User login
Vaginal hysterectomy: 6 challenges, an arsenal of solutions
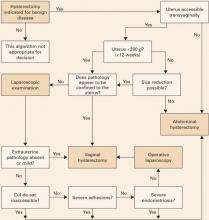
Newer codes for vaginal hysterectomy capture the work of removing larger uteri without laparoscopy
True or false: When it comes to hysterectomy, surgeons tend to use the route that is safest, least invasive, and most economical.
Sadly, the statement is false. Although vaginal hysterectomy tops all 3 categories, it is the least utilized of surgical routes. The number of vaginal hysterectomies may have increased slightly over the past decade, likely due to the incorporation of laparoscopically assisted vaginal hysterectomy into the mainstream and increased practice with the vaginal component, but fewer than 30% of hysterectomies are performed vaginally.
This article addresses 6 common challenges at vaginal hysterectomy and offers strategies to overcome them.
Laparoscopic strategies ease vaginal hysterectomy, too
Laparoscopic hysterectomy became widely accepted when surgical instruments were developed to overcome the technical challenges inherent in operating with limited access. By incorporating some of the techniques we routinely use for laparoscopic surgery, we can overcome many of the challenges faced during difficult vaginal surgery.
VAGINAL HYSTERECTOMY CHALLENGE 1: Obesity
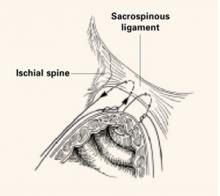
Unfortunately, our population is increasingly rotund. This is not only a significant risk factor for the patient’s health in general, but it poses some unique challenges for surgeons. I must say that, as tough as it may be to complete a hysterectomy vaginally in a morbidly obese woman, I would much rather approach her pelvic organs through the cul-de-sac, which contains no fat cells, than through the abdominal wall—either laparoscopically or abdominally! The trick is gaining access to the posterior cul-de-sac.
How to enter the cul-de-sac
It seems to be a perverse rule of nature, but a tight upper vaginal ring seems almost universal in obese women. Added to the redundant sidewalls and the large buttocks, this tightness makes entry into the anterior or posterior cul-de-sac problematic. Several tricks make peritoneal access possible:
Position the patient to increase access, with the buttocks well over the edge of the operating table. This brings the operative field a bit closer to the surgeon, and permits the use of long-handled retractors posteriorly.
Use candy-cane stirrups to allow assistants better access to the operative field. Adequate assistance is essential in attempting vaginal surgery in the morbidly obese.
Avoid the Trendelenburg position. Although it might seem that this position would facilitate visualization and placement of a posterior weighted speculum, all it does is allow the patient to slide up on the table, making placement of alternate retractors difficult.
Use the right tools. If the posterior weighted speculum will not stay in place or does not afford access to the cul-de-sac due to an upper vaginal ring, use a narrow Deaver retractor posteriorly (without sidewall or anterior retraction). Use a Jacob’s tenaculum on the posterior lip of the cervix and have your assistant pull straight up on the tenaculum while using the Deaver retractor to see the area between the uterosacral ligaments.
Use the uterosacral ligaments as a guide. Another perversity in morbidly obese women: Despite multiparity, they seem to have little or no apical prolapse but lots of vaginal wall redundancy. The cervix is often elongated, but the uterosacral ligaments are sky high.
I palpate these ligaments, injecting them with a combination of vasopressin diluted 1:5 with bupivacaine and epinephrine (for enhanced hemostasis and preemptive analgesia), then use a pencil electrosurgical electrode to rapidly open the vaginal epithelium between the ligaments.
I then use a long, toothed tissue forceps to tent the peritoneum at 90 degrees to the plane of the posterior cul-de-sac and use Mayo scissors to enter the peritoneal cavity. Usually there is a spurt of fluid to mark appropriate entry into the peritoneum.
I then use the blades of my scissors to stretch the peritoneum between the ligaments and place a moistened 4×4 sponge into the incision.
At the onset of the procedure, inject indigo carmine dye intravenously so that any injury to the bladder will be immediately recognized. I have the circulating nurse empty the bladder while she is prepping the patient, but do not leave an indwelling catheter in place during the operation. I find it cumbersome to work around the catheter.
Problematic entries
When entry into the posterior cul-de-sac is difficult, I stop dissection, place a 4×4 sponge into the incision to reduce bleeding from the vagina, and proceed to attempt anterior entry.
I place the Deaver retractor into the anterior space and move the tenaculum to the anterior lip of the cervix. This gives maximal space for downward traction on the cervix while anterior entry is attempted.
Once again, I inject the tissue with the bupivacaine solution before incising the vaginal epithelium at the level of the internal os. I use sharp dissection only when creating a plane between the lower uterine segment and the bladder.
Ensuring room to move and good visualization
If neither the anterior nor the posterior cul-de-sac can be accessed, it may be time to rethink the vaginal approach—but there is no harm in taking the uterosacral and cardinal ligaments extraperitoneally in an effort to gain some mobility. Hugging the uterus and leaving the anterior retractor in place to lift the bladder superiorly are essential steps to protect the ureters. The cervix can then be split in the midline (12 to 6 o’clock) to easily identify the peritoneal reflections.
Other tips
Sidewall retractors are rarely needed. They significantly impair placement of clamps and sutures by creating a long, narrow, parallel passageway. An alternative trick is to use the suction tip to retract the vaginal sidewall as the surgeon is working.
A disposable, fiberoptic, lighted suction irrigator is another option. The light can be directed precisely where it is needed, and the irrigation helps keep the field clean and tidy, simplifying identification of anatomy.
Skip the sutures whenever possible. Because it is difficult to place sutures with precision in a tight, poorly illuminated space, I use a vessel sealer for all pedicles above the uterosacral ligaments. Some of these instruments were designed specifically for vaginal hysterectomy in the same shape and size as Heaney clamps. They are remarkably efficient and permit the completion of a vaginal procedure when suture placement is difficult.
Use a Heaney needleholder, with the suture loaded precisely in the center of the needle curve, along with the lighted suction irrigator to retract redundant tissue away from the track of the needle, to facilitate suturing high in the pelvis.
VAGINAL HYSTERECTOMY CHALLENGE 2: Nulliparity
Many of us are reluctant to attempt vaginal hysterectomy in a woman who has never had children. Although this situation can be challenging at times, in my experience, the access issues tend to be more difficult in obese, multiparous women.
The same tricks and techniques addressed above will permit the vaginal approach in almost all nulliparous women.
VAGINAL HYSTERECTOMY CHALLENGE 3: Previous cesarean section
A prior cesarean delivery is sometimes considered an indication for laparoscopic hysterectomy. There are 2 concerns here: The patient may have a small pelvis, and there may be significant scar tissue and difficulty gaining access to the anterior cul-de-sac, as a result of repeated dissection between the lower uterine segment and the bladder. Several tricks may be useful:
Examine the patient under anesthesia to ensure that the fundus is not stuck to the anterior abdominal wall. This can occur if the peritoneum was not closed during the last cesarean section.
Empty the bladder before beginning the hysterectomy, and inject indigo carmine dye intravenously with the induction of anesthesia.
Use careful sharp dissection between the bladder and lower uterine segment using fine Metzenbaum scissors, with the tips pointed toward the uterus. Dissect only as far as you can easily see.
Secure the uterosacral, cardinal, and broad ligaments, if necessary, before pursuing entry into the anterior cul-de-sac. It is not essential to gain anterior access before taking these pedicles. The additional mobility and descensus enable safe sharp dissection.
If pedicles have been secured up to the fundus, and the anterior cul-de-sac remains difficult to assess, flip the fundus through the posterior cul-de-sac and reach your finger or an instrument around the top of the fundus to identify the peritoneum anteriorly, then incise it under direct vision.
VAGINAL HYSTERECTOMY CHALLENGE 4: Previous abdominal/pelvic surgery
This history is another often-cited rationale for avoiding the vaginal approach. In reality, the adhesions created from prior surgery tend to arise between the anterior abdominal wall and the omentum or small bowel. This situation makes laparoscopic or open abdominal entry riskier than vaginal peritoneal access through the cul-de-sac.
Two possible exceptions: a patient who has had surgery for deeply infiltrating endometriosis in the cul-de-sac or a woman who has undergone myomectomies with posterior incisions. These patients may have dense scarring in the cul-de-sac, which would preclude a vaginal approach. Whatever the surgical route, appropriate bowel preparation is necessary to permit simple closure of any intestinal injury at the time of hysterectomy. Why not begin vaginally if the exam under anesthesia demonstrates an accessible and reasonably free cul-de-sac?
VAGINAL HYSTERECTOMY CHALLENGE 5: Large myomatous uterus
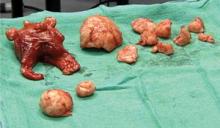
A uterus of any size can be removed vaginally as long as there is mobility with access to the uterine arteries. The one exception: a patient with a small, normal uterus and a massive pedunculated myoma arising from the top of the fundus. If the fibroid cannot be pulled into the true pelvis for morcellation, it cannot be removed transvaginally. Fortunately, this situation is quite rare.
Keep the uterine serosa intact to maintain orientation.
Split the uterus from 12 to 6 o’clock (bivalving). Protect the bladder with a Deaver retractor anteriorly, and protect the posterior vaginal epithelium with the weighted speculum posteriorly.
Remove chunks of tissue from the inside of the specimen. Orient your scalpel blade so that you are always cutting toward the center of the specimen. That way, if the blade slips, you will not accidentally injure tissue on the pelvic sidewall.
Use Lahey thyroid clamps to place the tissue you plan to remove under tension. Finding the capsule of each myoma and gently separating it from the surrounding myometrium facilitates delivery of larger fibroids into the endometrial cavity. Some myomas require morcellation themselves for removal.
Replace the scalpel blade periodically to keep it sharp. Calcified fibroids can dull the blade rapidly.
Work systematically to remove as much central tissue as possible. Try to keep a clean, sharp margin of tissue around the edges for easy grasping. Torn, irregular tissue is very difficult to grab and may cause significant frustration.
If access becomes limited, try clamping additional pedicles on each side of the specimen. A tiny amount of additional descensus can make a huge difference.
Do not administer GnRH agonists prior to surgery. The uterus may shrink, but the myomas tend to become quite soft and difficult to remove. If the patient is seriously anemic, give norethindrone acetate, 5 to 20 mg daily, to stop bleeding and allow the patient’s red blood cell volume to improve before elective surgery.
Use a vessel-sealing instrument to control the pedicles. This strategy produces optimal hemostasis to permit a dry field during morcellation. Moreover, the seals do not get disrupted when the large uterus is pulled past them. Placing suture around pedicles when there is a large, bulky uterus in the pelvis is challenging at best, and it is frustrating to see significant bleeding after removal of the specimen. This problem does not seem to occur with the sealing devices.
Know when to quit! We should not promise any patient a minimally invasive operation. If there is uncontrolled bleeding or no progress after 5 to 10 minutes, convert to a laparoscopic or abdominal approach.
I schedule cases I know will be challenging as “possible” laparoscopic or open hysterectomy. This alerts the OR staff to have additional equipment ready and nearby should we need it. It is not a surgical failure or complication to convert a minimally invasive hysterectomy to a more invasive technique when appropriate. Better to have tried and failed than never to have tried at all!
VAGINAL HYSTERECTOMY CHALLENGE 6: Avoiding complications
The most common complications of vaginal hysterectomy are bleeding, infection, and injury to the bladder. Ureteral injury is less common at vaginal hysterectomy than with the abdominal or laparoscopic approaches. Thus, I do not think routine cystoscopy is essential after uncomplicated vaginal hysterectomy, although I recommend intravenous administration of indigo carmine dye at the beginning of the procedure to enable rapid recognition of even a small bladder laceration. Sharp, careful dissection of the bladder off the lower uterine segment and the avoidance of finger dissection (especially with a gauze sponge) keep these injuries to a minimum.
Minimize bleeding by using newer vessel-sealing technologies rather than suture for most of the pedicles. I attach the uterosacral–cardinal ligament pedicles to the vaginal cuff at closure with suture. I suture the first pedicle once I have entered the posterior cul-de-sac and hold that suture to stay oriented.
Pay attention to patient positioning. Careful positioning will help you avoid neurological injuries. Avoid hyperflexion at the hips, which stretches the femoral nerve. Large nerves have comparatively little blood supply, so stretching them for prolonged periods can cause hypoxic injury. Although such injuries are almost always rapidly reversible, they are disconcerting for both the patient and her surgeon.
When operating on a very thin woman with a bony sacrum, I like to place egg-crate foam beneath the buttocks to provide some cushioning. I am also very careful with these women to keep their legs in a neutral position, and I watch my surgical assistants to be sure they are not leaning on the patient during the procedure.
Prophylactic antibiotics are a must to avoid postoperative vaginal cuff infections and pelvic abscess. Smokers and women with preexisting bacterial vaginosis are at highest risk for infection. I ask women to discontinue smoking at least 2 weeks prior to surgery and inform all smokers that their risk of infection is heightened. I treat anaerobic overgrowth in the vagina prior to surgery to help prevent infections in women with bacterial vaginosis.
The timing of prophylactic antibiotics is important. Intravenous first-generation cephalosporins must be administered within 60 minutes of the initial incision, but it is important to give them early enough for them to adequately disseminate to tissue before the colpotomy incision.
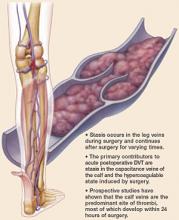
DVT prophylaxis is especially important for women with large uteri. Routine use of sequential compression stockings is both cost-effective and equivalent to the prophylactic use of subcutaneous heparin, so I use them for all patients undergoing vaginal hysterectomy. Early ambulation (usually within 2 hours of surgery) is also helpful in avoiding thromboses.
90% of hysterectomies can be performed vaginally
Using the techniques described in this article, I have been able to perform over 90% of the hysterectomies in my practice vaginally. More than 50% of my patients are either morbidly obese, nulliparous, or have had previous abdominal surgery of some type.
The instruments I find most useful are the lighted suction irrigator and the vessel-sealing Heaney-type clamp.
Establishing a routine and approaching technically challenging cases with a systematic and standardized set of techniques make the vaginal route possible for the vast majority of patients with benign disease.
Dr. Levy has served as a consultant to ValleyLab.
SUGGESTED READING
1. Abostini A, Vejux N, Colette E, et al. Risk of bladder injury during vaginal hysterectomy in women with a previous cesarean section. J Reprod Med. 2005;50:940-942.
2. Clave H, Barr H, Niccolai P. Painless vaginal hysterectomy with thermal hemostasis (results of a series of 152 cases). Gynecol Surg. 2005;2:101-105.
3. Isik-Akbay EF, Harmanli OH, Panganamamula UR, et al. Hysterectomy in obese women: a comparison of abdominal and vaginal routes. Obstet Gynecol. 2004;104:710-714.
4. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
5. O’Neal MG, Beste T, Shackelford DP. Utility of preemptive local analgesia in vaginal hysterectomy. Am J Obstet Gynecol. 2003;189:1539-1542.
6. Johnson N, Barlow D, Lethaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2005;(1):CD003677.-
Newer codes for vaginal hysterectomy capture the work of removing larger uteri without laparoscopy
True or false: When it comes to hysterectomy, surgeons tend to use the route that is safest, least invasive, and most economical.
Sadly, the statement is false. Although vaginal hysterectomy tops all 3 categories, it is the least utilized of surgical routes. The number of vaginal hysterectomies may have increased slightly over the past decade, likely due to the incorporation of laparoscopically assisted vaginal hysterectomy into the mainstream and increased practice with the vaginal component, but fewer than 30% of hysterectomies are performed vaginally.
This article addresses 6 common challenges at vaginal hysterectomy and offers strategies to overcome them.
Laparoscopic strategies ease vaginal hysterectomy, too
Laparoscopic hysterectomy became widely accepted when surgical instruments were developed to overcome the technical challenges inherent in operating with limited access. By incorporating some of the techniques we routinely use for laparoscopic surgery, we can overcome many of the challenges faced during difficult vaginal surgery.
VAGINAL HYSTERECTOMY CHALLENGE 1: Obesity
Unfortunately, our population is increasingly rotund. This is not only a significant risk factor for the patient’s health in general, but it poses some unique challenges for surgeons. I must say that, as tough as it may be to complete a hysterectomy vaginally in a morbidly obese woman, I would much rather approach her pelvic organs through the cul-de-sac, which contains no fat cells, than through the abdominal wall—either laparoscopically or abdominally! The trick is gaining access to the posterior cul-de-sac.
How to enter the cul-de-sac
It seems to be a perverse rule of nature, but a tight upper vaginal ring seems almost universal in obese women. Added to the redundant sidewalls and the large buttocks, this tightness makes entry into the anterior or posterior cul-de-sac problematic. Several tricks make peritoneal access possible:
Position the patient to increase access, with the buttocks well over the edge of the operating table. This brings the operative field a bit closer to the surgeon, and permits the use of long-handled retractors posteriorly.
Use candy-cane stirrups to allow assistants better access to the operative field. Adequate assistance is essential in attempting vaginal surgery in the morbidly obese.
Avoid the Trendelenburg position. Although it might seem that this position would facilitate visualization and placement of a posterior weighted speculum, all it does is allow the patient to slide up on the table, making placement of alternate retractors difficult.
Use the right tools. If the posterior weighted speculum will not stay in place or does not afford access to the cul-de-sac due to an upper vaginal ring, use a narrow Deaver retractor posteriorly (without sidewall or anterior retraction). Use a Jacob’s tenaculum on the posterior lip of the cervix and have your assistant pull straight up on the tenaculum while using the Deaver retractor to see the area between the uterosacral ligaments.
Use the uterosacral ligaments as a guide. Another perversity in morbidly obese women: Despite multiparity, they seem to have little or no apical prolapse but lots of vaginal wall redundancy. The cervix is often elongated, but the uterosacral ligaments are sky high.
I palpate these ligaments, injecting them with a combination of vasopressin diluted 1:5 with bupivacaine and epinephrine (for enhanced hemostasis and preemptive analgesia), then use a pencil electrosurgical electrode to rapidly open the vaginal epithelium between the ligaments.
I then use a long, toothed tissue forceps to tent the peritoneum at 90 degrees to the plane of the posterior cul-de-sac and use Mayo scissors to enter the peritoneal cavity. Usually there is a spurt of fluid to mark appropriate entry into the peritoneum.
I then use the blades of my scissors to stretch the peritoneum between the ligaments and place a moistened 4×4 sponge into the incision.
At the onset of the procedure, inject indigo carmine dye intravenously so that any injury to the bladder will be immediately recognized. I have the circulating nurse empty the bladder while she is prepping the patient, but do not leave an indwelling catheter in place during the operation. I find it cumbersome to work around the catheter.
Problematic entries
When entry into the posterior cul-de-sac is difficult, I stop dissection, place a 4×4 sponge into the incision to reduce bleeding from the vagina, and proceed to attempt anterior entry.
I place the Deaver retractor into the anterior space and move the tenaculum to the anterior lip of the cervix. This gives maximal space for downward traction on the cervix while anterior entry is attempted.
Once again, I inject the tissue with the bupivacaine solution before incising the vaginal epithelium at the level of the internal os. I use sharp dissection only when creating a plane between the lower uterine segment and the bladder.
Ensuring room to move and good visualization
If neither the anterior nor the posterior cul-de-sac can be accessed, it may be time to rethink the vaginal approach—but there is no harm in taking the uterosacral and cardinal ligaments extraperitoneally in an effort to gain some mobility. Hugging the uterus and leaving the anterior retractor in place to lift the bladder superiorly are essential steps to protect the ureters. The cervix can then be split in the midline (12 to 6 o’clock) to easily identify the peritoneal reflections.
Other tips
Sidewall retractors are rarely needed. They significantly impair placement of clamps and sutures by creating a long, narrow, parallel passageway. An alternative trick is to use the suction tip to retract the vaginal sidewall as the surgeon is working.
A disposable, fiberoptic, lighted suction irrigator is another option. The light can be directed precisely where it is needed, and the irrigation helps keep the field clean and tidy, simplifying identification of anatomy.
Skip the sutures whenever possible. Because it is difficult to place sutures with precision in a tight, poorly illuminated space, I use a vessel sealer for all pedicles above the uterosacral ligaments. Some of these instruments were designed specifically for vaginal hysterectomy in the same shape and size as Heaney clamps. They are remarkably efficient and permit the completion of a vaginal procedure when suture placement is difficult.
Use a Heaney needleholder, with the suture loaded precisely in the center of the needle curve, along with the lighted suction irrigator to retract redundant tissue away from the track of the needle, to facilitate suturing high in the pelvis.
VAGINAL HYSTERECTOMY CHALLENGE 2: Nulliparity
Many of us are reluctant to attempt vaginal hysterectomy in a woman who has never had children. Although this situation can be challenging at times, in my experience, the access issues tend to be more difficult in obese, multiparous women.
The same tricks and techniques addressed above will permit the vaginal approach in almost all nulliparous women.
VAGINAL HYSTERECTOMY CHALLENGE 3: Previous cesarean section
A prior cesarean delivery is sometimes considered an indication for laparoscopic hysterectomy. There are 2 concerns here: The patient may have a small pelvis, and there may be significant scar tissue and difficulty gaining access to the anterior cul-de-sac, as a result of repeated dissection between the lower uterine segment and the bladder. Several tricks may be useful:
Examine the patient under anesthesia to ensure that the fundus is not stuck to the anterior abdominal wall. This can occur if the peritoneum was not closed during the last cesarean section.
Empty the bladder before beginning the hysterectomy, and inject indigo carmine dye intravenously with the induction of anesthesia.
Use careful sharp dissection between the bladder and lower uterine segment using fine Metzenbaum scissors, with the tips pointed toward the uterus. Dissect only as far as you can easily see.
Secure the uterosacral, cardinal, and broad ligaments, if necessary, before pursuing entry into the anterior cul-de-sac. It is not essential to gain anterior access before taking these pedicles. The additional mobility and descensus enable safe sharp dissection.
If pedicles have been secured up to the fundus, and the anterior cul-de-sac remains difficult to assess, flip the fundus through the posterior cul-de-sac and reach your finger or an instrument around the top of the fundus to identify the peritoneum anteriorly, then incise it under direct vision.
VAGINAL HYSTERECTOMY CHALLENGE 4: Previous abdominal/pelvic surgery
This history is another often-cited rationale for avoiding the vaginal approach. In reality, the adhesions created from prior surgery tend to arise between the anterior abdominal wall and the omentum or small bowel. This situation makes laparoscopic or open abdominal entry riskier than vaginal peritoneal access through the cul-de-sac.
Two possible exceptions: a patient who has had surgery for deeply infiltrating endometriosis in the cul-de-sac or a woman who has undergone myomectomies with posterior incisions. These patients may have dense scarring in the cul-de-sac, which would preclude a vaginal approach. Whatever the surgical route, appropriate bowel preparation is necessary to permit simple closure of any intestinal injury at the time of hysterectomy. Why not begin vaginally if the exam under anesthesia demonstrates an accessible and reasonably free cul-de-sac?
VAGINAL HYSTERECTOMY CHALLENGE 5: Large myomatous uterus
A uterus of any size can be removed vaginally as long as there is mobility with access to the uterine arteries. The one exception: a patient with a small, normal uterus and a massive pedunculated myoma arising from the top of the fundus. If the fibroid cannot be pulled into the true pelvis for morcellation, it cannot be removed transvaginally. Fortunately, this situation is quite rare.
Keep the uterine serosa intact to maintain orientation.
Split the uterus from 12 to 6 o’clock (bivalving). Protect the bladder with a Deaver retractor anteriorly, and protect the posterior vaginal epithelium with the weighted speculum posteriorly.
Remove chunks of tissue from the inside of the specimen. Orient your scalpel blade so that you are always cutting toward the center of the specimen. That way, if the blade slips, you will not accidentally injure tissue on the pelvic sidewall.
Use Lahey thyroid clamps to place the tissue you plan to remove under tension. Finding the capsule of each myoma and gently separating it from the surrounding myometrium facilitates delivery of larger fibroids into the endometrial cavity. Some myomas require morcellation themselves for removal.
Replace the scalpel blade periodically to keep it sharp. Calcified fibroids can dull the blade rapidly.
Work systematically to remove as much central tissue as possible. Try to keep a clean, sharp margin of tissue around the edges for easy grasping. Torn, irregular tissue is very difficult to grab and may cause significant frustration.
If access becomes limited, try clamping additional pedicles on each side of the specimen. A tiny amount of additional descensus can make a huge difference.
Do not administer GnRH agonists prior to surgery. The uterus may shrink, but the myomas tend to become quite soft and difficult to remove. If the patient is seriously anemic, give norethindrone acetate, 5 to 20 mg daily, to stop bleeding and allow the patient’s red blood cell volume to improve before elective surgery.
Use a vessel-sealing instrument to control the pedicles. This strategy produces optimal hemostasis to permit a dry field during morcellation. Moreover, the seals do not get disrupted when the large uterus is pulled past them. Placing suture around pedicles when there is a large, bulky uterus in the pelvis is challenging at best, and it is frustrating to see significant bleeding after removal of the specimen. This problem does not seem to occur with the sealing devices.
Know when to quit! We should not promise any patient a minimally invasive operation. If there is uncontrolled bleeding or no progress after 5 to 10 minutes, convert to a laparoscopic or abdominal approach.
I schedule cases I know will be challenging as “possible” laparoscopic or open hysterectomy. This alerts the OR staff to have additional equipment ready and nearby should we need it. It is not a surgical failure or complication to convert a minimally invasive hysterectomy to a more invasive technique when appropriate. Better to have tried and failed than never to have tried at all!
VAGINAL HYSTERECTOMY CHALLENGE 6: Avoiding complications
The most common complications of vaginal hysterectomy are bleeding, infection, and injury to the bladder. Ureteral injury is less common at vaginal hysterectomy than with the abdominal or laparoscopic approaches. Thus, I do not think routine cystoscopy is essential after uncomplicated vaginal hysterectomy, although I recommend intravenous administration of indigo carmine dye at the beginning of the procedure to enable rapid recognition of even a small bladder laceration. Sharp, careful dissection of the bladder off the lower uterine segment and the avoidance of finger dissection (especially with a gauze sponge) keep these injuries to a minimum.
Minimize bleeding by using newer vessel-sealing technologies rather than suture for most of the pedicles. I attach the uterosacral–cardinal ligament pedicles to the vaginal cuff at closure with suture. I suture the first pedicle once I have entered the posterior cul-de-sac and hold that suture to stay oriented.
Pay attention to patient positioning. Careful positioning will help you avoid neurological injuries. Avoid hyperflexion at the hips, which stretches the femoral nerve. Large nerves have comparatively little blood supply, so stretching them for prolonged periods can cause hypoxic injury. Although such injuries are almost always rapidly reversible, they are disconcerting for both the patient and her surgeon.
When operating on a very thin woman with a bony sacrum, I like to place egg-crate foam beneath the buttocks to provide some cushioning. I am also very careful with these women to keep their legs in a neutral position, and I watch my surgical assistants to be sure they are not leaning on the patient during the procedure.
Prophylactic antibiotics are a must to avoid postoperative vaginal cuff infections and pelvic abscess. Smokers and women with preexisting bacterial vaginosis are at highest risk for infection. I ask women to discontinue smoking at least 2 weeks prior to surgery and inform all smokers that their risk of infection is heightened. I treat anaerobic overgrowth in the vagina prior to surgery to help prevent infections in women with bacterial vaginosis.
The timing of prophylactic antibiotics is important. Intravenous first-generation cephalosporins must be administered within 60 minutes of the initial incision, but it is important to give them early enough for them to adequately disseminate to tissue before the colpotomy incision.
DVT prophylaxis is especially important for women with large uteri. Routine use of sequential compression stockings is both cost-effective and equivalent to the prophylactic use of subcutaneous heparin, so I use them for all patients undergoing vaginal hysterectomy. Early ambulation (usually within 2 hours of surgery) is also helpful in avoiding thromboses.
90% of hysterectomies can be performed vaginally
Using the techniques described in this article, I have been able to perform over 90% of the hysterectomies in my practice vaginally. More than 50% of my patients are either morbidly obese, nulliparous, or have had previous abdominal surgery of some type.
The instruments I find most useful are the lighted suction irrigator and the vessel-sealing Heaney-type clamp.
Establishing a routine and approaching technically challenging cases with a systematic and standardized set of techniques make the vaginal route possible for the vast majority of patients with benign disease.
Dr. Levy has served as a consultant to ValleyLab.
Newer codes for vaginal hysterectomy capture the work of removing larger uteri without laparoscopy
True or false: When it comes to hysterectomy, surgeons tend to use the route that is safest, least invasive, and most economical.
Sadly, the statement is false. Although vaginal hysterectomy tops all 3 categories, it is the least utilized of surgical routes. The number of vaginal hysterectomies may have increased slightly over the past decade, likely due to the incorporation of laparoscopically assisted vaginal hysterectomy into the mainstream and increased practice with the vaginal component, but fewer than 30% of hysterectomies are performed vaginally.
This article addresses 6 common challenges at vaginal hysterectomy and offers strategies to overcome them.
Laparoscopic strategies ease vaginal hysterectomy, too
Laparoscopic hysterectomy became widely accepted when surgical instruments were developed to overcome the technical challenges inherent in operating with limited access. By incorporating some of the techniques we routinely use for laparoscopic surgery, we can overcome many of the challenges faced during difficult vaginal surgery.
VAGINAL HYSTERECTOMY CHALLENGE 1: Obesity
Unfortunately, our population is increasingly rotund. This is not only a significant risk factor for the patient’s health in general, but it poses some unique challenges for surgeons. I must say that, as tough as it may be to complete a hysterectomy vaginally in a morbidly obese woman, I would much rather approach her pelvic organs through the cul-de-sac, which contains no fat cells, than through the abdominal wall—either laparoscopically or abdominally! The trick is gaining access to the posterior cul-de-sac.
How to enter the cul-de-sac
It seems to be a perverse rule of nature, but a tight upper vaginal ring seems almost universal in obese women. Added to the redundant sidewalls and the large buttocks, this tightness makes entry into the anterior or posterior cul-de-sac problematic. Several tricks make peritoneal access possible:
Position the patient to increase access, with the buttocks well over the edge of the operating table. This brings the operative field a bit closer to the surgeon, and permits the use of long-handled retractors posteriorly.
Use candy-cane stirrups to allow assistants better access to the operative field. Adequate assistance is essential in attempting vaginal surgery in the morbidly obese.
Avoid the Trendelenburg position. Although it might seem that this position would facilitate visualization and placement of a posterior weighted speculum, all it does is allow the patient to slide up on the table, making placement of alternate retractors difficult.
Use the right tools. If the posterior weighted speculum will not stay in place or does not afford access to the cul-de-sac due to an upper vaginal ring, use a narrow Deaver retractor posteriorly (without sidewall or anterior retraction). Use a Jacob’s tenaculum on the posterior lip of the cervix and have your assistant pull straight up on the tenaculum while using the Deaver retractor to see the area between the uterosacral ligaments.
Use the uterosacral ligaments as a guide. Another perversity in morbidly obese women: Despite multiparity, they seem to have little or no apical prolapse but lots of vaginal wall redundancy. The cervix is often elongated, but the uterosacral ligaments are sky high.
I palpate these ligaments, injecting them with a combination of vasopressin diluted 1:5 with bupivacaine and epinephrine (for enhanced hemostasis and preemptive analgesia), then use a pencil electrosurgical electrode to rapidly open the vaginal epithelium between the ligaments.
I then use a long, toothed tissue forceps to tent the peritoneum at 90 degrees to the plane of the posterior cul-de-sac and use Mayo scissors to enter the peritoneal cavity. Usually there is a spurt of fluid to mark appropriate entry into the peritoneum.
I then use the blades of my scissors to stretch the peritoneum between the ligaments and place a moistened 4×4 sponge into the incision.
At the onset of the procedure, inject indigo carmine dye intravenously so that any injury to the bladder will be immediately recognized. I have the circulating nurse empty the bladder while she is prepping the patient, but do not leave an indwelling catheter in place during the operation. I find it cumbersome to work around the catheter.
Problematic entries
When entry into the posterior cul-de-sac is difficult, I stop dissection, place a 4×4 sponge into the incision to reduce bleeding from the vagina, and proceed to attempt anterior entry.
I place the Deaver retractor into the anterior space and move the tenaculum to the anterior lip of the cervix. This gives maximal space for downward traction on the cervix while anterior entry is attempted.
Once again, I inject the tissue with the bupivacaine solution before incising the vaginal epithelium at the level of the internal os. I use sharp dissection only when creating a plane between the lower uterine segment and the bladder.
Ensuring room to move and good visualization
If neither the anterior nor the posterior cul-de-sac can be accessed, it may be time to rethink the vaginal approach—but there is no harm in taking the uterosacral and cardinal ligaments extraperitoneally in an effort to gain some mobility. Hugging the uterus and leaving the anterior retractor in place to lift the bladder superiorly are essential steps to protect the ureters. The cervix can then be split in the midline (12 to 6 o’clock) to easily identify the peritoneal reflections.
Other tips
Sidewall retractors are rarely needed. They significantly impair placement of clamps and sutures by creating a long, narrow, parallel passageway. An alternative trick is to use the suction tip to retract the vaginal sidewall as the surgeon is working.
A disposable, fiberoptic, lighted suction irrigator is another option. The light can be directed precisely where it is needed, and the irrigation helps keep the field clean and tidy, simplifying identification of anatomy.
Skip the sutures whenever possible. Because it is difficult to place sutures with precision in a tight, poorly illuminated space, I use a vessel sealer for all pedicles above the uterosacral ligaments. Some of these instruments were designed specifically for vaginal hysterectomy in the same shape and size as Heaney clamps. They are remarkably efficient and permit the completion of a vaginal procedure when suture placement is difficult.
Use a Heaney needleholder, with the suture loaded precisely in the center of the needle curve, along with the lighted suction irrigator to retract redundant tissue away from the track of the needle, to facilitate suturing high in the pelvis.
VAGINAL HYSTERECTOMY CHALLENGE 2: Nulliparity
Many of us are reluctant to attempt vaginal hysterectomy in a woman who has never had children. Although this situation can be challenging at times, in my experience, the access issues tend to be more difficult in obese, multiparous women.
The same tricks and techniques addressed above will permit the vaginal approach in almost all nulliparous women.
VAGINAL HYSTERECTOMY CHALLENGE 3: Previous cesarean section
A prior cesarean delivery is sometimes considered an indication for laparoscopic hysterectomy. There are 2 concerns here: The patient may have a small pelvis, and there may be significant scar tissue and difficulty gaining access to the anterior cul-de-sac, as a result of repeated dissection between the lower uterine segment and the bladder. Several tricks may be useful:
Examine the patient under anesthesia to ensure that the fundus is not stuck to the anterior abdominal wall. This can occur if the peritoneum was not closed during the last cesarean section.
Empty the bladder before beginning the hysterectomy, and inject indigo carmine dye intravenously with the induction of anesthesia.
Use careful sharp dissection between the bladder and lower uterine segment using fine Metzenbaum scissors, with the tips pointed toward the uterus. Dissect only as far as you can easily see.
Secure the uterosacral, cardinal, and broad ligaments, if necessary, before pursuing entry into the anterior cul-de-sac. It is not essential to gain anterior access before taking these pedicles. The additional mobility and descensus enable safe sharp dissection.
If pedicles have been secured up to the fundus, and the anterior cul-de-sac remains difficult to assess, flip the fundus through the posterior cul-de-sac and reach your finger or an instrument around the top of the fundus to identify the peritoneum anteriorly, then incise it under direct vision.
VAGINAL HYSTERECTOMY CHALLENGE 4: Previous abdominal/pelvic surgery
This history is another often-cited rationale for avoiding the vaginal approach. In reality, the adhesions created from prior surgery tend to arise between the anterior abdominal wall and the omentum or small bowel. This situation makes laparoscopic or open abdominal entry riskier than vaginal peritoneal access through the cul-de-sac.
Two possible exceptions: a patient who has had surgery for deeply infiltrating endometriosis in the cul-de-sac or a woman who has undergone myomectomies with posterior incisions. These patients may have dense scarring in the cul-de-sac, which would preclude a vaginal approach. Whatever the surgical route, appropriate bowel preparation is necessary to permit simple closure of any intestinal injury at the time of hysterectomy. Why not begin vaginally if the exam under anesthesia demonstrates an accessible and reasonably free cul-de-sac?
VAGINAL HYSTERECTOMY CHALLENGE 5: Large myomatous uterus
A uterus of any size can be removed vaginally as long as there is mobility with access to the uterine arteries. The one exception: a patient with a small, normal uterus and a massive pedunculated myoma arising from the top of the fundus. If the fibroid cannot be pulled into the true pelvis for morcellation, it cannot be removed transvaginally. Fortunately, this situation is quite rare.
Keep the uterine serosa intact to maintain orientation.
Split the uterus from 12 to 6 o’clock (bivalving). Protect the bladder with a Deaver retractor anteriorly, and protect the posterior vaginal epithelium with the weighted speculum posteriorly.
Remove chunks of tissue from the inside of the specimen. Orient your scalpel blade so that you are always cutting toward the center of the specimen. That way, if the blade slips, you will not accidentally injure tissue on the pelvic sidewall.
Use Lahey thyroid clamps to place the tissue you plan to remove under tension. Finding the capsule of each myoma and gently separating it from the surrounding myometrium facilitates delivery of larger fibroids into the endometrial cavity. Some myomas require morcellation themselves for removal.
Replace the scalpel blade periodically to keep it sharp. Calcified fibroids can dull the blade rapidly.
Work systematically to remove as much central tissue as possible. Try to keep a clean, sharp margin of tissue around the edges for easy grasping. Torn, irregular tissue is very difficult to grab and may cause significant frustration.
If access becomes limited, try clamping additional pedicles on each side of the specimen. A tiny amount of additional descensus can make a huge difference.
Do not administer GnRH agonists prior to surgery. The uterus may shrink, but the myomas tend to become quite soft and difficult to remove. If the patient is seriously anemic, give norethindrone acetate, 5 to 20 mg daily, to stop bleeding and allow the patient’s red blood cell volume to improve before elective surgery.
Use a vessel-sealing instrument to control the pedicles. This strategy produces optimal hemostasis to permit a dry field during morcellation. Moreover, the seals do not get disrupted when the large uterus is pulled past them. Placing suture around pedicles when there is a large, bulky uterus in the pelvis is challenging at best, and it is frustrating to see significant bleeding after removal of the specimen. This problem does not seem to occur with the sealing devices.
Know when to quit! We should not promise any patient a minimally invasive operation. If there is uncontrolled bleeding or no progress after 5 to 10 minutes, convert to a laparoscopic or abdominal approach.
I schedule cases I know will be challenging as “possible” laparoscopic or open hysterectomy. This alerts the OR staff to have additional equipment ready and nearby should we need it. It is not a surgical failure or complication to convert a minimally invasive hysterectomy to a more invasive technique when appropriate. Better to have tried and failed than never to have tried at all!
VAGINAL HYSTERECTOMY CHALLENGE 6: Avoiding complications
The most common complications of vaginal hysterectomy are bleeding, infection, and injury to the bladder. Ureteral injury is less common at vaginal hysterectomy than with the abdominal or laparoscopic approaches. Thus, I do not think routine cystoscopy is essential after uncomplicated vaginal hysterectomy, although I recommend intravenous administration of indigo carmine dye at the beginning of the procedure to enable rapid recognition of even a small bladder laceration. Sharp, careful dissection of the bladder off the lower uterine segment and the avoidance of finger dissection (especially with a gauze sponge) keep these injuries to a minimum.
Minimize bleeding by using newer vessel-sealing technologies rather than suture for most of the pedicles. I attach the uterosacral–cardinal ligament pedicles to the vaginal cuff at closure with suture. I suture the first pedicle once I have entered the posterior cul-de-sac and hold that suture to stay oriented.
Pay attention to patient positioning. Careful positioning will help you avoid neurological injuries. Avoid hyperflexion at the hips, which stretches the femoral nerve. Large nerves have comparatively little blood supply, so stretching them for prolonged periods can cause hypoxic injury. Although such injuries are almost always rapidly reversible, they are disconcerting for both the patient and her surgeon.
When operating on a very thin woman with a bony sacrum, I like to place egg-crate foam beneath the buttocks to provide some cushioning. I am also very careful with these women to keep their legs in a neutral position, and I watch my surgical assistants to be sure they are not leaning on the patient during the procedure.
Prophylactic antibiotics are a must to avoid postoperative vaginal cuff infections and pelvic abscess. Smokers and women with preexisting bacterial vaginosis are at highest risk for infection. I ask women to discontinue smoking at least 2 weeks prior to surgery and inform all smokers that their risk of infection is heightened. I treat anaerobic overgrowth in the vagina prior to surgery to help prevent infections in women with bacterial vaginosis.
The timing of prophylactic antibiotics is important. Intravenous first-generation cephalosporins must be administered within 60 minutes of the initial incision, but it is important to give them early enough for them to adequately disseminate to tissue before the colpotomy incision.
DVT prophylaxis is especially important for women with large uteri. Routine use of sequential compression stockings is both cost-effective and equivalent to the prophylactic use of subcutaneous heparin, so I use them for all patients undergoing vaginal hysterectomy. Early ambulation (usually within 2 hours of surgery) is also helpful in avoiding thromboses.
90% of hysterectomies can be performed vaginally
Using the techniques described in this article, I have been able to perform over 90% of the hysterectomies in my practice vaginally. More than 50% of my patients are either morbidly obese, nulliparous, or have had previous abdominal surgery of some type.
The instruments I find most useful are the lighted suction irrigator and the vessel-sealing Heaney-type clamp.
Establishing a routine and approaching technically challenging cases with a systematic and standardized set of techniques make the vaginal route possible for the vast majority of patients with benign disease.
Dr. Levy has served as a consultant to ValleyLab.
SUGGESTED READING
1. Abostini A, Vejux N, Colette E, et al. Risk of bladder injury during vaginal hysterectomy in women with a previous cesarean section. J Reprod Med. 2005;50:940-942.
2. Clave H, Barr H, Niccolai P. Painless vaginal hysterectomy with thermal hemostasis (results of a series of 152 cases). Gynecol Surg. 2005;2:101-105.
3. Isik-Akbay EF, Harmanli OH, Panganamamula UR, et al. Hysterectomy in obese women: a comparison of abdominal and vaginal routes. Obstet Gynecol. 2004;104:710-714.
4. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
5. O’Neal MG, Beste T, Shackelford DP. Utility of preemptive local analgesia in vaginal hysterectomy. Am J Obstet Gynecol. 2003;189:1539-1542.
6. Johnson N, Barlow D, Lethaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2005;(1):CD003677.-
SUGGESTED READING
1. Abostini A, Vejux N, Colette E, et al. Risk of bladder injury during vaginal hysterectomy in women with a previous cesarean section. J Reprod Med. 2005;50:940-942.
2. Clave H, Barr H, Niccolai P. Painless vaginal hysterectomy with thermal hemostasis (results of a series of 152 cases). Gynecol Surg. 2005;2:101-105.
3. Isik-Akbay EF, Harmanli OH, Panganamamula UR, et al. Hysterectomy in obese women: a comparison of abdominal and vaginal routes. Obstet Gynecol. 2004;104:710-714.
4. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
5. O’Neal MG, Beste T, Shackelford DP. Utility of preemptive local analgesia in vaginal hysterectomy. Am J Obstet Gynecol. 2003;189:1539-1542.
6. Johnson N, Barlow D, Lethaby A, Tavender E, Curr E, Garry R. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2005;(1):CD003677.-
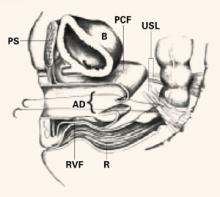
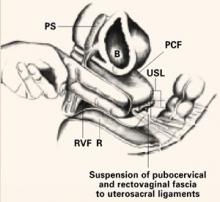
The Retroperitoneal Space: Keeping vital structures out of harm’s way
The accomplished gynecologic surgeon must know the anatomy of the retroperitoneal space in order to avoid damage to normal structures, as well as remove pathology. Many disease processes involve the pelvic peritoneum, uterosacral ligaments, rectosigmoid or ovarian pedicles, and require the surgeon to enter the retroperitoneal space to identify the ureters and blood vessels and keep them out of harm’s way. The challenges are complex:
- Badly distorted anatomy and the anterior and posterior cul-de-sac necessitate mobilization of the rectosigmoid and bladder.
- Intraligamentous fibroids require knowledge of the blood supply in the retroperitoneal space. Malignant disorders mandate that the lymph nodes be dissected to determine extent of disease and as part of treatment.
Is training adequate?
Every training program should teach the surgical anatomy of the retroperitoneal space, since every surgeon needs to be comfortable exposing the anatomy, both to prevent injury and to accomplish the needed surgery. Videotapes and cadaver courses can prepare the resident for the operating room.
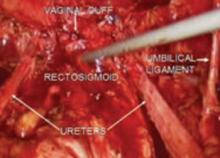
The “landmark” umbilical ligament
The umbilical ligament was the umbilical artery in fetal life and courses along the edge of the bladder to the anterior abdominal wall up to the umbilicus. It is a useful guide into the perivesicle space. Lateral to it are the iliac vessels, and medial is the bladder. It is also a good marker for finding the right spot to open the round ligament.
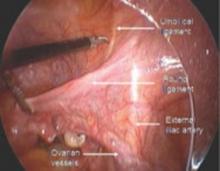
FIGURE 1 Opening the round ligament over the umbilical ligament
The round ligament is the key to exposing the retroperitoneal space. It should be open at the pelvic sidewall, just medial to the external iliac vessels. The umbilical ligament can be used as a landmark.
FIGURE 2 Opening lateral to the ovarian vessels
The divided round ligament is retracted ventrally and medially to place the ovarian vessels under traction. The peritoneum lateral to the ovarian vessels is divided up to the pelvic brim.
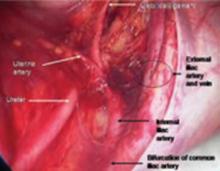
FIGURE 3 Right pelvic sidewall anatomy
Medial traction of the peritoneum around the ovarian vessels at the pelvic brim will expose the ureter coming over the iliac vessels at their bifurcation into external and internal branches.
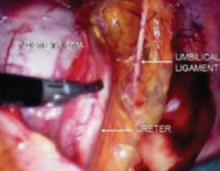
FIGURE 4 Relationship of ureter to the umbilical ligament
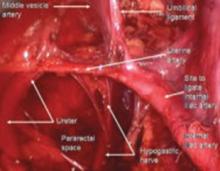
The ureter comes off medial with the fold of peritoneum. Once the space is developed, the operator’s finger or the laparoscopic probe can be introduced along the medial side of the internal iliac artery and ventral to the curve of the sacrum. This will open the pararectal space. The ureter and the anterior branch of the internal iliac artery are nearly parallel as they course through the pelvis.
FIGURE 5 Hypogastric nerve
The anterior branch of the internal iliac artery gives off the uterine artery and the middle and superior vesicle arteries before continuing on as the umbilical ligament.
Endometriosis may imperil the ureter
Endometriomas and peritoneal implants are among the most common reasons for accessing the retroperitoneal space. Frequently the peritoneum between the ovarian vessels and the uterosacral ligaments (ovarian fossa) is thickened and retracted within the endometriosis implants. This alters the pelvic anatomy and puts the ureter at risk for injury.
Definitive surgical treatment for endometriosis includes removal of this diseased peritoneum. The ureter is best identified using the technique described, and then dissected off the peritoneum down to the uterine artery.
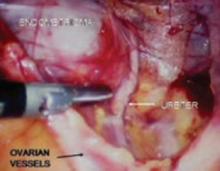
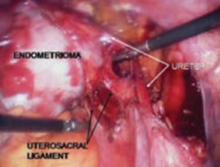
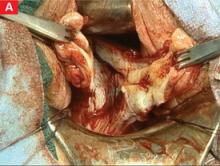
FIGURE 6 Finding the ureter lateral to an endometrioma
FIGURE 7 Relationship of ureter to the umbilical ligament
FIGURE 8 Dissecting the ureter out of the uterosacral ligament
The ureter may be placed on a Penrose drain to better isolate it and keep it under direct visualization. The cul-de-sac of Douglas is another site of endometrial implants. The fundus of the uterus is often adhesed to the rectosigmoid reflection and even the sigmoid colon. Nodules of endometriosis may infiltrate the uterosacral ligaments and extend into the rectovaginal space. To manage these implants, isolate the ureter to the point where it passes under the uterine artery.
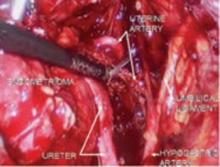
FIGURE 9 Divide uterine artery lateral to endometrioma
Here, the uterine artery is taken lateral to the mass, enabling the surgeon to remove all of the uterosacral implants. The bladder flap will be developed and the bladder advanced caudad. By dissecting medial to the ureter as it courses under the uterine artery, it will be retracted laterally and provide the space necessary to resect the uterosacral implants.
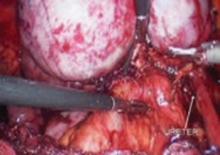
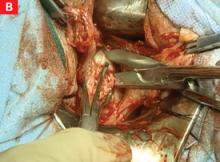
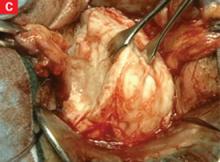
FIGURE 10 Rectosigmoid reflection with endometrioma nodule
FIGURE 11 Postoperative appearance of endometriosis resection
As dissection proceeds medially, use the perirectal fatty plane to remove implants between the uterosacral area and rectum. In the midline, remove implants below the rectosigmoid peritoneal reflexion, taking care not to injure the rectum.
Implants above the peritoneal reflexion are often attached to the sigmoid tinea coli and cannot be removed without taking a portion of the bowel. Thus, patients with extensive endometriosis should have a bowel prep with two 10-ounce bottles of magnesium citrate the day before surgery.
If bowel resection is necessary, consult a general surgeon or gynecologic oncologist. A history of painful defecation or a finding of nodules in the cul-de-sac with rectal dimpling warrants preoperative consultation with a specialist skilled at bowel resection.
Ureteral injuries occur in 0.5% to 2.5% of women undergoing gynecologic surgery.1 The most common predisposing condition is previous pelvic surgery.2,3 The usual sites of injury are at the pelvic rim close to the ovarian vessels, at the level of the uterine artery, and lateral to the vaginal cuff. Neither preoperative excretory urograms nor placement of ureteral catheters preoperatively have been found to be effective prevention measures.2,4-6 Using the steps described above, the ureter can be identified and mobilized.
Blood supply to the ureter comes from the plexus of vessels that form a network along the length of the ureter. This plexus is fed by arteries from the renal pelvis, common iliac, internal iliac, uterine artery, and the base of the bladder. Complete mobilization of the ureter away from its peritoneal attachments and these lateral blood supply sources can be accomplished as long as the vascular plexus is not disrupted by cautery, crushing, or tearing. A clean transection can be re-anastomosed or reimplanted with the expectation of normal healing. Innervation of the ureter is from the inferior mesenteric plexus superiorly and the inferior hypogastric plexus in the pelvis. Ureteral peristalsis will continue, even if the ureter is completely divided or ligated.
Protecting pelvic blood vessels
The majority of gynecologic operations involve the ovarian vessels and the uterine vessels. The operator rarely needs to explore the lateral pelvis to identify the rest of the vessels. When faced with a large endometrioma or cancer, knowledge of the anatomy of the lateral blood vessels is vital.
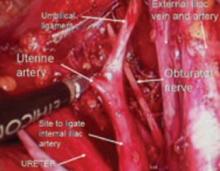
Since the pathology is usually deeper in the pelvis, it is wise to identify the anatomy of the pelvis starting at the pelvic brim, where the common iliac bifurcates into the external and internal iliacs. There is a safe dissection plane medial to the internal iliac artery all the way to the uterine artery. This exposes the pararectal space, which can be opened without risk of major bleeding (FIGURE 5).
The obliterated umbilical vessel is the other friendly marker just distal to the uterine artery. It can be placed on traction and the uterine artery isolated. The inferior and superior vesical arteries are generally not dissected, as they are adjacent to the bladder and the surgery takes place medial to them in the prevesical fascial space.
Hypogastric artery ligations are rarely performed today, as interventional radiology is the standard of care for patients with postoperative and postpartum bleeding. When it becomes necessary, the hypogastric artery can be isolated and tied using the right-angle clamp to pass the tie. The superior gluteal artery branches so close to the bifurcation of the common iliac artery that it is not visualized. The inferior gluteal artery is the largest distal branch, which might be visualized during the ligation. It is not necessary to identify it.
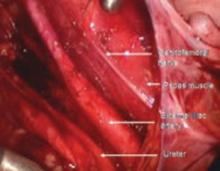
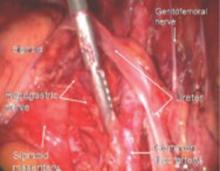
FIGURE 12 Right genitofemoral nerve
The genitofemoral nerve runs along the medial aspect of the body of the psoas muscle. It is sometimes injured by the self-retaining retractors placed at the time of laparotomy. This leads to some numbness and burning of the skin of the anterior thigh.
FIGURE 13 Right pelvic sidewall anatomy
The obturator nerve is in the obturator space and typically far lateral to the usual dissection. Metastatic cancer to the obturator lymph nodes may entrap it, or it may be injured during a node dissection, causing loss of internal rotation of the anterior thigh.
The sciatic nerve is seen only during exenterative surgery. Pressure on the lateral pelvis by advanced pelvic tumors can lead to sciatic pain and motor weakness—even loss of motor function to the lower leg, which commonly leads to foot drop.
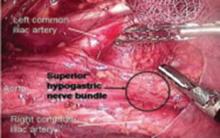
FIGURE 14 Superior hypogastric nerve bundle
The hypogastric plexus of nerves is sometimes damaged during surgery for endometriosis or for malignancy. The superior hypergastric plexus can be identified between the 2 common iliac arteries at the sacral promontory. The left common iliac vein runs underneath it.
FIGURE 15 Hypogastric nerve descending into the right pelvis
The right and left hypogastric nerves leave the hypogastric plexus and descend into the pelvis parallel to the ureter and 2 cm medial. It passes dorsal to the ureter as it goes through the cardinal ligament (FIGURE 5).
This plexus then supplies autonomic innervation of the bladder, rectum, uterus, and ureter. Complete disruption of the hypogastric nerve will lead to a hypertonic, noncontractile bladder and the necessity for self-catheterization to eliminate urine. Preservation of this nerve during radical hysterectomy or endometriosis resection is a high priority.
Laparoscopic uterosacral nerve ablation procedures divide the uterosacral ligament medial and caudad to the ureter and do not disrupt the main hypogastric nerve. Only the medial branches to the uterus are affected. Successful uterosacral nerve ablation has been reported in approximately 44% of women who have dysmenorrhea without visible endometriosis and approximately 62% of women who have visible endometriosis.7,8 The efficacy of this procedure is controversial, however. Removal of the superior hypogastric plexus (presacral neurectomy) has not proved to be more effective in controlling pelvic pain than conservative surgery that only destroys endometrial implants. Presacral neurectomy is no longer advised.9
1. American College of Obstetricians and Gynecologists. Educational Bulletin #238: Lower Urinary Tract Operative Injuries. Washington, DC: ACOG; 1985:1.
2. Daly JW, Higgins KA. Injury to the ureter during gynecologic surgical procedures. Surg Gynecol Obstet. 1988;167:19-22.
3. Selzman AA, Spirnak JP. Iatrogenic ureteral injuries: a 20-year experience in treating 165 injuries. J Urol. 1996;155:878-881.
4. Symmonds RE. Ureteral injuries associated with gynecologic surgery: prevention and management. Clin Obstet Gynecol. 19676;19:623-644.
5. Higgins CC. Ureteral injuries during surgery: a review of 87 cases. JAMA 1967;199:82-88.
6. Fry DE, Milholen L, Harbrecht PJ. Iatrogenic ureteral injury. Options in management. Arch Surg. 1983;118:454-457.
7. Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. J Reprod Med. 1987;32:37-41.
8. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696.-
9. Candiani GB, Fedele L, Vercellini P, Bianchi S, Di Nola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
Dr. Hatch receives research/grant support from Merck; is a consultant to Ethicon, Merck, and Quest Laboratory; and is a speaker for Cytyc, Digene, Ethicon, and Merck.
The accomplished gynecologic surgeon must know the anatomy of the retroperitoneal space in order to avoid damage to normal structures, as well as remove pathology. Many disease processes involve the pelvic peritoneum, uterosacral ligaments, rectosigmoid or ovarian pedicles, and require the surgeon to enter the retroperitoneal space to identify the ureters and blood vessels and keep them out of harm’s way. The challenges are complex:
- Badly distorted anatomy and the anterior and posterior cul-de-sac necessitate mobilization of the rectosigmoid and bladder.
- Intraligamentous fibroids require knowledge of the blood supply in the retroperitoneal space. Malignant disorders mandate that the lymph nodes be dissected to determine extent of disease and as part of treatment.
Is training adequate?
Every training program should teach the surgical anatomy of the retroperitoneal space, since every surgeon needs to be comfortable exposing the anatomy, both to prevent injury and to accomplish the needed surgery. Videotapes and cadaver courses can prepare the resident for the operating room.
The “landmark” umbilical ligament
The umbilical ligament was the umbilical artery in fetal life and courses along the edge of the bladder to the anterior abdominal wall up to the umbilicus. It is a useful guide into the perivesicle space. Lateral to it are the iliac vessels, and medial is the bladder. It is also a good marker for finding the right spot to open the round ligament.
FIGURE 1 Opening the round ligament over the umbilical ligament
The round ligament is the key to exposing the retroperitoneal space. It should be open at the pelvic sidewall, just medial to the external iliac vessels. The umbilical ligament can be used as a landmark.
FIGURE 2 Opening lateral to the ovarian vessels
The divided round ligament is retracted ventrally and medially to place the ovarian vessels under traction. The peritoneum lateral to the ovarian vessels is divided up to the pelvic brim.
FIGURE 3 Right pelvic sidewall anatomy
Medial traction of the peritoneum around the ovarian vessels at the pelvic brim will expose the ureter coming over the iliac vessels at their bifurcation into external and internal branches.
FIGURE 4 Relationship of ureter to the umbilical ligament
The ureter comes off medial with the fold of peritoneum. Once the space is developed, the operator’s finger or the laparoscopic probe can be introduced along the medial side of the internal iliac artery and ventral to the curve of the sacrum. This will open the pararectal space. The ureter and the anterior branch of the internal iliac artery are nearly parallel as they course through the pelvis.
FIGURE 5 Hypogastric nerve
The anterior branch of the internal iliac artery gives off the uterine artery and the middle and superior vesicle arteries before continuing on as the umbilical ligament.
Endometriosis may imperil the ureter
Endometriomas and peritoneal implants are among the most common reasons for accessing the retroperitoneal space. Frequently the peritoneum between the ovarian vessels and the uterosacral ligaments (ovarian fossa) is thickened and retracted within the endometriosis implants. This alters the pelvic anatomy and puts the ureter at risk for injury.
Definitive surgical treatment for endometriosis includes removal of this diseased peritoneum. The ureter is best identified using the technique described, and then dissected off the peritoneum down to the uterine artery.
FIGURE 6 Finding the ureter lateral to an endometrioma
FIGURE 7 Relationship of ureter to the umbilical ligament
FIGURE 8 Dissecting the ureter out of the uterosacral ligament
The ureter may be placed on a Penrose drain to better isolate it and keep it under direct visualization. The cul-de-sac of Douglas is another site of endometrial implants. The fundus of the uterus is often adhesed to the rectosigmoid reflection and even the sigmoid colon. Nodules of endometriosis may infiltrate the uterosacral ligaments and extend into the rectovaginal space. To manage these implants, isolate the ureter to the point where it passes under the uterine artery.
FIGURE 9 Divide uterine artery lateral to endometrioma
Here, the uterine artery is taken lateral to the mass, enabling the surgeon to remove all of the uterosacral implants. The bladder flap will be developed and the bladder advanced caudad. By dissecting medial to the ureter as it courses under the uterine artery, it will be retracted laterally and provide the space necessary to resect the uterosacral implants.
FIGURE 10 Rectosigmoid reflection with endometrioma nodule
FIGURE 11 Postoperative appearance of endometriosis resection
As dissection proceeds medially, use the perirectal fatty plane to remove implants between the uterosacral area and rectum. In the midline, remove implants below the rectosigmoid peritoneal reflexion, taking care not to injure the rectum.
Implants above the peritoneal reflexion are often attached to the sigmoid tinea coli and cannot be removed without taking a portion of the bowel. Thus, patients with extensive endometriosis should have a bowel prep with two 10-ounce bottles of magnesium citrate the day before surgery.
If bowel resection is necessary, consult a general surgeon or gynecologic oncologist. A history of painful defecation or a finding of nodules in the cul-de-sac with rectal dimpling warrants preoperative consultation with a specialist skilled at bowel resection.
Ureteral injuries occur in 0.5% to 2.5% of women undergoing gynecologic surgery.1 The most common predisposing condition is previous pelvic surgery.2,3 The usual sites of injury are at the pelvic rim close to the ovarian vessels, at the level of the uterine artery, and lateral to the vaginal cuff. Neither preoperative excretory urograms nor placement of ureteral catheters preoperatively have been found to be effective prevention measures.2,4-6 Using the steps described above, the ureter can be identified and mobilized.
Blood supply to the ureter comes from the plexus of vessels that form a network along the length of the ureter. This plexus is fed by arteries from the renal pelvis, common iliac, internal iliac, uterine artery, and the base of the bladder. Complete mobilization of the ureter away from its peritoneal attachments and these lateral blood supply sources can be accomplished as long as the vascular plexus is not disrupted by cautery, crushing, or tearing. A clean transection can be re-anastomosed or reimplanted with the expectation of normal healing. Innervation of the ureter is from the inferior mesenteric plexus superiorly and the inferior hypogastric plexus in the pelvis. Ureteral peristalsis will continue, even if the ureter is completely divided or ligated.
Protecting pelvic blood vessels
The majority of gynecologic operations involve the ovarian vessels and the uterine vessels. The operator rarely needs to explore the lateral pelvis to identify the rest of the vessels. When faced with a large endometrioma or cancer, knowledge of the anatomy of the lateral blood vessels is vital.
Since the pathology is usually deeper in the pelvis, it is wise to identify the anatomy of the pelvis starting at the pelvic brim, where the common iliac bifurcates into the external and internal iliacs. There is a safe dissection plane medial to the internal iliac artery all the way to the uterine artery. This exposes the pararectal space, which can be opened without risk of major bleeding (FIGURE 5).
The obliterated umbilical vessel is the other friendly marker just distal to the uterine artery. It can be placed on traction and the uterine artery isolated. The inferior and superior vesical arteries are generally not dissected, as they are adjacent to the bladder and the surgery takes place medial to them in the prevesical fascial space.
Hypogastric artery ligations are rarely performed today, as interventional radiology is the standard of care for patients with postoperative and postpartum bleeding. When it becomes necessary, the hypogastric artery can be isolated and tied using the right-angle clamp to pass the tie. The superior gluteal artery branches so close to the bifurcation of the common iliac artery that it is not visualized. The inferior gluteal artery is the largest distal branch, which might be visualized during the ligation. It is not necessary to identify it.
FIGURE 12 Right genitofemoral nerve
The genitofemoral nerve runs along the medial aspect of the body of the psoas muscle. It is sometimes injured by the self-retaining retractors placed at the time of laparotomy. This leads to some numbness and burning of the skin of the anterior thigh.
FIGURE 13 Right pelvic sidewall anatomy
The obturator nerve is in the obturator space and typically far lateral to the usual dissection. Metastatic cancer to the obturator lymph nodes may entrap it, or it may be injured during a node dissection, causing loss of internal rotation of the anterior thigh.
The sciatic nerve is seen only during exenterative surgery. Pressure on the lateral pelvis by advanced pelvic tumors can lead to sciatic pain and motor weakness—even loss of motor function to the lower leg, which commonly leads to foot drop.
FIGURE 14 Superior hypogastric nerve bundle
The hypogastric plexus of nerves is sometimes damaged during surgery for endometriosis or for malignancy. The superior hypergastric plexus can be identified between the 2 common iliac arteries at the sacral promontory. The left common iliac vein runs underneath it.
FIGURE 15 Hypogastric nerve descending into the right pelvis
The right and left hypogastric nerves leave the hypogastric plexus and descend into the pelvis parallel to the ureter and 2 cm medial. It passes dorsal to the ureter as it goes through the cardinal ligament (FIGURE 5).
This plexus then supplies autonomic innervation of the bladder, rectum, uterus, and ureter. Complete disruption of the hypogastric nerve will lead to a hypertonic, noncontractile bladder and the necessity for self-catheterization to eliminate urine. Preservation of this nerve during radical hysterectomy or endometriosis resection is a high priority.
Laparoscopic uterosacral nerve ablation procedures divide the uterosacral ligament medial and caudad to the ureter and do not disrupt the main hypogastric nerve. Only the medial branches to the uterus are affected. Successful uterosacral nerve ablation has been reported in approximately 44% of women who have dysmenorrhea without visible endometriosis and approximately 62% of women who have visible endometriosis.7,8 The efficacy of this procedure is controversial, however. Removal of the superior hypogastric plexus (presacral neurectomy) has not proved to be more effective in controlling pelvic pain than conservative surgery that only destroys endometrial implants. Presacral neurectomy is no longer advised.9
The accomplished gynecologic surgeon must know the anatomy of the retroperitoneal space in order to avoid damage to normal structures, as well as remove pathology. Many disease processes involve the pelvic peritoneum, uterosacral ligaments, rectosigmoid or ovarian pedicles, and require the surgeon to enter the retroperitoneal space to identify the ureters and blood vessels and keep them out of harm’s way. The challenges are complex:
- Badly distorted anatomy and the anterior and posterior cul-de-sac necessitate mobilization of the rectosigmoid and bladder.
- Intraligamentous fibroids require knowledge of the blood supply in the retroperitoneal space. Malignant disorders mandate that the lymph nodes be dissected to determine extent of disease and as part of treatment.
Is training adequate?
Every training program should teach the surgical anatomy of the retroperitoneal space, since every surgeon needs to be comfortable exposing the anatomy, both to prevent injury and to accomplish the needed surgery. Videotapes and cadaver courses can prepare the resident for the operating room.
The “landmark” umbilical ligament
The umbilical ligament was the umbilical artery in fetal life and courses along the edge of the bladder to the anterior abdominal wall up to the umbilicus. It is a useful guide into the perivesicle space. Lateral to it are the iliac vessels, and medial is the bladder. It is also a good marker for finding the right spot to open the round ligament.
FIGURE 1 Opening the round ligament over the umbilical ligament
The round ligament is the key to exposing the retroperitoneal space. It should be open at the pelvic sidewall, just medial to the external iliac vessels. The umbilical ligament can be used as a landmark.
FIGURE 2 Opening lateral to the ovarian vessels
The divided round ligament is retracted ventrally and medially to place the ovarian vessels under traction. The peritoneum lateral to the ovarian vessels is divided up to the pelvic brim.
FIGURE 3 Right pelvic sidewall anatomy
Medial traction of the peritoneum around the ovarian vessels at the pelvic brim will expose the ureter coming over the iliac vessels at their bifurcation into external and internal branches.
FIGURE 4 Relationship of ureter to the umbilical ligament
The ureter comes off medial with the fold of peritoneum. Once the space is developed, the operator’s finger or the laparoscopic probe can be introduced along the medial side of the internal iliac artery and ventral to the curve of the sacrum. This will open the pararectal space. The ureter and the anterior branch of the internal iliac artery are nearly parallel as they course through the pelvis.
FIGURE 5 Hypogastric nerve
The anterior branch of the internal iliac artery gives off the uterine artery and the middle and superior vesicle arteries before continuing on as the umbilical ligament.
Endometriosis may imperil the ureter
Endometriomas and peritoneal implants are among the most common reasons for accessing the retroperitoneal space. Frequently the peritoneum between the ovarian vessels and the uterosacral ligaments (ovarian fossa) is thickened and retracted within the endometriosis implants. This alters the pelvic anatomy and puts the ureter at risk for injury.
Definitive surgical treatment for endometriosis includes removal of this diseased peritoneum. The ureter is best identified using the technique described, and then dissected off the peritoneum down to the uterine artery.
FIGURE 6 Finding the ureter lateral to an endometrioma
FIGURE 7 Relationship of ureter to the umbilical ligament
FIGURE 8 Dissecting the ureter out of the uterosacral ligament
The ureter may be placed on a Penrose drain to better isolate it and keep it under direct visualization. The cul-de-sac of Douglas is another site of endometrial implants. The fundus of the uterus is often adhesed to the rectosigmoid reflection and even the sigmoid colon. Nodules of endometriosis may infiltrate the uterosacral ligaments and extend into the rectovaginal space. To manage these implants, isolate the ureter to the point where it passes under the uterine artery.
FIGURE 9 Divide uterine artery lateral to endometrioma
Here, the uterine artery is taken lateral to the mass, enabling the surgeon to remove all of the uterosacral implants. The bladder flap will be developed and the bladder advanced caudad. By dissecting medial to the ureter as it courses under the uterine artery, it will be retracted laterally and provide the space necessary to resect the uterosacral implants.
FIGURE 10 Rectosigmoid reflection with endometrioma nodule
FIGURE 11 Postoperative appearance of endometriosis resection
As dissection proceeds medially, use the perirectal fatty plane to remove implants between the uterosacral area and rectum. In the midline, remove implants below the rectosigmoid peritoneal reflexion, taking care not to injure the rectum.
Implants above the peritoneal reflexion are often attached to the sigmoid tinea coli and cannot be removed without taking a portion of the bowel. Thus, patients with extensive endometriosis should have a bowel prep with two 10-ounce bottles of magnesium citrate the day before surgery.
If bowel resection is necessary, consult a general surgeon or gynecologic oncologist. A history of painful defecation or a finding of nodules in the cul-de-sac with rectal dimpling warrants preoperative consultation with a specialist skilled at bowel resection.
Ureteral injuries occur in 0.5% to 2.5% of women undergoing gynecologic surgery.1 The most common predisposing condition is previous pelvic surgery.2,3 The usual sites of injury are at the pelvic rim close to the ovarian vessels, at the level of the uterine artery, and lateral to the vaginal cuff. Neither preoperative excretory urograms nor placement of ureteral catheters preoperatively have been found to be effective prevention measures.2,4-6 Using the steps described above, the ureter can be identified and mobilized.
Blood supply to the ureter comes from the plexus of vessels that form a network along the length of the ureter. This plexus is fed by arteries from the renal pelvis, common iliac, internal iliac, uterine artery, and the base of the bladder. Complete mobilization of the ureter away from its peritoneal attachments and these lateral blood supply sources can be accomplished as long as the vascular plexus is not disrupted by cautery, crushing, or tearing. A clean transection can be re-anastomosed or reimplanted with the expectation of normal healing. Innervation of the ureter is from the inferior mesenteric plexus superiorly and the inferior hypogastric plexus in the pelvis. Ureteral peristalsis will continue, even if the ureter is completely divided or ligated.
Protecting pelvic blood vessels
The majority of gynecologic operations involve the ovarian vessels and the uterine vessels. The operator rarely needs to explore the lateral pelvis to identify the rest of the vessels. When faced with a large endometrioma or cancer, knowledge of the anatomy of the lateral blood vessels is vital.
Since the pathology is usually deeper in the pelvis, it is wise to identify the anatomy of the pelvis starting at the pelvic brim, where the common iliac bifurcates into the external and internal iliacs. There is a safe dissection plane medial to the internal iliac artery all the way to the uterine artery. This exposes the pararectal space, which can be opened without risk of major bleeding (FIGURE 5).
The obliterated umbilical vessel is the other friendly marker just distal to the uterine artery. It can be placed on traction and the uterine artery isolated. The inferior and superior vesical arteries are generally not dissected, as they are adjacent to the bladder and the surgery takes place medial to them in the prevesical fascial space.
Hypogastric artery ligations are rarely performed today, as interventional radiology is the standard of care for patients with postoperative and postpartum bleeding. When it becomes necessary, the hypogastric artery can be isolated and tied using the right-angle clamp to pass the tie. The superior gluteal artery branches so close to the bifurcation of the common iliac artery that it is not visualized. The inferior gluteal artery is the largest distal branch, which might be visualized during the ligation. It is not necessary to identify it.
FIGURE 12 Right genitofemoral nerve
The genitofemoral nerve runs along the medial aspect of the body of the psoas muscle. It is sometimes injured by the self-retaining retractors placed at the time of laparotomy. This leads to some numbness and burning of the skin of the anterior thigh.
FIGURE 13 Right pelvic sidewall anatomy
The obturator nerve is in the obturator space and typically far lateral to the usual dissection. Metastatic cancer to the obturator lymph nodes may entrap it, or it may be injured during a node dissection, causing loss of internal rotation of the anterior thigh.
The sciatic nerve is seen only during exenterative surgery. Pressure on the lateral pelvis by advanced pelvic tumors can lead to sciatic pain and motor weakness—even loss of motor function to the lower leg, which commonly leads to foot drop.
FIGURE 14 Superior hypogastric nerve bundle
The hypogastric plexus of nerves is sometimes damaged during surgery for endometriosis or for malignancy. The superior hypergastric plexus can be identified between the 2 common iliac arteries at the sacral promontory. The left common iliac vein runs underneath it.
FIGURE 15 Hypogastric nerve descending into the right pelvis
The right and left hypogastric nerves leave the hypogastric plexus and descend into the pelvis parallel to the ureter and 2 cm medial. It passes dorsal to the ureter as it goes through the cardinal ligament (FIGURE 5).
This plexus then supplies autonomic innervation of the bladder, rectum, uterus, and ureter. Complete disruption of the hypogastric nerve will lead to a hypertonic, noncontractile bladder and the necessity for self-catheterization to eliminate urine. Preservation of this nerve during radical hysterectomy or endometriosis resection is a high priority.
Laparoscopic uterosacral nerve ablation procedures divide the uterosacral ligament medial and caudad to the ureter and do not disrupt the main hypogastric nerve. Only the medial branches to the uterus are affected. Successful uterosacral nerve ablation has been reported in approximately 44% of women who have dysmenorrhea without visible endometriosis and approximately 62% of women who have visible endometriosis.7,8 The efficacy of this procedure is controversial, however. Removal of the superior hypogastric plexus (presacral neurectomy) has not proved to be more effective in controlling pelvic pain than conservative surgery that only destroys endometrial implants. Presacral neurectomy is no longer advised.9
1. American College of Obstetricians and Gynecologists. Educational Bulletin #238: Lower Urinary Tract Operative Injuries. Washington, DC: ACOG; 1985:1.
2. Daly JW, Higgins KA. Injury to the ureter during gynecologic surgical procedures. Surg Gynecol Obstet. 1988;167:19-22.
3. Selzman AA, Spirnak JP. Iatrogenic ureteral injuries: a 20-year experience in treating 165 injuries. J Urol. 1996;155:878-881.
4. Symmonds RE. Ureteral injuries associated with gynecologic surgery: prevention and management. Clin Obstet Gynecol. 19676;19:623-644.
5. Higgins CC. Ureteral injuries during surgery: a review of 87 cases. JAMA 1967;199:82-88.
6. Fry DE, Milholen L, Harbrecht PJ. Iatrogenic ureteral injury. Options in management. Arch Surg. 1983;118:454-457.
7. Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. J Reprod Med. 1987;32:37-41.
8. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696.-
9. Candiani GB, Fedele L, Vercellini P, Bianchi S, Di Nola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
Dr. Hatch receives research/grant support from Merck; is a consultant to Ethicon, Merck, and Quest Laboratory; and is a speaker for Cytyc, Digene, Ethicon, and Merck.
1. American College of Obstetricians and Gynecologists. Educational Bulletin #238: Lower Urinary Tract Operative Injuries. Washington, DC: ACOG; 1985:1.
2. Daly JW, Higgins KA. Injury to the ureter during gynecologic surgical procedures. Surg Gynecol Obstet. 1988;167:19-22.
3. Selzman AA, Spirnak JP. Iatrogenic ureteral injuries: a 20-year experience in treating 165 injuries. J Urol. 1996;155:878-881.
4. Symmonds RE. Ureteral injuries associated with gynecologic surgery: prevention and management. Clin Obstet Gynecol. 19676;19:623-644.
5. Higgins CC. Ureteral injuries during surgery: a review of 87 cases. JAMA 1967;199:82-88.
6. Fry DE, Milholen L, Harbrecht PJ. Iatrogenic ureteral injury. Options in management. Arch Surg. 1983;118:454-457.
7. Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. J Reprod Med. 1987;32:37-41.
8. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696.-
9. Candiani GB, Fedele L, Vercellini P, Bianchi S, Di Nola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
Dr. Hatch receives research/grant support from Merck; is a consultant to Ethicon, Merck, and Quest Laboratory; and is a speaker for Cytyc, Digene, Ethicon, and Merck.
Things go better with Burch
- Moderator Neeraj Kohli, MD, MBA OBG Management Board of Editors Director, Division of Urogynecology, Brigham and Women’s Hospital, and Assistant Professor, Harvard Medical School, Boston.
- Lead investigator of the CARE trial Linda Brubaker, MD, MS, Professor and Director, Division of Female Pelvic Medicine and Reconstructive Pelvic Surgery, Departments of Obstetrics and Gynecology and Urology, Loyola University Chicago.
- Mark D. Walters, MD, Head, Section of General Gynecology, Urogynecology, and Pelvic Reconstructive Surgery, Department of Obstetrics and Gynecology, Cleveland Clinic, Cleveland, Ohio.
- Anne M. Weber, MD, MS, Program Officer, Pelvic Floor Disorders Network, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md. Dr. Weber is an investigator in the CARE Trial.
Why should we care about the CARE (Colpopexy and Urinary Reduction Efforts) trial?
Because pelvic organ prolapse and urinary incontinence are already major problems facing women as they age, and will become even more pervasive as the baby boomer generation moves through menopause and beyond.
Because the risk that a woman will experience stress incontinence after prolapse surgery ranges from 8% to 60%.1-6
Because roughly one third of women who undergo prolapse or incontinence surgery require a second operation.
These are just a few of the factors that spurred the Pelvic Floor Disorders Network to undertake the CARE trial, published April 13 in the New England Journal of Medicine. OBG Management convened a panel of experts in female pelvic medicine, including 2 CARE trial investigators, to discuss the findings of this landmark study, its long-term implications, and the future of research into pelvic floor disorders.
How the trial was conducted
The CARE trial involved 322 women who required surgery to correct pelvic organ prolapse (POP) but lacked symptoms of stress urinary incontinence. All these women underwent sacrocolpopexy, an abdominal procedure in which graft material is attached between the vagina and sacrum to support the vagina and correct the prolapse. These women were randomized to undergo Burch colposuspension at the time of the sacrocolpopexy, or to undergo sacrocolpopexy only. The Burch procedure is performed through the same incision as the sacrocolpopexy and involves suturing the periurethral vaginal tissue to the iliopectineal ligaments on each side, providing urethral support.
Enrollment in the trial was halted after the first of 2 planned interim analyses because the frequency of postoperative stress incontinence was significantly lower in the group undergoing Burch colposuspension: 23.8% and 44.1% of women in the Burch and no-Burch groups, respectively, experienced stress symptoms by 3 months after the surgery.
Why the CARE trial is an epochal event
- First randomized trial of preventive incontinence surgery in women with prolapse
- Randomized design establishes cause and effect
- Subjects will be followed for 2 years
BRUBAKER: First, it is a well-designed, randomized, controlled trial and thus provides the highest level of evidence for clinical practice. Although there is no perfect study, this one minimized the risk of bias by involving multiple centers (7) and using multiple surgeons, making the findings more generalizable than would be the case in a single-surgeon case series.
In addition, the use of blinded urodynamic testing lent strength, because the ability of urodynamic testing to predict the need for a concomitant continence procedure was not known before the trial. Our follow-up manuscript, containing data presented at the recent Society of Gynecologic Surgeons meeting, will provide more details on this aspect of the trial.
WEBER: Randomized trials are held in such high esteem—provided all other aspects of study design and implementation are performed properly—because they support conclusions of cause and effect. The conclusion that Burch colposuspension prevents stress incontinence when performed at the time of abdominal sacrocolpopexy could only be drawn from a randomized trial.
Trial design standardized key elements
Many types of bias confound the results of nonrandomized studies, particularly selection bias (eg, when surgeons select which procedure to perform on the basis of patient characteristics), and valid conclusions of cause and effect cannot be drawn. However, with a randomized trial, subjects are separated into groups by chance and no other factor. Thus, the groups are equivalent at baseline—provided the sample size is large enough (and allowing for random differences)—and therefore any changes measured after the experimental intervention can be confidently attributed to the intervention itself.
Another strength of the trial is standardization. The subjects were “standardized” by rather broad inclusion and exclusion criteria to constitute an important clinical population and to ensure they were sufficiently similar so that the treatment (abdominal sacrocolpopexy) was appropriate for all. In addition, surgeons at the multiple participating sites agreed to standardization of the technical details of the Burch colposuspension so that the subjects received the same intervention regardless of site. And data collection in follow-up was performed in a standard way by research staff who were blinded to the subjects’ group assignment (intervention versus control), so the data were as free of bias as possible.
Homogeneous study population may be a weakness
KOHLI: I agree that the methodology of this well-designed study is its major strength. What are its weaknesses?
WEBER: No doubt there are several, only some of which may be apparent at this time. For example, most women in the study were Caucasian, and very few were Hispanic, Asian, or black. Although we have no scientific reason to believe that Burch colposuspension has different responses in women of different racial and ethnic backgrounds, the trial’s subjects are not diverse enough to analyze the data by subgroups to confirm or refute the hypothesis that response to the Burch procedure is independent of race or ethnicity.
BRUBAKER: Another weakness: Because this study was closed after the first interim analysis, some of our secondary analyses will be underpowered, although we clearly demonstrated a difference in our primary endpoint.
It is important to remember that this study is not “finished.” Our participants are still in active follow-up for 2 years following surgery. It will be interesting to see what happens during the longer follow-up, especially with regard to prolapse and incontinence. We are also doing additional in-depth analyses of urodynamic and other parameters.
KOHLI: Again, I think the study design and analysis were well thought out. It would have been interesting to see how the results broke down according to site, to see if there was variation—which could indicate variation in surgical technique.
BRUBAKER: We have not done this analysis and do not plan to at this time.
Why paravaginal repairs were allowed
KOHLI: What about the decision to include surgeries that involved paravaginal repair?
WEBER: That generated a fair amount of discussion during trial design, as there was no clear “right” answer. Perhaps it would have been “cleaner” to eliminate the option of performing paravaginal repair, but when the trial was designed, we lacked unequivocal evidence that paravaginal repair at the time of abdominal sacrocolpopexy provides additional support for the anterior vagina. Therefore, we decided to allow the decision to be based on surgeon judgment.
Some surgeons perform paravaginal repair with abdominal sacrocolpopexy in almost all women because they believe quite strongly that this reduces the risk of recurrent anterior vaginal prolapse. Others never perform paravaginal repair with abdominal sacrocolpopexy and feel just as strongly that their patients are adequately treated and protected from subsequent anterior vaginal prolapse.
Investigators feared paravaginal repairs could dilute Burch effects
Study surgeons did agree that paravaginal repair reduces the likelihood of postoperative stress incontinence, although not as effectively as Burch colposuspension. Thus, our dilemma: If paravaginal repairs were performed in a large number of subjects, thereby improving their postoperative continence status regardless of whether Burch was performed, the effect of Burch could be so diluted as to be lost. On the other hand, if paravaginal repairs were completely excluded, that would restrict some surgeons’ practices and potentially reduce the number of women who would be offered participation in the study if their surgeons felt their anterior vaginal prolapse would be potentially undertreated.
We resolved the dilemma as follows:
- A relatively low proportion—about one quarter—of surgeons performed paravaginal repairs regularly with abdominal sacrocolpopexy, so the potential impact in the trial would not be great.
- Paravaginal repairs were allowed, but only when declared necessary by the surgeon before randomization; this step prevented surgeons from changing their minds about the necessity of paravaginal repair if the subject was assigned to the Burch group (ie, the woman would be receiving additional anterior vaginal support by way of the Burch).
- We stratified for paravaginal repair in the randomization, so women with paravaginal repair were equally distributed between the intervention and control groups.
Are subjective or objective measures better?
- Subjective measures convey a patient’s foremost concerns and how she is doing clinically
- Correlating symptoms with objective measures yields valuable insights into treatment
BRUBAKER: I prefer subjective measures because I think they reflect what is most important to patients in quality-of-life disorders. However, I believe we need to understand the relationship between subjective outcomes and traditional “objective” outcomes.
WEBER: I think the research community is reaching a consensus that “subjective” measures—better described as patient-oriented outcomes—are more important than objective measures, particularly for conditions that affect patients in “subjective” ways, ie, ways that affect their health-related quality of life, rather than quantity of life. This does not mean that objective measures are useless—although we should first evaluate each measure critically to make that determination on the basis of evidence.
Nevertheless, when a patient seeks and receives treatment based on symptoms and how those symptoms impact her daily life, I think it is incumbent upon researchers and clinicians to ensure that the treatment that is considered most effective actually results in a change that the patient finds worthwhile.
What is “success”?
WALTERS: When it comes to incontinence, for which there is an imperfect correlation between various objective and subjective measures, I think both types of measures are valuable and important. Gathering several different types of outcomes for each patient helps us better understand the nuances of how well an intervention works.
I can understand why some clinicians and researchers place greater reliance on subjective measures of incontinence, such as a diary of incontinence episodes and quality-of-life measures, because these measures tell us exactly how the patient is doing clinically and how she feels about the intervention. If she reports that she is completely cured and “perfect,” then objective measures are irrelevant. However, for any subjective outcome short of perfect, correlation with the objective measures such as cough stress test, physical examination, and urodynamic tests can help investigators understand the reason for the imperfect outcome and point to areas of possible improvement.
KOHLI: In my practice, some women who continue to leak slightly after an incontinence procedure consider their surgery a complete success, whereas, as a surgeon, I consider it a suboptimal result. Both objective and subjective results are important.
Putting the CARE trial into practice
- Data relate directly only to women undergoing abdominal sacrocolpopexy
- Patient education, medicolegal, and reimbursement may also relate
- Results reflect the high prevalence of pelvic floor disorders and the need to routinely ask about them
BRUBAKER: I routinely counsel patients who are planning sacrocolpopexy but who do not have stress incontinence to consider a concomitant Burch procedure. I do not have them undergo urodynamic testing because, at this time, the results of that testing would not change my clinical practice.
WALTERS: I have always been liberal when it comes to adding retropubic colposuspension to abdominal sacrocolpopexy, even in women who do not have preoperative stress incontinence. The reason? Patients who are continent preoperatively, but become stress-incontinent postoperatively, are particularly unhappy with their outcome, especially if they need another surgery within a year to treat the stress incontinence. So this study verified what I was already doing.
What I didn’t learn is whether a paravaginal defect repair helps or hurts the Burch procedure from an anatomic and functional perspective.
It also appears that preoperative urodynamic testing has little value, although that was not the point of this study. I am glad it will be addressed in future studies.
KOHLI: I think the findings apply to those select patients undergoing abdominal sacrocolpopexy for prolapse. It would be dangerous to extrapolate these results to other abdominal vault suspension procedures or vaginal prolapse procedures. Based on the CARE trial, I plan to counsel patients about the risks and benefits of “optional” Burch colposuspension at the time of planned sacrocolpopexy. In reality, however, I have almost completely switched to minimally invasive midurethral slings, even in the case of abdominal prolapse procedures, because of their high cure rates, low complication rates, and ease of postoperative adjustment.
Clinical implications depend on surgeon’s routine
KOHLI: What are the implications for the majority of ObGyns?
WEBER: It depends on what ObGyns are doing for women with prolapse.
For ObGyns who are confident and competent, through training and experience, to perform abdominal sacrocolpopexy for women with advanced prolapse, the CARE trial results have a direct effect. Women with prolapse who are stress continent with no contraindications, can be reassured that they will benefit from a 50% reduction of postoperative stress incontinence with the Burch procedure.
For ObGyns who do not perform sacrocolpopexy, the CARE trial will have no direct clinical effects. Nevertheless, these clinicians need to be aware of the findings so they can discuss the options with patients before decisions on route or type of prolapse surgery are made.
The CARE trial and its results remind us of the high prevalence of pelvic floor disorders in women, potentially even after corrective surgery—and the need to actively screen all women for pelvic dysfunction.
Warn of potential incontinence even with the Burch
KOHLI: How does this study affect counseling of candidates for prolapse surgery?
BRUBAKER: I would offer stress-continent women a Burch procedure at the time of sacrocolpopexy. That much is clear. The interesting discussions come from “similar” clinical scenarios, where data are not yet available. For example, should a stress-continent woman facing a suspension via the vaginal route undergo a concomitant continence procedure?
WEBER: It is important to keep in mind that even when Burch colposuspension was performed, a number of women still experienced urinary incontinence (some stress, some urge, some mixed) after surgery; and the vast majority of women have urinary symptoms of some kind both before and after surgery. So preoperative counseling should include the information that urinary symptoms are very common after abdominal sacrocolpopexy—some as persistent or recurrent, and some as new symptoms.
As longer follow-up data from the CARE trial become available, we will learn how many women have urinary symptoms that are temporary versus long-lasting.
Is routine Burch best?
- When not all women benefit, should a procedure be offered prophylactically? In this case, experts say, “Yes”
- Some physicians favor other incontinence procedures
- Final decision rests with the patient
WALTERS: It is an easy decision for me. As I said earlier, women are particularly unhappy if they go from continent to incontinent after a surgery. In fact, some women are more displeased with that outcome than with a failure of the prolapse surgery. Because most women with prolapse have substantial anterior vaginal wall prolapse, the Burch procedure—with or without a paravaginal defect repair—also serves as part of the prolapse repair of the anterior wall. And now we know it also improves postoperative urinary function.
BRUBAKER: Doing a Burch procedure at the time of sacrocolpopexy is a time-efficient and low-morbidity addition, so it is worthwhile for me and my patients. It is clearly not the same as doing a secondary, standalone procedure for new symptoms.
Over the next 2 years, we will see how many women who were moderately or greatly bothered by stress incontinence went on to a surgical treatment.
WEBER: Please note a careful distinction: I would be willing to recommend Burch colposuspension to 100 stress-continent women who are planning to undergo abdominal sacrocolpopexy for prolapse, with the expectation that this will prevent postoperative stress incontinence in roughly half the women who would have developed it otherwise. It is up to the patient to accept this recommendation or not.
Based on the CARE trial results, 44 of 100 previously continent women after only abdominal sacrocolpopexy develop postoperative stress incontinence, compared with about 24 of 100 women after Burch and abdominal sacrocolpopexy. Even more striking is the difference in women affected by bothersome stress incontinence: almost 25% in the control group versus 6% in the Burch group. Women in the Burch group did not experience an excess of adverse events, or a clinically significant difference in operative time or estimated blood loss, compared with women in the control group.
Is “wait and see” better?
KOHLI: Because I favor minimally invasive midurethral sling procedures, which can often be performed on an outpatient basis under local anesthesia, I counsel women undergoing prolapse surgery via an abdominal or vaginal route that it is best to treat the incontinence postoperatively if it occurs. Obviously, this applies to women who have no incontinence and do not demonstrate potential stress incontinence on urodynamic testing preoperatively.
Anecdotally, I have not found a high rate of new-onset urinary incontinence following prolapse procedures. We may retrospectively look at these patients more critically in light of this new data.
WALTERS: I think most women would be dissatisfied with a 44% risk of postoperative stress incontinence requiring a second surgery. Even if you counseled them appropriately, many women would ask that you try to manage everything at the first surgery.
What are nonclinical effects of the trial?
- Need for patient and physician education is great, and the CARE trial offers a valuable opportunity
- Potential for medicolegal risk if complications develop
- Payers may not be willing to reimburse for a prophylactic procedure
WEBER: Given how extensively the trial’s results were disseminated by the lay press, I think we have an important opportunity to educate both patients and health-care providers.
First, patients: For women who may be directly affected by the trial, knowledgeable clinicians should explain its results and limitations to help them reach a decision about their treatment.
For women who hear the trial’s results described incompletely or incorrectly (eg, “…2 stitches prevent incontinence…”), clinicians should take this opportunity to correct misunderstandings and educate women about incontinence, prolapse, and pelvic health in general.
For health-care providers, this trial reminds us of the extraordinarily high prevalence of pelvic floor disorders. Although current treatments are not perfect, virtually all women with pelvic floor disorders can be treated to substantially alleviate, if not eliminate, bothersome symptoms.
All clinicians should routinely inquire about pelvic symptoms and be prepared to initiate an evaluation or provide a referral.
Medicolegal fallout?
KOHLI: There is the question of medicolegal risk if complications occur after colposuspension when the patient had no complaints or evidence of stress incontinence at the time of preoperative urodynamic testing. I am not aware of any legal precedent in which a clinical study or data provided solid protection from a jury verdict. The study did not show increased risk or complication with the addition of the Burch procedure, but that may not be true for some surgeons and some patients.
Billing and coding
In terms of billing, how should we code for the Burch colposuspension when the patient had no demonstrable stress incontinence? Payment denials in this scenario seem likely. This may create a line of separation between what may be clinically indicated for the patient and what insurance companies are willing to pay for.
There may be the option to use the urethral hypermobility code (ICD 599.81) for the Burch colposuspension, but only time will tell if this will be reimbursed. I would be curious to hear the panel’s experience with reimbursement for a prophylactic procedure based on scientific data. Obviously, what is best for the patient is most important.
BRUBAKER: All these patients had urethral hypermobility, which is also an indication for a Burch colposuspension.
Is preoperative urodynamic testing useful?
- CARE trial data still to come
- Basic testing is probably helpful
WEBER: In the CARE trial, the relative level of protection from postoperative stress incontinence provided by the Burch procedure did not depend on the stress-test component (with prolapse reduced) of preoperative urodynamic testing. About 50% fewer women had postoperative stress incontinence after Burch, whether preoperative urodynamic testing showed positive or negative stress tests with prolapse reduction.
Although subsequent analyses (presented at the Society of Gynecologic Surgeons 2006 meeting; manuscript under review) focused on urodynamic testing and postoperative outcomes, the CARE trial was not designed primarily to determine whether women planning prolapse surgery benefit from preoperative urodynamic testing. Therefore, conclusions about the “need” for urodynamic testing should not be based only on the CARE trial.
Should urodynamic testing determine treatment?
Randomized trials that directly address the cost-benefit of urodynamic testing are urgently needed. For now, as is standard in good clinical practice, a test should be performed only if results will change recommendations or provide reliable and clinically important prognostic information about a patient’s outcome after intervention. Clinicians should determine whether urodynamic testing meets even 1 of these 2 minimum criteria.
I want to point out that the CARE trial did not address the utility of urodynamic testing.
BRUBAKER: It is clear that some women have stress incontinence despite the concomitant Burch colposuspension. If we learn that an alternative operation can perform better and that any urodynamic (or other clinical measure) can predict improved outcomes, I would consider resuming urodynamic testing.
WALTERS: At first glance, it appears that complex urodynamic testing is definitely not necessary if the goal is to improve outcomes of surgery. However, I believe the patient should undergo some components of urodynamic testing such as a void with a post-void residual urine volume and a basic bladder-filling study noting sensation and capacity. I also do a cough stress test with the prolapse reduced, although we may find that this does not predict postoperative function.
KOHLI: The results of this study are very procedure-specific. If similar results are borne out when other approaches to prolapse and incontinence are analyzed, the value and utility of preoperative uro-dynamic testing in all patients may be questionable.
However, in my practice, I use the results of preoperative urodynamic testing not only for diagnosis, but also to make subtle adjustments when performing incontinence procedures—especially in regard to suburethral slings.
What if you prefer midurethral slings?
- Surgeons should be comfortable with more than 1 incontinence procedure
- We should not jump to untested conclusions
WEBER: Ideally, well trained and experienced gynecologic, urologic, or urogynecologic surgeons perform more than 1 type of incontinence procedure, to meet the needs of different patients.
As yet, we have no direct, evidence-based answers to issues such as these:
Can a midurethral sling be substituted for a Burch colposuspension and have the same average results in preventing post-operative stress incontinence without increasing urgency symptoms…
- …when abdominal sacrocolpopexy is performed for prolapse in a preoperatively stress-continent patient?
- …when vaginal apical suspension is performed for prolapse in a preoperatively stress-continent patient?
Although it is tempting to jump 1 or 2 steps ahead and apply CARE trial data to situations that have not been tested directly, I would be cautious. We want to avoid creating long-lasting or refractory urgency symptoms—especially in a woman who had no such symptoms before surgery—because of a prophylactic procedure.
I think this is especially true because it is relatively easy to salvage patients who do develop bothersome stress incontinence after prolapse surgery.
Bonus: Burch helps anterior vaginal prolapse
WALTERS: I wonder whether prophylactic placement of a midurethral sling would yield the same results as a prophylactic Burch procedure. If your midurethral sling of choice is a tension-free vaginal tape (TVT), I would be cautious about placing it prophylactically, because the TVT has a 2% to 3% risk of prolonged voiding dysfunction requiring transection of the tape.
However, it is possible that prophylactic placement of a transobturator sling, which is associated with much less voiding dysfunction and fewer major surgical complications, might have a different outcome—though this requires further study.
In addition, midurethral slings would not be as effective as Burch colposuspension in treating anterior vaginal prolapse, so I would expect to see more anterior wall prolapse failures if slings replaced colposuspension.
What if you prefer the vaginal approach?
- Further study is needed
WALTERS: I wonder whether a prophylactic transobturator midurethral sling at the time of transvaginal prolapse repair would yield similar results. I do cystocele repair with suburethral (“Kelly”) plication, which seems to work well at stabilizing the urethra in women without stress incontinence. But this approach is not as popular these days, and future studies may demonstrate that a prophylactic midurethral sling will result in better long-term function without significantly increasing the long-term risk.
WEBER: The CARE trial results are relevant to all pelvic surgeons because they demonstrate the need for and benefit from well-designed randomized surgical trials. Another benefit will be extended follow-up in what will become a prospective cohort study of women with advanced prolapse treated by abdominal sacrocolpopexy—providing higher-quality evidence than retrospective case series. Although not as valuable as randomized trials, these data can help guide clinical recommendations.
If long-term results support the effectiveness and durability of abdominal prolapse repair, then gynecologists can reflect on the evidence and choose the approach that best fits the patient’s needs.
Need for other studies?
- Randomized, multicenter trials addressing almost any surgical treatment of prolapse and incontinence are sorely needed
BRUBAKER: Any and all high-quality, well-designed trial can improve our care of women with incontinence and/or prolapse.
WALTERS: I look forward to the follow-up studies from the CARE trial on the value of paravaginal defect repair, preoperative urodynamic testing, and the efficacy of various prolapse-reduction maneuvers in predicting surgical outcomes.
It would also seem logical to repeat this type of study using transvaginal prolapse repair with or without a prophylactic midurethral sling. Another option: anterior colporrhaphy with suburethral plication versus a prophylactic midurethral sling.
KOHLI: I look forward to data on surgical procedures currently being performed with greater frequency despite a lack of good-quality data. These include the transobturator suburethral midurethral sling procedures, laparoscopic sacrocolpopexy, and vaginal mesh augmentation for prolapse.
The Pelvic Floor Disorders Network affords a unique opportunity to perform well-designed multicenter trials to address the rapidly changing landscape of surgical treatment for prolapse and incontinence.
1. Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
2. Cross CA, Cespedes RD, McGuire EJ. Treatment results using pubovaginal slings in patients with large cystoceles and stress incontinence. J Urol. 1997;158:431-434.
3. FitzGerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189:1241-1244.
4. Klutke JJ, Ramos S. Urodynamic outcome after surgery for severe prolapse and potential stress incontinence. Am J Obstet Gynecol. 2000;182:1378-1381.
5. Meschia M, Pifarotti P, Spennacchio M, Buonaguidi A, Gattei U, Somigliana E. A randomized comparison of tension-free vaginal tape and endopelvic fascia plication in women with genital prolapse and occult stress urinary incontinence. Am J Obstet Gynecol. 2004;190:609-613.
6. Gordon D, Gold RS, Pauzner D, Lessing JB, Groutz A. Combined genitourinary prolapse repair and prophylactic tension-free vaginal tape in women with severe prolapse and occult stress urinary incontinence: preliminary results. Urology. 2001;58:547-550.
Dr. Brubaker, Dr. Kohli, and Dr. Weber report no financial relationships relevant to this article. Dr. Walters is a speaker for American Medical Systems.
- Moderator Neeraj Kohli, MD, MBA OBG Management Board of Editors Director, Division of Urogynecology, Brigham and Women’s Hospital, and Assistant Professor, Harvard Medical School, Boston.
- Lead investigator of the CARE trial Linda Brubaker, MD, MS, Professor and Director, Division of Female Pelvic Medicine and Reconstructive Pelvic Surgery, Departments of Obstetrics and Gynecology and Urology, Loyola University Chicago.
- Mark D. Walters, MD, Head, Section of General Gynecology, Urogynecology, and Pelvic Reconstructive Surgery, Department of Obstetrics and Gynecology, Cleveland Clinic, Cleveland, Ohio.
- Anne M. Weber, MD, MS, Program Officer, Pelvic Floor Disorders Network, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md. Dr. Weber is an investigator in the CARE Trial.
Why should we care about the CARE (Colpopexy and Urinary Reduction Efforts) trial?
Because pelvic organ prolapse and urinary incontinence are already major problems facing women as they age, and will become even more pervasive as the baby boomer generation moves through menopause and beyond.
Because the risk that a woman will experience stress incontinence after prolapse surgery ranges from 8% to 60%.1-6
Because roughly one third of women who undergo prolapse or incontinence surgery require a second operation.
These are just a few of the factors that spurred the Pelvic Floor Disorders Network to undertake the CARE trial, published April 13 in the New England Journal of Medicine. OBG Management convened a panel of experts in female pelvic medicine, including 2 CARE trial investigators, to discuss the findings of this landmark study, its long-term implications, and the future of research into pelvic floor disorders.
How the trial was conducted
The CARE trial involved 322 women who required surgery to correct pelvic organ prolapse (POP) but lacked symptoms of stress urinary incontinence. All these women underwent sacrocolpopexy, an abdominal procedure in which graft material is attached between the vagina and sacrum to support the vagina and correct the prolapse. These women were randomized to undergo Burch colposuspension at the time of the sacrocolpopexy, or to undergo sacrocolpopexy only. The Burch procedure is performed through the same incision as the sacrocolpopexy and involves suturing the periurethral vaginal tissue to the iliopectineal ligaments on each side, providing urethral support.
Enrollment in the trial was halted after the first of 2 planned interim analyses because the frequency of postoperative stress incontinence was significantly lower in the group undergoing Burch colposuspension: 23.8% and 44.1% of women in the Burch and no-Burch groups, respectively, experienced stress symptoms by 3 months after the surgery.
Why the CARE trial is an epochal event
- First randomized trial of preventive incontinence surgery in women with prolapse
- Randomized design establishes cause and effect
- Subjects will be followed for 2 years
BRUBAKER: First, it is a well-designed, randomized, controlled trial and thus provides the highest level of evidence for clinical practice. Although there is no perfect study, this one minimized the risk of bias by involving multiple centers (7) and using multiple surgeons, making the findings more generalizable than would be the case in a single-surgeon case series.
In addition, the use of blinded urodynamic testing lent strength, because the ability of urodynamic testing to predict the need for a concomitant continence procedure was not known before the trial. Our follow-up manuscript, containing data presented at the recent Society of Gynecologic Surgeons meeting, will provide more details on this aspect of the trial.
WEBER: Randomized trials are held in such high esteem—provided all other aspects of study design and implementation are performed properly—because they support conclusions of cause and effect. The conclusion that Burch colposuspension prevents stress incontinence when performed at the time of abdominal sacrocolpopexy could only be drawn from a randomized trial.
Trial design standardized key elements
Many types of bias confound the results of nonrandomized studies, particularly selection bias (eg, when surgeons select which procedure to perform on the basis of patient characteristics), and valid conclusions of cause and effect cannot be drawn. However, with a randomized trial, subjects are separated into groups by chance and no other factor. Thus, the groups are equivalent at baseline—provided the sample size is large enough (and allowing for random differences)—and therefore any changes measured after the experimental intervention can be confidently attributed to the intervention itself.
Another strength of the trial is standardization. The subjects were “standardized” by rather broad inclusion and exclusion criteria to constitute an important clinical population and to ensure they were sufficiently similar so that the treatment (abdominal sacrocolpopexy) was appropriate for all. In addition, surgeons at the multiple participating sites agreed to standardization of the technical details of the Burch colposuspension so that the subjects received the same intervention regardless of site. And data collection in follow-up was performed in a standard way by research staff who were blinded to the subjects’ group assignment (intervention versus control), so the data were as free of bias as possible.
Homogeneous study population may be a weakness
KOHLI: I agree that the methodology of this well-designed study is its major strength. What are its weaknesses?
WEBER: No doubt there are several, only some of which may be apparent at this time. For example, most women in the study were Caucasian, and very few were Hispanic, Asian, or black. Although we have no scientific reason to believe that Burch colposuspension has different responses in women of different racial and ethnic backgrounds, the trial’s subjects are not diverse enough to analyze the data by subgroups to confirm or refute the hypothesis that response to the Burch procedure is independent of race or ethnicity.
BRUBAKER: Another weakness: Because this study was closed after the first interim analysis, some of our secondary analyses will be underpowered, although we clearly demonstrated a difference in our primary endpoint.
It is important to remember that this study is not “finished.” Our participants are still in active follow-up for 2 years following surgery. It will be interesting to see what happens during the longer follow-up, especially with regard to prolapse and incontinence. We are also doing additional in-depth analyses of urodynamic and other parameters.
KOHLI: Again, I think the study design and analysis were well thought out. It would have been interesting to see how the results broke down according to site, to see if there was variation—which could indicate variation in surgical technique.
BRUBAKER: We have not done this analysis and do not plan to at this time.
Why paravaginal repairs were allowed
KOHLI: What about the decision to include surgeries that involved paravaginal repair?
WEBER: That generated a fair amount of discussion during trial design, as there was no clear “right” answer. Perhaps it would have been “cleaner” to eliminate the option of performing paravaginal repair, but when the trial was designed, we lacked unequivocal evidence that paravaginal repair at the time of abdominal sacrocolpopexy provides additional support for the anterior vagina. Therefore, we decided to allow the decision to be based on surgeon judgment.
Some surgeons perform paravaginal repair with abdominal sacrocolpopexy in almost all women because they believe quite strongly that this reduces the risk of recurrent anterior vaginal prolapse. Others never perform paravaginal repair with abdominal sacrocolpopexy and feel just as strongly that their patients are adequately treated and protected from subsequent anterior vaginal prolapse.
Investigators feared paravaginal repairs could dilute Burch effects
Study surgeons did agree that paravaginal repair reduces the likelihood of postoperative stress incontinence, although not as effectively as Burch colposuspension. Thus, our dilemma: If paravaginal repairs were performed in a large number of subjects, thereby improving their postoperative continence status regardless of whether Burch was performed, the effect of Burch could be so diluted as to be lost. On the other hand, if paravaginal repairs were completely excluded, that would restrict some surgeons’ practices and potentially reduce the number of women who would be offered participation in the study if their surgeons felt their anterior vaginal prolapse would be potentially undertreated.
We resolved the dilemma as follows:
- A relatively low proportion—about one quarter—of surgeons performed paravaginal repairs regularly with abdominal sacrocolpopexy, so the potential impact in the trial would not be great.
- Paravaginal repairs were allowed, but only when declared necessary by the surgeon before randomization; this step prevented surgeons from changing their minds about the necessity of paravaginal repair if the subject was assigned to the Burch group (ie, the woman would be receiving additional anterior vaginal support by way of the Burch).
- We stratified for paravaginal repair in the randomization, so women with paravaginal repair were equally distributed between the intervention and control groups.
Are subjective or objective measures better?
- Subjective measures convey a patient’s foremost concerns and how she is doing clinically
- Correlating symptoms with objective measures yields valuable insights into treatment
BRUBAKER: I prefer subjective measures because I think they reflect what is most important to patients in quality-of-life disorders. However, I believe we need to understand the relationship between subjective outcomes and traditional “objective” outcomes.
WEBER: I think the research community is reaching a consensus that “subjective” measures—better described as patient-oriented outcomes—are more important than objective measures, particularly for conditions that affect patients in “subjective” ways, ie, ways that affect their health-related quality of life, rather than quantity of life. This does not mean that objective measures are useless—although we should first evaluate each measure critically to make that determination on the basis of evidence.
Nevertheless, when a patient seeks and receives treatment based on symptoms and how those symptoms impact her daily life, I think it is incumbent upon researchers and clinicians to ensure that the treatment that is considered most effective actually results in a change that the patient finds worthwhile.
What is “success”?
WALTERS: When it comes to incontinence, for which there is an imperfect correlation between various objective and subjective measures, I think both types of measures are valuable and important. Gathering several different types of outcomes for each patient helps us better understand the nuances of how well an intervention works.
I can understand why some clinicians and researchers place greater reliance on subjective measures of incontinence, such as a diary of incontinence episodes and quality-of-life measures, because these measures tell us exactly how the patient is doing clinically and how she feels about the intervention. If she reports that she is completely cured and “perfect,” then objective measures are irrelevant. However, for any subjective outcome short of perfect, correlation with the objective measures such as cough stress test, physical examination, and urodynamic tests can help investigators understand the reason for the imperfect outcome and point to areas of possible improvement.
KOHLI: In my practice, some women who continue to leak slightly after an incontinence procedure consider their surgery a complete success, whereas, as a surgeon, I consider it a suboptimal result. Both objective and subjective results are important.
Putting the CARE trial into practice
- Data relate directly only to women undergoing abdominal sacrocolpopexy
- Patient education, medicolegal, and reimbursement may also relate
- Results reflect the high prevalence of pelvic floor disorders and the need to routinely ask about them
BRUBAKER: I routinely counsel patients who are planning sacrocolpopexy but who do not have stress incontinence to consider a concomitant Burch procedure. I do not have them undergo urodynamic testing because, at this time, the results of that testing would not change my clinical practice.
WALTERS: I have always been liberal when it comes to adding retropubic colposuspension to abdominal sacrocolpopexy, even in women who do not have preoperative stress incontinence. The reason? Patients who are continent preoperatively, but become stress-incontinent postoperatively, are particularly unhappy with their outcome, especially if they need another surgery within a year to treat the stress incontinence. So this study verified what I was already doing.
What I didn’t learn is whether a paravaginal defect repair helps or hurts the Burch procedure from an anatomic and functional perspective.
It also appears that preoperative urodynamic testing has little value, although that was not the point of this study. I am glad it will be addressed in future studies.
KOHLI: I think the findings apply to those select patients undergoing abdominal sacrocolpopexy for prolapse. It would be dangerous to extrapolate these results to other abdominal vault suspension procedures or vaginal prolapse procedures. Based on the CARE trial, I plan to counsel patients about the risks and benefits of “optional” Burch colposuspension at the time of planned sacrocolpopexy. In reality, however, I have almost completely switched to minimally invasive midurethral slings, even in the case of abdominal prolapse procedures, because of their high cure rates, low complication rates, and ease of postoperative adjustment.
Clinical implications depend on surgeon’s routine
KOHLI: What are the implications for the majority of ObGyns?
WEBER: It depends on what ObGyns are doing for women with prolapse.
For ObGyns who are confident and competent, through training and experience, to perform abdominal sacrocolpopexy for women with advanced prolapse, the CARE trial results have a direct effect. Women with prolapse who are stress continent with no contraindications, can be reassured that they will benefit from a 50% reduction of postoperative stress incontinence with the Burch procedure.
For ObGyns who do not perform sacrocolpopexy, the CARE trial will have no direct clinical effects. Nevertheless, these clinicians need to be aware of the findings so they can discuss the options with patients before decisions on route or type of prolapse surgery are made.
The CARE trial and its results remind us of the high prevalence of pelvic floor disorders in women, potentially even after corrective surgery—and the need to actively screen all women for pelvic dysfunction.
Warn of potential incontinence even with the Burch
KOHLI: How does this study affect counseling of candidates for prolapse surgery?
BRUBAKER: I would offer stress-continent women a Burch procedure at the time of sacrocolpopexy. That much is clear. The interesting discussions come from “similar” clinical scenarios, where data are not yet available. For example, should a stress-continent woman facing a suspension via the vaginal route undergo a concomitant continence procedure?
WEBER: It is important to keep in mind that even when Burch colposuspension was performed, a number of women still experienced urinary incontinence (some stress, some urge, some mixed) after surgery; and the vast majority of women have urinary symptoms of some kind both before and after surgery. So preoperative counseling should include the information that urinary symptoms are very common after abdominal sacrocolpopexy—some as persistent or recurrent, and some as new symptoms.
As longer follow-up data from the CARE trial become available, we will learn how many women have urinary symptoms that are temporary versus long-lasting.
Is routine Burch best?
- When not all women benefit, should a procedure be offered prophylactically? In this case, experts say, “Yes”
- Some physicians favor other incontinence procedures
- Final decision rests with the patient
WALTERS: It is an easy decision for me. As I said earlier, women are particularly unhappy if they go from continent to incontinent after a surgery. In fact, some women are more displeased with that outcome than with a failure of the prolapse surgery. Because most women with prolapse have substantial anterior vaginal wall prolapse, the Burch procedure—with or without a paravaginal defect repair—also serves as part of the prolapse repair of the anterior wall. And now we know it also improves postoperative urinary function.
BRUBAKER: Doing a Burch procedure at the time of sacrocolpopexy is a time-efficient and low-morbidity addition, so it is worthwhile for me and my patients. It is clearly not the same as doing a secondary, standalone procedure for new symptoms.
Over the next 2 years, we will see how many women who were moderately or greatly bothered by stress incontinence went on to a surgical treatment.
WEBER: Please note a careful distinction: I would be willing to recommend Burch colposuspension to 100 stress-continent women who are planning to undergo abdominal sacrocolpopexy for prolapse, with the expectation that this will prevent postoperative stress incontinence in roughly half the women who would have developed it otherwise. It is up to the patient to accept this recommendation or not.
Based on the CARE trial results, 44 of 100 previously continent women after only abdominal sacrocolpopexy develop postoperative stress incontinence, compared with about 24 of 100 women after Burch and abdominal sacrocolpopexy. Even more striking is the difference in women affected by bothersome stress incontinence: almost 25% in the control group versus 6% in the Burch group. Women in the Burch group did not experience an excess of adverse events, or a clinically significant difference in operative time or estimated blood loss, compared with women in the control group.
Is “wait and see” better?
KOHLI: Because I favor minimally invasive midurethral sling procedures, which can often be performed on an outpatient basis under local anesthesia, I counsel women undergoing prolapse surgery via an abdominal or vaginal route that it is best to treat the incontinence postoperatively if it occurs. Obviously, this applies to women who have no incontinence and do not demonstrate potential stress incontinence on urodynamic testing preoperatively.
Anecdotally, I have not found a high rate of new-onset urinary incontinence following prolapse procedures. We may retrospectively look at these patients more critically in light of this new data.
WALTERS: I think most women would be dissatisfied with a 44% risk of postoperative stress incontinence requiring a second surgery. Even if you counseled them appropriately, many women would ask that you try to manage everything at the first surgery.
What are nonclinical effects of the trial?
- Need for patient and physician education is great, and the CARE trial offers a valuable opportunity
- Potential for medicolegal risk if complications develop
- Payers may not be willing to reimburse for a prophylactic procedure
WEBER: Given how extensively the trial’s results were disseminated by the lay press, I think we have an important opportunity to educate both patients and health-care providers.
First, patients: For women who may be directly affected by the trial, knowledgeable clinicians should explain its results and limitations to help them reach a decision about their treatment.
For women who hear the trial’s results described incompletely or incorrectly (eg, “…2 stitches prevent incontinence…”), clinicians should take this opportunity to correct misunderstandings and educate women about incontinence, prolapse, and pelvic health in general.
For health-care providers, this trial reminds us of the extraordinarily high prevalence of pelvic floor disorders. Although current treatments are not perfect, virtually all women with pelvic floor disorders can be treated to substantially alleviate, if not eliminate, bothersome symptoms.
All clinicians should routinely inquire about pelvic symptoms and be prepared to initiate an evaluation or provide a referral.
Medicolegal fallout?
KOHLI: There is the question of medicolegal risk if complications occur after colposuspension when the patient had no complaints or evidence of stress incontinence at the time of preoperative urodynamic testing. I am not aware of any legal precedent in which a clinical study or data provided solid protection from a jury verdict. The study did not show increased risk or complication with the addition of the Burch procedure, but that may not be true for some surgeons and some patients.
Billing and coding
In terms of billing, how should we code for the Burch colposuspension when the patient had no demonstrable stress incontinence? Payment denials in this scenario seem likely. This may create a line of separation between what may be clinically indicated for the patient and what insurance companies are willing to pay for.
There may be the option to use the urethral hypermobility code (ICD 599.81) for the Burch colposuspension, but only time will tell if this will be reimbursed. I would be curious to hear the panel’s experience with reimbursement for a prophylactic procedure based on scientific data. Obviously, what is best for the patient is most important.
BRUBAKER: All these patients had urethral hypermobility, which is also an indication for a Burch colposuspension.
Is preoperative urodynamic testing useful?
- CARE trial data still to come
- Basic testing is probably helpful
WEBER: In the CARE trial, the relative level of protection from postoperative stress incontinence provided by the Burch procedure did not depend on the stress-test component (with prolapse reduced) of preoperative urodynamic testing. About 50% fewer women had postoperative stress incontinence after Burch, whether preoperative urodynamic testing showed positive or negative stress tests with prolapse reduction.
Although subsequent analyses (presented at the Society of Gynecologic Surgeons 2006 meeting; manuscript under review) focused on urodynamic testing and postoperative outcomes, the CARE trial was not designed primarily to determine whether women planning prolapse surgery benefit from preoperative urodynamic testing. Therefore, conclusions about the “need” for urodynamic testing should not be based only on the CARE trial.
Should urodynamic testing determine treatment?
Randomized trials that directly address the cost-benefit of urodynamic testing are urgently needed. For now, as is standard in good clinical practice, a test should be performed only if results will change recommendations or provide reliable and clinically important prognostic information about a patient’s outcome after intervention. Clinicians should determine whether urodynamic testing meets even 1 of these 2 minimum criteria.
I want to point out that the CARE trial did not address the utility of urodynamic testing.
BRUBAKER: It is clear that some women have stress incontinence despite the concomitant Burch colposuspension. If we learn that an alternative operation can perform better and that any urodynamic (or other clinical measure) can predict improved outcomes, I would consider resuming urodynamic testing.
WALTERS: At first glance, it appears that complex urodynamic testing is definitely not necessary if the goal is to improve outcomes of surgery. However, I believe the patient should undergo some components of urodynamic testing such as a void with a post-void residual urine volume and a basic bladder-filling study noting sensation and capacity. I also do a cough stress test with the prolapse reduced, although we may find that this does not predict postoperative function.
KOHLI: The results of this study are very procedure-specific. If similar results are borne out when other approaches to prolapse and incontinence are analyzed, the value and utility of preoperative uro-dynamic testing in all patients may be questionable.
However, in my practice, I use the results of preoperative urodynamic testing not only for diagnosis, but also to make subtle adjustments when performing incontinence procedures—especially in regard to suburethral slings.
What if you prefer midurethral slings?
- Surgeons should be comfortable with more than 1 incontinence procedure
- We should not jump to untested conclusions
WEBER: Ideally, well trained and experienced gynecologic, urologic, or urogynecologic surgeons perform more than 1 type of incontinence procedure, to meet the needs of different patients.
As yet, we have no direct, evidence-based answers to issues such as these:
Can a midurethral sling be substituted for a Burch colposuspension and have the same average results in preventing post-operative stress incontinence without increasing urgency symptoms…
- …when abdominal sacrocolpopexy is performed for prolapse in a preoperatively stress-continent patient?
- …when vaginal apical suspension is performed for prolapse in a preoperatively stress-continent patient?
Although it is tempting to jump 1 or 2 steps ahead and apply CARE trial data to situations that have not been tested directly, I would be cautious. We want to avoid creating long-lasting or refractory urgency symptoms—especially in a woman who had no such symptoms before surgery—because of a prophylactic procedure.
I think this is especially true because it is relatively easy to salvage patients who do develop bothersome stress incontinence after prolapse surgery.
Bonus: Burch helps anterior vaginal prolapse
WALTERS: I wonder whether prophylactic placement of a midurethral sling would yield the same results as a prophylactic Burch procedure. If your midurethral sling of choice is a tension-free vaginal tape (TVT), I would be cautious about placing it prophylactically, because the TVT has a 2% to 3% risk of prolonged voiding dysfunction requiring transection of the tape.
However, it is possible that prophylactic placement of a transobturator sling, which is associated with much less voiding dysfunction and fewer major surgical complications, might have a different outcome—though this requires further study.
In addition, midurethral slings would not be as effective as Burch colposuspension in treating anterior vaginal prolapse, so I would expect to see more anterior wall prolapse failures if slings replaced colposuspension.
What if you prefer the vaginal approach?
- Further study is needed
WALTERS: I wonder whether a prophylactic transobturator midurethral sling at the time of transvaginal prolapse repair would yield similar results. I do cystocele repair with suburethral (“Kelly”) plication, which seems to work well at stabilizing the urethra in women without stress incontinence. But this approach is not as popular these days, and future studies may demonstrate that a prophylactic midurethral sling will result in better long-term function without significantly increasing the long-term risk.
WEBER: The CARE trial results are relevant to all pelvic surgeons because they demonstrate the need for and benefit from well-designed randomized surgical trials. Another benefit will be extended follow-up in what will become a prospective cohort study of women with advanced prolapse treated by abdominal sacrocolpopexy—providing higher-quality evidence than retrospective case series. Although not as valuable as randomized trials, these data can help guide clinical recommendations.
If long-term results support the effectiveness and durability of abdominal prolapse repair, then gynecologists can reflect on the evidence and choose the approach that best fits the patient’s needs.
Need for other studies?
- Randomized, multicenter trials addressing almost any surgical treatment of prolapse and incontinence are sorely needed
BRUBAKER: Any and all high-quality, well-designed trial can improve our care of women with incontinence and/or prolapse.
WALTERS: I look forward to the follow-up studies from the CARE trial on the value of paravaginal defect repair, preoperative urodynamic testing, and the efficacy of various prolapse-reduction maneuvers in predicting surgical outcomes.
It would also seem logical to repeat this type of study using transvaginal prolapse repair with or without a prophylactic midurethral sling. Another option: anterior colporrhaphy with suburethral plication versus a prophylactic midurethral sling.
KOHLI: I look forward to data on surgical procedures currently being performed with greater frequency despite a lack of good-quality data. These include the transobturator suburethral midurethral sling procedures, laparoscopic sacrocolpopexy, and vaginal mesh augmentation for prolapse.
The Pelvic Floor Disorders Network affords a unique opportunity to perform well-designed multicenter trials to address the rapidly changing landscape of surgical treatment for prolapse and incontinence.
- Moderator Neeraj Kohli, MD, MBA OBG Management Board of Editors Director, Division of Urogynecology, Brigham and Women’s Hospital, and Assistant Professor, Harvard Medical School, Boston.
- Lead investigator of the CARE trial Linda Brubaker, MD, MS, Professor and Director, Division of Female Pelvic Medicine and Reconstructive Pelvic Surgery, Departments of Obstetrics and Gynecology and Urology, Loyola University Chicago.
- Mark D. Walters, MD, Head, Section of General Gynecology, Urogynecology, and Pelvic Reconstructive Surgery, Department of Obstetrics and Gynecology, Cleveland Clinic, Cleveland, Ohio.
- Anne M. Weber, MD, MS, Program Officer, Pelvic Floor Disorders Network, National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Md. Dr. Weber is an investigator in the CARE Trial.
Why should we care about the CARE (Colpopexy and Urinary Reduction Efforts) trial?
Because pelvic organ prolapse and urinary incontinence are already major problems facing women as they age, and will become even more pervasive as the baby boomer generation moves through menopause and beyond.
Because the risk that a woman will experience stress incontinence after prolapse surgery ranges from 8% to 60%.1-6
Because roughly one third of women who undergo prolapse or incontinence surgery require a second operation.
These are just a few of the factors that spurred the Pelvic Floor Disorders Network to undertake the CARE trial, published April 13 in the New England Journal of Medicine. OBG Management convened a panel of experts in female pelvic medicine, including 2 CARE trial investigators, to discuss the findings of this landmark study, its long-term implications, and the future of research into pelvic floor disorders.
How the trial was conducted
The CARE trial involved 322 women who required surgery to correct pelvic organ prolapse (POP) but lacked symptoms of stress urinary incontinence. All these women underwent sacrocolpopexy, an abdominal procedure in which graft material is attached between the vagina and sacrum to support the vagina and correct the prolapse. These women were randomized to undergo Burch colposuspension at the time of the sacrocolpopexy, or to undergo sacrocolpopexy only. The Burch procedure is performed through the same incision as the sacrocolpopexy and involves suturing the periurethral vaginal tissue to the iliopectineal ligaments on each side, providing urethral support.
Enrollment in the trial was halted after the first of 2 planned interim analyses because the frequency of postoperative stress incontinence was significantly lower in the group undergoing Burch colposuspension: 23.8% and 44.1% of women in the Burch and no-Burch groups, respectively, experienced stress symptoms by 3 months after the surgery.
Why the CARE trial is an epochal event
- First randomized trial of preventive incontinence surgery in women with prolapse
- Randomized design establishes cause and effect
- Subjects will be followed for 2 years
BRUBAKER: First, it is a well-designed, randomized, controlled trial and thus provides the highest level of evidence for clinical practice. Although there is no perfect study, this one minimized the risk of bias by involving multiple centers (7) and using multiple surgeons, making the findings more generalizable than would be the case in a single-surgeon case series.
In addition, the use of blinded urodynamic testing lent strength, because the ability of urodynamic testing to predict the need for a concomitant continence procedure was not known before the trial. Our follow-up manuscript, containing data presented at the recent Society of Gynecologic Surgeons meeting, will provide more details on this aspect of the trial.
WEBER: Randomized trials are held in such high esteem—provided all other aspects of study design and implementation are performed properly—because they support conclusions of cause and effect. The conclusion that Burch colposuspension prevents stress incontinence when performed at the time of abdominal sacrocolpopexy could only be drawn from a randomized trial.
Trial design standardized key elements
Many types of bias confound the results of nonrandomized studies, particularly selection bias (eg, when surgeons select which procedure to perform on the basis of patient characteristics), and valid conclusions of cause and effect cannot be drawn. However, with a randomized trial, subjects are separated into groups by chance and no other factor. Thus, the groups are equivalent at baseline—provided the sample size is large enough (and allowing for random differences)—and therefore any changes measured after the experimental intervention can be confidently attributed to the intervention itself.
Another strength of the trial is standardization. The subjects were “standardized” by rather broad inclusion and exclusion criteria to constitute an important clinical population and to ensure they were sufficiently similar so that the treatment (abdominal sacrocolpopexy) was appropriate for all. In addition, surgeons at the multiple participating sites agreed to standardization of the technical details of the Burch colposuspension so that the subjects received the same intervention regardless of site. And data collection in follow-up was performed in a standard way by research staff who were blinded to the subjects’ group assignment (intervention versus control), so the data were as free of bias as possible.
Homogeneous study population may be a weakness
KOHLI: I agree that the methodology of this well-designed study is its major strength. What are its weaknesses?
WEBER: No doubt there are several, only some of which may be apparent at this time. For example, most women in the study were Caucasian, and very few were Hispanic, Asian, or black. Although we have no scientific reason to believe that Burch colposuspension has different responses in women of different racial and ethnic backgrounds, the trial’s subjects are not diverse enough to analyze the data by subgroups to confirm or refute the hypothesis that response to the Burch procedure is independent of race or ethnicity.
BRUBAKER: Another weakness: Because this study was closed after the first interim analysis, some of our secondary analyses will be underpowered, although we clearly demonstrated a difference in our primary endpoint.
It is important to remember that this study is not “finished.” Our participants are still in active follow-up for 2 years following surgery. It will be interesting to see what happens during the longer follow-up, especially with regard to prolapse and incontinence. We are also doing additional in-depth analyses of urodynamic and other parameters.
KOHLI: Again, I think the study design and analysis were well thought out. It would have been interesting to see how the results broke down according to site, to see if there was variation—which could indicate variation in surgical technique.
BRUBAKER: We have not done this analysis and do not plan to at this time.
Why paravaginal repairs were allowed
KOHLI: What about the decision to include surgeries that involved paravaginal repair?
WEBER: That generated a fair amount of discussion during trial design, as there was no clear “right” answer. Perhaps it would have been “cleaner” to eliminate the option of performing paravaginal repair, but when the trial was designed, we lacked unequivocal evidence that paravaginal repair at the time of abdominal sacrocolpopexy provides additional support for the anterior vagina. Therefore, we decided to allow the decision to be based on surgeon judgment.
Some surgeons perform paravaginal repair with abdominal sacrocolpopexy in almost all women because they believe quite strongly that this reduces the risk of recurrent anterior vaginal prolapse. Others never perform paravaginal repair with abdominal sacrocolpopexy and feel just as strongly that their patients are adequately treated and protected from subsequent anterior vaginal prolapse.
Investigators feared paravaginal repairs could dilute Burch effects
Study surgeons did agree that paravaginal repair reduces the likelihood of postoperative stress incontinence, although not as effectively as Burch colposuspension. Thus, our dilemma: If paravaginal repairs were performed in a large number of subjects, thereby improving their postoperative continence status regardless of whether Burch was performed, the effect of Burch could be so diluted as to be lost. On the other hand, if paravaginal repairs were completely excluded, that would restrict some surgeons’ practices and potentially reduce the number of women who would be offered participation in the study if their surgeons felt their anterior vaginal prolapse would be potentially undertreated.
We resolved the dilemma as follows:
- A relatively low proportion—about one quarter—of surgeons performed paravaginal repairs regularly with abdominal sacrocolpopexy, so the potential impact in the trial would not be great.
- Paravaginal repairs were allowed, but only when declared necessary by the surgeon before randomization; this step prevented surgeons from changing their minds about the necessity of paravaginal repair if the subject was assigned to the Burch group (ie, the woman would be receiving additional anterior vaginal support by way of the Burch).
- We stratified for paravaginal repair in the randomization, so women with paravaginal repair were equally distributed between the intervention and control groups.
Are subjective or objective measures better?
- Subjective measures convey a patient’s foremost concerns and how she is doing clinically
- Correlating symptoms with objective measures yields valuable insights into treatment
BRUBAKER: I prefer subjective measures because I think they reflect what is most important to patients in quality-of-life disorders. However, I believe we need to understand the relationship between subjective outcomes and traditional “objective” outcomes.
WEBER: I think the research community is reaching a consensus that “subjective” measures—better described as patient-oriented outcomes—are more important than objective measures, particularly for conditions that affect patients in “subjective” ways, ie, ways that affect their health-related quality of life, rather than quantity of life. This does not mean that objective measures are useless—although we should first evaluate each measure critically to make that determination on the basis of evidence.
Nevertheless, when a patient seeks and receives treatment based on symptoms and how those symptoms impact her daily life, I think it is incumbent upon researchers and clinicians to ensure that the treatment that is considered most effective actually results in a change that the patient finds worthwhile.
What is “success”?
WALTERS: When it comes to incontinence, for which there is an imperfect correlation between various objective and subjective measures, I think both types of measures are valuable and important. Gathering several different types of outcomes for each patient helps us better understand the nuances of how well an intervention works.
I can understand why some clinicians and researchers place greater reliance on subjective measures of incontinence, such as a diary of incontinence episodes and quality-of-life measures, because these measures tell us exactly how the patient is doing clinically and how she feels about the intervention. If she reports that she is completely cured and “perfect,” then objective measures are irrelevant. However, for any subjective outcome short of perfect, correlation with the objective measures such as cough stress test, physical examination, and urodynamic tests can help investigators understand the reason for the imperfect outcome and point to areas of possible improvement.
KOHLI: In my practice, some women who continue to leak slightly after an incontinence procedure consider their surgery a complete success, whereas, as a surgeon, I consider it a suboptimal result. Both objective and subjective results are important.
Putting the CARE trial into practice
- Data relate directly only to women undergoing abdominal sacrocolpopexy
- Patient education, medicolegal, and reimbursement may also relate
- Results reflect the high prevalence of pelvic floor disorders and the need to routinely ask about them
BRUBAKER: I routinely counsel patients who are planning sacrocolpopexy but who do not have stress incontinence to consider a concomitant Burch procedure. I do not have them undergo urodynamic testing because, at this time, the results of that testing would not change my clinical practice.
WALTERS: I have always been liberal when it comes to adding retropubic colposuspension to abdominal sacrocolpopexy, even in women who do not have preoperative stress incontinence. The reason? Patients who are continent preoperatively, but become stress-incontinent postoperatively, are particularly unhappy with their outcome, especially if they need another surgery within a year to treat the stress incontinence. So this study verified what I was already doing.
What I didn’t learn is whether a paravaginal defect repair helps or hurts the Burch procedure from an anatomic and functional perspective.
It also appears that preoperative urodynamic testing has little value, although that was not the point of this study. I am glad it will be addressed in future studies.
KOHLI: I think the findings apply to those select patients undergoing abdominal sacrocolpopexy for prolapse. It would be dangerous to extrapolate these results to other abdominal vault suspension procedures or vaginal prolapse procedures. Based on the CARE trial, I plan to counsel patients about the risks and benefits of “optional” Burch colposuspension at the time of planned sacrocolpopexy. In reality, however, I have almost completely switched to minimally invasive midurethral slings, even in the case of abdominal prolapse procedures, because of their high cure rates, low complication rates, and ease of postoperative adjustment.
Clinical implications depend on surgeon’s routine
KOHLI: What are the implications for the majority of ObGyns?
WEBER: It depends on what ObGyns are doing for women with prolapse.
For ObGyns who are confident and competent, through training and experience, to perform abdominal sacrocolpopexy for women with advanced prolapse, the CARE trial results have a direct effect. Women with prolapse who are stress continent with no contraindications, can be reassured that they will benefit from a 50% reduction of postoperative stress incontinence with the Burch procedure.
For ObGyns who do not perform sacrocolpopexy, the CARE trial will have no direct clinical effects. Nevertheless, these clinicians need to be aware of the findings so they can discuss the options with patients before decisions on route or type of prolapse surgery are made.
The CARE trial and its results remind us of the high prevalence of pelvic floor disorders in women, potentially even after corrective surgery—and the need to actively screen all women for pelvic dysfunction.
Warn of potential incontinence even with the Burch
KOHLI: How does this study affect counseling of candidates for prolapse surgery?
BRUBAKER: I would offer stress-continent women a Burch procedure at the time of sacrocolpopexy. That much is clear. The interesting discussions come from “similar” clinical scenarios, where data are not yet available. For example, should a stress-continent woman facing a suspension via the vaginal route undergo a concomitant continence procedure?
WEBER: It is important to keep in mind that even when Burch colposuspension was performed, a number of women still experienced urinary incontinence (some stress, some urge, some mixed) after surgery; and the vast majority of women have urinary symptoms of some kind both before and after surgery. So preoperative counseling should include the information that urinary symptoms are very common after abdominal sacrocolpopexy—some as persistent or recurrent, and some as new symptoms.
As longer follow-up data from the CARE trial become available, we will learn how many women have urinary symptoms that are temporary versus long-lasting.
Is routine Burch best?
- When not all women benefit, should a procedure be offered prophylactically? In this case, experts say, “Yes”
- Some physicians favor other incontinence procedures
- Final decision rests with the patient
WALTERS: It is an easy decision for me. As I said earlier, women are particularly unhappy if they go from continent to incontinent after a surgery. In fact, some women are more displeased with that outcome than with a failure of the prolapse surgery. Because most women with prolapse have substantial anterior vaginal wall prolapse, the Burch procedure—with or without a paravaginal defect repair—also serves as part of the prolapse repair of the anterior wall. And now we know it also improves postoperative urinary function.
BRUBAKER: Doing a Burch procedure at the time of sacrocolpopexy is a time-efficient and low-morbidity addition, so it is worthwhile for me and my patients. It is clearly not the same as doing a secondary, standalone procedure for new symptoms.
Over the next 2 years, we will see how many women who were moderately or greatly bothered by stress incontinence went on to a surgical treatment.
WEBER: Please note a careful distinction: I would be willing to recommend Burch colposuspension to 100 stress-continent women who are planning to undergo abdominal sacrocolpopexy for prolapse, with the expectation that this will prevent postoperative stress incontinence in roughly half the women who would have developed it otherwise. It is up to the patient to accept this recommendation or not.
Based on the CARE trial results, 44 of 100 previously continent women after only abdominal sacrocolpopexy develop postoperative stress incontinence, compared with about 24 of 100 women after Burch and abdominal sacrocolpopexy. Even more striking is the difference in women affected by bothersome stress incontinence: almost 25% in the control group versus 6% in the Burch group. Women in the Burch group did not experience an excess of adverse events, or a clinically significant difference in operative time or estimated blood loss, compared with women in the control group.
Is “wait and see” better?
KOHLI: Because I favor minimally invasive midurethral sling procedures, which can often be performed on an outpatient basis under local anesthesia, I counsel women undergoing prolapse surgery via an abdominal or vaginal route that it is best to treat the incontinence postoperatively if it occurs. Obviously, this applies to women who have no incontinence and do not demonstrate potential stress incontinence on urodynamic testing preoperatively.
Anecdotally, I have not found a high rate of new-onset urinary incontinence following prolapse procedures. We may retrospectively look at these patients more critically in light of this new data.
WALTERS: I think most women would be dissatisfied with a 44% risk of postoperative stress incontinence requiring a second surgery. Even if you counseled them appropriately, many women would ask that you try to manage everything at the first surgery.
What are nonclinical effects of the trial?
- Need for patient and physician education is great, and the CARE trial offers a valuable opportunity
- Potential for medicolegal risk if complications develop
- Payers may not be willing to reimburse for a prophylactic procedure
WEBER: Given how extensively the trial’s results were disseminated by the lay press, I think we have an important opportunity to educate both patients and health-care providers.
First, patients: For women who may be directly affected by the trial, knowledgeable clinicians should explain its results and limitations to help them reach a decision about their treatment.
For women who hear the trial’s results described incompletely or incorrectly (eg, “…2 stitches prevent incontinence…”), clinicians should take this opportunity to correct misunderstandings and educate women about incontinence, prolapse, and pelvic health in general.
For health-care providers, this trial reminds us of the extraordinarily high prevalence of pelvic floor disorders. Although current treatments are not perfect, virtually all women with pelvic floor disorders can be treated to substantially alleviate, if not eliminate, bothersome symptoms.
All clinicians should routinely inquire about pelvic symptoms and be prepared to initiate an evaluation or provide a referral.
Medicolegal fallout?
KOHLI: There is the question of medicolegal risk if complications occur after colposuspension when the patient had no complaints or evidence of stress incontinence at the time of preoperative urodynamic testing. I am not aware of any legal precedent in which a clinical study or data provided solid protection from a jury verdict. The study did not show increased risk or complication with the addition of the Burch procedure, but that may not be true for some surgeons and some patients.
Billing and coding
In terms of billing, how should we code for the Burch colposuspension when the patient had no demonstrable stress incontinence? Payment denials in this scenario seem likely. This may create a line of separation between what may be clinically indicated for the patient and what insurance companies are willing to pay for.
There may be the option to use the urethral hypermobility code (ICD 599.81) for the Burch colposuspension, but only time will tell if this will be reimbursed. I would be curious to hear the panel’s experience with reimbursement for a prophylactic procedure based on scientific data. Obviously, what is best for the patient is most important.
BRUBAKER: All these patients had urethral hypermobility, which is also an indication for a Burch colposuspension.
Is preoperative urodynamic testing useful?
- CARE trial data still to come
- Basic testing is probably helpful
WEBER: In the CARE trial, the relative level of protection from postoperative stress incontinence provided by the Burch procedure did not depend on the stress-test component (with prolapse reduced) of preoperative urodynamic testing. About 50% fewer women had postoperative stress incontinence after Burch, whether preoperative urodynamic testing showed positive or negative stress tests with prolapse reduction.
Although subsequent analyses (presented at the Society of Gynecologic Surgeons 2006 meeting; manuscript under review) focused on urodynamic testing and postoperative outcomes, the CARE trial was not designed primarily to determine whether women planning prolapse surgery benefit from preoperative urodynamic testing. Therefore, conclusions about the “need” for urodynamic testing should not be based only on the CARE trial.
Should urodynamic testing determine treatment?
Randomized trials that directly address the cost-benefit of urodynamic testing are urgently needed. For now, as is standard in good clinical practice, a test should be performed only if results will change recommendations or provide reliable and clinically important prognostic information about a patient’s outcome after intervention. Clinicians should determine whether urodynamic testing meets even 1 of these 2 minimum criteria.
I want to point out that the CARE trial did not address the utility of urodynamic testing.
BRUBAKER: It is clear that some women have stress incontinence despite the concomitant Burch colposuspension. If we learn that an alternative operation can perform better and that any urodynamic (or other clinical measure) can predict improved outcomes, I would consider resuming urodynamic testing.
WALTERS: At first glance, it appears that complex urodynamic testing is definitely not necessary if the goal is to improve outcomes of surgery. However, I believe the patient should undergo some components of urodynamic testing such as a void with a post-void residual urine volume and a basic bladder-filling study noting sensation and capacity. I also do a cough stress test with the prolapse reduced, although we may find that this does not predict postoperative function.
KOHLI: The results of this study are very procedure-specific. If similar results are borne out when other approaches to prolapse and incontinence are analyzed, the value and utility of preoperative uro-dynamic testing in all patients may be questionable.
However, in my practice, I use the results of preoperative urodynamic testing not only for diagnosis, but also to make subtle adjustments when performing incontinence procedures—especially in regard to suburethral slings.
What if you prefer midurethral slings?
- Surgeons should be comfortable with more than 1 incontinence procedure
- We should not jump to untested conclusions
WEBER: Ideally, well trained and experienced gynecologic, urologic, or urogynecologic surgeons perform more than 1 type of incontinence procedure, to meet the needs of different patients.
As yet, we have no direct, evidence-based answers to issues such as these:
Can a midurethral sling be substituted for a Burch colposuspension and have the same average results in preventing post-operative stress incontinence without increasing urgency symptoms…
- …when abdominal sacrocolpopexy is performed for prolapse in a preoperatively stress-continent patient?
- …when vaginal apical suspension is performed for prolapse in a preoperatively stress-continent patient?
Although it is tempting to jump 1 or 2 steps ahead and apply CARE trial data to situations that have not been tested directly, I would be cautious. We want to avoid creating long-lasting or refractory urgency symptoms—especially in a woman who had no such symptoms before surgery—because of a prophylactic procedure.
I think this is especially true because it is relatively easy to salvage patients who do develop bothersome stress incontinence after prolapse surgery.
Bonus: Burch helps anterior vaginal prolapse
WALTERS: I wonder whether prophylactic placement of a midurethral sling would yield the same results as a prophylactic Burch procedure. If your midurethral sling of choice is a tension-free vaginal tape (TVT), I would be cautious about placing it prophylactically, because the TVT has a 2% to 3% risk of prolonged voiding dysfunction requiring transection of the tape.
However, it is possible that prophylactic placement of a transobturator sling, which is associated with much less voiding dysfunction and fewer major surgical complications, might have a different outcome—though this requires further study.
In addition, midurethral slings would not be as effective as Burch colposuspension in treating anterior vaginal prolapse, so I would expect to see more anterior wall prolapse failures if slings replaced colposuspension.
What if you prefer the vaginal approach?
- Further study is needed
WALTERS: I wonder whether a prophylactic transobturator midurethral sling at the time of transvaginal prolapse repair would yield similar results. I do cystocele repair with suburethral (“Kelly”) plication, which seems to work well at stabilizing the urethra in women without stress incontinence. But this approach is not as popular these days, and future studies may demonstrate that a prophylactic midurethral sling will result in better long-term function without significantly increasing the long-term risk.
WEBER: The CARE trial results are relevant to all pelvic surgeons because they demonstrate the need for and benefit from well-designed randomized surgical trials. Another benefit will be extended follow-up in what will become a prospective cohort study of women with advanced prolapse treated by abdominal sacrocolpopexy—providing higher-quality evidence than retrospective case series. Although not as valuable as randomized trials, these data can help guide clinical recommendations.
If long-term results support the effectiveness and durability of abdominal prolapse repair, then gynecologists can reflect on the evidence and choose the approach that best fits the patient’s needs.
Need for other studies?
- Randomized, multicenter trials addressing almost any surgical treatment of prolapse and incontinence are sorely needed
BRUBAKER: Any and all high-quality, well-designed trial can improve our care of women with incontinence and/or prolapse.
WALTERS: I look forward to the follow-up studies from the CARE trial on the value of paravaginal defect repair, preoperative urodynamic testing, and the efficacy of various prolapse-reduction maneuvers in predicting surgical outcomes.
It would also seem logical to repeat this type of study using transvaginal prolapse repair with or without a prophylactic midurethral sling. Another option: anterior colporrhaphy with suburethral plication versus a prophylactic midurethral sling.
KOHLI: I look forward to data on surgical procedures currently being performed with greater frequency despite a lack of good-quality data. These include the transobturator suburethral midurethral sling procedures, laparoscopic sacrocolpopexy, and vaginal mesh augmentation for prolapse.
The Pelvic Floor Disorders Network affords a unique opportunity to perform well-designed multicenter trials to address the rapidly changing landscape of surgical treatment for prolapse and incontinence.
1. Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
2. Cross CA, Cespedes RD, McGuire EJ. Treatment results using pubovaginal slings in patients with large cystoceles and stress incontinence. J Urol. 1997;158:431-434.
3. FitzGerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189:1241-1244.
4. Klutke JJ, Ramos S. Urodynamic outcome after surgery for severe prolapse and potential stress incontinence. Am J Obstet Gynecol. 2000;182:1378-1381.
5. Meschia M, Pifarotti P, Spennacchio M, Buonaguidi A, Gattei U, Somigliana E. A randomized comparison of tension-free vaginal tape and endopelvic fascia plication in women with genital prolapse and occult stress urinary incontinence. Am J Obstet Gynecol. 2004;190:609-613.
6. Gordon D, Gold RS, Pauzner D, Lessing JB, Groutz A. Combined genitourinary prolapse repair and prophylactic tension-free vaginal tape in women with severe prolapse and occult stress urinary incontinence: preliminary results. Urology. 2001;58:547-550.
Dr. Brubaker, Dr. Kohli, and Dr. Weber report no financial relationships relevant to this article. Dr. Walters is a speaker for American Medical Systems.
1. Chaikin DC, Groutz A, Blaivas JG. Predicting the need for anti-incontinence surgery in continent women undergoing repair of severe urogenital prolapse. J Urol. 2000;163:531-534.
2. Cross CA, Cespedes RD, McGuire EJ. Treatment results using pubovaginal slings in patients with large cystoceles and stress incontinence. J Urol. 1997;158:431-434.
3. FitzGerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189:1241-1244.
4. Klutke JJ, Ramos S. Urodynamic outcome after surgery for severe prolapse and potential stress incontinence. Am J Obstet Gynecol. 2000;182:1378-1381.
5. Meschia M, Pifarotti P, Spennacchio M, Buonaguidi A, Gattei U, Somigliana E. A randomized comparison of tension-free vaginal tape and endopelvic fascia plication in women with genital prolapse and occult stress urinary incontinence. Am J Obstet Gynecol. 2004;190:609-613.
6. Gordon D, Gold RS, Pauzner D, Lessing JB, Groutz A. Combined genitourinary prolapse repair and prophylactic tension-free vaginal tape in women with severe prolapse and occult stress urinary incontinence: preliminary results. Urology. 2001;58:547-550.
Dr. Brubaker, Dr. Kohli, and Dr. Weber report no financial relationships relevant to this article. Dr. Walters is a speaker for American Medical Systems.
Cystocele and rectocele repair: More success with mesh?
CASE Symptoms point to yet another prolapse recurrence
A 52-year-old woman presents with a bulge and pressure in her vagina. She has undergone 2 prior reconstructive surgeries. The first was a vaginal hysterectomy, anterior and posterior repair, and sling; the second was an abdominal procedure that included a sacrocolpopexy and paravaginal repair.
A physical examination reveals a recurrent 4th-degree cystocele that protrudes 2 cm beyond the hymenal ring. The vault and posterior compartment are well supported, and the patient reports no incontinence, a fact confirmed by urodynamics testing. She asks that you do everything in your power to prevent further recurrence.
How do you proceed?
This patient ultimately underwent anterior colporrhaphy and vaginal paravaginal repair using a decellularized dermal cadaveric implant. She was still doing well 1 year later, with no recurrence.
Despite success stories like this one, the use of graft materials to repair cystoceles and rectoceles is controversial. One reason is the difficulty of interpreting published data, since studies lack uniformity in technique, patient characteristics, graft shape, type of material, attachment sites, and duration of follow-up. Level I evidence that augmented repairs have a clear benefit over traditional repairs is sparse.
Advocates of graft materials argue that native tissue is already compromised—hence, the prolapse—making surgical failure likely.1 They claim graft materials help strengthen repairs, especially in the case of cystoceles. They also point out that adjuvant materials have been used in burns, plastic surgery, and orthopedics for more than 10 years and are generally well tolerated. Their success in hernia repairs prompted their consideration for the pelvic floor.
A pervasive problem, but only 10% to 20% seek help
Roughly 1 of 2 parous women lose pelvic support as they age, but only 10% to 20% seek medical care, with a lifetime risk of surgery for pelvic organ prolapse (POP) of 11% by age 80.2
With women living longer than ever and remaining active later in life, this percentage is likely to rise. Unfortunately, few alternatives to surgical treatment exist, and the reoperation rate for recurrence is 29%, according to a 1995 review.2 If surgical management is the only hope of cure, how can we lower the 29% recurrence rate?
Graft materials may provide part or all of the solution.
Elements of prolapse
Anterior compartment
Central and/or lateral defects can occur in the anterior compartment.
Lateral (paravaginal) defects indicate that the endopelvic connective tissue has separated from the arcus tendineus fascia pelvis. Lateral defects can be repaired vaginally or abdominally.
One study3 found that 67% of women with anterior wall prolapse had paravaginal defects, but no randomized trials have evaluated the clinical benefit of repairing these defects, compared with traditional colporrhaphies.
Central defects involve site-specific defects and/or general attenuation of the endopelvic connective tissue. These are usually repaired vaginally.
Recurrence rates for lateral and central defects range from 3% to 70%.4-8
Two large series of vaginal paravaginal repairs noted the following recurrence rates:
- Shull et al6 found a recurrence rate of 7% to the hymenal ring or beyond.
- Young et al7 observed a recurrence rate for lateral defects of 2%, with recurrence rates as high as 22% for central defects.
In a comparison of 3 techniques for vaginal repair of central defects, using strict criteria to assess anatomic outcomes, Weber et al4 found recurrence rates of 54% to 70%. Other studies show symptomatic recurrence rates of 3% to 22% for cystoceles.5,8
With grafts, both paravaginal and central defects can be repaired. Vaginal paravaginal repairs are not popular due to the technical difficulty involved. With the use of grafts, however, both paravaginal and central defects can be addressed simultaneously with relative ease.
Posterior compartment
Defects in the posterior compartment are less likely to recur. Reported success rates range from 80% to 90%.9,10
Posterior compartment defects include general attenuation of Denonvillier’s fascia or a tear anywhere along the fascia or any of its attachments.
Recurrence rates. Site-specific repairs are thought to minimize complications such as dyspareunia. However, few studies have compared the efficacy of site-specific repairs with that of traditional colporrhaphies. At our institution, women who underwent traditional colporrhaphy had fewer recurrences than controls (33% vs 14%), with no differences in postoperative symptoms such as dyspareunia, constipation, and fecal incontinence.11
Graft materials of questionable benefit. In the posterior compartment, these materials have not been shown to be beneficial, compared with traditional or site-specific repairs. Sand et al12 found no benefit for repairs in which absorbable Vicryl mesh was imbricated, but this randomized trial may have lacked sufficient power to show statistical significance. Large cohorts would be needed to show significant benefit of meshes in the posterior compartment.
A complex web of support
In the normal pelvis, support of reproductive organs depends on a complex web of muscles, fascia, and connective tissue. To ensure success, prolapse repairs should correct any separation or attenuation of tissue and preserve or enhance tissue resilience.
Risk factors for recurrent prolapse
- Poor tissue (assess tissue quality before and during surgery)
- Impaired healing
- Chronic increases in intraabdominal pressure due to obstructive pulmonary disease, asthma, or constipation
- High-grade cystocele
- Age 60 or above13
Patients with these conditions may benefit from the use of adjuvant materials in the anterior compartment.
Note that women who have had recurrences after earlier repairs may experience repeat recurrence.
Advantages of grafts
Using graft materials, the surgeon can repair all vaginal defects faster and with less effort. In the anterior compartment, a graft can be placed and anchored bilaterally from arcus to arcus tendineus, and posteriorly to the level of the spine, recreating level I support. Graft materials also offer the potential to treat stress urinary incontinence concomitantly using different shaped materials. Two authors have already described their success performing this type of repair.14
Nevertheless, great care and consideration should be devoted to actual and theoretical short- and long-term risks, many of which have not been fully elucidated.
Once a successful material is identified or developed, it may decrease operating time and morbidity in vaginal surgeries. It may also reduce the higher hospital costs normally associated with abdominal procedures.
Types of graft materials
There are 2 types of materials: synthetic or biologic. Synthetic materials can be further classified into permanent or absorbable.
The most widely used biologic materials include allografts such as human freeze-dried or solvent-dehydrated fascia lata (Tutoplast), decellularized human cadaveric dermis (Alloderm, Repliform), porcine dermal xenografts such as Pelvicol or Intexene, and bovine pericardial implants (Veritas).
Soft polypropylene meshes such as Gynemesh and Atrium are commonly used permanent materials, and polyglactin 910 is an absorbable material (TABLE).
TABLE
How successful are adjuvant materials in cystocele and rectocele repairs?
| MATERIAL (SIZE IN CM) | AUTHOR | NO. IN STUDY | RECURRENCE RATE (%) | SITE OF ATTACHMENT | FOLLOW-UP (MONTHS) | COMPLICATIONS |
|---|---|---|---|---|---|---|
| BIOLOGIC MATERIALS | ||||||
| Alloderm 3×7 patch with concomitant sling | Chung29 | 19 | 16 | Pubocervical fascia | 28 | None |
| Intexene 6×8 with sling | Gomelsky et al 200420 | 70 | 9 stage II 4 stage III | Arcus tendineus fascia pelvis | 24 | 1 wound separation |
| Solvent-dehydrated cadaveric fascia lata patch with sling | Gandhi et al 200521 | 76 patch vs 72 no patch | 21 vs 29, respectively (P=.23) | Overlay | 13 | None |
| Alloderm 3×7 trapezoid | Clemons et al 200322 | 33 | 41 stage II 3 symptomatic | Arcus tendineus fascia pelvis | 18 | None |
| SYNTHETIC MATERIALS WITH CONCOMITANT SLINGS* | ||||||
| Marlex 10×3×5 | Nicita 199823 | 44 | 0 | Arcus tendineus fascia pelvis | 13 | 1 vaginal erosion |
| Polyglactin 910 absorbable mesh | Sand et al12 | 80 mesh vs 80 no mesh | 25 vs 43 stage II cystoceles, respectively(P=.02) | Insert in the anterior and posterior colporrhaphy suture line | 12 | None |
| Polyglactin 910 absorbable mesh | Weber et al4 | 26 with mesh + standard repair; 24 with ultra-lateral repair; 33 with standard repair | 58 vs 54 vs 70 stage II, respectively (P=.58) | Overlay | 23 | None |
| SYNTHETIC PERMANENT GRAFTS WITHOUT CONCOMITANT SLINGS | ||||||
| Marlex trapezoid | Julian 199619 | 12 with 12 without | 0 vs 33, respectively | Arcus tendineus fascia pelvis | 24 | 3 vaginal erosions |
| Mixed-fiber mesh (polyglactin 910 and polyester 5×5) | Migliari and Usai 199924 | 12 | 25 | Pubourethral and cardinal ligaments | 20 | None |
| Prolene (Atrium) | Dwyer and O’Reilly25 | 64 anterior 50 posterior | 6 grade II | Tension-free | 29 | 8% vaginal erosion 1 rectovaginal fistula |
| Gynemesh 6×15 | de Tayrac et al 200526 | 87 | 7 stage II 2 stage III | Tension-free | 24 | 8% vaginal erosion |
| Prolene mesh patch | Milani et al 200527 | 32 anterior 31 posterior | 6 stage II | Fixed to endopelvic connective tissue | 17 | 20% anterior, 63% posterior dyspareunia; 13% vaginal erosion (anterior); 1 pelvic abscess (posterior) |
| Prolene mesh (double-wing shape) | Natale et al 2000 28 | 138 | 3 | Tension-free | 18 | 9% vaginal erosion 7% dyspareunia 1 hematoma |
| *Absorbable and permanent. | ||||||
Classification of synthetic materials
- Type 1 grafts are totally macroporous (>75 μm), which allows fibroblast, macrophage, and collagen penetration with angiogenesis. Examples include Prolene and Marlex meshes.
- Type 2 mesh is microporous (<10 μm in 1 dimension). This prevents penetration of fibroblasts, macrophages, or collagen. Gore-Tex is an example of a Type 2 mesh.
- Type 3 mesh is macroporous (>75 μm) with multifilamentous or microporous components. Examples include Mersilene (braided Dacron mesh), Teflon (polytetrafluoroethylene [PTFE]), Surgipro (braided polypropylene mesh), and MycroMesh (perforated PTFE patch).
- Type 4 mesh has a submicron pore size that prevents penetration. Examples include Silastic, Cellgard (polypropylene sheeting), and Preclude pericardial membrane/Preclude dura-substitute.1
2 other important properties are composition of fibers (multifilamentous materials commonly have interstices less than 10 microns) and flexibility (which has a bearing on erosion of the material).1
Bacteria can penetrate pores smaller than 1 μm, whereas polymorphonuclear white blood cells and macrophages need a pore size larger than 10 μm, and capillary ingrowth requires a size larger than 75 microns. Thus, Type 1 offers the advantages of larger pore size and monofilamentous interstices to allow for capillary ingrowth.
Which material is best?
Although the literature is difficult to interpret because of the diversity of studies and other factors, some findings are worth noting:
- Tutoplast and Alloderm appear to have the best tensile strength, maximum load to capacity, and microscopic architecture similar to the original tissue.15-17 However, these qualities were documented prior to implantation in vivo.
- Slings appear to help prevent cystocele recurrences, according to a study by Goldberg et al.18
- A fascial patch had no benefit when placed as an overlay in the anterior compartment in a randomized, controlled trial (involving 162 women) by Sand et al.12
- Marlex. One group of women with recurrent prolapse underwent synthetic graft (Marlex) augmentation with bilateral ATFP attachment, while the other group had anterior colporrhaphy only.19 None of the women who received grafts had further recurrence, while 33% of the control group did. However, 25% of the women with the graft had vaginal erosions.
- Polyglactin 910 had a protective effect when embedded in the plication, according to Sand et al.12 However, it had no benefit when used as an overlay to a traditional repair in a study by Weber et al.4 The discrepancy may be related to small sample size; the study by Weber et al was powered to detect only a 30% difference. However, these studies suggest that it is not only the type of graft that is important, but how it is used or attached.
In general, synthetic grafts may have slightly higher success rates, whereas biologic materials appear to be better tolerated.
Prospective, comparative trials of these materials are desperately needed.
1. Cervigni M, Natale F. The use of synthetics in the treatment of pelvic organ prolapse. Curr Opin Urol. 2001;11:429-435.
2. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
3. Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-573.
4. Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
5. Porges RF, Smilen SW. Long-term analysis of the surgical management of pelvic support defects. Am J Obstet Gynecol. 1994;171:1518-1526.
6. Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal segment: an analysis of support defects, operative morbidity, and anatomic outcome. Am J Obstet Gynecol. 1994;171:1429-1436.
7. Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001;185:1360-1366.
8. Macer GA. Transabdominal repair of cystocele, a 20-year experience, compared with the traditional vaginal approach. Trans Pac Coast Obstet Gynecol Soc. 1978;45:116-120.
9. Cundiff GW, Weidner AC, Visco AG, Addison WA, Bump RC. An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol. 1998;179:1451-1456.
10. Paraiso MF, Ballard LA, Walters MD, Lee JC, et al. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996;175:1423-1430.
11. Abramov Y, et al. Site-specific rectocele repair compared with standard posterior colporrhaphy. Obstet Gynecol. 2005;105:314-318.
12. Sand PK, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol. 2001;184:1357-1362.
13. Whitesides JL, Weber AM, Meyn LA, Walters MD. Risk factors for prolapse recurrence after vaginal repair. Obstet Gynecol Surv. 2005;60:164-165.
14. Kobashi KC, Mee SL, Leach GE. A new technique for cystocele repair and transvaginal sling: the cadaveric prolapse repair and sling (CAPS). Urology. 2000;56:9-14.
15. Lemer ML, Chaikin DC, Blaivas JG. Tissue strength analysis of autologous and cadaveric allografts for the pubovaginal sling. Neurourol Urodyn. 1999;18:497-503.
16. Choe JM, Kothandapani R, et al. Autologous, cadaveric, and synthetic materials used in sling surgery: comparative biomechanical analysis. Urology. 2001;58:482-486.
17. Scalfani AP. Biophysical and microscopic analysis of homologous dermal and fascial materials for facial aesthetic and reconstructive uses. Arch Facial Plast Surg. 2002;4:164-171.
18. Goldberg RP, et al. Protective effect of suburethral slings on postoperative cystocele recurrence after reconstructive pelvic operation. Am J Obstet Gynecol. 2001;185:1307-1312.
19. Julian TM. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472-1475.
20. Gomelsky A, Rudy DC, Dmochowski RR. Porcine dermis interposition graft for repair of high grade anterior compartment defects with or without concomitant pelvic organ prolapse procedures. J Urol. 2004;171:1581-1584.
21. Gandhi S, et al. A prospective randomized trial of solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Am J Obstet Gynecol. 2005;192:1649-1654.
22. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618.
23. Nicita G. A new operation for genitourinary prolapse. J Urol. 1998;160:741-745.
24. Migliari R, Usai E. Treatment results using a mixed fiber mesh in patients with grade IV cystocele. J Urol. 1999;161:1255-1258.
25. Dwyer PL, O’Reilly BA. Transvaginal repair of anterior and posterior compartment prolapse with Atrium polypropylene mesh. BJOG. 2004;111:831-836.
26. de Tayrac R, Gervaise A, Chauveaud A, Fernandez H. Tension-free polypropylene mesh for vaginal repair of anterior vaginal wall prolapse. J Reprod Med. 2005;50:75-80.
27. Milani R, et al. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with prolene mesh. BJOG. 2005;112:107-111.
28. Natale F, Marziali S, Cervigni M. Tension-free cystocele repair (TCR): long-term follow-up. Proceedings of the 25th annual meeting of the International Urogynecological Association. 2000;22-25.
29. Chung SY, et al. Technique of combined pubovaginal sling and cystocele using a single piece of cadaveric dermal graft. Urology. 2002;59:538-541.
Dr. Botros has no financial relationships relevant to this article. Dr. Sand receives grant/research support from Boston Scientific, and is a consultant and speaker for American Medical Systems and Boston Scientific.
CASE Symptoms point to yet another prolapse recurrence
A 52-year-old woman presents with a bulge and pressure in her vagina. She has undergone 2 prior reconstructive surgeries. The first was a vaginal hysterectomy, anterior and posterior repair, and sling; the second was an abdominal procedure that included a sacrocolpopexy and paravaginal repair.
A physical examination reveals a recurrent 4th-degree cystocele that protrudes 2 cm beyond the hymenal ring. The vault and posterior compartment are well supported, and the patient reports no incontinence, a fact confirmed by urodynamics testing. She asks that you do everything in your power to prevent further recurrence.
How do you proceed?
This patient ultimately underwent anterior colporrhaphy and vaginal paravaginal repair using a decellularized dermal cadaveric implant. She was still doing well 1 year later, with no recurrence.
Despite success stories like this one, the use of graft materials to repair cystoceles and rectoceles is controversial. One reason is the difficulty of interpreting published data, since studies lack uniformity in technique, patient characteristics, graft shape, type of material, attachment sites, and duration of follow-up. Level I evidence that augmented repairs have a clear benefit over traditional repairs is sparse.
Advocates of graft materials argue that native tissue is already compromised—hence, the prolapse—making surgical failure likely.1 They claim graft materials help strengthen repairs, especially in the case of cystoceles. They also point out that adjuvant materials have been used in burns, plastic surgery, and orthopedics for more than 10 years and are generally well tolerated. Their success in hernia repairs prompted their consideration for the pelvic floor.
A pervasive problem, but only 10% to 20% seek help
Roughly 1 of 2 parous women lose pelvic support as they age, but only 10% to 20% seek medical care, with a lifetime risk of surgery for pelvic organ prolapse (POP) of 11% by age 80.2
With women living longer than ever and remaining active later in life, this percentage is likely to rise. Unfortunately, few alternatives to surgical treatment exist, and the reoperation rate for recurrence is 29%, according to a 1995 review.2 If surgical management is the only hope of cure, how can we lower the 29% recurrence rate?
Graft materials may provide part or all of the solution.
Elements of prolapse
Anterior compartment
Central and/or lateral defects can occur in the anterior compartment.
Lateral (paravaginal) defects indicate that the endopelvic connective tissue has separated from the arcus tendineus fascia pelvis. Lateral defects can be repaired vaginally or abdominally.
One study3 found that 67% of women with anterior wall prolapse had paravaginal defects, but no randomized trials have evaluated the clinical benefit of repairing these defects, compared with traditional colporrhaphies.
Central defects involve site-specific defects and/or general attenuation of the endopelvic connective tissue. These are usually repaired vaginally.
Recurrence rates for lateral and central defects range from 3% to 70%.4-8
Two large series of vaginal paravaginal repairs noted the following recurrence rates:
- Shull et al6 found a recurrence rate of 7% to the hymenal ring or beyond.
- Young et al7 observed a recurrence rate for lateral defects of 2%, with recurrence rates as high as 22% for central defects.
In a comparison of 3 techniques for vaginal repair of central defects, using strict criteria to assess anatomic outcomes, Weber et al4 found recurrence rates of 54% to 70%. Other studies show symptomatic recurrence rates of 3% to 22% for cystoceles.5,8
With grafts, both paravaginal and central defects can be repaired. Vaginal paravaginal repairs are not popular due to the technical difficulty involved. With the use of grafts, however, both paravaginal and central defects can be addressed simultaneously with relative ease.
Posterior compartment
Defects in the posterior compartment are less likely to recur. Reported success rates range from 80% to 90%.9,10
Posterior compartment defects include general attenuation of Denonvillier’s fascia or a tear anywhere along the fascia or any of its attachments.
Recurrence rates. Site-specific repairs are thought to minimize complications such as dyspareunia. However, few studies have compared the efficacy of site-specific repairs with that of traditional colporrhaphies. At our institution, women who underwent traditional colporrhaphy had fewer recurrences than controls (33% vs 14%), with no differences in postoperative symptoms such as dyspareunia, constipation, and fecal incontinence.11
Graft materials of questionable benefit. In the posterior compartment, these materials have not been shown to be beneficial, compared with traditional or site-specific repairs. Sand et al12 found no benefit for repairs in which absorbable Vicryl mesh was imbricated, but this randomized trial may have lacked sufficient power to show statistical significance. Large cohorts would be needed to show significant benefit of meshes in the posterior compartment.
A complex web of support
In the normal pelvis, support of reproductive organs depends on a complex web of muscles, fascia, and connective tissue. To ensure success, prolapse repairs should correct any separation or attenuation of tissue and preserve or enhance tissue resilience.
Risk factors for recurrent prolapse
- Poor tissue (assess tissue quality before and during surgery)
- Impaired healing
- Chronic increases in intraabdominal pressure due to obstructive pulmonary disease, asthma, or constipation
- High-grade cystocele
- Age 60 or above13
Patients with these conditions may benefit from the use of adjuvant materials in the anterior compartment.
Note that women who have had recurrences after earlier repairs may experience repeat recurrence.
Advantages of grafts
Using graft materials, the surgeon can repair all vaginal defects faster and with less effort. In the anterior compartment, a graft can be placed and anchored bilaterally from arcus to arcus tendineus, and posteriorly to the level of the spine, recreating level I support. Graft materials also offer the potential to treat stress urinary incontinence concomitantly using different shaped materials. Two authors have already described their success performing this type of repair.14
Nevertheless, great care and consideration should be devoted to actual and theoretical short- and long-term risks, many of which have not been fully elucidated.
Once a successful material is identified or developed, it may decrease operating time and morbidity in vaginal surgeries. It may also reduce the higher hospital costs normally associated with abdominal procedures.
Types of graft materials
There are 2 types of materials: synthetic or biologic. Synthetic materials can be further classified into permanent or absorbable.
The most widely used biologic materials include allografts such as human freeze-dried or solvent-dehydrated fascia lata (Tutoplast), decellularized human cadaveric dermis (Alloderm, Repliform), porcine dermal xenografts such as Pelvicol or Intexene, and bovine pericardial implants (Veritas).
Soft polypropylene meshes such as Gynemesh and Atrium are commonly used permanent materials, and polyglactin 910 is an absorbable material (TABLE).
TABLE
How successful are adjuvant materials in cystocele and rectocele repairs?
| MATERIAL (SIZE IN CM) | AUTHOR | NO. IN STUDY | RECURRENCE RATE (%) | SITE OF ATTACHMENT | FOLLOW-UP (MONTHS) | COMPLICATIONS |
|---|---|---|---|---|---|---|
| BIOLOGIC MATERIALS | ||||||
| Alloderm 3×7 patch with concomitant sling | Chung29 | 19 | 16 | Pubocervical fascia | 28 | None |
| Intexene 6×8 with sling | Gomelsky et al 200420 | 70 | 9 stage II 4 stage III | Arcus tendineus fascia pelvis | 24 | 1 wound separation |
| Solvent-dehydrated cadaveric fascia lata patch with sling | Gandhi et al 200521 | 76 patch vs 72 no patch | 21 vs 29, respectively (P=.23) | Overlay | 13 | None |
| Alloderm 3×7 trapezoid | Clemons et al 200322 | 33 | 41 stage II 3 symptomatic | Arcus tendineus fascia pelvis | 18 | None |
| SYNTHETIC MATERIALS WITH CONCOMITANT SLINGS* | ||||||
| Marlex 10×3×5 | Nicita 199823 | 44 | 0 | Arcus tendineus fascia pelvis | 13 | 1 vaginal erosion |
| Polyglactin 910 absorbable mesh | Sand et al12 | 80 mesh vs 80 no mesh | 25 vs 43 stage II cystoceles, respectively(P=.02) | Insert in the anterior and posterior colporrhaphy suture line | 12 | None |
| Polyglactin 910 absorbable mesh | Weber et al4 | 26 with mesh + standard repair; 24 with ultra-lateral repair; 33 with standard repair | 58 vs 54 vs 70 stage II, respectively (P=.58) | Overlay | 23 | None |
| SYNTHETIC PERMANENT GRAFTS WITHOUT CONCOMITANT SLINGS | ||||||
| Marlex trapezoid | Julian 199619 | 12 with 12 without | 0 vs 33, respectively | Arcus tendineus fascia pelvis | 24 | 3 vaginal erosions |
| Mixed-fiber mesh (polyglactin 910 and polyester 5×5) | Migliari and Usai 199924 | 12 | 25 | Pubourethral and cardinal ligaments | 20 | None |
| Prolene (Atrium) | Dwyer and O’Reilly25 | 64 anterior 50 posterior | 6 grade II | Tension-free | 29 | 8% vaginal erosion 1 rectovaginal fistula |
| Gynemesh 6×15 | de Tayrac et al 200526 | 87 | 7 stage II 2 stage III | Tension-free | 24 | 8% vaginal erosion |
| Prolene mesh patch | Milani et al 200527 | 32 anterior 31 posterior | 6 stage II | Fixed to endopelvic connective tissue | 17 | 20% anterior, 63% posterior dyspareunia; 13% vaginal erosion (anterior); 1 pelvic abscess (posterior) |
| Prolene mesh (double-wing shape) | Natale et al 2000 28 | 138 | 3 | Tension-free | 18 | 9% vaginal erosion 7% dyspareunia 1 hematoma |
| *Absorbable and permanent. | ||||||
Classification of synthetic materials
- Type 1 grafts are totally macroporous (>75 μm), which allows fibroblast, macrophage, and collagen penetration with angiogenesis. Examples include Prolene and Marlex meshes.
- Type 2 mesh is microporous (<10 μm in 1 dimension). This prevents penetration of fibroblasts, macrophages, or collagen. Gore-Tex is an example of a Type 2 mesh.
- Type 3 mesh is macroporous (>75 μm) with multifilamentous or microporous components. Examples include Mersilene (braided Dacron mesh), Teflon (polytetrafluoroethylene [PTFE]), Surgipro (braided polypropylene mesh), and MycroMesh (perforated PTFE patch).
- Type 4 mesh has a submicron pore size that prevents penetration. Examples include Silastic, Cellgard (polypropylene sheeting), and Preclude pericardial membrane/Preclude dura-substitute.1
2 other important properties are composition of fibers (multifilamentous materials commonly have interstices less than 10 microns) and flexibility (which has a bearing on erosion of the material).1
Bacteria can penetrate pores smaller than 1 μm, whereas polymorphonuclear white blood cells and macrophages need a pore size larger than 10 μm, and capillary ingrowth requires a size larger than 75 microns. Thus, Type 1 offers the advantages of larger pore size and monofilamentous interstices to allow for capillary ingrowth.
Which material is best?
Although the literature is difficult to interpret because of the diversity of studies and other factors, some findings are worth noting:
- Tutoplast and Alloderm appear to have the best tensile strength, maximum load to capacity, and microscopic architecture similar to the original tissue.15-17 However, these qualities were documented prior to implantation in vivo.
- Slings appear to help prevent cystocele recurrences, according to a study by Goldberg et al.18
- A fascial patch had no benefit when placed as an overlay in the anterior compartment in a randomized, controlled trial (involving 162 women) by Sand et al.12
- Marlex. One group of women with recurrent prolapse underwent synthetic graft (Marlex) augmentation with bilateral ATFP attachment, while the other group had anterior colporrhaphy only.19 None of the women who received grafts had further recurrence, while 33% of the control group did. However, 25% of the women with the graft had vaginal erosions.
- Polyglactin 910 had a protective effect when embedded in the plication, according to Sand et al.12 However, it had no benefit when used as an overlay to a traditional repair in a study by Weber et al.4 The discrepancy may be related to small sample size; the study by Weber et al was powered to detect only a 30% difference. However, these studies suggest that it is not only the type of graft that is important, but how it is used or attached.
In general, synthetic grafts may have slightly higher success rates, whereas biologic materials appear to be better tolerated.
Prospective, comparative trials of these materials are desperately needed.
CASE Symptoms point to yet another prolapse recurrence
A 52-year-old woman presents with a bulge and pressure in her vagina. She has undergone 2 prior reconstructive surgeries. The first was a vaginal hysterectomy, anterior and posterior repair, and sling; the second was an abdominal procedure that included a sacrocolpopexy and paravaginal repair.
A physical examination reveals a recurrent 4th-degree cystocele that protrudes 2 cm beyond the hymenal ring. The vault and posterior compartment are well supported, and the patient reports no incontinence, a fact confirmed by urodynamics testing. She asks that you do everything in your power to prevent further recurrence.
How do you proceed?
This patient ultimately underwent anterior colporrhaphy and vaginal paravaginal repair using a decellularized dermal cadaveric implant. She was still doing well 1 year later, with no recurrence.
Despite success stories like this one, the use of graft materials to repair cystoceles and rectoceles is controversial. One reason is the difficulty of interpreting published data, since studies lack uniformity in technique, patient characteristics, graft shape, type of material, attachment sites, and duration of follow-up. Level I evidence that augmented repairs have a clear benefit over traditional repairs is sparse.
Advocates of graft materials argue that native tissue is already compromised—hence, the prolapse—making surgical failure likely.1 They claim graft materials help strengthen repairs, especially in the case of cystoceles. They also point out that adjuvant materials have been used in burns, plastic surgery, and orthopedics for more than 10 years and are generally well tolerated. Their success in hernia repairs prompted their consideration for the pelvic floor.
A pervasive problem, but only 10% to 20% seek help
Roughly 1 of 2 parous women lose pelvic support as they age, but only 10% to 20% seek medical care, with a lifetime risk of surgery for pelvic organ prolapse (POP) of 11% by age 80.2
With women living longer than ever and remaining active later in life, this percentage is likely to rise. Unfortunately, few alternatives to surgical treatment exist, and the reoperation rate for recurrence is 29%, according to a 1995 review.2 If surgical management is the only hope of cure, how can we lower the 29% recurrence rate?
Graft materials may provide part or all of the solution.
Elements of prolapse
Anterior compartment
Central and/or lateral defects can occur in the anterior compartment.
Lateral (paravaginal) defects indicate that the endopelvic connective tissue has separated from the arcus tendineus fascia pelvis. Lateral defects can be repaired vaginally or abdominally.
One study3 found that 67% of women with anterior wall prolapse had paravaginal defects, but no randomized trials have evaluated the clinical benefit of repairing these defects, compared with traditional colporrhaphies.
Central defects involve site-specific defects and/or general attenuation of the endopelvic connective tissue. These are usually repaired vaginally.
Recurrence rates for lateral and central defects range from 3% to 70%.4-8
Two large series of vaginal paravaginal repairs noted the following recurrence rates:
- Shull et al6 found a recurrence rate of 7% to the hymenal ring or beyond.
- Young et al7 observed a recurrence rate for lateral defects of 2%, with recurrence rates as high as 22% for central defects.
In a comparison of 3 techniques for vaginal repair of central defects, using strict criteria to assess anatomic outcomes, Weber et al4 found recurrence rates of 54% to 70%. Other studies show symptomatic recurrence rates of 3% to 22% for cystoceles.5,8
With grafts, both paravaginal and central defects can be repaired. Vaginal paravaginal repairs are not popular due to the technical difficulty involved. With the use of grafts, however, both paravaginal and central defects can be addressed simultaneously with relative ease.
Posterior compartment
Defects in the posterior compartment are less likely to recur. Reported success rates range from 80% to 90%.9,10
Posterior compartment defects include general attenuation of Denonvillier’s fascia or a tear anywhere along the fascia or any of its attachments.
Recurrence rates. Site-specific repairs are thought to minimize complications such as dyspareunia. However, few studies have compared the efficacy of site-specific repairs with that of traditional colporrhaphies. At our institution, women who underwent traditional colporrhaphy had fewer recurrences than controls (33% vs 14%), with no differences in postoperative symptoms such as dyspareunia, constipation, and fecal incontinence.11
Graft materials of questionable benefit. In the posterior compartment, these materials have not been shown to be beneficial, compared with traditional or site-specific repairs. Sand et al12 found no benefit for repairs in which absorbable Vicryl mesh was imbricated, but this randomized trial may have lacked sufficient power to show statistical significance. Large cohorts would be needed to show significant benefit of meshes in the posterior compartment.
A complex web of support
In the normal pelvis, support of reproductive organs depends on a complex web of muscles, fascia, and connective tissue. To ensure success, prolapse repairs should correct any separation or attenuation of tissue and preserve or enhance tissue resilience.
Risk factors for recurrent prolapse
- Poor tissue (assess tissue quality before and during surgery)
- Impaired healing
- Chronic increases in intraabdominal pressure due to obstructive pulmonary disease, asthma, or constipation
- High-grade cystocele
- Age 60 or above13
Patients with these conditions may benefit from the use of adjuvant materials in the anterior compartment.
Note that women who have had recurrences after earlier repairs may experience repeat recurrence.
Advantages of grafts
Using graft materials, the surgeon can repair all vaginal defects faster and with less effort. In the anterior compartment, a graft can be placed and anchored bilaterally from arcus to arcus tendineus, and posteriorly to the level of the spine, recreating level I support. Graft materials also offer the potential to treat stress urinary incontinence concomitantly using different shaped materials. Two authors have already described their success performing this type of repair.14
Nevertheless, great care and consideration should be devoted to actual and theoretical short- and long-term risks, many of which have not been fully elucidated.
Once a successful material is identified or developed, it may decrease operating time and morbidity in vaginal surgeries. It may also reduce the higher hospital costs normally associated with abdominal procedures.
Types of graft materials
There are 2 types of materials: synthetic or biologic. Synthetic materials can be further classified into permanent or absorbable.
The most widely used biologic materials include allografts such as human freeze-dried or solvent-dehydrated fascia lata (Tutoplast), decellularized human cadaveric dermis (Alloderm, Repliform), porcine dermal xenografts such as Pelvicol or Intexene, and bovine pericardial implants (Veritas).
Soft polypropylene meshes such as Gynemesh and Atrium are commonly used permanent materials, and polyglactin 910 is an absorbable material (TABLE).
TABLE
How successful are adjuvant materials in cystocele and rectocele repairs?
| MATERIAL (SIZE IN CM) | AUTHOR | NO. IN STUDY | RECURRENCE RATE (%) | SITE OF ATTACHMENT | FOLLOW-UP (MONTHS) | COMPLICATIONS |
|---|---|---|---|---|---|---|
| BIOLOGIC MATERIALS | ||||||
| Alloderm 3×7 patch with concomitant sling | Chung29 | 19 | 16 | Pubocervical fascia | 28 | None |
| Intexene 6×8 with sling | Gomelsky et al 200420 | 70 | 9 stage II 4 stage III | Arcus tendineus fascia pelvis | 24 | 1 wound separation |
| Solvent-dehydrated cadaveric fascia lata patch with sling | Gandhi et al 200521 | 76 patch vs 72 no patch | 21 vs 29, respectively (P=.23) | Overlay | 13 | None |
| Alloderm 3×7 trapezoid | Clemons et al 200322 | 33 | 41 stage II 3 symptomatic | Arcus tendineus fascia pelvis | 18 | None |
| SYNTHETIC MATERIALS WITH CONCOMITANT SLINGS* | ||||||
| Marlex 10×3×5 | Nicita 199823 | 44 | 0 | Arcus tendineus fascia pelvis | 13 | 1 vaginal erosion |
| Polyglactin 910 absorbable mesh | Sand et al12 | 80 mesh vs 80 no mesh | 25 vs 43 stage II cystoceles, respectively(P=.02) | Insert in the anterior and posterior colporrhaphy suture line | 12 | None |
| Polyglactin 910 absorbable mesh | Weber et al4 | 26 with mesh + standard repair; 24 with ultra-lateral repair; 33 with standard repair | 58 vs 54 vs 70 stage II, respectively (P=.58) | Overlay | 23 | None |
| SYNTHETIC PERMANENT GRAFTS WITHOUT CONCOMITANT SLINGS | ||||||
| Marlex trapezoid | Julian 199619 | 12 with 12 without | 0 vs 33, respectively | Arcus tendineus fascia pelvis | 24 | 3 vaginal erosions |
| Mixed-fiber mesh (polyglactin 910 and polyester 5×5) | Migliari and Usai 199924 | 12 | 25 | Pubourethral and cardinal ligaments | 20 | None |
| Prolene (Atrium) | Dwyer and O’Reilly25 | 64 anterior 50 posterior | 6 grade II | Tension-free | 29 | 8% vaginal erosion 1 rectovaginal fistula |
| Gynemesh 6×15 | de Tayrac et al 200526 | 87 | 7 stage II 2 stage III | Tension-free | 24 | 8% vaginal erosion |
| Prolene mesh patch | Milani et al 200527 | 32 anterior 31 posterior | 6 stage II | Fixed to endopelvic connective tissue | 17 | 20% anterior, 63% posterior dyspareunia; 13% vaginal erosion (anterior); 1 pelvic abscess (posterior) |
| Prolene mesh (double-wing shape) | Natale et al 2000 28 | 138 | 3 | Tension-free | 18 | 9% vaginal erosion 7% dyspareunia 1 hematoma |
| *Absorbable and permanent. | ||||||
Classification of synthetic materials
- Type 1 grafts are totally macroporous (>75 μm), which allows fibroblast, macrophage, and collagen penetration with angiogenesis. Examples include Prolene and Marlex meshes.
- Type 2 mesh is microporous (<10 μm in 1 dimension). This prevents penetration of fibroblasts, macrophages, or collagen. Gore-Tex is an example of a Type 2 mesh.
- Type 3 mesh is macroporous (>75 μm) with multifilamentous or microporous components. Examples include Mersilene (braided Dacron mesh), Teflon (polytetrafluoroethylene [PTFE]), Surgipro (braided polypropylene mesh), and MycroMesh (perforated PTFE patch).
- Type 4 mesh has a submicron pore size that prevents penetration. Examples include Silastic, Cellgard (polypropylene sheeting), and Preclude pericardial membrane/Preclude dura-substitute.1
2 other important properties are composition of fibers (multifilamentous materials commonly have interstices less than 10 microns) and flexibility (which has a bearing on erosion of the material).1
Bacteria can penetrate pores smaller than 1 μm, whereas polymorphonuclear white blood cells and macrophages need a pore size larger than 10 μm, and capillary ingrowth requires a size larger than 75 microns. Thus, Type 1 offers the advantages of larger pore size and monofilamentous interstices to allow for capillary ingrowth.
Which material is best?
Although the literature is difficult to interpret because of the diversity of studies and other factors, some findings are worth noting:
- Tutoplast and Alloderm appear to have the best tensile strength, maximum load to capacity, and microscopic architecture similar to the original tissue.15-17 However, these qualities were documented prior to implantation in vivo.
- Slings appear to help prevent cystocele recurrences, according to a study by Goldberg et al.18
- A fascial patch had no benefit when placed as an overlay in the anterior compartment in a randomized, controlled trial (involving 162 women) by Sand et al.12
- Marlex. One group of women with recurrent prolapse underwent synthetic graft (Marlex) augmentation with bilateral ATFP attachment, while the other group had anterior colporrhaphy only.19 None of the women who received grafts had further recurrence, while 33% of the control group did. However, 25% of the women with the graft had vaginal erosions.
- Polyglactin 910 had a protective effect when embedded in the plication, according to Sand et al.12 However, it had no benefit when used as an overlay to a traditional repair in a study by Weber et al.4 The discrepancy may be related to small sample size; the study by Weber et al was powered to detect only a 30% difference. However, these studies suggest that it is not only the type of graft that is important, but how it is used or attached.
In general, synthetic grafts may have slightly higher success rates, whereas biologic materials appear to be better tolerated.
Prospective, comparative trials of these materials are desperately needed.
1. Cervigni M, Natale F. The use of synthetics in the treatment of pelvic organ prolapse. Curr Opin Urol. 2001;11:429-435.
2. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
3. Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-573.
4. Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
5. Porges RF, Smilen SW. Long-term analysis of the surgical management of pelvic support defects. Am J Obstet Gynecol. 1994;171:1518-1526.
6. Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal segment: an analysis of support defects, operative morbidity, and anatomic outcome. Am J Obstet Gynecol. 1994;171:1429-1436.
7. Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001;185:1360-1366.
8. Macer GA. Transabdominal repair of cystocele, a 20-year experience, compared with the traditional vaginal approach. Trans Pac Coast Obstet Gynecol Soc. 1978;45:116-120.
9. Cundiff GW, Weidner AC, Visco AG, Addison WA, Bump RC. An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol. 1998;179:1451-1456.
10. Paraiso MF, Ballard LA, Walters MD, Lee JC, et al. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996;175:1423-1430.
11. Abramov Y, et al. Site-specific rectocele repair compared with standard posterior colporrhaphy. Obstet Gynecol. 2005;105:314-318.
12. Sand PK, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol. 2001;184:1357-1362.
13. Whitesides JL, Weber AM, Meyn LA, Walters MD. Risk factors for prolapse recurrence after vaginal repair. Obstet Gynecol Surv. 2005;60:164-165.
14. Kobashi KC, Mee SL, Leach GE. A new technique for cystocele repair and transvaginal sling: the cadaveric prolapse repair and sling (CAPS). Urology. 2000;56:9-14.
15. Lemer ML, Chaikin DC, Blaivas JG. Tissue strength analysis of autologous and cadaveric allografts for the pubovaginal sling. Neurourol Urodyn. 1999;18:497-503.
16. Choe JM, Kothandapani R, et al. Autologous, cadaveric, and synthetic materials used in sling surgery: comparative biomechanical analysis. Urology. 2001;58:482-486.
17. Scalfani AP. Biophysical and microscopic analysis of homologous dermal and fascial materials for facial aesthetic and reconstructive uses. Arch Facial Plast Surg. 2002;4:164-171.
18. Goldberg RP, et al. Protective effect of suburethral slings on postoperative cystocele recurrence after reconstructive pelvic operation. Am J Obstet Gynecol. 2001;185:1307-1312.
19. Julian TM. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472-1475.
20. Gomelsky A, Rudy DC, Dmochowski RR. Porcine dermis interposition graft for repair of high grade anterior compartment defects with or without concomitant pelvic organ prolapse procedures. J Urol. 2004;171:1581-1584.
21. Gandhi S, et al. A prospective randomized trial of solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Am J Obstet Gynecol. 2005;192:1649-1654.
22. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618.
23. Nicita G. A new operation for genitourinary prolapse. J Urol. 1998;160:741-745.
24. Migliari R, Usai E. Treatment results using a mixed fiber mesh in patients with grade IV cystocele. J Urol. 1999;161:1255-1258.
25. Dwyer PL, O’Reilly BA. Transvaginal repair of anterior and posterior compartment prolapse with Atrium polypropylene mesh. BJOG. 2004;111:831-836.
26. de Tayrac R, Gervaise A, Chauveaud A, Fernandez H. Tension-free polypropylene mesh for vaginal repair of anterior vaginal wall prolapse. J Reprod Med. 2005;50:75-80.
27. Milani R, et al. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with prolene mesh. BJOG. 2005;112:107-111.
28. Natale F, Marziali S, Cervigni M. Tension-free cystocele repair (TCR): long-term follow-up. Proceedings of the 25th annual meeting of the International Urogynecological Association. 2000;22-25.
29. Chung SY, et al. Technique of combined pubovaginal sling and cystocele using a single piece of cadaveric dermal graft. Urology. 2002;59:538-541.
Dr. Botros has no financial relationships relevant to this article. Dr. Sand receives grant/research support from Boston Scientific, and is a consultant and speaker for American Medical Systems and Boston Scientific.
1. Cervigni M, Natale F. The use of synthetics in the treatment of pelvic organ prolapse. Curr Opin Urol. 2001;11:429-435.
2. Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
3. Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568-573.
4. Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299-1304.
5. Porges RF, Smilen SW. Long-term analysis of the surgical management of pelvic support defects. Am J Obstet Gynecol. 1994;171:1518-1526.
6. Shull BL, Benn SJ, Kuehl TJ. Surgical management of prolapse of the anterior vaginal segment: an analysis of support defects, operative morbidity, and anatomic outcome. Am J Obstet Gynecol. 1994;171:1429-1436.
7. Young SB, Daman JJ, Bony LG. Vaginal paravaginal repair: one-year outcomes. Am J Obstet Gynecol. 2001;185:1360-1366.
8. Macer GA. Transabdominal repair of cystocele, a 20-year experience, compared with the traditional vaginal approach. Trans Pac Coast Obstet Gynecol Soc. 1978;45:116-120.
9. Cundiff GW, Weidner AC, Visco AG, Addison WA, Bump RC. An anatomic and functional assessment of the discrete defect rectocele repair. Am J Obstet Gynecol. 1998;179:1451-1456.
10. Paraiso MF, Ballard LA, Walters MD, Lee JC, et al. Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1996;175:1423-1430.
11. Abramov Y, et al. Site-specific rectocele repair compared with standard posterior colporrhaphy. Obstet Gynecol. 2005;105:314-318.
12. Sand PK, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol. 2001;184:1357-1362.
13. Whitesides JL, Weber AM, Meyn LA, Walters MD. Risk factors for prolapse recurrence after vaginal repair. Obstet Gynecol Surv. 2005;60:164-165.
14. Kobashi KC, Mee SL, Leach GE. A new technique for cystocele repair and transvaginal sling: the cadaveric prolapse repair and sling (CAPS). Urology. 2000;56:9-14.
15. Lemer ML, Chaikin DC, Blaivas JG. Tissue strength analysis of autologous and cadaveric allografts for the pubovaginal sling. Neurourol Urodyn. 1999;18:497-503.
16. Choe JM, Kothandapani R, et al. Autologous, cadaveric, and synthetic materials used in sling surgery: comparative biomechanical analysis. Urology. 2001;58:482-486.
17. Scalfani AP. Biophysical and microscopic analysis of homologous dermal and fascial materials for facial aesthetic and reconstructive uses. Arch Facial Plast Surg. 2002;4:164-171.
18. Goldberg RP, et al. Protective effect of suburethral slings on postoperative cystocele recurrence after reconstructive pelvic operation. Am J Obstet Gynecol. 2001;185:1307-1312.
19. Julian TM. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472-1475.
20. Gomelsky A, Rudy DC, Dmochowski RR. Porcine dermis interposition graft for repair of high grade anterior compartment defects with or without concomitant pelvic organ prolapse procedures. J Urol. 2004;171:1581-1584.
21. Gandhi S, et al. A prospective randomized trial of solvent dehydrated fascia lata for the prevention of recurrent anterior vaginal wall prolapse. Am J Obstet Gynecol. 2005;192:1649-1654.
22. Clemons JL, Myers DL, Aguilar VC, Arya LA. Vaginal paravaginal repair with an AlloDerm graft. Am J Obstet Gynecol. 2003;189:1612-1618.
23. Nicita G. A new operation for genitourinary prolapse. J Urol. 1998;160:741-745.
24. Migliari R, Usai E. Treatment results using a mixed fiber mesh in patients with grade IV cystocele. J Urol. 1999;161:1255-1258.
25. Dwyer PL, O’Reilly BA. Transvaginal repair of anterior and posterior compartment prolapse with Atrium polypropylene mesh. BJOG. 2004;111:831-836.
26. de Tayrac R, Gervaise A, Chauveaud A, Fernandez H. Tension-free polypropylene mesh for vaginal repair of anterior vaginal wall prolapse. J Reprod Med. 2005;50:75-80.
27. Milani R, et al. Functional and anatomical outcome of anterior and posterior vaginal prolapse repair with prolene mesh. BJOG. 2005;112:107-111.
28. Natale F, Marziali S, Cervigni M. Tension-free cystocele repair (TCR): long-term follow-up. Proceedings of the 25th annual meeting of the International Urogynecological Association. 2000;22-25.
29. Chung SY, et al. Technique of combined pubovaginal sling and cystocele using a single piece of cadaveric dermal graft. Urology. 2002;59:538-541.
Dr. Botros has no financial relationships relevant to this article. Dr. Sand receives grant/research support from Boston Scientific, and is a consultant and speaker for American Medical Systems and Boston Scientific.
IN THIS ARTICLE
Pelvic organ prolapse: Which operation for which patient?
The more numerous the choices of surgical techniques for pelvic organ prolapse, the less agreement there is on which operation is best. Further complicating the picture is the industry’s push to consider augmentation with synthetic or biologic materials on an almost routine basis.
Few scientific comparisons of the various approaches have been performed, however. To help shed some light on surgical decision-making, we convened an expert panel to review published data and explore our experience with selected procedures.
What to consider before choosing a procedure
- A woman’s desires regarding sexual activity are a critical piece of information, just as are her general health and history of pelvic surgery.
- It also helps to know which symptoms of her prolapse and related pelvic floor disorders she finds most bothersome.
KARRAM: When a woman with symptomatic pelvic organ prolapse desires surgical correction, what factors do you explore before deciding which procedure to use?
BRUBAKER: I make an effort to determine the woman’s readiness to undergo surgery and her expectations for it, as well as any concomitant pelvic floor or medical/surgical conditions.
Other important factors that I consider include her pelvic surgical history—specifically, whether she has undergone earlier continence and/or prolapse repairs—and the presence of any materials in the proposed surgical site, especially foreign bodies that may limit dissection planes or have eroded into pelvic viscera.
I also consider her desire (or lack of it) for sexual activity, and her preferred route of surgical access.
Patient’s lifestyle should sway surgical decision
PARAISO: I take into account her age and stage of prolapse; vaginal length; innervation of the pelvic floor; hormonal status; desire for uterine preservation and coitus; symptoms of sexual, urinary, or bowel dysfunction; and any comorbidities that influence her eligibility for anesthesia or chronically increase intra-abdominal pressure. Connective tissue disorders are also important, as are any coexisting medical conditions that impede healing.
Lifestyle has an impact, too, especially if she regularly performs heavy manual labor.
After assessing the patient’s history and performing an examination, I target the prolapse and functional symptoms and correlating anatomic defects that exacerbate her quality of life. I tailor her surgical therapy in order to correct her symptoms and minimize compensatory defects and de novo dysfunction.
Ask her to prioritize her complaints
SHULL: I have the patient list her complaints in order of their severity and impact on her lifestyle.
Next, I complete a detailed pelvic exam, including use of a mirror to demonstrate the findings to her. If appropriate, I test bladder or bowel function.
At that point, we discuss what I think are appropriate options, although in some situations I may not be able to treat all her complaints with equal success.
KARRAM: I think prioritizing the patient’s complaints is a good idea. My foremost aim is to determine what the woman is most bothered by. If it is prolapse symptoms such as pressure and tissue protrusion, with no functional derangements, I try to ensure that my surgical repair provides durable support but does not create de novo derangements such as stress incontinence. So, for example, I try to determine whether she has preexisting stress incontinence that is masked by the prolapse.
Correlation between prolapse and dysfunction can be weak
Obviously, if the patient has many functional derangements associated with the prolapse symptoms, the preoperative consultation becomes much more complicated. Although the complexity may not change my surgical approach, I think it is important for the patient to understand that the correlation between anatomic descent and the functional derangement may not be very good.
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati.
- Linda Brubaker, MD, MS, Assistant Dean of Clinical and Translational Research, and Professor and Director, Division of Female Pelvic Medicine and Reconstructive Pelvic Surgery, Departments of Obstetrics & Gynecology and Urology, Loyola University Health System, Chicago.
- Marie Fidela Paraiso, MD, Co-Director of Female Pelvic Medicine and Reconstructive Surgery, Department of Obstetrics and Gynecology and the Urological Institute, The Cleveland Clinic, Cleveland, Ohio.
- Bob L. Shull, MD, Vice Chairman, Department of Obstetrics and Gynecology, and Chief, Section of Female Pelvic Medicine and Pelvic Reconstructive Surgery, Scott & White Health Care System, Temple, Tex.
As previously mentioned, I make a point to ask about sexual function. If the woman is elderly and has no intention of being sexually active again, I may consider a very tight or obliterative repair because these are much less invasive than conventional repairs.
Is one surgical route superior?
- There is no consensus among experts as to the preferred route of surgery for advanced pelvic organ prolapse.
KARRAM: Numerous vaginal, abdominal, and laparoscopic procedures have been described. Which route do you prefer?
BRUBAKER: I don’t prefer any laparoscopic procedures, but I am flexible about vaginal or abdominal approaches.
Among vaginal procedures, I prefer uterosacral suspension at the time of hysterectomy, or the Michigan modification of sacrospinous ligament suspension when the patient has already undergone hysterectomy.
As for abdominal procedures, I prefer sacrocolpopexy with Mersilene mesh.
In my hands, these reconstructive procedures give predictable results that allow me to appropriately counsel patients preoperatively.
KARRAM: Why do you dislike the laparoscopic approach?
BRUBAKER: It is not a matter of “dislike,” but a matter of getting the most reliable result for my patient. When scientific evidence from well-done clinical trials demonstrates the equivalency of laparoscopic procedures, I fully anticipate incorporating them into my practice. Similarly, the novel use of the robot may be useful in reconstructive pelvic surgery.
Laparoscopic repair can produce good results in the right hands
PARAISO: I prefer the laparoscopic and vaginal routes. In fact, I have converted most abdominal procedures to laparoscopic access. I have nearly 10 years of experience with laparoscopic sacrocolpopexy, with excellent success.
My colleagues and I did a cohort study that showed equal cure rates for this procedure, compared with open sacrocolpopexy.1 I also have had great success with the vaginal route when performing uterosacral vaginal vault suspensions.
Patients are referred to me or seek me out specifically for minimally invasive procedures, so the majority of operations I perform are laparoscopic procedures with or without vaginal procedures, or vaginal procedures alone.
Vaginal approach is possible in high percentage of cases
SHULL: I probably perform 98% of reconstructive cases transvaginally. If the woman has urinary incontinence as well as prolapse, I usually perform a midurethral sling procedure along with the repair.
KARRAM: I do roughly 90% of prolapse repairs transvaginally. For the last 6 to 8 years, my colleagues and I have utilized a high uterosacral vaginal vault suspension to support the vaginal cuff. We do so in conjunction with a modified internal McCall-type procedure to obliterate the cul-de-sac. We also do site-specific anterior and posterior colporrhaphy as needed, and a synthetic midurethral sling if the patient has stress incontinence.
In very young patients (under 35 years of age) or those who have substantial recurrent prolapse or a prolapsed foreshortened vagina, we consider abdominal sacrocolpopexy with synthetic mesh as our primary operation. In such cases, we commonly perform retropubic repair for incontinence and paravaginal defects, as well as posterior repair and perineorrhaphy.
I have very little experience with laparoscopic prolapse repairs.
Abdominal sacrocolpopexy is anatomically superior
KARRAM: Dr. Brubaker, you just chaired a consensus panel on pelvic organ prolapse for the International Consultation on Incontinence. This panel reviewed all the published literature on the topic. What conclusions did it reach about the various surgical procedures for pelvic organ prolapse?
BRUBAKER: The “big picture” findings were that abdominal sacrocolpopexy is anatomically superior to the other procedures, but carries a higher rate of short-term morbidity than transvaginal procedures. Since that panel, a review on sacrocolpopexy by Nygaard et al2 highlighted the strengths, weaknesses, and uncertainties of this procedure.
We found no indications for routine use of ancillary materials when performing primary transvaginal repairs.
What is the best operation for advanced prolapse?
- The best procedure depends on the patient’s health, type and extent of prolapse, and sexual activity. Surgical history also is key.
KARRAM: Let’s say a 60-year-old woman with advanced, symptomatic, primary pelvic organ prolapse presents to you for surgical treatment. The findings include posthysterectomy vaginal vault prolapse with a large cystocele, large rectocele, and an enterocele. What operation would you perform?
SHULL: I would probably elect a transvaginal approach using the uterosacral ligaments to suspend the cuff and reapproximate the connective tissue of the anterior and posterior compartments. My colleagues and I described this technique.3
PARAISO: If the patient is physically and sexually active and willing to undergo synthetic graft implantation, I would perform laparoscopic sacrocolpopexy, especially if previous transvaginal apical suspension has failed, if she has a foreshortened vagina, or if she has denervation of her pelvic floor.
Check for defecatory dysfunction
If it is necessary for her to manually digitate her vagina or splint her perineum to defecate, I would perform a rectocele repair and perineorrhaphy.
If she is not a candidate for laparoscopic or abdominal surgery because of a history of multiple procedures for inflammatory bowel disease or severe adhesions, has not had a previous transvaginal apical suspension, and has intact pelvic floor innervation, I would perform either uterosacral vaginal vault suspension or sacrospinous ligament suspension with concomitant anterior and posterior repair.
I would consider offering this patient a tension-free vaginal mesh “kit” procedure (with synthetic mesh) if she:
- has failed previous vaginal procedures,
- has multiple comorbidities,
- is not a candidate for laparoscopic or abdominal surgery,
- desires to remain sexually active, and
- is willing to use and has no contraindications to intravaginal estrogen therapy.
If she does not wish to remain sexually active and is not a good operative candidate, I would offer colpectomy and colpocleisis with perineorrhaphy.
Which circumstances pose special challenges?
- Apical suspension is a critical factor in success and durability of the surgery.
KARRAM: Which segment of the pelvic floor do you find most challenging when correcting advanced pelvic organ prolapse?
SHULL: My colleagues and I have reported our experience with several techniques of vaginal repair for prolapse, including sacrospinous ligament suspension, iliococcygeus fascial suspension, and uterosacral ligament suspension. When we analyzed specific sites in the vagina, the anterior compartment always had the greatest percentage of persistent or recurrent loss of support.
Our best success has been with uterosacral ligament suspension.
Vaginal apex is key to success
PARAISO: I also find the anterior segment challenging. However, if I am able to suspend the vaginal apex well, management of the anterior vaginal wall is less challenging. The anterior wall fails because treatment of high transverse cystoceles and anterior enteroceles (less commonly seen) depends on the apical suspension. Many of these defects go untreated because they are often not detected.
BRUBAKER: I agree with Dr. Paraiso. If you get the apex up solidly, you’re usually home free.
KARRAM: Yes. If one can get good, high, durable support to the apex, the other segments of the pelvic floor are much more likely to endure.
Are unaugmented repairs doomed to fail?
- Despite claims to the contrary, reoperation rates are low for most conventional repairs.
- Surgeons may be tempted to adopt graft augmentation techniques to keep up with “Dr. Jones.”
KARRAM: As you know, there has been a recent push to consider augmenting most pelvic organ prolapse repairs with either biologic or synthetic mesh. This approach is based on a perception that conventional repairs without augmentation inevitably will fail. Do you agree with this perception?
SHULL: Not based on my own experience. Mesh has been effectively and safely used for midurethral slings and abdominal sacrocolpopexies, but there are not enough data on the use of allografts, xenografts, or meshes to be able to counsel a patient properly about their safety, efficacy, or long-term effects.
PARAISO: I agree that this perception is being promoted, prompting many physicians to adopt graft augmentation techniques to keep up with “Dr. Jones” or to offer their patients “cutting-edge” treatment. Despite the fact that conventional procedures are often described as having high failure rates, the reoperation rates in most series are low. Nevertheless, augmentation with biologic grafts has been widely adopted without prior investigation or data.
Traditional and site-specific repairs versus graft augmentation
My colleagues and I just presented a manuscript on traditional posterior colporrhaphy, site-specific rectocele repair, and site-specific repair with graft augmentation using a porcine small intestinal submucosa bioengineered collagen matrix.
The anatomic cure rate was substantially higher in the traditional and site-specific groups when compared with the graft augmentation arm, with cure rates of 86% and 78% versus 54%, respectively (P=.02).4
Currently, my indications for a mesh-augmented prolapse repair are:
- Nonexistent or suboptimal autologous tissue
- Need to augment weak or absent endopelvic tissue
- Connective tissue disorder
- Unavoidable stress on the repair (eg, chronic lifting, chronic obstructive pulmonary disease, chronic straining to defecate, obesity)
- Need to bridge a space such as sacral colpopexy
- Concern about vaginal length or caliber
- Denervated pelvic floor
- Recurrent prolapse
Surgeons should not believe that graft augmentation compensates for surgical mediocrity or patient risk factors for pelvic organ prolapse.
The key to success: Maintain the vaginal axis
KARRAM: I don’t believe all traditional repairs are bound to fail. Many factors play into recurrent prolapse. I think most people overlook the fact that the vagina is very sensitive to its axis. Any operation that alters the vaginal axis will seriously weaken the vagina opposite the distorted axis.
For example, we know that sacrospinous ligament suspension retroverts the vagina and sets women up for recurrence or development of anterior vaginal wall prolapse. Another example is a Burch colposuspension that anteverts a portion of the vagina and sets patients up for posterior vaginal wall defects in the form of a rectocele and enterocele.
Too much simplification
I also think surgeons and device manufacturers have attempted to simplify what, in reality, is a very complicated clinical picture. So many factors are involved in the identification and appropriate utilization of support structures for a durable prolapse repair.
Since Dr. Shull’s popularization of a high uterosacral suspension, we have had very good long-term success with transvaginal vault repair. Also, over time I have realized that it is possible to mobilize a substantial amount of durable fascial tissue—which is nothing more than the muscular lining of the vagina—to appropriately support the anterior and posterior vaginal walls.
That said, the results are far from perfect. I would estimate our anatomic failure rate at 15% to 20% over the long term.
Does augmentation add complications?
When it comes to mesh, we have to ask: Is it truly going to increase durability? If it is, is that going to be at the expense of a new set of complications such as mesh erosion or extrusion and dyspareunia?
The only way to answer these questions is with a randomized trial with long-term follow-up. At this time, such data are not available.
Are tension-free repair kits the wave of the future?
- It’s not yet time to make these kits the standard, although preliminary data are promising.
KARRAM: Do you think the synthetic mesh repairs now being promoted as tension-free repairs utilizing numerous industry-created “kits” will be the future of prolapse repair?
BRUBAKER: I hope not.
KARRAM: At present, I would say the answer to that question is “no.” However, I was very reluctant to accept synthetic midurethral slings, and they have turned out to be the standard of care.
SHULL: These products are the future for surgeons who allow industry to dictate their practice styles. For those of us who are more skeptical, we will change only after there is adequate scientific information to do so.
Though unproven, kits do have advantages
PARAISO: I agree. Even so, in many ways, these kits make sense. Operative time is greatly reduced and incisions are small, thus offering the advantage of minimally invasive procedures. Preliminary data at 6 months show excellent anatomic outcomes. However, the graft extrusion rate is high with the kit procedures, compared with existing evidence on synthetic mesh erosion associated with abdominal and laparoscopic sacral colpopexy.
In addition, current synthetic materials are not ideal. Long-term sequelae of transvaginal implantation of these meshes are not known. Nor do we have long-term data on sexual function.
By and large, these procedures are blind and involve the transobturator and transgluteal (ischiorectal fossa) spaces—uncharted waters for many gynecologic surgeons. Further, many gynecologic surgeons lack extensive training or experience in sacrospinous ligament suspension, iliococcygeus fascia suspension, and vaginal paravaginal defect repair, which are prerequisites for the kit procedures.
Matching the kit procedure to the patient
As for patient selection, women for whom previous anterior repair (with or without biologic graft), paravaginal defect repair, and apical suspension have failed, and who continue to have asymptomatic anterior vaginal wall prolapse are the best candidates for anterior kit procedures. The best candidates for posterior and apical segment kit procedures are women in whom transvaginal apical suspension has failed, and who are not suited for laparoscopic or abdominal procedures.
The only impediments to widespread adoption of these procedures for years to come will be adverse events or technology so advanced it makes gene modification possible, rendering surgery obsolete.
KARRAM: I think we need better and longer follow-up. Most of the surgeons currently using these procedures are proponents of the repairs, in my opinion, but until results from comparative trials become available, we won’t really know how they compare to conventional repairs.
Bringing up the next generation
- Residents need as much hands-on experience as possible, including cadaveric dissections, urodynamic labs, and urogynecologic clinics—even virtual-reality models.
KARRAM: How do we best train residents in the appropriate evaluation and surgical management of these very common pelvic floor disorders?
BRUBAKER: Carefully and ethically. Encourage them to be good consumers of surgical literature and to resist the urge to constantly demonstrate the “latest and greatest” until we have solid evidence.
PARAISO: Residents can learn from discussions of surgical indications prior to pelvic reconstructive procedures in which they are involved, attendance at urogynecologic clinics, urodynamic lab rotations, and study of urogynecologic learning modules and current clinical textbooks that focus on these surgeries.
Given the decrease in resident work hours, which translates to fewer cases, cadaver labs are also helpful.
Virtual-reality models are being developed and will be available this decade.
Golden rule of surgery: Do unto others…
SHULL: I would advise residents to treat every woman as you would your wife, mother, sister, daughter, or yourself. That may mean using a consultant for some of your patients.
Those of us who are teachers must use all available resources, including didactic instruction, video clips, cadaver dissection, simulators, and hands-on supervision in the operating room.
Those who are learning new procedures must be willing to accept constructive comments and critically evaluate their own skills.
KARRAM: I think it is important to continue training residents in the basics of pelvic floor support and anatomy. If the future involves passing needles into blind spaces, the outcomes will be disastrous if the surgeon is not comfortable with the relevant anatomy.
Secondly, surgeons should maintain their skills in proven operations such as abdominal sacrocolpopexy and sacrospinous ligament suspension. As we gain experience and long-term data, other procedures can be added more easily if we have a good understanding of the conventional repairs.
1. Paraiso MFR, Walters MD, Rackley RR, Melek S, Hugney C. Laparoscopic and open sacral colpopexies: a cohort study. Am J Obstet Gynecol. 2005;192:1752-1758.
2. Nygaard IE, McCreery R, Brubaker L, et al. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
3. Shull BL, Bachofen CG, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse using uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
4. Paraiso MFR, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006 [in press].
Dr. Karram receives grant/research support from American Medical Systems, Carbon Medical, Gynecare, and Pfizer; is a consultant to Gynecare; and is a speaker for Gynecare, Indevus, and Ortho-McNeil. Dr. Paraiso has received grant/research support from American Medical Systems and Organogenesis Inc.; and is a consultant to American Medical Systems, and Ethicon Women’s Health and Urology. Dr. Brubaker and Dr. Shull report no financial relationships relevant to this article.
The more numerous the choices of surgical techniques for pelvic organ prolapse, the less agreement there is on which operation is best. Further complicating the picture is the industry’s push to consider augmentation with synthetic or biologic materials on an almost routine basis.
Few scientific comparisons of the various approaches have been performed, however. To help shed some light on surgical decision-making, we convened an expert panel to review published data and explore our experience with selected procedures.
What to consider before choosing a procedure
- A woman’s desires regarding sexual activity are a critical piece of information, just as are her general health and history of pelvic surgery.
- It also helps to know which symptoms of her prolapse and related pelvic floor disorders she finds most bothersome.
KARRAM: When a woman with symptomatic pelvic organ prolapse desires surgical correction, what factors do you explore before deciding which procedure to use?
BRUBAKER: I make an effort to determine the woman’s readiness to undergo surgery and her expectations for it, as well as any concomitant pelvic floor or medical/surgical conditions.
Other important factors that I consider include her pelvic surgical history—specifically, whether she has undergone earlier continence and/or prolapse repairs—and the presence of any materials in the proposed surgical site, especially foreign bodies that may limit dissection planes or have eroded into pelvic viscera.
I also consider her desire (or lack of it) for sexual activity, and her preferred route of surgical access.
Patient’s lifestyle should sway surgical decision
PARAISO: I take into account her age and stage of prolapse; vaginal length; innervation of the pelvic floor; hormonal status; desire for uterine preservation and coitus; symptoms of sexual, urinary, or bowel dysfunction; and any comorbidities that influence her eligibility for anesthesia or chronically increase intra-abdominal pressure. Connective tissue disorders are also important, as are any coexisting medical conditions that impede healing.
Lifestyle has an impact, too, especially if she regularly performs heavy manual labor.
After assessing the patient’s history and performing an examination, I target the prolapse and functional symptoms and correlating anatomic defects that exacerbate her quality of life. I tailor her surgical therapy in order to correct her symptoms and minimize compensatory defects and de novo dysfunction.
Ask her to prioritize her complaints
SHULL: I have the patient list her complaints in order of their severity and impact on her lifestyle.
Next, I complete a detailed pelvic exam, including use of a mirror to demonstrate the findings to her. If appropriate, I test bladder or bowel function.
At that point, we discuss what I think are appropriate options, although in some situations I may not be able to treat all her complaints with equal success.
KARRAM: I think prioritizing the patient’s complaints is a good idea. My foremost aim is to determine what the woman is most bothered by. If it is prolapse symptoms such as pressure and tissue protrusion, with no functional derangements, I try to ensure that my surgical repair provides durable support but does not create de novo derangements such as stress incontinence. So, for example, I try to determine whether she has preexisting stress incontinence that is masked by the prolapse.
Correlation between prolapse and dysfunction can be weak
Obviously, if the patient has many functional derangements associated with the prolapse symptoms, the preoperative consultation becomes much more complicated. Although the complexity may not change my surgical approach, I think it is important for the patient to understand that the correlation between anatomic descent and the functional derangement may not be very good.
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati.
- Linda Brubaker, MD, MS, Assistant Dean of Clinical and Translational Research, and Professor and Director, Division of Female Pelvic Medicine and Reconstructive Pelvic Surgery, Departments of Obstetrics & Gynecology and Urology, Loyola University Health System, Chicago.
- Marie Fidela Paraiso, MD, Co-Director of Female Pelvic Medicine and Reconstructive Surgery, Department of Obstetrics and Gynecology and the Urological Institute, The Cleveland Clinic, Cleveland, Ohio.
- Bob L. Shull, MD, Vice Chairman, Department of Obstetrics and Gynecology, and Chief, Section of Female Pelvic Medicine and Pelvic Reconstructive Surgery, Scott & White Health Care System, Temple, Tex.
As previously mentioned, I make a point to ask about sexual function. If the woman is elderly and has no intention of being sexually active again, I may consider a very tight or obliterative repair because these are much less invasive than conventional repairs.
Is one surgical route superior?
- There is no consensus among experts as to the preferred route of surgery for advanced pelvic organ prolapse.
KARRAM: Numerous vaginal, abdominal, and laparoscopic procedures have been described. Which route do you prefer?
BRUBAKER: I don’t prefer any laparoscopic procedures, but I am flexible about vaginal or abdominal approaches.
Among vaginal procedures, I prefer uterosacral suspension at the time of hysterectomy, or the Michigan modification of sacrospinous ligament suspension when the patient has already undergone hysterectomy.
As for abdominal procedures, I prefer sacrocolpopexy with Mersilene mesh.
In my hands, these reconstructive procedures give predictable results that allow me to appropriately counsel patients preoperatively.
KARRAM: Why do you dislike the laparoscopic approach?
BRUBAKER: It is not a matter of “dislike,” but a matter of getting the most reliable result for my patient. When scientific evidence from well-done clinical trials demonstrates the equivalency of laparoscopic procedures, I fully anticipate incorporating them into my practice. Similarly, the novel use of the robot may be useful in reconstructive pelvic surgery.
Laparoscopic repair can produce good results in the right hands
PARAISO: I prefer the laparoscopic and vaginal routes. In fact, I have converted most abdominal procedures to laparoscopic access. I have nearly 10 years of experience with laparoscopic sacrocolpopexy, with excellent success.
My colleagues and I did a cohort study that showed equal cure rates for this procedure, compared with open sacrocolpopexy.1 I also have had great success with the vaginal route when performing uterosacral vaginal vault suspensions.
Patients are referred to me or seek me out specifically for minimally invasive procedures, so the majority of operations I perform are laparoscopic procedures with or without vaginal procedures, or vaginal procedures alone.
Vaginal approach is possible in high percentage of cases
SHULL: I probably perform 98% of reconstructive cases transvaginally. If the woman has urinary incontinence as well as prolapse, I usually perform a midurethral sling procedure along with the repair.
KARRAM: I do roughly 90% of prolapse repairs transvaginally. For the last 6 to 8 years, my colleagues and I have utilized a high uterosacral vaginal vault suspension to support the vaginal cuff. We do so in conjunction with a modified internal McCall-type procedure to obliterate the cul-de-sac. We also do site-specific anterior and posterior colporrhaphy as needed, and a synthetic midurethral sling if the patient has stress incontinence.
In very young patients (under 35 years of age) or those who have substantial recurrent prolapse or a prolapsed foreshortened vagina, we consider abdominal sacrocolpopexy with synthetic mesh as our primary operation. In such cases, we commonly perform retropubic repair for incontinence and paravaginal defects, as well as posterior repair and perineorrhaphy.
I have very little experience with laparoscopic prolapse repairs.
Abdominal sacrocolpopexy is anatomically superior
KARRAM: Dr. Brubaker, you just chaired a consensus panel on pelvic organ prolapse for the International Consultation on Incontinence. This panel reviewed all the published literature on the topic. What conclusions did it reach about the various surgical procedures for pelvic organ prolapse?
BRUBAKER: The “big picture” findings were that abdominal sacrocolpopexy is anatomically superior to the other procedures, but carries a higher rate of short-term morbidity than transvaginal procedures. Since that panel, a review on sacrocolpopexy by Nygaard et al2 highlighted the strengths, weaknesses, and uncertainties of this procedure.
We found no indications for routine use of ancillary materials when performing primary transvaginal repairs.
What is the best operation for advanced prolapse?
- The best procedure depends on the patient’s health, type and extent of prolapse, and sexual activity. Surgical history also is key.
KARRAM: Let’s say a 60-year-old woman with advanced, symptomatic, primary pelvic organ prolapse presents to you for surgical treatment. The findings include posthysterectomy vaginal vault prolapse with a large cystocele, large rectocele, and an enterocele. What operation would you perform?
SHULL: I would probably elect a transvaginal approach using the uterosacral ligaments to suspend the cuff and reapproximate the connective tissue of the anterior and posterior compartments. My colleagues and I described this technique.3
PARAISO: If the patient is physically and sexually active and willing to undergo synthetic graft implantation, I would perform laparoscopic sacrocolpopexy, especially if previous transvaginal apical suspension has failed, if she has a foreshortened vagina, or if she has denervation of her pelvic floor.
Check for defecatory dysfunction
If it is necessary for her to manually digitate her vagina or splint her perineum to defecate, I would perform a rectocele repair and perineorrhaphy.
If she is not a candidate for laparoscopic or abdominal surgery because of a history of multiple procedures for inflammatory bowel disease or severe adhesions, has not had a previous transvaginal apical suspension, and has intact pelvic floor innervation, I would perform either uterosacral vaginal vault suspension or sacrospinous ligament suspension with concomitant anterior and posterior repair.
I would consider offering this patient a tension-free vaginal mesh “kit” procedure (with synthetic mesh) if she:
- has failed previous vaginal procedures,
- has multiple comorbidities,
- is not a candidate for laparoscopic or abdominal surgery,
- desires to remain sexually active, and
- is willing to use and has no contraindications to intravaginal estrogen therapy.
If she does not wish to remain sexually active and is not a good operative candidate, I would offer colpectomy and colpocleisis with perineorrhaphy.
Which circumstances pose special challenges?
- Apical suspension is a critical factor in success and durability of the surgery.
KARRAM: Which segment of the pelvic floor do you find most challenging when correcting advanced pelvic organ prolapse?
SHULL: My colleagues and I have reported our experience with several techniques of vaginal repair for prolapse, including sacrospinous ligament suspension, iliococcygeus fascial suspension, and uterosacral ligament suspension. When we analyzed specific sites in the vagina, the anterior compartment always had the greatest percentage of persistent or recurrent loss of support.
Our best success has been with uterosacral ligament suspension.
Vaginal apex is key to success
PARAISO: I also find the anterior segment challenging. However, if I am able to suspend the vaginal apex well, management of the anterior vaginal wall is less challenging. The anterior wall fails because treatment of high transverse cystoceles and anterior enteroceles (less commonly seen) depends on the apical suspension. Many of these defects go untreated because they are often not detected.
BRUBAKER: I agree with Dr. Paraiso. If you get the apex up solidly, you’re usually home free.
KARRAM: Yes. If one can get good, high, durable support to the apex, the other segments of the pelvic floor are much more likely to endure.
Are unaugmented repairs doomed to fail?
- Despite claims to the contrary, reoperation rates are low for most conventional repairs.
- Surgeons may be tempted to adopt graft augmentation techniques to keep up with “Dr. Jones.”
KARRAM: As you know, there has been a recent push to consider augmenting most pelvic organ prolapse repairs with either biologic or synthetic mesh. This approach is based on a perception that conventional repairs without augmentation inevitably will fail. Do you agree with this perception?
SHULL: Not based on my own experience. Mesh has been effectively and safely used for midurethral slings and abdominal sacrocolpopexies, but there are not enough data on the use of allografts, xenografts, or meshes to be able to counsel a patient properly about their safety, efficacy, or long-term effects.
PARAISO: I agree that this perception is being promoted, prompting many physicians to adopt graft augmentation techniques to keep up with “Dr. Jones” or to offer their patients “cutting-edge” treatment. Despite the fact that conventional procedures are often described as having high failure rates, the reoperation rates in most series are low. Nevertheless, augmentation with biologic grafts has been widely adopted without prior investigation or data.
Traditional and site-specific repairs versus graft augmentation
My colleagues and I just presented a manuscript on traditional posterior colporrhaphy, site-specific rectocele repair, and site-specific repair with graft augmentation using a porcine small intestinal submucosa bioengineered collagen matrix.
The anatomic cure rate was substantially higher in the traditional and site-specific groups when compared with the graft augmentation arm, with cure rates of 86% and 78% versus 54%, respectively (P=.02).4
Currently, my indications for a mesh-augmented prolapse repair are:
- Nonexistent or suboptimal autologous tissue
- Need to augment weak or absent endopelvic tissue
- Connective tissue disorder
- Unavoidable stress on the repair (eg, chronic lifting, chronic obstructive pulmonary disease, chronic straining to defecate, obesity)
- Need to bridge a space such as sacral colpopexy
- Concern about vaginal length or caliber
- Denervated pelvic floor
- Recurrent prolapse
Surgeons should not believe that graft augmentation compensates for surgical mediocrity or patient risk factors for pelvic organ prolapse.
The key to success: Maintain the vaginal axis
KARRAM: I don’t believe all traditional repairs are bound to fail. Many factors play into recurrent prolapse. I think most people overlook the fact that the vagina is very sensitive to its axis. Any operation that alters the vaginal axis will seriously weaken the vagina opposite the distorted axis.
For example, we know that sacrospinous ligament suspension retroverts the vagina and sets women up for recurrence or development of anterior vaginal wall prolapse. Another example is a Burch colposuspension that anteverts a portion of the vagina and sets patients up for posterior vaginal wall defects in the form of a rectocele and enterocele.
Too much simplification
I also think surgeons and device manufacturers have attempted to simplify what, in reality, is a very complicated clinical picture. So many factors are involved in the identification and appropriate utilization of support structures for a durable prolapse repair.
Since Dr. Shull’s popularization of a high uterosacral suspension, we have had very good long-term success with transvaginal vault repair. Also, over time I have realized that it is possible to mobilize a substantial amount of durable fascial tissue—which is nothing more than the muscular lining of the vagina—to appropriately support the anterior and posterior vaginal walls.
That said, the results are far from perfect. I would estimate our anatomic failure rate at 15% to 20% over the long term.
Does augmentation add complications?
When it comes to mesh, we have to ask: Is it truly going to increase durability? If it is, is that going to be at the expense of a new set of complications such as mesh erosion or extrusion and dyspareunia?
The only way to answer these questions is with a randomized trial with long-term follow-up. At this time, such data are not available.
Are tension-free repair kits the wave of the future?
- It’s not yet time to make these kits the standard, although preliminary data are promising.
KARRAM: Do you think the synthetic mesh repairs now being promoted as tension-free repairs utilizing numerous industry-created “kits” will be the future of prolapse repair?
BRUBAKER: I hope not.
KARRAM: At present, I would say the answer to that question is “no.” However, I was very reluctant to accept synthetic midurethral slings, and they have turned out to be the standard of care.
SHULL: These products are the future for surgeons who allow industry to dictate their practice styles. For those of us who are more skeptical, we will change only after there is adequate scientific information to do so.
Though unproven, kits do have advantages
PARAISO: I agree. Even so, in many ways, these kits make sense. Operative time is greatly reduced and incisions are small, thus offering the advantage of minimally invasive procedures. Preliminary data at 6 months show excellent anatomic outcomes. However, the graft extrusion rate is high with the kit procedures, compared with existing evidence on synthetic mesh erosion associated with abdominal and laparoscopic sacral colpopexy.
In addition, current synthetic materials are not ideal. Long-term sequelae of transvaginal implantation of these meshes are not known. Nor do we have long-term data on sexual function.
By and large, these procedures are blind and involve the transobturator and transgluteal (ischiorectal fossa) spaces—uncharted waters for many gynecologic surgeons. Further, many gynecologic surgeons lack extensive training or experience in sacrospinous ligament suspension, iliococcygeus fascia suspension, and vaginal paravaginal defect repair, which are prerequisites for the kit procedures.
Matching the kit procedure to the patient
As for patient selection, women for whom previous anterior repair (with or without biologic graft), paravaginal defect repair, and apical suspension have failed, and who continue to have asymptomatic anterior vaginal wall prolapse are the best candidates for anterior kit procedures. The best candidates for posterior and apical segment kit procedures are women in whom transvaginal apical suspension has failed, and who are not suited for laparoscopic or abdominal procedures.
The only impediments to widespread adoption of these procedures for years to come will be adverse events or technology so advanced it makes gene modification possible, rendering surgery obsolete.
KARRAM: I think we need better and longer follow-up. Most of the surgeons currently using these procedures are proponents of the repairs, in my opinion, but until results from comparative trials become available, we won’t really know how they compare to conventional repairs.
Bringing up the next generation
- Residents need as much hands-on experience as possible, including cadaveric dissections, urodynamic labs, and urogynecologic clinics—even virtual-reality models.
KARRAM: How do we best train residents in the appropriate evaluation and surgical management of these very common pelvic floor disorders?
BRUBAKER: Carefully and ethically. Encourage them to be good consumers of surgical literature and to resist the urge to constantly demonstrate the “latest and greatest” until we have solid evidence.
PARAISO: Residents can learn from discussions of surgical indications prior to pelvic reconstructive procedures in which they are involved, attendance at urogynecologic clinics, urodynamic lab rotations, and study of urogynecologic learning modules and current clinical textbooks that focus on these surgeries.
Given the decrease in resident work hours, which translates to fewer cases, cadaver labs are also helpful.
Virtual-reality models are being developed and will be available this decade.
Golden rule of surgery: Do unto others…
SHULL: I would advise residents to treat every woman as you would your wife, mother, sister, daughter, or yourself. That may mean using a consultant for some of your patients.
Those of us who are teachers must use all available resources, including didactic instruction, video clips, cadaver dissection, simulators, and hands-on supervision in the operating room.
Those who are learning new procedures must be willing to accept constructive comments and critically evaluate their own skills.
KARRAM: I think it is important to continue training residents in the basics of pelvic floor support and anatomy. If the future involves passing needles into blind spaces, the outcomes will be disastrous if the surgeon is not comfortable with the relevant anatomy.
Secondly, surgeons should maintain their skills in proven operations such as abdominal sacrocolpopexy and sacrospinous ligament suspension. As we gain experience and long-term data, other procedures can be added more easily if we have a good understanding of the conventional repairs.
The more numerous the choices of surgical techniques for pelvic organ prolapse, the less agreement there is on which operation is best. Further complicating the picture is the industry’s push to consider augmentation with synthetic or biologic materials on an almost routine basis.
Few scientific comparisons of the various approaches have been performed, however. To help shed some light on surgical decision-making, we convened an expert panel to review published data and explore our experience with selected procedures.
What to consider before choosing a procedure
- A woman’s desires regarding sexual activity are a critical piece of information, just as are her general health and history of pelvic surgery.
- It also helps to know which symptoms of her prolapse and related pelvic floor disorders she finds most bothersome.
KARRAM: When a woman with symptomatic pelvic organ prolapse desires surgical correction, what factors do you explore before deciding which procedure to use?
BRUBAKER: I make an effort to determine the woman’s readiness to undergo surgery and her expectations for it, as well as any concomitant pelvic floor or medical/surgical conditions.
Other important factors that I consider include her pelvic surgical history—specifically, whether she has undergone earlier continence and/or prolapse repairs—and the presence of any materials in the proposed surgical site, especially foreign bodies that may limit dissection planes or have eroded into pelvic viscera.
I also consider her desire (or lack of it) for sexual activity, and her preferred route of surgical access.
Patient’s lifestyle should sway surgical decision
PARAISO: I take into account her age and stage of prolapse; vaginal length; innervation of the pelvic floor; hormonal status; desire for uterine preservation and coitus; symptoms of sexual, urinary, or bowel dysfunction; and any comorbidities that influence her eligibility for anesthesia or chronically increase intra-abdominal pressure. Connective tissue disorders are also important, as are any coexisting medical conditions that impede healing.
Lifestyle has an impact, too, especially if she regularly performs heavy manual labor.
After assessing the patient’s history and performing an examination, I target the prolapse and functional symptoms and correlating anatomic defects that exacerbate her quality of life. I tailor her surgical therapy in order to correct her symptoms and minimize compensatory defects and de novo dysfunction.
Ask her to prioritize her complaints
SHULL: I have the patient list her complaints in order of their severity and impact on her lifestyle.
Next, I complete a detailed pelvic exam, including use of a mirror to demonstrate the findings to her. If appropriate, I test bladder or bowel function.
At that point, we discuss what I think are appropriate options, although in some situations I may not be able to treat all her complaints with equal success.
KARRAM: I think prioritizing the patient’s complaints is a good idea. My foremost aim is to determine what the woman is most bothered by. If it is prolapse symptoms such as pressure and tissue protrusion, with no functional derangements, I try to ensure that my surgical repair provides durable support but does not create de novo derangements such as stress incontinence. So, for example, I try to determine whether she has preexisting stress incontinence that is masked by the prolapse.
Correlation between prolapse and dysfunction can be weak
Obviously, if the patient has many functional derangements associated with the prolapse symptoms, the preoperative consultation becomes much more complicated. Although the complexity may not change my surgical approach, I think it is important for the patient to understand that the correlation between anatomic descent and the functional derangement may not be very good.
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati.
- Linda Brubaker, MD, MS, Assistant Dean of Clinical and Translational Research, and Professor and Director, Division of Female Pelvic Medicine and Reconstructive Pelvic Surgery, Departments of Obstetrics & Gynecology and Urology, Loyola University Health System, Chicago.
- Marie Fidela Paraiso, MD, Co-Director of Female Pelvic Medicine and Reconstructive Surgery, Department of Obstetrics and Gynecology and the Urological Institute, The Cleveland Clinic, Cleveland, Ohio.
- Bob L. Shull, MD, Vice Chairman, Department of Obstetrics and Gynecology, and Chief, Section of Female Pelvic Medicine and Pelvic Reconstructive Surgery, Scott & White Health Care System, Temple, Tex.
As previously mentioned, I make a point to ask about sexual function. If the woman is elderly and has no intention of being sexually active again, I may consider a very tight or obliterative repair because these are much less invasive than conventional repairs.
Is one surgical route superior?
- There is no consensus among experts as to the preferred route of surgery for advanced pelvic organ prolapse.
KARRAM: Numerous vaginal, abdominal, and laparoscopic procedures have been described. Which route do you prefer?
BRUBAKER: I don’t prefer any laparoscopic procedures, but I am flexible about vaginal or abdominal approaches.
Among vaginal procedures, I prefer uterosacral suspension at the time of hysterectomy, or the Michigan modification of sacrospinous ligament suspension when the patient has already undergone hysterectomy.
As for abdominal procedures, I prefer sacrocolpopexy with Mersilene mesh.
In my hands, these reconstructive procedures give predictable results that allow me to appropriately counsel patients preoperatively.
KARRAM: Why do you dislike the laparoscopic approach?
BRUBAKER: It is not a matter of “dislike,” but a matter of getting the most reliable result for my patient. When scientific evidence from well-done clinical trials demonstrates the equivalency of laparoscopic procedures, I fully anticipate incorporating them into my practice. Similarly, the novel use of the robot may be useful in reconstructive pelvic surgery.
Laparoscopic repair can produce good results in the right hands
PARAISO: I prefer the laparoscopic and vaginal routes. In fact, I have converted most abdominal procedures to laparoscopic access. I have nearly 10 years of experience with laparoscopic sacrocolpopexy, with excellent success.
My colleagues and I did a cohort study that showed equal cure rates for this procedure, compared with open sacrocolpopexy.1 I also have had great success with the vaginal route when performing uterosacral vaginal vault suspensions.
Patients are referred to me or seek me out specifically for minimally invasive procedures, so the majority of operations I perform are laparoscopic procedures with or without vaginal procedures, or vaginal procedures alone.
Vaginal approach is possible in high percentage of cases
SHULL: I probably perform 98% of reconstructive cases transvaginally. If the woman has urinary incontinence as well as prolapse, I usually perform a midurethral sling procedure along with the repair.
KARRAM: I do roughly 90% of prolapse repairs transvaginally. For the last 6 to 8 years, my colleagues and I have utilized a high uterosacral vaginal vault suspension to support the vaginal cuff. We do so in conjunction with a modified internal McCall-type procedure to obliterate the cul-de-sac. We also do site-specific anterior and posterior colporrhaphy as needed, and a synthetic midurethral sling if the patient has stress incontinence.
In very young patients (under 35 years of age) or those who have substantial recurrent prolapse or a prolapsed foreshortened vagina, we consider abdominal sacrocolpopexy with synthetic mesh as our primary operation. In such cases, we commonly perform retropubic repair for incontinence and paravaginal defects, as well as posterior repair and perineorrhaphy.
I have very little experience with laparoscopic prolapse repairs.
Abdominal sacrocolpopexy is anatomically superior
KARRAM: Dr. Brubaker, you just chaired a consensus panel on pelvic organ prolapse for the International Consultation on Incontinence. This panel reviewed all the published literature on the topic. What conclusions did it reach about the various surgical procedures for pelvic organ prolapse?
BRUBAKER: The “big picture” findings were that abdominal sacrocolpopexy is anatomically superior to the other procedures, but carries a higher rate of short-term morbidity than transvaginal procedures. Since that panel, a review on sacrocolpopexy by Nygaard et al2 highlighted the strengths, weaknesses, and uncertainties of this procedure.
We found no indications for routine use of ancillary materials when performing primary transvaginal repairs.
What is the best operation for advanced prolapse?
- The best procedure depends on the patient’s health, type and extent of prolapse, and sexual activity. Surgical history also is key.
KARRAM: Let’s say a 60-year-old woman with advanced, symptomatic, primary pelvic organ prolapse presents to you for surgical treatment. The findings include posthysterectomy vaginal vault prolapse with a large cystocele, large rectocele, and an enterocele. What operation would you perform?
SHULL: I would probably elect a transvaginal approach using the uterosacral ligaments to suspend the cuff and reapproximate the connective tissue of the anterior and posterior compartments. My colleagues and I described this technique.3
PARAISO: If the patient is physically and sexually active and willing to undergo synthetic graft implantation, I would perform laparoscopic sacrocolpopexy, especially if previous transvaginal apical suspension has failed, if she has a foreshortened vagina, or if she has denervation of her pelvic floor.
Check for defecatory dysfunction
If it is necessary for her to manually digitate her vagina or splint her perineum to defecate, I would perform a rectocele repair and perineorrhaphy.
If she is not a candidate for laparoscopic or abdominal surgery because of a history of multiple procedures for inflammatory bowel disease or severe adhesions, has not had a previous transvaginal apical suspension, and has intact pelvic floor innervation, I would perform either uterosacral vaginal vault suspension or sacrospinous ligament suspension with concomitant anterior and posterior repair.
I would consider offering this patient a tension-free vaginal mesh “kit” procedure (with synthetic mesh) if she:
- has failed previous vaginal procedures,
- has multiple comorbidities,
- is not a candidate for laparoscopic or abdominal surgery,
- desires to remain sexually active, and
- is willing to use and has no contraindications to intravaginal estrogen therapy.
If she does not wish to remain sexually active and is not a good operative candidate, I would offer colpectomy and colpocleisis with perineorrhaphy.
Which circumstances pose special challenges?
- Apical suspension is a critical factor in success and durability of the surgery.
KARRAM: Which segment of the pelvic floor do you find most challenging when correcting advanced pelvic organ prolapse?
SHULL: My colleagues and I have reported our experience with several techniques of vaginal repair for prolapse, including sacrospinous ligament suspension, iliococcygeus fascial suspension, and uterosacral ligament suspension. When we analyzed specific sites in the vagina, the anterior compartment always had the greatest percentage of persistent or recurrent loss of support.
Our best success has been with uterosacral ligament suspension.
Vaginal apex is key to success
PARAISO: I also find the anterior segment challenging. However, if I am able to suspend the vaginal apex well, management of the anterior vaginal wall is less challenging. The anterior wall fails because treatment of high transverse cystoceles and anterior enteroceles (less commonly seen) depends on the apical suspension. Many of these defects go untreated because they are often not detected.
BRUBAKER: I agree with Dr. Paraiso. If you get the apex up solidly, you’re usually home free.
KARRAM: Yes. If one can get good, high, durable support to the apex, the other segments of the pelvic floor are much more likely to endure.
Are unaugmented repairs doomed to fail?
- Despite claims to the contrary, reoperation rates are low for most conventional repairs.
- Surgeons may be tempted to adopt graft augmentation techniques to keep up with “Dr. Jones.”
KARRAM: As you know, there has been a recent push to consider augmenting most pelvic organ prolapse repairs with either biologic or synthetic mesh. This approach is based on a perception that conventional repairs without augmentation inevitably will fail. Do you agree with this perception?
SHULL: Not based on my own experience. Mesh has been effectively and safely used for midurethral slings and abdominal sacrocolpopexies, but there are not enough data on the use of allografts, xenografts, or meshes to be able to counsel a patient properly about their safety, efficacy, or long-term effects.
PARAISO: I agree that this perception is being promoted, prompting many physicians to adopt graft augmentation techniques to keep up with “Dr. Jones” or to offer their patients “cutting-edge” treatment. Despite the fact that conventional procedures are often described as having high failure rates, the reoperation rates in most series are low. Nevertheless, augmentation with biologic grafts has been widely adopted without prior investigation or data.
Traditional and site-specific repairs versus graft augmentation
My colleagues and I just presented a manuscript on traditional posterior colporrhaphy, site-specific rectocele repair, and site-specific repair with graft augmentation using a porcine small intestinal submucosa bioengineered collagen matrix.
The anatomic cure rate was substantially higher in the traditional and site-specific groups when compared with the graft augmentation arm, with cure rates of 86% and 78% versus 54%, respectively (P=.02).4
Currently, my indications for a mesh-augmented prolapse repair are:
- Nonexistent or suboptimal autologous tissue
- Need to augment weak or absent endopelvic tissue
- Connective tissue disorder
- Unavoidable stress on the repair (eg, chronic lifting, chronic obstructive pulmonary disease, chronic straining to defecate, obesity)
- Need to bridge a space such as sacral colpopexy
- Concern about vaginal length or caliber
- Denervated pelvic floor
- Recurrent prolapse
Surgeons should not believe that graft augmentation compensates for surgical mediocrity or patient risk factors for pelvic organ prolapse.
The key to success: Maintain the vaginal axis
KARRAM: I don’t believe all traditional repairs are bound to fail. Many factors play into recurrent prolapse. I think most people overlook the fact that the vagina is very sensitive to its axis. Any operation that alters the vaginal axis will seriously weaken the vagina opposite the distorted axis.
For example, we know that sacrospinous ligament suspension retroverts the vagina and sets women up for recurrence or development of anterior vaginal wall prolapse. Another example is a Burch colposuspension that anteverts a portion of the vagina and sets patients up for posterior vaginal wall defects in the form of a rectocele and enterocele.
Too much simplification
I also think surgeons and device manufacturers have attempted to simplify what, in reality, is a very complicated clinical picture. So many factors are involved in the identification and appropriate utilization of support structures for a durable prolapse repair.
Since Dr. Shull’s popularization of a high uterosacral suspension, we have had very good long-term success with transvaginal vault repair. Also, over time I have realized that it is possible to mobilize a substantial amount of durable fascial tissue—which is nothing more than the muscular lining of the vagina—to appropriately support the anterior and posterior vaginal walls.
That said, the results are far from perfect. I would estimate our anatomic failure rate at 15% to 20% over the long term.
Does augmentation add complications?
When it comes to mesh, we have to ask: Is it truly going to increase durability? If it is, is that going to be at the expense of a new set of complications such as mesh erosion or extrusion and dyspareunia?
The only way to answer these questions is with a randomized trial with long-term follow-up. At this time, such data are not available.
Are tension-free repair kits the wave of the future?
- It’s not yet time to make these kits the standard, although preliminary data are promising.
KARRAM: Do you think the synthetic mesh repairs now being promoted as tension-free repairs utilizing numerous industry-created “kits” will be the future of prolapse repair?
BRUBAKER: I hope not.
KARRAM: At present, I would say the answer to that question is “no.” However, I was very reluctant to accept synthetic midurethral slings, and they have turned out to be the standard of care.
SHULL: These products are the future for surgeons who allow industry to dictate their practice styles. For those of us who are more skeptical, we will change only after there is adequate scientific information to do so.
Though unproven, kits do have advantages
PARAISO: I agree. Even so, in many ways, these kits make sense. Operative time is greatly reduced and incisions are small, thus offering the advantage of minimally invasive procedures. Preliminary data at 6 months show excellent anatomic outcomes. However, the graft extrusion rate is high with the kit procedures, compared with existing evidence on synthetic mesh erosion associated with abdominal and laparoscopic sacral colpopexy.
In addition, current synthetic materials are not ideal. Long-term sequelae of transvaginal implantation of these meshes are not known. Nor do we have long-term data on sexual function.
By and large, these procedures are blind and involve the transobturator and transgluteal (ischiorectal fossa) spaces—uncharted waters for many gynecologic surgeons. Further, many gynecologic surgeons lack extensive training or experience in sacrospinous ligament suspension, iliococcygeus fascia suspension, and vaginal paravaginal defect repair, which are prerequisites for the kit procedures.
Matching the kit procedure to the patient
As for patient selection, women for whom previous anterior repair (with or without biologic graft), paravaginal defect repair, and apical suspension have failed, and who continue to have asymptomatic anterior vaginal wall prolapse are the best candidates for anterior kit procedures. The best candidates for posterior and apical segment kit procedures are women in whom transvaginal apical suspension has failed, and who are not suited for laparoscopic or abdominal procedures.
The only impediments to widespread adoption of these procedures for years to come will be adverse events or technology so advanced it makes gene modification possible, rendering surgery obsolete.
KARRAM: I think we need better and longer follow-up. Most of the surgeons currently using these procedures are proponents of the repairs, in my opinion, but until results from comparative trials become available, we won’t really know how they compare to conventional repairs.
Bringing up the next generation
- Residents need as much hands-on experience as possible, including cadaveric dissections, urodynamic labs, and urogynecologic clinics—even virtual-reality models.
KARRAM: How do we best train residents in the appropriate evaluation and surgical management of these very common pelvic floor disorders?
BRUBAKER: Carefully and ethically. Encourage them to be good consumers of surgical literature and to resist the urge to constantly demonstrate the “latest and greatest” until we have solid evidence.
PARAISO: Residents can learn from discussions of surgical indications prior to pelvic reconstructive procedures in which they are involved, attendance at urogynecologic clinics, urodynamic lab rotations, and study of urogynecologic learning modules and current clinical textbooks that focus on these surgeries.
Given the decrease in resident work hours, which translates to fewer cases, cadaver labs are also helpful.
Virtual-reality models are being developed and will be available this decade.
Golden rule of surgery: Do unto others…
SHULL: I would advise residents to treat every woman as you would your wife, mother, sister, daughter, or yourself. That may mean using a consultant for some of your patients.
Those of us who are teachers must use all available resources, including didactic instruction, video clips, cadaver dissection, simulators, and hands-on supervision in the operating room.
Those who are learning new procedures must be willing to accept constructive comments and critically evaluate their own skills.
KARRAM: I think it is important to continue training residents in the basics of pelvic floor support and anatomy. If the future involves passing needles into blind spaces, the outcomes will be disastrous if the surgeon is not comfortable with the relevant anatomy.
Secondly, surgeons should maintain their skills in proven operations such as abdominal sacrocolpopexy and sacrospinous ligament suspension. As we gain experience and long-term data, other procedures can be added more easily if we have a good understanding of the conventional repairs.
1. Paraiso MFR, Walters MD, Rackley RR, Melek S, Hugney C. Laparoscopic and open sacral colpopexies: a cohort study. Am J Obstet Gynecol. 2005;192:1752-1758.
2. Nygaard IE, McCreery R, Brubaker L, et al. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
3. Shull BL, Bachofen CG, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse using uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
4. Paraiso MFR, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006 [in press].
Dr. Karram receives grant/research support from American Medical Systems, Carbon Medical, Gynecare, and Pfizer; is a consultant to Gynecare; and is a speaker for Gynecare, Indevus, and Ortho-McNeil. Dr. Paraiso has received grant/research support from American Medical Systems and Organogenesis Inc.; and is a consultant to American Medical Systems, and Ethicon Women’s Health and Urology. Dr. Brubaker and Dr. Shull report no financial relationships relevant to this article.
1. Paraiso MFR, Walters MD, Rackley RR, Melek S, Hugney C. Laparoscopic and open sacral colpopexies: a cohort study. Am J Obstet Gynecol. 2005;192:1752-1758.
2. Nygaard IE, McCreery R, Brubaker L, et al. Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol. 2004;104:805-823.
3. Shull BL, Bachofen CG, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse using uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
4. Paraiso MFR, Barber MD, Muir TW, Walters MD. Rectocele repair: a randomized trial of three surgical techniques including graft augmentation. Am J Obstet Gynecol. 2006 [in press].
Dr. Karram receives grant/research support from American Medical Systems, Carbon Medical, Gynecare, and Pfizer; is a consultant to Gynecare; and is a speaker for Gynecare, Indevus, and Ortho-McNeil. Dr. Paraiso has received grant/research support from American Medical Systems and Organogenesis Inc.; and is a consultant to American Medical Systems, and Ethicon Women’s Health and Urology. Dr. Brubaker and Dr. Shull report no financial relationships relevant to this article.
Preventing VTE: Evidence-based perioperative tactics
Pulmonary embolism is a master of disguises. It can appear with classic symptoms such as pleuritic chest pain, hemoptysis, and tachycardia—or it can arrive more insidiously, apparent only as a slight elevation in the respiratory rate.
This matters because 40% of all deaths following gynecologic surgery are directly attributable to pulmonary emboli,1 and pulmonary emboli are the most frequent cause of postoperative death in women with uterine or cervical carcinoma.2
Deep venous thrombosis (DVT) is almost as evasive. We know the signs and symptoms of DVT of the lower extremities—pain, edema, erythema, and a prominent vascular pattern of the superficial veins—but 50% to 80% of patients with these symptoms do not have DVT, and 80% of patients with symptomatic pulmonary embolism have no antecedent signs of thrombosis in the lower extremities.2 Morbidity and expense rise dramatically with DVT, especially when postphlebitic syndrome occurs.
How can we minimize these risks?
A good outcome is most likely when we:
- recognize risk factors,
- provide appropriate perioperative prophylaxis, and
- diagnose and treat venous thromboembolism (VTE) quickly.
This article looks in detail at each of these strategies.
3 factors set the stage for thrombogenesis
- Hypercoagulable state
- Venous stasis
- Vessel endothelial injury
These factors, known as Virchow’s triad, are especially likely at the time of major surgery, or when the patient is advanced in age or has a history of DVT, cancer, lower extremity edema, or venous stasis.
Intraoperative risk factors for postoperative DVT include increased anesthesia time, greater blood loss, and need for transfusion.
Some preventive methods come close to ideal
Being aware of risk factors is vital to provide the appropriate level of prophylaxis (TABLES 1 AND 2).3,4 The first step is identifying high-risk patients and tailoring the regimen to meet their individual needs. The perfect prophylactic method is not yet devised, but would be effective, free of significant side effects, well accepted by the patient and nursing staff, widely applicable to most patient groups, and inexpensive. A number of methods come close.
TABLE 1
Risk factors for thromboembolism
| Major gynecologic surgery |
| Age >40 years |
| Malignancy |
| Previous venous thrombosis (DVT or pulmonary embolism) |
| Obesity |
| Immobility |
| Pregnancy and the postpartum period |
| Oral contraceptives, hormone therapy, or tamoxifen |
| Varicose veins |
| Inherited or acquired thrombophilia (eg, Factor V Leiden) |
| Prolonged surgical procedure |
| Radical vulvectomy, inguinal-femoral lymphadenectomy, or pelvic exenteration |
TABLE 2
Match the preventive strategy to the surgery
| SURGERY | STRATEGY | DURATION OF PROPHYLAXIS* |
|---|---|---|
| Procedures <30 min for benign disease | Prophylaxis not needed | — |
| Laparoscopic gynecologic procedures in women with additional risk factors | Unfractionated heparin, 5,000 bid or | Until hospital discharge |
| LMWH, ≤3,400 U/day or | ||
| External pneumatic compression or | ||
| Graduated compression stockings | ||
| Major surgery for benign disease without additional risk factors | Unfractionated heparin, 5,000 U bid or | Until hospital discharge |
| LMWH, <3,400 U/day or | ||
| External pneumatic compression | ||
| Extensive major surgery in women with cancer or additional risk factors | Unfractionated heparin, 5,000 U tid or | Until hospital discharge |
| LMWH, >3,400 U/day or | ||
| External pneumatic compression | ||
| *For women at particularly high risk (eg, cancer surgery, age >60 years, prior VTE), continue prophylaxis for 2–4 weeks after hospital discharge. | ||
| Modified from Geerts WH, et al20 | ||
Low-dose unfractionated heparin
The most extensively studied prophylactic method is the use of small, subcutaneous doses of heparin. More than 25 controlled trials have shown that, when heparin is given subcutaneously 2 hours before surgery and every 8 to 12 hours afterward, the incidence of DVT diminishes substantially.
The value of low-dose heparin in preventing pulmonary emboli was established by a randomized, controlled, multicenter, international trial, in which fatal postoperative pulmonary emboli declined significantly in general surgery patients given the drug every 8 hours after surgery.5 In gynecologic surgical patients, postoperative DVT also declined significantly.
Increase in minor bleeding complications. Although low-dose heparin is thought to have no measurable effect on coagulation, most large series have noted an increase in minor bleeding complications such as wound hematoma. Up to 10% to 15% of otherwise healthy patients develop transiently prolonged activated partial thromboplastin time (APTT) after 5,000 U of heparin are given subcutaneously.6
Although relatively rare, thrombocytopenia is associated with the use of low-dose heparin. It has been found in 6% of women after gynecologic surgery.6 Therefore, it is reasonable to measure platelets in any patient taking low-dose heparin longer than 4 days to screen for heparin-induced thrombocytopenia.
Fear of major bleeding complications is unsubstantiated. There is ample evidence from placebo-controlled, blinded trials and meta-analysis that the risk of clinically important bleeding does not increase. Moreover, detailed analysis demonstrates that low-dose heparin has a good risk-to-benefit ratio and is cost-effective.
Low-molecular-weight heparins
These drugs are fragments of unfractionated heparin that vary in size from 4,500 to 6,500 daltons. Low-molecular-weight heparin (LMWH) has more anti-Xa and less antithrombin activity than unfractionated heparin and thus has less of an effect on partial thromboplastin time. LMWH may also lead to fewer bleeding complications.7
Once-daily dosing is possible. An increased half-life of 4 hours for LMWH produces greater bioavailability than with low-dose heparin. This allows once-daily dosing.
Pick one: Convenience or cost
Randomized controlled trials have compared LMWH to unfractionated heparin in gynecologic surgical patients. In all studies, DVT occurred in similar, low numbers of women regardless of the heparin used. Bleeding complications also were similar.8
A meta-analysis of general surgery and gynecologic surgery patients from 32 trials likewise found daily LMWH to be as effective as unfractionated heparin in DVT prophylaxis, without any difference in hemorrhagic complications.9
The choice of drugs often boils down to convenience versus cost: Prophylactic LMWH can be given once a day (compared with 2 or 3 times for unfractionated heparin), but is much more expensive.
Mechanical prophylactic methods
External pneumatic compression rivals low-dose heparin. The largest body of literature on mechanical methods to reduce postoperative venous stasis involves intermittent leg compression by pneumatically inflated sleeves placed around the calf or leg during surgery and after. A number of devices and sleeve designs are available, none of which has proven to be superior to the others.
In my experience, calf compression during and after gynecologic surgery lowers the incidence of DVT to a level seen with low-dose heparin. Besides increasing venous flow and pulsatile emptying of the calf veins, pneumatic compression appears to augment endogenous fibrinolysis, which may stimulate lysis of very early thrombi.10
How long is best for external compression? The optimal duration of postoperative external pneumatic compression is unclear. It may be effective when used in the operating room and for the first 24 hours postoperatively in patients with benign conditions who will ambulate on the first day after surgery.11,12
In women undergoing major surgery for gynecologic malignancy, it reduces the incidence of postoperative venous thromboemboli by nearly 3-fold, but only if calf compression is applied intraoperatively and for the first 5 postoperative days.13,14 These women may remain at risk because of stasis and a hypercoagulable state for a longer time than general surgical patients.
External pneumatic leg compression has no serious side effects or risks and is slightly more cost-effective than prophylactic drugs.15 However, to be fully effective, this method must be used consistently, in compliance with the protocol, when the patient is not ambulating.
Stockings can be a help or hazard. Controlled studies of graduated pressure stockings are limited but suggest modest benefit with careful fitting.16 Poorly fitted stockings that roll down the leg may create a tourniquet effect at the knee or mid-thigh. Another disadvantage of the stockings: The limited sizes available do not allow a perfect fit for all patients. This is especially true in obese patients.
The simplicity of elastic stockings and the absence of serious side effects are probably why stockings are often included in routine postoperative care.
Don’t overlook basic precautions. Although they may offer only modest benefit, short preoperative hospital stays and early postoperative ambulation are recommended.
Another basic strategy: elevating the foot of the bed to raise the calf above heart level. This allows gravity to drain the calf veins and should further reduce stasis.
How to detect VTE
DVT has nonspecific signs and symptoms
When DVT occurs in the lower extremities, harbingers such as pain, edema, and erythema are relatively nonspecific; 50% to 80% of patients exhibiting them do not have DVT. Conversely, approximately 80% of patients with symptomatic pulmonary emboli have no signs or symptoms of thrombosis in the lower extremities.
Because of this lack of specificity, additional tests are needed to establish DVT.
Diagnostic studies
A definitive diagnosis of DVT and pulmonary embolism is mandatory because diagnosis based on clinical symptoms and signs alone is frequently wrong. Strategies to reduce the use of ultrasound or spiral CT scanning have been put forward. These studies have evaluated outpatients using algorithms that utilize clinical probability (“clinical decision rule”) and D-dimer levels.
This strategy has been very accurate and avoids the use of ultrasound or spiral CT in low-risk patients. For example, individuals with a low probability score have an incidence of DVT below 5%, so ultrasound is unnecessary. This diagnostic strategy relies on the recognition of elevated D-dimer levels. Unfortunately, D-dimer is increased by a variety of nonthrombotic disorders, including recent surgery, hemorrhage, trauma, pregnancy, and cancer. Therefore, we cannot recommend the use of this strategy for the postoperative gynecologic surgery patient.17,18
Venography no longer the gold standard. Other diagnostic studies may be more useful. Venography has fallen from favor because it is moderately uncomfortable, requires injection of a contrast material that may trigger an allergic reaction or renal injury, and causes phlebitis in approximately 5% of patients.2 Newer, noninvasive diagnostic tests have been developed, fortunately.
Doppler ultrasound. B-mode duplex Doppler imaging is the most common technique to diagnose symptomatic venous thrombosis, especially when it arises in the proximal lower extremity. With duplex Doppler imaging, the femoral vein can be visualized, and clots may be seen directly. Compression of the vein with the tip of the ultrasound probe makes it possible to assess venous collapsibility, which is diminished when a thrombus is present.
Doppler imaging is less accurate when evaluating the calf and pelvic veins.
Magnetic resonance venography (MRV) sensitivity and specificity are comparable to venography. In addition, MRV may detect thrombi in pelvic veins that are not imaged by venography. The primary drawback is the time required to examine the lower extremity and pelvis. Further, MRV rarely identifies calf thrombi (most often not life-threatening, but potentially symptomatic) and is considerably more expensive than ultrasound.
Which prevention strategy works best?
We now consider low-molecular-weight heparin and external pneumatic compression the best choices
Because low-dose unfractionated heparin, low-molecular-weight heparin (LMWH), and external pneumatic compression all reduce the incidence of postoperative venous thromboembolism in high-risk gynecologic surgical patients, the question is: Which strategy is best?
We conducted 2 randomized clinical trials to answer this question.
Trial 1 Low-dose heparin vs pneumatic compression
Women were randomized to receive either low-dose heparin (5,000 U subcutaneously preoperatively and every 8 hours after surgery until hospital discharge) or external pneumatic compression of the calf prior to surgery and until hospital discharge.1
The incidence of DVT was identical in both groups, and no patients developed a pulmonary embolus throughout 30 days of follow-up. However, bleeding complications occurred more often in the group randomized to low-dose heparin. Specifically, nearly 25% had APTT levels in the “therapeutic” range, and significantly more patients required blood transfusions. After this trial, our institution decided to use external pneumatic compression because of its more favorable risk profile.1
Trial 2 LMWH vs pneumatic compression
The question of the best therapy arose again with the advent of LMWH, because of the possibility that these drugs carried a lower risk of bleeding complications. We therefore conducted a second trial to compare LMWH with external pneumatic compression.2
Because higher doses of LMWH had already proven to be more effective in cancer patients, we gave women in the trial 5,000 U dalteparin (Fragmin) preoperatively and 5,000 U daily postoperatively until hospital discharge.
In this trial, external pneumatic compression and LMWH produced similar low frequencies of DVT and no pulmonary emboli throughout 30 days of follow-up. We also found no association between LMWH and bleeding complications or transfusion requirements. Compliance and patient satisfaction were similar for both modalities.2
Bottom line
We now consider LMWH and external pneumatic compression the best choices for prophylaxis in gynecologic surgical patients.
REFERENCES
1. Clarke-Pearson DL, Synan IS, Dodge R, Soper JT, Berchuck A, Coleman RE. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1154.
2. Maxwell GL, Synan I, Dodge R, Carroll B, Clarke-Pearson DI. Pneumatic compression versus low molecular weight heparin in gynecologic oncology surgery: a randomized trial. Obstet Gynecol. 2001;98:989-995.
Increasing use of laparoscopic surgery raises an important question: What is the thromboembolic risk of laparoscopy itself? On one hand, many laparoscopic surgeries are prolonged, and intraperitoneal pressure from the pneumoperitoneum reduces venous flow. On the other hand, many patients who have laparoscopy have shorter hospital stays and return sooner to normal activities than those who have open procedures.
Although the risks of venous thromboembolism (VTE) have not been studied as thoroughly as other aspects of laparoscopy, they appear to be low. To date, there are no randomized trials of VTE prophylaxis among women undergoing gynecologic laparoscopy.
The prudent course
Nevertheless, it would seem prudent to consider prophylaxis when women with additional risk factors undergo extensive laparoscopic procedures.
Pulmonary embolism is often stealthy
Many of the typical signs and symptoms of pulmonary embolism are associated with other, more common pulmonary complications following surgery. Classic findings that should alert the physician to the possibility of pulmonary embolism include:
- pleuritic chest pain
- hemoptysis
- shortness of breath
- tachycardia
- tachypnea
Often, however, the signs are subtle and may include only persistent tachycardia or a slight elevation in respiration.
When pulmonary embolism is suspected, a chest x-ray, electrocardiography, and arterial blood gas assessment are warranted. Any abnormality justifies further evaluation by ventilation-perfusion lung scan or a spiral computed tomography scan of the chest. Unfortunately, a high percentage of lung scans are interpreted as “indeterminate.” In such cases, careful clinical evaluation and judgment are needed to determine whether pulmonary arteriography is necessary to document or exclude pulmonary embolism.
Immediate, aggressive therapy is crucial
The treatment of postoperative DVT requires immediate anticoagulant therapy using either unfractionated heparin or LMWH, followed by 6 months of oral anticoagulant therapy with warfarin.
Treatment strategy: Unfractionated heparin
Once VTE is diagnosed, start unfractionated heparin to prevent proximal propagation of the thrombus and allow physiologic thrombolytic pathways to dissolve the clot. After an initial IV bolus of 5,000 U, give the patient a continuous infusion of 30,000 U daily, and adjust the dose to maintain APTT levels at a therapeutic level that is 1.5 to 2.5 times the control value.
Subtherapeutic APTT levels in the first 24 hours mean a risk of recurrent thromboembolism 15 times greater than the risk in patients with appropriate levels. Therefore, aggressive management is warranted to achieve prompt anticoagulation.
Start an oral anticoagulant (warfarin) on the first day of heparin infusion, and monitor the international normalized ratio (INR) daily until a therapeutic level is achieved. The change in the INR after warfarin administration often precedes the anticoagulant effect by about 2 days, during which time low protein C levels are associated with a transient hypercoagulable state. Therefore, it is important to continue the heparin until the INR has been maintained in a therapeutic range for at least 2 days to confirm the proper warfarin dose. Intravenous heparin can be discontinued after 5 days if an adequate INR level has been established.
Alternative strategy: LMWH
A meta-analysis involving more than 1,000 patients from 19 trials suggests that LMWH is more effective, safer, and less costly than unfractionated heparin in preventing recurrent thromboembolism.19 The lower cost derives from the ability to use the drugs in an outpatient setting.
Dosages are unique and weight-adjusted according to each LMWH preparation. Because LMWH has a minimal effect on APTT, serial laboratory monitoring of APTT levels is unnecessary. Nor is monitoring of anti-Xa activity of significant benefit in the dose adjustment of LMWH.
Basic treatment of pulmonary embolism
In most cases, immediate anticoagulant therapy identical to that outlined for DVT is sufficient to prevent repeat thrombosis and embolism and to allow the patient’s endogenous thrombolytic mechanisms to lyse the pulmonary embolus.
Other interventions include:
- Respiratory support, including oxygen, bronchodilators, and intensive care.
- Although massive pulmonary emboli are usually quickly fatal, pulmonary embolectomy has been successful on rare occasions.
- Pulmonary artery catheterization and administration of thrombolytic agents may be important in patients with massive pulmonary embolism.
- Vena cava interruption may be necessary when anticoagulant therapy does not prevent rethrombosis and the formation of emboli from the lower extremities or pelvis. A vena cava umbrella or filter may be inserted percutaneously above the level of the thrombosis and caudad to the renal veins.
Take-home points
- Identify risk factors preoperatively
- VTE prophylaxis is warranted for most gynecologic surgery patients and can reduce the incidence of VTE by at least 60% with appropriate use! Plan prophylaxis in women at moderate, high, and highest risk, and remember that individuals at high and highest risk require more intense prophylaxis to realize a benefit.
- Maintain a high level of suspicion in women with signs and symptoms of DVT or pulmonary embolism in the first postoperative month. It is better to over-evaluate than to miss a potentially fatal complication.
- Treat women with VTE immediately with heparin or LMWH.
1. Jeffcoate TN, Tindall VR. Venous thrombosis and embolism in obstetrics and gynecology. Aust N Z J Obstet Gynecol. 1965;5:119-130.
2. Clarke-Pearson DL, Jelovsek FR, Creasman WT. Thromboembolism complicating surgery for cervical and uterine malignancy: incidence, risk factors, and prophylaxis. Obstet Gynecol. 1983;61:87-94.
3. Clayton JK, Anderson JA, McNicol GP. Preoperative prediction of postoperative deep vein thrombosis. BMJ. 1976;2:910-912.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Prevention of fatal postoperative pulmonary embolism by low-dose heparin. An international multicentre trial. Lancet. 1975;2:45-51.
6. Clarke-Pearson DL, DeLong ER, Synan IS, Creasman WT. Complications of low-dose heparin prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 1984;64:689-694.
7. Tapson VF, Hull RD. Management of venous thromboembolic disease. The impact of low-molecular-weight heparin. Clin Chest Med. 1995;16:281-294.
8. Borstad E, Urdal K, Handeland G, Abildgaard U. Comparison of low molecular weight heparin vs. unfractionated heparin in gynecological surgery. II: Reduced dose of low molecular weight heparin. Acta Obstet Gynecol Scand. 1992;71:471-475.
9. Jorgensen LN, Wille-Jorgensen P, Hauch O. Prophylaxis of postoperative thromboembolism with low molecular weight heparins. Br J Surg. 1993;80:689-704.
10. Allenby F, Boardman L, Pflug JJ, Calnan JS. Effects of external pneumatic intermittent compression on fibrinolysis in man. Lancet. 1973;2:1412-1414.
11. Salzman EW, Ploetz J, Bettmann M, Skillman J, Klein L. Intraoperative external pneumatic calf compression to afford long-term prophylaxis against deep vein thrombosis in urological patients. Surgery. 1980;87:239-242.
12. Nicolaides AN, Fernandes e Fernandes J, Pollock AV. Intermittent sequential pneumatic compression of the legs in the prevention of venous stasis and postoperative deep venous thrombosis. Surgery. 1980;87:69-76.
13. Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63:92-98.
14. Clarke-Pearson DL, Creasman WT, Coleman RE, Synan IS, Hinshaw WM. Perioperative external pneumatic calf compression as thromboembolism prophylaxis in gynecologic oncology: report of a randomized controlled trial. Gynecol Oncol. 1984;18:226-232.
15. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
16. Scurr JH, Ibrahim SZ, Faber RG, Le Quesne LP. The efficacy of graduated compression stockings in the prevention of deep vein thrombosis. Br J Surg. 1977;64:371-373.
17. Wells PS, Owen C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA. 2006;295:199-207.
18. Writing Group for the Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical D-dimer testing and computed tomography. JAMA. 2006;295:172-179.
19. Buller HR, Kucher N, Kipfmueller F, et al. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:401S-428S.
20. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
The author reports no financial relationships relevant to this article.
Pulmonary embolism is a master of disguises. It can appear with classic symptoms such as pleuritic chest pain, hemoptysis, and tachycardia—or it can arrive more insidiously, apparent only as a slight elevation in the respiratory rate.
This matters because 40% of all deaths following gynecologic surgery are directly attributable to pulmonary emboli,1 and pulmonary emboli are the most frequent cause of postoperative death in women with uterine or cervical carcinoma.2
Deep venous thrombosis (DVT) is almost as evasive. We know the signs and symptoms of DVT of the lower extremities—pain, edema, erythema, and a prominent vascular pattern of the superficial veins—but 50% to 80% of patients with these symptoms do not have DVT, and 80% of patients with symptomatic pulmonary embolism have no antecedent signs of thrombosis in the lower extremities.2 Morbidity and expense rise dramatically with DVT, especially when postphlebitic syndrome occurs.
How can we minimize these risks?
A good outcome is most likely when we:
- recognize risk factors,
- provide appropriate perioperative prophylaxis, and
- diagnose and treat venous thromboembolism (VTE) quickly.
This article looks in detail at each of these strategies.
3 factors set the stage for thrombogenesis
- Hypercoagulable state
- Venous stasis
- Vessel endothelial injury
These factors, known as Virchow’s triad, are especially likely at the time of major surgery, or when the patient is advanced in age or has a history of DVT, cancer, lower extremity edema, or venous stasis.
Intraoperative risk factors for postoperative DVT include increased anesthesia time, greater blood loss, and need for transfusion.
Some preventive methods come close to ideal
Being aware of risk factors is vital to provide the appropriate level of prophylaxis (TABLES 1 AND 2).3,4 The first step is identifying high-risk patients and tailoring the regimen to meet their individual needs. The perfect prophylactic method is not yet devised, but would be effective, free of significant side effects, well accepted by the patient and nursing staff, widely applicable to most patient groups, and inexpensive. A number of methods come close.
TABLE 1
Risk factors for thromboembolism
| Major gynecologic surgery |
| Age >40 years |
| Malignancy |
| Previous venous thrombosis (DVT or pulmonary embolism) |
| Obesity |
| Immobility |
| Pregnancy and the postpartum period |
| Oral contraceptives, hormone therapy, or tamoxifen |
| Varicose veins |
| Inherited or acquired thrombophilia (eg, Factor V Leiden) |
| Prolonged surgical procedure |
| Radical vulvectomy, inguinal-femoral lymphadenectomy, or pelvic exenteration |
TABLE 2
Match the preventive strategy to the surgery
| SURGERY | STRATEGY | DURATION OF PROPHYLAXIS* |
|---|---|---|
| Procedures <30 min for benign disease | Prophylaxis not needed | — |
| Laparoscopic gynecologic procedures in women with additional risk factors | Unfractionated heparin, 5,000 bid or | Until hospital discharge |
| LMWH, ≤3,400 U/day or | ||
| External pneumatic compression or | ||
| Graduated compression stockings | ||
| Major surgery for benign disease without additional risk factors | Unfractionated heparin, 5,000 U bid or | Until hospital discharge |
| LMWH, <3,400 U/day or | ||
| External pneumatic compression | ||
| Extensive major surgery in women with cancer or additional risk factors | Unfractionated heparin, 5,000 U tid or | Until hospital discharge |
| LMWH, >3,400 U/day or | ||
| External pneumatic compression | ||
| *For women at particularly high risk (eg, cancer surgery, age >60 years, prior VTE), continue prophylaxis for 2–4 weeks after hospital discharge. | ||
| Modified from Geerts WH, et al20 | ||
Low-dose unfractionated heparin
The most extensively studied prophylactic method is the use of small, subcutaneous doses of heparin. More than 25 controlled trials have shown that, when heparin is given subcutaneously 2 hours before surgery and every 8 to 12 hours afterward, the incidence of DVT diminishes substantially.
The value of low-dose heparin in preventing pulmonary emboli was established by a randomized, controlled, multicenter, international trial, in which fatal postoperative pulmonary emboli declined significantly in general surgery patients given the drug every 8 hours after surgery.5 In gynecologic surgical patients, postoperative DVT also declined significantly.
Increase in minor bleeding complications. Although low-dose heparin is thought to have no measurable effect on coagulation, most large series have noted an increase in minor bleeding complications such as wound hematoma. Up to 10% to 15% of otherwise healthy patients develop transiently prolonged activated partial thromboplastin time (APTT) after 5,000 U of heparin are given subcutaneously.6
Although relatively rare, thrombocytopenia is associated with the use of low-dose heparin. It has been found in 6% of women after gynecologic surgery.6 Therefore, it is reasonable to measure platelets in any patient taking low-dose heparin longer than 4 days to screen for heparin-induced thrombocytopenia.
Fear of major bleeding complications is unsubstantiated. There is ample evidence from placebo-controlled, blinded trials and meta-analysis that the risk of clinically important bleeding does not increase. Moreover, detailed analysis demonstrates that low-dose heparin has a good risk-to-benefit ratio and is cost-effective.
Low-molecular-weight heparins
These drugs are fragments of unfractionated heparin that vary in size from 4,500 to 6,500 daltons. Low-molecular-weight heparin (LMWH) has more anti-Xa and less antithrombin activity than unfractionated heparin and thus has less of an effect on partial thromboplastin time. LMWH may also lead to fewer bleeding complications.7
Once-daily dosing is possible. An increased half-life of 4 hours for LMWH produces greater bioavailability than with low-dose heparin. This allows once-daily dosing.
Pick one: Convenience or cost
Randomized controlled trials have compared LMWH to unfractionated heparin in gynecologic surgical patients. In all studies, DVT occurred in similar, low numbers of women regardless of the heparin used. Bleeding complications also were similar.8
A meta-analysis of general surgery and gynecologic surgery patients from 32 trials likewise found daily LMWH to be as effective as unfractionated heparin in DVT prophylaxis, without any difference in hemorrhagic complications.9
The choice of drugs often boils down to convenience versus cost: Prophylactic LMWH can be given once a day (compared with 2 or 3 times for unfractionated heparin), but is much more expensive.
Mechanical prophylactic methods
External pneumatic compression rivals low-dose heparin. The largest body of literature on mechanical methods to reduce postoperative venous stasis involves intermittent leg compression by pneumatically inflated sleeves placed around the calf or leg during surgery and after. A number of devices and sleeve designs are available, none of which has proven to be superior to the others.
In my experience, calf compression during and after gynecologic surgery lowers the incidence of DVT to a level seen with low-dose heparin. Besides increasing venous flow and pulsatile emptying of the calf veins, pneumatic compression appears to augment endogenous fibrinolysis, which may stimulate lysis of very early thrombi.10
How long is best for external compression? The optimal duration of postoperative external pneumatic compression is unclear. It may be effective when used in the operating room and for the first 24 hours postoperatively in patients with benign conditions who will ambulate on the first day after surgery.11,12
In women undergoing major surgery for gynecologic malignancy, it reduces the incidence of postoperative venous thromboemboli by nearly 3-fold, but only if calf compression is applied intraoperatively and for the first 5 postoperative days.13,14 These women may remain at risk because of stasis and a hypercoagulable state for a longer time than general surgical patients.
External pneumatic leg compression has no serious side effects or risks and is slightly more cost-effective than prophylactic drugs.15 However, to be fully effective, this method must be used consistently, in compliance with the protocol, when the patient is not ambulating.
Stockings can be a help or hazard. Controlled studies of graduated pressure stockings are limited but suggest modest benefit with careful fitting.16 Poorly fitted stockings that roll down the leg may create a tourniquet effect at the knee or mid-thigh. Another disadvantage of the stockings: The limited sizes available do not allow a perfect fit for all patients. This is especially true in obese patients.
The simplicity of elastic stockings and the absence of serious side effects are probably why stockings are often included in routine postoperative care.
Don’t overlook basic precautions. Although they may offer only modest benefit, short preoperative hospital stays and early postoperative ambulation are recommended.
Another basic strategy: elevating the foot of the bed to raise the calf above heart level. This allows gravity to drain the calf veins and should further reduce stasis.
How to detect VTE
DVT has nonspecific signs and symptoms
When DVT occurs in the lower extremities, harbingers such as pain, edema, and erythema are relatively nonspecific; 50% to 80% of patients exhibiting them do not have DVT. Conversely, approximately 80% of patients with symptomatic pulmonary emboli have no signs or symptoms of thrombosis in the lower extremities.
Because of this lack of specificity, additional tests are needed to establish DVT.
Diagnostic studies
A definitive diagnosis of DVT and pulmonary embolism is mandatory because diagnosis based on clinical symptoms and signs alone is frequently wrong. Strategies to reduce the use of ultrasound or spiral CT scanning have been put forward. These studies have evaluated outpatients using algorithms that utilize clinical probability (“clinical decision rule”) and D-dimer levels.
This strategy has been very accurate and avoids the use of ultrasound or spiral CT in low-risk patients. For example, individuals with a low probability score have an incidence of DVT below 5%, so ultrasound is unnecessary. This diagnostic strategy relies on the recognition of elevated D-dimer levels. Unfortunately, D-dimer is increased by a variety of nonthrombotic disorders, including recent surgery, hemorrhage, trauma, pregnancy, and cancer. Therefore, we cannot recommend the use of this strategy for the postoperative gynecologic surgery patient.17,18
Venography no longer the gold standard. Other diagnostic studies may be more useful. Venography has fallen from favor because it is moderately uncomfortable, requires injection of a contrast material that may trigger an allergic reaction or renal injury, and causes phlebitis in approximately 5% of patients.2 Newer, noninvasive diagnostic tests have been developed, fortunately.
Doppler ultrasound. B-mode duplex Doppler imaging is the most common technique to diagnose symptomatic venous thrombosis, especially when it arises in the proximal lower extremity. With duplex Doppler imaging, the femoral vein can be visualized, and clots may be seen directly. Compression of the vein with the tip of the ultrasound probe makes it possible to assess venous collapsibility, which is diminished when a thrombus is present.
Doppler imaging is less accurate when evaluating the calf and pelvic veins.
Magnetic resonance venography (MRV) sensitivity and specificity are comparable to venography. In addition, MRV may detect thrombi in pelvic veins that are not imaged by venography. The primary drawback is the time required to examine the lower extremity and pelvis. Further, MRV rarely identifies calf thrombi (most often not life-threatening, but potentially symptomatic) and is considerably more expensive than ultrasound.
Which prevention strategy works best?
We now consider low-molecular-weight heparin and external pneumatic compression the best choices
Because low-dose unfractionated heparin, low-molecular-weight heparin (LMWH), and external pneumatic compression all reduce the incidence of postoperative venous thromboembolism in high-risk gynecologic surgical patients, the question is: Which strategy is best?
We conducted 2 randomized clinical trials to answer this question.
Trial 1 Low-dose heparin vs pneumatic compression
Women were randomized to receive either low-dose heparin (5,000 U subcutaneously preoperatively and every 8 hours after surgery until hospital discharge) or external pneumatic compression of the calf prior to surgery and until hospital discharge.1
The incidence of DVT was identical in both groups, and no patients developed a pulmonary embolus throughout 30 days of follow-up. However, bleeding complications occurred more often in the group randomized to low-dose heparin. Specifically, nearly 25% had APTT levels in the “therapeutic” range, and significantly more patients required blood transfusions. After this trial, our institution decided to use external pneumatic compression because of its more favorable risk profile.1
Trial 2 LMWH vs pneumatic compression
The question of the best therapy arose again with the advent of LMWH, because of the possibility that these drugs carried a lower risk of bleeding complications. We therefore conducted a second trial to compare LMWH with external pneumatic compression.2
Because higher doses of LMWH had already proven to be more effective in cancer patients, we gave women in the trial 5,000 U dalteparin (Fragmin) preoperatively and 5,000 U daily postoperatively until hospital discharge.
In this trial, external pneumatic compression and LMWH produced similar low frequencies of DVT and no pulmonary emboli throughout 30 days of follow-up. We also found no association between LMWH and bleeding complications or transfusion requirements. Compliance and patient satisfaction were similar for both modalities.2
Bottom line
We now consider LMWH and external pneumatic compression the best choices for prophylaxis in gynecologic surgical patients.
REFERENCES
1. Clarke-Pearson DL, Synan IS, Dodge R, Soper JT, Berchuck A, Coleman RE. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1154.
2. Maxwell GL, Synan I, Dodge R, Carroll B, Clarke-Pearson DI. Pneumatic compression versus low molecular weight heparin in gynecologic oncology surgery: a randomized trial. Obstet Gynecol. 2001;98:989-995.
Increasing use of laparoscopic surgery raises an important question: What is the thromboembolic risk of laparoscopy itself? On one hand, many laparoscopic surgeries are prolonged, and intraperitoneal pressure from the pneumoperitoneum reduces venous flow. On the other hand, many patients who have laparoscopy have shorter hospital stays and return sooner to normal activities than those who have open procedures.
Although the risks of venous thromboembolism (VTE) have not been studied as thoroughly as other aspects of laparoscopy, they appear to be low. To date, there are no randomized trials of VTE prophylaxis among women undergoing gynecologic laparoscopy.
The prudent course
Nevertheless, it would seem prudent to consider prophylaxis when women with additional risk factors undergo extensive laparoscopic procedures.
Pulmonary embolism is often stealthy
Many of the typical signs and symptoms of pulmonary embolism are associated with other, more common pulmonary complications following surgery. Classic findings that should alert the physician to the possibility of pulmonary embolism include:
- pleuritic chest pain
- hemoptysis
- shortness of breath
- tachycardia
- tachypnea
Often, however, the signs are subtle and may include only persistent tachycardia or a slight elevation in respiration.
When pulmonary embolism is suspected, a chest x-ray, electrocardiography, and arterial blood gas assessment are warranted. Any abnormality justifies further evaluation by ventilation-perfusion lung scan or a spiral computed tomography scan of the chest. Unfortunately, a high percentage of lung scans are interpreted as “indeterminate.” In such cases, careful clinical evaluation and judgment are needed to determine whether pulmonary arteriography is necessary to document or exclude pulmonary embolism.
Immediate, aggressive therapy is crucial
The treatment of postoperative DVT requires immediate anticoagulant therapy using either unfractionated heparin or LMWH, followed by 6 months of oral anticoagulant therapy with warfarin.
Treatment strategy: Unfractionated heparin
Once VTE is diagnosed, start unfractionated heparin to prevent proximal propagation of the thrombus and allow physiologic thrombolytic pathways to dissolve the clot. After an initial IV bolus of 5,000 U, give the patient a continuous infusion of 30,000 U daily, and adjust the dose to maintain APTT levels at a therapeutic level that is 1.5 to 2.5 times the control value.
Subtherapeutic APTT levels in the first 24 hours mean a risk of recurrent thromboembolism 15 times greater than the risk in patients with appropriate levels. Therefore, aggressive management is warranted to achieve prompt anticoagulation.
Start an oral anticoagulant (warfarin) on the first day of heparin infusion, and monitor the international normalized ratio (INR) daily until a therapeutic level is achieved. The change in the INR after warfarin administration often precedes the anticoagulant effect by about 2 days, during which time low protein C levels are associated with a transient hypercoagulable state. Therefore, it is important to continue the heparin until the INR has been maintained in a therapeutic range for at least 2 days to confirm the proper warfarin dose. Intravenous heparin can be discontinued after 5 days if an adequate INR level has been established.
Alternative strategy: LMWH
A meta-analysis involving more than 1,000 patients from 19 trials suggests that LMWH is more effective, safer, and less costly than unfractionated heparin in preventing recurrent thromboembolism.19 The lower cost derives from the ability to use the drugs in an outpatient setting.
Dosages are unique and weight-adjusted according to each LMWH preparation. Because LMWH has a minimal effect on APTT, serial laboratory monitoring of APTT levels is unnecessary. Nor is monitoring of anti-Xa activity of significant benefit in the dose adjustment of LMWH.
Basic treatment of pulmonary embolism
In most cases, immediate anticoagulant therapy identical to that outlined for DVT is sufficient to prevent repeat thrombosis and embolism and to allow the patient’s endogenous thrombolytic mechanisms to lyse the pulmonary embolus.
Other interventions include:
- Respiratory support, including oxygen, bronchodilators, and intensive care.
- Although massive pulmonary emboli are usually quickly fatal, pulmonary embolectomy has been successful on rare occasions.
- Pulmonary artery catheterization and administration of thrombolytic agents may be important in patients with massive pulmonary embolism.
- Vena cava interruption may be necessary when anticoagulant therapy does not prevent rethrombosis and the formation of emboli from the lower extremities or pelvis. A vena cava umbrella or filter may be inserted percutaneously above the level of the thrombosis and caudad to the renal veins.
Take-home points
- Identify risk factors preoperatively
- VTE prophylaxis is warranted for most gynecologic surgery patients and can reduce the incidence of VTE by at least 60% with appropriate use! Plan prophylaxis in women at moderate, high, and highest risk, and remember that individuals at high and highest risk require more intense prophylaxis to realize a benefit.
- Maintain a high level of suspicion in women with signs and symptoms of DVT or pulmonary embolism in the first postoperative month. It is better to over-evaluate than to miss a potentially fatal complication.
- Treat women with VTE immediately with heparin or LMWH.
Pulmonary embolism is a master of disguises. It can appear with classic symptoms such as pleuritic chest pain, hemoptysis, and tachycardia—or it can arrive more insidiously, apparent only as a slight elevation in the respiratory rate.
This matters because 40% of all deaths following gynecologic surgery are directly attributable to pulmonary emboli,1 and pulmonary emboli are the most frequent cause of postoperative death in women with uterine or cervical carcinoma.2
Deep venous thrombosis (DVT) is almost as evasive. We know the signs and symptoms of DVT of the lower extremities—pain, edema, erythema, and a prominent vascular pattern of the superficial veins—but 50% to 80% of patients with these symptoms do not have DVT, and 80% of patients with symptomatic pulmonary embolism have no antecedent signs of thrombosis in the lower extremities.2 Morbidity and expense rise dramatically with DVT, especially when postphlebitic syndrome occurs.
How can we minimize these risks?
A good outcome is most likely when we:
- recognize risk factors,
- provide appropriate perioperative prophylaxis, and
- diagnose and treat venous thromboembolism (VTE) quickly.
This article looks in detail at each of these strategies.
3 factors set the stage for thrombogenesis
- Hypercoagulable state
- Venous stasis
- Vessel endothelial injury
These factors, known as Virchow’s triad, are especially likely at the time of major surgery, or when the patient is advanced in age or has a history of DVT, cancer, lower extremity edema, or venous stasis.
Intraoperative risk factors for postoperative DVT include increased anesthesia time, greater blood loss, and need for transfusion.
Some preventive methods come close to ideal
Being aware of risk factors is vital to provide the appropriate level of prophylaxis (TABLES 1 AND 2).3,4 The first step is identifying high-risk patients and tailoring the regimen to meet their individual needs. The perfect prophylactic method is not yet devised, but would be effective, free of significant side effects, well accepted by the patient and nursing staff, widely applicable to most patient groups, and inexpensive. A number of methods come close.
TABLE 1
Risk factors for thromboembolism
| Major gynecologic surgery |
| Age >40 years |
| Malignancy |
| Previous venous thrombosis (DVT or pulmonary embolism) |
| Obesity |
| Immobility |
| Pregnancy and the postpartum period |
| Oral contraceptives, hormone therapy, or tamoxifen |
| Varicose veins |
| Inherited or acquired thrombophilia (eg, Factor V Leiden) |
| Prolonged surgical procedure |
| Radical vulvectomy, inguinal-femoral lymphadenectomy, or pelvic exenteration |
TABLE 2
Match the preventive strategy to the surgery
| SURGERY | STRATEGY | DURATION OF PROPHYLAXIS* |
|---|---|---|
| Procedures <30 min for benign disease | Prophylaxis not needed | — |
| Laparoscopic gynecologic procedures in women with additional risk factors | Unfractionated heparin, 5,000 bid or | Until hospital discharge |
| LMWH, ≤3,400 U/day or | ||
| External pneumatic compression or | ||
| Graduated compression stockings | ||
| Major surgery for benign disease without additional risk factors | Unfractionated heparin, 5,000 U bid or | Until hospital discharge |
| LMWH, <3,400 U/day or | ||
| External pneumatic compression | ||
| Extensive major surgery in women with cancer or additional risk factors | Unfractionated heparin, 5,000 U tid or | Until hospital discharge |
| LMWH, >3,400 U/day or | ||
| External pneumatic compression | ||
| *For women at particularly high risk (eg, cancer surgery, age >60 years, prior VTE), continue prophylaxis for 2–4 weeks after hospital discharge. | ||
| Modified from Geerts WH, et al20 | ||
Low-dose unfractionated heparin
The most extensively studied prophylactic method is the use of small, subcutaneous doses of heparin. More than 25 controlled trials have shown that, when heparin is given subcutaneously 2 hours before surgery and every 8 to 12 hours afterward, the incidence of DVT diminishes substantially.
The value of low-dose heparin in preventing pulmonary emboli was established by a randomized, controlled, multicenter, international trial, in which fatal postoperative pulmonary emboli declined significantly in general surgery patients given the drug every 8 hours after surgery.5 In gynecologic surgical patients, postoperative DVT also declined significantly.
Increase in minor bleeding complications. Although low-dose heparin is thought to have no measurable effect on coagulation, most large series have noted an increase in minor bleeding complications such as wound hematoma. Up to 10% to 15% of otherwise healthy patients develop transiently prolonged activated partial thromboplastin time (APTT) after 5,000 U of heparin are given subcutaneously.6
Although relatively rare, thrombocytopenia is associated with the use of low-dose heparin. It has been found in 6% of women after gynecologic surgery.6 Therefore, it is reasonable to measure platelets in any patient taking low-dose heparin longer than 4 days to screen for heparin-induced thrombocytopenia.
Fear of major bleeding complications is unsubstantiated. There is ample evidence from placebo-controlled, blinded trials and meta-analysis that the risk of clinically important bleeding does not increase. Moreover, detailed analysis demonstrates that low-dose heparin has a good risk-to-benefit ratio and is cost-effective.
Low-molecular-weight heparins
These drugs are fragments of unfractionated heparin that vary in size from 4,500 to 6,500 daltons. Low-molecular-weight heparin (LMWH) has more anti-Xa and less antithrombin activity than unfractionated heparin and thus has less of an effect on partial thromboplastin time. LMWH may also lead to fewer bleeding complications.7
Once-daily dosing is possible. An increased half-life of 4 hours for LMWH produces greater bioavailability than with low-dose heparin. This allows once-daily dosing.
Pick one: Convenience or cost
Randomized controlled trials have compared LMWH to unfractionated heparin in gynecologic surgical patients. In all studies, DVT occurred in similar, low numbers of women regardless of the heparin used. Bleeding complications also were similar.8
A meta-analysis of general surgery and gynecologic surgery patients from 32 trials likewise found daily LMWH to be as effective as unfractionated heparin in DVT prophylaxis, without any difference in hemorrhagic complications.9
The choice of drugs often boils down to convenience versus cost: Prophylactic LMWH can be given once a day (compared with 2 or 3 times for unfractionated heparin), but is much more expensive.
Mechanical prophylactic methods
External pneumatic compression rivals low-dose heparin. The largest body of literature on mechanical methods to reduce postoperative venous stasis involves intermittent leg compression by pneumatically inflated sleeves placed around the calf or leg during surgery and after. A number of devices and sleeve designs are available, none of which has proven to be superior to the others.
In my experience, calf compression during and after gynecologic surgery lowers the incidence of DVT to a level seen with low-dose heparin. Besides increasing venous flow and pulsatile emptying of the calf veins, pneumatic compression appears to augment endogenous fibrinolysis, which may stimulate lysis of very early thrombi.10
How long is best for external compression? The optimal duration of postoperative external pneumatic compression is unclear. It may be effective when used in the operating room and for the first 24 hours postoperatively in patients with benign conditions who will ambulate on the first day after surgery.11,12
In women undergoing major surgery for gynecologic malignancy, it reduces the incidence of postoperative venous thromboemboli by nearly 3-fold, but only if calf compression is applied intraoperatively and for the first 5 postoperative days.13,14 These women may remain at risk because of stasis and a hypercoagulable state for a longer time than general surgical patients.
External pneumatic leg compression has no serious side effects or risks and is slightly more cost-effective than prophylactic drugs.15 However, to be fully effective, this method must be used consistently, in compliance with the protocol, when the patient is not ambulating.
Stockings can be a help or hazard. Controlled studies of graduated pressure stockings are limited but suggest modest benefit with careful fitting.16 Poorly fitted stockings that roll down the leg may create a tourniquet effect at the knee or mid-thigh. Another disadvantage of the stockings: The limited sizes available do not allow a perfect fit for all patients. This is especially true in obese patients.
The simplicity of elastic stockings and the absence of serious side effects are probably why stockings are often included in routine postoperative care.
Don’t overlook basic precautions. Although they may offer only modest benefit, short preoperative hospital stays and early postoperative ambulation are recommended.
Another basic strategy: elevating the foot of the bed to raise the calf above heart level. This allows gravity to drain the calf veins and should further reduce stasis.
How to detect VTE
DVT has nonspecific signs and symptoms
When DVT occurs in the lower extremities, harbingers such as pain, edema, and erythema are relatively nonspecific; 50% to 80% of patients exhibiting them do not have DVT. Conversely, approximately 80% of patients with symptomatic pulmonary emboli have no signs or symptoms of thrombosis in the lower extremities.
Because of this lack of specificity, additional tests are needed to establish DVT.
Diagnostic studies
A definitive diagnosis of DVT and pulmonary embolism is mandatory because diagnosis based on clinical symptoms and signs alone is frequently wrong. Strategies to reduce the use of ultrasound or spiral CT scanning have been put forward. These studies have evaluated outpatients using algorithms that utilize clinical probability (“clinical decision rule”) and D-dimer levels.
This strategy has been very accurate and avoids the use of ultrasound or spiral CT in low-risk patients. For example, individuals with a low probability score have an incidence of DVT below 5%, so ultrasound is unnecessary. This diagnostic strategy relies on the recognition of elevated D-dimer levels. Unfortunately, D-dimer is increased by a variety of nonthrombotic disorders, including recent surgery, hemorrhage, trauma, pregnancy, and cancer. Therefore, we cannot recommend the use of this strategy for the postoperative gynecologic surgery patient.17,18
Venography no longer the gold standard. Other diagnostic studies may be more useful. Venography has fallen from favor because it is moderately uncomfortable, requires injection of a contrast material that may trigger an allergic reaction or renal injury, and causes phlebitis in approximately 5% of patients.2 Newer, noninvasive diagnostic tests have been developed, fortunately.
Doppler ultrasound. B-mode duplex Doppler imaging is the most common technique to diagnose symptomatic venous thrombosis, especially when it arises in the proximal lower extremity. With duplex Doppler imaging, the femoral vein can be visualized, and clots may be seen directly. Compression of the vein with the tip of the ultrasound probe makes it possible to assess venous collapsibility, which is diminished when a thrombus is present.
Doppler imaging is less accurate when evaluating the calf and pelvic veins.
Magnetic resonance venography (MRV) sensitivity and specificity are comparable to venography. In addition, MRV may detect thrombi in pelvic veins that are not imaged by venography. The primary drawback is the time required to examine the lower extremity and pelvis. Further, MRV rarely identifies calf thrombi (most often not life-threatening, but potentially symptomatic) and is considerably more expensive than ultrasound.
Which prevention strategy works best?
We now consider low-molecular-weight heparin and external pneumatic compression the best choices
Because low-dose unfractionated heparin, low-molecular-weight heparin (LMWH), and external pneumatic compression all reduce the incidence of postoperative venous thromboembolism in high-risk gynecologic surgical patients, the question is: Which strategy is best?
We conducted 2 randomized clinical trials to answer this question.
Trial 1 Low-dose heparin vs pneumatic compression
Women were randomized to receive either low-dose heparin (5,000 U subcutaneously preoperatively and every 8 hours after surgery until hospital discharge) or external pneumatic compression of the calf prior to surgery and until hospital discharge.1
The incidence of DVT was identical in both groups, and no patients developed a pulmonary embolus throughout 30 days of follow-up. However, bleeding complications occurred more often in the group randomized to low-dose heparin. Specifically, nearly 25% had APTT levels in the “therapeutic” range, and significantly more patients required blood transfusions. After this trial, our institution decided to use external pneumatic compression because of its more favorable risk profile.1
Trial 2 LMWH vs pneumatic compression
The question of the best therapy arose again with the advent of LMWH, because of the possibility that these drugs carried a lower risk of bleeding complications. We therefore conducted a second trial to compare LMWH with external pneumatic compression.2
Because higher doses of LMWH had already proven to be more effective in cancer patients, we gave women in the trial 5,000 U dalteparin (Fragmin) preoperatively and 5,000 U daily postoperatively until hospital discharge.
In this trial, external pneumatic compression and LMWH produced similar low frequencies of DVT and no pulmonary emboli throughout 30 days of follow-up. We also found no association between LMWH and bleeding complications or transfusion requirements. Compliance and patient satisfaction were similar for both modalities.2
Bottom line
We now consider LMWH and external pneumatic compression the best choices for prophylaxis in gynecologic surgical patients.
REFERENCES
1. Clarke-Pearson DL, Synan IS, Dodge R, Soper JT, Berchuck A, Coleman RE. A randomized trial of low-dose heparin and intermittent pneumatic calf compression for the prevention of deep venous thrombosis after gynecologic oncology surgery. Am J Obstet Gynecol. 1993;168:1146-1154.
2. Maxwell GL, Synan I, Dodge R, Carroll B, Clarke-Pearson DI. Pneumatic compression versus low molecular weight heparin in gynecologic oncology surgery: a randomized trial. Obstet Gynecol. 2001;98:989-995.
Increasing use of laparoscopic surgery raises an important question: What is the thromboembolic risk of laparoscopy itself? On one hand, many laparoscopic surgeries are prolonged, and intraperitoneal pressure from the pneumoperitoneum reduces venous flow. On the other hand, many patients who have laparoscopy have shorter hospital stays and return sooner to normal activities than those who have open procedures.
Although the risks of venous thromboembolism (VTE) have not been studied as thoroughly as other aspects of laparoscopy, they appear to be low. To date, there are no randomized trials of VTE prophylaxis among women undergoing gynecologic laparoscopy.
The prudent course
Nevertheless, it would seem prudent to consider prophylaxis when women with additional risk factors undergo extensive laparoscopic procedures.
Pulmonary embolism is often stealthy
Many of the typical signs and symptoms of pulmonary embolism are associated with other, more common pulmonary complications following surgery. Classic findings that should alert the physician to the possibility of pulmonary embolism include:
- pleuritic chest pain
- hemoptysis
- shortness of breath
- tachycardia
- tachypnea
Often, however, the signs are subtle and may include only persistent tachycardia or a slight elevation in respiration.
When pulmonary embolism is suspected, a chest x-ray, electrocardiography, and arterial blood gas assessment are warranted. Any abnormality justifies further evaluation by ventilation-perfusion lung scan or a spiral computed tomography scan of the chest. Unfortunately, a high percentage of lung scans are interpreted as “indeterminate.” In such cases, careful clinical evaluation and judgment are needed to determine whether pulmonary arteriography is necessary to document or exclude pulmonary embolism.
Immediate, aggressive therapy is crucial
The treatment of postoperative DVT requires immediate anticoagulant therapy using either unfractionated heparin or LMWH, followed by 6 months of oral anticoagulant therapy with warfarin.
Treatment strategy: Unfractionated heparin
Once VTE is diagnosed, start unfractionated heparin to prevent proximal propagation of the thrombus and allow physiologic thrombolytic pathways to dissolve the clot. After an initial IV bolus of 5,000 U, give the patient a continuous infusion of 30,000 U daily, and adjust the dose to maintain APTT levels at a therapeutic level that is 1.5 to 2.5 times the control value.
Subtherapeutic APTT levels in the first 24 hours mean a risk of recurrent thromboembolism 15 times greater than the risk in patients with appropriate levels. Therefore, aggressive management is warranted to achieve prompt anticoagulation.
Start an oral anticoagulant (warfarin) on the first day of heparin infusion, and monitor the international normalized ratio (INR) daily until a therapeutic level is achieved. The change in the INR after warfarin administration often precedes the anticoagulant effect by about 2 days, during which time low protein C levels are associated with a transient hypercoagulable state. Therefore, it is important to continue the heparin until the INR has been maintained in a therapeutic range for at least 2 days to confirm the proper warfarin dose. Intravenous heparin can be discontinued after 5 days if an adequate INR level has been established.
Alternative strategy: LMWH
A meta-analysis involving more than 1,000 patients from 19 trials suggests that LMWH is more effective, safer, and less costly than unfractionated heparin in preventing recurrent thromboembolism.19 The lower cost derives from the ability to use the drugs in an outpatient setting.
Dosages are unique and weight-adjusted according to each LMWH preparation. Because LMWH has a minimal effect on APTT, serial laboratory monitoring of APTT levels is unnecessary. Nor is monitoring of anti-Xa activity of significant benefit in the dose adjustment of LMWH.
Basic treatment of pulmonary embolism
In most cases, immediate anticoagulant therapy identical to that outlined for DVT is sufficient to prevent repeat thrombosis and embolism and to allow the patient’s endogenous thrombolytic mechanisms to lyse the pulmonary embolus.
Other interventions include:
- Respiratory support, including oxygen, bronchodilators, and intensive care.
- Although massive pulmonary emboli are usually quickly fatal, pulmonary embolectomy has been successful on rare occasions.
- Pulmonary artery catheterization and administration of thrombolytic agents may be important in patients with massive pulmonary embolism.
- Vena cava interruption may be necessary when anticoagulant therapy does not prevent rethrombosis and the formation of emboli from the lower extremities or pelvis. A vena cava umbrella or filter may be inserted percutaneously above the level of the thrombosis and caudad to the renal veins.
Take-home points
- Identify risk factors preoperatively
- VTE prophylaxis is warranted for most gynecologic surgery patients and can reduce the incidence of VTE by at least 60% with appropriate use! Plan prophylaxis in women at moderate, high, and highest risk, and remember that individuals at high and highest risk require more intense prophylaxis to realize a benefit.
- Maintain a high level of suspicion in women with signs and symptoms of DVT or pulmonary embolism in the first postoperative month. It is better to over-evaluate than to miss a potentially fatal complication.
- Treat women with VTE immediately with heparin or LMWH.
1. Jeffcoate TN, Tindall VR. Venous thrombosis and embolism in obstetrics and gynecology. Aust N Z J Obstet Gynecol. 1965;5:119-130.
2. Clarke-Pearson DL, Jelovsek FR, Creasman WT. Thromboembolism complicating surgery for cervical and uterine malignancy: incidence, risk factors, and prophylaxis. Obstet Gynecol. 1983;61:87-94.
3. Clayton JK, Anderson JA, McNicol GP. Preoperative prediction of postoperative deep vein thrombosis. BMJ. 1976;2:910-912.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Prevention of fatal postoperative pulmonary embolism by low-dose heparin. An international multicentre trial. Lancet. 1975;2:45-51.
6. Clarke-Pearson DL, DeLong ER, Synan IS, Creasman WT. Complications of low-dose heparin prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 1984;64:689-694.
7. Tapson VF, Hull RD. Management of venous thromboembolic disease. The impact of low-molecular-weight heparin. Clin Chest Med. 1995;16:281-294.
8. Borstad E, Urdal K, Handeland G, Abildgaard U. Comparison of low molecular weight heparin vs. unfractionated heparin in gynecological surgery. II: Reduced dose of low molecular weight heparin. Acta Obstet Gynecol Scand. 1992;71:471-475.
9. Jorgensen LN, Wille-Jorgensen P, Hauch O. Prophylaxis of postoperative thromboembolism with low molecular weight heparins. Br J Surg. 1993;80:689-704.
10. Allenby F, Boardman L, Pflug JJ, Calnan JS. Effects of external pneumatic intermittent compression on fibrinolysis in man. Lancet. 1973;2:1412-1414.
11. Salzman EW, Ploetz J, Bettmann M, Skillman J, Klein L. Intraoperative external pneumatic calf compression to afford long-term prophylaxis against deep vein thrombosis in urological patients. Surgery. 1980;87:239-242.
12. Nicolaides AN, Fernandes e Fernandes J, Pollock AV. Intermittent sequential pneumatic compression of the legs in the prevention of venous stasis and postoperative deep venous thrombosis. Surgery. 1980;87:69-76.
13. Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63:92-98.
14. Clarke-Pearson DL, Creasman WT, Coleman RE, Synan IS, Hinshaw WM. Perioperative external pneumatic calf compression as thromboembolism prophylaxis in gynecologic oncology: report of a randomized controlled trial. Gynecol Oncol. 1984;18:226-232.
15. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
16. Scurr JH, Ibrahim SZ, Faber RG, Le Quesne LP. The efficacy of graduated compression stockings in the prevention of deep vein thrombosis. Br J Surg. 1977;64:371-373.
17. Wells PS, Owen C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA. 2006;295:199-207.
18. Writing Group for the Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical D-dimer testing and computed tomography. JAMA. 2006;295:172-179.
19. Buller HR, Kucher N, Kipfmueller F, et al. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:401S-428S.
20. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
The author reports no financial relationships relevant to this article.
1. Jeffcoate TN, Tindall VR. Venous thrombosis and embolism in obstetrics and gynecology. Aust N Z J Obstet Gynecol. 1965;5:119-130.
2. Clarke-Pearson DL, Jelovsek FR, Creasman WT. Thromboembolism complicating surgery for cervical and uterine malignancy: incidence, risk factors, and prophylaxis. Obstet Gynecol. 1983;61:87-94.
3. Clayton JK, Anderson JA, McNicol GP. Preoperative prediction of postoperative deep vein thrombosis. BMJ. 1976;2:910-912.
4. Clarke-Pearson DL, DeLong ER, Synan IS, Coleman RE, Creasman WT. Variables associated with postoperative deep venous thrombosis: a prospective study of 411 gynecology patients and creation of a prognostic model. Obstet Gynecol. 1987;69:146-150.
5. Prevention of fatal postoperative pulmonary embolism by low-dose heparin. An international multicentre trial. Lancet. 1975;2:45-51.
6. Clarke-Pearson DL, DeLong ER, Synan IS, Creasman WT. Complications of low-dose heparin prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 1984;64:689-694.
7. Tapson VF, Hull RD. Management of venous thromboembolic disease. The impact of low-molecular-weight heparin. Clin Chest Med. 1995;16:281-294.
8. Borstad E, Urdal K, Handeland G, Abildgaard U. Comparison of low molecular weight heparin vs. unfractionated heparin in gynecological surgery. II: Reduced dose of low molecular weight heparin. Acta Obstet Gynecol Scand. 1992;71:471-475.
9. Jorgensen LN, Wille-Jorgensen P, Hauch O. Prophylaxis of postoperative thromboembolism with low molecular weight heparins. Br J Surg. 1993;80:689-704.
10. Allenby F, Boardman L, Pflug JJ, Calnan JS. Effects of external pneumatic intermittent compression on fibrinolysis in man. Lancet. 1973;2:1412-1414.
11. Salzman EW, Ploetz J, Bettmann M, Skillman J, Klein L. Intraoperative external pneumatic calf compression to afford long-term prophylaxis against deep vein thrombosis in urological patients. Surgery. 1980;87:239-242.
12. Nicolaides AN, Fernandes e Fernandes J, Pollock AV. Intermittent sequential pneumatic compression of the legs in the prevention of venous stasis and postoperative deep venous thrombosis. Surgery. 1980;87:69-76.
13. Clarke-Pearson DL, Synan IS, Hinshaw WM, Coleman RE, Creasman WT. Prevention of postoperative venous thromboembolism by external pneumatic calf compression in patients with gynecologic malignancy. Obstet Gynecol. 1984;63:92-98.
14. Clarke-Pearson DL, Creasman WT, Coleman RE, Synan IS, Hinshaw WM. Perioperative external pneumatic calf compression as thromboembolism prophylaxis in gynecologic oncology: report of a randomized controlled trial. Gynecol Oncol. 1984;18:226-232.
15. Maxwell GL, Myers ER, Clarke-Pearson DL. Cost-effectiveness of deep venous thrombosis prophylaxis in gynecologic oncology surgery. Obstet Gynecol. 2000;95:206-214.
16. Scurr JH, Ibrahim SZ, Faber RG, Le Quesne LP. The efficacy of graduated compression stockings in the prevention of deep vein thrombosis. Br J Surg. 1977;64:371-373.
17. Wells PS, Owen C, Doucette S, et al. Does this patient have deep vein thrombosis? JAMA. 2006;295:199-207.
18. Writing Group for the Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical D-dimer testing and computed tomography. JAMA. 2006;295:172-179.
19. Buller HR, Kucher N, Kipfmueller F, et al. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:401S-428S.
20. Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(suppl):338S-400S.
The author reports no financial relationships relevant to this article.
Vaginal hysterectomy: Is skill the limiting factor?
CASE Bleeding, a large uterus, and no response to hormones
“M.G.,” a 42-year-old nullipara, complains of menstrual periods that last 10 days and occur on a 28-day cycle. She says the bleeding is extremely heavy, with frequent, copious clotting. She routinely avoids planning social activities around the time of her period and occasionally cancels nonessential engagements because of it. Over the past year, this professional woman has missed 6 days of work because of the problem with her menses.
When you ask about her history, she reports that another gynecologist first palpated an enlarged and irregular uterus 5 years earlier, and an ultrasound at that time revealed a multinodular fundus of approximately 12 weeks’ size. Oral contraceptives were prescribed, but the problem returned to pretreatment levels over the next 3 years. Oral medroxyprogesterone acetate was added to the regimen without success. Hysteroscopy and a dilation and curettage revealed no submucous fibroids, but by then the uterus had enlarged to 14 weeks’ size. M.G. was counseled about continued conservative management, uterine artery embolization, endometrial ablation, and vaginal hysterectomy. She now wants to go ahead with total vaginal hysterectomy and ovarian preservation.
Is the vaginal approach feasible?
Vaginal hysterectomy is not only feasible, it is preferred. Although laparoscopic surgeons are fond of using the phrase “minimally invasive surgery” to describe their procedures, when it comes to hysterectomy, only the vaginal route qualifies for this superlative description. And although uterine size does sometimes limit use of the vaginal route, it need do so in only a minority of cases.
This article describes surgical techniques for vaginal removal of the large uterus, using morcellation, coring, cervicectomy, and other strategies.
Is the vaginal approach always best?
Guidelines addressing this question were developed by the Society of Pelvic Reconstructive Surgeons and evaluated by Kovac et al (FIGURE 1).1 These guidelines, widely used around the world, recommend the vaginal approach for the small, mobile uterus in a pelvis that has no substantial, identifiable pathology. The guidelines recommend the abdominal route when an adnexal mass of unknown character is present or malignancy is suspected. They suggest the use of laparoscopy to identify or quantify pathology and to help convert cases from the abdominal route to laparoscopically assisted vaginal hysterectomy.
I view these guidelines as a minimum standard of care. As a surgeon’s confidence and skills increase, wider application of the vaginal route should be possible in progressively challenging cases. In the personal series of expert surgeons, use of the vaginal route often exceeds 90%. In contrast, the overall US average is 25%, including laparoscopically assisted procedures.2,3
The indications for salpingo-oophorectomy remain the same regardless of route. At present, the adnexa are removed in only 10% of vaginal hysterectomies and in 60% of abdominal procedures.2,3 However, successful routine removal of the adnexa through the vagina is well documented in the literature.4
Contraindications. There are few absolute contraindications to vaginal hysterectomy beyond known or suspected malignancy, but some conditions do increase the technical skill required (TABLE). Nor do complications increase, provided the surgeon has the proper skill and instrumentations.
FIGURE 1 How to choose a hysterectomy route
Source: Kovac SR et al1; used by permission of the American Journal of Obstetrics and Gynecology.TABLE
Contraindications to vaginal hysterectomy
| ABSOLUTE |
|
| RELATIVE |
|
Technique
Every procedure involves 3 basic tasks
Before the uterus can be removed vaginally, the surgeon must:
- enter the peritoneal cavity,
- divide the uterosacral, cardinal, and pubourethral ligamentous attachments of the paracolpium, and
- ligate the uterine artery.
Posterior entry is usually easier
Although peritoneal entry may be anterior or posterior, the latter is almost always easier. Apply an Allis clamp to the vaginal epithelium over the posterior cul-de-sac approximately 2 to 4 cm behind the cervix. Apply a small amount of traction to the clamp to create a vertical crease, which denotes the proper location for colpotomy. Palpate the crease manually to ensure no bowel is present. Then make a full-thickness incision with sharp scissors to enter the peritoneal cavity. Incomplete incisions and blunt dissection simply slow the process.
Once the peritoneum is entered, bluntly extend the incision laterally to the uterosacral ligaments, and place a weighted Steiner-Auvard retractor in the incision.
Dissect first, then divide the ligaments
When leiomyomata are present, anatomical distortion tends to be limited to the fundus; cervical anatomy remains relatively unaltered. After completing the circular cervical incision, dissect the vesicocervical and vesicouterine spaces. Some sharp dissection is usually required at the level of the pericervical ring—the supravaginal septum—which consists of dense fibroelastic connective tissue. Place a Heaney or Breisky-Navratil retractor within the anterior incision to obtain full cervical access.
Next, sequentially clamp, divide, and ligate the uterosacral, cardinal, and pubourethral ligaments. Once this task is done, the vascular bundle containing the uterine artery and veins becomes accessible; divide it as well.
If the anterior peritoneum has not been passively entered, it can now be easily incised.
Now that the suspensory apparatus and the major blood supply have been divided, uteroreductive techniques can be employed.
No absolute size limit
There is no objective limit to the size of a uterus that can be safely removed using reductive techniques. Generally, extra skill and experience are needed to remove an organ larger than 12 weeks’ size (approximately 280 g). Numerous reports document the safe removal of enlarged uteri, even those larger than 1,000 g.5-17
Uterine size is reduced in 3 ways: morcellation, coring, and/or cervicectomy.
The best method varies from case to case, depending on the specific uterine anatomy and the surgeon’s skill. Often, more than 1 debulking technique is used in a single case.
Do not begin debulking until peritoneal access is attained, the paracolpium is divided, and the uterine artery is ligated.
Morcellation is well suited to multiple fibroids
First, sharply divide the cervix, cutting vertically in the midline, and extend the incision into the uterine fundus using the endocervical canal and endometrial cavity as visual guides for the incision (FIGURE 2A). As leiomyomata are encountered, grasp each with a Myotome grasper (Marina Medical, Hollywood, Fla) and remove them with Myotomes (Marina Medical). Both the spoon-tipped and chisel-tipped Myotomes have dissecting tips that allow rapid and precise enucleation of tumors. The tip is sharp enough to dissect the capsule of the myomata, but not so sharp that it endangers adjacent structures.
Continue to remove the fibroids as they become accessible. If incisions into the serosa of the uterus are necessary to remove palpable subserosal tumors, make the incisions under direct vision. Access to the serosa usually is greater on the posterior surface of the uterus. With adequate retraction, the anterior surface can also be incised.
If a bulky uterus prevents immediate access to leiomyomata, one strategy is to remove elliptical wedges of myometrium adjacent to the uterine bisecting incision. The Martin myomectomy scissors (Marina Medical) have serrated edges originally designed by orthopedists to cut cartilage, as well as sharp tips that can be inserted into a tumor prior to cutting. They help debulk large myomata (FIGURE 2B) and can be used to quickly remove wedges of myometrium. After sufficient debulking, large myomata can be removed safely (FIGURE 2C).
This morcellation method minimizes the need to use a knife in the “invisible” upper reaches of the fundus. Continuous downward traction on the divided cervix prevents bleeding, and the gradual reduction in size of the debulked fundus allows for sufficient descent of the uterus; it also permits posterior rotation. Ultimately, it becomes possible to clamp the utero-ovarian pedicles and to completely remove the uterus.
In most cases, the uterine serosa can be left intact using morcellation. The size of the uterus that can be removed using this technique is limited only by the experience of the surgeon (FIGURE 3).
FIGURE 2 Expose, debulk, and remove the dominant myoma
Incise the cervix along the midline to gain access to the fundus and expose the dominant myoma.
Insert the sharp tip of the scissors into the myoma prior to cutting.
When the myoma has been sufficiently debulked, remove it through the vagina.
FIGURE 3 Massive uteri can be removed vaginally
This uterus and multiple myomata were removed from a single patient using the vaginal route.
Use coring for moderately enlarged uteri
This is a useful technique when the uterus contains multiple small leiomyomata, a single dominant tumor, or cicatrized adenomyosis. Begin by making a circular incision into the fundus of the uterus just above the isthmus. The incision should be parallel to the central axis of the endometrial cavity.18
Apply firm downward traction on the cervix to allow the portion of the uterus central to the incision to evert. Continue to incise the uterus in a circular pattern to allow more of the bulk of the fundus to descend.
This technique converts the globular, anatomically distorted fundus into a cylinder. Be sure to make the encircling incision under direct vision to reduce the risk of injuring adjacent structures.
In most cases, coring has the advantage of leaving the endometrial cavity and serosa intact. With practice, this technique can quickly and reliably reduce a large uterus to a manageable size.
In some cases, you may have to remove the cervix before debulking
If the cervix is particularly bulky or prevents access to the fundus, perform cervicectomy prior to debulking. This type of debulking is not highly technical, and it may make the remainder of the procedure easier to accomplish. As debulking proceeds, use the endocervical canal or endometrial cavity for orientation.
When complete removal is impossible
Occasionally, it is not possible to complete vaginal removal of the large uterus. While this scenario is not ideal, no evidence exists that conversion to the abdominal or laparoscopic route endangers the patient, especially if the decision is made in a timely and judicious manner. Perform cervicectomy before converting to the abdominal or laparoscopic route. The cervical cuff may also be closed prior to conversion.
Be sure to weigh the specimen
Inform the pathologist of the reason for the morcellated specimen so that an accurate total weight can be determined. This is important because CPT codes for uteri larger than 250 g carry more relative value units than the codes for smaller uteri, based on the extra time in the OR as well as the greater technical skill required.
CASE 610-g uterus safely removed
M.G. undergoes vaginal hysterectomy with uteroreductive morcellation. Estimated blood loss is 200 cc.
The morning after her surgery, M.G. voids after removal of the urinary catheter, and is able to tolerate a regular diet. She walks without difficulty and is discharged home. Seven days after her surgery, she returns to work. Her job allows her the freedom to define her own responsibilities, and she has no manual duties.
The pathology report reveals that her uterus weighed 610 g, with multiple leiomyomata. The largest myoma was 8.0×5.5×4.0 cm. No other abnormalities were present.
One year later, M.G. reports a substantially improved lifestyle and expresses satisfaction with her decision to undergo vaginal hysterectomy.
1. Kovac SR, Barhan S, Lister M, Tucker L, Bishop M, Das A. Guidelines for the selection of the route of hysterectomy: application in a resident clinic population. Am J Obstet Gynecol. 2002;187:1521-1527.
2. Kovac SR. Guidelines to determine the route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
3. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
4. Sheth S, Malpani A. Routine prophylactic oophorectomy at the time of vaginal hysterectomy in postmenopausal women. Arch Gynecol Obstet. 1992;251:87-91.
5. El-Lamie IK. Vaginal hysterectomy for uteri weighing 250 grams or more. J Pelvic Surg. 2001;7:140-146.
6. Grody MH. Vaginal hysterectomy: the large uterus. J Gynecol Surg. 1989;5:301-312.
7. Grody MH. Vaginal hysterectomy: the enlarged uterus. Operative Tech Gynecol Surg. 1999;4:53-61.
8. Grody MH, Pruzbylko K, Pagano AM. A practical method for removal of the huge benign fibromyomatous uterus through the vaginal route. J Pelvic Surg. 2000;6:39-44.
9. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico’s experience. Obstet Gynecol. 1996;88:560-563.
10. Larson SL. Uterine morcellation-review of 443 cases. Obstet Gynecol. 1999;4:61S.-
11. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
12. Lash AF. Technique for removal of abnormally large uteri without entering cavities. Clin Obstet Gynecol. 1961;4:210-216.
13. Magos A, Bournas N, Sinha R, et al. Vaginal hysterectomy for the large uterus. Br J Obstet Gynecol. 1996;103:246-251.
14. Moen MD, Webb MJ, Wilson TO. Vaginal hysterectomy in patients with benign uterine enlargement. J Pelvic Surg. 1995;4:197-203.
15. Peham H, Amreich I, Ferguson L. Operative Gynecology. Philadelphia: JB Lippincott; 1934.
16. Pelosi MA, II, Pelosi MA, III. Should uterine size alone require laparoscopic assistance? Vaginal hysterectomy for a 2,003-g uterus. J Lapendo Adv Surg Tech. 1998;8:99-103.
17. Pratt JH, Gunnlaugsson GH. Vaginal hysterectomy by morcellation. Mayo Clin Proc. 1970;45:374-387.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-134.
Dr. Zimmerman reports that he is a consultant and instrument designer for Marina Medical, Inc.
CASE Bleeding, a large uterus, and no response to hormones
“M.G.,” a 42-year-old nullipara, complains of menstrual periods that last 10 days and occur on a 28-day cycle. She says the bleeding is extremely heavy, with frequent, copious clotting. She routinely avoids planning social activities around the time of her period and occasionally cancels nonessential engagements because of it. Over the past year, this professional woman has missed 6 days of work because of the problem with her menses.
When you ask about her history, she reports that another gynecologist first palpated an enlarged and irregular uterus 5 years earlier, and an ultrasound at that time revealed a multinodular fundus of approximately 12 weeks’ size. Oral contraceptives were prescribed, but the problem returned to pretreatment levels over the next 3 years. Oral medroxyprogesterone acetate was added to the regimen without success. Hysteroscopy and a dilation and curettage revealed no submucous fibroids, but by then the uterus had enlarged to 14 weeks’ size. M.G. was counseled about continued conservative management, uterine artery embolization, endometrial ablation, and vaginal hysterectomy. She now wants to go ahead with total vaginal hysterectomy and ovarian preservation.
Is the vaginal approach feasible?
Vaginal hysterectomy is not only feasible, it is preferred. Although laparoscopic surgeons are fond of using the phrase “minimally invasive surgery” to describe their procedures, when it comes to hysterectomy, only the vaginal route qualifies for this superlative description. And although uterine size does sometimes limit use of the vaginal route, it need do so in only a minority of cases.
This article describes surgical techniques for vaginal removal of the large uterus, using morcellation, coring, cervicectomy, and other strategies.
Is the vaginal approach always best?
Guidelines addressing this question were developed by the Society of Pelvic Reconstructive Surgeons and evaluated by Kovac et al (FIGURE 1).1 These guidelines, widely used around the world, recommend the vaginal approach for the small, mobile uterus in a pelvis that has no substantial, identifiable pathology. The guidelines recommend the abdominal route when an adnexal mass of unknown character is present or malignancy is suspected. They suggest the use of laparoscopy to identify or quantify pathology and to help convert cases from the abdominal route to laparoscopically assisted vaginal hysterectomy.
I view these guidelines as a minimum standard of care. As a surgeon’s confidence and skills increase, wider application of the vaginal route should be possible in progressively challenging cases. In the personal series of expert surgeons, use of the vaginal route often exceeds 90%. In contrast, the overall US average is 25%, including laparoscopically assisted procedures.2,3
The indications for salpingo-oophorectomy remain the same regardless of route. At present, the adnexa are removed in only 10% of vaginal hysterectomies and in 60% of abdominal procedures.2,3 However, successful routine removal of the adnexa through the vagina is well documented in the literature.4
Contraindications. There are few absolute contraindications to vaginal hysterectomy beyond known or suspected malignancy, but some conditions do increase the technical skill required (TABLE). Nor do complications increase, provided the surgeon has the proper skill and instrumentations.
FIGURE 1 How to choose a hysterectomy route
Source: Kovac SR et al1; used by permission of the American Journal of Obstetrics and Gynecology.TABLE
Contraindications to vaginal hysterectomy
| ABSOLUTE |
|
| RELATIVE |
|
Technique
Every procedure involves 3 basic tasks
Before the uterus can be removed vaginally, the surgeon must:
- enter the peritoneal cavity,
- divide the uterosacral, cardinal, and pubourethral ligamentous attachments of the paracolpium, and
- ligate the uterine artery.
Posterior entry is usually easier
Although peritoneal entry may be anterior or posterior, the latter is almost always easier. Apply an Allis clamp to the vaginal epithelium over the posterior cul-de-sac approximately 2 to 4 cm behind the cervix. Apply a small amount of traction to the clamp to create a vertical crease, which denotes the proper location for colpotomy. Palpate the crease manually to ensure no bowel is present. Then make a full-thickness incision with sharp scissors to enter the peritoneal cavity. Incomplete incisions and blunt dissection simply slow the process.
Once the peritoneum is entered, bluntly extend the incision laterally to the uterosacral ligaments, and place a weighted Steiner-Auvard retractor in the incision.
Dissect first, then divide the ligaments
When leiomyomata are present, anatomical distortion tends to be limited to the fundus; cervical anatomy remains relatively unaltered. After completing the circular cervical incision, dissect the vesicocervical and vesicouterine spaces. Some sharp dissection is usually required at the level of the pericervical ring—the supravaginal septum—which consists of dense fibroelastic connective tissue. Place a Heaney or Breisky-Navratil retractor within the anterior incision to obtain full cervical access.
Next, sequentially clamp, divide, and ligate the uterosacral, cardinal, and pubourethral ligaments. Once this task is done, the vascular bundle containing the uterine artery and veins becomes accessible; divide it as well.
If the anterior peritoneum has not been passively entered, it can now be easily incised.
Now that the suspensory apparatus and the major blood supply have been divided, uteroreductive techniques can be employed.
No absolute size limit
There is no objective limit to the size of a uterus that can be safely removed using reductive techniques. Generally, extra skill and experience are needed to remove an organ larger than 12 weeks’ size (approximately 280 g). Numerous reports document the safe removal of enlarged uteri, even those larger than 1,000 g.5-17
Uterine size is reduced in 3 ways: morcellation, coring, and/or cervicectomy.
The best method varies from case to case, depending on the specific uterine anatomy and the surgeon’s skill. Often, more than 1 debulking technique is used in a single case.
Do not begin debulking until peritoneal access is attained, the paracolpium is divided, and the uterine artery is ligated.
Morcellation is well suited to multiple fibroids
First, sharply divide the cervix, cutting vertically in the midline, and extend the incision into the uterine fundus using the endocervical canal and endometrial cavity as visual guides for the incision (FIGURE 2A). As leiomyomata are encountered, grasp each with a Myotome grasper (Marina Medical, Hollywood, Fla) and remove them with Myotomes (Marina Medical). Both the spoon-tipped and chisel-tipped Myotomes have dissecting tips that allow rapid and precise enucleation of tumors. The tip is sharp enough to dissect the capsule of the myomata, but not so sharp that it endangers adjacent structures.
Continue to remove the fibroids as they become accessible. If incisions into the serosa of the uterus are necessary to remove palpable subserosal tumors, make the incisions under direct vision. Access to the serosa usually is greater on the posterior surface of the uterus. With adequate retraction, the anterior surface can also be incised.
If a bulky uterus prevents immediate access to leiomyomata, one strategy is to remove elliptical wedges of myometrium adjacent to the uterine bisecting incision. The Martin myomectomy scissors (Marina Medical) have serrated edges originally designed by orthopedists to cut cartilage, as well as sharp tips that can be inserted into a tumor prior to cutting. They help debulk large myomata (FIGURE 2B) and can be used to quickly remove wedges of myometrium. After sufficient debulking, large myomata can be removed safely (FIGURE 2C).
This morcellation method minimizes the need to use a knife in the “invisible” upper reaches of the fundus. Continuous downward traction on the divided cervix prevents bleeding, and the gradual reduction in size of the debulked fundus allows for sufficient descent of the uterus; it also permits posterior rotation. Ultimately, it becomes possible to clamp the utero-ovarian pedicles and to completely remove the uterus.
In most cases, the uterine serosa can be left intact using morcellation. The size of the uterus that can be removed using this technique is limited only by the experience of the surgeon (FIGURE 3).
FIGURE 2 Expose, debulk, and remove the dominant myoma
Incise the cervix along the midline to gain access to the fundus and expose the dominant myoma.
Insert the sharp tip of the scissors into the myoma prior to cutting.
When the myoma has been sufficiently debulked, remove it through the vagina.
FIGURE 3 Massive uteri can be removed vaginally
This uterus and multiple myomata were removed from a single patient using the vaginal route.
Use coring for moderately enlarged uteri
This is a useful technique when the uterus contains multiple small leiomyomata, a single dominant tumor, or cicatrized adenomyosis. Begin by making a circular incision into the fundus of the uterus just above the isthmus. The incision should be parallel to the central axis of the endometrial cavity.18
Apply firm downward traction on the cervix to allow the portion of the uterus central to the incision to evert. Continue to incise the uterus in a circular pattern to allow more of the bulk of the fundus to descend.
This technique converts the globular, anatomically distorted fundus into a cylinder. Be sure to make the encircling incision under direct vision to reduce the risk of injuring adjacent structures.
In most cases, coring has the advantage of leaving the endometrial cavity and serosa intact. With practice, this technique can quickly and reliably reduce a large uterus to a manageable size.
In some cases, you may have to remove the cervix before debulking
If the cervix is particularly bulky or prevents access to the fundus, perform cervicectomy prior to debulking. This type of debulking is not highly technical, and it may make the remainder of the procedure easier to accomplish. As debulking proceeds, use the endocervical canal or endometrial cavity for orientation.
When complete removal is impossible
Occasionally, it is not possible to complete vaginal removal of the large uterus. While this scenario is not ideal, no evidence exists that conversion to the abdominal or laparoscopic route endangers the patient, especially if the decision is made in a timely and judicious manner. Perform cervicectomy before converting to the abdominal or laparoscopic route. The cervical cuff may also be closed prior to conversion.
Be sure to weigh the specimen
Inform the pathologist of the reason for the morcellated specimen so that an accurate total weight can be determined. This is important because CPT codes for uteri larger than 250 g carry more relative value units than the codes for smaller uteri, based on the extra time in the OR as well as the greater technical skill required.
CASE 610-g uterus safely removed
M.G. undergoes vaginal hysterectomy with uteroreductive morcellation. Estimated blood loss is 200 cc.
The morning after her surgery, M.G. voids after removal of the urinary catheter, and is able to tolerate a regular diet. She walks without difficulty and is discharged home. Seven days after her surgery, she returns to work. Her job allows her the freedom to define her own responsibilities, and she has no manual duties.
The pathology report reveals that her uterus weighed 610 g, with multiple leiomyomata. The largest myoma was 8.0×5.5×4.0 cm. No other abnormalities were present.
One year later, M.G. reports a substantially improved lifestyle and expresses satisfaction with her decision to undergo vaginal hysterectomy.
CASE Bleeding, a large uterus, and no response to hormones
“M.G.,” a 42-year-old nullipara, complains of menstrual periods that last 10 days and occur on a 28-day cycle. She says the bleeding is extremely heavy, with frequent, copious clotting. She routinely avoids planning social activities around the time of her period and occasionally cancels nonessential engagements because of it. Over the past year, this professional woman has missed 6 days of work because of the problem with her menses.
When you ask about her history, she reports that another gynecologist first palpated an enlarged and irregular uterus 5 years earlier, and an ultrasound at that time revealed a multinodular fundus of approximately 12 weeks’ size. Oral contraceptives were prescribed, but the problem returned to pretreatment levels over the next 3 years. Oral medroxyprogesterone acetate was added to the regimen without success. Hysteroscopy and a dilation and curettage revealed no submucous fibroids, but by then the uterus had enlarged to 14 weeks’ size. M.G. was counseled about continued conservative management, uterine artery embolization, endometrial ablation, and vaginal hysterectomy. She now wants to go ahead with total vaginal hysterectomy and ovarian preservation.
Is the vaginal approach feasible?
Vaginal hysterectomy is not only feasible, it is preferred. Although laparoscopic surgeons are fond of using the phrase “minimally invasive surgery” to describe their procedures, when it comes to hysterectomy, only the vaginal route qualifies for this superlative description. And although uterine size does sometimes limit use of the vaginal route, it need do so in only a minority of cases.
This article describes surgical techniques for vaginal removal of the large uterus, using morcellation, coring, cervicectomy, and other strategies.
Is the vaginal approach always best?
Guidelines addressing this question were developed by the Society of Pelvic Reconstructive Surgeons and evaluated by Kovac et al (FIGURE 1).1 These guidelines, widely used around the world, recommend the vaginal approach for the small, mobile uterus in a pelvis that has no substantial, identifiable pathology. The guidelines recommend the abdominal route when an adnexal mass of unknown character is present or malignancy is suspected. They suggest the use of laparoscopy to identify or quantify pathology and to help convert cases from the abdominal route to laparoscopically assisted vaginal hysterectomy.
I view these guidelines as a minimum standard of care. As a surgeon’s confidence and skills increase, wider application of the vaginal route should be possible in progressively challenging cases. In the personal series of expert surgeons, use of the vaginal route often exceeds 90%. In contrast, the overall US average is 25%, including laparoscopically assisted procedures.2,3
The indications for salpingo-oophorectomy remain the same regardless of route. At present, the adnexa are removed in only 10% of vaginal hysterectomies and in 60% of abdominal procedures.2,3 However, successful routine removal of the adnexa through the vagina is well documented in the literature.4
Contraindications. There are few absolute contraindications to vaginal hysterectomy beyond known or suspected malignancy, but some conditions do increase the technical skill required (TABLE). Nor do complications increase, provided the surgeon has the proper skill and instrumentations.
FIGURE 1 How to choose a hysterectomy route
Source: Kovac SR et al1; used by permission of the American Journal of Obstetrics and Gynecology.TABLE
Contraindications to vaginal hysterectomy
| ABSOLUTE |
|
| RELATIVE |
|
Technique
Every procedure involves 3 basic tasks
Before the uterus can be removed vaginally, the surgeon must:
- enter the peritoneal cavity,
- divide the uterosacral, cardinal, and pubourethral ligamentous attachments of the paracolpium, and
- ligate the uterine artery.
Posterior entry is usually easier
Although peritoneal entry may be anterior or posterior, the latter is almost always easier. Apply an Allis clamp to the vaginal epithelium over the posterior cul-de-sac approximately 2 to 4 cm behind the cervix. Apply a small amount of traction to the clamp to create a vertical crease, which denotes the proper location for colpotomy. Palpate the crease manually to ensure no bowel is present. Then make a full-thickness incision with sharp scissors to enter the peritoneal cavity. Incomplete incisions and blunt dissection simply slow the process.
Once the peritoneum is entered, bluntly extend the incision laterally to the uterosacral ligaments, and place a weighted Steiner-Auvard retractor in the incision.
Dissect first, then divide the ligaments
When leiomyomata are present, anatomical distortion tends to be limited to the fundus; cervical anatomy remains relatively unaltered. After completing the circular cervical incision, dissect the vesicocervical and vesicouterine spaces. Some sharp dissection is usually required at the level of the pericervical ring—the supravaginal septum—which consists of dense fibroelastic connective tissue. Place a Heaney or Breisky-Navratil retractor within the anterior incision to obtain full cervical access.
Next, sequentially clamp, divide, and ligate the uterosacral, cardinal, and pubourethral ligaments. Once this task is done, the vascular bundle containing the uterine artery and veins becomes accessible; divide it as well.
If the anterior peritoneum has not been passively entered, it can now be easily incised.
Now that the suspensory apparatus and the major blood supply have been divided, uteroreductive techniques can be employed.
No absolute size limit
There is no objective limit to the size of a uterus that can be safely removed using reductive techniques. Generally, extra skill and experience are needed to remove an organ larger than 12 weeks’ size (approximately 280 g). Numerous reports document the safe removal of enlarged uteri, even those larger than 1,000 g.5-17
Uterine size is reduced in 3 ways: morcellation, coring, and/or cervicectomy.
The best method varies from case to case, depending on the specific uterine anatomy and the surgeon’s skill. Often, more than 1 debulking technique is used in a single case.
Do not begin debulking until peritoneal access is attained, the paracolpium is divided, and the uterine artery is ligated.
Morcellation is well suited to multiple fibroids
First, sharply divide the cervix, cutting vertically in the midline, and extend the incision into the uterine fundus using the endocervical canal and endometrial cavity as visual guides for the incision (FIGURE 2A). As leiomyomata are encountered, grasp each with a Myotome grasper (Marina Medical, Hollywood, Fla) and remove them with Myotomes (Marina Medical). Both the spoon-tipped and chisel-tipped Myotomes have dissecting tips that allow rapid and precise enucleation of tumors. The tip is sharp enough to dissect the capsule of the myomata, but not so sharp that it endangers adjacent structures.
Continue to remove the fibroids as they become accessible. If incisions into the serosa of the uterus are necessary to remove palpable subserosal tumors, make the incisions under direct vision. Access to the serosa usually is greater on the posterior surface of the uterus. With adequate retraction, the anterior surface can also be incised.
If a bulky uterus prevents immediate access to leiomyomata, one strategy is to remove elliptical wedges of myometrium adjacent to the uterine bisecting incision. The Martin myomectomy scissors (Marina Medical) have serrated edges originally designed by orthopedists to cut cartilage, as well as sharp tips that can be inserted into a tumor prior to cutting. They help debulk large myomata (FIGURE 2B) and can be used to quickly remove wedges of myometrium. After sufficient debulking, large myomata can be removed safely (FIGURE 2C).
This morcellation method minimizes the need to use a knife in the “invisible” upper reaches of the fundus. Continuous downward traction on the divided cervix prevents bleeding, and the gradual reduction in size of the debulked fundus allows for sufficient descent of the uterus; it also permits posterior rotation. Ultimately, it becomes possible to clamp the utero-ovarian pedicles and to completely remove the uterus.
In most cases, the uterine serosa can be left intact using morcellation. The size of the uterus that can be removed using this technique is limited only by the experience of the surgeon (FIGURE 3).
FIGURE 2 Expose, debulk, and remove the dominant myoma
Incise the cervix along the midline to gain access to the fundus and expose the dominant myoma.
Insert the sharp tip of the scissors into the myoma prior to cutting.
When the myoma has been sufficiently debulked, remove it through the vagina.
FIGURE 3 Massive uteri can be removed vaginally
This uterus and multiple myomata were removed from a single patient using the vaginal route.
Use coring for moderately enlarged uteri
This is a useful technique when the uterus contains multiple small leiomyomata, a single dominant tumor, or cicatrized adenomyosis. Begin by making a circular incision into the fundus of the uterus just above the isthmus. The incision should be parallel to the central axis of the endometrial cavity.18
Apply firm downward traction on the cervix to allow the portion of the uterus central to the incision to evert. Continue to incise the uterus in a circular pattern to allow more of the bulk of the fundus to descend.
This technique converts the globular, anatomically distorted fundus into a cylinder. Be sure to make the encircling incision under direct vision to reduce the risk of injuring adjacent structures.
In most cases, coring has the advantage of leaving the endometrial cavity and serosa intact. With practice, this technique can quickly and reliably reduce a large uterus to a manageable size.
In some cases, you may have to remove the cervix before debulking
If the cervix is particularly bulky or prevents access to the fundus, perform cervicectomy prior to debulking. This type of debulking is not highly technical, and it may make the remainder of the procedure easier to accomplish. As debulking proceeds, use the endocervical canal or endometrial cavity for orientation.
When complete removal is impossible
Occasionally, it is not possible to complete vaginal removal of the large uterus. While this scenario is not ideal, no evidence exists that conversion to the abdominal or laparoscopic route endangers the patient, especially if the decision is made in a timely and judicious manner. Perform cervicectomy before converting to the abdominal or laparoscopic route. The cervical cuff may also be closed prior to conversion.
Be sure to weigh the specimen
Inform the pathologist of the reason for the morcellated specimen so that an accurate total weight can be determined. This is important because CPT codes for uteri larger than 250 g carry more relative value units than the codes for smaller uteri, based on the extra time in the OR as well as the greater technical skill required.
CASE 610-g uterus safely removed
M.G. undergoes vaginal hysterectomy with uteroreductive morcellation. Estimated blood loss is 200 cc.
The morning after her surgery, M.G. voids after removal of the urinary catheter, and is able to tolerate a regular diet. She walks without difficulty and is discharged home. Seven days after her surgery, she returns to work. Her job allows her the freedom to define her own responsibilities, and she has no manual duties.
The pathology report reveals that her uterus weighed 610 g, with multiple leiomyomata. The largest myoma was 8.0×5.5×4.0 cm. No other abnormalities were present.
One year later, M.G. reports a substantially improved lifestyle and expresses satisfaction with her decision to undergo vaginal hysterectomy.
1. Kovac SR, Barhan S, Lister M, Tucker L, Bishop M, Das A. Guidelines for the selection of the route of hysterectomy: application in a resident clinic population. Am J Obstet Gynecol. 2002;187:1521-1527.
2. Kovac SR. Guidelines to determine the route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
3. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
4. Sheth S, Malpani A. Routine prophylactic oophorectomy at the time of vaginal hysterectomy in postmenopausal women. Arch Gynecol Obstet. 1992;251:87-91.
5. El-Lamie IK. Vaginal hysterectomy for uteri weighing 250 grams or more. J Pelvic Surg. 2001;7:140-146.
6. Grody MH. Vaginal hysterectomy: the large uterus. J Gynecol Surg. 1989;5:301-312.
7. Grody MH. Vaginal hysterectomy: the enlarged uterus. Operative Tech Gynecol Surg. 1999;4:53-61.
8. Grody MH, Pruzbylko K, Pagano AM. A practical method for removal of the huge benign fibromyomatous uterus through the vaginal route. J Pelvic Surg. 2000;6:39-44.
9. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico’s experience. Obstet Gynecol. 1996;88:560-563.
10. Larson SL. Uterine morcellation-review of 443 cases. Obstet Gynecol. 1999;4:61S.-
11. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
12. Lash AF. Technique for removal of abnormally large uteri without entering cavities. Clin Obstet Gynecol. 1961;4:210-216.
13. Magos A, Bournas N, Sinha R, et al. Vaginal hysterectomy for the large uterus. Br J Obstet Gynecol. 1996;103:246-251.
14. Moen MD, Webb MJ, Wilson TO. Vaginal hysterectomy in patients with benign uterine enlargement. J Pelvic Surg. 1995;4:197-203.
15. Peham H, Amreich I, Ferguson L. Operative Gynecology. Philadelphia: JB Lippincott; 1934.
16. Pelosi MA, II, Pelosi MA, III. Should uterine size alone require laparoscopic assistance? Vaginal hysterectomy for a 2,003-g uterus. J Lapendo Adv Surg Tech. 1998;8:99-103.
17. Pratt JH, Gunnlaugsson GH. Vaginal hysterectomy by morcellation. Mayo Clin Proc. 1970;45:374-387.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-134.
Dr. Zimmerman reports that he is a consultant and instrument designer for Marina Medical, Inc.
1. Kovac SR, Barhan S, Lister M, Tucker L, Bishop M, Das A. Guidelines for the selection of the route of hysterectomy: application in a resident clinic population. Am J Obstet Gynecol. 2002;187:1521-1527.
2. Kovac SR. Guidelines to determine the route of hysterectomy. Obstet Gynecol. 1995;85:18-23.
3. Kovac SR. Clinical opinion: guidelines for hysterectomy. Am J Obstet Gynecol. 2004;191:635-640.
4. Sheth S, Malpani A. Routine prophylactic oophorectomy at the time of vaginal hysterectomy in postmenopausal women. Arch Gynecol Obstet. 1992;251:87-91.
5. El-Lamie IK. Vaginal hysterectomy for uteri weighing 250 grams or more. J Pelvic Surg. 2001;7:140-146.
6. Grody MH. Vaginal hysterectomy: the large uterus. J Gynecol Surg. 1989;5:301-312.
7. Grody MH. Vaginal hysterectomy: the enlarged uterus. Operative Tech Gynecol Surg. 1999;4:53-61.
8. Grody MH, Pruzbylko K, Pagano AM. A practical method for removal of the huge benign fibromyomatous uterus through the vaginal route. J Pelvic Surg. 2000;6:39-44.
9. Kammerer-Doak D, Mao J. Vaginal hysterectomy with and without morcellation: the University of New Mexico’s experience. Obstet Gynecol. 1996;88:560-563.
10. Larson SL. Uterine morcellation-review of 443 cases. Obstet Gynecol. 1999;4:61S.-
11. Lash AF. A method for reducing the size of the uterus in vaginal hysterectomy. Am J Obstet Gynecol. 1941;42:452-459.
12. Lash AF. Technique for removal of abnormally large uteri without entering cavities. Clin Obstet Gynecol. 1961;4:210-216.
13. Magos A, Bournas N, Sinha R, et al. Vaginal hysterectomy for the large uterus. Br J Obstet Gynecol. 1996;103:246-251.
14. Moen MD, Webb MJ, Wilson TO. Vaginal hysterectomy in patients with benign uterine enlargement. J Pelvic Surg. 1995;4:197-203.
15. Peham H, Amreich I, Ferguson L. Operative Gynecology. Philadelphia: JB Lippincott; 1934.
16. Pelosi MA, II, Pelosi MA, III. Should uterine size alone require laparoscopic assistance? Vaginal hysterectomy for a 2,003-g uterus. J Lapendo Adv Surg Tech. 1998;8:99-103.
17. Pratt JH, Gunnlaugsson GH. Vaginal hysterectomy by morcellation. Mayo Clin Proc. 1970;45:374-387.
18. Kovac SR. Intramyometrial coring as an adjunct to vaginal hysterectomy. Obstet Gynecol. 1986;67:131-134.
Dr. Zimmerman reports that he is a consultant and instrument designer for Marina Medical, Inc.
Hysterectomy: Which route for which patient?
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati, Ohio.
- Tommaso Falcone, MD, Professor and Chairman, Department of Obstetrics and Gynecology, Cleveland Clinic, Cleveland, Ohio.
- Thomas Herzog, MD, Director, Division of Gynecologic Oncology, Physicians and Surgeons Alumni Professor, Columbia University Medical Center, New York City.
- Barbara S. Levy, MD, Medical Director, Women’s Health Center, Franciscan Health System, Federal Way, Wash. Dr. Levy serves on the OBG MANAGEMENT Board of Editors.
Why the continued reliance on the abdominal approach despite convincing evidence that vaginal and laparoscopicassisted vaginal hysterectomy (LAVH) offer faster recovery, better cosmesis, and, in many cases, a shorter operation with fewer complications?
OBG Management convened a panel of experts in different aspects of gynecologic surgery to explore this issue. They discuss the reasons most physicians prefer the abdominal approach, how residency programs affect the choice of hysterectomy route, indications for LAVH and supracervical hysterectomy, the issue of ovarian conservation, and management of uterine fibroids.
Why the abdominal route remains the old standby
Physicians use the procedure they are most comfortable with, and residents lack sufficient hands-on experience with laparoscopic and vaginal surgery. Medicolegal risk and reimbursement also have an impact.
KARRAM: Hysterectomy is one of the most widely performed surgeries in the United States, but approximately 60% to 80% of these surgeries still involve the abdominal route.2 Why do you think that is?
FALCONE: Most physicians practice in the manner they were trained, and most residency programs train residents to perform abdominal hysterectomy.
LEVY: I agree. Residents in obstetrics and gynecology have a limited time frame in which to learn and become facile with surgical gynecology. The requirements for primary care training and continuity clinics leave little time for the resident to become comfortable with endoscopic and vaginal surgery. However, they do get substantial exposure to abdominal surgery, both in obstetrics (with cesarean sections constituting 27.5% of all deliveries in the United States3) and gynecologic oncology rotations.
Furthermore, the volume of benign gynecologic surgery is low and the technical skills required for laparoscopic and vaginal surgery are more challenging than “slash and gash” abdominal surgery, so residents don’t get enough exposure to develop a comfort level with these procedures.
HERZOG: This trend is likely to change in the future because recent and current trainees have much more exposure to the laparoscopic approach than in the past, and the equipment has continued to improve. However, I have serious doubts about whether adequate amounts of vaginal surgery are performed in training programs to educate the next generation of vaginal surgeons.
LEVY: Another factor is the type of practice physicians enter after training. Most join practices in which the bulk of their income for many years derives from obstetrics. Without a mentor in the practice who is skilled at minimally invasive surgery, most of these young physicians appropriately resort to the hysterectomy approach for which they have the most comfort and skill: the abdominal route.
HERZOG: Secondary barriers to nonabdominal procedures are lower reimbursement and heightened medicolegal risk, since time and complications are greater for laparoscopic surgery and, to a lesser degree, vaginal procedures. Surgeons are not adequately compensated for either the increased time or risk.
Patients also tend to have higher expectations when the planned approach is minimally invasive. When conversion to laparotomy is necessary, the patient and her family may have trouble understanding why.
KARRAM: I agree that there is a serious lack of training in simple and complicated vaginal hysterectomy. Many inaccurate perceptions have been handed down over the years about its absolute and relative contraindications, such as the belief that any history of pelvic infection, endometriosis, or cesarean section is a contraindication for the vaginal approach.
When is laparoscopic assistance appropriate?
At a fundamental level, its value lies in converting abdominal hysterectomy intovaginal hysterectomy.
KARRAM: In my experience, LAVH is appropriate in the presence of benign adnexal pathology: The adnexa can be evaluated and detached laparoscopically followed by vaginal hysterectomy and vaginal removal of the adnexa. It also is appropriate in any situation that involves excessive pelvic adhesions. The uterus can be mobilized laparoscopically, followed by removal through the vagina.
FALCONE: Any patient who is not a candidate for vaginal hysterectomy should be considered for laparoscopic assistance. The general rationale for the surgery is to convert an abdominal hysterectomy into a vaginal one, so the surgeon should start laparoscopically and then switch to the vaginal approach as soon as possible. Of course, it is impossible to proceed vaginally in some cases. When it is, the entire case can be performed laparoscopically.
LEVY: In my hands, patients with an unidentified adnexal mass who also need or request hysterectomy are appropriate candidates for laparoscopic abdominal exploration followed by vaginal hysterectomy if appropriate. Women with a very contracted pelvis, which precludes transvaginal access to the uterine vasculature, may also be candidates for the laparoscopic approach. However, as surgeons become more skilled at vaginal surgery and learn to use newer instrumentation, the need for laparoscopy to access a tight pelvis will diminish.
Using a laparoscopic approach for patients with fibroids wedged into the pelvis carries serious risk to the pelvic sidewall. It is very difficult to access the sidewall safely laparoscopically in the presence of a large lower segment or cervical myomas.
HERZOG: When LAVH was introduced, many clinicians challenged the utility of combined laparoscopic and vaginal surgery, with some referring to this surgical exercise as a procedure looking for an indication. However, as operative laparoscopy has gained acceptance, some benefits of LAVH have become apparent. The greatest advantage is the potential to convert a procedure that would have been performed abdominally into a vaginal hysterectomy.
The most commonly cited indications for LAVH are to lyse adhesions secondary to prior abdominopelvic surgery, substantial endometriosis, or a pelvic mass.
Is oophorectomy an indication for LAVH?
The need to remove the ovaries does not mean laparoscopic assistance is imperative.
HERZOG: Simple removal of the ovaries can often be performed using the vaginal route, and is not in itself an indication for LAVH.
LEVY: I agree. The ovaries can usually be accessed transvaginally, especially with good fiberoptic lighting and vessel-sealing technology.
FALCONE: Several studies, most notably the one by Ballard and Walters,4 demonstrate that oophorectomy can be carried out vaginally in most cases. In their study, they did not use special instruments.
HERZOG: But LAVH is indicated to facilitate complete removal of the ovaries in riskreduction surgery for documented or suspected BrCa 1 or 2 mutations. The entire ovary and as much of the tube as possible must be removed in these women. Thus, if the patient has other indications for hysterectomy, LAVH may be the preferred route to assure that the blood supply is taken proximally enough to remove absolutely all ovarian tissue. Simply clamping directly along the side of the ovary is not an adequate removal technique for these patients, since an ovarian remnant may become a fatal oversight.
LEVY: Yes, laparoscopy is indicated for riskreducing salpingo-oophorectomy in order to adequately assess the entire peritoneal cavity. Up to 2% of these patients will have occult invasive ovarian or peritoneal carcinoma at the time of their prophylactic surgery, so full surgical abdominal exploration is mandatory and can be nicely accomplished via laparoscopy.5
How endometriosis history affects choice of route
In some women, laparoscopic surgery is preferred over the vaginal route.
FALCONE: Hysterectomy with or without salpingo-oophorectomy can be considered in women whose endometriosis fails to respond to conservative management and who do not desire fertility. Most studies have shown substantial pain relief with definitive surgery.6,7 Although ovarian conservation may be advisable in younger women, in some women it increases the probability of recurrent pain and the need for reoperation. The main concern is whether the endometriosis is completely removed during hysterectomy.
A history of cul-de-sac obliteration or extensive pelvic adhesions from endometriosis is an indication for laparoscopic hysterectomy rather than vaginal hysterectomy. Women with less severe disease will benefit from a diagnostic laparoscopy prior to a vaginal hysterectomy to evaluate the pelvis and excise any endometriosis.
Is there any benefit to leaving the cervix?
There is no evidence of any benefit except in selected cases of heavy bleeding, postpartum hemorrhage, advanced endometriosis, or ovarian cancer surgery.
KARRAM: What about supracervical hysterectomy? The only time I have performed one was at the time of a cesareanhysterectomy because blood loss was significant and dissection of the cervix could have led to more morbidity. What are the indications for this procedure?
HERZOG: It is unclear whether there are any definitive indications for supracervical hysterectomy. A number of benefits have been proposed, such as better support and improved sexual function. Some of the perceived benefits have been supported by nonrandomized trials, but critical analysis of the randomized data has failed to support most of these contentions.8,9 However, the procedure can be a valuable intervention to decrease critical blood loss intraoperatively, as you point out, or to simplify complicated pelvic surgery in selected cases, such as postpartum hemorrhage, advanced endometriosis, ovarian cancer debulking (when the cervix is not involved), or significant bleeding in patients who object to transfusion on ethical or religious grounds.
FALCONE: There are clear contraindications to supracervical hysterectomy, namely the presence of a malignant or premalignant condition of the uterine corpus or cervix, but no indications, except perhaps for an unstable patient undergoing hysterectomy in whom you want to finish quickly. None of the randomized clinical trials have shown supracervical hysterectomy to be superior to total hysterectomy. The randomized trials involved the abdominal route; the time to complete a laparoscopic supracervical hysterectomy is less than for a laparoscopic total hysterectomy.
Nevertheless, many patients ask for the procedure. After I present the risks and benefits, I leave the choice up to them. Of course, it is important to explain that total hysterectomy implies removal of the cervix and not the ovaries.
LEVY: In rare cases of immunocompromised patients or women with widely disseminated intraperitoneal carcinoma, one could make an argument for avoiding entry into the vagina to reduce infectious risk, speed healing, or avoid tumor seeding. Otherwise, there is absolutely no evidence to support an indication for supracervical hysterectomy.
Does leaving the cervix affect long-term function?
The residual cervix can become the site of later neoplasia or disease.
HERZOG: Supracervical hysterectomy can be associated with several problems with longterm implications. One is the potential for cervical intraepithelial neoplasia. Another concern relates to bleeding from a portion of active endometrium at the top of the endocervical canal. Rarer problems include the development of endometriosis or invasive cancer in the residual cervix. These potential drawbacks need to be strongly considered and included in patient counseling.
KARRAM: One study followed 67 patients for 66 months after supracervical hysterectomy; trachelectomy was ultimately required in 22.8% of patients.10
The $64,0000 question: Remove the ovaries?
Overall, the decision should be made case by case.
KARRAM: Should routine oophorectomy be performed at the time of hysterectomy in postmenopausal women to decrease the potential for ovarian cancer later in life?
HERZOG: This is a very important question. Conventional thinking used to be that, for women over the age of 45, and certainly for women older than 50, ovarian removal should be strongly considered to reduce the risk of cancer of the ovary or fallopian tubes at a later date. Studies focusing on the number of ovarian cancer cases possibly prevented with routine oophorectomy at the time of hysterectomy reinforced this concept. One single-institution study showed that more than 60 cases of ovarian cancer would have been prevented over a 14-year period if ovaries were routinely removed in women older than 40 undergoing hysterectomy. By extrapolation, that would result in more than 1,000 cases prevented annually in the United States.11
Recent data refute the rationale for routine oophorectomy. One study that used statistical modeling with Markov decision analysis to determine life expectancy concluded that, at least until the age of 65, women are best served with ovarian conservation if their risk of developing ovarian cancer is average or less.12 Researchers found that women who underwent oophorectomy before age 55 experienced 8.6% excess mortality by age 80. The validity of certain assumptions used to construct this model has been challenged; nevertheless, this cogent study certainly challenges previous concepts regarding agebased routine prophylactic oophorectomy. Until further study results are reported, it is important to counsel women who are considering having their ovaries removed about the potential risks and benefits. Furthermore, these decisions must be made on a case-by-case basis, with special deliberation given to women at any increased risk for breast or ovarian cancer.
What route is preferred when fibroids are present?
Assuming hysterectomy is the optimal treatment, the vaginal route is feasible.
KARRAM: Uterine fibroids are still the No. 1 indication for hysterectomy. Are minimally invasive laparoscopic procedures with morcellation techniques the best way to manage these women?
FALCONE: The management of symptomatic leiomyomas depends on the patient’s desire to preserve her fertility. If she does not have an interest in future fertility and there are no myomas that are largely submucous or pedunculated, then uterine fibroid embolization is the treatment of choice. Many studies have shown an excellent response, few complications, and rapid return to work.
If the woman wants to preserve fertility, myomectomy is the treatment of choice. If there are few myomas of moderate size, laparoscopic myomectomy is as effective as laparotomy in the hands of experienced laparoscopists who have the ability to suture.
HERZOG: I also want to stress that we now have a number of options, including uterine artery embolization, medications, and surgery, available for women with symptomatic fibroids. The surgical approaches are numerous and include hysteroscopic and/or laparoscopic myomectomy with or without morcellation, as well as hysterectomy. The approach should be determined by the symptoms, size, and distribution of the fibroids, as well as by individual patient characteristics such as prior surgeries, body mass index, and so on. Just because a procedure is technically feasible does not mean it is the preferred method, and this tenet certainly applies to morcellation. In some instances, women with very large fibroids may be better served by laparotomy to decrease blood loss and the duration of surgery while optimizing uterine wall reconstruction, especially when future fertility is an important consideration. Once again, proper patient selection is paramount in achieving favorable outcomes, especially for those who may be undergoing morcellation.
LEVY: For women who have completed childbearing and who desire hysterectomy, I always attempt a vaginal approach first. Most uteri, regardless of size, can be safely and efficiently removed vaginally as long as there is access to the uterine vasculature. Morcellation is easily performed vaginally once hemostasis is assured. For the rare patient with a large fundal myoma that cannot be brought into the pelvis for morcellation, minilaparotomy or laparoscopic approaches are appropriate.
KARRAM: I think randomized trials are needed in this area. It is important to remember that most cases performed laparoscopically result in supracervical hysterectomies and that significant costs are accrued from the equipment required for morcellation. These factors need to be weighed against potential advantages over abdominal hysterectomy, which include shorter hospital stay, potentially decreased morbidity, and faster recovery. The only way to make any objective conclusions about the options would be a randomized trial with appropriate power involving surgeons equally skilled in laparoscopic and open techniques.
Are residents adequately trained?
It depends on the program but, on the whole, more concentrated experience in minimally invasive surgery is needed.
KARRAM: Let’s focus on residency training for a moment. We seem to agree there is a lack of it in vaginal hysterectomy. It seems to me that the lack of training increases as time goes on. Because the current generation of gynecologists-in-training is ultimately the next generation of teachers, it bodes ill for the future when they are reluctant to attempt vaginal hysterectomy, except in the simplest and most straightforward cases. The medicolegal climate also plays a role, as Dr. Herzog mentioned.
Any other thoughts?
FALCONE: The training across residency programs is not homogenous. Some institutions promote vaginal hysterectomy as the primary access, and others do not.
HERZOG: I agree that some institutions do provide an adequate volume of cases, but many others offer a paucity of vaginal surgeries. Many reasons have combined to cause this shortage of training cases over the past 15 years, including a decrease in the number of hysterectomies performed overall, thanks to a number of nonsurgical or less radical surgical treatments for the most common indications for hysterectomy. These approaches generally are mandated by third-party payers prior to invasive surgery. These mandates were not as rigidly enforced in the past.
In a survey of gynecologic oncologists—the vast majority of whom were at academic training centers—the consensus was that residents had fewer surgical experiences and were less skillful than their predecessors over a 5-year period. More than 80% of respondents thought residents needed more surgical experience to achieve competence.13
Compounding the problem, resident work hours have been restricted and additional educational objectives and nonsurgical rotations have been added to the curriculum without any lengthening of the residency tenure. The adverse effects of these factors on residency case volume has prompted some educators to propose major changes in the residency curriculum, either by lengthening training or developing distinct tracks that facilitate early concentration on an area of interest, thereby allowing residents who choose a surgical track to gain increased training and volume.
Until substantive changes occur, educators must rely on surgical simulators and other in vitro models, especially for laparoscopic training. These have benefit but are not a perfect substitute for actual operative experience. A recent study explored the value of a surgical bench skills training program and concluded that, while residents showed definite improvement in bench laboratory tasks, this improvement did not translate into statistically significant improvement in global skills intraoperatively.14 Clearly, educators must continue to explore options to enhance surgical training, especially for vaginal surgery, or this route will become nearly obsolete in the gynecologic generalist’s armamentarium.
Dr. Karram reports that he receives grant/research support from Gynecare, American Medical Systems, and Pfizer and is a speaker for Gynecare, Ortho-McNeil, and Watson. Dr. Falcone, Dr. Herzog, and Dr. Levy have no financial relationships relevant to this article.
1. Kovac SR. Transvaginal hysterectomy: rationale and surgical approach. Obstet Gynecol. 2004;103:1321-1325.
2. Baggish MS. Total and subtotal abdominal hysterectomy. Best Pract Res Clin Obstet Gynaecol. 2005;19:333-356.
3. National Center for Health Statistics. Births—method of delivery. Available at: www.cdc.gov/nchs/fastats/ delivery.htm. Accessed January 18, 2006.
4. Ballard LA, Walters MD. Transvaginal mobilization and removal of ovaries and fallopian tubes after vaginal hysterectomy. Obstet Gynecol. 1996;87:35-39.
5. Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58-64.
6. Dmowski WP, Radwanska E, Rana N. Recurrent endometriosis following hysterectomy and oophorectomy: the role of residual ovarian fragments. Int J Gynecol Obstet. 1988;26:93-103.
7. Namnoun AB, Hickman NT, Goodman SB, Gehlbach DL, Rock JA. Incidence of symptom recurrence after hysterectomy for endometriosis. Fertil Steril. 1995;64:898-902.
8. Kuppermann M, Summitt RL, Jr, Varner RE, et al. Total or Supracervical Hysterectomy Research Group. Sexual functioning after total compared with supracervical hysterectomy: a randomized trial. Obstet Gynecol. 2005;105:1309-1318.
9. Learman LA, Summitt RL, Jr, Varner RE, et al. Total or Supracervical Hysterectomy (TOSH) Research Group. A randomized comparison of total or supracervical hysterectomy: surgical complications and clinical outcomes. Obstet Gynecol. 2003;102:453-462.
10. Okaro EO, Jones KD, Sutton C. Long-term outcome following laparoscopic supracervical hysterectomy. BJOG. 2001;108:1017-1020.
11. Sightler SE, Boike GM, Estape RE, Averette HE. Ovarian cancer in women with prior hysterectomy: a 14-year experience at the University of Miami. Obstet Gynecol. 1991;78:681-684.
12. Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226.
13. Sorosky JI, Anderson B. Surgical experiences and training of residents: perspective of experienced gynecologic oncologists. Gynecol Oncol. 1999;75:222-223.
14. Lentz GM, Mandel LS, Goff BA. A six-year study of surgical teaching and skills evaluation for obstetric/gynecologic residents in porcine and inanimate surgical models. Am J Obstet Gynecol. 2005;193:2056-2061.
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati, Ohio.
- Tommaso Falcone, MD, Professor and Chairman, Department of Obstetrics and Gynecology, Cleveland Clinic, Cleveland, Ohio.
- Thomas Herzog, MD, Director, Division of Gynecologic Oncology, Physicians and Surgeons Alumni Professor, Columbia University Medical Center, New York City.
- Barbara S. Levy, MD, Medical Director, Women’s Health Center, Franciscan Health System, Federal Way, Wash. Dr. Levy serves on the OBG MANAGEMENT Board of Editors.
Why the continued reliance on the abdominal approach despite convincing evidence that vaginal and laparoscopicassisted vaginal hysterectomy (LAVH) offer faster recovery, better cosmesis, and, in many cases, a shorter operation with fewer complications?
OBG Management convened a panel of experts in different aspects of gynecologic surgery to explore this issue. They discuss the reasons most physicians prefer the abdominal approach, how residency programs affect the choice of hysterectomy route, indications for LAVH and supracervical hysterectomy, the issue of ovarian conservation, and management of uterine fibroids.
Why the abdominal route remains the old standby
Physicians use the procedure they are most comfortable with, and residents lack sufficient hands-on experience with laparoscopic and vaginal surgery. Medicolegal risk and reimbursement also have an impact.
KARRAM: Hysterectomy is one of the most widely performed surgeries in the United States, but approximately 60% to 80% of these surgeries still involve the abdominal route.2 Why do you think that is?
FALCONE: Most physicians practice in the manner they were trained, and most residency programs train residents to perform abdominal hysterectomy.
LEVY: I agree. Residents in obstetrics and gynecology have a limited time frame in which to learn and become facile with surgical gynecology. The requirements for primary care training and continuity clinics leave little time for the resident to become comfortable with endoscopic and vaginal surgery. However, they do get substantial exposure to abdominal surgery, both in obstetrics (with cesarean sections constituting 27.5% of all deliveries in the United States3) and gynecologic oncology rotations.
Furthermore, the volume of benign gynecologic surgery is low and the technical skills required for laparoscopic and vaginal surgery are more challenging than “slash and gash” abdominal surgery, so residents don’t get enough exposure to develop a comfort level with these procedures.
HERZOG: This trend is likely to change in the future because recent and current trainees have much more exposure to the laparoscopic approach than in the past, and the equipment has continued to improve. However, I have serious doubts about whether adequate amounts of vaginal surgery are performed in training programs to educate the next generation of vaginal surgeons.
LEVY: Another factor is the type of practice physicians enter after training. Most join practices in which the bulk of their income for many years derives from obstetrics. Without a mentor in the practice who is skilled at minimally invasive surgery, most of these young physicians appropriately resort to the hysterectomy approach for which they have the most comfort and skill: the abdominal route.
HERZOG: Secondary barriers to nonabdominal procedures are lower reimbursement and heightened medicolegal risk, since time and complications are greater for laparoscopic surgery and, to a lesser degree, vaginal procedures. Surgeons are not adequately compensated for either the increased time or risk.
Patients also tend to have higher expectations when the planned approach is minimally invasive. When conversion to laparotomy is necessary, the patient and her family may have trouble understanding why.
KARRAM: I agree that there is a serious lack of training in simple and complicated vaginal hysterectomy. Many inaccurate perceptions have been handed down over the years about its absolute and relative contraindications, such as the belief that any history of pelvic infection, endometriosis, or cesarean section is a contraindication for the vaginal approach.
When is laparoscopic assistance appropriate?
At a fundamental level, its value lies in converting abdominal hysterectomy intovaginal hysterectomy.
KARRAM: In my experience, LAVH is appropriate in the presence of benign adnexal pathology: The adnexa can be evaluated and detached laparoscopically followed by vaginal hysterectomy and vaginal removal of the adnexa. It also is appropriate in any situation that involves excessive pelvic adhesions. The uterus can be mobilized laparoscopically, followed by removal through the vagina.
FALCONE: Any patient who is not a candidate for vaginal hysterectomy should be considered for laparoscopic assistance. The general rationale for the surgery is to convert an abdominal hysterectomy into a vaginal one, so the surgeon should start laparoscopically and then switch to the vaginal approach as soon as possible. Of course, it is impossible to proceed vaginally in some cases. When it is, the entire case can be performed laparoscopically.
LEVY: In my hands, patients with an unidentified adnexal mass who also need or request hysterectomy are appropriate candidates for laparoscopic abdominal exploration followed by vaginal hysterectomy if appropriate. Women with a very contracted pelvis, which precludes transvaginal access to the uterine vasculature, may also be candidates for the laparoscopic approach. However, as surgeons become more skilled at vaginal surgery and learn to use newer instrumentation, the need for laparoscopy to access a tight pelvis will diminish.
Using a laparoscopic approach for patients with fibroids wedged into the pelvis carries serious risk to the pelvic sidewall. It is very difficult to access the sidewall safely laparoscopically in the presence of a large lower segment or cervical myomas.
HERZOG: When LAVH was introduced, many clinicians challenged the utility of combined laparoscopic and vaginal surgery, with some referring to this surgical exercise as a procedure looking for an indication. However, as operative laparoscopy has gained acceptance, some benefits of LAVH have become apparent. The greatest advantage is the potential to convert a procedure that would have been performed abdominally into a vaginal hysterectomy.
The most commonly cited indications for LAVH are to lyse adhesions secondary to prior abdominopelvic surgery, substantial endometriosis, or a pelvic mass.
Is oophorectomy an indication for LAVH?
The need to remove the ovaries does not mean laparoscopic assistance is imperative.
HERZOG: Simple removal of the ovaries can often be performed using the vaginal route, and is not in itself an indication for LAVH.
LEVY: I agree. The ovaries can usually be accessed transvaginally, especially with good fiberoptic lighting and vessel-sealing technology.
FALCONE: Several studies, most notably the one by Ballard and Walters,4 demonstrate that oophorectomy can be carried out vaginally in most cases. In their study, they did not use special instruments.
HERZOG: But LAVH is indicated to facilitate complete removal of the ovaries in riskreduction surgery for documented or suspected BrCa 1 or 2 mutations. The entire ovary and as much of the tube as possible must be removed in these women. Thus, if the patient has other indications for hysterectomy, LAVH may be the preferred route to assure that the blood supply is taken proximally enough to remove absolutely all ovarian tissue. Simply clamping directly along the side of the ovary is not an adequate removal technique for these patients, since an ovarian remnant may become a fatal oversight.
LEVY: Yes, laparoscopy is indicated for riskreducing salpingo-oophorectomy in order to adequately assess the entire peritoneal cavity. Up to 2% of these patients will have occult invasive ovarian or peritoneal carcinoma at the time of their prophylactic surgery, so full surgical abdominal exploration is mandatory and can be nicely accomplished via laparoscopy.5
How endometriosis history affects choice of route
In some women, laparoscopic surgery is preferred over the vaginal route.
FALCONE: Hysterectomy with or without salpingo-oophorectomy can be considered in women whose endometriosis fails to respond to conservative management and who do not desire fertility. Most studies have shown substantial pain relief with definitive surgery.6,7 Although ovarian conservation may be advisable in younger women, in some women it increases the probability of recurrent pain and the need for reoperation. The main concern is whether the endometriosis is completely removed during hysterectomy.
A history of cul-de-sac obliteration or extensive pelvic adhesions from endometriosis is an indication for laparoscopic hysterectomy rather than vaginal hysterectomy. Women with less severe disease will benefit from a diagnostic laparoscopy prior to a vaginal hysterectomy to evaluate the pelvis and excise any endometriosis.
Is there any benefit to leaving the cervix?
There is no evidence of any benefit except in selected cases of heavy bleeding, postpartum hemorrhage, advanced endometriosis, or ovarian cancer surgery.
KARRAM: What about supracervical hysterectomy? The only time I have performed one was at the time of a cesareanhysterectomy because blood loss was significant and dissection of the cervix could have led to more morbidity. What are the indications for this procedure?
HERZOG: It is unclear whether there are any definitive indications for supracervical hysterectomy. A number of benefits have been proposed, such as better support and improved sexual function. Some of the perceived benefits have been supported by nonrandomized trials, but critical analysis of the randomized data has failed to support most of these contentions.8,9 However, the procedure can be a valuable intervention to decrease critical blood loss intraoperatively, as you point out, or to simplify complicated pelvic surgery in selected cases, such as postpartum hemorrhage, advanced endometriosis, ovarian cancer debulking (when the cervix is not involved), or significant bleeding in patients who object to transfusion on ethical or religious grounds.
FALCONE: There are clear contraindications to supracervical hysterectomy, namely the presence of a malignant or premalignant condition of the uterine corpus or cervix, but no indications, except perhaps for an unstable patient undergoing hysterectomy in whom you want to finish quickly. None of the randomized clinical trials have shown supracervical hysterectomy to be superior to total hysterectomy. The randomized trials involved the abdominal route; the time to complete a laparoscopic supracervical hysterectomy is less than for a laparoscopic total hysterectomy.
Nevertheless, many patients ask for the procedure. After I present the risks and benefits, I leave the choice up to them. Of course, it is important to explain that total hysterectomy implies removal of the cervix and not the ovaries.
LEVY: In rare cases of immunocompromised patients or women with widely disseminated intraperitoneal carcinoma, one could make an argument for avoiding entry into the vagina to reduce infectious risk, speed healing, or avoid tumor seeding. Otherwise, there is absolutely no evidence to support an indication for supracervical hysterectomy.
Does leaving the cervix affect long-term function?
The residual cervix can become the site of later neoplasia or disease.
HERZOG: Supracervical hysterectomy can be associated with several problems with longterm implications. One is the potential for cervical intraepithelial neoplasia. Another concern relates to bleeding from a portion of active endometrium at the top of the endocervical canal. Rarer problems include the development of endometriosis or invasive cancer in the residual cervix. These potential drawbacks need to be strongly considered and included in patient counseling.
KARRAM: One study followed 67 patients for 66 months after supracervical hysterectomy; trachelectomy was ultimately required in 22.8% of patients.10
The $64,0000 question: Remove the ovaries?
Overall, the decision should be made case by case.
KARRAM: Should routine oophorectomy be performed at the time of hysterectomy in postmenopausal women to decrease the potential for ovarian cancer later in life?
HERZOG: This is a very important question. Conventional thinking used to be that, for women over the age of 45, and certainly for women older than 50, ovarian removal should be strongly considered to reduce the risk of cancer of the ovary or fallopian tubes at a later date. Studies focusing on the number of ovarian cancer cases possibly prevented with routine oophorectomy at the time of hysterectomy reinforced this concept. One single-institution study showed that more than 60 cases of ovarian cancer would have been prevented over a 14-year period if ovaries were routinely removed in women older than 40 undergoing hysterectomy. By extrapolation, that would result in more than 1,000 cases prevented annually in the United States.11
Recent data refute the rationale for routine oophorectomy. One study that used statistical modeling with Markov decision analysis to determine life expectancy concluded that, at least until the age of 65, women are best served with ovarian conservation if their risk of developing ovarian cancer is average or less.12 Researchers found that women who underwent oophorectomy before age 55 experienced 8.6% excess mortality by age 80. The validity of certain assumptions used to construct this model has been challenged; nevertheless, this cogent study certainly challenges previous concepts regarding agebased routine prophylactic oophorectomy. Until further study results are reported, it is important to counsel women who are considering having their ovaries removed about the potential risks and benefits. Furthermore, these decisions must be made on a case-by-case basis, with special deliberation given to women at any increased risk for breast or ovarian cancer.
What route is preferred when fibroids are present?
Assuming hysterectomy is the optimal treatment, the vaginal route is feasible.
KARRAM: Uterine fibroids are still the No. 1 indication for hysterectomy. Are minimally invasive laparoscopic procedures with morcellation techniques the best way to manage these women?
FALCONE: The management of symptomatic leiomyomas depends on the patient’s desire to preserve her fertility. If she does not have an interest in future fertility and there are no myomas that are largely submucous or pedunculated, then uterine fibroid embolization is the treatment of choice. Many studies have shown an excellent response, few complications, and rapid return to work.
If the woman wants to preserve fertility, myomectomy is the treatment of choice. If there are few myomas of moderate size, laparoscopic myomectomy is as effective as laparotomy in the hands of experienced laparoscopists who have the ability to suture.
HERZOG: I also want to stress that we now have a number of options, including uterine artery embolization, medications, and surgery, available for women with symptomatic fibroids. The surgical approaches are numerous and include hysteroscopic and/or laparoscopic myomectomy with or without morcellation, as well as hysterectomy. The approach should be determined by the symptoms, size, and distribution of the fibroids, as well as by individual patient characteristics such as prior surgeries, body mass index, and so on. Just because a procedure is technically feasible does not mean it is the preferred method, and this tenet certainly applies to morcellation. In some instances, women with very large fibroids may be better served by laparotomy to decrease blood loss and the duration of surgery while optimizing uterine wall reconstruction, especially when future fertility is an important consideration. Once again, proper patient selection is paramount in achieving favorable outcomes, especially for those who may be undergoing morcellation.
LEVY: For women who have completed childbearing and who desire hysterectomy, I always attempt a vaginal approach first. Most uteri, regardless of size, can be safely and efficiently removed vaginally as long as there is access to the uterine vasculature. Morcellation is easily performed vaginally once hemostasis is assured. For the rare patient with a large fundal myoma that cannot be brought into the pelvis for morcellation, minilaparotomy or laparoscopic approaches are appropriate.
KARRAM: I think randomized trials are needed in this area. It is important to remember that most cases performed laparoscopically result in supracervical hysterectomies and that significant costs are accrued from the equipment required for morcellation. These factors need to be weighed against potential advantages over abdominal hysterectomy, which include shorter hospital stay, potentially decreased morbidity, and faster recovery. The only way to make any objective conclusions about the options would be a randomized trial with appropriate power involving surgeons equally skilled in laparoscopic and open techniques.
Are residents adequately trained?
It depends on the program but, on the whole, more concentrated experience in minimally invasive surgery is needed.
KARRAM: Let’s focus on residency training for a moment. We seem to agree there is a lack of it in vaginal hysterectomy. It seems to me that the lack of training increases as time goes on. Because the current generation of gynecologists-in-training is ultimately the next generation of teachers, it bodes ill for the future when they are reluctant to attempt vaginal hysterectomy, except in the simplest and most straightforward cases. The medicolegal climate also plays a role, as Dr. Herzog mentioned.
Any other thoughts?
FALCONE: The training across residency programs is not homogenous. Some institutions promote vaginal hysterectomy as the primary access, and others do not.
HERZOG: I agree that some institutions do provide an adequate volume of cases, but many others offer a paucity of vaginal surgeries. Many reasons have combined to cause this shortage of training cases over the past 15 years, including a decrease in the number of hysterectomies performed overall, thanks to a number of nonsurgical or less radical surgical treatments for the most common indications for hysterectomy. These approaches generally are mandated by third-party payers prior to invasive surgery. These mandates were not as rigidly enforced in the past.
In a survey of gynecologic oncologists—the vast majority of whom were at academic training centers—the consensus was that residents had fewer surgical experiences and were less skillful than their predecessors over a 5-year period. More than 80% of respondents thought residents needed more surgical experience to achieve competence.13
Compounding the problem, resident work hours have been restricted and additional educational objectives and nonsurgical rotations have been added to the curriculum without any lengthening of the residency tenure. The adverse effects of these factors on residency case volume has prompted some educators to propose major changes in the residency curriculum, either by lengthening training or developing distinct tracks that facilitate early concentration on an area of interest, thereby allowing residents who choose a surgical track to gain increased training and volume.
Until substantive changes occur, educators must rely on surgical simulators and other in vitro models, especially for laparoscopic training. These have benefit but are not a perfect substitute for actual operative experience. A recent study explored the value of a surgical bench skills training program and concluded that, while residents showed definite improvement in bench laboratory tasks, this improvement did not translate into statistically significant improvement in global skills intraoperatively.14 Clearly, educators must continue to explore options to enhance surgical training, especially for vaginal surgery, or this route will become nearly obsolete in the gynecologic generalist’s armamentarium.
Dr. Karram reports that he receives grant/research support from Gynecare, American Medical Systems, and Pfizer and is a speaker for Gynecare, Ortho-McNeil, and Watson. Dr. Falcone, Dr. Herzog, and Dr. Levy have no financial relationships relevant to this article.
- Moderator Mickey Karram, MD, Director of Urogynecology, Good Samaritan Hospital, Cincinnati, and Professor of Obstetrics and Gynecology, University of Cincinnati, Ohio.
- Tommaso Falcone, MD, Professor and Chairman, Department of Obstetrics and Gynecology, Cleveland Clinic, Cleveland, Ohio.
- Thomas Herzog, MD, Director, Division of Gynecologic Oncology, Physicians and Surgeons Alumni Professor, Columbia University Medical Center, New York City.
- Barbara S. Levy, MD, Medical Director, Women’s Health Center, Franciscan Health System, Federal Way, Wash. Dr. Levy serves on the OBG MANAGEMENT Board of Editors.
Why the continued reliance on the abdominal approach despite convincing evidence that vaginal and laparoscopicassisted vaginal hysterectomy (LAVH) offer faster recovery, better cosmesis, and, in many cases, a shorter operation with fewer complications?
OBG Management convened a panel of experts in different aspects of gynecologic surgery to explore this issue. They discuss the reasons most physicians prefer the abdominal approach, how residency programs affect the choice of hysterectomy route, indications for LAVH and supracervical hysterectomy, the issue of ovarian conservation, and management of uterine fibroids.
Why the abdominal route remains the old standby
Physicians use the procedure they are most comfortable with, and residents lack sufficient hands-on experience with laparoscopic and vaginal surgery. Medicolegal risk and reimbursement also have an impact.
KARRAM: Hysterectomy is one of the most widely performed surgeries in the United States, but approximately 60% to 80% of these surgeries still involve the abdominal route.2 Why do you think that is?
FALCONE: Most physicians practice in the manner they were trained, and most residency programs train residents to perform abdominal hysterectomy.
LEVY: I agree. Residents in obstetrics and gynecology have a limited time frame in which to learn and become facile with surgical gynecology. The requirements for primary care training and continuity clinics leave little time for the resident to become comfortable with endoscopic and vaginal surgery. However, they do get substantial exposure to abdominal surgery, both in obstetrics (with cesarean sections constituting 27.5% of all deliveries in the United States3) and gynecologic oncology rotations.
Furthermore, the volume of benign gynecologic surgery is low and the technical skills required for laparoscopic and vaginal surgery are more challenging than “slash and gash” abdominal surgery, so residents don’t get enough exposure to develop a comfort level with these procedures.
HERZOG: This trend is likely to change in the future because recent and current trainees have much more exposure to the laparoscopic approach than in the past, and the equipment has continued to improve. However, I have serious doubts about whether adequate amounts of vaginal surgery are performed in training programs to educate the next generation of vaginal surgeons.
LEVY: Another factor is the type of practice physicians enter after training. Most join practices in which the bulk of their income for many years derives from obstetrics. Without a mentor in the practice who is skilled at minimally invasive surgery, most of these young physicians appropriately resort to the hysterectomy approach for which they have the most comfort and skill: the abdominal route.
HERZOG: Secondary barriers to nonabdominal procedures are lower reimbursement and heightened medicolegal risk, since time and complications are greater for laparoscopic surgery and, to a lesser degree, vaginal procedures. Surgeons are not adequately compensated for either the increased time or risk.
Patients also tend to have higher expectations when the planned approach is minimally invasive. When conversion to laparotomy is necessary, the patient and her family may have trouble understanding why.
KARRAM: I agree that there is a serious lack of training in simple and complicated vaginal hysterectomy. Many inaccurate perceptions have been handed down over the years about its absolute and relative contraindications, such as the belief that any history of pelvic infection, endometriosis, or cesarean section is a contraindication for the vaginal approach.
When is laparoscopic assistance appropriate?
At a fundamental level, its value lies in converting abdominal hysterectomy intovaginal hysterectomy.
KARRAM: In my experience, LAVH is appropriate in the presence of benign adnexal pathology: The adnexa can be evaluated and detached laparoscopically followed by vaginal hysterectomy and vaginal removal of the adnexa. It also is appropriate in any situation that involves excessive pelvic adhesions. The uterus can be mobilized laparoscopically, followed by removal through the vagina.
FALCONE: Any patient who is not a candidate for vaginal hysterectomy should be considered for laparoscopic assistance. The general rationale for the surgery is to convert an abdominal hysterectomy into a vaginal one, so the surgeon should start laparoscopically and then switch to the vaginal approach as soon as possible. Of course, it is impossible to proceed vaginally in some cases. When it is, the entire case can be performed laparoscopically.
LEVY: In my hands, patients with an unidentified adnexal mass who also need or request hysterectomy are appropriate candidates for laparoscopic abdominal exploration followed by vaginal hysterectomy if appropriate. Women with a very contracted pelvis, which precludes transvaginal access to the uterine vasculature, may also be candidates for the laparoscopic approach. However, as surgeons become more skilled at vaginal surgery and learn to use newer instrumentation, the need for laparoscopy to access a tight pelvis will diminish.
Using a laparoscopic approach for patients with fibroids wedged into the pelvis carries serious risk to the pelvic sidewall. It is very difficult to access the sidewall safely laparoscopically in the presence of a large lower segment or cervical myomas.
HERZOG: When LAVH was introduced, many clinicians challenged the utility of combined laparoscopic and vaginal surgery, with some referring to this surgical exercise as a procedure looking for an indication. However, as operative laparoscopy has gained acceptance, some benefits of LAVH have become apparent. The greatest advantage is the potential to convert a procedure that would have been performed abdominally into a vaginal hysterectomy.
The most commonly cited indications for LAVH are to lyse adhesions secondary to prior abdominopelvic surgery, substantial endometriosis, or a pelvic mass.
Is oophorectomy an indication for LAVH?
The need to remove the ovaries does not mean laparoscopic assistance is imperative.
HERZOG: Simple removal of the ovaries can often be performed using the vaginal route, and is not in itself an indication for LAVH.
LEVY: I agree. The ovaries can usually be accessed transvaginally, especially with good fiberoptic lighting and vessel-sealing technology.
FALCONE: Several studies, most notably the one by Ballard and Walters,4 demonstrate that oophorectomy can be carried out vaginally in most cases. In their study, they did not use special instruments.
HERZOG: But LAVH is indicated to facilitate complete removal of the ovaries in riskreduction surgery for documented or suspected BrCa 1 or 2 mutations. The entire ovary and as much of the tube as possible must be removed in these women. Thus, if the patient has other indications for hysterectomy, LAVH may be the preferred route to assure that the blood supply is taken proximally enough to remove absolutely all ovarian tissue. Simply clamping directly along the side of the ovary is not an adequate removal technique for these patients, since an ovarian remnant may become a fatal oversight.
LEVY: Yes, laparoscopy is indicated for riskreducing salpingo-oophorectomy in order to adequately assess the entire peritoneal cavity. Up to 2% of these patients will have occult invasive ovarian or peritoneal carcinoma at the time of their prophylactic surgery, so full surgical abdominal exploration is mandatory and can be nicely accomplished via laparoscopy.5
How endometriosis history affects choice of route
In some women, laparoscopic surgery is preferred over the vaginal route.
FALCONE: Hysterectomy with or without salpingo-oophorectomy can be considered in women whose endometriosis fails to respond to conservative management and who do not desire fertility. Most studies have shown substantial pain relief with definitive surgery.6,7 Although ovarian conservation may be advisable in younger women, in some women it increases the probability of recurrent pain and the need for reoperation. The main concern is whether the endometriosis is completely removed during hysterectomy.
A history of cul-de-sac obliteration or extensive pelvic adhesions from endometriosis is an indication for laparoscopic hysterectomy rather than vaginal hysterectomy. Women with less severe disease will benefit from a diagnostic laparoscopy prior to a vaginal hysterectomy to evaluate the pelvis and excise any endometriosis.
Is there any benefit to leaving the cervix?
There is no evidence of any benefit except in selected cases of heavy bleeding, postpartum hemorrhage, advanced endometriosis, or ovarian cancer surgery.
KARRAM: What about supracervical hysterectomy? The only time I have performed one was at the time of a cesareanhysterectomy because blood loss was significant and dissection of the cervix could have led to more morbidity. What are the indications for this procedure?
HERZOG: It is unclear whether there are any definitive indications for supracervical hysterectomy. A number of benefits have been proposed, such as better support and improved sexual function. Some of the perceived benefits have been supported by nonrandomized trials, but critical analysis of the randomized data has failed to support most of these contentions.8,9 However, the procedure can be a valuable intervention to decrease critical blood loss intraoperatively, as you point out, or to simplify complicated pelvic surgery in selected cases, such as postpartum hemorrhage, advanced endometriosis, ovarian cancer debulking (when the cervix is not involved), or significant bleeding in patients who object to transfusion on ethical or religious grounds.
FALCONE: There are clear contraindications to supracervical hysterectomy, namely the presence of a malignant or premalignant condition of the uterine corpus or cervix, but no indications, except perhaps for an unstable patient undergoing hysterectomy in whom you want to finish quickly. None of the randomized clinical trials have shown supracervical hysterectomy to be superior to total hysterectomy. The randomized trials involved the abdominal route; the time to complete a laparoscopic supracervical hysterectomy is less than for a laparoscopic total hysterectomy.
Nevertheless, many patients ask for the procedure. After I present the risks and benefits, I leave the choice up to them. Of course, it is important to explain that total hysterectomy implies removal of the cervix and not the ovaries.
LEVY: In rare cases of immunocompromised patients or women with widely disseminated intraperitoneal carcinoma, one could make an argument for avoiding entry into the vagina to reduce infectious risk, speed healing, or avoid tumor seeding. Otherwise, there is absolutely no evidence to support an indication for supracervical hysterectomy.
Does leaving the cervix affect long-term function?
The residual cervix can become the site of later neoplasia or disease.
HERZOG: Supracervical hysterectomy can be associated with several problems with longterm implications. One is the potential for cervical intraepithelial neoplasia. Another concern relates to bleeding from a portion of active endometrium at the top of the endocervical canal. Rarer problems include the development of endometriosis or invasive cancer in the residual cervix. These potential drawbacks need to be strongly considered and included in patient counseling.
KARRAM: One study followed 67 patients for 66 months after supracervical hysterectomy; trachelectomy was ultimately required in 22.8% of patients.10
The $64,0000 question: Remove the ovaries?
Overall, the decision should be made case by case.
KARRAM: Should routine oophorectomy be performed at the time of hysterectomy in postmenopausal women to decrease the potential for ovarian cancer later in life?
HERZOG: This is a very important question. Conventional thinking used to be that, for women over the age of 45, and certainly for women older than 50, ovarian removal should be strongly considered to reduce the risk of cancer of the ovary or fallopian tubes at a later date. Studies focusing on the number of ovarian cancer cases possibly prevented with routine oophorectomy at the time of hysterectomy reinforced this concept. One single-institution study showed that more than 60 cases of ovarian cancer would have been prevented over a 14-year period if ovaries were routinely removed in women older than 40 undergoing hysterectomy. By extrapolation, that would result in more than 1,000 cases prevented annually in the United States.11
Recent data refute the rationale for routine oophorectomy. One study that used statistical modeling with Markov decision analysis to determine life expectancy concluded that, at least until the age of 65, women are best served with ovarian conservation if their risk of developing ovarian cancer is average or less.12 Researchers found that women who underwent oophorectomy before age 55 experienced 8.6% excess mortality by age 80. The validity of certain assumptions used to construct this model has been challenged; nevertheless, this cogent study certainly challenges previous concepts regarding agebased routine prophylactic oophorectomy. Until further study results are reported, it is important to counsel women who are considering having their ovaries removed about the potential risks and benefits. Furthermore, these decisions must be made on a case-by-case basis, with special deliberation given to women at any increased risk for breast or ovarian cancer.
What route is preferred when fibroids are present?
Assuming hysterectomy is the optimal treatment, the vaginal route is feasible.
KARRAM: Uterine fibroids are still the No. 1 indication for hysterectomy. Are minimally invasive laparoscopic procedures with morcellation techniques the best way to manage these women?
FALCONE: The management of symptomatic leiomyomas depends on the patient’s desire to preserve her fertility. If she does not have an interest in future fertility and there are no myomas that are largely submucous or pedunculated, then uterine fibroid embolization is the treatment of choice. Many studies have shown an excellent response, few complications, and rapid return to work.
If the woman wants to preserve fertility, myomectomy is the treatment of choice. If there are few myomas of moderate size, laparoscopic myomectomy is as effective as laparotomy in the hands of experienced laparoscopists who have the ability to suture.
HERZOG: I also want to stress that we now have a number of options, including uterine artery embolization, medications, and surgery, available for women with symptomatic fibroids. The surgical approaches are numerous and include hysteroscopic and/or laparoscopic myomectomy with or without morcellation, as well as hysterectomy. The approach should be determined by the symptoms, size, and distribution of the fibroids, as well as by individual patient characteristics such as prior surgeries, body mass index, and so on. Just because a procedure is technically feasible does not mean it is the preferred method, and this tenet certainly applies to morcellation. In some instances, women with very large fibroids may be better served by laparotomy to decrease blood loss and the duration of surgery while optimizing uterine wall reconstruction, especially when future fertility is an important consideration. Once again, proper patient selection is paramount in achieving favorable outcomes, especially for those who may be undergoing morcellation.
LEVY: For women who have completed childbearing and who desire hysterectomy, I always attempt a vaginal approach first. Most uteri, regardless of size, can be safely and efficiently removed vaginally as long as there is access to the uterine vasculature. Morcellation is easily performed vaginally once hemostasis is assured. For the rare patient with a large fundal myoma that cannot be brought into the pelvis for morcellation, minilaparotomy or laparoscopic approaches are appropriate.
KARRAM: I think randomized trials are needed in this area. It is important to remember that most cases performed laparoscopically result in supracervical hysterectomies and that significant costs are accrued from the equipment required for morcellation. These factors need to be weighed against potential advantages over abdominal hysterectomy, which include shorter hospital stay, potentially decreased morbidity, and faster recovery. The only way to make any objective conclusions about the options would be a randomized trial with appropriate power involving surgeons equally skilled in laparoscopic and open techniques.
Are residents adequately trained?
It depends on the program but, on the whole, more concentrated experience in minimally invasive surgery is needed.
KARRAM: Let’s focus on residency training for a moment. We seem to agree there is a lack of it in vaginal hysterectomy. It seems to me that the lack of training increases as time goes on. Because the current generation of gynecologists-in-training is ultimately the next generation of teachers, it bodes ill for the future when they are reluctant to attempt vaginal hysterectomy, except in the simplest and most straightforward cases. The medicolegal climate also plays a role, as Dr. Herzog mentioned.
Any other thoughts?
FALCONE: The training across residency programs is not homogenous. Some institutions promote vaginal hysterectomy as the primary access, and others do not.
HERZOG: I agree that some institutions do provide an adequate volume of cases, but many others offer a paucity of vaginal surgeries. Many reasons have combined to cause this shortage of training cases over the past 15 years, including a decrease in the number of hysterectomies performed overall, thanks to a number of nonsurgical or less radical surgical treatments for the most common indications for hysterectomy. These approaches generally are mandated by third-party payers prior to invasive surgery. These mandates were not as rigidly enforced in the past.
In a survey of gynecologic oncologists—the vast majority of whom were at academic training centers—the consensus was that residents had fewer surgical experiences and were less skillful than their predecessors over a 5-year period. More than 80% of respondents thought residents needed more surgical experience to achieve competence.13
Compounding the problem, resident work hours have been restricted and additional educational objectives and nonsurgical rotations have been added to the curriculum without any lengthening of the residency tenure. The adverse effects of these factors on residency case volume has prompted some educators to propose major changes in the residency curriculum, either by lengthening training or developing distinct tracks that facilitate early concentration on an area of interest, thereby allowing residents who choose a surgical track to gain increased training and volume.
Until substantive changes occur, educators must rely on surgical simulators and other in vitro models, especially for laparoscopic training. These have benefit but are not a perfect substitute for actual operative experience. A recent study explored the value of a surgical bench skills training program and concluded that, while residents showed definite improvement in bench laboratory tasks, this improvement did not translate into statistically significant improvement in global skills intraoperatively.14 Clearly, educators must continue to explore options to enhance surgical training, especially for vaginal surgery, or this route will become nearly obsolete in the gynecologic generalist’s armamentarium.
Dr. Karram reports that he receives grant/research support from Gynecare, American Medical Systems, and Pfizer and is a speaker for Gynecare, Ortho-McNeil, and Watson. Dr. Falcone, Dr. Herzog, and Dr. Levy have no financial relationships relevant to this article.
1. Kovac SR. Transvaginal hysterectomy: rationale and surgical approach. Obstet Gynecol. 2004;103:1321-1325.
2. Baggish MS. Total and subtotal abdominal hysterectomy. Best Pract Res Clin Obstet Gynaecol. 2005;19:333-356.
3. National Center for Health Statistics. Births—method of delivery. Available at: www.cdc.gov/nchs/fastats/ delivery.htm. Accessed January 18, 2006.
4. Ballard LA, Walters MD. Transvaginal mobilization and removal of ovaries and fallopian tubes after vaginal hysterectomy. Obstet Gynecol. 1996;87:35-39.
5. Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58-64.
6. Dmowski WP, Radwanska E, Rana N. Recurrent endometriosis following hysterectomy and oophorectomy: the role of residual ovarian fragments. Int J Gynecol Obstet. 1988;26:93-103.
7. Namnoun AB, Hickman NT, Goodman SB, Gehlbach DL, Rock JA. Incidence of symptom recurrence after hysterectomy for endometriosis. Fertil Steril. 1995;64:898-902.
8. Kuppermann M, Summitt RL, Jr, Varner RE, et al. Total or Supracervical Hysterectomy Research Group. Sexual functioning after total compared with supracervical hysterectomy: a randomized trial. Obstet Gynecol. 2005;105:1309-1318.
9. Learman LA, Summitt RL, Jr, Varner RE, et al. Total or Supracervical Hysterectomy (TOSH) Research Group. A randomized comparison of total or supracervical hysterectomy: surgical complications and clinical outcomes. Obstet Gynecol. 2003;102:453-462.
10. Okaro EO, Jones KD, Sutton C. Long-term outcome following laparoscopic supracervical hysterectomy. BJOG. 2001;108:1017-1020.
11. Sightler SE, Boike GM, Estape RE, Averette HE. Ovarian cancer in women with prior hysterectomy: a 14-year experience at the University of Miami. Obstet Gynecol. 1991;78:681-684.
12. Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226.
13. Sorosky JI, Anderson B. Surgical experiences and training of residents: perspective of experienced gynecologic oncologists. Gynecol Oncol. 1999;75:222-223.
14. Lentz GM, Mandel LS, Goff BA. A six-year study of surgical teaching and skills evaluation for obstetric/gynecologic residents in porcine and inanimate surgical models. Am J Obstet Gynecol. 2005;193:2056-2061.
1. Kovac SR. Transvaginal hysterectomy: rationale and surgical approach. Obstet Gynecol. 2004;103:1321-1325.
2. Baggish MS. Total and subtotal abdominal hysterectomy. Best Pract Res Clin Obstet Gynaecol. 2005;19:333-356.
3. National Center for Health Statistics. Births—method of delivery. Available at: www.cdc.gov/nchs/fastats/ delivery.htm. Accessed January 18, 2006.
4. Ballard LA, Walters MD. Transvaginal mobilization and removal of ovaries and fallopian tubes after vaginal hysterectomy. Obstet Gynecol. 1996;87:35-39.
5. Finch A, Shaw P, Rosen B, et al. Clinical and pathologic findings of prophylactic salpingo-oophorectomies in 159 BRCA1 and BRCA2 carriers. Gynecol Oncol. 2006;100:58-64.
6. Dmowski WP, Radwanska E, Rana N. Recurrent endometriosis following hysterectomy and oophorectomy: the role of residual ovarian fragments. Int J Gynecol Obstet. 1988;26:93-103.
7. Namnoun AB, Hickman NT, Goodman SB, Gehlbach DL, Rock JA. Incidence of symptom recurrence after hysterectomy for endometriosis. Fertil Steril. 1995;64:898-902.
8. Kuppermann M, Summitt RL, Jr, Varner RE, et al. Total or Supracervical Hysterectomy Research Group. Sexual functioning after total compared with supracervical hysterectomy: a randomized trial. Obstet Gynecol. 2005;105:1309-1318.
9. Learman LA, Summitt RL, Jr, Varner RE, et al. Total or Supracervical Hysterectomy (TOSH) Research Group. A randomized comparison of total or supracervical hysterectomy: surgical complications and clinical outcomes. Obstet Gynecol. 2003;102:453-462.
10. Okaro EO, Jones KD, Sutton C. Long-term outcome following laparoscopic supracervical hysterectomy. BJOG. 2001;108:1017-1020.
11. Sightler SE, Boike GM, Estape RE, Averette HE. Ovarian cancer in women with prior hysterectomy: a 14-year experience at the University of Miami. Obstet Gynecol. 1991;78:681-684.
12. Parker WH, Broder MS, Liu Z, Shoupe D, Farquhar C, Berek JS. Ovarian conservation at the time of hysterectomy for benign disease. Obstet Gynecol. 2005;106:219-226.
13. Sorosky JI, Anderson B. Surgical experiences and training of residents: perspective of experienced gynecologic oncologists. Gynecol Oncol. 1999;75:222-223.
14. Lentz GM, Mandel LS, Goff BA. A six-year study of surgical teaching and skills evaluation for obstetric/gynecologic residents in porcine and inanimate surgical models. Am J Obstet Gynecol. 2005;193:2056-2061.
IN THIS ARTICLE
- Editorial We are at the tipping point
Abdominal techniques for surgical management of vaginal vault prolapse
A range of clinical conditions can suggest an abdominal approach for vaginal vault prolapse procedures.
These include, but are not limited to:
- prior unsuccessful vaginal attempts
- obligate need for adnexal access
- markedly foreshortened vagina
- pelvic bony architectural limitations
- high risk for surgical failure (eg, athleticism, obesity, chronic obstructive pulmonary disease, congenital connective tissue disorder)
- desire for uterine preservation
In Part 1 (November 2005) of this 2-part article, we reviewed the most widely used and the newest vaginal techniques. Part 2 focuses on the abdominal approach, and compares vaginal and abdominal approaches.
High uterosacral ligament suspension
Surgical technique for this procedure for mild to moderate vaginal vault prolapse (stage I or II), using a vaginal approach, was described in Part 1, in the November issue of OBG Management. Abdominal repair involves the same concepts; like the vaginal approach, it is applicable only to the patient with mild to moderate vault prolapse. It will be less successful if it is performed to address complete vault prolapse.
Technique
Identify and tag the remnants of the uterosacral ligaments at the level of the ischial spines. Once the ureters are identified and isolated, address the enterocele by obliterating the cul-de-sac via Halban’s culdoplasty or abdominal McCall’s culdoplasty.
Open the peritoneum over the vaginal apex and trim it back to the level of the endopelvic fascia of the vaginal wall. After excising the redundant peritoneum of the vaginal apex, identify and reapproximate the pubocervical fascia of the anterior vaginal wall and the rectovaginal fascia of the posterior vaginal wall using interrupted or running nonabsorbable suture.
Then use nonabsorbable sutures to suspend each corner of the prolapsed vagina from its respective ipsilateral uterosacral ligament.
Abdominal sacral colpopexy
Abdominal sacral colpopexy was first popularized by Addison and Timmons in the 1980s, and is the abdominal standard of apical prolapse repair due to its long-term durability.
Abdominal sacral colpopexy can be performed with or without uterine extirpation. When a hysterectomy is performed concomitantly, some surgeons prefer a supracervical approach, provided there is no history of cervical dysplasia, because, theoretically, the cervical stump serves as a firm and substantial point of fixation for the synthetic mesh that will be used to perform the repair. This in turn may diminish the likelihood of postoperative mesh erosion.
Technique
Reflect the sigmoid colon as far as possible into the left lateral pelvis to expose the sacral promontory. If it has not already been done, free all adhesions between the colon and pelvic peritoneum to fully mobilize the colon and permit its maximal retraction out of the pelvic field prior to making the peritoneal incision.
Also make it a point to identify all structures at risk during this portion of the procedure—namely, the common iliac vessels, ureters, and middle sacral artery and vein. The left common iliac vein is medial to the left common iliac artery and is particularly susceptible to injury during this phase of the procedure.
Make a longitudinal incision in the peritoneum overlying the sacral promontory and extend it approximately 6 cm from the promontory dorsally into the cul-de-sac, opening the retrorectal space (FIGURE 1, TOP). Using a fine tonsil forceps and cautery, very gently dissect the retroareolar filmy tissue overlying the anterior longitudinal ligament away from S1 in thin layers until the white periosteum of the anterior longitudinal ligament overlying S1 is clearly exposed. It now becomes very easy to visualize the course of the middle sacral artery and vein. With these vessels under direct visualization, place 2 permanent #0 sutures through the periosteum of S1.
Do not attempt to place these sutures deeper in the presacral space than the S1 vertebral body, or life-threatening and uncontrollable bleeding may result.
If there is no uterus, insert a probe such as an end-to-end anastomotic sizer or handheld Harrington retractor into the vagina and extend it, elongating and elevating the vaginal cylinder. It now becomes much easier to identify the interface between the bladder and vagina prior to making the peritoneal incision.
If the interface remains indistinct, instill 150 cc of saline into the bladder to delineate its boundaries. Then elevate and incise the vesicouterine peritoneum overlying the junction between the bladder and vaginal apex; this provides access to the vesicocervical space. Dissect the bladder off the anterior vaginal wall in a caudal direction until the pubocervical fascia can be identified. Do not dissect away the peritoneum over the posterior vaginal wall, but leave it intact.
FIGURE 1Abdominal sacral colpopexy technique
Reconstructive materials
Although many different materials have been described, none have undergone rigorous comparisons. In our institution, we use soft polypropylene (Surgipro; US Surgical, Norwalk, Conn).
Fold a piece of 5-inch mesh over onto itself and suture the layers together to create a double-thickness configuration. Then “fishmouth” the caudal end of this mesh prosthesis, producing both an anterior and posterior leaf. With the obturator still within the vaginal cylinder stretching the vaginal apex, secure the posterior leaf of the mesh to the posterior vaginal wall with 3 to 5 nonabsorbable #0 sutures.
Suture placement. Thread each suture initially through the posterior leaf of the mesh, placed deeply through the fibromuscular thickness of the posterior vaginal wall, then bring it back out through the mesh at the same point. Place the sutures in a transverse line 1 to 2 cm apart and 3 to 4 cm distal to the vaginal apex.
Once all sutures have been placed, tie each of them, thereby securing the posterior leaf of the mesh to the posterior vaginal wall. Now perform a “mirror” procedure to secure the anterior leaf of the mesh prosthesis to the anterior vaginal wall. Then firmly attach the mesh prosthesis to the vaginal apex using several interrupted, permanent sutures.
At this point, the sutures previously placed through the periosteum of the sacral promontory are threaded through the apex of the mesh prosthesis at a point that will allow the vagina to rest comfortably within the pelvis, without undue tension or traction once the sutures are tied into place (FIGURE 1, MIDDLE).
Trim any excess mesh, and close the 6-cm longitudinal peritoneal incision previously created in the cul-de-sac. Close the incision over the top of the mesh, retroperitonealizing the mesh prosthesis (FIGURE 1, BOTTOM).
Paravaginal defect repair
The bladder base is intimately associated with the anterior vaginal wall via a triangular sheet of pubocervical fascia attached to and extending from the arcus tendineus fascia pelvis bilaterally. When the apex of the vagina prolapses through the introitus, as in total vault prolapse, the base of the bladder is torn free from these fascial attachments in the pelvis and herniates through the introitus along with the vaginal apex. By definition, a bilateral paravaginal defect will result. Thus, surgical repair of total vaginal vault prolapse almost invariably requires paravaginal defect repair as well.
The goal of paravaginal defect repair is to reattach, bilaterally, the anterolateral vaginal sulcus and its overlying endopelvic fascia to the pubococcygeus and obturator internus muscles and fascia at the level of the arcus tendineus fascia pelvis.
Technique
Enter and gently develop the retropubic space of Retzius, taking care not to disrupt the myriad venous anastomotic networks of the plexus of Santorini, located on and around the bladder. Bluntly mobilize the bladder bilaterally, exposing the lateral retropubic spaces, the pubococcygeus and obturator internus muscles, and the obturator neurovascular bundles. Within each retropubic space, palpate the ischial spine. Then visualize the arcus, seen as a white ligamentous band, as it courses from the ischial spine caudally toward the ipsilateral posterior pubic symphysis. A lateral paravaginal defect representing avulsion of the vagina off the arcus tendineus fascia pelvis, or of the arcus tendineus fascia pelvis off the obturator internus muscle, can now be visualized.
While gently reflecting the bladder medially with a wide ribbon, insert a few fingers of the nondominant hand into the vagina and elevate the ipsilateral anterolateral vaginal sulcus. Then place a suture through the fibromuscular thickness of the lateral vaginal apex, just above the uplifting fingers in the vagina, and then slightly cephalad into the arcus tendineus fascia pelvis or obturator internus fascia on the pelvic sidewall, at a point 1 to 2 cm distal to the ischial spine. Place 3 to 5 additional sutures in a similar fashion at 1-cm intervals. The most distal suture should be placed as close as possible to the pubic ramus into the pubourethral ligament. If necessary, repeat the procedure on the contralateral side. Then tie all sutures into place, thereby completing the repair.
When a patient has complete vault prolapse, she typically has defects in all 3 levels of pelvic support, and thus may need to undergo several different procedures to correct all anatomic defects and restore function.
In other words, vaginal vault prolapse rarely presents as an isolated defect. It more commonly occurs in conjunction with a cystocele, rectocele, enterocele, or some combination of these.1 Richter reported that 72% of patients with vaginal vault prolapse had a combination of other pelvic floor defects as well.2
If all vaginal support defects are repaired at the time of sacral colpopexy, recurrent vault prolapse is rare. Failures can be minimized by suturing the suspensory mesh to the posterior vagina and anterior vaginal apex over as extended an area as possible. Also test the sutures once they are placed within the periosteum of the sacral promontory to ensure they will not pull free.
Plan on occult incontinence
Total vaginal vault prolapse is commonly associated with some degree of urethral kinking, with subsequent outflow tract obstruction. As a result, most patients with complete vault prolapse do not complain of incontinence at the initial presentation. However, once the anatomic axis of the vagina is restored and the bladder is replaced within the pelvis with subsequent straightening of the urethra, occult incontinence often is uncovered. Although the patient may have a wonderful anatomic repair of severe vault prolapse at the completion of the surgical procedure, she will not be satisfied if she suddenly finds herself floridly incontinent.
Consider formal multichannel cystometrics prior to surgery in all women undergoing repair of total vault prolapse. If genuine stress urinary incontinence is present when the prolapse is reduced, an anti-incontinence procedure can be scheduled at the same time as the surgical repair. A Burch procedure can be performed for type IIA or IIB genuine stress incontinence, or a pubovaginal sling procedure can be performed for type III stress incontinence.
Posterior colporrhaphy/perineorrhaphy
These procedures are now performed to treat the remaining rectocele and perineal defect, when present.
Vaginal vs abdominal route
Somewhat surprisingly, the abdominal route appears to produce better long-term results. In a prospective, randomized controlled trial comparing both routes for the repair of total vault prolapse, Benson et al3 found that, after 5 years of follow-up, women managed vaginally had a 6-fold increased incidence of recurrent vault prolapse, a 3-fold increased incidence of recurrent cystocele, and twice the reoperation rate, compared with women whose initial repair was abdominal.
In the study, 48 women with total vault prolapse underwent vaginal bilateral sacrospinous fixation and paravaginal defect repair, and 40 underwent abdominal sacral colpopexy and paravaginal defect repair. Although the vaginal approach was associated with a shorter operative time and decreased hospital stay in the short term, it necessitated longer postoperative catheter use and was associated with more urinary tract infections and postoperative incontinence and a higher overall failure rate.
Sze and colleagues4 addressed a similar question in retrospective fashion, reviewing the medical records of 117 women surgically treated for total vault prolapse. Sixty-one women underwent vaginal sacrospinous ligament fixation and Raz urethropexy, while 56 underwent abdominal sacral colpopexy and Burch urethropexy. After a mean follow-up of 24 months, 33% of the women managed vaginally developed recurrent pelvic organ prolapse, compared with only 19% of the women managed abdominally. In addition, 26% of the women managed vaginally had recurrent urinary incontinence, compared with only 13% of the women managed abdominally
A separate randomized, prospective study by Maher et al5 compared abdominal sacral colpopexy (n=47) and vaginal sacrospinous ligament fixation (n=48) for stage II to IV vault prolapse. After a mean follow-up of 2 years, subjective and objective success rates did not differ significantly between the 2 routes.
Why is the abdominal route more durable?
Any number of reasons may apply:
- The traditional surgical procedure for vaginal management of total vault prolapse—sacrospinous ligament fixation—distorts the axis of the vagina.
- Native tissues are not as strong as synthetic materials. In postmenopausal women, a repair in which the thin, atrophic vaginal apex is secured to the sacrospinous ligament will not have the same durability as a repair involving mesh.
- In vaginal paravaginal repair, the extensive periurethral dissection required can damage fine branches of the pudendal nerve that innervate and control the urethral sphincter. Such extensive dissection is not required for paravaginal repair from the abdominal approach.
- In the vaginal approach, it can be difficult to gain adequate exposure high in the retroperitoneum to reattach the endopelvic fascia of the vaginal apex to the arcus at its origin just distal to the ischial spine.
The long view
The surgical options described in this article have varying degrees of risk and benefit. Multicenter, prospective surgical trials are needed to clarify these risks and benefits and provide physicians and their patients with reliable information. Ultimately, pursuit of a surgical “cure” will be supplanted by sustainable forms of disease prevention. Until then, decisions about prolapse surgery are best left to the judgment of the surgeon and the desires of his or her patient.
The authors report no financial relationships relevant to this article.
1. Herbst A, Mishell D, Stenchever M. Disorders of the abdominal wall and pelvic support. In: Comprehensive Gynecology. 2nd ed. Philadelphia: Mosby Year Book; 1992:594–612.
2. Richter K. Massive eversion of the vagina: pathogenesis, diagnosis and therapy of the true prolapse of the vaginal stump. Clin Obstet Gynecol. 1982;25:897-912.
3. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1422.
4. Sze EH, Kohli N, Miklos JR, Roat T, Karram MM. A retrospective comparison of abdominal sacrocolpopexy with Burch colposuspension versus sacrospinous fixation with transvaginal needle suspension for the management of vaginal vault prolapse and coexisting stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:390-393.
5. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal SCP or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
A range of clinical conditions can suggest an abdominal approach for vaginal vault prolapse procedures.
These include, but are not limited to:
- prior unsuccessful vaginal attempts
- obligate need for adnexal access
- markedly foreshortened vagina
- pelvic bony architectural limitations
- high risk for surgical failure (eg, athleticism, obesity, chronic obstructive pulmonary disease, congenital connective tissue disorder)
- desire for uterine preservation
In Part 1 (November 2005) of this 2-part article, we reviewed the most widely used and the newest vaginal techniques. Part 2 focuses on the abdominal approach, and compares vaginal and abdominal approaches.
High uterosacral ligament suspension
Surgical technique for this procedure for mild to moderate vaginal vault prolapse (stage I or II), using a vaginal approach, was described in Part 1, in the November issue of OBG Management. Abdominal repair involves the same concepts; like the vaginal approach, it is applicable only to the patient with mild to moderate vault prolapse. It will be less successful if it is performed to address complete vault prolapse.
Technique
Identify and tag the remnants of the uterosacral ligaments at the level of the ischial spines. Once the ureters are identified and isolated, address the enterocele by obliterating the cul-de-sac via Halban’s culdoplasty or abdominal McCall’s culdoplasty.
Open the peritoneum over the vaginal apex and trim it back to the level of the endopelvic fascia of the vaginal wall. After excising the redundant peritoneum of the vaginal apex, identify and reapproximate the pubocervical fascia of the anterior vaginal wall and the rectovaginal fascia of the posterior vaginal wall using interrupted or running nonabsorbable suture.
Then use nonabsorbable sutures to suspend each corner of the prolapsed vagina from its respective ipsilateral uterosacral ligament.
Abdominal sacral colpopexy
Abdominal sacral colpopexy was first popularized by Addison and Timmons in the 1980s, and is the abdominal standard of apical prolapse repair due to its long-term durability.
Abdominal sacral colpopexy can be performed with or without uterine extirpation. When a hysterectomy is performed concomitantly, some surgeons prefer a supracervical approach, provided there is no history of cervical dysplasia, because, theoretically, the cervical stump serves as a firm and substantial point of fixation for the synthetic mesh that will be used to perform the repair. This in turn may diminish the likelihood of postoperative mesh erosion.
Technique
Reflect the sigmoid colon as far as possible into the left lateral pelvis to expose the sacral promontory. If it has not already been done, free all adhesions between the colon and pelvic peritoneum to fully mobilize the colon and permit its maximal retraction out of the pelvic field prior to making the peritoneal incision.
Also make it a point to identify all structures at risk during this portion of the procedure—namely, the common iliac vessels, ureters, and middle sacral artery and vein. The left common iliac vein is medial to the left common iliac artery and is particularly susceptible to injury during this phase of the procedure.
Make a longitudinal incision in the peritoneum overlying the sacral promontory and extend it approximately 6 cm from the promontory dorsally into the cul-de-sac, opening the retrorectal space (FIGURE 1, TOP). Using a fine tonsil forceps and cautery, very gently dissect the retroareolar filmy tissue overlying the anterior longitudinal ligament away from S1 in thin layers until the white periosteum of the anterior longitudinal ligament overlying S1 is clearly exposed. It now becomes very easy to visualize the course of the middle sacral artery and vein. With these vessels under direct visualization, place 2 permanent #0 sutures through the periosteum of S1.
Do not attempt to place these sutures deeper in the presacral space than the S1 vertebral body, or life-threatening and uncontrollable bleeding may result.
If there is no uterus, insert a probe such as an end-to-end anastomotic sizer or handheld Harrington retractor into the vagina and extend it, elongating and elevating the vaginal cylinder. It now becomes much easier to identify the interface between the bladder and vagina prior to making the peritoneal incision.
If the interface remains indistinct, instill 150 cc of saline into the bladder to delineate its boundaries. Then elevate and incise the vesicouterine peritoneum overlying the junction between the bladder and vaginal apex; this provides access to the vesicocervical space. Dissect the bladder off the anterior vaginal wall in a caudal direction until the pubocervical fascia can be identified. Do not dissect away the peritoneum over the posterior vaginal wall, but leave it intact.
FIGURE 1Abdominal sacral colpopexy technique
Reconstructive materials
Although many different materials have been described, none have undergone rigorous comparisons. In our institution, we use soft polypropylene (Surgipro; US Surgical, Norwalk, Conn).
Fold a piece of 5-inch mesh over onto itself and suture the layers together to create a double-thickness configuration. Then “fishmouth” the caudal end of this mesh prosthesis, producing both an anterior and posterior leaf. With the obturator still within the vaginal cylinder stretching the vaginal apex, secure the posterior leaf of the mesh to the posterior vaginal wall with 3 to 5 nonabsorbable #0 sutures.
Suture placement. Thread each suture initially through the posterior leaf of the mesh, placed deeply through the fibromuscular thickness of the posterior vaginal wall, then bring it back out through the mesh at the same point. Place the sutures in a transverse line 1 to 2 cm apart and 3 to 4 cm distal to the vaginal apex.
Once all sutures have been placed, tie each of them, thereby securing the posterior leaf of the mesh to the posterior vaginal wall. Now perform a “mirror” procedure to secure the anterior leaf of the mesh prosthesis to the anterior vaginal wall. Then firmly attach the mesh prosthesis to the vaginal apex using several interrupted, permanent sutures.
At this point, the sutures previously placed through the periosteum of the sacral promontory are threaded through the apex of the mesh prosthesis at a point that will allow the vagina to rest comfortably within the pelvis, without undue tension or traction once the sutures are tied into place (FIGURE 1, MIDDLE).
Trim any excess mesh, and close the 6-cm longitudinal peritoneal incision previously created in the cul-de-sac. Close the incision over the top of the mesh, retroperitonealizing the mesh prosthesis (FIGURE 1, BOTTOM).
Paravaginal defect repair
The bladder base is intimately associated with the anterior vaginal wall via a triangular sheet of pubocervical fascia attached to and extending from the arcus tendineus fascia pelvis bilaterally. When the apex of the vagina prolapses through the introitus, as in total vault prolapse, the base of the bladder is torn free from these fascial attachments in the pelvis and herniates through the introitus along with the vaginal apex. By definition, a bilateral paravaginal defect will result. Thus, surgical repair of total vaginal vault prolapse almost invariably requires paravaginal defect repair as well.
The goal of paravaginal defect repair is to reattach, bilaterally, the anterolateral vaginal sulcus and its overlying endopelvic fascia to the pubococcygeus and obturator internus muscles and fascia at the level of the arcus tendineus fascia pelvis.
Technique
Enter and gently develop the retropubic space of Retzius, taking care not to disrupt the myriad venous anastomotic networks of the plexus of Santorini, located on and around the bladder. Bluntly mobilize the bladder bilaterally, exposing the lateral retropubic spaces, the pubococcygeus and obturator internus muscles, and the obturator neurovascular bundles. Within each retropubic space, palpate the ischial spine. Then visualize the arcus, seen as a white ligamentous band, as it courses from the ischial spine caudally toward the ipsilateral posterior pubic symphysis. A lateral paravaginal defect representing avulsion of the vagina off the arcus tendineus fascia pelvis, or of the arcus tendineus fascia pelvis off the obturator internus muscle, can now be visualized.
While gently reflecting the bladder medially with a wide ribbon, insert a few fingers of the nondominant hand into the vagina and elevate the ipsilateral anterolateral vaginal sulcus. Then place a suture through the fibromuscular thickness of the lateral vaginal apex, just above the uplifting fingers in the vagina, and then slightly cephalad into the arcus tendineus fascia pelvis or obturator internus fascia on the pelvic sidewall, at a point 1 to 2 cm distal to the ischial spine. Place 3 to 5 additional sutures in a similar fashion at 1-cm intervals. The most distal suture should be placed as close as possible to the pubic ramus into the pubourethral ligament. If necessary, repeat the procedure on the contralateral side. Then tie all sutures into place, thereby completing the repair.
When a patient has complete vault prolapse, she typically has defects in all 3 levels of pelvic support, and thus may need to undergo several different procedures to correct all anatomic defects and restore function.
In other words, vaginal vault prolapse rarely presents as an isolated defect. It more commonly occurs in conjunction with a cystocele, rectocele, enterocele, or some combination of these.1 Richter reported that 72% of patients with vaginal vault prolapse had a combination of other pelvic floor defects as well.2
If all vaginal support defects are repaired at the time of sacral colpopexy, recurrent vault prolapse is rare. Failures can be minimized by suturing the suspensory mesh to the posterior vagina and anterior vaginal apex over as extended an area as possible. Also test the sutures once they are placed within the periosteum of the sacral promontory to ensure they will not pull free.
Plan on occult incontinence
Total vaginal vault prolapse is commonly associated with some degree of urethral kinking, with subsequent outflow tract obstruction. As a result, most patients with complete vault prolapse do not complain of incontinence at the initial presentation. However, once the anatomic axis of the vagina is restored and the bladder is replaced within the pelvis with subsequent straightening of the urethra, occult incontinence often is uncovered. Although the patient may have a wonderful anatomic repair of severe vault prolapse at the completion of the surgical procedure, she will not be satisfied if she suddenly finds herself floridly incontinent.
Consider formal multichannel cystometrics prior to surgery in all women undergoing repair of total vault prolapse. If genuine stress urinary incontinence is present when the prolapse is reduced, an anti-incontinence procedure can be scheduled at the same time as the surgical repair. A Burch procedure can be performed for type IIA or IIB genuine stress incontinence, or a pubovaginal sling procedure can be performed for type III stress incontinence.
Posterior colporrhaphy/perineorrhaphy
These procedures are now performed to treat the remaining rectocele and perineal defect, when present.
Vaginal vs abdominal route
Somewhat surprisingly, the abdominal route appears to produce better long-term results. In a prospective, randomized controlled trial comparing both routes for the repair of total vault prolapse, Benson et al3 found that, after 5 years of follow-up, women managed vaginally had a 6-fold increased incidence of recurrent vault prolapse, a 3-fold increased incidence of recurrent cystocele, and twice the reoperation rate, compared with women whose initial repair was abdominal.
In the study, 48 women with total vault prolapse underwent vaginal bilateral sacrospinous fixation and paravaginal defect repair, and 40 underwent abdominal sacral colpopexy and paravaginal defect repair. Although the vaginal approach was associated with a shorter operative time and decreased hospital stay in the short term, it necessitated longer postoperative catheter use and was associated with more urinary tract infections and postoperative incontinence and a higher overall failure rate.
Sze and colleagues4 addressed a similar question in retrospective fashion, reviewing the medical records of 117 women surgically treated for total vault prolapse. Sixty-one women underwent vaginal sacrospinous ligament fixation and Raz urethropexy, while 56 underwent abdominal sacral colpopexy and Burch urethropexy. After a mean follow-up of 24 months, 33% of the women managed vaginally developed recurrent pelvic organ prolapse, compared with only 19% of the women managed abdominally. In addition, 26% of the women managed vaginally had recurrent urinary incontinence, compared with only 13% of the women managed abdominally
A separate randomized, prospective study by Maher et al5 compared abdominal sacral colpopexy (n=47) and vaginal sacrospinous ligament fixation (n=48) for stage II to IV vault prolapse. After a mean follow-up of 2 years, subjective and objective success rates did not differ significantly between the 2 routes.
Why is the abdominal route more durable?
Any number of reasons may apply:
- The traditional surgical procedure for vaginal management of total vault prolapse—sacrospinous ligament fixation—distorts the axis of the vagina.
- Native tissues are not as strong as synthetic materials. In postmenopausal women, a repair in which the thin, atrophic vaginal apex is secured to the sacrospinous ligament will not have the same durability as a repair involving mesh.
- In vaginal paravaginal repair, the extensive periurethral dissection required can damage fine branches of the pudendal nerve that innervate and control the urethral sphincter. Such extensive dissection is not required for paravaginal repair from the abdominal approach.
- In the vaginal approach, it can be difficult to gain adequate exposure high in the retroperitoneum to reattach the endopelvic fascia of the vaginal apex to the arcus at its origin just distal to the ischial spine.
The long view
The surgical options described in this article have varying degrees of risk and benefit. Multicenter, prospective surgical trials are needed to clarify these risks and benefits and provide physicians and their patients with reliable information. Ultimately, pursuit of a surgical “cure” will be supplanted by sustainable forms of disease prevention. Until then, decisions about prolapse surgery are best left to the judgment of the surgeon and the desires of his or her patient.
The authors report no financial relationships relevant to this article.
A range of clinical conditions can suggest an abdominal approach for vaginal vault prolapse procedures.
These include, but are not limited to:
- prior unsuccessful vaginal attempts
- obligate need for adnexal access
- markedly foreshortened vagina
- pelvic bony architectural limitations
- high risk for surgical failure (eg, athleticism, obesity, chronic obstructive pulmonary disease, congenital connective tissue disorder)
- desire for uterine preservation
In Part 1 (November 2005) of this 2-part article, we reviewed the most widely used and the newest vaginal techniques. Part 2 focuses on the abdominal approach, and compares vaginal and abdominal approaches.
High uterosacral ligament suspension
Surgical technique for this procedure for mild to moderate vaginal vault prolapse (stage I or II), using a vaginal approach, was described in Part 1, in the November issue of OBG Management. Abdominal repair involves the same concepts; like the vaginal approach, it is applicable only to the patient with mild to moderate vault prolapse. It will be less successful if it is performed to address complete vault prolapse.
Technique
Identify and tag the remnants of the uterosacral ligaments at the level of the ischial spines. Once the ureters are identified and isolated, address the enterocele by obliterating the cul-de-sac via Halban’s culdoplasty or abdominal McCall’s culdoplasty.
Open the peritoneum over the vaginal apex and trim it back to the level of the endopelvic fascia of the vaginal wall. After excising the redundant peritoneum of the vaginal apex, identify and reapproximate the pubocervical fascia of the anterior vaginal wall and the rectovaginal fascia of the posterior vaginal wall using interrupted or running nonabsorbable suture.
Then use nonabsorbable sutures to suspend each corner of the prolapsed vagina from its respective ipsilateral uterosacral ligament.
Abdominal sacral colpopexy
Abdominal sacral colpopexy was first popularized by Addison and Timmons in the 1980s, and is the abdominal standard of apical prolapse repair due to its long-term durability.
Abdominal sacral colpopexy can be performed with or without uterine extirpation. When a hysterectomy is performed concomitantly, some surgeons prefer a supracervical approach, provided there is no history of cervical dysplasia, because, theoretically, the cervical stump serves as a firm and substantial point of fixation for the synthetic mesh that will be used to perform the repair. This in turn may diminish the likelihood of postoperative mesh erosion.
Technique
Reflect the sigmoid colon as far as possible into the left lateral pelvis to expose the sacral promontory. If it has not already been done, free all adhesions between the colon and pelvic peritoneum to fully mobilize the colon and permit its maximal retraction out of the pelvic field prior to making the peritoneal incision.
Also make it a point to identify all structures at risk during this portion of the procedure—namely, the common iliac vessels, ureters, and middle sacral artery and vein. The left common iliac vein is medial to the left common iliac artery and is particularly susceptible to injury during this phase of the procedure.
Make a longitudinal incision in the peritoneum overlying the sacral promontory and extend it approximately 6 cm from the promontory dorsally into the cul-de-sac, opening the retrorectal space (FIGURE 1, TOP). Using a fine tonsil forceps and cautery, very gently dissect the retroareolar filmy tissue overlying the anterior longitudinal ligament away from S1 in thin layers until the white periosteum of the anterior longitudinal ligament overlying S1 is clearly exposed. It now becomes very easy to visualize the course of the middle sacral artery and vein. With these vessels under direct visualization, place 2 permanent #0 sutures through the periosteum of S1.
Do not attempt to place these sutures deeper in the presacral space than the S1 vertebral body, or life-threatening and uncontrollable bleeding may result.
If there is no uterus, insert a probe such as an end-to-end anastomotic sizer or handheld Harrington retractor into the vagina and extend it, elongating and elevating the vaginal cylinder. It now becomes much easier to identify the interface between the bladder and vagina prior to making the peritoneal incision.
If the interface remains indistinct, instill 150 cc of saline into the bladder to delineate its boundaries. Then elevate and incise the vesicouterine peritoneum overlying the junction between the bladder and vaginal apex; this provides access to the vesicocervical space. Dissect the bladder off the anterior vaginal wall in a caudal direction until the pubocervical fascia can be identified. Do not dissect away the peritoneum over the posterior vaginal wall, but leave it intact.
FIGURE 1Abdominal sacral colpopexy technique
Reconstructive materials
Although many different materials have been described, none have undergone rigorous comparisons. In our institution, we use soft polypropylene (Surgipro; US Surgical, Norwalk, Conn).
Fold a piece of 5-inch mesh over onto itself and suture the layers together to create a double-thickness configuration. Then “fishmouth” the caudal end of this mesh prosthesis, producing both an anterior and posterior leaf. With the obturator still within the vaginal cylinder stretching the vaginal apex, secure the posterior leaf of the mesh to the posterior vaginal wall with 3 to 5 nonabsorbable #0 sutures.
Suture placement. Thread each suture initially through the posterior leaf of the mesh, placed deeply through the fibromuscular thickness of the posterior vaginal wall, then bring it back out through the mesh at the same point. Place the sutures in a transverse line 1 to 2 cm apart and 3 to 4 cm distal to the vaginal apex.
Once all sutures have been placed, tie each of them, thereby securing the posterior leaf of the mesh to the posterior vaginal wall. Now perform a “mirror” procedure to secure the anterior leaf of the mesh prosthesis to the anterior vaginal wall. Then firmly attach the mesh prosthesis to the vaginal apex using several interrupted, permanent sutures.
At this point, the sutures previously placed through the periosteum of the sacral promontory are threaded through the apex of the mesh prosthesis at a point that will allow the vagina to rest comfortably within the pelvis, without undue tension or traction once the sutures are tied into place (FIGURE 1, MIDDLE).
Trim any excess mesh, and close the 6-cm longitudinal peritoneal incision previously created in the cul-de-sac. Close the incision over the top of the mesh, retroperitonealizing the mesh prosthesis (FIGURE 1, BOTTOM).
Paravaginal defect repair
The bladder base is intimately associated with the anterior vaginal wall via a triangular sheet of pubocervical fascia attached to and extending from the arcus tendineus fascia pelvis bilaterally. When the apex of the vagina prolapses through the introitus, as in total vault prolapse, the base of the bladder is torn free from these fascial attachments in the pelvis and herniates through the introitus along with the vaginal apex. By definition, a bilateral paravaginal defect will result. Thus, surgical repair of total vaginal vault prolapse almost invariably requires paravaginal defect repair as well.
The goal of paravaginal defect repair is to reattach, bilaterally, the anterolateral vaginal sulcus and its overlying endopelvic fascia to the pubococcygeus and obturator internus muscles and fascia at the level of the arcus tendineus fascia pelvis.
Technique
Enter and gently develop the retropubic space of Retzius, taking care not to disrupt the myriad venous anastomotic networks of the plexus of Santorini, located on and around the bladder. Bluntly mobilize the bladder bilaterally, exposing the lateral retropubic spaces, the pubococcygeus and obturator internus muscles, and the obturator neurovascular bundles. Within each retropubic space, palpate the ischial spine. Then visualize the arcus, seen as a white ligamentous band, as it courses from the ischial spine caudally toward the ipsilateral posterior pubic symphysis. A lateral paravaginal defect representing avulsion of the vagina off the arcus tendineus fascia pelvis, or of the arcus tendineus fascia pelvis off the obturator internus muscle, can now be visualized.
While gently reflecting the bladder medially with a wide ribbon, insert a few fingers of the nondominant hand into the vagina and elevate the ipsilateral anterolateral vaginal sulcus. Then place a suture through the fibromuscular thickness of the lateral vaginal apex, just above the uplifting fingers in the vagina, and then slightly cephalad into the arcus tendineus fascia pelvis or obturator internus fascia on the pelvic sidewall, at a point 1 to 2 cm distal to the ischial spine. Place 3 to 5 additional sutures in a similar fashion at 1-cm intervals. The most distal suture should be placed as close as possible to the pubic ramus into the pubourethral ligament. If necessary, repeat the procedure on the contralateral side. Then tie all sutures into place, thereby completing the repair.
When a patient has complete vault prolapse, she typically has defects in all 3 levels of pelvic support, and thus may need to undergo several different procedures to correct all anatomic defects and restore function.
In other words, vaginal vault prolapse rarely presents as an isolated defect. It more commonly occurs in conjunction with a cystocele, rectocele, enterocele, or some combination of these.1 Richter reported that 72% of patients with vaginal vault prolapse had a combination of other pelvic floor defects as well.2
If all vaginal support defects are repaired at the time of sacral colpopexy, recurrent vault prolapse is rare. Failures can be minimized by suturing the suspensory mesh to the posterior vagina and anterior vaginal apex over as extended an area as possible. Also test the sutures once they are placed within the periosteum of the sacral promontory to ensure they will not pull free.
Plan on occult incontinence
Total vaginal vault prolapse is commonly associated with some degree of urethral kinking, with subsequent outflow tract obstruction. As a result, most patients with complete vault prolapse do not complain of incontinence at the initial presentation. However, once the anatomic axis of the vagina is restored and the bladder is replaced within the pelvis with subsequent straightening of the urethra, occult incontinence often is uncovered. Although the patient may have a wonderful anatomic repair of severe vault prolapse at the completion of the surgical procedure, she will not be satisfied if she suddenly finds herself floridly incontinent.
Consider formal multichannel cystometrics prior to surgery in all women undergoing repair of total vault prolapse. If genuine stress urinary incontinence is present when the prolapse is reduced, an anti-incontinence procedure can be scheduled at the same time as the surgical repair. A Burch procedure can be performed for type IIA or IIB genuine stress incontinence, or a pubovaginal sling procedure can be performed for type III stress incontinence.
Posterior colporrhaphy/perineorrhaphy
These procedures are now performed to treat the remaining rectocele and perineal defect, when present.
Vaginal vs abdominal route
Somewhat surprisingly, the abdominal route appears to produce better long-term results. In a prospective, randomized controlled trial comparing both routes for the repair of total vault prolapse, Benson et al3 found that, after 5 years of follow-up, women managed vaginally had a 6-fold increased incidence of recurrent vault prolapse, a 3-fold increased incidence of recurrent cystocele, and twice the reoperation rate, compared with women whose initial repair was abdominal.
In the study, 48 women with total vault prolapse underwent vaginal bilateral sacrospinous fixation and paravaginal defect repair, and 40 underwent abdominal sacral colpopexy and paravaginal defect repair. Although the vaginal approach was associated with a shorter operative time and decreased hospital stay in the short term, it necessitated longer postoperative catheter use and was associated with more urinary tract infections and postoperative incontinence and a higher overall failure rate.
Sze and colleagues4 addressed a similar question in retrospective fashion, reviewing the medical records of 117 women surgically treated for total vault prolapse. Sixty-one women underwent vaginal sacrospinous ligament fixation and Raz urethropexy, while 56 underwent abdominal sacral colpopexy and Burch urethropexy. After a mean follow-up of 24 months, 33% of the women managed vaginally developed recurrent pelvic organ prolapse, compared with only 19% of the women managed abdominally. In addition, 26% of the women managed vaginally had recurrent urinary incontinence, compared with only 13% of the women managed abdominally
A separate randomized, prospective study by Maher et al5 compared abdominal sacral colpopexy (n=47) and vaginal sacrospinous ligament fixation (n=48) for stage II to IV vault prolapse. After a mean follow-up of 2 years, subjective and objective success rates did not differ significantly between the 2 routes.
Why is the abdominal route more durable?
Any number of reasons may apply:
- The traditional surgical procedure for vaginal management of total vault prolapse—sacrospinous ligament fixation—distorts the axis of the vagina.
- Native tissues are not as strong as synthetic materials. In postmenopausal women, a repair in which the thin, atrophic vaginal apex is secured to the sacrospinous ligament will not have the same durability as a repair involving mesh.
- In vaginal paravaginal repair, the extensive periurethral dissection required can damage fine branches of the pudendal nerve that innervate and control the urethral sphincter. Such extensive dissection is not required for paravaginal repair from the abdominal approach.
- In the vaginal approach, it can be difficult to gain adequate exposure high in the retroperitoneum to reattach the endopelvic fascia of the vaginal apex to the arcus at its origin just distal to the ischial spine.
The long view
The surgical options described in this article have varying degrees of risk and benefit. Multicenter, prospective surgical trials are needed to clarify these risks and benefits and provide physicians and their patients with reliable information. Ultimately, pursuit of a surgical “cure” will be supplanted by sustainable forms of disease prevention. Until then, decisions about prolapse surgery are best left to the judgment of the surgeon and the desires of his or her patient.
The authors report no financial relationships relevant to this article.
1. Herbst A, Mishell D, Stenchever M. Disorders of the abdominal wall and pelvic support. In: Comprehensive Gynecology. 2nd ed. Philadelphia: Mosby Year Book; 1992:594–612.
2. Richter K. Massive eversion of the vagina: pathogenesis, diagnosis and therapy of the true prolapse of the vaginal stump. Clin Obstet Gynecol. 1982;25:897-912.
3. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1422.
4. Sze EH, Kohli N, Miklos JR, Roat T, Karram MM. A retrospective comparison of abdominal sacrocolpopexy with Burch colposuspension versus sacrospinous fixation with transvaginal needle suspension for the management of vaginal vault prolapse and coexisting stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:390-393.
5. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal SCP or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
1. Herbst A, Mishell D, Stenchever M. Disorders of the abdominal wall and pelvic support. In: Comprehensive Gynecology. 2nd ed. Philadelphia: Mosby Year Book; 1992:594–612.
2. Richter K. Massive eversion of the vagina: pathogenesis, diagnosis and therapy of the true prolapse of the vaginal stump. Clin Obstet Gynecol. 1982;25:897-912.
3. Benson JT, Lucente V, McClellan E. Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long term outcome evaluation. Am J Obstet Gynecol. 1996;175:1418-1422.
4. Sze EH, Kohli N, Miklos JR, Roat T, Karram MM. A retrospective comparison of abdominal sacrocolpopexy with Burch colposuspension versus sacrospinous fixation with transvaginal needle suspension for the management of vaginal vault prolapse and coexisting stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 1999;10:390-393.
5. Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ. Abdominal SCP or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol. 2004;190:20-26.
Surgical management of vaginal vault prolapse
First, the good news: We have numerous techniques to choose from to repair prolapse of the vaginal vault, which affects as many as 50% of parous women.1 The bad news: Most of the data on these techniques are anecdotal or retrospective, not the result of randomized, controlled trials. Few investigators have compared the vaginal and abdominal approaches.
So how should we decide on a procedure? It is a judgment call, ultimately. After taking into account the patient’s age, functional status, comorbidities, desire for coitus, and surgical history, the surgeon must weigh the risks and benefits of the procedures that seem most appropriate. Part 1 of this 2-part article reviews what is known about the most widely used and newest vaginal techniques:
- sacrospinous ligament fixation,
- iliococcygeal fixation,
- modified McCall culdoplasty,
- high uterosacral ligament suspension with fascial reconstruction, and
- posterior intravaginal slingplasty (infracoccygeal sacropexy).
In Part 2, next month, we focus on the abdominal approach, and survey the data comparing vaginal and abdominal repairs.
Unfortunately, success and failure rates are still poorly defined because of a lack of standardization, and because techniques and materials continually change. This underscores the need for better understanding of the pathophysiology of genital prolapse, improved preoperative assessment, and more effective and durable repair techniques.
Why prolapse occurs
Pelvic support involves a complex interplay of anatomic, histologic, genetic, and electrophysiologic factors that, although incompletely understood, are frequently disrupted. For example, MacLennan et al2 reported that 46.2% of women aged 15 to 97 years experience pelvic floor dysfunction; a large retrospective study by Olsen and colleagues3 found that 11.1% of women undergo surgery for prolapse by the age of 80, and 29.2% of these women require repeat surgery.
Here’s what we know about the anatomy of pelvic support:
Ligaments serve as secondary supports
The uterus and upper third of the vagina are held in place over the levator plate by the fibers of the parametrium (cardinal and uterosacral ligaments) and paracolpium. These fibers arise from a broad area on the pelvic sidewall overlying the fascia of the piriformis muscle, the sacroiliac joint, and lateral sacrum. The fibers represent condensations of the endopelvic fascia of the pelvis, acting as suspensory ligaments that run in a predominantly vertical direction to insert into the lateral upper third of the vagina and lateral and posterolateral aspect of the cervical portion of the uterus.
In the normal, healthy pelvis, these suspensory ligaments represent secondary support mechanisms and are not routinely under tension.
Pelvic-floor muscles play leading role
Gosling4 argued that pelvic floor muscle tone is more crucial to normal positioning of the pelvic viscera than are the fascial and ligamentous supports of the pelvic organs. Specifically, the pubococcygeus, iliococcygeus, and puborectalis muscles collectively define the levator ani of the pelvic floor. Fusion of the right and left bellies of the levator ani, behind the rectum and anterior to the coccyx, creates a muscular platform known as the levator plate. This plate provides indirect support for the upper genital tract by acting as a platform against which the upper vagina and other pelvic viscera are compressed during increases in intra-abdominal pressure.
Contraction of the levator ani pulls the levator plate toward the posterior symphysis pubis, minimizing the size of the urogenital hiatus through which the rectum, vagina, and urethra exit the pelvis on their way to the perineum. Weakness in the muscular pelvic floor—whether caused by disuse, pudendal nerve damage, or muscular trauma—increases the size of the urogenital hiatus, and the pelvic organs begin to prolapse through it.
Ultimately, constant tension on the ligamentous supports of the pelvic organs exceeds their tensile strength, and pelvic organ prolapse results.
Goals of surgery
Successful surgery achieves effective and sustained vault support, obliterates any enterocele sac, and repairs the cystocele and rectocele that occur in approximately two thirds of women with vault prolapse.
The broader goals: anatomic and functional restoration of the lower female genital tract and improvement in quality of life.
Surgery can be reconstructive or obliterative. Reconstructive surgery can be performed vaginally, abdominally, or a combination of both.
Vaginal techniques
Proponents of the vaginal approach argue that, by avoiding the need for laparotomy, it results in fewer complications, less blood loss and postoperative discomfort, a shorter hospital stay, and less expense.5
Sacrospinous ligament fixation
The sacrospinous ligaments extend from the ischial spines on either side to the lower portion of the sacrum and coccyx. Fixation of the vaginal apex to 1 or both of the sacrospinous ligaments is an option for posthysterectomy vault prolapse.
Technique. Nichols6 described the need to penetrate the right rectal pillar into the pararectal space near the ischial spine. The next step is grasping the ligament and muscle with a long Babcock clamp. Place two #2 polyglycolic sutures through the sacrospinous ligament, 1.5 to 2 fingerbreadths medial to the ischial spine. Attach 1 end of the suture to the undersurface of the posterior vaginal wall at the apical area. When the posterior colporrhaphy reaches the midportion of the vagina, tie the sacrospinous suspension sutures, firmly attaching the vaginal apex to the surface of the coccygeal-sacrospinous ligament complex with no intervening suture bridge (FIGURE 1).
Modifications. The most notable modification is the Miyazaki technique, which substitutes the Miya hook for the DesChamps ligature carrier. The Miya hook is reportedly safer for the pudendal complex, which may lie up to 5.5 cm medial to the ischial spine.7 The path of the Miya hook avoids Alcock’s canal and its neurovascular pudendal bundle.
The Caprio ligature carrier (Boston Scientific, Boston, Mass) is also useful for the placement of sacrospinous sutures; unlike the Miya hook, however, the Caprio ligature carrier is a disposable instrument and thus is not reusable.
Potential complications of sacrospinous ligament fixation include hemorrhage, pudendal nerve injury, rectal or bladder injury, and recurrent anterior vaginal wall prolapse.
Outcomes. Follow-up studies in women undergoing this procedure report a roughly 20% incidence of recurrent or persistent anterior vaginal wall relaxation, or symptomatic cystocele, within 1 year after the surgery. Alteration of the vaginal axis in an exaggerated posterolateral direction after this procedure is thought to place undue tension on the anterior segment of the vaginal wall and predispose women to prolapse at a site opposite the repair.8,9
FIGURE 1 2 “pulley stitches” secure the apex
Place 2 nonabsorbable monofilament “pulley stitches” to secure the vaginal apex to the ligament.
Iliococcygeal fixation
Inmon10 was the first to describe a technique in which the everted vaginal apex is secured to the iliococcygeal fascia bilaterally, just below the ischial spine. He performed this technique successfully in 3 women with atrophied uterosacral ligaments.
Technique. Open the posterior vaginal wall in the midline, as if preparing to perform a posterior colporrhaphy. Develop the rectovaginal spaces bluntly and bilaterally—laterally toward the levator muscles and posteriorally toward the ischial spines. Use the nondominant hand to depress the rectum downward and medially, and place a single #0 polyglycolic suture deep into the iliococcygeus muscle and fascia at a point 1 to 2 cm caudad and posterior to the ischial spine. Then pass both ends of the suture through the ipsilateral posterior vaginal apex and hold them with a hemostat. Repeat the procedure contralaterally.
Usually no vaginal epithelium needs excision because the upper vagina is attached bilaterally, resulting in good vaginal length and circumference. When posterior colporrhaphy is completed and the posterior vaginal wall is closed, tie both iliococcygeal-fixation sutures in place.
Complications. Shull and colleagues11 studied 42 women who underwent suspension of the vaginal cuff to iliococcygeus fascia and repair of coexisting pelvic support defects. Of these women, 2 (5%) had recurrence of their cuff prolapse during follow-up, one of whom required further surgery (she also had recurrence of an inguinal hernia that had been repaired at the original surgery). The other patient, who had undergone 5 previous pelvic procedures, developed asymptomatic prolapse of the cuff halfway to the hymen. Six additional patients had loss of support at other sites in the follow-up period, one of whom required repeat surgery. Ninety-five percent of women experienced no persistence or recurrence of cuff prolapse 6 weeks to 5 years after the procedure.
Meeks and colleagues12 also applied the Inmon technique in 110 women with posthysterectomy vault prolapse or total uterine procidentia. In both studies, the most commonly reported complications included hemorrhage (1.2%), bladder/rectal perforation (1.2%), and recurrent vault prolapse (8%).
Benefits. In comparison with sacrospinous ligament fixation, iliococcygeus fixation is technically easier and places less tension on the anterior vaginal wall.
Modified McCall culdoplasty
Symmonds and colleagues13 described this approach to symptomatic vaginal vault prolapse.
Technique. Excise an elliptical wedge of mucosa from the anterior and posterior walls of the prolapsed vagina to narrow the vault and allow access to the lateral fascial supports of the vagina and rectum. The width and length of the excised wedges are determined by the desired dimensions of the reconstructed vagina.
After isolating and excising the enterocele sac, place up to 3 modified McCall stitches, each one slightly higher than its predecessor. Each suture should incorporate the full thickness of the posterior vaginal wall, the cul-de-sac peritoneum, the remains of the uterosacral-cardinal complex bilaterally, and the fascial tissue lateral and posterior to the upper vagina and rectum (FIGURE 2).
Once they are in place, tie the sutures in the opposite order in which they were placed. These stitches fix the prolapsed vaginal vault to the uppermost portion of the endopelvic fascia at the same time as they accomplish a high closure of the culdesac peritoneum.
The evidence. Sze and Karram5 found an 11.5% incidence of recurrent vault prolapse and an associated 22% incidence of new-onset dyspareunia.
FIGURE 2 Classic vs modified McCall culdoplasty
In the classic McCall culdoplasty shown here, only the distal-most suture incorporates the posterior vaginal wall. With the “modified” technique, however, all sutures incorporate the full thickness of the posterior vaginal wall, as well as the cul-de-sac peritoneum, the remnants of the uterosacral-cardinal complex bilaterally, and the fascial tissue lateral and posterior to the upper vagina and rectum.
High uterosacral ligament suspension with fascial reconstruction
This approach is based on the observations of Richardson,14 who suggested that the endopelvic fascial supports (uterosacral/cardinal complex) do not stretch and attenuate over time, as some have hypothesized, but break at definable points. By identifying these points, the surgeon can reattach the prolapsed vagina to the intact uterosacral complex cephalad to the break.
Technique. Grasp the vaginal apex with 2 Allis clamps and incise it with a scalpel. If an enterocele sac is present, dissect it off the vaginal epithelium to the neck of the hernia, open it, and then excise it. Place a delayed absorbable or permanent pursestring suture about the neck of the hernia to close the peritoneal defect. If an anterior colporrhaphy or sling is required, perform them at this time.
Place a moist laparotomy pad in the cul-de-sac, and insert and elevate a Deaver retractor to remove the intestines from the cul-de-sac and improve exposure. Next, palpate the ischial spines transperitoneally.
Once the spines are identified, the remnants of the uterosacral ligaments can be identified posterior and medial to the spines and can be palpated transperitoneally or transrectally. Remember that the ureters are also quite close to the ischial spines at this location, running along the lateral pelvic sidewall 2 to 5 cm ventral and lateral to the ischial spines.
After identifying the uterosacral ligaments, grasp their remnants with Allis clamps and place 2 to 3 delayed absorbable or permanent sutures through the rectovaginal fascia of the inner posterior vaginal wall epithelium at one lateral vaginal apex, through the ipsilateral plicated uterosacral ligament complex, and then through the pubocervical fascia of the anterior vaginal wall of the ipsilateral vaginal apex. Hold these sutures while performing the same procedure contralaterally (FIGURE 3). Close the apex and tie these sutures in place, suspending the corners of the vaginal apex from the uterosacral complex bilaterally, and restoring the continuity of the paracervical ring (FIGURES 4, 5).
Benefits of this technique include:
- creation of an anatomically appropriate and correctly positioned midline vaginal axis,
- preservation of adequate vaginal length,
- reduced risk of nerve injury, and
- restored continuity of the paracervical ring when the pelvic pararectal, uterosacral, and pubocervical fascia are reapproximated circumferentially.
Risks include the potential for ureteral kinking or obstruction. Thus, it is prudent to perform cystoscopy after this procedure to rule out occult injury.
FIGURE 3 Suspend apical corners bilaterally
The corners of the vaginal apex are suspended from the cardinal-uterosacral complex bilaterally, with all sutures placed posterior and medial to the ischial spines. Copyright 1998 by C.G. Bachofen.
FIGURE 4 Suture placement penetrates multiple layers
Sagittal view of correct suture placement. PS=pubic symphysis, B=bladder, PCF=pubocervical fascia, USL=uterosacral ligaments, AD=apical defect, RVF=rectovaginal fascia, R=rectum. Copyright 1998 by C.G. Bachofen.
FIGURE 5 Suspend the fascia from uterosacral ligaments
Sagittal view after tying of sutures. Note restoration of the normal anatomic axis. PS=pubic symphysis, B=bladder, PCF=pubocervical fascia, USL=uterosacral ligaments, RVF=rectovaginal fascia, R=rectum. Copyright 1998 by C.G. Bachofen.
Posterior intravaginal slingplasty
This investigative technique, also known as infracoccygeal sacropexy, is a minimally invasive, transperineal approach to vaginal vault prolapse. The anatomic and physiologic concepts are similar to those of the tension-free vaginal tape (Gynecare, Somerville, NJ) in the treatment of stress incontinence. However, because it is a new procedure, further evaluation is needed before it can be adopted into clinical practice.
This is an outpatient surgery.
Technique. Use a narrow tunneling device to pass a synthetic nonabsorbable tape through each pararectal space via a small perineal incision, and make a small vaginal incision to secure the tape to the vault.
The evidence. In the initial case series of 93 patients,15 1 rectal perforation and 1 rectal tape erosion were noted. The cure rate was 91% in short-term follow-up in a small number of patients.
The authors report no financial relationships relevant to this article.
1. Beck RP. Pelvic relaxational prolapse. In: Principles and Practice of Clinical Gynecology. New York: John Wiley and Sons; 1983;667-685.
2. MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity, and mode of delivery. Br J Obstet Gynecol. 2000;107:1460-1470.
3. Olsen AL, Smith VJ, Bergstrom JO. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
4. Gosling JA. The structure of the bladder neck, urethra and pelvic floor in relation to female urinary incontinence. Int Urogynecol J. 1996;7:177-178.
5. Sze EHM, Karram MM. Transvaginal repair of vault prolapse: a review. Obstet Gynecol. 1997;89:466-475.
6. Nichols D. Sacrospinous fixation for massive eversion of the vagina. Am J Obstet Gynecol. 1982;142:901-904.
7. Miyazaki FS. Miya hook ligature carrier for sacrospinous ligament suspension. Obstet Gynecol. 1987;70:286-288.
8. Morley G, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872-878.
9. Shull BL, Capen CV, Riggs MW. Preoperative analysis of site specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
10. Inmon WB. Pelvic relaxation and repair including prolapse of vagina following hysterectomy. South Med J. 1963;56:577-582.
11. Shull BT, Capen CV, Riggs MW. Bilateral attachment of the vaginal cuff to iliococcygeus fascia: an effective method of cuff suspension. Am J Obstet Gynecol. 193;168:1669-1673.
12. Meeks GR, Washburne JF, McGehrer RP. Repair of vaginal vault prolapse by suspension of the vagina to iliococcygeus (prespinous) fascia. Am J Obstet Gynecol. 1994;171:1444-1449.
13. Symmonds RE, Williams TJ, Lee RA, Webbs MJ. Posthysterectomy enterocoele and vaginal vault prolapse. Am J Obstet Gynecol. 1981;140:852-859.
14. Richardson AL. The anatomic defects in rectocoele and enterocoele. J Pelvic Surg. 1995;1:214-218.
15. Farnsworth BN. Posterior intravaginal slingplasty (infracoccygeal sacropexy) for severe posthysterectomy vaginal vault prolapse: a preliminary report on efficacy and safety. Int Urogynecol J. 2002;13:4-8.
First, the good news: We have numerous techniques to choose from to repair prolapse of the vaginal vault, which affects as many as 50% of parous women.1 The bad news: Most of the data on these techniques are anecdotal or retrospective, not the result of randomized, controlled trials. Few investigators have compared the vaginal and abdominal approaches.
So how should we decide on a procedure? It is a judgment call, ultimately. After taking into account the patient’s age, functional status, comorbidities, desire for coitus, and surgical history, the surgeon must weigh the risks and benefits of the procedures that seem most appropriate. Part 1 of this 2-part article reviews what is known about the most widely used and newest vaginal techniques:
- sacrospinous ligament fixation,
- iliococcygeal fixation,
- modified McCall culdoplasty,
- high uterosacral ligament suspension with fascial reconstruction, and
- posterior intravaginal slingplasty (infracoccygeal sacropexy).
In Part 2, next month, we focus on the abdominal approach, and survey the data comparing vaginal and abdominal repairs.
Unfortunately, success and failure rates are still poorly defined because of a lack of standardization, and because techniques and materials continually change. This underscores the need for better understanding of the pathophysiology of genital prolapse, improved preoperative assessment, and more effective and durable repair techniques.
Why prolapse occurs
Pelvic support involves a complex interplay of anatomic, histologic, genetic, and electrophysiologic factors that, although incompletely understood, are frequently disrupted. For example, MacLennan et al2 reported that 46.2% of women aged 15 to 97 years experience pelvic floor dysfunction; a large retrospective study by Olsen and colleagues3 found that 11.1% of women undergo surgery for prolapse by the age of 80, and 29.2% of these women require repeat surgery.
Here’s what we know about the anatomy of pelvic support:
Ligaments serve as secondary supports
The uterus and upper third of the vagina are held in place over the levator plate by the fibers of the parametrium (cardinal and uterosacral ligaments) and paracolpium. These fibers arise from a broad area on the pelvic sidewall overlying the fascia of the piriformis muscle, the sacroiliac joint, and lateral sacrum. The fibers represent condensations of the endopelvic fascia of the pelvis, acting as suspensory ligaments that run in a predominantly vertical direction to insert into the lateral upper third of the vagina and lateral and posterolateral aspect of the cervical portion of the uterus.
In the normal, healthy pelvis, these suspensory ligaments represent secondary support mechanisms and are not routinely under tension.
Pelvic-floor muscles play leading role
Gosling4 argued that pelvic floor muscle tone is more crucial to normal positioning of the pelvic viscera than are the fascial and ligamentous supports of the pelvic organs. Specifically, the pubococcygeus, iliococcygeus, and puborectalis muscles collectively define the levator ani of the pelvic floor. Fusion of the right and left bellies of the levator ani, behind the rectum and anterior to the coccyx, creates a muscular platform known as the levator plate. This plate provides indirect support for the upper genital tract by acting as a platform against which the upper vagina and other pelvic viscera are compressed during increases in intra-abdominal pressure.
Contraction of the levator ani pulls the levator plate toward the posterior symphysis pubis, minimizing the size of the urogenital hiatus through which the rectum, vagina, and urethra exit the pelvis on their way to the perineum. Weakness in the muscular pelvic floor—whether caused by disuse, pudendal nerve damage, or muscular trauma—increases the size of the urogenital hiatus, and the pelvic organs begin to prolapse through it.
Ultimately, constant tension on the ligamentous supports of the pelvic organs exceeds their tensile strength, and pelvic organ prolapse results.
Goals of surgery
Successful surgery achieves effective and sustained vault support, obliterates any enterocele sac, and repairs the cystocele and rectocele that occur in approximately two thirds of women with vault prolapse.
The broader goals: anatomic and functional restoration of the lower female genital tract and improvement in quality of life.
Surgery can be reconstructive or obliterative. Reconstructive surgery can be performed vaginally, abdominally, or a combination of both.
Vaginal techniques
Proponents of the vaginal approach argue that, by avoiding the need for laparotomy, it results in fewer complications, less blood loss and postoperative discomfort, a shorter hospital stay, and less expense.5
Sacrospinous ligament fixation
The sacrospinous ligaments extend from the ischial spines on either side to the lower portion of the sacrum and coccyx. Fixation of the vaginal apex to 1 or both of the sacrospinous ligaments is an option for posthysterectomy vault prolapse.
Technique. Nichols6 described the need to penetrate the right rectal pillar into the pararectal space near the ischial spine. The next step is grasping the ligament and muscle with a long Babcock clamp. Place two #2 polyglycolic sutures through the sacrospinous ligament, 1.5 to 2 fingerbreadths medial to the ischial spine. Attach 1 end of the suture to the undersurface of the posterior vaginal wall at the apical area. When the posterior colporrhaphy reaches the midportion of the vagina, tie the sacrospinous suspension sutures, firmly attaching the vaginal apex to the surface of the coccygeal-sacrospinous ligament complex with no intervening suture bridge (FIGURE 1).
Modifications. The most notable modification is the Miyazaki technique, which substitutes the Miya hook for the DesChamps ligature carrier. The Miya hook is reportedly safer for the pudendal complex, which may lie up to 5.5 cm medial to the ischial spine.7 The path of the Miya hook avoids Alcock’s canal and its neurovascular pudendal bundle.
The Caprio ligature carrier (Boston Scientific, Boston, Mass) is also useful for the placement of sacrospinous sutures; unlike the Miya hook, however, the Caprio ligature carrier is a disposable instrument and thus is not reusable.
Potential complications of sacrospinous ligament fixation include hemorrhage, pudendal nerve injury, rectal or bladder injury, and recurrent anterior vaginal wall prolapse.
Outcomes. Follow-up studies in women undergoing this procedure report a roughly 20% incidence of recurrent or persistent anterior vaginal wall relaxation, or symptomatic cystocele, within 1 year after the surgery. Alteration of the vaginal axis in an exaggerated posterolateral direction after this procedure is thought to place undue tension on the anterior segment of the vaginal wall and predispose women to prolapse at a site opposite the repair.8,9
FIGURE 1 2 “pulley stitches” secure the apex
Place 2 nonabsorbable monofilament “pulley stitches” to secure the vaginal apex to the ligament.
Iliococcygeal fixation
Inmon10 was the first to describe a technique in which the everted vaginal apex is secured to the iliococcygeal fascia bilaterally, just below the ischial spine. He performed this technique successfully in 3 women with atrophied uterosacral ligaments.
Technique. Open the posterior vaginal wall in the midline, as if preparing to perform a posterior colporrhaphy. Develop the rectovaginal spaces bluntly and bilaterally—laterally toward the levator muscles and posteriorally toward the ischial spines. Use the nondominant hand to depress the rectum downward and medially, and place a single #0 polyglycolic suture deep into the iliococcygeus muscle and fascia at a point 1 to 2 cm caudad and posterior to the ischial spine. Then pass both ends of the suture through the ipsilateral posterior vaginal apex and hold them with a hemostat. Repeat the procedure contralaterally.
Usually no vaginal epithelium needs excision because the upper vagina is attached bilaterally, resulting in good vaginal length and circumference. When posterior colporrhaphy is completed and the posterior vaginal wall is closed, tie both iliococcygeal-fixation sutures in place.
Complications. Shull and colleagues11 studied 42 women who underwent suspension of the vaginal cuff to iliococcygeus fascia and repair of coexisting pelvic support defects. Of these women, 2 (5%) had recurrence of their cuff prolapse during follow-up, one of whom required further surgery (she also had recurrence of an inguinal hernia that had been repaired at the original surgery). The other patient, who had undergone 5 previous pelvic procedures, developed asymptomatic prolapse of the cuff halfway to the hymen. Six additional patients had loss of support at other sites in the follow-up period, one of whom required repeat surgery. Ninety-five percent of women experienced no persistence or recurrence of cuff prolapse 6 weeks to 5 years after the procedure.
Meeks and colleagues12 also applied the Inmon technique in 110 women with posthysterectomy vault prolapse or total uterine procidentia. In both studies, the most commonly reported complications included hemorrhage (1.2%), bladder/rectal perforation (1.2%), and recurrent vault prolapse (8%).
Benefits. In comparison with sacrospinous ligament fixation, iliococcygeus fixation is technically easier and places less tension on the anterior vaginal wall.
Modified McCall culdoplasty
Symmonds and colleagues13 described this approach to symptomatic vaginal vault prolapse.
Technique. Excise an elliptical wedge of mucosa from the anterior and posterior walls of the prolapsed vagina to narrow the vault and allow access to the lateral fascial supports of the vagina and rectum. The width and length of the excised wedges are determined by the desired dimensions of the reconstructed vagina.
After isolating and excising the enterocele sac, place up to 3 modified McCall stitches, each one slightly higher than its predecessor. Each suture should incorporate the full thickness of the posterior vaginal wall, the cul-de-sac peritoneum, the remains of the uterosacral-cardinal complex bilaterally, and the fascial tissue lateral and posterior to the upper vagina and rectum (FIGURE 2).
Once they are in place, tie the sutures in the opposite order in which they were placed. These stitches fix the prolapsed vaginal vault to the uppermost portion of the endopelvic fascia at the same time as they accomplish a high closure of the culdesac peritoneum.
The evidence. Sze and Karram5 found an 11.5% incidence of recurrent vault prolapse and an associated 22% incidence of new-onset dyspareunia.
FIGURE 2 Classic vs modified McCall culdoplasty
In the classic McCall culdoplasty shown here, only the distal-most suture incorporates the posterior vaginal wall. With the “modified” technique, however, all sutures incorporate the full thickness of the posterior vaginal wall, as well as the cul-de-sac peritoneum, the remnants of the uterosacral-cardinal complex bilaterally, and the fascial tissue lateral and posterior to the upper vagina and rectum.
High uterosacral ligament suspension with fascial reconstruction
This approach is based on the observations of Richardson,14 who suggested that the endopelvic fascial supports (uterosacral/cardinal complex) do not stretch and attenuate over time, as some have hypothesized, but break at definable points. By identifying these points, the surgeon can reattach the prolapsed vagina to the intact uterosacral complex cephalad to the break.
Technique. Grasp the vaginal apex with 2 Allis clamps and incise it with a scalpel. If an enterocele sac is present, dissect it off the vaginal epithelium to the neck of the hernia, open it, and then excise it. Place a delayed absorbable or permanent pursestring suture about the neck of the hernia to close the peritoneal defect. If an anterior colporrhaphy or sling is required, perform them at this time.
Place a moist laparotomy pad in the cul-de-sac, and insert and elevate a Deaver retractor to remove the intestines from the cul-de-sac and improve exposure. Next, palpate the ischial spines transperitoneally.
Once the spines are identified, the remnants of the uterosacral ligaments can be identified posterior and medial to the spines and can be palpated transperitoneally or transrectally. Remember that the ureters are also quite close to the ischial spines at this location, running along the lateral pelvic sidewall 2 to 5 cm ventral and lateral to the ischial spines.
After identifying the uterosacral ligaments, grasp their remnants with Allis clamps and place 2 to 3 delayed absorbable or permanent sutures through the rectovaginal fascia of the inner posterior vaginal wall epithelium at one lateral vaginal apex, through the ipsilateral plicated uterosacral ligament complex, and then through the pubocervical fascia of the anterior vaginal wall of the ipsilateral vaginal apex. Hold these sutures while performing the same procedure contralaterally (FIGURE 3). Close the apex and tie these sutures in place, suspending the corners of the vaginal apex from the uterosacral complex bilaterally, and restoring the continuity of the paracervical ring (FIGURES 4, 5).
Benefits of this technique include:
- creation of an anatomically appropriate and correctly positioned midline vaginal axis,
- preservation of adequate vaginal length,
- reduced risk of nerve injury, and
- restored continuity of the paracervical ring when the pelvic pararectal, uterosacral, and pubocervical fascia are reapproximated circumferentially.
Risks include the potential for ureteral kinking or obstruction. Thus, it is prudent to perform cystoscopy after this procedure to rule out occult injury.
FIGURE 3 Suspend apical corners bilaterally
The corners of the vaginal apex are suspended from the cardinal-uterosacral complex bilaterally, with all sutures placed posterior and medial to the ischial spines. Copyright 1998 by C.G. Bachofen.
FIGURE 4 Suture placement penetrates multiple layers
Sagittal view of correct suture placement. PS=pubic symphysis, B=bladder, PCF=pubocervical fascia, USL=uterosacral ligaments, AD=apical defect, RVF=rectovaginal fascia, R=rectum. Copyright 1998 by C.G. Bachofen.
FIGURE 5 Suspend the fascia from uterosacral ligaments
Sagittal view after tying of sutures. Note restoration of the normal anatomic axis. PS=pubic symphysis, B=bladder, PCF=pubocervical fascia, USL=uterosacral ligaments, RVF=rectovaginal fascia, R=rectum. Copyright 1998 by C.G. Bachofen.
Posterior intravaginal slingplasty
This investigative technique, also known as infracoccygeal sacropexy, is a minimally invasive, transperineal approach to vaginal vault prolapse. The anatomic and physiologic concepts are similar to those of the tension-free vaginal tape (Gynecare, Somerville, NJ) in the treatment of stress incontinence. However, because it is a new procedure, further evaluation is needed before it can be adopted into clinical practice.
This is an outpatient surgery.
Technique. Use a narrow tunneling device to pass a synthetic nonabsorbable tape through each pararectal space via a small perineal incision, and make a small vaginal incision to secure the tape to the vault.
The evidence. In the initial case series of 93 patients,15 1 rectal perforation and 1 rectal tape erosion were noted. The cure rate was 91% in short-term follow-up in a small number of patients.
The authors report no financial relationships relevant to this article.
First, the good news: We have numerous techniques to choose from to repair prolapse of the vaginal vault, which affects as many as 50% of parous women.1 The bad news: Most of the data on these techniques are anecdotal or retrospective, not the result of randomized, controlled trials. Few investigators have compared the vaginal and abdominal approaches.
So how should we decide on a procedure? It is a judgment call, ultimately. After taking into account the patient’s age, functional status, comorbidities, desire for coitus, and surgical history, the surgeon must weigh the risks and benefits of the procedures that seem most appropriate. Part 1 of this 2-part article reviews what is known about the most widely used and newest vaginal techniques:
- sacrospinous ligament fixation,
- iliococcygeal fixation,
- modified McCall culdoplasty,
- high uterosacral ligament suspension with fascial reconstruction, and
- posterior intravaginal slingplasty (infracoccygeal sacropexy).
In Part 2, next month, we focus on the abdominal approach, and survey the data comparing vaginal and abdominal repairs.
Unfortunately, success and failure rates are still poorly defined because of a lack of standardization, and because techniques and materials continually change. This underscores the need for better understanding of the pathophysiology of genital prolapse, improved preoperative assessment, and more effective and durable repair techniques.
Why prolapse occurs
Pelvic support involves a complex interplay of anatomic, histologic, genetic, and electrophysiologic factors that, although incompletely understood, are frequently disrupted. For example, MacLennan et al2 reported that 46.2% of women aged 15 to 97 years experience pelvic floor dysfunction; a large retrospective study by Olsen and colleagues3 found that 11.1% of women undergo surgery for prolapse by the age of 80, and 29.2% of these women require repeat surgery.
Here’s what we know about the anatomy of pelvic support:
Ligaments serve as secondary supports
The uterus and upper third of the vagina are held in place over the levator plate by the fibers of the parametrium (cardinal and uterosacral ligaments) and paracolpium. These fibers arise from a broad area on the pelvic sidewall overlying the fascia of the piriformis muscle, the sacroiliac joint, and lateral sacrum. The fibers represent condensations of the endopelvic fascia of the pelvis, acting as suspensory ligaments that run in a predominantly vertical direction to insert into the lateral upper third of the vagina and lateral and posterolateral aspect of the cervical portion of the uterus.
In the normal, healthy pelvis, these suspensory ligaments represent secondary support mechanisms and are not routinely under tension.
Pelvic-floor muscles play leading role
Gosling4 argued that pelvic floor muscle tone is more crucial to normal positioning of the pelvic viscera than are the fascial and ligamentous supports of the pelvic organs. Specifically, the pubococcygeus, iliococcygeus, and puborectalis muscles collectively define the levator ani of the pelvic floor. Fusion of the right and left bellies of the levator ani, behind the rectum and anterior to the coccyx, creates a muscular platform known as the levator plate. This plate provides indirect support for the upper genital tract by acting as a platform against which the upper vagina and other pelvic viscera are compressed during increases in intra-abdominal pressure.
Contraction of the levator ani pulls the levator plate toward the posterior symphysis pubis, minimizing the size of the urogenital hiatus through which the rectum, vagina, and urethra exit the pelvis on their way to the perineum. Weakness in the muscular pelvic floor—whether caused by disuse, pudendal nerve damage, or muscular trauma—increases the size of the urogenital hiatus, and the pelvic organs begin to prolapse through it.
Ultimately, constant tension on the ligamentous supports of the pelvic organs exceeds their tensile strength, and pelvic organ prolapse results.
Goals of surgery
Successful surgery achieves effective and sustained vault support, obliterates any enterocele sac, and repairs the cystocele and rectocele that occur in approximately two thirds of women with vault prolapse.
The broader goals: anatomic and functional restoration of the lower female genital tract and improvement in quality of life.
Surgery can be reconstructive or obliterative. Reconstructive surgery can be performed vaginally, abdominally, or a combination of both.
Vaginal techniques
Proponents of the vaginal approach argue that, by avoiding the need for laparotomy, it results in fewer complications, less blood loss and postoperative discomfort, a shorter hospital stay, and less expense.5
Sacrospinous ligament fixation
The sacrospinous ligaments extend from the ischial spines on either side to the lower portion of the sacrum and coccyx. Fixation of the vaginal apex to 1 or both of the sacrospinous ligaments is an option for posthysterectomy vault prolapse.
Technique. Nichols6 described the need to penetrate the right rectal pillar into the pararectal space near the ischial spine. The next step is grasping the ligament and muscle with a long Babcock clamp. Place two #2 polyglycolic sutures through the sacrospinous ligament, 1.5 to 2 fingerbreadths medial to the ischial spine. Attach 1 end of the suture to the undersurface of the posterior vaginal wall at the apical area. When the posterior colporrhaphy reaches the midportion of the vagina, tie the sacrospinous suspension sutures, firmly attaching the vaginal apex to the surface of the coccygeal-sacrospinous ligament complex with no intervening suture bridge (FIGURE 1).
Modifications. The most notable modification is the Miyazaki technique, which substitutes the Miya hook for the DesChamps ligature carrier. The Miya hook is reportedly safer for the pudendal complex, which may lie up to 5.5 cm medial to the ischial spine.7 The path of the Miya hook avoids Alcock’s canal and its neurovascular pudendal bundle.
The Caprio ligature carrier (Boston Scientific, Boston, Mass) is also useful for the placement of sacrospinous sutures; unlike the Miya hook, however, the Caprio ligature carrier is a disposable instrument and thus is not reusable.
Potential complications of sacrospinous ligament fixation include hemorrhage, pudendal nerve injury, rectal or bladder injury, and recurrent anterior vaginal wall prolapse.
Outcomes. Follow-up studies in women undergoing this procedure report a roughly 20% incidence of recurrent or persistent anterior vaginal wall relaxation, or symptomatic cystocele, within 1 year after the surgery. Alteration of the vaginal axis in an exaggerated posterolateral direction after this procedure is thought to place undue tension on the anterior segment of the vaginal wall and predispose women to prolapse at a site opposite the repair.8,9
FIGURE 1 2 “pulley stitches” secure the apex
Place 2 nonabsorbable monofilament “pulley stitches” to secure the vaginal apex to the ligament.
Iliococcygeal fixation
Inmon10 was the first to describe a technique in which the everted vaginal apex is secured to the iliococcygeal fascia bilaterally, just below the ischial spine. He performed this technique successfully in 3 women with atrophied uterosacral ligaments.
Technique. Open the posterior vaginal wall in the midline, as if preparing to perform a posterior colporrhaphy. Develop the rectovaginal spaces bluntly and bilaterally—laterally toward the levator muscles and posteriorally toward the ischial spines. Use the nondominant hand to depress the rectum downward and medially, and place a single #0 polyglycolic suture deep into the iliococcygeus muscle and fascia at a point 1 to 2 cm caudad and posterior to the ischial spine. Then pass both ends of the suture through the ipsilateral posterior vaginal apex and hold them with a hemostat. Repeat the procedure contralaterally.
Usually no vaginal epithelium needs excision because the upper vagina is attached bilaterally, resulting in good vaginal length and circumference. When posterior colporrhaphy is completed and the posterior vaginal wall is closed, tie both iliococcygeal-fixation sutures in place.
Complications. Shull and colleagues11 studied 42 women who underwent suspension of the vaginal cuff to iliococcygeus fascia and repair of coexisting pelvic support defects. Of these women, 2 (5%) had recurrence of their cuff prolapse during follow-up, one of whom required further surgery (she also had recurrence of an inguinal hernia that had been repaired at the original surgery). The other patient, who had undergone 5 previous pelvic procedures, developed asymptomatic prolapse of the cuff halfway to the hymen. Six additional patients had loss of support at other sites in the follow-up period, one of whom required repeat surgery. Ninety-five percent of women experienced no persistence or recurrence of cuff prolapse 6 weeks to 5 years after the procedure.
Meeks and colleagues12 also applied the Inmon technique in 110 women with posthysterectomy vault prolapse or total uterine procidentia. In both studies, the most commonly reported complications included hemorrhage (1.2%), bladder/rectal perforation (1.2%), and recurrent vault prolapse (8%).
Benefits. In comparison with sacrospinous ligament fixation, iliococcygeus fixation is technically easier and places less tension on the anterior vaginal wall.
Modified McCall culdoplasty
Symmonds and colleagues13 described this approach to symptomatic vaginal vault prolapse.
Technique. Excise an elliptical wedge of mucosa from the anterior and posterior walls of the prolapsed vagina to narrow the vault and allow access to the lateral fascial supports of the vagina and rectum. The width and length of the excised wedges are determined by the desired dimensions of the reconstructed vagina.
After isolating and excising the enterocele sac, place up to 3 modified McCall stitches, each one slightly higher than its predecessor. Each suture should incorporate the full thickness of the posterior vaginal wall, the cul-de-sac peritoneum, the remains of the uterosacral-cardinal complex bilaterally, and the fascial tissue lateral and posterior to the upper vagina and rectum (FIGURE 2).
Once they are in place, tie the sutures in the opposite order in which they were placed. These stitches fix the prolapsed vaginal vault to the uppermost portion of the endopelvic fascia at the same time as they accomplish a high closure of the culdesac peritoneum.
The evidence. Sze and Karram5 found an 11.5% incidence of recurrent vault prolapse and an associated 22% incidence of new-onset dyspareunia.
FIGURE 2 Classic vs modified McCall culdoplasty
In the classic McCall culdoplasty shown here, only the distal-most suture incorporates the posterior vaginal wall. With the “modified” technique, however, all sutures incorporate the full thickness of the posterior vaginal wall, as well as the cul-de-sac peritoneum, the remnants of the uterosacral-cardinal complex bilaterally, and the fascial tissue lateral and posterior to the upper vagina and rectum.
High uterosacral ligament suspension with fascial reconstruction
This approach is based on the observations of Richardson,14 who suggested that the endopelvic fascial supports (uterosacral/cardinal complex) do not stretch and attenuate over time, as some have hypothesized, but break at definable points. By identifying these points, the surgeon can reattach the prolapsed vagina to the intact uterosacral complex cephalad to the break.
Technique. Grasp the vaginal apex with 2 Allis clamps and incise it with a scalpel. If an enterocele sac is present, dissect it off the vaginal epithelium to the neck of the hernia, open it, and then excise it. Place a delayed absorbable or permanent pursestring suture about the neck of the hernia to close the peritoneal defect. If an anterior colporrhaphy or sling is required, perform them at this time.
Place a moist laparotomy pad in the cul-de-sac, and insert and elevate a Deaver retractor to remove the intestines from the cul-de-sac and improve exposure. Next, palpate the ischial spines transperitoneally.
Once the spines are identified, the remnants of the uterosacral ligaments can be identified posterior and medial to the spines and can be palpated transperitoneally or transrectally. Remember that the ureters are also quite close to the ischial spines at this location, running along the lateral pelvic sidewall 2 to 5 cm ventral and lateral to the ischial spines.
After identifying the uterosacral ligaments, grasp their remnants with Allis clamps and place 2 to 3 delayed absorbable or permanent sutures through the rectovaginal fascia of the inner posterior vaginal wall epithelium at one lateral vaginal apex, through the ipsilateral plicated uterosacral ligament complex, and then through the pubocervical fascia of the anterior vaginal wall of the ipsilateral vaginal apex. Hold these sutures while performing the same procedure contralaterally (FIGURE 3). Close the apex and tie these sutures in place, suspending the corners of the vaginal apex from the uterosacral complex bilaterally, and restoring the continuity of the paracervical ring (FIGURES 4, 5).
Benefits of this technique include:
- creation of an anatomically appropriate and correctly positioned midline vaginal axis,
- preservation of adequate vaginal length,
- reduced risk of nerve injury, and
- restored continuity of the paracervical ring when the pelvic pararectal, uterosacral, and pubocervical fascia are reapproximated circumferentially.
Risks include the potential for ureteral kinking or obstruction. Thus, it is prudent to perform cystoscopy after this procedure to rule out occult injury.
FIGURE 3 Suspend apical corners bilaterally
The corners of the vaginal apex are suspended from the cardinal-uterosacral complex bilaterally, with all sutures placed posterior and medial to the ischial spines. Copyright 1998 by C.G. Bachofen.
FIGURE 4 Suture placement penetrates multiple layers
Sagittal view of correct suture placement. PS=pubic symphysis, B=bladder, PCF=pubocervical fascia, USL=uterosacral ligaments, AD=apical defect, RVF=rectovaginal fascia, R=rectum. Copyright 1998 by C.G. Bachofen.
FIGURE 5 Suspend the fascia from uterosacral ligaments
Sagittal view after tying of sutures. Note restoration of the normal anatomic axis. PS=pubic symphysis, B=bladder, PCF=pubocervical fascia, USL=uterosacral ligaments, RVF=rectovaginal fascia, R=rectum. Copyright 1998 by C.G. Bachofen.
Posterior intravaginal slingplasty
This investigative technique, also known as infracoccygeal sacropexy, is a minimally invasive, transperineal approach to vaginal vault prolapse. The anatomic and physiologic concepts are similar to those of the tension-free vaginal tape (Gynecare, Somerville, NJ) in the treatment of stress incontinence. However, because it is a new procedure, further evaluation is needed before it can be adopted into clinical practice.
This is an outpatient surgery.
Technique. Use a narrow tunneling device to pass a synthetic nonabsorbable tape through each pararectal space via a small perineal incision, and make a small vaginal incision to secure the tape to the vault.
The evidence. In the initial case series of 93 patients,15 1 rectal perforation and 1 rectal tape erosion were noted. The cure rate was 91% in short-term follow-up in a small number of patients.
The authors report no financial relationships relevant to this article.
1. Beck RP. Pelvic relaxational prolapse. In: Principles and Practice of Clinical Gynecology. New York: John Wiley and Sons; 1983;667-685.
2. MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity, and mode of delivery. Br J Obstet Gynecol. 2000;107:1460-1470.
3. Olsen AL, Smith VJ, Bergstrom JO. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
4. Gosling JA. The structure of the bladder neck, urethra and pelvic floor in relation to female urinary incontinence. Int Urogynecol J. 1996;7:177-178.
5. Sze EHM, Karram MM. Transvaginal repair of vault prolapse: a review. Obstet Gynecol. 1997;89:466-475.
6. Nichols D. Sacrospinous fixation for massive eversion of the vagina. Am J Obstet Gynecol. 1982;142:901-904.
7. Miyazaki FS. Miya hook ligature carrier for sacrospinous ligament suspension. Obstet Gynecol. 1987;70:286-288.
8. Morley G, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872-878.
9. Shull BL, Capen CV, Riggs MW. Preoperative analysis of site specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
10. Inmon WB. Pelvic relaxation and repair including prolapse of vagina following hysterectomy. South Med J. 1963;56:577-582.
11. Shull BT, Capen CV, Riggs MW. Bilateral attachment of the vaginal cuff to iliococcygeus fascia: an effective method of cuff suspension. Am J Obstet Gynecol. 193;168:1669-1673.
12. Meeks GR, Washburne JF, McGehrer RP. Repair of vaginal vault prolapse by suspension of the vagina to iliococcygeus (prespinous) fascia. Am J Obstet Gynecol. 1994;171:1444-1449.
13. Symmonds RE, Williams TJ, Lee RA, Webbs MJ. Posthysterectomy enterocoele and vaginal vault prolapse. Am J Obstet Gynecol. 1981;140:852-859.
14. Richardson AL. The anatomic defects in rectocoele and enterocoele. J Pelvic Surg. 1995;1:214-218.
15. Farnsworth BN. Posterior intravaginal slingplasty (infracoccygeal sacropexy) for severe posthysterectomy vaginal vault prolapse: a preliminary report on efficacy and safety. Int Urogynecol J. 2002;13:4-8.
1. Beck RP. Pelvic relaxational prolapse. In: Principles and Practice of Clinical Gynecology. New York: John Wiley and Sons; 1983;667-685.
2. MacLennan AH, Taylor AW, Wilson DH, Wilson D. The prevalence of pelvic floor disorders and their relationship to gender, age, parity, and mode of delivery. Br J Obstet Gynecol. 2000;107:1460-1470.
3. Olsen AL, Smith VJ, Bergstrom JO. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
4. Gosling JA. The structure of the bladder neck, urethra and pelvic floor in relation to female urinary incontinence. Int Urogynecol J. 1996;7:177-178.
5. Sze EHM, Karram MM. Transvaginal repair of vault prolapse: a review. Obstet Gynecol. 1997;89:466-475.
6. Nichols D. Sacrospinous fixation for massive eversion of the vagina. Am J Obstet Gynecol. 1982;142:901-904.
7. Miyazaki FS. Miya hook ligature carrier for sacrospinous ligament suspension. Obstet Gynecol. 1987;70:286-288.
8. Morley G, DeLancey JO. Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol. 1988;158:872-878.
9. Shull BL, Capen CV, Riggs MW. Preoperative analysis of site specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol. 1992;166:1764-1771.
10. Inmon WB. Pelvic relaxation and repair including prolapse of vagina following hysterectomy. South Med J. 1963;56:577-582.
11. Shull BT, Capen CV, Riggs MW. Bilateral attachment of the vaginal cuff to iliococcygeus fascia: an effective method of cuff suspension. Am J Obstet Gynecol. 193;168:1669-1673.
12. Meeks GR, Washburne JF, McGehrer RP. Repair of vaginal vault prolapse by suspension of the vagina to iliococcygeus (prespinous) fascia. Am J Obstet Gynecol. 1994;171:1444-1449.
13. Symmonds RE, Williams TJ, Lee RA, Webbs MJ. Posthysterectomy enterocoele and vaginal vault prolapse. Am J Obstet Gynecol. 1981;140:852-859.
14. Richardson AL. The anatomic defects in rectocoele and enterocoele. J Pelvic Surg. 1995;1:214-218.
15. Farnsworth BN. Posterior intravaginal slingplasty (infracoccygeal sacropexy) for severe posthysterectomy vaginal vault prolapse: a preliminary report on efficacy and safety. Int Urogynecol J. 2002;13:4-8.