User login
Staphylococcal Scalded Skin Syndrome in Pregnancy
To the Editor:
Staphylococcal scalded skin syndrome (SSSS) is a superficial blistering disorder mediated by Staphylococcus aureus exfoliative toxins (ETs).1 It is rare in adults, but when diagnosed, it is often associated with renal failure, immunodeficiency, or overwhelming staphylococcal infection.2 We present a unique case of a pregnant woman with chronic atopic dermatitis (AD) who developed SSSS.
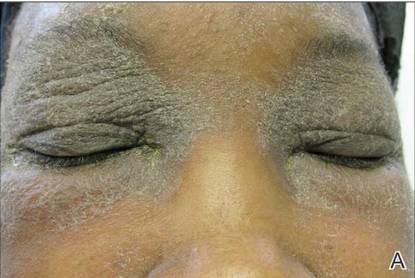
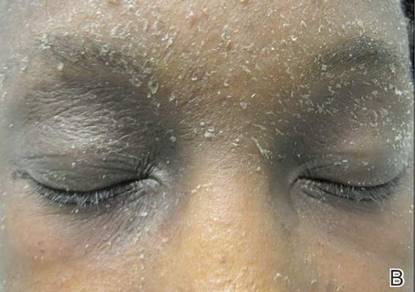
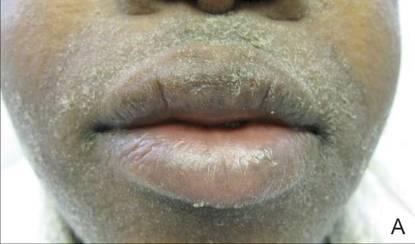
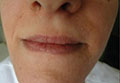
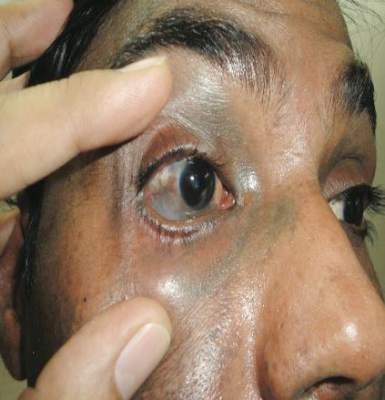
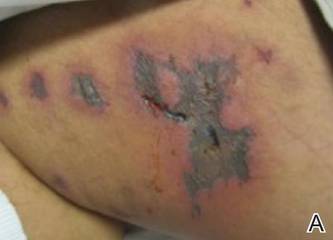
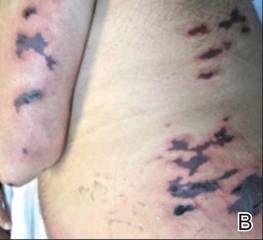
A 21-year-old gravida 3, para 2, aborta 0pregnant woman (29 weeks’ gestation) with a history of chronic AD who was hospitalized with facial edema, purulent ocular discharge, and substantial worsening of AD presented for a dermatology consultation. Her AD was previously managed with topical steroids but had been complicated by multiple methicillin-resistant Staphylococcus aureus (MRSA) infections. On physical examination, she had substantial periorbital edema with purulent discharge from both eyes (Figure 1A), perioral crust with radial fissures (Figure 2A), and mild generalized facial swelling and desquamation (Figure 3). However, the oral cavity was not involved. She had diffuse desquamation in addition to chronic lichenified plaques of the arms, legs, and trunk and SSSS was clinically diagnosed. Cultures of conjunctival discharge were positive for MRSA. The patient was treated with intravenous vancomycin and had a full recovery (Figures 1B and 2B). She delivered a healthy newborn with Apgar scores of 9 and 9 at 1 and 5 minutes, respectively, at 36 weeks and 6 days’ gestation by cesarean delivery; however, her postoperative care was complicated by preeclampsia, which was treated with magnesium sulfate. The newborn showed no evidence of infection or blistering at birth or during the hospital stay.
|
| ||
Figure 1. Periorbital edema with purulent ocular discharge before (A) and after (B) treatment. | Figure 2. Perioral desquamation and radial fissuring before (A) and after (B) treatment. |

Staphylococcal scalded skin syndrome is a superficial blistering disorder that ranges in severity from localized blisters to generalized exfoliation.1 Exfoliative toxin is the major virulence factor responsible for SSSS. Exfoliative toxin is a serine protease that targets desmoglein 1, resulting in intraepidermal separation of keratinocytes.3 Two serologically distinct exfoliative toxins—ETA and ETB—have been associated with human disease.4 Although ETA is encoded on a phage genome, ETB is encoded on a large plasmid.3 Initially it was thought that only strains of S aureus carrying lytic group II phages were responsible for ET production; however, it is now accepted that all phage groups are capable of producing ET and causing SSSS.1
Staphylococcal scalded skin syndrome is most common in infants and children and rare in adults. Although it has been occasionally described in otherwise healthy adults,5 it is most often diagnosed in patients with renal failure (decreased toxin excretion), immunodeficiency (lack of antibodies against toxins), and overwhelming staphylococcal infection (excessive toxin).2 Mortality in treated children is low, but it can reach almost 60% in adults1; therefore, defining risk factors that may aid in early diagnosis are exceedingly important.
We believe that both our patient’s history of AD and her pregnancy contributed to the development of SSSS. The patient had a history of multiple MRSA infections prior to this hospitalization, suggesting MRSA colonization, which is a common complication of AD with more than 75% of AD patients colonized with S aureus.6 Additionally, S aureus superantigen stimulation can result in the loss of regulatory T cells’ natural immunosuppression. Regulatory T cells are remarkably increased in patients with AD; therefore, the inflammatory response to S aureus is likely amplified in an atopic patient, as there is more native immunosuppressive capacity to be affected.4 Furthermore, we believe that pregnancy and its associated immunomodulation is a risk for SSSS. Immune changes in pregnancy are still not well understood; however, it is known that there are alterations to allow symbiosis between the mother and fetus. Anti-ET IgG antibodies are thought to play an important role in protecting against SSSS. Historically, studies on serum immunoglobulin levels during pregnancy have had conflicting findings. They have shown that IgG is either unchanged or decreased, while IgA, IgE, and IgM can be increased, decreased, or unchanged.7 In a study of immunoglobulins in pregnancy, Bahna et al7 showed that IgE is unchanged over the course of pregnancy, but their analysis did not address IgG levels. If IgG levels in fact decrease during pregnancy, the mother could be at risk for SSSS due to her inability to neutralize toxins. Even if total IgG levels remain unchanged, it is possible that specific antitoxin antibodies are decreased. Additionally, there is a documented suppression and alteration in T-cell response to prevent fetal rejection during pregnancy.8 Adult SSSS has been documented several times in human immunodeficiency virus–positive patients, suggesting there may be some association between T-cell suppression and SSSS susceptibility.9 Interestingly, pregnancy, similar to AD, results in an increase in immunosuppressive T cells,10 which, if deactivated by superantigens, could potentially contribute to an increased inflammatory response. All of these immune system alterations likely leave the mother vulnerable to toxin-mediated events such as SSSS.
We believe this case highlights the importance of considering SSSS in both atopic and pregnant patients with desquamating eruptions. In the case of pregnant patients, it is important to consider the risks and benefits of any medical treatments for both the mother and infant. Vancomycin is a pregnancy category B drug and was chosen for its known effectiveness and safety in pregnancy. One study compared 10 babies with mothers who were treated with vancomycin during the second and third trimesters for MRSA to 20 babies with mothers who did not receive vancomycin and did not find an increased risk for sensorineural hearing loss or nephrotoxicity.11 There is no known increased risk for preeclampsia with vancomycin, but some studies have suggested that maternal infection independently increases the risk for preeclampsia.12 Other treatment options were not as safe as vancomycin in this case: doxycycline is contraindicated (pregnancy category D) due to the potential for staining of deciduous teeth and skeletal growth impairment, trimethoprim-sulfamethoxazole is a pregnancy category D drug during the third trimester due to the risk of kernicterus, and linezolid is a pregnancy category C drug.13
1. Ladhani S. Recent developments in staphylococcal scalded skin syndrome. Clin Microbiol Infect. 2001;7:301-307.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kato F, Kadomoto N, Iwamoto Y, et al. Regulatory mechanism for exfoliative toxin production in Staphylococcus aureus. Infect Immun. 2011;79:1660-1670.
4. Iwatsuki K, Yamasaki O, Morizane S, et al. Staphylococcal cutaneous infections: invasion, evasion and aggression. J Dermatol Sci. 2006;42:203-214.
5. Opal SM, Johnson-Winegar AD, Cross AS. Staphylococcal scalded skin syndrome in two immunocompetent adults caused by exfoliation B-producing Staphylococcus aureus. J Clin Microbiol. 1988;26:1283-1286.
6. Hill SE, Yung A, Rademaker M. Prevalence of Staphylococcus aureus and antibiotic resistance in children with atopic dermatitis: a New Zealand experience. Australas J Dermatol. 2011;52:27-31.
7. Bahna SL, Woo CK, Manuel PV, et al. Serum total IgE level during pregnancy and postpartum. Allergol Immunopathol (Madr). 2011;39:291-294.
8. Poole JA, Claman HN. Immunology of pregnancy: implications for the mother. Clin Rev Allergy Immunol. 2004;26:161-170.
9. Farrell AM, Ross JS, Umasankar S, et al. Staphylococcal scalded skin syndrome in an HIV-1 seropositive man. Br J Dermatol. 1996;134:962-965.
10. Somerset DA, Zheng Y, Kilby MD, et al. Normal human pregnancy is associated with an elevation in the immune suppressive CD251 CD41 regulatory T-cell subset. Immunology. 2004;112:38-43.
11. Reyes MP, Ostrea EM Jr, Carbinian AE, et al. Vancomycin during pregnancy: does it cause hearing loss or nephrotoxicity in the infant? Am J Obstet Gynecol. 1989;161:977-981.
12. Rustveldt LO, Kelsey SF, Sharma, R. Associations between maternal infections and preeclampsia: a systemic review of epidemiologic studies. Matern Child Health J. 2008;12: 223-242.
13. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Barcelona, Spain: Elsevier Limited; 2008.
To the Editor:
Staphylococcal scalded skin syndrome (SSSS) is a superficial blistering disorder mediated by Staphylococcus aureus exfoliative toxins (ETs).1 It is rare in adults, but when diagnosed, it is often associated with renal failure, immunodeficiency, or overwhelming staphylococcal infection.2 We present a unique case of a pregnant woman with chronic atopic dermatitis (AD) who developed SSSS.
A 21-year-old gravida 3, para 2, aborta 0pregnant woman (29 weeks’ gestation) with a history of chronic AD who was hospitalized with facial edema, purulent ocular discharge, and substantial worsening of AD presented for a dermatology consultation. Her AD was previously managed with topical steroids but had been complicated by multiple methicillin-resistant Staphylococcus aureus (MRSA) infections. On physical examination, she had substantial periorbital edema with purulent discharge from both eyes (Figure 1A), perioral crust with radial fissures (Figure 2A), and mild generalized facial swelling and desquamation (Figure 3). However, the oral cavity was not involved. She had diffuse desquamation in addition to chronic lichenified plaques of the arms, legs, and trunk and SSSS was clinically diagnosed. Cultures of conjunctival discharge were positive for MRSA. The patient was treated with intravenous vancomycin and had a full recovery (Figures 1B and 2B). She delivered a healthy newborn with Apgar scores of 9 and 9 at 1 and 5 minutes, respectively, at 36 weeks and 6 days’ gestation by cesarean delivery; however, her postoperative care was complicated by preeclampsia, which was treated with magnesium sulfate. The newborn showed no evidence of infection or blistering at birth or during the hospital stay.
|
| ||
Figure 1. Periorbital edema with purulent ocular discharge before (A) and after (B) treatment. | Figure 2. Perioral desquamation and radial fissuring before (A) and after (B) treatment. |

Staphylococcal scalded skin syndrome is a superficial blistering disorder that ranges in severity from localized blisters to generalized exfoliation.1 Exfoliative toxin is the major virulence factor responsible for SSSS. Exfoliative toxin is a serine protease that targets desmoglein 1, resulting in intraepidermal separation of keratinocytes.3 Two serologically distinct exfoliative toxins—ETA and ETB—have been associated with human disease.4 Although ETA is encoded on a phage genome, ETB is encoded on a large plasmid.3 Initially it was thought that only strains of S aureus carrying lytic group II phages were responsible for ET production; however, it is now accepted that all phage groups are capable of producing ET and causing SSSS.1
Staphylococcal scalded skin syndrome is most common in infants and children and rare in adults. Although it has been occasionally described in otherwise healthy adults,5 it is most often diagnosed in patients with renal failure (decreased toxin excretion), immunodeficiency (lack of antibodies against toxins), and overwhelming staphylococcal infection (excessive toxin).2 Mortality in treated children is low, but it can reach almost 60% in adults1; therefore, defining risk factors that may aid in early diagnosis are exceedingly important.
We believe that both our patient’s history of AD and her pregnancy contributed to the development of SSSS. The patient had a history of multiple MRSA infections prior to this hospitalization, suggesting MRSA colonization, which is a common complication of AD with more than 75% of AD patients colonized with S aureus.6 Additionally, S aureus superantigen stimulation can result in the loss of regulatory T cells’ natural immunosuppression. Regulatory T cells are remarkably increased in patients with AD; therefore, the inflammatory response to S aureus is likely amplified in an atopic patient, as there is more native immunosuppressive capacity to be affected.4 Furthermore, we believe that pregnancy and its associated immunomodulation is a risk for SSSS. Immune changes in pregnancy are still not well understood; however, it is known that there are alterations to allow symbiosis between the mother and fetus. Anti-ET IgG antibodies are thought to play an important role in protecting against SSSS. Historically, studies on serum immunoglobulin levels during pregnancy have had conflicting findings. They have shown that IgG is either unchanged or decreased, while IgA, IgE, and IgM can be increased, decreased, or unchanged.7 In a study of immunoglobulins in pregnancy, Bahna et al7 showed that IgE is unchanged over the course of pregnancy, but their analysis did not address IgG levels. If IgG levels in fact decrease during pregnancy, the mother could be at risk for SSSS due to her inability to neutralize toxins. Even if total IgG levels remain unchanged, it is possible that specific antitoxin antibodies are decreased. Additionally, there is a documented suppression and alteration in T-cell response to prevent fetal rejection during pregnancy.8 Adult SSSS has been documented several times in human immunodeficiency virus–positive patients, suggesting there may be some association between T-cell suppression and SSSS susceptibility.9 Interestingly, pregnancy, similar to AD, results in an increase in immunosuppressive T cells,10 which, if deactivated by superantigens, could potentially contribute to an increased inflammatory response. All of these immune system alterations likely leave the mother vulnerable to toxin-mediated events such as SSSS.
We believe this case highlights the importance of considering SSSS in both atopic and pregnant patients with desquamating eruptions. In the case of pregnant patients, it is important to consider the risks and benefits of any medical treatments for both the mother and infant. Vancomycin is a pregnancy category B drug and was chosen for its known effectiveness and safety in pregnancy. One study compared 10 babies with mothers who were treated with vancomycin during the second and third trimesters for MRSA to 20 babies with mothers who did not receive vancomycin and did not find an increased risk for sensorineural hearing loss or nephrotoxicity.11 There is no known increased risk for preeclampsia with vancomycin, but some studies have suggested that maternal infection independently increases the risk for preeclampsia.12 Other treatment options were not as safe as vancomycin in this case: doxycycline is contraindicated (pregnancy category D) due to the potential for staining of deciduous teeth and skeletal growth impairment, trimethoprim-sulfamethoxazole is a pregnancy category D drug during the third trimester due to the risk of kernicterus, and linezolid is a pregnancy category C drug.13
To the Editor:
Staphylococcal scalded skin syndrome (SSSS) is a superficial blistering disorder mediated by Staphylococcus aureus exfoliative toxins (ETs).1 It is rare in adults, but when diagnosed, it is often associated with renal failure, immunodeficiency, or overwhelming staphylococcal infection.2 We present a unique case of a pregnant woman with chronic atopic dermatitis (AD) who developed SSSS.
A 21-year-old gravida 3, para 2, aborta 0pregnant woman (29 weeks’ gestation) with a history of chronic AD who was hospitalized with facial edema, purulent ocular discharge, and substantial worsening of AD presented for a dermatology consultation. Her AD was previously managed with topical steroids but had been complicated by multiple methicillin-resistant Staphylococcus aureus (MRSA) infections. On physical examination, she had substantial periorbital edema with purulent discharge from both eyes (Figure 1A), perioral crust with radial fissures (Figure 2A), and mild generalized facial swelling and desquamation (Figure 3). However, the oral cavity was not involved. She had diffuse desquamation in addition to chronic lichenified plaques of the arms, legs, and trunk and SSSS was clinically diagnosed. Cultures of conjunctival discharge were positive for MRSA. The patient was treated with intravenous vancomycin and had a full recovery (Figures 1B and 2B). She delivered a healthy newborn with Apgar scores of 9 and 9 at 1 and 5 minutes, respectively, at 36 weeks and 6 days’ gestation by cesarean delivery; however, her postoperative care was complicated by preeclampsia, which was treated with magnesium sulfate. The newborn showed no evidence of infection or blistering at birth or during the hospital stay.
|
| ||
Figure 1. Periorbital edema with purulent ocular discharge before (A) and after (B) treatment. | Figure 2. Perioral desquamation and radial fissuring before (A) and after (B) treatment. |

Staphylococcal scalded skin syndrome is a superficial blistering disorder that ranges in severity from localized blisters to generalized exfoliation.1 Exfoliative toxin is the major virulence factor responsible for SSSS. Exfoliative toxin is a serine protease that targets desmoglein 1, resulting in intraepidermal separation of keratinocytes.3 Two serologically distinct exfoliative toxins—ETA and ETB—have been associated with human disease.4 Although ETA is encoded on a phage genome, ETB is encoded on a large plasmid.3 Initially it was thought that only strains of S aureus carrying lytic group II phages were responsible for ET production; however, it is now accepted that all phage groups are capable of producing ET and causing SSSS.1
Staphylococcal scalded skin syndrome is most common in infants and children and rare in adults. Although it has been occasionally described in otherwise healthy adults,5 it is most often diagnosed in patients with renal failure (decreased toxin excretion), immunodeficiency (lack of antibodies against toxins), and overwhelming staphylococcal infection (excessive toxin).2 Mortality in treated children is low, but it can reach almost 60% in adults1; therefore, defining risk factors that may aid in early diagnosis are exceedingly important.
We believe that both our patient’s history of AD and her pregnancy contributed to the development of SSSS. The patient had a history of multiple MRSA infections prior to this hospitalization, suggesting MRSA colonization, which is a common complication of AD with more than 75% of AD patients colonized with S aureus.6 Additionally, S aureus superantigen stimulation can result in the loss of regulatory T cells’ natural immunosuppression. Regulatory T cells are remarkably increased in patients with AD; therefore, the inflammatory response to S aureus is likely amplified in an atopic patient, as there is more native immunosuppressive capacity to be affected.4 Furthermore, we believe that pregnancy and its associated immunomodulation is a risk for SSSS. Immune changes in pregnancy are still not well understood; however, it is known that there are alterations to allow symbiosis between the mother and fetus. Anti-ET IgG antibodies are thought to play an important role in protecting against SSSS. Historically, studies on serum immunoglobulin levels during pregnancy have had conflicting findings. They have shown that IgG is either unchanged or decreased, while IgA, IgE, and IgM can be increased, decreased, or unchanged.7 In a study of immunoglobulins in pregnancy, Bahna et al7 showed that IgE is unchanged over the course of pregnancy, but their analysis did not address IgG levels. If IgG levels in fact decrease during pregnancy, the mother could be at risk for SSSS due to her inability to neutralize toxins. Even if total IgG levels remain unchanged, it is possible that specific antitoxin antibodies are decreased. Additionally, there is a documented suppression and alteration in T-cell response to prevent fetal rejection during pregnancy.8 Adult SSSS has been documented several times in human immunodeficiency virus–positive patients, suggesting there may be some association between T-cell suppression and SSSS susceptibility.9 Interestingly, pregnancy, similar to AD, results in an increase in immunosuppressive T cells,10 which, if deactivated by superantigens, could potentially contribute to an increased inflammatory response. All of these immune system alterations likely leave the mother vulnerable to toxin-mediated events such as SSSS.
We believe this case highlights the importance of considering SSSS in both atopic and pregnant patients with desquamating eruptions. In the case of pregnant patients, it is important to consider the risks and benefits of any medical treatments for both the mother and infant. Vancomycin is a pregnancy category B drug and was chosen for its known effectiveness and safety in pregnancy. One study compared 10 babies with mothers who were treated with vancomycin during the second and third trimesters for MRSA to 20 babies with mothers who did not receive vancomycin and did not find an increased risk for sensorineural hearing loss or nephrotoxicity.11 There is no known increased risk for preeclampsia with vancomycin, but some studies have suggested that maternal infection independently increases the risk for preeclampsia.12 Other treatment options were not as safe as vancomycin in this case: doxycycline is contraindicated (pregnancy category D) due to the potential for staining of deciduous teeth and skeletal growth impairment, trimethoprim-sulfamethoxazole is a pregnancy category D drug during the third trimester due to the risk of kernicterus, and linezolid is a pregnancy category C drug.13
1. Ladhani S. Recent developments in staphylococcal scalded skin syndrome. Clin Microbiol Infect. 2001;7:301-307.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kato F, Kadomoto N, Iwamoto Y, et al. Regulatory mechanism for exfoliative toxin production in Staphylococcus aureus. Infect Immun. 2011;79:1660-1670.
4. Iwatsuki K, Yamasaki O, Morizane S, et al. Staphylococcal cutaneous infections: invasion, evasion and aggression. J Dermatol Sci. 2006;42:203-214.
5. Opal SM, Johnson-Winegar AD, Cross AS. Staphylococcal scalded skin syndrome in two immunocompetent adults caused by exfoliation B-producing Staphylococcus aureus. J Clin Microbiol. 1988;26:1283-1286.
6. Hill SE, Yung A, Rademaker M. Prevalence of Staphylococcus aureus and antibiotic resistance in children with atopic dermatitis: a New Zealand experience. Australas J Dermatol. 2011;52:27-31.
7. Bahna SL, Woo CK, Manuel PV, et al. Serum total IgE level during pregnancy and postpartum. Allergol Immunopathol (Madr). 2011;39:291-294.
8. Poole JA, Claman HN. Immunology of pregnancy: implications for the mother. Clin Rev Allergy Immunol. 2004;26:161-170.
9. Farrell AM, Ross JS, Umasankar S, et al. Staphylococcal scalded skin syndrome in an HIV-1 seropositive man. Br J Dermatol. 1996;134:962-965.
10. Somerset DA, Zheng Y, Kilby MD, et al. Normal human pregnancy is associated with an elevation in the immune suppressive CD251 CD41 regulatory T-cell subset. Immunology. 2004;112:38-43.
11. Reyes MP, Ostrea EM Jr, Carbinian AE, et al. Vancomycin during pregnancy: does it cause hearing loss or nephrotoxicity in the infant? Am J Obstet Gynecol. 1989;161:977-981.
12. Rustveldt LO, Kelsey SF, Sharma, R. Associations between maternal infections and preeclampsia: a systemic review of epidemiologic studies. Matern Child Health J. 2008;12: 223-242.
13. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Barcelona, Spain: Elsevier Limited; 2008.
1. Ladhani S. Recent developments in staphylococcal scalded skin syndrome. Clin Microbiol Infect. 2001;7:301-307.
2. Ladhani S, Joannou CL, Lochrie DP, et al. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin Microbiol Rev. 1999;12:224-242.
3. Kato F, Kadomoto N, Iwamoto Y, et al. Regulatory mechanism for exfoliative toxin production in Staphylococcus aureus. Infect Immun. 2011;79:1660-1670.
4. Iwatsuki K, Yamasaki O, Morizane S, et al. Staphylococcal cutaneous infections: invasion, evasion and aggression. J Dermatol Sci. 2006;42:203-214.
5. Opal SM, Johnson-Winegar AD, Cross AS. Staphylococcal scalded skin syndrome in two immunocompetent adults caused by exfoliation B-producing Staphylococcus aureus. J Clin Microbiol. 1988;26:1283-1286.
6. Hill SE, Yung A, Rademaker M. Prevalence of Staphylococcus aureus and antibiotic resistance in children with atopic dermatitis: a New Zealand experience. Australas J Dermatol. 2011;52:27-31.
7. Bahna SL, Woo CK, Manuel PV, et al. Serum total IgE level during pregnancy and postpartum. Allergol Immunopathol (Madr). 2011;39:291-294.
8. Poole JA, Claman HN. Immunology of pregnancy: implications for the mother. Clin Rev Allergy Immunol. 2004;26:161-170.
9. Farrell AM, Ross JS, Umasankar S, et al. Staphylococcal scalded skin syndrome in an HIV-1 seropositive man. Br J Dermatol. 1996;134:962-965.
10. Somerset DA, Zheng Y, Kilby MD, et al. Normal human pregnancy is associated with an elevation in the immune suppressive CD251 CD41 regulatory T-cell subset. Immunology. 2004;112:38-43.
11. Reyes MP, Ostrea EM Jr, Carbinian AE, et al. Vancomycin during pregnancy: does it cause hearing loss or nephrotoxicity in the infant? Am J Obstet Gynecol. 1989;161:977-981.
12. Rustveldt LO, Kelsey SF, Sharma, R. Associations between maternal infections and preeclampsia: a systemic review of epidemiologic studies. Matern Child Health J. 2008;12: 223-242.
13. Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. Vol 2. 2nd ed. Barcelona, Spain: Elsevier Limited; 2008.
Nevus of Ota/Oculodermal Melancytosis: A Rare Report of an Oral Mucosal Lesion Involving the Hard Palate
To the Editor:
Nevus of Ota, also known as oculodermal melanocytosis or nevus fuscoceruleus ophthalmomaxillaris, is a hamartoma of dermal melanocytes that is characterized by a unilateral or bilateral blue-brown, speckled patch usually involving the malar, periorbital, temple, and/or forehead regions of the face.1 It also may affect the sclera, conjunctiva, retinas, corneas, ocular muscles, periosteum, and retrobulbar fat corresponding to the distribution of the ophthalmic (V1) and maxillary (V2) divisions of the trigeminal nerve.
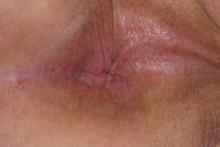
Examination of the oral cavity in the setting of nevus of Ota is imperative, as it can present as a developmental lesion of the oral mucosa.2 Involvement of the hard palate is rare but has been observed.3-5 We present a case of blue-pigmented macules in the upper right periorbital region with involvement of the hard palate that were diagnosed as nevus of Ota.
A 34-year-old Indian man presented with progressive, asymptomatic, ashy blue macules in the upper right periorbital region that had been present since birth. The pigmented macules had gradually increased to cover the infraorbital, maxillary, and temporal regions of the right side of the face with involvement of the conjunctiva and sclera (Figure 1).
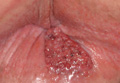
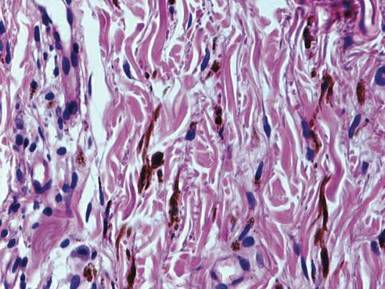
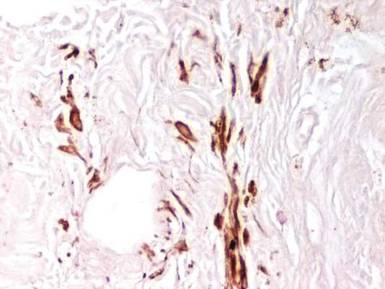
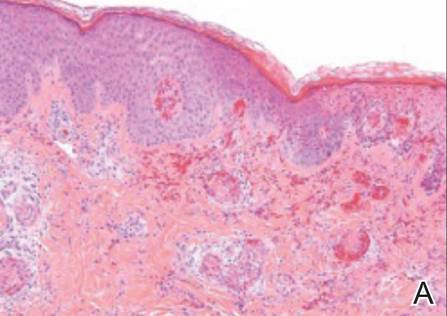
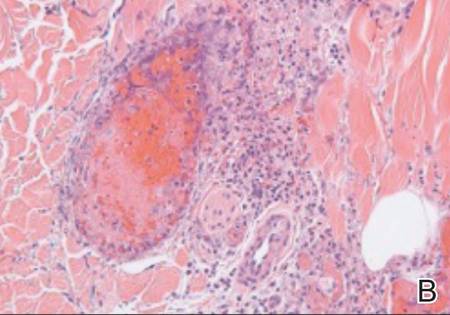
Examination of the mucous membrane of the hard palate revealed several blue-pigmented macules with ill-defined borders merging into the surrounding mucosa (Figure 2). Ocular tension was normal and slit-lamp examination of the right eye did not reveal any abnormalities. Hematoxylin and eosin–stained sections prepared from a biopsy of the oral mucosa on the hard palate showed numerous elongated, fusiform, dendritic melanocytes in small aggregates scattered widely between the bundles of collagen in the papillary to midreticular dermis (Figure 3). On histology, the melanocytes stained positive for S100 protein (Figure 4) and human melanoma black 45. No evidence indicative of malignancy was found. The stratified squamous epithelium was unremarkable except for the presence of mild perivascular lymphocytic infiltrate in the subepithelial tissue. A diagnosis of nevus of Ota with involvement of the hard palate was made.
Cutaneous macules may enlarge slowly, become deeper in color, and persist throughout the patient’s life. Its pathogenesis is not known, but it is speculated that nevus of Ota is caused by faulty migration of melanoblasts from the neural crest to the skin. Nevus of Ito also is a dermal melanocytic aberration that exclusively affects the shoulders and often occurs in association with nevus of Ota.1
Ashy or slate-blue pigmentation in individuals with skin of color (eg, Fitzpatrick skin type V) is uncommon, as this discoloration usually is seen in fair-skinned individuals (eg, Fitzpatrick skin type II).6 Occasionally, blue-pigmented lesions of the oral mucosa may be seen in nevus of Ota (as in our patient) and are considered developmental; therefore, examination of the oral cavity is suggested when patients present with blue-pigmented lesions in the facial region. Although this finding is rare, several other cases of blue-pigmented macules on the palatal mucosa have been reported.3-5
The diagnosis of nevus of Ota should be confirmed by histopathology and can be classified into 5 types according to the distribution of melanocytes, including (1) superficial, (2) superficial dominant, (3) diffuse, (4) deep dominant, and (5) deep.7 The diagnosis of nevus of Ota can be made based on its characteristic morphology; however, nevus of Ito, Mongolian spots, melanoma, fixed drug eruptions,8 and lichen planus pigmantosus should also be ruled out.9
Nevus of Ota is a well-established entity that should be considered when ashy or slate-blue pigmentation is noted along the branches of the ophthalmic and maxillary divisions of the trigeminal nerve. Diagnosis is largely clinical, but should be confirmed on histopathology and immunohistochemistry. Possible concomitant involvement of the buccal mucosa and/or the hard palate warrants a thorough examination of the oral cavity in the setting of nevus of Ota to identify oral mucosal lesions. Histopathology is essential to confirm its status as well as to exclude melanoma.
- Ito M. Studies on melanin XXII. Nevus fuscocaeruleus acromio-deltoideus. Tohoku J Exper Med. 1954;60:10.
- Syed NH, Sehgal VN, Aggarwal A, et al. Oral mucosal lesions, institutional study of 200 consecutive patients in dermatologic practice. Int J Dermatol. In press.
- Rathi SK. Bilateral nevus of ota with oral mucosal involvement. Indian J Dermatol Venereol Leprol. 2002;68:104.
- Kannan SK. Oculodermal melanocytosis—nevus of Ota (with palatal pigmentation). Indian J Dent Res. 2003;14: 230-233.
- Shetty SR, Subhas BG, Rao KA, et al. Nevus of Ota with buccal mucosal pigmentation: a rare case. Dent Res J (Isfahan). 2011;8:52-55.
- Fitzpatrick TB, Pathak MA, Parrish JA. Protection of human skin against the effects of the sunburn ultraviolet (290–320 nm). In: Pathak MA, Harber LC, Seiji M, et al, eds. Sunlight and Man: Normal and Abnormal Photobiological Responses. Tokyo, Japan: University of Tokyo Press; 1974:751-765.
- Hirayama T, Suzuki T. A new classification of Ota’s nevus based on histopathological features. Dermatologica. 1991;183:169-172.
- Sehgal VN, Verma P, Bhattacharya SN, et al. Lichen planus pigmentosus. Skinmed. 2013;11:96-103.
- Sehgal VN, Srivastava G. Fixed drug eruption (FDE): changing scenario of incriminating drugs. Int J Dermatol. 2006;45:897-908.
To the Editor:
Nevus of Ota, also known as oculodermal melanocytosis or nevus fuscoceruleus ophthalmomaxillaris, is a hamartoma of dermal melanocytes that is characterized by a unilateral or bilateral blue-brown, speckled patch usually involving the malar, periorbital, temple, and/or forehead regions of the face.1 It also may affect the sclera, conjunctiva, retinas, corneas, ocular muscles, periosteum, and retrobulbar fat corresponding to the distribution of the ophthalmic (V1) and maxillary (V2) divisions of the trigeminal nerve.
Examination of the oral cavity in the setting of nevus of Ota is imperative, as it can present as a developmental lesion of the oral mucosa.2 Involvement of the hard palate is rare but has been observed.3-5 We present a case of blue-pigmented macules in the upper right periorbital region with involvement of the hard palate that were diagnosed as nevus of Ota.
A 34-year-old Indian man presented with progressive, asymptomatic, ashy blue macules in the upper right periorbital region that had been present since birth. The pigmented macules had gradually increased to cover the infraorbital, maxillary, and temporal regions of the right side of the face with involvement of the conjunctiva and sclera (Figure 1).

Examination of the mucous membrane of the hard palate revealed several blue-pigmented macules with ill-defined borders merging into the surrounding mucosa (Figure 2). Ocular tension was normal and slit-lamp examination of the right eye did not reveal any abnormalities. Hematoxylin and eosin–stained sections prepared from a biopsy of the oral mucosa on the hard palate showed numerous elongated, fusiform, dendritic melanocytes in small aggregates scattered widely between the bundles of collagen in the papillary to midreticular dermis (Figure 3). On histology, the melanocytes stained positive for S100 protein (Figure 4) and human melanoma black 45. No evidence indicative of malignancy was found. The stratified squamous epithelium was unremarkable except for the presence of mild perivascular lymphocytic infiltrate in the subepithelial tissue. A diagnosis of nevus of Ota with involvement of the hard palate was made.



Cutaneous macules may enlarge slowly, become deeper in color, and persist throughout the patient’s life. Its pathogenesis is not known, but it is speculated that nevus of Ota is caused by faulty migration of melanoblasts from the neural crest to the skin. Nevus of Ito also is a dermal melanocytic aberration that exclusively affects the shoulders and often occurs in association with nevus of Ota.1
Ashy or slate-blue pigmentation in individuals with skin of color (eg, Fitzpatrick skin type V) is uncommon, as this discoloration usually is seen in fair-skinned individuals (eg, Fitzpatrick skin type II).6 Occasionally, blue-pigmented lesions of the oral mucosa may be seen in nevus of Ota (as in our patient) and are considered developmental; therefore, examination of the oral cavity is suggested when patients present with blue-pigmented lesions in the facial region. Although this finding is rare, several other cases of blue-pigmented macules on the palatal mucosa have been reported.3-5
The diagnosis of nevus of Ota should be confirmed by histopathology and can be classified into 5 types according to the distribution of melanocytes, including (1) superficial, (2) superficial dominant, (3) diffuse, (4) deep dominant, and (5) deep.7 The diagnosis of nevus of Ota can be made based on its characteristic morphology; however, nevus of Ito, Mongolian spots, melanoma, fixed drug eruptions,8 and lichen planus pigmantosus should also be ruled out.9
Nevus of Ota is a well-established entity that should be considered when ashy or slate-blue pigmentation is noted along the branches of the ophthalmic and maxillary divisions of the trigeminal nerve. Diagnosis is largely clinical, but should be confirmed on histopathology and immunohistochemistry. Possible concomitant involvement of the buccal mucosa and/or the hard palate warrants a thorough examination of the oral cavity in the setting of nevus of Ota to identify oral mucosal lesions. Histopathology is essential to confirm its status as well as to exclude melanoma.
To the Editor:
Nevus of Ota, also known as oculodermal melanocytosis or nevus fuscoceruleus ophthalmomaxillaris, is a hamartoma of dermal melanocytes that is characterized by a unilateral or bilateral blue-brown, speckled patch usually involving the malar, periorbital, temple, and/or forehead regions of the face.1 It also may affect the sclera, conjunctiva, retinas, corneas, ocular muscles, periosteum, and retrobulbar fat corresponding to the distribution of the ophthalmic (V1) and maxillary (V2) divisions of the trigeminal nerve.
Examination of the oral cavity in the setting of nevus of Ota is imperative, as it can present as a developmental lesion of the oral mucosa.2 Involvement of the hard palate is rare but has been observed.3-5 We present a case of blue-pigmented macules in the upper right periorbital region with involvement of the hard palate that were diagnosed as nevus of Ota.
A 34-year-old Indian man presented with progressive, asymptomatic, ashy blue macules in the upper right periorbital region that had been present since birth. The pigmented macules had gradually increased to cover the infraorbital, maxillary, and temporal regions of the right side of the face with involvement of the conjunctiva and sclera (Figure 1).

Examination of the mucous membrane of the hard palate revealed several blue-pigmented macules with ill-defined borders merging into the surrounding mucosa (Figure 2). Ocular tension was normal and slit-lamp examination of the right eye did not reveal any abnormalities. Hematoxylin and eosin–stained sections prepared from a biopsy of the oral mucosa on the hard palate showed numerous elongated, fusiform, dendritic melanocytes in small aggregates scattered widely between the bundles of collagen in the papillary to midreticular dermis (Figure 3). On histology, the melanocytes stained positive for S100 protein (Figure 4) and human melanoma black 45. No evidence indicative of malignancy was found. The stratified squamous epithelium was unremarkable except for the presence of mild perivascular lymphocytic infiltrate in the subepithelial tissue. A diagnosis of nevus of Ota with involvement of the hard palate was made.



Cutaneous macules may enlarge slowly, become deeper in color, and persist throughout the patient’s life. Its pathogenesis is not known, but it is speculated that nevus of Ota is caused by faulty migration of melanoblasts from the neural crest to the skin. Nevus of Ito also is a dermal melanocytic aberration that exclusively affects the shoulders and often occurs in association with nevus of Ota.1
Ashy or slate-blue pigmentation in individuals with skin of color (eg, Fitzpatrick skin type V) is uncommon, as this discoloration usually is seen in fair-skinned individuals (eg, Fitzpatrick skin type II).6 Occasionally, blue-pigmented lesions of the oral mucosa may be seen in nevus of Ota (as in our patient) and are considered developmental; therefore, examination of the oral cavity is suggested when patients present with blue-pigmented lesions in the facial region. Although this finding is rare, several other cases of blue-pigmented macules on the palatal mucosa have been reported.3-5
The diagnosis of nevus of Ota should be confirmed by histopathology and can be classified into 5 types according to the distribution of melanocytes, including (1) superficial, (2) superficial dominant, (3) diffuse, (4) deep dominant, and (5) deep.7 The diagnosis of nevus of Ota can be made based on its characteristic morphology; however, nevus of Ito, Mongolian spots, melanoma, fixed drug eruptions,8 and lichen planus pigmantosus should also be ruled out.9
Nevus of Ota is a well-established entity that should be considered when ashy or slate-blue pigmentation is noted along the branches of the ophthalmic and maxillary divisions of the trigeminal nerve. Diagnosis is largely clinical, but should be confirmed on histopathology and immunohistochemistry. Possible concomitant involvement of the buccal mucosa and/or the hard palate warrants a thorough examination of the oral cavity in the setting of nevus of Ota to identify oral mucosal lesions. Histopathology is essential to confirm its status as well as to exclude melanoma.
- Ito M. Studies on melanin XXII. Nevus fuscocaeruleus acromio-deltoideus. Tohoku J Exper Med. 1954;60:10.
- Syed NH, Sehgal VN, Aggarwal A, et al. Oral mucosal lesions, institutional study of 200 consecutive patients in dermatologic practice. Int J Dermatol. In press.
- Rathi SK. Bilateral nevus of ota with oral mucosal involvement. Indian J Dermatol Venereol Leprol. 2002;68:104.
- Kannan SK. Oculodermal melanocytosis—nevus of Ota (with palatal pigmentation). Indian J Dent Res. 2003;14: 230-233.
- Shetty SR, Subhas BG, Rao KA, et al. Nevus of Ota with buccal mucosal pigmentation: a rare case. Dent Res J (Isfahan). 2011;8:52-55.
- Fitzpatrick TB, Pathak MA, Parrish JA. Protection of human skin against the effects of the sunburn ultraviolet (290–320 nm). In: Pathak MA, Harber LC, Seiji M, et al, eds. Sunlight and Man: Normal and Abnormal Photobiological Responses. Tokyo, Japan: University of Tokyo Press; 1974:751-765.
- Hirayama T, Suzuki T. A new classification of Ota’s nevus based on histopathological features. Dermatologica. 1991;183:169-172.
- Sehgal VN, Verma P, Bhattacharya SN, et al. Lichen planus pigmentosus. Skinmed. 2013;11:96-103.
- Sehgal VN, Srivastava G. Fixed drug eruption (FDE): changing scenario of incriminating drugs. Int J Dermatol. 2006;45:897-908.
- Ito M. Studies on melanin XXII. Nevus fuscocaeruleus acromio-deltoideus. Tohoku J Exper Med. 1954;60:10.
- Syed NH, Sehgal VN, Aggarwal A, et al. Oral mucosal lesions, institutional study of 200 consecutive patients in dermatologic practice. Int J Dermatol. In press.
- Rathi SK. Bilateral nevus of ota with oral mucosal involvement. Indian J Dermatol Venereol Leprol. 2002;68:104.
- Kannan SK. Oculodermal melanocytosis—nevus of Ota (with palatal pigmentation). Indian J Dent Res. 2003;14: 230-233.
- Shetty SR, Subhas BG, Rao KA, et al. Nevus of Ota with buccal mucosal pigmentation: a rare case. Dent Res J (Isfahan). 2011;8:52-55.
- Fitzpatrick TB, Pathak MA, Parrish JA. Protection of human skin against the effects of the sunburn ultraviolet (290–320 nm). In: Pathak MA, Harber LC, Seiji M, et al, eds. Sunlight and Man: Normal and Abnormal Photobiological Responses. Tokyo, Japan: University of Tokyo Press; 1974:751-765.
- Hirayama T, Suzuki T. A new classification of Ota’s nevus based on histopathological features. Dermatologica. 1991;183:169-172.
- Sehgal VN, Verma P, Bhattacharya SN, et al. Lichen planus pigmentosus. Skinmed. 2013;11:96-103.
- Sehgal VN, Srivastava G. Fixed drug eruption (FDE): changing scenario of incriminating drugs. Int J Dermatol. 2006;45:897-908.
Occupational Contact Dermatitis From Carbapenems
To the Editor:
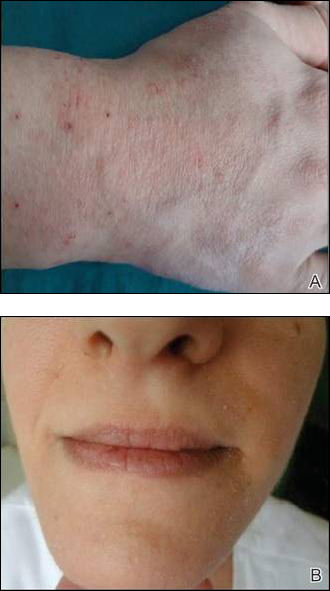
Contact sensitivity to drugs that are systemically administered can occur among health care workers.1 We report the case of a 28-year-old nurse who developed eczema on the dorsal aspect of the hand (Figure 1A) and the face (Figure 1B) in the workplace. The nurse was working in the hematology department where she usually handled and administered antibiotics such as imipenem, ertapenem, piperacillin, vancomycin, anidulafungin, teicoplanin, and ciprofloxacin. She was moved to a different department where she did not have contact with the suspicious drugs and the dermatitis completely resolved.
One month after the resolution of the eczema she was referred to our allergy department for an allergological evaluation. A dermatologic evaluation was made and a skin biopsy was performed from a lesional area of the left hand. The patient underwent delayed skin test and patch tests with many β-lactam compounds including penicilloyl polylysine, minor determinant mixture, penicillin G, penicillin V, ampicillin, amoxicillin, bacampicillin, piperacillin, mezlocillin and ticarcillin, imipenem-cilastatin, aztreonam, meropenem, ertapenem, and cephalosporins (eg, cephalexin, cefaclor, cefalotin, cefadroxil, cephradine, cefuroxime, ceftriaxone, cefixime, cefoperazone, cefamandole, ceftazidime, cefotaxime). Undiluted solutions of commercial drugs (parenteral drugs when available were used) were used for skin prick test, and if negative, they were tested intradermally as described by Schiavino et al.2 The concentrations used for the skin test and for the patch test are reported in the Table. Histamine (10 mg/mL) and saline were employed as positive and negative controls, respectively. Immediate reactions of at least 3 mm greater in diameter compared to the control for the skin prick test and 5 mm greater for intradermal tests were considered positive. Immediate-type skin tests were read after 20 minutes and also after 48 hours should any delayed reaction occur. An infiltrated erythema with a diameter greater than 5 mm was considered a delayed positive reaction.
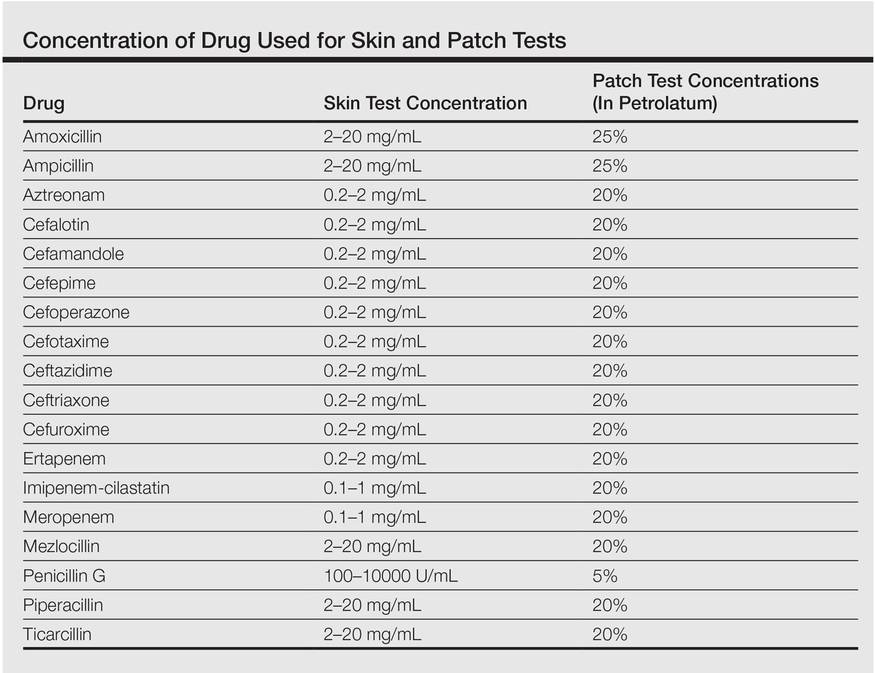
Patch tests were applied to the interscapular region using acrylate adhesive strips with small plates. They were evaluated at 48 and 72 hours. Positivity was assessed according to the indications of the European Network for Drug Allergy.3 Patch tests were carried out using the same drugs as the skin test. All drugs were mixed in petrolatum at 25% wt/wt for ampicillin and amoxicillin, 5% for penicillin G, and 20% for the other drugs as recommended by Schiavino et al.2 We also performed patch tests with ertapenem in 20 healthy controls.
A skin biopsy from lesional skin showed a perivascular infiltrate of the upper dermis with spongiosis of the lesional area similar to eczema. Patch tests and intradermal tests were positive for ertapenem after 48 hours (Figure 2). Imipenem-cilastatin, ampicillin, piperacillin, mezlocillin, and meropenem showed a positive reaction for patch tests. We concluded that the patient had delayed hypersensitivity to carbapenems (ertapenem, imipenem-cilastatin, and meropenem) and semisynthetic penicillins (piperacillin, mezlocillin, and ampicillin).

Drug sensitization in nurses and in health care workers can occur. Natural and semisynthetic penicillin can cause allergic contact dermatitis in health care workers. We report a case of occupational allergy to ertapenem, which is a 1-β-methyl-carbapenem that is administered as a single agent. It is highly active in vitro against bacteria that are generally associated with community-acquired and mixed aerobic and anaerobic infections.4 Occupational contact allergy to other carbapenems such as meropenem also was reported.5 The contact sensitization potential of imipenem has been confirmed in the guinea pig.6 Carbapenems have a bicyclic nucleus composed by a β-lactam ring with an associated 5-membered ring. In our patient, patch tests for ertapenem, imipenem, and meropenem were positive. Although the cross-reactivity between imipenem and penicillin has been demonstrated,2 data on the cross-reactivity between the carbapenems are not strong. Bauer et al7 reported a case of an allergy to imipenem-cilastatin that tolerated treatment with meropenem, but our case showed a complete cross-reactivity between carbapenems. Patch tests for ampicillin, mezlocillin, and piperacillin also were positive; therefore, it can be hypothesized that in our patient, the β-lactam ring was the main epitope recognized by T lymphocytes. Gielen and Goossens1 reported in a study on work-related dermatitis that the most common sensitizers were antibiotics such as penicillins, cephalosporins, and aminoglycosides.
Health care workers should protect their hands with gloves during the preparation of drugs because they have the risk for developing an occupational contact allergy. Detailed allergological and dermatological evaluation is mandatory to confirm or exclude occupational contact allergy.
- Gielen K, Goossens A. Occupational allergic contact dermatitis from drugs in healthcare workers. Contact Dermatitis. 2001;45:273-279.
- Schiavino D, Nucera E, Lombardo C, et al. Cross-reactivity and tolerability of imipenem in patients with delayed-type, cell-mediated hypersensitivity to beta-lactams. Allergy. 2009;64:1644-1648.
- Romano A, Blanca M, Torres MJ, et al. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy. 2004;59:1153-1160.
- Teppler H, Gesser RM, Friedland IR, et al. Safety and tolerability of ertapenem. J Antimicrob Chemother. 2004;53(suppl 2):75-81.
- Yesudian PD, King CM. Occupational allergic contact dermatitis from meropenem. Contact Dermatitis. 2001;45:53.
- Nagakura N, Souma S, Shimizu T, et al. Comparison of cross-reactivities of imipenem and other beta-lactam antibiotics by delayed-type hypersensitivity reaction in guinea pigs. Chem Pharm Bull. 1991;39:765-768.
- Bauer SL, Wall GC, Skoglund K, et al. Lack of cross-reactivity to meropenem in a patient with an allergy to imipenem-cilastatin. J Allergy Clin Immunol. 2004;113:173-175.
To the Editor:
Contact sensitivity to drugs that are systemically administered can occur among health care workers.1 We report the case of a 28-year-old nurse who developed eczema on the dorsal aspect of the hand (Figure 1A) and the face (Figure 1B) in the workplace. The nurse was working in the hematology department where she usually handled and administered antibiotics such as imipenem, ertapenem, piperacillin, vancomycin, anidulafungin, teicoplanin, and ciprofloxacin. She was moved to a different department where she did not have contact with the suspicious drugs and the dermatitis completely resolved.

One month after the resolution of the eczema she was referred to our allergy department for an allergological evaluation. A dermatologic evaluation was made and a skin biopsy was performed from a lesional area of the left hand. The patient underwent delayed skin test and patch tests with many β-lactam compounds including penicilloyl polylysine, minor determinant mixture, penicillin G, penicillin V, ampicillin, amoxicillin, bacampicillin, piperacillin, mezlocillin and ticarcillin, imipenem-cilastatin, aztreonam, meropenem, ertapenem, and cephalosporins (eg, cephalexin, cefaclor, cefalotin, cefadroxil, cephradine, cefuroxime, ceftriaxone, cefixime, cefoperazone, cefamandole, ceftazidime, cefotaxime). Undiluted solutions of commercial drugs (parenteral drugs when available were used) were used for skin prick test, and if negative, they were tested intradermally as described by Schiavino et al.2 The concentrations used for the skin test and for the patch test are reported in the Table. Histamine (10 mg/mL) and saline were employed as positive and negative controls, respectively. Immediate reactions of at least 3 mm greater in diameter compared to the control for the skin prick test and 5 mm greater for intradermal tests were considered positive. Immediate-type skin tests were read after 20 minutes and also after 48 hours should any delayed reaction occur. An infiltrated erythema with a diameter greater than 5 mm was considered a delayed positive reaction.

Patch tests were applied to the interscapular region using acrylate adhesive strips with small plates. They were evaluated at 48 and 72 hours. Positivity was assessed according to the indications of the European Network for Drug Allergy.3 Patch tests were carried out using the same drugs as the skin test. All drugs were mixed in petrolatum at 25% wt/wt for ampicillin and amoxicillin, 5% for penicillin G, and 20% for the other drugs as recommended by Schiavino et al.2 We also performed patch tests with ertapenem in 20 healthy controls.
A skin biopsy from lesional skin showed a perivascular infiltrate of the upper dermis with spongiosis of the lesional area similar to eczema. Patch tests and intradermal tests were positive for ertapenem after 48 hours (Figure 2). Imipenem-cilastatin, ampicillin, piperacillin, mezlocillin, and meropenem showed a positive reaction for patch tests. We concluded that the patient had delayed hypersensitivity to carbapenems (ertapenem, imipenem-cilastatin, and meropenem) and semisynthetic penicillins (piperacillin, mezlocillin, and ampicillin).

Drug sensitization in nurses and in health care workers can occur. Natural and semisynthetic penicillin can cause allergic contact dermatitis in health care workers. We report a case of occupational allergy to ertapenem, which is a 1-β-methyl-carbapenem that is administered as a single agent. It is highly active in vitro against bacteria that are generally associated with community-acquired and mixed aerobic and anaerobic infections.4 Occupational contact allergy to other carbapenems such as meropenem also was reported.5 The contact sensitization potential of imipenem has been confirmed in the guinea pig.6 Carbapenems have a bicyclic nucleus composed by a β-lactam ring with an associated 5-membered ring. In our patient, patch tests for ertapenem, imipenem, and meropenem were positive. Although the cross-reactivity between imipenem and penicillin has been demonstrated,2 data on the cross-reactivity between the carbapenems are not strong. Bauer et al7 reported a case of an allergy to imipenem-cilastatin that tolerated treatment with meropenem, but our case showed a complete cross-reactivity between carbapenems. Patch tests for ampicillin, mezlocillin, and piperacillin also were positive; therefore, it can be hypothesized that in our patient, the β-lactam ring was the main epitope recognized by T lymphocytes. Gielen and Goossens1 reported in a study on work-related dermatitis that the most common sensitizers were antibiotics such as penicillins, cephalosporins, and aminoglycosides.
Health care workers should protect their hands with gloves during the preparation of drugs because they have the risk for developing an occupational contact allergy. Detailed allergological and dermatological evaluation is mandatory to confirm or exclude occupational contact allergy.
To the Editor:
Contact sensitivity to drugs that are systemically administered can occur among health care workers.1 We report the case of a 28-year-old nurse who developed eczema on the dorsal aspect of the hand (Figure 1A) and the face (Figure 1B) in the workplace. The nurse was working in the hematology department where she usually handled and administered antibiotics such as imipenem, ertapenem, piperacillin, vancomycin, anidulafungin, teicoplanin, and ciprofloxacin. She was moved to a different department where she did not have contact with the suspicious drugs and the dermatitis completely resolved.

One month after the resolution of the eczema she was referred to our allergy department for an allergological evaluation. A dermatologic evaluation was made and a skin biopsy was performed from a lesional area of the left hand. The patient underwent delayed skin test and patch tests with many β-lactam compounds including penicilloyl polylysine, minor determinant mixture, penicillin G, penicillin V, ampicillin, amoxicillin, bacampicillin, piperacillin, mezlocillin and ticarcillin, imipenem-cilastatin, aztreonam, meropenem, ertapenem, and cephalosporins (eg, cephalexin, cefaclor, cefalotin, cefadroxil, cephradine, cefuroxime, ceftriaxone, cefixime, cefoperazone, cefamandole, ceftazidime, cefotaxime). Undiluted solutions of commercial drugs (parenteral drugs when available were used) were used for skin prick test, and if negative, they were tested intradermally as described by Schiavino et al.2 The concentrations used for the skin test and for the patch test are reported in the Table. Histamine (10 mg/mL) and saline were employed as positive and negative controls, respectively. Immediate reactions of at least 3 mm greater in diameter compared to the control for the skin prick test and 5 mm greater for intradermal tests were considered positive. Immediate-type skin tests were read after 20 minutes and also after 48 hours should any delayed reaction occur. An infiltrated erythema with a diameter greater than 5 mm was considered a delayed positive reaction.

Patch tests were applied to the interscapular region using acrylate adhesive strips with small plates. They were evaluated at 48 and 72 hours. Positivity was assessed according to the indications of the European Network for Drug Allergy.3 Patch tests were carried out using the same drugs as the skin test. All drugs were mixed in petrolatum at 25% wt/wt for ampicillin and amoxicillin, 5% for penicillin G, and 20% for the other drugs as recommended by Schiavino et al.2 We also performed patch tests with ertapenem in 20 healthy controls.
A skin biopsy from lesional skin showed a perivascular infiltrate of the upper dermis with spongiosis of the lesional area similar to eczema. Patch tests and intradermal tests were positive for ertapenem after 48 hours (Figure 2). Imipenem-cilastatin, ampicillin, piperacillin, mezlocillin, and meropenem showed a positive reaction for patch tests. We concluded that the patient had delayed hypersensitivity to carbapenems (ertapenem, imipenem-cilastatin, and meropenem) and semisynthetic penicillins (piperacillin, mezlocillin, and ampicillin).

Drug sensitization in nurses and in health care workers can occur. Natural and semisynthetic penicillin can cause allergic contact dermatitis in health care workers. We report a case of occupational allergy to ertapenem, which is a 1-β-methyl-carbapenem that is administered as a single agent. It is highly active in vitro against bacteria that are generally associated with community-acquired and mixed aerobic and anaerobic infections.4 Occupational contact allergy to other carbapenems such as meropenem also was reported.5 The contact sensitization potential of imipenem has been confirmed in the guinea pig.6 Carbapenems have a bicyclic nucleus composed by a β-lactam ring with an associated 5-membered ring. In our patient, patch tests for ertapenem, imipenem, and meropenem were positive. Although the cross-reactivity between imipenem and penicillin has been demonstrated,2 data on the cross-reactivity between the carbapenems are not strong. Bauer et al7 reported a case of an allergy to imipenem-cilastatin that tolerated treatment with meropenem, but our case showed a complete cross-reactivity between carbapenems. Patch tests for ampicillin, mezlocillin, and piperacillin also were positive; therefore, it can be hypothesized that in our patient, the β-lactam ring was the main epitope recognized by T lymphocytes. Gielen and Goossens1 reported in a study on work-related dermatitis that the most common sensitizers were antibiotics such as penicillins, cephalosporins, and aminoglycosides.
Health care workers should protect their hands with gloves during the preparation of drugs because they have the risk for developing an occupational contact allergy. Detailed allergological and dermatological evaluation is mandatory to confirm or exclude occupational contact allergy.
- Gielen K, Goossens A. Occupational allergic contact dermatitis from drugs in healthcare workers. Contact Dermatitis. 2001;45:273-279.
- Schiavino D, Nucera E, Lombardo C, et al. Cross-reactivity and tolerability of imipenem in patients with delayed-type, cell-mediated hypersensitivity to beta-lactams. Allergy. 2009;64:1644-1648.
- Romano A, Blanca M, Torres MJ, et al. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy. 2004;59:1153-1160.
- Teppler H, Gesser RM, Friedland IR, et al. Safety and tolerability of ertapenem. J Antimicrob Chemother. 2004;53(suppl 2):75-81.
- Yesudian PD, King CM. Occupational allergic contact dermatitis from meropenem. Contact Dermatitis. 2001;45:53.
- Nagakura N, Souma S, Shimizu T, et al. Comparison of cross-reactivities of imipenem and other beta-lactam antibiotics by delayed-type hypersensitivity reaction in guinea pigs. Chem Pharm Bull. 1991;39:765-768.
- Bauer SL, Wall GC, Skoglund K, et al. Lack of cross-reactivity to meropenem in a patient with an allergy to imipenem-cilastatin. J Allergy Clin Immunol. 2004;113:173-175.
- Gielen K, Goossens A. Occupational allergic contact dermatitis from drugs in healthcare workers. Contact Dermatitis. 2001;45:273-279.
- Schiavino D, Nucera E, Lombardo C, et al. Cross-reactivity and tolerability of imipenem in patients with delayed-type, cell-mediated hypersensitivity to beta-lactams. Allergy. 2009;64:1644-1648.
- Romano A, Blanca M, Torres MJ, et al. Diagnosis of nonimmediate reactions to beta-lactam antibiotics. Allergy. 2004;59:1153-1160.
- Teppler H, Gesser RM, Friedland IR, et al. Safety and tolerability of ertapenem. J Antimicrob Chemother. 2004;53(suppl 2):75-81.
- Yesudian PD, King CM. Occupational allergic contact dermatitis from meropenem. Contact Dermatitis. 2001;45:53.
- Nagakura N, Souma S, Shimizu T, et al. Comparison of cross-reactivities of imipenem and other beta-lactam antibiotics by delayed-type hypersensitivity reaction in guinea pigs. Chem Pharm Bull. 1991;39:765-768.
- Bauer SL, Wall GC, Skoglund K, et al. Lack of cross-reactivity to meropenem in a patient with an allergy to imipenem-cilastatin. J Allergy Clin Immunol. 2004;113:173-175.
Dermatoses of Pregnancy
After, test your knowledge by answering the 5 practice questions.
Practice Questions
1. Which dermatosis of pregnancy occurs during the third trimester and is associated with multiple gestation pregnancies?
a. atopic eruption of pregnancy
b. gestational pemphigoid
c. intrahepatic cholestasis of pregnancy
d. prurigo of pregnancy
e. pruritic urticarial papules and plaques of pregnancy
2. Which dermatosis of pregnancy frequently flares after delivery?
a. atopic eruption of pregnancy
b. gestational pemphigoid
c. polymorphic eruption of pregnancy
d. prurigo gravidarum
e. prurigo of pregnancy
3. Which dermatosis of pregnancy has lesions that have a predilection for the abdominal striae?
a. cholestasis of pregnancy
b. gestational pemphigoid
c. prurigo gestationis
d. prurigo of pregnancy
e. pruritic urticarial papules and plaques of pregnancy
4. Which dermatosis of pregnancy has a risk for the development of hydatidiform moles and choriocarcinomas?
a. atopic eruption of pregnancy
b. cholestasis of pregnancy
c. gestational pemphigoid
d. pruritic urticarial papules and plaques of pregnancy
e. toxic erythema of pregnancy
5. Intrahepatic cholestasis of pregnancy has been associated with:
a. fetal mortality as high as 13%
b. jaundice in 20% of cases
c. onset in the third trimester of pregnancy
d. recurrence in subsequent pregnancies
e. all of the above
The answers appear on the next page.
1. Which dermatosis of pregnancy occurs during the third trimester and is associated with multiple gestation pregnancies?
a. atopic eruption of pregnancy
b. gestational pemphigoid
c. intrahepatic cholestasis of pregnancy
d. prurigo of pregnancy
e. pruritic urticarial papules and plaques of pregnancy
2. Which dermatosis of pregnancy frequently flares after delivery?
a. atopic eruption of pregnancy
b. gestational pemphigoid
c. polymorphic eruption of pregnancy
d. prurigo gravidarum
e. prurigo of pregnancy
3. Which dermatosis of pregnancy has lesions that have a predilection for the abdominal striae?
a. cholestasis of pregnancy
b. gestational pemphigoid
c. prurigo gestationis
d. prurigo of pregnancy
e. pruritic urticarial papules and plaques of pregnancy
4. Which dermatosis of pregnancy has a risk for the development of hydatidiform moles and choriocarcinomas?
a. atopic eruption of pregnancy
b. cholestasis of pregnancy
c. gestational pemphigoid
d. pruritic urticarial papules and plaques of pregnancy
e. toxic erythema of pregnancy
5. Intrahepatic cholestasis of pregnancy has been associated with:
a. fetal mortality as high as 13%
b. jaundice in 20% of cases
c. onset in the third trimester of pregnancy
d. recurrence in subsequent pregnancies
e. all of the above
After, test your knowledge by answering the 5 practice questions.
Practice Questions
1. Which dermatosis of pregnancy occurs during the third trimester and is associated with multiple gestation pregnancies?
a. atopic eruption of pregnancy
b. gestational pemphigoid
c. intrahepatic cholestasis of pregnancy
d. prurigo of pregnancy
e. pruritic urticarial papules and plaques of pregnancy
2. Which dermatosis of pregnancy frequently flares after delivery?
a. atopic eruption of pregnancy
b. gestational pemphigoid
c. polymorphic eruption of pregnancy
d. prurigo gravidarum
e. prurigo of pregnancy
3. Which dermatosis of pregnancy has lesions that have a predilection for the abdominal striae?
a. cholestasis of pregnancy
b. gestational pemphigoid
c. prurigo gestationis
d. prurigo of pregnancy
e. pruritic urticarial papules and plaques of pregnancy
4. Which dermatosis of pregnancy has a risk for the development of hydatidiform moles and choriocarcinomas?
a. atopic eruption of pregnancy
b. cholestasis of pregnancy
c. gestational pemphigoid
d. pruritic urticarial papules and plaques of pregnancy
e. toxic erythema of pregnancy
5. Intrahepatic cholestasis of pregnancy has been associated with:
a. fetal mortality as high as 13%
b. jaundice in 20% of cases
c. onset in the third trimester of pregnancy
d. recurrence in subsequent pregnancies
e. all of the above
The answers appear on the next page.
1. Which dermatosis of pregnancy occurs during the third trimester and is associated with multiple gestation pregnancies?
a. atopic eruption of pregnancy
b. gestational pemphigoid
c. intrahepatic cholestasis of pregnancy
d. prurigo of pregnancy
e. pruritic urticarial papules and plaques of pregnancy
2. Which dermatosis of pregnancy frequently flares after delivery?
a. atopic eruption of pregnancy
b. gestational pemphigoid
c. polymorphic eruption of pregnancy
d. prurigo gravidarum
e. prurigo of pregnancy
3. Which dermatosis of pregnancy has lesions that have a predilection for the abdominal striae?
a. cholestasis of pregnancy
b. gestational pemphigoid
c. prurigo gestationis
d. prurigo of pregnancy
e. pruritic urticarial papules and plaques of pregnancy
4. Which dermatosis of pregnancy has a risk for the development of hydatidiform moles and choriocarcinomas?
a. atopic eruption of pregnancy
b. cholestasis of pregnancy
c. gestational pemphigoid
d. pruritic urticarial papules and plaques of pregnancy
e. toxic erythema of pregnancy
5. Intrahepatic cholestasis of pregnancy has been associated with:
a. fetal mortality as high as 13%
b. jaundice in 20% of cases
c. onset in the third trimester of pregnancy
d. recurrence in subsequent pregnancies
e. all of the above
After, test your knowledge by answering the 5 practice questions.
Practice Questions
1. Which dermatosis of pregnancy occurs during the third trimester and is associated with multiple gestation pregnancies?
a. atopic eruption of pregnancy
b. gestational pemphigoid
c. intrahepatic cholestasis of pregnancy
d. prurigo of pregnancy
e. pruritic urticarial papules and plaques of pregnancy
2. Which dermatosis of pregnancy frequently flares after delivery?
a. atopic eruption of pregnancy
b. gestational pemphigoid
c. polymorphic eruption of pregnancy
d. prurigo gravidarum
e. prurigo of pregnancy
3. Which dermatosis of pregnancy has lesions that have a predilection for the abdominal striae?
a. cholestasis of pregnancy
b. gestational pemphigoid
c. prurigo gestationis
d. prurigo of pregnancy
e. pruritic urticarial papules and plaques of pregnancy
4. Which dermatosis of pregnancy has a risk for the development of hydatidiform moles and choriocarcinomas?
a. atopic eruption of pregnancy
b. cholestasis of pregnancy
c. gestational pemphigoid
d. pruritic urticarial papules and plaques of pregnancy
e. toxic erythema of pregnancy
5. Intrahepatic cholestasis of pregnancy has been associated with:
a. fetal mortality as high as 13%
b. jaundice in 20% of cases
c. onset in the third trimester of pregnancy
d. recurrence in subsequent pregnancies
e. all of the above
The answers appear on the next page.
1. Which dermatosis of pregnancy occurs during the third trimester and is associated with multiple gestation pregnancies?
a. atopic eruption of pregnancy
b. gestational pemphigoid
c. intrahepatic cholestasis of pregnancy
d. prurigo of pregnancy
e. pruritic urticarial papules and plaques of pregnancy
2. Which dermatosis of pregnancy frequently flares after delivery?
a. atopic eruption of pregnancy
b. gestational pemphigoid
c. polymorphic eruption of pregnancy
d. prurigo gravidarum
e. prurigo of pregnancy
3. Which dermatosis of pregnancy has lesions that have a predilection for the abdominal striae?
a. cholestasis of pregnancy
b. gestational pemphigoid
c. prurigo gestationis
d. prurigo of pregnancy
e. pruritic urticarial papules and plaques of pregnancy
4. Which dermatosis of pregnancy has a risk for the development of hydatidiform moles and choriocarcinomas?
a. atopic eruption of pregnancy
b. cholestasis of pregnancy
c. gestational pemphigoid
d. pruritic urticarial papules and plaques of pregnancy
e. toxic erythema of pregnancy
5. Intrahepatic cholestasis of pregnancy has been associated with:
a. fetal mortality as high as 13%
b. jaundice in 20% of cases
c. onset in the third trimester of pregnancy
d. recurrence in subsequent pregnancies
e. all of the above
Extensive Skin Necrosis From Suspected Levamisole-Contaminated Cocaine
To the Editor:
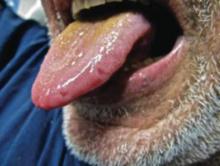
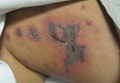
A 52-year-old man presented to the emergency department with skin pain. Although he felt well overall, he reported that he had developed skin sores 3 weeks prior to presentation that were progressively causing skin pain and sleep loss. He acknowledged smoking cigarettes and snorting cocaine but denied intravenous use of cocaine or using any other drugs. His usual medications were lisinopril and tramadol, and he had no known drug allergies. His history was remarkable for methicillin-resistant Staphylococcus aureus (MRSA) septic arthritis of the shoulder and MRSA prepatellar bursitis within the last 2 years. During examination in the emergency department he was alert, afebrile, nontoxic, generally healthy, and in no acute distress. Extensive necrotic skin lesions were present on the trunk, extremities, and both ears. The lesions were large necrotic patches with irregular, sharply angulated borders with thin or ulcerated epidermis surrounded by a bright halo of erythema (Figure 1). Ulcers were noted on the tongue (Figure 2).

|
| Figure 1. Extensive skin necrosis on the leg from levamisole-contaminated cocaine (A). Necrotic skin lesions also were present on the trunk, arm (B), and ear (C). |
The clinical diagnosis was probable thrombosis of skin vessels with skin necrosis due to cocaine that was likely contaminated with levamisole. Pertinent laboratory results included the following: mild anemia and mild leukopenia; values within reference range for liver function, serum protein electrophoresis, hepatitis profile, human immunodeficiency virus 1 and 2, rapid plasma reagin, and antinuclear antibody; normal thrombotic studies for antithrombin III, protein C, protein S, factor V Leiden, prothrombin mutation G20210A, anticardiolipin IgG, IgM, and IgA; erythrocyte sedimentation rate of 26 mm/h (reference range, 0–15 mm/h); perinuclear antineutrophil cytoplasmic antibody greater than 1:320 (reference range, <1:20) with normal proteinase 3 and myeloperoxidase antibodies; urine positive for cocaine but blood negative for cocaine; normal chest radiograph; and normal electrocardiogram.
The patient was stable with good family support and was discharged from the emergency department to be followed in our dermatology office. The following day his skin biopsies were interpreted as neutrophilic vasculitis with extensive intravascular early and organizing thrombi involving all small- and medium-sized blood vessels consistent with levamisole-induced necrosis or septic vasculitis (Figure 3). With his history of MRSA septic arthritis and bursitis, he was hospitalized for treatment with intravenous vancomycin pending further studies. Skin biopsy for direct immunofluorescence revealed granular deposits of IgM and linear deposits of C3 at the dermoepidermal junction and in blood vessel walls. Two tissue cultures for bacteria and fungi were negative and 2 blood cultures were negative. An echocardiogram was normal and without evidence of emboli. The patient remained stable and antibiotics were discontinued. He was released from the hospital and his skin lesions healed satisfactorily with showering and mupirocin ointment.
|
| Figure 3. Thrombotic occlusion of blood vessels was seen on histopathology (A and B)(H&E, original magnifications ×100 and ×400).
|
Cocaine is a white powder that is primarily derived from the leaves of the coca plant in South America. It is ingested orally; injected intravenously; snorted intranasally; chewed; eaten; used as a suppository; or dissolved in water and baking soda then heated to crystallization for smoking, which is the most addictive method and known as freebasing. When smoked, crack cocaine produces a crackling sound. Cocaine stimulates the central nervous system similar to amphetamine but may harm any body organ through vasoconstriction/vasospasm and cause skin necrosis without any additive. Perhaps less known is its ability to produce smooth muscle hyperplasia of small vessels and premature atherosclerosis.1
Levamisole has been used to treat worms, cancer, and stimulation of the immune system but currently is used only by veterinarians because of agranulocytosis and vasculitis in humans. As of July 2009, the Drug Enforcement Agency reported that 69% of seized cocaine lots coming into the United States contained levamisole.2 By January 2010, 73.2% of seized cocaine exhibits contained levamisole according to the California Poison Control System, with reports of contamination rates from across the country ranging from 40% to 90%.3 Levamisole is an inexpensive additive to cocaine and may increase the release of brain dopamine.4 It is difficult to detect levamisole in urine due to its short half-life of 5.6 hours and only 2% to 5% of the parent compound being found in the urine.5
Skin necrosis due to cocaine-contaminated levamisole usually occurs in younger individuals who have characteristic skin lesions and a history of cocaine use. Skin lesions usually are multiple, purpuric or necrotic with irregular angulated edges and a halo of erythema. Ear involvement is common but not invariable.6 Descriptive adjectives include branched, netlike, retiform, and stellate, all revealing the compromised underlying dermal and subcutaneous vascular anatomy. Supportive evidence includes a decreased white blood cell count (neutropenia in up to 50%),5 positive antineutrophilic cytoplasmic antibodies,5,7 and/or positive drug screen. Skin biopsy may reveal thrombosis,4 fibrin thrombi without vasculitis,8 or leukocytoclastic vasculitis,4,5 or may suggest septic vasculitis.9 Direct immunofluorescence may suggest an immune complex-mediated vasculitis.5
The differential diagnosis for a patient with purpuric/necrotic skin lesions should be broad and include vasculitis (eg, inflammatory, antineutrophil cytoplasmic antibody positive, septic), hypercoagulopathy (eg, antiphospholipid syndrome, antithrombin III, prothrombin mutation G20210A, factor V Leiden, protein C, protein S), drugs (eg, heparin, warfarin, cocaine with or without levamisole, intravenous drug use, hydroxyurea, ergotamine, propylthiouracil10), calciphylaxis, cold-induced thrombosis, emboli (eg, atheroma, cholesterol, endocarditis, myxoma, aortic angiosarcoma, marantic), febrile ulceronecrotic Mucha-Habermann disease, infection especially if immunosuppressed (eg, disseminated Acanthamoeba/Candida/histoplasmosis/strongyloides/varicella-zoster virus, S aureus, streptococcus, ecthyma gangrenosum, gas gangrene, hemorrhagic smallpox, lues maligna with human immunodeficiency virus, Meleney ulcer, Rocky Mountain spotted fever, Vibrio vulnificus), idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, thrombocythemia, Waldenström hyperglobulinemic purpura, pyoderma gangrenosum, cancer (eg, paraneoplastic arterial thrombi), oxalosis, paraproteinemia (eg, multiple myeloma), and lupus with generalized coagulopathy. Less likely diagnoses might include Degos disease, factitial dermatitis, foreign bodies, multiple spider bites, paroxysmal nocturnal hemoglobinuria, sickle cell anemia, Buruli ulcer, or thromboangiitis obliterans. Branched, angulated, retiform lesions are an important finding, and some of these diagnostic possibilities are not classically retiform. However, clinical findings are not always classical, and astute physicians want to be circumspect. Had more ominous findings been present in our patient (eg, fever, hemodynamic instability, progressive skin lesions, systemic organ involvement), prompt hospitalization and additional considerations would have been necessary, such as septicemia (eg, meningococcemia, bubonic plague [Black Death], necrotizing fasciitis, purpura fulminans), catastrophic antiphospholipid syndrome, or disseminated intra- vascular coagulation.
The prognosis for skin necrosis caused by levamisole-contaminated cocaine generally is good without long-term sequelae.5 Autoantibody serologies normalize within weeks to months after stopping levamisole.5,8 Our patient recovered with conservative measures.
1. Dhawan SS, Wang BW. Four-extremity gangrene associated with crack cocaine abuse [published online ahead of print October 23, 2006]. Ann Emerg Med. 2007;49:186-189.
2. Centers for Disease Control and Prevention. Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Buchanan J; California Poison Control System. Levamisole-contaminated cocaine. Call Us… December 3, 2014. http://www.calpoison.org/hcp/2014/ callusvol12no3.htm. Accessed September 1, 2015.
4. Mouzakis J, Somboonwit C, Lakshmi S, et al. Levamisole induced necrosis of the skin and neutropenia following intranasal cocaine use: a newly recognized syndrome. J Drugs Dermatology. 2011;10:1204-1207.
5. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine [published online ahead of print June 11, 2011]. J Am Acad Dermatol. 2011;65:722-725.
6. Farhat EK, Muirhead TT, Chaffins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;46:1320-1321.
7. Geller L, Whang TB, Mercer SE. Retiform purpura: a new stigmata of illicit drug use? Dermatol Online J. 2011;17:7.
8. Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol. 2010;63:530-535.
9. Reutemann P, Grenier N, Telang GH. Occlusive vasculopathy with vascular and skin necrosis secondary to smoking crack cocaine. J Am Acad Dermatol. 2011;64:1004-1006.
10. Mahmood T, Delacerda A, Fiala K. Painful purpura on bilateral helices. JAMA Dermatol. 2015;151:551-552.
To the Editor:
A 52-year-old man presented to the emergency department with skin pain. Although he felt well overall, he reported that he had developed skin sores 3 weeks prior to presentation that were progressively causing skin pain and sleep loss. He acknowledged smoking cigarettes and snorting cocaine but denied intravenous use of cocaine or using any other drugs. His usual medications were lisinopril and tramadol, and he had no known drug allergies. His history was remarkable for methicillin-resistant Staphylococcus aureus (MRSA) septic arthritis of the shoulder and MRSA prepatellar bursitis within the last 2 years. During examination in the emergency department he was alert, afebrile, nontoxic, generally healthy, and in no acute distress. Extensive necrotic skin lesions were present on the trunk, extremities, and both ears. The lesions were large necrotic patches with irregular, sharply angulated borders with thin or ulcerated epidermis surrounded by a bright halo of erythema (Figure 1). Ulcers were noted on the tongue (Figure 2).



|
| Figure 1. Extensive skin necrosis on the leg from levamisole-contaminated cocaine (A). Necrotic skin lesions also were present on the trunk, arm (B), and ear (C). |
The clinical diagnosis was probable thrombosis of skin vessels with skin necrosis due to cocaine that was likely contaminated with levamisole. Pertinent laboratory results included the following: mild anemia and mild leukopenia; values within reference range for liver function, serum protein electrophoresis, hepatitis profile, human immunodeficiency virus 1 and 2, rapid plasma reagin, and antinuclear antibody; normal thrombotic studies for antithrombin III, protein C, protein S, factor V Leiden, prothrombin mutation G20210A, anticardiolipin IgG, IgM, and IgA; erythrocyte sedimentation rate of 26 mm/h (reference range, 0–15 mm/h); perinuclear antineutrophil cytoplasmic antibody greater than 1:320 (reference range, <1:20) with normal proteinase 3 and myeloperoxidase antibodies; urine positive for cocaine but blood negative for cocaine; normal chest radiograph; and normal electrocardiogram.
The patient was stable with good family support and was discharged from the emergency department to be followed in our dermatology office. The following day his skin biopsies were interpreted as neutrophilic vasculitis with extensive intravascular early and organizing thrombi involving all small- and medium-sized blood vessels consistent with levamisole-induced necrosis or septic vasculitis (Figure 3). With his history of MRSA septic arthritis and bursitis, he was hospitalized for treatment with intravenous vancomycin pending further studies. Skin biopsy for direct immunofluorescence revealed granular deposits of IgM and linear deposits of C3 at the dermoepidermal junction and in blood vessel walls. Two tissue cultures for bacteria and fungi were negative and 2 blood cultures were negative. An echocardiogram was normal and without evidence of emboli. The patient remained stable and antibiotics were discontinued. He was released from the hospital and his skin lesions healed satisfactorily with showering and mupirocin ointment.


|
| Figure 3. Thrombotic occlusion of blood vessels was seen on histopathology (A and B)(H&E, original magnifications ×100 and ×400).
|
Cocaine is a white powder that is primarily derived from the leaves of the coca plant in South America. It is ingested orally; injected intravenously; snorted intranasally; chewed; eaten; used as a suppository; or dissolved in water and baking soda then heated to crystallization for smoking, which is the most addictive method and known as freebasing. When smoked, crack cocaine produces a crackling sound. Cocaine stimulates the central nervous system similar to amphetamine but may harm any body organ through vasoconstriction/vasospasm and cause skin necrosis without any additive. Perhaps less known is its ability to produce smooth muscle hyperplasia of small vessels and premature atherosclerosis.1
Levamisole has been used to treat worms, cancer, and stimulation of the immune system but currently is used only by veterinarians because of agranulocytosis and vasculitis in humans. As of July 2009, the Drug Enforcement Agency reported that 69% of seized cocaine lots coming into the United States contained levamisole.2 By January 2010, 73.2% of seized cocaine exhibits contained levamisole according to the California Poison Control System, with reports of contamination rates from across the country ranging from 40% to 90%.3 Levamisole is an inexpensive additive to cocaine and may increase the release of brain dopamine.4 It is difficult to detect levamisole in urine due to its short half-life of 5.6 hours and only 2% to 5% of the parent compound being found in the urine.5
Skin necrosis due to cocaine-contaminated levamisole usually occurs in younger individuals who have characteristic skin lesions and a history of cocaine use. Skin lesions usually are multiple, purpuric or necrotic with irregular angulated edges and a halo of erythema. Ear involvement is common but not invariable.6 Descriptive adjectives include branched, netlike, retiform, and stellate, all revealing the compromised underlying dermal and subcutaneous vascular anatomy. Supportive evidence includes a decreased white blood cell count (neutropenia in up to 50%),5 positive antineutrophilic cytoplasmic antibodies,5,7 and/or positive drug screen. Skin biopsy may reveal thrombosis,4 fibrin thrombi without vasculitis,8 or leukocytoclastic vasculitis,4,5 or may suggest septic vasculitis.9 Direct immunofluorescence may suggest an immune complex-mediated vasculitis.5
The differential diagnosis for a patient with purpuric/necrotic skin lesions should be broad and include vasculitis (eg, inflammatory, antineutrophil cytoplasmic antibody positive, septic), hypercoagulopathy (eg, antiphospholipid syndrome, antithrombin III, prothrombin mutation G20210A, factor V Leiden, protein C, protein S), drugs (eg, heparin, warfarin, cocaine with or without levamisole, intravenous drug use, hydroxyurea, ergotamine, propylthiouracil10), calciphylaxis, cold-induced thrombosis, emboli (eg, atheroma, cholesterol, endocarditis, myxoma, aortic angiosarcoma, marantic), febrile ulceronecrotic Mucha-Habermann disease, infection especially if immunosuppressed (eg, disseminated Acanthamoeba/Candida/histoplasmosis/strongyloides/varicella-zoster virus, S aureus, streptococcus, ecthyma gangrenosum, gas gangrene, hemorrhagic smallpox, lues maligna with human immunodeficiency virus, Meleney ulcer, Rocky Mountain spotted fever, Vibrio vulnificus), idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, thrombocythemia, Waldenström hyperglobulinemic purpura, pyoderma gangrenosum, cancer (eg, paraneoplastic arterial thrombi), oxalosis, paraproteinemia (eg, multiple myeloma), and lupus with generalized coagulopathy. Less likely diagnoses might include Degos disease, factitial dermatitis, foreign bodies, multiple spider bites, paroxysmal nocturnal hemoglobinuria, sickle cell anemia, Buruli ulcer, or thromboangiitis obliterans. Branched, angulated, retiform lesions are an important finding, and some of these diagnostic possibilities are not classically retiform. However, clinical findings are not always classical, and astute physicians want to be circumspect. Had more ominous findings been present in our patient (eg, fever, hemodynamic instability, progressive skin lesions, systemic organ involvement), prompt hospitalization and additional considerations would have been necessary, such as septicemia (eg, meningococcemia, bubonic plague [Black Death], necrotizing fasciitis, purpura fulminans), catastrophic antiphospholipid syndrome, or disseminated intra- vascular coagulation.
The prognosis for skin necrosis caused by levamisole-contaminated cocaine generally is good without long-term sequelae.5 Autoantibody serologies normalize within weeks to months after stopping levamisole.5,8 Our patient recovered with conservative measures.
To the Editor:
A 52-year-old man presented to the emergency department with skin pain. Although he felt well overall, he reported that he had developed skin sores 3 weeks prior to presentation that were progressively causing skin pain and sleep loss. He acknowledged smoking cigarettes and snorting cocaine but denied intravenous use of cocaine or using any other drugs. His usual medications were lisinopril and tramadol, and he had no known drug allergies. His history was remarkable for methicillin-resistant Staphylococcus aureus (MRSA) septic arthritis of the shoulder and MRSA prepatellar bursitis within the last 2 years. During examination in the emergency department he was alert, afebrile, nontoxic, generally healthy, and in no acute distress. Extensive necrotic skin lesions were present on the trunk, extremities, and both ears. The lesions were large necrotic patches with irregular, sharply angulated borders with thin or ulcerated epidermis surrounded by a bright halo of erythema (Figure 1). Ulcers were noted on the tongue (Figure 2).



|
| Figure 1. Extensive skin necrosis on the leg from levamisole-contaminated cocaine (A). Necrotic skin lesions also were present on the trunk, arm (B), and ear (C). |
The clinical diagnosis was probable thrombosis of skin vessels with skin necrosis due to cocaine that was likely contaminated with levamisole. Pertinent laboratory results included the following: mild anemia and mild leukopenia; values within reference range for liver function, serum protein electrophoresis, hepatitis profile, human immunodeficiency virus 1 and 2, rapid plasma reagin, and antinuclear antibody; normal thrombotic studies for antithrombin III, protein C, protein S, factor V Leiden, prothrombin mutation G20210A, anticardiolipin IgG, IgM, and IgA; erythrocyte sedimentation rate of 26 mm/h (reference range, 0–15 mm/h); perinuclear antineutrophil cytoplasmic antibody greater than 1:320 (reference range, <1:20) with normal proteinase 3 and myeloperoxidase antibodies; urine positive for cocaine but blood negative for cocaine; normal chest radiograph; and normal electrocardiogram.
The patient was stable with good family support and was discharged from the emergency department to be followed in our dermatology office. The following day his skin biopsies were interpreted as neutrophilic vasculitis with extensive intravascular early and organizing thrombi involving all small- and medium-sized blood vessels consistent with levamisole-induced necrosis or septic vasculitis (Figure 3). With his history of MRSA septic arthritis and bursitis, he was hospitalized for treatment with intravenous vancomycin pending further studies. Skin biopsy for direct immunofluorescence revealed granular deposits of IgM and linear deposits of C3 at the dermoepidermal junction and in blood vessel walls. Two tissue cultures for bacteria and fungi were negative and 2 blood cultures were negative. An echocardiogram was normal and without evidence of emboli. The patient remained stable and antibiotics were discontinued. He was released from the hospital and his skin lesions healed satisfactorily with showering and mupirocin ointment.


|
| Figure 3. Thrombotic occlusion of blood vessels was seen on histopathology (A and B)(H&E, original magnifications ×100 and ×400).
|
Cocaine is a white powder that is primarily derived from the leaves of the coca plant in South America. It is ingested orally; injected intravenously; snorted intranasally; chewed; eaten; used as a suppository; or dissolved in water and baking soda then heated to crystallization for smoking, which is the most addictive method and known as freebasing. When smoked, crack cocaine produces a crackling sound. Cocaine stimulates the central nervous system similar to amphetamine but may harm any body organ through vasoconstriction/vasospasm and cause skin necrosis without any additive. Perhaps less known is its ability to produce smooth muscle hyperplasia of small vessels and premature atherosclerosis.1
Levamisole has been used to treat worms, cancer, and stimulation of the immune system but currently is used only by veterinarians because of agranulocytosis and vasculitis in humans. As of July 2009, the Drug Enforcement Agency reported that 69% of seized cocaine lots coming into the United States contained levamisole.2 By January 2010, 73.2% of seized cocaine exhibits contained levamisole according to the California Poison Control System, with reports of contamination rates from across the country ranging from 40% to 90%.3 Levamisole is an inexpensive additive to cocaine and may increase the release of brain dopamine.4 It is difficult to detect levamisole in urine due to its short half-life of 5.6 hours and only 2% to 5% of the parent compound being found in the urine.5
Skin necrosis due to cocaine-contaminated levamisole usually occurs in younger individuals who have characteristic skin lesions and a history of cocaine use. Skin lesions usually are multiple, purpuric or necrotic with irregular angulated edges and a halo of erythema. Ear involvement is common but not invariable.6 Descriptive adjectives include branched, netlike, retiform, and stellate, all revealing the compromised underlying dermal and subcutaneous vascular anatomy. Supportive evidence includes a decreased white blood cell count (neutropenia in up to 50%),5 positive antineutrophilic cytoplasmic antibodies,5,7 and/or positive drug screen. Skin biopsy may reveal thrombosis,4 fibrin thrombi without vasculitis,8 or leukocytoclastic vasculitis,4,5 or may suggest septic vasculitis.9 Direct immunofluorescence may suggest an immune complex-mediated vasculitis.5
The differential diagnosis for a patient with purpuric/necrotic skin lesions should be broad and include vasculitis (eg, inflammatory, antineutrophil cytoplasmic antibody positive, septic), hypercoagulopathy (eg, antiphospholipid syndrome, antithrombin III, prothrombin mutation G20210A, factor V Leiden, protein C, protein S), drugs (eg, heparin, warfarin, cocaine with or without levamisole, intravenous drug use, hydroxyurea, ergotamine, propylthiouracil10), calciphylaxis, cold-induced thrombosis, emboli (eg, atheroma, cholesterol, endocarditis, myxoma, aortic angiosarcoma, marantic), febrile ulceronecrotic Mucha-Habermann disease, infection especially if immunosuppressed (eg, disseminated Acanthamoeba/Candida/histoplasmosis/strongyloides/varicella-zoster virus, S aureus, streptococcus, ecthyma gangrenosum, gas gangrene, hemorrhagic smallpox, lues maligna with human immunodeficiency virus, Meleney ulcer, Rocky Mountain spotted fever, Vibrio vulnificus), idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura, thrombocythemia, Waldenström hyperglobulinemic purpura, pyoderma gangrenosum, cancer (eg, paraneoplastic arterial thrombi), oxalosis, paraproteinemia (eg, multiple myeloma), and lupus with generalized coagulopathy. Less likely diagnoses might include Degos disease, factitial dermatitis, foreign bodies, multiple spider bites, paroxysmal nocturnal hemoglobinuria, sickle cell anemia, Buruli ulcer, or thromboangiitis obliterans. Branched, angulated, retiform lesions are an important finding, and some of these diagnostic possibilities are not classically retiform. However, clinical findings are not always classical, and astute physicians want to be circumspect. Had more ominous findings been present in our patient (eg, fever, hemodynamic instability, progressive skin lesions, systemic organ involvement), prompt hospitalization and additional considerations would have been necessary, such as septicemia (eg, meningococcemia, bubonic plague [Black Death], necrotizing fasciitis, purpura fulminans), catastrophic antiphospholipid syndrome, or disseminated intra- vascular coagulation.
The prognosis for skin necrosis caused by levamisole-contaminated cocaine generally is good without long-term sequelae.5 Autoantibody serologies normalize within weeks to months after stopping levamisole.5,8 Our patient recovered with conservative measures.
1. Dhawan SS, Wang BW. Four-extremity gangrene associated with crack cocaine abuse [published online ahead of print October 23, 2006]. Ann Emerg Med. 2007;49:186-189.
2. Centers for Disease Control and Prevention. Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Buchanan J; California Poison Control System. Levamisole-contaminated cocaine. Call Us… December 3, 2014. http://www.calpoison.org/hcp/2014/ callusvol12no3.htm. Accessed September 1, 2015.
4. Mouzakis J, Somboonwit C, Lakshmi S, et al. Levamisole induced necrosis of the skin and neutropenia following intranasal cocaine use: a newly recognized syndrome. J Drugs Dermatology. 2011;10:1204-1207.
5. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine [published online ahead of print June 11, 2011]. J Am Acad Dermatol. 2011;65:722-725.
6. Farhat EK, Muirhead TT, Chaffins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;46:1320-1321.
7. Geller L, Whang TB, Mercer SE. Retiform purpura: a new stigmata of illicit drug use? Dermatol Online J. 2011;17:7.
8. Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol. 2010;63:530-535.
9. Reutemann P, Grenier N, Telang GH. Occlusive vasculopathy with vascular and skin necrosis secondary to smoking crack cocaine. J Am Acad Dermatol. 2011;64:1004-1006.
10. Mahmood T, Delacerda A, Fiala K. Painful purpura on bilateral helices. JAMA Dermatol. 2015;151:551-552.
1. Dhawan SS, Wang BW. Four-extremity gangrene associated with crack cocaine abuse [published online ahead of print October 23, 2006]. Ann Emerg Med. 2007;49:186-189.
2. Centers for Disease Control and Prevention. Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
3. Buchanan J; California Poison Control System. Levamisole-contaminated cocaine. Call Us… December 3, 2014. http://www.calpoison.org/hcp/2014/ callusvol12no3.htm. Accessed September 1, 2015.
4. Mouzakis J, Somboonwit C, Lakshmi S, et al. Levamisole induced necrosis of the skin and neutropenia following intranasal cocaine use: a newly recognized syndrome. J Drugs Dermatology. 2011;10:1204-1207.
5. Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine [published online ahead of print June 11, 2011]. J Am Acad Dermatol. 2011;65:722-725.
6. Farhat EK, Muirhead TT, Chaffins ML, et al. Levamisole-induced cutaneous necrosis mimicking coagulopathy. Arch Dermatol. 2010;46:1320-1321.
7. Geller L, Whang TB, Mercer SE. Retiform purpura: a new stigmata of illicit drug use? Dermatol Online J. 2011;17:7.
8. Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol. 2010;63:530-535.
9. Reutemann P, Grenier N, Telang GH. Occlusive vasculopathy with vascular and skin necrosis secondary to smoking crack cocaine. J Am Acad Dermatol. 2011;64:1004-1006.
10. Mahmood T, Delacerda A, Fiala K. Painful purpura on bilateral helices. JAMA Dermatol. 2015;151:551-552.
Painful Skin Lesions on the Hands Following Black Henna Application
The Diagnosis: Allergic Contact Dermatitis to Para-phenylenediamine
To darken the color of henna and increase penetrance and staining, para-phenylenediamine (PPD) is added.1 Allergic contact dermatitis is the most common type of hypersensitivity to PPD.2 A retrospective study that examined severe adverse events from applying henna dyes in children found that angioedema of mucosal tissues was the most common severe adverse event; others included renal failure and shock.3
Black henna is associated with multiple cultural practices. For example, Indian weddings contain a henna decoration ceremony for the bride based on the belief that the longer the henna lasts, the longer the marriage lasts. Black henna is favored for this practice, as it lasts longer than red henna.

Henna (Lawsonia inermis) is a plant that contains the molecule lawsone (naphthoquinone). Lawsone has an intense affinity for keratin; as a result, lawsone is frequently added to temporary body tattoos and hair dyes to create a relatively permanent change in skin or hair color.4 Henna is mixed with hennotannic acid to release the lawsone from the plant. Lawsone and hennotannic acid rarely cause allergic reactions.1,5-7 Once applied to skin, henna takes a few hours to dry, and the resulting color is orange to red.8 Often, PPD is added to henna paste to create a black color, to speed up the drying process, and to increase its longevity.
Para-phenylenediamine has been repeatedly reported to cause allergic contact dermatitis. We describe a case of allergic contact dermatitis secondary to PPD in black henna. Our patient is a clear example that PPD is the allergen in black henna given that there was no reaction to the natural red henna tattoo that was applied at the same time to the palmar surfaces of the hands (Figure). Aside from the bullous reaction to black henna dye described here, other reported presentations include erythema multiforme–like and exudative erythema reactions.9,10
Contact dermatitis lesions from black henna dye can be treated with topical corticosteroids. Patients may develop residual postinflammatory hyperpigmentation or hypopigmentation, leukoderma, keloids, or scars.1,11,12
- Onder M, Atahan CA, Oztas P, et al. Temporary henna tattoo reactions in children. Int J Dermatol. 2001;40:577-579.
- Marcoux D, Couture-Trudel PM, Rboulet-Delmas G, et al. Sensitization to paraphenylenediame from a streetside temporary tattoo. Pediatr Dermatol. 2002;19:498-502.
- Hashim S, Hamza Y, Yahia B, et al. Poisoning from henna dye and para-phenylenediamine mixtures in children in Khartoum. Ann Trop Paediatr. 1992;12:3-6.
- Hijji Y, Barare B, Zhang Y. Lawsone (2- hydroxy-1, 4-naphthoquinone) as a sensitive cyanide and acetate sensor. Sensors and Actuators B: Chemical. 2012;169:106-112.
- Neri I, Guareschi E, Savoia F, et al. Childhood allergic contact dermatitis from henna tattoo. Pediatr Dermatol. 2002;19:503-505.
- Evans CC, Fleming JD. Allergic contact dermatitis from a henna tattoo. N Engl J Med. 2008;359:627.
- Belhadjali H, Akkari H, Youssef M, et al. Bullous allergic contact dermatitis to pure henna in a 3-year-old girl. Pediatr Dermatol. 2011;28:580-581.
- Najem N, Bagher Zadeh V. Allergic contact dermatitis to black henna. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:87-88.
- Sidwell RU, Francis ND, Basarab T, et al. Vesicular erythema multiforme-like reaction to para-phenylenediamine in a henna tattoo. Pediatr Dermatol. 2008;25:201-204.
- Jovanovic DL, Slavkovic-Jovanovic MR. Allergic contact dermatitis from temporary henna tattoo. J Dermatol. 2009;36:63-65.
- Valsecchi R, Leghissa P, Di Landro A, et al. Persistent leukoderma after henna tattoo. Contact Dermatitis. 2007;56:108-109.
- Gunasti S, Aksungur VL. Severe inflammatory and keloidal, allergic reaction due to para-phenylenediamine in temporary tattoos. Indian J Dermatol Venereol Leprol. 2010;76:165-167.
The Diagnosis: Allergic Contact Dermatitis to Para-phenylenediamine
To darken the color of henna and increase penetrance and staining, para-phenylenediamine (PPD) is added.1 Allergic contact dermatitis is the most common type of hypersensitivity to PPD.2 A retrospective study that examined severe adverse events from applying henna dyes in children found that angioedema of mucosal tissues was the most common severe adverse event; others included renal failure and shock.3
Black henna is associated with multiple cultural practices. For example, Indian weddings contain a henna decoration ceremony for the bride based on the belief that the longer the henna lasts, the longer the marriage lasts. Black henna is favored for this practice, as it lasts longer than red henna.

Henna (Lawsonia inermis) is a plant that contains the molecule lawsone (naphthoquinone). Lawsone has an intense affinity for keratin; as a result, lawsone is frequently added to temporary body tattoos and hair dyes to create a relatively permanent change in skin or hair color.4 Henna is mixed with hennotannic acid to release the lawsone from the plant. Lawsone and hennotannic acid rarely cause allergic reactions.1,5-7 Once applied to skin, henna takes a few hours to dry, and the resulting color is orange to red.8 Often, PPD is added to henna paste to create a black color, to speed up the drying process, and to increase its longevity.
Para-phenylenediamine has been repeatedly reported to cause allergic contact dermatitis. We describe a case of allergic contact dermatitis secondary to PPD in black henna. Our patient is a clear example that PPD is the allergen in black henna given that there was no reaction to the natural red henna tattoo that was applied at the same time to the palmar surfaces of the hands (Figure). Aside from the bullous reaction to black henna dye described here, other reported presentations include erythema multiforme–like and exudative erythema reactions.9,10
Contact dermatitis lesions from black henna dye can be treated with topical corticosteroids. Patients may develop residual postinflammatory hyperpigmentation or hypopigmentation, leukoderma, keloids, or scars.1,11,12
The Diagnosis: Allergic Contact Dermatitis to Para-phenylenediamine
To darken the color of henna and increase penetrance and staining, para-phenylenediamine (PPD) is added.1 Allergic contact dermatitis is the most common type of hypersensitivity to PPD.2 A retrospective study that examined severe adverse events from applying henna dyes in children found that angioedema of mucosal tissues was the most common severe adverse event; others included renal failure and shock.3
Black henna is associated with multiple cultural practices. For example, Indian weddings contain a henna decoration ceremony for the bride based on the belief that the longer the henna lasts, the longer the marriage lasts. Black henna is favored for this practice, as it lasts longer than red henna.

Henna (Lawsonia inermis) is a plant that contains the molecule lawsone (naphthoquinone). Lawsone has an intense affinity for keratin; as a result, lawsone is frequently added to temporary body tattoos and hair dyes to create a relatively permanent change in skin or hair color.4 Henna is mixed with hennotannic acid to release the lawsone from the plant. Lawsone and hennotannic acid rarely cause allergic reactions.1,5-7 Once applied to skin, henna takes a few hours to dry, and the resulting color is orange to red.8 Often, PPD is added to henna paste to create a black color, to speed up the drying process, and to increase its longevity.
Para-phenylenediamine has been repeatedly reported to cause allergic contact dermatitis. We describe a case of allergic contact dermatitis secondary to PPD in black henna. Our patient is a clear example that PPD is the allergen in black henna given that there was no reaction to the natural red henna tattoo that was applied at the same time to the palmar surfaces of the hands (Figure). Aside from the bullous reaction to black henna dye described here, other reported presentations include erythema multiforme–like and exudative erythema reactions.9,10
Contact dermatitis lesions from black henna dye can be treated with topical corticosteroids. Patients may develop residual postinflammatory hyperpigmentation or hypopigmentation, leukoderma, keloids, or scars.1,11,12
- Onder M, Atahan CA, Oztas P, et al. Temporary henna tattoo reactions in children. Int J Dermatol. 2001;40:577-579.
- Marcoux D, Couture-Trudel PM, Rboulet-Delmas G, et al. Sensitization to paraphenylenediame from a streetside temporary tattoo. Pediatr Dermatol. 2002;19:498-502.
- Hashim S, Hamza Y, Yahia B, et al. Poisoning from henna dye and para-phenylenediamine mixtures in children in Khartoum. Ann Trop Paediatr. 1992;12:3-6.
- Hijji Y, Barare B, Zhang Y. Lawsone (2- hydroxy-1, 4-naphthoquinone) as a sensitive cyanide and acetate sensor. Sensors and Actuators B: Chemical. 2012;169:106-112.
- Neri I, Guareschi E, Savoia F, et al. Childhood allergic contact dermatitis from henna tattoo. Pediatr Dermatol. 2002;19:503-505.
- Evans CC, Fleming JD. Allergic contact dermatitis from a henna tattoo. N Engl J Med. 2008;359:627.
- Belhadjali H, Akkari H, Youssef M, et al. Bullous allergic contact dermatitis to pure henna in a 3-year-old girl. Pediatr Dermatol. 2011;28:580-581.
- Najem N, Bagher Zadeh V. Allergic contact dermatitis to black henna. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:87-88.
- Sidwell RU, Francis ND, Basarab T, et al. Vesicular erythema multiforme-like reaction to para-phenylenediamine in a henna tattoo. Pediatr Dermatol. 2008;25:201-204.
- Jovanovic DL, Slavkovic-Jovanovic MR. Allergic contact dermatitis from temporary henna tattoo. J Dermatol. 2009;36:63-65.
- Valsecchi R, Leghissa P, Di Landro A, et al. Persistent leukoderma after henna tattoo. Contact Dermatitis. 2007;56:108-109.
- Gunasti S, Aksungur VL. Severe inflammatory and keloidal, allergic reaction due to para-phenylenediamine in temporary tattoos. Indian J Dermatol Venereol Leprol. 2010;76:165-167.
- Onder M, Atahan CA, Oztas P, et al. Temporary henna tattoo reactions in children. Int J Dermatol. 2001;40:577-579.
- Marcoux D, Couture-Trudel PM, Rboulet-Delmas G, et al. Sensitization to paraphenylenediame from a streetside temporary tattoo. Pediatr Dermatol. 2002;19:498-502.
- Hashim S, Hamza Y, Yahia B, et al. Poisoning from henna dye and para-phenylenediamine mixtures in children in Khartoum. Ann Trop Paediatr. 1992;12:3-6.
- Hijji Y, Barare B, Zhang Y. Lawsone (2- hydroxy-1, 4-naphthoquinone) as a sensitive cyanide and acetate sensor. Sensors and Actuators B: Chemical. 2012;169:106-112.
- Neri I, Guareschi E, Savoia F, et al. Childhood allergic contact dermatitis from henna tattoo. Pediatr Dermatol. 2002;19:503-505.
- Evans CC, Fleming JD. Allergic contact dermatitis from a henna tattoo. N Engl J Med. 2008;359:627.
- Belhadjali H, Akkari H, Youssef M, et al. Bullous allergic contact dermatitis to pure henna in a 3-year-old girl. Pediatr Dermatol. 2011;28:580-581.
- Najem N, Bagher Zadeh V. Allergic contact dermatitis to black henna. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:87-88.
- Sidwell RU, Francis ND, Basarab T, et al. Vesicular erythema multiforme-like reaction to para-phenylenediamine in a henna tattoo. Pediatr Dermatol. 2008;25:201-204.
- Jovanovic DL, Slavkovic-Jovanovic MR. Allergic contact dermatitis from temporary henna tattoo. J Dermatol. 2009;36:63-65.
- Valsecchi R, Leghissa P, Di Landro A, et al. Persistent leukoderma after henna tattoo. Contact Dermatitis. 2007;56:108-109.
- Gunasti S, Aksungur VL. Severe inflammatory and keloidal, allergic reaction due to para-phenylenediamine in temporary tattoos. Indian J Dermatol Venereol Leprol. 2010;76:165-167.

A 14-year-old adolescent girl presented with painful skin lesions on the dorsal aspect of the hands of 10 days’ duration. She reported having received red henna tattoo on the palmar surface of the hands and black henna tattoo on the dorsal surface of the hands 1 day prior to development of the lesions. Within 1 day of receiving the tattoo, she developed pruritus, blisters, and pain on the dorsal aspect of the hands. The palms were unaffected. Physical examination revealed erythematous, brown to black bullae and crusts that followed the contours of the henna design on the dorsal aspect of the hands. There were orange and brown henna designs on the patient’s palms, but no erythema, bullae, or induration was noted.
The Use of Sodium Sulfacetamide in Dermatology
Sodium sulfacetamide has various uses in the field of dermatology due to its anti-inflammatory and antibacterial properties. It has been shown to be effective in the management of a variety of inflammatory facial dermatoses, including papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis. We review the mechanism of action, pharmacology and formulations, clinical uses, and adverse effects of sodium sulfacetamide as a dermatologic treatment.
Mechanism of Action
Sodium sulfacetamide is a sulfonamide-type antibacterial agent. Its mechanism of action is the inhibition of bacterial dihydropteroate synthetase, which prevents the conversion of p-aminobenzoic acid to folic acid. This process causes a bacteriostatic effect on the growth of several gram-negative and gram-positive organisms, including Propionibacterium acnes.1,2
The effectiveness of sodium sulfacetamide is increased when used in combination with sulfur, which has keratolytic, antibacterial, antifungal, and antiparasitic effects. The addition of hydrocortisone has been reported to increase the effectiveness of both agents.3
Pharmacology
Sodium sulfacetamide is highly soluble at the physiologic pH of 7.4, which contributes to its high level of penetration and absorption.4 An in vitro study showed percutaneous absorption of sodium sulfacetamide to be around 4%.5 Sulfonamides are metabolized mainly by the liver and are excreted by the kidneys.
Formulations
The most common concentrations of sodium sulfacetamide and sulfur are 10% and 5%, respectively. A wide variety of sulfacetamide-containing products are available, many of which are marketed to treat specific conditions depending on additional ingredients or the type of delivery system.
Clinical Uses
Topical formulations of sodium sulfacetamide and sulfur have proven to be efficacious in the management of rosacea, with a typical regimen consisting of twice-daily application for 8 weeks.6 The sulfur in the formulation has the additional benefit of targeting Demodex mites, which are implicated as a contributing factor in some cases of rosacea.7 Sodium sulfacetamide 10%–sulfur 5% lotion was more effective in improving the erythema, papulopustules, and overall severity of rosacea as compared to metronidazole gel 0.75%.8 Other studies have reported increased efficacy when sodium sulfacetamide and topical sulfur are used along with metronidazole.9,10
Sodium sulfacetamide also has shown efficacy against acne. Its antibacterial and drying properties have been shown to decrease the number of inflammatory lesions and comedones, and in the treatment of acne vulgaris, no sensitivity reactions have been observed.2 Also, unlike topical antibiotics, cases of P acnes resistance to topical sulfur products have not been widely reported. Studies have demonstrated that twice-daily use of sodium sulfacetamide 10%–sulfur 5% for 12 weeks decreases inflammatory acne lesions by 80.4% to 83%.11,12
Seborrheic dermatitis is a common chronic infection of the skin caused by Malassezia species. One study investigated the use of sodium sulfacetamide ointment and soap to treat seborrheic dermatitis and found that the condition was either improved or completely controlled in 93% (71/76) of cases.4 Sodium sulfacetamide lotion was an effective treatment of seborrheic dermatitis in 89% (54/61) of patients with scalp involvement and 68% (30/44) of patients with glabrous skin involvement.13
Perioral dermatitis is characterized by groups of erythematous papules and pustules localized around the mouth. The use of topical sodium sulfacetamide along with oral tetracyclines has been demonstrated to consistently clear lesions in most patients with perioral dermatitis.14 Sodium sulfacetamide is unique in that it is not associated with the excessive erythema and irritation often found with retinoic acid and benzoyl peroxide.15 Unfortunately, however, there have been no well-controlled trials to compare the efficacy of sodium sulfacetamide to other topical therapies for this condition.
Adverse Effects
Adverse effects from sodium sulfacetamide are rare and generally are limited to cutaneous reactions including dryness, erythema, pruritus, and discomfort.1 Periocular use of sodium sulfacetamide can cause conjunctival irritation. One study reported that 19% (6/31) of patients experienced local reactions but most were considered mild.9 Rare but serious reactions including erythema multiforme and Stevens-Johnson syndrome have been reported from ophthalmic use.16,17
A common limiting factor to sodium sulfacetamide preparations that include elemental sulfur is the offensive smell, which has hindered patient compliance in the past; however, pharmaceutical companies have attempted to create more tolerable products without the odor.10 One study found that the tolerability of a sodium sulfacetamide 10%–sulfur 5% foam using a rinse-off method of application was excellent, with only 33% (8/24) of participants commenting on the smell.18 Another limiting factor of sodium sulfacetamide preparations containing sulfur is orange-brown discoloration when combined with benzoyl peroxide, which does not affect the skin but may stain clothing.19
Sodium sulfacetamide is rendered less effective when combined with silver-containing products.20 No other notable drug interactions are known; however, oral sulfonamides are known to interact with several drugs, including cyclosporine and phenytoin.21,22
Contraindications
Sodium sulfacetamide is contraindicated in patients with known hypersensitivity to sulfonamides, sulfur, or any other component of the preparation. It is a pregnancy category C drug, and pregnant women should only use sodium sulfacetamide if it is the only modality to treat the condition or the benefits outweigh the risks. Although there are no known reports of problems related to topical sodium sulfacetamide during pregnancy, the use of oral sulfonamides during pregnancy can increase the risk for neonatal jaundice.23 Likewise, caution should be exercised in prescribing this product to nursing women, as systemic sulfonamide antibacterials are well known to cause kernicterus in nursing neonates.1
Conclusion
The efficacy and safety of sodium sulfacetamide, used alone or in combination with sulfur, has been demonstrated in the treatment of rosacea, acne, seborrheic dermatitis, and perioral dermatitis. Advances in formulation technology to decrease odor and irritation have allowed for more use of this product. Further studies will help elucidate the role that sodium sulfacetamide should play in the treatment of inflammatory dermatoses in comparison to other available products.
1. Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. Am J Clin Dermatol. 2003;4:473-492.
2. Gupta AK, Nicol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
3. Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders; 2012:445-459.
4. Duemling WM. Sodium sulfacetamide in topical therapy. AMA Arch Derm Syphilol. 1954;69:75-82.
5. Sodium sulfacetamide. Drugs.com Web site. http://drugs.com/pro/sodium-sulfacetamide.html. Revised December 2012. Accessed June 16, 2015.
6. Sauder DN, Miller R, Gratton D, et al. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double-blind study. J Dermatol Treat. 1997;8:79-85.
7. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
8. Lebwohl MG, Medansky RS, Russo CL, et al. The comparative efficacy of sodium sulfacetamide 10%/sulfur 5% lotion and metronidazole 0.75% gel in the treatment of rosacea. J Geriatr Dermatol. 1995;3:183-185.
9. Nally JB, Berson DS. Topical therapies for rosacea. J Drugs Dermatol. 2006;5:23-26.
10. Pelle MT, Crawford GH, James WD. Rosacea II: therapy. J Am Acad Dermatol. 2004;51:499-512.
11. Tarimci N, Sener S, Kilinç T. Topical sodium sulfacetamide/sulfur lotion. J Clin Pharm Ther. 1997;22:301.
12. Breneman DL, Ariano MC. Successful treatment of acne vulgaris in women with a new topical sodium sulfacetamide/sulfur lotion. Int J Dermatol. 1993;32:365-367.
13. Whelan ST. Sodium sulfacetamide for seborrheic dermatitis. AMA Arch Derm. 1955;71:724.
14. Bendl BJ. Perioral dermatitis: etiology and treatment. Cutis. 1976;17:903-908.
15. Olansky S. Old drug—in a new system—revisited. Cutis. 1977;19:852-854.
16. Genvert GI, Cohen EJ, Donnenfeld ED, et al. Erythema multiforme after use of topical sulfacetamide. Am J Ophthalmol. 1985;99:465-468.
17. Rubin Z. Ophthalmic sulfonamide-induced Stevens-Johnson syndrome. Arch Dermatol. 1977;113:235-236.
18. Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-246.
19. Dubina MI, Fleischer AB. Interaction of topical sulfacetamide and topical dapsone with benzoyl peroxide. Arch Dermatol. 2009;145:1027-1029.
20. Sodium sulfacetamide – sulfacetamide sodium liquid. DailyMed Web site. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0d92c55b-5b54-4f5d-8921-24e4e877ae50. Accessed June 17, 2015.
21. Spes CH, Angermann CE, Stempfle HU, et al. Sulfadiazine therapy for toxoplasmosis in heart transplant recipients decreases cyclosporine concentration. Clin Investig. 1992;70:752-754.
22. Hansen JM, Kampmann JP, Siersbaek-Nielsen K, et al. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand Suppl. 1979;624:106-110.
23. Bradley JS, Sauberan JB. Antimicrobial agents. In: Long SS, Pickering LK, Prober CG. Principles and Practices of Pediatric Infectious Diseases. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1453-1483.
Sodium sulfacetamide has various uses in the field of dermatology due to its anti-inflammatory and antibacterial properties. It has been shown to be effective in the management of a variety of inflammatory facial dermatoses, including papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis. We review the mechanism of action, pharmacology and formulations, clinical uses, and adverse effects of sodium sulfacetamide as a dermatologic treatment.
Mechanism of Action
Sodium sulfacetamide is a sulfonamide-type antibacterial agent. Its mechanism of action is the inhibition of bacterial dihydropteroate synthetase, which prevents the conversion of p-aminobenzoic acid to folic acid. This process causes a bacteriostatic effect on the growth of several gram-negative and gram-positive organisms, including Propionibacterium acnes.1,2
The effectiveness of sodium sulfacetamide is increased when used in combination with sulfur, which has keratolytic, antibacterial, antifungal, and antiparasitic effects. The addition of hydrocortisone has been reported to increase the effectiveness of both agents.3
Pharmacology
Sodium sulfacetamide is highly soluble at the physiologic pH of 7.4, which contributes to its high level of penetration and absorption.4 An in vitro study showed percutaneous absorption of sodium sulfacetamide to be around 4%.5 Sulfonamides are metabolized mainly by the liver and are excreted by the kidneys.
Formulations
The most common concentrations of sodium sulfacetamide and sulfur are 10% and 5%, respectively. A wide variety of sulfacetamide-containing products are available, many of which are marketed to treat specific conditions depending on additional ingredients or the type of delivery system.
Clinical Uses
Topical formulations of sodium sulfacetamide and sulfur have proven to be efficacious in the management of rosacea, with a typical regimen consisting of twice-daily application for 8 weeks.6 The sulfur in the formulation has the additional benefit of targeting Demodex mites, which are implicated as a contributing factor in some cases of rosacea.7 Sodium sulfacetamide 10%–sulfur 5% lotion was more effective in improving the erythema, papulopustules, and overall severity of rosacea as compared to metronidazole gel 0.75%.8 Other studies have reported increased efficacy when sodium sulfacetamide and topical sulfur are used along with metronidazole.9,10
Sodium sulfacetamide also has shown efficacy against acne. Its antibacterial and drying properties have been shown to decrease the number of inflammatory lesions and comedones, and in the treatment of acne vulgaris, no sensitivity reactions have been observed.2 Also, unlike topical antibiotics, cases of P acnes resistance to topical sulfur products have not been widely reported. Studies have demonstrated that twice-daily use of sodium sulfacetamide 10%–sulfur 5% for 12 weeks decreases inflammatory acne lesions by 80.4% to 83%.11,12
Seborrheic dermatitis is a common chronic infection of the skin caused by Malassezia species. One study investigated the use of sodium sulfacetamide ointment and soap to treat seborrheic dermatitis and found that the condition was either improved or completely controlled in 93% (71/76) of cases.4 Sodium sulfacetamide lotion was an effective treatment of seborrheic dermatitis in 89% (54/61) of patients with scalp involvement and 68% (30/44) of patients with glabrous skin involvement.13
Perioral dermatitis is characterized by groups of erythematous papules and pustules localized around the mouth. The use of topical sodium sulfacetamide along with oral tetracyclines has been demonstrated to consistently clear lesions in most patients with perioral dermatitis.14 Sodium sulfacetamide is unique in that it is not associated with the excessive erythema and irritation often found with retinoic acid and benzoyl peroxide.15 Unfortunately, however, there have been no well-controlled trials to compare the efficacy of sodium sulfacetamide to other topical therapies for this condition.
Adverse Effects
Adverse effects from sodium sulfacetamide are rare and generally are limited to cutaneous reactions including dryness, erythema, pruritus, and discomfort.1 Periocular use of sodium sulfacetamide can cause conjunctival irritation. One study reported that 19% (6/31) of patients experienced local reactions but most were considered mild.9 Rare but serious reactions including erythema multiforme and Stevens-Johnson syndrome have been reported from ophthalmic use.16,17
A common limiting factor to sodium sulfacetamide preparations that include elemental sulfur is the offensive smell, which has hindered patient compliance in the past; however, pharmaceutical companies have attempted to create more tolerable products without the odor.10 One study found that the tolerability of a sodium sulfacetamide 10%–sulfur 5% foam using a rinse-off method of application was excellent, with only 33% (8/24) of participants commenting on the smell.18 Another limiting factor of sodium sulfacetamide preparations containing sulfur is orange-brown discoloration when combined with benzoyl peroxide, which does not affect the skin but may stain clothing.19
Sodium sulfacetamide is rendered less effective when combined with silver-containing products.20 No other notable drug interactions are known; however, oral sulfonamides are known to interact with several drugs, including cyclosporine and phenytoin.21,22
Contraindications
Sodium sulfacetamide is contraindicated in patients with known hypersensitivity to sulfonamides, sulfur, or any other component of the preparation. It is a pregnancy category C drug, and pregnant women should only use sodium sulfacetamide if it is the only modality to treat the condition or the benefits outweigh the risks. Although there are no known reports of problems related to topical sodium sulfacetamide during pregnancy, the use of oral sulfonamides during pregnancy can increase the risk for neonatal jaundice.23 Likewise, caution should be exercised in prescribing this product to nursing women, as systemic sulfonamide antibacterials are well known to cause kernicterus in nursing neonates.1
Conclusion
The efficacy and safety of sodium sulfacetamide, used alone or in combination with sulfur, has been demonstrated in the treatment of rosacea, acne, seborrheic dermatitis, and perioral dermatitis. Advances in formulation technology to decrease odor and irritation have allowed for more use of this product. Further studies will help elucidate the role that sodium sulfacetamide should play in the treatment of inflammatory dermatoses in comparison to other available products.
Sodium sulfacetamide has various uses in the field of dermatology due to its anti-inflammatory and antibacterial properties. It has been shown to be effective in the management of a variety of inflammatory facial dermatoses, including papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis. We review the mechanism of action, pharmacology and formulations, clinical uses, and adverse effects of sodium sulfacetamide as a dermatologic treatment.
Mechanism of Action
Sodium sulfacetamide is a sulfonamide-type antibacterial agent. Its mechanism of action is the inhibition of bacterial dihydropteroate synthetase, which prevents the conversion of p-aminobenzoic acid to folic acid. This process causes a bacteriostatic effect on the growth of several gram-negative and gram-positive organisms, including Propionibacterium acnes.1,2
The effectiveness of sodium sulfacetamide is increased when used in combination with sulfur, which has keratolytic, antibacterial, antifungal, and antiparasitic effects. The addition of hydrocortisone has been reported to increase the effectiveness of both agents.3
Pharmacology
Sodium sulfacetamide is highly soluble at the physiologic pH of 7.4, which contributes to its high level of penetration and absorption.4 An in vitro study showed percutaneous absorption of sodium sulfacetamide to be around 4%.5 Sulfonamides are metabolized mainly by the liver and are excreted by the kidneys.
Formulations
The most common concentrations of sodium sulfacetamide and sulfur are 10% and 5%, respectively. A wide variety of sulfacetamide-containing products are available, many of which are marketed to treat specific conditions depending on additional ingredients or the type of delivery system.
Clinical Uses
Topical formulations of sodium sulfacetamide and sulfur have proven to be efficacious in the management of rosacea, with a typical regimen consisting of twice-daily application for 8 weeks.6 The sulfur in the formulation has the additional benefit of targeting Demodex mites, which are implicated as a contributing factor in some cases of rosacea.7 Sodium sulfacetamide 10%–sulfur 5% lotion was more effective in improving the erythema, papulopustules, and overall severity of rosacea as compared to metronidazole gel 0.75%.8 Other studies have reported increased efficacy when sodium sulfacetamide and topical sulfur are used along with metronidazole.9,10
Sodium sulfacetamide also has shown efficacy against acne. Its antibacterial and drying properties have been shown to decrease the number of inflammatory lesions and comedones, and in the treatment of acne vulgaris, no sensitivity reactions have been observed.2 Also, unlike topical antibiotics, cases of P acnes resistance to topical sulfur products have not been widely reported. Studies have demonstrated that twice-daily use of sodium sulfacetamide 10%–sulfur 5% for 12 weeks decreases inflammatory acne lesions by 80.4% to 83%.11,12
Seborrheic dermatitis is a common chronic infection of the skin caused by Malassezia species. One study investigated the use of sodium sulfacetamide ointment and soap to treat seborrheic dermatitis and found that the condition was either improved or completely controlled in 93% (71/76) of cases.4 Sodium sulfacetamide lotion was an effective treatment of seborrheic dermatitis in 89% (54/61) of patients with scalp involvement and 68% (30/44) of patients with glabrous skin involvement.13
Perioral dermatitis is characterized by groups of erythematous papules and pustules localized around the mouth. The use of topical sodium sulfacetamide along with oral tetracyclines has been demonstrated to consistently clear lesions in most patients with perioral dermatitis.14 Sodium sulfacetamide is unique in that it is not associated with the excessive erythema and irritation often found with retinoic acid and benzoyl peroxide.15 Unfortunately, however, there have been no well-controlled trials to compare the efficacy of sodium sulfacetamide to other topical therapies for this condition.
Adverse Effects
Adverse effects from sodium sulfacetamide are rare and generally are limited to cutaneous reactions including dryness, erythema, pruritus, and discomfort.1 Periocular use of sodium sulfacetamide can cause conjunctival irritation. One study reported that 19% (6/31) of patients experienced local reactions but most were considered mild.9 Rare but serious reactions including erythema multiforme and Stevens-Johnson syndrome have been reported from ophthalmic use.16,17
A common limiting factor to sodium sulfacetamide preparations that include elemental sulfur is the offensive smell, which has hindered patient compliance in the past; however, pharmaceutical companies have attempted to create more tolerable products without the odor.10 One study found that the tolerability of a sodium sulfacetamide 10%–sulfur 5% foam using a rinse-off method of application was excellent, with only 33% (8/24) of participants commenting on the smell.18 Another limiting factor of sodium sulfacetamide preparations containing sulfur is orange-brown discoloration when combined with benzoyl peroxide, which does not affect the skin but may stain clothing.19
Sodium sulfacetamide is rendered less effective when combined with silver-containing products.20 No other notable drug interactions are known; however, oral sulfonamides are known to interact with several drugs, including cyclosporine and phenytoin.21,22
Contraindications
Sodium sulfacetamide is contraindicated in patients with known hypersensitivity to sulfonamides, sulfur, or any other component of the preparation. It is a pregnancy category C drug, and pregnant women should only use sodium sulfacetamide if it is the only modality to treat the condition or the benefits outweigh the risks. Although there are no known reports of problems related to topical sodium sulfacetamide during pregnancy, the use of oral sulfonamides during pregnancy can increase the risk for neonatal jaundice.23 Likewise, caution should be exercised in prescribing this product to nursing women, as systemic sulfonamide antibacterials are well known to cause kernicterus in nursing neonates.1
Conclusion
The efficacy and safety of sodium sulfacetamide, used alone or in combination with sulfur, has been demonstrated in the treatment of rosacea, acne, seborrheic dermatitis, and perioral dermatitis. Advances in formulation technology to decrease odor and irritation have allowed for more use of this product. Further studies will help elucidate the role that sodium sulfacetamide should play in the treatment of inflammatory dermatoses in comparison to other available products.
1. Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. Am J Clin Dermatol. 2003;4:473-492.
2. Gupta AK, Nicol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
3. Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders; 2012:445-459.
4. Duemling WM. Sodium sulfacetamide in topical therapy. AMA Arch Derm Syphilol. 1954;69:75-82.
5. Sodium sulfacetamide. Drugs.com Web site. http://drugs.com/pro/sodium-sulfacetamide.html. Revised December 2012. Accessed June 16, 2015.
6. Sauder DN, Miller R, Gratton D, et al. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double-blind study. J Dermatol Treat. 1997;8:79-85.
7. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
8. Lebwohl MG, Medansky RS, Russo CL, et al. The comparative efficacy of sodium sulfacetamide 10%/sulfur 5% lotion and metronidazole 0.75% gel in the treatment of rosacea. J Geriatr Dermatol. 1995;3:183-185.
9. Nally JB, Berson DS. Topical therapies for rosacea. J Drugs Dermatol. 2006;5:23-26.
10. Pelle MT, Crawford GH, James WD. Rosacea II: therapy. J Am Acad Dermatol. 2004;51:499-512.
11. Tarimci N, Sener S, Kilinç T. Topical sodium sulfacetamide/sulfur lotion. J Clin Pharm Ther. 1997;22:301.
12. Breneman DL, Ariano MC. Successful treatment of acne vulgaris in women with a new topical sodium sulfacetamide/sulfur lotion. Int J Dermatol. 1993;32:365-367.
13. Whelan ST. Sodium sulfacetamide for seborrheic dermatitis. AMA Arch Derm. 1955;71:724.
14. Bendl BJ. Perioral dermatitis: etiology and treatment. Cutis. 1976;17:903-908.
15. Olansky S. Old drug—in a new system—revisited. Cutis. 1977;19:852-854.
16. Genvert GI, Cohen EJ, Donnenfeld ED, et al. Erythema multiforme after use of topical sulfacetamide. Am J Ophthalmol. 1985;99:465-468.
17. Rubin Z. Ophthalmic sulfonamide-induced Stevens-Johnson syndrome. Arch Dermatol. 1977;113:235-236.
18. Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-246.
19. Dubina MI, Fleischer AB. Interaction of topical sulfacetamide and topical dapsone with benzoyl peroxide. Arch Dermatol. 2009;145:1027-1029.
20. Sodium sulfacetamide – sulfacetamide sodium liquid. DailyMed Web site. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0d92c55b-5b54-4f5d-8921-24e4e877ae50. Accessed June 17, 2015.
21. Spes CH, Angermann CE, Stempfle HU, et al. Sulfadiazine therapy for toxoplasmosis in heart transplant recipients decreases cyclosporine concentration. Clin Investig. 1992;70:752-754.
22. Hansen JM, Kampmann JP, Siersbaek-Nielsen K, et al. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand Suppl. 1979;624:106-110.
23. Bradley JS, Sauberan JB. Antimicrobial agents. In: Long SS, Pickering LK, Prober CG. Principles and Practices of Pediatric Infectious Diseases. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1453-1483.
1. Akhavan A, Bershad S. Topical acne drugs: review of clinical properties, systemic exposure, and safety. Am J Clin Dermatol. 2003;4:473-492.
2. Gupta AK, Nicol K. The use of sulfur in dermatology. J Drugs Dermatol. 2004;3:427-431.
3. Motaparthi K, Hsu S. Topical antibacterial agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders; 2012:445-459.
4. Duemling WM. Sodium sulfacetamide in topical therapy. AMA Arch Derm Syphilol. 1954;69:75-82.
5. Sodium sulfacetamide. Drugs.com Web site. http://drugs.com/pro/sodium-sulfacetamide.html. Revised December 2012. Accessed June 16, 2015.
6. Sauder DN, Miller R, Gratton D, et al. The treatment of rosacea: the safety and efficacy of sodium sulfacetamide 10% and sulfur 5% lotion (Novacet) is demonstrated in a double-blind study. J Dermatol Treat. 1997;8:79-85.
7. Trumbore MW, Goldstein JA, Gurge RM. Treatment of papulopustular rosacea with sodium sulfacetamide 10%/sulfur 5% emollient foam. J Drugs Dermatol. 2009;8:299-304.
8. Lebwohl MG, Medansky RS, Russo CL, et al. The comparative efficacy of sodium sulfacetamide 10%/sulfur 5% lotion and metronidazole 0.75% gel in the treatment of rosacea. J Geriatr Dermatol. 1995;3:183-185.
9. Nally JB, Berson DS. Topical therapies for rosacea. J Drugs Dermatol. 2006;5:23-26.
10. Pelle MT, Crawford GH, James WD. Rosacea II: therapy. J Am Acad Dermatol. 2004;51:499-512.
11. Tarimci N, Sener S, Kilinç T. Topical sodium sulfacetamide/sulfur lotion. J Clin Pharm Ther. 1997;22:301.
12. Breneman DL, Ariano MC. Successful treatment of acne vulgaris in women with a new topical sodium sulfacetamide/sulfur lotion. Int J Dermatol. 1993;32:365-367.
13. Whelan ST. Sodium sulfacetamide for seborrheic dermatitis. AMA Arch Derm. 1955;71:724.
14. Bendl BJ. Perioral dermatitis: etiology and treatment. Cutis. 1976;17:903-908.
15. Olansky S. Old drug—in a new system—revisited. Cutis. 1977;19:852-854.
16. Genvert GI, Cohen EJ, Donnenfeld ED, et al. Erythema multiforme after use of topical sulfacetamide. Am J Ophthalmol. 1985;99:465-468.
17. Rubin Z. Ophthalmic sulfonamide-induced Stevens-Johnson syndrome. Arch Dermatol. 1977;113:235-236.
18. Draelos ZD. The multifunctionality of 10% sodium sulfacetamide, 5% sulfur emollient foam in the treatment of inflammatory facial dermatoses. J Drugs Dermatol. 2010;9:234-246.
19. Dubina MI, Fleischer AB. Interaction of topical sulfacetamide and topical dapsone with benzoyl peroxide. Arch Dermatol. 2009;145:1027-1029.
20. Sodium sulfacetamide – sulfacetamide sodium liquid. DailyMed Web site. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0d92c55b-5b54-4f5d-8921-24e4e877ae50. Accessed June 17, 2015.
21. Spes CH, Angermann CE, Stempfle HU, et al. Sulfadiazine therapy for toxoplasmosis in heart transplant recipients decreases cyclosporine concentration. Clin Investig. 1992;70:752-754.
22. Hansen JM, Kampmann JP, Siersbaek-Nielsen K, et al. The effect of different sulfonamides on phenytoin metabolism in man. Acta Med Scand Suppl. 1979;624:106-110.
23. Bradley JS, Sauberan JB. Antimicrobial agents. In: Long SS, Pickering LK, Prober CG. Principles and Practices of Pediatric Infectious Diseases. 4th ed. Philadelphia, PA: Elsevier Saunders; 2012:1453-1483.
Practice Points
- Sodium sulfacetamide is a useful agent in the management of papulopustular rosacea, acne vulgaris, seborrheic dermatitis, and perioral dermatitis.
- Adverse effects are rare and generally are limited to dryness, erythema, pruritus, and discomfort.
Stratum Corneum Absorption Kinetics of 2 Potent Topical Corticosteroid Formulations: A Pilot Study
The active ingredient of any pharmaceutical product is responsible for the agent’s efficacy and safety profile. This ingredient is extensively studied in clinical trials and evaluated by the US Food and Drug Administration before the product is commercially available. In dermatologic products, especially those for treating dermatoses, the vehicle in which the active ingredient is formulated also plays a role in drug delivery and indirectly impacts therapeutic outcomes, unlike excipients in oral medications. Topical vehicles must be stable, provide a suitable environment that will not degrade the active ingredient or affect its efficacy, and be cosmetically acceptable.1
Topical vehicles are formulated to maintain the stability of the active ingredient and allow it to readily penetrate the skin and reach its target area with minimal absorption into the bloodstream, thus avoiding systemic adverse events. A variety of vehicles can exist for a single active ingredient to accommodate different phases of disease and different anatomical sites where the disease may occur.2 For example, alcohol-based vehicles, sprays, and foams are preferred for the scalp where evaporation of the vehicle is beneficial to prevent greasiness of the hair, while ointments may be preferred due to their occlusive nature for areas with xerotic or thick skin from dermatoses.
Cosmetic acceptability of the vehicle may influence patient adherence to therapy. Housman et al3 assessed a variety of products formulated in different vehicles (ie, solutions, foams, emollients, gels, creams, ointments) for the treatment of psoriasis. Patients with psoriasis applied each test product to a quarter-sized area of normal skin on the forearm using a cotton swab and completed a preference questionnaire. By far, respondents significantly preferred solutions and foams over creams, gels, and ointments (P<.01). Side effects were rated to be the most important characteristics of topical therapy, followed by time needed for application, ease of application, and messiness.3 Presumably, if patients are frustrated with the topical product that they are using, adherence to the prescribed dosage and application instructions will diminish over time, leading to suboptimal steady-state levels of the product. If appropriate levels of the drug are not present at the target site, treatment will not be successful.
Steady-state levels of a topical drug at the site of action also are maintained via appropriate application frequency, most commonly once to 4 times daily for dermatologic products. Fluocinonide and halcinonide are class II (potent) corticosteroids indicated for the relief of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses and usually are administered at least twice daily. In double-blind clinical studies comparing both products in the treatment of psoriasis, halcinonide resulted in more improved outcomes than fluocinonide.4-6 Sudilovsky and Clewe4 studied 140 patients with moderate to severe psoriasis. After 3 weeks of treatment, 44% showed superior results with halcinonide, 27% showed superior results with fluocinonide, 26% showed equal results with both products, and 3% showed no relief.4 Similarly, Close5 reported that 61% of patients showed superior results with halcinonide, 25% showed superior results with fluocinonide, 10% showed equal results with both products, and 4% showed no relief (N=50). Lynfield and Watsky6 reported that 56% of patients with severe psoriasis who were treated with halcinonide for 2 weeks showed improvement to normal or slight inflammation compared to 44% of patients treated with fluocinonide (N=59). All 3 studies used cream formulations of halcinonide and fluocinonide.
Recently, halcinonide cream was shown to have an immediate release into the stratum corneum that peaked within 1 hour of application and remained elevated for 6 hours before beginning to decline.7 These results support a biphasic release of halcinonide, which is in agreement with its formulation—that halcinonide exists in both a solution phase for immediate release into the skin and in a suspension phase that allows a sustained release after equilibrium is reached between the solution and suspension phases.8 Fluocinonide is not known to be formulated in a similar way. Its vehicle composition and penetration into the skin could explain the superior efficacy of halcinonide versus fluocinonide.
The current pilot study was conducted to compare the release pattern of fluocinonide cream versus halcinonide cream into the stratum corneum using an in vivo, noninvasive method. Results for halcin-onide have been previously published.7
Methods
Participants were sequestered in a controlled environment for the entire day to allow the skin to equilibrate prior to product application. The methodology for the application and quantification of halcinonide cream 0.1% into the stratum corneum of 5 participants using a tape-stripping protocol has been described elsewhere.7 Concordia Clinical Research institutional review board (Cedar Knolls, New Jersey) approved this study, which was conducted at Dermatology Consulting Services (High Point, North Carolina).
A 0.1-g dose of generic fluocinonide cream 0.05% was applied to four 2.5-cm circular sites on the forearm in 5 participants with normal skin until completely absorbed. Circular tape strips were subsequently placed on the application site at 1, 3, 6, and 9 hours posttreatment and were held for 10 seconds with a controlled pressure plunger to ensure adequate and consistent contact between the tape strip and the skin. The tape strip was removed with forceps, rolled with the skin scale inside, and placed in a glass vial. This procedure was repeated 6 times at 1 of 4 sites with a new tape strip at each time point to obtain samples from deeper skin layers. A total of 24 tape strips were collected from each participant.
All vials were frozen at -20°C and were shipped overnight to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University (Flagstaff, Arizona) for mass spectroscopy evaluation. Once received at the outside facility, the vials were stored at -20°C until analysis. Each sample was spiked with a known quantity of an appropriate reference standard and extracted with 1 mL acetonitrile at room temperature for 1 minute with agitation. New unused tape strips were spiked with a small amount of fluocinonide reference standard for extraction efficiency.
Extracts were evaporated to dryness under nitrogen gas, resuspended in 200 µL chromatography solvent, and quantified using liquid chromatography–mass spectrometry. To remove the skin scale from the tape strips, 10 mL of a solvent solution of 0.1 mg/mL fludrocortisone acetate in acetonitrile was dispensed into a 4 dram vial containing the tape strip. The vials were ultrasonicated and shaken for 10 to 15 minutes, and the samples were further diluted to 100-fold and were inverted several times to ensure complete dissolution of fluocinonide before liquid chromatography–mass spectrometry.
A standard curve ranging from the lower limit of quantification to the upper limit of quantification for the fluocinonide reference was used to determine the quantity of fluocinonide in each of the tape strips. Once the lower limit of quantification was reached in a given set of tape strip samples (1-, 3-, 6-, and 9-hour samples), the next 2 sequential tape strips in that set were analyzed to confirm fluocinonide was not detectable in deeper layers. Standard quality controls were analyzed to ensure run-to-run and sample-to-sample accuracy.
Each sample was analyzed in duplicate; 10 mg fluocinonide was used as a reference standard. The minimum detectable concentration of fluocinonide was 1 ng/mL.
Results
As expected, tape strip 1 from each participant contained the highest concentration of fluocinonide. This strip corresponded to the most superficial layer of skin. Concentrations decreased in deeper skin layers, as detected in strips 2 to 6.
In general, the average concentration of fluocin-onide in strip 1 for all 5 participants was highest at hour 1, with a subsequent decline at hours 3, 6, and 9; however, participant 1 showed a second peak in fluocinonide concentration at hour 6 (Figure 1). When the fluocinonide concentration in strips 1 to 6 was averaged for each participant at each time point, similar results were obtained: a general decline after hour 1, but a second prominent peak at hour 6 in participant 1 only. In participant 1, the average fluocinonide concentration for strips 1 to 6 was 393 ng/mL at hour 1 and declined to 208 ng/mL at hour 3; it increased to 451 ng/mL at hour 6 before declining again to 202 ng/mL at hour 9.
Because participant 1 was the only one to exhibit a second peak of fluocinonide concentration, it appears that measurements obtained from this participant may be outliers. When removing partici-pant 1 from the analysis of fluocinonide concentration in strip 1 at each time point, a clear decline is evident from hour 1 to hour 9 (Figure 2A, red line [partici-pants 2–5] vs blue line [participants 1–5]).
When the average concentration of fluocinonide was calculated in strips 1 to 6 from all participants, there was a general steady decline after hour 1 with a slight increase of 25 ng/mL at hour 6 (Figure 2B, blue line). This increase is due to the measurements obtained from participant 1; however, if partici-pant 1 is removed from the analysis, a constant decline is observed from hour 1 to hour 9 (Figure 2B, red line).
|
A prior study evaluated the penetration and absorption of halcinonide in the stratum corneum.7 In summary, halcinonide concentration peaked at hour 1 following application and remained elevated to hour 6, before beginning a slow decline. The average concentration of halcinonide from all participants in strips 1 to 6 reached 1350 ng/mL at hour 1, remained within 93% to 97% of this level (1253–1303 ng/mL) for the next 5 hours, and declined only 29% from the peak at hour 1 to hour 9 (958 ng/mL)(Figure 3, blue line).7 In contrast, the fluocinonide concentration in participants 2 to 5 from the current study reached 190 ng/mL at hour 1 and steadily declined 53% to 89 ng/mL by hour 9 (Figure 3, red line).
Two participants from the prior halcinonide study also were enrolled in the current fluocinonide study (referred to as participant A and B). In general, halcinonide levels in both participants remained elevated for 6 hours after application and declined 27.5% and 35.5%, respectively, by hour 9 (Figure 4). Participant A experienced a 20.5% dip in halcinonide concentration at hour 3 followed by an increase at hour 6; however, the halcinonide concentration at hour 9 was similar to hour 3.7 In contrast, fluocin-onide concentrations for these participants peaked at 1 hour and clearly declined approximately 60% over the next 8 hours.
Comment
The release of both fluocinonide and halcinonide into the skin was evaluated using dermal tape stripping on 4 sites on the forearms of healthy individuals. Cream formulations of each corticosteroid were evaluated in 5 participants, with 2 participants receiving both formulations during different study periods. In the prior study with halcinonide, the stratum corneum exhibited the highest concentration of the corticosteroid, with substantial declines beyond strip 6 (ie, strips 7–20).7 For this reason, only strips 1 to 6 were evaluated for corticosteroid penetration and absorption.
Results from strip 1 indicated immediate absorption of corticosteroid (fluocinonide and halcinonide) into the skin. Unlike the release of halcinonide, which demonstrated a clear sustained release over 6 hours before decreasing,7 fluocinonide concentrations began declining immediately after peaking at hour 1 and continued to decline up to hour 9. Only participant 1 exhibited a second peak of fluocinonide concentration at hour 6; the rest of the participants did not. This second peak is most likely an anomaly due to the small number of participants rather than a true elevation.
Given the rapid decline of fluocinonide concentration over the 9 hours compared with the more gradual decline of halcinonide concentration, there appears to be no evidence of a biphasic sustained release of fluocinonide from its vehicle. This difference in release pattern from each corticosteroid’s respective vehicle may explain in part the different clinical outcomes in comparative studies.4-6
It is known that vehicle composition affects corticosteroid diffusion from the vehicle to the skin surface and subsequent penetration into the skin.9 Either process can determine the overall effectiveness of the product. Ayres and Hooper10 evaluated the penetration of 4 topical preparations of cortisol. Product 1 delivered 16 times more cortisol to the skin than product 2, 8 times more than product 3, and 3 times more than product 4. Because all the preparations contained cortisol-free alcohol, these differences were attributed to the vehicle in which the cortisol was formulated. Products 1 and 4 both contained 10% urea, but the urea in product 1 was a powder in a cream base and the urea in product 4 was in a stabilizing emulsified base. Product 2 contained a propylene glycol/water base and product 3 was a water-miscible cream.10
Generic corticosteroid products have been observed in clinical practice and have been shown in vasoconstriction assays to be less and more potent than their brand-name equivalents.2,11 Vasoconstriction assays are the standard for assessing the potency of topical corticosteroids and predicting their clinical efficacy.2 One study reported significant differences in therapeutic effectiveness between generic formulations and their brand-name equivalents.12 Kenalog cream 0.1% (multiple manufacturers) was significantly more potent than any of the generic triamcinolone creams tested (P<.05); in fact, Kenalog cream 0.025% (multiple manufacturers) was statistically superior to all the generic triamcinolone creams 0.1%. Moreover, Artistocort A ointment 0.1% (Lederele Laboratories) and Valisone cream 0.1% (Schering Corporation) also were more potent than their generics at the same concentration in the same vehicle type.12 A second study also observed that 2 of 6 generic formulations had significantly less vasoconstriction than their respective brand-name formulations.11 A brand-name betamethasone valerate cream produced significantly greater vasoconstriction than its generic equivalent, and a brand-name betamethasone dipropionate cream produced greater vasoconstriction than one generic and equal vasoconstriction to another generic. Additionally, the vasoconstriction measured with Diprosone was greater than that measured with Diprolene, another brand-name product of betamethasone dipropionate.11 Diprosone and Diprolene differ in their vehicle content. The latter, a class I corticosteroid, contains a modified vehicle high in propylene glycol, whereas the former contains less propylene glycol and thus is classified as a class III corticosteroid. Propylene glycol allows hydrophobic molecules such as corticosteroids to dissolve more fully in the vehicle.12
Ostrenga et al1 studied the solubility of corticosteroids in different vehicles and, as expected, corticosteroids that fully solubilized in the vehicle exhibited better penetration into the skin on assessment with vasoconstriction assays. Corticosteroids in a suspension, on the other hand, showed slower penetration into the skin.1,13 A balance between the solution and suspension phase would allow a drug to rapidly penetrate the skin upon application, and when this pool of solubilized drug was depleted, additional drug could penetrate into the skin from the suspension phase. Based on the tape strip results from the current study it appears that halcinonide, which is manufactured in a biphasic formulation, follows this pattern of penetration and absorption into the stratum corneum. In contrast, fluocinonide appears to exist in a soluble state without much, if any, amount in a suspension phase because it had no sustained release during the 9 hours after application.
Common belief among dermatologists is that long-term use of corticosteroids leads to tachyphylaxis,14 which can be attributed to poor patient adherence. If patients skip doses, then the steady state of the product at the target site is not maintained. It is interesting to speculate that using agents with more sustained release beyond the time of application (such as halcinonide) may preserve steady-state levels even when patients are neglectful of the next medication application. Corticosteroids that work in 2 phases such as halcinonide may minimize tachyphylaxis experienced with prolonged use of corticosteroids.
Fluocinonide and halcinonide are both class II high-potency corticosteroids as shown on outcomes from vasoconstrictor assays, which assess the extent to which a corticosteroid causes cutaneous vasoconstriction or blanching in normal healthy individuals.15 The assay depends on the molecule diffusing from the vehicle, penetrating the skin, and causing a reaction (blanching) that is then evaluated. The assay cannot effectively evaluate the rate of continued diffusion and skin penetration beyond the appearance of blanching. In contrast, the tape-stripping method provides an inside look at the extent of penetration of the corticosteroid beyond the skin surface and the rate of its clearance from different skin layers. In the current study, the levels of fluocinonide declined after peaking at 1 hour after application, but the levels of halcinonide clearly remained elevated after peaking at the same time point. Most likely, vasoconstrictor studies would not be able to differentiate between the concentrations of the 2 products in the stratum corneum beyond the first hour after application.
Tape stripping, or dermatopharmacokinetics, has advantages over vasoconstriction assays in studying corticosteroid penetration and clearance from the stratum corneum. At one point, the US Food and Drug Administration had included tape stripping in its preliminary guidelines for generic topical bioequivalence studies until data from the same formulation generated from 2 different laboratories produced different results.16 Since that time, much work has been done with tape stripping to ensure its consistency. Weigmann et al17 demonstrated equivalent results with clobetasol using vasoconstriction and tape stripping, and Wiedersberg et al18 demonstrated the same with betamethasone. For the current study, the fluocinonide and halcinonide formulations were weighed prior to application so that the same dose was tested in all participants. A plunger was used to produce consistent pressure at all application sites to control for the amount of skin that was stripped off with the tape. Results for both corticosteroids were consistent between the participants. Variability in the data was detected; however, this observation is most likely due to the small number of participants in the studies.
Conclusion
In summary, this pilot study demonstrated that fluocinonide concentration in the stratum corneum peaks within the first hour of application before beginning a steady general decline. There was no evidence of sustained release. In contrast, halcin-onide demonstrated a sustained release for 6 hours after application. Halcinonide is formulated in a cream base in which the corticosteroid is present in a solution and suspension phase that allows for sustained delivery in skin over time. Fluocinonide does not appear to be formulated in the same way, and its concentrations in the stratum corneum begin to decline 1 hour after application.
Acknowledgement
Thank you to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University, Flagstaff, for conducting the liquid chromatography–mass spectrometry.
1. Ostrenga J, Haleblian J, Poulsen B, et al. Vehicle design for a new topical steroid, fluocinonide. J Invest Dermatol. 1971;56:392-399.
2. Rathi SK, D’Souza P. Rational and ethical use of topical corticosteroids based on safety and efficacy. Indian J Dermatol. 2012;57:251-259.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Sudilovsky A, Clewe TH. Comparative efficacy of halcin-onide and fluocinonide creams in psoriasis and eczematous dermatoses. J Clin Pharmacol. 1975;15:779-784.
5. Close JE. Double-blind comparison of topical halcinonide and fluocinonide in the treatment of psoriasis. Int J Dermatol. 1976;15:534-537.
6. Lynfield Y, Watsky M. Psoriasis: topical corticosteroid therapy. Cutis. 1976;18:133, 136-137.
7. Draelos ZD. Demonstration of the biphasic release of 0.1% halcinonide cream. J Drugs Dermatol. 2015;14:89-90.
8. Bagatell FK. Halcinonide: a new potent topical anti-inflammatory drug. Cutis. 1974;14:459-462.
9. Ostrenga J, Steinmetz C, Poulsen B. Significance of vehicle composition. I. relationship between topical vehicle composition, skin penetrability, and clinical efficacy. J Pharm Sci. 1971;60:1175-1179.
10. Ayres PJ, Hooper G. Assessment of the skin penetration properties of different carrier vehicles for topically applied cortisol. Br J Dermatol. 1978;99:307-317.
11. Olsen EA. Double-blind controlled comparison of generic and trade-name topical steroids using the vasoconstriction assay. Arch Dermatol. 1991;127:197-201.
12. Stoughton RB. Are generic formulations equivalent to trade name topical glucocorticoids? Arch Dermatol. 1987;123:1312-1314.
13. Poulsen BJ, Young E, Coquilla V, et al. Effect of topical vehicle composition on the in vitro release of fluocinolone acetonide and its acetate ester. J Pharm Sci. 1968;57:928-933.
14. Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids: what is the evidence? Dermatol Online J. 2013;19:18954.
15. Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician. 2009;79:135-140.
16. Pershing LK, Nelson JL, Corlett JL, et al. Assessment of dermatopharmacokinetic approach in the bioequivalence determination of topical tretinoin gel products. J Am Acad Dermatol. 2003;48:740-751.
17. Weigmann H, Lademann J, v Pelchrzim R, et al. Bioavailability of clobetasol propionate-quantification of drug concentrations in the stratum corneum by dermatopharmacokinetics using tape stripping. Skin Pharmacol Appl Skin Physiol. 1999;12:46-53.
18. Wiedersberg S, Naik A, Leopold CS, et al. Pharmacodynamics and dermatopharmacokinetics of betamethasone 17-valerate: assessment of topical bioavailability. Br J Dermatol. 2009;160:676-686.
The active ingredient of any pharmaceutical product is responsible for the agent’s efficacy and safety profile. This ingredient is extensively studied in clinical trials and evaluated by the US Food and Drug Administration before the product is commercially available. In dermatologic products, especially those for treating dermatoses, the vehicle in which the active ingredient is formulated also plays a role in drug delivery and indirectly impacts therapeutic outcomes, unlike excipients in oral medications. Topical vehicles must be stable, provide a suitable environment that will not degrade the active ingredient or affect its efficacy, and be cosmetically acceptable.1
Topical vehicles are formulated to maintain the stability of the active ingredient and allow it to readily penetrate the skin and reach its target area with minimal absorption into the bloodstream, thus avoiding systemic adverse events. A variety of vehicles can exist for a single active ingredient to accommodate different phases of disease and different anatomical sites where the disease may occur.2 For example, alcohol-based vehicles, sprays, and foams are preferred for the scalp where evaporation of the vehicle is beneficial to prevent greasiness of the hair, while ointments may be preferred due to their occlusive nature for areas with xerotic or thick skin from dermatoses.
Cosmetic acceptability of the vehicle may influence patient adherence to therapy. Housman et al3 assessed a variety of products formulated in different vehicles (ie, solutions, foams, emollients, gels, creams, ointments) for the treatment of psoriasis. Patients with psoriasis applied each test product to a quarter-sized area of normal skin on the forearm using a cotton swab and completed a preference questionnaire. By far, respondents significantly preferred solutions and foams over creams, gels, and ointments (P<.01). Side effects were rated to be the most important characteristics of topical therapy, followed by time needed for application, ease of application, and messiness.3 Presumably, if patients are frustrated with the topical product that they are using, adherence to the prescribed dosage and application instructions will diminish over time, leading to suboptimal steady-state levels of the product. If appropriate levels of the drug are not present at the target site, treatment will not be successful.
Steady-state levels of a topical drug at the site of action also are maintained via appropriate application frequency, most commonly once to 4 times daily for dermatologic products. Fluocinonide and halcinonide are class II (potent) corticosteroids indicated for the relief of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses and usually are administered at least twice daily. In double-blind clinical studies comparing both products in the treatment of psoriasis, halcinonide resulted in more improved outcomes than fluocinonide.4-6 Sudilovsky and Clewe4 studied 140 patients with moderate to severe psoriasis. After 3 weeks of treatment, 44% showed superior results with halcinonide, 27% showed superior results with fluocinonide, 26% showed equal results with both products, and 3% showed no relief.4 Similarly, Close5 reported that 61% of patients showed superior results with halcinonide, 25% showed superior results with fluocinonide, 10% showed equal results with both products, and 4% showed no relief (N=50). Lynfield and Watsky6 reported that 56% of patients with severe psoriasis who were treated with halcinonide for 2 weeks showed improvement to normal or slight inflammation compared to 44% of patients treated with fluocinonide (N=59). All 3 studies used cream formulations of halcinonide and fluocinonide.
Recently, halcinonide cream was shown to have an immediate release into the stratum corneum that peaked within 1 hour of application and remained elevated for 6 hours before beginning to decline.7 These results support a biphasic release of halcinonide, which is in agreement with its formulation—that halcinonide exists in both a solution phase for immediate release into the skin and in a suspension phase that allows a sustained release after equilibrium is reached between the solution and suspension phases.8 Fluocinonide is not known to be formulated in a similar way. Its vehicle composition and penetration into the skin could explain the superior efficacy of halcinonide versus fluocinonide.
The current pilot study was conducted to compare the release pattern of fluocinonide cream versus halcinonide cream into the stratum corneum using an in vivo, noninvasive method. Results for halcin-onide have been previously published.7
Methods
Participants were sequestered in a controlled environment for the entire day to allow the skin to equilibrate prior to product application. The methodology for the application and quantification of halcinonide cream 0.1% into the stratum corneum of 5 participants using a tape-stripping protocol has been described elsewhere.7 Concordia Clinical Research institutional review board (Cedar Knolls, New Jersey) approved this study, which was conducted at Dermatology Consulting Services (High Point, North Carolina).
A 0.1-g dose of generic fluocinonide cream 0.05% was applied to four 2.5-cm circular sites on the forearm in 5 participants with normal skin until completely absorbed. Circular tape strips were subsequently placed on the application site at 1, 3, 6, and 9 hours posttreatment and were held for 10 seconds with a controlled pressure plunger to ensure adequate and consistent contact between the tape strip and the skin. The tape strip was removed with forceps, rolled with the skin scale inside, and placed in a glass vial. This procedure was repeated 6 times at 1 of 4 sites with a new tape strip at each time point to obtain samples from deeper skin layers. A total of 24 tape strips were collected from each participant.
All vials were frozen at -20°C and were shipped overnight to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University (Flagstaff, Arizona) for mass spectroscopy evaluation. Once received at the outside facility, the vials were stored at -20°C until analysis. Each sample was spiked with a known quantity of an appropriate reference standard and extracted with 1 mL acetonitrile at room temperature for 1 minute with agitation. New unused tape strips were spiked with a small amount of fluocinonide reference standard for extraction efficiency.
Extracts were evaporated to dryness under nitrogen gas, resuspended in 200 µL chromatography solvent, and quantified using liquid chromatography–mass spectrometry. To remove the skin scale from the tape strips, 10 mL of a solvent solution of 0.1 mg/mL fludrocortisone acetate in acetonitrile was dispensed into a 4 dram vial containing the tape strip. The vials were ultrasonicated and shaken for 10 to 15 minutes, and the samples were further diluted to 100-fold and were inverted several times to ensure complete dissolution of fluocinonide before liquid chromatography–mass spectrometry.
A standard curve ranging from the lower limit of quantification to the upper limit of quantification for the fluocinonide reference was used to determine the quantity of fluocinonide in each of the tape strips. Once the lower limit of quantification was reached in a given set of tape strip samples (1-, 3-, 6-, and 9-hour samples), the next 2 sequential tape strips in that set were analyzed to confirm fluocinonide was not detectable in deeper layers. Standard quality controls were analyzed to ensure run-to-run and sample-to-sample accuracy.
Each sample was analyzed in duplicate; 10 mg fluocinonide was used as a reference standard. The minimum detectable concentration of fluocinonide was 1 ng/mL.
Results
As expected, tape strip 1 from each participant contained the highest concentration of fluocinonide. This strip corresponded to the most superficial layer of skin. Concentrations decreased in deeper skin layers, as detected in strips 2 to 6.
In general, the average concentration of fluocin-onide in strip 1 for all 5 participants was highest at hour 1, with a subsequent decline at hours 3, 6, and 9; however, participant 1 showed a second peak in fluocinonide concentration at hour 6 (Figure 1). When the fluocinonide concentration in strips 1 to 6 was averaged for each participant at each time point, similar results were obtained: a general decline after hour 1, but a second prominent peak at hour 6 in participant 1 only. In participant 1, the average fluocinonide concentration for strips 1 to 6 was 393 ng/mL at hour 1 and declined to 208 ng/mL at hour 3; it increased to 451 ng/mL at hour 6 before declining again to 202 ng/mL at hour 9.
Because participant 1 was the only one to exhibit a second peak of fluocinonide concentration, it appears that measurements obtained from this participant may be outliers. When removing partici-pant 1 from the analysis of fluocinonide concentration in strip 1 at each time point, a clear decline is evident from hour 1 to hour 9 (Figure 2A, red line [partici-pants 2–5] vs blue line [participants 1–5]).
When the average concentration of fluocinonide was calculated in strips 1 to 6 from all participants, there was a general steady decline after hour 1 with a slight increase of 25 ng/mL at hour 6 (Figure 2B, blue line). This increase is due to the measurements obtained from participant 1; however, if partici-pant 1 is removed from the analysis, a constant decline is observed from hour 1 to hour 9 (Figure 2B, red line).
|
A prior study evaluated the penetration and absorption of halcinonide in the stratum corneum.7 In summary, halcinonide concentration peaked at hour 1 following application and remained elevated to hour 6, before beginning a slow decline. The average concentration of halcinonide from all participants in strips 1 to 6 reached 1350 ng/mL at hour 1, remained within 93% to 97% of this level (1253–1303 ng/mL) for the next 5 hours, and declined only 29% from the peak at hour 1 to hour 9 (958 ng/mL)(Figure 3, blue line).7 In contrast, the fluocinonide concentration in participants 2 to 5 from the current study reached 190 ng/mL at hour 1 and steadily declined 53% to 89 ng/mL by hour 9 (Figure 3, red line).
Two participants from the prior halcinonide study also were enrolled in the current fluocinonide study (referred to as participant A and B). In general, halcinonide levels in both participants remained elevated for 6 hours after application and declined 27.5% and 35.5%, respectively, by hour 9 (Figure 4). Participant A experienced a 20.5% dip in halcinonide concentration at hour 3 followed by an increase at hour 6; however, the halcinonide concentration at hour 9 was similar to hour 3.7 In contrast, fluocin-onide concentrations for these participants peaked at 1 hour and clearly declined approximately 60% over the next 8 hours.
Comment
The release of both fluocinonide and halcinonide into the skin was evaluated using dermal tape stripping on 4 sites on the forearms of healthy individuals. Cream formulations of each corticosteroid were evaluated in 5 participants, with 2 participants receiving both formulations during different study periods. In the prior study with halcinonide, the stratum corneum exhibited the highest concentration of the corticosteroid, with substantial declines beyond strip 6 (ie, strips 7–20).7 For this reason, only strips 1 to 6 were evaluated for corticosteroid penetration and absorption.
Results from strip 1 indicated immediate absorption of corticosteroid (fluocinonide and halcinonide) into the skin. Unlike the release of halcinonide, which demonstrated a clear sustained release over 6 hours before decreasing,7 fluocinonide concentrations began declining immediately after peaking at hour 1 and continued to decline up to hour 9. Only participant 1 exhibited a second peak of fluocinonide concentration at hour 6; the rest of the participants did not. This second peak is most likely an anomaly due to the small number of participants rather than a true elevation.
Given the rapid decline of fluocinonide concentration over the 9 hours compared with the more gradual decline of halcinonide concentration, there appears to be no evidence of a biphasic sustained release of fluocinonide from its vehicle. This difference in release pattern from each corticosteroid’s respective vehicle may explain in part the different clinical outcomes in comparative studies.4-6
It is known that vehicle composition affects corticosteroid diffusion from the vehicle to the skin surface and subsequent penetration into the skin.9 Either process can determine the overall effectiveness of the product. Ayres and Hooper10 evaluated the penetration of 4 topical preparations of cortisol. Product 1 delivered 16 times more cortisol to the skin than product 2, 8 times more than product 3, and 3 times more than product 4. Because all the preparations contained cortisol-free alcohol, these differences were attributed to the vehicle in which the cortisol was formulated. Products 1 and 4 both contained 10% urea, but the urea in product 1 was a powder in a cream base and the urea in product 4 was in a stabilizing emulsified base. Product 2 contained a propylene glycol/water base and product 3 was a water-miscible cream.10
Generic corticosteroid products have been observed in clinical practice and have been shown in vasoconstriction assays to be less and more potent than their brand-name equivalents.2,11 Vasoconstriction assays are the standard for assessing the potency of topical corticosteroids and predicting their clinical efficacy.2 One study reported significant differences in therapeutic effectiveness between generic formulations and their brand-name equivalents.12 Kenalog cream 0.1% (multiple manufacturers) was significantly more potent than any of the generic triamcinolone creams tested (P<.05); in fact, Kenalog cream 0.025% (multiple manufacturers) was statistically superior to all the generic triamcinolone creams 0.1%. Moreover, Artistocort A ointment 0.1% (Lederele Laboratories) and Valisone cream 0.1% (Schering Corporation) also were more potent than their generics at the same concentration in the same vehicle type.12 A second study also observed that 2 of 6 generic formulations had significantly less vasoconstriction than their respective brand-name formulations.11 A brand-name betamethasone valerate cream produced significantly greater vasoconstriction than its generic equivalent, and a brand-name betamethasone dipropionate cream produced greater vasoconstriction than one generic and equal vasoconstriction to another generic. Additionally, the vasoconstriction measured with Diprosone was greater than that measured with Diprolene, another brand-name product of betamethasone dipropionate.11 Diprosone and Diprolene differ in their vehicle content. The latter, a class I corticosteroid, contains a modified vehicle high in propylene glycol, whereas the former contains less propylene glycol and thus is classified as a class III corticosteroid. Propylene glycol allows hydrophobic molecules such as corticosteroids to dissolve more fully in the vehicle.12
Ostrenga et al1 studied the solubility of corticosteroids in different vehicles and, as expected, corticosteroids that fully solubilized in the vehicle exhibited better penetration into the skin on assessment with vasoconstriction assays. Corticosteroids in a suspension, on the other hand, showed slower penetration into the skin.1,13 A balance between the solution and suspension phase would allow a drug to rapidly penetrate the skin upon application, and when this pool of solubilized drug was depleted, additional drug could penetrate into the skin from the suspension phase. Based on the tape strip results from the current study it appears that halcinonide, which is manufactured in a biphasic formulation, follows this pattern of penetration and absorption into the stratum corneum. In contrast, fluocinonide appears to exist in a soluble state without much, if any, amount in a suspension phase because it had no sustained release during the 9 hours after application.
Common belief among dermatologists is that long-term use of corticosteroids leads to tachyphylaxis,14 which can be attributed to poor patient adherence. If patients skip doses, then the steady state of the product at the target site is not maintained. It is interesting to speculate that using agents with more sustained release beyond the time of application (such as halcinonide) may preserve steady-state levels even when patients are neglectful of the next medication application. Corticosteroids that work in 2 phases such as halcinonide may minimize tachyphylaxis experienced with prolonged use of corticosteroids.
Fluocinonide and halcinonide are both class II high-potency corticosteroids as shown on outcomes from vasoconstrictor assays, which assess the extent to which a corticosteroid causes cutaneous vasoconstriction or blanching in normal healthy individuals.15 The assay depends on the molecule diffusing from the vehicle, penetrating the skin, and causing a reaction (blanching) that is then evaluated. The assay cannot effectively evaluate the rate of continued diffusion and skin penetration beyond the appearance of blanching. In contrast, the tape-stripping method provides an inside look at the extent of penetration of the corticosteroid beyond the skin surface and the rate of its clearance from different skin layers. In the current study, the levels of fluocinonide declined after peaking at 1 hour after application, but the levels of halcinonide clearly remained elevated after peaking at the same time point. Most likely, vasoconstrictor studies would not be able to differentiate between the concentrations of the 2 products in the stratum corneum beyond the first hour after application.
Tape stripping, or dermatopharmacokinetics, has advantages over vasoconstriction assays in studying corticosteroid penetration and clearance from the stratum corneum. At one point, the US Food and Drug Administration had included tape stripping in its preliminary guidelines for generic topical bioequivalence studies until data from the same formulation generated from 2 different laboratories produced different results.16 Since that time, much work has been done with tape stripping to ensure its consistency. Weigmann et al17 demonstrated equivalent results with clobetasol using vasoconstriction and tape stripping, and Wiedersberg et al18 demonstrated the same with betamethasone. For the current study, the fluocinonide and halcinonide formulations were weighed prior to application so that the same dose was tested in all participants. A plunger was used to produce consistent pressure at all application sites to control for the amount of skin that was stripped off with the tape. Results for both corticosteroids were consistent between the participants. Variability in the data was detected; however, this observation is most likely due to the small number of participants in the studies.
Conclusion
In summary, this pilot study demonstrated that fluocinonide concentration in the stratum corneum peaks within the first hour of application before beginning a steady general decline. There was no evidence of sustained release. In contrast, halcin-onide demonstrated a sustained release for 6 hours after application. Halcinonide is formulated in a cream base in which the corticosteroid is present in a solution and suspension phase that allows for sustained delivery in skin over time. Fluocinonide does not appear to be formulated in the same way, and its concentrations in the stratum corneum begin to decline 1 hour after application.
Acknowledgement
Thank you to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University, Flagstaff, for conducting the liquid chromatography–mass spectrometry.
The active ingredient of any pharmaceutical product is responsible for the agent’s efficacy and safety profile. This ingredient is extensively studied in clinical trials and evaluated by the US Food and Drug Administration before the product is commercially available. In dermatologic products, especially those for treating dermatoses, the vehicle in which the active ingredient is formulated also plays a role in drug delivery and indirectly impacts therapeutic outcomes, unlike excipients in oral medications. Topical vehicles must be stable, provide a suitable environment that will not degrade the active ingredient or affect its efficacy, and be cosmetically acceptable.1
Topical vehicles are formulated to maintain the stability of the active ingredient and allow it to readily penetrate the skin and reach its target area with minimal absorption into the bloodstream, thus avoiding systemic adverse events. A variety of vehicles can exist for a single active ingredient to accommodate different phases of disease and different anatomical sites where the disease may occur.2 For example, alcohol-based vehicles, sprays, and foams are preferred for the scalp where evaporation of the vehicle is beneficial to prevent greasiness of the hair, while ointments may be preferred due to their occlusive nature for areas with xerotic or thick skin from dermatoses.
Cosmetic acceptability of the vehicle may influence patient adherence to therapy. Housman et al3 assessed a variety of products formulated in different vehicles (ie, solutions, foams, emollients, gels, creams, ointments) for the treatment of psoriasis. Patients with psoriasis applied each test product to a quarter-sized area of normal skin on the forearm using a cotton swab and completed a preference questionnaire. By far, respondents significantly preferred solutions and foams over creams, gels, and ointments (P<.01). Side effects were rated to be the most important characteristics of topical therapy, followed by time needed for application, ease of application, and messiness.3 Presumably, if patients are frustrated with the topical product that they are using, adherence to the prescribed dosage and application instructions will diminish over time, leading to suboptimal steady-state levels of the product. If appropriate levels of the drug are not present at the target site, treatment will not be successful.
Steady-state levels of a topical drug at the site of action also are maintained via appropriate application frequency, most commonly once to 4 times daily for dermatologic products. Fluocinonide and halcinonide are class II (potent) corticosteroids indicated for the relief of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses and usually are administered at least twice daily. In double-blind clinical studies comparing both products in the treatment of psoriasis, halcinonide resulted in more improved outcomes than fluocinonide.4-6 Sudilovsky and Clewe4 studied 140 patients with moderate to severe psoriasis. After 3 weeks of treatment, 44% showed superior results with halcinonide, 27% showed superior results with fluocinonide, 26% showed equal results with both products, and 3% showed no relief.4 Similarly, Close5 reported that 61% of patients showed superior results with halcinonide, 25% showed superior results with fluocinonide, 10% showed equal results with both products, and 4% showed no relief (N=50). Lynfield and Watsky6 reported that 56% of patients with severe psoriasis who were treated with halcinonide for 2 weeks showed improvement to normal or slight inflammation compared to 44% of patients treated with fluocinonide (N=59). All 3 studies used cream formulations of halcinonide and fluocinonide.
Recently, halcinonide cream was shown to have an immediate release into the stratum corneum that peaked within 1 hour of application and remained elevated for 6 hours before beginning to decline.7 These results support a biphasic release of halcinonide, which is in agreement with its formulation—that halcinonide exists in both a solution phase for immediate release into the skin and in a suspension phase that allows a sustained release after equilibrium is reached between the solution and suspension phases.8 Fluocinonide is not known to be formulated in a similar way. Its vehicle composition and penetration into the skin could explain the superior efficacy of halcinonide versus fluocinonide.
The current pilot study was conducted to compare the release pattern of fluocinonide cream versus halcinonide cream into the stratum corneum using an in vivo, noninvasive method. Results for halcin-onide have been previously published.7
Methods
Participants were sequestered in a controlled environment for the entire day to allow the skin to equilibrate prior to product application. The methodology for the application and quantification of halcinonide cream 0.1% into the stratum corneum of 5 participants using a tape-stripping protocol has been described elsewhere.7 Concordia Clinical Research institutional review board (Cedar Knolls, New Jersey) approved this study, which was conducted at Dermatology Consulting Services (High Point, North Carolina).
A 0.1-g dose of generic fluocinonide cream 0.05% was applied to four 2.5-cm circular sites on the forearm in 5 participants with normal skin until completely absorbed. Circular tape strips were subsequently placed on the application site at 1, 3, 6, and 9 hours posttreatment and were held for 10 seconds with a controlled pressure plunger to ensure adequate and consistent contact between the tape strip and the skin. The tape strip was removed with forceps, rolled with the skin scale inside, and placed in a glass vial. This procedure was repeated 6 times at 1 of 4 sites with a new tape strip at each time point to obtain samples from deeper skin layers. A total of 24 tape strips were collected from each participant.
All vials were frozen at -20°C and were shipped overnight to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University (Flagstaff, Arizona) for mass spectroscopy evaluation. Once received at the outside facility, the vials were stored at -20°C until analysis. Each sample was spiked with a known quantity of an appropriate reference standard and extracted with 1 mL acetonitrile at room temperature for 1 minute with agitation. New unused tape strips were spiked with a small amount of fluocinonide reference standard for extraction efficiency.
Extracts were evaporated to dryness under nitrogen gas, resuspended in 200 µL chromatography solvent, and quantified using liquid chromatography–mass spectrometry. To remove the skin scale from the tape strips, 10 mL of a solvent solution of 0.1 mg/mL fludrocortisone acetate in acetonitrile was dispensed into a 4 dram vial containing the tape strip. The vials were ultrasonicated and shaken for 10 to 15 minutes, and the samples were further diluted to 100-fold and were inverted several times to ensure complete dissolution of fluocinonide before liquid chromatography–mass spectrometry.
A standard curve ranging from the lower limit of quantification to the upper limit of quantification for the fluocinonide reference was used to determine the quantity of fluocinonide in each of the tape strips. Once the lower limit of quantification was reached in a given set of tape strip samples (1-, 3-, 6-, and 9-hour samples), the next 2 sequential tape strips in that set were analyzed to confirm fluocinonide was not detectable in deeper layers. Standard quality controls were analyzed to ensure run-to-run and sample-to-sample accuracy.
Each sample was analyzed in duplicate; 10 mg fluocinonide was used as a reference standard. The minimum detectable concentration of fluocinonide was 1 ng/mL.
Results
As expected, tape strip 1 from each participant contained the highest concentration of fluocinonide. This strip corresponded to the most superficial layer of skin. Concentrations decreased in deeper skin layers, as detected in strips 2 to 6.
In general, the average concentration of fluocin-onide in strip 1 for all 5 participants was highest at hour 1, with a subsequent decline at hours 3, 6, and 9; however, participant 1 showed a second peak in fluocinonide concentration at hour 6 (Figure 1). When the fluocinonide concentration in strips 1 to 6 was averaged for each participant at each time point, similar results were obtained: a general decline after hour 1, but a second prominent peak at hour 6 in participant 1 only. In participant 1, the average fluocinonide concentration for strips 1 to 6 was 393 ng/mL at hour 1 and declined to 208 ng/mL at hour 3; it increased to 451 ng/mL at hour 6 before declining again to 202 ng/mL at hour 9.
Because participant 1 was the only one to exhibit a second peak of fluocinonide concentration, it appears that measurements obtained from this participant may be outliers. When removing partici-pant 1 from the analysis of fluocinonide concentration in strip 1 at each time point, a clear decline is evident from hour 1 to hour 9 (Figure 2A, red line [partici-pants 2–5] vs blue line [participants 1–5]).
When the average concentration of fluocinonide was calculated in strips 1 to 6 from all participants, there was a general steady decline after hour 1 with a slight increase of 25 ng/mL at hour 6 (Figure 2B, blue line). This increase is due to the measurements obtained from participant 1; however, if partici-pant 1 is removed from the analysis, a constant decline is observed from hour 1 to hour 9 (Figure 2B, red line).
|
A prior study evaluated the penetration and absorption of halcinonide in the stratum corneum.7 In summary, halcinonide concentration peaked at hour 1 following application and remained elevated to hour 6, before beginning a slow decline. The average concentration of halcinonide from all participants in strips 1 to 6 reached 1350 ng/mL at hour 1, remained within 93% to 97% of this level (1253–1303 ng/mL) for the next 5 hours, and declined only 29% from the peak at hour 1 to hour 9 (958 ng/mL)(Figure 3, blue line).7 In contrast, the fluocinonide concentration in participants 2 to 5 from the current study reached 190 ng/mL at hour 1 and steadily declined 53% to 89 ng/mL by hour 9 (Figure 3, red line).
Two participants from the prior halcinonide study also were enrolled in the current fluocinonide study (referred to as participant A and B). In general, halcinonide levels in both participants remained elevated for 6 hours after application and declined 27.5% and 35.5%, respectively, by hour 9 (Figure 4). Participant A experienced a 20.5% dip in halcinonide concentration at hour 3 followed by an increase at hour 6; however, the halcinonide concentration at hour 9 was similar to hour 3.7 In contrast, fluocin-onide concentrations for these participants peaked at 1 hour and clearly declined approximately 60% over the next 8 hours.
Comment
The release of both fluocinonide and halcinonide into the skin was evaluated using dermal tape stripping on 4 sites on the forearms of healthy individuals. Cream formulations of each corticosteroid were evaluated in 5 participants, with 2 participants receiving both formulations during different study periods. In the prior study with halcinonide, the stratum corneum exhibited the highest concentration of the corticosteroid, with substantial declines beyond strip 6 (ie, strips 7–20).7 For this reason, only strips 1 to 6 were evaluated for corticosteroid penetration and absorption.
Results from strip 1 indicated immediate absorption of corticosteroid (fluocinonide and halcinonide) into the skin. Unlike the release of halcinonide, which demonstrated a clear sustained release over 6 hours before decreasing,7 fluocinonide concentrations began declining immediately after peaking at hour 1 and continued to decline up to hour 9. Only participant 1 exhibited a second peak of fluocinonide concentration at hour 6; the rest of the participants did not. This second peak is most likely an anomaly due to the small number of participants rather than a true elevation.
Given the rapid decline of fluocinonide concentration over the 9 hours compared with the more gradual decline of halcinonide concentration, there appears to be no evidence of a biphasic sustained release of fluocinonide from its vehicle. This difference in release pattern from each corticosteroid’s respective vehicle may explain in part the different clinical outcomes in comparative studies.4-6
It is known that vehicle composition affects corticosteroid diffusion from the vehicle to the skin surface and subsequent penetration into the skin.9 Either process can determine the overall effectiveness of the product. Ayres and Hooper10 evaluated the penetration of 4 topical preparations of cortisol. Product 1 delivered 16 times more cortisol to the skin than product 2, 8 times more than product 3, and 3 times more than product 4. Because all the preparations contained cortisol-free alcohol, these differences were attributed to the vehicle in which the cortisol was formulated. Products 1 and 4 both contained 10% urea, but the urea in product 1 was a powder in a cream base and the urea in product 4 was in a stabilizing emulsified base. Product 2 contained a propylene glycol/water base and product 3 was a water-miscible cream.10
Generic corticosteroid products have been observed in clinical practice and have been shown in vasoconstriction assays to be less and more potent than their brand-name equivalents.2,11 Vasoconstriction assays are the standard for assessing the potency of topical corticosteroids and predicting their clinical efficacy.2 One study reported significant differences in therapeutic effectiveness between generic formulations and their brand-name equivalents.12 Kenalog cream 0.1% (multiple manufacturers) was significantly more potent than any of the generic triamcinolone creams tested (P<.05); in fact, Kenalog cream 0.025% (multiple manufacturers) was statistically superior to all the generic triamcinolone creams 0.1%. Moreover, Artistocort A ointment 0.1% (Lederele Laboratories) and Valisone cream 0.1% (Schering Corporation) also were more potent than their generics at the same concentration in the same vehicle type.12 A second study also observed that 2 of 6 generic formulations had significantly less vasoconstriction than their respective brand-name formulations.11 A brand-name betamethasone valerate cream produced significantly greater vasoconstriction than its generic equivalent, and a brand-name betamethasone dipropionate cream produced greater vasoconstriction than one generic and equal vasoconstriction to another generic. Additionally, the vasoconstriction measured with Diprosone was greater than that measured with Diprolene, another brand-name product of betamethasone dipropionate.11 Diprosone and Diprolene differ in their vehicle content. The latter, a class I corticosteroid, contains a modified vehicle high in propylene glycol, whereas the former contains less propylene glycol and thus is classified as a class III corticosteroid. Propylene glycol allows hydrophobic molecules such as corticosteroids to dissolve more fully in the vehicle.12
Ostrenga et al1 studied the solubility of corticosteroids in different vehicles and, as expected, corticosteroids that fully solubilized in the vehicle exhibited better penetration into the skin on assessment with vasoconstriction assays. Corticosteroids in a suspension, on the other hand, showed slower penetration into the skin.1,13 A balance between the solution and suspension phase would allow a drug to rapidly penetrate the skin upon application, and when this pool of solubilized drug was depleted, additional drug could penetrate into the skin from the suspension phase. Based on the tape strip results from the current study it appears that halcinonide, which is manufactured in a biphasic formulation, follows this pattern of penetration and absorption into the stratum corneum. In contrast, fluocinonide appears to exist in a soluble state without much, if any, amount in a suspension phase because it had no sustained release during the 9 hours after application.
Common belief among dermatologists is that long-term use of corticosteroids leads to tachyphylaxis,14 which can be attributed to poor patient adherence. If patients skip doses, then the steady state of the product at the target site is not maintained. It is interesting to speculate that using agents with more sustained release beyond the time of application (such as halcinonide) may preserve steady-state levels even when patients are neglectful of the next medication application. Corticosteroids that work in 2 phases such as halcinonide may minimize tachyphylaxis experienced with prolonged use of corticosteroids.
Fluocinonide and halcinonide are both class II high-potency corticosteroids as shown on outcomes from vasoconstrictor assays, which assess the extent to which a corticosteroid causes cutaneous vasoconstriction or blanching in normal healthy individuals.15 The assay depends on the molecule diffusing from the vehicle, penetrating the skin, and causing a reaction (blanching) that is then evaluated. The assay cannot effectively evaluate the rate of continued diffusion and skin penetration beyond the appearance of blanching. In contrast, the tape-stripping method provides an inside look at the extent of penetration of the corticosteroid beyond the skin surface and the rate of its clearance from different skin layers. In the current study, the levels of fluocinonide declined after peaking at 1 hour after application, but the levels of halcinonide clearly remained elevated after peaking at the same time point. Most likely, vasoconstrictor studies would not be able to differentiate between the concentrations of the 2 products in the stratum corneum beyond the first hour after application.
Tape stripping, or dermatopharmacokinetics, has advantages over vasoconstriction assays in studying corticosteroid penetration and clearance from the stratum corneum. At one point, the US Food and Drug Administration had included tape stripping in its preliminary guidelines for generic topical bioequivalence studies until data from the same formulation generated from 2 different laboratories produced different results.16 Since that time, much work has been done with tape stripping to ensure its consistency. Weigmann et al17 demonstrated equivalent results with clobetasol using vasoconstriction and tape stripping, and Wiedersberg et al18 demonstrated the same with betamethasone. For the current study, the fluocinonide and halcinonide formulations were weighed prior to application so that the same dose was tested in all participants. A plunger was used to produce consistent pressure at all application sites to control for the amount of skin that was stripped off with the tape. Results for both corticosteroids were consistent between the participants. Variability in the data was detected; however, this observation is most likely due to the small number of participants in the studies.
Conclusion
In summary, this pilot study demonstrated that fluocinonide concentration in the stratum corneum peaks within the first hour of application before beginning a steady general decline. There was no evidence of sustained release. In contrast, halcin-onide demonstrated a sustained release for 6 hours after application. Halcinonide is formulated in a cream base in which the corticosteroid is present in a solution and suspension phase that allows for sustained delivery in skin over time. Fluocinonide does not appear to be formulated in the same way, and its concentrations in the stratum corneum begin to decline 1 hour after application.
Acknowledgement
Thank you to Robert Kellar, PhD, at the Center for Bioengineering Innovation at Northern Arizona University, Flagstaff, for conducting the liquid chromatography–mass spectrometry.
1. Ostrenga J, Haleblian J, Poulsen B, et al. Vehicle design for a new topical steroid, fluocinonide. J Invest Dermatol. 1971;56:392-399.
2. Rathi SK, D’Souza P. Rational and ethical use of topical corticosteroids based on safety and efficacy. Indian J Dermatol. 2012;57:251-259.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Sudilovsky A, Clewe TH. Comparative efficacy of halcin-onide and fluocinonide creams in psoriasis and eczematous dermatoses. J Clin Pharmacol. 1975;15:779-784.
5. Close JE. Double-blind comparison of topical halcinonide and fluocinonide in the treatment of psoriasis. Int J Dermatol. 1976;15:534-537.
6. Lynfield Y, Watsky M. Psoriasis: topical corticosteroid therapy. Cutis. 1976;18:133, 136-137.
7. Draelos ZD. Demonstration of the biphasic release of 0.1% halcinonide cream. J Drugs Dermatol. 2015;14:89-90.
8. Bagatell FK. Halcinonide: a new potent topical anti-inflammatory drug. Cutis. 1974;14:459-462.
9. Ostrenga J, Steinmetz C, Poulsen B. Significance of vehicle composition. I. relationship between topical vehicle composition, skin penetrability, and clinical efficacy. J Pharm Sci. 1971;60:1175-1179.
10. Ayres PJ, Hooper G. Assessment of the skin penetration properties of different carrier vehicles for topically applied cortisol. Br J Dermatol. 1978;99:307-317.
11. Olsen EA. Double-blind controlled comparison of generic and trade-name topical steroids using the vasoconstriction assay. Arch Dermatol. 1991;127:197-201.
12. Stoughton RB. Are generic formulations equivalent to trade name topical glucocorticoids? Arch Dermatol. 1987;123:1312-1314.
13. Poulsen BJ, Young E, Coquilla V, et al. Effect of topical vehicle composition on the in vitro release of fluocinolone acetonide and its acetate ester. J Pharm Sci. 1968;57:928-933.
14. Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids: what is the evidence? Dermatol Online J. 2013;19:18954.
15. Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician. 2009;79:135-140.
16. Pershing LK, Nelson JL, Corlett JL, et al. Assessment of dermatopharmacokinetic approach in the bioequivalence determination of topical tretinoin gel products. J Am Acad Dermatol. 2003;48:740-751.
17. Weigmann H, Lademann J, v Pelchrzim R, et al. Bioavailability of clobetasol propionate-quantification of drug concentrations in the stratum corneum by dermatopharmacokinetics using tape stripping. Skin Pharmacol Appl Skin Physiol. 1999;12:46-53.
18. Wiedersberg S, Naik A, Leopold CS, et al. Pharmacodynamics and dermatopharmacokinetics of betamethasone 17-valerate: assessment of topical bioavailability. Br J Dermatol. 2009;160:676-686.
1. Ostrenga J, Haleblian J, Poulsen B, et al. Vehicle design for a new topical steroid, fluocinonide. J Invest Dermatol. 1971;56:392-399.
2. Rathi SK, D’Souza P. Rational and ethical use of topical corticosteroids based on safety and efficacy. Indian J Dermatol. 2012;57:251-259.
3. Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
4. Sudilovsky A, Clewe TH. Comparative efficacy of halcin-onide and fluocinonide creams in psoriasis and eczematous dermatoses. J Clin Pharmacol. 1975;15:779-784.
5. Close JE. Double-blind comparison of topical halcinonide and fluocinonide in the treatment of psoriasis. Int J Dermatol. 1976;15:534-537.
6. Lynfield Y, Watsky M. Psoriasis: topical corticosteroid therapy. Cutis. 1976;18:133, 136-137.
7. Draelos ZD. Demonstration of the biphasic release of 0.1% halcinonide cream. J Drugs Dermatol. 2015;14:89-90.
8. Bagatell FK. Halcinonide: a new potent topical anti-inflammatory drug. Cutis. 1974;14:459-462.
9. Ostrenga J, Steinmetz C, Poulsen B. Significance of vehicle composition. I. relationship between topical vehicle composition, skin penetrability, and clinical efficacy. J Pharm Sci. 1971;60:1175-1179.
10. Ayres PJ, Hooper G. Assessment of the skin penetration properties of different carrier vehicles for topically applied cortisol. Br J Dermatol. 1978;99:307-317.
11. Olsen EA. Double-blind controlled comparison of generic and trade-name topical steroids using the vasoconstriction assay. Arch Dermatol. 1991;127:197-201.
12. Stoughton RB. Are generic formulations equivalent to trade name topical glucocorticoids? Arch Dermatol. 1987;123:1312-1314.
13. Poulsen BJ, Young E, Coquilla V, et al. Effect of topical vehicle composition on the in vitro release of fluocinolone acetonide and its acetate ester. J Pharm Sci. 1968;57:928-933.
14. Taheri A, Cantrell J, Feldman SR. Tachyphylaxis to topical glucocorticoids: what is the evidence? Dermatol Online J. 2013;19:18954.
15. Ference JD, Last AR. Choosing topical corticosteroids. Am Fam Physician. 2009;79:135-140.
16. Pershing LK, Nelson JL, Corlett JL, et al. Assessment of dermatopharmacokinetic approach in the bioequivalence determination of topical tretinoin gel products. J Am Acad Dermatol. 2003;48:740-751.
17. Weigmann H, Lademann J, v Pelchrzim R, et al. Bioavailability of clobetasol propionate-quantification of drug concentrations in the stratum corneum by dermatopharmacokinetics using tape stripping. Skin Pharmacol Appl Skin Physiol. 1999;12:46-53.
18. Wiedersberg S, Naik A, Leopold CS, et al. Pharmacodynamics and dermatopharmacokinetics of betamethasone 17-valerate: assessment of topical bioavailability. Br J Dermatol. 2009;160:676-686.
Practice Points
- Fluocinonide concentration in the stratum corneum peaks within the first hour of application and then begins a steady decline.
- Halcinonide concentration also peaks within the first hour of application and remains elevated for 6 hours after application.
- Halcinonide, rather than fluocinonide, may provide clinical benefits in between doses because of its sustained release hours after application.
Perianal North American Blastomycosis
Cutaneous North American blastomycosis is a deep fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus that is endemic to the Great Lakes region as well as the Mississippi and Ohio River valleys where it thrives in moist acidic soil enriched with organic material.1,2 In humans, the annual incidence rate is estimated to be 0.6 cases per million,3 though it may be as high as 42 cases per 100,000 in endemic areas.4 Infection typically results from the inhalation of conidia and manifests as either acute or chronic pneumonia.5 Most patients with acute disease present with nonspecific flulike symptoms and a nonproductive cough.
Dissemination occurs in approximately 25% of cases,6 most commonly affecting the skin. Other potential sites of dissemination include bone, the genitourinary tract, and the central nervous system. Cutaneous lesions, which may be either verrucous or ulcerative plaques, often occur on or around orifices contiguous to the respiratory tract.7 Verrucous lesions tend to have an irregular shape with well-defined borders and surface crusting. Ulcerative lesions have heaped-up borders and often have an exudative base.8 The differential diagnosis of cutaneous North American blastomycosis lesions includes squamous cell carcinoma, giant keratoacanthoma, verrucae, basal cell carcinoma, scrofuloderma, lupus vulgaris, nocardiosis, syphilis, bromoderma, iododerma, granuloma inguinale, tuberculosis verrucosa cutis, mycetoma, and actinomycosis.7,8
Although periorificial cutaneous manifestations of disseminated blastomycosis are common, perianal lesions are rare. The differential diagnosis of perianal verrucous plaques includes condyloma acuminatum, squamous cell carcinoma, adenocarcinoma, Buschke-Löwenstein tumor, actinomycosis, and localized fungal infections such as blastomycosis.9
Case Report
A 57-year-old man presented with a palpable perianal mass that produced small amounts of blood in his underwear and on toilet paper. The patient reported no history of hemorrhoids, anoreceptive intercourse, or sexually transmitted disease. Four months prior to presentation, he had a prolonged upper respiratory tract illness with a subjective fever and productive cough of 2 months’ duration. The patient described himself as an avid outdoorsman who worked at a summer resort and spent a great deal of time in the forests of central Wisconsin last autumn. Physical examination revealed a well-demarcated, firm, moist plaque with a verrucous surface that measured 3.5×2.7 cm and extended from the anal verge to the perianal skin (Figure 1).
Potassium hydroxide preparation of a biopsy specimen (Figure 2), a punch biopsy of the lesion (Figure 3), and Gomori methenamine-silver staining (Figure 4) revealed scattered yeast spores, some demonstrating broad-based budding, with pseudoepitheliomatous hyperplasia, dermal neutrophils, and intraepithelial microabscesses. The patient’s urine was positive for Blastomyces antigen (1.04 ng/mL). Chest radiography demonstrated a localized infiltrate in the right hilum with possible mass effect. Computed tomography showed a consolidative opacity measuring 4.0×3.4 cm in the upper lobe of the right lung (Figure 5).
| 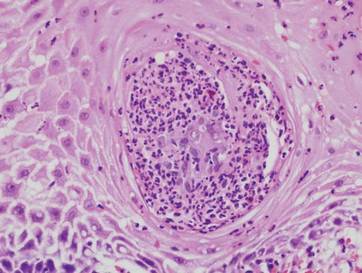
|
The patient was diagnosed with cutaneous North American blastomycosis and prescribed a 6-month course of oral itraconazole 200 mg twice daily. At his 3-month follow-up visit, the perianal plaque hadalmost completely resolved (Figure 6). However, because the patient had increasing lower extremity edema, subjective hearing loss, and abnormal liver function tests, itraconazole treatment was discontinued and replaced with oral fluconazole 400 mg daily for the next 3 months. The right hilar mass had visibly improved on follow-up chest radiography 2 months after the patient started antifungal therapy with itraconazole and had resolved within another 3 months of treatment.
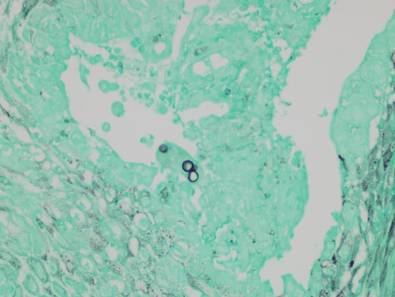
| 
|
Comment
Cutaneous blastomycosis results most often from the hematogenous spread of B dermatitidis from the lungs and rarely from direct inoculation.5,10 Skin lesions tend to occur on exposed areas, such as the face, scalp, hands, wrists, feet, and ankles.7,11-13 Dissemination to the perianal skin is rare, though it has been reported in 2 other patients; both patients, similar to our patient, had evidence of pulmonary involvement at some point in their clinical course.9,14
Diagnosis is based on identification of B dermatitidis by microscopy or culture. Potassium hydroxide preparation of biopsy specimens typically shows broad-based budding yeast.13 Characteristic findings of histopathologic studies include pseudo-epitheliomatous hyperplasia, intraepidermal abscesses, and a dermal infiltrate of polymorphonuclear leukocytes.15 On fungal culture, B dermatitidis is slow growing and may require a 2- to 4-week incubation period. Serologic tests are available, but sensitivity is low, at 9%, 28%, and 77% for complement fixation, immunodiffusion, and enzyme immunoassay, respectively.16
Conclusion
North American blastomycosis should be considered in patients who have verrucous or ulcerative perianal lesions and have lived in or traveled to endemic regions, especially if they have recent or ongoing pulmonary symptoms. Potassium hydroxide preparation and fungal staining of biopsy specimens can aid in diagnosis.
Acknowledgment
The authors thank the Marshfield Clinic Research Foundation’s Office of Scientific Writing and Publication (Marshfield, Wisconsin) for editorial assistance in the preparation of this manuscript.
1. Klein BS, Vergeront JM, Davis JP. Epidemiologic aspects of blastomycosis, the enigmatic systemic mycosis. Semin Respir Infect. 1986;1:29-39.
2. Klein BS, Vergeront JM, Weeks RJ, et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314:529-534.
3. Reingold AL, Lu XD, Plikaytis BD, et al. Systemic mycoses in the United States, 1980-1982. J Med Vet Mycol. 1986;24:433-436.
4. Centers for Disease Control and Prevention (CDC). Blastomycosis—Wisconsin, 1986-1995. MMWR Morb Mortal Wkly Rep. 1996;45:601-603.
5. Smith JA, Kauffman CA. Blastomycosis. Proc Am Thorac Soc. 2010;7:173-180.
6. Goldman M, Johnson PC, Sarosi GA. Fungal pneumonias. the endemic mycoses. Clin Chest Med. 1999;20:507-519.
7. Mercurio MG, Elewski BE. Cutaneous blastomycosis. Cutis. 1992;50:422-424.
8. Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-381.
9. Ricciardi R, Alavi K, Filice GA, et al. Blastomyces dermatitidis of the perianal skin: report of a case. Dis Colon Rectum. 2007;50:118-121.
10. Gray NA, Baddour LM. Cutaneous inoculation blastomycosis [published online ahead of print April 17, 2002]. Clin Infect Dis. 2002;34:e44-e49.
11. Kisso B, Mahmoud F, Thakkar JR. Blastomycosis presenting as recurrent tender cutaneous nodules. S D Med. 2006;59:255-259.
12. Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010.
13. Mason AR, Cortes GY, Cook J, et al. Cutaneous blastomycosis: a diagnostic challenge. Int J Dermatol. 2008;47:824-830.
14. Linn JE. Pseudo-epitheliomatous lesions of the perirectal tissue: report of a case of squamous epithelioma due to blastomycosis. South Med J. 1958;51:1101-1104.
15. Woofter MJ, Cripps DJ, Warner TF. Verrucous plaques on the face. North American blastomycosis. Arch Dermatol. 2000;136:547, 550.
16. Klein BS, Vergeront JM, Kaufman L, et al. Serological tests for blastomycosis: assessments during a large point-source outbreak in Wisconsin. J Infect Dis. 1987;155:262-268.
Cutaneous North American blastomycosis is a deep fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus that is endemic to the Great Lakes region as well as the Mississippi and Ohio River valleys where it thrives in moist acidic soil enriched with organic material.1,2 In humans, the annual incidence rate is estimated to be 0.6 cases per million,3 though it may be as high as 42 cases per 100,000 in endemic areas.4 Infection typically results from the inhalation of conidia and manifests as either acute or chronic pneumonia.5 Most patients with acute disease present with nonspecific flulike symptoms and a nonproductive cough.
Dissemination occurs in approximately 25% of cases,6 most commonly affecting the skin. Other potential sites of dissemination include bone, the genitourinary tract, and the central nervous system. Cutaneous lesions, which may be either verrucous or ulcerative plaques, often occur on or around orifices contiguous to the respiratory tract.7 Verrucous lesions tend to have an irregular shape with well-defined borders and surface crusting. Ulcerative lesions have heaped-up borders and often have an exudative base.8 The differential diagnosis of cutaneous North American blastomycosis lesions includes squamous cell carcinoma, giant keratoacanthoma, verrucae, basal cell carcinoma, scrofuloderma, lupus vulgaris, nocardiosis, syphilis, bromoderma, iododerma, granuloma inguinale, tuberculosis verrucosa cutis, mycetoma, and actinomycosis.7,8
Although periorificial cutaneous manifestations of disseminated blastomycosis are common, perianal lesions are rare. The differential diagnosis of perianal verrucous plaques includes condyloma acuminatum, squamous cell carcinoma, adenocarcinoma, Buschke-Löwenstein tumor, actinomycosis, and localized fungal infections such as blastomycosis.9
Case Report
A 57-year-old man presented with a palpable perianal mass that produced small amounts of blood in his underwear and on toilet paper. The patient reported no history of hemorrhoids, anoreceptive intercourse, or sexually transmitted disease. Four months prior to presentation, he had a prolonged upper respiratory tract illness with a subjective fever and productive cough of 2 months’ duration. The patient described himself as an avid outdoorsman who worked at a summer resort and spent a great deal of time in the forests of central Wisconsin last autumn. Physical examination revealed a well-demarcated, firm, moist plaque with a verrucous surface that measured 3.5×2.7 cm and extended from the anal verge to the perianal skin (Figure 1).
Potassium hydroxide preparation of a biopsy specimen (Figure 2), a punch biopsy of the lesion (Figure 3), and Gomori methenamine-silver staining (Figure 4) revealed scattered yeast spores, some demonstrating broad-based budding, with pseudoepitheliomatous hyperplasia, dermal neutrophils, and intraepithelial microabscesses. The patient’s urine was positive for Blastomyces antigen (1.04 ng/mL). Chest radiography demonstrated a localized infiltrate in the right hilum with possible mass effect. Computed tomography showed a consolidative opacity measuring 4.0×3.4 cm in the upper lobe of the right lung (Figure 5).

| 
|
The patient was diagnosed with cutaneous North American blastomycosis and prescribed a 6-month course of oral itraconazole 200 mg twice daily. At his 3-month follow-up visit, the perianal plaque hadalmost completely resolved (Figure 6). However, because the patient had increasing lower extremity edema, subjective hearing loss, and abnormal liver function tests, itraconazole treatment was discontinued and replaced with oral fluconazole 400 mg daily for the next 3 months. The right hilar mass had visibly improved on follow-up chest radiography 2 months after the patient started antifungal therapy with itraconazole and had resolved within another 3 months of treatment.

| 
|
Comment
Cutaneous blastomycosis results most often from the hematogenous spread of B dermatitidis from the lungs and rarely from direct inoculation.5,10 Skin lesions tend to occur on exposed areas, such as the face, scalp, hands, wrists, feet, and ankles.7,11-13 Dissemination to the perianal skin is rare, though it has been reported in 2 other patients; both patients, similar to our patient, had evidence of pulmonary involvement at some point in their clinical course.9,14
Diagnosis is based on identification of B dermatitidis by microscopy or culture. Potassium hydroxide preparation of biopsy specimens typically shows broad-based budding yeast.13 Characteristic findings of histopathologic studies include pseudo-epitheliomatous hyperplasia, intraepidermal abscesses, and a dermal infiltrate of polymorphonuclear leukocytes.15 On fungal culture, B dermatitidis is slow growing and may require a 2- to 4-week incubation period. Serologic tests are available, but sensitivity is low, at 9%, 28%, and 77% for complement fixation, immunodiffusion, and enzyme immunoassay, respectively.16
Conclusion
North American blastomycosis should be considered in patients who have verrucous or ulcerative perianal lesions and have lived in or traveled to endemic regions, especially if they have recent or ongoing pulmonary symptoms. Potassium hydroxide preparation and fungal staining of biopsy specimens can aid in diagnosis.
Acknowledgment
The authors thank the Marshfield Clinic Research Foundation’s Office of Scientific Writing and Publication (Marshfield, Wisconsin) for editorial assistance in the preparation of this manuscript.
Cutaneous North American blastomycosis is a deep fungal infection caused by Blastomyces dermatitidis, a thermally dimorphic fungus that is endemic to the Great Lakes region as well as the Mississippi and Ohio River valleys where it thrives in moist acidic soil enriched with organic material.1,2 In humans, the annual incidence rate is estimated to be 0.6 cases per million,3 though it may be as high as 42 cases per 100,000 in endemic areas.4 Infection typically results from the inhalation of conidia and manifests as either acute or chronic pneumonia.5 Most patients with acute disease present with nonspecific flulike symptoms and a nonproductive cough.
Dissemination occurs in approximately 25% of cases,6 most commonly affecting the skin. Other potential sites of dissemination include bone, the genitourinary tract, and the central nervous system. Cutaneous lesions, which may be either verrucous or ulcerative plaques, often occur on or around orifices contiguous to the respiratory tract.7 Verrucous lesions tend to have an irregular shape with well-defined borders and surface crusting. Ulcerative lesions have heaped-up borders and often have an exudative base.8 The differential diagnosis of cutaneous North American blastomycosis lesions includes squamous cell carcinoma, giant keratoacanthoma, verrucae, basal cell carcinoma, scrofuloderma, lupus vulgaris, nocardiosis, syphilis, bromoderma, iododerma, granuloma inguinale, tuberculosis verrucosa cutis, mycetoma, and actinomycosis.7,8
Although periorificial cutaneous manifestations of disseminated blastomycosis are common, perianal lesions are rare. The differential diagnosis of perianal verrucous plaques includes condyloma acuminatum, squamous cell carcinoma, adenocarcinoma, Buschke-Löwenstein tumor, actinomycosis, and localized fungal infections such as blastomycosis.9
Case Report
A 57-year-old man presented with a palpable perianal mass that produced small amounts of blood in his underwear and on toilet paper. The patient reported no history of hemorrhoids, anoreceptive intercourse, or sexually transmitted disease. Four months prior to presentation, he had a prolonged upper respiratory tract illness with a subjective fever and productive cough of 2 months’ duration. The patient described himself as an avid outdoorsman who worked at a summer resort and spent a great deal of time in the forests of central Wisconsin last autumn. Physical examination revealed a well-demarcated, firm, moist plaque with a verrucous surface that measured 3.5×2.7 cm and extended from the anal verge to the perianal skin (Figure 1).
Potassium hydroxide preparation of a biopsy specimen (Figure 2), a punch biopsy of the lesion (Figure 3), and Gomori methenamine-silver staining (Figure 4) revealed scattered yeast spores, some demonstrating broad-based budding, with pseudoepitheliomatous hyperplasia, dermal neutrophils, and intraepithelial microabscesses. The patient’s urine was positive for Blastomyces antigen (1.04 ng/mL). Chest radiography demonstrated a localized infiltrate in the right hilum with possible mass effect. Computed tomography showed a consolidative opacity measuring 4.0×3.4 cm in the upper lobe of the right lung (Figure 5).

| 
|
The patient was diagnosed with cutaneous North American blastomycosis and prescribed a 6-month course of oral itraconazole 200 mg twice daily. At his 3-month follow-up visit, the perianal plaque hadalmost completely resolved (Figure 6). However, because the patient had increasing lower extremity edema, subjective hearing loss, and abnormal liver function tests, itraconazole treatment was discontinued and replaced with oral fluconazole 400 mg daily for the next 3 months. The right hilar mass had visibly improved on follow-up chest radiography 2 months after the patient started antifungal therapy with itraconazole and had resolved within another 3 months of treatment.

| 
|
Comment
Cutaneous blastomycosis results most often from the hematogenous spread of B dermatitidis from the lungs and rarely from direct inoculation.5,10 Skin lesions tend to occur on exposed areas, such as the face, scalp, hands, wrists, feet, and ankles.7,11-13 Dissemination to the perianal skin is rare, though it has been reported in 2 other patients; both patients, similar to our patient, had evidence of pulmonary involvement at some point in their clinical course.9,14
Diagnosis is based on identification of B dermatitidis by microscopy or culture. Potassium hydroxide preparation of biopsy specimens typically shows broad-based budding yeast.13 Characteristic findings of histopathologic studies include pseudo-epitheliomatous hyperplasia, intraepidermal abscesses, and a dermal infiltrate of polymorphonuclear leukocytes.15 On fungal culture, B dermatitidis is slow growing and may require a 2- to 4-week incubation period. Serologic tests are available, but sensitivity is low, at 9%, 28%, and 77% for complement fixation, immunodiffusion, and enzyme immunoassay, respectively.16
Conclusion
North American blastomycosis should be considered in patients who have verrucous or ulcerative perianal lesions and have lived in or traveled to endemic regions, especially if they have recent or ongoing pulmonary symptoms. Potassium hydroxide preparation and fungal staining of biopsy specimens can aid in diagnosis.
Acknowledgment
The authors thank the Marshfield Clinic Research Foundation’s Office of Scientific Writing and Publication (Marshfield, Wisconsin) for editorial assistance in the preparation of this manuscript.
1. Klein BS, Vergeront JM, Davis JP. Epidemiologic aspects of blastomycosis, the enigmatic systemic mycosis. Semin Respir Infect. 1986;1:29-39.
2. Klein BS, Vergeront JM, Weeks RJ, et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314:529-534.
3. Reingold AL, Lu XD, Plikaytis BD, et al. Systemic mycoses in the United States, 1980-1982. J Med Vet Mycol. 1986;24:433-436.
4. Centers for Disease Control and Prevention (CDC). Blastomycosis—Wisconsin, 1986-1995. MMWR Morb Mortal Wkly Rep. 1996;45:601-603.
5. Smith JA, Kauffman CA. Blastomycosis. Proc Am Thorac Soc. 2010;7:173-180.
6. Goldman M, Johnson PC, Sarosi GA. Fungal pneumonias. the endemic mycoses. Clin Chest Med. 1999;20:507-519.
7. Mercurio MG, Elewski BE. Cutaneous blastomycosis. Cutis. 1992;50:422-424.
8. Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-381.
9. Ricciardi R, Alavi K, Filice GA, et al. Blastomyces dermatitidis of the perianal skin: report of a case. Dis Colon Rectum. 2007;50:118-121.
10. Gray NA, Baddour LM. Cutaneous inoculation blastomycosis [published online ahead of print April 17, 2002]. Clin Infect Dis. 2002;34:e44-e49.
11. Kisso B, Mahmoud F, Thakkar JR. Blastomycosis presenting as recurrent tender cutaneous nodules. S D Med. 2006;59:255-259.
12. Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010.
13. Mason AR, Cortes GY, Cook J, et al. Cutaneous blastomycosis: a diagnostic challenge. Int J Dermatol. 2008;47:824-830.
14. Linn JE. Pseudo-epitheliomatous lesions of the perirectal tissue: report of a case of squamous epithelioma due to blastomycosis. South Med J. 1958;51:1101-1104.
15. Woofter MJ, Cripps DJ, Warner TF. Verrucous plaques on the face. North American blastomycosis. Arch Dermatol. 2000;136:547, 550.
16. Klein BS, Vergeront JM, Kaufman L, et al. Serological tests for blastomycosis: assessments during a large point-source outbreak in Wisconsin. J Infect Dis. 1987;155:262-268.
1. Klein BS, Vergeront JM, Davis JP. Epidemiologic aspects of blastomycosis, the enigmatic systemic mycosis. Semin Respir Infect. 1986;1:29-39.
2. Klein BS, Vergeront JM, Weeks RJ, et al. Isolation of Blastomyces dermatitidis in soil associated with a large outbreak of blastomycosis in Wisconsin. N Engl J Med. 1986;314:529-534.
3. Reingold AL, Lu XD, Plikaytis BD, et al. Systemic mycoses in the United States, 1980-1982. J Med Vet Mycol. 1986;24:433-436.
4. Centers for Disease Control and Prevention (CDC). Blastomycosis—Wisconsin, 1986-1995. MMWR Morb Mortal Wkly Rep. 1996;45:601-603.
5. Smith JA, Kauffman CA. Blastomycosis. Proc Am Thorac Soc. 2010;7:173-180.
6. Goldman M, Johnson PC, Sarosi GA. Fungal pneumonias. the endemic mycoses. Clin Chest Med. 1999;20:507-519.
7. Mercurio MG, Elewski BE. Cutaneous blastomycosis. Cutis. 1992;50:422-424.
8. Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23:367-381.
9. Ricciardi R, Alavi K, Filice GA, et al. Blastomyces dermatitidis of the perianal skin: report of a case. Dis Colon Rectum. 2007;50:118-121.
10. Gray NA, Baddour LM. Cutaneous inoculation blastomycosis [published online ahead of print April 17, 2002]. Clin Infect Dis. 2002;34:e44-e49.
11. Kisso B, Mahmoud F, Thakkar JR. Blastomycosis presenting as recurrent tender cutaneous nodules. S D Med. 2006;59:255-259.
12. Mandell GL, Bennett JE, Dolin R. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2010.
13. Mason AR, Cortes GY, Cook J, et al. Cutaneous blastomycosis: a diagnostic challenge. Int J Dermatol. 2008;47:824-830.
14. Linn JE. Pseudo-epitheliomatous lesions of the perirectal tissue: report of a case of squamous epithelioma due to blastomycosis. South Med J. 1958;51:1101-1104.
15. Woofter MJ, Cripps DJ, Warner TF. Verrucous plaques on the face. North American blastomycosis. Arch Dermatol. 2000;136:547, 550.
16. Klein BS, Vergeront JM, Kaufman L, et al. Serological tests for blastomycosis: assessments during a large point-source outbreak in Wisconsin. J Infect Dis. 1987;155:262-268.
Practice Points
- Cutaneous North American blastomycosis usually occurs in a periorificial distribution.
- The perianal region should be included in the periorificial regions considered in North American blastomycosis infections.
Cosmetic Corner: Dermatologists Weigh in on Products for Sensitive Skin
To improve patient care and outcomes, leading dermatologists offered their recommendations on top products for sensitive skin. Consideration must be given to:
- Aveeno Eczema Therapy Moisturizing Cream
- Cetaphil Restoraderm
- PRESCRIBEDsolutions Don’t Be So Sensitive Post-Procedure Cleanser
- Rosaliac AR Intense
- Vanicream
Cutis invites readers to send us their recommendations. Skin care products for babies, men’s shaving products, eye creams, and OTC dandruff treatments will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on top products for sensitive skin. Consideration must be given to:
- Aveeno Eczema Therapy Moisturizing Cream
- Cetaphil Restoraderm
- PRESCRIBEDsolutions Don’t Be So Sensitive Post-Procedure Cleanser
- Rosaliac AR Intense
- Vanicream
Cutis invites readers to send us their recommendations. Skin care products for babies, men’s shaving products, eye creams, and OTC dandruff treatments will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.
To improve patient care and outcomes, leading dermatologists offered their recommendations on top products for sensitive skin. Consideration must be given to:
- Aveeno Eczema Therapy Moisturizing Cream
- Cetaphil Restoraderm
- PRESCRIBEDsolutions Don’t Be So Sensitive Post-Procedure Cleanser
- Rosaliac AR Intense
- Vanicream
Cutis invites readers to send us their recommendations. Skin care products for babies, men’s shaving products, eye creams, and OTC dandruff treatments will be featured in upcoming editions of Cosmetic Corner. Please e-mail your recommendation(s) to the Editorial Office.
Disclaimer: Opinions expressed herein do not necessarily reflect those of Cutis or Frontline Medical Communications Inc. and shall not be used for product endorsement purposes. Any reference made to a specific commercial product does not indicate or imply that Cutis or Frontline Medical Communications Inc. endorses, recommends, or favors the product mentioned. No guarantee is given to the effects of recommended products.