User login
Allergic Contact Dermatitis for Residents
Allergic contact dermatitis (ACD) is a common inflammatory skin condition that affects more than 14 million Americans each year.1 It has been estimated that the economic burden of ACD is nearly $3 billion per year due to school absences, work time lost, and medical expenditures.1,2 In fact, skin diseases rank second to traumatic injuries as the most common type of occupational disease.3 As dermatology residents, we will encounter many patients with ACD, a potentially debilitating skin condition. In this column, I will discuss the different types of ACD as well as their differential diagnoses and management options according to the American Academy of Allergy, Asthma & Immunology’s updated practice parameter for contact dermatitis.4 The 2015 American Contact Dermatitis Society (ACDS) Allergen of the Year and the ACDS’s Contact Allergen Management Program also will be discussed.
Clinical Presentation and Pathophysiology
Allergic contact dermatitis is a widespread skin condition characterized by erythematous and pruritic skin lesions that occur after contact with external stimuli.5 It is caused by a type IV, T cell–mediated, delayed hypersensitivity reaction in which a foreign substance comes into contact with the skin and forms an antigen complex that subsequently leads to sensitization. Upon reexposure to the antigen, the sensitized T cells induce an inflammatory cascade causing the skin changes associated with ACD. Clinical presentations of ACD include vesicles and bullae with distinct angles, lines, and borders.6
Differential Diagnosis
In contrast to ACD, irritant contact dermatitis (the more common form of contact dermatitis) is a non–immune-modulated skin reaction that occurs when an individual is exposed to a substance that causes irritation and damage to the keratinocytes.6,7 It can be an acute reaction to a household cleaning product or a chronic reaction to soap if the patient has had exposure to the product for a prolonged period of time.7 The clinical presentation of irritant contact dermatitis includes dry and fissured skin with less distinct borders and negative patch test results.6
Some other skin diseases that should be considered in the differential diagnosis for suspected ACD include atopic dermatitis, dyshidrotic eczema, inverse psoriasis, latex allergy, palmoplantar psoriasis, scabies, and tinea pedis.5 When ACD is suspected, our diagnostic approach as dermatology residents should be based on a combination of the following factors: the clinical features of the skin reaction (eg, morphology, location, symptoms), the patient’s history of exposure to an alleged allergen and lack of exposure after treatment and/or avoidance, patch test results, laboratory test results, and/or histopathologic examination to exclude other disorders with similar clinical features.8
Management
Localized acute lesions of ACD can be successfully treated with mid- or high-potency topical steroids such as triamcinolone 0.1% or clobetasol 0.05%. If an extensive area of the skin (>20%) is affected, systemic steroid therapy often is required, generally offering relief within 12 to 24 hours. Caution should be taken when prescribing oral prednisone, such as for poison ivy, as it should be tapered over a few weeks to prevent rebound dermatitis. If treatment fails and the diagnosis or specific allergen remains unknown, patch testing should be performed.3,5
Updated Practice Parameter
Practice parameters for contact dermatitis were updated in 2015, as commissioned by the Joint Task Force on Practice Parameters, to address recent advances in the field of contact dermatitis and the most recommended methods for diagnosis and management based on the current scientific literature.4 Prior to this update, the most recent recommendations were from 2006.3
Since the publication of the original practice parameter, new questions have been addressed related to emerging clinical problems such as preoperative screening and postimplantation patch testing for metal allergy in patients undergoing joint replacement surgery. In the updated practice parameter, statements have been added that more comprehensively address evaluation and management of occupational contact dermatitis.4 The potential benefits and limitations of drug patch testing in patients with maculopapular rashes, erythroderma, and nonimmediate cutaneous reactions also have been addressed. New summary statements have been included that make recommendations on the management of ACD, particularly avoidance and prevention.4
ACDS Allergen of the Year
The purpose of this “award” is to recognize the agents that cause the most remarkable clinical effects, those that draw less attention, or those that exhibit exposure patterns that have changed. The ACDS’s 2015 Allergen of the Year is formaldehyde, an inexpensive biocidal preservative used in a wide range of products such as tissue specimen and cadaveric preservation solutions, nail polish, hair-smoothing treatments, and wrinkle-free fabrics.9
Formaldehyde-releasing preservatives (FRPs) are among the leading contact allergens and are found in many personal hygiene products, medications, and household cleansers.8 Specific sources of FRPs include shampoos, bodywashes, hand soaps, lotions, creams, baby wipes, mascara, disinfectants, fabric softeners, topical wart remedies, adhesives, and tissue specimen preservation solutions.10-13 According to de Groot et al,14 the US Food and Drug Administration’s Voluntary Cosmetic Registration Program database has estimated that approximately 20% of personal hygiene products and cosmetics contain an FRP, with imidazolidinyl urea as the most common.
It is important for patients to be aware of sources of formaldehyde exposure and understand that many products containing formaldehyde or FRPs may not list this information on their labels. In fact, one study reported that 33% of 67 moisturizers evaluated did not have proper labeling with regard to their formaldehyde/FRP content.15
Contact Allergen Management Program
During medical school I served as the Dermatology Interest Group Contact Dermatitis Awareness Chair at the University of Texas Medical Branch (Galveston, Texas) and was fortunate to have attended the annual meeting of the ACDS where I learned about the ACDS Contact Allergen Management Program (CAMP), an online resource for dermatologists to access that provides patients a printout list of allergen and cross-reactivity information for more than 1200 products (http://www.contactderm.org/i4a/pages/indexcfm?pageID=3489). This information helps consumers to choose the right products based on their allergies.
Final Thoughts
A thorough review of a patient’s medical history and, if needed, skin patch testing can identify the responsible allergen and initiate an appropriate avoidance plan for the patient. With appropriate avoidance, patients can achieve resolution of their dermatitis and prevent further episodes to substantially improve their quality of life and decrease health care costs.1 If left untreated, ACD can evolve from an acute form to a subacute form and eventually chronic eczematous dermatitis or progression to systemic disease.16,17 Allergic contact dermatitis can negatively impact an individual’s health-related quality of life, particularly in social functioning and psychological well-being.18,19 Therefore, it is pertinent in our role as dermatology residents to recognize ACD before its progression to a chronic state.
1. Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
2. Jacob SE. The lanolin-wool wax alcohol update. The Dermatologist. February 2014;22. http://www.the-dermatologist.com/content/lanolin-wool-wax-alcohol-update. Accessed June 26, 2015.
3. Beltrani VS, Bernstein IL, Cohen DE, et al. Contact dermatitis: a practice parameter [published correction appears in Ann Allergy Asthma Immunol. 2006;97:819]. Ann Allergy Asthma Immunol. 2006;97(3, suppl 2):S1-S38.
4. Fonacier L, Bernstein DI, Pacheco K, et al. Contact dermatitis: a practice parameter—update 2015. J Allergy Clin Immunol Pract. 2015;3(suppl 3):S1-S39.
5. Usatine R, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
6. Usatine RP. Contact dermatitis. In: Usatine RP, Smith M, Mayeaux EJ Jr, et al, eds. Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009. http://accessmedicine.mhmedical.com/content.aspx?bookid=378&Sectionid=40419504. Accessed June 26, 2015.
7. Vazirnia A, Jacob SE. Review of ACDS’ allergen of the year 2010-2015. The Dermatologist. November 2014;22. http://www.the-dermatologist.com/content/review-acds%E2%80%99-allergen-od-year-2000-2015. Accessed June 26, 2015.
8. Yiannias J. Clinical features and diagnosis of allergic contact dermatitis. UpToDate Web site. http://www.uptodate.com/contents/clinical-features-and-diagnosis-of-allergic-contact-dermatitis?source=search_result&search=allergic+contact+dermatitis&selectedTitle=2~142#. Updated May 20, 2014. Accessed June 18, 2015.
9. Pontén A, Bruze M. Formaldehyde. Dermatitis. 2015;26:3-6.
10. Maier LE, Lampel HP, Bhutani T, et al. Hand dermatitis: a focus on allergic contact dermatitis to biocides. Dermatol Clin. 2009;27:251-264.
11. Marks JG, Elsner P, DeLeo VA. Contact & Occupational Dermatology. 3rd ed. St. Louis, MO: Mosby; 2002.
12. Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 6th ed. Hamilton, ON: BC Decker Inc; 2008.
13. Sasseville D. Hypersensitivity to preservatives. Dermatol Ther. 2004;17:251-263.
14. de Groot AC, White IR, Flyvholm MA, et al. Formaldehyde-releasers in cosmetics: relationship to formaldehyde contact allergy. part 1. characterization, frequency and relevance of sensitization, and frequency of use in cosmetics. Contact Dermatitis. 2010;62:2-17.
15. Rastogi SC. Analytical control of preservative labeling on skin creams. Contact Dermatitis. 2000;43:339-343.
16. Hsu JW, Matiz C, Jacob SE. Nickel allergy: localized, id, and systemic manifestations in children. Pediatr Dermatol. 2011;28:276-280.
17. Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
18. Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
19. Hutchings CV, Shum KW, Gawkrodger DJ. Occupational contact dermatitis has an appreciable impact on quality of life. Contact Dermatitis. 2001;45:17-20.
Allergic contact dermatitis (ACD) is a common inflammatory skin condition that affects more than 14 million Americans each year.1 It has been estimated that the economic burden of ACD is nearly $3 billion per year due to school absences, work time lost, and medical expenditures.1,2 In fact, skin diseases rank second to traumatic injuries as the most common type of occupational disease.3 As dermatology residents, we will encounter many patients with ACD, a potentially debilitating skin condition. In this column, I will discuss the different types of ACD as well as their differential diagnoses and management options according to the American Academy of Allergy, Asthma & Immunology’s updated practice parameter for contact dermatitis.4 The 2015 American Contact Dermatitis Society (ACDS) Allergen of the Year and the ACDS’s Contact Allergen Management Program also will be discussed.
Clinical Presentation and Pathophysiology
Allergic contact dermatitis is a widespread skin condition characterized by erythematous and pruritic skin lesions that occur after contact with external stimuli.5 It is caused by a type IV, T cell–mediated, delayed hypersensitivity reaction in which a foreign substance comes into contact with the skin and forms an antigen complex that subsequently leads to sensitization. Upon reexposure to the antigen, the sensitized T cells induce an inflammatory cascade causing the skin changes associated with ACD. Clinical presentations of ACD include vesicles and bullae with distinct angles, lines, and borders.6
Differential Diagnosis
In contrast to ACD, irritant contact dermatitis (the more common form of contact dermatitis) is a non–immune-modulated skin reaction that occurs when an individual is exposed to a substance that causes irritation and damage to the keratinocytes.6,7 It can be an acute reaction to a household cleaning product or a chronic reaction to soap if the patient has had exposure to the product for a prolonged period of time.7 The clinical presentation of irritant contact dermatitis includes dry and fissured skin with less distinct borders and negative patch test results.6
Some other skin diseases that should be considered in the differential diagnosis for suspected ACD include atopic dermatitis, dyshidrotic eczema, inverse psoriasis, latex allergy, palmoplantar psoriasis, scabies, and tinea pedis.5 When ACD is suspected, our diagnostic approach as dermatology residents should be based on a combination of the following factors: the clinical features of the skin reaction (eg, morphology, location, symptoms), the patient’s history of exposure to an alleged allergen and lack of exposure after treatment and/or avoidance, patch test results, laboratory test results, and/or histopathologic examination to exclude other disorders with similar clinical features.8
Management
Localized acute lesions of ACD can be successfully treated with mid- or high-potency topical steroids such as triamcinolone 0.1% or clobetasol 0.05%. If an extensive area of the skin (>20%) is affected, systemic steroid therapy often is required, generally offering relief within 12 to 24 hours. Caution should be taken when prescribing oral prednisone, such as for poison ivy, as it should be tapered over a few weeks to prevent rebound dermatitis. If treatment fails and the diagnosis or specific allergen remains unknown, patch testing should be performed.3,5
Updated Practice Parameter
Practice parameters for contact dermatitis were updated in 2015, as commissioned by the Joint Task Force on Practice Parameters, to address recent advances in the field of contact dermatitis and the most recommended methods for diagnosis and management based on the current scientific literature.4 Prior to this update, the most recent recommendations were from 2006.3
Since the publication of the original practice parameter, new questions have been addressed related to emerging clinical problems such as preoperative screening and postimplantation patch testing for metal allergy in patients undergoing joint replacement surgery. In the updated practice parameter, statements have been added that more comprehensively address evaluation and management of occupational contact dermatitis.4 The potential benefits and limitations of drug patch testing in patients with maculopapular rashes, erythroderma, and nonimmediate cutaneous reactions also have been addressed. New summary statements have been included that make recommendations on the management of ACD, particularly avoidance and prevention.4
ACDS Allergen of the Year
The purpose of this “award” is to recognize the agents that cause the most remarkable clinical effects, those that draw less attention, or those that exhibit exposure patterns that have changed. The ACDS’s 2015 Allergen of the Year is formaldehyde, an inexpensive biocidal preservative used in a wide range of products such as tissue specimen and cadaveric preservation solutions, nail polish, hair-smoothing treatments, and wrinkle-free fabrics.9
Formaldehyde-releasing preservatives (FRPs) are among the leading contact allergens and are found in many personal hygiene products, medications, and household cleansers.8 Specific sources of FRPs include shampoos, bodywashes, hand soaps, lotions, creams, baby wipes, mascara, disinfectants, fabric softeners, topical wart remedies, adhesives, and tissue specimen preservation solutions.10-13 According to de Groot et al,14 the US Food and Drug Administration’s Voluntary Cosmetic Registration Program database has estimated that approximately 20% of personal hygiene products and cosmetics contain an FRP, with imidazolidinyl urea as the most common.
It is important for patients to be aware of sources of formaldehyde exposure and understand that many products containing formaldehyde or FRPs may not list this information on their labels. In fact, one study reported that 33% of 67 moisturizers evaluated did not have proper labeling with regard to their formaldehyde/FRP content.15
Contact Allergen Management Program
During medical school I served as the Dermatology Interest Group Contact Dermatitis Awareness Chair at the University of Texas Medical Branch (Galveston, Texas) and was fortunate to have attended the annual meeting of the ACDS where I learned about the ACDS Contact Allergen Management Program (CAMP), an online resource for dermatologists to access that provides patients a printout list of allergen and cross-reactivity information for more than 1200 products (http://www.contactderm.org/i4a/pages/indexcfm?pageID=3489). This information helps consumers to choose the right products based on their allergies.
Final Thoughts
A thorough review of a patient’s medical history and, if needed, skin patch testing can identify the responsible allergen and initiate an appropriate avoidance plan for the patient. With appropriate avoidance, patients can achieve resolution of their dermatitis and prevent further episodes to substantially improve their quality of life and decrease health care costs.1 If left untreated, ACD can evolve from an acute form to a subacute form and eventually chronic eczematous dermatitis or progression to systemic disease.16,17 Allergic contact dermatitis can negatively impact an individual’s health-related quality of life, particularly in social functioning and psychological well-being.18,19 Therefore, it is pertinent in our role as dermatology residents to recognize ACD before its progression to a chronic state.
Allergic contact dermatitis (ACD) is a common inflammatory skin condition that affects more than 14 million Americans each year.1 It has been estimated that the economic burden of ACD is nearly $3 billion per year due to school absences, work time lost, and medical expenditures.1,2 In fact, skin diseases rank second to traumatic injuries as the most common type of occupational disease.3 As dermatology residents, we will encounter many patients with ACD, a potentially debilitating skin condition. In this column, I will discuss the different types of ACD as well as their differential diagnoses and management options according to the American Academy of Allergy, Asthma & Immunology’s updated practice parameter for contact dermatitis.4 The 2015 American Contact Dermatitis Society (ACDS) Allergen of the Year and the ACDS’s Contact Allergen Management Program also will be discussed.
Clinical Presentation and Pathophysiology
Allergic contact dermatitis is a widespread skin condition characterized by erythematous and pruritic skin lesions that occur after contact with external stimuli.5 It is caused by a type IV, T cell–mediated, delayed hypersensitivity reaction in which a foreign substance comes into contact with the skin and forms an antigen complex that subsequently leads to sensitization. Upon reexposure to the antigen, the sensitized T cells induce an inflammatory cascade causing the skin changes associated with ACD. Clinical presentations of ACD include vesicles and bullae with distinct angles, lines, and borders.6
Differential Diagnosis
In contrast to ACD, irritant contact dermatitis (the more common form of contact dermatitis) is a non–immune-modulated skin reaction that occurs when an individual is exposed to a substance that causes irritation and damage to the keratinocytes.6,7 It can be an acute reaction to a household cleaning product or a chronic reaction to soap if the patient has had exposure to the product for a prolonged period of time.7 The clinical presentation of irritant contact dermatitis includes dry and fissured skin with less distinct borders and negative patch test results.6
Some other skin diseases that should be considered in the differential diagnosis for suspected ACD include atopic dermatitis, dyshidrotic eczema, inverse psoriasis, latex allergy, palmoplantar psoriasis, scabies, and tinea pedis.5 When ACD is suspected, our diagnostic approach as dermatology residents should be based on a combination of the following factors: the clinical features of the skin reaction (eg, morphology, location, symptoms), the patient’s history of exposure to an alleged allergen and lack of exposure after treatment and/or avoidance, patch test results, laboratory test results, and/or histopathologic examination to exclude other disorders with similar clinical features.8
Management
Localized acute lesions of ACD can be successfully treated with mid- or high-potency topical steroids such as triamcinolone 0.1% or clobetasol 0.05%. If an extensive area of the skin (>20%) is affected, systemic steroid therapy often is required, generally offering relief within 12 to 24 hours. Caution should be taken when prescribing oral prednisone, such as for poison ivy, as it should be tapered over a few weeks to prevent rebound dermatitis. If treatment fails and the diagnosis or specific allergen remains unknown, patch testing should be performed.3,5
Updated Practice Parameter
Practice parameters for contact dermatitis were updated in 2015, as commissioned by the Joint Task Force on Practice Parameters, to address recent advances in the field of contact dermatitis and the most recommended methods for diagnosis and management based on the current scientific literature.4 Prior to this update, the most recent recommendations were from 2006.3
Since the publication of the original practice parameter, new questions have been addressed related to emerging clinical problems such as preoperative screening and postimplantation patch testing for metal allergy in patients undergoing joint replacement surgery. In the updated practice parameter, statements have been added that more comprehensively address evaluation and management of occupational contact dermatitis.4 The potential benefits and limitations of drug patch testing in patients with maculopapular rashes, erythroderma, and nonimmediate cutaneous reactions also have been addressed. New summary statements have been included that make recommendations on the management of ACD, particularly avoidance and prevention.4
ACDS Allergen of the Year
The purpose of this “award” is to recognize the agents that cause the most remarkable clinical effects, those that draw less attention, or those that exhibit exposure patterns that have changed. The ACDS’s 2015 Allergen of the Year is formaldehyde, an inexpensive biocidal preservative used in a wide range of products such as tissue specimen and cadaveric preservation solutions, nail polish, hair-smoothing treatments, and wrinkle-free fabrics.9
Formaldehyde-releasing preservatives (FRPs) are among the leading contact allergens and are found in many personal hygiene products, medications, and household cleansers.8 Specific sources of FRPs include shampoos, bodywashes, hand soaps, lotions, creams, baby wipes, mascara, disinfectants, fabric softeners, topical wart remedies, adhesives, and tissue specimen preservation solutions.10-13 According to de Groot et al,14 the US Food and Drug Administration’s Voluntary Cosmetic Registration Program database has estimated that approximately 20% of personal hygiene products and cosmetics contain an FRP, with imidazolidinyl urea as the most common.
It is important for patients to be aware of sources of formaldehyde exposure and understand that many products containing formaldehyde or FRPs may not list this information on their labels. In fact, one study reported that 33% of 67 moisturizers evaluated did not have proper labeling with regard to their formaldehyde/FRP content.15
Contact Allergen Management Program
During medical school I served as the Dermatology Interest Group Contact Dermatitis Awareness Chair at the University of Texas Medical Branch (Galveston, Texas) and was fortunate to have attended the annual meeting of the ACDS where I learned about the ACDS Contact Allergen Management Program (CAMP), an online resource for dermatologists to access that provides patients a printout list of allergen and cross-reactivity information for more than 1200 products (http://www.contactderm.org/i4a/pages/indexcfm?pageID=3489). This information helps consumers to choose the right products based on their allergies.
Final Thoughts
A thorough review of a patient’s medical history and, if needed, skin patch testing can identify the responsible allergen and initiate an appropriate avoidance plan for the patient. With appropriate avoidance, patients can achieve resolution of their dermatitis and prevent further episodes to substantially improve their quality of life and decrease health care costs.1 If left untreated, ACD can evolve from an acute form to a subacute form and eventually chronic eczematous dermatitis or progression to systemic disease.16,17 Allergic contact dermatitis can negatively impact an individual’s health-related quality of life, particularly in social functioning and psychological well-being.18,19 Therefore, it is pertinent in our role as dermatology residents to recognize ACD before its progression to a chronic state.
1. Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
2. Jacob SE. The lanolin-wool wax alcohol update. The Dermatologist. February 2014;22. http://www.the-dermatologist.com/content/lanolin-wool-wax-alcohol-update. Accessed June 26, 2015.
3. Beltrani VS, Bernstein IL, Cohen DE, et al. Contact dermatitis: a practice parameter [published correction appears in Ann Allergy Asthma Immunol. 2006;97:819]. Ann Allergy Asthma Immunol. 2006;97(3, suppl 2):S1-S38.
4. Fonacier L, Bernstein DI, Pacheco K, et al. Contact dermatitis: a practice parameter—update 2015. J Allergy Clin Immunol Pract. 2015;3(suppl 3):S1-S39.
5. Usatine R, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
6. Usatine RP. Contact dermatitis. In: Usatine RP, Smith M, Mayeaux EJ Jr, et al, eds. Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009. http://accessmedicine.mhmedical.com/content.aspx?bookid=378&Sectionid=40419504. Accessed June 26, 2015.
7. Vazirnia A, Jacob SE. Review of ACDS’ allergen of the year 2010-2015. The Dermatologist. November 2014;22. http://www.the-dermatologist.com/content/review-acds%E2%80%99-allergen-od-year-2000-2015. Accessed June 26, 2015.
8. Yiannias J. Clinical features and diagnosis of allergic contact dermatitis. UpToDate Web site. http://www.uptodate.com/contents/clinical-features-and-diagnosis-of-allergic-contact-dermatitis?source=search_result&search=allergic+contact+dermatitis&selectedTitle=2~142#. Updated May 20, 2014. Accessed June 18, 2015.
9. Pontén A, Bruze M. Formaldehyde. Dermatitis. 2015;26:3-6.
10. Maier LE, Lampel HP, Bhutani T, et al. Hand dermatitis: a focus on allergic contact dermatitis to biocides. Dermatol Clin. 2009;27:251-264.
11. Marks JG, Elsner P, DeLeo VA. Contact & Occupational Dermatology. 3rd ed. St. Louis, MO: Mosby; 2002.
12. Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 6th ed. Hamilton, ON: BC Decker Inc; 2008.
13. Sasseville D. Hypersensitivity to preservatives. Dermatol Ther. 2004;17:251-263.
14. de Groot AC, White IR, Flyvholm MA, et al. Formaldehyde-releasers in cosmetics: relationship to formaldehyde contact allergy. part 1. characterization, frequency and relevance of sensitization, and frequency of use in cosmetics. Contact Dermatitis. 2010;62:2-17.
15. Rastogi SC. Analytical control of preservative labeling on skin creams. Contact Dermatitis. 2000;43:339-343.
16. Hsu JW, Matiz C, Jacob SE. Nickel allergy: localized, id, and systemic manifestations in children. Pediatr Dermatol. 2011;28:276-280.
17. Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
18. Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
19. Hutchings CV, Shum KW, Gawkrodger DJ. Occupational contact dermatitis has an appreciable impact on quality of life. Contact Dermatitis. 2001;45:17-20.
1. Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
2. Jacob SE. The lanolin-wool wax alcohol update. The Dermatologist. February 2014;22. http://www.the-dermatologist.com/content/lanolin-wool-wax-alcohol-update. Accessed June 26, 2015.
3. Beltrani VS, Bernstein IL, Cohen DE, et al. Contact dermatitis: a practice parameter [published correction appears in Ann Allergy Asthma Immunol. 2006;97:819]. Ann Allergy Asthma Immunol. 2006;97(3, suppl 2):S1-S38.
4. Fonacier L, Bernstein DI, Pacheco K, et al. Contact dermatitis: a practice parameter—update 2015. J Allergy Clin Immunol Pract. 2015;3(suppl 3):S1-S39.
5. Usatine R, Riojas M. Diagnosis and management of contact dermatitis. Am Fam Physician. 2010;82:249-255.
6. Usatine RP. Contact dermatitis. In: Usatine RP, Smith M, Mayeaux EJ Jr, et al, eds. Color Atlas of Family Medicine. New York, NY: McGraw-Hill; 2009. http://accessmedicine.mhmedical.com/content.aspx?bookid=378&Sectionid=40419504. Accessed June 26, 2015.
7. Vazirnia A, Jacob SE. Review of ACDS’ allergen of the year 2010-2015. The Dermatologist. November 2014;22. http://www.the-dermatologist.com/content/review-acds%E2%80%99-allergen-od-year-2000-2015. Accessed June 26, 2015.
8. Yiannias J. Clinical features and diagnosis of allergic contact dermatitis. UpToDate Web site. http://www.uptodate.com/contents/clinical-features-and-diagnosis-of-allergic-contact-dermatitis?source=search_result&search=allergic+contact+dermatitis&selectedTitle=2~142#. Updated May 20, 2014. Accessed June 18, 2015.
9. Pontén A, Bruze M. Formaldehyde. Dermatitis. 2015;26:3-6.
10. Maier LE, Lampel HP, Bhutani T, et al. Hand dermatitis: a focus on allergic contact dermatitis to biocides. Dermatol Clin. 2009;27:251-264.
11. Marks JG, Elsner P, DeLeo VA. Contact & Occupational Dermatology. 3rd ed. St. Louis, MO: Mosby; 2002.
12. Rietschel RL, Fowler JF Jr, eds. Fisher’s Contact Dermatitis. 6th ed. Hamilton, ON: BC Decker Inc; 2008.
13. Sasseville D. Hypersensitivity to preservatives. Dermatol Ther. 2004;17:251-263.
14. de Groot AC, White IR, Flyvholm MA, et al. Formaldehyde-releasers in cosmetics: relationship to formaldehyde contact allergy. part 1. characterization, frequency and relevance of sensitization, and frequency of use in cosmetics. Contact Dermatitis. 2010;62:2-17.
15. Rastogi SC. Analytical control of preservative labeling on skin creams. Contact Dermatitis. 2000;43:339-343.
16. Hsu JW, Matiz C, Jacob SE. Nickel allergy: localized, id, and systemic manifestations in children. Pediatr Dermatol. 2011;28:276-280.
17. Salam TN, Fowler JF Jr. Balsam-related systemic contact dermatitis. J Am Acad Dermatol. 2001;45:377-381.
18. Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158.
19. Hutchings CV, Shum KW, Gawkrodger DJ. Occupational contact dermatitis has an appreciable impact on quality of life. Contact Dermatitis. 2001;45:17-20.
Nephrogenic Systemic Fibrosis Following Gadolinium Administration
To the Editor:
Nephrogenic systemic fibrosis (NSF) is an emerging medical entity in patients with renal disease, which results in progressive cutaneous and systemic fibrosis. It is a rare disorder that has been recognized in patients with renal impairment since 2000.1 Patients with NSF demonstrate symmetric dermal and subcutaneous fibrosis evidenced by increasing skin induration on clinical examination. Nephrogenic systemic fibrosis most commonly involves the lower extremities, and after extending to the upper extremities and trunk, it sporadically involves the head and neck.
The clinical manifestation of NSF begins with edema, followed by marked dermal induration, sclerotic plaques, and joint contractures that can lead to considerable disability. Pathogenesis remains to be elucidated; it has been hypothesized that it could be related to gadolinium (Gd). Currently, there is no treatment of this unremitting disease.1-3 We report the case of a patient affected by NSF after administration of Gd for magnetic resonance angiography.
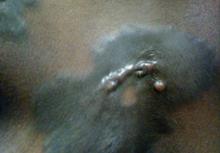
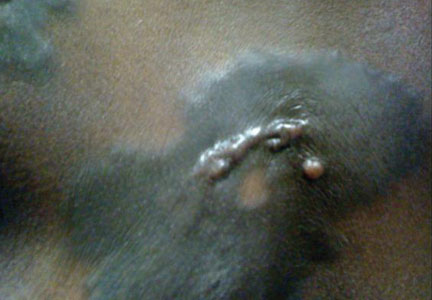
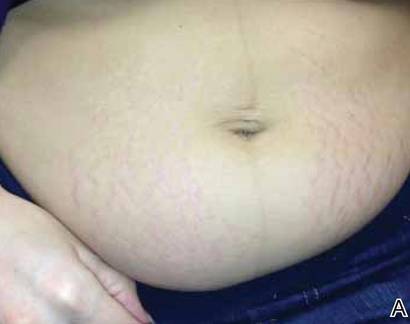
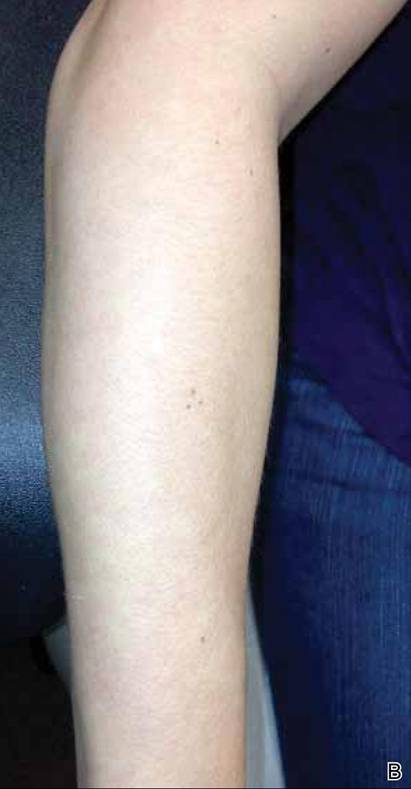
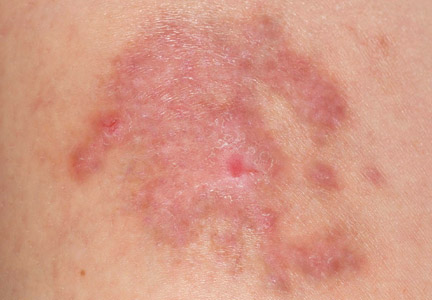
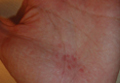
A 56-year-old woman was referred to the department of dermatology at the University of Maryland (Baltimore, Maryland) with persistent swelling of the lower legs, forearms, and trunk of 5 months’ duration. She had end-stage renal disease of nonspecific origin. Five months prior to presentation, she had magnetic resonance angiography, during which 10 mmol of Gd was administered. After, she developed a persistent rash and swelling of the lower legs. On presentation, physical examination revealed symmetric, shiny, pigmented papules and plaques on the forearms, buttocks, thighs, and legs, with no facial involvement (Figure).
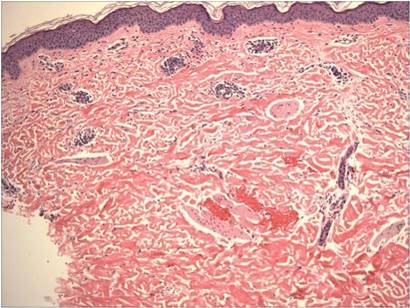
A skin biopsy of thigh lesions showed a diffuse dermal proliferation of bland spindle cells associated with dermal fibrosis. A CD4+ cellular infiltrate showed extension into the subcutaneous tissue. Deposition of Gd also was noted in the skin. She was treated with corticosteroid therapy, and after 2 months she reported softening of the affected skin. On 6-month follow-up, her skin lesions did not progress and there was no evidence of systemic involvement. Additionally, renal function had improved.
Nephrogenic systemic fibrosis, also known as nephrogenic fibrosing dermopathy, was first described by Cowper et al1 in 2000. Since then, more than 215 cases have been reported in the literature. Clinically, it is characterized by acute onset of cutaneous hardening and thickening of the extremities and the trunk, often resulting in flexion contractures. There may be varying surface changes such as pigmentation, peau d’orange texture, and shiny sclerosis. Patients often experience unpleasant symptoms such as pain, pruritus, stiffness, and paresthesia. Systemic involvement has been documented in the heart, lung, tendons, muscle, testes, and lamina dura.1-4
Histologic findings of NSF are diffuse dermal fibrosis with increased cellular infiltrates comprised of bland spindled fibrocytes. These fibrocytes express CD34 and type I procollagen. Collagen bundles are thickened but retain clefting, and elastic fibers often are prominent. This fibrotic pattern typically extends to the subcutaneous fat septa, which are widened and collagenous. The epidermis generally is uninvolved. Other findings include dermal mucin deposition, calcification of collagen and vessels, increased CD68+ histiocytes, increased factor XIIIa and dendritic cells, and neoangiogenesis. Rarely, multinucleated giant cells and Miescher radial granulomas with lymphocytic aggregates mimicking erythema nodosum have been described.2-4
Dermatologic entities with similar clinical and histopathologic features, including scleroderma, scleromyxedema, lipodermatosclerosis, erythema nodosum, eosinophilic fasciitis, and spindle cell neoplasms, should be excluded.1-4 The exact pathogenetic mechanisms of NSF have yet to be determined, but there is strong evidence that Gd plays an important causative role.1 In fact, almost all patients with NSF have been exposed to Gd. Gadolinium has been documented in affected skin of patients with NSF and has been shown to induce NSF-like changes in rat models.
Other clinical factors that have been associated with NSF include erythropoietin, elevated serum calcium and phosphate levels, vascular injury or surgery, iron metabolic abnormalities, and metabolic acidosis. It is likely that many factors in the unique physiologic state of patients with renal failure contribute to the abnormal fibrotic reaction to Gd-containing contrast agents in NSF. Gadolinium is a member of a group of 15 elemental metals termed lanthanoids and has been used extensively worldwide in magnetic resonance imaging as a component of intravenously administered contrast agents. Currently, 6 such agents are approved for use in the United States: gadopentetate dimeglumine, gadoteridol, gadodiamide, gadoversetamide, gadobenate dimeglumine, and gadoxetate sodium. All are chelated Gd products, with the chelate serving to prevent toxicity from free Gd ions.
In patients with no renal function abnormalities, the biologic half-life of Gd-based magnetic resonance contrast agents (GBCAs) is 1.5 to 2.0 hours. However, in patients with abnormal kidney function, this half-life is inversely prolonged, proportional to the glomerular filtration rate.5-7 The link between GBCA administration and NSF is compelling, though other etiologic associations have been reported. Surgical or vascular procedures, history of a hypercoagulable state, erythropoietin administration, and immune suppression have been proposed as triggering factors in NSF. The proposed mechanisms responsible for fibrosis in NSF have centered on a collagen-producing cell in the peripheral blood termed the circulating fibrocyte. These cells express CD34 and CD45RO antigens and are capable of producing type I collagen.
Circulating fibrocytes traffic to areas of chronic antigenic stimulation promoting wound repair and fibrotic reactions. Some authors have proposed that materials deposited in the skin might serve as targets for circulating fibrocytes.8 Circulating fibrocytes also are known to produce inflammatory cytokines including IL-1 and chemokines such as platelet-derived growth factor, transforming growth factor b, and others capable of propagating fibrotic responses. Increased expression of transforming growth factor has been reported in dendritic cells in NSF lesions and Parsons et al9 postulated that transglutaminase-2 activation of this protein may be responsible for inciting fibrosis in NSF. Transglutaminases also are known to be directly activated by Gd.10,11
Transmetalation has been proposed as a possible operative phenomenon responsible for NSF. Several cations including zinc, copper, iron, and carbon are known to compete with Gd and may displace it from the ligand, with anions such as OHe, PO4 3e, and CO3 2e binding the resultant free Gd. Some GBCAs contain excess ligand to diminish potential free Gd concentrations. In fact, substantial elevations of serum calcium and phosphorus in patients with NSF have been noted in a large series of patients with NSF. Calciphylaxis, an often catastrophic condition arising in patients with renal failure, has been described in association with NSF, and sodium thiosulfate has been used with success in treating both conditions.10 In addition, Sanyal et al12 noted a substantially higher serum calcium in NSF cases compared with controls.
Gadolinium plays an important role in the pathology of NSF and is confirmed by the presence of Gd in skin biopsies.
1. Cowper SE, Robin HS, Steinberg SM, et al. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000-1001.
2. Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathologiocal definition and workup recommendations [published online ahead of print July 2, 2011]. J Am Acad Dermatol. 2011;65:1095-1106.
3. Gupta A, Shamseddin MK, Khaira A. Pathomechanisms of nephrogenic systemic fibrosis: new insights [published online ahead of print July 25, 2011]. Clin Exp Dermatol. 2011;36:763-768.
4. Zou Z, Ma L. Nephrogenic systemic fibrosis: review of 408 biopsy-confirmed cases. Indian J Dermatol. 2011;56:65-73.
5. Pan D, Schmieder AH, Wickline SA, et al. Manganese-based MRI contrast agents: past, present and future. Tetrahedron. 2011;67:8431-8444.
6. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18:188-198.
7. Wang Y, Alkasab TK, Narin O, et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelines [published online ahead of print May 17, 2011]. Radiology. 2011;260:105-111.
8. Ortonne N, Lipsker D, Chantrel F, et al. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150:1050-1052.
9. Parsons AC, Yosipovitch G, Sheehan DJ, et al. Transglutaminases: the missing link in nephrogenic systemic fibrosis. Am J Dermatopathol. 2007;29:433-436.
10. Wahba IM, Simpson EL, White K. Gadolinium is not the only trigger for nephrogenic systemic fibrosis: insights from two cases and review of the recent literature [published online ahead of print August 16, 2007]. Am J Transplant. 2007;7:2425-2432.
11. Goveia M, Chan BP, Patel PR. Evaluating the role of recombinant erythropoietin in nephrogenic systemic fibrosis [published online ahead of print August 8, 2007]. J Am Acad Dermatol. 2007;57:725-727.
12. Sanyal S, Marckmann P, Scherer S, et al. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis–an autopsy-based review [published online ahead of print March 25, 2011]. Nephrol Dial Transplant. 2011;26:3616-3626.
To the Editor:
Nephrogenic systemic fibrosis (NSF) is an emerging medical entity in patients with renal disease, which results in progressive cutaneous and systemic fibrosis. It is a rare disorder that has been recognized in patients with renal impairment since 2000.1 Patients with NSF demonstrate symmetric dermal and subcutaneous fibrosis evidenced by increasing skin induration on clinical examination. Nephrogenic systemic fibrosis most commonly involves the lower extremities, and after extending to the upper extremities and trunk, it sporadically involves the head and neck.
The clinical manifestation of NSF begins with edema, followed by marked dermal induration, sclerotic plaques, and joint contractures that can lead to considerable disability. Pathogenesis remains to be elucidated; it has been hypothesized that it could be related to gadolinium (Gd). Currently, there is no treatment of this unremitting disease.1-3 We report the case of a patient affected by NSF after administration of Gd for magnetic resonance angiography.
A 56-year-old woman was referred to the department of dermatology at the University of Maryland (Baltimore, Maryland) with persistent swelling of the lower legs, forearms, and trunk of 5 months’ duration. She had end-stage renal disease of nonspecific origin. Five months prior to presentation, she had magnetic resonance angiography, during which 10 mmol of Gd was administered. After, she developed a persistent rash and swelling of the lower legs. On presentation, physical examination revealed symmetric, shiny, pigmented papules and plaques on the forearms, buttocks, thighs, and legs, with no facial involvement (Figure).
A skin biopsy of thigh lesions showed a diffuse dermal proliferation of bland spindle cells associated with dermal fibrosis. A CD4+ cellular infiltrate showed extension into the subcutaneous tissue. Deposition of Gd also was noted in the skin. She was treated with corticosteroid therapy, and after 2 months she reported softening of the affected skin. On 6-month follow-up, her skin lesions did not progress and there was no evidence of systemic involvement. Additionally, renal function had improved.
Nephrogenic systemic fibrosis, also known as nephrogenic fibrosing dermopathy, was first described by Cowper et al1 in 2000. Since then, more than 215 cases have been reported in the literature. Clinically, it is characterized by acute onset of cutaneous hardening and thickening of the extremities and the trunk, often resulting in flexion contractures. There may be varying surface changes such as pigmentation, peau d’orange texture, and shiny sclerosis. Patients often experience unpleasant symptoms such as pain, pruritus, stiffness, and paresthesia. Systemic involvement has been documented in the heart, lung, tendons, muscle, testes, and lamina dura.1-4
Histologic findings of NSF are diffuse dermal fibrosis with increased cellular infiltrates comprised of bland spindled fibrocytes. These fibrocytes express CD34 and type I procollagen. Collagen bundles are thickened but retain clefting, and elastic fibers often are prominent. This fibrotic pattern typically extends to the subcutaneous fat septa, which are widened and collagenous. The epidermis generally is uninvolved. Other findings include dermal mucin deposition, calcification of collagen and vessels, increased CD68+ histiocytes, increased factor XIIIa and dendritic cells, and neoangiogenesis. Rarely, multinucleated giant cells and Miescher radial granulomas with lymphocytic aggregates mimicking erythema nodosum have been described.2-4
Dermatologic entities with similar clinical and histopathologic features, including scleroderma, scleromyxedema, lipodermatosclerosis, erythema nodosum, eosinophilic fasciitis, and spindle cell neoplasms, should be excluded.1-4 The exact pathogenetic mechanisms of NSF have yet to be determined, but there is strong evidence that Gd plays an important causative role.1 In fact, almost all patients with NSF have been exposed to Gd. Gadolinium has been documented in affected skin of patients with NSF and has been shown to induce NSF-like changes in rat models.
Other clinical factors that have been associated with NSF include erythropoietin, elevated serum calcium and phosphate levels, vascular injury or surgery, iron metabolic abnormalities, and metabolic acidosis. It is likely that many factors in the unique physiologic state of patients with renal failure contribute to the abnormal fibrotic reaction to Gd-containing contrast agents in NSF. Gadolinium is a member of a group of 15 elemental metals termed lanthanoids and has been used extensively worldwide in magnetic resonance imaging as a component of intravenously administered contrast agents. Currently, 6 such agents are approved for use in the United States: gadopentetate dimeglumine, gadoteridol, gadodiamide, gadoversetamide, gadobenate dimeglumine, and gadoxetate sodium. All are chelated Gd products, with the chelate serving to prevent toxicity from free Gd ions.
In patients with no renal function abnormalities, the biologic half-life of Gd-based magnetic resonance contrast agents (GBCAs) is 1.5 to 2.0 hours. However, in patients with abnormal kidney function, this half-life is inversely prolonged, proportional to the glomerular filtration rate.5-7 The link between GBCA administration and NSF is compelling, though other etiologic associations have been reported. Surgical or vascular procedures, history of a hypercoagulable state, erythropoietin administration, and immune suppression have been proposed as triggering factors in NSF. The proposed mechanisms responsible for fibrosis in NSF have centered on a collagen-producing cell in the peripheral blood termed the circulating fibrocyte. These cells express CD34 and CD45RO antigens and are capable of producing type I collagen.
Circulating fibrocytes traffic to areas of chronic antigenic stimulation promoting wound repair and fibrotic reactions. Some authors have proposed that materials deposited in the skin might serve as targets for circulating fibrocytes.8 Circulating fibrocytes also are known to produce inflammatory cytokines including IL-1 and chemokines such as platelet-derived growth factor, transforming growth factor b, and others capable of propagating fibrotic responses. Increased expression of transforming growth factor has been reported in dendritic cells in NSF lesions and Parsons et al9 postulated that transglutaminase-2 activation of this protein may be responsible for inciting fibrosis in NSF. Transglutaminases also are known to be directly activated by Gd.10,11
Transmetalation has been proposed as a possible operative phenomenon responsible for NSF. Several cations including zinc, copper, iron, and carbon are known to compete with Gd and may displace it from the ligand, with anions such as OHe, PO4 3e, and CO3 2e binding the resultant free Gd. Some GBCAs contain excess ligand to diminish potential free Gd concentrations. In fact, substantial elevations of serum calcium and phosphorus in patients with NSF have been noted in a large series of patients with NSF. Calciphylaxis, an often catastrophic condition arising in patients with renal failure, has been described in association with NSF, and sodium thiosulfate has been used with success in treating both conditions.10 In addition, Sanyal et al12 noted a substantially higher serum calcium in NSF cases compared with controls.
Gadolinium plays an important role in the pathology of NSF and is confirmed by the presence of Gd in skin biopsies.
To the Editor:
Nephrogenic systemic fibrosis (NSF) is an emerging medical entity in patients with renal disease, which results in progressive cutaneous and systemic fibrosis. It is a rare disorder that has been recognized in patients with renal impairment since 2000.1 Patients with NSF demonstrate symmetric dermal and subcutaneous fibrosis evidenced by increasing skin induration on clinical examination. Nephrogenic systemic fibrosis most commonly involves the lower extremities, and after extending to the upper extremities and trunk, it sporadically involves the head and neck.
The clinical manifestation of NSF begins with edema, followed by marked dermal induration, sclerotic plaques, and joint contractures that can lead to considerable disability. Pathogenesis remains to be elucidated; it has been hypothesized that it could be related to gadolinium (Gd). Currently, there is no treatment of this unremitting disease.1-3 We report the case of a patient affected by NSF after administration of Gd for magnetic resonance angiography.
A 56-year-old woman was referred to the department of dermatology at the University of Maryland (Baltimore, Maryland) with persistent swelling of the lower legs, forearms, and trunk of 5 months’ duration. She had end-stage renal disease of nonspecific origin. Five months prior to presentation, she had magnetic resonance angiography, during which 10 mmol of Gd was administered. After, she developed a persistent rash and swelling of the lower legs. On presentation, physical examination revealed symmetric, shiny, pigmented papules and plaques on the forearms, buttocks, thighs, and legs, with no facial involvement (Figure).
A skin biopsy of thigh lesions showed a diffuse dermal proliferation of bland spindle cells associated with dermal fibrosis. A CD4+ cellular infiltrate showed extension into the subcutaneous tissue. Deposition of Gd also was noted in the skin. She was treated with corticosteroid therapy, and after 2 months she reported softening of the affected skin. On 6-month follow-up, her skin lesions did not progress and there was no evidence of systemic involvement. Additionally, renal function had improved.
Nephrogenic systemic fibrosis, also known as nephrogenic fibrosing dermopathy, was first described by Cowper et al1 in 2000. Since then, more than 215 cases have been reported in the literature. Clinically, it is characterized by acute onset of cutaneous hardening and thickening of the extremities and the trunk, often resulting in flexion contractures. There may be varying surface changes such as pigmentation, peau d’orange texture, and shiny sclerosis. Patients often experience unpleasant symptoms such as pain, pruritus, stiffness, and paresthesia. Systemic involvement has been documented in the heart, lung, tendons, muscle, testes, and lamina dura.1-4
Histologic findings of NSF are diffuse dermal fibrosis with increased cellular infiltrates comprised of bland spindled fibrocytes. These fibrocytes express CD34 and type I procollagen. Collagen bundles are thickened but retain clefting, and elastic fibers often are prominent. This fibrotic pattern typically extends to the subcutaneous fat septa, which are widened and collagenous. The epidermis generally is uninvolved. Other findings include dermal mucin deposition, calcification of collagen and vessels, increased CD68+ histiocytes, increased factor XIIIa and dendritic cells, and neoangiogenesis. Rarely, multinucleated giant cells and Miescher radial granulomas with lymphocytic aggregates mimicking erythema nodosum have been described.2-4
Dermatologic entities with similar clinical and histopathologic features, including scleroderma, scleromyxedema, lipodermatosclerosis, erythema nodosum, eosinophilic fasciitis, and spindle cell neoplasms, should be excluded.1-4 The exact pathogenetic mechanisms of NSF have yet to be determined, but there is strong evidence that Gd plays an important causative role.1 In fact, almost all patients with NSF have been exposed to Gd. Gadolinium has been documented in affected skin of patients with NSF and has been shown to induce NSF-like changes in rat models.
Other clinical factors that have been associated with NSF include erythropoietin, elevated serum calcium and phosphate levels, vascular injury or surgery, iron metabolic abnormalities, and metabolic acidosis. It is likely that many factors in the unique physiologic state of patients with renal failure contribute to the abnormal fibrotic reaction to Gd-containing contrast agents in NSF. Gadolinium is a member of a group of 15 elemental metals termed lanthanoids and has been used extensively worldwide in magnetic resonance imaging as a component of intravenously administered contrast agents. Currently, 6 such agents are approved for use in the United States: gadopentetate dimeglumine, gadoteridol, gadodiamide, gadoversetamide, gadobenate dimeglumine, and gadoxetate sodium. All are chelated Gd products, with the chelate serving to prevent toxicity from free Gd ions.
In patients with no renal function abnormalities, the biologic half-life of Gd-based magnetic resonance contrast agents (GBCAs) is 1.5 to 2.0 hours. However, in patients with abnormal kidney function, this half-life is inversely prolonged, proportional to the glomerular filtration rate.5-7 The link between GBCA administration and NSF is compelling, though other etiologic associations have been reported. Surgical or vascular procedures, history of a hypercoagulable state, erythropoietin administration, and immune suppression have been proposed as triggering factors in NSF. The proposed mechanisms responsible for fibrosis in NSF have centered on a collagen-producing cell in the peripheral blood termed the circulating fibrocyte. These cells express CD34 and CD45RO antigens and are capable of producing type I collagen.
Circulating fibrocytes traffic to areas of chronic antigenic stimulation promoting wound repair and fibrotic reactions. Some authors have proposed that materials deposited in the skin might serve as targets for circulating fibrocytes.8 Circulating fibrocytes also are known to produce inflammatory cytokines including IL-1 and chemokines such as platelet-derived growth factor, transforming growth factor b, and others capable of propagating fibrotic responses. Increased expression of transforming growth factor has been reported in dendritic cells in NSF lesions and Parsons et al9 postulated that transglutaminase-2 activation of this protein may be responsible for inciting fibrosis in NSF. Transglutaminases also are known to be directly activated by Gd.10,11
Transmetalation has been proposed as a possible operative phenomenon responsible for NSF. Several cations including zinc, copper, iron, and carbon are known to compete with Gd and may displace it from the ligand, with anions such as OHe, PO4 3e, and CO3 2e binding the resultant free Gd. Some GBCAs contain excess ligand to diminish potential free Gd concentrations. In fact, substantial elevations of serum calcium and phosphorus in patients with NSF have been noted in a large series of patients with NSF. Calciphylaxis, an often catastrophic condition arising in patients with renal failure, has been described in association with NSF, and sodium thiosulfate has been used with success in treating both conditions.10 In addition, Sanyal et al12 noted a substantially higher serum calcium in NSF cases compared with controls.
Gadolinium plays an important role in the pathology of NSF and is confirmed by the presence of Gd in skin biopsies.
1. Cowper SE, Robin HS, Steinberg SM, et al. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000-1001.
2. Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathologiocal definition and workup recommendations [published online ahead of print July 2, 2011]. J Am Acad Dermatol. 2011;65:1095-1106.
3. Gupta A, Shamseddin MK, Khaira A. Pathomechanisms of nephrogenic systemic fibrosis: new insights [published online ahead of print July 25, 2011]. Clin Exp Dermatol. 2011;36:763-768.
4. Zou Z, Ma L. Nephrogenic systemic fibrosis: review of 408 biopsy-confirmed cases. Indian J Dermatol. 2011;56:65-73.
5. Pan D, Schmieder AH, Wickline SA, et al. Manganese-based MRI contrast agents: past, present and future. Tetrahedron. 2011;67:8431-8444.
6. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18:188-198.
7. Wang Y, Alkasab TK, Narin O, et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelines [published online ahead of print May 17, 2011]. Radiology. 2011;260:105-111.
8. Ortonne N, Lipsker D, Chantrel F, et al. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150:1050-1052.
9. Parsons AC, Yosipovitch G, Sheehan DJ, et al. Transglutaminases: the missing link in nephrogenic systemic fibrosis. Am J Dermatopathol. 2007;29:433-436.
10. Wahba IM, Simpson EL, White K. Gadolinium is not the only trigger for nephrogenic systemic fibrosis: insights from two cases and review of the recent literature [published online ahead of print August 16, 2007]. Am J Transplant. 2007;7:2425-2432.
11. Goveia M, Chan BP, Patel PR. Evaluating the role of recombinant erythropoietin in nephrogenic systemic fibrosis [published online ahead of print August 8, 2007]. J Am Acad Dermatol. 2007;57:725-727.
12. Sanyal S, Marckmann P, Scherer S, et al. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis–an autopsy-based review [published online ahead of print March 25, 2011]. Nephrol Dial Transplant. 2011;26:3616-3626.
1. Cowper SE, Robin HS, Steinberg SM, et al. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000-1001.
2. Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathologiocal definition and workup recommendations [published online ahead of print July 2, 2011]. J Am Acad Dermatol. 2011;65:1095-1106.
3. Gupta A, Shamseddin MK, Khaira A. Pathomechanisms of nephrogenic systemic fibrosis: new insights [published online ahead of print July 25, 2011]. Clin Exp Dermatol. 2011;36:763-768.
4. Zou Z, Ma L. Nephrogenic systemic fibrosis: review of 408 biopsy-confirmed cases. Indian J Dermatol. 2011;56:65-73.
5. Pan D, Schmieder AH, Wickline SA, et al. Manganese-based MRI contrast agents: past, present and future. Tetrahedron. 2011;67:8431-8444.
6. Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18:188-198.
7. Wang Y, Alkasab TK, Narin O, et al. Incidence of nephrogenic systemic fibrosis after adoption of restrictive gadolinium-based contrast agent guidelines [published online ahead of print May 17, 2011]. Radiology. 2011;260:105-111.
8. Ortonne N, Lipsker D, Chantrel F, et al. Presence of CD45RO+ CD34+ cells with collagen synthesis activity in nephrogenic fibrosing dermopathy: a new pathogenic hypothesis. Br J Dermatol. 2004;150:1050-1052.
9. Parsons AC, Yosipovitch G, Sheehan DJ, et al. Transglutaminases: the missing link in nephrogenic systemic fibrosis. Am J Dermatopathol. 2007;29:433-436.
10. Wahba IM, Simpson EL, White K. Gadolinium is not the only trigger for nephrogenic systemic fibrosis: insights from two cases and review of the recent literature [published online ahead of print August 16, 2007]. Am J Transplant. 2007;7:2425-2432.
11. Goveia M, Chan BP, Patel PR. Evaluating the role of recombinant erythropoietin in nephrogenic systemic fibrosis [published online ahead of print August 8, 2007]. J Am Acad Dermatol. 2007;57:725-727.
12. Sanyal S, Marckmann P, Scherer S, et al. Multiorgan gadolinium (Gd) deposition and fibrosis in a patient with nephrogenic systemic fibrosis–an autopsy-based review [published online ahead of print March 25, 2011]. Nephrol Dial Transplant. 2011;26:3616-3626.
Cutaneous Infection With Mycobacterium kansasii in a Patient With Myelodysplastic Syndrome and Sweet Syndrome
To the Editor:
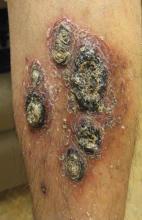
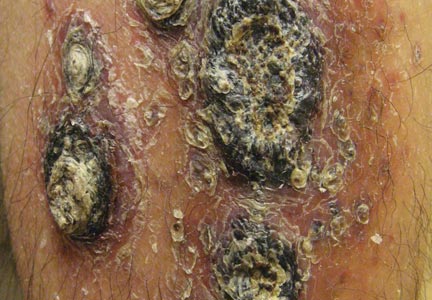
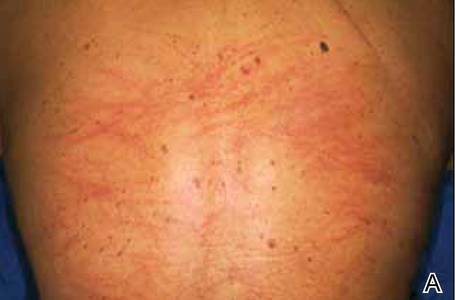
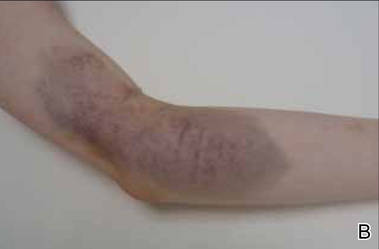
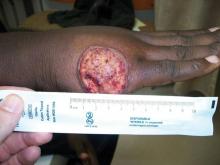
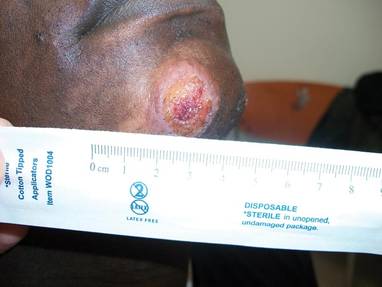
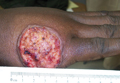
A 68-year-old man with a history of myelodysplastic syndrome and recurrent Sweet syndrome presented with left leg lesions of 3 months’ duration. The lesions originated as a solitary nodule on the left calf and subsequently developed into multiple nonpainful, nonpruritic, erythematous plaques of varying sizes with violaceous coloration and overlying necrotic eschar, occupying the entire anterior aspect of the left lower leg and left popliteal fossa (Figure). The patient denied any trauma or associated symptoms but had a history of Sweet syndrome that manifested as lesions on the arms and legs for which he took 6 mg of prednisone daily to prevent recurrence.
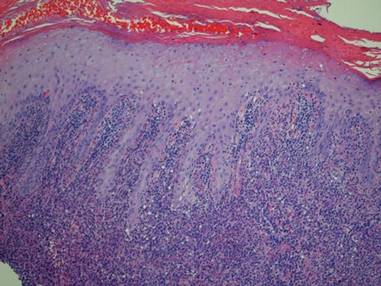
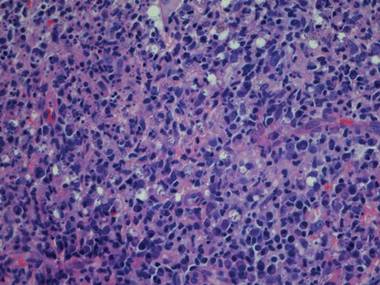
Histologic examination revealed nodular and diffuse chronic granulomatous and acute inflammatory infiltrate. Stains for bacteria, fungi, and acid-fast bacilli were negative. Cultures subsequently grew Mycobacterium kansasii, and the patient was started on isoniazid 300 mg daily, rifampin 600 mg daily, ethambutol 800 mg daily, and pyridoxine 50 mg daily. Chest radiograph and computed tomography showed no evidence of pulmonary disease and 2 blood cultures were negative for growth. The patient subsequently developed weakness that he attributed to the antibiotics and he decided to discontinue all treatment.
At 11 months the lesions showed no change; however, magnetic resonance imaging of the leg was suggestive of osteomyelitis. The patient was started on clarithromycin 500 mg twice daily with planned addition of isoniazid. The patient refused any additional antibiotics but agreed to continue the clarithromycin treatment for one year. He was subsequently lost to dermatology follow-up.
Nontuberculous mycobacteria (NTM) infection is a rare sequela of hematologic malignancy, seen in only 1.5% of patients.1 The NTM most commonly seen in hematologic malignancy are generally the fast-growing species Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium fortuitum, or Mycobacterium phlei, rather than slow growers Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium kansasii, Mycobacterium marinum, and Mycobacterium xenopi. Mycobacterium kansasii infection, such as seen in our patient, accounts for only 18% of cases.1 This case is further distinguished by the fact that cutaneous infections with NTM also are generally caused by fast-growing organisms such as Mycobacterium abscessus-chelonae complex and M fortuitum, rather than the slow-growing M kansasii.2,3
Mycobacterium kansasii is a slow-growing, acid-fast bacillus found in local water reservoirs, swimming pools, sewers, and tap water where it can live for up to 12 months.2,4,5Mycobacterium kansasii is traditionally considered the most virulent NTM.3,6 It most frequently causes a pulmonary infection in the immunosuppressed and patients with chronic bronchopulmonary disease.6,7 Disseminated disease is less common and is primarily seen in immunocompromised patients, particularly in human immunodeficiency virus–positive patients, transplant recipients, and patients with hematologic malignancies.1,6,8 Disseminated disease rarely has been seen in patients with normal immune function.2,3
Cutaneous M kansasii infection has only infrequently been described. Most patients tend to be middle-aged men, with a median affected age of 43 years.2,7,9,10 One review of cutaneous cases found that 72% had some form of altered immunity and more than 50% of those patients were on chronic steroids. The same review found that of the cases of cutaneous M kansasii in patients with altered immunity, only 30% had disseminated disease.10 Our patient was immunocompromised but showed no evidence of disseminated disease, as displayed by negative chest radiograph and computed tomography, lack of pulmonary symptoms, and negative blood cultures. As a 68-year-old man with myelodysplastic syndrome on chronic steroids with no disseminated disease, our patient fits well into these demographics, aside from his advanced age.
Cutaneous M kansasii infection has a variable presentation, manifesting as solitary lesions, nodules, pustules, seromas, erythematous plaques, verrucous lesions, ulcers, and as cellulitis.5,7,9-12 Immune competent individuals were more likely to present with raised lesions or ulcers, whereas immune compromised individuals had a more diffuse presentation of cellulitis or seromas with variable histology.6,8 Our patient, though immune compromised, presented with multiple erythematous plaques with eschars, which further endorses having a high clinical suspicion, as the lesions display marked heterogeneity.
Treatment of M kansasii infection consists of at least 1 year of isoniazid 300 mg daily, rifampin 600 mg daily, and ethambutol 15 mg/kg daily, with possible addition of streptomycin.8,13Mycobacterium kansasii infection necessitates multidrug treatment due to the broad range of resistance exhibited by different isolated strains.14
Response to treatment in cutaneous M kansasii greatly depends on the underlying disease state of the individual. Generally, immune competent individuals do very well, while the course in immune compromised patients depends on their degree of illness. Patients with disseminated disease generally do poorly.4,7,10 In at least one case of cutaneous disease, dissemination developed as a sequela, thus suggesting treatment is needed even in stable lesions.2 Dissemination was a concern with our patient given the magnetic resonance imaging findings suggestive of osteomyelitis. Although treatment generally consists of triple therapy with isoniazid, rifampin, and ethambutol, given the high frequency of adverse effects due to isoniazid or rifampin, as was seen in our patient, the drug regimen might have to be altered to suit the patient. Susceptibility testing is desirable to aid in tailoring the treatment.8,13 Furthermore, as the duration of treatment is at least 1 year, diligent follow-up must be maintained to avoid incomplete treatment.
The unpredictable presentation of cutaneous M kansasii infection coupled with the variable history necessitates a high level of clinical suspicion and a low threshold for culturing lesions. Furthermore, the long duration and complexity of the antibiotic regimen and the high incidence of adverse reactions demands strict follow-up, especially given the risk for progression to disseminated disease.
1. Chen CY, Sheng WH, Lai CC. Mycobacterial infections in adult patients with hematological malignancy. Eur J Clin Microbiol Infect Dis. 2012;31:1059-1066.
2. Han SH, Kim KM, Chin BS, et al. Disseminated Mycobacterium kansasii infection associated with skin lesions: a case report and comprehensive review of the literature. J Korean Med Sci. 2010;25:304-308.
3. Razavi B, Cleveland MG. Cutaneous infection due to Mycobacterium kansasii. Diagn Microbiol Infect Dis. 2000;38:173-175.
4. Portaels F. Epidemiology of mycobacterial diseases. Clin Dermatol. 1995;13:207-222.
5. Nomura Y, Nishie W, Shibaki A, et al. Disseminated cutaneous Mycobacterium kansasii infection in a patient infected with the human immunodeficiency virus. Clin Exp Dermatol. 2009;34:625-626.
6. Bloch KC, Zwerling L, Pletcher MJ, et al. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Intern Med. 1998;129:698-704.
7. Breathnach A, Levell N, Munro C, et al. Cutaneous Mycobacterium kansasii infection: case report and review. Clin Infect Dis. 1995;20:812-817.
8. Pintado V, Gómez-Mampaso E, Martín-Dávila P. Mycobacterium kansasii infection in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis. 1999;18:582-586.
9. Stengem J, Grande KK, Hsu S. Localized primary cutaneous Mycobacterium kansasii infection in an immunocompromised patient. J Am Acad Dermatol. 1999;41(5, pt 2):854-856.
10. Czelusta A, Moore AY. Cutaneous Mycobacterium kansasii infection in a patient with systemic lupus erythematosus: case report and review. J Am Acad Dermatol. 1999;40(2, pt 2):359-363.
11. Curcó N, Pagerols X, Gómez L, et al. Mycobacterium kansasii infection limited to the skin in a patient with AIDS. Br J Dermatol. 1996;135:324-326.
12. Hanke CW, Temofeew RK, Slama SL. Mycobacterium kansasii infection with multiple cutaneous lesions. J Am Acad Dermatol. 1987;16(5, pt 2):1122-1128.
13. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculousmycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
14. da Silva Telles MA, Chimara E, Ferrazoli L, Riley LW. Mycobacterium kansasii: antibiotic susceptibility and PCR-restriction analysis of clinical isolates. J Med Microbiol. 2005;54:975-979.
To the Editor:
A 68-year-old man with a history of myelodysplastic syndrome and recurrent Sweet syndrome presented with left leg lesions of 3 months’ duration. The lesions originated as a solitary nodule on the left calf and subsequently developed into multiple nonpainful, nonpruritic, erythematous plaques of varying sizes with violaceous coloration and overlying necrotic eschar, occupying the entire anterior aspect of the left lower leg and left popliteal fossa (Figure). The patient denied any trauma or associated symptoms but had a history of Sweet syndrome that manifested as lesions on the arms and legs for which he took 6 mg of prednisone daily to prevent recurrence.
Histologic examination revealed nodular and diffuse chronic granulomatous and acute inflammatory infiltrate. Stains for bacteria, fungi, and acid-fast bacilli were negative. Cultures subsequently grew Mycobacterium kansasii, and the patient was started on isoniazid 300 mg daily, rifampin 600 mg daily, ethambutol 800 mg daily, and pyridoxine 50 mg daily. Chest radiograph and computed tomography showed no evidence of pulmonary disease and 2 blood cultures were negative for growth. The patient subsequently developed weakness that he attributed to the antibiotics and he decided to discontinue all treatment.
At 11 months the lesions showed no change; however, magnetic resonance imaging of the leg was suggestive of osteomyelitis. The patient was started on clarithromycin 500 mg twice daily with planned addition of isoniazid. The patient refused any additional antibiotics but agreed to continue the clarithromycin treatment for one year. He was subsequently lost to dermatology follow-up.
Nontuberculous mycobacteria (NTM) infection is a rare sequela of hematologic malignancy, seen in only 1.5% of patients.1 The NTM most commonly seen in hematologic malignancy are generally the fast-growing species Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium fortuitum, or Mycobacterium phlei, rather than slow growers Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium kansasii, Mycobacterium marinum, and Mycobacterium xenopi. Mycobacterium kansasii infection, such as seen in our patient, accounts for only 18% of cases.1 This case is further distinguished by the fact that cutaneous infections with NTM also are generally caused by fast-growing organisms such as Mycobacterium abscessus-chelonae complex and M fortuitum, rather than the slow-growing M kansasii.2,3
Mycobacterium kansasii is a slow-growing, acid-fast bacillus found in local water reservoirs, swimming pools, sewers, and tap water where it can live for up to 12 months.2,4,5Mycobacterium kansasii is traditionally considered the most virulent NTM.3,6 It most frequently causes a pulmonary infection in the immunosuppressed and patients with chronic bronchopulmonary disease.6,7 Disseminated disease is less common and is primarily seen in immunocompromised patients, particularly in human immunodeficiency virus–positive patients, transplant recipients, and patients with hematologic malignancies.1,6,8 Disseminated disease rarely has been seen in patients with normal immune function.2,3
Cutaneous M kansasii infection has only infrequently been described. Most patients tend to be middle-aged men, with a median affected age of 43 years.2,7,9,10 One review of cutaneous cases found that 72% had some form of altered immunity and more than 50% of those patients were on chronic steroids. The same review found that of the cases of cutaneous M kansasii in patients with altered immunity, only 30% had disseminated disease.10 Our patient was immunocompromised but showed no evidence of disseminated disease, as displayed by negative chest radiograph and computed tomography, lack of pulmonary symptoms, and negative blood cultures. As a 68-year-old man with myelodysplastic syndrome on chronic steroids with no disseminated disease, our patient fits well into these demographics, aside from his advanced age.
Cutaneous M kansasii infection has a variable presentation, manifesting as solitary lesions, nodules, pustules, seromas, erythematous plaques, verrucous lesions, ulcers, and as cellulitis.5,7,9-12 Immune competent individuals were more likely to present with raised lesions or ulcers, whereas immune compromised individuals had a more diffuse presentation of cellulitis or seromas with variable histology.6,8 Our patient, though immune compromised, presented with multiple erythematous plaques with eschars, which further endorses having a high clinical suspicion, as the lesions display marked heterogeneity.
Treatment of M kansasii infection consists of at least 1 year of isoniazid 300 mg daily, rifampin 600 mg daily, and ethambutol 15 mg/kg daily, with possible addition of streptomycin.8,13Mycobacterium kansasii infection necessitates multidrug treatment due to the broad range of resistance exhibited by different isolated strains.14
Response to treatment in cutaneous M kansasii greatly depends on the underlying disease state of the individual. Generally, immune competent individuals do very well, while the course in immune compromised patients depends on their degree of illness. Patients with disseminated disease generally do poorly.4,7,10 In at least one case of cutaneous disease, dissemination developed as a sequela, thus suggesting treatment is needed even in stable lesions.2 Dissemination was a concern with our patient given the magnetic resonance imaging findings suggestive of osteomyelitis. Although treatment generally consists of triple therapy with isoniazid, rifampin, and ethambutol, given the high frequency of adverse effects due to isoniazid or rifampin, as was seen in our patient, the drug regimen might have to be altered to suit the patient. Susceptibility testing is desirable to aid in tailoring the treatment.8,13 Furthermore, as the duration of treatment is at least 1 year, diligent follow-up must be maintained to avoid incomplete treatment.
The unpredictable presentation of cutaneous M kansasii infection coupled with the variable history necessitates a high level of clinical suspicion and a low threshold for culturing lesions. Furthermore, the long duration and complexity of the antibiotic regimen and the high incidence of adverse reactions demands strict follow-up, especially given the risk for progression to disseminated disease.
To the Editor:
A 68-year-old man with a history of myelodysplastic syndrome and recurrent Sweet syndrome presented with left leg lesions of 3 months’ duration. The lesions originated as a solitary nodule on the left calf and subsequently developed into multiple nonpainful, nonpruritic, erythematous plaques of varying sizes with violaceous coloration and overlying necrotic eschar, occupying the entire anterior aspect of the left lower leg and left popliteal fossa (Figure). The patient denied any trauma or associated symptoms but had a history of Sweet syndrome that manifested as lesions on the arms and legs for which he took 6 mg of prednisone daily to prevent recurrence.
Histologic examination revealed nodular and diffuse chronic granulomatous and acute inflammatory infiltrate. Stains for bacteria, fungi, and acid-fast bacilli were negative. Cultures subsequently grew Mycobacterium kansasii, and the patient was started on isoniazid 300 mg daily, rifampin 600 mg daily, ethambutol 800 mg daily, and pyridoxine 50 mg daily. Chest radiograph and computed tomography showed no evidence of pulmonary disease and 2 blood cultures were negative for growth. The patient subsequently developed weakness that he attributed to the antibiotics and he decided to discontinue all treatment.
At 11 months the lesions showed no change; however, magnetic resonance imaging of the leg was suggestive of osteomyelitis. The patient was started on clarithromycin 500 mg twice daily with planned addition of isoniazid. The patient refused any additional antibiotics but agreed to continue the clarithromycin treatment for one year. He was subsequently lost to dermatology follow-up.
Nontuberculous mycobacteria (NTM) infection is a rare sequela of hematologic malignancy, seen in only 1.5% of patients.1 The NTM most commonly seen in hematologic malignancy are generally the fast-growing species Mycobacterium abscessus, Mycobacterium chelonae, Mycobacterium fortuitum, or Mycobacterium phlei, rather than slow growers Mycobacterium avium, Mycobacterium intracellulare, Mycobacterium kansasii, Mycobacterium marinum, and Mycobacterium xenopi. Mycobacterium kansasii infection, such as seen in our patient, accounts for only 18% of cases.1 This case is further distinguished by the fact that cutaneous infections with NTM also are generally caused by fast-growing organisms such as Mycobacterium abscessus-chelonae complex and M fortuitum, rather than the slow-growing M kansasii.2,3
Mycobacterium kansasii is a slow-growing, acid-fast bacillus found in local water reservoirs, swimming pools, sewers, and tap water where it can live for up to 12 months.2,4,5Mycobacterium kansasii is traditionally considered the most virulent NTM.3,6 It most frequently causes a pulmonary infection in the immunosuppressed and patients with chronic bronchopulmonary disease.6,7 Disseminated disease is less common and is primarily seen in immunocompromised patients, particularly in human immunodeficiency virus–positive patients, transplant recipients, and patients with hematologic malignancies.1,6,8 Disseminated disease rarely has been seen in patients with normal immune function.2,3
Cutaneous M kansasii infection has only infrequently been described. Most patients tend to be middle-aged men, with a median affected age of 43 years.2,7,9,10 One review of cutaneous cases found that 72% had some form of altered immunity and more than 50% of those patients were on chronic steroids. The same review found that of the cases of cutaneous M kansasii in patients with altered immunity, only 30% had disseminated disease.10 Our patient was immunocompromised but showed no evidence of disseminated disease, as displayed by negative chest radiograph and computed tomography, lack of pulmonary symptoms, and negative blood cultures. As a 68-year-old man with myelodysplastic syndrome on chronic steroids with no disseminated disease, our patient fits well into these demographics, aside from his advanced age.
Cutaneous M kansasii infection has a variable presentation, manifesting as solitary lesions, nodules, pustules, seromas, erythematous plaques, verrucous lesions, ulcers, and as cellulitis.5,7,9-12 Immune competent individuals were more likely to present with raised lesions or ulcers, whereas immune compromised individuals had a more diffuse presentation of cellulitis or seromas with variable histology.6,8 Our patient, though immune compromised, presented with multiple erythematous plaques with eschars, which further endorses having a high clinical suspicion, as the lesions display marked heterogeneity.
Treatment of M kansasii infection consists of at least 1 year of isoniazid 300 mg daily, rifampin 600 mg daily, and ethambutol 15 mg/kg daily, with possible addition of streptomycin.8,13Mycobacterium kansasii infection necessitates multidrug treatment due to the broad range of resistance exhibited by different isolated strains.14
Response to treatment in cutaneous M kansasii greatly depends on the underlying disease state of the individual. Generally, immune competent individuals do very well, while the course in immune compromised patients depends on their degree of illness. Patients with disseminated disease generally do poorly.4,7,10 In at least one case of cutaneous disease, dissemination developed as a sequela, thus suggesting treatment is needed even in stable lesions.2 Dissemination was a concern with our patient given the magnetic resonance imaging findings suggestive of osteomyelitis. Although treatment generally consists of triple therapy with isoniazid, rifampin, and ethambutol, given the high frequency of adverse effects due to isoniazid or rifampin, as was seen in our patient, the drug regimen might have to be altered to suit the patient. Susceptibility testing is desirable to aid in tailoring the treatment.8,13 Furthermore, as the duration of treatment is at least 1 year, diligent follow-up must be maintained to avoid incomplete treatment.
The unpredictable presentation of cutaneous M kansasii infection coupled with the variable history necessitates a high level of clinical suspicion and a low threshold for culturing lesions. Furthermore, the long duration and complexity of the antibiotic regimen and the high incidence of adverse reactions demands strict follow-up, especially given the risk for progression to disseminated disease.
1. Chen CY, Sheng WH, Lai CC. Mycobacterial infections in adult patients with hematological malignancy. Eur J Clin Microbiol Infect Dis. 2012;31:1059-1066.
2. Han SH, Kim KM, Chin BS, et al. Disseminated Mycobacterium kansasii infection associated with skin lesions: a case report and comprehensive review of the literature. J Korean Med Sci. 2010;25:304-308.
3. Razavi B, Cleveland MG. Cutaneous infection due to Mycobacterium kansasii. Diagn Microbiol Infect Dis. 2000;38:173-175.
4. Portaels F. Epidemiology of mycobacterial diseases. Clin Dermatol. 1995;13:207-222.
5. Nomura Y, Nishie W, Shibaki A, et al. Disseminated cutaneous Mycobacterium kansasii infection in a patient infected with the human immunodeficiency virus. Clin Exp Dermatol. 2009;34:625-626.
6. Bloch KC, Zwerling L, Pletcher MJ, et al. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Intern Med. 1998;129:698-704.
7. Breathnach A, Levell N, Munro C, et al. Cutaneous Mycobacterium kansasii infection: case report and review. Clin Infect Dis. 1995;20:812-817.
8. Pintado V, Gómez-Mampaso E, Martín-Dávila P. Mycobacterium kansasii infection in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis. 1999;18:582-586.
9. Stengem J, Grande KK, Hsu S. Localized primary cutaneous Mycobacterium kansasii infection in an immunocompromised patient. J Am Acad Dermatol. 1999;41(5, pt 2):854-856.
10. Czelusta A, Moore AY. Cutaneous Mycobacterium kansasii infection in a patient with systemic lupus erythematosus: case report and review. J Am Acad Dermatol. 1999;40(2, pt 2):359-363.
11. Curcó N, Pagerols X, Gómez L, et al. Mycobacterium kansasii infection limited to the skin in a patient with AIDS. Br J Dermatol. 1996;135:324-326.
12. Hanke CW, Temofeew RK, Slama SL. Mycobacterium kansasii infection with multiple cutaneous lesions. J Am Acad Dermatol. 1987;16(5, pt 2):1122-1128.
13. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculousmycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
14. da Silva Telles MA, Chimara E, Ferrazoli L, Riley LW. Mycobacterium kansasii: antibiotic susceptibility and PCR-restriction analysis of clinical isolates. J Med Microbiol. 2005;54:975-979.
1. Chen CY, Sheng WH, Lai CC. Mycobacterial infections in adult patients with hematological malignancy. Eur J Clin Microbiol Infect Dis. 2012;31:1059-1066.
2. Han SH, Kim KM, Chin BS, et al. Disseminated Mycobacterium kansasii infection associated with skin lesions: a case report and comprehensive review of the literature. J Korean Med Sci. 2010;25:304-308.
3. Razavi B, Cleveland MG. Cutaneous infection due to Mycobacterium kansasii. Diagn Microbiol Infect Dis. 2000;38:173-175.
4. Portaels F. Epidemiology of mycobacterial diseases. Clin Dermatol. 1995;13:207-222.
5. Nomura Y, Nishie W, Shibaki A, et al. Disseminated cutaneous Mycobacterium kansasii infection in a patient infected with the human immunodeficiency virus. Clin Exp Dermatol. 2009;34:625-626.
6. Bloch KC, Zwerling L, Pletcher MJ, et al. Incidence and clinical implications of isolation of Mycobacterium kansasii: results of a 5-year, population-based study. Ann Intern Med. 1998;129:698-704.
7. Breathnach A, Levell N, Munro C, et al. Cutaneous Mycobacterium kansasii infection: case report and review. Clin Infect Dis. 1995;20:812-817.
8. Pintado V, Gómez-Mampaso E, Martín-Dávila P. Mycobacterium kansasii infection in patients infected with the human immunodeficiency virus. Eur J Clin Microbiol Infect Dis. 1999;18:582-586.
9. Stengem J, Grande KK, Hsu S. Localized primary cutaneous Mycobacterium kansasii infection in an immunocompromised patient. J Am Acad Dermatol. 1999;41(5, pt 2):854-856.
10. Czelusta A, Moore AY. Cutaneous Mycobacterium kansasii infection in a patient with systemic lupus erythematosus: case report and review. J Am Acad Dermatol. 1999;40(2, pt 2):359-363.
11. Curcó N, Pagerols X, Gómez L, et al. Mycobacterium kansasii infection limited to the skin in a patient with AIDS. Br J Dermatol. 1996;135:324-326.
12. Hanke CW, Temofeew RK, Slama SL. Mycobacterium kansasii infection with multiple cutaneous lesions. J Am Acad Dermatol. 1987;16(5, pt 2):1122-1128.
13. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculousmycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416.
14. da Silva Telles MA, Chimara E, Ferrazoli L, Riley LW. Mycobacterium kansasii: antibiotic susceptibility and PCR-restriction analysis of clinical isolates. J Med Microbiol. 2005;54:975-979.
Pruritic Urticarial Papules and Plaques of Pregnancy Occurring Postpartum
The cutaneous effects of pregnancy are variable and numerous. We all have likely seen the pigmentary changes induced by pregnancy as well as both exacerbation and complete resolution of preexisting skin conditions. The dermatoses of pregnancy are classified as a group of inflammatory skin conditions exclusively seen in pregnant women, the most common being pruritic urticarial papules and plaques of pregnancy (PUPPP).1 Also known as polymorphic eruption of pregnancy in Europe, PUPPP was first recognized in 1979 as a distinct entity that manifested as an intense pruritic eruption unique to women in the third trimester of pregnancy.2 The condition usually is self-limited, with the majority of cases spontaneously resolving within 4 to 6 weeks after delivery.3,4 Presentation of PUPPP in the postpartum period is rare.1-4 We report a biopsy-proven case of PUPPP in a 30-year-old woman who presented 2 weeks postpartum with an intensely pruritic generalized eruption. A PubMed search of articles indexed for MEDLINE using the search terms pruritic urticarial papules and plaques of pregnancy or polymorphic eruption of pregnancy and postpartum revealed only 5 reports of PUPPP or polymorphic eruption of pregnancy occurring in the postpartum period, 2 occurring in the United States.5-9
Case Report
A 30-year-old woman who was 2 weeks postpartum presented to our dermatology clinic with an intensely pruritic generalized rash. Within 24 hours of delivery of her first child, the patient developed an itchy rash on the abdomen and was started on oral corticosteroids and antihistamines in the hospital. On discharge, she was instructed to follow up with the dermatology department if the rash did not resolve. After leaving the hospital, she reported that the eruption had progressively spread to the buttocks, legs, and arms, and the itching seemed to be worse despite finishing the course of oral corticosteroids and antihistamines.
The patient’s prenatal course was uneventful. She gained 16 kg during pregnancy, with a prepregnancy weight of 50 kg. A healthy male neonate was delivered at 38 weeks’ gestation without complication. The patient’s medical history was unremarkable. Her current medications included prenatal vitamins, oral prednisone, and loratadine, and she reported no known drug allergies.
On physical examination, the patient was afebrile and her blood pressure was normal. Examination of the skin revealed erythematous papules and urticarial plaques involving the abdominal striae with periumbilical sparing (Figure 1A). Similar lesions were noted on the legs, buttocks, and arms (Figure 1B). The face, palms, and soles were uninvolved. No vesicles or pustules were noted. The oral mucosa was pink, moist, and unremarkable.

Figure 1. Initial presentation of urticarial plaques involving the abdominal striae with periumbilical sparing (A) and the left arm (B). |
Based on the patient’s clinical presentation, the differential diagnosis included pemphigoid gestationis, a hypersensitivity reaction, cutaneous lupus, cholestasis of pregnancy, and PUPPP. Pruritic urticarial papules and plaques of pregnancy was considered to be unlikely because of the uncharacteristic postpartum presentation of the eruption.
Two 4-mm punch biopsies were performed on the left upper arm and were sent for histopathologic examination and direct immunofluorescence. Laboratory studies including complete blood cell count with differential, complete metabolic panel, antinuclear antibodies, and IgE levels were conducted. The patient was started on triamcinolone cream 0.1% twice daily and her antihistamine was switched from loratadine to cetirizine.
Histopathologic examination revealed a mixed perivascular infiltrate in the superficial dermis consisting of lymphocytes, mast cells, and eosinophils (Figures 2 and 3), which was consistent with a diagnosis of PUPPP. Direct immunofluorescence was negative. Laboratory studies were within reference range and antinuclear antibodies and IgE levels were negative. A diagnosis of postpartum PUPPP was made. Complete resolution of the eruption was experienced by 2-week follow-up (Figures 4A and 4B). The patient noted that her symptoms improved within 2 days of starting topical therapy.
|
|
Comment
Pruritic urticarial papules and plaques of pregnancy complicates 1 of 160 to 1 of 300 pregnancies.1 As seen in our case, the majority of cases of PUPPP are diagnosed in women who are nulliparous or primigravida.10 A study by Aronson et al10 reported that of 57 cases of PUPPP, 24 (42%) patients were primigravida, 16 (28%) were gravida 2, 9 (16%) were gravida 3, 4 (7%) were gravida 4, 3 (5%) were gravida 6, and 1 (2%) was gravida 7. Thirty-nine (68%) patients were nulliparous.10 The average onset of symptoms is approximately 35 weeks’ gestation.9
Classical presentation of PUPPP starts with erythematous papules within the abdominal striae, sparing the periumbilical skin.1 The abdominal striae are most commonly affected, and in some women, it may be the only site affected.10 The lesions then may pro-gress to urticarial plaques involving the extremities, while the face, palms, and soles usually are spared.11 However, clinical manifestations of PUPPP can vary, with reports of targetlike lesions with a surrounding halo resembling erythema multiforme as well as involvement of the face and palmoplantar skin.10-13 Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.14 Histologically, PUPPP demonstrates variable epidermal spongiosis and a nonspecific superficial perivascular infiltrate in the dermis composed of lymphocytes with eosinophils or neutrophils, and there may be dermal edema.10,15 Direct immunofluorescence usually is negative in PUPPP; however, 31% of cases have demonstrated deposition of C3 and IgM or IgA, either perivascularly or at the dermoepidermal junction.1,10,15
There are no systemic alterations seen in PUPPP; however, all patients report severe pruritus.12 Pruritic urticarial papules and plaques of pregnancy typically affects women in the third trimester, and delivery is curative in most patients.13 Recurrence of PUPPP usually is not seen with subsequent pregnancies, and the long-term prognosis is excellent.15
The pathogenesis of PUPPP is not well understood and likely is multifactorial. Ohel et al12 found PUPPP to be strongly associated with hypertensive disorders, multiple gestation pregnancies, excessive maternal weight gain, excessive stretching of the abdominal skin, and nulliparity.13 One theory suggests that abdominal skin stretching, if drastic, can damage underlying connective tissue, resulting in the release of antigens that can trigger a reactive inflammatory response.16 The majority of maternal weight gain occurs during the third trimester, which may explain why most cases of PUPPP present in the third trimester.17 Alternative theories have suggested that PUPPP may represent an immunologic response to circulating fetal antigens.18 It is possible, as in our case, that certain nulliparous women who have a healthy weight prior to pregnancy (as determined by a body mass index of 18.5 to 24.9) in combination with excessive weight gain during the third trimester and drastic hormone fluctuations associated with labor and delivery may be at greater risk for developing PUPPP. Another theory may be related to the degree of skin stretching during the third trimester and the abrupt decrease in the stretching of the skin that occurs with delivery.16
Conclusion
Pruritic urticarial papules and plaques of pregnancy can present in a variety of ways, most commonly in the third trimester but also in the postpartum period. When a patient presents in the postpartum period with a pruritic eruption, PUPPP should be included in the differential diagnosis. The pathogenesis of PUPPP is multifactorial and not well understood, and additional research in the field may lead to improved prediction of who may be at risk and what we can do to prevent it.
1. Pomeranz MK. Dermatoses of pregnancy. UpToDate Web site. http://www.uptodate.com/contents/dermatoses-of-pregnancy. Updated December 22, 2014. Accessed May 5, 2015.
2. Lawley TJ, Hertz KC, Wade TR, et al. Pruritic urticarial papules and plaques of pregnancy. JAMA. 1979;241:1696-1699.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
4. Callen JP, Hanno R. Pruritic urticarial papules and plaques of pregnancy (PUPPP): a clinicopathologic study. J Am Acad Dermatol.1981;5:401-405.
5. Ozcan D, Ozcakmak B, Aydogan FC. J Obstet Gynaecol Res. 2011;37:1158-1161.
6. Journet-Tollhupp J, Tchen T, Remy-Leroux V, et al. Polymorphic eruption of pregnancy and acquired hemophilia A [in French]. Ann Dermatol Venereol. 2010;137:713-717.
7. Buccolo LS, Viera AJ. Pruritic urticarial papules and plaques of pregnancy presenting in the postpartum period: a case report. J Reprod Med. 2005;50:61-63.
8. Kirkup ME, Dunnill MG. Polymorphic eruption of pregnancy developing in the puerrperium. Clin Exp Dermatol. 2002;27:657-660.
9. Yancy KB, Hall RP, Lawley TJ. Pruritic urticarial papules and plaques of pregnancy: clinical experience in twenty-five patients. J Am Acad Dermatol. 1984;10:473-480.
10. Aronson IK, Bond S, Fiedler VC, et al. Pruritic urticarial papules and plaques of pregnancy: clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol. 1998;39:933-939.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic dermatoses of pregnancy: a prospective study of 3129 women. Arch Dermatol. 1994;130:734-739.
12. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Matern Fetal Neonatal Med. 2006;19:305-308.
13. Elling SV, McKenna P, Pawell FC. Pruritic urticarial papules and plaques of pregnancy in twin and triplet pregnancies. J Eur Acad Dermatol Venereol. 2000;14:378-381.
14. Scheinfeld N. Pruritic urticarial papules and plaques of pregnancy wholly abated with one week twice daily application of fluticasone propionate lotion: a case report and review of the literature. Dermatol Online J. 2008;14:4.
15. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
16. Cohen LM, Capeless EL, Krusinski PA, et al. Pruritic urticarial papules and plaques of pregnancy and its relationship to maternal-fetal weight gain and twin pregnancy. Arch Dermatol. 1989;125:1534-1536.
17. Drehmer M, Duncan BB, Kac G, et al. Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One. 2013;8:e54704.
18. Aractingi S, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
The cutaneous effects of pregnancy are variable and numerous. We all have likely seen the pigmentary changes induced by pregnancy as well as both exacerbation and complete resolution of preexisting skin conditions. The dermatoses of pregnancy are classified as a group of inflammatory skin conditions exclusively seen in pregnant women, the most common being pruritic urticarial papules and plaques of pregnancy (PUPPP).1 Also known as polymorphic eruption of pregnancy in Europe, PUPPP was first recognized in 1979 as a distinct entity that manifested as an intense pruritic eruption unique to women in the third trimester of pregnancy.2 The condition usually is self-limited, with the majority of cases spontaneously resolving within 4 to 6 weeks after delivery.3,4 Presentation of PUPPP in the postpartum period is rare.1-4 We report a biopsy-proven case of PUPPP in a 30-year-old woman who presented 2 weeks postpartum with an intensely pruritic generalized eruption. A PubMed search of articles indexed for MEDLINE using the search terms pruritic urticarial papules and plaques of pregnancy or polymorphic eruption of pregnancy and postpartum revealed only 5 reports of PUPPP or polymorphic eruption of pregnancy occurring in the postpartum period, 2 occurring in the United States.5-9
Case Report
A 30-year-old woman who was 2 weeks postpartum presented to our dermatology clinic with an intensely pruritic generalized rash. Within 24 hours of delivery of her first child, the patient developed an itchy rash on the abdomen and was started on oral corticosteroids and antihistamines in the hospital. On discharge, she was instructed to follow up with the dermatology department if the rash did not resolve. After leaving the hospital, she reported that the eruption had progressively spread to the buttocks, legs, and arms, and the itching seemed to be worse despite finishing the course of oral corticosteroids and antihistamines.
The patient’s prenatal course was uneventful. She gained 16 kg during pregnancy, with a prepregnancy weight of 50 kg. A healthy male neonate was delivered at 38 weeks’ gestation without complication. The patient’s medical history was unremarkable. Her current medications included prenatal vitamins, oral prednisone, and loratadine, and she reported no known drug allergies.
On physical examination, the patient was afebrile and her blood pressure was normal. Examination of the skin revealed erythematous papules and urticarial plaques involving the abdominal striae with periumbilical sparing (Figure 1A). Similar lesions were noted on the legs, buttocks, and arms (Figure 1B). The face, palms, and soles were uninvolved. No vesicles or pustules were noted. The oral mucosa was pink, moist, and unremarkable.


Figure 1. Initial presentation of urticarial plaques involving the abdominal striae with periumbilical sparing (A) and the left arm (B). |
Based on the patient’s clinical presentation, the differential diagnosis included pemphigoid gestationis, a hypersensitivity reaction, cutaneous lupus, cholestasis of pregnancy, and PUPPP. Pruritic urticarial papules and plaques of pregnancy was considered to be unlikely because of the uncharacteristic postpartum presentation of the eruption.
Two 4-mm punch biopsies were performed on the left upper arm and were sent for histopathologic examination and direct immunofluorescence. Laboratory studies including complete blood cell count with differential, complete metabolic panel, antinuclear antibodies, and IgE levels were conducted. The patient was started on triamcinolone cream 0.1% twice daily and her antihistamine was switched from loratadine to cetirizine.
Histopathologic examination revealed a mixed perivascular infiltrate in the superficial dermis consisting of lymphocytes, mast cells, and eosinophils (Figures 2 and 3), which was consistent with a diagnosis of PUPPP. Direct immunofluorescence was negative. Laboratory studies were within reference range and antinuclear antibodies and IgE levels were negative. A diagnosis of postpartum PUPPP was made. Complete resolution of the eruption was experienced by 2-week follow-up (Figures 4A and 4B). The patient noted that her symptoms improved within 2 days of starting topical therapy.
|
|
Comment
Pruritic urticarial papules and plaques of pregnancy complicates 1 of 160 to 1 of 300 pregnancies.1 As seen in our case, the majority of cases of PUPPP are diagnosed in women who are nulliparous or primigravida.10 A study by Aronson et al10 reported that of 57 cases of PUPPP, 24 (42%) patients were primigravida, 16 (28%) were gravida 2, 9 (16%) were gravida 3, 4 (7%) were gravida 4, 3 (5%) were gravida 6, and 1 (2%) was gravida 7. Thirty-nine (68%) patients were nulliparous.10 The average onset of symptoms is approximately 35 weeks’ gestation.9
Classical presentation of PUPPP starts with erythematous papules within the abdominal striae, sparing the periumbilical skin.1 The abdominal striae are most commonly affected, and in some women, it may be the only site affected.10 The lesions then may pro-gress to urticarial plaques involving the extremities, while the face, palms, and soles usually are spared.11 However, clinical manifestations of PUPPP can vary, with reports of targetlike lesions with a surrounding halo resembling erythema multiforme as well as involvement of the face and palmoplantar skin.10-13 Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.14 Histologically, PUPPP demonstrates variable epidermal spongiosis and a nonspecific superficial perivascular infiltrate in the dermis composed of lymphocytes with eosinophils or neutrophils, and there may be dermal edema.10,15 Direct immunofluorescence usually is negative in PUPPP; however, 31% of cases have demonstrated deposition of C3 and IgM or IgA, either perivascularly or at the dermoepidermal junction.1,10,15
There are no systemic alterations seen in PUPPP; however, all patients report severe pruritus.12 Pruritic urticarial papules and plaques of pregnancy typically affects women in the third trimester, and delivery is curative in most patients.13 Recurrence of PUPPP usually is not seen with subsequent pregnancies, and the long-term prognosis is excellent.15
The pathogenesis of PUPPP is not well understood and likely is multifactorial. Ohel et al12 found PUPPP to be strongly associated with hypertensive disorders, multiple gestation pregnancies, excessive maternal weight gain, excessive stretching of the abdominal skin, and nulliparity.13 One theory suggests that abdominal skin stretching, if drastic, can damage underlying connective tissue, resulting in the release of antigens that can trigger a reactive inflammatory response.16 The majority of maternal weight gain occurs during the third trimester, which may explain why most cases of PUPPP present in the third trimester.17 Alternative theories have suggested that PUPPP may represent an immunologic response to circulating fetal antigens.18 It is possible, as in our case, that certain nulliparous women who have a healthy weight prior to pregnancy (as determined by a body mass index of 18.5 to 24.9) in combination with excessive weight gain during the third trimester and drastic hormone fluctuations associated with labor and delivery may be at greater risk for developing PUPPP. Another theory may be related to the degree of skin stretching during the third trimester and the abrupt decrease in the stretching of the skin that occurs with delivery.16
Conclusion
Pruritic urticarial papules and plaques of pregnancy can present in a variety of ways, most commonly in the third trimester but also in the postpartum period. When a patient presents in the postpartum period with a pruritic eruption, PUPPP should be included in the differential diagnosis. The pathogenesis of PUPPP is multifactorial and not well understood, and additional research in the field may lead to improved prediction of who may be at risk and what we can do to prevent it.
The cutaneous effects of pregnancy are variable and numerous. We all have likely seen the pigmentary changes induced by pregnancy as well as both exacerbation and complete resolution of preexisting skin conditions. The dermatoses of pregnancy are classified as a group of inflammatory skin conditions exclusively seen in pregnant women, the most common being pruritic urticarial papules and plaques of pregnancy (PUPPP).1 Also known as polymorphic eruption of pregnancy in Europe, PUPPP was first recognized in 1979 as a distinct entity that manifested as an intense pruritic eruption unique to women in the third trimester of pregnancy.2 The condition usually is self-limited, with the majority of cases spontaneously resolving within 4 to 6 weeks after delivery.3,4 Presentation of PUPPP in the postpartum period is rare.1-4 We report a biopsy-proven case of PUPPP in a 30-year-old woman who presented 2 weeks postpartum with an intensely pruritic generalized eruption. A PubMed search of articles indexed for MEDLINE using the search terms pruritic urticarial papules and plaques of pregnancy or polymorphic eruption of pregnancy and postpartum revealed only 5 reports of PUPPP or polymorphic eruption of pregnancy occurring in the postpartum period, 2 occurring in the United States.5-9
Case Report
A 30-year-old woman who was 2 weeks postpartum presented to our dermatology clinic with an intensely pruritic generalized rash. Within 24 hours of delivery of her first child, the patient developed an itchy rash on the abdomen and was started on oral corticosteroids and antihistamines in the hospital. On discharge, she was instructed to follow up with the dermatology department if the rash did not resolve. After leaving the hospital, she reported that the eruption had progressively spread to the buttocks, legs, and arms, and the itching seemed to be worse despite finishing the course of oral corticosteroids and antihistamines.
The patient’s prenatal course was uneventful. She gained 16 kg during pregnancy, with a prepregnancy weight of 50 kg. A healthy male neonate was delivered at 38 weeks’ gestation without complication. The patient’s medical history was unremarkable. Her current medications included prenatal vitamins, oral prednisone, and loratadine, and she reported no known drug allergies.
On physical examination, the patient was afebrile and her blood pressure was normal. Examination of the skin revealed erythematous papules and urticarial plaques involving the abdominal striae with periumbilical sparing (Figure 1A). Similar lesions were noted on the legs, buttocks, and arms (Figure 1B). The face, palms, and soles were uninvolved. No vesicles or pustules were noted. The oral mucosa was pink, moist, and unremarkable.


Figure 1. Initial presentation of urticarial plaques involving the abdominal striae with periumbilical sparing (A) and the left arm (B). |
Based on the patient’s clinical presentation, the differential diagnosis included pemphigoid gestationis, a hypersensitivity reaction, cutaneous lupus, cholestasis of pregnancy, and PUPPP. Pruritic urticarial papules and plaques of pregnancy was considered to be unlikely because of the uncharacteristic postpartum presentation of the eruption.
Two 4-mm punch biopsies were performed on the left upper arm and were sent for histopathologic examination and direct immunofluorescence. Laboratory studies including complete blood cell count with differential, complete metabolic panel, antinuclear antibodies, and IgE levels were conducted. The patient was started on triamcinolone cream 0.1% twice daily and her antihistamine was switched from loratadine to cetirizine.
Histopathologic examination revealed a mixed perivascular infiltrate in the superficial dermis consisting of lymphocytes, mast cells, and eosinophils (Figures 2 and 3), which was consistent with a diagnosis of PUPPP. Direct immunofluorescence was negative. Laboratory studies were within reference range and antinuclear antibodies and IgE levels were negative. A diagnosis of postpartum PUPPP was made. Complete resolution of the eruption was experienced by 2-week follow-up (Figures 4A and 4B). The patient noted that her symptoms improved within 2 days of starting topical therapy.
|
|
Comment
Pruritic urticarial papules and plaques of pregnancy complicates 1 of 160 to 1 of 300 pregnancies.1 As seen in our case, the majority of cases of PUPPP are diagnosed in women who are nulliparous or primigravida.10 A study by Aronson et al10 reported that of 57 cases of PUPPP, 24 (42%) patients were primigravida, 16 (28%) were gravida 2, 9 (16%) were gravida 3, 4 (7%) were gravida 4, 3 (5%) were gravida 6, and 1 (2%) was gravida 7. Thirty-nine (68%) patients were nulliparous.10 The average onset of symptoms is approximately 35 weeks’ gestation.9
Classical presentation of PUPPP starts with erythematous papules within the abdominal striae, sparing the periumbilical skin.1 The abdominal striae are most commonly affected, and in some women, it may be the only site affected.10 The lesions then may pro-gress to urticarial plaques involving the extremities, while the face, palms, and soles usually are spared.11 However, clinical manifestations of PUPPP can vary, with reports of targetlike lesions with a surrounding halo resembling erythema multiforme as well as involvement of the face and palmoplantar skin.10-13 Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.14 Histologically, PUPPP demonstrates variable epidermal spongiosis and a nonspecific superficial perivascular infiltrate in the dermis composed of lymphocytes with eosinophils or neutrophils, and there may be dermal edema.10,15 Direct immunofluorescence usually is negative in PUPPP; however, 31% of cases have demonstrated deposition of C3 and IgM or IgA, either perivascularly or at the dermoepidermal junction.1,10,15
There are no systemic alterations seen in PUPPP; however, all patients report severe pruritus.12 Pruritic urticarial papules and plaques of pregnancy typically affects women in the third trimester, and delivery is curative in most patients.13 Recurrence of PUPPP usually is not seen with subsequent pregnancies, and the long-term prognosis is excellent.15
The pathogenesis of PUPPP is not well understood and likely is multifactorial. Ohel et al12 found PUPPP to be strongly associated with hypertensive disorders, multiple gestation pregnancies, excessive maternal weight gain, excessive stretching of the abdominal skin, and nulliparity.13 One theory suggests that abdominal skin stretching, if drastic, can damage underlying connective tissue, resulting in the release of antigens that can trigger a reactive inflammatory response.16 The majority of maternal weight gain occurs during the third trimester, which may explain why most cases of PUPPP present in the third trimester.17 Alternative theories have suggested that PUPPP may represent an immunologic response to circulating fetal antigens.18 It is possible, as in our case, that certain nulliparous women who have a healthy weight prior to pregnancy (as determined by a body mass index of 18.5 to 24.9) in combination with excessive weight gain during the third trimester and drastic hormone fluctuations associated with labor and delivery may be at greater risk for developing PUPPP. Another theory may be related to the degree of skin stretching during the third trimester and the abrupt decrease in the stretching of the skin that occurs with delivery.16
Conclusion
Pruritic urticarial papules and plaques of pregnancy can present in a variety of ways, most commonly in the third trimester but also in the postpartum period. When a patient presents in the postpartum period with a pruritic eruption, PUPPP should be included in the differential diagnosis. The pathogenesis of PUPPP is multifactorial and not well understood, and additional research in the field may lead to improved prediction of who may be at risk and what we can do to prevent it.
1. Pomeranz MK. Dermatoses of pregnancy. UpToDate Web site. http://www.uptodate.com/contents/dermatoses-of-pregnancy. Updated December 22, 2014. Accessed May 5, 2015.
2. Lawley TJ, Hertz KC, Wade TR, et al. Pruritic urticarial papules and plaques of pregnancy. JAMA. 1979;241:1696-1699.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
4. Callen JP, Hanno R. Pruritic urticarial papules and plaques of pregnancy (PUPPP): a clinicopathologic study. J Am Acad Dermatol.1981;5:401-405.
5. Ozcan D, Ozcakmak B, Aydogan FC. J Obstet Gynaecol Res. 2011;37:1158-1161.
6. Journet-Tollhupp J, Tchen T, Remy-Leroux V, et al. Polymorphic eruption of pregnancy and acquired hemophilia A [in French]. Ann Dermatol Venereol. 2010;137:713-717.
7. Buccolo LS, Viera AJ. Pruritic urticarial papules and plaques of pregnancy presenting in the postpartum period: a case report. J Reprod Med. 2005;50:61-63.
8. Kirkup ME, Dunnill MG. Polymorphic eruption of pregnancy developing in the puerrperium. Clin Exp Dermatol. 2002;27:657-660.
9. Yancy KB, Hall RP, Lawley TJ. Pruritic urticarial papules and plaques of pregnancy: clinical experience in twenty-five patients. J Am Acad Dermatol. 1984;10:473-480.
10. Aronson IK, Bond S, Fiedler VC, et al. Pruritic urticarial papules and plaques of pregnancy: clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol. 1998;39:933-939.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic dermatoses of pregnancy: a prospective study of 3129 women. Arch Dermatol. 1994;130:734-739.
12. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Matern Fetal Neonatal Med. 2006;19:305-308.
13. Elling SV, McKenna P, Pawell FC. Pruritic urticarial papules and plaques of pregnancy in twin and triplet pregnancies. J Eur Acad Dermatol Venereol. 2000;14:378-381.
14. Scheinfeld N. Pruritic urticarial papules and plaques of pregnancy wholly abated with one week twice daily application of fluticasone propionate lotion: a case report and review of the literature. Dermatol Online J. 2008;14:4.
15. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
16. Cohen LM, Capeless EL, Krusinski PA, et al. Pruritic urticarial papules and plaques of pregnancy and its relationship to maternal-fetal weight gain and twin pregnancy. Arch Dermatol. 1989;125:1534-1536.
17. Drehmer M, Duncan BB, Kac G, et al. Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One. 2013;8:e54704.
18. Aractingi S, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
1. Pomeranz MK. Dermatoses of pregnancy. UpToDate Web site. http://www.uptodate.com/contents/dermatoses-of-pregnancy. Updated December 22, 2014. Accessed May 5, 2015.
2. Lawley TJ, Hertz KC, Wade TR, et al. Pruritic urticarial papules and plaques of pregnancy. JAMA. 1979;241:1696-1699.
3. Kroumpouzos G, Cohen LM. Specific dermatoses of pregnancy: an evidence-based systematic review. Am J Obstet Gynecol. 2003;188:1083-1092.
4. Callen JP, Hanno R. Pruritic urticarial papules and plaques of pregnancy (PUPPP): a clinicopathologic study. J Am Acad Dermatol.1981;5:401-405.
5. Ozcan D, Ozcakmak B, Aydogan FC. J Obstet Gynaecol Res. 2011;37:1158-1161.
6. Journet-Tollhupp J, Tchen T, Remy-Leroux V, et al. Polymorphic eruption of pregnancy and acquired hemophilia A [in French]. Ann Dermatol Venereol. 2010;137:713-717.
7. Buccolo LS, Viera AJ. Pruritic urticarial papules and plaques of pregnancy presenting in the postpartum period: a case report. J Reprod Med. 2005;50:61-63.
8. Kirkup ME, Dunnill MG. Polymorphic eruption of pregnancy developing in the puerrperium. Clin Exp Dermatol. 2002;27:657-660.
9. Yancy KB, Hall RP, Lawley TJ. Pruritic urticarial papules and plaques of pregnancy: clinical experience in twenty-five patients. J Am Acad Dermatol. 1984;10:473-480.
10. Aronson IK, Bond S, Fiedler VC, et al. Pruritic urticarial papules and plaques of pregnancy: clinical and immunopathologic observations in 57 patients. J Am Acad Dermatol. 1998;39:933-939.
11. Roger D, Vaillant L, Fignon A, et al. Specific pruritic dermatoses of pregnancy: a prospective study of 3129 women. Arch Dermatol. 1994;130:734-739.
12. Ohel I, Levy A, Silberstein T, et al. Pregnancy outcome of patients with pruritic urticarial papules and plaques of pregnancy. J Matern Fetal Neonatal Med. 2006;19:305-308.
13. Elling SV, McKenna P, Pawell FC. Pruritic urticarial papules and plaques of pregnancy in twin and triplet pregnancies. J Eur Acad Dermatol Venereol. 2000;14:378-381.
14. Scheinfeld N. Pruritic urticarial papules and plaques of pregnancy wholly abated with one week twice daily application of fluticasone propionate lotion: a case report and review of the literature. Dermatol Online J. 2008;14:4.
15. Shornick JK. Dermatoses of pregnancy. Semin Cutan Med Surg. 1998;17:172-181.
16. Cohen LM, Capeless EL, Krusinski PA, et al. Pruritic urticarial papules and plaques of pregnancy and its relationship to maternal-fetal weight gain and twin pregnancy. Arch Dermatol. 1989;125:1534-1536.
17. Drehmer M, Duncan BB, Kac G, et al. Association of second and third trimester weight gain in pregnancy with maternal and fetal outcomes. PLoS One. 2013;8:e54704.
18. Aractingi S, Berkane N, Bertheau P, et al. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898-1901.
Practice Points
- Pruritic urticarial papules and plaques of pregnancy (PUPPP) is an intensely pruritic eruption that typically affects women during the third trimester of pregnancy.
- Because clinical manifestations can vary, PUPPP should be considered in the differential diagnosis when patients present in the postpartum period with a pruritic eruption.
- Histologic findings are not diagnostic but can help distinguish PUPPP from other pregnancy-associated dermatoses.
Prevalence and Impact of Health-Related Internet and Smartphone Use Among Dermatology Patients
Patients increasingly use the Internet and/or smartphone applications (apps) to seek health information and track personal health data,1,2 typically in the spirit of being a more educated consumer. However, many patients use the Internet in an attempt to self-diagnose and independently find treatment options, thus avoiding (in their opinion) the need to seek in-person medical care. Additionally, electronic access to health information has expanded beyond computers to smartphones with apps that can provide users with a simple interface to personalize the health information they seek and receive.
Prior studies have shown that seeking online health information and health-related social media is more common among women, younger patients, those with a college education, and those with a higher income.3,4 However, the prevalence of health-related Internet and smartphone use among dermatology patients as well as how patients ultimately use this information is not well studied. This information about patient behavior is important because of the potential harm that may come from patient self-diagnosis, which may delay or prevent treatment, as well as the benefits of patient self-education, which may expedite diagnosis and treatment.5 We surveyed a heterogeneous patient population at 2 dermatology offices in a major academic medical center to assess the prevalence and predictors of Internet and smartphone use to obtain both general medical and dermatologic information among dermatology patients. We also evaluated the impact that health information obtained from online sources has on a patient’s degree of concern about cutaneous disease and the likelihood of seeing a dermatologist for a skin problem.
Methods
Survey and Participants
This study was approved by the institutional review board at the University of Pittsburgh, Pennsylvania. All patients aged 18 years or older who presented to the department of dermatology at 2 offices of the University of Pittsburgh Medical Center from September 2013 through July 2014 were invited to participate in an anonymous 33-question survey regarding their use of the Internet and smartphone apps to obtain health information and make health care decisions. Patients were asked to complete the survey prior to seeing a health care provider and return it to a locked box by the front desk before leaving the office. Survey questions were designed by physicians with content expertise (J.A.W. and L.K.F.) and were reviewed by a statistician with survey expertise (D.G.W.). The survey included questions about patient demographics, Internet and smartphone use (both general and health related), and specific sources accessed. The survey also inquired about the impact of health information obtained via the Internet and smartphone apps on respondents’ degree of worry about a hypothetical skin condition or lesion using a 5-point Likert scale (1=no worry; 5=very worried). Respondents also were asked which skin conditions they previously researched online and whether their findings impacted their decision to see a dermatologist. Additionally, respondents were asked to list the smartphone apps and other online health resources they had used within the last 3 months. Prior to distribution, the survey was piloted with 10 participants and no issues with comprehensibility were noted.
Statistical Analysis
We described demographic traits (eg, age, sex, race/ethnicity, level of education, income) and factors associated with access to health care (eg, specialist co-pay, travel time from dermatology office) of respondents using proportions. We evaluated respondents’ access to and use of Internet- and smartphone-based health information using proportions and used χ² tests to quantify differences by sex and age (<50 years and ≥50 years).
We analyzed the impact of Internet and smartphone-based health information on patient worry about skin conditions by obtaining median worry on a 5-point Likert scale. Due to the nonparametric nature of the data, we used the Mann-Whitney U test to quantify differences by sex and age (<50 and ≥50 years). We used multiple logistic regression to identify factors associated with 3 outcomes: (1) using the Internet to self-diagnose a dermatologic disease, (2) using the Internet to obtain dermatology-related information within the last 3 months, (3) and previously refraining from visiting a dermatologist based on reassurance from online resources. Predictors included the aforementioned demographic and health-care access–related traits. We also categorized smartphone apps used by respondents (ie, fitness/nutrition, reference, self-help, health monitoring, diagnostic aids, electronic medical record) and calculated the proportion of respondents with 1 or more of each type of app on their smartphones. Analyses were conducted in Stata 13.1 and IBM SPSS 22.0.
Results
Of 1000 patients who were invited to participate in the study, a total of 775 respondents completed the survey, yielding a response rate of 77.5%. The majority of respondents were aged 30 to 60 years (mean age [standard deviation], 44.5 [17.2] years; median age [interquartile range], 44 [29–59] years), female (66.7%), and non-Hispanic white (83.3%)(Table 1). The majority of respondents (88.8%) had completed at least some college. Nearly all respondents had medical insurance (97.8%), but annual household income and insurance co-pay varied considerably. Only 10.8% of respondents traveled more than an hour to our offices.
The majority of respondents had access to home Internet and owned a smartphone (Table 2). Use of the Internet to obtain health-related information in the 3 months prior to presentation was more common among females (77.9% vs 70.1%; P=.03) and respondents younger than 50 years (83.4% vs 62.5%; P<.001); the same was true for dermatology-related infor-mation (females: 43.2% vs 31.0%; P=.003; aged <50 years, 51.6% vs 22.2%; P<.001). The majority of respondents indicated that they use the Internet to obtain health-related information both before and after they see their doctor. Most respondents indicated that they sometimes discuss health-related information found on the Internet with a physician. Smartphone use to obtain health-related information was more common among respondents younger than 50 years versus those who were 50 years or older (55.5% vs 24.1%; P<.001), as was smartphone use to diagnose skin problems (20.0% vs 6.3%; P<.001).
In multivariable analysis, use of the Internet or a smartphone to obtain health-related information was associated with younger age (<50 years) and a higher level of education (both P<.001). Use of the Internet to obtain dermatology-related information (P<.001) and use of a smartphone to help diagnose a skin problem (P=.001) was associated with younger age (<50 years) only. Income, sex, co-pay to see a dermatologist, and travel time to the dermatology office were not associated with use of online resources for general or dermatology-specific health-related information or assistance with diagnosing a skin problem.
Of 204 respondents who indicated that they previously attempted to self-diagnose a skin condition using the Internet, the most commonly researched condition was skin cancer/moles/unknown spots (64.7%), followed by rashes (40.7%), acne (20.6%), cosmetic issues (16.2%), psoriasis (12.7%), dermatitis (3.4%), warts (1.5%), tick bites (1.0%), and lupus (1.0%)(some respondents selected more than one condition). Only 7.0% of respondents indicated that they previously had refrained from visiting a dermatologist based on reassurance from online resources. Compared to the rest of the surveyed population, these respondents were younger (P=.001), but there were no significant differences in sex, highest level of education, household income, or travel time to the dermatology office. The most commonly researched condition among these respondents was acne (12 respondents), and 11 respondents indicated that they had attempted to self-diagnose a mole or potential cancer using online sources.
Of 557 respondents who owned a smartphone, 31.8% reported using at least 1 health-related app (mean number of health apps per respondent, 1.5). Of the apps that respondents used, 45.9% focused on fitness/nutrition, 28.7% provided reference information, 13.4% were a patient portal for receiving information from their electronic medical record, 8.6% provided a health monitoring function, 1.9% served as a diagnostic aid, and 1.5% provided coping assistance and emotional support for individuals with cognitive or emotional conditions; only 1 respondent reported using an app related to dermatology.
All respondents were asked to rate their anticipated degree of worry if the Internet or a smartphone app suggested that a skin lesion was benign versus dangerous on a 5-point scale. Overall, the median worry rating increased from 3 to 5 when information accessed via the Internet or a smartphone app suggested a lesion was dangerous rather than benign. A change in worry of 2 or more points was seen in 36.1% of females and 49.1% of males (P=.002) when information obtained via the Internet indicated a lesion was dangerous and in 47.5% of females and 58.8% of males (P=.006) when a smartphone app indicated that a lesion was dangerous. When information obtained via the Internet indicated a lesion was dangerous, a change in worry of 2 or more points was seen in 41.8% of respondents who were younger than 50 years and in 41.1% of those who were 50 years or older (P=.93). When a smartphone app indicated a lesion was dangerous, a change in worry of 2 or more points was seen in 50.2% of respondents who were younger than 50 years and in 52.2% of those who were 50 years or older (P=.61).
Discussion
In this cross-sectional study, we found that health-related Internet and smartphone use among dermatology patients is common and may impact both patients’ degree of concern about a skin lesion as well as the likelihood of seeking in-person medical care if they are reassured by the results of their online findings. Age and level of education were associated with Internet and smartphone use to obtain dermatology-related health information but not factors related to health care access. More patients used the Internet or a smartphone to obtain general medical information versus dermatology-related information. Respondents who indicated that they used the Internet to obtain health-related information tended to do so before visiting their physician.
Our finding that a patient’s level of worry about a hypothetical skin condition or lesion is influenced by health information obtained via the Internet or a smartphone app is concerning. One study found that participants who used a popular search engine to look for information about vaccine safety and dangers were directed to Web sites with inaccurate information more than 50% of the time, and 65% of the information they obtained from these sites was false.6 In our study, approximately 25% of respondents had previously consulted online resources to attempt toself-diagnose a skin condition. Online sources about dermatologic conditions were consulted most frequently for information about potential skin cancers, moles, and unknown spots. A prior study showed that smartphone apps that claim to aid patients in determining whether a skin lesion is low or high risk for melanoma often are inaccurate and are associated with a high rate of missed melanomas.5 Even though we surveyed patients who did end up seeing a dermatologist, some respondents had previously opted out of seeing a dermatologist based on information they had found online. Because our study was conducted among patients who chose to seek care at a dermatology office, the problem is likely greater than estimated from our findings because we had no way of reaching individuals who decided to completely forgo a visit with a dermatologist.
Although use of the Internet to obtain health-related information was common among older adults in our population, it was nearly universal in younger adults. Health-related smartphone use was more than twice as common in younger versus older adults, which could be due to an increased comfort with technology and its integration into daily life. The fact that age and education were associated with Internet use for dermatology-related health information but not household income or travel time to the dermatology office suggests that information seeking is not due to lack of resources limiting access to dermatologic care but rather to the greater role that rapid access to online information plays in patients’ lives. Our findings are similar to another study that examined the use of online sources for general health information.7
This study has several limitations. First, there may have been some selection bias. We specifically aimed to understand the health-related Internet and smartphone use among dermatology patients, thus restricting our sample to this population. By doing so, we were unable to assess the use of such resources by the general population, particularly those individuals who chose not to see a dermatologist at all based on their own online research. Our findings may not apply to other practices and regions of the country, as we implemented our study in one geographic location and in offices of an academic practice. Although our sample size and diversity with regard to income, education, and age suggest that our results are likely generalizable to many settings, it is important to note that nearly all respondents in this study had health insurance and our findings are thus not necessarily applicable to those individuals who are uninsured.
Conclusion
Our findings suggest that the availability of online health information regarding dermatologic conditions provides dermatologists with both opportunities and challenges. Many patients consult online resources for health information, and the popularity of this practice is likely to increase with time, particularly as newer smartphones with features designed to allow users to monitor their health are developed with health-conscious consumers in mind. Most large health care systems provide patients with resources to view laboratory results and communicate with physicians online. It is important for dermatologists to be involved in the development of high-quality online content that educates the public while also emphasizing the need to seek in-person medical care, particularly in potential cases of skin cancer. It also is important for patients to be involved in the content development process to ensure that the messages they take away from online resources are the ones physicians wish to convey. Ideally, online forms of education will increase patients’ sense of self-efficacy while encouraging appropriate consultation for potentially harmful skin conditions.
1. Atkinson NL, Saperstein SL, Pleis J. Using the Internet for health-related activities: findings from a national probability sample. J Med Internet Res. 2009;11:e4.
2. Ybarra M, Suman M. Reasons, assessments and actions taken: sex and age differences in uses of Internet health information. Health Educ Res. 2008;23:512-521.
3. Bhandari N, Shi Y, Jung K. Seeking health information online: does limited healthcare access matter? J Am Med Inform Assoc. 2014;21:1113-1117.
4. Thackeray R, Crookston BT, West JH. Correlates of health-related social media use among adults. J Med Internet Res. 2013;15:e21.
5. Wolf JA, Moreau JF, Akilov O, et al. Diagnostic inaccuracy of smartphone applications for melanoma detection. JAMA Dermatol. 2013;149:422-426.
6. Kortum P, Edwards C, Richards-Kortum R. The impact of inaccurate Internet health information in a secondary school learning environment. J Med Internet Res. 2008;10:e17.
7. Mead N, Varnam R, Rogers A, et al. What predicts patients’ interest in the internet as a health resource in primary care in England? J Health Serv Res Policy. 2003;8:33-39.
Patients increasingly use the Internet and/or smartphone applications (apps) to seek health information and track personal health data,1,2 typically in the spirit of being a more educated consumer. However, many patients use the Internet in an attempt to self-diagnose and independently find treatment options, thus avoiding (in their opinion) the need to seek in-person medical care. Additionally, electronic access to health information has expanded beyond computers to smartphones with apps that can provide users with a simple interface to personalize the health information they seek and receive.
Prior studies have shown that seeking online health information and health-related social media is more common among women, younger patients, those with a college education, and those with a higher income.3,4 However, the prevalence of health-related Internet and smartphone use among dermatology patients as well as how patients ultimately use this information is not well studied. This information about patient behavior is important because of the potential harm that may come from patient self-diagnosis, which may delay or prevent treatment, as well as the benefits of patient self-education, which may expedite diagnosis and treatment.5 We surveyed a heterogeneous patient population at 2 dermatology offices in a major academic medical center to assess the prevalence and predictors of Internet and smartphone use to obtain both general medical and dermatologic information among dermatology patients. We also evaluated the impact that health information obtained from online sources has on a patient’s degree of concern about cutaneous disease and the likelihood of seeing a dermatologist for a skin problem.
Methods
Survey and Participants
This study was approved by the institutional review board at the University of Pittsburgh, Pennsylvania. All patients aged 18 years or older who presented to the department of dermatology at 2 offices of the University of Pittsburgh Medical Center from September 2013 through July 2014 were invited to participate in an anonymous 33-question survey regarding their use of the Internet and smartphone apps to obtain health information and make health care decisions. Patients were asked to complete the survey prior to seeing a health care provider and return it to a locked box by the front desk before leaving the office. Survey questions were designed by physicians with content expertise (J.A.W. and L.K.F.) and were reviewed by a statistician with survey expertise (D.G.W.). The survey included questions about patient demographics, Internet and smartphone use (both general and health related), and specific sources accessed. The survey also inquired about the impact of health information obtained via the Internet and smartphone apps on respondents’ degree of worry about a hypothetical skin condition or lesion using a 5-point Likert scale (1=no worry; 5=very worried). Respondents also were asked which skin conditions they previously researched online and whether their findings impacted their decision to see a dermatologist. Additionally, respondents were asked to list the smartphone apps and other online health resources they had used within the last 3 months. Prior to distribution, the survey was piloted with 10 participants and no issues with comprehensibility were noted.
Statistical Analysis
We described demographic traits (eg, age, sex, race/ethnicity, level of education, income) and factors associated with access to health care (eg, specialist co-pay, travel time from dermatology office) of respondents using proportions. We evaluated respondents’ access to and use of Internet- and smartphone-based health information using proportions and used χ² tests to quantify differences by sex and age (<50 years and ≥50 years).
We analyzed the impact of Internet and smartphone-based health information on patient worry about skin conditions by obtaining median worry on a 5-point Likert scale. Due to the nonparametric nature of the data, we used the Mann-Whitney U test to quantify differences by sex and age (<50 and ≥50 years). We used multiple logistic regression to identify factors associated with 3 outcomes: (1) using the Internet to self-diagnose a dermatologic disease, (2) using the Internet to obtain dermatology-related information within the last 3 months, (3) and previously refraining from visiting a dermatologist based on reassurance from online resources. Predictors included the aforementioned demographic and health-care access–related traits. We also categorized smartphone apps used by respondents (ie, fitness/nutrition, reference, self-help, health monitoring, diagnostic aids, electronic medical record) and calculated the proportion of respondents with 1 or more of each type of app on their smartphones. Analyses were conducted in Stata 13.1 and IBM SPSS 22.0.
Results
Of 1000 patients who were invited to participate in the study, a total of 775 respondents completed the survey, yielding a response rate of 77.5%. The majority of respondents were aged 30 to 60 years (mean age [standard deviation], 44.5 [17.2] years; median age [interquartile range], 44 [29–59] years), female (66.7%), and non-Hispanic white (83.3%)(Table 1). The majority of respondents (88.8%) had completed at least some college. Nearly all respondents had medical insurance (97.8%), but annual household income and insurance co-pay varied considerably. Only 10.8% of respondents traveled more than an hour to our offices.
The majority of respondents had access to home Internet and owned a smartphone (Table 2). Use of the Internet to obtain health-related information in the 3 months prior to presentation was more common among females (77.9% vs 70.1%; P=.03) and respondents younger than 50 years (83.4% vs 62.5%; P<.001); the same was true for dermatology-related infor-mation (females: 43.2% vs 31.0%; P=.003; aged <50 years, 51.6% vs 22.2%; P<.001). The majority of respondents indicated that they use the Internet to obtain health-related information both before and after they see their doctor. Most respondents indicated that they sometimes discuss health-related information found on the Internet with a physician. Smartphone use to obtain health-related information was more common among respondents younger than 50 years versus those who were 50 years or older (55.5% vs 24.1%; P<.001), as was smartphone use to diagnose skin problems (20.0% vs 6.3%; P<.001).
In multivariable analysis, use of the Internet or a smartphone to obtain health-related information was associated with younger age (<50 years) and a higher level of education (both P<.001). Use of the Internet to obtain dermatology-related information (P<.001) and use of a smartphone to help diagnose a skin problem (P=.001) was associated with younger age (<50 years) only. Income, sex, co-pay to see a dermatologist, and travel time to the dermatology office were not associated with use of online resources for general or dermatology-specific health-related information or assistance with diagnosing a skin problem.
Of 204 respondents who indicated that they previously attempted to self-diagnose a skin condition using the Internet, the most commonly researched condition was skin cancer/moles/unknown spots (64.7%), followed by rashes (40.7%), acne (20.6%), cosmetic issues (16.2%), psoriasis (12.7%), dermatitis (3.4%), warts (1.5%), tick bites (1.0%), and lupus (1.0%)(some respondents selected more than one condition). Only 7.0% of respondents indicated that they previously had refrained from visiting a dermatologist based on reassurance from online resources. Compared to the rest of the surveyed population, these respondents were younger (P=.001), but there were no significant differences in sex, highest level of education, household income, or travel time to the dermatology office. The most commonly researched condition among these respondents was acne (12 respondents), and 11 respondents indicated that they had attempted to self-diagnose a mole or potential cancer using online sources.
Of 557 respondents who owned a smartphone, 31.8% reported using at least 1 health-related app (mean number of health apps per respondent, 1.5). Of the apps that respondents used, 45.9% focused on fitness/nutrition, 28.7% provided reference information, 13.4% were a patient portal for receiving information from their electronic medical record, 8.6% provided a health monitoring function, 1.9% served as a diagnostic aid, and 1.5% provided coping assistance and emotional support for individuals with cognitive or emotional conditions; only 1 respondent reported using an app related to dermatology.
All respondents were asked to rate their anticipated degree of worry if the Internet or a smartphone app suggested that a skin lesion was benign versus dangerous on a 5-point scale. Overall, the median worry rating increased from 3 to 5 when information accessed via the Internet or a smartphone app suggested a lesion was dangerous rather than benign. A change in worry of 2 or more points was seen in 36.1% of females and 49.1% of males (P=.002) when information obtained via the Internet indicated a lesion was dangerous and in 47.5% of females and 58.8% of males (P=.006) when a smartphone app indicated that a lesion was dangerous. When information obtained via the Internet indicated a lesion was dangerous, a change in worry of 2 or more points was seen in 41.8% of respondents who were younger than 50 years and in 41.1% of those who were 50 years or older (P=.93). When a smartphone app indicated a lesion was dangerous, a change in worry of 2 or more points was seen in 50.2% of respondents who were younger than 50 years and in 52.2% of those who were 50 years or older (P=.61).
Discussion
In this cross-sectional study, we found that health-related Internet and smartphone use among dermatology patients is common and may impact both patients’ degree of concern about a skin lesion as well as the likelihood of seeking in-person medical care if they are reassured by the results of their online findings. Age and level of education were associated with Internet and smartphone use to obtain dermatology-related health information but not factors related to health care access. More patients used the Internet or a smartphone to obtain general medical information versus dermatology-related information. Respondents who indicated that they used the Internet to obtain health-related information tended to do so before visiting their physician.
Our finding that a patient’s level of worry about a hypothetical skin condition or lesion is influenced by health information obtained via the Internet or a smartphone app is concerning. One study found that participants who used a popular search engine to look for information about vaccine safety and dangers were directed to Web sites with inaccurate information more than 50% of the time, and 65% of the information they obtained from these sites was false.6 In our study, approximately 25% of respondents had previously consulted online resources to attempt toself-diagnose a skin condition. Online sources about dermatologic conditions were consulted most frequently for information about potential skin cancers, moles, and unknown spots. A prior study showed that smartphone apps that claim to aid patients in determining whether a skin lesion is low or high risk for melanoma often are inaccurate and are associated with a high rate of missed melanomas.5 Even though we surveyed patients who did end up seeing a dermatologist, some respondents had previously opted out of seeing a dermatologist based on information they had found online. Because our study was conducted among patients who chose to seek care at a dermatology office, the problem is likely greater than estimated from our findings because we had no way of reaching individuals who decided to completely forgo a visit with a dermatologist.
Although use of the Internet to obtain health-related information was common among older adults in our population, it was nearly universal in younger adults. Health-related smartphone use was more than twice as common in younger versus older adults, which could be due to an increased comfort with technology and its integration into daily life. The fact that age and education were associated with Internet use for dermatology-related health information but not household income or travel time to the dermatology office suggests that information seeking is not due to lack of resources limiting access to dermatologic care but rather to the greater role that rapid access to online information plays in patients’ lives. Our findings are similar to another study that examined the use of online sources for general health information.7
This study has several limitations. First, there may have been some selection bias. We specifically aimed to understand the health-related Internet and smartphone use among dermatology patients, thus restricting our sample to this population. By doing so, we were unable to assess the use of such resources by the general population, particularly those individuals who chose not to see a dermatologist at all based on their own online research. Our findings may not apply to other practices and regions of the country, as we implemented our study in one geographic location and in offices of an academic practice. Although our sample size and diversity with regard to income, education, and age suggest that our results are likely generalizable to many settings, it is important to note that nearly all respondents in this study had health insurance and our findings are thus not necessarily applicable to those individuals who are uninsured.
Conclusion
Our findings suggest that the availability of online health information regarding dermatologic conditions provides dermatologists with both opportunities and challenges. Many patients consult online resources for health information, and the popularity of this practice is likely to increase with time, particularly as newer smartphones with features designed to allow users to monitor their health are developed with health-conscious consumers in mind. Most large health care systems provide patients with resources to view laboratory results and communicate with physicians online. It is important for dermatologists to be involved in the development of high-quality online content that educates the public while also emphasizing the need to seek in-person medical care, particularly in potential cases of skin cancer. It also is important for patients to be involved in the content development process to ensure that the messages they take away from online resources are the ones physicians wish to convey. Ideally, online forms of education will increase patients’ sense of self-efficacy while encouraging appropriate consultation for potentially harmful skin conditions.
Patients increasingly use the Internet and/or smartphone applications (apps) to seek health information and track personal health data,1,2 typically in the spirit of being a more educated consumer. However, many patients use the Internet in an attempt to self-diagnose and independently find treatment options, thus avoiding (in their opinion) the need to seek in-person medical care. Additionally, electronic access to health information has expanded beyond computers to smartphones with apps that can provide users with a simple interface to personalize the health information they seek and receive.
Prior studies have shown that seeking online health information and health-related social media is more common among women, younger patients, those with a college education, and those with a higher income.3,4 However, the prevalence of health-related Internet and smartphone use among dermatology patients as well as how patients ultimately use this information is not well studied. This information about patient behavior is important because of the potential harm that may come from patient self-diagnosis, which may delay or prevent treatment, as well as the benefits of patient self-education, which may expedite diagnosis and treatment.5 We surveyed a heterogeneous patient population at 2 dermatology offices in a major academic medical center to assess the prevalence and predictors of Internet and smartphone use to obtain both general medical and dermatologic information among dermatology patients. We also evaluated the impact that health information obtained from online sources has on a patient’s degree of concern about cutaneous disease and the likelihood of seeing a dermatologist for a skin problem.
Methods
Survey and Participants
This study was approved by the institutional review board at the University of Pittsburgh, Pennsylvania. All patients aged 18 years or older who presented to the department of dermatology at 2 offices of the University of Pittsburgh Medical Center from September 2013 through July 2014 were invited to participate in an anonymous 33-question survey regarding their use of the Internet and smartphone apps to obtain health information and make health care decisions. Patients were asked to complete the survey prior to seeing a health care provider and return it to a locked box by the front desk before leaving the office. Survey questions were designed by physicians with content expertise (J.A.W. and L.K.F.) and were reviewed by a statistician with survey expertise (D.G.W.). The survey included questions about patient demographics, Internet and smartphone use (both general and health related), and specific sources accessed. The survey also inquired about the impact of health information obtained via the Internet and smartphone apps on respondents’ degree of worry about a hypothetical skin condition or lesion using a 5-point Likert scale (1=no worry; 5=very worried). Respondents also were asked which skin conditions they previously researched online and whether their findings impacted their decision to see a dermatologist. Additionally, respondents were asked to list the smartphone apps and other online health resources they had used within the last 3 months. Prior to distribution, the survey was piloted with 10 participants and no issues with comprehensibility were noted.
Statistical Analysis
We described demographic traits (eg, age, sex, race/ethnicity, level of education, income) and factors associated with access to health care (eg, specialist co-pay, travel time from dermatology office) of respondents using proportions. We evaluated respondents’ access to and use of Internet- and smartphone-based health information using proportions and used χ² tests to quantify differences by sex and age (<50 years and ≥50 years).
We analyzed the impact of Internet and smartphone-based health information on patient worry about skin conditions by obtaining median worry on a 5-point Likert scale. Due to the nonparametric nature of the data, we used the Mann-Whitney U test to quantify differences by sex and age (<50 and ≥50 years). We used multiple logistic regression to identify factors associated with 3 outcomes: (1) using the Internet to self-diagnose a dermatologic disease, (2) using the Internet to obtain dermatology-related information within the last 3 months, (3) and previously refraining from visiting a dermatologist based on reassurance from online resources. Predictors included the aforementioned demographic and health-care access–related traits. We also categorized smartphone apps used by respondents (ie, fitness/nutrition, reference, self-help, health monitoring, diagnostic aids, electronic medical record) and calculated the proportion of respondents with 1 or more of each type of app on their smartphones. Analyses were conducted in Stata 13.1 and IBM SPSS 22.0.
Results
Of 1000 patients who were invited to participate in the study, a total of 775 respondents completed the survey, yielding a response rate of 77.5%. The majority of respondents were aged 30 to 60 years (mean age [standard deviation], 44.5 [17.2] years; median age [interquartile range], 44 [29–59] years), female (66.7%), and non-Hispanic white (83.3%)(Table 1). The majority of respondents (88.8%) had completed at least some college. Nearly all respondents had medical insurance (97.8%), but annual household income and insurance co-pay varied considerably. Only 10.8% of respondents traveled more than an hour to our offices.
The majority of respondents had access to home Internet and owned a smartphone (Table 2). Use of the Internet to obtain health-related information in the 3 months prior to presentation was more common among females (77.9% vs 70.1%; P=.03) and respondents younger than 50 years (83.4% vs 62.5%; P<.001); the same was true for dermatology-related infor-mation (females: 43.2% vs 31.0%; P=.003; aged <50 years, 51.6% vs 22.2%; P<.001). The majority of respondents indicated that they use the Internet to obtain health-related information both before and after they see their doctor. Most respondents indicated that they sometimes discuss health-related information found on the Internet with a physician. Smartphone use to obtain health-related information was more common among respondents younger than 50 years versus those who were 50 years or older (55.5% vs 24.1%; P<.001), as was smartphone use to diagnose skin problems (20.0% vs 6.3%; P<.001).
In multivariable analysis, use of the Internet or a smartphone to obtain health-related information was associated with younger age (<50 years) and a higher level of education (both P<.001). Use of the Internet to obtain dermatology-related information (P<.001) and use of a smartphone to help diagnose a skin problem (P=.001) was associated with younger age (<50 years) only. Income, sex, co-pay to see a dermatologist, and travel time to the dermatology office were not associated with use of online resources for general or dermatology-specific health-related information or assistance with diagnosing a skin problem.
Of 204 respondents who indicated that they previously attempted to self-diagnose a skin condition using the Internet, the most commonly researched condition was skin cancer/moles/unknown spots (64.7%), followed by rashes (40.7%), acne (20.6%), cosmetic issues (16.2%), psoriasis (12.7%), dermatitis (3.4%), warts (1.5%), tick bites (1.0%), and lupus (1.0%)(some respondents selected more than one condition). Only 7.0% of respondents indicated that they previously had refrained from visiting a dermatologist based on reassurance from online resources. Compared to the rest of the surveyed population, these respondents were younger (P=.001), but there were no significant differences in sex, highest level of education, household income, or travel time to the dermatology office. The most commonly researched condition among these respondents was acne (12 respondents), and 11 respondents indicated that they had attempted to self-diagnose a mole or potential cancer using online sources.
Of 557 respondents who owned a smartphone, 31.8% reported using at least 1 health-related app (mean number of health apps per respondent, 1.5). Of the apps that respondents used, 45.9% focused on fitness/nutrition, 28.7% provided reference information, 13.4% were a patient portal for receiving information from their electronic medical record, 8.6% provided a health monitoring function, 1.9% served as a diagnostic aid, and 1.5% provided coping assistance and emotional support for individuals with cognitive or emotional conditions; only 1 respondent reported using an app related to dermatology.
All respondents were asked to rate their anticipated degree of worry if the Internet or a smartphone app suggested that a skin lesion was benign versus dangerous on a 5-point scale. Overall, the median worry rating increased from 3 to 5 when information accessed via the Internet or a smartphone app suggested a lesion was dangerous rather than benign. A change in worry of 2 or more points was seen in 36.1% of females and 49.1% of males (P=.002) when information obtained via the Internet indicated a lesion was dangerous and in 47.5% of females and 58.8% of males (P=.006) when a smartphone app indicated that a lesion was dangerous. When information obtained via the Internet indicated a lesion was dangerous, a change in worry of 2 or more points was seen in 41.8% of respondents who were younger than 50 years and in 41.1% of those who were 50 years or older (P=.93). When a smartphone app indicated a lesion was dangerous, a change in worry of 2 or more points was seen in 50.2% of respondents who were younger than 50 years and in 52.2% of those who were 50 years or older (P=.61).
Discussion
In this cross-sectional study, we found that health-related Internet and smartphone use among dermatology patients is common and may impact both patients’ degree of concern about a skin lesion as well as the likelihood of seeking in-person medical care if they are reassured by the results of their online findings. Age and level of education were associated with Internet and smartphone use to obtain dermatology-related health information but not factors related to health care access. More patients used the Internet or a smartphone to obtain general medical information versus dermatology-related information. Respondents who indicated that they used the Internet to obtain health-related information tended to do so before visiting their physician.
Our finding that a patient’s level of worry about a hypothetical skin condition or lesion is influenced by health information obtained via the Internet or a smartphone app is concerning. One study found that participants who used a popular search engine to look for information about vaccine safety and dangers were directed to Web sites with inaccurate information more than 50% of the time, and 65% of the information they obtained from these sites was false.6 In our study, approximately 25% of respondents had previously consulted online resources to attempt toself-diagnose a skin condition. Online sources about dermatologic conditions were consulted most frequently for information about potential skin cancers, moles, and unknown spots. A prior study showed that smartphone apps that claim to aid patients in determining whether a skin lesion is low or high risk for melanoma often are inaccurate and are associated with a high rate of missed melanomas.5 Even though we surveyed patients who did end up seeing a dermatologist, some respondents had previously opted out of seeing a dermatologist based on information they had found online. Because our study was conducted among patients who chose to seek care at a dermatology office, the problem is likely greater than estimated from our findings because we had no way of reaching individuals who decided to completely forgo a visit with a dermatologist.
Although use of the Internet to obtain health-related information was common among older adults in our population, it was nearly universal in younger adults. Health-related smartphone use was more than twice as common in younger versus older adults, which could be due to an increased comfort with technology and its integration into daily life. The fact that age and education were associated with Internet use for dermatology-related health information but not household income or travel time to the dermatology office suggests that information seeking is not due to lack of resources limiting access to dermatologic care but rather to the greater role that rapid access to online information plays in patients’ lives. Our findings are similar to another study that examined the use of online sources for general health information.7
This study has several limitations. First, there may have been some selection bias. We specifically aimed to understand the health-related Internet and smartphone use among dermatology patients, thus restricting our sample to this population. By doing so, we were unable to assess the use of such resources by the general population, particularly those individuals who chose not to see a dermatologist at all based on their own online research. Our findings may not apply to other practices and regions of the country, as we implemented our study in one geographic location and in offices of an academic practice. Although our sample size and diversity with regard to income, education, and age suggest that our results are likely generalizable to many settings, it is important to note that nearly all respondents in this study had health insurance and our findings are thus not necessarily applicable to those individuals who are uninsured.
Conclusion
Our findings suggest that the availability of online health information regarding dermatologic conditions provides dermatologists with both opportunities and challenges. Many patients consult online resources for health information, and the popularity of this practice is likely to increase with time, particularly as newer smartphones with features designed to allow users to monitor their health are developed with health-conscious consumers in mind. Most large health care systems provide patients with resources to view laboratory results and communicate with physicians online. It is important for dermatologists to be involved in the development of high-quality online content that educates the public while also emphasizing the need to seek in-person medical care, particularly in potential cases of skin cancer. It also is important for patients to be involved in the content development process to ensure that the messages they take away from online resources are the ones physicians wish to convey. Ideally, online forms of education will increase patients’ sense of self-efficacy while encouraging appropriate consultation for potentially harmful skin conditions.
1. Atkinson NL, Saperstein SL, Pleis J. Using the Internet for health-related activities: findings from a national probability sample. J Med Internet Res. 2009;11:e4.
2. Ybarra M, Suman M. Reasons, assessments and actions taken: sex and age differences in uses of Internet health information. Health Educ Res. 2008;23:512-521.
3. Bhandari N, Shi Y, Jung K. Seeking health information online: does limited healthcare access matter? J Am Med Inform Assoc. 2014;21:1113-1117.
4. Thackeray R, Crookston BT, West JH. Correlates of health-related social media use among adults. J Med Internet Res. 2013;15:e21.
5. Wolf JA, Moreau JF, Akilov O, et al. Diagnostic inaccuracy of smartphone applications for melanoma detection. JAMA Dermatol. 2013;149:422-426.
6. Kortum P, Edwards C, Richards-Kortum R. The impact of inaccurate Internet health information in a secondary school learning environment. J Med Internet Res. 2008;10:e17.
7. Mead N, Varnam R, Rogers A, et al. What predicts patients’ interest in the internet as a health resource in primary care in England? J Health Serv Res Policy. 2003;8:33-39.
1. Atkinson NL, Saperstein SL, Pleis J. Using the Internet for health-related activities: findings from a national probability sample. J Med Internet Res. 2009;11:e4.
2. Ybarra M, Suman M. Reasons, assessments and actions taken: sex and age differences in uses of Internet health information. Health Educ Res. 2008;23:512-521.
3. Bhandari N, Shi Y, Jung K. Seeking health information online: does limited healthcare access matter? J Am Med Inform Assoc. 2014;21:1113-1117.
4. Thackeray R, Crookston BT, West JH. Correlates of health-related social media use among adults. J Med Internet Res. 2013;15:e21.
5. Wolf JA, Moreau JF, Akilov O, et al. Diagnostic inaccuracy of smartphone applications for melanoma detection. JAMA Dermatol. 2013;149:422-426.
6. Kortum P, Edwards C, Richards-Kortum R. The impact of inaccurate Internet health information in a secondary school learning environment. J Med Internet Res. 2008;10:e17.
7. Mead N, Varnam R, Rogers A, et al. What predicts patients’ interest in the internet as a health resource in primary care in England? J Health Serv Res Policy. 2003;8:33-39.
Black Salve and Bloodroot Extract in Dermatologic Conditions
Black salve is composed of various ingredients, many of which are inert; however, some black salves contain escharotics, the 2 most common are zinc chloride and bloodroot (Sanguinaria canadensis) extract. In high doses, such as those contained in most black salve products, these corrosive agents can indiscriminately damage both healthy and diseased tissue.1 Nevertheless, many black salve products currently are advertised as safe and natural methods for curing skin cancer2-4 or treating a variety of other skin conditions (eg, moles, warts, skin tags, boils, abscesses, bee stings, other minor wounds)1,5 and even nondermatologic conditions such as a sore throat.6 Despite the information and testimonials that are widely available on the Internet, black salve use has not been validated by rigorous studies. Black salve is not regulated by the US Food and Drug Administration, resulting in poor quality control and inconsistent user instructions. We report the case of application of black salve to a biopsy site of a compound nevus with moderate atypia that resulted in the formation of a dermatitis plaque with subsequent scarring and basal layer pigmentation.
Case Report
A 35-year-old woman with a family history of melanoma presented for follow-up of a compound nevus with moderate atypia on the right anterior thigh that had been biopsied 6 months prior. Complete excision of the lesion was recommended at the initial presentation but was not performed due to scheduling conflicts. The patient reported applying black salve to the biopsy site and also to the left thigh 3 months later. There was no reaction on the left thigh after one 24-hour application of black salve, but an area around the biopsy site on the right thigh became thickened and irritated with superficial erosion of the skin following 2 applications of black salve, each of 24 hours’ duration. Physical examination revealed a granulomatous plaque at the biopsy site that was approximately 5 cm in diameter (Figure 1A). One year later the lesion had completely healed (Figure 1B) and a biopsy revealed scarring with basal layer pigmentation (Figure 2).
| 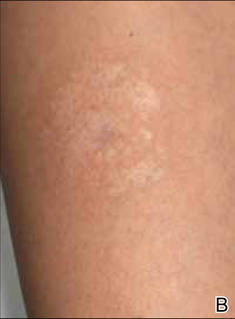
| 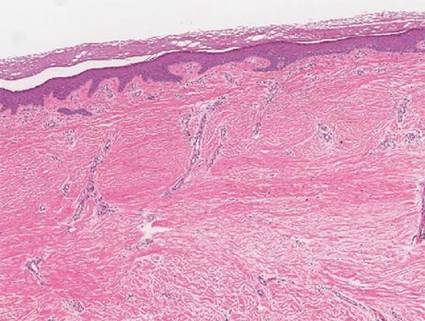
| |||
| Figure 1. A 5-cm granulomatous reaction surrounding a biopsy site on the right anterior thigh 3 months after application of black salve (A). One year later, the lesion had completely healed (B). | Figure 2. A biopsy one year following application of black salve demonstrated scarring with basal layer pigmentation (H&E, original magnification ×4). | ||||
Comment
A Web search using the term black salve yields a large number of products labeled as skin cancer salves, many showing glowing reviews and some being sold by major US retailers. The ingredients in black salves often vary in the innocuous substances they contain, but most products include the escharotics zinc chloride and bloodroot extract, which is derived from the plant S canadensis.1,3 For example, the ingredients of one popular black salve product include zinc chloride, chaparral (active ingredient is nordihydroguaiaretic acid), graviola leaf extract, oleander leaf extract, bloodroot extract, and glycerine,7 while another product includes bloodroot extract, zinc chloride, chaparral, cayenne pepper, red clover, birch bark, dimethyl sulfoxide, and burdock root.4
Bloodroot extract’s antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory effects derive from its benzylisoquinoline alkaloids including sanguinarine, allocryptopine, berberine, coptisine, protopine, and stylopine.3,8 Bloodroot extract possesses some degree of tumoricidal potency, with one study finding that it selectively targets cancer cells.9 However, this differential response is seen only at low doses and not at the high concentrations contained in most black salve products.1 According to fluorometric assays, sanguinarine is not selective for tumor cells and therefore damages healthy tissue in addition to the unwanted lesions.6,10,11 The US Food and Drug Administration includes black salve products on its list of fake cancer cures that consumers should avoid.12 Reports of extensive damage from black salve use include skin ulceration2,10 and complete loss of a naris1 and nasal ala.5 Our case suggests the possible association between black salve use and an irritant reaction and erosion of the skin.
Furthermore, reliance on black salve alone in the treatment of skin cancer poses the threat of recurrence or metastasis of cancer because there is no way to know if the salve completely removed the cancer without a biopsy. Self-treatment can delay more effective therapy and may require further treatments.
Black salve should be subject to standarddrug regulations and its use discouraged by dermatologists due to the associated harmful effects and the availability of safer treatments. To better treat and inform their patients, dermatologists should be aware that patients may be attracted to alternative treatments such as black salves.
1. Eastman KL, McFarland LV, Raugi GJ. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289.
2. Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:e154-e155.
3. Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80.
4. McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
5. Payne CE. ‘Black Salve’ and melanomas [published online ahead of print August 11, 2010]. J Plast Reconstr Aesthet Surg. 2011;64:422.
6. Cienki JJ, Zaret L. An Internet misadventure: bloodroot salve toxicity. J Altern Complement Med. 2010;16:1125-1127.
7. Cansema and escharotics. Alpha Omega Labs Web site. http://www.altcancer.com/faqcan.htm. Accessed May 6, 2015.
8. Vlachojannis C, Magora F, Chrubasik S. Rise and fall of oral health products with Canadian bloodroot extract. Phytother Res. 2012;26:1423-1426.
9. Ahmad N, Gupta S, Husain MM, et al. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin Cancer Res. 2000;6:1524-1528.
10. Saltzberg F, Barron G, Fenske N. Deforming self-treatment with herbal “black salve.” Dermatol Surg. 2009;35:1152-1154.
11. Debiton E, Madelmont JC, Legault J, et al. Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother Pharmacol. 2003;51:474-482.
12. 187 fake cancer “cures” consumers should avoid. US Food and Drug Administration Web site. http://www.fda.gov/Drugs/GuidanceCompliance RegulatoryInformation/EnforcementActivitiesbyFDA/ucm171057.htm. Updated July 9, 2009. Accessed May 6, 2015.
Black salve is composed of various ingredients, many of which are inert; however, some black salves contain escharotics, the 2 most common are zinc chloride and bloodroot (Sanguinaria canadensis) extract. In high doses, such as those contained in most black salve products, these corrosive agents can indiscriminately damage both healthy and diseased tissue.1 Nevertheless, many black salve products currently are advertised as safe and natural methods for curing skin cancer2-4 or treating a variety of other skin conditions (eg, moles, warts, skin tags, boils, abscesses, bee stings, other minor wounds)1,5 and even nondermatologic conditions such as a sore throat.6 Despite the information and testimonials that are widely available on the Internet, black salve use has not been validated by rigorous studies. Black salve is not regulated by the US Food and Drug Administration, resulting in poor quality control and inconsistent user instructions. We report the case of application of black salve to a biopsy site of a compound nevus with moderate atypia that resulted in the formation of a dermatitis plaque with subsequent scarring and basal layer pigmentation.
Case Report
A 35-year-old woman with a family history of melanoma presented for follow-up of a compound nevus with moderate atypia on the right anterior thigh that had been biopsied 6 months prior. Complete excision of the lesion was recommended at the initial presentation but was not performed due to scheduling conflicts. The patient reported applying black salve to the biopsy site and also to the left thigh 3 months later. There was no reaction on the left thigh after one 24-hour application of black salve, but an area around the biopsy site on the right thigh became thickened and irritated with superficial erosion of the skin following 2 applications of black salve, each of 24 hours’ duration. Physical examination revealed a granulomatous plaque at the biopsy site that was approximately 5 cm in diameter (Figure 1A). One year later the lesion had completely healed (Figure 1B) and a biopsy revealed scarring with basal layer pigmentation (Figure 2).

| 
| 
| |||
| Figure 1. A 5-cm granulomatous reaction surrounding a biopsy site on the right anterior thigh 3 months after application of black salve (A). One year later, the lesion had completely healed (B). | Figure 2. A biopsy one year following application of black salve demonstrated scarring with basal layer pigmentation (H&E, original magnification ×4). | ||||
Comment
A Web search using the term black salve yields a large number of products labeled as skin cancer salves, many showing glowing reviews and some being sold by major US retailers. The ingredients in black salves often vary in the innocuous substances they contain, but most products include the escharotics zinc chloride and bloodroot extract, which is derived from the plant S canadensis.1,3 For example, the ingredients of one popular black salve product include zinc chloride, chaparral (active ingredient is nordihydroguaiaretic acid), graviola leaf extract, oleander leaf extract, bloodroot extract, and glycerine,7 while another product includes bloodroot extract, zinc chloride, chaparral, cayenne pepper, red clover, birch bark, dimethyl sulfoxide, and burdock root.4
Bloodroot extract’s antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory effects derive from its benzylisoquinoline alkaloids including sanguinarine, allocryptopine, berberine, coptisine, protopine, and stylopine.3,8 Bloodroot extract possesses some degree of tumoricidal potency, with one study finding that it selectively targets cancer cells.9 However, this differential response is seen only at low doses and not at the high concentrations contained in most black salve products.1 According to fluorometric assays, sanguinarine is not selective for tumor cells and therefore damages healthy tissue in addition to the unwanted lesions.6,10,11 The US Food and Drug Administration includes black salve products on its list of fake cancer cures that consumers should avoid.12 Reports of extensive damage from black salve use include skin ulceration2,10 and complete loss of a naris1 and nasal ala.5 Our case suggests the possible association between black salve use and an irritant reaction and erosion of the skin.
Furthermore, reliance on black salve alone in the treatment of skin cancer poses the threat of recurrence or metastasis of cancer because there is no way to know if the salve completely removed the cancer without a biopsy. Self-treatment can delay more effective therapy and may require further treatments.
Black salve should be subject to standarddrug regulations and its use discouraged by dermatologists due to the associated harmful effects and the availability of safer treatments. To better treat and inform their patients, dermatologists should be aware that patients may be attracted to alternative treatments such as black salves.
Black salve is composed of various ingredients, many of which are inert; however, some black salves contain escharotics, the 2 most common are zinc chloride and bloodroot (Sanguinaria canadensis) extract. In high doses, such as those contained in most black salve products, these corrosive agents can indiscriminately damage both healthy and diseased tissue.1 Nevertheless, many black salve products currently are advertised as safe and natural methods for curing skin cancer2-4 or treating a variety of other skin conditions (eg, moles, warts, skin tags, boils, abscesses, bee stings, other minor wounds)1,5 and even nondermatologic conditions such as a sore throat.6 Despite the information and testimonials that are widely available on the Internet, black salve use has not been validated by rigorous studies. Black salve is not regulated by the US Food and Drug Administration, resulting in poor quality control and inconsistent user instructions. We report the case of application of black salve to a biopsy site of a compound nevus with moderate atypia that resulted in the formation of a dermatitis plaque with subsequent scarring and basal layer pigmentation.
Case Report
A 35-year-old woman with a family history of melanoma presented for follow-up of a compound nevus with moderate atypia on the right anterior thigh that had been biopsied 6 months prior. Complete excision of the lesion was recommended at the initial presentation but was not performed due to scheduling conflicts. The patient reported applying black salve to the biopsy site and also to the left thigh 3 months later. There was no reaction on the left thigh after one 24-hour application of black salve, but an area around the biopsy site on the right thigh became thickened and irritated with superficial erosion of the skin following 2 applications of black salve, each of 24 hours’ duration. Physical examination revealed a granulomatous plaque at the biopsy site that was approximately 5 cm in diameter (Figure 1A). One year later the lesion had completely healed (Figure 1B) and a biopsy revealed scarring with basal layer pigmentation (Figure 2).

| 
| 
| |||
| Figure 1. A 5-cm granulomatous reaction surrounding a biopsy site on the right anterior thigh 3 months after application of black salve (A). One year later, the lesion had completely healed (B). | Figure 2. A biopsy one year following application of black salve demonstrated scarring with basal layer pigmentation (H&E, original magnification ×4). | ||||
Comment
A Web search using the term black salve yields a large number of products labeled as skin cancer salves, many showing glowing reviews and some being sold by major US retailers. The ingredients in black salves often vary in the innocuous substances they contain, but most products include the escharotics zinc chloride and bloodroot extract, which is derived from the plant S canadensis.1,3 For example, the ingredients of one popular black salve product include zinc chloride, chaparral (active ingredient is nordihydroguaiaretic acid), graviola leaf extract, oleander leaf extract, bloodroot extract, and glycerine,7 while another product includes bloodroot extract, zinc chloride, chaparral, cayenne pepper, red clover, birch bark, dimethyl sulfoxide, and burdock root.4
Bloodroot extract’s antimicrobial, anti-inflammatory, antioxidant, and immunomodulatory effects derive from its benzylisoquinoline alkaloids including sanguinarine, allocryptopine, berberine, coptisine, protopine, and stylopine.3,8 Bloodroot extract possesses some degree of tumoricidal potency, with one study finding that it selectively targets cancer cells.9 However, this differential response is seen only at low doses and not at the high concentrations contained in most black salve products.1 According to fluorometric assays, sanguinarine is not selective for tumor cells and therefore damages healthy tissue in addition to the unwanted lesions.6,10,11 The US Food and Drug Administration includes black salve products on its list of fake cancer cures that consumers should avoid.12 Reports of extensive damage from black salve use include skin ulceration2,10 and complete loss of a naris1 and nasal ala.5 Our case suggests the possible association between black salve use and an irritant reaction and erosion of the skin.
Furthermore, reliance on black salve alone in the treatment of skin cancer poses the threat of recurrence or metastasis of cancer because there is no way to know if the salve completely removed the cancer without a biopsy. Self-treatment can delay more effective therapy and may require further treatments.
Black salve should be subject to standarddrug regulations and its use discouraged by dermatologists due to the associated harmful effects and the availability of safer treatments. To better treat and inform their patients, dermatologists should be aware that patients may be attracted to alternative treatments such as black salves.
1. Eastman KL, McFarland LV, Raugi GJ. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289.
2. Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:e154-e155.
3. Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80.
4. McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
5. Payne CE. ‘Black Salve’ and melanomas [published online ahead of print August 11, 2010]. J Plast Reconstr Aesthet Surg. 2011;64:422.
6. Cienki JJ, Zaret L. An Internet misadventure: bloodroot salve toxicity. J Altern Complement Med. 2010;16:1125-1127.
7. Cansema and escharotics. Alpha Omega Labs Web site. http://www.altcancer.com/faqcan.htm. Accessed May 6, 2015.
8. Vlachojannis C, Magora F, Chrubasik S. Rise and fall of oral health products with Canadian bloodroot extract. Phytother Res. 2012;26:1423-1426.
9. Ahmad N, Gupta S, Husain MM, et al. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin Cancer Res. 2000;6:1524-1528.
10. Saltzberg F, Barron G, Fenske N. Deforming self-treatment with herbal “black salve.” Dermatol Surg. 2009;35:1152-1154.
11. Debiton E, Madelmont JC, Legault J, et al. Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother Pharmacol. 2003;51:474-482.
12. 187 fake cancer “cures” consumers should avoid. US Food and Drug Administration Web site. http://www.fda.gov/Drugs/GuidanceCompliance RegulatoryInformation/EnforcementActivitiesbyFDA/ucm171057.htm. Updated July 9, 2009. Accessed May 6, 2015.
1. Eastman KL, McFarland LV, Raugi GJ. A review of topical corrosive black salve. J Altern Complement Med. 2014;20:284-289.
2. Eastman KL, McFarland LV, Raugi GJ. Buyer beware: a black salve caution. J Am Acad Dermatol. 2011;65:e154-e155.
3. Sivyer GW, Rosendahl C. Application of black salve to a thin melanoma that subsequently progressed to metastatic melanoma: a case study. Dermatol Pract Concept. 2014;4:77-80.
4. McDaniel S, Goldman GD. Consequences of using escharotic agents as primary treatment for nonmelanoma skin cancer. Arch Dermatol. 2002;138:1593-1596.
5. Payne CE. ‘Black Salve’ and melanomas [published online ahead of print August 11, 2010]. J Plast Reconstr Aesthet Surg. 2011;64:422.
6. Cienki JJ, Zaret L. An Internet misadventure: bloodroot salve toxicity. J Altern Complement Med. 2010;16:1125-1127.
7. Cansema and escharotics. Alpha Omega Labs Web site. http://www.altcancer.com/faqcan.htm. Accessed May 6, 2015.
8. Vlachojannis C, Magora F, Chrubasik S. Rise and fall of oral health products with Canadian bloodroot extract. Phytother Res. 2012;26:1423-1426.
9. Ahmad N, Gupta S, Husain MM, et al. Differential antiproliferative and apoptotic response of sanguinarine for cancer cells versus normal cells. Clin Cancer Res. 2000;6:1524-1528.
10. Saltzberg F, Barron G, Fenske N. Deforming self-treatment with herbal “black salve.” Dermatol Surg. 2009;35:1152-1154.
11. Debiton E, Madelmont JC, Legault J, et al. Sanguinarine-induced apoptosis is associated with an early and severe cellular glutathione depletion. Cancer Chemother Pharmacol. 2003;51:474-482.
12. 187 fake cancer “cures” consumers should avoid. US Food and Drug Administration Web site. http://www.fda.gov/Drugs/GuidanceCompliance RegulatoryInformation/EnforcementActivitiesbyFDA/ucm171057.htm. Updated July 9, 2009. Accessed May 6, 2015.
Practice Points
- Clinicians should be aware that black salve containing bloodroot extract is a popular alternative treatment used to cure a variety of skin ailments.
- Black salve containing bloodroot extract is not selective for tumor cells. Various case reports have shown that black salve can result in extensive tissue damage and recurrence or metastasis of skin cancer.
- Damage to healthy tissue can occur with as few as 2 applications of black salve.
Shiitake Mushroom Dermatitis
To the Editor:
The shiitake mushroom (Lentinula edodes) is a popular Asian food and represents the second most consumed mushroom in the world. It is known for having a range of strong health benefits including antihypertensive, anti-inflammatory, and immunomodulatory effects. Especially in Asia, this mushroom has been used in patients with cancers of the gastrointestinal tract and also may be helpful in the treatment of human immunodeficiency virus.1,2 The source of these effects is lentinan, a polysaccharide in the mushroom. However, lentinan also can cause a toxic reaction of the skin when the mushrooms are eaten raw or undercooked. These reactions are mainly reported in Asia, but more cases have been published in the last decade in Europe and the United States, evidence that the incidence of this adverse effect has increased in the Western world.
A 65-year-old woman with no notable medical history presented to our outpatient practice with sudden onset of a pruritic, erythematous, papular eruption on the neck. The eruption began that morning. The diagnosis of eczematous dermatitis was made and hydrocortisone cream 2.5% was started. Three days later, she returned with spread of the rash to the trunk, arms, and legs despite the topical treatment. She denied fevers, chills, or constitutional symptoms. The patient also denied recent travel or bug bites. However, she reported that she recently had started using raw shiitake mushrooms in her salad; the first time was 3 days before the symptoms appeared. Physical examination revealed erythematous skin with long flagellate streaks composed of petechiae, papules, and vesicles involving the trunk, arms, and legs (Figure). Oral and nasal mucosae were uninvolved. Dermatographism was negative. The diagnosis of flagellate dermatitis from shiitake mushrooms was made given the patient’s history and the unique clinical findings of the skin. Blood work and a biopsy were not performed. Instead, the patient was advised to avoid shiitake mushrooms and use clobetasol propionate cream 0.05% twice daily for 2 weeks on the affected areas. The symptoms resolved within 10 days.
|
The first known case of toxicoderma to shiitake mushrooms was reported in Japan by Nakamura3 in 1977. Since this seminal report, numerous cases have followed. This disorder is mainly seen in Asia.
Patients usually present with linear groups of pruritic, papular, petechial, and vesicular lesions in a flagellate pattern, most commonly localized on the trunk, arms, and legs. Oral and nasal mucosae usually are not involved, and fever and malaise may be associated. All symptoms typically occur 1 to 2 days after ingestion of the mushrooms. The patient’s history and typical clinical findings lead to a diagnosis; however, blood tests may show inflammation with leukocytosis and elevated C-reactive protein levels. Biopsy of the skin shows lymphocytic dermal infiltrates with spongiosis and necrotic cells within the epidermis.4
Differential diagnoses include flagellate dermatitis associated with bleomycin, a glycopeptide antibiotic produced by the bacterium Streptomyces verticillus. Because it causes breaks in the DNA, bleomycin is commonly used as a chemotherapeutic agent in treating Hodgkin lymphoma and other malignancies. It presents with linear postinflammatory hyperpigmentation of the skin. However, unlike shiitake dermatitis, there is a lack of papules. Another differential diagnosis includes herpes zoster virus, which should be ruled out clinically.
All symptoms in shiitake dermatitis usually resolve within 1 to 8 weeks of avoidance of the culprit food. Topical steroids and antihistamines can be given.
The underlying pathology is a toxic reaction to the polysaccharide lentinan in the mushrooms, which is known as a thermolabile agent.5 Therefore, it may only cause a toxic reaction when the mushrooms are consumed raw or undercooked. Prick testing is usually negative in these patients, which suggests a toxic and not an immunologic reaction of the human body.6 Other forms of reaction to shiitake mushrooms include contact dermatitis after skin contact and allergic alveolitis after occupational exposure to mushroom spores, mainly in individuals cultivating shiitake mushrooms (mushroom worker’s lung). In these forms of the disease, prick testing may be positive.7,8
Flagellate dermatitis caused by shiitake mushrooms is still an uncommon dermatologic phenomenon in the Western world. Future studies and cases should be reported to increase the awareness of this disorder. Although the patients present with typical clinical findings, the diagnosis can be missed if history is not carefully considered.
1. Ng ML, Yap AT. Inhibition of human colon carcinoma development by lentinan from shiitake mushrooms (Lentinus edodes). J Altern Complement Med. 2002;8:581-589.
2. Gordon M, Bihari B, Goosby E, et al. A placebo-controlled trial of the immune modulator, lentinan, in HIV-positive patients: a phase I/II trial. J Med. 1998;29:305-330.
3. Nakamura T. Toxicoderma caused by shiitake. Jpn J Clin Dermatol. 1977;31:65-68.
4. Hanada K, Hashimoto I. Flagellate mushroom (shiitake) dermatitis and photosensitivity. Dermatology. 1998;197:255-257.
5. Lippert U, Martin V, Schwertfeger C, et al. Shiitake dermatitis. Br J Dermatol. 2003;148:178-179.
6. Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:6570.
7. Ueda A, Obama K, Aoyama K, et al. Allergic contact dermatitis in shiitake (Lentinus edodes (Berk) Sing) growers. Contact Dermatitis. 1992;26:228-233.
8. Ampere A, Delhaes L, Soots J, et al. Hypersensitivity pneumonitis induced by shiitake mushroom spores. Med Mycol. 2012;50:654-657.
To the Editor:
The shiitake mushroom (Lentinula edodes) is a popular Asian food and represents the second most consumed mushroom in the world. It is known for having a range of strong health benefits including antihypertensive, anti-inflammatory, and immunomodulatory effects. Especially in Asia, this mushroom has been used in patients with cancers of the gastrointestinal tract and also may be helpful in the treatment of human immunodeficiency virus.1,2 The source of these effects is lentinan, a polysaccharide in the mushroom. However, lentinan also can cause a toxic reaction of the skin when the mushrooms are eaten raw or undercooked. These reactions are mainly reported in Asia, but more cases have been published in the last decade in Europe and the United States, evidence that the incidence of this adverse effect has increased in the Western world.
A 65-year-old woman with no notable medical history presented to our outpatient practice with sudden onset of a pruritic, erythematous, papular eruption on the neck. The eruption began that morning. The diagnosis of eczematous dermatitis was made and hydrocortisone cream 2.5% was started. Three days later, she returned with spread of the rash to the trunk, arms, and legs despite the topical treatment. She denied fevers, chills, or constitutional symptoms. The patient also denied recent travel or bug bites. However, she reported that she recently had started using raw shiitake mushrooms in her salad; the first time was 3 days before the symptoms appeared. Physical examination revealed erythematous skin with long flagellate streaks composed of petechiae, papules, and vesicles involving the trunk, arms, and legs (Figure). Oral and nasal mucosae were uninvolved. Dermatographism was negative. The diagnosis of flagellate dermatitis from shiitake mushrooms was made given the patient’s history and the unique clinical findings of the skin. Blood work and a biopsy were not performed. Instead, the patient was advised to avoid shiitake mushrooms and use clobetasol propionate cream 0.05% twice daily for 2 weeks on the affected areas. The symptoms resolved within 10 days.
|
The first known case of toxicoderma to shiitake mushrooms was reported in Japan by Nakamura3 in 1977. Since this seminal report, numerous cases have followed. This disorder is mainly seen in Asia.
Patients usually present with linear groups of pruritic, papular, petechial, and vesicular lesions in a flagellate pattern, most commonly localized on the trunk, arms, and legs. Oral and nasal mucosae usually are not involved, and fever and malaise may be associated. All symptoms typically occur 1 to 2 days after ingestion of the mushrooms. The patient’s history and typical clinical findings lead to a diagnosis; however, blood tests may show inflammation with leukocytosis and elevated C-reactive protein levels. Biopsy of the skin shows lymphocytic dermal infiltrates with spongiosis and necrotic cells within the epidermis.4
Differential diagnoses include flagellate dermatitis associated with bleomycin, a glycopeptide antibiotic produced by the bacterium Streptomyces verticillus. Because it causes breaks in the DNA, bleomycin is commonly used as a chemotherapeutic agent in treating Hodgkin lymphoma and other malignancies. It presents with linear postinflammatory hyperpigmentation of the skin. However, unlike shiitake dermatitis, there is a lack of papules. Another differential diagnosis includes herpes zoster virus, which should be ruled out clinically.
All symptoms in shiitake dermatitis usually resolve within 1 to 8 weeks of avoidance of the culprit food. Topical steroids and antihistamines can be given.
The underlying pathology is a toxic reaction to the polysaccharide lentinan in the mushrooms, which is known as a thermolabile agent.5 Therefore, it may only cause a toxic reaction when the mushrooms are consumed raw or undercooked. Prick testing is usually negative in these patients, which suggests a toxic and not an immunologic reaction of the human body.6 Other forms of reaction to shiitake mushrooms include contact dermatitis after skin contact and allergic alveolitis after occupational exposure to mushroom spores, mainly in individuals cultivating shiitake mushrooms (mushroom worker’s lung). In these forms of the disease, prick testing may be positive.7,8
Flagellate dermatitis caused by shiitake mushrooms is still an uncommon dermatologic phenomenon in the Western world. Future studies and cases should be reported to increase the awareness of this disorder. Although the patients present with typical clinical findings, the diagnosis can be missed if history is not carefully considered.
To the Editor:
The shiitake mushroom (Lentinula edodes) is a popular Asian food and represents the second most consumed mushroom in the world. It is known for having a range of strong health benefits including antihypertensive, anti-inflammatory, and immunomodulatory effects. Especially in Asia, this mushroom has been used in patients with cancers of the gastrointestinal tract and also may be helpful in the treatment of human immunodeficiency virus.1,2 The source of these effects is lentinan, a polysaccharide in the mushroom. However, lentinan also can cause a toxic reaction of the skin when the mushrooms are eaten raw or undercooked. These reactions are mainly reported in Asia, but more cases have been published in the last decade in Europe and the United States, evidence that the incidence of this adverse effect has increased in the Western world.
A 65-year-old woman with no notable medical history presented to our outpatient practice with sudden onset of a pruritic, erythematous, papular eruption on the neck. The eruption began that morning. The diagnosis of eczematous dermatitis was made and hydrocortisone cream 2.5% was started. Three days later, she returned with spread of the rash to the trunk, arms, and legs despite the topical treatment. She denied fevers, chills, or constitutional symptoms. The patient also denied recent travel or bug bites. However, she reported that she recently had started using raw shiitake mushrooms in her salad; the first time was 3 days before the symptoms appeared. Physical examination revealed erythematous skin with long flagellate streaks composed of petechiae, papules, and vesicles involving the trunk, arms, and legs (Figure). Oral and nasal mucosae were uninvolved. Dermatographism was negative. The diagnosis of flagellate dermatitis from shiitake mushrooms was made given the patient’s history and the unique clinical findings of the skin. Blood work and a biopsy were not performed. Instead, the patient was advised to avoid shiitake mushrooms and use clobetasol propionate cream 0.05% twice daily for 2 weeks on the affected areas. The symptoms resolved within 10 days.
|
The first known case of toxicoderma to shiitake mushrooms was reported in Japan by Nakamura3 in 1977. Since this seminal report, numerous cases have followed. This disorder is mainly seen in Asia.
Patients usually present with linear groups of pruritic, papular, petechial, and vesicular lesions in a flagellate pattern, most commonly localized on the trunk, arms, and legs. Oral and nasal mucosae usually are not involved, and fever and malaise may be associated. All symptoms typically occur 1 to 2 days after ingestion of the mushrooms. The patient’s history and typical clinical findings lead to a diagnosis; however, blood tests may show inflammation with leukocytosis and elevated C-reactive protein levels. Biopsy of the skin shows lymphocytic dermal infiltrates with spongiosis and necrotic cells within the epidermis.4
Differential diagnoses include flagellate dermatitis associated with bleomycin, a glycopeptide antibiotic produced by the bacterium Streptomyces verticillus. Because it causes breaks in the DNA, bleomycin is commonly used as a chemotherapeutic agent in treating Hodgkin lymphoma and other malignancies. It presents with linear postinflammatory hyperpigmentation of the skin. However, unlike shiitake dermatitis, there is a lack of papules. Another differential diagnosis includes herpes zoster virus, which should be ruled out clinically.
All symptoms in shiitake dermatitis usually resolve within 1 to 8 weeks of avoidance of the culprit food. Topical steroids and antihistamines can be given.
The underlying pathology is a toxic reaction to the polysaccharide lentinan in the mushrooms, which is known as a thermolabile agent.5 Therefore, it may only cause a toxic reaction when the mushrooms are consumed raw or undercooked. Prick testing is usually negative in these patients, which suggests a toxic and not an immunologic reaction of the human body.6 Other forms of reaction to shiitake mushrooms include contact dermatitis after skin contact and allergic alveolitis after occupational exposure to mushroom spores, mainly in individuals cultivating shiitake mushrooms (mushroom worker’s lung). In these forms of the disease, prick testing may be positive.7,8
Flagellate dermatitis caused by shiitake mushrooms is still an uncommon dermatologic phenomenon in the Western world. Future studies and cases should be reported to increase the awareness of this disorder. Although the patients present with typical clinical findings, the diagnosis can be missed if history is not carefully considered.
1. Ng ML, Yap AT. Inhibition of human colon carcinoma development by lentinan from shiitake mushrooms (Lentinus edodes). J Altern Complement Med. 2002;8:581-589.
2. Gordon M, Bihari B, Goosby E, et al. A placebo-controlled trial of the immune modulator, lentinan, in HIV-positive patients: a phase I/II trial. J Med. 1998;29:305-330.
3. Nakamura T. Toxicoderma caused by shiitake. Jpn J Clin Dermatol. 1977;31:65-68.
4. Hanada K, Hashimoto I. Flagellate mushroom (shiitake) dermatitis and photosensitivity. Dermatology. 1998;197:255-257.
5. Lippert U, Martin V, Schwertfeger C, et al. Shiitake dermatitis. Br J Dermatol. 2003;148:178-179.
6. Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:6570.
7. Ueda A, Obama K, Aoyama K, et al. Allergic contact dermatitis in shiitake (Lentinus edodes (Berk) Sing) growers. Contact Dermatitis. 1992;26:228-233.
8. Ampere A, Delhaes L, Soots J, et al. Hypersensitivity pneumonitis induced by shiitake mushroom spores. Med Mycol. 2012;50:654-657.
1. Ng ML, Yap AT. Inhibition of human colon carcinoma development by lentinan from shiitake mushrooms (Lentinus edodes). J Altern Complement Med. 2002;8:581-589.
2. Gordon M, Bihari B, Goosby E, et al. A placebo-controlled trial of the immune modulator, lentinan, in HIV-positive patients: a phase I/II trial. J Med. 1998;29:305-330.
3. Nakamura T. Toxicoderma caused by shiitake. Jpn J Clin Dermatol. 1977;31:65-68.
4. Hanada K, Hashimoto I. Flagellate mushroom (shiitake) dermatitis and photosensitivity. Dermatology. 1998;197:255-257.
5. Lippert U, Martin V, Schwertfeger C, et al. Shiitake dermatitis. Br J Dermatol. 2003;148:178-179.
6. Nakamura T. Shiitake (Lentinus edodes) dermatitis. Contact Dermatitis. 1992;27:6570.
7. Ueda A, Obama K, Aoyama K, et al. Allergic contact dermatitis in shiitake (Lentinus edodes (Berk) Sing) growers. Contact Dermatitis. 1992;26:228-233.
8. Ampere A, Delhaes L, Soots J, et al. Hypersensitivity pneumonitis induced by shiitake mushroom spores. Med Mycol. 2012;50:654-657.
Sports Purpura From Floorball, Indoor Climbing, and Archery
To the Editor:
Sports purpura can be broken down into different types including traumatic purpura,1 exercise-induced cutaneous vasculitis,2 occurrence of coincidental systemic purpura,3 and other conditions.4-6 Traumatic purpura results from brutal contact with an opponent, the court, the equipment, or the ball. Three cases of sports purpura related to equipment and balls are reported.
An otherwise healthy 27-year-old woman presented with multiple ecchymotic round patches on her legs. The largest patch was 70 mm and displayed a heterogeneous Swiss cheese–like pattern with discrete whiter round areas within the patch (Figure 1). She reported that she played as a defender in a second division floorball team weekly, acknowledging frequent body contacts and being hit on the legs with the sticks and balls. Purpura was diagnosed due to hits from the floorball.
A 32-year-old healthy man presented with purpuric petechiae of the left palm after indoor climbing. He had been regularly climbing indoors for 3 years and denied a history of similar eruptions. The lesions were painless, noninfiltrated, and did not disappear after pressure (Figure 2). Lesions presumably were due to repeated friction on the climbing hold. Petechiae took a transiently golden hue before resolving within a week.
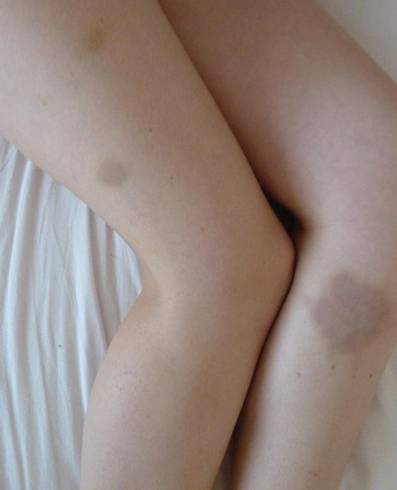
| 
|
A 26-year-old right-handed woman injured the left forearm while practicing target archery. She was not wearing an arm guard at the time of the injury. Once released, the bowstring scraped the volar aspect of the forearm, causing a painful warm ecchymotic and swollen plaque. She denied neurologic or vascular symptoms. The hematoma rapidly evolved from red to blue (Figure 3) and spontaneously resolved within weeks.
|
|
Purpura related to the high-velocity impact of sport balls has been previously reported with ping-pong,7 paintball,8,9 racquetball, squash,10 and baseball. Floorball, one of the most popular team sports in Finland, is played indoors and resembles ice hockey. The players use graphite compound sticks and a light hollow plastic ball. Except for the goalkeeper, players do not wear specific protective gear. Accidental body contact, including a direct hit from the floorball stick or ball, are frequent.11 The ball weighs 23 g, measures 72 mm in diameter, and has 26 holes that are 11 mm in diameter. The fastest shot was recorded at 127 miles per hour.12 The cutaneous imprint from the ball impact on bare skin, as shown with patient 1, initially is annular,8-10 but the bruise later takes an unusual design due to the peculiar shape of the ball. This complication is no stranger to floorball players but has been rarely reported. The diagnosis is easy, the condition is benign and asymptomatic, and it resolves when the season is over; therefore, players commonly will not seek medical attention. Of note, lower limb injuries, including joint sprains, muscle strains, and soft-tissue contusions, are frequent in female athletes.11 Additional causes of purpura include collision with another player or with boards and stick hits.
Palmar petechiae from indoor climbing is similar to black palm from weight lifting.13 Although the typical black discoloration is absent, the mechanisms of friction and brutal trauma, clinical presentation, and evolution are similar.
Lastly, archery-induced hematomas are caused by the absence of an arm guard, which protects the wrist and forearm when the string snaps back.14 This complication is not often reported but is known by archers. Because archers usually wear protective gear, these injuries are expected to occur in novices or when safety measures are not respected.
1. Aguayo-Leiva I, Vano-Galvan S, Arrazola JM. A purpuric rash. Aust Fam Physician. 2009;38:889-890.
2. Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
3. Leonard JC, Rieger M. Idiopathic thrombocytopenic purpura presenting in a high school football player: a case report. J Athl Train. 1998;33:269-270.
4. Nordlind K, Bondesson L, Johansson SG, et al. Purpura provoked by cold exposure in a skier. Dermatologica. 1983;167:101-103.
5. Latenser BA, Hempstead RW. Exercise-associated solar purpura in an atypical location. Cutis. 1985;35:365-366.
6. Allan SJ, Humphreys F, Buxton PK. Annular purpura and step aerobics. Clin Exp Dermatol. 1994;19:418.
7. Scott MJ Jr, Scott MJ 3rd. Ping pong patches. Cutis. 1989;43:363-364.
8. Aboutalebi S, Stetson CL. Paintball purpura. J Am Acad Dermatol. 2005;53:901-902.
9. Levsky ME, Crowe M. What is your diagnosis? paintball purpura. Cutis. 2005;75:148, 157-158.
10. Barazi H, Adams BB. Sports purpura. Int J Dermatol. 2006;45:1443.
11. Pasanen K, Parkkari J, Kannus P, et al. Injury risk in female floorball: a prospective one-season follow-up [published online ahead of print May 9, 2007]. Scand J Med Sci Sports. 2008;18:49-54.
12. New world record. Floorball Central Web site. http://www.floorballcentral.com/2010/11/new-world -record.html. Published November 5, 2010. Accessed April 8, 2015.
13. Izumi AK. Letter: pigmented palmar petechiae (black palm). Arch Dermatol. 1974;109:261.
14. Rayan GM. Archery-related injuries of the hand, forearm, and elbow. South Med J. 1992;85:961-964.
To the Editor:
Sports purpura can be broken down into different types including traumatic purpura,1 exercise-induced cutaneous vasculitis,2 occurrence of coincidental systemic purpura,3 and other conditions.4-6 Traumatic purpura results from brutal contact with an opponent, the court, the equipment, or the ball. Three cases of sports purpura related to equipment and balls are reported.
An otherwise healthy 27-year-old woman presented with multiple ecchymotic round patches on her legs. The largest patch was 70 mm and displayed a heterogeneous Swiss cheese–like pattern with discrete whiter round areas within the patch (Figure 1). She reported that she played as a defender in a second division floorball team weekly, acknowledging frequent body contacts and being hit on the legs with the sticks and balls. Purpura was diagnosed due to hits from the floorball.
A 32-year-old healthy man presented with purpuric petechiae of the left palm after indoor climbing. He had been regularly climbing indoors for 3 years and denied a history of similar eruptions. The lesions were painless, noninfiltrated, and did not disappear after pressure (Figure 2). Lesions presumably were due to repeated friction on the climbing hold. Petechiae took a transiently golden hue before resolving within a week.

| 
|
A 26-year-old right-handed woman injured the left forearm while practicing target archery. She was not wearing an arm guard at the time of the injury. Once released, the bowstring scraped the volar aspect of the forearm, causing a painful warm ecchymotic and swollen plaque. She denied neurologic or vascular symptoms. The hematoma rapidly evolved from red to blue (Figure 3) and spontaneously resolved within weeks.
|
|
Purpura related to the high-velocity impact of sport balls has been previously reported with ping-pong,7 paintball,8,9 racquetball, squash,10 and baseball. Floorball, one of the most popular team sports in Finland, is played indoors and resembles ice hockey. The players use graphite compound sticks and a light hollow plastic ball. Except for the goalkeeper, players do not wear specific protective gear. Accidental body contact, including a direct hit from the floorball stick or ball, are frequent.11 The ball weighs 23 g, measures 72 mm in diameter, and has 26 holes that are 11 mm in diameter. The fastest shot was recorded at 127 miles per hour.12 The cutaneous imprint from the ball impact on bare skin, as shown with patient 1, initially is annular,8-10 but the bruise later takes an unusual design due to the peculiar shape of the ball. This complication is no stranger to floorball players but has been rarely reported. The diagnosis is easy, the condition is benign and asymptomatic, and it resolves when the season is over; therefore, players commonly will not seek medical attention. Of note, lower limb injuries, including joint sprains, muscle strains, and soft-tissue contusions, are frequent in female athletes.11 Additional causes of purpura include collision with another player or with boards and stick hits.
Palmar petechiae from indoor climbing is similar to black palm from weight lifting.13 Although the typical black discoloration is absent, the mechanisms of friction and brutal trauma, clinical presentation, and evolution are similar.
Lastly, archery-induced hematomas are caused by the absence of an arm guard, which protects the wrist and forearm when the string snaps back.14 This complication is not often reported but is known by archers. Because archers usually wear protective gear, these injuries are expected to occur in novices or when safety measures are not respected.
To the Editor:
Sports purpura can be broken down into different types including traumatic purpura,1 exercise-induced cutaneous vasculitis,2 occurrence of coincidental systemic purpura,3 and other conditions.4-6 Traumatic purpura results from brutal contact with an opponent, the court, the equipment, or the ball. Three cases of sports purpura related to equipment and balls are reported.
An otherwise healthy 27-year-old woman presented with multiple ecchymotic round patches on her legs. The largest patch was 70 mm and displayed a heterogeneous Swiss cheese–like pattern with discrete whiter round areas within the patch (Figure 1). She reported that she played as a defender in a second division floorball team weekly, acknowledging frequent body contacts and being hit on the legs with the sticks and balls. Purpura was diagnosed due to hits from the floorball.
A 32-year-old healthy man presented with purpuric petechiae of the left palm after indoor climbing. He had been regularly climbing indoors for 3 years and denied a history of similar eruptions. The lesions were painless, noninfiltrated, and did not disappear after pressure (Figure 2). Lesions presumably were due to repeated friction on the climbing hold. Petechiae took a transiently golden hue before resolving within a week.

| 
|
A 26-year-old right-handed woman injured the left forearm while practicing target archery. She was not wearing an arm guard at the time of the injury. Once released, the bowstring scraped the volar aspect of the forearm, causing a painful warm ecchymotic and swollen plaque. She denied neurologic or vascular symptoms. The hematoma rapidly evolved from red to blue (Figure 3) and spontaneously resolved within weeks.
|
|
Purpura related to the high-velocity impact of sport balls has been previously reported with ping-pong,7 paintball,8,9 racquetball, squash,10 and baseball. Floorball, one of the most popular team sports in Finland, is played indoors and resembles ice hockey. The players use graphite compound sticks and a light hollow plastic ball. Except for the goalkeeper, players do not wear specific protective gear. Accidental body contact, including a direct hit from the floorball stick or ball, are frequent.11 The ball weighs 23 g, measures 72 mm in diameter, and has 26 holes that are 11 mm in diameter. The fastest shot was recorded at 127 miles per hour.12 The cutaneous imprint from the ball impact on bare skin, as shown with patient 1, initially is annular,8-10 but the bruise later takes an unusual design due to the peculiar shape of the ball. This complication is no stranger to floorball players but has been rarely reported. The diagnosis is easy, the condition is benign and asymptomatic, and it resolves when the season is over; therefore, players commonly will not seek medical attention. Of note, lower limb injuries, including joint sprains, muscle strains, and soft-tissue contusions, are frequent in female athletes.11 Additional causes of purpura include collision with another player or with boards and stick hits.
Palmar petechiae from indoor climbing is similar to black palm from weight lifting.13 Although the typical black discoloration is absent, the mechanisms of friction and brutal trauma, clinical presentation, and evolution are similar.
Lastly, archery-induced hematomas are caused by the absence of an arm guard, which protects the wrist and forearm when the string snaps back.14 This complication is not often reported but is known by archers. Because archers usually wear protective gear, these injuries are expected to occur in novices or when safety measures are not respected.
1. Aguayo-Leiva I, Vano-Galvan S, Arrazola JM. A purpuric rash. Aust Fam Physician. 2009;38:889-890.
2. Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
3. Leonard JC, Rieger M. Idiopathic thrombocytopenic purpura presenting in a high school football player: a case report. J Athl Train. 1998;33:269-270.
4. Nordlind K, Bondesson L, Johansson SG, et al. Purpura provoked by cold exposure in a skier. Dermatologica. 1983;167:101-103.
5. Latenser BA, Hempstead RW. Exercise-associated solar purpura in an atypical location. Cutis. 1985;35:365-366.
6. Allan SJ, Humphreys F, Buxton PK. Annular purpura and step aerobics. Clin Exp Dermatol. 1994;19:418.
7. Scott MJ Jr, Scott MJ 3rd. Ping pong patches. Cutis. 1989;43:363-364.
8. Aboutalebi S, Stetson CL. Paintball purpura. J Am Acad Dermatol. 2005;53:901-902.
9. Levsky ME, Crowe M. What is your diagnosis? paintball purpura. Cutis. 2005;75:148, 157-158.
10. Barazi H, Adams BB. Sports purpura. Int J Dermatol. 2006;45:1443.
11. Pasanen K, Parkkari J, Kannus P, et al. Injury risk in female floorball: a prospective one-season follow-up [published online ahead of print May 9, 2007]. Scand J Med Sci Sports. 2008;18:49-54.
12. New world record. Floorball Central Web site. http://www.floorballcentral.com/2010/11/new-world -record.html. Published November 5, 2010. Accessed April 8, 2015.
13. Izumi AK. Letter: pigmented palmar petechiae (black palm). Arch Dermatol. 1974;109:261.
14. Rayan GM. Archery-related injuries of the hand, forearm, and elbow. South Med J. 1992;85:961-964.
1. Aguayo-Leiva I, Vano-Galvan S, Arrazola JM. A purpuric rash. Aust Fam Physician. 2009;38:889-890.
2. Ramelet AA. Exercise-induced vasculitis. J Eur Acad Dermatol Venereol. 2006;20:423-427.
3. Leonard JC, Rieger M. Idiopathic thrombocytopenic purpura presenting in a high school football player: a case report. J Athl Train. 1998;33:269-270.
4. Nordlind K, Bondesson L, Johansson SG, et al. Purpura provoked by cold exposure in a skier. Dermatologica. 1983;167:101-103.
5. Latenser BA, Hempstead RW. Exercise-associated solar purpura in an atypical location. Cutis. 1985;35:365-366.
6. Allan SJ, Humphreys F, Buxton PK. Annular purpura and step aerobics. Clin Exp Dermatol. 1994;19:418.
7. Scott MJ Jr, Scott MJ 3rd. Ping pong patches. Cutis. 1989;43:363-364.
8. Aboutalebi S, Stetson CL. Paintball purpura. J Am Acad Dermatol. 2005;53:901-902.
9. Levsky ME, Crowe M. What is your diagnosis? paintball purpura. Cutis. 2005;75:148, 157-158.
10. Barazi H, Adams BB. Sports purpura. Int J Dermatol. 2006;45:1443.
11. Pasanen K, Parkkari J, Kannus P, et al. Injury risk in female floorball: a prospective one-season follow-up [published online ahead of print May 9, 2007]. Scand J Med Sci Sports. 2008;18:49-54.
12. New world record. Floorball Central Web site. http://www.floorballcentral.com/2010/11/new-world -record.html. Published November 5, 2010. Accessed April 8, 2015.
13. Izumi AK. Letter: pigmented palmar petechiae (black palm). Arch Dermatol. 1974;109:261.
14. Rayan GM. Archery-related injuries of the hand, forearm, and elbow. South Med J. 1992;85:961-964.
Yoga for Dermatologic Conditions
Regardless of its spiritual origins, yoga has become a popular way of reaching mind and body well-being with nearly 30 million people practicing regularly worldwide.1 Yoga, which is the combination of physical postures, controlled breathing, and meditation or mindfulness, has long been used in complementary and alternative medicine around the world and recently has gained popularity as a therapeutic practice, with nearly 14 million Americans reporting that yoga was recommended to them by a physician or therapist.2,3 Studies suggest that people who participate in even brief yoga programs may see improvements in anxiety, somatic stress and discomfort, health-related quality of life, and self-rated sleep quality, all benefits that can help medical conditions, especially those that are dermatologic in nature.4,5
Stress and Dermatologic Conditions
The interaction between the mind, skin, and body is well known. Research in psychoneuroimmunology, the interaction between psychological processes and the nervous and immune systems, has examined the role of neuropeptides, hormones, and neurotransmitters in psychodermatological disorders. The correlation between neuroimmunological pathways and skin inflammation is now well recognized, specifically the interactions between the brain and skin underlying many dermatological diseases (eg, acne, alopecia areata, various types of eczema and dermatitis, oral and genital herpes, hyperhidrosis, pruritus, psoriasis, rosacea, urticaria, warts, breaking or ridging of the nails).6-9
Two biological systems are known to be affected by the systemic stress response: (1) the hypothalamic-pituitary-adrenal axis, which regulates the release of adrenocorticotropin, ß-endorphin, and cortisol, and (2) the sympathoadrenal medullary system, which regulates the release of catecholamines (eg, epinephrine, norepinephrine).7 Cortisol and catecholamines have been shown to have potent effects on the immune system as well as the inflammatory response.9 Additionally, it has been shown that cutaneous sensory nerve terminals release neuropeptides, including calcitonin gene-related peptide and substance P, both of which have different effects on the local inflammatory response.10,11
Psychological stress is well known to trigger many dermatologic conditions, but it also may lead to abnormal skin barrier function.12 The mechanism in which skin barrier function is affected appears to involve a stress-induced increase of endogenous glucocorticoids, which may consequently disrupt skin barrier function and recovery rates, stratum corneum cohesion, and epidermal antimicrobial function.13,14
Atopic dermatitis, for example, is classified as a psychophysiological disorder. Although it is not caused by stress, atopic dermatitis has been described to be precipitated or exacerbated by stress in patients.15 In fact, it was found that stressful life events preceded the onset of itching in more than 70% of patients with atopic dermatitis,16 which is especially relevant, as there is no cure and patients often experience a lifelong struggle with the condition. Additionally, stress mediates the degranulation of mast cells via corticotropin-releasing hormone and neuropeptides, and the upregulation of mast cell corticotropin-releasing hormone receptors supporting its putative role in the pathogenesis of urticaria.9,17 Furthermore, the increase in cortisol also has been described in the exacerbation of acne during times of stress.18
Psychological factors affect the management of skin conditions in more than one-third of reported dermatology patients; therefore, it is important to consider these factors in the treatment of chronic dermatological conditions, especially when they are inquired by the patient.19,20
Yoga Benefits in the Literature
The therapeutic potential of yoga has been explored in a growing number of randomized controlled trials to date.21 A recently published bibliometric analysis provided a comprehensive review of the characteristics of the randomized yoga trials available in the literature.22 The review included 366 full-text articles, with the 2 earliest studies published in 1975 and nearly 90% published within the last decade. In addition to healthy patients, it was found these randomized controlled yoga trials most commonly enrolled patients with breast cancer, depression, asthma, and type 2 diabetes mellitus.22 Another study examined psychological (eg, self-rated stress and stress behavior, anger, exhaustion, quality of life) and physiological (eg, blood pressure, heart rate, urinary catecholamines, salivary cortisol) measurements obtained before and after a 10-session yoga program that participants completed over a 4-month period, with results showing significant improvements (P<.05) on almost all stress-related subjective and physiological variables. Results were comparable with cognitive behavioral therapy.23
Not only has it been shown that yoga helps patients on a psychological level, but a recent study reported that 90-minute sessions of mindfulness meditation and gentle Hatha yoga over an 8-week period led to observable benefits on a cellular level, as telomere length was maintained in distressed breast cancer survivors compared to decreases in telomere length in the control group with patients who solely participated in a stress management seminar.24 To date, there are no known studies examining the effects of yoga on patients with skin cancer. However, a few studies have specifically examined the effect of yoga in managing non–cancer-related dermatologic issues. Specifically, one small study of psoriasis patients found that those who listened to mindfulness meditation tapes while undergoing standard phototherapy (psoralen plus UVA) healed faster than those who underwent phototherapy treatment alone.25
Because some dermatologic problems have comorbidities and increased risk factors of other medical problems, such as psoriasis with psoriatic arthritis and metabolic diseases (eg, abdominal obesity, diabetes, nonalcoholic fatty liver disease, dyslipidemia, metabolic syndrome, chronic kidney disease), it is even more pertinent to recommend approaches for healthy mind and body well-being as a supplement to medical care.26
Final Thoughts
With accurate diagnosis by a dermatologist, appropriate conventional treatments can improve dermatologic problems. These treatments alone can reduce patients’ stress and improve skin, hair, and nail conditions; however, if it is clear that stress is interfering with a patient’s overall well-being and ability to cope with his/her dermatologic condition, concurrent stress management interventions may be warranted. In some instances, recommending yoga sessions, mindful meditation, or breathing exercises may help, while in others referral to a mental health professional may be necessary.
Beyond the direct physiological effects of stress, it also is worth mentioning that patients who deal with stress also tend to scratch, pick, or irritate their skin more and often lack the motivation to adhere to skin care regimens or treatments, again supporting the idea that our approach in managing these patients must be multifaceted. As dermatologists in training, residents should be cognizant of the potential psychological sequelae of some dermatologic problems and be aware of the possible use of supplemental interventions by our patients.
1. Dangerfield A. Yoga wars. BBC News. http://news.bbc.co.uk/1/hi/7844691.stm. Published January 23, 2009. Accessed March 25, 2015.
2. Yoga Journal releases 2012 yoga in America market study [press release]. San Francisco, CA: Yoga Journal; December 6, 2012.
3. De Michaelis E. A History of Modern Yoga: Patanjali and Western Esotericism. London, United Kingdom: A&C Black; 2005.
4. Telles S, Singh N, Yadav A, et al. Effect of yoga on different aspects of mental health. Indian J Physiol Pharmacol. 2012;56:245-254.
5. Rodriguez-Vallecillo E, Woodbury-Fariña MA. Dermatological manifestations of stress in normal and psychiatric populations. Psychiatr Clin North Am. 2014;37:625-651.
6. Stander S, Raap U, Weisshaar E, et al. Pathogenesis of pruritus. J Dtsch Dermatol Ges. 2011;9:456-463.
7. Arck PC, Slominski A, Theoharides TC, et al. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697-1704.
8. Recognizing the mind-skin connection. Harvard Health Publications Web site. http://www.health.harvard.edu/newsletter_article/Recognizing_the_mind-skin_connection. Published November 1, 2006. Accessed March 31, 2015.
9. Tausk F, Elenkov I, Moynihan J. Psychoneuroimmunology. Dermatol Ther. 2008;21:22-31.
10. Pavlovic S, Liezmann C, Blois SM, et al. Substance P is a key mediator of stress-induced protection from allergic sensitization via modified antigen presentation. J Immunol. 2011;186:848-855.
11. Toyoda M, Nakamura M, Makino T, et al. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br J Dermatol. 2002;147:71-79.
12. Koo JYM, Lee CS. General approach to evaluating psychodermatological disorders. In: Koo JYM, Lee CS, eds. Psychocutaneous Medicine. New York, NY: Marcel Dekker; 2003:1-29.
13. Garg A, Chren MM, Sands LP, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137:53-59.
14. Elias PM, Sun R, Eder AR, et al. Treating atopic dermatitis at the source: corrective barrier repair therapy based upon new pathogenic insights. Expert Rev Dermatol. 2013;8:27-36.
15. Morren MA, Przybilla B, Bamelis M, et al. Atopic dermatitis: triggering factors. J Am Acad Dermatol. 1994;31:467-473.
16. Faulstich ME, Williamson DA. An overview of atopic dermatitis: toward a bio-behavioural integration. J Psychosom Res. 1985;29:647-654.
17. Theoharides TC, Donelan JM, Papadopoulou N, et al. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563-568.
18. Suh DH, Kwon HH. What’s new in the physiopathology of acne [published online ahead of print Jan 24, 2015]? Br J Dermatol. doi:10.1111/bjd.13634.
19. Picardi A, Mazzotti E, Pasquini P. Prevalence and correlates of suicidal ideation among patients with skin disease. J Am Acad Dermatol. 2006;54:420-426.
20. Ponarovsky B, Amital D, Lazarov A, et al. Anxiety and depression in patients with allergic and non-allergic cutaneous disorders. Int J Dermatol. 2011;50:1217-1222.
21. Khalsa SB. Yoga as a therapeutic intervention: a bibliometric analysis of published research studies. Indian J Physiol Pharmacol. 2004;48:269-285.
22. Cramer H, Lauche R, Dobos G. Characteristics of randomized controlled trials of yoga: a bibliometric analysis. BMC Complement Altern Med. 2014;14:328.
23. Granath J, Ingvarsson S, von Thiele U, et al. Stress management: a randomized study of cognitive behavioural therapy and yoga. Cogn Behav Ther. 2006;35:3-10.
24. Carlson LE, Beattie TL, Giese-Davis J, et al. Mindfulness-based cancer recovery and supportive-expressive therapy maintain telomere length relative to controls in distressed breast cancer survivors. Cancer. 2015;121:476-484.
25. Kabat-Zinn J, Wheeler E, Light T, et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA). Psychosom Med. 1998;60:625-632.
26. Gisondi P, Galvan A, Idolazzi L, et al. Management of moderate to severe psoriasis in patients with metabolic comorbidities. Front Med (Lausanne). 2015;2:1.
Regardless of its spiritual origins, yoga has become a popular way of reaching mind and body well-being with nearly 30 million people practicing regularly worldwide.1 Yoga, which is the combination of physical postures, controlled breathing, and meditation or mindfulness, has long been used in complementary and alternative medicine around the world and recently has gained popularity as a therapeutic practice, with nearly 14 million Americans reporting that yoga was recommended to them by a physician or therapist.2,3 Studies suggest that people who participate in even brief yoga programs may see improvements in anxiety, somatic stress and discomfort, health-related quality of life, and self-rated sleep quality, all benefits that can help medical conditions, especially those that are dermatologic in nature.4,5
Stress and Dermatologic Conditions
The interaction between the mind, skin, and body is well known. Research in psychoneuroimmunology, the interaction between psychological processes and the nervous and immune systems, has examined the role of neuropeptides, hormones, and neurotransmitters in psychodermatological disorders. The correlation between neuroimmunological pathways and skin inflammation is now well recognized, specifically the interactions between the brain and skin underlying many dermatological diseases (eg, acne, alopecia areata, various types of eczema and dermatitis, oral and genital herpes, hyperhidrosis, pruritus, psoriasis, rosacea, urticaria, warts, breaking or ridging of the nails).6-9
Two biological systems are known to be affected by the systemic stress response: (1) the hypothalamic-pituitary-adrenal axis, which regulates the release of adrenocorticotropin, ß-endorphin, and cortisol, and (2) the sympathoadrenal medullary system, which regulates the release of catecholamines (eg, epinephrine, norepinephrine).7 Cortisol and catecholamines have been shown to have potent effects on the immune system as well as the inflammatory response.9 Additionally, it has been shown that cutaneous sensory nerve terminals release neuropeptides, including calcitonin gene-related peptide and substance P, both of which have different effects on the local inflammatory response.10,11
Psychological stress is well known to trigger many dermatologic conditions, but it also may lead to abnormal skin barrier function.12 The mechanism in which skin barrier function is affected appears to involve a stress-induced increase of endogenous glucocorticoids, which may consequently disrupt skin barrier function and recovery rates, stratum corneum cohesion, and epidermal antimicrobial function.13,14
Atopic dermatitis, for example, is classified as a psychophysiological disorder. Although it is not caused by stress, atopic dermatitis has been described to be precipitated or exacerbated by stress in patients.15 In fact, it was found that stressful life events preceded the onset of itching in more than 70% of patients with atopic dermatitis,16 which is especially relevant, as there is no cure and patients often experience a lifelong struggle with the condition. Additionally, stress mediates the degranulation of mast cells via corticotropin-releasing hormone and neuropeptides, and the upregulation of mast cell corticotropin-releasing hormone receptors supporting its putative role in the pathogenesis of urticaria.9,17 Furthermore, the increase in cortisol also has been described in the exacerbation of acne during times of stress.18
Psychological factors affect the management of skin conditions in more than one-third of reported dermatology patients; therefore, it is important to consider these factors in the treatment of chronic dermatological conditions, especially when they are inquired by the patient.19,20
Yoga Benefits in the Literature
The therapeutic potential of yoga has been explored in a growing number of randomized controlled trials to date.21 A recently published bibliometric analysis provided a comprehensive review of the characteristics of the randomized yoga trials available in the literature.22 The review included 366 full-text articles, with the 2 earliest studies published in 1975 and nearly 90% published within the last decade. In addition to healthy patients, it was found these randomized controlled yoga trials most commonly enrolled patients with breast cancer, depression, asthma, and type 2 diabetes mellitus.22 Another study examined psychological (eg, self-rated stress and stress behavior, anger, exhaustion, quality of life) and physiological (eg, blood pressure, heart rate, urinary catecholamines, salivary cortisol) measurements obtained before and after a 10-session yoga program that participants completed over a 4-month period, with results showing significant improvements (P<.05) on almost all stress-related subjective and physiological variables. Results were comparable with cognitive behavioral therapy.23
Not only has it been shown that yoga helps patients on a psychological level, but a recent study reported that 90-minute sessions of mindfulness meditation and gentle Hatha yoga over an 8-week period led to observable benefits on a cellular level, as telomere length was maintained in distressed breast cancer survivors compared to decreases in telomere length in the control group with patients who solely participated in a stress management seminar.24 To date, there are no known studies examining the effects of yoga on patients with skin cancer. However, a few studies have specifically examined the effect of yoga in managing non–cancer-related dermatologic issues. Specifically, one small study of psoriasis patients found that those who listened to mindfulness meditation tapes while undergoing standard phototherapy (psoralen plus UVA) healed faster than those who underwent phototherapy treatment alone.25
Because some dermatologic problems have comorbidities and increased risk factors of other medical problems, such as psoriasis with psoriatic arthritis and metabolic diseases (eg, abdominal obesity, diabetes, nonalcoholic fatty liver disease, dyslipidemia, metabolic syndrome, chronic kidney disease), it is even more pertinent to recommend approaches for healthy mind and body well-being as a supplement to medical care.26
Final Thoughts
With accurate diagnosis by a dermatologist, appropriate conventional treatments can improve dermatologic problems. These treatments alone can reduce patients’ stress and improve skin, hair, and nail conditions; however, if it is clear that stress is interfering with a patient’s overall well-being and ability to cope with his/her dermatologic condition, concurrent stress management interventions may be warranted. In some instances, recommending yoga sessions, mindful meditation, or breathing exercises may help, while in others referral to a mental health professional may be necessary.
Beyond the direct physiological effects of stress, it also is worth mentioning that patients who deal with stress also tend to scratch, pick, or irritate their skin more and often lack the motivation to adhere to skin care regimens or treatments, again supporting the idea that our approach in managing these patients must be multifaceted. As dermatologists in training, residents should be cognizant of the potential psychological sequelae of some dermatologic problems and be aware of the possible use of supplemental interventions by our patients.
Regardless of its spiritual origins, yoga has become a popular way of reaching mind and body well-being with nearly 30 million people practicing regularly worldwide.1 Yoga, which is the combination of physical postures, controlled breathing, and meditation or mindfulness, has long been used in complementary and alternative medicine around the world and recently has gained popularity as a therapeutic practice, with nearly 14 million Americans reporting that yoga was recommended to them by a physician or therapist.2,3 Studies suggest that people who participate in even brief yoga programs may see improvements in anxiety, somatic stress and discomfort, health-related quality of life, and self-rated sleep quality, all benefits that can help medical conditions, especially those that are dermatologic in nature.4,5
Stress and Dermatologic Conditions
The interaction between the mind, skin, and body is well known. Research in psychoneuroimmunology, the interaction between psychological processes and the nervous and immune systems, has examined the role of neuropeptides, hormones, and neurotransmitters in psychodermatological disorders. The correlation between neuroimmunological pathways and skin inflammation is now well recognized, specifically the interactions between the brain and skin underlying many dermatological diseases (eg, acne, alopecia areata, various types of eczema and dermatitis, oral and genital herpes, hyperhidrosis, pruritus, psoriasis, rosacea, urticaria, warts, breaking or ridging of the nails).6-9
Two biological systems are known to be affected by the systemic stress response: (1) the hypothalamic-pituitary-adrenal axis, which regulates the release of adrenocorticotropin, ß-endorphin, and cortisol, and (2) the sympathoadrenal medullary system, which regulates the release of catecholamines (eg, epinephrine, norepinephrine).7 Cortisol and catecholamines have been shown to have potent effects on the immune system as well as the inflammatory response.9 Additionally, it has been shown that cutaneous sensory nerve terminals release neuropeptides, including calcitonin gene-related peptide and substance P, both of which have different effects on the local inflammatory response.10,11
Psychological stress is well known to trigger many dermatologic conditions, but it also may lead to abnormal skin barrier function.12 The mechanism in which skin barrier function is affected appears to involve a stress-induced increase of endogenous glucocorticoids, which may consequently disrupt skin barrier function and recovery rates, stratum corneum cohesion, and epidermal antimicrobial function.13,14
Atopic dermatitis, for example, is classified as a psychophysiological disorder. Although it is not caused by stress, atopic dermatitis has been described to be precipitated or exacerbated by stress in patients.15 In fact, it was found that stressful life events preceded the onset of itching in more than 70% of patients with atopic dermatitis,16 which is especially relevant, as there is no cure and patients often experience a lifelong struggle with the condition. Additionally, stress mediates the degranulation of mast cells via corticotropin-releasing hormone and neuropeptides, and the upregulation of mast cell corticotropin-releasing hormone receptors supporting its putative role in the pathogenesis of urticaria.9,17 Furthermore, the increase in cortisol also has been described in the exacerbation of acne during times of stress.18
Psychological factors affect the management of skin conditions in more than one-third of reported dermatology patients; therefore, it is important to consider these factors in the treatment of chronic dermatological conditions, especially when they are inquired by the patient.19,20
Yoga Benefits in the Literature
The therapeutic potential of yoga has been explored in a growing number of randomized controlled trials to date.21 A recently published bibliometric analysis provided a comprehensive review of the characteristics of the randomized yoga trials available in the literature.22 The review included 366 full-text articles, with the 2 earliest studies published in 1975 and nearly 90% published within the last decade. In addition to healthy patients, it was found these randomized controlled yoga trials most commonly enrolled patients with breast cancer, depression, asthma, and type 2 diabetes mellitus.22 Another study examined psychological (eg, self-rated stress and stress behavior, anger, exhaustion, quality of life) and physiological (eg, blood pressure, heart rate, urinary catecholamines, salivary cortisol) measurements obtained before and after a 10-session yoga program that participants completed over a 4-month period, with results showing significant improvements (P<.05) on almost all stress-related subjective and physiological variables. Results were comparable with cognitive behavioral therapy.23
Not only has it been shown that yoga helps patients on a psychological level, but a recent study reported that 90-minute sessions of mindfulness meditation and gentle Hatha yoga over an 8-week period led to observable benefits on a cellular level, as telomere length was maintained in distressed breast cancer survivors compared to decreases in telomere length in the control group with patients who solely participated in a stress management seminar.24 To date, there are no known studies examining the effects of yoga on patients with skin cancer. However, a few studies have specifically examined the effect of yoga in managing non–cancer-related dermatologic issues. Specifically, one small study of psoriasis patients found that those who listened to mindfulness meditation tapes while undergoing standard phototherapy (psoralen plus UVA) healed faster than those who underwent phototherapy treatment alone.25
Because some dermatologic problems have comorbidities and increased risk factors of other medical problems, such as psoriasis with psoriatic arthritis and metabolic diseases (eg, abdominal obesity, diabetes, nonalcoholic fatty liver disease, dyslipidemia, metabolic syndrome, chronic kidney disease), it is even more pertinent to recommend approaches for healthy mind and body well-being as a supplement to medical care.26
Final Thoughts
With accurate diagnosis by a dermatologist, appropriate conventional treatments can improve dermatologic problems. These treatments alone can reduce patients’ stress and improve skin, hair, and nail conditions; however, if it is clear that stress is interfering with a patient’s overall well-being and ability to cope with his/her dermatologic condition, concurrent stress management interventions may be warranted. In some instances, recommending yoga sessions, mindful meditation, or breathing exercises may help, while in others referral to a mental health professional may be necessary.
Beyond the direct physiological effects of stress, it also is worth mentioning that patients who deal with stress also tend to scratch, pick, or irritate their skin more and often lack the motivation to adhere to skin care regimens or treatments, again supporting the idea that our approach in managing these patients must be multifaceted. As dermatologists in training, residents should be cognizant of the potential psychological sequelae of some dermatologic problems and be aware of the possible use of supplemental interventions by our patients.
1. Dangerfield A. Yoga wars. BBC News. http://news.bbc.co.uk/1/hi/7844691.stm. Published January 23, 2009. Accessed March 25, 2015.
2. Yoga Journal releases 2012 yoga in America market study [press release]. San Francisco, CA: Yoga Journal; December 6, 2012.
3. De Michaelis E. A History of Modern Yoga: Patanjali and Western Esotericism. London, United Kingdom: A&C Black; 2005.
4. Telles S, Singh N, Yadav A, et al. Effect of yoga on different aspects of mental health. Indian J Physiol Pharmacol. 2012;56:245-254.
5. Rodriguez-Vallecillo E, Woodbury-Fariña MA. Dermatological manifestations of stress in normal and psychiatric populations. Psychiatr Clin North Am. 2014;37:625-651.
6. Stander S, Raap U, Weisshaar E, et al. Pathogenesis of pruritus. J Dtsch Dermatol Ges. 2011;9:456-463.
7. Arck PC, Slominski A, Theoharides TC, et al. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697-1704.
8. Recognizing the mind-skin connection. Harvard Health Publications Web site. http://www.health.harvard.edu/newsletter_article/Recognizing_the_mind-skin_connection. Published November 1, 2006. Accessed March 31, 2015.
9. Tausk F, Elenkov I, Moynihan J. Psychoneuroimmunology. Dermatol Ther. 2008;21:22-31.
10. Pavlovic S, Liezmann C, Blois SM, et al. Substance P is a key mediator of stress-induced protection from allergic sensitization via modified antigen presentation. J Immunol. 2011;186:848-855.
11. Toyoda M, Nakamura M, Makino T, et al. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br J Dermatol. 2002;147:71-79.
12. Koo JYM, Lee CS. General approach to evaluating psychodermatological disorders. In: Koo JYM, Lee CS, eds. Psychocutaneous Medicine. New York, NY: Marcel Dekker; 2003:1-29.
13. Garg A, Chren MM, Sands LP, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137:53-59.
14. Elias PM, Sun R, Eder AR, et al. Treating atopic dermatitis at the source: corrective barrier repair therapy based upon new pathogenic insights. Expert Rev Dermatol. 2013;8:27-36.
15. Morren MA, Przybilla B, Bamelis M, et al. Atopic dermatitis: triggering factors. J Am Acad Dermatol. 1994;31:467-473.
16. Faulstich ME, Williamson DA. An overview of atopic dermatitis: toward a bio-behavioural integration. J Psychosom Res. 1985;29:647-654.
17. Theoharides TC, Donelan JM, Papadopoulou N, et al. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563-568.
18. Suh DH, Kwon HH. What’s new in the physiopathology of acne [published online ahead of print Jan 24, 2015]? Br J Dermatol. doi:10.1111/bjd.13634.
19. Picardi A, Mazzotti E, Pasquini P. Prevalence and correlates of suicidal ideation among patients with skin disease. J Am Acad Dermatol. 2006;54:420-426.
20. Ponarovsky B, Amital D, Lazarov A, et al. Anxiety and depression in patients with allergic and non-allergic cutaneous disorders. Int J Dermatol. 2011;50:1217-1222.
21. Khalsa SB. Yoga as a therapeutic intervention: a bibliometric analysis of published research studies. Indian J Physiol Pharmacol. 2004;48:269-285.
22. Cramer H, Lauche R, Dobos G. Characteristics of randomized controlled trials of yoga: a bibliometric analysis. BMC Complement Altern Med. 2014;14:328.
23. Granath J, Ingvarsson S, von Thiele U, et al. Stress management: a randomized study of cognitive behavioural therapy and yoga. Cogn Behav Ther. 2006;35:3-10.
24. Carlson LE, Beattie TL, Giese-Davis J, et al. Mindfulness-based cancer recovery and supportive-expressive therapy maintain telomere length relative to controls in distressed breast cancer survivors. Cancer. 2015;121:476-484.
25. Kabat-Zinn J, Wheeler E, Light T, et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA). Psychosom Med. 1998;60:625-632.
26. Gisondi P, Galvan A, Idolazzi L, et al. Management of moderate to severe psoriasis in patients with metabolic comorbidities. Front Med (Lausanne). 2015;2:1.
1. Dangerfield A. Yoga wars. BBC News. http://news.bbc.co.uk/1/hi/7844691.stm. Published January 23, 2009. Accessed March 25, 2015.
2. Yoga Journal releases 2012 yoga in America market study [press release]. San Francisco, CA: Yoga Journal; December 6, 2012.
3. De Michaelis E. A History of Modern Yoga: Patanjali and Western Esotericism. London, United Kingdom: A&C Black; 2005.
4. Telles S, Singh N, Yadav A, et al. Effect of yoga on different aspects of mental health. Indian J Physiol Pharmacol. 2012;56:245-254.
5. Rodriguez-Vallecillo E, Woodbury-Fariña MA. Dermatological manifestations of stress in normal and psychiatric populations. Psychiatr Clin North Am. 2014;37:625-651.
6. Stander S, Raap U, Weisshaar E, et al. Pathogenesis of pruritus. J Dtsch Dermatol Ges. 2011;9:456-463.
7. Arck PC, Slominski A, Theoharides TC, et al. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697-1704.
8. Recognizing the mind-skin connection. Harvard Health Publications Web site. http://www.health.harvard.edu/newsletter_article/Recognizing_the_mind-skin_connection. Published November 1, 2006. Accessed March 31, 2015.
9. Tausk F, Elenkov I, Moynihan J. Psychoneuroimmunology. Dermatol Ther. 2008;21:22-31.
10. Pavlovic S, Liezmann C, Blois SM, et al. Substance P is a key mediator of stress-induced protection from allergic sensitization via modified antigen presentation. J Immunol. 2011;186:848-855.
11. Toyoda M, Nakamura M, Makino T, et al. Nerve growth factor and substance P are useful plasma markers of disease activity in atopic dermatitis. Br J Dermatol. 2002;147:71-79.
12. Koo JYM, Lee CS. General approach to evaluating psychodermatological disorders. In: Koo JYM, Lee CS, eds. Psychocutaneous Medicine. New York, NY: Marcel Dekker; 2003:1-29.
13. Garg A, Chren MM, Sands LP, et al. Psychological stress perturbs epidermal permeability barrier homeostasis: implications for the pathogenesis of stress-associated skin disorders. Arch Dermatol. 2001;137:53-59.
14. Elias PM, Sun R, Eder AR, et al. Treating atopic dermatitis at the source: corrective barrier repair therapy based upon new pathogenic insights. Expert Rev Dermatol. 2013;8:27-36.
15. Morren MA, Przybilla B, Bamelis M, et al. Atopic dermatitis: triggering factors. J Am Acad Dermatol. 1994;31:467-473.
16. Faulstich ME, Williamson DA. An overview of atopic dermatitis: toward a bio-behavioural integration. J Psychosom Res. 1985;29:647-654.
17. Theoharides TC, Donelan JM, Papadopoulou N, et al. Mast cells as targets of corticotropin-releasing factor and related peptides. Trends Pharmacol Sci. 2004;25:563-568.
18. Suh DH, Kwon HH. What’s new in the physiopathology of acne [published online ahead of print Jan 24, 2015]? Br J Dermatol. doi:10.1111/bjd.13634.
19. Picardi A, Mazzotti E, Pasquini P. Prevalence and correlates of suicidal ideation among patients with skin disease. J Am Acad Dermatol. 2006;54:420-426.
20. Ponarovsky B, Amital D, Lazarov A, et al. Anxiety and depression in patients with allergic and non-allergic cutaneous disorders. Int J Dermatol. 2011;50:1217-1222.
21. Khalsa SB. Yoga as a therapeutic intervention: a bibliometric analysis of published research studies. Indian J Physiol Pharmacol. 2004;48:269-285.
22. Cramer H, Lauche R, Dobos G. Characteristics of randomized controlled trials of yoga: a bibliometric analysis. BMC Complement Altern Med. 2014;14:328.
23. Granath J, Ingvarsson S, von Thiele U, et al. Stress management: a randomized study of cognitive behavioural therapy and yoga. Cogn Behav Ther. 2006;35:3-10.
24. Carlson LE, Beattie TL, Giese-Davis J, et al. Mindfulness-based cancer recovery and supportive-expressive therapy maintain telomere length relative to controls in distressed breast cancer survivors. Cancer. 2015;121:476-484.
25. Kabat-Zinn J, Wheeler E, Light T, et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA). Psychosom Med. 1998;60:625-632.
26. Gisondi P, Galvan A, Idolazzi L, et al. Management of moderate to severe psoriasis in patients with metabolic comorbidities. Front Med (Lausanne). 2015;2:1.
What Is Your Diagnosis? New World Cutaneous Leishmaniasis
A 40-year-old man presented with a nonhealing ulcer on the right hand of 2 months’ duration. The lesion had started as a pruritic papule while he was visiting Guyana 2 months prior. The area had slowly enlarged with progressive ulceration. He denied any systemic signs including fever, chills, or weight loss, and his medical history was unremarkable. Physical examination revealed a 4-cm fungating ulceration with heaped-up borders on the dorsal aspect of the right hand.
The Diagnosis: New World Cutaneous Leishmaniasis
In addition to the ulceration on the right hand, a 2-cm ulcerated plaque also had developed on the right side of the chin a few days later (Figure 1). A biopsy was obtained from the lesion on the hand. Histopathologic examination revealed granulomatous inflammation with numerous histiocytes containing intracellular organisms (Figure 2). The microorganisms had pale pink nuclei with basophilic kinetoplasts (Figure 3). Tissue culture showed a mixed growth of gram-positive and gram-negative organisms but no predominant organism. Fungal and mycobacterial cultures were negative. A diagnosis of New World cutaneous leishmaniasis (CL) was made due to visualization of intracellular microorganisms containing basophilic kinetoplasts. Polymerase chain reaction on the tissue block confirmed the presence of Leishmania guyanensis.
|
Cutaneous leishmaniasis is caused by protozoa from the Leishmania species and is transmitted by the bite of the female sandfly. There are 2 classifications for the disease: Old World and New World. Old World CL is transmitted by the sandfly of the genus Phlebotomus, which is endemic in Asia, Africa, the Mediterranean, and the Middle East. New World CL is transmitted by the sandflies of the genus Lutzomyia, which are endemic in Mexico, Central America, and South America. There have been occasional cases of autochthonous transmission reported in Texas and Oklahoma,1 but there has been no known transmission of CL in Australia or the Pacific Islands.2 Human infection can be transmitted by 21 species of Leishmania and can be speciated by designated laboratories such as the US Centers for Disease Control and Prevention (CDC) and the Walter Reed Army Institute of Research (Silver Spring, Maryland) using tissue culture and polymerase chain reaction.1 The CDC can assist with the collection of specimens and supply of culture medium for cases occurring in the United States. Identification of the species is important because there are associated implications for treatment and prognosis.
There are 3 major forms of leishmaniasis: cutaneous, mucocutaneous, and visceral. Cutaneous leishmaniasis is the most common form. There are approximately 1.5 million new cases of CL each year worldwide,3 and more than 90% of these cases occur in Afghanistan, Algeria, Iran, Iraq, Saudi Arabia, Syria, Brazil, and Peru.1 New World CL is caused by 2 species complexes: Leishmania mexicana and Leishmania viannia, including the subspecies L guyanensis, which was seen in our patient.
Cutaneous leishmaniasis usually begins as a small, well-defined papule at the site of the insect bite that then enlarges and becomes a nodule or plaque. Next, the lesion becomes ulcerated with raised borders (Figure 1). The ulcer typically is painless unless there is secondary bacterial or fungal infection.3,4 The incubation period usually is 2 to 8 weeks and multiple lesions may be present, as seen in our patient.4
Old World CL can resolve without treatment, but New World CL is less likely to spontaneously resolve. Additionally, there is a greater risk for spread of the infection to mucous membranes or for systemic dissemination if New World CL is left untreated.2,5 Patients with multiple lesions (ie, ≥3); large lesions (ie, >2.5 cm); lesions on the face, hands, feet, or joints; and those who are immunocompromised should be treated promptly.2 Pentavalent antimonials (eg, meglumine antimoniate, sodium stibogluconate) are the treatment of choice for New World CL, except for infections caused by L guyanensis. The most common pentavalent antimonial agent used in the United States is sodium stibogluconate and is given at a standard intravenous dose of 20 mg antimony/kg daily for 20 days.2 The drug is only available through the CDC’s Drug Service under an Investigational New Drug protocol.1
Intramuscular pentamidine (3 mg/kg daily every other day for 4 doses) is the first-line treatment of CL caused by L guyanensis because systemic antimony usually is not effective.2,6,7 Intralesional injection with pentavalent antimonials usually is not recommended for treatment of New World CL because of the possibility of disseminated disease.2 Liposomal amphotericin B has mainly been used to treat visceral and mucosal leishmaniasis, but there have been some small studies and case reports that have showed it to be successful in treating CL.8-10 Larger controlled studies need to be performed. Oral antifungal drugs (eg, fluconazole, ketoconazole, itraconazole) also have been used to treat CL with variable results depending on the Leishmania species.11
There currently are no vaccines or drugs available to prevent against leishmaniasis. Preventive measures such as avoiding outdoor activities from dusk to dawn when sandflies are the most active, wearing protective clothing, and applying insect repellent that contains DEET (diethyltoluamide) can help reduce a traveler’s risk for becoming infected. Mosquito nets also should be treated with permethrin, which acts as an insect repellent, as sandflies are so small that they can penetrate mosquito nets.1,3,11
Acknowledgements—We would like to thank Francis Steurer, MS, and Barbara Herwaldt, MD, MPH, at the CDC in Atlanta, Georgia, for their help with the identification of the Leishmania species.
1. Herwaldt BL, Magill AJ. Infectious diseases related to travel: leishmaniasis, cutaneous. Centers for Disease Control and Prevention Web site. http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-5/cutaneous-leish maniasis.htm. Published August 1, 2013. Accessed March 5, 2015.
2. Mitropolos P, Konidas P, Durkin-Konidas M. New World cutaneous leishmaniasis: updated review of current and future diagnosis and treatment. J Am Acad Dermatol. 2010;63:309-322.
3. Hepburn NC. Cutaneous leishmaniasis: an overview. J Postgrad Med. 2003;49:50-54.
4. Markle WH, Makhoul K. Cutaneous leishmaniasis: recognition and treatment. Am Fam Physician. 2004;69:1455-1460.
5. Couppié P, Clyti E, Sainte Marie D, et al. Disseminated cutaneous leishmaniasis due to Leishmania guyanensis: case of a patient with 425 lesions. Am J Trop Med Hyg. 2004;71:558-560.
6. Nacher M, Carme B, Sainte Marie D, et al. Influence of clinical presentation on the efficacy of a short course of pentamidine in the treatment of cutaneous leishmaniasis in French Guiana. Ann Trop Med Parasitol. 2001;95:331-336.
7. Minodier P, Parola P. Cutaneous leishmaniasis treatment. Trav Med Inf Dis. 2007;5:150-158.
8. Solomon M, Baum S, Barzilai A, et al. Liposomal amphotericin B in comparison to sodium stibogluconate for cutaneous infection due to Leishmania braziliensis. J Am Acad Dermatol. 2007;56:612-616.
9. Konecny P, Stark DJ. An Australian case of New World cutaneous leishmaniasis. Med J Aust. 2007;186:315-317.
10. Brown M, Noursadeghi M, Boyle J, et al. Successful liposomal amphotericin B treatment of Leishmania braziliensis cutaneous leishmaniasis. Br J Dermatol. 2005;153:203-205.
11. Parasites: leishmaniasis. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/parasites/leishmaniasis/index.html. Updated January 10, 2013. Accessed March 5, 2015.
A 40-year-old man presented with a nonhealing ulcer on the right hand of 2 months’ duration. The lesion had started as a pruritic papule while he was visiting Guyana 2 months prior. The area had slowly enlarged with progressive ulceration. He denied any systemic signs including fever, chills, or weight loss, and his medical history was unremarkable. Physical examination revealed a 4-cm fungating ulceration with heaped-up borders on the dorsal aspect of the right hand.
The Diagnosis: New World Cutaneous Leishmaniasis
In addition to the ulceration on the right hand, a 2-cm ulcerated plaque also had developed on the right side of the chin a few days later (Figure 1). A biopsy was obtained from the lesion on the hand. Histopathologic examination revealed granulomatous inflammation with numerous histiocytes containing intracellular organisms (Figure 2). The microorganisms had pale pink nuclei with basophilic kinetoplasts (Figure 3). Tissue culture showed a mixed growth of gram-positive and gram-negative organisms but no predominant organism. Fungal and mycobacterial cultures were negative. A diagnosis of New World cutaneous leishmaniasis (CL) was made due to visualization of intracellular microorganisms containing basophilic kinetoplasts. Polymerase chain reaction on the tissue block confirmed the presence of Leishmania guyanensis.
|
Cutaneous leishmaniasis is caused by protozoa from the Leishmania species and is transmitted by the bite of the female sandfly. There are 2 classifications for the disease: Old World and New World. Old World CL is transmitted by the sandfly of the genus Phlebotomus, which is endemic in Asia, Africa, the Mediterranean, and the Middle East. New World CL is transmitted by the sandflies of the genus Lutzomyia, which are endemic in Mexico, Central America, and South America. There have been occasional cases of autochthonous transmission reported in Texas and Oklahoma,1 but there has been no known transmission of CL in Australia or the Pacific Islands.2 Human infection can be transmitted by 21 species of Leishmania and can be speciated by designated laboratories such as the US Centers for Disease Control and Prevention (CDC) and the Walter Reed Army Institute of Research (Silver Spring, Maryland) using tissue culture and polymerase chain reaction.1 The CDC can assist with the collection of specimens and supply of culture medium for cases occurring in the United States. Identification of the species is important because there are associated implications for treatment and prognosis.
There are 3 major forms of leishmaniasis: cutaneous, mucocutaneous, and visceral. Cutaneous leishmaniasis is the most common form. There are approximately 1.5 million new cases of CL each year worldwide,3 and more than 90% of these cases occur in Afghanistan, Algeria, Iran, Iraq, Saudi Arabia, Syria, Brazil, and Peru.1 New World CL is caused by 2 species complexes: Leishmania mexicana and Leishmania viannia, including the subspecies L guyanensis, which was seen in our patient.
Cutaneous leishmaniasis usually begins as a small, well-defined papule at the site of the insect bite that then enlarges and becomes a nodule or plaque. Next, the lesion becomes ulcerated with raised borders (Figure 1). The ulcer typically is painless unless there is secondary bacterial or fungal infection.3,4 The incubation period usually is 2 to 8 weeks and multiple lesions may be present, as seen in our patient.4
Old World CL can resolve without treatment, but New World CL is less likely to spontaneously resolve. Additionally, there is a greater risk for spread of the infection to mucous membranes or for systemic dissemination if New World CL is left untreated.2,5 Patients with multiple lesions (ie, ≥3); large lesions (ie, >2.5 cm); lesions on the face, hands, feet, or joints; and those who are immunocompromised should be treated promptly.2 Pentavalent antimonials (eg, meglumine antimoniate, sodium stibogluconate) are the treatment of choice for New World CL, except for infections caused by L guyanensis. The most common pentavalent antimonial agent used in the United States is sodium stibogluconate and is given at a standard intravenous dose of 20 mg antimony/kg daily for 20 days.2 The drug is only available through the CDC’s Drug Service under an Investigational New Drug protocol.1
Intramuscular pentamidine (3 mg/kg daily every other day for 4 doses) is the first-line treatment of CL caused by L guyanensis because systemic antimony usually is not effective.2,6,7 Intralesional injection with pentavalent antimonials usually is not recommended for treatment of New World CL because of the possibility of disseminated disease.2 Liposomal amphotericin B has mainly been used to treat visceral and mucosal leishmaniasis, but there have been some small studies and case reports that have showed it to be successful in treating CL.8-10 Larger controlled studies need to be performed. Oral antifungal drugs (eg, fluconazole, ketoconazole, itraconazole) also have been used to treat CL with variable results depending on the Leishmania species.11
There currently are no vaccines or drugs available to prevent against leishmaniasis. Preventive measures such as avoiding outdoor activities from dusk to dawn when sandflies are the most active, wearing protective clothing, and applying insect repellent that contains DEET (diethyltoluamide) can help reduce a traveler’s risk for becoming infected. Mosquito nets also should be treated with permethrin, which acts as an insect repellent, as sandflies are so small that they can penetrate mosquito nets.1,3,11
Acknowledgements—We would like to thank Francis Steurer, MS, and Barbara Herwaldt, MD, MPH, at the CDC in Atlanta, Georgia, for their help with the identification of the Leishmania species.
A 40-year-old man presented with a nonhealing ulcer on the right hand of 2 months’ duration. The lesion had started as a pruritic papule while he was visiting Guyana 2 months prior. The area had slowly enlarged with progressive ulceration. He denied any systemic signs including fever, chills, or weight loss, and his medical history was unremarkable. Physical examination revealed a 4-cm fungating ulceration with heaped-up borders on the dorsal aspect of the right hand.
The Diagnosis: New World Cutaneous Leishmaniasis
In addition to the ulceration on the right hand, a 2-cm ulcerated plaque also had developed on the right side of the chin a few days later (Figure 1). A biopsy was obtained from the lesion on the hand. Histopathologic examination revealed granulomatous inflammation with numerous histiocytes containing intracellular organisms (Figure 2). The microorganisms had pale pink nuclei with basophilic kinetoplasts (Figure 3). Tissue culture showed a mixed growth of gram-positive and gram-negative organisms but no predominant organism. Fungal and mycobacterial cultures were negative. A diagnosis of New World cutaneous leishmaniasis (CL) was made due to visualization of intracellular microorganisms containing basophilic kinetoplasts. Polymerase chain reaction on the tissue block confirmed the presence of Leishmania guyanensis.
|
Cutaneous leishmaniasis is caused by protozoa from the Leishmania species and is transmitted by the bite of the female sandfly. There are 2 classifications for the disease: Old World and New World. Old World CL is transmitted by the sandfly of the genus Phlebotomus, which is endemic in Asia, Africa, the Mediterranean, and the Middle East. New World CL is transmitted by the sandflies of the genus Lutzomyia, which are endemic in Mexico, Central America, and South America. There have been occasional cases of autochthonous transmission reported in Texas and Oklahoma,1 but there has been no known transmission of CL in Australia or the Pacific Islands.2 Human infection can be transmitted by 21 species of Leishmania and can be speciated by designated laboratories such as the US Centers for Disease Control and Prevention (CDC) and the Walter Reed Army Institute of Research (Silver Spring, Maryland) using tissue culture and polymerase chain reaction.1 The CDC can assist with the collection of specimens and supply of culture medium for cases occurring in the United States. Identification of the species is important because there are associated implications for treatment and prognosis.
There are 3 major forms of leishmaniasis: cutaneous, mucocutaneous, and visceral. Cutaneous leishmaniasis is the most common form. There are approximately 1.5 million new cases of CL each year worldwide,3 and more than 90% of these cases occur in Afghanistan, Algeria, Iran, Iraq, Saudi Arabia, Syria, Brazil, and Peru.1 New World CL is caused by 2 species complexes: Leishmania mexicana and Leishmania viannia, including the subspecies L guyanensis, which was seen in our patient.
Cutaneous leishmaniasis usually begins as a small, well-defined papule at the site of the insect bite that then enlarges and becomes a nodule or plaque. Next, the lesion becomes ulcerated with raised borders (Figure 1). The ulcer typically is painless unless there is secondary bacterial or fungal infection.3,4 The incubation period usually is 2 to 8 weeks and multiple lesions may be present, as seen in our patient.4
Old World CL can resolve without treatment, but New World CL is less likely to spontaneously resolve. Additionally, there is a greater risk for spread of the infection to mucous membranes or for systemic dissemination if New World CL is left untreated.2,5 Patients with multiple lesions (ie, ≥3); large lesions (ie, >2.5 cm); lesions on the face, hands, feet, or joints; and those who are immunocompromised should be treated promptly.2 Pentavalent antimonials (eg, meglumine antimoniate, sodium stibogluconate) are the treatment of choice for New World CL, except for infections caused by L guyanensis. The most common pentavalent antimonial agent used in the United States is sodium stibogluconate and is given at a standard intravenous dose of 20 mg antimony/kg daily for 20 days.2 The drug is only available through the CDC’s Drug Service under an Investigational New Drug protocol.1
Intramuscular pentamidine (3 mg/kg daily every other day for 4 doses) is the first-line treatment of CL caused by L guyanensis because systemic antimony usually is not effective.2,6,7 Intralesional injection with pentavalent antimonials usually is not recommended for treatment of New World CL because of the possibility of disseminated disease.2 Liposomal amphotericin B has mainly been used to treat visceral and mucosal leishmaniasis, but there have been some small studies and case reports that have showed it to be successful in treating CL.8-10 Larger controlled studies need to be performed. Oral antifungal drugs (eg, fluconazole, ketoconazole, itraconazole) also have been used to treat CL with variable results depending on the Leishmania species.11
There currently are no vaccines or drugs available to prevent against leishmaniasis. Preventive measures such as avoiding outdoor activities from dusk to dawn when sandflies are the most active, wearing protective clothing, and applying insect repellent that contains DEET (diethyltoluamide) can help reduce a traveler’s risk for becoming infected. Mosquito nets also should be treated with permethrin, which acts as an insect repellent, as sandflies are so small that they can penetrate mosquito nets.1,3,11
Acknowledgements—We would like to thank Francis Steurer, MS, and Barbara Herwaldt, MD, MPH, at the CDC in Atlanta, Georgia, for their help with the identification of the Leishmania species.
1. Herwaldt BL, Magill AJ. Infectious diseases related to travel: leishmaniasis, cutaneous. Centers for Disease Control and Prevention Web site. http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-5/cutaneous-leish maniasis.htm. Published August 1, 2013. Accessed March 5, 2015.
2. Mitropolos P, Konidas P, Durkin-Konidas M. New World cutaneous leishmaniasis: updated review of current and future diagnosis and treatment. J Am Acad Dermatol. 2010;63:309-322.
3. Hepburn NC. Cutaneous leishmaniasis: an overview. J Postgrad Med. 2003;49:50-54.
4. Markle WH, Makhoul K. Cutaneous leishmaniasis: recognition and treatment. Am Fam Physician. 2004;69:1455-1460.
5. Couppié P, Clyti E, Sainte Marie D, et al. Disseminated cutaneous leishmaniasis due to Leishmania guyanensis: case of a patient with 425 lesions. Am J Trop Med Hyg. 2004;71:558-560.
6. Nacher M, Carme B, Sainte Marie D, et al. Influence of clinical presentation on the efficacy of a short course of pentamidine in the treatment of cutaneous leishmaniasis in French Guiana. Ann Trop Med Parasitol. 2001;95:331-336.
7. Minodier P, Parola P. Cutaneous leishmaniasis treatment. Trav Med Inf Dis. 2007;5:150-158.
8. Solomon M, Baum S, Barzilai A, et al. Liposomal amphotericin B in comparison to sodium stibogluconate for cutaneous infection due to Leishmania braziliensis. J Am Acad Dermatol. 2007;56:612-616.
9. Konecny P, Stark DJ. An Australian case of New World cutaneous leishmaniasis. Med J Aust. 2007;186:315-317.
10. Brown M, Noursadeghi M, Boyle J, et al. Successful liposomal amphotericin B treatment of Leishmania braziliensis cutaneous leishmaniasis. Br J Dermatol. 2005;153:203-205.
11. Parasites: leishmaniasis. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/parasites/leishmaniasis/index.html. Updated January 10, 2013. Accessed March 5, 2015.
1. Herwaldt BL, Magill AJ. Infectious diseases related to travel: leishmaniasis, cutaneous. Centers for Disease Control and Prevention Web site. http://wwwnc.cdc.gov/travel/yellowbook/2010/chapter-5/cutaneous-leish maniasis.htm. Published August 1, 2013. Accessed March 5, 2015.
2. Mitropolos P, Konidas P, Durkin-Konidas M. New World cutaneous leishmaniasis: updated review of current and future diagnosis and treatment. J Am Acad Dermatol. 2010;63:309-322.
3. Hepburn NC. Cutaneous leishmaniasis: an overview. J Postgrad Med. 2003;49:50-54.
4. Markle WH, Makhoul K. Cutaneous leishmaniasis: recognition and treatment. Am Fam Physician. 2004;69:1455-1460.
5. Couppié P, Clyti E, Sainte Marie D, et al. Disseminated cutaneous leishmaniasis due to Leishmania guyanensis: case of a patient with 425 lesions. Am J Trop Med Hyg. 2004;71:558-560.
6. Nacher M, Carme B, Sainte Marie D, et al. Influence of clinical presentation on the efficacy of a short course of pentamidine in the treatment of cutaneous leishmaniasis in French Guiana. Ann Trop Med Parasitol. 2001;95:331-336.
7. Minodier P, Parola P. Cutaneous leishmaniasis treatment. Trav Med Inf Dis. 2007;5:150-158.
8. Solomon M, Baum S, Barzilai A, et al. Liposomal amphotericin B in comparison to sodium stibogluconate for cutaneous infection due to Leishmania braziliensis. J Am Acad Dermatol. 2007;56:612-616.
9. Konecny P, Stark DJ. An Australian case of New World cutaneous leishmaniasis. Med J Aust. 2007;186:315-317.
10. Brown M, Noursadeghi M, Boyle J, et al. Successful liposomal amphotericin B treatment of Leishmania braziliensis cutaneous leishmaniasis. Br J Dermatol. 2005;153:203-205.
11. Parasites: leishmaniasis. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/parasites/leishmaniasis/index.html. Updated January 10, 2013. Accessed March 5, 2015.