifer Gwazdauskas, MBA, Research Triangle Park, North Carolina, of Stiefel, a GSK company, for her assistance with manuscript review.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient p for topical antibiotic treatment for acne. a randomised controlled trial. Br J Dermatol. 2006;154:524-532.
- Trookman NS, Rizer RL, Ho ET, et al. The importance of vehicle properties to patients with atopic dermatitis. Cutis. 2011;88:13-17.
- Weiss S, Wyres M, Brundage T. A novel foam vehicle is consistently preferred by patients for dermatologic conditions. J Am Acad Dermatol. 2011;64(suppl 1):AB50.
The skin care market includes topical product formulations (eg, foams, lotions, ointments, creams) for a number of dermatologic therapeutic targets; however, there is limited information available regarding patient preference for product vehicles by specific dermatologic disease. There are few studies in the literature examining patient adherence to topical medications.1 In the current study, 6 focus groups comprised of patients with 3 dermatologic conditions of interest—acne, atopic dermatitis (AD), or plaque psoriasis (PP)—were surveyed to gain a better understanding of treatment preferences among patients and attributes of various formulations that are most desirable and important to this patient population.
Patient preference for a vehicle is relevant to treatment adherence in patients with conditions such as acne, AD, and PP because noncompliance is a major factor in the high rates of failure that have been associated with topical dermatologic treatments.2 The aesthetic attributes of a given vehicle formulation depend on the disease state being treated, the site of application, and the length of treatment.3 A limited number of studies have linked patient preferences to the attributes of topical medications. In one study, participants preferred an aqueous gel formulation compared with previously used topical treatments for AD.3 In another study, participants ranked the following properties of a hypothetical topical medication as most important: gel formulation, room temperature storage, product life of up to 18 months once opened, application with fingers, and a once-daily regimen.2 A third study found that foams were preferred among other formulations (ie, creams, gels, and ointments) for a variety of disease states, including AD, PP, and seborrheic dermatitis.4 The current study uses a qualitative analysis to further explore patient preferences of vehicle attributes for the topical treatment of acne, AD, and PP.
Methods
Study Participants
Six focus groups were conducted with patients who were using topical prescription medications for the treatment of acne, AD, or PP from March to April 2012. Participants were recruited from Raleigh, North Carolina, and New York, New York, with 3 focus groups (1 for each condition) from each city.
Following institutional review board approval of the study processes and recruitment materials, participants meeting the following criteria were included in the study: 18 years or older; diagnosed with acne, AD, or PP by a physician (participants who currently reported more than 1 of these conditions were not eligible); diagnosed with the respective skin condition at least 6 months prior to screening; current or prior use of topical prescription medications in at least 2 different vehicle formulations (eg, cream and foam, ointment and gel); use of at least 1 topical prescription treatment 5 or more times per month; and English speaking and able to provide written consent.
Study Design
A semistructured discussion guide was developed to ensure consistency in the topics surveyed among all 6 focus groups, and the same 2 moderators conducted the discussion for all 6 groups. At the beginning of the study, after providing written informed consent, participants were given an overview of the study and were asked general questions intended to get the participants talking about their experiences with their respective conditions. To avoid or minimize bias, participants were only asked open-ended questions designed to ascertain what symptoms they experienced in relation to their respective conditions. Finally, the discussions were focused on the topical prescription treatments that participants had tried and the properties of each treatment they liked and disliked.
The focus groups were recorded (audio) and transcribed; transcriptions were then verified through an iterative process of technical and editorial review. Analysis of the results was conducted by evaluation and review of the field notes as well as the transcripts from the focus groups.
Results
A total of 54 participants were surveyed (average age, 40.9 years). Although the average age of participants in the acne and AD groups was generally the same (35.2 and 35.4 years, respectively), the average age of participants in the PP group was higher (52.2 years). The majority of participants were white females who had at least a college degree. On average, participants had been diagnosed with their respective condition approximately 15.5 years prior to screening. Participant demographics and clinical characteristics are presented in more detail in Table 1.

At the time of screening, participants reported prior or present use of topical prescription medications in various formulations for treatment of their respective conditions. The most commonly reported vehicles across all 3 conditions were creams and ointments, followed by lotions, gels, and foams.
Symptoms Across Conditions
At the beginning of the study, participants reported symptoms they experienced in association with their respective skin conditions. Itching and redness were the only symptoms reported across all 3 conditions in all 6 focus groups. Dry skin/dryness, flaking/scaling/peeling, and pain were reported by at least 1 participant in 5 of 6 focus groups. Other common symptoms reported in at least 3 focus groups included pimples/bumps/boils, sensitivity, bleeding, discoloration of skin, cracking/cuts/skin breaking, and buildup of dead or thickened skin/chunks of skin/plaque. As shown in Table 2, there was more variability in the symptoms reported across the 2 acne groups compared with those reported by the AD and PP groups. Table 2 displays all symptoms spontaneously reported across each of the 6 focus groups. Symptoms are listed according to the terms/descriptions provided by focus group participants.


Results by Condition
Unless otherwise noted, participant responses generally were consistent across the North Carolina and New York focus groups. Results from the study, which included a total of 54 participants, suggested a high degree of similarity among preferences for topical treatment attributes across the 3 conditions. Moisturizing was the single attribute that was mentioned across all 3 conditions in all 6 focus groups as an important characteristic in a topical dermatologic treatment. Other attributes mentioned by at least 1 participant in 5 of 6 focus groups included the following: absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy/oily, is not sticky/tacky, is long lasting/long acting/stays on/lasts through sweating or hand washing, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
A few condition-specific attributes also were noted. An attribute was considered condition specific if it was mentioned by at least 1 participant in both groups for any condition. Preferred properties for topical medications that were specific to acne patients included the following: easy to dispense/dispenses right amount, nondrying, product goes on/spreads smoothly, container (eg, tube, pump) is not easily broken/does not leak, and creamy. Preferred properties that were specific to AD patients included the following: is not noticeable to others/conceals area, good consistency, and cooling. Preferred properties that were specific to 2 conditions included no residue among acne and AD patients and soothing among AD and PP patients. Preferred properties for topical medications across all focus groups are described in further detail in Table 3.



Regarding vehicle-type preferences, acne patients tended to prefer washes, creams, and lotions; AD patients preferred creams; and psoriasis patients preferred creams, ointments, and foams (particularly for the scalp).
Participants across all 3 conditions reported that during daytime hours (versus at night), they would be less likely to use products that are oily, shiny, thick (eg, ointments, oils); bleach or stain the skin/clothing; interfere with makeup, work, or other activities; or are visible to others. Rather, a majority of participants noted that they were more likely to use thinner, less oily products (eg, creams, lotions) during daytime hours, in social situations, or during certain activities (eg, exercise). Participants across all 3 conditions noted that they only used prescription shampoos when they had enough time to repeatedly rinse their hair to eliminate the smell. Participants across all 6 groups noted that they tended to choose creams or lotions over ointments (when available) during the summer because they considered these products to be lighter and less greasy.
Overall, participants indicated that condition-specific symptoms did not influence their preference for topical formulations; rather, they used whatever prescriptions they currently had. However, in some instances, participants noted that the location of the affected area might influence the type of product selected. For example, some participants tended not to use ointments on the scalp or in locations where their clothes might come into contact with the medicated area to avoid clothes sticking to medicated areas. Other participants used lighter, less stringent products on the face versus other locations. Participants noted that foams were preferred for more discrete localized areas.
Comment
Despite the variability in symptoms and conditions across the study population as well as participants’ varying experience with and access to topical treatments, the attributes participants valued most in topical prescription treatments were relatively consistent across the 3 conditions: moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time. Preferred vehicle attributes were generally consistent across the 3 conditions, but preferences for vehicle types tended to vary by condition. Although some vehicles were more closely associated with specific attributes (eg, the majority considered lotions to be moisturizing), no one vehicle was associated with the complete list of attributes for the ideal topical medication. Regardless of the symptoms experienced or particular situations/activities, participants noted that they tended to use the topical medications that were available to them, even if intended for different locations of the body, and nearly all reported using more than 1 type of topical treatment.
Caution should be used in interpreting these findings, as participant preferences are largely dependent on prior experience with different vehicle types. The small number of participants across the 3 conditions in this analysis also limits conclusions that can be drawn from the data sets, as each condition has different though somewhat overlapping treatment algorithms. A focused inquiry into each of these conditions through separate evaluations may provide for a more robust analysis. Future research might employ questionnaire methodology to develop items assessing the attributes of interest (eg, moisturizing ability, rate of absorption, greasiness/oiliness, stickiness/tackiness, fragrance/odor). The final set of desirable attributes may vary depending on the condition (eg, a moisturizing product may be more important to PP and AD patients than acne patients) as well as comparator products.
Acknowledgment—The authors would like to thank Jennifer Gwazdauskas, MBA, Research Triangle Park, North Carolina, of Stiefel, a GSK company, for her assistance with manuscript review.
The skin care market includes topical product formulations (eg, foams, lotions, ointments, creams) for a number of dermatologic therapeutic targets; however, there is limited information available regarding patient preference for product vehicles by specific dermatologic disease. There are few studies in the literature examining patient adherence to topical medications.1 In the current study, 6 focus groups comprised of patients with 3 dermatologic conditions of interest—acne, atopic dermatitis (AD), or plaque psoriasis (PP)—were surveyed to gain a better understanding of treatment preferences among patients and attributes of various formulations that are most desirable and important to this patient population.
Patient preference for a vehicle is relevant to treatment adherence in patients with conditions such as acne, AD, and PP because noncompliance is a major factor in the high rates of failure that have been associated with topical dermatologic treatments.2 The aesthetic attributes of a given vehicle formulation depend on the disease state being treated, the site of application, and the length of treatment.3 A limited number of studies have linked patient preferences to the attributes of topical medications. In one study, participants preferred an aqueous gel formulation compared with previously used topical treatments for AD.3 In another study, participants ranked the following properties of a hypothetical topical medication as most important: gel formulation, room temperature storage, product life of up to 18 months once opened, application with fingers, and a once-daily regimen.2 A third study found that foams were preferred among other formulations (ie, creams, gels, and ointments) for a variety of disease states, including AD, PP, and seborrheic dermatitis.4 The current study uses a qualitative analysis to further explore patient preferences of vehicle attributes for the topical treatment of acne, AD, and PP.
Methods
Study Participants
Six focus groups were conducted with patients who were using topical prescription medications for the treatment of acne, AD, or PP from March to April 2012. Participants were recruited from Raleigh, North Carolina, and New York, New York, with 3 focus groups (1 for each condition) from each city.
Following institutional review board approval of the study processes and recruitment materials, participants meeting the following criteria were included in the study: 18 years or older; diagnosed with acne, AD, or PP by a physician (participants who currently reported more than 1 of these conditions were not eligible); diagnosed with the respective skin condition at least 6 months prior to screening; current or prior use of topical prescription medications in at least 2 different vehicle formulations (eg, cream and foam, ointment and gel); use of at least 1 topical prescription treatment 5 or more times per month; and English speaking and able to provide written consent.
Study Design
A semistructured discussion guide was developed to ensure consistency in the topics surveyed among all 6 focus groups, and the same 2 moderators conducted the discussion for all 6 groups. At the beginning of the study, after providing written informed consent, participants were given an overview of the study and were asked general questions intended to get the participants talking about their experiences with their respective conditions. To avoid or minimize bias, participants were only asked open-ended questions designed to ascertain what symptoms they experienced in relation to their respective conditions. Finally, the discussions were focused on the topical prescription treatments that participants had tried and the properties of each treatment they liked and disliked.
The focus groups were recorded (audio) and transcribed; transcriptions were then verified through an iterative process of technical and editorial review. Analysis of the results was conducted by evaluation and review of the field notes as well as the transcripts from the focus groups.
Results
A total of 54 participants were surveyed (average age, 40.9 years). Although the average age of participants in the acne and AD groups was generally the same (35.2 and 35.4 years, respectively), the average age of participants in the PP group was higher (52.2 years). The majority of participants were white females who had at least a college degree. On average, participants had been diagnosed with their respective condition approximately 15.5 years prior to screening. Participant demographics and clinical characteristics are presented in more detail in Table 1.

At the time of screening, participants reported prior or present use of topical prescription medications in various formulations for treatment of their respective conditions. The most commonly reported vehicles across all 3 conditions were creams and ointments, followed by lotions, gels, and foams.
Symptoms Across Conditions
At the beginning of the study, participants reported symptoms they experienced in association with their respective skin conditions. Itching and redness were the only symptoms reported across all 3 conditions in all 6 focus groups. Dry skin/dryness, flaking/scaling/peeling, and pain were reported by at least 1 participant in 5 of 6 focus groups. Other common symptoms reported in at least 3 focus groups included pimples/bumps/boils, sensitivity, bleeding, discoloration of skin, cracking/cuts/skin breaking, and buildup of dead or thickened skin/chunks of skin/plaque. As shown in Table 2, there was more variability in the symptoms reported across the 2 acne groups compared with those reported by the AD and PP groups. Table 2 displays all symptoms spontaneously reported across each of the 6 focus groups. Symptoms are listed according to the terms/descriptions provided by focus group participants.


Results by Condition
Unless otherwise noted, participant responses generally were consistent across the North Carolina and New York focus groups. Results from the study, which included a total of 54 participants, suggested a high degree of similarity among preferences for topical treatment attributes across the 3 conditions. Moisturizing was the single attribute that was mentioned across all 3 conditions in all 6 focus groups as an important characteristic in a topical dermatologic treatment. Other attributes mentioned by at least 1 participant in 5 of 6 focus groups included the following: absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy/oily, is not sticky/tacky, is long lasting/long acting/stays on/lasts through sweating or hand washing, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
A few condition-specific attributes also were noted. An attribute was considered condition specific if it was mentioned by at least 1 participant in both groups for any condition. Preferred properties for topical medications that were specific to acne patients included the following: easy to dispense/dispenses right amount, nondrying, product goes on/spreads smoothly, container (eg, tube, pump) is not easily broken/does not leak, and creamy. Preferred properties that were specific to AD patients included the following: is not noticeable to others/conceals area, good consistency, and cooling. Preferred properties that were specific to 2 conditions included no residue among acne and AD patients and soothing among AD and PP patients. Preferred properties for topical medications across all focus groups are described in further detail in Table 3.



Regarding vehicle-type preferences, acne patients tended to prefer washes, creams, and lotions; AD patients preferred creams; and psoriasis patients preferred creams, ointments, and foams (particularly for the scalp).
Participants across all 3 conditions reported that during daytime hours (versus at night), they would be less likely to use products that are oily, shiny, thick (eg, ointments, oils); bleach or stain the skin/clothing; interfere with makeup, work, or other activities; or are visible to others. Rather, a majority of participants noted that they were more likely to use thinner, less oily products (eg, creams, lotions) during daytime hours, in social situations, or during certain activities (eg, exercise). Participants across all 3 conditions noted that they only used prescription shampoos when they had enough time to repeatedly rinse their hair to eliminate the smell. Participants across all 6 groups noted that they tended to choose creams or lotions over ointments (when available) during the summer because they considered these products to be lighter and less greasy.
Overall, participants indicated that condition-specific symptoms did not influence their preference for topical formulations; rather, they used whatever prescriptions they currently had. However, in some instances, participants noted that the location of the affected area might influence the type of product selected. For example, some participants tended not to use ointments on the scalp or in locations where their clothes might come into contact with the medicated area to avoid clothes sticking to medicated areas. Other participants used lighter, less stringent products on the face versus other locations. Participants noted that foams were preferred for more discrete localized areas.
Comment
Despite the variability in symptoms and conditions across the study population as well as participants’ varying experience with and access to topical treatments, the attributes participants valued most in topical prescription treatments were relatively consistent across the 3 conditions: moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time. Preferred vehicle attributes were generally consistent across the 3 conditions, but preferences for vehicle types tended to vary by condition. Although some vehicles were more closely associated with specific attributes (eg, the majority considered lotions to be moisturizing), no one vehicle was associated with the complete list of attributes for the ideal topical medication. Regardless of the symptoms experienced or particular situations/activities, participants noted that they tended to use the topical medications that were available to them, even if intended for different locations of the body, and nearly all reported using more than 1 type of topical treatment.
Caution should be used in interpreting these findings, as participant preferences are largely dependent on prior experience with different vehicle types. The small number of participants across the 3 conditions in this analysis also limits conclusions that can be drawn from the data sets, as each condition has different though somewhat overlapping treatment algorithms. A focused inquiry into each of these conditions through separate evaluations may provide for a more robust analysis. Future research might employ questionnaire methodology to develop items assessing the attributes of interest (eg, moisturizing ability, rate of absorption, greasiness/oiliness, stickiness/tackiness, fragrance/odor). The final set of desirable attributes may vary depending on the condition (eg, a moisturizing product may be more important to PP and AD patients than acne patients) as well as comparator products.
Acknowledgment—The authors would like to thank Jennifer Gwazdauskas, MBA, Research Triangle Park, North Carolina, of Stiefel, a GSK company, for her assistance with manuscript review.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient p for topical antibiotic treatment for acne. a randomised controlled trial. Br J Dermatol. 2006;154:524-532.
- Trookman NS, Rizer RL, Ho ET, et al. The importance of vehicle properties to patients with atopic dermatitis. Cutis. 2011;88:13-17.
- Weiss S, Wyres M, Brundage T. A novel foam vehicle is consistently preferred by patients for dermatologic conditions. J Am Acad Dermatol. 2011;64(suppl 1):AB50.
- Tan X, Feldman SR, Chang J, et al. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263-1271.
- Kellett N, West F, Finlay AY. Conjoint analysis: a novel, rigorous tool for determining patient p for topical antibiotic treatment for acne. a randomised controlled trial. Br J Dermatol. 2006;154:524-532.
- Trookman NS, Rizer RL, Ho ET, et al. The importance of vehicle properties to patients with atopic dermatitis. Cutis. 2011;88:13-17.
- Weiss S, Wyres M, Brundage T. A novel foam vehicle is consistently preferred by patients for dermatologic conditions. J Am Acad Dermatol. 2011;64(suppl 1):AB50.
- Patient preference for topical product formulation varies by dermatologic condition being treated.
- Most important vehicle attributes cited across study conditions include moisturizing, absorbs/disappears/dries quickly, available in various formulations, does not bleach or stain skin/hair/clothing, is not greasy or oily, is not sticky or tacky, is long lasting/long acting, is fragrance or odor free, is easy to apply/simple to use, and can use all the time.
- Patient preference for product vehicle is relevant to treatment adherence and may vary depending on the condition being treated.
Cutaneous Signs of Piety
Religious practices can lead to cutaneous changes, and awareness of these changes is of paramount importance in establishing the cause. We review the cutaneous changes related to religious practices, including the Semitic religions, Hinduism, and Sikhism (Table). The most widely followed Semitic religions are Christianity, Islam, and Judaism. Christianity and Islam collectively account for more than half of the world’s population.1

Christianity
Christian individuals are prone to blisters that develop below the knees due to repeated kneeling in prayer.2 A case of allergic contact dermatitis to a wooden cross made from Dalbergia nigra has been reported.3 Localized swelling with hypertrichosis due to muscular hypertrophy in the lower neck above the interscapular region has been described in well-built men who lift weights to bear pasos (floats with wooden sculptures) during Holy Week in Seville, Spain.4
Islam
Cutaneous signs of piety have been well documented in Muslim individuals. The most common presentation is hyperpigmentation of the forehead, usually noted as a secondary finding in patients seeking treatment of unrelated symptoms.5 Cutaneous changes in this region correspond with the area of the forehead that rests on the carpet during prayer. Macules typically present on the upper central aspect of the forehead close to the hairline and/or in pairs above the medial ends of the eyebrows; sometimes 3 or 4 lesions may be present in this area with involvement of the nasion (Figure 1).6
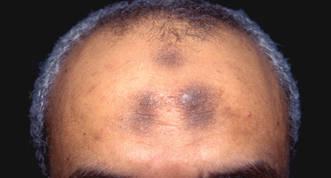
In Saudi Arabia where Sunni Islam predominates, Muslim individuals observe prayer 5 times per day. Calluses have been observed in areas of the body that are frequently subject to friction during this practice.7 For instance, calluses are more prominent on the right knee (Figure 2) and the left ankle, which bear the individual’s weight during prayer, and typically become nodular over time (Figure 3). In Arabic, these calluses are referred to as zabiba.8

A notable finding in followers of Shia Islam, which predominates in Iran, is the development of small nodules on the forehead, possibly caused by rubbing the forehead on a flat disclike prayer stone called a mohr during daily prayer,9 which is said to enhance public esteem.10 The nodules generally are asymptomatic, but some individuals experience minimal pain on pressure.8 Ulceration of the nodules has rarely been observed.7
Limited access to thick and soft carpets and rarely bony exostoses or obesity are factors associated with prayer that can lead to skin changes (known as prayer signs), as they render the skin sensitive to pressure. Localized alopecia may occur on the forehead in individuals with low or pointed hairlines. An unexplained finding noted by one of the authors (K.A.) in some elderly Muslim individuals is that hair located on the forehead at the point of pressure during prayer remains pigmented, while the rest of the hair on the scalp turns white. Hyperpigmentation of the knuckles may be seen in individuals who use closed fists to rise from the ground following prayer. Except for mild hyperpigmentation of the knees,7 Muslim women rarely develop these changes, as they either do not pray,10 particularly during menstruation or puerperium, or they have more subcutaneous fat for protection.7 Some Muslim individuals who pray regularly at home may be conscious of these skin changes and therefore use a soft pillow to rest the forehead during prayer.
The histopathologic findings of prayer signs depend on the extent of lichenification and typically show compact hyperkeratosis or orthokeratosis, hypergranulosis, acanthosis, and mild dermal inflammation.8 Increased dermal vascularization and papillary fibrosis unlike that seen in lichen simplex chronicus have been described from skin changes in the lower limbs due to prayer practices.7 Additional findings in forehead biopsies include multiple comedones and epidermoid cysts in elderly patients showing a foreign body granulomatous reaction to hair fragments.10 Deposition of mucinous material in the dermal collagen in a prayer nodule on the forehead has been described in a Shiite individual, possibly due to repetitive microtrauma from the use of a prayer stone.9 Infections developed from sharing communal facilities or performing ritual sacrifices (eg, tinea,11 orf12) are prevalent during the yearly Hajj pilgrimage at Makkah, Saudi Arabia, in addition to other infectious and noninfectious dermatoses.13 Muslim women wearing headscarves secured at the neck with a safety pin have developed vitiligo at that site due to friction.14 Occasionally, Muslim individuals may apply perfumes before prayers, which may cause allergic contact dermatitis.
Judaism
Hyperpigmentation has been described in Jewish men at Talmudic seminaries due to the practice of reciting scriptures, which involves a rocking motion known as daven that leads to friction on the back.15 Lesions associated with this practice typically appear as isolated macules or a continuous linear patch over the skin of the bony protuberances of the inferior thoracic and lumbar vertebrae. Allergic contact dermatitis has been reported in Jewish individuals due to exposure to a variety of agents during religious practices, such as potassium dichromate, which is present in the leather used to make phylacteries or tefillin (boxes containing scripture that are secured to the forehead with straps that are then tied to the left arm during prayer). This finding has been noted in some or all areas of contact including the forehead, scalp, neck, left wrist, and waist.16
It is customary for both Orthodox Jewish and Muslim women to be concealed by clothing, which predisposes them to vitamin D deficiency17,18 but also protects them from developing malignant melanoma.19 Neonates have developed genital herpetic infections following circumcision due to the ancient practice of having the mohel (the person who performs the Jewish circumcision) suck on the wound until the bleeding stops.20
Hinduism
Hinduism espouses an eclectic philosophy of life subsuming numerous beliefs involving guardian deities, invoked by sacred marks, symbols, and rituals. Marks generally are placed on the forehead or other specified sites on the body. Sandalwood paste as well as vibhuti and kumkum powders most commonly are used, which can cause allergic contact dermatitis. Vibhuti is holy ash prepared by burning balls of dried cow dung in a fire pit with rice husk and clarified butter. Kumkum is prepared by alkalinizing turmeric powder, which turns red in color. A case of contact allergic dermatitis was reported in a Hindu priest who regularly used sandalwood paste on the forehead and as a balm for an ailment of the hands and feet.21 In our experience, vibhuti also has caused dermatitis on the forehead as well as on the neck and arms. The main difference between the 2 eruptions is that sandalwood dermatitis generally is localized to the center of the forehead as a circular or vertical mark or often in the center of the left palm, which is used to mix sandalwood powder with water to make a paste (Figure 4), while vibhuti contact dermatitis typically presents as a broad horizontal patch on the forehead because the powder is smeared with the middle 3 fingers (Figure 5). Perfumes used by some Muslim individuals before prayer that are applied on the clothes can mimic this type of contact dermatitis, but eruptions typically are confined to the fingers and palms.22 Contact dermatitis caused by necklaces made with beads of the stem of the Ocimum sanctum (holy basil) plant and seeds of the evergreen tree Elaeocarpus ganitrus have been reported.23 Calluses are sometimes seen in individuals who meditate for long hours while sitting in a cross-legged position and usually occur on or uncommonly below the lateral malleolus of the right foot, similar to practitioners of yoga.24
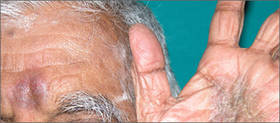
Hemorrhaging and crusting below the lateral malleolus of the right foot have been reported in Buddhist monks due to sitting in a cross-legged position for prolonged periods of meditation.25 Hyperpigmentation of the knees, ankles, and interphalangeal joints of the feet has been seen after sitting in the traditional Japanese meditative position.26 Tattoos of Hindu gods are common, while tattoos are forbidden in Islam and Judaism. Attributes of prominent deities branded on the body may be seen. Discrete sarcoidlike nodules along the axillae and chest wall have been attributed to a Hindu ritual (kavadi) that is performed annually as a form of self-inflicted punishment for their sins in which devotees pierce the chest wall with spokes to form a base over a heavy cage in which offerings are carried, and skewers passed through the cheeks have resulted in similar nodules in the oral cavity.27,28 Consumption of cow’s urine during rituals may induce acute urticaria.29 Lichen planus of the trunk30 and leukoderma of the waist31 may be induced by köbnerization or contact allergy from wearing sacred threads, respectively.
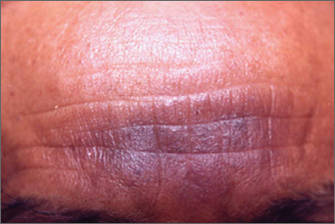
Sikhism
Sikhism, a religion founded in the 15th century, epitomizes the high-water mark of the syncretism between Hinduism and Islam. Men must abstain from cutting their hair; pulling and knotting the hair to maintain a coiffure can cause traction alopecia in the submandibular region and the frontal and parietal areas of the scalp as well as ridging and furrowing of the scalp resembling cutis verticis gyrata. Fixer, a product used to keep the beard intact, can cause contact dermatitis. The tight broad band of cloth (known as a ribbon) that is worn around the head to keep hair intact beneath a turban may cause forehead lesions. Discoid lupus erythematosus–like lesions or painful chondrodermatitis of the pinnae due to pressure from wearing a starched turban have been observed, also called “turban ear” from prominence of both anthelices.32,33 A case of a Sikh man who developed oral sarcoidal lesions from body piercing has been reported.28
Conclusion
Knowledge of the religious practices of patients would help in recognizing puzzling and peculiar dermatoses. It may not be possible to eliminate the causes of these conditions, but methods to reduce their effects on the skin can be discussed with patients.
Acknowledgments—We are grateful to the valuable help rendered by Joginder Kumar, MD, New Delhi, India, and C. Indira, MD, Hyderabad, India.
- The Pew Forum on Religion & Public Life. The Global Religious Landscape: A Report on the Size and Distribution of the World’s Major Religious Groups as of 2010. Washington, DC: The Pew Forum on Religion & Public Life, The Pew Research Center; 2012.
- Goodheart HP. “Devotional dermatoses”: a new nosologic entity? J Am Acad Dermatol. 2001;44:543.
- Fisher AA, Bikowski J. Allergic dermatitis due to a wooden cross made of Dalbergia nigra. Contact Dermatitis. 1981;7:45-46.
- Camacho F. Acquired circumscribed hypertrichosis in the ‘costaleros’ who bear the ‘pasos’ during Holy Week in Seville, Spain. Arch Dermatol. 1995;131:361-363.
- Mishriki YY. Skin commotion from repetitive devotion. prayer callus. Postgrad Med. 1999;105:153-154.
- Barankin B. Prayer marks. Int J Dermatol. 2004;43:985-986.
- Abanmi AA, Al Zouman AY, Al Hussaini H, et al. Prayer marks. Int J Dermatol. 2002;41:411-414.
- Kahana M, Cohen M, Ronnen M, et al. Prayer nodules in Moslem men. Cutis. 1986;38:281-282.
- O’Goshi KI, Aoyama H, Tagami H. Mucin deposition in a prayer nodule on the forehead. Dermatology. 1998;196:364.
- Vollum DI, Azadeh B. Prayer nodules. Clin Exp Dermatol. 1979;4:39-47.
- Arrese JE, Piérard-Franchimont C, Piérard GE. Scytalidium dimidiatum melanonychia and scaly plantar skin in four patients from the Maghreb: imported disease or outbreak in a Belgian mosque? Dermatology. 2001;202:183-185.
- Malik M, Bharier M, Tahan S, et al. Orf acquired during religious observance. Arch Dermatol. 2009;145:606-608.
- Mimesh SA, Al-Khenaizan S, Memish ZA. Dermatologic challenge of pilgrimage. Clin Dermatol. 2008;26:52-61.
- El-Din Anbar T, Abdel-Rahman AT, El-Khayyat MA, et al. Vitiligo on anterior aspect of neck in Muslim females: case series. Int J Dermatol. 2008;47:178-179.
- Naimer SA, Trattner A, Biton A, et al. Davener’s dermatosis: a variant of friction hypermelanosis. J Am Acad Dermatol. 2000;42:442-445.
- Feit NE, Weinberg JM, DeLeo VA. Cutaneous disease and religious practice: case of allergic contact dermatitis to tefillin and review of the literature. Int J Dermatol. 2004;43:886-888.
- Mukamel MN, Weisman Y, Somech R, et al. Vitamin D deficiency and insufficiency in Orthodox and non-Orthodox Jewish mothers in Israel. Isr Med Assoc J. 2001;3:419-421.
- Hatun S, Islam O, Cizmecioglu F, et al. Subclinical vitamin D deficiency is increased in adolescent girls who wear concealing clothing. J Nutr. 2005;135:218-222.
- Vardi G, Modan B, Golan R, et al. Orthodox Jews have a lower incidence of malignant melanoma. a note on the potentially protective role of traditional clothing. Int J Cancer. 1993;53:771-773.
- Gesundheit B, Grisaru-Soen G, Greenberg G, et al. Neonatal genital herpes virus type 1 infection after Jewish ritual circumcision: modern medicine and religious tradition. Pediatrics. 2004;114:e259-e263.
- Pasricha JS, Ramam M. Contact dermatitis due to sandalwood (Santalum album Linn). Indian J Dermatol Venereol Leprol. 1986;52:232-233.
- Carmichael AJ, Foulds IS. Sensitization as a result of a religious ritual. Br J Dermatol. 1990;123:846.
- Bajaj AK, Saraswat A. Contact dermatitis. In: Valia RG, Valia AR, eds. Textbook of Dermatology. 3rd ed. Mumbai, India: Bhalani Publishing House; 2008:545-549.
- Verma SB, Wollina U. Callosities of cross-legged sitting: “yoga sign”—an under-recognized cultural cutaneous presentation. Int J Dermatol. 2008;47:1212-1214.
- Rehman H, Asfour NA. Clinical images: prayer nodules [published online ahead of print November 16, 2009]. CMAJ. 2010;182:e19.
- Ruhnke WG, Serizawa Y. Viral pericarditis. BMJ. 2010;340:b5579.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Ng KH, Siar CH, Ganesapillai T. Sarcoid-like foreign body reaction in body piercing: a report of two cases. Oral Surg Oral Med Oral Pathol Radiol Endod. 1997;84:28-31.
- Bhalla M, Thami GP. Acute urticaria following ‘gomutra’ (cow’s urine) gargles. Clin Exp Dermatol. 2005;30:722-723.
- Joshi A, Agarwalla A, Agrawal S, et al. Köbner phenomenon due to sacred thread in lichen planus. J Dermatol. 2000;27:129-130.
- Banerjee K, Banerjee R, Mandal B. Amulet string contact leukoderma and its differentiation from vitiligo. Indian J Dermatol Venereol Leprol. 2004;70:180-181.
- Kanwar AJ, Kaur S. Some dermatoses peculiar to Sikh men. Int J Dermatol. 1990;29:739-740.
- Williams HC. Turban ear. Arch Dermatol. 1994;130:117-119.
Religious practices can lead to cutaneous changes, and awareness of these changes is of paramount importance in establishing the cause. We review the cutaneous changes related to religious practices, including the Semitic religions, Hinduism, and Sikhism (Table). The most widely followed Semitic religions are Christianity, Islam, and Judaism. Christianity and Islam collectively account for more than half of the world’s population.1

Christianity
Christian individuals are prone to blisters that develop below the knees due to repeated kneeling in prayer.2 A case of allergic contact dermatitis to a wooden cross made from Dalbergia nigra has been reported.3 Localized swelling with hypertrichosis due to muscular hypertrophy in the lower neck above the interscapular region has been described in well-built men who lift weights to bear pasos (floats with wooden sculptures) during Holy Week in Seville, Spain.4
Islam
Cutaneous signs of piety have been well documented in Muslim individuals. The most common presentation is hyperpigmentation of the forehead, usually noted as a secondary finding in patients seeking treatment of unrelated symptoms.5 Cutaneous changes in this region correspond with the area of the forehead that rests on the carpet during prayer. Macules typically present on the upper central aspect of the forehead close to the hairline and/or in pairs above the medial ends of the eyebrows; sometimes 3 or 4 lesions may be present in this area with involvement of the nasion (Figure 1).6

In Saudi Arabia where Sunni Islam predominates, Muslim individuals observe prayer 5 times per day. Calluses have been observed in areas of the body that are frequently subject to friction during this practice.7 For instance, calluses are more prominent on the right knee (Figure 2) and the left ankle, which bear the individual’s weight during prayer, and typically become nodular over time (Figure 3). In Arabic, these calluses are referred to as zabiba.8

A notable finding in followers of Shia Islam, which predominates in Iran, is the development of small nodules on the forehead, possibly caused by rubbing the forehead on a flat disclike prayer stone called a mohr during daily prayer,9 which is said to enhance public esteem.10 The nodules generally are asymptomatic, but some individuals experience minimal pain on pressure.8 Ulceration of the nodules has rarely been observed.7

Limited access to thick and soft carpets and rarely bony exostoses or obesity are factors associated with prayer that can lead to skin changes (known as prayer signs), as they render the skin sensitive to pressure. Localized alopecia may occur on the forehead in individuals with low or pointed hairlines. An unexplained finding noted by one of the authors (K.A.) in some elderly Muslim individuals is that hair located on the forehead at the point of pressure during prayer remains pigmented, while the rest of the hair on the scalp turns white. Hyperpigmentation of the knuckles may be seen in individuals who use closed fists to rise from the ground following prayer. Except for mild hyperpigmentation of the knees,7 Muslim women rarely develop these changes, as they either do not pray,10 particularly during menstruation or puerperium, or they have more subcutaneous fat for protection.7 Some Muslim individuals who pray regularly at home may be conscious of these skin changes and therefore use a soft pillow to rest the forehead during prayer.
The histopathologic findings of prayer signs depend on the extent of lichenification and typically show compact hyperkeratosis or orthokeratosis, hypergranulosis, acanthosis, and mild dermal inflammation.8 Increased dermal vascularization and papillary fibrosis unlike that seen in lichen simplex chronicus have been described from skin changes in the lower limbs due to prayer practices.7 Additional findings in forehead biopsies include multiple comedones and epidermoid cysts in elderly patients showing a foreign body granulomatous reaction to hair fragments.10 Deposition of mucinous material in the dermal collagen in a prayer nodule on the forehead has been described in a Shiite individual, possibly due to repetitive microtrauma from the use of a prayer stone.9 Infections developed from sharing communal facilities or performing ritual sacrifices (eg, tinea,11 orf12) are prevalent during the yearly Hajj pilgrimage at Makkah, Saudi Arabia, in addition to other infectious and noninfectious dermatoses.13 Muslim women wearing headscarves secured at the neck with a safety pin have developed vitiligo at that site due to friction.14 Occasionally, Muslim individuals may apply perfumes before prayers, which may cause allergic contact dermatitis.
Judaism
Hyperpigmentation has been described in Jewish men at Talmudic seminaries due to the practice of reciting scriptures, which involves a rocking motion known as daven that leads to friction on the back.15 Lesions associated with this practice typically appear as isolated macules or a continuous linear patch over the skin of the bony protuberances of the inferior thoracic and lumbar vertebrae. Allergic contact dermatitis has been reported in Jewish individuals due to exposure to a variety of agents during religious practices, such as potassium dichromate, which is present in the leather used to make phylacteries or tefillin (boxes containing scripture that are secured to the forehead with straps that are then tied to the left arm during prayer). This finding has been noted in some or all areas of contact including the forehead, scalp, neck, left wrist, and waist.16
It is customary for both Orthodox Jewish and Muslim women to be concealed by clothing, which predisposes them to vitamin D deficiency17,18 but also protects them from developing malignant melanoma.19 Neonates have developed genital herpetic infections following circumcision due to the ancient practice of having the mohel (the person who performs the Jewish circumcision) suck on the wound until the bleeding stops.20
Hinduism
Hinduism espouses an eclectic philosophy of life subsuming numerous beliefs involving guardian deities, invoked by sacred marks, symbols, and rituals. Marks generally are placed on the forehead or other specified sites on the body. Sandalwood paste as well as vibhuti and kumkum powders most commonly are used, which can cause allergic contact dermatitis. Vibhuti is holy ash prepared by burning balls of dried cow dung in a fire pit with rice husk and clarified butter. Kumkum is prepared by alkalinizing turmeric powder, which turns red in color. A case of contact allergic dermatitis was reported in a Hindu priest who regularly used sandalwood paste on the forehead and as a balm for an ailment of the hands and feet.21 In our experience, vibhuti also has caused dermatitis on the forehead as well as on the neck and arms. The main difference between the 2 eruptions is that sandalwood dermatitis generally is localized to the center of the forehead as a circular or vertical mark or often in the center of the left palm, which is used to mix sandalwood powder with water to make a paste (Figure 4), while vibhuti contact dermatitis typically presents as a broad horizontal patch on the forehead because the powder is smeared with the middle 3 fingers (Figure 5). Perfumes used by some Muslim individuals before prayer that are applied on the clothes can mimic this type of contact dermatitis, but eruptions typically are confined to the fingers and palms.22 Contact dermatitis caused by necklaces made with beads of the stem of the Ocimum sanctum (holy basil) plant and seeds of the evergreen tree Elaeocarpus ganitrus have been reported.23 Calluses are sometimes seen in individuals who meditate for long hours while sitting in a cross-legged position and usually occur on or uncommonly below the lateral malleolus of the right foot, similar to practitioners of yoga.24

Hemorrhaging and crusting below the lateral malleolus of the right foot have been reported in Buddhist monks due to sitting in a cross-legged position for prolonged periods of meditation.25 Hyperpigmentation of the knees, ankles, and interphalangeal joints of the feet has been seen after sitting in the traditional Japanese meditative position.26 Tattoos of Hindu gods are common, while tattoos are forbidden in Islam and Judaism. Attributes of prominent deities branded on the body may be seen. Discrete sarcoidlike nodules along the axillae and chest wall have been attributed to a Hindu ritual (kavadi) that is performed annually as a form of self-inflicted punishment for their sins in which devotees pierce the chest wall with spokes to form a base over a heavy cage in which offerings are carried, and skewers passed through the cheeks have resulted in similar nodules in the oral cavity.27,28 Consumption of cow’s urine during rituals may induce acute urticaria.29 Lichen planus of the trunk30 and leukoderma of the waist31 may be induced by köbnerization or contact allergy from wearing sacred threads, respectively.

Sikhism
Sikhism, a religion founded in the 15th century, epitomizes the high-water mark of the syncretism between Hinduism and Islam. Men must abstain from cutting their hair; pulling and knotting the hair to maintain a coiffure can cause traction alopecia in the submandibular region and the frontal and parietal areas of the scalp as well as ridging and furrowing of the scalp resembling cutis verticis gyrata. Fixer, a product used to keep the beard intact, can cause contact dermatitis. The tight broad band of cloth (known as a ribbon) that is worn around the head to keep hair intact beneath a turban may cause forehead lesions. Discoid lupus erythematosus–like lesions or painful chondrodermatitis of the pinnae due to pressure from wearing a starched turban have been observed, also called “turban ear” from prominence of both anthelices.32,33 A case of a Sikh man who developed oral sarcoidal lesions from body piercing has been reported.28
Conclusion
Knowledge of the religious practices of patients would help in recognizing puzzling and peculiar dermatoses. It may not be possible to eliminate the causes of these conditions, but methods to reduce their effects on the skin can be discussed with patients.
Acknowledgments—We are grateful to the valuable help rendered by Joginder Kumar, MD, New Delhi, India, and C. Indira, MD, Hyderabad, India.
Religious practices can lead to cutaneous changes, and awareness of these changes is of paramount importance in establishing the cause. We review the cutaneous changes related to religious practices, including the Semitic religions, Hinduism, and Sikhism (Table). The most widely followed Semitic religions are Christianity, Islam, and Judaism. Christianity and Islam collectively account for more than half of the world’s population.1

Christianity
Christian individuals are prone to blisters that develop below the knees due to repeated kneeling in prayer.2 A case of allergic contact dermatitis to a wooden cross made from Dalbergia nigra has been reported.3 Localized swelling with hypertrichosis due to muscular hypertrophy in the lower neck above the interscapular region has been described in well-built men who lift weights to bear pasos (floats with wooden sculptures) during Holy Week in Seville, Spain.4
Islam
Cutaneous signs of piety have been well documented in Muslim individuals. The most common presentation is hyperpigmentation of the forehead, usually noted as a secondary finding in patients seeking treatment of unrelated symptoms.5 Cutaneous changes in this region correspond with the area of the forehead that rests on the carpet during prayer. Macules typically present on the upper central aspect of the forehead close to the hairline and/or in pairs above the medial ends of the eyebrows; sometimes 3 or 4 lesions may be present in this area with involvement of the nasion (Figure 1).6

In Saudi Arabia where Sunni Islam predominates, Muslim individuals observe prayer 5 times per day. Calluses have been observed in areas of the body that are frequently subject to friction during this practice.7 For instance, calluses are more prominent on the right knee (Figure 2) and the left ankle, which bear the individual’s weight during prayer, and typically become nodular over time (Figure 3). In Arabic, these calluses are referred to as zabiba.8

A notable finding in followers of Shia Islam, which predominates in Iran, is the development of small nodules on the forehead, possibly caused by rubbing the forehead on a flat disclike prayer stone called a mohr during daily prayer,9 which is said to enhance public esteem.10 The nodules generally are asymptomatic, but some individuals experience minimal pain on pressure.8 Ulceration of the nodules has rarely been observed.7

Limited access to thick and soft carpets and rarely bony exostoses or obesity are factors associated with prayer that can lead to skin changes (known as prayer signs), as they render the skin sensitive to pressure. Localized alopecia may occur on the forehead in individuals with low or pointed hairlines. An unexplained finding noted by one of the authors (K.A.) in some elderly Muslim individuals is that hair located on the forehead at the point of pressure during prayer remains pigmented, while the rest of the hair on the scalp turns white. Hyperpigmentation of the knuckles may be seen in individuals who use closed fists to rise from the ground following prayer. Except for mild hyperpigmentation of the knees,7 Muslim women rarely develop these changes, as they either do not pray,10 particularly during menstruation or puerperium, or they have more subcutaneous fat for protection.7 Some Muslim individuals who pray regularly at home may be conscious of these skin changes and therefore use a soft pillow to rest the forehead during prayer.
The histopathologic findings of prayer signs depend on the extent of lichenification and typically show compact hyperkeratosis or orthokeratosis, hypergranulosis, acanthosis, and mild dermal inflammation.8 Increased dermal vascularization and papillary fibrosis unlike that seen in lichen simplex chronicus have been described from skin changes in the lower limbs due to prayer practices.7 Additional findings in forehead biopsies include multiple comedones and epidermoid cysts in elderly patients showing a foreign body granulomatous reaction to hair fragments.10 Deposition of mucinous material in the dermal collagen in a prayer nodule on the forehead has been described in a Shiite individual, possibly due to repetitive microtrauma from the use of a prayer stone.9 Infections developed from sharing communal facilities or performing ritual sacrifices (eg, tinea,11 orf12) are prevalent during the yearly Hajj pilgrimage at Makkah, Saudi Arabia, in addition to other infectious and noninfectious dermatoses.13 Muslim women wearing headscarves secured at the neck with a safety pin have developed vitiligo at that site due to friction.14 Occasionally, Muslim individuals may apply perfumes before prayers, which may cause allergic contact dermatitis.
Judaism
Hyperpigmentation has been described in Jewish men at Talmudic seminaries due to the practice of reciting scriptures, which involves a rocking motion known as daven that leads to friction on the back.15 Lesions associated with this practice typically appear as isolated macules or a continuous linear patch over the skin of the bony protuberances of the inferior thoracic and lumbar vertebrae. Allergic contact dermatitis has been reported in Jewish individuals due to exposure to a variety of agents during religious practices, such as potassium dichromate, which is present in the leather used to make phylacteries or tefillin (boxes containing scripture that are secured to the forehead with straps that are then tied to the left arm during prayer). This finding has been noted in some or all areas of contact including the forehead, scalp, neck, left wrist, and waist.16
It is customary for both Orthodox Jewish and Muslim women to be concealed by clothing, which predisposes them to vitamin D deficiency17,18 but also protects them from developing malignant melanoma.19 Neonates have developed genital herpetic infections following circumcision due to the ancient practice of having the mohel (the person who performs the Jewish circumcision) suck on the wound until the bleeding stops.20
Hinduism
Hinduism espouses an eclectic philosophy of life subsuming numerous beliefs involving guardian deities, invoked by sacred marks, symbols, and rituals. Marks generally are placed on the forehead or other specified sites on the body. Sandalwood paste as well as vibhuti and kumkum powders most commonly are used, which can cause allergic contact dermatitis. Vibhuti is holy ash prepared by burning balls of dried cow dung in a fire pit with rice husk and clarified butter. Kumkum is prepared by alkalinizing turmeric powder, which turns red in color. A case of contact allergic dermatitis was reported in a Hindu priest who regularly used sandalwood paste on the forehead and as a balm for an ailment of the hands and feet.21 In our experience, vibhuti also has caused dermatitis on the forehead as well as on the neck and arms. The main difference between the 2 eruptions is that sandalwood dermatitis generally is localized to the center of the forehead as a circular or vertical mark or often in the center of the left palm, which is used to mix sandalwood powder with water to make a paste (Figure 4), while vibhuti contact dermatitis typically presents as a broad horizontal patch on the forehead because the powder is smeared with the middle 3 fingers (Figure 5). Perfumes used by some Muslim individuals before prayer that are applied on the clothes can mimic this type of contact dermatitis, but eruptions typically are confined to the fingers and palms.22 Contact dermatitis caused by necklaces made with beads of the stem of the Ocimum sanctum (holy basil) plant and seeds of the evergreen tree Elaeocarpus ganitrus have been reported.23 Calluses are sometimes seen in individuals who meditate for long hours while sitting in a cross-legged position and usually occur on or uncommonly below the lateral malleolus of the right foot, similar to practitioners of yoga.24

Hemorrhaging and crusting below the lateral malleolus of the right foot have been reported in Buddhist monks due to sitting in a cross-legged position for prolonged periods of meditation.25 Hyperpigmentation of the knees, ankles, and interphalangeal joints of the feet has been seen after sitting in the traditional Japanese meditative position.26 Tattoos of Hindu gods are common, while tattoos are forbidden in Islam and Judaism. Attributes of prominent deities branded on the body may be seen. Discrete sarcoidlike nodules along the axillae and chest wall have been attributed to a Hindu ritual (kavadi) that is performed annually as a form of self-inflicted punishment for their sins in which devotees pierce the chest wall with spokes to form a base over a heavy cage in which offerings are carried, and skewers passed through the cheeks have resulted in similar nodules in the oral cavity.27,28 Consumption of cow’s urine during rituals may induce acute urticaria.29 Lichen planus of the trunk30 and leukoderma of the waist31 may be induced by köbnerization or contact allergy from wearing sacred threads, respectively.

Sikhism
Sikhism, a religion founded in the 15th century, epitomizes the high-water mark of the syncretism between Hinduism and Islam. Men must abstain from cutting their hair; pulling and knotting the hair to maintain a coiffure can cause traction alopecia in the submandibular region and the frontal and parietal areas of the scalp as well as ridging and furrowing of the scalp resembling cutis verticis gyrata. Fixer, a product used to keep the beard intact, can cause contact dermatitis. The tight broad band of cloth (known as a ribbon) that is worn around the head to keep hair intact beneath a turban may cause forehead lesions. Discoid lupus erythematosus–like lesions or painful chondrodermatitis of the pinnae due to pressure from wearing a starched turban have been observed, also called “turban ear” from prominence of both anthelices.32,33 A case of a Sikh man who developed oral sarcoidal lesions from body piercing has been reported.28
Conclusion
Knowledge of the religious practices of patients would help in recognizing puzzling and peculiar dermatoses. It may not be possible to eliminate the causes of these conditions, but methods to reduce their effects on the skin can be discussed with patients.
Acknowledgments—We are grateful to the valuable help rendered by Joginder Kumar, MD, New Delhi, India, and C. Indira, MD, Hyderabad, India.
- The Pew Forum on Religion & Public Life. The Global Religious Landscape: A Report on the Size and Distribution of the World’s Major Religious Groups as of 2010. Washington, DC: The Pew Forum on Religion & Public Life, The Pew Research Center; 2012.
- Goodheart HP. “Devotional dermatoses”: a new nosologic entity? J Am Acad Dermatol. 2001;44:543.
- Fisher AA, Bikowski J. Allergic dermatitis due to a wooden cross made of Dalbergia nigra. Contact Dermatitis. 1981;7:45-46.
- Camacho F. Acquired circumscribed hypertrichosis in the ‘costaleros’ who bear the ‘pasos’ during Holy Week in Seville, Spain. Arch Dermatol. 1995;131:361-363.
- Mishriki YY. Skin commotion from repetitive devotion. prayer callus. Postgrad Med. 1999;105:153-154.
- Barankin B. Prayer marks. Int J Dermatol. 2004;43:985-986.
- Abanmi AA, Al Zouman AY, Al Hussaini H, et al. Prayer marks. Int J Dermatol. 2002;41:411-414.
- Kahana M, Cohen M, Ronnen M, et al. Prayer nodules in Moslem men. Cutis. 1986;38:281-282.
- O’Goshi KI, Aoyama H, Tagami H. Mucin deposition in a prayer nodule on the forehead. Dermatology. 1998;196:364.
- Vollum DI, Azadeh B. Prayer nodules. Clin Exp Dermatol. 1979;4:39-47.
- Arrese JE, Piérard-Franchimont C, Piérard GE. Scytalidium dimidiatum melanonychia and scaly plantar skin in four patients from the Maghreb: imported disease or outbreak in a Belgian mosque? Dermatology. 2001;202:183-185.
- Malik M, Bharier M, Tahan S, et al. Orf acquired during religious observance. Arch Dermatol. 2009;145:606-608.
- Mimesh SA, Al-Khenaizan S, Memish ZA. Dermatologic challenge of pilgrimage. Clin Dermatol. 2008;26:52-61.
- El-Din Anbar T, Abdel-Rahman AT, El-Khayyat MA, et al. Vitiligo on anterior aspect of neck in Muslim females: case series. Int J Dermatol. 2008;47:178-179.
- Naimer SA, Trattner A, Biton A, et al. Davener’s dermatosis: a variant of friction hypermelanosis. J Am Acad Dermatol. 2000;42:442-445.
- Feit NE, Weinberg JM, DeLeo VA. Cutaneous disease and religious practice: case of allergic contact dermatitis to tefillin and review of the literature. Int J Dermatol. 2004;43:886-888.
- Mukamel MN, Weisman Y, Somech R, et al. Vitamin D deficiency and insufficiency in Orthodox and non-Orthodox Jewish mothers in Israel. Isr Med Assoc J. 2001;3:419-421.
- Hatun S, Islam O, Cizmecioglu F, et al. Subclinical vitamin D deficiency is increased in adolescent girls who wear concealing clothing. J Nutr. 2005;135:218-222.
- Vardi G, Modan B, Golan R, et al. Orthodox Jews have a lower incidence of malignant melanoma. a note on the potentially protective role of traditional clothing. Int J Cancer. 1993;53:771-773.
- Gesundheit B, Grisaru-Soen G, Greenberg G, et al. Neonatal genital herpes virus type 1 infection after Jewish ritual circumcision: modern medicine and religious tradition. Pediatrics. 2004;114:e259-e263.
- Pasricha JS, Ramam M. Contact dermatitis due to sandalwood (Santalum album Linn). Indian J Dermatol Venereol Leprol. 1986;52:232-233.
- Carmichael AJ, Foulds IS. Sensitization as a result of a religious ritual. Br J Dermatol. 1990;123:846.
- Bajaj AK, Saraswat A. Contact dermatitis. In: Valia RG, Valia AR, eds. Textbook of Dermatology. 3rd ed. Mumbai, India: Bhalani Publishing House; 2008:545-549.
- Verma SB, Wollina U. Callosities of cross-legged sitting: “yoga sign”—an under-recognized cultural cutaneous presentation. Int J Dermatol. 2008;47:1212-1214.
- Rehman H, Asfour NA. Clinical images: prayer nodules [published online ahead of print November 16, 2009]. CMAJ. 2010;182:e19.
- Ruhnke WG, Serizawa Y. Viral pericarditis. BMJ. 2010;340:b5579.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Ng KH, Siar CH, Ganesapillai T. Sarcoid-like foreign body reaction in body piercing: a report of two cases. Oral Surg Oral Med Oral Pathol Radiol Endod. 1997;84:28-31.
- Bhalla M, Thami GP. Acute urticaria following ‘gomutra’ (cow’s urine) gargles. Clin Exp Dermatol. 2005;30:722-723.
- Joshi A, Agarwalla A, Agrawal S, et al. Köbner phenomenon due to sacred thread in lichen planus. J Dermatol. 2000;27:129-130.
- Banerjee K, Banerjee R, Mandal B. Amulet string contact leukoderma and its differentiation from vitiligo. Indian J Dermatol Venereol Leprol. 2004;70:180-181.
- Kanwar AJ, Kaur S. Some dermatoses peculiar to Sikh men. Int J Dermatol. 1990;29:739-740.
- Williams HC. Turban ear. Arch Dermatol. 1994;130:117-119.
- The Pew Forum on Religion & Public Life. The Global Religious Landscape: A Report on the Size and Distribution of the World’s Major Religious Groups as of 2010. Washington, DC: The Pew Forum on Religion & Public Life, The Pew Research Center; 2012.
- Goodheart HP. “Devotional dermatoses”: a new nosologic entity? J Am Acad Dermatol. 2001;44:543.
- Fisher AA, Bikowski J. Allergic dermatitis due to a wooden cross made of Dalbergia nigra. Contact Dermatitis. 1981;7:45-46.
- Camacho F. Acquired circumscribed hypertrichosis in the ‘costaleros’ who bear the ‘pasos’ during Holy Week in Seville, Spain. Arch Dermatol. 1995;131:361-363.
- Mishriki YY. Skin commotion from repetitive devotion. prayer callus. Postgrad Med. 1999;105:153-154.
- Barankin B. Prayer marks. Int J Dermatol. 2004;43:985-986.
- Abanmi AA, Al Zouman AY, Al Hussaini H, et al. Prayer marks. Int J Dermatol. 2002;41:411-414.
- Kahana M, Cohen M, Ronnen M, et al. Prayer nodules in Moslem men. Cutis. 1986;38:281-282.
- O’Goshi KI, Aoyama H, Tagami H. Mucin deposition in a prayer nodule on the forehead. Dermatology. 1998;196:364.
- Vollum DI, Azadeh B. Prayer nodules. Clin Exp Dermatol. 1979;4:39-47.
- Arrese JE, Piérard-Franchimont C, Piérard GE. Scytalidium dimidiatum melanonychia and scaly plantar skin in four patients from the Maghreb: imported disease or outbreak in a Belgian mosque? Dermatology. 2001;202:183-185.
- Malik M, Bharier M, Tahan S, et al. Orf acquired during religious observance. Arch Dermatol. 2009;145:606-608.
- Mimesh SA, Al-Khenaizan S, Memish ZA. Dermatologic challenge of pilgrimage. Clin Dermatol. 2008;26:52-61.
- El-Din Anbar T, Abdel-Rahman AT, El-Khayyat MA, et al. Vitiligo on anterior aspect of neck in Muslim females: case series. Int J Dermatol. 2008;47:178-179.
- Naimer SA, Trattner A, Biton A, et al. Davener’s dermatosis: a variant of friction hypermelanosis. J Am Acad Dermatol. 2000;42:442-445.
- Feit NE, Weinberg JM, DeLeo VA. Cutaneous disease and religious practice: case of allergic contact dermatitis to tefillin and review of the literature. Int J Dermatol. 2004;43:886-888.
- Mukamel MN, Weisman Y, Somech R, et al. Vitamin D deficiency and insufficiency in Orthodox and non-Orthodox Jewish mothers in Israel. Isr Med Assoc J. 2001;3:419-421.
- Hatun S, Islam O, Cizmecioglu F, et al. Subclinical vitamin D deficiency is increased in adolescent girls who wear concealing clothing. J Nutr. 2005;135:218-222.
- Vardi G, Modan B, Golan R, et al. Orthodox Jews have a lower incidence of malignant melanoma. a note on the potentially protective role of traditional clothing. Int J Cancer. 1993;53:771-773.
- Gesundheit B, Grisaru-Soen G, Greenberg G, et al. Neonatal genital herpes virus type 1 infection after Jewish ritual circumcision: modern medicine and religious tradition. Pediatrics. 2004;114:e259-e263.
- Pasricha JS, Ramam M. Contact dermatitis due to sandalwood (Santalum album Linn). Indian J Dermatol Venereol Leprol. 1986;52:232-233.
- Carmichael AJ, Foulds IS. Sensitization as a result of a religious ritual. Br J Dermatol. 1990;123:846.
- Bajaj AK, Saraswat A. Contact dermatitis. In: Valia RG, Valia AR, eds. Textbook of Dermatology. 3rd ed. Mumbai, India: Bhalani Publishing House; 2008:545-549.
- Verma SB, Wollina U. Callosities of cross-legged sitting: “yoga sign”—an under-recognized cultural cutaneous presentation. Int J Dermatol. 2008;47:1212-1214.
- Rehman H, Asfour NA. Clinical images: prayer nodules [published online ahead of print November 16, 2009]. CMAJ. 2010;182:e19.
- Ruhnke WG, Serizawa Y. Viral pericarditis. BMJ. 2010;340:b5579.
- Nayar M. Sarcoidosis on ritual scarification. Int J Dermatol. 1993;32:116-118.
- Ng KH, Siar CH, Ganesapillai T. Sarcoid-like foreign body reaction in body piercing: a report of two cases. Oral Surg Oral Med Oral Pathol Radiol Endod. 1997;84:28-31.
- Bhalla M, Thami GP. Acute urticaria following ‘gomutra’ (cow’s urine) gargles. Clin Exp Dermatol. 2005;30:722-723.
- Joshi A, Agarwalla A, Agrawal S, et al. Köbner phenomenon due to sacred thread in lichen planus. J Dermatol. 2000;27:129-130.
- Banerjee K, Banerjee R, Mandal B. Amulet string contact leukoderma and its differentiation from vitiligo. Indian J Dermatol Venereol Leprol. 2004;70:180-181.
- Kanwar AJ, Kaur S. Some dermatoses peculiar to Sikh men. Int J Dermatol. 1990;29:739-740.
- Williams HC. Turban ear. Arch Dermatol. 1994;130:117-119.
Practice Points
- Cutaneous changes may be seen in specified areas of the skin following regular worship in almost all major religions of the world.
- Cutaneous lesions are most commonly associated with friction from praying, along with contact allergic dermatitis from products and substances commonly used in worshipping and granulomas due to practices such as tattoos and skin piercing.
- Uncommon skin manifestations include urticaria and leukoderma.
- Some religious practices may render individuals prone to infections that manifest on the skin.
Frostbite on the Hand of a Homeless Man
To the Editor:
A 58-year-old homeless man presented to the emergency department after being found wandering in the middle of winter in Detroit, Michigan, with altered mental status. A workup for his mental incapacitation uncovered severe electrolyte disturbances, hyperglycemia, and acute renal failure, as well as both alcohol and drug intoxication. After 1 day of admission the patient reported progressive swelling, blistering, and pain in the right hand. The pain was stabbing in nature, worse with movement, and graded 10 of 10 (1=minimal; 10=severe). His medical history was notable for diabetes mellitus with peripheral neuropathy, hypertension, hyperlipidemia, and alcohol and drug abuse. The patient was not taking any medications for these conditions.
Physical examination revealed 2+ moderate pitting edema in all distal extremities, with increased edema of the dorsal aspect of the right hand. The right hand also demonstrated patchy erythema and was warm to touch. The dorsal aspect of the right ring finger had a dusky tip and was studded with several tense blisters (Figure). Vital signs were stable. Based on the patient’s history and physical examination findings, a diagnosis of frostbite was made. Our treatment process involved several modalities including immersion of the affected site in a warm water bath, surgical debridement of blistered sites, tetanus toxoid, penicillin to prevent infection, and oral ibuprofen for pain management. At 3-day follow-up, the patient’s condition substantially improved with a decreased amount of erythema, edema, and pain. All affected sites were successfully preserved with no evidence of focal, motor, or sensory impairment.

Frostbite is a form of localized tissue injury due to extreme cold that most commonly affects the hands and feet, with the greatest incidence occurring in adults aged 30 to 49 years.1,2 Other sites commonly affected include the ears, nose, cheeks, and penis. Frostbite injuries can be categorized into 4 degrees of severity that correlate with the clinical presentation.1,3 Rewarming the affected site is necessary to properly classify the injury, as the initial appearance may be similar among the different degrees of injury. A first-degree injury classically shows a central white plaque with peripheral erythema and is extremely cold to touch. Second-degree injuries display tense blisters filled with clear or milky fluid surrounded by erythema and edema within the first 24 hours. Third-degree injuries are associated with hemorrhagic blisters. Fourth-degree injuries involve complete tissue loss and necrosis.1 Frostbite injuries also may be classified as superficial or deep; the former affects skin and subcutaneous tissue, while the latter affects bones, joints, and tendons.3,4 The superficial form exhibits clear blisters, whereas hemorrhagic blisters demonstrate deep frostbite.
Factors such as the surrounding temperature, length of exposure, and alcohol consumption may exacerbate frostbite injuries.1 Conditions such as atherosclerosis and diabetes mellitus, which can cause neuropathy and peripheral vascular disease, also are potential risks. Psychiatric patients also are at risk for frostbite given the propensity for eccentric behavior as well as the homeless due to inadequate clothing or shelter. Diagnosis often can be made based on medical history and physical examination, though techniques such as radiography, angiography, digital plethysmography, Doppler ultrasonography, and bone scintigraphy (technetium-99) also have been utilized to determine severity and prognosis.2 Differential diagnoses of frostbite are listed in the Table.
Frostbite treatment begins with removal of wet clothing and region protection. Rewarming the site should not begin until refreezing is unlikely to occur and involves placing the injured area in water (temperature, 40°C–42°C) for 15 to 30 minutes to minimize tissue loss.1,2 Analgesics, tetanus toxoid, oral ibuprofen, and benzylpenicillin also are indicated, along with daily hydrotherapy.1,2 White blisters should be debrided, while hemorrhagic blisters should be left intact. Amputation and aggressive debridement typically are delayed until complete ischemia occurs and final demarcation is determined, usually over 1 to 3 months.1 Combination therapy allowed for a positive outcome in our patient.
Frostnip is a mild form of cold injury characterized by localized pain, pallor, and possible numbness.3 Warming the cold area restores the function and sensation with no loss of tissue. Chilblain or pernio refers to a localized cold injury that typically presents as pruritic red-purple papules, macules, plaques, or nodules on the face, anterior tibial surface, or dorsum and tips of the hands and feet.3 The primary cause is repeated exposure to cold, not freezing, temperatures.
Trench foot or cold immersion foot (or hand) is a nonfreezing injury to the hands or feet caused by chronic exposure to wet conditions and temperatures above freezing.3 Painful burning and dysesthesia as well as tissue damage involving edema, blistering, redness, ecchymosis, and ulceration are common. Cellulitis is a bacterial infection of the skin and underlying tissues that can occur anywhere on the body, but the legs are most commonly affected. Typical presentation involves erythema, warmth, swelling, and pain in the infected area.
Although the conditions described above may be considered in the differential diagnosis, physical examination and the patient’s clinical history typically will allow for the distinction of frostbite from these other disease processes.
- Petrone P, Kuncir EJ, Asensio JA. Surgical management and strategies in the treatment of hypothermia and cold injury. Emerg Med Clin North Am. 2003;21:1165-1178.
- Reamy BV. Frostbite: review and current concepts. J Am Board Fam Pract. 1998;11:34-40.
- Jurkovich GJ. Environmental cold-induced injury. Surg Clin North Am. 2007;87:247-267, viii.
- Biem J, Koehncke N, Classen D, et al. Out of the cold: management of hypothermia and frostbite. CMAJ. 2003;168:305-311.
To the Editor:
A 58-year-old homeless man presented to the emergency department after being found wandering in the middle of winter in Detroit, Michigan, with altered mental status. A workup for his mental incapacitation uncovered severe electrolyte disturbances, hyperglycemia, and acute renal failure, as well as both alcohol and drug intoxication. After 1 day of admission the patient reported progressive swelling, blistering, and pain in the right hand. The pain was stabbing in nature, worse with movement, and graded 10 of 10 (1=minimal; 10=severe). His medical history was notable for diabetes mellitus with peripheral neuropathy, hypertension, hyperlipidemia, and alcohol and drug abuse. The patient was not taking any medications for these conditions.
Physical examination revealed 2+ moderate pitting edema in all distal extremities, with increased edema of the dorsal aspect of the right hand. The right hand also demonstrated patchy erythema and was warm to touch. The dorsal aspect of the right ring finger had a dusky tip and was studded with several tense blisters (Figure). Vital signs were stable. Based on the patient’s history and physical examination findings, a diagnosis of frostbite was made. Our treatment process involved several modalities including immersion of the affected site in a warm water bath, surgical debridement of blistered sites, tetanus toxoid, penicillin to prevent infection, and oral ibuprofen for pain management. At 3-day follow-up, the patient’s condition substantially improved with a decreased amount of erythema, edema, and pain. All affected sites were successfully preserved with no evidence of focal, motor, or sensory impairment.

Frostbite is a form of localized tissue injury due to extreme cold that most commonly affects the hands and feet, with the greatest incidence occurring in adults aged 30 to 49 years.1,2 Other sites commonly affected include the ears, nose, cheeks, and penis. Frostbite injuries can be categorized into 4 degrees of severity that correlate with the clinical presentation.1,3 Rewarming the affected site is necessary to properly classify the injury, as the initial appearance may be similar among the different degrees of injury. A first-degree injury classically shows a central white plaque with peripheral erythema and is extremely cold to touch. Second-degree injuries display tense blisters filled with clear or milky fluid surrounded by erythema and edema within the first 24 hours. Third-degree injuries are associated with hemorrhagic blisters. Fourth-degree injuries involve complete tissue loss and necrosis.1 Frostbite injuries also may be classified as superficial or deep; the former affects skin and subcutaneous tissue, while the latter affects bones, joints, and tendons.3,4 The superficial form exhibits clear blisters, whereas hemorrhagic blisters demonstrate deep frostbite.
Factors such as the surrounding temperature, length of exposure, and alcohol consumption may exacerbate frostbite injuries.1 Conditions such as atherosclerosis and diabetes mellitus, which can cause neuropathy and peripheral vascular disease, also are potential risks. Psychiatric patients also are at risk for frostbite given the propensity for eccentric behavior as well as the homeless due to inadequate clothing or shelter. Diagnosis often can be made based on medical history and physical examination, though techniques such as radiography, angiography, digital plethysmography, Doppler ultrasonography, and bone scintigraphy (technetium-99) also have been utilized to determine severity and prognosis.2 Differential diagnoses of frostbite are listed in the Table.

Frostbite treatment begins with removal of wet clothing and region protection. Rewarming the site should not begin until refreezing is unlikely to occur and involves placing the injured area in water (temperature, 40°C–42°C) for 15 to 30 minutes to minimize tissue loss.1,2 Analgesics, tetanus toxoid, oral ibuprofen, and benzylpenicillin also are indicated, along with daily hydrotherapy.1,2 White blisters should be debrided, while hemorrhagic blisters should be left intact. Amputation and aggressive debridement typically are delayed until complete ischemia occurs and final demarcation is determined, usually over 1 to 3 months.1 Combination therapy allowed for a positive outcome in our patient.
Frostnip is a mild form of cold injury characterized by localized pain, pallor, and possible numbness.3 Warming the cold area restores the function and sensation with no loss of tissue. Chilblain or pernio refers to a localized cold injury that typically presents as pruritic red-purple papules, macules, plaques, or nodules on the face, anterior tibial surface, or dorsum and tips of the hands and feet.3 The primary cause is repeated exposure to cold, not freezing, temperatures.
Trench foot or cold immersion foot (or hand) is a nonfreezing injury to the hands or feet caused by chronic exposure to wet conditions and temperatures above freezing.3 Painful burning and dysesthesia as well as tissue damage involving edema, blistering, redness, ecchymosis, and ulceration are common. Cellulitis is a bacterial infection of the skin and underlying tissues that can occur anywhere on the body, but the legs are most commonly affected. Typical presentation involves erythema, warmth, swelling, and pain in the infected area.
Although the conditions described above may be considered in the differential diagnosis, physical examination and the patient’s clinical history typically will allow for the distinction of frostbite from these other disease processes.
To the Editor:
A 58-year-old homeless man presented to the emergency department after being found wandering in the middle of winter in Detroit, Michigan, with altered mental status. A workup for his mental incapacitation uncovered severe electrolyte disturbances, hyperglycemia, and acute renal failure, as well as both alcohol and drug intoxication. After 1 day of admission the patient reported progressive swelling, blistering, and pain in the right hand. The pain was stabbing in nature, worse with movement, and graded 10 of 10 (1=minimal; 10=severe). His medical history was notable for diabetes mellitus with peripheral neuropathy, hypertension, hyperlipidemia, and alcohol and drug abuse. The patient was not taking any medications for these conditions.
Physical examination revealed 2+ moderate pitting edema in all distal extremities, with increased edema of the dorsal aspect of the right hand. The right hand also demonstrated patchy erythema and was warm to touch. The dorsal aspect of the right ring finger had a dusky tip and was studded with several tense blisters (Figure). Vital signs were stable. Based on the patient’s history and physical examination findings, a diagnosis of frostbite was made. Our treatment process involved several modalities including immersion of the affected site in a warm water bath, surgical debridement of blistered sites, tetanus toxoid, penicillin to prevent infection, and oral ibuprofen for pain management. At 3-day follow-up, the patient’s condition substantially improved with a decreased amount of erythema, edema, and pain. All affected sites were successfully preserved with no evidence of focal, motor, or sensory impairment.

Frostbite is a form of localized tissue injury due to extreme cold that most commonly affects the hands and feet, with the greatest incidence occurring in adults aged 30 to 49 years.1,2 Other sites commonly affected include the ears, nose, cheeks, and penis. Frostbite injuries can be categorized into 4 degrees of severity that correlate with the clinical presentation.1,3 Rewarming the affected site is necessary to properly classify the injury, as the initial appearance may be similar among the different degrees of injury. A first-degree injury classically shows a central white plaque with peripheral erythema and is extremely cold to touch. Second-degree injuries display tense blisters filled with clear or milky fluid surrounded by erythema and edema within the first 24 hours. Third-degree injuries are associated with hemorrhagic blisters. Fourth-degree injuries involve complete tissue loss and necrosis.1 Frostbite injuries also may be classified as superficial or deep; the former affects skin and subcutaneous tissue, while the latter affects bones, joints, and tendons.3,4 The superficial form exhibits clear blisters, whereas hemorrhagic blisters demonstrate deep frostbite.
Factors such as the surrounding temperature, length of exposure, and alcohol consumption may exacerbate frostbite injuries.1 Conditions such as atherosclerosis and diabetes mellitus, which can cause neuropathy and peripheral vascular disease, also are potential risks. Psychiatric patients also are at risk for frostbite given the propensity for eccentric behavior as well as the homeless due to inadequate clothing or shelter. Diagnosis often can be made based on medical history and physical examination, though techniques such as radiography, angiography, digital plethysmography, Doppler ultrasonography, and bone scintigraphy (technetium-99) also have been utilized to determine severity and prognosis.2 Differential diagnoses of frostbite are listed in the Table.

Frostbite treatment begins with removal of wet clothing and region protection. Rewarming the site should not begin until refreezing is unlikely to occur and involves placing the injured area in water (temperature, 40°C–42°C) for 15 to 30 minutes to minimize tissue loss.1,2 Analgesics, tetanus toxoid, oral ibuprofen, and benzylpenicillin also are indicated, along with daily hydrotherapy.1,2 White blisters should be debrided, while hemorrhagic blisters should be left intact. Amputation and aggressive debridement typically are delayed until complete ischemia occurs and final demarcation is determined, usually over 1 to 3 months.1 Combination therapy allowed for a positive outcome in our patient.
Frostnip is a mild form of cold injury characterized by localized pain, pallor, and possible numbness.3 Warming the cold area restores the function and sensation with no loss of tissue. Chilblain or pernio refers to a localized cold injury that typically presents as pruritic red-purple papules, macules, plaques, or nodules on the face, anterior tibial surface, or dorsum and tips of the hands and feet.3 The primary cause is repeated exposure to cold, not freezing, temperatures.
Trench foot or cold immersion foot (or hand) is a nonfreezing injury to the hands or feet caused by chronic exposure to wet conditions and temperatures above freezing.3 Painful burning and dysesthesia as well as tissue damage involving edema, blistering, redness, ecchymosis, and ulceration are common. Cellulitis is a bacterial infection of the skin and underlying tissues that can occur anywhere on the body, but the legs are most commonly affected. Typical presentation involves erythema, warmth, swelling, and pain in the infected area.
Although the conditions described above may be considered in the differential diagnosis, physical examination and the patient’s clinical history typically will allow for the distinction of frostbite from these other disease processes.
- Petrone P, Kuncir EJ, Asensio JA. Surgical management and strategies in the treatment of hypothermia and cold injury. Emerg Med Clin North Am. 2003;21:1165-1178.
- Reamy BV. Frostbite: review and current concepts. J Am Board Fam Pract. 1998;11:34-40.
- Jurkovich GJ. Environmental cold-induced injury. Surg Clin North Am. 2007;87:247-267, viii.
- Biem J, Koehncke N, Classen D, et al. Out of the cold: management of hypothermia and frostbite. CMAJ. 2003;168:305-311.
- Petrone P, Kuncir EJ, Asensio JA. Surgical management and strategies in the treatment of hypothermia and cold injury. Emerg Med Clin North Am. 2003;21:1165-1178.
- Reamy BV. Frostbite: review and current concepts. J Am Board Fam Pract. 1998;11:34-40.
- Jurkovich GJ. Environmental cold-induced injury. Surg Clin North Am. 2007;87:247-267, viii.
- Biem J, Koehncke N, Classen D, et al. Out of the cold: management of hypothermia and frostbite. CMAJ. 2003;168:305-311.
Photoinduced Classic Sweet Syndrome Presenting as Hemorrhagic Bullae
To the Editor:
Sweet syndrome (SS) is characterized by fever; acute onset of painful erythematous papules, plaques, or nodules; peripheral neutrophilic leukocytosis; and histologic findings of a dense neutrophilic infiltrate without evidence of primary vasculitis.1 We report a rare case of classic SS presenting with hemorrhagic bullae over photoexposed areas. Our case is notable because of the unusual nature of the clinical manifestation.
A 45-year-old woman presented with painful, fluid-filled lesions on the upper extremities of 1 week’s duration. Lesions were acute in onset and associated with fever. The patient had a history of diabetes mellitus and hypertension, which were well controlled. She also had a history of minimal itching on sun exposure as well as an upper respiratory tract infection 2 months prior to presentation. There was no history of muscle weakness, pain and/or discoloration of the fingertips, or treatment with topical or systemic agents.
On physical examination, the patient was well nourished with an average build. She was febrile (temperature, 38.5°C) with a pulse of 82 beats per minute, a blood pressure of 130/80, and a respiratory rate of 14 breaths per minute. On cutaneous examination multiple erythematous plaques with central large hemorrhagic bullae were present on the extensor aspect of the forearms and dorsum of the left hand. The smallest plaque measured 4×8 cm and the largest measured 8×15 cm (Figure 1). The lesions were tender, and Nikolsky sign was negative. Considering the clinical features, a differential diagnosis of bullous systemic lupus erythematosus, polymorphic light eruption, Jessner lymphocytic infiltrate, and SS were considered. Complete blood cell count demonstrated a hemoglobin level of 12.1 g/dL (reference range, 14.0–17.5 g/dL), total leukocyte count of 13,280/μL (reference range, 4500–11,000/μL), neutrophil count of 80% (reference range, 56%), lymphocyte count of 13% (reference range, 34%), monocyte count of 8% (reference range, 4%), and an erythrocyte sedimentation rate of 40 mm/h (reference range, 0–20 mm/h). Serum creatinine levels were 0.9 mg/dL (reference range, 0.6–1.2 mg/dL) and urea nitrogen levels were 26 mg/dL (reference range, 8–23 mg/dL). C-reactive protein was positive, antinuclear antibody was negative, and double-stranded DNA was negative. Ultrasonography of the abdomen and pelvis and a chest radiograph revealed no abnormalities.
Histopathology from a lesion on the forearm revealed a dense, predominantly neutrophilic infiltrate located in the superficial dermis as well as prominent papillary dermal edema infiltration in the dermis with vasodilatation in some areas without leukocytoclastic vasculitis features (Figures 2 and 3). Considering the clinical and histopathologic features, a diagnosis of SS was made, and the patient was started on intravenous dexamethasone (4 mg twice daily) with a dramatic response, as the lesions almost cleared within 4 to 5 days of treatment (Figure 4).
Sweet syndrome was first described in 1964 as acute febrile neutrophilic dermatosis.2 Sweet syndrome can be subdivided into 3 groups depending on the clinical setting: classic or idiopathic, malignancy associated, and drug induced.3 Classic or idiopathic SS typically affects women in the third to fifth decades of life,4 as seen in our case. The proposed diagnostic criteria for SS state that patients must meet both of the 2 major criteria and 2 of 4 minor criteria for the diagnosis.3 The major criteria include acute onset of typical skin lesions and histopathologic findings consistent with SS. The minor criteria include fever (temperature >38°C) or general malaise; association with malignancy, inflammatory disease, pregnancy, or antecedent respiratory or gastrointestinal tract infection; excellent response to treatment with systemic corticosteroids or potassium iodide; and abnormal laboratory values at presentation (3 of 4 required: erythrocyte sedimentation rate >20 mm/h; leukocyte count >8000/μL; neutrophil count >70%; positive C-reactive protein). Our patient fulfilled both the major criteria and 3 of 4 minor criteria. Although the exact etiology of SS is unknown, it is widely believed that SS may be a hypersensitivity response to underlying bacterial infections such as Yersinia enterocolitica, viral infections, or tumors.5
Cytokine dysregulation also has been indicated in the pathogenesis of SS, an imbalance of cytokine secretion from helper T cells such as IL-2 and IFN-γ, which may stimulate the cytokine cascade leading to activation of neutrophils and release of toxic metabolites.6 The cutaneous manifestations of SS consist of erythematous to violaceous tender papules or nodules that often coalesce to form irregular plaques.7 Rare clinical manifestations include bullous lesions; oral involvement; glomerulonephritis; myositis; and ocular manifestations including conjunctivitis, episcleritis, and iridocyclitis.5,8 Photoaggravated and photoinduced syndromes also have been reported.9
Bullous-type SS has been reported, but all the known cases were secondary to malignancy or were drug induced8,10; they did not present in a classic or idiopathic variant. Our case is unique in that it is a report of the classic SS variant with lesions including bullae over photoexposed areas, possibly indicating a causal association of sun exposure and the development of SS.
The diagnostic histopathologic features of SS include a dense, predominantly neutrophilic infiltrate located in the superficial dermis as well as prominent papillary dermal edema, which occasionally may lead to subepidermal vesiculation.11,12 The epidermis often is normal but spongiosis may be present, and rarely neutrophils may extend into the epidermis to form subcorneal pustules.13 Severe edema in the papillary dermis may cause subepidermal blistering and bullous lesions.10 Systemic steroids are the therapeutic mainstay in SS. Other treatment options include methylprednisolone, potassium iodide, colchicine, indomethacin, cyclosporine, and dapsone.
1. Von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556.
2. Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;74:349-356.
3. Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
4. Cohen PR. Pregnancy-associated Sweet’s syndrome: world literature review. Obstet Gynecol Surv. 1993;48:584-587.
5. Cohen PR, Hönigsmann H, Kurzrock R. Acute febrile neutrophilic dermatosis (Sweet syndrome). In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2008:289-295.
6. Giasuddin AS, El-Orfi AH, Ziu MM, et al. Sweet’s syndrome: is the pathogenesis mediated by helper T cell type 1 cytokines? J Am Acad Dermatol. 1998;39:940-943.
7. Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
8. Lund JJ, Stratman EJ, Jose D, et al. Drug-induced bullous Sweet syndrome with multiple autoimmune features. Autoimmune Dis. 2010;2011:176749.
9. Bessis D, Dereure O, Peyron JL, et al. Photoinduced Sweet syndrome. Arch Dermatol. 2003;137:1106-1108.
10. Bielsa S, Baradad M, Martí RM, et al. Sweet’s syndrome with bullous lesions [in Spanish]. Actas Dermosifiliogr. 2005;96:315-316.
11. Jordaan HF. Acute febrile neutrophilic dermatosis: a histopathological study of 37 cases and a review of the literature. Am J Dermatopathol. 1989;11:99-111.
12. Kemmett D, Hunter JA. Sweet’s syndrome: a clinicopathologic review of twenty-nine cases. J Am Acad Dermatol. 1990;23(3, pt 1):503-507.
13. Wallach D. Neutrophilic disease [in French]. Rev Prat. 1999;49:356-358.
To the Editor:
Sweet syndrome (SS) is characterized by fever; acute onset of painful erythematous papules, plaques, or nodules; peripheral neutrophilic leukocytosis; and histologic findings of a dense neutrophilic infiltrate without evidence of primary vasculitis.1 We report a rare case of classic SS presenting with hemorrhagic bullae over photoexposed areas. Our case is notable because of the unusual nature of the clinical manifestation.
A 45-year-old woman presented with painful, fluid-filled lesions on the upper extremities of 1 week’s duration. Lesions were acute in onset and associated with fever. The patient had a history of diabetes mellitus and hypertension, which were well controlled. She also had a history of minimal itching on sun exposure as well as an upper respiratory tract infection 2 months prior to presentation. There was no history of muscle weakness, pain and/or discoloration of the fingertips, or treatment with topical or systemic agents.
On physical examination, the patient was well nourished with an average build. She was febrile (temperature, 38.5°C) with a pulse of 82 beats per minute, a blood pressure of 130/80, and a respiratory rate of 14 breaths per minute. On cutaneous examination multiple erythematous plaques with central large hemorrhagic bullae were present on the extensor aspect of the forearms and dorsum of the left hand. The smallest plaque measured 4×8 cm and the largest measured 8×15 cm (Figure 1). The lesions were tender, and Nikolsky sign was negative. Considering the clinical features, a differential diagnosis of bullous systemic lupus erythematosus, polymorphic light eruption, Jessner lymphocytic infiltrate, and SS were considered. Complete blood cell count demonstrated a hemoglobin level of 12.1 g/dL (reference range, 14.0–17.5 g/dL), total leukocyte count of 13,280/μL (reference range, 4500–11,000/μL), neutrophil count of 80% (reference range, 56%), lymphocyte count of 13% (reference range, 34%), monocyte count of 8% (reference range, 4%), and an erythrocyte sedimentation rate of 40 mm/h (reference range, 0–20 mm/h). Serum creatinine levels were 0.9 mg/dL (reference range, 0.6–1.2 mg/dL) and urea nitrogen levels were 26 mg/dL (reference range, 8–23 mg/dL). C-reactive protein was positive, antinuclear antibody was negative, and double-stranded DNA was negative. Ultrasonography of the abdomen and pelvis and a chest radiograph revealed no abnormalities.
Histopathology from a lesion on the forearm revealed a dense, predominantly neutrophilic infiltrate located in the superficial dermis as well as prominent papillary dermal edema infiltration in the dermis with vasodilatation in some areas without leukocytoclastic vasculitis features (Figures 2 and 3). Considering the clinical and histopathologic features, a diagnosis of SS was made, and the patient was started on intravenous dexamethasone (4 mg twice daily) with a dramatic response, as the lesions almost cleared within 4 to 5 days of treatment (Figure 4).
Sweet syndrome was first described in 1964 as acute febrile neutrophilic dermatosis.2 Sweet syndrome can be subdivided into 3 groups depending on the clinical setting: classic or idiopathic, malignancy associated, and drug induced.3 Classic or idiopathic SS typically affects women in the third to fifth decades of life,4 as seen in our case. The proposed diagnostic criteria for SS state that patients must meet both of the 2 major criteria and 2 of 4 minor criteria for the diagnosis.3 The major criteria include acute onset of typical skin lesions and histopathologic findings consistent with SS. The minor criteria include fever (temperature >38°C) or general malaise; association with malignancy, inflammatory disease, pregnancy, or antecedent respiratory or gastrointestinal tract infection; excellent response to treatment with systemic corticosteroids or potassium iodide; and abnormal laboratory values at presentation (3 of 4 required: erythrocyte sedimentation rate >20 mm/h; leukocyte count >8000/μL; neutrophil count >70%; positive C-reactive protein). Our patient fulfilled both the major criteria and 3 of 4 minor criteria. Although the exact etiology of SS is unknown, it is widely believed that SS may be a hypersensitivity response to underlying bacterial infections such as Yersinia enterocolitica, viral infections, or tumors.5
Cytokine dysregulation also has been indicated in the pathogenesis of SS, an imbalance of cytokine secretion from helper T cells such as IL-2 and IFN-γ, which may stimulate the cytokine cascade leading to activation of neutrophils and release of toxic metabolites.6 The cutaneous manifestations of SS consist of erythematous to violaceous tender papules or nodules that often coalesce to form irregular plaques.7 Rare clinical manifestations include bullous lesions; oral involvement; glomerulonephritis; myositis; and ocular manifestations including conjunctivitis, episcleritis, and iridocyclitis.5,8 Photoaggravated and photoinduced syndromes also have been reported.9
Bullous-type SS has been reported, but all the known cases were secondary to malignancy or were drug induced8,10; they did not present in a classic or idiopathic variant. Our case is unique in that it is a report of the classic SS variant with lesions including bullae over photoexposed areas, possibly indicating a causal association of sun exposure and the development of SS.
The diagnostic histopathologic features of SS include a dense, predominantly neutrophilic infiltrate located in the superficial dermis as well as prominent papillary dermal edema, which occasionally may lead to subepidermal vesiculation.11,12 The epidermis often is normal but spongiosis may be present, and rarely neutrophils may extend into the epidermis to form subcorneal pustules.13 Severe edema in the papillary dermis may cause subepidermal blistering and bullous lesions.10 Systemic steroids are the therapeutic mainstay in SS. Other treatment options include methylprednisolone, potassium iodide, colchicine, indomethacin, cyclosporine, and dapsone.
To the Editor:
Sweet syndrome (SS) is characterized by fever; acute onset of painful erythematous papules, plaques, or nodules; peripheral neutrophilic leukocytosis; and histologic findings of a dense neutrophilic infiltrate without evidence of primary vasculitis.1 We report a rare case of classic SS presenting with hemorrhagic bullae over photoexposed areas. Our case is notable because of the unusual nature of the clinical manifestation.
A 45-year-old woman presented with painful, fluid-filled lesions on the upper extremities of 1 week’s duration. Lesions were acute in onset and associated with fever. The patient had a history of diabetes mellitus and hypertension, which were well controlled. She also had a history of minimal itching on sun exposure as well as an upper respiratory tract infection 2 months prior to presentation. There was no history of muscle weakness, pain and/or discoloration of the fingertips, or treatment with topical or systemic agents.
On physical examination, the patient was well nourished with an average build. She was febrile (temperature, 38.5°C) with a pulse of 82 beats per minute, a blood pressure of 130/80, and a respiratory rate of 14 breaths per minute. On cutaneous examination multiple erythematous plaques with central large hemorrhagic bullae were present on the extensor aspect of the forearms and dorsum of the left hand. The smallest plaque measured 4×8 cm and the largest measured 8×15 cm (Figure 1). The lesions were tender, and Nikolsky sign was negative. Considering the clinical features, a differential diagnosis of bullous systemic lupus erythematosus, polymorphic light eruption, Jessner lymphocytic infiltrate, and SS were considered. Complete blood cell count demonstrated a hemoglobin level of 12.1 g/dL (reference range, 14.0–17.5 g/dL), total leukocyte count of 13,280/μL (reference range, 4500–11,000/μL), neutrophil count of 80% (reference range, 56%), lymphocyte count of 13% (reference range, 34%), monocyte count of 8% (reference range, 4%), and an erythrocyte sedimentation rate of 40 mm/h (reference range, 0–20 mm/h). Serum creatinine levels were 0.9 mg/dL (reference range, 0.6–1.2 mg/dL) and urea nitrogen levels were 26 mg/dL (reference range, 8–23 mg/dL). C-reactive protein was positive, antinuclear antibody was negative, and double-stranded DNA was negative. Ultrasonography of the abdomen and pelvis and a chest radiograph revealed no abnormalities.
Histopathology from a lesion on the forearm revealed a dense, predominantly neutrophilic infiltrate located in the superficial dermis as well as prominent papillary dermal edema infiltration in the dermis with vasodilatation in some areas without leukocytoclastic vasculitis features (Figures 2 and 3). Considering the clinical and histopathologic features, a diagnosis of SS was made, and the patient was started on intravenous dexamethasone (4 mg twice daily) with a dramatic response, as the lesions almost cleared within 4 to 5 days of treatment (Figure 4).
Sweet syndrome was first described in 1964 as acute febrile neutrophilic dermatosis.2 Sweet syndrome can be subdivided into 3 groups depending on the clinical setting: classic or idiopathic, malignancy associated, and drug induced.3 Classic or idiopathic SS typically affects women in the third to fifth decades of life,4 as seen in our case. The proposed diagnostic criteria for SS state that patients must meet both of the 2 major criteria and 2 of 4 minor criteria for the diagnosis.3 The major criteria include acute onset of typical skin lesions and histopathologic findings consistent with SS. The minor criteria include fever (temperature >38°C) or general malaise; association with malignancy, inflammatory disease, pregnancy, or antecedent respiratory or gastrointestinal tract infection; excellent response to treatment with systemic corticosteroids or potassium iodide; and abnormal laboratory values at presentation (3 of 4 required: erythrocyte sedimentation rate >20 mm/h; leukocyte count >8000/μL; neutrophil count >70%; positive C-reactive protein). Our patient fulfilled both the major criteria and 3 of 4 minor criteria. Although the exact etiology of SS is unknown, it is widely believed that SS may be a hypersensitivity response to underlying bacterial infections such as Yersinia enterocolitica, viral infections, or tumors.5
Cytokine dysregulation also has been indicated in the pathogenesis of SS, an imbalance of cytokine secretion from helper T cells such as IL-2 and IFN-γ, which may stimulate the cytokine cascade leading to activation of neutrophils and release of toxic metabolites.6 The cutaneous manifestations of SS consist of erythematous to violaceous tender papules or nodules that often coalesce to form irregular plaques.7 Rare clinical manifestations include bullous lesions; oral involvement; glomerulonephritis; myositis; and ocular manifestations including conjunctivitis, episcleritis, and iridocyclitis.5,8 Photoaggravated and photoinduced syndromes also have been reported.9
Bullous-type SS has been reported, but all the known cases were secondary to malignancy or were drug induced8,10; they did not present in a classic or idiopathic variant. Our case is unique in that it is a report of the classic SS variant with lesions including bullae over photoexposed areas, possibly indicating a causal association of sun exposure and the development of SS.
The diagnostic histopathologic features of SS include a dense, predominantly neutrophilic infiltrate located in the superficial dermis as well as prominent papillary dermal edema, which occasionally may lead to subepidermal vesiculation.11,12 The epidermis often is normal but spongiosis may be present, and rarely neutrophils may extend into the epidermis to form subcorneal pustules.13 Severe edema in the papillary dermis may cause subepidermal blistering and bullous lesions.10 Systemic steroids are the therapeutic mainstay in SS. Other treatment options include methylprednisolone, potassium iodide, colchicine, indomethacin, cyclosporine, and dapsone.
1. Von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556.
2. Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;74:349-356.
3. Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
4. Cohen PR. Pregnancy-associated Sweet’s syndrome: world literature review. Obstet Gynecol Surv. 1993;48:584-587.
5. Cohen PR, Hönigsmann H, Kurzrock R. Acute febrile neutrophilic dermatosis (Sweet syndrome). In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2008:289-295.
6. Giasuddin AS, El-Orfi AH, Ziu MM, et al. Sweet’s syndrome: is the pathogenesis mediated by helper T cell type 1 cytokines? J Am Acad Dermatol. 1998;39:940-943.
7. Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
8. Lund JJ, Stratman EJ, Jose D, et al. Drug-induced bullous Sweet syndrome with multiple autoimmune features. Autoimmune Dis. 2010;2011:176749.
9. Bessis D, Dereure O, Peyron JL, et al. Photoinduced Sweet syndrome. Arch Dermatol. 2003;137:1106-1108.
10. Bielsa S, Baradad M, Martí RM, et al. Sweet’s syndrome with bullous lesions [in Spanish]. Actas Dermosifiliogr. 2005;96:315-316.
11. Jordaan HF. Acute febrile neutrophilic dermatosis: a histopathological study of 37 cases and a review of the literature. Am J Dermatopathol. 1989;11:99-111.
12. Kemmett D, Hunter JA. Sweet’s syndrome: a clinicopathologic review of twenty-nine cases. J Am Acad Dermatol. 1990;23(3, pt 1):503-507.
13. Wallach D. Neutrophilic disease [in French]. Rev Prat. 1999;49:356-358.
1. Von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol. 1994;31:535-556.
2. Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;74:349-356.
3. Su WP, Liu HN. Diagnostic criteria for Sweet’s syndrome. Cutis. 1986;37:167-174.
4. Cohen PR. Pregnancy-associated Sweet’s syndrome: world literature review. Obstet Gynecol Surv. 1993;48:584-587.
5. Cohen PR, Hönigsmann H, Kurzrock R. Acute febrile neutrophilic dermatosis (Sweet syndrome). In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2008:289-295.
6. Giasuddin AS, El-Orfi AH, Ziu MM, et al. Sweet’s syndrome: is the pathogenesis mediated by helper T cell type 1 cytokines? J Am Acad Dermatol. 1998;39:940-943.
7. Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
8. Lund JJ, Stratman EJ, Jose D, et al. Drug-induced bullous Sweet syndrome with multiple autoimmune features. Autoimmune Dis. 2010;2011:176749.
9. Bessis D, Dereure O, Peyron JL, et al. Photoinduced Sweet syndrome. Arch Dermatol. 2003;137:1106-1108.
10. Bielsa S, Baradad M, Martí RM, et al. Sweet’s syndrome with bullous lesions [in Spanish]. Actas Dermosifiliogr. 2005;96:315-316.
11. Jordaan HF. Acute febrile neutrophilic dermatosis: a histopathological study of 37 cases and a review of the literature. Am J Dermatopathol. 1989;11:99-111.
12. Kemmett D, Hunter JA. Sweet’s syndrome: a clinicopathologic review of twenty-nine cases. J Am Acad Dermatol. 1990;23(3, pt 1):503-507.
13. Wallach D. Neutrophilic disease [in French]. Rev Prat. 1999;49:356-358.
Late-Onset Acrokeratosis Paraneoplastica of Bazex Associated With Metastatic Adenocarcinoma of the Colon
To the Editor:
Acrokeratosis paraneoplastica (AP) of Bazex is a rare but distinctive acral psoriasiform dermatosis associated with internal malignancy, usually squamous cell carcinoma (SCC), of the upper aerodigestive tract.1,2 Recognizing this paraneoplastic condition is paramount because cutaneous findings often precede the onset of symptoms associated with an occult malignancy.3
A 76-year-old woman with adenocarcinoma of the transverse colon of 3 years’ duration was referred to the dermatology department. She had a hemicolectomy and was doing well until tumor recurrence with peritoneal metastasis was detected following an exploratory laparotomy 1 year prior to presentation to us. She underwent a total hysterectomy and bilateral salpingo-oophorectomy. Palliative chemotherapy was initiated, and she completed 5 cycles of capecitabine and oxaliplatin. Long-term medications for diabetes mellitus, hypertension, and hyperlipidemia included glibenclamide, nifedipine, hydrochlorothiazide, and losartan. The patient had a progressive pruritic rash of 6 months’ duration that started on the hands and forearms and spread to involve the feet and lower limbs as well as the ears and face. She also experienced progressive thickening of the palms and soles. She had been treated with topical steroids and emollients with no improvement. Clinical examination revealed erythematous scaly patches on all of her limbs, especially on the elbows and knees, and on the ear helices and nose. There also was notable palmoplantar keratoderma with central sparing (Figure 1), onycholysis, and subungual hyperkeratosis. A skin biopsy of the forearm was performed, and histology revealed orthokeratosis, hypergranulosis and basal vacuolar alteration, and superficial perivascular lymphohistiocytic infiltrate with melanophages (Figure 2). Direct immunofluorescence studies and fungal cultures were negative. The cutaneous features of a treatment-resistant acral dermatosis supported the clinical diagnosis of AP, especially in the setting of an internal malignancy. The patient was started on palliative radiotherapy with no notable resolution of the cutaneous lesions. She was lost to follow-up.
First described by Bazex et al1 in 1965, AP is an uncommon but well-recognized paraneoplastic dermatosis associated with an underlying neoplasm. Fewer than 160 cases have been reported, and the majority of cases have been men older than 40 years. The most commonly associated malignancy was SCC (at least 50% of reported cases) involving mainly the oropharynx and larynx, with lung and esophageal SCC also described.2-4 Only a few cases of adenocarcinoma-associated AP have been described, such as adenocarcinoma of the lung, prostate, and stomach.3-5 In a reported case of AP associated with early colon adenocarcinoma, the patient had remarkable cutaneous resolution following successful tumor resection.6 Other reported rare hematologic associations included Hodgkin disease, peripheral T-cell lymphoma, and multiple myeloma.3-5 Karabulut et al4 described a case associated with cholangiocarcinoma and studied the primary sites of malignancies in another 133 patients with AP (118 patients with documented cell type): oropharynx and larynx in 55 (41%); lung in 23 (17%); unknown location in 20 (15%); esophagus in 14 (11%); prostate in 3 (2%); stomach in 3 (2%); and isolated cases involving the liver, thymus, uterus, vulva, breast, urinary bladder, lymph nodes, and bone marrow.
A review of 113 cases of AP showed that only 15% developed cutaneous lesions after the malignancy had been discovered; in the majority of cases (67%), cutaneous lesions were present for an average of 1 year preceding the diagnosis of malignancy.3 In our patient, the late-onset cutaneous involvement corresponded to the progression of the underlying colon adenocarcinoma. In the absence of initial cutaneous involvement or when successful treatment of a tumor results in cutaneous resolution, subsequent emergence of cutaneous lesions of AP signifies tumor progression. The evolution of cutaneous features in AP was well described by Bazex et al.1 Stage 1 of the disease shows initial erythema and psoriasiform scaling of the fingers and toes, spreading to the nose and ear helices (hence acrokeratosis); the tumor frequently remains asymptomatic or undetected. Stage 2 shows palmoplantar keratoderma with central sparing and more extensive facial lesions; progression to stage 3 occurs if the tumor remains undetected or untreated, with further spread of psoriasiform lesions to the elbows, knees, trunk, and scalp.1 Nail involvement occurs in nearly 75% of cases5; typical changes include subungual hyperkeratosis, onycholysis, longitudinal streaks, yellow pigmentation, and rarely onychomadesis. A high index of suspicion of AP is paramount when evaluating any recalcitrant acral dermatosis that fails to respond to appropriate therapy, especially in the presence of constitutional symptoms, typical bulbous enlargement of distal phalanges, or isolated involvement of helices. These findings should prompt physicians to perform an extensive search for an underlying malignancy with complete physical examination, particularly of the head and neck region, with appropriate endoscopic assessment and imaging studies.
A myriad of nonspecific histologic features of AP commonly reported include hyperkeratosis, parakeratosis, acanthosis, and dermal perivascular lymphohistiocytic infiltrate.7 Less common features include dyskeratotic keratinocytes, vacuolar degeneration, bandlike infiltrate, and melanin incontinence.7 The pathogenesis of AP remains elusive. A postulated immunologic mechanism is based on reports of immunoglobulins (IgG, IgA, IgM) and complement (C3) deposition along the basement membrane zone.8 Association with autoimmune disorders such as alopecia areata and vitiligo also has been reported.9 Another possible mechanism is cross-reactivity between antigens found in the tumor and skin, resulting in a T-cell–mediated immune response to tumorlike antigens in the epidermis, or secretion of tumor-originating growth factors responsible for the hyperkeratotic skin changes, such as epidermal growth factor, transforming growth factor a, or insulinlike growth factor 1.3,7,10,11
Spontaneous remission of cutaneous lesions in untreated underlying malignancy is rare. Isolated reports of treatment using topical and systemic steroids, salicylic acid, topical vitamin D analogues, etretinate, and psoralen plus UVA showed minimal improvement.5,7 The mainstay in attaining cutaneous resolution is to detect and eradicate the underlying neoplasm with surgery, chemotherapy, or radiotherapy, or combination therapy.
Our case is noteworthy because of the patient’s gender (female), underlying malignancy (adenocarcinoma of the colon), and late onset of cutaneous involvement, which are all uncommon associations related to paraneoplastic syndrome. The clinical features of AP should be recognized early to facilitate an extensive search for an occult malignancy, and late-onset cutaneous involvement also should be recognized as a marker of tumor relapse or progression.
1. Bazex A, Salvador R, Dupré A, et al. Late symptomatic hepatic porphyria developing to the picture of lipoidoproteinsosis [in French]. Bull Soc Fr Dermatol Syphiligr. 1965;72:182.
2. Witkowski JA, Parish LC. Bazex’s syndrome. paraneoplastic acrokeratosis. JAMA. 1982;248:2883-2884.
3. Bolognia JL. Bazex syndrome: acrokeratosis paraneoplastica. Semin Dermatol. 1995;14:84-89.
4. Karabulut AA, Sahin S, Sahin M, et al. Paraneoplastic acrokeratosis of Bazex (Bazex’s syndrome): report of a female case associated with cholangiocarcinoma and review of the published work. J Dermatol. 2006;33:850-854.
5. Valdivielso M, Longo I, Suárez R. Acrokeratosis paraneoplastica: Bazex syndrome. J Eur Acad Dermatol Venereol. 2005;19:340-344.
6. Hsu YS, Lien GS, Lai HH, et al. Acrokeratosis paraneoplastica (Bazex syndrome) with adenocarcinoma of the colon: report of a case and review of the literature. J Gastroenterol. 2000;35:460-464.
7. Bolognia JL, Brewer YP, Cooper DL. Bazex syndrome (acrokeratosis paraneoplastica). an analytic review. Medicine (Baltimore). 1991;70:269-280.
8. Mutasim DF, Meiri G. Bazex syndrome mimicking a primary autoimmune bullous disorder. J Am Acad Dermatol. 1999;40(5, pt 2):822-825.
9. Hara M, Hunayama M, Aiba S, et al. Acrokeratosis paraneoplastica (Bazex syndrome) associated with primary cutaneous squamous cell carcinoma of the lower leg, vitiligo and alopecia areata. Br J Dermatol. 1995;133:121-124.
10. Stone SP, Buescher LS. Life-threatening paraneoplastic cutaneous syndromes. Clin Dermatol. 2005;23:301-306.
11. Politi Y, Ophir J, Brenner S. Cutaneous paraneoplastic syndromes. Acta Derm Venereol. 1993;73:161-170.
To the Editor:
Acrokeratosis paraneoplastica (AP) of Bazex is a rare but distinctive acral psoriasiform dermatosis associated with internal malignancy, usually squamous cell carcinoma (SCC), of the upper aerodigestive tract.1,2 Recognizing this paraneoplastic condition is paramount because cutaneous findings often precede the onset of symptoms associated with an occult malignancy.3
A 76-year-old woman with adenocarcinoma of the transverse colon of 3 years’ duration was referred to the dermatology department. She had a hemicolectomy and was doing well until tumor recurrence with peritoneal metastasis was detected following an exploratory laparotomy 1 year prior to presentation to us. She underwent a total hysterectomy and bilateral salpingo-oophorectomy. Palliative chemotherapy was initiated, and she completed 5 cycles of capecitabine and oxaliplatin. Long-term medications for diabetes mellitus, hypertension, and hyperlipidemia included glibenclamide, nifedipine, hydrochlorothiazide, and losartan. The patient had a progressive pruritic rash of 6 months’ duration that started on the hands and forearms and spread to involve the feet and lower limbs as well as the ears and face. She also experienced progressive thickening of the palms and soles. She had been treated with topical steroids and emollients with no improvement. Clinical examination revealed erythematous scaly patches on all of her limbs, especially on the elbows and knees, and on the ear helices and nose. There also was notable palmoplantar keratoderma with central sparing (Figure 1), onycholysis, and subungual hyperkeratosis. A skin biopsy of the forearm was performed, and histology revealed orthokeratosis, hypergranulosis and basal vacuolar alteration, and superficial perivascular lymphohistiocytic infiltrate with melanophages (Figure 2). Direct immunofluorescence studies and fungal cultures were negative. The cutaneous features of a treatment-resistant acral dermatosis supported the clinical diagnosis of AP, especially in the setting of an internal malignancy. The patient was started on palliative radiotherapy with no notable resolution of the cutaneous lesions. She was lost to follow-up.
First described by Bazex et al1 in 1965, AP is an uncommon but well-recognized paraneoplastic dermatosis associated with an underlying neoplasm. Fewer than 160 cases have been reported, and the majority of cases have been men older than 40 years. The most commonly associated malignancy was SCC (at least 50% of reported cases) involving mainly the oropharynx and larynx, with lung and esophageal SCC also described.2-4 Only a few cases of adenocarcinoma-associated AP have been described, such as adenocarcinoma of the lung, prostate, and stomach.3-5 In a reported case of AP associated with early colon adenocarcinoma, the patient had remarkable cutaneous resolution following successful tumor resection.6 Other reported rare hematologic associations included Hodgkin disease, peripheral T-cell lymphoma, and multiple myeloma.3-5 Karabulut et al4 described a case associated with cholangiocarcinoma and studied the primary sites of malignancies in another 133 patients with AP (118 patients with documented cell type): oropharynx and larynx in 55 (41%); lung in 23 (17%); unknown location in 20 (15%); esophagus in 14 (11%); prostate in 3 (2%); stomach in 3 (2%); and isolated cases involving the liver, thymus, uterus, vulva, breast, urinary bladder, lymph nodes, and bone marrow.
A review of 113 cases of AP showed that only 15% developed cutaneous lesions after the malignancy had been discovered; in the majority of cases (67%), cutaneous lesions were present for an average of 1 year preceding the diagnosis of malignancy.3 In our patient, the late-onset cutaneous involvement corresponded to the progression of the underlying colon adenocarcinoma. In the absence of initial cutaneous involvement or when successful treatment of a tumor results in cutaneous resolution, subsequent emergence of cutaneous lesions of AP signifies tumor progression. The evolution of cutaneous features in AP was well described by Bazex et al.1 Stage 1 of the disease shows initial erythema and psoriasiform scaling of the fingers and toes, spreading to the nose and ear helices (hence acrokeratosis); the tumor frequently remains asymptomatic or undetected. Stage 2 shows palmoplantar keratoderma with central sparing and more extensive facial lesions; progression to stage 3 occurs if the tumor remains undetected or untreated, with further spread of psoriasiform lesions to the elbows, knees, trunk, and scalp.1 Nail involvement occurs in nearly 75% of cases5; typical changes include subungual hyperkeratosis, onycholysis, longitudinal streaks, yellow pigmentation, and rarely onychomadesis. A high index of suspicion of AP is paramount when evaluating any recalcitrant acral dermatosis that fails to respond to appropriate therapy, especially in the presence of constitutional symptoms, typical bulbous enlargement of distal phalanges, or isolated involvement of helices. These findings should prompt physicians to perform an extensive search for an underlying malignancy with complete physical examination, particularly of the head and neck region, with appropriate endoscopic assessment and imaging studies.
A myriad of nonspecific histologic features of AP commonly reported include hyperkeratosis, parakeratosis, acanthosis, and dermal perivascular lymphohistiocytic infiltrate.7 Less common features include dyskeratotic keratinocytes, vacuolar degeneration, bandlike infiltrate, and melanin incontinence.7 The pathogenesis of AP remains elusive. A postulated immunologic mechanism is based on reports of immunoglobulins (IgG, IgA, IgM) and complement (C3) deposition along the basement membrane zone.8 Association with autoimmune disorders such as alopecia areata and vitiligo also has been reported.9 Another possible mechanism is cross-reactivity between antigens found in the tumor and skin, resulting in a T-cell–mediated immune response to tumorlike antigens in the epidermis, or secretion of tumor-originating growth factors responsible for the hyperkeratotic skin changes, such as epidermal growth factor, transforming growth factor a, or insulinlike growth factor 1.3,7,10,11
Spontaneous remission of cutaneous lesions in untreated underlying malignancy is rare. Isolated reports of treatment using topical and systemic steroids, salicylic acid, topical vitamin D analogues, etretinate, and psoralen plus UVA showed minimal improvement.5,7 The mainstay in attaining cutaneous resolution is to detect and eradicate the underlying neoplasm with surgery, chemotherapy, or radiotherapy, or combination therapy.
Our case is noteworthy because of the patient’s gender (female), underlying malignancy (adenocarcinoma of the colon), and late onset of cutaneous involvement, which are all uncommon associations related to paraneoplastic syndrome. The clinical features of AP should be recognized early to facilitate an extensive search for an occult malignancy, and late-onset cutaneous involvement also should be recognized as a marker of tumor relapse or progression.
To the Editor:
Acrokeratosis paraneoplastica (AP) of Bazex is a rare but distinctive acral psoriasiform dermatosis associated with internal malignancy, usually squamous cell carcinoma (SCC), of the upper aerodigestive tract.1,2 Recognizing this paraneoplastic condition is paramount because cutaneous findings often precede the onset of symptoms associated with an occult malignancy.3
A 76-year-old woman with adenocarcinoma of the transverse colon of 3 years’ duration was referred to the dermatology department. She had a hemicolectomy and was doing well until tumor recurrence with peritoneal metastasis was detected following an exploratory laparotomy 1 year prior to presentation to us. She underwent a total hysterectomy and bilateral salpingo-oophorectomy. Palliative chemotherapy was initiated, and she completed 5 cycles of capecitabine and oxaliplatin. Long-term medications for diabetes mellitus, hypertension, and hyperlipidemia included glibenclamide, nifedipine, hydrochlorothiazide, and losartan. The patient had a progressive pruritic rash of 6 months’ duration that started on the hands and forearms and spread to involve the feet and lower limbs as well as the ears and face. She also experienced progressive thickening of the palms and soles. She had been treated with topical steroids and emollients with no improvement. Clinical examination revealed erythematous scaly patches on all of her limbs, especially on the elbows and knees, and on the ear helices and nose. There also was notable palmoplantar keratoderma with central sparing (Figure 1), onycholysis, and subungual hyperkeratosis. A skin biopsy of the forearm was performed, and histology revealed orthokeratosis, hypergranulosis and basal vacuolar alteration, and superficial perivascular lymphohistiocytic infiltrate with melanophages (Figure 2). Direct immunofluorescence studies and fungal cultures were negative. The cutaneous features of a treatment-resistant acral dermatosis supported the clinical diagnosis of AP, especially in the setting of an internal malignancy. The patient was started on palliative radiotherapy with no notable resolution of the cutaneous lesions. She was lost to follow-up.
First described by Bazex et al1 in 1965, AP is an uncommon but well-recognized paraneoplastic dermatosis associated with an underlying neoplasm. Fewer than 160 cases have been reported, and the majority of cases have been men older than 40 years. The most commonly associated malignancy was SCC (at least 50% of reported cases) involving mainly the oropharynx and larynx, with lung and esophageal SCC also described.2-4 Only a few cases of adenocarcinoma-associated AP have been described, such as adenocarcinoma of the lung, prostate, and stomach.3-5 In a reported case of AP associated with early colon adenocarcinoma, the patient had remarkable cutaneous resolution following successful tumor resection.6 Other reported rare hematologic associations included Hodgkin disease, peripheral T-cell lymphoma, and multiple myeloma.3-5 Karabulut et al4 described a case associated with cholangiocarcinoma and studied the primary sites of malignancies in another 133 patients with AP (118 patients with documented cell type): oropharynx and larynx in 55 (41%); lung in 23 (17%); unknown location in 20 (15%); esophagus in 14 (11%); prostate in 3 (2%); stomach in 3 (2%); and isolated cases involving the liver, thymus, uterus, vulva, breast, urinary bladder, lymph nodes, and bone marrow.
A review of 113 cases of AP showed that only 15% developed cutaneous lesions after the malignancy had been discovered; in the majority of cases (67%), cutaneous lesions were present for an average of 1 year preceding the diagnosis of malignancy.3 In our patient, the late-onset cutaneous involvement corresponded to the progression of the underlying colon adenocarcinoma. In the absence of initial cutaneous involvement or when successful treatment of a tumor results in cutaneous resolution, subsequent emergence of cutaneous lesions of AP signifies tumor progression. The evolution of cutaneous features in AP was well described by Bazex et al.1 Stage 1 of the disease shows initial erythema and psoriasiform scaling of the fingers and toes, spreading to the nose and ear helices (hence acrokeratosis); the tumor frequently remains asymptomatic or undetected. Stage 2 shows palmoplantar keratoderma with central sparing and more extensive facial lesions; progression to stage 3 occurs if the tumor remains undetected or untreated, with further spread of psoriasiform lesions to the elbows, knees, trunk, and scalp.1 Nail involvement occurs in nearly 75% of cases5; typical changes include subungual hyperkeratosis, onycholysis, longitudinal streaks, yellow pigmentation, and rarely onychomadesis. A high index of suspicion of AP is paramount when evaluating any recalcitrant acral dermatosis that fails to respond to appropriate therapy, especially in the presence of constitutional symptoms, typical bulbous enlargement of distal phalanges, or isolated involvement of helices. These findings should prompt physicians to perform an extensive search for an underlying malignancy with complete physical examination, particularly of the head and neck region, with appropriate endoscopic assessment and imaging studies.
A myriad of nonspecific histologic features of AP commonly reported include hyperkeratosis, parakeratosis, acanthosis, and dermal perivascular lymphohistiocytic infiltrate.7 Less common features include dyskeratotic keratinocytes, vacuolar degeneration, bandlike infiltrate, and melanin incontinence.7 The pathogenesis of AP remains elusive. A postulated immunologic mechanism is based on reports of immunoglobulins (IgG, IgA, IgM) and complement (C3) deposition along the basement membrane zone.8 Association with autoimmune disorders such as alopecia areata and vitiligo also has been reported.9 Another possible mechanism is cross-reactivity between antigens found in the tumor and skin, resulting in a T-cell–mediated immune response to tumorlike antigens in the epidermis, or secretion of tumor-originating growth factors responsible for the hyperkeratotic skin changes, such as epidermal growth factor, transforming growth factor a, or insulinlike growth factor 1.3,7,10,11
Spontaneous remission of cutaneous lesions in untreated underlying malignancy is rare. Isolated reports of treatment using topical and systemic steroids, salicylic acid, topical vitamin D analogues, etretinate, and psoralen plus UVA showed minimal improvement.5,7 The mainstay in attaining cutaneous resolution is to detect and eradicate the underlying neoplasm with surgery, chemotherapy, or radiotherapy, or combination therapy.
Our case is noteworthy because of the patient’s gender (female), underlying malignancy (adenocarcinoma of the colon), and late onset of cutaneous involvement, which are all uncommon associations related to paraneoplastic syndrome. The clinical features of AP should be recognized early to facilitate an extensive search for an occult malignancy, and late-onset cutaneous involvement also should be recognized as a marker of tumor relapse or progression.
1. Bazex A, Salvador R, Dupré A, et al. Late symptomatic hepatic porphyria developing to the picture of lipoidoproteinsosis [in French]. Bull Soc Fr Dermatol Syphiligr. 1965;72:182.
2. Witkowski JA, Parish LC. Bazex’s syndrome. paraneoplastic acrokeratosis. JAMA. 1982;248:2883-2884.
3. Bolognia JL. Bazex syndrome: acrokeratosis paraneoplastica. Semin Dermatol. 1995;14:84-89.
4. Karabulut AA, Sahin S, Sahin M, et al. Paraneoplastic acrokeratosis of Bazex (Bazex’s syndrome): report of a female case associated with cholangiocarcinoma and review of the published work. J Dermatol. 2006;33:850-854.
5. Valdivielso M, Longo I, Suárez R. Acrokeratosis paraneoplastica: Bazex syndrome. J Eur Acad Dermatol Venereol. 2005;19:340-344.
6. Hsu YS, Lien GS, Lai HH, et al. Acrokeratosis paraneoplastica (Bazex syndrome) with adenocarcinoma of the colon: report of a case and review of the literature. J Gastroenterol. 2000;35:460-464.
7. Bolognia JL, Brewer YP, Cooper DL. Bazex syndrome (acrokeratosis paraneoplastica). an analytic review. Medicine (Baltimore). 1991;70:269-280.
8. Mutasim DF, Meiri G. Bazex syndrome mimicking a primary autoimmune bullous disorder. J Am Acad Dermatol. 1999;40(5, pt 2):822-825.
9. Hara M, Hunayama M, Aiba S, et al. Acrokeratosis paraneoplastica (Bazex syndrome) associated with primary cutaneous squamous cell carcinoma of the lower leg, vitiligo and alopecia areata. Br J Dermatol. 1995;133:121-124.
10. Stone SP, Buescher LS. Life-threatening paraneoplastic cutaneous syndromes. Clin Dermatol. 2005;23:301-306.
11. Politi Y, Ophir J, Brenner S. Cutaneous paraneoplastic syndromes. Acta Derm Venereol. 1993;73:161-170.
1. Bazex A, Salvador R, Dupré A, et al. Late symptomatic hepatic porphyria developing to the picture of lipoidoproteinsosis [in French]. Bull Soc Fr Dermatol Syphiligr. 1965;72:182.
2. Witkowski JA, Parish LC. Bazex’s syndrome. paraneoplastic acrokeratosis. JAMA. 1982;248:2883-2884.
3. Bolognia JL. Bazex syndrome: acrokeratosis paraneoplastica. Semin Dermatol. 1995;14:84-89.
4. Karabulut AA, Sahin S, Sahin M, et al. Paraneoplastic acrokeratosis of Bazex (Bazex’s syndrome): report of a female case associated with cholangiocarcinoma and review of the published work. J Dermatol. 2006;33:850-854.
5. Valdivielso M, Longo I, Suárez R. Acrokeratosis paraneoplastica: Bazex syndrome. J Eur Acad Dermatol Venereol. 2005;19:340-344.
6. Hsu YS, Lien GS, Lai HH, et al. Acrokeratosis paraneoplastica (Bazex syndrome) with adenocarcinoma of the colon: report of a case and review of the literature. J Gastroenterol. 2000;35:460-464.
7. Bolognia JL, Brewer YP, Cooper DL. Bazex syndrome (acrokeratosis paraneoplastica). an analytic review. Medicine (Baltimore). 1991;70:269-280.
8. Mutasim DF, Meiri G. Bazex syndrome mimicking a primary autoimmune bullous disorder. J Am Acad Dermatol. 1999;40(5, pt 2):822-825.
9. Hara M, Hunayama M, Aiba S, et al. Acrokeratosis paraneoplastica (Bazex syndrome) associated with primary cutaneous squamous cell carcinoma of the lower leg, vitiligo and alopecia areata. Br J Dermatol. 1995;133:121-124.
10. Stone SP, Buescher LS. Life-threatening paraneoplastic cutaneous syndromes. Clin Dermatol. 2005;23:301-306.
11. Politi Y, Ophir J, Brenner S. Cutaneous paraneoplastic syndromes. Acta Derm Venereol. 1993;73:161-170.