User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Prenatal dietary folate not enough to offset AEDs’ effect on kids’ cognition
New research underscores the importance of folic acid supplementation for pregnant women with epilepsy who are taking antiepileptic drugs (AEDs).
Dietary folate alone, even in the United States, where food is fortified with folic acid, is “not sufficient” to improve cognitive outcomes for children of women who take AEDs during pregnancy, the researchers report.
“We found that dietary folate was not related to outcomes,” study investigator Kimford Meador, MD, professor of neurology and neurologic sciences, Stanford (Calif.) University, told this news organization.
“Only when the mother was taking extra folate did we see an improvement in child outcomes,” he added.
The findings were published online Feb. 23 in Epilepsy and Behavior.
Cognitive boost
“Daily folate is recommended to women in the general populations to reduce congenital malformations,” Dr. Meador said. In addition, periconceptional use of folate has been shown in previous research to improve neurodevelopmental outcomes for children of mothers with epilepsy who are taking AEDs.
Whether folate-fortified food alone, without supplements, has any effect on cognitive outcomes in this population of children has not been examined previously.
To investigate, the researchers assessed 117 children from the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, a prospective, observational study of women with epilepsy who were taking one of four AEDs: carbamazepine, lamotrigine, phenytoin, or valproate.
Results showed that dietary folate from fortified food alone, without supplements, had no significant impact on IQ at age 6 years among children with prenatal exposure to AEDs.
In contrast, use of periconceptual folate supplements was significantly associated with a 10-point higher IQ at age 6 in the adjusted analyses (95% confidence interval, 5.2-15.0; P < .001).
These six other nutrients from food and supplements had no significant association with IQ at age 6 years: vitamins C, D, and E, omega-3, gamma tocopherol, and vitamin B12.
Optimal dose unclear
The findings indicate that folates, including natural folate and folic acid, in food do not have positive cognitive effects for children of women with epilepsy who take AEDs, the researchers write.
Dr. Meador noted that the optimal dose of folic acid supplementation to provide a cognitive benefit remains unclear.
The U.S. Centers for Disease Control recommends 0.4 mg/d for the general population of women of childbearing age. In Europe, the recommendation is 1 mg/d.
“Higher doses are recommended if there is a personal or family history of spina bifida in prior pregnancies, but there is some concern that very high doses of folate may be detrimental,” Dr. Meador said.
For women with epilepsy, he would recommend “at least 1 mg/d and not more than 4 mg/d.”
Proves a point?
Commenting on the study for this news organization, Derek Chong, MD, vice chair of neurology and director of epilepsy at Lenox Hill Hospital, New York, said the finding that folate fortification of food alone is not adequate for women with epilepsy is “not groundbreaking” but does prove something previously thought.
“Folic acid is important for all women, but it does seem like folic acid may be even more important in the epilepsy population,” said Dr. Chong, who was not involved with the research.
He cautioned that the current analysis included only four medications, three of which are not used very often anymore.
“Lamotrigine is probably the most commonly used one now. It’s unfortunate that this study did not include Keppra [levetiracetam], which probably is the number one medication that we use now,” Dr. Chong said.
The research was supported by the National Institutes of Health. Dr. Meador and Dr. Chong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research underscores the importance of folic acid supplementation for pregnant women with epilepsy who are taking antiepileptic drugs (AEDs).
Dietary folate alone, even in the United States, where food is fortified with folic acid, is “not sufficient” to improve cognitive outcomes for children of women who take AEDs during pregnancy, the researchers report.
“We found that dietary folate was not related to outcomes,” study investigator Kimford Meador, MD, professor of neurology and neurologic sciences, Stanford (Calif.) University, told this news organization.
“Only when the mother was taking extra folate did we see an improvement in child outcomes,” he added.
The findings were published online Feb. 23 in Epilepsy and Behavior.
Cognitive boost
“Daily folate is recommended to women in the general populations to reduce congenital malformations,” Dr. Meador said. In addition, periconceptional use of folate has been shown in previous research to improve neurodevelopmental outcomes for children of mothers with epilepsy who are taking AEDs.
Whether folate-fortified food alone, without supplements, has any effect on cognitive outcomes in this population of children has not been examined previously.
To investigate, the researchers assessed 117 children from the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, a prospective, observational study of women with epilepsy who were taking one of four AEDs: carbamazepine, lamotrigine, phenytoin, or valproate.
Results showed that dietary folate from fortified food alone, without supplements, had no significant impact on IQ at age 6 years among children with prenatal exposure to AEDs.
In contrast, use of periconceptual folate supplements was significantly associated with a 10-point higher IQ at age 6 in the adjusted analyses (95% confidence interval, 5.2-15.0; P < .001).
These six other nutrients from food and supplements had no significant association with IQ at age 6 years: vitamins C, D, and E, omega-3, gamma tocopherol, and vitamin B12.
Optimal dose unclear
The findings indicate that folates, including natural folate and folic acid, in food do not have positive cognitive effects for children of women with epilepsy who take AEDs, the researchers write.
Dr. Meador noted that the optimal dose of folic acid supplementation to provide a cognitive benefit remains unclear.
The U.S. Centers for Disease Control recommends 0.4 mg/d for the general population of women of childbearing age. In Europe, the recommendation is 1 mg/d.
“Higher doses are recommended if there is a personal or family history of spina bifida in prior pregnancies, but there is some concern that very high doses of folate may be detrimental,” Dr. Meador said.
For women with epilepsy, he would recommend “at least 1 mg/d and not more than 4 mg/d.”
Proves a point?
Commenting on the study for this news organization, Derek Chong, MD, vice chair of neurology and director of epilepsy at Lenox Hill Hospital, New York, said the finding that folate fortification of food alone is not adequate for women with epilepsy is “not groundbreaking” but does prove something previously thought.
“Folic acid is important for all women, but it does seem like folic acid may be even more important in the epilepsy population,” said Dr. Chong, who was not involved with the research.
He cautioned that the current analysis included only four medications, three of which are not used very often anymore.
“Lamotrigine is probably the most commonly used one now. It’s unfortunate that this study did not include Keppra [levetiracetam], which probably is the number one medication that we use now,” Dr. Chong said.
The research was supported by the National Institutes of Health. Dr. Meador and Dr. Chong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research underscores the importance of folic acid supplementation for pregnant women with epilepsy who are taking antiepileptic drugs (AEDs).
Dietary folate alone, even in the United States, where food is fortified with folic acid, is “not sufficient” to improve cognitive outcomes for children of women who take AEDs during pregnancy, the researchers report.
“We found that dietary folate was not related to outcomes,” study investigator Kimford Meador, MD, professor of neurology and neurologic sciences, Stanford (Calif.) University, told this news organization.
“Only when the mother was taking extra folate did we see an improvement in child outcomes,” he added.
The findings were published online Feb. 23 in Epilepsy and Behavior.
Cognitive boost
“Daily folate is recommended to women in the general populations to reduce congenital malformations,” Dr. Meador said. In addition, periconceptional use of folate has been shown in previous research to improve neurodevelopmental outcomes for children of mothers with epilepsy who are taking AEDs.
Whether folate-fortified food alone, without supplements, has any effect on cognitive outcomes in this population of children has not been examined previously.
To investigate, the researchers assessed 117 children from the Neurodevelopmental Effects of Antiepileptic Drugs (NEAD) study, a prospective, observational study of women with epilepsy who were taking one of four AEDs: carbamazepine, lamotrigine, phenytoin, or valproate.
Results showed that dietary folate from fortified food alone, without supplements, had no significant impact on IQ at age 6 years among children with prenatal exposure to AEDs.
In contrast, use of periconceptual folate supplements was significantly associated with a 10-point higher IQ at age 6 in the adjusted analyses (95% confidence interval, 5.2-15.0; P < .001).
These six other nutrients from food and supplements had no significant association with IQ at age 6 years: vitamins C, D, and E, omega-3, gamma tocopherol, and vitamin B12.
Optimal dose unclear
The findings indicate that folates, including natural folate and folic acid, in food do not have positive cognitive effects for children of women with epilepsy who take AEDs, the researchers write.
Dr. Meador noted that the optimal dose of folic acid supplementation to provide a cognitive benefit remains unclear.
The U.S. Centers for Disease Control recommends 0.4 mg/d for the general population of women of childbearing age. In Europe, the recommendation is 1 mg/d.
“Higher doses are recommended if there is a personal or family history of spina bifida in prior pregnancies, but there is some concern that very high doses of folate may be detrimental,” Dr. Meador said.
For women with epilepsy, he would recommend “at least 1 mg/d and not more than 4 mg/d.”
Proves a point?
Commenting on the study for this news organization, Derek Chong, MD, vice chair of neurology and director of epilepsy at Lenox Hill Hospital, New York, said the finding that folate fortification of food alone is not adequate for women with epilepsy is “not groundbreaking” but does prove something previously thought.
“Folic acid is important for all women, but it does seem like folic acid may be even more important in the epilepsy population,” said Dr. Chong, who was not involved with the research.
He cautioned that the current analysis included only four medications, three of which are not used very often anymore.
“Lamotrigine is probably the most commonly used one now. It’s unfortunate that this study did not include Keppra [levetiracetam], which probably is the number one medication that we use now,” Dr. Chong said.
The research was supported by the National Institutes of Health. Dr. Meador and Dr. Chong have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Ruxolitinib cream for atopic dermatitis is in regulatory home stretch
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
, demonstrated a dual mechanism of action in two pivotal phase 3 trials: antipruritic and anti-inflammatory, Kim A. Papp, MD, PhD, said at Innovations in Dermatology: Virtual Spring Conference 2021. He presented a pooled analysis of the TRuE-AD1 and TRuE-AD2 trials, in which 1,249 patients with AD affecting 3%-20% of the body surface area were randomized 2:2:1 double-blind to ruxolitinib cream 0.75%, 1.5%, or vehicle twice daily for 8 weeks.
Striking evidence of the drug’s antipruritic effect comes from the finding that patient-reported itch scores separated significantly from the vehicle controls within just 12 hours after the first application. The margin of difference grew over time such that at 4 weeks, 48.5% of patients on ruxolitinib 1.5% experienced a clinically meaningful reduction in itch – defined by at least a 4-point improvement on the itch numeric rating scale – as did 30.1% of those on ruxolitinib 0.75% and 6.1% of controls. By week 8, these figures had further improved to 51.5%, 41.5%, and 15.8%, respectively, noted Dr. Papp, a dermatologist and president of Probity Medical Research in Waterloo, Ont.
Ruxolitinib’s anti-inflammatory mechanism of action was on display in the primary study endpoint, which was the proportion of patients achieving an Investigator Global Assessment score of 0 or 1 with at least a 2-grade improvement from baseline at week 8. The rates were 52.6% with ruxolitinib 1.5% and 44.7% at the lower dose, both significantly better than the 11.5% rate with vehicle.
For the secondary endpoint of at least a 75% improvement in Eczema Area and Severity Index score at week 8, the rates were 62% with ruxolitinib 1.5% and 53.8% at the 0.75% concentration, compared with 19.7% with vehicle.
The topical JAK inhibitor also showed superior efficacy in terms of improvement on the Patient-Reported Outcomes Measurement Information System Sleep Disturbance Score, with a clinically meaningful 6-point or greater improvement in 23.9% and 20.9% of patients in the high- and low-dose ruxolitinib groups, versus 14.2% in controls.
Plasma drug levels remained consistently low and near-flat throughout the study.
Session comoderator Lawrence F. Eichenfield, MD, was struck by what he termed the “incredibly low” rates of irritancy, burning, and stinging in the ruxolitinib-treated patients: 7 cases of application-site burning in 999 treated patients, compared with 11 cases in 250 vehicle-treated patients, and 4 cases of application-site pruritus in nearly 1,000 patients on ruxolitinib, versus 6 cases in one-fourth as many controls.
“If that’s really true in clinical practice, it would be tremendous to have a nonsteroid that doesn’t have stinging and burning and may have that efficacy,” said Dr. Eichenfield, professor of dermatology and pediatrics and vice-chair of dermatology at the University of California, San Diego.
“I think the fast action is an exciting aspect of this,” said comoderator Jonathan I. Silverberg, MD, PhD, MBA, director of clinical research and contact dermatitis in the department of dermatology at George Washington University in Washington.
He noted that in an earlier phase 2 study, ruxolitinib cream was at least as efficacious as 0.1% triamcinolone cream, providing dermatologists with a rough yardstick as to where the topical JAK inhibitor lies on the potency spectrum for AD treatment.
The FDA is expected to issue a decision on the application for approval of ruxolitinib cream in June. Dr. Eichenfield expects the drug to easily win approval. The big unanswered question is whether the regulatory agency will require boxed safety warnings, as it does for the oral JAK inhibitors approved for various indications, even though safety issues haven’t arisen with the topical agent in the clinical trials.
Dr. Papp reported receiving research grants from and serving as a consultant to Incyte Corp., which funded the ruxolitinib studies, as well as numerous other pharmaceutical companies. MedscapeLive and this news organization are owned by the same parent company.
FROM INNOVATIONS IN DERMATOLOGY
THC persists in breast milk 6 weeks after quitting cannabis
Delta-9-Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, remains detectable in breast milk even after weeks of abstinence, new data show. The estimated half-life of THC in breast milk is 17 days, according to the study results, with a projected time to elimination of more than 6 weeks. The clinical importance of the remaining THC is up for debate, according to some experts.
“To limit THC effects on fetal brain development and promote safe breastfeeding, it is critical to emphasize marijuana abstention both early in pregnancy and post partum,” Erica M. Wymore, MD, MPH, an assistant professor of pediatrics and neonatology at the University of Colorado at Denver, Aurora, and colleagues wrote. The group published their results online March 8, 2021, in JAMA Pediatrics.
And while the study was a pharmacokinetic analysis rather than a safety investigation, Dr. Wymore said in an interview that the detectable levels of THC suggest any use is of concern and no safety thresholds have been established. “We wish we had more data on the potential effects on the neurocognitive development of children, but for now we must discourage any use in prepregnancy, pregnancy, and breastfeeding, as our national guidelines recommend.”
Therefore, the findings support current guidelines discouraging any cannabis use in mothers-to-be and breast-feeding mothers issued by national organizations, including those from the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the Academy of Breastfeeding Medicine.
Furthermore, the difficulties many mothers face in abstaining from marijuana, a commonly used drug in pregnancy, and the persistence of THC in maternal milk led the authors to question the feasibility of having women who use marijuana simply discard their breast milk until THC is cleared.
“We report challenges in abstention and prolonged excretion of THC in breast milk greater than 6 weeks among women with prenatal marijuana use,” they wrote. “These findings make the recommendations for mothers to discard breast milk until THC is undetectable unrealistic for mothers committed to breastfeeding.”
However, not all experts are equally concerned about low THC concentrations in breast milk. Neonatal pharmacologist Thomas R. Hale, PhD, a professor of pediatrics at Texas Tech University, Lubbock, said a previous study by his group showed that THC levels in maternal milk peaked within 60 minutes of a moderate dose of inhaled marijuana and fell to quite low levels over the next 4 hours. The highest concentration in maternal milk occurred shortly after the peak in plasma.
“So you can see that, just because a mom is drug screen positive, the clinical dose transferred to the infant is probably exceedingly low,” he said in an interview.
Dr. Hale also stressed that judgments about drugs in this context should weigh the risk of the drug against the risk of not breastfeeding. “All of us caution women not to use cannabis when pregnant or breastfeeding,” Dr. Hale said. “But when the decision has to be made as to whether a mom breastfeeds or not if she is drug screen positive, a lot of other factors must be analyzed to make such a decision.”
Study cohort
For the study, Dr. Wymore and colleagues screened 394 women who gave birth between Nov. 1, 2016, and June 30, 2019. Of those, 25 women, with a median age of 26 years, were eligible and enrolled. Inclusion criteria included known prenatal marijuana use, intention to breastfeed, and self-reported abstinence. Prenatal use primarily involved inhaling cannabis more than twice a week.
Of the 25 enrolled mothers, 12 who self-reported marijuana abstinence were in fact found to be abstinent according to the results of plasma analysis. Those who continued to use the substance were younger than the overall sample, with a median age of 21, and were less likely to have attended college (23%) than abstainers (58%).
The researchers prospectively collected data on self-reported marijuana usage and paired maternal plasma and breast milk samples several times a week. All participants had detectable THC in breast milk throughout the study. Initial median THC concentrations were 3.2 ng/mL (interquartile range, 1.2-6.8) within the first week after delivery. These increased to 5.5 ng/mL (IQR, 4.4-16.0) at 2 weeks and declined to 1.9 ng/mL (IQR, 1.1-4.3) at 6 weeks. In terms of ratio, the milk:plasma partition coefficient for THC was approximately 6:1 (IQR, 3.8:1-8.1:1).
Dr. Hale noted that, although THC was detectable in milk, the levels were exceedingly low. “This is where the risk assessment comes in. There’s a lot of hysteria in the cannabis field right now, and we’re going to need time and a lot more studies to really be able to predict any untoward complications.”
Dr. Wymore, however, countered that THC levels were low only in those who abstained and that her concerns relate not just to postpartum breast milk levels but the health effects on children of mothers’ cannabis use over the course of prepregnancy, pregnancy, and lactation. “[Dr. Hale’s] message makes it difficult for clinicians to counsel mothers since it goes against national guidelines,” she said. “We need to be consistent.”
But Dr. Wymore and other experts acknowledge the dilemma faced in that breast milk clearly offers substantial benefits for infant and child health. “The risks of an infant’s exposure to marijuana versus the benefit of breast milk must be considered,” said Amy B. Hair, MD, assistant professor of pediatrics and neonatal medicine at Baylor College of Medicine, Houston, who was not involved in the Colorado study. “And it’s unrealistic, as the study suggests, for mothers to discard breast milk for 6 weeks.”
Nevertheless, calling the findings of THC persistence after abstinence “troublesome,” Dr. Hair said the legalization of marijuana in some states gives the public the impression it’s safe to use marijuana even during pregnancy and lactation. “Research studies, however, are concerning for potential detrimental effects on brain growth and development in infants whose mothers use marijuana during pregnancy and breastfeeding,” she added.
Dr. Wymore stressed that more U.S. cannabis dispensaries must engage in rigorous point-of-sale counseling to women on the potential harms during pregnancy. This is the case in Canada, she noted, where recreational and medicinal cannabis has been legal since 2018 and more than 90% of outlets (vs. two thirds of their U.S. counterparts) advise women not to use cannabis during pregnancy or lactation, even for nausea.
“This is where many women are getting their information on cannabis,” she said. “We learned the hard way with alcohol and we don’t want to make the same mistake with marijuana.”
The study was funded by the Colorado Department of Public Health and Environment, the Children’s Hospital Colorado Research Institute, the Colorado Fetal Care Center, the Colorado Perinatal Clinical and Translational Research Center, and the Children’s Colorado Research Institute. Two study coauthors disclosed relationships with the private sector outside the submitted work. Dr. Hale and Dr. Hair have disclosed no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Delta-9-Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, remains detectable in breast milk even after weeks of abstinence, new data show. The estimated half-life of THC in breast milk is 17 days, according to the study results, with a projected time to elimination of more than 6 weeks. The clinical importance of the remaining THC is up for debate, according to some experts.
“To limit THC effects on fetal brain development and promote safe breastfeeding, it is critical to emphasize marijuana abstention both early in pregnancy and post partum,” Erica M. Wymore, MD, MPH, an assistant professor of pediatrics and neonatology at the University of Colorado at Denver, Aurora, and colleagues wrote. The group published their results online March 8, 2021, in JAMA Pediatrics.
And while the study was a pharmacokinetic analysis rather than a safety investigation, Dr. Wymore said in an interview that the detectable levels of THC suggest any use is of concern and no safety thresholds have been established. “We wish we had more data on the potential effects on the neurocognitive development of children, but for now we must discourage any use in prepregnancy, pregnancy, and breastfeeding, as our national guidelines recommend.”
Therefore, the findings support current guidelines discouraging any cannabis use in mothers-to-be and breast-feeding mothers issued by national organizations, including those from the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the Academy of Breastfeeding Medicine.
Furthermore, the difficulties many mothers face in abstaining from marijuana, a commonly used drug in pregnancy, and the persistence of THC in maternal milk led the authors to question the feasibility of having women who use marijuana simply discard their breast milk until THC is cleared.
“We report challenges in abstention and prolonged excretion of THC in breast milk greater than 6 weeks among women with prenatal marijuana use,” they wrote. “These findings make the recommendations for mothers to discard breast milk until THC is undetectable unrealistic for mothers committed to breastfeeding.”
However, not all experts are equally concerned about low THC concentrations in breast milk. Neonatal pharmacologist Thomas R. Hale, PhD, a professor of pediatrics at Texas Tech University, Lubbock, said a previous study by his group showed that THC levels in maternal milk peaked within 60 minutes of a moderate dose of inhaled marijuana and fell to quite low levels over the next 4 hours. The highest concentration in maternal milk occurred shortly after the peak in plasma.
“So you can see that, just because a mom is drug screen positive, the clinical dose transferred to the infant is probably exceedingly low,” he said in an interview.
Dr. Hale also stressed that judgments about drugs in this context should weigh the risk of the drug against the risk of not breastfeeding. “All of us caution women not to use cannabis when pregnant or breastfeeding,” Dr. Hale said. “But when the decision has to be made as to whether a mom breastfeeds or not if she is drug screen positive, a lot of other factors must be analyzed to make such a decision.”
Study cohort
For the study, Dr. Wymore and colleagues screened 394 women who gave birth between Nov. 1, 2016, and June 30, 2019. Of those, 25 women, with a median age of 26 years, were eligible and enrolled. Inclusion criteria included known prenatal marijuana use, intention to breastfeed, and self-reported abstinence. Prenatal use primarily involved inhaling cannabis more than twice a week.
Of the 25 enrolled mothers, 12 who self-reported marijuana abstinence were in fact found to be abstinent according to the results of plasma analysis. Those who continued to use the substance were younger than the overall sample, with a median age of 21, and were less likely to have attended college (23%) than abstainers (58%).
The researchers prospectively collected data on self-reported marijuana usage and paired maternal plasma and breast milk samples several times a week. All participants had detectable THC in breast milk throughout the study. Initial median THC concentrations were 3.2 ng/mL (interquartile range, 1.2-6.8) within the first week after delivery. These increased to 5.5 ng/mL (IQR, 4.4-16.0) at 2 weeks and declined to 1.9 ng/mL (IQR, 1.1-4.3) at 6 weeks. In terms of ratio, the milk:plasma partition coefficient for THC was approximately 6:1 (IQR, 3.8:1-8.1:1).
Dr. Hale noted that, although THC was detectable in milk, the levels were exceedingly low. “This is where the risk assessment comes in. There’s a lot of hysteria in the cannabis field right now, and we’re going to need time and a lot more studies to really be able to predict any untoward complications.”
Dr. Wymore, however, countered that THC levels were low only in those who abstained and that her concerns relate not just to postpartum breast milk levels but the health effects on children of mothers’ cannabis use over the course of prepregnancy, pregnancy, and lactation. “[Dr. Hale’s] message makes it difficult for clinicians to counsel mothers since it goes against national guidelines,” she said. “We need to be consistent.”
But Dr. Wymore and other experts acknowledge the dilemma faced in that breast milk clearly offers substantial benefits for infant and child health. “The risks of an infant’s exposure to marijuana versus the benefit of breast milk must be considered,” said Amy B. Hair, MD, assistant professor of pediatrics and neonatal medicine at Baylor College of Medicine, Houston, who was not involved in the Colorado study. “And it’s unrealistic, as the study suggests, for mothers to discard breast milk for 6 weeks.”
Nevertheless, calling the findings of THC persistence after abstinence “troublesome,” Dr. Hair said the legalization of marijuana in some states gives the public the impression it’s safe to use marijuana even during pregnancy and lactation. “Research studies, however, are concerning for potential detrimental effects on brain growth and development in infants whose mothers use marijuana during pregnancy and breastfeeding,” she added.
Dr. Wymore stressed that more U.S. cannabis dispensaries must engage in rigorous point-of-sale counseling to women on the potential harms during pregnancy. This is the case in Canada, she noted, where recreational and medicinal cannabis has been legal since 2018 and more than 90% of outlets (vs. two thirds of their U.S. counterparts) advise women not to use cannabis during pregnancy or lactation, even for nausea.
“This is where many women are getting their information on cannabis,” she said. “We learned the hard way with alcohol and we don’t want to make the same mistake with marijuana.”
The study was funded by the Colorado Department of Public Health and Environment, the Children’s Hospital Colorado Research Institute, the Colorado Fetal Care Center, the Colorado Perinatal Clinical and Translational Research Center, and the Children’s Colorado Research Institute. Two study coauthors disclosed relationships with the private sector outside the submitted work. Dr. Hale and Dr. Hair have disclosed no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Delta-9-Tetrahydrocannabinol (THC), the main psychoactive component of cannabis, remains detectable in breast milk even after weeks of abstinence, new data show. The estimated half-life of THC in breast milk is 17 days, according to the study results, with a projected time to elimination of more than 6 weeks. The clinical importance of the remaining THC is up for debate, according to some experts.
“To limit THC effects on fetal brain development and promote safe breastfeeding, it is critical to emphasize marijuana abstention both early in pregnancy and post partum,” Erica M. Wymore, MD, MPH, an assistant professor of pediatrics and neonatology at the University of Colorado at Denver, Aurora, and colleagues wrote. The group published their results online March 8, 2021, in JAMA Pediatrics.
And while the study was a pharmacokinetic analysis rather than a safety investigation, Dr. Wymore said in an interview that the detectable levels of THC suggest any use is of concern and no safety thresholds have been established. “We wish we had more data on the potential effects on the neurocognitive development of children, but for now we must discourage any use in prepregnancy, pregnancy, and breastfeeding, as our national guidelines recommend.”
Therefore, the findings support current guidelines discouraging any cannabis use in mothers-to-be and breast-feeding mothers issued by national organizations, including those from the American Academy of Pediatrics, the American College of Obstetricians and Gynecologists, and the Academy of Breastfeeding Medicine.
Furthermore, the difficulties many mothers face in abstaining from marijuana, a commonly used drug in pregnancy, and the persistence of THC in maternal milk led the authors to question the feasibility of having women who use marijuana simply discard their breast milk until THC is cleared.
“We report challenges in abstention and prolonged excretion of THC in breast milk greater than 6 weeks among women with prenatal marijuana use,” they wrote. “These findings make the recommendations for mothers to discard breast milk until THC is undetectable unrealistic for mothers committed to breastfeeding.”
However, not all experts are equally concerned about low THC concentrations in breast milk. Neonatal pharmacologist Thomas R. Hale, PhD, a professor of pediatrics at Texas Tech University, Lubbock, said a previous study by his group showed that THC levels in maternal milk peaked within 60 minutes of a moderate dose of inhaled marijuana and fell to quite low levels over the next 4 hours. The highest concentration in maternal milk occurred shortly after the peak in plasma.
“So you can see that, just because a mom is drug screen positive, the clinical dose transferred to the infant is probably exceedingly low,” he said in an interview.
Dr. Hale also stressed that judgments about drugs in this context should weigh the risk of the drug against the risk of not breastfeeding. “All of us caution women not to use cannabis when pregnant or breastfeeding,” Dr. Hale said. “But when the decision has to be made as to whether a mom breastfeeds or not if she is drug screen positive, a lot of other factors must be analyzed to make such a decision.”
Study cohort
For the study, Dr. Wymore and colleagues screened 394 women who gave birth between Nov. 1, 2016, and June 30, 2019. Of those, 25 women, with a median age of 26 years, were eligible and enrolled. Inclusion criteria included known prenatal marijuana use, intention to breastfeed, and self-reported abstinence. Prenatal use primarily involved inhaling cannabis more than twice a week.
Of the 25 enrolled mothers, 12 who self-reported marijuana abstinence were in fact found to be abstinent according to the results of plasma analysis. Those who continued to use the substance were younger than the overall sample, with a median age of 21, and were less likely to have attended college (23%) than abstainers (58%).
The researchers prospectively collected data on self-reported marijuana usage and paired maternal plasma and breast milk samples several times a week. All participants had detectable THC in breast milk throughout the study. Initial median THC concentrations were 3.2 ng/mL (interquartile range, 1.2-6.8) within the first week after delivery. These increased to 5.5 ng/mL (IQR, 4.4-16.0) at 2 weeks and declined to 1.9 ng/mL (IQR, 1.1-4.3) at 6 weeks. In terms of ratio, the milk:plasma partition coefficient for THC was approximately 6:1 (IQR, 3.8:1-8.1:1).
Dr. Hale noted that, although THC was detectable in milk, the levels were exceedingly low. “This is where the risk assessment comes in. There’s a lot of hysteria in the cannabis field right now, and we’re going to need time and a lot more studies to really be able to predict any untoward complications.”
Dr. Wymore, however, countered that THC levels were low only in those who abstained and that her concerns relate not just to postpartum breast milk levels but the health effects on children of mothers’ cannabis use over the course of prepregnancy, pregnancy, and lactation. “[Dr. Hale’s] message makes it difficult for clinicians to counsel mothers since it goes against national guidelines,” she said. “We need to be consistent.”
But Dr. Wymore and other experts acknowledge the dilemma faced in that breast milk clearly offers substantial benefits for infant and child health. “The risks of an infant’s exposure to marijuana versus the benefit of breast milk must be considered,” said Amy B. Hair, MD, assistant professor of pediatrics and neonatal medicine at Baylor College of Medicine, Houston, who was not involved in the Colorado study. “And it’s unrealistic, as the study suggests, for mothers to discard breast milk for 6 weeks.”
Nevertheless, calling the findings of THC persistence after abstinence “troublesome,” Dr. Hair said the legalization of marijuana in some states gives the public the impression it’s safe to use marijuana even during pregnancy and lactation. “Research studies, however, are concerning for potential detrimental effects on brain growth and development in infants whose mothers use marijuana during pregnancy and breastfeeding,” she added.
Dr. Wymore stressed that more U.S. cannabis dispensaries must engage in rigorous point-of-sale counseling to women on the potential harms during pregnancy. This is the case in Canada, she noted, where recreational and medicinal cannabis has been legal since 2018 and more than 90% of outlets (vs. two thirds of their U.S. counterparts) advise women not to use cannabis during pregnancy or lactation, even for nausea.
“This is where many women are getting their information on cannabis,” she said. “We learned the hard way with alcohol and we don’t want to make the same mistake with marijuana.”
The study was funded by the Colorado Department of Public Health and Environment, the Children’s Hospital Colorado Research Institute, the Colorado Fetal Care Center, the Colorado Perinatal Clinical and Translational Research Center, and the Children’s Colorado Research Institute. Two study coauthors disclosed relationships with the private sector outside the submitted work. Dr. Hale and Dr. Hair have disclosed no competing interests with regard to their comments.
A version of this article first appeared on Medscape.com.
Comic books help explain type 1 diabetes to all ages
Overcoming the challenges in managing type 1 diabetes can sometimes feel like an unappreciated “superpower.” That was part of the thinking behind the creation of a comic book trilogy that aims to educate people of all ages – including health care providers – about the realities of living with this condition.
The series was initially launched by a team from Portsmouth (England) Hospitals University National Health Service Trust and University Hospital Southampton NHS Foundation Trust. It is now officially backed by the NHS. The first book in the trilogy, published in 2016, visually illustrates the challenges faced by a teenage boy who had recently been diagnosed with type 1 diabetes. The second volume, released in 2018, follows a young girl who is hospitalized with diabetic ketoacidosis. The third, published in December 2020, explores the stigma associated with diabetes and delves into hypoglycemia.
Available for free online, the three comic books are meant for adults, children, health care professionals, and laypeople. This news organization spoke with series cocreator Partha Kar, MBBS, MD, national specialty adviser, Diabetes for NHS England, about the series. This interview has been edited for length and clarity.
How did the idea for a comic book series about type 1 diabetes come about?Dr. Kar: My Southampton colleague Mayank Patel, BM, DM, FRCP, and I were discussing ways of reaching different audiences to raise awareness about type 1 diabetes, and we had the idea of comic books. After all, comic book movies are among the biggest blockbusters if one looks at popular culture, because it’s not just kids watching them.
One of our patients made an interesting observation that really resonated. He said having type 1 diabetes was like the Marvel Comics superhero Hulk.
Several scenes in the first publication, Type 1: Origins, were based on the Hulk, a scientist who gets a radioactive dose by accident. He doesn’t like turning green when he’s angry, even though he also becomes very strong. He basically spends the rest of his life trying to find the cure for himself, but he eventually makes the best of his two worlds – Professor and Hulk – rather than constantly fighting his situation.
The story line was primarily written by a group of patients with type 1 diabetes based on their own experiences. Mayank and I were mostly just supervising and financing the project. The graphics and layout were done by Revolve Comics, a publisher specializing in health education via the comic book medium.
Our aim was to bring awareness of type 1 diabetes to people who don’t have diabetes, including teachers, family members, and friends. At the end of Origins, we provide a list of online resources for more information and for social connection.
Since it launched in October 2016, Origins has been downloaded nearly 10,000 times. Lots of local charities and schools have picked it up. Parents and kids have come to us asking for more and giving us ideas. That’s what prompted the next one.
The second volume, Type 1: Attack of the Ketones, is more technical and somewhat surprising in that it portrays some hospital staff members as not well-informed about type 1 diabetes. Are they part of the intended audience?
Yes, this one was directed a little bit more towards professionals, hospitals, and staff. It’s also informed by patient feedback, and dovetails with my efforts to improve hospital care for people with type 1 diabetes. But of course, patients and interested laypeople can also learn from it.
A theme in volume 2 comes from another Marvel Comics superhero, Iron Man. In the movie, when Tony Stark’s heart is severely damaged with shrapnel, he acquires an arc reactor that keeps him alive and also powers the suit that gives him superpowers. After the reactor is taken away, he devises a way to replace the missing part and reassemble the suit.
Similarly, in type 1 diabetes, the ability to produce insulin has been taken away without permission. But what is missing can thankfully be replaced, albeit imperfectly. As we illustrate, things don’t always go to plan despite best efforts to administer insulin in the right dose at the right time.
At the end of Attack of the Ketones, we provide two pages of text about recognizing and managing hyperglycemia and preventing diabetic ketoacidosis. This volume was funded by NHS England and then backed by JDRF and Diabetes UK, and many hospitals picked it up. It has had about 8,000 downloads.
In Volume 3, you explore stigma and the issue of language regarding type 1 diabetes. How did those topics come about?
Kar: Type 1 Mission 3: S.T.I.G.M.A. was also based on patient feedback, with input from some Indian diabetes groups I’ve worked with. Here, the protagonist is a young man with type 1 diabetes who goes on holiday to India, where diabetes stigma is widespread. The characters address language problems such as use of the word “diabetic” to label a person, and they counter misconceptions such as that diabetes is contagious. There’s an Indian comic book version of this volume out now.
The main character of this volume experiences severe hypoglycemia and is saved by a glucagon injection from a colleague, one of several presented as superheroes who help in the fight to end diabetes stigma. They are referred to as Guardians of the Glucose, a take on yet another Marvel franchise, Guardians of the Galaxy.
At the end of this volume, we provide two pages of text about recognizing, managing, and preventing hypoglycemia. Again, we hope to educate as wide an audience as possible.
At the end of volume 3, you also briefly mention the COVID-19 pandemic. Will there be a fourth volume dealing with that, or other topics, such as diabetes technology?
We’ve left it open. We want to see how volume 3 lands. Depending on that, we might take it forward. There are certainly plenty of topics to tackle. We’ve also discussed moving into gaming or virtual reality. Overall, we hope to educate people by engaging them in different ways.
Dr. Kar has been a consultant diabetologist/endocrinologist within the NHS since 2008. He disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Overcoming the challenges in managing type 1 diabetes can sometimes feel like an unappreciated “superpower.” That was part of the thinking behind the creation of a comic book trilogy that aims to educate people of all ages – including health care providers – about the realities of living with this condition.
The series was initially launched by a team from Portsmouth (England) Hospitals University National Health Service Trust and University Hospital Southampton NHS Foundation Trust. It is now officially backed by the NHS. The first book in the trilogy, published in 2016, visually illustrates the challenges faced by a teenage boy who had recently been diagnosed with type 1 diabetes. The second volume, released in 2018, follows a young girl who is hospitalized with diabetic ketoacidosis. The third, published in December 2020, explores the stigma associated with diabetes and delves into hypoglycemia.
Available for free online, the three comic books are meant for adults, children, health care professionals, and laypeople. This news organization spoke with series cocreator Partha Kar, MBBS, MD, national specialty adviser, Diabetes for NHS England, about the series. This interview has been edited for length and clarity.
How did the idea for a comic book series about type 1 diabetes come about?Dr. Kar: My Southampton colleague Mayank Patel, BM, DM, FRCP, and I were discussing ways of reaching different audiences to raise awareness about type 1 diabetes, and we had the idea of comic books. After all, comic book movies are among the biggest blockbusters if one looks at popular culture, because it’s not just kids watching them.
One of our patients made an interesting observation that really resonated. He said having type 1 diabetes was like the Marvel Comics superhero Hulk.
Several scenes in the first publication, Type 1: Origins, were based on the Hulk, a scientist who gets a radioactive dose by accident. He doesn’t like turning green when he’s angry, even though he also becomes very strong. He basically spends the rest of his life trying to find the cure for himself, but he eventually makes the best of his two worlds – Professor and Hulk – rather than constantly fighting his situation.
The story line was primarily written by a group of patients with type 1 diabetes based on their own experiences. Mayank and I were mostly just supervising and financing the project. The graphics and layout were done by Revolve Comics, a publisher specializing in health education via the comic book medium.
Our aim was to bring awareness of type 1 diabetes to people who don’t have diabetes, including teachers, family members, and friends. At the end of Origins, we provide a list of online resources for more information and for social connection.
Since it launched in October 2016, Origins has been downloaded nearly 10,000 times. Lots of local charities and schools have picked it up. Parents and kids have come to us asking for more and giving us ideas. That’s what prompted the next one.
The second volume, Type 1: Attack of the Ketones, is more technical and somewhat surprising in that it portrays some hospital staff members as not well-informed about type 1 diabetes. Are they part of the intended audience?
Yes, this one was directed a little bit more towards professionals, hospitals, and staff. It’s also informed by patient feedback, and dovetails with my efforts to improve hospital care for people with type 1 diabetes. But of course, patients and interested laypeople can also learn from it.
A theme in volume 2 comes from another Marvel Comics superhero, Iron Man. In the movie, when Tony Stark’s heart is severely damaged with shrapnel, he acquires an arc reactor that keeps him alive and also powers the suit that gives him superpowers. After the reactor is taken away, he devises a way to replace the missing part and reassemble the suit.
Similarly, in type 1 diabetes, the ability to produce insulin has been taken away without permission. But what is missing can thankfully be replaced, albeit imperfectly. As we illustrate, things don’t always go to plan despite best efforts to administer insulin in the right dose at the right time.
At the end of Attack of the Ketones, we provide two pages of text about recognizing and managing hyperglycemia and preventing diabetic ketoacidosis. This volume was funded by NHS England and then backed by JDRF and Diabetes UK, and many hospitals picked it up. It has had about 8,000 downloads.
In Volume 3, you explore stigma and the issue of language regarding type 1 diabetes. How did those topics come about?
Kar: Type 1 Mission 3: S.T.I.G.M.A. was also based on patient feedback, with input from some Indian diabetes groups I’ve worked with. Here, the protagonist is a young man with type 1 diabetes who goes on holiday to India, where diabetes stigma is widespread. The characters address language problems such as use of the word “diabetic” to label a person, and they counter misconceptions such as that diabetes is contagious. There’s an Indian comic book version of this volume out now.
The main character of this volume experiences severe hypoglycemia and is saved by a glucagon injection from a colleague, one of several presented as superheroes who help in the fight to end diabetes stigma. They are referred to as Guardians of the Glucose, a take on yet another Marvel franchise, Guardians of the Galaxy.
At the end of this volume, we provide two pages of text about recognizing, managing, and preventing hypoglycemia. Again, we hope to educate as wide an audience as possible.
At the end of volume 3, you also briefly mention the COVID-19 pandemic. Will there be a fourth volume dealing with that, or other topics, such as diabetes technology?
We’ve left it open. We want to see how volume 3 lands. Depending on that, we might take it forward. There are certainly plenty of topics to tackle. We’ve also discussed moving into gaming or virtual reality. Overall, we hope to educate people by engaging them in different ways.
Dr. Kar has been a consultant diabetologist/endocrinologist within the NHS since 2008. He disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Overcoming the challenges in managing type 1 diabetes can sometimes feel like an unappreciated “superpower.” That was part of the thinking behind the creation of a comic book trilogy that aims to educate people of all ages – including health care providers – about the realities of living with this condition.
The series was initially launched by a team from Portsmouth (England) Hospitals University National Health Service Trust and University Hospital Southampton NHS Foundation Trust. It is now officially backed by the NHS. The first book in the trilogy, published in 2016, visually illustrates the challenges faced by a teenage boy who had recently been diagnosed with type 1 diabetes. The second volume, released in 2018, follows a young girl who is hospitalized with diabetic ketoacidosis. The third, published in December 2020, explores the stigma associated with diabetes and delves into hypoglycemia.
Available for free online, the three comic books are meant for adults, children, health care professionals, and laypeople. This news organization spoke with series cocreator Partha Kar, MBBS, MD, national specialty adviser, Diabetes for NHS England, about the series. This interview has been edited for length and clarity.
How did the idea for a comic book series about type 1 diabetes come about?Dr. Kar: My Southampton colleague Mayank Patel, BM, DM, FRCP, and I were discussing ways of reaching different audiences to raise awareness about type 1 diabetes, and we had the idea of comic books. After all, comic book movies are among the biggest blockbusters if one looks at popular culture, because it’s not just kids watching them.
One of our patients made an interesting observation that really resonated. He said having type 1 diabetes was like the Marvel Comics superhero Hulk.
Several scenes in the first publication, Type 1: Origins, were based on the Hulk, a scientist who gets a radioactive dose by accident. He doesn’t like turning green when he’s angry, even though he also becomes very strong. He basically spends the rest of his life trying to find the cure for himself, but he eventually makes the best of his two worlds – Professor and Hulk – rather than constantly fighting his situation.
The story line was primarily written by a group of patients with type 1 diabetes based on their own experiences. Mayank and I were mostly just supervising and financing the project. The graphics and layout were done by Revolve Comics, a publisher specializing in health education via the comic book medium.
Our aim was to bring awareness of type 1 diabetes to people who don’t have diabetes, including teachers, family members, and friends. At the end of Origins, we provide a list of online resources for more information and for social connection.
Since it launched in October 2016, Origins has been downloaded nearly 10,000 times. Lots of local charities and schools have picked it up. Parents and kids have come to us asking for more and giving us ideas. That’s what prompted the next one.
The second volume, Type 1: Attack of the Ketones, is more technical and somewhat surprising in that it portrays some hospital staff members as not well-informed about type 1 diabetes. Are they part of the intended audience?
Yes, this one was directed a little bit more towards professionals, hospitals, and staff. It’s also informed by patient feedback, and dovetails with my efforts to improve hospital care for people with type 1 diabetes. But of course, patients and interested laypeople can also learn from it.
A theme in volume 2 comes from another Marvel Comics superhero, Iron Man. In the movie, when Tony Stark’s heart is severely damaged with shrapnel, he acquires an arc reactor that keeps him alive and also powers the suit that gives him superpowers. After the reactor is taken away, he devises a way to replace the missing part and reassemble the suit.
Similarly, in type 1 diabetes, the ability to produce insulin has been taken away without permission. But what is missing can thankfully be replaced, albeit imperfectly. As we illustrate, things don’t always go to plan despite best efforts to administer insulin in the right dose at the right time.
At the end of Attack of the Ketones, we provide two pages of text about recognizing and managing hyperglycemia and preventing diabetic ketoacidosis. This volume was funded by NHS England and then backed by JDRF and Diabetes UK, and many hospitals picked it up. It has had about 8,000 downloads.
In Volume 3, you explore stigma and the issue of language regarding type 1 diabetes. How did those topics come about?
Kar: Type 1 Mission 3: S.T.I.G.M.A. was also based on patient feedback, with input from some Indian diabetes groups I’ve worked with. Here, the protagonist is a young man with type 1 diabetes who goes on holiday to India, where diabetes stigma is widespread. The characters address language problems such as use of the word “diabetic” to label a person, and they counter misconceptions such as that diabetes is contagious. There’s an Indian comic book version of this volume out now.
The main character of this volume experiences severe hypoglycemia and is saved by a glucagon injection from a colleague, one of several presented as superheroes who help in the fight to end diabetes stigma. They are referred to as Guardians of the Glucose, a take on yet another Marvel franchise, Guardians of the Galaxy.
At the end of this volume, we provide two pages of text about recognizing, managing, and preventing hypoglycemia. Again, we hope to educate as wide an audience as possible.
At the end of volume 3, you also briefly mention the COVID-19 pandemic. Will there be a fourth volume dealing with that, or other topics, such as diabetes technology?
We’ve left it open. We want to see how volume 3 lands. Depending on that, we might take it forward. There are certainly plenty of topics to tackle. We’ve also discussed moving into gaming or virtual reality. Overall, we hope to educate people by engaging them in different ways.
Dr. Kar has been a consultant diabetologist/endocrinologist within the NHS since 2008. He disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
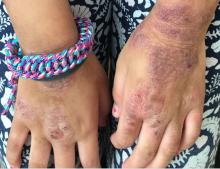
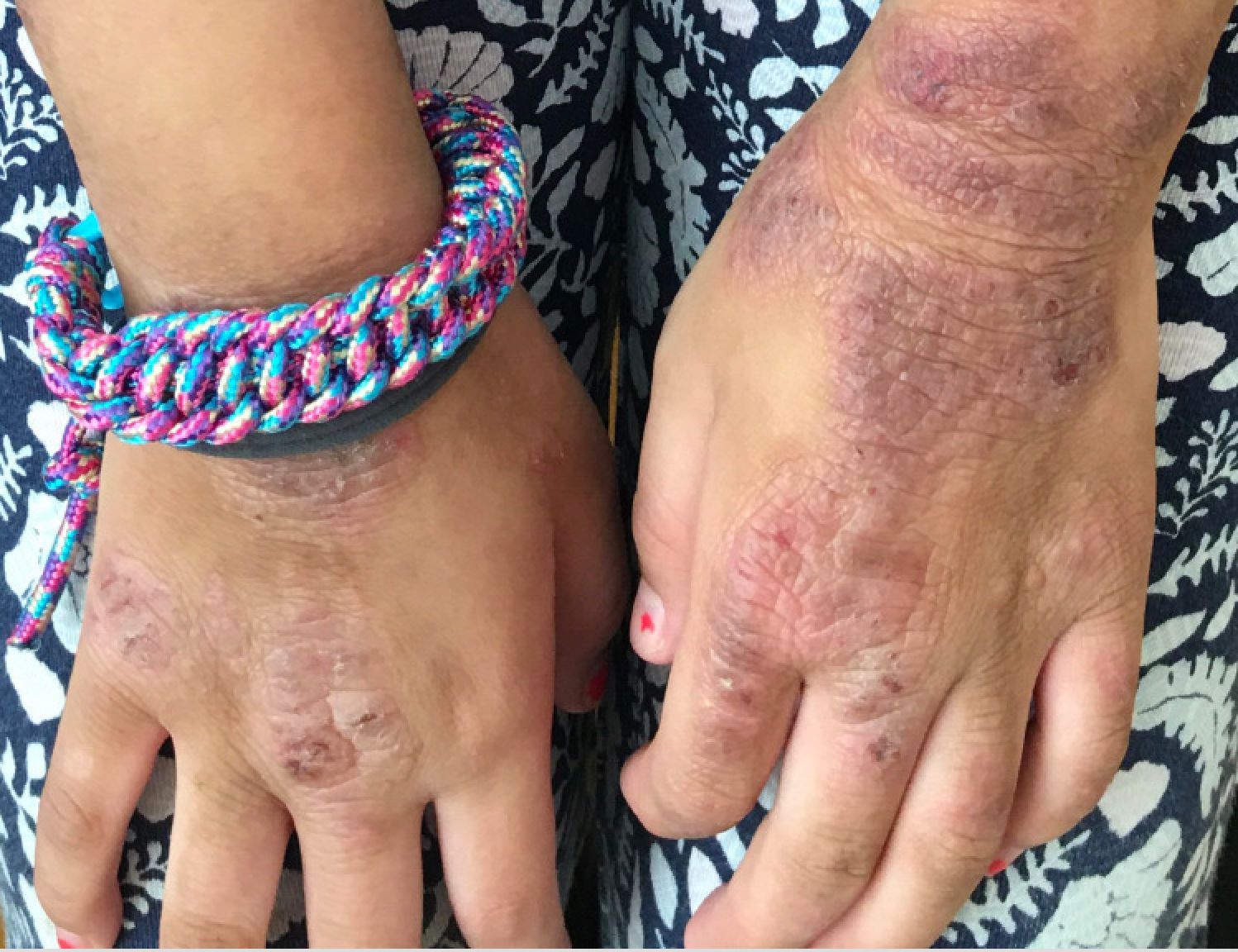
A young girl presents with ‘itchy, rashy’ hands
Given the presence of erythema, lichenification, fissuring, and scale of the hands over the course of more than 3 months with the absence of nail findings is most consistent with a diagnosis of chronic hand eczema.
Chronic hand eczema (CHE) is an inflammatory dermatitis of the hands or wrists that persists for longer than 3 months or recurs twice or more in a 12-month timespan.1,2 Hand eczema can be a manifestation of atopic dermatitis, allergic contact dermatitis, or irritant contact dermatitis. Its multifactorial pathogenesis includes epidermal injury and disturbed epidermal barrier function from exogenous factors such as irritants or contact allergens, as well as endogenous factors including atopic dermatitis.3 In pediatrics, it often presents after an acute phase of hand dermatitis with chronic pruritus, erythema, and dry skin with scale.4 Examination findings vary widely with erythema, vesicles, scale, fissures, crusting, hyperkeratosis, and/or lichenification.3,5 Diagnosis is often achieved with careful history, asking about potential exposures that may induce lesions, and physical exam of the entire skin, including the feet. Based upon clinical history or persistent dermatitis, allergic contact dermatitis patch testing should be considered.2
What’s the treatment plan?
Given that CHE is an inflammatory disease process, the goal of treatment is to reduce inflammation and allow for skin barrier repair. Unfortunately, only one study has investigated therapeutics for pediatric CHE,6 with the remainder of the literature based on adult CHE. Current CHE guidelines recommend avoidance of allergens, irritants, or other triggers of the disease as well as liberal and regular use of emollients. Because of the relative thickness of hand skin, higher-potency topical corticosteroids are often used as first-line therapy, with lower-strength topical steroids, calcineurin inhibitors, or crisaborole used as maintenance therapy. Other treatment options include phototherapy, and rarely, systemic therapies are utilized for atopic dermatitis.
What’s the differential diagnosis?
The differential diagnosis of CHE includes other scaling or hyperkeratotic skin conditions including psoriasis and tinea manuum. Other skin conditions that localize to extremities including scabies and hand-foot-and-mouth disease are discussed below.
Psoriasis can present on the hands with erythematous, well-demarcated, silver scaling plaques. However, additional plaques may be found on the elbows, knees, scalp, umbilicus, and sacrum. Nails can demonstrate pitting, oil drops, splinter hemorrhages, or onycholysis. First-line treatment includes a combination of topical steroids, topical vitamin D analogues, and keratolytics.
Tinea mannum is a dermatophyte infection of the skin of the hands. Typically, only one hand is affected with concomitant bilateral tinea pedis. It results in a white scaly plaque with dorsal hand involvement demonstrating an annular appearance, elevated edge, and central clearing. KOH prep will demonstrate septate hyphae, and cultures will grow dermatophyte colonies. Treatment includes topical antifungals or systemic antifungals for recalcitrant disease.
Scabies presents with short linear hypopigmented lesions with a black dot on one end as well as erythematous pruritic papules. These appear on the interdigital web spaces, wrists, axilla, buttocks, and genital region. Skin scraping prep with mineral oil can show mites and eggs. All individuals in an affected household should be treated with either topical permethrin or oral ivermectin to avoid reinfection or parasitic spread. All contacted linens must be cleaned with hot water and dried on high heat.
Hand-foot-and-mouth disease, classically caused by coxsackievirus, is an acute viral illness that results in an eruption of erythematous macules, papules, and vesicles on the ventral hands, soles of the feet, and oral mucosa. Diagnosis is achieved clinically and treatment is symptomatic as the lesions are self-limited.
Our patient underwent patch testing but did not return positive to any allergens. She was started on potent topical corticosteroids, educated on trigger avoidance, and gradually achieved good disease control.
Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
Michael Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is a 4th year medical student at the University of Rochester (N.Y.). Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital.
References
1. Diepgen TL et al. Br J Dermatol. 2009;160(2):353-8.
2. Diepgen TL et al. J Dtsch Dermatol Ges. 2015;13(1):e1-22.
3. Agner T and Elsner P. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:4-12.
4. Mortz CG et al. Br J Dermatol. 2001;144(3):523-32.
5. Silvestre Salvador JF et al. Actas Dermosifiliogr. 2020;111(1):26-40.
6. Luchsinger I et al. J Eur Acad Dermatol Venereol. 2020;34(5):1037-42.
7. English J et al. Clin Exp Dermatol. 2009;34(7):761-9.
8. Elsner P and Agner T. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:13-21.
Given the presence of erythema, lichenification, fissuring, and scale of the hands over the course of more than 3 months with the absence of nail findings is most consistent with a diagnosis of chronic hand eczema.
Chronic hand eczema (CHE) is an inflammatory dermatitis of the hands or wrists that persists for longer than 3 months or recurs twice or more in a 12-month timespan.1,2 Hand eczema can be a manifestation of atopic dermatitis, allergic contact dermatitis, or irritant contact dermatitis. Its multifactorial pathogenesis includes epidermal injury and disturbed epidermal barrier function from exogenous factors such as irritants or contact allergens, as well as endogenous factors including atopic dermatitis.3 In pediatrics, it often presents after an acute phase of hand dermatitis with chronic pruritus, erythema, and dry skin with scale.4 Examination findings vary widely with erythema, vesicles, scale, fissures, crusting, hyperkeratosis, and/or lichenification.3,5 Diagnosis is often achieved with careful history, asking about potential exposures that may induce lesions, and physical exam of the entire skin, including the feet. Based upon clinical history or persistent dermatitis, allergic contact dermatitis patch testing should be considered.2
What’s the treatment plan?
Given that CHE is an inflammatory disease process, the goal of treatment is to reduce inflammation and allow for skin barrier repair. Unfortunately, only one study has investigated therapeutics for pediatric CHE,6 with the remainder of the literature based on adult CHE. Current CHE guidelines recommend avoidance of allergens, irritants, or other triggers of the disease as well as liberal and regular use of emollients. Because of the relative thickness of hand skin, higher-potency topical corticosteroids are often used as first-line therapy, with lower-strength topical steroids, calcineurin inhibitors, or crisaborole used as maintenance therapy. Other treatment options include phototherapy, and rarely, systemic therapies are utilized for atopic dermatitis.
What’s the differential diagnosis?
The differential diagnosis of CHE includes other scaling or hyperkeratotic skin conditions including psoriasis and tinea manuum. Other skin conditions that localize to extremities including scabies and hand-foot-and-mouth disease are discussed below.
Psoriasis can present on the hands with erythematous, well-demarcated, silver scaling plaques. However, additional plaques may be found on the elbows, knees, scalp, umbilicus, and sacrum. Nails can demonstrate pitting, oil drops, splinter hemorrhages, or onycholysis. First-line treatment includes a combination of topical steroids, topical vitamin D analogues, and keratolytics.
Tinea mannum is a dermatophyte infection of the skin of the hands. Typically, only one hand is affected with concomitant bilateral tinea pedis. It results in a white scaly plaque with dorsal hand involvement demonstrating an annular appearance, elevated edge, and central clearing. KOH prep will demonstrate septate hyphae, and cultures will grow dermatophyte colonies. Treatment includes topical antifungals or systemic antifungals for recalcitrant disease.
Scabies presents with short linear hypopigmented lesions with a black dot on one end as well as erythematous pruritic papules. These appear on the interdigital web spaces, wrists, axilla, buttocks, and genital region. Skin scraping prep with mineral oil can show mites and eggs. All individuals in an affected household should be treated with either topical permethrin or oral ivermectin to avoid reinfection or parasitic spread. All contacted linens must be cleaned with hot water and dried on high heat.
Hand-foot-and-mouth disease, classically caused by coxsackievirus, is an acute viral illness that results in an eruption of erythematous macules, papules, and vesicles on the ventral hands, soles of the feet, and oral mucosa. Diagnosis is achieved clinically and treatment is symptomatic as the lesions are self-limited.
Our patient underwent patch testing but did not return positive to any allergens. She was started on potent topical corticosteroids, educated on trigger avoidance, and gradually achieved good disease control.
Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
Michael Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is a 4th year medical student at the University of Rochester (N.Y.). Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital.
References
1. Diepgen TL et al. Br J Dermatol. 2009;160(2):353-8.
2. Diepgen TL et al. J Dtsch Dermatol Ges. 2015;13(1):e1-22.
3. Agner T and Elsner P. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:4-12.
4. Mortz CG et al. Br J Dermatol. 2001;144(3):523-32.
5. Silvestre Salvador JF et al. Actas Dermosifiliogr. 2020;111(1):26-40.
6. Luchsinger I et al. J Eur Acad Dermatol Venereol. 2020;34(5):1037-42.
7. English J et al. Clin Exp Dermatol. 2009;34(7):761-9.
8. Elsner P and Agner T. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:13-21.
Given the presence of erythema, lichenification, fissuring, and scale of the hands over the course of more than 3 months with the absence of nail findings is most consistent with a diagnosis of chronic hand eczema.
Chronic hand eczema (CHE) is an inflammatory dermatitis of the hands or wrists that persists for longer than 3 months or recurs twice or more in a 12-month timespan.1,2 Hand eczema can be a manifestation of atopic dermatitis, allergic contact dermatitis, or irritant contact dermatitis. Its multifactorial pathogenesis includes epidermal injury and disturbed epidermal barrier function from exogenous factors such as irritants or contact allergens, as well as endogenous factors including atopic dermatitis.3 In pediatrics, it often presents after an acute phase of hand dermatitis with chronic pruritus, erythema, and dry skin with scale.4 Examination findings vary widely with erythema, vesicles, scale, fissures, crusting, hyperkeratosis, and/or lichenification.3,5 Diagnosis is often achieved with careful history, asking about potential exposures that may induce lesions, and physical exam of the entire skin, including the feet. Based upon clinical history or persistent dermatitis, allergic contact dermatitis patch testing should be considered.2
What’s the treatment plan?
Given that CHE is an inflammatory disease process, the goal of treatment is to reduce inflammation and allow for skin barrier repair. Unfortunately, only one study has investigated therapeutics for pediatric CHE,6 with the remainder of the literature based on adult CHE. Current CHE guidelines recommend avoidance of allergens, irritants, or other triggers of the disease as well as liberal and regular use of emollients. Because of the relative thickness of hand skin, higher-potency topical corticosteroids are often used as first-line therapy, with lower-strength topical steroids, calcineurin inhibitors, or crisaborole used as maintenance therapy. Other treatment options include phototherapy, and rarely, systemic therapies are utilized for atopic dermatitis.
What’s the differential diagnosis?
The differential diagnosis of CHE includes other scaling or hyperkeratotic skin conditions including psoriasis and tinea manuum. Other skin conditions that localize to extremities including scabies and hand-foot-and-mouth disease are discussed below.
Psoriasis can present on the hands with erythematous, well-demarcated, silver scaling plaques. However, additional plaques may be found on the elbows, knees, scalp, umbilicus, and sacrum. Nails can demonstrate pitting, oil drops, splinter hemorrhages, or onycholysis. First-line treatment includes a combination of topical steroids, topical vitamin D analogues, and keratolytics.
Tinea mannum is a dermatophyte infection of the skin of the hands. Typically, only one hand is affected with concomitant bilateral tinea pedis. It results in a white scaly plaque with dorsal hand involvement demonstrating an annular appearance, elevated edge, and central clearing. KOH prep will demonstrate septate hyphae, and cultures will grow dermatophyte colonies. Treatment includes topical antifungals or systemic antifungals for recalcitrant disease.
Scabies presents with short linear hypopigmented lesions with a black dot on one end as well as erythematous pruritic papules. These appear on the interdigital web spaces, wrists, axilla, buttocks, and genital region. Skin scraping prep with mineral oil can show mites and eggs. All individuals in an affected household should be treated with either topical permethrin or oral ivermectin to avoid reinfection or parasitic spread. All contacted linens must be cleaned with hot water and dried on high heat.
Hand-foot-and-mouth disease, classically caused by coxsackievirus, is an acute viral illness that results in an eruption of erythematous macules, papules, and vesicles on the ventral hands, soles of the feet, and oral mucosa. Diagnosis is achieved clinically and treatment is symptomatic as the lesions are self-limited.
Our patient underwent patch testing but did not return positive to any allergens. She was started on potent topical corticosteroids, educated on trigger avoidance, and gradually achieved good disease control.
Neither Mr. Haft nor Dr. Eichenfield have any relevant financial disclosures.
Michael Haft is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. He is a 4th year medical student at the University of Rochester (N.Y.). Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital.
References
1. Diepgen TL et al. Br J Dermatol. 2009;160(2):353-8.
2. Diepgen TL et al. J Dtsch Dermatol Ges. 2015;13(1):e1-22.
3. Agner T and Elsner P. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:4-12.
4. Mortz CG et al. Br J Dermatol. 2001;144(3):523-32.
5. Silvestre Salvador JF et al. Actas Dermosifiliogr. 2020;111(1):26-40.
6. Luchsinger I et al. J Eur Acad Dermatol Venereol. 2020;34(5):1037-42.
7. English J et al. Clin Exp Dermatol. 2009;34(7):761-9.
8. Elsner P and Agner T. J Eur Acad Dermatol Venereol. 2020;34 Suppl 1:13-21.
Examination findings of the bilateral hands and wrists demonstrate plaques of erythema, lichenification, and scale of the dorsal surfaces of the hands and digits. Closer inspection reveals fissuring and erythematous crust of the affected skin but normal nails. The rest of the skin exam is unremarkable.
Update: U.S. regulators question AstraZeneca vaccine trial data
Federal regulators on March 23 said they were “concerned” that drug maker AstraZeneca included “outdated information” in its announcement the previous day that the company’s COVID-19 vaccine was effective.
The federal Data and Safety Monitoring Board shared those concerns with the company as well as with the National Institute of Allergy and Infectious Diseases, and the U.S. Biomedical Advanced Research and Development Authority, according to a statement from NIAID issued early March 23.
“We urge the company to work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible,” the agency said.
The NIAID statement does not say what data may have been outdated or how it may have changed the results. The company said March 22 it plans to see U.S. authorization for the vaccine in April.
The statement from NIAID comes a day after AstraZeneca said the interim results of their phase III U.S. study found it was 79% effective against symptomatic COVID-19, 80% effective in people 65 years and older, and 100% effective against severe or critical disease and hospitalization.
Company officials and clinical trial investigators on March 22 also addressed the recent concerns about blood clots, how well the vaccine will perform against variants, and provided a timeline for seeking regulatory approval.
“There are many countries in Europe and throughout the world that have already authorized this. The fact that a United States-run study has confirmed the efficacy and safety of this vaccine, I think is an important contribution to global health in general,” Anthony Fauci, MD, chief medical advisor to President Joe Biden, said during a White House press briefing March 22.
Andy Slavitt, White House senior advisor for the COVID-19 Response Team, had a more tempered reaction.
“It’s important to remind everyone we cannot and will not get ahead of the FDA,” he said. “While we would certainly call today’s news encouraging, it’s the kind of thing we like to see, we have a rigorous process that will come once an EUA is submitted and that will give us more information.”
With 30 million doses at the ready, the company plans to file for FDA emergency use authorization “within weeks,” Menelas Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said during a media briefing March 22.
Risk of thrombosis addressed
Regarding highly publicized reports of problems with blood clots from the AstraZeneca vaccine, the World Health Organization found the vaccine creates no greater risks, as did the European Medicines Agency
“We’ve had absolute confidence in the efficacy of the vaccine. Seeing this data now I hope gives others increased confidence that this is a very safe and effective vaccine,” Mr. Pangalos said.
“We’re glad this is being investigated really thoroughly,” Magda Sobieszczyk, MD, an infectious disease specialist at Columbia University In New York City, said. “It’s incredibly reassuring that the regulatory agencies have looked at the data thoroughly and there is no enhanced signal above what is seen in the population.”
“There were no concerning signals noted in the U.S. data,” she added.
Regarding the risk of blood clots, “These data are therefore timely in further addressing any safety concerns that could undermine vaccine uptake.” Andrew Garrett, PhD, executive vice president of scientific operations at ICON Clinical Research, agreed.
The vaccine was well-tolerated, the company reported, with no serious adverse events. Temporary pain and tenderness at the injection site, mild-to-moderate headaches, fatigue, chills, fever, muscle aches. and malaise were among the reported reactions.
The phase III interim results show 141 cases of symptomatic COVID-19 in the study of 32,449 adults. “We don’t have the whole breakdown yet . . . these are the high-level results we just got this week,” Mr. Pangalos said. Further information on rates of mild to moderate COVID-19 illness between groups is not yet available, for example.
The company explained that participants were randomly assigned to vaccine or placebo, with twice as many receiving the actual vaccine.
The trial is ongoing, so the FDA will receive information on more than the 141 COVID-19 symptomatic cases when the company submits a full primary analysis to the agency, Mr. Pangalos said.
In the phase III study, patients received two doses 4 weeks apart.
Beyond the U.S. study, the company has additional information, including real-world data from the United Kingdom, that it intends to submit to the FDA. Part of this evidence suggests increased efficacy when a second dose is administered at 3 months
‘Robust’ findings
“This is a large study, so these results can be expected to be robust. They could be expected to be even more so if there were more cases to compare between the groups, but 141 is still a substantial number of cases,” said Peter English, MD, of Horsham, United Kingdom, who is immediate past chair of the British Medical Association Public Health Medicine Committee.
Experts welcomed the 80% efficacy in people 65 and older in particular. “Importantly, the trial provides further support for efficacy in the elderly where previous clinical trial data, other than immunologic data, had been lacking,” Dr. Garrett said.
“It is clear this vaccine has very good efficacy. Remember that 60% was, prior to any trials being started, regarded as a good target,” said Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine. “This efficacy does not show a notable decline at older ages. This was expected and the speculation that it was ineffective or quasi-ineffective at older ages was totally unjustified.
“This is good news for the global community and one hopes that any political statements around this good news are avoided,” he added.
Efficacy against variants?
Regarding virus variants, Mr. Pangalos noted the study was conducted when several variants of concern were in circulation.
“What I can say is given this study was conducted much later in terms of timing, it’s very encouraging that we’ve got such high efficacy numbers when undoubtedly there are variants of concern in circulation in this study,” Mr. Pangalos said.
“It also highlights why we believe that against severe disease, our vaccine will be effective against all variants of concern,” he added.
Once the company submits its EUA to the FDA, the company is ready to immediately distribute 30 million doses of the vaccine and expects to ship 50 million total within the first month, Ruud Dobber, PhD, AstraZeneca executive vice president and president of the AZ Biopharmaceuticals Business Unit, said during the briefing.
The vaccine can be stored at 2 to 8 degrees Celsius for at least 6 months. Like other COVID-19 vaccines already authorized for emergency use, the duration of protection with the AstraZeneca product remains unknown.
This article was updated March 23, 2021.
A version of this article first appeared on WebMD.com.
Federal regulators on March 23 said they were “concerned” that drug maker AstraZeneca included “outdated information” in its announcement the previous day that the company’s COVID-19 vaccine was effective.
The federal Data and Safety Monitoring Board shared those concerns with the company as well as with the National Institute of Allergy and Infectious Diseases, and the U.S. Biomedical Advanced Research and Development Authority, according to a statement from NIAID issued early March 23.
“We urge the company to work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible,” the agency said.
The NIAID statement does not say what data may have been outdated or how it may have changed the results. The company said March 22 it plans to see U.S. authorization for the vaccine in April.
The statement from NIAID comes a day after AstraZeneca said the interim results of their phase III U.S. study found it was 79% effective against symptomatic COVID-19, 80% effective in people 65 years and older, and 100% effective against severe or critical disease and hospitalization.
Company officials and clinical trial investigators on March 22 also addressed the recent concerns about blood clots, how well the vaccine will perform against variants, and provided a timeline for seeking regulatory approval.
“There are many countries in Europe and throughout the world that have already authorized this. The fact that a United States-run study has confirmed the efficacy and safety of this vaccine, I think is an important contribution to global health in general,” Anthony Fauci, MD, chief medical advisor to President Joe Biden, said during a White House press briefing March 22.
Andy Slavitt, White House senior advisor for the COVID-19 Response Team, had a more tempered reaction.
“It’s important to remind everyone we cannot and will not get ahead of the FDA,” he said. “While we would certainly call today’s news encouraging, it’s the kind of thing we like to see, we have a rigorous process that will come once an EUA is submitted and that will give us more information.”
With 30 million doses at the ready, the company plans to file for FDA emergency use authorization “within weeks,” Menelas Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said during a media briefing March 22.
Risk of thrombosis addressed
Regarding highly publicized reports of problems with blood clots from the AstraZeneca vaccine, the World Health Organization found the vaccine creates no greater risks, as did the European Medicines Agency
“We’ve had absolute confidence in the efficacy of the vaccine. Seeing this data now I hope gives others increased confidence that this is a very safe and effective vaccine,” Mr. Pangalos said.
“We’re glad this is being investigated really thoroughly,” Magda Sobieszczyk, MD, an infectious disease specialist at Columbia University In New York City, said. “It’s incredibly reassuring that the regulatory agencies have looked at the data thoroughly and there is no enhanced signal above what is seen in the population.”
“There were no concerning signals noted in the U.S. data,” she added.
Regarding the risk of blood clots, “These data are therefore timely in further addressing any safety concerns that could undermine vaccine uptake.” Andrew Garrett, PhD, executive vice president of scientific operations at ICON Clinical Research, agreed.
The vaccine was well-tolerated, the company reported, with no serious adverse events. Temporary pain and tenderness at the injection site, mild-to-moderate headaches, fatigue, chills, fever, muscle aches. and malaise were among the reported reactions.
The phase III interim results show 141 cases of symptomatic COVID-19 in the study of 32,449 adults. “We don’t have the whole breakdown yet . . . these are the high-level results we just got this week,” Mr. Pangalos said. Further information on rates of mild to moderate COVID-19 illness between groups is not yet available, for example.
The company explained that participants were randomly assigned to vaccine or placebo, with twice as many receiving the actual vaccine.
The trial is ongoing, so the FDA will receive information on more than the 141 COVID-19 symptomatic cases when the company submits a full primary analysis to the agency, Mr. Pangalos said.
In the phase III study, patients received two doses 4 weeks apart.
Beyond the U.S. study, the company has additional information, including real-world data from the United Kingdom, that it intends to submit to the FDA. Part of this evidence suggests increased efficacy when a second dose is administered at 3 months
‘Robust’ findings
“This is a large study, so these results can be expected to be robust. They could be expected to be even more so if there were more cases to compare between the groups, but 141 is still a substantial number of cases,” said Peter English, MD, of Horsham, United Kingdom, who is immediate past chair of the British Medical Association Public Health Medicine Committee.
Experts welcomed the 80% efficacy in people 65 and older in particular. “Importantly, the trial provides further support for efficacy in the elderly where previous clinical trial data, other than immunologic data, had been lacking,” Dr. Garrett said.
“It is clear this vaccine has very good efficacy. Remember that 60% was, prior to any trials being started, regarded as a good target,” said Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine. “This efficacy does not show a notable decline at older ages. This was expected and the speculation that it was ineffective or quasi-ineffective at older ages was totally unjustified.
“This is good news for the global community and one hopes that any political statements around this good news are avoided,” he added.
Efficacy against variants?
Regarding virus variants, Mr. Pangalos noted the study was conducted when several variants of concern were in circulation.
“What I can say is given this study was conducted much later in terms of timing, it’s very encouraging that we’ve got such high efficacy numbers when undoubtedly there are variants of concern in circulation in this study,” Mr. Pangalos said.
“It also highlights why we believe that against severe disease, our vaccine will be effective against all variants of concern,” he added.
Once the company submits its EUA to the FDA, the company is ready to immediately distribute 30 million doses of the vaccine and expects to ship 50 million total within the first month, Ruud Dobber, PhD, AstraZeneca executive vice president and president of the AZ Biopharmaceuticals Business Unit, said during the briefing.
The vaccine can be stored at 2 to 8 degrees Celsius for at least 6 months. Like other COVID-19 vaccines already authorized for emergency use, the duration of protection with the AstraZeneca product remains unknown.
This article was updated March 23, 2021.
A version of this article first appeared on WebMD.com.
Federal regulators on March 23 said they were “concerned” that drug maker AstraZeneca included “outdated information” in its announcement the previous day that the company’s COVID-19 vaccine was effective.
The federal Data and Safety Monitoring Board shared those concerns with the company as well as with the National Institute of Allergy and Infectious Diseases, and the U.S. Biomedical Advanced Research and Development Authority, according to a statement from NIAID issued early March 23.
“We urge the company to work with the DSMB to review the efficacy data and ensure the most accurate, up-to-date efficacy data be made public as quickly as possible,” the agency said.
The NIAID statement does not say what data may have been outdated or how it may have changed the results. The company said March 22 it plans to see U.S. authorization for the vaccine in April.
The statement from NIAID comes a day after AstraZeneca said the interim results of their phase III U.S. study found it was 79% effective against symptomatic COVID-19, 80% effective in people 65 years and older, and 100% effective against severe or critical disease and hospitalization.
Company officials and clinical trial investigators on March 22 also addressed the recent concerns about blood clots, how well the vaccine will perform against variants, and provided a timeline for seeking regulatory approval.
“There are many countries in Europe and throughout the world that have already authorized this. The fact that a United States-run study has confirmed the efficacy and safety of this vaccine, I think is an important contribution to global health in general,” Anthony Fauci, MD, chief medical advisor to President Joe Biden, said during a White House press briefing March 22.
Andy Slavitt, White House senior advisor for the COVID-19 Response Team, had a more tempered reaction.
“It’s important to remind everyone we cannot and will not get ahead of the FDA,” he said. “While we would certainly call today’s news encouraging, it’s the kind of thing we like to see, we have a rigorous process that will come once an EUA is submitted and that will give us more information.”
With 30 million doses at the ready, the company plans to file for FDA emergency use authorization “within weeks,” Menelas Pangalos, executive vice president of biopharmaceuticals research and development at AstraZeneca, said during a media briefing March 22.
Risk of thrombosis addressed
Regarding highly publicized reports of problems with blood clots from the AstraZeneca vaccine, the World Health Organization found the vaccine creates no greater risks, as did the European Medicines Agency
“We’ve had absolute confidence in the efficacy of the vaccine. Seeing this data now I hope gives others increased confidence that this is a very safe and effective vaccine,” Mr. Pangalos said.
“We’re glad this is being investigated really thoroughly,” Magda Sobieszczyk, MD, an infectious disease specialist at Columbia University In New York City, said. “It’s incredibly reassuring that the regulatory agencies have looked at the data thoroughly and there is no enhanced signal above what is seen in the population.”
“There were no concerning signals noted in the U.S. data,” she added.
Regarding the risk of blood clots, “These data are therefore timely in further addressing any safety concerns that could undermine vaccine uptake.” Andrew Garrett, PhD, executive vice president of scientific operations at ICON Clinical Research, agreed.
The vaccine was well-tolerated, the company reported, with no serious adverse events. Temporary pain and tenderness at the injection site, mild-to-moderate headaches, fatigue, chills, fever, muscle aches. and malaise were among the reported reactions.
The phase III interim results show 141 cases of symptomatic COVID-19 in the study of 32,449 adults. “We don’t have the whole breakdown yet . . . these are the high-level results we just got this week,” Mr. Pangalos said. Further information on rates of mild to moderate COVID-19 illness between groups is not yet available, for example.
The company explained that participants were randomly assigned to vaccine or placebo, with twice as many receiving the actual vaccine.
The trial is ongoing, so the FDA will receive information on more than the 141 COVID-19 symptomatic cases when the company submits a full primary analysis to the agency, Mr. Pangalos said.
In the phase III study, patients received two doses 4 weeks apart.
Beyond the U.S. study, the company has additional information, including real-world data from the United Kingdom, that it intends to submit to the FDA. Part of this evidence suggests increased efficacy when a second dose is administered at 3 months
‘Robust’ findings
“This is a large study, so these results can be expected to be robust. They could be expected to be even more so if there were more cases to compare between the groups, but 141 is still a substantial number of cases,” said Peter English, MD, of Horsham, United Kingdom, who is immediate past chair of the British Medical Association Public Health Medicine Committee.
Experts welcomed the 80% efficacy in people 65 and older in particular. “Importantly, the trial provides further support for efficacy in the elderly where previous clinical trial data, other than immunologic data, had been lacking,” Dr. Garrett said.
“It is clear this vaccine has very good efficacy. Remember that 60% was, prior to any trials being started, regarded as a good target,” said Stephen Evans, professor of pharmacoepidemiology at the London School of Hygiene & Tropical Medicine. “This efficacy does not show a notable decline at older ages. This was expected and the speculation that it was ineffective or quasi-ineffective at older ages was totally unjustified.
“This is good news for the global community and one hopes that any political statements around this good news are avoided,” he added.
Efficacy against variants?
Regarding virus variants, Mr. Pangalos noted the study was conducted when several variants of concern were in circulation.
“What I can say is given this study was conducted much later in terms of timing, it’s very encouraging that we’ve got such high efficacy numbers when undoubtedly there are variants of concern in circulation in this study,” Mr. Pangalos said.
“It also highlights why we believe that against severe disease, our vaccine will be effective against all variants of concern,” he added.
Once the company submits its EUA to the FDA, the company is ready to immediately distribute 30 million doses of the vaccine and expects to ship 50 million total within the first month, Ruud Dobber, PhD, AstraZeneca executive vice president and president of the AZ Biopharmaceuticals Business Unit, said during the briefing.
The vaccine can be stored at 2 to 8 degrees Celsius for at least 6 months. Like other COVID-19 vaccines already authorized for emergency use, the duration of protection with the AstraZeneca product remains unknown.
This article was updated March 23, 2021.
A version of this article first appeared on WebMD.com.
How family medicine has changed over the past half century
From my residency training graduation date, June 1978, many changes to the family medicine specialty have occurred. These are not due to certification requirements but to the dilution of physician control in health care.
The need to provide more affordable health care by insurance companies while maintaining quality prompted more changes. Additionally, employer-based decisions to change insurance plans, since they were the payer for employer-based health insurance, sometimes yearly, prompted mandatory changes in health insurance.
To achieve hospital-based goals and cost containment the advent and use of hospitalists and the expanded use of physician extenders emerged. While I have some support for these changes, they have redefined elements of the Folsom report, which concluded that every American should have a personal physician to care for them and help integrate them into the health care system.
Changes in the health care delivery system and insurance companies’ need to contain costs, while expanding preventative medicine, coupled with a decreasing number of trained family medicine physicians, represents the background of some of the changes in family medicine over the past 50 years. Managed health care, I believe, was certainly part of the answer to implementing the following recommendation of the Folsom report: every American should have a physician-manager for their health care.
Despite the continual output of new family physicians, a shortage of physicians trained in this specialty remained. Advances in health care, which lengthened life expectancy and the fact that most health insurance companies required its members to name a primary care physician expanded the population requiring primary health care services. This only exacerbated the shortage of family physicians and lowered earning power for doctors practicing family medicine, and it created greater professional demands on family physicians, compared with those in other, more limited-scope specialties. The primary care physician shortage needed to be addressed, prompting a redefinition in the traditional nurse practitioner role.
The expansion of nurse practitioners and physician assistants’ roles
The nursing profession began training advanced-placement nurses and instituted a Doctor of Nurse Practitioner degree. At the same time physician assistants, a program that began while I was a resident, had a further role expansion, including training confined to a single specialty area of medicine. These roles were expanded by state legislators who added them to the list of primary care providers, in some locations, permitting independent practice and placing the physician assistant under the state medical boards and the nurse practitioner and Doctor of Nurse Practitioners under the nursing boards, for expanded regulations and the implementation of the new provider requirements for licensure.
The effects of insurance companies on primary care physicians and patients
When I started practicing medicine the physician was truly the manager of a person’s health care. With the advent of managed health care, that has changed. Physicians are no longer the managers; an uninvited marriage between physician, physician extender, insurance company, employer, and patient jointly controls health care.
Patients are opting for less care at the cheapest price based on incentives driven by cost and abetted by insurance companies and employers. The cost of medications has increased and provider services, coupled with medication and specialty costs have nearly priced many beyond their economic limits to pay. As a result, the patient is not always as committed as their provider to meeting the metrics of their insurance company, especially if that is increasing their out-of-pocket cost.
In addition to usual services, the primary care physician is required to demonstrate the adequacy of services provided through meeting certain practice quality metrics for nearly all insurance carriers, including Medicare and Medicaid. Because meeting these metrics carries a significant economic incentive many practices are retaining fewer noncompliant patients and have opted to bolster their bottom line with the more complaint. This adversely impacts the delivery of primary care to a significant portion of the population.
Patients that reside in poorer neighborhoods, rural areas, as well the marginalized compose a significant portion of many primary care provider’s practices and make up a significant percentage of noncompliant patients. Recognizing that the primary care physician’s overhead is high, coupled with the amount of financial and personal resources put into place to meet metrics, it costs much more to care for the marginalized, poor, and rural populations than easier-to-care for patient groups. This creates a disparity in health care.
A study that revisited the Folsom report concluded that “the 21st century primary care physician must be a true public health professional, forming partnerships and assisting data sharing with community organizations to facilitate healthy changes.” These observations have redefined primary care. This type of medicine is no longer tied to a physician; it is tied to a fairly expensive team of providers, which includes a nurse manager, physician, physician extender, social worker, and in some cases, a pharmacist. The days of mostly solo practitioners are waning and the days of the traditional family medicine residency training requires continuous nuancing, to accommodate the expanded list of practice-related responsibilities assigned to the family doctor.
Low reimbursements rates and high office overhead
The last change I have observed in the practice of family medicine over the past 50 years is a decline in the ratio of reimbursement rate for services to practice expenses. Many practitioners opt out of Medicaid or have certainly curtailed the number of Medicaid recipients on their panel because of its unacceptably low reimbursement rates combined with their high office overhead. The requirements for organizing community resources, including nursing agencies and church and community groups, carry no reimbursement for time invested. The primary care provider is responsible and evaluated on patient outcomes despite the noncompliant behavior of the patient.
What is the future of the primary care physician or provider?
The factors that determine this answer lie in what will be required of the provider and the role of the insurance company in assisting the provider of services. Insurance companies have a responsibility because they receive money to pay for metrics while remaining profitable. They must be brought into the success formula and assist the provider in order for the latter to survive. Currently the primary care provider, in an abundance of caution, is required to seek more specialty services, which drives up the cost of health care. Instead, the insurance company should allow the primary care provider to direct the health care and stop being the manager, approving or disapproving services. In summary, much has happened in family medicine over the past 50 years. The ongoing personal doctor-patient relationship has turned into a doctor-patient-insurance company relationship. The introduction of the third party has created an economic incentive for the physician to meet practice metrics, which sometimes, from the patient’s economic perspective, creates economic hardship.
Some patients enlist a primary care physician in name only but continue to drive their health care by the older model, thanks to the advent of the urgent care centers. These patients see participating in the crisis-care model as resulting in lower out-of-pocket costs. Insurance companies should enlist patient support by expanding their patient education to include the benefits of health, the benefits of meeting quality metrics by their physician, and the necessity of maintaining a compliant doctor-patient relationship. Just as they offer incentives to the primary care practitioner for meeting quality metrics incentives should be offered to those patients that meet quality metrics as well.
In the 21st century, a new model of health care emerged, which includes a primary care practitioner, nurse manager-educator, social worker, and a pharmacist. To deliver quality health care one person can’t be responsible for this burden and do it effectively. Many family practice residencies already use this model and most likely advise their graduates to seek employment where this model exists. Additionally, I am sure that family practice residencies are continually nuanced to achieve the teaching mantra required for successful postgraduate employment and good patient outcomes.
What is the future of family medicine?
The family medicine specialty is represented by a practice that looks at outcome metrics primarily without an incentive for helping the marginalized, poor, homeless, and displaced members of our society.
Urban family medicine, much like what I have practiced in this my 43rd year, is different. My practice community includes every segment of society and my approach lies in the improvement of outcomes from all that I serve. It is my impression that the future of family medicine education must include all members of our society and train residents to effectively care for all, irrespective of economic status, and evolve ways to improve the health outcomes for all.
The federal government, through reimbursement and incentive programs, needs to include such efforts in the model of care for these individuals to reduce the expense burden on the practitioner achieving better practice success and less burnout.
Dr. Betton practices family medicine in Little Rock, Ark. He also serves on the editorial advisory board of Family Practice News.
From my residency training graduation date, June 1978, many changes to the family medicine specialty have occurred. These are not due to certification requirements but to the dilution of physician control in health care.
The need to provide more affordable health care by insurance companies while maintaining quality prompted more changes. Additionally, employer-based decisions to change insurance plans, since they were the payer for employer-based health insurance, sometimes yearly, prompted mandatory changes in health insurance.
To achieve hospital-based goals and cost containment the advent and use of hospitalists and the expanded use of physician extenders emerged. While I have some support for these changes, they have redefined elements of the Folsom report, which concluded that every American should have a personal physician to care for them and help integrate them into the health care system.
Changes in the health care delivery system and insurance companies’ need to contain costs, while expanding preventative medicine, coupled with a decreasing number of trained family medicine physicians, represents the background of some of the changes in family medicine over the past 50 years. Managed health care, I believe, was certainly part of the answer to implementing the following recommendation of the Folsom report: every American should have a physician-manager for their health care.
Despite the continual output of new family physicians, a shortage of physicians trained in this specialty remained. Advances in health care, which lengthened life expectancy and the fact that most health insurance companies required its members to name a primary care physician expanded the population requiring primary health care services. This only exacerbated the shortage of family physicians and lowered earning power for doctors practicing family medicine, and it created greater professional demands on family physicians, compared with those in other, more limited-scope specialties. The primary care physician shortage needed to be addressed, prompting a redefinition in the traditional nurse practitioner role.
The expansion of nurse practitioners and physician assistants’ roles
The nursing profession began training advanced-placement nurses and instituted a Doctor of Nurse Practitioner degree. At the same time physician assistants, a program that began while I was a resident, had a further role expansion, including training confined to a single specialty area of medicine. These roles were expanded by state legislators who added them to the list of primary care providers, in some locations, permitting independent practice and placing the physician assistant under the state medical boards and the nurse practitioner and Doctor of Nurse Practitioners under the nursing boards, for expanded regulations and the implementation of the new provider requirements for licensure.
The effects of insurance companies on primary care physicians and patients
When I started practicing medicine the physician was truly the manager of a person’s health care. With the advent of managed health care, that has changed. Physicians are no longer the managers; an uninvited marriage between physician, physician extender, insurance company, employer, and patient jointly controls health care.
Patients are opting for less care at the cheapest price based on incentives driven by cost and abetted by insurance companies and employers. The cost of medications has increased and provider services, coupled with medication and specialty costs have nearly priced many beyond their economic limits to pay. As a result, the patient is not always as committed as their provider to meeting the metrics of their insurance company, especially if that is increasing their out-of-pocket cost.
In addition to usual services, the primary care physician is required to demonstrate the adequacy of services provided through meeting certain practice quality metrics for nearly all insurance carriers, including Medicare and Medicaid. Because meeting these metrics carries a significant economic incentive many practices are retaining fewer noncompliant patients and have opted to bolster their bottom line with the more complaint. This adversely impacts the delivery of primary care to a significant portion of the population.
Patients that reside in poorer neighborhoods, rural areas, as well the marginalized compose a significant portion of many primary care provider’s practices and make up a significant percentage of noncompliant patients. Recognizing that the primary care physician’s overhead is high, coupled with the amount of financial and personal resources put into place to meet metrics, it costs much more to care for the marginalized, poor, and rural populations than easier-to-care for patient groups. This creates a disparity in health care.
A study that revisited the Folsom report concluded that “the 21st century primary care physician must be a true public health professional, forming partnerships and assisting data sharing with community organizations to facilitate healthy changes.” These observations have redefined primary care. This type of medicine is no longer tied to a physician; it is tied to a fairly expensive team of providers, which includes a nurse manager, physician, physician extender, social worker, and in some cases, a pharmacist. The days of mostly solo practitioners are waning and the days of the traditional family medicine residency training requires continuous nuancing, to accommodate the expanded list of practice-related responsibilities assigned to the family doctor.
Low reimbursements rates and high office overhead
The last change I have observed in the practice of family medicine over the past 50 years is a decline in the ratio of reimbursement rate for services to practice expenses. Many practitioners opt out of Medicaid or have certainly curtailed the number of Medicaid recipients on their panel because of its unacceptably low reimbursement rates combined with their high office overhead. The requirements for organizing community resources, including nursing agencies and church and community groups, carry no reimbursement for time invested. The primary care provider is responsible and evaluated on patient outcomes despite the noncompliant behavior of the patient.
What is the future of the primary care physician or provider?
The factors that determine this answer lie in what will be required of the provider and the role of the insurance company in assisting the provider of services. Insurance companies have a responsibility because they receive money to pay for metrics while remaining profitable. They must be brought into the success formula and assist the provider in order for the latter to survive. Currently the primary care provider, in an abundance of caution, is required to seek more specialty services, which drives up the cost of health care. Instead, the insurance company should allow the primary care provider to direct the health care and stop being the manager, approving or disapproving services. In summary, much has happened in family medicine over the past 50 years. The ongoing personal doctor-patient relationship has turned into a doctor-patient-insurance company relationship. The introduction of the third party has created an economic incentive for the physician to meet practice metrics, which sometimes, from the patient’s economic perspective, creates economic hardship.
Some patients enlist a primary care physician in name only but continue to drive their health care by the older model, thanks to the advent of the urgent care centers. These patients see participating in the crisis-care model as resulting in lower out-of-pocket costs. Insurance companies should enlist patient support by expanding their patient education to include the benefits of health, the benefits of meeting quality metrics by their physician, and the necessity of maintaining a compliant doctor-patient relationship. Just as they offer incentives to the primary care practitioner for meeting quality metrics incentives should be offered to those patients that meet quality metrics as well.
In the 21st century, a new model of health care emerged, which includes a primary care practitioner, nurse manager-educator, social worker, and a pharmacist. To deliver quality health care one person can’t be responsible for this burden and do it effectively. Many family practice residencies already use this model and most likely advise their graduates to seek employment where this model exists. Additionally, I am sure that family practice residencies are continually nuanced to achieve the teaching mantra required for successful postgraduate employment and good patient outcomes.
What is the future of family medicine?
The family medicine specialty is represented by a practice that looks at outcome metrics primarily without an incentive for helping the marginalized, poor, homeless, and displaced members of our society.
Urban family medicine, much like what I have practiced in this my 43rd year, is different. My practice community includes every segment of society and my approach lies in the improvement of outcomes from all that I serve. It is my impression that the future of family medicine education must include all members of our society and train residents to effectively care for all, irrespective of economic status, and evolve ways to improve the health outcomes for all.
The federal government, through reimbursement and incentive programs, needs to include such efforts in the model of care for these individuals to reduce the expense burden on the practitioner achieving better practice success and less burnout.
Dr. Betton practices family medicine in Little Rock, Ark. He also serves on the editorial advisory board of Family Practice News.
From my residency training graduation date, June 1978, many changes to the family medicine specialty have occurred. These are not due to certification requirements but to the dilution of physician control in health care.
The need to provide more affordable health care by insurance companies while maintaining quality prompted more changes. Additionally, employer-based decisions to change insurance plans, since they were the payer for employer-based health insurance, sometimes yearly, prompted mandatory changes in health insurance.
To achieve hospital-based goals and cost containment the advent and use of hospitalists and the expanded use of physician extenders emerged. While I have some support for these changes, they have redefined elements of the Folsom report, which concluded that every American should have a personal physician to care for them and help integrate them into the health care system.
Changes in the health care delivery system and insurance companies’ need to contain costs, while expanding preventative medicine, coupled with a decreasing number of trained family medicine physicians, represents the background of some of the changes in family medicine over the past 50 years. Managed health care, I believe, was certainly part of the answer to implementing the following recommendation of the Folsom report: every American should have a physician-manager for their health care.
Despite the continual output of new family physicians, a shortage of physicians trained in this specialty remained. Advances in health care, which lengthened life expectancy and the fact that most health insurance companies required its members to name a primary care physician expanded the population requiring primary health care services. This only exacerbated the shortage of family physicians and lowered earning power for doctors practicing family medicine, and it created greater professional demands on family physicians, compared with those in other, more limited-scope specialties. The primary care physician shortage needed to be addressed, prompting a redefinition in the traditional nurse practitioner role.
The expansion of nurse practitioners and physician assistants’ roles
The nursing profession began training advanced-placement nurses and instituted a Doctor of Nurse Practitioner degree. At the same time physician assistants, a program that began while I was a resident, had a further role expansion, including training confined to a single specialty area of medicine. These roles were expanded by state legislators who added them to the list of primary care providers, in some locations, permitting independent practice and placing the physician assistant under the state medical boards and the nurse practitioner and Doctor of Nurse Practitioners under the nursing boards, for expanded regulations and the implementation of the new provider requirements for licensure.
The effects of insurance companies on primary care physicians and patients
When I started practicing medicine the physician was truly the manager of a person’s health care. With the advent of managed health care, that has changed. Physicians are no longer the managers; an uninvited marriage between physician, physician extender, insurance company, employer, and patient jointly controls health care.
Patients are opting for less care at the cheapest price based on incentives driven by cost and abetted by insurance companies and employers. The cost of medications has increased and provider services, coupled with medication and specialty costs have nearly priced many beyond their economic limits to pay. As a result, the patient is not always as committed as their provider to meeting the metrics of their insurance company, especially if that is increasing their out-of-pocket cost.
In addition to usual services, the primary care physician is required to demonstrate the adequacy of services provided through meeting certain practice quality metrics for nearly all insurance carriers, including Medicare and Medicaid. Because meeting these metrics carries a significant economic incentive many practices are retaining fewer noncompliant patients and have opted to bolster their bottom line with the more complaint. This adversely impacts the delivery of primary care to a significant portion of the population.
Patients that reside in poorer neighborhoods, rural areas, as well the marginalized compose a significant portion of many primary care provider’s practices and make up a significant percentage of noncompliant patients. Recognizing that the primary care physician’s overhead is high, coupled with the amount of financial and personal resources put into place to meet metrics, it costs much more to care for the marginalized, poor, and rural populations than easier-to-care for patient groups. This creates a disparity in health care.
A study that revisited the Folsom report concluded that “the 21st century primary care physician must be a true public health professional, forming partnerships and assisting data sharing with community organizations to facilitate healthy changes.” These observations have redefined primary care. This type of medicine is no longer tied to a physician; it is tied to a fairly expensive team of providers, which includes a nurse manager, physician, physician extender, social worker, and in some cases, a pharmacist. The days of mostly solo practitioners are waning and the days of the traditional family medicine residency training requires continuous nuancing, to accommodate the expanded list of practice-related responsibilities assigned to the family doctor.
Low reimbursements rates and high office overhead
The last change I have observed in the practice of family medicine over the past 50 years is a decline in the ratio of reimbursement rate for services to practice expenses. Many practitioners opt out of Medicaid or have certainly curtailed the number of Medicaid recipients on their panel because of its unacceptably low reimbursement rates combined with their high office overhead. The requirements for organizing community resources, including nursing agencies and church and community groups, carry no reimbursement for time invested. The primary care provider is responsible and evaluated on patient outcomes despite the noncompliant behavior of the patient.
What is the future of the primary care physician or provider?
The factors that determine this answer lie in what will be required of the provider and the role of the insurance company in assisting the provider of services. Insurance companies have a responsibility because they receive money to pay for metrics while remaining profitable. They must be brought into the success formula and assist the provider in order for the latter to survive. Currently the primary care provider, in an abundance of caution, is required to seek more specialty services, which drives up the cost of health care. Instead, the insurance company should allow the primary care provider to direct the health care and stop being the manager, approving or disapproving services. In summary, much has happened in family medicine over the past 50 years. The ongoing personal doctor-patient relationship has turned into a doctor-patient-insurance company relationship. The introduction of the third party has created an economic incentive for the physician to meet practice metrics, which sometimes, from the patient’s economic perspective, creates economic hardship.
Some patients enlist a primary care physician in name only but continue to drive their health care by the older model, thanks to the advent of the urgent care centers. These patients see participating in the crisis-care model as resulting in lower out-of-pocket costs. Insurance companies should enlist patient support by expanding their patient education to include the benefits of health, the benefits of meeting quality metrics by their physician, and the necessity of maintaining a compliant doctor-patient relationship. Just as they offer incentives to the primary care practitioner for meeting quality metrics incentives should be offered to those patients that meet quality metrics as well.
In the 21st century, a new model of health care emerged, which includes a primary care practitioner, nurse manager-educator, social worker, and a pharmacist. To deliver quality health care one person can’t be responsible for this burden and do it effectively. Many family practice residencies already use this model and most likely advise their graduates to seek employment where this model exists. Additionally, I am sure that family practice residencies are continually nuanced to achieve the teaching mantra required for successful postgraduate employment and good patient outcomes.
What is the future of family medicine?
The family medicine specialty is represented by a practice that looks at outcome metrics primarily without an incentive for helping the marginalized, poor, homeless, and displaced members of our society.
Urban family medicine, much like what I have practiced in this my 43rd year, is different. My practice community includes every segment of society and my approach lies in the improvement of outcomes from all that I serve. It is my impression that the future of family medicine education must include all members of our society and train residents to effectively care for all, irrespective of economic status, and evolve ways to improve the health outcomes for all.
The federal government, through reimbursement and incentive programs, needs to include such efforts in the model of care for these individuals to reduce the expense burden on the practitioner achieving better practice success and less burnout.
Dr. Betton practices family medicine in Little Rock, Ark. He also serves on the editorial advisory board of Family Practice News.
Success in achondroplasia spurs testing vosoritide in more growth disorders
On the basis of the quality of sustained bone growth achieved with vosoritide in dwarfism, studies are underway or being considered for more diseases that impair bone growth, according to discussion that followed the presentation of a phase 3 trial extension study at the annual meeting of the Endocrine Society.
After 1 year on active therapy in the randomized trial and another year in the extension study, patients in the vosoritide group had sustained growth velocity while placebo group patients who crossed over to active therapy caught up, reported Ravi Savarirayan, MD, Murdoch Children’s Research Institute, University of Melbourne, Australia.
Moreover, the quality and type of bone growth, such as the improvement in body segment ratios over the second year of the study, support a durable benefit. Dr. Savarirayan said that improvements in activities of daily living are expected from this improvement in upper-to-lower body segment ratios, as well as the growth seen in the limbs.
Currently there is no approved pharmacologic therapy for achondroplasia in the United States. Growth hormone has been approved in Japan, but Dr. Savarirayan said its effects have been limited. Surgery such as limb lengthening is another option, but this approach is not uniformly effective and carries risks.
The 52-week results from the multinational phase 3 trial with vosoritide, which stimulates bone growth, were published last year in The Lancet. In that trial, 121 patients between the ages of 5 and 18 years with achondroplasia were randomized to vosoritide at a dose of 15 μg/kg once daily or placebo.
Relative to those in the placebo arm, which did not experience any change in growth, the median growth at the end of 52 weeks was 1.75 cm/year greater (6.71 vs. 3.99 cm).
After crossover, placebo patients catch up
In the extension study, the placebo patients were crossed over to the active therapy and both groups were followed for an additional 52 weeks. Over this period, velocity declined modestly in those in the group initially randomized to vosoritide but climbed steeply in the placebo group so that rates after 1 year were nearly identical (5.57 vs. 5.65 cm, respectively).
“The results suggest this medication may well have a durable effect,” said Dr. Savarirayan, who believes that the benefit is derived from stimulation of the growth plates. Based on the very similar efficacy observed in the placebo group once switched to active therapy, the response to vosoritide appears to be predictable.
Of the 60 patients initially randomized to vosoritide, 58 entered the extension. Of the patients who did not remain in the study, two left due to discomfort from injection-site reactions. All 61 patients initially assigned to placebo crossed over.
“We did not see any evidence of tachyphylaxis in the randomized study or in the extension,” Dr. Savarirayan said.
Although two more patients initiated on vosoritide discontinued treatment before the end of 2 years, there were no new adverse events observed. Rather, injection-site pain, which self-resolved in all patients, appears to be the most significant side effect.
“In children, the daily subcutaneous injections can be an issue,” Dr. Savarirayan acknowledged.
Injection site reactions most common adverse event
In a detailed evaluation of safety in a previously published dose-finding phase 2 study, injection-site reactions were also the most common of treatment-related adverse events, but there were no episodes of anaphylaxis or other grade 3 or higher hypersensitivity reactions (N Engl J Med. 2019 Jul 4;381:25-35).
Prior to clinical trials, continuous infusion of endogenous C-type natriuretic peptide demonstrated an ability to stimulate long-bone growth in experimental studies. Vosoritide, a recombinant analogue of C-type natriuretic peptide, appears to provide the same activity but offers a longer half-life.
Based on the benefits observed in achondroplasia, other applications are now being explored.
“When you evaluate the quality of the bone growth associated with vosoritide, it is normal,” said Melita Irving, MD, a consultant in clinical genetics at the Guy’s and St .Thomas’ NHS Trust, London. Dr. Irving has been involved in other research initiatives with this therapy and she cited a variety of evidence that has supported healthy bone development, including favorable changes in markers of bone growth such as type 10 collagen.
As a result, vosoritide, which is now under review by the U.S. Food and Drug Administration for treatment of dwarfism, is being pursued for several other diseases that result in abnormal bone growth, such as hypochondroplasia. Not least, clinical studies in idiopathic short stature have reached “early stages,” Dr. Irving said.
Dr. Savarirayan and Dr. Irving report no relevant conflicts of interest.
On the basis of the quality of sustained bone growth achieved with vosoritide in dwarfism, studies are underway or being considered for more diseases that impair bone growth, according to discussion that followed the presentation of a phase 3 trial extension study at the annual meeting of the Endocrine Society.
After 1 year on active therapy in the randomized trial and another year in the extension study, patients in the vosoritide group had sustained growth velocity while placebo group patients who crossed over to active therapy caught up, reported Ravi Savarirayan, MD, Murdoch Children’s Research Institute, University of Melbourne, Australia.
Moreover, the quality and type of bone growth, such as the improvement in body segment ratios over the second year of the study, support a durable benefit. Dr. Savarirayan said that improvements in activities of daily living are expected from this improvement in upper-to-lower body segment ratios, as well as the growth seen in the limbs.
Currently there is no approved pharmacologic therapy for achondroplasia in the United States. Growth hormone has been approved in Japan, but Dr. Savarirayan said its effects have been limited. Surgery such as limb lengthening is another option, but this approach is not uniformly effective and carries risks.
The 52-week results from the multinational phase 3 trial with vosoritide, which stimulates bone growth, were published last year in The Lancet. In that trial, 121 patients between the ages of 5 and 18 years with achondroplasia were randomized to vosoritide at a dose of 15 μg/kg once daily or placebo.
Relative to those in the placebo arm, which did not experience any change in growth, the median growth at the end of 52 weeks was 1.75 cm/year greater (6.71 vs. 3.99 cm).
After crossover, placebo patients catch up
In the extension study, the placebo patients were crossed over to the active therapy and both groups were followed for an additional 52 weeks. Over this period, velocity declined modestly in those in the group initially randomized to vosoritide but climbed steeply in the placebo group so that rates after 1 year were nearly identical (5.57 vs. 5.65 cm, respectively).
“The results suggest this medication may well have a durable effect,” said Dr. Savarirayan, who believes that the benefit is derived from stimulation of the growth plates. Based on the very similar efficacy observed in the placebo group once switched to active therapy, the response to vosoritide appears to be predictable.
Of the 60 patients initially randomized to vosoritide, 58 entered the extension. Of the patients who did not remain in the study, two left due to discomfort from injection-site reactions. All 61 patients initially assigned to placebo crossed over.
“We did not see any evidence of tachyphylaxis in the randomized study or in the extension,” Dr. Savarirayan said.
Although two more patients initiated on vosoritide discontinued treatment before the end of 2 years, there were no new adverse events observed. Rather, injection-site pain, which self-resolved in all patients, appears to be the most significant side effect.
“In children, the daily subcutaneous injections can be an issue,” Dr. Savarirayan acknowledged.
Injection site reactions most common adverse event
In a detailed evaluation of safety in a previously published dose-finding phase 2 study, injection-site reactions were also the most common of treatment-related adverse events, but there were no episodes of anaphylaxis or other grade 3 or higher hypersensitivity reactions (N Engl J Med. 2019 Jul 4;381:25-35).
Prior to clinical trials, continuous infusion of endogenous C-type natriuretic peptide demonstrated an ability to stimulate long-bone growth in experimental studies. Vosoritide, a recombinant analogue of C-type natriuretic peptide, appears to provide the same activity but offers a longer half-life.
Based on the benefits observed in achondroplasia, other applications are now being explored.
“When you evaluate the quality of the bone growth associated with vosoritide, it is normal,” said Melita Irving, MD, a consultant in clinical genetics at the Guy’s and St .Thomas’ NHS Trust, London. Dr. Irving has been involved in other research initiatives with this therapy and she cited a variety of evidence that has supported healthy bone development, including favorable changes in markers of bone growth such as type 10 collagen.
As a result, vosoritide, which is now under review by the U.S. Food and Drug Administration for treatment of dwarfism, is being pursued for several other diseases that result in abnormal bone growth, such as hypochondroplasia. Not least, clinical studies in idiopathic short stature have reached “early stages,” Dr. Irving said.
Dr. Savarirayan and Dr. Irving report no relevant conflicts of interest.
On the basis of the quality of sustained bone growth achieved with vosoritide in dwarfism, studies are underway or being considered for more diseases that impair bone growth, according to discussion that followed the presentation of a phase 3 trial extension study at the annual meeting of the Endocrine Society.
After 1 year on active therapy in the randomized trial and another year in the extension study, patients in the vosoritide group had sustained growth velocity while placebo group patients who crossed over to active therapy caught up, reported Ravi Savarirayan, MD, Murdoch Children’s Research Institute, University of Melbourne, Australia.
Moreover, the quality and type of bone growth, such as the improvement in body segment ratios over the second year of the study, support a durable benefit. Dr. Savarirayan said that improvements in activities of daily living are expected from this improvement in upper-to-lower body segment ratios, as well as the growth seen in the limbs.
Currently there is no approved pharmacologic therapy for achondroplasia in the United States. Growth hormone has been approved in Japan, but Dr. Savarirayan said its effects have been limited. Surgery such as limb lengthening is another option, but this approach is not uniformly effective and carries risks.
The 52-week results from the multinational phase 3 trial with vosoritide, which stimulates bone growth, were published last year in The Lancet. In that trial, 121 patients between the ages of 5 and 18 years with achondroplasia were randomized to vosoritide at a dose of 15 μg/kg once daily or placebo.
Relative to those in the placebo arm, which did not experience any change in growth, the median growth at the end of 52 weeks was 1.75 cm/year greater (6.71 vs. 3.99 cm).
After crossover, placebo patients catch up
In the extension study, the placebo patients were crossed over to the active therapy and both groups were followed for an additional 52 weeks. Over this period, velocity declined modestly in those in the group initially randomized to vosoritide but climbed steeply in the placebo group so that rates after 1 year were nearly identical (5.57 vs. 5.65 cm, respectively).
“The results suggest this medication may well have a durable effect,” said Dr. Savarirayan, who believes that the benefit is derived from stimulation of the growth plates. Based on the very similar efficacy observed in the placebo group once switched to active therapy, the response to vosoritide appears to be predictable.
Of the 60 patients initially randomized to vosoritide, 58 entered the extension. Of the patients who did not remain in the study, two left due to discomfort from injection-site reactions. All 61 patients initially assigned to placebo crossed over.
“We did not see any evidence of tachyphylaxis in the randomized study or in the extension,” Dr. Savarirayan said.
Although two more patients initiated on vosoritide discontinued treatment before the end of 2 years, there were no new adverse events observed. Rather, injection-site pain, which self-resolved in all patients, appears to be the most significant side effect.
“In children, the daily subcutaneous injections can be an issue,” Dr. Savarirayan acknowledged.
Injection site reactions most common adverse event
In a detailed evaluation of safety in a previously published dose-finding phase 2 study, injection-site reactions were also the most common of treatment-related adverse events, but there were no episodes of anaphylaxis or other grade 3 or higher hypersensitivity reactions (N Engl J Med. 2019 Jul 4;381:25-35).
Prior to clinical trials, continuous infusion of endogenous C-type natriuretic peptide demonstrated an ability to stimulate long-bone growth in experimental studies. Vosoritide, a recombinant analogue of C-type natriuretic peptide, appears to provide the same activity but offers a longer half-life.
Based on the benefits observed in achondroplasia, other applications are now being explored.
“When you evaluate the quality of the bone growth associated with vosoritide, it is normal,” said Melita Irving, MD, a consultant in clinical genetics at the Guy’s and St .Thomas’ NHS Trust, London. Dr. Irving has been involved in other research initiatives with this therapy and she cited a variety of evidence that has supported healthy bone development, including favorable changes in markers of bone growth such as type 10 collagen.
As a result, vosoritide, which is now under review by the U.S. Food and Drug Administration for treatment of dwarfism, is being pursued for several other diseases that result in abnormal bone growth, such as hypochondroplasia. Not least, clinical studies in idiopathic short stature have reached “early stages,” Dr. Irving said.
Dr. Savarirayan and Dr. Irving report no relevant conflicts of interest.
FROM ENDO 2021
Children with increased suicide risk are falling through the cracks
Children in the welfare system who died by suicide were twice as likely to receive mental health services within the 6 months before their death, according to a recent study published in Pediatrics.
“Health care settings that provide more robust mental health screening and suicide risk assessment are needed for youth with child welfare system involvement,” study author Donna Ruch, PhD, a research scientist at the Nationwide Children’s Hospital, said in an interview.
Researchers noted that integrating suicide prevention strategies in primary care and providing access to effective health services for this vulnerable group could be beneficial.
At-risk kids are falling through the cracks
Suicide is the second leading cause of death in children, adolescents, and young adults between the ages of 15 and 24 years old. Children in the welfare system are four times more likely to have attempted suicide; however, research on suicide rates in this population is minimal.
“Kids in the child welfare system are so understudied and yet at such a high risk for suicide,” said Lisa Horowitz, PhD, clinical psychologist at the National Institutes of Health, who was not involved in the study. “A lot of kids pass through the health care system undetected.”
In an attempt to understand and prevent suicide in this group, Dr. Ruch and her team examined health service utilization patterns of children in the welfare system who committed suicide, compared with those in the system who did not die by suicide.
Researchers collected data on 120 deceased youth between the ages of 5 and 21 years old who had an open case in Ohio’s Statewide Automated Child Welfare Information System between 2010 and 2017. For the purpose of the study, open cases were defined as investigated child maltreatment where the family received services or the child was removed from the home.
Researchers matched each child who died by suicide with 10 controls – children in the welfare system who did not commit suicide – based on sex, race, and ethnicity.
The findings revealed that 59.2% of suicide decedents had a diagnosed mental health condition, compared with 31.3% of the control group. Researchers also found that the suicide decedent group was more likely to have multiple mental health diagnoses, with a quarter of them having at least three diagnosed conditions.
Children who died by suicide were also more likely to have a history of self-harm and to have been placed in foster or kinship care.
“Existing research also suggests that known risk factors for youth suicide are more common in youth involved with the child welfare system. This includes mental health conditions, developmental delays, problematic family-related issues, and trauma,” said Dr. Ruch. “All of these factors may be compounded for youth who are removed from their homes.”
Dr. Ruch said it is likely that children who are removed from their homes and placed in foster care may not have consistent access to necessary health services, such as therapy, which may place them at an increased risk for suicidal behavior.
Robust prevention strategies needed
Researchers also found that 90% of children who died by suicide had a health care visit within 6 months of their deaths, compared with 69.4% of controls; 48% of those visits occurred 1 month before they died.
The frequency of health care services used by suicide decedents suggests that prevention strategies for children in the welfare system should be embedded in routine medical and mental health care.
“If we as mental health counselors allow these kids to pass through the health care system, it’s really further neglect,” said Dr. Horowitz, who wrote an accompanying commentary. “And these children already deal with abuse and neglect – we don’t need to further neglect them.”
Dr. Horowitz said health care providers could go over coping strategies and discuss how children deal with hard times and make sure they have access to suicide prevention resources, such as the suicide hotline.
Additionally, better coordination with health care systems and the child welfare system is necessary to make sure there are follow-ups and screenings for suicide and other mental health conditions.
It’s not one size fits all: There may be tailored suicide prevention strategies that work better,” Dr. Horowitz explained.
Dr. Ruch and her team also believe suicide prevention strategies such as the Zero Suicide approach – an initiative that aims to embed suicide prevention health and behavioral health care systems – as well as interventions focused on family preservation to reduce the chance of a child being removed from their home could also benefit children in the welfare system.
Dr. Ruch, the other authors of the study, and Dr. Horowitz disclosed no relevant financial conflicts,
Children in the welfare system who died by suicide were twice as likely to receive mental health services within the 6 months before their death, according to a recent study published in Pediatrics.
“Health care settings that provide more robust mental health screening and suicide risk assessment are needed for youth with child welfare system involvement,” study author Donna Ruch, PhD, a research scientist at the Nationwide Children’s Hospital, said in an interview.
Researchers noted that integrating suicide prevention strategies in primary care and providing access to effective health services for this vulnerable group could be beneficial.
At-risk kids are falling through the cracks
Suicide is the second leading cause of death in children, adolescents, and young adults between the ages of 15 and 24 years old. Children in the welfare system are four times more likely to have attempted suicide; however, research on suicide rates in this population is minimal.
“Kids in the child welfare system are so understudied and yet at such a high risk for suicide,” said Lisa Horowitz, PhD, clinical psychologist at the National Institutes of Health, who was not involved in the study. “A lot of kids pass through the health care system undetected.”
In an attempt to understand and prevent suicide in this group, Dr. Ruch and her team examined health service utilization patterns of children in the welfare system who committed suicide, compared with those in the system who did not die by suicide.
Researchers collected data on 120 deceased youth between the ages of 5 and 21 years old who had an open case in Ohio’s Statewide Automated Child Welfare Information System between 2010 and 2017. For the purpose of the study, open cases were defined as investigated child maltreatment where the family received services or the child was removed from the home.
Researchers matched each child who died by suicide with 10 controls – children in the welfare system who did not commit suicide – based on sex, race, and ethnicity.
The findings revealed that 59.2% of suicide decedents had a diagnosed mental health condition, compared with 31.3% of the control group. Researchers also found that the suicide decedent group was more likely to have multiple mental health diagnoses, with a quarter of them having at least three diagnosed conditions.
Children who died by suicide were also more likely to have a history of self-harm and to have been placed in foster or kinship care.
“Existing research also suggests that known risk factors for youth suicide are more common in youth involved with the child welfare system. This includes mental health conditions, developmental delays, problematic family-related issues, and trauma,” said Dr. Ruch. “All of these factors may be compounded for youth who are removed from their homes.”
Dr. Ruch said it is likely that children who are removed from their homes and placed in foster care may not have consistent access to necessary health services, such as therapy, which may place them at an increased risk for suicidal behavior.
Robust prevention strategies needed
Researchers also found that 90% of children who died by suicide had a health care visit within 6 months of their deaths, compared with 69.4% of controls; 48% of those visits occurred 1 month before they died.
The frequency of health care services used by suicide decedents suggests that prevention strategies for children in the welfare system should be embedded in routine medical and mental health care.
“If we as mental health counselors allow these kids to pass through the health care system, it’s really further neglect,” said Dr. Horowitz, who wrote an accompanying commentary. “And these children already deal with abuse and neglect – we don’t need to further neglect them.”
Dr. Horowitz said health care providers could go over coping strategies and discuss how children deal with hard times and make sure they have access to suicide prevention resources, such as the suicide hotline.
Additionally, better coordination with health care systems and the child welfare system is necessary to make sure there are follow-ups and screenings for suicide and other mental health conditions.
It’s not one size fits all: There may be tailored suicide prevention strategies that work better,” Dr. Horowitz explained.
Dr. Ruch and her team also believe suicide prevention strategies such as the Zero Suicide approach – an initiative that aims to embed suicide prevention health and behavioral health care systems – as well as interventions focused on family preservation to reduce the chance of a child being removed from their home could also benefit children in the welfare system.
Dr. Ruch, the other authors of the study, and Dr. Horowitz disclosed no relevant financial conflicts,
Children in the welfare system who died by suicide were twice as likely to receive mental health services within the 6 months before their death, according to a recent study published in Pediatrics.
“Health care settings that provide more robust mental health screening and suicide risk assessment are needed for youth with child welfare system involvement,” study author Donna Ruch, PhD, a research scientist at the Nationwide Children’s Hospital, said in an interview.
Researchers noted that integrating suicide prevention strategies in primary care and providing access to effective health services for this vulnerable group could be beneficial.
At-risk kids are falling through the cracks
Suicide is the second leading cause of death in children, adolescents, and young adults between the ages of 15 and 24 years old. Children in the welfare system are four times more likely to have attempted suicide; however, research on suicide rates in this population is minimal.
“Kids in the child welfare system are so understudied and yet at such a high risk for suicide,” said Lisa Horowitz, PhD, clinical psychologist at the National Institutes of Health, who was not involved in the study. “A lot of kids pass through the health care system undetected.”
In an attempt to understand and prevent suicide in this group, Dr. Ruch and her team examined health service utilization patterns of children in the welfare system who committed suicide, compared with those in the system who did not die by suicide.
Researchers collected data on 120 deceased youth between the ages of 5 and 21 years old who had an open case in Ohio’s Statewide Automated Child Welfare Information System between 2010 and 2017. For the purpose of the study, open cases were defined as investigated child maltreatment where the family received services or the child was removed from the home.
Researchers matched each child who died by suicide with 10 controls – children in the welfare system who did not commit suicide – based on sex, race, and ethnicity.
The findings revealed that 59.2% of suicide decedents had a diagnosed mental health condition, compared with 31.3% of the control group. Researchers also found that the suicide decedent group was more likely to have multiple mental health diagnoses, with a quarter of them having at least three diagnosed conditions.
Children who died by suicide were also more likely to have a history of self-harm and to have been placed in foster or kinship care.
“Existing research also suggests that known risk factors for youth suicide are more common in youth involved with the child welfare system. This includes mental health conditions, developmental delays, problematic family-related issues, and trauma,” said Dr. Ruch. “All of these factors may be compounded for youth who are removed from their homes.”
Dr. Ruch said it is likely that children who are removed from their homes and placed in foster care may not have consistent access to necessary health services, such as therapy, which may place them at an increased risk for suicidal behavior.
Robust prevention strategies needed
Researchers also found that 90% of children who died by suicide had a health care visit within 6 months of their deaths, compared with 69.4% of controls; 48% of those visits occurred 1 month before they died.
The frequency of health care services used by suicide decedents suggests that prevention strategies for children in the welfare system should be embedded in routine medical and mental health care.
“If we as mental health counselors allow these kids to pass through the health care system, it’s really further neglect,” said Dr. Horowitz, who wrote an accompanying commentary. “And these children already deal with abuse and neglect – we don’t need to further neglect them.”
Dr. Horowitz said health care providers could go over coping strategies and discuss how children deal with hard times and make sure they have access to suicide prevention resources, such as the suicide hotline.
Additionally, better coordination with health care systems and the child welfare system is necessary to make sure there are follow-ups and screenings for suicide and other mental health conditions.
It’s not one size fits all: There may be tailored suicide prevention strategies that work better,” Dr. Horowitz explained.
Dr. Ruch and her team also believe suicide prevention strategies such as the Zero Suicide approach – an initiative that aims to embed suicide prevention health and behavioral health care systems – as well as interventions focused on family preservation to reduce the chance of a child being removed from their home could also benefit children in the welfare system.
Dr. Ruch, the other authors of the study, and Dr. Horowitz disclosed no relevant financial conflicts,
2021 match sets records: Who matched and who didn’t?
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.
A total of 38,106 positions were offered, up 850 spots (2.3%) from 2020. Of those, 35,194 were first-year (PGY-1) positions, which was 928 more than the previous year (2.7%). A record 5,915 programs were part of the Match, 88 more than 2020.
“The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP president and CEO, said in a new release.
The report comes amid a year of Zoom interview fatigue, canceled testing, and virus fears and work-arounds, which the NMRP has never had to wrestle with since it was established in 1952.
Despite challenges, fill rates increased across the board. Of the 38,106 total positions offered, 36,179 were filled, representing a 2.6% increase over 2020. Of the 35,194 first-year positions available, 33,535 were filled, representing a 2.9% increase.
Those rates drove the percentage of all positions filled to 94.9% (up from 94.6%) and the percentage of PGY-1 positions filled to 94.8% (also up from 94.6%). There were 1,927 unfilled positions, a decline of 71 (3.6%) from 2020.
Primary care results strong
Of the first-year positions offered, 17,649 (49.6%) were in family medicine, internal medicine, and pediatrics. That’s an increase of 514 positions (3%) over 2020.
Of first-year positions offered in 2021, 16,860 (95.5%) were filled. U.S. seniors took 11,013 (65.3%) of those slots; that represents a slight decline (0.3%) from 2020. Family medicine saw a gain of 63 U.S. MD seniors who matched, and internal medicine saw a gain of 93 U.S. DO seniors who matched.
Some specialties filled all positions
PGY-1 specialties with 30 positions or more that filled all available positions include dermatology, medicine – emergency medicine, medicine – pediatrics, neurologic surgery, otolaryngology, integrated plastic surgery, and vascular surgery.*
PGY-1 specialties with 30 positions or more that filled more than 90% with U.S. seniors include dermatology (100%), medicine – emergency medicine (93.6%), medicine – pediatrics (93.5%), otolaryngology (93.2%), orthopedic surgery (92.8%), and integrated plastic surgery (90.4%).*
PGY-1 specialties with at least 30 positions that filled less than 50% with U.S. seniors include pathology (41.4 %) and surgery–preliminary (28%).
The number of U.S. citizen international medical graduates who submitted rank-ordered lists was 5,295, an increase of 128 (2.5%) over 2020 and the highest in 6 years; 3,152 of them matched to first-year positions, down two PGY-1 matched applicants over last year.
Full data are available on the NRMP’s website.
Correction, 3/22/21: An earlier version of this article misstated the affected specialties.
A version of this article first appeared on Medscape.com.