User login
Major vascular injury during laparoscopy: Pearls to cope
The author reports no financial relationships relevant to this article.
- Strategies that may help you avoid MVI
- Tips on how to recognize it
- What to do when MVI occurs
CASE Trocar insertion, then a bleed
Sophia, a 29-year-old nulliparous patient, undergoes diagnostic laparoscopy after unsuccessful medical therapy for chronic pelvic pain. After insufflation, a reusable 11-mm trocar with obturator is placed. A survey of the pelvis and abdomen reveals a 10-cm retroperitoneal hematoma in the midline just below the umbilicus.
What is the best way to manage this hemorrhage?
Contrary to widespread belief, gynecologic laparoscopy has a rate of major complication similar to, not higher than, that of laparotomy—but it has a higher rate of major vascular injury (MVI), defined as laceration of the aorta, inferior vena cava, or common iliac or external iliac vessels.1 In fact, MVI appears to be somewhat exclusive to laparoscopy.
In a large review of 29,966 gynecologic laparoscopies, investigators found the surgeon’s level of experience to be an important variable in the overall complication rate but not in the incidence of MVI.2 This means patients are at risk of MVI regardless of how many or how few laparoscopic procedures you perform.
What can a surgeon do to avert this type of injury? The most important strategy is to be conversant with variables that clearly increase the risk, as well as behaviors and protocols that can minimize it. This article offers five pearls—from prevention to postop management.
1. Pay attention to subtlety, starting at the preop visit
When a marriage counselor was asked which significant actions can improve a relationship, he responded: “The little things are the big things.”
To minimize the risk of vascular injury, a meticulous surgeon begins with the “little things” at the preoperative evaluation. Important considerations include the patient’s height, weight, and body mass index (BMI). Does she have a history of abdominal surgery or pelvic disease that could suggest intra-abdominal adhesions and anatomic distortion?
It is important to discuss the risk of bowel or vascular injury, as well as the need to convert to an open procedure, with all patients, but particularly with those who are thin or who have abnormal abdominal findings.
A recent randomized clinical trial found that routine mechanical bowel preparation did not facilitate surgery or decrease the incidence of complications, but may be helpful in select cases.3
2. Don’t undervalue that ounce of prevention
A commuter commented to her friend that she thought the bus driver had exceptional driving skill. The friend couldn’t understand this remark. “The driver hasn’t been challenged yet,” the friend argued. “She hasn’t needed to demonstrate any action in the face of an emergency—so how do you know what level of skill she has?”
“That is precisely the point,” the commuter replied. “The driver has exercised extreme judgment and caution so as not to require using her superior skill in an emergency.”
It is always better to avoid a potentially catastrophic complication than to manage it. The first requirement is vigilance in preparing for laparoscopy. It is important to ensure that the patient is in the supine position during initial entry and that her arms are comfortably tucked on both sides. In 85% of cases, the aorta can be palpated.4
Select an entry technique wisely
Base this decision on experience, patient characteristics, the surgical procedure, and availability of equipment. No entry technique or device can be considered completely safe.
Most vascular injuries occur during the initial phase of laparoscopy:
- As many as 39% are related to placement of the Veress needle
- As many as 37.9% are related to placement of the primary trocar.5
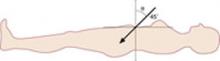
The classic “closed” method of laparoscopy has been used successfully for decades. In thin patients, insert the Veress needle toward the uterus. In computed tomography studies, an angle of at least 34 degrees has been shown to avoid the aorta and its bifurcation (FIGURE 1).6
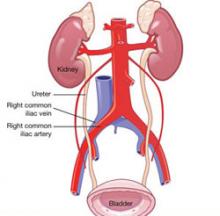
The right common iliac vessel is most commonly involved in injury, given its anatomic relationship overriding the inferior vena cava (FIGURE 2). When you stand at the patient’s left, the Veress needle can inadvertently be directed off to the right of midline, in harm’s way of the right common iliac vessels.
FIGURE 1 In thin women, insert the Veress needle toward the uterus
If the angle of insertion is 45° (≥34°) in the sagittal plane at the umbilicus, puncture safely avoids the aortic bifurcation.
FIGURE 2 At risk: The right common iliac artery
The right common iliac artery overlies its corresponding vein, which puts the artery at risk of injury at initial insertion of the Veress needle.
Tips to facilitate entry
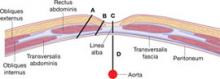
After insufflation, the distance from the base of the navel to the peritoneum is approximately 1.5 to 2.0 cm (FIGURE 3).7 It is neither necessary nor prudent to bury the needle to its hub when placing the initial trocar. Some authors report that over-inflation to 25 mm Hg lowers the risk of vascular injury, creating a longer distance to the retroperitoneal structures.8 In addition, the reduction of tenting means that less force is required.
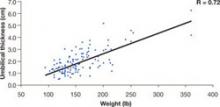
Patients with a high BMI are likely to be protected from MVI, given the greater distances to major vessels.9 The distance between the entry trocar and retroperitoneal vessels increases directly with BMI, commensurate with increasing abdominal-wall thickness (FIGURE 4).
FIGURE 3 The umbilicus is at its thinnest at low body weight
The distance (cm) into the peritoneal cavity after insufflation correlates with a patient’s weight.
SOURCE: Milad and Terkildsen.7
FIGURE 4 How far is it from umbilical skin to the peritoneum and vascular structures?
SOURCE: Hurd et al.9
Even optical trocars have been associated with MVI. Between 1994 and 2002, 79 serious complications, including 37 MVIs, were associated with use of these trocars.10
Direct insertion, open technique, and optical trocars have not been studied in comparable numbers. Although the Hasson cannula was not associated with MVI in two large trials involving 10,840 and 5,284 procedures, respectively, there have been case reports to the contrary.11-14
Rubinstein and colleagues15 evaluated the efficacy and safety of the radial dilating trocar VersaStep (US Surgical) for laparoscopic access. This trocar system has a blunt tip and circumferentially dilates rather than directly incising the fascia of the abdominal wall. Use of the blunt-tip trocar may decrease the incidence of initial access injury.
3. Don’t be the King or Queen of denial
Because MVI is estimated to occur in one in every 1,000 to one in every 10,000 procedures, and a typical gynecologic surgeon performs roughly 12 laparoscopies a year, it is unlikely that he or she will encounter more than one MVI over the course of a career. Nevertheless, when a retroperitoneal hematoma or brisk bleeding is visualized at laparoscopy, an MVI should immediately be suspected.
Do not enter into a state of denial and attempt to manage this complication conservatively. The earlier the MVI can be diagnosed, the better the outcome. A retroperitoneal hematoma, dark venous blood pooling in the abdomen, and bright red pulsatile blood are all signs of MVI. Do not wait for systemic changes such as hypotension or cardiac arrhythmias, as these are late findings.
4. No man is an island. Get help when you need it!
As residents, we had the shoulder-dystocia drill burned into memory, the first step being: “Call for help.” Regrettably, there is much less emphasis placed on the need to call for help in a surgical emergency.
All members of the surgical team should be notified of the situation and its associated gravity. The anesthesia team will need to ensure that there are adequate intravenous (IV) access, IV fluids, and blood products for resuscitation. The circulator will need to recruit help so that there will be additional hands to call consults, obtain blood, bring in abdominal trays, scrub in and retract, and so on.
5. Identify, secure, and control the site of injury
Just as a pilot must memorize the early response to an emergency, so must a surgeon be prepared for MVI. In the setting of potential catastrophic hemorrhage, time is of the essence.
The first task is to stay calm and avoid panic. Your role as the surgeon is to identify, secure, and control the site of injury while other team members work on their duties.
Laparoscopy usually not an option 2
It is the rare laparoscopic surgeon who can manage catastrophic hemorrhage endoscopically. It usually is best to remove the trocar from the vessel wall and convert to laparotomy. Attempting to leave a trocar in a vessel may extend the injury and create greater trauma to the vessel.
It is likely that patients will need to undergo secondary procedures if traditional laparoscopic interventions are used.
A vertical skin incision is best
The vertical incision is preferable because it allows greater access and visibility. Sadly, that information does not seem to translate into common practice. One study reported that 27 of 31 women with vascular injury were opened with a Pfannenstiel incision for emergency laparotomy.16
Control the bleeding
Apply pressure in an atraumatic fashion. Call for vascular instruments and a second suction device.
Control the bleeding digitally at first, followed by proximal and distal occlusion. Never use traumatic clamps because of the high rate of intimal damage associated with thrombi and secondary occlusion. Clips, staplers, or electrosurgery may cause vascular occlusion, thrombi, claudication, or lower-extremity edema.
Debride the blood and clots and identify the defect.
The frequency of MVI is thought to range from one in every 1,000 to one in every 10,000 gynecologic laparoscopic procedures.17-19 In a large review of 29,966 gynecologic laparoscopies, the risk of MVI was 0.02%.2
Polypropylene (6-0) can be used to close the defect in an interrupted fashion with sutures running parallel to the line of the vascular structure.
A vascular surgeon can be very helpful in the repair of vessels and should be consulted in the event of MVI. If extensive intimal damage has occurred, primary closure may not be possible and the vascular surgeon may perform a graft angioplasty repair or place a short interposition graft. Proximal and distal control is achieved by applying atraumatic clamps proximal and distal to the injury. Intravenous anticoagulation (5,000 U of heparin) is common but not mandatory. A longitudinal arteriotomy is extended, and any areas of thrombi are removed. The damaged intima is endarterectomized, and tacking sutures, using 7-0 Prolene, can be used to prevent propagation of the intimal dissection. Last, a venous or synthetic patch is sewn into place using 6-0 Prolene.
Patients typically remain on aspirin for 3 to 6 weeks.
CASE Resolved
Sophia’s procedure is immediately converted to a midline vertical laparotomy, a vascular surgery consult is obtained, and exploration reveals a 5-mm defect in the right common iliac artery consistent with an extended Veress needle injury. A vascular patch is used for the vessel repair, and the patient is discharged 4 days later in stable condition. She takes clopidogrel bisulfate (Plavix) for 6 weeks and has no lower-limb compromise.
1. Chapron C, Fauconnier A, Goffinet F, Breart G, Dubuisson JB. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Hum Reprod. 2002;17:1334-1342.
2. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynaecological laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
3. Muzii L, Bellati F, Zullo MA, Manci N, Angioli R, Panici PB. Mechanical bowel preparation before gynecologic laparoscopy: a randomized, single-blind, controlled trial. Fertil Steril. 2006;85:689-693.
4. Polyzos D, Papadopoulos N, Chapman L, et al. Where is the aorta? Is it worth palpating the aorta prior to laparoscopy? Acta Obstet Gynaecol Scand. 2007;86:235-239.
5. Philips PA, Amaral JF. Abdominal access complications in laparoscopic surgery. J Am Coll Surg. 2001;192:525-536.
6. Sriprasad S, Yu DF, Muir GH, Poulsen J, Sidhu PS. Positional anatomy of vessels that may be damaged at laparoscopy: new access criteria based on CT and ultrasonography to avoid vascular injury. J Endourol. 2006;20:498-503.
7. Milad MP, Terkildsen MF. The spinal needle test effectively measures the abdominal wall thickness prior to trocar placement at laparoscopy. J Am Assoc Gynecol Laparosc. 2002;9:514-518.
8. Reich H, Ribeiro SC, Rasmussen C, Rosenberg J, Vidali A. High-pressure trocar insertion technique. J Soc Laparoendosc Surg. 1999;3:45-48.
9. Hurd WH, Bude RO, DeLancey JO, Gauvin JM, Aisen AM. Abdominal wall characterization with magnetic resonance imaging and computed tomography. The effect of obesity on the laparoscopic approach. J Re-prod Med. 1991-36:473-476.
10. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette C, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
11. Hasson HM, Rotman C, Rana N, Kumari NA. Open laparoscopy: 29-year experience. Obstet Gynecol. 2000;96:763-766.
12. Penfield AJ. How to prevent complications of open laparoscopy. J Reprod Med. 1985;30:660-663.
13. Hanney RM, Carmalt HL, Merrett N, Tait N. Use of the Hasson cannula producing major vascular injury at laparoscopy. Surg Endosc. 1999;13:1238-1240.
14. Pring CM. Aortic injury using the Hasson trocar: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W3-5.
15. Rubenstein JN, Blunt LW, Jr, Lin WW, User HM, Nadler RB, Gonzalez CM. Safety and efficacy of 12-mm radial dilating ports for laparoscopic access. BJU Int. 2003;92:327-329.
16. Baggish MS. Analysis of 31 cases of major vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
17. Saville LE, Woods MS. Laparoscopy and major retroperitoneal vascular injuries (MRVI). Surg Endosc. 1995;9:1099-1101.
18. Ridel HH, Lehmann-Willenbrock E, Conrad P, Semm K. German pelviscopic statistics for the years 1978–1982. Endoscopy. 1986;18:219-222.
19. Harkki-Siren P, Sjoberg J, Kurki T. Major complications of laparoscopy: a follow-up Finnish study. Obstet Gynecol. 1999;94:94-98.
The author reports no financial relationships relevant to this article.
- Strategies that may help you avoid MVI
- Tips on how to recognize it
- What to do when MVI occurs
CASE Trocar insertion, then a bleed
Sophia, a 29-year-old nulliparous patient, undergoes diagnostic laparoscopy after unsuccessful medical therapy for chronic pelvic pain. After insufflation, a reusable 11-mm trocar with obturator is placed. A survey of the pelvis and abdomen reveals a 10-cm retroperitoneal hematoma in the midline just below the umbilicus.
What is the best way to manage this hemorrhage?
Contrary to widespread belief, gynecologic laparoscopy has a rate of major complication similar to, not higher than, that of laparotomy—but it has a higher rate of major vascular injury (MVI), defined as laceration of the aorta, inferior vena cava, or common iliac or external iliac vessels.1 In fact, MVI appears to be somewhat exclusive to laparoscopy.
In a large review of 29,966 gynecologic laparoscopies, investigators found the surgeon’s level of experience to be an important variable in the overall complication rate but not in the incidence of MVI.2 This means patients are at risk of MVI regardless of how many or how few laparoscopic procedures you perform.
What can a surgeon do to avert this type of injury? The most important strategy is to be conversant with variables that clearly increase the risk, as well as behaviors and protocols that can minimize it. This article offers five pearls—from prevention to postop management.
1. Pay attention to subtlety, starting at the preop visit
When a marriage counselor was asked which significant actions can improve a relationship, he responded: “The little things are the big things.”
To minimize the risk of vascular injury, a meticulous surgeon begins with the “little things” at the preoperative evaluation. Important considerations include the patient’s height, weight, and body mass index (BMI). Does she have a history of abdominal surgery or pelvic disease that could suggest intra-abdominal adhesions and anatomic distortion?
It is important to discuss the risk of bowel or vascular injury, as well as the need to convert to an open procedure, with all patients, but particularly with those who are thin or who have abnormal abdominal findings.
A recent randomized clinical trial found that routine mechanical bowel preparation did not facilitate surgery or decrease the incidence of complications, but may be helpful in select cases.3
2. Don’t undervalue that ounce of prevention
A commuter commented to her friend that she thought the bus driver had exceptional driving skill. The friend couldn’t understand this remark. “The driver hasn’t been challenged yet,” the friend argued. “She hasn’t needed to demonstrate any action in the face of an emergency—so how do you know what level of skill she has?”
“That is precisely the point,” the commuter replied. “The driver has exercised extreme judgment and caution so as not to require using her superior skill in an emergency.”
It is always better to avoid a potentially catastrophic complication than to manage it. The first requirement is vigilance in preparing for laparoscopy. It is important to ensure that the patient is in the supine position during initial entry and that her arms are comfortably tucked on both sides. In 85% of cases, the aorta can be palpated.4
Select an entry technique wisely
Base this decision on experience, patient characteristics, the surgical procedure, and availability of equipment. No entry technique or device can be considered completely safe.
Most vascular injuries occur during the initial phase of laparoscopy:
- As many as 39% are related to placement of the Veress needle
- As many as 37.9% are related to placement of the primary trocar.5
The classic “closed” method of laparoscopy has been used successfully for decades. In thin patients, insert the Veress needle toward the uterus. In computed tomography studies, an angle of at least 34 degrees has been shown to avoid the aorta and its bifurcation (FIGURE 1).6
The right common iliac vessel is most commonly involved in injury, given its anatomic relationship overriding the inferior vena cava (FIGURE 2). When you stand at the patient’s left, the Veress needle can inadvertently be directed off to the right of midline, in harm’s way of the right common iliac vessels.
FIGURE 1 In thin women, insert the Veress needle toward the uterus
If the angle of insertion is 45° (≥34°) in the sagittal plane at the umbilicus, puncture safely avoids the aortic bifurcation.
FIGURE 2 At risk: The right common iliac artery
The right common iliac artery overlies its corresponding vein, which puts the artery at risk of injury at initial insertion of the Veress needle.
Tips to facilitate entry
After insufflation, the distance from the base of the navel to the peritoneum is approximately 1.5 to 2.0 cm (FIGURE 3).7 It is neither necessary nor prudent to bury the needle to its hub when placing the initial trocar. Some authors report that over-inflation to 25 mm Hg lowers the risk of vascular injury, creating a longer distance to the retroperitoneal structures.8 In addition, the reduction of tenting means that less force is required.
Patients with a high BMI are likely to be protected from MVI, given the greater distances to major vessels.9 The distance between the entry trocar and retroperitoneal vessels increases directly with BMI, commensurate with increasing abdominal-wall thickness (FIGURE 4).
FIGURE 3 The umbilicus is at its thinnest at low body weight
The distance (cm) into the peritoneal cavity after insufflation correlates with a patient’s weight.
SOURCE: Milad and Terkildsen.7
FIGURE 4 How far is it from umbilical skin to the peritoneum and vascular structures?
SOURCE: Hurd et al.9
Even optical trocars have been associated with MVI. Between 1994 and 2002, 79 serious complications, including 37 MVIs, were associated with use of these trocars.10
Direct insertion, open technique, and optical trocars have not been studied in comparable numbers. Although the Hasson cannula was not associated with MVI in two large trials involving 10,840 and 5,284 procedures, respectively, there have been case reports to the contrary.11-14
Rubinstein and colleagues15 evaluated the efficacy and safety of the radial dilating trocar VersaStep (US Surgical) for laparoscopic access. This trocar system has a blunt tip and circumferentially dilates rather than directly incising the fascia of the abdominal wall. Use of the blunt-tip trocar may decrease the incidence of initial access injury.
3. Don’t be the King or Queen of denial
Because MVI is estimated to occur in one in every 1,000 to one in every 10,000 procedures, and a typical gynecologic surgeon performs roughly 12 laparoscopies a year, it is unlikely that he or she will encounter more than one MVI over the course of a career. Nevertheless, when a retroperitoneal hematoma or brisk bleeding is visualized at laparoscopy, an MVI should immediately be suspected.
Do not enter into a state of denial and attempt to manage this complication conservatively. The earlier the MVI can be diagnosed, the better the outcome. A retroperitoneal hematoma, dark venous blood pooling in the abdomen, and bright red pulsatile blood are all signs of MVI. Do not wait for systemic changes such as hypotension or cardiac arrhythmias, as these are late findings.
4. No man is an island. Get help when you need it!
As residents, we had the shoulder-dystocia drill burned into memory, the first step being: “Call for help.” Regrettably, there is much less emphasis placed on the need to call for help in a surgical emergency.
All members of the surgical team should be notified of the situation and its associated gravity. The anesthesia team will need to ensure that there are adequate intravenous (IV) access, IV fluids, and blood products for resuscitation. The circulator will need to recruit help so that there will be additional hands to call consults, obtain blood, bring in abdominal trays, scrub in and retract, and so on.
5. Identify, secure, and control the site of injury
Just as a pilot must memorize the early response to an emergency, so must a surgeon be prepared for MVI. In the setting of potential catastrophic hemorrhage, time is of the essence.
The first task is to stay calm and avoid panic. Your role as the surgeon is to identify, secure, and control the site of injury while other team members work on their duties.
Laparoscopy usually not an option 2
It is the rare laparoscopic surgeon who can manage catastrophic hemorrhage endoscopically. It usually is best to remove the trocar from the vessel wall and convert to laparotomy. Attempting to leave a trocar in a vessel may extend the injury and create greater trauma to the vessel.
It is likely that patients will need to undergo secondary procedures if traditional laparoscopic interventions are used.
A vertical skin incision is best
The vertical incision is preferable because it allows greater access and visibility. Sadly, that information does not seem to translate into common practice. One study reported that 27 of 31 women with vascular injury were opened with a Pfannenstiel incision for emergency laparotomy.16
Control the bleeding
Apply pressure in an atraumatic fashion. Call for vascular instruments and a second suction device.
Control the bleeding digitally at first, followed by proximal and distal occlusion. Never use traumatic clamps because of the high rate of intimal damage associated with thrombi and secondary occlusion. Clips, staplers, or electrosurgery may cause vascular occlusion, thrombi, claudication, or lower-extremity edema.
Debride the blood and clots and identify the defect.
The frequency of MVI is thought to range from one in every 1,000 to one in every 10,000 gynecologic laparoscopic procedures.17-19 In a large review of 29,966 gynecologic laparoscopies, the risk of MVI was 0.02%.2
Polypropylene (6-0) can be used to close the defect in an interrupted fashion with sutures running parallel to the line of the vascular structure.
A vascular surgeon can be very helpful in the repair of vessels and should be consulted in the event of MVI. If extensive intimal damage has occurred, primary closure may not be possible and the vascular surgeon may perform a graft angioplasty repair or place a short interposition graft. Proximal and distal control is achieved by applying atraumatic clamps proximal and distal to the injury. Intravenous anticoagulation (5,000 U of heparin) is common but not mandatory. A longitudinal arteriotomy is extended, and any areas of thrombi are removed. The damaged intima is endarterectomized, and tacking sutures, using 7-0 Prolene, can be used to prevent propagation of the intimal dissection. Last, a venous or synthetic patch is sewn into place using 6-0 Prolene.
Patients typically remain on aspirin for 3 to 6 weeks.
CASE Resolved
Sophia’s procedure is immediately converted to a midline vertical laparotomy, a vascular surgery consult is obtained, and exploration reveals a 5-mm defect in the right common iliac artery consistent with an extended Veress needle injury. A vascular patch is used for the vessel repair, and the patient is discharged 4 days later in stable condition. She takes clopidogrel bisulfate (Plavix) for 6 weeks and has no lower-limb compromise.
The author reports no financial relationships relevant to this article.
- Strategies that may help you avoid MVI
- Tips on how to recognize it
- What to do when MVI occurs
CASE Trocar insertion, then a bleed
Sophia, a 29-year-old nulliparous patient, undergoes diagnostic laparoscopy after unsuccessful medical therapy for chronic pelvic pain. After insufflation, a reusable 11-mm trocar with obturator is placed. A survey of the pelvis and abdomen reveals a 10-cm retroperitoneal hematoma in the midline just below the umbilicus.
What is the best way to manage this hemorrhage?
Contrary to widespread belief, gynecologic laparoscopy has a rate of major complication similar to, not higher than, that of laparotomy—but it has a higher rate of major vascular injury (MVI), defined as laceration of the aorta, inferior vena cava, or common iliac or external iliac vessels.1 In fact, MVI appears to be somewhat exclusive to laparoscopy.
In a large review of 29,966 gynecologic laparoscopies, investigators found the surgeon’s level of experience to be an important variable in the overall complication rate but not in the incidence of MVI.2 This means patients are at risk of MVI regardless of how many or how few laparoscopic procedures you perform.
What can a surgeon do to avert this type of injury? The most important strategy is to be conversant with variables that clearly increase the risk, as well as behaviors and protocols that can minimize it. This article offers five pearls—from prevention to postop management.
1. Pay attention to subtlety, starting at the preop visit
When a marriage counselor was asked which significant actions can improve a relationship, he responded: “The little things are the big things.”
To minimize the risk of vascular injury, a meticulous surgeon begins with the “little things” at the preoperative evaluation. Important considerations include the patient’s height, weight, and body mass index (BMI). Does she have a history of abdominal surgery or pelvic disease that could suggest intra-abdominal adhesions and anatomic distortion?
It is important to discuss the risk of bowel or vascular injury, as well as the need to convert to an open procedure, with all patients, but particularly with those who are thin or who have abnormal abdominal findings.
A recent randomized clinical trial found that routine mechanical bowel preparation did not facilitate surgery or decrease the incidence of complications, but may be helpful in select cases.3
2. Don’t undervalue that ounce of prevention
A commuter commented to her friend that she thought the bus driver had exceptional driving skill. The friend couldn’t understand this remark. “The driver hasn’t been challenged yet,” the friend argued. “She hasn’t needed to demonstrate any action in the face of an emergency—so how do you know what level of skill she has?”
“That is precisely the point,” the commuter replied. “The driver has exercised extreme judgment and caution so as not to require using her superior skill in an emergency.”
It is always better to avoid a potentially catastrophic complication than to manage it. The first requirement is vigilance in preparing for laparoscopy. It is important to ensure that the patient is in the supine position during initial entry and that her arms are comfortably tucked on both sides. In 85% of cases, the aorta can be palpated.4
Select an entry technique wisely
Base this decision on experience, patient characteristics, the surgical procedure, and availability of equipment. No entry technique or device can be considered completely safe.
Most vascular injuries occur during the initial phase of laparoscopy:
- As many as 39% are related to placement of the Veress needle
- As many as 37.9% are related to placement of the primary trocar.5
The classic “closed” method of laparoscopy has been used successfully for decades. In thin patients, insert the Veress needle toward the uterus. In computed tomography studies, an angle of at least 34 degrees has been shown to avoid the aorta and its bifurcation (FIGURE 1).6
The right common iliac vessel is most commonly involved in injury, given its anatomic relationship overriding the inferior vena cava (FIGURE 2). When you stand at the patient’s left, the Veress needle can inadvertently be directed off to the right of midline, in harm’s way of the right common iliac vessels.
FIGURE 1 In thin women, insert the Veress needle toward the uterus
If the angle of insertion is 45° (≥34°) in the sagittal plane at the umbilicus, puncture safely avoids the aortic bifurcation.
FIGURE 2 At risk: The right common iliac artery
The right common iliac artery overlies its corresponding vein, which puts the artery at risk of injury at initial insertion of the Veress needle.
Tips to facilitate entry
After insufflation, the distance from the base of the navel to the peritoneum is approximately 1.5 to 2.0 cm (FIGURE 3).7 It is neither necessary nor prudent to bury the needle to its hub when placing the initial trocar. Some authors report that over-inflation to 25 mm Hg lowers the risk of vascular injury, creating a longer distance to the retroperitoneal structures.8 In addition, the reduction of tenting means that less force is required.
Patients with a high BMI are likely to be protected from MVI, given the greater distances to major vessels.9 The distance between the entry trocar and retroperitoneal vessels increases directly with BMI, commensurate with increasing abdominal-wall thickness (FIGURE 4).
FIGURE 3 The umbilicus is at its thinnest at low body weight
The distance (cm) into the peritoneal cavity after insufflation correlates with a patient’s weight.
SOURCE: Milad and Terkildsen.7
FIGURE 4 How far is it from umbilical skin to the peritoneum and vascular structures?
SOURCE: Hurd et al.9
Even optical trocars have been associated with MVI. Between 1994 and 2002, 79 serious complications, including 37 MVIs, were associated with use of these trocars.10
Direct insertion, open technique, and optical trocars have not been studied in comparable numbers. Although the Hasson cannula was not associated with MVI in two large trials involving 10,840 and 5,284 procedures, respectively, there have been case reports to the contrary.11-14
Rubinstein and colleagues15 evaluated the efficacy and safety of the radial dilating trocar VersaStep (US Surgical) for laparoscopic access. This trocar system has a blunt tip and circumferentially dilates rather than directly incising the fascia of the abdominal wall. Use of the blunt-tip trocar may decrease the incidence of initial access injury.
3. Don’t be the King or Queen of denial
Because MVI is estimated to occur in one in every 1,000 to one in every 10,000 procedures, and a typical gynecologic surgeon performs roughly 12 laparoscopies a year, it is unlikely that he or she will encounter more than one MVI over the course of a career. Nevertheless, when a retroperitoneal hematoma or brisk bleeding is visualized at laparoscopy, an MVI should immediately be suspected.
Do not enter into a state of denial and attempt to manage this complication conservatively. The earlier the MVI can be diagnosed, the better the outcome. A retroperitoneal hematoma, dark venous blood pooling in the abdomen, and bright red pulsatile blood are all signs of MVI. Do not wait for systemic changes such as hypotension or cardiac arrhythmias, as these are late findings.
4. No man is an island. Get help when you need it!
As residents, we had the shoulder-dystocia drill burned into memory, the first step being: “Call for help.” Regrettably, there is much less emphasis placed on the need to call for help in a surgical emergency.
All members of the surgical team should be notified of the situation and its associated gravity. The anesthesia team will need to ensure that there are adequate intravenous (IV) access, IV fluids, and blood products for resuscitation. The circulator will need to recruit help so that there will be additional hands to call consults, obtain blood, bring in abdominal trays, scrub in and retract, and so on.
5. Identify, secure, and control the site of injury
Just as a pilot must memorize the early response to an emergency, so must a surgeon be prepared for MVI. In the setting of potential catastrophic hemorrhage, time is of the essence.
The first task is to stay calm and avoid panic. Your role as the surgeon is to identify, secure, and control the site of injury while other team members work on their duties.
Laparoscopy usually not an option 2
It is the rare laparoscopic surgeon who can manage catastrophic hemorrhage endoscopically. It usually is best to remove the trocar from the vessel wall and convert to laparotomy. Attempting to leave a trocar in a vessel may extend the injury and create greater trauma to the vessel.
It is likely that patients will need to undergo secondary procedures if traditional laparoscopic interventions are used.
A vertical skin incision is best
The vertical incision is preferable because it allows greater access and visibility. Sadly, that information does not seem to translate into common practice. One study reported that 27 of 31 women with vascular injury were opened with a Pfannenstiel incision for emergency laparotomy.16
Control the bleeding
Apply pressure in an atraumatic fashion. Call for vascular instruments and a second suction device.
Control the bleeding digitally at first, followed by proximal and distal occlusion. Never use traumatic clamps because of the high rate of intimal damage associated with thrombi and secondary occlusion. Clips, staplers, or electrosurgery may cause vascular occlusion, thrombi, claudication, or lower-extremity edema.
Debride the blood and clots and identify the defect.
The frequency of MVI is thought to range from one in every 1,000 to one in every 10,000 gynecologic laparoscopic procedures.17-19 In a large review of 29,966 gynecologic laparoscopies, the risk of MVI was 0.02%.2
Polypropylene (6-0) can be used to close the defect in an interrupted fashion with sutures running parallel to the line of the vascular structure.
A vascular surgeon can be very helpful in the repair of vessels and should be consulted in the event of MVI. If extensive intimal damage has occurred, primary closure may not be possible and the vascular surgeon may perform a graft angioplasty repair or place a short interposition graft. Proximal and distal control is achieved by applying atraumatic clamps proximal and distal to the injury. Intravenous anticoagulation (5,000 U of heparin) is common but not mandatory. A longitudinal arteriotomy is extended, and any areas of thrombi are removed. The damaged intima is endarterectomized, and tacking sutures, using 7-0 Prolene, can be used to prevent propagation of the intimal dissection. Last, a venous or synthetic patch is sewn into place using 6-0 Prolene.
Patients typically remain on aspirin for 3 to 6 weeks.
CASE Resolved
Sophia’s procedure is immediately converted to a midline vertical laparotomy, a vascular surgery consult is obtained, and exploration reveals a 5-mm defect in the right common iliac artery consistent with an extended Veress needle injury. A vascular patch is used for the vessel repair, and the patient is discharged 4 days later in stable condition. She takes clopidogrel bisulfate (Plavix) for 6 weeks and has no lower-limb compromise.
1. Chapron C, Fauconnier A, Goffinet F, Breart G, Dubuisson JB. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Hum Reprod. 2002;17:1334-1342.
2. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynaecological laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
3. Muzii L, Bellati F, Zullo MA, Manci N, Angioli R, Panici PB. Mechanical bowel preparation before gynecologic laparoscopy: a randomized, single-blind, controlled trial. Fertil Steril. 2006;85:689-693.
4. Polyzos D, Papadopoulos N, Chapman L, et al. Where is the aorta? Is it worth palpating the aorta prior to laparoscopy? Acta Obstet Gynaecol Scand. 2007;86:235-239.
5. Philips PA, Amaral JF. Abdominal access complications in laparoscopic surgery. J Am Coll Surg. 2001;192:525-536.
6. Sriprasad S, Yu DF, Muir GH, Poulsen J, Sidhu PS. Positional anatomy of vessels that may be damaged at laparoscopy: new access criteria based on CT and ultrasonography to avoid vascular injury. J Endourol. 2006;20:498-503.
7. Milad MP, Terkildsen MF. The spinal needle test effectively measures the abdominal wall thickness prior to trocar placement at laparoscopy. J Am Assoc Gynecol Laparosc. 2002;9:514-518.
8. Reich H, Ribeiro SC, Rasmussen C, Rosenberg J, Vidali A. High-pressure trocar insertion technique. J Soc Laparoendosc Surg. 1999;3:45-48.
9. Hurd WH, Bude RO, DeLancey JO, Gauvin JM, Aisen AM. Abdominal wall characterization with magnetic resonance imaging and computed tomography. The effect of obesity on the laparoscopic approach. J Re-prod Med. 1991-36:473-476.
10. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette C, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
11. Hasson HM, Rotman C, Rana N, Kumari NA. Open laparoscopy: 29-year experience. Obstet Gynecol. 2000;96:763-766.
12. Penfield AJ. How to prevent complications of open laparoscopy. J Reprod Med. 1985;30:660-663.
13. Hanney RM, Carmalt HL, Merrett N, Tait N. Use of the Hasson cannula producing major vascular injury at laparoscopy. Surg Endosc. 1999;13:1238-1240.
14. Pring CM. Aortic injury using the Hasson trocar: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W3-5.
15. Rubenstein JN, Blunt LW, Jr, Lin WW, User HM, Nadler RB, Gonzalez CM. Safety and efficacy of 12-mm radial dilating ports for laparoscopic access. BJU Int. 2003;92:327-329.
16. Baggish MS. Analysis of 31 cases of major vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
17. Saville LE, Woods MS. Laparoscopy and major retroperitoneal vascular injuries (MRVI). Surg Endosc. 1995;9:1099-1101.
18. Ridel HH, Lehmann-Willenbrock E, Conrad P, Semm K. German pelviscopic statistics for the years 1978–1982. Endoscopy. 1986;18:219-222.
19. Harkki-Siren P, Sjoberg J, Kurki T. Major complications of laparoscopy: a follow-up Finnish study. Obstet Gynecol. 1999;94:94-98.
1. Chapron C, Fauconnier A, Goffinet F, Breart G, Dubuisson JB. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Hum Reprod. 2002;17:1334-1342.
2. Chapron C, Querleu D, Bruhat MA, et al. Surgical complications of diagnostic and operative gynaecological laparoscopy: a series of 29,966 cases. Hum Reprod. 1998;13:867-872.
3. Muzii L, Bellati F, Zullo MA, Manci N, Angioli R, Panici PB. Mechanical bowel preparation before gynecologic laparoscopy: a randomized, single-blind, controlled trial. Fertil Steril. 2006;85:689-693.
4. Polyzos D, Papadopoulos N, Chapman L, et al. Where is the aorta? Is it worth palpating the aorta prior to laparoscopy? Acta Obstet Gynaecol Scand. 2007;86:235-239.
5. Philips PA, Amaral JF. Abdominal access complications in laparoscopic surgery. J Am Coll Surg. 2001;192:525-536.
6. Sriprasad S, Yu DF, Muir GH, Poulsen J, Sidhu PS. Positional anatomy of vessels that may be damaged at laparoscopy: new access criteria based on CT and ultrasonography to avoid vascular injury. J Endourol. 2006;20:498-503.
7. Milad MP, Terkildsen MF. The spinal needle test effectively measures the abdominal wall thickness prior to trocar placement at laparoscopy. J Am Assoc Gynecol Laparosc. 2002;9:514-518.
8. Reich H, Ribeiro SC, Rasmussen C, Rosenberg J, Vidali A. High-pressure trocar insertion technique. J Soc Laparoendosc Surg. 1999;3:45-48.
9. Hurd WH, Bude RO, DeLancey JO, Gauvin JM, Aisen AM. Abdominal wall characterization with magnetic resonance imaging and computed tomography. The effect of obesity on the laparoscopic approach. J Re-prod Med. 1991-36:473-476.
10. Sharp HT, Dodson MK, Draper ML, Watts DA, Doucette C, Hurd WW. Complications associated with optical-access laparoscopic trocars. Obstet Gynecol. 2002;99:553-555.
11. Hasson HM, Rotman C, Rana N, Kumari NA. Open laparoscopy: 29-year experience. Obstet Gynecol. 2000;96:763-766.
12. Penfield AJ. How to prevent complications of open laparoscopy. J Reprod Med. 1985;30:660-663.
13. Hanney RM, Carmalt HL, Merrett N, Tait N. Use of the Hasson cannula producing major vascular injury at laparoscopy. Surg Endosc. 1999;13:1238-1240.
14. Pring CM. Aortic injury using the Hasson trocar: a case report and review of the literature. Ann R Coll Surg Engl. 2007;89:W3-5.
15. Rubenstein JN, Blunt LW, Jr, Lin WW, User HM, Nadler RB, Gonzalez CM. Safety and efficacy of 12-mm radial dilating ports for laparoscopic access. BJU Int. 2003;92:327-329.
16. Baggish MS. Analysis of 31 cases of major vessel injury associated with gynecologic laparoscopy operations. J Gynecol Surg. 2003;19:63-73.
17. Saville LE, Woods MS. Laparoscopy and major retroperitoneal vascular injuries (MRVI). Surg Endosc. 1995;9:1099-1101.
18. Ridel HH, Lehmann-Willenbrock E, Conrad P, Semm K. German pelviscopic statistics for the years 1978–1982. Endoscopy. 1986;18:219-222.
19. Harkki-Siren P, Sjoberg J, Kurki T. Major complications of laparoscopy: a follow-up Finnish study. Obstet Gynecol. 1999;94:94-98.