User login
To the Editor:
Liposomal doxorubicin hydrochloride is an anthracycline topoisomerase inhibitor indicated for ovarian cancer, AIDS-related Kaposi sarcoma, and multiple myeloma.1 It also has been used with limited success in a clinical trial of previously treated patients with endometrial cancer.2 The most common adverse reactions include asthenia, fatigue, fever, anorexia, nausea, vomiting, stomatitis, diarrhea, constipation, hand-and-foot syndrome, rash, neutropenia, thrombocytopenia, and anemia.1
A 58-year-old woman with a history of stage IIIA endometrial cancer underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy soon after diagnosis. She then completed 5 high-dose-rate brachytherapy treatments and 6 cycles of paclitaxel and carboplatin. Follow-up imaging revealed pulmonary metastasis. The patient was then enrolled in a clinical trial but was switched to 40 mg/m2 liposomal doxorubicin given once every 28 days for 5 cycles after progression of disease.
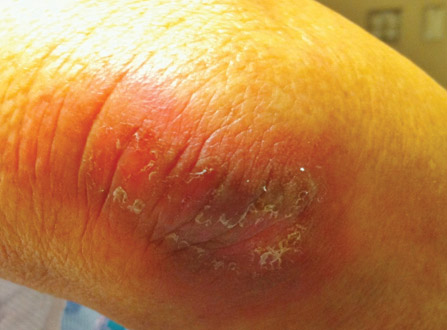
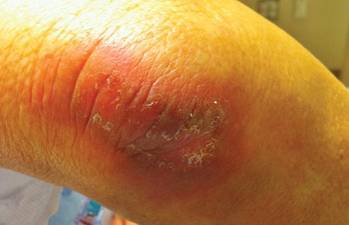
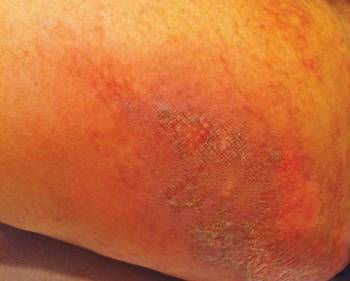
After each dose of doxorubicin, she developed redness of the palms and soles. Following the third cycle of doxorubicin, a painful rash involving the thighs and axilla appeared with some desquamation in the left axilla. Three weeks after the fourth dose of doxorubicin, she presented with severe worsening of the rash to involve the extensor elbows (Figure 1), back, and lower legs with bilateral axillary desquamation. The bilateral medial thighs were erythematous with maceration that was tender and blanchable (Figure 2). The total affected body surface area was 10% to 15%. There was no involvement of the mucosa. She was treated with hydrogel sheet dressings and silver sulfadiazine cream 1%.
|
|
The patient’s rash was thought to be due to doxorubicin toxicity; however, a 4-mm punch biopsy specimen from the left thigh was taken for culture and hemotoxylin and eosin stain to rule out other possibilities. Biopsy was consistent with a drug reaction, revealing superficial perivascular dermatitis with keratinocyte atypia of the epidermis. Doxorubicin was discontinued and the rash resolved completely within 2 weeks, except for some thickening of the skin on the palms, soles, and thighs. After a delay of approximately 1 week, doxorubicin was resumed at a lower dose of 30 mg/m2. No dermatologic symptoms followed treatment at this dose.
Four clinical patterns of doxorubicin toxicity are recognized. The most common pattern is acral erythema, also known as hand-and-foot syndrome, which is followed by desquamation of the palms and soles, occurring in approximately 50% of patients. Ten percent of patients experience a diffuse follicular rash with mild, diffuse, scaly erythema and follicular accentuation that often occurs over the lateral limbs but also may occur over the trunk. New melanotic macules may appear on the trunk or extremities including palms and soles.3 Finally, an intertrigolike eruption exacerbated by friction with erythematous patches over skin folds or in areas of friction also has been described.3-5 Our patient presented with a combination of dermatologic toxicities including acral erythema and intertrigolike eruption. Acral erythema occurred in 24 of 60 patients and intertrigolike eruption occurred in 5 of 60 patients in one study.3 Another report documented both occurring together.5
Treatment of doxorubicin skin toxicity consists of reduction of the dose of doxorubicin, supportive care, and patient education. Specific treatments include topical wound care, emollient creams, and pain management with analgesics. Other interventions include wearing loose clothing, avoiding vigorous exercise, and sitting on padded surfaces.6
Doxorubicin skin toxicity presents in several clinical patterns. Although acral erythema is the most common pattern, severe intertrigolike eruptions similar to our case may occur. Physicians caring for patients receiving doxorubicin should be aware of the variety of presentations of skin toxicity and the possible need for dose reduction to decrease symptoms.
1. Doxil [package insert]. Horsham, PA: Janssen Products, LP; 2014.
2. Muggia FM, Blessing JA, Sorosky J, et al. Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2002;20:2360-2364.
3. Lotem M, Hubert A, Lyass O, et al. Skin toxic effects of polyethylene glycol-coated liposomal doxorubicin. Arch Dermatol. 2000;136:1475-1480.
4. Korver GE, Ronald H, Petersen MJ. An intertrigo-like eruption from pegylated liposomal doxorubicin. J Drugs Dermatol. 2006;5:901-902.
5. Sánchez Henarejos P, Ros Martinez S, Marín Zafra GR,
et al. Intertrigo-like eruption caused by pegylated liposomal doxorubicin (PLD). Clin Transl Oncol. 2009;11:486-487.
6. von Moos R, Thuerlimann BJ, Aapro M, et al. Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts [published online ahead of print March 10, 2008]. Eur J Cancer. 2008;44:781-790.
To the Editor:
Liposomal doxorubicin hydrochloride is an anthracycline topoisomerase inhibitor indicated for ovarian cancer, AIDS-related Kaposi sarcoma, and multiple myeloma.1 It also has been used with limited success in a clinical trial of previously treated patients with endometrial cancer.2 The most common adverse reactions include asthenia, fatigue, fever, anorexia, nausea, vomiting, stomatitis, diarrhea, constipation, hand-and-foot syndrome, rash, neutropenia, thrombocytopenia, and anemia.1
A 58-year-old woman with a history of stage IIIA endometrial cancer underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy soon after diagnosis. She then completed 5 high-dose-rate brachytherapy treatments and 6 cycles of paclitaxel and carboplatin. Follow-up imaging revealed pulmonary metastasis. The patient was then enrolled in a clinical trial but was switched to 40 mg/m2 liposomal doxorubicin given once every 28 days for 5 cycles after progression of disease.
After each dose of doxorubicin, she developed redness of the palms and soles. Following the third cycle of doxorubicin, a painful rash involving the thighs and axilla appeared with some desquamation in the left axilla. Three weeks after the fourth dose of doxorubicin, she presented with severe worsening of the rash to involve the extensor elbows (Figure 1), back, and lower legs with bilateral axillary desquamation. The bilateral medial thighs were erythematous with maceration that was tender and blanchable (Figure 2). The total affected body surface area was 10% to 15%. There was no involvement of the mucosa. She was treated with hydrogel sheet dressings and silver sulfadiazine cream 1%.

|

|
The patient’s rash was thought to be due to doxorubicin toxicity; however, a 4-mm punch biopsy specimen from the left thigh was taken for culture and hemotoxylin and eosin stain to rule out other possibilities. Biopsy was consistent with a drug reaction, revealing superficial perivascular dermatitis with keratinocyte atypia of the epidermis. Doxorubicin was discontinued and the rash resolved completely within 2 weeks, except for some thickening of the skin on the palms, soles, and thighs. After a delay of approximately 1 week, doxorubicin was resumed at a lower dose of 30 mg/m2. No dermatologic symptoms followed treatment at this dose.
Four clinical patterns of doxorubicin toxicity are recognized. The most common pattern is acral erythema, also known as hand-and-foot syndrome, which is followed by desquamation of the palms and soles, occurring in approximately 50% of patients. Ten percent of patients experience a diffuse follicular rash with mild, diffuse, scaly erythema and follicular accentuation that often occurs over the lateral limbs but also may occur over the trunk. New melanotic macules may appear on the trunk or extremities including palms and soles.3 Finally, an intertrigolike eruption exacerbated by friction with erythematous patches over skin folds or in areas of friction also has been described.3-5 Our patient presented with a combination of dermatologic toxicities including acral erythema and intertrigolike eruption. Acral erythema occurred in 24 of 60 patients and intertrigolike eruption occurred in 5 of 60 patients in one study.3 Another report documented both occurring together.5
Treatment of doxorubicin skin toxicity consists of reduction of the dose of doxorubicin, supportive care, and patient education. Specific treatments include topical wound care, emollient creams, and pain management with analgesics. Other interventions include wearing loose clothing, avoiding vigorous exercise, and sitting on padded surfaces.6
Doxorubicin skin toxicity presents in several clinical patterns. Although acral erythema is the most common pattern, severe intertrigolike eruptions similar to our case may occur. Physicians caring for patients receiving doxorubicin should be aware of the variety of presentations of skin toxicity and the possible need for dose reduction to decrease symptoms.
To the Editor:
Liposomal doxorubicin hydrochloride is an anthracycline topoisomerase inhibitor indicated for ovarian cancer, AIDS-related Kaposi sarcoma, and multiple myeloma.1 It also has been used with limited success in a clinical trial of previously treated patients with endometrial cancer.2 The most common adverse reactions include asthenia, fatigue, fever, anorexia, nausea, vomiting, stomatitis, diarrhea, constipation, hand-and-foot syndrome, rash, neutropenia, thrombocytopenia, and anemia.1
A 58-year-old woman with a history of stage IIIA endometrial cancer underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy soon after diagnosis. She then completed 5 high-dose-rate brachytherapy treatments and 6 cycles of paclitaxel and carboplatin. Follow-up imaging revealed pulmonary metastasis. The patient was then enrolled in a clinical trial but was switched to 40 mg/m2 liposomal doxorubicin given once every 28 days for 5 cycles after progression of disease.
After each dose of doxorubicin, she developed redness of the palms and soles. Following the third cycle of doxorubicin, a painful rash involving the thighs and axilla appeared with some desquamation in the left axilla. Three weeks after the fourth dose of doxorubicin, she presented with severe worsening of the rash to involve the extensor elbows (Figure 1), back, and lower legs with bilateral axillary desquamation. The bilateral medial thighs were erythematous with maceration that was tender and blanchable (Figure 2). The total affected body surface area was 10% to 15%. There was no involvement of the mucosa. She was treated with hydrogel sheet dressings and silver sulfadiazine cream 1%.

|

|
The patient’s rash was thought to be due to doxorubicin toxicity; however, a 4-mm punch biopsy specimen from the left thigh was taken for culture and hemotoxylin and eosin stain to rule out other possibilities. Biopsy was consistent with a drug reaction, revealing superficial perivascular dermatitis with keratinocyte atypia of the epidermis. Doxorubicin was discontinued and the rash resolved completely within 2 weeks, except for some thickening of the skin on the palms, soles, and thighs. After a delay of approximately 1 week, doxorubicin was resumed at a lower dose of 30 mg/m2. No dermatologic symptoms followed treatment at this dose.
Four clinical patterns of doxorubicin toxicity are recognized. The most common pattern is acral erythema, also known as hand-and-foot syndrome, which is followed by desquamation of the palms and soles, occurring in approximately 50% of patients. Ten percent of patients experience a diffuse follicular rash with mild, diffuse, scaly erythema and follicular accentuation that often occurs over the lateral limbs but also may occur over the trunk. New melanotic macules may appear on the trunk or extremities including palms and soles.3 Finally, an intertrigolike eruption exacerbated by friction with erythematous patches over skin folds or in areas of friction also has been described.3-5 Our patient presented with a combination of dermatologic toxicities including acral erythema and intertrigolike eruption. Acral erythema occurred in 24 of 60 patients and intertrigolike eruption occurred in 5 of 60 patients in one study.3 Another report documented both occurring together.5
Treatment of doxorubicin skin toxicity consists of reduction of the dose of doxorubicin, supportive care, and patient education. Specific treatments include topical wound care, emollient creams, and pain management with analgesics. Other interventions include wearing loose clothing, avoiding vigorous exercise, and sitting on padded surfaces.6
Doxorubicin skin toxicity presents in several clinical patterns. Although acral erythema is the most common pattern, severe intertrigolike eruptions similar to our case may occur. Physicians caring for patients receiving doxorubicin should be aware of the variety of presentations of skin toxicity and the possible need for dose reduction to decrease symptoms.
1. Doxil [package insert]. Horsham, PA: Janssen Products, LP; 2014.
2. Muggia FM, Blessing JA, Sorosky J, et al. Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2002;20:2360-2364.
3. Lotem M, Hubert A, Lyass O, et al. Skin toxic effects of polyethylene glycol-coated liposomal doxorubicin. Arch Dermatol. 2000;136:1475-1480.
4. Korver GE, Ronald H, Petersen MJ. An intertrigo-like eruption from pegylated liposomal doxorubicin. J Drugs Dermatol. 2006;5:901-902.
5. Sánchez Henarejos P, Ros Martinez S, Marín Zafra GR,
et al. Intertrigo-like eruption caused by pegylated liposomal doxorubicin (PLD). Clin Transl Oncol. 2009;11:486-487.
6. von Moos R, Thuerlimann BJ, Aapro M, et al. Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts [published online ahead of print March 10, 2008]. Eur J Cancer. 2008;44:781-790.
1. Doxil [package insert]. Horsham, PA: Janssen Products, LP; 2014.
2. Muggia FM, Blessing JA, Sorosky J, et al. Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2002;20:2360-2364.
3. Lotem M, Hubert A, Lyass O, et al. Skin toxic effects of polyethylene glycol-coated liposomal doxorubicin. Arch Dermatol. 2000;136:1475-1480.
4. Korver GE, Ronald H, Petersen MJ. An intertrigo-like eruption from pegylated liposomal doxorubicin. J Drugs Dermatol. 2006;5:901-902.
5. Sánchez Henarejos P, Ros Martinez S, Marín Zafra GR,
et al. Intertrigo-like eruption caused by pegylated liposomal doxorubicin (PLD). Clin Transl Oncol. 2009;11:486-487.
6. von Moos R, Thuerlimann BJ, Aapro M, et al. Pegylated liposomal doxorubicin-associated hand-foot syndrome: recommendations of an international panel of experts [published online ahead of print March 10, 2008]. Eur J Cancer. 2008;44:781-790.