User login
Prostate cancer drug shortage leaves some with uncertainty
according to the Food and Drug Administration.
The therapy lutetium Lu 177 vipivotide tetraxetan (Pluvicto), approved in March 2022, will remain in limited supply until the drug’s manufacturer, Novartis, can ramp up production of the drug over the next 12 months.
In a letter in February, Novartis said it is giving priority to patients who have already started the regimen so they can “appropriately complete their course of therapy.” The manufacturer will not be taking any orders for new patients over the next 4-6 months, as they work to increase supply.
“We are operating our production site at full capacity to treat as many patients as possible, as quickly as possible,” Novartis said. “However, with a nuclear medicine like Pluvicto, there is no backup supply that we can draw from when we experience a delay.”
Pluvicto is currently made in small batches in the company’s manufacturing facility in Italy. The drug only has a 5-day window to reach its intended patient, after which time it cannot be used. Any disruption in the production or shipping process can create a delay.
Novartis said the facility in Italy is currently operating at full capacity and the company is “working to increase production capacity and supply” of the drug over the next 12 months at two new manufacturing sites in the United States.
The company also encountered supply problems with Pluvicto in 2022 after quality issues were discovered in the manufacturing process.
Currently, patients who are waiting for their first dose of Pluvicto will need to be rescheduled. The manufacturer will be reaching out to health care professionals with options for rescheduling.
Jonathan McConathy, MD, PhD, told The Wall Street Journal that “people will die from this shortage, for sure.”
Dr. McConathy, a radiologist at the University of Alabama at Birmingham who has consulted for Novartis, explained that some patients who would have benefited from the drug likely won’t receive it in time.
A version of this article first appeared on Medscape.com.
according to the Food and Drug Administration.
The therapy lutetium Lu 177 vipivotide tetraxetan (Pluvicto), approved in March 2022, will remain in limited supply until the drug’s manufacturer, Novartis, can ramp up production of the drug over the next 12 months.
In a letter in February, Novartis said it is giving priority to patients who have already started the regimen so they can “appropriately complete their course of therapy.” The manufacturer will not be taking any orders for new patients over the next 4-6 months, as they work to increase supply.
“We are operating our production site at full capacity to treat as many patients as possible, as quickly as possible,” Novartis said. “However, with a nuclear medicine like Pluvicto, there is no backup supply that we can draw from when we experience a delay.”
Pluvicto is currently made in small batches in the company’s manufacturing facility in Italy. The drug only has a 5-day window to reach its intended patient, after which time it cannot be used. Any disruption in the production or shipping process can create a delay.
Novartis said the facility in Italy is currently operating at full capacity and the company is “working to increase production capacity and supply” of the drug over the next 12 months at two new manufacturing sites in the United States.
The company also encountered supply problems with Pluvicto in 2022 after quality issues were discovered in the manufacturing process.
Currently, patients who are waiting for their first dose of Pluvicto will need to be rescheduled. The manufacturer will be reaching out to health care professionals with options for rescheduling.
Jonathan McConathy, MD, PhD, told The Wall Street Journal that “people will die from this shortage, for sure.”
Dr. McConathy, a radiologist at the University of Alabama at Birmingham who has consulted for Novartis, explained that some patients who would have benefited from the drug likely won’t receive it in time.
A version of this article first appeared on Medscape.com.
according to the Food and Drug Administration.
The therapy lutetium Lu 177 vipivotide tetraxetan (Pluvicto), approved in March 2022, will remain in limited supply until the drug’s manufacturer, Novartis, can ramp up production of the drug over the next 12 months.
In a letter in February, Novartis said it is giving priority to patients who have already started the regimen so they can “appropriately complete their course of therapy.” The manufacturer will not be taking any orders for new patients over the next 4-6 months, as they work to increase supply.
“We are operating our production site at full capacity to treat as many patients as possible, as quickly as possible,” Novartis said. “However, with a nuclear medicine like Pluvicto, there is no backup supply that we can draw from when we experience a delay.”
Pluvicto is currently made in small batches in the company’s manufacturing facility in Italy. The drug only has a 5-day window to reach its intended patient, after which time it cannot be used. Any disruption in the production or shipping process can create a delay.
Novartis said the facility in Italy is currently operating at full capacity and the company is “working to increase production capacity and supply” of the drug over the next 12 months at two new manufacturing sites in the United States.
The company also encountered supply problems with Pluvicto in 2022 after quality issues were discovered in the manufacturing process.
Currently, patients who are waiting for their first dose of Pluvicto will need to be rescheduled. The manufacturer will be reaching out to health care professionals with options for rescheduling.
Jonathan McConathy, MD, PhD, told The Wall Street Journal that “people will die from this shortage, for sure.”
Dr. McConathy, a radiologist at the University of Alabama at Birmingham who has consulted for Novartis, explained that some patients who would have benefited from the drug likely won’t receive it in time.
A version of this article first appeared on Medscape.com.
Cases of potentially deadly fungus jump 200%: CDC
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
After the Match: Next steps for new residents, unmatched
Medical school graduates around the US took to social media after last week's Match Day to share their joy ― or explore their options if they did not match.
Take this post March 19 on Twitter: “I went unmatched this year; looking for research position at any institute for internal medicine.”
including an international medical graduate who matched into his chosen specialty after multiple disappointments.
“I’ve waited for this email for 8 years,” Sahil Bawa, MD, posted on Twitter on March 13. A few days later, when he learned about his residency position, he posted: “I’m beyond grateful. Will be moving to Alabama soon #familymedicine.”
Dr. Bawa, who matched into UAB Medicine Selma (Ala.), graduated from medical school in India in 2014. He said in an interview that he has visited the United States periodically since then to pass medical tests, obtain letters of recommendation, and participate in research.
Over the years he watched his Indian colleagues give up on becoming American doctors, find alternative careers, or resolve to practice in their native country. But he held onto the few success stories he saw on social media. “There were always one to two every year. It kept me going. If they can do it, I can do it.”
International medical graduates (IMGs) like Dr. Bawa applied in record numbers to Match2023, according to the National Resident Matching Program (NRMP), which announced the results on March 13 of its main residency match and the Supplemental Offer and Acceptance Program (SOAP) for unfilled positions or unmatched applicants.
Overall, 48,156 total applicants registered for the match, which was driven by the increase of non-U.S. IMG applicants and U.S. DO seniors over the past year, NRMP stated in its release. U.S. MD seniors had a match rate of nearly 94%, and U.S. DO seniors, nearly 92%. U.S. IMGs had a match rate of nearly 68%, an “all-time high,” and non-U.S. IMGs, nearly 60%, NRMP stated.
Three specialties that filled all of their 30 or more available positions were orthopedic surgery, plastic surgery (integrated), radiology – diagnostic, and thoracic surgery. Specialties with 30 or more positions that filled with the highest percentage of U.S. MD and DO seniors were plastic surgery (integrated), internal medicine-pediatrics, ob.gyn., and orthopedic surgery.
The number of available primary care positions increased slightly, NRMP reported. Considering “a serious and growing shortage of primary care physicians across the U.S.,” there were 571 more primary care positions than 2022. That’s an increase of about 3% over last year and 17% over the past 5 years. Primary care positions filled at a rate of 94%, which remained steady from 2022.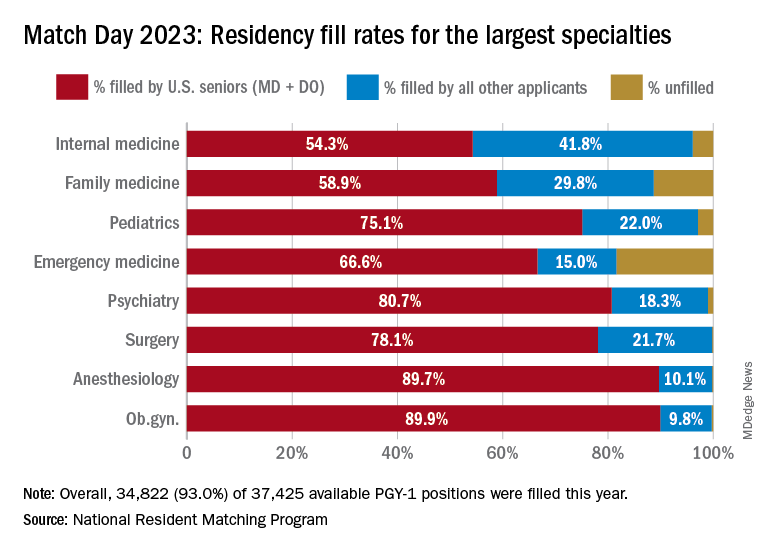
NRMP also pointed out specialties with increases in the number of positions filled by U.S. MD seniors of more than 10% and 10 positions in the past 5 years: anesthesiology, child neurology, interventional radiology, neurology, pathology, physical medicine and rehabilitation, plastic surgery (integrated), psychiatry, radiology-diagnostic, transitional year, and vascular surgery.
Bryan Carmody, MD, MPH, a pediatric nephrologist known for his medical school commentaries, said in an interview that the most competitive specialties he noted in 2023 were radiology, pathology, and neurology.
“The surgical specialties are always competitive, so it wasn’t a surprise that orthopedics, plastic surgery, and thoracic surgery filled all of their positions. But I was surprised to see diagnostic radiology fill every single one of their positions in the match. And although pathology and neurology aren’t typically considered extremely competitive specialties, they filled over 99% of their positions in the Match this year.”
On Dr. Carmody’s blog about the winners and losers of Match Day, he said that despite the record number of primary care positions offered, family medicine programs suffered. “Only 89% of family medicine programs filled in the Match, and graduating U.S. MD and DO students only filled a little more than half of all the available positions,” he wrote.
For a record number of applicants that match each year, and “the most favorable ratio in the past 2 decades” of applicants-to-positions in 2023, there are still a lot unmatched, Dr. Carmody said. “It’s a tough thing to talk about. The reality is the number of residency positions should be determined by the number of physicians needed.”
One student, Asim Ansari, didn’t match into a traditional residency or through SOAP. It was his fifth attempt. He was serving a transitional-year residency at Merit Health Wesley in Hattiesburg, Miss., and when he didn’t match, he accepted a child and adolescent psychiatry fellowship at the University of Kansas Medical Center, Kansas City.
He said he was “relieved and excited” to have found a program in his chosen specialty. Still, in 2 years, Mr. Ansari must again try to match into a traditional psychiatry residency.
Meanwhile, Dr. Bawa will prepare for his 3-year residency in Alabama after completing his interim research year in the surgery department at Wayne State University, Detroit, in May.
Despite his years in limbo, Dr. Bawa said, “I have no regrets, no complaints. I am still very happy.”
A version of this article originally appeared on Medscape.com.
Medical school graduates around the US took to social media after last week's Match Day to share their joy ― or explore their options if they did not match.
Take this post March 19 on Twitter: “I went unmatched this year; looking for research position at any institute for internal medicine.”
including an international medical graduate who matched into his chosen specialty after multiple disappointments.
“I’ve waited for this email for 8 years,” Sahil Bawa, MD, posted on Twitter on March 13. A few days later, when he learned about his residency position, he posted: “I’m beyond grateful. Will be moving to Alabama soon #familymedicine.”
Dr. Bawa, who matched into UAB Medicine Selma (Ala.), graduated from medical school in India in 2014. He said in an interview that he has visited the United States periodically since then to pass medical tests, obtain letters of recommendation, and participate in research.
Over the years he watched his Indian colleagues give up on becoming American doctors, find alternative careers, or resolve to practice in their native country. But he held onto the few success stories he saw on social media. “There were always one to two every year. It kept me going. If they can do it, I can do it.”
International medical graduates (IMGs) like Dr. Bawa applied in record numbers to Match2023, according to the National Resident Matching Program (NRMP), which announced the results on March 13 of its main residency match and the Supplemental Offer and Acceptance Program (SOAP) for unfilled positions or unmatched applicants.
Overall, 48,156 total applicants registered for the match, which was driven by the increase of non-U.S. IMG applicants and U.S. DO seniors over the past year, NRMP stated in its release. U.S. MD seniors had a match rate of nearly 94%, and U.S. DO seniors, nearly 92%. U.S. IMGs had a match rate of nearly 68%, an “all-time high,” and non-U.S. IMGs, nearly 60%, NRMP stated.
Three specialties that filled all of their 30 or more available positions were orthopedic surgery, plastic surgery (integrated), radiology – diagnostic, and thoracic surgery. Specialties with 30 or more positions that filled with the highest percentage of U.S. MD and DO seniors were plastic surgery (integrated), internal medicine-pediatrics, ob.gyn., and orthopedic surgery.
The number of available primary care positions increased slightly, NRMP reported. Considering “a serious and growing shortage of primary care physicians across the U.S.,” there were 571 more primary care positions than 2022. That’s an increase of about 3% over last year and 17% over the past 5 years. Primary care positions filled at a rate of 94%, which remained steady from 2022.
NRMP also pointed out specialties with increases in the number of positions filled by U.S. MD seniors of more than 10% and 10 positions in the past 5 years: anesthesiology, child neurology, interventional radiology, neurology, pathology, physical medicine and rehabilitation, plastic surgery (integrated), psychiatry, radiology-diagnostic, transitional year, and vascular surgery.
Bryan Carmody, MD, MPH, a pediatric nephrologist known for his medical school commentaries, said in an interview that the most competitive specialties he noted in 2023 were radiology, pathology, and neurology.
“The surgical specialties are always competitive, so it wasn’t a surprise that orthopedics, plastic surgery, and thoracic surgery filled all of their positions. But I was surprised to see diagnostic radiology fill every single one of their positions in the match. And although pathology and neurology aren’t typically considered extremely competitive specialties, they filled over 99% of their positions in the Match this year.”
On Dr. Carmody’s blog about the winners and losers of Match Day, he said that despite the record number of primary care positions offered, family medicine programs suffered. “Only 89% of family medicine programs filled in the Match, and graduating U.S. MD and DO students only filled a little more than half of all the available positions,” he wrote.
For a record number of applicants that match each year, and “the most favorable ratio in the past 2 decades” of applicants-to-positions in 2023, there are still a lot unmatched, Dr. Carmody said. “It’s a tough thing to talk about. The reality is the number of residency positions should be determined by the number of physicians needed.”
One student, Asim Ansari, didn’t match into a traditional residency or through SOAP. It was his fifth attempt. He was serving a transitional-year residency at Merit Health Wesley in Hattiesburg, Miss., and when he didn’t match, he accepted a child and adolescent psychiatry fellowship at the University of Kansas Medical Center, Kansas City.
He said he was “relieved and excited” to have found a program in his chosen specialty. Still, in 2 years, Mr. Ansari must again try to match into a traditional psychiatry residency.
Meanwhile, Dr. Bawa will prepare for his 3-year residency in Alabama after completing his interim research year in the surgery department at Wayne State University, Detroit, in May.
Despite his years in limbo, Dr. Bawa said, “I have no regrets, no complaints. I am still very happy.”
A version of this article originally appeared on Medscape.com.
Medical school graduates around the US took to social media after last week's Match Day to share their joy ― or explore their options if they did not match.
Take this post March 19 on Twitter: “I went unmatched this year; looking for research position at any institute for internal medicine.”
including an international medical graduate who matched into his chosen specialty after multiple disappointments.
“I’ve waited for this email for 8 years,” Sahil Bawa, MD, posted on Twitter on March 13. A few days later, when he learned about his residency position, he posted: “I’m beyond grateful. Will be moving to Alabama soon #familymedicine.”
Dr. Bawa, who matched into UAB Medicine Selma (Ala.), graduated from medical school in India in 2014. He said in an interview that he has visited the United States periodically since then to pass medical tests, obtain letters of recommendation, and participate in research.
Over the years he watched his Indian colleagues give up on becoming American doctors, find alternative careers, or resolve to practice in their native country. But he held onto the few success stories he saw on social media. “There were always one to two every year. It kept me going. If they can do it, I can do it.”
International medical graduates (IMGs) like Dr. Bawa applied in record numbers to Match2023, according to the National Resident Matching Program (NRMP), which announced the results on March 13 of its main residency match and the Supplemental Offer and Acceptance Program (SOAP) for unfilled positions or unmatched applicants.
Overall, 48,156 total applicants registered for the match, which was driven by the increase of non-U.S. IMG applicants and U.S. DO seniors over the past year, NRMP stated in its release. U.S. MD seniors had a match rate of nearly 94%, and U.S. DO seniors, nearly 92%. U.S. IMGs had a match rate of nearly 68%, an “all-time high,” and non-U.S. IMGs, nearly 60%, NRMP stated.
Three specialties that filled all of their 30 or more available positions were orthopedic surgery, plastic surgery (integrated), radiology – diagnostic, and thoracic surgery. Specialties with 30 or more positions that filled with the highest percentage of U.S. MD and DO seniors were plastic surgery (integrated), internal medicine-pediatrics, ob.gyn., and orthopedic surgery.
The number of available primary care positions increased slightly, NRMP reported. Considering “a serious and growing shortage of primary care physicians across the U.S.,” there were 571 more primary care positions than 2022. That’s an increase of about 3% over last year and 17% over the past 5 years. Primary care positions filled at a rate of 94%, which remained steady from 2022.
NRMP also pointed out specialties with increases in the number of positions filled by U.S. MD seniors of more than 10% and 10 positions in the past 5 years: anesthesiology, child neurology, interventional radiology, neurology, pathology, physical medicine and rehabilitation, plastic surgery (integrated), psychiatry, radiology-diagnostic, transitional year, and vascular surgery.
Bryan Carmody, MD, MPH, a pediatric nephrologist known for his medical school commentaries, said in an interview that the most competitive specialties he noted in 2023 were radiology, pathology, and neurology.
“The surgical specialties are always competitive, so it wasn’t a surprise that orthopedics, plastic surgery, and thoracic surgery filled all of their positions. But I was surprised to see diagnostic radiology fill every single one of their positions in the match. And although pathology and neurology aren’t typically considered extremely competitive specialties, they filled over 99% of their positions in the Match this year.”
On Dr. Carmody’s blog about the winners and losers of Match Day, he said that despite the record number of primary care positions offered, family medicine programs suffered. “Only 89% of family medicine programs filled in the Match, and graduating U.S. MD and DO students only filled a little more than half of all the available positions,” he wrote.
For a record number of applicants that match each year, and “the most favorable ratio in the past 2 decades” of applicants-to-positions in 2023, there are still a lot unmatched, Dr. Carmody said. “It’s a tough thing to talk about. The reality is the number of residency positions should be determined by the number of physicians needed.”
One student, Asim Ansari, didn’t match into a traditional residency or through SOAP. It was his fifth attempt. He was serving a transitional-year residency at Merit Health Wesley in Hattiesburg, Miss., and when he didn’t match, he accepted a child and adolescent psychiatry fellowship at the University of Kansas Medical Center, Kansas City.
He said he was “relieved and excited” to have found a program in his chosen specialty. Still, in 2 years, Mr. Ansari must again try to match into a traditional psychiatry residency.
Meanwhile, Dr. Bawa will prepare for his 3-year residency in Alabama after completing his interim research year in the surgery department at Wayne State University, Detroit, in May.
Despite his years in limbo, Dr. Bawa said, “I have no regrets, no complaints. I am still very happy.”
A version of this article originally appeared on Medscape.com.
Old-school printer helps scientists quickly spot bacteria in blood
When a bacterial infection reaches the bloodstream, every second is critical. The person’s life is on the line. Yet blood tests to identify bacteria take hours to days. While waiting, doctors often prescribe broad-spectrum antibiotics in hopes of killing whatever bug may be at fault.
Someday soon, that wait time could shrink significantly, allowing health care providers to more quickly zero in on the best antibiotic for each infection – thanks to an innovation from Stanford (Calif.) University that identifies bacteria in seconds.
The cutting-edge method relies on old-school tech: an inkjet printer similar the kind you might have at home – except this one has been modified to print blood instead of ink.
The very small sample size – each drop is two trillionths of a liter, or about a billion times smaller than a raindrop – make spotting bacteria easier. Smaller samples mean fewer cells, so lab techs can more swiftly separate the bacterial spectra from other components, like red blood cells and white blood cells.
To boost efficiency even more, the researchers added gold nanoparticles, which attach to the bacteria, serving like antennas to focus the light. Machine learning – a type of artificial intelligence – helps interpret the spectrum of light and identify which fingerprint goes with which bacteria.
“It kind of wound up being this really interesting historical period where we could put the pieces together from different technologies, including nanophotonics, printing, and artificial intelligence, to help accelerate identification of bacteria in these complex samples,” says study author Jennifer Dionne, PhD, associate professor of materials science and engineering at Stanford.
Compare that to blood culture testing in hospitals, where it takes days for bacterial cells to grow and multiply inside a large machine that looks like a refrigerator. For some bacteria, like the kinds that cause tuberculosis, cultures take weeks.
Then further testing is needed to identify which antibiotics will quell the infection. The new technology from Stanford could accelerate this process, too.
“The promise of our technique is that you don’t need to have a culture of cells to put the antibiotic on top,” says Dr. Dionne. “What we’re finding is that from the Raman scattering, we can use that to identify – even without incubating with antibiotics – which drug the bacteria would respond to, and that’s really exciting.”
If patients can receive the antibiotic best suited for their infection, they will likely have better outcomes.
“Blood cultures can typically take 48-72 hours to come back, and then you base your clinical decisions and adjusting antibiotics based on those blood cultures,” says Richard Watkins, MD, an infectious disease physician and professor of medicine at the Northeastern Ohio Universities, Rootstown. Dr. Watkins was not involved in the study.
“Sometimes, despite your best guess, you’re wrong,” Dr. Watkins says, “and obviously, the patient could have an adverse outcome. So, if you can diagnose the pathogen sooner, that is ideal. Whatever technology enables clinicians to do that is definitely progress and a step forward.”
On a global scale, this technology could help reduce the overuse of broad-spectrum antibiotics, which contributes to antimicrobial resistance, an emerging health threat, says Dr. Dionne.
The team is working to develop the technology further into an instrument the size of a shoebox and, with further testing, commercialize the product. That could take a few years.
This technology has potential beyond bloodstream infections, too. It could be used to identify bacteria in other fluids, such as in wastewater or contaminated food.
A version of this article originally appeared on WebMD.com.
When a bacterial infection reaches the bloodstream, every second is critical. The person’s life is on the line. Yet blood tests to identify bacteria take hours to days. While waiting, doctors often prescribe broad-spectrum antibiotics in hopes of killing whatever bug may be at fault.
Someday soon, that wait time could shrink significantly, allowing health care providers to more quickly zero in on the best antibiotic for each infection – thanks to an innovation from Stanford (Calif.) University that identifies bacteria in seconds.
The cutting-edge method relies on old-school tech: an inkjet printer similar the kind you might have at home – except this one has been modified to print blood instead of ink.
The very small sample size – each drop is two trillionths of a liter, or about a billion times smaller than a raindrop – make spotting bacteria easier. Smaller samples mean fewer cells, so lab techs can more swiftly separate the bacterial spectra from other components, like red blood cells and white blood cells.
To boost efficiency even more, the researchers added gold nanoparticles, which attach to the bacteria, serving like antennas to focus the light. Machine learning – a type of artificial intelligence – helps interpret the spectrum of light and identify which fingerprint goes with which bacteria.
“It kind of wound up being this really interesting historical period where we could put the pieces together from different technologies, including nanophotonics, printing, and artificial intelligence, to help accelerate identification of bacteria in these complex samples,” says study author Jennifer Dionne, PhD, associate professor of materials science and engineering at Stanford.
Compare that to blood culture testing in hospitals, where it takes days for bacterial cells to grow and multiply inside a large machine that looks like a refrigerator. For some bacteria, like the kinds that cause tuberculosis, cultures take weeks.
Then further testing is needed to identify which antibiotics will quell the infection. The new technology from Stanford could accelerate this process, too.
“The promise of our technique is that you don’t need to have a culture of cells to put the antibiotic on top,” says Dr. Dionne. “What we’re finding is that from the Raman scattering, we can use that to identify – even without incubating with antibiotics – which drug the bacteria would respond to, and that’s really exciting.”
If patients can receive the antibiotic best suited for their infection, they will likely have better outcomes.
“Blood cultures can typically take 48-72 hours to come back, and then you base your clinical decisions and adjusting antibiotics based on those blood cultures,” says Richard Watkins, MD, an infectious disease physician and professor of medicine at the Northeastern Ohio Universities, Rootstown. Dr. Watkins was not involved in the study.
“Sometimes, despite your best guess, you’re wrong,” Dr. Watkins says, “and obviously, the patient could have an adverse outcome. So, if you can diagnose the pathogen sooner, that is ideal. Whatever technology enables clinicians to do that is definitely progress and a step forward.”
On a global scale, this technology could help reduce the overuse of broad-spectrum antibiotics, which contributes to antimicrobial resistance, an emerging health threat, says Dr. Dionne.
The team is working to develop the technology further into an instrument the size of a shoebox and, with further testing, commercialize the product. That could take a few years.
This technology has potential beyond bloodstream infections, too. It could be used to identify bacteria in other fluids, such as in wastewater or contaminated food.
A version of this article originally appeared on WebMD.com.
When a bacterial infection reaches the bloodstream, every second is critical. The person’s life is on the line. Yet blood tests to identify bacteria take hours to days. While waiting, doctors often prescribe broad-spectrum antibiotics in hopes of killing whatever bug may be at fault.
Someday soon, that wait time could shrink significantly, allowing health care providers to more quickly zero in on the best antibiotic for each infection – thanks to an innovation from Stanford (Calif.) University that identifies bacteria in seconds.
The cutting-edge method relies on old-school tech: an inkjet printer similar the kind you might have at home – except this one has been modified to print blood instead of ink.
The very small sample size – each drop is two trillionths of a liter, or about a billion times smaller than a raindrop – make spotting bacteria easier. Smaller samples mean fewer cells, so lab techs can more swiftly separate the bacterial spectra from other components, like red blood cells and white blood cells.
To boost efficiency even more, the researchers added gold nanoparticles, which attach to the bacteria, serving like antennas to focus the light. Machine learning – a type of artificial intelligence – helps interpret the spectrum of light and identify which fingerprint goes with which bacteria.
“It kind of wound up being this really interesting historical period where we could put the pieces together from different technologies, including nanophotonics, printing, and artificial intelligence, to help accelerate identification of bacteria in these complex samples,” says study author Jennifer Dionne, PhD, associate professor of materials science and engineering at Stanford.
Compare that to blood culture testing in hospitals, where it takes days for bacterial cells to grow and multiply inside a large machine that looks like a refrigerator. For some bacteria, like the kinds that cause tuberculosis, cultures take weeks.
Then further testing is needed to identify which antibiotics will quell the infection. The new technology from Stanford could accelerate this process, too.
“The promise of our technique is that you don’t need to have a culture of cells to put the antibiotic on top,” says Dr. Dionne. “What we’re finding is that from the Raman scattering, we can use that to identify – even without incubating with antibiotics – which drug the bacteria would respond to, and that’s really exciting.”
If patients can receive the antibiotic best suited for their infection, they will likely have better outcomes.
“Blood cultures can typically take 48-72 hours to come back, and then you base your clinical decisions and adjusting antibiotics based on those blood cultures,” says Richard Watkins, MD, an infectious disease physician and professor of medicine at the Northeastern Ohio Universities, Rootstown. Dr. Watkins was not involved in the study.
“Sometimes, despite your best guess, you’re wrong,” Dr. Watkins says, “and obviously, the patient could have an adverse outcome. So, if you can diagnose the pathogen sooner, that is ideal. Whatever technology enables clinicians to do that is definitely progress and a step forward.”
On a global scale, this technology could help reduce the overuse of broad-spectrum antibiotics, which contributes to antimicrobial resistance, an emerging health threat, says Dr. Dionne.
The team is working to develop the technology further into an instrument the size of a shoebox and, with further testing, commercialize the product. That could take a few years.
This technology has potential beyond bloodstream infections, too. It could be used to identify bacteria in other fluids, such as in wastewater or contaminated food.
A version of this article originally appeared on WebMD.com.
State medical board chair steps down amid Medicaid fraud accusations
He has stepped down as board chair, and state officials have suspended all Medicaid payments to Dr. Hyatt and his practice, Pinnacle Premier Psychiatry in Rogers, Arkansas.
Dr. Hyatt billed 99.95% of the claims for his patients’ hospital care to Medicaid at the highest severity level, according to an affidavit filed by an investigator with the Medicaid Fraud Control Unit, Arkansas Attorney General’s Office. Other Arkansas psychiatrists billed that same level in only about 39% of claims, the affidavit states.
The possible upcoding alleged in the affidavit was a red flag that prompted the state to temporarily suspend Dr. Hyatt’s Medicaid payments.
Dr. Hyatt has until this Friday to file an appeal. He did not respond to requests from this news organization for comment.
The affidavit pointed to other concerns. For example, a whistleblower who worked at the Northwest Medical Center where Dr. Hyatt admitted patients claimed that Dr. Hyatt was only on the floor a few minutes a day and that he had no contact with patients. A review of hundreds of hours of video by state investigators revealed that Dr. Hyatt did not enter patients’ rooms, nor did he have any contact with patients, according to the affidavit. Dr. Hyatt served as the hospital’s behavioral unit director from 2018 until his contract was abruptly terminated in May 2022, according to the affidavit.
However, Dr. Hyatt claimed to have conducted daily face-to-face evaluation and management with patients, according to the affidavit. In addition, the whistleblower claimed that Dr. Hyatt did not want patients to know his name and instructed staff to cover up his name on patient armbands.
Detaining patients
Dr. Hyatt also faces accusations that he held patients against their will, according to civil lawsuits filed in Washington County, Ark., reports the Arkansas Advocate.
Karla Adrian-Caceres filed suit on Jan. 17. Ms. Adrian-Caceres also named Brooke Green, Northwest Arkansas Hospitals, and 25 unidentified hospital employees as defendants.
According to the complaint, Ms. Adrian-Caceres, an engineering student at the University of Arkansas, arrived at the Northwest Medical Emergency Department after accidentally taking too many Tylenol on Jan. 18, 2022. She was then taken by ambulance to a Northwest psychiatric facility in Springdale, court records show.
According to the complaint, Ms. Adrian-Caceres said that she was given a sedative and asked to sign consent for admission while on the way to Northwest. She said that she “signed some documents without being able to read or understand them at the time.”
When she asked when she could go home, Ms. Adrian-Caceres said, “more than one employee told her there was a minimum stay and that if she asked to leave, they would take her to court where a judge would give her a longer stay because the judge always sides with Dr. Hyatt and Northwest,” according to court documents. Northwest employees stripped Ms. Adrian-Caceres, searched her body, took all of her possessions from her and issued underwear and a uniform, according to the lawsuit.
Ms. Adrian-Caceres’ mother, Katty Caceres, claimed in the lawsuit that she was prohibited from seeing her daughter. Ms. Caceres spoke with five different employees, four of whom had only their first names on their badges. Each of them reportedly said that they could not help, or that the plaintiff “would be in there for some time” and that it was Dr. Hyatt’s decision regarding how long that would be, according to court documents.
Katty Caceres hired a local attorney named Aaron Cash to represent her daughter. On Jan. 20, 2022, Mr. Cash faxed a letter to the hospital demanding her release. When Ms. Caceres arrived to pick up her daughter, she claimed that staff members indicated that the daughter was there voluntarily and refused to release her “at the direction of Dr Hyatt.” During a phone call later that day, the plaintiff told her mother that her status was being changed to an involuntary hold, court documents show.
“At one point she was threatened with the longer time in there if she kept asking to leave,” Mr. Cash told this news organization. In addition, staff members reportedly told Ms. Adrian-Caceres that the “judge always sided with Dr Hyatt” and she “would get way longer there, 30-45 days if [she] went before the judge,” according to Mr. Cash.
Mr. Cash said nine other patients have contacted his firm with similar allegations against Dr. Hyatt.
“We’ve talked to many people that have experienced the same threats,” Mr. Cash said. “When they’re asking to leave, they get these threats, they get coerced … and they’re never taken to court. They’re never given opportunity to talk to a judge or to have a public defender appointed.”
A version of this article first appeared on Medscape.com.
He has stepped down as board chair, and state officials have suspended all Medicaid payments to Dr. Hyatt and his practice, Pinnacle Premier Psychiatry in Rogers, Arkansas.
Dr. Hyatt billed 99.95% of the claims for his patients’ hospital care to Medicaid at the highest severity level, according to an affidavit filed by an investigator with the Medicaid Fraud Control Unit, Arkansas Attorney General’s Office. Other Arkansas psychiatrists billed that same level in only about 39% of claims, the affidavit states.
The possible upcoding alleged in the affidavit was a red flag that prompted the state to temporarily suspend Dr. Hyatt’s Medicaid payments.
Dr. Hyatt has until this Friday to file an appeal. He did not respond to requests from this news organization for comment.
The affidavit pointed to other concerns. For example, a whistleblower who worked at the Northwest Medical Center where Dr. Hyatt admitted patients claimed that Dr. Hyatt was only on the floor a few minutes a day and that he had no contact with patients. A review of hundreds of hours of video by state investigators revealed that Dr. Hyatt did not enter patients’ rooms, nor did he have any contact with patients, according to the affidavit. Dr. Hyatt served as the hospital’s behavioral unit director from 2018 until his contract was abruptly terminated in May 2022, according to the affidavit.
However, Dr. Hyatt claimed to have conducted daily face-to-face evaluation and management with patients, according to the affidavit. In addition, the whistleblower claimed that Dr. Hyatt did not want patients to know his name and instructed staff to cover up his name on patient armbands.
Detaining patients
Dr. Hyatt also faces accusations that he held patients against their will, according to civil lawsuits filed in Washington County, Ark., reports the Arkansas Advocate.
Karla Adrian-Caceres filed suit on Jan. 17. Ms. Adrian-Caceres also named Brooke Green, Northwest Arkansas Hospitals, and 25 unidentified hospital employees as defendants.
According to the complaint, Ms. Adrian-Caceres, an engineering student at the University of Arkansas, arrived at the Northwest Medical Emergency Department after accidentally taking too many Tylenol on Jan. 18, 2022. She was then taken by ambulance to a Northwest psychiatric facility in Springdale, court records show.
According to the complaint, Ms. Adrian-Caceres said that she was given a sedative and asked to sign consent for admission while on the way to Northwest. She said that she “signed some documents without being able to read or understand them at the time.”
When she asked when she could go home, Ms. Adrian-Caceres said, “more than one employee told her there was a minimum stay and that if she asked to leave, they would take her to court where a judge would give her a longer stay because the judge always sides with Dr. Hyatt and Northwest,” according to court documents. Northwest employees stripped Ms. Adrian-Caceres, searched her body, took all of her possessions from her and issued underwear and a uniform, according to the lawsuit.
Ms. Adrian-Caceres’ mother, Katty Caceres, claimed in the lawsuit that she was prohibited from seeing her daughter. Ms. Caceres spoke with five different employees, four of whom had only their first names on their badges. Each of them reportedly said that they could not help, or that the plaintiff “would be in there for some time” and that it was Dr. Hyatt’s decision regarding how long that would be, according to court documents.
Katty Caceres hired a local attorney named Aaron Cash to represent her daughter. On Jan. 20, 2022, Mr. Cash faxed a letter to the hospital demanding her release. When Ms. Caceres arrived to pick up her daughter, she claimed that staff members indicated that the daughter was there voluntarily and refused to release her “at the direction of Dr Hyatt.” During a phone call later that day, the plaintiff told her mother that her status was being changed to an involuntary hold, court documents show.
“At one point she was threatened with the longer time in there if she kept asking to leave,” Mr. Cash told this news organization. In addition, staff members reportedly told Ms. Adrian-Caceres that the “judge always sided with Dr Hyatt” and she “would get way longer there, 30-45 days if [she] went before the judge,” according to Mr. Cash.
Mr. Cash said nine other patients have contacted his firm with similar allegations against Dr. Hyatt.
“We’ve talked to many people that have experienced the same threats,” Mr. Cash said. “When they’re asking to leave, they get these threats, they get coerced … and they’re never taken to court. They’re never given opportunity to talk to a judge or to have a public defender appointed.”
A version of this article first appeared on Medscape.com.
He has stepped down as board chair, and state officials have suspended all Medicaid payments to Dr. Hyatt and his practice, Pinnacle Premier Psychiatry in Rogers, Arkansas.
Dr. Hyatt billed 99.95% of the claims for his patients’ hospital care to Medicaid at the highest severity level, according to an affidavit filed by an investigator with the Medicaid Fraud Control Unit, Arkansas Attorney General’s Office. Other Arkansas psychiatrists billed that same level in only about 39% of claims, the affidavit states.
The possible upcoding alleged in the affidavit was a red flag that prompted the state to temporarily suspend Dr. Hyatt’s Medicaid payments.
Dr. Hyatt has until this Friday to file an appeal. He did not respond to requests from this news organization for comment.
The affidavit pointed to other concerns. For example, a whistleblower who worked at the Northwest Medical Center where Dr. Hyatt admitted patients claimed that Dr. Hyatt was only on the floor a few minutes a day and that he had no contact with patients. A review of hundreds of hours of video by state investigators revealed that Dr. Hyatt did not enter patients’ rooms, nor did he have any contact with patients, according to the affidavit. Dr. Hyatt served as the hospital’s behavioral unit director from 2018 until his contract was abruptly terminated in May 2022, according to the affidavit.
However, Dr. Hyatt claimed to have conducted daily face-to-face evaluation and management with patients, according to the affidavit. In addition, the whistleblower claimed that Dr. Hyatt did not want patients to know his name and instructed staff to cover up his name on patient armbands.
Detaining patients
Dr. Hyatt also faces accusations that he held patients against their will, according to civil lawsuits filed in Washington County, Ark., reports the Arkansas Advocate.
Karla Adrian-Caceres filed suit on Jan. 17. Ms. Adrian-Caceres also named Brooke Green, Northwest Arkansas Hospitals, and 25 unidentified hospital employees as defendants.
According to the complaint, Ms. Adrian-Caceres, an engineering student at the University of Arkansas, arrived at the Northwest Medical Emergency Department after accidentally taking too many Tylenol on Jan. 18, 2022. She was then taken by ambulance to a Northwest psychiatric facility in Springdale, court records show.
According to the complaint, Ms. Adrian-Caceres said that she was given a sedative and asked to sign consent for admission while on the way to Northwest. She said that she “signed some documents without being able to read or understand them at the time.”
When she asked when she could go home, Ms. Adrian-Caceres said, “more than one employee told her there was a minimum stay and that if she asked to leave, they would take her to court where a judge would give her a longer stay because the judge always sides with Dr. Hyatt and Northwest,” according to court documents. Northwest employees stripped Ms. Adrian-Caceres, searched her body, took all of her possessions from her and issued underwear and a uniform, according to the lawsuit.
Ms. Adrian-Caceres’ mother, Katty Caceres, claimed in the lawsuit that she was prohibited from seeing her daughter. Ms. Caceres spoke with five different employees, four of whom had only their first names on their badges. Each of them reportedly said that they could not help, or that the plaintiff “would be in there for some time” and that it was Dr. Hyatt’s decision regarding how long that would be, according to court documents.
Katty Caceres hired a local attorney named Aaron Cash to represent her daughter. On Jan. 20, 2022, Mr. Cash faxed a letter to the hospital demanding her release. When Ms. Caceres arrived to pick up her daughter, she claimed that staff members indicated that the daughter was there voluntarily and refused to release her “at the direction of Dr Hyatt.” During a phone call later that day, the plaintiff told her mother that her status was being changed to an involuntary hold, court documents show.
“At one point she was threatened with the longer time in there if she kept asking to leave,” Mr. Cash told this news organization. In addition, staff members reportedly told Ms. Adrian-Caceres that the “judge always sided with Dr Hyatt” and she “would get way longer there, 30-45 days if [she] went before the judge,” according to Mr. Cash.
Mr. Cash said nine other patients have contacted his firm with similar allegations against Dr. Hyatt.
“We’ve talked to many people that have experienced the same threats,” Mr. Cash said. “When they’re asking to leave, they get these threats, they get coerced … and they’re never taken to court. They’re never given opportunity to talk to a judge or to have a public defender appointed.”
A version of this article first appeared on Medscape.com.
Liquid albuterol shortage effects reduced by alternative drugs, similar shortages may be increasingly common
The shortage of 0.5% albuterol sulfate inhalation solution, first reported by the FDA last October, gained increasing attention earlier this month when Akorn Pharmaceuticals – one of just two companies making the product – shut down after years of financial and regulatory troubles.
The other manufacturer, Nephron Pharmaceuticals, is producing 0.5% albuterol “as fast as possible” to overcome the shortage, CEO Lou Kennedy said in a written comment.
Meanwhile, the more commonly used version of liquid albuterol, with a concentration of 0.083%, remains in “good supply from several manufacturers,” according to an FDA spokesperson.
Still, headlines concerning the shortage have caused “a bit of a panic” for patients with asthma and parents with asthmatic children, according to David R. Stukus, MD, professor of clinical pediatrics in the division of allergy and immunology at Nationwide Children’s, Columbus, Ohio.
Much of the media coverage has lacked context, causing unnecessary worry, he said, as the shortage only affects one type of albuterol generally reserved for inpatient and emergency use.
“The shortage has not impacted our albuterol inhalers thus far,” Dr. Stukus said in an interview. “So I certainly don’t want people with asthma to panic that they’re going to run out of their inhaler anytime soon.”
Even infants and toddlers can use inhalers
Although Dr. Stukus noted that certain patients do require nebulizers, such as those with conditions that physically limit their breathing, like muscular dystrophy, most patients can use inhalers just fine. He said it’s a “pretty common misconception, even among medical professionals,” that infants and toddlers need nebulizers instead.
“In our institution, for example, we rarely ever start babies on a nebulizer when we diagnose them with asthma,” Dr. Stukus said. “We often just start right away with an inhaler with a spacer and a face mask.”
The shortage of liquid albuterol may therefore have a silver lining, he suggested, as it prompts clinicians to reconsider their routine practice.
“When situations like this arise, it’s a great opportunity for all of us to just take a step back and reevaluate the way we do things,” Dr. Stukus said. “Sometimes we just get caught up with inertia and we continue to do things the same way even though new options are available, or evidence has changed to the contrary.”
Nathan Rabinovitch, MD, professor of pediatrics in the division of pediatric allergy and clinical immunology at National Jewish Health, Denver, said that his center had trouble obtaining liquid albuterol about 2 weeks ago, so they pivoted to the more expensive levalbuterol for about a week and a half, until their albuterol supply was restored.
While Dr. Rabinovitch agreed that most children don’t need a nebulizer, he said about 5%-10% of kids with severe asthma should have one on hand in case their inhaler fails to control an exacerbation.
Personal preferences may also considered, he added.
“If [a parent] says, ‘I like to use the nebulizer. The kid likes it,’ I’m fine if they just use a nebulizer.”
One possible downside of relying on a nebulizer, however, is portability, according to Kelly O’Shea, MD, assistant professor in the division of allergy and clinical immunology at the University of Michigan, Ann Arbor.
“If you’re out at the park or out at a soccer game with your kids, and they are having trouble breathing ... and they need their albuterol, you don’t have that ability if you are tied to a nebulizer,” Dr. O’Shea said in an interview. “As long as a parent feels comfortable – they feel like [their child] can get deep breaths in, I agree that you can use [an inhaler] in the infant and toddler population.”
She also agreed that a nebulizer may serve as a kind of second step if an inhaler isn’t controlling an exacerbation; however, she emphasized that a nebulizer should not be considered a replacement for professional care, and should not give a false sense of security.
“I caution parents to make sure that when they need it, they also take the next step and head over to the emergency room,” Dr. O’Shea said.
Generic drug shortages becoming more common
While the present scarcity of liquid albuterol appears relatively mild in terms of clinical impact, it brings up broader concerns about generic drug supply, and why shortages like this are becoming more common, according to Katie J. Suda, PharmD, MS, professor of medicine and pharmacy, and associate director, center for pharmaceutical policy and prescribing at the University of Pittsburgh.
“Drug shortages continue to increase in frequency, and the duration and severity of the shortages are also getting worse,” Dr. Suda said in an interview.
The reasons for these shortages can be elusive, according to 2022 report by the American Society of Health-System Pharmacists, which found that more than half of shortages came with no explanation from manufacturers.
The same report showed that only 5% of shortages were due to a “business decision,” but this factor is likely more central than publicly stated.
A recent FDA analysis on drug shortages, for instance, lists “lack of incentives to produce less profitable drugs,” as the first “root cause,” and Dr. Suda agrees.
“It’s important that we have generic medicines to decrease costs to our health systems, as well as for our patients,” Dr. Suda said. “But frequently, with those generic products, the price is driven so low that it increases the risk of a shortage.”
The drive to maintain profit margins may motivate companies to cut corners in production, Dr. Suda explained. She emphasized that this connection is speculative, because motivations are effectively unknowable, but the rationale is supported by past and present shortages.
Akorn Pharmaceuticals, for example, received a warning letter from the FDA in 2019 because of a variety of manufacturing issues, including defective bottles, questionable data, and metal shavings on aseptic filling equipment.
When a manufacturer like Akorn fails, the effects can be far-reaching, Dr. Suda said, noting their broad catalog of agents. Beyond liquid albuterol, Akorn was producing cardiac drugs, antibiotics, vitamins, local anesthetics, eye products, and others.
Drug shortages cause “a significant strain on our health care system,” Dr. Suda said, and substituting other medications increases risk of medical errors.
Fortunately, the increasing number of drug shortages is not going unnoticed, according to Dr. Suda. The FDA and multiple other organizations, including the ASHP, American Medical Association, and National Academies of Sciences, Engineering, and Medicine, are all taking steps to ensure that essential medicines are in steady supply, including moves to gather more data from manufacturers.
“I hope that a lot of the efforts that are moving forward ... will help us decrease the impact of shortages on our patients,” Dr. Suda said.
Lou Kennedy is the CEO of Nephron Pharmaceuticals, which commercially produces liquid albuterol. The other interviewees disclosed no relevant conflicts of interest.
The shortage of 0.5% albuterol sulfate inhalation solution, first reported by the FDA last October, gained increasing attention earlier this month when Akorn Pharmaceuticals – one of just two companies making the product – shut down after years of financial and regulatory troubles.
The other manufacturer, Nephron Pharmaceuticals, is producing 0.5% albuterol “as fast as possible” to overcome the shortage, CEO Lou Kennedy said in a written comment.
Meanwhile, the more commonly used version of liquid albuterol, with a concentration of 0.083%, remains in “good supply from several manufacturers,” according to an FDA spokesperson.
Still, headlines concerning the shortage have caused “a bit of a panic” for patients with asthma and parents with asthmatic children, according to David R. Stukus, MD, professor of clinical pediatrics in the division of allergy and immunology at Nationwide Children’s, Columbus, Ohio.
Much of the media coverage has lacked context, causing unnecessary worry, he said, as the shortage only affects one type of albuterol generally reserved for inpatient and emergency use.
“The shortage has not impacted our albuterol inhalers thus far,” Dr. Stukus said in an interview. “So I certainly don’t want people with asthma to panic that they’re going to run out of their inhaler anytime soon.”
Even infants and toddlers can use inhalers
Although Dr. Stukus noted that certain patients do require nebulizers, such as those with conditions that physically limit their breathing, like muscular dystrophy, most patients can use inhalers just fine. He said it’s a “pretty common misconception, even among medical professionals,” that infants and toddlers need nebulizers instead.
“In our institution, for example, we rarely ever start babies on a nebulizer when we diagnose them with asthma,” Dr. Stukus said. “We often just start right away with an inhaler with a spacer and a face mask.”
The shortage of liquid albuterol may therefore have a silver lining, he suggested, as it prompts clinicians to reconsider their routine practice.
“When situations like this arise, it’s a great opportunity for all of us to just take a step back and reevaluate the way we do things,” Dr. Stukus said. “Sometimes we just get caught up with inertia and we continue to do things the same way even though new options are available, or evidence has changed to the contrary.”
Nathan Rabinovitch, MD, professor of pediatrics in the division of pediatric allergy and clinical immunology at National Jewish Health, Denver, said that his center had trouble obtaining liquid albuterol about 2 weeks ago, so they pivoted to the more expensive levalbuterol for about a week and a half, until their albuterol supply was restored.
While Dr. Rabinovitch agreed that most children don’t need a nebulizer, he said about 5%-10% of kids with severe asthma should have one on hand in case their inhaler fails to control an exacerbation.
Personal preferences may also considered, he added.
“If [a parent] says, ‘I like to use the nebulizer. The kid likes it,’ I’m fine if they just use a nebulizer.”
One possible downside of relying on a nebulizer, however, is portability, according to Kelly O’Shea, MD, assistant professor in the division of allergy and clinical immunology at the University of Michigan, Ann Arbor.
“If you’re out at the park or out at a soccer game with your kids, and they are having trouble breathing ... and they need their albuterol, you don’t have that ability if you are tied to a nebulizer,” Dr. O’Shea said in an interview. “As long as a parent feels comfortable – they feel like [their child] can get deep breaths in, I agree that you can use [an inhaler] in the infant and toddler population.”
She also agreed that a nebulizer may serve as a kind of second step if an inhaler isn’t controlling an exacerbation; however, she emphasized that a nebulizer should not be considered a replacement for professional care, and should not give a false sense of security.
“I caution parents to make sure that when they need it, they also take the next step and head over to the emergency room,” Dr. O’Shea said.
Generic drug shortages becoming more common
While the present scarcity of liquid albuterol appears relatively mild in terms of clinical impact, it brings up broader concerns about generic drug supply, and why shortages like this are becoming more common, according to Katie J. Suda, PharmD, MS, professor of medicine and pharmacy, and associate director, center for pharmaceutical policy and prescribing at the University of Pittsburgh.
“Drug shortages continue to increase in frequency, and the duration and severity of the shortages are also getting worse,” Dr. Suda said in an interview.
The reasons for these shortages can be elusive, according to 2022 report by the American Society of Health-System Pharmacists, which found that more than half of shortages came with no explanation from manufacturers.
The same report showed that only 5% of shortages were due to a “business decision,” but this factor is likely more central than publicly stated.
A recent FDA analysis on drug shortages, for instance, lists “lack of incentives to produce less profitable drugs,” as the first “root cause,” and Dr. Suda agrees.
“It’s important that we have generic medicines to decrease costs to our health systems, as well as for our patients,” Dr. Suda said. “But frequently, with those generic products, the price is driven so low that it increases the risk of a shortage.”
The drive to maintain profit margins may motivate companies to cut corners in production, Dr. Suda explained. She emphasized that this connection is speculative, because motivations are effectively unknowable, but the rationale is supported by past and present shortages.
Akorn Pharmaceuticals, for example, received a warning letter from the FDA in 2019 because of a variety of manufacturing issues, including defective bottles, questionable data, and metal shavings on aseptic filling equipment.
When a manufacturer like Akorn fails, the effects can be far-reaching, Dr. Suda said, noting their broad catalog of agents. Beyond liquid albuterol, Akorn was producing cardiac drugs, antibiotics, vitamins, local anesthetics, eye products, and others.
Drug shortages cause “a significant strain on our health care system,” Dr. Suda said, and substituting other medications increases risk of medical errors.
Fortunately, the increasing number of drug shortages is not going unnoticed, according to Dr. Suda. The FDA and multiple other organizations, including the ASHP, American Medical Association, and National Academies of Sciences, Engineering, and Medicine, are all taking steps to ensure that essential medicines are in steady supply, including moves to gather more data from manufacturers.
“I hope that a lot of the efforts that are moving forward ... will help us decrease the impact of shortages on our patients,” Dr. Suda said.
Lou Kennedy is the CEO of Nephron Pharmaceuticals, which commercially produces liquid albuterol. The other interviewees disclosed no relevant conflicts of interest.
The shortage of 0.5% albuterol sulfate inhalation solution, first reported by the FDA last October, gained increasing attention earlier this month when Akorn Pharmaceuticals – one of just two companies making the product – shut down after years of financial and regulatory troubles.
The other manufacturer, Nephron Pharmaceuticals, is producing 0.5% albuterol “as fast as possible” to overcome the shortage, CEO Lou Kennedy said in a written comment.
Meanwhile, the more commonly used version of liquid albuterol, with a concentration of 0.083%, remains in “good supply from several manufacturers,” according to an FDA spokesperson.
Still, headlines concerning the shortage have caused “a bit of a panic” for patients with asthma and parents with asthmatic children, according to David R. Stukus, MD, professor of clinical pediatrics in the division of allergy and immunology at Nationwide Children’s, Columbus, Ohio.
Much of the media coverage has lacked context, causing unnecessary worry, he said, as the shortage only affects one type of albuterol generally reserved for inpatient and emergency use.
“The shortage has not impacted our albuterol inhalers thus far,” Dr. Stukus said in an interview. “So I certainly don’t want people with asthma to panic that they’re going to run out of their inhaler anytime soon.”
Even infants and toddlers can use inhalers
Although Dr. Stukus noted that certain patients do require nebulizers, such as those with conditions that physically limit their breathing, like muscular dystrophy, most patients can use inhalers just fine. He said it’s a “pretty common misconception, even among medical professionals,” that infants and toddlers need nebulizers instead.
“In our institution, for example, we rarely ever start babies on a nebulizer when we diagnose them with asthma,” Dr. Stukus said. “We often just start right away with an inhaler with a spacer and a face mask.”
The shortage of liquid albuterol may therefore have a silver lining, he suggested, as it prompts clinicians to reconsider their routine practice.
“When situations like this arise, it’s a great opportunity for all of us to just take a step back and reevaluate the way we do things,” Dr. Stukus said. “Sometimes we just get caught up with inertia and we continue to do things the same way even though new options are available, or evidence has changed to the contrary.”
Nathan Rabinovitch, MD, professor of pediatrics in the division of pediatric allergy and clinical immunology at National Jewish Health, Denver, said that his center had trouble obtaining liquid albuterol about 2 weeks ago, so they pivoted to the more expensive levalbuterol for about a week and a half, until their albuterol supply was restored.
While Dr. Rabinovitch agreed that most children don’t need a nebulizer, he said about 5%-10% of kids with severe asthma should have one on hand in case their inhaler fails to control an exacerbation.
Personal preferences may also considered, he added.
“If [a parent] says, ‘I like to use the nebulizer. The kid likes it,’ I’m fine if they just use a nebulizer.”
One possible downside of relying on a nebulizer, however, is portability, according to Kelly O’Shea, MD, assistant professor in the division of allergy and clinical immunology at the University of Michigan, Ann Arbor.
“If you’re out at the park or out at a soccer game with your kids, and they are having trouble breathing ... and they need their albuterol, you don’t have that ability if you are tied to a nebulizer,” Dr. O’Shea said in an interview. “As long as a parent feels comfortable – they feel like [their child] can get deep breaths in, I agree that you can use [an inhaler] in the infant and toddler population.”
She also agreed that a nebulizer may serve as a kind of second step if an inhaler isn’t controlling an exacerbation; however, she emphasized that a nebulizer should not be considered a replacement for professional care, and should not give a false sense of security.
“I caution parents to make sure that when they need it, they also take the next step and head over to the emergency room,” Dr. O’Shea said.
Generic drug shortages becoming more common
While the present scarcity of liquid albuterol appears relatively mild in terms of clinical impact, it brings up broader concerns about generic drug supply, and why shortages like this are becoming more common, according to Katie J. Suda, PharmD, MS, professor of medicine and pharmacy, and associate director, center for pharmaceutical policy and prescribing at the University of Pittsburgh.
“Drug shortages continue to increase in frequency, and the duration and severity of the shortages are also getting worse,” Dr. Suda said in an interview.
The reasons for these shortages can be elusive, according to 2022 report by the American Society of Health-System Pharmacists, which found that more than half of shortages came with no explanation from manufacturers.
The same report showed that only 5% of shortages were due to a “business decision,” but this factor is likely more central than publicly stated.
A recent FDA analysis on drug shortages, for instance, lists “lack of incentives to produce less profitable drugs,” as the first “root cause,” and Dr. Suda agrees.
“It’s important that we have generic medicines to decrease costs to our health systems, as well as for our patients,” Dr. Suda said. “But frequently, with those generic products, the price is driven so low that it increases the risk of a shortage.”
The drive to maintain profit margins may motivate companies to cut corners in production, Dr. Suda explained. She emphasized that this connection is speculative, because motivations are effectively unknowable, but the rationale is supported by past and present shortages.
Akorn Pharmaceuticals, for example, received a warning letter from the FDA in 2019 because of a variety of manufacturing issues, including defective bottles, questionable data, and metal shavings on aseptic filling equipment.
When a manufacturer like Akorn fails, the effects can be far-reaching, Dr. Suda said, noting their broad catalog of agents. Beyond liquid albuterol, Akorn was producing cardiac drugs, antibiotics, vitamins, local anesthetics, eye products, and others.
Drug shortages cause “a significant strain on our health care system,” Dr. Suda said, and substituting other medications increases risk of medical errors.
Fortunately, the increasing number of drug shortages is not going unnoticed, according to Dr. Suda. The FDA and multiple other organizations, including the ASHP, American Medical Association, and National Academies of Sciences, Engineering, and Medicine, are all taking steps to ensure that essential medicines are in steady supply, including moves to gather more data from manufacturers.
“I hope that a lot of the efforts that are moving forward ... will help us decrease the impact of shortages on our patients,” Dr. Suda said.
Lou Kennedy is the CEO of Nephron Pharmaceuticals, which commercially produces liquid albuterol. The other interviewees disclosed no relevant conflicts of interest.
New hope for MDS, with AML treatments
Until just over a year ago, Pat Trueman, an 82-year-old in New Hampshire, had always been a “go-go-go” kind of person. Then she started feeling tired easily, even while doing basic housework.
“I had no stamina,” Ms. Trueman said. “I didn’t feel that bad, but I just couldn’t do anything.” She had also begun noticing black and blue bruises appearing on her body, so she met with her cardiologist. But when switching medications and getting a pacemaker didn’t rid Ms. Trueman of the symptoms, her doctor referred her to a hematologist oncologist.
A bone marrow biopsy eventually revealed that Ms. Trueman had myelodysplastic neoplasms, or MDS, a blood cancer affecting an estimated 60,000-170,000 people in the United States, mostly over age 60. MDS includes several bone marrow disorders in which the bone marrow does not produce enough healthy, normal blood cells. Cytopenias are therefore a key feature of MDS, whether it’s anemia (in Ms. Trueman’s case), neutropenia, or thrombocytopenia.
Jamie Koprivnikar, MD, a hematologist oncologist at Hackensack (N.J) University Medical Center, describes the condition to her patients using a factory metaphor: “Our bone marrow is the factory where the red blood cells, white blood cells, and platelets are made, and MDS is where the machinery of the factory is broken, so the factory is making defective parts and not enough parts.”
The paradox of MDS is that too many cells are in the bone marrow while too few are in the blood, since most in the marrow die before reaching the blood, explained Azra Raza, MD, a professor of medicine and director of the MDS Center at Columbia University Medical Center, New York, and author of The First Cell (New York: Basic Books, 2019).
“We’re looking at taking a lot of the therapies that we’ve used to treat AML and then trying to apply them to MDS,” Dr. Koprivnikar said. “With all the improvement that we’re seeing there with leukemia, we’re definitely expecting this trickle-down effect to also help our high-risk MDS patients.”
Workup begins with risk stratification
While different types of MDS exist, based on morphology of the blood cells, after diagnosis the most important determination to make is of the patient’s risk level, based on the International Prognostic Scoring System–Revised (IPSS-R), updated in 2022.
While there are six MDS risk levels, patients generally fall into the high-risk and low-risk categories. The risk-level workup includes “a bone marrow biopsy with morphology, looking at how many blasts they have, looking for dysplasia, cytogenetics, and a full spectrum myeloid mutation testing, or molecular testing,” according to Anna Halpern, MD, an assistant professor of hematology in the clinical research division at Fred Hutchinson Cancer Center, Seattle. ”I use that information and along with their age, in some cases to calculate an IPSS-M or IPPS-R score, and what goes into that risk stratification includes how low their blood counts are as well as any adverse risks features we might see in their marrow, like adverse risk genetics, adverse risk mutations or increased blasts.”
Treatment decisions then turn on whether a patient is high risk – about a third of MDS patients – or low risk, because those treatment goals differ.
“With low-risk, the goal is to improve quality of life,” Dr. Raza said. “For higher-risk MDS, the goal is to prolong survival and delay progression to acute leukemia” since nearly a third of MDS patients will eventually develop AML.
More specifically, the aim with low-risk MDS is “to foster transfusion independence, either to prevent transfusions or to decrease the need for transfusions in people already receiving them,” explained Ellen Ritchie, MD, an assistant professor of medicine and hematologist-oncologist at Weill Cornell Medicine, New York. “We’re not hoping so much to cure the myelofibrosis at that point, but rather to improve blood counts.”
Sometimes, Dr. Halpern said, such treatment means active surveillance monitoring of blood counts, and at other times, it means treating cytopenia – most often anemia. The erythropoiesis-stimulating agents used to treat anemia are epoetin alfa (Epogen/Procrit) or darbepoetin alfa (Aranesp).
Ms. Trueman, whose MDS is low risk, started taking Aranesp, but she didn’t feel well on the drug and didn’t think it was helping much. She was taken off that drug and now relies only on transfusions for treatment, when her blood counts fall too low.
A newer anemia medication, luspatercept (Reblozyl), was approved in 2020 but is reserved primarily for those who fail one of the other erythropoiesis-stimulating agents and have a subtype of MDS with ring sideroblasts. Although white blood cell and platelet growth factors exist for other cytopenias, they’re rarely used because they offer little survival benefit and carry risks, Dr. Halpern said. The only other medication typically used for low-risk MDS is lenalidomide (Revlimid), which is reserved only for those with 5q-deletion syndrome.
The goal of treating high-risk MDS, on the other hand, is to cure it – when possible.
“The only curative approach for MDS is an allogeneic stem cell transplant or bone marrow transplant,” Dr. Halpern said, but transplants carry high rates of morbidity and mortality and therefore require a base level of physical fitness for a patient to consider it.
Dr. Koprivnikar observed that “MDS is certainly a disease of the elderly, and with each increasing decade of life, incidence increases. So there are a lot of patients who do not qualify for transplant.”
Age is not the sole determining factor, however. Dr. Ritchie noted that transplants can be offered to patients up to age 75 and sometimes older, depending on their physical condition. “It all depends upon the patient, their fitness, how much caretaker support they have, and what their comorbid illnesses are.”
If a transplant isn’t an option, Dr. Halpern and Dr. Raza said, they steer patients toward clinical trial participation. Otherwise, the first-line treatment is chemotherapy with hypomethylating agents to hopefully put patients in remission, Dr. Ritchie said.
The main chemo agents for high-risk patients ineligible for transplant are azacitidine (Vidaza) or decitabine (Dacogen), offered indefinitely until patients stop responding or experience progression or intolerance, Dr. Koprivnikar said. The only recently approved drug in this space is Inqovi, which is not a new agent, but it provides decitabine and cedazuridine in an oral pill form, so that patients can avoid infusions.
Treatment gaps
Few treatments options currently exist for patients with MDS, beyond erythropoiesis-stimulating agents for low-risk MDS and chemotherapy or transplant for high-risk MDS, as well as lenalidomide and luspatercept for specific subpopulations. With few breakthroughs occurring, Dr. Halpern expects that progress will only happen gradually, with new treatments coming primarily in the form of AML therapies.
“The biggest gap in our MDS regimen is treatment that can successfully treat or alter the natural history of TP53-mutated disease,” said Dr. Halpern, referring to an adverse risk mutation that can occur spontaneously or as a result of exposure to chemotherapy or radiation. “TP53-mutated MDS is very challenging to treat, and we have not had any successful therapy, so that is the biggest area of need.”
The most promising possibility in that area is an anti-CD47 drug called magrolimab, a drug being tested in a trial of which Dr. Halpern is a principal investigator. Not yet approved, magrolimab has been showing promise for AML when given with azacitidine (Vidaza) and venetoclax (Venclexta).
Venetoclax, currently used for AML, is another drug that Dr. Halpern expects to be approved for MDS soon. A phase 1b trial presented at the 2021 annual meeting of the American Hematology Society found that more than three-quarters of patients with high-risk MDS responded to the combination of venetoclax and azacitidine.
Unlike so many other cancers, MDS has seen little success with immunotherapy, which tends to have too much toxicity for patients with MDS. While Dr. Halpern sees potential for more exploration in this realm, she doesn’t anticipate immunotherapy or chimeric antigen receptor T-cell therapy becoming treatments for MDS in the near future.
“What I do think is, hopefully, we will have better treatment for TP53-mutated disease,” she said, while adding that there are currently no standard options for patients who stopped responding or don’t respond to hypomethylating agents.
Similarly, few new treatments have emerged for low-risk MDS, but there a couple of possibilities on the horizon.
“For a while, low-risk, transfusion-dependent MDS was an area that was being overlooked, and we are starting to see more activity in that area as well, with more drugs being developed,” Dr. Koprivnikar said. Drugs showing promise include imetelstat – an investigative telomerase inhibitor – and IRAK inhibitors. A phase 3 trial of imetelstat recently met its primary endpoint of 8 weeks of transfusion independence in low-risk MDS patients who aren’t responding to or cannot take erythropoiesis-stimulating agents, like Ms. Trueman. If effective and approved, a drug like imetelstat may allow patients like Ms. Trueman to resume some activities that she misses now.
“I have so much energy in my head, and I want to do so much, but I can’t,” Ms. Trueman said. “Now I think I’m getting lazy and I don’t like it because I’m not that kind of person. It’s pretty hard.”
Dr. Raza disclosed relationships with Epizyme, Grail, Vor, Taiho, RareCells, and TFC Therapeutics. Dr Ritchie reported ties with Jazz Pharmaceuticals, Novartis, Takeda, Incyte, AbbVie, Astellas, and Imago Biosciences. Dr. Halpern disclosed relationships with AbbVie, Notable Labs, Imago, Bayer, Gilead, Jazz, Incyte, Karyopharm, and Disc Medicine.
Until just over a year ago, Pat Trueman, an 82-year-old in New Hampshire, had always been a “go-go-go” kind of person. Then she started feeling tired easily, even while doing basic housework.
“I had no stamina,” Ms. Trueman said. “I didn’t feel that bad, but I just couldn’t do anything.” She had also begun noticing black and blue bruises appearing on her body, so she met with her cardiologist. But when switching medications and getting a pacemaker didn’t rid Ms. Trueman of the symptoms, her doctor referred her to a hematologist oncologist.
A bone marrow biopsy eventually revealed that Ms. Trueman had myelodysplastic neoplasms, or MDS, a blood cancer affecting an estimated 60,000-170,000 people in the United States, mostly over age 60. MDS includes several bone marrow disorders in which the bone marrow does not produce enough healthy, normal blood cells. Cytopenias are therefore a key feature of MDS, whether it’s anemia (in Ms. Trueman’s case), neutropenia, or thrombocytopenia.
Jamie Koprivnikar, MD, a hematologist oncologist at Hackensack (N.J) University Medical Center, describes the condition to her patients using a factory metaphor: “Our bone marrow is the factory where the red blood cells, white blood cells, and platelets are made, and MDS is where the machinery of the factory is broken, so the factory is making defective parts and not enough parts.”
The paradox of MDS is that too many cells are in the bone marrow while too few are in the blood, since most in the marrow die before reaching the blood, explained Azra Raza, MD, a professor of medicine and director of the MDS Center at Columbia University Medical Center, New York, and author of The First Cell (New York: Basic Books, 2019).
“We’re looking at taking a lot of the therapies that we’ve used to treat AML and then trying to apply them to MDS,” Dr. Koprivnikar said. “With all the improvement that we’re seeing there with leukemia, we’re definitely expecting this trickle-down effect to also help our high-risk MDS patients.”
Workup begins with risk stratification
While different types of MDS exist, based on morphology of the blood cells, after diagnosis the most important determination to make is of the patient’s risk level, based on the International Prognostic Scoring System–Revised (IPSS-R), updated in 2022.
While there are six MDS risk levels, patients generally fall into the high-risk and low-risk categories. The risk-level workup includes “a bone marrow biopsy with morphology, looking at how many blasts they have, looking for dysplasia, cytogenetics, and a full spectrum myeloid mutation testing, or molecular testing,” according to Anna Halpern, MD, an assistant professor of hematology in the clinical research division at Fred Hutchinson Cancer Center, Seattle. ”I use that information and along with their age, in some cases to calculate an IPSS-M or IPPS-R score, and what goes into that risk stratification includes how low their blood counts are as well as any adverse risks features we might see in their marrow, like adverse risk genetics, adverse risk mutations or increased blasts.”
Treatment decisions then turn on whether a patient is high risk – about a third of MDS patients – or low risk, because those treatment goals differ.
“With low-risk, the goal is to improve quality of life,” Dr. Raza said. “For higher-risk MDS, the goal is to prolong survival and delay progression to acute leukemia” since nearly a third of MDS patients will eventually develop AML.
More specifically, the aim with low-risk MDS is “to foster transfusion independence, either to prevent transfusions or to decrease the need for transfusions in people already receiving them,” explained Ellen Ritchie, MD, an assistant professor of medicine and hematologist-oncologist at Weill Cornell Medicine, New York. “We’re not hoping so much to cure the myelofibrosis at that point, but rather to improve blood counts.”
Sometimes, Dr. Halpern said, such treatment means active surveillance monitoring of blood counts, and at other times, it means treating cytopenia – most often anemia. The erythropoiesis-stimulating agents used to treat anemia are epoetin alfa (Epogen/Procrit) or darbepoetin alfa (Aranesp).
Ms. Trueman, whose MDS is low risk, started taking Aranesp, but she didn’t feel well on the drug and didn’t think it was helping much. She was taken off that drug and now relies only on transfusions for treatment, when her blood counts fall too low.
A newer anemia medication, luspatercept (Reblozyl), was approved in 2020 but is reserved primarily for those who fail one of the other erythropoiesis-stimulating agents and have a subtype of MDS with ring sideroblasts. Although white blood cell and platelet growth factors exist for other cytopenias, they’re rarely used because they offer little survival benefit and carry risks, Dr. Halpern said. The only other medication typically used for low-risk MDS is lenalidomide (Revlimid), which is reserved only for those with 5q-deletion syndrome.
The goal of treating high-risk MDS, on the other hand, is to cure it – when possible.
“The only curative approach for MDS is an allogeneic stem cell transplant or bone marrow transplant,” Dr. Halpern said, but transplants carry high rates of morbidity and mortality and therefore require a base level of physical fitness for a patient to consider it.
Dr. Koprivnikar observed that “MDS is certainly a disease of the elderly, and with each increasing decade of life, incidence increases. So there are a lot of patients who do not qualify for transplant.”
Age is not the sole determining factor, however. Dr. Ritchie noted that transplants can be offered to patients up to age 75 and sometimes older, depending on their physical condition. “It all depends upon the patient, their fitness, how much caretaker support they have, and what their comorbid illnesses are.”
If a transplant isn’t an option, Dr. Halpern and Dr. Raza said, they steer patients toward clinical trial participation. Otherwise, the first-line treatment is chemotherapy with hypomethylating agents to hopefully put patients in remission, Dr. Ritchie said.
The main chemo agents for high-risk patients ineligible for transplant are azacitidine (Vidaza) or decitabine (Dacogen), offered indefinitely until patients stop responding or experience progression or intolerance, Dr. Koprivnikar said. The only recently approved drug in this space is Inqovi, which is not a new agent, but it provides decitabine and cedazuridine in an oral pill form, so that patients can avoid infusions.
Treatment gaps
Few treatments options currently exist for patients with MDS, beyond erythropoiesis-stimulating agents for low-risk MDS and chemotherapy or transplant for high-risk MDS, as well as lenalidomide and luspatercept for specific subpopulations. With few breakthroughs occurring, Dr. Halpern expects that progress will only happen gradually, with new treatments coming primarily in the form of AML therapies.
“The biggest gap in our MDS regimen is treatment that can successfully treat or alter the natural history of TP53-mutated disease,” said Dr. Halpern, referring to an adverse risk mutation that can occur spontaneously or as a result of exposure to chemotherapy or radiation. “TP53-mutated MDS is very challenging to treat, and we have not had any successful therapy, so that is the biggest area of need.”
The most promising possibility in that area is an anti-CD47 drug called magrolimab, a drug being tested in a trial of which Dr. Halpern is a principal investigator. Not yet approved, magrolimab has been showing promise for AML when given with azacitidine (Vidaza) and venetoclax (Venclexta).
Venetoclax, currently used for AML, is another drug that Dr. Halpern expects to be approved for MDS soon. A phase 1b trial presented at the 2021 annual meeting of the American Hematology Society found that more than three-quarters of patients with high-risk MDS responded to the combination of venetoclax and azacitidine.
Unlike so many other cancers, MDS has seen little success with immunotherapy, which tends to have too much toxicity for patients with MDS. While Dr. Halpern sees potential for more exploration in this realm, she doesn’t anticipate immunotherapy or chimeric antigen receptor T-cell therapy becoming treatments for MDS in the near future.
“What I do think is, hopefully, we will have better treatment for TP53-mutated disease,” she said, while adding that there are currently no standard options for patients who stopped responding or don’t respond to hypomethylating agents.
Similarly, few new treatments have emerged for low-risk MDS, but there a couple of possibilities on the horizon.
“For a while, low-risk, transfusion-dependent MDS was an area that was being overlooked, and we are starting to see more activity in that area as well, with more drugs being developed,” Dr. Koprivnikar said. Drugs showing promise include imetelstat – an investigative telomerase inhibitor – and IRAK inhibitors. A phase 3 trial of imetelstat recently met its primary endpoint of 8 weeks of transfusion independence in low-risk MDS patients who aren’t responding to or cannot take erythropoiesis-stimulating agents, like Ms. Trueman. If effective and approved, a drug like imetelstat may allow patients like Ms. Trueman to resume some activities that she misses now.
“I have so much energy in my head, and I want to do so much, but I can’t,” Ms. Trueman said. “Now I think I’m getting lazy and I don’t like it because I’m not that kind of person. It’s pretty hard.”
Dr. Raza disclosed relationships with Epizyme, Grail, Vor, Taiho, RareCells, and TFC Therapeutics. Dr Ritchie reported ties with Jazz Pharmaceuticals, Novartis, Takeda, Incyte, AbbVie, Astellas, and Imago Biosciences. Dr. Halpern disclosed relationships with AbbVie, Notable Labs, Imago, Bayer, Gilead, Jazz, Incyte, Karyopharm, and Disc Medicine.
Until just over a year ago, Pat Trueman, an 82-year-old in New Hampshire, had always been a “go-go-go” kind of person. Then she started feeling tired easily, even while doing basic housework.
“I had no stamina,” Ms. Trueman said. “I didn’t feel that bad, but I just couldn’t do anything.” She had also begun noticing black and blue bruises appearing on her body, so she met with her cardiologist. But when switching medications and getting a pacemaker didn’t rid Ms. Trueman of the symptoms, her doctor referred her to a hematologist oncologist.
A bone marrow biopsy eventually revealed that Ms. Trueman had myelodysplastic neoplasms, or MDS, a blood cancer affecting an estimated 60,000-170,000 people in the United States, mostly over age 60. MDS includes several bone marrow disorders in which the bone marrow does not produce enough healthy, normal blood cells. Cytopenias are therefore a key feature of MDS, whether it’s anemia (in Ms. Trueman’s case), neutropenia, or thrombocytopenia.
Jamie Koprivnikar, MD, a hematologist oncologist at Hackensack (N.J) University Medical Center, describes the condition to her patients using a factory metaphor: “Our bone marrow is the factory where the red blood cells, white blood cells, and platelets are made, and MDS is where the machinery of the factory is broken, so the factory is making defective parts and not enough parts.”
The paradox of MDS is that too many cells are in the bone marrow while too few are in the blood, since most in the marrow die before reaching the blood, explained Azra Raza, MD, a professor of medicine and director of the MDS Center at Columbia University Medical Center, New York, and author of The First Cell (New York: Basic Books, 2019).
“We’re looking at taking a lot of the therapies that we’ve used to treat AML and then trying to apply them to MDS,” Dr. Koprivnikar said. “With all the improvement that we’re seeing there with leukemia, we’re definitely expecting this trickle-down effect to also help our high-risk MDS patients.”
Workup begins with risk stratification
While different types of MDS exist, based on morphology of the blood cells, after diagnosis the most important determination to make is of the patient’s risk level, based on the International Prognostic Scoring System–Revised (IPSS-R), updated in 2022.
While there are six MDS risk levels, patients generally fall into the high-risk and low-risk categories. The risk-level workup includes “a bone marrow biopsy with morphology, looking at how many blasts they have, looking for dysplasia, cytogenetics, and a full spectrum myeloid mutation testing, or molecular testing,” according to Anna Halpern, MD, an assistant professor of hematology in the clinical research division at Fred Hutchinson Cancer Center, Seattle. ”I use that information and along with their age, in some cases to calculate an IPSS-M or IPPS-R score, and what goes into that risk stratification includes how low their blood counts are as well as any adverse risks features we might see in their marrow, like adverse risk genetics, adverse risk mutations or increased blasts.”
Treatment decisions then turn on whether a patient is high risk – about a third of MDS patients – or low risk, because those treatment goals differ.
“With low-risk, the goal is to improve quality of life,” Dr. Raza said. “For higher-risk MDS, the goal is to prolong survival and delay progression to acute leukemia” since nearly a third of MDS patients will eventually develop AML.
More specifically, the aim with low-risk MDS is “to foster transfusion independence, either to prevent transfusions or to decrease the need for transfusions in people already receiving them,” explained Ellen Ritchie, MD, an assistant professor of medicine and hematologist-oncologist at Weill Cornell Medicine, New York. “We’re not hoping so much to cure the myelofibrosis at that point, but rather to improve blood counts.”
Sometimes, Dr. Halpern said, such treatment means active surveillance monitoring of blood counts, and at other times, it means treating cytopenia – most often anemia. The erythropoiesis-stimulating agents used to treat anemia are epoetin alfa (Epogen/Procrit) or darbepoetin alfa (Aranesp).
Ms. Trueman, whose MDS is low risk, started taking Aranesp, but she didn’t feel well on the drug and didn’t think it was helping much. She was taken off that drug and now relies only on transfusions for treatment, when her blood counts fall too low.
A newer anemia medication, luspatercept (Reblozyl), was approved in 2020 but is reserved primarily for those who fail one of the other erythropoiesis-stimulating agents and have a subtype of MDS with ring sideroblasts. Although white blood cell and platelet growth factors exist for other cytopenias, they’re rarely used because they offer little survival benefit and carry risks, Dr. Halpern said. The only other medication typically used for low-risk MDS is lenalidomide (Revlimid), which is reserved only for those with 5q-deletion syndrome.
The goal of treating high-risk MDS, on the other hand, is to cure it – when possible.
“The only curative approach for MDS is an allogeneic stem cell transplant or bone marrow transplant,” Dr. Halpern said, but transplants carry high rates of morbidity and mortality and therefore require a base level of physical fitness for a patient to consider it.
Dr. Koprivnikar observed that “MDS is certainly a disease of the elderly, and with each increasing decade of life, incidence increases. So there are a lot of patients who do not qualify for transplant.”
Age is not the sole determining factor, however. Dr. Ritchie noted that transplants can be offered to patients up to age 75 and sometimes older, depending on their physical condition. “It all depends upon the patient, their fitness, how much caretaker support they have, and what their comorbid illnesses are.”
If a transplant isn’t an option, Dr. Halpern and Dr. Raza said, they steer patients toward clinical trial participation. Otherwise, the first-line treatment is chemotherapy with hypomethylating agents to hopefully put patients in remission, Dr. Ritchie said.
The main chemo agents for high-risk patients ineligible for transplant are azacitidine (Vidaza) or decitabine (Dacogen), offered indefinitely until patients stop responding or experience progression or intolerance, Dr. Koprivnikar said. The only recently approved drug in this space is Inqovi, which is not a new agent, but it provides decitabine and cedazuridine in an oral pill form, so that patients can avoid infusions.
Treatment gaps
Few treatments options currently exist for patients with MDS, beyond erythropoiesis-stimulating agents for low-risk MDS and chemotherapy or transplant for high-risk MDS, as well as lenalidomide and luspatercept for specific subpopulations. With few breakthroughs occurring, Dr. Halpern expects that progress will only happen gradually, with new treatments coming primarily in the form of AML therapies.
“The biggest gap in our MDS regimen is treatment that can successfully treat or alter the natural history of TP53-mutated disease,” said Dr. Halpern, referring to an adverse risk mutation that can occur spontaneously or as a result of exposure to chemotherapy or radiation. “TP53-mutated MDS is very challenging to treat, and we have not had any successful therapy, so that is the biggest area of need.”
The most promising possibility in that area is an anti-CD47 drug called magrolimab, a drug being tested in a trial of which Dr. Halpern is a principal investigator. Not yet approved, magrolimab has been showing promise for AML when given with azacitidine (Vidaza) and venetoclax (Venclexta).
Venetoclax, currently used for AML, is another drug that Dr. Halpern expects to be approved for MDS soon. A phase 1b trial presented at the 2021 annual meeting of the American Hematology Society found that more than three-quarters of patients with high-risk MDS responded to the combination of venetoclax and azacitidine.
Unlike so many other cancers, MDS has seen little success with immunotherapy, which tends to have too much toxicity for patients with MDS. While Dr. Halpern sees potential for more exploration in this realm, she doesn’t anticipate immunotherapy or chimeric antigen receptor T-cell therapy becoming treatments for MDS in the near future.
“What I do think is, hopefully, we will have better treatment for TP53-mutated disease,” she said, while adding that there are currently no standard options for patients who stopped responding or don’t respond to hypomethylating agents.
Similarly, few new treatments have emerged for low-risk MDS, but there a couple of possibilities on the horizon.
“For a while, low-risk, transfusion-dependent MDS was an area that was being overlooked, and we are starting to see more activity in that area as well, with more drugs being developed,” Dr. Koprivnikar said. Drugs showing promise include imetelstat – an investigative telomerase inhibitor – and IRAK inhibitors. A phase 3 trial of imetelstat recently met its primary endpoint of 8 weeks of transfusion independence in low-risk MDS patients who aren’t responding to or cannot take erythropoiesis-stimulating agents, like Ms. Trueman. If effective and approved, a drug like imetelstat may allow patients like Ms. Trueman to resume some activities that she misses now.
“I have so much energy in my head, and I want to do so much, but I can’t,” Ms. Trueman said. “Now I think I’m getting lazy and I don’t like it because I’m not that kind of person. It’s pretty hard.”
Dr. Raza disclosed relationships with Epizyme, Grail, Vor, Taiho, RareCells, and TFC Therapeutics. Dr Ritchie reported ties with Jazz Pharmaceuticals, Novartis, Takeda, Incyte, AbbVie, Astellas, and Imago Biosciences. Dr. Halpern disclosed relationships with AbbVie, Notable Labs, Imago, Bayer, Gilead, Jazz, Incyte, Karyopharm, and Disc Medicine.
California picks generic drug company Civica to produce low-cost insulin
Gov. Gavin Newsom on March 18 announced the selection of Utah-based generic drug manufacturer Civica to produce low-cost insulin for California, an unprecedented move that makes good on his promise to put state government in direct competition with the brand-name drug companies that dominate the market.
“People should not be forced to go into debt to get lifesaving prescriptions,” Gov. Newsom said. “Californians will have access to some of the most inexpensive insulin available, helping them save thousands of dollars each year.”
The contract, with an initial cost of $50 million that Gov. Newsom and his fellow Democratic lawmakers approved last year, calls for Civica to manufacture state-branded insulin and make the lifesaving drug available to any Californian who needs it, regardless of insurance coverage, by mail order and at local pharmacies. But insulin is just the beginning. Gov. Newsom said the state will also look to produce the opioid overdose reversal drug naloxone.
Allan Coukell, Civica’s senior vice president of public policy, said in an interview that the nonprofit drugmaker is also in talks with the Newsom administration to potentially produce other generic medications, but he declined to elaborate, saying the company is focused on making cheap insulin widely available first.
“We are very excited about this partnership with the state of California,” Mr. Coukell said. “We’re not looking to have 100% of the market, but we do want 100% of people to have access to fair insulin prices.”
As insulin costs for consumers have soared, Democratic lawmakers and activists have called on the industry to rein in prices. Just weeks after President Joe Biden attacked Big Pharma for jacking up insulin prices, the three drugmakers that control the insulin market – Eli Lilly, Novo Nordisk, and Sanofi – announced they would slash the list prices of some products.
Gov. Newsom, who has previously accused the pharmaceutical industry of gouging Californians with “sky-high prices,” argued that the launch of the state’s generic drug label, CalRx, will add competition and apply pressure on the industry. Administration officials declined to say when California’s insulin products would be available, but experts say it could be as soon as 2025. Mr. Coukell said the state-branded medication will still require approval from the Food and Drug Administration, which can take roughly 10 months.
The Pharmaceutical Research and Manufacturers of America, which lobbies on behalf of brand-name companies, blasted California’s move. Reid Porter, senior director of state public affairs for PhRMA, said Gov. Newsom just “wants to score political points.”
“If the governor wants to impact what patients pay for insulins and other medicines meaningfully, he should expand his focus to others in the system that often make patients pay more than they do for medicines,” Mr. Porter said, blaming pharmaceutical go-between companies, known as pharmacy benefit managers, that negotiate with manufacturers on behalf of insurers for rebates and discounts on drugs.
The Pharmaceutical Care Management Association, which represents pharmacy benefit managers argued in turn that it’s pharmaceutical companies that are to blame for high prices.
Drug pricing experts, however, say pharmacy benefit managers and drugmakers share the blame.
Gov. Newsom administration officials say that inflated insulin costs force some to pay as much as $300 per vial or $500 for a box of injectable pens, and that too many Californians with diabetes skip or ration their medication. Doing so can lead to blindness, amputations, and life-threatening conditions such as heart disease and kidney failure. Nearly 10% of California adults have diabetes.
Civica is developing three types of generic insulin, known as a biosimilar, which will be available both in vials and in injectable pens. They are expected to be interchangeable with brand-name products including Lantus, Humalog, and NovoLog. Mr. Coukell said the company would make the drug available for no more than $30 a vial, or $55 for five injectable pens.
Gov. Newsom said the state’s insulin will save many patients $2,000-$4,000 a year, though critical questions about how California would get the products into the hands of consumers remain unanswered, including how it would persuade pharmacies, insurers, and retailers to distribute the drugs.
In 2022, Gov. Newsom also secured $50 million in seed money to build a facility to manufacture insulin; Mr. Coukell said Civica is exploring building a plant in California.
California’s move, though never previously tried by a state government, could be blunted by recent industry decisions to lower insulin prices. In March, Lilly, Novo Nordisk, and Sanofi vowed to cut prices, with Lilly offering a vial at $25 per month, Novo Nordisk promising major reductions that would bring the price of a particular generic vial to $48, and Sanofi pegging one vial at $64.
The governor’s office said it will cost the state $30 per vial to manufacture and distribute insulin and it will be sold at that price. Doing so, the administration argued, “will prevent the egregious cost-shifting that happens in traditional pharmaceutical price games.”
Drug pricing experts said generic production in California could further lower costs for insulin, and benefit people with high-deductible health insurance plans or no insurance.
“This is an extraordinary move in the pharmaceutical industry, not just for insulin but potentially for all kinds of drugs,” said Robin Feldman, a professor at the University of California, San Francisco. “It’s a very difficult industry to disrupt, but California is poised to do just that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Gov. Gavin Newsom on March 18 announced the selection of Utah-based generic drug manufacturer Civica to produce low-cost insulin for California, an unprecedented move that makes good on his promise to put state government in direct competition with the brand-name drug companies that dominate the market.
“People should not be forced to go into debt to get lifesaving prescriptions,” Gov. Newsom said. “Californians will have access to some of the most inexpensive insulin available, helping them save thousands of dollars each year.”
The contract, with an initial cost of $50 million that Gov. Newsom and his fellow Democratic lawmakers approved last year, calls for Civica to manufacture state-branded insulin and make the lifesaving drug available to any Californian who needs it, regardless of insurance coverage, by mail order and at local pharmacies. But insulin is just the beginning. Gov. Newsom said the state will also look to produce the opioid overdose reversal drug naloxone.
Allan Coukell, Civica’s senior vice president of public policy, said in an interview that the nonprofit drugmaker is also in talks with the Newsom administration to potentially produce other generic medications, but he declined to elaborate, saying the company is focused on making cheap insulin widely available first.
“We are very excited about this partnership with the state of California,” Mr. Coukell said. “We’re not looking to have 100% of the market, but we do want 100% of people to have access to fair insulin prices.”
As insulin costs for consumers have soared, Democratic lawmakers and activists have called on the industry to rein in prices. Just weeks after President Joe Biden attacked Big Pharma for jacking up insulin prices, the three drugmakers that control the insulin market – Eli Lilly, Novo Nordisk, and Sanofi – announced they would slash the list prices of some products.
Gov. Newsom, who has previously accused the pharmaceutical industry of gouging Californians with “sky-high prices,” argued that the launch of the state’s generic drug label, CalRx, will add competition and apply pressure on the industry. Administration officials declined to say when California’s insulin products would be available, but experts say it could be as soon as 2025. Mr. Coukell said the state-branded medication will still require approval from the Food and Drug Administration, which can take roughly 10 months.
The Pharmaceutical Research and Manufacturers of America, which lobbies on behalf of brand-name companies, blasted California’s move. Reid Porter, senior director of state public affairs for PhRMA, said Gov. Newsom just “wants to score political points.”
“If the governor wants to impact what patients pay for insulins and other medicines meaningfully, he should expand his focus to others in the system that often make patients pay more than they do for medicines,” Mr. Porter said, blaming pharmaceutical go-between companies, known as pharmacy benefit managers, that negotiate with manufacturers on behalf of insurers for rebates and discounts on drugs.
The Pharmaceutical Care Management Association, which represents pharmacy benefit managers argued in turn that it’s pharmaceutical companies that are to blame for high prices.
Drug pricing experts, however, say pharmacy benefit managers and drugmakers share the blame.
Gov. Newsom administration officials say that inflated insulin costs force some to pay as much as $300 per vial or $500 for a box of injectable pens, and that too many Californians with diabetes skip or ration their medication. Doing so can lead to blindness, amputations, and life-threatening conditions such as heart disease and kidney failure. Nearly 10% of California adults have diabetes.
Civica is developing three types of generic insulin, known as a biosimilar, which will be available both in vials and in injectable pens. They are expected to be interchangeable with brand-name products including Lantus, Humalog, and NovoLog. Mr. Coukell said the company would make the drug available for no more than $30 a vial, or $55 for five injectable pens.
Gov. Newsom said the state’s insulin will save many patients $2,000-$4,000 a year, though critical questions about how California would get the products into the hands of consumers remain unanswered, including how it would persuade pharmacies, insurers, and retailers to distribute the drugs.
In 2022, Gov. Newsom also secured $50 million in seed money to build a facility to manufacture insulin; Mr. Coukell said Civica is exploring building a plant in California.
California’s move, though never previously tried by a state government, could be blunted by recent industry decisions to lower insulin prices. In March, Lilly, Novo Nordisk, and Sanofi vowed to cut prices, with Lilly offering a vial at $25 per month, Novo Nordisk promising major reductions that would bring the price of a particular generic vial to $48, and Sanofi pegging one vial at $64.
The governor’s office said it will cost the state $30 per vial to manufacture and distribute insulin and it will be sold at that price. Doing so, the administration argued, “will prevent the egregious cost-shifting that happens in traditional pharmaceutical price games.”
Drug pricing experts said generic production in California could further lower costs for insulin, and benefit people with high-deductible health insurance plans or no insurance.
“This is an extraordinary move in the pharmaceutical industry, not just for insulin but potentially for all kinds of drugs,” said Robin Feldman, a professor at the University of California, San Francisco. “It’s a very difficult industry to disrupt, but California is poised to do just that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Gov. Gavin Newsom on March 18 announced the selection of Utah-based generic drug manufacturer Civica to produce low-cost insulin for California, an unprecedented move that makes good on his promise to put state government in direct competition with the brand-name drug companies that dominate the market.
“People should not be forced to go into debt to get lifesaving prescriptions,” Gov. Newsom said. “Californians will have access to some of the most inexpensive insulin available, helping them save thousands of dollars each year.”
The contract, with an initial cost of $50 million that Gov. Newsom and his fellow Democratic lawmakers approved last year, calls for Civica to manufacture state-branded insulin and make the lifesaving drug available to any Californian who needs it, regardless of insurance coverage, by mail order and at local pharmacies. But insulin is just the beginning. Gov. Newsom said the state will also look to produce the opioid overdose reversal drug naloxone.
Allan Coukell, Civica’s senior vice president of public policy, said in an interview that the nonprofit drugmaker is also in talks with the Newsom administration to potentially produce other generic medications, but he declined to elaborate, saying the company is focused on making cheap insulin widely available first.
“We are very excited about this partnership with the state of California,” Mr. Coukell said. “We’re not looking to have 100% of the market, but we do want 100% of people to have access to fair insulin prices.”
As insulin costs for consumers have soared, Democratic lawmakers and activists have called on the industry to rein in prices. Just weeks after President Joe Biden attacked Big Pharma for jacking up insulin prices, the three drugmakers that control the insulin market – Eli Lilly, Novo Nordisk, and Sanofi – announced they would slash the list prices of some products.
Gov. Newsom, who has previously accused the pharmaceutical industry of gouging Californians with “sky-high prices,” argued that the launch of the state’s generic drug label, CalRx, will add competition and apply pressure on the industry. Administration officials declined to say when California’s insulin products would be available, but experts say it could be as soon as 2025. Mr. Coukell said the state-branded medication will still require approval from the Food and Drug Administration, which can take roughly 10 months.
The Pharmaceutical Research and Manufacturers of America, which lobbies on behalf of brand-name companies, blasted California’s move. Reid Porter, senior director of state public affairs for PhRMA, said Gov. Newsom just “wants to score political points.”
“If the governor wants to impact what patients pay for insulins and other medicines meaningfully, he should expand his focus to others in the system that often make patients pay more than they do for medicines,” Mr. Porter said, blaming pharmaceutical go-between companies, known as pharmacy benefit managers, that negotiate with manufacturers on behalf of insurers for rebates and discounts on drugs.
The Pharmaceutical Care Management Association, which represents pharmacy benefit managers argued in turn that it’s pharmaceutical companies that are to blame for high prices.
Drug pricing experts, however, say pharmacy benefit managers and drugmakers share the blame.
Gov. Newsom administration officials say that inflated insulin costs force some to pay as much as $300 per vial or $500 for a box of injectable pens, and that too many Californians with diabetes skip or ration their medication. Doing so can lead to blindness, amputations, and life-threatening conditions such as heart disease and kidney failure. Nearly 10% of California adults have diabetes.
Civica is developing three types of generic insulin, known as a biosimilar, which will be available both in vials and in injectable pens. They are expected to be interchangeable with brand-name products including Lantus, Humalog, and NovoLog. Mr. Coukell said the company would make the drug available for no more than $30 a vial, or $55 for five injectable pens.
Gov. Newsom said the state’s insulin will save many patients $2,000-$4,000 a year, though critical questions about how California would get the products into the hands of consumers remain unanswered, including how it would persuade pharmacies, insurers, and retailers to distribute the drugs.
In 2022, Gov. Newsom also secured $50 million in seed money to build a facility to manufacture insulin; Mr. Coukell said Civica is exploring building a plant in California.
California’s move, though never previously tried by a state government, could be blunted by recent industry decisions to lower insulin prices. In March, Lilly, Novo Nordisk, and Sanofi vowed to cut prices, with Lilly offering a vial at $25 per month, Novo Nordisk promising major reductions that would bring the price of a particular generic vial to $48, and Sanofi pegging one vial at $64.
The governor’s office said it will cost the state $30 per vial to manufacture and distribute insulin and it will be sold at that price. Doing so, the administration argued, “will prevent the egregious cost-shifting that happens in traditional pharmaceutical price games.”
Drug pricing experts said generic production in California could further lower costs for insulin, and benefit people with high-deductible health insurance plans or no insurance.
“This is an extraordinary move in the pharmaceutical industry, not just for insulin but potentially for all kinds of drugs,” said Robin Feldman, a professor at the University of California, San Francisco. “It’s a very difficult industry to disrupt, but California is poised to do just that.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Match Day: Record number of residencies offered
Baily Nagle, vice president of her graduating class at Harvard Medical School, Boston, celebrated “the luck of the Irish” on St. Patrick’s Day that allowed her to match into her chosen specialty and top choice of residency programs: anesthesia at Brigham and Women’s Hospital.
“I am feeling very excited and relieved – I matched,” she said in an interview upon hearing her good fortune on Match Monday, March 13. She had a similar reaction on Match Day, March 17. “After a lot of long nights and hard work, happy to have it pay off.”
Ms. Nagle was so determined to match into her specialty that she didn’t have any other specialties in mind as a backup.
The annual process of matching medical school graduates with compatible residency programs is an emotional roller coaster for all applicants, their personal March Madness, so to speak. But Ms. Nagle was one of the more fortunate applicants. She didn’t have to confront the heartbreak other applicants felt when the National Resident Matching Program (NRMP) announced results of the main residency match and the Supplemental Offer and Acceptance Program (SOAP), which offers alternate programs for unfilled positions or unmatched applicants.
During the 2023 Match process, this news organization has been following a handful of students, checking in with them periodically for updates on their progress. Most of them matched successfully, but at least one international medical graduate (IMG) did not. What the others have in common is that their hearts were set on a chosen specialty. Like Ms. Nagle, another student banked on landing his chosen specialty without a backup plan, whereas another said that she’d continue through the SOAP if she didn’t match successfully.
Overall, Match Day resulted in a record number of residency positions offered, most notably in primary care, which “hit an all-time high,” according to NRMP President and CEO Donna L. Lamb, DHSc, MBA, BSN. The number of positions has “consistently increased over the past 5 years, and most importantly the fill rate for primary care has remained steady,” Dr.. Lamb noted in the NRMP release of Match Day results. The release coincided with students learning through emails at noon Eastern Time to which residency or supplemental programs they were matched.
Though more applicants registered for the Match in 2023 than in 2022 – driven primarily by non-U.S. IMGs – the NRMP stated that it was surprised by the decrease in U.S. MD senior applicants.
U.S. MD seniors had a nearly 94% Match rate, a small increase over 2022. U.S. citizen IMGs saw a nearly 68% Match rate, which NRMP reported as an “all-time high” and about six percentage points over in 2022, whereas non-U.S. IMGs had a nearly 60% Match rate, a 1.3 percentage point increase over 2022.
Among the specialties that filled all available positions in 2023 were orthopedic surgery, plastic surgery (integrated), and radiology – diagnostic and thoracic surgery.
Not everyone matches
On March 13, the American College of Emergency Physicians issued a joint statement with other emergency medicine (EM) organizations about a high rate of unfilled EM positions expected in 2023.
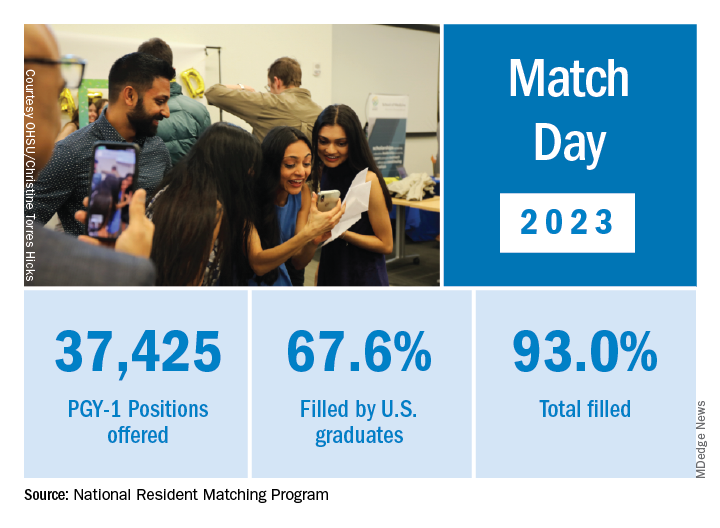
NRMP acknowledged March 17 that 554 positions remained unfilled, an increase of 335 more unfilled positions than 2022. NRMP attributed the increase in unfilled positions in part to a decrease in the number of U.S. MD and U.S. DO seniors who submitted ranks for the specialty, which “could reflect changing applicant interests or projections about workforce opportunities post residency.”
Applicants who didn’t match usually try to obtain an unfilled position through SOAP. In 2023, 2,685 positions were unfilled after the matching algorithm was processed, an increase of nearly 19% over 2022. The vast majority of those positions were placed in SOAP, an increase of 17.5% over 2022.
Asim Ansari was one of the unlucky ones. Mr. Ansari was trying to match for the fifth time. He was unsuccessful in doing so again in 2023 in the Match and SOAP. Still, he was offered and accepted a child and adolescent psychiatry fellowship at Kansas University Medical Center in Kansas City. Psychiatry was his chosen specialty, so he was “feeling good. It’s a nice place to go to do the next 2 years.”
Mr. Ansari, who started the #MatchMadness support group for unmatched doctors on Twitter Spaces, was quick to cheer on his fellow matching peers on March 13 while revealing his own fate: “Congratulations to everyone who matched!!! Y’all are amazing. So proud of each one of you!!! I didn’t.”
Soon after the results, #MatchMadness held a #Soap2023 support session, and Mr. Ansari sought advice for those willing to review SOAP applications. Elsewhere on Twitter Match Day threads, a few doctors offered their support to those who planned to SOAP, students announced their matches, and others either congratulated or encouraged those still trying to match.
Couples match
Not everyone who matched considered the alternative. Before March 13, William Boyer said that he hadn’t given much thought to what would happen if he didn’t match because he was “optimistically confident” he would match into his chosen EM specialty. But he did and got his top choice of programs: Yale New Haven (Conn.) Hospital.
“I feel great,” he said in an interview. “I was definitely nervous opening the envelope” that revealed his residency program, “but there was a rush of relief” when he saw he landed Yale.
Earlier in the match cycle, he said in an interview that he “interviewed at a few ‘reach’ programs, so I hope I don’t match lower than expected on my rank list.”
Mr. Boyer considers himself “a mature applicant,” entering the University of South Carolina, Columbia, after 4 years as an insurance broker.
“I am celebrating today by playing pickleball with a few close medical friends who also matched this morning,” Mr. Boyer said on March 13. “I definitely had periods of nervousness leading up to this morning though that quickly turned into joy and relief” after learning he matched.
Mr. Boyer believes that his professional experience in the insurance industry and health care lobbying efforts with the National Association of Health Underwriters set him apart from other applicants.
“I changed careers to pursue this aspiration, which demonstrates my full dedication to the medical profession.”
He applied to 48 programs and was offered interviews to nearly half. Mr. Boyer visited the majority of those virtually. He said he targeted programs close to where his and his partner’s families are located: Massachusetts, North Carolina, and Texas. “My partner, who I met in medical school, matched into ortho as well so the whole household is very happy,” Mr. Boyer said.
She matched into her top choice as well on March 17, though a distance away at UT Health in San Antonio, he said. “We are both ecstatic. We both got our no. 1 choice. That was the plan going into it. We will make it work. I have 4 weeks of vacation.”
In his program choices, Mr. Boyer prioritized access to nature, minimal leadership turnover, a mix of clinical training sites, and adequate elective rotations and fellowship opportunities, such as in wilderness medicine and health policy.
NRMP reported that there were 1,239 couples participating in the Match; 1,095 had both partners match, and 114 had one partner match to residency training programs for a match rate of 93%.
Like Mr. Boyer, Hannah Hedriana matched into EM, one of the more popular despite the reported unfilled positions. In the past few years, it has consistently been one of the fastest-growing specialties, according to the NRMP.
Still Ms. Hedriana had a fall-back plan. “If I don’t match, then I do plan on going through SOAP. With the number of EM spots that were unfilled in 2022, there’s a chance I could still be an EM physician, but if not, then that’s okay with me.”
Her reaction on March 13, after learning she matched? “Super excited, celebrating with my friends right now.” On Match Day, she said she was “ecstatic” to be matched into Lakeland (Fla.) Regional Health. “This was my first choice so now I can stay close to family and friends,” she said in an interview soon after the results were released.
A first-generation, Filipino American student from the University of South Florida, Tampa, Ms. Hedriana comes from a family of health care professionals. Her father is a respiratory therapist turned physical therapist; her mother a registered nurse. Her sister is a patient care technician applying to nursing school.
Ms. Hedriana applied to 70 programs and interviewed mostly online with 24. Her goal was to stay on the East Coast.
“My partner is a licensed dentist in the state of Florida, and so for his career it would be more practical to stay in state, rather than get relicensed in another state, which could take months,” she said earlier in the matching cycle. “However, when we discussed choosing a residency program, he ultimately left it up to me and wanted me to pick where I thought I’d flourish best,” Ms. Hedriana said, adding that her family lives in Florida, too.
She said she sought a residency program that values family and teamwork.
“A program gets more points in my book if they have sites at nonprofit hospitals or has residents that regularly volunteer throughout their communities or participate in DEI [diversity, equity, and inclusion] initiatives.”
Ms. Hedriana noted that some specialties exclusively offered virtual interviews in 2023, whereas other specialties favored in-person interviews. “This year, many of my classmates were able to do multiple away rotations, which they saw as a positive regarding their chances of matching.” During COVID, in-person visits were limited.
“However, I’ve noticed that many of my classmates are not fond of the signaling aspect that was present for this year’s cycle,” she said. Signaling is a relatively new process that allows applicants to indicate interest in a limited number of residency programs. Not all residencies participate, but it’s growing in popularity among specialties, according to the American Medical Association.
‘Extremely competitive’
Ms. Nagle, a second lieutenant in the U.S. Air Force, applied to 12 programs and interviewed with half of them online. She said that she wasn’t targeting any specific type of program through the match.
“I believe you can get phenomenal training anywhere where you mesh with the residents and leadership. My ultimate priority is to (1) be near good people, (2) be near good food (Indian and Thai are a must), and (3) be near an international airport so I can flee the country during breaks.”
Meanwhile, she said that she found the application process, in which students have to articulate their entire medical school experience, extremely competitive. “I think this process is so easy to get wound up in and the anxiety can be palpable,” Ms. Nagle said. “People around you match your energy. So if you are a ball of anxiety then so are your attendings and residents – and that doesn’t bode well for passing the ‘do I want to be on call with them’ test.”
Looking back at medical school, Ms. Nagle recalled having a baby named after her during her first anesthesia rotation and being featured on The Kelly Clarkson Show. Ms. Nagle said that she had walked into the delivery room where new parents had been debating names of babies beginning with the letter B. “And when I introduced myself, they looked at each other and said, ‘Yep, that’s the one.’”
Mr. Boyer recounted how the majority of his medical school experience involved online education. “Roughly two-thirds of my first year was in-person prior to the pandemic. However, from spring break first year to in-person clinical rotations at the beginning of third year, we were all virtual. While I missed interacting with my classmates, I benefited from the virtual learning environment as I learn more efficiently from reading and visual aids than auditory lectures.”
Ms. Hedriana cited the friends and memories she made while learning to be a doctor. “Medical school was hard, but I wouldn’t have changed a thing.”
A version of this article first appeared on Medscape.com.
Baily Nagle, vice president of her graduating class at Harvard Medical School, Boston, celebrated “the luck of the Irish” on St. Patrick’s Day that allowed her to match into her chosen specialty and top choice of residency programs: anesthesia at Brigham and Women’s Hospital.
“I am feeling very excited and relieved – I matched,” she said in an interview upon hearing her good fortune on Match Monday, March 13. She had a similar reaction on Match Day, March 17. “After a lot of long nights and hard work, happy to have it pay off.”
Ms. Nagle was so determined to match into her specialty that she didn’t have any other specialties in mind as a backup.
The annual process of matching medical school graduates with compatible residency programs is an emotional roller coaster for all applicants, their personal March Madness, so to speak. But Ms. Nagle was one of the more fortunate applicants. She didn’t have to confront the heartbreak other applicants felt when the National Resident Matching Program (NRMP) announced results of the main residency match and the Supplemental Offer and Acceptance Program (SOAP), which offers alternate programs for unfilled positions or unmatched applicants.
During the 2023 Match process, this news organization has been following a handful of students, checking in with them periodically for updates on their progress. Most of them matched successfully, but at least one international medical graduate (IMG) did not. What the others have in common is that their hearts were set on a chosen specialty. Like Ms. Nagle, another student banked on landing his chosen specialty without a backup plan, whereas another said that she’d continue through the SOAP if she didn’t match successfully.
Overall, Match Day resulted in a record number of residency positions offered, most notably in primary care, which “hit an all-time high,” according to NRMP President and CEO Donna L. Lamb, DHSc, MBA, BSN. The number of positions has “consistently increased over the past 5 years, and most importantly the fill rate for primary care has remained steady,” Dr.. Lamb noted in the NRMP release of Match Day results. The release coincided with students learning through emails at noon Eastern Time to which residency or supplemental programs they were matched.
Though more applicants registered for the Match in 2023 than in 2022 – driven primarily by non-U.S. IMGs – the NRMP stated that it was surprised by the decrease in U.S. MD senior applicants.
U.S. MD seniors had a nearly 94% Match rate, a small increase over 2022. U.S. citizen IMGs saw a nearly 68% Match rate, which NRMP reported as an “all-time high” and about six percentage points over in 2022, whereas non-U.S. IMGs had a nearly 60% Match rate, a 1.3 percentage point increase over 2022.
Among the specialties that filled all available positions in 2023 were orthopedic surgery, plastic surgery (integrated), and radiology – diagnostic and thoracic surgery.
Not everyone matches
On March 13, the American College of Emergency Physicians issued a joint statement with other emergency medicine (EM) organizations about a high rate of unfilled EM positions expected in 2023.

NRMP acknowledged March 17 that 554 positions remained unfilled, an increase of 335 more unfilled positions than 2022. NRMP attributed the increase in unfilled positions in part to a decrease in the number of U.S. MD and U.S. DO seniors who submitted ranks for the specialty, which “could reflect changing applicant interests or projections about workforce opportunities post residency.”
Applicants who didn’t match usually try to obtain an unfilled position through SOAP. In 2023, 2,685 positions were unfilled after the matching algorithm was processed, an increase of nearly 19% over 2022. The vast majority of those positions were placed in SOAP, an increase of 17.5% over 2022.
Asim Ansari was one of the unlucky ones. Mr. Ansari was trying to match for the fifth time. He was unsuccessful in doing so again in 2023 in the Match and SOAP. Still, he was offered and accepted a child and adolescent psychiatry fellowship at Kansas University Medical Center in Kansas City. Psychiatry was his chosen specialty, so he was “feeling good. It’s a nice place to go to do the next 2 years.”
Mr. Ansari, who started the #MatchMadness support group for unmatched doctors on Twitter Spaces, was quick to cheer on his fellow matching peers on March 13 while revealing his own fate: “Congratulations to everyone who matched!!! Y’all are amazing. So proud of each one of you!!! I didn’t.”
Soon after the results, #MatchMadness held a #Soap2023 support session, and Mr. Ansari sought advice for those willing to review SOAP applications. Elsewhere on Twitter Match Day threads, a few doctors offered their support to those who planned to SOAP, students announced their matches, and others either congratulated or encouraged those still trying to match.
Couples match
Not everyone who matched considered the alternative. Before March 13, William Boyer said that he hadn’t given much thought to what would happen if he didn’t match because he was “optimistically confident” he would match into his chosen EM specialty. But he did and got his top choice of programs: Yale New Haven (Conn.) Hospital.
“I feel great,” he said in an interview. “I was definitely nervous opening the envelope” that revealed his residency program, “but there was a rush of relief” when he saw he landed Yale.
Earlier in the match cycle, he said in an interview that he “interviewed at a few ‘reach’ programs, so I hope I don’t match lower than expected on my rank list.”
Mr. Boyer considers himself “a mature applicant,” entering the University of South Carolina, Columbia, after 4 years as an insurance broker.
“I am celebrating today by playing pickleball with a few close medical friends who also matched this morning,” Mr. Boyer said on March 13. “I definitely had periods of nervousness leading up to this morning though that quickly turned into joy and relief” after learning he matched.
Mr. Boyer believes that his professional experience in the insurance industry and health care lobbying efforts with the National Association of Health Underwriters set him apart from other applicants.
“I changed careers to pursue this aspiration, which demonstrates my full dedication to the medical profession.”
He applied to 48 programs and was offered interviews to nearly half. Mr. Boyer visited the majority of those virtually. He said he targeted programs close to where his and his partner’s families are located: Massachusetts, North Carolina, and Texas. “My partner, who I met in medical school, matched into ortho as well so the whole household is very happy,” Mr. Boyer said.
She matched into her top choice as well on March 17, though a distance away at UT Health in San Antonio, he said. “We are both ecstatic. We both got our no. 1 choice. That was the plan going into it. We will make it work. I have 4 weeks of vacation.”
In his program choices, Mr. Boyer prioritized access to nature, minimal leadership turnover, a mix of clinical training sites, and adequate elective rotations and fellowship opportunities, such as in wilderness medicine and health policy.
NRMP reported that there were 1,239 couples participating in the Match; 1,095 had both partners match, and 114 had one partner match to residency training programs for a match rate of 93%.
Like Mr. Boyer, Hannah Hedriana matched into EM, one of the more popular despite the reported unfilled positions. In the past few years, it has consistently been one of the fastest-growing specialties, according to the NRMP.
Still Ms. Hedriana had a fall-back plan. “If I don’t match, then I do plan on going through SOAP. With the number of EM spots that were unfilled in 2022, there’s a chance I could still be an EM physician, but if not, then that’s okay with me.”
Her reaction on March 13, after learning she matched? “Super excited, celebrating with my friends right now.” On Match Day, she said she was “ecstatic” to be matched into Lakeland (Fla.) Regional Health. “This was my first choice so now I can stay close to family and friends,” she said in an interview soon after the results were released.
A first-generation, Filipino American student from the University of South Florida, Tampa, Ms. Hedriana comes from a family of health care professionals. Her father is a respiratory therapist turned physical therapist; her mother a registered nurse. Her sister is a patient care technician applying to nursing school.
Ms. Hedriana applied to 70 programs and interviewed mostly online with 24. Her goal was to stay on the East Coast.
“My partner is a licensed dentist in the state of Florida, and so for his career it would be more practical to stay in state, rather than get relicensed in another state, which could take months,” she said earlier in the matching cycle. “However, when we discussed choosing a residency program, he ultimately left it up to me and wanted me to pick where I thought I’d flourish best,” Ms. Hedriana said, adding that her family lives in Florida, too.
She said she sought a residency program that values family and teamwork.
“A program gets more points in my book if they have sites at nonprofit hospitals or has residents that regularly volunteer throughout their communities or participate in DEI [diversity, equity, and inclusion] initiatives.”
Ms. Hedriana noted that some specialties exclusively offered virtual interviews in 2023, whereas other specialties favored in-person interviews. “This year, many of my classmates were able to do multiple away rotations, which they saw as a positive regarding their chances of matching.” During COVID, in-person visits were limited.
“However, I’ve noticed that many of my classmates are not fond of the signaling aspect that was present for this year’s cycle,” she said. Signaling is a relatively new process that allows applicants to indicate interest in a limited number of residency programs. Not all residencies participate, but it’s growing in popularity among specialties, according to the American Medical Association.
‘Extremely competitive’
Ms. Nagle, a second lieutenant in the U.S. Air Force, applied to 12 programs and interviewed with half of them online. She said that she wasn’t targeting any specific type of program through the match.
“I believe you can get phenomenal training anywhere where you mesh with the residents and leadership. My ultimate priority is to (1) be near good people, (2) be near good food (Indian and Thai are a must), and (3) be near an international airport so I can flee the country during breaks.”
Meanwhile, she said that she found the application process, in which students have to articulate their entire medical school experience, extremely competitive. “I think this process is so easy to get wound up in and the anxiety can be palpable,” Ms. Nagle said. “People around you match your energy. So if you are a ball of anxiety then so are your attendings and residents – and that doesn’t bode well for passing the ‘do I want to be on call with them’ test.”
Looking back at medical school, Ms. Nagle recalled having a baby named after her during her first anesthesia rotation and being featured on The Kelly Clarkson Show. Ms. Nagle said that she had walked into the delivery room where new parents had been debating names of babies beginning with the letter B. “And when I introduced myself, they looked at each other and said, ‘Yep, that’s the one.’”
Mr. Boyer recounted how the majority of his medical school experience involved online education. “Roughly two-thirds of my first year was in-person prior to the pandemic. However, from spring break first year to in-person clinical rotations at the beginning of third year, we were all virtual. While I missed interacting with my classmates, I benefited from the virtual learning environment as I learn more efficiently from reading and visual aids than auditory lectures.”
Ms. Hedriana cited the friends and memories she made while learning to be a doctor. “Medical school was hard, but I wouldn’t have changed a thing.”
A version of this article first appeared on Medscape.com.
Baily Nagle, vice president of her graduating class at Harvard Medical School, Boston, celebrated “the luck of the Irish” on St. Patrick’s Day that allowed her to match into her chosen specialty and top choice of residency programs: anesthesia at Brigham and Women’s Hospital.
“I am feeling very excited and relieved – I matched,” she said in an interview upon hearing her good fortune on Match Monday, March 13. She had a similar reaction on Match Day, March 17. “After a lot of long nights and hard work, happy to have it pay off.”
Ms. Nagle was so determined to match into her specialty that she didn’t have any other specialties in mind as a backup.
The annual process of matching medical school graduates with compatible residency programs is an emotional roller coaster for all applicants, their personal March Madness, so to speak. But Ms. Nagle was one of the more fortunate applicants. She didn’t have to confront the heartbreak other applicants felt when the National Resident Matching Program (NRMP) announced results of the main residency match and the Supplemental Offer and Acceptance Program (SOAP), which offers alternate programs for unfilled positions or unmatched applicants.
During the 2023 Match process, this news organization has been following a handful of students, checking in with them periodically for updates on their progress. Most of them matched successfully, but at least one international medical graduate (IMG) did not. What the others have in common is that their hearts were set on a chosen specialty. Like Ms. Nagle, another student banked on landing his chosen specialty without a backup plan, whereas another said that she’d continue through the SOAP if she didn’t match successfully.
Overall, Match Day resulted in a record number of residency positions offered, most notably in primary care, which “hit an all-time high,” according to NRMP President and CEO Donna L. Lamb, DHSc, MBA, BSN. The number of positions has “consistently increased over the past 5 years, and most importantly the fill rate for primary care has remained steady,” Dr.. Lamb noted in the NRMP release of Match Day results. The release coincided with students learning through emails at noon Eastern Time to which residency or supplemental programs they were matched.
Though more applicants registered for the Match in 2023 than in 2022 – driven primarily by non-U.S. IMGs – the NRMP stated that it was surprised by the decrease in U.S. MD senior applicants.
U.S. MD seniors had a nearly 94% Match rate, a small increase over 2022. U.S. citizen IMGs saw a nearly 68% Match rate, which NRMP reported as an “all-time high” and about six percentage points over in 2022, whereas non-U.S. IMGs had a nearly 60% Match rate, a 1.3 percentage point increase over 2022.
Among the specialties that filled all available positions in 2023 were orthopedic surgery, plastic surgery (integrated), and radiology – diagnostic and thoracic surgery.
Not everyone matches
On March 13, the American College of Emergency Physicians issued a joint statement with other emergency medicine (EM) organizations about a high rate of unfilled EM positions expected in 2023.

NRMP acknowledged March 17 that 554 positions remained unfilled, an increase of 335 more unfilled positions than 2022. NRMP attributed the increase in unfilled positions in part to a decrease in the number of U.S. MD and U.S. DO seniors who submitted ranks for the specialty, which “could reflect changing applicant interests or projections about workforce opportunities post residency.”
Applicants who didn’t match usually try to obtain an unfilled position through SOAP. In 2023, 2,685 positions were unfilled after the matching algorithm was processed, an increase of nearly 19% over 2022. The vast majority of those positions were placed in SOAP, an increase of 17.5% over 2022.
Asim Ansari was one of the unlucky ones. Mr. Ansari was trying to match for the fifth time. He was unsuccessful in doing so again in 2023 in the Match and SOAP. Still, he was offered and accepted a child and adolescent psychiatry fellowship at Kansas University Medical Center in Kansas City. Psychiatry was his chosen specialty, so he was “feeling good. It’s a nice place to go to do the next 2 years.”
Mr. Ansari, who started the #MatchMadness support group for unmatched doctors on Twitter Spaces, was quick to cheer on his fellow matching peers on March 13 while revealing his own fate: “Congratulations to everyone who matched!!! Y’all are amazing. So proud of each one of you!!! I didn’t.”
Soon after the results, #MatchMadness held a #Soap2023 support session, and Mr. Ansari sought advice for those willing to review SOAP applications. Elsewhere on Twitter Match Day threads, a few doctors offered their support to those who planned to SOAP, students announced their matches, and others either congratulated or encouraged those still trying to match.
Couples match
Not everyone who matched considered the alternative. Before March 13, William Boyer said that he hadn’t given much thought to what would happen if he didn’t match because he was “optimistically confident” he would match into his chosen EM specialty. But he did and got his top choice of programs: Yale New Haven (Conn.) Hospital.
“I feel great,” he said in an interview. “I was definitely nervous opening the envelope” that revealed his residency program, “but there was a rush of relief” when he saw he landed Yale.
Earlier in the match cycle, he said in an interview that he “interviewed at a few ‘reach’ programs, so I hope I don’t match lower than expected on my rank list.”
Mr. Boyer considers himself “a mature applicant,” entering the University of South Carolina, Columbia, after 4 years as an insurance broker.
“I am celebrating today by playing pickleball with a few close medical friends who also matched this morning,” Mr. Boyer said on March 13. “I definitely had periods of nervousness leading up to this morning though that quickly turned into joy and relief” after learning he matched.
Mr. Boyer believes that his professional experience in the insurance industry and health care lobbying efforts with the National Association of Health Underwriters set him apart from other applicants.
“I changed careers to pursue this aspiration, which demonstrates my full dedication to the medical profession.”
He applied to 48 programs and was offered interviews to nearly half. Mr. Boyer visited the majority of those virtually. He said he targeted programs close to where his and his partner’s families are located: Massachusetts, North Carolina, and Texas. “My partner, who I met in medical school, matched into ortho as well so the whole household is very happy,” Mr. Boyer said.
She matched into her top choice as well on March 17, though a distance away at UT Health in San Antonio, he said. “We are both ecstatic. We both got our no. 1 choice. That was the plan going into it. We will make it work. I have 4 weeks of vacation.”
In his program choices, Mr. Boyer prioritized access to nature, minimal leadership turnover, a mix of clinical training sites, and adequate elective rotations and fellowship opportunities, such as in wilderness medicine and health policy.
NRMP reported that there were 1,239 couples participating in the Match; 1,095 had both partners match, and 114 had one partner match to residency training programs for a match rate of 93%.
Like Mr. Boyer, Hannah Hedriana matched into EM, one of the more popular despite the reported unfilled positions. In the past few years, it has consistently been one of the fastest-growing specialties, according to the NRMP.
Still Ms. Hedriana had a fall-back plan. “If I don’t match, then I do plan on going through SOAP. With the number of EM spots that were unfilled in 2022, there’s a chance I could still be an EM physician, but if not, then that’s okay with me.”
Her reaction on March 13, after learning she matched? “Super excited, celebrating with my friends right now.” On Match Day, she said she was “ecstatic” to be matched into Lakeland (Fla.) Regional Health. “This was my first choice so now I can stay close to family and friends,” she said in an interview soon after the results were released.
A first-generation, Filipino American student from the University of South Florida, Tampa, Ms. Hedriana comes from a family of health care professionals. Her father is a respiratory therapist turned physical therapist; her mother a registered nurse. Her sister is a patient care technician applying to nursing school.
Ms. Hedriana applied to 70 programs and interviewed mostly online with 24. Her goal was to stay on the East Coast.
“My partner is a licensed dentist in the state of Florida, and so for his career it would be more practical to stay in state, rather than get relicensed in another state, which could take months,” she said earlier in the matching cycle. “However, when we discussed choosing a residency program, he ultimately left it up to me and wanted me to pick where I thought I’d flourish best,” Ms. Hedriana said, adding that her family lives in Florida, too.
She said she sought a residency program that values family and teamwork.
“A program gets more points in my book if they have sites at nonprofit hospitals or has residents that regularly volunteer throughout their communities or participate in DEI [diversity, equity, and inclusion] initiatives.”
Ms. Hedriana noted that some specialties exclusively offered virtual interviews in 2023, whereas other specialties favored in-person interviews. “This year, many of my classmates were able to do multiple away rotations, which they saw as a positive regarding their chances of matching.” During COVID, in-person visits were limited.
“However, I’ve noticed that many of my classmates are not fond of the signaling aspect that was present for this year’s cycle,” she said. Signaling is a relatively new process that allows applicants to indicate interest in a limited number of residency programs. Not all residencies participate, but it’s growing in popularity among specialties, according to the American Medical Association.
‘Extremely competitive’
Ms. Nagle, a second lieutenant in the U.S. Air Force, applied to 12 programs and interviewed with half of them online. She said that she wasn’t targeting any specific type of program through the match.
“I believe you can get phenomenal training anywhere where you mesh with the residents and leadership. My ultimate priority is to (1) be near good people, (2) be near good food (Indian and Thai are a must), and (3) be near an international airport so I can flee the country during breaks.”
Meanwhile, she said that she found the application process, in which students have to articulate their entire medical school experience, extremely competitive. “I think this process is so easy to get wound up in and the anxiety can be palpable,” Ms. Nagle said. “People around you match your energy. So if you are a ball of anxiety then so are your attendings and residents – and that doesn’t bode well for passing the ‘do I want to be on call with them’ test.”
Looking back at medical school, Ms. Nagle recalled having a baby named after her during her first anesthesia rotation and being featured on The Kelly Clarkson Show. Ms. Nagle said that she had walked into the delivery room where new parents had been debating names of babies beginning with the letter B. “And when I introduced myself, they looked at each other and said, ‘Yep, that’s the one.’”
Mr. Boyer recounted how the majority of his medical school experience involved online education. “Roughly two-thirds of my first year was in-person prior to the pandemic. However, from spring break first year to in-person clinical rotations at the beginning of third year, we were all virtual. While I missed interacting with my classmates, I benefited from the virtual learning environment as I learn more efficiently from reading and visual aids than auditory lectures.”
Ms. Hedriana cited the friends and memories she made while learning to be a doctor. “Medical school was hard, but I wouldn’t have changed a thing.”
A version of this article first appeared on Medscape.com.
Increased cancer in military pilots and ground crew: Pentagon
“Military aircrew and ground crew were overall more likely to be diagnosed with cancer, but less likely to die from cancer compared to the U.S. population,” the report concludes.
The study involved 156,050 aircrew and 737,891 ground crew. Participants were followed between 1992 and 2017. Both groups were predominantly male and non-Hispanic.
Data on cancer incidence and mortality for these two groups were compared with data from groups of similar age in the general population through use of the Surveillance, Epidemiology, and End Results (SEER) Database of the National Cancer Institute.
For aircrew, the study found an 87% higher rate of melanoma, a 39% higher rate of thyroid cancer, a 16% higher rate of prostate cancer, and a 24% higher rate of cancer for all sites combined.
A higher rate of melanoma and prostate cancer among aircrew has been reported previously, but the increased rate of thyroid cancer is a new finding, the authors note.
The uptick in melanoma has also been reported in studies of civilian pilots and cabin crew. It has been attributed to exposure to hazardous ultraviolet and cosmic radiation.
For ground crew members, the analysis found a 19% higher rate of cancers of the brain and nervous system, a 15% higher rate of thyroid cancer, a 9% higher rate of melanoma and of kidney and renal pelvis cancers, and a 3% higher rate of cancer for all sites combined.
There is little to compare these findings with: This is the first time that cancer risk has been evaluated in such a large population of military ground crew.
Lower rates of cancer mortality
In contrast to the increase in cancer incidence, the report found a decrease in cancer mortality.
When compared with a demographically similar U.S. population, the mortality rate among aircrew was 56% lower for all cancer sites; for ground crew, the mortality rate was 35% lower.
However, the report authors emphasize that “it is important to note that the military study population was relatively young.”
The median age at the end of follow-up for the cancer incidence analysis was 41 years for aircrew and 26 years for ground crew. The median age at the end of follow-up for the cancer mortality analysis was 48 years for aircrew and 41 years for ground crew.
“Results may have differed if additional older former Service members had been included in the study, since cancer risk and mortality rates increase with age,” the authors comment.
Other studies have found an increase in deaths from melanoma as well as an increase in the incidence of melanoma. A meta-analysis published in 2019 in the British Journal of Dermatology found that airline pilots and cabin crew have about twice the risk of melanoma and other skin cancers than the general population. Pilots are also more likely to die from melanoma.
Further study underway
The findings on military air and ground crew come from phase 1 of a study that was required by Congress in the 2021 defense bill. Because the investigators found an increase in the incidence of cancer, phase 2 of the study is now necessary.
The report authors explain that phase 2 will consist of identifying the carcinogenic toxicants or hazardous materials associated with military flight operations; identifying operating environments that could be associated with increased amounts of ionizing and nonionizing radiation; identifying specific duties, dates of service, and types of aircraft flown that could have increased the risk for cancer; identifying duty locations associated with a higher incidence of cancers; identifying potential exposures through military service that are not related to aviation; and determining the appropriate age to begin screening military aircrew and ground crew for cancers.
A version of this article first appeared on Medscape.com.
“Military aircrew and ground crew were overall more likely to be diagnosed with cancer, but less likely to die from cancer compared to the U.S. population,” the report concludes.
The study involved 156,050 aircrew and 737,891 ground crew. Participants were followed between 1992 and 2017. Both groups were predominantly male and non-Hispanic.
Data on cancer incidence and mortality for these two groups were compared with data from groups of similar age in the general population through use of the Surveillance, Epidemiology, and End Results (SEER) Database of the National Cancer Institute.
For aircrew, the study found an 87% higher rate of melanoma, a 39% higher rate of thyroid cancer, a 16% higher rate of prostate cancer, and a 24% higher rate of cancer for all sites combined.
A higher rate of melanoma and prostate cancer among aircrew has been reported previously, but the increased rate of thyroid cancer is a new finding, the authors note.
The uptick in melanoma has also been reported in studies of civilian pilots and cabin crew. It has been attributed to exposure to hazardous ultraviolet and cosmic radiation.
For ground crew members, the analysis found a 19% higher rate of cancers of the brain and nervous system, a 15% higher rate of thyroid cancer, a 9% higher rate of melanoma and of kidney and renal pelvis cancers, and a 3% higher rate of cancer for all sites combined.
There is little to compare these findings with: This is the first time that cancer risk has been evaluated in such a large population of military ground crew.
Lower rates of cancer mortality
In contrast to the increase in cancer incidence, the report found a decrease in cancer mortality.
When compared with a demographically similar U.S. population, the mortality rate among aircrew was 56% lower for all cancer sites; for ground crew, the mortality rate was 35% lower.
However, the report authors emphasize that “it is important to note that the military study population was relatively young.”
The median age at the end of follow-up for the cancer incidence analysis was 41 years for aircrew and 26 years for ground crew. The median age at the end of follow-up for the cancer mortality analysis was 48 years for aircrew and 41 years for ground crew.
“Results may have differed if additional older former Service members had been included in the study, since cancer risk and mortality rates increase with age,” the authors comment.
Other studies have found an increase in deaths from melanoma as well as an increase in the incidence of melanoma. A meta-analysis published in 2019 in the British Journal of Dermatology found that airline pilots and cabin crew have about twice the risk of melanoma and other skin cancers than the general population. Pilots are also more likely to die from melanoma.
Further study underway
The findings on military air and ground crew come from phase 1 of a study that was required by Congress in the 2021 defense bill. Because the investigators found an increase in the incidence of cancer, phase 2 of the study is now necessary.
The report authors explain that phase 2 will consist of identifying the carcinogenic toxicants or hazardous materials associated with military flight operations; identifying operating environments that could be associated with increased amounts of ionizing and nonionizing radiation; identifying specific duties, dates of service, and types of aircraft flown that could have increased the risk for cancer; identifying duty locations associated with a higher incidence of cancers; identifying potential exposures through military service that are not related to aviation; and determining the appropriate age to begin screening military aircrew and ground crew for cancers.
A version of this article first appeared on Medscape.com.
“Military aircrew and ground crew were overall more likely to be diagnosed with cancer, but less likely to die from cancer compared to the U.S. population,” the report concludes.
The study involved 156,050 aircrew and 737,891 ground crew. Participants were followed between 1992 and 2017. Both groups were predominantly male and non-Hispanic.
Data on cancer incidence and mortality for these two groups were compared with data from groups of similar age in the general population through use of the Surveillance, Epidemiology, and End Results (SEER) Database of the National Cancer Institute.
For aircrew, the study found an 87% higher rate of melanoma, a 39% higher rate of thyroid cancer, a 16% higher rate of prostate cancer, and a 24% higher rate of cancer for all sites combined.
A higher rate of melanoma and prostate cancer among aircrew has been reported previously, but the increased rate of thyroid cancer is a new finding, the authors note.
The uptick in melanoma has also been reported in studies of civilian pilots and cabin crew. It has been attributed to exposure to hazardous ultraviolet and cosmic radiation.
For ground crew members, the analysis found a 19% higher rate of cancers of the brain and nervous system, a 15% higher rate of thyroid cancer, a 9% higher rate of melanoma and of kidney and renal pelvis cancers, and a 3% higher rate of cancer for all sites combined.
There is little to compare these findings with: This is the first time that cancer risk has been evaluated in such a large population of military ground crew.
Lower rates of cancer mortality
In contrast to the increase in cancer incidence, the report found a decrease in cancer mortality.
When compared with a demographically similar U.S. population, the mortality rate among aircrew was 56% lower for all cancer sites; for ground crew, the mortality rate was 35% lower.
However, the report authors emphasize that “it is important to note that the military study population was relatively young.”
The median age at the end of follow-up for the cancer incidence analysis was 41 years for aircrew and 26 years for ground crew. The median age at the end of follow-up for the cancer mortality analysis was 48 years for aircrew and 41 years for ground crew.
“Results may have differed if additional older former Service members had been included in the study, since cancer risk and mortality rates increase with age,” the authors comment.
Other studies have found an increase in deaths from melanoma as well as an increase in the incidence of melanoma. A meta-analysis published in 2019 in the British Journal of Dermatology found that airline pilots and cabin crew have about twice the risk of melanoma and other skin cancers than the general population. Pilots are also more likely to die from melanoma.
Further study underway
The findings on military air and ground crew come from phase 1 of a study that was required by Congress in the 2021 defense bill. Because the investigators found an increase in the incidence of cancer, phase 2 of the study is now necessary.
The report authors explain that phase 2 will consist of identifying the carcinogenic toxicants or hazardous materials associated with military flight operations; identifying operating environments that could be associated with increased amounts of ionizing and nonionizing radiation; identifying specific duties, dates of service, and types of aircraft flown that could have increased the risk for cancer; identifying duty locations associated with a higher incidence of cancers; identifying potential exposures through military service that are not related to aviation; and determining the appropriate age to begin screening military aircrew and ground crew for cancers.
A version of this article first appeared on Medscape.com.






