User login
CASE 1 What procedures should accompany hysterectomy?
A.E., 44, mother of one, complains of heavy irregular bleeding with no sensation of a vaginal bulge. She has tried oral contraceptives, but they did not improve her bleeding pattern. She also has undergone dilatation and curettage and hysteroscopy (benign findings), also with no improvement.
Examination reveals a 9- to 10-week-size fibroid uterus, which is confirmed by ultrasonography. Pelvic support appears to be excellent.
After a discussion of the options, the patient elects to undergo vaginal hysterectomy. Are other procedures warranted?
Ask a gynecologic surgeon to name the most significant challenges he or she faces, and the answer is likely to include preventing pelvic organ prolapse after surgical intervention. Approximately one third of operations for pelvic organ prolapse involve patients whose prolapse has recurred after previous surgery.1 Although we have advanced our understanding of the anatomy of pelvic support and the pathophysiology of support defects, the various surgical strategies remain largely untested and unproven.
Even women with good pelvic support who are undergoing hysterectomy—like the patient described above—are vulnerable. One particular area of concern: the risk of enterocele or vaginal apical prolapse, or both, after hysterectomy. In this article, I describe a technique to reduce the risk of these defects after vaginal hysterectomy: high uterosacral suspension, or modified McCall culdoplasty.
Enterocele and apical prolapse do not always coexist
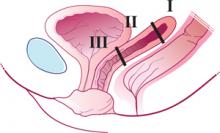
Enterocele and apical prolapse are distinct entities. The latter represents a deficiency in the level I supporting structures described by DeLancey2—primarily the uterosacral and cardinal ligaments (FIGURE 1). Enterocele, or peritoneocele, is a herniation of the cul-de-sac peritoneum, with or without intestinal contents. In women who have undergone hysterectomy, enterocele is usually caused by a lack of continuity of level II fibers, namely, the failure to approximate the pubocervical and rectovaginal connective tissues at the time of hysterectomy.3 Careful attention to the vaginal cuff and cul-de-sac at the time of hysterectomy is therefore imperative.
FIGURE 1 Three levels of support
The endopelvic fascia of a posthysterectomy patient divided into DeLancey’s biomechanical levels: level I—proximal suspension, level II—lateral attachment, and level III—distal fusion.
The McCall culdoplasty: 50 years “young”
In 1957, Milton McCall, MD, described a technique to manage the cul-de-sac at the time of vaginal hysterectomy.4 The McCall technique of posterior culdoplasty differs from other approaches by omitting dissection and excision of the hernia sac, or excess cul-de-sac peritoneum. The original McCall culdoplasty begins with the placement of several rows (average of 3) of nonabsorbable suture (“internal” McCall sutures), starting at the left uterosacral ligament about 2 cm above its cut edge, and proceeding across the redundant cul-de-sac to terminate in the right uterosacral ligament. Each subsequent row is placed superior to the first, by applying traction to the previously placed sutures.
Prior to the tying of these sutures, 3 “external” absorbable sutures are placed. These sutures incorporate posterior vaginal epithelium, each uterosacral ligament, and the contralateral vaginal epithelium in a mirror image of the first pass through the vagina. Again, several rows are placed, each more superior to the last, to move the newly created vaginal apex to the highest point on the uterosacral ligaments once all the sutures are tied.
Tying the internal sutures not only creates a firm, shelf-like midline structure, but obliterates the redundant cul-de-sac. The external sutures move the vaginal apex to the uterosacral bridge and are tied at the conclusion of the procedure (FIGURES 2 and 3).
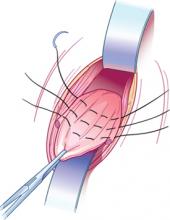
FIGURE 2 Internal McCall sutures
Traction on the most dependent portion of the cul-de-sac and posterior vaginal epithelium allows placement of 3 rows of sutures across the cul-de-sac from one uterosacral ligament to the other.
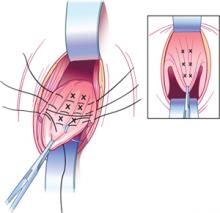
FIGURE 3 External McCall sutures
Three additional rows of absorbable sutures incorporate vaginal epithelium and uterosacral ligaments to move the vaginal cuff superiorly.
Modifications enhance durability and support
When the surgical indication is significant apical vaginal prolapse, the efficacy of the McCall procedure as both treatment and prevention is uncertain, because we lack adequate studies in this population. However, assuming that identifiable defects or breaks in the uterosacral ligaments lead to apical prolapse,3 use of the portion of the uterosacral ligament nearest the vagina appears unlikely to create a durable repair.
Thus, the concept of a “high” uterosacral attachment came to be proposed to provide a strong midline site of support for the vaginal apex.5,6 Further modifications include attachment of the uterosacral ligaments to pubocervical and rectovaginal connective tissues to create continuity of these level II fibers and prevent subsequent enterocele.7
With a high uterosacral attachment, the uterosacral ligaments need not be brought together in the midline.
Technique for modified approach
To locate each ligament, place traction on the vaginal apex toward the contralateral side. Palpate the pelvic structures posterior and medial to the ischial spines, at the 4 and 8 o’clock positions, to identify the strong tissue emanating from the sacrum.
Place several nonabsorbable sutures through the medial aspect of each uterosacral ligament, working from lateral to medial to minimize the risk of ureteral trauma. Then place 1 strand of each suture through the pubocervical and rectovaginal connective tissues. Tie the sutures to move the vaginal apex to the proximal segment of the uterosacral ligament (near the sacrum) and establish continuity of the pubocervical and rectovaginal connective tissues (FIGURES 4-6).
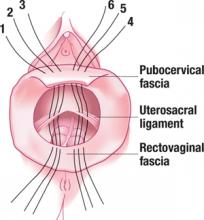
FIGURE 4 Suture placement is bilateral
Three sutures are placed in the uterosacral ligament pedicles on each side, with 1 arm of each suture placed in the transverse portion of the pubocervical and rectovaginal fascia.
FIGURE 5 Suspensory suture
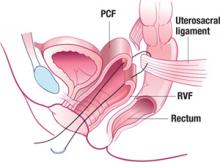
Sagittal view of a suspensory suture in left uterosacral ligament with 1 arm through pubocervical fascia (PCF) and 1 arm through rectovaginal fascia (RVF).
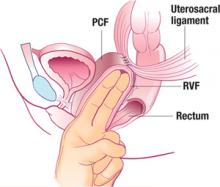
FIGURE 6 The suspended vaginal vault
Sagittal view of pubocervical fascia (PCF) and rectovaginal fascia (RVF) suspended from uterosacral ligaments.
Main concern is ureteral injury
The ureter lies near the anterior margin of the uterosacral ligament, with a mean distance of 4.1±0.6 cm at the level of the sacrum and 2.3±0.9 cm at the level of the ischial spine.8 In 1 series of high uterosacral ligament suspension with site-specific endopelvic fascia defect repair, ureteral complications occurred in 11% of patients.5 Other series have reported rates of ureteral trauma in the range of 0.7%9 to 2.4%.6
Evaluate ureteral patency
After performing this procedure, the surgeon should ensure ureteral patency. For this reason, I believe that only surgeons skilled in cystoscopy and able to treat ureteral injury (or with ready access to those capable of treating this complication) should undertake high uterosacral suspension.
How the McCall procedure compares with other approaches
Cruikshank SH, Kovac SR. Randomized comparison of three surgical methods used at the time of vaginal hysterectomy to prevent posterior enterocele. Am J Obstet Gynecol. 1999;180:859–865
Although many techniques and modifications have been described for management of the cul-de-sac and vaginal cuff, few comparative data exist. In McCall’s original series,4 43 patients undergoing vaginal hysterectomy with posterior culdoplasty were followed for a minimum of 3 months and a maximum of 3 years. Mean and median lengths of follow-up were not provided, nor were the indications for hysterectomy or the method of assessing the patient for recurrent defects. McCall reported that no patients developed an enterocele after the surgery.
The first and only randomized study
Cruikshank and Kovac performed the only prospective, randomized comparison of procedures used at the time of hysterectomy to prevent enterocele. In their study, 100 patients undergoing vaginal hysterectomy for various indications (excluding prolapse of the posterior superior segment of the vagina) were randomized to 1 of 3 surgical methods to prevent enterocele:
- Moschcowitz-type closure (n=33), in which the peritoneum was closed using a purse-string technique, incorporating the distal ends of the uterosacral and cardinal ligaments and thereby drawing these structures to the midline
- modified McCall culdoplasty (n=33), in which a higher purse-string closure of the peritoneum was performed superior to the “yellow fat line,” incorporating the uterosacral-cardinal ligament complex (thus drawing these structures to the midline) and including the vagina (similar to the external McCall sutures) to move the apex superiorly
- simple closure of the peritoneum with a purse-string suture (n=34), with none of the uterosacral-cardinal ligaments incorporated into the repair.
Three years of follow-up
Of the 100 patients, 98 were followed with serial examinations for 3 years, and the outcomes at all vaginal segments were documented, with particular attention to the posterior superior segment, using staging from the Pelvic Organ Prolapse Quantification (POP-Q) system.10
McCall procedure was most effective
Overall, 11 patients (11.2%) were found to have stage II prolapse of the posterior superior vagina, none of them in the McCall group. An additional 14 patients (14.3%) had stage I prolapse, with only 2 (2%) of these in the McCall group.
The McCall repair was significantly more effective than the other 2 types of repair, with a 6.1% risk of subsequent prolapse, versus 30.3% in women who had a Moschcowitz-type closure and 39.4% in those who underwent simple closure of the peritoneum. No patients in any group had prolapse greater than stage II at follow-up.
Limitations of the study
Although Cruikshank and Kovac designed their study to analyze appropriate prophylaxis against enterocele in patients without prolapse, several patients did have some form of prolapse—although it was unrelated to the posterior superior vagina. Therefore, several patients underwent concomitant reconstructive procedures that included: anterior colporrhaphy (13), posterior colporrhaphy (4), sacrospinous ligament fixation (3), bilateral paravaginal repair (10), and anti-incontinence procedures (10).
It is not clear which groups these patients fell into and whether the distribution was similar across all groups.
CASE 2 Is McCall procedure appropriate?
B.D., 57, complains of increasing pelvic pressure and a noticeable vaginal bulge. Her 2 children were delivered vaginally, the largest weighing 8 lb. B.D. reports that she remains sexually active.
Physical examination reveals the cervix to be at the level of the introitus, but it descends 2 cm beyond the introitus when the patient performs the valsalva maneuver. Although there is also some descent of the anterior and posterior vaginal walls (1 cm superior to the hymen with strain; Pelvic Organ Prolapse Quantification [POP-Q] value=-1), the predominant component of prolapse is an elongated cervix. The posterior vaginal fornix (POP-Q point D), representing apical support, descends to 7 cm superior to the hymen with strain, with a total vaginal length of 9 cm.
At surgery, the uterosacral ligaments do not appear to be attenuated. After vaginal hysterectomy, the apex of the vaginal vault is superior to the level of the ischial spines.
How do you proceed?
Given the relatively good support at the apex, this patient is a good candidate for a McCall-type culdoplasty. Whether or not this procedure will be truly prophylactic (because there is already some descent of the apex, albeit mild) is perhaps only a matter of semantics.
The author reports no financial relationships relevant to this article.
1. Olson AL, Smith VJ, Bergstrom JO, Coiling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
2. DeLancey JOL. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717-1728.
3. Richardson AC. The anatomic defects in rectocele and enterocele. J Pelvic Surg. 1995;1:214-221.
4. McCall ML. Posterior culdoplasty: surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10:595-602.
5. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1402-1411.
6. Karram M, Goldwasser S, Kleeman S, et al. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185:1339-1342.
7. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
8. Buller JL, Thompson JR, Cundiff GW, et al. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol. 2001;97:873-879.
9. Aronson MP, Aronson PK, Howard AE, et al. Low risk of ureteral obstruction with “deep” (dorsal/posterior) uterosacral ligament suture placement for transvaginal apical suspension. Am J Obstet Gynecol. 2005;192:1530-1536.
10. Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10-17.
CASE 1 What procedures should accompany hysterectomy?
A.E., 44, mother of one, complains of heavy irregular bleeding with no sensation of a vaginal bulge. She has tried oral contraceptives, but they did not improve her bleeding pattern. She also has undergone dilatation and curettage and hysteroscopy (benign findings), also with no improvement.
Examination reveals a 9- to 10-week-size fibroid uterus, which is confirmed by ultrasonography. Pelvic support appears to be excellent.
After a discussion of the options, the patient elects to undergo vaginal hysterectomy. Are other procedures warranted?
Ask a gynecologic surgeon to name the most significant challenges he or she faces, and the answer is likely to include preventing pelvic organ prolapse after surgical intervention. Approximately one third of operations for pelvic organ prolapse involve patients whose prolapse has recurred after previous surgery.1 Although we have advanced our understanding of the anatomy of pelvic support and the pathophysiology of support defects, the various surgical strategies remain largely untested and unproven.
Even women with good pelvic support who are undergoing hysterectomy—like the patient described above—are vulnerable. One particular area of concern: the risk of enterocele or vaginal apical prolapse, or both, after hysterectomy. In this article, I describe a technique to reduce the risk of these defects after vaginal hysterectomy: high uterosacral suspension, or modified McCall culdoplasty.
Enterocele and apical prolapse do not always coexist
Enterocele and apical prolapse are distinct entities. The latter represents a deficiency in the level I supporting structures described by DeLancey2—primarily the uterosacral and cardinal ligaments (FIGURE 1). Enterocele, or peritoneocele, is a herniation of the cul-de-sac peritoneum, with or without intestinal contents. In women who have undergone hysterectomy, enterocele is usually caused by a lack of continuity of level II fibers, namely, the failure to approximate the pubocervical and rectovaginal connective tissues at the time of hysterectomy.3 Careful attention to the vaginal cuff and cul-de-sac at the time of hysterectomy is therefore imperative.
FIGURE 1 Three levels of support
The endopelvic fascia of a posthysterectomy patient divided into DeLancey’s biomechanical levels: level I—proximal suspension, level II—lateral attachment, and level III—distal fusion.
The McCall culdoplasty: 50 years “young”
In 1957, Milton McCall, MD, described a technique to manage the cul-de-sac at the time of vaginal hysterectomy.4 The McCall technique of posterior culdoplasty differs from other approaches by omitting dissection and excision of the hernia sac, or excess cul-de-sac peritoneum. The original McCall culdoplasty begins with the placement of several rows (average of 3) of nonabsorbable suture (“internal” McCall sutures), starting at the left uterosacral ligament about 2 cm above its cut edge, and proceeding across the redundant cul-de-sac to terminate in the right uterosacral ligament. Each subsequent row is placed superior to the first, by applying traction to the previously placed sutures.
Prior to the tying of these sutures, 3 “external” absorbable sutures are placed. These sutures incorporate posterior vaginal epithelium, each uterosacral ligament, and the contralateral vaginal epithelium in a mirror image of the first pass through the vagina. Again, several rows are placed, each more superior to the last, to move the newly created vaginal apex to the highest point on the uterosacral ligaments once all the sutures are tied.
Tying the internal sutures not only creates a firm, shelf-like midline structure, but obliterates the redundant cul-de-sac. The external sutures move the vaginal apex to the uterosacral bridge and are tied at the conclusion of the procedure (FIGURES 2 and 3).
FIGURE 2 Internal McCall sutures
Traction on the most dependent portion of the cul-de-sac and posterior vaginal epithelium allows placement of 3 rows of sutures across the cul-de-sac from one uterosacral ligament to the other.
FIGURE 3 External McCall sutures
Three additional rows of absorbable sutures incorporate vaginal epithelium and uterosacral ligaments to move the vaginal cuff superiorly.
Modifications enhance durability and support
When the surgical indication is significant apical vaginal prolapse, the efficacy of the McCall procedure as both treatment and prevention is uncertain, because we lack adequate studies in this population. However, assuming that identifiable defects or breaks in the uterosacral ligaments lead to apical prolapse,3 use of the portion of the uterosacral ligament nearest the vagina appears unlikely to create a durable repair.
Thus, the concept of a “high” uterosacral attachment came to be proposed to provide a strong midline site of support for the vaginal apex.5,6 Further modifications include attachment of the uterosacral ligaments to pubocervical and rectovaginal connective tissues to create continuity of these level II fibers and prevent subsequent enterocele.7
With a high uterosacral attachment, the uterosacral ligaments need not be brought together in the midline.
Technique for modified approach
To locate each ligament, place traction on the vaginal apex toward the contralateral side. Palpate the pelvic structures posterior and medial to the ischial spines, at the 4 and 8 o’clock positions, to identify the strong tissue emanating from the sacrum.
Place several nonabsorbable sutures through the medial aspect of each uterosacral ligament, working from lateral to medial to minimize the risk of ureteral trauma. Then place 1 strand of each suture through the pubocervical and rectovaginal connective tissues. Tie the sutures to move the vaginal apex to the proximal segment of the uterosacral ligament (near the sacrum) and establish continuity of the pubocervical and rectovaginal connective tissues (FIGURES 4-6).
FIGURE 4 Suture placement is bilateral
Three sutures are placed in the uterosacral ligament pedicles on each side, with 1 arm of each suture placed in the transverse portion of the pubocervical and rectovaginal fascia.
FIGURE 5 Suspensory suture
Sagittal view of a suspensory suture in left uterosacral ligament with 1 arm through pubocervical fascia (PCF) and 1 arm through rectovaginal fascia (RVF).
FIGURE 6 The suspended vaginal vault
Sagittal view of pubocervical fascia (PCF) and rectovaginal fascia (RVF) suspended from uterosacral ligaments.
Main concern is ureteral injury
The ureter lies near the anterior margin of the uterosacral ligament, with a mean distance of 4.1±0.6 cm at the level of the sacrum and 2.3±0.9 cm at the level of the ischial spine.8 In 1 series of high uterosacral ligament suspension with site-specific endopelvic fascia defect repair, ureteral complications occurred in 11% of patients.5 Other series have reported rates of ureteral trauma in the range of 0.7%9 to 2.4%.6
Evaluate ureteral patency
After performing this procedure, the surgeon should ensure ureteral patency. For this reason, I believe that only surgeons skilled in cystoscopy and able to treat ureteral injury (or with ready access to those capable of treating this complication) should undertake high uterosacral suspension.
How the McCall procedure compares with other approaches
Cruikshank SH, Kovac SR. Randomized comparison of three surgical methods used at the time of vaginal hysterectomy to prevent posterior enterocele. Am J Obstet Gynecol. 1999;180:859–865
Although many techniques and modifications have been described for management of the cul-de-sac and vaginal cuff, few comparative data exist. In McCall’s original series,4 43 patients undergoing vaginal hysterectomy with posterior culdoplasty were followed for a minimum of 3 months and a maximum of 3 years. Mean and median lengths of follow-up were not provided, nor were the indications for hysterectomy or the method of assessing the patient for recurrent defects. McCall reported that no patients developed an enterocele after the surgery.
The first and only randomized study
Cruikshank and Kovac performed the only prospective, randomized comparison of procedures used at the time of hysterectomy to prevent enterocele. In their study, 100 patients undergoing vaginal hysterectomy for various indications (excluding prolapse of the posterior superior segment of the vagina) were randomized to 1 of 3 surgical methods to prevent enterocele:
- Moschcowitz-type closure (n=33), in which the peritoneum was closed using a purse-string technique, incorporating the distal ends of the uterosacral and cardinal ligaments and thereby drawing these structures to the midline
- modified McCall culdoplasty (n=33), in which a higher purse-string closure of the peritoneum was performed superior to the “yellow fat line,” incorporating the uterosacral-cardinal ligament complex (thus drawing these structures to the midline) and including the vagina (similar to the external McCall sutures) to move the apex superiorly
- simple closure of the peritoneum with a purse-string suture (n=34), with none of the uterosacral-cardinal ligaments incorporated into the repair.
Three years of follow-up
Of the 100 patients, 98 were followed with serial examinations for 3 years, and the outcomes at all vaginal segments were documented, with particular attention to the posterior superior segment, using staging from the Pelvic Organ Prolapse Quantification (POP-Q) system.10
McCall procedure was most effective
Overall, 11 patients (11.2%) were found to have stage II prolapse of the posterior superior vagina, none of them in the McCall group. An additional 14 patients (14.3%) had stage I prolapse, with only 2 (2%) of these in the McCall group.
The McCall repair was significantly more effective than the other 2 types of repair, with a 6.1% risk of subsequent prolapse, versus 30.3% in women who had a Moschcowitz-type closure and 39.4% in those who underwent simple closure of the peritoneum. No patients in any group had prolapse greater than stage II at follow-up.
Limitations of the study
Although Cruikshank and Kovac designed their study to analyze appropriate prophylaxis against enterocele in patients without prolapse, several patients did have some form of prolapse—although it was unrelated to the posterior superior vagina. Therefore, several patients underwent concomitant reconstructive procedures that included: anterior colporrhaphy (13), posterior colporrhaphy (4), sacrospinous ligament fixation (3), bilateral paravaginal repair (10), and anti-incontinence procedures (10).
It is not clear which groups these patients fell into and whether the distribution was similar across all groups.
CASE 2 Is McCall procedure appropriate?
B.D., 57, complains of increasing pelvic pressure and a noticeable vaginal bulge. Her 2 children were delivered vaginally, the largest weighing 8 lb. B.D. reports that she remains sexually active.
Physical examination reveals the cervix to be at the level of the introitus, but it descends 2 cm beyond the introitus when the patient performs the valsalva maneuver. Although there is also some descent of the anterior and posterior vaginal walls (1 cm superior to the hymen with strain; Pelvic Organ Prolapse Quantification [POP-Q] value=-1), the predominant component of prolapse is an elongated cervix. The posterior vaginal fornix (POP-Q point D), representing apical support, descends to 7 cm superior to the hymen with strain, with a total vaginal length of 9 cm.
At surgery, the uterosacral ligaments do not appear to be attenuated. After vaginal hysterectomy, the apex of the vaginal vault is superior to the level of the ischial spines.
How do you proceed?
Given the relatively good support at the apex, this patient is a good candidate for a McCall-type culdoplasty. Whether or not this procedure will be truly prophylactic (because there is already some descent of the apex, albeit mild) is perhaps only a matter of semantics.
The author reports no financial relationships relevant to this article.
CASE 1 What procedures should accompany hysterectomy?
A.E., 44, mother of one, complains of heavy irregular bleeding with no sensation of a vaginal bulge. She has tried oral contraceptives, but they did not improve her bleeding pattern. She also has undergone dilatation and curettage and hysteroscopy (benign findings), also with no improvement.
Examination reveals a 9- to 10-week-size fibroid uterus, which is confirmed by ultrasonography. Pelvic support appears to be excellent.
After a discussion of the options, the patient elects to undergo vaginal hysterectomy. Are other procedures warranted?
Ask a gynecologic surgeon to name the most significant challenges he or she faces, and the answer is likely to include preventing pelvic organ prolapse after surgical intervention. Approximately one third of operations for pelvic organ prolapse involve patients whose prolapse has recurred after previous surgery.1 Although we have advanced our understanding of the anatomy of pelvic support and the pathophysiology of support defects, the various surgical strategies remain largely untested and unproven.
Even women with good pelvic support who are undergoing hysterectomy—like the patient described above—are vulnerable. One particular area of concern: the risk of enterocele or vaginal apical prolapse, or both, after hysterectomy. In this article, I describe a technique to reduce the risk of these defects after vaginal hysterectomy: high uterosacral suspension, or modified McCall culdoplasty.
Enterocele and apical prolapse do not always coexist
Enterocele and apical prolapse are distinct entities. The latter represents a deficiency in the level I supporting structures described by DeLancey2—primarily the uterosacral and cardinal ligaments (FIGURE 1). Enterocele, or peritoneocele, is a herniation of the cul-de-sac peritoneum, with or without intestinal contents. In women who have undergone hysterectomy, enterocele is usually caused by a lack of continuity of level II fibers, namely, the failure to approximate the pubocervical and rectovaginal connective tissues at the time of hysterectomy.3 Careful attention to the vaginal cuff and cul-de-sac at the time of hysterectomy is therefore imperative.
FIGURE 1 Three levels of support
The endopelvic fascia of a posthysterectomy patient divided into DeLancey’s biomechanical levels: level I—proximal suspension, level II—lateral attachment, and level III—distal fusion.
The McCall culdoplasty: 50 years “young”
In 1957, Milton McCall, MD, described a technique to manage the cul-de-sac at the time of vaginal hysterectomy.4 The McCall technique of posterior culdoplasty differs from other approaches by omitting dissection and excision of the hernia sac, or excess cul-de-sac peritoneum. The original McCall culdoplasty begins with the placement of several rows (average of 3) of nonabsorbable suture (“internal” McCall sutures), starting at the left uterosacral ligament about 2 cm above its cut edge, and proceeding across the redundant cul-de-sac to terminate in the right uterosacral ligament. Each subsequent row is placed superior to the first, by applying traction to the previously placed sutures.
Prior to the tying of these sutures, 3 “external” absorbable sutures are placed. These sutures incorporate posterior vaginal epithelium, each uterosacral ligament, and the contralateral vaginal epithelium in a mirror image of the first pass through the vagina. Again, several rows are placed, each more superior to the last, to move the newly created vaginal apex to the highest point on the uterosacral ligaments once all the sutures are tied.
Tying the internal sutures not only creates a firm, shelf-like midline structure, but obliterates the redundant cul-de-sac. The external sutures move the vaginal apex to the uterosacral bridge and are tied at the conclusion of the procedure (FIGURES 2 and 3).
FIGURE 2 Internal McCall sutures
Traction on the most dependent portion of the cul-de-sac and posterior vaginal epithelium allows placement of 3 rows of sutures across the cul-de-sac from one uterosacral ligament to the other.
FIGURE 3 External McCall sutures
Three additional rows of absorbable sutures incorporate vaginal epithelium and uterosacral ligaments to move the vaginal cuff superiorly.
Modifications enhance durability and support
When the surgical indication is significant apical vaginal prolapse, the efficacy of the McCall procedure as both treatment and prevention is uncertain, because we lack adequate studies in this population. However, assuming that identifiable defects or breaks in the uterosacral ligaments lead to apical prolapse,3 use of the portion of the uterosacral ligament nearest the vagina appears unlikely to create a durable repair.
Thus, the concept of a “high” uterosacral attachment came to be proposed to provide a strong midline site of support for the vaginal apex.5,6 Further modifications include attachment of the uterosacral ligaments to pubocervical and rectovaginal connective tissues to create continuity of these level II fibers and prevent subsequent enterocele.7
With a high uterosacral attachment, the uterosacral ligaments need not be brought together in the midline.
Technique for modified approach
To locate each ligament, place traction on the vaginal apex toward the contralateral side. Palpate the pelvic structures posterior and medial to the ischial spines, at the 4 and 8 o’clock positions, to identify the strong tissue emanating from the sacrum.
Place several nonabsorbable sutures through the medial aspect of each uterosacral ligament, working from lateral to medial to minimize the risk of ureteral trauma. Then place 1 strand of each suture through the pubocervical and rectovaginal connective tissues. Tie the sutures to move the vaginal apex to the proximal segment of the uterosacral ligament (near the sacrum) and establish continuity of the pubocervical and rectovaginal connective tissues (FIGURES 4-6).
FIGURE 4 Suture placement is bilateral
Three sutures are placed in the uterosacral ligament pedicles on each side, with 1 arm of each suture placed in the transverse portion of the pubocervical and rectovaginal fascia.
FIGURE 5 Suspensory suture
Sagittal view of a suspensory suture in left uterosacral ligament with 1 arm through pubocervical fascia (PCF) and 1 arm through rectovaginal fascia (RVF).
FIGURE 6 The suspended vaginal vault
Sagittal view of pubocervical fascia (PCF) and rectovaginal fascia (RVF) suspended from uterosacral ligaments.
Main concern is ureteral injury
The ureter lies near the anterior margin of the uterosacral ligament, with a mean distance of 4.1±0.6 cm at the level of the sacrum and 2.3±0.9 cm at the level of the ischial spine.8 In 1 series of high uterosacral ligament suspension with site-specific endopelvic fascia defect repair, ureteral complications occurred in 11% of patients.5 Other series have reported rates of ureteral trauma in the range of 0.7%9 to 2.4%.6
Evaluate ureteral patency
After performing this procedure, the surgeon should ensure ureteral patency. For this reason, I believe that only surgeons skilled in cystoscopy and able to treat ureteral injury (or with ready access to those capable of treating this complication) should undertake high uterosacral suspension.
How the McCall procedure compares with other approaches
Cruikshank SH, Kovac SR. Randomized comparison of three surgical methods used at the time of vaginal hysterectomy to prevent posterior enterocele. Am J Obstet Gynecol. 1999;180:859–865
Although many techniques and modifications have been described for management of the cul-de-sac and vaginal cuff, few comparative data exist. In McCall’s original series,4 43 patients undergoing vaginal hysterectomy with posterior culdoplasty were followed for a minimum of 3 months and a maximum of 3 years. Mean and median lengths of follow-up were not provided, nor were the indications for hysterectomy or the method of assessing the patient for recurrent defects. McCall reported that no patients developed an enterocele after the surgery.
The first and only randomized study
Cruikshank and Kovac performed the only prospective, randomized comparison of procedures used at the time of hysterectomy to prevent enterocele. In their study, 100 patients undergoing vaginal hysterectomy for various indications (excluding prolapse of the posterior superior segment of the vagina) were randomized to 1 of 3 surgical methods to prevent enterocele:
- Moschcowitz-type closure (n=33), in which the peritoneum was closed using a purse-string technique, incorporating the distal ends of the uterosacral and cardinal ligaments and thereby drawing these structures to the midline
- modified McCall culdoplasty (n=33), in which a higher purse-string closure of the peritoneum was performed superior to the “yellow fat line,” incorporating the uterosacral-cardinal ligament complex (thus drawing these structures to the midline) and including the vagina (similar to the external McCall sutures) to move the apex superiorly
- simple closure of the peritoneum with a purse-string suture (n=34), with none of the uterosacral-cardinal ligaments incorporated into the repair.
Three years of follow-up
Of the 100 patients, 98 were followed with serial examinations for 3 years, and the outcomes at all vaginal segments were documented, with particular attention to the posterior superior segment, using staging from the Pelvic Organ Prolapse Quantification (POP-Q) system.10
McCall procedure was most effective
Overall, 11 patients (11.2%) were found to have stage II prolapse of the posterior superior vagina, none of them in the McCall group. An additional 14 patients (14.3%) had stage I prolapse, with only 2 (2%) of these in the McCall group.
The McCall repair was significantly more effective than the other 2 types of repair, with a 6.1% risk of subsequent prolapse, versus 30.3% in women who had a Moschcowitz-type closure and 39.4% in those who underwent simple closure of the peritoneum. No patients in any group had prolapse greater than stage II at follow-up.
Limitations of the study
Although Cruikshank and Kovac designed their study to analyze appropriate prophylaxis against enterocele in patients without prolapse, several patients did have some form of prolapse—although it was unrelated to the posterior superior vagina. Therefore, several patients underwent concomitant reconstructive procedures that included: anterior colporrhaphy (13), posterior colporrhaphy (4), sacrospinous ligament fixation (3), bilateral paravaginal repair (10), and anti-incontinence procedures (10).
It is not clear which groups these patients fell into and whether the distribution was similar across all groups.
CASE 2 Is McCall procedure appropriate?
B.D., 57, complains of increasing pelvic pressure and a noticeable vaginal bulge. Her 2 children were delivered vaginally, the largest weighing 8 lb. B.D. reports that she remains sexually active.
Physical examination reveals the cervix to be at the level of the introitus, but it descends 2 cm beyond the introitus when the patient performs the valsalva maneuver. Although there is also some descent of the anterior and posterior vaginal walls (1 cm superior to the hymen with strain; Pelvic Organ Prolapse Quantification [POP-Q] value=-1), the predominant component of prolapse is an elongated cervix. The posterior vaginal fornix (POP-Q point D), representing apical support, descends to 7 cm superior to the hymen with strain, with a total vaginal length of 9 cm.
At surgery, the uterosacral ligaments do not appear to be attenuated. After vaginal hysterectomy, the apex of the vaginal vault is superior to the level of the ischial spines.
How do you proceed?
Given the relatively good support at the apex, this patient is a good candidate for a McCall-type culdoplasty. Whether or not this procedure will be truly prophylactic (because there is already some descent of the apex, albeit mild) is perhaps only a matter of semantics.
The author reports no financial relationships relevant to this article.
1. Olson AL, Smith VJ, Bergstrom JO, Coiling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
2. DeLancey JOL. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717-1728.
3. Richardson AC. The anatomic defects in rectocele and enterocele. J Pelvic Surg. 1995;1:214-221.
4. McCall ML. Posterior culdoplasty: surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10:595-602.
5. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1402-1411.
6. Karram M, Goldwasser S, Kleeman S, et al. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185:1339-1342.
7. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
8. Buller JL, Thompson JR, Cundiff GW, et al. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol. 2001;97:873-879.
9. Aronson MP, Aronson PK, Howard AE, et al. Low risk of ureteral obstruction with “deep” (dorsal/posterior) uterosacral ligament suture placement for transvaginal apical suspension. Am J Obstet Gynecol. 2005;192:1530-1536.
10. Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10-17.
1. Olson AL, Smith VJ, Bergstrom JO, Coiling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501-506.
2. DeLancey JOL. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166:1717-1728.
3. Richardson AC. The anatomic defects in rectocele and enterocele. J Pelvic Surg. 1995;1:214-221.
4. McCall ML. Posterior culdoplasty: surgical correction of enterocele during vaginal hysterectomy; a preliminary report. Obstet Gynecol. 1957;10:595-602.
5. Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol. 2000;183:1402-1411.
6. Karram M, Goldwasser S, Kleeman S, et al. High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol. 2001;185:1339-1342.
7. Shull BL, Bachofen C, Coates KW, Kuehl TJ. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol. 2000;183:1365-1374.
8. Buller JL, Thompson JR, Cundiff GW, et al. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol. 2001;97:873-879.
9. Aronson MP, Aronson PK, Howard AE, et al. Low risk of ureteral obstruction with “deep” (dorsal/posterior) uterosacral ligament suture placement for transvaginal apical suspension. Am J Obstet Gynecol. 2005;192:1530-1536.
10. Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10-17.
IN THIS ARTICLE