User login
Concomitant Sensitization to Inhaled Budesonide and Oral Nystatin Presenting as Allergic Contact Stomatitis and Systemic Allergic Contact Dermatitis
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
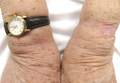
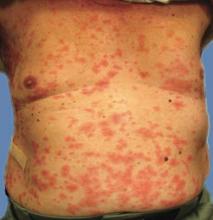
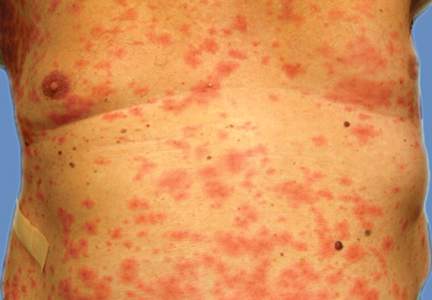
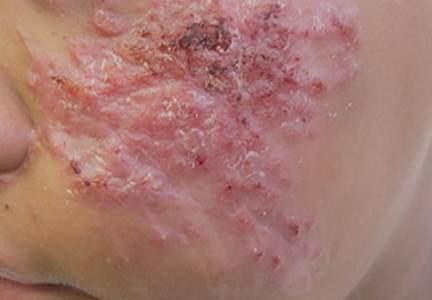
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
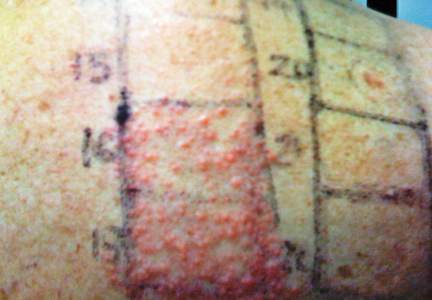
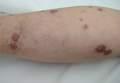
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.
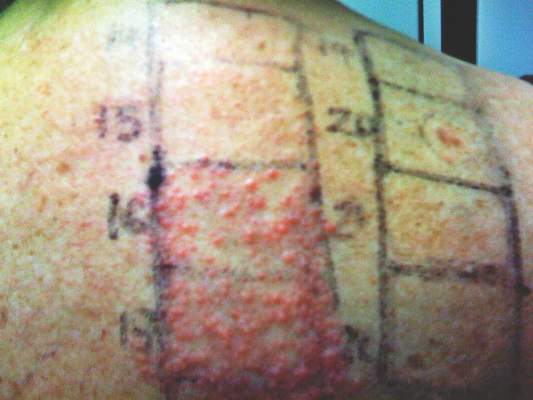
Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8

In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.

Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8

In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
The development of concomitant allergic reactions to multiple drugs is uncommon. Dermatitis induced by topical or inhaled corticosteroids (eg, budesonide) is rare,1 and allergic reactions associated with oral nystatin, a macrolide antifungal drug, also are unusual.2 We present the case of concomitant sensitization to inhaled budesonide and oral nystatin presenting as allergic contact stomatitis and systemic allergic contact dermatitis. Concomitant allergic reactions to these treatments are rare and may result in diagnostic challenges for the physician.
Case Report
A 66-year-old woman presented to the Allergy Department for evaluation of painful erosions on the oral mucosa that had developed 72 hours after she started treatment with inhaled budesonide (400 mcg every 12 hours) prescribed by her general practitioner for a nonproductive cough. Budesonide inhalation was discontinued due to suspected oral candidiasis and treatment with oral nystatin (500,000 IU every 8 hours) was started, but the erosions did not resolve. After 2 days of treatment with oral nystatin, the patient presented with erythematous macules on the abdomen and thighs as well as a larger erythematous and edematous lesion with papules and vesicles on the hypothenar eminence of the right hand. Nystatin was discontinued and the lesions turned desquamative and healed spontaneously 7 days later. The oral lesions resolved after 15 days with no further treatment.
Patch testing was conducted using a commercially standard series of contact allergens, all of which showed negative results at 48 and 96 hours except for budesonide and triamcinolone, which led to the diagnosis of allergic contact stomatitis from the inhaled budesonide. Patch testing with other corticosteroids was negative. Challenge tests with alternative corticosteroids (ie, oral methylprednisolone, parenteral betamethasone, topical mometasone furoate, inhaled fluticasone) were negative.
In order to rule out involvement of oral nystatin, a single-blind, placebo-controlled oral challenge test was performed. Eight hours after taking oral nystatin (500,000 IU), erythematous macules developed on the patient’s abdomen along with an erythematous, 3×4-cm lesion with papules on the hypothenar eminence of the right hand that was similar in appearance to the original presentation. The lesion on the hand was biopsied and histologic examination revealed spongiosis, edema of the superficial dermis, perivascular lymphocytic infiltrates, and extravasated erythrocytes with no vasculitis. Further patch testing subsequently was conducted with antifungal and antibiotic macrolides in different vehicles (ie, petrolatum, water, polyethylene glycol), as well as with excipients of the oral nystatin formulation that had been tested (Figure). Patch testing was positive with nystatin 10% in petrolatum and nystatin 30,000 IU and 90,000 IU in polyethylene glycol. Testing also were conducted in 7 healthy volunteers to rule out an irritant reaction and showed negative results. Finally, challenge tests conducted in our patient with another antifungal macrolide (parenteral amphotericin B) and antibiotic macrolides (oral clarithromycin, erythromycin, and azithromycin) were negative.

Patch and challenge test results along with the histologic findings led to diagnosis of concomitant systemic allergic contact dermatitis from oral nystatin.
Comment
Our patient presented with 2 unusual delayed hypersensitivity reactions that occurred in the same medical episode: allergic contact stomatitis from inhaled budesonide and systemic allergic contact dermatitis from oral nystatin. It is noteworthy that, despite the poor intestinal absorption of nystatin, systemic contact dermatitis to this drug has been previously described.3 Patch testing with macrolides proved useful for diagnosis in our patient, and based on the results we concluded that polyethylene glycol seemed to be the optimal vehicle for patch testing macrolide drugs versus water or petrolatum, as has been previously suggested.4
When a diagnosis of drug allergy is established, it is important to rule out cross-reactivity with other similar drugs by assessing if they produce the same reaction despite differences in chemical structure. Possible cross-reactivity of nystatin with other macrolides (validated on patch testing) has been reported but the tolerability was not evaluated.5 Our patient showed good tolerability to other macrolide drugs, both antibiotics and antifungals. Therefore, nystatin does not seem to cross-react with other structurally related drugs belonging to the macrolide group based on our results.
Corticosteroid allergies are more common than those associated with macrolides, especially contact dermatitis. Nonhalogenated corticosteroids (eg, hydrocortisone, budesonide) are most frequently associated with allergic reactions,6 and patch testing remains the diagnostic method of choice for the detection of delayed hypersensitivity to corticosteroids. In Europe, standard series include budesonide and tixocortol pivalate, and in the United States they include hydrocortisone 17–butyrate, triamcinolone acetonide, and clobetasol 17–propionate.6
To assess cross-reactivity among topical corticosteroids, patch testing with other steroids should be performed. In 1989, Coopman et al7 established a classification system for corticosteroids based on molecular structure, thus dividing them into 4 empirical groups: group A, hydrocortisone type; group B, acetonide type; group C, betamethasone type; and group D, ester type. The investigators hypothesized that allergic contact reactions occurred more frequently with corticosteroids belonging to the same group, while cross-reactions were uncommon between groups; however, cross-reactivity is known to occur among corticosteroids belonging to different groups in standard clinical practice, which conflicts with this claim.
Due to distinctively different behaviors among certain compounds in group D, Matura et al8 proposed subdividing the ester steroids into 2 groups: group D1, containing C16 methyl substitution and halogenation on the B ring, and group D2, comprising the labile ester steroids that lack both substitutions. A modified classification system including these subdivided groups is presented in the Table.8

In recent years, new corticosteroid drugs such as deflazacort, fluticasone propionate, and mometasone furoate have been developed, but classification of these agents has been difficult due to differences in their chemical structure, although mometasone furoate and fluticasone propionate have been included in group D1.9 Futhermore, the structural differences of these new steroids may mean less cross-reactivity with other steroids, which would facilitate their use in patients who are allergic to classic steroids. However, cross-reactivity between mometasone furoate and corticosteroids belonging to group B has already been described,10 which may restrict its use in patients who are allergic to other corticosteroids.
The classification of corticosteroids can provide useful information about cross-reactivity, which may help physicians in choosing an alternative drug in patients with an allergy to topical corticosteroids, but this advice about cross-reactivity does not seem to apply to systemic allergic dermatitis or immediate-type reactions to corticosteroids.11 Therefore, in these types of reactions, an individualized evaluation of the sensitization profile is needed, performing wider studies with alternative corticosteroids by skin tests with late readings and challenge tests.
It is important to emphasize that hypersensitivity to corticosteroids should always be considered in the differential diagnosis along with oral candidiasis when oropharyngeal symptoms appear during inhaled corticosteroid along with oral candidiasis. We recommend that all drugs involved in a presumed allergic reaction must be systematically evaluated because an unexpected concomitant sensitization to multiple drugs could be present.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
- English JS. Corticosteroid-induced contact dermatitis: a pragmatic approach. Clin Exp Dermatol. 2000;25:261-264.
- Martínez FV, Muñoz Pamplona MP, García EC, et al. Delayed hypersensitivity to oral nystatin. Contact Dermatitis. 2007;57:200-201.
- Quirce S, Parra F, Lázaro M, et al. Generalized dermatitis due to oral nystatin. Contact Dermatitis. 1991;25:197-198.
- de Groot AC, Conemans JM. Nystatin allergy: petrolatum is not the optimal vehicle for patch testing. Dermatol Clin. 1990;8:153-155.
- Barranco R, Tornero P, de Barrio M, et al. Type IV hypersensitivity to oral nystatin. Contact Dermatitis. 2001;45:60.
- Jacob SE, Steele T. Corticosteroid classes: a quick reference guide including patch test substances and cross-reactivity. J Am Acad Dermatol. 2006;54:723-727.
- Coopman S, Degreef H, Dooms-Goossens A. Identification of cross-reaction patterns in allergic contact dermatitis from topical corticosteroids. Br J Dermatol. 1989;121:27-34.
- Matura M, Goossens A. Contact allergy to corticosteroids. Allergy. 2000;55:698-704.
- Baeck M, Chamelle JA, Goossens A, et al. Corticosteroid cross-reactivity: clinical and molecular modeling tools. Allergy. 2011;66:1367-1374.
- Seyfarth F, Elsner P, Tittelbach J, et al. Contact allergy to mometasone furoate with cross-reactivity to group B corticosteroids. Contact Dermatitis. 2008;58:180-181.
- Torres MJ, Canto G. Hypersensitivity reactions to corticosteroids. Curr Opin Allergy Clin Immunol. 2010;10:273-279.
Practice Points
- When lesions develop in the oral cavity during treatment with inhaled corticosteroids, delayed contact allergy should be considered in the differential diagnosis along with fungal infection.
- Although it generally is not considered to be allergenic due to its poor intestinal absorption, oral nystatin may induce systemic allergic disorders.
- All drugs involved in a presumed allergic reaction must be evaluated since concomitant sensitization to multiple drugs could be present. Patch and challenge testing should be conducted to diagnose allergic contact dermatitis and assess drug cross-reactivity.
Differentiation of Latex Allergy From Irritant Contact Dermatitis
Latex allergy is an all-encompassing term used to describe hypersensitivity reactions to products containing natural rubber latex from the Hevea brasiliensis tree and affects approximately 1% to 2% of the general population.1 Although latex gloves are the most widely known culprits, several other commonly used products can contain natural rubber latex, including adhesive tape, balloons, condoms, rubber bands, paint, tourniquets, electrode pads, and Foley catheters.2 The term latex allergy often is used as a general diagnosis, but there are in fact 3 distinct mechanisms by which individuals may develop an adverse reaction to latex-containing products: irritant contact dermatitis, allergic contact dermatitis (type IV hypersensitivity) and true latex allergy (type I hypersensitivity).
Irritant Contact Dermatitis
Irritant contact dermatitis, a nonimmunologic reaction, occurs due to mechanical factors (eg, friction) or contact with chemicals, which can have irritating and dehydrating effects. Individuals with irritant contact dermatitis do not have true latex allergy and will not necessarily develop a reaction to products containing natural rubber latex. Incorrectly attributing these irritant contact dermatitis reactions to latex allergy and simply advising patients to avoid all latex products (eg, use nitrile gloves rather than latex gloves) will not address the underlying problem. Rather, these patients must be informed that the dermatitis is a result of a disruption to the natural, protective skin barrier and not an allergic reaction.
Allergic Contact Dermatitis
Allergic contact dermatitis to rubber is caused by a type IV (delayed) hypersensitivity reaction and is the result of exposure to the accelerators present in rubber products in sensitive individuals. Individuals experiencing this type of reaction typically develop localized erythema, pruritus, and urticarial lesions 48 hours after exposure.3 Incorrectly labeling this problem as latex allergy and recommending nonlatex rubber substitutes (eg, hypoallergenic gloves) likely will not be effective, as these nonlatex replacement products contain the same accelerators as do latex gloves.
True Latex Allergy
The most severe form of latex allergy, often referred to as true latex allergy, is caused by a type I (immediate) hypersensitivity reaction mediated by immunoglobulin E (IgE) antibodies. Individuals experiencing this type of reaction have a systemic response to latex proteins that may result in fulminant anaphylaxis. Individuals with true latex allergy must absolutely avoid latex products, and substituting nonlatex products is the most effective approach.
Latex Reactions in Medical Practice
The varying propensity of certain populations to develop latex allergy has been well documented; for example, the prevalence of hypersensitivity in patients with spina bifida ranges from 20% to 65%, figures that are much higher than those reported in the general population.3 This hypersensitivity in patients with spina bifida most likely results from repeated exposure to latex products during corrective surgeries and diagnostic procedures early in life. Atopic individuals, such as those with allergic rhinitis, eczema, and asthma, have a 4-fold increased risk for developing latex allergy compared to nonatopic individuals.4 The risk of latex allergy among health care workers is increased due to increased exposure to rubber products. One study found that the risk of latex sensitization among health care workers exposed to products containing latex was 4.3%, while the risk in the general population was only 1.37%.1 Those at highest risk for sensitization include dental assistants, operating room personnel, hospital housekeeping staff, and paramedics or emergency medical technicians.3 However, sensitization documented on laboratory assessment does not reliably correlate with symptomatic allergy, as many patients with a positive IgE test do not show clinical symptoms. Schmid et al4 demonstrated that a 1.3% prevalence of clinically symptomatic latex allergy among health care workers may approximate the prevalence of latex allergy in the general population. In a study by Brown et al,5 although 12.5% of anesthesiologists were found to be sensitized to latex, only 2.4% had clinically symptomatic allergic reactions.
Testing for Latex Allergy
Several diagnostic tests are available to establish a diagnosis of type I sensitization or true latex allergy. Skin prick testing is an in vivo assay and is the gold standard for diagnosing IgE-mediated type I hypersensitivity to latex. The test involves pricking the skin of the forearm and applying a commercial extract of nonammoniated latex to monitor for development of a wheal within several minutes. The skin prick test should be performed in a health care setting equipped with oxygen, epinephrine, and latex-free resuscitation equipment in case of anaphylaxis following exposure. Although latex skin prick testing is the gold standard, it is rarely performed in the United States because there is no US Food and Drug Administration–approved natural rubber latex reagent.3 Consequently, physicians who wish to perform skin prick testing for latex allergy are forced to develop improvised reagents from the H brasiliensis tree itself or from highly allergenic latex gloves. Standardized latex allergens are commercially available in Europe.
The most noninvasive method of latex allergy testing is an in vitro assay for latex-specific IgE antibodies, which can be detected by either a radioallergosorbent test (RAST) or enzyme-linked immunosorbent assay (ELISA). The presence of antilatex IgE antibodies confirms sensitization but does not necessarily mean the patient will develop a symptomatic reaction following exposure. Due to the unavailability of a standardized reagent for the skin prick test in the United States, evaluation of latex-specific serum IgE levels may be the best alternative. While the skin prick test has the highest sensitivity, the sensitivity and specificity of latex-specific serum IgE testing are 50% to 90% and 80% to 87%, respectively.6
The wear test (also known as the use or glove provocation test), can be used to diagnose clinically symptomatic latex allergy when there is a discrepancy between the patient’s clinical history and results from skin prick or serum IgE antibody testing. To perform the wear test, place a natural rubber latex glove on one of the patient’s fingers for 15 minutes and monitor the area for development of urticaria. If there is no evidence of allergic reaction within 15 minutes, place the glove on the whole hand for an additional 15 minutes. The patient is said to be nonreactive if a latex glove can be placed on the entire hand for 15 minutes without evidence of reaction.3
Lastly, patch testing can differentiate between irritant contact and allergic contact (type IV hypersensitivity) dermatitis. Apply a small amount of each substance of interest onto a separate disc and place the discs in direct contact with the skin using hypoallergenic tape. With type IV latex hypersensitivity, the skin underneath the disc will become erythematous with developing papulovesicles, starting between 2 and 5 days after exposure. The Figure outlines the differentiation of true latex allergy from irritant and allergic contact dermatitis and identifies methods for making these diagnoses.
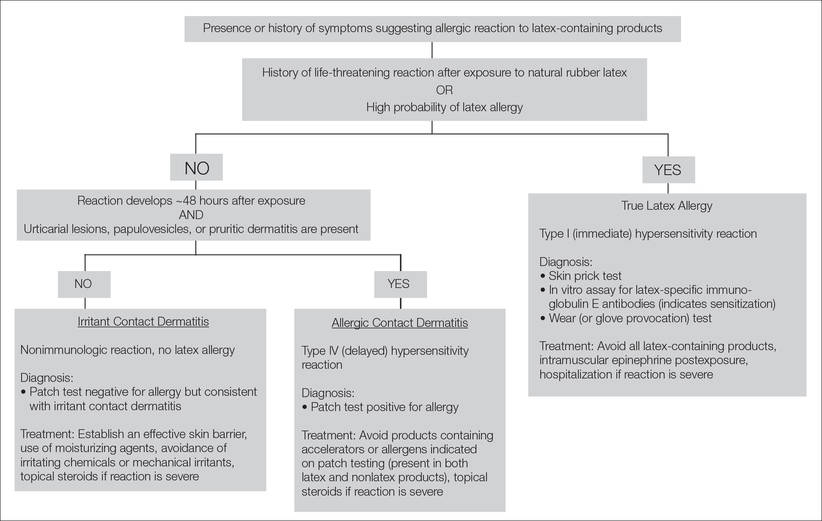
General Medical Protocol With Latex Reactions
To reduce the incidence of latex allergic reactions among health care workers and patients, Kumar2 recommends putting a protocol in place to document steps in preventing, diagnosing, and treating latex allergy. This protocol includes employee and patient education about the risks for developing latex allergy and the signs and symptoms of a reaction; available diagnostic testing; and alternative products (eg, hypoallergenic gloves) that are available to individuals with a known or suspected allergy. At-risk health care workers who have not been sensitized should be advised to avoid latex-containing products.3 Routine questioning and diagnostic testing may be necessary as part of every preoperative assessment, as there have been reported cases of anaphylaxis in patients with undocumented allergies.7 Anaphylaxis caused by latex allergy is the second leading cause of perioperative anaphylaxis, accounting for as many as 20% of cases.8 With the use of preventative measures and early identification of at-risk patients, the incidence of latex-related anaphylaxis is decreasing.8 Ascertaining valuable information about the patient’s medical history, such as known allergies to foods that have cross-reactivity to latex (eg, bananas, mango, kiwi, avocado), is one simple way of identifying a patient who should be tested for possible underlying latex allergy.8 Total avoidance of latex-containing products (eg, in the workplace) can further reduce the incidence of allergic reactions by decreasing primary sensitization and risk of exposure.
Conclusion
Patients claiming to be allergic to latex without documentation should be tested. The diagnostic testing available in the United States includes patch testing, wear (or glove provocation) testing, or assessment of IgE antibody titer. Accurate differentiation among irritant contact dermatitis, allergic contact dermatitis, and true latex allergy is paramount for properly educating patients and effectively treating these conditions. Additionally, distinguishing patients with true latex allergy from those who have been misdiagnosed can save resources and reduce health care costs.
- Bousquet J, Flahault A, Vandenplas O, et al. Natural rubber latex allergy among health care workers: a systematic review of the evidence. J Allergy Clin Immunol. 2006;118:447-454.
- Kumar RP. Latex allergy in clinical practice. Indian J Dermatol. 2012;57:66-70.
- Taylor JS, Erkek E. Latex allergy: diagnosis and management. Dermatol Ther. 2004;17:289-301.
- Schmid K, Christoph Broding H, Niklas D, et al. Latex sensitization in dental students using powder-free gloves low in latex protein: a cross-sectional study. Contact Dermatitis. 2002;47:103-108.
- Brown RH, Schauble JF, Hamilton RG. Prevalence of latex allergy among anesthesiologists: identification of sensitized but asymptomatic individuals. Anesthesiology. 1998;89:292-299.
- Pollart SM, Warniment C, Mori T. Latex allergy. Am Fam Physician. 2009;80:1413-1418.
- Duger C, Kol IO, Kaygusuz K, et al. A perioperative anaphylactic reaction caused by latex in a patient with no history of allergy. Anaesth Pain Intensive Care. 2012;16:71-73.
- Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003;97:1381-1395.
Latex allergy is an all-encompassing term used to describe hypersensitivity reactions to products containing natural rubber latex from the Hevea brasiliensis tree and affects approximately 1% to 2% of the general population.1 Although latex gloves are the most widely known culprits, several other commonly used products can contain natural rubber latex, including adhesive tape, balloons, condoms, rubber bands, paint, tourniquets, electrode pads, and Foley catheters.2 The term latex allergy often is used as a general diagnosis, but there are in fact 3 distinct mechanisms by which individuals may develop an adverse reaction to latex-containing products: irritant contact dermatitis, allergic contact dermatitis (type IV hypersensitivity) and true latex allergy (type I hypersensitivity).
Irritant Contact Dermatitis
Irritant contact dermatitis, a nonimmunologic reaction, occurs due to mechanical factors (eg, friction) or contact with chemicals, which can have irritating and dehydrating effects. Individuals with irritant contact dermatitis do not have true latex allergy and will not necessarily develop a reaction to products containing natural rubber latex. Incorrectly attributing these irritant contact dermatitis reactions to latex allergy and simply advising patients to avoid all latex products (eg, use nitrile gloves rather than latex gloves) will not address the underlying problem. Rather, these patients must be informed that the dermatitis is a result of a disruption to the natural, protective skin barrier and not an allergic reaction.
Allergic Contact Dermatitis
Allergic contact dermatitis to rubber is caused by a type IV (delayed) hypersensitivity reaction and is the result of exposure to the accelerators present in rubber products in sensitive individuals. Individuals experiencing this type of reaction typically develop localized erythema, pruritus, and urticarial lesions 48 hours after exposure.3 Incorrectly labeling this problem as latex allergy and recommending nonlatex rubber substitutes (eg, hypoallergenic gloves) likely will not be effective, as these nonlatex replacement products contain the same accelerators as do latex gloves.
True Latex Allergy
The most severe form of latex allergy, often referred to as true latex allergy, is caused by a type I (immediate) hypersensitivity reaction mediated by immunoglobulin E (IgE) antibodies. Individuals experiencing this type of reaction have a systemic response to latex proteins that may result in fulminant anaphylaxis. Individuals with true latex allergy must absolutely avoid latex products, and substituting nonlatex products is the most effective approach.
Latex Reactions in Medical Practice
The varying propensity of certain populations to develop latex allergy has been well documented; for example, the prevalence of hypersensitivity in patients with spina bifida ranges from 20% to 65%, figures that are much higher than those reported in the general population.3 This hypersensitivity in patients with spina bifida most likely results from repeated exposure to latex products during corrective surgeries and diagnostic procedures early in life. Atopic individuals, such as those with allergic rhinitis, eczema, and asthma, have a 4-fold increased risk for developing latex allergy compared to nonatopic individuals.4 The risk of latex allergy among health care workers is increased due to increased exposure to rubber products. One study found that the risk of latex sensitization among health care workers exposed to products containing latex was 4.3%, while the risk in the general population was only 1.37%.1 Those at highest risk for sensitization include dental assistants, operating room personnel, hospital housekeeping staff, and paramedics or emergency medical technicians.3 However, sensitization documented on laboratory assessment does not reliably correlate with symptomatic allergy, as many patients with a positive IgE test do not show clinical symptoms. Schmid et al4 demonstrated that a 1.3% prevalence of clinically symptomatic latex allergy among health care workers may approximate the prevalence of latex allergy in the general population. In a study by Brown et al,5 although 12.5% of anesthesiologists were found to be sensitized to latex, only 2.4% had clinically symptomatic allergic reactions.
Testing for Latex Allergy
Several diagnostic tests are available to establish a diagnosis of type I sensitization or true latex allergy. Skin prick testing is an in vivo assay and is the gold standard for diagnosing IgE-mediated type I hypersensitivity to latex. The test involves pricking the skin of the forearm and applying a commercial extract of nonammoniated latex to monitor for development of a wheal within several minutes. The skin prick test should be performed in a health care setting equipped with oxygen, epinephrine, and latex-free resuscitation equipment in case of anaphylaxis following exposure. Although latex skin prick testing is the gold standard, it is rarely performed in the United States because there is no US Food and Drug Administration–approved natural rubber latex reagent.3 Consequently, physicians who wish to perform skin prick testing for latex allergy are forced to develop improvised reagents from the H brasiliensis tree itself or from highly allergenic latex gloves. Standardized latex allergens are commercially available in Europe.
The most noninvasive method of latex allergy testing is an in vitro assay for latex-specific IgE antibodies, which can be detected by either a radioallergosorbent test (RAST) or enzyme-linked immunosorbent assay (ELISA). The presence of antilatex IgE antibodies confirms sensitization but does not necessarily mean the patient will develop a symptomatic reaction following exposure. Due to the unavailability of a standardized reagent for the skin prick test in the United States, evaluation of latex-specific serum IgE levels may be the best alternative. While the skin prick test has the highest sensitivity, the sensitivity and specificity of latex-specific serum IgE testing are 50% to 90% and 80% to 87%, respectively.6
The wear test (also known as the use or glove provocation test), can be used to diagnose clinically symptomatic latex allergy when there is a discrepancy between the patient’s clinical history and results from skin prick or serum IgE antibody testing. To perform the wear test, place a natural rubber latex glove on one of the patient’s fingers for 15 minutes and monitor the area for development of urticaria. If there is no evidence of allergic reaction within 15 minutes, place the glove on the whole hand for an additional 15 minutes. The patient is said to be nonreactive if a latex glove can be placed on the entire hand for 15 minutes without evidence of reaction.3
Lastly, patch testing can differentiate between irritant contact and allergic contact (type IV hypersensitivity) dermatitis. Apply a small amount of each substance of interest onto a separate disc and place the discs in direct contact with the skin using hypoallergenic tape. With type IV latex hypersensitivity, the skin underneath the disc will become erythematous with developing papulovesicles, starting between 2 and 5 days after exposure. The Figure outlines the differentiation of true latex allergy from irritant and allergic contact dermatitis and identifies methods for making these diagnoses.

General Medical Protocol With Latex Reactions
To reduce the incidence of latex allergic reactions among health care workers and patients, Kumar2 recommends putting a protocol in place to document steps in preventing, diagnosing, and treating latex allergy. This protocol includes employee and patient education about the risks for developing latex allergy and the signs and symptoms of a reaction; available diagnostic testing; and alternative products (eg, hypoallergenic gloves) that are available to individuals with a known or suspected allergy. At-risk health care workers who have not been sensitized should be advised to avoid latex-containing products.3 Routine questioning and diagnostic testing may be necessary as part of every preoperative assessment, as there have been reported cases of anaphylaxis in patients with undocumented allergies.7 Anaphylaxis caused by latex allergy is the second leading cause of perioperative anaphylaxis, accounting for as many as 20% of cases.8 With the use of preventative measures and early identification of at-risk patients, the incidence of latex-related anaphylaxis is decreasing.8 Ascertaining valuable information about the patient’s medical history, such as known allergies to foods that have cross-reactivity to latex (eg, bananas, mango, kiwi, avocado), is one simple way of identifying a patient who should be tested for possible underlying latex allergy.8 Total avoidance of latex-containing products (eg, in the workplace) can further reduce the incidence of allergic reactions by decreasing primary sensitization and risk of exposure.
Conclusion
Patients claiming to be allergic to latex without documentation should be tested. The diagnostic testing available in the United States includes patch testing, wear (or glove provocation) testing, or assessment of IgE antibody titer. Accurate differentiation among irritant contact dermatitis, allergic contact dermatitis, and true latex allergy is paramount for properly educating patients and effectively treating these conditions. Additionally, distinguishing patients with true latex allergy from those who have been misdiagnosed can save resources and reduce health care costs.
Latex allergy is an all-encompassing term used to describe hypersensitivity reactions to products containing natural rubber latex from the Hevea brasiliensis tree and affects approximately 1% to 2% of the general population.1 Although latex gloves are the most widely known culprits, several other commonly used products can contain natural rubber latex, including adhesive tape, balloons, condoms, rubber bands, paint, tourniquets, electrode pads, and Foley catheters.2 The term latex allergy often is used as a general diagnosis, but there are in fact 3 distinct mechanisms by which individuals may develop an adverse reaction to latex-containing products: irritant contact dermatitis, allergic contact dermatitis (type IV hypersensitivity) and true latex allergy (type I hypersensitivity).
Irritant Contact Dermatitis
Irritant contact dermatitis, a nonimmunologic reaction, occurs due to mechanical factors (eg, friction) or contact with chemicals, which can have irritating and dehydrating effects. Individuals with irritant contact dermatitis do not have true latex allergy and will not necessarily develop a reaction to products containing natural rubber latex. Incorrectly attributing these irritant contact dermatitis reactions to latex allergy and simply advising patients to avoid all latex products (eg, use nitrile gloves rather than latex gloves) will not address the underlying problem. Rather, these patients must be informed that the dermatitis is a result of a disruption to the natural, protective skin barrier and not an allergic reaction.
Allergic Contact Dermatitis
Allergic contact dermatitis to rubber is caused by a type IV (delayed) hypersensitivity reaction and is the result of exposure to the accelerators present in rubber products in sensitive individuals. Individuals experiencing this type of reaction typically develop localized erythema, pruritus, and urticarial lesions 48 hours after exposure.3 Incorrectly labeling this problem as latex allergy and recommending nonlatex rubber substitutes (eg, hypoallergenic gloves) likely will not be effective, as these nonlatex replacement products contain the same accelerators as do latex gloves.
True Latex Allergy
The most severe form of latex allergy, often referred to as true latex allergy, is caused by a type I (immediate) hypersensitivity reaction mediated by immunoglobulin E (IgE) antibodies. Individuals experiencing this type of reaction have a systemic response to latex proteins that may result in fulminant anaphylaxis. Individuals with true latex allergy must absolutely avoid latex products, and substituting nonlatex products is the most effective approach.
Latex Reactions in Medical Practice
The varying propensity of certain populations to develop latex allergy has been well documented; for example, the prevalence of hypersensitivity in patients with spina bifida ranges from 20% to 65%, figures that are much higher than those reported in the general population.3 This hypersensitivity in patients with spina bifida most likely results from repeated exposure to latex products during corrective surgeries and diagnostic procedures early in life. Atopic individuals, such as those with allergic rhinitis, eczema, and asthma, have a 4-fold increased risk for developing latex allergy compared to nonatopic individuals.4 The risk of latex allergy among health care workers is increased due to increased exposure to rubber products. One study found that the risk of latex sensitization among health care workers exposed to products containing latex was 4.3%, while the risk in the general population was only 1.37%.1 Those at highest risk for sensitization include dental assistants, operating room personnel, hospital housekeeping staff, and paramedics or emergency medical technicians.3 However, sensitization documented on laboratory assessment does not reliably correlate with symptomatic allergy, as many patients with a positive IgE test do not show clinical symptoms. Schmid et al4 demonstrated that a 1.3% prevalence of clinically symptomatic latex allergy among health care workers may approximate the prevalence of latex allergy in the general population. In a study by Brown et al,5 although 12.5% of anesthesiologists were found to be sensitized to latex, only 2.4% had clinically symptomatic allergic reactions.
Testing for Latex Allergy
Several diagnostic tests are available to establish a diagnosis of type I sensitization or true latex allergy. Skin prick testing is an in vivo assay and is the gold standard for diagnosing IgE-mediated type I hypersensitivity to latex. The test involves pricking the skin of the forearm and applying a commercial extract of nonammoniated latex to monitor for development of a wheal within several minutes. The skin prick test should be performed in a health care setting equipped with oxygen, epinephrine, and latex-free resuscitation equipment in case of anaphylaxis following exposure. Although latex skin prick testing is the gold standard, it is rarely performed in the United States because there is no US Food and Drug Administration–approved natural rubber latex reagent.3 Consequently, physicians who wish to perform skin prick testing for latex allergy are forced to develop improvised reagents from the H brasiliensis tree itself or from highly allergenic latex gloves. Standardized latex allergens are commercially available in Europe.
The most noninvasive method of latex allergy testing is an in vitro assay for latex-specific IgE antibodies, which can be detected by either a radioallergosorbent test (RAST) or enzyme-linked immunosorbent assay (ELISA). The presence of antilatex IgE antibodies confirms sensitization but does not necessarily mean the patient will develop a symptomatic reaction following exposure. Due to the unavailability of a standardized reagent for the skin prick test in the United States, evaluation of latex-specific serum IgE levels may be the best alternative. While the skin prick test has the highest sensitivity, the sensitivity and specificity of latex-specific serum IgE testing are 50% to 90% and 80% to 87%, respectively.6
The wear test (also known as the use or glove provocation test), can be used to diagnose clinically symptomatic latex allergy when there is a discrepancy between the patient’s clinical history and results from skin prick or serum IgE antibody testing. To perform the wear test, place a natural rubber latex glove on one of the patient’s fingers for 15 minutes and monitor the area for development of urticaria. If there is no evidence of allergic reaction within 15 minutes, place the glove on the whole hand for an additional 15 minutes. The patient is said to be nonreactive if a latex glove can be placed on the entire hand for 15 minutes without evidence of reaction.3
Lastly, patch testing can differentiate between irritant contact and allergic contact (type IV hypersensitivity) dermatitis. Apply a small amount of each substance of interest onto a separate disc and place the discs in direct contact with the skin using hypoallergenic tape. With type IV latex hypersensitivity, the skin underneath the disc will become erythematous with developing papulovesicles, starting between 2 and 5 days after exposure. The Figure outlines the differentiation of true latex allergy from irritant and allergic contact dermatitis and identifies methods for making these diagnoses.

General Medical Protocol With Latex Reactions
To reduce the incidence of latex allergic reactions among health care workers and patients, Kumar2 recommends putting a protocol in place to document steps in preventing, diagnosing, and treating latex allergy. This protocol includes employee and patient education about the risks for developing latex allergy and the signs and symptoms of a reaction; available diagnostic testing; and alternative products (eg, hypoallergenic gloves) that are available to individuals with a known or suspected allergy. At-risk health care workers who have not been sensitized should be advised to avoid latex-containing products.3 Routine questioning and diagnostic testing may be necessary as part of every preoperative assessment, as there have been reported cases of anaphylaxis in patients with undocumented allergies.7 Anaphylaxis caused by latex allergy is the second leading cause of perioperative anaphylaxis, accounting for as many as 20% of cases.8 With the use of preventative measures and early identification of at-risk patients, the incidence of latex-related anaphylaxis is decreasing.8 Ascertaining valuable information about the patient’s medical history, such as known allergies to foods that have cross-reactivity to latex (eg, bananas, mango, kiwi, avocado), is one simple way of identifying a patient who should be tested for possible underlying latex allergy.8 Total avoidance of latex-containing products (eg, in the workplace) can further reduce the incidence of allergic reactions by decreasing primary sensitization and risk of exposure.
Conclusion
Patients claiming to be allergic to latex without documentation should be tested. The diagnostic testing available in the United States includes patch testing, wear (or glove provocation) testing, or assessment of IgE antibody titer. Accurate differentiation among irritant contact dermatitis, allergic contact dermatitis, and true latex allergy is paramount for properly educating patients and effectively treating these conditions. Additionally, distinguishing patients with true latex allergy from those who have been misdiagnosed can save resources and reduce health care costs.
- Bousquet J, Flahault A, Vandenplas O, et al. Natural rubber latex allergy among health care workers: a systematic review of the evidence. J Allergy Clin Immunol. 2006;118:447-454.
- Kumar RP. Latex allergy in clinical practice. Indian J Dermatol. 2012;57:66-70.
- Taylor JS, Erkek E. Latex allergy: diagnosis and management. Dermatol Ther. 2004;17:289-301.
- Schmid K, Christoph Broding H, Niklas D, et al. Latex sensitization in dental students using powder-free gloves low in latex protein: a cross-sectional study. Contact Dermatitis. 2002;47:103-108.
- Brown RH, Schauble JF, Hamilton RG. Prevalence of latex allergy among anesthesiologists: identification of sensitized but asymptomatic individuals. Anesthesiology. 1998;89:292-299.
- Pollart SM, Warniment C, Mori T. Latex allergy. Am Fam Physician. 2009;80:1413-1418.
- Duger C, Kol IO, Kaygusuz K, et al. A perioperative anaphylactic reaction caused by latex in a patient with no history of allergy. Anaesth Pain Intensive Care. 2012;16:71-73.
- Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003;97:1381-1395.
- Bousquet J, Flahault A, Vandenplas O, et al. Natural rubber latex allergy among health care workers: a systematic review of the evidence. J Allergy Clin Immunol. 2006;118:447-454.
- Kumar RP. Latex allergy in clinical practice. Indian J Dermatol. 2012;57:66-70.
- Taylor JS, Erkek E. Latex allergy: diagnosis and management. Dermatol Ther. 2004;17:289-301.
- Schmid K, Christoph Broding H, Niklas D, et al. Latex sensitization in dental students using powder-free gloves low in latex protein: a cross-sectional study. Contact Dermatitis. 2002;47:103-108.
- Brown RH, Schauble JF, Hamilton RG. Prevalence of latex allergy among anesthesiologists: identification of sensitized but asymptomatic individuals. Anesthesiology. 1998;89:292-299.
- Pollart SM, Warniment C, Mori T. Latex allergy. Am Fam Physician. 2009;80:1413-1418.
- Duger C, Kol IO, Kaygusuz K, et al. A perioperative anaphylactic reaction caused by latex in a patient with no history of allergy. Anaesth Pain Intensive Care. 2012;16:71-73.
- Hepner DL, Castells MC. Anaphylaxis during the perioperative period. Anesth Analg. 2003;97:1381-1395.
Practice Points
- The term latex allergy often is used as a general diagnosis to describe 3 types of reactions to natural rubber latex, including irritant contact dermatitis, allergic contact dermatitis (type IV hypersensitivity reaction), and true latex allergy (type I hypersensitivity reaction).
- The latex skin prick test is considered the gold standard for diagnosis of true latex allergy, but this method is not available in the United States. In vitro assay for latex-specific immunoglobulin E antibodies is the best alternative.
Inside Out or Outside In: Does Atopic Dermatitis Disrupt Barrier Function or Does Disruption of Barrier Function Trigger Atopic Dermatitis?
Atopic dermatitis (AD) is a multifactorial inflammatory disorder with an estimated prevalence of 279,889,120 cases worldwide.1 Most cases of AD begin in early childhood (with almost 85% developing by 5 years of age),2 but recent studies have found that 40% to over 80% of cases persist into adulthood.1,3,4 Although a previous study focused largely on T helper type 1/T helper type 2 (Th2) immune dysregulation as the pathogenesis of the disease,5 disruption of the skin barrier and systemic inflammation are at the center of current AD research. In AD, breakdown of the skin barrier results in increased transepidermal water loss, reduced skin hydration, and increased antigen presentation by Langerhans cells initiating inflammation.6-8 The cascade largely activated is the Th2 and T helper type 22 cascade with resultant cytokine release (ie, IL-4, IL-13, IL-2, IL-8, IL-10, IL-17, IL-22, tumor necrosis factor α, interferon γ).9,10 In active AD, Th2 inflammation and barrier breakdown result in reduced filaggrin and claudin 1 expression, resulting in further exacerbation of the barrier defect and enhancing the risk of development of asthma and hay fever as well as transcutaneous sensitization to a variety of food allergens (eg, peanuts).9,11,12 Although all of these immunologic features are well established in AD, controversy remains as to whether AD is caused by systemic inflammation triggering barrier dysfunction (the “inside-out” hypothesis) or from the epidermal skin barrier disruption triggering immunologic imbalance (the “outside-in” hypothesis).
Inside-Out Hypothesis
While barrier impairment appears to occur in all patients with AD, it still is unclear how AD begins. The inside-out hypothesis suggests that cutaneous inflammation precedes barrier impairment and in fact may result in an impaired skin barrier. It has previously been reported that inflammatory states weaken the barrier by downregulating filaggrin production in the skin.13 Barrier disruption may be accompanied by transcutaneous penetration of allergens and increased Staphylococcus aureus counts. Recently, mutations and polymorphisms of inflammatory genes have been linked to AD (eg, single nucleotide polymorphisms of the IL4RA [interleukin 4 receptor, alpha] and CD14 [cluster of differentiation 14] genes, the serine protease inhibitor SPINK5 [serine peptidase inhibitor, Kazal type 5], RANTES [chemokine (C-C motif) ligand 5], IL-4, IL-13).14 These alterations highlight the role of systemic inflammation in triggering AD.
Outside-In Hypothesis
The outside-in hypothesis suggests that the impaired skin barrier precedes AD and is required for immune dysregulation to occur. This hypothesis was largely advanced by a study demonstrating that deactivating mutations of the filaggrin gene were linked to nearly 20% of AD cases in Northern European populations.15 Filaggrin (chromosome 1q21.3) performs an essential function in the skin barrier through its differential cleavage and the breakdown and release of natural moisturizing factor.16 Filaggrin gene mutations are associated with persistent AD, and it has been posited that environmental factors such as temperature and humidity also can affect filaggrin production as it relates to barrier function.17-19 Skin barrier disruption results in increased cutaneous and systemic Th2 responses (ie, IL-4/13), with thymic stromal lymphopoietin as the potential mechanism of Th2 cell recruitment.10,20 Inflammatory Th2 cells triggered by an impaired skin barrier also may predispose patients to the development of allergic diseases such as asthma, in line with Atopic March, or the progression of AD to other forms of atopy (eg, food allergy, asthma).5,7,21-23
The outside-in hypothesis may only explain the root pathogenesis of AD in a subset of patients, however, as only 1 in 5 cases of AD in Northern European and Asian populations are associated with underlying filaggrin mutations (which are only present in about 10% of those who are unaffected by AD).15 Filaggrin does not appear to account for the basis of AD in all cases. In a study of 762 newborns in Cincinnati, Ohio, 39% of children with at least one parent with atopy developed AD by 3 years of age, about quadruple of what would be projected based on filaggrin defects in general population studies, which are noted in only about 10% of white individuals.24 Furthermore, less than 5% of patients of African descent have mutations of the filaggrin 1 gene.25
Implications for the Prevention and Treatment of Atopic Dermatitis
Preventative strategies for AD currently are in development. Atopic dermatitis may be unpreventable because the in utero environment triggers some of the barrier alterations, which can be noted as early as 2 days following birth and will predict early-onset AD. The putative mechanism is via Th2 cytokines (IL-4, IL-13).26
Certainly, application of over-the-counter and prescription emollients are mainstays of treatment for AD and may suffice as monotherapy in cases of mild disease. In a recent randomized trial in the United States and the United Kingdom, emollients were used in newborns considered at high risk for AD (family history of atopy) until 6 months of age.27 The risk of AD development was reduced by half, irrespective of the emollient used. Unfortunately, 21.8% of children without a family history of atopy will develop AD; therefore, not all cases can be prevented if use of emollients is limited to newborns with a family history of atopy.28 Long-term follow-up is needed to track whether emollient use in newborns will prevent AD indefinitely.
Prevention of AD onset using systemic interventions has also been investigated. Probiotics have been suggested as a means to modify the gut microbiota and reduce systemic and mucosal inflammation. Lactobacillus reuteri taken prenatally by pregnant women and by newborns has shown mild benefit in preventing some forms of AD.29 Although they are not approved by the US Food and Drug Administration for this indication, systemic interventions for moderate-to-severe AD such as methotrexate and cyclosporine certainly have shown benefit in managing ongoing illness and breaking the cycle of disease.30 The efficacy of these agents points to the role of systemic inflammation in ongoing AD activity. Moreover, the inside-out hypothesis recently has led to the proliferation of promising new therapeutic agents in the pipeline to treat the systemic Th2 inflammation that occurs in severe AD (eg, anti–IL-4/13 receptor antibody, anti–IL-13 antibodies, and biologics targeting IL-12/23, IL-22, and IL-31 receptors).31
Final Thoughts
Atopic dermatitis is a multifactorial disease associated with barrier disruption and intense systemic inflammation. It is likely that both the inside-out and outside-in hypotheses hold true in different subsets of AD patients. It is clear that some individuals are born with filaggrin defects that sufficiently trigger systemic inflammation, resulting in AD. On the other hand, there are clearly some individuals with inflammatory dysregulation that results in systemic inflammation and secondary barrier disruption. Until we can determine the genomic triggering or promoting event in each individual patient, large-scale introduction of active prevention and severity reduction strategies may not be realistic. In the meantime, we can approach AD in childhood from the inside out, through appropriate treatment of systemic inflammation of AD, and from the outside in, with treatment and prevention via emollient use in newborns.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
- Margolis JS, Abuabara K, Bilker W, et al. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150:593-600.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Zheng T, Jinho Y, Oh MH, et al. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011;3:67-73.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care. Clin Dermatol. 2015;33:271-280.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
- Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
- Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Brown SJ, Irvine AD. Atopic eczema and the filaggrin story. Semin Cutan Med Surg. 2008;27:128-137.
- Harding CR, Aho S, Bosko CA. Filaggrin—revisited. Int J Cosmet Sci. 2013;35:412-423.
- Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29-40.
- Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease and increased heart attacks in 3 population-based studies [published online ahead of print July 4, 2015]. Allergy. doi:10.1111/all.12685.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
- Kelleher M, Dunn-Galvin A, Hourihane JO, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015;135:930-935.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Parazzini F, Cipriani S, Zinetti C, et al. Perinatal factors and the risk of atopic dermatitis: a cohort study. Pediatr Allergy Immunol. 2014;25:43-50.
- Abrahamsson TR, Jakobsson T, Böttcher MF, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1174-1180.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Eczema drugs in development. National Eczema Association Web site. https://nationaleczema.org/research/phases-drug-development/. Accessed August 18, 2015.
Atopic dermatitis (AD) is a multifactorial inflammatory disorder with an estimated prevalence of 279,889,120 cases worldwide.1 Most cases of AD begin in early childhood (with almost 85% developing by 5 years of age),2 but recent studies have found that 40% to over 80% of cases persist into adulthood.1,3,4 Although a previous study focused largely on T helper type 1/T helper type 2 (Th2) immune dysregulation as the pathogenesis of the disease,5 disruption of the skin barrier and systemic inflammation are at the center of current AD research. In AD, breakdown of the skin barrier results in increased transepidermal water loss, reduced skin hydration, and increased antigen presentation by Langerhans cells initiating inflammation.6-8 The cascade largely activated is the Th2 and T helper type 22 cascade with resultant cytokine release (ie, IL-4, IL-13, IL-2, IL-8, IL-10, IL-17, IL-22, tumor necrosis factor α, interferon γ).9,10 In active AD, Th2 inflammation and barrier breakdown result in reduced filaggrin and claudin 1 expression, resulting in further exacerbation of the barrier defect and enhancing the risk of development of asthma and hay fever as well as transcutaneous sensitization to a variety of food allergens (eg, peanuts).9,11,12 Although all of these immunologic features are well established in AD, controversy remains as to whether AD is caused by systemic inflammation triggering barrier dysfunction (the “inside-out” hypothesis) or from the epidermal skin barrier disruption triggering immunologic imbalance (the “outside-in” hypothesis).
Inside-Out Hypothesis
While barrier impairment appears to occur in all patients with AD, it still is unclear how AD begins. The inside-out hypothesis suggests that cutaneous inflammation precedes barrier impairment and in fact may result in an impaired skin barrier. It has previously been reported that inflammatory states weaken the barrier by downregulating filaggrin production in the skin.13 Barrier disruption may be accompanied by transcutaneous penetration of allergens and increased Staphylococcus aureus counts. Recently, mutations and polymorphisms of inflammatory genes have been linked to AD (eg, single nucleotide polymorphisms of the IL4RA [interleukin 4 receptor, alpha] and CD14 [cluster of differentiation 14] genes, the serine protease inhibitor SPINK5 [serine peptidase inhibitor, Kazal type 5], RANTES [chemokine (C-C motif) ligand 5], IL-4, IL-13).14 These alterations highlight the role of systemic inflammation in triggering AD.
Outside-In Hypothesis
The outside-in hypothesis suggests that the impaired skin barrier precedes AD and is required for immune dysregulation to occur. This hypothesis was largely advanced by a study demonstrating that deactivating mutations of the filaggrin gene were linked to nearly 20% of AD cases in Northern European populations.15 Filaggrin (chromosome 1q21.3) performs an essential function in the skin barrier through its differential cleavage and the breakdown and release of natural moisturizing factor.16 Filaggrin gene mutations are associated with persistent AD, and it has been posited that environmental factors such as temperature and humidity also can affect filaggrin production as it relates to barrier function.17-19 Skin barrier disruption results in increased cutaneous and systemic Th2 responses (ie, IL-4/13), with thymic stromal lymphopoietin as the potential mechanism of Th2 cell recruitment.10,20 Inflammatory Th2 cells triggered by an impaired skin barrier also may predispose patients to the development of allergic diseases such as asthma, in line with Atopic March, or the progression of AD to other forms of atopy (eg, food allergy, asthma).5,7,21-23
The outside-in hypothesis may only explain the root pathogenesis of AD in a subset of patients, however, as only 1 in 5 cases of AD in Northern European and Asian populations are associated with underlying filaggrin mutations (which are only present in about 10% of those who are unaffected by AD).15 Filaggrin does not appear to account for the basis of AD in all cases. In a study of 762 newborns in Cincinnati, Ohio, 39% of children with at least one parent with atopy developed AD by 3 years of age, about quadruple of what would be projected based on filaggrin defects in general population studies, which are noted in only about 10% of white individuals.24 Furthermore, less than 5% of patients of African descent have mutations of the filaggrin 1 gene.25
Implications for the Prevention and Treatment of Atopic Dermatitis
Preventative strategies for AD currently are in development. Atopic dermatitis may be unpreventable because the in utero environment triggers some of the barrier alterations, which can be noted as early as 2 days following birth and will predict early-onset AD. The putative mechanism is via Th2 cytokines (IL-4, IL-13).26
Certainly, application of over-the-counter and prescription emollients are mainstays of treatment for AD and may suffice as monotherapy in cases of mild disease. In a recent randomized trial in the United States and the United Kingdom, emollients were used in newborns considered at high risk for AD (family history of atopy) until 6 months of age.27 The risk of AD development was reduced by half, irrespective of the emollient used. Unfortunately, 21.8% of children without a family history of atopy will develop AD; therefore, not all cases can be prevented if use of emollients is limited to newborns with a family history of atopy.28 Long-term follow-up is needed to track whether emollient use in newborns will prevent AD indefinitely.
Prevention of AD onset using systemic interventions has also been investigated. Probiotics have been suggested as a means to modify the gut microbiota and reduce systemic and mucosal inflammation. Lactobacillus reuteri taken prenatally by pregnant women and by newborns has shown mild benefit in preventing some forms of AD.29 Although they are not approved by the US Food and Drug Administration for this indication, systemic interventions for moderate-to-severe AD such as methotrexate and cyclosporine certainly have shown benefit in managing ongoing illness and breaking the cycle of disease.30 The efficacy of these agents points to the role of systemic inflammation in ongoing AD activity. Moreover, the inside-out hypothesis recently has led to the proliferation of promising new therapeutic agents in the pipeline to treat the systemic Th2 inflammation that occurs in severe AD (eg, anti–IL-4/13 receptor antibody, anti–IL-13 antibodies, and biologics targeting IL-12/23, IL-22, and IL-31 receptors).31
Final Thoughts
Atopic dermatitis is a multifactorial disease associated with barrier disruption and intense systemic inflammation. It is likely that both the inside-out and outside-in hypotheses hold true in different subsets of AD patients. It is clear that some individuals are born with filaggrin defects that sufficiently trigger systemic inflammation, resulting in AD. On the other hand, there are clearly some individuals with inflammatory dysregulation that results in systemic inflammation and secondary barrier disruption. Until we can determine the genomic triggering or promoting event in each individual patient, large-scale introduction of active prevention and severity reduction strategies may not be realistic. In the meantime, we can approach AD in childhood from the inside out, through appropriate treatment of systemic inflammation of AD, and from the outside in, with treatment and prevention via emollient use in newborns.
Atopic dermatitis (AD) is a multifactorial inflammatory disorder with an estimated prevalence of 279,889,120 cases worldwide.1 Most cases of AD begin in early childhood (with almost 85% developing by 5 years of age),2 but recent studies have found that 40% to over 80% of cases persist into adulthood.1,3,4 Although a previous study focused largely on T helper type 1/T helper type 2 (Th2) immune dysregulation as the pathogenesis of the disease,5 disruption of the skin barrier and systemic inflammation are at the center of current AD research. In AD, breakdown of the skin barrier results in increased transepidermal water loss, reduced skin hydration, and increased antigen presentation by Langerhans cells initiating inflammation.6-8 The cascade largely activated is the Th2 and T helper type 22 cascade with resultant cytokine release (ie, IL-4, IL-13, IL-2, IL-8, IL-10, IL-17, IL-22, tumor necrosis factor α, interferon γ).9,10 In active AD, Th2 inflammation and barrier breakdown result in reduced filaggrin and claudin 1 expression, resulting in further exacerbation of the barrier defect and enhancing the risk of development of asthma and hay fever as well as transcutaneous sensitization to a variety of food allergens (eg, peanuts).9,11,12 Although all of these immunologic features are well established in AD, controversy remains as to whether AD is caused by systemic inflammation triggering barrier dysfunction (the “inside-out” hypothesis) or from the epidermal skin barrier disruption triggering immunologic imbalance (the “outside-in” hypothesis).
Inside-Out Hypothesis
While barrier impairment appears to occur in all patients with AD, it still is unclear how AD begins. The inside-out hypothesis suggests that cutaneous inflammation precedes barrier impairment and in fact may result in an impaired skin barrier. It has previously been reported that inflammatory states weaken the barrier by downregulating filaggrin production in the skin.13 Barrier disruption may be accompanied by transcutaneous penetration of allergens and increased Staphylococcus aureus counts. Recently, mutations and polymorphisms of inflammatory genes have been linked to AD (eg, single nucleotide polymorphisms of the IL4RA [interleukin 4 receptor, alpha] and CD14 [cluster of differentiation 14] genes, the serine protease inhibitor SPINK5 [serine peptidase inhibitor, Kazal type 5], RANTES [chemokine (C-C motif) ligand 5], IL-4, IL-13).14 These alterations highlight the role of systemic inflammation in triggering AD.
Outside-In Hypothesis
The outside-in hypothesis suggests that the impaired skin barrier precedes AD and is required for immune dysregulation to occur. This hypothesis was largely advanced by a study demonstrating that deactivating mutations of the filaggrin gene were linked to nearly 20% of AD cases in Northern European populations.15 Filaggrin (chromosome 1q21.3) performs an essential function in the skin barrier through its differential cleavage and the breakdown and release of natural moisturizing factor.16 Filaggrin gene mutations are associated with persistent AD, and it has been posited that environmental factors such as temperature and humidity also can affect filaggrin production as it relates to barrier function.17-19 Skin barrier disruption results in increased cutaneous and systemic Th2 responses (ie, IL-4/13), with thymic stromal lymphopoietin as the potential mechanism of Th2 cell recruitment.10,20 Inflammatory Th2 cells triggered by an impaired skin barrier also may predispose patients to the development of allergic diseases such as asthma, in line with Atopic March, or the progression of AD to other forms of atopy (eg, food allergy, asthma).5,7,21-23
The outside-in hypothesis may only explain the root pathogenesis of AD in a subset of patients, however, as only 1 in 5 cases of AD in Northern European and Asian populations are associated with underlying filaggrin mutations (which are only present in about 10% of those who are unaffected by AD).15 Filaggrin does not appear to account for the basis of AD in all cases. In a study of 762 newborns in Cincinnati, Ohio, 39% of children with at least one parent with atopy developed AD by 3 years of age, about quadruple of what would be projected based on filaggrin defects in general population studies, which are noted in only about 10% of white individuals.24 Furthermore, less than 5% of patients of African descent have mutations of the filaggrin 1 gene.25
Implications for the Prevention and Treatment of Atopic Dermatitis
Preventative strategies for AD currently are in development. Atopic dermatitis may be unpreventable because the in utero environment triggers some of the barrier alterations, which can be noted as early as 2 days following birth and will predict early-onset AD. The putative mechanism is via Th2 cytokines (IL-4, IL-13).26
Certainly, application of over-the-counter and prescription emollients are mainstays of treatment for AD and may suffice as monotherapy in cases of mild disease. In a recent randomized trial in the United States and the United Kingdom, emollients were used in newborns considered at high risk for AD (family history of atopy) until 6 months of age.27 The risk of AD development was reduced by half, irrespective of the emollient used. Unfortunately, 21.8% of children without a family history of atopy will develop AD; therefore, not all cases can be prevented if use of emollients is limited to newborns with a family history of atopy.28 Long-term follow-up is needed to track whether emollient use in newborns will prevent AD indefinitely.
Prevention of AD onset using systemic interventions has also been investigated. Probiotics have been suggested as a means to modify the gut microbiota and reduce systemic and mucosal inflammation. Lactobacillus reuteri taken prenatally by pregnant women and by newborns has shown mild benefit in preventing some forms of AD.29 Although they are not approved by the US Food and Drug Administration for this indication, systemic interventions for moderate-to-severe AD such as methotrexate and cyclosporine certainly have shown benefit in managing ongoing illness and breaking the cycle of disease.30 The efficacy of these agents points to the role of systemic inflammation in ongoing AD activity. Moreover, the inside-out hypothesis recently has led to the proliferation of promising new therapeutic agents in the pipeline to treat the systemic Th2 inflammation that occurs in severe AD (eg, anti–IL-4/13 receptor antibody, anti–IL-13 antibodies, and biologics targeting IL-12/23, IL-22, and IL-31 receptors).31
Final Thoughts
Atopic dermatitis is a multifactorial disease associated with barrier disruption and intense systemic inflammation. It is likely that both the inside-out and outside-in hypotheses hold true in different subsets of AD patients. It is clear that some individuals are born with filaggrin defects that sufficiently trigger systemic inflammation, resulting in AD. On the other hand, there are clearly some individuals with inflammatory dysregulation that results in systemic inflammation and secondary barrier disruption. Until we can determine the genomic triggering or promoting event in each individual patient, large-scale introduction of active prevention and severity reduction strategies may not be realistic. In the meantime, we can approach AD in childhood from the inside out, through appropriate treatment of systemic inflammation of AD, and from the outside in, with treatment and prevention via emollient use in newborns.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
- Margolis JS, Abuabara K, Bilker W, et al. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150:593-600.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Zheng T, Jinho Y, Oh MH, et al. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011;3:67-73.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care. Clin Dermatol. 2015;33:271-280.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
- Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
- Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Brown SJ, Irvine AD. Atopic eczema and the filaggrin story. Semin Cutan Med Surg. 2008;27:128-137.
- Harding CR, Aho S, Bosko CA. Filaggrin—revisited. Int J Cosmet Sci. 2013;35:412-423.
- Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29-40.
- Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease and increased heart attacks in 3 population-based studies [published online ahead of print July 4, 2015]. Allergy. doi:10.1111/all.12685.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
- Kelleher M, Dunn-Galvin A, Hourihane JO, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015;135:930-935.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Parazzini F, Cipriani S, Zinetti C, et al. Perinatal factors and the risk of atopic dermatitis: a cohort study. Pediatr Allergy Immunol. 2014;25:43-50.
- Abrahamsson TR, Jakobsson T, Böttcher MF, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1174-1180.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Eczema drugs in development. National Eczema Association Web site. https://nationaleczema.org/research/phases-drug-development/. Accessed August 18, 2015.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
- Margolis JS, Abuabara K, Bilker W, et al. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150:593-600.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Zheng T, Jinho Y, Oh MH, et al. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011;3:67-73.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care. Clin Dermatol. 2015;33:271-280.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
- Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
- Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Brown SJ, Irvine AD. Atopic eczema and the filaggrin story. Semin Cutan Med Surg. 2008;27:128-137.
- Harding CR, Aho S, Bosko CA. Filaggrin—revisited. Int J Cosmet Sci. 2013;35:412-423.
- Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29-40.
- Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease and increased heart attacks in 3 population-based studies [published online ahead of print July 4, 2015]. Allergy. doi:10.1111/all.12685.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
- Kelleher M, Dunn-Galvin A, Hourihane JO, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015;135:930-935.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Parazzini F, Cipriani S, Zinetti C, et al. Perinatal factors and the risk of atopic dermatitis: a cohort study. Pediatr Allergy Immunol. 2014;25:43-50.
- Abrahamsson TR, Jakobsson T, Böttcher MF, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1174-1180.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Eczema drugs in development. National Eczema Association Web site. https://nationaleczema.org/research/phases-drug-development/. Accessed August 18, 2015.
Cutaneous Leishmaniasis: An Emerging Infectious Disease in Travelers
Leishmaniasis describes any disease caused by protozoan parasites of the genus Leishmania1 and can manifest in 3 different forms: cutaneous (the most common); mucosal, a destructive metastatic sequela of the cutaneous form; and visceral, which is potentially fatal.2 According to the World Health Organization, the leishmaniases are endemic in 88 countries.3 It is estimated that 95% of cutaneous cases occur in the Americas (most notably Central and South America), the Mediterranean basin, the Middle East, and Central Asia.2 Most cutaneous cases diagnosed among nonmilitary personnel in the United States are acquired in Mexico and Central America.4 In Central and South America, the causative human pathogens include species of the Leishmania (Viannia) complex (eg, Leishmania panamensis, Leishmania braziliensis, Leishmania guyanensis, Leishmania peruviana) and the Leishmania mexicana complex (eg, Leishmania mexicana, Leishmania amazonensis, Leishmania venezuelensis). All of these species can cause localized cutaneous lesions, but only L panamensis, L braziliensis, and L guyanensis are associated with metastatic mucosal lesions. In Central and South Americas, only Leishmaniasis chagasi (also known as Leishmaniasis infantum) is known to cause visceral leishmaniasis.5
Case Report
A 26-year-old man was referred to the dermatology clinic by his primary care provider for evaluation of a nonhealing sore on the left volar forearm of 6 weeks’ duration. The patient described the initial lesion as a red bump resembling a mosquito bite. Over 6 weeks the papule evolved into an indurated plaque with painless ulceration. The patient’s primary care provider had prescribed antibiotics for a presumed Staphylococcus aureus infection of the skin 5 weeks prior to presentation; however, the lesion continued to enlarge in size, resulting in referral to our dermatology clinic.
Skin examination revealed a solitary, 4-cm, painless, ulcerated plaque on the left volar forearm (Figure 1). No lymphadenopathy was noted. The patient reported that he had returned from a mission trip to rural Costa Rica 2 weeks prior to the appearance of the lesion. His medical history was otherwise unremarkable and his vital signs were within normal limits. Our initial differential diagnosis included pyoderma gangrenosum, Sweet syndrome, cutaneous leishmaniasis, and an insect bite.
Histopathologic study of a 5-mm punch biopsy specimen from the lesion showed a dense nodular and diffuse lymphohistiocytic infiltrate containing foci of suppuration. Within these suppurative foci were histiocytes parasitized by intracellular organisms that appeared to be of uniform size and shape on Giemsa staining, all of which are considered to be pathognomonic features of cutaneous leishmaniasis6 (Figures 2 and 3). The dermatopathologist’s diagnosis of cutaneous leishmaniasis was confirmed by the Centers for Disease Control and Prevention. The species was identified by polymerase chain reaction (PCR) as L panamensis.
The patient was treated with intravenous sodium stibogluconate 20 mg/kg for 20 consecutive days as recommended by expert consensus. The decision to treat a frequently self-limited cutaneous lesion with a highly toxic systemic drug was based on the small but real risk of metastatic mucosal lesions, which is caused by the Viannia subgenus, including L panamensis. Of note, sodium stibogluconate and other antimony drugs are not sold in the United States. Sodium stibogluconate is approved by the US Food and Drug Administration to be distributed by the Centers for Disease Control and Prevention under a protocol requiring baseline and weekly electrocardiograms and monitoring of patients’ creatinine, transaminase, lipase, amylase, and complete blood count levels.7 Our patient tolerated treatment but experienced mild to moderate flulike symptoms. The patient experienced no remarkable sequelae other than scarring in the affected area. He was warned to notify his health care providers of any persistent nasal symptoms, including nasal stuffiness, mucosal bleeding, and increased secretions, heralding the possibility of mucosal metastasis.
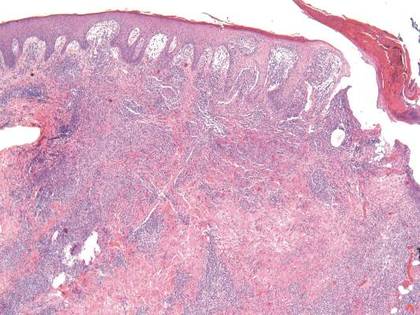
| 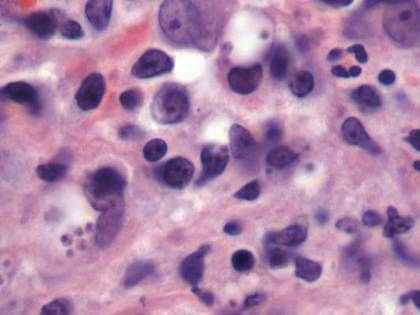
| |
Figure 2. Dense nodular and diffuse lymphohistiocytic infiltrate containing foci of suppuration (H&E, original magnification ×10). | Figure 3. Histiocytes parasitized by intracellular organisms of uniform shape and size on Giemsa staining (original magnification ×1000). |
Comment
The true incidence of cutaneous leishmaniasis in American travelers returning from Mexico and South and Central Americas is not known. The best incidence estimates are based on the number of physician requests for sodium stibogluconate and travel surveillance data collected by the Centers for Disease Control and Prevention. One study estimated the incidence of cutaneous leishmaniasis in Americans to be 1 case per every 100,000 travelers to Mexico.9 Data on the incidence of cutaneous leishmaniasis in American travelers seen in travel clinics for skin lesions gives a different perspective.10 Leishmaniasis is one of the most common dermatologic diseases seen in patients (European, North American, and other) returning from South America, accounting for 143 of every 1000 patients diagnosed with a skin disease acquired in South America.
Although males are thought to be at higher risk for cutaneous leishmaniasis infection than females, other demographic and behavioral risk factors are not well defined. In a case series of US travelers diagnosed with cutaneous leishmaniasis between January 1985 and April 1990, Herwaldt et al9 found that 46% (27/59) were conducting field studies, while 39% (23/59) were tourists, visitors, or tour guides. At least 15 of the 58 travelers interviewed (26%) were in forested areas for 1 week or less, and of these 15 respondents, at least 6 had a maximum exposure of 2 days.9
Evidence suggests that cutaneous leishmaniasis is inefficiently diagnosed in the United States. One study showed that some patients may consult up to 7 physicians before a definitive diagnosis is made, and the median time from noticing eruption of the lesions to definitive treatment was 112 days.9 Several factors may contribute to delays and inefficiencies in diagnosis. First, the lesions of cutaneous leishmaniasis are varied in morphology, and although ulcers are thought to be the most commonly presenting lesions,11 there are no specific morphologic features that are pathognomonic for cutaneous leishmaniasis. Second, the temporal association with travel to endemic countries is not necessarily apparent, with lesions developing gradually or weeks after the patient returns home. In the one study, 17% (10/58) of patients were home for more than 1 month before they noticed skin lesions.9 Finally, definitive diagnosis requires biopsy or scraping of the lesion followed by PCR, special histopathological staining (Giemsa), or culture. Polymerase chain reaction is currently the best means of identifying the causative Leishmania species.12-14 However, since skin biopsies are not routine in primary care settings and few practitioners are familiar with PCR for identification of leishmaniasis, diagnosis is typically made only after referral to a specialist.
Leishmaniasis transmission occurs in diverse geographical settings though a variety of mechanisms (Figure 4). The morphology of cutaneous leishmaniasis varies and may include papules, nodules, psoriasiform plaques, or ulcers. The differential diagnosis may include staphylococcal skin infection, insect bite, cutaneous neoplasm, pyoderma gangrenosum, sporotrichosis, blastomycosis, chromomycosis, lobomycosis, cutaneous tuberculosis, atypical mycobacterial infection, syphilis, yaws, leprosy, Hansen disease, and sarcoidosis. A definitive diagnosis can be made only after identifying the causative parasite. A scraping or punch biopsy taken from a cleaned lesion provides an adequate sample. Identification can then be accomplished by histopathology, tissue culture, or PCR.5
We present a rhyme that can be used to promote greater awareness of cutaneous leishmaniasis among US health care practitioners:
And on his leg finds an ulcerated plaque.
The possibilities are many,
Numbering far more than 20.
Leishmaniasis is a lurking issue,
So the savvy physician tests the tissue.
Although clinical resolution of cutaneous leishmaniasis usually occurs over months to years, the unsightly appearance of the lesions as well as the potential for scarring and mucosal metastasis (associated with some species) drives medical treatment.15 Pentavalent antimonial drugs, which have been the mainstay of treatment for more than 50 years, remain the most popular treatment for cutaneous leishmaniasis. Two antimony compounds, sodium stibogluconate and meglumine antimoniate, often lead to clinical cure in less than 1 month7; however, these drugs are far from ideal because of the inconvenience of obtaining them, emerging parasite resistance, long treatment course, parenteral route of administration, and serious side effects including infusion reactions, arrhythmias, pancreatitis, and liver toxicity. Moreover, the subclinical persistence of cutaneous leishmaniasis years after treatment and clinical cure is common. There have been reports of spontaneous disease reactivation in immunocompromised individuals, and Leishmania has been detected in old cutaneous leishmaniasis scars on PCR testing.16-18 Other therapies that have been used to treat cutaneous leishmaniasis include allopurinol, aminosidine sulphate, amphotericin B, the Bacillus Calmette–Guérin vaccine, cotrimoxazole, cryotherapy, dapsone, fluconazole, itraconazole, ketoconazole, laser therapy, metronidazole, miltefosine, paromomycin, pentamidine, pentoxifylline, photodynamic therapy, rifampicin, and surgical excision of the entire lesion.8 A 2009 Cochrane review of the various treatments for cutaneous leishmaniasis concluded that “no general consensus on optimal treatment has been achieved” and suggested “the creation of an international platform to improve the quality and standardization of future trials in order to develop a better evidence-based approach.”8
Conclusion
Cutaneous leishmaniasis should be included in the differential diagnosis for travelers returning from endemic areas who present with new skin lesions. Since no specific lesion types are pathognomonic for cutaneous leishmaniasis, tissue biopsy for histopathology and PCR are essential for diagnosis. Prevention of cutaneous leishmaniasis hinges on appropriate counseling of travelers to endemic regions.
1. Etymologia-Leishmaniasis. Emerg Infect Dis. 2008;14:666.
2. Burden and distribution. World Health Organization Web site. http://www.who.int/leishmaniasis/burden/en/. Accessed November 10, 2015.
3. Emergencies preparedness, response. World Health Organization Web site. http://www.who.int/csr/resources/publications/CSR_ISR_2000_1leish/en/. Accessed November 3, 2015.
4. Pavli A, Maltezou HC. Leishmaniasis, an emerging infection in travelers. Int J Infect Dis. 2010;14:e1032-e1039.
5. Magill AJ. Leishmania species: visceral (Kala-Azar), cutaneous, and mucosal leishmaniasis. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone; 2009:3463-3480.
6. Mysore V. Invisible dermatoses. Indian J Dermatol Venereol Leprol. 2010;76:239-248.
7. Parasites – Leishmaniasis. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/parasites/leishmaniasis/health_professionals/. Updated September 14, 2015. Accessed November 13, 2015.
8. González U, Pinart M, Rengifo-Pardo M, et al. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst Rev. 2009;15:CD004834.
9. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med. 1993;118:779-784.
10. Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
11. El Hajj L, Thellier M, Carriere J, et al. Localized cutaneous leishmaniasis imported into Paris: a review of 39 cases. Int J Dermatol. 2004;43:120-125.
12. Harris E, Kropp G, Belli A, et al. Single-step multiplex PCR assay for characterization of New World Leishmania complexes. J Clin Microbiol. 1998;36:1989-1995.
13. Marfurt J, Niederwieser I, Makia D, et al. Diagnostic genotyping of Old and New World Leishmania species by PCR-RFLP. Diagn Microbiol Infect Dis. 2003;46:115-124.
14. Schonian G, Nasereddin A, Dinse N, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47:349-358.
15. Reithinger R, Aadil K, Kolaczinski J, et al. Social impact of leishmaniasis, Afghanistan. Emerg Infect Dis. 2005;11:634-636.
16. Morales MA, Cruz I, Rubio JM, et al. Relapses versus reinfections in patients coinfected with Leishmania infantum and human immunodeficiency virus type 1 [published online ahead of print April 22, 2002]. J Infect Dis. 2002;185:1533-1537.
17. Coutinho SG, Pirmez C, Da-Cruz AM. Parasitological and immunological follow-up of American tegumentary leishmaniasis patients. Trans R Soc Trop Med Hyg. 2002;96(suppl 1):S173-S178.
18. Mendonça MG, de Brito ME, Rodrigues EH, et al. Persistance of leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: is there a sterile cure [published online ahead of print March 2, 2004]? J Infect Dis. 2004;189:1018-1023.
Leishmaniasis describes any disease caused by protozoan parasites of the genus Leishmania1 and can manifest in 3 different forms: cutaneous (the most common); mucosal, a destructive metastatic sequela of the cutaneous form; and visceral, which is potentially fatal.2 According to the World Health Organization, the leishmaniases are endemic in 88 countries.3 It is estimated that 95% of cutaneous cases occur in the Americas (most notably Central and South America), the Mediterranean basin, the Middle East, and Central Asia.2 Most cutaneous cases diagnosed among nonmilitary personnel in the United States are acquired in Mexico and Central America.4 In Central and South America, the causative human pathogens include species of the Leishmania (Viannia) complex (eg, Leishmania panamensis, Leishmania braziliensis, Leishmania guyanensis, Leishmania peruviana) and the Leishmania mexicana complex (eg, Leishmania mexicana, Leishmania amazonensis, Leishmania venezuelensis). All of these species can cause localized cutaneous lesions, but only L panamensis, L braziliensis, and L guyanensis are associated with metastatic mucosal lesions. In Central and South Americas, only Leishmaniasis chagasi (also known as Leishmaniasis infantum) is known to cause visceral leishmaniasis.5
Case Report
A 26-year-old man was referred to the dermatology clinic by his primary care provider for evaluation of a nonhealing sore on the left volar forearm of 6 weeks’ duration. The patient described the initial lesion as a red bump resembling a mosquito bite. Over 6 weeks the papule evolved into an indurated plaque with painless ulceration. The patient’s primary care provider had prescribed antibiotics for a presumed Staphylococcus aureus infection of the skin 5 weeks prior to presentation; however, the lesion continued to enlarge in size, resulting in referral to our dermatology clinic.
Skin examination revealed a solitary, 4-cm, painless, ulcerated plaque on the left volar forearm (Figure 1). No lymphadenopathy was noted. The patient reported that he had returned from a mission trip to rural Costa Rica 2 weeks prior to the appearance of the lesion. His medical history was otherwise unremarkable and his vital signs were within normal limits. Our initial differential diagnosis included pyoderma gangrenosum, Sweet syndrome, cutaneous leishmaniasis, and an insect bite.
Histopathologic study of a 5-mm punch biopsy specimen from the lesion showed a dense nodular and diffuse lymphohistiocytic infiltrate containing foci of suppuration. Within these suppurative foci were histiocytes parasitized by intracellular organisms that appeared to be of uniform size and shape on Giemsa staining, all of which are considered to be pathognomonic features of cutaneous leishmaniasis6 (Figures 2 and 3). The dermatopathologist’s diagnosis of cutaneous leishmaniasis was confirmed by the Centers for Disease Control and Prevention. The species was identified by polymerase chain reaction (PCR) as L panamensis.
The patient was treated with intravenous sodium stibogluconate 20 mg/kg for 20 consecutive days as recommended by expert consensus. The decision to treat a frequently self-limited cutaneous lesion with a highly toxic systemic drug was based on the small but real risk of metastatic mucosal lesions, which is caused by the Viannia subgenus, including L panamensis. Of note, sodium stibogluconate and other antimony drugs are not sold in the United States. Sodium stibogluconate is approved by the US Food and Drug Administration to be distributed by the Centers for Disease Control and Prevention under a protocol requiring baseline and weekly electrocardiograms and monitoring of patients’ creatinine, transaminase, lipase, amylase, and complete blood count levels.7 Our patient tolerated treatment but experienced mild to moderate flulike symptoms. The patient experienced no remarkable sequelae other than scarring in the affected area. He was warned to notify his health care providers of any persistent nasal symptoms, including nasal stuffiness, mucosal bleeding, and increased secretions, heralding the possibility of mucosal metastasis.

| 
| |
Figure 2. Dense nodular and diffuse lymphohistiocytic infiltrate containing foci of suppuration (H&E, original magnification ×10). | Figure 3. Histiocytes parasitized by intracellular organisms of uniform shape and size on Giemsa staining (original magnification ×1000). |
Comment
The true incidence of cutaneous leishmaniasis in American travelers returning from Mexico and South and Central Americas is not known. The best incidence estimates are based on the number of physician requests for sodium stibogluconate and travel surveillance data collected by the Centers for Disease Control and Prevention. One study estimated the incidence of cutaneous leishmaniasis in Americans to be 1 case per every 100,000 travelers to Mexico.9 Data on the incidence of cutaneous leishmaniasis in American travelers seen in travel clinics for skin lesions gives a different perspective.10 Leishmaniasis is one of the most common dermatologic diseases seen in patients (European, North American, and other) returning from South America, accounting for 143 of every 1000 patients diagnosed with a skin disease acquired in South America.
Although males are thought to be at higher risk for cutaneous leishmaniasis infection than females, other demographic and behavioral risk factors are not well defined. In a case series of US travelers diagnosed with cutaneous leishmaniasis between January 1985 and April 1990, Herwaldt et al9 found that 46% (27/59) were conducting field studies, while 39% (23/59) were tourists, visitors, or tour guides. At least 15 of the 58 travelers interviewed (26%) were in forested areas for 1 week or less, and of these 15 respondents, at least 6 had a maximum exposure of 2 days.9
Evidence suggests that cutaneous leishmaniasis is inefficiently diagnosed in the United States. One study showed that some patients may consult up to 7 physicians before a definitive diagnosis is made, and the median time from noticing eruption of the lesions to definitive treatment was 112 days.9 Several factors may contribute to delays and inefficiencies in diagnosis. First, the lesions of cutaneous leishmaniasis are varied in morphology, and although ulcers are thought to be the most commonly presenting lesions,11 there are no specific morphologic features that are pathognomonic for cutaneous leishmaniasis. Second, the temporal association with travel to endemic countries is not necessarily apparent, with lesions developing gradually or weeks after the patient returns home. In the one study, 17% (10/58) of patients were home for more than 1 month before they noticed skin lesions.9 Finally, definitive diagnosis requires biopsy or scraping of the lesion followed by PCR, special histopathological staining (Giemsa), or culture. Polymerase chain reaction is currently the best means of identifying the causative Leishmania species.12-14 However, since skin biopsies are not routine in primary care settings and few practitioners are familiar with PCR for identification of leishmaniasis, diagnosis is typically made only after referral to a specialist.
Leishmaniasis transmission occurs in diverse geographical settings though a variety of mechanisms (Figure 4). The morphology of cutaneous leishmaniasis varies and may include papules, nodules, psoriasiform plaques, or ulcers. The differential diagnosis may include staphylococcal skin infection, insect bite, cutaneous neoplasm, pyoderma gangrenosum, sporotrichosis, blastomycosis, chromomycosis, lobomycosis, cutaneous tuberculosis, atypical mycobacterial infection, syphilis, yaws, leprosy, Hansen disease, and sarcoidosis. A definitive diagnosis can be made only after identifying the causative parasite. A scraping or punch biopsy taken from a cleaned lesion provides an adequate sample. Identification can then be accomplished by histopathology, tissue culture, or PCR.5
We present a rhyme that can be used to promote greater awareness of cutaneous leishmaniasis among US health care practitioners:
And on his leg finds an ulcerated plaque.
The possibilities are many,
Numbering far more than 20.
Leishmaniasis is a lurking issue,
So the savvy physician tests the tissue.
Although clinical resolution of cutaneous leishmaniasis usually occurs over months to years, the unsightly appearance of the lesions as well as the potential for scarring and mucosal metastasis (associated with some species) drives medical treatment.15 Pentavalent antimonial drugs, which have been the mainstay of treatment for more than 50 years, remain the most popular treatment for cutaneous leishmaniasis. Two antimony compounds, sodium stibogluconate and meglumine antimoniate, often lead to clinical cure in less than 1 month7; however, these drugs are far from ideal because of the inconvenience of obtaining them, emerging parasite resistance, long treatment course, parenteral route of administration, and serious side effects including infusion reactions, arrhythmias, pancreatitis, and liver toxicity. Moreover, the subclinical persistence of cutaneous leishmaniasis years after treatment and clinical cure is common. There have been reports of spontaneous disease reactivation in immunocompromised individuals, and Leishmania has been detected in old cutaneous leishmaniasis scars on PCR testing.16-18 Other therapies that have been used to treat cutaneous leishmaniasis include allopurinol, aminosidine sulphate, amphotericin B, the Bacillus Calmette–Guérin vaccine, cotrimoxazole, cryotherapy, dapsone, fluconazole, itraconazole, ketoconazole, laser therapy, metronidazole, miltefosine, paromomycin, pentamidine, pentoxifylline, photodynamic therapy, rifampicin, and surgical excision of the entire lesion.8 A 2009 Cochrane review of the various treatments for cutaneous leishmaniasis concluded that “no general consensus on optimal treatment has been achieved” and suggested “the creation of an international platform to improve the quality and standardization of future trials in order to develop a better evidence-based approach.”8
Conclusion
Cutaneous leishmaniasis should be included in the differential diagnosis for travelers returning from endemic areas who present with new skin lesions. Since no specific lesion types are pathognomonic for cutaneous leishmaniasis, tissue biopsy for histopathology and PCR are essential for diagnosis. Prevention of cutaneous leishmaniasis hinges on appropriate counseling of travelers to endemic regions.
Leishmaniasis describes any disease caused by protozoan parasites of the genus Leishmania1 and can manifest in 3 different forms: cutaneous (the most common); mucosal, a destructive metastatic sequela of the cutaneous form; and visceral, which is potentially fatal.2 According to the World Health Organization, the leishmaniases are endemic in 88 countries.3 It is estimated that 95% of cutaneous cases occur in the Americas (most notably Central and South America), the Mediterranean basin, the Middle East, and Central Asia.2 Most cutaneous cases diagnosed among nonmilitary personnel in the United States are acquired in Mexico and Central America.4 In Central and South America, the causative human pathogens include species of the Leishmania (Viannia) complex (eg, Leishmania panamensis, Leishmania braziliensis, Leishmania guyanensis, Leishmania peruviana) and the Leishmania mexicana complex (eg, Leishmania mexicana, Leishmania amazonensis, Leishmania venezuelensis). All of these species can cause localized cutaneous lesions, but only L panamensis, L braziliensis, and L guyanensis are associated with metastatic mucosal lesions. In Central and South Americas, only Leishmaniasis chagasi (also known as Leishmaniasis infantum) is known to cause visceral leishmaniasis.5
Case Report
A 26-year-old man was referred to the dermatology clinic by his primary care provider for evaluation of a nonhealing sore on the left volar forearm of 6 weeks’ duration. The patient described the initial lesion as a red bump resembling a mosquito bite. Over 6 weeks the papule evolved into an indurated plaque with painless ulceration. The patient’s primary care provider had prescribed antibiotics for a presumed Staphylococcus aureus infection of the skin 5 weeks prior to presentation; however, the lesion continued to enlarge in size, resulting in referral to our dermatology clinic.
Skin examination revealed a solitary, 4-cm, painless, ulcerated plaque on the left volar forearm (Figure 1). No lymphadenopathy was noted. The patient reported that he had returned from a mission trip to rural Costa Rica 2 weeks prior to the appearance of the lesion. His medical history was otherwise unremarkable and his vital signs were within normal limits. Our initial differential diagnosis included pyoderma gangrenosum, Sweet syndrome, cutaneous leishmaniasis, and an insect bite.
Histopathologic study of a 5-mm punch biopsy specimen from the lesion showed a dense nodular and diffuse lymphohistiocytic infiltrate containing foci of suppuration. Within these suppurative foci were histiocytes parasitized by intracellular organisms that appeared to be of uniform size and shape on Giemsa staining, all of which are considered to be pathognomonic features of cutaneous leishmaniasis6 (Figures 2 and 3). The dermatopathologist’s diagnosis of cutaneous leishmaniasis was confirmed by the Centers for Disease Control and Prevention. The species was identified by polymerase chain reaction (PCR) as L panamensis.
The patient was treated with intravenous sodium stibogluconate 20 mg/kg for 20 consecutive days as recommended by expert consensus. The decision to treat a frequently self-limited cutaneous lesion with a highly toxic systemic drug was based on the small but real risk of metastatic mucosal lesions, which is caused by the Viannia subgenus, including L panamensis. Of note, sodium stibogluconate and other antimony drugs are not sold in the United States. Sodium stibogluconate is approved by the US Food and Drug Administration to be distributed by the Centers for Disease Control and Prevention under a protocol requiring baseline and weekly electrocardiograms and monitoring of patients’ creatinine, transaminase, lipase, amylase, and complete blood count levels.7 Our patient tolerated treatment but experienced mild to moderate flulike symptoms. The patient experienced no remarkable sequelae other than scarring in the affected area. He was warned to notify his health care providers of any persistent nasal symptoms, including nasal stuffiness, mucosal bleeding, and increased secretions, heralding the possibility of mucosal metastasis.

| 
| |
Figure 2. Dense nodular and diffuse lymphohistiocytic infiltrate containing foci of suppuration (H&E, original magnification ×10). | Figure 3. Histiocytes parasitized by intracellular organisms of uniform shape and size on Giemsa staining (original magnification ×1000). |
Comment
The true incidence of cutaneous leishmaniasis in American travelers returning from Mexico and South and Central Americas is not known. The best incidence estimates are based on the number of physician requests for sodium stibogluconate and travel surveillance data collected by the Centers for Disease Control and Prevention. One study estimated the incidence of cutaneous leishmaniasis in Americans to be 1 case per every 100,000 travelers to Mexico.9 Data on the incidence of cutaneous leishmaniasis in American travelers seen in travel clinics for skin lesions gives a different perspective.10 Leishmaniasis is one of the most common dermatologic diseases seen in patients (European, North American, and other) returning from South America, accounting for 143 of every 1000 patients diagnosed with a skin disease acquired in South America.
Although males are thought to be at higher risk for cutaneous leishmaniasis infection than females, other demographic and behavioral risk factors are not well defined. In a case series of US travelers diagnosed with cutaneous leishmaniasis between January 1985 and April 1990, Herwaldt et al9 found that 46% (27/59) were conducting field studies, while 39% (23/59) were tourists, visitors, or tour guides. At least 15 of the 58 travelers interviewed (26%) were in forested areas for 1 week or less, and of these 15 respondents, at least 6 had a maximum exposure of 2 days.9
Evidence suggests that cutaneous leishmaniasis is inefficiently diagnosed in the United States. One study showed that some patients may consult up to 7 physicians before a definitive diagnosis is made, and the median time from noticing eruption of the lesions to definitive treatment was 112 days.9 Several factors may contribute to delays and inefficiencies in diagnosis. First, the lesions of cutaneous leishmaniasis are varied in morphology, and although ulcers are thought to be the most commonly presenting lesions,11 there are no specific morphologic features that are pathognomonic for cutaneous leishmaniasis. Second, the temporal association with travel to endemic countries is not necessarily apparent, with lesions developing gradually or weeks after the patient returns home. In the one study, 17% (10/58) of patients were home for more than 1 month before they noticed skin lesions.9 Finally, definitive diagnosis requires biopsy or scraping of the lesion followed by PCR, special histopathological staining (Giemsa), or culture. Polymerase chain reaction is currently the best means of identifying the causative Leishmania species.12-14 However, since skin biopsies are not routine in primary care settings and few practitioners are familiar with PCR for identification of leishmaniasis, diagnosis is typically made only after referral to a specialist.
Leishmaniasis transmission occurs in diverse geographical settings though a variety of mechanisms (Figure 4). The morphology of cutaneous leishmaniasis varies and may include papules, nodules, psoriasiform plaques, or ulcers. The differential diagnosis may include staphylococcal skin infection, insect bite, cutaneous neoplasm, pyoderma gangrenosum, sporotrichosis, blastomycosis, chromomycosis, lobomycosis, cutaneous tuberculosis, atypical mycobacterial infection, syphilis, yaws, leprosy, Hansen disease, and sarcoidosis. A definitive diagnosis can be made only after identifying the causative parasite. A scraping or punch biopsy taken from a cleaned lesion provides an adequate sample. Identification can then be accomplished by histopathology, tissue culture, or PCR.5
We present a rhyme that can be used to promote greater awareness of cutaneous leishmaniasis among US health care practitioners:
And on his leg finds an ulcerated plaque.
The possibilities are many,
Numbering far more than 20.
Leishmaniasis is a lurking issue,
So the savvy physician tests the tissue.
Although clinical resolution of cutaneous leishmaniasis usually occurs over months to years, the unsightly appearance of the lesions as well as the potential for scarring and mucosal metastasis (associated with some species) drives medical treatment.15 Pentavalent antimonial drugs, which have been the mainstay of treatment for more than 50 years, remain the most popular treatment for cutaneous leishmaniasis. Two antimony compounds, sodium stibogluconate and meglumine antimoniate, often lead to clinical cure in less than 1 month7; however, these drugs are far from ideal because of the inconvenience of obtaining them, emerging parasite resistance, long treatment course, parenteral route of administration, and serious side effects including infusion reactions, arrhythmias, pancreatitis, and liver toxicity. Moreover, the subclinical persistence of cutaneous leishmaniasis years after treatment and clinical cure is common. There have been reports of spontaneous disease reactivation in immunocompromised individuals, and Leishmania has been detected in old cutaneous leishmaniasis scars on PCR testing.16-18 Other therapies that have been used to treat cutaneous leishmaniasis include allopurinol, aminosidine sulphate, amphotericin B, the Bacillus Calmette–Guérin vaccine, cotrimoxazole, cryotherapy, dapsone, fluconazole, itraconazole, ketoconazole, laser therapy, metronidazole, miltefosine, paromomycin, pentamidine, pentoxifylline, photodynamic therapy, rifampicin, and surgical excision of the entire lesion.8 A 2009 Cochrane review of the various treatments for cutaneous leishmaniasis concluded that “no general consensus on optimal treatment has been achieved” and suggested “the creation of an international platform to improve the quality and standardization of future trials in order to develop a better evidence-based approach.”8
Conclusion
Cutaneous leishmaniasis should be included in the differential diagnosis for travelers returning from endemic areas who present with new skin lesions. Since no specific lesion types are pathognomonic for cutaneous leishmaniasis, tissue biopsy for histopathology and PCR are essential for diagnosis. Prevention of cutaneous leishmaniasis hinges on appropriate counseling of travelers to endemic regions.
1. Etymologia-Leishmaniasis. Emerg Infect Dis. 2008;14:666.
2. Burden and distribution. World Health Organization Web site. http://www.who.int/leishmaniasis/burden/en/. Accessed November 10, 2015.
3. Emergencies preparedness, response. World Health Organization Web site. http://www.who.int/csr/resources/publications/CSR_ISR_2000_1leish/en/. Accessed November 3, 2015.
4. Pavli A, Maltezou HC. Leishmaniasis, an emerging infection in travelers. Int J Infect Dis. 2010;14:e1032-e1039.
5. Magill AJ. Leishmania species: visceral (Kala-Azar), cutaneous, and mucosal leishmaniasis. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone; 2009:3463-3480.
6. Mysore V. Invisible dermatoses. Indian J Dermatol Venereol Leprol. 2010;76:239-248.
7. Parasites – Leishmaniasis. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/parasites/leishmaniasis/health_professionals/. Updated September 14, 2015. Accessed November 13, 2015.
8. González U, Pinart M, Rengifo-Pardo M, et al. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst Rev. 2009;15:CD004834.
9. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med. 1993;118:779-784.
10. Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
11. El Hajj L, Thellier M, Carriere J, et al. Localized cutaneous leishmaniasis imported into Paris: a review of 39 cases. Int J Dermatol. 2004;43:120-125.
12. Harris E, Kropp G, Belli A, et al. Single-step multiplex PCR assay for characterization of New World Leishmania complexes. J Clin Microbiol. 1998;36:1989-1995.
13. Marfurt J, Niederwieser I, Makia D, et al. Diagnostic genotyping of Old and New World Leishmania species by PCR-RFLP. Diagn Microbiol Infect Dis. 2003;46:115-124.
14. Schonian G, Nasereddin A, Dinse N, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47:349-358.
15. Reithinger R, Aadil K, Kolaczinski J, et al. Social impact of leishmaniasis, Afghanistan. Emerg Infect Dis. 2005;11:634-636.
16. Morales MA, Cruz I, Rubio JM, et al. Relapses versus reinfections in patients coinfected with Leishmania infantum and human immunodeficiency virus type 1 [published online ahead of print April 22, 2002]. J Infect Dis. 2002;185:1533-1537.
17. Coutinho SG, Pirmez C, Da-Cruz AM. Parasitological and immunological follow-up of American tegumentary leishmaniasis patients. Trans R Soc Trop Med Hyg. 2002;96(suppl 1):S173-S178.
18. Mendonça MG, de Brito ME, Rodrigues EH, et al. Persistance of leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: is there a sterile cure [published online ahead of print March 2, 2004]? J Infect Dis. 2004;189:1018-1023.
1. Etymologia-Leishmaniasis. Emerg Infect Dis. 2008;14:666.
2. Burden and distribution. World Health Organization Web site. http://www.who.int/leishmaniasis/burden/en/. Accessed November 10, 2015.
3. Emergencies preparedness, response. World Health Organization Web site. http://www.who.int/csr/resources/publications/CSR_ISR_2000_1leish/en/. Accessed November 3, 2015.
4. Pavli A, Maltezou HC. Leishmaniasis, an emerging infection in travelers. Int J Infect Dis. 2010;14:e1032-e1039.
5. Magill AJ. Leishmania species: visceral (Kala-Azar), cutaneous, and mucosal leishmaniasis. In: Mandell GL, Bennett JE, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, PA: Churchill Livingstone; 2009:3463-3480.
6. Mysore V. Invisible dermatoses. Indian J Dermatol Venereol Leprol. 2010;76:239-248.
7. Parasites – Leishmaniasis. Centers for Disease Control and Prevention Web site. http://www.cdc.gov/parasites/leishmaniasis/health_professionals/. Updated September 14, 2015. Accessed November 13, 2015.
8. González U, Pinart M, Rengifo-Pardo M, et al. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst Rev. 2009;15:CD004834.
9. Herwaldt BL, Stokes SL, Juranek DD. American cutaneous leishmaniasis in U.S. travelers. Ann Intern Med. 1993;118:779-784.
10. Freedman DO, Weld LH, Kozarsky PE, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. New Engl J Med. 2006;354:119-130.
11. El Hajj L, Thellier M, Carriere J, et al. Localized cutaneous leishmaniasis imported into Paris: a review of 39 cases. Int J Dermatol. 2004;43:120-125.
12. Harris E, Kropp G, Belli A, et al. Single-step multiplex PCR assay for characterization of New World Leishmania complexes. J Clin Microbiol. 1998;36:1989-1995.
13. Marfurt J, Niederwieser I, Makia D, et al. Diagnostic genotyping of Old and New World Leishmania species by PCR-RFLP. Diagn Microbiol Infect Dis. 2003;46:115-124.
14. Schonian G, Nasereddin A, Dinse N, et al. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diagn Microbiol Infect Dis. 2003;47:349-358.
15. Reithinger R, Aadil K, Kolaczinski J, et al. Social impact of leishmaniasis, Afghanistan. Emerg Infect Dis. 2005;11:634-636.
16. Morales MA, Cruz I, Rubio JM, et al. Relapses versus reinfections in patients coinfected with Leishmania infantum and human immunodeficiency virus type 1 [published online ahead of print April 22, 2002]. J Infect Dis. 2002;185:1533-1537.
17. Coutinho SG, Pirmez C, Da-Cruz AM. Parasitological and immunological follow-up of American tegumentary leishmaniasis patients. Trans R Soc Trop Med Hyg. 2002;96(suppl 1):S173-S178.
18. Mendonça MG, de Brito ME, Rodrigues EH, et al. Persistance of leishmania parasites in scars after clinical cure of American cutaneous leishmaniasis: is there a sterile cure [published online ahead of print March 2, 2004]? J Infect Dis. 2004;189:1018-1023.
Practice Points
- Cutaneous leishmaniasis is an emerging infectious disease that may be misdiagnosed due to its rarity and varied clinical presentation as well as the limited use of tissue biopsy in general practice.
- United States health care practitioners who evaluate patients with new isolated skin lesions and a history of recent travel to Mexico or South or Central Americas should consider cutaneous leishmaniasis in the differential diagnosis.
- Whenever possible, travelers to rural areas of Mexico and South and Central Americas should be educated about strategies to avoid arthropod bites, such as wearing protective clothing and using insect repellents.
What Is Your Diagnosis? Tinea Corporis
The Diagnosis: Tinea Corporis
Although contact dermatitis from the metal on the back of the watch was suspected, many modern wrist watches are made with stainless steel rather than nickel, which is a common contact allergen; therefore, other diagnoses were considered in the differential including irritant contact dermatitis, psoriasis, and tinea infection. A potassium hydroxide (KOH) preparation was performed to rule out tinea infection. Unexpectedly, the KOH preparation was positive for fungal hyphae, confirming a diagnosis of tinea corporis. The patient was treated with clotrimazole cream 1% twice daily for 3 weeks during which time the rash completely resolved.
We present this case to stress the importance of performing KOH preparations even when the likelihood of tinea infection seems remote. At our institution, we teach our residents, “If it’s scaly, scrape it.” This adage has served us well. Tinea corporis may be mistaken for many other skin diseases, including eczema, psoriasis, and seborrheic dermatitis.1 A KOH preparation often is a helpful tool in confirming the diagnosis and should be performed when a dermatophyte infection is suspected. The KOH preparation is the most sensitive diagnostic test used to confirm dermatophyte infection, with 90% of infections showing positive results.2,3
Tinea infections may occur anywhere on the body, but areas that are prone to excessive heat and/or moisture are particularly susceptible.4 Dermatophyte infections typically present as annular, scaly, pruritic patches or plaques often with central clearing and an active border.1 In our patient, the lesion showed characteristics that were suggestive of a dermatophyte infection but was somewhat atypical in appearance, as it lacked central clearing (Figure). The 3 genera of dermatophytes—Trichophyton, Microsporum, and Epidermophyton—are common causes of fungal infections.2 The pathogenesis of dermatophytosis is the synthesis of keratinases that digest keratin and sustain the presence of the fungi. Local factors such as sweating and occlusion facilitate the activity of these organisms.2 In our case, the pathogenesis was believed to be due to the entrapment of moisture behind the patient’s watch, creating a favorable environment for fungal growth.
- Ely JW, Rosenfeld S, Seabury SM. Diagnosis and management of tinea infections. Am Fam Physician. 2014:90:702-710.
- Wolff K, Saavedra AP, Fitzpatrick TB. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill Medical; 2013.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis (published online ahead of print June 22, 2010). Dermatol Res Pract. doi:10.1155/2010/764843.
- Gupta AK, Chaudhry M, Elewski B. Tinea corporis, tinea cruris, tinea nigra and piedra. Dermatol Clin. 2003;21:395-400.
The Diagnosis: Tinea Corporis
Although contact dermatitis from the metal on the back of the watch was suspected, many modern wrist watches are made with stainless steel rather than nickel, which is a common contact allergen; therefore, other diagnoses were considered in the differential including irritant contact dermatitis, psoriasis, and tinea infection. A potassium hydroxide (KOH) preparation was performed to rule out tinea infection. Unexpectedly, the KOH preparation was positive for fungal hyphae, confirming a diagnosis of tinea corporis. The patient was treated with clotrimazole cream 1% twice daily for 3 weeks during which time the rash completely resolved.

We present this case to stress the importance of performing KOH preparations even when the likelihood of tinea infection seems remote. At our institution, we teach our residents, “If it’s scaly, scrape it.” This adage has served us well. Tinea corporis may be mistaken for many other skin diseases, including eczema, psoriasis, and seborrheic dermatitis.1 A KOH preparation often is a helpful tool in confirming the diagnosis and should be performed when a dermatophyte infection is suspected. The KOH preparation is the most sensitive diagnostic test used to confirm dermatophyte infection, with 90% of infections showing positive results.2,3
Tinea infections may occur anywhere on the body, but areas that are prone to excessive heat and/or moisture are particularly susceptible.4 Dermatophyte infections typically present as annular, scaly, pruritic patches or plaques often with central clearing and an active border.1 In our patient, the lesion showed characteristics that were suggestive of a dermatophyte infection but was somewhat atypical in appearance, as it lacked central clearing (Figure). The 3 genera of dermatophytes—Trichophyton, Microsporum, and Epidermophyton—are common causes of fungal infections.2 The pathogenesis of dermatophytosis is the synthesis of keratinases that digest keratin and sustain the presence of the fungi. Local factors such as sweating and occlusion facilitate the activity of these organisms.2 In our case, the pathogenesis was believed to be due to the entrapment of moisture behind the patient’s watch, creating a favorable environment for fungal growth.
The Diagnosis: Tinea Corporis
Although contact dermatitis from the metal on the back of the watch was suspected, many modern wrist watches are made with stainless steel rather than nickel, which is a common contact allergen; therefore, other diagnoses were considered in the differential including irritant contact dermatitis, psoriasis, and tinea infection. A potassium hydroxide (KOH) preparation was performed to rule out tinea infection. Unexpectedly, the KOH preparation was positive for fungal hyphae, confirming a diagnosis of tinea corporis. The patient was treated with clotrimazole cream 1% twice daily for 3 weeks during which time the rash completely resolved.

We present this case to stress the importance of performing KOH preparations even when the likelihood of tinea infection seems remote. At our institution, we teach our residents, “If it’s scaly, scrape it.” This adage has served us well. Tinea corporis may be mistaken for many other skin diseases, including eczema, psoriasis, and seborrheic dermatitis.1 A KOH preparation often is a helpful tool in confirming the diagnosis and should be performed when a dermatophyte infection is suspected. The KOH preparation is the most sensitive diagnostic test used to confirm dermatophyte infection, with 90% of infections showing positive results.2,3
Tinea infections may occur anywhere on the body, but areas that are prone to excessive heat and/or moisture are particularly susceptible.4 Dermatophyte infections typically present as annular, scaly, pruritic patches or plaques often with central clearing and an active border.1 In our patient, the lesion showed characteristics that were suggestive of a dermatophyte infection but was somewhat atypical in appearance, as it lacked central clearing (Figure). The 3 genera of dermatophytes—Trichophyton, Microsporum, and Epidermophyton—are common causes of fungal infections.2 The pathogenesis of dermatophytosis is the synthesis of keratinases that digest keratin and sustain the presence of the fungi. Local factors such as sweating and occlusion facilitate the activity of these organisms.2 In our case, the pathogenesis was believed to be due to the entrapment of moisture behind the patient’s watch, creating a favorable environment for fungal growth.
- Ely JW, Rosenfeld S, Seabury SM. Diagnosis and management of tinea infections. Am Fam Physician. 2014:90:702-710.
- Wolff K, Saavedra AP, Fitzpatrick TB. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill Medical; 2013.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis (published online ahead of print June 22, 2010). Dermatol Res Pract. doi:10.1155/2010/764843.
- Gupta AK, Chaudhry M, Elewski B. Tinea corporis, tinea cruris, tinea nigra and piedra. Dermatol Clin. 2003;21:395-400.
- Ely JW, Rosenfeld S, Seabury SM. Diagnosis and management of tinea infections. Am Fam Physician. 2014:90:702-710.
- Wolff K, Saavedra AP, Fitzpatrick TB. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill Medical; 2013.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis (published online ahead of print June 22, 2010). Dermatol Res Pract. doi:10.1155/2010/764843.
- Gupta AK, Chaudhry M, Elewski B. Tinea corporis, tinea cruris, tinea nigra and piedra. Dermatol Clin. 2003;21:395-400.
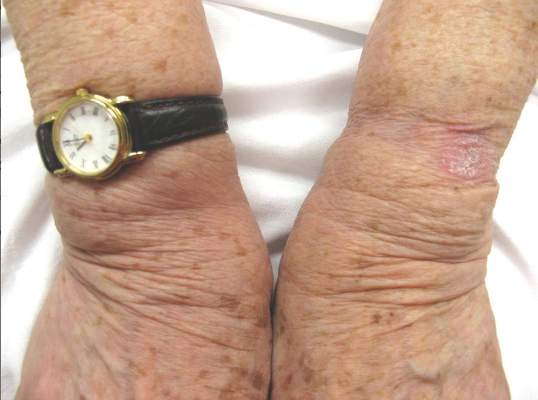
An 81-year-old woman presented with a 2-cm erythematous, scaly, pruritic rash on the left dorsal wrist localized to the skin under her watch. The patient first noticed the lesion 2 months prior. She moved the watch to the right wrist a few days prior to presentation and no symptoms developed in that location. No other areas of the skin were affected. She had no known allergies and was otherwise in good health.
Disseminated Cutaneous Infection with Mycobacterium chelonae in a Renal Transplant Recipient
Mycobacterium chelonae, along with Mycobacterium fortuitum and Mycobacterium abscessus, belongs to a rapidly growing group of nontuberculous mycobacteria (NTM), which are classified as environmental saprophytes found in soil, water, and dust. Under certain circumstances, NTM can cause infection in humans. Nontuberculous mycobacteria are known to cause infection in immunosuppressed patients (such as in the setting of AIDS or immunotherapy following solid organ transplantation); however, they can also cause serious morbidity in immunocompetent patients with certain predisposing factors (eg, recent history of a traumatic wound, recent drug injections, impaired cell-mediated immunity).1-4
We present the case of a patient who presented with multiple reddish blue, nodular, suppurative lesions on the bilateral legs of 1 month’s duration. The patient had a history of renal transplantation 6 years prior followed by immunosuppressive therapy. A punch biopsy of a sample nodule was performed, followed by histologic examination and culture of the biopsy specimen, but polymerase chain reaction (PCR) assay for genotyping of the specimen was necessary to determine the responsible Mycobacterium species.
Case Report
A 61-year-old woman was admitted to our hospital for evaluation and treatment of multiple subcutaneous nodules on the bilateral legs. The patient had undergone successful cadaveric renal transplantation 6 years prior due to polycystic kidney disease and was undergoing maintenance immunosuppressive combination therapy with tacrolimus 4 mg and methylprednisolone 4 mg daily. No other medications or concomitant diseases were reported.
Physical examination revealed multiple slightly tender, brown to purple papules and nodules on the lower legs ranging in size from 2 mm to 1 cm in diameter (Figure 1), some of which exhibited central necrosis (Figure 2). The patient did not recall any previous trauma to the lower legs. Her body temperature was measured at 37.9°C and no regional lymphadenopathy or any other physical abnormalities were observed. Multiple blood culture samples were negative for bacteria, fungi, and mycobacteria.
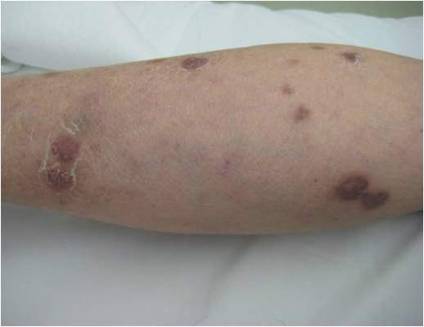
| 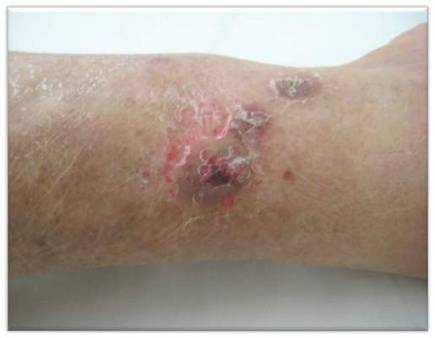
| |
Figure 1. Multiple slightly tender, brown to purple papules and nodules on the lower left leg. | Figure 2. A nodule on the lower right leg exhibited central necrosis. |
During her 2 weeks in the hospital, the patient’s tacrolimus and methylprednisolone dosages were decreased to 2 mg daily. Routine laboratory tests and serum chemistry were normal with the exception of elevated creatinine levels (1.88 mg/dL [reference range, 0.6 to 1.2 mg/dL]). Chest radiography and interferon-γ release assay were negative. A punch biopsy from a sample nodule was performed and revealed granulomatous inflammation surrounded by giant cells on histopathology. Microscopic examination of the specimen revealed alcohol- and acid-resistant bacilli on Ziehl-Neelsen staining. A biopsy specimen was cultured on Löwenstein-Jensen medium at 25°C, 37°C, and 42°C according to NTM detection protocol5 and showed growth of NTM at 37°C. On the basis of the positive culture, genetic analysis of the specimen was performed using a strip test that permits identification of 13 common species of NTM. The organism was identified as M chelonae.
While awaiting species identification and results of drug susceptibility testing, treatment with oral clarithromycin 250 mg twice daily was initiated and continued for 10 days until the patient developed gastrointestinal adverse effects, at which point oral ciprofloxacin 250 mg twice daily was substituted. In laboratory testing, the isolated M chelonae strain showed sensitivity to ciprofloxacin, clarithromycin, tobramycin, and amikacin at minimum inhibitory concentrations of less than 1, 2, 4, and 16, respectively. Treatment with ciprofloxacin 250 mg twice daily was continued for 6 months, which resulted in slow resolution of the lesions until the end of treatment (Figure 3). No recurrence of the lesions was noted at 24-month follow-up, but areas of hyperpigmentation were noted at the lesion sites (Figure 4).
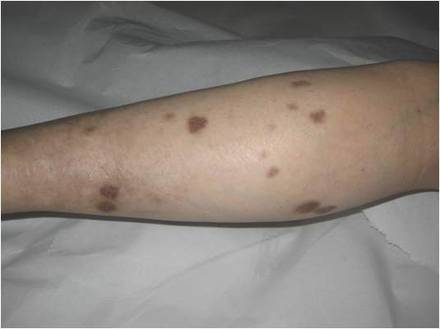
| 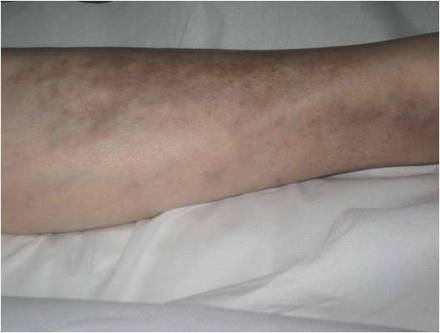
| ||
Figure 3. Following 6 months of treatment with oral ciprofloxacin 250 mg twice daily, nodules on the left leg had resolved and papules had decreased in size. | Figure 4. Skin lesions had resolved without recurrence at 24-month follow-up, although hyperpigmented areas remained. |
Comment
Mycobacterium chelonae, a member of the NTM group, grows rapidly on Löwenstein-Jensen medium, usually following incubation for 5 to 7 days at temperatures of 28°C to 32°C, and is characterized by its lack of pigmentation. Nontuberculous mycobacteria, which are resistant to standard disinfectants such as chlorine, organomercurials, and alkaline glutaraldehydes, may cause nosocomial outbreaks, infecting otherwise healthy individuals receiving any type of injection (eg, in cosmetic procedures), as well as those with suppressed immunity.6
In addition to cutaneous manifestations, NTM may cause various extracutaneous diseases, such as osteomyelitis, infective bronchiectasis, endocarditis, pericarditis, lymphadenopathy, and ocular infections.1-4 The species M chelonae may cause localized skin infections, soft tissue lesions (eg, granulomatous nodules, ulcers, abscesses, sporotrichoid lesions), and cutaneous disseminated infections.
Immunosuppression associated with treatment following renal transplantation was the primary cause of M chelonae infection in our patient, as has previously been reported in the literature.3-4 This was further supported by the lack of prior trauma or invasive procedure (eg, mesotherapy) in the affected areas. Specifically, our patient had more than 5 lesions on the lower legs; in accordance with a previous comprehensive study,1 the presence of more than 5 lesions indicates a disseminated cutaneous infection, which usually is correlated with immunosuppression (such as in our patient). Localized infections generally are observed in immunocompetent hosts.1
The exact pathogenetic mechanism of M chelonae infection in our patient is not clear. In patients with suppressed immunity, the variable clinical presentation of infection with NTM often impedes diagnosis. Cutaneous M chelonae lesions may be mistakenly diagnosed as Kaposi sarcoma or rarely as pyoderma gangrenosum. The differential diagnosis of subcutaneous nodules includes histoplasmosis, cryptococcosis, blastomycosis, coccidioidomycosis, nocardiosis, mycetoma, sporotrichosis, actinomycosis, and tuberculosis. In our patient, approximately 2 months elapsed between presentation of symptoms and definitive diagnosis, which was less than that reported in previously published cases.2,7-9
Histology and tissue culture followed by proper genetic analysis remains the gold standard for diagnosing NTM infection.10,11 In the interest of patients, time-consuming biochemical analyses should be replaced by molecular genetic diagnostic strip tests, which are fast, exact, and available in commercial kits for both common mycobacteria and additional species.12
Once the diagnosis of NTM infection has been established, sensitivity testing is mandatory to guide targeted therapy; however, clinicians should bear in mind that susceptibility testing does not guarantee clinical success, as correlations of susceptibility testing and clinical response have not been assessed.8 Standard antituberculous drugs (eg, isoniazid, rifampin, pyrazinamide) have no role in the treatment of M chelonae infection. The first-line antibiotics are clarithromycin, tobramycin, and linezolid, followed by imipenem, amikacin, clofazimine, doxycycline, and ciprofloxacin.10 Optimal outcomes have been reported in patients treated both with antibiotics and with surgical debridement. Although monotherapy with quinolones is not recommended for treatment of infection with NTM due to the high risk of mutational resistance, our patient received long-term antibiotic treatment with ciprofloxacin over a 6-month period and showed no recurrence at 24-month follow-up.
Conclusion
Clinicians who treat patients with chronic skin or soft tissue infections should consider infection with NTM in the differential diagnosis, particularly in patients with suppressed immunity, but also in immunocompetent patients following any invasive procedure. Detailed medical history and skin biopsy followed by histology and culture are recommended for the diagnosis. Infection with NTM requires rapid action. Sensitivity testing is necessary in choosing an effective treatment. New molecular genetic diagnostic strip tests can differentiate species of NTM sooner than biochemical analyses, thereby helping clinicians initiate appropriate antimicrobial treatment in a timely fashion.
1. Wallace RJ Jr, Brown BA, Onyi GO. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance of oral antimicrobials other than clarithromycin. J Infect Dis. 1992;166:405-412.
2. Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
3. Alexander S, John GT, Jesudason M, et al. Infections with atypical mycobacteria in renal transplant recipients. Indian J Pathol Microbiol. 2007;50:482-484.
4. Dorman S, Subramanian A; AST Infectious Diseases Community of Practice. Nontuberculous mycobacteria in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S63-S69.
5. Whitman WB, Goodfellow M, Kämpfer P, et al, eds. Bergey’s Manual of Systematic Bacteriology. 2nd ed. New York, NY: Springer-Verlag; 2012. The Actinobacteria; vol 5.
6. Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria [published online ahead of print September 5, 2001]. Clin Infect Dis. 2001;33:1363-1374.
7. Dodiuk-Gad R, Dyachenko P, Ziv M, et al. Nontuberculous mycobacterial infections of the skin: a retrospective study of 25 cases [published online ahead of print March 26, 2007]. J Am Acad Dermatol. 2007;57:413-420.
8. Regnier S, Cambau E, Meningaud JP, et al. Clinical management of rapidly growing mycobacterial cutaneous infections in patients after mesotherapy. Clin Infect Dis. 2009;49:1358-1364.
9. Somily AM, AL-Anazi AR, Babay HA, et al. Mycobacterium chelonae complex bacteremia from a post-renal transplant patient: case report and literature review. Jpn J Infect Dis. 2010;63:61-64.
10. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction in Am J Respir Crit Care Med. 2007;175:744-745]. Am J Respir Crit Care Med. 2007;175:367-416.
11. Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases [published online ahead of print September 6, 2010]. J Dermatol. 2010;37:965-972.
12. Lee AS, Jelfs P, Sintchenko V, et al. Identification of non-tuberculous mycobacteria: utility of the GenoType Mycobacterium CM/AS assay compared with HPLC and 16S rRNA gene sequencing [published online ahead of print June 5, 2009]. J of Med Microb. 2009;58(pt 7):900-904.
Mycobacterium chelonae, along with Mycobacterium fortuitum and Mycobacterium abscessus, belongs to a rapidly growing group of nontuberculous mycobacteria (NTM), which are classified as environmental saprophytes found in soil, water, and dust. Under certain circumstances, NTM can cause infection in humans. Nontuberculous mycobacteria are known to cause infection in immunosuppressed patients (such as in the setting of AIDS or immunotherapy following solid organ transplantation); however, they can also cause serious morbidity in immunocompetent patients with certain predisposing factors (eg, recent history of a traumatic wound, recent drug injections, impaired cell-mediated immunity).1-4
We present the case of a patient who presented with multiple reddish blue, nodular, suppurative lesions on the bilateral legs of 1 month’s duration. The patient had a history of renal transplantation 6 years prior followed by immunosuppressive therapy. A punch biopsy of a sample nodule was performed, followed by histologic examination and culture of the biopsy specimen, but polymerase chain reaction (PCR) assay for genotyping of the specimen was necessary to determine the responsible Mycobacterium species.
Case Report
A 61-year-old woman was admitted to our hospital for evaluation and treatment of multiple subcutaneous nodules on the bilateral legs. The patient had undergone successful cadaveric renal transplantation 6 years prior due to polycystic kidney disease and was undergoing maintenance immunosuppressive combination therapy with tacrolimus 4 mg and methylprednisolone 4 mg daily. No other medications or concomitant diseases were reported.
Physical examination revealed multiple slightly tender, brown to purple papules and nodules on the lower legs ranging in size from 2 mm to 1 cm in diameter (Figure 1), some of which exhibited central necrosis (Figure 2). The patient did not recall any previous trauma to the lower legs. Her body temperature was measured at 37.9°C and no regional lymphadenopathy or any other physical abnormalities were observed. Multiple blood culture samples were negative for bacteria, fungi, and mycobacteria.

| 
| |
Figure 1. Multiple slightly tender, brown to purple papules and nodules on the lower left leg. | Figure 2. A nodule on the lower right leg exhibited central necrosis. |
During her 2 weeks in the hospital, the patient’s tacrolimus and methylprednisolone dosages were decreased to 2 mg daily. Routine laboratory tests and serum chemistry were normal with the exception of elevated creatinine levels (1.88 mg/dL [reference range, 0.6 to 1.2 mg/dL]). Chest radiography and interferon-γ release assay were negative. A punch biopsy from a sample nodule was performed and revealed granulomatous inflammation surrounded by giant cells on histopathology. Microscopic examination of the specimen revealed alcohol- and acid-resistant bacilli on Ziehl-Neelsen staining. A biopsy specimen was cultured on Löwenstein-Jensen medium at 25°C, 37°C, and 42°C according to NTM detection protocol5 and showed growth of NTM at 37°C. On the basis of the positive culture, genetic analysis of the specimen was performed using a strip test that permits identification of 13 common species of NTM. The organism was identified as M chelonae.
While awaiting species identification and results of drug susceptibility testing, treatment with oral clarithromycin 250 mg twice daily was initiated and continued for 10 days until the patient developed gastrointestinal adverse effects, at which point oral ciprofloxacin 250 mg twice daily was substituted. In laboratory testing, the isolated M chelonae strain showed sensitivity to ciprofloxacin, clarithromycin, tobramycin, and amikacin at minimum inhibitory concentrations of less than 1, 2, 4, and 16, respectively. Treatment with ciprofloxacin 250 mg twice daily was continued for 6 months, which resulted in slow resolution of the lesions until the end of treatment (Figure 3). No recurrence of the lesions was noted at 24-month follow-up, but areas of hyperpigmentation were noted at the lesion sites (Figure 4).

| 
| ||
Figure 3. Following 6 months of treatment with oral ciprofloxacin 250 mg twice daily, nodules on the left leg had resolved and papules had decreased in size. | Figure 4. Skin lesions had resolved without recurrence at 24-month follow-up, although hyperpigmented areas remained. |
Comment
Mycobacterium chelonae, a member of the NTM group, grows rapidly on Löwenstein-Jensen medium, usually following incubation for 5 to 7 days at temperatures of 28°C to 32°C, and is characterized by its lack of pigmentation. Nontuberculous mycobacteria, which are resistant to standard disinfectants such as chlorine, organomercurials, and alkaline glutaraldehydes, may cause nosocomial outbreaks, infecting otherwise healthy individuals receiving any type of injection (eg, in cosmetic procedures), as well as those with suppressed immunity.6
In addition to cutaneous manifestations, NTM may cause various extracutaneous diseases, such as osteomyelitis, infective bronchiectasis, endocarditis, pericarditis, lymphadenopathy, and ocular infections.1-4 The species M chelonae may cause localized skin infections, soft tissue lesions (eg, granulomatous nodules, ulcers, abscesses, sporotrichoid lesions), and cutaneous disseminated infections.
Immunosuppression associated with treatment following renal transplantation was the primary cause of M chelonae infection in our patient, as has previously been reported in the literature.3-4 This was further supported by the lack of prior trauma or invasive procedure (eg, mesotherapy) in the affected areas. Specifically, our patient had more than 5 lesions on the lower legs; in accordance with a previous comprehensive study,1 the presence of more than 5 lesions indicates a disseminated cutaneous infection, which usually is correlated with immunosuppression (such as in our patient). Localized infections generally are observed in immunocompetent hosts.1
The exact pathogenetic mechanism of M chelonae infection in our patient is not clear. In patients with suppressed immunity, the variable clinical presentation of infection with NTM often impedes diagnosis. Cutaneous M chelonae lesions may be mistakenly diagnosed as Kaposi sarcoma or rarely as pyoderma gangrenosum. The differential diagnosis of subcutaneous nodules includes histoplasmosis, cryptococcosis, blastomycosis, coccidioidomycosis, nocardiosis, mycetoma, sporotrichosis, actinomycosis, and tuberculosis. In our patient, approximately 2 months elapsed between presentation of symptoms and definitive diagnosis, which was less than that reported in previously published cases.2,7-9
Histology and tissue culture followed by proper genetic analysis remains the gold standard for diagnosing NTM infection.10,11 In the interest of patients, time-consuming biochemical analyses should be replaced by molecular genetic diagnostic strip tests, which are fast, exact, and available in commercial kits for both common mycobacteria and additional species.12
Once the diagnosis of NTM infection has been established, sensitivity testing is mandatory to guide targeted therapy; however, clinicians should bear in mind that susceptibility testing does not guarantee clinical success, as correlations of susceptibility testing and clinical response have not been assessed.8 Standard antituberculous drugs (eg, isoniazid, rifampin, pyrazinamide) have no role in the treatment of M chelonae infection. The first-line antibiotics are clarithromycin, tobramycin, and linezolid, followed by imipenem, amikacin, clofazimine, doxycycline, and ciprofloxacin.10 Optimal outcomes have been reported in patients treated both with antibiotics and with surgical debridement. Although monotherapy with quinolones is not recommended for treatment of infection with NTM due to the high risk of mutational resistance, our patient received long-term antibiotic treatment with ciprofloxacin over a 6-month period and showed no recurrence at 24-month follow-up.
Conclusion
Clinicians who treat patients with chronic skin or soft tissue infections should consider infection with NTM in the differential diagnosis, particularly in patients with suppressed immunity, but also in immunocompetent patients following any invasive procedure. Detailed medical history and skin biopsy followed by histology and culture are recommended for the diagnosis. Infection with NTM requires rapid action. Sensitivity testing is necessary in choosing an effective treatment. New molecular genetic diagnostic strip tests can differentiate species of NTM sooner than biochemical analyses, thereby helping clinicians initiate appropriate antimicrobial treatment in a timely fashion.
Mycobacterium chelonae, along with Mycobacterium fortuitum and Mycobacterium abscessus, belongs to a rapidly growing group of nontuberculous mycobacteria (NTM), which are classified as environmental saprophytes found in soil, water, and dust. Under certain circumstances, NTM can cause infection in humans. Nontuberculous mycobacteria are known to cause infection in immunosuppressed patients (such as in the setting of AIDS or immunotherapy following solid organ transplantation); however, they can also cause serious morbidity in immunocompetent patients with certain predisposing factors (eg, recent history of a traumatic wound, recent drug injections, impaired cell-mediated immunity).1-4
We present the case of a patient who presented with multiple reddish blue, nodular, suppurative lesions on the bilateral legs of 1 month’s duration. The patient had a history of renal transplantation 6 years prior followed by immunosuppressive therapy. A punch biopsy of a sample nodule was performed, followed by histologic examination and culture of the biopsy specimen, but polymerase chain reaction (PCR) assay for genotyping of the specimen was necessary to determine the responsible Mycobacterium species.
Case Report
A 61-year-old woman was admitted to our hospital for evaluation and treatment of multiple subcutaneous nodules on the bilateral legs. The patient had undergone successful cadaveric renal transplantation 6 years prior due to polycystic kidney disease and was undergoing maintenance immunosuppressive combination therapy with tacrolimus 4 mg and methylprednisolone 4 mg daily. No other medications or concomitant diseases were reported.
Physical examination revealed multiple slightly tender, brown to purple papules and nodules on the lower legs ranging in size from 2 mm to 1 cm in diameter (Figure 1), some of which exhibited central necrosis (Figure 2). The patient did not recall any previous trauma to the lower legs. Her body temperature was measured at 37.9°C and no regional lymphadenopathy or any other physical abnormalities were observed. Multiple blood culture samples were negative for bacteria, fungi, and mycobacteria.

| 
| |
Figure 1. Multiple slightly tender, brown to purple papules and nodules on the lower left leg. | Figure 2. A nodule on the lower right leg exhibited central necrosis. |
During her 2 weeks in the hospital, the patient’s tacrolimus and methylprednisolone dosages were decreased to 2 mg daily. Routine laboratory tests and serum chemistry were normal with the exception of elevated creatinine levels (1.88 mg/dL [reference range, 0.6 to 1.2 mg/dL]). Chest radiography and interferon-γ release assay were negative. A punch biopsy from a sample nodule was performed and revealed granulomatous inflammation surrounded by giant cells on histopathology. Microscopic examination of the specimen revealed alcohol- and acid-resistant bacilli on Ziehl-Neelsen staining. A biopsy specimen was cultured on Löwenstein-Jensen medium at 25°C, 37°C, and 42°C according to NTM detection protocol5 and showed growth of NTM at 37°C. On the basis of the positive culture, genetic analysis of the specimen was performed using a strip test that permits identification of 13 common species of NTM. The organism was identified as M chelonae.
While awaiting species identification and results of drug susceptibility testing, treatment with oral clarithromycin 250 mg twice daily was initiated and continued for 10 days until the patient developed gastrointestinal adverse effects, at which point oral ciprofloxacin 250 mg twice daily was substituted. In laboratory testing, the isolated M chelonae strain showed sensitivity to ciprofloxacin, clarithromycin, tobramycin, and amikacin at minimum inhibitory concentrations of less than 1, 2, 4, and 16, respectively. Treatment with ciprofloxacin 250 mg twice daily was continued for 6 months, which resulted in slow resolution of the lesions until the end of treatment (Figure 3). No recurrence of the lesions was noted at 24-month follow-up, but areas of hyperpigmentation were noted at the lesion sites (Figure 4).

| 
| ||
Figure 3. Following 6 months of treatment with oral ciprofloxacin 250 mg twice daily, nodules on the left leg had resolved and papules had decreased in size. | Figure 4. Skin lesions had resolved without recurrence at 24-month follow-up, although hyperpigmented areas remained. |
Comment
Mycobacterium chelonae, a member of the NTM group, grows rapidly on Löwenstein-Jensen medium, usually following incubation for 5 to 7 days at temperatures of 28°C to 32°C, and is characterized by its lack of pigmentation. Nontuberculous mycobacteria, which are resistant to standard disinfectants such as chlorine, organomercurials, and alkaline glutaraldehydes, may cause nosocomial outbreaks, infecting otherwise healthy individuals receiving any type of injection (eg, in cosmetic procedures), as well as those with suppressed immunity.6
In addition to cutaneous manifestations, NTM may cause various extracutaneous diseases, such as osteomyelitis, infective bronchiectasis, endocarditis, pericarditis, lymphadenopathy, and ocular infections.1-4 The species M chelonae may cause localized skin infections, soft tissue lesions (eg, granulomatous nodules, ulcers, abscesses, sporotrichoid lesions), and cutaneous disseminated infections.
Immunosuppression associated with treatment following renal transplantation was the primary cause of M chelonae infection in our patient, as has previously been reported in the literature.3-4 This was further supported by the lack of prior trauma or invasive procedure (eg, mesotherapy) in the affected areas. Specifically, our patient had more than 5 lesions on the lower legs; in accordance with a previous comprehensive study,1 the presence of more than 5 lesions indicates a disseminated cutaneous infection, which usually is correlated with immunosuppression (such as in our patient). Localized infections generally are observed in immunocompetent hosts.1
The exact pathogenetic mechanism of M chelonae infection in our patient is not clear. In patients with suppressed immunity, the variable clinical presentation of infection with NTM often impedes diagnosis. Cutaneous M chelonae lesions may be mistakenly diagnosed as Kaposi sarcoma or rarely as pyoderma gangrenosum. The differential diagnosis of subcutaneous nodules includes histoplasmosis, cryptococcosis, blastomycosis, coccidioidomycosis, nocardiosis, mycetoma, sporotrichosis, actinomycosis, and tuberculosis. In our patient, approximately 2 months elapsed between presentation of symptoms and definitive diagnosis, which was less than that reported in previously published cases.2,7-9
Histology and tissue culture followed by proper genetic analysis remains the gold standard for diagnosing NTM infection.10,11 In the interest of patients, time-consuming biochemical analyses should be replaced by molecular genetic diagnostic strip tests, which are fast, exact, and available in commercial kits for both common mycobacteria and additional species.12
Once the diagnosis of NTM infection has been established, sensitivity testing is mandatory to guide targeted therapy; however, clinicians should bear in mind that susceptibility testing does not guarantee clinical success, as correlations of susceptibility testing and clinical response have not been assessed.8 Standard antituberculous drugs (eg, isoniazid, rifampin, pyrazinamide) have no role in the treatment of M chelonae infection. The first-line antibiotics are clarithromycin, tobramycin, and linezolid, followed by imipenem, amikacin, clofazimine, doxycycline, and ciprofloxacin.10 Optimal outcomes have been reported in patients treated both with antibiotics and with surgical debridement. Although monotherapy with quinolones is not recommended for treatment of infection with NTM due to the high risk of mutational resistance, our patient received long-term antibiotic treatment with ciprofloxacin over a 6-month period and showed no recurrence at 24-month follow-up.
Conclusion
Clinicians who treat patients with chronic skin or soft tissue infections should consider infection with NTM in the differential diagnosis, particularly in patients with suppressed immunity, but also in immunocompetent patients following any invasive procedure. Detailed medical history and skin biopsy followed by histology and culture are recommended for the diagnosis. Infection with NTM requires rapid action. Sensitivity testing is necessary in choosing an effective treatment. New molecular genetic diagnostic strip tests can differentiate species of NTM sooner than biochemical analyses, thereby helping clinicians initiate appropriate antimicrobial treatment in a timely fashion.
1. Wallace RJ Jr, Brown BA, Onyi GO. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance of oral antimicrobials other than clarithromycin. J Infect Dis. 1992;166:405-412.
2. Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
3. Alexander S, John GT, Jesudason M, et al. Infections with atypical mycobacteria in renal transplant recipients. Indian J Pathol Microbiol. 2007;50:482-484.
4. Dorman S, Subramanian A; AST Infectious Diseases Community of Practice. Nontuberculous mycobacteria in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S63-S69.
5. Whitman WB, Goodfellow M, Kämpfer P, et al, eds. Bergey’s Manual of Systematic Bacteriology. 2nd ed. New York, NY: Springer-Verlag; 2012. The Actinobacteria; vol 5.
6. Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria [published online ahead of print September 5, 2001]. Clin Infect Dis. 2001;33:1363-1374.
7. Dodiuk-Gad R, Dyachenko P, Ziv M, et al. Nontuberculous mycobacterial infections of the skin: a retrospective study of 25 cases [published online ahead of print March 26, 2007]. J Am Acad Dermatol. 2007;57:413-420.
8. Regnier S, Cambau E, Meningaud JP, et al. Clinical management of rapidly growing mycobacterial cutaneous infections in patients after mesotherapy. Clin Infect Dis. 2009;49:1358-1364.
9. Somily AM, AL-Anazi AR, Babay HA, et al. Mycobacterium chelonae complex bacteremia from a post-renal transplant patient: case report and literature review. Jpn J Infect Dis. 2010;63:61-64.
10. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction in Am J Respir Crit Care Med. 2007;175:744-745]. Am J Respir Crit Care Med. 2007;175:367-416.
11. Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases [published online ahead of print September 6, 2010]. J Dermatol. 2010;37:965-972.
12. Lee AS, Jelfs P, Sintchenko V, et al. Identification of non-tuberculous mycobacteria: utility of the GenoType Mycobacterium CM/AS assay compared with HPLC and 16S rRNA gene sequencing [published online ahead of print June 5, 2009]. J of Med Microb. 2009;58(pt 7):900-904.
1. Wallace RJ Jr, Brown BA, Onyi GO. Skin, soft tissue, and bone infections due to Mycobacterium chelonae chelonae: importance of prior corticosteroid therapy, frequency of disseminated infections, and resistance of oral antimicrobials other than clarithromycin. J Infect Dis. 1992;166:405-412.
2. Uslan DZ, Kowalski TJ, Wengenack NL, et al. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142:1287-1292.
3. Alexander S, John GT, Jesudason M, et al. Infections with atypical mycobacteria in renal transplant recipients. Indian J Pathol Microbiol. 2007;50:482-484.
4. Dorman S, Subramanian A; AST Infectious Diseases Community of Practice. Nontuberculous mycobacteria in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S63-S69.
5. Whitman WB, Goodfellow M, Kämpfer P, et al, eds. Bergey’s Manual of Systematic Bacteriology. 2nd ed. New York, NY: Springer-Verlag; 2012. The Actinobacteria; vol 5.
6. Phillips MS, von Reyn CF. Nosocomial infections due to nontuberculous mycobacteria [published online ahead of print September 5, 2001]. Clin Infect Dis. 2001;33:1363-1374.
7. Dodiuk-Gad R, Dyachenko P, Ziv M, et al. Nontuberculous mycobacterial infections of the skin: a retrospective study of 25 cases [published online ahead of print March 26, 2007]. J Am Acad Dermatol. 2007;57:413-420.
8. Regnier S, Cambau E, Meningaud JP, et al. Clinical management of rapidly growing mycobacterial cutaneous infections in patients after mesotherapy. Clin Infect Dis. 2009;49:1358-1364.
9. Somily AM, AL-Anazi AR, Babay HA, et al. Mycobacterium chelonae complex bacteremia from a post-renal transplant patient: case report and literature review. Jpn J Infect Dis. 2010;63:61-64.
10. Griffith DE, Aksamit T, Brown-Elliott BA, et al; ATS Mycobacterial Diseases Subcommittee; American Thoracic Society; Infectious Diseases Society of America. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases [published correction in Am J Respir Crit Care Med. 2007;175:744-745]. Am J Respir Crit Care Med. 2007;175:367-416.
11. Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases [published online ahead of print September 6, 2010]. J Dermatol. 2010;37:965-972.
12. Lee AS, Jelfs P, Sintchenko V, et al. Identification of non-tuberculous mycobacteria: utility of the GenoType Mycobacterium CM/AS assay compared with HPLC and 16S rRNA gene sequencing [published online ahead of print June 5, 2009]. J of Med Microb. 2009;58(pt 7):900-904.
Practice Points
- Nontuberculous mycobacteria (NTM) are environmental saprophytes that can cause infection in immunosuppressed individuals as well as immunocompetent individuals with certain predisposing factors.
- It is important for clinicians to consider NTM in the differential diagnosis for patients who present with chronic skin or soft tissue infections.
- Histologic examination and culture of a biopsy specimen followed by polymerase chain reaction assay for genotyping of the specimen are recommended to determine the responsible Mycobacterium species.
- New molecular genetic strip tests can differentiate NTM species more quickly.
Acute Generalized Exanthematous Pustulosis Associated With Ranolazine
Acute generalized exanthematous pustulosis (AGEP) is a potentially widespread, pustular, cutaneous eruption. In 90% of cases, AGEP results from drug administration.1,2 It manifests as numerous subcorneal, nonfollicular, sterile pustules of rapid onset on an erythematous base,2 often in conjunction with fever, peripheral leukocytosis, and neutrophilia.3 Numerous drug therapies have been implicated in the etiology of AGEP, most commonly the β-lactam antibiotics, such as the penicillin derivatives and cephalosporins.2 Typically, AGEP occurs soon after drug ingestion and resolves spontaneously, shortly after the causative drug is discontinued.
Ranolazine is an antianginal, anti-ischemic medication with an undetermined mechanism of action. Its antianginal and anti-ischemic effects do not depend on reduced heart rate or blood pressure. At therapeutic levels, it inhibits the cardiac late sodium current (INa), reducing the sodium-induced calcium overload in ischemic cardiac myocytes. Severe adverse reactions include angioedema; paresthesia; pancytopenia; and, in animal studies, tumorigenicity.4 Herein we report a case of AGEP associated with the use of ranolazine.
Case Report
An 83-year-old man presented with a generalized rash of approximately 12 days’ duration. The patient reported that the small “pimple-like” bumps initially erupted on the back of the neck but gradually spread to the chest, back, and extremities. The lesions were asymptomatic at the outset and became pruritic over time. For the last several years, the patient had been taking tamsulosin for benign prostatic hypertrophy and rosuvastatin for hyperlipidemia. Twelve days prior to the exanthem, he had started taking ranolazine for symptomatic ischemia until coronary angiography could be performed. He reported having no associated fevers, chills, or malaise and had no personal history of psoriasis, though he had a maternal history of the disorder.
Examination revealed numerous nonfollicular-based pustules on diffuse erythematous patches (Figure 1). There was no mucosal involvement and the skin was negative for the Nikolsky sign. Spongiform intracorneal collections of neutrophils were visible on punch biopsy (Figures 2 and 3). Periodic acid–Schiff stains for fungi were negative.
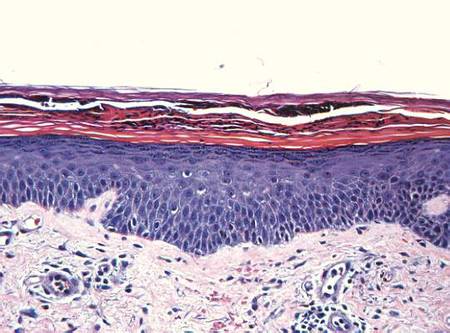
| 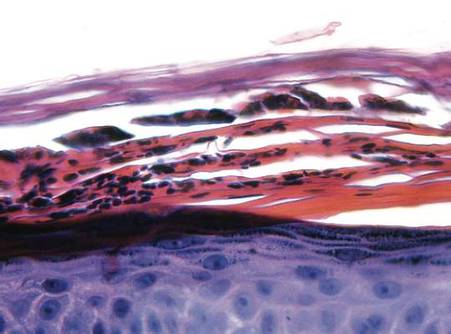
| |
Figure 2. A punch biopsy showed spongiform intracorneal collections of neutrophils (H&E, original magnification ×200). | Figure 3. A cornified layer of epidermis with neutrophils, as visible on punch biopsy (H&E, original magnification ×630). |
The patient’s primary care physician had initiated a course of oral prednisone 5 mg daily, 3 days before he presented to our outpatient dermatology clinic, but it had little effect on the rash. Upon dermatologic evaluation, we discontinued ranolazine therapy and prescribed the following tapered course of oral prednisone: 60 mg daily for 4 days; 40 mg daily for 3 days; 30 mg daily for 3 days; 20 mg daily for 3 days; 10 mg daily for 3 days; and 5 mg daily for 3 days). Within a week after this regimen was initiated, the rash showed improvement with eventual resolution and desquamation (Figure 4). Subsequently, the patient underwent successful angioplasty and multiple stent placement, which ultimately alleviated his angina.
Comment
Since its original description in 1968,5 AGEP has been misdiagnosed and underreported. Due to its rarity and clinical resemblance to more common pustular eruptions, such as exanthematous pustular psoriasis, the typical characteristics of AGEP were not clearly delineated until Beylot et al3 coined the term AGEP in 1980. Since that time, formalized criteria for the diagnosis and characterization of AGEP have been published.1,2,6-8
Numerous drug therapies have been implicated in the etiology of AGEP, most commonly antimicrobial agents, such as β-lactam antibiotics. Many other drugs, however, also have been identified as potential causative agents,8 including but not limited to antifungal, anticonvulsant, and antihypertensive agents. Other less common etiologies include viral infections,6,9-11 UV radiation, contrast media, heavy metal exposure (eg, to mercury), ingestion of urushiol (eg, in lacquered chicken), and spider bites.2,8,12-16 Nevertheless, more than 90% of AGEP cases are attributed to drug exposure, with 80% of drug-induced cases believed to be caused by antibiotics.1,8
The incidence of AGEP is estimated to be between 1 and 5 cases per million per year, using inclusion criteria from the EuroSCAR study, a multinational, case-controlled, pharmacoepidemiologic study of severe cutaneous adverse reactions.8,16 The condition seems to affect males and females equally.1,4 There are no reports of age or racial predilection.1,6,17 It has been suggested that those with AGEP may have some form of psoriatic background.1 Our patient had no personal history of inflammatory skin disease, although his mother had psoriasis.
The dermatitis presents as the sudden onset of a diffuse exanthematous eruption, which typically produces dozens to hundreds of sterile, nonfollicular, superficial pustules on an erythematous and possibly edematous base. Atypical presentations include target lesions, purpura, and vesicles. The reaction usually begins on the face or intertriginous areas of flexural surfaces and quickly disseminates. Patients may experience burning or pruritus. Acute generalized exanthematous pustulosis may involve mucous membranes but is usually limited to 1 location, most often the oral mucosa.1,8,16,18 Systemic signs and symptoms include fever, lymphadenopathy, pharyngitis, and hepatosplenomegaly. Unlike most drug allergies that demonstrate eosinophilia, AGEP is associated with leukocytosis and neutrophilic predominance. Only 25% of affected patients exhibit eosinophilia.1 Approximately 30% of patients in a retrospective analysis demonstrated abnormal renal function,2 and there have been reports of mildly elevated transaminases.8,19
In the EuroSCAR study, for reasons that were not apparent, symptoms developed within 24 hours of exposure to triggering antibiotics, whereas the median time to rash onset in response to non–anti-infective agents was 11 days.8 This finding is consistent with the delayed onset of symptoms experienced by our patient after initiating ranolazine therapy.
The differential diagnosis of AGEP primarily includes pustular psoriasis, subcorneal pustulosis, pustular folliculitis, DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, bullous impetigo, and occasionally erythema multiforme and toxic epidermal necrolysis, with the latter typically characterized by more mucous membrane involvement.20 Biopsy does not always support a definitive diagnosis; clinical correlation is often necessary. Because of the EuroSCAR study, Sidoroff et al8 devised a clinical validation score based on morphology (presence of pustules and erythema, distribution, and eventual desquamation), histopathology (presence of intraepidermal pustules, spongiosis, and papillary edema), and disease course (duration of symptoms, neutrophilia, fever, acute onset, and time to resolution). A definitive score is 8 to 12 (out of 12), and our patient’s score was 10; the score may have been higher had blood work been performed, but by the time the diagnosis was made the patient’s condition had improved enough to make laboratory workup unnecessary.
Several theories have been proposed to explain the pathophysiology of AGEP. Some hold that the causative agent induces the formation of antigen-antibody complexes, thereby activating the complement system, which in turn produces neutrophil chemotaxis.3,21 A more recent theory suggests that drug exposure causes drug-specific CD4 and CD8 cells to migrate into dermal and epidermal layers of the skin.17 Both T cells and keratinocytes express IL-8, which attracts polymorphonuclear leukocytes, causing them to accumulate in the dermis and then the epidermis. The different clinical presentations of AGEP may be attributed to other cytokines and interleukins that T cells express during this process. In the epidermis, CD8 cells kill keratinocytes, causing focal necrosis and prompting the formation of subcorneal vesicles filled primarily with CD4 cells. CD4 and CD8 cells are then localized to the dermis where neutrophils enter the vesicles, transforming them into sterile pustules.6,16,17
Acute generalized exanthematous pustulosis has been characterized as a type IV delayed hypersensitivity reaction, with affected patients often demonstrating positive patch testing or a history of prior sensitization to the perpetrating agent.18,19,21 Although there have been reports of positive patch testing for certain drugs, the unknown sensitivity and specificity of such testing as well as preparation-dependent variables may limit the diagnostic utility of this approach.21 The additional risk for inducing AGEP by patch testing the suspected drug also is a consideration. Due to our patient’s definitive clinical validation score, we did not perform this test.21
The AGEP eruption is typically self-limited and tends to resolve within 4 to 10 days after cessation of the triggering agent. Postpustular desquamation often occurs upon resolution of the primary lesions. Treatment usually involves discontinuation of the suspected causative agent and the use of antihistamines, antipyretics, topical corticosteroids, and emollients. Although there are reports of AGEP responsiveness to oral and intravenous steroids, such treatment rarely is required.8,16,22 We prescribed a tapered course of oral prednisone due to our patient’s imminent need for angioplasty.
Conclusion
This case of AGEP induced by ranolazine is notable. Given the potential widespread use of this antianginal medication and the severity of this potential adverse reaction, it is important for clinicians to recognize AGEP, discontinue ranolazine if determined to be a causative agent, and then initiate an appropriate alternative antianginal therapy.
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Sidoroff A, Halevy S, Bavnick JN, et al. Acute generalized exanthematous pustulosis (AGEP)–a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
3. Beylot C, Bioulac P, Doutre MS. Acute generalized exanthematic pustuloses (four cases) [in French]. Ann Dermatol Venereol. 1980;107:37-48.
4. Ranexa [package insert]. Foster City, CA: Gilead Sciences, Inc; December 2013.
5. Baker H, Ryan TJ. Generalized pustular psoriasis. a clinical and epidemiological study of 104 cases. Br J Dermatol. 1968;80:771-793.
6. Guevara-Gutierrez E, Uribe-Jimenez E, Diaz-Canchola M, et al. Acute generalized exanthematous pustulosis: report of 12 cases and literature review. Int J Dermatol. 2009;48:253-258.
7. Chang SL, Huang YH, Yang CH, et al. Clinical manifestations and characteristics of patients with acute generalized exanthematous pustulosis in Asia. Acta Derm Venereol. 2008;88:363-365.
8. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) [published online ahead of print September 13, 2007]. Br J Dermatol. 2007;157:989-996.
9. Rouchouse B, Bonnefoy M, Pallot B, et al. Acute generalized exanthematous pustular dermatitis and viral infection. Dermatologica. 1986;173:180-184.
10. Naides SJ, Piette W, Veach LA, et al. Human parvovirus B19-induced vesiculopustular skin eruption. Am J Med. 1988;84:968-972.
11. Feio AB, Apetato M, Costa MM, et al. Acute generalized exanthematous pustulosis due to Coxsackie B4 virus [in Portuguese]. Acta Med Port. 1997;10:487-491.
12. Goh TK, Pang SM, Thirumoorthy T, et al. Acute generalised exanthematous pustulosis and toxic epidermal necrolysis induced by carbamazepine. Singapore Med J. 2008;49:507-510.
13. Ofuji S, Yamamoto O. Acute generalized exanthematous pustulosis associated with a human parvovirus B19 infection. J Dermatol. 2007;34:121-123.
14. Davidovici BB, Pavel D, Cagnano E, et al. Acute generalized exanthematous pustulosis following a spider bite: report of 3 cases. J Am Acad Dermatol. 2006;55:525-529.
15. Park YM, Park JG, Kang H, et al. Acute generalized exanthematous pustulosis induced by ingestion of lacquer chicken. Br J Dermatol. 2000;143:230-232.
16. Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis: a case report. Arch Dermatol. 2009;145:683-687.17. Halevy S. Acute generalized exanthematous pustulosis. Curr Opin Allergy Clin Immunol. 2009;9:322-328.
18. Kim HJ, Jung KD, Lee KT, et al. Acute generalized exanthematous pustulosis caused by diltiazem [published online ahead of print February 28, 2011]. Ann Dermatol. 2011;23:108-110.
19. Speck LM, Wilkerson MG, Perri AJ, et al. Acute generalized exanthematous pustulosis caused by terazosin hydrochloride. J Drugs Dermatol. 2008;7:395-397.
20. Sidoroff A. Acute generalized exanthematous pustulosis (AGEP). UpToDate Web site. http://www.uptodate.com /contents/acute-generalized-exanthematous-pustulosis -agep?source=search_result&search=agep&selected Title=1~85. Updated March 18, 2015. Accessed October 6, 2015.
21. Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003;139:1181-1183.
22. Ibrahimi O, Gunawardane N, Sepehr A, et al. Terbinafine-induced acute generalized exanthematous pustulosis (AGEP) responsive to high dose intravenous corticosteroid. Dermatol Online J. 2009;15:8.
Acute generalized exanthematous pustulosis (AGEP) is a potentially widespread, pustular, cutaneous eruption. In 90% of cases, AGEP results from drug administration.1,2 It manifests as numerous subcorneal, nonfollicular, sterile pustules of rapid onset on an erythematous base,2 often in conjunction with fever, peripheral leukocytosis, and neutrophilia.3 Numerous drug therapies have been implicated in the etiology of AGEP, most commonly the β-lactam antibiotics, such as the penicillin derivatives and cephalosporins.2 Typically, AGEP occurs soon after drug ingestion and resolves spontaneously, shortly after the causative drug is discontinued.
Ranolazine is an antianginal, anti-ischemic medication with an undetermined mechanism of action. Its antianginal and anti-ischemic effects do not depend on reduced heart rate or blood pressure. At therapeutic levels, it inhibits the cardiac late sodium current (INa), reducing the sodium-induced calcium overload in ischemic cardiac myocytes. Severe adverse reactions include angioedema; paresthesia; pancytopenia; and, in animal studies, tumorigenicity.4 Herein we report a case of AGEP associated with the use of ranolazine.
Case Report
An 83-year-old man presented with a generalized rash of approximately 12 days’ duration. The patient reported that the small “pimple-like” bumps initially erupted on the back of the neck but gradually spread to the chest, back, and extremities. The lesions were asymptomatic at the outset and became pruritic over time. For the last several years, the patient had been taking tamsulosin for benign prostatic hypertrophy and rosuvastatin for hyperlipidemia. Twelve days prior to the exanthem, he had started taking ranolazine for symptomatic ischemia until coronary angiography could be performed. He reported having no associated fevers, chills, or malaise and had no personal history of psoriasis, though he had a maternal history of the disorder.
Examination revealed numerous nonfollicular-based pustules on diffuse erythematous patches (Figure 1). There was no mucosal involvement and the skin was negative for the Nikolsky sign. Spongiform intracorneal collections of neutrophils were visible on punch biopsy (Figures 2 and 3). Periodic acid–Schiff stains for fungi were negative.

| 
| |
Figure 2. A punch biopsy showed spongiform intracorneal collections of neutrophils (H&E, original magnification ×200). | Figure 3. A cornified layer of epidermis with neutrophils, as visible on punch biopsy (H&E, original magnification ×630). |
The patient’s primary care physician had initiated a course of oral prednisone 5 mg daily, 3 days before he presented to our outpatient dermatology clinic, but it had little effect on the rash. Upon dermatologic evaluation, we discontinued ranolazine therapy and prescribed the following tapered course of oral prednisone: 60 mg daily for 4 days; 40 mg daily for 3 days; 30 mg daily for 3 days; 20 mg daily for 3 days; 10 mg daily for 3 days; and 5 mg daily for 3 days). Within a week after this regimen was initiated, the rash showed improvement with eventual resolution and desquamation (Figure 4). Subsequently, the patient underwent successful angioplasty and multiple stent placement, which ultimately alleviated his angina.
Comment
Since its original description in 1968,5 AGEP has been misdiagnosed and underreported. Due to its rarity and clinical resemblance to more common pustular eruptions, such as exanthematous pustular psoriasis, the typical characteristics of AGEP were not clearly delineated until Beylot et al3 coined the term AGEP in 1980. Since that time, formalized criteria for the diagnosis and characterization of AGEP have been published.1,2,6-8
Numerous drug therapies have been implicated in the etiology of AGEP, most commonly antimicrobial agents, such as β-lactam antibiotics. Many other drugs, however, also have been identified as potential causative agents,8 including but not limited to antifungal, anticonvulsant, and antihypertensive agents. Other less common etiologies include viral infections,6,9-11 UV radiation, contrast media, heavy metal exposure (eg, to mercury), ingestion of urushiol (eg, in lacquered chicken), and spider bites.2,8,12-16 Nevertheless, more than 90% of AGEP cases are attributed to drug exposure, with 80% of drug-induced cases believed to be caused by antibiotics.1,8
The incidence of AGEP is estimated to be between 1 and 5 cases per million per year, using inclusion criteria from the EuroSCAR study, a multinational, case-controlled, pharmacoepidemiologic study of severe cutaneous adverse reactions.8,16 The condition seems to affect males and females equally.1,4 There are no reports of age or racial predilection.1,6,17 It has been suggested that those with AGEP may have some form of psoriatic background.1 Our patient had no personal history of inflammatory skin disease, although his mother had psoriasis.
The dermatitis presents as the sudden onset of a diffuse exanthematous eruption, which typically produces dozens to hundreds of sterile, nonfollicular, superficial pustules on an erythematous and possibly edematous base. Atypical presentations include target lesions, purpura, and vesicles. The reaction usually begins on the face or intertriginous areas of flexural surfaces and quickly disseminates. Patients may experience burning or pruritus. Acute generalized exanthematous pustulosis may involve mucous membranes but is usually limited to 1 location, most often the oral mucosa.1,8,16,18 Systemic signs and symptoms include fever, lymphadenopathy, pharyngitis, and hepatosplenomegaly. Unlike most drug allergies that demonstrate eosinophilia, AGEP is associated with leukocytosis and neutrophilic predominance. Only 25% of affected patients exhibit eosinophilia.1 Approximately 30% of patients in a retrospective analysis demonstrated abnormal renal function,2 and there have been reports of mildly elevated transaminases.8,19
In the EuroSCAR study, for reasons that were not apparent, symptoms developed within 24 hours of exposure to triggering antibiotics, whereas the median time to rash onset in response to non–anti-infective agents was 11 days.8 This finding is consistent with the delayed onset of symptoms experienced by our patient after initiating ranolazine therapy.
The differential diagnosis of AGEP primarily includes pustular psoriasis, subcorneal pustulosis, pustular folliculitis, DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, bullous impetigo, and occasionally erythema multiforme and toxic epidermal necrolysis, with the latter typically characterized by more mucous membrane involvement.20 Biopsy does not always support a definitive diagnosis; clinical correlation is often necessary. Because of the EuroSCAR study, Sidoroff et al8 devised a clinical validation score based on morphology (presence of pustules and erythema, distribution, and eventual desquamation), histopathology (presence of intraepidermal pustules, spongiosis, and papillary edema), and disease course (duration of symptoms, neutrophilia, fever, acute onset, and time to resolution). A definitive score is 8 to 12 (out of 12), and our patient’s score was 10; the score may have been higher had blood work been performed, but by the time the diagnosis was made the patient’s condition had improved enough to make laboratory workup unnecessary.
Several theories have been proposed to explain the pathophysiology of AGEP. Some hold that the causative agent induces the formation of antigen-antibody complexes, thereby activating the complement system, which in turn produces neutrophil chemotaxis.3,21 A more recent theory suggests that drug exposure causes drug-specific CD4 and CD8 cells to migrate into dermal and epidermal layers of the skin.17 Both T cells and keratinocytes express IL-8, which attracts polymorphonuclear leukocytes, causing them to accumulate in the dermis and then the epidermis. The different clinical presentations of AGEP may be attributed to other cytokines and interleukins that T cells express during this process. In the epidermis, CD8 cells kill keratinocytes, causing focal necrosis and prompting the formation of subcorneal vesicles filled primarily with CD4 cells. CD4 and CD8 cells are then localized to the dermis where neutrophils enter the vesicles, transforming them into sterile pustules.6,16,17
Acute generalized exanthematous pustulosis has been characterized as a type IV delayed hypersensitivity reaction, with affected patients often demonstrating positive patch testing or a history of prior sensitization to the perpetrating agent.18,19,21 Although there have been reports of positive patch testing for certain drugs, the unknown sensitivity and specificity of such testing as well as preparation-dependent variables may limit the diagnostic utility of this approach.21 The additional risk for inducing AGEP by patch testing the suspected drug also is a consideration. Due to our patient’s definitive clinical validation score, we did not perform this test.21
The AGEP eruption is typically self-limited and tends to resolve within 4 to 10 days after cessation of the triggering agent. Postpustular desquamation often occurs upon resolution of the primary lesions. Treatment usually involves discontinuation of the suspected causative agent and the use of antihistamines, antipyretics, topical corticosteroids, and emollients. Although there are reports of AGEP responsiveness to oral and intravenous steroids, such treatment rarely is required.8,16,22 We prescribed a tapered course of oral prednisone due to our patient’s imminent need for angioplasty.
Conclusion
This case of AGEP induced by ranolazine is notable. Given the potential widespread use of this antianginal medication and the severity of this potential adverse reaction, it is important for clinicians to recognize AGEP, discontinue ranolazine if determined to be a causative agent, and then initiate an appropriate alternative antianginal therapy.
Acute generalized exanthematous pustulosis (AGEP) is a potentially widespread, pustular, cutaneous eruption. In 90% of cases, AGEP results from drug administration.1,2 It manifests as numerous subcorneal, nonfollicular, sterile pustules of rapid onset on an erythematous base,2 often in conjunction with fever, peripheral leukocytosis, and neutrophilia.3 Numerous drug therapies have been implicated in the etiology of AGEP, most commonly the β-lactam antibiotics, such as the penicillin derivatives and cephalosporins.2 Typically, AGEP occurs soon after drug ingestion and resolves spontaneously, shortly after the causative drug is discontinued.
Ranolazine is an antianginal, anti-ischemic medication with an undetermined mechanism of action. Its antianginal and anti-ischemic effects do not depend on reduced heart rate or blood pressure. At therapeutic levels, it inhibits the cardiac late sodium current (INa), reducing the sodium-induced calcium overload in ischemic cardiac myocytes. Severe adverse reactions include angioedema; paresthesia; pancytopenia; and, in animal studies, tumorigenicity.4 Herein we report a case of AGEP associated with the use of ranolazine.
Case Report
An 83-year-old man presented with a generalized rash of approximately 12 days’ duration. The patient reported that the small “pimple-like” bumps initially erupted on the back of the neck but gradually spread to the chest, back, and extremities. The lesions were asymptomatic at the outset and became pruritic over time. For the last several years, the patient had been taking tamsulosin for benign prostatic hypertrophy and rosuvastatin for hyperlipidemia. Twelve days prior to the exanthem, he had started taking ranolazine for symptomatic ischemia until coronary angiography could be performed. He reported having no associated fevers, chills, or malaise and had no personal history of psoriasis, though he had a maternal history of the disorder.
Examination revealed numerous nonfollicular-based pustules on diffuse erythematous patches (Figure 1). There was no mucosal involvement and the skin was negative for the Nikolsky sign. Spongiform intracorneal collections of neutrophils were visible on punch biopsy (Figures 2 and 3). Periodic acid–Schiff stains for fungi were negative.

| 
| |
Figure 2. A punch biopsy showed spongiform intracorneal collections of neutrophils (H&E, original magnification ×200). | Figure 3. A cornified layer of epidermis with neutrophils, as visible on punch biopsy (H&E, original magnification ×630). |
The patient’s primary care physician had initiated a course of oral prednisone 5 mg daily, 3 days before he presented to our outpatient dermatology clinic, but it had little effect on the rash. Upon dermatologic evaluation, we discontinued ranolazine therapy and prescribed the following tapered course of oral prednisone: 60 mg daily for 4 days; 40 mg daily for 3 days; 30 mg daily for 3 days; 20 mg daily for 3 days; 10 mg daily for 3 days; and 5 mg daily for 3 days). Within a week after this regimen was initiated, the rash showed improvement with eventual resolution and desquamation (Figure 4). Subsequently, the patient underwent successful angioplasty and multiple stent placement, which ultimately alleviated his angina.
Comment
Since its original description in 1968,5 AGEP has been misdiagnosed and underreported. Due to its rarity and clinical resemblance to more common pustular eruptions, such as exanthematous pustular psoriasis, the typical characteristics of AGEP were not clearly delineated until Beylot et al3 coined the term AGEP in 1980. Since that time, formalized criteria for the diagnosis and characterization of AGEP have been published.1,2,6-8
Numerous drug therapies have been implicated in the etiology of AGEP, most commonly antimicrobial agents, such as β-lactam antibiotics. Many other drugs, however, also have been identified as potential causative agents,8 including but not limited to antifungal, anticonvulsant, and antihypertensive agents. Other less common etiologies include viral infections,6,9-11 UV radiation, contrast media, heavy metal exposure (eg, to mercury), ingestion of urushiol (eg, in lacquered chicken), and spider bites.2,8,12-16 Nevertheless, more than 90% of AGEP cases are attributed to drug exposure, with 80% of drug-induced cases believed to be caused by antibiotics.1,8
The incidence of AGEP is estimated to be between 1 and 5 cases per million per year, using inclusion criteria from the EuroSCAR study, a multinational, case-controlled, pharmacoepidemiologic study of severe cutaneous adverse reactions.8,16 The condition seems to affect males and females equally.1,4 There are no reports of age or racial predilection.1,6,17 It has been suggested that those with AGEP may have some form of psoriatic background.1 Our patient had no personal history of inflammatory skin disease, although his mother had psoriasis.
The dermatitis presents as the sudden onset of a diffuse exanthematous eruption, which typically produces dozens to hundreds of sterile, nonfollicular, superficial pustules on an erythematous and possibly edematous base. Atypical presentations include target lesions, purpura, and vesicles. The reaction usually begins on the face or intertriginous areas of flexural surfaces and quickly disseminates. Patients may experience burning or pruritus. Acute generalized exanthematous pustulosis may involve mucous membranes but is usually limited to 1 location, most often the oral mucosa.1,8,16,18 Systemic signs and symptoms include fever, lymphadenopathy, pharyngitis, and hepatosplenomegaly. Unlike most drug allergies that demonstrate eosinophilia, AGEP is associated with leukocytosis and neutrophilic predominance. Only 25% of affected patients exhibit eosinophilia.1 Approximately 30% of patients in a retrospective analysis demonstrated abnormal renal function,2 and there have been reports of mildly elevated transaminases.8,19
In the EuroSCAR study, for reasons that were not apparent, symptoms developed within 24 hours of exposure to triggering antibiotics, whereas the median time to rash onset in response to non–anti-infective agents was 11 days.8 This finding is consistent with the delayed onset of symptoms experienced by our patient after initiating ranolazine therapy.
The differential diagnosis of AGEP primarily includes pustular psoriasis, subcorneal pustulosis, pustular folliculitis, DRESS (drug reaction with eosinophilia and systemic symptoms) syndrome, bullous impetigo, and occasionally erythema multiforme and toxic epidermal necrolysis, with the latter typically characterized by more mucous membrane involvement.20 Biopsy does not always support a definitive diagnosis; clinical correlation is often necessary. Because of the EuroSCAR study, Sidoroff et al8 devised a clinical validation score based on morphology (presence of pustules and erythema, distribution, and eventual desquamation), histopathology (presence of intraepidermal pustules, spongiosis, and papillary edema), and disease course (duration of symptoms, neutrophilia, fever, acute onset, and time to resolution). A definitive score is 8 to 12 (out of 12), and our patient’s score was 10; the score may have been higher had blood work been performed, but by the time the diagnosis was made the patient’s condition had improved enough to make laboratory workup unnecessary.
Several theories have been proposed to explain the pathophysiology of AGEP. Some hold that the causative agent induces the formation of antigen-antibody complexes, thereby activating the complement system, which in turn produces neutrophil chemotaxis.3,21 A more recent theory suggests that drug exposure causes drug-specific CD4 and CD8 cells to migrate into dermal and epidermal layers of the skin.17 Both T cells and keratinocytes express IL-8, which attracts polymorphonuclear leukocytes, causing them to accumulate in the dermis and then the epidermis. The different clinical presentations of AGEP may be attributed to other cytokines and interleukins that T cells express during this process. In the epidermis, CD8 cells kill keratinocytes, causing focal necrosis and prompting the formation of subcorneal vesicles filled primarily with CD4 cells. CD4 and CD8 cells are then localized to the dermis where neutrophils enter the vesicles, transforming them into sterile pustules.6,16,17
Acute generalized exanthematous pustulosis has been characterized as a type IV delayed hypersensitivity reaction, with affected patients often demonstrating positive patch testing or a history of prior sensitization to the perpetrating agent.18,19,21 Although there have been reports of positive patch testing for certain drugs, the unknown sensitivity and specificity of such testing as well as preparation-dependent variables may limit the diagnostic utility of this approach.21 The additional risk for inducing AGEP by patch testing the suspected drug also is a consideration. Due to our patient’s definitive clinical validation score, we did not perform this test.21
The AGEP eruption is typically self-limited and tends to resolve within 4 to 10 days after cessation of the triggering agent. Postpustular desquamation often occurs upon resolution of the primary lesions. Treatment usually involves discontinuation of the suspected causative agent and the use of antihistamines, antipyretics, topical corticosteroids, and emollients. Although there are reports of AGEP responsiveness to oral and intravenous steroids, such treatment rarely is required.8,16,22 We prescribed a tapered course of oral prednisone due to our patient’s imminent need for angioplasty.
Conclusion
This case of AGEP induced by ranolazine is notable. Given the potential widespread use of this antianginal medication and the severity of this potential adverse reaction, it is important for clinicians to recognize AGEP, discontinue ranolazine if determined to be a causative agent, and then initiate an appropriate alternative antianginal therapy.
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Sidoroff A, Halevy S, Bavnick JN, et al. Acute generalized exanthematous pustulosis (AGEP)–a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
3. Beylot C, Bioulac P, Doutre MS. Acute generalized exanthematic pustuloses (four cases) [in French]. Ann Dermatol Venereol. 1980;107:37-48.
4. Ranexa [package insert]. Foster City, CA: Gilead Sciences, Inc; December 2013.
5. Baker H, Ryan TJ. Generalized pustular psoriasis. a clinical and epidemiological study of 104 cases. Br J Dermatol. 1968;80:771-793.
6. Guevara-Gutierrez E, Uribe-Jimenez E, Diaz-Canchola M, et al. Acute generalized exanthematous pustulosis: report of 12 cases and literature review. Int J Dermatol. 2009;48:253-258.
7. Chang SL, Huang YH, Yang CH, et al. Clinical manifestations and characteristics of patients with acute generalized exanthematous pustulosis in Asia. Acta Derm Venereol. 2008;88:363-365.
8. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) [published online ahead of print September 13, 2007]. Br J Dermatol. 2007;157:989-996.
9. Rouchouse B, Bonnefoy M, Pallot B, et al. Acute generalized exanthematous pustular dermatitis and viral infection. Dermatologica. 1986;173:180-184.
10. Naides SJ, Piette W, Veach LA, et al. Human parvovirus B19-induced vesiculopustular skin eruption. Am J Med. 1988;84:968-972.
11. Feio AB, Apetato M, Costa MM, et al. Acute generalized exanthematous pustulosis due to Coxsackie B4 virus [in Portuguese]. Acta Med Port. 1997;10:487-491.
12. Goh TK, Pang SM, Thirumoorthy T, et al. Acute generalised exanthematous pustulosis and toxic epidermal necrolysis induced by carbamazepine. Singapore Med J. 2008;49:507-510.
13. Ofuji S, Yamamoto O. Acute generalized exanthematous pustulosis associated with a human parvovirus B19 infection. J Dermatol. 2007;34:121-123.
14. Davidovici BB, Pavel D, Cagnano E, et al. Acute generalized exanthematous pustulosis following a spider bite: report of 3 cases. J Am Acad Dermatol. 2006;55:525-529.
15. Park YM, Park JG, Kang H, et al. Acute generalized exanthematous pustulosis induced by ingestion of lacquer chicken. Br J Dermatol. 2000;143:230-232.
16. Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis: a case report. Arch Dermatol. 2009;145:683-687.17. Halevy S. Acute generalized exanthematous pustulosis. Curr Opin Allergy Clin Immunol. 2009;9:322-328.
18. Kim HJ, Jung KD, Lee KT, et al. Acute generalized exanthematous pustulosis caused by diltiazem [published online ahead of print February 28, 2011]. Ann Dermatol. 2011;23:108-110.
19. Speck LM, Wilkerson MG, Perri AJ, et al. Acute generalized exanthematous pustulosis caused by terazosin hydrochloride. J Drugs Dermatol. 2008;7:395-397.
20. Sidoroff A. Acute generalized exanthematous pustulosis (AGEP). UpToDate Web site. http://www.uptodate.com /contents/acute-generalized-exanthematous-pustulosis -agep?source=search_result&search=agep&selected Title=1~85. Updated March 18, 2015. Accessed October 6, 2015.
21. Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003;139:1181-1183.
22. Ibrahimi O, Gunawardane N, Sepehr A, et al. Terbinafine-induced acute generalized exanthematous pustulosis (AGEP) responsive to high dose intravenous corticosteroid. Dermatol Online J. 2009;15:8.
1. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. analysis of 63 cases. Arch Dermatol. 1991;127:1333-1338.
2. Sidoroff A, Halevy S, Bavnick JN, et al. Acute generalized exanthematous pustulosis (AGEP)–a clinical reaction pattern. J Cutan Pathol. 2001;28:113-119.
3. Beylot C, Bioulac P, Doutre MS. Acute generalized exanthematic pustuloses (four cases) [in French]. Ann Dermatol Venereol. 1980;107:37-48.
4. Ranexa [package insert]. Foster City, CA: Gilead Sciences, Inc; December 2013.
5. Baker H, Ryan TJ. Generalized pustular psoriasis. a clinical and epidemiological study of 104 cases. Br J Dermatol. 1968;80:771-793.
6. Guevara-Gutierrez E, Uribe-Jimenez E, Diaz-Canchola M, et al. Acute generalized exanthematous pustulosis: report of 12 cases and literature review. Int J Dermatol. 2009;48:253-258.
7. Chang SL, Huang YH, Yang CH, et al. Clinical manifestations and characteristics of patients with acute generalized exanthematous pustulosis in Asia. Acta Derm Venereol. 2008;88:363-365.
8. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR) [published online ahead of print September 13, 2007]. Br J Dermatol. 2007;157:989-996.
9. Rouchouse B, Bonnefoy M, Pallot B, et al. Acute generalized exanthematous pustular dermatitis and viral infection. Dermatologica. 1986;173:180-184.
10. Naides SJ, Piette W, Veach LA, et al. Human parvovirus B19-induced vesiculopustular skin eruption. Am J Med. 1988;84:968-972.
11. Feio AB, Apetato M, Costa MM, et al. Acute generalized exanthematous pustulosis due to Coxsackie B4 virus [in Portuguese]. Acta Med Port. 1997;10:487-491.
12. Goh TK, Pang SM, Thirumoorthy T, et al. Acute generalised exanthematous pustulosis and toxic epidermal necrolysis induced by carbamazepine. Singapore Med J. 2008;49:507-510.
13. Ofuji S, Yamamoto O. Acute generalized exanthematous pustulosis associated with a human parvovirus B19 infection. J Dermatol. 2007;34:121-123.
14. Davidovici BB, Pavel D, Cagnano E, et al. Acute generalized exanthematous pustulosis following a spider bite: report of 3 cases. J Am Acad Dermatol. 2006;55:525-529.
15. Park YM, Park JG, Kang H, et al. Acute generalized exanthematous pustulosis induced by ingestion of lacquer chicken. Br J Dermatol. 2000;143:230-232.
16. Hammerbeck AA, Daniels NH, Callen JP. Ioversol-induced acute generalized exanthematous pustulosis: a case report. Arch Dermatol. 2009;145:683-687.17. Halevy S. Acute generalized exanthematous pustulosis. Curr Opin Allergy Clin Immunol. 2009;9:322-328.
18. Kim HJ, Jung KD, Lee KT, et al. Acute generalized exanthematous pustulosis caused by diltiazem [published online ahead of print February 28, 2011]. Ann Dermatol. 2011;23:108-110.
19. Speck LM, Wilkerson MG, Perri AJ, et al. Acute generalized exanthematous pustulosis caused by terazosin hydrochloride. J Drugs Dermatol. 2008;7:395-397.
20. Sidoroff A. Acute generalized exanthematous pustulosis (AGEP). UpToDate Web site. http://www.uptodate.com /contents/acute-generalized-exanthematous-pustulosis -agep?source=search_result&search=agep&selected Title=1~85. Updated March 18, 2015. Accessed October 6, 2015.
21. Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003;139:1181-1183.
22. Ibrahimi O, Gunawardane N, Sepehr A, et al. Terbinafine-induced acute generalized exanthematous pustulosis (AGEP) responsive to high dose intravenous corticosteroid. Dermatol Online J. 2009;15:8.
Practice Points
- Encountering an acute pustular reaction pattern should trigger the clinician to rule out acute generalized exanthematous pustulosis (AGEP).
- Ranolazine, a new antianginal therapy, has been associated with AGEP.
- Upon confirmation of AGEP, the patient’s recent medication history should be reviewed so the potential causative agent can be identified and withdrawn.
Tolerance of Fragranced and Fragrance-Free Facial Cleansers in Adults With Clinically Sensitive Skin
For thousands of years, humans have used fragrances to change or affect their mood and enhance an “aura of beauty.”1 Fragrance is a primary driver in consumer choice and purchasing decisions, especially when considering personal care products.2 In addition to fragrance, consumers choose cleanser products based on compatibility with skin, cleansing properties, and sensory attributes such as viscosity and foaming.3,4 However, fragrance sensitivity is among the most common causes of allergic contact dermatitis from cosmetics and personal care products,5 and estimates of the prevalence of fragrance sensitivity range from 1.8% to 4.2%.6
A panel of 26 fragrance ingredients that frequently induce contact dermatitis in sensitive individuals has been identified.7 Since 2003, regulatory authorities in the European Union require these compounds to be listed on the labels of consumer products to protect presensitized consumers.7,8 However, manufacturers of cosmetics are not required to specify allergenic fragrance ingredients outside the European Union, and therefore it is difficult for consumers in the United States to avoid fragrance allergens.
Creation of a fragranced product for fragrance-sensitive individuals begins with careful selection of ingredients and extensive formulation testing and evaluation. This process usually is followed by testing in normal individuals to confirm that the fragranced product is well accepted and then evaluation is done in clinically confirmed fragrance-sensitive patients and those with a compromised skin barrier from atopic dermatitis, rosacea, or eczema.
Sensitive skin may be due to increased immune responsiveness, altered neurosensory input, and/or decreased skin barrier function, and presents a complex challenge for dermatologists.9 Subjective perceptions of sensitive skin include stinging, burning, pruritus, and tightness following product application. Clinically sensitive skin is defined by the presence of erythema, stratum corneum desquamation, papules, pustules, wheals, vesicles, bullae, and/or erosions.9 Although some of these symptoms may be observed immediately, others may be delayed by minutes, hours, or days following the use of an irritating product. Patients who present with subjective symptoms of sensitive skin may or may not show objective symptoms.
Gentle skin cleansing is particularly important for patients with compromised skin barrier integrity, such as those with acne, atopic dermatitis, eczema, or rosacea. Standard alkaline surfactants in skin cleansers help to remove dirt and oily soil and produce lather but can impair the skin barrier function and facilitate development of irritation.10-13 The tolerability of a cleanser is influenced by its pH, the type and amount of surfactant ingredients, the presence of moisturizing agents, and the amount of residue left on the skin after washing.11,12 Mild cleansers have been developed for patients with sensitive skin conditions and are expected to provide cleansing benefits without negatively affecting the hydration and viscoelastic properties of skin.11 Mild cleansers interact minimally with skin proteins and lipids because they usually contain nonionic synthetic surfactant mixtures; they also have a pH value close to the slightly acidic pH of normal skin, contain moisturizing agents,11,14,15 and usually produce less foam.10,16 In patients with sensitive skin, mild and fragrance-free cleansers often are recommended.17,18 Because fragrances often affect consumers’ perception of product performance19 and enhance the cleaning experience of the user, consumer compliance with clinical recommendations to use fragrance-free cleansers often is poor.
Low–molecular-weight, water-soluble, hydrophobically modified polymers (HMPs) have been used to create gentle foaming cleansers with reduced impact on the skin barrier.12,16,20 In the presence of HMPs, surfactants assemble into larger, more stable polymer-surfactant structures that are less likely to penetrate the skin.16 Hydrophobically modified polymers can potentially reduce skin irritation by lowering the concentration of free micelles in solution. Additionally, both HMPs and HMP-surfactant complexes stabilize newly formed air-water interfaces, leading to thicker, denser, and longer-lasting foams.16 A gentle, fragrance-free, foaming liquid facial test cleanser with HMPs has been shown to be well tolerated in women with sensitive skin.20
This report describes 2 studies of a new mild, HMP-containing, foaming facial cleanser with a fragrance that was free of common allergens and irritating essential oils in patients with sensitive skin. Study 1 was designed to evaluate the tolerance and acceptability of 2 variations of the HMP-containing cleanser—one fragrance free and the other with fragrance—in a small sample of healthy adults with clinically diagnosed fragrance-sensitive skin. Study 2 was a large, 2-center study of the tolerability and effectiveness of the fragranced HMP-containing cleanser compared with a benchmark dermatologist-recommended, gentle, fragrance-free, nonfoaming cleanser in women with clinically diagnosed sensitive skin.
Methods
Study 1 Design
The primary objective of this prospective, randomized, single-center, crossover study was to evaluate the tolerability of fragranced versus fragrance-free formulations of a mild, HMP-containing liquid facial cleanser in healthy male and female adults with Fitzpatrick skin types I to IV who were clinically diagnosed as having fragrance sensitivity. Fragrance sensitivity was defined as a history of positive reactions to a fragrance mixture of 8 components (fragrance mixture I) and/or a fragrance mixture of 14 fragrances (fragrance mixture II) that included balsam of Peru (Myroxylonpereirae), geraniol, jasmine oil, and oakmoss.5 All participants provided written informed consent prior to enrolling in the study, and both the study protocol and informed consent agreement were approved by an institutional review board.
Participants were instructed to wash their face twice daily, noting the time of cleansing and providing commentary about their cleansing experience in a diary. The liquid facial test cleansers contained the HMP potassium acrylates copolymer, glycerin, and a surfactant system primarily containing cocamidopropyl betaine and lauryl glucoside prepared without added fragrance (as previously described20) or with a fragrance free of common allergens and irritating essential oils.
Half of the participants used the fragranced test cleanser and half used the fragrance-free test cleanser for a 3-week treatment period (weeks 1–3). Each treatment group subsequently switched to the other test cleanser for a second 3-week treatment period (weeks 4–6). Clinicians assessed global disease severity (an overall assessment of skin condition that was independent of other evaluation criteria), itching/burning, visible irritation, erythema, and desquamation at weekly time points throughout the study and graded each clinical tolerance attribute on a 5-point scale (0=none; 1=minimal; 2=mild; 3=moderate; 4=severe). Ordinal scores at baseline and at weeks 1 and 3 were used to calculate change from baseline.
A 7-item questionnaire also was administered to participants at each visit to assess skin condition, smoothness, softness, cleanliness, radiance, satisfaction with cleansing experience, and lathering. Each item was scored on a 5-point ordinal scale (0=none; 1=minimal; 2=good; 3=excellent; 4=superior). The scores for all parameters were statistically compared with baseline values using a paired t test with a significance level of P≤.05.
Study 2 Design
This prospective, 3-week, double-blind, randomized, comparative, 2-center study to evaluate the tolerability of the fragranced, HMP-containing test cleanser from study 1 versus a benchmark gentle, fragrance-free, nonfoaming cleanser in a large population of otherwise healthy females who had been clinically diagnosed with sensitive skin (not limited to fragrance sensitivity). The study sponsor provided blinded test materials, and neither the examiner nor the recorder knew which investigational product was administered to which participants. Additionally, personnel who dispensed the test cleansers to participants or supervised their use did not participate in the evaluation to minimize potential bias. All participants provided written informed consent prior to enrolling in the study, and the study protocol and informed consent agreement were approved by an institutional review board.
Participants included women aged 18 to 65 years with mild to moderate clinical symptoms of atopic dermatitis, eczema, acne, or rosacea within the 90 days prior to the study period. They were randomized into 2 balanced treatment groups: group 1 received the mild, fragranced, HMP-containing liquid facial cleanser from study 1 and group 2 received a leading, dermatologist-recommended, gentle, fragrance-free, nonfoaming cleanser. Each treatment group used the test cleansers at least once daily for 3 weeks.
Clinicians evaluated facial skin for softness and smoothness, global disease severity (rated visually by the investigator as an overall assessment of skin condition that was independent of other evaluation criteria [as previously described20]), itching, irritation, erythema, and desquamation at baseline and at weeks 1 and 3. The effectiveness of each product to remove facial dirt, cosmetics, and sebum also was assessed; clinical grading was performed as described for study 1 using the same grading scale as in study 1 and percentage change from baseline (improvement) was calculated.
The study also included a self-assessment of skin irritation in which participants responded yes or no to the following question: Have you experienced irritation using this product? Participants also completed a questionnaire in which they were asked to select the most appropriate answer—agree strongly, agree somewhat, neither, disagree somewhat, and disagree strongly— to the following statements: the cleanser leaves no residue; cleanses deep to remove dirt, oil, and makeup; the cleanser effectively removes makeup; the cleanser leaves my skin smooth; the cleanser leaves my skin soft; the cleanser rinses completely clean; cleanser does not over dry my skin; and my skin is completely clean.
The statistical analysis was performed using a nonparametric, 2-tailed, paired Mann-Whitney U test, and statistical significance was set at P≤.05.
Results
Study 1 Assessment
Eight female participants aged 22 to 60 years with clinically diagnosed fragrance sensitivity were enrolled in the study. After 3 weeks of use, clinician assessment showed that both the fragranced and fragrance-free test cleansers with HMPs improved several skin tolerance attributes, including global disease severity, irritation, and erythema (Figure 1). No notable differences in skin tolerance attributes were reported in the fragranced versus the fragrance-free formulations.
There were no reported differences in participant-reported cleanser effectiveness for the fragranced versus the fragrance-free cleanser either at baseline or weeks 1 or 3 (data not shown).
Study 2 Assessment
A total of 153 women aged 25 to 54 years with sensitive skin were enrolled in the study. Seventy-three participants were randomized to receive the fragranced test cleanser and 80 were randomized to receive the benchmark fragrance-free cleanser.
At week 3, there were no differences between the fragranced test cleanser and the benchmark cleanser in any of the clinician-assessed skin parameters (Figure 2). Of the parameters assessed, itching, irritation, and desquamation were the most improved from baseline in both treatment groups. Similar results were observed at week 1 (data not shown).
There were no apparent differences in subjective self-assessment of skin irritation between the test and benchmark cleansers at week 1 (15.7% vs 13.0%) or week 3 (24.3% vs 12.3%). When asked to respond to a series of 8 statements related to cleanser effectiveness, most participants either agreed strongly or agreed somewhat with the statements (Figure 3). There were no statistically significant differences between treatment groups, and responses to all statements indicated that participants were as satisfied with the test cleanser as they were with the benchmark cleanser.
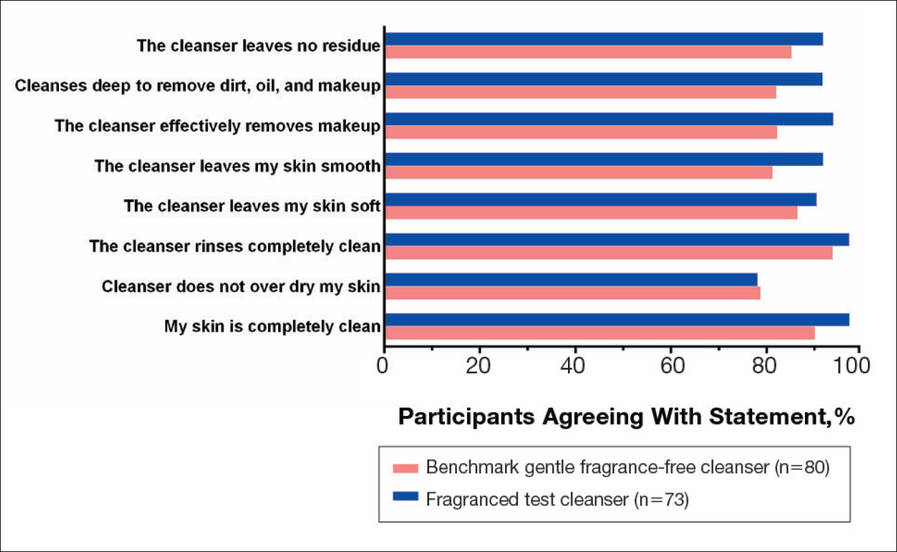
Comment
Consumers value cleansing, fragrance, viscosity, and foaming attributes in skin care products very highly.3,4,10 Fragrances are added to personal care products to positively affect consumers’ perception of product performance and to add emotional benefits by implying social or economic prestige to the use of a product.19 In one study, shampoo formulations that varied only in the added fragrance received different consumer evaluations for cleansing effectiveness and foaming.4
Although mild nonfoaming cleansers can be effective, adult consumers generally use cleansers that foam10,16 and often judge the performance of a cleansing product based on its foaming properties.3,10 Mild cleansers with HMPs maintain the ability to foam while also reducing the likelihood of skin irritation.16 One study showed that a mild, fragrance-free, foaming cleanser containing HMPs was as effective, well tolerated, and nonirritating in patients with sensitive skin as a benchmark nonfoaming gentle cleanser.20
Results from study 1 presented here show that fragranced and fragrance-free formulations of a mild, HMP-containing cleanser are equally efficacious and well tolerated in a small sample of participants with clinically diagnosed fragrance sensitivity. Skin tolerance attributes improved with both cleansers over a 3-week period, particularly global disease severity, irritation, and erythema. These results suggest that a fragrance free of common allergens and irritating essential oils could be introduced into a mild foaming cleanser containing HMPs without causing adverse reactions, even in patients who are fragrance sensitive.
Although the populations of studies 1 and 2 both included female participants with sensitive skin, they were not identical. While study 1 assessed a limited number of participants with clinically diagnosed fragrance sensitivity, study 2 was larger and included a broader range of participants with clinically diagnosed skin sensitivity, which could include fragrance sensitivity. The well-chosen fragrance of the test cleanser containing HMPs was well tolerated; however, this does not imply that any other fragrances added to this cleanser formulation would be as well tolerated.
Conclusion
The current studies indicate that a gentle fragranced foaming cleanser with HMPs was well tolerated in a small population of participants with clinically diagnosed fragrance sensitivity. In a larger population of female participants with sensitive skin, the gentle fragranced foaming cleanser with HMPs was as effective as a leading dermatologist-recommended, fragrance-free, gentle, nonfoaming cleanser. The gentle, HMP-containing, foaming cleanser with a fragrance that does not contain common allergens and irritating essential oils offers a new cleansing option for adults with sensitive skin who may prefer to use a fragranced and foaming product.
Acknowledgments—The authors are grateful to the patients and clinicians who participated in these studies. Editorial and medical writing support was provided by Tove Anderson, PhD, and Alex Loeb, PhD, both from Evidence Scientific Solutions, Inc, Philadelphia, Pennsylvania, and was funded by Johnson & Johnson Consumer Inc.
- Draelos ZD. To smell or not to smell? that is the question! J Cosmet Dermatol. 2013;12:1-2.
- Milotic D. The impact of fragrance on consumer choice. J Consumer Behaviour. 2003;3:179-191.
- Klein K. Evaluating shampoo foam. Cosmetics & Toiletries. 2004;119:32-36.
- Herman S. Skin care: the importance of feel. GCI Magazine. December 2007:70-74.
- Larsen WG. How to test for fragrance allergy. Cutis. 2000;65:39-41.
- Schnuch A, Uter W, Geier J, et al. Epidemiology of contact allergy: an estimation of morbidity employing the clinical epidemiology and drug-utilization research (CE-DUR) approach. Contact Dermatitis. 2002;47:32-39.
- Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products. Official Journal of the European Communities. 2003;L66:26-35.
- Guidance note: labelling of ingredients in Cosmetics Directive 76/768/EEC. European Commission Web site. http: //ec.europa.eu/consumers/sectors/cosmetics/files/doc/guide _labelling200802_en.pdf. Updated February 2008. Accessed September 2, 2015.
- Draelos ZD. Sensitive skin: perceptions, evaluation, and treatment. Am J Contact Dermatitis. 1997;8:67-78.
- Abbas S, Goldberg JW, Massaro M. Personal cleanser technology and clinical performance. Dermatol Ther. 2004;17(suppl 1):35-42.
- Ananthapadmanabhan KP, Moore DJ, Subramanyan K, et al. Cleansing without compromise: the impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol Ther. 2004;17(suppl 1):16-25.
- Walters RM, Mao G, Gunn ET, et al. Cleansing formulations that respect skin barrier integrity. Dermatol Res Pract. 2012;2012:495917.
- Saad P, Flach CR, Walters RM, et al. Infrared spectroscopic studies of sodium dodecyl sulphate permeation and interaction with stratum corneum lipids in skin. Int J Cosmet Sci. 2012;34:36-43.
- Bikowski J. The use of cleansers as therapeutic concomitants in various dermatologic disorders. Cutis. 2001;68(suppl 5):12-19.
- Walters RM, Fevola MJ, LiBrizzi JJ, et al. Designing cleansers for the unique needs of baby skin. Cosmetics & Toiletries. 2008;123:53-60.
- Fevola MJ, Walters RM, LiBrizzi JJ. A new approach to formulating mild cleansers: hydrophobically-modified polymers for irritation mitigation. In: Morgan SE, Lochhead RY, eds. Polymeric Delivery of Therapeutics. Vol 1053. Washington, DC: American Chemical Society; 2011:221-242.
- Nelson SA, Yiannias JA. Relevance and avoidance of skin-care product allergens: pearls and pitfalls. Dermatol Clin. 2009;27:329-336.
- Arribas MP, Soro P, Silvestre JF. Allergic contact dermatitis to fragrances: part 2. Actas Dermosifiliogr. 2013;104:29-37.
- Schroeder W. Understanding fragrance in personal care. Cosmetics & Toiletries. 2009;124:36-44.
- Draelos Z, Hornby S, Walters RM, et al. Hydrophobically-modified polymers can minimize skin irritation potential caused by surfactant-based cleansers. J Cosmet Dermatol. 2013;12:314-321.
For thousands of years, humans have used fragrances to change or affect their mood and enhance an “aura of beauty.”1 Fragrance is a primary driver in consumer choice and purchasing decisions, especially when considering personal care products.2 In addition to fragrance, consumers choose cleanser products based on compatibility with skin, cleansing properties, and sensory attributes such as viscosity and foaming.3,4 However, fragrance sensitivity is among the most common causes of allergic contact dermatitis from cosmetics and personal care products,5 and estimates of the prevalence of fragrance sensitivity range from 1.8% to 4.2%.6
A panel of 26 fragrance ingredients that frequently induce contact dermatitis in sensitive individuals has been identified.7 Since 2003, regulatory authorities in the European Union require these compounds to be listed on the labels of consumer products to protect presensitized consumers.7,8 However, manufacturers of cosmetics are not required to specify allergenic fragrance ingredients outside the European Union, and therefore it is difficult for consumers in the United States to avoid fragrance allergens.
Creation of a fragranced product for fragrance-sensitive individuals begins with careful selection of ingredients and extensive formulation testing and evaluation. This process usually is followed by testing in normal individuals to confirm that the fragranced product is well accepted and then evaluation is done in clinically confirmed fragrance-sensitive patients and those with a compromised skin barrier from atopic dermatitis, rosacea, or eczema.
Sensitive skin may be due to increased immune responsiveness, altered neurosensory input, and/or decreased skin barrier function, and presents a complex challenge for dermatologists.9 Subjective perceptions of sensitive skin include stinging, burning, pruritus, and tightness following product application. Clinically sensitive skin is defined by the presence of erythema, stratum corneum desquamation, papules, pustules, wheals, vesicles, bullae, and/or erosions.9 Although some of these symptoms may be observed immediately, others may be delayed by minutes, hours, or days following the use of an irritating product. Patients who present with subjective symptoms of sensitive skin may or may not show objective symptoms.
Gentle skin cleansing is particularly important for patients with compromised skin barrier integrity, such as those with acne, atopic dermatitis, eczema, or rosacea. Standard alkaline surfactants in skin cleansers help to remove dirt and oily soil and produce lather but can impair the skin barrier function and facilitate development of irritation.10-13 The tolerability of a cleanser is influenced by its pH, the type and amount of surfactant ingredients, the presence of moisturizing agents, and the amount of residue left on the skin after washing.11,12 Mild cleansers have been developed for patients with sensitive skin conditions and are expected to provide cleansing benefits without negatively affecting the hydration and viscoelastic properties of skin.11 Mild cleansers interact minimally with skin proteins and lipids because they usually contain nonionic synthetic surfactant mixtures; they also have a pH value close to the slightly acidic pH of normal skin, contain moisturizing agents,11,14,15 and usually produce less foam.10,16 In patients with sensitive skin, mild and fragrance-free cleansers often are recommended.17,18 Because fragrances often affect consumers’ perception of product performance19 and enhance the cleaning experience of the user, consumer compliance with clinical recommendations to use fragrance-free cleansers often is poor.
Low–molecular-weight, water-soluble, hydrophobically modified polymers (HMPs) have been used to create gentle foaming cleansers with reduced impact on the skin barrier.12,16,20 In the presence of HMPs, surfactants assemble into larger, more stable polymer-surfactant structures that are less likely to penetrate the skin.16 Hydrophobically modified polymers can potentially reduce skin irritation by lowering the concentration of free micelles in solution. Additionally, both HMPs and HMP-surfactant complexes stabilize newly formed air-water interfaces, leading to thicker, denser, and longer-lasting foams.16 A gentle, fragrance-free, foaming liquid facial test cleanser with HMPs has been shown to be well tolerated in women with sensitive skin.20
This report describes 2 studies of a new mild, HMP-containing, foaming facial cleanser with a fragrance that was free of common allergens and irritating essential oils in patients with sensitive skin. Study 1 was designed to evaluate the tolerance and acceptability of 2 variations of the HMP-containing cleanser—one fragrance free and the other with fragrance—in a small sample of healthy adults with clinically diagnosed fragrance-sensitive skin. Study 2 was a large, 2-center study of the tolerability and effectiveness of the fragranced HMP-containing cleanser compared with a benchmark dermatologist-recommended, gentle, fragrance-free, nonfoaming cleanser in women with clinically diagnosed sensitive skin.
Methods
Study 1 Design
The primary objective of this prospective, randomized, single-center, crossover study was to evaluate the tolerability of fragranced versus fragrance-free formulations of a mild, HMP-containing liquid facial cleanser in healthy male and female adults with Fitzpatrick skin types I to IV who were clinically diagnosed as having fragrance sensitivity. Fragrance sensitivity was defined as a history of positive reactions to a fragrance mixture of 8 components (fragrance mixture I) and/or a fragrance mixture of 14 fragrances (fragrance mixture II) that included balsam of Peru (Myroxylonpereirae), geraniol, jasmine oil, and oakmoss.5 All participants provided written informed consent prior to enrolling in the study, and both the study protocol and informed consent agreement were approved by an institutional review board.
Participants were instructed to wash their face twice daily, noting the time of cleansing and providing commentary about their cleansing experience in a diary. The liquid facial test cleansers contained the HMP potassium acrylates copolymer, glycerin, and a surfactant system primarily containing cocamidopropyl betaine and lauryl glucoside prepared without added fragrance (as previously described20) or with a fragrance free of common allergens and irritating essential oils.
Half of the participants used the fragranced test cleanser and half used the fragrance-free test cleanser for a 3-week treatment period (weeks 1–3). Each treatment group subsequently switched to the other test cleanser for a second 3-week treatment period (weeks 4–6). Clinicians assessed global disease severity (an overall assessment of skin condition that was independent of other evaluation criteria), itching/burning, visible irritation, erythema, and desquamation at weekly time points throughout the study and graded each clinical tolerance attribute on a 5-point scale (0=none; 1=minimal; 2=mild; 3=moderate; 4=severe). Ordinal scores at baseline and at weeks 1 and 3 were used to calculate change from baseline.
A 7-item questionnaire also was administered to participants at each visit to assess skin condition, smoothness, softness, cleanliness, radiance, satisfaction with cleansing experience, and lathering. Each item was scored on a 5-point ordinal scale (0=none; 1=minimal; 2=good; 3=excellent; 4=superior). The scores for all parameters were statistically compared with baseline values using a paired t test with a significance level of P≤.05.
Study 2 Design
This prospective, 3-week, double-blind, randomized, comparative, 2-center study to evaluate the tolerability of the fragranced, HMP-containing test cleanser from study 1 versus a benchmark gentle, fragrance-free, nonfoaming cleanser in a large population of otherwise healthy females who had been clinically diagnosed with sensitive skin (not limited to fragrance sensitivity). The study sponsor provided blinded test materials, and neither the examiner nor the recorder knew which investigational product was administered to which participants. Additionally, personnel who dispensed the test cleansers to participants or supervised their use did not participate in the evaluation to minimize potential bias. All participants provided written informed consent prior to enrolling in the study, and the study protocol and informed consent agreement were approved by an institutional review board.
Participants included women aged 18 to 65 years with mild to moderate clinical symptoms of atopic dermatitis, eczema, acne, or rosacea within the 90 days prior to the study period. They were randomized into 2 balanced treatment groups: group 1 received the mild, fragranced, HMP-containing liquid facial cleanser from study 1 and group 2 received a leading, dermatologist-recommended, gentle, fragrance-free, nonfoaming cleanser. Each treatment group used the test cleansers at least once daily for 3 weeks.
Clinicians evaluated facial skin for softness and smoothness, global disease severity (rated visually by the investigator as an overall assessment of skin condition that was independent of other evaluation criteria [as previously described20]), itching, irritation, erythema, and desquamation at baseline and at weeks 1 and 3. The effectiveness of each product to remove facial dirt, cosmetics, and sebum also was assessed; clinical grading was performed as described for study 1 using the same grading scale as in study 1 and percentage change from baseline (improvement) was calculated.
The study also included a self-assessment of skin irritation in which participants responded yes or no to the following question: Have you experienced irritation using this product? Participants also completed a questionnaire in which they were asked to select the most appropriate answer—agree strongly, agree somewhat, neither, disagree somewhat, and disagree strongly— to the following statements: the cleanser leaves no residue; cleanses deep to remove dirt, oil, and makeup; the cleanser effectively removes makeup; the cleanser leaves my skin smooth; the cleanser leaves my skin soft; the cleanser rinses completely clean; cleanser does not over dry my skin; and my skin is completely clean.
The statistical analysis was performed using a nonparametric, 2-tailed, paired Mann-Whitney U test, and statistical significance was set at P≤.05.
Results
Study 1 Assessment
Eight female participants aged 22 to 60 years with clinically diagnosed fragrance sensitivity were enrolled in the study. After 3 weeks of use, clinician assessment showed that both the fragranced and fragrance-free test cleansers with HMPs improved several skin tolerance attributes, including global disease severity, irritation, and erythema (Figure 1). No notable differences in skin tolerance attributes were reported in the fragranced versus the fragrance-free formulations.

There were no reported differences in participant-reported cleanser effectiveness for the fragranced versus the fragrance-free cleanser either at baseline or weeks 1 or 3 (data not shown).
Study 2 Assessment
A total of 153 women aged 25 to 54 years with sensitive skin were enrolled in the study. Seventy-three participants were randomized to receive the fragranced test cleanser and 80 were randomized to receive the benchmark fragrance-free cleanser.
At week 3, there were no differences between the fragranced test cleanser and the benchmark cleanser in any of the clinician-assessed skin parameters (Figure 2). Of the parameters assessed, itching, irritation, and desquamation were the most improved from baseline in both treatment groups. Similar results were observed at week 1 (data not shown).
There were no apparent differences in subjective self-assessment of skin irritation between the test and benchmark cleansers at week 1 (15.7% vs 13.0%) or week 3 (24.3% vs 12.3%). When asked to respond to a series of 8 statements related to cleanser effectiveness, most participants either agreed strongly or agreed somewhat with the statements (Figure 3). There were no statistically significant differences between treatment groups, and responses to all statements indicated that participants were as satisfied with the test cleanser as they were with the benchmark cleanser.

Comment
Consumers value cleansing, fragrance, viscosity, and foaming attributes in skin care products very highly.3,4,10 Fragrances are added to personal care products to positively affect consumers’ perception of product performance and to add emotional benefits by implying social or economic prestige to the use of a product.19 In one study, shampoo formulations that varied only in the added fragrance received different consumer evaluations for cleansing effectiveness and foaming.4
Although mild nonfoaming cleansers can be effective, adult consumers generally use cleansers that foam10,16 and often judge the performance of a cleansing product based on its foaming properties.3,10 Mild cleansers with HMPs maintain the ability to foam while also reducing the likelihood of skin irritation.16 One study showed that a mild, fragrance-free, foaming cleanser containing HMPs was as effective, well tolerated, and nonirritating in patients with sensitive skin as a benchmark nonfoaming gentle cleanser.20
Results from study 1 presented here show that fragranced and fragrance-free formulations of a mild, HMP-containing cleanser are equally efficacious and well tolerated in a small sample of participants with clinically diagnosed fragrance sensitivity. Skin tolerance attributes improved with both cleansers over a 3-week period, particularly global disease severity, irritation, and erythema. These results suggest that a fragrance free of common allergens and irritating essential oils could be introduced into a mild foaming cleanser containing HMPs without causing adverse reactions, even in patients who are fragrance sensitive.
Although the populations of studies 1 and 2 both included female participants with sensitive skin, they were not identical. While study 1 assessed a limited number of participants with clinically diagnosed fragrance sensitivity, study 2 was larger and included a broader range of participants with clinically diagnosed skin sensitivity, which could include fragrance sensitivity. The well-chosen fragrance of the test cleanser containing HMPs was well tolerated; however, this does not imply that any other fragrances added to this cleanser formulation would be as well tolerated.
Conclusion
The current studies indicate that a gentle fragranced foaming cleanser with HMPs was well tolerated in a small population of participants with clinically diagnosed fragrance sensitivity. In a larger population of female participants with sensitive skin, the gentle fragranced foaming cleanser with HMPs was as effective as a leading dermatologist-recommended, fragrance-free, gentle, nonfoaming cleanser. The gentle, HMP-containing, foaming cleanser with a fragrance that does not contain common allergens and irritating essential oils offers a new cleansing option for adults with sensitive skin who may prefer to use a fragranced and foaming product.
Acknowledgments—The authors are grateful to the patients and clinicians who participated in these studies. Editorial and medical writing support was provided by Tove Anderson, PhD, and Alex Loeb, PhD, both from Evidence Scientific Solutions, Inc, Philadelphia, Pennsylvania, and was funded by Johnson & Johnson Consumer Inc.
For thousands of years, humans have used fragrances to change or affect their mood and enhance an “aura of beauty.”1 Fragrance is a primary driver in consumer choice and purchasing decisions, especially when considering personal care products.2 In addition to fragrance, consumers choose cleanser products based on compatibility with skin, cleansing properties, and sensory attributes such as viscosity and foaming.3,4 However, fragrance sensitivity is among the most common causes of allergic contact dermatitis from cosmetics and personal care products,5 and estimates of the prevalence of fragrance sensitivity range from 1.8% to 4.2%.6
A panel of 26 fragrance ingredients that frequently induce contact dermatitis in sensitive individuals has been identified.7 Since 2003, regulatory authorities in the European Union require these compounds to be listed on the labels of consumer products to protect presensitized consumers.7,8 However, manufacturers of cosmetics are not required to specify allergenic fragrance ingredients outside the European Union, and therefore it is difficult for consumers in the United States to avoid fragrance allergens.
Creation of a fragranced product for fragrance-sensitive individuals begins with careful selection of ingredients and extensive formulation testing and evaluation. This process usually is followed by testing in normal individuals to confirm that the fragranced product is well accepted and then evaluation is done in clinically confirmed fragrance-sensitive patients and those with a compromised skin barrier from atopic dermatitis, rosacea, or eczema.
Sensitive skin may be due to increased immune responsiveness, altered neurosensory input, and/or decreased skin barrier function, and presents a complex challenge for dermatologists.9 Subjective perceptions of sensitive skin include stinging, burning, pruritus, and tightness following product application. Clinically sensitive skin is defined by the presence of erythema, stratum corneum desquamation, papules, pustules, wheals, vesicles, bullae, and/or erosions.9 Although some of these symptoms may be observed immediately, others may be delayed by minutes, hours, or days following the use of an irritating product. Patients who present with subjective symptoms of sensitive skin may or may not show objective symptoms.
Gentle skin cleansing is particularly important for patients with compromised skin barrier integrity, such as those with acne, atopic dermatitis, eczema, or rosacea. Standard alkaline surfactants in skin cleansers help to remove dirt and oily soil and produce lather but can impair the skin barrier function and facilitate development of irritation.10-13 The tolerability of a cleanser is influenced by its pH, the type and amount of surfactant ingredients, the presence of moisturizing agents, and the amount of residue left on the skin after washing.11,12 Mild cleansers have been developed for patients with sensitive skin conditions and are expected to provide cleansing benefits without negatively affecting the hydration and viscoelastic properties of skin.11 Mild cleansers interact minimally with skin proteins and lipids because they usually contain nonionic synthetic surfactant mixtures; they also have a pH value close to the slightly acidic pH of normal skin, contain moisturizing agents,11,14,15 and usually produce less foam.10,16 In patients with sensitive skin, mild and fragrance-free cleansers often are recommended.17,18 Because fragrances often affect consumers’ perception of product performance19 and enhance the cleaning experience of the user, consumer compliance with clinical recommendations to use fragrance-free cleansers often is poor.
Low–molecular-weight, water-soluble, hydrophobically modified polymers (HMPs) have been used to create gentle foaming cleansers with reduced impact on the skin barrier.12,16,20 In the presence of HMPs, surfactants assemble into larger, more stable polymer-surfactant structures that are less likely to penetrate the skin.16 Hydrophobically modified polymers can potentially reduce skin irritation by lowering the concentration of free micelles in solution. Additionally, both HMPs and HMP-surfactant complexes stabilize newly formed air-water interfaces, leading to thicker, denser, and longer-lasting foams.16 A gentle, fragrance-free, foaming liquid facial test cleanser with HMPs has been shown to be well tolerated in women with sensitive skin.20
This report describes 2 studies of a new mild, HMP-containing, foaming facial cleanser with a fragrance that was free of common allergens and irritating essential oils in patients with sensitive skin. Study 1 was designed to evaluate the tolerance and acceptability of 2 variations of the HMP-containing cleanser—one fragrance free and the other with fragrance—in a small sample of healthy adults with clinically diagnosed fragrance-sensitive skin. Study 2 was a large, 2-center study of the tolerability and effectiveness of the fragranced HMP-containing cleanser compared with a benchmark dermatologist-recommended, gentle, fragrance-free, nonfoaming cleanser in women with clinically diagnosed sensitive skin.
Methods
Study 1 Design
The primary objective of this prospective, randomized, single-center, crossover study was to evaluate the tolerability of fragranced versus fragrance-free formulations of a mild, HMP-containing liquid facial cleanser in healthy male and female adults with Fitzpatrick skin types I to IV who were clinically diagnosed as having fragrance sensitivity. Fragrance sensitivity was defined as a history of positive reactions to a fragrance mixture of 8 components (fragrance mixture I) and/or a fragrance mixture of 14 fragrances (fragrance mixture II) that included balsam of Peru (Myroxylonpereirae), geraniol, jasmine oil, and oakmoss.5 All participants provided written informed consent prior to enrolling in the study, and both the study protocol and informed consent agreement were approved by an institutional review board.
Participants were instructed to wash their face twice daily, noting the time of cleansing and providing commentary about their cleansing experience in a diary. The liquid facial test cleansers contained the HMP potassium acrylates copolymer, glycerin, and a surfactant system primarily containing cocamidopropyl betaine and lauryl glucoside prepared without added fragrance (as previously described20) or with a fragrance free of common allergens and irritating essential oils.
Half of the participants used the fragranced test cleanser and half used the fragrance-free test cleanser for a 3-week treatment period (weeks 1–3). Each treatment group subsequently switched to the other test cleanser for a second 3-week treatment period (weeks 4–6). Clinicians assessed global disease severity (an overall assessment of skin condition that was independent of other evaluation criteria), itching/burning, visible irritation, erythema, and desquamation at weekly time points throughout the study and graded each clinical tolerance attribute on a 5-point scale (0=none; 1=minimal; 2=mild; 3=moderate; 4=severe). Ordinal scores at baseline and at weeks 1 and 3 were used to calculate change from baseline.
A 7-item questionnaire also was administered to participants at each visit to assess skin condition, smoothness, softness, cleanliness, radiance, satisfaction with cleansing experience, and lathering. Each item was scored on a 5-point ordinal scale (0=none; 1=minimal; 2=good; 3=excellent; 4=superior). The scores for all parameters were statistically compared with baseline values using a paired t test with a significance level of P≤.05.
Study 2 Design
This prospective, 3-week, double-blind, randomized, comparative, 2-center study to evaluate the tolerability of the fragranced, HMP-containing test cleanser from study 1 versus a benchmark gentle, fragrance-free, nonfoaming cleanser in a large population of otherwise healthy females who had been clinically diagnosed with sensitive skin (not limited to fragrance sensitivity). The study sponsor provided blinded test materials, and neither the examiner nor the recorder knew which investigational product was administered to which participants. Additionally, personnel who dispensed the test cleansers to participants or supervised their use did not participate in the evaluation to minimize potential bias. All participants provided written informed consent prior to enrolling in the study, and the study protocol and informed consent agreement were approved by an institutional review board.
Participants included women aged 18 to 65 years with mild to moderate clinical symptoms of atopic dermatitis, eczema, acne, or rosacea within the 90 days prior to the study period. They were randomized into 2 balanced treatment groups: group 1 received the mild, fragranced, HMP-containing liquid facial cleanser from study 1 and group 2 received a leading, dermatologist-recommended, gentle, fragrance-free, nonfoaming cleanser. Each treatment group used the test cleansers at least once daily for 3 weeks.
Clinicians evaluated facial skin for softness and smoothness, global disease severity (rated visually by the investigator as an overall assessment of skin condition that was independent of other evaluation criteria [as previously described20]), itching, irritation, erythema, and desquamation at baseline and at weeks 1 and 3. The effectiveness of each product to remove facial dirt, cosmetics, and sebum also was assessed; clinical grading was performed as described for study 1 using the same grading scale as in study 1 and percentage change from baseline (improvement) was calculated.
The study also included a self-assessment of skin irritation in which participants responded yes or no to the following question: Have you experienced irritation using this product? Participants also completed a questionnaire in which they were asked to select the most appropriate answer—agree strongly, agree somewhat, neither, disagree somewhat, and disagree strongly— to the following statements: the cleanser leaves no residue; cleanses deep to remove dirt, oil, and makeup; the cleanser effectively removes makeup; the cleanser leaves my skin smooth; the cleanser leaves my skin soft; the cleanser rinses completely clean; cleanser does not over dry my skin; and my skin is completely clean.
The statistical analysis was performed using a nonparametric, 2-tailed, paired Mann-Whitney U test, and statistical significance was set at P≤.05.
Results
Study 1 Assessment
Eight female participants aged 22 to 60 years with clinically diagnosed fragrance sensitivity were enrolled in the study. After 3 weeks of use, clinician assessment showed that both the fragranced and fragrance-free test cleansers with HMPs improved several skin tolerance attributes, including global disease severity, irritation, and erythema (Figure 1). No notable differences in skin tolerance attributes were reported in the fragranced versus the fragrance-free formulations.

There were no reported differences in participant-reported cleanser effectiveness for the fragranced versus the fragrance-free cleanser either at baseline or weeks 1 or 3 (data not shown).
Study 2 Assessment
A total of 153 women aged 25 to 54 years with sensitive skin were enrolled in the study. Seventy-three participants were randomized to receive the fragranced test cleanser and 80 were randomized to receive the benchmark fragrance-free cleanser.
At week 3, there were no differences between the fragranced test cleanser and the benchmark cleanser in any of the clinician-assessed skin parameters (Figure 2). Of the parameters assessed, itching, irritation, and desquamation were the most improved from baseline in both treatment groups. Similar results were observed at week 1 (data not shown).
There were no apparent differences in subjective self-assessment of skin irritation between the test and benchmark cleansers at week 1 (15.7% vs 13.0%) or week 3 (24.3% vs 12.3%). When asked to respond to a series of 8 statements related to cleanser effectiveness, most participants either agreed strongly or agreed somewhat with the statements (Figure 3). There were no statistically significant differences between treatment groups, and responses to all statements indicated that participants were as satisfied with the test cleanser as they were with the benchmark cleanser.

Comment
Consumers value cleansing, fragrance, viscosity, and foaming attributes in skin care products very highly.3,4,10 Fragrances are added to personal care products to positively affect consumers’ perception of product performance and to add emotional benefits by implying social or economic prestige to the use of a product.19 In one study, shampoo formulations that varied only in the added fragrance received different consumer evaluations for cleansing effectiveness and foaming.4
Although mild nonfoaming cleansers can be effective, adult consumers generally use cleansers that foam10,16 and often judge the performance of a cleansing product based on its foaming properties.3,10 Mild cleansers with HMPs maintain the ability to foam while also reducing the likelihood of skin irritation.16 One study showed that a mild, fragrance-free, foaming cleanser containing HMPs was as effective, well tolerated, and nonirritating in patients with sensitive skin as a benchmark nonfoaming gentle cleanser.20
Results from study 1 presented here show that fragranced and fragrance-free formulations of a mild, HMP-containing cleanser are equally efficacious and well tolerated in a small sample of participants with clinically diagnosed fragrance sensitivity. Skin tolerance attributes improved with both cleansers over a 3-week period, particularly global disease severity, irritation, and erythema. These results suggest that a fragrance free of common allergens and irritating essential oils could be introduced into a mild foaming cleanser containing HMPs without causing adverse reactions, even in patients who are fragrance sensitive.
Although the populations of studies 1 and 2 both included female participants with sensitive skin, they were not identical. While study 1 assessed a limited number of participants with clinically diagnosed fragrance sensitivity, study 2 was larger and included a broader range of participants with clinically diagnosed skin sensitivity, which could include fragrance sensitivity. The well-chosen fragrance of the test cleanser containing HMPs was well tolerated; however, this does not imply that any other fragrances added to this cleanser formulation would be as well tolerated.
Conclusion
The current studies indicate that a gentle fragranced foaming cleanser with HMPs was well tolerated in a small population of participants with clinically diagnosed fragrance sensitivity. In a larger population of female participants with sensitive skin, the gentle fragranced foaming cleanser with HMPs was as effective as a leading dermatologist-recommended, fragrance-free, gentle, nonfoaming cleanser. The gentle, HMP-containing, foaming cleanser with a fragrance that does not contain common allergens and irritating essential oils offers a new cleansing option for adults with sensitive skin who may prefer to use a fragranced and foaming product.
Acknowledgments—The authors are grateful to the patients and clinicians who participated in these studies. Editorial and medical writing support was provided by Tove Anderson, PhD, and Alex Loeb, PhD, both from Evidence Scientific Solutions, Inc, Philadelphia, Pennsylvania, and was funded by Johnson & Johnson Consumer Inc.
- Draelos ZD. To smell or not to smell? that is the question! J Cosmet Dermatol. 2013;12:1-2.
- Milotic D. The impact of fragrance on consumer choice. J Consumer Behaviour. 2003;3:179-191.
- Klein K. Evaluating shampoo foam. Cosmetics & Toiletries. 2004;119:32-36.
- Herman S. Skin care: the importance of feel. GCI Magazine. December 2007:70-74.
- Larsen WG. How to test for fragrance allergy. Cutis. 2000;65:39-41.
- Schnuch A, Uter W, Geier J, et al. Epidemiology of contact allergy: an estimation of morbidity employing the clinical epidemiology and drug-utilization research (CE-DUR) approach. Contact Dermatitis. 2002;47:32-39.
- Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products. Official Journal of the European Communities. 2003;L66:26-35.
- Guidance note: labelling of ingredients in Cosmetics Directive 76/768/EEC. European Commission Web site. http: //ec.europa.eu/consumers/sectors/cosmetics/files/doc/guide _labelling200802_en.pdf. Updated February 2008. Accessed September 2, 2015.
- Draelos ZD. Sensitive skin: perceptions, evaluation, and treatment. Am J Contact Dermatitis. 1997;8:67-78.
- Abbas S, Goldberg JW, Massaro M. Personal cleanser technology and clinical performance. Dermatol Ther. 2004;17(suppl 1):35-42.
- Ananthapadmanabhan KP, Moore DJ, Subramanyan K, et al. Cleansing without compromise: the impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol Ther. 2004;17(suppl 1):16-25.
- Walters RM, Mao G, Gunn ET, et al. Cleansing formulations that respect skin barrier integrity. Dermatol Res Pract. 2012;2012:495917.
- Saad P, Flach CR, Walters RM, et al. Infrared spectroscopic studies of sodium dodecyl sulphate permeation and interaction with stratum corneum lipids in skin. Int J Cosmet Sci. 2012;34:36-43.
- Bikowski J. The use of cleansers as therapeutic concomitants in various dermatologic disorders. Cutis. 2001;68(suppl 5):12-19.
- Walters RM, Fevola MJ, LiBrizzi JJ, et al. Designing cleansers for the unique needs of baby skin. Cosmetics & Toiletries. 2008;123:53-60.
- Fevola MJ, Walters RM, LiBrizzi JJ. A new approach to formulating mild cleansers: hydrophobically-modified polymers for irritation mitigation. In: Morgan SE, Lochhead RY, eds. Polymeric Delivery of Therapeutics. Vol 1053. Washington, DC: American Chemical Society; 2011:221-242.
- Nelson SA, Yiannias JA. Relevance and avoidance of skin-care product allergens: pearls and pitfalls. Dermatol Clin. 2009;27:329-336.
- Arribas MP, Soro P, Silvestre JF. Allergic contact dermatitis to fragrances: part 2. Actas Dermosifiliogr. 2013;104:29-37.
- Schroeder W. Understanding fragrance in personal care. Cosmetics & Toiletries. 2009;124:36-44.
- Draelos Z, Hornby S, Walters RM, et al. Hydrophobically-modified polymers can minimize skin irritation potential caused by surfactant-based cleansers. J Cosmet Dermatol. 2013;12:314-321.
- Draelos ZD. To smell or not to smell? that is the question! J Cosmet Dermatol. 2013;12:1-2.
- Milotic D. The impact of fragrance on consumer choice. J Consumer Behaviour. 2003;3:179-191.
- Klein K. Evaluating shampoo foam. Cosmetics & Toiletries. 2004;119:32-36.
- Herman S. Skin care: the importance of feel. GCI Magazine. December 2007:70-74.
- Larsen WG. How to test for fragrance allergy. Cutis. 2000;65:39-41.
- Schnuch A, Uter W, Geier J, et al. Epidemiology of contact allergy: an estimation of morbidity employing the clinical epidemiology and drug-utilization research (CE-DUR) approach. Contact Dermatitis. 2002;47:32-39.
- Directive 2003/15/EC of the European Parliament and of the Council of 27 February 2003 amending Council Directive 76/768/EEC on the approximation of the laws of the Member States relating to cosmetic products. Official Journal of the European Communities. 2003;L66:26-35.
- Guidance note: labelling of ingredients in Cosmetics Directive 76/768/EEC. European Commission Web site. http: //ec.europa.eu/consumers/sectors/cosmetics/files/doc/guide _labelling200802_en.pdf. Updated February 2008. Accessed September 2, 2015.
- Draelos ZD. Sensitive skin: perceptions, evaluation, and treatment. Am J Contact Dermatitis. 1997;8:67-78.
- Abbas S, Goldberg JW, Massaro M. Personal cleanser technology and clinical performance. Dermatol Ther. 2004;17(suppl 1):35-42.
- Ananthapadmanabhan KP, Moore DJ, Subramanyan K, et al. Cleansing without compromise: the impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol Ther. 2004;17(suppl 1):16-25.
- Walters RM, Mao G, Gunn ET, et al. Cleansing formulations that respect skin barrier integrity. Dermatol Res Pract. 2012;2012:495917.
- Saad P, Flach CR, Walters RM, et al. Infrared spectroscopic studies of sodium dodecyl sulphate permeation and interaction with stratum corneum lipids in skin. Int J Cosmet Sci. 2012;34:36-43.
- Bikowski J. The use of cleansers as therapeutic concomitants in various dermatologic disorders. Cutis. 2001;68(suppl 5):12-19.
- Walters RM, Fevola MJ, LiBrizzi JJ, et al. Designing cleansers for the unique needs of baby skin. Cosmetics & Toiletries. 2008;123:53-60.
- Fevola MJ, Walters RM, LiBrizzi JJ. A new approach to formulating mild cleansers: hydrophobically-modified polymers for irritation mitigation. In: Morgan SE, Lochhead RY, eds. Polymeric Delivery of Therapeutics. Vol 1053. Washington, DC: American Chemical Society; 2011:221-242.
- Nelson SA, Yiannias JA. Relevance and avoidance of skin-care product allergens: pearls and pitfalls. Dermatol Clin. 2009;27:329-336.
- Arribas MP, Soro P, Silvestre JF. Allergic contact dermatitis to fragrances: part 2. Actas Dermosifiliogr. 2013;104:29-37.
- Schroeder W. Understanding fragrance in personal care. Cosmetics & Toiletries. 2009;124:36-44.
- Draelos Z, Hornby S, Walters RM, et al. Hydrophobically-modified polymers can minimize skin irritation potential caused by surfactant-based cleansers. J Cosmet Dermatol. 2013;12:314-321.
Practice Points
- Fragranced and fragrance-free versions of a gentle foaming cleanser with hydrophobically modified polymers (HMPs) were similarly well tolerated in participants with clinically diagnosed fragrance sensitivity.
- In a large population of female participants with sensitive skin, the fragranced gentle foaming cleanser with HMPs was as effective as a leading dermatologist-recommended, fragrance-free, gentle, nonfoaming cleanser.
- The gentle, HMP-containing, foaming cleanser with a fragrance offers a new cleansing option for adults with sensitive skin who may prefer to use a fragranced and foaming product.
What Is Your Diagnosis? Fixed Cutaneous Sporotrichosis
The Diagnosis: Fixed Cutaneous Sporotrichosis
On further questioning at our dermatology clinic, the patient reported having landed face-first into rocks and gravel during the all-terrain vehicle accident. After his medical history was noted and a physical examination was completed, bacterial and fungal cultures of the wound were taken. The fungal culture was positive for Sporothrix schenckii. The patient was prescribed itraconazole 200 mg 3 times daily for 3 days, then 200 mg twice daily for an additional 4 weeks after the lesions completely resolved. An ophthalmologist was immediately consulted to rule out sinus and periorbital involvement. After computed tomography revealed possible preseptal cellulitis with frontal sinus involvement, the patient was admitted and intravenous amphotericin B was administered. Following consultations with infectious disease specialists and radiologists, amphotericin B was discontinued and the patient was discharged on itraconazole 200 mg twice daily with close monitoring. At 3-month follow-up, the sporotrichosis infection had completely cleared (Figure).
Deep fungal infections comprise 2 distinct groups: systemic and subcutaneous mycoses. Individuals with subcutaneous mycoses present with skin involvement as the primary feature. Sporotrichosis is the most common cause of this type of mycosis1 and is caused by the dimorphic fungus S schenckii, an environmental saprophyte often residing in soil. Sporothrix schenckii exists as mold in a natural environment but exists as yeast in host tissue, thus causing ensuing infection.
Epidemiology
Sporotrichosis occurs worldwide but most frequently in temperate tropical and subtropical regions. The majority of cases are reported in Mexico and Central and South America1; however, cases have been seen in the southern United States, Japan, and Australia.2 In the United States, sporotrichosis is most commonly found in river valleys of the Midwest.
Sporothrix schenckii is most commonly isolated in hay, sphagnum moss, thorny plants, and soil, but it also has been described in other manifold host environments. Unusual origins of inoculation include an old and rust-stained camping tent in Mexico,3 crawl space joists of a house in Indiana,4 and hay bales used as props in a haunted house in Oklahoma.5
The incidence of infection is primarily sporadic; however, outbreaks among individuals who share a common environment favorable for the growth of S schenckii are at risk. Those identified to be at risk include rose gardeners, berry pickers, those who work in tree nurseries, horticulturists, landscapers, and miners.
Pathogenesis
As a dimorphic fungus, infection occurs when a conidium in the mold phase is introduced into the skin, usually by traumatic skin injury, and is converted to the yeast form in vivo. Distribution of infection by this organism is most commonly localized to the cutaneous, subcutaneous, and lymphocutaneous regions in healthy hosts but can involve visceral and osteoarticular structures in immunocompromised hosts.1,6 Pulmonary and disseminated forms are rare but can occur when S schenckii conidia are inhaled. Zoonotic transmission of the fungus also can occur with exposure to infected animals. Sporothrix schenckii has been reported to occur in cats, dogs, horses, donkeys, squirrels, armadillos, and dolphins.7-11
Pathology
Sporothrix schenckii is typically not visualized on microscopic examination due to the small number of microorganisms present; however, cultures grow rapidly (3–5 days) on Sabouraud agar. The fungus most commonly develops as white or off-white compact colonies that progressively darken with age, transitioning to gray and then black.1 Microscopically, the hyphae produce oval or pyriform conidia, which are assembled in a typical bouquetlike manner. Conversion of the organism to yeast on enriched medium such as brain-heart infusion agar or blood-cysteine-glucose agar confirms the diagnosis.
Acute lesions typically show a nonspecific mixed infiltrate, but established lesions may reveal granulomatous formation and neutrophilic microabscesses.1,2 Asteroid bodies, which are cigar-shaped yeasts surrounded by eosinophilic coronae radiata, may be found. Organisms are sparsely distributed within the lesions, necessitating a thorough examination of the culture for identification.
Clinical Features
Sporotrichosis has 3 main classifications: lymphocutaneous, fixed cutaneous, and disseminated. Lymphocutaneous sporotrichosis is the most common form of the infection.2 The disease presents with a small indurated papule occurring approximately 7 to 30 days after inoculation into the skin. The papule slowly enlarges, forms a nodule, and then frequently ulcerates. Over time, draining lymphatics become edematous and inflammatory, and a chain of secondary nodules begins to appear proximal to the initial lesion. The primary and secondary nodules may continue to ulcerate; alternately, they may heal or become chronic.
In fixed cutaneous sporotrichosis, the infection remains localized to one region and a granuloma may develop, which also may ulcerate. Satellite nodules may appear along the periphery of the lesion. Lymphatic spread is not observed in this form of the disease.
The disseminated form is a result of hematogenous spread from the primary inoculation site and typically occurs in an immunocompromised host. This form can present as pulmonary disease, sinusitis, and meningitis.1
Differential Diagnosis
The differential diagnosis for sporotrichosis includes atypical mycobacteria, nocardiosis, blastomycosis, pyogenic bacteria, leishmaniasis, tularemia, and tuberculosis.
Treatment
Treatment of sporotrichosis is always required. A saturated solution of potassium iodide has classically been used; however, it is frequently associated with side effects and can be problematic to administer.12 Given its low cost and traditional efficacy, it may still be used in some parts of the world.
Currently, the treatment of choice for fixed cutaneous and lymphocutaneous sporotrichosis is itraconazole 100 to 200 mg once daily for 3 to 6 months.1 The recommended treatment of osteoarticular sporotrichosis is itraconazole, but prolonged therapy is required.
Heat therapy is an alternative treatment option, as certain strains of S schenckii do not grow at temperatures higher than 35°C. Hot compresses must be used for at least 1 hour a day for several months, which may affect patient compliance.
Immunocompromised patients often have disseminated infection and require lifelong suppressive therapy with itraconazole and may require initial treatment with amphotericin B.13
Conclusion
Subcutaneous sporotrichosis can develop in patients with a traumatic injury involving vegetation, soil, or animals. Although some patients may develop more invasive disease, most infections in immunocompetent patients will resolve after 3 to 6 months of itraconazole 100 to 200 mg once daily.1
- De Araujo T, Marques AC, Kerdel F. Sporotrichosis. Int J Dermatol. 2001;40:737-742.
- Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 2. 6th ed. New York, NY: McGraw-Hill; 2003.
- Campos P, Arenas R, Coronado H. Epidemic cutaneous sporotrichosis. Int J Dermatol. 1994;33:38-41.
- Dillon GP, Lehmann PF, Talanin NY. Handyperson’s hazard: crawl space sporotrichosis. JAMA. 1995;274: 1673-1674.
- Dooley DP, Bostic PS, Beckius ML. Spook house sporotrichosis: a point-source outbreak of sporotrichosis associated with hay bale props in a Halloween haunted house. Arch Int Med. 1997;157:1885-1887.
- Kauffman CA. Sporotrichosis. Clin Infect Dis. 1999;29:231-236.
- Migaki G, Font RL, Kaplan W, et al. Sporotrichosis in a Pacific white-sided dolphin (Lagenorhynchus obliquidens). Am J Vet Res. 1978;39:1916-1919.
- Crothers SL, White SD, Ihrke PJ, et al. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007). Vet Dermatol. 2009;20:249-259.
- Saravanakumar PS, Eslami P, Zar FA. Lymphocutaneous sporotrichosis associated with a squirrel bite: case reports and review. Clin Infect Dis. 1996;23:647-648.
- Wenker CJ, Kaufman L, Bacciarini LN, et al. Sporotrichosis in a nine-banded armadillo (Dasypus novemcinctus). J Zoo Wildl Med. 1998;29:474-478.
- Barros MB, Schubach Ade O, do Valle AC, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529-535.
- Kauffman CA. Old and new therapies for sporotrichosis. Clin Infect Dis. 1995;21:981-985.
- Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the managements of patients with sporotrichosis. Clin Infect Dis. 2000;30:684-687.
The Diagnosis: Fixed Cutaneous Sporotrichosis
On further questioning at our dermatology clinic, the patient reported having landed face-first into rocks and gravel during the all-terrain vehicle accident. After his medical history was noted and a physical examination was completed, bacterial and fungal cultures of the wound were taken. The fungal culture was positive for Sporothrix schenckii. The patient was prescribed itraconazole 200 mg 3 times daily for 3 days, then 200 mg twice daily for an additional 4 weeks after the lesions completely resolved. An ophthalmologist was immediately consulted to rule out sinus and periorbital involvement. After computed tomography revealed possible preseptal cellulitis with frontal sinus involvement, the patient was admitted and intravenous amphotericin B was administered. Following consultations with infectious disease specialists and radiologists, amphotericin B was discontinued and the patient was discharged on itraconazole 200 mg twice daily with close monitoring. At 3-month follow-up, the sporotrichosis infection had completely cleared (Figure).
Deep fungal infections comprise 2 distinct groups: systemic and subcutaneous mycoses. Individuals with subcutaneous mycoses present with skin involvement as the primary feature. Sporotrichosis is the most common cause of this type of mycosis1 and is caused by the dimorphic fungus S schenckii, an environmental saprophyte often residing in soil. Sporothrix schenckii exists as mold in a natural environment but exists as yeast in host tissue, thus causing ensuing infection.
Epidemiology
Sporotrichosis occurs worldwide but most frequently in temperate tropical and subtropical regions. The majority of cases are reported in Mexico and Central and South America1; however, cases have been seen in the southern United States, Japan, and Australia.2 In the United States, sporotrichosis is most commonly found in river valleys of the Midwest.
Sporothrix schenckii is most commonly isolated in hay, sphagnum moss, thorny plants, and soil, but it also has been described in other manifold host environments. Unusual origins of inoculation include an old and rust-stained camping tent in Mexico,3 crawl space joists of a house in Indiana,4 and hay bales used as props in a haunted house in Oklahoma.5
The incidence of infection is primarily sporadic; however, outbreaks among individuals who share a common environment favorable for the growth of S schenckii are at risk. Those identified to be at risk include rose gardeners, berry pickers, those who work in tree nurseries, horticulturists, landscapers, and miners.
Pathogenesis
As a dimorphic fungus, infection occurs when a conidium in the mold phase is introduced into the skin, usually by traumatic skin injury, and is converted to the yeast form in vivo. Distribution of infection by this organism is most commonly localized to the cutaneous, subcutaneous, and lymphocutaneous regions in healthy hosts but can involve visceral and osteoarticular structures in immunocompromised hosts.1,6 Pulmonary and disseminated forms are rare but can occur when S schenckii conidia are inhaled. Zoonotic transmission of the fungus also can occur with exposure to infected animals. Sporothrix schenckii has been reported to occur in cats, dogs, horses, donkeys, squirrels, armadillos, and dolphins.7-11
Pathology
Sporothrix schenckii is typically not visualized on microscopic examination due to the small number of microorganisms present; however, cultures grow rapidly (3–5 days) on Sabouraud agar. The fungus most commonly develops as white or off-white compact colonies that progressively darken with age, transitioning to gray and then black.1 Microscopically, the hyphae produce oval or pyriform conidia, which are assembled in a typical bouquetlike manner. Conversion of the organism to yeast on enriched medium such as brain-heart infusion agar or blood-cysteine-glucose agar confirms the diagnosis.
Acute lesions typically show a nonspecific mixed infiltrate, but established lesions may reveal granulomatous formation and neutrophilic microabscesses.1,2 Asteroid bodies, which are cigar-shaped yeasts surrounded by eosinophilic coronae radiata, may be found. Organisms are sparsely distributed within the lesions, necessitating a thorough examination of the culture for identification.
Clinical Features
Sporotrichosis has 3 main classifications: lymphocutaneous, fixed cutaneous, and disseminated. Lymphocutaneous sporotrichosis is the most common form of the infection.2 The disease presents with a small indurated papule occurring approximately 7 to 30 days after inoculation into the skin. The papule slowly enlarges, forms a nodule, and then frequently ulcerates. Over time, draining lymphatics become edematous and inflammatory, and a chain of secondary nodules begins to appear proximal to the initial lesion. The primary and secondary nodules may continue to ulcerate; alternately, they may heal or become chronic.
In fixed cutaneous sporotrichosis, the infection remains localized to one region and a granuloma may develop, which also may ulcerate. Satellite nodules may appear along the periphery of the lesion. Lymphatic spread is not observed in this form of the disease.
The disseminated form is a result of hematogenous spread from the primary inoculation site and typically occurs in an immunocompromised host. This form can present as pulmonary disease, sinusitis, and meningitis.1
Differential Diagnosis
The differential diagnosis for sporotrichosis includes atypical mycobacteria, nocardiosis, blastomycosis, pyogenic bacteria, leishmaniasis, tularemia, and tuberculosis.
Treatment
Treatment of sporotrichosis is always required. A saturated solution of potassium iodide has classically been used; however, it is frequently associated with side effects and can be problematic to administer.12 Given its low cost and traditional efficacy, it may still be used in some parts of the world.
Currently, the treatment of choice for fixed cutaneous and lymphocutaneous sporotrichosis is itraconazole 100 to 200 mg once daily for 3 to 6 months.1 The recommended treatment of osteoarticular sporotrichosis is itraconazole, but prolonged therapy is required.
Heat therapy is an alternative treatment option, as certain strains of S schenckii do not grow at temperatures higher than 35°C. Hot compresses must be used for at least 1 hour a day for several months, which may affect patient compliance.
Immunocompromised patients often have disseminated infection and require lifelong suppressive therapy with itraconazole and may require initial treatment with amphotericin B.13
Conclusion
Subcutaneous sporotrichosis can develop in patients with a traumatic injury involving vegetation, soil, or animals. Although some patients may develop more invasive disease, most infections in immunocompetent patients will resolve after 3 to 6 months of itraconazole 100 to 200 mg once daily.1
The Diagnosis: Fixed Cutaneous Sporotrichosis
On further questioning at our dermatology clinic, the patient reported having landed face-first into rocks and gravel during the all-terrain vehicle accident. After his medical history was noted and a physical examination was completed, bacterial and fungal cultures of the wound were taken. The fungal culture was positive for Sporothrix schenckii. The patient was prescribed itraconazole 200 mg 3 times daily for 3 days, then 200 mg twice daily for an additional 4 weeks after the lesions completely resolved. An ophthalmologist was immediately consulted to rule out sinus and periorbital involvement. After computed tomography revealed possible preseptal cellulitis with frontal sinus involvement, the patient was admitted and intravenous amphotericin B was administered. Following consultations with infectious disease specialists and radiologists, amphotericin B was discontinued and the patient was discharged on itraconazole 200 mg twice daily with close monitoring. At 3-month follow-up, the sporotrichosis infection had completely cleared (Figure).
Deep fungal infections comprise 2 distinct groups: systemic and subcutaneous mycoses. Individuals with subcutaneous mycoses present with skin involvement as the primary feature. Sporotrichosis is the most common cause of this type of mycosis1 and is caused by the dimorphic fungus S schenckii, an environmental saprophyte often residing in soil. Sporothrix schenckii exists as mold in a natural environment but exists as yeast in host tissue, thus causing ensuing infection.
Epidemiology
Sporotrichosis occurs worldwide but most frequently in temperate tropical and subtropical regions. The majority of cases are reported in Mexico and Central and South America1; however, cases have been seen in the southern United States, Japan, and Australia.2 In the United States, sporotrichosis is most commonly found in river valleys of the Midwest.
Sporothrix schenckii is most commonly isolated in hay, sphagnum moss, thorny plants, and soil, but it also has been described in other manifold host environments. Unusual origins of inoculation include an old and rust-stained camping tent in Mexico,3 crawl space joists of a house in Indiana,4 and hay bales used as props in a haunted house in Oklahoma.5
The incidence of infection is primarily sporadic; however, outbreaks among individuals who share a common environment favorable for the growth of S schenckii are at risk. Those identified to be at risk include rose gardeners, berry pickers, those who work in tree nurseries, horticulturists, landscapers, and miners.
Pathogenesis
As a dimorphic fungus, infection occurs when a conidium in the mold phase is introduced into the skin, usually by traumatic skin injury, and is converted to the yeast form in vivo. Distribution of infection by this organism is most commonly localized to the cutaneous, subcutaneous, and lymphocutaneous regions in healthy hosts but can involve visceral and osteoarticular structures in immunocompromised hosts.1,6 Pulmonary and disseminated forms are rare but can occur when S schenckii conidia are inhaled. Zoonotic transmission of the fungus also can occur with exposure to infected animals. Sporothrix schenckii has been reported to occur in cats, dogs, horses, donkeys, squirrels, armadillos, and dolphins.7-11
Pathology
Sporothrix schenckii is typically not visualized on microscopic examination due to the small number of microorganisms present; however, cultures grow rapidly (3–5 days) on Sabouraud agar. The fungus most commonly develops as white or off-white compact colonies that progressively darken with age, transitioning to gray and then black.1 Microscopically, the hyphae produce oval or pyriform conidia, which are assembled in a typical bouquetlike manner. Conversion of the organism to yeast on enriched medium such as brain-heart infusion agar or blood-cysteine-glucose agar confirms the diagnosis.
Acute lesions typically show a nonspecific mixed infiltrate, but established lesions may reveal granulomatous formation and neutrophilic microabscesses.1,2 Asteroid bodies, which are cigar-shaped yeasts surrounded by eosinophilic coronae radiata, may be found. Organisms are sparsely distributed within the lesions, necessitating a thorough examination of the culture for identification.
Clinical Features
Sporotrichosis has 3 main classifications: lymphocutaneous, fixed cutaneous, and disseminated. Lymphocutaneous sporotrichosis is the most common form of the infection.2 The disease presents with a small indurated papule occurring approximately 7 to 30 days after inoculation into the skin. The papule slowly enlarges, forms a nodule, and then frequently ulcerates. Over time, draining lymphatics become edematous and inflammatory, and a chain of secondary nodules begins to appear proximal to the initial lesion. The primary and secondary nodules may continue to ulcerate; alternately, they may heal or become chronic.
In fixed cutaneous sporotrichosis, the infection remains localized to one region and a granuloma may develop, which also may ulcerate. Satellite nodules may appear along the periphery of the lesion. Lymphatic spread is not observed in this form of the disease.
The disseminated form is a result of hematogenous spread from the primary inoculation site and typically occurs in an immunocompromised host. This form can present as pulmonary disease, sinusitis, and meningitis.1
Differential Diagnosis
The differential diagnosis for sporotrichosis includes atypical mycobacteria, nocardiosis, blastomycosis, pyogenic bacteria, leishmaniasis, tularemia, and tuberculosis.
Treatment
Treatment of sporotrichosis is always required. A saturated solution of potassium iodide has classically been used; however, it is frequently associated with side effects and can be problematic to administer.12 Given its low cost and traditional efficacy, it may still be used in some parts of the world.
Currently, the treatment of choice for fixed cutaneous and lymphocutaneous sporotrichosis is itraconazole 100 to 200 mg once daily for 3 to 6 months.1 The recommended treatment of osteoarticular sporotrichosis is itraconazole, but prolonged therapy is required.
Heat therapy is an alternative treatment option, as certain strains of S schenckii do not grow at temperatures higher than 35°C. Hot compresses must be used for at least 1 hour a day for several months, which may affect patient compliance.
Immunocompromised patients often have disseminated infection and require lifelong suppressive therapy with itraconazole and may require initial treatment with amphotericin B.13
Conclusion
Subcutaneous sporotrichosis can develop in patients with a traumatic injury involving vegetation, soil, or animals. Although some patients may develop more invasive disease, most infections in immunocompetent patients will resolve after 3 to 6 months of itraconazole 100 to 200 mg once daily.1
- De Araujo T, Marques AC, Kerdel F. Sporotrichosis. Int J Dermatol. 2001;40:737-742.
- Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 2. 6th ed. New York, NY: McGraw-Hill; 2003.
- Campos P, Arenas R, Coronado H. Epidemic cutaneous sporotrichosis. Int J Dermatol. 1994;33:38-41.
- Dillon GP, Lehmann PF, Talanin NY. Handyperson’s hazard: crawl space sporotrichosis. JAMA. 1995;274: 1673-1674.
- Dooley DP, Bostic PS, Beckius ML. Spook house sporotrichosis: a point-source outbreak of sporotrichosis associated with hay bale props in a Halloween haunted house. Arch Int Med. 1997;157:1885-1887.
- Kauffman CA. Sporotrichosis. Clin Infect Dis. 1999;29:231-236.
- Migaki G, Font RL, Kaplan W, et al. Sporotrichosis in a Pacific white-sided dolphin (Lagenorhynchus obliquidens). Am J Vet Res. 1978;39:1916-1919.
- Crothers SL, White SD, Ihrke PJ, et al. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007). Vet Dermatol. 2009;20:249-259.
- Saravanakumar PS, Eslami P, Zar FA. Lymphocutaneous sporotrichosis associated with a squirrel bite: case reports and review. Clin Infect Dis. 1996;23:647-648.
- Wenker CJ, Kaufman L, Bacciarini LN, et al. Sporotrichosis in a nine-banded armadillo (Dasypus novemcinctus). J Zoo Wildl Med. 1998;29:474-478.
- Barros MB, Schubach Ade O, do Valle AC, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529-535.
- Kauffman CA. Old and new therapies for sporotrichosis. Clin Infect Dis. 1995;21:981-985.
- Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the managements of patients with sporotrichosis. Clin Infect Dis. 2000;30:684-687.
- De Araujo T, Marques AC, Kerdel F. Sporotrichosis. Int J Dermatol. 2001;40:737-742.
- Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 2. 6th ed. New York, NY: McGraw-Hill; 2003.
- Campos P, Arenas R, Coronado H. Epidemic cutaneous sporotrichosis. Int J Dermatol. 1994;33:38-41.
- Dillon GP, Lehmann PF, Talanin NY. Handyperson’s hazard: crawl space sporotrichosis. JAMA. 1995;274: 1673-1674.
- Dooley DP, Bostic PS, Beckius ML. Spook house sporotrichosis: a point-source outbreak of sporotrichosis associated with hay bale props in a Halloween haunted house. Arch Int Med. 1997;157:1885-1887.
- Kauffman CA. Sporotrichosis. Clin Infect Dis. 1999;29:231-236.
- Migaki G, Font RL, Kaplan W, et al. Sporotrichosis in a Pacific white-sided dolphin (Lagenorhynchus obliquidens). Am J Vet Res. 1978;39:1916-1919.
- Crothers SL, White SD, Ihrke PJ, et al. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007). Vet Dermatol. 2009;20:249-259.
- Saravanakumar PS, Eslami P, Zar FA. Lymphocutaneous sporotrichosis associated with a squirrel bite: case reports and review. Clin Infect Dis. 1996;23:647-648.
- Wenker CJ, Kaufman L, Bacciarini LN, et al. Sporotrichosis in a nine-banded armadillo (Dasypus novemcinctus). J Zoo Wildl Med. 1998;29:474-478.
- Barros MB, Schubach Ade O, do Valle AC, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529-535.
- Kauffman CA. Old and new therapies for sporotrichosis. Clin Infect Dis. 1995;21:981-985.
- Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the managements of patients with sporotrichosis. Clin Infect Dis. 2000;30:684-687.
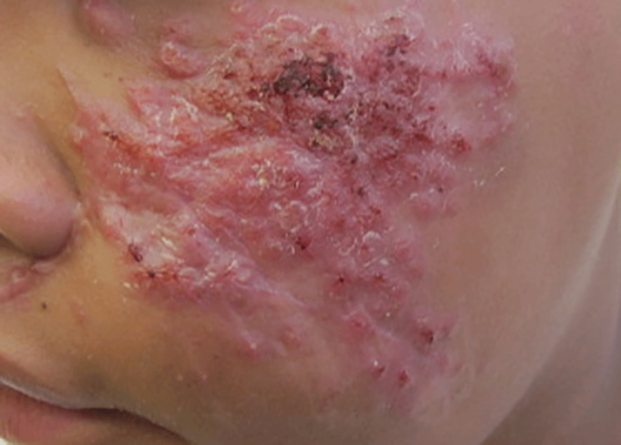
A 13-year-old adolescent boy presented with erythematous, tender, scaly, indurated nodules coalescing into plaques on the left cheek and periocular region. He denied any vision changes, the extraocular muscles were intact, and he was afebrile. Two weeks prior to presentation, the patient was hospitalized after an all-terrain vehicle accident that resulted in an extensive midfacial avulsion of the left cheek. The wound was cleaned and repaired by an otorhinolaryngologist. Three days later, he developed swelling and erythema of the left cheek, which was treated by his primary care provider with oral cephalexin, then trimethoprim-sulfamethoxazole for postsurgical wound infection. After completing his antibiotic course, he noticed continued worsening of the wound with increased edema, erythema, and tenderness. He was then referred to our clinic for further evaluation.
What’s Eating You? Ant-Induced Alopecia (Pheidole)
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
Case Report
An 18-year-old Iranian man presented to the dermatology clinic with hair loss of 1 night’s duration. He denied pruritus, pain, discharge, or flaking. The patient had no notable personal, family, or surgical history and was not currently taking any medications. He denied recent travel. The patient reported that he found hair on his pillow upon waking up in the morning prior to coming to the clinic. On physical examination, 2 ants (Figure 1) were found on the scalp and alopecia with a vertical linear distribution was noted (Figure 2). Hairs of various lengths were found on the scalp within the distribution of the alopecia. No excoriations, crusting, seborrhea, or other areas of hair loss were detected. Wood lamp examination was negative. Based on these findings, which were concordant with similar findings from prior reports,1-4 a diagnosis of ant-induced alopecia was made. Hair regrowth was noted within 1 week with full appearance of normal-length hair within 2.5 weeks.


Comment
Ant-induced alopecia is a form of localized hair loss caused by the Pheidole genus, the second largest genus of ants in the world.5 These ants can be found worldwide, but most cases of ant-induced alopecia have been from Iran, with at least 1 reported case from Turkey.1-4,6 An early case series of ant-induced alopecia was reported in 1999,6 but the causative species was not described at that time.
The majority of reported cases of ant-induced alopecia are attributed to the barber ant (Pheidole pallidula). This type of alopecia is caused by worker ants within the species hierarchy.1,4,6 The P pallidula worker ants are dimorphic and are classified as major and minor workers.7 Major workers have body lengths ranging up to 6 mm, whereas minor workers have body lengths ranging up to 4 mm. Major workers have larger heads and mandibles than minor workers and also have up to 2 pairs of denticles on the cranium.5 The minor workers are foragers and mainly collect food, whereas the major workers defend the nest and store food.8 These ants have widespread habitats with the ability to live in indoor and outdoor environments.
The presentation of hair loss caused by these ants is acute. Hair loss usually is confined to one specific area. Some patients may report pruritus or may present with erythematous lesions from ant stings or manual scratching.5 None of these signs or symptoms were seen in our patient. Some investigators have suggested that the barber ant is attracted to the hair of individuals with seborrheic dermatitis,1 but our patient had no medical history of seborrheic dermatitis. Most likely, ants are attracted to excess sebum on the scalp in select individuals in their search for food and cause localized hair destruction.
Localized hair loss, as depicted in our case, should warrant a thorough evaluation for alopecia areata, trichotillomania, and tinea capitis.9 Alopecia areata should be considered in individuals with multiple focal patches of hair loss that have a positive hair pull test from peripheral sites of active lesions. Tinea capitis usually has localized sites of hair loss with underlying scaling, crusting, pruritus, erythema, and discharge from lesions, with positive potassium hydroxide preparations or fungal cultures. Trichotillomania typically presents with a spared peripheral fringe of hair. Remaining hairs may be thick and hyperpigmented as a response to repeated pulling, and biopsy often demonstrates fracture or degeneration of the hair shaft. A psychiatric evaluation may be warranted in cases of trichotillomania. Other cases of arthropod-induced hair loss include tick bite alopecia10,11 and hair loss induced by numerous honeybee stings,12 and these diagnoses should be suspected in patients with a history of ants on their pillow or in those from endemic areas.
No specific treatment is indicated in cases of ant-induced alopecia because hair usually regrows to its normal length without intervention.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
- Shamsadini S. Localized scalp hair shedding caused by Pheidole ants and overview of similar case reports. Dermatol Online J. 2003;9:12.
- Aghaei S, Sodaifi M. Circumscribed scalp hair loss following multiple hair-cutter ant invasion. Dermatol Online J. 2004;10:14.
- Mortazavi M, Mansouri P. Ant-induced alopecia: report of 2 cases and review of the literature. Dermatol Online J. 2004;10:19.
- Kapdağli S, Seçkin D, Baba M, et al. Localized hair breakage caused by ants. Pediatr Dermatol. 2006;23:519-520.
- Ogata K. Toxonomy and biology of the genus Pheidole of Japan. Nature and Insects. 1981;16:17-22.
- Radmanesh M, Mousavipour M. Alopecia induced by ants. Trans R Soc Trop Med Hyg. 1999;93:427.
- Hölldobler B, Wilson EO. The Ants. Cambridge, MA: Harvard University Press; 1990.
- Wilson EO. Pheidole in the New World: A Dominant Hyperdiverse Ant Genus. Cambridge MA: Harvard University Press; 2003.
- Veraldi S, Lunardon L, Francia C, et al. Alopecia caused by the “barber ant” Pheidole pallidula. Int J Dermatol. 2008;47:1329-1330.
- Marshall J. Alopecia after tick bite. S Afr Med J. 1966;40: 555-556.
- Heyl T. Tick bite alopecia. Clin Exp Dermatol. 1982;7: 537-542.
- Sharma AK, Sharma RC, Sharma NL. Diffuse hair loss following multiple honeybee stings. Dermatology. 1997;195:305.
Practice Points
- Ant-induced alopecia should be considered in the differential diagnosis for patients from endemic regions (eg, Iran, Turkey) with new-onset localized hair loss or in patients recently visiting those areas with a concordant history.
- Ant-induced alopecia is thought to result from mechanical and/or chemical breakage, most commonly caused by Pheidole ants, leaving follicles intact and allowing for hair regrowth without treatment through the normal hair cycle.