User login
Practice Question Answers: Allergic Contact Dermatitis, Part 1
1. Patients with a documented contact allergy to caine mix should avoid all of the following except:
a. benzocaine
b. butacaine
c. lidocaine
d. procaine
e. tetracaine
2. A patient with atopic dermatitis whose condition is worsening with the use of topical steroids is referred for patch testing and found to have positivity to budesonide. Which of the following would be an appropriate topical steroid to prescribe to this patient?
a. desonide
b. desoximetasone
c. fluocinolone
d. fluocinonide
e. triamcinolone
3. A patient with a documented contact allergy to carba mix should avoid which of the following systemic medications?
a. ciprofloxacin
b. disulfiram
c. gold sodium thiomalate
d. hydroxyzine
e. piroxicam
4. Bronopol (2-bromo-2-nitropropane-1,3-diol) can cross-react with all of the following except:
a. diazolidinyl urea
b. DMDM hydantoin
c. imidazolidinyl urea
d. p-aminobenzoic acid
e. quaternium-15
5. Allergy to cocamidopropyl betaine is thought to be secondary to which of the following?
a. amidoamine
b. benzoic acid
c. bronopol
d. Myroxylon pereirae
e. N-isopropyl-N'-phenyl parapheylenediamine
1. Patients with a documented contact allergy to caine mix should avoid all of the following except:
a. benzocaine
b. butacaine
c. lidocaine
d. procaine
e. tetracaine
2. A patient with atopic dermatitis whose condition is worsening with the use of topical steroids is referred for patch testing and found to have positivity to budesonide. Which of the following would be an appropriate topical steroid to prescribe to this patient?
a. desonide
b. desoximetasone
c. fluocinolone
d. fluocinonide
e. triamcinolone
3. A patient with a documented contact allergy to carba mix should avoid which of the following systemic medications?
a. ciprofloxacin
b. disulfiram
c. gold sodium thiomalate
d. hydroxyzine
e. piroxicam
4. Bronopol (2-bromo-2-nitropropane-1,3-diol) can cross-react with all of the following except:
a. diazolidinyl urea
b. DMDM hydantoin
c. imidazolidinyl urea
d. p-aminobenzoic acid
e. quaternium-15
5. Allergy to cocamidopropyl betaine is thought to be secondary to which of the following?
a. amidoamine
b. benzoic acid
c. bronopol
d. Myroxylon pereirae
e. N-isopropyl-N'-phenyl parapheylenediamine
1. Patients with a documented contact allergy to caine mix should avoid all of the following except:
a. benzocaine
b. butacaine
c. lidocaine
d. procaine
e. tetracaine
2. A patient with atopic dermatitis whose condition is worsening with the use of topical steroids is referred for patch testing and found to have positivity to budesonide. Which of the following would be an appropriate topical steroid to prescribe to this patient?
a. desonide
b. desoximetasone
c. fluocinolone
d. fluocinonide
e. triamcinolone
3. A patient with a documented contact allergy to carba mix should avoid which of the following systemic medications?
a. ciprofloxacin
b. disulfiram
c. gold sodium thiomalate
d. hydroxyzine
e. piroxicam
4. Bronopol (2-bromo-2-nitropropane-1,3-diol) can cross-react with all of the following except:
a. diazolidinyl urea
b. DMDM hydantoin
c. imidazolidinyl urea
d. p-aminobenzoic acid
e. quaternium-15
5. Allergy to cocamidopropyl betaine is thought to be secondary to which of the following?
a. amidoamine
b. benzoic acid
c. bronopol
d. Myroxylon pereirae
e. N-isopropyl-N'-phenyl parapheylenediamine
Allergic Contact Dermatitis, Part 1
Midodrine-Induced Acute Generalized Exanthematous Pustulosis
Lichenoid Photosensitivity: An Unusual Reaction to Doxycycline and an Unusual Response
New Developments in Comorbidities of Atopic Dermatitis
Practice Question Answers: Medications in Dermatology, Part 2
1. A 40-year-old woman is diagnosed with systemic lupus erythematosus. You discuss treatment options and decide to start hydroxychloroquine. What laboratory tests and monitoring are required prior to starting this medication?
a. complete blood cell count with differential and glucose-6-phosphate dehydrogenase
b. complete blood cell count with differential and complete metabolic profile
c. ophthalmology evaluation and glucose-6-phosphate dehydrogenase
d. b and c
2. Two months ago you saw a 30-year-old woman with a history of severe atopic dermatitis. She had been using topical steroids with not much improvement. You decided to start a systemic medication. Within 1 month of drug initiation, she called your office to tell you that she is much better but has noticed unwanted hair on her face lately. Which medication is most likely implicated?
a. cyclosporine
b. dapsone
c. hydroxychloroquine
d. methotrexate
3. A 70-year-old man with type 2 diabetes mellitus who drinks 10 cans of beer per week presents to the emergency department with a 3-day history of diffuse tense bullae and pruritus on the legs and trunk. Direct immunofluorescence displayed linear deposition of IgG and C3 at the dermoepidermal junction, confirming your clinical diagnosis. What is the best long-term treatment option for this patient?
a. combination of oral steroids plus methotrexate
b. oral steroids and mycophenolate mofetil
c. oral steroids only
d. topical steroids only
4. A 45-year-old Venezuelan man presents with painful nodules on his bilateral lower legs. A biopsy demonstrates acid-fast bacilli, and a multidrug regimen is initiated for erythema nodosum leprosum. Which of the following is the mechanism of action of the treatment that is US Food and Drug Administration approved for this condition?
a. inhibits chemotaxis
b. inhibits dihydrofolate reductase
c. inhibits tumor necrosis factor α
d. suppresses T-cell function and B-cell antibody production
5. A patient consults her physician because of several side effects from a medication she started 2 weeks ago due to erythematous to violaceous papules on the legs from palpable purpura. She reports diarrhea, abdominal pain, and fatigue. Which medication is she taking?
a. azathioprine
b. colchicine
c. dapsone
d. methotrexate
1. A 40-year-old woman is diagnosed with systemic lupus erythematosus. You discuss treatment options and decide to start hydroxychloroquine. What laboratory tests and monitoring are required prior to starting this medication?
a. complete blood cell count with differential and glucose-6-phosphate dehydrogenase
b. complete blood cell count with differential and complete metabolic profile
c. ophthalmology evaluation and glucose-6-phosphate dehydrogenase
d. b and c
2. Two months ago you saw a 30-year-old woman with a history of severe atopic dermatitis. She had been using topical steroids with not much improvement. You decided to start a systemic medication. Within 1 month of drug initiation, she called your office to tell you that she is much better but has noticed unwanted hair on her face lately. Which medication is most likely implicated?
a. cyclosporine
b. dapsone
c. hydroxychloroquine
d. methotrexate
3. A 70-year-old man with type 2 diabetes mellitus who drinks 10 cans of beer per week presents to the emergency department with a 3-day history of diffuse tense bullae and pruritus on the legs and trunk. Direct immunofluorescence displayed linear deposition of IgG and C3 at the dermoepidermal junction, confirming your clinical diagnosis. What is the best long-term treatment option for this patient?
a. combination of oral steroids plus methotrexate
b. oral steroids and mycophenolate mofetil
c. oral steroids only
d. topical steroids only
4. A 45-year-old Venezuelan man presents with painful nodules on his bilateral lower legs. A biopsy demonstrates acid-fast bacilli, and a multidrug regimen is initiated for erythema nodosum leprosum. Which of the following is the mechanism of action of the treatment that is US Food and Drug Administration approved for this condition?
a. inhibits chemotaxis
b. inhibits dihydrofolate reductase
c. inhibits tumor necrosis factor α
d. suppresses T-cell function and B-cell antibody production
5. A patient consults her physician because of several side effects from a medication she started 2 weeks ago due to erythematous to violaceous papules on the legs from palpable purpura. She reports diarrhea, abdominal pain, and fatigue. Which medication is she taking?
a. azathioprine
b. colchicine
c. dapsone
d. methotrexate
1. A 40-year-old woman is diagnosed with systemic lupus erythematosus. You discuss treatment options and decide to start hydroxychloroquine. What laboratory tests and monitoring are required prior to starting this medication?
a. complete blood cell count with differential and glucose-6-phosphate dehydrogenase
b. complete blood cell count with differential and complete metabolic profile
c. ophthalmology evaluation and glucose-6-phosphate dehydrogenase
d. b and c
2. Two months ago you saw a 30-year-old woman with a history of severe atopic dermatitis. She had been using topical steroids with not much improvement. You decided to start a systemic medication. Within 1 month of drug initiation, she called your office to tell you that she is much better but has noticed unwanted hair on her face lately. Which medication is most likely implicated?
a. cyclosporine
b. dapsone
c. hydroxychloroquine
d. methotrexate
3. A 70-year-old man with type 2 diabetes mellitus who drinks 10 cans of beer per week presents to the emergency department with a 3-day history of diffuse tense bullae and pruritus on the legs and trunk. Direct immunofluorescence displayed linear deposition of IgG and C3 at the dermoepidermal junction, confirming your clinical diagnosis. What is the best long-term treatment option for this patient?
a. combination of oral steroids plus methotrexate
b. oral steroids and mycophenolate mofetil
c. oral steroids only
d. topical steroids only
4. A 45-year-old Venezuelan man presents with painful nodules on his bilateral lower legs. A biopsy demonstrates acid-fast bacilli, and a multidrug regimen is initiated for erythema nodosum leprosum. Which of the following is the mechanism of action of the treatment that is US Food and Drug Administration approved for this condition?
a. inhibits chemotaxis
b. inhibits dihydrofolate reductase
c. inhibits tumor necrosis factor α
d. suppresses T-cell function and B-cell antibody production
5. A patient consults her physician because of several side effects from a medication she started 2 weeks ago due to erythematous to violaceous papules on the legs from palpable purpura. She reports diarrhea, abdominal pain, and fatigue. Which medication is she taking?
a. azathioprine
b. colchicine
c. dapsone
d. methotrexate
Medications in Dermatology, Part 2: Immunosuppressives
Counterphobia and Poor Sun Protection Practices in First-Degree Relatives of Melanoma Patients
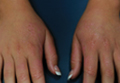
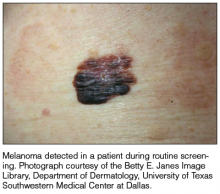
It is widely accepted that there are several factors that may independently elevate an individual’s risk for melanoma, such as a history of childhood sunburns, family history of melanoma, and poor sun protection practices. Several studies have examined risk behaviors in melanoma patients following their diagnosis and have reported findings such as increased UV exposure patterns, persistent tanning bed use, and sun-protective behaviors similar to those of the general population (Figure).1-4
Although first-degree relatives (FDRs) of melanoma patients are at an increased risk for melanoma, they also have been found to exhibit surprisingly poor sun protection practices. In one retrospective analysis, Geller et al5 found that frequent sunburns, high rates of tanning bed use, and low rates of sunscreen use were common among children of health care workers who reported a personal or family history of skin cancer. An independent study reported that merely 37% (37/100) of FDRs of melanoma patients use sunscreen more than half of the time, and considerably fewer wear protective clothing or seek shade while outdoors.6 Given their increased risk for developing melanoma, it is likely to be assumed that FDRs of melanoma patients practice diligent sun protection. The underlying reasons for the failure of this at-risk population to adhere strongly to sun protection practices warrants special attention.
Manne et al7 conducted a survey in a group of FDRs of melanoma patients with self-reported poor sun protection practices to evaluate the demographic, medical, psychological, educational (knowledge of sun protection guidelines), and social influences that correlate with sun protection and sunbathing practices. More effective sun protective behaviors were identified in FDRs with higher education, fewer perceived benefits of sunbathing, more prominent photoaging concerns, and greater sunscreen self-efficacy. The authors concluded that sun-protective behavior in FDRs was not associated with prior knowledge about sunscreen or UV exposure, their relative’s melanoma stage, or physician recommendations for sun protection.7
Factors that have been documented as influencing sun-protective behavior in the general population include knowledge of the benefits of sun protection; attitudes toward tanning and sun protection; subjective norms regarding the beauty and perceived health of a tan; and optimistic bias, which is a cognitive mechanism that causes a person to believe that he/she is at lesser risk for experiencing a negative outcome compared to others. Additionally, sun protection behaviors are influenced by the immediacy of getting the reward (the perceived benefits of tanning) versus the delayed punishment (development of skin cancer).6 Although all of these elements may be important for some individuals, we believe that a subset of FDRs of melanoma patients may be susceptible to the phenomenon known as counterphobia.
Counterphobia is a neurotic response to anxiety in which an individual actively pursues situations that heighten his/her fear rather than fleeing from a feared object or behavior.8 Most insight into counterphobia has come from the experiences of children who have parent(s) with a debilitating or fatal diagnosis. Due to their immature coping mechanisms, some children are at risk for maladaptive behavioral responses. The loss of a parent typically produces severe psychological trauma in all children, but in those who develop counterphobia, it manifests as a heightened fear of death and vulnerability to their parent’s illness. This maladaptive response is dependent on self-identification with the parent, especially among daughters of lost mothers and sons of lost fathers, and this fear remains with the child through adulthood. A survey of 154 motherless daughters found that women aged 19 to 35 years have the highest level of obsessive thoughts of mortality and more than 75% believe they will succumb to their mother’s illness (92% in the case of cancer).9 Despite this fear, children may exhibit health-compromising behaviors related to the diagnoses that led to their parents’ deaths; for example, counterphobia has been identified as a pathologic factor behind sexually promiscuous practices in the children of patients with AIDS, and it also may explain high-risk drinking behavior in a child whose parent died from hepatocellular carcinoma due to a history of alcoholic cirrhosis. Similarly, counterphobia can manifest as the deliberate refusal to undergo a mammogram in a woman whose mother died of breast cancer.9 Psychologists have hypothesized that counterphobic pursuits may result from attempts to master the anxiety associated with fear of injury or death as well as from the notion that attempts at risk-factor reduction are futile, as their death is certain.10
The strong influence of counterphobia on perspectives of health and mortality among individuals affected by early loss of a parent is well documented. An assessment of the subjective life expectancy, death anxiety, and health-related behaviors of college students who lost a parent revealed that these individuals estimated their own life spans to be shorter than college students with 2 living parents.11 Moreover, when students were explicitly instructed to predict their life expectancy based on a purely objective mentality rather than one influenced by personal feelings, the exclusion of emotion yielded a longer projected life span. This finding highlights the magnitude of the psychological forces influencing the ethos of individuals affected by premature parental loss. In the same study, individuals who had experienced early loss of a parent believed they would die of the same condition that caused their parent’s death, a finding accompanied by notably poorer diet and smoking behaviors, which might be expected among those with counterphobic defenses.11
Although Manne et al7 did not find an association between melanoma disease severity and sun-protective behavior in FDRs, the study design did not allow for assessment of potential counterphobic responses, which are most likely to develop in younger individuals who strongly identify with the family member whose disease was disabling or fatal. For example, the study included adult relatives (mean age, 46 years) of melanoma patients diagnosed in the preceding 4 years. Furthermore, fewer than 20% (108/545) of the patients had stage III or IV melanoma, and it was not known if melanoma patients communicated the diagnosis to their family members.7
A practice gap exists in FDRs of melanoma patients who are largely assumed to be practicing adequate, if not heightened, sun protection practices. Given that this group demonstrates poor sun protection practices, it is important to identify reasons for such behavior that may extend beyond what is currently known and may include counterphobia. Based on research performed in other medical conditions, the individuals most at risk for counterphobic responses are young children of patients diagnosed with a disabling or fatal condition, but whether in cases of melanoma counterphobia exists as a maladaptive response and whether such a response may occur in all close relatives, not just offspring, is unknown. Currently, the type of measure(s) that may mitigate poor risk factor modification due to counterphobia, including sun protection practices, is unknown. However, physician knowledge of counterphobic responses as a possibility in close relatives of melanoma patients may improve physician efforts to modify behavior in this unique, high-risk population.
The multimodal pathway of melanoma development suggests that individuals with an underlying genetic predisposition for melanoma who also neglect sun-protective measures are an especially high-risk group.12 As such, targeted education and screening of this patient population may be warranted (Table 1). Although it is incumbent on physicians to incorporate concerted screening, counseling, and focused interventions for newly diagnosed melanoma patients, taking similar measures to counsel and educate immediate relatives who may be at high risk for poor sun protection practices also is encouraged (Table 2).
We believe that recognition of counterphobic behavior is critical in the evaluation of FDRs of melanoma patients. Heightened awareness may bolster primary prevention efforts, especially in our patients with genetic diatheses toward melanoma development.
1. Idorn L, Datta P, Heydenreich J, et al. A 3-year follow-up of sun behavior in patients with cutaneous malignant melanoma [published online ahead of print October 2, 2013]. JAMA Dermatol. doi:10.1001/jamadermatol.2013.5098.
2. Idorn LW, Datta P, Heydenreich J, et al. Sun behaviour after cutaneous malignant melanoma: a study based on ultraviolet radiation measurements and sun diary data [published online ahead of print]. Br J Dermatol. 2013;168:367-373.
3. Mayer D, Layman A, Carlson J. Sun-protection behaviors of melanoma survivors. J Am Acad Dermatol. 2012;66:e9-e10.
4. Lee TK, Brazier AS, Shoveller JA, et al. Sun-related behavior after a diagnosis of cutaneous malignant melanoma. Melanoma Res. 2007;17:51-55.
5. Geller AC, Brooks DR, Colditz GA, et al. Sun protection practices among offspring of women with personal or family history of skin cancer. Pediatrics. 2006;117:e688-e694.
6. Azzarello LM, Dessureault S, Jacobsen PB. Sun-protective behavior among individuals with a family history of melanoma. Cancer Epidemiol Boomarkers Prev. 2006;15:142-145.
7. Manne SL, Coups EJ, Jacobsen PB, et al. Sun protection and sunbathing practices among at-risk family members of patients with melanoma. BMC Public Health. 2011;11:122.
8. Fenichel O. The Psychoanalytic Theory of Neurosis. Oxford, United Kingdom: Taylor & Francis; 1999.
9. Edelman H. Motherless Daughters: The Legacy of Loss. 2nd ed. Cambridge, MA: Da Capo Press; 2006.
10. Poznanski E, Arthur B. The counterphobic defense in children. Child Psychiatry Hum Dev. 1971;1:178-191.
11. Denes-Raj V, Ehrlichman H. Effects of premature parental death on subjective life expectancy, death anxiety, and health behavior. Omega: Journal of Death and Dying. 1991;23:309-321.
12. Hayward NK. Genetics of melanoma predisposition. Oncogene. 2003;22:3053-3062.
13. Arthey S, Clarke VA. Suntanning and sun protection: a review of the psychologial literature. Soc Sci Med. 1995;40:265-274.
It is widely accepted that there are several factors that may independently elevate an individual’s risk for melanoma, such as a history of childhood sunburns, family history of melanoma, and poor sun protection practices. Several studies have examined risk behaviors in melanoma patients following their diagnosis and have reported findings such as increased UV exposure patterns, persistent tanning bed use, and sun-protective behaviors similar to those of the general population (Figure).1-4
Although first-degree relatives (FDRs) of melanoma patients are at an increased risk for melanoma, they also have been found to exhibit surprisingly poor sun protection practices. In one retrospective analysis, Geller et al5 found that frequent sunburns, high rates of tanning bed use, and low rates of sunscreen use were common among children of health care workers who reported a personal or family history of skin cancer. An independent study reported that merely 37% (37/100) of FDRs of melanoma patients use sunscreen more than half of the time, and considerably fewer wear protective clothing or seek shade while outdoors.6 Given their increased risk for developing melanoma, it is likely to be assumed that FDRs of melanoma patients practice diligent sun protection. The underlying reasons for the failure of this at-risk population to adhere strongly to sun protection practices warrants special attention.
Manne et al7 conducted a survey in a group of FDRs of melanoma patients with self-reported poor sun protection practices to evaluate the demographic, medical, psychological, educational (knowledge of sun protection guidelines), and social influences that correlate with sun protection and sunbathing practices. More effective sun protective behaviors were identified in FDRs with higher education, fewer perceived benefits of sunbathing, more prominent photoaging concerns, and greater sunscreen self-efficacy. The authors concluded that sun-protective behavior in FDRs was not associated with prior knowledge about sunscreen or UV exposure, their relative’s melanoma stage, or physician recommendations for sun protection.7
Factors that have been documented as influencing sun-protective behavior in the general population include knowledge of the benefits of sun protection; attitudes toward tanning and sun protection; subjective norms regarding the beauty and perceived health of a tan; and optimistic bias, which is a cognitive mechanism that causes a person to believe that he/she is at lesser risk for experiencing a negative outcome compared to others. Additionally, sun protection behaviors are influenced by the immediacy of getting the reward (the perceived benefits of tanning) versus the delayed punishment (development of skin cancer).6 Although all of these elements may be important for some individuals, we believe that a subset of FDRs of melanoma patients may be susceptible to the phenomenon known as counterphobia.
Counterphobia is a neurotic response to anxiety in which an individual actively pursues situations that heighten his/her fear rather than fleeing from a feared object or behavior.8 Most insight into counterphobia has come from the experiences of children who have parent(s) with a debilitating or fatal diagnosis. Due to their immature coping mechanisms, some children are at risk for maladaptive behavioral responses. The loss of a parent typically produces severe psychological trauma in all children, but in those who develop counterphobia, it manifests as a heightened fear of death and vulnerability to their parent’s illness. This maladaptive response is dependent on self-identification with the parent, especially among daughters of lost mothers and sons of lost fathers, and this fear remains with the child through adulthood. A survey of 154 motherless daughters found that women aged 19 to 35 years have the highest level of obsessive thoughts of mortality and more than 75% believe they will succumb to their mother’s illness (92% in the case of cancer).9 Despite this fear, children may exhibit health-compromising behaviors related to the diagnoses that led to their parents’ deaths; for example, counterphobia has been identified as a pathologic factor behind sexually promiscuous practices in the children of patients with AIDS, and it also may explain high-risk drinking behavior in a child whose parent died from hepatocellular carcinoma due to a history of alcoholic cirrhosis. Similarly, counterphobia can manifest as the deliberate refusal to undergo a mammogram in a woman whose mother died of breast cancer.9 Psychologists have hypothesized that counterphobic pursuits may result from attempts to master the anxiety associated with fear of injury or death as well as from the notion that attempts at risk-factor reduction are futile, as their death is certain.10
The strong influence of counterphobia on perspectives of health and mortality among individuals affected by early loss of a parent is well documented. An assessment of the subjective life expectancy, death anxiety, and health-related behaviors of college students who lost a parent revealed that these individuals estimated their own life spans to be shorter than college students with 2 living parents.11 Moreover, when students were explicitly instructed to predict their life expectancy based on a purely objective mentality rather than one influenced by personal feelings, the exclusion of emotion yielded a longer projected life span. This finding highlights the magnitude of the psychological forces influencing the ethos of individuals affected by premature parental loss. In the same study, individuals who had experienced early loss of a parent believed they would die of the same condition that caused their parent’s death, a finding accompanied by notably poorer diet and smoking behaviors, which might be expected among those with counterphobic defenses.11
Although Manne et al7 did not find an association between melanoma disease severity and sun-protective behavior in FDRs, the study design did not allow for assessment of potential counterphobic responses, which are most likely to develop in younger individuals who strongly identify with the family member whose disease was disabling or fatal. For example, the study included adult relatives (mean age, 46 years) of melanoma patients diagnosed in the preceding 4 years. Furthermore, fewer than 20% (108/545) of the patients had stage III or IV melanoma, and it was not known if melanoma patients communicated the diagnosis to their family members.7
A practice gap exists in FDRs of melanoma patients who are largely assumed to be practicing adequate, if not heightened, sun protection practices. Given that this group demonstrates poor sun protection practices, it is important to identify reasons for such behavior that may extend beyond what is currently known and may include counterphobia. Based on research performed in other medical conditions, the individuals most at risk for counterphobic responses are young children of patients diagnosed with a disabling or fatal condition, but whether in cases of melanoma counterphobia exists as a maladaptive response and whether such a response may occur in all close relatives, not just offspring, is unknown. Currently, the type of measure(s) that may mitigate poor risk factor modification due to counterphobia, including sun protection practices, is unknown. However, physician knowledge of counterphobic responses as a possibility in close relatives of melanoma patients may improve physician efforts to modify behavior in this unique, high-risk population.
The multimodal pathway of melanoma development suggests that individuals with an underlying genetic predisposition for melanoma who also neglect sun-protective measures are an especially high-risk group.12 As such, targeted education and screening of this patient population may be warranted (Table 1). Although it is incumbent on physicians to incorporate concerted screening, counseling, and focused interventions for newly diagnosed melanoma patients, taking similar measures to counsel and educate immediate relatives who may be at high risk for poor sun protection practices also is encouraged (Table 2).
We believe that recognition of counterphobic behavior is critical in the evaluation of FDRs of melanoma patients. Heightened awareness may bolster primary prevention efforts, especially in our patients with genetic diatheses toward melanoma development.
It is widely accepted that there are several factors that may independently elevate an individual’s risk for melanoma, such as a history of childhood sunburns, family history of melanoma, and poor sun protection practices. Several studies have examined risk behaviors in melanoma patients following their diagnosis and have reported findings such as increased UV exposure patterns, persistent tanning bed use, and sun-protective behaviors similar to those of the general population (Figure).1-4
Although first-degree relatives (FDRs) of melanoma patients are at an increased risk for melanoma, they also have been found to exhibit surprisingly poor sun protection practices. In one retrospective analysis, Geller et al5 found that frequent sunburns, high rates of tanning bed use, and low rates of sunscreen use were common among children of health care workers who reported a personal or family history of skin cancer. An independent study reported that merely 37% (37/100) of FDRs of melanoma patients use sunscreen more than half of the time, and considerably fewer wear protective clothing or seek shade while outdoors.6 Given their increased risk for developing melanoma, it is likely to be assumed that FDRs of melanoma patients practice diligent sun protection. The underlying reasons for the failure of this at-risk population to adhere strongly to sun protection practices warrants special attention.
Manne et al7 conducted a survey in a group of FDRs of melanoma patients with self-reported poor sun protection practices to evaluate the demographic, medical, psychological, educational (knowledge of sun protection guidelines), and social influences that correlate with sun protection and sunbathing practices. More effective sun protective behaviors were identified in FDRs with higher education, fewer perceived benefits of sunbathing, more prominent photoaging concerns, and greater sunscreen self-efficacy. The authors concluded that sun-protective behavior in FDRs was not associated with prior knowledge about sunscreen or UV exposure, their relative’s melanoma stage, or physician recommendations for sun protection.7
Factors that have been documented as influencing sun-protective behavior in the general population include knowledge of the benefits of sun protection; attitudes toward tanning and sun protection; subjective norms regarding the beauty and perceived health of a tan; and optimistic bias, which is a cognitive mechanism that causes a person to believe that he/she is at lesser risk for experiencing a negative outcome compared to others. Additionally, sun protection behaviors are influenced by the immediacy of getting the reward (the perceived benefits of tanning) versus the delayed punishment (development of skin cancer).6 Although all of these elements may be important for some individuals, we believe that a subset of FDRs of melanoma patients may be susceptible to the phenomenon known as counterphobia.
Counterphobia is a neurotic response to anxiety in which an individual actively pursues situations that heighten his/her fear rather than fleeing from a feared object or behavior.8 Most insight into counterphobia has come from the experiences of children who have parent(s) with a debilitating or fatal diagnosis. Due to their immature coping mechanisms, some children are at risk for maladaptive behavioral responses. The loss of a parent typically produces severe psychological trauma in all children, but in those who develop counterphobia, it manifests as a heightened fear of death and vulnerability to their parent’s illness. This maladaptive response is dependent on self-identification with the parent, especially among daughters of lost mothers and sons of lost fathers, and this fear remains with the child through adulthood. A survey of 154 motherless daughters found that women aged 19 to 35 years have the highest level of obsessive thoughts of mortality and more than 75% believe they will succumb to their mother’s illness (92% in the case of cancer).9 Despite this fear, children may exhibit health-compromising behaviors related to the diagnoses that led to their parents’ deaths; for example, counterphobia has been identified as a pathologic factor behind sexually promiscuous practices in the children of patients with AIDS, and it also may explain high-risk drinking behavior in a child whose parent died from hepatocellular carcinoma due to a history of alcoholic cirrhosis. Similarly, counterphobia can manifest as the deliberate refusal to undergo a mammogram in a woman whose mother died of breast cancer.9 Psychologists have hypothesized that counterphobic pursuits may result from attempts to master the anxiety associated with fear of injury or death as well as from the notion that attempts at risk-factor reduction are futile, as their death is certain.10
The strong influence of counterphobia on perspectives of health and mortality among individuals affected by early loss of a parent is well documented. An assessment of the subjective life expectancy, death anxiety, and health-related behaviors of college students who lost a parent revealed that these individuals estimated their own life spans to be shorter than college students with 2 living parents.11 Moreover, when students were explicitly instructed to predict their life expectancy based on a purely objective mentality rather than one influenced by personal feelings, the exclusion of emotion yielded a longer projected life span. This finding highlights the magnitude of the psychological forces influencing the ethos of individuals affected by premature parental loss. In the same study, individuals who had experienced early loss of a parent believed they would die of the same condition that caused their parent’s death, a finding accompanied by notably poorer diet and smoking behaviors, which might be expected among those with counterphobic defenses.11
Although Manne et al7 did not find an association between melanoma disease severity and sun-protective behavior in FDRs, the study design did not allow for assessment of potential counterphobic responses, which are most likely to develop in younger individuals who strongly identify with the family member whose disease was disabling or fatal. For example, the study included adult relatives (mean age, 46 years) of melanoma patients diagnosed in the preceding 4 years. Furthermore, fewer than 20% (108/545) of the patients had stage III or IV melanoma, and it was not known if melanoma patients communicated the diagnosis to their family members.7
A practice gap exists in FDRs of melanoma patients who are largely assumed to be practicing adequate, if not heightened, sun protection practices. Given that this group demonstrates poor sun protection practices, it is important to identify reasons for such behavior that may extend beyond what is currently known and may include counterphobia. Based on research performed in other medical conditions, the individuals most at risk for counterphobic responses are young children of patients diagnosed with a disabling or fatal condition, but whether in cases of melanoma counterphobia exists as a maladaptive response and whether such a response may occur in all close relatives, not just offspring, is unknown. Currently, the type of measure(s) that may mitigate poor risk factor modification due to counterphobia, including sun protection practices, is unknown. However, physician knowledge of counterphobic responses as a possibility in close relatives of melanoma patients may improve physician efforts to modify behavior in this unique, high-risk population.
The multimodal pathway of melanoma development suggests that individuals with an underlying genetic predisposition for melanoma who also neglect sun-protective measures are an especially high-risk group.12 As such, targeted education and screening of this patient population may be warranted (Table 1). Although it is incumbent on physicians to incorporate concerted screening, counseling, and focused interventions for newly diagnosed melanoma patients, taking similar measures to counsel and educate immediate relatives who may be at high risk for poor sun protection practices also is encouraged (Table 2).
We believe that recognition of counterphobic behavior is critical in the evaluation of FDRs of melanoma patients. Heightened awareness may bolster primary prevention efforts, especially in our patients with genetic diatheses toward melanoma development.
1. Idorn L, Datta P, Heydenreich J, et al. A 3-year follow-up of sun behavior in patients with cutaneous malignant melanoma [published online ahead of print October 2, 2013]. JAMA Dermatol. doi:10.1001/jamadermatol.2013.5098.
2. Idorn LW, Datta P, Heydenreich J, et al. Sun behaviour after cutaneous malignant melanoma: a study based on ultraviolet radiation measurements and sun diary data [published online ahead of print]. Br J Dermatol. 2013;168:367-373.
3. Mayer D, Layman A, Carlson J. Sun-protection behaviors of melanoma survivors. J Am Acad Dermatol. 2012;66:e9-e10.
4. Lee TK, Brazier AS, Shoveller JA, et al. Sun-related behavior after a diagnosis of cutaneous malignant melanoma. Melanoma Res. 2007;17:51-55.
5. Geller AC, Brooks DR, Colditz GA, et al. Sun protection practices among offspring of women with personal or family history of skin cancer. Pediatrics. 2006;117:e688-e694.
6. Azzarello LM, Dessureault S, Jacobsen PB. Sun-protective behavior among individuals with a family history of melanoma. Cancer Epidemiol Boomarkers Prev. 2006;15:142-145.
7. Manne SL, Coups EJ, Jacobsen PB, et al. Sun protection and sunbathing practices among at-risk family members of patients with melanoma. BMC Public Health. 2011;11:122.
8. Fenichel O. The Psychoanalytic Theory of Neurosis. Oxford, United Kingdom: Taylor & Francis; 1999.
9. Edelman H. Motherless Daughters: The Legacy of Loss. 2nd ed. Cambridge, MA: Da Capo Press; 2006.
10. Poznanski E, Arthur B. The counterphobic defense in children. Child Psychiatry Hum Dev. 1971;1:178-191.
11. Denes-Raj V, Ehrlichman H. Effects of premature parental death on subjective life expectancy, death anxiety, and health behavior. Omega: Journal of Death and Dying. 1991;23:309-321.
12. Hayward NK. Genetics of melanoma predisposition. Oncogene. 2003;22:3053-3062.
13. Arthey S, Clarke VA. Suntanning and sun protection: a review of the psychologial literature. Soc Sci Med. 1995;40:265-274.
1. Idorn L, Datta P, Heydenreich J, et al. A 3-year follow-up of sun behavior in patients with cutaneous malignant melanoma [published online ahead of print October 2, 2013]. JAMA Dermatol. doi:10.1001/jamadermatol.2013.5098.
2. Idorn LW, Datta P, Heydenreich J, et al. Sun behaviour after cutaneous malignant melanoma: a study based on ultraviolet radiation measurements and sun diary data [published online ahead of print]. Br J Dermatol. 2013;168:367-373.
3. Mayer D, Layman A, Carlson J. Sun-protection behaviors of melanoma survivors. J Am Acad Dermatol. 2012;66:e9-e10.
4. Lee TK, Brazier AS, Shoveller JA, et al. Sun-related behavior after a diagnosis of cutaneous malignant melanoma. Melanoma Res. 2007;17:51-55.
5. Geller AC, Brooks DR, Colditz GA, et al. Sun protection practices among offspring of women with personal or family history of skin cancer. Pediatrics. 2006;117:e688-e694.
6. Azzarello LM, Dessureault S, Jacobsen PB. Sun-protective behavior among individuals with a family history of melanoma. Cancer Epidemiol Boomarkers Prev. 2006;15:142-145.
7. Manne SL, Coups EJ, Jacobsen PB, et al. Sun protection and sunbathing practices among at-risk family members of patients with melanoma. BMC Public Health. 2011;11:122.
8. Fenichel O. The Psychoanalytic Theory of Neurosis. Oxford, United Kingdom: Taylor & Francis; 1999.
9. Edelman H. Motherless Daughters: The Legacy of Loss. 2nd ed. Cambridge, MA: Da Capo Press; 2006.
10. Poznanski E, Arthur B. The counterphobic defense in children. Child Psychiatry Hum Dev. 1971;1:178-191.
11. Denes-Raj V, Ehrlichman H. Effects of premature parental death on subjective life expectancy, death anxiety, and health behavior. Omega: Journal of Death and Dying. 1991;23:309-321.
12. Hayward NK. Genetics of melanoma predisposition. Oncogene. 2003;22:3053-3062.
13. Arthey S, Clarke VA. Suntanning and sun protection: a review of the psychologial literature. Soc Sci Med. 1995;40:265-274.
Double-Positive CD4+CD8+ Sézary Syndrome: An Unusual Phenotype With an Aggressive Clinical Course
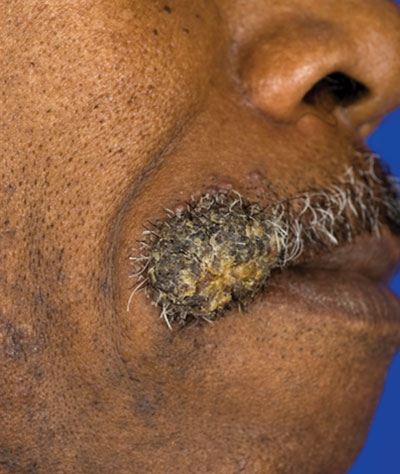
Verrucous Nodule on the Upper Lip
The Diagnosis: Disseminated Coccidioidomycosis
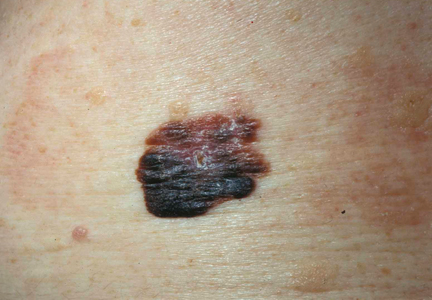
Fungi of the genus Coccidioides cause coccidioidomycosis and live in the soil of endemic areas including the southwestern United States (eg, Arizona, New Mexico, California) and Mexico. Coccidioides is a dimorphic fungus with parasitic and infectious saprophytic phases. Each year there are approximately 150,000 new infections of coccidioidomycosis in the United States, almost exclusively in the southwest.1 Coccidioidomycosis typically is an asymptomatic or mild infection in an immunocompetent patient. Although the lungs are nearly always the primary sites of infection, common sites of dissemination include the skin, meninges, bones, and joints. The skin is the most common site of disseminated, or secondary, coccidioidomycosis.2 Less commonly and usually caused by traumatic implantation, the skin is the site of primary infection.
Disseminated coccidioidomycosis occurs in approximately 1 in 200 infected individuals.2,3 Certain populations of patients are more likely to be affected by disseminated coccidioidomycosis, including specific ethnic groups such as black individuals, Filipinos, and Mexicans4,5; pregnant women6; and immunosuppressed patients such as those with human immunodeficiency virus, hematogenous malignancy, or organ transplantation.7-9 When skin lesions are present, they usually develop after the initial lung manifestation. The location of the lesion in cutaneous disseminated disease can be highly variable, but the face and head are the most common locations (30%).10
Cutaneous manifestations of coccidioidomycosis may be classified as being caused by the presence of the organism in the skin (organism specific) or a reactive process. Organism-specific cutaneous lesions are commonly due to systemic disease with secondary skin involvement, but they also may be due to a primary infection. These organism-specific lesions can present as papules, nodules, macules, verrucous plaques, abscesses, or pustules. Reactive cutaneous manifestations are only associated with disseminated disease; do not contain any organisms; and include manifestations such as erythema nodosum, acute exanthem, erythema multiforme, and possibly Sweet syndrome.11
The clinical differential diagnosis of cutaneous coccidioidomycosis includes other
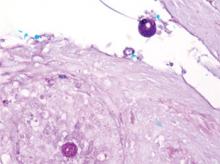
mycoses such as histoplasmosis and blastomycosis, as well as tuberculosis, sarcoidosis, basal cell and squamous cell carcinoma, and verruca vulgaris. The diagnosis of cutaneous coccidioidomycosis can be made with skin biopsy, culture, and serologic tests. The characteristic spherules can be visualized on routine hematoxylin and eosin stain or more readily with fungal stains (Figure). Spherules are thick walled and distinguishable from other fungi because of the characteristic endospores inside as well as their larger size. Organisms also may be detected via culture within 2 to 5 days.
Distinguishing primary cutaneous from disseminated skin lesions can be challenging but can have notable treatment implications. Although histology typically cannot distinguish primary from disseminated cutaneous infections, clinical history and serologic studies have been found to be useful. In disseminated disease, IgG antibodies are elevated, while in primary cutaneous disease, IgM antibodies are elevated but typically not IgG.12 Therefore, tube precipitin and latex particle agglutination tests that detect IgM antibodies should be positive in primary infections.13 Primary lesions can spontaneously resolve within months to years and may not require treatment if symptomatic, while secondary lesions must be therapeutically addressed.12 Despite the lack of treatment needed, primary cutaneous infections often are treated with azoles.14 In contrast, disseminated cutaneous infection requires systemic therapy. Treatment of disseminated infection with cutaneous coccidioidomycosis typically includes amphotericin B until a clinical response is achieved and antibody titers decline. Amphotericin B can then be replaced with an oral azole such as itraconozole or fluconazole.15
The patient discussed in this case demonstrates the typical clinical presentation of disseminated coccidioidomycosis with classical and diagnostic pathology. This case also highlights the importance of a detailed travel history; in the era of globalization, patients often present with diseases in nonendemic areas. Clinicians must consider the diagnosis of coccidioidomycosis, even in immunocompetent patients in nonendemic areas when their history and presentation are appropriate. The diagnosis should be confirmed with biopsy, culture, and/or serology.
1. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis [published online ahead of print September 20, 2005]. Clin Infect Dis. 2005;41:1217-1223.
2. Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am. 2003;17:41-57, vii.
3. Rance BR, Elston DM. Disseminated coccidioidomyclosis discovered during routine skin cancer screening. Cutis. 2002;70:70-72.
4. Einstein HE, Johnson RH. Coccidioidomycosis: new aspects of epidemiology and therapy [comment in Clin Infect Dis. 1994;18:470]. Clin Infect Dis. 1993;16:349- 354.
5. Crum NF, Ballon-Landa G. Coccidioidomycosis in pregnancy: case report and review of the literature. Am J Med. 2006;119:e11-e17.
6. Caldwell JW, Arsura EL, Kilgore WB, et al. Coccidioidomycosis in pregnancy during an epidemic in California. Obstet Gynecol. 2000;95:236-239.
7. Singh VR, Smith DK, Lawerence J, et al. Coccidioidomy-cosis in patients infected with human immunodeficiency virus: review of 91 cases at a single institution. Clin Infect Dis. 1996;23:563-568.
8. Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation [published online ahead of print October 4, 2001]. Clin Infect Dis. 2001;33:1536-1544.
9. Riley DK, Galgiani JN, O’Donnell MR, et al. Coccidioidomycosis in bone marrow transplant recipients. Transplantation. 1993;56:1531-1533.
10. Carpenter JB, Feldman JS, Leyva WH, et al. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol. 2010;62:831-837.
11. DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-945.
12. Chang A, Tung RC, McGillis TS, et al. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol. 2003;49:944-949.
13. Wilson JW, Smith CE, Plunkett OA. Primary cutaneous coccidioidomycosis: the criteria for diagnosis and report of a case. Calif Med. 1953;79:233-239.
14. Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections [published online ahead of print March 1, 2007]. Ann N Y Acad Sci. 2007;1111:411-421.
15. Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guidelines for the treatment of coccidioidomycosis. Infectious Diseases Society of America [published online ahead of print April 20, 2000]. Clin Infect Dis. 2000;30:658-661.
The Diagnosis: Disseminated Coccidioidomycosis
Fungi of the genus Coccidioides cause coccidioidomycosis and live in the soil of endemic areas including the southwestern United States (eg, Arizona, New Mexico, California) and Mexico. Coccidioides is a dimorphic fungus with parasitic and infectious saprophytic phases. Each year there are approximately 150,000 new infections of coccidioidomycosis in the United States, almost exclusively in the southwest.1 Coccidioidomycosis typically is an asymptomatic or mild infection in an immunocompetent patient. Although the lungs are nearly always the primary sites of infection, common sites of dissemination include the skin, meninges, bones, and joints. The skin is the most common site of disseminated, or secondary, coccidioidomycosis.2 Less commonly and usually caused by traumatic implantation, the skin is the site of primary infection.
Disseminated coccidioidomycosis occurs in approximately 1 in 200 infected individuals.2,3 Certain populations of patients are more likely to be affected by disseminated coccidioidomycosis, including specific ethnic groups such as black individuals, Filipinos, and Mexicans4,5; pregnant women6; and immunosuppressed patients such as those with human immunodeficiency virus, hematogenous malignancy, or organ transplantation.7-9 When skin lesions are present, they usually develop after the initial lung manifestation. The location of the lesion in cutaneous disseminated disease can be highly variable, but the face and head are the most common locations (30%).10
Cutaneous manifestations of coccidioidomycosis may be classified as being caused by the presence of the organism in the skin (organism specific) or a reactive process. Organism-specific cutaneous lesions are commonly due to systemic disease with secondary skin involvement, but they also may be due to a primary infection. These organism-specific lesions can present as papules, nodules, macules, verrucous plaques, abscesses, or pustules. Reactive cutaneous manifestations are only associated with disseminated disease; do not contain any organisms; and include manifestations such as erythema nodosum, acute exanthem, erythema multiforme, and possibly Sweet syndrome.11
The clinical differential diagnosis of cutaneous coccidioidomycosis includes other
mycoses such as histoplasmosis and blastomycosis, as well as tuberculosis, sarcoidosis, basal cell and squamous cell carcinoma, and verruca vulgaris. The diagnosis of cutaneous coccidioidomycosis can be made with skin biopsy, culture, and serologic tests. The characteristic spherules can be visualized on routine hematoxylin and eosin stain or more readily with fungal stains (Figure). Spherules are thick walled and distinguishable from other fungi because of the characteristic endospores inside as well as their larger size. Organisms also may be detected via culture within 2 to 5 days.
Distinguishing primary cutaneous from disseminated skin lesions can be challenging but can have notable treatment implications. Although histology typically cannot distinguish primary from disseminated cutaneous infections, clinical history and serologic studies have been found to be useful. In disseminated disease, IgG antibodies are elevated, while in primary cutaneous disease, IgM antibodies are elevated but typically not IgG.12 Therefore, tube precipitin and latex particle agglutination tests that detect IgM antibodies should be positive in primary infections.13 Primary lesions can spontaneously resolve within months to years and may not require treatment if symptomatic, while secondary lesions must be therapeutically addressed.12 Despite the lack of treatment needed, primary cutaneous infections often are treated with azoles.14 In contrast, disseminated cutaneous infection requires systemic therapy. Treatment of disseminated infection with cutaneous coccidioidomycosis typically includes amphotericin B until a clinical response is achieved and antibody titers decline. Amphotericin B can then be replaced with an oral azole such as itraconozole or fluconazole.15
The patient discussed in this case demonstrates the typical clinical presentation of disseminated coccidioidomycosis with classical and diagnostic pathology. This case also highlights the importance of a detailed travel history; in the era of globalization, patients often present with diseases in nonendemic areas. Clinicians must consider the diagnosis of coccidioidomycosis, even in immunocompetent patients in nonendemic areas when their history and presentation are appropriate. The diagnosis should be confirmed with biopsy, culture, and/or serology.
The Diagnosis: Disseminated Coccidioidomycosis
Fungi of the genus Coccidioides cause coccidioidomycosis and live in the soil of endemic areas including the southwestern United States (eg, Arizona, New Mexico, California) and Mexico. Coccidioides is a dimorphic fungus with parasitic and infectious saprophytic phases. Each year there are approximately 150,000 new infections of coccidioidomycosis in the United States, almost exclusively in the southwest.1 Coccidioidomycosis typically is an asymptomatic or mild infection in an immunocompetent patient. Although the lungs are nearly always the primary sites of infection, common sites of dissemination include the skin, meninges, bones, and joints. The skin is the most common site of disseminated, or secondary, coccidioidomycosis.2 Less commonly and usually caused by traumatic implantation, the skin is the site of primary infection.
Disseminated coccidioidomycosis occurs in approximately 1 in 200 infected individuals.2,3 Certain populations of patients are more likely to be affected by disseminated coccidioidomycosis, including specific ethnic groups such as black individuals, Filipinos, and Mexicans4,5; pregnant women6; and immunosuppressed patients such as those with human immunodeficiency virus, hematogenous malignancy, or organ transplantation.7-9 When skin lesions are present, they usually develop after the initial lung manifestation. The location of the lesion in cutaneous disseminated disease can be highly variable, but the face and head are the most common locations (30%).10
Cutaneous manifestations of coccidioidomycosis may be classified as being caused by the presence of the organism in the skin (organism specific) or a reactive process. Organism-specific cutaneous lesions are commonly due to systemic disease with secondary skin involvement, but they also may be due to a primary infection. These organism-specific lesions can present as papules, nodules, macules, verrucous plaques, abscesses, or pustules. Reactive cutaneous manifestations are only associated with disseminated disease; do not contain any organisms; and include manifestations such as erythema nodosum, acute exanthem, erythema multiforme, and possibly Sweet syndrome.11
The clinical differential diagnosis of cutaneous coccidioidomycosis includes other
mycoses such as histoplasmosis and blastomycosis, as well as tuberculosis, sarcoidosis, basal cell and squamous cell carcinoma, and verruca vulgaris. The diagnosis of cutaneous coccidioidomycosis can be made with skin biopsy, culture, and serologic tests. The characteristic spherules can be visualized on routine hematoxylin and eosin stain or more readily with fungal stains (Figure). Spherules are thick walled and distinguishable from other fungi because of the characteristic endospores inside as well as their larger size. Organisms also may be detected via culture within 2 to 5 days.
Distinguishing primary cutaneous from disseminated skin lesions can be challenging but can have notable treatment implications. Although histology typically cannot distinguish primary from disseminated cutaneous infections, clinical history and serologic studies have been found to be useful. In disseminated disease, IgG antibodies are elevated, while in primary cutaneous disease, IgM antibodies are elevated but typically not IgG.12 Therefore, tube precipitin and latex particle agglutination tests that detect IgM antibodies should be positive in primary infections.13 Primary lesions can spontaneously resolve within months to years and may not require treatment if symptomatic, while secondary lesions must be therapeutically addressed.12 Despite the lack of treatment needed, primary cutaneous infections often are treated with azoles.14 In contrast, disseminated cutaneous infection requires systemic therapy. Treatment of disseminated infection with cutaneous coccidioidomycosis typically includes amphotericin B until a clinical response is achieved and antibody titers decline. Amphotericin B can then be replaced with an oral azole such as itraconozole or fluconazole.15
The patient discussed in this case demonstrates the typical clinical presentation of disseminated coccidioidomycosis with classical and diagnostic pathology. This case also highlights the importance of a detailed travel history; in the era of globalization, patients often present with diseases in nonendemic areas. Clinicians must consider the diagnosis of coccidioidomycosis, even in immunocompetent patients in nonendemic areas when their history and presentation are appropriate. The diagnosis should be confirmed with biopsy, culture, and/or serology.
1. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis [published online ahead of print September 20, 2005]. Clin Infect Dis. 2005;41:1217-1223.
2. Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am. 2003;17:41-57, vii.
3. Rance BR, Elston DM. Disseminated coccidioidomyclosis discovered during routine skin cancer screening. Cutis. 2002;70:70-72.
4. Einstein HE, Johnson RH. Coccidioidomycosis: new aspects of epidemiology and therapy [comment in Clin Infect Dis. 1994;18:470]. Clin Infect Dis. 1993;16:349- 354.
5. Crum NF, Ballon-Landa G. Coccidioidomycosis in pregnancy: case report and review of the literature. Am J Med. 2006;119:e11-e17.
6. Caldwell JW, Arsura EL, Kilgore WB, et al. Coccidioidomycosis in pregnancy during an epidemic in California. Obstet Gynecol. 2000;95:236-239.
7. Singh VR, Smith DK, Lawerence J, et al. Coccidioidomy-cosis in patients infected with human immunodeficiency virus: review of 91 cases at a single institution. Clin Infect Dis. 1996;23:563-568.
8. Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation [published online ahead of print October 4, 2001]. Clin Infect Dis. 2001;33:1536-1544.
9. Riley DK, Galgiani JN, O’Donnell MR, et al. Coccidioidomycosis in bone marrow transplant recipients. Transplantation. 1993;56:1531-1533.
10. Carpenter JB, Feldman JS, Leyva WH, et al. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol. 2010;62:831-837.
11. DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-945.
12. Chang A, Tung RC, McGillis TS, et al. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol. 2003;49:944-949.
13. Wilson JW, Smith CE, Plunkett OA. Primary cutaneous coccidioidomycosis: the criteria for diagnosis and report of a case. Calif Med. 1953;79:233-239.
14. Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections [published online ahead of print March 1, 2007]. Ann N Y Acad Sci. 2007;1111:411-421.
15. Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guidelines for the treatment of coccidioidomycosis. Infectious Diseases Society of America [published online ahead of print April 20, 2000]. Clin Infect Dis. 2000;30:658-661.
1. Galgiani JN, Ampel NM, Blair JE, et al. Coccidioidomycosis [published online ahead of print September 20, 2005]. Clin Infect Dis. 2005;41:1217-1223.
2. Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am. 2003;17:41-57, vii.
3. Rance BR, Elston DM. Disseminated coccidioidomyclosis discovered during routine skin cancer screening. Cutis. 2002;70:70-72.
4. Einstein HE, Johnson RH. Coccidioidomycosis: new aspects of epidemiology and therapy [comment in Clin Infect Dis. 1994;18:470]. Clin Infect Dis. 1993;16:349- 354.
5. Crum NF, Ballon-Landa G. Coccidioidomycosis in pregnancy: case report and review of the literature. Am J Med. 2006;119:e11-e17.
6. Caldwell JW, Arsura EL, Kilgore WB, et al. Coccidioidomycosis in pregnancy during an epidemic in California. Obstet Gynecol. 2000;95:236-239.
7. Singh VR, Smith DK, Lawerence J, et al. Coccidioidomy-cosis in patients infected with human immunodeficiency virus: review of 91 cases at a single institution. Clin Infect Dis. 1996;23:563-568.
8. Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation [published online ahead of print October 4, 2001]. Clin Infect Dis. 2001;33:1536-1544.
9. Riley DK, Galgiani JN, O’Donnell MR, et al. Coccidioidomycosis in bone marrow transplant recipients. Transplantation. 1993;56:1531-1533.
10. Carpenter JB, Feldman JS, Leyva WH, et al. Clinical and pathologic characteristics of disseminated cutaneous coccidioidomycosis. J Am Acad Dermatol. 2010;62:831-837.
11. DiCaudo DJ. Coccidioidomycosis: a review and update. J Am Acad Dermatol. 2006;55:929-942; quiz 943-945.
12. Chang A, Tung RC, McGillis TS, et al. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol. 2003;49:944-949.
13. Wilson JW, Smith CE, Plunkett OA. Primary cutaneous coccidioidomycosis: the criteria for diagnosis and report of a case. Calif Med. 1953;79:233-239.
14. Blair JE. State-of-the-art treatment of coccidioidomycosis: skin and soft-tissue infections [published online ahead of print March 1, 2007]. Ann N Y Acad Sci. 2007;1111:411-421.
15. Galgiani JN, Ampel NM, Catanzaro A, et al. Practice guidelines for the treatment of coccidioidomycosis. Infectious Diseases Society of America [published online ahead of print April 20, 2000]. Clin Infect Dis. 2000;30:658-661.
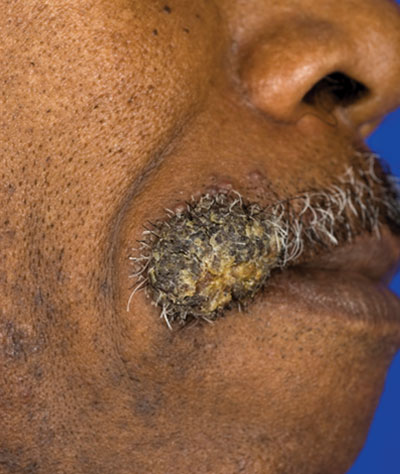
A 62-year-old black man presented to a dermatologist in the northeastern United States with a verrucous nontender nodule on the upper lip after traveling to southern California 1 month prior. The patient was not immunocompromised but reported a recent febrile upper respiratory illness.