User login
Lessons in timely recognition of laparoscopy-related bowel injury
In Part 1 of this article, I outlined circumstances in which abdominal adhesions should be anticipated and described strategies to prevent intestinal injury during operative procedures. Here, I describe ways to identify intestinal injury as soon as possible after it occurs, which is vital to prevent serious sequelae such as sepsis and even death.
During operative laparoscopy, a quick search for injury through the laparoscope cannot assure any surgeon that the intestinal wall has not been seriously denuded. A damaged muscularis—even if it is not recognized as transmural injury—may subsequently rupture if it is not appropriately repaired intraoperatively.
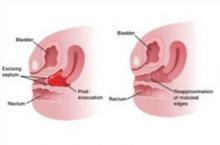
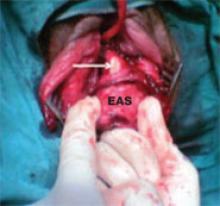
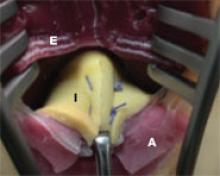
Following dissection of adhesions, irrigate the neighboring intestine with sterile saline, and perform a detailed inspection of the intestine to ascertain integrity of the bowel wall. The color of the intestine is important, as it can indicate whether the abundant vascular supply has been compromised. Include a detailed description of the intestines in the operative note.
Avoid stapling or vascular clips when repairing any wound; careful suturing is preferred.
Why early diagnosis is critical
The most favorable time to diagnose an iatrogenic intestinal perforation is within the intraoperative period. Prompt recognition and repair of bowel perforation offers several advantages:
- A second or third operation is less likely (
- The risk of abdominal sepsis is decreased.
- The volume of peripheral injury to the intestine is reduced.
- The patient can be followed for subsequent complications more precisely, permitting earlier diagnosis, more timely and effective treatment, and lower morbidity.
The 130 intestinal injuries reported by Baggish reflect the clinical significance of timely diagnosis.1 Seventy percent of small bowel and 51% of large bowel perforations were correctly diagnosed more than 48 hours postoperatively. Sepsis was present in a majority of these cases at the time of diagnosis.
Infection, fluid-electrolyte imbalance, sepsis syndrome
The principal derangements that arise as a result of bowel perforation are infection and fluid-electrolyte imbalance and their sequelae. Intestinal fluid and feces contain a variety of bacteria, such as Escherichia coli, Enterococcus, Klebsiella, Proteus, Pseudomonas, and Clostridium, to name a few. These bacteria produce toxins that facilitate entry of bacteria into the circulation and contribute to a downward spiral of events, referred to as sepsis syndrome, as well as intra-abdominal abscess:
- Contamination of the abdominal cavity leads to inflammation of the peritoneum
- In turn, subperitoneal blood vessels become porous, causing interstitial fluid to leak into the third space
- Paralytic ileus and an accumulation of intra-abdominal fluid push the diaphragm upward, lowering the capacity for lung expansion within the thorax and contributing to partial lung collapse
- Fluid of inflammatory origin may accumulate in the chest as pleural cavity effusion.
A number of progressive complications are predictable, but may occur at variable intervals after the initial perforation. The most frequent complications associated with colonic injuries are:
- peritonitis (98% of cases)
- ileus (92%)
- pleural effusion (84%)
- colostomy (80%)
- intra-abdominal abscess (78%).
The most common sequelae after small-bowel perforation are:
- peritonitis (100% of cases)
- ileus (89%)
- intra-abdominal abscess (63%)
- pleural effusion (59%).1
Reasons for diagnostic delay
- The gynecologic surgeon fails to place intestinal injury at the top of the differential diagnosis.
- A surgical consultant is delayed in making a correct diagnosis. Surgeons have less experience with perforation than do gynecologists, and invariably consider the postoperative abdominal problem to be ileus or intestinal obstruction. The presence of postoperative pneumoperitoneum is incorrectly thought to be lingering CO2 gas from the initial laparoscopy rather than air from a perforated viscus.
- Ancillary diagnosis confuses the primary physician. Pleural effusion, chest pain, and tachypnea are usually thought to indicate pulmonary embolism; as a result, the gynecologist and consulting pulmonologist focus on pulmonary embolus and deep-vein thrombosis. Only a spiral computed tomography (CT) scan, a ventilation perfusion (VQ) scan, or arteriogram quickly rules pulmonary embolus in or out. Peritonitis associated with ileus or third-space fluid leakage resulting in diaphragmatic elevation also creates pleural effusion, tachypnea, and dyspnea.
Presumptive diagnosis is critical
Definitive diagnosis of intestinal perforation happens at the operating table under direct vision and is corroborated by the pathology laboratory if bowel resection is performed. However, presumptive diagnosis helps overcome inertia and gets the patient to the operating room sooner.
The process by which the presumptive diagnosis is made is the most important issue in this article. The shorter the process, the lower the patient’s morbidity, and vice versa.
Look for steady improvement. Worry when it is absent
After any laparoscopic operation, the postoperative course should be one of steady clinical improvement. When a patient deviates from this model, the foremost presumptive diagnosis should be laparoscopy-associated injury, and the intestine should top the list of organs that may be injured. Other diagnoses should be subordinate to the principal presumptive diagnosis; these include ileus, bowel obstruction, pulmonary embolus, gastroenteritis, and hematoma, to name a few.
I do not mean to imply that a potentially life-threatening complication such as pulmonary embolus should not be ruled in or out, but that the necessary imaging should be performed in a timely fashion. The abdominal-pelvic CT scan will offer clues to the presence of free air, free fluid, air-fluid levels, and foreign bodies. It also is useful in detecting intra-abdominal—specifically, subphrenic—abscess. If necessary, a VQ scan or spiral CT scan can then be performed without delaying the diagnosis of the primary intra-abdominal catastrophe responsible for the pulmonary symptoms.
In the opening case, before making an improbable presumptive diagnosis, the surgeon should have questioned why an otherwise healthy woman would coincidentally develop gastroenteritis after laparoscopic surgery. The same can be said for diagnoses of intestinal obstruction or vascular thrombosis involving the intestinal blood supply.
Typical presentation of the injured patient
An injured patient does not experience daily improvement and a return to normal activity. Instead, the postoperative period is marked by persistent and worsening pain, often compounded by nausea or vomiting, or both. The patient may complain of fever, chills, weakness, or simply not feeling normal. Breathing may be labored. As time elapses, the symptoms become worse.
Reports of more than one visit to an emergency care facility are not uncommon. When examined, the patient exhibits direct or rebound tenderness, or both. The abdomen may or may not be distended, but usually is increased in girth. Bowel sounds are diminished or absent.
Vital signs initially reveal normal, low-grade, or subnormal temperature, and tachycardia, tachypnea, and normal blood pressure are typical. As time and sepsis progress, however, fever and hypotension develop. Most other symptoms and signs become progressively more abnormal in direct proportion to the length of time the diagnosis is delayed.
Seminal laboratory values for sepsis include a lower than normal white blood cell (WBC) count, elevated immature white-cell elements (e.g., “bandemia”), elevated liver chemistries, and an elevated serum creatinine level.
Mortality is most often the result of overwhelming and prolonged sepsis, leading to multiorgan failure, bleeding diathesis, and adult respiratory distress syndrome.
Among 130 laparoscopic surgical cases complicated by bowel injury and reported by Baggish, sepsis was diagnosed in 100% of colonic perforations and 50% of small-bowel perforations when the diagnosis was delayed more than 48 hours after surgery.1
TABLE 1 lists the signs and frequency of sepsis in these 100 cases, and TABLE 2 collates the signs and symptoms that were observed. Peritoneal cultures obtained at the time of exploratory laparotomy revealed multiple organisms (polymicrobial) in 60% of cases.
TABLE 1
Frequency of signs of sepsis among 130 patients with colon or small-bowel injury
| Sign | Colon (49 patients) | Small bowel (81 patients) |
|---|---|---|
| Normal or subnormal temperature | 30* | 41* |
| Fever | 19 | 40 |
| Tachycardia | 31 | 44 |
| Tachypnea | 30 | 40 |
| Hypotension | 21 | 15 |
| Anemia | 38 | 51 |
| Depressed WBC count | 20 | 18 |
| Elevated WBC count | 24 | 32 |
| Bandemia | 25 | 30 |
| Elevated creatinine and blood urea nitrogen levels | 12 | 5 |
| *Number of patients. | ||
| Source: Baggish1 | ||
Watch for signs and symptoms of intestinal injury
| Symptom | Sign |
|---|---|
| Abdominal pain | Direct or rebound tenderness |
| Bloating | Abdominal distension |
| Nausea, vomiting | Diminished bowel sounds |
| Fever, chills | Elevated or subnormal temperature |
| Difficulty breathing | Tachypnea, tachycardia |
| Weakness | Pallor, hypotension, diminished consciousness |
| Source: Baggish1 | |
Concurrent injuries to neighboring structures
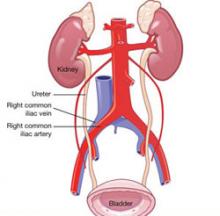
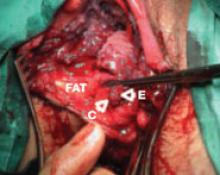
A number of collateral injuries may occur in conjunction with intestinal perforation, depending on the location of the trauma. The most dangerous combination includes indirect laceration of one of the major retroperitoneal vessels. A through-and-through perforation of the cecum can also involve one or more of the right iliac vessels. A trocar perforation of the ileum may continue directly into the presacral space or pass above it and penetrate the left common iliac vein or aorta. Similarly, perforation of the sigmoid colon may penetrate the left iliac vessels.
Careful inspection of the posterior peritoneum for tears and evidence of retroperitoneal hematoma is required to avoid missing a serious collateral injury. More likely, however, is a penetrating injury to the small bowel presenting with collateral mesenteric damage and compromise of the blood supply of an entire segment of bowel. The ureter and bladder may also be injured when dissection along the pelvic sidewall, or a trocar thrust, deviates to the right or left of midline. In thin patients, the stomach may be perforated as well as the small intestine or transverse colon.
In one memorable case, a primary trocar penetrated the omentum, injuring several underlying structures. In its transit, the trocar passed through both the anterior and posterior walls of the duodenum and finally entered the superior mesenteric artery. The gynecologic surgeon performing the laparoscopic tubal ligation failed to recognize any of these injuries. The patient went into shock in the recovery room and was returned to the operating room. Fortunately, a transplant surgeon from a neighboring theater was immediately available to consult and repair the damage.
Another danger: intestinal ischemic necrosis
Abnormalities in splanchnic blood flow are sometimes caused by elevations in intra-abdominal pressure. Caldwell and Ricotta inflated the abdomens of nine dogs and reported a significant reduction of blood flow to omentum, stomach, duodenum, jejunum, ileum, colon, pancreas, liver, and spleen, but not to the adrenal glands.2 The splanchnic flow reductions essentially shunted blood away from abdominal viscera with auto-transfusion to the heart, lungs, and systemic circulation.
Eleftheriadis and colleagues studied 16 women randomized to laparoscopic versus open cholecystectomy.3 Significant depression of the hepatic microcirculation during the period of CO2 gas insufflation was noted in the laparoscopy cohort but not in the control group. Gastric mucosal ischemia also was observed in the laparoscopy group.
Several case reports of catastrophic intestinal ischemia have appeared in the literature (1994–1995).4-7 These articles have mainly involved laparoscopic upper abdominal operations in elderly people.
Recently, however, Hasson and colleagues reported a case of possible ischemic necrosis of the small intestine following laparoscopic adhesiolysis and bipolar myolysis.8 The authors emphasized that CO2 pneumoperitoneum reduces splanchnic blood flow, predisposing the patient to ischemia, but that ischemia with infarction requires an underlying vasculopathy or inciting factors such as traction on a short mesentery, atherosclerosis, or thrombosis.
A high index of suspicion for bowel ischemia following laparoscopic surgery should occur when, postoperatively, a patient experiences inordinately severe abdominal pain associated with tachypnea, tachycardia, and alterations in the WBC count. A paucity of physical abdominal signs in the early phases of this disorder should alert the clinician to the possibility of bowel ischemia.
Diagnosing and treating ischemia
A CT scan with contrast can suggest ischemia, but angiography is usually required for definitive diagnosis.
Treatment begins with infusion of papaverine into the intestinal vasculature via angiography cannula. In some cases, anticoagulation may be indicated. Surgery by laparotomy is clearly indicated for patients who fail to respond to vasodilatation measures.
This condition can be ameliorated by intermittent intraoperative decompression of the abdomen. Avoiding prolonged CO2 pneumoperitoneum and a lengthy laparoscopic operation also may diminish the risk of intestinal ischemia.
1. Baggish MS. One hundred and thirty small and large bowel injuries associated with gynecologic laparoscopic operations. J Gynecol Surg. 2007;23:83-95.
2. Caldwell CB, Ricotta JJ. Changes in visceral blood flow with elevated intra-abdominal pressure. J Surg Res. 1987;43:14-20.
3. Eleftheriadis E, Kotzampassi K, Botsios D, Tzartinoglu E, Farmakis H, Dadoukis J. Splanchnic ischemia during laparoscopic cholecystectomy. Surg Endosc. 1996;10:324-326.
4. Schorr RT. Laparoscopic upper abdominal operations and mesenteric infarction. J Laparoendosc Surg. 1995;5:389-392.
5. Mitchell PC, Jamieson GG. Coeliac axis and mesenteric arterial thrombosis following laparoscopic Nissen fundoplication. Aust N Z J Surg. 1994;64:728-730.
6. Dwerryhouse SJ, Melsom DS, Burton PA, Thompson MH. Acute intestinal ischaemia after laparoscopic cholecystectomy. Br J Surg. 1995;82:1413.-
7. Jaffe V, Russell RCG. Fatal intestinal ischaemia following laparoscopic cholecystectomy. Br J Surg. 1994;81:1827-1828.
8. Hasson HM, Galanopoulos C, Lanferman A. Ischemic necrosis of small bowel following laparoscopic surgery. JSLS. 2004;8:159-163.
In Part 1 of this article, I outlined circumstances in which abdominal adhesions should be anticipated and described strategies to prevent intestinal injury during operative procedures. Here, I describe ways to identify intestinal injury as soon as possible after it occurs, which is vital to prevent serious sequelae such as sepsis and even death.
During operative laparoscopy, a quick search for injury through the laparoscope cannot assure any surgeon that the intestinal wall has not been seriously denuded. A damaged muscularis—even if it is not recognized as transmural injury—may subsequently rupture if it is not appropriately repaired intraoperatively.
Following dissection of adhesions, irrigate the neighboring intestine with sterile saline, and perform a detailed inspection of the intestine to ascertain integrity of the bowel wall. The color of the intestine is important, as it can indicate whether the abundant vascular supply has been compromised. Include a detailed description of the intestines in the operative note.
Avoid stapling or vascular clips when repairing any wound; careful suturing is preferred.
Why early diagnosis is critical
The most favorable time to diagnose an iatrogenic intestinal perforation is within the intraoperative period. Prompt recognition and repair of bowel perforation offers several advantages:
- A second or third operation is less likely (
- The risk of abdominal sepsis is decreased.
- The volume of peripheral injury to the intestine is reduced.
- The patient can be followed for subsequent complications more precisely, permitting earlier diagnosis, more timely and effective treatment, and lower morbidity.
The 130 intestinal injuries reported by Baggish reflect the clinical significance of timely diagnosis.1 Seventy percent of small bowel and 51% of large bowel perforations were correctly diagnosed more than 48 hours postoperatively. Sepsis was present in a majority of these cases at the time of diagnosis.
Infection, fluid-electrolyte imbalance, sepsis syndrome
The principal derangements that arise as a result of bowel perforation are infection and fluid-electrolyte imbalance and their sequelae. Intestinal fluid and feces contain a variety of bacteria, such as Escherichia coli, Enterococcus, Klebsiella, Proteus, Pseudomonas, and Clostridium, to name a few. These bacteria produce toxins that facilitate entry of bacteria into the circulation and contribute to a downward spiral of events, referred to as sepsis syndrome, as well as intra-abdominal abscess:
- Contamination of the abdominal cavity leads to inflammation of the peritoneum
- In turn, subperitoneal blood vessels become porous, causing interstitial fluid to leak into the third space
- Paralytic ileus and an accumulation of intra-abdominal fluid push the diaphragm upward, lowering the capacity for lung expansion within the thorax and contributing to partial lung collapse
- Fluid of inflammatory origin may accumulate in the chest as pleural cavity effusion.
A number of progressive complications are predictable, but may occur at variable intervals after the initial perforation. The most frequent complications associated with colonic injuries are:
- peritonitis (98% of cases)
- ileus (92%)
- pleural effusion (84%)
- colostomy (80%)
- intra-abdominal abscess (78%).
The most common sequelae after small-bowel perforation are:
- peritonitis (100% of cases)
- ileus (89%)
- intra-abdominal abscess (63%)
- pleural effusion (59%).1
Reasons for diagnostic delay
- The gynecologic surgeon fails to place intestinal injury at the top of the differential diagnosis.
- A surgical consultant is delayed in making a correct diagnosis. Surgeons have less experience with perforation than do gynecologists, and invariably consider the postoperative abdominal problem to be ileus or intestinal obstruction. The presence of postoperative pneumoperitoneum is incorrectly thought to be lingering CO2 gas from the initial laparoscopy rather than air from a perforated viscus.
- Ancillary diagnosis confuses the primary physician. Pleural effusion, chest pain, and tachypnea are usually thought to indicate pulmonary embolism; as a result, the gynecologist and consulting pulmonologist focus on pulmonary embolus and deep-vein thrombosis. Only a spiral computed tomography (CT) scan, a ventilation perfusion (VQ) scan, or arteriogram quickly rules pulmonary embolus in or out. Peritonitis associated with ileus or third-space fluid leakage resulting in diaphragmatic elevation also creates pleural effusion, tachypnea, and dyspnea.
Presumptive diagnosis is critical
Definitive diagnosis of intestinal perforation happens at the operating table under direct vision and is corroborated by the pathology laboratory if bowel resection is performed. However, presumptive diagnosis helps overcome inertia and gets the patient to the operating room sooner.
The process by which the presumptive diagnosis is made is the most important issue in this article. The shorter the process, the lower the patient’s morbidity, and vice versa.
Look for steady improvement. Worry when it is absent
After any laparoscopic operation, the postoperative course should be one of steady clinical improvement. When a patient deviates from this model, the foremost presumptive diagnosis should be laparoscopy-associated injury, and the intestine should top the list of organs that may be injured. Other diagnoses should be subordinate to the principal presumptive diagnosis; these include ileus, bowel obstruction, pulmonary embolus, gastroenteritis, and hematoma, to name a few.
I do not mean to imply that a potentially life-threatening complication such as pulmonary embolus should not be ruled in or out, but that the necessary imaging should be performed in a timely fashion. The abdominal-pelvic CT scan will offer clues to the presence of free air, free fluid, air-fluid levels, and foreign bodies. It also is useful in detecting intra-abdominal—specifically, subphrenic—abscess. If necessary, a VQ scan or spiral CT scan can then be performed without delaying the diagnosis of the primary intra-abdominal catastrophe responsible for the pulmonary symptoms.
In the opening case, before making an improbable presumptive diagnosis, the surgeon should have questioned why an otherwise healthy woman would coincidentally develop gastroenteritis after laparoscopic surgery. The same can be said for diagnoses of intestinal obstruction or vascular thrombosis involving the intestinal blood supply.
Typical presentation of the injured patient
An injured patient does not experience daily improvement and a return to normal activity. Instead, the postoperative period is marked by persistent and worsening pain, often compounded by nausea or vomiting, or both. The patient may complain of fever, chills, weakness, or simply not feeling normal. Breathing may be labored. As time elapses, the symptoms become worse.
Reports of more than one visit to an emergency care facility are not uncommon. When examined, the patient exhibits direct or rebound tenderness, or both. The abdomen may or may not be distended, but usually is increased in girth. Bowel sounds are diminished or absent.
Vital signs initially reveal normal, low-grade, or subnormal temperature, and tachycardia, tachypnea, and normal blood pressure are typical. As time and sepsis progress, however, fever and hypotension develop. Most other symptoms and signs become progressively more abnormal in direct proportion to the length of time the diagnosis is delayed.
Seminal laboratory values for sepsis include a lower than normal white blood cell (WBC) count, elevated immature white-cell elements (e.g., “bandemia”), elevated liver chemistries, and an elevated serum creatinine level.
Mortality is most often the result of overwhelming and prolonged sepsis, leading to multiorgan failure, bleeding diathesis, and adult respiratory distress syndrome.
Among 130 laparoscopic surgical cases complicated by bowel injury and reported by Baggish, sepsis was diagnosed in 100% of colonic perforations and 50% of small-bowel perforations when the diagnosis was delayed more than 48 hours after surgery.1
TABLE 1 lists the signs and frequency of sepsis in these 100 cases, and TABLE 2 collates the signs and symptoms that were observed. Peritoneal cultures obtained at the time of exploratory laparotomy revealed multiple organisms (polymicrobial) in 60% of cases.
TABLE 1
Frequency of signs of sepsis among 130 patients with colon or small-bowel injury
| Sign | Colon (49 patients) | Small bowel (81 patients) |
|---|---|---|
| Normal or subnormal temperature | 30* | 41* |
| Fever | 19 | 40 |
| Tachycardia | 31 | 44 |
| Tachypnea | 30 | 40 |
| Hypotension | 21 | 15 |
| Anemia | 38 | 51 |
| Depressed WBC count | 20 | 18 |
| Elevated WBC count | 24 | 32 |
| Bandemia | 25 | 30 |
| Elevated creatinine and blood urea nitrogen levels | 12 | 5 |
| *Number of patients. | ||
| Source: Baggish1 | ||
Watch for signs and symptoms of intestinal injury
| Symptom | Sign |
|---|---|
| Abdominal pain | Direct or rebound tenderness |
| Bloating | Abdominal distension |
| Nausea, vomiting | Diminished bowel sounds |
| Fever, chills | Elevated or subnormal temperature |
| Difficulty breathing | Tachypnea, tachycardia |
| Weakness | Pallor, hypotension, diminished consciousness |
| Source: Baggish1 | |
Concurrent injuries to neighboring structures
A number of collateral injuries may occur in conjunction with intestinal perforation, depending on the location of the trauma. The most dangerous combination includes indirect laceration of one of the major retroperitoneal vessels. A through-and-through perforation of the cecum can also involve one or more of the right iliac vessels. A trocar perforation of the ileum may continue directly into the presacral space or pass above it and penetrate the left common iliac vein or aorta. Similarly, perforation of the sigmoid colon may penetrate the left iliac vessels.
Careful inspection of the posterior peritoneum for tears and evidence of retroperitoneal hematoma is required to avoid missing a serious collateral injury. More likely, however, is a penetrating injury to the small bowel presenting with collateral mesenteric damage and compromise of the blood supply of an entire segment of bowel. The ureter and bladder may also be injured when dissection along the pelvic sidewall, or a trocar thrust, deviates to the right or left of midline. In thin patients, the stomach may be perforated as well as the small intestine or transverse colon.
In one memorable case, a primary trocar penetrated the omentum, injuring several underlying structures. In its transit, the trocar passed through both the anterior and posterior walls of the duodenum and finally entered the superior mesenteric artery. The gynecologic surgeon performing the laparoscopic tubal ligation failed to recognize any of these injuries. The patient went into shock in the recovery room and was returned to the operating room. Fortunately, a transplant surgeon from a neighboring theater was immediately available to consult and repair the damage.
Another danger: intestinal ischemic necrosis
Abnormalities in splanchnic blood flow are sometimes caused by elevations in intra-abdominal pressure. Caldwell and Ricotta inflated the abdomens of nine dogs and reported a significant reduction of blood flow to omentum, stomach, duodenum, jejunum, ileum, colon, pancreas, liver, and spleen, but not to the adrenal glands.2 The splanchnic flow reductions essentially shunted blood away from abdominal viscera with auto-transfusion to the heart, lungs, and systemic circulation.
Eleftheriadis and colleagues studied 16 women randomized to laparoscopic versus open cholecystectomy.3 Significant depression of the hepatic microcirculation during the period of CO2 gas insufflation was noted in the laparoscopy cohort but not in the control group. Gastric mucosal ischemia also was observed in the laparoscopy group.
Several case reports of catastrophic intestinal ischemia have appeared in the literature (1994–1995).4-7 These articles have mainly involved laparoscopic upper abdominal operations in elderly people.
Recently, however, Hasson and colleagues reported a case of possible ischemic necrosis of the small intestine following laparoscopic adhesiolysis and bipolar myolysis.8 The authors emphasized that CO2 pneumoperitoneum reduces splanchnic blood flow, predisposing the patient to ischemia, but that ischemia with infarction requires an underlying vasculopathy or inciting factors such as traction on a short mesentery, atherosclerosis, or thrombosis.
A high index of suspicion for bowel ischemia following laparoscopic surgery should occur when, postoperatively, a patient experiences inordinately severe abdominal pain associated with tachypnea, tachycardia, and alterations in the WBC count. A paucity of physical abdominal signs in the early phases of this disorder should alert the clinician to the possibility of bowel ischemia.
Diagnosing and treating ischemia
A CT scan with contrast can suggest ischemia, but angiography is usually required for definitive diagnosis.
Treatment begins with infusion of papaverine into the intestinal vasculature via angiography cannula. In some cases, anticoagulation may be indicated. Surgery by laparotomy is clearly indicated for patients who fail to respond to vasodilatation measures.
This condition can be ameliorated by intermittent intraoperative decompression of the abdomen. Avoiding prolonged CO2 pneumoperitoneum and a lengthy laparoscopic operation also may diminish the risk of intestinal ischemia.
In Part 1 of this article, I outlined circumstances in which abdominal adhesions should be anticipated and described strategies to prevent intestinal injury during operative procedures. Here, I describe ways to identify intestinal injury as soon as possible after it occurs, which is vital to prevent serious sequelae such as sepsis and even death.
During operative laparoscopy, a quick search for injury through the laparoscope cannot assure any surgeon that the intestinal wall has not been seriously denuded. A damaged muscularis—even if it is not recognized as transmural injury—may subsequently rupture if it is not appropriately repaired intraoperatively.
Following dissection of adhesions, irrigate the neighboring intestine with sterile saline, and perform a detailed inspection of the intestine to ascertain integrity of the bowel wall. The color of the intestine is important, as it can indicate whether the abundant vascular supply has been compromised. Include a detailed description of the intestines in the operative note.
Avoid stapling or vascular clips when repairing any wound; careful suturing is preferred.
Why early diagnosis is critical
The most favorable time to diagnose an iatrogenic intestinal perforation is within the intraoperative period. Prompt recognition and repair of bowel perforation offers several advantages:
- A second or third operation is less likely (
- The risk of abdominal sepsis is decreased.
- The volume of peripheral injury to the intestine is reduced.
- The patient can be followed for subsequent complications more precisely, permitting earlier diagnosis, more timely and effective treatment, and lower morbidity.
The 130 intestinal injuries reported by Baggish reflect the clinical significance of timely diagnosis.1 Seventy percent of small bowel and 51% of large bowel perforations were correctly diagnosed more than 48 hours postoperatively. Sepsis was present in a majority of these cases at the time of diagnosis.
Infection, fluid-electrolyte imbalance, sepsis syndrome
The principal derangements that arise as a result of bowel perforation are infection and fluid-electrolyte imbalance and their sequelae. Intestinal fluid and feces contain a variety of bacteria, such as Escherichia coli, Enterococcus, Klebsiella, Proteus, Pseudomonas, and Clostridium, to name a few. These bacteria produce toxins that facilitate entry of bacteria into the circulation and contribute to a downward spiral of events, referred to as sepsis syndrome, as well as intra-abdominal abscess:
- Contamination of the abdominal cavity leads to inflammation of the peritoneum
- In turn, subperitoneal blood vessels become porous, causing interstitial fluid to leak into the third space
- Paralytic ileus and an accumulation of intra-abdominal fluid push the diaphragm upward, lowering the capacity for lung expansion within the thorax and contributing to partial lung collapse
- Fluid of inflammatory origin may accumulate in the chest as pleural cavity effusion.
A number of progressive complications are predictable, but may occur at variable intervals after the initial perforation. The most frequent complications associated with colonic injuries are:
- peritonitis (98% of cases)
- ileus (92%)
- pleural effusion (84%)
- colostomy (80%)
- intra-abdominal abscess (78%).
The most common sequelae after small-bowel perforation are:
- peritonitis (100% of cases)
- ileus (89%)
- intra-abdominal abscess (63%)
- pleural effusion (59%).1
Reasons for diagnostic delay
- The gynecologic surgeon fails to place intestinal injury at the top of the differential diagnosis.
- A surgical consultant is delayed in making a correct diagnosis. Surgeons have less experience with perforation than do gynecologists, and invariably consider the postoperative abdominal problem to be ileus or intestinal obstruction. The presence of postoperative pneumoperitoneum is incorrectly thought to be lingering CO2 gas from the initial laparoscopy rather than air from a perforated viscus.
- Ancillary diagnosis confuses the primary physician. Pleural effusion, chest pain, and tachypnea are usually thought to indicate pulmonary embolism; as a result, the gynecologist and consulting pulmonologist focus on pulmonary embolus and deep-vein thrombosis. Only a spiral computed tomography (CT) scan, a ventilation perfusion (VQ) scan, or arteriogram quickly rules pulmonary embolus in or out. Peritonitis associated with ileus or third-space fluid leakage resulting in diaphragmatic elevation also creates pleural effusion, tachypnea, and dyspnea.
Presumptive diagnosis is critical
Definitive diagnosis of intestinal perforation happens at the operating table under direct vision and is corroborated by the pathology laboratory if bowel resection is performed. However, presumptive diagnosis helps overcome inertia and gets the patient to the operating room sooner.
The process by which the presumptive diagnosis is made is the most important issue in this article. The shorter the process, the lower the patient’s morbidity, and vice versa.
Look for steady improvement. Worry when it is absent
After any laparoscopic operation, the postoperative course should be one of steady clinical improvement. When a patient deviates from this model, the foremost presumptive diagnosis should be laparoscopy-associated injury, and the intestine should top the list of organs that may be injured. Other diagnoses should be subordinate to the principal presumptive diagnosis; these include ileus, bowel obstruction, pulmonary embolus, gastroenteritis, and hematoma, to name a few.
I do not mean to imply that a potentially life-threatening complication such as pulmonary embolus should not be ruled in or out, but that the necessary imaging should be performed in a timely fashion. The abdominal-pelvic CT scan will offer clues to the presence of free air, free fluid, air-fluid levels, and foreign bodies. It also is useful in detecting intra-abdominal—specifically, subphrenic—abscess. If necessary, a VQ scan or spiral CT scan can then be performed without delaying the diagnosis of the primary intra-abdominal catastrophe responsible for the pulmonary symptoms.
In the opening case, before making an improbable presumptive diagnosis, the surgeon should have questioned why an otherwise healthy woman would coincidentally develop gastroenteritis after laparoscopic surgery. The same can be said for diagnoses of intestinal obstruction or vascular thrombosis involving the intestinal blood supply.
Typical presentation of the injured patient
An injured patient does not experience daily improvement and a return to normal activity. Instead, the postoperative period is marked by persistent and worsening pain, often compounded by nausea or vomiting, or both. The patient may complain of fever, chills, weakness, or simply not feeling normal. Breathing may be labored. As time elapses, the symptoms become worse.
Reports of more than one visit to an emergency care facility are not uncommon. When examined, the patient exhibits direct or rebound tenderness, or both. The abdomen may or may not be distended, but usually is increased in girth. Bowel sounds are diminished or absent.
Vital signs initially reveal normal, low-grade, or subnormal temperature, and tachycardia, tachypnea, and normal blood pressure are typical. As time and sepsis progress, however, fever and hypotension develop. Most other symptoms and signs become progressively more abnormal in direct proportion to the length of time the diagnosis is delayed.
Seminal laboratory values for sepsis include a lower than normal white blood cell (WBC) count, elevated immature white-cell elements (e.g., “bandemia”), elevated liver chemistries, and an elevated serum creatinine level.
Mortality is most often the result of overwhelming and prolonged sepsis, leading to multiorgan failure, bleeding diathesis, and adult respiratory distress syndrome.
Among 130 laparoscopic surgical cases complicated by bowel injury and reported by Baggish, sepsis was diagnosed in 100% of colonic perforations and 50% of small-bowel perforations when the diagnosis was delayed more than 48 hours after surgery.1
TABLE 1 lists the signs and frequency of sepsis in these 100 cases, and TABLE 2 collates the signs and symptoms that were observed. Peritoneal cultures obtained at the time of exploratory laparotomy revealed multiple organisms (polymicrobial) in 60% of cases.
TABLE 1
Frequency of signs of sepsis among 130 patients with colon or small-bowel injury
| Sign | Colon (49 patients) | Small bowel (81 patients) |
|---|---|---|
| Normal or subnormal temperature | 30* | 41* |
| Fever | 19 | 40 |
| Tachycardia | 31 | 44 |
| Tachypnea | 30 | 40 |
| Hypotension | 21 | 15 |
| Anemia | 38 | 51 |
| Depressed WBC count | 20 | 18 |
| Elevated WBC count | 24 | 32 |
| Bandemia | 25 | 30 |
| Elevated creatinine and blood urea nitrogen levels | 12 | 5 |
| *Number of patients. | ||
| Source: Baggish1 | ||
Watch for signs and symptoms of intestinal injury
| Symptom | Sign |
|---|---|
| Abdominal pain | Direct or rebound tenderness |
| Bloating | Abdominal distension |
| Nausea, vomiting | Diminished bowel sounds |
| Fever, chills | Elevated or subnormal temperature |
| Difficulty breathing | Tachypnea, tachycardia |
| Weakness | Pallor, hypotension, diminished consciousness |
| Source: Baggish1 | |
Concurrent injuries to neighboring structures
A number of collateral injuries may occur in conjunction with intestinal perforation, depending on the location of the trauma. The most dangerous combination includes indirect laceration of one of the major retroperitoneal vessels. A through-and-through perforation of the cecum can also involve one or more of the right iliac vessels. A trocar perforation of the ileum may continue directly into the presacral space or pass above it and penetrate the left common iliac vein or aorta. Similarly, perforation of the sigmoid colon may penetrate the left iliac vessels.
Careful inspection of the posterior peritoneum for tears and evidence of retroperitoneal hematoma is required to avoid missing a serious collateral injury. More likely, however, is a penetrating injury to the small bowel presenting with collateral mesenteric damage and compromise of the blood supply of an entire segment of bowel. The ureter and bladder may also be injured when dissection along the pelvic sidewall, or a trocar thrust, deviates to the right or left of midline. In thin patients, the stomach may be perforated as well as the small intestine or transverse colon.
In one memorable case, a primary trocar penetrated the omentum, injuring several underlying structures. In its transit, the trocar passed through both the anterior and posterior walls of the duodenum and finally entered the superior mesenteric artery. The gynecologic surgeon performing the laparoscopic tubal ligation failed to recognize any of these injuries. The patient went into shock in the recovery room and was returned to the operating room. Fortunately, a transplant surgeon from a neighboring theater was immediately available to consult and repair the damage.
Another danger: intestinal ischemic necrosis
Abnormalities in splanchnic blood flow are sometimes caused by elevations in intra-abdominal pressure. Caldwell and Ricotta inflated the abdomens of nine dogs and reported a significant reduction of blood flow to omentum, stomach, duodenum, jejunum, ileum, colon, pancreas, liver, and spleen, but not to the adrenal glands.2 The splanchnic flow reductions essentially shunted blood away from abdominal viscera with auto-transfusion to the heart, lungs, and systemic circulation.
Eleftheriadis and colleagues studied 16 women randomized to laparoscopic versus open cholecystectomy.3 Significant depression of the hepatic microcirculation during the period of CO2 gas insufflation was noted in the laparoscopy cohort but not in the control group. Gastric mucosal ischemia also was observed in the laparoscopy group.
Several case reports of catastrophic intestinal ischemia have appeared in the literature (1994–1995).4-7 These articles have mainly involved laparoscopic upper abdominal operations in elderly people.
Recently, however, Hasson and colleagues reported a case of possible ischemic necrosis of the small intestine following laparoscopic adhesiolysis and bipolar myolysis.8 The authors emphasized that CO2 pneumoperitoneum reduces splanchnic blood flow, predisposing the patient to ischemia, but that ischemia with infarction requires an underlying vasculopathy or inciting factors such as traction on a short mesentery, atherosclerosis, or thrombosis.
A high index of suspicion for bowel ischemia following laparoscopic surgery should occur when, postoperatively, a patient experiences inordinately severe abdominal pain associated with tachypnea, tachycardia, and alterations in the WBC count. A paucity of physical abdominal signs in the early phases of this disorder should alert the clinician to the possibility of bowel ischemia.
Diagnosing and treating ischemia
A CT scan with contrast can suggest ischemia, but angiography is usually required for definitive diagnosis.
Treatment begins with infusion of papaverine into the intestinal vasculature via angiography cannula. In some cases, anticoagulation may be indicated. Surgery by laparotomy is clearly indicated for patients who fail to respond to vasodilatation measures.
This condition can be ameliorated by intermittent intraoperative decompression of the abdomen. Avoiding prolonged CO2 pneumoperitoneum and a lengthy laparoscopic operation also may diminish the risk of intestinal ischemia.
1. Baggish MS. One hundred and thirty small and large bowel injuries associated with gynecologic laparoscopic operations. J Gynecol Surg. 2007;23:83-95.
2. Caldwell CB, Ricotta JJ. Changes in visceral blood flow with elevated intra-abdominal pressure. J Surg Res. 1987;43:14-20.
3. Eleftheriadis E, Kotzampassi K, Botsios D, Tzartinoglu E, Farmakis H, Dadoukis J. Splanchnic ischemia during laparoscopic cholecystectomy. Surg Endosc. 1996;10:324-326.
4. Schorr RT. Laparoscopic upper abdominal operations and mesenteric infarction. J Laparoendosc Surg. 1995;5:389-392.
5. Mitchell PC, Jamieson GG. Coeliac axis and mesenteric arterial thrombosis following laparoscopic Nissen fundoplication. Aust N Z J Surg. 1994;64:728-730.
6. Dwerryhouse SJ, Melsom DS, Burton PA, Thompson MH. Acute intestinal ischaemia after laparoscopic cholecystectomy. Br J Surg. 1995;82:1413.-
7. Jaffe V, Russell RCG. Fatal intestinal ischaemia following laparoscopic cholecystectomy. Br J Surg. 1994;81:1827-1828.
8. Hasson HM, Galanopoulos C, Lanferman A. Ischemic necrosis of small bowel following laparoscopic surgery. JSLS. 2004;8:159-163.
1. Baggish MS. One hundred and thirty small and large bowel injuries associated with gynecologic laparoscopic operations. J Gynecol Surg. 2007;23:83-95.
2. Caldwell CB, Ricotta JJ. Changes in visceral blood flow with elevated intra-abdominal pressure. J Surg Res. 1987;43:14-20.
3. Eleftheriadis E, Kotzampassi K, Botsios D, Tzartinoglu E, Farmakis H, Dadoukis J. Splanchnic ischemia during laparoscopic cholecystectomy. Surg Endosc. 1996;10:324-326.
4. Schorr RT. Laparoscopic upper abdominal operations and mesenteric infarction. J Laparoendosc Surg. 1995;5:389-392.
5. Mitchell PC, Jamieson GG. Coeliac axis and mesenteric arterial thrombosis following laparoscopic Nissen fundoplication. Aust N Z J Surg. 1994;64:728-730.
6. Dwerryhouse SJ, Melsom DS, Burton PA, Thompson MH. Acute intestinal ischaemia after laparoscopic cholecystectomy. Br J Surg. 1995;82:1413.-
7. Jaffe V, Russell RCG. Fatal intestinal ischaemia following laparoscopic cholecystectomy. Br J Surg. 1994;81:1827-1828.
8. Hasson HM, Galanopoulos C, Lanferman A. Ischemic necrosis of small bowel following laparoscopic surgery. JSLS. 2004;8:159-163.
When necessity calls for treating uterine fibroids
The author reports no financial relationships relevant to this article.
Part 1 of this article, in the May 2008 issue, discusses how to counsel patients who are found to have a uterine fibroid.
CASE 1 Menorrhagia with anemia
G.L. is a 44-year-old G2P2 who comes to the office for a second opinion on treatment for menorrhagia and a 10-weeks–size fibroid uterus. She reports that her periods last 8 days, and that for 3 of those days she changes a pad once an hour. Her most recent hemoglobin level was 10.2 g/dL. Her regular gynecologist has recommended abdominal hysterectomy. She would like to avoid major surgery and asks about alternatives. What therapies do you tell her are appropriate?
Most women who have uterine fibroids are asymptomatic or mildly symptomatic; they do not require treatment. In one study, 77% of women choosing observation for their fibroids had no significant changes in bleeding, pain, bothersome symptoms, mental health, general health, or activity after 1 year.1 After menopause, fibroids shrink, and the rate of surgery decreases greatly.2 For women such as these, “watchful waiting” may allow them to avoid treatment indefinitely.
For such women as G.L., however, who develop severe anemia from fibroid-related menorrhagia, treatment is necessary. It also is indicated in the rare case of hydro-nephrosis due to obstruction of the ureter(s) by fibroids, or when menorrhagia, pelvic pain or pressure, or urinary frequency or incontinence compromises quality of life.
The distress experienced by women with symptoms such as these can be severe. In one study, women who chose hysterectomy for fibroid-related symptoms assessed their quality of life as worse than that of women who suffered hypertension, heart disease, chronic lung disease, or arthritis.3
Nevertheless, when symptomatic women were offered hysterectomy as a first and sometimes sole treatment, some chose to adapt to symptoms and stop seeking treatment. In fact, hysterectomy is not the only option. A number of alternatives are available, including:
- medical therapy
- the progesterone-releasing IUD
- endometrial ablation
- hysteroscopic, laparoscopic, and abdominal myomectomy
- uterine artery embolization (UAE).4
With the exception of medical therapy, all of these modalities are described here.
- Most uterine fibroids are asymptomatic, require no treatment, and can be managed by watchful waiting.
- Treatment is indicated when fibroids cause severe anemia and when symptoms interfere with quality of life.
- Hysterectomy is not the only treatment option; alternatives include medical therapy, the progesterone-releasing intrauterine system, endometrial ablation, myomectomy (hysteroscopic, laparoscopic, or abdominal), uterine artery embolization (UAE), and focused ultrasound.
- Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent treatment with gonadotropin-releasing hormone agonists, previous iliac or uterine artery occlusion, or postmenopausal status.
- Myomectomy may be considered even for women who have large uterine fibroids who wish to retain their uterus. Surgical techniques available for abdominal or laparoscopic myomectomy make this procedure safe.
- Women who have intractable symptoms and who have not been helped by other therapies may benefit from hysterectomy. Laparoscopic hysterectomy has the benefits of less postoperative pain, shorter hospital stay, and quicker recovery. If a vaginal hysterectomy is feasible, however, there is no benefit to a laparoscopic hysterectomy.
Progesterone-releasing intrauterine system
In a woman who has fibroids no larger than 12-weeks size and a normal uterine cavity, the levonorgestrel-releasing intrauterine system (IUS) (brand name, Mirena) has been shown to substantially reduce menstrual bleeding.5 Within 3 months, 22 of 26 (85%) women with documented menorrhagia treated in this way had normal bleeding and, by 12 months, 40% of all 76 women studied were amenorrheic.
CASE 1 CONTINUED
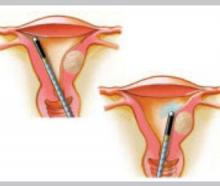
You perform an office hysteroscopy on G.L., which reveals a 3-cm, type 1 submucosal fibroid, suggesting, by its size, that the levonorgestrel-releasing IUS is unlikely to relieve her bleeding. What other treatments might be appropriate?
Studies show a reduction in bleeding following hysteroscopic resection of submucous fibroids. One hundred ninety-six consecutive women who had menorrhagia and one or more submucous myomas were followed for an average of 73 months after hysteroscopic myomectomy.6 Sixty-eight percent reported “satisfaction and ability to lead a normal life,” and 32% considered results unsatisfactory.
In a report of 285 consecutive women treated with hysteroscopic myomectomy for menorrhagia or metrorrhagia, additional surgery was necessary for 9.5% by 2 years, 10.8% by 5 years, and 26.7% by 8 years.7
Endometrial ablation
In women who do not desire future childbearing, endometrial ablation with or without hysteroscopic myomectomy may be an option. One study that used pad counts as an objective measure found that abnormal bleeding resolved in 48 of 51 women (94%) following endometrial ablation, after an average follow-up of 2 years.8
A study of 33 women who had uterine myomas and total uterine volume smaller than 16-weeks size, and who were followed for a mean of 8 months after Nd:YAG laser ablation of the endometrium, reported amenorrhea in 16 women (49%) and eumenorrhea or hypomenorrhea in the other 17.9
Hydrothermal ablation was used to treat 22 women who had submucous myomas as large as 4 cm in diameter, with 91% reporting amenorrhea, hypomenorrhea, or eumenorrhea after a minimum of 12 months of follow-up.10
Sixty-five women who suffered from menometrorrhagia with hysteroscopically confirmed type I or type II submucous myomas as large as 3 cm had endometrial ablation with the NovaSure System.11 After 1 year, 95% had a reduction in bleeding to a normal degree; 69% had amenorrhea. No intraoperative or postoperative complications occurred.
Uterine artery embolization
UAE appears to be an effective treatment for some women who have fibroids. At the moment, the effect of UAE on premature ovarian failure, fertility, and pregnancy is not clear; most interventional radiologists advise against the procedure for women who want to become pregnant. Although very rare, complications of UAE may necessitate lifesaving hysterectomy, and women who would not accept hysterectomy even under these circumstances should not undergo UAE.
Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous (IV) contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent gonadotropin-releasing hormone (GnRH) agonist treatment, previous iliac or uterine artery occlusion, and postmenopausal status.12
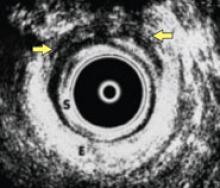
How UAE works
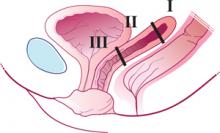
In UAE, a trained interventional radiologist performs percutaneous cannulation of the femoral artery. Embolization of the uterine artery and its branches (FIGURE 1) is accomplished with gelatin sponges, polyvinyl alcohol particles (PVA), or tris-acryl gelatin microspheres under fluoroscopic guidance. Total radiation exposure is equivalent to one to two computed tomography (CT) scans.
Postprocedural pain usually requires pain management in the hospital overnight, but most women are discharged the next day on a nonsteroidal anti-inflammatory drug (NSAID). Most women can return to normal activity in 1 to 3 weeks, although about 5% to 10% of women experience a longer bout of pain.
Postembolization syndrome requires admission for treatment with IV fluids, an NSAID, and pain management. It usually resolves in 48 to 72 hours. Persistent fever should be managed with antibiotics, but a failure to respond to antibiotics may indicate sepsis, indicating the need for aggressive management with hysterectomy. ACOG recommends that women considering UAE have a thorough evaluation with a gynecologist to help facilitate collaboration with the interventional radiologist, and that protocols be in place to establish the responsibility of caring for the patient at all times.13
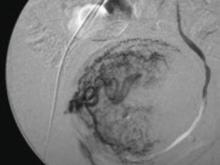
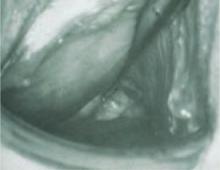
FIGURE 1 Target: blood supply
Arteriogram showing blood supply to fibroid to be targeted during uterine artery embolization.
What the data show
The largest prospective study of UAE included 555 women, 18 to 59 years old, 40% of whom had required time off from work for fibroid-related symptoms. Three months after UAE, the largest myomas were reduced by a mean of 33%. Menorrhagia had improved in 83%; dysmenorrhea, in 77%; and urinary frequency, in 86%.14 Interestingly, improvement in menorrhagia was not related to pre-UAE uterine volume or the volume reduction attained.
Hysterectomy was performed for complications in 1.5% of women: two for infection, four for persistent postembolization pain, one for prolapsed myoma, and one for continued vaginal bleeding. Of 400 women followed for a mean of 16.7 months, 74% were considered a clinical success.15
More than 50,000 UAE procedures have been performed worldwide. Five deaths have been reported: two from septic shock, one from a pulmonary embolus, and two from uncertain causes. This compares favorably with the mortality of 3 for every 10,000 hysterectomies in a similar group of women, which was reported in the national inpatient sample of the Healthcare Cost and Utilization Project (HCUP) database of the Agency for HealthCare Research and Quality, available at http://hcup.ahrq.gov/HCUPnet.asp.
Effects on fertility
Following UAE, amenorrhea has been reported in 3% of women under 40 but in 41% of women over 50.16 Although normal follicle-stimulating hormone (FSH), estradiol, ovarian volume, and antral follicle counts have been found in most women shortly after UAE, such testing is unable to predict the onset of menopause.
Loss of follicles as a result of misembolization to the ovarian vessels and decreased ovarian perfusion might cause ovarian failure at an earlier age than expected (Robert Vogetzang, MD, personal communication, 2007). Long-term follow-up of women who have had UAE will be necessary to answer this important question.
CASE 1 RESOLVED
G.L. chose hysteroscopic myomectomy and endometrial ablation for her menorrhagia. Twelve months later, she remains amenorrheic.
CASE 2 Large fibroids; options other than hysterectomy?
A.M., a 39-year-old G2P2, complains of pelvic pressure and urinary frequency. On examination, you find a 14-weeks–size fibroid uterus. She has not given up hope for giving birth to one more child, and wants to avoid hysterectomy. Ultrasonography shows two fundal fibroids, both about 7 cm in diameter. A.M. asks what treatment options are available for her. What can you offer this patient?
Abdominal myomectomy
Myomectomy is used less often than hysterectomy. In 1999, when one third of the 598,000 hysterectomies performed annually were performed for fibroids, only 30,000 myomectomies were performed.17
As long ago as 1931, Victor Bonney advocated abdominal myomectomy because he believed that the procedure best served what should be the “ultimate goal of surgical treatment, the restoration and maintenance of physiologic function.” Yet women are still being told that hysterectomy is safer, associated with less blood loss—or that myomectomy is inappropriate because sarcoma may be present. Recent reports do not support these concerns.
Uterine fibroids are extremely common. By age 50, 80% of African-American and 70% of Caucasian women have fibroids.1 Fibroids were the primary indication for surgery in the United States in 1997, accounting for 199,000 hysterectomies and 30,000 myomectomies at a cost of $2.1 billion.1 The costs of alternative surgical therapies, medical treatments, and time away from work or family add significantly to the expense associated with fibroids.2
References
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229-234.
2. Myers E, Barber M, Couchman G, et al. Management of Uterine Fibroids. AHRQ Evidence Reports. Vol. 1, No. 34. Washington, DC: AHRQ; 2001.
Myomectomy vs hysterectomy
A review of 197 women who underwent myomectomy and 197 women who underwent hysterectomy with similar uterine size (14.4 vs 15.6 weeks) found the risks of hemorrhage, fever, unintended surgical procedures, life-threatening events, and rehospitalization equivalent between the two procedures.18 Women in the hysterectomy group had more surgical blood loss (484 mL vs 227 mL) and suffered more complications (13%), including one cystotomy, one ureteral injury, three bowel injuries, eight cases of ileus, and six cases of pelvic abscess.19
In contrast, only 5% of the myomectomy patients had a complication, which included one cystotomy, two reoperations for small bowel obstruction, and six cases of ileus. The authors concluded that myomectomy is a safe alternative to hysterectomy.
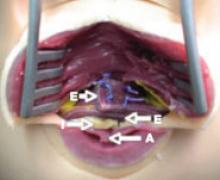
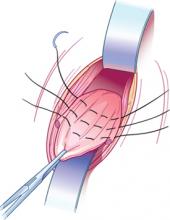
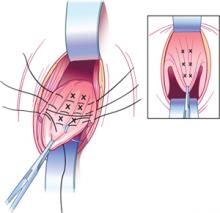
Myomectomy may be feasible even with large fibroids
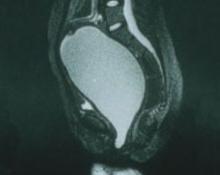
Abdominal myomectomy may be considered even for women who have large uterine fibroids (FIGURE 2) and who wish to retain their uterus. A study of 91 women who had uterine size larger than 16 weeks (range, 16 to 36 weeks) and underwent abdominal myomectomy reported no instance of conversion to hysterectomy. Complications included one bowel injury, one bladder injury, and one reoperation for bowel obstruction.20
In the past, enlarging fibroids have been deemed an indication that hysterectomy should be performed because leiomyosarcoma may be present. This concern is unfounded. A study of 371 women with a “rapidly growing uterus” found leiomyosarcoma in only one.21
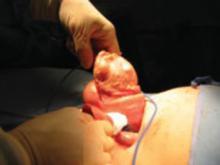
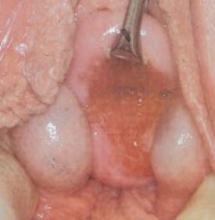
FIGURE 2 Abdominal myomectomy
Myomectomy may be appropriate even for women who have large fibroids who wish to retain their uterus.
Removing large fibroids safely
Surgical techniques available for myomectomy allow safe removal of even large fibroids. Tourniquets and vasoconstrictive substances (vasopressin [off-label use]) may be used to limit blood loss. Continuing the uterine incisions through the myometrium and entire pseudocapsule until the fibroid is clearly seen exposes a less vascular surgical plane, which is deeper than commonly appreciated. Vascular corrosion casting shows that fibroids are totally surrounded by a dense vascular layer and that no distinct “vascular pedicle” exists at the base of the myoma.22
Fibroids that are near dominant fibroids can be removed through the same uterine incision, but avoid tunneling through the myometrium to remove distant fibroids; many myometrial tunnels are hard to close and can continue to bleed. Promptly closing each incision allows immediate hemostasis and, although multiple uterine incisions may be needed, adhesion barriers may help limit formation of adhesions.23
Avoiding heterologous transfusion
Cell-saver technology has been used extensively in orthopedic, cardiac, and neurologic surgery; consider it during myomectomy (or hysterectomy).
The cell saver suctions blood from the operative field and mixes it with heparinized saline. If blood reinfusion is necessary, the blood is washed with saline, filtered, centrifuged to a hematocrit of approximately 50%, and given back to the patient via an IV line. The need for preoperative autologous blood donation or heterologous blood transfusion can therefore often be avoided, eliminating the risk of infection and transfusion reaction.24
Seventy of 91 women who underwent myomectomy for uterine size of 16 to 36 weeks had cell-saver blood reinfused (mean volume, 355 mL); only seven women required heterologous transfusion.20
Laparoscopic myomectomy
Instrumentation makes laparoscopic myomectomy feasible, although the application of this approach is limited by the size and number of fibroids that can be reasonably removed and by the difficulty of laparoscopic suturing. However, a study of 131 women randomized to abdominal and laparoscopic myomectomy for nonpedunculated large myomas (mean diameter, 7 cm) found a higher postoperative hemoglobin level, lower incidence of postoperative fever, and shorter hospital stays with laparoscopic myomectomy.25
A case series of 144 women (largest fibroid, 18 cm [mean, 7.8 cm]) reported that only two (1.4%) women required conversion to laparotomy.26
Myomas do not recur
Once individual myomas are removed, they do not recur, although new myomas may appear. Most women require no additional treatment. If the first myomectomy is performed for one fibroid, 11% of women require subsequent surgery (mean follow-up, 7.6 years). If multiple fibroids are removed initially, 26% require subsequent surgery.27
The appearance of a new myoma may reflect the persistence of fibroids not removed initially—as ultrasonography has demonstrated in 29% of women after myomectomy.28
CASE 2 RESOLVED
A.M. underwent pelvic magnetic resonance imaging, which revealed two 7-cm intramural fibroids and four other intramural fibroids between 2 cm and 4 cm in size. She chose abdominal myomectomy and is now attempting pregnancy.
CASE 3 Patient asks for hysterectomy
S.L. is a 44-year-old G2P2 who complains of missing a few days of work every month because of heavy menstrual bleeding and fatigue. Her hemoglobin level is now 8.2 g/dL. She underwent myomectomy about 10 years ago, successfully followed by two pregnancies, but her uterus is now about 12-weeks size. She is not interested in getting pregnant again and wants to be able to work without bleeding through her clothes. She has explored other options, but has decided to have a hysterectomy. She asks whether laparoscopic supracervical hysterectomy is appropriate for her situation. What do you advise?
Treating preoperative anemia
The first step for this patient is to treat her anemia.
Erythropoietin alfa and epoetin have been shown to increase preoperative hemoglobin concentrations in cardiac, orthopedic, and neurologic surgery. They should be considered more often, when appropriate, before gynecologic surgery.29 A randomized study showed that approximately 15,000 U of epoetin a week for 3 weeks before surgery raised the hemoglobin concentration by 1.6 g/dL and significantly reduced the transfusion rate when compared with controls.30 No side effects were reported.
GnRH agonists have been shown to reduce uterine volume, fibroid volume, and bleeding; these benefits may be limited, however, by side effects and risks. Reduction in uterine size occurs mostly within the first 3 months of treatment; after 6 months, fibroid volume is reduced by 30% and total uterine volume by 35%.31,32 Heavy bleeding responds well to GnRH agonists; in one study, 37 of 38 women had resolution by 6 months.
Side effects generally do not deter treatment
Side effects are common with GnRH agonists: 78% experience hot flushes; 32%, vaginal dryness; and 55%, transient headache. Arthralgia, myalgia, insomnia, emotional lability, and decreased libido are reported less often. However, only 8% of women terminate treatment because of side effects.33
Bone loss is significant after 6 months of a GnRH agonist.34
A Cochrane review found that women who have myomas and who were treated preoperatively with 3 to 4 months of a GnRH agonist had significantly reduced uterine volume and uterine size; an improved preoperative hemoglobin level; and reduced operating times and hospital stay.35 Although operative blood loss was less for both abdominal hysterectomy and abdominal myomectomy patients, there was no significant difference in the transfusion rate.
Hysterectomy
Fibroids were the indication for hysterectomy in 40% of abdominal, 17% of vaginal, and 29% of laparoscopic hysterectomies, according to a review in the United States.17 Women with intractable symptoms who have not been helped by other therapies may benefit from hysterectomy. The Maine Women’s Health Study found that, following hysterectomy (35% of which were performed for myomas) for moderate or severe symptoms, 72% of women felt “much better,” 16% felt a “little better,” and 3% felt worse than they did before surgery.1
Laparoscopic hysterectomy
Either total or supracervical laparoscopic hysterectomy is feasible. Benefits include less postoperative pain, short hospital stay, and quick recovery. However, if a vaginal hysterectomy is feasible, there is no benefit to laparoscopic hysterectomy.36
What the data show
A prospective, randomized, multicenter study concluded that laparoscopic-assisted hysterectomy offered the benefits of less invasive surgery without increased risk.37 Eighty women whose uterus was between 280 g and 700 g were randomized to laparoscopic-assisted vaginal and abdominal hysterectomy. Estimated blood loss, postoperative day 1 hemoglobin level, pain, and hospital stay were all significantly better for the laparoscopic-assisted group. Complications in the abdominal hysterectomy group included one woman who had a cuff hematoma and who required transfusion; one who had bleeding requiring reoperation and transfusion; and five who had fever. The only complication in the laparoscopic group was postoperative fever in two women.
Even large fibroids may benefit from laparoscopy
In experienced hands, the benefits of laparoscopic hysterectomy may extend to women who have large fibroids. A retrospective cohort study compared laparoscopic hysterectomy in 34 women who had a uterine weight greater than 500 g (range, 500 to 1,230 g) with 68 women whose uterus weighed less than 300 g.38 Operating time was significantly shorter in women with smaller uteri, but no difference was observed in complications, blood loss, hospital stay, or recovery, and no patient required conversion to laparotomy.
CASE 3 RESOLVED
S.L. underwent laparoscopic supracervical hysterectomy, which involved a 1-night hospital stay, and returned to work in 2 weeks. She is happy to be free of monthly bleeding and believes she made the right treatment decision.
Just as there are multiple options for removing myomas, so are there multiple coding possibilities for this service. Note that some procedures require special documentation of the clinical circumstances to ensure correct payment and that other treatments may be considered investigational by payers.
Surgical removal of uterine fibroids can be accomplished vaginally (58145), abdominally (58140, 58146), hysteroscopically (58561), and laparoscopically (58545–58546). Except for the hysteroscopic approach, all require documentation of the number and weight of the fibroids, to ensure that payment reflects how much work was done. When five or more fibroids are removed, or when the combined weight of all fibroids removed exceeds 250 g, the CPT codes that represent these services will reimburse at a higher rate. When endometrial ablation is the treatment of choice, you must choose between hysteroscopic (58563) and nonhysteroscopic (58353) methods when selecting a code.
Insertion of the levonorgestrel-releasing intrauterine system (Mirena) requires that you report more than one code. Report insertion 58300 (S4981 for Blue Cross and Blue Shield carriers). Bill for the device itself with J7302, or with J7306 (the system and supplies).
Last, some payers consider uterine artery embolization investigational, even though it has its own CPT code (37210).—MELANIE WITT, RN, CPC-OGS, MA
1. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566-572.
2. Cramer SF, Marchetti C, Freedman J, Padela A. Relationship of myoma cell size and menopausal status in small uterine leiomyomas. Arch Pathol Lab Med. 2000;124:1448-1453.
3. Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93:915-921.
4. Parker W. Uterine myomas: management. Fertil Steril. 2007;88:255-271.
5. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
6. Cravello L. [Indications and modalities of surgical treatment for sub-mucosal myomas]. J Gynecol Obstet Biol Reprod (Paris). 1999;28:748-752.
7. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93:743-748.
8. Indman PD. Hysteroscopic treatment of menorrhagia associated with uterine leiomyomas. Obstet Gynecol. 1993;81:716-720.
9. Lomano J. Endometrial ablation for the treatment of menorrhagia: a comparison of patients with normal, enlarged, and fibroid uteri. Lasers Surg Med. 1991;11:8-12.
10. Glasser MH, Zimmerman JD. The HydroThermAblator system for management of menorrhagia in women with submucous myomas: 12- to 20-month follow-up. J Am Assoc Gynecol Laparosc. 2003;10:521-527.
11. Sabbah R, Desaulniers G. Use of the NovaSure Impedance Controlled Endometrial Ablation System in patients with intracavitary disease: 12-month follow-up results of a prospective, single-arm clinical study. J Minim Invasive Gynecol. 2006;13:467-471.
12. Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines. Uterine fibroid embolization (UFE). Number 150, October 2004. Int. J Gynaecol Obstet. 2005;89:305-318.
13. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Uterine artery embolization. Obstet Gynecol. 2004;103:403-404.
14. Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
15. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109:1262-1272.
16. Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
17. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
18. Iverson RE, Jr, Chelmow D, Strohbehn K, Waldman L, Evantash EG. Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996;88:415-419.
19. Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183:1448-1455.
20. West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85:36-39.
21. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
22. Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088-1093.
23. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904-910.
24. Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59:233-236.
25. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
26. Malzoni M, Rotond M, Perone C, et al. Fertility after laparoscopic myomectomy of large uterine myomas: operative technique and preliminary results. Eur J Gynaecol Oncol. 2003;24:79-82.
27. Malone L. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34:200-203.
28. Fedele L, Parazzini F, Luchini L, Mezzopane R, Tozzi L, Villa L. Recurrence of fibroids after myomectomy: a transvaginal ultrasonographic study. Hum Reprod. 1995;10:1795-1796.
29. Sesti F, Ticconi C, Bonifacio S, Piccione E. Preoperative administration of recombinant human erythropoietin in patients undergoing gynecologic surgery. Gynecol Obstet Invest. 2002;54:1-5.
30. Wurnig C, Schatz K, Noske H, et al. Collaborative Study Group. Subcutaneous low-dose epoetin beta for the avoidance of transfusion in patients scheduled for elective surgery not eligible for autologous blood donation. Eur Surg Res. 2001;33:303-310.
31. Schlaff WD, Zerhouni EA, Huth JA, Chen J, Damewood MD, Rock JA. A placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (leuprolide) in the treatment of uterine leiomyomata. Obstet Gynecol. 1989;74:856-862.
32. Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet Gynecol. 1991;77:720-725.
33. Letterie GS, Coddington CC, Winkel CA, Shawker TH, Loriaux DL, Collins RL. Efficacy of a gonadotropin-releasing hormone agonist in the treatment of uterine leiomyomata: long-term follow-up. Fertil Steril. 1989;51:951-956.
34. Leather AT, Studd JW, Watson NR, Holland EF. The prevention of bone loss in young women treated with GnRH analogues with “add-back” estrogen therapy. Obstet Gynecol. 1993;81:104-107.
35. Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG. 2002;109:1097-1108.
36. Stovall TG, Summitt RL Jr, Bran DF, Ling FW. Outpatient vaginal hysterectomy: a pilot study. Obstet Gynecol. 1992;80:145-149.
37. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multi-center study. Am J Obstet Gynecol. 1999;180:270-275.
38. Wattiez A, Soriano D, Fiaccavento A, et al. Total laparoscopic hysterectomy for very enlarged uteri. J Am Assoc Gynecol Laparosc. 2002;9:125-130.
The author reports no financial relationships relevant to this article.
Part 1 of this article, in the May 2008 issue, discusses how to counsel patients who are found to have a uterine fibroid.
CASE 1 Menorrhagia with anemia
G.L. is a 44-year-old G2P2 who comes to the office for a second opinion on treatment for menorrhagia and a 10-weeks–size fibroid uterus. She reports that her periods last 8 days, and that for 3 of those days she changes a pad once an hour. Her most recent hemoglobin level was 10.2 g/dL. Her regular gynecologist has recommended abdominal hysterectomy. She would like to avoid major surgery and asks about alternatives. What therapies do you tell her are appropriate?
Most women who have uterine fibroids are asymptomatic or mildly symptomatic; they do not require treatment. In one study, 77% of women choosing observation for their fibroids had no significant changes in bleeding, pain, bothersome symptoms, mental health, general health, or activity after 1 year.1 After menopause, fibroids shrink, and the rate of surgery decreases greatly.2 For women such as these, “watchful waiting” may allow them to avoid treatment indefinitely.
For such women as G.L., however, who develop severe anemia from fibroid-related menorrhagia, treatment is necessary. It also is indicated in the rare case of hydro-nephrosis due to obstruction of the ureter(s) by fibroids, or when menorrhagia, pelvic pain or pressure, or urinary frequency or incontinence compromises quality of life.
The distress experienced by women with symptoms such as these can be severe. In one study, women who chose hysterectomy for fibroid-related symptoms assessed their quality of life as worse than that of women who suffered hypertension, heart disease, chronic lung disease, or arthritis.3
Nevertheless, when symptomatic women were offered hysterectomy as a first and sometimes sole treatment, some chose to adapt to symptoms and stop seeking treatment. In fact, hysterectomy is not the only option. A number of alternatives are available, including:
- medical therapy
- the progesterone-releasing IUD
- endometrial ablation
- hysteroscopic, laparoscopic, and abdominal myomectomy
- uterine artery embolization (UAE).4
With the exception of medical therapy, all of these modalities are described here.
- Most uterine fibroids are asymptomatic, require no treatment, and can be managed by watchful waiting.
- Treatment is indicated when fibroids cause severe anemia and when symptoms interfere with quality of life.
- Hysterectomy is not the only treatment option; alternatives include medical therapy, the progesterone-releasing intrauterine system, endometrial ablation, myomectomy (hysteroscopic, laparoscopic, or abdominal), uterine artery embolization (UAE), and focused ultrasound.
- Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent treatment with gonadotropin-releasing hormone agonists, previous iliac or uterine artery occlusion, or postmenopausal status.
- Myomectomy may be considered even for women who have large uterine fibroids who wish to retain their uterus. Surgical techniques available for abdominal or laparoscopic myomectomy make this procedure safe.
- Women who have intractable symptoms and who have not been helped by other therapies may benefit from hysterectomy. Laparoscopic hysterectomy has the benefits of less postoperative pain, shorter hospital stay, and quicker recovery. If a vaginal hysterectomy is feasible, however, there is no benefit to a laparoscopic hysterectomy.
Progesterone-releasing intrauterine system
In a woman who has fibroids no larger than 12-weeks size and a normal uterine cavity, the levonorgestrel-releasing intrauterine system (IUS) (brand name, Mirena) has been shown to substantially reduce menstrual bleeding.5 Within 3 months, 22 of 26 (85%) women with documented menorrhagia treated in this way had normal bleeding and, by 12 months, 40% of all 76 women studied were amenorrheic.
CASE 1 CONTINUED
You perform an office hysteroscopy on G.L., which reveals a 3-cm, type 1 submucosal fibroid, suggesting, by its size, that the levonorgestrel-releasing IUS is unlikely to relieve her bleeding. What other treatments might be appropriate?
Studies show a reduction in bleeding following hysteroscopic resection of submucous fibroids. One hundred ninety-six consecutive women who had menorrhagia and one or more submucous myomas were followed for an average of 73 months after hysteroscopic myomectomy.6 Sixty-eight percent reported “satisfaction and ability to lead a normal life,” and 32% considered results unsatisfactory.
In a report of 285 consecutive women treated with hysteroscopic myomectomy for menorrhagia or metrorrhagia, additional surgery was necessary for 9.5% by 2 years, 10.8% by 5 years, and 26.7% by 8 years.7
Endometrial ablation
In women who do not desire future childbearing, endometrial ablation with or without hysteroscopic myomectomy may be an option. One study that used pad counts as an objective measure found that abnormal bleeding resolved in 48 of 51 women (94%) following endometrial ablation, after an average follow-up of 2 years.8
A study of 33 women who had uterine myomas and total uterine volume smaller than 16-weeks size, and who were followed for a mean of 8 months after Nd:YAG laser ablation of the endometrium, reported amenorrhea in 16 women (49%) and eumenorrhea or hypomenorrhea in the other 17.9
Hydrothermal ablation was used to treat 22 women who had submucous myomas as large as 4 cm in diameter, with 91% reporting amenorrhea, hypomenorrhea, or eumenorrhea after a minimum of 12 months of follow-up.10
Sixty-five women who suffered from menometrorrhagia with hysteroscopically confirmed type I or type II submucous myomas as large as 3 cm had endometrial ablation with the NovaSure System.11 After 1 year, 95% had a reduction in bleeding to a normal degree; 69% had amenorrhea. No intraoperative or postoperative complications occurred.
Uterine artery embolization
UAE appears to be an effective treatment for some women who have fibroids. At the moment, the effect of UAE on premature ovarian failure, fertility, and pregnancy is not clear; most interventional radiologists advise against the procedure for women who want to become pregnant. Although very rare, complications of UAE may necessitate lifesaving hysterectomy, and women who would not accept hysterectomy even under these circumstances should not undergo UAE.
Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous (IV) contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent gonadotropin-releasing hormone (GnRH) agonist treatment, previous iliac or uterine artery occlusion, and postmenopausal status.12
How UAE works
In UAE, a trained interventional radiologist performs percutaneous cannulation of the femoral artery. Embolization of the uterine artery and its branches (FIGURE 1) is accomplished with gelatin sponges, polyvinyl alcohol particles (PVA), or tris-acryl gelatin microspheres under fluoroscopic guidance. Total radiation exposure is equivalent to one to two computed tomography (CT) scans.
Postprocedural pain usually requires pain management in the hospital overnight, but most women are discharged the next day on a nonsteroidal anti-inflammatory drug (NSAID). Most women can return to normal activity in 1 to 3 weeks, although about 5% to 10% of women experience a longer bout of pain.
Postembolization syndrome requires admission for treatment with IV fluids, an NSAID, and pain management. It usually resolves in 48 to 72 hours. Persistent fever should be managed with antibiotics, but a failure to respond to antibiotics may indicate sepsis, indicating the need for aggressive management with hysterectomy. ACOG recommends that women considering UAE have a thorough evaluation with a gynecologist to help facilitate collaboration with the interventional radiologist, and that protocols be in place to establish the responsibility of caring for the patient at all times.13
FIGURE 1 Target: blood supply
Arteriogram showing blood supply to fibroid to be targeted during uterine artery embolization.
What the data show
The largest prospective study of UAE included 555 women, 18 to 59 years old, 40% of whom had required time off from work for fibroid-related symptoms. Three months after UAE, the largest myomas were reduced by a mean of 33%. Menorrhagia had improved in 83%; dysmenorrhea, in 77%; and urinary frequency, in 86%.14 Interestingly, improvement in menorrhagia was not related to pre-UAE uterine volume or the volume reduction attained.
Hysterectomy was performed for complications in 1.5% of women: two for infection, four for persistent postembolization pain, one for prolapsed myoma, and one for continued vaginal bleeding. Of 400 women followed for a mean of 16.7 months, 74% were considered a clinical success.15
More than 50,000 UAE procedures have been performed worldwide. Five deaths have been reported: two from septic shock, one from a pulmonary embolus, and two from uncertain causes. This compares favorably with the mortality of 3 for every 10,000 hysterectomies in a similar group of women, which was reported in the national inpatient sample of the Healthcare Cost and Utilization Project (HCUP) database of the Agency for HealthCare Research and Quality, available at http://hcup.ahrq.gov/HCUPnet.asp.
Effects on fertility
Following UAE, amenorrhea has been reported in 3% of women under 40 but in 41% of women over 50.16 Although normal follicle-stimulating hormone (FSH), estradiol, ovarian volume, and antral follicle counts have been found in most women shortly after UAE, such testing is unable to predict the onset of menopause.
Loss of follicles as a result of misembolization to the ovarian vessels and decreased ovarian perfusion might cause ovarian failure at an earlier age than expected (Robert Vogetzang, MD, personal communication, 2007). Long-term follow-up of women who have had UAE will be necessary to answer this important question.
CASE 1 RESOLVED
G.L. chose hysteroscopic myomectomy and endometrial ablation for her menorrhagia. Twelve months later, she remains amenorrheic.
CASE 2 Large fibroids; options other than hysterectomy?
A.M., a 39-year-old G2P2, complains of pelvic pressure and urinary frequency. On examination, you find a 14-weeks–size fibroid uterus. She has not given up hope for giving birth to one more child, and wants to avoid hysterectomy. Ultrasonography shows two fundal fibroids, both about 7 cm in diameter. A.M. asks what treatment options are available for her. What can you offer this patient?
Abdominal myomectomy
Myomectomy is used less often than hysterectomy. In 1999, when one third of the 598,000 hysterectomies performed annually were performed for fibroids, only 30,000 myomectomies were performed.17
As long ago as 1931, Victor Bonney advocated abdominal myomectomy because he believed that the procedure best served what should be the “ultimate goal of surgical treatment, the restoration and maintenance of physiologic function.” Yet women are still being told that hysterectomy is safer, associated with less blood loss—or that myomectomy is inappropriate because sarcoma may be present. Recent reports do not support these concerns.
Uterine fibroids are extremely common. By age 50, 80% of African-American and 70% of Caucasian women have fibroids.1 Fibroids were the primary indication for surgery in the United States in 1997, accounting for 199,000 hysterectomies and 30,000 myomectomies at a cost of $2.1 billion.1 The costs of alternative surgical therapies, medical treatments, and time away from work or family add significantly to the expense associated with fibroids.2
References
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229-234.
2. Myers E, Barber M, Couchman G, et al. Management of Uterine Fibroids. AHRQ Evidence Reports. Vol. 1, No. 34. Washington, DC: AHRQ; 2001.
Myomectomy vs hysterectomy
A review of 197 women who underwent myomectomy and 197 women who underwent hysterectomy with similar uterine size (14.4 vs 15.6 weeks) found the risks of hemorrhage, fever, unintended surgical procedures, life-threatening events, and rehospitalization equivalent between the two procedures.18 Women in the hysterectomy group had more surgical blood loss (484 mL vs 227 mL) and suffered more complications (13%), including one cystotomy, one ureteral injury, three bowel injuries, eight cases of ileus, and six cases of pelvic abscess.19
In contrast, only 5% of the myomectomy patients had a complication, which included one cystotomy, two reoperations for small bowel obstruction, and six cases of ileus. The authors concluded that myomectomy is a safe alternative to hysterectomy.
Myomectomy may be feasible even with large fibroids
Abdominal myomectomy may be considered even for women who have large uterine fibroids (FIGURE 2) and who wish to retain their uterus. A study of 91 women who had uterine size larger than 16 weeks (range, 16 to 36 weeks) and underwent abdominal myomectomy reported no instance of conversion to hysterectomy. Complications included one bowel injury, one bladder injury, and one reoperation for bowel obstruction.20
In the past, enlarging fibroids have been deemed an indication that hysterectomy should be performed because leiomyosarcoma may be present. This concern is unfounded. A study of 371 women with a “rapidly growing uterus” found leiomyosarcoma in only one.21
FIGURE 2 Abdominal myomectomy
Myomectomy may be appropriate even for women who have large fibroids who wish to retain their uterus.
Removing large fibroids safely
Surgical techniques available for myomectomy allow safe removal of even large fibroids. Tourniquets and vasoconstrictive substances (vasopressin [off-label use]) may be used to limit blood loss. Continuing the uterine incisions through the myometrium and entire pseudocapsule until the fibroid is clearly seen exposes a less vascular surgical plane, which is deeper than commonly appreciated. Vascular corrosion casting shows that fibroids are totally surrounded by a dense vascular layer and that no distinct “vascular pedicle” exists at the base of the myoma.22
Fibroids that are near dominant fibroids can be removed through the same uterine incision, but avoid tunneling through the myometrium to remove distant fibroids; many myometrial tunnels are hard to close and can continue to bleed. Promptly closing each incision allows immediate hemostasis and, although multiple uterine incisions may be needed, adhesion barriers may help limit formation of adhesions.23
Avoiding heterologous transfusion
Cell-saver technology has been used extensively in orthopedic, cardiac, and neurologic surgery; consider it during myomectomy (or hysterectomy).
The cell saver suctions blood from the operative field and mixes it with heparinized saline. If blood reinfusion is necessary, the blood is washed with saline, filtered, centrifuged to a hematocrit of approximately 50%, and given back to the patient via an IV line. The need for preoperative autologous blood donation or heterologous blood transfusion can therefore often be avoided, eliminating the risk of infection and transfusion reaction.24
Seventy of 91 women who underwent myomectomy for uterine size of 16 to 36 weeks had cell-saver blood reinfused (mean volume, 355 mL); only seven women required heterologous transfusion.20
Laparoscopic myomectomy
Instrumentation makes laparoscopic myomectomy feasible, although the application of this approach is limited by the size and number of fibroids that can be reasonably removed and by the difficulty of laparoscopic suturing. However, a study of 131 women randomized to abdominal and laparoscopic myomectomy for nonpedunculated large myomas (mean diameter, 7 cm) found a higher postoperative hemoglobin level, lower incidence of postoperative fever, and shorter hospital stays with laparoscopic myomectomy.25
A case series of 144 women (largest fibroid, 18 cm [mean, 7.8 cm]) reported that only two (1.4%) women required conversion to laparotomy.26
Myomas do not recur
Once individual myomas are removed, they do not recur, although new myomas may appear. Most women require no additional treatment. If the first myomectomy is performed for one fibroid, 11% of women require subsequent surgery (mean follow-up, 7.6 years). If multiple fibroids are removed initially, 26% require subsequent surgery.27
The appearance of a new myoma may reflect the persistence of fibroids not removed initially—as ultrasonography has demonstrated in 29% of women after myomectomy.28
CASE 2 RESOLVED
A.M. underwent pelvic magnetic resonance imaging, which revealed two 7-cm intramural fibroids and four other intramural fibroids between 2 cm and 4 cm in size. She chose abdominal myomectomy and is now attempting pregnancy.
CASE 3 Patient asks for hysterectomy
S.L. is a 44-year-old G2P2 who complains of missing a few days of work every month because of heavy menstrual bleeding and fatigue. Her hemoglobin level is now 8.2 g/dL. She underwent myomectomy about 10 years ago, successfully followed by two pregnancies, but her uterus is now about 12-weeks size. She is not interested in getting pregnant again and wants to be able to work without bleeding through her clothes. She has explored other options, but has decided to have a hysterectomy. She asks whether laparoscopic supracervical hysterectomy is appropriate for her situation. What do you advise?
Treating preoperative anemia
The first step for this patient is to treat her anemia.
Erythropoietin alfa and epoetin have been shown to increase preoperative hemoglobin concentrations in cardiac, orthopedic, and neurologic surgery. They should be considered more often, when appropriate, before gynecologic surgery.29 A randomized study showed that approximately 15,000 U of epoetin a week for 3 weeks before surgery raised the hemoglobin concentration by 1.6 g/dL and significantly reduced the transfusion rate when compared with controls.30 No side effects were reported.
GnRH agonists have been shown to reduce uterine volume, fibroid volume, and bleeding; these benefits may be limited, however, by side effects and risks. Reduction in uterine size occurs mostly within the first 3 months of treatment; after 6 months, fibroid volume is reduced by 30% and total uterine volume by 35%.31,32 Heavy bleeding responds well to GnRH agonists; in one study, 37 of 38 women had resolution by 6 months.
Side effects generally do not deter treatment
Side effects are common with GnRH agonists: 78% experience hot flushes; 32%, vaginal dryness; and 55%, transient headache. Arthralgia, myalgia, insomnia, emotional lability, and decreased libido are reported less often. However, only 8% of women terminate treatment because of side effects.33
Bone loss is significant after 6 months of a GnRH agonist.34
A Cochrane review found that women who have myomas and who were treated preoperatively with 3 to 4 months of a GnRH agonist had significantly reduced uterine volume and uterine size; an improved preoperative hemoglobin level; and reduced operating times and hospital stay.35 Although operative blood loss was less for both abdominal hysterectomy and abdominal myomectomy patients, there was no significant difference in the transfusion rate.
Hysterectomy
Fibroids were the indication for hysterectomy in 40% of abdominal, 17% of vaginal, and 29% of laparoscopic hysterectomies, according to a review in the United States.17 Women with intractable symptoms who have not been helped by other therapies may benefit from hysterectomy. The Maine Women’s Health Study found that, following hysterectomy (35% of which were performed for myomas) for moderate or severe symptoms, 72% of women felt “much better,” 16% felt a “little better,” and 3% felt worse than they did before surgery.1
Laparoscopic hysterectomy
Either total or supracervical laparoscopic hysterectomy is feasible. Benefits include less postoperative pain, short hospital stay, and quick recovery. However, if a vaginal hysterectomy is feasible, there is no benefit to laparoscopic hysterectomy.36
What the data show
A prospective, randomized, multicenter study concluded that laparoscopic-assisted hysterectomy offered the benefits of less invasive surgery without increased risk.37 Eighty women whose uterus was between 280 g and 700 g were randomized to laparoscopic-assisted vaginal and abdominal hysterectomy. Estimated blood loss, postoperative day 1 hemoglobin level, pain, and hospital stay were all significantly better for the laparoscopic-assisted group. Complications in the abdominal hysterectomy group included one woman who had a cuff hematoma and who required transfusion; one who had bleeding requiring reoperation and transfusion; and five who had fever. The only complication in the laparoscopic group was postoperative fever in two women.
Even large fibroids may benefit from laparoscopy
In experienced hands, the benefits of laparoscopic hysterectomy may extend to women who have large fibroids. A retrospective cohort study compared laparoscopic hysterectomy in 34 women who had a uterine weight greater than 500 g (range, 500 to 1,230 g) with 68 women whose uterus weighed less than 300 g.38 Operating time was significantly shorter in women with smaller uteri, but no difference was observed in complications, blood loss, hospital stay, or recovery, and no patient required conversion to laparotomy.
CASE 3 RESOLVED
S.L. underwent laparoscopic supracervical hysterectomy, which involved a 1-night hospital stay, and returned to work in 2 weeks. She is happy to be free of monthly bleeding and believes she made the right treatment decision.
Just as there are multiple options for removing myomas, so are there multiple coding possibilities for this service. Note that some procedures require special documentation of the clinical circumstances to ensure correct payment and that other treatments may be considered investigational by payers.
Surgical removal of uterine fibroids can be accomplished vaginally (58145), abdominally (58140, 58146), hysteroscopically (58561), and laparoscopically (58545–58546). Except for the hysteroscopic approach, all require documentation of the number and weight of the fibroids, to ensure that payment reflects how much work was done. When five or more fibroids are removed, or when the combined weight of all fibroids removed exceeds 250 g, the CPT codes that represent these services will reimburse at a higher rate. When endometrial ablation is the treatment of choice, you must choose between hysteroscopic (58563) and nonhysteroscopic (58353) methods when selecting a code.
Insertion of the levonorgestrel-releasing intrauterine system (Mirena) requires that you report more than one code. Report insertion 58300 (S4981 for Blue Cross and Blue Shield carriers). Bill for the device itself with J7302, or with J7306 (the system and supplies).
Last, some payers consider uterine artery embolization investigational, even though it has its own CPT code (37210).—MELANIE WITT, RN, CPC-OGS, MA
The author reports no financial relationships relevant to this article.
Part 1 of this article, in the May 2008 issue, discusses how to counsel patients who are found to have a uterine fibroid.
CASE 1 Menorrhagia with anemia
G.L. is a 44-year-old G2P2 who comes to the office for a second opinion on treatment for menorrhagia and a 10-weeks–size fibroid uterus. She reports that her periods last 8 days, and that for 3 of those days she changes a pad once an hour. Her most recent hemoglobin level was 10.2 g/dL. Her regular gynecologist has recommended abdominal hysterectomy. She would like to avoid major surgery and asks about alternatives. What therapies do you tell her are appropriate?
Most women who have uterine fibroids are asymptomatic or mildly symptomatic; they do not require treatment. In one study, 77% of women choosing observation for their fibroids had no significant changes in bleeding, pain, bothersome symptoms, mental health, general health, or activity after 1 year.1 After menopause, fibroids shrink, and the rate of surgery decreases greatly.2 For women such as these, “watchful waiting” may allow them to avoid treatment indefinitely.
For such women as G.L., however, who develop severe anemia from fibroid-related menorrhagia, treatment is necessary. It also is indicated in the rare case of hydro-nephrosis due to obstruction of the ureter(s) by fibroids, or when menorrhagia, pelvic pain or pressure, or urinary frequency or incontinence compromises quality of life.
The distress experienced by women with symptoms such as these can be severe. In one study, women who chose hysterectomy for fibroid-related symptoms assessed their quality of life as worse than that of women who suffered hypertension, heart disease, chronic lung disease, or arthritis.3
Nevertheless, when symptomatic women were offered hysterectomy as a first and sometimes sole treatment, some chose to adapt to symptoms and stop seeking treatment. In fact, hysterectomy is not the only option. A number of alternatives are available, including:
- medical therapy
- the progesterone-releasing IUD
- endometrial ablation
- hysteroscopic, laparoscopic, and abdominal myomectomy
- uterine artery embolization (UAE).4
With the exception of medical therapy, all of these modalities are described here.
- Most uterine fibroids are asymptomatic, require no treatment, and can be managed by watchful waiting.
- Treatment is indicated when fibroids cause severe anemia and when symptoms interfere with quality of life.
- Hysterectomy is not the only treatment option; alternatives include medical therapy, the progesterone-releasing intrauterine system, endometrial ablation, myomectomy (hysteroscopic, laparoscopic, or abdominal), uterine artery embolization (UAE), and focused ultrasound.
- Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent treatment with gonadotropin-releasing hormone agonists, previous iliac or uterine artery occlusion, or postmenopausal status.
- Myomectomy may be considered even for women who have large uterine fibroids who wish to retain their uterus. Surgical techniques available for abdominal or laparoscopic myomectomy make this procedure safe.
- Women who have intractable symptoms and who have not been helped by other therapies may benefit from hysterectomy. Laparoscopic hysterectomy has the benefits of less postoperative pain, shorter hospital stay, and quicker recovery. If a vaginal hysterectomy is feasible, however, there is no benefit to a laparoscopic hysterectomy.
Progesterone-releasing intrauterine system
In a woman who has fibroids no larger than 12-weeks size and a normal uterine cavity, the levonorgestrel-releasing intrauterine system (IUS) (brand name, Mirena) has been shown to substantially reduce menstrual bleeding.5 Within 3 months, 22 of 26 (85%) women with documented menorrhagia treated in this way had normal bleeding and, by 12 months, 40% of all 76 women studied were amenorrheic.
CASE 1 CONTINUED
You perform an office hysteroscopy on G.L., which reveals a 3-cm, type 1 submucosal fibroid, suggesting, by its size, that the levonorgestrel-releasing IUS is unlikely to relieve her bleeding. What other treatments might be appropriate?
Studies show a reduction in bleeding following hysteroscopic resection of submucous fibroids. One hundred ninety-six consecutive women who had menorrhagia and one or more submucous myomas were followed for an average of 73 months after hysteroscopic myomectomy.6 Sixty-eight percent reported “satisfaction and ability to lead a normal life,” and 32% considered results unsatisfactory.
In a report of 285 consecutive women treated with hysteroscopic myomectomy for menorrhagia or metrorrhagia, additional surgery was necessary for 9.5% by 2 years, 10.8% by 5 years, and 26.7% by 8 years.7
Endometrial ablation
In women who do not desire future childbearing, endometrial ablation with or without hysteroscopic myomectomy may be an option. One study that used pad counts as an objective measure found that abnormal bleeding resolved in 48 of 51 women (94%) following endometrial ablation, after an average follow-up of 2 years.8
A study of 33 women who had uterine myomas and total uterine volume smaller than 16-weeks size, and who were followed for a mean of 8 months after Nd:YAG laser ablation of the endometrium, reported amenorrhea in 16 women (49%) and eumenorrhea or hypomenorrhea in the other 17.9
Hydrothermal ablation was used to treat 22 women who had submucous myomas as large as 4 cm in diameter, with 91% reporting amenorrhea, hypomenorrhea, or eumenorrhea after a minimum of 12 months of follow-up.10
Sixty-five women who suffered from menometrorrhagia with hysteroscopically confirmed type I or type II submucous myomas as large as 3 cm had endometrial ablation with the NovaSure System.11 After 1 year, 95% had a reduction in bleeding to a normal degree; 69% had amenorrhea. No intraoperative or postoperative complications occurred.
Uterine artery embolization
UAE appears to be an effective treatment for some women who have fibroids. At the moment, the effect of UAE on premature ovarian failure, fertility, and pregnancy is not clear; most interventional radiologists advise against the procedure for women who want to become pregnant. Although very rare, complications of UAE may necessitate lifesaving hysterectomy, and women who would not accept hysterectomy even under these circumstances should not undergo UAE.
Contraindications to UAE include active genitourinary infection, genital tract malignancy, reduced immune status, severe vascular disease, allergy to intravenous (IV) contrast, or impaired renal function. Relative contraindications include large submucous myomas, pedunculated myomas, recent gonadotropin-releasing hormone (GnRH) agonist treatment, previous iliac or uterine artery occlusion, and postmenopausal status.12
How UAE works
In UAE, a trained interventional radiologist performs percutaneous cannulation of the femoral artery. Embolization of the uterine artery and its branches (FIGURE 1) is accomplished with gelatin sponges, polyvinyl alcohol particles (PVA), or tris-acryl gelatin microspheres under fluoroscopic guidance. Total radiation exposure is equivalent to one to two computed tomography (CT) scans.
Postprocedural pain usually requires pain management in the hospital overnight, but most women are discharged the next day on a nonsteroidal anti-inflammatory drug (NSAID). Most women can return to normal activity in 1 to 3 weeks, although about 5% to 10% of women experience a longer bout of pain.
Postembolization syndrome requires admission for treatment with IV fluids, an NSAID, and pain management. It usually resolves in 48 to 72 hours. Persistent fever should be managed with antibiotics, but a failure to respond to antibiotics may indicate sepsis, indicating the need for aggressive management with hysterectomy. ACOG recommends that women considering UAE have a thorough evaluation with a gynecologist to help facilitate collaboration with the interventional radiologist, and that protocols be in place to establish the responsibility of caring for the patient at all times.13
FIGURE 1 Target: blood supply
Arteriogram showing blood supply to fibroid to be targeted during uterine artery embolization.
What the data show
The largest prospective study of UAE included 555 women, 18 to 59 years old, 40% of whom had required time off from work for fibroid-related symptoms. Three months after UAE, the largest myomas were reduced by a mean of 33%. Menorrhagia had improved in 83%; dysmenorrhea, in 77%; and urinary frequency, in 86%.14 Interestingly, improvement in menorrhagia was not related to pre-UAE uterine volume or the volume reduction attained.
Hysterectomy was performed for complications in 1.5% of women: two for infection, four for persistent postembolization pain, one for prolapsed myoma, and one for continued vaginal bleeding. Of 400 women followed for a mean of 16.7 months, 74% were considered a clinical success.15
More than 50,000 UAE procedures have been performed worldwide. Five deaths have been reported: two from septic shock, one from a pulmonary embolus, and two from uncertain causes. This compares favorably with the mortality of 3 for every 10,000 hysterectomies in a similar group of women, which was reported in the national inpatient sample of the Healthcare Cost and Utilization Project (HCUP) database of the Agency for HealthCare Research and Quality, available at http://hcup.ahrq.gov/HCUPnet.asp.
Effects on fertility
Following UAE, amenorrhea has been reported in 3% of women under 40 but in 41% of women over 50.16 Although normal follicle-stimulating hormone (FSH), estradiol, ovarian volume, and antral follicle counts have been found in most women shortly after UAE, such testing is unable to predict the onset of menopause.
Loss of follicles as a result of misembolization to the ovarian vessels and decreased ovarian perfusion might cause ovarian failure at an earlier age than expected (Robert Vogetzang, MD, personal communication, 2007). Long-term follow-up of women who have had UAE will be necessary to answer this important question.
CASE 1 RESOLVED
G.L. chose hysteroscopic myomectomy and endometrial ablation for her menorrhagia. Twelve months later, she remains amenorrheic.
CASE 2 Large fibroids; options other than hysterectomy?
A.M., a 39-year-old G2P2, complains of pelvic pressure and urinary frequency. On examination, you find a 14-weeks–size fibroid uterus. She has not given up hope for giving birth to one more child, and wants to avoid hysterectomy. Ultrasonography shows two fundal fibroids, both about 7 cm in diameter. A.M. asks what treatment options are available for her. What can you offer this patient?
Abdominal myomectomy
Myomectomy is used less often than hysterectomy. In 1999, when one third of the 598,000 hysterectomies performed annually were performed for fibroids, only 30,000 myomectomies were performed.17
As long ago as 1931, Victor Bonney advocated abdominal myomectomy because he believed that the procedure best served what should be the “ultimate goal of surgical treatment, the restoration and maintenance of physiologic function.” Yet women are still being told that hysterectomy is safer, associated with less blood loss—or that myomectomy is inappropriate because sarcoma may be present. Recent reports do not support these concerns.
Uterine fibroids are extremely common. By age 50, 80% of African-American and 70% of Caucasian women have fibroids.1 Fibroids were the primary indication for surgery in the United States in 1997, accounting for 199,000 hysterectomies and 30,000 myomectomies at a cost of $2.1 billion.1 The costs of alternative surgical therapies, medical treatments, and time away from work or family add significantly to the expense associated with fibroids.2
References
1. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229-234.
2. Myers E, Barber M, Couchman G, et al. Management of Uterine Fibroids. AHRQ Evidence Reports. Vol. 1, No. 34. Washington, DC: AHRQ; 2001.
Myomectomy vs hysterectomy
A review of 197 women who underwent myomectomy and 197 women who underwent hysterectomy with similar uterine size (14.4 vs 15.6 weeks) found the risks of hemorrhage, fever, unintended surgical procedures, life-threatening events, and rehospitalization equivalent between the two procedures.18 Women in the hysterectomy group had more surgical blood loss (484 mL vs 227 mL) and suffered more complications (13%), including one cystotomy, one ureteral injury, three bowel injuries, eight cases of ileus, and six cases of pelvic abscess.19
In contrast, only 5% of the myomectomy patients had a complication, which included one cystotomy, two reoperations for small bowel obstruction, and six cases of ileus. The authors concluded that myomectomy is a safe alternative to hysterectomy.
Myomectomy may be feasible even with large fibroids
Abdominal myomectomy may be considered even for women who have large uterine fibroids (FIGURE 2) and who wish to retain their uterus. A study of 91 women who had uterine size larger than 16 weeks (range, 16 to 36 weeks) and underwent abdominal myomectomy reported no instance of conversion to hysterectomy. Complications included one bowel injury, one bladder injury, and one reoperation for bowel obstruction.20
In the past, enlarging fibroids have been deemed an indication that hysterectomy should be performed because leiomyosarcoma may be present. This concern is unfounded. A study of 371 women with a “rapidly growing uterus” found leiomyosarcoma in only one.21
FIGURE 2 Abdominal myomectomy
Myomectomy may be appropriate even for women who have large fibroids who wish to retain their uterus.
Removing large fibroids safely
Surgical techniques available for myomectomy allow safe removal of even large fibroids. Tourniquets and vasoconstrictive substances (vasopressin [off-label use]) may be used to limit blood loss. Continuing the uterine incisions through the myometrium and entire pseudocapsule until the fibroid is clearly seen exposes a less vascular surgical plane, which is deeper than commonly appreciated. Vascular corrosion casting shows that fibroids are totally surrounded by a dense vascular layer and that no distinct “vascular pedicle” exists at the base of the myoma.22
Fibroids that are near dominant fibroids can be removed through the same uterine incision, but avoid tunneling through the myometrium to remove distant fibroids; many myometrial tunnels are hard to close and can continue to bleed. Promptly closing each incision allows immediate hemostasis and, although multiple uterine incisions may be needed, adhesion barriers may help limit formation of adhesions.23
Avoiding heterologous transfusion
Cell-saver technology has been used extensively in orthopedic, cardiac, and neurologic surgery; consider it during myomectomy (or hysterectomy).
The cell saver suctions blood from the operative field and mixes it with heparinized saline. If blood reinfusion is necessary, the blood is washed with saline, filtered, centrifuged to a hematocrit of approximately 50%, and given back to the patient via an IV line. The need for preoperative autologous blood donation or heterologous blood transfusion can therefore often be avoided, eliminating the risk of infection and transfusion reaction.24
Seventy of 91 women who underwent myomectomy for uterine size of 16 to 36 weeks had cell-saver blood reinfused (mean volume, 355 mL); only seven women required heterologous transfusion.20
Laparoscopic myomectomy
Instrumentation makes laparoscopic myomectomy feasible, although the application of this approach is limited by the size and number of fibroids that can be reasonably removed and by the difficulty of laparoscopic suturing. However, a study of 131 women randomized to abdominal and laparoscopic myomectomy for nonpedunculated large myomas (mean diameter, 7 cm) found a higher postoperative hemoglobin level, lower incidence of postoperative fever, and shorter hospital stays with laparoscopic myomectomy.25
A case series of 144 women (largest fibroid, 18 cm [mean, 7.8 cm]) reported that only two (1.4%) women required conversion to laparotomy.26
Myomas do not recur
Once individual myomas are removed, they do not recur, although new myomas may appear. Most women require no additional treatment. If the first myomectomy is performed for one fibroid, 11% of women require subsequent surgery (mean follow-up, 7.6 years). If multiple fibroids are removed initially, 26% require subsequent surgery.27
The appearance of a new myoma may reflect the persistence of fibroids not removed initially—as ultrasonography has demonstrated in 29% of women after myomectomy.28
CASE 2 RESOLVED
A.M. underwent pelvic magnetic resonance imaging, which revealed two 7-cm intramural fibroids and four other intramural fibroids between 2 cm and 4 cm in size. She chose abdominal myomectomy and is now attempting pregnancy.
CASE 3 Patient asks for hysterectomy
S.L. is a 44-year-old G2P2 who complains of missing a few days of work every month because of heavy menstrual bleeding and fatigue. Her hemoglobin level is now 8.2 g/dL. She underwent myomectomy about 10 years ago, successfully followed by two pregnancies, but her uterus is now about 12-weeks size. She is not interested in getting pregnant again and wants to be able to work without bleeding through her clothes. She has explored other options, but has decided to have a hysterectomy. She asks whether laparoscopic supracervical hysterectomy is appropriate for her situation. What do you advise?
Treating preoperative anemia
The first step for this patient is to treat her anemia.
Erythropoietin alfa and epoetin have been shown to increase preoperative hemoglobin concentrations in cardiac, orthopedic, and neurologic surgery. They should be considered more often, when appropriate, before gynecologic surgery.29 A randomized study showed that approximately 15,000 U of epoetin a week for 3 weeks before surgery raised the hemoglobin concentration by 1.6 g/dL and significantly reduced the transfusion rate when compared with controls.30 No side effects were reported.
GnRH agonists have been shown to reduce uterine volume, fibroid volume, and bleeding; these benefits may be limited, however, by side effects and risks. Reduction in uterine size occurs mostly within the first 3 months of treatment; after 6 months, fibroid volume is reduced by 30% and total uterine volume by 35%.31,32 Heavy bleeding responds well to GnRH agonists; in one study, 37 of 38 women had resolution by 6 months.
Side effects generally do not deter treatment
Side effects are common with GnRH agonists: 78% experience hot flushes; 32%, vaginal dryness; and 55%, transient headache. Arthralgia, myalgia, insomnia, emotional lability, and decreased libido are reported less often. However, only 8% of women terminate treatment because of side effects.33
Bone loss is significant after 6 months of a GnRH agonist.34
A Cochrane review found that women who have myomas and who were treated preoperatively with 3 to 4 months of a GnRH agonist had significantly reduced uterine volume and uterine size; an improved preoperative hemoglobin level; and reduced operating times and hospital stay.35 Although operative blood loss was less for both abdominal hysterectomy and abdominal myomectomy patients, there was no significant difference in the transfusion rate.
Hysterectomy
Fibroids were the indication for hysterectomy in 40% of abdominal, 17% of vaginal, and 29% of laparoscopic hysterectomies, according to a review in the United States.17 Women with intractable symptoms who have not been helped by other therapies may benefit from hysterectomy. The Maine Women’s Health Study found that, following hysterectomy (35% of which were performed for myomas) for moderate or severe symptoms, 72% of women felt “much better,” 16% felt a “little better,” and 3% felt worse than they did before surgery.1
Laparoscopic hysterectomy
Either total or supracervical laparoscopic hysterectomy is feasible. Benefits include less postoperative pain, short hospital stay, and quick recovery. However, if a vaginal hysterectomy is feasible, there is no benefit to laparoscopic hysterectomy.36
What the data show
A prospective, randomized, multicenter study concluded that laparoscopic-assisted hysterectomy offered the benefits of less invasive surgery without increased risk.37 Eighty women whose uterus was between 280 g and 700 g were randomized to laparoscopic-assisted vaginal and abdominal hysterectomy. Estimated blood loss, postoperative day 1 hemoglobin level, pain, and hospital stay were all significantly better for the laparoscopic-assisted group. Complications in the abdominal hysterectomy group included one woman who had a cuff hematoma and who required transfusion; one who had bleeding requiring reoperation and transfusion; and five who had fever. The only complication in the laparoscopic group was postoperative fever in two women.
Even large fibroids may benefit from laparoscopy
In experienced hands, the benefits of laparoscopic hysterectomy may extend to women who have large fibroids. A retrospective cohort study compared laparoscopic hysterectomy in 34 women who had a uterine weight greater than 500 g (range, 500 to 1,230 g) with 68 women whose uterus weighed less than 300 g.38 Operating time was significantly shorter in women with smaller uteri, but no difference was observed in complications, blood loss, hospital stay, or recovery, and no patient required conversion to laparotomy.
CASE 3 RESOLVED
S.L. underwent laparoscopic supracervical hysterectomy, which involved a 1-night hospital stay, and returned to work in 2 weeks. She is happy to be free of monthly bleeding and believes she made the right treatment decision.
Just as there are multiple options for removing myomas, so are there multiple coding possibilities for this service. Note that some procedures require special documentation of the clinical circumstances to ensure correct payment and that other treatments may be considered investigational by payers.
Surgical removal of uterine fibroids can be accomplished vaginally (58145), abdominally (58140, 58146), hysteroscopically (58561), and laparoscopically (58545–58546). Except for the hysteroscopic approach, all require documentation of the number and weight of the fibroids, to ensure that payment reflects how much work was done. When five or more fibroids are removed, or when the combined weight of all fibroids removed exceeds 250 g, the CPT codes that represent these services will reimburse at a higher rate. When endometrial ablation is the treatment of choice, you must choose between hysteroscopic (58563) and nonhysteroscopic (58353) methods when selecting a code.
Insertion of the levonorgestrel-releasing intrauterine system (Mirena) requires that you report more than one code. Report insertion 58300 (S4981 for Blue Cross and Blue Shield carriers). Bill for the device itself with J7302, or with J7306 (the system and supplies).
Last, some payers consider uterine artery embolization investigational, even though it has its own CPT code (37210).—MELANIE WITT, RN, CPC-OGS, MA
1. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566-572.
2. Cramer SF, Marchetti C, Freedman J, Padela A. Relationship of myoma cell size and menopausal status in small uterine leiomyomas. Arch Pathol Lab Med. 2000;124:1448-1453.
3. Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93:915-921.
4. Parker W. Uterine myomas: management. Fertil Steril. 2007;88:255-271.
5. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
6. Cravello L. [Indications and modalities of surgical treatment for sub-mucosal myomas]. J Gynecol Obstet Biol Reprod (Paris). 1999;28:748-752.
7. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93:743-748.
8. Indman PD. Hysteroscopic treatment of menorrhagia associated with uterine leiomyomas. Obstet Gynecol. 1993;81:716-720.
9. Lomano J. Endometrial ablation for the treatment of menorrhagia: a comparison of patients with normal, enlarged, and fibroid uteri. Lasers Surg Med. 1991;11:8-12.
10. Glasser MH, Zimmerman JD. The HydroThermAblator system for management of menorrhagia in women with submucous myomas: 12- to 20-month follow-up. J Am Assoc Gynecol Laparosc. 2003;10:521-527.
11. Sabbah R, Desaulniers G. Use of the NovaSure Impedance Controlled Endometrial Ablation System in patients with intracavitary disease: 12-month follow-up results of a prospective, single-arm clinical study. J Minim Invasive Gynecol. 2006;13:467-471.
12. Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines. Uterine fibroid embolization (UFE). Number 150, October 2004. Int. J Gynaecol Obstet. 2005;89:305-318.
13. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Uterine artery embolization. Obstet Gynecol. 2004;103:403-404.
14. Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
15. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109:1262-1272.
16. Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
17. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
18. Iverson RE, Jr, Chelmow D, Strohbehn K, Waldman L, Evantash EG. Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996;88:415-419.
19. Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183:1448-1455.
20. West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85:36-39.
21. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
22. Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088-1093.
23. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904-910.
24. Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59:233-236.
25. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
26. Malzoni M, Rotond M, Perone C, et al. Fertility after laparoscopic myomectomy of large uterine myomas: operative technique and preliminary results. Eur J Gynaecol Oncol. 2003;24:79-82.
27. Malone L. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34:200-203.
28. Fedele L, Parazzini F, Luchini L, Mezzopane R, Tozzi L, Villa L. Recurrence of fibroids after myomectomy: a transvaginal ultrasonographic study. Hum Reprod. 1995;10:1795-1796.
29. Sesti F, Ticconi C, Bonifacio S, Piccione E. Preoperative administration of recombinant human erythropoietin in patients undergoing gynecologic surgery. Gynecol Obstet Invest. 2002;54:1-5.
30. Wurnig C, Schatz K, Noske H, et al. Collaborative Study Group. Subcutaneous low-dose epoetin beta for the avoidance of transfusion in patients scheduled for elective surgery not eligible for autologous blood donation. Eur Surg Res. 2001;33:303-310.
31. Schlaff WD, Zerhouni EA, Huth JA, Chen J, Damewood MD, Rock JA. A placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (leuprolide) in the treatment of uterine leiomyomata. Obstet Gynecol. 1989;74:856-862.
32. Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet Gynecol. 1991;77:720-725.
33. Letterie GS, Coddington CC, Winkel CA, Shawker TH, Loriaux DL, Collins RL. Efficacy of a gonadotropin-releasing hormone agonist in the treatment of uterine leiomyomata: long-term follow-up. Fertil Steril. 1989;51:951-956.
34. Leather AT, Studd JW, Watson NR, Holland EF. The prevention of bone loss in young women treated with GnRH analogues with “add-back” estrogen therapy. Obstet Gynecol. 1993;81:104-107.
35. Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG. 2002;109:1097-1108.
36. Stovall TG, Summitt RL Jr, Bran DF, Ling FW. Outpatient vaginal hysterectomy: a pilot study. Obstet Gynecol. 1992;80:145-149.
37. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multi-center study. Am J Obstet Gynecol. 1999;180:270-275.
38. Wattiez A, Soriano D, Fiaccavento A, et al. Total laparoscopic hysterectomy for very enlarged uteri. J Am Assoc Gynecol Laparosc. 2002;9:125-130.
1. Carlson KJ, Miller BA, Fowler FJ, Jr. The Maine Women’s Health Study: II. Outcomes of nonsurgical management of leiomyomas, abnormal bleeding, and chronic pelvic pain. Obstet Gynecol. 1994;83:566-572.
2. Cramer SF, Marchetti C, Freedman J, Padela A. Relationship of myoma cell size and menopausal status in small uterine leiomyomas. Arch Pathol Lab Med. 2000;124:1448-1453.
3. Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93:915-921.
4. Parker W. Uterine myomas: management. Fertil Steril. 2007;88:255-271.
5. Grigorieva V, Chen-Mok M, Tarasova M, Mikhailov A. Use of a levonorgestrel-releasing intrauterine system to treat bleeding related to uterine leiomyomas. Fertil Steril. 2003;79:1194-1198.
6. Cravello L. [Indications and modalities of surgical treatment for sub-mucosal myomas]. J Gynecol Obstet Biol Reprod (Paris). 1999;28:748-752.
7. Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol. 1999;93:743-748.
8. Indman PD. Hysteroscopic treatment of menorrhagia associated with uterine leiomyomas. Obstet Gynecol. 1993;81:716-720.
9. Lomano J. Endometrial ablation for the treatment of menorrhagia: a comparison of patients with normal, enlarged, and fibroid uteri. Lasers Surg Med. 1991;11:8-12.
10. Glasser MH, Zimmerman JD. The HydroThermAblator system for management of menorrhagia in women with submucous myomas: 12- to 20-month follow-up. J Am Assoc Gynecol Laparosc. 2003;10:521-527.
11. Sabbah R, Desaulniers G. Use of the NovaSure Impedance Controlled Endometrial Ablation System in patients with intracavitary disease: 12-month follow-up results of a prospective, single-arm clinical study. J Minim Invasive Gynecol. 2006;13:467-471.
12. Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines. Uterine fibroid embolization (UFE). Number 150, October 2004. Int. J Gynaecol Obstet. 2005;89:305-318.
13. Committee on Gynecologic Practice, American College of Obstetricians and Gynecologists. ACOG Committee Opinion. Uterine artery embolization. Obstet Gynecol. 2004;103:403-404.
14. Pron G, Mocarski E, Bennett J, Vilos G, Common A, Vanderburgh L. Ontario UFE Collaborative Group. Pregnancy after uterine artery embolization for leiomyomata: the Ontario multicenter trial. Obstet Gynecol. 2005;105:67-76.
15. Walker WJ, Pelage JP. Uterine artery embolisation for symptomatic fibroids: clinical results in 400 women with imaging follow up. BJOG. 2002;109:1262-1272.
16. Pron G, Bennett J, Common A, Wall J, Asch M, Sniderman K. The Ontario Uterine Fibroid Embolization Trial. Part 2. Uterine fibroid reduction and symptom relief after uterine artery embolization for fibroids. Fertil Steril. 2003;79:120-127.
17. Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990-1997. Obstet Gynecol. 2002;99:229-234.
18. Iverson RE, Jr, Chelmow D, Strohbehn K, Waldman L, Evantash EG. Relative morbidity of abdominal hysterectomy and myomectomy for management of uterine leiomyomas. Obstet Gynecol. 1996;88:415-419.
19. Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183:1448-1455.
20. West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85:36-39.
21. Parker WH, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83:414-418.
22. Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18:1088-1093.
23. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66:904-910.
24. Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59:233-236.
25. Seracchioli R, Rossi S, Govoni F, et al. Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: a randomized comparison with abdominal myomectomy. Hum Reprod. 2000;15:2663-2668.
26. Malzoni M, Rotond M, Perone C, et al. Fertility after laparoscopic myomectomy of large uterine myomas: operative technique and preliminary results. Eur J Gynaecol Oncol. 2003;24:79-82.
27. Malone L. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34:200-203.
28. Fedele L, Parazzini F, Luchini L, Mezzopane R, Tozzi L, Villa L. Recurrence of fibroids after myomectomy: a transvaginal ultrasonographic study. Hum Reprod. 1995;10:1795-1796.
29. Sesti F, Ticconi C, Bonifacio S, Piccione E. Preoperative administration of recombinant human erythropoietin in patients undergoing gynecologic surgery. Gynecol Obstet Invest. 2002;54:1-5.
30. Wurnig C, Schatz K, Noske H, et al. Collaborative Study Group. Subcutaneous low-dose epoetin beta for the avoidance of transfusion in patients scheduled for elective surgery not eligible for autologous blood donation. Eur Surg Res. 2001;33:303-310.
31. Schlaff WD, Zerhouni EA, Huth JA, Chen J, Damewood MD, Rock JA. A placebo-controlled trial of a depot gonadotropin-releasing hormone analogue (leuprolide) in the treatment of uterine leiomyomata. Obstet Gynecol. 1989;74:856-862.
32. Friedman AJ, Hoffman DI, Comite F, Browneller RW, Miller JD. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet Gynecol. 1991;77:720-725.
33. Letterie GS, Coddington CC, Winkel CA, Shawker TH, Loriaux DL, Collins RL. Efficacy of a gonadotropin-releasing hormone agonist in the treatment of uterine leiomyomata: long-term follow-up. Fertil Steril. 1989;51:951-956.
34. Leather AT, Studd JW, Watson NR, Holland EF. The prevention of bone loss in young women treated with GnRH analogues with “add-back” estrogen therapy. Obstet Gynecol. 1993;81:104-107.
35. Lethaby A, Vollenhoven B, Sowter M. Efficacy of pre-operative gonadotrophin hormone releasing analogues for women with uterine fibroids undergoing hysterectomy or myomectomy: a systematic review. BJOG. 2002;109:1097-1108.
36. Stovall TG, Summitt RL Jr, Bran DF, Ling FW. Outpatient vaginal hysterectomy: a pilot study. Obstet Gynecol. 1992;80:145-149.
37. Marana R, Busacca M, Zupi E, Garcea N, Paparella P, Catalano GF. Laparoscopically assisted vaginal hysterectomy versus total abdominal hysterectomy: a prospective, randomized, multi-center study. Am J Obstet Gynecol. 1999;180:270-275.
38. Wattiez A, Soriano D, Fiaccavento A, et al. Total laparoscopic hysterectomy for very enlarged uteri. J Am Assoc Gynecol Laparosc. 2002;9:125-130.
Major vascular injury during laparoscopy: Pearls to cope
The author reports no financial relationships relevant to this article.
- Strategies that may help you avoid MVI
- Tips on how to recognize it
- What to do when MVI occurs
CASE Trocar insertion, then a bleed
Sophia, a 29-year-old nulliparous patient, undergoes diagnostic laparoscopy after unsuccessful medical therapy for chronic pelvic pain. After insufflation, a reusable 11-mm trocar with obturator is placed. A survey of the pelvis and abdomen reveals a 10-cm retroperitoneal hematoma in the midline just below the umbilicus.
What is the best way to manage this hemorrhage?
Contrary to widespread belief, gynecologic laparoscopy has a rate of major complication similar to, not higher than, that of laparotomy—but it has a higher rate of major vascular injury (MVI), defined as laceration of the aorta, inferior vena cava, or common iliac or external iliac vessels.1 In fact, MVI appears to be somewhat exclusive to laparoscopy.
In a large review of 29,966 gynecologic laparoscopies, investigators found the surgeon’s level of experience to be an important variable in the overall complication rate but not in the incidence of MVI.2 This means patients are at risk of MVI regardless of how many or how few laparoscopic procedures you perform.
What can a surgeon do to avert this type of injury? The most important strategy is to be conversant with variables that clearly increase the risk, as well as behaviors and protocols that can minimize it. This article offers five pearls—from prevention to postop management.
1. Pay attention to subtlety, starting at the preop visit
When a marriage counselor was asked which significant actions can improve a relationship, he responded: “The little things are the big things.”
To minimize the risk of vascular injury, a meticulous surgeon begins with the “little things” at the preoperative evaluation. Important considerations include the patient’s height, weight, and body mass index (BMI). Does she have a history of abdominal surgery or pelvic disease that could suggest intra-abdominal adhesions and anatomic distortion?
It is important to discuss the risk of bowel or vascular injury, as well as the need to convert to an open procedure, with all patients, but particularly with those who are thin or who have abnormal abdominal findings.
A recent randomized clinical trial found that routine mechanical bowel preparation did not facilitate surgery or decrease the incidence of complications, but may be helpful in select cases.3
2. Don’t undervalue that ounce of prevention
A commuter commented to her friend that she thought the bus driver had exceptional driving skill. The friend couldn’t understand this remark. “The driver hasn’t been challenged yet,” the friend argued. “She hasn’t needed to demonstrate any action in the face of an emergency—so how do you know what level of skill she has?”
“That is precisely the point,” the commuter replied. “The driver has exercised extreme judgment and caution so as not to require using her superior skill in an emergency.”
It is always better to avoid a potentially catastrophic complication than to manage it. The first requirement is vigilance in preparing for laparoscopy. It is important to ensure that the patient is in the supine position during initial entry and that her arms are comfortably tucked on both sides. In 85% of cases, the aorta can be palpated.4
Select an entry technique wisely
Base this decision on experience, patient characteristics, the surgical procedure, and availability of equipment. No entry technique or device can be considered completely safe.
Most vascular injuries occur during the initial phase of laparoscopy:
- As many as 39% are related to placement of the Veress needle
- As many as 37.9% are related to placement of the primary trocar.5
The classic “closed” method of laparoscopy has been used successfully for decades. In thin patients, insert the Veress needle toward the uterus. In computed tomography studies, an angle of at least 34 degrees has been shown to avoid the aorta and its bifurcation (FIGURE 1).6
The right common iliac vessel is most commonly involved in injury, given its anatomic relationship overriding the inferior vena cava (FIGURE 2). When you stand at the patient’s left, the Veress needle can inadvertently be directed off to the right of midline, in harm’s way of the right common iliac vessels.
FIGURE 1 In thin women, insert the Veress needle toward the uterus
If the angle of insertion is 45° (≥34°) in the sagittal plane at the umbilicus, puncture safely avoids the aortic bifurcation.
FIGURE 2 At risk: The right common iliac artery
The right common iliac artery overlies its corresponding vein, which puts the artery at risk of injury at initial insertion of the Veress needle.
Tips to facilitate entry
After insufflation, the distance from the base of the navel to the peritoneum is approximately 1.5 to 2.0 cm (FIGURE 3).7 It is neither necessary nor prudent to bury the needle to its hub when placing the initial trocar. Some authors report that over-inflation to 25 mm Hg lowers the risk of vascular injury, creating a longer distance to the retroperitoneal structures.8 In addition, the reduction of tenting means that less force is required.
Patients with a high BMI are likely to be protected from MVI, given the greater distances to major vessels.9 The distance between the entry trocar and retroperitoneal vessels increases directly with BMI, commensurate with increasing abdominal-wall thickness (FIGURE 4).
FIGURE 3 The umbilicus is at its thinnest at low body weight
The distance (cm) into the peritoneal cavity after insufflation correlates with a patient’s weight.
SOURCE: Milad and Terkildsen.7
FIGURE 4 How far is it from umbilical skin to the peritoneum and vascular structures?
SOURCE: Hurd et al.9
Even optical trocars have been associated with MVI. Between 1994 and 2002, 79 serious complications, including 37 MVIs, were associated with use of these trocars.10
Direct insertion, open technique, and optical trocars have not been studied in comparable numbers. Although the Hasson cannula was not associated with MVI in two large trials involving 10,840 and 5,284 procedures, respectively, there have been case reports to the contrary.11-14
Rubinstein and colleagues15 evaluated the efficacy and safety of the radial dilating trocar VersaStep (US Surgical) for laparoscopic access. This trocar system has a blunt tip and circumferentially dilates rather than directly incising the fascia of the abdominal wall. Use of the blunt-tip trocar may decrease the incidence of initial access injury.
3. Don’t be the King or Queen of denial
Because MVI is estimated to occur in one in every 1,000 to one in every 10,000 procedures, and a typical gynecologic surgeon performs roughly 12 laparoscopies a year, it is unlikely that he or she will encounter more than one MVI over the course of a career. Nevertheless, when a retroperitoneal hematoma or brisk bleeding is visualized at laparoscopy, an MVI should immediately be suspected.
Do not enter into a state of denial and attempt to manage this complication conservatively. The earlier the MVI can be diagnosed, the better the outcome. A retroperitoneal hematoma, dark venous blood pooling in the abdomen, and bright red pulsatile blood are all signs of MVI. Do not wait for systemic changes such as hypotension or cardiac arrhythmias, as these are late findings.
4. No man is an island. Get help when you need it!
As residents, we had the shoulder-dystocia drill burned into memory, the first step being: “Call for help.” Regrettably, there is much less emphasis placed on the need to call for help in a surgical emergency.
All members of the surgical team should be notified of the situation and its associated gravity. The anesthesia team will need to ensure that there are adequate intravenous (IV) access, IV fluids, and blood products for resuscitation. The circulator will need to recruit help so that there will be additional hands to call consults, obtain blood, bring in abdominal trays, scrub in and retract, and so on.
5. Identify, secure, and control the site of injury
Just as a pilot must memorize the early response to an emergency, so must a surgeon be prepared for MVI. In the setting of potential catastrophic hemorrhage, time is of the essence.
The first task is to stay calm and avoid panic. Your role as the surgeon is to identify, secure, and control the site of injury while other team members work on their duties.
Laparoscopy usually not an option 2
It is the rare laparoscopic surgeon who can manage catastrophic hemorrhage endoscopically. It usually is best to remove the trocar from the vessel wall and convert to laparotomy. Attempting to leave a trocar in a vessel may extend the injury and create greater trauma to the vessel.
It is likely that patients will need to undergo secondary procedures if traditional laparoscopic interventions are used.
A vertical skin incision is best
The vertical incision is preferable because it allows greater access and visibility. Sadly, that information does not seem to translate into common practice. One study reported that 27 of 31 women with vascular injury were opened with a Pfannenstiel incision for emergency laparotomy.16
Control the bleeding
Apply pressure in an atraumatic fashion. Call for vascular instruments and a second suction device.
Control the bleeding digitally at first, followed by proximal and distal occlusion. Never use traumatic clamps because of the high rate of intimal damage associated with thrombi and secondary occlusion. Clips, staplers, or electrosurgery may cause vascular occlusion, thrombi, claudication, or lower-extremity edema.
Debride the blood and clots and identify the defect.
The frequency of MVI is thought to range from one in every 1,000 to one in every 10,000 gynecologic laparoscopic procedures.17-19 In a large review of 29,966 gynecologic laparoscopies, the risk of MVI was 0.02%.2
Polypropylene (6-0) can be used to close the defect in an interrupted fashion with sutures running parallel to the line of the vascular structure.
A vascular surgeon can be very helpful in the repair of vessels and should be consulted in the event of MVI. If extensive intimal damage has occurred, primary closure may not be possible and the vascular surgeon may perform a graft angioplasty repair or place a short interposition graft. Proximal and distal control is achieved by applying atraumatic clamps proximal and distal to the injury. Intravenous anticoagulation (5,000 U of heparin) is common but not mandatory. A longitudinal arteriotomy is extended, and any areas of thrombi are removed. The damaged intima is endarterectomized, and tacking sutures, using 7-0 Prolene, can be used to prevent propagation of the intimal dissection. Last, a venous or synthetic patch is sewn into place using 6-0 Prolene.
Patients typically remain on aspirin for 3 to 6 weeks.
CASE Resolved
Sophia’s procedure is immediately converted to a midline vertical laparotomy, a vascular surgery consult is obtained, and exploration reveals a 5-mm defect in the right common iliac artery consistent with an extended Veress needle injury. A vascular patch is used for the vessel repair, and the patient is discharged 4 days later in stable condition. She takes clopidogrel bisulfate (Plavix) for 6 weeks and has no lower-limb compromise.
1. Chapron C, Fauconnier A, Goffinet F, Breart G, Dubuisson JB. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Hum Reprod. 2002;17:1334-1342.
2. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynaecological laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
3. Muzii L, Bellati F, Zullo MA, Manci N, Angioli R, Panici PB. Mechanical bowel preparation before gynecologic laparoscopy: a randomized, single-blind, controlled trial. Fertil Steril. 2006;85:689-693.
4. Polyzos D, Papadopoulos N, Chapman L, et al. Where is the aorta? Is it worth palpating the aorta prior to laparoscopy? Acta Obstet Gynaecol Scand. 2007;86:235-239.
5. Philips PA, Amaral JF. Abdominal access complications in laparoscopic surgery. J Am Coll Surg. 2001;192:525-536.
6. Sriprasad S, Yu DF, Muir GH, Poulsen J, Sidhu PS. Positional anatomy of vessels that may be damaged at laparoscopy: new access criteria based on CT and ultrasonography to avoid vascular injury. J Endourol. 2006;20:498-503.
7. Milad MP, Terkildsen MF. The spinal needle test effectively measures the abdominal wall thickness prior to trocar placement at laparoscopy. J Am Assoc Gynecol Laparosc. 2002;9:514-518.
8. Reich H, Ribeiro SC, Rasmussen C, Rosenberg J, Vidali A. High-pressure trocar insertion technique. J Soc Laparoendosc Surg. 1999;3:45-48.
9. Hurd WH, Bude RO, DeLancey JO, Gauvin JM, Aisen AM. Abdominal wall characterization with magnetic resonance imaging and computed tomography. The effect of obesity on the laparoscopic approach. J Re-prod Med. 1991-36:473-476.
10. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette C, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
11. Hasson HM, Rotman C, Rana N, Kumari NA. Open laparoscopy: 29-year experience. Obstet Gynecol. 2000;96:763-766.
12. Penfield AJ. How to prevent complications of open laparoscopy. J Reprod Med. 1985;30:660-663.
13. Hanney RM, Carmalt HL, Merrett N, Tait N. Use of the Hasson cannula producing major vascular injury at laparoscopy. Surg Endosc. 1999;13:1238-1240.
14. Pring CM. Aortic injury using the Hasson trocar: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W3-5.
15. Rubenstein JN, Blunt LW, Jr, Lin WW, User HM, Nadler RB, Gonzalez CM. Safety and efficacy of 12-mm radial dilating ports for laparoscopic access. BJU Int. 2003;92:327-329.
16. Baggish MS. Analysis of 31 cases of major vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
17. Saville LE, Woods MS. Laparoscopy and major retroperitoneal vascular injuries (MRVI). Surg Endosc. 1995;9:1099-1101.
18. Ridel HH, Lehmann-Willenbrock E, Conrad P, Semm K. German pelviscopic statistics for the years 1978–1982. Endoscopy. 1986;18:219-222.
19. Harkki-Siren P, Sjoberg J, Kurki T. Major complications of laparoscopy: a follow-up Finnish study. Obstet Gynecol. 1999;94:94-98.
The author reports no financial relationships relevant to this article.
- Strategies that may help you avoid MVI
- Tips on how to recognize it
- What to do when MVI occurs
CASE Trocar insertion, then a bleed
Sophia, a 29-year-old nulliparous patient, undergoes diagnostic laparoscopy after unsuccessful medical therapy for chronic pelvic pain. After insufflation, a reusable 11-mm trocar with obturator is placed. A survey of the pelvis and abdomen reveals a 10-cm retroperitoneal hematoma in the midline just below the umbilicus.
What is the best way to manage this hemorrhage?
Contrary to widespread belief, gynecologic laparoscopy has a rate of major complication similar to, not higher than, that of laparotomy—but it has a higher rate of major vascular injury (MVI), defined as laceration of the aorta, inferior vena cava, or common iliac or external iliac vessels.1 In fact, MVI appears to be somewhat exclusive to laparoscopy.
In a large review of 29,966 gynecologic laparoscopies, investigators found the surgeon’s level of experience to be an important variable in the overall complication rate but not in the incidence of MVI.2 This means patients are at risk of MVI regardless of how many or how few laparoscopic procedures you perform.
What can a surgeon do to avert this type of injury? The most important strategy is to be conversant with variables that clearly increase the risk, as well as behaviors and protocols that can minimize it. This article offers five pearls—from prevention to postop management.
1. Pay attention to subtlety, starting at the preop visit
When a marriage counselor was asked which significant actions can improve a relationship, he responded: “The little things are the big things.”
To minimize the risk of vascular injury, a meticulous surgeon begins with the “little things” at the preoperative evaluation. Important considerations include the patient’s height, weight, and body mass index (BMI). Does she have a history of abdominal surgery or pelvic disease that could suggest intra-abdominal adhesions and anatomic distortion?
It is important to discuss the risk of bowel or vascular injury, as well as the need to convert to an open procedure, with all patients, but particularly with those who are thin or who have abnormal abdominal findings.
A recent randomized clinical trial found that routine mechanical bowel preparation did not facilitate surgery or decrease the incidence of complications, but may be helpful in select cases.3
2. Don’t undervalue that ounce of prevention
A commuter commented to her friend that she thought the bus driver had exceptional driving skill. The friend couldn’t understand this remark. “The driver hasn’t been challenged yet,” the friend argued. “She hasn’t needed to demonstrate any action in the face of an emergency—so how do you know what level of skill she has?”
“That is precisely the point,” the commuter replied. “The driver has exercised extreme judgment and caution so as not to require using her superior skill in an emergency.”
It is always better to avoid a potentially catastrophic complication than to manage it. The first requirement is vigilance in preparing for laparoscopy. It is important to ensure that the patient is in the supine position during initial entry and that her arms are comfortably tucked on both sides. In 85% of cases, the aorta can be palpated.4
Select an entry technique wisely
Base this decision on experience, patient characteristics, the surgical procedure, and availability of equipment. No entry technique or device can be considered completely safe.
Most vascular injuries occur during the initial phase of laparoscopy:
- As many as 39% are related to placement of the Veress needle
- As many as 37.9% are related to placement of the primary trocar.5
The classic “closed” method of laparoscopy has been used successfully for decades. In thin patients, insert the Veress needle toward the uterus. In computed tomography studies, an angle of at least 34 degrees has been shown to avoid the aorta and its bifurcation (FIGURE 1).6
The right common iliac vessel is most commonly involved in injury, given its anatomic relationship overriding the inferior vena cava (FIGURE 2). When you stand at the patient’s left, the Veress needle can inadvertently be directed off to the right of midline, in harm’s way of the right common iliac vessels.
FIGURE 1 In thin women, insert the Veress needle toward the uterus
If the angle of insertion is 45° (≥34°) in the sagittal plane at the umbilicus, puncture safely avoids the aortic bifurcation.
FIGURE 2 At risk: The right common iliac artery
The right common iliac artery overlies its corresponding vein, which puts the artery at risk of injury at initial insertion of the Veress needle.
Tips to facilitate entry
After insufflation, the distance from the base of the navel to the peritoneum is approximately 1.5 to 2.0 cm (FIGURE 3).7 It is neither necessary nor prudent to bury the needle to its hub when placing the initial trocar. Some authors report that over-inflation to 25 mm Hg lowers the risk of vascular injury, creating a longer distance to the retroperitoneal structures.8 In addition, the reduction of tenting means that less force is required.
Patients with a high BMI are likely to be protected from MVI, given the greater distances to major vessels.9 The distance between the entry trocar and retroperitoneal vessels increases directly with BMI, commensurate with increasing abdominal-wall thickness (FIGURE 4).
FIGURE 3 The umbilicus is at its thinnest at low body weight
The distance (cm) into the peritoneal cavity after insufflation correlates with a patient’s weight.
SOURCE: Milad and Terkildsen.7
FIGURE 4 How far is it from umbilical skin to the peritoneum and vascular structures?
SOURCE: Hurd et al.9
Even optical trocars have been associated with MVI. Between 1994 and 2002, 79 serious complications, including 37 MVIs, were associated with use of these trocars.10
Direct insertion, open technique, and optical trocars have not been studied in comparable numbers. Although the Hasson cannula was not associated with MVI in two large trials involving 10,840 and 5,284 procedures, respectively, there have been case reports to the contrary.11-14
Rubinstein and colleagues15 evaluated the efficacy and safety of the radial dilating trocar VersaStep (US Surgical) for laparoscopic access. This trocar system has a blunt tip and circumferentially dilates rather than directly incising the fascia of the abdominal wall. Use of the blunt-tip trocar may decrease the incidence of initial access injury.
3. Don’t be the King or Queen of denial
Because MVI is estimated to occur in one in every 1,000 to one in every 10,000 procedures, and a typical gynecologic surgeon performs roughly 12 laparoscopies a year, it is unlikely that he or she will encounter more than one MVI over the course of a career. Nevertheless, when a retroperitoneal hematoma or brisk bleeding is visualized at laparoscopy, an MVI should immediately be suspected.
Do not enter into a state of denial and attempt to manage this complication conservatively. The earlier the MVI can be diagnosed, the better the outcome. A retroperitoneal hematoma, dark venous blood pooling in the abdomen, and bright red pulsatile blood are all signs of MVI. Do not wait for systemic changes such as hypotension or cardiac arrhythmias, as these are late findings.
4. No man is an island. Get help when you need it!
As residents, we had the shoulder-dystocia drill burned into memory, the first step being: “Call for help.” Regrettably, there is much less emphasis placed on the need to call for help in a surgical emergency.
All members of the surgical team should be notified of the situation and its associated gravity. The anesthesia team will need to ensure that there are adequate intravenous (IV) access, IV fluids, and blood products for resuscitation. The circulator will need to recruit help so that there will be additional hands to call consults, obtain blood, bring in abdominal trays, scrub in and retract, and so on.
5. Identify, secure, and control the site of injury
Just as a pilot must memorize the early response to an emergency, so must a surgeon be prepared for MVI. In the setting of potential catastrophic hemorrhage, time is of the essence.
The first task is to stay calm and avoid panic. Your role as the surgeon is to identify, secure, and control the site of injury while other team members work on their duties.
Laparoscopy usually not an option 2
It is the rare laparoscopic surgeon who can manage catastrophic hemorrhage endoscopically. It usually is best to remove the trocar from the vessel wall and convert to laparotomy. Attempting to leave a trocar in a vessel may extend the injury and create greater trauma to the vessel.
It is likely that patients will need to undergo secondary procedures if traditional laparoscopic interventions are used.
A vertical skin incision is best
The vertical incision is preferable because it allows greater access and visibility. Sadly, that information does not seem to translate into common practice. One study reported that 27 of 31 women with vascular injury were opened with a Pfannenstiel incision for emergency laparotomy.16
Control the bleeding
Apply pressure in an atraumatic fashion. Call for vascular instruments and a second suction device.
Control the bleeding digitally at first, followed by proximal and distal occlusion. Never use traumatic clamps because of the high rate of intimal damage associated with thrombi and secondary occlusion. Clips, staplers, or electrosurgery may cause vascular occlusion, thrombi, claudication, or lower-extremity edema.
Debride the blood and clots and identify the defect.
The frequency of MVI is thought to range from one in every 1,000 to one in every 10,000 gynecologic laparoscopic procedures.17-19 In a large review of 29,966 gynecologic laparoscopies, the risk of MVI was 0.02%.2
Polypropylene (6-0) can be used to close the defect in an interrupted fashion with sutures running parallel to the line of the vascular structure.
A vascular surgeon can be very helpful in the repair of vessels and should be consulted in the event of MVI. If extensive intimal damage has occurred, primary closure may not be possible and the vascular surgeon may perform a graft angioplasty repair or place a short interposition graft. Proximal and distal control is achieved by applying atraumatic clamps proximal and distal to the injury. Intravenous anticoagulation (5,000 U of heparin) is common but not mandatory. A longitudinal arteriotomy is extended, and any areas of thrombi are removed. The damaged intima is endarterectomized, and tacking sutures, using 7-0 Prolene, can be used to prevent propagation of the intimal dissection. Last, a venous or synthetic patch is sewn into place using 6-0 Prolene.
Patients typically remain on aspirin for 3 to 6 weeks.
CASE Resolved
Sophia’s procedure is immediately converted to a midline vertical laparotomy, a vascular surgery consult is obtained, and exploration reveals a 5-mm defect in the right common iliac artery consistent with an extended Veress needle injury. A vascular patch is used for the vessel repair, and the patient is discharged 4 days later in stable condition. She takes clopidogrel bisulfate (Plavix) for 6 weeks and has no lower-limb compromise.
The author reports no financial relationships relevant to this article.
- Strategies that may help you avoid MVI
- Tips on how to recognize it
- What to do when MVI occurs
CASE Trocar insertion, then a bleed
Sophia, a 29-year-old nulliparous patient, undergoes diagnostic laparoscopy after unsuccessful medical therapy for chronic pelvic pain. After insufflation, a reusable 11-mm trocar with obturator is placed. A survey of the pelvis and abdomen reveals a 10-cm retroperitoneal hematoma in the midline just below the umbilicus.
What is the best way to manage this hemorrhage?
Contrary to widespread belief, gynecologic laparoscopy has a rate of major complication similar to, not higher than, that of laparotomy—but it has a higher rate of major vascular injury (MVI), defined as laceration of the aorta, inferior vena cava, or common iliac or external iliac vessels.1 In fact, MVI appears to be somewhat exclusive to laparoscopy.
In a large review of 29,966 gynecologic laparoscopies, investigators found the surgeon’s level of experience to be an important variable in the overall complication rate but not in the incidence of MVI.2 This means patients are at risk of MVI regardless of how many or how few laparoscopic procedures you perform.
What can a surgeon do to avert this type of injury? The most important strategy is to be conversant with variables that clearly increase the risk, as well as behaviors and protocols that can minimize it. This article offers five pearls—from prevention to postop management.
1. Pay attention to subtlety, starting at the preop visit
When a marriage counselor was asked which significant actions can improve a relationship, he responded: “The little things are the big things.”
To minimize the risk of vascular injury, a meticulous surgeon begins with the “little things” at the preoperative evaluation. Important considerations include the patient’s height, weight, and body mass index (BMI). Does she have a history of abdominal surgery or pelvic disease that could suggest intra-abdominal adhesions and anatomic distortion?
It is important to discuss the risk of bowel or vascular injury, as well as the need to convert to an open procedure, with all patients, but particularly with those who are thin or who have abnormal abdominal findings.
A recent randomized clinical trial found that routine mechanical bowel preparation did not facilitate surgery or decrease the incidence of complications, but may be helpful in select cases.3
2. Don’t undervalue that ounce of prevention
A commuter commented to her friend that she thought the bus driver had exceptional driving skill. The friend couldn’t understand this remark. “The driver hasn’t been challenged yet,” the friend argued. “She hasn’t needed to demonstrate any action in the face of an emergency—so how do you know what level of skill she has?”
“That is precisely the point,” the commuter replied. “The driver has exercised extreme judgment and caution so as not to require using her superior skill in an emergency.”
It is always better to avoid a potentially catastrophic complication than to manage it. The first requirement is vigilance in preparing for laparoscopy. It is important to ensure that the patient is in the supine position during initial entry and that her arms are comfortably tucked on both sides. In 85% of cases, the aorta can be palpated.4
Select an entry technique wisely
Base this decision on experience, patient characteristics, the surgical procedure, and availability of equipment. No entry technique or device can be considered completely safe.
Most vascular injuries occur during the initial phase of laparoscopy:
- As many as 39% are related to placement of the Veress needle
- As many as 37.9% are related to placement of the primary trocar.5
The classic “closed” method of laparoscopy has been used successfully for decades. In thin patients, insert the Veress needle toward the uterus. In computed tomography studies, an angle of at least 34 degrees has been shown to avoid the aorta and its bifurcation (FIGURE 1).6
The right common iliac vessel is most commonly involved in injury, given its anatomic relationship overriding the inferior vena cava (FIGURE 2). When you stand at the patient’s left, the Veress needle can inadvertently be directed off to the right of midline, in harm’s way of the right common iliac vessels.
FIGURE 1 In thin women, insert the Veress needle toward the uterus
If the angle of insertion is 45° (≥34°) in the sagittal plane at the umbilicus, puncture safely avoids the aortic bifurcation.
FIGURE 2 At risk: The right common iliac artery
The right common iliac artery overlies its corresponding vein, which puts the artery at risk of injury at initial insertion of the Veress needle.
Tips to facilitate entry
After insufflation, the distance from the base of the navel to the peritoneum is approximately 1.5 to 2.0 cm (FIGURE 3).7 It is neither necessary nor prudent to bury the needle to its hub when placing the initial trocar. Some authors report that over-inflation to 25 mm Hg lowers the risk of vascular injury, creating a longer distance to the retroperitoneal structures.8 In addition, the reduction of tenting means that less force is required.
Patients with a high BMI are likely to be protected from MVI, given the greater distances to major vessels.9 The distance between the entry trocar and retroperitoneal vessels increases directly with BMI, commensurate with increasing abdominal-wall thickness (FIGURE 4).
FIGURE 3 The umbilicus is at its thinnest at low body weight
The distance (cm) into the peritoneal cavity after insufflation correlates with a patient’s weight.
SOURCE: Milad and Terkildsen.7
FIGURE 4 How far is it from umbilical skin to the peritoneum and vascular structures?
SOURCE: Hurd et al.9
Even optical trocars have been associated with MVI. Between 1994 and 2002, 79 serious complications, including 37 MVIs, were associated with use of these trocars.10
Direct insertion, open technique, and optical trocars have not been studied in comparable numbers. Although the Hasson cannula was not associated with MVI in two large trials involving 10,840 and 5,284 procedures, respectively, there have been case reports to the contrary.11-14
Rubinstein and colleagues15 evaluated the efficacy and safety of the radial dilating trocar VersaStep (US Surgical) for laparoscopic access. This trocar system has a blunt tip and circumferentially dilates rather than directly incising the fascia of the abdominal wall. Use of the blunt-tip trocar may decrease the incidence of initial access injury.
3. Don’t be the King or Queen of denial
Because MVI is estimated to occur in one in every 1,000 to one in every 10,000 procedures, and a typical gynecologic surgeon performs roughly 12 laparoscopies a year, it is unlikely that he or she will encounter more than one MVI over the course of a career. Nevertheless, when a retroperitoneal hematoma or brisk bleeding is visualized at laparoscopy, an MVI should immediately be suspected.
Do not enter into a state of denial and attempt to manage this complication conservatively. The earlier the MVI can be diagnosed, the better the outcome. A retroperitoneal hematoma, dark venous blood pooling in the abdomen, and bright red pulsatile blood are all signs of MVI. Do not wait for systemic changes such as hypotension or cardiac arrhythmias, as these are late findings.
4. No man is an island. Get help when you need it!
As residents, we had the shoulder-dystocia drill burned into memory, the first step being: “Call for help.” Regrettably, there is much less emphasis placed on the need to call for help in a surgical emergency.
All members of the surgical team should be notified of the situation and its associated gravity. The anesthesia team will need to ensure that there are adequate intravenous (IV) access, IV fluids, and blood products for resuscitation. The circulator will need to recruit help so that there will be additional hands to call consults, obtain blood, bring in abdominal trays, scrub in and retract, and so on.
5. Identify, secure, and control the site of injury
Just as a pilot must memorize the early response to an emergency, so must a surgeon be prepared for MVI. In the setting of potential catastrophic hemorrhage, time is of the essence.
The first task is to stay calm and avoid panic. Your role as the surgeon is to identify, secure, and control the site of injury while other team members work on their duties.
Laparoscopy usually not an option 2
It is the rare laparoscopic surgeon who can manage catastrophic hemorrhage endoscopically. It usually is best to remove the trocar from the vessel wall and convert to laparotomy. Attempting to leave a trocar in a vessel may extend the injury and create greater trauma to the vessel.
It is likely that patients will need to undergo secondary procedures if traditional laparoscopic interventions are used.
A vertical skin incision is best
The vertical incision is preferable because it allows greater access and visibility. Sadly, that information does not seem to translate into common practice. One study reported that 27 of 31 women with vascular injury were opened with a Pfannenstiel incision for emergency laparotomy.16
Control the bleeding
Apply pressure in an atraumatic fashion. Call for vascular instruments and a second suction device.
Control the bleeding digitally at first, followed by proximal and distal occlusion. Never use traumatic clamps because of the high rate of intimal damage associated with thrombi and secondary occlusion. Clips, staplers, or electrosurgery may cause vascular occlusion, thrombi, claudication, or lower-extremity edema.
Debride the blood and clots and identify the defect.
The frequency of MVI is thought to range from one in every 1,000 to one in every 10,000 gynecologic laparoscopic procedures.17-19 In a large review of 29,966 gynecologic laparoscopies, the risk of MVI was 0.02%.2
Polypropylene (6-0) can be used to close the defect in an interrupted fashion with sutures running parallel to the line of the vascular structure.
A vascular surgeon can be very helpful in the repair of vessels and should be consulted in the event of MVI. If extensive intimal damage has occurred, primary closure may not be possible and the vascular surgeon may perform a graft angioplasty repair or place a short interposition graft. Proximal and distal control is achieved by applying atraumatic clamps proximal and distal to the injury. Intravenous anticoagulation (5,000 U of heparin) is common but not mandatory. A longitudinal arteriotomy is extended, and any areas of thrombi are removed. The damaged intima is endarterectomized, and tacking sutures, using 7-0 Prolene, can be used to prevent propagation of the intimal dissection. Last, a venous or synthetic patch is sewn into place using 6-0 Prolene.
Patients typically remain on aspirin for 3 to 6 weeks.
CASE Resolved
Sophia’s procedure is immediately converted to a midline vertical laparotomy, a vascular surgery consult is obtained, and exploration reveals a 5-mm defect in the right common iliac artery consistent with an extended Veress needle injury. A vascular patch is used for the vessel repair, and the patient is discharged 4 days later in stable condition. She takes clopidogrel bisulfate (Plavix) for 6 weeks and has no lower-limb compromise.
1. Chapron C, Fauconnier A, Goffinet F, Breart G, Dubuisson JB. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Hum Reprod. 2002;17:1334-1342.
2. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynaecological laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
3. Muzii L, Bellati F, Zullo MA, Manci N, Angioli R, Panici PB. Mechanical bowel preparation before gynecologic laparoscopy: a randomized, single-blind, controlled trial. Fertil Steril. 2006;85:689-693.
4. Polyzos D, Papadopoulos N, Chapman L, et al. Where is the aorta? Is it worth palpating the aorta prior to laparoscopy? Acta Obstet Gynaecol Scand. 2007;86:235-239.
5. Philips PA, Amaral JF. Abdominal access complications in laparoscopic surgery. J Am Coll Surg. 2001;192:525-536.
6. Sriprasad S, Yu DF, Muir GH, Poulsen J, Sidhu PS. Positional anatomy of vessels that may be damaged at laparoscopy: new access criteria based on CT and ultrasonography to avoid vascular injury. J Endourol. 2006;20:498-503.
7. Milad MP, Terkildsen MF. The spinal needle test effectively measures the abdominal wall thickness prior to trocar placement at laparoscopy. J Am Assoc Gynecol Laparosc. 2002;9:514-518.
8. Reich H, Ribeiro SC, Rasmussen C, Rosenberg J, Vidali A. High-pressure trocar insertion technique. J Soc Laparoendosc Surg. 1999;3:45-48.
9. Hurd WH, Bude RO, DeLancey JO, Gauvin JM, Aisen AM. Abdominal wall characterization with magnetic resonance imaging and computed tomography. The effect of obesity on the laparoscopic approach. J Re-prod Med. 1991-36:473-476.
10. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette C, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
11. Hasson HM, Rotman C, Rana N, Kumari NA. Open laparoscopy: 29-year experience. Obstet Gynecol. 2000;96:763-766.
12. Penfield AJ. How to prevent complications of open laparoscopy. J Reprod Med. 1985;30:660-663.
13. Hanney RM, Carmalt HL, Merrett N, Tait N. Use of the Hasson cannula producing major vascular injury at laparoscopy. Surg Endosc. 1999;13:1238-1240.
14. Pring CM. Aortic injury using the Hasson trocar: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W3-5.
15. Rubenstein JN, Blunt LW, Jr, Lin WW, User HM, Nadler RB, Gonzalez CM. Safety and efficacy of 12-mm radial dilating ports for laparoscopic access. BJU Int. 2003;92:327-329.
16. Baggish MS. Analysis of 31 cases of major vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
17. Saville LE, Woods MS. Laparoscopy and major retroperitoneal vascular injuries (MRVI). Surg Endosc. 1995;9:1099-1101.
18. Ridel HH, Lehmann-Willenbrock E, Conrad P, Semm K. German pelviscopic statistics for the years 1978–1982. Endoscopy. 1986;18:219-222.
19. Harkki-Siren P, Sjoberg J, Kurki T. Major complications of laparoscopy: a follow-up Finnish study. Obstet Gynecol. 1999;94:94-98.
1. Chapron C, Fauconnier A, Goffinet F, Breart G, Dubuisson JB. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Hum Reprod. 2002;17:1334-1342.
2. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynaecological laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
3. Muzii L, Bellati F, Zullo MA, Manci N, Angioli R, Panici PB. Mechanical bowel preparation before gynecologic laparoscopy: a randomized, single-blind, controlled trial. Fertil Steril. 2006;85:689-693.
4. Polyzos D, Papadopoulos N, Chapman L, et al. Where is the aorta? Is it worth palpating the aorta prior to laparoscopy? Acta Obstet Gynaecol Scand. 2007;86:235-239.
5. Philips PA, Amaral JF. Abdominal access complications in laparoscopic surgery. J Am Coll Surg. 2001;192:525-536.
6. Sriprasad S, Yu DF, Muir GH, Poulsen J, Sidhu PS. Positional anatomy of vessels that may be damaged at laparoscopy: new access criteria based on CT and ultrasonography to avoid vascular injury. J Endourol. 2006;20:498-503.
7. Milad MP, Terkildsen MF. The spinal needle test effectively measures the abdominal wall thickness prior to trocar placement at laparoscopy. J Am Assoc Gynecol Laparosc. 2002;9:514-518.
8. Reich H, Ribeiro SC, Rasmussen C, Rosenberg J, Vidali A. High-pressure trocar insertion technique. J Soc Laparoendosc Surg. 1999;3:45-48.
9. Hurd WH, Bude RO, DeLancey JO, Gauvin JM, Aisen AM. Abdominal wall characterization with magnetic resonance imaging and computed tomography. The effect of obesity on the laparoscopic approach. J Re-prod Med. 1991-36:473-476.
10. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette C, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
11. Hasson HM, Rotman C, Rana N, Kumari NA. Open laparoscopy: 29-year experience. Obstet Gynecol. 2000;96:763-766.
12. Penfield AJ. How to prevent complications of open laparoscopy. J Reprod Med. 1985;30:660-663.
13. Hanney RM, Carmalt HL, Merrett N, Tait N. Use of the Hasson cannula producing major vascular injury at laparoscopy. Surg Endosc. 1999;13:1238-1240.
14. Pring CM. Aortic injury using the Hasson trocar: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W3-5.
15. Rubenstein JN, Blunt LW, Jr, Lin WW, User HM, Nadler RB, Gonzalez CM. Safety and efficacy of 12-mm radial dilating ports for laparoscopic access. BJU Int. 2003;92:327-329.
16. Baggish MS. Analysis of 31 cases of major vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
17. Saville LE, Woods MS. Laparoscopy and major retroperitoneal vascular injuries (MRVI). Surg Endosc. 1995;9:1099-1101.
18. Ridel HH, Lehmann-Willenbrock E, Conrad P, Semm K. German pelviscopic statistics for the years 1978–1982. Endoscopy. 1986;18:219-222.
19. Harkki-Siren P, Sjoberg J, Kurki T. Major complications of laparoscopy: a follow-up Finnish study. Obstet Gynecol. 1999;94:94-98.
How to diagnose and repair congenital uterovaginal malformations
The author reports no financial relationships relevant to this article.
CASE 1 Fluid-filled abdominal mass
A 14-year-old girl complains of crampy, episodic, lower abdominal pain of 3 months’ duration and acute retention of urine. She also reports back pain and dyschezia. She has never menstruated, but her breasts began developing 18 months earlier. Examination reveals normal Tanner stage 3 breast development and female hair distribution and a large abdominal mass. No fetal heart tones are apparent. The external genitalia appear to be normal except for a bulging mass at the introitus.
Imaging (FIGURE 1) reveals that the mass is fluid-filled. The uterus, tubes, and ovaries are present at the dome of the mass. The kidneys are normal.
What is the mass?
FIGURE 1 No exit for menstrual products
An MRI shows a large hematocolpos that develops after menarche in women who have outflow obstruction, such as imperforate hymen—as this patient had.A list of the processes involved in normal development of the female reproductive tract highlights its precarious complexity: cellular differentiation, migration, canalization, fusion, and programmed cell death. A failure in any of these processes can cause a malformation. When that malformation becomes apparent depends on the stage of life of the patient and the nature of the abnormality. As you might imagine, diagnosis and treatment are not always straightforward.
In the patient just described, the likely diagnosis is imperforate hymen or transverse vaginal septum due to failed canalization of the vaginal plate. The patient has been menstruating internally for many months and now has a large hematocolpos. Urinary retention develops when the amount of retained blood in the vagina causes acute angulation of the urethrovesical junction. Evacuation of the blood restores the physiologic angle, enabling the patient to void normally.
Most anomalies involving the external genitalia are apparent at birth (clitoromegaly, imperforate hymen), whereas obstructive and nonobstructive malformations may become evident at birth; during childhood, puberty, or adolescence; or with menarche or childbearing.
Treatment of these abnormalities has changed significantly over the past few years—largely due to refinements in diagnostic imaging, surgical and nonsurgical techniques, and instrumentation. These advances have improved reproductive function and enhanced the psychosexual attitude of these patients.
In this article, I review the most common abnormalities and describe their evaluation and management.
Vaginal canalization
The sinovaginal bulbs are two solid evaginations originating from the urogenital sinus at the distal aspect of the müllerian tubercle, as shown at right (A). The sinovaginal bulbs proliferate into the caudal end of the uterovaginal canal to become the solid vaginal plate (B). The lumen of the lower vagina is formed by degeneration of the central cells of this vaginal plate, which occurs in a cephalad direction (C). This process of canalization is complete by 20 weeks’ gestation.
Hymen usually ruptures before birth
The vaginal hymen is separated from the urogenital sinus by the hymeneal membrane. The hymen usually ruptures before birth with the degeneration of central epithelial cells (i.e., canalization), leaving a thin fold of mucous membrane around the vaginal introitus.
Uterus, fallopian tubes develop from solid tissue
The müllerian ducts are first identifiable at approximately 6 weeks of gestation, when they begin to elongate caudally and cross the metanephric ducts medially to meet in the midline. By the seventh week, the urorectal septum has developed, separating the rectum from the urogenital sinus. Around 12 weeks’ gestation, the caudal portion of the müllerian ducts fuses to form the uterovaginal canal, which inserts into the dorsal wall of the urogenital sinus at Müller’s tubercle.
The two müllerian ducts are initially solid tissue and lie side by side. Subsequently, internal canalization of each duct produces two channels divided by a septum that is reabsorbed in a cephalad direction by 20 weeks. The cranial unfused portions of the müllerian ducts develop into the fimbria and fallopian tubes, whereas the caudal, fused portions form the uterus and upper vagina.
Impaired canalization
Imperforate hymen
The most common obstructive lesion of the female reproductive tract is an imperforate hymen. It can be diagnosed at birth with careful examination to ensure patency of both the rectum and vagina. Not infrequently, the infant presents with a bulging introitus due to mucocolpos from vaginal secretions stimulated by maternal estrogen (FIGURE 2). If diagnosis is delayed, the mucus usually resorbs and the child remains asymptomatic until menarche, when she presents with a bulging hymen and a large hematocolpos, as in the opening case. Management of imperforate hymen is feasible for the generalist. Repair can be performed in a patient of any age, but is facilitated if the tissue has undergone estrogen stimulation. Surgery is, therefore, ideal in the neonatal, postpubertal, and premenarchal periods.
Repair involves incision of the membrane near the hymeneal ring, followed by evacuation of accumulated blood. The cervix may appear to be flush with the vaginal apex, owing to compression, but this condition usually corrects itself within 2 or 3 weeks. Extra hymeneal tissue is excised to create an orifice of normal size, and the vaginal mucosa is intermittently sutured to the hymeneal ring using absorbable material (FIGURE 3).
FIGURE 2 Imperforate hymen
Bulging hymen due to mucocolpos in a newborn (top) and hematocolpos in an adolescent (bottom).
FIGURE 3 Hymenectomy restores vaginal orifice
Extra hymeneal tissue is excised to create an orifice of normal size. The vaginal mucosa is intermittently sutured to the hymeneal ring.
Incomplete hymeneal fenestration
This condition often goes undiagnosed until the patient seeks evaluation for an inability to insert tampons or medication or because of coital difficulty. Young girls with microperforate hymen may present with postmenstrual spotting or malodorous discharge due to partial obstruction and poor drainage.
Treatment of microperforate, cribriform, or septate hymen entails resection of excess hymeneal tissue to create a functional hymeneal ring.
Transverse vaginal septum
A transverse septum represents failed canalization of the vaginal plate. This condition occurs in approximately one of every 18,000 to 30,000 women.1,2 The septum can be located at any of various levels in the vagina; approximately 46% are found in the upper vagina, 35% to 40% in the middle portion, and 15% to 20% in the lower vagina.1,2 Lower septa appear to be thinner than those in the proximal vagina; thinness may be due to the pressure of accumulated fluid. If a small perforation is present, the patient will experience irregular spotting.
Examination. The external genitalia appear to be normal, but the vagina is shortened. Children may present with mucocolpos, whereas adolescents develop hematocolpos. If a small perforation is present, pyohematocolpos may result from ascending infection. A mass can be palpated on rectal examination.
Ultrasonography (US) or magnetic resonance imaging (MRI) helps define the location and thickness of the septum and distinguish a high septum from congenital absence of the cervix.
Treatment. Resect a small septum and perform end-to-end anastomosis of the upper and lower edges of the vaginal wall (FIGURE 4). Only an experienced surgeon should excise a thick septum. Reanastomosis is easier if the upper vagina is distended with menstrual blood. To ensure healing without stricture, mobilize the vaginal edge circumferentially before suturing the raw edges.
Outflow obstruction caused by a transverse septum in the distal vagina almost never leads to hematometra or hematosalpinx. However, septa that arise in the proximal vagina are usually thicker and, if left untreated, can cause reflux that damages the upper reproductive tract. These cases are almost always associated with severe dysmenorrhea and should be referred to an experienced vaginal surgeon. Pelvic MRI is recommended to assess the thickness and precise anatomy of the defect before repair.
FIGURE 4 Removing a transverse vaginal septum
The septum is resected from the distal vagina. This is followed by end-to-end anastomosis of mucosal edges.
Uterine septum
A septate uterus has a normal external surface but two endometrial cavities. It is caused by a defect in fusion or resorption of the midline septum between the two müllerian ducts. The degree of septation varies from a small midline septum to a total failure of resorption that produces a septate uterus with a longitudinal vaginal septum. A slight midline septum with minimal fundal indentation is classified by some as a septate uterus; by others, as a bicornuate uterus.
A higher risk of recurrent miscarriage is associated with longer septa.
Surgical repair with hysteroscopic resection of the septum yields excellent results.
CASE 2 The problem of agenesis
An 18-year-old patient consults a gynecologist because she has never menstruated and is unable to have sexual intercourse. She appears to be phenotypically normal, with normal labia upon examination, but no hymeneal opening is found. Pelvic US reveals absence of the uterus with ovaries that appear to be normal.
How should her condition be managed?
Uterovaginal agenesis, also known as müllerian aplasia or Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome, refers to congenital absence of the vagina with variable uterine development (FIGURE 5). It arises from agenesis of the
müllerian duct system, although the underlying cause is unknown. Vaginal agenesis is usually accompanied by cervical and uterine agenesis. However, 7% to 12% of patients with this syndrome have a normal but obstructed or rudimentary uterus with functional endometrium. This condition occurs in roughly one of every 5,000 females.1,3
In addition, these girls often exhibit extragenital anomalies.1,4 Approximately 25% to 50% have a urologic anomaly, such as unilateral renal agenesis, pelvic or horseshoe kidneys, or irregularities of the collecting systems; 10% to 15% have another type of anomaly, such as congenital heart disease, abnormalities of the hands, congenital deafness, cleft palate, and inguinal or femoral hernia.
The genetic basis of MRKH syndrome is unknown. These patients have normal female karyotypes and phenotypes with normal ovaries and ovarian function—although there is evidence that they are prone to early menopause. They develop secondary sexual characteristics. They usually present with primary amenorrhea at 15 to 17 years of age.
Vaginal agenesis is the second most common cause of primary amenorrhea after gonadal dysgenesis. Most patients have a rudimentary, nonfunctioning uterus, but 2% to 7% have a uterus with functioning endometrium and present with cyclic or chronic abdominopelvic pain secondary to obstruction or endometriosis, hematocolpos, or hematometra.
Imaging. US examination facilitates evaluation of the kidneys and confirms the presence of ovaries and absence of the uterus. MRI may be useful to confirm the presence of functioning endometrium.
The differential diagnosis of vaginal agenesis includes androgen insensitivity and low-lying transverse vaginal septum. Treatment includes psychosocial support to reassure the patient that a normal sex life will be possible after treatment.5 Construction of a neovagina, using vaginal dilators (the first-line strategy) or surgical placement of split-thickness skin graft or artificial skin (Abbe–McIndoe procedure), is the treatment. In one case series, more than 90% of patients with vaginal agenesis were successfully treated using vaginal dilation.6
The American College of Obstetricians and Gynecologists (ACOG) recommends that patients be referred to centers with expertise in surgical creation of a neovagina.5
FIGURE 5 Uterine agenesis
This depiction of uterine agenesis shows small, nonfunctioning müllerian remnants and normal tubes and ovaries.
Agenesis of the lower vagina
This malformation is caused by a failure of canalization of the sinovaginal bulbs and vaginal plate. The tubes, ovaries, cervix, and upper vagina are normal, but the lower vagina is replaced by fibrous tissue. Adolescents present with primary amenorrhea accompanied by cyclic pelvic pain. A vaginal dimple may be apparent on external inspection.
Because these girls have normal cyclic pituitary–ovarian–endometrial function at menarche, the upper vagina will distend with blood and secretions, and a mass can be palpated on rectoabdominal examination. US confirms the presence of a fluid-filled mass, and MRI further defines the length of obstruction.
Treatment. Surgery (by an experienced vaginal surgeon) is best performed when a large hematocolpos is present because distention increases the amount of tissue available for pull-through.
CASE 3 Fusion failure
A 17-year-old woman complains of lower abdominal pain and an enlarging abdomen. Menarche occurred at 13 years. The patient began to notice right-sided lower abdominal pain and abdominal swelling about 2 years ago, but denies any other symptoms.
Examination reveals a mass arising from the right lateral vaginal wall. The cervix appears to be normal. US shows a large, predominantly right-sided, fluid-filled mass arising from the pelvis. The uterus appears to be didelphic, with normal ovaries and an absent right kidney.
What is the mass?
This is a classic description of uterovaginal duplication that arises when the müllerian ducts fail to fuse (FIGURE 6).
In this case, the right-sided vagina failed to canalize as well, causing a large hematocolpos. Generally, duplication is limited to the uterus and cervix (uterus didelphys bicollis), although duplication of the vulva, bladder, urethra, vagina, and anus have been described.
Women with a didelphic uterus often have a good reproductive outcome. A septated vagina occurs in 75% of cases and may cause difficulty with sexual intercourse or vaginal delivery. Symptomatic women usually opt for resection of the vaginal septum.
Women who have an obstructed hemivagina and ipsilateral renal agenesis have regular menses because menstrual blood from one uterus can exit through its unobstructed side.
Treatment involves resection of the wall of the obstructed vagina, creating a single vaginal vault. If both vaginas have failed to canalize, the patient will present much earlier, with primary amenorrhea and cyclic pelvic pain—a presentation similar to that of transverse septum. Reconstructive surgery of the uterus is not recommended for complete duplication of the uterus.1,7
FIGURE 6 Uterus didelphys
This patient has uterus didelphys with an obstructed hemivagina.
Unicornuate and bicornuate uterus
A unicornuate uterus represents an asymmetric lateral fusion defect. One cavity is usually normal, with a fallopian tube and cervix, but the failed müllerian duct can have various configurations. The affected müllerian duct may fail to develop, or it may develop partially as a horn on the uterus or a separate structure that may or may not communicate with the uterus.
Most rudimentary horns are asymptomatic, but others contain functional endometrium that is shed cyclically. If the rudimentary horn is obstructed, as is usually the case, the woman develops cyclic or chronic abdominopelvic pain that necessitates surgical excision of the horn. Endometriosis, a result of reflux, is common.
In a bicornuate uterus, the fundus is indented and the vagina is normal. This anomaly occurs when the müllerian ducts fuse only partially. The result is varying degrees of separation of the uterine horns.
Pregnancy outcomes appear to be similar to those of the general population. However, some women develop early pregnancy loss, preterm labor, incompetent cervix, or malpresentation.
Treatment is open surgical reunification.
CASE 4 Embryonic rest cells
A 55-year-old woman complains of “fullness” in the vagina, especially during intercourse. Upon examination, a 4- to 5-cm fluid-filled cyst is visible at the right posterior fornix.
What type of cyst is it?
Gartner’s duct cysts from embryonic rest cells arise from remnants of the metanephric duct system. They usually develop on the lateral vaginal walls or fornix (FIGURE 7), unlike epidermal inclusion cysts, which are generally located on the posterior wall or at the cervical–vesical junction. Although they sometimes cause dyspareunia or make it difficult for the patient to insert a tampon, they are usually asymptomatic unless they are large. Intervention is not indicated unless the patient is symptomatic.
Treatment. Marsupialization rather than excision is the treatment of choice because these embryonic rest cells may extend deep into the retroperitoneum.
FIGURE 7 Gartner’s duct cysts
These bilateral cysts developed from embryonic rest cells.
1. Emans SJ, Laufer MR, Goldstein DP. Pediatric and Adolescent Gynecology. 5th ed. Chapter 10. Philadelphia: Lippincott Williams & Wilkins;. 2005:334-416.
2. Acién P. Incidence of Müllerian defects in fertile and infertile women. Hum Reprod. 1997;12:1372-1376.
3. Evans TN, Poland ML, Boving RL. Vaginal malformations. Am J Obstet Gynecol. 1981;141:910-920.
4. Fedele L, Bianchi S, Agnoli B, Tozzi L, Vignali M. Urinary tract anomalies associated with unicornuate uterus. J Urol. 1996;155:847-858.
5. Vaginal agenesis: diagnosis, management, and routine care. Committee Opinion No. 355. Washington, DC: ACOG; December 2006.
6. Roberts CP, Haber MJ, Rock JA. Vaginal creation for müllerian agenesis. Am J Obstet Gynecol. 2001;185:1349-1353.
7. Stassart JP, Nagel TC, Prem KA, Phipps WR. Uterus didelphys, obstructed hemivagina, and ipsilateral renal agenesis: the University of Minnesota experience. Fertil Steril. 1992;57:756-761.
The author reports no financial relationships relevant to this article.
CASE 1 Fluid-filled abdominal mass
A 14-year-old girl complains of crampy, episodic, lower abdominal pain of 3 months’ duration and acute retention of urine. She also reports back pain and dyschezia. She has never menstruated, but her breasts began developing 18 months earlier. Examination reveals normal Tanner stage 3 breast development and female hair distribution and a large abdominal mass. No fetal heart tones are apparent. The external genitalia appear to be normal except for a bulging mass at the introitus.
Imaging (FIGURE 1) reveals that the mass is fluid-filled. The uterus, tubes, and ovaries are present at the dome of the mass. The kidneys are normal.
What is the mass?
FIGURE 1 No exit for menstrual products
An MRI shows a large hematocolpos that develops after menarche in women who have outflow obstruction, such as imperforate hymen—as this patient had.A list of the processes involved in normal development of the female reproductive tract highlights its precarious complexity: cellular differentiation, migration, canalization, fusion, and programmed cell death. A failure in any of these processes can cause a malformation. When that malformation becomes apparent depends on the stage of life of the patient and the nature of the abnormality. As you might imagine, diagnosis and treatment are not always straightforward.
In the patient just described, the likely diagnosis is imperforate hymen or transverse vaginal septum due to failed canalization of the vaginal plate. The patient has been menstruating internally for many months and now has a large hematocolpos. Urinary retention develops when the amount of retained blood in the vagina causes acute angulation of the urethrovesical junction. Evacuation of the blood restores the physiologic angle, enabling the patient to void normally.
Most anomalies involving the external genitalia are apparent at birth (clitoromegaly, imperforate hymen), whereas obstructive and nonobstructive malformations may become evident at birth; during childhood, puberty, or adolescence; or with menarche or childbearing.
Treatment of these abnormalities has changed significantly over the past few years—largely due to refinements in diagnostic imaging, surgical and nonsurgical techniques, and instrumentation. These advances have improved reproductive function and enhanced the psychosexual attitude of these patients.
In this article, I review the most common abnormalities and describe their evaluation and management.
Vaginal canalization
The sinovaginal bulbs are two solid evaginations originating from the urogenital sinus at the distal aspect of the müllerian tubercle, as shown at right (A). The sinovaginal bulbs proliferate into the caudal end of the uterovaginal canal to become the solid vaginal plate (B). The lumen of the lower vagina is formed by degeneration of the central cells of this vaginal plate, which occurs in a cephalad direction (C). This process of canalization is complete by 20 weeks’ gestation.
Hymen usually ruptures before birth
The vaginal hymen is separated from the urogenital sinus by the hymeneal membrane. The hymen usually ruptures before birth with the degeneration of central epithelial cells (i.e., canalization), leaving a thin fold of mucous membrane around the vaginal introitus.
Uterus, fallopian tubes develop from solid tissue
The müllerian ducts are first identifiable at approximately 6 weeks of gestation, when they begin to elongate caudally and cross the metanephric ducts medially to meet in the midline. By the seventh week, the urorectal septum has developed, separating the rectum from the urogenital sinus. Around 12 weeks’ gestation, the caudal portion of the müllerian ducts fuses to form the uterovaginal canal, which inserts into the dorsal wall of the urogenital sinus at Müller’s tubercle.
The two müllerian ducts are initially solid tissue and lie side by side. Subsequently, internal canalization of each duct produces two channels divided by a septum that is reabsorbed in a cephalad direction by 20 weeks. The cranial unfused portions of the müllerian ducts develop into the fimbria and fallopian tubes, whereas the caudal, fused portions form the uterus and upper vagina.
Impaired canalization
Imperforate hymen
The most common obstructive lesion of the female reproductive tract is an imperforate hymen. It can be diagnosed at birth with careful examination to ensure patency of both the rectum and vagina. Not infrequently, the infant presents with a bulging introitus due to mucocolpos from vaginal secretions stimulated by maternal estrogen (FIGURE 2). If diagnosis is delayed, the mucus usually resorbs and the child remains asymptomatic until menarche, when she presents with a bulging hymen and a large hematocolpos, as in the opening case. Management of imperforate hymen is feasible for the generalist. Repair can be performed in a patient of any age, but is facilitated if the tissue has undergone estrogen stimulation. Surgery is, therefore, ideal in the neonatal, postpubertal, and premenarchal periods.
Repair involves incision of the membrane near the hymeneal ring, followed by evacuation of accumulated blood. The cervix may appear to be flush with the vaginal apex, owing to compression, but this condition usually corrects itself within 2 or 3 weeks. Extra hymeneal tissue is excised to create an orifice of normal size, and the vaginal mucosa is intermittently sutured to the hymeneal ring using absorbable material (FIGURE 3).
FIGURE 2 Imperforate hymen
Bulging hymen due to mucocolpos in a newborn (top) and hematocolpos in an adolescent (bottom).
FIGURE 3 Hymenectomy restores vaginal orifice
Extra hymeneal tissue is excised to create an orifice of normal size. The vaginal mucosa is intermittently sutured to the hymeneal ring.
Incomplete hymeneal fenestration
This condition often goes undiagnosed until the patient seeks evaluation for an inability to insert tampons or medication or because of coital difficulty. Young girls with microperforate hymen may present with postmenstrual spotting or malodorous discharge due to partial obstruction and poor drainage.
Treatment of microperforate, cribriform, or septate hymen entails resection of excess hymeneal tissue to create a functional hymeneal ring.
Transverse vaginal septum
A transverse septum represents failed canalization of the vaginal plate. This condition occurs in approximately one of every 18,000 to 30,000 women.1,2 The septum can be located at any of various levels in the vagina; approximately 46% are found in the upper vagina, 35% to 40% in the middle portion, and 15% to 20% in the lower vagina.1,2 Lower septa appear to be thinner than those in the proximal vagina; thinness may be due to the pressure of accumulated fluid. If a small perforation is present, the patient will experience irregular spotting.
Examination. The external genitalia appear to be normal, but the vagina is shortened. Children may present with mucocolpos, whereas adolescents develop hematocolpos. If a small perforation is present, pyohematocolpos may result from ascending infection. A mass can be palpated on rectal examination.
Ultrasonography (US) or magnetic resonance imaging (MRI) helps define the location and thickness of the septum and distinguish a high septum from congenital absence of the cervix.
Treatment. Resect a small septum and perform end-to-end anastomosis of the upper and lower edges of the vaginal wall (FIGURE 4). Only an experienced surgeon should excise a thick septum. Reanastomosis is easier if the upper vagina is distended with menstrual blood. To ensure healing without stricture, mobilize the vaginal edge circumferentially before suturing the raw edges.
Outflow obstruction caused by a transverse septum in the distal vagina almost never leads to hematometra or hematosalpinx. However, septa that arise in the proximal vagina are usually thicker and, if left untreated, can cause reflux that damages the upper reproductive tract. These cases are almost always associated with severe dysmenorrhea and should be referred to an experienced vaginal surgeon. Pelvic MRI is recommended to assess the thickness and precise anatomy of the defect before repair.
FIGURE 4 Removing a transverse vaginal septum
The septum is resected from the distal vagina. This is followed by end-to-end anastomosis of mucosal edges.
Uterine septum
A septate uterus has a normal external surface but two endometrial cavities. It is caused by a defect in fusion or resorption of the midline septum between the two müllerian ducts. The degree of septation varies from a small midline septum to a total failure of resorption that produces a septate uterus with a longitudinal vaginal septum. A slight midline septum with minimal fundal indentation is classified by some as a septate uterus; by others, as a bicornuate uterus.
A higher risk of recurrent miscarriage is associated with longer septa.
Surgical repair with hysteroscopic resection of the septum yields excellent results.
CASE 2 The problem of agenesis
An 18-year-old patient consults a gynecologist because she has never menstruated and is unable to have sexual intercourse. She appears to be phenotypically normal, with normal labia upon examination, but no hymeneal opening is found. Pelvic US reveals absence of the uterus with ovaries that appear to be normal.
How should her condition be managed?
Uterovaginal agenesis, also known as müllerian aplasia or Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome, refers to congenital absence of the vagina with variable uterine development (FIGURE 5). It arises from agenesis of the
müllerian duct system, although the underlying cause is unknown. Vaginal agenesis is usually accompanied by cervical and uterine agenesis. However, 7% to 12% of patients with this syndrome have a normal but obstructed or rudimentary uterus with functional endometrium. This condition occurs in roughly one of every 5,000 females.1,3
In addition, these girls often exhibit extragenital anomalies.1,4 Approximately 25% to 50% have a urologic anomaly, such as unilateral renal agenesis, pelvic or horseshoe kidneys, or irregularities of the collecting systems; 10% to 15% have another type of anomaly, such as congenital heart disease, abnormalities of the hands, congenital deafness, cleft palate, and inguinal or femoral hernia.
The genetic basis of MRKH syndrome is unknown. These patients have normal female karyotypes and phenotypes with normal ovaries and ovarian function—although there is evidence that they are prone to early menopause. They develop secondary sexual characteristics. They usually present with primary amenorrhea at 15 to 17 years of age.
Vaginal agenesis is the second most common cause of primary amenorrhea after gonadal dysgenesis. Most patients have a rudimentary, nonfunctioning uterus, but 2% to 7% have a uterus with functioning endometrium and present with cyclic or chronic abdominopelvic pain secondary to obstruction or endometriosis, hematocolpos, or hematometra.
Imaging. US examination facilitates evaluation of the kidneys and confirms the presence of ovaries and absence of the uterus. MRI may be useful to confirm the presence of functioning endometrium.
The differential diagnosis of vaginal agenesis includes androgen insensitivity and low-lying transverse vaginal septum. Treatment includes psychosocial support to reassure the patient that a normal sex life will be possible after treatment.5 Construction of a neovagina, using vaginal dilators (the first-line strategy) or surgical placement of split-thickness skin graft or artificial skin (Abbe–McIndoe procedure), is the treatment. In one case series, more than 90% of patients with vaginal agenesis were successfully treated using vaginal dilation.6
The American College of Obstetricians and Gynecologists (ACOG) recommends that patients be referred to centers with expertise in surgical creation of a neovagina.5
FIGURE 5 Uterine agenesis
This depiction of uterine agenesis shows small, nonfunctioning müllerian remnants and normal tubes and ovaries.
Agenesis of the lower vagina
This malformation is caused by a failure of canalization of the sinovaginal bulbs and vaginal plate. The tubes, ovaries, cervix, and upper vagina are normal, but the lower vagina is replaced by fibrous tissue. Adolescents present with primary amenorrhea accompanied by cyclic pelvic pain. A vaginal dimple may be apparent on external inspection.
Because these girls have normal cyclic pituitary–ovarian–endometrial function at menarche, the upper vagina will distend with blood and secretions, and a mass can be palpated on rectoabdominal examination. US confirms the presence of a fluid-filled mass, and MRI further defines the length of obstruction.
Treatment. Surgery (by an experienced vaginal surgeon) is best performed when a large hematocolpos is present because distention increases the amount of tissue available for pull-through.
CASE 3 Fusion failure
A 17-year-old woman complains of lower abdominal pain and an enlarging abdomen. Menarche occurred at 13 years. The patient began to notice right-sided lower abdominal pain and abdominal swelling about 2 years ago, but denies any other symptoms.
Examination reveals a mass arising from the right lateral vaginal wall. The cervix appears to be normal. US shows a large, predominantly right-sided, fluid-filled mass arising from the pelvis. The uterus appears to be didelphic, with normal ovaries and an absent right kidney.
What is the mass?
This is a classic description of uterovaginal duplication that arises when the müllerian ducts fail to fuse (FIGURE 6).
In this case, the right-sided vagina failed to canalize as well, causing a large hematocolpos. Generally, duplication is limited to the uterus and cervix (uterus didelphys bicollis), although duplication of the vulva, bladder, urethra, vagina, and anus have been described.
Women with a didelphic uterus often have a good reproductive outcome. A septated vagina occurs in 75% of cases and may cause difficulty with sexual intercourse or vaginal delivery. Symptomatic women usually opt for resection of the vaginal septum.
Women who have an obstructed hemivagina and ipsilateral renal agenesis have regular menses because menstrual blood from one uterus can exit through its unobstructed side.
Treatment involves resection of the wall of the obstructed vagina, creating a single vaginal vault. If both vaginas have failed to canalize, the patient will present much earlier, with primary amenorrhea and cyclic pelvic pain—a presentation similar to that of transverse septum. Reconstructive surgery of the uterus is not recommended for complete duplication of the uterus.1,7
FIGURE 6 Uterus didelphys
This patient has uterus didelphys with an obstructed hemivagina.
Unicornuate and bicornuate uterus
A unicornuate uterus represents an asymmetric lateral fusion defect. One cavity is usually normal, with a fallopian tube and cervix, but the failed müllerian duct can have various configurations. The affected müllerian duct may fail to develop, or it may develop partially as a horn on the uterus or a separate structure that may or may not communicate with the uterus.
Most rudimentary horns are asymptomatic, but others contain functional endometrium that is shed cyclically. If the rudimentary horn is obstructed, as is usually the case, the woman develops cyclic or chronic abdominopelvic pain that necessitates surgical excision of the horn. Endometriosis, a result of reflux, is common.
In a bicornuate uterus, the fundus is indented and the vagina is normal. This anomaly occurs when the müllerian ducts fuse only partially. The result is varying degrees of separation of the uterine horns.
Pregnancy outcomes appear to be similar to those of the general population. However, some women develop early pregnancy loss, preterm labor, incompetent cervix, or malpresentation.
Treatment is open surgical reunification.
CASE 4 Embryonic rest cells
A 55-year-old woman complains of “fullness” in the vagina, especially during intercourse. Upon examination, a 4- to 5-cm fluid-filled cyst is visible at the right posterior fornix.
What type of cyst is it?
Gartner’s duct cysts from embryonic rest cells arise from remnants of the metanephric duct system. They usually develop on the lateral vaginal walls or fornix (FIGURE 7), unlike epidermal inclusion cysts, which are generally located on the posterior wall or at the cervical–vesical junction. Although they sometimes cause dyspareunia or make it difficult for the patient to insert a tampon, they are usually asymptomatic unless they are large. Intervention is not indicated unless the patient is symptomatic.
Treatment. Marsupialization rather than excision is the treatment of choice because these embryonic rest cells may extend deep into the retroperitoneum.
FIGURE 7 Gartner’s duct cysts
These bilateral cysts developed from embryonic rest cells.
The author reports no financial relationships relevant to this article.
CASE 1 Fluid-filled abdominal mass
A 14-year-old girl complains of crampy, episodic, lower abdominal pain of 3 months’ duration and acute retention of urine. She also reports back pain and dyschezia. She has never menstruated, but her breasts began developing 18 months earlier. Examination reveals normal Tanner stage 3 breast development and female hair distribution and a large abdominal mass. No fetal heart tones are apparent. The external genitalia appear to be normal except for a bulging mass at the introitus.
Imaging (FIGURE 1) reveals that the mass is fluid-filled. The uterus, tubes, and ovaries are present at the dome of the mass. The kidneys are normal.
What is the mass?
FIGURE 1 No exit for menstrual products
An MRI shows a large hematocolpos that develops after menarche in women who have outflow obstruction, such as imperforate hymen—as this patient had.A list of the processes involved in normal development of the female reproductive tract highlights its precarious complexity: cellular differentiation, migration, canalization, fusion, and programmed cell death. A failure in any of these processes can cause a malformation. When that malformation becomes apparent depends on the stage of life of the patient and the nature of the abnormality. As you might imagine, diagnosis and treatment are not always straightforward.
In the patient just described, the likely diagnosis is imperforate hymen or transverse vaginal septum due to failed canalization of the vaginal plate. The patient has been menstruating internally for many months and now has a large hematocolpos. Urinary retention develops when the amount of retained blood in the vagina causes acute angulation of the urethrovesical junction. Evacuation of the blood restores the physiologic angle, enabling the patient to void normally.
Most anomalies involving the external genitalia are apparent at birth (clitoromegaly, imperforate hymen), whereas obstructive and nonobstructive malformations may become evident at birth; during childhood, puberty, or adolescence; or with menarche or childbearing.
Treatment of these abnormalities has changed significantly over the past few years—largely due to refinements in diagnostic imaging, surgical and nonsurgical techniques, and instrumentation. These advances have improved reproductive function and enhanced the psychosexual attitude of these patients.
In this article, I review the most common abnormalities and describe their evaluation and management.
Vaginal canalization
The sinovaginal bulbs are two solid evaginations originating from the urogenital sinus at the distal aspect of the müllerian tubercle, as shown at right (A). The sinovaginal bulbs proliferate into the caudal end of the uterovaginal canal to become the solid vaginal plate (B). The lumen of the lower vagina is formed by degeneration of the central cells of this vaginal plate, which occurs in a cephalad direction (C). This process of canalization is complete by 20 weeks’ gestation.
Hymen usually ruptures before birth
The vaginal hymen is separated from the urogenital sinus by the hymeneal membrane. The hymen usually ruptures before birth with the degeneration of central epithelial cells (i.e., canalization), leaving a thin fold of mucous membrane around the vaginal introitus.
Uterus, fallopian tubes develop from solid tissue
The müllerian ducts are first identifiable at approximately 6 weeks of gestation, when they begin to elongate caudally and cross the metanephric ducts medially to meet in the midline. By the seventh week, the urorectal septum has developed, separating the rectum from the urogenital sinus. Around 12 weeks’ gestation, the caudal portion of the müllerian ducts fuses to form the uterovaginal canal, which inserts into the dorsal wall of the urogenital sinus at Müller’s tubercle.
The two müllerian ducts are initially solid tissue and lie side by side. Subsequently, internal canalization of each duct produces two channels divided by a septum that is reabsorbed in a cephalad direction by 20 weeks. The cranial unfused portions of the müllerian ducts develop into the fimbria and fallopian tubes, whereas the caudal, fused portions form the uterus and upper vagina.
Impaired canalization
Imperforate hymen
The most common obstructive lesion of the female reproductive tract is an imperforate hymen. It can be diagnosed at birth with careful examination to ensure patency of both the rectum and vagina. Not infrequently, the infant presents with a bulging introitus due to mucocolpos from vaginal secretions stimulated by maternal estrogen (FIGURE 2). If diagnosis is delayed, the mucus usually resorbs and the child remains asymptomatic until menarche, when she presents with a bulging hymen and a large hematocolpos, as in the opening case. Management of imperforate hymen is feasible for the generalist. Repair can be performed in a patient of any age, but is facilitated if the tissue has undergone estrogen stimulation. Surgery is, therefore, ideal in the neonatal, postpubertal, and premenarchal periods.
Repair involves incision of the membrane near the hymeneal ring, followed by evacuation of accumulated blood. The cervix may appear to be flush with the vaginal apex, owing to compression, but this condition usually corrects itself within 2 or 3 weeks. Extra hymeneal tissue is excised to create an orifice of normal size, and the vaginal mucosa is intermittently sutured to the hymeneal ring using absorbable material (FIGURE 3).
FIGURE 2 Imperforate hymen
Bulging hymen due to mucocolpos in a newborn (top) and hematocolpos in an adolescent (bottom).
FIGURE 3 Hymenectomy restores vaginal orifice
Extra hymeneal tissue is excised to create an orifice of normal size. The vaginal mucosa is intermittently sutured to the hymeneal ring.
Incomplete hymeneal fenestration
This condition often goes undiagnosed until the patient seeks evaluation for an inability to insert tampons or medication or because of coital difficulty. Young girls with microperforate hymen may present with postmenstrual spotting or malodorous discharge due to partial obstruction and poor drainage.
Treatment of microperforate, cribriform, or septate hymen entails resection of excess hymeneal tissue to create a functional hymeneal ring.
Transverse vaginal septum
A transverse septum represents failed canalization of the vaginal plate. This condition occurs in approximately one of every 18,000 to 30,000 women.1,2 The septum can be located at any of various levels in the vagina; approximately 46% are found in the upper vagina, 35% to 40% in the middle portion, and 15% to 20% in the lower vagina.1,2 Lower septa appear to be thinner than those in the proximal vagina; thinness may be due to the pressure of accumulated fluid. If a small perforation is present, the patient will experience irregular spotting.
Examination. The external genitalia appear to be normal, but the vagina is shortened. Children may present with mucocolpos, whereas adolescents develop hematocolpos. If a small perforation is present, pyohematocolpos may result from ascending infection. A mass can be palpated on rectal examination.
Ultrasonography (US) or magnetic resonance imaging (MRI) helps define the location and thickness of the septum and distinguish a high septum from congenital absence of the cervix.
Treatment. Resect a small septum and perform end-to-end anastomosis of the upper and lower edges of the vaginal wall (FIGURE 4). Only an experienced surgeon should excise a thick septum. Reanastomosis is easier if the upper vagina is distended with menstrual blood. To ensure healing without stricture, mobilize the vaginal edge circumferentially before suturing the raw edges.
Outflow obstruction caused by a transverse septum in the distal vagina almost never leads to hematometra or hematosalpinx. However, septa that arise in the proximal vagina are usually thicker and, if left untreated, can cause reflux that damages the upper reproductive tract. These cases are almost always associated with severe dysmenorrhea and should be referred to an experienced vaginal surgeon. Pelvic MRI is recommended to assess the thickness and precise anatomy of the defect before repair.
FIGURE 4 Removing a transverse vaginal septum
The septum is resected from the distal vagina. This is followed by end-to-end anastomosis of mucosal edges.
Uterine septum
A septate uterus has a normal external surface but two endometrial cavities. It is caused by a defect in fusion or resorption of the midline septum between the two müllerian ducts. The degree of septation varies from a small midline septum to a total failure of resorption that produces a septate uterus with a longitudinal vaginal septum. A slight midline septum with minimal fundal indentation is classified by some as a septate uterus; by others, as a bicornuate uterus.
A higher risk of recurrent miscarriage is associated with longer septa.
Surgical repair with hysteroscopic resection of the septum yields excellent results.
CASE 2 The problem of agenesis
An 18-year-old patient consults a gynecologist because she has never menstruated and is unable to have sexual intercourse. She appears to be phenotypically normal, with normal labia upon examination, but no hymeneal opening is found. Pelvic US reveals absence of the uterus with ovaries that appear to be normal.
How should her condition be managed?
Uterovaginal agenesis, also known as müllerian aplasia or Mayer–Rokitansky–Küster–Hauser (MRKH) syndrome, refers to congenital absence of the vagina with variable uterine development (FIGURE 5). It arises from agenesis of the
müllerian duct system, although the underlying cause is unknown. Vaginal agenesis is usually accompanied by cervical and uterine agenesis. However, 7% to 12% of patients with this syndrome have a normal but obstructed or rudimentary uterus with functional endometrium. This condition occurs in roughly one of every 5,000 females.1,3
In addition, these girls often exhibit extragenital anomalies.1,4 Approximately 25% to 50% have a urologic anomaly, such as unilateral renal agenesis, pelvic or horseshoe kidneys, or irregularities of the collecting systems; 10% to 15% have another type of anomaly, such as congenital heart disease, abnormalities of the hands, congenital deafness, cleft palate, and inguinal or femoral hernia.
The genetic basis of MRKH syndrome is unknown. These patients have normal female karyotypes and phenotypes with normal ovaries and ovarian function—although there is evidence that they are prone to early menopause. They develop secondary sexual characteristics. They usually present with primary amenorrhea at 15 to 17 years of age.
Vaginal agenesis is the second most common cause of primary amenorrhea after gonadal dysgenesis. Most patients have a rudimentary, nonfunctioning uterus, but 2% to 7% have a uterus with functioning endometrium and present with cyclic or chronic abdominopelvic pain secondary to obstruction or endometriosis, hematocolpos, or hematometra.
Imaging. US examination facilitates evaluation of the kidneys and confirms the presence of ovaries and absence of the uterus. MRI may be useful to confirm the presence of functioning endometrium.
The differential diagnosis of vaginal agenesis includes androgen insensitivity and low-lying transverse vaginal septum. Treatment includes psychosocial support to reassure the patient that a normal sex life will be possible after treatment.5 Construction of a neovagina, using vaginal dilators (the first-line strategy) or surgical placement of split-thickness skin graft or artificial skin (Abbe–McIndoe procedure), is the treatment. In one case series, more than 90% of patients with vaginal agenesis were successfully treated using vaginal dilation.6
The American College of Obstetricians and Gynecologists (ACOG) recommends that patients be referred to centers with expertise in surgical creation of a neovagina.5
FIGURE 5 Uterine agenesis
This depiction of uterine agenesis shows small, nonfunctioning müllerian remnants and normal tubes and ovaries.
Agenesis of the lower vagina
This malformation is caused by a failure of canalization of the sinovaginal bulbs and vaginal plate. The tubes, ovaries, cervix, and upper vagina are normal, but the lower vagina is replaced by fibrous tissue. Adolescents present with primary amenorrhea accompanied by cyclic pelvic pain. A vaginal dimple may be apparent on external inspection.
Because these girls have normal cyclic pituitary–ovarian–endometrial function at menarche, the upper vagina will distend with blood and secretions, and a mass can be palpated on rectoabdominal examination. US confirms the presence of a fluid-filled mass, and MRI further defines the length of obstruction.
Treatment. Surgery (by an experienced vaginal surgeon) is best performed when a large hematocolpos is present because distention increases the amount of tissue available for pull-through.
CASE 3 Fusion failure
A 17-year-old woman complains of lower abdominal pain and an enlarging abdomen. Menarche occurred at 13 years. The patient began to notice right-sided lower abdominal pain and abdominal swelling about 2 years ago, but denies any other symptoms.
Examination reveals a mass arising from the right lateral vaginal wall. The cervix appears to be normal. US shows a large, predominantly right-sided, fluid-filled mass arising from the pelvis. The uterus appears to be didelphic, with normal ovaries and an absent right kidney.
What is the mass?
This is a classic description of uterovaginal duplication that arises when the müllerian ducts fail to fuse (FIGURE 6).
In this case, the right-sided vagina failed to canalize as well, causing a large hematocolpos. Generally, duplication is limited to the uterus and cervix (uterus didelphys bicollis), although duplication of the vulva, bladder, urethra, vagina, and anus have been described.
Women with a didelphic uterus often have a good reproductive outcome. A septated vagina occurs in 75% of cases and may cause difficulty with sexual intercourse or vaginal delivery. Symptomatic women usually opt for resection of the vaginal septum.
Women who have an obstructed hemivagina and ipsilateral renal agenesis have regular menses because menstrual blood from one uterus can exit through its unobstructed side.
Treatment involves resection of the wall of the obstructed vagina, creating a single vaginal vault. If both vaginas have failed to canalize, the patient will present much earlier, with primary amenorrhea and cyclic pelvic pain—a presentation similar to that of transverse septum. Reconstructive surgery of the uterus is not recommended for complete duplication of the uterus.1,7
FIGURE 6 Uterus didelphys
This patient has uterus didelphys with an obstructed hemivagina.
Unicornuate and bicornuate uterus
A unicornuate uterus represents an asymmetric lateral fusion defect. One cavity is usually normal, with a fallopian tube and cervix, but the failed müllerian duct can have various configurations. The affected müllerian duct may fail to develop, or it may develop partially as a horn on the uterus or a separate structure that may or may not communicate with the uterus.
Most rudimentary horns are asymptomatic, but others contain functional endometrium that is shed cyclically. If the rudimentary horn is obstructed, as is usually the case, the woman develops cyclic or chronic abdominopelvic pain that necessitates surgical excision of the horn. Endometriosis, a result of reflux, is common.
In a bicornuate uterus, the fundus is indented and the vagina is normal. This anomaly occurs when the müllerian ducts fuse only partially. The result is varying degrees of separation of the uterine horns.
Pregnancy outcomes appear to be similar to those of the general population. However, some women develop early pregnancy loss, preterm labor, incompetent cervix, or malpresentation.
Treatment is open surgical reunification.
CASE 4 Embryonic rest cells
A 55-year-old woman complains of “fullness” in the vagina, especially during intercourse. Upon examination, a 4- to 5-cm fluid-filled cyst is visible at the right posterior fornix.
What type of cyst is it?
Gartner’s duct cysts from embryonic rest cells arise from remnants of the metanephric duct system. They usually develop on the lateral vaginal walls or fornix (FIGURE 7), unlike epidermal inclusion cysts, which are generally located on the posterior wall or at the cervical–vesical junction. Although they sometimes cause dyspareunia or make it difficult for the patient to insert a tampon, they are usually asymptomatic unless they are large. Intervention is not indicated unless the patient is symptomatic.
Treatment. Marsupialization rather than excision is the treatment of choice because these embryonic rest cells may extend deep into the retroperitoneum.
FIGURE 7 Gartner’s duct cysts
These bilateral cysts developed from embryonic rest cells.
1. Emans SJ, Laufer MR, Goldstein DP. Pediatric and Adolescent Gynecology. 5th ed. Chapter 10. Philadelphia: Lippincott Williams & Wilkins;. 2005:334-416.
2. Acién P. Incidence of Müllerian defects in fertile and infertile women. Hum Reprod. 1997;12:1372-1376.
3. Evans TN, Poland ML, Boving RL. Vaginal malformations. Am J Obstet Gynecol. 1981;141:910-920.
4. Fedele L, Bianchi S, Agnoli B, Tozzi L, Vignali M. Urinary tract anomalies associated with unicornuate uterus. J Urol. 1996;155:847-858.
5. Vaginal agenesis: diagnosis, management, and routine care. Committee Opinion No. 355. Washington, DC: ACOG; December 2006.
6. Roberts CP, Haber MJ, Rock JA. Vaginal creation for müllerian agenesis. Am J Obstet Gynecol. 2001;185:1349-1353.
7. Stassart JP, Nagel TC, Prem KA, Phipps WR. Uterus didelphys, obstructed hemivagina, and ipsilateral renal agenesis: the University of Minnesota experience. Fertil Steril. 1992;57:756-761.
1. Emans SJ, Laufer MR, Goldstein DP. Pediatric and Adolescent Gynecology. 5th ed. Chapter 10. Philadelphia: Lippincott Williams & Wilkins;. 2005:334-416.
2. Acién P. Incidence of Müllerian defects in fertile and infertile women. Hum Reprod. 1997;12:1372-1376.
3. Evans TN, Poland ML, Boving RL. Vaginal malformations. Am J Obstet Gynecol. 1981;141:910-920.
4. Fedele L, Bianchi S, Agnoli B, Tozzi L, Vignali M. Urinary tract anomalies associated with unicornuate uterus. J Urol. 1996;155:847-858.
5. Vaginal agenesis: diagnosis, management, and routine care. Committee Opinion No. 355. Washington, DC: ACOG; December 2006.
6. Roberts CP, Haber MJ, Rock JA. Vaginal creation for müllerian agenesis. Am J Obstet Gynecol. 2001;185:1349-1353.
7. Stassart JP, Nagel TC, Prem KA, Phipps WR. Uterus didelphys, obstructed hemivagina, and ipsilateral renal agenesis: the University of Minnesota experience. Fertil Steril. 1992;57:756-761.
Obstetric anal sphincter injury: 7 critical questions about care
The authors report no financial relationships relevant to this article.
CASE Large baby, extensive tear
A 28-year-old primigravida undergoes a forceps delivery with a midline episiotomy for failure to progress in the second stage of labor. At birth, the infant weighs 4 kg (8.8 lb), and the episiotomy extends to the anal verge. The resident who delivered the child is uncertain whether the anal sphincter is involved in the injury and asks a consultant to examine the perineum.
What should this examination entail?
The obstetrician is rarely culpable when a third- or fourth-degree obstetric anal sphincter injury (OASIS) occurs—but there is little excuse for letting one go undetected.
To minimize the risk of undiagnosed OASIS, a digital anorectal examination is warranted—before any suturing—in every woman who delivers vaginally. This practice can help you avoid missing isolated tears, such as “buttonhole” of the rectal mucosa, which can occur even when the anal sphincter remains intact (FIGURE 1), or a third- or fourth-degree tear that can sometimes be present behind apparently intact perineal skin (FIGURE 2).1
Clinical training of physicians and midwives also needs to improve.
Every labor room should have a protocol for management of anal sphincter injury2; this article describes detection, diagnosis, and management, focusing on seven critical questions.
Only a physician formally trained in primary anal sphincter repair (or under supervision) should repair OASIS.
FIGURE 1 Buttonhole tear
A buttonhole tear of the rectal mucosa (arrow) with an intact external anal sphincter (EAS) demonstrated during a digital rectal examination. SOURCE: Sultan AH3 (used with permission).
FIGURE 2 Injury obscured by intact skin
(A) Intact perineum on visual examination. (B) Anal sphincter trauma detected after rectal examination. SOURCE: Sultan AH, Kettle C1 (used with permission).
1. When (and how) should the torn perineum be examined?
The first requisite is informed consent for vaginal and rectal examination immediately after delivery. Also vital are adequate exposure of the perineum, good lighting, and, if necessary, sufficient analgesia to prevent pain-related restriction of the evaluation. It may be advisable to place the patient in the lithotomy position to improve exposure.
After visual examination of the perineum, part the labia and examine the vagina to establish the full extent of the tear. Always identify the apex of the vaginal laceration.
Next, perform a rectal examination to exclude injury to the anorectal mucosa and anal sphincter.3
Palpation is necessary to confirm OASIS
Insert the index finger into the anal canal and the thumb into the vagina and perform a pill-rolling motion to palpate the anal sphincter. If this technique is inconclusive, ask the woman to contract her anal sphincter with your fingers still in place. When the sphincter is disrupted, you feel a distinct gap anteriorly. If the perineal skin is intact, there may be an absence of puckering on the perianal skin over any underlying defect that may not be evident under regional or general anesthesia.
Because the external anal sphincter (EAS) is in a state of tonic contraction, the sphincter ends will retract when it is disrupted. These ends need to be grasped and retrieved at the time of repair.
Also identify the internal anal sphincter (IAS). It is a circular smooth muscle (FIGURE 3) that is paler in appearance (similar to the flesh of raw fish) than the striated EAS (similar to raw red meat).4 Under normal circumstances, the distal end of the IAS lies a few millimeters proximal to the distal end of the EAS (FIGURE 4). However, if the EAS is relaxed due to regional or general anesthesia, the distal end of the IAS will appear to be at a lower level. If the IAS or anal epithelium is torn, the EAS is, invariably, torn, too.
General or regional (spinal, epidural, caudal) anesthesia provides analgesia and muscle relaxation and enables proper evaluation of the full extent of the injury.
FIGURE 3 Grade 3b tear
Grade 3b tear with an intact internal anal sphincter (IAS). The external sphincter (EAS) is being grasped with Allis forceps. Note the difference in appearance of the paler IAS and darker EAS. SOURCE: Sultan AH, Kettle C1 (used with permission).
FIGURE 4 Classification of anal sphincter injury
First- and second-degree injuries are described below.
2. Is endoanal US helpful to detect OASIS?
Endoanal ultrasonography (US) to identify OASIS requires specific expertise, particularly in the immediate postpartum period, when the anal canal is lax (especially after an epidural). Ultimately, however, the diagnosis rests on clinical assessment and a rectal examination because, even if a defect is seen on US, it has to be clinically apparent to be repaired.
In a study by Faltin and colleagues, in which routine postpartum endoanal US was used as the gold standard for diagnosis of OASIS, five of 21 women had unnecessary intervention because the sonographic defect was not clinically visible despite exploration of the anal sphincter.5 As a result of this unnecessary exploration based on endoanal US, 20% of these women developed severe fecal incontinence. Therefore, we believe that OASIS is best detected clinically immediately after delivery, provided the physician performs a careful examination with palpation of the anal sphincter.6 In such a scenario, endoanal US is of limited value.
3. How is obstetric anal sphincter trauma classified?
To standardize the classification of perineal trauma, Sultan proposed the following system, which has been adopted by the Royal College of Obstetricians and Gynaecologists and internationally7-9:
First degree: Laceration of the vaginal epithelium or perineal skin only
Second degree: Involvement of the perineal muscles, but not the anal sphincter
Third degree: Disruption of the anal sphincter muscles (FIGURE 4):
- 3a: Less than 50% thickness of the external sphincter is torn
- 3b: More than 50% thickness of the external sphincter is torn
- 3c: Internal sphincter is also torn
Fourth degree: A third-degree anal tear with disruption of the anal epithelium (FIGURE 4).
If there is any ambiguity about grading of the injury, the higher grade should be selected. For example, if there is uncertainty between grades 3a and 3b, the injury should be classified as Grade 3b.
4. Is an operating room necessary?
OASIS should be repaired in the operating theater, where there is access to good lighting, appropriate equipment, and aseptic conditions. In our unit, we have a specially prepared instrument tray containing:
- a Weislander self-retaining retractor
- 4 Allis tissue forceps
- McIndoe scissors
- tooth forceps
- 4 artery forceps
- stitch scissors
- a needle holder.
In addition, deep retractors (e.g., Deavers) are useful when there are associated paravaginal tears.
5. What surgical technique is recommended?
Buttonhole injury
This type of injury can occur in the rectum without disrupting the anal sphincter or perineum. It is best repaired transvaginally using interrupted Vicryl (polyglactin) sutures.
To minimize the risk of persistent rectovaginal fistula, interpose a second layer of tissue between the rectum and vagina by approximating the rectovaginal fascia. A colostomy is rarely indicated unless a large tear extends above the pelvic floor or there is gross fecal contamination of the wound.
Fourth-degree tear
Repair torn anal epithelium with interrupted Vicryl 3-0 sutures, with the knots tied in the anal lumen. Proponents of this widely described technique argue that it reduces the quantity of foreign body (knots) within the tissue and lowers the risk of infection. Concern about a foreign body probably applies to the use of catgut, which dissolves by proteolysis, rather than to newer synthetic material such as Vicryl or Dexon (polyglycolic acid), which dissolves by hydrolysis.
Subcuticular repair of anal epithelium using a transvaginal approach has also been described and could be equally effective if the terminal knots are secure.10
Sphincter muscles
Repair these muscles using 3-0 polydioxanone (PDS) dyed sutures. Compared with braided sutures, monofilament sutures are believed to lessen the risk of infection, although a randomized controlled trial revealed no difference in suture-related morbidity between Vicryl and PDS at 6 weeks postpartum.11 Complete absorption of PDS takes longer than with Vicryl, with 50% tensile strength lasting more than 3 months, compared with 3 weeks for Vicryl.11 To minimize suture migration, cut suture ends short and ensure that they are covered by the overlying superficial perineal muscles.
Internal anal sphincter. Repair the IAS separately from the EAS. Grasp the ends of the torn muscle using Allis forceps and perform an end-to-end repair with interrupted or mattress 3-0 PDS sutures (FIGURE 5). Overlapping repair can be technically difficult.
There is some evidence that repair of an isolated IAS defect benefits patients with established anal incontinence.
External anal sphincter. Because the EAS is normally under tonic contraction, it tends to retract when torn. Therefore, repair requires identification and grasping of the torn ends using Allis tissue forceps (FIGURE 6).
When the EAS is only partially torn (Grade 3a and some cases of Grade 3b), perform an end-to end repair using 2 or 3 mattress sutures, similar to repair of IAS injury, instead of hemostatic “figure of eight” sutures.
For a full-thickness tear (some cases of Grade 3b or 3c, or Grade 4), overlapping repair may be preferable in experienced hands. The EAS may need to be mobilized by dissecting it free of the ischioanal fat laterally using a pair of McIndoe scissors. The torn ends of the EAS can then be overlapped in “double-breasted” fashion (FIGURE 7) using PDS 3-0 sutures. Proper overlap is possible only when the full length of the torn ends is identified.
Overlapping the ends of the sphincter allows for greater surface area of contact between muscle. In contrast, end-to-end repair can be performed without identifying the full length of the EAS and may give rise to incomplete apposition. Fernando and colleagues demonstrated that, in experienced hands, early primary overlap repair carries a lower risk of fecal urgency and anal incontinence than does immediate primary end-to-end repair.12,13
FIGURE 5 End-to-end repair
Internal anal sphincter (I) repair using mattress sutures, demonstrated on the latex Sultan model, used for training (www.perineum.net) (E, external sphincter; A, anal epithelium). SOURCE: Sultan AH, Thakar R2 (used with permission).
FIGURE 6 Locating the external anal sphincter
The external sphincter (E), grasped with Allis forceps, is surrounded by the capsule (C) and lies medial to the ischioanal fat. SOURCE: Sultan AH, Thakar R2 (used with permission).
FIGURE 7 Overlapping sphincter repair
Repair of a fourth degree tear (demonstrated on the Sultan model) using the overlap repair technique on the external sphincter (E). The anal epithelium (A) and the internal sphincter (I) have also been repaired. SOURCE: Sultan AH, Thakar R2 (used with permission).
Perineal muscles
After repair of the sphincter, suture the perineal muscles to reconstruct the perineal body and provide support to the repaired anal sphincter. A short, deficient perineum would leave the anal sphincter more vulnerable to trauma during a subsequent vaginal delivery.
Next, suture the vaginal skin and approximate the perineal skin using Vicryl Rapide 2-0 subcuticular suture.
Examine, and document, the repair
Perform a rectal and vaginal examination to confirm adequate repair and ensure that no other tears have been missed—and that all tampons or swabs have been removed.
Make detailed notes of the findings and repair. A pro forma pictorial representation of the tears proves very useful when notes are reviewed following complications or during audit or litigation.
6. What does postoperative care entail?
Prophylactic antibiotics are common
No randomized trials have substantiated the benefits of intraoperative and postoperative antibiotics after repair of OASIS. Nevertheless, these drugs are commonly prescribed, especially after fourth- degree tears, because infection and wound breakdown could jeopardize the repair and lead to incontinence or fistula.10,14
We prescribe intravenous broad-spectrum antibiotics such as cefuroxime and metronidazole intraoperatively and continue the drugs orally for 5 days.
Bladder catheterization is recommended
Severe perineal discomfort, especially after instrumental delivery, is a known cause of urinary retention. Moreover, after administration of regional anesthesia, it can take up to 12 hours before bladder sensation returns.
We recommend insertion of a Foley catheter for approximately 24 hours, unless medical staff can ensure that spontaneous voiding occurs at least every 3 to 4 hours without bladder overdistension.
Pain may persist after severe injury
The degree of pain following perineal trauma is related to the extent of the injury. OASIS is frequently associated with other more extensive injuries such as paravaginal tears. In one study, 91% of women continued to complain of severe perineal pain 7 days after OASIS.15
In a systematic review, Hedayati and associates found rectal analgesia, such as diclofenac sodium, to be effective at reducing pain from perineal trauma within the first 24 hours after birth; they also found that women used less additional analgesia within the first 48 hours after birth.16 Diclofenac is almost completely bound to protein, so excretion in breast milk is negligible.17
In women who have undergone repair of a fourth-degree tear, administer oral diclofenac; suppositories may be uncomfortable, and there is a theoretical risk of poor healing associated with local anti-inflammatory agents.
Avoid codeine-based preparations because they may cause constipation and lead to excessive straining and disruption of the repair.
Recommend a stool softener
It is vital that constipation be avoided as the patient heals; passage of constipated stool or fecal impaction can disrupt the repair. We prescribe a stool softener (lactulose, 15 mL twice daily) for 10 to 14 days and have encountered no problem with bowel evacuation.18
We recommend that the patient telephone a healthcare provider 24 to 48 hours after hospital discharge to confirm that bowel evacuation has occurred. If it hasn’t, we add mineral oil, magnesium hydroxide, or another oral bowel stimulant to the stool softener and bulking agent.
Mahoney and colleagues conducted a randomized trial (n=105) of constipating versus laxative regimens and found the latter to be associated with earlier and less painful first bowel motion and earlier hospital discharge.19 Nineteen percent of women following the constipating regimen had troublesome constipation (two required hospitalization for fecal impaction), compared with 5% of women receiving a laxative. There were no significant differences in continence scores, anal manometry, and endoanal US findings.
Give the patient adequate information
Before the patient is discharged from the hospital, we give her a booklet that describes the implications of OASIS and explains when and where to seek help if symptoms of infection or incontinence develop. All women also complete a validated bowel-health and quality-of-life questionnaire regarding conditions prior to the delivery. We also recommend pelvic floor and anal sphincter exercises as soon as her discomfort resolves.
Perform a comprehensive follow-up exam
All women who sustain OASIS should be assessed by a senior obstetrician 6 to 8 weeks after delivery. In our practice, these women are seen in a dedicated perineal clinic.20 The clinic provides a supportive environment and increases the patient’s confidence in the team.21
At the clinic, each woman completes the same symptom questionnaire that she was given before hospital discharge. She then undergoes a genital examination in which the physician checks the degree of scarring, residual granulation tissue, and tenderness; ensures that the patient understands the circumstances surrounding the delivery and injury; and addresses any concerns. All women then undergo anal manometry and endoanal US (FIGURE 8). Each patient is encouraged to continue pelvic floor exercises. If she has minimal sphincter contractility, she may need electrical stimulation.
If a dedicated perineal clinic is unavailable, the patient should be given clear instructions, preferably in writing, before leaving the hospital. During the 6 weeks immediately after delivery, she should be instructed to look for signs of infection or wound dehiscence and to telephone the physician to report any increase in pain or swelling, rectal bleeding, or purulent discharge. Any incontinence of stool or flatus also should be reported.
FIGURE 8 Defect visible on US
Endoanal sonogram showing a defect in the external anal sphincter between 11 o’clock and 1 o’clock (between the yellow arrows) (S, subepithelium; E, external anal sphincter). SOURCE: Sultan AH, Thakar R2 (used with permission).
7. Is vaginal delivery advisable after OASIS?
No randomized trials have determined the most appropriate mode of delivery after a third- or fourth-degree tear. We base our counseling of the patient on a completed symptom questionnaire and findings from manometry and endoanal US (FIGURE 8). If vaginal delivery is contemplated, these tests should be performed during the current pregnancy unless they were abnormal at an earlier date. FIGURE 9 is a simple flow diagram from our unit that illustrates management of subsequent delivery after OASIS.
When determining the mode of delivery, thorough counseling and clear documentation of that counseling are extremely important.
FIGURE 9 How do you determine the mode of delivery after OASIS?
Vaginal delivery is possible unless anal sphincter function is impaired
One study found that when a large sonographic defect (more than one quadrant) is present, or the squeeze-pressure increment (above resting pressure) is less than 20 mm Hg, the risk of impaired continence after a subsequent delivery increases dramatically.22
Based on these findings, we conducted a prospective study that found no deterioration of sphincter function or increase in symptoms after vaginal delivery unless the patient had significant compromise of anal sphincter function before the pregnancy.23 Therefore, we encourage asymptomatic women who have minimal compromise of anal sphincter function to undergo vaginal delivery.
Routine episiotomy is not protective
There is no evidence that routine episiotomy prevents recurrent OASIS. If episiotomy is deemed to be necessary—e.g., for a thick inelastic or scarred perineum—mediolateral episiotomy is preferred.
High likelihood of success in some women
Women who have minimal compromise of anal sphincter function should be counseled that they have an 88% (in centers practicing midline episiotomy) to 95% (in centers practicing mediolateral episiotomy) chance of delivering without sustaining another OASIS.24,25 This should reassure them if they have misgivings about vaginal delivery.
Threshold for C-section is lower if additional risk factors are present
If traumatic delivery is anticipated, as in the presence of one or more additional risk factors (macrosomia, shoulder dystocia, prolonged labor, difficult instrumental delivery), cesarean section may be appropriate.
Consider emotional needs
Some women who have sustained OASIS may be scarred emotionally as well as physically and may find it difficult to cope with the thought of another vaginal delivery. These women deserve sympathy, psychological support, and consideration of their request for cesarean section.
When cesarean is a good idea
Women who have a minor degree of incontinence (e.g., fecal urgency or flatus incontinence) may be managed with dietary advice, constipating agents (loperamide or codeine phosphate), and physiotherapy or biofeedback. These women who have some degree of anal sphincter compromise but whose symptoms are controlled should be counseled that cesarean delivery is recommended (FIGURE 9).
Women who have sustained a previous third- or fourth-degree tear with subsequent severe incontinence should be offered secondary sphincter repair by a colorectal surgeon or urogynecologist with expertise in secondary sphincter repair. All subsequent deliveries by these women should be by cesarean section.
Some women with fecal incontinence may choose to complete their family before embarking on anal sphincter surgery. It remains unclear whether these women should be allowed a vaginal delivery, but it is likely that most damage has already occurred and that the risk of further injury is minimal and possibly insignificant. The benefit of cesarean delivery, if any, should be weighed against its risks for all subsequent pregnancies.
Women who have undergone a previous successful secondary sphincter repair for fecal incontinence should be delivered by cesarean delivery.9
Not all women fit neatly into one category
There are going to be women who do not entirely fit any of the categories described—such as those who have isolated internal sphincter defects or irritable bowel syndrome. Management of these women should be individualized, with the mode of delivery determined by mutual agreement after taking into account symptoms and clinical and other findings.
If there are no facilities for anal manometry and US, the physician should base management on symptoms and clinical evaluation. Asymptomatic women who do not have clinical evidence of sphincter compromise during anal tone assessment may be allowed to undergo vaginal delivery. All women who are symptomatic should be referred to a center with facilities for anorectal assessment to establish the ideal management and mode of delivery.
Pay attention to modifiable risk factors
In the case described at the beginning of this article, two risk factors could have been modified to minimize the patient’s risk of OASIS—namely, midline episiotomy and forceps delivery. In a quasirandomized study by Coats, involving 407 nulliparous women, which compared mediolateral and midline episiotomy (when episiotomy was necessary), tears into or through the anal sphincter occurred in 12% of women undergoing midline episiotomy and 2% of those undergoing mediolateral episiotomy.26
If operative vaginal delivery is required, vacuum extraction is preferred. In a meta-analysis of randomized studies, Thakar and Eason found that fewer women have anal sphincter trauma with vacuum delivery than with forceps.27 One anal sphincter tear is avoided for every 18 women delivered by vacuum extraction instead of forceps. A randomized trial conducted in the United Kingdom involving mediolateral episiotomy found severe vaginal laceration in 17% of forceps deliveries and 11% of vacuum deliveries.28 A randomized controlled trial in Canada involving midline episiotomy found third- or fourth-degree tears in 29% of forceps deliveries, versus 12% of vacuum deliveries.29
Q. What is the proper code for reporting an anal sphincter injury incurred in pregnancy?
A. That depends—on when the tear occurred, whether the patient is currently pregnant, and whether there were additional lacerations of the perineum.
ICD-9-CM offers four codes in this setting. Choose one, as follows:
- If you note an anal tear at the time of, or after, delivery but there is no perineal laceration, report 664.6×. This code takes a fifth digit: “1,” for the patient who has just delivered, or “4,” if you are treating the tear after she has been discharged.
- If the tear is noted in addition to a third-degree perineal tear, report 664.2× instead; fifth-digit choices for this code are also “1” and “4.”
- If the patient had an anal tear before delivery, from a prior pregnancy, code 654.8× [congenital or acquired abnormality of the vulva].
- Last, if you are treating the patient for an old anal tear and she is not pregnant at the moment, report 569.43 and add any additional codes that have resulted from the tear, such as fecal incontinence (787.6).
—Melanie Witt, RN, CPC-OGS, MA
1. Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:13-19.
2. Sultan AH, Thakar R. Third and fourth degree tears. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:33-51.
3. Sultan AH. Primary repair of obstetric anal sphincter injury. In: Staskin DR, Cardozo L, ed. Textbook of Female Urology and Urogynaecology. London: ISIS Medical Media; 2006.
4. Thakar R, Fenner DE. Anatomy of the perineum and the anal sphincter. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:1-12.
5. Faltin DL, Boulvain M, Floris LA, Irion O. Diagnosis of anal sphincter tears to prevent fecal incontinence: a randomized controlled trial. Obstet Gynecol. 2005;106:6-13.
6. Andrews V, Thakar R, Sultan AH. Occult anal sphincter injuries—myth or reality. Br J Obstet Gynaecol. 2006;113:195-200.
7. Sultan AH. Obstetric perineal injury and anal incontinence. Clin Risk. 1999;5:193-196.
8. Royal College of Obstetricians and Gynaecologists. Management of third and fourth degree perineal tears following vaginal delivery. Guideline 29. London: RCOG Press; 2001.
9. Norton C, Christensen J, Butler U, et al. Anal Incontinence. 2nd ed. Plymouth: Health Publication Ltd; 2005:985-1044.
10. Sultan AH, Thakar R. Lower genital tract and anal sphincter trauma. Best Pract Res Clin Obstet Gynaecol. 2002;16:99-116.
11. Williams A, Adams EJ, Tincello DG, Alfirevic Z, Walkinshaw SA, Richmond DH. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
12. Fernando RJ, Sultan AH, Kettle C, Radley S, Jones P, O’Brien PMS. Repair techniques for obstetric anal sphincter injuries. A randomized controlled trial. Obstet Gynecol. 2006;107:1261-1268.
13. Fernando R, Sultan AH, Kettle C, Thakar R, Radley S. Methods of repair for obstetric anal sphincter injury. Cochrane Database Syst Rev. 2006;3:CD002866.-
14. Fernando RJ, Sultan AH, Radley S, Jones PW, Johanson RB. Management of obstetric anal sphincter injury: a systematic review and national practice survey. BMC Health Serv Res. 2002;2:9.-
15. MacArthur AJ, MacArthur C. Incidence, severity, and determinants of perineal pain after vaginal delivery: a prospective cohort study. Am J Obstet Gynecol. 2004;191:1199-1204.
16. Hedayati H, Parsons J, Crowther CA. Rectal analgesia for pain from perineal trauma following childbirth. Cochrane Database Syst Rev. 2003;(3):CD003931.-
17. Kettle C, Hills RK, Jones P, Darby L, Gray R, Johanson R. Continuous versus interrupted perineal repair with standard or rapidly absorbed sutures after spontaneous vaginal birth: a randomised controlled trial. Lancet. 2002;359:2217-2223.
18. Sultan AH, Monga AK, Kumar D, Stanton SL. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
19. Mahony R, Behan M, O’Herlihy C, O’Connell PR. Randomized, clinical trial of bowel confinement vs. laxative use after primary repair of a third-degree obstetric anal sphincter tear. Dis Colon Rectum. 2004;47:12-17.
20. Thakar R, Sultan A. Postpartum problems and the role of a perineal clinic. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:65-79.
21. Williams A, Lavender T, Richmond DH, Tincello DG. Women’s experiences after a third-degree obstetric anal sphincter tear: a qualitative study. Birth. 2005;32:129-136.
22. Fynes M, Donnelly V, Behan M, O’Connell PR, O’Herlihy C. Effect of second vaginal delivery on anorectal physiology and faecal continence: a prospective study. Lancet. 1999;354:983-986.
23. Scheer I, Thakar R, Sultan A. Should women who sustained obstetric anal sphincter injuries be allowed a vaginal delivery? Neurourol Urodynam. 2006;25:512-513.
24. Peleg D, Kennedy CM, Merrill D, Zlatnik FJ. Risk of repetition of a severe perineal laceration. Obstet Gynecol. 1999;93:1021-1024.
25. Harkin R, Fitzpatrick M, O’Connell PR, O’Herlihy C. Anal sphincter disruption at vaginal delivery: is recurrence predictable? Eur J Obstet Gynaecol Reprod Biol. 2003;109:149-152.
26. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
27. Thakar R, Eason E. Prevention of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:52-64.
28. Johanson RB, Rice C, Doyle M. A randomised prospective study comparing the new vacuum extractor policy with forceps delivery. Br J Obstet Gynaecol. 1993;100:524-530.
29. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol. 1996;175:1325-1330.
The authors report no financial relationships relevant to this article.
CASE Large baby, extensive tear
A 28-year-old primigravida undergoes a forceps delivery with a midline episiotomy for failure to progress in the second stage of labor. At birth, the infant weighs 4 kg (8.8 lb), and the episiotomy extends to the anal verge. The resident who delivered the child is uncertain whether the anal sphincter is involved in the injury and asks a consultant to examine the perineum.
What should this examination entail?
The obstetrician is rarely culpable when a third- or fourth-degree obstetric anal sphincter injury (OASIS) occurs—but there is little excuse for letting one go undetected.
To minimize the risk of undiagnosed OASIS, a digital anorectal examination is warranted—before any suturing—in every woman who delivers vaginally. This practice can help you avoid missing isolated tears, such as “buttonhole” of the rectal mucosa, which can occur even when the anal sphincter remains intact (FIGURE 1), or a third- or fourth-degree tear that can sometimes be present behind apparently intact perineal skin (FIGURE 2).1
Clinical training of physicians and midwives also needs to improve.
Every labor room should have a protocol for management of anal sphincter injury2; this article describes detection, diagnosis, and management, focusing on seven critical questions.
Only a physician formally trained in primary anal sphincter repair (or under supervision) should repair OASIS.
FIGURE 1 Buttonhole tear
A buttonhole tear of the rectal mucosa (arrow) with an intact external anal sphincter (EAS) demonstrated during a digital rectal examination. SOURCE: Sultan AH3 (used with permission).
FIGURE 2 Injury obscured by intact skin
(A) Intact perineum on visual examination. (B) Anal sphincter trauma detected after rectal examination. SOURCE: Sultan AH, Kettle C1 (used with permission).
1. When (and how) should the torn perineum be examined?
The first requisite is informed consent for vaginal and rectal examination immediately after delivery. Also vital are adequate exposure of the perineum, good lighting, and, if necessary, sufficient analgesia to prevent pain-related restriction of the evaluation. It may be advisable to place the patient in the lithotomy position to improve exposure.
After visual examination of the perineum, part the labia and examine the vagina to establish the full extent of the tear. Always identify the apex of the vaginal laceration.
Next, perform a rectal examination to exclude injury to the anorectal mucosa and anal sphincter.3
Palpation is necessary to confirm OASIS
Insert the index finger into the anal canal and the thumb into the vagina and perform a pill-rolling motion to palpate the anal sphincter. If this technique is inconclusive, ask the woman to contract her anal sphincter with your fingers still in place. When the sphincter is disrupted, you feel a distinct gap anteriorly. If the perineal skin is intact, there may be an absence of puckering on the perianal skin over any underlying defect that may not be evident under regional or general anesthesia.
Because the external anal sphincter (EAS) is in a state of tonic contraction, the sphincter ends will retract when it is disrupted. These ends need to be grasped and retrieved at the time of repair.
Also identify the internal anal sphincter (IAS). It is a circular smooth muscle (FIGURE 3) that is paler in appearance (similar to the flesh of raw fish) than the striated EAS (similar to raw red meat).4 Under normal circumstances, the distal end of the IAS lies a few millimeters proximal to the distal end of the EAS (FIGURE 4). However, if the EAS is relaxed due to regional or general anesthesia, the distal end of the IAS will appear to be at a lower level. If the IAS or anal epithelium is torn, the EAS is, invariably, torn, too.
General or regional (spinal, epidural, caudal) anesthesia provides analgesia and muscle relaxation and enables proper evaluation of the full extent of the injury.
FIGURE 3 Grade 3b tear
Grade 3b tear with an intact internal anal sphincter (IAS). The external sphincter (EAS) is being grasped with Allis forceps. Note the difference in appearance of the paler IAS and darker EAS. SOURCE: Sultan AH, Kettle C1 (used with permission).
FIGURE 4 Classification of anal sphincter injury
First- and second-degree injuries are described below.
2. Is endoanal US helpful to detect OASIS?
Endoanal ultrasonography (US) to identify OASIS requires specific expertise, particularly in the immediate postpartum period, when the anal canal is lax (especially after an epidural). Ultimately, however, the diagnosis rests on clinical assessment and a rectal examination because, even if a defect is seen on US, it has to be clinically apparent to be repaired.
In a study by Faltin and colleagues, in which routine postpartum endoanal US was used as the gold standard for diagnosis of OASIS, five of 21 women had unnecessary intervention because the sonographic defect was not clinically visible despite exploration of the anal sphincter.5 As a result of this unnecessary exploration based on endoanal US, 20% of these women developed severe fecal incontinence. Therefore, we believe that OASIS is best detected clinically immediately after delivery, provided the physician performs a careful examination with palpation of the anal sphincter.6 In such a scenario, endoanal US is of limited value.
3. How is obstetric anal sphincter trauma classified?
To standardize the classification of perineal trauma, Sultan proposed the following system, which has been adopted by the Royal College of Obstetricians and Gynaecologists and internationally7-9:
First degree: Laceration of the vaginal epithelium or perineal skin only
Second degree: Involvement of the perineal muscles, but not the anal sphincter
Third degree: Disruption of the anal sphincter muscles (FIGURE 4):
- 3a: Less than 50% thickness of the external sphincter is torn
- 3b: More than 50% thickness of the external sphincter is torn
- 3c: Internal sphincter is also torn
Fourth degree: A third-degree anal tear with disruption of the anal epithelium (FIGURE 4).
If there is any ambiguity about grading of the injury, the higher grade should be selected. For example, if there is uncertainty between grades 3a and 3b, the injury should be classified as Grade 3b.
4. Is an operating room necessary?
OASIS should be repaired in the operating theater, where there is access to good lighting, appropriate equipment, and aseptic conditions. In our unit, we have a specially prepared instrument tray containing:
- a Weislander self-retaining retractor
- 4 Allis tissue forceps
- McIndoe scissors
- tooth forceps
- 4 artery forceps
- stitch scissors
- a needle holder.
In addition, deep retractors (e.g., Deavers) are useful when there are associated paravaginal tears.
5. What surgical technique is recommended?
Buttonhole injury
This type of injury can occur in the rectum without disrupting the anal sphincter or perineum. It is best repaired transvaginally using interrupted Vicryl (polyglactin) sutures.
To minimize the risk of persistent rectovaginal fistula, interpose a second layer of tissue between the rectum and vagina by approximating the rectovaginal fascia. A colostomy is rarely indicated unless a large tear extends above the pelvic floor or there is gross fecal contamination of the wound.
Fourth-degree tear
Repair torn anal epithelium with interrupted Vicryl 3-0 sutures, with the knots tied in the anal lumen. Proponents of this widely described technique argue that it reduces the quantity of foreign body (knots) within the tissue and lowers the risk of infection. Concern about a foreign body probably applies to the use of catgut, which dissolves by proteolysis, rather than to newer synthetic material such as Vicryl or Dexon (polyglycolic acid), which dissolves by hydrolysis.
Subcuticular repair of anal epithelium using a transvaginal approach has also been described and could be equally effective if the terminal knots are secure.10
Sphincter muscles
Repair these muscles using 3-0 polydioxanone (PDS) dyed sutures. Compared with braided sutures, monofilament sutures are believed to lessen the risk of infection, although a randomized controlled trial revealed no difference in suture-related morbidity between Vicryl and PDS at 6 weeks postpartum.11 Complete absorption of PDS takes longer than with Vicryl, with 50% tensile strength lasting more than 3 months, compared with 3 weeks for Vicryl.11 To minimize suture migration, cut suture ends short and ensure that they are covered by the overlying superficial perineal muscles.
Internal anal sphincter. Repair the IAS separately from the EAS. Grasp the ends of the torn muscle using Allis forceps and perform an end-to-end repair with interrupted or mattress 3-0 PDS sutures (FIGURE 5). Overlapping repair can be technically difficult.
There is some evidence that repair of an isolated IAS defect benefits patients with established anal incontinence.
External anal sphincter. Because the EAS is normally under tonic contraction, it tends to retract when torn. Therefore, repair requires identification and grasping of the torn ends using Allis tissue forceps (FIGURE 6).
When the EAS is only partially torn (Grade 3a and some cases of Grade 3b), perform an end-to end repair using 2 or 3 mattress sutures, similar to repair of IAS injury, instead of hemostatic “figure of eight” sutures.
For a full-thickness tear (some cases of Grade 3b or 3c, or Grade 4), overlapping repair may be preferable in experienced hands. The EAS may need to be mobilized by dissecting it free of the ischioanal fat laterally using a pair of McIndoe scissors. The torn ends of the EAS can then be overlapped in “double-breasted” fashion (FIGURE 7) using PDS 3-0 sutures. Proper overlap is possible only when the full length of the torn ends is identified.
Overlapping the ends of the sphincter allows for greater surface area of contact between muscle. In contrast, end-to-end repair can be performed without identifying the full length of the EAS and may give rise to incomplete apposition. Fernando and colleagues demonstrated that, in experienced hands, early primary overlap repair carries a lower risk of fecal urgency and anal incontinence than does immediate primary end-to-end repair.12,13
FIGURE 5 End-to-end repair
Internal anal sphincter (I) repair using mattress sutures, demonstrated on the latex Sultan model, used for training (www.perineum.net) (E, external sphincter; A, anal epithelium). SOURCE: Sultan AH, Thakar R2 (used with permission).
FIGURE 6 Locating the external anal sphincter
The external sphincter (E), grasped with Allis forceps, is surrounded by the capsule (C) and lies medial to the ischioanal fat. SOURCE: Sultan AH, Thakar R2 (used with permission).
FIGURE 7 Overlapping sphincter repair
Repair of a fourth degree tear (demonstrated on the Sultan model) using the overlap repair technique on the external sphincter (E). The anal epithelium (A) and the internal sphincter (I) have also been repaired. SOURCE: Sultan AH, Thakar R2 (used with permission).
Perineal muscles
After repair of the sphincter, suture the perineal muscles to reconstruct the perineal body and provide support to the repaired anal sphincter. A short, deficient perineum would leave the anal sphincter more vulnerable to trauma during a subsequent vaginal delivery.
Next, suture the vaginal skin and approximate the perineal skin using Vicryl Rapide 2-0 subcuticular suture.
Examine, and document, the repair
Perform a rectal and vaginal examination to confirm adequate repair and ensure that no other tears have been missed—and that all tampons or swabs have been removed.
Make detailed notes of the findings and repair. A pro forma pictorial representation of the tears proves very useful when notes are reviewed following complications or during audit or litigation.
6. What does postoperative care entail?
Prophylactic antibiotics are common
No randomized trials have substantiated the benefits of intraoperative and postoperative antibiotics after repair of OASIS. Nevertheless, these drugs are commonly prescribed, especially after fourth- degree tears, because infection and wound breakdown could jeopardize the repair and lead to incontinence or fistula.10,14
We prescribe intravenous broad-spectrum antibiotics such as cefuroxime and metronidazole intraoperatively and continue the drugs orally for 5 days.
Bladder catheterization is recommended
Severe perineal discomfort, especially after instrumental delivery, is a known cause of urinary retention. Moreover, after administration of regional anesthesia, it can take up to 12 hours before bladder sensation returns.
We recommend insertion of a Foley catheter for approximately 24 hours, unless medical staff can ensure that spontaneous voiding occurs at least every 3 to 4 hours without bladder overdistension.
Pain may persist after severe injury
The degree of pain following perineal trauma is related to the extent of the injury. OASIS is frequently associated with other more extensive injuries such as paravaginal tears. In one study, 91% of women continued to complain of severe perineal pain 7 days after OASIS.15
In a systematic review, Hedayati and associates found rectal analgesia, such as diclofenac sodium, to be effective at reducing pain from perineal trauma within the first 24 hours after birth; they also found that women used less additional analgesia within the first 48 hours after birth.16 Diclofenac is almost completely bound to protein, so excretion in breast milk is negligible.17
In women who have undergone repair of a fourth-degree tear, administer oral diclofenac; suppositories may be uncomfortable, and there is a theoretical risk of poor healing associated with local anti-inflammatory agents.
Avoid codeine-based preparations because they may cause constipation and lead to excessive straining and disruption of the repair.
Recommend a stool softener
It is vital that constipation be avoided as the patient heals; passage of constipated stool or fecal impaction can disrupt the repair. We prescribe a stool softener (lactulose, 15 mL twice daily) for 10 to 14 days and have encountered no problem with bowel evacuation.18
We recommend that the patient telephone a healthcare provider 24 to 48 hours after hospital discharge to confirm that bowel evacuation has occurred. If it hasn’t, we add mineral oil, magnesium hydroxide, or another oral bowel stimulant to the stool softener and bulking agent.
Mahoney and colleagues conducted a randomized trial (n=105) of constipating versus laxative regimens and found the latter to be associated with earlier and less painful first bowel motion and earlier hospital discharge.19 Nineteen percent of women following the constipating regimen had troublesome constipation (two required hospitalization for fecal impaction), compared with 5% of women receiving a laxative. There were no significant differences in continence scores, anal manometry, and endoanal US findings.
Give the patient adequate information
Before the patient is discharged from the hospital, we give her a booklet that describes the implications of OASIS and explains when and where to seek help if symptoms of infection or incontinence develop. All women also complete a validated bowel-health and quality-of-life questionnaire regarding conditions prior to the delivery. We also recommend pelvic floor and anal sphincter exercises as soon as her discomfort resolves.
Perform a comprehensive follow-up exam
All women who sustain OASIS should be assessed by a senior obstetrician 6 to 8 weeks after delivery. In our practice, these women are seen in a dedicated perineal clinic.20 The clinic provides a supportive environment and increases the patient’s confidence in the team.21
At the clinic, each woman completes the same symptom questionnaire that she was given before hospital discharge. She then undergoes a genital examination in which the physician checks the degree of scarring, residual granulation tissue, and tenderness; ensures that the patient understands the circumstances surrounding the delivery and injury; and addresses any concerns. All women then undergo anal manometry and endoanal US (FIGURE 8). Each patient is encouraged to continue pelvic floor exercises. If she has minimal sphincter contractility, she may need electrical stimulation.
If a dedicated perineal clinic is unavailable, the patient should be given clear instructions, preferably in writing, before leaving the hospital. During the 6 weeks immediately after delivery, she should be instructed to look for signs of infection or wound dehiscence and to telephone the physician to report any increase in pain or swelling, rectal bleeding, or purulent discharge. Any incontinence of stool or flatus also should be reported.
FIGURE 8 Defect visible on US
Endoanal sonogram showing a defect in the external anal sphincter between 11 o’clock and 1 o’clock (between the yellow arrows) (S, subepithelium; E, external anal sphincter). SOURCE: Sultan AH, Thakar R2 (used with permission).
7. Is vaginal delivery advisable after OASIS?
No randomized trials have determined the most appropriate mode of delivery after a third- or fourth-degree tear. We base our counseling of the patient on a completed symptom questionnaire and findings from manometry and endoanal US (FIGURE 8). If vaginal delivery is contemplated, these tests should be performed during the current pregnancy unless they were abnormal at an earlier date. FIGURE 9 is a simple flow diagram from our unit that illustrates management of subsequent delivery after OASIS.
When determining the mode of delivery, thorough counseling and clear documentation of that counseling are extremely important.
FIGURE 9 How do you determine the mode of delivery after OASIS?
Vaginal delivery is possible unless anal sphincter function is impaired
One study found that when a large sonographic defect (more than one quadrant) is present, or the squeeze-pressure increment (above resting pressure) is less than 20 mm Hg, the risk of impaired continence after a subsequent delivery increases dramatically.22
Based on these findings, we conducted a prospective study that found no deterioration of sphincter function or increase in symptoms after vaginal delivery unless the patient had significant compromise of anal sphincter function before the pregnancy.23 Therefore, we encourage asymptomatic women who have minimal compromise of anal sphincter function to undergo vaginal delivery.
Routine episiotomy is not protective
There is no evidence that routine episiotomy prevents recurrent OASIS. If episiotomy is deemed to be necessary—e.g., for a thick inelastic or scarred perineum—mediolateral episiotomy is preferred.
High likelihood of success in some women
Women who have minimal compromise of anal sphincter function should be counseled that they have an 88% (in centers practicing midline episiotomy) to 95% (in centers practicing mediolateral episiotomy) chance of delivering without sustaining another OASIS.24,25 This should reassure them if they have misgivings about vaginal delivery.
Threshold for C-section is lower if additional risk factors are present
If traumatic delivery is anticipated, as in the presence of one or more additional risk factors (macrosomia, shoulder dystocia, prolonged labor, difficult instrumental delivery), cesarean section may be appropriate.
Consider emotional needs
Some women who have sustained OASIS may be scarred emotionally as well as physically and may find it difficult to cope with the thought of another vaginal delivery. These women deserve sympathy, psychological support, and consideration of their request for cesarean section.
When cesarean is a good idea
Women who have a minor degree of incontinence (e.g., fecal urgency or flatus incontinence) may be managed with dietary advice, constipating agents (loperamide or codeine phosphate), and physiotherapy or biofeedback. These women who have some degree of anal sphincter compromise but whose symptoms are controlled should be counseled that cesarean delivery is recommended (FIGURE 9).
Women who have sustained a previous third- or fourth-degree tear with subsequent severe incontinence should be offered secondary sphincter repair by a colorectal surgeon or urogynecologist with expertise in secondary sphincter repair. All subsequent deliveries by these women should be by cesarean section.
Some women with fecal incontinence may choose to complete their family before embarking on anal sphincter surgery. It remains unclear whether these women should be allowed a vaginal delivery, but it is likely that most damage has already occurred and that the risk of further injury is minimal and possibly insignificant. The benefit of cesarean delivery, if any, should be weighed against its risks for all subsequent pregnancies.
Women who have undergone a previous successful secondary sphincter repair for fecal incontinence should be delivered by cesarean delivery.9
Not all women fit neatly into one category
There are going to be women who do not entirely fit any of the categories described—such as those who have isolated internal sphincter defects or irritable bowel syndrome. Management of these women should be individualized, with the mode of delivery determined by mutual agreement after taking into account symptoms and clinical and other findings.
If there are no facilities for anal manometry and US, the physician should base management on symptoms and clinical evaluation. Asymptomatic women who do not have clinical evidence of sphincter compromise during anal tone assessment may be allowed to undergo vaginal delivery. All women who are symptomatic should be referred to a center with facilities for anorectal assessment to establish the ideal management and mode of delivery.
Pay attention to modifiable risk factors
In the case described at the beginning of this article, two risk factors could have been modified to minimize the patient’s risk of OASIS—namely, midline episiotomy and forceps delivery. In a quasirandomized study by Coats, involving 407 nulliparous women, which compared mediolateral and midline episiotomy (when episiotomy was necessary), tears into or through the anal sphincter occurred in 12% of women undergoing midline episiotomy and 2% of those undergoing mediolateral episiotomy.26
If operative vaginal delivery is required, vacuum extraction is preferred. In a meta-analysis of randomized studies, Thakar and Eason found that fewer women have anal sphincter trauma with vacuum delivery than with forceps.27 One anal sphincter tear is avoided for every 18 women delivered by vacuum extraction instead of forceps. A randomized trial conducted in the United Kingdom involving mediolateral episiotomy found severe vaginal laceration in 17% of forceps deliveries and 11% of vacuum deliveries.28 A randomized controlled trial in Canada involving midline episiotomy found third- or fourth-degree tears in 29% of forceps deliveries, versus 12% of vacuum deliveries.29
Q. What is the proper code for reporting an anal sphincter injury incurred in pregnancy?
A. That depends—on when the tear occurred, whether the patient is currently pregnant, and whether there were additional lacerations of the perineum.
ICD-9-CM offers four codes in this setting. Choose one, as follows:
- If you note an anal tear at the time of, or after, delivery but there is no perineal laceration, report 664.6×. This code takes a fifth digit: “1,” for the patient who has just delivered, or “4,” if you are treating the tear after she has been discharged.
- If the tear is noted in addition to a third-degree perineal tear, report 664.2× instead; fifth-digit choices for this code are also “1” and “4.”
- If the patient had an anal tear before delivery, from a prior pregnancy, code 654.8× [congenital or acquired abnormality of the vulva].
- Last, if you are treating the patient for an old anal tear and she is not pregnant at the moment, report 569.43 and add any additional codes that have resulted from the tear, such as fecal incontinence (787.6).
—Melanie Witt, RN, CPC-OGS, MA
The authors report no financial relationships relevant to this article.
CASE Large baby, extensive tear
A 28-year-old primigravida undergoes a forceps delivery with a midline episiotomy for failure to progress in the second stage of labor. At birth, the infant weighs 4 kg (8.8 lb), and the episiotomy extends to the anal verge. The resident who delivered the child is uncertain whether the anal sphincter is involved in the injury and asks a consultant to examine the perineum.
What should this examination entail?
The obstetrician is rarely culpable when a third- or fourth-degree obstetric anal sphincter injury (OASIS) occurs—but there is little excuse for letting one go undetected.
To minimize the risk of undiagnosed OASIS, a digital anorectal examination is warranted—before any suturing—in every woman who delivers vaginally. This practice can help you avoid missing isolated tears, such as “buttonhole” of the rectal mucosa, which can occur even when the anal sphincter remains intact (FIGURE 1), or a third- or fourth-degree tear that can sometimes be present behind apparently intact perineal skin (FIGURE 2).1
Clinical training of physicians and midwives also needs to improve.
Every labor room should have a protocol for management of anal sphincter injury2; this article describes detection, diagnosis, and management, focusing on seven critical questions.
Only a physician formally trained in primary anal sphincter repair (or under supervision) should repair OASIS.
FIGURE 1 Buttonhole tear
A buttonhole tear of the rectal mucosa (arrow) with an intact external anal sphincter (EAS) demonstrated during a digital rectal examination. SOURCE: Sultan AH3 (used with permission).
FIGURE 2 Injury obscured by intact skin
(A) Intact perineum on visual examination. (B) Anal sphincter trauma detected after rectal examination. SOURCE: Sultan AH, Kettle C1 (used with permission).
1. When (and how) should the torn perineum be examined?
The first requisite is informed consent for vaginal and rectal examination immediately after delivery. Also vital are adequate exposure of the perineum, good lighting, and, if necessary, sufficient analgesia to prevent pain-related restriction of the evaluation. It may be advisable to place the patient in the lithotomy position to improve exposure.
After visual examination of the perineum, part the labia and examine the vagina to establish the full extent of the tear. Always identify the apex of the vaginal laceration.
Next, perform a rectal examination to exclude injury to the anorectal mucosa and anal sphincter.3
Palpation is necessary to confirm OASIS
Insert the index finger into the anal canal and the thumb into the vagina and perform a pill-rolling motion to palpate the anal sphincter. If this technique is inconclusive, ask the woman to contract her anal sphincter with your fingers still in place. When the sphincter is disrupted, you feel a distinct gap anteriorly. If the perineal skin is intact, there may be an absence of puckering on the perianal skin over any underlying defect that may not be evident under regional or general anesthesia.
Because the external anal sphincter (EAS) is in a state of tonic contraction, the sphincter ends will retract when it is disrupted. These ends need to be grasped and retrieved at the time of repair.
Also identify the internal anal sphincter (IAS). It is a circular smooth muscle (FIGURE 3) that is paler in appearance (similar to the flesh of raw fish) than the striated EAS (similar to raw red meat).4 Under normal circumstances, the distal end of the IAS lies a few millimeters proximal to the distal end of the EAS (FIGURE 4). However, if the EAS is relaxed due to regional or general anesthesia, the distal end of the IAS will appear to be at a lower level. If the IAS or anal epithelium is torn, the EAS is, invariably, torn, too.
General or regional (spinal, epidural, caudal) anesthesia provides analgesia and muscle relaxation and enables proper evaluation of the full extent of the injury.
FIGURE 3 Grade 3b tear
Grade 3b tear with an intact internal anal sphincter (IAS). The external sphincter (EAS) is being grasped with Allis forceps. Note the difference in appearance of the paler IAS and darker EAS. SOURCE: Sultan AH, Kettle C1 (used with permission).
FIGURE 4 Classification of anal sphincter injury
First- and second-degree injuries are described below.
2. Is endoanal US helpful to detect OASIS?
Endoanal ultrasonography (US) to identify OASIS requires specific expertise, particularly in the immediate postpartum period, when the anal canal is lax (especially after an epidural). Ultimately, however, the diagnosis rests on clinical assessment and a rectal examination because, even if a defect is seen on US, it has to be clinically apparent to be repaired.
In a study by Faltin and colleagues, in which routine postpartum endoanal US was used as the gold standard for diagnosis of OASIS, five of 21 women had unnecessary intervention because the sonographic defect was not clinically visible despite exploration of the anal sphincter.5 As a result of this unnecessary exploration based on endoanal US, 20% of these women developed severe fecal incontinence. Therefore, we believe that OASIS is best detected clinically immediately after delivery, provided the physician performs a careful examination with palpation of the anal sphincter.6 In such a scenario, endoanal US is of limited value.
3. How is obstetric anal sphincter trauma classified?
To standardize the classification of perineal trauma, Sultan proposed the following system, which has been adopted by the Royal College of Obstetricians and Gynaecologists and internationally7-9:
First degree: Laceration of the vaginal epithelium or perineal skin only
Second degree: Involvement of the perineal muscles, but not the anal sphincter
Third degree: Disruption of the anal sphincter muscles (FIGURE 4):
- 3a: Less than 50% thickness of the external sphincter is torn
- 3b: More than 50% thickness of the external sphincter is torn
- 3c: Internal sphincter is also torn
Fourth degree: A third-degree anal tear with disruption of the anal epithelium (FIGURE 4).
If there is any ambiguity about grading of the injury, the higher grade should be selected. For example, if there is uncertainty between grades 3a and 3b, the injury should be classified as Grade 3b.
4. Is an operating room necessary?
OASIS should be repaired in the operating theater, where there is access to good lighting, appropriate equipment, and aseptic conditions. In our unit, we have a specially prepared instrument tray containing:
- a Weislander self-retaining retractor
- 4 Allis tissue forceps
- McIndoe scissors
- tooth forceps
- 4 artery forceps
- stitch scissors
- a needle holder.
In addition, deep retractors (e.g., Deavers) are useful when there are associated paravaginal tears.
5. What surgical technique is recommended?
Buttonhole injury
This type of injury can occur in the rectum without disrupting the anal sphincter or perineum. It is best repaired transvaginally using interrupted Vicryl (polyglactin) sutures.
To minimize the risk of persistent rectovaginal fistula, interpose a second layer of tissue between the rectum and vagina by approximating the rectovaginal fascia. A colostomy is rarely indicated unless a large tear extends above the pelvic floor or there is gross fecal contamination of the wound.
Fourth-degree tear
Repair torn anal epithelium with interrupted Vicryl 3-0 sutures, with the knots tied in the anal lumen. Proponents of this widely described technique argue that it reduces the quantity of foreign body (knots) within the tissue and lowers the risk of infection. Concern about a foreign body probably applies to the use of catgut, which dissolves by proteolysis, rather than to newer synthetic material such as Vicryl or Dexon (polyglycolic acid), which dissolves by hydrolysis.
Subcuticular repair of anal epithelium using a transvaginal approach has also been described and could be equally effective if the terminal knots are secure.10
Sphincter muscles
Repair these muscles using 3-0 polydioxanone (PDS) dyed sutures. Compared with braided sutures, monofilament sutures are believed to lessen the risk of infection, although a randomized controlled trial revealed no difference in suture-related morbidity between Vicryl and PDS at 6 weeks postpartum.11 Complete absorption of PDS takes longer than with Vicryl, with 50% tensile strength lasting more than 3 months, compared with 3 weeks for Vicryl.11 To minimize suture migration, cut suture ends short and ensure that they are covered by the overlying superficial perineal muscles.
Internal anal sphincter. Repair the IAS separately from the EAS. Grasp the ends of the torn muscle using Allis forceps and perform an end-to-end repair with interrupted or mattress 3-0 PDS sutures (FIGURE 5). Overlapping repair can be technically difficult.
There is some evidence that repair of an isolated IAS defect benefits patients with established anal incontinence.
External anal sphincter. Because the EAS is normally under tonic contraction, it tends to retract when torn. Therefore, repair requires identification and grasping of the torn ends using Allis tissue forceps (FIGURE 6).
When the EAS is only partially torn (Grade 3a and some cases of Grade 3b), perform an end-to end repair using 2 or 3 mattress sutures, similar to repair of IAS injury, instead of hemostatic “figure of eight” sutures.
For a full-thickness tear (some cases of Grade 3b or 3c, or Grade 4), overlapping repair may be preferable in experienced hands. The EAS may need to be mobilized by dissecting it free of the ischioanal fat laterally using a pair of McIndoe scissors. The torn ends of the EAS can then be overlapped in “double-breasted” fashion (FIGURE 7) using PDS 3-0 sutures. Proper overlap is possible only when the full length of the torn ends is identified.
Overlapping the ends of the sphincter allows for greater surface area of contact between muscle. In contrast, end-to-end repair can be performed without identifying the full length of the EAS and may give rise to incomplete apposition. Fernando and colleagues demonstrated that, in experienced hands, early primary overlap repair carries a lower risk of fecal urgency and anal incontinence than does immediate primary end-to-end repair.12,13
FIGURE 5 End-to-end repair
Internal anal sphincter (I) repair using mattress sutures, demonstrated on the latex Sultan model, used for training (www.perineum.net) (E, external sphincter; A, anal epithelium). SOURCE: Sultan AH, Thakar R2 (used with permission).
FIGURE 6 Locating the external anal sphincter
The external sphincter (E), grasped with Allis forceps, is surrounded by the capsule (C) and lies medial to the ischioanal fat. SOURCE: Sultan AH, Thakar R2 (used with permission).
FIGURE 7 Overlapping sphincter repair
Repair of a fourth degree tear (demonstrated on the Sultan model) using the overlap repair technique on the external sphincter (E). The anal epithelium (A) and the internal sphincter (I) have also been repaired. SOURCE: Sultan AH, Thakar R2 (used with permission).
Perineal muscles
After repair of the sphincter, suture the perineal muscles to reconstruct the perineal body and provide support to the repaired anal sphincter. A short, deficient perineum would leave the anal sphincter more vulnerable to trauma during a subsequent vaginal delivery.
Next, suture the vaginal skin and approximate the perineal skin using Vicryl Rapide 2-0 subcuticular suture.
Examine, and document, the repair
Perform a rectal and vaginal examination to confirm adequate repair and ensure that no other tears have been missed—and that all tampons or swabs have been removed.
Make detailed notes of the findings and repair. A pro forma pictorial representation of the tears proves very useful when notes are reviewed following complications or during audit or litigation.
6. What does postoperative care entail?
Prophylactic antibiotics are common
No randomized trials have substantiated the benefits of intraoperative and postoperative antibiotics after repair of OASIS. Nevertheless, these drugs are commonly prescribed, especially after fourth- degree tears, because infection and wound breakdown could jeopardize the repair and lead to incontinence or fistula.10,14
We prescribe intravenous broad-spectrum antibiotics such as cefuroxime and metronidazole intraoperatively and continue the drugs orally for 5 days.
Bladder catheterization is recommended
Severe perineal discomfort, especially after instrumental delivery, is a known cause of urinary retention. Moreover, after administration of regional anesthesia, it can take up to 12 hours before bladder sensation returns.
We recommend insertion of a Foley catheter for approximately 24 hours, unless medical staff can ensure that spontaneous voiding occurs at least every 3 to 4 hours without bladder overdistension.
Pain may persist after severe injury
The degree of pain following perineal trauma is related to the extent of the injury. OASIS is frequently associated with other more extensive injuries such as paravaginal tears. In one study, 91% of women continued to complain of severe perineal pain 7 days after OASIS.15
In a systematic review, Hedayati and associates found rectal analgesia, such as diclofenac sodium, to be effective at reducing pain from perineal trauma within the first 24 hours after birth; they also found that women used less additional analgesia within the first 48 hours after birth.16 Diclofenac is almost completely bound to protein, so excretion in breast milk is negligible.17
In women who have undergone repair of a fourth-degree tear, administer oral diclofenac; suppositories may be uncomfortable, and there is a theoretical risk of poor healing associated with local anti-inflammatory agents.
Avoid codeine-based preparations because they may cause constipation and lead to excessive straining and disruption of the repair.
Recommend a stool softener
It is vital that constipation be avoided as the patient heals; passage of constipated stool or fecal impaction can disrupt the repair. We prescribe a stool softener (lactulose, 15 mL twice daily) for 10 to 14 days and have encountered no problem with bowel evacuation.18
We recommend that the patient telephone a healthcare provider 24 to 48 hours after hospital discharge to confirm that bowel evacuation has occurred. If it hasn’t, we add mineral oil, magnesium hydroxide, or another oral bowel stimulant to the stool softener and bulking agent.
Mahoney and colleagues conducted a randomized trial (n=105) of constipating versus laxative regimens and found the latter to be associated with earlier and less painful first bowel motion and earlier hospital discharge.19 Nineteen percent of women following the constipating regimen had troublesome constipation (two required hospitalization for fecal impaction), compared with 5% of women receiving a laxative. There were no significant differences in continence scores, anal manometry, and endoanal US findings.
Give the patient adequate information
Before the patient is discharged from the hospital, we give her a booklet that describes the implications of OASIS and explains when and where to seek help if symptoms of infection or incontinence develop. All women also complete a validated bowel-health and quality-of-life questionnaire regarding conditions prior to the delivery. We also recommend pelvic floor and anal sphincter exercises as soon as her discomfort resolves.
Perform a comprehensive follow-up exam
All women who sustain OASIS should be assessed by a senior obstetrician 6 to 8 weeks after delivery. In our practice, these women are seen in a dedicated perineal clinic.20 The clinic provides a supportive environment and increases the patient’s confidence in the team.21
At the clinic, each woman completes the same symptom questionnaire that she was given before hospital discharge. She then undergoes a genital examination in which the physician checks the degree of scarring, residual granulation tissue, and tenderness; ensures that the patient understands the circumstances surrounding the delivery and injury; and addresses any concerns. All women then undergo anal manometry and endoanal US (FIGURE 8). Each patient is encouraged to continue pelvic floor exercises. If she has minimal sphincter contractility, she may need electrical stimulation.
If a dedicated perineal clinic is unavailable, the patient should be given clear instructions, preferably in writing, before leaving the hospital. During the 6 weeks immediately after delivery, she should be instructed to look for signs of infection or wound dehiscence and to telephone the physician to report any increase in pain or swelling, rectal bleeding, or purulent discharge. Any incontinence of stool or flatus also should be reported.
FIGURE 8 Defect visible on US
Endoanal sonogram showing a defect in the external anal sphincter between 11 o’clock and 1 o’clock (between the yellow arrows) (S, subepithelium; E, external anal sphincter). SOURCE: Sultan AH, Thakar R2 (used with permission).
7. Is vaginal delivery advisable after OASIS?
No randomized trials have determined the most appropriate mode of delivery after a third- or fourth-degree tear. We base our counseling of the patient on a completed symptom questionnaire and findings from manometry and endoanal US (FIGURE 8). If vaginal delivery is contemplated, these tests should be performed during the current pregnancy unless they were abnormal at an earlier date. FIGURE 9 is a simple flow diagram from our unit that illustrates management of subsequent delivery after OASIS.
When determining the mode of delivery, thorough counseling and clear documentation of that counseling are extremely important.
FIGURE 9 How do you determine the mode of delivery after OASIS?
Vaginal delivery is possible unless anal sphincter function is impaired
One study found that when a large sonographic defect (more than one quadrant) is present, or the squeeze-pressure increment (above resting pressure) is less than 20 mm Hg, the risk of impaired continence after a subsequent delivery increases dramatically.22
Based on these findings, we conducted a prospective study that found no deterioration of sphincter function or increase in symptoms after vaginal delivery unless the patient had significant compromise of anal sphincter function before the pregnancy.23 Therefore, we encourage asymptomatic women who have minimal compromise of anal sphincter function to undergo vaginal delivery.
Routine episiotomy is not protective
There is no evidence that routine episiotomy prevents recurrent OASIS. If episiotomy is deemed to be necessary—e.g., for a thick inelastic or scarred perineum—mediolateral episiotomy is preferred.
High likelihood of success in some women
Women who have minimal compromise of anal sphincter function should be counseled that they have an 88% (in centers practicing midline episiotomy) to 95% (in centers practicing mediolateral episiotomy) chance of delivering without sustaining another OASIS.24,25 This should reassure them if they have misgivings about vaginal delivery.
Threshold for C-section is lower if additional risk factors are present
If traumatic delivery is anticipated, as in the presence of one or more additional risk factors (macrosomia, shoulder dystocia, prolonged labor, difficult instrumental delivery), cesarean section may be appropriate.
Consider emotional needs
Some women who have sustained OASIS may be scarred emotionally as well as physically and may find it difficult to cope with the thought of another vaginal delivery. These women deserve sympathy, psychological support, and consideration of their request for cesarean section.
When cesarean is a good idea
Women who have a minor degree of incontinence (e.g., fecal urgency or flatus incontinence) may be managed with dietary advice, constipating agents (loperamide or codeine phosphate), and physiotherapy or biofeedback. These women who have some degree of anal sphincter compromise but whose symptoms are controlled should be counseled that cesarean delivery is recommended (FIGURE 9).
Women who have sustained a previous third- or fourth-degree tear with subsequent severe incontinence should be offered secondary sphincter repair by a colorectal surgeon or urogynecologist with expertise in secondary sphincter repair. All subsequent deliveries by these women should be by cesarean section.
Some women with fecal incontinence may choose to complete their family before embarking on anal sphincter surgery. It remains unclear whether these women should be allowed a vaginal delivery, but it is likely that most damage has already occurred and that the risk of further injury is minimal and possibly insignificant. The benefit of cesarean delivery, if any, should be weighed against its risks for all subsequent pregnancies.
Women who have undergone a previous successful secondary sphincter repair for fecal incontinence should be delivered by cesarean delivery.9
Not all women fit neatly into one category
There are going to be women who do not entirely fit any of the categories described—such as those who have isolated internal sphincter defects or irritable bowel syndrome. Management of these women should be individualized, with the mode of delivery determined by mutual agreement after taking into account symptoms and clinical and other findings.
If there are no facilities for anal manometry and US, the physician should base management on symptoms and clinical evaluation. Asymptomatic women who do not have clinical evidence of sphincter compromise during anal tone assessment may be allowed to undergo vaginal delivery. All women who are symptomatic should be referred to a center with facilities for anorectal assessment to establish the ideal management and mode of delivery.
Pay attention to modifiable risk factors
In the case described at the beginning of this article, two risk factors could have been modified to minimize the patient’s risk of OASIS—namely, midline episiotomy and forceps delivery. In a quasirandomized study by Coats, involving 407 nulliparous women, which compared mediolateral and midline episiotomy (when episiotomy was necessary), tears into or through the anal sphincter occurred in 12% of women undergoing midline episiotomy and 2% of those undergoing mediolateral episiotomy.26
If operative vaginal delivery is required, vacuum extraction is preferred. In a meta-analysis of randomized studies, Thakar and Eason found that fewer women have anal sphincter trauma with vacuum delivery than with forceps.27 One anal sphincter tear is avoided for every 18 women delivered by vacuum extraction instead of forceps. A randomized trial conducted in the United Kingdom involving mediolateral episiotomy found severe vaginal laceration in 17% of forceps deliveries and 11% of vacuum deliveries.28 A randomized controlled trial in Canada involving midline episiotomy found third- or fourth-degree tears in 29% of forceps deliveries, versus 12% of vacuum deliveries.29
Q. What is the proper code for reporting an anal sphincter injury incurred in pregnancy?
A. That depends—on when the tear occurred, whether the patient is currently pregnant, and whether there were additional lacerations of the perineum.
ICD-9-CM offers four codes in this setting. Choose one, as follows:
- If you note an anal tear at the time of, or after, delivery but there is no perineal laceration, report 664.6×. This code takes a fifth digit: “1,” for the patient who has just delivered, or “4,” if you are treating the tear after she has been discharged.
- If the tear is noted in addition to a third-degree perineal tear, report 664.2× instead; fifth-digit choices for this code are also “1” and “4.”
- If the patient had an anal tear before delivery, from a prior pregnancy, code 654.8× [congenital or acquired abnormality of the vulva].
- Last, if you are treating the patient for an old anal tear and she is not pregnant at the moment, report 569.43 and add any additional codes that have resulted from the tear, such as fecal incontinence (787.6).
—Melanie Witt, RN, CPC-OGS, MA
1. Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:13-19.
2. Sultan AH, Thakar R. Third and fourth degree tears. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:33-51.
3. Sultan AH. Primary repair of obstetric anal sphincter injury. In: Staskin DR, Cardozo L, ed. Textbook of Female Urology and Urogynaecology. London: ISIS Medical Media; 2006.
4. Thakar R, Fenner DE. Anatomy of the perineum and the anal sphincter. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:1-12.
5. Faltin DL, Boulvain M, Floris LA, Irion O. Diagnosis of anal sphincter tears to prevent fecal incontinence: a randomized controlled trial. Obstet Gynecol. 2005;106:6-13.
6. Andrews V, Thakar R, Sultan AH. Occult anal sphincter injuries—myth or reality. Br J Obstet Gynaecol. 2006;113:195-200.
7. Sultan AH. Obstetric perineal injury and anal incontinence. Clin Risk. 1999;5:193-196.
8. Royal College of Obstetricians and Gynaecologists. Management of third and fourth degree perineal tears following vaginal delivery. Guideline 29. London: RCOG Press; 2001.
9. Norton C, Christensen J, Butler U, et al. Anal Incontinence. 2nd ed. Plymouth: Health Publication Ltd; 2005:985-1044.
10. Sultan AH, Thakar R. Lower genital tract and anal sphincter trauma. Best Pract Res Clin Obstet Gynaecol. 2002;16:99-116.
11. Williams A, Adams EJ, Tincello DG, Alfirevic Z, Walkinshaw SA, Richmond DH. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
12. Fernando RJ, Sultan AH, Kettle C, Radley S, Jones P, O’Brien PMS. Repair techniques for obstetric anal sphincter injuries. A randomized controlled trial. Obstet Gynecol. 2006;107:1261-1268.
13. Fernando R, Sultan AH, Kettle C, Thakar R, Radley S. Methods of repair for obstetric anal sphincter injury. Cochrane Database Syst Rev. 2006;3:CD002866.-
14. Fernando RJ, Sultan AH, Radley S, Jones PW, Johanson RB. Management of obstetric anal sphincter injury: a systematic review and national practice survey. BMC Health Serv Res. 2002;2:9.-
15. MacArthur AJ, MacArthur C. Incidence, severity, and determinants of perineal pain after vaginal delivery: a prospective cohort study. Am J Obstet Gynecol. 2004;191:1199-1204.
16. Hedayati H, Parsons J, Crowther CA. Rectal analgesia for pain from perineal trauma following childbirth. Cochrane Database Syst Rev. 2003;(3):CD003931.-
17. Kettle C, Hills RK, Jones P, Darby L, Gray R, Johanson R. Continuous versus interrupted perineal repair with standard or rapidly absorbed sutures after spontaneous vaginal birth: a randomised controlled trial. Lancet. 2002;359:2217-2223.
18. Sultan AH, Monga AK, Kumar D, Stanton SL. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
19. Mahony R, Behan M, O’Herlihy C, O’Connell PR. Randomized, clinical trial of bowel confinement vs. laxative use after primary repair of a third-degree obstetric anal sphincter tear. Dis Colon Rectum. 2004;47:12-17.
20. Thakar R, Sultan A. Postpartum problems and the role of a perineal clinic. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:65-79.
21. Williams A, Lavender T, Richmond DH, Tincello DG. Women’s experiences after a third-degree obstetric anal sphincter tear: a qualitative study. Birth. 2005;32:129-136.
22. Fynes M, Donnelly V, Behan M, O’Connell PR, O’Herlihy C. Effect of second vaginal delivery on anorectal physiology and faecal continence: a prospective study. Lancet. 1999;354:983-986.
23. Scheer I, Thakar R, Sultan A. Should women who sustained obstetric anal sphincter injuries be allowed a vaginal delivery? Neurourol Urodynam. 2006;25:512-513.
24. Peleg D, Kennedy CM, Merrill D, Zlatnik FJ. Risk of repetition of a severe perineal laceration. Obstet Gynecol. 1999;93:1021-1024.
25. Harkin R, Fitzpatrick M, O’Connell PR, O’Herlihy C. Anal sphincter disruption at vaginal delivery: is recurrence predictable? Eur J Obstet Gynaecol Reprod Biol. 2003;109:149-152.
26. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
27. Thakar R, Eason E. Prevention of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:52-64.
28. Johanson RB, Rice C, Doyle M. A randomised prospective study comparing the new vacuum extractor policy with forceps delivery. Br J Obstet Gynaecol. 1993;100:524-530.
29. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol. 1996;175:1325-1330.
1. Sultan AH, Kettle C. Diagnosis of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:13-19.
2. Sultan AH, Thakar R. Third and fourth degree tears. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:33-51.
3. Sultan AH. Primary repair of obstetric anal sphincter injury. In: Staskin DR, Cardozo L, ed. Textbook of Female Urology and Urogynaecology. London: ISIS Medical Media; 2006.
4. Thakar R, Fenner DE. Anatomy of the perineum and the anal sphincter. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:1-12.
5. Faltin DL, Boulvain M, Floris LA, Irion O. Diagnosis of anal sphincter tears to prevent fecal incontinence: a randomized controlled trial. Obstet Gynecol. 2005;106:6-13.
6. Andrews V, Thakar R, Sultan AH. Occult anal sphincter injuries—myth or reality. Br J Obstet Gynaecol. 2006;113:195-200.
7. Sultan AH. Obstetric perineal injury and anal incontinence. Clin Risk. 1999;5:193-196.
8. Royal College of Obstetricians and Gynaecologists. Management of third and fourth degree perineal tears following vaginal delivery. Guideline 29. London: RCOG Press; 2001.
9. Norton C, Christensen J, Butler U, et al. Anal Incontinence. 2nd ed. Plymouth: Health Publication Ltd; 2005:985-1044.
10. Sultan AH, Thakar R. Lower genital tract and anal sphincter trauma. Best Pract Res Clin Obstet Gynaecol. 2002;16:99-116.
11. Williams A, Adams EJ, Tincello DG, Alfirevic Z, Walkinshaw SA, Richmond DH. How to repair an anal sphincter injury after vaginal delivery: results of a randomised controlled trial. BJOG. 2006;113:201-207.
12. Fernando RJ, Sultan AH, Kettle C, Radley S, Jones P, O’Brien PMS. Repair techniques for obstetric anal sphincter injuries. A randomized controlled trial. Obstet Gynecol. 2006;107:1261-1268.
13. Fernando R, Sultan AH, Kettle C, Thakar R, Radley S. Methods of repair for obstetric anal sphincter injury. Cochrane Database Syst Rev. 2006;3:CD002866.-
14. Fernando RJ, Sultan AH, Radley S, Jones PW, Johanson RB. Management of obstetric anal sphincter injury: a systematic review and national practice survey. BMC Health Serv Res. 2002;2:9.-
15. MacArthur AJ, MacArthur C. Incidence, severity, and determinants of perineal pain after vaginal delivery: a prospective cohort study. Am J Obstet Gynecol. 2004;191:1199-1204.
16. Hedayati H, Parsons J, Crowther CA. Rectal analgesia for pain from perineal trauma following childbirth. Cochrane Database Syst Rev. 2003;(3):CD003931.-
17. Kettle C, Hills RK, Jones P, Darby L, Gray R, Johanson R. Continuous versus interrupted perineal repair with standard or rapidly absorbed sutures after spontaneous vaginal birth: a randomised controlled trial. Lancet. 2002;359:2217-2223.
18. Sultan AH, Monga AK, Kumar D, Stanton SL. Primary repair of obstetric anal sphincter rupture using the overlap technique. Br J Obstet Gynaecol. 1999;106:318-323.
19. Mahony R, Behan M, O’Herlihy C, O’Connell PR. Randomized, clinical trial of bowel confinement vs. laxative use after primary repair of a third-degree obstetric anal sphincter tear. Dis Colon Rectum. 2004;47:12-17.
20. Thakar R, Sultan A. Postpartum problems and the role of a perineal clinic. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:65-79.
21. Williams A, Lavender T, Richmond DH, Tincello DG. Women’s experiences after a third-degree obstetric anal sphincter tear: a qualitative study. Birth. 2005;32:129-136.
22. Fynes M, Donnelly V, Behan M, O’Connell PR, O’Herlihy C. Effect of second vaginal delivery on anorectal physiology and faecal continence: a prospective study. Lancet. 1999;354:983-986.
23. Scheer I, Thakar R, Sultan A. Should women who sustained obstetric anal sphincter injuries be allowed a vaginal delivery? Neurourol Urodynam. 2006;25:512-513.
24. Peleg D, Kennedy CM, Merrill D, Zlatnik FJ. Risk of repetition of a severe perineal laceration. Obstet Gynecol. 1999;93:1021-1024.
25. Harkin R, Fitzpatrick M, O’Connell PR, O’Herlihy C. Anal sphincter disruption at vaginal delivery: is recurrence predictable? Eur J Obstet Gynaecol Reprod Biol. 2003;109:149-152.
26. Coats PM, Chan KK, Wilkins M, Beard RJ. A comparison between midline and mediolateral episiotomies. Br J Obstet Gynaecol. 1980;87:408-412.
27. Thakar R, Eason E. Prevention of perineal trauma. In: Sultan AH, Thakar R, Fenner DE, eds. Perineal and Anal Sphincter Trauma. London: Springer; 2007:52-64.
28. Johanson RB, Rice C, Doyle M. A randomised prospective study comparing the new vacuum extractor policy with forceps delivery. Br J Obstet Gynaecol. 1993;100:524-530.
29. Bofill JA, Rust OA, Schorr SJ, et al. A randomized prospective trial of the obstetric forceps versus the M-cup vacuum extractor. Am J Obstet Gynecol. 1996;175:1325-1330.
Risks and remedies when your surgical patient is obese
The authors report no financial relationships relevant to this article.
The adverse consequences of obesity go far beyond aesthetic and psychosocial concerns. Patients who are markedly overweight face a real risk of developing severe health conditions—not just cardiac disease, diabetes mellitus, and hypertension, but also sleep apnea, venous thromboembolism, certain cancers (particularly breast and uterine), and biliary tract disease. Obesity also contributes to menstrual abnormalities and infertility and may complicate pregnancy.
Surgery in these patients poses a number of challenges. Not only does obesity frequently compromise the technical aspects of a procedure, it requires the surgeon to use certain measures in the preoperative and postoperative phases of management, such as counseling the patient extensively about the risks and potential complications she faces, initiating antibiotic prophylaxis, and ensuring early ambulation. These and other measures are especially important when uncontrolled, coexisting disease is present.
Not every obese patient is a significant surgical risk, so care should be individualized and use a team approach involving the gynecologist, anesthesiologist, primary care physician, and other appropriate subspecialists.
This article outlines the parameters of good surgical care in the obese patient, defined here as having a body mass index (BMI) of 30 kg/m2 or above, or 35 kg/m2 or above for morbid obesity. Whenever possible, we draw our recommendations from the published literature. In the absence of data, we base them on our surgical experience in the obese population.
Risks of surgery
It is imperative for the gynecologic surgeon to discuss the special risks of surgery with the obese patient well in advance of the operation and to formulate a systematic plan for evaluation, utilizing other members of the team when necessary. If the surgeon keeps the following risks in mind and is proactive, complications can be kept to a minimum.
Poor wound healing
Wound healing is a complex process involving several concurrent phases; an abnormality in any phase may impair healing. Those phases are:
- inflammatory phase, in which fluid and cells are released to clean the wound and prepare for the next phase of healing
- fibroplastic (proliferative) phase, in which fibroblasts accumulate and form collagen, the building block of connective tissue. This stage is marked by neovascularization and increased formation of granulation tissue
- wound contraction
- remodeling/maturation, in which new collagen is laid down as old collagen is broken down, resulting in scar formation.
- Obtain a chemistry panel: complete blood count, prothrombin time, activated partial thromboplastin time, and arterial blood gas studies. Type and cross-match if significant blood loss is expected
- Order a chest radiograph and electrocardiogram
- Test pulmonary function only if the patient has a history or suspected history of obstructive lung disease
- Order echocardiography only if the electrocardiogram or history suggests compromised cardiac function
- Instruct the patient on the use of incentive spirometry
- Prescribe a mechanical bowel-cleansing regimen if inadvertent injury is likely
- Notify anesthesiology and operating room personnel before the patient’s arrival
- Give 1 g of cefoxitin or another cephalosporin 60 minutes before the start of the procedure3
- Give 5,000 U of subcutaneous unfractionated heparin at least 2 hours before the start of surgery, and administer it every 8 hours until discharge.6 Alternative regimens: 5,000 U of dalteparin, a low-molecular-weight heparin, 12 hours before beginning the procedure and every 12 hours until discharge, or 40 mg of enoxaparin 12 hours before beginning surgery and every 12 hours until discharge
- Apply a pneumatic calf-compression apparatus in the operating room
Obese patients possess a thick layer of adipose tissue, which by its nature and location is minimally vascularized. This tissue essentially becomes dead space, an ideal medium for bacterial growth. Many obese patients also have diabetes mellitus, malignancy, or other comorbidity that further impairs healing.
As a result, obese patients are at increased risk of wound complications, breakdown, and subsequent dehiscence and evisceration. This translates into increased febrile morbidity, prolonged hospitalization, and higher cost.
What the data show. A number of studies have documented a higher incidence of wound complications in obese patients. In one retrospective review, Gallup1 observed an increased risk of wound complications in obese patients, but the incidence diminished after implementation of a protocol of meticulous cleansing, subcutaneous heparin, and modified incision and closure techniques. In a similar retrospective study of 300 obese patients, Pitkin2 reported wound complications among approximately one third of patients and postoperative fever among more than three quarters. Surgical-site infections are thought to occur in as many as 5% of patients.
Prophylaxis may be effective in some patients, but can be challenging. It entails meticulous skin cleansing and careful consideration of where the incision is placed, type of closure, use of a drain, and antibiotic administration.
Antibiotic prophylaxis is based on the theory that its presence in host tissues will alter natural defense mechanisms and kill bacteria that inoculate the wound. Because the window of efficacy is narrow, antibiotics should be administered shortly before the time of inoculation (ie, shortly before the time of incision, vaginal entry, etc.). Current guidelines suggest the use of broad-spectrum agents, including a cephalosporin, approximately 60 minutes before the incision. Redosing is recommended for procedures that last longer than 3 hours, as well as for those that involve significant blood loss (>1,500 mL).
For surgical procedures other than hysterectomy and laparotomy, prophylaxis may not be warranted.3 However, when the patient is markedly obese, many surgeons, including me (Dr. Perkins), sometimes opt to give antibiotics anyway—except for laparoscopic procedures—primarily for wound healing.
Compromised operative exposure
One of the main challenges of surgery in the obese is achieving adequate exposure; when it is inadequate, inadvertent injury may occur.
In addition to a thick abdominal wall composed largely of adipose tissue, these patients frequently have significant accumulations of fat in the mesenteries of the bowel, omentum, and pelvic peritoneum. These accumulations make it difficult to navigate around what becomes a narrow operative field.
Exposure can also be limited in vaginal surgery, because many obese women have very large thighs, buttocks, and accumulations of perineal fat.
Because exposure is a key element of successful surgery, modification of the procedure often becomes necessary—eg, focusing on a single area of the operative field at a time.
Use of a special retractor may help. A self-retaining retractor can be extremely useful. The Bookwalter retractor, first described in 1980, is a commonly used, table-fixed system that attaches to the side rail and can be assembled in minutes (FIGURE 1).4 The variety of rings and blades allows for excellent exposure. Although several complications have been associated with use of the Bookwalter retractor (primarily colon perforation and neuropathy5), they are infrequent and can be minimized by selecting the appropriate blade size and periodically repositioning the blades.
When this or other table-fixed retractors are unavailable, two Balfour retractors, placed at opposite poles of the field to obtain satisfactory exposure, may suffice. With a morbidly obese patient, multiple assistants may still be required to facilitate optimal exposure.
FIGURE 1 A tool to increase exposure
This self-retaining Bookwalter retractor is fixed to the surgical table and features a variety of rings and blades to facilitate exposure.
Thromboembolism
Venous thromboembolism is a major cause of mortality and morbidity in hospitalized patients, causing approximately 60,000 deaths every year.6 Obesity increases the risk of deep venous thrombosis in patients undergoing pelvic surgery. Because of their weight—and, often, coexisting conditions such as cardiorespiratory disease—many obese women are inactive or minimally active postoperatively, increasing the risk of thromboembolism, which remains heightened as long as 3 weeks after discharge. Older women are also at high risk, as are those with a malignancy.
Current guidelines call for the application of a pneumatic calf-compression device in the operating room, with removal after the patient is fully ambulatory. I (Dr. Perkins) also advocate simultaneous use of low-dose heparin, which should be given before surgery and continued until discharge.
Although the use of unfractionated heparin in conjunction with spinal or epidural anesthesia is not a major concern, the use of low-molecular-weight heparin warrants consultation with the anesthesiologist. Unfractionated heparin is preferred because it is metabolized much more rapidly than low-molecular weight heparin.7 The main concern with use of heparin in this setting (regardless of the patient’s weight) is spinal hematoma formation.
Inadvertent injury
Because exposure tends to be limited in obese patients, there is an ever-present risk of injury to bowel, bladder, ureter, and vascular structures. In obese women with a history of abdominal surgery, adhesions are likely, and the risk of bowel injury is increased. Similarly, in obese patients undergoing a laparoscopic procedure, many surface landmarks and vessels may be hard to discern.
Consider preoperative bowel preparation when there is a high risk of intestinal injury.8
Use a scalpel to incise the skin and delineate the area to be excised. That area typically consists of a large wedge of abdominal skin and subcutaneous fat.
Although the addition of panniculectomy to gynecologic surgery in the morbidly obese patient is a fairly recent strategy to increase exposure, the procedure itself has roots in the 19th century. In 1910, Howard Kelly of The Johns Hopkins Hospital reported a lipectomy that involved excision of a large wedge of abdominal skin and fat,21 although indications for that lipectomy appear to have been grounded in cosmesis and personal comfort for the patient.
Technique
Panniculectomy involves excision of a large portion of abdominal skin and subcutaneous fat down to, but not including, the rectus fascia, to gain much greater exposure to the lower abdominal cavity and pelvis.
Once the segment to be excised is delineated, it is mobilized using electrocautery to achieve meticulous hemostasis. After all surgical procedures are completed, the abdominal wall is closed in multiple layers, and drains are placed in the subcutaneous layer. Surgeons who have performed this procedure report significantly increased exposure and access to pelvic structures.
Once the skin is incised, the wedge is mobilized using electrocautery, down to, but not including, the rectus fascia.
Risks
The most important risk is impaired healing of the abdominal wound. One study of this procedure in patients on a gynecologic oncology service noted wound complications in 35% of patients, as well as significant blood loss (up to 1,800 mL in one case).22 Another study by Hopkins and associates,23 however, reported minimal blood loss and wound complications.
Caveats
The procedure requires an experienced surgeon, particularly if no plastic surgeon is readily available. Also, when counseling the patient about this procedure, it is important to emphasize that its primary indication is to maximize exposure; cosmetic benefits are secondary.
Impaired cardiorespiratory function
Pulmonary function typically is compromised in the markedly obese, with restrictive lung disease and reduced functional residual capacity. If the patient smokes or has chronic obstructive lung disease, her pulmonary function is compromised even further, and her condition should be relayed to the anesthesiologist.
In addition, many obese patients have preexisting heart disease or conditions such as hyperlipidemia that put them at risk for heart disease. When evaluating an obese surgical patient, also ask about less apparent disorders, such as sleep apnea, which, if not addressed, may have grave postoperative consequences.
Preoperative evaluation and preparation
Goal: Assess and minimize risks
This process begins in the office or clinic with a discussion with the patient of any concerns and risks. The importance of early mobilization and ambulation after surgery should be emphasized. Any patient with uncontrolled diabetes or hypertension should continue to be monitored by her primary care physician. A patient who has not seen a physician recently should be assessed by an internist to ensure that no conditions go undetected before surgery.
Routine testing to start, but additional assessment may be justified. An obese patient should undergo the same routine testing as a woman of normal weight, but further testing may be warranted for any coexisting disorder. Because the obese patient may have a restrictive lung pattern by virtue of her body habitus, pulmonary-function testing is unlikely to yield new information and is probably not indicated—unless she smokes or has a history of chronic obstructive pulmonary disease. In that case, tests will clarify the obstructive component and bronchodilator response and are useful in postoperative management. Measurement of arterial blood gases is useful, however, because levels reflect respiratory function on a day-to-day basis.
Additional tests of cardiac status probably are not indicated on the basis of obesity alone. However, if initial tests (eg, the electrocardiogram) and the history suggest compromised cardiac function, two-dimensional echocardiography should be performed to determine the ejection fraction. Any concerns regarding cardiac function should be discussed with the anesthesiologist and cardiologist.
Because compromised pulmonary function is likely, I (Dr. Perkins) instruct each patient on the use of incentive spirometry before she undergoes anesthesia so that she has realistic expectations about the postoperative course. I also administer heparin at least 2 hours before induction (8 hours before induction if unfractionated heparin is used).6
If a hysterectomy or prolonged laparotomy is planned, prophylactic antibiotics are recommended.3 Thigh-high compression stockings or a pneumatic calf-compression device should be applied upon arrival in the operating room.
Anesthesia-related issues
Anticipate challenges involving the airway
The anesthesiologist’s primary concern in regard to the obese patient is establishment and maintenance of an airway to promote oxygenation. In morbidly and extremely morbidly obese patients, anatomic factors such as large breasts; a short, thick neck; large tongue; decreased mobility of the cervical spine; limited mouth opening; and greater amount of adipose tissue in the face and cheeks can render mask ventilation and intubation extremely difficult or impossible. Decreased functional residual capacity and tidal volume in the range of closing capacity may lead to extremely rapid oxygen desaturation when the patient is apneic.9
If the patient is pregnant, factors such as excess adipose tissue in the face become even more pronounced and increase the potential for catastrophe.
Appreciate mechanical concerns
The morbidly obese patient may exceed the weight limit of the operating table. In addition, placing her in a steep Trendelenburg position or rotating her laterally may compromise the integrity of the bed.
Coexisting disease, such as sleep apnea and acid reflux disease, should also be kept in mind. Compromised respiratory mechanics (eg, restrictive lung pattern) may cause further deterioration and make mechanical ventilation more difficult.
It also may be hard to establish vascular access, necessitating central venous line placement and introducing its associated risks.
Keep the anesthesiologist in the loop
In the postoperative period, obese patients face a heightened risk of complications related to diminished pulmonary function, such as oxygen desaturation, hypoventilation, and airway obstruction, which may lead to atelectasis, pneumonia, and pulmonary edema.10,11 For these reasons, early consultation with the anesthesiologist is recommended, especially if the initial evaluation suggests potential difficulties in securing the airway. In turn, the anesthesiologist should understand that, in some obese patients, even establishing a surgical airway may be difficult. Regional anesthesia should be considered when feasible.
Antacids and drugs that increase gastric motility have proved to be useful in minimizing aspiration-related risks.12,13
Surgical technique
Begin abdominal procedures by carefully choosing an incision
Do not base the decision solely on the degree of obesity, but also consider any additional procedures that are planned, such as lymph-node sampling. A vertical incision does permit greater exposure than is afforded by a transverse incision, but in some cases the latter may be more appropriate—even if the patient is morbidly obese.
Do not place the incision below the panniculus, in the crease just above the suprapubic mound, though it may be tempting to do so when the panniculus is large and thick. This area is a warm, moist, anaerobic environment that promotes the proliferation of numerous micro-organisms, creating a bacterial cesspool. It is the worst place to make an incision.
If a transverse incision is selected, place caudal traction on the panniculus (which may be facilitated by applying two towel clips to the tissue fold), and incise through the fold at a point approximately three to four fingerbreadths above the symphysis (FIGURE 2).
If a midline incision is selected, a similar technique is appropriate, with downward traction applied to the panniculus and the incision begun at the lower pole of this fold up to the umbilicus—or through it and above, should more room be required.14
After incising the superficial fascia, greater exposure may be gained by incising the rectus sheath beneath the pannicular fold and extending it down to the symphysis. After entering the abdominal cavity, the surgeon may encounter a redundant, fat-laden layer of peritoneum. The edges of this tissue may be temporarily sutured to the edges of the skin incision to remove it from the operative field and obtain better visualization.
FIGURE 2 Incision placement can be counterintuitive
Avoid placing the skin incision beneath the panniculus, an anaerobic area ripe for infection. Instead, retract the panniculus caudally and incise the skin above the fold, as shown.
A few remedies can help when exposure is limited
Exposure is one of the most important elements of successful surgery, but it is often restricted when marked obesity is present. Fortunately, numerous adjuncts are available to address this problem, such as the table-fixed retractor systems described earlier in this article, or one or more of the following tactics:
- Do not try to expose the entire pelvic basin. One technique to ease exploration—especially when more than one procedure is planned—is to refrain from exposing the entire pelvic basin at one time. Instead, focus on obtaining adequate visualization in the immediate area and, once work in that area is finished, concentrate on the next.
- Use extra-long instruments in an extremely large patient, especially if she has a “deep” pelvis.
- Abbreviate the procedure, if possible. Because these cases are technically more difficult and frequently involve excessive blood loss, it may be wiser to perform an “incomplete” procedure, such as supracervical hysterectomy instead of total hysterectomy, depending on the indication.14
- Use a cell-saver blood-collection system if significant blood loss is anticipated. Also remember to give a second dose of prophylactic antibiotic.
Strategies for effective wound closure
The best method of abdominal wound closure has been a subject of debate among both gynecologic and general surgeons. In the obese patient, the key variables are the rather thick subcutaneous fat layer within the abdominal wall and the impact of intra-abdominal pressure on the incision.
What the data show. A number of studies, including one by Montz and colleagues,15 have demonstrated that the running mass-closure technique using delayed-absorbable or permanent suture is just as effective as interrupted suture placement (eg, Smead-Jones closure) but is faster, with less suture deposited in the wound.
The following considerations may also be helpful:
- Approximate the subcutaneous fascia? There has been some debate about whether the subcutaneous fascia must be approximated. My (Dr. Perkins) personal preference in morbidly obese patients is to place several interrupted sutures to obliterate much of the dead space, facilitate skin closure, and minimize tension on the wound; I have noted no significant increase in wound complications using this technique. Placement of a closed-suction drain (eg, Jackson Pratt, Hemovac) is a good alternative.
- Is a drain useful? Some have questioned whether use of a drain increases the likelihood of wound complications,16 but this concern is irrelevant because, in most—if not all—cases, the drainage tubing is exteriorized via a separate stab wound remote from the incision.
- Consider retention sutures. A morbidly obese patient may benefit from through-and-through retention sutures using 0 or #1 permanent material along with rubber bolsters to minimize cutting of the suture into the skin, especially if increased intra-abdominal pressure is likely (FIGURE 3).17 These sutures can be removed on the 10th to 12th postoperative day.
FIGURE 3 Minimize tension on the wound
A morbidly obese patient may benefit from through-and-through retention sutures along with rubber bolsters to minimize cutting of the suture into the skin.
Vaginal surgery does not increase complication rate in the obese
The vaginal approach can be extremely challenging in the morbidly obese patient, especially when hysterectomy is performed. Exposure is often compromised by the large folds about the thighs and buttocks, limiting access to the perineum and vaginal vault. If the patient also has a narrow, contracted pelvis, the difficulty is compounded. Because of these and other concerns about morbidity, many gynecologists hesitate to perform hysterectomy via the vaginal route when the patient is obese.
What the data show. Several studies have addressed these issues, including one by Pratt and Daikoku18 and another by Rafii and colleagues,19 both of which demonstrated that obese patients have a complication rate roughly equivalent to that observed in patients of normal weight, although obese patients have a greater decrease in hemoglobin level and a slightly higher incidence of postoperative fever.
Similarly, in a retrospective study, Pitkin20 found no significant difference between the complication rates of obese and nonobese patients.
Another argument for the self-retaining retractor. From a technical standpoint, achieving good exposure is the primary challenge of vaginal surgery and usually requires two or more assistants—who themselves have limited or no direct view of the field—who must stand for long periods. Again, a viable alternative is use of a self-retaining retractor. One in particular, the MiniOmni retractor, is a small, uncumbersome, table-fixed system that can be maneuvered so that vaginal and perineal structures are readily accessible.
Choice of stirrups is also relevant. Exposure can be affected by the type of stirrups used. “Candy cane” stirrups facilitate exposure more than fixed stirrups (eg, Allen stirrups) do. Regardless of the stirrups selected, however, it is important to avoid excessive or prolonged hip flexion, or nerve injury may result.
How to minimize postoperative complications
After surgery, an obese patient requires close and continuous monitoring to avert complications and detect any that occur. Consider the following measures:
- A stint in the intensive care unit. In the morbidly obese patient, massive fluid shift (eg, extensive blood loss, prolonged surgery with losses from the peritoneum, etc.) or concern about sleep apnea may justify close monitoring in an intensive care unit or similar setting—at least briefly. Later, as the patient recovers, sleep studies may indicate whether apnea is present.
- Document fluid intake and output, especially in the elderly and in women with cardiorespiratory disease.
- Give analgesics in an amount sufficient to control pain and minimize activity that might place excessive tension on the abdominal incision, but also allows the patient to remain alert enough to ambulate effectively and perform pulmonary toileting.
- Begin ambulation on the first postoperative day—or on the evening after surgery, if circumstances permit. This helps clear secretions from the respiratory tract, reduces the risk of thromboembolism, and speeds the return of normal bowel function. Hourly incentive spirometry is also recommended for the first few days after surgery.
- Continue heparin. In patients at moderate or high risk of thromboembolism, continue low-dose heparin until discharge or for 7 days, whichever comes first.
- Keep other physicians involved. If the patient has a significant comorbidity, such as cardiorespiratory disease or uncontrolled diabetes, she should remain under the care of her internist or other primary care provider.
- Closely monitor the surgical wound for early signs of infection, which include inflammation and collections of serous fluid, blood, pus, or a mixture of these. If retention sutures were placed, check them frequently to ensure that they are not cutting into the skin of the abdomen.
- Strongly recommend weight loss. At the time of the last postoperative visit, tell the patient in clear language that obesity is extremely bad for her health and strongly encourage her to lose weight under the supervision of her primary care provider. If she has no such provider, make the appropriate referral.
1. Gallup DG. Modifications of celiotomy techniques to decrease morbidity in obese gynecologic patients. Am J Obstet Gynecol. 1984;150:171-178.
2. Pitkin RM. Abdominal hysterectomy in obese women. Surg Gynecol Obstet. 1976;142:532-536.
3. Antibiotic prophylaxis for gynecologic procedures. ACOG Practice Bulletin No. 74. Washington, DC: American College of Obstetricians and Gynecologists; July 2006.
4. Bookwalter JR. A new table-fixed retractor. Surg Clin North Am. 1980;60:399-405.
5. Noldus J, Graefen M, Huland H. Major postoperative complications secondary to use of the Bookwalter self-retaining retractor. Urology. 2002;60:964-967.
6. Prevention of deep vein thrombosis and pulmonary embolism. ACOG Practice Bulletin No. 21. Washington, DC: American College of Obstetricians and Gynecologists; October 2000.
7. Vandermeulen EP, Van Aken H, Vermylen J. Anticoagulants and spinal-epidural anesthesia. Anesth Analg. 1994;79:1165-1177.
8. Perkins JD, Dent LL. Avoiding and repairing bowel injury in gynecologic surgery. OBG Management. 2004;16(8):15-28.
9. Cressey DM, Berthoud MC, Reilly CS. Effectiveness of continuous airway pressure to enhance preoxygenation in morbidly obese women. Anesthesia. 2001;56:670-689.
10. Jordan H, Perlow MD, Mark A, Morgan MD. Massive maternal obesity and perioperative cesarean morbidity. Am J Obstet Gynecol. 1994;170:560-565.
11. Hood DD, Dewan DN. Anesthetic and obstetric outcome in morbidly obese parturients. Anesthesiology. 1993;79:1210-1218.
12. James CF, Gibbs CP, Banner T. Postpartum perioperative risk of aspiration pneumonia. Anesthesiology. 1984;61:756-759.
13. Manchikanti L, Colliver JA, Marrero TC, Roush JR. Ranitidine and metoclopramide for prophylaxis of aspiration pneumonitis in elective surgery. Anesth Analg. 1984;63:903-910.
14. Morrow CP, Hernandez WL, Townsend DE, DiSaia PJ. Pelvic celiotomy in the obese patient. Am J Obstet Gynecol. 1977;127:335-339.
15. Montz FJ, Creasman WT, Eddy G, DiSaia PJ. Running mass closure of abdominal wounds using absorbable looped suture. J Gynecol Surg. 1991;7:107-110.
16. Ramsey PS, White AM, Guinn DA, et al. Subcutaneous tissue reapproximation, alone or in combination with drain, in obese women undergoing cesarean delivery. Obstet Gynecol. 2005;105:967-973.
17. Soisson AP, Olt G, Soper JT, Berchuck A, Rodriguez G, Clarke-Pearson DL. Prevention of superficial wound separation with subcutaneous retention sutures. Gynecol Oncol. 1993;51:330-334.
18. Pratt JH, Daikoku NH. Obesity and vaginal hysterectomy. J Reprod Med. 1990;35:945-949.
19. Rafii A, Samain E, Levardon M, Darai E, Deval B. Vaginal hysterectomy for benign disorders in obese women: a prospective study. Br J Obstet Gynaecol. 2005;111:223-227.
20. Pitkin RM. Vaginal hysterectomy in obese women. Obstet Gynecol. 1977;49:567-569.
21. Kelly HA. Excision of the fat of the abdominal wall—lipectomy. Surg Gynecol Obstet. 1910;10:229-231.
22. Wright JD, Rosenbush EJ, Powell MA, et al. Longterm outcome of women who undergo panniculectomy at the time of gynecologic surgery. Gynecol Oncol. 2006;102:86-91.
23. Hopkins MP, Shriner AM, Parker MG, Scott L. Panniculectomy at the time of gynecologic surgery in morbidly obese patients. Am J Obstet Gynecol. 2000;182:1502-1505.
The authors report no financial relationships relevant to this article.
The adverse consequences of obesity go far beyond aesthetic and psychosocial concerns. Patients who are markedly overweight face a real risk of developing severe health conditions—not just cardiac disease, diabetes mellitus, and hypertension, but also sleep apnea, venous thromboembolism, certain cancers (particularly breast and uterine), and biliary tract disease. Obesity also contributes to menstrual abnormalities and infertility and may complicate pregnancy.
Surgery in these patients poses a number of challenges. Not only does obesity frequently compromise the technical aspects of a procedure, it requires the surgeon to use certain measures in the preoperative and postoperative phases of management, such as counseling the patient extensively about the risks and potential complications she faces, initiating antibiotic prophylaxis, and ensuring early ambulation. These and other measures are especially important when uncontrolled, coexisting disease is present.
Not every obese patient is a significant surgical risk, so care should be individualized and use a team approach involving the gynecologist, anesthesiologist, primary care physician, and other appropriate subspecialists.
This article outlines the parameters of good surgical care in the obese patient, defined here as having a body mass index (BMI) of 30 kg/m2 or above, or 35 kg/m2 or above for morbid obesity. Whenever possible, we draw our recommendations from the published literature. In the absence of data, we base them on our surgical experience in the obese population.
Risks of surgery
It is imperative for the gynecologic surgeon to discuss the special risks of surgery with the obese patient well in advance of the operation and to formulate a systematic plan for evaluation, utilizing other members of the team when necessary. If the surgeon keeps the following risks in mind and is proactive, complications can be kept to a minimum.
Poor wound healing
Wound healing is a complex process involving several concurrent phases; an abnormality in any phase may impair healing. Those phases are:
- inflammatory phase, in which fluid and cells are released to clean the wound and prepare for the next phase of healing
- fibroplastic (proliferative) phase, in which fibroblasts accumulate and form collagen, the building block of connective tissue. This stage is marked by neovascularization and increased formation of granulation tissue
- wound contraction
- remodeling/maturation, in which new collagen is laid down as old collagen is broken down, resulting in scar formation.
- Obtain a chemistry panel: complete blood count, prothrombin time, activated partial thromboplastin time, and arterial blood gas studies. Type and cross-match if significant blood loss is expected
- Order a chest radiograph and electrocardiogram
- Test pulmonary function only if the patient has a history or suspected history of obstructive lung disease
- Order echocardiography only if the electrocardiogram or history suggests compromised cardiac function
- Instruct the patient on the use of incentive spirometry
- Prescribe a mechanical bowel-cleansing regimen if inadvertent injury is likely
- Notify anesthesiology and operating room personnel before the patient’s arrival
- Give 1 g of cefoxitin or another cephalosporin 60 minutes before the start of the procedure3
- Give 5,000 U of subcutaneous unfractionated heparin at least 2 hours before the start of surgery, and administer it every 8 hours until discharge.6 Alternative regimens: 5,000 U of dalteparin, a low-molecular-weight heparin, 12 hours before beginning the procedure and every 12 hours until discharge, or 40 mg of enoxaparin 12 hours before beginning surgery and every 12 hours until discharge
- Apply a pneumatic calf-compression apparatus in the operating room
Obese patients possess a thick layer of adipose tissue, which by its nature and location is minimally vascularized. This tissue essentially becomes dead space, an ideal medium for bacterial growth. Many obese patients also have diabetes mellitus, malignancy, or other comorbidity that further impairs healing.
As a result, obese patients are at increased risk of wound complications, breakdown, and subsequent dehiscence and evisceration. This translates into increased febrile morbidity, prolonged hospitalization, and higher cost.
What the data show. A number of studies have documented a higher incidence of wound complications in obese patients. In one retrospective review, Gallup1 observed an increased risk of wound complications in obese patients, but the incidence diminished after implementation of a protocol of meticulous cleansing, subcutaneous heparin, and modified incision and closure techniques. In a similar retrospective study of 300 obese patients, Pitkin2 reported wound complications among approximately one third of patients and postoperative fever among more than three quarters. Surgical-site infections are thought to occur in as many as 5% of patients.
Prophylaxis may be effective in some patients, but can be challenging. It entails meticulous skin cleansing and careful consideration of where the incision is placed, type of closure, use of a drain, and antibiotic administration.
Antibiotic prophylaxis is based on the theory that its presence in host tissues will alter natural defense mechanisms and kill bacteria that inoculate the wound. Because the window of efficacy is narrow, antibiotics should be administered shortly before the time of inoculation (ie, shortly before the time of incision, vaginal entry, etc.). Current guidelines suggest the use of broad-spectrum agents, including a cephalosporin, approximately 60 minutes before the incision. Redosing is recommended for procedures that last longer than 3 hours, as well as for those that involve significant blood loss (>1,500 mL).
For surgical procedures other than hysterectomy and laparotomy, prophylaxis may not be warranted.3 However, when the patient is markedly obese, many surgeons, including me (Dr. Perkins), sometimes opt to give antibiotics anyway—except for laparoscopic procedures—primarily for wound healing.
Compromised operative exposure
One of the main challenges of surgery in the obese is achieving adequate exposure; when it is inadequate, inadvertent injury may occur.
In addition to a thick abdominal wall composed largely of adipose tissue, these patients frequently have significant accumulations of fat in the mesenteries of the bowel, omentum, and pelvic peritoneum. These accumulations make it difficult to navigate around what becomes a narrow operative field.
Exposure can also be limited in vaginal surgery, because many obese women have very large thighs, buttocks, and accumulations of perineal fat.
Because exposure is a key element of successful surgery, modification of the procedure often becomes necessary—eg, focusing on a single area of the operative field at a time.
Use of a special retractor may help. A self-retaining retractor can be extremely useful. The Bookwalter retractor, first described in 1980, is a commonly used, table-fixed system that attaches to the side rail and can be assembled in minutes (FIGURE 1).4 The variety of rings and blades allows for excellent exposure. Although several complications have been associated with use of the Bookwalter retractor (primarily colon perforation and neuropathy5), they are infrequent and can be minimized by selecting the appropriate blade size and periodically repositioning the blades.
When this or other table-fixed retractors are unavailable, two Balfour retractors, placed at opposite poles of the field to obtain satisfactory exposure, may suffice. With a morbidly obese patient, multiple assistants may still be required to facilitate optimal exposure.
FIGURE 1 A tool to increase exposure
This self-retaining Bookwalter retractor is fixed to the surgical table and features a variety of rings and blades to facilitate exposure.
Thromboembolism
Venous thromboembolism is a major cause of mortality and morbidity in hospitalized patients, causing approximately 60,000 deaths every year.6 Obesity increases the risk of deep venous thrombosis in patients undergoing pelvic surgery. Because of their weight—and, often, coexisting conditions such as cardiorespiratory disease—many obese women are inactive or minimally active postoperatively, increasing the risk of thromboembolism, which remains heightened as long as 3 weeks after discharge. Older women are also at high risk, as are those with a malignancy.
Current guidelines call for the application of a pneumatic calf-compression device in the operating room, with removal after the patient is fully ambulatory. I (Dr. Perkins) also advocate simultaneous use of low-dose heparin, which should be given before surgery and continued until discharge.
Although the use of unfractionated heparin in conjunction with spinal or epidural anesthesia is not a major concern, the use of low-molecular-weight heparin warrants consultation with the anesthesiologist. Unfractionated heparin is preferred because it is metabolized much more rapidly than low-molecular weight heparin.7 The main concern with use of heparin in this setting (regardless of the patient’s weight) is spinal hematoma formation.
Inadvertent injury
Because exposure tends to be limited in obese patients, there is an ever-present risk of injury to bowel, bladder, ureter, and vascular structures. In obese women with a history of abdominal surgery, adhesions are likely, and the risk of bowel injury is increased. Similarly, in obese patients undergoing a laparoscopic procedure, many surface landmarks and vessels may be hard to discern.
Consider preoperative bowel preparation when there is a high risk of intestinal injury.8
Use a scalpel to incise the skin and delineate the area to be excised. That area typically consists of a large wedge of abdominal skin and subcutaneous fat.
Although the addition of panniculectomy to gynecologic surgery in the morbidly obese patient is a fairly recent strategy to increase exposure, the procedure itself has roots in the 19th century. In 1910, Howard Kelly of The Johns Hopkins Hospital reported a lipectomy that involved excision of a large wedge of abdominal skin and fat,21 although indications for that lipectomy appear to have been grounded in cosmesis and personal comfort for the patient.
Technique
Panniculectomy involves excision of a large portion of abdominal skin and subcutaneous fat down to, but not including, the rectus fascia, to gain much greater exposure to the lower abdominal cavity and pelvis.
Once the segment to be excised is delineated, it is mobilized using electrocautery to achieve meticulous hemostasis. After all surgical procedures are completed, the abdominal wall is closed in multiple layers, and drains are placed in the subcutaneous layer. Surgeons who have performed this procedure report significantly increased exposure and access to pelvic structures.
Once the skin is incised, the wedge is mobilized using electrocautery, down to, but not including, the rectus fascia.
Risks
The most important risk is impaired healing of the abdominal wound. One study of this procedure in patients on a gynecologic oncology service noted wound complications in 35% of patients, as well as significant blood loss (up to 1,800 mL in one case).22 Another study by Hopkins and associates,23 however, reported minimal blood loss and wound complications.
Caveats
The procedure requires an experienced surgeon, particularly if no plastic surgeon is readily available. Also, when counseling the patient about this procedure, it is important to emphasize that its primary indication is to maximize exposure; cosmetic benefits are secondary.
Impaired cardiorespiratory function
Pulmonary function typically is compromised in the markedly obese, with restrictive lung disease and reduced functional residual capacity. If the patient smokes or has chronic obstructive lung disease, her pulmonary function is compromised even further, and her condition should be relayed to the anesthesiologist.
In addition, many obese patients have preexisting heart disease or conditions such as hyperlipidemia that put them at risk for heart disease. When evaluating an obese surgical patient, also ask about less apparent disorders, such as sleep apnea, which, if not addressed, may have grave postoperative consequences.
Preoperative evaluation and preparation
Goal: Assess and minimize risks
This process begins in the office or clinic with a discussion with the patient of any concerns and risks. The importance of early mobilization and ambulation after surgery should be emphasized. Any patient with uncontrolled diabetes or hypertension should continue to be monitored by her primary care physician. A patient who has not seen a physician recently should be assessed by an internist to ensure that no conditions go undetected before surgery.
Routine testing to start, but additional assessment may be justified. An obese patient should undergo the same routine testing as a woman of normal weight, but further testing may be warranted for any coexisting disorder. Because the obese patient may have a restrictive lung pattern by virtue of her body habitus, pulmonary-function testing is unlikely to yield new information and is probably not indicated—unless she smokes or has a history of chronic obstructive pulmonary disease. In that case, tests will clarify the obstructive component and bronchodilator response and are useful in postoperative management. Measurement of arterial blood gases is useful, however, because levels reflect respiratory function on a day-to-day basis.
Additional tests of cardiac status probably are not indicated on the basis of obesity alone. However, if initial tests (eg, the electrocardiogram) and the history suggest compromised cardiac function, two-dimensional echocardiography should be performed to determine the ejection fraction. Any concerns regarding cardiac function should be discussed with the anesthesiologist and cardiologist.
Because compromised pulmonary function is likely, I (Dr. Perkins) instruct each patient on the use of incentive spirometry before she undergoes anesthesia so that she has realistic expectations about the postoperative course. I also administer heparin at least 2 hours before induction (8 hours before induction if unfractionated heparin is used).6
If a hysterectomy or prolonged laparotomy is planned, prophylactic antibiotics are recommended.3 Thigh-high compression stockings or a pneumatic calf-compression device should be applied upon arrival in the operating room.
Anesthesia-related issues
Anticipate challenges involving the airway
The anesthesiologist’s primary concern in regard to the obese patient is establishment and maintenance of an airway to promote oxygenation. In morbidly and extremely morbidly obese patients, anatomic factors such as large breasts; a short, thick neck; large tongue; decreased mobility of the cervical spine; limited mouth opening; and greater amount of adipose tissue in the face and cheeks can render mask ventilation and intubation extremely difficult or impossible. Decreased functional residual capacity and tidal volume in the range of closing capacity may lead to extremely rapid oxygen desaturation when the patient is apneic.9
If the patient is pregnant, factors such as excess adipose tissue in the face become even more pronounced and increase the potential for catastrophe.
Appreciate mechanical concerns
The morbidly obese patient may exceed the weight limit of the operating table. In addition, placing her in a steep Trendelenburg position or rotating her laterally may compromise the integrity of the bed.
Coexisting disease, such as sleep apnea and acid reflux disease, should also be kept in mind. Compromised respiratory mechanics (eg, restrictive lung pattern) may cause further deterioration and make mechanical ventilation more difficult.
It also may be hard to establish vascular access, necessitating central venous line placement and introducing its associated risks.
Keep the anesthesiologist in the loop
In the postoperative period, obese patients face a heightened risk of complications related to diminished pulmonary function, such as oxygen desaturation, hypoventilation, and airway obstruction, which may lead to atelectasis, pneumonia, and pulmonary edema.10,11 For these reasons, early consultation with the anesthesiologist is recommended, especially if the initial evaluation suggests potential difficulties in securing the airway. In turn, the anesthesiologist should understand that, in some obese patients, even establishing a surgical airway may be difficult. Regional anesthesia should be considered when feasible.
Antacids and drugs that increase gastric motility have proved to be useful in minimizing aspiration-related risks.12,13
Surgical technique
Begin abdominal procedures by carefully choosing an incision
Do not base the decision solely on the degree of obesity, but also consider any additional procedures that are planned, such as lymph-node sampling. A vertical incision does permit greater exposure than is afforded by a transverse incision, but in some cases the latter may be more appropriate—even if the patient is morbidly obese.
Do not place the incision below the panniculus, in the crease just above the suprapubic mound, though it may be tempting to do so when the panniculus is large and thick. This area is a warm, moist, anaerobic environment that promotes the proliferation of numerous micro-organisms, creating a bacterial cesspool. It is the worst place to make an incision.
If a transverse incision is selected, place caudal traction on the panniculus (which may be facilitated by applying two towel clips to the tissue fold), and incise through the fold at a point approximately three to four fingerbreadths above the symphysis (FIGURE 2).
If a midline incision is selected, a similar technique is appropriate, with downward traction applied to the panniculus and the incision begun at the lower pole of this fold up to the umbilicus—or through it and above, should more room be required.14
After incising the superficial fascia, greater exposure may be gained by incising the rectus sheath beneath the pannicular fold and extending it down to the symphysis. After entering the abdominal cavity, the surgeon may encounter a redundant, fat-laden layer of peritoneum. The edges of this tissue may be temporarily sutured to the edges of the skin incision to remove it from the operative field and obtain better visualization.
FIGURE 2 Incision placement can be counterintuitive
Avoid placing the skin incision beneath the panniculus, an anaerobic area ripe for infection. Instead, retract the panniculus caudally and incise the skin above the fold, as shown.
A few remedies can help when exposure is limited
Exposure is one of the most important elements of successful surgery, but it is often restricted when marked obesity is present. Fortunately, numerous adjuncts are available to address this problem, such as the table-fixed retractor systems described earlier in this article, or one or more of the following tactics:
- Do not try to expose the entire pelvic basin. One technique to ease exploration—especially when more than one procedure is planned—is to refrain from exposing the entire pelvic basin at one time. Instead, focus on obtaining adequate visualization in the immediate area and, once work in that area is finished, concentrate on the next.
- Use extra-long instruments in an extremely large patient, especially if she has a “deep” pelvis.
- Abbreviate the procedure, if possible. Because these cases are technically more difficult and frequently involve excessive blood loss, it may be wiser to perform an “incomplete” procedure, such as supracervical hysterectomy instead of total hysterectomy, depending on the indication.14
- Use a cell-saver blood-collection system if significant blood loss is anticipated. Also remember to give a second dose of prophylactic antibiotic.
Strategies for effective wound closure
The best method of abdominal wound closure has been a subject of debate among both gynecologic and general surgeons. In the obese patient, the key variables are the rather thick subcutaneous fat layer within the abdominal wall and the impact of intra-abdominal pressure on the incision.
What the data show. A number of studies, including one by Montz and colleagues,15 have demonstrated that the running mass-closure technique using delayed-absorbable or permanent suture is just as effective as interrupted suture placement (eg, Smead-Jones closure) but is faster, with less suture deposited in the wound.
The following considerations may also be helpful:
- Approximate the subcutaneous fascia? There has been some debate about whether the subcutaneous fascia must be approximated. My (Dr. Perkins) personal preference in morbidly obese patients is to place several interrupted sutures to obliterate much of the dead space, facilitate skin closure, and minimize tension on the wound; I have noted no significant increase in wound complications using this technique. Placement of a closed-suction drain (eg, Jackson Pratt, Hemovac) is a good alternative.
- Is a drain useful? Some have questioned whether use of a drain increases the likelihood of wound complications,16 but this concern is irrelevant because, in most—if not all—cases, the drainage tubing is exteriorized via a separate stab wound remote from the incision.
- Consider retention sutures. A morbidly obese patient may benefit from through-and-through retention sutures using 0 or #1 permanent material along with rubber bolsters to minimize cutting of the suture into the skin, especially if increased intra-abdominal pressure is likely (FIGURE 3).17 These sutures can be removed on the 10th to 12th postoperative day.
FIGURE 3 Minimize tension on the wound
A morbidly obese patient may benefit from through-and-through retention sutures along with rubber bolsters to minimize cutting of the suture into the skin.
Vaginal surgery does not increase complication rate in the obese
The vaginal approach can be extremely challenging in the morbidly obese patient, especially when hysterectomy is performed. Exposure is often compromised by the large folds about the thighs and buttocks, limiting access to the perineum and vaginal vault. If the patient also has a narrow, contracted pelvis, the difficulty is compounded. Because of these and other concerns about morbidity, many gynecologists hesitate to perform hysterectomy via the vaginal route when the patient is obese.
What the data show. Several studies have addressed these issues, including one by Pratt and Daikoku18 and another by Rafii and colleagues,19 both of which demonstrated that obese patients have a complication rate roughly equivalent to that observed in patients of normal weight, although obese patients have a greater decrease in hemoglobin level and a slightly higher incidence of postoperative fever.
Similarly, in a retrospective study, Pitkin20 found no significant difference between the complication rates of obese and nonobese patients.
Another argument for the self-retaining retractor. From a technical standpoint, achieving good exposure is the primary challenge of vaginal surgery and usually requires two or more assistants—who themselves have limited or no direct view of the field—who must stand for long periods. Again, a viable alternative is use of a self-retaining retractor. One in particular, the MiniOmni retractor, is a small, uncumbersome, table-fixed system that can be maneuvered so that vaginal and perineal structures are readily accessible.
Choice of stirrups is also relevant. Exposure can be affected by the type of stirrups used. “Candy cane” stirrups facilitate exposure more than fixed stirrups (eg, Allen stirrups) do. Regardless of the stirrups selected, however, it is important to avoid excessive or prolonged hip flexion, or nerve injury may result.
How to minimize postoperative complications
After surgery, an obese patient requires close and continuous monitoring to avert complications and detect any that occur. Consider the following measures:
- A stint in the intensive care unit. In the morbidly obese patient, massive fluid shift (eg, extensive blood loss, prolonged surgery with losses from the peritoneum, etc.) or concern about sleep apnea may justify close monitoring in an intensive care unit or similar setting—at least briefly. Later, as the patient recovers, sleep studies may indicate whether apnea is present.
- Document fluid intake and output, especially in the elderly and in women with cardiorespiratory disease.
- Give analgesics in an amount sufficient to control pain and minimize activity that might place excessive tension on the abdominal incision, but also allows the patient to remain alert enough to ambulate effectively and perform pulmonary toileting.
- Begin ambulation on the first postoperative day—or on the evening after surgery, if circumstances permit. This helps clear secretions from the respiratory tract, reduces the risk of thromboembolism, and speeds the return of normal bowel function. Hourly incentive spirometry is also recommended for the first few days after surgery.
- Continue heparin. In patients at moderate or high risk of thromboembolism, continue low-dose heparin until discharge or for 7 days, whichever comes first.
- Keep other physicians involved. If the patient has a significant comorbidity, such as cardiorespiratory disease or uncontrolled diabetes, she should remain under the care of her internist or other primary care provider.
- Closely monitor the surgical wound for early signs of infection, which include inflammation and collections of serous fluid, blood, pus, or a mixture of these. If retention sutures were placed, check them frequently to ensure that they are not cutting into the skin of the abdomen.
- Strongly recommend weight loss. At the time of the last postoperative visit, tell the patient in clear language that obesity is extremely bad for her health and strongly encourage her to lose weight under the supervision of her primary care provider. If she has no such provider, make the appropriate referral.
The authors report no financial relationships relevant to this article.
The adverse consequences of obesity go far beyond aesthetic and psychosocial concerns. Patients who are markedly overweight face a real risk of developing severe health conditions—not just cardiac disease, diabetes mellitus, and hypertension, but also sleep apnea, venous thromboembolism, certain cancers (particularly breast and uterine), and biliary tract disease. Obesity also contributes to menstrual abnormalities and infertility and may complicate pregnancy.
Surgery in these patients poses a number of challenges. Not only does obesity frequently compromise the technical aspects of a procedure, it requires the surgeon to use certain measures in the preoperative and postoperative phases of management, such as counseling the patient extensively about the risks and potential complications she faces, initiating antibiotic prophylaxis, and ensuring early ambulation. These and other measures are especially important when uncontrolled, coexisting disease is present.
Not every obese patient is a significant surgical risk, so care should be individualized and use a team approach involving the gynecologist, anesthesiologist, primary care physician, and other appropriate subspecialists.
This article outlines the parameters of good surgical care in the obese patient, defined here as having a body mass index (BMI) of 30 kg/m2 or above, or 35 kg/m2 or above for morbid obesity. Whenever possible, we draw our recommendations from the published literature. In the absence of data, we base them on our surgical experience in the obese population.
Risks of surgery
It is imperative for the gynecologic surgeon to discuss the special risks of surgery with the obese patient well in advance of the operation and to formulate a systematic plan for evaluation, utilizing other members of the team when necessary. If the surgeon keeps the following risks in mind and is proactive, complications can be kept to a minimum.
Poor wound healing
Wound healing is a complex process involving several concurrent phases; an abnormality in any phase may impair healing. Those phases are:
- inflammatory phase, in which fluid and cells are released to clean the wound and prepare for the next phase of healing
- fibroplastic (proliferative) phase, in which fibroblasts accumulate and form collagen, the building block of connective tissue. This stage is marked by neovascularization and increased formation of granulation tissue
- wound contraction
- remodeling/maturation, in which new collagen is laid down as old collagen is broken down, resulting in scar formation.
- Obtain a chemistry panel: complete blood count, prothrombin time, activated partial thromboplastin time, and arterial blood gas studies. Type and cross-match if significant blood loss is expected
- Order a chest radiograph and electrocardiogram
- Test pulmonary function only if the patient has a history or suspected history of obstructive lung disease
- Order echocardiography only if the electrocardiogram or history suggests compromised cardiac function
- Instruct the patient on the use of incentive spirometry
- Prescribe a mechanical bowel-cleansing regimen if inadvertent injury is likely
- Notify anesthesiology and operating room personnel before the patient’s arrival
- Give 1 g of cefoxitin or another cephalosporin 60 minutes before the start of the procedure3
- Give 5,000 U of subcutaneous unfractionated heparin at least 2 hours before the start of surgery, and administer it every 8 hours until discharge.6 Alternative regimens: 5,000 U of dalteparin, a low-molecular-weight heparin, 12 hours before beginning the procedure and every 12 hours until discharge, or 40 mg of enoxaparin 12 hours before beginning surgery and every 12 hours until discharge
- Apply a pneumatic calf-compression apparatus in the operating room
Obese patients possess a thick layer of adipose tissue, which by its nature and location is minimally vascularized. This tissue essentially becomes dead space, an ideal medium for bacterial growth. Many obese patients also have diabetes mellitus, malignancy, or other comorbidity that further impairs healing.
As a result, obese patients are at increased risk of wound complications, breakdown, and subsequent dehiscence and evisceration. This translates into increased febrile morbidity, prolonged hospitalization, and higher cost.
What the data show. A number of studies have documented a higher incidence of wound complications in obese patients. In one retrospective review, Gallup1 observed an increased risk of wound complications in obese patients, but the incidence diminished after implementation of a protocol of meticulous cleansing, subcutaneous heparin, and modified incision and closure techniques. In a similar retrospective study of 300 obese patients, Pitkin2 reported wound complications among approximately one third of patients and postoperative fever among more than three quarters. Surgical-site infections are thought to occur in as many as 5% of patients.
Prophylaxis may be effective in some patients, but can be challenging. It entails meticulous skin cleansing and careful consideration of where the incision is placed, type of closure, use of a drain, and antibiotic administration.
Antibiotic prophylaxis is based on the theory that its presence in host tissues will alter natural defense mechanisms and kill bacteria that inoculate the wound. Because the window of efficacy is narrow, antibiotics should be administered shortly before the time of inoculation (ie, shortly before the time of incision, vaginal entry, etc.). Current guidelines suggest the use of broad-spectrum agents, including a cephalosporin, approximately 60 minutes before the incision. Redosing is recommended for procedures that last longer than 3 hours, as well as for those that involve significant blood loss (>1,500 mL).
For surgical procedures other than hysterectomy and laparotomy, prophylaxis may not be warranted.3 However, when the patient is markedly obese, many surgeons, including me (Dr. Perkins), sometimes opt to give antibiotics anyway—except for laparoscopic procedures—primarily for wound healing.
Compromised operative exposure
One of the main challenges of surgery in the obese is achieving adequate exposure; when it is inadequate, inadvertent injury may occur.
In addition to a thick abdominal wall composed largely of adipose tissue, these patients frequently have significant accumulations of fat in the mesenteries of the bowel, omentum, and pelvic peritoneum. These accumulations make it difficult to navigate around what becomes a narrow operative field.
Exposure can also be limited in vaginal surgery, because many obese women have very large thighs, buttocks, and accumulations of perineal fat.
Because exposure is a key element of successful surgery, modification of the procedure often becomes necessary—eg, focusing on a single area of the operative field at a time.
Use of a special retractor may help. A self-retaining retractor can be extremely useful. The Bookwalter retractor, first described in 1980, is a commonly used, table-fixed system that attaches to the side rail and can be assembled in minutes (FIGURE 1).4 The variety of rings and blades allows for excellent exposure. Although several complications have been associated with use of the Bookwalter retractor (primarily colon perforation and neuropathy5), they are infrequent and can be minimized by selecting the appropriate blade size and periodically repositioning the blades.
When this or other table-fixed retractors are unavailable, two Balfour retractors, placed at opposite poles of the field to obtain satisfactory exposure, may suffice. With a morbidly obese patient, multiple assistants may still be required to facilitate optimal exposure.
FIGURE 1 A tool to increase exposure
This self-retaining Bookwalter retractor is fixed to the surgical table and features a variety of rings and blades to facilitate exposure.
Thromboembolism
Venous thromboembolism is a major cause of mortality and morbidity in hospitalized patients, causing approximately 60,000 deaths every year.6 Obesity increases the risk of deep venous thrombosis in patients undergoing pelvic surgery. Because of their weight—and, often, coexisting conditions such as cardiorespiratory disease—many obese women are inactive or minimally active postoperatively, increasing the risk of thromboembolism, which remains heightened as long as 3 weeks after discharge. Older women are also at high risk, as are those with a malignancy.
Current guidelines call for the application of a pneumatic calf-compression device in the operating room, with removal after the patient is fully ambulatory. I (Dr. Perkins) also advocate simultaneous use of low-dose heparin, which should be given before surgery and continued until discharge.
Although the use of unfractionated heparin in conjunction with spinal or epidural anesthesia is not a major concern, the use of low-molecular-weight heparin warrants consultation with the anesthesiologist. Unfractionated heparin is preferred because it is metabolized much more rapidly than low-molecular weight heparin.7 The main concern with use of heparin in this setting (regardless of the patient’s weight) is spinal hematoma formation.
Inadvertent injury
Because exposure tends to be limited in obese patients, there is an ever-present risk of injury to bowel, bladder, ureter, and vascular structures. In obese women with a history of abdominal surgery, adhesions are likely, and the risk of bowel injury is increased. Similarly, in obese patients undergoing a laparoscopic procedure, many surface landmarks and vessels may be hard to discern.
Consider preoperative bowel preparation when there is a high risk of intestinal injury.8
Use a scalpel to incise the skin and delineate the area to be excised. That area typically consists of a large wedge of abdominal skin and subcutaneous fat.
Although the addition of panniculectomy to gynecologic surgery in the morbidly obese patient is a fairly recent strategy to increase exposure, the procedure itself has roots in the 19th century. In 1910, Howard Kelly of The Johns Hopkins Hospital reported a lipectomy that involved excision of a large wedge of abdominal skin and fat,21 although indications for that lipectomy appear to have been grounded in cosmesis and personal comfort for the patient.
Technique
Panniculectomy involves excision of a large portion of abdominal skin and subcutaneous fat down to, but not including, the rectus fascia, to gain much greater exposure to the lower abdominal cavity and pelvis.
Once the segment to be excised is delineated, it is mobilized using electrocautery to achieve meticulous hemostasis. After all surgical procedures are completed, the abdominal wall is closed in multiple layers, and drains are placed in the subcutaneous layer. Surgeons who have performed this procedure report significantly increased exposure and access to pelvic structures.
Once the skin is incised, the wedge is mobilized using electrocautery, down to, but not including, the rectus fascia.
Risks
The most important risk is impaired healing of the abdominal wound. One study of this procedure in patients on a gynecologic oncology service noted wound complications in 35% of patients, as well as significant blood loss (up to 1,800 mL in one case).22 Another study by Hopkins and associates,23 however, reported minimal blood loss and wound complications.
Caveats
The procedure requires an experienced surgeon, particularly if no plastic surgeon is readily available. Also, when counseling the patient about this procedure, it is important to emphasize that its primary indication is to maximize exposure; cosmetic benefits are secondary.
Impaired cardiorespiratory function
Pulmonary function typically is compromised in the markedly obese, with restrictive lung disease and reduced functional residual capacity. If the patient smokes or has chronic obstructive lung disease, her pulmonary function is compromised even further, and her condition should be relayed to the anesthesiologist.
In addition, many obese patients have preexisting heart disease or conditions such as hyperlipidemia that put them at risk for heart disease. When evaluating an obese surgical patient, also ask about less apparent disorders, such as sleep apnea, which, if not addressed, may have grave postoperative consequences.
Preoperative evaluation and preparation
Goal: Assess and minimize risks
This process begins in the office or clinic with a discussion with the patient of any concerns and risks. The importance of early mobilization and ambulation after surgery should be emphasized. Any patient with uncontrolled diabetes or hypertension should continue to be monitored by her primary care physician. A patient who has not seen a physician recently should be assessed by an internist to ensure that no conditions go undetected before surgery.
Routine testing to start, but additional assessment may be justified. An obese patient should undergo the same routine testing as a woman of normal weight, but further testing may be warranted for any coexisting disorder. Because the obese patient may have a restrictive lung pattern by virtue of her body habitus, pulmonary-function testing is unlikely to yield new information and is probably not indicated—unless she smokes or has a history of chronic obstructive pulmonary disease. In that case, tests will clarify the obstructive component and bronchodilator response and are useful in postoperative management. Measurement of arterial blood gases is useful, however, because levels reflect respiratory function on a day-to-day basis.
Additional tests of cardiac status probably are not indicated on the basis of obesity alone. However, if initial tests (eg, the electrocardiogram) and the history suggest compromised cardiac function, two-dimensional echocardiography should be performed to determine the ejection fraction. Any concerns regarding cardiac function should be discussed with the anesthesiologist and cardiologist.
Because compromised pulmonary function is likely, I (Dr. Perkins) instruct each patient on the use of incentive spirometry before she undergoes anesthesia so that she has realistic expectations about the postoperative course. I also administer heparin at least 2 hours before induction (8 hours before induction if unfractionated heparin is used).6
If a hysterectomy or prolonged laparotomy is planned, prophylactic antibiotics are recommended.3 Thigh-high compression stockings or a pneumatic calf-compression device should be applied upon arrival in the operating room.
Anesthesia-related issues
Anticipate challenges involving the airway
The anesthesiologist’s primary concern in regard to the obese patient is establishment and maintenance of an airway to promote oxygenation. In morbidly and extremely morbidly obese patients, anatomic factors such as large breasts; a short, thick neck; large tongue; decreased mobility of the cervical spine; limited mouth opening; and greater amount of adipose tissue in the face and cheeks can render mask ventilation and intubation extremely difficult or impossible. Decreased functional residual capacity and tidal volume in the range of closing capacity may lead to extremely rapid oxygen desaturation when the patient is apneic.9
If the patient is pregnant, factors such as excess adipose tissue in the face become even more pronounced and increase the potential for catastrophe.
Appreciate mechanical concerns
The morbidly obese patient may exceed the weight limit of the operating table. In addition, placing her in a steep Trendelenburg position or rotating her laterally may compromise the integrity of the bed.
Coexisting disease, such as sleep apnea and acid reflux disease, should also be kept in mind. Compromised respiratory mechanics (eg, restrictive lung pattern) may cause further deterioration and make mechanical ventilation more difficult.
It also may be hard to establish vascular access, necessitating central venous line placement and introducing its associated risks.
Keep the anesthesiologist in the loop
In the postoperative period, obese patients face a heightened risk of complications related to diminished pulmonary function, such as oxygen desaturation, hypoventilation, and airway obstruction, which may lead to atelectasis, pneumonia, and pulmonary edema.10,11 For these reasons, early consultation with the anesthesiologist is recommended, especially if the initial evaluation suggests potential difficulties in securing the airway. In turn, the anesthesiologist should understand that, in some obese patients, even establishing a surgical airway may be difficult. Regional anesthesia should be considered when feasible.
Antacids and drugs that increase gastric motility have proved to be useful in minimizing aspiration-related risks.12,13
Surgical technique
Begin abdominal procedures by carefully choosing an incision
Do not base the decision solely on the degree of obesity, but also consider any additional procedures that are planned, such as lymph-node sampling. A vertical incision does permit greater exposure than is afforded by a transverse incision, but in some cases the latter may be more appropriate—even if the patient is morbidly obese.
Do not place the incision below the panniculus, in the crease just above the suprapubic mound, though it may be tempting to do so when the panniculus is large and thick. This area is a warm, moist, anaerobic environment that promotes the proliferation of numerous micro-organisms, creating a bacterial cesspool. It is the worst place to make an incision.
If a transverse incision is selected, place caudal traction on the panniculus (which may be facilitated by applying two towel clips to the tissue fold), and incise through the fold at a point approximately three to four fingerbreadths above the symphysis (FIGURE 2).
If a midline incision is selected, a similar technique is appropriate, with downward traction applied to the panniculus and the incision begun at the lower pole of this fold up to the umbilicus—or through it and above, should more room be required.14
After incising the superficial fascia, greater exposure may be gained by incising the rectus sheath beneath the pannicular fold and extending it down to the symphysis. After entering the abdominal cavity, the surgeon may encounter a redundant, fat-laden layer of peritoneum. The edges of this tissue may be temporarily sutured to the edges of the skin incision to remove it from the operative field and obtain better visualization.
FIGURE 2 Incision placement can be counterintuitive
Avoid placing the skin incision beneath the panniculus, an anaerobic area ripe for infection. Instead, retract the panniculus caudally and incise the skin above the fold, as shown.
A few remedies can help when exposure is limited
Exposure is one of the most important elements of successful surgery, but it is often restricted when marked obesity is present. Fortunately, numerous adjuncts are available to address this problem, such as the table-fixed retractor systems described earlier in this article, or one or more of the following tactics:
- Do not try to expose the entire pelvic basin. One technique to ease exploration—especially when more than one procedure is planned—is to refrain from exposing the entire pelvic basin at one time. Instead, focus on obtaining adequate visualization in the immediate area and, once work in that area is finished, concentrate on the next.
- Use extra-long instruments in an extremely large patient, especially if she has a “deep” pelvis.
- Abbreviate the procedure, if possible. Because these cases are technically more difficult and frequently involve excessive blood loss, it may be wiser to perform an “incomplete” procedure, such as supracervical hysterectomy instead of total hysterectomy, depending on the indication.14
- Use a cell-saver blood-collection system if significant blood loss is anticipated. Also remember to give a second dose of prophylactic antibiotic.
Strategies for effective wound closure
The best method of abdominal wound closure has been a subject of debate among both gynecologic and general surgeons. In the obese patient, the key variables are the rather thick subcutaneous fat layer within the abdominal wall and the impact of intra-abdominal pressure on the incision.
What the data show. A number of studies, including one by Montz and colleagues,15 have demonstrated that the running mass-closure technique using delayed-absorbable or permanent suture is just as effective as interrupted suture placement (eg, Smead-Jones closure) but is faster, with less suture deposited in the wound.
The following considerations may also be helpful:
- Approximate the subcutaneous fascia? There has been some debate about whether the subcutaneous fascia must be approximated. My (Dr. Perkins) personal preference in morbidly obese patients is to place several interrupted sutures to obliterate much of the dead space, facilitate skin closure, and minimize tension on the wound; I have noted no significant increase in wound complications using this technique. Placement of a closed-suction drain (eg, Jackson Pratt, Hemovac) is a good alternative.
- Is a drain useful? Some have questioned whether use of a drain increases the likelihood of wound complications,16 but this concern is irrelevant because, in most—if not all—cases, the drainage tubing is exteriorized via a separate stab wound remote from the incision.
- Consider retention sutures. A morbidly obese patient may benefit from through-and-through retention sutures using 0 or #1 permanent material along with rubber bolsters to minimize cutting of the suture into the skin, especially if increased intra-abdominal pressure is likely (FIGURE 3).17 These sutures can be removed on the 10th to 12th postoperative day.
FIGURE 3 Minimize tension on the wound
A morbidly obese patient may benefit from through-and-through retention sutures along with rubber bolsters to minimize cutting of the suture into the skin.
Vaginal surgery does not increase complication rate in the obese
The vaginal approach can be extremely challenging in the morbidly obese patient, especially when hysterectomy is performed. Exposure is often compromised by the large folds about the thighs and buttocks, limiting access to the perineum and vaginal vault. If the patient also has a narrow, contracted pelvis, the difficulty is compounded. Because of these and other concerns about morbidity, many gynecologists hesitate to perform hysterectomy via the vaginal route when the patient is obese.
What the data show. Several studies have addressed these issues, including one by Pratt and Daikoku18 and another by Rafii and colleagues,19 both of which demonstrated that obese patients have a complication rate roughly equivalent to that observed in patients of normal weight, although obese patients have a greater decrease in hemoglobin level and a slightly higher incidence of postoperative fever.
Similarly, in a retrospective study, Pitkin20 found no significant difference between the complication rates of obese and nonobese patients.
Another argument for the self-retaining retractor. From a technical standpoint, achieving good exposure is the primary challenge of vaginal surgery and usually requires two or more assistants—who themselves have limited or no direct view of the field—who must stand for long periods. Again, a viable alternative is use of a self-retaining retractor. One in particular, the MiniOmni retractor, is a small, uncumbersome, table-fixed system that can be maneuvered so that vaginal and perineal structures are readily accessible.
Choice of stirrups is also relevant. Exposure can be affected by the type of stirrups used. “Candy cane” stirrups facilitate exposure more than fixed stirrups (eg, Allen stirrups) do. Regardless of the stirrups selected, however, it is important to avoid excessive or prolonged hip flexion, or nerve injury may result.
How to minimize postoperative complications
After surgery, an obese patient requires close and continuous monitoring to avert complications and detect any that occur. Consider the following measures:
- A stint in the intensive care unit. In the morbidly obese patient, massive fluid shift (eg, extensive blood loss, prolonged surgery with losses from the peritoneum, etc.) or concern about sleep apnea may justify close monitoring in an intensive care unit or similar setting—at least briefly. Later, as the patient recovers, sleep studies may indicate whether apnea is present.
- Document fluid intake and output, especially in the elderly and in women with cardiorespiratory disease.
- Give analgesics in an amount sufficient to control pain and minimize activity that might place excessive tension on the abdominal incision, but also allows the patient to remain alert enough to ambulate effectively and perform pulmonary toileting.
- Begin ambulation on the first postoperative day—or on the evening after surgery, if circumstances permit. This helps clear secretions from the respiratory tract, reduces the risk of thromboembolism, and speeds the return of normal bowel function. Hourly incentive spirometry is also recommended for the first few days after surgery.
- Continue heparin. In patients at moderate or high risk of thromboembolism, continue low-dose heparin until discharge or for 7 days, whichever comes first.
- Keep other physicians involved. If the patient has a significant comorbidity, such as cardiorespiratory disease or uncontrolled diabetes, she should remain under the care of her internist or other primary care provider.
- Closely monitor the surgical wound for early signs of infection, which include inflammation and collections of serous fluid, blood, pus, or a mixture of these. If retention sutures were placed, check them frequently to ensure that they are not cutting into the skin of the abdomen.
- Strongly recommend weight loss. At the time of the last postoperative visit, tell the patient in clear language that obesity is extremely bad for her health and strongly encourage her to lose weight under the supervision of her primary care provider. If she has no such provider, make the appropriate referral.
1. Gallup DG. Modifications of celiotomy techniques to decrease morbidity in obese gynecologic patients. Am J Obstet Gynecol. 1984;150:171-178.
2. Pitkin RM. Abdominal hysterectomy in obese women. Surg Gynecol Obstet. 1976;142:532-536.
3. Antibiotic prophylaxis for gynecologic procedures. ACOG Practice Bulletin No. 74. Washington, DC: American College of Obstetricians and Gynecologists; July 2006.
4. Bookwalter JR. A new table-fixed retractor. Surg Clin North Am. 1980;60:399-405.
5. Noldus J, Graefen M, Huland H. Major postoperative complications secondary to use of the Bookwalter self-retaining retractor. Urology. 2002;60:964-967.
6. Prevention of deep vein thrombosis and pulmonary embolism. ACOG Practice Bulletin No. 21. Washington, DC: American College of Obstetricians and Gynecologists; October 2000.
7. Vandermeulen EP, Van Aken H, Vermylen J. Anticoagulants and spinal-epidural anesthesia. Anesth Analg. 1994;79:1165-1177.
8. Perkins JD, Dent LL. Avoiding and repairing bowel injury in gynecologic surgery. OBG Management. 2004;16(8):15-28.
9. Cressey DM, Berthoud MC, Reilly CS. Effectiveness of continuous airway pressure to enhance preoxygenation in morbidly obese women. Anesthesia. 2001;56:670-689.
10. Jordan H, Perlow MD, Mark A, Morgan MD. Massive maternal obesity and perioperative cesarean morbidity. Am J Obstet Gynecol. 1994;170:560-565.
11. Hood DD, Dewan DN. Anesthetic and obstetric outcome in morbidly obese parturients. Anesthesiology. 1993;79:1210-1218.
12. James CF, Gibbs CP, Banner T. Postpartum perioperative risk of aspiration pneumonia. Anesthesiology. 1984;61:756-759.
13. Manchikanti L, Colliver JA, Marrero TC, Roush JR. Ranitidine and metoclopramide for prophylaxis of aspiration pneumonitis in elective surgery. Anesth Analg. 1984;63:903-910.
14. Morrow CP, Hernandez WL, Townsend DE, DiSaia PJ. Pelvic celiotomy in the obese patient. Am J Obstet Gynecol. 1977;127:335-339.
15. Montz FJ, Creasman WT, Eddy G, DiSaia PJ. Running mass closure of abdominal wounds using absorbable looped suture. J Gynecol Surg. 1991;7:107-110.
16. Ramsey PS, White AM, Guinn DA, et al. Subcutaneous tissue reapproximation, alone or in combination with drain, in obese women undergoing cesarean delivery. Obstet Gynecol. 2005;105:967-973.
17. Soisson AP, Olt G, Soper JT, Berchuck A, Rodriguez G, Clarke-Pearson DL. Prevention of superficial wound separation with subcutaneous retention sutures. Gynecol Oncol. 1993;51:330-334.
18. Pratt JH, Daikoku NH. Obesity and vaginal hysterectomy. J Reprod Med. 1990;35:945-949.
19. Rafii A, Samain E, Levardon M, Darai E, Deval B. Vaginal hysterectomy for benign disorders in obese women: a prospective study. Br J Obstet Gynaecol. 2005;111:223-227.
20. Pitkin RM. Vaginal hysterectomy in obese women. Obstet Gynecol. 1977;49:567-569.
21. Kelly HA. Excision of the fat of the abdominal wall—lipectomy. Surg Gynecol Obstet. 1910;10:229-231.
22. Wright JD, Rosenbush EJ, Powell MA, et al. Longterm outcome of women who undergo panniculectomy at the time of gynecologic surgery. Gynecol Oncol. 2006;102:86-91.
23. Hopkins MP, Shriner AM, Parker MG, Scott L. Panniculectomy at the time of gynecologic surgery in morbidly obese patients. Am J Obstet Gynecol. 2000;182:1502-1505.
1. Gallup DG. Modifications of celiotomy techniques to decrease morbidity in obese gynecologic patients. Am J Obstet Gynecol. 1984;150:171-178.
2. Pitkin RM. Abdominal hysterectomy in obese women. Surg Gynecol Obstet. 1976;142:532-536.
3. Antibiotic prophylaxis for gynecologic procedures. ACOG Practice Bulletin No. 74. Washington, DC: American College of Obstetricians and Gynecologists; July 2006.
4. Bookwalter JR. A new table-fixed retractor. Surg Clin North Am. 1980;60:399-405.
5. Noldus J, Graefen M, Huland H. Major postoperative complications secondary to use of the Bookwalter self-retaining retractor. Urology. 2002;60:964-967.
6. Prevention of deep vein thrombosis and pulmonary embolism. ACOG Practice Bulletin No. 21. Washington, DC: American College of Obstetricians and Gynecologists; October 2000.
7. Vandermeulen EP, Van Aken H, Vermylen J. Anticoagulants and spinal-epidural anesthesia. Anesth Analg. 1994;79:1165-1177.
8. Perkins JD, Dent LL. Avoiding and repairing bowel injury in gynecologic surgery. OBG Management. 2004;16(8):15-28.
9. Cressey DM, Berthoud MC, Reilly CS. Effectiveness of continuous airway pressure to enhance preoxygenation in morbidly obese women. Anesthesia. 2001;56:670-689.
10. Jordan H, Perlow MD, Mark A, Morgan MD. Massive maternal obesity and perioperative cesarean morbidity. Am J Obstet Gynecol. 1994;170:560-565.
11. Hood DD, Dewan DN. Anesthetic and obstetric outcome in morbidly obese parturients. Anesthesiology. 1993;79:1210-1218.
12. James CF, Gibbs CP, Banner T. Postpartum perioperative risk of aspiration pneumonia. Anesthesiology. 1984;61:756-759.
13. Manchikanti L, Colliver JA, Marrero TC, Roush JR. Ranitidine and metoclopramide for prophylaxis of aspiration pneumonitis in elective surgery. Anesth Analg. 1984;63:903-910.
14. Morrow CP, Hernandez WL, Townsend DE, DiSaia PJ. Pelvic celiotomy in the obese patient. Am J Obstet Gynecol. 1977;127:335-339.
15. Montz FJ, Creasman WT, Eddy G, DiSaia PJ. Running mass closure of abdominal wounds using absorbable looped suture. J Gynecol Surg. 1991;7:107-110.
16. Ramsey PS, White AM, Guinn DA, et al. Subcutaneous tissue reapproximation, alone or in combination with drain, in obese women undergoing cesarean delivery. Obstet Gynecol. 2005;105:967-973.
17. Soisson AP, Olt G, Soper JT, Berchuck A, Rodriguez G, Clarke-Pearson DL. Prevention of superficial wound separation with subcutaneous retention sutures. Gynecol Oncol. 1993;51:330-334.
18. Pratt JH, Daikoku NH. Obesity and vaginal hysterectomy. J Reprod Med. 1990;35:945-949.
19. Rafii A, Samain E, Levardon M, Darai E, Deval B. Vaginal hysterectomy for benign disorders in obese women: a prospective study. Br J Obstet Gynaecol. 2005;111:223-227.
20. Pitkin RM. Vaginal hysterectomy in obese women. Obstet Gynecol. 1977;49:567-569.
21. Kelly HA. Excision of the fat of the abdominal wall—lipectomy. Surg Gynecol Obstet. 1910;10:229-231.
22. Wright JD, Rosenbush EJ, Powell MA, et al. Longterm outcome of women who undergo panniculectomy at the time of gynecologic surgery. Gynecol Oncol. 2006;102:86-91.
23. Hopkins MP, Shriner AM, Parker MG, Scott L. Panniculectomy at the time of gynecologic surgery in morbidly obese patients. Am J Obstet Gynecol. 2000;182:1502-1505.
Endometrial ablation devices: How to make them truly safe
CASE: Leaking fluid causes intraoperative burns
G.S. is a 45-year-old mother of three who is admitted for surgery for persistent menorrhagia. She has experienced at least two menstrual periods every month for several months, each of them associated with heavy bleeding. She has a history of hypothyroidism and hypertension, but no serious disease or surgery, and considers herself to be in good physical and mental health.
G.S. undergoes endometrial hydrothermablation (HTA) under general inhalation anesthesia. After the HTA mechanism is primed, the heating cycle is started, with a good seal and no fluid leaking from the cervix.
Approximately 8 minutes into the procedure, a 5-mL fluid deficit is noted, and a small amount of hot fluid is observed to be leaking from the cervical os. Examination reveals a thermal injury to the cervix and anterior vaginal wall. The wound is irrigated with cool, sterile saline, and silver sulfadiazine cream is applied. The patient is discharged.
Could this injury have been avoided? Is further treatment warranted?
A minimally invasive operation does not necessarily translate to minimal risk of serious complications. Although few studies of nonhysteroscopic endometrial ablation techniques report any complications,1,2 Baggish and Savells3 found a number of injuries when they searched hospital records and the Food and Drug Administration (FDA) database (TABLE). They identified serious complications associated with the following devices:
- HydroThermablator (Boston Scientific), which utilizes a modified operating hysteroscope to deliver 10 to 12 mL of preheated saline into the uterus under low pressure.4 Complications: 16 adverse events were reported to the FDA, 13 of which involved the retrograde leakage of hot water, causing burns to the cervix, vagina, and vulva. Six additional injuries not reported to the FDA were identified at a single institution.
- Novasure (Cytyc), which employs bipolar electrodes that cover a porous bag.5,6 Complications: 32 injuries, 26 of them uterine perforations.
- Thermachoice (Gynecare), a fluid-distended balloon ablator.7 Complications: 22 injuries included retrograde leakage of hot water after balloon failure and transmural thermal injury, with spread to, and injury of, proximal structures. One death was reported.
- Microsulis (MEA), which uses microwave energy to ablate the endometrium.8-10 Complications: 19 injuries, including 13 thermal injuries to the intestines.
Baggish and Savells3 initiated this study after discovering six adverse events within their own hospital system utilizing a single device (HTA). Because these injuries were not reported to the FDA, the overall number of complications is likely higher than the figures given here.
This article describes the proper use of nonhysteroscopic endometrial ablation devices, the best ways to avert serious injury, and optimal treatment when complication occurs.
TABLE
Complications associated with 4 endometrial ablation devices
| COMPLICATION | HYDRO THERMABLATOR* | THERMACHOICE | NOVASURE | MICROSULIS |
|---|---|---|---|---|
| Uterine perforation | 2 | 3 | 26 | 19 |
| Intestinal injury | 1† | 1† | – | 13† |
| Retrograde leakage burn | 19 | 6 | – | – |
| Infection/sepsis | – | 1† | 2 | 1 |
| Fistula/sinus | – | 1† | 1 | – |
| Transmural uterine burn | – | 1 | – | – |
| Cervical stenosis | – | 8 | 1 | – |
| Cardiac arrest | 1 | – | 1 | – |
| Death | – | 1 | – | – |
| Other major | – | 3 | 1 | 4† |
| Total | 22 | 22 | 32 | 20 |
| * Includes author’s data; 6 retrograde leaks | ||||
| † Collateral injury | ||||
CASE continued: Patient opts for hysterectomy
In the case just described, G.S. was examined 1 week after surgery and found to have an exophytic burn over the entire right half of the cervix, extending into the vagina. She was readmitted for 3 days of intravenous (IV) antibiotic treatment and wound care. Computed tomography imaging showed gas formation within the damaged cervix.
Six weeks after surgery, the patient was still menstruating heavily, but her cervix and vagina had healed. Six months later, she underwent total abdominal hysterectomy for continued menorrhagia.
When is endometrial ablation an option?
Indications for endometrial ablation using a nonhysteroscopic, minimally invasive technique are no different from those for hysteroscopic ablation.11 Abnormal, or dysfunctional, uterine bleeding is the principal reason for this operation. Dysfunctional bleeding is heavy or prolonged menses over 6 months or longer that fail to respond to conservative measures and occur in the absence of tumor, pregnancy, or inflammation (ie, infection).
A woman who meets these criteria should have a desire to retain her uterus if she is to be a candidate for a nonhysteroscopic, minimally invasive technique. She also should understand that ablation can render pregnancy unlikely and even pathologic. Her understanding of this consequence should be documented in the chart! Last, she should be informed that ablation will not necessarily render her sterile, so contraception or sterilization will be required to avoid pregnancy. This should also be clearly documented in the medical record.
Endometrial ablation may also be an alternative to hysterectomy for a mentally retarded woman who is unable to manage menses. Abnormal uterine bleeding in conjunction with bleeding diathesis, significant obesity, or serious medical disorders can also be treated by endometrial ablation.
Avoid endometrial ablation in certain circumstances
These circumstances include the presence of endometrial hyperplasia, endometrial cancer, endocervical neoplasia, cervical stenosis, an undiagnosed adnexal mass, moderate to severe dysmenorrhea, adenomyosis, or a uterine cavity larger than 10 cm.12-15
Valle and Baggish15 reported eight cases in which women developed endometrial carcinoma following ablation, and identified the following major risk factors for postablation cancer:
- endometrial hyperplasia unresponsive to progesterone or progestin therapy
- complex endometrial hyperplasia
- atypical hyperplasia.
These conditions are contraindications to endometrial ablation.
Avoid a rush to ablation
The growing popularity of office-based, minimally invasive, nonhysteroscopic techniques, coupled with an increasing desire for and acceptance of elective cessation of menses, may stretch the indications listed above and cut short the discovery of contraindications. Clearly, thorough endometrial sampling and precise histopathologic interpretation are required before embarking on any type of endometrial ablation, to minimize the risk of complications.
How to prevent injury
Reduce the risk of perforation
Uterine perforation occurs for a variety of reasons:
- position of the uterus is unknown
- uterus has not been gently and carefully sounded
- cervix is insufficiently dilated to permit passage of the probe
- device is too long (large) to be accommodated in an individual patient’s uterus
- uterine cavity is distorted by pathology, such as adhesions, myomas, etc.
Attention to these details before surgery can prevent perforation.
When uterine injury occurs, the bowel is also at risk
The intestines can be injured following perforation or transmural injury of the uterus. Bowel injury has been reported with hysteroscopic ablation and resection as well as with Nd-YAG laser ablation.16-18
Do not activate hot water or electrosurgical energy unless you are 100% certain that the device is within the uterine cavity.
Ideally, manufacturers’ safety studies should guarantee no risk of transtubal spillage of hot liquid.
Hot fluid adds to risk of burns
Devices that permit retrograde leakage of hot fluid, such as the HTA, should be modified to ensure sealing at the level of the external and internal cervical os. The Enable device (Innerdyne), no longer marketed in the United States, had such a sealing mechanism, which minimized retrograde leakage of hot water.
Balloon failure may be an unavoidable injury, but pretesting of the device and careful attention to pressure readings—particularly in a small uterus—may mitigate the risk.
Be alert for electrical leakage
The microwave device operates at the megahertz range of frequency. At this high frequency, the risk of leakage is much greater than with devices that operate in the kilohertz range. Therefore, it is important to pay close attention to grounding sites, such as cardiovascular-monitoring electrodes.
High-power monopolar devices, prolonged application of energy to tissue, and high generator frequency are all associated with leakage and subsequent burns.
- Keep the success rate above 90%
- Minimize complications by proper technique and instrument selection
- Press the market to develop a range of device sizes that will individualize the procedure
- Keep the price of a procedure under $1,000
- Establish and adhere to careful patient selection criteria
Early recognition and treatment are vital to ensure the patient’s safety and reduce the risk of medicolegal liability. I recommend the following steps:
- Stop the procedure immediately if perforation is suspected. If you suspect that hot water has been dispersed within the abdominal cavity, switch to laparotomy and consult a general surgeon to inspect the entire intestine for injury. If perforation occurs during the use of electrosurgical energy, the same action is warranted. If uterine perforation occurs in isolation (ie, there is no thermal energy compounding the problem), admit the patient for careful observation, appropriate blood chemistries and hematologic studies, and radiologic examination.
- When hot liquids are spilled, switch to retrograde flow immediately and generously flush the vulva, vagina, and cervix with cold water. Cleanse the entire area with a soapless detergent, and apply clindamycin cream to the vagina and silver sulfadiazine cream to the vulva. Admit the patient for application of cold compresses, ice packs, and burn therapy, and obtain baseline cultures and hematologic studies and a plastic surgery consult. If third-degree (full thickness) burns are suspected, treat any suspected wound infection aggressively after obtaining cultures. Severe and inordinate pain should be investigated as a possible sign of necrotizing fasciitis. After discharge, follow the patient’s progress at weekly intervals.
- Talk to the patient and her family. It is a good idea to explain the complication in very clear terms. I believe it is reasonable to explain how the complication occurred, without speculation or theatrical explanations. Also be sure to document this conversation, including date and time. It may be useful to have a neutral witness present during the conversation. By and large, the patient and her family are likely to appreciate an honest account of how the complication occurred. Hiding data or attempting to cover up the injury may motivate the patient to seek legal representation.
The long-term success of endometrial ablation devices as a whole depends on several conditions. Foremost, the entire class of devices should demonstrate efficacy on par with hysteroscopic ablation. Currently, efficacy ranges from 80% to 95% (short-term follow-up).11 The goal of minimally invasive procedures should be a sustainable 92% rate of amenorrhea, hypomenorrhea, or light, periodic menses. A long-term failure rate of 25% is unacceptable.22-24 If the devices can, by their simplicity, be adapted to more or less universal office application and attain a 5-year success rate of 90% or higher, they will become the standard of care.
One size does not really fit all
Serious complications from endometrial ablation devices occur with regular frequency and must be eliminated or greatly reduced. Perforation is a significant problem and may be related to the “one-size-fits-all” design of the device. Perhaps a range of sizes needs to be produced and fitted to the individual uterine cavity.
If such complications as perforation and burns to the bowel, cervix, vagina, and vulva can be eliminated or relegated to rarity, then a happy future for these procedures lies beyond the horizon.
Price ceiling should be set at $1,000
If an operation can consistently be performed for less than $1,000 total cost—the cost of in-hospital endometrial ablation—it will gain mass appeal. In hospitals and so-called surgicenters, ablations are expensive and, therefore, less attractive to self- or third-party payers. If fees are based on the volume of cases, then a procedure may be price-efficient.
Outcome depends on patient selection
Poorly screened patients who have underlying hyperplasia may develop postablation carcinoma. Women who have dysmenorrhea before the procedure can be predicted to suffer from it afterward. Older women (ie, 40 years or older) will have better long-term success than younger women. And women with a large uterus or myomas will have a higher failure rate than women with smaller cavities (ie, less than 10 cm in length).
What this means for the individual surgeon
Although minimally invasive techniques are relatively easy to perform and simple to learn, each part of the procedure requires careful application and great attention to detail. Perforation of the uterus and leakage of scalding hot liquid must be avoided. If these complications occur, prompt diagnosis and appropriate treatment are critical. The removal of these procedures from the operating room to the office as well as competitive pricing of instrumentation will make nonhysteroscopic, minimally invasive endometrial ablation more cost-effective.
The modern era of practical endometrial ablation began in 1981, when Goldrath and colleagues19 reported Nd-YAG laser photovaporization of the endometrium via hysteroscopy for treatment of excessive uterine bleeding. Two years later, DeCherney and Polan20 reported hysteroscopic control of abnormal uterine bleeding using the urologic resectoscope.
Over succeeding years, Baggish and Baltoyannis21 and Baggish and Sze22 reported extensive experience with hysteroscopic endometrial ablation in both high- and average-risk patients, including long-term follow-up of 568 cases over 11 years. Garry and colleagues23 reported a large series of 600 cases from the United Kingdom. Not only did these laser techniques prove to be effective, achieving amenorrhea rates ranging from 30% to 60%, but overall control of abnormal bleeding exceeded 90%. In the large series involving approximately 1,200 cases, no uterine perforations were reported.21-23 The major complication: Fluid overload secondary to vascular uptake of distension medium.
In Europe and the United Kingdom, most hysteroscopic treatment of abnormal bleeding involved endometrial resection using the cutting loop of the resectoscope. In the United States, ablation with the ball electrode of the resectoscope largely replaced the Nd-YAG laser because the resectoscopic trigger mechanism required less skill and hand–eye coordination than the hand–finger-controlled movement of the 600- to 1,000-micron laser fiber.24-26
A search for more benign techniques
A 1997 UK survey analyzed 10,686 cases of hysteroscopic endometrial destruction and identified 474 complications.27 Resection alone had a complication rate of 10.9% and an emergency hysterectomy rate of 13 for every 1,000 patients. Laser ablation had a complication rate of 5.5% and an emergency hysterectomy rate of 2 for every 1,000 patients, and the corresponding figures for rollerball ablation were a 4.5% complication rate and 3 emergency hysterectomies for every 1,000 patients. Two deaths occurred (in 10,000 cases) and were associated with loop excision.
Published data indicated that:
- Successful outcomes after endometrial ablation or resection were directly proportional to the skill of the surgeon
- Complications, particularly serious complications, were related to the experience and skill of the surgeon
- Infusion of uterine distension medium, particularly hypo-osmolar solutions, was associated with serious complications when fluid deficits exceeded approximately 500 to 1,000 mL.
As a result, a number of investigators sought to develop new surgical techniques to control abnormal uterine bleeding that would minimize the skill required by the surgeon (requiring only insertion of a cannula into the uterus and a “cookbook” ablation procedure), eliminate the need for distension medium and general anesthesia, and attain efficacy equivalent to earlier techniques.
A quartet of options
Among the devices that resulted were:
- A microwave technique, described by several investigators.8-10 Its chief drawback: High-frequency electrical leakage with the potential to cause thermal burns.
- An intrauterine balloon device distended with sterile water or saline is heated in situ to 85° to 90° Celsius, thereby cooking the endometrium.
- An electrode-bearing device that features an array of monopolar electrodes over the endometrium-facing aspect of a balloon or bipolar electrodes over a porous bag.
- Devices that circulate a small volume of hot saline freely within the uterine cavity. Hydrothermablation delivers 10 to 12 mL of preheated saline into the uterus under low pressure. A similar technique delivers 10 to 12 mL of cool water or saline into the uterus through a sealed cannula, followed by in situ heating and circulation of the fluid at low pressure via a computer-controlled device.
Safety studies were required by the FDA and were performed on all these devices, and the risk of complications appeared to be negligible.1,2 As this article illustrates, that is not the case.
Four devices, four ways of achieving ablation
Since the advent of nonhysteroscopic, minimally invasive endometrial ablation devices, four distinct techniques have gained widespread use
Hydrothermablation
The closed-loop system (HTA) ablates the lining of the endometrium under hysteroscopic visualization by recirculating heated saline within the uterus. The modified hysteroscope allows the operator to view the ablation as it occurs within the uterine cavity.
Balloon ablation
Balloon ablation (Thermachoice) features a double-dip balloon construction that conforms to the contours of the uterine cavity. The saline or water in the balloon is heated in situ. This device requires an undistorted uterine cavity, relies on the integrity of the balloon to prevent forward or retrograde spillage of scalding water, and is time-controlled.
Radiofrequency technology
The three-dimensional gold-plated bipolar mesh electrode (NovaSure) is inserted into the uterine cavity and advanced toward the fundus. Once it is properly positioned (above, left), the system is activated to produce 180 W of bipolar power. A moisture-transport vacuum system draws the endometrium into contact with the mesh to enhance tissue vaporization and evacuate debris.
Microwave energy
Microwave energy is emitted from the tip of the device (Microsulis), which is moved back and forth in a sweeping manner, from the fundus to the lower uterine segment. The device directly heats tissue to a depth of 3 mm, with conductive heating of adjacent tissue for an additional 2 to 3 mm. The total 5- to 6-mm depth ensures coagulation and destruction of the basal layer. Microwave energy does not require direct contact with the tissue, as it will “fill the gap” caused by cornual and fibroid distortions.
1. Bustos-Lopez H, Baggish MS, Valle RF, et al. Assessment of the safety of intrauterine instillation of heated saline for endometrial ablation. Fertil Steril. 1998;69:155-160.
2. Baggish MS, Paraiso M, Breznock EM, et al. A computer-controlled, continuously circulating, hot irrigating system for endometrial ablation. Am J Obstet Gynecol. 1995;173:1842-1848.
3. Baggish MS, Savells A. Complications associated with minimally invasive non-hysteroscopic endometrial ablation techniques. J Gynecol Surg. 2007;23:7-12.
4. Goldrath MH. Evaluation of HydroThermablator and rollerball endometrial ablation for menorrhagia: 3 years after treatment. J Am Assoc Gynecol Laparosc. 2003;10:505-511.
5. Abbott J, Hawe J, Hunter D, et al. A double-blind randomized trial comparing the cavaterm and the Novasure endometrial ablation systems for the treatment of dysfunctional uterine bleeding. Fertil Steril. 2003;80:203-208.
6. Cooper J, Gimpelson R, Laberge P, et al. A randomized, multi-center trial of safety and efficacy of the Novasure system in the treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:418-428.
7. Loffer FD, Grainger D. Five-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:429-435.
8. Phipps JH, Lewis BV, Roberts T, et al. Treatment of functional menorrhagia with radiofrequency endometrial ablation. Lancet. 1990;335:374-376.
9. Sharp N, Cronin N, Feldberg I, et al. Microwaves for menorrhagia: a new fast technique for endometrial ablation. Lancet. 1995;346:1003-1004.
10. Thijssen RFA. Radiofrequency-induced endometrial ablation: an update. Br J Obstet Gynaecol. 1997;104:608-613.
11. Baggish MS. Minimally invasive non-hysteroscopic methods for endometrial ablation. In: Baggish MS, Valle RF, Guedj H, eds. Hysteroscopy: Visual Perspectives of Uterine Anatomy, Physiology, and Pathology. 3rd ed. Philadelphia: Lippincott, Williams and Wilkins; 2007:405-415.
12. Dwyer N, Hutton J, Stirrat GM. Randomized controlled trial comparing endometrial resection with abdominal hysterectomy for the surgical treatment of menorrhagia. Br J Obstet Gynaecol. 1993;100:237-243.
13. Raiga J, Mage G, Glowaczower E, et al. Factors affecting risk of failure after endometrial resection. J Gynecol Surg. 1995;11:1-5.
14. Shelly-Jones D, Mooney P, Garry R. Factors influencing the outcome of endometrial laser ablation. J Gynecol Surg. 1994;10:211-215.
15. Valle RF, Baggish MS. Endometrial carcinoma after endometrial ablation: high-risk factors predicting its occurrence. Am J Obstet Gynecol. 1998;176:569-572.
16. Kanter MH, Kivnick S. Bowel injury from rollerball ablation of the endometrium. Obstet Gynecol. 1992;79:833-835.
17. Perry CP, Daniell JF, Gimpelson RJ. Bowel injury from Nd-YAG endometrial ablation. J Gynecol Surg. 1990;6:1999-2003.
18. Scottish Hysteroscopy Audit Group. A Scottish audit of hysteroscopic surgery for menorrhagia: complications and follow-up. Br J Obstet Gynaecol. 1995;102:239-254.
19. Goldrath MH, Fuller TA, Segal S. Laser photovaporization of the endometrium for the treatment of menorrhagia. Am J Obstet Gynecol. 1981;40:14-19.
20. DeCherney A, Polan ML. Hysteroscopic management of intrauterine lesions and intractable uterine bleeding. Obstet Gynecol. 1983;61:392-396.
21. Baggish MS, Baltoyannis P. New techniques for laser ablation of the endometrium in high-risk patients. Am J Obstet Gynecol. 1988;159:287-292.
22. Baggish MS, Sze EHM. Endometrial ablation: a series of 568 patients treated over an 11-year period. Am J Obstet Gynecol. 1996;174:908-913.
23. Garry R, Shelly-Jones D, Mooney P, et al. Six hundred endometrial laser ablations. Obstet Gynecol. 1995;85:24-29.
24. Magos AL, Bauman R, Lockwood GM, et al. Experience with the first 250 endometrial resections for menorrhagia. Lancet. 1991;337:1074-1078.
25. Wortman M, Daggett A. Hysteroscopic endometrial resection: a new technique for the treatment of menorrhagia. Obstet Gynecol. 1994;83:295-298.
26. Townsend DE, Richart RM, Paskowitz, et al. Rollerball coagulation of the endometrium. Obstet Gynecol. 1990;76:310-313.
27. Overton C, Hargreaves J, Maresh M. A national survey of the complications of endometrial destruction for menstrual disorders: the mistletoe study. Br J Obstet Gynaecol. 1997;104:1351-1359.
CASE: Leaking fluid causes intraoperative burns
G.S. is a 45-year-old mother of three who is admitted for surgery for persistent menorrhagia. She has experienced at least two menstrual periods every month for several months, each of them associated with heavy bleeding. She has a history of hypothyroidism and hypertension, but no serious disease or surgery, and considers herself to be in good physical and mental health.
G.S. undergoes endometrial hydrothermablation (HTA) under general inhalation anesthesia. After the HTA mechanism is primed, the heating cycle is started, with a good seal and no fluid leaking from the cervix.
Approximately 8 minutes into the procedure, a 5-mL fluid deficit is noted, and a small amount of hot fluid is observed to be leaking from the cervical os. Examination reveals a thermal injury to the cervix and anterior vaginal wall. The wound is irrigated with cool, sterile saline, and silver sulfadiazine cream is applied. The patient is discharged.
Could this injury have been avoided? Is further treatment warranted?
A minimally invasive operation does not necessarily translate to minimal risk of serious complications. Although few studies of nonhysteroscopic endometrial ablation techniques report any complications,1,2 Baggish and Savells3 found a number of injuries when they searched hospital records and the Food and Drug Administration (FDA) database (TABLE). They identified serious complications associated with the following devices:
- HydroThermablator (Boston Scientific), which utilizes a modified operating hysteroscope to deliver 10 to 12 mL of preheated saline into the uterus under low pressure.4 Complications: 16 adverse events were reported to the FDA, 13 of which involved the retrograde leakage of hot water, causing burns to the cervix, vagina, and vulva. Six additional injuries not reported to the FDA were identified at a single institution.
- Novasure (Cytyc), which employs bipolar electrodes that cover a porous bag.5,6 Complications: 32 injuries, 26 of them uterine perforations.
- Thermachoice (Gynecare), a fluid-distended balloon ablator.7 Complications: 22 injuries included retrograde leakage of hot water after balloon failure and transmural thermal injury, with spread to, and injury of, proximal structures. One death was reported.
- Microsulis (MEA), which uses microwave energy to ablate the endometrium.8-10 Complications: 19 injuries, including 13 thermal injuries to the intestines.
Baggish and Savells3 initiated this study after discovering six adverse events within their own hospital system utilizing a single device (HTA). Because these injuries were not reported to the FDA, the overall number of complications is likely higher than the figures given here.
This article describes the proper use of nonhysteroscopic endometrial ablation devices, the best ways to avert serious injury, and optimal treatment when complication occurs.
TABLE
Complications associated with 4 endometrial ablation devices
| COMPLICATION | HYDRO THERMABLATOR* | THERMACHOICE | NOVASURE | MICROSULIS |
|---|---|---|---|---|
| Uterine perforation | 2 | 3 | 26 | 19 |
| Intestinal injury | 1† | 1† | – | 13† |
| Retrograde leakage burn | 19 | 6 | – | – |
| Infection/sepsis | – | 1† | 2 | 1 |
| Fistula/sinus | – | 1† | 1 | – |
| Transmural uterine burn | – | 1 | – | – |
| Cervical stenosis | – | 8 | 1 | – |
| Cardiac arrest | 1 | – | 1 | – |
| Death | – | 1 | – | – |
| Other major | – | 3 | 1 | 4† |
| Total | 22 | 22 | 32 | 20 |
| * Includes author’s data; 6 retrograde leaks | ||||
| † Collateral injury | ||||
CASE continued: Patient opts for hysterectomy
In the case just described, G.S. was examined 1 week after surgery and found to have an exophytic burn over the entire right half of the cervix, extending into the vagina. She was readmitted for 3 days of intravenous (IV) antibiotic treatment and wound care. Computed tomography imaging showed gas formation within the damaged cervix.
Six weeks after surgery, the patient was still menstruating heavily, but her cervix and vagina had healed. Six months later, she underwent total abdominal hysterectomy for continued menorrhagia.
When is endometrial ablation an option?
Indications for endometrial ablation using a nonhysteroscopic, minimally invasive technique are no different from those for hysteroscopic ablation.11 Abnormal, or dysfunctional, uterine bleeding is the principal reason for this operation. Dysfunctional bleeding is heavy or prolonged menses over 6 months or longer that fail to respond to conservative measures and occur in the absence of tumor, pregnancy, or inflammation (ie, infection).
A woman who meets these criteria should have a desire to retain her uterus if she is to be a candidate for a nonhysteroscopic, minimally invasive technique. She also should understand that ablation can render pregnancy unlikely and even pathologic. Her understanding of this consequence should be documented in the chart! Last, she should be informed that ablation will not necessarily render her sterile, so contraception or sterilization will be required to avoid pregnancy. This should also be clearly documented in the medical record.
Endometrial ablation may also be an alternative to hysterectomy for a mentally retarded woman who is unable to manage menses. Abnormal uterine bleeding in conjunction with bleeding diathesis, significant obesity, or serious medical disorders can also be treated by endometrial ablation.
Avoid endometrial ablation in certain circumstances
These circumstances include the presence of endometrial hyperplasia, endometrial cancer, endocervical neoplasia, cervical stenosis, an undiagnosed adnexal mass, moderate to severe dysmenorrhea, adenomyosis, or a uterine cavity larger than 10 cm.12-15
Valle and Baggish15 reported eight cases in which women developed endometrial carcinoma following ablation, and identified the following major risk factors for postablation cancer:
- endometrial hyperplasia unresponsive to progesterone or progestin therapy
- complex endometrial hyperplasia
- atypical hyperplasia.
These conditions are contraindications to endometrial ablation.
Avoid a rush to ablation
The growing popularity of office-based, minimally invasive, nonhysteroscopic techniques, coupled with an increasing desire for and acceptance of elective cessation of menses, may stretch the indications listed above and cut short the discovery of contraindications. Clearly, thorough endometrial sampling and precise histopathologic interpretation are required before embarking on any type of endometrial ablation, to minimize the risk of complications.
How to prevent injury
Reduce the risk of perforation
Uterine perforation occurs for a variety of reasons:
- position of the uterus is unknown
- uterus has not been gently and carefully sounded
- cervix is insufficiently dilated to permit passage of the probe
- device is too long (large) to be accommodated in an individual patient’s uterus
- uterine cavity is distorted by pathology, such as adhesions, myomas, etc.
Attention to these details before surgery can prevent perforation.
When uterine injury occurs, the bowel is also at risk
The intestines can be injured following perforation or transmural injury of the uterus. Bowel injury has been reported with hysteroscopic ablation and resection as well as with Nd-YAG laser ablation.16-18
Do not activate hot water or electrosurgical energy unless you are 100% certain that the device is within the uterine cavity.
Ideally, manufacturers’ safety studies should guarantee no risk of transtubal spillage of hot liquid.
Hot fluid adds to risk of burns
Devices that permit retrograde leakage of hot fluid, such as the HTA, should be modified to ensure sealing at the level of the external and internal cervical os. The Enable device (Innerdyne), no longer marketed in the United States, had such a sealing mechanism, which minimized retrograde leakage of hot water.
Balloon failure may be an unavoidable injury, but pretesting of the device and careful attention to pressure readings—particularly in a small uterus—may mitigate the risk.
Be alert for electrical leakage
The microwave device operates at the megahertz range of frequency. At this high frequency, the risk of leakage is much greater than with devices that operate in the kilohertz range. Therefore, it is important to pay close attention to grounding sites, such as cardiovascular-monitoring electrodes.
High-power monopolar devices, prolonged application of energy to tissue, and high generator frequency are all associated with leakage and subsequent burns.
- Keep the success rate above 90%
- Minimize complications by proper technique and instrument selection
- Press the market to develop a range of device sizes that will individualize the procedure
- Keep the price of a procedure under $1,000
- Establish and adhere to careful patient selection criteria
Early recognition and treatment are vital to ensure the patient’s safety and reduce the risk of medicolegal liability. I recommend the following steps:
- Stop the procedure immediately if perforation is suspected. If you suspect that hot water has been dispersed within the abdominal cavity, switch to laparotomy and consult a general surgeon to inspect the entire intestine for injury. If perforation occurs during the use of electrosurgical energy, the same action is warranted. If uterine perforation occurs in isolation (ie, there is no thermal energy compounding the problem), admit the patient for careful observation, appropriate blood chemistries and hematologic studies, and radiologic examination.
- When hot liquids are spilled, switch to retrograde flow immediately and generously flush the vulva, vagina, and cervix with cold water. Cleanse the entire area with a soapless detergent, and apply clindamycin cream to the vagina and silver sulfadiazine cream to the vulva. Admit the patient for application of cold compresses, ice packs, and burn therapy, and obtain baseline cultures and hematologic studies and a plastic surgery consult. If third-degree (full thickness) burns are suspected, treat any suspected wound infection aggressively after obtaining cultures. Severe and inordinate pain should be investigated as a possible sign of necrotizing fasciitis. After discharge, follow the patient’s progress at weekly intervals.
- Talk to the patient and her family. It is a good idea to explain the complication in very clear terms. I believe it is reasonable to explain how the complication occurred, without speculation or theatrical explanations. Also be sure to document this conversation, including date and time. It may be useful to have a neutral witness present during the conversation. By and large, the patient and her family are likely to appreciate an honest account of how the complication occurred. Hiding data or attempting to cover up the injury may motivate the patient to seek legal representation.
The long-term success of endometrial ablation devices as a whole depends on several conditions. Foremost, the entire class of devices should demonstrate efficacy on par with hysteroscopic ablation. Currently, efficacy ranges from 80% to 95% (short-term follow-up).11 The goal of minimally invasive procedures should be a sustainable 92% rate of amenorrhea, hypomenorrhea, or light, periodic menses. A long-term failure rate of 25% is unacceptable.22-24 If the devices can, by their simplicity, be adapted to more or less universal office application and attain a 5-year success rate of 90% or higher, they will become the standard of care.
One size does not really fit all
Serious complications from endometrial ablation devices occur with regular frequency and must be eliminated or greatly reduced. Perforation is a significant problem and may be related to the “one-size-fits-all” design of the device. Perhaps a range of sizes needs to be produced and fitted to the individual uterine cavity.
If such complications as perforation and burns to the bowel, cervix, vagina, and vulva can be eliminated or relegated to rarity, then a happy future for these procedures lies beyond the horizon.
Price ceiling should be set at $1,000
If an operation can consistently be performed for less than $1,000 total cost—the cost of in-hospital endometrial ablation—it will gain mass appeal. In hospitals and so-called surgicenters, ablations are expensive and, therefore, less attractive to self- or third-party payers. If fees are based on the volume of cases, then a procedure may be price-efficient.
Outcome depends on patient selection
Poorly screened patients who have underlying hyperplasia may develop postablation carcinoma. Women who have dysmenorrhea before the procedure can be predicted to suffer from it afterward. Older women (ie, 40 years or older) will have better long-term success than younger women. And women with a large uterus or myomas will have a higher failure rate than women with smaller cavities (ie, less than 10 cm in length).
What this means for the individual surgeon
Although minimally invasive techniques are relatively easy to perform and simple to learn, each part of the procedure requires careful application and great attention to detail. Perforation of the uterus and leakage of scalding hot liquid must be avoided. If these complications occur, prompt diagnosis and appropriate treatment are critical. The removal of these procedures from the operating room to the office as well as competitive pricing of instrumentation will make nonhysteroscopic, minimally invasive endometrial ablation more cost-effective.
The modern era of practical endometrial ablation began in 1981, when Goldrath and colleagues19 reported Nd-YAG laser photovaporization of the endometrium via hysteroscopy for treatment of excessive uterine bleeding. Two years later, DeCherney and Polan20 reported hysteroscopic control of abnormal uterine bleeding using the urologic resectoscope.
Over succeeding years, Baggish and Baltoyannis21 and Baggish and Sze22 reported extensive experience with hysteroscopic endometrial ablation in both high- and average-risk patients, including long-term follow-up of 568 cases over 11 years. Garry and colleagues23 reported a large series of 600 cases from the United Kingdom. Not only did these laser techniques prove to be effective, achieving amenorrhea rates ranging from 30% to 60%, but overall control of abnormal bleeding exceeded 90%. In the large series involving approximately 1,200 cases, no uterine perforations were reported.21-23 The major complication: Fluid overload secondary to vascular uptake of distension medium.
In Europe and the United Kingdom, most hysteroscopic treatment of abnormal bleeding involved endometrial resection using the cutting loop of the resectoscope. In the United States, ablation with the ball electrode of the resectoscope largely replaced the Nd-YAG laser because the resectoscopic trigger mechanism required less skill and hand–eye coordination than the hand–finger-controlled movement of the 600- to 1,000-micron laser fiber.24-26
A search for more benign techniques
A 1997 UK survey analyzed 10,686 cases of hysteroscopic endometrial destruction and identified 474 complications.27 Resection alone had a complication rate of 10.9% and an emergency hysterectomy rate of 13 for every 1,000 patients. Laser ablation had a complication rate of 5.5% and an emergency hysterectomy rate of 2 for every 1,000 patients, and the corresponding figures for rollerball ablation were a 4.5% complication rate and 3 emergency hysterectomies for every 1,000 patients. Two deaths occurred (in 10,000 cases) and were associated with loop excision.
Published data indicated that:
- Successful outcomes after endometrial ablation or resection were directly proportional to the skill of the surgeon
- Complications, particularly serious complications, were related to the experience and skill of the surgeon
- Infusion of uterine distension medium, particularly hypo-osmolar solutions, was associated with serious complications when fluid deficits exceeded approximately 500 to 1,000 mL.
As a result, a number of investigators sought to develop new surgical techniques to control abnormal uterine bleeding that would minimize the skill required by the surgeon (requiring only insertion of a cannula into the uterus and a “cookbook” ablation procedure), eliminate the need for distension medium and general anesthesia, and attain efficacy equivalent to earlier techniques.
A quartet of options
Among the devices that resulted were:
- A microwave technique, described by several investigators.8-10 Its chief drawback: High-frequency electrical leakage with the potential to cause thermal burns.
- An intrauterine balloon device distended with sterile water or saline is heated in situ to 85° to 90° Celsius, thereby cooking the endometrium.
- An electrode-bearing device that features an array of monopolar electrodes over the endometrium-facing aspect of a balloon or bipolar electrodes over a porous bag.
- Devices that circulate a small volume of hot saline freely within the uterine cavity. Hydrothermablation delivers 10 to 12 mL of preheated saline into the uterus under low pressure. A similar technique delivers 10 to 12 mL of cool water or saline into the uterus through a sealed cannula, followed by in situ heating and circulation of the fluid at low pressure via a computer-controlled device.
Safety studies were required by the FDA and were performed on all these devices, and the risk of complications appeared to be negligible.1,2 As this article illustrates, that is not the case.
Four devices, four ways of achieving ablation
Since the advent of nonhysteroscopic, minimally invasive endometrial ablation devices, four distinct techniques have gained widespread use
Hydrothermablation
The closed-loop system (HTA) ablates the lining of the endometrium under hysteroscopic visualization by recirculating heated saline within the uterus. The modified hysteroscope allows the operator to view the ablation as it occurs within the uterine cavity.
Balloon ablation
Balloon ablation (Thermachoice) features a double-dip balloon construction that conforms to the contours of the uterine cavity. The saline or water in the balloon is heated in situ. This device requires an undistorted uterine cavity, relies on the integrity of the balloon to prevent forward or retrograde spillage of scalding water, and is time-controlled.
Radiofrequency technology
The three-dimensional gold-plated bipolar mesh electrode (NovaSure) is inserted into the uterine cavity and advanced toward the fundus. Once it is properly positioned (above, left), the system is activated to produce 180 W of bipolar power. A moisture-transport vacuum system draws the endometrium into contact with the mesh to enhance tissue vaporization and evacuate debris.
Microwave energy
Microwave energy is emitted from the tip of the device (Microsulis), which is moved back and forth in a sweeping manner, from the fundus to the lower uterine segment. The device directly heats tissue to a depth of 3 mm, with conductive heating of adjacent tissue for an additional 2 to 3 mm. The total 5- to 6-mm depth ensures coagulation and destruction of the basal layer. Microwave energy does not require direct contact with the tissue, as it will “fill the gap” caused by cornual and fibroid distortions.
CASE: Leaking fluid causes intraoperative burns
G.S. is a 45-year-old mother of three who is admitted for surgery for persistent menorrhagia. She has experienced at least two menstrual periods every month for several months, each of them associated with heavy bleeding. She has a history of hypothyroidism and hypertension, but no serious disease or surgery, and considers herself to be in good physical and mental health.
G.S. undergoes endometrial hydrothermablation (HTA) under general inhalation anesthesia. After the HTA mechanism is primed, the heating cycle is started, with a good seal and no fluid leaking from the cervix.
Approximately 8 minutes into the procedure, a 5-mL fluid deficit is noted, and a small amount of hot fluid is observed to be leaking from the cervical os. Examination reveals a thermal injury to the cervix and anterior vaginal wall. The wound is irrigated with cool, sterile saline, and silver sulfadiazine cream is applied. The patient is discharged.
Could this injury have been avoided? Is further treatment warranted?
A minimally invasive operation does not necessarily translate to minimal risk of serious complications. Although few studies of nonhysteroscopic endometrial ablation techniques report any complications,1,2 Baggish and Savells3 found a number of injuries when they searched hospital records and the Food and Drug Administration (FDA) database (TABLE). They identified serious complications associated with the following devices:
- HydroThermablator (Boston Scientific), which utilizes a modified operating hysteroscope to deliver 10 to 12 mL of preheated saline into the uterus under low pressure.4 Complications: 16 adverse events were reported to the FDA, 13 of which involved the retrograde leakage of hot water, causing burns to the cervix, vagina, and vulva. Six additional injuries not reported to the FDA were identified at a single institution.
- Novasure (Cytyc), which employs bipolar electrodes that cover a porous bag.5,6 Complications: 32 injuries, 26 of them uterine perforations.
- Thermachoice (Gynecare), a fluid-distended balloon ablator.7 Complications: 22 injuries included retrograde leakage of hot water after balloon failure and transmural thermal injury, with spread to, and injury of, proximal structures. One death was reported.
- Microsulis (MEA), which uses microwave energy to ablate the endometrium.8-10 Complications: 19 injuries, including 13 thermal injuries to the intestines.
Baggish and Savells3 initiated this study after discovering six adverse events within their own hospital system utilizing a single device (HTA). Because these injuries were not reported to the FDA, the overall number of complications is likely higher than the figures given here.
This article describes the proper use of nonhysteroscopic endometrial ablation devices, the best ways to avert serious injury, and optimal treatment when complication occurs.
TABLE
Complications associated with 4 endometrial ablation devices
| COMPLICATION | HYDRO THERMABLATOR* | THERMACHOICE | NOVASURE | MICROSULIS |
|---|---|---|---|---|
| Uterine perforation | 2 | 3 | 26 | 19 |
| Intestinal injury | 1† | 1† | – | 13† |
| Retrograde leakage burn | 19 | 6 | – | – |
| Infection/sepsis | – | 1† | 2 | 1 |
| Fistula/sinus | – | 1† | 1 | – |
| Transmural uterine burn | – | 1 | – | – |
| Cervical stenosis | – | 8 | 1 | – |
| Cardiac arrest | 1 | – | 1 | – |
| Death | – | 1 | – | – |
| Other major | – | 3 | 1 | 4† |
| Total | 22 | 22 | 32 | 20 |
| * Includes author’s data; 6 retrograde leaks | ||||
| † Collateral injury | ||||
CASE continued: Patient opts for hysterectomy
In the case just described, G.S. was examined 1 week after surgery and found to have an exophytic burn over the entire right half of the cervix, extending into the vagina. She was readmitted for 3 days of intravenous (IV) antibiotic treatment and wound care. Computed tomography imaging showed gas formation within the damaged cervix.
Six weeks after surgery, the patient was still menstruating heavily, but her cervix and vagina had healed. Six months later, she underwent total abdominal hysterectomy for continued menorrhagia.
When is endometrial ablation an option?
Indications for endometrial ablation using a nonhysteroscopic, minimally invasive technique are no different from those for hysteroscopic ablation.11 Abnormal, or dysfunctional, uterine bleeding is the principal reason for this operation. Dysfunctional bleeding is heavy or prolonged menses over 6 months or longer that fail to respond to conservative measures and occur in the absence of tumor, pregnancy, or inflammation (ie, infection).
A woman who meets these criteria should have a desire to retain her uterus if she is to be a candidate for a nonhysteroscopic, minimally invasive technique. She also should understand that ablation can render pregnancy unlikely and even pathologic. Her understanding of this consequence should be documented in the chart! Last, she should be informed that ablation will not necessarily render her sterile, so contraception or sterilization will be required to avoid pregnancy. This should also be clearly documented in the medical record.
Endometrial ablation may also be an alternative to hysterectomy for a mentally retarded woman who is unable to manage menses. Abnormal uterine bleeding in conjunction with bleeding diathesis, significant obesity, or serious medical disorders can also be treated by endometrial ablation.
Avoid endometrial ablation in certain circumstances
These circumstances include the presence of endometrial hyperplasia, endometrial cancer, endocervical neoplasia, cervical stenosis, an undiagnosed adnexal mass, moderate to severe dysmenorrhea, adenomyosis, or a uterine cavity larger than 10 cm.12-15
Valle and Baggish15 reported eight cases in which women developed endometrial carcinoma following ablation, and identified the following major risk factors for postablation cancer:
- endometrial hyperplasia unresponsive to progesterone or progestin therapy
- complex endometrial hyperplasia
- atypical hyperplasia.
These conditions are contraindications to endometrial ablation.
Avoid a rush to ablation
The growing popularity of office-based, minimally invasive, nonhysteroscopic techniques, coupled with an increasing desire for and acceptance of elective cessation of menses, may stretch the indications listed above and cut short the discovery of contraindications. Clearly, thorough endometrial sampling and precise histopathologic interpretation are required before embarking on any type of endometrial ablation, to minimize the risk of complications.
How to prevent injury
Reduce the risk of perforation
Uterine perforation occurs for a variety of reasons:
- position of the uterus is unknown
- uterus has not been gently and carefully sounded
- cervix is insufficiently dilated to permit passage of the probe
- device is too long (large) to be accommodated in an individual patient’s uterus
- uterine cavity is distorted by pathology, such as adhesions, myomas, etc.
Attention to these details before surgery can prevent perforation.
When uterine injury occurs, the bowel is also at risk
The intestines can be injured following perforation or transmural injury of the uterus. Bowel injury has been reported with hysteroscopic ablation and resection as well as with Nd-YAG laser ablation.16-18
Do not activate hot water or electrosurgical energy unless you are 100% certain that the device is within the uterine cavity.
Ideally, manufacturers’ safety studies should guarantee no risk of transtubal spillage of hot liquid.
Hot fluid adds to risk of burns
Devices that permit retrograde leakage of hot fluid, such as the HTA, should be modified to ensure sealing at the level of the external and internal cervical os. The Enable device (Innerdyne), no longer marketed in the United States, had such a sealing mechanism, which minimized retrograde leakage of hot water.
Balloon failure may be an unavoidable injury, but pretesting of the device and careful attention to pressure readings—particularly in a small uterus—may mitigate the risk.
Be alert for electrical leakage
The microwave device operates at the megahertz range of frequency. At this high frequency, the risk of leakage is much greater than with devices that operate in the kilohertz range. Therefore, it is important to pay close attention to grounding sites, such as cardiovascular-monitoring electrodes.
High-power monopolar devices, prolonged application of energy to tissue, and high generator frequency are all associated with leakage and subsequent burns.
- Keep the success rate above 90%
- Minimize complications by proper technique and instrument selection
- Press the market to develop a range of device sizes that will individualize the procedure
- Keep the price of a procedure under $1,000
- Establish and adhere to careful patient selection criteria
Early recognition and treatment are vital to ensure the patient’s safety and reduce the risk of medicolegal liability. I recommend the following steps:
- Stop the procedure immediately if perforation is suspected. If you suspect that hot water has been dispersed within the abdominal cavity, switch to laparotomy and consult a general surgeon to inspect the entire intestine for injury. If perforation occurs during the use of electrosurgical energy, the same action is warranted. If uterine perforation occurs in isolation (ie, there is no thermal energy compounding the problem), admit the patient for careful observation, appropriate blood chemistries and hematologic studies, and radiologic examination.
- When hot liquids are spilled, switch to retrograde flow immediately and generously flush the vulva, vagina, and cervix with cold water. Cleanse the entire area with a soapless detergent, and apply clindamycin cream to the vagina and silver sulfadiazine cream to the vulva. Admit the patient for application of cold compresses, ice packs, and burn therapy, and obtain baseline cultures and hematologic studies and a plastic surgery consult. If third-degree (full thickness) burns are suspected, treat any suspected wound infection aggressively after obtaining cultures. Severe and inordinate pain should be investigated as a possible sign of necrotizing fasciitis. After discharge, follow the patient’s progress at weekly intervals.
- Talk to the patient and her family. It is a good idea to explain the complication in very clear terms. I believe it is reasonable to explain how the complication occurred, without speculation or theatrical explanations. Also be sure to document this conversation, including date and time. It may be useful to have a neutral witness present during the conversation. By and large, the patient and her family are likely to appreciate an honest account of how the complication occurred. Hiding data or attempting to cover up the injury may motivate the patient to seek legal representation.
The long-term success of endometrial ablation devices as a whole depends on several conditions. Foremost, the entire class of devices should demonstrate efficacy on par with hysteroscopic ablation. Currently, efficacy ranges from 80% to 95% (short-term follow-up).11 The goal of minimally invasive procedures should be a sustainable 92% rate of amenorrhea, hypomenorrhea, or light, periodic menses. A long-term failure rate of 25% is unacceptable.22-24 If the devices can, by their simplicity, be adapted to more or less universal office application and attain a 5-year success rate of 90% or higher, they will become the standard of care.
One size does not really fit all
Serious complications from endometrial ablation devices occur with regular frequency and must be eliminated or greatly reduced. Perforation is a significant problem and may be related to the “one-size-fits-all” design of the device. Perhaps a range of sizes needs to be produced and fitted to the individual uterine cavity.
If such complications as perforation and burns to the bowel, cervix, vagina, and vulva can be eliminated or relegated to rarity, then a happy future for these procedures lies beyond the horizon.
Price ceiling should be set at $1,000
If an operation can consistently be performed for less than $1,000 total cost—the cost of in-hospital endometrial ablation—it will gain mass appeal. In hospitals and so-called surgicenters, ablations are expensive and, therefore, less attractive to self- or third-party payers. If fees are based on the volume of cases, then a procedure may be price-efficient.
Outcome depends on patient selection
Poorly screened patients who have underlying hyperplasia may develop postablation carcinoma. Women who have dysmenorrhea before the procedure can be predicted to suffer from it afterward. Older women (ie, 40 years or older) will have better long-term success than younger women. And women with a large uterus or myomas will have a higher failure rate than women with smaller cavities (ie, less than 10 cm in length).
What this means for the individual surgeon
Although minimally invasive techniques are relatively easy to perform and simple to learn, each part of the procedure requires careful application and great attention to detail. Perforation of the uterus and leakage of scalding hot liquid must be avoided. If these complications occur, prompt diagnosis and appropriate treatment are critical. The removal of these procedures from the operating room to the office as well as competitive pricing of instrumentation will make nonhysteroscopic, minimally invasive endometrial ablation more cost-effective.
The modern era of practical endometrial ablation began in 1981, when Goldrath and colleagues19 reported Nd-YAG laser photovaporization of the endometrium via hysteroscopy for treatment of excessive uterine bleeding. Two years later, DeCherney and Polan20 reported hysteroscopic control of abnormal uterine bleeding using the urologic resectoscope.
Over succeeding years, Baggish and Baltoyannis21 and Baggish and Sze22 reported extensive experience with hysteroscopic endometrial ablation in both high- and average-risk patients, including long-term follow-up of 568 cases over 11 years. Garry and colleagues23 reported a large series of 600 cases from the United Kingdom. Not only did these laser techniques prove to be effective, achieving amenorrhea rates ranging from 30% to 60%, but overall control of abnormal bleeding exceeded 90%. In the large series involving approximately 1,200 cases, no uterine perforations were reported.21-23 The major complication: Fluid overload secondary to vascular uptake of distension medium.
In Europe and the United Kingdom, most hysteroscopic treatment of abnormal bleeding involved endometrial resection using the cutting loop of the resectoscope. In the United States, ablation with the ball electrode of the resectoscope largely replaced the Nd-YAG laser because the resectoscopic trigger mechanism required less skill and hand–eye coordination than the hand–finger-controlled movement of the 600- to 1,000-micron laser fiber.24-26
A search for more benign techniques
A 1997 UK survey analyzed 10,686 cases of hysteroscopic endometrial destruction and identified 474 complications.27 Resection alone had a complication rate of 10.9% and an emergency hysterectomy rate of 13 for every 1,000 patients. Laser ablation had a complication rate of 5.5% and an emergency hysterectomy rate of 2 for every 1,000 patients, and the corresponding figures for rollerball ablation were a 4.5% complication rate and 3 emergency hysterectomies for every 1,000 patients. Two deaths occurred (in 10,000 cases) and were associated with loop excision.
Published data indicated that:
- Successful outcomes after endometrial ablation or resection were directly proportional to the skill of the surgeon
- Complications, particularly serious complications, were related to the experience and skill of the surgeon
- Infusion of uterine distension medium, particularly hypo-osmolar solutions, was associated with serious complications when fluid deficits exceeded approximately 500 to 1,000 mL.
As a result, a number of investigators sought to develop new surgical techniques to control abnormal uterine bleeding that would minimize the skill required by the surgeon (requiring only insertion of a cannula into the uterus and a “cookbook” ablation procedure), eliminate the need for distension medium and general anesthesia, and attain efficacy equivalent to earlier techniques.
A quartet of options
Among the devices that resulted were:
- A microwave technique, described by several investigators.8-10 Its chief drawback: High-frequency electrical leakage with the potential to cause thermal burns.
- An intrauterine balloon device distended with sterile water or saline is heated in situ to 85° to 90° Celsius, thereby cooking the endometrium.
- An electrode-bearing device that features an array of monopolar electrodes over the endometrium-facing aspect of a balloon or bipolar electrodes over a porous bag.
- Devices that circulate a small volume of hot saline freely within the uterine cavity. Hydrothermablation delivers 10 to 12 mL of preheated saline into the uterus under low pressure. A similar technique delivers 10 to 12 mL of cool water or saline into the uterus through a sealed cannula, followed by in situ heating and circulation of the fluid at low pressure via a computer-controlled device.
Safety studies were required by the FDA and were performed on all these devices, and the risk of complications appeared to be negligible.1,2 As this article illustrates, that is not the case.
Four devices, four ways of achieving ablation
Since the advent of nonhysteroscopic, minimally invasive endometrial ablation devices, four distinct techniques have gained widespread use
Hydrothermablation
The closed-loop system (HTA) ablates the lining of the endometrium under hysteroscopic visualization by recirculating heated saline within the uterus. The modified hysteroscope allows the operator to view the ablation as it occurs within the uterine cavity.
Balloon ablation
Balloon ablation (Thermachoice) features a double-dip balloon construction that conforms to the contours of the uterine cavity. The saline or water in the balloon is heated in situ. This device requires an undistorted uterine cavity, relies on the integrity of the balloon to prevent forward or retrograde spillage of scalding water, and is time-controlled.
Radiofrequency technology
The three-dimensional gold-plated bipolar mesh electrode (NovaSure) is inserted into the uterine cavity and advanced toward the fundus. Once it is properly positioned (above, left), the system is activated to produce 180 W of bipolar power. A moisture-transport vacuum system draws the endometrium into contact with the mesh to enhance tissue vaporization and evacuate debris.
Microwave energy
Microwave energy is emitted from the tip of the device (Microsulis), which is moved back and forth in a sweeping manner, from the fundus to the lower uterine segment. The device directly heats tissue to a depth of 3 mm, with conductive heating of adjacent tissue for an additional 2 to 3 mm. The total 5- to 6-mm depth ensures coagulation and destruction of the basal layer. Microwave energy does not require direct contact with the tissue, as it will “fill the gap” caused by cornual and fibroid distortions.
1. Bustos-Lopez H, Baggish MS, Valle RF, et al. Assessment of the safety of intrauterine instillation of heated saline for endometrial ablation. Fertil Steril. 1998;69:155-160.
2. Baggish MS, Paraiso M, Breznock EM, et al. A computer-controlled, continuously circulating, hot irrigating system for endometrial ablation. Am J Obstet Gynecol. 1995;173:1842-1848.
3. Baggish MS, Savells A. Complications associated with minimally invasive non-hysteroscopic endometrial ablation techniques. J Gynecol Surg. 2007;23:7-12.
4. Goldrath MH. Evaluation of HydroThermablator and rollerball endometrial ablation for menorrhagia: 3 years after treatment. J Am Assoc Gynecol Laparosc. 2003;10:505-511.
5. Abbott J, Hawe J, Hunter D, et al. A double-blind randomized trial comparing the cavaterm and the Novasure endometrial ablation systems for the treatment of dysfunctional uterine bleeding. Fertil Steril. 2003;80:203-208.
6. Cooper J, Gimpelson R, Laberge P, et al. A randomized, multi-center trial of safety and efficacy of the Novasure system in the treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:418-428.
7. Loffer FD, Grainger D. Five-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:429-435.
8. Phipps JH, Lewis BV, Roberts T, et al. Treatment of functional menorrhagia with radiofrequency endometrial ablation. Lancet. 1990;335:374-376.
9. Sharp N, Cronin N, Feldberg I, et al. Microwaves for menorrhagia: a new fast technique for endometrial ablation. Lancet. 1995;346:1003-1004.
10. Thijssen RFA. Radiofrequency-induced endometrial ablation: an update. Br J Obstet Gynaecol. 1997;104:608-613.
11. Baggish MS. Minimally invasive non-hysteroscopic methods for endometrial ablation. In: Baggish MS, Valle RF, Guedj H, eds. Hysteroscopy: Visual Perspectives of Uterine Anatomy, Physiology, and Pathology. 3rd ed. Philadelphia: Lippincott, Williams and Wilkins; 2007:405-415.
12. Dwyer N, Hutton J, Stirrat GM. Randomized controlled trial comparing endometrial resection with abdominal hysterectomy for the surgical treatment of menorrhagia. Br J Obstet Gynaecol. 1993;100:237-243.
13. Raiga J, Mage G, Glowaczower E, et al. Factors affecting risk of failure after endometrial resection. J Gynecol Surg. 1995;11:1-5.
14. Shelly-Jones D, Mooney P, Garry R. Factors influencing the outcome of endometrial laser ablation. J Gynecol Surg. 1994;10:211-215.
15. Valle RF, Baggish MS. Endometrial carcinoma after endometrial ablation: high-risk factors predicting its occurrence. Am J Obstet Gynecol. 1998;176:569-572.
16. Kanter MH, Kivnick S. Bowel injury from rollerball ablation of the endometrium. Obstet Gynecol. 1992;79:833-835.
17. Perry CP, Daniell JF, Gimpelson RJ. Bowel injury from Nd-YAG endometrial ablation. J Gynecol Surg. 1990;6:1999-2003.
18. Scottish Hysteroscopy Audit Group. A Scottish audit of hysteroscopic surgery for menorrhagia: complications and follow-up. Br J Obstet Gynaecol. 1995;102:239-254.
19. Goldrath MH, Fuller TA, Segal S. Laser photovaporization of the endometrium for the treatment of menorrhagia. Am J Obstet Gynecol. 1981;40:14-19.
20. DeCherney A, Polan ML. Hysteroscopic management of intrauterine lesions and intractable uterine bleeding. Obstet Gynecol. 1983;61:392-396.
21. Baggish MS, Baltoyannis P. New techniques for laser ablation of the endometrium in high-risk patients. Am J Obstet Gynecol. 1988;159:287-292.
22. Baggish MS, Sze EHM. Endometrial ablation: a series of 568 patients treated over an 11-year period. Am J Obstet Gynecol. 1996;174:908-913.
23. Garry R, Shelly-Jones D, Mooney P, et al. Six hundred endometrial laser ablations. Obstet Gynecol. 1995;85:24-29.
24. Magos AL, Bauman R, Lockwood GM, et al. Experience with the first 250 endometrial resections for menorrhagia. Lancet. 1991;337:1074-1078.
25. Wortman M, Daggett A. Hysteroscopic endometrial resection: a new technique for the treatment of menorrhagia. Obstet Gynecol. 1994;83:295-298.
26. Townsend DE, Richart RM, Paskowitz, et al. Rollerball coagulation of the endometrium. Obstet Gynecol. 1990;76:310-313.
27. Overton C, Hargreaves J, Maresh M. A national survey of the complications of endometrial destruction for menstrual disorders: the mistletoe study. Br J Obstet Gynaecol. 1997;104:1351-1359.
1. Bustos-Lopez H, Baggish MS, Valle RF, et al. Assessment of the safety of intrauterine instillation of heated saline for endometrial ablation. Fertil Steril. 1998;69:155-160.
2. Baggish MS, Paraiso M, Breznock EM, et al. A computer-controlled, continuously circulating, hot irrigating system for endometrial ablation. Am J Obstet Gynecol. 1995;173:1842-1848.
3. Baggish MS, Savells A. Complications associated with minimally invasive non-hysteroscopic endometrial ablation techniques. J Gynecol Surg. 2007;23:7-12.
4. Goldrath MH. Evaluation of HydroThermablator and rollerball endometrial ablation for menorrhagia: 3 years after treatment. J Am Assoc Gynecol Laparosc. 2003;10:505-511.
5. Abbott J, Hawe J, Hunter D, et al. A double-blind randomized trial comparing the cavaterm and the Novasure endometrial ablation systems for the treatment of dysfunctional uterine bleeding. Fertil Steril. 2003;80:203-208.
6. Cooper J, Gimpelson R, Laberge P, et al. A randomized, multi-center trial of safety and efficacy of the Novasure system in the treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:418-428.
7. Loffer FD, Grainger D. Five-year follow-up of patients participating in a randomized trial of uterine balloon therapy versus rollerball ablation for treatment of menorrhagia. J Am Assoc Gynecol Laparosc. 2002;9:429-435.
8. Phipps JH, Lewis BV, Roberts T, et al. Treatment of functional menorrhagia with radiofrequency endometrial ablation. Lancet. 1990;335:374-376.
9. Sharp N, Cronin N, Feldberg I, et al. Microwaves for menorrhagia: a new fast technique for endometrial ablation. Lancet. 1995;346:1003-1004.
10. Thijssen RFA. Radiofrequency-induced endometrial ablation: an update. Br J Obstet Gynaecol. 1997;104:608-613.
11. Baggish MS. Minimally invasive non-hysteroscopic methods for endometrial ablation. In: Baggish MS, Valle RF, Guedj H, eds. Hysteroscopy: Visual Perspectives of Uterine Anatomy, Physiology, and Pathology. 3rd ed. Philadelphia: Lippincott, Williams and Wilkins; 2007:405-415.
12. Dwyer N, Hutton J, Stirrat GM. Randomized controlled trial comparing endometrial resection with abdominal hysterectomy for the surgical treatment of menorrhagia. Br J Obstet Gynaecol. 1993;100:237-243.
13. Raiga J, Mage G, Glowaczower E, et al. Factors affecting risk of failure after endometrial resection. J Gynecol Surg. 1995;11:1-5.
14. Shelly-Jones D, Mooney P, Garry R. Factors influencing the outcome of endometrial laser ablation. J Gynecol Surg. 1994;10:211-215.
15. Valle RF, Baggish MS. Endometrial carcinoma after endometrial ablation: high-risk factors predicting its occurrence. Am J Obstet Gynecol. 1998;176:569-572.
16. Kanter MH, Kivnick S. Bowel injury from rollerball ablation of the endometrium. Obstet Gynecol. 1992;79:833-835.
17. Perry CP, Daniell JF, Gimpelson RJ. Bowel injury from Nd-YAG endometrial ablation. J Gynecol Surg. 1990;6:1999-2003.
18. Scottish Hysteroscopy Audit Group. A Scottish audit of hysteroscopic surgery for menorrhagia: complications and follow-up. Br J Obstet Gynaecol. 1995;102:239-254.
19. Goldrath MH, Fuller TA, Segal S. Laser photovaporization of the endometrium for the treatment of menorrhagia. Am J Obstet Gynecol. 1981;40:14-19.
20. DeCherney A, Polan ML. Hysteroscopic management of intrauterine lesions and intractable uterine bleeding. Obstet Gynecol. 1983;61:392-396.
21. Baggish MS, Baltoyannis P. New techniques for laser ablation of the endometrium in high-risk patients. Am J Obstet Gynecol. 1988;159:287-292.
22. Baggish MS, Sze EHM. Endometrial ablation: a series of 568 patients treated over an 11-year period. Am J Obstet Gynecol. 1996;174:908-913.
23. Garry R, Shelly-Jones D, Mooney P, et al. Six hundred endometrial laser ablations. Obstet Gynecol. 1995;85:24-29.
24. Magos AL, Bauman R, Lockwood GM, et al. Experience with the first 250 endometrial resections for menorrhagia. Lancet. 1991;337:1074-1078.
25. Wortman M, Daggett A. Hysteroscopic endometrial resection: a new technique for the treatment of menorrhagia. Obstet Gynecol. 1994;83:295-298.
26. Townsend DE, Richart RM, Paskowitz, et al. Rollerball coagulation of the endometrium. Obstet Gynecol. 1990;76:310-313.
27. Overton C, Hargreaves J, Maresh M. A national survey of the complications of endometrial destruction for menstrual disorders: the mistletoe study. Br J Obstet Gynaecol. 1997;104:1351-1359.
Voices of experience weigh in: Do electronic medical records make for a better practice?
MODERATOR

G. William Bates, MD, MBA
Vanderbilt University Medical Center, Nashville, Tenn
PANELISTS
Have introduced EMR to their practice

B. David Hall, MD, FACOG
Rowan OB/GYN Associates, Salisbury, NC

Don Shuwarger, MD, FACOG
Forest Women’s Center, Forest, Va
PANELISTS
Have not introduced EMR

Frank O. Page, MD, FACOG
Henderson Walton Women’s Center, Birmingham, Ala

Mark A. VanMeter
Group Practice Manager, Columbus Obstetricians– Gynecologists, Inc., Columbus, Ohio
Are your colleagues in private practice who have made the transition to a system of electronic medical records (EMR) satisfied with their decision and experience? Yes and, on some points, less than yes.
For practices that—perhaps, like yours—haven’t made the leap, the question is: What’s holding them back?
In this concluding installment of a two-part article on EMR, a panel of three ObGyns and one ObGyn practice administrator talk with Moderator G. William Bates, MD, MBA, about, in the case of two practices, the work of bringing EMR into their offices. Two other panelists describe their practices’ calculated reluctance to discard paper processes right now.
Why have you and your partners adopted EMR?
Shuwarger: Our practice quickly identified the direct and indirect benefits of bringing technology to bear on our processes. Paper records were often illegible, misplaced, or being used by another staff member. We recognized that to meet our internal goals for growth, increasing patient safety, and streamlining processes, we would have to adopt an EMR solution that met those needs.
Hall: Our practice was drowning in paperwork. An exam room was recently converted to hold more charts, and two warehouses held our overflow. Employees were constantly searching for records, and telephone messages were delayed for hours or days until the chart could be reviewed. Notoriously bad handwriting and incomplete documentation hampered good communication and good medical care. Transcription costs were out of control. Forms helped but added to ongoing costs and storage problems.
What efficiency gains have you achieved?
Shuwarger: Forest Women’s Center is able to see more patients in the day because our ObGyn-specific EMR system has a “Patient Portal” that enables patients to enter all their history and complaint-specific information in advance of a visit. Another efficiency is the time gained by never searching for lost or misplaced charts. We also like the ability to access our records 24-7-365.
Hall: The patient’s chart is readily available. Hours of searching have been eliminated, and patients’ questions, lab reports, and prescription refills can be managed with very few steps. The physician can record recommendations and treatment plans, which the staff relays to the patient. Records take about the same time to finish, but they are much more complete and legible, with dramatic gains in safety for the patient and improved liability protection for the physician.
Which features provide the greatest value?
Shuwarger: The patient portal that I mentioned is a great time saver for us. We were amazed at the acceptance and rapid adoption. Even our octogenarians love it. Universal access to data is of incalculable value. One of our physicians loves to go home early, have dinner, and then review his charts from home. EMR improves my recordkeeping, makes encounter documentation more complete, and helps me avoid medication errors. Our billing staff loves the thorough documentation when it is time to file or appeal claims.
Hall: Immediate access to a clear, legible, and complete patient record provides a solid foundation for our medical decision making.
How have your patients reacted to your conversion from paper to EMR?
Shuwarger: At the beginning, there were people who resisted the patient portal, but when they saw for themselves how it enhances the visit experience and helps their physician address their needs, they became vocal proponents.
Hall: Our patients are impressed with our knowledge of their history, with the fact that reports are immediately available, and with how responsive our staff is to their needs. Rather than creating a barrier to communication, TabletPCs allow them to see images of their own procedures, illustrations, treatment outlines, and even education videos. Flow sheets help mark their progress or encourage them to better adherence. Many seem pleased that their medical records are so cutting-edge. Their confidence in our medical skills appears enhanced.
Has your vendor met expectations?
Shuwarger: No—our vendor exceeded our expectations. We had experience with technology vendors before—“We’ll overpromise and underdeliver” was their mantra! With our EMR vendor, however, our preparation was outstanding, the training was thorough, and implementation went better than any we had experienced. Our uptime has exceeded expectations. Enhancements have been well thought out.
And customer support was good at first but now is even better.
Hall: The program is extremely powerful, with an excellent architecture, but its flexibility is also its main limitation. Recently, core clinical content for primary care medicine has been added, but specialty content remains severely limited. Value-added vendors have developed—at additional cost—excellent form-editing tools and specialty forms, and a vigorous users’ community is generous in sharing forms and workflows. But untold hours were required to develop clinical and office workflows, document templates, and just to discover all the options in the system. The learning curve was huge, and further automation requires the skills of a computer programmer.
Our EMR and practice management systems are interfaced but not integrated—even though the same vendor developed them. The problem is that the interface requires several translation programs and multiple servers to implement. Our dependence on our network engineering firm to maintain our bank of servers and interfaces is worrisome— and costly.
Training on our system was inadequate. The basics of the system were covered but, beyond that, we are just now able to shift into second gear. Much of the system’s potential remains untapped.
What is your approximate return on investment?
Shuwarger: We’ve grown receipts by 20%, year over year, since going with our ObGyn-specific EMR system. The rise in revenue is related directly to increased productivity, a reduction in lost charges, and improved collection from third-party payers because we can provide better documentation. At the least, our EMR system has returned $3 for every $1 spent, not counting intangibles.
Hall: Charge capture is much more complete and accurate, with readily available codes and guidelines. The greatest savings are in chart transcription, management, and storage.
Ongoing maintenance and upgrade costs, including hardware and networking software, have gone far beyond our initial investment, however. Problems with training and initial workflow design have slowed our return on investment. But we’re making progress in that direction.
- Streamlined history-taking and complaint-reporting may mean greater productivity in a practice—and a resulting ability to see more patients in a day
- A so-called patient portal gives patients easier access to providers and the varied resources and services of a practice, which boosts satisfaction
- Caveat emptor! Shop carefully when selecting a system vendor—the experiences of practices from installation through system maintenance range very widely
- Interconnectivity between an EMR system and other databases is not a given
- For a large, multisite practice, the cost of hardware alone may have a chilling effect on implementing an EMR system
- All physicians in a practice must buy into an EMR system that’s being put into place—and a range of ages, attitudes, and practice patterns may be a cause for disagreement on how the system is to be best used
- There is concern among some that the federal government may shape the future of EMR by mandating that all systems in private practices interface with hospitals, insurers, and other providers.
Are features lacking that would bring greater efficiency?
Shuwarger: Our labor suite wants data from our ACOG obstetric record to flow into its system to avoid the need to reenter data manually. And our practice’s physicians want the labor and delivery summary to populate our EMR. These issues of interconnection will be worked out as CCHIT certification (see “EMR certifying body arises from the private sector,” page 62) brings disparate systems into proximity.
Hall: Physicians aren’t computer programmers. We practice medicine, not EMR system development, and we are rarely on top of the “best practices” in practice workflow. Many of us who work with EMR may wish to customize a system to the way we practice, but that is not the best way to proceed. A robust and comprehensive specialty-specific set of clinical content that can be loaded as a unit and easily updated is going to provide far greater efficiency than an infinitely customizable basic program.
I look forward to being able to integrate our private medical record with a central data repository, in which interactions with other specialists and medical centers—not the faulty memory of patients—provide a more accurate background and reduce costly duplication of our increasingly stretched medical resources.
In 2004, President George W. Bush set a goal: nationwide adoption of EMR—to include all medical practices—within a decade. Subsequently, the US Department of Health and Human Services (HHS) established the Office of the National Coordinator for Health Information Technology and the American Health Information Community. The sweeping goal of these bodies? Better health care by application of information technology and creation of standards for certifying EMR systems that provide core functionality.
In response, three private-sector health information management groups jointly formed the Certification Commission for Healthcare Information Technology (CCHIT; www.cchit.org). In 2005, this independent private-sector entity entered into a contract with HHS, to, in the commission’s words, “develop and evaluate certification criteria and create a voluntary inspection process for healthcare information technology” in three areas:
- Ambulatory EMR for offices
- Inpatient EMR for hospitals and health systems
- The network components through which EMR share information.
The work of CCHIT is ongoing; the commission provides voluntary certification of EMR systems, publishes a list of certified EMR systems, provides consultative services to providers and payers through its Web site, and even offers a bank of resources for patients on the intricacies and legalities of medical-record-keeping.
Why haven’t you and your partners adopted EMR?
Page: We recently converted to a new practice management software system, and we want to have all systems working properly and efficiently before implementing an EMR system. All options and processes must be reviewed before we implement EMR for the practice. These options include voice-activation software integrated with the EMR, practice process changes, and practice workflow adaptation.
VanMeter: For our independent practice, with five locations, the initial cost of hardware and software is clearly an early concern. With a rapidly changing hardware environment, once a decision is made, the technology that was proposed may be obsolete before being implemented. Then the continuing cost of hardware and software upgrades—read: “the newest gadget”—and maintenance is also a major budgetary item that we need to consider.
As with most medical practices, our organizational structure is flat. If we were to implement a client-server application, we’d need a systems administrator—and that again increases the cost to the practice. Then we’re faced with the question of how we best utilize this person. Or do we outsource this function? And outsourcing then raises a concern of timely responsiveness to major system problems that may extend downtime, prohibiting the use of your EMR system.
Today, telecommunication costs have plummeted, so the costs of a T-1 line [for high-volume Internet access] and high-speed Internet service are not as onerous as they once were. But a major expense will be to retrofit all our offices (wiring, etc.) to adapt to an electronic environment.
Overall, this is a young industry. I compare it to what we saw with video-tape technology in the 1970s: You had to choose between Beta and VHS formats. Once you made that decision, you paid a premium for the early technology.
Similarly, no one knows which EMR system will prevail over time. The early players are paying for the cost of startup and research and development. As time goes on, we all know that costs should fall—significantly.
Another concern that we have is the long-term viability of the software vendor. Until recently, most applications were developed by small independent firms. Their product was a proprietary one—for which only they have the code and only they could manipulate. If that vendor goes out of business, we’d be left to find a new system, and incur all those implementation costs again.
I think we’ll see a major consolidation of vendors over the next several years— one that leaves only premier vendors with superior products in the market.
As a final concern, and perhaps most important, the role of the federal government weighs heavily on our minds. We believe that, very soon, Washington will mandate EMR and how they are to be accomplished. We also believe that the feds will require integration of medical practice EMR systems with the systems of hospitals, third-party payers, and other medical providers. Our belief is that money may become available—like the funding recently authorized for hospitals to subsidize software and maintenance costs—that will defray the cost of implementing an EMR system in our practice. When this comes to pass, we don’t want to have to reinvent the wheel.
What economic barriers does EMR present?
Page: The economic barrier is really not capital expense but the perception that, for a significant period, EMR will require additional time from the medical staff, which reduces the number of patients seen by a physician and, therefore, affects compensation.”
VanMeter: It seems that, when you purchase an EMR system, you have to comply with the way it works. The tail wags the dog. More flexibility in how a system works at the level of the individual provider would make it more economical in terms of productivity.
What features are lacking that causes you to delay adoption?
Page: Successful voice activation and complete handwriting functionality from laptop to chart.
Are there political barriers to adoption?
Page: EMR represents change, and this is always difficult for larger physician groups. Some physicians are still hesitant to make the transition to an EMR from a paper chart, even when the benefit of EMR is proven. Others are hesitant because they are not acclimated to using a computer in the setting of a patient visit.
VanMeter: First, and foremost, the buy-in of all physicians in a group is needed. In my group of 16 physicians and two nurse practitioners, this is tough—especially when age ranges from 31 to 67 years (four in their 60s and close to retirement). Finding consensus on a system will be difficult for that reason alone.
Second, for physicians who are in the twilight of their career, there’s hesitancy to spend a large sum on a new system that, for them, is going to have a relatively short life span.
Third, and last, I am concerned about up-coding. Although an EMR system may allow you to document a level-4 or level-5 service, is that truly necessary for the patient’s problem? With a yeast infection, for example, is a level-4 or level-5 service appropriate, even if the documentation supports it?
Did this roundtable—or the descriptive article on EMR in the July 2007 issue of OBG Management—leave you with questions on what electronic medical records can do for your practice? Write to the Editors at [email protected] and tell us what you still need to know. Your question may become part of upcoming coverage of the topic in these pages.
Dr. Bates is founder and chief executive officer of digiChart, Inc., an electronic medical records system for ObGyn practices.
Dr. Shuwarger is a current user of digiChart’s electronic medical records system for ObGyns. He pays for his service and received no consideration for this article from digiChart.
Dr. Hall, Dr. Page, and Mr. VanMeter report no financial relationships relevant to this article.
MODERATOR

G. William Bates, MD, MBA
Vanderbilt University Medical Center, Nashville, Tenn
PANELISTS
Have introduced EMR to their practice

B. David Hall, MD, FACOG
Rowan OB/GYN Associates, Salisbury, NC

Don Shuwarger, MD, FACOG
Forest Women’s Center, Forest, Va
PANELISTS
Have not introduced EMR

Frank O. Page, MD, FACOG
Henderson Walton Women’s Center, Birmingham, Ala

Mark A. VanMeter
Group Practice Manager, Columbus Obstetricians– Gynecologists, Inc., Columbus, Ohio
Are your colleagues in private practice who have made the transition to a system of electronic medical records (EMR) satisfied with their decision and experience? Yes and, on some points, less than yes.
For practices that—perhaps, like yours—haven’t made the leap, the question is: What’s holding them back?
In this concluding installment of a two-part article on EMR, a panel of three ObGyns and one ObGyn practice administrator talk with Moderator G. William Bates, MD, MBA, about, in the case of two practices, the work of bringing EMR into their offices. Two other panelists describe their practices’ calculated reluctance to discard paper processes right now.
Why have you and your partners adopted EMR?
Shuwarger: Our practice quickly identified the direct and indirect benefits of bringing technology to bear on our processes. Paper records were often illegible, misplaced, or being used by another staff member. We recognized that to meet our internal goals for growth, increasing patient safety, and streamlining processes, we would have to adopt an EMR solution that met those needs.
Hall: Our practice was drowning in paperwork. An exam room was recently converted to hold more charts, and two warehouses held our overflow. Employees were constantly searching for records, and telephone messages were delayed for hours or days until the chart could be reviewed. Notoriously bad handwriting and incomplete documentation hampered good communication and good medical care. Transcription costs were out of control. Forms helped but added to ongoing costs and storage problems.
What efficiency gains have you achieved?
Shuwarger: Forest Women’s Center is able to see more patients in the day because our ObGyn-specific EMR system has a “Patient Portal” that enables patients to enter all their history and complaint-specific information in advance of a visit. Another efficiency is the time gained by never searching for lost or misplaced charts. We also like the ability to access our records 24-7-365.
Hall: The patient’s chart is readily available. Hours of searching have been eliminated, and patients’ questions, lab reports, and prescription refills can be managed with very few steps. The physician can record recommendations and treatment plans, which the staff relays to the patient. Records take about the same time to finish, but they are much more complete and legible, with dramatic gains in safety for the patient and improved liability protection for the physician.
Which features provide the greatest value?
Shuwarger: The patient portal that I mentioned is a great time saver for us. We were amazed at the acceptance and rapid adoption. Even our octogenarians love it. Universal access to data is of incalculable value. One of our physicians loves to go home early, have dinner, and then review his charts from home. EMR improves my recordkeeping, makes encounter documentation more complete, and helps me avoid medication errors. Our billing staff loves the thorough documentation when it is time to file or appeal claims.
Hall: Immediate access to a clear, legible, and complete patient record provides a solid foundation for our medical decision making.
How have your patients reacted to your conversion from paper to EMR?
Shuwarger: At the beginning, there were people who resisted the patient portal, but when they saw for themselves how it enhances the visit experience and helps their physician address their needs, they became vocal proponents.
Hall: Our patients are impressed with our knowledge of their history, with the fact that reports are immediately available, and with how responsive our staff is to their needs. Rather than creating a barrier to communication, TabletPCs allow them to see images of their own procedures, illustrations, treatment outlines, and even education videos. Flow sheets help mark their progress or encourage them to better adherence. Many seem pleased that their medical records are so cutting-edge. Their confidence in our medical skills appears enhanced.
Has your vendor met expectations?
Shuwarger: No—our vendor exceeded our expectations. We had experience with technology vendors before—“We’ll overpromise and underdeliver” was their mantra! With our EMR vendor, however, our preparation was outstanding, the training was thorough, and implementation went better than any we had experienced. Our uptime has exceeded expectations. Enhancements have been well thought out.
And customer support was good at first but now is even better.
Hall: The program is extremely powerful, with an excellent architecture, but its flexibility is also its main limitation. Recently, core clinical content for primary care medicine has been added, but specialty content remains severely limited. Value-added vendors have developed—at additional cost—excellent form-editing tools and specialty forms, and a vigorous users’ community is generous in sharing forms and workflows. But untold hours were required to develop clinical and office workflows, document templates, and just to discover all the options in the system. The learning curve was huge, and further automation requires the skills of a computer programmer.
Our EMR and practice management systems are interfaced but not integrated—even though the same vendor developed them. The problem is that the interface requires several translation programs and multiple servers to implement. Our dependence on our network engineering firm to maintain our bank of servers and interfaces is worrisome— and costly.
Training on our system was inadequate. The basics of the system were covered but, beyond that, we are just now able to shift into second gear. Much of the system’s potential remains untapped.
What is your approximate return on investment?
Shuwarger: We’ve grown receipts by 20%, year over year, since going with our ObGyn-specific EMR system. The rise in revenue is related directly to increased productivity, a reduction in lost charges, and improved collection from third-party payers because we can provide better documentation. At the least, our EMR system has returned $3 for every $1 spent, not counting intangibles.
Hall: Charge capture is much more complete and accurate, with readily available codes and guidelines. The greatest savings are in chart transcription, management, and storage.
Ongoing maintenance and upgrade costs, including hardware and networking software, have gone far beyond our initial investment, however. Problems with training and initial workflow design have slowed our return on investment. But we’re making progress in that direction.
- Streamlined history-taking and complaint-reporting may mean greater productivity in a practice—and a resulting ability to see more patients in a day
- A so-called patient portal gives patients easier access to providers and the varied resources and services of a practice, which boosts satisfaction
- Caveat emptor! Shop carefully when selecting a system vendor—the experiences of practices from installation through system maintenance range very widely
- Interconnectivity between an EMR system and other databases is not a given
- For a large, multisite practice, the cost of hardware alone may have a chilling effect on implementing an EMR system
- All physicians in a practice must buy into an EMR system that’s being put into place—and a range of ages, attitudes, and practice patterns may be a cause for disagreement on how the system is to be best used
- There is concern among some that the federal government may shape the future of EMR by mandating that all systems in private practices interface with hospitals, insurers, and other providers.
Are features lacking that would bring greater efficiency?
Shuwarger: Our labor suite wants data from our ACOG obstetric record to flow into its system to avoid the need to reenter data manually. And our practice’s physicians want the labor and delivery summary to populate our EMR. These issues of interconnection will be worked out as CCHIT certification (see “EMR certifying body arises from the private sector,” page 62) brings disparate systems into proximity.
Hall: Physicians aren’t computer programmers. We practice medicine, not EMR system development, and we are rarely on top of the “best practices” in practice workflow. Many of us who work with EMR may wish to customize a system to the way we practice, but that is not the best way to proceed. A robust and comprehensive specialty-specific set of clinical content that can be loaded as a unit and easily updated is going to provide far greater efficiency than an infinitely customizable basic program.
I look forward to being able to integrate our private medical record with a central data repository, in which interactions with other specialists and medical centers—not the faulty memory of patients—provide a more accurate background and reduce costly duplication of our increasingly stretched medical resources.
In 2004, President George W. Bush set a goal: nationwide adoption of EMR—to include all medical practices—within a decade. Subsequently, the US Department of Health and Human Services (HHS) established the Office of the National Coordinator for Health Information Technology and the American Health Information Community. The sweeping goal of these bodies? Better health care by application of information technology and creation of standards for certifying EMR systems that provide core functionality.
In response, three private-sector health information management groups jointly formed the Certification Commission for Healthcare Information Technology (CCHIT; www.cchit.org). In 2005, this independent private-sector entity entered into a contract with HHS, to, in the commission’s words, “develop and evaluate certification criteria and create a voluntary inspection process for healthcare information technology” in three areas:
- Ambulatory EMR for offices
- Inpatient EMR for hospitals and health systems
- The network components through which EMR share information.
The work of CCHIT is ongoing; the commission provides voluntary certification of EMR systems, publishes a list of certified EMR systems, provides consultative services to providers and payers through its Web site, and even offers a bank of resources for patients on the intricacies and legalities of medical-record-keeping.
Why haven’t you and your partners adopted EMR?
Page: We recently converted to a new practice management software system, and we want to have all systems working properly and efficiently before implementing an EMR system. All options and processes must be reviewed before we implement EMR for the practice. These options include voice-activation software integrated with the EMR, practice process changes, and practice workflow adaptation.
VanMeter: For our independent practice, with five locations, the initial cost of hardware and software is clearly an early concern. With a rapidly changing hardware environment, once a decision is made, the technology that was proposed may be obsolete before being implemented. Then the continuing cost of hardware and software upgrades—read: “the newest gadget”—and maintenance is also a major budgetary item that we need to consider.
As with most medical practices, our organizational structure is flat. If we were to implement a client-server application, we’d need a systems administrator—and that again increases the cost to the practice. Then we’re faced with the question of how we best utilize this person. Or do we outsource this function? And outsourcing then raises a concern of timely responsiveness to major system problems that may extend downtime, prohibiting the use of your EMR system.
Today, telecommunication costs have plummeted, so the costs of a T-1 line [for high-volume Internet access] and high-speed Internet service are not as onerous as they once were. But a major expense will be to retrofit all our offices (wiring, etc.) to adapt to an electronic environment.
Overall, this is a young industry. I compare it to what we saw with video-tape technology in the 1970s: You had to choose between Beta and VHS formats. Once you made that decision, you paid a premium for the early technology.
Similarly, no one knows which EMR system will prevail over time. The early players are paying for the cost of startup and research and development. As time goes on, we all know that costs should fall—significantly.
Another concern that we have is the long-term viability of the software vendor. Until recently, most applications were developed by small independent firms. Their product was a proprietary one—for which only they have the code and only they could manipulate. If that vendor goes out of business, we’d be left to find a new system, and incur all those implementation costs again.
I think we’ll see a major consolidation of vendors over the next several years— one that leaves only premier vendors with superior products in the market.
As a final concern, and perhaps most important, the role of the federal government weighs heavily on our minds. We believe that, very soon, Washington will mandate EMR and how they are to be accomplished. We also believe that the feds will require integration of medical practice EMR systems with the systems of hospitals, third-party payers, and other medical providers. Our belief is that money may become available—like the funding recently authorized for hospitals to subsidize software and maintenance costs—that will defray the cost of implementing an EMR system in our practice. When this comes to pass, we don’t want to have to reinvent the wheel.
What economic barriers does EMR present?
Page: The economic barrier is really not capital expense but the perception that, for a significant period, EMR will require additional time from the medical staff, which reduces the number of patients seen by a physician and, therefore, affects compensation.”
VanMeter: It seems that, when you purchase an EMR system, you have to comply with the way it works. The tail wags the dog. More flexibility in how a system works at the level of the individual provider would make it more economical in terms of productivity.
What features are lacking that causes you to delay adoption?
Page: Successful voice activation and complete handwriting functionality from laptop to chart.
Are there political barriers to adoption?
Page: EMR represents change, and this is always difficult for larger physician groups. Some physicians are still hesitant to make the transition to an EMR from a paper chart, even when the benefit of EMR is proven. Others are hesitant because they are not acclimated to using a computer in the setting of a patient visit.
VanMeter: First, and foremost, the buy-in of all physicians in a group is needed. In my group of 16 physicians and two nurse practitioners, this is tough—especially when age ranges from 31 to 67 years (four in their 60s and close to retirement). Finding consensus on a system will be difficult for that reason alone.
Second, for physicians who are in the twilight of their career, there’s hesitancy to spend a large sum on a new system that, for them, is going to have a relatively short life span.
Third, and last, I am concerned about up-coding. Although an EMR system may allow you to document a level-4 or level-5 service, is that truly necessary for the patient’s problem? With a yeast infection, for example, is a level-4 or level-5 service appropriate, even if the documentation supports it?
Did this roundtable—or the descriptive article on EMR in the July 2007 issue of OBG Management—leave you with questions on what electronic medical records can do for your practice? Write to the Editors at [email protected] and tell us what you still need to know. Your question may become part of upcoming coverage of the topic in these pages.
MODERATOR

G. William Bates, MD, MBA
Vanderbilt University Medical Center, Nashville, Tenn
PANELISTS
Have introduced EMR to their practice

B. David Hall, MD, FACOG
Rowan OB/GYN Associates, Salisbury, NC

Don Shuwarger, MD, FACOG
Forest Women’s Center, Forest, Va
PANELISTS
Have not introduced EMR

Frank O. Page, MD, FACOG
Henderson Walton Women’s Center, Birmingham, Ala

Mark A. VanMeter
Group Practice Manager, Columbus Obstetricians– Gynecologists, Inc., Columbus, Ohio
Are your colleagues in private practice who have made the transition to a system of electronic medical records (EMR) satisfied with their decision and experience? Yes and, on some points, less than yes.
For practices that—perhaps, like yours—haven’t made the leap, the question is: What’s holding them back?
In this concluding installment of a two-part article on EMR, a panel of three ObGyns and one ObGyn practice administrator talk with Moderator G. William Bates, MD, MBA, about, in the case of two practices, the work of bringing EMR into their offices. Two other panelists describe their practices’ calculated reluctance to discard paper processes right now.
Why have you and your partners adopted EMR?
Shuwarger: Our practice quickly identified the direct and indirect benefits of bringing technology to bear on our processes. Paper records were often illegible, misplaced, or being used by another staff member. We recognized that to meet our internal goals for growth, increasing patient safety, and streamlining processes, we would have to adopt an EMR solution that met those needs.
Hall: Our practice was drowning in paperwork. An exam room was recently converted to hold more charts, and two warehouses held our overflow. Employees were constantly searching for records, and telephone messages were delayed for hours or days until the chart could be reviewed. Notoriously bad handwriting and incomplete documentation hampered good communication and good medical care. Transcription costs were out of control. Forms helped but added to ongoing costs and storage problems.
What efficiency gains have you achieved?
Shuwarger: Forest Women’s Center is able to see more patients in the day because our ObGyn-specific EMR system has a “Patient Portal” that enables patients to enter all their history and complaint-specific information in advance of a visit. Another efficiency is the time gained by never searching for lost or misplaced charts. We also like the ability to access our records 24-7-365.
Hall: The patient’s chart is readily available. Hours of searching have been eliminated, and patients’ questions, lab reports, and prescription refills can be managed with very few steps. The physician can record recommendations and treatment plans, which the staff relays to the patient. Records take about the same time to finish, but they are much more complete and legible, with dramatic gains in safety for the patient and improved liability protection for the physician.
Which features provide the greatest value?
Shuwarger: The patient portal that I mentioned is a great time saver for us. We were amazed at the acceptance and rapid adoption. Even our octogenarians love it. Universal access to data is of incalculable value. One of our physicians loves to go home early, have dinner, and then review his charts from home. EMR improves my recordkeeping, makes encounter documentation more complete, and helps me avoid medication errors. Our billing staff loves the thorough documentation when it is time to file or appeal claims.
Hall: Immediate access to a clear, legible, and complete patient record provides a solid foundation for our medical decision making.
How have your patients reacted to your conversion from paper to EMR?
Shuwarger: At the beginning, there were people who resisted the patient portal, but when they saw for themselves how it enhances the visit experience and helps their physician address their needs, they became vocal proponents.
Hall: Our patients are impressed with our knowledge of their history, with the fact that reports are immediately available, and with how responsive our staff is to their needs. Rather than creating a barrier to communication, TabletPCs allow them to see images of their own procedures, illustrations, treatment outlines, and even education videos. Flow sheets help mark their progress or encourage them to better adherence. Many seem pleased that their medical records are so cutting-edge. Their confidence in our medical skills appears enhanced.
Has your vendor met expectations?
Shuwarger: No—our vendor exceeded our expectations. We had experience with technology vendors before—“We’ll overpromise and underdeliver” was their mantra! With our EMR vendor, however, our preparation was outstanding, the training was thorough, and implementation went better than any we had experienced. Our uptime has exceeded expectations. Enhancements have been well thought out.
And customer support was good at first but now is even better.
Hall: The program is extremely powerful, with an excellent architecture, but its flexibility is also its main limitation. Recently, core clinical content for primary care medicine has been added, but specialty content remains severely limited. Value-added vendors have developed—at additional cost—excellent form-editing tools and specialty forms, and a vigorous users’ community is generous in sharing forms and workflows. But untold hours were required to develop clinical and office workflows, document templates, and just to discover all the options in the system. The learning curve was huge, and further automation requires the skills of a computer programmer.
Our EMR and practice management systems are interfaced but not integrated—even though the same vendor developed them. The problem is that the interface requires several translation programs and multiple servers to implement. Our dependence on our network engineering firm to maintain our bank of servers and interfaces is worrisome— and costly.
Training on our system was inadequate. The basics of the system were covered but, beyond that, we are just now able to shift into second gear. Much of the system’s potential remains untapped.
What is your approximate return on investment?
Shuwarger: We’ve grown receipts by 20%, year over year, since going with our ObGyn-specific EMR system. The rise in revenue is related directly to increased productivity, a reduction in lost charges, and improved collection from third-party payers because we can provide better documentation. At the least, our EMR system has returned $3 for every $1 spent, not counting intangibles.
Hall: Charge capture is much more complete and accurate, with readily available codes and guidelines. The greatest savings are in chart transcription, management, and storage.
Ongoing maintenance and upgrade costs, including hardware and networking software, have gone far beyond our initial investment, however. Problems with training and initial workflow design have slowed our return on investment. But we’re making progress in that direction.
- Streamlined history-taking and complaint-reporting may mean greater productivity in a practice—and a resulting ability to see more patients in a day
- A so-called patient portal gives patients easier access to providers and the varied resources and services of a practice, which boosts satisfaction
- Caveat emptor! Shop carefully when selecting a system vendor—the experiences of practices from installation through system maintenance range very widely
- Interconnectivity between an EMR system and other databases is not a given
- For a large, multisite practice, the cost of hardware alone may have a chilling effect on implementing an EMR system
- All physicians in a practice must buy into an EMR system that’s being put into place—and a range of ages, attitudes, and practice patterns may be a cause for disagreement on how the system is to be best used
- There is concern among some that the federal government may shape the future of EMR by mandating that all systems in private practices interface with hospitals, insurers, and other providers.
Are features lacking that would bring greater efficiency?
Shuwarger: Our labor suite wants data from our ACOG obstetric record to flow into its system to avoid the need to reenter data manually. And our practice’s physicians want the labor and delivery summary to populate our EMR. These issues of interconnection will be worked out as CCHIT certification (see “EMR certifying body arises from the private sector,” page 62) brings disparate systems into proximity.
Hall: Physicians aren’t computer programmers. We practice medicine, not EMR system development, and we are rarely on top of the “best practices” in practice workflow. Many of us who work with EMR may wish to customize a system to the way we practice, but that is not the best way to proceed. A robust and comprehensive specialty-specific set of clinical content that can be loaded as a unit and easily updated is going to provide far greater efficiency than an infinitely customizable basic program.
I look forward to being able to integrate our private medical record with a central data repository, in which interactions with other specialists and medical centers—not the faulty memory of patients—provide a more accurate background and reduce costly duplication of our increasingly stretched medical resources.
In 2004, President George W. Bush set a goal: nationwide adoption of EMR—to include all medical practices—within a decade. Subsequently, the US Department of Health and Human Services (HHS) established the Office of the National Coordinator for Health Information Technology and the American Health Information Community. The sweeping goal of these bodies? Better health care by application of information technology and creation of standards for certifying EMR systems that provide core functionality.
In response, three private-sector health information management groups jointly formed the Certification Commission for Healthcare Information Technology (CCHIT; www.cchit.org). In 2005, this independent private-sector entity entered into a contract with HHS, to, in the commission’s words, “develop and evaluate certification criteria and create a voluntary inspection process for healthcare information technology” in three areas:
- Ambulatory EMR for offices
- Inpatient EMR for hospitals and health systems
- The network components through which EMR share information.
The work of CCHIT is ongoing; the commission provides voluntary certification of EMR systems, publishes a list of certified EMR systems, provides consultative services to providers and payers through its Web site, and even offers a bank of resources for patients on the intricacies and legalities of medical-record-keeping.
Why haven’t you and your partners adopted EMR?
Page: We recently converted to a new practice management software system, and we want to have all systems working properly and efficiently before implementing an EMR system. All options and processes must be reviewed before we implement EMR for the practice. These options include voice-activation software integrated with the EMR, practice process changes, and practice workflow adaptation.
VanMeter: For our independent practice, with five locations, the initial cost of hardware and software is clearly an early concern. With a rapidly changing hardware environment, once a decision is made, the technology that was proposed may be obsolete before being implemented. Then the continuing cost of hardware and software upgrades—read: “the newest gadget”—and maintenance is also a major budgetary item that we need to consider.
As with most medical practices, our organizational structure is flat. If we were to implement a client-server application, we’d need a systems administrator—and that again increases the cost to the practice. Then we’re faced with the question of how we best utilize this person. Or do we outsource this function? And outsourcing then raises a concern of timely responsiveness to major system problems that may extend downtime, prohibiting the use of your EMR system.
Today, telecommunication costs have plummeted, so the costs of a T-1 line [for high-volume Internet access] and high-speed Internet service are not as onerous as they once were. But a major expense will be to retrofit all our offices (wiring, etc.) to adapt to an electronic environment.
Overall, this is a young industry. I compare it to what we saw with video-tape technology in the 1970s: You had to choose between Beta and VHS formats. Once you made that decision, you paid a premium for the early technology.
Similarly, no one knows which EMR system will prevail over time. The early players are paying for the cost of startup and research and development. As time goes on, we all know that costs should fall—significantly.
Another concern that we have is the long-term viability of the software vendor. Until recently, most applications were developed by small independent firms. Their product was a proprietary one—for which only they have the code and only they could manipulate. If that vendor goes out of business, we’d be left to find a new system, and incur all those implementation costs again.
I think we’ll see a major consolidation of vendors over the next several years— one that leaves only premier vendors with superior products in the market.
As a final concern, and perhaps most important, the role of the federal government weighs heavily on our minds. We believe that, very soon, Washington will mandate EMR and how they are to be accomplished. We also believe that the feds will require integration of medical practice EMR systems with the systems of hospitals, third-party payers, and other medical providers. Our belief is that money may become available—like the funding recently authorized for hospitals to subsidize software and maintenance costs—that will defray the cost of implementing an EMR system in our practice. When this comes to pass, we don’t want to have to reinvent the wheel.
What economic barriers does EMR present?
Page: The economic barrier is really not capital expense but the perception that, for a significant period, EMR will require additional time from the medical staff, which reduces the number of patients seen by a physician and, therefore, affects compensation.”
VanMeter: It seems that, when you purchase an EMR system, you have to comply with the way it works. The tail wags the dog. More flexibility in how a system works at the level of the individual provider would make it more economical in terms of productivity.
What features are lacking that causes you to delay adoption?
Page: Successful voice activation and complete handwriting functionality from laptop to chart.
Are there political barriers to adoption?
Page: EMR represents change, and this is always difficult for larger physician groups. Some physicians are still hesitant to make the transition to an EMR from a paper chart, even when the benefit of EMR is proven. Others are hesitant because they are not acclimated to using a computer in the setting of a patient visit.
VanMeter: First, and foremost, the buy-in of all physicians in a group is needed. In my group of 16 physicians and two nurse practitioners, this is tough—especially when age ranges from 31 to 67 years (four in their 60s and close to retirement). Finding consensus on a system will be difficult for that reason alone.
Second, for physicians who are in the twilight of their career, there’s hesitancy to spend a large sum on a new system that, for them, is going to have a relatively short life span.
Third, and last, I am concerned about up-coding. Although an EMR system may allow you to document a level-4 or level-5 service, is that truly necessary for the patient’s problem? With a yeast infection, for example, is a level-4 or level-5 service appropriate, even if the documentation supports it?
Did this roundtable—or the descriptive article on EMR in the July 2007 issue of OBG Management—leave you with questions on what electronic medical records can do for your practice? Write to the Editors at [email protected] and tell us what you still need to know. Your question may become part of upcoming coverage of the topic in these pages.
Dr. Bates is founder and chief executive officer of digiChart, Inc., an electronic medical records system for ObGyn practices.
Dr. Shuwarger is a current user of digiChart’s electronic medical records system for ObGyns. He pays for his service and received no consideration for this article from digiChart.
Dr. Hall, Dr. Page, and Mr. VanMeter report no financial relationships relevant to this article.
Dr. Bates is founder and chief executive officer of digiChart, Inc., an electronic medical records system for ObGyn practices.
Dr. Shuwarger is a current user of digiChart’s electronic medical records system for ObGyns. He pays for his service and received no consideration for this article from digiChart.
Dr. Hall, Dr. Page, and Mr. VanMeter report no financial relationships relevant to this article.
How to manage the cuff at vaginal hysterectomy
CASE 1 What procedures should accompany hysterectomy?
A.E., 44, mother of one, complains of heavy irregular bleeding with no sensation of a vaginal bulge. She has tried oral contraceptives, but they did not improve her bleeding pattern. She also has undergone dilatation and curettage and hysteroscopy (benign findings), also with no improvement.
Examination reveals a 9- to 10-week-size fibroid uterus, which is confirmed by ultrasonography. Pelvic support appears to be excellent.
After a discussion of the options, the patient elects to undergo vaginal hysterectomy. Are other procedures warranted?
Ask a gynecologic surgeon to name the most significant challenges he or she faces, and the answer is likely to include preventing pelvic organ prolapse after surgical intervention. Approximately one third of operations for pelvic organ prolapse involve patients whose prolapse has recurred after previous surgery.1 Although we have advanced our understanding of the anatomy of pelvic support and the pathophysiology of support defects, the various surgical strategies remain largely untested and unproven.
Even women with good pelvic support who are undergoing hysterectomy—like the patient described above—are vulnerable. One particular area of concern: the risk of enterocele or vaginal apical prolapse, or both, after hysterectomy. In this article, I describe a technique to reduce the risk of these defects after vaginal hysterectomy: high uterosacral suspension, or modified McCall culdoplasty.
Enterocele and apical prolapse do not always coexist
Enterocele and apical prolapse are distinct entities. The latter represents a deficiency in the level I supporting structures described by DeLancey2—primarily the uterosacral and cardinal ligaments (FIGURE 1). Enterocele, or peritoneocele, is a herniation of the cul-de-sac peritoneum, with or without intestinal contents. In women who have undergone hysterectomy, enterocele is usually caused by a lack of continuity of level II fibers, namely, the failure to approximate the pubocervical and rectovaginal connective tissues at the time of hysterectomy.3 Careful attention to the vaginal cuff and cul-de-sac at the time of hysterectomy is therefore imperative.
FIGURE 1 Three levels of support
The endopelvic fascia of a posthysterectomy patient divided into DeLancey’s biomechanical levels: level I—proximal suspension, level II—lateral attachment, and level III—distal fusion.
The McCall culdoplasty: 50 years “young”
In 1957, Milton McCall, MD, described a technique to manage the cul-de-sac at the time of vaginal hysterectomy.4 The McCall technique of posterior culdoplasty differs from other approaches by omitting dissection and excision of the hernia sac, or excess cul-de-sac peritoneum. The original McCall culdoplasty begins with the placement of several rows (average of 3) of nonabsorbable suture (“internal” McCall sutures), starting at the left uterosacral ligament about 2 cm above its cut edge, and proceeding across the redundant cul-de-sac to terminate in the right uterosacral ligament. Each subsequent row is placed superior to the first, by applying traction to the previously placed sutures.
Prior to the tying of these sutures, 3 “external” absorbable sutures are placed. These sutures incorporate posterior vaginal epithelium, each uterosacral ligament, and the contralateral vaginal epithelium in a mirror image of the first pass through the vagina. Again, several rows are placed, each more superior to the last, to move the newly created vaginal apex to the highest point on the uterosacral ligaments once all the sutures are tied.
Tying the internal sutures not only creates a firm, shelf-like midline structure, but obliterates the redundant cul-de-sac. The external sutures move the vaginal apex to the uterosacral bridge and are tied at the conclusion of the procedure (FIGURES 2 and 3).
FIGURE 2 Internal McCall sutures
Traction on the most dependent portion of the cul-de-sac and posterior vaginal epithelium allows placement of 3 rows of sutures across the cul-de-sac from one uterosacral ligament to the other.
FIGURE 3 External McCall sutures
Three additional rows of absorbable sutures incorporate vaginal epithelium and uterosacral ligaments to move the vaginal cuff superiorly.
Modifications enhance durability and support
When the surgical indication is significant apical vaginal prolapse, the efficacy of the McCall procedure as both treatment and prevention is uncertain, because we lack adequate studies in this population. However, assuming that identifiable defects or breaks in the uterosacral ligaments lead to apical prolapse,3 use of the portion of the uterosacral ligament nearest the vagina appears unlikely to create a durable repair.
Thus, the concept of a “high” uterosacral attachment came to be proposed to provide a strong midline site of support for the vaginal apex.5,6 Further modifications include attachment of the uterosacral ligaments to pubocervical and rectovaginal connective tissues to create continuity of these level II fibers and prevent subsequent enterocele.7
With a high uterosacral attachment, the uterosacral ligaments need not be brought together in the midline.
Technique for modified approach
To locate each ligament, place traction on the vaginal apex toward the contralateral side. Palpate the pelvic structures posterior and medial to the ischial spines, at the 4 and 8 o’clock positions, to identify the strong tissue emanating from the sacrum.
Place several nonabsorbable sutures through the medial aspect of each uterosacral ligament, working from lateral to medial to minimize the risk of ureteral trauma. Then place 1 strand of each suture through the pubocervical and rectovaginal connective tissues. Tie the sutures to move the vaginal apex to the proximal segment of the uterosacral ligament (near the sacrum) and establish continuity of the pubocervical and rectovaginal connective tissues (FIGURES 4-6).
FIGURE 4 Suture placement is bilateral
Three sutures are placed in the uterosacral ligament pedicles on each side, with 1 arm of each suture placed in the transverse portion of the pubocervical and rectovaginal fascia.
FIGURE 5 Suspensory suture
Sagittal view of a suspensory suture in left uterosacral ligament with 1 arm through pubocervical fascia (PCF) and 1 arm through rectovaginal fascia (RVF).
FIGURE 6 The suspended vaginal vault
Sagittal view of pubocervical fascia (PCF) and rectovaginal fascia (RVF) suspended from uterosacral ligaments.
Main concern is ureteral injury
The ureter lies near the anterior margin of the uterosacral ligament, with a mean distance of 4.1±0.6 cm at the level of the sacrum and 2.3±0.9 cm at the level of the ischial spine.8 In 1 series of high uterosacral ligament suspension with site-specific endopelvic fascia defect repair, ureteral complications occurred in 11% of patients.5 Other series have reported rates of ureteral trauma in the range of 0.7%9 to 2.4%.6
Evaluate ureteral patency
After performing this procedure, the surgeon should ensure ureteral patency. For this reason, I believe that only surgeons skilled in cystoscopy and able to treat ureteral injury (or with ready access to those capable of treating this complication) should undertake high uterosacral suspension.
How the McCall procedure compares with other approaches
Cruikshank SH, Kovac SR. Randomized comparison of three surgical methods used at the time of vaginal hysterectomy to prevent posterior enterocele. Am J Obstet Gynecol. 1999;180:859–865
Although many techniques and modifications have been described for management of the cul-de-sac and vaginal cuff, few comparative data exist. In McCall’s original series,4 43 patients undergoing vaginal hysterectomy with posterior culdoplasty were followed for a minimum of 3 months and a maximum of 3 years. Mean and median lengths of follow-up were not provided, nor were the indications for hysterectomy or the method of assessing the patient for recurrent defects. McCall reported that no patients developed an enterocele after the surgery.
The first and only randomized study
Cruikshank and Kovac performed the only prospective, randomized comparison of procedures used at the time of hysterectomy to prevent enterocele. In their study, 100 patients undergoing vaginal hysterectomy for various indications (excluding prolapse of the posterior superior segment of the vagina) were randomized to 1 of 3 surgical methods to prevent enterocele:
- Moschcowitz-type closure (n=33), in which the peritoneum was closed using a purse-string technique, incorporating the distal ends of the uterosacral and cardinal ligaments and thereby drawing these structures to the midline
- modified McCall culdoplasty (n=33), in which a higher purse-string closure of the peritoneum was performed superior to the “yellow fat line,” incorporating the uterosacral-cardinal ligament complex (thus drawing these structures to the midline) and including the vagina (similar to the external McCall sutures) to move the apex superiorly
- simple closure of the peritoneum with a purse-string suture (n=34), with none of the uterosacral-cardinal ligaments incorporated into the repair.
Three years of follow-up
Of the 100 patients, 98 were followed with serial examinations for 3 years, and the outcomes at all vaginal segments were documented, with particular attention to the posterior superior segment, using staging from the Pelvic Organ Prolapse Quantification (POP-Q) system.10
McCall procedure was most effective
Overall, 11 patients (11.2%) were found to have stage II prolapse of the posterior superior vagina, none of them in the McCall group. An additional 14 patients (14.3%) had stage I prolapse, with only 2 (2%) of these in the McCall group.
The McCall repair was significantly more effective than the other 2 types of repair, with a 6.1% risk of subsequent prolapse, versus 30.3% in women who had a Moschcowitz-type closure and 39.4% in those who underwent simple closure of the peritoneum. No patients in any group had prolapse greater than stage II at follow-up.
Limitations of the study
Although Cruikshank and Kovac designed their study to analyze appropriate prophylaxis against enterocele in patients without prolapse, several patients did have some form of prolapse—although it was unrelated to the posterior superior vagina. Therefore, several patients underwent concomitant reconstructive procedures that included: anterior colporrhaphy (13), posterior colporrhaphy (4), sacrospinous ligament fixation (3), bilateral paravaginal repair (10), and anti-incontinence procedures (10).
It is not clear which groups these patients fell into and whether the distribution was similar across all groups.
CASE 2 Is McCall procedure appropriate?
B.D., 57, complains of increasing pelvic pressure and a noticeable vaginal bulge. Her 2 children were delivered vaginally, the largest weighing 8 lb. B.D. reports that she remains sexually active.
Physical examination reveals the cervix to be at the level of the introitus, but it descends 2 cm beyond the introitus when the patient performs the valsalva maneuver. Although there is also some descent of the anterior and posterior vaginal walls (1 cm superior to the hymen with strain; Pelvic Organ Prolapse Quantification [POP-Q] value=-1), the predominant component of prolapse is an elongated cervix. The posterior vaginal fornix (POP-Q point D), representing apical support, descends to 7 cm superior to the hymen with strain, with a total vaginal length of 9 cm.
At surgery, the uterosacral ligaments do not appear to be attenuated. After vaginal hysterectomy, the apex of the vaginal vault is superior to the level of the ischial spines.
How do you proceed?
Given the relatively good support at the apex, this patient is a good candidate for a McCall-type culdoplasty. Whether or not this procedure will be truly prophylactic (because there is already some descent of the apex, albeit mild) is perhaps only a matter of semantics.
The author reports no financial relationships relevant to this article.
1. Olson AL, Smith VJ, Bergstrom JO, Coiling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
2. DeLancey JOL. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717-1728.
3. Richardson AC. The anatomic defects in rectocele and enterocele. J Pelvic Surg. 1995;1:214-221.
4. McCall ML. Posterior culdoplasty: surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10:595-602.
5. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1402-1411.
6. Karram M, Goldwasser S, Kleeman S, et al. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185:1339-1342.
7. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
8. Buller JL, Thompson JR, Cundiff GW, et al. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol. 2001;97:873-879.
9. Aronson MP, Aronson PK, Howard AE, et al. Low risk of ureteral obstruction with “deep” (dorsal/posterior) uterosacral ligament suture placement for transvaginal apical suspension. Am J Obstet Gynecol. 2005;192:1530-1536.
10. Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10-17.
CASE 1 What procedures should accompany hysterectomy?
A.E., 44, mother of one, complains of heavy irregular bleeding with no sensation of a vaginal bulge. She has tried oral contraceptives, but they did not improve her bleeding pattern. She also has undergone dilatation and curettage and hysteroscopy (benign findings), also with no improvement.
Examination reveals a 9- to 10-week-size fibroid uterus, which is confirmed by ultrasonography. Pelvic support appears to be excellent.
After a discussion of the options, the patient elects to undergo vaginal hysterectomy. Are other procedures warranted?
Ask a gynecologic surgeon to name the most significant challenges he or she faces, and the answer is likely to include preventing pelvic organ prolapse after surgical intervention. Approximately one third of operations for pelvic organ prolapse involve patients whose prolapse has recurred after previous surgery.1 Although we have advanced our understanding of the anatomy of pelvic support and the pathophysiology of support defects, the various surgical strategies remain largely untested and unproven.
Even women with good pelvic support who are undergoing hysterectomy—like the patient described above—are vulnerable. One particular area of concern: the risk of enterocele or vaginal apical prolapse, or both, after hysterectomy. In this article, I describe a technique to reduce the risk of these defects after vaginal hysterectomy: high uterosacral suspension, or modified McCall culdoplasty.
Enterocele and apical prolapse do not always coexist
Enterocele and apical prolapse are distinct entities. The latter represents a deficiency in the level I supporting structures described by DeLancey2—primarily the uterosacral and cardinal ligaments (FIGURE 1). Enterocele, or peritoneocele, is a herniation of the cul-de-sac peritoneum, with or without intestinal contents. In women who have undergone hysterectomy, enterocele is usually caused by a lack of continuity of level II fibers, namely, the failure to approximate the pubocervical and rectovaginal connective tissues at the time of hysterectomy.3 Careful attention to the vaginal cuff and cul-de-sac at the time of hysterectomy is therefore imperative.
FIGURE 1 Three levels of support
The endopelvic fascia of a posthysterectomy patient divided into DeLancey’s biomechanical levels: level I—proximal suspension, level II—lateral attachment, and level III—distal fusion.
The McCall culdoplasty: 50 years “young”
In 1957, Milton McCall, MD, described a technique to manage the cul-de-sac at the time of vaginal hysterectomy.4 The McCall technique of posterior culdoplasty differs from other approaches by omitting dissection and excision of the hernia sac, or excess cul-de-sac peritoneum. The original McCall culdoplasty begins with the placement of several rows (average of 3) of nonabsorbable suture (“internal” McCall sutures), starting at the left uterosacral ligament about 2 cm above its cut edge, and proceeding across the redundant cul-de-sac to terminate in the right uterosacral ligament. Each subsequent row is placed superior to the first, by applying traction to the previously placed sutures.
Prior to the tying of these sutures, 3 “external” absorbable sutures are placed. These sutures incorporate posterior vaginal epithelium, each uterosacral ligament, and the contralateral vaginal epithelium in a mirror image of the first pass through the vagina. Again, several rows are placed, each more superior to the last, to move the newly created vaginal apex to the highest point on the uterosacral ligaments once all the sutures are tied.
Tying the internal sutures not only creates a firm, shelf-like midline structure, but obliterates the redundant cul-de-sac. The external sutures move the vaginal apex to the uterosacral bridge and are tied at the conclusion of the procedure (FIGURES 2 and 3).
FIGURE 2 Internal McCall sutures
Traction on the most dependent portion of the cul-de-sac and posterior vaginal epithelium allows placement of 3 rows of sutures across the cul-de-sac from one uterosacral ligament to the other.
FIGURE 3 External McCall sutures
Three additional rows of absorbable sutures incorporate vaginal epithelium and uterosacral ligaments to move the vaginal cuff superiorly.
Modifications enhance durability and support
When the surgical indication is significant apical vaginal prolapse, the efficacy of the McCall procedure as both treatment and prevention is uncertain, because we lack adequate studies in this population. However, assuming that identifiable defects or breaks in the uterosacral ligaments lead to apical prolapse,3 use of the portion of the uterosacral ligament nearest the vagina appears unlikely to create a durable repair.
Thus, the concept of a “high” uterosacral attachment came to be proposed to provide a strong midline site of support for the vaginal apex.5,6 Further modifications include attachment of the uterosacral ligaments to pubocervical and rectovaginal connective tissues to create continuity of these level II fibers and prevent subsequent enterocele.7
With a high uterosacral attachment, the uterosacral ligaments need not be brought together in the midline.
Technique for modified approach
To locate each ligament, place traction on the vaginal apex toward the contralateral side. Palpate the pelvic structures posterior and medial to the ischial spines, at the 4 and 8 o’clock positions, to identify the strong tissue emanating from the sacrum.
Place several nonabsorbable sutures through the medial aspect of each uterosacral ligament, working from lateral to medial to minimize the risk of ureteral trauma. Then place 1 strand of each suture through the pubocervical and rectovaginal connective tissues. Tie the sutures to move the vaginal apex to the proximal segment of the uterosacral ligament (near the sacrum) and establish continuity of the pubocervical and rectovaginal connective tissues (FIGURES 4-6).
FIGURE 4 Suture placement is bilateral
Three sutures are placed in the uterosacral ligament pedicles on each side, with 1 arm of each suture placed in the transverse portion of the pubocervical and rectovaginal fascia.
FIGURE 5 Suspensory suture
Sagittal view of a suspensory suture in left uterosacral ligament with 1 arm through pubocervical fascia (PCF) and 1 arm through rectovaginal fascia (RVF).
FIGURE 6 The suspended vaginal vault
Sagittal view of pubocervical fascia (PCF) and rectovaginal fascia (RVF) suspended from uterosacral ligaments.
Main concern is ureteral injury
The ureter lies near the anterior margin of the uterosacral ligament, with a mean distance of 4.1±0.6 cm at the level of the sacrum and 2.3±0.9 cm at the level of the ischial spine.8 In 1 series of high uterosacral ligament suspension with site-specific endopelvic fascia defect repair, ureteral complications occurred in 11% of patients.5 Other series have reported rates of ureteral trauma in the range of 0.7%9 to 2.4%.6
Evaluate ureteral patency
After performing this procedure, the surgeon should ensure ureteral patency. For this reason, I believe that only surgeons skilled in cystoscopy and able to treat ureteral injury (or with ready access to those capable of treating this complication) should undertake high uterosacral suspension.
How the McCall procedure compares with other approaches
Cruikshank SH, Kovac SR. Randomized comparison of three surgical methods used at the time of vaginal hysterectomy to prevent posterior enterocele. Am J Obstet Gynecol. 1999;180:859–865
Although many techniques and modifications have been described for management of the cul-de-sac and vaginal cuff, few comparative data exist. In McCall’s original series,4 43 patients undergoing vaginal hysterectomy with posterior culdoplasty were followed for a minimum of 3 months and a maximum of 3 years. Mean and median lengths of follow-up were not provided, nor were the indications for hysterectomy or the method of assessing the patient for recurrent defects. McCall reported that no patients developed an enterocele after the surgery.
The first and only randomized study
Cruikshank and Kovac performed the only prospective, randomized comparison of procedures used at the time of hysterectomy to prevent enterocele. In their study, 100 patients undergoing vaginal hysterectomy for various indications (excluding prolapse of the posterior superior segment of the vagina) were randomized to 1 of 3 surgical methods to prevent enterocele:
- Moschcowitz-type closure (n=33), in which the peritoneum was closed using a purse-string technique, incorporating the distal ends of the uterosacral and cardinal ligaments and thereby drawing these structures to the midline
- modified McCall culdoplasty (n=33), in which a higher purse-string closure of the peritoneum was performed superior to the “yellow fat line,” incorporating the uterosacral-cardinal ligament complex (thus drawing these structures to the midline) and including the vagina (similar to the external McCall sutures) to move the apex superiorly
- simple closure of the peritoneum with a purse-string suture (n=34), with none of the uterosacral-cardinal ligaments incorporated into the repair.
Three years of follow-up
Of the 100 patients, 98 were followed with serial examinations for 3 years, and the outcomes at all vaginal segments were documented, with particular attention to the posterior superior segment, using staging from the Pelvic Organ Prolapse Quantification (POP-Q) system.10
McCall procedure was most effective
Overall, 11 patients (11.2%) were found to have stage II prolapse of the posterior superior vagina, none of them in the McCall group. An additional 14 patients (14.3%) had stage I prolapse, with only 2 (2%) of these in the McCall group.
The McCall repair was significantly more effective than the other 2 types of repair, with a 6.1% risk of subsequent prolapse, versus 30.3% in women who had a Moschcowitz-type closure and 39.4% in those who underwent simple closure of the peritoneum. No patients in any group had prolapse greater than stage II at follow-up.
Limitations of the study
Although Cruikshank and Kovac designed their study to analyze appropriate prophylaxis against enterocele in patients without prolapse, several patients did have some form of prolapse—although it was unrelated to the posterior superior vagina. Therefore, several patients underwent concomitant reconstructive procedures that included: anterior colporrhaphy (13), posterior colporrhaphy (4), sacrospinous ligament fixation (3), bilateral paravaginal repair (10), and anti-incontinence procedures (10).
It is not clear which groups these patients fell into and whether the distribution was similar across all groups.
CASE 2 Is McCall procedure appropriate?
B.D., 57, complains of increasing pelvic pressure and a noticeable vaginal bulge. Her 2 children were delivered vaginally, the largest weighing 8 lb. B.D. reports that she remains sexually active.
Physical examination reveals the cervix to be at the level of the introitus, but it descends 2 cm beyond the introitus when the patient performs the valsalva maneuver. Although there is also some descent of the anterior and posterior vaginal walls (1 cm superior to the hymen with strain; Pelvic Organ Prolapse Quantification [POP-Q] value=-1), the predominant component of prolapse is an elongated cervix. The posterior vaginal fornix (POP-Q point D), representing apical support, descends to 7 cm superior to the hymen with strain, with a total vaginal length of 9 cm.
At surgery, the uterosacral ligaments do not appear to be attenuated. After vaginal hysterectomy, the apex of the vaginal vault is superior to the level of the ischial spines.
How do you proceed?
Given the relatively good support at the apex, this patient is a good candidate for a McCall-type culdoplasty. Whether or not this procedure will be truly prophylactic (because there is already some descent of the apex, albeit mild) is perhaps only a matter of semantics.
The author reports no financial relationships relevant to this article.
CASE 1 What procedures should accompany hysterectomy?
A.E., 44, mother of one, complains of heavy irregular bleeding with no sensation of a vaginal bulge. She has tried oral contraceptives, but they did not improve her bleeding pattern. She also has undergone dilatation and curettage and hysteroscopy (benign findings), also with no improvement.
Examination reveals a 9- to 10-week-size fibroid uterus, which is confirmed by ultrasonography. Pelvic support appears to be excellent.
After a discussion of the options, the patient elects to undergo vaginal hysterectomy. Are other procedures warranted?
Ask a gynecologic surgeon to name the most significant challenges he or she faces, and the answer is likely to include preventing pelvic organ prolapse after surgical intervention. Approximately one third of operations for pelvic organ prolapse involve patients whose prolapse has recurred after previous surgery.1 Although we have advanced our understanding of the anatomy of pelvic support and the pathophysiology of support defects, the various surgical strategies remain largely untested and unproven.
Even women with good pelvic support who are undergoing hysterectomy—like the patient described above—are vulnerable. One particular area of concern: the risk of enterocele or vaginal apical prolapse, or both, after hysterectomy. In this article, I describe a technique to reduce the risk of these defects after vaginal hysterectomy: high uterosacral suspension, or modified McCall culdoplasty.
Enterocele and apical prolapse do not always coexist
Enterocele and apical prolapse are distinct entities. The latter represents a deficiency in the level I supporting structures described by DeLancey2—primarily the uterosacral and cardinal ligaments (FIGURE 1). Enterocele, or peritoneocele, is a herniation of the cul-de-sac peritoneum, with or without intestinal contents. In women who have undergone hysterectomy, enterocele is usually caused by a lack of continuity of level II fibers, namely, the failure to approximate the pubocervical and rectovaginal connective tissues at the time of hysterectomy.3 Careful attention to the vaginal cuff and cul-de-sac at the time of hysterectomy is therefore imperative.
FIGURE 1 Three levels of support
The endopelvic fascia of a posthysterectomy patient divided into DeLancey’s biomechanical levels: level I—proximal suspension, level II—lateral attachment, and level III—distal fusion.
The McCall culdoplasty: 50 years “young”
In 1957, Milton McCall, MD, described a technique to manage the cul-de-sac at the time of vaginal hysterectomy.4 The McCall technique of posterior culdoplasty differs from other approaches by omitting dissection and excision of the hernia sac, or excess cul-de-sac peritoneum. The original McCall culdoplasty begins with the placement of several rows (average of 3) of nonabsorbable suture (“internal” McCall sutures), starting at the left uterosacral ligament about 2 cm above its cut edge, and proceeding across the redundant cul-de-sac to terminate in the right uterosacral ligament. Each subsequent row is placed superior to the first, by applying traction to the previously placed sutures.
Prior to the tying of these sutures, 3 “external” absorbable sutures are placed. These sutures incorporate posterior vaginal epithelium, each uterosacral ligament, and the contralateral vaginal epithelium in a mirror image of the first pass through the vagina. Again, several rows are placed, each more superior to the last, to move the newly created vaginal apex to the highest point on the uterosacral ligaments once all the sutures are tied.
Tying the internal sutures not only creates a firm, shelf-like midline structure, but obliterates the redundant cul-de-sac. The external sutures move the vaginal apex to the uterosacral bridge and are tied at the conclusion of the procedure (FIGURES 2 and 3).
FIGURE 2 Internal McCall sutures
Traction on the most dependent portion of the cul-de-sac and posterior vaginal epithelium allows placement of 3 rows of sutures across the cul-de-sac from one uterosacral ligament to the other.
FIGURE 3 External McCall sutures
Three additional rows of absorbable sutures incorporate vaginal epithelium and uterosacral ligaments to move the vaginal cuff superiorly.
Modifications enhance durability and support
When the surgical indication is significant apical vaginal prolapse, the efficacy of the McCall procedure as both treatment and prevention is uncertain, because we lack adequate studies in this population. However, assuming that identifiable defects or breaks in the uterosacral ligaments lead to apical prolapse,3 use of the portion of the uterosacral ligament nearest the vagina appears unlikely to create a durable repair.
Thus, the concept of a “high” uterosacral attachment came to be proposed to provide a strong midline site of support for the vaginal apex.5,6 Further modifications include attachment of the uterosacral ligaments to pubocervical and rectovaginal connective tissues to create continuity of these level II fibers and prevent subsequent enterocele.7
With a high uterosacral attachment, the uterosacral ligaments need not be brought together in the midline.
Technique for modified approach
To locate each ligament, place traction on the vaginal apex toward the contralateral side. Palpate the pelvic structures posterior and medial to the ischial spines, at the 4 and 8 o’clock positions, to identify the strong tissue emanating from the sacrum.
Place several nonabsorbable sutures through the medial aspect of each uterosacral ligament, working from lateral to medial to minimize the risk of ureteral trauma. Then place 1 strand of each suture through the pubocervical and rectovaginal connective tissues. Tie the sutures to move the vaginal apex to the proximal segment of the uterosacral ligament (near the sacrum) and establish continuity of the pubocervical and rectovaginal connective tissues (FIGURES 4-6).
FIGURE 4 Suture placement is bilateral
Three sutures are placed in the uterosacral ligament pedicles on each side, with 1 arm of each suture placed in the transverse portion of the pubocervical and rectovaginal fascia.
FIGURE 5 Suspensory suture
Sagittal view of a suspensory suture in left uterosacral ligament with 1 arm through pubocervical fascia (PCF) and 1 arm through rectovaginal fascia (RVF).
FIGURE 6 The suspended vaginal vault
Sagittal view of pubocervical fascia (PCF) and rectovaginal fascia (RVF) suspended from uterosacral ligaments.
Main concern is ureteral injury
The ureter lies near the anterior margin of the uterosacral ligament, with a mean distance of 4.1±0.6 cm at the level of the sacrum and 2.3±0.9 cm at the level of the ischial spine.8 In 1 series of high uterosacral ligament suspension with site-specific endopelvic fascia defect repair, ureteral complications occurred in 11% of patients.5 Other series have reported rates of ureteral trauma in the range of 0.7%9 to 2.4%.6
Evaluate ureteral patency
After performing this procedure, the surgeon should ensure ureteral patency. For this reason, I believe that only surgeons skilled in cystoscopy and able to treat ureteral injury (or with ready access to those capable of treating this complication) should undertake high uterosacral suspension.
How the McCall procedure compares with other approaches
Cruikshank SH, Kovac SR. Randomized comparison of three surgical methods used at the time of vaginal hysterectomy to prevent posterior enterocele. Am J Obstet Gynecol. 1999;180:859–865
Although many techniques and modifications have been described for management of the cul-de-sac and vaginal cuff, few comparative data exist. In McCall’s original series,4 43 patients undergoing vaginal hysterectomy with posterior culdoplasty were followed for a minimum of 3 months and a maximum of 3 years. Mean and median lengths of follow-up were not provided, nor were the indications for hysterectomy or the method of assessing the patient for recurrent defects. McCall reported that no patients developed an enterocele after the surgery.
The first and only randomized study
Cruikshank and Kovac performed the only prospective, randomized comparison of procedures used at the time of hysterectomy to prevent enterocele. In their study, 100 patients undergoing vaginal hysterectomy for various indications (excluding prolapse of the posterior superior segment of the vagina) were randomized to 1 of 3 surgical methods to prevent enterocele:
- Moschcowitz-type closure (n=33), in which the peritoneum was closed using a purse-string technique, incorporating the distal ends of the uterosacral and cardinal ligaments and thereby drawing these structures to the midline
- modified McCall culdoplasty (n=33), in which a higher purse-string closure of the peritoneum was performed superior to the “yellow fat line,” incorporating the uterosacral-cardinal ligament complex (thus drawing these structures to the midline) and including the vagina (similar to the external McCall sutures) to move the apex superiorly
- simple closure of the peritoneum with a purse-string suture (n=34), with none of the uterosacral-cardinal ligaments incorporated into the repair.
Three years of follow-up
Of the 100 patients, 98 were followed with serial examinations for 3 years, and the outcomes at all vaginal segments were documented, with particular attention to the posterior superior segment, using staging from the Pelvic Organ Prolapse Quantification (POP-Q) system.10
McCall procedure was most effective
Overall, 11 patients (11.2%) were found to have stage II prolapse of the posterior superior vagina, none of them in the McCall group. An additional 14 patients (14.3%) had stage I prolapse, with only 2 (2%) of these in the McCall group.
The McCall repair was significantly more effective than the other 2 types of repair, with a 6.1% risk of subsequent prolapse, versus 30.3% in women who had a Moschcowitz-type closure and 39.4% in those who underwent simple closure of the peritoneum. No patients in any group had prolapse greater than stage II at follow-up.
Limitations of the study
Although Cruikshank and Kovac designed their study to analyze appropriate prophylaxis against enterocele in patients without prolapse, several patients did have some form of prolapse—although it was unrelated to the posterior superior vagina. Therefore, several patients underwent concomitant reconstructive procedures that included: anterior colporrhaphy (13), posterior colporrhaphy (4), sacrospinous ligament fixation (3), bilateral paravaginal repair (10), and anti-incontinence procedures (10).
It is not clear which groups these patients fell into and whether the distribution was similar across all groups.
CASE 2 Is McCall procedure appropriate?
B.D., 57, complains of increasing pelvic pressure and a noticeable vaginal bulge. Her 2 children were delivered vaginally, the largest weighing 8 lb. B.D. reports that she remains sexually active.
Physical examination reveals the cervix to be at the level of the introitus, but it descends 2 cm beyond the introitus when the patient performs the valsalva maneuver. Although there is also some descent of the anterior and posterior vaginal walls (1 cm superior to the hymen with strain; Pelvic Organ Prolapse Quantification [POP-Q] value=-1), the predominant component of prolapse is an elongated cervix. The posterior vaginal fornix (POP-Q point D), representing apical support, descends to 7 cm superior to the hymen with strain, with a total vaginal length of 9 cm.
At surgery, the uterosacral ligaments do not appear to be attenuated. After vaginal hysterectomy, the apex of the vaginal vault is superior to the level of the ischial spines.
How do you proceed?
Given the relatively good support at the apex, this patient is a good candidate for a McCall-type culdoplasty. Whether or not this procedure will be truly prophylactic (because there is already some descent of the apex, albeit mild) is perhaps only a matter of semantics.
The author reports no financial relationships relevant to this article.
1. Olson AL, Smith VJ, Bergstrom JO, Coiling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
2. DeLancey JOL. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717-1728.
3. Richardson AC. The anatomic defects in rectocele and enterocele. J Pelvic Surg. 1995;1:214-221.
4. McCall ML. Posterior culdoplasty: surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10:595-602.
5. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1402-1411.
6. Karram M, Goldwasser S, Kleeman S, et al. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185:1339-1342.
7. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
8. Buller JL, Thompson JR, Cundiff GW, et al. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol. 2001;97:873-879.
9. Aronson MP, Aronson PK, Howard AE, et al. Low risk of ureteral obstruction with “deep” (dorsal/posterior) uterosacral ligament suture placement for transvaginal apical suspension. Am J Obstet Gynecol. 2005;192:1530-1536.
10. Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10-17.
1. Olson AL, Smith VJ, Bergstrom JO, Coiling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
2. DeLancey JOL. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717-1728.
3. Richardson AC. The anatomic defects in rectocele and enterocele. J Pelvic Surg. 1995;1:214-221.
4. McCall ML. Posterior culdoplasty: surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10:595-602.
5. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1402-1411.
6. Karram M, Goldwasser S, Kleeman S, et al. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185:1339-1342.
7. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
8. Buller JL, Thompson JR, Cundiff GW, et al. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol. 2001;97:873-879.
9. Aronson MP, Aronson PK, Howard AE, et al. Low risk of ureteral obstruction with “deep” (dorsal/posterior) uterosacral ligament suture placement for transvaginal apical suspension. Am J Obstet Gynecol. 2005;192:1530-1536.
10. Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10-17.
IN THIS ARTICLE
Secrets to successful vaginal hysterectomy
M.K. is a 43-year-old gravida 2 para 2 who is undergoing a vaginal hysterectomy for menorrhagia. A preoperative pelvic exam and ultrasound suggested a 12-week-size uterus with several small leiomyomata. Her gynecologist estimates the uterine weight at 240 g and notes that the uterus is mobile. M.K. asks that her ovaries be removed at the time of hysterectomy because of a family history of ovarian cancer.
During the initial dissection, the surgeon is unable to enter the anterior cul-de-sac due to distortion created by an anterior fibroid. The surgeon has entered the posterior cul-de-sac, but the uterus is too large to manipulate a finger around anteriorly to identify the peritoneal fold. Although he feels confident that the bladder has been adequately mobilized from the cervix, the surgeon is strongly considering abandoning the vaginal approach and completing the hysterectomy abdominally.
How should he proceed?
Entry into the peritoneal cavity through the anterior or posterior cul-de-sac can sometimes be challenging, as this case illustrates. However, there is no need for the surgeon to abandon the vaginal approach just yet. In my experience, the anterior peritoneal fold can be high or distorted by fibroids in some women. The key to successful surgery is a pause in activity to consider the case at hand and determine whether additional progress can be made safely without changing the approach.
Avoid blind entry at all costs
No less an authority than Heaney1 advised against blind attempts to enter the anterior cul-de-sac. Such attempts are often frustrating, can involve bleeding, and raise the risk of injury to the bladder. However, once the surgeon is confident that the bladder is free and retracted out of the way, he or she can proceed without intraperitoneal entry. This is especially true if the posterior cul-de-sac has been entered safely.
The “climb up” technique
In some cases, the surgeon may safely proceed extraperitoneally even if neither cul-de-sac has been opened. Krige2 coined the term “climb up” to describe the extraperitoneal approach to the inaccessible posterior cul-de-sac. He performed extensive extraperitoneal dissection that, if necessary, included both uterosacral and cardinal ligaments as well as uterine vessels. A surgeon may carry a total extraperitoneal dissection completely to the uterine fundus as long as the bladder and rectum are free.3
In M.K.’s case, the surgeon should proceed to take the uterosacral and cardinal ligaments posteriorly without swinging the clamps around to the anterior aspect of the cervix, if possible. Once these ligaments are taken, the uterus often descends enough that the anterior peritoneal fold becomes accessible. Once it is identified, the anterior cul-de-sac can be entered safely.
If safe entry still is not possible, the surgeon can take the uterine vessels if he or she is confident that the bladder is out of harm’s way. If the fold still cannot be identified after this bite, proceed with broad-ligament clamps, which usually lead to eventual opening of the peritoneal fold.
CASE 1 Some progress, then surgery stalls
The surgeon proceeds to operate extraperitoneally, as described above, and successfully enters the anterior cul-de-sac after the uterine vessels are ligated. However, after several additional bites of broad ligament on each side, progress stalls because of uterine size. The surgeon seems to be stuck and is growing increasingly frustrated.
What is the best way around this impasse?
Morcellation can involve a range of techniques
Whenever a large uterus prevents further progress, and the uterine vessels have been ligated, uterine morcellation can be performed. Morcellation techniques originated when vaginal hysterectomy was the archetypal gynecologic operation,4-7 and include uterine bisection,8-11 Lash intramyometrial coring,6,8,9 myomectomy,10,11 and wedge debulking.9 Although every surgeon has a favorite, some or all of these procedures may be necessary in the same patient.12-15 In all cases it is mandatory that the uterine vessels be ligated before any morcellation procedure is initiated.
In my experience, a uterus in the range of 240 g usually lends itself very nicely to Lash intramyometrial coring. This technique is a nearly bloodless procedure that does not violate the endometrial cavity when it is performed properly. In addition, any intramyometrial fibroids can be easily removed.
If coring does not decompress the uterus enough for safe delivery, the core can be cut off and the remaining uterus can be further morcellated by removing wedges of myometrium or by bivalving the uterus. Since there is usually more room in the posterior vagina than in the anterior vagina, as much of the wedge morcellation as possible should be done posteriorly.
CASE 1 Ovaries appear out of reach
After Lash intramyometrial coring, the surgeon successfully removes the uterus. He then turns his attention to the bilateral adnexectomy. Unfortunately, the ovaries are higher than anticipated, and he once again considers switching to the abdominal route to remove them.
Is a change in route the best option?
Focus on the round ligaments
The key to safe removal of the adnexa, especially in difficult cases, is the separate transection and ligation of the round ligaments. Many authors have reported high success rates for vaginal oophorectomy using this technique, especially in premenopausal women.16-19
Separate transection of the round ligament allows the surgeon to accomplish 2 very important tasks:
In many hysterectomy cases when oophorectomy is planned, this maneuver can be carried out prior to removal of the uterus. Once the round ligaments have been reached, the surgeon can deliver the uterine fundus anteriorly, allowing the round ligaments to be clamped and cut. It is not uncommon to be able to remove the uterus with both adnexa still attached.
With a large uterus, it may be necessary to clamp and transect the round ligament after the uterus is out. This does not preclude identification and transection of the round ligament to carry out the maneuvers described above.
Consider your tools
In very difficult cases, specialized clamps or sutures may be necessary. I find long, sturdy, right-angle clamps to be most useful. In addition, endoloop-type sutures often facilitate ligation of the vascular pedicle. The use of newer specialized bipolar electrosurgical instruments may be helpful, although I have no personal experience using them in vaginal surgery.
CASE 1 At closure, concerns about injury
After successful removal of both adnexa using the round-ligament technique, the surgeon is satisfied that he has achieved hemostasis and proceeds with his usual closure. However, he has nagging concerns about the possibility of undetected complications, because this case turned out to be more of a challenge than he had expected. He wonders if there is anything else he can do to ensure that everything is OK.
What would you do?
Besides ensuring satisfactory hemostasis, confirming the integrity of the urinary tract is the most important goal to achieve before leaving the operating room. Unrecognized injuries to the bladder or ureters are unacceptable and will lead to significant morbidity for the patient. I would certainly recommend that the surgeon in M.K.’s case perform cystoscopy after giving the patient intravenous indigo carmine to assure both ureteral patency and integrity of the bladder. I perform cystoscopy after all vaginal hysterectomies.
CASE 2 History of cesarean delivery
C.S. is a 38-year-old gravida 3 para 3 who presents with menometrorrhagia and dysmenorrhea unresponsive to medical therapy. Her first pregnancy resulted in vaginal delivery of a full-term infant without complications. Her second child was delivered via low-segment transverse cesarean section due to a persistent breech presentation at term. Her last child was delivered vaginally, also at term. Two years later C.S. underwent a laparoscopic tubal ligation without complications. That was 4 years ago. She began seeing her current gynecologist 2 years ago, when she moved to a new community.
Pelvic examination reveals a 6-week–size uterus and normal adnexa. Her uterus is mobile, and there does not appear to be any ventral fixation of the uterus to the abdominal wall from the cesarean section. Endometrial biopsy reveals proliferative endometrium only. Saline ultrasound demonstrates a 2-cm submucosal leiomyoma.
C.S. refuses hysteroscopic resection of the myoma and prefers hysterectomy as definitive therapy. She is the business manager for her family’s construction business, and she would like to be able to return to work as soon as possible after her surgery. She requests vaginal hysterectomy with conservation of her ovaries.
What is the best way to proceed at this point?
Many gynecologic surgeons regard previous pelvic surgery, including cesarean delivery, as a relative contraindication to vaginal hysterectomy. Although the major concern seems to be a potential for bladder injury during the bladder dissection, other problems such as ventral fixation of the uterus to the previous abdominal incision also are possible.
Vaginal hysterectomy requires a mobile uterus
All patients who will be undergoing vaginal hysterectomy must have demonstrated mobility of the uterus upon pelvic examination. This is particularly important in the case of prior pelvic surgery. In this case, the gynecologist also should make every attempt to obtain her surgical records—especially those from her laparoscopic tubal ligation—to exclude major adhesive disease in the pelvis.
Laparoscopic adhesiolysis may facilitate vaginal hysterectomy
If there is any concern that the uterus is fixed to the abdominal wall, abdominal hysterectomy should be considered. Even more preferable is laparoscopic adhesiolysis, which can make it possible to proceed with vaginal hysterectomy. I have used this approach in women with as many as 5 previous cesarean deliveries and severe ventral fixation of the uterus.20 After adhesiolysis, the remainder of the hysterectomy can usually be performed solely through the vaginal route.
CASE 2 Medical records suggest the vaginal route is feasible
The gynecologist obtains C.S.’s previous medical records, which confirm that the cesarean delivery was uncomplicated. They also indicate that, at the time of the sterilization procedure, there was no evidence of ventral fixation of the uterus or other major adhesive disease.
The physician decides to proceed with vaginal hysterectomy, but remains very concerned about the possibility of bladder injury. How can she avoid inadvertent cystotomy?
Difficulty identifying and safely dissecting the bladder—because of distortion of the vesicouterine space from the previous cesarean delivery—is a legitimate concern. However, injury to a scarred and densely adherent bladder is a risk even with abdominal dissection.
The vaginal approach to the distal vesicouterine space has a clear advantage: The vesicouterine space closest to the initial vaginal dissection is unaffected by the previous operation on the lower uterine segment. In contrast, with the abdominal approach, dissection begins in the area of scar, and only after penetrating the scar does one find the unaffected space. With the vaginal approach, dissection begins in the correct surgical plane, which aids in identification of the location of the bladder and cesarean scar.
Sharp dissection is a must to protect the bladder
Once the correct surgical plane is encountered, sharp dissection is necessary to prevent tears of the adherent bladder, which can occur with blunt dissection.
Although sharp dissection is the key to success under these circumstances, other maneuvers may be helpful in some cases.
Nichols21 suggested performing dissection of the bladder after it has been filled with a dilute indigo carmine solution to stain the bladder tissues and help prevent bladder injury.
Hoffman and Jaeger22 describe the placement of a bent uterine sound in the posterior cul-de-sac. The sound is then brought around to the anterior cul-de-sac as an aid to dissection of the bladder and the vesicouterine peritoneal fold.
Sheth and Malpani23 describe developing a lateral “window” through the broad ligament to the bladder dissection when there are dense midline adhesions.
Although these are all valuable suggestions, I have found that they are rarely needed with careful sharp dissection. Remember, it is essential to avoid the temptation of blunt dissection when performing vaginal hysterectomy in women with a prior cesarean delivery.
CASE 2 Procedure is a success
The vaginal hysterectomy is carried out without incident, and cystoscopy following the hysterectomy is negative for any bladder injury; both ureteral orifices promptly efflux indigo carmine.
The surgeon encountered little difficulty during the bladder dissection, which was performed sharply. In fact, she was surprised at how well she could actually identify the hysterotomy scar and bladder. The patient goes home after 24 hours and is back at work in 2 weeks.
As noted in both cases presented here, the gynecologic surgeon must be certain that the urinary tract is intact and uninjured prior to leaving the operating room. This includes careful inspection of the bladder grossly for any sign of injury, as well as cystoscopy.
Most bladder injuries that occur with hysterectomy—either vaginal or abdominal—are usually well above the trigone and can be carefully repaired by the gynecologic surgeon. Complex injuries to the bladder involving the trigone or ureters usually require urologic intraoperative consultation.
The author reports no financial relationships relevant to this article.
REFERNCES
1. Heaney NS. Vaginal hysterectomy-its indications and technique. Am J Surg. 1940;48:284-288.
2. Krige CF. Vaginal hysterectomy and genital prolapse repair. Johannesburg, South Africa: Witwatersrand University Press; 1965: 57-70.
3. Unger JB. The extraperitoneal approach to vaginal hysterectomy. J Pelvic Surg. 1997;3:240-245.
4. Garceau E. Vaginal hysterectomy as done in France. Am J Obstet Dis Women Child. 1895;31:305-346.
5. Heaney NS. A report of 565 vaginal hysterectomies performed for benign disease. Am J Obstet Gynecol. 1934;28:751-755.
6. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
7. Allen E, Peterson LF. Versatility of vaginal hysterectomy technic. Obstet Gynecol. 1954;3:240-247.
8. Nichols DH, Randall CL. Vaginal Surgery. 4th ed. Baltimore: Williams and Wilkins;1996.
9. Stovall TG. Vaginal, abdominal, and laparoscopic-assisted hysterectomy. In: Mann WJ, Stovall TG, eds. Gynecologic Surgery. New York: Churchill Livingstone;1996: 403-404.
10. Lee RA. Atlas of Gynecologic Surgery. Philadelphia: WB Saunders; 1992.
11. Reiffenstuhl G, Platzer W, Knapstein PG, Imig JR. Vaginal operations–surgical anatomy and technique. 2nd ed. Baltimore: Williams and Wilkins; 1996.
12. Unger JB. Vaginal hysterectomy for the woman with a moderately enlarged uterus weighing 200 to 700 grams. Am J Obstet Gynecol. 1999;180:1337-1344.
13. Magos A, Bournas N, Sinha R, Richardson RE, O’Connor H. Vaginal hysterectomy for the large uterus. Br J Obstet Gynaecol. 1996;103:246-251.
14. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico Hospital’s experience. Obstet Gynecol. 1996;88:560-563.
15. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, nonmorbid procedure. Obstet Gynecol. 1995;86:60-64.
16. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
17. Ballard LA, Walters MD. Transvaginal mobilization and removal of the ovaries and fallopian tubes after vaginal hysterectomy. Obstet Gynecol. 1996;87:35-39.
18. Davies A, O’Connor H, Magos AL. A prospective study to evaluate oophorectomy at the time of vaginal hysterectomy. Br J Obstet Gynaecol. 1996;103:915-920.
19. Unger JB. Planned prophylactic oophorectomy at vaginal hysterectomy: clamp technique with separate division of the round and infundibulopelvic ligaments. J Pelvic Surg. 1999;5:151-155.
20. Unger JB, Meeks GR. Vaginal hysterectomy in women with history of previous cesarean delivery. Am J Obstet Gynecol. 1998;179:1473-1478.
21. Nichols DH. Vaginal versus abdominal hysterectomy. In: Stovall TG, ed. Current topics in obstetrics and gynecology: hysterectomy. New York: Elsevier; 1993:27-33.
22. Hoffman MS, Jaeger M. A new method for gaining entry into the scarred anterior cul-de-sac during transvaginal hysterectomy. Am J Obstet Gynecol. 1990;162:1269-1270.
23. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynecol Obstet. 1995;50:165-169.
M.K. is a 43-year-old gravida 2 para 2 who is undergoing a vaginal hysterectomy for menorrhagia. A preoperative pelvic exam and ultrasound suggested a 12-week-size uterus with several small leiomyomata. Her gynecologist estimates the uterine weight at 240 g and notes that the uterus is mobile. M.K. asks that her ovaries be removed at the time of hysterectomy because of a family history of ovarian cancer.
During the initial dissection, the surgeon is unable to enter the anterior cul-de-sac due to distortion created by an anterior fibroid. The surgeon has entered the posterior cul-de-sac, but the uterus is too large to manipulate a finger around anteriorly to identify the peritoneal fold. Although he feels confident that the bladder has been adequately mobilized from the cervix, the surgeon is strongly considering abandoning the vaginal approach and completing the hysterectomy abdominally.
How should he proceed?
Entry into the peritoneal cavity through the anterior or posterior cul-de-sac can sometimes be challenging, as this case illustrates. However, there is no need for the surgeon to abandon the vaginal approach just yet. In my experience, the anterior peritoneal fold can be high or distorted by fibroids in some women. The key to successful surgery is a pause in activity to consider the case at hand and determine whether additional progress can be made safely without changing the approach.
Avoid blind entry at all costs
No less an authority than Heaney1 advised against blind attempts to enter the anterior cul-de-sac. Such attempts are often frustrating, can involve bleeding, and raise the risk of injury to the bladder. However, once the surgeon is confident that the bladder is free and retracted out of the way, he or she can proceed without intraperitoneal entry. This is especially true if the posterior cul-de-sac has been entered safely.
The “climb up” technique
In some cases, the surgeon may safely proceed extraperitoneally even if neither cul-de-sac has been opened. Krige2 coined the term “climb up” to describe the extraperitoneal approach to the inaccessible posterior cul-de-sac. He performed extensive extraperitoneal dissection that, if necessary, included both uterosacral and cardinal ligaments as well as uterine vessels. A surgeon may carry a total extraperitoneal dissection completely to the uterine fundus as long as the bladder and rectum are free.3
In M.K.’s case, the surgeon should proceed to take the uterosacral and cardinal ligaments posteriorly without swinging the clamps around to the anterior aspect of the cervix, if possible. Once these ligaments are taken, the uterus often descends enough that the anterior peritoneal fold becomes accessible. Once it is identified, the anterior cul-de-sac can be entered safely.
If safe entry still is not possible, the surgeon can take the uterine vessels if he or she is confident that the bladder is out of harm’s way. If the fold still cannot be identified after this bite, proceed with broad-ligament clamps, which usually lead to eventual opening of the peritoneal fold.
CASE 1 Some progress, then surgery stalls
The surgeon proceeds to operate extraperitoneally, as described above, and successfully enters the anterior cul-de-sac after the uterine vessels are ligated. However, after several additional bites of broad ligament on each side, progress stalls because of uterine size. The surgeon seems to be stuck and is growing increasingly frustrated.
What is the best way around this impasse?
Morcellation can involve a range of techniques
Whenever a large uterus prevents further progress, and the uterine vessels have been ligated, uterine morcellation can be performed. Morcellation techniques originated when vaginal hysterectomy was the archetypal gynecologic operation,4-7 and include uterine bisection,8-11 Lash intramyometrial coring,6,8,9 myomectomy,10,11 and wedge debulking.9 Although every surgeon has a favorite, some or all of these procedures may be necessary in the same patient.12-15 In all cases it is mandatory that the uterine vessels be ligated before any morcellation procedure is initiated.
In my experience, a uterus in the range of 240 g usually lends itself very nicely to Lash intramyometrial coring. This technique is a nearly bloodless procedure that does not violate the endometrial cavity when it is performed properly. In addition, any intramyometrial fibroids can be easily removed.
If coring does not decompress the uterus enough for safe delivery, the core can be cut off and the remaining uterus can be further morcellated by removing wedges of myometrium or by bivalving the uterus. Since there is usually more room in the posterior vagina than in the anterior vagina, as much of the wedge morcellation as possible should be done posteriorly.
CASE 1 Ovaries appear out of reach
After Lash intramyometrial coring, the surgeon successfully removes the uterus. He then turns his attention to the bilateral adnexectomy. Unfortunately, the ovaries are higher than anticipated, and he once again considers switching to the abdominal route to remove them.
Is a change in route the best option?
Focus on the round ligaments
The key to safe removal of the adnexa, especially in difficult cases, is the separate transection and ligation of the round ligaments. Many authors have reported high success rates for vaginal oophorectomy using this technique, especially in premenopausal women.16-19
Separate transection of the round ligament allows the surgeon to accomplish 2 very important tasks:
In many hysterectomy cases when oophorectomy is planned, this maneuver can be carried out prior to removal of the uterus. Once the round ligaments have been reached, the surgeon can deliver the uterine fundus anteriorly, allowing the round ligaments to be clamped and cut. It is not uncommon to be able to remove the uterus with both adnexa still attached.
With a large uterus, it may be necessary to clamp and transect the round ligament after the uterus is out. This does not preclude identification and transection of the round ligament to carry out the maneuvers described above.
Consider your tools
In very difficult cases, specialized clamps or sutures may be necessary. I find long, sturdy, right-angle clamps to be most useful. In addition, endoloop-type sutures often facilitate ligation of the vascular pedicle. The use of newer specialized bipolar electrosurgical instruments may be helpful, although I have no personal experience using them in vaginal surgery.
CASE 1 At closure, concerns about injury
After successful removal of both adnexa using the round-ligament technique, the surgeon is satisfied that he has achieved hemostasis and proceeds with his usual closure. However, he has nagging concerns about the possibility of undetected complications, because this case turned out to be more of a challenge than he had expected. He wonders if there is anything else he can do to ensure that everything is OK.
What would you do?
Besides ensuring satisfactory hemostasis, confirming the integrity of the urinary tract is the most important goal to achieve before leaving the operating room. Unrecognized injuries to the bladder or ureters are unacceptable and will lead to significant morbidity for the patient. I would certainly recommend that the surgeon in M.K.’s case perform cystoscopy after giving the patient intravenous indigo carmine to assure both ureteral patency and integrity of the bladder. I perform cystoscopy after all vaginal hysterectomies.
CASE 2 History of cesarean delivery
C.S. is a 38-year-old gravida 3 para 3 who presents with menometrorrhagia and dysmenorrhea unresponsive to medical therapy. Her first pregnancy resulted in vaginal delivery of a full-term infant without complications. Her second child was delivered via low-segment transverse cesarean section due to a persistent breech presentation at term. Her last child was delivered vaginally, also at term. Two years later C.S. underwent a laparoscopic tubal ligation without complications. That was 4 years ago. She began seeing her current gynecologist 2 years ago, when she moved to a new community.
Pelvic examination reveals a 6-week–size uterus and normal adnexa. Her uterus is mobile, and there does not appear to be any ventral fixation of the uterus to the abdominal wall from the cesarean section. Endometrial biopsy reveals proliferative endometrium only. Saline ultrasound demonstrates a 2-cm submucosal leiomyoma.
C.S. refuses hysteroscopic resection of the myoma and prefers hysterectomy as definitive therapy. She is the business manager for her family’s construction business, and she would like to be able to return to work as soon as possible after her surgery. She requests vaginal hysterectomy with conservation of her ovaries.
What is the best way to proceed at this point?
Many gynecologic surgeons regard previous pelvic surgery, including cesarean delivery, as a relative contraindication to vaginal hysterectomy. Although the major concern seems to be a potential for bladder injury during the bladder dissection, other problems such as ventral fixation of the uterus to the previous abdominal incision also are possible.
Vaginal hysterectomy requires a mobile uterus
All patients who will be undergoing vaginal hysterectomy must have demonstrated mobility of the uterus upon pelvic examination. This is particularly important in the case of prior pelvic surgery. In this case, the gynecologist also should make every attempt to obtain her surgical records—especially those from her laparoscopic tubal ligation—to exclude major adhesive disease in the pelvis.
Laparoscopic adhesiolysis may facilitate vaginal hysterectomy
If there is any concern that the uterus is fixed to the abdominal wall, abdominal hysterectomy should be considered. Even more preferable is laparoscopic adhesiolysis, which can make it possible to proceed with vaginal hysterectomy. I have used this approach in women with as many as 5 previous cesarean deliveries and severe ventral fixation of the uterus.20 After adhesiolysis, the remainder of the hysterectomy can usually be performed solely through the vaginal route.
CASE 2 Medical records suggest the vaginal route is feasible
The gynecologist obtains C.S.’s previous medical records, which confirm that the cesarean delivery was uncomplicated. They also indicate that, at the time of the sterilization procedure, there was no evidence of ventral fixation of the uterus or other major adhesive disease.
The physician decides to proceed with vaginal hysterectomy, but remains very concerned about the possibility of bladder injury. How can she avoid inadvertent cystotomy?
Difficulty identifying and safely dissecting the bladder—because of distortion of the vesicouterine space from the previous cesarean delivery—is a legitimate concern. However, injury to a scarred and densely adherent bladder is a risk even with abdominal dissection.
The vaginal approach to the distal vesicouterine space has a clear advantage: The vesicouterine space closest to the initial vaginal dissection is unaffected by the previous operation on the lower uterine segment. In contrast, with the abdominal approach, dissection begins in the area of scar, and only after penetrating the scar does one find the unaffected space. With the vaginal approach, dissection begins in the correct surgical plane, which aids in identification of the location of the bladder and cesarean scar.
Sharp dissection is a must to protect the bladder
Once the correct surgical plane is encountered, sharp dissection is necessary to prevent tears of the adherent bladder, which can occur with blunt dissection.
Although sharp dissection is the key to success under these circumstances, other maneuvers may be helpful in some cases.
Nichols21 suggested performing dissection of the bladder after it has been filled with a dilute indigo carmine solution to stain the bladder tissues and help prevent bladder injury.
Hoffman and Jaeger22 describe the placement of a bent uterine sound in the posterior cul-de-sac. The sound is then brought around to the anterior cul-de-sac as an aid to dissection of the bladder and the vesicouterine peritoneal fold.
Sheth and Malpani23 describe developing a lateral “window” through the broad ligament to the bladder dissection when there are dense midline adhesions.
Although these are all valuable suggestions, I have found that they are rarely needed with careful sharp dissection. Remember, it is essential to avoid the temptation of blunt dissection when performing vaginal hysterectomy in women with a prior cesarean delivery.
CASE 2 Procedure is a success
The vaginal hysterectomy is carried out without incident, and cystoscopy following the hysterectomy is negative for any bladder injury; both ureteral orifices promptly efflux indigo carmine.
The surgeon encountered little difficulty during the bladder dissection, which was performed sharply. In fact, she was surprised at how well she could actually identify the hysterotomy scar and bladder. The patient goes home after 24 hours and is back at work in 2 weeks.
As noted in both cases presented here, the gynecologic surgeon must be certain that the urinary tract is intact and uninjured prior to leaving the operating room. This includes careful inspection of the bladder grossly for any sign of injury, as well as cystoscopy.
Most bladder injuries that occur with hysterectomy—either vaginal or abdominal—are usually well above the trigone and can be carefully repaired by the gynecologic surgeon. Complex injuries to the bladder involving the trigone or ureters usually require urologic intraoperative consultation.
The author reports no financial relationships relevant to this article.
M.K. is a 43-year-old gravida 2 para 2 who is undergoing a vaginal hysterectomy for menorrhagia. A preoperative pelvic exam and ultrasound suggested a 12-week-size uterus with several small leiomyomata. Her gynecologist estimates the uterine weight at 240 g and notes that the uterus is mobile. M.K. asks that her ovaries be removed at the time of hysterectomy because of a family history of ovarian cancer.
During the initial dissection, the surgeon is unable to enter the anterior cul-de-sac due to distortion created by an anterior fibroid. The surgeon has entered the posterior cul-de-sac, but the uterus is too large to manipulate a finger around anteriorly to identify the peritoneal fold. Although he feels confident that the bladder has been adequately mobilized from the cervix, the surgeon is strongly considering abandoning the vaginal approach and completing the hysterectomy abdominally.
How should he proceed?
Entry into the peritoneal cavity through the anterior or posterior cul-de-sac can sometimes be challenging, as this case illustrates. However, there is no need for the surgeon to abandon the vaginal approach just yet. In my experience, the anterior peritoneal fold can be high or distorted by fibroids in some women. The key to successful surgery is a pause in activity to consider the case at hand and determine whether additional progress can be made safely without changing the approach.
Avoid blind entry at all costs
No less an authority than Heaney1 advised against blind attempts to enter the anterior cul-de-sac. Such attempts are often frustrating, can involve bleeding, and raise the risk of injury to the bladder. However, once the surgeon is confident that the bladder is free and retracted out of the way, he or she can proceed without intraperitoneal entry. This is especially true if the posterior cul-de-sac has been entered safely.
The “climb up” technique
In some cases, the surgeon may safely proceed extraperitoneally even if neither cul-de-sac has been opened. Krige2 coined the term “climb up” to describe the extraperitoneal approach to the inaccessible posterior cul-de-sac. He performed extensive extraperitoneal dissection that, if necessary, included both uterosacral and cardinal ligaments as well as uterine vessels. A surgeon may carry a total extraperitoneal dissection completely to the uterine fundus as long as the bladder and rectum are free.3
In M.K.’s case, the surgeon should proceed to take the uterosacral and cardinal ligaments posteriorly without swinging the clamps around to the anterior aspect of the cervix, if possible. Once these ligaments are taken, the uterus often descends enough that the anterior peritoneal fold becomes accessible. Once it is identified, the anterior cul-de-sac can be entered safely.
If safe entry still is not possible, the surgeon can take the uterine vessels if he or she is confident that the bladder is out of harm’s way. If the fold still cannot be identified after this bite, proceed with broad-ligament clamps, which usually lead to eventual opening of the peritoneal fold.
CASE 1 Some progress, then surgery stalls
The surgeon proceeds to operate extraperitoneally, as described above, and successfully enters the anterior cul-de-sac after the uterine vessels are ligated. However, after several additional bites of broad ligament on each side, progress stalls because of uterine size. The surgeon seems to be stuck and is growing increasingly frustrated.
What is the best way around this impasse?
Morcellation can involve a range of techniques
Whenever a large uterus prevents further progress, and the uterine vessels have been ligated, uterine morcellation can be performed. Morcellation techniques originated when vaginal hysterectomy was the archetypal gynecologic operation,4-7 and include uterine bisection,8-11 Lash intramyometrial coring,6,8,9 myomectomy,10,11 and wedge debulking.9 Although every surgeon has a favorite, some or all of these procedures may be necessary in the same patient.12-15 In all cases it is mandatory that the uterine vessels be ligated before any morcellation procedure is initiated.
In my experience, a uterus in the range of 240 g usually lends itself very nicely to Lash intramyometrial coring. This technique is a nearly bloodless procedure that does not violate the endometrial cavity when it is performed properly. In addition, any intramyometrial fibroids can be easily removed.
If coring does not decompress the uterus enough for safe delivery, the core can be cut off and the remaining uterus can be further morcellated by removing wedges of myometrium or by bivalving the uterus. Since there is usually more room in the posterior vagina than in the anterior vagina, as much of the wedge morcellation as possible should be done posteriorly.
CASE 1 Ovaries appear out of reach
After Lash intramyometrial coring, the surgeon successfully removes the uterus. He then turns his attention to the bilateral adnexectomy. Unfortunately, the ovaries are higher than anticipated, and he once again considers switching to the abdominal route to remove them.
Is a change in route the best option?
Focus on the round ligaments
The key to safe removal of the adnexa, especially in difficult cases, is the separate transection and ligation of the round ligaments. Many authors have reported high success rates for vaginal oophorectomy using this technique, especially in premenopausal women.16-19
Separate transection of the round ligament allows the surgeon to accomplish 2 very important tasks:
In many hysterectomy cases when oophorectomy is planned, this maneuver can be carried out prior to removal of the uterus. Once the round ligaments have been reached, the surgeon can deliver the uterine fundus anteriorly, allowing the round ligaments to be clamped and cut. It is not uncommon to be able to remove the uterus with both adnexa still attached.
With a large uterus, it may be necessary to clamp and transect the round ligament after the uterus is out. This does not preclude identification and transection of the round ligament to carry out the maneuvers described above.
Consider your tools
In very difficult cases, specialized clamps or sutures may be necessary. I find long, sturdy, right-angle clamps to be most useful. In addition, endoloop-type sutures often facilitate ligation of the vascular pedicle. The use of newer specialized bipolar electrosurgical instruments may be helpful, although I have no personal experience using them in vaginal surgery.
CASE 1 At closure, concerns about injury
After successful removal of both adnexa using the round-ligament technique, the surgeon is satisfied that he has achieved hemostasis and proceeds with his usual closure. However, he has nagging concerns about the possibility of undetected complications, because this case turned out to be more of a challenge than he had expected. He wonders if there is anything else he can do to ensure that everything is OK.
What would you do?
Besides ensuring satisfactory hemostasis, confirming the integrity of the urinary tract is the most important goal to achieve before leaving the operating room. Unrecognized injuries to the bladder or ureters are unacceptable and will lead to significant morbidity for the patient. I would certainly recommend that the surgeon in M.K.’s case perform cystoscopy after giving the patient intravenous indigo carmine to assure both ureteral patency and integrity of the bladder. I perform cystoscopy after all vaginal hysterectomies.
CASE 2 History of cesarean delivery
C.S. is a 38-year-old gravida 3 para 3 who presents with menometrorrhagia and dysmenorrhea unresponsive to medical therapy. Her first pregnancy resulted in vaginal delivery of a full-term infant without complications. Her second child was delivered via low-segment transverse cesarean section due to a persistent breech presentation at term. Her last child was delivered vaginally, also at term. Two years later C.S. underwent a laparoscopic tubal ligation without complications. That was 4 years ago. She began seeing her current gynecologist 2 years ago, when she moved to a new community.
Pelvic examination reveals a 6-week–size uterus and normal adnexa. Her uterus is mobile, and there does not appear to be any ventral fixation of the uterus to the abdominal wall from the cesarean section. Endometrial biopsy reveals proliferative endometrium only. Saline ultrasound demonstrates a 2-cm submucosal leiomyoma.
C.S. refuses hysteroscopic resection of the myoma and prefers hysterectomy as definitive therapy. She is the business manager for her family’s construction business, and she would like to be able to return to work as soon as possible after her surgery. She requests vaginal hysterectomy with conservation of her ovaries.
What is the best way to proceed at this point?
Many gynecologic surgeons regard previous pelvic surgery, including cesarean delivery, as a relative contraindication to vaginal hysterectomy. Although the major concern seems to be a potential for bladder injury during the bladder dissection, other problems such as ventral fixation of the uterus to the previous abdominal incision also are possible.
Vaginal hysterectomy requires a mobile uterus
All patients who will be undergoing vaginal hysterectomy must have demonstrated mobility of the uterus upon pelvic examination. This is particularly important in the case of prior pelvic surgery. In this case, the gynecologist also should make every attempt to obtain her surgical records—especially those from her laparoscopic tubal ligation—to exclude major adhesive disease in the pelvis.
Laparoscopic adhesiolysis may facilitate vaginal hysterectomy
If there is any concern that the uterus is fixed to the abdominal wall, abdominal hysterectomy should be considered. Even more preferable is laparoscopic adhesiolysis, which can make it possible to proceed with vaginal hysterectomy. I have used this approach in women with as many as 5 previous cesarean deliveries and severe ventral fixation of the uterus.20 After adhesiolysis, the remainder of the hysterectomy can usually be performed solely through the vaginal route.
CASE 2 Medical records suggest the vaginal route is feasible
The gynecologist obtains C.S.’s previous medical records, which confirm that the cesarean delivery was uncomplicated. They also indicate that, at the time of the sterilization procedure, there was no evidence of ventral fixation of the uterus or other major adhesive disease.
The physician decides to proceed with vaginal hysterectomy, but remains very concerned about the possibility of bladder injury. How can she avoid inadvertent cystotomy?
Difficulty identifying and safely dissecting the bladder—because of distortion of the vesicouterine space from the previous cesarean delivery—is a legitimate concern. However, injury to a scarred and densely adherent bladder is a risk even with abdominal dissection.
The vaginal approach to the distal vesicouterine space has a clear advantage: The vesicouterine space closest to the initial vaginal dissection is unaffected by the previous operation on the lower uterine segment. In contrast, with the abdominal approach, dissection begins in the area of scar, and only after penetrating the scar does one find the unaffected space. With the vaginal approach, dissection begins in the correct surgical plane, which aids in identification of the location of the bladder and cesarean scar.
Sharp dissection is a must to protect the bladder
Once the correct surgical plane is encountered, sharp dissection is necessary to prevent tears of the adherent bladder, which can occur with blunt dissection.
Although sharp dissection is the key to success under these circumstances, other maneuvers may be helpful in some cases.
Nichols21 suggested performing dissection of the bladder after it has been filled with a dilute indigo carmine solution to stain the bladder tissues and help prevent bladder injury.
Hoffman and Jaeger22 describe the placement of a bent uterine sound in the posterior cul-de-sac. The sound is then brought around to the anterior cul-de-sac as an aid to dissection of the bladder and the vesicouterine peritoneal fold.
Sheth and Malpani23 describe developing a lateral “window” through the broad ligament to the bladder dissection when there are dense midline adhesions.
Although these are all valuable suggestions, I have found that they are rarely needed with careful sharp dissection. Remember, it is essential to avoid the temptation of blunt dissection when performing vaginal hysterectomy in women with a prior cesarean delivery.
CASE 2 Procedure is a success
The vaginal hysterectomy is carried out without incident, and cystoscopy following the hysterectomy is negative for any bladder injury; both ureteral orifices promptly efflux indigo carmine.
The surgeon encountered little difficulty during the bladder dissection, which was performed sharply. In fact, she was surprised at how well she could actually identify the hysterotomy scar and bladder. The patient goes home after 24 hours and is back at work in 2 weeks.
As noted in both cases presented here, the gynecologic surgeon must be certain that the urinary tract is intact and uninjured prior to leaving the operating room. This includes careful inspection of the bladder grossly for any sign of injury, as well as cystoscopy.
Most bladder injuries that occur with hysterectomy—either vaginal or abdominal—are usually well above the trigone and can be carefully repaired by the gynecologic surgeon. Complex injuries to the bladder involving the trigone or ureters usually require urologic intraoperative consultation.
The author reports no financial relationships relevant to this article.
REFERNCES
1. Heaney NS. Vaginal hysterectomy-its indications and technique. Am J Surg. 1940;48:284-288.
2. Krige CF. Vaginal hysterectomy and genital prolapse repair. Johannesburg, South Africa: Witwatersrand University Press; 1965: 57-70.
3. Unger JB. The extraperitoneal approach to vaginal hysterectomy. J Pelvic Surg. 1997;3:240-245.
4. Garceau E. Vaginal hysterectomy as done in France. Am J Obstet Dis Women Child. 1895;31:305-346.
5. Heaney NS. A report of 565 vaginal hysterectomies performed for benign disease. Am J Obstet Gynecol. 1934;28:751-755.
6. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
7. Allen E, Peterson LF. Versatility of vaginal hysterectomy technic. Obstet Gynecol. 1954;3:240-247.
8. Nichols DH, Randall CL. Vaginal Surgery. 4th ed. Baltimore: Williams and Wilkins;1996.
9. Stovall TG. Vaginal, abdominal, and laparoscopic-assisted hysterectomy. In: Mann WJ, Stovall TG, eds. Gynecologic Surgery. New York: Churchill Livingstone;1996: 403-404.
10. Lee RA. Atlas of Gynecologic Surgery. Philadelphia: WB Saunders; 1992.
11. Reiffenstuhl G, Platzer W, Knapstein PG, Imig JR. Vaginal operations–surgical anatomy and technique. 2nd ed. Baltimore: Williams and Wilkins; 1996.
12. Unger JB. Vaginal hysterectomy for the woman with a moderately enlarged uterus weighing 200 to 700 grams. Am J Obstet Gynecol. 1999;180:1337-1344.
13. Magos A, Bournas N, Sinha R, Richardson RE, O’Connor H. Vaginal hysterectomy for the large uterus. Br J Obstet Gynaecol. 1996;103:246-251.
14. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico Hospital’s experience. Obstet Gynecol. 1996;88:560-563.
15. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, nonmorbid procedure. Obstet Gynecol. 1995;86:60-64.
16. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
17. Ballard LA, Walters MD. Transvaginal mobilization and removal of the ovaries and fallopian tubes after vaginal hysterectomy. Obstet Gynecol. 1996;87:35-39.
18. Davies A, O’Connor H, Magos AL. A prospective study to evaluate oophorectomy at the time of vaginal hysterectomy. Br J Obstet Gynaecol. 1996;103:915-920.
19. Unger JB. Planned prophylactic oophorectomy at vaginal hysterectomy: clamp technique with separate division of the round and infundibulopelvic ligaments. J Pelvic Surg. 1999;5:151-155.
20. Unger JB, Meeks GR. Vaginal hysterectomy in women with history of previous cesarean delivery. Am J Obstet Gynecol. 1998;179:1473-1478.
21. Nichols DH. Vaginal versus abdominal hysterectomy. In: Stovall TG, ed. Current topics in obstetrics and gynecology: hysterectomy. New York: Elsevier; 1993:27-33.
22. Hoffman MS, Jaeger M. A new method for gaining entry into the scarred anterior cul-de-sac during transvaginal hysterectomy. Am J Obstet Gynecol. 1990;162:1269-1270.
23. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynecol Obstet. 1995;50:165-169.
REFERNCES
1. Heaney NS. Vaginal hysterectomy-its indications and technique. Am J Surg. 1940;48:284-288.
2. Krige CF. Vaginal hysterectomy and genital prolapse repair. Johannesburg, South Africa: Witwatersrand University Press; 1965: 57-70.
3. Unger JB. The extraperitoneal approach to vaginal hysterectomy. J Pelvic Surg. 1997;3:240-245.
4. Garceau E. Vaginal hysterectomy as done in France. Am J Obstet Dis Women Child. 1895;31:305-346.
5. Heaney NS. A report of 565 vaginal hysterectomies performed for benign disease. Am J Obstet Gynecol. 1934;28:751-755.
6. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
7. Allen E, Peterson LF. Versatility of vaginal hysterectomy technic. Obstet Gynecol. 1954;3:240-247.
8. Nichols DH, Randall CL. Vaginal Surgery. 4th ed. Baltimore: Williams and Wilkins;1996.
9. Stovall TG. Vaginal, abdominal, and laparoscopic-assisted hysterectomy. In: Mann WJ, Stovall TG, eds. Gynecologic Surgery. New York: Churchill Livingstone;1996: 403-404.
10. Lee RA. Atlas of Gynecologic Surgery. Philadelphia: WB Saunders; 1992.
11. Reiffenstuhl G, Platzer W, Knapstein PG, Imig JR. Vaginal operations–surgical anatomy and technique. 2nd ed. Baltimore: Williams and Wilkins; 1996.
12. Unger JB. Vaginal hysterectomy for the woman with a moderately enlarged uterus weighing 200 to 700 grams. Am J Obstet Gynecol. 1999;180:1337-1344.
13. Magos A, Bournas N, Sinha R, Richardson RE, O’Connor H. Vaginal hysterectomy for the large uterus. Br J Obstet Gynaecol. 1996;103:246-251.
14. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico Hospital’s experience. Obstet Gynecol. 1996;88:560-563.
15. Mazdisnian F, Kurzel RB, Coe S, Bosuk M, Montz F. Vaginal hysterectomy by uterine morcellation: an efficient, nonmorbid procedure. Obstet Gynecol. 1995;86:60-64.
16. Sheth SS. The place of oophorectomy at vaginal hysterectomy. Br J Obstet Gynaecol. 1991;98:662-666.
17. Ballard LA, Walters MD. Transvaginal mobilization and removal of the ovaries and fallopian tubes after vaginal hysterectomy. Obstet Gynecol. 1996;87:35-39.
18. Davies A, O’Connor H, Magos AL. A prospective study to evaluate oophorectomy at the time of vaginal hysterectomy. Br J Obstet Gynaecol. 1996;103:915-920.
19. Unger JB. Planned prophylactic oophorectomy at vaginal hysterectomy: clamp technique with separate division of the round and infundibulopelvic ligaments. J Pelvic Surg. 1999;5:151-155.
20. Unger JB, Meeks GR. Vaginal hysterectomy in women with history of previous cesarean delivery. Am J Obstet Gynecol. 1998;179:1473-1478.
21. Nichols DH. Vaginal versus abdominal hysterectomy. In: Stovall TG, ed. Current topics in obstetrics and gynecology: hysterectomy. New York: Elsevier; 1993:27-33.
22. Hoffman MS, Jaeger M. A new method for gaining entry into the scarred anterior cul-de-sac during transvaginal hysterectomy. Am J Obstet Gynecol. 1990;162:1269-1270.
23. Sheth SS, Malpani AN. Vaginal hysterectomy following previous cesarean section. Int J Gynecol Obstet. 1995;50:165-169.
IN THIS ARTICLE