User login
How an office theft can change your habits
Last week, my secretary was checking a patient out when I went into the little galley area across from her desk to get coffee. Unfortunately, I knocked the pot over and it broke, sending glass and hot coffee everywhere.
My secretary asked the patient to wait a minute, grabbed a roll of paper towels that was behind her, and ran over to help me clean up. She was with me for 1-2 minutes, then returned to finish signing the patient out while I picked up glass shards.
A while later, we realized that somewhere in that 2 minutes an envelope containing roughly $200 in copays had disappeared from her desk drawer. It had been there 30 minutes before when another patient had paid a copay in cash, and now it was gone.
My secretary? No. She’s been with me for more than 15 years. She’s never stolen from the practice before, so why would she start now? I trust her.
The only people who had access to the drawer in that time were the patient, her, and me. While the money was out of sight, it was within reach of anyone who leaned over the counter, opened the drawer to look through it, and grabbed it.
I admit I probably should have gone to the bank sooner. Normally, we only have $20-$40 in small bills on hand, which we use for change. Most people prefer credit cards. But in the 2-3 weeks before this, we had had an unusual number of people using cash for copays. Combined with a crazier schedule than usual, I just hadn’t had a chance to deposit the bills.
Obviously, I’m not going to do that again.
Generally, no one has a chance to reach over and grab the drawer, either. When a patient is checking out, my secretary is always there making the transaction. But this one time, we had an unexpected distraction and she left the desk to help me.
She’s not going to do that again with someone standing there, either.
$200 isn’t, even in a small practice, a make-or-break amount. It stings, but I’ll still be able to make payroll and pay the rent. At the end of the year, it will have to come out of my own salary, because that’s the nature of owning a business. I can’t (and wouldn’t) charge the next 200 patients a $1 “administrative fee” to cover it.
Of course, it’s possible I’m accusing the wrong person. But there wasn’t anyone in the office besides me, my secretary, and the patient in that time frame. I don’t have any actual proof, like a video, though, so I certainly can’t press charges. She didn’t schedule a follow-up visit, either, so doubt she’ll be coming back.
Why would a patient steal from a doctor who’s trying to help her? Money is the simple answer. She had an opportunity to look and take it, and she did. Her moral compass must be skewed toward dishonesty, and she took advantage of the situation. I doubt it was anything personal against me, or doctors, or the situation in general. She’s a thief, and in her mind, it was a business decision.
Of course, I could be wrong on that point. Maybe she did rationalize it by the incorrect, but widespread, belief that doctors are “rich.” In her mind, she may have thought I’d never notice it, therefore there’s nothing wrong with stealing from me.
Do I hold it against future patients? No. In 20 years this is the first time one has stolen anything of significant financial value from my office (we’ve lost pens, magazines, a stapler, and a snowman-shaped candy dish in the past). The vast majority of my patients are decent people who wouldn’t do something like this.
But it does cast a pall over new patients we don’t know. Next time I need help while someone’s being checked out, my secretary won’t be able to give it. Any amount over a few small bills for change will be promptly taken to the bank.
It’s a bitter pill that leaves a bad taste in my mouth. Not harmful in the grand scheme of things, but certainly unpleasant. My job is based on the idea that people trust me to do my best for them, and in return, I trust them to be honest with me in return.
But one morning last week, it was just a one-way street.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Last week, my secretary was checking a patient out when I went into the little galley area across from her desk to get coffee. Unfortunately, I knocked the pot over and it broke, sending glass and hot coffee everywhere.
My secretary asked the patient to wait a minute, grabbed a roll of paper towels that was behind her, and ran over to help me clean up. She was with me for 1-2 minutes, then returned to finish signing the patient out while I picked up glass shards.
A while later, we realized that somewhere in that 2 minutes an envelope containing roughly $200 in copays had disappeared from her desk drawer. It had been there 30 minutes before when another patient had paid a copay in cash, and now it was gone.
My secretary? No. She’s been with me for more than 15 years. She’s never stolen from the practice before, so why would she start now? I trust her.
The only people who had access to the drawer in that time were the patient, her, and me. While the money was out of sight, it was within reach of anyone who leaned over the counter, opened the drawer to look through it, and grabbed it.
I admit I probably should have gone to the bank sooner. Normally, we only have $20-$40 in small bills on hand, which we use for change. Most people prefer credit cards. But in the 2-3 weeks before this, we had had an unusual number of people using cash for copays. Combined with a crazier schedule than usual, I just hadn’t had a chance to deposit the bills.
Obviously, I’m not going to do that again.
Generally, no one has a chance to reach over and grab the drawer, either. When a patient is checking out, my secretary is always there making the transaction. But this one time, we had an unexpected distraction and she left the desk to help me.
She’s not going to do that again with someone standing there, either.
$200 isn’t, even in a small practice, a make-or-break amount. It stings, but I’ll still be able to make payroll and pay the rent. At the end of the year, it will have to come out of my own salary, because that’s the nature of owning a business. I can’t (and wouldn’t) charge the next 200 patients a $1 “administrative fee” to cover it.
Of course, it’s possible I’m accusing the wrong person. But there wasn’t anyone in the office besides me, my secretary, and the patient in that time frame. I don’t have any actual proof, like a video, though, so I certainly can’t press charges. She didn’t schedule a follow-up visit, either, so doubt she’ll be coming back.
Why would a patient steal from a doctor who’s trying to help her? Money is the simple answer. She had an opportunity to look and take it, and she did. Her moral compass must be skewed toward dishonesty, and she took advantage of the situation. I doubt it was anything personal against me, or doctors, or the situation in general. She’s a thief, and in her mind, it was a business decision.
Of course, I could be wrong on that point. Maybe she did rationalize it by the incorrect, but widespread, belief that doctors are “rich.” In her mind, she may have thought I’d never notice it, therefore there’s nothing wrong with stealing from me.
Do I hold it against future patients? No. In 20 years this is the first time one has stolen anything of significant financial value from my office (we’ve lost pens, magazines, a stapler, and a snowman-shaped candy dish in the past). The vast majority of my patients are decent people who wouldn’t do something like this.
But it does cast a pall over new patients we don’t know. Next time I need help while someone’s being checked out, my secretary won’t be able to give it. Any amount over a few small bills for change will be promptly taken to the bank.
It’s a bitter pill that leaves a bad taste in my mouth. Not harmful in the grand scheme of things, but certainly unpleasant. My job is based on the idea that people trust me to do my best for them, and in return, I trust them to be honest with me in return.
But one morning last week, it was just a one-way street.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Last week, my secretary was checking a patient out when I went into the little galley area across from her desk to get coffee. Unfortunately, I knocked the pot over and it broke, sending glass and hot coffee everywhere.
My secretary asked the patient to wait a minute, grabbed a roll of paper towels that was behind her, and ran over to help me clean up. She was with me for 1-2 minutes, then returned to finish signing the patient out while I picked up glass shards.
A while later, we realized that somewhere in that 2 minutes an envelope containing roughly $200 in copays had disappeared from her desk drawer. It had been there 30 minutes before when another patient had paid a copay in cash, and now it was gone.
My secretary? No. She’s been with me for more than 15 years. She’s never stolen from the practice before, so why would she start now? I trust her.
The only people who had access to the drawer in that time were the patient, her, and me. While the money was out of sight, it was within reach of anyone who leaned over the counter, opened the drawer to look through it, and grabbed it.
I admit I probably should have gone to the bank sooner. Normally, we only have $20-$40 in small bills on hand, which we use for change. Most people prefer credit cards. But in the 2-3 weeks before this, we had had an unusual number of people using cash for copays. Combined with a crazier schedule than usual, I just hadn’t had a chance to deposit the bills.
Obviously, I’m not going to do that again.
Generally, no one has a chance to reach over and grab the drawer, either. When a patient is checking out, my secretary is always there making the transaction. But this one time, we had an unexpected distraction and she left the desk to help me.
She’s not going to do that again with someone standing there, either.
$200 isn’t, even in a small practice, a make-or-break amount. It stings, but I’ll still be able to make payroll and pay the rent. At the end of the year, it will have to come out of my own salary, because that’s the nature of owning a business. I can’t (and wouldn’t) charge the next 200 patients a $1 “administrative fee” to cover it.
Of course, it’s possible I’m accusing the wrong person. But there wasn’t anyone in the office besides me, my secretary, and the patient in that time frame. I don’t have any actual proof, like a video, though, so I certainly can’t press charges. She didn’t schedule a follow-up visit, either, so doubt she’ll be coming back.
Why would a patient steal from a doctor who’s trying to help her? Money is the simple answer. She had an opportunity to look and take it, and she did. Her moral compass must be skewed toward dishonesty, and she took advantage of the situation. I doubt it was anything personal against me, or doctors, or the situation in general. She’s a thief, and in her mind, it was a business decision.
Of course, I could be wrong on that point. Maybe she did rationalize it by the incorrect, but widespread, belief that doctors are “rich.” In her mind, she may have thought I’d never notice it, therefore there’s nothing wrong with stealing from me.
Do I hold it against future patients? No. In 20 years this is the first time one has stolen anything of significant financial value from my office (we’ve lost pens, magazines, a stapler, and a snowman-shaped candy dish in the past). The vast majority of my patients are decent people who wouldn’t do something like this.
But it does cast a pall over new patients we don’t know. Next time I need help while someone’s being checked out, my secretary won’t be able to give it. Any amount over a few small bills for change will be promptly taken to the bank.
It’s a bitter pill that leaves a bad taste in my mouth. Not harmful in the grand scheme of things, but certainly unpleasant. My job is based on the idea that people trust me to do my best for them, and in return, I trust them to be honest with me in return.
But one morning last week, it was just a one-way street.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
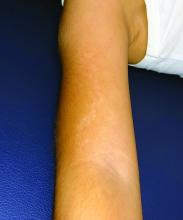
A 5-year-old boy with a papular rash on his arm
Lichen striatus (LS) is a common benign skin condition that presents in children between the ages of 5 and 15 years.1 The rash is typically unilateral and most frequently on the extremities, although it may appear on the face, trunk, or buttocks. The lesions start as pink or skin-colored asymptomatic papules in a linear orientation following the lines of Blaschko. There may be residual postinflammatory hypo- or hyperpigmentation which often improves within a few years.
Of note, there are subsets of lichen striatus: Hypopigmented lichen striatus with minimal papules has been termed “lichen striatus albus.” Nail lichen striatus may present as onycholysis or fissuring of nails, present as an isolated finding, or more commonly in association with concurrent affected skin. Nail lichen striatus typically resolves on its own, however there are case reports of improvement with intralesional steroids.2
There is no established etiology for LS. Autoimmune disease, viruses, immunizations, medications, and hypersensitivity reactions have been associated with triggering LS in various case reports, although strength of the associations is low. Children have been reported to have LS following scarlet fever and Candida vulvitis.3 Diagnosis usually is clinical, although biopsy may be helpful for histopathologic confirmation. No work-up for associated infections or conditions is warranted.
The differential for linear papular lesions includes inflammatory linear verrucous epidermal nevus (ILVEN), blaschkitis, or linear morphea. ILVEN is a hamartoma that usually is congenital or presents in early childhood; presents with linear or whorled, hyperkeratotic papules and plaque in similar linear “line of Blaschko” patterns; and represents cutaneous mosaicism. It is often difficult to differentiate between lichen striatus and ILVEN, however lichen striatus is not congenital, and is a self-limited condition. Under dermoscopy (polarized light systems) findings of LS more frequently demonstrate gray granular pigmentation. ILVEN is more frequently associated with cerebriform pattern.4 Blaschkitis is a term for a blaschkoid inflammation of the skin that presents with more eczematous findings and histology of spongiosis, unlike the lichenoid findings of LS. It is typically accompanied by noticeable pruritus and broader bands of involved area, and has older age of onset than LS. Linear morphea is a deeper inflammatory process of the dermis or subcutaneous fat, presenting with sclerotic skin, and typically has associated atrophy.
Treatment need not be pursued for lichen striatus because it is a benign condition. The lesions typically self-resolve without any residual scarring. If patients have associated pruritus then low- to midpotency topical steroids can be used for symptomatic relief.
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Gupta D, Mathes E. Lichen Striatus. (Levy ML ed.) 2019: UpToDate.
2. Dermatol Ther. 2018 Nov;31(6):e12713.
3. Int J Dermatol. 2018 Sep;57(9):1118-9.
4. J Dermatol. 2017 Dec;44(12):e355-6.
Lichen striatus (LS) is a common benign skin condition that presents in children between the ages of 5 and 15 years.1 The rash is typically unilateral and most frequently on the extremities, although it may appear on the face, trunk, or buttocks. The lesions start as pink or skin-colored asymptomatic papules in a linear orientation following the lines of Blaschko. There may be residual postinflammatory hypo- or hyperpigmentation which often improves within a few years.
Of note, there are subsets of lichen striatus: Hypopigmented lichen striatus with minimal papules has been termed “lichen striatus albus.” Nail lichen striatus may present as onycholysis or fissuring of nails, present as an isolated finding, or more commonly in association with concurrent affected skin. Nail lichen striatus typically resolves on its own, however there are case reports of improvement with intralesional steroids.2
There is no established etiology for LS. Autoimmune disease, viruses, immunizations, medications, and hypersensitivity reactions have been associated with triggering LS in various case reports, although strength of the associations is low. Children have been reported to have LS following scarlet fever and Candida vulvitis.3 Diagnosis usually is clinical, although biopsy may be helpful for histopathologic confirmation. No work-up for associated infections or conditions is warranted.
The differential for linear papular lesions includes inflammatory linear verrucous epidermal nevus (ILVEN), blaschkitis, or linear morphea. ILVEN is a hamartoma that usually is congenital or presents in early childhood; presents with linear or whorled, hyperkeratotic papules and plaque in similar linear “line of Blaschko” patterns; and represents cutaneous mosaicism. It is often difficult to differentiate between lichen striatus and ILVEN, however lichen striatus is not congenital, and is a self-limited condition. Under dermoscopy (polarized light systems) findings of LS more frequently demonstrate gray granular pigmentation. ILVEN is more frequently associated with cerebriform pattern.4 Blaschkitis is a term for a blaschkoid inflammation of the skin that presents with more eczematous findings and histology of spongiosis, unlike the lichenoid findings of LS. It is typically accompanied by noticeable pruritus and broader bands of involved area, and has older age of onset than LS. Linear morphea is a deeper inflammatory process of the dermis or subcutaneous fat, presenting with sclerotic skin, and typically has associated atrophy.
Treatment need not be pursued for lichen striatus because it is a benign condition. The lesions typically self-resolve without any residual scarring. If patients have associated pruritus then low- to midpotency topical steroids can be used for symptomatic relief.
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Gupta D, Mathes E. Lichen Striatus. (Levy ML ed.) 2019: UpToDate.
2. Dermatol Ther. 2018 Nov;31(6):e12713.
3. Int J Dermatol. 2018 Sep;57(9):1118-9.
4. J Dermatol. 2017 Dec;44(12):e355-6.
Lichen striatus (LS) is a common benign skin condition that presents in children between the ages of 5 and 15 years.1 The rash is typically unilateral and most frequently on the extremities, although it may appear on the face, trunk, or buttocks. The lesions start as pink or skin-colored asymptomatic papules in a linear orientation following the lines of Blaschko. There may be residual postinflammatory hypo- or hyperpigmentation which often improves within a few years.
Of note, there are subsets of lichen striatus: Hypopigmented lichen striatus with minimal papules has been termed “lichen striatus albus.” Nail lichen striatus may present as onycholysis or fissuring of nails, present as an isolated finding, or more commonly in association with concurrent affected skin. Nail lichen striatus typically resolves on its own, however there are case reports of improvement with intralesional steroids.2
There is no established etiology for LS. Autoimmune disease, viruses, immunizations, medications, and hypersensitivity reactions have been associated with triggering LS in various case reports, although strength of the associations is low. Children have been reported to have LS following scarlet fever and Candida vulvitis.3 Diagnosis usually is clinical, although biopsy may be helpful for histopathologic confirmation. No work-up for associated infections or conditions is warranted.
The differential for linear papular lesions includes inflammatory linear verrucous epidermal nevus (ILVEN), blaschkitis, or linear morphea. ILVEN is a hamartoma that usually is congenital or presents in early childhood; presents with linear or whorled, hyperkeratotic papules and plaque in similar linear “line of Blaschko” patterns; and represents cutaneous mosaicism. It is often difficult to differentiate between lichen striatus and ILVEN, however lichen striatus is not congenital, and is a self-limited condition. Under dermoscopy (polarized light systems) findings of LS more frequently demonstrate gray granular pigmentation. ILVEN is more frequently associated with cerebriform pattern.4 Blaschkitis is a term for a blaschkoid inflammation of the skin that presents with more eczematous findings and histology of spongiosis, unlike the lichenoid findings of LS. It is typically accompanied by noticeable pruritus and broader bands of involved area, and has older age of onset than LS. Linear morphea is a deeper inflammatory process of the dermis or subcutaneous fat, presenting with sclerotic skin, and typically has associated atrophy.
Treatment need not be pursued for lichen striatus because it is a benign condition. The lesions typically self-resolve without any residual scarring. If patients have associated pruritus then low- to midpotency topical steroids can be used for symptomatic relief.
Dr. Kaushik is with the division of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego, and Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. There are no conflicts of interest or financial disclosures for Dr. Kaushik or Dr. Eichenfield. Email them at [email protected].
References
1. Gupta D, Mathes E. Lichen Striatus. (Levy ML ed.) 2019: UpToDate.
2. Dermatol Ther. 2018 Nov;31(6):e12713.
3. Int J Dermatol. 2018 Sep;57(9):1118-9.
4. J Dermatol. 2017 Dec;44(12):e355-6.
Young children with neuromuscular disease are vulnerable to respiratory viruses
This highlights the need for new vaccines
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.
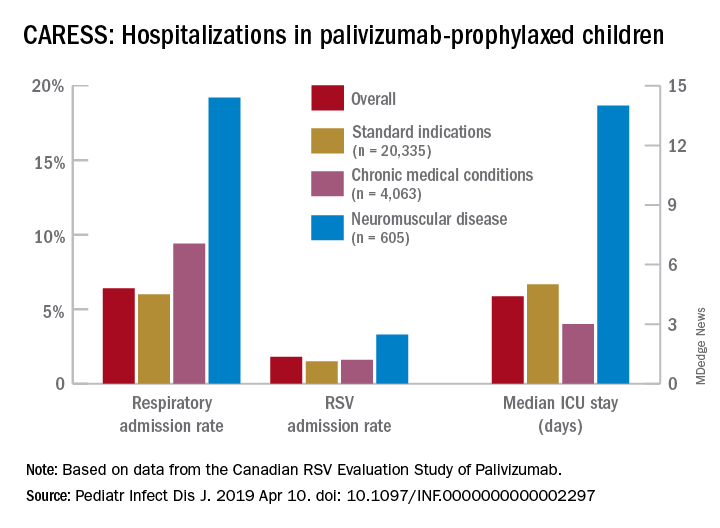
RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.
This highlights the need for new vaccines
This highlights the need for new vaccines
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.

RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.
Influenza gets a lot of attention each winter, but respiratory syncytial virus (RSV) and other respiratory viruses have as much or more impact on pediatric populations, particularly certain high-risk groups. But currently there are no vaccines for noninfluenza respiratory viruses. That said, several are under development, for RSV and parainfluenza.
Which groups are likely to get the most benefit from these newer vaccines?
We all are aware of the extra vulnerability to respiratory viruses (RSV being the most frequent) in premature infants, those with chronic lung disease, or those with congenital heart syndromes; such vulnerable patients are not infrequently seen in routine practice. A recent report shined a brighter light on such a group.
Real-world data from a nationwide Canadian surveillance system (CARESS) was used to analyze relative risks of categories of young children who are thought to be vulnerable to respiratory viruses, with a particular focus on those with neuromuscular disease. The CARESS investigators analyzed 12 years’ data on respiratory hospitalizations from among palivizumab-prophylaxed patients (including specific data on RSV when patients were tested for RSV per standard of care).1 Unfortunately, RSV testing was not universal despite hospitalization, so the true incidence of RSV-specific hospitalizations was likely underestimated.
Nevertheless, more than 25,000 children from 2005 through 2017 were grouped into three categories of palivizumab-prophylaxed high-risk children: standard indications (SI), n = 20,335; chronic medical conditions (CMD), n = 4,063; and neuromuscular disease (NMD), n = 605. This study is notable for having a relatively large number of neuromuscular disease subjects. Two-thirds of each group were fully palivizumab adherent.
The SI group included the standard American Academy of Pediatrics–recommended groups, such as premature infants, congenital heart disease, etc.
The CMD group included conditions that lead clinicians to use palivizumab off label, such as cystic fibrosis, congenital airway anomalies, immunodeficiency, and pulmonary disorders.
The NMD participants were subdivided into two groups. Group 1 comprised general hypotonic neuromuscular diseases such as hypoxic-ischemic encephalopathy, Prader-Willi syndrome, chromosomal disorders, and migration/demyelinating diseases. Group 2 included more severe infantile neuromuscular disorders, such as spinal muscular atrophy, myotonic dystrophy, centronuclear and nemaline myopathy, mitochondrial and glycogen storage myopathies, or arthrogryposis.
Overall, 6.9% of CARESS RSV-prophylaxed subjects were hospitalized. About one in five hospitalized patients from each group was hospitalized more than once. Specific respiratory hospitalization rates for each group were 6% (n = 1,228) for SI subjects and 9.4% (n = 380) for CMD, compared with 19.2% (n = 116) for NMD subjects.
It is unclear what proportion underwent RSV testing, but a total of 334 were confirmed RSV positive: 261 were SI, 54 were CMD and 19 were NMD. The RSV-test-positive rate was 1.5% for SI, 1.6% for CMD and 3.3% for NMD; so while a higher number of SI children were RSV positive, the rate of RSV positivity was actually highest with NMD.

RSV-positive subjects needing ICU care among NMD patients also had longer ICU stays (median 14 days), compared with RSV-positive CMD or SI subjects (median 3 and 5 days, respectively). Further, hospitalized RSV-positive NMD subjects presented more frequently with pneumonia (42% vs. 30% for CMD and 20% for SI) while hospitalized RSV-positive SI subjects more often had apnea (17% vs. 10% for NMD and 5% for CMD, P less than .05).
These differences in the courses of NMD patients raise the question as to whether the NMD group was somehow different from the SI and CMD groups, other than muscular weakness that likely leads to less ability to clear secretions and a less efficient cough. It turns out that NMD children were older and had worse neonatal medical courses (longer hospital stays, more often ventilated, and used oxygen longer). It could be argued that these differences may have been in part due to the muscular weakness inherent in their underlying disease, but they appear to be predictors of worse respiratory infectious disease than other vulnerable populations as the NMD children get older.
Indeed, the overall risk of any respiratory admission among NMD subjects was nearly twice as high, compared with SI (hazard ratio, 1.90, P less than .0005); but the somewhat higher risk for NMD vs. CMD was not significant (HR, 1.33, P = .090). However, when looking specifically at RSV confirmed admissions, NMD had more than twice the hospitalization risk than either other group (HR, 2.26, P = .001 vs. SI; and HR, 2.74, P = .001 vs. CMD).
Further, an NMD subgroup analysis showed 1.69 times the overall respiratory hospitalization risk among the more severe vs. less severe NMD group, but a similar risk of RSV admission. The authors point out that one reason for this discrepancy may be a higher probability of aspiration causing hospitalization because of more dramatic acute events during respiratory infections in patients with more severe NMD. It also may be that palivizumab evened the playing field for RSV but not for other viruses such as parainfluenza, adenovirus, or even rhinovirus.
Nevertheless, these data tell us that risk of respiratory disease severe enough to need hospitalization continues to an older age in NMD than SI or CMD patients, well past 2 years of age. And the risk is not only from RSV. That said, RSV remains a player in some patients (particularly NMD patients) despite palivizumab prophylaxis, highlighting the need for RSV as well as parainfluenza vaccines. While these vaccines should help all young children, they seem likely to be even more beneficial for high-risk children including those with NMD, and particularly those with more severe NMD.
Eleven among 60 total candidate RSV vaccines (live attenuated, particle based, or vector based) are currently in clinical trials.2 Fewer parainfluenza vaccines are in the pipeline, but clinical trials also are underway.3-5 Approval of such vaccines is not expected until the mid-2020s, so at present we are left with providing palivizumab to our vulnerable patients while emphasizing nonmedical strategies that may help prevent respiratory viruses. These only partially successful preventive interventions include breastfeeding, avoiding secondhand smoke, and avoiding known high-risk exposures, such as large day care centers.
My hope is for quicker than projected progress on the vaccine front so that winter admissions for respiratory viruses might decrease in numbers similar to the decrease we have noted with another vaccine successful against a seasonally active pathogen – rotavirus.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospital–Kansas City, Mo. Children’s Mercy Hospital receives grant funding to study two candidate RSV vaccines. The hospital also receives CDC funding under the New Vaccine Surveillance Network for multicenter surveillance of acute respiratory infections, including influenza, RSV, and parainfluenza virus. Email Dr. Harrison at [email protected].
References
1. Pediatr Infect Dis J. 2019 Apr 10. doi: 10.1097/INF.0000000000002297.
2. “Advances in RSV Vaccine Research and Development – A Global Agenda.”
3. J Pediatric Infect Dis Soc. 2015 Dec;4(4): e143-6.
4. J Virol. 2015 Oct;89(20):10319-32.
5. Vaccine. 2017 Dec 18;35(51):7139-46.
Handling defamatory online reviews
In my last column, I gave you some options for handling those inevitable negative online reviews without violating patient confidentiality or pouring fuel on the fire. Your options in such cases are limited by HIPAA rules (among others) and by the patient’s right to free expression under the First Amendment.
– although you need to carefully consider the situation before going to war with a critic.
Critics have legislative protection in many states, called anti-SLAPP (Strategic Lawsuit Against Public Participation) laws, which allow judges to summarily dismiss lawsuits that they consider retaliatory or intended to intimidate and silence citizens speaking out on issues of public interest – such as health care. Federal courts recently nullified anti-SLAPP laws in Washington and Minnesota as unconstitutional; but as I write this, similar laws remain on the books in 28 other states, plus Washington, D.C., and Guam.
There is also a federal law – the Consumer Review Freedom Act of 2016 – which prohibits any attempt to prevent consumers from giving “honest” reviews about products or services. No law protects demonstrably false statements, of course.
The first thing to do before taking any action is to determine whether that defamatory review is, in fact, defamatory. Defamation is generally defined as the act of making false statements “with malice” – that is, in a deliberate attempt to damage someone’s reputation. The main issue in most defamation cases is whether the statements in question are merely strong opinions, which are protected by the First Amendment; or “assertions of verifiable fact”, which are not.
For example, “Dr. ____’s office does not clean its instruments properly” is a statement that can be proven true or false. Therefore, it is an assertion of fact, not an opinion, and if false, vulnerable to a defamation suit. The only unimpeachable defense in such a suit would be to prove that the assertion is true.
It is worth noting that attempting to disguise assertions of fact as opinions, simply by calling them opinions – for example, “In my opinion, Dr. ____’s office does not clean its instruments properly” – does not make them unverifiable or immunize them from litigation.
Once you have determined that the review fits the legal definition of defamation, the usual first step is to contact the website where the review is posted. Most rating sites are loath to intercede in arguments. (for example, Yelp’s official position: “We don’t typically take sides in factual disputes, and generally allow Yelpers to stand behind their reviews.”) They also have their own legal shield: The U.S. Communications Decency Act, which prohibits lawsuits against websites for publishing reviews, comments, and other third-party content, unless the site itself changes or somehow alters the meaning of the original post.
Even so, websites have their own reputations to protect; they don’t want to be used as venues for acts of defamation, nor be seen as perpetuating false or misleading information, and can sometimes be persuaded to take down really egregious hatchet jobs. It is certainly worth a try – but it may take a lawyer’s letter to get their attention.
If the site won’t remove it, you’ll have to try to persuade the patient to do so. Most attorneys recommend sending a “cease-and-desist” letter, explaining why the review is defamatory and demanding its removal. You should carefully consider the situation before sending such a letter; it may fuel the patient’s anger and trigger additional online attacks.
If a cease-and-desist letter is ineffective, your only further option is to file a lawsuit. Such cases are rare, and success even rarer: Of the 29 health care–related defamation cases that I was able to find in the public record, 19 were summarily dismissed; in 6 of those cases, the plaintiff was ordered to pay the defendant’s court costs. The other 10 were settled on undisclosed terms; only one, apparently, involved a cash payment to the plaintiff.
If you believe that the defamation is causing you real, monetary damage – enough to outweigh the costs of litigation – and you can prove that the allegations against you are false, it might be worth the considerable time, money, and emotional energy that litigation demands to pursue it.
As always, never venture into the litigation jungle without the support and guidance of an experienced attorney.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In my last column, I gave you some options for handling those inevitable negative online reviews without violating patient confidentiality or pouring fuel on the fire. Your options in such cases are limited by HIPAA rules (among others) and by the patient’s right to free expression under the First Amendment.
– although you need to carefully consider the situation before going to war with a critic.
Critics have legislative protection in many states, called anti-SLAPP (Strategic Lawsuit Against Public Participation) laws, which allow judges to summarily dismiss lawsuits that they consider retaliatory or intended to intimidate and silence citizens speaking out on issues of public interest – such as health care. Federal courts recently nullified anti-SLAPP laws in Washington and Minnesota as unconstitutional; but as I write this, similar laws remain on the books in 28 other states, plus Washington, D.C., and Guam.
There is also a federal law – the Consumer Review Freedom Act of 2016 – which prohibits any attempt to prevent consumers from giving “honest” reviews about products or services. No law protects demonstrably false statements, of course.
The first thing to do before taking any action is to determine whether that defamatory review is, in fact, defamatory. Defamation is generally defined as the act of making false statements “with malice” – that is, in a deliberate attempt to damage someone’s reputation. The main issue in most defamation cases is whether the statements in question are merely strong opinions, which are protected by the First Amendment; or “assertions of verifiable fact”, which are not.
For example, “Dr. ____’s office does not clean its instruments properly” is a statement that can be proven true or false. Therefore, it is an assertion of fact, not an opinion, and if false, vulnerable to a defamation suit. The only unimpeachable defense in such a suit would be to prove that the assertion is true.
It is worth noting that attempting to disguise assertions of fact as opinions, simply by calling them opinions – for example, “In my opinion, Dr. ____’s office does not clean its instruments properly” – does not make them unverifiable or immunize them from litigation.
Once you have determined that the review fits the legal definition of defamation, the usual first step is to contact the website where the review is posted. Most rating sites are loath to intercede in arguments. (for example, Yelp’s official position: “We don’t typically take sides in factual disputes, and generally allow Yelpers to stand behind their reviews.”) They also have their own legal shield: The U.S. Communications Decency Act, which prohibits lawsuits against websites for publishing reviews, comments, and other third-party content, unless the site itself changes or somehow alters the meaning of the original post.
Even so, websites have their own reputations to protect; they don’t want to be used as venues for acts of defamation, nor be seen as perpetuating false or misleading information, and can sometimes be persuaded to take down really egregious hatchet jobs. It is certainly worth a try – but it may take a lawyer’s letter to get their attention.
If the site won’t remove it, you’ll have to try to persuade the patient to do so. Most attorneys recommend sending a “cease-and-desist” letter, explaining why the review is defamatory and demanding its removal. You should carefully consider the situation before sending such a letter; it may fuel the patient’s anger and trigger additional online attacks.
If a cease-and-desist letter is ineffective, your only further option is to file a lawsuit. Such cases are rare, and success even rarer: Of the 29 health care–related defamation cases that I was able to find in the public record, 19 were summarily dismissed; in 6 of those cases, the plaintiff was ordered to pay the defendant’s court costs. The other 10 were settled on undisclosed terms; only one, apparently, involved a cash payment to the plaintiff.
If you believe that the defamation is causing you real, monetary damage – enough to outweigh the costs of litigation – and you can prove that the allegations against you are false, it might be worth the considerable time, money, and emotional energy that litigation demands to pursue it.
As always, never venture into the litigation jungle without the support and guidance of an experienced attorney.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
In my last column, I gave you some options for handling those inevitable negative online reviews without violating patient confidentiality or pouring fuel on the fire. Your options in such cases are limited by HIPAA rules (among others) and by the patient’s right to free expression under the First Amendment.
– although you need to carefully consider the situation before going to war with a critic.
Critics have legislative protection in many states, called anti-SLAPP (Strategic Lawsuit Against Public Participation) laws, which allow judges to summarily dismiss lawsuits that they consider retaliatory or intended to intimidate and silence citizens speaking out on issues of public interest – such as health care. Federal courts recently nullified anti-SLAPP laws in Washington and Minnesota as unconstitutional; but as I write this, similar laws remain on the books in 28 other states, plus Washington, D.C., and Guam.
There is also a federal law – the Consumer Review Freedom Act of 2016 – which prohibits any attempt to prevent consumers from giving “honest” reviews about products or services. No law protects demonstrably false statements, of course.
The first thing to do before taking any action is to determine whether that defamatory review is, in fact, defamatory. Defamation is generally defined as the act of making false statements “with malice” – that is, in a deliberate attempt to damage someone’s reputation. The main issue in most defamation cases is whether the statements in question are merely strong opinions, which are protected by the First Amendment; or “assertions of verifiable fact”, which are not.
For example, “Dr. ____’s office does not clean its instruments properly” is a statement that can be proven true or false. Therefore, it is an assertion of fact, not an opinion, and if false, vulnerable to a defamation suit. The only unimpeachable defense in such a suit would be to prove that the assertion is true.
It is worth noting that attempting to disguise assertions of fact as opinions, simply by calling them opinions – for example, “In my opinion, Dr. ____’s office does not clean its instruments properly” – does not make them unverifiable or immunize them from litigation.
Once you have determined that the review fits the legal definition of defamation, the usual first step is to contact the website where the review is posted. Most rating sites are loath to intercede in arguments. (for example, Yelp’s official position: “We don’t typically take sides in factual disputes, and generally allow Yelpers to stand behind their reviews.”) They also have their own legal shield: The U.S. Communications Decency Act, which prohibits lawsuits against websites for publishing reviews, comments, and other third-party content, unless the site itself changes or somehow alters the meaning of the original post.
Even so, websites have their own reputations to protect; they don’t want to be used as venues for acts of defamation, nor be seen as perpetuating false or misleading information, and can sometimes be persuaded to take down really egregious hatchet jobs. It is certainly worth a try – but it may take a lawyer’s letter to get their attention.
If the site won’t remove it, you’ll have to try to persuade the patient to do so. Most attorneys recommend sending a “cease-and-desist” letter, explaining why the review is defamatory and demanding its removal. You should carefully consider the situation before sending such a letter; it may fuel the patient’s anger and trigger additional online attacks.
If a cease-and-desist letter is ineffective, your only further option is to file a lawsuit. Such cases are rare, and success even rarer: Of the 29 health care–related defamation cases that I was able to find in the public record, 19 were summarily dismissed; in 6 of those cases, the plaintiff was ordered to pay the defendant’s court costs. The other 10 were settled on undisclosed terms; only one, apparently, involved a cash payment to the plaintiff.
If you believe that the defamation is causing you real, monetary damage – enough to outweigh the costs of litigation – and you can prove that the allegations against you are false, it might be worth the considerable time, money, and emotional energy that litigation demands to pursue it.
As always, never venture into the litigation jungle without the support and guidance of an experienced attorney.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
How medical providers can observe LGBT Pride Month
June is Pride Month in the United States. It is a time in which people take a stand against discrimination and violence against lesbian, gay, bisexual, and transgender (LGBT) people and promote dignity, equality, and visibility of this community. During this time, many cities will be holding events ranging from rallies to parades to not only celebrate sexual diversity and gender variance, but also to serve as a reminder of the work that needs to be done to foster equal treatment for LGBT people. As a medical provider, you have the unique role of advancing this cause – from educating your colleagues on the health needs of this population to advocating for policies that protect their health and well-being. If you’re interested in serving the LGBT community as a medical provider, here are some ways you can show this community your commitment to their health and well-being.
Be visible
There will be numerous LGBT Pride events occurring the month of June and even throughout the summer in the United States. They can occur in cities big and small, and they can even be in the city you work in. Visibility matters for LGBT youth. Eight percent of lesbian, gay, and bisexual people report that a health care provider refused to see them because of their sexual orientation and 29% of transgender people report that their health care providers refused to see them because of their gender identity or expression.1 Therefore, LGBT people will expect discrimination everywhere they go.2
Being present at a Pride event signals to the community that you are willing to serve LGBT people. Many Pride events will allow hospitals and clinics to have a table at the event, but keep in mind that many will prioritize organizations that specifically cater to the LGBT community or that are owned and operated by members of the community. Another way to show the community that you will treat LGBT people with dignity and respect is to list your practice in a database for LGBT-friendly providers. The Gay and Lesbian Medical Association keeps a database of LGBT-friendly medical providers, and many Pride events will advertise businesses and organizations that serve the LGBT community. You may want to consider having your clinic or hospital participate in the Human Rights Campaign (HRC) Health Equality Index (HEI). The HEI is a list of best practices for hospitals and clinics to use that affirm and support LGBT health (such as having gender-neutral bathrooms in facilities). Hospitals and clinics that endorse a high amount or all of these practices are listed as committed to the health and well-being of the LGBT community on the HRC website.
Be a part of LGBT Pride
Many LGBT Pride events are supported by local community organizations, most of which are nonprofits. They will need the necessary resources to keep holding these events every year. These resources can include both time and money. Consider donating your time by volunteering at these Pride events. For example, many Pride events hold health screenings, and you can use your skills and knowledge to promote the well-being of the LGBT community. At the same time, make sure that the PRIDE event is created to help serve the community. There is controversy over the commercialization of LGBT Pride events, as some corporate sponsors have been inconsistent in advocating for the LGBT community. Some feel that the commercialization of LGBT Pride ignores the original purpose of the event as a political movement.3 Do some research to make sure that your donation is going to an LGBT Pride event that serves the whole community, not just certain segments of it, and if you feel that it is not, you may consider donating to other LGBT-serving organizations in your community.
Educate yourself
There are many medical providers who have made it their life’s work doing this. Consider learning more about the role medical providers have played in the health and well-being of the LGBT community, which may serve as an inspiration for your work. The list is long, and includes pioneers such as Ben Barres, MD, PhD, a transgender neurobiologist and physician who transitioned from female to male mid-career and was known for his work on interaction between neurons and glial cells in the nervous system, and Rachel Levine, MD, a physician who became the first transgender woman to serve as Physician General, then Secretary of State, of Pennsylvania.
Other providers have tackled health problems that plagued the LGBT community. Joel D. Weisman, DO, was one of the first physicians to identify the AIDS epidemic and became an advocate for AIDS research, treatment, and prevention, whereas Kevin A. Fenton, MD, PhD, a gay black man, was the director for the National Center for HIV/AIDS at the Centers for Disease Control and Prevention; he helped cultivate strategies to combat the HIV epidemic among gay black men.4 Finally, there is Nanette Gartrell, MD, a psychiatrist and researcher who leads the U.S. National Longitudinal Lesbian Family Study. This ongoing, prospective, and influential study was the first to identify that children raised by lesbian mothers had higher levels of social and school/academic competence and significantly lower levels of social problems, rule-breaking behaviors, and aggressive behaviors, compared with children raised by opposite sex parents.5
LGBT Pride is a time to recognize the achievements the LGBT community has made in the last couple of decades, and at the same time, it is a reminder that the work to promote health equity for this community remains unfinished. Health care providers have an important responsibility in fostering this work in a responsible and ethical matter. Many medical providers have dedicated their lives to this movement, and even when the LGBT Pride season is over, their mission will continue.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. “Discrimination Prevents LGBTQ People from Accessing Health Care,” Center for American Progress, Jan. 18, 2018.
2. Psychol Bull. 2003 Sep;129(5): 674-97.
3. “How LGBTQ Pride Month became a branded holiday,” Vox, Jun 25, 2018.
4. “Dr. Kevin Fenton stepping down after 8 years,” The Georgia Voice, Dec 7, 2012.
5. Pediatrics. 2010;126(1):28-36.
June is Pride Month in the United States. It is a time in which people take a stand against discrimination and violence against lesbian, gay, bisexual, and transgender (LGBT) people and promote dignity, equality, and visibility of this community. During this time, many cities will be holding events ranging from rallies to parades to not only celebrate sexual diversity and gender variance, but also to serve as a reminder of the work that needs to be done to foster equal treatment for LGBT people. As a medical provider, you have the unique role of advancing this cause – from educating your colleagues on the health needs of this population to advocating for policies that protect their health and well-being. If you’re interested in serving the LGBT community as a medical provider, here are some ways you can show this community your commitment to their health and well-being.
Be visible
There will be numerous LGBT Pride events occurring the month of June and even throughout the summer in the United States. They can occur in cities big and small, and they can even be in the city you work in. Visibility matters for LGBT youth. Eight percent of lesbian, gay, and bisexual people report that a health care provider refused to see them because of their sexual orientation and 29% of transgender people report that their health care providers refused to see them because of their gender identity or expression.1 Therefore, LGBT people will expect discrimination everywhere they go.2
Being present at a Pride event signals to the community that you are willing to serve LGBT people. Many Pride events will allow hospitals and clinics to have a table at the event, but keep in mind that many will prioritize organizations that specifically cater to the LGBT community or that are owned and operated by members of the community. Another way to show the community that you will treat LGBT people with dignity and respect is to list your practice in a database for LGBT-friendly providers. The Gay and Lesbian Medical Association keeps a database of LGBT-friendly medical providers, and many Pride events will advertise businesses and organizations that serve the LGBT community. You may want to consider having your clinic or hospital participate in the Human Rights Campaign (HRC) Health Equality Index (HEI). The HEI is a list of best practices for hospitals and clinics to use that affirm and support LGBT health (such as having gender-neutral bathrooms in facilities). Hospitals and clinics that endorse a high amount or all of these practices are listed as committed to the health and well-being of the LGBT community on the HRC website.
Be a part of LGBT Pride
Many LGBT Pride events are supported by local community organizations, most of which are nonprofits. They will need the necessary resources to keep holding these events every year. These resources can include both time and money. Consider donating your time by volunteering at these Pride events. For example, many Pride events hold health screenings, and you can use your skills and knowledge to promote the well-being of the LGBT community. At the same time, make sure that the PRIDE event is created to help serve the community. There is controversy over the commercialization of LGBT Pride events, as some corporate sponsors have been inconsistent in advocating for the LGBT community. Some feel that the commercialization of LGBT Pride ignores the original purpose of the event as a political movement.3 Do some research to make sure that your donation is going to an LGBT Pride event that serves the whole community, not just certain segments of it, and if you feel that it is not, you may consider donating to other LGBT-serving organizations in your community.
Educate yourself
There are many medical providers who have made it their life’s work doing this. Consider learning more about the role medical providers have played in the health and well-being of the LGBT community, which may serve as an inspiration for your work. The list is long, and includes pioneers such as Ben Barres, MD, PhD, a transgender neurobiologist and physician who transitioned from female to male mid-career and was known for his work on interaction between neurons and glial cells in the nervous system, and Rachel Levine, MD, a physician who became the first transgender woman to serve as Physician General, then Secretary of State, of Pennsylvania.
Other providers have tackled health problems that plagued the LGBT community. Joel D. Weisman, DO, was one of the first physicians to identify the AIDS epidemic and became an advocate for AIDS research, treatment, and prevention, whereas Kevin A. Fenton, MD, PhD, a gay black man, was the director for the National Center for HIV/AIDS at the Centers for Disease Control and Prevention; he helped cultivate strategies to combat the HIV epidemic among gay black men.4 Finally, there is Nanette Gartrell, MD, a psychiatrist and researcher who leads the U.S. National Longitudinal Lesbian Family Study. This ongoing, prospective, and influential study was the first to identify that children raised by lesbian mothers had higher levels of social and school/academic competence and significantly lower levels of social problems, rule-breaking behaviors, and aggressive behaviors, compared with children raised by opposite sex parents.5
LGBT Pride is a time to recognize the achievements the LGBT community has made in the last couple of decades, and at the same time, it is a reminder that the work to promote health equity for this community remains unfinished. Health care providers have an important responsibility in fostering this work in a responsible and ethical matter. Many medical providers have dedicated their lives to this movement, and even when the LGBT Pride season is over, their mission will continue.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. “Discrimination Prevents LGBTQ People from Accessing Health Care,” Center for American Progress, Jan. 18, 2018.
2. Psychol Bull. 2003 Sep;129(5): 674-97.
3. “How LGBTQ Pride Month became a branded holiday,” Vox, Jun 25, 2018.
4. “Dr. Kevin Fenton stepping down after 8 years,” The Georgia Voice, Dec 7, 2012.
5. Pediatrics. 2010;126(1):28-36.
June is Pride Month in the United States. It is a time in which people take a stand against discrimination and violence against lesbian, gay, bisexual, and transgender (LGBT) people and promote dignity, equality, and visibility of this community. During this time, many cities will be holding events ranging from rallies to parades to not only celebrate sexual diversity and gender variance, but also to serve as a reminder of the work that needs to be done to foster equal treatment for LGBT people. As a medical provider, you have the unique role of advancing this cause – from educating your colleagues on the health needs of this population to advocating for policies that protect their health and well-being. If you’re interested in serving the LGBT community as a medical provider, here are some ways you can show this community your commitment to their health and well-being.
Be visible
There will be numerous LGBT Pride events occurring the month of June and even throughout the summer in the United States. They can occur in cities big and small, and they can even be in the city you work in. Visibility matters for LGBT youth. Eight percent of lesbian, gay, and bisexual people report that a health care provider refused to see them because of their sexual orientation and 29% of transgender people report that their health care providers refused to see them because of their gender identity or expression.1 Therefore, LGBT people will expect discrimination everywhere they go.2
Being present at a Pride event signals to the community that you are willing to serve LGBT people. Many Pride events will allow hospitals and clinics to have a table at the event, but keep in mind that many will prioritize organizations that specifically cater to the LGBT community or that are owned and operated by members of the community. Another way to show the community that you will treat LGBT people with dignity and respect is to list your practice in a database for LGBT-friendly providers. The Gay and Lesbian Medical Association keeps a database of LGBT-friendly medical providers, and many Pride events will advertise businesses and organizations that serve the LGBT community. You may want to consider having your clinic or hospital participate in the Human Rights Campaign (HRC) Health Equality Index (HEI). The HEI is a list of best practices for hospitals and clinics to use that affirm and support LGBT health (such as having gender-neutral bathrooms in facilities). Hospitals and clinics that endorse a high amount or all of these practices are listed as committed to the health and well-being of the LGBT community on the HRC website.
Be a part of LGBT Pride
Many LGBT Pride events are supported by local community organizations, most of which are nonprofits. They will need the necessary resources to keep holding these events every year. These resources can include both time and money. Consider donating your time by volunteering at these Pride events. For example, many Pride events hold health screenings, and you can use your skills and knowledge to promote the well-being of the LGBT community. At the same time, make sure that the PRIDE event is created to help serve the community. There is controversy over the commercialization of LGBT Pride events, as some corporate sponsors have been inconsistent in advocating for the LGBT community. Some feel that the commercialization of LGBT Pride ignores the original purpose of the event as a political movement.3 Do some research to make sure that your donation is going to an LGBT Pride event that serves the whole community, not just certain segments of it, and if you feel that it is not, you may consider donating to other LGBT-serving organizations in your community.
Educate yourself
There are many medical providers who have made it their life’s work doing this. Consider learning more about the role medical providers have played in the health and well-being of the LGBT community, which may serve as an inspiration for your work. The list is long, and includes pioneers such as Ben Barres, MD, PhD, a transgender neurobiologist and physician who transitioned from female to male mid-career and was known for his work on interaction between neurons and glial cells in the nervous system, and Rachel Levine, MD, a physician who became the first transgender woman to serve as Physician General, then Secretary of State, of Pennsylvania.
Other providers have tackled health problems that plagued the LGBT community. Joel D. Weisman, DO, was one of the first physicians to identify the AIDS epidemic and became an advocate for AIDS research, treatment, and prevention, whereas Kevin A. Fenton, MD, PhD, a gay black man, was the director for the National Center for HIV/AIDS at the Centers for Disease Control and Prevention; he helped cultivate strategies to combat the HIV epidemic among gay black men.4 Finally, there is Nanette Gartrell, MD, a psychiatrist and researcher who leads the U.S. National Longitudinal Lesbian Family Study. This ongoing, prospective, and influential study was the first to identify that children raised by lesbian mothers had higher levels of social and school/academic competence and significantly lower levels of social problems, rule-breaking behaviors, and aggressive behaviors, compared with children raised by opposite sex parents.5
LGBT Pride is a time to recognize the achievements the LGBT community has made in the last couple of decades, and at the same time, it is a reminder that the work to promote health equity for this community remains unfinished. Health care providers have an important responsibility in fostering this work in a responsible and ethical matter. Many medical providers have dedicated their lives to this movement, and even when the LGBT Pride season is over, their mission will continue.
Dr. Montano is an assistant professor of pediatrics at the University of Pittsburgh and an adolescent medicine physician at Children’s Hospital of Pittsburgh of UPMC. Email him at [email protected].
References
1. “Discrimination Prevents LGBTQ People from Accessing Health Care,” Center for American Progress, Jan. 18, 2018.
2. Psychol Bull. 2003 Sep;129(5): 674-97.
3. “How LGBTQ Pride Month became a branded holiday,” Vox, Jun 25, 2018.
4. “Dr. Kevin Fenton stepping down after 8 years,” The Georgia Voice, Dec 7, 2012.
5. Pediatrics. 2010;126(1):28-36.
Whatever “my last doctor” did, I don’t take the bait
In car repair, there’s a mysterious bogeyman known as “the last guy.”
“The last guy put it in wrong.”
“The last guy didn’t use the right part.”
“I have no idea what the last guy was thinking.”
In medicine, there’s “my last doctor.”
“My last doctor ordered the wrong test.”
“The medication, from my last doctor, almost killed me.”
“My last doctor didn’t know what he was doing.”
I don’t say anything, I just listen. Most of the time I’m not convinced the other doctor did anything wrong, and even if I were, I’d stay silent. Every doctor makes mistakes. It’s inevitable in any job.
Sometimes the patients mention this in passing, at other times they seem to be hoping for a response from me. I don’t give them one. Bashing other doctors is common enough as it is, and I’m not going to join in. My job is to do my best to help them, which is what the last doctor was trying to do, too.
The fact is that you can’t make everyone happy. Outside competency and human errors, there are too many variables in human relationships – the chemistry between people – to know what went wrong. Some patients have legitimate grievances, others may just be nitpicking and looking for trouble. It’s not my role to address it. If the patients came here for that, they’re at the wrong place. Most of the time, I happen to know their previous physicians, and think they’re decent neurologists.
The problem with these types of things is that it propagates. Even if I do everything right, and try my best, there’s a good chance that, in a few months, I’ll be “my last doctor.” I can only hope the next doctor feels the same way about me as I do about the last one.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In car repair, there’s a mysterious bogeyman known as “the last guy.”
“The last guy put it in wrong.”
“The last guy didn’t use the right part.”
“I have no idea what the last guy was thinking.”
In medicine, there’s “my last doctor.”
“My last doctor ordered the wrong test.”
“The medication, from my last doctor, almost killed me.”
“My last doctor didn’t know what he was doing.”
I don’t say anything, I just listen. Most of the time I’m not convinced the other doctor did anything wrong, and even if I were, I’d stay silent. Every doctor makes mistakes. It’s inevitable in any job.
Sometimes the patients mention this in passing, at other times they seem to be hoping for a response from me. I don’t give them one. Bashing other doctors is common enough as it is, and I’m not going to join in. My job is to do my best to help them, which is what the last doctor was trying to do, too.
The fact is that you can’t make everyone happy. Outside competency and human errors, there are too many variables in human relationships – the chemistry between people – to know what went wrong. Some patients have legitimate grievances, others may just be nitpicking and looking for trouble. It’s not my role to address it. If the patients came here for that, they’re at the wrong place. Most of the time, I happen to know their previous physicians, and think they’re decent neurologists.
The problem with these types of things is that it propagates. Even if I do everything right, and try my best, there’s a good chance that, in a few months, I’ll be “my last doctor.” I can only hope the next doctor feels the same way about me as I do about the last one.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In car repair, there’s a mysterious bogeyman known as “the last guy.”
“The last guy put it in wrong.”
“The last guy didn’t use the right part.”
“I have no idea what the last guy was thinking.”
In medicine, there’s “my last doctor.”
“My last doctor ordered the wrong test.”
“The medication, from my last doctor, almost killed me.”
“My last doctor didn’t know what he was doing.”
I don’t say anything, I just listen. Most of the time I’m not convinced the other doctor did anything wrong, and even if I were, I’d stay silent. Every doctor makes mistakes. It’s inevitable in any job.
Sometimes the patients mention this in passing, at other times they seem to be hoping for a response from me. I don’t give them one. Bashing other doctors is common enough as it is, and I’m not going to join in. My job is to do my best to help them, which is what the last doctor was trying to do, too.
The fact is that you can’t make everyone happy. Outside competency and human errors, there are too many variables in human relationships – the chemistry between people – to know what went wrong. Some patients have legitimate grievances, others may just be nitpicking and looking for trouble. It’s not my role to address it. If the patients came here for that, they’re at the wrong place. Most of the time, I happen to know their previous physicians, and think they’re decent neurologists.
The problem with these types of things is that it propagates. Even if I do everything right, and try my best, there’s a good chance that, in a few months, I’ll be “my last doctor.” I can only hope the next doctor feels the same way about me as I do about the last one.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Is it measles? – Diagnosis and management for the pediatric provider
The mother of an 8-month-old calls your office and is hysterical. Her daughter has had cough for a few days with high fevers and now has developed a full body rash. She is worried about measles and is on her way to your office.
We are in the middle of a measles epidemic, there’s no denying it. Measles was declared eliminated in 2000, but reported cases in the United States have been on the rise, and are now at the highest number since 2014. Five months into 2019, there have been 839 reported cases as of May 13). Measles outbreaks (defined by the Centers for Disease Control and Prevention as three or more cases) have been reported in California, Georgia, Maryland, Michigan, New Jersey, New York, and Pennsylvania. When vaccination rates fall, it is easy for measles to spread. The virus is highly contagious in nonimmune people, because of its airborne spread and its persistence in the environment for hours.
First – is it really measles?
It can be difficult to distinguish the maculopapular rash of measles from similar rashes that occur with more benign viral illnesses. Adding to the challenge, the last major measles outbreak in the United States was over 2 decades ago, and many practicing pediatricians have never seen a single case. So, what clinical features can help distinguish measles from other febrile illnesses?
The prodromal phase of measles lasts approximately 2-4 days and children have high fevers (103°-105° F), anorexia, and malaise. Conjunctivitis, coryza, and cough develop during this phase, and precede any rash. Koplik spots appear during the prodromal phase, but are not seen in all cases. These spots are 1- to 3-mm blue-white lesions on an erythematous base on the buccal mucosa, classically opposite the first molar. The spots often slough once the rash appears. The rash appears 2-4 days after the onset of fever, and is initially maculopapular and blanching. The first lesions appear on the face and neck, and the rash spreads cranial to caudal, typically sparing palms and soles. After days 3-4, the rash will no longer blanch. High fevers persist for 2-4 more days with rash, ongoing respiratory symptoms, conjunctivitis, and pharyngitis. Note that the fever will persist even with development of the rash, unlike in roseola.
It is not only important to diagnosis measles from a public health standpoint, but also because measles can have severe complications, especially in infants and children under 5 years. During the 1989-1991 outbreak, the mortality rate was 2.2 deaths per 1,000 cases (J Infect Dis. 2004 May 1. doi: 10.1086/377694).
Six percent of patients develop pneumonia, which in infants and toddlers can lead to respiratory distress or failure requiring hospitalization. Pneumonia is responsible for 60% of measles deaths, according to the CDC “Pink Book,” Epidemiology and Prevention of Vaccine-Preventable Diseases, chapter 13 on measles, 13th Ed., 2015. Ocular complications include keratitis and corneal ulceration. Measles also can cause serious neurologic complications. Encephalitis, seen in 1 per 1,000 cases, usually arises several days after the rash and may present with seizure or encephalopathy. Acute disseminated encephalomyelitis (ADEM), an inflammatory demyelinating disease of the central nervous system, occurs in approximately 1 per 1,000 cases, typically presents during the recovery phase (1-2 weeks after rash), and can have long-term sequelae. Subacute sclerosing panencephalitis (SSPE) is a progressive and fatal neurodegenerative disorder, and presents 7-10 years after measles infection.
Should you transfer the patient to a hospital?
Unless there is a medical need for the child to be admitted, sending a patient with potential measles to the hospital is not necessary, and can cause exposure to a large group of medical personnel, and patients who cannot be vaccinated (such as infants, immunocompromised patients, and pregnant women). However, if there is concern for complications such as seizures, encephalitis, or pneumonia, then transfer is indicated. Call the accepting hospital in advance so the staff can prepare for the patient. During transfer, place a standard face mask on the patient and instruct the patient not to remove it.
For hospitals accepting a suspected measles case, meet the patient outside of the facility and ensure that the patient is wearing a standard face mask. All staff interacting with the patient should practice contact and airborne precautions (N95 respirator mask). Take the patient directly to an isolation room with negative airflow. Caution pregnant staff that they should not have contact with the patient.
Which diagnostic tests should you use?
Diagnosis can be made based on serum antibody tests (measles IgM and IgG), throat or urine viral cultures, and nasopharyngeal and throat specimen polymerase chain reaction (PCR) testing. The CDC recommends obtaining a serum sample for measles IgM testing and a throat swab for PCR in all suspected cases, but local health departments vary in their specific testing recommendations. Familiarize yourself with the tests recommended by your local department of health, and where they prefer testing on outpatients to be done. Confirmed measles should be reported to your department of health.
What are considerations for community pediatric offices?
Update families in emails to call ahead if they suspect measles. This way the office can prepare a room for the family, and have the family immediately brought back without exposing staff and other families in the waiting area. It may be more prudent to examine these children at the end of the clinic day as the virus can persist for up to 2 hours on fomites and in the air. Therefore, all waiting areas and shared air spaces (including those with shared air ducts) should be cleared for 2 hours after the patient leaves.
When should you provide prophylaxis after exposure?
A patient with suspected measles does not require immediate vaccination. If it is measles, it is already too late to vaccinate. If measles is ruled out, the child should follow the standard measles vaccination guidelines.
Individuals are contagious from 4 days before to 4 days after the rash appears.
If measles is confirmed, all people who are unvaccinated or undervaccinated and were exposed to the confirmed case during the contagious period should be vaccinated within 72 hours of exposure. Infants 6 months or older may safely receive the MMR vaccine. However, infants vaccinated with MMR before their first birthday must be vaccinated again at age 12-15 months (greater than 28 days after prior vaccine) and at 4-6 years. Immunoglobulin prophylaxis should be given intramuscularly in exposed infants ages birth to less than 6 months, and in those ages 6-12 months who present beyond the 72-hour window. Unvaccinated or undervaccinated, exposed individuals at high risk for complications from measles (immunocompromised, pregnant) also should receive immunoglobulin.
What should you tell traveling families?
Several countries have large, ongoing measles outbreaks, including Israel, Ukraine, and the Philippines. Before international travel, infants 6-11 months should receive one dose of MMR vaccine, and children 12 months and older need two doses separated by at least 28 days. For unvaccinated or undervaccinated children, consider advising families to hold off travel to high-risk countries, or understand the indications to vaccinate a child upon return.
Dr. Angelica DesPain is a pediatric emergency medicine fellow at Children’s National Medical Center in Washington. She said she has no relevant financial disclosures. Dr. Emily Willner is a pediatric emergency medicine attending at Children’s National Medical Center, and an assistant professor of pediatrics and emergency medicine at George Washington University, Washington. She has no relevant financial disclosures.
The mother of an 8-month-old calls your office and is hysterical. Her daughter has had cough for a few days with high fevers and now has developed a full body rash. She is worried about measles and is on her way to your office.
We are in the middle of a measles epidemic, there’s no denying it. Measles was declared eliminated in 2000, but reported cases in the United States have been on the rise, and are now at the highest number since 2014. Five months into 2019, there have been 839 reported cases as of May 13). Measles outbreaks (defined by the Centers for Disease Control and Prevention as three or more cases) have been reported in California, Georgia, Maryland, Michigan, New Jersey, New York, and Pennsylvania. When vaccination rates fall, it is easy for measles to spread. The virus is highly contagious in nonimmune people, because of its airborne spread and its persistence in the environment for hours.
First – is it really measles?
It can be difficult to distinguish the maculopapular rash of measles from similar rashes that occur with more benign viral illnesses. Adding to the challenge, the last major measles outbreak in the United States was over 2 decades ago, and many practicing pediatricians have never seen a single case. So, what clinical features can help distinguish measles from other febrile illnesses?
The prodromal phase of measles lasts approximately 2-4 days and children have high fevers (103°-105° F), anorexia, and malaise. Conjunctivitis, coryza, and cough develop during this phase, and precede any rash. Koplik spots appear during the prodromal phase, but are not seen in all cases. These spots are 1- to 3-mm blue-white lesions on an erythematous base on the buccal mucosa, classically opposite the first molar. The spots often slough once the rash appears. The rash appears 2-4 days after the onset of fever, and is initially maculopapular and blanching. The first lesions appear on the face and neck, and the rash spreads cranial to caudal, typically sparing palms and soles. After days 3-4, the rash will no longer blanch. High fevers persist for 2-4 more days with rash, ongoing respiratory symptoms, conjunctivitis, and pharyngitis. Note that the fever will persist even with development of the rash, unlike in roseola.
It is not only important to diagnosis measles from a public health standpoint, but also because measles can have severe complications, especially in infants and children under 5 years. During the 1989-1991 outbreak, the mortality rate was 2.2 deaths per 1,000 cases (J Infect Dis. 2004 May 1. doi: 10.1086/377694).
Six percent of patients develop pneumonia, which in infants and toddlers can lead to respiratory distress or failure requiring hospitalization. Pneumonia is responsible for 60% of measles deaths, according to the CDC “Pink Book,” Epidemiology and Prevention of Vaccine-Preventable Diseases, chapter 13 on measles, 13th Ed., 2015. Ocular complications include keratitis and corneal ulceration. Measles also can cause serious neurologic complications. Encephalitis, seen in 1 per 1,000 cases, usually arises several days after the rash and may present with seizure or encephalopathy. Acute disseminated encephalomyelitis (ADEM), an inflammatory demyelinating disease of the central nervous system, occurs in approximately 1 per 1,000 cases, typically presents during the recovery phase (1-2 weeks after rash), and can have long-term sequelae. Subacute sclerosing panencephalitis (SSPE) is a progressive and fatal neurodegenerative disorder, and presents 7-10 years after measles infection.
Should you transfer the patient to a hospital?
Unless there is a medical need for the child to be admitted, sending a patient with potential measles to the hospital is not necessary, and can cause exposure to a large group of medical personnel, and patients who cannot be vaccinated (such as infants, immunocompromised patients, and pregnant women). However, if there is concern for complications such as seizures, encephalitis, or pneumonia, then transfer is indicated. Call the accepting hospital in advance so the staff can prepare for the patient. During transfer, place a standard face mask on the patient and instruct the patient not to remove it.
For hospitals accepting a suspected measles case, meet the patient outside of the facility and ensure that the patient is wearing a standard face mask. All staff interacting with the patient should practice contact and airborne precautions (N95 respirator mask). Take the patient directly to an isolation room with negative airflow. Caution pregnant staff that they should not have contact with the patient.
Which diagnostic tests should you use?
Diagnosis can be made based on serum antibody tests (measles IgM and IgG), throat or urine viral cultures, and nasopharyngeal and throat specimen polymerase chain reaction (PCR) testing. The CDC recommends obtaining a serum sample for measles IgM testing and a throat swab for PCR in all suspected cases, but local health departments vary in their specific testing recommendations. Familiarize yourself with the tests recommended by your local department of health, and where they prefer testing on outpatients to be done. Confirmed measles should be reported to your department of health.
What are considerations for community pediatric offices?
Update families in emails to call ahead if they suspect measles. This way the office can prepare a room for the family, and have the family immediately brought back without exposing staff and other families in the waiting area. It may be more prudent to examine these children at the end of the clinic day as the virus can persist for up to 2 hours on fomites and in the air. Therefore, all waiting areas and shared air spaces (including those with shared air ducts) should be cleared for 2 hours after the patient leaves.
When should you provide prophylaxis after exposure?
A patient with suspected measles does not require immediate vaccination. If it is measles, it is already too late to vaccinate. If measles is ruled out, the child should follow the standard measles vaccination guidelines.
Individuals are contagious from 4 days before to 4 days after the rash appears.
If measles is confirmed, all people who are unvaccinated or undervaccinated and were exposed to the confirmed case during the contagious period should be vaccinated within 72 hours of exposure. Infants 6 months or older may safely receive the MMR vaccine. However, infants vaccinated with MMR before their first birthday must be vaccinated again at age 12-15 months (greater than 28 days after prior vaccine) and at 4-6 years. Immunoglobulin prophylaxis should be given intramuscularly in exposed infants ages birth to less than 6 months, and in those ages 6-12 months who present beyond the 72-hour window. Unvaccinated or undervaccinated, exposed individuals at high risk for complications from measles (immunocompromised, pregnant) also should receive immunoglobulin.
What should you tell traveling families?
Several countries have large, ongoing measles outbreaks, including Israel, Ukraine, and the Philippines. Before international travel, infants 6-11 months should receive one dose of MMR vaccine, and children 12 months and older need two doses separated by at least 28 days. For unvaccinated or undervaccinated children, consider advising families to hold off travel to high-risk countries, or understand the indications to vaccinate a child upon return.
Dr. Angelica DesPain is a pediatric emergency medicine fellow at Children’s National Medical Center in Washington. She said she has no relevant financial disclosures. Dr. Emily Willner is a pediatric emergency medicine attending at Children’s National Medical Center, and an assistant professor of pediatrics and emergency medicine at George Washington University, Washington. She has no relevant financial disclosures.
The mother of an 8-month-old calls your office and is hysterical. Her daughter has had cough for a few days with high fevers and now has developed a full body rash. She is worried about measles and is on her way to your office.
We are in the middle of a measles epidemic, there’s no denying it. Measles was declared eliminated in 2000, but reported cases in the United States have been on the rise, and are now at the highest number since 2014. Five months into 2019, there have been 839 reported cases as of May 13). Measles outbreaks (defined by the Centers for Disease Control and Prevention as three or more cases) have been reported in California, Georgia, Maryland, Michigan, New Jersey, New York, and Pennsylvania. When vaccination rates fall, it is easy for measles to spread. The virus is highly contagious in nonimmune people, because of its airborne spread and its persistence in the environment for hours.
First – is it really measles?
It can be difficult to distinguish the maculopapular rash of measles from similar rashes that occur with more benign viral illnesses. Adding to the challenge, the last major measles outbreak in the United States was over 2 decades ago, and many practicing pediatricians have never seen a single case. So, what clinical features can help distinguish measles from other febrile illnesses?
The prodromal phase of measles lasts approximately 2-4 days and children have high fevers (103°-105° F), anorexia, and malaise. Conjunctivitis, coryza, and cough develop during this phase, and precede any rash. Koplik spots appear during the prodromal phase, but are not seen in all cases. These spots are 1- to 3-mm blue-white lesions on an erythematous base on the buccal mucosa, classically opposite the first molar. The spots often slough once the rash appears. The rash appears 2-4 days after the onset of fever, and is initially maculopapular and blanching. The first lesions appear on the face and neck, and the rash spreads cranial to caudal, typically sparing palms and soles. After days 3-4, the rash will no longer blanch. High fevers persist for 2-4 more days with rash, ongoing respiratory symptoms, conjunctivitis, and pharyngitis. Note that the fever will persist even with development of the rash, unlike in roseola.
It is not only important to diagnosis measles from a public health standpoint, but also because measles can have severe complications, especially in infants and children under 5 years. During the 1989-1991 outbreak, the mortality rate was 2.2 deaths per 1,000 cases (J Infect Dis. 2004 May 1. doi: 10.1086/377694).
Six percent of patients develop pneumonia, which in infants and toddlers can lead to respiratory distress or failure requiring hospitalization. Pneumonia is responsible for 60% of measles deaths, according to the CDC “Pink Book,” Epidemiology and Prevention of Vaccine-Preventable Diseases, chapter 13 on measles, 13th Ed., 2015. Ocular complications include keratitis and corneal ulceration. Measles also can cause serious neurologic complications. Encephalitis, seen in 1 per 1,000 cases, usually arises several days after the rash and may present with seizure or encephalopathy. Acute disseminated encephalomyelitis (ADEM), an inflammatory demyelinating disease of the central nervous system, occurs in approximately 1 per 1,000 cases, typically presents during the recovery phase (1-2 weeks after rash), and can have long-term sequelae. Subacute sclerosing panencephalitis (SSPE) is a progressive and fatal neurodegenerative disorder, and presents 7-10 years after measles infection.
Should you transfer the patient to a hospital?
Unless there is a medical need for the child to be admitted, sending a patient with potential measles to the hospital is not necessary, and can cause exposure to a large group of medical personnel, and patients who cannot be vaccinated (such as infants, immunocompromised patients, and pregnant women). However, if there is concern for complications such as seizures, encephalitis, or pneumonia, then transfer is indicated. Call the accepting hospital in advance so the staff can prepare for the patient. During transfer, place a standard face mask on the patient and instruct the patient not to remove it.
For hospitals accepting a suspected measles case, meet the patient outside of the facility and ensure that the patient is wearing a standard face mask. All staff interacting with the patient should practice contact and airborne precautions (N95 respirator mask). Take the patient directly to an isolation room with negative airflow. Caution pregnant staff that they should not have contact with the patient.
Which diagnostic tests should you use?
Diagnosis can be made based on serum antibody tests (measles IgM and IgG), throat or urine viral cultures, and nasopharyngeal and throat specimen polymerase chain reaction (PCR) testing. The CDC recommends obtaining a serum sample for measles IgM testing and a throat swab for PCR in all suspected cases, but local health departments vary in their specific testing recommendations. Familiarize yourself with the tests recommended by your local department of health, and where they prefer testing on outpatients to be done. Confirmed measles should be reported to your department of health.
What are considerations for community pediatric offices?
Update families in emails to call ahead if they suspect measles. This way the office can prepare a room for the family, and have the family immediately brought back without exposing staff and other families in the waiting area. It may be more prudent to examine these children at the end of the clinic day as the virus can persist for up to 2 hours on fomites and in the air. Therefore, all waiting areas and shared air spaces (including those with shared air ducts) should be cleared for 2 hours after the patient leaves.
When should you provide prophylaxis after exposure?
A patient with suspected measles does not require immediate vaccination. If it is measles, it is already too late to vaccinate. If measles is ruled out, the child should follow the standard measles vaccination guidelines.
Individuals are contagious from 4 days before to 4 days after the rash appears.
If measles is confirmed, all people who are unvaccinated or undervaccinated and were exposed to the confirmed case during the contagious period should be vaccinated within 72 hours of exposure. Infants 6 months or older may safely receive the MMR vaccine. However, infants vaccinated with MMR before their first birthday must be vaccinated again at age 12-15 months (greater than 28 days after prior vaccine) and at 4-6 years. Immunoglobulin prophylaxis should be given intramuscularly in exposed infants ages birth to less than 6 months, and in those ages 6-12 months who present beyond the 72-hour window. Unvaccinated or undervaccinated, exposed individuals at high risk for complications from measles (immunocompromised, pregnant) also should receive immunoglobulin.
What should you tell traveling families?
Several countries have large, ongoing measles outbreaks, including Israel, Ukraine, and the Philippines. Before international travel, infants 6-11 months should receive one dose of MMR vaccine, and children 12 months and older need two doses separated by at least 28 days. For unvaccinated or undervaccinated children, consider advising families to hold off travel to high-risk countries, or understand the indications to vaccinate a child upon return.
Dr. Angelica DesPain is a pediatric emergency medicine fellow at Children’s National Medical Center in Washington. She said she has no relevant financial disclosures. Dr. Emily Willner is a pediatric emergency medicine attending at Children’s National Medical Center, and an assistant professor of pediatrics and emergency medicine at George Washington University, Washington. She has no relevant financial disclosures.
Evaluation, treatment of anxiety in children and adolescents with autism spectrum disorder
1 As ASD by definition involves deficits in communication and interaction, as well as restricted, repetitive patterns of behavior, interests, or activities, diagnosis and treatment of anxiety disorders in this population can present a significant challenge.
martinedoucet/E+/Getty Images
Clinical vignette
Sean is a 9-year-old boy in the fourth grade diagnosed with ASD. He is in a regular education classroom setting. Until this year, his grades have been above average. This year his mother has been getting calls from the teachers reporting that he is disruptive in class, and is having difficulty paying attention unless the subject relates to a specific interest of his. At home, his mother has been struggling to get him to do chores and homework, and even sitting at the dinner table is now a battle. He is significantly more irritable than usual. While he always preferred routines and familiar activities, deviations from them now trigger strong reactions and sometimes tantrums. He has started to insist on staying up late, and refuses to go to bed without his mother present. Notably his mother reports that she and Sean’s father recently separated, and that she believes he is very upset by this, although he refuses to talk about it.
Discussion
This case highlights the diagnostic complexity with which children with ASD may present. With the overlap between some of the core symptoms of ASD and anxiety, as well as the potential for other co-occurring disorders, a number of factors need to be explored before arriving at a treatment plan.
In evaluating behavior changes in children with ASD, I find it most helpful to start by looking for any medical or environmental factors. Medical problems such as illness or gastrointestinal difficulties may contribute to behavioral challenges and anxiety. Also, be sure to inquire if there are any precipitating events or change in the environment which might correlate with the change in behavior. In this case, we do have a situation – namely Sean’s parents separating – that may be contributing. While addressing Sean’s thoughts and feelings about this remains challenging, awareness of this factor certainly is important.
Understanding the educational setting and supports of a child with ASD is of significant importance. Academic challenges may result from learning or language difficulties, which can result in significant stress. While the vignette mentions that Sean’s grades had previously been above average, it is possible that increased complexity of material is contributing to his school struggles.
Next, it is worth looking at the question of whether Sean meets criteria for ADHD, which is estimated to occur in 30%-61% of people with ASD. In the case vignette, the mention of disruptions and attentional difficulties in the classroom warrant further investigation.
Finally, the question of whether insistence on routine, strong reactions to unfamiliar circumstances, disruptive behavior, and irritability meet criteria for an anxiety disorder is a complex one. Children with ASD may have difficulty communicating that they are anxious, making the behavioral observations of those around them especially important. An advantage pediatric primary care providers have in this circumstance is longitudinal experience with the child and family, which can help confirm whether the problem perceived as anxiety is a manifestation of core autism symptoms, or newer-onset phenomena. Assessing the severity and settings of the behavior also is necessary to guide treatment decisions. In the vignette, Sean’s irritability, acting out, and bedtime difficulties all are of relatively new onset, and occurring across multiple settings with significant functional consequences, making a diagnosis of an anxiety disorder the likely explanation.
As for treatment, cognitive behavioral therapy has been shown to be effective for anxiety in children with high functioning ASD.2 If a clinician with experience with this population is available, that certainly is preferred. If medication is being considered, there are no randomized controlled trials that have demonstrated efficacy of medication for anxiety specifically in children with co-occurring ASD. Treatment recommendations are taken from studies in typically developing children,3 where the SSRIs fluoxetine and sertraline have demonstrated efficacy in treatment of anxiety. When opting for pharmacotherapy in children with ASD, starting low, going slow, and carefully monitoring for side effects is recommended. Regardless of the method of treatment, a clear definition of the target symptoms ahead of time is critical for monitoring response and evaluating treatment effect.
Dr. Hoffnung is a pediatric psychiatrist at the University of Vermont Children’s Hospital and an assistant professor of psychiatry at the Robert Larner, M.D. College of Medicine at the University of Vermont, both in Burlington. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Clin Child Fam Psychol Rev. 2011 Sep;14(3):302-17.
2. Child Psychiatry Hum Dev. 2015 Aug;46(4):533-47.
3. Pediatrics. 2016 Feb;137 Suppl 2:S115-23.
1 As ASD by definition involves deficits in communication and interaction, as well as restricted, repetitive patterns of behavior, interests, or activities, diagnosis and treatment of anxiety disorders in this population can present a significant challenge.
martinedoucet/E+/Getty Images
Clinical vignette
Sean is a 9-year-old boy in the fourth grade diagnosed with ASD. He is in a regular education classroom setting. Until this year, his grades have been above average. This year his mother has been getting calls from the teachers reporting that he is disruptive in class, and is having difficulty paying attention unless the subject relates to a specific interest of his. At home, his mother has been struggling to get him to do chores and homework, and even sitting at the dinner table is now a battle. He is significantly more irritable than usual. While he always preferred routines and familiar activities, deviations from them now trigger strong reactions and sometimes tantrums. He has started to insist on staying up late, and refuses to go to bed without his mother present. Notably his mother reports that she and Sean’s father recently separated, and that she believes he is very upset by this, although he refuses to talk about it.
Discussion
This case highlights the diagnostic complexity with which children with ASD may present. With the overlap between some of the core symptoms of ASD and anxiety, as well as the potential for other co-occurring disorders, a number of factors need to be explored before arriving at a treatment plan.
In evaluating behavior changes in children with ASD, I find it most helpful to start by looking for any medical or environmental factors. Medical problems such as illness or gastrointestinal difficulties may contribute to behavioral challenges and anxiety. Also, be sure to inquire if there are any precipitating events or change in the environment which might correlate with the change in behavior. In this case, we do have a situation – namely Sean’s parents separating – that may be contributing. While addressing Sean’s thoughts and feelings about this remains challenging, awareness of this factor certainly is important.
Understanding the educational setting and supports of a child with ASD is of significant importance. Academic challenges may result from learning or language difficulties, which can result in significant stress. While the vignette mentions that Sean’s grades had previously been above average, it is possible that increased complexity of material is contributing to his school struggles.
Next, it is worth looking at the question of whether Sean meets criteria for ADHD, which is estimated to occur in 30%-61% of people with ASD. In the case vignette, the mention of disruptions and attentional difficulties in the classroom warrant further investigation.
Finally, the question of whether insistence on routine, strong reactions to unfamiliar circumstances, disruptive behavior, and irritability meet criteria for an anxiety disorder is a complex one. Children with ASD may have difficulty communicating that they are anxious, making the behavioral observations of those around them especially important. An advantage pediatric primary care providers have in this circumstance is longitudinal experience with the child and family, which can help confirm whether the problem perceived as anxiety is a manifestation of core autism symptoms, or newer-onset phenomena. Assessing the severity and settings of the behavior also is necessary to guide treatment decisions. In the vignette, Sean’s irritability, acting out, and bedtime difficulties all are of relatively new onset, and occurring across multiple settings with significant functional consequences, making a diagnosis of an anxiety disorder the likely explanation.
As for treatment, cognitive behavioral therapy has been shown to be effective for anxiety in children with high functioning ASD.2 If a clinician with experience with this population is available, that certainly is preferred. If medication is being considered, there are no randomized controlled trials that have demonstrated efficacy of medication for anxiety specifically in children with co-occurring ASD. Treatment recommendations are taken from studies in typically developing children,3 where the SSRIs fluoxetine and sertraline have demonstrated efficacy in treatment of anxiety. When opting for pharmacotherapy in children with ASD, starting low, going slow, and carefully monitoring for side effects is recommended. Regardless of the method of treatment, a clear definition of the target symptoms ahead of time is critical for monitoring response and evaluating treatment effect.
Dr. Hoffnung is a pediatric psychiatrist at the University of Vermont Children’s Hospital and an assistant professor of psychiatry at the Robert Larner, M.D. College of Medicine at the University of Vermont, both in Burlington. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Clin Child Fam Psychol Rev. 2011 Sep;14(3):302-17.
2. Child Psychiatry Hum Dev. 2015 Aug;46(4):533-47.
3. Pediatrics. 2016 Feb;137 Suppl 2:S115-23.
1 As ASD by definition involves deficits in communication and interaction, as well as restricted, repetitive patterns of behavior, interests, or activities, diagnosis and treatment of anxiety disorders in this population can present a significant challenge.
martinedoucet/E+/Getty Images
Clinical vignette
Sean is a 9-year-old boy in the fourth grade diagnosed with ASD. He is in a regular education classroom setting. Until this year, his grades have been above average. This year his mother has been getting calls from the teachers reporting that he is disruptive in class, and is having difficulty paying attention unless the subject relates to a specific interest of his. At home, his mother has been struggling to get him to do chores and homework, and even sitting at the dinner table is now a battle. He is significantly more irritable than usual. While he always preferred routines and familiar activities, deviations from them now trigger strong reactions and sometimes tantrums. He has started to insist on staying up late, and refuses to go to bed without his mother present. Notably his mother reports that she and Sean’s father recently separated, and that she believes he is very upset by this, although he refuses to talk about it.
Discussion
This case highlights the diagnostic complexity with which children with ASD may present. With the overlap between some of the core symptoms of ASD and anxiety, as well as the potential for other co-occurring disorders, a number of factors need to be explored before arriving at a treatment plan.
In evaluating behavior changes in children with ASD, I find it most helpful to start by looking for any medical or environmental factors. Medical problems such as illness or gastrointestinal difficulties may contribute to behavioral challenges and anxiety. Also, be sure to inquire if there are any precipitating events or change in the environment which might correlate with the change in behavior. In this case, we do have a situation – namely Sean’s parents separating – that may be contributing. While addressing Sean’s thoughts and feelings about this remains challenging, awareness of this factor certainly is important.
Understanding the educational setting and supports of a child with ASD is of significant importance. Academic challenges may result from learning or language difficulties, which can result in significant stress. While the vignette mentions that Sean’s grades had previously been above average, it is possible that increased complexity of material is contributing to his school struggles.
Next, it is worth looking at the question of whether Sean meets criteria for ADHD, which is estimated to occur in 30%-61% of people with ASD. In the case vignette, the mention of disruptions and attentional difficulties in the classroom warrant further investigation.
Finally, the question of whether insistence on routine, strong reactions to unfamiliar circumstances, disruptive behavior, and irritability meet criteria for an anxiety disorder is a complex one. Children with ASD may have difficulty communicating that they are anxious, making the behavioral observations of those around them especially important. An advantage pediatric primary care providers have in this circumstance is longitudinal experience with the child and family, which can help confirm whether the problem perceived as anxiety is a manifestation of core autism symptoms, or newer-onset phenomena. Assessing the severity and settings of the behavior also is necessary to guide treatment decisions. In the vignette, Sean’s irritability, acting out, and bedtime difficulties all are of relatively new onset, and occurring across multiple settings with significant functional consequences, making a diagnosis of an anxiety disorder the likely explanation.
As for treatment, cognitive behavioral therapy has been shown to be effective for anxiety in children with high functioning ASD.2 If a clinician with experience with this population is available, that certainly is preferred. If medication is being considered, there are no randomized controlled trials that have demonstrated efficacy of medication for anxiety specifically in children with co-occurring ASD. Treatment recommendations are taken from studies in typically developing children,3 where the SSRIs fluoxetine and sertraline have demonstrated efficacy in treatment of anxiety. When opting for pharmacotherapy in children with ASD, starting low, going slow, and carefully monitoring for side effects is recommended. Regardless of the method of treatment, a clear definition of the target symptoms ahead of time is critical for monitoring response and evaluating treatment effect.
Dr. Hoffnung is a pediatric psychiatrist at the University of Vermont Children’s Hospital and an assistant professor of psychiatry at the Robert Larner, M.D. College of Medicine at the University of Vermont, both in Burlington. He said he had no relevant financial disclosures. Email him at [email protected].
References
1. Clin Child Fam Psychol Rev. 2011 Sep;14(3):302-17.
2. Child Psychiatry Hum Dev. 2015 Aug;46(4):533-47.
3. Pediatrics. 2016 Feb;137 Suppl 2:S115-23.
Is there an epidemic of anxiety and depression among today’s adolescents?
It seems that every week there are fresh headlines about a mental health crisis in children and adolescents, reporting exploding rates of severe anxiety and depression in youth. These reports raise the question of whether or not there has been a significant change in their incidence: Are more children developing depressive and anxiety disorders? Are they having greater difficulty accessing care? Are the disorders more severe than they were in the past? Or are young people failing to develop appropriate skills to manage anxiety, sadness, and other forms of distress that are a normal (if unpleasant) part of life? These are important questions, as they will help us to advocate for the proper services to address the public health challenge that underlies this “epidemic.”

What do the data show?
It is important to start by noting that epidemiologic data on child psychiatry in the United States are not as robust as we might like. It was only in 1999 that the Surgeon General’s Report on Mental Health articulated that there was a need for a more systematic approach to collecting epidemiologic data on psychiatric illness in children and adolescents. At that time, the consensus was that approximately one in five children would develop a psychiatric illness by the age of 18 and that approximately 5% of all children would experience a severe or persistent mental illness.1 In the 2 decades since then there have been expanded efforts to collect data, including the addition of an adolescent supplement to the National Comorbidity Survey sponsored by the National Institute of Mental Health, although our current estimates still are based on representative surveys of thousands of U.S. children and teenagers, often with questionnaires filled out by their parents. Thus, we may have overestimates of some behavioral disorders that are obvious and of concern to parents or underestimates of certain internalizing disorders such as depression that can remain unstated and contained in the mind of the adolescent. And even with accurate current estimates, our ability to make statements about trends or changes in rates of disease is limited by the very short period of time in which we have been studying these disease rates in U.S. youth, some changes in definitions, and the unknown impact of increasing recognition rather than true change in incidence.
What is unequivocally clear is that psychiatric illnesses usually present in youth and that these illnesses are among the most common illnesses of youth. Current estimates are that nearly one in four young people will have a psychiatric illness (by The Diagnostic and Statistical Manual of Mental Disorders [DSM], Fifth Edition criteria) by the time they turn 18,2 although only 10% of youth will experience an illness that meets the Substance Abuse and Mental Health Services Administration criteria for a serious emotional disturbance, or one that has a substantial impact on a child’s ability to function socially, emotionally, and academically.3
While it once was believed that children did not experience psychiatric illness, we now know that the majority of psychiatric illnesses present during childhood, adolescence, and young adulthood. The Centers for Disease Control and Prevention estimates that 50% of lifetime psychiatric illness has presented by the age of 15 years and 75% by the age of 24. Only one-quarter of all lifetime psychiatric illnesses emerge in full adulthood, or after the age of 24. Early diagnosis and treatment can make a significant difference in the overall impact of serious illnesses such as schizophrenia and bipolar disorder. We also can state with confidence that anxiety disorders are the most common psychiatric illnesses of youth, making up over 30% of all diagnoses, followed by disorders of behavior (19%), mood (14%), and then substance use (11%).4 Even compared with asthma (with a prevalence of approximately 11%), widely considered to be among the most common disease of childhood, psychiatric illnesses are the most common in youth.
The question then is whether these numbers are changing. The National Comorbidity Survey conducted in 2014 found that the incidence of major depressive episodes in adolescents had increased significantly between 2005 and 2014, from 9% to 11%.5 This is a survey of nearly 200,000 youth across the United States, interviewed by phone with a structured questionnaire assessing their (self-reported) DSM criteria for a major depressive episode, along with other illnesses. During this time frame, access to specialty mental health providers increased among adolescents, alongside their rate of use of psychiatric medications and inpatient hospitalization.
In Europe, where they have more robust epidemiological data, there also has been a public perception of an increase in depression in adolescents. Studies there have suggested that prevalence rates have not changed significantly, and that the problem actually may be a function of a growing population, greater public awareness, and higher rates of psychological distress.6
In the United States, it is difficult to place the prevalence rates in a meaningful context, given the shorter time frame during which we have been following these rates in young people. It is worth highlighting that although the rates at which young people are gaining access to mental health clinicians, being prescribed medications, and being admitted to psychiatric hospitals all have increased, there has not been an associated decrease in the rate of illness or in the severity of symptoms. It certainly is possible that the increase in use of services by youth is being driven by the increased prevalence of this diagnosis, or it may be that other factors, such as those detailed in international studies, are driving this increase in the incidence of depression.
What about the suicide rate?
Another statistic that addressed the question of whether there may be an epidemic of anxiety and depression in adolescents is the recent increase in the suicide rate. While the rate of completed suicide in 15- to 24-year-olds has been trending upward over the last decade, it is worth noting that this phenomenon appears to be occurring across age groups and is not isolated to adolescents. While adolescents may have a unique underlying set of issues driving the increase, it also may be that factors affecting the entire population (access to firearms, the epidemic of opioid addiction) may be at the core of this worrisome trend.
What about the role of stress?
It is worth noting that there is evidence of an increased rate of psychological distress in adolescents and young adults separate from any increase in the rate of psychiatric illness. Surveys of adolescents in high school and entering college demonstrate higher self-reported rates of severe stress and anxiety. One survey from the American Psychological Association from August 2018 found teenagers reporting higher levels of stress and related sadness and anxiety than the levels among the adults who were surveyed. So more young people are struggling with feelings of anxiety and sadness, without necessarily meeting criteria for a psychiatric illness. This suggests that levels of external stressors may have increased, or that the establishment of healthy coping skills has somehow been compromised in young people, or both.
What can you do as a clinician?
While the broader question of whether actual incidence rates of depression are on the rise will not be settled any time soon, when a patient of yours complains of high levels of stress, anxiety, or feelings of depression, it is very possible that the individual has a psychiatric diagnosis. A quick screening evaluation, using a questionnaire such as the Pediatric Symptom Checklist and/or a brief interview, can indicate if the patient may benefit from a referral.
In addition, all children, including those who have a psychiatric diagnosis, will benefit from a calm, patient, supportive adult who is interested in their distress. It would be very helpful if you are ready to talk about healthy coping skills, and how they are developed over time and only in the setting of actually struggling with some adversity. Help them frame their source of stress as a challenge rather than a threat. Help them identify their meaningful supports, particularly adults who know them well, and offer concrete and practical advice and motivation. And remind them about how self-care is essential to managing the normal stress of adolescence. Have handouts (or virtual ones) ready on good sleep hygiene, the value of exercise, and fact-based nutritional guidance. Offer strategies to manage screen time so that it is a recharging break and not a time sink. Support their identification of other strategies to decompress and manage stress: Are they recharged by time with friends? Exercise? Playing music? Listening to music? Playing video games? They should be building their personalized list, and it should include more active as well as passive strategies. Educate them about the risks of using drugs and alcohol “to relax,” or only having one way of unwinding. Educate your patients and parents about the special value of a mindfulness practice, whether meditation, yoga, or any activity in which they practice a nonjudgmental observation and acceptance of strong emotions.
Accurate prevalence rates can help us consider the statistical probability of a psychiatric diagnosis. By talking with your patients about stressful feelings, you can consider the individual need for a fuller psychiatric evaluation while also helping them reframe their approach to stress to one that is more empowering, adaptive, and healthy.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Mental Health: A Report of the Surgeon General, National Institutes of Mental Health (1999).
2. Prevalence of psychiatric disorders in childhood and adolescence, in “Mental Health Services: A Public Health Perspective,” 2nd ed. (Oxford, UK: Oxford University Press; 2004, pp. 111-28).
3. Public Health Rep. 2006 May-Jun;121(3):303-10.
4. J Am Acad Child Adolesc Psychiatry. 2010 Oct;49(10):980-9.
5. Pediatrics. 2016 Dec;138(6):e20161878.
6. Depress Anxiety. 2014 Jun;31(6):506-16.
It seems that every week there are fresh headlines about a mental health crisis in children and adolescents, reporting exploding rates of severe anxiety and depression in youth. These reports raise the question of whether or not there has been a significant change in their incidence: Are more children developing depressive and anxiety disorders? Are they having greater difficulty accessing care? Are the disorders more severe than they were in the past? Or are young people failing to develop appropriate skills to manage anxiety, sadness, and other forms of distress that are a normal (if unpleasant) part of life? These are important questions, as they will help us to advocate for the proper services to address the public health challenge that underlies this “epidemic.”

What do the data show?
It is important to start by noting that epidemiologic data on child psychiatry in the United States are not as robust as we might like. It was only in 1999 that the Surgeon General’s Report on Mental Health articulated that there was a need for a more systematic approach to collecting epidemiologic data on psychiatric illness in children and adolescents. At that time, the consensus was that approximately one in five children would develop a psychiatric illness by the age of 18 and that approximately 5% of all children would experience a severe or persistent mental illness.1 In the 2 decades since then there have been expanded efforts to collect data, including the addition of an adolescent supplement to the National Comorbidity Survey sponsored by the National Institute of Mental Health, although our current estimates still are based on representative surveys of thousands of U.S. children and teenagers, often with questionnaires filled out by their parents. Thus, we may have overestimates of some behavioral disorders that are obvious and of concern to parents or underestimates of certain internalizing disorders such as depression that can remain unstated and contained in the mind of the adolescent. And even with accurate current estimates, our ability to make statements about trends or changes in rates of disease is limited by the very short period of time in which we have been studying these disease rates in U.S. youth, some changes in definitions, and the unknown impact of increasing recognition rather than true change in incidence.
What is unequivocally clear is that psychiatric illnesses usually present in youth and that these illnesses are among the most common illnesses of youth. Current estimates are that nearly one in four young people will have a psychiatric illness (by The Diagnostic and Statistical Manual of Mental Disorders [DSM], Fifth Edition criteria) by the time they turn 18,2 although only 10% of youth will experience an illness that meets the Substance Abuse and Mental Health Services Administration criteria for a serious emotional disturbance, or one that has a substantial impact on a child’s ability to function socially, emotionally, and academically.3
While it once was believed that children did not experience psychiatric illness, we now know that the majority of psychiatric illnesses present during childhood, adolescence, and young adulthood. The Centers for Disease Control and Prevention estimates that 50% of lifetime psychiatric illness has presented by the age of 15 years and 75% by the age of 24. Only one-quarter of all lifetime psychiatric illnesses emerge in full adulthood, or after the age of 24. Early diagnosis and treatment can make a significant difference in the overall impact of serious illnesses such as schizophrenia and bipolar disorder. We also can state with confidence that anxiety disorders are the most common psychiatric illnesses of youth, making up over 30% of all diagnoses, followed by disorders of behavior (19%), mood (14%), and then substance use (11%).4 Even compared with asthma (with a prevalence of approximately 11%), widely considered to be among the most common disease of childhood, psychiatric illnesses are the most common in youth.
The question then is whether these numbers are changing. The National Comorbidity Survey conducted in 2014 found that the incidence of major depressive episodes in adolescents had increased significantly between 2005 and 2014, from 9% to 11%.5 This is a survey of nearly 200,000 youth across the United States, interviewed by phone with a structured questionnaire assessing their (self-reported) DSM criteria for a major depressive episode, along with other illnesses. During this time frame, access to specialty mental health providers increased among adolescents, alongside their rate of use of psychiatric medications and inpatient hospitalization.
In Europe, where they have more robust epidemiological data, there also has been a public perception of an increase in depression in adolescents. Studies there have suggested that prevalence rates have not changed significantly, and that the problem actually may be a function of a growing population, greater public awareness, and higher rates of psychological distress.6
In the United States, it is difficult to place the prevalence rates in a meaningful context, given the shorter time frame during which we have been following these rates in young people. It is worth highlighting that although the rates at which young people are gaining access to mental health clinicians, being prescribed medications, and being admitted to psychiatric hospitals all have increased, there has not been an associated decrease in the rate of illness or in the severity of symptoms. It certainly is possible that the increase in use of services by youth is being driven by the increased prevalence of this diagnosis, or it may be that other factors, such as those detailed in international studies, are driving this increase in the incidence of depression.
What about the suicide rate?
Another statistic that addressed the question of whether there may be an epidemic of anxiety and depression in adolescents is the recent increase in the suicide rate. While the rate of completed suicide in 15- to 24-year-olds has been trending upward over the last decade, it is worth noting that this phenomenon appears to be occurring across age groups and is not isolated to adolescents. While adolescents may have a unique underlying set of issues driving the increase, it also may be that factors affecting the entire population (access to firearms, the epidemic of opioid addiction) may be at the core of this worrisome trend.
What about the role of stress?
It is worth noting that there is evidence of an increased rate of psychological distress in adolescents and young adults separate from any increase in the rate of psychiatric illness. Surveys of adolescents in high school and entering college demonstrate higher self-reported rates of severe stress and anxiety. One survey from the American Psychological Association from August 2018 found teenagers reporting higher levels of stress and related sadness and anxiety than the levels among the adults who were surveyed. So more young people are struggling with feelings of anxiety and sadness, without necessarily meeting criteria for a psychiatric illness. This suggests that levels of external stressors may have increased, or that the establishment of healthy coping skills has somehow been compromised in young people, or both.
What can you do as a clinician?
While the broader question of whether actual incidence rates of depression are on the rise will not be settled any time soon, when a patient of yours complains of high levels of stress, anxiety, or feelings of depression, it is very possible that the individual has a psychiatric diagnosis. A quick screening evaluation, using a questionnaire such as the Pediatric Symptom Checklist and/or a brief interview, can indicate if the patient may benefit from a referral.
In addition, all children, including those who have a psychiatric diagnosis, will benefit from a calm, patient, supportive adult who is interested in their distress. It would be very helpful if you are ready to talk about healthy coping skills, and how they are developed over time and only in the setting of actually struggling with some adversity. Help them frame their source of stress as a challenge rather than a threat. Help them identify their meaningful supports, particularly adults who know them well, and offer concrete and practical advice and motivation. And remind them about how self-care is essential to managing the normal stress of adolescence. Have handouts (or virtual ones) ready on good sleep hygiene, the value of exercise, and fact-based nutritional guidance. Offer strategies to manage screen time so that it is a recharging break and not a time sink. Support their identification of other strategies to decompress and manage stress: Are they recharged by time with friends? Exercise? Playing music? Listening to music? Playing video games? They should be building their personalized list, and it should include more active as well as passive strategies. Educate them about the risks of using drugs and alcohol “to relax,” or only having one way of unwinding. Educate your patients and parents about the special value of a mindfulness practice, whether meditation, yoga, or any activity in which they practice a nonjudgmental observation and acceptance of strong emotions.
Accurate prevalence rates can help us consider the statistical probability of a psychiatric diagnosis. By talking with your patients about stressful feelings, you can consider the individual need for a fuller psychiatric evaluation while also helping them reframe their approach to stress to one that is more empowering, adaptive, and healthy.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Mental Health: A Report of the Surgeon General, National Institutes of Mental Health (1999).
2. Prevalence of psychiatric disorders in childhood and adolescence, in “Mental Health Services: A Public Health Perspective,” 2nd ed. (Oxford, UK: Oxford University Press; 2004, pp. 111-28).
3. Public Health Rep. 2006 May-Jun;121(3):303-10.
4. J Am Acad Child Adolesc Psychiatry. 2010 Oct;49(10):980-9.
5. Pediatrics. 2016 Dec;138(6):e20161878.
6. Depress Anxiety. 2014 Jun;31(6):506-16.
It seems that every week there are fresh headlines about a mental health crisis in children and adolescents, reporting exploding rates of severe anxiety and depression in youth. These reports raise the question of whether or not there has been a significant change in their incidence: Are more children developing depressive and anxiety disorders? Are they having greater difficulty accessing care? Are the disorders more severe than they were in the past? Or are young people failing to develop appropriate skills to manage anxiety, sadness, and other forms of distress that are a normal (if unpleasant) part of life? These are important questions, as they will help us to advocate for the proper services to address the public health challenge that underlies this “epidemic.”

What do the data show?
It is important to start by noting that epidemiologic data on child psychiatry in the United States are not as robust as we might like. It was only in 1999 that the Surgeon General’s Report on Mental Health articulated that there was a need for a more systematic approach to collecting epidemiologic data on psychiatric illness in children and adolescents. At that time, the consensus was that approximately one in five children would develop a psychiatric illness by the age of 18 and that approximately 5% of all children would experience a severe or persistent mental illness.1 In the 2 decades since then there have been expanded efforts to collect data, including the addition of an adolescent supplement to the National Comorbidity Survey sponsored by the National Institute of Mental Health, although our current estimates still are based on representative surveys of thousands of U.S. children and teenagers, often with questionnaires filled out by their parents. Thus, we may have overestimates of some behavioral disorders that are obvious and of concern to parents or underestimates of certain internalizing disorders such as depression that can remain unstated and contained in the mind of the adolescent. And even with accurate current estimates, our ability to make statements about trends or changes in rates of disease is limited by the very short period of time in which we have been studying these disease rates in U.S. youth, some changes in definitions, and the unknown impact of increasing recognition rather than true change in incidence.
What is unequivocally clear is that psychiatric illnesses usually present in youth and that these illnesses are among the most common illnesses of youth. Current estimates are that nearly one in four young people will have a psychiatric illness (by The Diagnostic and Statistical Manual of Mental Disorders [DSM], Fifth Edition criteria) by the time they turn 18,2 although only 10% of youth will experience an illness that meets the Substance Abuse and Mental Health Services Administration criteria for a serious emotional disturbance, or one that has a substantial impact on a child’s ability to function socially, emotionally, and academically.3
While it once was believed that children did not experience psychiatric illness, we now know that the majority of psychiatric illnesses present during childhood, adolescence, and young adulthood. The Centers for Disease Control and Prevention estimates that 50% of lifetime psychiatric illness has presented by the age of 15 years and 75% by the age of 24. Only one-quarter of all lifetime psychiatric illnesses emerge in full adulthood, or after the age of 24. Early diagnosis and treatment can make a significant difference in the overall impact of serious illnesses such as schizophrenia and bipolar disorder. We also can state with confidence that anxiety disorders are the most common psychiatric illnesses of youth, making up over 30% of all diagnoses, followed by disorders of behavior (19%), mood (14%), and then substance use (11%).4 Even compared with asthma (with a prevalence of approximately 11%), widely considered to be among the most common disease of childhood, psychiatric illnesses are the most common in youth.
The question then is whether these numbers are changing. The National Comorbidity Survey conducted in 2014 found that the incidence of major depressive episodes in adolescents had increased significantly between 2005 and 2014, from 9% to 11%.5 This is a survey of nearly 200,000 youth across the United States, interviewed by phone with a structured questionnaire assessing their (self-reported) DSM criteria for a major depressive episode, along with other illnesses. During this time frame, access to specialty mental health providers increased among adolescents, alongside their rate of use of psychiatric medications and inpatient hospitalization.
In Europe, where they have more robust epidemiological data, there also has been a public perception of an increase in depression in adolescents. Studies there have suggested that prevalence rates have not changed significantly, and that the problem actually may be a function of a growing population, greater public awareness, and higher rates of psychological distress.6
In the United States, it is difficult to place the prevalence rates in a meaningful context, given the shorter time frame during which we have been following these rates in young people. It is worth highlighting that although the rates at which young people are gaining access to mental health clinicians, being prescribed medications, and being admitted to psychiatric hospitals all have increased, there has not been an associated decrease in the rate of illness or in the severity of symptoms. It certainly is possible that the increase in use of services by youth is being driven by the increased prevalence of this diagnosis, or it may be that other factors, such as those detailed in international studies, are driving this increase in the incidence of depression.
What about the suicide rate?
Another statistic that addressed the question of whether there may be an epidemic of anxiety and depression in adolescents is the recent increase in the suicide rate. While the rate of completed suicide in 15- to 24-year-olds has been trending upward over the last decade, it is worth noting that this phenomenon appears to be occurring across age groups and is not isolated to adolescents. While adolescents may have a unique underlying set of issues driving the increase, it also may be that factors affecting the entire population (access to firearms, the epidemic of opioid addiction) may be at the core of this worrisome trend.
What about the role of stress?
It is worth noting that there is evidence of an increased rate of psychological distress in adolescents and young adults separate from any increase in the rate of psychiatric illness. Surveys of adolescents in high school and entering college demonstrate higher self-reported rates of severe stress and anxiety. One survey from the American Psychological Association from August 2018 found teenagers reporting higher levels of stress and related sadness and anxiety than the levels among the adults who were surveyed. So more young people are struggling with feelings of anxiety and sadness, without necessarily meeting criteria for a psychiatric illness. This suggests that levels of external stressors may have increased, or that the establishment of healthy coping skills has somehow been compromised in young people, or both.
What can you do as a clinician?
While the broader question of whether actual incidence rates of depression are on the rise will not be settled any time soon, when a patient of yours complains of high levels of stress, anxiety, or feelings of depression, it is very possible that the individual has a psychiatric diagnosis. A quick screening evaluation, using a questionnaire such as the Pediatric Symptom Checklist and/or a brief interview, can indicate if the patient may benefit from a referral.
In addition, all children, including those who have a psychiatric diagnosis, will benefit from a calm, patient, supportive adult who is interested in their distress. It would be very helpful if you are ready to talk about healthy coping skills, and how they are developed over time and only in the setting of actually struggling with some adversity. Help them frame their source of stress as a challenge rather than a threat. Help them identify their meaningful supports, particularly adults who know them well, and offer concrete and practical advice and motivation. And remind them about how self-care is essential to managing the normal stress of adolescence. Have handouts (or virtual ones) ready on good sleep hygiene, the value of exercise, and fact-based nutritional guidance. Offer strategies to manage screen time so that it is a recharging break and not a time sink. Support their identification of other strategies to decompress and manage stress: Are they recharged by time with friends? Exercise? Playing music? Listening to music? Playing video games? They should be building their personalized list, and it should include more active as well as passive strategies. Educate them about the risks of using drugs and alcohol “to relax,” or only having one way of unwinding. Educate your patients and parents about the special value of a mindfulness practice, whether meditation, yoga, or any activity in which they practice a nonjudgmental observation and acceptance of strong emotions.
Accurate prevalence rates can help us consider the statistical probability of a psychiatric diagnosis. By talking with your patients about stressful feelings, you can consider the individual need for a fuller psychiatric evaluation while also helping them reframe their approach to stress to one that is more empowering, adaptive, and healthy.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
References
1. Mental Health: A Report of the Surgeon General, National Institutes of Mental Health (1999).
2. Prevalence of psychiatric disorders in childhood and adolescence, in “Mental Health Services: A Public Health Perspective,” 2nd ed. (Oxford, UK: Oxford University Press; 2004, pp. 111-28).
3. Public Health Rep. 2006 May-Jun;121(3):303-10.
4. J Am Acad Child Adolesc Psychiatry. 2010 Oct;49(10):980-9.
5. Pediatrics. 2016 Dec;138(6):e20161878.
6. Depress Anxiety. 2014 Jun;31(6):506-16.
A breath of objectivity
How many minutes do you spend each day trying to coax new parents off the guilt train? They have delayed their childbearing until they felt comfortable economically and emotionally ready to raise a child. Convinced that up to this point they have done enough correctly to be considered successful, they see no reason that they won’t be able to tackle parenthood just as easily. Their black lab is a model of obedience. Housebreaking him was a breeze. They are skilled at using the Internet and social media to gather the information they will need for raising a child.
However, at some point in the first 72 hours after the birth of their child, most parents are going to hit the wall of reality. It may be that breastfeeding doesn’t work as well their cousin told them it would or simply that babies cry, often for no discernible reason. Desperately wanting to do what’s right for their child, guilt creeps in as the little failures and fatigue begin to accumulate.
In their search for answers, new parents naturally come to us as pediatricians and family practitioners for the facts, but they also will search the Internet, talk to lactation consultants, and be bombarded by unsolicited advice from family members and neighbors. Every source they turn to, including physicians, will be filtered through its own bias.
I recently came across the most sensible advice for new parents I have read in a long time, and it came not from a pediatrician but from an economics professor at Brown University. Emily Oster, PhD, writing in the New York Times, examines the available data on the topics of breastfeeding, sleep training, and parents working out of the home with the objectivity of an economist and the sensitivity of a mother who has been there and done that (“The Data All Guilt-Ridden Parents Need,” New York Times, April 19, 2019).
For example, she observes that many of the benefits of breastfeeding are supported by some evidence, “just not always especially good evidence. And even when the evidence is good, the benefits are smaller than many people realize.” She points out that “most studies of breastfeeding are biased by the fact that women who breastfeed are typically different from those who do not.” I will leave it to you to read her full discussion that includes a comparison of random trials versus observational studies. But she concludes that, if one relies on only good evidence, the only demonstrable benefit of breastfeeding is for mothers who nurse longer than 12 months who may have a 20%-30% decrease in breast cancer risk.
Using the same kind of careful analysis, Dr. Oster finds that sleep training may have a short-term benefit for parents who will have improved sleep and less maternal depression, but in the long run children who were sleep trained were no different than those that weren’t.
She also finds that, when it comes to the “optimal configuration of adult work hours” for a household, there is “no compelling evidence that proves that having a stay-at-home parent affects child outcomes, positively or negatively.” It is up to each household what works best for all it members, not just the child.
I found it particularly helpful as a practitioner who has often felt shackled, or at least disadvantaged, by the American Academy of Pediatrics’ overly simplistic and sometimes biased recommendations on issues that send my patients’ parents on unfortunate and avoidable guilt trips.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
How many minutes do you spend each day trying to coax new parents off the guilt train? They have delayed their childbearing until they felt comfortable economically and emotionally ready to raise a child. Convinced that up to this point they have done enough correctly to be considered successful, they see no reason that they won’t be able to tackle parenthood just as easily. Their black lab is a model of obedience. Housebreaking him was a breeze. They are skilled at using the Internet and social media to gather the information they will need for raising a child.
However, at some point in the first 72 hours after the birth of their child, most parents are going to hit the wall of reality. It may be that breastfeeding doesn’t work as well their cousin told them it would or simply that babies cry, often for no discernible reason. Desperately wanting to do what’s right for their child, guilt creeps in as the little failures and fatigue begin to accumulate.
In their search for answers, new parents naturally come to us as pediatricians and family practitioners for the facts, but they also will search the Internet, talk to lactation consultants, and be bombarded by unsolicited advice from family members and neighbors. Every source they turn to, including physicians, will be filtered through its own bias.
I recently came across the most sensible advice for new parents I have read in a long time, and it came not from a pediatrician but from an economics professor at Brown University. Emily Oster, PhD, writing in the New York Times, examines the available data on the topics of breastfeeding, sleep training, and parents working out of the home with the objectivity of an economist and the sensitivity of a mother who has been there and done that (“The Data All Guilt-Ridden Parents Need,” New York Times, April 19, 2019).
For example, she observes that many of the benefits of breastfeeding are supported by some evidence, “just not always especially good evidence. And even when the evidence is good, the benefits are smaller than many people realize.” She points out that “most studies of breastfeeding are biased by the fact that women who breastfeed are typically different from those who do not.” I will leave it to you to read her full discussion that includes a comparison of random trials versus observational studies. But she concludes that, if one relies on only good evidence, the only demonstrable benefit of breastfeeding is for mothers who nurse longer than 12 months who may have a 20%-30% decrease in breast cancer risk.
Using the same kind of careful analysis, Dr. Oster finds that sleep training may have a short-term benefit for parents who will have improved sleep and less maternal depression, but in the long run children who were sleep trained were no different than those that weren’t.
She also finds that, when it comes to the “optimal configuration of adult work hours” for a household, there is “no compelling evidence that proves that having a stay-at-home parent affects child outcomes, positively or negatively.” It is up to each household what works best for all it members, not just the child.
I found it particularly helpful as a practitioner who has often felt shackled, or at least disadvantaged, by the American Academy of Pediatrics’ overly simplistic and sometimes biased recommendations on issues that send my patients’ parents on unfortunate and avoidable guilt trips.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
How many minutes do you spend each day trying to coax new parents off the guilt train? They have delayed their childbearing until they felt comfortable economically and emotionally ready to raise a child. Convinced that up to this point they have done enough correctly to be considered successful, they see no reason that they won’t be able to tackle parenthood just as easily. Their black lab is a model of obedience. Housebreaking him was a breeze. They are skilled at using the Internet and social media to gather the information they will need for raising a child.
However, at some point in the first 72 hours after the birth of their child, most parents are going to hit the wall of reality. It may be that breastfeeding doesn’t work as well their cousin told them it would or simply that babies cry, often for no discernible reason. Desperately wanting to do what’s right for their child, guilt creeps in as the little failures and fatigue begin to accumulate.
In their search for answers, new parents naturally come to us as pediatricians and family practitioners for the facts, but they also will search the Internet, talk to lactation consultants, and be bombarded by unsolicited advice from family members and neighbors. Every source they turn to, including physicians, will be filtered through its own bias.
I recently came across the most sensible advice for new parents I have read in a long time, and it came not from a pediatrician but from an economics professor at Brown University. Emily Oster, PhD, writing in the New York Times, examines the available data on the topics of breastfeeding, sleep training, and parents working out of the home with the objectivity of an economist and the sensitivity of a mother who has been there and done that (“The Data All Guilt-Ridden Parents Need,” New York Times, April 19, 2019).
For example, she observes that many of the benefits of breastfeeding are supported by some evidence, “just not always especially good evidence. And even when the evidence is good, the benefits are smaller than many people realize.” She points out that “most studies of breastfeeding are biased by the fact that women who breastfeed are typically different from those who do not.” I will leave it to you to read her full discussion that includes a comparison of random trials versus observational studies. But she concludes that, if one relies on only good evidence, the only demonstrable benefit of breastfeeding is for mothers who nurse longer than 12 months who may have a 20%-30% decrease in breast cancer risk.
Using the same kind of careful analysis, Dr. Oster finds that sleep training may have a short-term benefit for parents who will have improved sleep and less maternal depression, but in the long run children who were sleep trained were no different than those that weren’t.
She also finds that, when it comes to the “optimal configuration of adult work hours” for a household, there is “no compelling evidence that proves that having a stay-at-home parent affects child outcomes, positively or negatively.” It is up to each household what works best for all it members, not just the child.
I found it particularly helpful as a practitioner who has often felt shackled, or at least disadvantaged, by the American Academy of Pediatrics’ overly simplistic and sometimes biased recommendations on issues that send my patients’ parents on unfortunate and avoidable guilt trips.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].