User login
The Retroperitoneal Space: Keeping vital structures out of harm’s way
The accomplished gynecologic surgeon must know the anatomy of the retroperitoneal space in order to avoid damage to normal structures, as well as remove pathology. Many disease processes involve the pelvic peritoneum, uterosacral ligaments, rectosigmoid or ovarian pedicles, and require the surgeon to enter the retroperitoneal space to identify the ureters and blood vessels and keep them out of harm’s way. The challenges are complex:
- Badly distorted anatomy and the anterior and posterior cul-de-sac necessitate mobilization of the rectosigmoid and bladder.
- Intraligamentous fibroids require knowledge of the blood supply in the retroperitoneal space. Malignant disorders mandate that the lymph nodes be dissected to determine extent of disease and as part of treatment.
Is training adequate?
Every training program should teach the surgical anatomy of the retroperitoneal space, since every surgeon needs to be comfortable exposing the anatomy, both to prevent injury and to accomplish the needed surgery. Videotapes and cadaver courses can prepare the resident for the operating room.
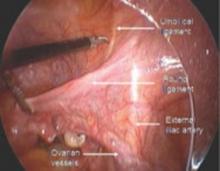
The “landmark” umbilical ligament
The umbilical ligament was the umbilical artery in fetal life and courses along the edge of the bladder to the anterior abdominal wall up to the umbilicus. It is a useful guide into the perivesicle space. Lateral to it are the iliac vessels, and medial is the bladder. It is also a good marker for finding the right spot to open the round ligament.
FIGURE 1 Opening the round ligament over the umbilical ligament
The round ligament is the key to exposing the retroperitoneal space. It should be open at the pelvic sidewall, just medial to the external iliac vessels. The umbilical ligament can be used as a landmark.
FIGURE 2 Opening lateral to the ovarian vessels
The divided round ligament is retracted ventrally and medially to place the ovarian vessels under traction. The peritoneum lateral to the ovarian vessels is divided up to the pelvic brim.
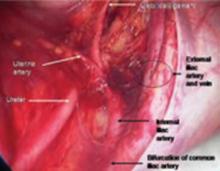
FIGURE 3 Right pelvic sidewall anatomy
Medial traction of the peritoneum around the ovarian vessels at the pelvic brim will expose the ureter coming over the iliac vessels at their bifurcation into external and internal branches.
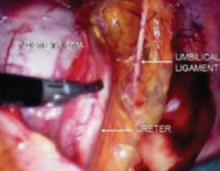
FIGURE 4 Relationship of ureter to the umbilical ligament
The ureter comes off medial with the fold of peritoneum. Once the space is developed, the operator’s finger or the laparoscopic probe can be introduced along the medial side of the internal iliac artery and ventral to the curve of the sacrum. This will open the pararectal space. The ureter and the anterior branch of the internal iliac artery are nearly parallel as they course through the pelvis.
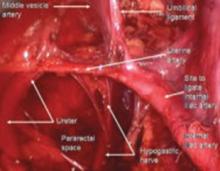
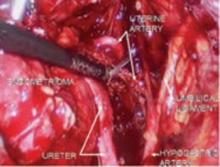
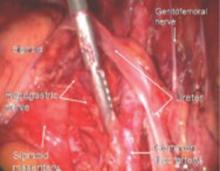
FIGURE 5 Hypogastric nerve
The anterior branch of the internal iliac artery gives off the uterine artery and the middle and superior vesicle arteries before continuing on as the umbilical ligament.
Endometriosis may imperil the ureter
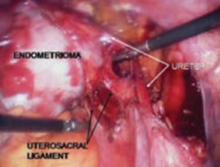
Endometriomas and peritoneal implants are among the most common reasons for accessing the retroperitoneal space. Frequently the peritoneum between the ovarian vessels and the uterosacral ligaments (ovarian fossa) is thickened and retracted within the endometriosis implants. This alters the pelvic anatomy and puts the ureter at risk for injury.
Definitive surgical treatment for endometriosis includes removal of this diseased peritoneum. The ureter is best identified using the technique described, and then dissected off the peritoneum down to the uterine artery.
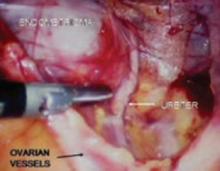
FIGURE 6 Finding the ureter lateral to an endometrioma
FIGURE 7 Relationship of ureter to the umbilical ligament
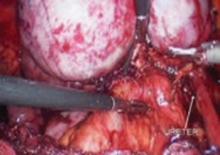
FIGURE 8 Dissecting the ureter out of the uterosacral ligament
The ureter may be placed on a Penrose drain to better isolate it and keep it under direct visualization. The cul-de-sac of Douglas is another site of endometrial implants. The fundus of the uterus is often adhesed to the rectosigmoid reflection and even the sigmoid colon. Nodules of endometriosis may infiltrate the uterosacral ligaments and extend into the rectovaginal space. To manage these implants, isolate the ureter to the point where it passes under the uterine artery.
FIGURE 9 Divide uterine artery lateral to endometrioma
Here, the uterine artery is taken lateral to the mass, enabling the surgeon to remove all of the uterosacral implants. The bladder flap will be developed and the bladder advanced caudad. By dissecting medial to the ureter as it courses under the uterine artery, it will be retracted laterally and provide the space necessary to resect the uterosacral implants.
FIGURE 10 Rectosigmoid reflection with endometrioma nodule
FIGURE 11 Postoperative appearance of endometriosis resection
As dissection proceeds medially, use the perirectal fatty plane to remove implants between the uterosacral area and rectum. In the midline, remove implants below the rectosigmoid peritoneal reflexion, taking care not to injure the rectum.
Implants above the peritoneal reflexion are often attached to the sigmoid tinea coli and cannot be removed without taking a portion of the bowel. Thus, patients with extensive endometriosis should have a bowel prep with two 10-ounce bottles of magnesium citrate the day before surgery.
If bowel resection is necessary, consult a general surgeon or gynecologic oncologist. A history of painful defecation or a finding of nodules in the cul-de-sac with rectal dimpling warrants preoperative consultation with a specialist skilled at bowel resection.
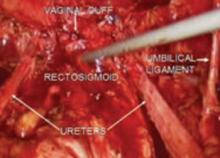
Ureteral injuries occur in 0.5% to 2.5% of women undergoing gynecologic surgery.1 The most common predisposing condition is previous pelvic surgery.2,3 The usual sites of injury are at the pelvic rim close to the ovarian vessels, at the level of the uterine artery, and lateral to the vaginal cuff. Neither preoperative excretory urograms nor placement of ureteral catheters preoperatively have been found to be effective prevention measures.2,4-6 Using the steps described above, the ureter can be identified and mobilized.
Blood supply to the ureter comes from the plexus of vessels that form a network along the length of the ureter. This plexus is fed by arteries from the renal pelvis, common iliac, internal iliac, uterine artery, and the base of the bladder. Complete mobilization of the ureter away from its peritoneal attachments and these lateral blood supply sources can be accomplished as long as the vascular plexus is not disrupted by cautery, crushing, or tearing. A clean transection can be re-anastomosed or reimplanted with the expectation of normal healing. Innervation of the ureter is from the inferior mesenteric plexus superiorly and the inferior hypogastric plexus in the pelvis. Ureteral peristalsis will continue, even if the ureter is completely divided or ligated.
Protecting pelvic blood vessels
The majority of gynecologic operations involve the ovarian vessels and the uterine vessels. The operator rarely needs to explore the lateral pelvis to identify the rest of the vessels. When faced with a large endometrioma or cancer, knowledge of the anatomy of the lateral blood vessels is vital.
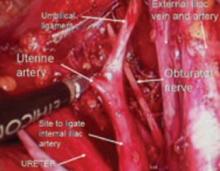
Since the pathology is usually deeper in the pelvis, it is wise to identify the anatomy of the pelvis starting at the pelvic brim, where the common iliac bifurcates into the external and internal iliacs. There is a safe dissection plane medial to the internal iliac artery all the way to the uterine artery. This exposes the pararectal space, which can be opened without risk of major bleeding (FIGURE 5).
The obliterated umbilical vessel is the other friendly marker just distal to the uterine artery. It can be placed on traction and the uterine artery isolated. The inferior and superior vesical arteries are generally not dissected, as they are adjacent to the bladder and the surgery takes place medial to them in the prevesical fascial space.
Hypogastric artery ligations are rarely performed today, as interventional radiology is the standard of care for patients with postoperative and postpartum bleeding. When it becomes necessary, the hypogastric artery can be isolated and tied using the right-angle clamp to pass the tie. The superior gluteal artery branches so close to the bifurcation of the common iliac artery that it is not visualized. The inferior gluteal artery is the largest distal branch, which might be visualized during the ligation. It is not necessary to identify it.
FIGURE 12 Right genitofemoral nerve
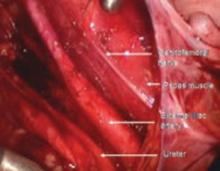
The genitofemoral nerve runs along the medial aspect of the body of the psoas muscle. It is sometimes injured by the self-retaining retractors placed at the time of laparotomy. This leads to some numbness and burning of the skin of the anterior thigh.
FIGURE 13 Right pelvic sidewall anatomy
The obturator nerve is in the obturator space and typically far lateral to the usual dissection. Metastatic cancer to the obturator lymph nodes may entrap it, or it may be injured during a node dissection, causing loss of internal rotation of the anterior thigh.
The sciatic nerve is seen only during exenterative surgery. Pressure on the lateral pelvis by advanced pelvic tumors can lead to sciatic pain and motor weakness—even loss of motor function to the lower leg, which commonly leads to foot drop.
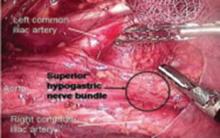
FIGURE 14 Superior hypogastric nerve bundle
The hypogastric plexus of nerves is sometimes damaged during surgery for endometriosis or for malignancy. The superior hypergastric plexus can be identified between the 2 common iliac arteries at the sacral promontory. The left common iliac vein runs underneath it.
FIGURE 15 Hypogastric nerve descending into the right pelvis
The right and left hypogastric nerves leave the hypogastric plexus and descend into the pelvis parallel to the ureter and 2 cm medial. It passes dorsal to the ureter as it goes through the cardinal ligament (FIGURE 5).
This plexus then supplies autonomic innervation of the bladder, rectum, uterus, and ureter. Complete disruption of the hypogastric nerve will lead to a hypertonic, noncontractile bladder and the necessity for self-catheterization to eliminate urine. Preservation of this nerve during radical hysterectomy or endometriosis resection is a high priority.
Laparoscopic uterosacral nerve ablation procedures divide the uterosacral ligament medial and caudad to the ureter and do not disrupt the main hypogastric nerve. Only the medial branches to the uterus are affected. Successful uterosacral nerve ablation has been reported in approximately 44% of women who have dysmenorrhea without visible endometriosis and approximately 62% of women who have visible endometriosis.7,8 The efficacy of this procedure is controversial, however. Removal of the superior hypogastric plexus (presacral neurectomy) has not proved to be more effective in controlling pelvic pain than conservative surgery that only destroys endometrial implants. Presacral neurectomy is no longer advised.9
1. American College of Obstetricians and Gynecologists. Educational Bulletin #238: Lower Urinary Tract Operative Injuries. Washington, DC: ACOG; 1985:1.
2. Daly JW, Higgins KA. Injury to the ureter during gynecologic surgical procedures. Surg Gynecol Obstet. 1988;167:19-22.
3. Selzman AA, Spirnak JP. Iatrogenic ureteral injuries: a 20-year experience in treating 165 injuries. J Urol. 1996;155:878-881.
4. Symmonds RE. Ureteral injuries associated with gynecologic surgery: prevention and management. Clin Obstet Gynecol. 19676;19:623-644.
5. Higgins CC. Ureteral injuries during surgery: a review of 87 cases. JAMA 1967;199:82-88.
6. Fry DE, Milholen L, Harbrecht PJ. Iatrogenic ureteral injury. Options in management. Arch Surg. 1983;118:454-457.
7. Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. J Reprod Med. 1987;32:37-41.
8. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696.-
9. Candiani GB, Fedele L, Vercellini P, Bianchi S, Di Nola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
Dr. Hatch receives research/grant support from Merck; is a consultant to Ethicon, Merck, and Quest Laboratory; and is a speaker for Cytyc, Digene, Ethicon, and Merck.
The accomplished gynecologic surgeon must know the anatomy of the retroperitoneal space in order to avoid damage to normal structures, as well as remove pathology. Many disease processes involve the pelvic peritoneum, uterosacral ligaments, rectosigmoid or ovarian pedicles, and require the surgeon to enter the retroperitoneal space to identify the ureters and blood vessels and keep them out of harm’s way. The challenges are complex:
- Badly distorted anatomy and the anterior and posterior cul-de-sac necessitate mobilization of the rectosigmoid and bladder.
- Intraligamentous fibroids require knowledge of the blood supply in the retroperitoneal space. Malignant disorders mandate that the lymph nodes be dissected to determine extent of disease and as part of treatment.
Is training adequate?
Every training program should teach the surgical anatomy of the retroperitoneal space, since every surgeon needs to be comfortable exposing the anatomy, both to prevent injury and to accomplish the needed surgery. Videotapes and cadaver courses can prepare the resident for the operating room.
The “landmark” umbilical ligament
The umbilical ligament was the umbilical artery in fetal life and courses along the edge of the bladder to the anterior abdominal wall up to the umbilicus. It is a useful guide into the perivesicle space. Lateral to it are the iliac vessels, and medial is the bladder. It is also a good marker for finding the right spot to open the round ligament.
FIGURE 1 Opening the round ligament over the umbilical ligament
The round ligament is the key to exposing the retroperitoneal space. It should be open at the pelvic sidewall, just medial to the external iliac vessels. The umbilical ligament can be used as a landmark.
FIGURE 2 Opening lateral to the ovarian vessels
The divided round ligament is retracted ventrally and medially to place the ovarian vessels under traction. The peritoneum lateral to the ovarian vessels is divided up to the pelvic brim.
FIGURE 3 Right pelvic sidewall anatomy
Medial traction of the peritoneum around the ovarian vessels at the pelvic brim will expose the ureter coming over the iliac vessels at their bifurcation into external and internal branches.
FIGURE 4 Relationship of ureter to the umbilical ligament
The ureter comes off medial with the fold of peritoneum. Once the space is developed, the operator’s finger or the laparoscopic probe can be introduced along the medial side of the internal iliac artery and ventral to the curve of the sacrum. This will open the pararectal space. The ureter and the anterior branch of the internal iliac artery are nearly parallel as they course through the pelvis.
FIGURE 5 Hypogastric nerve
The anterior branch of the internal iliac artery gives off the uterine artery and the middle and superior vesicle arteries before continuing on as the umbilical ligament.
Endometriosis may imperil the ureter
Endometriomas and peritoneal implants are among the most common reasons for accessing the retroperitoneal space. Frequently the peritoneum between the ovarian vessels and the uterosacral ligaments (ovarian fossa) is thickened and retracted within the endometriosis implants. This alters the pelvic anatomy and puts the ureter at risk for injury.
Definitive surgical treatment for endometriosis includes removal of this diseased peritoneum. The ureter is best identified using the technique described, and then dissected off the peritoneum down to the uterine artery.
FIGURE 6 Finding the ureter lateral to an endometrioma
FIGURE 7 Relationship of ureter to the umbilical ligament
FIGURE 8 Dissecting the ureter out of the uterosacral ligament
The ureter may be placed on a Penrose drain to better isolate it and keep it under direct visualization. The cul-de-sac of Douglas is another site of endometrial implants. The fundus of the uterus is often adhesed to the rectosigmoid reflection and even the sigmoid colon. Nodules of endometriosis may infiltrate the uterosacral ligaments and extend into the rectovaginal space. To manage these implants, isolate the ureter to the point where it passes under the uterine artery.
FIGURE 9 Divide uterine artery lateral to endometrioma
Here, the uterine artery is taken lateral to the mass, enabling the surgeon to remove all of the uterosacral implants. The bladder flap will be developed and the bladder advanced caudad. By dissecting medial to the ureter as it courses under the uterine artery, it will be retracted laterally and provide the space necessary to resect the uterosacral implants.
FIGURE 10 Rectosigmoid reflection with endometrioma nodule
FIGURE 11 Postoperative appearance of endometriosis resection
As dissection proceeds medially, use the perirectal fatty plane to remove implants between the uterosacral area and rectum. In the midline, remove implants below the rectosigmoid peritoneal reflexion, taking care not to injure the rectum.
Implants above the peritoneal reflexion are often attached to the sigmoid tinea coli and cannot be removed without taking a portion of the bowel. Thus, patients with extensive endometriosis should have a bowel prep with two 10-ounce bottles of magnesium citrate the day before surgery.
If bowel resection is necessary, consult a general surgeon or gynecologic oncologist. A history of painful defecation or a finding of nodules in the cul-de-sac with rectal dimpling warrants preoperative consultation with a specialist skilled at bowel resection.
Ureteral injuries occur in 0.5% to 2.5% of women undergoing gynecologic surgery.1 The most common predisposing condition is previous pelvic surgery.2,3 The usual sites of injury are at the pelvic rim close to the ovarian vessels, at the level of the uterine artery, and lateral to the vaginal cuff. Neither preoperative excretory urograms nor placement of ureteral catheters preoperatively have been found to be effective prevention measures.2,4-6 Using the steps described above, the ureter can be identified and mobilized.
Blood supply to the ureter comes from the plexus of vessels that form a network along the length of the ureter. This plexus is fed by arteries from the renal pelvis, common iliac, internal iliac, uterine artery, and the base of the bladder. Complete mobilization of the ureter away from its peritoneal attachments and these lateral blood supply sources can be accomplished as long as the vascular plexus is not disrupted by cautery, crushing, or tearing. A clean transection can be re-anastomosed or reimplanted with the expectation of normal healing. Innervation of the ureter is from the inferior mesenteric plexus superiorly and the inferior hypogastric plexus in the pelvis. Ureteral peristalsis will continue, even if the ureter is completely divided or ligated.
Protecting pelvic blood vessels
The majority of gynecologic operations involve the ovarian vessels and the uterine vessels. The operator rarely needs to explore the lateral pelvis to identify the rest of the vessels. When faced with a large endometrioma or cancer, knowledge of the anatomy of the lateral blood vessels is vital.
Since the pathology is usually deeper in the pelvis, it is wise to identify the anatomy of the pelvis starting at the pelvic brim, where the common iliac bifurcates into the external and internal iliacs. There is a safe dissection plane medial to the internal iliac artery all the way to the uterine artery. This exposes the pararectal space, which can be opened without risk of major bleeding (FIGURE 5).
The obliterated umbilical vessel is the other friendly marker just distal to the uterine artery. It can be placed on traction and the uterine artery isolated. The inferior and superior vesical arteries are generally not dissected, as they are adjacent to the bladder and the surgery takes place medial to them in the prevesical fascial space.
Hypogastric artery ligations are rarely performed today, as interventional radiology is the standard of care for patients with postoperative and postpartum bleeding. When it becomes necessary, the hypogastric artery can be isolated and tied using the right-angle clamp to pass the tie. The superior gluteal artery branches so close to the bifurcation of the common iliac artery that it is not visualized. The inferior gluteal artery is the largest distal branch, which might be visualized during the ligation. It is not necessary to identify it.
FIGURE 12 Right genitofemoral nerve
The genitofemoral nerve runs along the medial aspect of the body of the psoas muscle. It is sometimes injured by the self-retaining retractors placed at the time of laparotomy. This leads to some numbness and burning of the skin of the anterior thigh.
FIGURE 13 Right pelvic sidewall anatomy
The obturator nerve is in the obturator space and typically far lateral to the usual dissection. Metastatic cancer to the obturator lymph nodes may entrap it, or it may be injured during a node dissection, causing loss of internal rotation of the anterior thigh.
The sciatic nerve is seen only during exenterative surgery. Pressure on the lateral pelvis by advanced pelvic tumors can lead to sciatic pain and motor weakness—even loss of motor function to the lower leg, which commonly leads to foot drop.
FIGURE 14 Superior hypogastric nerve bundle
The hypogastric plexus of nerves is sometimes damaged during surgery for endometriosis or for malignancy. The superior hypergastric plexus can be identified between the 2 common iliac arteries at the sacral promontory. The left common iliac vein runs underneath it.
FIGURE 15 Hypogastric nerve descending into the right pelvis
The right and left hypogastric nerves leave the hypogastric plexus and descend into the pelvis parallel to the ureter and 2 cm medial. It passes dorsal to the ureter as it goes through the cardinal ligament (FIGURE 5).
This plexus then supplies autonomic innervation of the bladder, rectum, uterus, and ureter. Complete disruption of the hypogastric nerve will lead to a hypertonic, noncontractile bladder and the necessity for self-catheterization to eliminate urine. Preservation of this nerve during radical hysterectomy or endometriosis resection is a high priority.
Laparoscopic uterosacral nerve ablation procedures divide the uterosacral ligament medial and caudad to the ureter and do not disrupt the main hypogastric nerve. Only the medial branches to the uterus are affected. Successful uterosacral nerve ablation has been reported in approximately 44% of women who have dysmenorrhea without visible endometriosis and approximately 62% of women who have visible endometriosis.7,8 The efficacy of this procedure is controversial, however. Removal of the superior hypogastric plexus (presacral neurectomy) has not proved to be more effective in controlling pelvic pain than conservative surgery that only destroys endometrial implants. Presacral neurectomy is no longer advised.9
The accomplished gynecologic surgeon must know the anatomy of the retroperitoneal space in order to avoid damage to normal structures, as well as remove pathology. Many disease processes involve the pelvic peritoneum, uterosacral ligaments, rectosigmoid or ovarian pedicles, and require the surgeon to enter the retroperitoneal space to identify the ureters and blood vessels and keep them out of harm’s way. The challenges are complex:
- Badly distorted anatomy and the anterior and posterior cul-de-sac necessitate mobilization of the rectosigmoid and bladder.
- Intraligamentous fibroids require knowledge of the blood supply in the retroperitoneal space. Malignant disorders mandate that the lymph nodes be dissected to determine extent of disease and as part of treatment.
Is training adequate?
Every training program should teach the surgical anatomy of the retroperitoneal space, since every surgeon needs to be comfortable exposing the anatomy, both to prevent injury and to accomplish the needed surgery. Videotapes and cadaver courses can prepare the resident for the operating room.
The “landmark” umbilical ligament
The umbilical ligament was the umbilical artery in fetal life and courses along the edge of the bladder to the anterior abdominal wall up to the umbilicus. It is a useful guide into the perivesicle space. Lateral to it are the iliac vessels, and medial is the bladder. It is also a good marker for finding the right spot to open the round ligament.
FIGURE 1 Opening the round ligament over the umbilical ligament
The round ligament is the key to exposing the retroperitoneal space. It should be open at the pelvic sidewall, just medial to the external iliac vessels. The umbilical ligament can be used as a landmark.
FIGURE 2 Opening lateral to the ovarian vessels
The divided round ligament is retracted ventrally and medially to place the ovarian vessels under traction. The peritoneum lateral to the ovarian vessels is divided up to the pelvic brim.
FIGURE 3 Right pelvic sidewall anatomy
Medial traction of the peritoneum around the ovarian vessels at the pelvic brim will expose the ureter coming over the iliac vessels at their bifurcation into external and internal branches.
FIGURE 4 Relationship of ureter to the umbilical ligament
The ureter comes off medial with the fold of peritoneum. Once the space is developed, the operator’s finger or the laparoscopic probe can be introduced along the medial side of the internal iliac artery and ventral to the curve of the sacrum. This will open the pararectal space. The ureter and the anterior branch of the internal iliac artery are nearly parallel as they course through the pelvis.
FIGURE 5 Hypogastric nerve
The anterior branch of the internal iliac artery gives off the uterine artery and the middle and superior vesicle arteries before continuing on as the umbilical ligament.
Endometriosis may imperil the ureter
Endometriomas and peritoneal implants are among the most common reasons for accessing the retroperitoneal space. Frequently the peritoneum between the ovarian vessels and the uterosacral ligaments (ovarian fossa) is thickened and retracted within the endometriosis implants. This alters the pelvic anatomy and puts the ureter at risk for injury.
Definitive surgical treatment for endometriosis includes removal of this diseased peritoneum. The ureter is best identified using the technique described, and then dissected off the peritoneum down to the uterine artery.
FIGURE 6 Finding the ureter lateral to an endometrioma
FIGURE 7 Relationship of ureter to the umbilical ligament
FIGURE 8 Dissecting the ureter out of the uterosacral ligament
The ureter may be placed on a Penrose drain to better isolate it and keep it under direct visualization. The cul-de-sac of Douglas is another site of endometrial implants. The fundus of the uterus is often adhesed to the rectosigmoid reflection and even the sigmoid colon. Nodules of endometriosis may infiltrate the uterosacral ligaments and extend into the rectovaginal space. To manage these implants, isolate the ureter to the point where it passes under the uterine artery.
FIGURE 9 Divide uterine artery lateral to endometrioma
Here, the uterine artery is taken lateral to the mass, enabling the surgeon to remove all of the uterosacral implants. The bladder flap will be developed and the bladder advanced caudad. By dissecting medial to the ureter as it courses under the uterine artery, it will be retracted laterally and provide the space necessary to resect the uterosacral implants.
FIGURE 10 Rectosigmoid reflection with endometrioma nodule
FIGURE 11 Postoperative appearance of endometriosis resection
As dissection proceeds medially, use the perirectal fatty plane to remove implants between the uterosacral area and rectum. In the midline, remove implants below the rectosigmoid peritoneal reflexion, taking care not to injure the rectum.
Implants above the peritoneal reflexion are often attached to the sigmoid tinea coli and cannot be removed without taking a portion of the bowel. Thus, patients with extensive endometriosis should have a bowel prep with two 10-ounce bottles of magnesium citrate the day before surgery.
If bowel resection is necessary, consult a general surgeon or gynecologic oncologist. A history of painful defecation or a finding of nodules in the cul-de-sac with rectal dimpling warrants preoperative consultation with a specialist skilled at bowel resection.
Ureteral injuries occur in 0.5% to 2.5% of women undergoing gynecologic surgery.1 The most common predisposing condition is previous pelvic surgery.2,3 The usual sites of injury are at the pelvic rim close to the ovarian vessels, at the level of the uterine artery, and lateral to the vaginal cuff. Neither preoperative excretory urograms nor placement of ureteral catheters preoperatively have been found to be effective prevention measures.2,4-6 Using the steps described above, the ureter can be identified and mobilized.
Blood supply to the ureter comes from the plexus of vessels that form a network along the length of the ureter. This plexus is fed by arteries from the renal pelvis, common iliac, internal iliac, uterine artery, and the base of the bladder. Complete mobilization of the ureter away from its peritoneal attachments and these lateral blood supply sources can be accomplished as long as the vascular plexus is not disrupted by cautery, crushing, or tearing. A clean transection can be re-anastomosed or reimplanted with the expectation of normal healing. Innervation of the ureter is from the inferior mesenteric plexus superiorly and the inferior hypogastric plexus in the pelvis. Ureteral peristalsis will continue, even if the ureter is completely divided or ligated.
Protecting pelvic blood vessels
The majority of gynecologic operations involve the ovarian vessels and the uterine vessels. The operator rarely needs to explore the lateral pelvis to identify the rest of the vessels. When faced with a large endometrioma or cancer, knowledge of the anatomy of the lateral blood vessels is vital.
Since the pathology is usually deeper in the pelvis, it is wise to identify the anatomy of the pelvis starting at the pelvic brim, where the common iliac bifurcates into the external and internal iliacs. There is a safe dissection plane medial to the internal iliac artery all the way to the uterine artery. This exposes the pararectal space, which can be opened without risk of major bleeding (FIGURE 5).
The obliterated umbilical vessel is the other friendly marker just distal to the uterine artery. It can be placed on traction and the uterine artery isolated. The inferior and superior vesical arteries are generally not dissected, as they are adjacent to the bladder and the surgery takes place medial to them in the prevesical fascial space.
Hypogastric artery ligations are rarely performed today, as interventional radiology is the standard of care for patients with postoperative and postpartum bleeding. When it becomes necessary, the hypogastric artery can be isolated and tied using the right-angle clamp to pass the tie. The superior gluteal artery branches so close to the bifurcation of the common iliac artery that it is not visualized. The inferior gluteal artery is the largest distal branch, which might be visualized during the ligation. It is not necessary to identify it.
FIGURE 12 Right genitofemoral nerve
The genitofemoral nerve runs along the medial aspect of the body of the psoas muscle. It is sometimes injured by the self-retaining retractors placed at the time of laparotomy. This leads to some numbness and burning of the skin of the anterior thigh.
FIGURE 13 Right pelvic sidewall anatomy
The obturator nerve is in the obturator space and typically far lateral to the usual dissection. Metastatic cancer to the obturator lymph nodes may entrap it, or it may be injured during a node dissection, causing loss of internal rotation of the anterior thigh.
The sciatic nerve is seen only during exenterative surgery. Pressure on the lateral pelvis by advanced pelvic tumors can lead to sciatic pain and motor weakness—even loss of motor function to the lower leg, which commonly leads to foot drop.
FIGURE 14 Superior hypogastric nerve bundle
The hypogastric plexus of nerves is sometimes damaged during surgery for endometriosis or for malignancy. The superior hypergastric plexus can be identified between the 2 common iliac arteries at the sacral promontory. The left common iliac vein runs underneath it.
FIGURE 15 Hypogastric nerve descending into the right pelvis
The right and left hypogastric nerves leave the hypogastric plexus and descend into the pelvis parallel to the ureter and 2 cm medial. It passes dorsal to the ureter as it goes through the cardinal ligament (FIGURE 5).
This plexus then supplies autonomic innervation of the bladder, rectum, uterus, and ureter. Complete disruption of the hypogastric nerve will lead to a hypertonic, noncontractile bladder and the necessity for self-catheterization to eliminate urine. Preservation of this nerve during radical hysterectomy or endometriosis resection is a high priority.
Laparoscopic uterosacral nerve ablation procedures divide the uterosacral ligament medial and caudad to the ureter and do not disrupt the main hypogastric nerve. Only the medial branches to the uterus are affected. Successful uterosacral nerve ablation has been reported in approximately 44% of women who have dysmenorrhea without visible endometriosis and approximately 62% of women who have visible endometriosis.7,8 The efficacy of this procedure is controversial, however. Removal of the superior hypogastric plexus (presacral neurectomy) has not proved to be more effective in controlling pelvic pain than conservative surgery that only destroys endometrial implants. Presacral neurectomy is no longer advised.9
1. American College of Obstetricians and Gynecologists. Educational Bulletin #238: Lower Urinary Tract Operative Injuries. Washington, DC: ACOG; 1985:1.
2. Daly JW, Higgins KA. Injury to the ureter during gynecologic surgical procedures. Surg Gynecol Obstet. 1988;167:19-22.
3. Selzman AA, Spirnak JP. Iatrogenic ureteral injuries: a 20-year experience in treating 165 injuries. J Urol. 1996;155:878-881.
4. Symmonds RE. Ureteral injuries associated with gynecologic surgery: prevention and management. Clin Obstet Gynecol. 19676;19:623-644.
5. Higgins CC. Ureteral injuries during surgery: a review of 87 cases. JAMA 1967;199:82-88.
6. Fry DE, Milholen L, Harbrecht PJ. Iatrogenic ureteral injury. Options in management. Arch Surg. 1983;118:454-457.
7. Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. J Reprod Med. 1987;32:37-41.
8. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696.-
9. Candiani GB, Fedele L, Vercellini P, Bianchi S, Di Nola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
Dr. Hatch receives research/grant support from Merck; is a consultant to Ethicon, Merck, and Quest Laboratory; and is a speaker for Cytyc, Digene, Ethicon, and Merck.
1. American College of Obstetricians and Gynecologists. Educational Bulletin #238: Lower Urinary Tract Operative Injuries. Washington, DC: ACOG; 1985:1.
2. Daly JW, Higgins KA. Injury to the ureter during gynecologic surgical procedures. Surg Gynecol Obstet. 1988;167:19-22.
3. Selzman AA, Spirnak JP. Iatrogenic ureteral injuries: a 20-year experience in treating 165 injuries. J Urol. 1996;155:878-881.
4. Symmonds RE. Ureteral injuries associated with gynecologic surgery: prevention and management. Clin Obstet Gynecol. 19676;19:623-644.
5. Higgins CC. Ureteral injuries during surgery: a review of 87 cases. JAMA 1967;199:82-88.
6. Fry DE, Milholen L, Harbrecht PJ. Iatrogenic ureteral injury. Options in management. Arch Surg. 1983;118:454-457.
7. Lichten EM, Bombard J. Surgical treatment of primary dysmenorrhea with laparoscopic uterine nerve ablation. J Reprod Med. 1987;32:37-41.
8. Sutton CJG, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696.-
9. Candiani GB, Fedele L, Vercellini P, Bianchi S, Di Nola G. Presacral neurectomy for the treatment of pelvic pain associated with endometriosis: a controlled study. Am J Obstet Gynecol. 1992;167:100-103.
Dr. Hatch receives research/grant support from Merck; is a consultant to Ethicon, Merck, and Quest Laboratory; and is a speaker for Cytyc, Digene, Ethicon, and Merck.