User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
COVID-19 maternal antibodies transferred to fetus, newborn from pregnant and lactating vaccine recipients
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY
Change is hard: Lessons from an EHR conversion
During this “go-live,” 5 hospitals and approximately 300 ambulatory service and physician practice locations made the transition, consolidating over 100 disparate electronic systems and dozens of interfaces into one world-class medical record.
If you’ve ever been part of such an event, you know it is anything but simple. On the contrary, it requires an enormous financial investment along with years of planning, hours of meetings, and months of training. No matter how much preparation goes into it, there are sure to be bumps along the way. It is a traumatic and stressful time for all involved, but the end result is well worth the effort. Still, there are lessons to be learned and wisdom to be gleaned, and this month we’d like to share a few that we found most important. We believe that many of these are useful lessons even to those who will never live through a go-live.
Safety always comes first
Patient safety is a term so often used that it has a tendency to be taken for granted. Health systems build processes and procedures to ensure safety – some even win awards and recognition for their efforts. But the best (and safest) health care institutions build patient safety into their cultures. More than just being taught to use checklists or buzzwords, the staff at these institutions are encouraged to put the welfare of patients first, making all other activities secondary to this pursuit. We had the opportunity to witness the benefits of such a culture during this go-live and were incredibly impressed with the results.
To be successful in an EHR transition of any magnitude, an organization needs to hold patient safety as a core value and provide its employees with the tools to execute on that value. This enables staff to prepare adequately and to identify risks and opportunities before the conversion takes place. Once go-live occurs, staff also must feel empowered to speak up when they identify problem areas that might jeopardize patients’ care. They also must be given a clear escalation path to ensure their voices can be heard. Most importantly, everyone must understand that the electronic health record itself is just one piece of a major operational change.
As workflows are modified to adapt to the new technology, unsafe processes should be called out and fixed quickly. While the EHR may offer the latest in decision support and system integration, no advancement in technology can make up for bad outcomes, nor justify processes that lead to patient harm.
Training is no substitute for good support
It takes a long time to train thousands of employees, especially when that training must occur during the era of social distancing in the midst of a pandemic. Still, even in the best of times, education should be married to hands-on experience in order to have a real impact. Unfortunately, this is extremely challenging.
Trainees forget much of what they’ve learned in the weeks or months between education and go-live, so they must be given immediately accessible support to bridge the gap. This is known as “at-the-elbow” (ATE) support, and as the name implies, it consists of individuals who are familiar with the new system and are always available to end users, answering their questions and helping them navigate. Since health care never sleeps, this support needs to be offered 24/7, and it should also be flexible and plentiful.
There are many areas that will require more support than anticipated to accommodate the number of clinical and other staff who will use the system, so support staff must be nimble and available for redeployment. In addition, ensuring high-quality support is essential. As many ATE experts are hired contractors, their knowledge base and communications skills can vary widely. Accountability is key, and end users should feel empowered to identify gaps in coverage and deficits in knowledge base in the ATE.
As employees become more familiar with the new system, the need for ATE will wane, but there will still be questions that arise for many weeks to months, and new EHR users will also be added all the time. A good after–go-live support system should remain available so clinical and clerical employees can get just-in-time assistance whenever they need it.
Users should be given clear expectations
Clinicians going through an EHR conversion may be frustrated to discover that the data transferred from their old system into the new one is not quite what they expected. While structured elements such as allergies and immunizations may transfer, unstructured patient histories may not come over at all.
There may be gaps in data, or the opposite may even be true: an overabundance of useless information may transfer over, leaving doctors with dozens of meaningless data points to sift through and eliminate to clean up the chart. This can be extremely time-consuming and discouraging and may jeopardize the success of the go-live.
Providers deserve clear expectations prior to conversion. They should be told what will and will not transfer and be informed that there will be extra work required for documentation at the outset. They may also want the option to preemptively reduce patient volumes to accommodate the additional effort involved in preparing charts. No matter what, this will be a heavy lift, and physicians should understand the implications long before go-live to prepare accordingly.
Old habits die hard
One of the most common complaints we’ve heard following EHR conversions is that “things just worked better in the old system.” We always respond with a question: “Were things better, or just different?” The truth may lie somewhere in the middle, but there is no question that muscle memory develops over many years, and change is difficult no matter how much better the new system is. Still, appropriate expectations, access to just-in-time support, and a continual focus on safety will ensure that the long-term benefits of a patient-centered and integrated electronic record will far outweigh the initial challenges of go-live.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
During this “go-live,” 5 hospitals and approximately 300 ambulatory service and physician practice locations made the transition, consolidating over 100 disparate electronic systems and dozens of interfaces into one world-class medical record.
If you’ve ever been part of such an event, you know it is anything but simple. On the contrary, it requires an enormous financial investment along with years of planning, hours of meetings, and months of training. No matter how much preparation goes into it, there are sure to be bumps along the way. It is a traumatic and stressful time for all involved, but the end result is well worth the effort. Still, there are lessons to be learned and wisdom to be gleaned, and this month we’d like to share a few that we found most important. We believe that many of these are useful lessons even to those who will never live through a go-live.
Safety always comes first
Patient safety is a term so often used that it has a tendency to be taken for granted. Health systems build processes and procedures to ensure safety – some even win awards and recognition for their efforts. But the best (and safest) health care institutions build patient safety into their cultures. More than just being taught to use checklists or buzzwords, the staff at these institutions are encouraged to put the welfare of patients first, making all other activities secondary to this pursuit. We had the opportunity to witness the benefits of such a culture during this go-live and were incredibly impressed with the results.
To be successful in an EHR transition of any magnitude, an organization needs to hold patient safety as a core value and provide its employees with the tools to execute on that value. This enables staff to prepare adequately and to identify risks and opportunities before the conversion takes place. Once go-live occurs, staff also must feel empowered to speak up when they identify problem areas that might jeopardize patients’ care. They also must be given a clear escalation path to ensure their voices can be heard. Most importantly, everyone must understand that the electronic health record itself is just one piece of a major operational change.
As workflows are modified to adapt to the new technology, unsafe processes should be called out and fixed quickly. While the EHR may offer the latest in decision support and system integration, no advancement in technology can make up for bad outcomes, nor justify processes that lead to patient harm.
Training is no substitute for good support
It takes a long time to train thousands of employees, especially when that training must occur during the era of social distancing in the midst of a pandemic. Still, even in the best of times, education should be married to hands-on experience in order to have a real impact. Unfortunately, this is extremely challenging.
Trainees forget much of what they’ve learned in the weeks or months between education and go-live, so they must be given immediately accessible support to bridge the gap. This is known as “at-the-elbow” (ATE) support, and as the name implies, it consists of individuals who are familiar with the new system and are always available to end users, answering their questions and helping them navigate. Since health care never sleeps, this support needs to be offered 24/7, and it should also be flexible and plentiful.
There are many areas that will require more support than anticipated to accommodate the number of clinical and other staff who will use the system, so support staff must be nimble and available for redeployment. In addition, ensuring high-quality support is essential. As many ATE experts are hired contractors, their knowledge base and communications skills can vary widely. Accountability is key, and end users should feel empowered to identify gaps in coverage and deficits in knowledge base in the ATE.
As employees become more familiar with the new system, the need for ATE will wane, but there will still be questions that arise for many weeks to months, and new EHR users will also be added all the time. A good after–go-live support system should remain available so clinical and clerical employees can get just-in-time assistance whenever they need it.
Users should be given clear expectations
Clinicians going through an EHR conversion may be frustrated to discover that the data transferred from their old system into the new one is not quite what they expected. While structured elements such as allergies and immunizations may transfer, unstructured patient histories may not come over at all.
There may be gaps in data, or the opposite may even be true: an overabundance of useless information may transfer over, leaving doctors with dozens of meaningless data points to sift through and eliminate to clean up the chart. This can be extremely time-consuming and discouraging and may jeopardize the success of the go-live.
Providers deserve clear expectations prior to conversion. They should be told what will and will not transfer and be informed that there will be extra work required for documentation at the outset. They may also want the option to preemptively reduce patient volumes to accommodate the additional effort involved in preparing charts. No matter what, this will be a heavy lift, and physicians should understand the implications long before go-live to prepare accordingly.
Old habits die hard
One of the most common complaints we’ve heard following EHR conversions is that “things just worked better in the old system.” We always respond with a question: “Were things better, or just different?” The truth may lie somewhere in the middle, but there is no question that muscle memory develops over many years, and change is difficult no matter how much better the new system is. Still, appropriate expectations, access to just-in-time support, and a continual focus on safety will ensure that the long-term benefits of a patient-centered and integrated electronic record will far outweigh the initial challenges of go-live.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
During this “go-live,” 5 hospitals and approximately 300 ambulatory service and physician practice locations made the transition, consolidating over 100 disparate electronic systems and dozens of interfaces into one world-class medical record.
If you’ve ever been part of such an event, you know it is anything but simple. On the contrary, it requires an enormous financial investment along with years of planning, hours of meetings, and months of training. No matter how much preparation goes into it, there are sure to be bumps along the way. It is a traumatic and stressful time for all involved, but the end result is well worth the effort. Still, there are lessons to be learned and wisdom to be gleaned, and this month we’d like to share a few that we found most important. We believe that many of these are useful lessons even to those who will never live through a go-live.
Safety always comes first
Patient safety is a term so often used that it has a tendency to be taken for granted. Health systems build processes and procedures to ensure safety – some even win awards and recognition for their efforts. But the best (and safest) health care institutions build patient safety into their cultures. More than just being taught to use checklists or buzzwords, the staff at these institutions are encouraged to put the welfare of patients first, making all other activities secondary to this pursuit. We had the opportunity to witness the benefits of such a culture during this go-live and were incredibly impressed with the results.
To be successful in an EHR transition of any magnitude, an organization needs to hold patient safety as a core value and provide its employees with the tools to execute on that value. This enables staff to prepare adequately and to identify risks and opportunities before the conversion takes place. Once go-live occurs, staff also must feel empowered to speak up when they identify problem areas that might jeopardize patients’ care. They also must be given a clear escalation path to ensure their voices can be heard. Most importantly, everyone must understand that the electronic health record itself is just one piece of a major operational change.
As workflows are modified to adapt to the new technology, unsafe processes should be called out and fixed quickly. While the EHR may offer the latest in decision support and system integration, no advancement in technology can make up for bad outcomes, nor justify processes that lead to patient harm.
Training is no substitute for good support
It takes a long time to train thousands of employees, especially when that training must occur during the era of social distancing in the midst of a pandemic. Still, even in the best of times, education should be married to hands-on experience in order to have a real impact. Unfortunately, this is extremely challenging.
Trainees forget much of what they’ve learned in the weeks or months between education and go-live, so they must be given immediately accessible support to bridge the gap. This is known as “at-the-elbow” (ATE) support, and as the name implies, it consists of individuals who are familiar with the new system and are always available to end users, answering their questions and helping them navigate. Since health care never sleeps, this support needs to be offered 24/7, and it should also be flexible and plentiful.
There are many areas that will require more support than anticipated to accommodate the number of clinical and other staff who will use the system, so support staff must be nimble and available for redeployment. In addition, ensuring high-quality support is essential. As many ATE experts are hired contractors, their knowledge base and communications skills can vary widely. Accountability is key, and end users should feel empowered to identify gaps in coverage and deficits in knowledge base in the ATE.
As employees become more familiar with the new system, the need for ATE will wane, but there will still be questions that arise for many weeks to months, and new EHR users will also be added all the time. A good after–go-live support system should remain available so clinical and clerical employees can get just-in-time assistance whenever they need it.
Users should be given clear expectations
Clinicians going through an EHR conversion may be frustrated to discover that the data transferred from their old system into the new one is not quite what they expected. While structured elements such as allergies and immunizations may transfer, unstructured patient histories may not come over at all.
There may be gaps in data, or the opposite may even be true: an overabundance of useless information may transfer over, leaving doctors with dozens of meaningless data points to sift through and eliminate to clean up the chart. This can be extremely time-consuming and discouraging and may jeopardize the success of the go-live.
Providers deserve clear expectations prior to conversion. They should be told what will and will not transfer and be informed that there will be extra work required for documentation at the outset. They may also want the option to preemptively reduce patient volumes to accommodate the additional effort involved in preparing charts. No matter what, this will be a heavy lift, and physicians should understand the implications long before go-live to prepare accordingly.
Old habits die hard
One of the most common complaints we’ve heard following EHR conversions is that “things just worked better in the old system.” We always respond with a question: “Were things better, or just different?” The truth may lie somewhere in the middle, but there is no question that muscle memory develops over many years, and change is difficult no matter how much better the new system is. Still, appropriate expectations, access to just-in-time support, and a continual focus on safety will ensure that the long-term benefits of a patient-centered and integrated electronic record will far outweigh the initial challenges of go-live.
Dr. Notte is a family physician and chief medical officer of Abington (Pa.) Hospital–Jefferson Health. Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Philadelphia, and associate director of the family medicine residency program at Abington Hospital–Jefferson Health. They have no conflicts related to the content of this piece.
Top JAMA editor on leave amid podcast investigation
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.
The American Medical Association’s Joint Oversight Committee announced that Howard Bauchner, MD, is on leave beginning at the end of the day on March 25. Dr. Bauchner is the top editor at JAMA, the journal of the AMA.
“The decision to place the editor-in-chief on administrative leave neither implicates nor exonerates individuals and is standard operating procedure for such investigations,” the committee said in a statement.
More than 2,000 people signed a petition on Change.org calling for an investigation at JAMA over the February podcast episode, called “Structural Racism for Doctors: What Is It?”
Already, Edward H. Livingston, MD, the host of the podcast, has resigned as deputy editor of the journal.
During the podcast, Dr. Livingston, who is White, said, “Structural racism is an unfortunate term. Personally, I think taking racism out of the conversation will help. Many of us are offended by the concept that we are racist.”
The audio of the podcast has been deleted from JAMA’s website. In its place is audio of a statement from Dr. Bauchner. In his statement, which he released in the week prior to his being on leave, he said the comments in the podcast, which also featured Mitch Katz, MD, were “inaccurate, offensive, hurtful, and inconsistent with the standards of JAMA.”
Also deleted was a JAMA tweet promoting the podcast episode. The tweet said: “No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast.”
This story will be updated.
A version of this article first appeared on WedMD.com.
Contact allergen of the year found in foam in shin guards, footwear
.
The announcement was made by Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, during a presentation at the annual meeting of the American Contact Dermatitis Society, held virtually this year. In his opinion, he said, the most exciting selections occur when international cooperation results in the identification of a new allergen that could become problematic, and acetophenone azine falls into this category.
The chemical formula of acetophenone azine is C16H16N2.
Acetophenone azine was highlighted as a contact allergen in a recent report in Dermatitis. The authors, Nadia Raison-Peyron, MD, from the department of dermatology at the University of Montpelier (France), and Denis Sasseville, MD, from the division of dermatology at McGill University Health Center, Quebec, described publications and reports of about 12 cases of severe allergic contact dermatitis secondary to shin pads or footwear, mainly in children and teens in Europe (one case was in Canada).
A common feature of these cases was the presence of a foam used for cushioning, made of ethyl vinyl acetate (EVA) used in the relevant products.
In one case, a 13-year-old boy who wore shin pads for soccer developed contact dermatitis on both shins that spread, and was described as severe. Patch testing revealed the EVA foam in the shin pads as the only positive reaction. Similar cases have been reported after exposure to EVA-containing products, including shin pads, sneakers, flip-flops, ski boots, insoles, swimming goggles, and bicycle seats, according to the authors.
In some reports, cases related to footwear presented as dyshidrosiform, vesiculobullous eczema, with or without palmar lesions, or presented as plantar hyperkeratotic dermatitis, they wrote. In other cases, patients experienced scarring and postinflammatory hypopigmentation.
The compound is likely not added to EVA intentionally, they added, but instead is thought to result from reactions between additives during the manufacturing process. The presence of acetophenone azine is not well explained, but the current theory is that it results from a combination of “the degradation of the initiator dicumylperoxide and hydrazine from the foaming agent azodicarbonamide,” the authors said.
In the paper, Dr. Raison-Peyron and Dr. Sasseville recommended a patch testing concentration of 0.1% in acetone or petrolatum, as acetophenone azine is not currently available from path test suppliers, although it can be obtained from chemical product distributors.
“Given the recent discovery of this allergen, it is presumed that cases of allergic contact dermatitis would have been missed and labeled irritant contact dermatitis or dyshidrosis,” they noted. To avoid missing more cases, acetophenone azine should be added to the patch testing shoe series, as well as plastics and glues series, they emphasized.
Although no cases of allergic reactions to acetophenone azine have been reported in the United States to date, it is an emerging allergen that should be on the radar for U.S. dermatologists, Amber Atwater, MD, outgoing ACDS president, said in an interview. The lack of reported cases may be in part attributed to the fact that acetophenone azine is not yet available to purchase for testing in the United States, and the allergen could be present in shin guards and other products identified in reported cases, added Dr. Atwater, associate professor of dermatology, Duke University, Durham, N.C.
.
The announcement was made by Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, during a presentation at the annual meeting of the American Contact Dermatitis Society, held virtually this year. In his opinion, he said, the most exciting selections occur when international cooperation results in the identification of a new allergen that could become problematic, and acetophenone azine falls into this category.
The chemical formula of acetophenone azine is C16H16N2.
Acetophenone azine was highlighted as a contact allergen in a recent report in Dermatitis. The authors, Nadia Raison-Peyron, MD, from the department of dermatology at the University of Montpelier (France), and Denis Sasseville, MD, from the division of dermatology at McGill University Health Center, Quebec, described publications and reports of about 12 cases of severe allergic contact dermatitis secondary to shin pads or footwear, mainly in children and teens in Europe (one case was in Canada).
A common feature of these cases was the presence of a foam used for cushioning, made of ethyl vinyl acetate (EVA) used in the relevant products.
In one case, a 13-year-old boy who wore shin pads for soccer developed contact dermatitis on both shins that spread, and was described as severe. Patch testing revealed the EVA foam in the shin pads as the only positive reaction. Similar cases have been reported after exposure to EVA-containing products, including shin pads, sneakers, flip-flops, ski boots, insoles, swimming goggles, and bicycle seats, according to the authors.
In some reports, cases related to footwear presented as dyshidrosiform, vesiculobullous eczema, with or without palmar lesions, or presented as plantar hyperkeratotic dermatitis, they wrote. In other cases, patients experienced scarring and postinflammatory hypopigmentation.
The compound is likely not added to EVA intentionally, they added, but instead is thought to result from reactions between additives during the manufacturing process. The presence of acetophenone azine is not well explained, but the current theory is that it results from a combination of “the degradation of the initiator dicumylperoxide and hydrazine from the foaming agent azodicarbonamide,” the authors said.
In the paper, Dr. Raison-Peyron and Dr. Sasseville recommended a patch testing concentration of 0.1% in acetone or petrolatum, as acetophenone azine is not currently available from path test suppliers, although it can be obtained from chemical product distributors.
“Given the recent discovery of this allergen, it is presumed that cases of allergic contact dermatitis would have been missed and labeled irritant contact dermatitis or dyshidrosis,” they noted. To avoid missing more cases, acetophenone azine should be added to the patch testing shoe series, as well as plastics and glues series, they emphasized.
Although no cases of allergic reactions to acetophenone azine have been reported in the United States to date, it is an emerging allergen that should be on the radar for U.S. dermatologists, Amber Atwater, MD, outgoing ACDS president, said in an interview. The lack of reported cases may be in part attributed to the fact that acetophenone azine is not yet available to purchase for testing in the United States, and the allergen could be present in shin guards and other products identified in reported cases, added Dr. Atwater, associate professor of dermatology, Duke University, Durham, N.C.
.
The announcement was made by Donald V. Belsito, MD, professor of dermatology, Columbia University, New York, during a presentation at the annual meeting of the American Contact Dermatitis Society, held virtually this year. In his opinion, he said, the most exciting selections occur when international cooperation results in the identification of a new allergen that could become problematic, and acetophenone azine falls into this category.
The chemical formula of acetophenone azine is C16H16N2.
Acetophenone azine was highlighted as a contact allergen in a recent report in Dermatitis. The authors, Nadia Raison-Peyron, MD, from the department of dermatology at the University of Montpelier (France), and Denis Sasseville, MD, from the division of dermatology at McGill University Health Center, Quebec, described publications and reports of about 12 cases of severe allergic contact dermatitis secondary to shin pads or footwear, mainly in children and teens in Europe (one case was in Canada).
A common feature of these cases was the presence of a foam used for cushioning, made of ethyl vinyl acetate (EVA) used in the relevant products.
In one case, a 13-year-old boy who wore shin pads for soccer developed contact dermatitis on both shins that spread, and was described as severe. Patch testing revealed the EVA foam in the shin pads as the only positive reaction. Similar cases have been reported after exposure to EVA-containing products, including shin pads, sneakers, flip-flops, ski boots, insoles, swimming goggles, and bicycle seats, according to the authors.
In some reports, cases related to footwear presented as dyshidrosiform, vesiculobullous eczema, with or without palmar lesions, or presented as plantar hyperkeratotic dermatitis, they wrote. In other cases, patients experienced scarring and postinflammatory hypopigmentation.
The compound is likely not added to EVA intentionally, they added, but instead is thought to result from reactions between additives during the manufacturing process. The presence of acetophenone azine is not well explained, but the current theory is that it results from a combination of “the degradation of the initiator dicumylperoxide and hydrazine from the foaming agent azodicarbonamide,” the authors said.
In the paper, Dr. Raison-Peyron and Dr. Sasseville recommended a patch testing concentration of 0.1% in acetone or petrolatum, as acetophenone azine is not currently available from path test suppliers, although it can be obtained from chemical product distributors.
“Given the recent discovery of this allergen, it is presumed that cases of allergic contact dermatitis would have been missed and labeled irritant contact dermatitis or dyshidrosis,” they noted. To avoid missing more cases, acetophenone azine should be added to the patch testing shoe series, as well as plastics and glues series, they emphasized.
Although no cases of allergic reactions to acetophenone azine have been reported in the United States to date, it is an emerging allergen that should be on the radar for U.S. dermatologists, Amber Atwater, MD, outgoing ACDS president, said in an interview. The lack of reported cases may be in part attributed to the fact that acetophenone azine is not yet available to purchase for testing in the United States, and the allergen could be present in shin guards and other products identified in reported cases, added Dr. Atwater, associate professor of dermatology, Duke University, Durham, N.C.
FROM ACDS 2021
COVID-19 variants now detected in more animals, may find hosts in mice
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.
The new SARS-CoV-2 variants are not just problems for humans.
New research shows they can also infect animals, and for the first time, variants have been able to infect mice, a development that may complicate efforts to rein in the global spread of the virus.
In addition, two new studies have implications for pets. Veterinarians in Texas and the United Kingdom have documented infections of B.1.1.7 – the fast-spreading variant first found in the United Kingdom – in dogs and cats. The animals in the U.K. study also had heart damage, but it’s unclear if the damage was caused by the virus or was already there and was found as a result of their infections.
Animal studies of SARS-CoV-2 and its emerging variants are urgent, said Sarah Hamer, DVM, PhD, a veterinarian and epidemiologist at Texas A&M University, College Station.
She’s part of a network of scientists who are swabbing the pets of people who are diagnosed with COVID-19 to find out how often the virus passes from people to animals.
The collaboration is part of the One Health initiative through the Centers for Disease Control and Prevention. One Health aims to tackle infectious diseases by recognizing that people can’t be fully protected from pathogens unless animals and the environment are also safeguarded. “Over 70% of emerging diseases of humans have their origins in animal populations,” Dr. Hamer said. “So if we are only focusing on studying disease as it emerges in humans and ignoring where those pathogens have been transmitted or circulating for years, then we might miss the ability to detect early emergence. We might miss the ability to control these diseases before they become problems for human health.”
Variants move to mice
In new work, researchers at the Institut Pasteur in Paris have shown that the B.1.351 and P.1 variants of concern, which were first identified in South Africa and Brazil, respectively, can infect mice, giving the virus a potential new host. Older versions of the virus couldn’t infect mice because they weren’t able bind to receptors on their cells. These two variants can.
On one hand, that’s a good thing, because it will help scientists more easily conduct experiments in mice. Before, if they wanted to do an experiment with SARS-CoV-2 in mice, they had to use a special strain of mouse that was bred to carry human ACE2 receptors on their lung cells. Now that mice can become naturally infected, any breed will do, making it less costly and time-consuming to study the virus in animals.
On the other hand, the idea that the virus could have more and different ways to spread isn’t good news.
“From the beginning of the epidemic and since human coronaviruses emerged from animals, it has been very important to establish in which species the virus can replicate, in particular the species that live close to humans,” said Xavier Montagutelli, DVM, PhD, head of the Mouse Genetics Laboratory at the Institut Pasteur. His study was published as a preprint ahead of peer review on BioRXIV.
Once a virus establishes itself within a population of animals, it will continue to spread and change and may eventually be passed back to humans. It’s the reason that birds and pigs are closely monitored for influenza viruses.
So far, with SARS-CoV-2, only one animal has been found to catch and spread the virus and pass it back to people – farmed mink. Researchers have also documented SARS-CoV-2 antibodies in escaped mink living near mink farms in Utah, suggesting the virus has the potential to be transmitted to wild populations.
And the move of the virus into mice suggests that SARS-CoV-2 could establish itself in a population of wild animals that live close to humans.
“At this point, we have no evidence that wild mice are infected, or can become infected from humans,” Dr. Montagutelli said. He added that his findings emphasize the need to regularly test animals for signs of the infection. He said these surveys will need to be updated as more variants emerge.
“So far, we’ve been lucky that our livestock species aren’t really susceptible to this,” said Scott Weese, DVM, a professor at Ontario Veterinary College at the University of Guelph, who studies emerging infectious diseases that pass between animals and people.
While the outbreaks on mink farms have been bad, imagine what would happen, Dr. Weese said, if the virus moved to pigs.
“If this infects a barn with a few thousand pigs – which is like the mink scenario – but we have a lot more pig farms than mink farms,” he said.
“With these variants, we have to reset,” he said. “We’ve figured all this about animals and how it spreads or how it doesn’t, but now we need to repeat all those studies to make sure it’s the same thing.”
Pets catch variants, too
Pets living with people who are infected with SARS-CoV-2 can catch it from their owners, and cats are particularly susceptible, Dr. Weese said.
Contact tracing studies, which also tested animals for signs of the virus, have found that about half of cats living with infected people have signs of infection, while 20%-30% of dogs were sick.
“It’s quite common,” for pets to get COVID, Dr. Weese said.
Now, two new studies have shown that pets can also be infected by the newer B.1.1.7 variant.
The first study, from researchers at Texas A&M, documented the variant in a dog and a cat from Brazos County, Texas. Neither the older black Lab mix or the older domestic shorthair cat had symptoms of COVID-19. They were tested as part of a project funded by the CDC.
Dr. Weese said pets are at risk by people who are infected, but they don’t seem to play a big role in spreading the disease to humans. So if you have pets, there’s no reason to worry that they could bring the virus home to you. You’re more likely to be a risk to them.
The second study, from a specialty animal hospital in southeast England, documented infection by the B.1.1.7 virus variant in 11 dogs and cats. Most of the pets had unusual symptoms, including inflamed hearts and heart damage.
Dr. Weese called this study interesting and said its findings deserve more investigation, but pointed out that the study can’t determine whether the infection caused the heart damage, or whether it was already there.
“This is a human virus. There’s no doubt about it. It can affect other species, but it likes people a lot better,” he said. “If you think about the big picture and what is the potential role of animals, pets are pretty low risk.”
A version of this article first appeared on Medscape.com.
Time is of the essence: DST up for debate again
Seasonal time change is now up for consideration in the U.S. Congress, prompting sleep medicine specialists to weigh in on the health impact of a major policy change.
As lawmakers in Washington propose an end to seasonal time changes by permanently establishing daylight saving time (DST), the American Academy of Sleep Medicine (AASM) is pushing for a Congressional hearing so scientists can present evidence in favor of converse legislation – to make standard time the new norm.
According to the AASM, ; however, the switch from standard time to DST incurs more risk.
“Current evidence best supports the adoption of year-round standard time, which aligns best with human circadian biology and provides distinct benefits for public health and safety,” the AASM noted in a 2020 position statement on DST.
The statement cites a number of studies that have reported associations between the switch to DST and acute, negative health outcomes, including higher rates of hospital admission, cardiovascular morbidity, atrial fibrillation, and stroke. The time shift has been associated with a spectrum of cellular, metabolic, and circadian derangements, from increased production of inflammatory markers, to higher blood pressure, and loss of sleep. These biological effects may have far-reaching consequences, including increased rates of fatal motor accidents in the days following the time change, and even increased volatility in the stock market, which may stem from cognitive deficits.
U.S. Senator Marco Rubio (R-Fla.) and others in the U.S. Congress have reintroduced the 2019 Sunshine Protection Act, legislation that would make DST permanent across the country. According to a statement on Sen. Rubio’s website, “The bill reflects the Florida legislature’s 2018 enactment of year-round DST; however, for Florida’s change to apply, a change in the federal statute is required. Fifteen other states – Arkansas, Alabama, California, Delaware, Georgia, Idaho, Louisiana, Maine, Ohio, Oregon, South Carolina, Tennessee, Utah, Washington, and Wyoming – have passed similar laws, resolutions, or voter initiatives, and dozens more are looking. The legislation, if enacted, would apply to those states [that] currently participate in DST, which most states observe for eight months out of the year.”
A stitch in time
“The sudden change in clock time disrupts sleep/wake patterns, decreasing total sleep time and sleep quality, leading to decrements in daytime cognition,” said Kannan Ramar, MBBS, MD, president of the AASM and a sleep medicine specialist at Mayo Clinic, Rochester, Minn.
Emphasizing this point, Dr. Ramar noted a recent study that reported an 18% increase in “patient safety-related incidents associated with human error” among health care workers within a week of the spring time change.
“Irregular bedtimes and wake times disrupt the timing of our circadian rhythms, which can lead to symptoms of insomnia or long-term, excessive daytime sleepiness. Lack of sleep can lead to numerous adverse effects on our minds, including decreased cognitive function, trouble concentrating, and general moodiness,” Dr. Ramar said.
He noted that these impacts may be more significant among certain individuals.
“The daylight saving time changes can be especially problematic for any populations that already experience chronic insufficient sleep or other sleep difficulties,” Dr. Ramar said. “Populations at greatest risk include teenagers, who tend to experience chronic sleep restriction during the school week, and night shift workers, who often struggle to sleep well during daytime hours.”
While fewer studies have evaluated the long-term effects of seasonal time changes, the AASM position statement cited evidence that “the body clock does not adjust to daylight saving time after several months,” possibly because “daylight saving time is less well-aligned with intrinsic human circadian physiology, and it disrupts the natural seasonal adjustment of the human clock due to the effect of late-evening light on the circadian rhythm.”
According to the AASM, permanent DST, as proposed by Sen. Rubio and colleagues, could “result in permanent phase delay, a condition that can also lead to a perpetual discrepancy between the innate biological clock and the extrinsic environmental clock, as well as chronic sleep loss due to early morning social demands that truncate the opportunity to sleep.” This mismatch between sleep/wake cycles and social demands, known as “social jet lag,” has been associated with chronic health risks, including metabolic syndrome, obesity, depression, and cardiovascular disease.
Cardiac impacts of seasonal time change
Muhammad Adeel Rishi, MD, a sleep specialist at Mayo Clinic, Eau Claire, Wis., and lead author of the AASM position statement, highlighted cardiovascular risks in a written statement for this article, noting increased rates of heart attack following the spring time change, and a higher risk of atrial fibrillation.
“Mayo Clinic has not taken a position on this issue,” Dr. Rishi noted. Still, he advocated for permanent standard time as the author of the AASM position statement and vice chair of the AASM public safety committee.
Jay Chudow, MD, and Andrew K. Krumerman, MD, of Montefiore Medical Center, New York, lead author and principal author, respectively, of a recent study that reported increased rates of atrial fibrillation admissions after DST transitions, had the same stance.
“We support elimination of seasonal time changes from a health perspective,” they wrote in a joint comment. “There is mounting evidence of a negative health impact with these seasonal time changes related to effects on sleep and circadian rhythm. Our work found the spring change was associated with more admissions for atrial fibrillation. This added to prior evidence of increased cardiovascular events related to these time changes. If physicians counsel patients on reducing risk factors for disease, shouldn’t we do the same as a society?”
Pros and cons
Not all sleep experts are convinced. Mary Jo Farmer, MD, PhD, FCCP, a sleep specialist and director of pulmonary hypertension services at Baystate Medical Center, and assistant professor of medicine at the University of Massachusetts, Springfield, considers perspectives from both sides of the issue.
“Daylight saving time promotes active lifestyles as people engage in more outdoor activities after work and school, [and] daylight saving time produces economic and safety benefits to society as retail revenues are higher and crimes are lower,” Dr. Farmer said. “Alternatively, moving the clocks forward is a cost burden to the U.S. economy when health issues, decreased productivity, and workplace injuries are considered.”
If one time system is permanently established, Dr. Farmer anticipates divided opinions from patients with sleep issues, regardless of which system is chosen.
“I can tell you, I have a cohort of sleep patients who prefer more evening light and look forward to the spring time change to daylight saving time,” she said. “However, they would not want the sun coming up at 9:00 a.m. in the winter months if we stayed on daylight saving time year-round. Similarly, patients would not want the sun coming up at 4:00 a.m. on the longest day of the year if we stayed on standard time all year round.”
Dr. Farmer called for more research before a decision is made.
“I suggest we need more information about the dangers of staying on daylight saving or standard time year-round because perhaps the current strategy of keeping morning light consistent is not so bad,” she said.
Time for a Congressional hearing?
According to Dr. Ramar, the time is now for a Congressional hearing, as lawmakers and the public need to be adequately informed when considering new legislation.
“There are public misconceptions about daylight saving time and standard time,” Dr. Ramar said. “People often like the idea of daylight saving time because they think it provides more light, and they dislike the concept of standard time because they think it provides more darkness. The reality is that neither time system provides more light or darkness than the other; it is only the timing that changes.”
Until new legislation is introduced, Dr. Ramar offered some practical advice for navigating seasonal time shifts.
“Beginning 2-3 days before the time change, it can be helpful to gradually adjust sleep and wake times, as well as other daily routines such as meal times,” he said. “After the time change, going outside for some morning light can help adjust the timing of your internal body clock.”
The investigators reported no conflicts of interest.
Seasonal time change is now up for consideration in the U.S. Congress, prompting sleep medicine specialists to weigh in on the health impact of a major policy change.
As lawmakers in Washington propose an end to seasonal time changes by permanently establishing daylight saving time (DST), the American Academy of Sleep Medicine (AASM) is pushing for a Congressional hearing so scientists can present evidence in favor of converse legislation – to make standard time the new norm.
According to the AASM, ; however, the switch from standard time to DST incurs more risk.
“Current evidence best supports the adoption of year-round standard time, which aligns best with human circadian biology and provides distinct benefits for public health and safety,” the AASM noted in a 2020 position statement on DST.
The statement cites a number of studies that have reported associations between the switch to DST and acute, negative health outcomes, including higher rates of hospital admission, cardiovascular morbidity, atrial fibrillation, and stroke. The time shift has been associated with a spectrum of cellular, metabolic, and circadian derangements, from increased production of inflammatory markers, to higher blood pressure, and loss of sleep. These biological effects may have far-reaching consequences, including increased rates of fatal motor accidents in the days following the time change, and even increased volatility in the stock market, which may stem from cognitive deficits.
U.S. Senator Marco Rubio (R-Fla.) and others in the U.S. Congress have reintroduced the 2019 Sunshine Protection Act, legislation that would make DST permanent across the country. According to a statement on Sen. Rubio’s website, “The bill reflects the Florida legislature’s 2018 enactment of year-round DST; however, for Florida’s change to apply, a change in the federal statute is required. Fifteen other states – Arkansas, Alabama, California, Delaware, Georgia, Idaho, Louisiana, Maine, Ohio, Oregon, South Carolina, Tennessee, Utah, Washington, and Wyoming – have passed similar laws, resolutions, or voter initiatives, and dozens more are looking. The legislation, if enacted, would apply to those states [that] currently participate in DST, which most states observe for eight months out of the year.”
A stitch in time
“The sudden change in clock time disrupts sleep/wake patterns, decreasing total sleep time and sleep quality, leading to decrements in daytime cognition,” said Kannan Ramar, MBBS, MD, president of the AASM and a sleep medicine specialist at Mayo Clinic, Rochester, Minn.
Emphasizing this point, Dr. Ramar noted a recent study that reported an 18% increase in “patient safety-related incidents associated with human error” among health care workers within a week of the spring time change.
“Irregular bedtimes and wake times disrupt the timing of our circadian rhythms, which can lead to symptoms of insomnia or long-term, excessive daytime sleepiness. Lack of sleep can lead to numerous adverse effects on our minds, including decreased cognitive function, trouble concentrating, and general moodiness,” Dr. Ramar said.
He noted that these impacts may be more significant among certain individuals.
“The daylight saving time changes can be especially problematic for any populations that already experience chronic insufficient sleep or other sleep difficulties,” Dr. Ramar said. “Populations at greatest risk include teenagers, who tend to experience chronic sleep restriction during the school week, and night shift workers, who often struggle to sleep well during daytime hours.”
While fewer studies have evaluated the long-term effects of seasonal time changes, the AASM position statement cited evidence that “the body clock does not adjust to daylight saving time after several months,” possibly because “daylight saving time is less well-aligned with intrinsic human circadian physiology, and it disrupts the natural seasonal adjustment of the human clock due to the effect of late-evening light on the circadian rhythm.”
According to the AASM, permanent DST, as proposed by Sen. Rubio and colleagues, could “result in permanent phase delay, a condition that can also lead to a perpetual discrepancy between the innate biological clock and the extrinsic environmental clock, as well as chronic sleep loss due to early morning social demands that truncate the opportunity to sleep.” This mismatch between sleep/wake cycles and social demands, known as “social jet lag,” has been associated with chronic health risks, including metabolic syndrome, obesity, depression, and cardiovascular disease.
Cardiac impacts of seasonal time change
Muhammad Adeel Rishi, MD, a sleep specialist at Mayo Clinic, Eau Claire, Wis., and lead author of the AASM position statement, highlighted cardiovascular risks in a written statement for this article, noting increased rates of heart attack following the spring time change, and a higher risk of atrial fibrillation.
“Mayo Clinic has not taken a position on this issue,” Dr. Rishi noted. Still, he advocated for permanent standard time as the author of the AASM position statement and vice chair of the AASM public safety committee.
Jay Chudow, MD, and Andrew K. Krumerman, MD, of Montefiore Medical Center, New York, lead author and principal author, respectively, of a recent study that reported increased rates of atrial fibrillation admissions after DST transitions, had the same stance.
“We support elimination of seasonal time changes from a health perspective,” they wrote in a joint comment. “There is mounting evidence of a negative health impact with these seasonal time changes related to effects on sleep and circadian rhythm. Our work found the spring change was associated with more admissions for atrial fibrillation. This added to prior evidence of increased cardiovascular events related to these time changes. If physicians counsel patients on reducing risk factors for disease, shouldn’t we do the same as a society?”
Pros and cons
Not all sleep experts are convinced. Mary Jo Farmer, MD, PhD, FCCP, a sleep specialist and director of pulmonary hypertension services at Baystate Medical Center, and assistant professor of medicine at the University of Massachusetts, Springfield, considers perspectives from both sides of the issue.
“Daylight saving time promotes active lifestyles as people engage in more outdoor activities after work and school, [and] daylight saving time produces economic and safety benefits to society as retail revenues are higher and crimes are lower,” Dr. Farmer said. “Alternatively, moving the clocks forward is a cost burden to the U.S. economy when health issues, decreased productivity, and workplace injuries are considered.”
If one time system is permanently established, Dr. Farmer anticipates divided opinions from patients with sleep issues, regardless of which system is chosen.
“I can tell you, I have a cohort of sleep patients who prefer more evening light and look forward to the spring time change to daylight saving time,” she said. “However, they would not want the sun coming up at 9:00 a.m. in the winter months if we stayed on daylight saving time year-round. Similarly, patients would not want the sun coming up at 4:00 a.m. on the longest day of the year if we stayed on standard time all year round.”
Dr. Farmer called for more research before a decision is made.
“I suggest we need more information about the dangers of staying on daylight saving or standard time year-round because perhaps the current strategy of keeping morning light consistent is not so bad,” she said.
Time for a Congressional hearing?
According to Dr. Ramar, the time is now for a Congressional hearing, as lawmakers and the public need to be adequately informed when considering new legislation.
“There are public misconceptions about daylight saving time and standard time,” Dr. Ramar said. “People often like the idea of daylight saving time because they think it provides more light, and they dislike the concept of standard time because they think it provides more darkness. The reality is that neither time system provides more light or darkness than the other; it is only the timing that changes.”
Until new legislation is introduced, Dr. Ramar offered some practical advice for navigating seasonal time shifts.
“Beginning 2-3 days before the time change, it can be helpful to gradually adjust sleep and wake times, as well as other daily routines such as meal times,” he said. “After the time change, going outside for some morning light can help adjust the timing of your internal body clock.”
The investigators reported no conflicts of interest.
Seasonal time change is now up for consideration in the U.S. Congress, prompting sleep medicine specialists to weigh in on the health impact of a major policy change.
As lawmakers in Washington propose an end to seasonal time changes by permanently establishing daylight saving time (DST), the American Academy of Sleep Medicine (AASM) is pushing for a Congressional hearing so scientists can present evidence in favor of converse legislation – to make standard time the new norm.
According to the AASM, ; however, the switch from standard time to DST incurs more risk.
“Current evidence best supports the adoption of year-round standard time, which aligns best with human circadian biology and provides distinct benefits for public health and safety,” the AASM noted in a 2020 position statement on DST.
The statement cites a number of studies that have reported associations between the switch to DST and acute, negative health outcomes, including higher rates of hospital admission, cardiovascular morbidity, atrial fibrillation, and stroke. The time shift has been associated with a spectrum of cellular, metabolic, and circadian derangements, from increased production of inflammatory markers, to higher blood pressure, and loss of sleep. These biological effects may have far-reaching consequences, including increased rates of fatal motor accidents in the days following the time change, and even increased volatility in the stock market, which may stem from cognitive deficits.
U.S. Senator Marco Rubio (R-Fla.) and others in the U.S. Congress have reintroduced the 2019 Sunshine Protection Act, legislation that would make DST permanent across the country. According to a statement on Sen. Rubio’s website, “The bill reflects the Florida legislature’s 2018 enactment of year-round DST; however, for Florida’s change to apply, a change in the federal statute is required. Fifteen other states – Arkansas, Alabama, California, Delaware, Georgia, Idaho, Louisiana, Maine, Ohio, Oregon, South Carolina, Tennessee, Utah, Washington, and Wyoming – have passed similar laws, resolutions, or voter initiatives, and dozens more are looking. The legislation, if enacted, would apply to those states [that] currently participate in DST, which most states observe for eight months out of the year.”
A stitch in time
“The sudden change in clock time disrupts sleep/wake patterns, decreasing total sleep time and sleep quality, leading to decrements in daytime cognition,” said Kannan Ramar, MBBS, MD, president of the AASM and a sleep medicine specialist at Mayo Clinic, Rochester, Minn.
Emphasizing this point, Dr. Ramar noted a recent study that reported an 18% increase in “patient safety-related incidents associated with human error” among health care workers within a week of the spring time change.
“Irregular bedtimes and wake times disrupt the timing of our circadian rhythms, which can lead to symptoms of insomnia or long-term, excessive daytime sleepiness. Lack of sleep can lead to numerous adverse effects on our minds, including decreased cognitive function, trouble concentrating, and general moodiness,” Dr. Ramar said.
He noted that these impacts may be more significant among certain individuals.
“The daylight saving time changes can be especially problematic for any populations that already experience chronic insufficient sleep or other sleep difficulties,” Dr. Ramar said. “Populations at greatest risk include teenagers, who tend to experience chronic sleep restriction during the school week, and night shift workers, who often struggle to sleep well during daytime hours.”
While fewer studies have evaluated the long-term effects of seasonal time changes, the AASM position statement cited evidence that “the body clock does not adjust to daylight saving time after several months,” possibly because “daylight saving time is less well-aligned with intrinsic human circadian physiology, and it disrupts the natural seasonal adjustment of the human clock due to the effect of late-evening light on the circadian rhythm.”
According to the AASM, permanent DST, as proposed by Sen. Rubio and colleagues, could “result in permanent phase delay, a condition that can also lead to a perpetual discrepancy between the innate biological clock and the extrinsic environmental clock, as well as chronic sleep loss due to early morning social demands that truncate the opportunity to sleep.” This mismatch between sleep/wake cycles and social demands, known as “social jet lag,” has been associated with chronic health risks, including metabolic syndrome, obesity, depression, and cardiovascular disease.
Cardiac impacts of seasonal time change
Muhammad Adeel Rishi, MD, a sleep specialist at Mayo Clinic, Eau Claire, Wis., and lead author of the AASM position statement, highlighted cardiovascular risks in a written statement for this article, noting increased rates of heart attack following the spring time change, and a higher risk of atrial fibrillation.
“Mayo Clinic has not taken a position on this issue,” Dr. Rishi noted. Still, he advocated for permanent standard time as the author of the AASM position statement and vice chair of the AASM public safety committee.
Jay Chudow, MD, and Andrew K. Krumerman, MD, of Montefiore Medical Center, New York, lead author and principal author, respectively, of a recent study that reported increased rates of atrial fibrillation admissions after DST transitions, had the same stance.
“We support elimination of seasonal time changes from a health perspective,” they wrote in a joint comment. “There is mounting evidence of a negative health impact with these seasonal time changes related to effects on sleep and circadian rhythm. Our work found the spring change was associated with more admissions for atrial fibrillation. This added to prior evidence of increased cardiovascular events related to these time changes. If physicians counsel patients on reducing risk factors for disease, shouldn’t we do the same as a society?”
Pros and cons
Not all sleep experts are convinced. Mary Jo Farmer, MD, PhD, FCCP, a sleep specialist and director of pulmonary hypertension services at Baystate Medical Center, and assistant professor of medicine at the University of Massachusetts, Springfield, considers perspectives from both sides of the issue.
“Daylight saving time promotes active lifestyles as people engage in more outdoor activities after work and school, [and] daylight saving time produces economic and safety benefits to society as retail revenues are higher and crimes are lower,” Dr. Farmer said. “Alternatively, moving the clocks forward is a cost burden to the U.S. economy when health issues, decreased productivity, and workplace injuries are considered.”
If one time system is permanently established, Dr. Farmer anticipates divided opinions from patients with sleep issues, regardless of which system is chosen.
“I can tell you, I have a cohort of sleep patients who prefer more evening light and look forward to the spring time change to daylight saving time,” she said. “However, they would not want the sun coming up at 9:00 a.m. in the winter months if we stayed on daylight saving time year-round. Similarly, patients would not want the sun coming up at 4:00 a.m. on the longest day of the year if we stayed on standard time all year round.”
Dr. Farmer called for more research before a decision is made.
“I suggest we need more information about the dangers of staying on daylight saving or standard time year-round because perhaps the current strategy of keeping morning light consistent is not so bad,” she said.
Time for a Congressional hearing?
According to Dr. Ramar, the time is now for a Congressional hearing, as lawmakers and the public need to be adequately informed when considering new legislation.
“There are public misconceptions about daylight saving time and standard time,” Dr. Ramar said. “People often like the idea of daylight saving time because they think it provides more light, and they dislike the concept of standard time because they think it provides more darkness. The reality is that neither time system provides more light or darkness than the other; it is only the timing that changes.”
Until new legislation is introduced, Dr. Ramar offered some practical advice for navigating seasonal time shifts.
“Beginning 2-3 days before the time change, it can be helpful to gradually adjust sleep and wake times, as well as other daily routines such as meal times,” he said. “After the time change, going outside for some morning light can help adjust the timing of your internal body clock.”
The investigators reported no conflicts of interest.
Less sleep, more burnout linked to higher COVID-19 risk, study shows
among health care workers considered to be at high risk for exposure to patients with COVID-19, new evidence reveals.
For each additional hour of sleep at night, for example, risk for COVID-19 dropped by 12% in a study of 2844 frontline health care workers.
Furthermore, those who reported experiencing work-related burnout every day were 2.6 times more likely to report having COVID-19, to report having COVID-19 for a longer time, and to experience COVID-19 of more severity.
“This study underscores the importance of non–hygiene-related risk factors for COVID-19 and supports a holistic approach to health – including optimal sleep and job stress reduction to protect our health care workers from this and future pandemics,” senior author Sara B. Seidelmann, MD, said in an interview.
“Our findings add to the literature that sleep duration at night, sleep problems, and burnout may be risk factors for viral illnesses like COVID-19,” wrote Dr. Seidelmann and colleagues.
This is the first study to link COVID-19 risk to sleep habits – including number of hours of sleep at night, daytime napping hours, and severe sleep problems – among health care workers across multiple countries.
The study was published online March 22 in BMJ Nutrition, Prevention, and Health.
The researchers surveyed health care professionals in specialties considered to place personnel at high risk for exposure to SARS-CoV-2: critical care, emergency care, and internal medicine.
The association between sleep and burnout risk factors and COVID-19 did not vary significantly by specialty. “We didn’t detect any significant interactions between age, sex, specialty, or country,” said Dr. Seidelmann, assistant professor of clinical medicine at Columbia University College of Physicians and Surgeons, New York, and an internist at Stamford (Conn.) Hospital.
In addition to the 12% lower risk associated with each additional hour of sleep at night, each 1 additional hour of daytime napping was linked with a 6% increased risk for COVID-19 in an adjusted analysis (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.01-1.12).
Daytime napping slightly increased risk for COVID-19 in five of the six countries included in the study: France, Germany, Italy, the United Kingdom, and the United States. In contrast, in Spain, napping had a nonsignificant protective effect.
The survey asked health care workers to recall nighttime sleep duration, sleep disorders, and burnout in the year prior to onset of the COVID-19 pandemic.
‘Significant, close contact’ with COVID-19?
Lead author Hyunju Kim, NP, Dr. Seidelmann, and colleagues conducted the population-based, case-control study from July 17 to Sept. 25, 2020. They identified health care workers from the SurveyHealthcareGlobus (SHG) network.
Of the respondents, 72% were men. The mean age of the participants was 48 years, and the study population was 77% White, 12% Asian, 6% mixed background, 2% Black, and 1% other. (The remainder preferred not to say).
The 568 health care workers considered to have COVID-19 were classified on the basis of self-reported symptoms. Control participants had no symptoms associated with COVID-19.
All 2,844 participants answered yes to a question about having “significant close contact” with COVID-19 patients in their workplace.
Compared to reporting no sleep problems, having three such problems – difficulty sleeping at night, poor sleep continuity, and frequent use of sleeping pills – was associated with 88% greater odds of COVID-19 (OR, 1.88; 95% CI, 1.17–3.01).
Having one sleep problem was not associated with COVID-19.
More burnout, greater risk
The health care workers reported the severity of any work-related burnout. “There was a significant dose-response relationship between frequency of burnout and COVID-19,” the researchers noted.
Those who reported having burnout rarely or weekly had a 1.3-1.4 greater chance of reporting COVID-19 compared to those who reported having no burnout, for example.
In addition, reporting a high level of burnout was linked to about three times the risk for having COVID-19 of longer duration and of greater severity.
What drives the association between sleep problems, burnout, and higher risk for COVID-19 and severe COVID-19 remains unknown.
“The mechanism underlying these associations isn’t clear, but suboptimal sleep, sleep disorders, and stress may result in immune system dysregulation, increased inflammation, and alterations in hormones such as cortisol and melatonin that may increase vulnerability to viral infections,” Dr. Seidelmann said.
Strengths and limitations
Using a large network of health care workers in the early phase of the pandemic is a strength of the study. How generalizable the findings are outside the SHG database of 1.5 million health care workers remains unknown.
Another limitation was reliance on self-reporting of COVID-19 patient exposure, outcomes, and covariates, which could have introduced bias.
“However,” the researchers noted, “health care workers are likely a reliable source of information.”
Insomnia a common challenge
A 2020 meta-analysis examined the effect of insomnia and psychological factors on COVID-19 risk among health care workers. Lead author Kavita Batra, PhD, of the University of Nevada, Las Vegas (UNLV), and colleagues found that the pooled prevalence of insomnia was almost 28%.
“The recent six-country study by Kim and colleagues also underscores this relationship between lack of sleep and having higher odds of COVID-19 infection,” Manoj Sharma, MBBS, PhD, professor of social and behavioral health in the UNLV department of environmental and occupational health, and one of the study authors, said in an interview.
More research is warranted to learn the direction of the association, he said. Does reduced sleep lower immunity and make a health care worker more susceptible to SARS-CoV-2 infection, or does the anxiety associated with COVID-19 contribute to insomnia?
“Practicing sleep hygiene is a must not only for health workers but also for everyone,” Dr. Sharma added. Recommendations include having fixed hours of going to bed, fixed hours of waking up, not overdoing naps, having at least 30 minutes of winding down before sleeping, having a dark bedroom devoid of all electronics and other disturbances, avoiding smoking, alcohol, and stimulants (such as caffeine) before sleeping, and practicing relaxation right before sleeping, he said.
“It is hard for some health care workers, especially those who work night shifts, but it must be a priority to follow as many sleep hygiene measures as possible,” Dr. Sharma said. “After all, if you do not take care of yourself how can you take care of others?”
Dr. Seidelmann, Dr. Batra, and Dr. Sharma have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
among health care workers considered to be at high risk for exposure to patients with COVID-19, new evidence reveals.
For each additional hour of sleep at night, for example, risk for COVID-19 dropped by 12% in a study of 2844 frontline health care workers.
Furthermore, those who reported experiencing work-related burnout every day were 2.6 times more likely to report having COVID-19, to report having COVID-19 for a longer time, and to experience COVID-19 of more severity.
“This study underscores the importance of non–hygiene-related risk factors for COVID-19 and supports a holistic approach to health – including optimal sleep and job stress reduction to protect our health care workers from this and future pandemics,” senior author Sara B. Seidelmann, MD, said in an interview.
“Our findings add to the literature that sleep duration at night, sleep problems, and burnout may be risk factors for viral illnesses like COVID-19,” wrote Dr. Seidelmann and colleagues.
This is the first study to link COVID-19 risk to sleep habits – including number of hours of sleep at night, daytime napping hours, and severe sleep problems – among health care workers across multiple countries.
The study was published online March 22 in BMJ Nutrition, Prevention, and Health.
The researchers surveyed health care professionals in specialties considered to place personnel at high risk for exposure to SARS-CoV-2: critical care, emergency care, and internal medicine.
The association between sleep and burnout risk factors and COVID-19 did not vary significantly by specialty. “We didn’t detect any significant interactions between age, sex, specialty, or country,” said Dr. Seidelmann, assistant professor of clinical medicine at Columbia University College of Physicians and Surgeons, New York, and an internist at Stamford (Conn.) Hospital.
In addition to the 12% lower risk associated with each additional hour of sleep at night, each 1 additional hour of daytime napping was linked with a 6% increased risk for COVID-19 in an adjusted analysis (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.01-1.12).
Daytime napping slightly increased risk for COVID-19 in five of the six countries included in the study: France, Germany, Italy, the United Kingdom, and the United States. In contrast, in Spain, napping had a nonsignificant protective effect.
The survey asked health care workers to recall nighttime sleep duration, sleep disorders, and burnout in the year prior to onset of the COVID-19 pandemic.
‘Significant, close contact’ with COVID-19?
Lead author Hyunju Kim, NP, Dr. Seidelmann, and colleagues conducted the population-based, case-control study from July 17 to Sept. 25, 2020. They identified health care workers from the SurveyHealthcareGlobus (SHG) network.
Of the respondents, 72% were men. The mean age of the participants was 48 years, and the study population was 77% White, 12% Asian, 6% mixed background, 2% Black, and 1% other. (The remainder preferred not to say).
The 568 health care workers considered to have COVID-19 were classified on the basis of self-reported symptoms. Control participants had no symptoms associated with COVID-19.
All 2,844 participants answered yes to a question about having “significant close contact” with COVID-19 patients in their workplace.
Compared to reporting no sleep problems, having three such problems – difficulty sleeping at night, poor sleep continuity, and frequent use of sleeping pills – was associated with 88% greater odds of COVID-19 (OR, 1.88; 95% CI, 1.17–3.01).
Having one sleep problem was not associated with COVID-19.
More burnout, greater risk
The health care workers reported the severity of any work-related burnout. “There was a significant dose-response relationship between frequency of burnout and COVID-19,” the researchers noted.
Those who reported having burnout rarely or weekly had a 1.3-1.4 greater chance of reporting COVID-19 compared to those who reported having no burnout, for example.
In addition, reporting a high level of burnout was linked to about three times the risk for having COVID-19 of longer duration and of greater severity.
What drives the association between sleep problems, burnout, and higher risk for COVID-19 and severe COVID-19 remains unknown.
“The mechanism underlying these associations isn’t clear, but suboptimal sleep, sleep disorders, and stress may result in immune system dysregulation, increased inflammation, and alterations in hormones such as cortisol and melatonin that may increase vulnerability to viral infections,” Dr. Seidelmann said.
Strengths and limitations
Using a large network of health care workers in the early phase of the pandemic is a strength of the study. How generalizable the findings are outside the SHG database of 1.5 million health care workers remains unknown.
Another limitation was reliance on self-reporting of COVID-19 patient exposure, outcomes, and covariates, which could have introduced bias.
“However,” the researchers noted, “health care workers are likely a reliable source of information.”
Insomnia a common challenge
A 2020 meta-analysis examined the effect of insomnia and psychological factors on COVID-19 risk among health care workers. Lead author Kavita Batra, PhD, of the University of Nevada, Las Vegas (UNLV), and colleagues found that the pooled prevalence of insomnia was almost 28%.
“The recent six-country study by Kim and colleagues also underscores this relationship between lack of sleep and having higher odds of COVID-19 infection,” Manoj Sharma, MBBS, PhD, professor of social and behavioral health in the UNLV department of environmental and occupational health, and one of the study authors, said in an interview.
More research is warranted to learn the direction of the association, he said. Does reduced sleep lower immunity and make a health care worker more susceptible to SARS-CoV-2 infection, or does the anxiety associated with COVID-19 contribute to insomnia?
“Practicing sleep hygiene is a must not only for health workers but also for everyone,” Dr. Sharma added. Recommendations include having fixed hours of going to bed, fixed hours of waking up, not overdoing naps, having at least 30 minutes of winding down before sleeping, having a dark bedroom devoid of all electronics and other disturbances, avoiding smoking, alcohol, and stimulants (such as caffeine) before sleeping, and practicing relaxation right before sleeping, he said.
“It is hard for some health care workers, especially those who work night shifts, but it must be a priority to follow as many sleep hygiene measures as possible,” Dr. Sharma said. “After all, if you do not take care of yourself how can you take care of others?”
Dr. Seidelmann, Dr. Batra, and Dr. Sharma have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
among health care workers considered to be at high risk for exposure to patients with COVID-19, new evidence reveals.
For each additional hour of sleep at night, for example, risk for COVID-19 dropped by 12% in a study of 2844 frontline health care workers.
Furthermore, those who reported experiencing work-related burnout every day were 2.6 times more likely to report having COVID-19, to report having COVID-19 for a longer time, and to experience COVID-19 of more severity.
“This study underscores the importance of non–hygiene-related risk factors for COVID-19 and supports a holistic approach to health – including optimal sleep and job stress reduction to protect our health care workers from this and future pandemics,” senior author Sara B. Seidelmann, MD, said in an interview.
“Our findings add to the literature that sleep duration at night, sleep problems, and burnout may be risk factors for viral illnesses like COVID-19,” wrote Dr. Seidelmann and colleagues.
This is the first study to link COVID-19 risk to sleep habits – including number of hours of sleep at night, daytime napping hours, and severe sleep problems – among health care workers across multiple countries.
The study was published online March 22 in BMJ Nutrition, Prevention, and Health.
The researchers surveyed health care professionals in specialties considered to place personnel at high risk for exposure to SARS-CoV-2: critical care, emergency care, and internal medicine.
The association between sleep and burnout risk factors and COVID-19 did not vary significantly by specialty. “We didn’t detect any significant interactions between age, sex, specialty, or country,” said Dr. Seidelmann, assistant professor of clinical medicine at Columbia University College of Physicians and Surgeons, New York, and an internist at Stamford (Conn.) Hospital.
In addition to the 12% lower risk associated with each additional hour of sleep at night, each 1 additional hour of daytime napping was linked with a 6% increased risk for COVID-19 in an adjusted analysis (odds ratio [OR], 1.06; 95% confidence interval [CI], 1.01-1.12).
Daytime napping slightly increased risk for COVID-19 in five of the six countries included in the study: France, Germany, Italy, the United Kingdom, and the United States. In contrast, in Spain, napping had a nonsignificant protective effect.
The survey asked health care workers to recall nighttime sleep duration, sleep disorders, and burnout in the year prior to onset of the COVID-19 pandemic.
‘Significant, close contact’ with COVID-19?
Lead author Hyunju Kim, NP, Dr. Seidelmann, and colleagues conducted the population-based, case-control study from July 17 to Sept. 25, 2020. They identified health care workers from the SurveyHealthcareGlobus (SHG) network.
Of the respondents, 72% were men. The mean age of the participants was 48 years, and the study population was 77% White, 12% Asian, 6% mixed background, 2% Black, and 1% other. (The remainder preferred not to say).
The 568 health care workers considered to have COVID-19 were classified on the basis of self-reported symptoms. Control participants had no symptoms associated with COVID-19.
All 2,844 participants answered yes to a question about having “significant close contact” with COVID-19 patients in their workplace.
Compared to reporting no sleep problems, having three such problems – difficulty sleeping at night, poor sleep continuity, and frequent use of sleeping pills – was associated with 88% greater odds of COVID-19 (OR, 1.88; 95% CI, 1.17–3.01).
Having one sleep problem was not associated with COVID-19.
More burnout, greater risk
The health care workers reported the severity of any work-related burnout. “There was a significant dose-response relationship between frequency of burnout and COVID-19,” the researchers noted.
Those who reported having burnout rarely or weekly had a 1.3-1.4 greater chance of reporting COVID-19 compared to those who reported having no burnout, for example.
In addition, reporting a high level of burnout was linked to about three times the risk for having COVID-19 of longer duration and of greater severity.
What drives the association between sleep problems, burnout, and higher risk for COVID-19 and severe COVID-19 remains unknown.
“The mechanism underlying these associations isn’t clear, but suboptimal sleep, sleep disorders, and stress may result in immune system dysregulation, increased inflammation, and alterations in hormones such as cortisol and melatonin that may increase vulnerability to viral infections,” Dr. Seidelmann said.
Strengths and limitations
Using a large network of health care workers in the early phase of the pandemic is a strength of the study. How generalizable the findings are outside the SHG database of 1.5 million health care workers remains unknown.
Another limitation was reliance on self-reporting of COVID-19 patient exposure, outcomes, and covariates, which could have introduced bias.
“However,” the researchers noted, “health care workers are likely a reliable source of information.”
Insomnia a common challenge
A 2020 meta-analysis examined the effect of insomnia and psychological factors on COVID-19 risk among health care workers. Lead author Kavita Batra, PhD, of the University of Nevada, Las Vegas (UNLV), and colleagues found that the pooled prevalence of insomnia was almost 28%.
“The recent six-country study by Kim and colleagues also underscores this relationship between lack of sleep and having higher odds of COVID-19 infection,” Manoj Sharma, MBBS, PhD, professor of social and behavioral health in the UNLV department of environmental and occupational health, and one of the study authors, said in an interview.
More research is warranted to learn the direction of the association, he said. Does reduced sleep lower immunity and make a health care worker more susceptible to SARS-CoV-2 infection, or does the anxiety associated with COVID-19 contribute to insomnia?
“Practicing sleep hygiene is a must not only for health workers but also for everyone,” Dr. Sharma added. Recommendations include having fixed hours of going to bed, fixed hours of waking up, not overdoing naps, having at least 30 minutes of winding down before sleeping, having a dark bedroom devoid of all electronics and other disturbances, avoiding smoking, alcohol, and stimulants (such as caffeine) before sleeping, and practicing relaxation right before sleeping, he said.
“It is hard for some health care workers, especially those who work night shifts, but it must be a priority to follow as many sleep hygiene measures as possible,” Dr. Sharma said. “After all, if you do not take care of yourself how can you take care of others?”
Dr. Seidelmann, Dr. Batra, and Dr. Sharma have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Here we go again? Rate of COVID-19 in children takes a turn for the worse
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
Match Day 2021: Pediatrics experiences slow, steady growth
Match Day 2021 was another record breaker, despite the pandemic, and pediatrics played its part, adding nearly 40 more slots than 2020 and filling nearly 50 more, according to the National Resident Matching Program (NRMP).
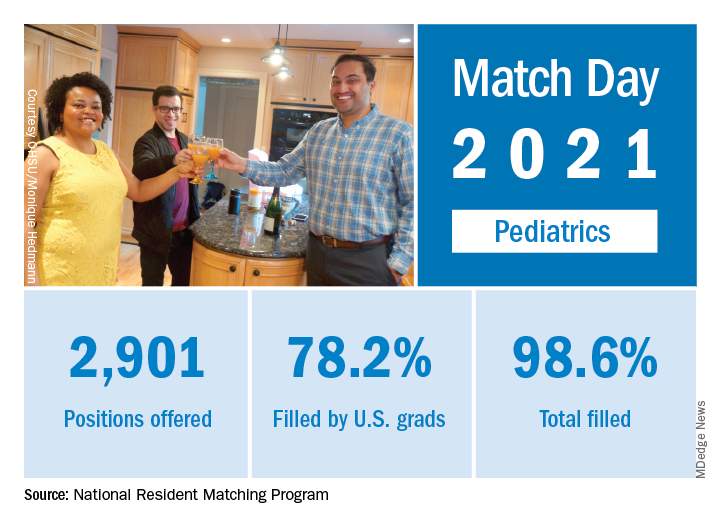
“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a press release. Overall, 35,194 first-year (PGY-1) slots were offered and 33,353 were filled, both more than ever before, for a fill rate of 94.8%, a slight increase from the 94.6% fill rate last year.
Pediatrics offered 2,901 slots in 2021, up from 2,864 in 2020, though the proportion of pediatrics slots in the overall total fell slightly to 8.2% from 8.4% in 2020. Of those 2,901 slots, 2,860 were filled, for a fill rate of 98.6%, up from 98.2% last year. Of those filled positions, 60.3% were filled by MD seniors, and 78.2% were filled by U.S. graduates.
Since 2017, pediatrics has offered more slots every year, rising from 2,738 in 2017 up to the 2,901 in 2021, an overall growth rate of just under 6%.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized, [as] growth in registration was seen in every applicant group,” the NRMP noted. Rank-order lists submissions in 2021 were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen international medical graduates, and 15.0% for non–U.S.-citizen IMGs, compared with 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. ... The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in the press release.
Match Day 2021 was another record breaker, despite the pandemic, and pediatrics played its part, adding nearly 40 more slots than 2020 and filling nearly 50 more, according to the National Resident Matching Program (NRMP).

“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a press release. Overall, 35,194 first-year (PGY-1) slots were offered and 33,353 were filled, both more than ever before, for a fill rate of 94.8%, a slight increase from the 94.6% fill rate last year.
Pediatrics offered 2,901 slots in 2021, up from 2,864 in 2020, though the proportion of pediatrics slots in the overall total fell slightly to 8.2% from 8.4% in 2020. Of those 2,901 slots, 2,860 were filled, for a fill rate of 98.6%, up from 98.2% last year. Of those filled positions, 60.3% were filled by MD seniors, and 78.2% were filled by U.S. graduates.
Since 2017, pediatrics has offered more slots every year, rising from 2,738 in 2017 up to the 2,901 in 2021, an overall growth rate of just under 6%.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized, [as] growth in registration was seen in every applicant group,” the NRMP noted. Rank-order lists submissions in 2021 were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen international medical graduates, and 15.0% for non–U.S.-citizen IMGs, compared with 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. ... The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in the press release.
Match Day 2021 was another record breaker, despite the pandemic, and pediatrics played its part, adding nearly 40 more slots than 2020 and filling nearly 50 more, according to the National Resident Matching Program (NRMP).

“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a press release. Overall, 35,194 first-year (PGY-1) slots were offered and 33,353 were filled, both more than ever before, for a fill rate of 94.8%, a slight increase from the 94.6% fill rate last year.
Pediatrics offered 2,901 slots in 2021, up from 2,864 in 2020, though the proportion of pediatrics slots in the overall total fell slightly to 8.2% from 8.4% in 2020. Of those 2,901 slots, 2,860 were filled, for a fill rate of 98.6%, up from 98.2% last year. Of those filled positions, 60.3% were filled by MD seniors, and 78.2% were filled by U.S. graduates.
Since 2017, pediatrics has offered more slots every year, rising from 2,738 in 2017 up to the 2,901 in 2021, an overall growth rate of just under 6%.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized, [as] growth in registration was seen in every applicant group,” the NRMP noted. Rank-order lists submissions in 2021 were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen international medical graduates, and 15.0% for non–U.S.-citizen IMGs, compared with 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. ... The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in the press release.
Black nonsmokers still at high risk for secondhand smoke exposure
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.
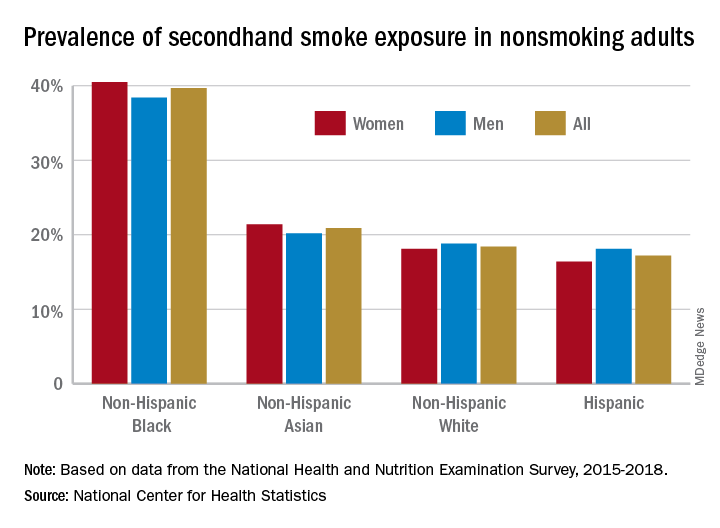
While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.

While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.

While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.













