User login
Windshield and UV exposure
As the summer draws to a close and I have finished my 20th road trip with my three children who frequently complain of “being too hot” or that the “sun is in my eyes” while in the car, I would like to particularly for passengers. As thoughtful parents, we lather our kids with sunscreen before going to the pool or beach, but do we really remember to do this, or to provide sunglasses before embarking on a 5-hour car ride? Many people do not. We must raise awareness of the risk of UV light in cars, and take better care of both our children and ourselves.
Windshield glass is federally regulated to allow in a maximum amount of light for visibility, but has no requirements for sun protection. Many people do not understand the difference between UVA and UVB protection, let alone that UVB radiation is blocked by the window glass, but UVA radiation is not, and reaches the skin and eyes through glass. By law, windshields must be made of laminated glass, which includes two 2.1-mm layers of glass separated by a 0.8-mm piece of plastic. The glass is made to break easily upon impact and the plastic then stretches to absorb the impact. The thin layer of plastic also helps windshields absorb nearly all of the sun’s UVA and UVB rays. Sunroofs also contain UV-protective technology, which blocks UVA and UVB radiation while also keeping the car cool and protecting against direct sun exposure. However, rear windows do not offer the same protection.
Side and rear windows are made of a cheaper tempered glass that does not include a plastic layer, thereby offering no UVA protection. In a study by Butler et al. reviewing 900 head and neck cancers, 53% were found on the left side, and those who spent more hours driving each week had a higher chance of getting a left-side skin cancer (J Am Acad Dermatol. 2010 Dec;63[6]:1006-10). Many automakers have not helped this problem; while there is higher-SPF glass that can be used, it is more costly for automobile manufacturers – and ultimately for consumers. A cheaper and more practical alternative is a UV film that can be applied to the glass; these films both improve UV protection and cool the car. In addition to providing sun protection, it can be assumed that the subsequent reduction of temperature within a car decreases the usage of air conditioners, thus improving both fuel economy and the environment.
Aftermarket window tinting and UV films can also be applied by glass-tinting companies and auto dealers for $150-$200. Companies like Solar Gard, LLUMAr, and 3M offer window films that can block UV rays. While these are available, the legal allowable tint limit varies from state to state. Visible light transmission (VLT) is the measurement of the percent of visible light that gets through a car’s window. The lower the VLT, the darker the tint. Most states prohibit less than 50% VLT for the driver and front passenger window, and 35% for the rear passenger, side, and rear windows.
To mitigate this, I offer patients with severe photo-dermitides a letter of medical necessity to the DMV to allow a higher percentage of tinting and recommend that they get aftermarket UV-protective films or tints on their vehicles. Regardless of whether higher tints are an option for them, sun protection of the skin and eyes is recommended for all passengers. Sunscreen with broad-spectrum coverage is recommended regardless of how long a car ride might be, and it is recommended that individuals keep the sunroof closed while driving for added UV protection. The use of polarized sunglasses for adults and children is also recommended to avoid UV damage to the eyes. Sunscreens and glasses with protection against blue light are also recommended for passengers who stare at screens and tablets during long car rides.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
As the summer draws to a close and I have finished my 20th road trip with my three children who frequently complain of “being too hot” or that the “sun is in my eyes” while in the car, I would like to particularly for passengers. As thoughtful parents, we lather our kids with sunscreen before going to the pool or beach, but do we really remember to do this, or to provide sunglasses before embarking on a 5-hour car ride? Many people do not. We must raise awareness of the risk of UV light in cars, and take better care of both our children and ourselves.
Windshield glass is federally regulated to allow in a maximum amount of light for visibility, but has no requirements for sun protection. Many people do not understand the difference between UVA and UVB protection, let alone that UVB radiation is blocked by the window glass, but UVA radiation is not, and reaches the skin and eyes through glass. By law, windshields must be made of laminated glass, which includes two 2.1-mm layers of glass separated by a 0.8-mm piece of plastic. The glass is made to break easily upon impact and the plastic then stretches to absorb the impact. The thin layer of plastic also helps windshields absorb nearly all of the sun’s UVA and UVB rays. Sunroofs also contain UV-protective technology, which blocks UVA and UVB radiation while also keeping the car cool and protecting against direct sun exposure. However, rear windows do not offer the same protection.
Side and rear windows are made of a cheaper tempered glass that does not include a plastic layer, thereby offering no UVA protection. In a study by Butler et al. reviewing 900 head and neck cancers, 53% were found on the left side, and those who spent more hours driving each week had a higher chance of getting a left-side skin cancer (J Am Acad Dermatol. 2010 Dec;63[6]:1006-10). Many automakers have not helped this problem; while there is higher-SPF glass that can be used, it is more costly for automobile manufacturers – and ultimately for consumers. A cheaper and more practical alternative is a UV film that can be applied to the glass; these films both improve UV protection and cool the car. In addition to providing sun protection, it can be assumed that the subsequent reduction of temperature within a car decreases the usage of air conditioners, thus improving both fuel economy and the environment.
Aftermarket window tinting and UV films can also be applied by glass-tinting companies and auto dealers for $150-$200. Companies like Solar Gard, LLUMAr, and 3M offer window films that can block UV rays. While these are available, the legal allowable tint limit varies from state to state. Visible light transmission (VLT) is the measurement of the percent of visible light that gets through a car’s window. The lower the VLT, the darker the tint. Most states prohibit less than 50% VLT for the driver and front passenger window, and 35% for the rear passenger, side, and rear windows.
To mitigate this, I offer patients with severe photo-dermitides a letter of medical necessity to the DMV to allow a higher percentage of tinting and recommend that they get aftermarket UV-protective films or tints on their vehicles. Regardless of whether higher tints are an option for them, sun protection of the skin and eyes is recommended for all passengers. Sunscreen with broad-spectrum coverage is recommended regardless of how long a car ride might be, and it is recommended that individuals keep the sunroof closed while driving for added UV protection. The use of polarized sunglasses for adults and children is also recommended to avoid UV damage to the eyes. Sunscreens and glasses with protection against blue light are also recommended for passengers who stare at screens and tablets during long car rides.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
As the summer draws to a close and I have finished my 20th road trip with my three children who frequently complain of “being too hot” or that the “sun is in my eyes” while in the car, I would like to particularly for passengers. As thoughtful parents, we lather our kids with sunscreen before going to the pool or beach, but do we really remember to do this, or to provide sunglasses before embarking on a 5-hour car ride? Many people do not. We must raise awareness of the risk of UV light in cars, and take better care of both our children and ourselves.
Windshield glass is federally regulated to allow in a maximum amount of light for visibility, but has no requirements for sun protection. Many people do not understand the difference between UVA and UVB protection, let alone that UVB radiation is blocked by the window glass, but UVA radiation is not, and reaches the skin and eyes through glass. By law, windshields must be made of laminated glass, which includes two 2.1-mm layers of glass separated by a 0.8-mm piece of plastic. The glass is made to break easily upon impact and the plastic then stretches to absorb the impact. The thin layer of plastic also helps windshields absorb nearly all of the sun’s UVA and UVB rays. Sunroofs also contain UV-protective technology, which blocks UVA and UVB radiation while also keeping the car cool and protecting against direct sun exposure. However, rear windows do not offer the same protection.
Side and rear windows are made of a cheaper tempered glass that does not include a plastic layer, thereby offering no UVA protection. In a study by Butler et al. reviewing 900 head and neck cancers, 53% were found on the left side, and those who spent more hours driving each week had a higher chance of getting a left-side skin cancer (J Am Acad Dermatol. 2010 Dec;63[6]:1006-10). Many automakers have not helped this problem; while there is higher-SPF glass that can be used, it is more costly for automobile manufacturers – and ultimately for consumers. A cheaper and more practical alternative is a UV film that can be applied to the glass; these films both improve UV protection and cool the car. In addition to providing sun protection, it can be assumed that the subsequent reduction of temperature within a car decreases the usage of air conditioners, thus improving both fuel economy and the environment.
Aftermarket window tinting and UV films can also be applied by glass-tinting companies and auto dealers for $150-$200. Companies like Solar Gard, LLUMAr, and 3M offer window films that can block UV rays. While these are available, the legal allowable tint limit varies from state to state. Visible light transmission (VLT) is the measurement of the percent of visible light that gets through a car’s window. The lower the VLT, the darker the tint. Most states prohibit less than 50% VLT for the driver and front passenger window, and 35% for the rear passenger, side, and rear windows.
To mitigate this, I offer patients with severe photo-dermitides a letter of medical necessity to the DMV to allow a higher percentage of tinting and recommend that they get aftermarket UV-protective films or tints on their vehicles. Regardless of whether higher tints are an option for them, sun protection of the skin and eyes is recommended for all passengers. Sunscreen with broad-spectrum coverage is recommended regardless of how long a car ride might be, and it is recommended that individuals keep the sunroof closed while driving for added UV protection. The use of polarized sunglasses for adults and children is also recommended to avoid UV damage to the eyes. Sunscreens and glasses with protection against blue light are also recommended for passengers who stare at screens and tablets during long car rides.
Dr. Talakoub and Dr. Wesley are cocontributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub. Write to them at [email protected]. They had no relevant disclosures.
Flying solo
Recently, a stiff-white-coat family medicine resident showed up eager for his first day in dermatology. Working with him reminded me how exciting the journey is from resident to attending. It also reminded me how slow residents are – we’re 10 minutes into a visit and not yet done injecting anesthesia. A simple biopsy and electrodesiccation of a basal cell carcinoma would take 3 minutes – easy, rote, like driving home and being surprised when you arrive. But this task is making my resident so fraught with fear he’s barely moving. He won’t be crawling for long; his journey from novice to confident physician will be quick. Like a student pilot learning to fly, in a few hundred hours he’ll be flying solo.
After a year, most residents are adept, exuding the temerity of an attending. But as any practicing physician knows, medicine can make cowards of us all without warning. An experienced pilot facing an unexpected gusty 25-knot crosswind landing can find himself or herself a trembling beginner just as an excellent clinician can be overwhelmed facing an unexpectedly sick patient.
Not long after my visiting resident, I was back to my own packed clinic. With one swoop of a dermablade, I intended to quickly extirpate a keratoacanthoma on the back of an elderly man’s hand. I hardly had to think about it. However, when I lifted the blade, dark blood pooled where a dorsal saphenous vein used to live. After much electrodesiccation (and sutures) this particular biopsy was safely landed. But it wasn’t without a bit of blood loss and inconvenience for the patient. What might I have done differently? Injected 5-fluorouracil instead? Done an incisional biopsy? Used a different blade? More importantly, what will I do next time?
To avoid adverse outcomes, it might seem like the best strategy is to avoid deteriorating conditions, whether flying or in clinic. That would be a mistake. The journey from apprehension to mastery must pass through discomfort. It is only by working through unease and successfully managing complications that expertise is forged. Our days are mostly routine and the longer the period without adversity, the greater the risk of complacency. Consider seeking difficulty once in awhile and learn how to work through it. No pilot wants to be in a situation he or she hasn’t practiced managing.
For some physicians and residents, an unexpected complication or adverse outcome can make them apprehensive and defensive. I’ve seen doctors choose not to treat complicated diseases or dire lesions because of a previous bad experience or adverse outcome. It is sometimes appropriate to transfer a patient to a different service, but as physicians, it’s also our job to take care of our patient. When it’s your plane, you’ll have to land it.
Much later (or so it seemed), my resident finally finished the electrodesiccation and curettage. He had a look of relief knowing he has landed safely. I hope he realizes that this is a trip that he must take over and over again. One is never done learning to fly.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Recently, a stiff-white-coat family medicine resident showed up eager for his first day in dermatology. Working with him reminded me how exciting the journey is from resident to attending. It also reminded me how slow residents are – we’re 10 minutes into a visit and not yet done injecting anesthesia. A simple biopsy and electrodesiccation of a basal cell carcinoma would take 3 minutes – easy, rote, like driving home and being surprised when you arrive. But this task is making my resident so fraught with fear he’s barely moving. He won’t be crawling for long; his journey from novice to confident physician will be quick. Like a student pilot learning to fly, in a few hundred hours he’ll be flying solo.
After a year, most residents are adept, exuding the temerity of an attending. But as any practicing physician knows, medicine can make cowards of us all without warning. An experienced pilot facing an unexpected gusty 25-knot crosswind landing can find himself or herself a trembling beginner just as an excellent clinician can be overwhelmed facing an unexpectedly sick patient.
Not long after my visiting resident, I was back to my own packed clinic. With one swoop of a dermablade, I intended to quickly extirpate a keratoacanthoma on the back of an elderly man’s hand. I hardly had to think about it. However, when I lifted the blade, dark blood pooled where a dorsal saphenous vein used to live. After much electrodesiccation (and sutures) this particular biopsy was safely landed. But it wasn’t without a bit of blood loss and inconvenience for the patient. What might I have done differently? Injected 5-fluorouracil instead? Done an incisional biopsy? Used a different blade? More importantly, what will I do next time?
To avoid adverse outcomes, it might seem like the best strategy is to avoid deteriorating conditions, whether flying or in clinic. That would be a mistake. The journey from apprehension to mastery must pass through discomfort. It is only by working through unease and successfully managing complications that expertise is forged. Our days are mostly routine and the longer the period without adversity, the greater the risk of complacency. Consider seeking difficulty once in awhile and learn how to work through it. No pilot wants to be in a situation he or she hasn’t practiced managing.
For some physicians and residents, an unexpected complication or adverse outcome can make them apprehensive and defensive. I’ve seen doctors choose not to treat complicated diseases or dire lesions because of a previous bad experience or adverse outcome. It is sometimes appropriate to transfer a patient to a different service, but as physicians, it’s also our job to take care of our patient. When it’s your plane, you’ll have to land it.
Much later (or so it seemed), my resident finally finished the electrodesiccation and curettage. He had a look of relief knowing he has landed safely. I hope he realizes that this is a trip that he must take over and over again. One is never done learning to fly.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Recently, a stiff-white-coat family medicine resident showed up eager for his first day in dermatology. Working with him reminded me how exciting the journey is from resident to attending. It also reminded me how slow residents are – we’re 10 minutes into a visit and not yet done injecting anesthesia. A simple biopsy and electrodesiccation of a basal cell carcinoma would take 3 minutes – easy, rote, like driving home and being surprised when you arrive. But this task is making my resident so fraught with fear he’s barely moving. He won’t be crawling for long; his journey from novice to confident physician will be quick. Like a student pilot learning to fly, in a few hundred hours he’ll be flying solo.
After a year, most residents are adept, exuding the temerity of an attending. But as any practicing physician knows, medicine can make cowards of us all without warning. An experienced pilot facing an unexpected gusty 25-knot crosswind landing can find himself or herself a trembling beginner just as an excellent clinician can be overwhelmed facing an unexpectedly sick patient.
Not long after my visiting resident, I was back to my own packed clinic. With one swoop of a dermablade, I intended to quickly extirpate a keratoacanthoma on the back of an elderly man’s hand. I hardly had to think about it. However, when I lifted the blade, dark blood pooled where a dorsal saphenous vein used to live. After much electrodesiccation (and sutures) this particular biopsy was safely landed. But it wasn’t without a bit of blood loss and inconvenience for the patient. What might I have done differently? Injected 5-fluorouracil instead? Done an incisional biopsy? Used a different blade? More importantly, what will I do next time?
To avoid adverse outcomes, it might seem like the best strategy is to avoid deteriorating conditions, whether flying or in clinic. That would be a mistake. The journey from apprehension to mastery must pass through discomfort. It is only by working through unease and successfully managing complications that expertise is forged. Our days are mostly routine and the longer the period without adversity, the greater the risk of complacency. Consider seeking difficulty once in awhile and learn how to work through it. No pilot wants to be in a situation he or she hasn’t practiced managing.
For some physicians and residents, an unexpected complication or adverse outcome can make them apprehensive and defensive. I’ve seen doctors choose not to treat complicated diseases or dire lesions because of a previous bad experience or adverse outcome. It is sometimes appropriate to transfer a patient to a different service, but as physicians, it’s also our job to take care of our patient. When it’s your plane, you’ll have to land it.
Much later (or so it seemed), my resident finally finished the electrodesiccation and curettage. He had a look of relief knowing he has landed safely. I hope he realizes that this is a trip that he must take over and over again. One is never done learning to fly.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
How thin should we go?
An 88-year-old man with hypertension, chronic obstructive pulmonary disease, and atrial fibrillation presents with severe cerebral palsy and is diagnosed with a non–ST-elevation MI. He is found to have 90% left anterior descending artery occlusion and receives a drug-eluting stent. His current medications include warfarin, tiotropium, amlodipine, aspirin, and lisinopril. What anticoagulant therapy should he receive?
A) Clopidogrel, warfarin, and aspirin
B) Clopidogrel and aspirin
C) Clopidogrel and warfarin
D) Warfarin
E) Warfarin and aspirin
This issue comes up frequently with our patients with atrial fibrillation who are on anticoagulation, then have a coronary event and have a stent placed. What is the best approach to anticoagulation? I think for this patient adding clopidogrel, continuing warfarin, and stopping aspirin would be the best of the options presented.
Elderly patients have a higher risk of bleeding. They also have a greater chance of accumulating cardiovascular disease (atrial fibrillation, cardiac allograft vasculopathy, and valvular disease) that requires anticoagulation. Dewilde et al. studied the difference in bleeding risk in patients who were on oral anticoagulants who then underwent a percutaneous coronary intervention.1 Patients were assigned clopidogrel alone or clopidogrel plus aspirin in addition to their oral anticoagulant (warfarin). There was a significant increase in all-cause mortality in the patients who received clopidogrel plus aspirin (P = .027), and no significant difference in cardiac mortality between the two groups. There was a much higher risk of bleeding (44.4%) in the patients receiving triple therapy, compared with the double-therapy group (19.4%; P less than .0001).
In a large meta-analysis of over 7,000 patients by D’Ascenzo et al., there was no difference in thrombotic risk between double and triple therapy, and lower bleeding risk in patients who received double therapy.2
In a recently published article, Lopes et al. looked at the benefits and risks of antithrombotic therapy after acute coronary syndrome or percutaneous coronary intervention in patients with atrial fibrillation.3 The study included 4,614 patients, all of whom received a P2Y12 inhibitor. In addition, they received either apixaban or warfarin, and either aspirin or placebo. The patients who received apixaban had a lower risk of bleeding than those receiving warfarin (P less than .001), and those receiving aspirin had a higher risk than those receiving placebo (hazard ratio, 1.89; P less than .001). Patients using the combination of apixaban plus placebo had the lowest event rate per 100 years (16.8), followed by warfarin plus placebo (26.7), then apixaban plus aspirin (33.6), with warfarin plus aspirin having the highest event rate (49.1). The conclusion for the study was that regimens with apixaban without aspirin had less bleeding and hospitalizations without increased ischemic events, compared with regimens of warfarin with or without aspirin.
Pearl: Avoid using triple anticoagulant therapy by eliminating aspirin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Dewilde WJ et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet. 2013 Mar 30;381(9872):1107-15.
2. D’Ascenzo F et al. Meta-analysis of randomized controlled trials and adjusted observational results of use of clopidogrel, aspirin, and oral anticoagulants in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2015 May 1;115(9):1185-93.
3. Lopes RD et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019 Apr 18;380(16):1509-24.
An 88-year-old man with hypertension, chronic obstructive pulmonary disease, and atrial fibrillation presents with severe cerebral palsy and is diagnosed with a non–ST-elevation MI. He is found to have 90% left anterior descending artery occlusion and receives a drug-eluting stent. His current medications include warfarin, tiotropium, amlodipine, aspirin, and lisinopril. What anticoagulant therapy should he receive?
A) Clopidogrel, warfarin, and aspirin
B) Clopidogrel and aspirin
C) Clopidogrel and warfarin
D) Warfarin
E) Warfarin and aspirin
This issue comes up frequently with our patients with atrial fibrillation who are on anticoagulation, then have a coronary event and have a stent placed. What is the best approach to anticoagulation? I think for this patient adding clopidogrel, continuing warfarin, and stopping aspirin would be the best of the options presented.
Elderly patients have a higher risk of bleeding. They also have a greater chance of accumulating cardiovascular disease (atrial fibrillation, cardiac allograft vasculopathy, and valvular disease) that requires anticoagulation. Dewilde et al. studied the difference in bleeding risk in patients who were on oral anticoagulants who then underwent a percutaneous coronary intervention.1 Patients were assigned clopidogrel alone or clopidogrel plus aspirin in addition to their oral anticoagulant (warfarin). There was a significant increase in all-cause mortality in the patients who received clopidogrel plus aspirin (P = .027), and no significant difference in cardiac mortality between the two groups. There was a much higher risk of bleeding (44.4%) in the patients receiving triple therapy, compared with the double-therapy group (19.4%; P less than .0001).
In a large meta-analysis of over 7,000 patients by D’Ascenzo et al., there was no difference in thrombotic risk between double and triple therapy, and lower bleeding risk in patients who received double therapy.2
In a recently published article, Lopes et al. looked at the benefits and risks of antithrombotic therapy after acute coronary syndrome or percutaneous coronary intervention in patients with atrial fibrillation.3 The study included 4,614 patients, all of whom received a P2Y12 inhibitor. In addition, they received either apixaban or warfarin, and either aspirin or placebo. The patients who received apixaban had a lower risk of bleeding than those receiving warfarin (P less than .001), and those receiving aspirin had a higher risk than those receiving placebo (hazard ratio, 1.89; P less than .001). Patients using the combination of apixaban plus placebo had the lowest event rate per 100 years (16.8), followed by warfarin plus placebo (26.7), then apixaban plus aspirin (33.6), with warfarin plus aspirin having the highest event rate (49.1). The conclusion for the study was that regimens with apixaban without aspirin had less bleeding and hospitalizations without increased ischemic events, compared with regimens of warfarin with or without aspirin.
Pearl: Avoid using triple anticoagulant therapy by eliminating aspirin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Dewilde WJ et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet. 2013 Mar 30;381(9872):1107-15.
2. D’Ascenzo F et al. Meta-analysis of randomized controlled trials and adjusted observational results of use of clopidogrel, aspirin, and oral anticoagulants in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2015 May 1;115(9):1185-93.
3. Lopes RD et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019 Apr 18;380(16):1509-24.
An 88-year-old man with hypertension, chronic obstructive pulmonary disease, and atrial fibrillation presents with severe cerebral palsy and is diagnosed with a non–ST-elevation MI. He is found to have 90% left anterior descending artery occlusion and receives a drug-eluting stent. His current medications include warfarin, tiotropium, amlodipine, aspirin, and lisinopril. What anticoagulant therapy should he receive?
A) Clopidogrel, warfarin, and aspirin
B) Clopidogrel and aspirin
C) Clopidogrel and warfarin
D) Warfarin
E) Warfarin and aspirin
This issue comes up frequently with our patients with atrial fibrillation who are on anticoagulation, then have a coronary event and have a stent placed. What is the best approach to anticoagulation? I think for this patient adding clopidogrel, continuing warfarin, and stopping aspirin would be the best of the options presented.
Elderly patients have a higher risk of bleeding. They also have a greater chance of accumulating cardiovascular disease (atrial fibrillation, cardiac allograft vasculopathy, and valvular disease) that requires anticoagulation. Dewilde et al. studied the difference in bleeding risk in patients who were on oral anticoagulants who then underwent a percutaneous coronary intervention.1 Patients were assigned clopidogrel alone or clopidogrel plus aspirin in addition to their oral anticoagulant (warfarin). There was a significant increase in all-cause mortality in the patients who received clopidogrel plus aspirin (P = .027), and no significant difference in cardiac mortality between the two groups. There was a much higher risk of bleeding (44.4%) in the patients receiving triple therapy, compared with the double-therapy group (19.4%; P less than .0001).
In a large meta-analysis of over 7,000 patients by D’Ascenzo et al., there was no difference in thrombotic risk between double and triple therapy, and lower bleeding risk in patients who received double therapy.2
In a recently published article, Lopes et al. looked at the benefits and risks of antithrombotic therapy after acute coronary syndrome or percutaneous coronary intervention in patients with atrial fibrillation.3 The study included 4,614 patients, all of whom received a P2Y12 inhibitor. In addition, they received either apixaban or warfarin, and either aspirin or placebo. The patients who received apixaban had a lower risk of bleeding than those receiving warfarin (P less than .001), and those receiving aspirin had a higher risk than those receiving placebo (hazard ratio, 1.89; P less than .001). Patients using the combination of apixaban plus placebo had the lowest event rate per 100 years (16.8), followed by warfarin plus placebo (26.7), then apixaban plus aspirin (33.6), with warfarin plus aspirin having the highest event rate (49.1). The conclusion for the study was that regimens with apixaban without aspirin had less bleeding and hospitalizations without increased ischemic events, compared with regimens of warfarin with or without aspirin.
Pearl: Avoid using triple anticoagulant therapy by eliminating aspirin.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Dewilde WJ et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: An open-label, randomised, controlled trial. Lancet. 2013 Mar 30;381(9872):1107-15.
2. D’Ascenzo F et al. Meta-analysis of randomized controlled trials and adjusted observational results of use of clopidogrel, aspirin, and oral anticoagulants in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2015 May 1;115(9):1185-93.
3. Lopes RD et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019 Apr 18;380(16):1509-24.
Clicking override when the EHR system argues about an order
The EHR system at the hospital occasionally argues with me about my orders.
I may order a brain MRI, or CT angiography, or pretty much anything, and when I click to submit it a box pops up, telling me I shouldn’t be ordering that.
Sometimes it’s based on cost, saying that the MRI is more expensive than a CT, and according to some internal algorithm I should do that instead. Other times it says the test isn’t appropriate given the patient’s condition, age, zodiac sign, whatever. It might also say the test is redundant, because the patient just had a brain MRI during an admission last month.
I ignore them. There’s an override button to close the box and order the test, and that’s what I always click.
I have no objection to a reasonable review, but neither the computer nor its algorithms went through medical school, or residency, or read journals regularly, or have 20 years of experience in this field. I’d like to think (or hope) I know what I’m doing.
I don’t take this job lightly. When I order a test it’s because I’m trying to do the right thing for the patient. To find out what’s going on. To see what I can do to treat them. In short, to help as much as I can within the limitations of modern medical practice. Sometimes those things don’t always involve saving the insurance company money, or trying to get by with a previous study’s results.
Medicine is not a cookbook. While guidelines can be useful, every patient is different, and treatment plans have to be adjusted accordingly. It would be nice if this was the one-size-fits-all world the computer algorithms would like, but patient care is anything but.
I’d also rather “overcare” than “undercare.” To me, that’s just good practice. If I follow the computer’s advice and provide less care than needed and miss something, I’m pretty sure “because the computer told me not to” isn’t going to stand up as a defense in court.
I’m going to just keep on practicing medicine using, as one of my past attendings would say, “clinical correlation” and keeping what’s best for the patient in mind. Anything less may be fine for the computer, but not for me, and certainly not for those I’m trying to help.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The EHR system at the hospital occasionally argues with me about my orders.
I may order a brain MRI, or CT angiography, or pretty much anything, and when I click to submit it a box pops up, telling me I shouldn’t be ordering that.
Sometimes it’s based on cost, saying that the MRI is more expensive than a CT, and according to some internal algorithm I should do that instead. Other times it says the test isn’t appropriate given the patient’s condition, age, zodiac sign, whatever. It might also say the test is redundant, because the patient just had a brain MRI during an admission last month.
I ignore them. There’s an override button to close the box and order the test, and that’s what I always click.
I have no objection to a reasonable review, but neither the computer nor its algorithms went through medical school, or residency, or read journals regularly, or have 20 years of experience in this field. I’d like to think (or hope) I know what I’m doing.
I don’t take this job lightly. When I order a test it’s because I’m trying to do the right thing for the patient. To find out what’s going on. To see what I can do to treat them. In short, to help as much as I can within the limitations of modern medical practice. Sometimes those things don’t always involve saving the insurance company money, or trying to get by with a previous study’s results.
Medicine is not a cookbook. While guidelines can be useful, every patient is different, and treatment plans have to be adjusted accordingly. It would be nice if this was the one-size-fits-all world the computer algorithms would like, but patient care is anything but.
I’d also rather “overcare” than “undercare.” To me, that’s just good practice. If I follow the computer’s advice and provide less care than needed and miss something, I’m pretty sure “because the computer told me not to” isn’t going to stand up as a defense in court.
I’m going to just keep on practicing medicine using, as one of my past attendings would say, “clinical correlation” and keeping what’s best for the patient in mind. Anything less may be fine for the computer, but not for me, and certainly not for those I’m trying to help.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The EHR system at the hospital occasionally argues with me about my orders.
I may order a brain MRI, or CT angiography, or pretty much anything, and when I click to submit it a box pops up, telling me I shouldn’t be ordering that.
Sometimes it’s based on cost, saying that the MRI is more expensive than a CT, and according to some internal algorithm I should do that instead. Other times it says the test isn’t appropriate given the patient’s condition, age, zodiac sign, whatever. It might also say the test is redundant, because the patient just had a brain MRI during an admission last month.
I ignore them. There’s an override button to close the box and order the test, and that’s what I always click.
I have no objection to a reasonable review, but neither the computer nor its algorithms went through medical school, or residency, or read journals regularly, or have 20 years of experience in this field. I’d like to think (or hope) I know what I’m doing.
I don’t take this job lightly. When I order a test it’s because I’m trying to do the right thing for the patient. To find out what’s going on. To see what I can do to treat them. In short, to help as much as I can within the limitations of modern medical practice. Sometimes those things don’t always involve saving the insurance company money, or trying to get by with a previous study’s results.
Medicine is not a cookbook. While guidelines can be useful, every patient is different, and treatment plans have to be adjusted accordingly. It would be nice if this was the one-size-fits-all world the computer algorithms would like, but patient care is anything but.
I’d also rather “overcare” than “undercare.” To me, that’s just good practice. If I follow the computer’s advice and provide less care than needed and miss something, I’m pretty sure “because the computer told me not to” isn’t going to stand up as a defense in court.
I’m going to just keep on practicing medicine using, as one of my past attendings would say, “clinical correlation” and keeping what’s best for the patient in mind. Anything less may be fine for the computer, but not for me, and certainly not for those I’m trying to help.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
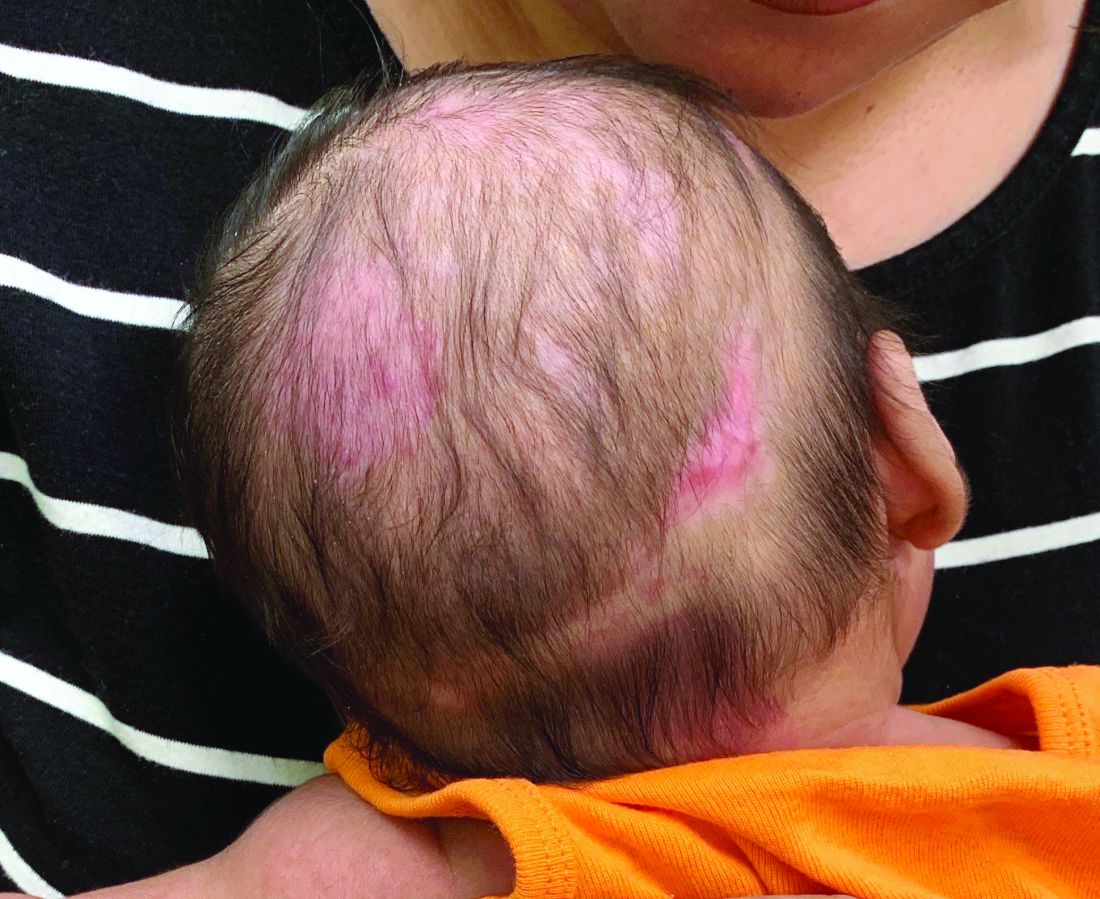
Is your office ready for a case of measles?
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.
Legal duty to nonpatients: Driving accidents
Question: Driver D strikes a pedestrian after losing control of his vehicle from insulin-induced hypoglycemia. Both Driver D and pedestrian were seriously injured. Driver D was recently diagnosed with diabetes, and his physician had started him on insulin, but did not warn of driving risks associated with hypoglycemia. The injured pedestrian is a total stranger to both Driver D and his doctor. Given these facts, which one of the following choices is correct?
A. Driver D can sue his doctor for failure to disclose hypoglycemic risk of insulin therapy under the doctrine of informed consent.
B. The pedestrian can sue Driver D for negligent driving.
C. The pedestrian may succeed in suing Driver D’s doctor for failure to warn of hypoglycemia.
D. The pedestrian’s lawsuit against Driver D’s doctor may fail in a jurisdiction that does not recognize a doctor’s legal duty to an unidentifiable, nonpatient third party.
E. All statements above are correct.
Answer: E. This legal duty grows out of the doctor-patient relationship, and is normally owed to the patient and to no one else. However, in limited circumstances, it may be extended to other individuals, so-called third parties, who may be total strangers. Injured nonpatient third parties from driving accidents have successfully sued doctors for failing to warn their patients that their medical conditions and/or medications can adversely affect driving ability.
Vizzoni v. Mulford-Dera is a New Jersey malpractice case that is currently before the state’s appellate court. The issue is whether Dr. Lerner, a psychiatrist, can be found negligent for the death of a bicyclist caused by the psychiatrist’s patient, Ms. Mulford-Dera, whose car struck and killed the cyclist. The decedent’s estate alleged that the physician should have warned the patient of the risks of driving while taking psychotropic medications. Dr. Lerner had been treating Ms. Mulford-Dera for psychological conditions, including major depression, panic disorder, and attention deficit disorder. As part of her treatment, Dr. Lerner prescribed several medications, allegedly without disclosing their potential adverse impact on driving. The trial court granted summary judgment and dismissed the case, ruling that the doctor owed no direct or indirect duty to the victim.
The case is currently on appeal. The AMA has filed an amicus brief in support of Dr. Lerner,1 pointing out that third-party claims had previously been rejected in New Jersey, where the injured victim is not readily identifiable. The brief emphasizes the folly of placing the physician or therapist in the untenable position of serving two potentially competing interests when a physician’s priority should be providing care to the patient. It referenced a similar case in Kansas, where a motorist who had fallen asleep at the wheel struck a bicyclist. The motorist was being treated by a neurologist for a sleep disorder.2 The Kansas Supreme Court held that there was no special relationship between the doctor and the cyclist that would impose a duty to warn the motorist about harming a third party.
Other jurisdictions have likewise rejected attempts at “derivative duties” in automobile accident cases. The Connecticut Supreme Court has ruled3 that doctors are immune from third party traffic accident lawsuits, as such litigation would detract from what’s best for the patient (“a physician’s desire to avoid lawsuits may result in far more restrictive advice than necessary for the patient’s well-being”). In that case, the defendant-gastroenterologist, Dr. Troncale, was treating a patient with hepatic encephalopathy and had not warned of the associated risk of driving. And an Illinois court dismissed a third party’s case against a hospital when one of its physicians fell asleep at the wheel after working excessive hours.4
In contrast, other jurisdictions have found a legal duty for physicians toward nonpatient victims. For example, in McKenzie v. Hawaii Permanente Medical Group,5 a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter. The driver alleged that the prescription medication, Prazosin, caused him to lose control of the car, and that the treating physician was negligent, first in prescribing an inappropriate type and dose of medication, and second in failing to warn of potential side effects that could affect driving ability. The Hawaii Supreme Court emphasized that the risk of tort liability to an individual physician already discourages negligent prescribing; therefore, a physician does not have a duty to third parties where the alleged negligence involves prescribing decisions, i.e., whether to prescribe medication at all, which medication to prescribe, and what dosage to use. On the other hand, physicians have a duty to their patients to warn of potential adverse effects and this responsibility should therefore extend to third parties. Thus, liability would attach to injuries of innocent third parties as a result of failing to warn of a medication’s effects on driving—unless a reasonable person could be expected to be aware of this risk without the warning.
A foreseeable and unreasonable risk of harm is an important but not the only decisive factor in construing the existence of legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of existing social values, customs, and policy considerations. In a Massachusetts case,6 a family physician had failed to warn his patient of the risk of diabetes drugs when operating a vehicle. Some 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court invoked the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist for public policy reasons.
Dr. Tan is professor of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Vizzoni v. Mulford-Dera, In the Superior Court of New Jersey Appellate Division, Docket No. A-001255-18T3.
2. Calwell v. Hassan, 925 P.2d 422, 430 (Kan. 1996).
3. Jarmie v. Troncale, 50 A.3d 802 (Conn. 2012).
4. Brewster v. Rush-Presbyterian-St. Luke’s Med. Ctr., 836 N.E.2d 635 (Ill. Ct. App. 2005).
5. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
6. Arsenault v. McConarty, 21 Mass. L. Rptr. 500 (2006).
Question: Driver D strikes a pedestrian after losing control of his vehicle from insulin-induced hypoglycemia. Both Driver D and pedestrian were seriously injured. Driver D was recently diagnosed with diabetes, and his physician had started him on insulin, but did not warn of driving risks associated with hypoglycemia. The injured pedestrian is a total stranger to both Driver D and his doctor. Given these facts, which one of the following choices is correct?
A. Driver D can sue his doctor for failure to disclose hypoglycemic risk of insulin therapy under the doctrine of informed consent.
B. The pedestrian can sue Driver D for negligent driving.
C. The pedestrian may succeed in suing Driver D’s doctor for failure to warn of hypoglycemia.
D. The pedestrian’s lawsuit against Driver D’s doctor may fail in a jurisdiction that does not recognize a doctor’s legal duty to an unidentifiable, nonpatient third party.
E. All statements above are correct.
Answer: E. This legal duty grows out of the doctor-patient relationship, and is normally owed to the patient and to no one else. However, in limited circumstances, it may be extended to other individuals, so-called third parties, who may be total strangers. Injured nonpatient third parties from driving accidents have successfully sued doctors for failing to warn their patients that their medical conditions and/or medications can adversely affect driving ability.
Vizzoni v. Mulford-Dera is a New Jersey malpractice case that is currently before the state’s appellate court. The issue is whether Dr. Lerner, a psychiatrist, can be found negligent for the death of a bicyclist caused by the psychiatrist’s patient, Ms. Mulford-Dera, whose car struck and killed the cyclist. The decedent’s estate alleged that the physician should have warned the patient of the risks of driving while taking psychotropic medications. Dr. Lerner had been treating Ms. Mulford-Dera for psychological conditions, including major depression, panic disorder, and attention deficit disorder. As part of her treatment, Dr. Lerner prescribed several medications, allegedly without disclosing their potential adverse impact on driving. The trial court granted summary judgment and dismissed the case, ruling that the doctor owed no direct or indirect duty to the victim.
The case is currently on appeal. The AMA has filed an amicus brief in support of Dr. Lerner,1 pointing out that third-party claims had previously been rejected in New Jersey, where the injured victim is not readily identifiable. The brief emphasizes the folly of placing the physician or therapist in the untenable position of serving two potentially competing interests when a physician’s priority should be providing care to the patient. It referenced a similar case in Kansas, where a motorist who had fallen asleep at the wheel struck a bicyclist. The motorist was being treated by a neurologist for a sleep disorder.2 The Kansas Supreme Court held that there was no special relationship between the doctor and the cyclist that would impose a duty to warn the motorist about harming a third party.
Other jurisdictions have likewise rejected attempts at “derivative duties” in automobile accident cases. The Connecticut Supreme Court has ruled3 that doctors are immune from third party traffic accident lawsuits, as such litigation would detract from what’s best for the patient (“a physician’s desire to avoid lawsuits may result in far more restrictive advice than necessary for the patient’s well-being”). In that case, the defendant-gastroenterologist, Dr. Troncale, was treating a patient with hepatic encephalopathy and had not warned of the associated risk of driving. And an Illinois court dismissed a third party’s case against a hospital when one of its physicians fell asleep at the wheel after working excessive hours.4
In contrast, other jurisdictions have found a legal duty for physicians toward nonpatient victims. For example, in McKenzie v. Hawaii Permanente Medical Group,5 a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter. The driver alleged that the prescription medication, Prazosin, caused him to lose control of the car, and that the treating physician was negligent, first in prescribing an inappropriate type and dose of medication, and second in failing to warn of potential side effects that could affect driving ability. The Hawaii Supreme Court emphasized that the risk of tort liability to an individual physician already discourages negligent prescribing; therefore, a physician does not have a duty to third parties where the alleged negligence involves prescribing decisions, i.e., whether to prescribe medication at all, which medication to prescribe, and what dosage to use. On the other hand, physicians have a duty to their patients to warn of potential adverse effects and this responsibility should therefore extend to third parties. Thus, liability would attach to injuries of innocent third parties as a result of failing to warn of a medication’s effects on driving—unless a reasonable person could be expected to be aware of this risk without the warning.
A foreseeable and unreasonable risk of harm is an important but not the only decisive factor in construing the existence of legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of existing social values, customs, and policy considerations. In a Massachusetts case,6 a family physician had failed to warn his patient of the risk of diabetes drugs when operating a vehicle. Some 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court invoked the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist for public policy reasons.
Dr. Tan is professor of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Vizzoni v. Mulford-Dera, In the Superior Court of New Jersey Appellate Division, Docket No. A-001255-18T3.
2. Calwell v. Hassan, 925 P.2d 422, 430 (Kan. 1996).
3. Jarmie v. Troncale, 50 A.3d 802 (Conn. 2012).
4. Brewster v. Rush-Presbyterian-St. Luke’s Med. Ctr., 836 N.E.2d 635 (Ill. Ct. App. 2005).
5. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
6. Arsenault v. McConarty, 21 Mass. L. Rptr. 500 (2006).
Question: Driver D strikes a pedestrian after losing control of his vehicle from insulin-induced hypoglycemia. Both Driver D and pedestrian were seriously injured. Driver D was recently diagnosed with diabetes, and his physician had started him on insulin, but did not warn of driving risks associated with hypoglycemia. The injured pedestrian is a total stranger to both Driver D and his doctor. Given these facts, which one of the following choices is correct?
A. Driver D can sue his doctor for failure to disclose hypoglycemic risk of insulin therapy under the doctrine of informed consent.
B. The pedestrian can sue Driver D for negligent driving.
C. The pedestrian may succeed in suing Driver D’s doctor for failure to warn of hypoglycemia.
D. The pedestrian’s lawsuit against Driver D’s doctor may fail in a jurisdiction that does not recognize a doctor’s legal duty to an unidentifiable, nonpatient third party.
E. All statements above are correct.
Answer: E. This legal duty grows out of the doctor-patient relationship, and is normally owed to the patient and to no one else. However, in limited circumstances, it may be extended to other individuals, so-called third parties, who may be total strangers. Injured nonpatient third parties from driving accidents have successfully sued doctors for failing to warn their patients that their medical conditions and/or medications can adversely affect driving ability.
Vizzoni v. Mulford-Dera is a New Jersey malpractice case that is currently before the state’s appellate court. The issue is whether Dr. Lerner, a psychiatrist, can be found negligent for the death of a bicyclist caused by the psychiatrist’s patient, Ms. Mulford-Dera, whose car struck and killed the cyclist. The decedent’s estate alleged that the physician should have warned the patient of the risks of driving while taking psychotropic medications. Dr. Lerner had been treating Ms. Mulford-Dera for psychological conditions, including major depression, panic disorder, and attention deficit disorder. As part of her treatment, Dr. Lerner prescribed several medications, allegedly without disclosing their potential adverse impact on driving. The trial court granted summary judgment and dismissed the case, ruling that the doctor owed no direct or indirect duty to the victim.
The case is currently on appeal. The AMA has filed an amicus brief in support of Dr. Lerner,1 pointing out that third-party claims had previously been rejected in New Jersey, where the injured victim is not readily identifiable. The brief emphasizes the folly of placing the physician or therapist in the untenable position of serving two potentially competing interests when a physician’s priority should be providing care to the patient. It referenced a similar case in Kansas, where a motorist who had fallen asleep at the wheel struck a bicyclist. The motorist was being treated by a neurologist for a sleep disorder.2 The Kansas Supreme Court held that there was no special relationship between the doctor and the cyclist that would impose a duty to warn the motorist about harming a third party.
Other jurisdictions have likewise rejected attempts at “derivative duties” in automobile accident cases. The Connecticut Supreme Court has ruled3 that doctors are immune from third party traffic accident lawsuits, as such litigation would detract from what’s best for the patient (“a physician’s desire to avoid lawsuits may result in far more restrictive advice than necessary for the patient’s well-being”). In that case, the defendant-gastroenterologist, Dr. Troncale, was treating a patient with hepatic encephalopathy and had not warned of the associated risk of driving. And an Illinois court dismissed a third party’s case against a hospital when one of its physicians fell asleep at the wheel after working excessive hours.4
In contrast, other jurisdictions have found a legal duty for physicians toward nonpatient victims. For example, in McKenzie v. Hawaii Permanente Medical Group,5 a car suddenly veered across five lanes of traffic, striking an 11-year-old girl and crushing her against a cement planter. The driver alleged that the prescription medication, Prazosin, caused him to lose control of the car, and that the treating physician was negligent, first in prescribing an inappropriate type and dose of medication, and second in failing to warn of potential side effects that could affect driving ability. The Hawaii Supreme Court emphasized that the risk of tort liability to an individual physician already discourages negligent prescribing; therefore, a physician does not have a duty to third parties where the alleged negligence involves prescribing decisions, i.e., whether to prescribe medication at all, which medication to prescribe, and what dosage to use. On the other hand, physicians have a duty to their patients to warn of potential adverse effects and this responsibility should therefore extend to third parties. Thus, liability would attach to injuries of innocent third parties as a result of failing to warn of a medication’s effects on driving—unless a reasonable person could be expected to be aware of this risk without the warning.
A foreseeable and unreasonable risk of harm is an important but not the only decisive factor in construing the existence of legal duty. Under some circumstances, the term “special relationship” has been employed based on a consideration of existing social values, customs, and policy considerations. In a Massachusetts case,6 a family physician had failed to warn his patient of the risk of diabetes drugs when operating a vehicle. Some 45 minutes after the patient’s discharge from the hospital, he developed hypoglycemia, losing consciousness and injuring a motorcyclist who then sued the doctor. The court invoked the “special relationship” rationale in ruling that the doctor owed a duty to the motorcyclist for public policy reasons.
Dr. Tan is professor of medicine and former adjunct professor of law at the University of Hawaii. This article is meant to be educational and does not constitute medical, ethical, or legal advice. For additional information, readers may contact the author at [email protected].
References
1. Vizzoni v. Mulford-Dera, In the Superior Court of New Jersey Appellate Division, Docket No. A-001255-18T3.
2. Calwell v. Hassan, 925 P.2d 422, 430 (Kan. 1996).
3. Jarmie v. Troncale, 50 A.3d 802 (Conn. 2012).
4. Brewster v. Rush-Presbyterian-St. Luke’s Med. Ctr., 836 N.E.2d 635 (Ill. Ct. App. 2005).
5. McKenzie v. Hawaii Permanente Medical Group, 47 P.3d 1209 (Haw. 2002).
6. Arsenault v. McConarty, 21 Mass. L. Rptr. 500 (2006).
Screening for pancreatic and lung cancers
In this edition of “How I will treat my next patient,” I examine the U.S. Preventive Services Task Force Reaffirmation Recommendation Statement regarding screening for pancreatic cancer in normal-risk populations. I also review newly published information regarding low-dose CT screening (LDCT) for lung cancer in a commonly screened population – individuals with chronic obstructive pulmonary disease (COPD). Both publications highlight the complexity of implementing shared decision making in clinicians’ efforts to find these highly lethal cancers in their earliest, most curable stages.
Pancreatic cancer screening
In their recommendation, the USPSTF considered data relevant to the benefits and harms (exclusive of costs) of screening for pancreatic cancer in the 85%-90% of individuals who are at normal risk because they lack a known familial or genetic syndrome and do not have at least two affected relatives or one first-degree affected relative (JAMA. 2019;322[5]:438-44).
After reviewing 13 cohort studies employing image-based technologies (CT, MRI, endoscopic ultrasound) and biomarkers for screening, the USPSTF reaffirmed its 2004 recommendation against pancreatic cancer screening. They found no new evidence of sufficient strength and quality to alter their previous “D grade” for screening (i.e., “Don’t do it.”). There were at least moderate harms of screening and subsequent treatment in normal-risk populations. These recommendations apply to asymptomatic individuals with new-onset diabetes mellitus, smokers, older adults, obese patients, and patients with a history of chronic pancreatitis.
What this means in practice
In the nicely written and comprehensive recommendation statement and in the two accompanying editorials (JAMA Surg. 2019 Aug 6. doi: 10.1001/jamasurg.2019.2832; JAMA. 2019;322[5]:407-8), the authors were explicit that the “D grade” for pancreatic cancer screening did not apply to individuals from familial pancreatic cancer kindreds and those with germline mutations and Lynch syndrome mismatch repair genes. For them, the relative risk of pancreatic cancer (greater than 5%) may justify the morbidity of available surveillance technologies, especially since U.S. and International screening studies in these high-risk individuals have generated data suggesting a benefit for treatment of screen-detected cancers.
The USPSTF and the editorial authors were strongly supportive of, and enthusiastic about, ongoing research efforts. Recently, a joint effort at the National Institutes of Health began recruiting centers for a study to assess the sensitivity of novel biomarkers in detecting pancreatic cancer among adults with new-onset diabetes (A211701).
To me, it is clear that the pathway to identifying effective screening for pancreatic cancer – which is forecast to become the second leading cause of cancer death in the United States by 2020 – will focus on high-risk populations first, enabling accurate determination of sensitivity and specificity before being applied to the general population. This is as it should be.
Lung cancer screening
Currently, guidelines from the National Comprehensive Cancer Network recommend LDCT screening annually for high-risk smokers, former smokers, and individuals with additional risk factors aged 55-77 years. The National Lung Screening Trial indicated that LDCT screening achieved a 20% relative reduction in lung cancer mortality and 6.7% relative reduction in overall mortality in a similar population. NCCN guidelines stress the importance of shared decision making and include a table of risks and benefits that should be considered.
Jonathan M. Iaccarino, MD, and colleagues quantified the risks of screening among COPD patients in a secondary analysis of the more than 75,000 LDCT scans that were performed among the more than 26,000 participants in the National Lung Screening Trial (Chest 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016). In comparison with participants who did not self-report a diagnosis of COPD, the 4,632 participants with self-reported COPD were significantly more likely to require further diagnostic studies, have an invasive procedure, have a complication of any type from the invasive procedure, and suffer a serious complication. The establishment of a lung cancer diagnosis from the invasive procedure, however, occurred in just 6.1% of COPD patients versus 3.6% of patients without COPD.
What this means in practice
At a consensus conference convened by the National Quality Forum, shared decision making was defined as a process of communication in which clinicians and patients work together to make decisions that align with what matters most to patients. Ideally, shared decision making requires clear, accurate, unbiased medical evidence about reasonable alternatives; tailored evidence for individual patients; and the incorporation of patient values, goals, informed preferences and concerns, including a discussion of treatment burdens. All of us wrestle with the challenge of conducting these conversations in a comprehensive and unbiased manner. I am not sure that I have ever achieved an ideal shared decision-making conversation in my practice.
Despite the limitations acknowledged by the authors – self-reported diagnosis of COPD, outcomes that were not the primary focus of the trial, failure to incorporate other important comorbid conditions – the study by Dr. Iaccarino and colleagues helps to quantify risks and benefits for a commonly screened population, specifically COPD patients. Most importantly, it focuses our attention on the key goal of all cancer-screening efforts – applying our personal and technological resources to patients who benefit the most and will suffer the least harm from our efforts.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I examine the U.S. Preventive Services Task Force Reaffirmation Recommendation Statement regarding screening for pancreatic cancer in normal-risk populations. I also review newly published information regarding low-dose CT screening (LDCT) for lung cancer in a commonly screened population – individuals with chronic obstructive pulmonary disease (COPD). Both publications highlight the complexity of implementing shared decision making in clinicians’ efforts to find these highly lethal cancers in their earliest, most curable stages.
Pancreatic cancer screening
In their recommendation, the USPSTF considered data relevant to the benefits and harms (exclusive of costs) of screening for pancreatic cancer in the 85%-90% of individuals who are at normal risk because they lack a known familial or genetic syndrome and do not have at least two affected relatives or one first-degree affected relative (JAMA. 2019;322[5]:438-44).
After reviewing 13 cohort studies employing image-based technologies (CT, MRI, endoscopic ultrasound) and biomarkers for screening, the USPSTF reaffirmed its 2004 recommendation against pancreatic cancer screening. They found no new evidence of sufficient strength and quality to alter their previous “D grade” for screening (i.e., “Don’t do it.”). There were at least moderate harms of screening and subsequent treatment in normal-risk populations. These recommendations apply to asymptomatic individuals with new-onset diabetes mellitus, smokers, older adults, obese patients, and patients with a history of chronic pancreatitis.
What this means in practice
In the nicely written and comprehensive recommendation statement and in the two accompanying editorials (JAMA Surg. 2019 Aug 6. doi: 10.1001/jamasurg.2019.2832; JAMA. 2019;322[5]:407-8), the authors were explicit that the “D grade” for pancreatic cancer screening did not apply to individuals from familial pancreatic cancer kindreds and those with germline mutations and Lynch syndrome mismatch repair genes. For them, the relative risk of pancreatic cancer (greater than 5%) may justify the morbidity of available surveillance technologies, especially since U.S. and International screening studies in these high-risk individuals have generated data suggesting a benefit for treatment of screen-detected cancers.
The USPSTF and the editorial authors were strongly supportive of, and enthusiastic about, ongoing research efforts. Recently, a joint effort at the National Institutes of Health began recruiting centers for a study to assess the sensitivity of novel biomarkers in detecting pancreatic cancer among adults with new-onset diabetes (A211701).
To me, it is clear that the pathway to identifying effective screening for pancreatic cancer – which is forecast to become the second leading cause of cancer death in the United States by 2020 – will focus on high-risk populations first, enabling accurate determination of sensitivity and specificity before being applied to the general population. This is as it should be.
Lung cancer screening
Currently, guidelines from the National Comprehensive Cancer Network recommend LDCT screening annually for high-risk smokers, former smokers, and individuals with additional risk factors aged 55-77 years. The National Lung Screening Trial indicated that LDCT screening achieved a 20% relative reduction in lung cancer mortality and 6.7% relative reduction in overall mortality in a similar population. NCCN guidelines stress the importance of shared decision making and include a table of risks and benefits that should be considered.
Jonathan M. Iaccarino, MD, and colleagues quantified the risks of screening among COPD patients in a secondary analysis of the more than 75,000 LDCT scans that were performed among the more than 26,000 participants in the National Lung Screening Trial (Chest 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016). In comparison with participants who did not self-report a diagnosis of COPD, the 4,632 participants with self-reported COPD were significantly more likely to require further diagnostic studies, have an invasive procedure, have a complication of any type from the invasive procedure, and suffer a serious complication. The establishment of a lung cancer diagnosis from the invasive procedure, however, occurred in just 6.1% of COPD patients versus 3.6% of patients without COPD.
What this means in practice
At a consensus conference convened by the National Quality Forum, shared decision making was defined as a process of communication in which clinicians and patients work together to make decisions that align with what matters most to patients. Ideally, shared decision making requires clear, accurate, unbiased medical evidence about reasonable alternatives; tailored evidence for individual patients; and the incorporation of patient values, goals, informed preferences and concerns, including a discussion of treatment burdens. All of us wrestle with the challenge of conducting these conversations in a comprehensive and unbiased manner. I am not sure that I have ever achieved an ideal shared decision-making conversation in my practice.
Despite the limitations acknowledged by the authors – self-reported diagnosis of COPD, outcomes that were not the primary focus of the trial, failure to incorporate other important comorbid conditions – the study by Dr. Iaccarino and colleagues helps to quantify risks and benefits for a commonly screened population, specifically COPD patients. Most importantly, it focuses our attention on the key goal of all cancer-screening efforts – applying our personal and technological resources to patients who benefit the most and will suffer the least harm from our efforts.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
In this edition of “How I will treat my next patient,” I examine the U.S. Preventive Services Task Force Reaffirmation Recommendation Statement regarding screening for pancreatic cancer in normal-risk populations. I also review newly published information regarding low-dose CT screening (LDCT) for lung cancer in a commonly screened population – individuals with chronic obstructive pulmonary disease (COPD). Both publications highlight the complexity of implementing shared decision making in clinicians’ efforts to find these highly lethal cancers in their earliest, most curable stages.
Pancreatic cancer screening
In their recommendation, the USPSTF considered data relevant to the benefits and harms (exclusive of costs) of screening for pancreatic cancer in the 85%-90% of individuals who are at normal risk because they lack a known familial or genetic syndrome and do not have at least two affected relatives or one first-degree affected relative (JAMA. 2019;322[5]:438-44).
After reviewing 13 cohort studies employing image-based technologies (CT, MRI, endoscopic ultrasound) and biomarkers for screening, the USPSTF reaffirmed its 2004 recommendation against pancreatic cancer screening. They found no new evidence of sufficient strength and quality to alter their previous “D grade” for screening (i.e., “Don’t do it.”). There were at least moderate harms of screening and subsequent treatment in normal-risk populations. These recommendations apply to asymptomatic individuals with new-onset diabetes mellitus, smokers, older adults, obese patients, and patients with a history of chronic pancreatitis.
What this means in practice
In the nicely written and comprehensive recommendation statement and in the two accompanying editorials (JAMA Surg. 2019 Aug 6. doi: 10.1001/jamasurg.2019.2832; JAMA. 2019;322[5]:407-8), the authors were explicit that the “D grade” for pancreatic cancer screening did not apply to individuals from familial pancreatic cancer kindreds and those with germline mutations and Lynch syndrome mismatch repair genes. For them, the relative risk of pancreatic cancer (greater than 5%) may justify the morbidity of available surveillance technologies, especially since U.S. and International screening studies in these high-risk individuals have generated data suggesting a benefit for treatment of screen-detected cancers.
The USPSTF and the editorial authors were strongly supportive of, and enthusiastic about, ongoing research efforts. Recently, a joint effort at the National Institutes of Health began recruiting centers for a study to assess the sensitivity of novel biomarkers in detecting pancreatic cancer among adults with new-onset diabetes (A211701).
To me, it is clear that the pathway to identifying effective screening for pancreatic cancer – which is forecast to become the second leading cause of cancer death in the United States by 2020 – will focus on high-risk populations first, enabling accurate determination of sensitivity and specificity before being applied to the general population. This is as it should be.
Lung cancer screening
Currently, guidelines from the National Comprehensive Cancer Network recommend LDCT screening annually for high-risk smokers, former smokers, and individuals with additional risk factors aged 55-77 years. The National Lung Screening Trial indicated that LDCT screening achieved a 20% relative reduction in lung cancer mortality and 6.7% relative reduction in overall mortality in a similar population. NCCN guidelines stress the importance of shared decision making and include a table of risks and benefits that should be considered.
Jonathan M. Iaccarino, MD, and colleagues quantified the risks of screening among COPD patients in a secondary analysis of the more than 75,000 LDCT scans that were performed among the more than 26,000 participants in the National Lung Screening Trial (Chest 2019 Jul 5. doi: 10.1016/j.chest.2019.06.016). In comparison with participants who did not self-report a diagnosis of COPD, the 4,632 participants with self-reported COPD were significantly more likely to require further diagnostic studies, have an invasive procedure, have a complication of any type from the invasive procedure, and suffer a serious complication. The establishment of a lung cancer diagnosis from the invasive procedure, however, occurred in just 6.1% of COPD patients versus 3.6% of patients without COPD.
What this means in practice
At a consensus conference convened by the National Quality Forum, shared decision making was defined as a process of communication in which clinicians and patients work together to make decisions that align with what matters most to patients. Ideally, shared decision making requires clear, accurate, unbiased medical evidence about reasonable alternatives; tailored evidence for individual patients; and the incorporation of patient values, goals, informed preferences and concerns, including a discussion of treatment burdens. All of us wrestle with the challenge of conducting these conversations in a comprehensive and unbiased manner. I am not sure that I have ever achieved an ideal shared decision-making conversation in my practice.
Despite the limitations acknowledged by the authors – self-reported diagnosis of COPD, outcomes that were not the primary focus of the trial, failure to incorporate other important comorbid conditions – the study by Dr. Iaccarino and colleagues helps to quantify risks and benefits for a commonly screened population, specifically COPD patients. Most importantly, it focuses our attention on the key goal of all cancer-screening efforts – applying our personal and technological resources to patients who benefit the most and will suffer the least harm from our efforts.
Dr. Lyss has been a community-based medical oncologist and clinical researcher for more than 35 years, practicing in St. Louis. His clinical and research interests are in the prevention, diagnosis, and treatment of breast and lung cancers and in expanding access to clinical trials to medically underserved populations.
A 2-month-old infant with a scalp rash that appeared after birth
With the perinatal history of prolonged labor and prolonged rupture of membranes, the diagnosis of halo scalp ring was made. This occurs secondary to prolonged pressure of the baby’s scalp with the mother’s pelvic bones, uterus, or cervical area, which causes decreased blood flow to the area, secondary ischemic damage, and in some cases scarring and hair loss.1
The degree of involvement is variable as some babies have mild alopecia and others have severe full-thickness necrosis and scarring. These lesions also can present with associated caput succedaneum and scalp molding, but these were not seen in our patient. Predisposing factors for halo scalp ring include caput succedaneum, prolonged or difficult labor, premature or prolonged rupture of membranes, vaginal delivery, vertex presentation, first delivery, as well as prematurity.2 On physical examination, a semicircular patch of alopecia with associated scarring, crusting, or erythema can be seen in some more severe cases.
The differential diagnosis includes aplasia cutis. In aplasia cutis, there is congenital loss of skin on the affected areas. The scalp usually is affected, but these lesions can occur in any other part of the body. Most patients with aplasia cutis have no other findings, but there are cases that can be associated with other cardiovascular, gastrointestinal, or central nervous system abnormalities. Neonatal lupus also can present with scarring lesions on the scalp, but they usually present a little after birth, mainly affecting the face. The mothers of this children usually have a diagnosis of connective tissue disease and may have positive antibodies to Sjögren’s syndrome antibody A, Sjögren’s syndrome antibody B, or antiribonucleoprotein antibody. Seborrheic dermatitis does not cause scarring alopecia. The lesions present as waxy scaly plaques on the scalp, erythematous waxy plaques behind the ears, face, and folds. Some patients can have hair loss secondary to the inflammation, but the hair grows back once the inflammation is controlled. Dissecting cellulitis is a type of scarring alopecia seen in pubescent and adult individuals. No cases of neonatal dissecting cellulitis have been described.
Halo scalp ring is not associated with any other systemic symptoms or syndromes. Extensive imaging and systemic work-up are not required unless the baby presents with other neurologic symptoms. The areas can be treated with petrolatum and observation as most lesions resolve.
In cases of extensive areas of scarring alopecia, referral to a plastic surgeon can be made to consider tissue expanders or scar revision prior to the child starting school if the lesions are causing psychological stressors.
The true prevalence of this condition is unknown. We believe halo ring alopecia is sometimes not diagnosed, and as lesions tend to resolve, most cases go unreported.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Pediatr Adolesc Med. 2010;164(7):673.
2. Pediatr Dermatol. 2009 Nov-Dec;26(6):706-8.
3. Dermatol Online J. 2016 Nov 15;22(11).pii:13030/qt7rt592tz.
With the perinatal history of prolonged labor and prolonged rupture of membranes, the diagnosis of halo scalp ring was made. This occurs secondary to prolonged pressure of the baby’s scalp with the mother’s pelvic bones, uterus, or cervical area, which causes decreased blood flow to the area, secondary ischemic damage, and in some cases scarring and hair loss.1
The degree of involvement is variable as some babies have mild alopecia and others have severe full-thickness necrosis and scarring. These lesions also can present with associated caput succedaneum and scalp molding, but these were not seen in our patient. Predisposing factors for halo scalp ring include caput succedaneum, prolonged or difficult labor, premature or prolonged rupture of membranes, vaginal delivery, vertex presentation, first delivery, as well as prematurity.2 On physical examination, a semicircular patch of alopecia with associated scarring, crusting, or erythema can be seen in some more severe cases.
The differential diagnosis includes aplasia cutis. In aplasia cutis, there is congenital loss of skin on the affected areas. The scalp usually is affected, but these lesions can occur in any other part of the body. Most patients with aplasia cutis have no other findings, but there are cases that can be associated with other cardiovascular, gastrointestinal, or central nervous system abnormalities. Neonatal lupus also can present with scarring lesions on the scalp, but they usually present a little after birth, mainly affecting the face. The mothers of this children usually have a diagnosis of connective tissue disease and may have positive antibodies to Sjögren’s syndrome antibody A, Sjögren’s syndrome antibody B, or antiribonucleoprotein antibody. Seborrheic dermatitis does not cause scarring alopecia. The lesions present as waxy scaly plaques on the scalp, erythematous waxy plaques behind the ears, face, and folds. Some patients can have hair loss secondary to the inflammation, but the hair grows back once the inflammation is controlled. Dissecting cellulitis is a type of scarring alopecia seen in pubescent and adult individuals. No cases of neonatal dissecting cellulitis have been described.
Halo scalp ring is not associated with any other systemic symptoms or syndromes. Extensive imaging and systemic work-up are not required unless the baby presents with other neurologic symptoms. The areas can be treated with petrolatum and observation as most lesions resolve.
In cases of extensive areas of scarring alopecia, referral to a plastic surgeon can be made to consider tissue expanders or scar revision prior to the child starting school if the lesions are causing psychological stressors.
The true prevalence of this condition is unknown. We believe halo ring alopecia is sometimes not diagnosed, and as lesions tend to resolve, most cases go unreported.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Pediatr Adolesc Med. 2010;164(7):673.
2. Pediatr Dermatol. 2009 Nov-Dec;26(6):706-8.
3. Dermatol Online J. 2016 Nov 15;22(11).pii:13030/qt7rt592tz.
With the perinatal history of prolonged labor and prolonged rupture of membranes, the diagnosis of halo scalp ring was made. This occurs secondary to prolonged pressure of the baby’s scalp with the mother’s pelvic bones, uterus, or cervical area, which causes decreased blood flow to the area, secondary ischemic damage, and in some cases scarring and hair loss.1
The degree of involvement is variable as some babies have mild alopecia and others have severe full-thickness necrosis and scarring. These lesions also can present with associated caput succedaneum and scalp molding, but these were not seen in our patient. Predisposing factors for halo scalp ring include caput succedaneum, prolonged or difficult labor, premature or prolonged rupture of membranes, vaginal delivery, vertex presentation, first delivery, as well as prematurity.2 On physical examination, a semicircular patch of alopecia with associated scarring, crusting, or erythema can be seen in some more severe cases.
The differential diagnosis includes aplasia cutis. In aplasia cutis, there is congenital loss of skin on the affected areas. The scalp usually is affected, but these lesions can occur in any other part of the body. Most patients with aplasia cutis have no other findings, but there are cases that can be associated with other cardiovascular, gastrointestinal, or central nervous system abnormalities. Neonatal lupus also can present with scarring lesions on the scalp, but they usually present a little after birth, mainly affecting the face. The mothers of this children usually have a diagnosis of connective tissue disease and may have positive antibodies to Sjögren’s syndrome antibody A, Sjögren’s syndrome antibody B, or antiribonucleoprotein antibody. Seborrheic dermatitis does not cause scarring alopecia. The lesions present as waxy scaly plaques on the scalp, erythematous waxy plaques behind the ears, face, and folds. Some patients can have hair loss secondary to the inflammation, but the hair grows back once the inflammation is controlled. Dissecting cellulitis is a type of scarring alopecia seen in pubescent and adult individuals. No cases of neonatal dissecting cellulitis have been described.
Halo scalp ring is not associated with any other systemic symptoms or syndromes. Extensive imaging and systemic work-up are not required unless the baby presents with other neurologic symptoms. The areas can be treated with petrolatum and observation as most lesions resolve.
In cases of extensive areas of scarring alopecia, referral to a plastic surgeon can be made to consider tissue expanders or scar revision prior to the child starting school if the lesions are causing psychological stressors.
The true prevalence of this condition is unknown. We believe halo ring alopecia is sometimes not diagnosed, and as lesions tend to resolve, most cases go unreported.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego. Email her at [email protected].
References
1. Arch Pediatr Adolesc Med. 2010;164(7):673.
2. Pediatr Dermatol. 2009 Nov-Dec;26(6):706-8.
3. Dermatol Online J. 2016 Nov 15;22(11).pii:13030/qt7rt592tz.
A 2-month-old male is referred to our pediatric dermatology clinic for evaluation of persistent seborrheic dermatitis. The mother reports that he presented with a rash on his scalp a few days after birth. She has been treating the crusted areas with clotrimazole and hydrocortisone and has noted improvement on the crusting, but now is worried that there is some scarring. The affected areas are not bleeding or tender. There are no other rashes elsewhere in the body.
He was born at 36 weeks from a 35-year-old gravida 1 para 0 woman with adequate prenatal care. The mother was diagnosed with preeclampsia and was induced. She had a prolonged labor and had premature rupture of membranes. The baby was delivered via cesarean section because of failure to progress and fetal distress; forceps, vacuum, and a scalp probe were not used during delivery. He was admitted to the neonatal unit for 5 days for sepsis work-up and respiratory distress. No intubation was needed.
Besides the preeclampsia, the mother denied any other medical conditions and was not taking any medications. He has met all developmental milestones for his age. He has no history of seizures.
On physical exam, there are semicircular patches of alopecia on the scalp. Some areas have pink, rubbery plaques with loss of hair follicles. On the frontal scalp, there are waxy plaques.
There is a blanchable violaceous patch on the occiput and there are some erythematous papules on the cheeks.
Consider hormones and mood in adolescent girls
Prior to puberty, the rate of mood disorders in males and females is roughly the same; however in adolescence, depression doubles in (biological) girls. While the association isn’t clear, it is reasonable to consider that hormones may be involved, at least for some. For instance, we know that menstrual cycle–related mood changes have been noted since the time of Hippocrates. In this article, I will discuss premenstrual dysphoric disorder (PMDD), as well as potential mood-related side effects of hormonal contraceptives. Because I am talking about physiology, I will be referring to individuals born as biological females in the absence of any hormonal gender treatments, regardless of identified gender.
Many young women will acknowledge somatic and/or psychological symptoms that occur in the luteal phase of their cycle, most commonly in the week before menses begins. The most common somatic symptom is bloating, and mood symptoms are irritability and mood lability.1 To meet criteria for premenstrual syndrome (PMS), a woman must endorse one symptom that causes impairment in their functioning and reoccurs over consecutive cycles. PMDD is more specific and involves five or more affective symptoms, at least one of which is consistent with depressed mood, irritability, anxiety, or mood lability. The other potential symptoms include impaired concentration, fatigue, insomnia or hypersomnia, anhedonia, and appetite issues, all of which are included as criteria for major depression. The population prevalence has been quoted between 2% and 5% and is relatively stable across cultures.1 It tends to be highly genetic, as well as highly comorbid with other psychiatric disorders.2 Girls and women with higher rates of trauma appear to be more likely to experience symptoms,3 which indicates there are environmental influences that can interact with genetic vulnerability.
Interestingly, studies have not found differences in serum hormone levels between those with PMDD and others, which leads to the hypothesis that the main difference is in a woman’s sensitivity to circulating hormones,4 and there has been some evidence of different concentrations of neurotransmitters between affected and unaffected women. Many hormone-neurotransmitter interactions have been described, but two that have received the most attention include the relationship between progesterone, its main metabolite allopregnanolone, and gamma-aminobutyric acid (GABA) receptors. Allopregnanolone, which interacts with GABA receptors similarly to benzodiazepines, tends to be higher in the luteal phase, rising with progesterone, and the concentration quickly recedes at the onset of menses as progesterone levels drop off. The other highly notable relationship is the positive association between estradiol and the expression of the serotonin transporter (SERT) genes, which can potentially lead to higher levels of circulating serotonin in the follicular phase.
When PMDD is suspected in an adolescent who presents with intermittent mood and anxiety symptoms that lessen or disappear at baseline and appear unrelated to circumstances, it is important to check in regarding monthly patterns. It can be challenging for adolescents to make this connection, and even more so prior to achieving cycle regularity. Observational studies suggest that by age 14 years, about 82% of girls have a regular cycle.5 The best way to help a patient make the connection is to suggest a period tracking app on their smartphone or tablet. There are many available period trackers that track mood as well and are free to download. Sometimes, simply the act of tracking and bringing awareness to the pattern is therapeutic in itself; sometimes, more formal treatment is needed.
Once the diagnosis of PMDD has been established, there are several options for treatment that range from supplements and herbal remedies to SSRIs, as well as psychotherapy. Treatment may begin with calcium supplements (1,200 mg have been effective in reducing symptoms)6 and referral for cognitive-behavioral therapy (CBT). CBT appears to be associated with a shift in the ability to attribute symptoms to hormones,7 which can help decrease hopelessness and reactivity. SSRIs are another effective strategy to treat PMDD, both taken daily and continuously and also taken in a pulsed fashion, starting with the onset of symptoms or 7-10 days before the period starts and stopping on the first day of menses. Low doses of sertraline, fluoxetine, paroxetine, escitalopram and citalopram have been studied.8 There is some low-quality evidence for herbal supplements as well, probably the most consistent finding is for Vitex taken the week prior to menses.9 Finally, certain oral contraceptives have been associated with PMDD symptom reduction, specifically formulations with 3 mg of drospirenone (a fourth generation progesterone) and 20 mcg of ethinyl estradiol.10 Other formulations, including progesterone-only pills, have not been helpful and have been demonstrated to have a negative effect on mood.11
The literature on hormonal contraceptives and mood can be confusing. While oral agents containing drospirenone have been helpful for premenstrual dysphoria, other studies outside of the PMDD literature have found positive associations between oral contraceptives and depression in adolescents.11 Girls taking combined oral contraceptives seem to be at a 1.8-fold risk of depression, while girls taking progesterone-only formulations were at 2.2 times the risk of developing depression, compared with girls who weren’t taking anything. These days, more pediatricians are recommending long acting reversible contraceptives (LARCs), and there is some thought that even these may carry some risk, but this remains to be studied.
In conclusion, As a provider, you are in a special position to help your patients by bringing nonjudgmental awareness to the potential contribution of their own cyclical hormones or that of exogenous hormones associated with contraceptive choices. Whether it means switching contraceptives, adding calcium or starting a low dose SSRI for 1 week a month, there are many ways to approach symptoms. Often, simply helping make the connection between physiology and mood can be empowering.
Dr. Guth is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and the University of Vermont, both in Burlington. Email her at [email protected].
References
1. Am J Psychiatry. 2012 May;169(5):465-75.
2. Arch Womens Ment Health. 2004 Feb;7(1):37-47.
3. Arch Womens Ment Health. 2011 Oct;14(5):383-93.
4. Curr Psychiatry Rep. 2015 Nov;17(11):87.
5. Ital J Pediatr. 2012 Aug 14;38:38.
6. Am J Obstet Gynecol. 1998 Aug;179(2):444-52.
7. J Clin Psychol Med Settings. 2012 Sep;19(3):308-19.
8. Cochrane Database Syst Rev. 2013 Jun 7;(6):CD001396.
9. J Psychosom Obstet Gynaecol. 2011 Mar;32(1):42-51.
10. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD006586.
11. PLoS One. 2018 Mar 22;13(3):e0194773.
Prior to puberty, the rate of mood disorders in males and females is roughly the same; however in adolescence, depression doubles in (biological) girls. While the association isn’t clear, it is reasonable to consider that hormones may be involved, at least for some. For instance, we know that menstrual cycle–related mood changes have been noted since the time of Hippocrates. In this article, I will discuss premenstrual dysphoric disorder (PMDD), as well as potential mood-related side effects of hormonal contraceptives. Because I am talking about physiology, I will be referring to individuals born as biological females in the absence of any hormonal gender treatments, regardless of identified gender.
Many young women will acknowledge somatic and/or psychological symptoms that occur in the luteal phase of their cycle, most commonly in the week before menses begins. The most common somatic symptom is bloating, and mood symptoms are irritability and mood lability.1 To meet criteria for premenstrual syndrome (PMS), a woman must endorse one symptom that causes impairment in their functioning and reoccurs over consecutive cycles. PMDD is more specific and involves five or more affective symptoms, at least one of which is consistent with depressed mood, irritability, anxiety, or mood lability. The other potential symptoms include impaired concentration, fatigue, insomnia or hypersomnia, anhedonia, and appetite issues, all of which are included as criteria for major depression. The population prevalence has been quoted between 2% and 5% and is relatively stable across cultures.1 It tends to be highly genetic, as well as highly comorbid with other psychiatric disorders.2 Girls and women with higher rates of trauma appear to be more likely to experience symptoms,3 which indicates there are environmental influences that can interact with genetic vulnerability.
Interestingly, studies have not found differences in serum hormone levels between those with PMDD and others, which leads to the hypothesis that the main difference is in a woman’s sensitivity to circulating hormones,4 and there has been some evidence of different concentrations of neurotransmitters between affected and unaffected women. Many hormone-neurotransmitter interactions have been described, but two that have received the most attention include the relationship between progesterone, its main metabolite allopregnanolone, and gamma-aminobutyric acid (GABA) receptors. Allopregnanolone, which interacts with GABA receptors similarly to benzodiazepines, tends to be higher in the luteal phase, rising with progesterone, and the concentration quickly recedes at the onset of menses as progesterone levels drop off. The other highly notable relationship is the positive association between estradiol and the expression of the serotonin transporter (SERT) genes, which can potentially lead to higher levels of circulating serotonin in the follicular phase.
When PMDD is suspected in an adolescent who presents with intermittent mood and anxiety symptoms that lessen or disappear at baseline and appear unrelated to circumstances, it is important to check in regarding monthly patterns. It can be challenging for adolescents to make this connection, and even more so prior to achieving cycle regularity. Observational studies suggest that by age 14 years, about 82% of girls have a regular cycle.5 The best way to help a patient make the connection is to suggest a period tracking app on their smartphone or tablet. There are many available period trackers that track mood as well and are free to download. Sometimes, simply the act of tracking and bringing awareness to the pattern is therapeutic in itself; sometimes, more formal treatment is needed.
Once the diagnosis of PMDD has been established, there are several options for treatment that range from supplements and herbal remedies to SSRIs, as well as psychotherapy. Treatment may begin with calcium supplements (1,200 mg have been effective in reducing symptoms)6 and referral for cognitive-behavioral therapy (CBT). CBT appears to be associated with a shift in the ability to attribute symptoms to hormones,7 which can help decrease hopelessness and reactivity. SSRIs are another effective strategy to treat PMDD, both taken daily and continuously and also taken in a pulsed fashion, starting with the onset of symptoms or 7-10 days before the period starts and stopping on the first day of menses. Low doses of sertraline, fluoxetine, paroxetine, escitalopram and citalopram have been studied.8 There is some low-quality evidence for herbal supplements as well, probably the most consistent finding is for Vitex taken the week prior to menses.9 Finally, certain oral contraceptives have been associated with PMDD symptom reduction, specifically formulations with 3 mg of drospirenone (a fourth generation progesterone) and 20 mcg of ethinyl estradiol.10 Other formulations, including progesterone-only pills, have not been helpful and have been demonstrated to have a negative effect on mood.11
The literature on hormonal contraceptives and mood can be confusing. While oral agents containing drospirenone have been helpful for premenstrual dysphoria, other studies outside of the PMDD literature have found positive associations between oral contraceptives and depression in adolescents.11 Girls taking combined oral contraceptives seem to be at a 1.8-fold risk of depression, while girls taking progesterone-only formulations were at 2.2 times the risk of developing depression, compared with girls who weren’t taking anything. These days, more pediatricians are recommending long acting reversible contraceptives (LARCs), and there is some thought that even these may carry some risk, but this remains to be studied.
In conclusion, As a provider, you are in a special position to help your patients by bringing nonjudgmental awareness to the potential contribution of their own cyclical hormones or that of exogenous hormones associated with contraceptive choices. Whether it means switching contraceptives, adding calcium or starting a low dose SSRI for 1 week a month, there are many ways to approach symptoms. Often, simply helping make the connection between physiology and mood can be empowering.
Dr. Guth is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and the University of Vermont, both in Burlington. Email her at [email protected].
References
1. Am J Psychiatry. 2012 May;169(5):465-75.
2. Arch Womens Ment Health. 2004 Feb;7(1):37-47.
3. Arch Womens Ment Health. 2011 Oct;14(5):383-93.
4. Curr Psychiatry Rep. 2015 Nov;17(11):87.
5. Ital J Pediatr. 2012 Aug 14;38:38.
6. Am J Obstet Gynecol. 1998 Aug;179(2):444-52.
7. J Clin Psychol Med Settings. 2012 Sep;19(3):308-19.
8. Cochrane Database Syst Rev. 2013 Jun 7;(6):CD001396.
9. J Psychosom Obstet Gynaecol. 2011 Mar;32(1):42-51.
10. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD006586.
11. PLoS One. 2018 Mar 22;13(3):e0194773.
Prior to puberty, the rate of mood disorders in males and females is roughly the same; however in adolescence, depression doubles in (biological) girls. While the association isn’t clear, it is reasonable to consider that hormones may be involved, at least for some. For instance, we know that menstrual cycle–related mood changes have been noted since the time of Hippocrates. In this article, I will discuss premenstrual dysphoric disorder (PMDD), as well as potential mood-related side effects of hormonal contraceptives. Because I am talking about physiology, I will be referring to individuals born as biological females in the absence of any hormonal gender treatments, regardless of identified gender.
Many young women will acknowledge somatic and/or psychological symptoms that occur in the luteal phase of their cycle, most commonly in the week before menses begins. The most common somatic symptom is bloating, and mood symptoms are irritability and mood lability.1 To meet criteria for premenstrual syndrome (PMS), a woman must endorse one symptom that causes impairment in their functioning and reoccurs over consecutive cycles. PMDD is more specific and involves five or more affective symptoms, at least one of which is consistent with depressed mood, irritability, anxiety, or mood lability. The other potential symptoms include impaired concentration, fatigue, insomnia or hypersomnia, anhedonia, and appetite issues, all of which are included as criteria for major depression. The population prevalence has been quoted between 2% and 5% and is relatively stable across cultures.1 It tends to be highly genetic, as well as highly comorbid with other psychiatric disorders.2 Girls and women with higher rates of trauma appear to be more likely to experience symptoms,3 which indicates there are environmental influences that can interact with genetic vulnerability.
Interestingly, studies have not found differences in serum hormone levels between those with PMDD and others, which leads to the hypothesis that the main difference is in a woman’s sensitivity to circulating hormones,4 and there has been some evidence of different concentrations of neurotransmitters between affected and unaffected women. Many hormone-neurotransmitter interactions have been described, but two that have received the most attention include the relationship between progesterone, its main metabolite allopregnanolone, and gamma-aminobutyric acid (GABA) receptors. Allopregnanolone, which interacts with GABA receptors similarly to benzodiazepines, tends to be higher in the luteal phase, rising with progesterone, and the concentration quickly recedes at the onset of menses as progesterone levels drop off. The other highly notable relationship is the positive association between estradiol and the expression of the serotonin transporter (SERT) genes, which can potentially lead to higher levels of circulating serotonin in the follicular phase.
When PMDD is suspected in an adolescent who presents with intermittent mood and anxiety symptoms that lessen or disappear at baseline and appear unrelated to circumstances, it is important to check in regarding monthly patterns. It can be challenging for adolescents to make this connection, and even more so prior to achieving cycle regularity. Observational studies suggest that by age 14 years, about 82% of girls have a regular cycle.5 The best way to help a patient make the connection is to suggest a period tracking app on their smartphone or tablet. There are many available period trackers that track mood as well and are free to download. Sometimes, simply the act of tracking and bringing awareness to the pattern is therapeutic in itself; sometimes, more formal treatment is needed.
Once the diagnosis of PMDD has been established, there are several options for treatment that range from supplements and herbal remedies to SSRIs, as well as psychotherapy. Treatment may begin with calcium supplements (1,200 mg have been effective in reducing symptoms)6 and referral for cognitive-behavioral therapy (CBT). CBT appears to be associated with a shift in the ability to attribute symptoms to hormones,7 which can help decrease hopelessness and reactivity. SSRIs are another effective strategy to treat PMDD, both taken daily and continuously and also taken in a pulsed fashion, starting with the onset of symptoms or 7-10 days before the period starts and stopping on the first day of menses. Low doses of sertraline, fluoxetine, paroxetine, escitalopram and citalopram have been studied.8 There is some low-quality evidence for herbal supplements as well, probably the most consistent finding is for Vitex taken the week prior to menses.9 Finally, certain oral contraceptives have been associated with PMDD symptom reduction, specifically formulations with 3 mg of drospirenone (a fourth generation progesterone) and 20 mcg of ethinyl estradiol.10 Other formulations, including progesterone-only pills, have not been helpful and have been demonstrated to have a negative effect on mood.11
The literature on hormonal contraceptives and mood can be confusing. While oral agents containing drospirenone have been helpful for premenstrual dysphoria, other studies outside of the PMDD literature have found positive associations between oral contraceptives and depression in adolescents.11 Girls taking combined oral contraceptives seem to be at a 1.8-fold risk of depression, while girls taking progesterone-only formulations were at 2.2 times the risk of developing depression, compared with girls who weren’t taking anything. These days, more pediatricians are recommending long acting reversible contraceptives (LARCs), and there is some thought that even these may carry some risk, but this remains to be studied.
In conclusion, As a provider, you are in a special position to help your patients by bringing nonjudgmental awareness to the potential contribution of their own cyclical hormones or that of exogenous hormones associated with contraceptive choices. Whether it means switching contraceptives, adding calcium or starting a low dose SSRI for 1 week a month, there are many ways to approach symptoms. Often, simply helping make the connection between physiology and mood can be empowering.
Dr. Guth is an assistant professor in the department of psychiatry at the University of Vermont Medical Center and the University of Vermont, both in Burlington. Email her at [email protected].
References
1. Am J Psychiatry. 2012 May;169(5):465-75.
2. Arch Womens Ment Health. 2004 Feb;7(1):37-47.
3. Arch Womens Ment Health. 2011 Oct;14(5):383-93.
4. Curr Psychiatry Rep. 2015 Nov;17(11):87.
5. Ital J Pediatr. 2012 Aug 14;38:38.
6. Am J Obstet Gynecol. 1998 Aug;179(2):444-52.
7. J Clin Psychol Med Settings. 2012 Sep;19(3):308-19.
8. Cochrane Database Syst Rev. 2013 Jun 7;(6):CD001396.
9. J Psychosom Obstet Gynaecol. 2011 Mar;32(1):42-51.
10. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD006586.
11. PLoS One. 2018 Mar 22;13(3):e0194773.
ALERT: Grandmother in the room!
One of the things that makes pediatrics challenging and potentially rewarding is that there are often multiple family members in the room for our visits.
With an expansion of our acknowledged role safeguarding and promoting a child’s current and future health to include asking about environmental and caregiving influences comes some tricky dynamics. The parent is in the room with a teen who has a secret love life. A father is in the room with the mother he threatens at home. The mother is in the room with the preschooler she has hit. There is risk but also opportunity for valuable discourse, discovery, and healing.
We now know that about 50% of the future morbidity of the child before us will be determined by the adverse childhood experiences (ACEs) they experience during the exact years (0-18) they are under our health supervision. Many of these risk factors come from home life and may be preventable or at least moderated. When we begin screening for ACEs we are asking the parents about bad things that happened when they were growing up.
The dilemma that came up recently was the grandmother in the room with the daughter she raised who is now caring for her own child. In this situation, you may be in the presence of the person whom the parent feels inflicted – or failed to prevent – the hurts being discussed.
“How are things at home?” you ask dutifully. Mother says “Fine,” but rolls her eyes. Grandma, sitting in the room looking at her phone, says something to the mother in a foreign language. You are in the dark, but sense friction.
Even in modern times, it is respectful to address the grandparent who is present first in a visit, introducing yourself, and finding out the relationship and living situation. Previsit questionnaires can help set visit priorities and alert you to what topics may be better discussed in private, perhaps including ACEs. It is important to ask the parent permission to discuss potentially sensitive topics when the grandparent is present or ask for the grandparent to step out of the room.
Why not just be sure the grandmother stays in the waiting room? If the hurts from the past still are affecting the mother now, moderating an open conversation about those experiences can be very valuable to reducing their impact on present parenting or dysfunctional coping strategies.
Some experts say that any enumeration of ACEs should include asking “Which of these are still bothering you now?” Saying, “Parenting often brings up memories of similar situations from when we were young that can tilt how we act toward our own children,” then asking, “Can we talk about those experiences you had?”
Eliciting the grandparent’s perspective with a question such as “What were those years of parenting like for you?” respects the significance of the grandparent’s role. The grandparents probably were young, stressed, and inexperienced when they were the mother’s primary caregivers. Or the grandparent may have gratefully erased memories of the tough moments but the parent clearly remembers the childhood pain, because hurts sear themselves into our brains more than positive or neutral experiences do. She or he may have been holding resentment against the grandparent and living it out for years and now in her/his parenting.
Suddenly, you and the parent may hear the grandparent reveal other factors that would never have been visible to the child then and may never have been brought up because the grandparent was working three jobs because the father was deployed; caring for a sick sibling; suffering from depression; being subject to an abusive alcoholic spouse; having to keep the children inside because of shooting in the neighborhood, etc. You may be able reframe what is said to point out that the grandparents “did the best they could at the time and with the resources and skills they possessed then.”
Mothers who had troubled relationships with their own mothers (e.g. insecure attachment) tend to pass these patterns down in their own parenting unless they have processed the experiences and come to a place of acceptance. People may process their past effectively on their own, some through mental health counseling or religion, but for others a brief “Ah ha!” moment may help settle the waters.
Ask the grandparent as well as the parent, “What are you hoping will go differently than when [parent] was growing up?” Some try to make up for what they see as their own mistakes by advocating coming down harder on their grandchildren than they did on their own. An example among my patients was the grandmother whose daughter had become a heroin user, who was very strict and critical in her determination that her granddaughter would not fall into the wrong crowd. Voicing this for her as a possible reason for her strident posture dramatically shifted her attitude.
Other times parents, maybe grateful for a place to live with the grandparents but also trapped there financially, are simmering with anger at the grandparents’ intrusions into their parenting choices. It can be useful to point out that grandparents, no longer caught up in careers, may feel the need to be useful and have a role by giving advice or bustling around cleaning and buying things for their grandchildren. Hey, any help can be welcome if you don’t take it as meaning you are not doing a good job! Although limits can be set.
Transitioning to regarding one’s grown child as an adult and letting them make their own choices and mistakes, waiting until asked for advice, can take a lot of tongue biting after years of active parenting. One essay I read about an adult’s fond memories of his father included the realization that some of the best parenting he recalled was when his father left things unsaid.
Cultural differences in parenting between grandparent and parent can be another source of stress. Assimilation happens fast in America. The newest generation is often trying very hard NOT to be like the “old country.” The parents’ turning away from the grandparents’ culture or religion can threaten their values and culture for which they may have sacrificed everything. Advising the parent to celebrate at least some traditions may reduce the tension at little cost.
As of 2016, 5% of U.S. children in two-parent families lived with a grandparent and 15% of single parents do. This is much higher in certain ethnic groups where living with your adult children is expected. Even if not living together, their influence may be great. Many parents report more stress than support from grandparents. Negative mother-in-law stories abound! That is why simply asking if the parent “has anyone to help them” as a way of assessing social support can be very misleading. You also may ask “Is that more a help or a problem?”
When grandparents undermine the parents’ rules, of course, a family meeting is in order. But grandparents, with the wisdom of years and reduced life pressure, tend to care for children more generously and less critically than when they were main caregivers, conveying unconditional love that can buffer stresses and be remembered by the beloved child forever.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
One of the things that makes pediatrics challenging and potentially rewarding is that there are often multiple family members in the room for our visits.
With an expansion of our acknowledged role safeguarding and promoting a child’s current and future health to include asking about environmental and caregiving influences comes some tricky dynamics. The parent is in the room with a teen who has a secret love life. A father is in the room with the mother he threatens at home. The mother is in the room with the preschooler she has hit. There is risk but also opportunity for valuable discourse, discovery, and healing.
We now know that about 50% of the future morbidity of the child before us will be determined by the adverse childhood experiences (ACEs) they experience during the exact years (0-18) they are under our health supervision. Many of these risk factors come from home life and may be preventable or at least moderated. When we begin screening for ACEs we are asking the parents about bad things that happened when they were growing up.
The dilemma that came up recently was the grandmother in the room with the daughter she raised who is now caring for her own child. In this situation, you may be in the presence of the person whom the parent feels inflicted – or failed to prevent – the hurts being discussed.
“How are things at home?” you ask dutifully. Mother says “Fine,” but rolls her eyes. Grandma, sitting in the room looking at her phone, says something to the mother in a foreign language. You are in the dark, but sense friction.
Even in modern times, it is respectful to address the grandparent who is present first in a visit, introducing yourself, and finding out the relationship and living situation. Previsit questionnaires can help set visit priorities and alert you to what topics may be better discussed in private, perhaps including ACEs. It is important to ask the parent permission to discuss potentially sensitive topics when the grandparent is present or ask for the grandparent to step out of the room.
Why not just be sure the grandmother stays in the waiting room? If the hurts from the past still are affecting the mother now, moderating an open conversation about those experiences can be very valuable to reducing their impact on present parenting or dysfunctional coping strategies.
Some experts say that any enumeration of ACEs should include asking “Which of these are still bothering you now?” Saying, “Parenting often brings up memories of similar situations from when we were young that can tilt how we act toward our own children,” then asking, “Can we talk about those experiences you had?”
Eliciting the grandparent’s perspective with a question such as “What were those years of parenting like for you?” respects the significance of the grandparent’s role. The grandparents probably were young, stressed, and inexperienced when they were the mother’s primary caregivers. Or the grandparent may have gratefully erased memories of the tough moments but the parent clearly remembers the childhood pain, because hurts sear themselves into our brains more than positive or neutral experiences do. She or he may have been holding resentment against the grandparent and living it out for years and now in her/his parenting.
Suddenly, you and the parent may hear the grandparent reveal other factors that would never have been visible to the child then and may never have been brought up because the grandparent was working three jobs because the father was deployed; caring for a sick sibling; suffering from depression; being subject to an abusive alcoholic spouse; having to keep the children inside because of shooting in the neighborhood, etc. You may be able reframe what is said to point out that the grandparents “did the best they could at the time and with the resources and skills they possessed then.”
Mothers who had troubled relationships with their own mothers (e.g. insecure attachment) tend to pass these patterns down in their own parenting unless they have processed the experiences and come to a place of acceptance. People may process their past effectively on their own, some through mental health counseling or religion, but for others a brief “Ah ha!” moment may help settle the waters.
Ask the grandparent as well as the parent, “What are you hoping will go differently than when [parent] was growing up?” Some try to make up for what they see as their own mistakes by advocating coming down harder on their grandchildren than they did on their own. An example among my patients was the grandmother whose daughter had become a heroin user, who was very strict and critical in her determination that her granddaughter would not fall into the wrong crowd. Voicing this for her as a possible reason for her strident posture dramatically shifted her attitude.
Other times parents, maybe grateful for a place to live with the grandparents but also trapped there financially, are simmering with anger at the grandparents’ intrusions into their parenting choices. It can be useful to point out that grandparents, no longer caught up in careers, may feel the need to be useful and have a role by giving advice or bustling around cleaning and buying things for their grandchildren. Hey, any help can be welcome if you don’t take it as meaning you are not doing a good job! Although limits can be set.
Transitioning to regarding one’s grown child as an adult and letting them make their own choices and mistakes, waiting until asked for advice, can take a lot of tongue biting after years of active parenting. One essay I read about an adult’s fond memories of his father included the realization that some of the best parenting he recalled was when his father left things unsaid.
Cultural differences in parenting between grandparent and parent can be another source of stress. Assimilation happens fast in America. The newest generation is often trying very hard NOT to be like the “old country.” The parents’ turning away from the grandparents’ culture or religion can threaten their values and culture for which they may have sacrificed everything. Advising the parent to celebrate at least some traditions may reduce the tension at little cost.
As of 2016, 5% of U.S. children in two-parent families lived with a grandparent and 15% of single parents do. This is much higher in certain ethnic groups where living with your adult children is expected. Even if not living together, their influence may be great. Many parents report more stress than support from grandparents. Negative mother-in-law stories abound! That is why simply asking if the parent “has anyone to help them” as a way of assessing social support can be very misleading. You also may ask “Is that more a help or a problem?”
When grandparents undermine the parents’ rules, of course, a family meeting is in order. But grandparents, with the wisdom of years and reduced life pressure, tend to care for children more generously and less critically than when they were main caregivers, conveying unconditional love that can buffer stresses and be remembered by the beloved child forever.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
One of the things that makes pediatrics challenging and potentially rewarding is that there are often multiple family members in the room for our visits.
With an expansion of our acknowledged role safeguarding and promoting a child’s current and future health to include asking about environmental and caregiving influences comes some tricky dynamics. The parent is in the room with a teen who has a secret love life. A father is in the room with the mother he threatens at home. The mother is in the room with the preschooler she has hit. There is risk but also opportunity for valuable discourse, discovery, and healing.
We now know that about 50% of the future morbidity of the child before us will be determined by the adverse childhood experiences (ACEs) they experience during the exact years (0-18) they are under our health supervision. Many of these risk factors come from home life and may be preventable or at least moderated. When we begin screening for ACEs we are asking the parents about bad things that happened when they were growing up.
The dilemma that came up recently was the grandmother in the room with the daughter she raised who is now caring for her own child. In this situation, you may be in the presence of the person whom the parent feels inflicted – or failed to prevent – the hurts being discussed.
“How are things at home?” you ask dutifully. Mother says “Fine,” but rolls her eyes. Grandma, sitting in the room looking at her phone, says something to the mother in a foreign language. You are in the dark, but sense friction.
Even in modern times, it is respectful to address the grandparent who is present first in a visit, introducing yourself, and finding out the relationship and living situation. Previsit questionnaires can help set visit priorities and alert you to what topics may be better discussed in private, perhaps including ACEs. It is important to ask the parent permission to discuss potentially sensitive topics when the grandparent is present or ask for the grandparent to step out of the room.
Why not just be sure the grandmother stays in the waiting room? If the hurts from the past still are affecting the mother now, moderating an open conversation about those experiences can be very valuable to reducing their impact on present parenting or dysfunctional coping strategies.
Some experts say that any enumeration of ACEs should include asking “Which of these are still bothering you now?” Saying, “Parenting often brings up memories of similar situations from when we were young that can tilt how we act toward our own children,” then asking, “Can we talk about those experiences you had?”
Eliciting the grandparent’s perspective with a question such as “What were those years of parenting like for you?” respects the significance of the grandparent’s role. The grandparents probably were young, stressed, and inexperienced when they were the mother’s primary caregivers. Or the grandparent may have gratefully erased memories of the tough moments but the parent clearly remembers the childhood pain, because hurts sear themselves into our brains more than positive or neutral experiences do. She or he may have been holding resentment against the grandparent and living it out for years and now in her/his parenting.
Suddenly, you and the parent may hear the grandparent reveal other factors that would never have been visible to the child then and may never have been brought up because the grandparent was working three jobs because the father was deployed; caring for a sick sibling; suffering from depression; being subject to an abusive alcoholic spouse; having to keep the children inside because of shooting in the neighborhood, etc. You may be able reframe what is said to point out that the grandparents “did the best they could at the time and with the resources and skills they possessed then.”
Mothers who had troubled relationships with their own mothers (e.g. insecure attachment) tend to pass these patterns down in their own parenting unless they have processed the experiences and come to a place of acceptance. People may process their past effectively on their own, some through mental health counseling or religion, but for others a brief “Ah ha!” moment may help settle the waters.
Ask the grandparent as well as the parent, “What are you hoping will go differently than when [parent] was growing up?” Some try to make up for what they see as their own mistakes by advocating coming down harder on their grandchildren than they did on their own. An example among my patients was the grandmother whose daughter had become a heroin user, who was very strict and critical in her determination that her granddaughter would not fall into the wrong crowd. Voicing this for her as a possible reason for her strident posture dramatically shifted her attitude.
Other times parents, maybe grateful for a place to live with the grandparents but also trapped there financially, are simmering with anger at the grandparents’ intrusions into their parenting choices. It can be useful to point out that grandparents, no longer caught up in careers, may feel the need to be useful and have a role by giving advice or bustling around cleaning and buying things for their grandchildren. Hey, any help can be welcome if you don’t take it as meaning you are not doing a good job! Although limits can be set.
Transitioning to regarding one’s grown child as an adult and letting them make their own choices and mistakes, waiting until asked for advice, can take a lot of tongue biting after years of active parenting. One essay I read about an adult’s fond memories of his father included the realization that some of the best parenting he recalled was when his father left things unsaid.
Cultural differences in parenting between grandparent and parent can be another source of stress. Assimilation happens fast in America. The newest generation is often trying very hard NOT to be like the “old country.” The parents’ turning away from the grandparents’ culture or religion can threaten their values and culture for which they may have sacrificed everything. Advising the parent to celebrate at least some traditions may reduce the tension at little cost.
As of 2016, 5% of U.S. children in two-parent families lived with a grandparent and 15% of single parents do. This is much higher in certain ethnic groups where living with your adult children is expected. Even if not living together, their influence may be great. Many parents report more stress than support from grandparents. Negative mother-in-law stories abound! That is why simply asking if the parent “has anyone to help them” as a way of assessing social support can be very misleading. You also may ask “Is that more a help or a problem?”
When grandparents undermine the parents’ rules, of course, a family meeting is in order. But grandparents, with the wisdom of years and reduced life pressure, tend to care for children more generously and less critically than when they were main caregivers, conveying unconditional love that can buffer stresses and be remembered by the beloved child forever.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].