User login
Adverse events reported in one-quarter of inpatient admissions
as indicated from data from 2,809 admissions at 11 hospitals.
The 1991 Harvard Medical Practice Study, which focused on medical injury and litigation, documented an adverse event rate of 3.7 events per 100 admissions; 28% of those events were attributed to negligence, write David W. Bates, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
Although patient safety has changed significantly since 1991, documenting improvements has been challenging, the researchers say. Several reports have shown a decrease in health care–associated infections. However, other aspects of safety – notably, adverse drug events, defined as injuries resulting from drugs taken – are not easily measured and tracked, the researchers say.
“We have not had good estimates of how much harm is being caused by care in hospitals in an ongoing way that looked across all types of adverse events,” and the current review is therefore important, Dr. Bates said in an interview.
In a study recently published in the New England Journal of Medicine, the researchers analyzed a random sample of 2,809 hospital admissions from 11 hospitals in Massachusetts during the 2018 calendar year. The hospitals ranged in size from fewer than 100 beds to more than 700 beds; all patients were aged 18 years and older. A panel of nine nurses reviewed the admissions records to identify potential adverse events, and eight physicians reviewed the adverse event summaries and either agreed or disagreed with the adverse event type. The severity of each event was ranked using a general severity scale into categories of significant, serious, life-threatening, or fatal.
Overall, at least one adverse event was identified in 23.6% of the hospital admissions. A total of 978 adverse events were deemed to have occurred during the index admission, and 222 of these (22.7%) were deemed preventable. Among the preventable adverse events, 19.7% were classified as serious, 3.3% as life-threatening, and 0.5% as fatal.
A total of 523 admissions (18.6%) involved at least one significant adverse event, defined as an event that caused unnecessary harm but from which recovery was rapid. A total of 211 admissions involved a serious adverse event, defined as harm resulting in substantial intervention or prolonged recovery; 34 included at least one life-threatening event; and seven admissions involved a fatal adverse event.
A total of 191 admissions involved at least one adverse event deemed preventable. Of those, 29 involved at least one preventable adverse event that was serious, life-threatening, or fatal, the researchers write. Of the seven deaths in the study population, one was deemed preventable.
The most common adverse events were adverse drug events, which accounted for 39.0% of the adverse events; surgical or other procedural events accounted for 30.4%; patient care events (including falls and pressure ulcers) accounted for 15.0%; and health care–associated infections accounted for 11.9%.
Overcoming barriers to better safety
“The overall level of harm, with nearly 1 in 4 patients suffering an adverse event, was higher than I expected it might be,” Dr. Bates told this news organization. However, techniques for identifying adverse events have improved, and “it is easier to find them in electronic records than in paper records,” he noted.
“Hospitals have many issues they are currently dealing with since COVID, and one issue is simply prioritization,” Dr. Bates said. “But it is now possible to measure harm for all patients using electronic tools, and if hospitals know how much harm they are having in specific areas, they can make choices about which ones to focus on.”
“We now have effective prevention strategies for most of the main kinds of harm,” he said. Generally, rates of harm are high because these strategies are not being used effectively, he said. “In addition, there are new tools that can be used – for example, to identify patients who are decompensating earlier,” he noted.
As for additional research, some specific types of harm that have been resistant to interventions, such as pressure ulcers, deserve more attention, said Dr. Bates. “In addition, diagnostic errors appear to cause a great deal of harm, but we don’t yet have good strategies for preventing these,” he said.
The study findings were limited by several factors, including the use of data from hospitals that might not represent hospitals at large and by the inclusion mainly of patients with private insurance, the researchers write. Other limitations include the likelihood that some adverse events were missed and the level of agreement on adverse events between adjudicators was only fair.
However, the findings serve as a reminder to health care professionals of the need for continued attention to improving patient safety, and measuring adverse events remains a critical part of guiding these improvements, the researchers conclude.
Timely reassessment and opportunities to improve
In the decades since the publication of the report, “To Err Is Human,” by the National Academies in 2000, significant attention has been paid to improving patient safety during hospitalizations, and health care systems have increased in both system and disease complexity, Said Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview. “Therefore, this study is important in reassessing the safety of inpatient care at the current time,” he said.
“The findings of this study showing preventable adverse events in approximately 7% of all admissions; while concerning, is not surprising, as it is consistent with other studies over time, as the authors have also noted in their discussion,” said Dr. Pal. The current findings “underscore the importance of continuous quality improvement efforts to increase the safety of patient care for hospitalized patients,” he noted.
“The increasing complexity of medical care, fragmentation of health care, structural inequities of health systems, and more recent widespread public health challenges such as the COVID-19 pandemic have been, in my opinion, barriers to improving patient safety,” Dr. Pal said. “The use of innovation and an interdisciplinary approach to patient safety and quality improvement in hospital-based care, such as the use of machine learning to monitor trends and predict the individualized risk of harm, could be a potential way out” to help reduce barriers and improve safety, he said.
“Additional research is needed to understand the key drivers of preventable harm for hospitalized patients in the United States,” said Dr. Pal. “When planning for change, keen attention must be paid to understanding how these [drivers] may differ for patients who have been historically marginalized or are otherwise underserved so as to not exacerbate health care inequities,” he added.
The study was funded by the Controlled Risk Insurance Company and the Risk Management Foundation of the Harvard Medical Institutions. Dr. Bates owns stock options with AESOP, Clew, FeelBetter, Guided Clinical Solutions, MDClone, and ValeraHealth and has grants/contracts from IBM Watson and EarlySense. He has also served as a consultant for CDI Negev. Dr. Pal has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
as indicated from data from 2,809 admissions at 11 hospitals.
The 1991 Harvard Medical Practice Study, which focused on medical injury and litigation, documented an adverse event rate of 3.7 events per 100 admissions; 28% of those events were attributed to negligence, write David W. Bates, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
Although patient safety has changed significantly since 1991, documenting improvements has been challenging, the researchers say. Several reports have shown a decrease in health care–associated infections. However, other aspects of safety – notably, adverse drug events, defined as injuries resulting from drugs taken – are not easily measured and tracked, the researchers say.
“We have not had good estimates of how much harm is being caused by care in hospitals in an ongoing way that looked across all types of adverse events,” and the current review is therefore important, Dr. Bates said in an interview.
In a study recently published in the New England Journal of Medicine, the researchers analyzed a random sample of 2,809 hospital admissions from 11 hospitals in Massachusetts during the 2018 calendar year. The hospitals ranged in size from fewer than 100 beds to more than 700 beds; all patients were aged 18 years and older. A panel of nine nurses reviewed the admissions records to identify potential adverse events, and eight physicians reviewed the adverse event summaries and either agreed or disagreed with the adverse event type. The severity of each event was ranked using a general severity scale into categories of significant, serious, life-threatening, or fatal.
Overall, at least one adverse event was identified in 23.6% of the hospital admissions. A total of 978 adverse events were deemed to have occurred during the index admission, and 222 of these (22.7%) were deemed preventable. Among the preventable adverse events, 19.7% were classified as serious, 3.3% as life-threatening, and 0.5% as fatal.
A total of 523 admissions (18.6%) involved at least one significant adverse event, defined as an event that caused unnecessary harm but from which recovery was rapid. A total of 211 admissions involved a serious adverse event, defined as harm resulting in substantial intervention or prolonged recovery; 34 included at least one life-threatening event; and seven admissions involved a fatal adverse event.
A total of 191 admissions involved at least one adverse event deemed preventable. Of those, 29 involved at least one preventable adverse event that was serious, life-threatening, or fatal, the researchers write. Of the seven deaths in the study population, one was deemed preventable.
The most common adverse events were adverse drug events, which accounted for 39.0% of the adverse events; surgical or other procedural events accounted for 30.4%; patient care events (including falls and pressure ulcers) accounted for 15.0%; and health care–associated infections accounted for 11.9%.
Overcoming barriers to better safety
“The overall level of harm, with nearly 1 in 4 patients suffering an adverse event, was higher than I expected it might be,” Dr. Bates told this news organization. However, techniques for identifying adverse events have improved, and “it is easier to find them in electronic records than in paper records,” he noted.
“Hospitals have many issues they are currently dealing with since COVID, and one issue is simply prioritization,” Dr. Bates said. “But it is now possible to measure harm for all patients using electronic tools, and if hospitals know how much harm they are having in specific areas, they can make choices about which ones to focus on.”
“We now have effective prevention strategies for most of the main kinds of harm,” he said. Generally, rates of harm are high because these strategies are not being used effectively, he said. “In addition, there are new tools that can be used – for example, to identify patients who are decompensating earlier,” he noted.
As for additional research, some specific types of harm that have been resistant to interventions, such as pressure ulcers, deserve more attention, said Dr. Bates. “In addition, diagnostic errors appear to cause a great deal of harm, but we don’t yet have good strategies for preventing these,” he said.
The study findings were limited by several factors, including the use of data from hospitals that might not represent hospitals at large and by the inclusion mainly of patients with private insurance, the researchers write. Other limitations include the likelihood that some adverse events were missed and the level of agreement on adverse events between adjudicators was only fair.
However, the findings serve as a reminder to health care professionals of the need for continued attention to improving patient safety, and measuring adverse events remains a critical part of guiding these improvements, the researchers conclude.
Timely reassessment and opportunities to improve
In the decades since the publication of the report, “To Err Is Human,” by the National Academies in 2000, significant attention has been paid to improving patient safety during hospitalizations, and health care systems have increased in both system and disease complexity, Said Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview. “Therefore, this study is important in reassessing the safety of inpatient care at the current time,” he said.
“The findings of this study showing preventable adverse events in approximately 7% of all admissions; while concerning, is not surprising, as it is consistent with other studies over time, as the authors have also noted in their discussion,” said Dr. Pal. The current findings “underscore the importance of continuous quality improvement efforts to increase the safety of patient care for hospitalized patients,” he noted.
“The increasing complexity of medical care, fragmentation of health care, structural inequities of health systems, and more recent widespread public health challenges such as the COVID-19 pandemic have been, in my opinion, barriers to improving patient safety,” Dr. Pal said. “The use of innovation and an interdisciplinary approach to patient safety and quality improvement in hospital-based care, such as the use of machine learning to monitor trends and predict the individualized risk of harm, could be a potential way out” to help reduce barriers and improve safety, he said.
“Additional research is needed to understand the key drivers of preventable harm for hospitalized patients in the United States,” said Dr. Pal. “When planning for change, keen attention must be paid to understanding how these [drivers] may differ for patients who have been historically marginalized or are otherwise underserved so as to not exacerbate health care inequities,” he added.
The study was funded by the Controlled Risk Insurance Company and the Risk Management Foundation of the Harvard Medical Institutions. Dr. Bates owns stock options with AESOP, Clew, FeelBetter, Guided Clinical Solutions, MDClone, and ValeraHealth and has grants/contracts from IBM Watson and EarlySense. He has also served as a consultant for CDI Negev. Dr. Pal has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
as indicated from data from 2,809 admissions at 11 hospitals.
The 1991 Harvard Medical Practice Study, which focused on medical injury and litigation, documented an adverse event rate of 3.7 events per 100 admissions; 28% of those events were attributed to negligence, write David W. Bates, MD, of Brigham and Women’s Hospital, Boston, and colleagues.
Although patient safety has changed significantly since 1991, documenting improvements has been challenging, the researchers say. Several reports have shown a decrease in health care–associated infections. However, other aspects of safety – notably, adverse drug events, defined as injuries resulting from drugs taken – are not easily measured and tracked, the researchers say.
“We have not had good estimates of how much harm is being caused by care in hospitals in an ongoing way that looked across all types of adverse events,” and the current review is therefore important, Dr. Bates said in an interview.
In a study recently published in the New England Journal of Medicine, the researchers analyzed a random sample of 2,809 hospital admissions from 11 hospitals in Massachusetts during the 2018 calendar year. The hospitals ranged in size from fewer than 100 beds to more than 700 beds; all patients were aged 18 years and older. A panel of nine nurses reviewed the admissions records to identify potential adverse events, and eight physicians reviewed the adverse event summaries and either agreed or disagreed with the adverse event type. The severity of each event was ranked using a general severity scale into categories of significant, serious, life-threatening, or fatal.
Overall, at least one adverse event was identified in 23.6% of the hospital admissions. A total of 978 adverse events were deemed to have occurred during the index admission, and 222 of these (22.7%) were deemed preventable. Among the preventable adverse events, 19.7% were classified as serious, 3.3% as life-threatening, and 0.5% as fatal.
A total of 523 admissions (18.6%) involved at least one significant adverse event, defined as an event that caused unnecessary harm but from which recovery was rapid. A total of 211 admissions involved a serious adverse event, defined as harm resulting in substantial intervention or prolonged recovery; 34 included at least one life-threatening event; and seven admissions involved a fatal adverse event.
A total of 191 admissions involved at least one adverse event deemed preventable. Of those, 29 involved at least one preventable adverse event that was serious, life-threatening, or fatal, the researchers write. Of the seven deaths in the study population, one was deemed preventable.
The most common adverse events were adverse drug events, which accounted for 39.0% of the adverse events; surgical or other procedural events accounted for 30.4%; patient care events (including falls and pressure ulcers) accounted for 15.0%; and health care–associated infections accounted for 11.9%.
Overcoming barriers to better safety
“The overall level of harm, with nearly 1 in 4 patients suffering an adverse event, was higher than I expected it might be,” Dr. Bates told this news organization. However, techniques for identifying adverse events have improved, and “it is easier to find them in electronic records than in paper records,” he noted.
“Hospitals have many issues they are currently dealing with since COVID, and one issue is simply prioritization,” Dr. Bates said. “But it is now possible to measure harm for all patients using electronic tools, and if hospitals know how much harm they are having in specific areas, they can make choices about which ones to focus on.”
“We now have effective prevention strategies for most of the main kinds of harm,” he said. Generally, rates of harm are high because these strategies are not being used effectively, he said. “In addition, there are new tools that can be used – for example, to identify patients who are decompensating earlier,” he noted.
As for additional research, some specific types of harm that have been resistant to interventions, such as pressure ulcers, deserve more attention, said Dr. Bates. “In addition, diagnostic errors appear to cause a great deal of harm, but we don’t yet have good strategies for preventing these,” he said.
The study findings were limited by several factors, including the use of data from hospitals that might not represent hospitals at large and by the inclusion mainly of patients with private insurance, the researchers write. Other limitations include the likelihood that some adverse events were missed and the level of agreement on adverse events between adjudicators was only fair.
However, the findings serve as a reminder to health care professionals of the need for continued attention to improving patient safety, and measuring adverse events remains a critical part of guiding these improvements, the researchers conclude.
Timely reassessment and opportunities to improve
In the decades since the publication of the report, “To Err Is Human,” by the National Academies in 2000, significant attention has been paid to improving patient safety during hospitalizations, and health care systems have increased in both system and disease complexity, Said Suman Pal, MBBS, a specialist in hospital medicine at the University of New Mexico, Albuquerque, said in an interview. “Therefore, this study is important in reassessing the safety of inpatient care at the current time,” he said.
“The findings of this study showing preventable adverse events in approximately 7% of all admissions; while concerning, is not surprising, as it is consistent with other studies over time, as the authors have also noted in their discussion,” said Dr. Pal. The current findings “underscore the importance of continuous quality improvement efforts to increase the safety of patient care for hospitalized patients,” he noted.
“The increasing complexity of medical care, fragmentation of health care, structural inequities of health systems, and more recent widespread public health challenges such as the COVID-19 pandemic have been, in my opinion, barriers to improving patient safety,” Dr. Pal said. “The use of innovation and an interdisciplinary approach to patient safety and quality improvement in hospital-based care, such as the use of machine learning to monitor trends and predict the individualized risk of harm, could be a potential way out” to help reduce barriers and improve safety, he said.
“Additional research is needed to understand the key drivers of preventable harm for hospitalized patients in the United States,” said Dr. Pal. “When planning for change, keen attention must be paid to understanding how these [drivers] may differ for patients who have been historically marginalized or are otherwise underserved so as to not exacerbate health care inequities,” he added.
The study was funded by the Controlled Risk Insurance Company and the Risk Management Foundation of the Harvard Medical Institutions. Dr. Bates owns stock options with AESOP, Clew, FeelBetter, Guided Clinical Solutions, MDClone, and ValeraHealth and has grants/contracts from IBM Watson and EarlySense. He has also served as a consultant for CDI Negev. Dr. Pal has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
How Well Does the Third Dose of COVID-19 Vaccine Work?
How effective are the COVID-19 vaccines, third time around? Researchers compared 2 large groups of veterans to find out how well a third dose protected against documented infection, symptomatic COVID-19, and COVID-19–related hospitalization, intensive care unit (ICU) admission, and death.
The research, published in Nature, used electronic health records of 65,196 veterans who received BNT162b2 (Pfizer-BioNTech) and 65,196 who received mRNA-1273 (Moderna). They chose to study the 16 weeks between October 20, 2021 and February 8, 2022, which included both Delta- and Omicron-variant waves.
During the follow-up (median, 77 days), 2994 COVID-19 infections were documented, of which 200 were detected as symptomatic, 194 required hospitalization, and 52 required ICU admission. Twenty-two patients died.
In a previous head-to-head trial comparing breakthrough COVID-19 outcomes after the first doses of the 2 vaccines (given when the Alpha and Delta variants were predominant), the researchers had found a low risk of documented infection and severe outcomes, but lower for the Moderna vaccine. They note that few head-to-head comparisons have been made of third-dose effectiveness.
As expected, in this trial, the researchers found a “nearly identical” pattern for the risk of the 2 vaccine groups. Although the risks for all of the measured outcomes over 16 weeks were low for both vaccines ≤ 4% for documented infection and < 0.03% for death in each group—those veterans who received the Pfizer-BioNTech vaccine had an excess of 45 documented infections and 11 hospitalizations per 10,000 persons, compared with the Moderna group. The Pfizer-BioNTech group also had a higher risk of documented infection over 9 weeks of follow-up, during which an Omicron-variant predominated.
Given the high effectiveness of a third dose of both vaccines, either vaccine is strongly recommended, the researchers conclude. They point to “evidence of clear and comparable benefits” for the most severe outcomes: The difference in estimated 16-week risk of death between the 2 groups was two-thousandths of 1 %.
They add that, while the differences in estimated risk for less severe outcomes between the 2 groups were small on the absolute scale, they may be meaningful when considering the population scale at which these vaccines are deployed.
How effective are the COVID-19 vaccines, third time around? Researchers compared 2 large groups of veterans to find out how well a third dose protected against documented infection, symptomatic COVID-19, and COVID-19–related hospitalization, intensive care unit (ICU) admission, and death.
The research, published in Nature, used electronic health records of 65,196 veterans who received BNT162b2 (Pfizer-BioNTech) and 65,196 who received mRNA-1273 (Moderna). They chose to study the 16 weeks between October 20, 2021 and February 8, 2022, which included both Delta- and Omicron-variant waves.
During the follow-up (median, 77 days), 2994 COVID-19 infections were documented, of which 200 were detected as symptomatic, 194 required hospitalization, and 52 required ICU admission. Twenty-two patients died.
In a previous head-to-head trial comparing breakthrough COVID-19 outcomes after the first doses of the 2 vaccines (given when the Alpha and Delta variants were predominant), the researchers had found a low risk of documented infection and severe outcomes, but lower for the Moderna vaccine. They note that few head-to-head comparisons have been made of third-dose effectiveness.
As expected, in this trial, the researchers found a “nearly identical” pattern for the risk of the 2 vaccine groups. Although the risks for all of the measured outcomes over 16 weeks were low for both vaccines ≤ 4% for documented infection and < 0.03% for death in each group—those veterans who received the Pfizer-BioNTech vaccine had an excess of 45 documented infections and 11 hospitalizations per 10,000 persons, compared with the Moderna group. The Pfizer-BioNTech group also had a higher risk of documented infection over 9 weeks of follow-up, during which an Omicron-variant predominated.
Given the high effectiveness of a third dose of both vaccines, either vaccine is strongly recommended, the researchers conclude. They point to “evidence of clear and comparable benefits” for the most severe outcomes: The difference in estimated 16-week risk of death between the 2 groups was two-thousandths of 1 %.
They add that, while the differences in estimated risk for less severe outcomes between the 2 groups were small on the absolute scale, they may be meaningful when considering the population scale at which these vaccines are deployed.
How effective are the COVID-19 vaccines, third time around? Researchers compared 2 large groups of veterans to find out how well a third dose protected against documented infection, symptomatic COVID-19, and COVID-19–related hospitalization, intensive care unit (ICU) admission, and death.
The research, published in Nature, used electronic health records of 65,196 veterans who received BNT162b2 (Pfizer-BioNTech) and 65,196 who received mRNA-1273 (Moderna). They chose to study the 16 weeks between October 20, 2021 and February 8, 2022, which included both Delta- and Omicron-variant waves.
During the follow-up (median, 77 days), 2994 COVID-19 infections were documented, of which 200 were detected as symptomatic, 194 required hospitalization, and 52 required ICU admission. Twenty-two patients died.
In a previous head-to-head trial comparing breakthrough COVID-19 outcomes after the first doses of the 2 vaccines (given when the Alpha and Delta variants were predominant), the researchers had found a low risk of documented infection and severe outcomes, but lower for the Moderna vaccine. They note that few head-to-head comparisons have been made of third-dose effectiveness.
As expected, in this trial, the researchers found a “nearly identical” pattern for the risk of the 2 vaccine groups. Although the risks for all of the measured outcomes over 16 weeks were low for both vaccines ≤ 4% for documented infection and < 0.03% for death in each group—those veterans who received the Pfizer-BioNTech vaccine had an excess of 45 documented infections and 11 hospitalizations per 10,000 persons, compared with the Moderna group. The Pfizer-BioNTech group also had a higher risk of documented infection over 9 weeks of follow-up, during which an Omicron-variant predominated.
Given the high effectiveness of a third dose of both vaccines, either vaccine is strongly recommended, the researchers conclude. They point to “evidence of clear and comparable benefits” for the most severe outcomes: The difference in estimated 16-week risk of death between the 2 groups was two-thousandths of 1 %.
They add that, while the differences in estimated risk for less severe outcomes between the 2 groups were small on the absolute scale, they may be meaningful when considering the population scale at which these vaccines are deployed.
Black Veterans Disproportionately Denied VA Benefits
Black veterans are less likely to have their benefits claims processed and paid than are their White peers because of systemic problems within the US Department of Veterans Affairs, according to a lawsuit filed against the agency.
“A Black veteran who served honorably can walk into the VA, file a disability claim, and be at a significantly higher likelihood of having that claim denied,” said Adam Henderson, a student working with the Yale Law School Veterans Legal Services Clinic, one of several groups connected to the lawsuit.
“The VA has denied countless meritorious applications of Black veterans and thus deprived them and their families of the support that they are entitled to.”
The suit, filed in federal court by the clinic on behalf of Vietnam War veteran Conley Monk Jr., asks for “redress for the harms caused by the failure of VA staff and leaders to administer these benefits programs in a manner free from racial discrimination against Black veterans.”
In a press conference announcing the lawsuit, the effort received backing from Sen. Richard Blumenthal (D, Connecticut) who called it an “unacceptable” situation.
“Black veterans are denied benefits at a very significantly disproportionate rate,” he said. “We know the results. We want to know the reason why.”
The suit stems from an analysis of VA claims records released by the department following an earlier legal action. Between 2001 and 2020, the average denial rate for disability claims filed for Black veterans was 29.5%, significantly above the 24.2% for White veterans.
Attorneys allege the problems date back even further and that VA officials should have known about the racial disparities in the system from previous complaints.
“The negligence of VA leadership, and their failure to train, supervise, monitor and instruct agency officials to take steps to identify and correct racial disparities, led to systematic benefits obstruction for Black veterans,” the suit states.
Monk is a Black disabled Marine Corps veteran who previously sued the military to overturn his less-than-honorable military discharge due to complications from undiagnosed posttraumatic stress disorder.
He was subsequently granted access to a host of veterans benefits but not to retroactive payouts for claims he was denied in the 1970s.
“They didn’t fully compensate me or my family,” he said. “I wasn’t able to give my kids my educational benefits. We should have been receiving checks while they were growing up.”
Along with potential past benefits for Monk, individuals involved with the lawsuit said the move could force the VA to reassess thousands of other unfairly dismissed cases. “For decades [the US government] has allowed racially discriminatory practices to obstruct Black veterans from easily accessing veterans housing, education, and health care benefits with wide-reaching economic consequences for Black veterans and their families,” said Richard Brookshire, executive director of the Black Veterans Project.
“This lawsuit reckons with the shameful history of racism by the Department of Veteran Affairs and seeks to redress long-standing improprieties reverberating across generations of Black military service.”
In a statement, VA press secretary Terrence Hayes did not directly respond to the lawsuit but noted that “throughout history, there have been unacceptable disparities in both VA benefits decisions and military discharge status due to racism, which have wrongly left Black veterans without access to VA care and benefits.”
“We are actively working to right these wrongs, and we will stop at nothing to ensure that all Black veterans get the VA services they have earned and deserve,” he said. “We are currently studying racial disparities in benefits claims decisions, and we will publish the results of that study as soon as they are available.”
Hayes said the department has already begun targeted outreach to Black veterans to help them with claims and is “taking steps to ensure that our claims process combats institutional racism, rather than perpetuating it.”
Black veterans are less likely to have their benefits claims processed and paid than are their White peers because of systemic problems within the US Department of Veterans Affairs, according to a lawsuit filed against the agency.
“A Black veteran who served honorably can walk into the VA, file a disability claim, and be at a significantly higher likelihood of having that claim denied,” said Adam Henderson, a student working with the Yale Law School Veterans Legal Services Clinic, one of several groups connected to the lawsuit.
“The VA has denied countless meritorious applications of Black veterans and thus deprived them and their families of the support that they are entitled to.”
The suit, filed in federal court by the clinic on behalf of Vietnam War veteran Conley Monk Jr., asks for “redress for the harms caused by the failure of VA staff and leaders to administer these benefits programs in a manner free from racial discrimination against Black veterans.”
In a press conference announcing the lawsuit, the effort received backing from Sen. Richard Blumenthal (D, Connecticut) who called it an “unacceptable” situation.
“Black veterans are denied benefits at a very significantly disproportionate rate,” he said. “We know the results. We want to know the reason why.”
The suit stems from an analysis of VA claims records released by the department following an earlier legal action. Between 2001 and 2020, the average denial rate for disability claims filed for Black veterans was 29.5%, significantly above the 24.2% for White veterans.
Attorneys allege the problems date back even further and that VA officials should have known about the racial disparities in the system from previous complaints.
“The negligence of VA leadership, and their failure to train, supervise, monitor and instruct agency officials to take steps to identify and correct racial disparities, led to systematic benefits obstruction for Black veterans,” the suit states.
Monk is a Black disabled Marine Corps veteran who previously sued the military to overturn his less-than-honorable military discharge due to complications from undiagnosed posttraumatic stress disorder.
He was subsequently granted access to a host of veterans benefits but not to retroactive payouts for claims he was denied in the 1970s.
“They didn’t fully compensate me or my family,” he said. “I wasn’t able to give my kids my educational benefits. We should have been receiving checks while they were growing up.”
Along with potential past benefits for Monk, individuals involved with the lawsuit said the move could force the VA to reassess thousands of other unfairly dismissed cases. “For decades [the US government] has allowed racially discriminatory practices to obstruct Black veterans from easily accessing veterans housing, education, and health care benefits with wide-reaching economic consequences for Black veterans and their families,” said Richard Brookshire, executive director of the Black Veterans Project.
“This lawsuit reckons with the shameful history of racism by the Department of Veteran Affairs and seeks to redress long-standing improprieties reverberating across generations of Black military service.”
In a statement, VA press secretary Terrence Hayes did not directly respond to the lawsuit but noted that “throughout history, there have been unacceptable disparities in both VA benefits decisions and military discharge status due to racism, which have wrongly left Black veterans without access to VA care and benefits.”
“We are actively working to right these wrongs, and we will stop at nothing to ensure that all Black veterans get the VA services they have earned and deserve,” he said. “We are currently studying racial disparities in benefits claims decisions, and we will publish the results of that study as soon as they are available.”
Hayes said the department has already begun targeted outreach to Black veterans to help them with claims and is “taking steps to ensure that our claims process combats institutional racism, rather than perpetuating it.”
Black veterans are less likely to have their benefits claims processed and paid than are their White peers because of systemic problems within the US Department of Veterans Affairs, according to a lawsuit filed against the agency.
“A Black veteran who served honorably can walk into the VA, file a disability claim, and be at a significantly higher likelihood of having that claim denied,” said Adam Henderson, a student working with the Yale Law School Veterans Legal Services Clinic, one of several groups connected to the lawsuit.
“The VA has denied countless meritorious applications of Black veterans and thus deprived them and their families of the support that they are entitled to.”
The suit, filed in federal court by the clinic on behalf of Vietnam War veteran Conley Monk Jr., asks for “redress for the harms caused by the failure of VA staff and leaders to administer these benefits programs in a manner free from racial discrimination against Black veterans.”
In a press conference announcing the lawsuit, the effort received backing from Sen. Richard Blumenthal (D, Connecticut) who called it an “unacceptable” situation.
“Black veterans are denied benefits at a very significantly disproportionate rate,” he said. “We know the results. We want to know the reason why.”
The suit stems from an analysis of VA claims records released by the department following an earlier legal action. Between 2001 and 2020, the average denial rate for disability claims filed for Black veterans was 29.5%, significantly above the 24.2% for White veterans.
Attorneys allege the problems date back even further and that VA officials should have known about the racial disparities in the system from previous complaints.
“The negligence of VA leadership, and their failure to train, supervise, monitor and instruct agency officials to take steps to identify and correct racial disparities, led to systematic benefits obstruction for Black veterans,” the suit states.
Monk is a Black disabled Marine Corps veteran who previously sued the military to overturn his less-than-honorable military discharge due to complications from undiagnosed posttraumatic stress disorder.
He was subsequently granted access to a host of veterans benefits but not to retroactive payouts for claims he was denied in the 1970s.
“They didn’t fully compensate me or my family,” he said. “I wasn’t able to give my kids my educational benefits. We should have been receiving checks while they were growing up.”
Along with potential past benefits for Monk, individuals involved with the lawsuit said the move could force the VA to reassess thousands of other unfairly dismissed cases. “For decades [the US government] has allowed racially discriminatory practices to obstruct Black veterans from easily accessing veterans housing, education, and health care benefits with wide-reaching economic consequences for Black veterans and their families,” said Richard Brookshire, executive director of the Black Veterans Project.
“This lawsuit reckons with the shameful history of racism by the Department of Veteran Affairs and seeks to redress long-standing improprieties reverberating across generations of Black military service.”
In a statement, VA press secretary Terrence Hayes did not directly respond to the lawsuit but noted that “throughout history, there have been unacceptable disparities in both VA benefits decisions and military discharge status due to racism, which have wrongly left Black veterans without access to VA care and benefits.”
“We are actively working to right these wrongs, and we will stop at nothing to ensure that all Black veterans get the VA services they have earned and deserve,” he said. “We are currently studying racial disparities in benefits claims decisions, and we will publish the results of that study as soon as they are available.”
Hayes said the department has already begun targeted outreach to Black veterans to help them with claims and is “taking steps to ensure that our claims process combats institutional racism, rather than perpetuating it.”
Sleep complaints in major depression flag risk for other psychiatric disorders
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.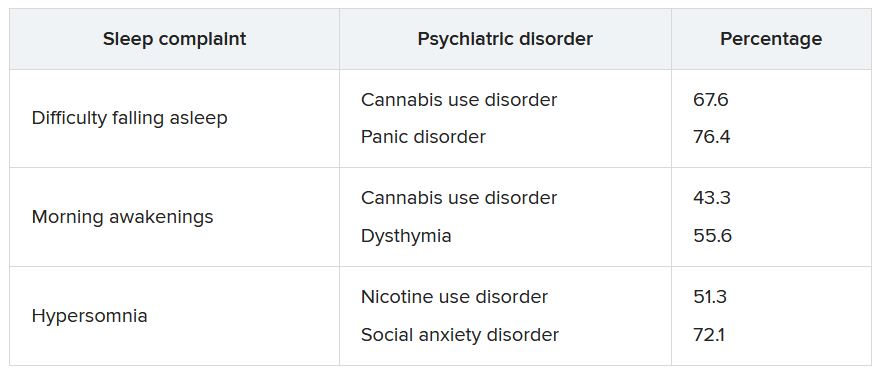
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
Investigators studied 3-year incidence rates of psychiatric disorders in almost 3,000 patients experiencing an MDE. Results showed that having a history of difficulty falling asleep, early morning awakening, and hypersomnia increased risk for incident psychiatric disorders.
“The findings of this study suggest the potential value of including insomnia and hypersomnia in clinical assessments of all psychiatric disorders,” write the investigators, led by Bénédicte Barbotin, MD, Département de Psychiatrie et d’Addictologie, Assistance Publique-Hôpitaux de Paris, Hôpital Bichat-Claude Bernard, France.
“Insomnia and hypersomnia symptoms may be prodromal transdiagnostic biomarkers and easily modifiable therapeutic targets for the prevention of psychiatric disorders,” they add.
The findings were published online recently in the Journal of Clinical Psychiatry.
Bidirectional association
The researchers note that sleep disturbance is “one of the most common symptoms” associated with major depressive disorder (MDD) and may be “both a consequence and a cause.”
Moreover, improving sleep disturbances for patients with an MDE “tends to improve depressive symptom and outcomes,” they add.
Although the possibility of a bidirectional association between MDEs and sleep disturbances “offers a new perspective that sleep complaints might be a predictive prodromal symptom,” the association of sleep complaints with the subsequent development of other psychiatric disorders in MDEs “remains poorly documented,” the investigators write.
The observation that sleep complaints are associated with psychiatric complications and adverse outcomes, such as suicidality and substance overdose, suggests that longitudinal studies “may help to better understand these relationships.”
To investigate these issues, the researchers examined three sleep complaints among patients with MDE: trouble falling asleep, early morning awakening, and hypersomnia. They adjusted for an array of variables, including antisocial personality disorders, use of sedatives or tranquilizers, sociodemographic characteristics, MDE severity, poverty, obesity, educational level, and stressful life events.
They also used a “bifactor latent variable approach” to “disentangle” a number of effects, including those shared by all psychiatric disorders; those specific to dimensions of psychopathology, such as internalizing dimension; and those specific to individual psychiatric disorders, such as dysthymia.
“To our knowledge, this is the most extensive prospective assessment [ever conducted] of associations between sleep complaints and incident psychiatric disorders,” the investigators write.
They drew on data from Waves 1 and 2 of the National Epidemiological Survey on Alcohol and Related Conditions, a large nationally representative survey conducted in 2001-2002 (Wave 1) and 2004-2005 (Wave 2) by the National Institute on Alcoholism and Alcohol Abuse.
The analysis included 2,864 participants who experienced MDE in the year prior to Wave 1 and who completed interviews at both waves.
Researchers assessed past-year DSM-IV Axis I disorders and baseline sleep complaints at Wave 1, as well as incident DSM-IV Axis I disorders between the two waves – including substance use, mood, and anxiety disorders.
Screening needed?
Results showed a wide range of incidence rates for psychiatric disorders between Wave 1 and Wave 2, ranging from 2.7% for cannabis use to 8.2% for generalized anxiety disorder.
The lifetime prevalence of sleep complaints was higher among participants who developed a psychiatric disorder between the two waves than among those who did not have sleep complaints. The range (from lowest to highest percentage) is shown in the accompanying table.
A higher number of sleep complaints was also associated with higher percentages of psychiatric disorders.
Hypersomnia, in particular, significantly increased the odds of having another psychiatric disorder. For patients with MDD who reported hypersomnia, the mean number of sleep disorders was significantly higher than for patients without hypersomnia (2.08 vs. 1.32; P < .001).
“This explains why hypersomnia appears more strongly associated with the incidence of psychiatric disorders,” the investigators write.
After adjusting for sociodemographic and clinical characteristics and antisocial personality disorder, the effects shared across all sleep complaints were “significantly associated with the incident general psychopathology factor, representing mechanisms that may lead to incidence of all psychiatric disorder in the model,” they add.
The researchers note that insomnia and hypersomnia can impair cognitive function, decision-making, problem-solving, and emotion processing networks, thereby increasing the onset of psychiatric disorders in vulnerable individuals.
Shared biological determinants, such as monoamine neurotransmitters that play a major role in depression, anxiety, substance use disorders, and the regulation of sleep stages, may also underlie both sleep disturbances and psychiatric disorders, they speculate.
“These results suggest the importance of systematically assessing insomnia and hypersomnia when evaluating psychiatric disorders and considering these symptoms as nonspecific prodromal or at-risk symptoms, also shared with suicidal behaviors,” the investigators write.
“In addition, since most individuals who developed a psychiatric disorder had at least one sleep complaint, all psychiatric disorders should be carefully screened among individuals with sleep complaints,” they add.
Transdiagnostic phenomenon
In a comment, Roger McIntyre, MD, professor of psychiatry and pharmacology at the University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, noted that the study replicates previous observations that a bidirectional relationship exists between sleep disturbances and mental disorders and that there “seems to be a relationship between sleep disturbance and suicidality that is bidirectional.”
He added that he appreciated the fact that the investigators “took this knowledge one step further; and what they are saying is that within the syndrome of depression, it is the sleep disturbance that is predicting future problems.”
Dr. McIntyre, who is also chairman and executive director of the Brain and Cognitive Discover Foundation in Toronto, was not involved with the study.
The data suggest that, “conceptually, sleep disturbance is a transdiagnostic phenomenon that may also be the nexus when multiple comorbid mental disorders occur,” he said.
“If this is the case, clinically, there is an opportunity here to prevent incident mental disorders in persons with depression and sleep disturbance, prioritizing sleep management in any patient with a mood disorder,” Dr. McIntyre added.
He noted that “the testable hypothesis” is how this is occurring mechanistically.
“I would conjecture that it could be inflammation and/or insulin resistance that is part of sleep disturbance that could predispose and portend other mental illnesses – and likely other medical conditions too, such as obesity and diabetes,” he said.
The study received no specific funding from any funding agency, commercial, or not-for-profit sectors. The investigators’ relevant financial relationships are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China and the Milken Institute; has received speaker/consultation fees from Lundbeck, Janssen, Alkermes,Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences; and is a CEO of Braxia Scientific Corp.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Long COVID comes into focus, showing older patients fare worse
These findings help define long COVID, guiding providers and patients through the recovery process, Barak Mizrahi, MSc, of KI Research Institute, Kfar Malal, Israel, and colleagues reported.
“To provide efficient continuous treatment and prevent adverse events related to potential long term effects and delayed symptoms of COVID-19, determining the magnitude and severity of this phenomenon and distinguishing it from similar clinical manifestations that occur normally or following infections with other pathogens is essential,” the investigators wrote in The BMJ.
To this end, they conducted a retrospective, nationwide cohort study involving 1,913,234 people who took a polymerase chain reaction test for SARS-CoV-2 between March 1, 2020, and Oct. 1, 2021. They compared a range of long-term outcomes at different intervals post infection, and compared these trends across subgroups sorted by age, sex, and variant. Outcomes ranged broadly, including respiratory disorders, cough, arthralgia, weakness, hair loss, and others.
The investigators compared hazard ratios for each of these outcomes among patients who tested positive versus those who tested negative at three intervals after testing: 30-90 days, 30-180 days, and 180-360 days. Statistically significant differences in the risks of these outcomes between infected versus uninfected groups suggested that COVID was playing a role.
“The health outcomes that represent long COVID showed a significant increase in both early and late phases,” the investigators wrote. These outcomes included anosmia and dysgeusia, cognitive impairment, dyspnea, weakness, and palpitations. In contrast, chest pain, myalgia, arthralgia, cough, and dizziness were associated with patients who were in the early phase, but not the late phase of long COVID.
“Vaccinated patients with a breakthrough SARS-CoV-2 infection had a lower risk for dyspnea and similar risk for other outcomes compared with unvaccinated infected patients,” the investigators noted.
For the long COVID outcomes, plots of risk differences over time showed that symptoms tended to get milder or resolve within a few months to a year. Patients 41-60 years were most likely to be impacted by long COVID outcomes, and show least improvement at 1 year, compared with other age groups.
“We believe that these findings will shed light on what is ‘long COVID’, support patients and doctors, and facilitate better and more efficient care,” Mr. Mizrahi and coauthor Maytal Bivas-Benita, PhD said in a joint written comment. “Primary care physicians (and patients) will now more clearly understand what are the symptoms that might be related to COVID and for how long they might linger. This would help physicians monitor the patients efficiently, ease their patients’ concerns and navigate a more efficient disease management.”
They suggested that the findings should hold consistent for future variants, although they could not “rule out the possibility of the emergence of new and more severe variants which will be more virulent and cause a more severe illness.”
One “major limitation” of the study, according to Monica Verduzco-Gutierrez, MD, a physiatrist and professor and chair of rehabilitation medicine at the University of Texas Health Science Center, San Antonio, is the lack of data for fatigue and dysautonomia, which are “the major presentations” that she sees in her long COVID clinic.
“The authors of the article focus on the primary damage being related to the lungs, though we know this is a systemic disease beyond the respiratory system, with endothelial dysfunction and immune dysregulation,” Dr. Verduzco-Gutierrez, who is also director of COVID recovery at the University of Texas Health Science Center, said in an interview.
Although it was reassuring to see that younger adults with long COVID trended toward improvement, she noted that patients 41-60 years “still had pretty significant symptoms” after 12 months.
“That [age group comprises] probably the majority of my patients that I’m seeing in the long COVID clinic,” Dr. Verduzco-Gutierrez said. “If you look at the whole thing, it looks better, but then when you drill down to that age group where you’re seeing patients, then it’s not.”
Dr. Verduzco-Gutierrez is so busy managing patients with long COVID that new appointments in her clinic are now delayed until May 31, so most patients will remain under the care of their primary care providers. She recommended that these physicians follow guidance from the American Academy of Physical Medicine and Rehabilitation, who offer consensus statements based on clinical characteristics, with separate recommendations for pediatric patients.
Our understanding of long COVID will continue to improve, and with it, available recommendations, she predicted, but further advances will require persistent effort.
“I think no matter what this [study] shows us, more research is needed,” Dr. Verduzco-Gutierrez said. “We can’t just forget about it, just because there is a population of people who get better. What about the ones who don’t?”
The investigators and Dr. Verduzco-Gutierrez disclosed no conflicts of interest.
These findings help define long COVID, guiding providers and patients through the recovery process, Barak Mizrahi, MSc, of KI Research Institute, Kfar Malal, Israel, and colleagues reported.
“To provide efficient continuous treatment and prevent adverse events related to potential long term effects and delayed symptoms of COVID-19, determining the magnitude and severity of this phenomenon and distinguishing it from similar clinical manifestations that occur normally or following infections with other pathogens is essential,” the investigators wrote in The BMJ.
To this end, they conducted a retrospective, nationwide cohort study involving 1,913,234 people who took a polymerase chain reaction test for SARS-CoV-2 between March 1, 2020, and Oct. 1, 2021. They compared a range of long-term outcomes at different intervals post infection, and compared these trends across subgroups sorted by age, sex, and variant. Outcomes ranged broadly, including respiratory disorders, cough, arthralgia, weakness, hair loss, and others.
The investigators compared hazard ratios for each of these outcomes among patients who tested positive versus those who tested negative at three intervals after testing: 30-90 days, 30-180 days, and 180-360 days. Statistically significant differences in the risks of these outcomes between infected versus uninfected groups suggested that COVID was playing a role.
“The health outcomes that represent long COVID showed a significant increase in both early and late phases,” the investigators wrote. These outcomes included anosmia and dysgeusia, cognitive impairment, dyspnea, weakness, and palpitations. In contrast, chest pain, myalgia, arthralgia, cough, and dizziness were associated with patients who were in the early phase, but not the late phase of long COVID.
“Vaccinated patients with a breakthrough SARS-CoV-2 infection had a lower risk for dyspnea and similar risk for other outcomes compared with unvaccinated infected patients,” the investigators noted.
For the long COVID outcomes, plots of risk differences over time showed that symptoms tended to get milder or resolve within a few months to a year. Patients 41-60 years were most likely to be impacted by long COVID outcomes, and show least improvement at 1 year, compared with other age groups.
“We believe that these findings will shed light on what is ‘long COVID’, support patients and doctors, and facilitate better and more efficient care,” Mr. Mizrahi and coauthor Maytal Bivas-Benita, PhD said in a joint written comment. “Primary care physicians (and patients) will now more clearly understand what are the symptoms that might be related to COVID and for how long they might linger. This would help physicians monitor the patients efficiently, ease their patients’ concerns and navigate a more efficient disease management.”
They suggested that the findings should hold consistent for future variants, although they could not “rule out the possibility of the emergence of new and more severe variants which will be more virulent and cause a more severe illness.”
One “major limitation” of the study, according to Monica Verduzco-Gutierrez, MD, a physiatrist and professor and chair of rehabilitation medicine at the University of Texas Health Science Center, San Antonio, is the lack of data for fatigue and dysautonomia, which are “the major presentations” that she sees in her long COVID clinic.
“The authors of the article focus on the primary damage being related to the lungs, though we know this is a systemic disease beyond the respiratory system, with endothelial dysfunction and immune dysregulation,” Dr. Verduzco-Gutierrez, who is also director of COVID recovery at the University of Texas Health Science Center, said in an interview.
Although it was reassuring to see that younger adults with long COVID trended toward improvement, she noted that patients 41-60 years “still had pretty significant symptoms” after 12 months.
“That [age group comprises] probably the majority of my patients that I’m seeing in the long COVID clinic,” Dr. Verduzco-Gutierrez said. “If you look at the whole thing, it looks better, but then when you drill down to that age group where you’re seeing patients, then it’s not.”
Dr. Verduzco-Gutierrez is so busy managing patients with long COVID that new appointments in her clinic are now delayed until May 31, so most patients will remain under the care of their primary care providers. She recommended that these physicians follow guidance from the American Academy of Physical Medicine and Rehabilitation, who offer consensus statements based on clinical characteristics, with separate recommendations for pediatric patients.
Our understanding of long COVID will continue to improve, and with it, available recommendations, she predicted, but further advances will require persistent effort.
“I think no matter what this [study] shows us, more research is needed,” Dr. Verduzco-Gutierrez said. “We can’t just forget about it, just because there is a population of people who get better. What about the ones who don’t?”
The investigators and Dr. Verduzco-Gutierrez disclosed no conflicts of interest.
These findings help define long COVID, guiding providers and patients through the recovery process, Barak Mizrahi, MSc, of KI Research Institute, Kfar Malal, Israel, and colleagues reported.
“To provide efficient continuous treatment and prevent adverse events related to potential long term effects and delayed symptoms of COVID-19, determining the magnitude and severity of this phenomenon and distinguishing it from similar clinical manifestations that occur normally or following infections with other pathogens is essential,” the investigators wrote in The BMJ.
To this end, they conducted a retrospective, nationwide cohort study involving 1,913,234 people who took a polymerase chain reaction test for SARS-CoV-2 between March 1, 2020, and Oct. 1, 2021. They compared a range of long-term outcomes at different intervals post infection, and compared these trends across subgroups sorted by age, sex, and variant. Outcomes ranged broadly, including respiratory disorders, cough, arthralgia, weakness, hair loss, and others.
The investigators compared hazard ratios for each of these outcomes among patients who tested positive versus those who tested negative at three intervals after testing: 30-90 days, 30-180 days, and 180-360 days. Statistically significant differences in the risks of these outcomes between infected versus uninfected groups suggested that COVID was playing a role.
“The health outcomes that represent long COVID showed a significant increase in both early and late phases,” the investigators wrote. These outcomes included anosmia and dysgeusia, cognitive impairment, dyspnea, weakness, and palpitations. In contrast, chest pain, myalgia, arthralgia, cough, and dizziness were associated with patients who were in the early phase, but not the late phase of long COVID.
“Vaccinated patients with a breakthrough SARS-CoV-2 infection had a lower risk for dyspnea and similar risk for other outcomes compared with unvaccinated infected patients,” the investigators noted.
For the long COVID outcomes, plots of risk differences over time showed that symptoms tended to get milder or resolve within a few months to a year. Patients 41-60 years were most likely to be impacted by long COVID outcomes, and show least improvement at 1 year, compared with other age groups.
“We believe that these findings will shed light on what is ‘long COVID’, support patients and doctors, and facilitate better and more efficient care,” Mr. Mizrahi and coauthor Maytal Bivas-Benita, PhD said in a joint written comment. “Primary care physicians (and patients) will now more clearly understand what are the symptoms that might be related to COVID and for how long they might linger. This would help physicians monitor the patients efficiently, ease their patients’ concerns and navigate a more efficient disease management.”
They suggested that the findings should hold consistent for future variants, although they could not “rule out the possibility of the emergence of new and more severe variants which will be more virulent and cause a more severe illness.”
One “major limitation” of the study, according to Monica Verduzco-Gutierrez, MD, a physiatrist and professor and chair of rehabilitation medicine at the University of Texas Health Science Center, San Antonio, is the lack of data for fatigue and dysautonomia, which are “the major presentations” that she sees in her long COVID clinic.
“The authors of the article focus on the primary damage being related to the lungs, though we know this is a systemic disease beyond the respiratory system, with endothelial dysfunction and immune dysregulation,” Dr. Verduzco-Gutierrez, who is also director of COVID recovery at the University of Texas Health Science Center, said in an interview.
Although it was reassuring to see that younger adults with long COVID trended toward improvement, she noted that patients 41-60 years “still had pretty significant symptoms” after 12 months.
“That [age group comprises] probably the majority of my patients that I’m seeing in the long COVID clinic,” Dr. Verduzco-Gutierrez said. “If you look at the whole thing, it looks better, but then when you drill down to that age group where you’re seeing patients, then it’s not.”
Dr. Verduzco-Gutierrez is so busy managing patients with long COVID that new appointments in her clinic are now delayed until May 31, so most patients will remain under the care of their primary care providers. She recommended that these physicians follow guidance from the American Academy of Physical Medicine and Rehabilitation, who offer consensus statements based on clinical characteristics, with separate recommendations for pediatric patients.
Our understanding of long COVID will continue to improve, and with it, available recommendations, she predicted, but further advances will require persistent effort.
“I think no matter what this [study] shows us, more research is needed,” Dr. Verduzco-Gutierrez said. “We can’t just forget about it, just because there is a population of people who get better. What about the ones who don’t?”
The investigators and Dr. Verduzco-Gutierrez disclosed no conflicts of interest.
FROM THE BMJ
PPI use in type 2 diabetes links with cardiovascular events
Among people with type 2 diabetes who self-reported regularly using a proton pump inhibitor (PPI), the incidence of cardiovascular disease (CVD) events as well as all-cause death was significantly increased in a study of more than 19,000 people with type 2 diabetes in a prospective U.K. database.
During median follow-up of about 11 years, regular use of a PPI by people with type 2 diabetes was significantly linked with a 27% relative increase in the incidence of coronary artery disease, compared with nonuse of a PPI, after full adjustment for potential confounding variables.
The results also show PPI use was significantly linked after full adjustment with a 34% relative increase in MI, a 35% relative increase in heart failure, and a 30% relative increase in all-cause death, say a team of Chinese researchers in a recent report in the Journal of Clinical Endocrinology and Metabolism.
PPIs are a medication class widely used in both over-the-counter and prescription formulations to reduce acid production in the stomach and to treat gastroesophageal reflux disease and other acid-related disorders. The PPI class includes such widely used agents as esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
The analyses in this report, which used data collected in the UK Biobank, are “rigorous,” and the findings of “a modest elevation of CVD risk are consistent with a growing number of observational studies in populations with and without diabetes,” commented Mary R. Rooney, PhD, an epidemiologist at Johns Hopkins University, Baltimore, who focuses on diabetes and cardiovascular diseases.
Prior observational reports
For example, a report from a prospective, observational study of more than 4300 U.S. residents published in 2021 that Dr. Rooney coauthored documented that cumulative PPI exposure for more than 5 years was significantly linked with a twofold increase in the rate of CVD events, compared with people who did not use a PPI. (This analysis did not examine a possible effect of diabetes status.)
And in a separate prospective, observational study of more than 1,000 Australians with type 2 diabetes, initiation of PPI treatment was significantly linked with a 3.6-fold increased incidence of CVD events, compared with PPI nonuse.
However, Dr. Rooney cautioned that the role of PPI use in raising CVD events “is still an unresolved question. It is too soon to tell if PPI use in people with diabetes should trigger additional caution.” Findings are needed from prospective, randomized trials to determine more definitively whether PPIs play a causal role in the incidence of CVD events, she said in an interview.
U.S. practice often results in unwarranted prolongation of PPI treatment, said the authors of an editorial that accompanied the 2021 report by Dr. Rooney and coauthors.
Long-term PPI use threatens harm
“The practice of initiating stress ulcer prophylaxis [by administering a PPI] in critical care is common,” wrote the authors of the 2021 editorial, Nitin Malik, MD, and William S. Weintraub, MD. “Although it is data driven and well intentioned, the possibility of causing harm – if it is continued on a long-term basis after resolution of the acute illness – is palpable.”
The new analyses using UK Biobank data included 19,229 adults with type 2 diabetes and no preexisting coronary artery disease, MI, heart failure, or stroke. The cohort included 15,954 people (83%) who did not report using a PPI and 3,275 who currently used PPIs regularly. Study limitations include self-report as the only verification of PPI use and lack of information on type of PPI, dose size, or use duration.
The findings remained consistent in several sensitivity analyses, including a propensity score–matched analysis and after further adjustment for use of histamine2 receptor antagonists, a drug class with indications similar to those for PPIs.
The authors of the report speculated that mechanisms that might link PPI use and increased CVD and mortality risk could include changes to the gut microbiota and possible interactions between PPIs and antiplatelet agents.
The study received no commercial funding. The authors and Dr. Rooney disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among people with type 2 diabetes who self-reported regularly using a proton pump inhibitor (PPI), the incidence of cardiovascular disease (CVD) events as well as all-cause death was significantly increased in a study of more than 19,000 people with type 2 diabetes in a prospective U.K. database.
During median follow-up of about 11 years, regular use of a PPI by people with type 2 diabetes was significantly linked with a 27% relative increase in the incidence of coronary artery disease, compared with nonuse of a PPI, after full adjustment for potential confounding variables.
The results also show PPI use was significantly linked after full adjustment with a 34% relative increase in MI, a 35% relative increase in heart failure, and a 30% relative increase in all-cause death, say a team of Chinese researchers in a recent report in the Journal of Clinical Endocrinology and Metabolism.
PPIs are a medication class widely used in both over-the-counter and prescription formulations to reduce acid production in the stomach and to treat gastroesophageal reflux disease and other acid-related disorders. The PPI class includes such widely used agents as esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
The analyses in this report, which used data collected in the UK Biobank, are “rigorous,” and the findings of “a modest elevation of CVD risk are consistent with a growing number of observational studies in populations with and without diabetes,” commented Mary R. Rooney, PhD, an epidemiologist at Johns Hopkins University, Baltimore, who focuses on diabetes and cardiovascular diseases.
Prior observational reports
For example, a report from a prospective, observational study of more than 4300 U.S. residents published in 2021 that Dr. Rooney coauthored documented that cumulative PPI exposure for more than 5 years was significantly linked with a twofold increase in the rate of CVD events, compared with people who did not use a PPI. (This analysis did not examine a possible effect of diabetes status.)
And in a separate prospective, observational study of more than 1,000 Australians with type 2 diabetes, initiation of PPI treatment was significantly linked with a 3.6-fold increased incidence of CVD events, compared with PPI nonuse.
However, Dr. Rooney cautioned that the role of PPI use in raising CVD events “is still an unresolved question. It is too soon to tell if PPI use in people with diabetes should trigger additional caution.” Findings are needed from prospective, randomized trials to determine more definitively whether PPIs play a causal role in the incidence of CVD events, she said in an interview.
U.S. practice often results in unwarranted prolongation of PPI treatment, said the authors of an editorial that accompanied the 2021 report by Dr. Rooney and coauthors.
Long-term PPI use threatens harm
“The practice of initiating stress ulcer prophylaxis [by administering a PPI] in critical care is common,” wrote the authors of the 2021 editorial, Nitin Malik, MD, and William S. Weintraub, MD. “Although it is data driven and well intentioned, the possibility of causing harm – if it is continued on a long-term basis after resolution of the acute illness – is palpable.”
The new analyses using UK Biobank data included 19,229 adults with type 2 diabetes and no preexisting coronary artery disease, MI, heart failure, or stroke. The cohort included 15,954 people (83%) who did not report using a PPI and 3,275 who currently used PPIs regularly. Study limitations include self-report as the only verification of PPI use and lack of information on type of PPI, dose size, or use duration.
The findings remained consistent in several sensitivity analyses, including a propensity score–matched analysis and after further adjustment for use of histamine2 receptor antagonists, a drug class with indications similar to those for PPIs.
The authors of the report speculated that mechanisms that might link PPI use and increased CVD and mortality risk could include changes to the gut microbiota and possible interactions between PPIs and antiplatelet agents.
The study received no commercial funding. The authors and Dr. Rooney disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Among people with type 2 diabetes who self-reported regularly using a proton pump inhibitor (PPI), the incidence of cardiovascular disease (CVD) events as well as all-cause death was significantly increased in a study of more than 19,000 people with type 2 diabetes in a prospective U.K. database.
During median follow-up of about 11 years, regular use of a PPI by people with type 2 diabetes was significantly linked with a 27% relative increase in the incidence of coronary artery disease, compared with nonuse of a PPI, after full adjustment for potential confounding variables.
The results also show PPI use was significantly linked after full adjustment with a 34% relative increase in MI, a 35% relative increase in heart failure, and a 30% relative increase in all-cause death, say a team of Chinese researchers in a recent report in the Journal of Clinical Endocrinology and Metabolism.
PPIs are a medication class widely used in both over-the-counter and prescription formulations to reduce acid production in the stomach and to treat gastroesophageal reflux disease and other acid-related disorders. The PPI class includes such widely used agents as esomeprazole (Nexium), lansoprazole (Prevacid), and omeprazole (Prilosec).
The analyses in this report, which used data collected in the UK Biobank, are “rigorous,” and the findings of “a modest elevation of CVD risk are consistent with a growing number of observational studies in populations with and without diabetes,” commented Mary R. Rooney, PhD, an epidemiologist at Johns Hopkins University, Baltimore, who focuses on diabetes and cardiovascular diseases.
Prior observational reports
For example, a report from a prospective, observational study of more than 4300 U.S. residents published in 2021 that Dr. Rooney coauthored documented that cumulative PPI exposure for more than 5 years was significantly linked with a twofold increase in the rate of CVD events, compared with people who did not use a PPI. (This analysis did not examine a possible effect of diabetes status.)
And in a separate prospective, observational study of more than 1,000 Australians with type 2 diabetes, initiation of PPI treatment was significantly linked with a 3.6-fold increased incidence of CVD events, compared with PPI nonuse.
However, Dr. Rooney cautioned that the role of PPI use in raising CVD events “is still an unresolved question. It is too soon to tell if PPI use in people with diabetes should trigger additional caution.” Findings are needed from prospective, randomized trials to determine more definitively whether PPIs play a causal role in the incidence of CVD events, she said in an interview.
U.S. practice often results in unwarranted prolongation of PPI treatment, said the authors of an editorial that accompanied the 2021 report by Dr. Rooney and coauthors.
Long-term PPI use threatens harm
“The practice of initiating stress ulcer prophylaxis [by administering a PPI] in critical care is common,” wrote the authors of the 2021 editorial, Nitin Malik, MD, and William S. Weintraub, MD. “Although it is data driven and well intentioned, the possibility of causing harm – if it is continued on a long-term basis after resolution of the acute illness – is palpable.”
The new analyses using UK Biobank data included 19,229 adults with type 2 diabetes and no preexisting coronary artery disease, MI, heart failure, or stroke. The cohort included 15,954 people (83%) who did not report using a PPI and 3,275 who currently used PPIs regularly. Study limitations include self-report as the only verification of PPI use and lack of information on type of PPI, dose size, or use duration.
The findings remained consistent in several sensitivity analyses, including a propensity score–matched analysis and after further adjustment for use of histamine2 receptor antagonists, a drug class with indications similar to those for PPIs.
The authors of the report speculated that mechanisms that might link PPI use and increased CVD and mortality risk could include changes to the gut microbiota and possible interactions between PPIs and antiplatelet agents.
The study received no commercial funding. The authors and Dr. Rooney disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Some BP meds tied to significantly lower risk for dementia, Alzheimer’s
Antihypertensive medications that stimulate rather than inhibit type 2 and 4 angiotensin II receptors can lower the rate of dementia among new users of these medications, new research suggests.
Results from a cohort study of more than 57,000 older Medicare beneficiaries showed that the initiation of antihypertensives that stimulate the receptors was linked to a 16% lower risk for incident Alzheimer’s disease and related dementia (ADRD) and an 18% lower risk for vascular dementia compared with those that inhibit the receptors.
“Achieving appropriate blood pressure control is essential for maximizing brain health, and this promising research suggests certain antihypertensives could yield brain benefit compared to others,” lead study author Zachary A. Marcum, PharmD, PhD, associate professor, University of Washington School of Pharmacy, Seattle, told this news organization.
The findings were published online in JAMA Network Open.
Medicare beneficiaries
Previous observational studies showed that antihypertensive medications that stimulate type 2 and 4 angiotensin II receptors, in comparison with those that don’t, were associated with lower rates of dementia. However, those studies included individuals with prevalent hypertension and were relatively small.
The new retrospective cohort study included a random sample of 57,773 Medicare beneficiaries aged at least 65 years with new-onset hypertension. The mean age of participants was 73.8 years, 62.9% were women, and 86.9% were White.
Over the course of the study, some participants filled at least one prescription for a stimulating angiotensin II receptor type 2 and 4, such as angiotensin II receptor type 1 blockers, dihydropyridine calcium channel blockers, and thiazide diuretics.
Others participants filled a prescription for an inhibiting type 2 and 4 angiotensin II receptors, including angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and nondihydropyridine calcium channel blockers.
“All these medications lower blood pressure, but they do it in different ways,” said Dr. Marcum.
The researchers were interested in the varying activity of these drugs at the type 2 and 4 angiotensin II receptors.
For each 30-day interval, they categorized beneficiaries into four groups: a stimulating medication group (n = 4,879) consisting of individuals mostly taking stimulating antihypertensives; an inhibiting medication group (n = 10,303) that mostly included individuals prescribed this type of antihypertensive; a mixed group (n = 2,179) that included a combination of the first two classifications; and a nonuser group (n = 40,413) of individuals who were not using either type of drug.
The primary outcome was time to first occurrence of ADRD. The secondary outcome was time to first occurrence of vascular dementia.
Researchers controlled for cardiovascular risk factors and sociodemographic characteristics, such as age, sex, race/ethnicity, and receipt of low-income subsidy.
Unanswered questions
After adjustments, results showed that initiation of an antihypertensive medication regimen that exclusively stimulates, rather than inhibits, type 2 and 4 angiotensin II receptors was associated with a 16% lower risk for incident ADRD over a follow-up of just under 7 years (hazard ratio, 0.84; 95% confidence interval, 0.79-0.90; P < .001).
The mixed regimen was also associated with statistically significant (P = .001) reduced odds of ADRD compared with the inhibiting medications.
As for vascular dementia, use of stimulating vs. inhibiting medications was associated with an 18% lower risk (HR, 0.82; 95% CI, 0.69-0.96; P = .02).
Again, use of the mixed regimen was associated with reduced risk of vascular dementia compared with the inhibiting medications (P = .03).
A variety of potential mechanisms might explain the superiority of stimulating agents when it comes to dementia risk, said Dr. Marcum. These could include, for example, increased blood flow to the brain and reduced amyloid.
“But more mechanistic work is needed as well as evaluation of dose responses, because that’s not something we looked at in this study,” Dr. Marcum said. “There are still a lot of unanswered questions.”
Stimulators instead of inhibitors?
The results of the current analysis come on the heels of some previous work showing the benefits of lowering blood pressure. For example, the Systolic Blood Pressure Intervention Trial (SPRINT) showed that targeting a systolic blood pressure below 120 mm Hg significantly reduces risk for heart disease, stroke, and death from these diseases.
But in contrast to previous research, the current study included only beneficiaries with incident hypertension and new use of antihypertensive medications, and it adjusted for time-varying confounding.
Prescribing stimulating instead of inhibiting treatments could make a difference at the population level, Dr. Marcum noted.
“If we could shift the prescribing a little bit from inhibiting to stimulating, that could possibly reduce dementia risk,” he said.
However, “we’re not suggesting [that all patients] have their regimen switched,” he added.
That’s because inhibiting medications still have an important place in the antihypertensive treatment armamentarium, Dr. Marcum noted. As an example, beta-blockers are used post heart attack.
As well, factors such as cost and side effects should be taken into consideration when prescribing an antihypertensive drug.
The new results could be used to set up a comparison in a future randomized controlled trial that would provide the strongest evidence for estimating causal effects of treatments, said Dr. Marcum.
‘More convincing’
Carlos G. Santos-Gallego, MD, Icahn School of Medicine at Mount Sinai, New York, said the study is “more convincing” than previous related research, as it has a larger sample size and a longer follow-up.
“And the exquisite statistical analysis gives more robustness, more solidity, to the hypothesis that drugs that stimulate type 2 and 4 angiotensin II receptors might be protective for dementia,” said Dr. Santos-Gallego, who was not involved with the research.
However, he noted that the retrospective study had some limitations, including the underdiagnosis of dementia. “The diagnosis of dementia is, honestly, very poorly done in the clinical setting,” he said.
As well, the study could be subject to “confounding by indication,” Dr. Santos-Gallego said. “There could be a third variable, another confounding factor, that’s responsible both for the dementia and for the prescription of these drugs,” he added.
For example, he noted that comorbidities such as atrial fibrillation, myocardial infarction, and heart failure might increase the risk of dementia.
He agreed with the investigators that a randomized clinical trial would address these limitations. “All comorbidities would be equally shared” in the randomized groups, and all participants would be given “a specific test for dementia at the same time,” Dr. Santos-Gallego said.
Still, he noted that the new results are in keeping with hypertension guidelines that recommend stimulating drugs.
“This trial definitely shows that the current hypertension guidelines are good treatment for our patients, not only to control blood pressure and not only to prevent infarction to prevent stroke but also to prevent dementia,” said Dr. Santos-Gallego.
Also commenting for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said the new data provide “clarity” on why previous research had differing results on the effect of antihypertensives on cognition.
Among the caveats of this new analysis is that “it’s unclear if the demographics in this study are fully representative of Medicare beneficiaries,” said Dr. Snyder.
She, too, said a clinical trial is important “to understand if there is a preventative and/or treatment potential in the medications that stimulate type 2 and 4 angiotensin II receptors.”
The study received funding from the National Institute on Aging. Dr. Marcum and Dr. Santos-Gallego have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antihypertensive medications that stimulate rather than inhibit type 2 and 4 angiotensin II receptors can lower the rate of dementia among new users of these medications, new research suggests.
Results from a cohort study of more than 57,000 older Medicare beneficiaries showed that the initiation of antihypertensives that stimulate the receptors was linked to a 16% lower risk for incident Alzheimer’s disease and related dementia (ADRD) and an 18% lower risk for vascular dementia compared with those that inhibit the receptors.
“Achieving appropriate blood pressure control is essential for maximizing brain health, and this promising research suggests certain antihypertensives could yield brain benefit compared to others,” lead study author Zachary A. Marcum, PharmD, PhD, associate professor, University of Washington School of Pharmacy, Seattle, told this news organization.
The findings were published online in JAMA Network Open.
Medicare beneficiaries
Previous observational studies showed that antihypertensive medications that stimulate type 2 and 4 angiotensin II receptors, in comparison with those that don’t, were associated with lower rates of dementia. However, those studies included individuals with prevalent hypertension and were relatively small.
The new retrospective cohort study included a random sample of 57,773 Medicare beneficiaries aged at least 65 years with new-onset hypertension. The mean age of participants was 73.8 years, 62.9% were women, and 86.9% were White.
Over the course of the study, some participants filled at least one prescription for a stimulating angiotensin II receptor type 2 and 4, such as angiotensin II receptor type 1 blockers, dihydropyridine calcium channel blockers, and thiazide diuretics.
Others participants filled a prescription for an inhibiting type 2 and 4 angiotensin II receptors, including angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and nondihydropyridine calcium channel blockers.
“All these medications lower blood pressure, but they do it in different ways,” said Dr. Marcum.
The researchers were interested in the varying activity of these drugs at the type 2 and 4 angiotensin II receptors.
For each 30-day interval, they categorized beneficiaries into four groups: a stimulating medication group (n = 4,879) consisting of individuals mostly taking stimulating antihypertensives; an inhibiting medication group (n = 10,303) that mostly included individuals prescribed this type of antihypertensive; a mixed group (n = 2,179) that included a combination of the first two classifications; and a nonuser group (n = 40,413) of individuals who were not using either type of drug.
The primary outcome was time to first occurrence of ADRD. The secondary outcome was time to first occurrence of vascular dementia.
Researchers controlled for cardiovascular risk factors and sociodemographic characteristics, such as age, sex, race/ethnicity, and receipt of low-income subsidy.
Unanswered questions
After adjustments, results showed that initiation of an antihypertensive medication regimen that exclusively stimulates, rather than inhibits, type 2 and 4 angiotensin II receptors was associated with a 16% lower risk for incident ADRD over a follow-up of just under 7 years (hazard ratio, 0.84; 95% confidence interval, 0.79-0.90; P < .001).
The mixed regimen was also associated with statistically significant (P = .001) reduced odds of ADRD compared with the inhibiting medications.
As for vascular dementia, use of stimulating vs. inhibiting medications was associated with an 18% lower risk (HR, 0.82; 95% CI, 0.69-0.96; P = .02).
Again, use of the mixed regimen was associated with reduced risk of vascular dementia compared with the inhibiting medications (P = .03).
A variety of potential mechanisms might explain the superiority of stimulating agents when it comes to dementia risk, said Dr. Marcum. These could include, for example, increased blood flow to the brain and reduced amyloid.
“But more mechanistic work is needed as well as evaluation of dose responses, because that’s not something we looked at in this study,” Dr. Marcum said. “There are still a lot of unanswered questions.”
Stimulators instead of inhibitors?
The results of the current analysis come on the heels of some previous work showing the benefits of lowering blood pressure. For example, the Systolic Blood Pressure Intervention Trial (SPRINT) showed that targeting a systolic blood pressure below 120 mm Hg significantly reduces risk for heart disease, stroke, and death from these diseases.
But in contrast to previous research, the current study included only beneficiaries with incident hypertension and new use of antihypertensive medications, and it adjusted for time-varying confounding.
Prescribing stimulating instead of inhibiting treatments could make a difference at the population level, Dr. Marcum noted.
“If we could shift the prescribing a little bit from inhibiting to stimulating, that could possibly reduce dementia risk,” he said.
However, “we’re not suggesting [that all patients] have their regimen switched,” he added.
That’s because inhibiting medications still have an important place in the antihypertensive treatment armamentarium, Dr. Marcum noted. As an example, beta-blockers are used post heart attack.
As well, factors such as cost and side effects should be taken into consideration when prescribing an antihypertensive drug.
The new results could be used to set up a comparison in a future randomized controlled trial that would provide the strongest evidence for estimating causal effects of treatments, said Dr. Marcum.
‘More convincing’
Carlos G. Santos-Gallego, MD, Icahn School of Medicine at Mount Sinai, New York, said the study is “more convincing” than previous related research, as it has a larger sample size and a longer follow-up.
“And the exquisite statistical analysis gives more robustness, more solidity, to the hypothesis that drugs that stimulate type 2 and 4 angiotensin II receptors might be protective for dementia,” said Dr. Santos-Gallego, who was not involved with the research.
However, he noted that the retrospective study had some limitations, including the underdiagnosis of dementia. “The diagnosis of dementia is, honestly, very poorly done in the clinical setting,” he said.
As well, the study could be subject to “confounding by indication,” Dr. Santos-Gallego said. “There could be a third variable, another confounding factor, that’s responsible both for the dementia and for the prescription of these drugs,” he added.
For example, he noted that comorbidities such as atrial fibrillation, myocardial infarction, and heart failure might increase the risk of dementia.
He agreed with the investigators that a randomized clinical trial would address these limitations. “All comorbidities would be equally shared” in the randomized groups, and all participants would be given “a specific test for dementia at the same time,” Dr. Santos-Gallego said.
Still, he noted that the new results are in keeping with hypertension guidelines that recommend stimulating drugs.
“This trial definitely shows that the current hypertension guidelines are good treatment for our patients, not only to control blood pressure and not only to prevent infarction to prevent stroke but also to prevent dementia,” said Dr. Santos-Gallego.
Also commenting for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said the new data provide “clarity” on why previous research had differing results on the effect of antihypertensives on cognition.
Among the caveats of this new analysis is that “it’s unclear if the demographics in this study are fully representative of Medicare beneficiaries,” said Dr. Snyder.
She, too, said a clinical trial is important “to understand if there is a preventative and/or treatment potential in the medications that stimulate type 2 and 4 angiotensin II receptors.”
The study received funding from the National Institute on Aging. Dr. Marcum and Dr. Santos-Gallego have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antihypertensive medications that stimulate rather than inhibit type 2 and 4 angiotensin II receptors can lower the rate of dementia among new users of these medications, new research suggests.
Results from a cohort study of more than 57,000 older Medicare beneficiaries showed that the initiation of antihypertensives that stimulate the receptors was linked to a 16% lower risk for incident Alzheimer’s disease and related dementia (ADRD) and an 18% lower risk for vascular dementia compared with those that inhibit the receptors.
“Achieving appropriate blood pressure control is essential for maximizing brain health, and this promising research suggests certain antihypertensives could yield brain benefit compared to others,” lead study author Zachary A. Marcum, PharmD, PhD, associate professor, University of Washington School of Pharmacy, Seattle, told this news organization.
The findings were published online in JAMA Network Open.
Medicare beneficiaries
Previous observational studies showed that antihypertensive medications that stimulate type 2 and 4 angiotensin II receptors, in comparison with those that don’t, were associated with lower rates of dementia. However, those studies included individuals with prevalent hypertension and were relatively small.
The new retrospective cohort study included a random sample of 57,773 Medicare beneficiaries aged at least 65 years with new-onset hypertension. The mean age of participants was 73.8 years, 62.9% were women, and 86.9% were White.
Over the course of the study, some participants filled at least one prescription for a stimulating angiotensin II receptor type 2 and 4, such as angiotensin II receptor type 1 blockers, dihydropyridine calcium channel blockers, and thiazide diuretics.
Others participants filled a prescription for an inhibiting type 2 and 4 angiotensin II receptors, including angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, and nondihydropyridine calcium channel blockers.
“All these medications lower blood pressure, but they do it in different ways,” said Dr. Marcum.
The researchers were interested in the varying activity of these drugs at the type 2 and 4 angiotensin II receptors.
For each 30-day interval, they categorized beneficiaries into four groups: a stimulating medication group (n = 4,879) consisting of individuals mostly taking stimulating antihypertensives; an inhibiting medication group (n = 10,303) that mostly included individuals prescribed this type of antihypertensive; a mixed group (n = 2,179) that included a combination of the first two classifications; and a nonuser group (n = 40,413) of individuals who were not using either type of drug.
The primary outcome was time to first occurrence of ADRD. The secondary outcome was time to first occurrence of vascular dementia.
Researchers controlled for cardiovascular risk factors and sociodemographic characteristics, such as age, sex, race/ethnicity, and receipt of low-income subsidy.
Unanswered questions
After adjustments, results showed that initiation of an antihypertensive medication regimen that exclusively stimulates, rather than inhibits, type 2 and 4 angiotensin II receptors was associated with a 16% lower risk for incident ADRD over a follow-up of just under 7 years (hazard ratio, 0.84; 95% confidence interval, 0.79-0.90; P < .001).
The mixed regimen was also associated with statistically significant (P = .001) reduced odds of ADRD compared with the inhibiting medications.
As for vascular dementia, use of stimulating vs. inhibiting medications was associated with an 18% lower risk (HR, 0.82; 95% CI, 0.69-0.96; P = .02).
Again, use of the mixed regimen was associated with reduced risk of vascular dementia compared with the inhibiting medications (P = .03).
A variety of potential mechanisms might explain the superiority of stimulating agents when it comes to dementia risk, said Dr. Marcum. These could include, for example, increased blood flow to the brain and reduced amyloid.
“But more mechanistic work is needed as well as evaluation of dose responses, because that’s not something we looked at in this study,” Dr. Marcum said. “There are still a lot of unanswered questions.”
Stimulators instead of inhibitors?
The results of the current analysis come on the heels of some previous work showing the benefits of lowering blood pressure. For example, the Systolic Blood Pressure Intervention Trial (SPRINT) showed that targeting a systolic blood pressure below 120 mm Hg significantly reduces risk for heart disease, stroke, and death from these diseases.
But in contrast to previous research, the current study included only beneficiaries with incident hypertension and new use of antihypertensive medications, and it adjusted for time-varying confounding.
Prescribing stimulating instead of inhibiting treatments could make a difference at the population level, Dr. Marcum noted.
“If we could shift the prescribing a little bit from inhibiting to stimulating, that could possibly reduce dementia risk,” he said.
However, “we’re not suggesting [that all patients] have their regimen switched,” he added.
That’s because inhibiting medications still have an important place in the antihypertensive treatment armamentarium, Dr. Marcum noted. As an example, beta-blockers are used post heart attack.
As well, factors such as cost and side effects should be taken into consideration when prescribing an antihypertensive drug.
The new results could be used to set up a comparison in a future randomized controlled trial that would provide the strongest evidence for estimating causal effects of treatments, said Dr. Marcum.
‘More convincing’
Carlos G. Santos-Gallego, MD, Icahn School of Medicine at Mount Sinai, New York, said the study is “more convincing” than previous related research, as it has a larger sample size and a longer follow-up.
“And the exquisite statistical analysis gives more robustness, more solidity, to the hypothesis that drugs that stimulate type 2 and 4 angiotensin II receptors might be protective for dementia,” said Dr. Santos-Gallego, who was not involved with the research.
However, he noted that the retrospective study had some limitations, including the underdiagnosis of dementia. “The diagnosis of dementia is, honestly, very poorly done in the clinical setting,” he said.
As well, the study could be subject to “confounding by indication,” Dr. Santos-Gallego said. “There could be a third variable, another confounding factor, that’s responsible both for the dementia and for the prescription of these drugs,” he added.
For example, he noted that comorbidities such as atrial fibrillation, myocardial infarction, and heart failure might increase the risk of dementia.
He agreed with the investigators that a randomized clinical trial would address these limitations. “All comorbidities would be equally shared” in the randomized groups, and all participants would be given “a specific test for dementia at the same time,” Dr. Santos-Gallego said.
Still, he noted that the new results are in keeping with hypertension guidelines that recommend stimulating drugs.
“This trial definitely shows that the current hypertension guidelines are good treatment for our patients, not only to control blood pressure and not only to prevent infarction to prevent stroke but also to prevent dementia,” said Dr. Santos-Gallego.
Also commenting for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations at the Alzheimer’s Association, said the new data provide “clarity” on why previous research had differing results on the effect of antihypertensives on cognition.
Among the caveats of this new analysis is that “it’s unclear if the demographics in this study are fully representative of Medicare beneficiaries,” said Dr. Snyder.
She, too, said a clinical trial is important “to understand if there is a preventative and/or treatment potential in the medications that stimulate type 2 and 4 angiotensin II receptors.”
The study received funding from the National Institute on Aging. Dr. Marcum and Dr. Santos-Gallego have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Kaposi’s sarcoma: Antiretroviral-related improvements in survival measured
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
than their uninfected counterparts, based on the first such analysis of the American College of Surgeons’ National Cancer Database.
One-year overall survival for all patients with Kaposi’s sarcoma (KS), 74.9% in 2004-2007, rose by 6.4 percentage points to 81.3% in 2016-2018, with the use of ART for HIV starting in 2008. Two-year survival was up by an even larger 8.3 percentage points: 68.0% to 76.3%, said Amar D. Desai of New Jersey Medical School, Newark, and Shari R. Lipner, MD, of Weill Cornell Medicine, New York.
Since HIV-infected patients represented a much lower 46.7% of the Kaposi’s population in 2016-2018 than in 2004-2007 (70.5%), “better outcomes for all KS patients likely reflects advancements in ART, preventing many HIV+ patients from progressing to AIDS, changes in clinical practice with earlier treatment start, and more off-label treatments,” they wrote in the Journal of the American Academy of Dermatology.
Overall survival rates for the 10,027 patients with KS with data available in the National Cancer Database were 77.9% at 1 year and 72.4% at 2 years. HIV status had a significant (P < .0074) effect over the entire study period: One-year survival rates were 88.9% for HIV-negative and 74.5% for HIV-positive patients, and 2-year rates were 83.0% (HIV-negative) and 69.3% (HIV-positive), the investigators reported in what they called “the largest analysis since the advent of antiretroviral therapy for HIV in 2008.”
The improvement in overall survival, along with the continued differences in survival between HIV infected and noninfected patients, indicate that “dermatologists, as part of a multidisciplinary team including oncologists and infectious disease physicians, can play significant roles in early KS diagnosis,” Mr. Desai and Dr. Lipner said.
Mr. Desai had no conflicts of interest to report. Dr. Lipner has served as a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus Corporation.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
LDL cholesterol triglycerides ‘robust’ ASCVD risk marker
High levels of triglyceride molecules in LDL cholesterol are “robustly” linked with an increased risk of atherosclerotic cardiovascular disease, according to a study that used two different methods in two separate cohorts from a large European population study plus a meta-analysis to verify the results.
“There have been some studies in the past, as you can see from our meta-analysis, that found a similar association, but I don’t think most people are convinced that there is really this relationship, and certainly I was not convinced,” lead investigator Børge G. Nordestgaard, MD, DMSc, professor at the University of Copenhagen, said in an interview.
The study enrolled 68,290 patients from the Copenhagen General Population study; 38,081 were assigned to direct automated assay to measure their LDL triglycerides and 30,208 had nuclear magnetic resonance (NMR) spectroscopy. Median follow-up was 3 and 9.2 years for the respective cohorts.
LDL triglycerides carry higher ASCVD risk
In the automated assay group, each 0.1-mmol/L (9 mg/dL)–higher direct LDL triglycerides carried a 22%-38% higher risk for the following outcomes: ASCVD (hazard ratio, 1.26; 95% confidence interval, 1.17-1.35); ischemic heart disease (HR, 1.27; 95% CI, 1.16-1.39); myocardial infarction (HR, 1.28; 95% CI, 1.11-1.48); ischemic stroke (HR, 1.22; 95% CI, 1.08-1.38); and peripheral artery disease (HR, 1.38; 95% CI, 1.21-1.58).
In the group that had NMR spectroscopy to measure LDL triglycerides, risks were similar, ranging from HRs of 1.13 (95% CI, 1.05-1.23) for ischemic stroke to 1.41 (95% CI, 1.31-1.52) for myocardial infarction. The investigators noted that apolipoprotein B levels didn’t entirely explain these results.
The meta-analysis included 18 studies that evaluated varying cardiovascular disease outcomes. It compared random-effects risk ratios for the highest quartile vs. the lowest quartile of LDL triglycerides. They were 1.50 (95% CI, 1.35-1.66) for ASCVD (four studies, 71,526 individuals, 8,576 events); 1.62 (95% CI, 1.37-1.93) for ischemic heart disease (six studies, 107,538 individuals, 9,734 events); 1.30 (95% CI, 1.13-1.49) for ischemic stroke (four studies, 78,026 individuals, 4,273 events); and 1.53 (95% CI, 1.29-1.81) for peripheral artery disease (four studies, 107,511 individuals, 1,848 events). The study was published online in the Journal of the American College of Cardiology.
Results confirm hypothesis the study sought to disprove
The purpose of the study was to actually disprove the hypothesis that the study ended up confirming, Dr. Nordestgaard said. “When we started this study, my idea was that we wanted to show that LDL triglyceride was not related to these diseases, because that didn’t make sense to me,” he said. “I’m so used to the thinking that the cholesterol content of these particles drive atherosclerosis and therefore atherosclerotic cardiovascular disease.”
He noted that LDL can carry both cholesterol and triglycerides, and that larger remnant lipoproteins can carry a substantial amount of triglycerides and a lesser amount of cholesterol. “Those remnants actually transfer into LDL, so they somewhat bring the triglycerides molecules into LDL,” Dr. Nordestgaard said.
The direct automated assay test used in the study to measure LDL triglycerides is not approved for use in the United States by the Food and Drug Administration, according to Denka, the manufacturer of the test.
The use of the Copenhagen General Population Study cohorts is a strength of the study because it has 100% follow-up with all patients, Dr. Nordestgaard said. The meta-analysis is another strength. “So we can show real clearly, not only in our two prospective studies, but also added to the former ones in the literature: All say exactly the same thing: High LDL triglycerides carry a high risk for ASCVD and its components.”
A limitation Dr. Nordestgaard acknowledged: The study doesn’t explain the causal relationship between high LDL triglycerides and ASCVD. But the study provides “very sound evidence that there’s a relationship,” he added. The study population was also a White, Danish population that lacked ethnic and racial diversity.
Next step is finding a treatment
The Danish study essentially confirms what the Atherosclerosis Risk in Community Study (ARIC) found with regard to LDL triglycerides, said Christie M. Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston, and an ARIC investigator.
This study is the “first step” to coming up with a test to identify risk, he said. “These data are pretty convincing, when you throw in the data in this study plus all the meta-analyses data, that LDL triglycerides, when they’re elevated, identify individuals at increased risk for an atherosclerotic cardiovascular event.”
The next step, he said, is coming up with a treatment for people with elevated HDL triglyceride. “That’s where we don’t have as much data because this test hasn’t been used. I’m pretty sure that statins are going to work fine for these people, because they lower LDL cholesterol and they also lower triglycerides, and some of the data have shown already that they reduce the LDL remnant,” Dr. Ballantyne said.
The Danish study provides enough of a basis for pursuing future studies to better understand the effect of statins on LDL triglyceride levels, Dr. Ballantyne added.
The study received funding from the Novo Nordisk Foundation and the Danish Heart Foundation, along with institutional support. Dr. Nordestgaard has no relevant disclosures. Dr. Ballantyne disclosed receiving research support from Denka.
High levels of triglyceride molecules in LDL cholesterol are “robustly” linked with an increased risk of atherosclerotic cardiovascular disease, according to a study that used two different methods in two separate cohorts from a large European population study plus a meta-analysis to verify the results.
“There have been some studies in the past, as you can see from our meta-analysis, that found a similar association, but I don’t think most people are convinced that there is really this relationship, and certainly I was not convinced,” lead investigator Børge G. Nordestgaard, MD, DMSc, professor at the University of Copenhagen, said in an interview.
The study enrolled 68,290 patients from the Copenhagen General Population study; 38,081 were assigned to direct automated assay to measure their LDL triglycerides and 30,208 had nuclear magnetic resonance (NMR) spectroscopy. Median follow-up was 3 and 9.2 years for the respective cohorts.
LDL triglycerides carry higher ASCVD risk
In the automated assay group, each 0.1-mmol/L (9 mg/dL)–higher direct LDL triglycerides carried a 22%-38% higher risk for the following outcomes: ASCVD (hazard ratio, 1.26; 95% confidence interval, 1.17-1.35); ischemic heart disease (HR, 1.27; 95% CI, 1.16-1.39); myocardial infarction (HR, 1.28; 95% CI, 1.11-1.48); ischemic stroke (HR, 1.22; 95% CI, 1.08-1.38); and peripheral artery disease (HR, 1.38; 95% CI, 1.21-1.58).
In the group that had NMR spectroscopy to measure LDL triglycerides, risks were similar, ranging from HRs of 1.13 (95% CI, 1.05-1.23) for ischemic stroke to 1.41 (95% CI, 1.31-1.52) for myocardial infarction. The investigators noted that apolipoprotein B levels didn’t entirely explain these results.
The meta-analysis included 18 studies that evaluated varying cardiovascular disease outcomes. It compared random-effects risk ratios for the highest quartile vs. the lowest quartile of LDL triglycerides. They were 1.50 (95% CI, 1.35-1.66) for ASCVD (four studies, 71,526 individuals, 8,576 events); 1.62 (95% CI, 1.37-1.93) for ischemic heart disease (six studies, 107,538 individuals, 9,734 events); 1.30 (95% CI, 1.13-1.49) for ischemic stroke (four studies, 78,026 individuals, 4,273 events); and 1.53 (95% CI, 1.29-1.81) for peripheral artery disease (four studies, 107,511 individuals, 1,848 events). The study was published online in the Journal of the American College of Cardiology.
Results confirm hypothesis the study sought to disprove
The purpose of the study was to actually disprove the hypothesis that the study ended up confirming, Dr. Nordestgaard said. “When we started this study, my idea was that we wanted to show that LDL triglyceride was not related to these diseases, because that didn’t make sense to me,” he said. “I’m so used to the thinking that the cholesterol content of these particles drive atherosclerosis and therefore atherosclerotic cardiovascular disease.”
He noted that LDL can carry both cholesterol and triglycerides, and that larger remnant lipoproteins can carry a substantial amount of triglycerides and a lesser amount of cholesterol. “Those remnants actually transfer into LDL, so they somewhat bring the triglycerides molecules into LDL,” Dr. Nordestgaard said.
The direct automated assay test used in the study to measure LDL triglycerides is not approved for use in the United States by the Food and Drug Administration, according to Denka, the manufacturer of the test.
The use of the Copenhagen General Population Study cohorts is a strength of the study because it has 100% follow-up with all patients, Dr. Nordestgaard said. The meta-analysis is another strength. “So we can show real clearly, not only in our two prospective studies, but also added to the former ones in the literature: All say exactly the same thing: High LDL triglycerides carry a high risk for ASCVD and its components.”
A limitation Dr. Nordestgaard acknowledged: The study doesn’t explain the causal relationship between high LDL triglycerides and ASCVD. But the study provides “very sound evidence that there’s a relationship,” he added. The study population was also a White, Danish population that lacked ethnic and racial diversity.
Next step is finding a treatment
The Danish study essentially confirms what the Atherosclerosis Risk in Community Study (ARIC) found with regard to LDL triglycerides, said Christie M. Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston, and an ARIC investigator.
This study is the “first step” to coming up with a test to identify risk, he said. “These data are pretty convincing, when you throw in the data in this study plus all the meta-analyses data, that LDL triglycerides, when they’re elevated, identify individuals at increased risk for an atherosclerotic cardiovascular event.”
The next step, he said, is coming up with a treatment for people with elevated HDL triglyceride. “That’s where we don’t have as much data because this test hasn’t been used. I’m pretty sure that statins are going to work fine for these people, because they lower LDL cholesterol and they also lower triglycerides, and some of the data have shown already that they reduce the LDL remnant,” Dr. Ballantyne said.
The Danish study provides enough of a basis for pursuing future studies to better understand the effect of statins on LDL triglyceride levels, Dr. Ballantyne added.
The study received funding from the Novo Nordisk Foundation and the Danish Heart Foundation, along with institutional support. Dr. Nordestgaard has no relevant disclosures. Dr. Ballantyne disclosed receiving research support from Denka.
High levels of triglyceride molecules in LDL cholesterol are “robustly” linked with an increased risk of atherosclerotic cardiovascular disease, according to a study that used two different methods in two separate cohorts from a large European population study plus a meta-analysis to verify the results.
“There have been some studies in the past, as you can see from our meta-analysis, that found a similar association, but I don’t think most people are convinced that there is really this relationship, and certainly I was not convinced,” lead investigator Børge G. Nordestgaard, MD, DMSc, professor at the University of Copenhagen, said in an interview.
The study enrolled 68,290 patients from the Copenhagen General Population study; 38,081 were assigned to direct automated assay to measure their LDL triglycerides and 30,208 had nuclear magnetic resonance (NMR) spectroscopy. Median follow-up was 3 and 9.2 years for the respective cohorts.
LDL triglycerides carry higher ASCVD risk
In the automated assay group, each 0.1-mmol/L (9 mg/dL)–higher direct LDL triglycerides carried a 22%-38% higher risk for the following outcomes: ASCVD (hazard ratio, 1.26; 95% confidence interval, 1.17-1.35); ischemic heart disease (HR, 1.27; 95% CI, 1.16-1.39); myocardial infarction (HR, 1.28; 95% CI, 1.11-1.48); ischemic stroke (HR, 1.22; 95% CI, 1.08-1.38); and peripheral artery disease (HR, 1.38; 95% CI, 1.21-1.58).
In the group that had NMR spectroscopy to measure LDL triglycerides, risks were similar, ranging from HRs of 1.13 (95% CI, 1.05-1.23) for ischemic stroke to 1.41 (95% CI, 1.31-1.52) for myocardial infarction. The investigators noted that apolipoprotein B levels didn’t entirely explain these results.
The meta-analysis included 18 studies that evaluated varying cardiovascular disease outcomes. It compared random-effects risk ratios for the highest quartile vs. the lowest quartile of LDL triglycerides. They were 1.50 (95% CI, 1.35-1.66) for ASCVD (four studies, 71,526 individuals, 8,576 events); 1.62 (95% CI, 1.37-1.93) for ischemic heart disease (six studies, 107,538 individuals, 9,734 events); 1.30 (95% CI, 1.13-1.49) for ischemic stroke (four studies, 78,026 individuals, 4,273 events); and 1.53 (95% CI, 1.29-1.81) for peripheral artery disease (four studies, 107,511 individuals, 1,848 events). The study was published online in the Journal of the American College of Cardiology.
Results confirm hypothesis the study sought to disprove
The purpose of the study was to actually disprove the hypothesis that the study ended up confirming, Dr. Nordestgaard said. “When we started this study, my idea was that we wanted to show that LDL triglyceride was not related to these diseases, because that didn’t make sense to me,” he said. “I’m so used to the thinking that the cholesterol content of these particles drive atherosclerosis and therefore atherosclerotic cardiovascular disease.”
He noted that LDL can carry both cholesterol and triglycerides, and that larger remnant lipoproteins can carry a substantial amount of triglycerides and a lesser amount of cholesterol. “Those remnants actually transfer into LDL, so they somewhat bring the triglycerides molecules into LDL,” Dr. Nordestgaard said.
The direct automated assay test used in the study to measure LDL triglycerides is not approved for use in the United States by the Food and Drug Administration, according to Denka, the manufacturer of the test.
The use of the Copenhagen General Population Study cohorts is a strength of the study because it has 100% follow-up with all patients, Dr. Nordestgaard said. The meta-analysis is another strength. “So we can show real clearly, not only in our two prospective studies, but also added to the former ones in the literature: All say exactly the same thing: High LDL triglycerides carry a high risk for ASCVD and its components.”
A limitation Dr. Nordestgaard acknowledged: The study doesn’t explain the causal relationship between high LDL triglycerides and ASCVD. But the study provides “very sound evidence that there’s a relationship,” he added. The study population was also a White, Danish population that lacked ethnic and racial diversity.
Next step is finding a treatment
The Danish study essentially confirms what the Atherosclerosis Risk in Community Study (ARIC) found with regard to LDL triglycerides, said Christie M. Ballantyne, MD, chief of cardiology at Baylor College of Medicine in Houston, and an ARIC investigator.
This study is the “first step” to coming up with a test to identify risk, he said. “These data are pretty convincing, when you throw in the data in this study plus all the meta-analyses data, that LDL triglycerides, when they’re elevated, identify individuals at increased risk for an atherosclerotic cardiovascular event.”
The next step, he said, is coming up with a treatment for people with elevated HDL triglyceride. “That’s where we don’t have as much data because this test hasn’t been used. I’m pretty sure that statins are going to work fine for these people, because they lower LDL cholesterol and they also lower triglycerides, and some of the data have shown already that they reduce the LDL remnant,” Dr. Ballantyne said.
The Danish study provides enough of a basis for pursuing future studies to better understand the effect of statins on LDL triglyceride levels, Dr. Ballantyne added.
The study received funding from the Novo Nordisk Foundation and the Danish Heart Foundation, along with institutional support. Dr. Nordestgaard has no relevant disclosures. Dr. Ballantyne disclosed receiving research support from Denka.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
New Omicron subvariant is ‘crazy infectious,’ COVID expert warns
“It’s crazy infectious,” said Paula Cannon, PhD, a virologist at the University of Southern California, Los Angeles. “All the things that have protected you for the past couple of years, I don’t think are going to protect you against this new crop of variants.”
XBB.1.5 is spreading quickly in the United States. It accounted for 27.6% of cases in the country in the week ending on Jan. 7, up from about 1% of cases at one point in December, according to the Centers for Disease Control and Prevention. It’s especially prevalent in the Northeast, now accounting for more than 70% of the cases in that region.
It’s spreading across the globe, too. Maria Van Kerkhove, PhD, technical lead of the World Health Organization, has called XBB.1.5 is “the most transmissible subvariant that has been detected yet.”
Ashish Jha, MD, the White House COVID-19 response coordinator, tweeted a few days ago that the spread of XBB.1.5 is “stunning” but cautioned that it’s unclear if the symptoms of infection will be more severe than for previous variants.
“Whether we’ll have an XBB.1.5 wave (and if yes, how big) will depend on many factors including immunity of the population, people’s actions, etc.,” he tweeted.
He urged people to get up to date on their boosters, wear a snug-fitting mask, and avoid crowded indoor spaces. He noted that people who haven’t been infected recently or haven’t gotten the bivalent booster likely have little protection against infection.
The symptoms for XBB.1.5 appear to be the same as for other versions of COVID-19. However, it’s less common for people infected with XBB.1.5 to report losing their sense of taste and smell, USA Today reported.
A version of this article first appeared on WebMD.com.
“It’s crazy infectious,” said Paula Cannon, PhD, a virologist at the University of Southern California, Los Angeles. “All the things that have protected you for the past couple of years, I don’t think are going to protect you against this new crop of variants.”
XBB.1.5 is spreading quickly in the United States. It accounted for 27.6% of cases in the country in the week ending on Jan. 7, up from about 1% of cases at one point in December, according to the Centers for Disease Control and Prevention. It’s especially prevalent in the Northeast, now accounting for more than 70% of the cases in that region.
It’s spreading across the globe, too. Maria Van Kerkhove, PhD, technical lead of the World Health Organization, has called XBB.1.5 is “the most transmissible subvariant that has been detected yet.”
Ashish Jha, MD, the White House COVID-19 response coordinator, tweeted a few days ago that the spread of XBB.1.5 is “stunning” but cautioned that it’s unclear if the symptoms of infection will be more severe than for previous variants.
“Whether we’ll have an XBB.1.5 wave (and if yes, how big) will depend on many factors including immunity of the population, people’s actions, etc.,” he tweeted.
He urged people to get up to date on their boosters, wear a snug-fitting mask, and avoid crowded indoor spaces. He noted that people who haven’t been infected recently or haven’t gotten the bivalent booster likely have little protection against infection.
The symptoms for XBB.1.5 appear to be the same as for other versions of COVID-19. However, it’s less common for people infected with XBB.1.5 to report losing their sense of taste and smell, USA Today reported.
A version of this article first appeared on WebMD.com.
“It’s crazy infectious,” said Paula Cannon, PhD, a virologist at the University of Southern California, Los Angeles. “All the things that have protected you for the past couple of years, I don’t think are going to protect you against this new crop of variants.”
XBB.1.5 is spreading quickly in the United States. It accounted for 27.6% of cases in the country in the week ending on Jan. 7, up from about 1% of cases at one point in December, according to the Centers for Disease Control and Prevention. It’s especially prevalent in the Northeast, now accounting for more than 70% of the cases in that region.
It’s spreading across the globe, too. Maria Van Kerkhove, PhD, technical lead of the World Health Organization, has called XBB.1.5 is “the most transmissible subvariant that has been detected yet.”
Ashish Jha, MD, the White House COVID-19 response coordinator, tweeted a few days ago that the spread of XBB.1.5 is “stunning” but cautioned that it’s unclear if the symptoms of infection will be more severe than for previous variants.
“Whether we’ll have an XBB.1.5 wave (and if yes, how big) will depend on many factors including immunity of the population, people’s actions, etc.,” he tweeted.
He urged people to get up to date on their boosters, wear a snug-fitting mask, and avoid crowded indoor spaces. He noted that people who haven’t been infected recently or haven’t gotten the bivalent booster likely have little protection against infection.
The symptoms for XBB.1.5 appear to be the same as for other versions of COVID-19. However, it’s less common for people infected with XBB.1.5 to report losing their sense of taste and smell, USA Today reported.
A version of this article first appeared on WebMD.com.






