User login
News and Views that Matter to Pediatricians
The leading independent newspaper covering news and commentary in pediatrics.
A technicality could keep RSV shots from kids in need
which has put an estimated 90,000 U.S. infants and small children in the hospital since the start of October.
But only one of the shots is designed to be given to babies, and a glitch in congressional language may make it difficult to allow children from low-income families to get it as readily as the well insured.
Since 1994, routine vaccination has been a childhood entitlement under the Vaccines for Children program, through which the federal government buys millions of vaccines and provides them free through pediatricians and clinics to children who are uninsured, underinsured, or on Medicaid – more than half of all American kids.
The 1993 law creating the program didn’t specifically include antibody shots, which were used only as rare emergency therapy at the time the bill was written.
But the first medication of its kind likely to be available to babies, called nirsevimab (it was approved in Europe in December, and Food and Drug Administration approval is expected in the summer of 2023), is not a vaccine but rather a monoclonal antibody that neutralizes RSV in the bloodstream.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is certain to recommend giving the antibody to infants, said Kelly Moore, MD, president of the advocacy group Immunize.org. The CDC is currently assessing whether nirsevimab would be eligible for the Vaccines for Children program, agency spokesperson Kristen Nordlund told KHN.
Failing to do so would “consign thousands upon thousands of infants to hospitalization and serious illness for semantic reasons despite existence of an immunization that functionally performs just like a seasonal vaccine,” Dr. Moore said.
Officials from Sanofi, which is producing the nirsevimab injection along with AstraZeneca, declined to state a price but said the range would be similar to that of a pediatric vaccine course. The CDC pays about $650 for the most expensive routine vaccine, the four shots against pneumococcal infection. In other words, FDA approval would make nirsevimab a blockbuster drug worth billions annually if it’s given to a large share of the 3.7 million or so children born in the U.S. each year.
Pfizer and GlaxoSmithKline are making traditional vaccines against RSV and expect FDA approval later in 2023. Pfizer’s shot initially would be given to pregnant women – to shield their babies from the disease – while GSK’s would be given to the elderly.
Vaccines designed for infants are in the pipeline, but some experts are still nervous about them. A 1966 RSV vaccine trial failed spectacularly, killing two toddlers, and immunologists aren’t totally in agreement over the cause, said Barney Graham, MD, PhD, the retired National Institutes of Health scientist whose studies of the episode contributed to successful COVID-19 and RSV vaccines.
After 2 years of COVID lockdowns and masking slowed its transmission, RSV exploded across the United States in 2023, swamping pediatric intensive care units.
Sanofi and AstraZeneca hope to have nirsevimab approved by the FDA, recommended by the CDC, and deployed nationwide by fall to prevent future RSV epidemics.
Their product is designed to be provided before a baby’s first winter RSV season. In clinical trials, the antibodies provided up to 5 months of protection. Most children wouldn’t need a second dose because the virus is not a mortal danger to healthy kids over a year old, said Jon Heinrichs, a senior member of Sanofi’s vaccines division.
If the antibody treatment is not accepted for the Vaccines for Children program, that will limit access to the shot for the uninsured and those on Medicaid, the majority of whom represent racial or ethnic minorities, Dr. Moore said. The drugmakers would have to negotiate with each state’s Medicaid program to get it on their formularies.
Excluding the shot from Vaccines for Children “would only worsen existing health disparities,” said Sean O’Leary, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora, and chair of the infectious diseases committee of the American Academy of Pediatrics.
RSV affects babies of all social classes but tends to hit poor, crowded households hardest, said Dr. Graham. “Family history of asthma or allergy makes it worse,” he said, and premature babies are also at higher risk.
While 2%-3% of U.S. infants are hospitalized with RSV each year, only a few hundred don’t survive. But as many as 10,000 people 65 and older perish because of an infection every year, and a little-discussed legal change will make RSV and other vaccines more available to this group.
A section of the 2022 Inflation Reduction Act that went into effect Jan. 1 ends out-of-pocket payments for all vaccines by Medicare patients – including RSV vaccines, if they are licensed for this group.
Before, “if you hadn’t met your deductible, it could be very expensive,” said Leonard Friedland, MD, vice president for scientific affairs and public health in GSK’s vaccines division, which also makes shingles and combination tetanus-diphtheria-whooping cough boosters covered by the new law. “It’s a tremendously important advance.”
Of course, high levels of vaccine hesitancy are likely to blunt uptake of the shots regardless of who pays, said Jennifer Reich, a sociologist at the University of Colorado who studies vaccination attitudes.
New types of shots, like the Sanofi-AstraZeneca antibodies, often alarm parents, and Pfizer’s shot for pregnant women is likely to push fear buttons as well, she said.
Public health officials “don’t seem very savvy about how to get ahead” of claims that vaccines undermine fertility or otherwise harm people, said Ms. Reich.
On the other hand, this winter’s RSV epidemic will be persuasive to many parents, said Heidi Larson, leader of the Vaccine Confidence Project and a professor of anthropology at the London School of Hygiene and Tropical Medicine.
“It’s a scary thing to have your kid hospitalized with RSV,” she said.
While unfortunate, “the high number of children who died or were admitted to the ICU in the past season with RSV – in some ways that’s helpful,” said Laura Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in her field haven’t really started talking about how to communicate with women about the vaccine, said Dr. Riley, who chairs the immunization group at the American College of Obstetricians and Gynecologists.
“Everyone’s been waiting to see if it gets approved,” she said. “The education has to start soon, but it’s hard to roll out education before you roll out the shot.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
which has put an estimated 90,000 U.S. infants and small children in the hospital since the start of October.
But only one of the shots is designed to be given to babies, and a glitch in congressional language may make it difficult to allow children from low-income families to get it as readily as the well insured.
Since 1994, routine vaccination has been a childhood entitlement under the Vaccines for Children program, through which the federal government buys millions of vaccines and provides them free through pediatricians and clinics to children who are uninsured, underinsured, or on Medicaid – more than half of all American kids.
The 1993 law creating the program didn’t specifically include antibody shots, which were used only as rare emergency therapy at the time the bill was written.
But the first medication of its kind likely to be available to babies, called nirsevimab (it was approved in Europe in December, and Food and Drug Administration approval is expected in the summer of 2023), is not a vaccine but rather a monoclonal antibody that neutralizes RSV in the bloodstream.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is certain to recommend giving the antibody to infants, said Kelly Moore, MD, president of the advocacy group Immunize.org. The CDC is currently assessing whether nirsevimab would be eligible for the Vaccines for Children program, agency spokesperson Kristen Nordlund told KHN.
Failing to do so would “consign thousands upon thousands of infants to hospitalization and serious illness for semantic reasons despite existence of an immunization that functionally performs just like a seasonal vaccine,” Dr. Moore said.
Officials from Sanofi, which is producing the nirsevimab injection along with AstraZeneca, declined to state a price but said the range would be similar to that of a pediatric vaccine course. The CDC pays about $650 for the most expensive routine vaccine, the four shots against pneumococcal infection. In other words, FDA approval would make nirsevimab a blockbuster drug worth billions annually if it’s given to a large share of the 3.7 million or so children born in the U.S. each year.
Pfizer and GlaxoSmithKline are making traditional vaccines against RSV and expect FDA approval later in 2023. Pfizer’s shot initially would be given to pregnant women – to shield their babies from the disease – while GSK’s would be given to the elderly.
Vaccines designed for infants are in the pipeline, but some experts are still nervous about them. A 1966 RSV vaccine trial failed spectacularly, killing two toddlers, and immunologists aren’t totally in agreement over the cause, said Barney Graham, MD, PhD, the retired National Institutes of Health scientist whose studies of the episode contributed to successful COVID-19 and RSV vaccines.
After 2 years of COVID lockdowns and masking slowed its transmission, RSV exploded across the United States in 2023, swamping pediatric intensive care units.
Sanofi and AstraZeneca hope to have nirsevimab approved by the FDA, recommended by the CDC, and deployed nationwide by fall to prevent future RSV epidemics.
Their product is designed to be provided before a baby’s first winter RSV season. In clinical trials, the antibodies provided up to 5 months of protection. Most children wouldn’t need a second dose because the virus is not a mortal danger to healthy kids over a year old, said Jon Heinrichs, a senior member of Sanofi’s vaccines division.
If the antibody treatment is not accepted for the Vaccines for Children program, that will limit access to the shot for the uninsured and those on Medicaid, the majority of whom represent racial or ethnic minorities, Dr. Moore said. The drugmakers would have to negotiate with each state’s Medicaid program to get it on their formularies.
Excluding the shot from Vaccines for Children “would only worsen existing health disparities,” said Sean O’Leary, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora, and chair of the infectious diseases committee of the American Academy of Pediatrics.
RSV affects babies of all social classes but tends to hit poor, crowded households hardest, said Dr. Graham. “Family history of asthma or allergy makes it worse,” he said, and premature babies are also at higher risk.
While 2%-3% of U.S. infants are hospitalized with RSV each year, only a few hundred don’t survive. But as many as 10,000 people 65 and older perish because of an infection every year, and a little-discussed legal change will make RSV and other vaccines more available to this group.
A section of the 2022 Inflation Reduction Act that went into effect Jan. 1 ends out-of-pocket payments for all vaccines by Medicare patients – including RSV vaccines, if they are licensed for this group.
Before, “if you hadn’t met your deductible, it could be very expensive,” said Leonard Friedland, MD, vice president for scientific affairs and public health in GSK’s vaccines division, which also makes shingles and combination tetanus-diphtheria-whooping cough boosters covered by the new law. “It’s a tremendously important advance.”
Of course, high levels of vaccine hesitancy are likely to blunt uptake of the shots regardless of who pays, said Jennifer Reich, a sociologist at the University of Colorado who studies vaccination attitudes.
New types of shots, like the Sanofi-AstraZeneca antibodies, often alarm parents, and Pfizer’s shot for pregnant women is likely to push fear buttons as well, she said.
Public health officials “don’t seem very savvy about how to get ahead” of claims that vaccines undermine fertility or otherwise harm people, said Ms. Reich.
On the other hand, this winter’s RSV epidemic will be persuasive to many parents, said Heidi Larson, leader of the Vaccine Confidence Project and a professor of anthropology at the London School of Hygiene and Tropical Medicine.
“It’s a scary thing to have your kid hospitalized with RSV,” she said.
While unfortunate, “the high number of children who died or were admitted to the ICU in the past season with RSV – in some ways that’s helpful,” said Laura Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in her field haven’t really started talking about how to communicate with women about the vaccine, said Dr. Riley, who chairs the immunization group at the American College of Obstetricians and Gynecologists.
“Everyone’s been waiting to see if it gets approved,” she said. “The education has to start soon, but it’s hard to roll out education before you roll out the shot.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
which has put an estimated 90,000 U.S. infants and small children in the hospital since the start of October.
But only one of the shots is designed to be given to babies, and a glitch in congressional language may make it difficult to allow children from low-income families to get it as readily as the well insured.
Since 1994, routine vaccination has been a childhood entitlement under the Vaccines for Children program, through which the federal government buys millions of vaccines and provides them free through pediatricians and clinics to children who are uninsured, underinsured, or on Medicaid – more than half of all American kids.
The 1993 law creating the program didn’t specifically include antibody shots, which were used only as rare emergency therapy at the time the bill was written.
But the first medication of its kind likely to be available to babies, called nirsevimab (it was approved in Europe in December, and Food and Drug Administration approval is expected in the summer of 2023), is not a vaccine but rather a monoclonal antibody that neutralizes RSV in the bloodstream.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices is certain to recommend giving the antibody to infants, said Kelly Moore, MD, president of the advocacy group Immunize.org. The CDC is currently assessing whether nirsevimab would be eligible for the Vaccines for Children program, agency spokesperson Kristen Nordlund told KHN.
Failing to do so would “consign thousands upon thousands of infants to hospitalization and serious illness for semantic reasons despite existence of an immunization that functionally performs just like a seasonal vaccine,” Dr. Moore said.
Officials from Sanofi, which is producing the nirsevimab injection along with AstraZeneca, declined to state a price but said the range would be similar to that of a pediatric vaccine course. The CDC pays about $650 for the most expensive routine vaccine, the four shots against pneumococcal infection. In other words, FDA approval would make nirsevimab a blockbuster drug worth billions annually if it’s given to a large share of the 3.7 million or so children born in the U.S. each year.
Pfizer and GlaxoSmithKline are making traditional vaccines against RSV and expect FDA approval later in 2023. Pfizer’s shot initially would be given to pregnant women – to shield their babies from the disease – while GSK’s would be given to the elderly.
Vaccines designed for infants are in the pipeline, but some experts are still nervous about them. A 1966 RSV vaccine trial failed spectacularly, killing two toddlers, and immunologists aren’t totally in agreement over the cause, said Barney Graham, MD, PhD, the retired National Institutes of Health scientist whose studies of the episode contributed to successful COVID-19 and RSV vaccines.
After 2 years of COVID lockdowns and masking slowed its transmission, RSV exploded across the United States in 2023, swamping pediatric intensive care units.
Sanofi and AstraZeneca hope to have nirsevimab approved by the FDA, recommended by the CDC, and deployed nationwide by fall to prevent future RSV epidemics.
Their product is designed to be provided before a baby’s first winter RSV season. In clinical trials, the antibodies provided up to 5 months of protection. Most children wouldn’t need a second dose because the virus is not a mortal danger to healthy kids over a year old, said Jon Heinrichs, a senior member of Sanofi’s vaccines division.
If the antibody treatment is not accepted for the Vaccines for Children program, that will limit access to the shot for the uninsured and those on Medicaid, the majority of whom represent racial or ethnic minorities, Dr. Moore said. The drugmakers would have to negotiate with each state’s Medicaid program to get it on their formularies.
Excluding the shot from Vaccines for Children “would only worsen existing health disparities,” said Sean O’Leary, MD, a professor of pediatrics at the University of Colorado at Denver, Aurora, and chair of the infectious diseases committee of the American Academy of Pediatrics.
RSV affects babies of all social classes but tends to hit poor, crowded households hardest, said Dr. Graham. “Family history of asthma or allergy makes it worse,” he said, and premature babies are also at higher risk.
While 2%-3% of U.S. infants are hospitalized with RSV each year, only a few hundred don’t survive. But as many as 10,000 people 65 and older perish because of an infection every year, and a little-discussed legal change will make RSV and other vaccines more available to this group.
A section of the 2022 Inflation Reduction Act that went into effect Jan. 1 ends out-of-pocket payments for all vaccines by Medicare patients – including RSV vaccines, if they are licensed for this group.
Before, “if you hadn’t met your deductible, it could be very expensive,” said Leonard Friedland, MD, vice president for scientific affairs and public health in GSK’s vaccines division, which also makes shingles and combination tetanus-diphtheria-whooping cough boosters covered by the new law. “It’s a tremendously important advance.”
Of course, high levels of vaccine hesitancy are likely to blunt uptake of the shots regardless of who pays, said Jennifer Reich, a sociologist at the University of Colorado who studies vaccination attitudes.
New types of shots, like the Sanofi-AstraZeneca antibodies, often alarm parents, and Pfizer’s shot for pregnant women is likely to push fear buttons as well, she said.
Public health officials “don’t seem very savvy about how to get ahead” of claims that vaccines undermine fertility or otherwise harm people, said Ms. Reich.
On the other hand, this winter’s RSV epidemic will be persuasive to many parents, said Heidi Larson, leader of the Vaccine Confidence Project and a professor of anthropology at the London School of Hygiene and Tropical Medicine.
“It’s a scary thing to have your kid hospitalized with RSV,” she said.
While unfortunate, “the high number of children who died or were admitted to the ICU in the past season with RSV – in some ways that’s helpful,” said Laura Riley, MD, chair of obstetrics and gynecology at Weill Cornell Medicine, New York.
Specialists in her field haven’t really started talking about how to communicate with women about the vaccine, said Dr. Riley, who chairs the immunization group at the American College of Obstetricians and Gynecologists.
“Everyone’s been waiting to see if it gets approved,” she said. “The education has to start soon, but it’s hard to roll out education before you roll out the shot.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
In adults with prediabetes, vitamin D cuts diabetes risk
Results of the analysis, led by Anastassios G. Pittas, MD, MS, with the division of endocrinology, diabetes, and metabolism at Tufts Medical Center, in Boston, were published online in Annals of Internal Medicine (2023 Feb 7. doi: 10.7326/M22-3018).
All three eligible trials included in the analysis were randomized, double blinded, and placebo controlled. The three eligible trials tested three oral formulations of Vitamin D: cholecalciferol, 20,000 IU (500 mcg) weekly; cholecalciferol, 4,000 IU (100 mcg) daily; or eldecalcitol, 0.75 mcg daily, against placebos.
The authors of the new paper found that vitamin D reduced the risk for diabetes in people with prediabetes by a statistically significant 15% in adjusted analyses. The 3-year absolute risk reduction was 3.3%.
They found no difference in the rate ratios for adverse events (kidney stones, 1.17, 95% confidence interval, 0.69-1.99; hypercalcemia, 2.34; 95% CI, 0.83-6.66]; hypercalciuria, 1.65; 95% CI, 0.83-3.28]; death, 0.85; 95% CI, 0.31-2.36]) when study participants got vitamin D instead of placebo.
Differences from previous analyses
The relationship between vitamin D levels and risk for type 2 diabetes has been studied in previous trials and results have been mixed.
The authors note that two previous meta-analyses included trials “that had relatively short durations for assessment of diabetes risk (for example, ≤ 1 year), had high risk of bias (for example, open-label trials), or were not specifically designed and conducted for primary prevention of type 2 diabetes, potentially undermining the validity of the results.”
Each of the trials in this meta-analysis had a low risk of bias as determined by the Cochrane risk-of-bias tool, Dr. Pittas and colleagues said.
“The present study does not reach an opposite conclusion from the D2d study,” said Dr. Pittas, who coauthored that paper as well. “Rather, it confirms the results of the D2d study. In D2d and two other similar vitamin D and diabetes prevention trials (one in Norway and one in Japan), vitamin D reduced the rate of progression to diabetes in adults with prediabetes, but the observed differences were not statistically significant because the reported relative risk reductions (10%-13%) were smaller than each trial was powered to detect (25%-36%).”
“Individual participant data meta-analyses increase the statistical power to detect an effect. After combining data, we found that vitamin D reduced the risk of progression from prediabetes to diabetes by 15% and this result was statistically significant. So, the conclusion of the meta-analysis is essentially the same conclusion as in D2d and the other two trials. The difference is that the result is now statistically significant,” Dr. Pittas added.
Small reduction but large population
The authors acknowledged that the absolute risk reduction number is small, especially when compared with the risk reduction seen with intensive lifestyle changes (58%) and metformin (31%), as reported in an article published in the New England of Journal of Medicine (2002 Feb 7;346:393-403). But “extrapolating to the more than 374 million adults worldwide who have prediabetes suggests that inexpensive vitamin D supplementation could delay the development of diabetes in more than 10 million people,” they said.
As for how high vitamin D levels need to be, the authors write that their research indicates that the optimal level of vitamin D in the blood needed to reduce diabetes risk may be higher than an Institute of Medicine committee recommendation in 2011.
“The blood 25-hydroxy vitamin D level needed to optimally reduce diabetes risk may be near and possibly above the range of 125-150 nmol/L (50-60 ng/mL) that the 2011 Institute of Medicine Committee to Review Dietary Reference Intakes for Calcium and Vitamin D provided as the range corresponding to the tolerable upper intake level (UL) of 4,000 IU/d for vitamin D,” the authors of the new paper said.
Editorialists urge caution
In an accompanying editorial also published in the Annals of Internal Medicine, Malachi J. McKenna, MD, with the department of clinical chemistry, at St. Vincent’s University Hospital, and Mary A.T. Flynn, PhD, RD, with the Food Safety Authority of Ireland in Dublin, urge caution regarding vitamin D dosing.
They write that there are important distinctions between vitamin D supplements and vitamin D therapy, and the potential harms of high-dose vitamin D are still unclear.
“Vitamin D supplementation of 10 to 20 mcg (400 to 800 IU) daily can be applied safely at the population level to prevent skeletal and possibly nonskeletal disease. Very-high-dose vitamin D therapy might prevent type 2 diabetes in some patients but may also cause harm,” they note.
Dr. Pittas said in an interview that there have been some studies with high-dose vitamin D (up to 500,000 IU a year in one study) that reported an increased fall risk in older adults who had high fall risk. “However, these findings are not generalizable to other populations that are younger and at low or average fall risk, such as the prediabetes population to which the results of this meta-analysis apply,” he noted.
“The benefit-to-risk ratio for vitamin D depends on the target population and medical condition,” Dr. Pittas said. “The editorial refers to the NAM (National Academy of Medicine) vitamin D guidelines for the general, healthy population to promote bone health. The guidelines should not be extrapolated to specific populations, for example [patients with] prediabetes,” where the vitamin D benefit-to-risk ratio would be different from that in the general population.
Dr. Pittas and colleagues caution that the people studied in this meta-analysis were at high risk for type 2 diabetes, so these results do not apply to the general healthy population. The results also should not be extrapolated to people at average risk for any type of diabetes, they add.
Several physicians either declined to comment or did not respond to requests for comment on this research.
Dr. Pittas reports the National Institutes of Health and the American Diabetes Association made payments to his institution to conduct Vitamin D-related research. He is an unpaid cochair of the Endocrine Society’s Evaluation, Treatment and Prevention of Vitamin D Deficiency Clinical Practice Guideline team.
Coauthor Dr. Jorde reports grants from Novo Nordisk Foundation, North Norwegian Regional Health Authorities, and the Research Council of Norway.
Dr. Dawson-Hughes reports she is on the DSMB for AgNovos Healthcare. AgNovos is developing a bone implant to reduce hip fracture risk and she gets a stipend from the company. She reports Helsinn Therapeutics provided anamorelin and matching placebo for an NIH-funded clinical trial.
Dr. Trikalinos was supported by the D2d study. He is a technical methodological consultant to Latham and Watkins, who is retained by Pacira Pharmaceuticals.
Dr. Angellotti has been employed by Takeda and owns stock in the company.
The editorialists report no relevant financial relationships.
Results of the analysis, led by Anastassios G. Pittas, MD, MS, with the division of endocrinology, diabetes, and metabolism at Tufts Medical Center, in Boston, were published online in Annals of Internal Medicine (2023 Feb 7. doi: 10.7326/M22-3018).
All three eligible trials included in the analysis were randomized, double blinded, and placebo controlled. The three eligible trials tested three oral formulations of Vitamin D: cholecalciferol, 20,000 IU (500 mcg) weekly; cholecalciferol, 4,000 IU (100 mcg) daily; or eldecalcitol, 0.75 mcg daily, against placebos.
The authors of the new paper found that vitamin D reduced the risk for diabetes in people with prediabetes by a statistically significant 15% in adjusted analyses. The 3-year absolute risk reduction was 3.3%.
They found no difference in the rate ratios for adverse events (kidney stones, 1.17, 95% confidence interval, 0.69-1.99; hypercalcemia, 2.34; 95% CI, 0.83-6.66]; hypercalciuria, 1.65; 95% CI, 0.83-3.28]; death, 0.85; 95% CI, 0.31-2.36]) when study participants got vitamin D instead of placebo.
Differences from previous analyses
The relationship between vitamin D levels and risk for type 2 diabetes has been studied in previous trials and results have been mixed.
The authors note that two previous meta-analyses included trials “that had relatively short durations for assessment of diabetes risk (for example, ≤ 1 year), had high risk of bias (for example, open-label trials), or were not specifically designed and conducted for primary prevention of type 2 diabetes, potentially undermining the validity of the results.”
Each of the trials in this meta-analysis had a low risk of bias as determined by the Cochrane risk-of-bias tool, Dr. Pittas and colleagues said.
“The present study does not reach an opposite conclusion from the D2d study,” said Dr. Pittas, who coauthored that paper as well. “Rather, it confirms the results of the D2d study. In D2d and two other similar vitamin D and diabetes prevention trials (one in Norway and one in Japan), vitamin D reduced the rate of progression to diabetes in adults with prediabetes, but the observed differences were not statistically significant because the reported relative risk reductions (10%-13%) were smaller than each trial was powered to detect (25%-36%).”
“Individual participant data meta-analyses increase the statistical power to detect an effect. After combining data, we found that vitamin D reduced the risk of progression from prediabetes to diabetes by 15% and this result was statistically significant. So, the conclusion of the meta-analysis is essentially the same conclusion as in D2d and the other two trials. The difference is that the result is now statistically significant,” Dr. Pittas added.
Small reduction but large population
The authors acknowledged that the absolute risk reduction number is small, especially when compared with the risk reduction seen with intensive lifestyle changes (58%) and metformin (31%), as reported in an article published in the New England of Journal of Medicine (2002 Feb 7;346:393-403). But “extrapolating to the more than 374 million adults worldwide who have prediabetes suggests that inexpensive vitamin D supplementation could delay the development of diabetes in more than 10 million people,” they said.
As for how high vitamin D levels need to be, the authors write that their research indicates that the optimal level of vitamin D in the blood needed to reduce diabetes risk may be higher than an Institute of Medicine committee recommendation in 2011.
“The blood 25-hydroxy vitamin D level needed to optimally reduce diabetes risk may be near and possibly above the range of 125-150 nmol/L (50-60 ng/mL) that the 2011 Institute of Medicine Committee to Review Dietary Reference Intakes for Calcium and Vitamin D provided as the range corresponding to the tolerable upper intake level (UL) of 4,000 IU/d for vitamin D,” the authors of the new paper said.
Editorialists urge caution
In an accompanying editorial also published in the Annals of Internal Medicine, Malachi J. McKenna, MD, with the department of clinical chemistry, at St. Vincent’s University Hospital, and Mary A.T. Flynn, PhD, RD, with the Food Safety Authority of Ireland in Dublin, urge caution regarding vitamin D dosing.
They write that there are important distinctions between vitamin D supplements and vitamin D therapy, and the potential harms of high-dose vitamin D are still unclear.
“Vitamin D supplementation of 10 to 20 mcg (400 to 800 IU) daily can be applied safely at the population level to prevent skeletal and possibly nonskeletal disease. Very-high-dose vitamin D therapy might prevent type 2 diabetes in some patients but may also cause harm,” they note.
Dr. Pittas said in an interview that there have been some studies with high-dose vitamin D (up to 500,000 IU a year in one study) that reported an increased fall risk in older adults who had high fall risk. “However, these findings are not generalizable to other populations that are younger and at low or average fall risk, such as the prediabetes population to which the results of this meta-analysis apply,” he noted.
“The benefit-to-risk ratio for vitamin D depends on the target population and medical condition,” Dr. Pittas said. “The editorial refers to the NAM (National Academy of Medicine) vitamin D guidelines for the general, healthy population to promote bone health. The guidelines should not be extrapolated to specific populations, for example [patients with] prediabetes,” where the vitamin D benefit-to-risk ratio would be different from that in the general population.
Dr. Pittas and colleagues caution that the people studied in this meta-analysis were at high risk for type 2 diabetes, so these results do not apply to the general healthy population. The results also should not be extrapolated to people at average risk for any type of diabetes, they add.
Several physicians either declined to comment or did not respond to requests for comment on this research.
Dr. Pittas reports the National Institutes of Health and the American Diabetes Association made payments to his institution to conduct Vitamin D-related research. He is an unpaid cochair of the Endocrine Society’s Evaluation, Treatment and Prevention of Vitamin D Deficiency Clinical Practice Guideline team.
Coauthor Dr. Jorde reports grants from Novo Nordisk Foundation, North Norwegian Regional Health Authorities, and the Research Council of Norway.
Dr. Dawson-Hughes reports she is on the DSMB for AgNovos Healthcare. AgNovos is developing a bone implant to reduce hip fracture risk and she gets a stipend from the company. She reports Helsinn Therapeutics provided anamorelin and matching placebo for an NIH-funded clinical trial.
Dr. Trikalinos was supported by the D2d study. He is a technical methodological consultant to Latham and Watkins, who is retained by Pacira Pharmaceuticals.
Dr. Angellotti has been employed by Takeda and owns stock in the company.
The editorialists report no relevant financial relationships.
Results of the analysis, led by Anastassios G. Pittas, MD, MS, with the division of endocrinology, diabetes, and metabolism at Tufts Medical Center, in Boston, were published online in Annals of Internal Medicine (2023 Feb 7. doi: 10.7326/M22-3018).
All three eligible trials included in the analysis were randomized, double blinded, and placebo controlled. The three eligible trials tested three oral formulations of Vitamin D: cholecalciferol, 20,000 IU (500 mcg) weekly; cholecalciferol, 4,000 IU (100 mcg) daily; or eldecalcitol, 0.75 mcg daily, against placebos.
The authors of the new paper found that vitamin D reduced the risk for diabetes in people with prediabetes by a statistically significant 15% in adjusted analyses. The 3-year absolute risk reduction was 3.3%.
They found no difference in the rate ratios for adverse events (kidney stones, 1.17, 95% confidence interval, 0.69-1.99; hypercalcemia, 2.34; 95% CI, 0.83-6.66]; hypercalciuria, 1.65; 95% CI, 0.83-3.28]; death, 0.85; 95% CI, 0.31-2.36]) when study participants got vitamin D instead of placebo.
Differences from previous analyses
The relationship between vitamin D levels and risk for type 2 diabetes has been studied in previous trials and results have been mixed.
The authors note that two previous meta-analyses included trials “that had relatively short durations for assessment of diabetes risk (for example, ≤ 1 year), had high risk of bias (for example, open-label trials), or were not specifically designed and conducted for primary prevention of type 2 diabetes, potentially undermining the validity of the results.”
Each of the trials in this meta-analysis had a low risk of bias as determined by the Cochrane risk-of-bias tool, Dr. Pittas and colleagues said.
“The present study does not reach an opposite conclusion from the D2d study,” said Dr. Pittas, who coauthored that paper as well. “Rather, it confirms the results of the D2d study. In D2d and two other similar vitamin D and diabetes prevention trials (one in Norway and one in Japan), vitamin D reduced the rate of progression to diabetes in adults with prediabetes, but the observed differences were not statistically significant because the reported relative risk reductions (10%-13%) were smaller than each trial was powered to detect (25%-36%).”
“Individual participant data meta-analyses increase the statistical power to detect an effect. After combining data, we found that vitamin D reduced the risk of progression from prediabetes to diabetes by 15% and this result was statistically significant. So, the conclusion of the meta-analysis is essentially the same conclusion as in D2d and the other two trials. The difference is that the result is now statistically significant,” Dr. Pittas added.
Small reduction but large population
The authors acknowledged that the absolute risk reduction number is small, especially when compared with the risk reduction seen with intensive lifestyle changes (58%) and metformin (31%), as reported in an article published in the New England of Journal of Medicine (2002 Feb 7;346:393-403). But “extrapolating to the more than 374 million adults worldwide who have prediabetes suggests that inexpensive vitamin D supplementation could delay the development of diabetes in more than 10 million people,” they said.
As for how high vitamin D levels need to be, the authors write that their research indicates that the optimal level of vitamin D in the blood needed to reduce diabetes risk may be higher than an Institute of Medicine committee recommendation in 2011.
“The blood 25-hydroxy vitamin D level needed to optimally reduce diabetes risk may be near and possibly above the range of 125-150 nmol/L (50-60 ng/mL) that the 2011 Institute of Medicine Committee to Review Dietary Reference Intakes for Calcium and Vitamin D provided as the range corresponding to the tolerable upper intake level (UL) of 4,000 IU/d for vitamin D,” the authors of the new paper said.
Editorialists urge caution
In an accompanying editorial also published in the Annals of Internal Medicine, Malachi J. McKenna, MD, with the department of clinical chemistry, at St. Vincent’s University Hospital, and Mary A.T. Flynn, PhD, RD, with the Food Safety Authority of Ireland in Dublin, urge caution regarding vitamin D dosing.
They write that there are important distinctions between vitamin D supplements and vitamin D therapy, and the potential harms of high-dose vitamin D are still unclear.
“Vitamin D supplementation of 10 to 20 mcg (400 to 800 IU) daily can be applied safely at the population level to prevent skeletal and possibly nonskeletal disease. Very-high-dose vitamin D therapy might prevent type 2 diabetes in some patients but may also cause harm,” they note.
Dr. Pittas said in an interview that there have been some studies with high-dose vitamin D (up to 500,000 IU a year in one study) that reported an increased fall risk in older adults who had high fall risk. “However, these findings are not generalizable to other populations that are younger and at low or average fall risk, such as the prediabetes population to which the results of this meta-analysis apply,” he noted.
“The benefit-to-risk ratio for vitamin D depends on the target population and medical condition,” Dr. Pittas said. “The editorial refers to the NAM (National Academy of Medicine) vitamin D guidelines for the general, healthy population to promote bone health. The guidelines should not be extrapolated to specific populations, for example [patients with] prediabetes,” where the vitamin D benefit-to-risk ratio would be different from that in the general population.
Dr. Pittas and colleagues caution that the people studied in this meta-analysis were at high risk for type 2 diabetes, so these results do not apply to the general healthy population. The results also should not be extrapolated to people at average risk for any type of diabetes, they add.
Several physicians either declined to comment or did not respond to requests for comment on this research.
Dr. Pittas reports the National Institutes of Health and the American Diabetes Association made payments to his institution to conduct Vitamin D-related research. He is an unpaid cochair of the Endocrine Society’s Evaluation, Treatment and Prevention of Vitamin D Deficiency Clinical Practice Guideline team.
Coauthor Dr. Jorde reports grants from Novo Nordisk Foundation, North Norwegian Regional Health Authorities, and the Research Council of Norway.
Dr. Dawson-Hughes reports she is on the DSMB for AgNovos Healthcare. AgNovos is developing a bone implant to reduce hip fracture risk and she gets a stipend from the company. She reports Helsinn Therapeutics provided anamorelin and matching placebo for an NIH-funded clinical trial.
Dr. Trikalinos was supported by the D2d study. He is a technical methodological consultant to Latham and Watkins, who is retained by Pacira Pharmaceuticals.
Dr. Angellotti has been employed by Takeda and owns stock in the company.
The editorialists report no relevant financial relationships.
FROM ANNALS OF INTERNAL MEDICINE
Primary care providers are increasingly addressing mental health concerns
particularly anxiety and stress-related diagnoses, based on a recent study.
These findings point to a sizable gap in psychiatric care that has likely been exacerbated by the pandemic, reported lead author Lisa S. Rotenstein, MD, MBA, assistant professor of medicine at Harvard Medical School and Medical Director of Population Health at Brigham and Women’s Hospital, both in Boston, and colleagues.
To ensure that PCPs can effectively manage this burden, innovative approaches are needed, such as value-based care models, billing codes for integrated behavioral health, and e-consultations with psychiatric colleagues, they added.
“Previous studies demonstrated that the rate of adult mental health outpatient visits increased between 1995 and 2010,” Dr. Rotenstein and colleagues wrote in Health Affairs. “However, more than a decade later, the extent to which the rate of primary care visits addressing mental health concerns has changed is unclear, with multiple health care delivery trends potentially influencing a further increase in prevalence.”
To address this knowledge gap, the investigators turned to the 2006-2018 National Ambulatory Medical Care Surveys, a nationally representative, serial, cross-sectional dataset. The present analysis included 109,898 visits representing 3,891,233,060 weighted visits.
Over the study period, the proportion of PCP visits that addressed mental health concerns rose from 10.7% to 15.9%.
This latter figure has probably increased since the onset of the pandemic, the investigators wrote, while availability of psychiatric care hasn’t kept pace, meaning PCPs are increasingly on the hook for managing mental illness.
“Even before the pandemic, one in five Americans lived with a mental health condition,” Dr. Rotenstein said in a written comment. “The COVID pandemic has only accelerated demand for mental health treatment. ... We know that there aren’t enough psychiatrists to meet this demand.”
Over the course of the study period, the rate of depression and affective disorders diagnoses slowed while anxiety and stress-related disorders were increasingly diagnosed.
“Particularly given the common co-occurrence of anxiety and depression, the trends we identified may represent physicians’ greater comfort over time with accurately diagnosing anxiety in the primary care setting, potentially for diagnoses that previously would have been classified as depression,” the investigators wrote, noting these findings align with a 2014 study by Olfson and colleagues.
Multiple factors associated with primary care mental health visits
Several variables were associated with significantly greater likelihood that a mental health concern would be addressed at a given visit, including female sex, younger age, payment via Medicare or Medicaid, and the physician being the patient’s regular physician.
“Our study demonstrated that mental health concerns were significantly more likely to be addressed in a visit with one’s usual primary care physician,” Dr. Rotenstein said. “This finding emphasizes the value of the longitudinal, supportive relationship developed in primary care for raising and addressing the full continuum of a patient’s needs, including mental health concerns.”
The investigators also observed significant associations between race/ethnicity and likelihood of addressing a mental health concern.
Compared with White patients, Black patients were 40% less likely to have a primary care visit with a mental health concern (odds ratio, 0.6; P less than .001). Similarly, Hispanic patients were 40% less likely than non-Hispanic patients to have a visit with a mental health concern (OR, 0.6; P less than .001).
“Unfortunately, our data don’t give us insight into why Black and Hispanic patients were less likely to have a mental health concern addressed in the context of a primary care visit,” Dr. Rotenstein said. “However, the data do suggest an urgent need to better understand and subsequently address the underlying causes of these disparities.”
She suggested several possible explanations, including differences in rates of screening, issues with access to care, insurance coverage disparities, and communication or cultural barriers.
Stuck in the reimbursement trap
Michael Klinkman, MD , professor of family medicine and learning health sciences at the University of Michigan Medical School, Ann Arbor, said the data align with his own clinical experience.
“The proportion of visits where depression was addresed went down, but the baseline is going up, so I don’t think we’re dealing with any less depression,” Dr. Klinkman said in an interview. “It’s just that there’s a lot more anxiety and stress that we’re finding and dealing with in primary care.”
While most family doctors are comfortable with best practices in managing these conditions, they may feel increasingly overburdened by the sheer number of patients with mental illness under their care alone, according to Dr. Klinkman.
“Primary care docs are increasingly feeling like they’re on their own in dealing with mental health problems,” he said.
While he agreed in theory with the interventions proposed by Dr. Rotenstein and colleagues, some solutions, like billing code changes, may ultimately worsen the burden on primary care providers.
“My fear in all of this, frankly, is that we’re going to create a better sense of the need for primary care practice in general to address mental health and social care issues, and we’re just going to create a lot more work and more widget-counting around doing that,” said Dr. Klinkman.
Value-based care appears to be a better solution, he said, since “we’re trying to take care of a human being, not the 1,050 pieces of that human being’s care that we’re trying to bundle up with different codes.”
A flat-fee, per-patient model, however, is unlikely to gain traction in the United States.
Dr. Klinkman has been involved in health care system reform up to the federal level, where he has encountered politicians who understood the issues but were incapable of helping because of partisan gridlock, he said. “It’s just politically near impossible to make changes in this basic health care business model.”
Policymakers advised Dr. Klinkman and his colleagues to strive for incremental changes, leaving them to grapple with increasingly complex reimbursement rules.
“We’re kind of stuck in this trap of trying to create new codes for services that we think ought to be better reimbursed,” Dr. Klinkman said. “We’re missing the person in all of this – the human being we’re trying to serve.”
The investigators, Dr. Cain, and Dr. Klinkman disclosed no conflicts of interest.
*This article was updated on 2/27/2023.
particularly anxiety and stress-related diagnoses, based on a recent study.
These findings point to a sizable gap in psychiatric care that has likely been exacerbated by the pandemic, reported lead author Lisa S. Rotenstein, MD, MBA, assistant professor of medicine at Harvard Medical School and Medical Director of Population Health at Brigham and Women’s Hospital, both in Boston, and colleagues.
To ensure that PCPs can effectively manage this burden, innovative approaches are needed, such as value-based care models, billing codes for integrated behavioral health, and e-consultations with psychiatric colleagues, they added.
“Previous studies demonstrated that the rate of adult mental health outpatient visits increased between 1995 and 2010,” Dr. Rotenstein and colleagues wrote in Health Affairs. “However, more than a decade later, the extent to which the rate of primary care visits addressing mental health concerns has changed is unclear, with multiple health care delivery trends potentially influencing a further increase in prevalence.”
To address this knowledge gap, the investigators turned to the 2006-2018 National Ambulatory Medical Care Surveys, a nationally representative, serial, cross-sectional dataset. The present analysis included 109,898 visits representing 3,891,233,060 weighted visits.
Over the study period, the proportion of PCP visits that addressed mental health concerns rose from 10.7% to 15.9%.
This latter figure has probably increased since the onset of the pandemic, the investigators wrote, while availability of psychiatric care hasn’t kept pace, meaning PCPs are increasingly on the hook for managing mental illness.
“Even before the pandemic, one in five Americans lived with a mental health condition,” Dr. Rotenstein said in a written comment. “The COVID pandemic has only accelerated demand for mental health treatment. ... We know that there aren’t enough psychiatrists to meet this demand.”
Over the course of the study period, the rate of depression and affective disorders diagnoses slowed while anxiety and stress-related disorders were increasingly diagnosed.
“Particularly given the common co-occurrence of anxiety and depression, the trends we identified may represent physicians’ greater comfort over time with accurately diagnosing anxiety in the primary care setting, potentially for diagnoses that previously would have been classified as depression,” the investigators wrote, noting these findings align with a 2014 study by Olfson and colleagues.
Multiple factors associated with primary care mental health visits
Several variables were associated with significantly greater likelihood that a mental health concern would be addressed at a given visit, including female sex, younger age, payment via Medicare or Medicaid, and the physician being the patient’s regular physician.
“Our study demonstrated that mental health concerns were significantly more likely to be addressed in a visit with one’s usual primary care physician,” Dr. Rotenstein said. “This finding emphasizes the value of the longitudinal, supportive relationship developed in primary care for raising and addressing the full continuum of a patient’s needs, including mental health concerns.”
The investigators also observed significant associations between race/ethnicity and likelihood of addressing a mental health concern.
Compared with White patients, Black patients were 40% less likely to have a primary care visit with a mental health concern (odds ratio, 0.6; P less than .001). Similarly, Hispanic patients were 40% less likely than non-Hispanic patients to have a visit with a mental health concern (OR, 0.6; P less than .001).
“Unfortunately, our data don’t give us insight into why Black and Hispanic patients were less likely to have a mental health concern addressed in the context of a primary care visit,” Dr. Rotenstein said. “However, the data do suggest an urgent need to better understand and subsequently address the underlying causes of these disparities.”
She suggested several possible explanations, including differences in rates of screening, issues with access to care, insurance coverage disparities, and communication or cultural barriers.
Stuck in the reimbursement trap
Michael Klinkman, MD , professor of family medicine and learning health sciences at the University of Michigan Medical School, Ann Arbor, said the data align with his own clinical experience.
“The proportion of visits where depression was addresed went down, but the baseline is going up, so I don’t think we’re dealing with any less depression,” Dr. Klinkman said in an interview. “It’s just that there’s a lot more anxiety and stress that we’re finding and dealing with in primary care.”
While most family doctors are comfortable with best practices in managing these conditions, they may feel increasingly overburdened by the sheer number of patients with mental illness under their care alone, according to Dr. Klinkman.
“Primary care docs are increasingly feeling like they’re on their own in dealing with mental health problems,” he said.
While he agreed in theory with the interventions proposed by Dr. Rotenstein and colleagues, some solutions, like billing code changes, may ultimately worsen the burden on primary care providers.
“My fear in all of this, frankly, is that we’re going to create a better sense of the need for primary care practice in general to address mental health and social care issues, and we’re just going to create a lot more work and more widget-counting around doing that,” said Dr. Klinkman.
Value-based care appears to be a better solution, he said, since “we’re trying to take care of a human being, not the 1,050 pieces of that human being’s care that we’re trying to bundle up with different codes.”
A flat-fee, per-patient model, however, is unlikely to gain traction in the United States.
Dr. Klinkman has been involved in health care system reform up to the federal level, where he has encountered politicians who understood the issues but were incapable of helping because of partisan gridlock, he said. “It’s just politically near impossible to make changes in this basic health care business model.”
Policymakers advised Dr. Klinkman and his colleagues to strive for incremental changes, leaving them to grapple with increasingly complex reimbursement rules.
“We’re kind of stuck in this trap of trying to create new codes for services that we think ought to be better reimbursed,” Dr. Klinkman said. “We’re missing the person in all of this – the human being we’re trying to serve.”
The investigators, Dr. Cain, and Dr. Klinkman disclosed no conflicts of interest.
*This article was updated on 2/27/2023.
particularly anxiety and stress-related diagnoses, based on a recent study.
These findings point to a sizable gap in psychiatric care that has likely been exacerbated by the pandemic, reported lead author Lisa S. Rotenstein, MD, MBA, assistant professor of medicine at Harvard Medical School and Medical Director of Population Health at Brigham and Women’s Hospital, both in Boston, and colleagues.
To ensure that PCPs can effectively manage this burden, innovative approaches are needed, such as value-based care models, billing codes for integrated behavioral health, and e-consultations with psychiatric colleagues, they added.
“Previous studies demonstrated that the rate of adult mental health outpatient visits increased between 1995 and 2010,” Dr. Rotenstein and colleagues wrote in Health Affairs. “However, more than a decade later, the extent to which the rate of primary care visits addressing mental health concerns has changed is unclear, with multiple health care delivery trends potentially influencing a further increase in prevalence.”
To address this knowledge gap, the investigators turned to the 2006-2018 National Ambulatory Medical Care Surveys, a nationally representative, serial, cross-sectional dataset. The present analysis included 109,898 visits representing 3,891,233,060 weighted visits.
Over the study period, the proportion of PCP visits that addressed mental health concerns rose from 10.7% to 15.9%.
This latter figure has probably increased since the onset of the pandemic, the investigators wrote, while availability of psychiatric care hasn’t kept pace, meaning PCPs are increasingly on the hook for managing mental illness.
“Even before the pandemic, one in five Americans lived with a mental health condition,” Dr. Rotenstein said in a written comment. “The COVID pandemic has only accelerated demand for mental health treatment. ... We know that there aren’t enough psychiatrists to meet this demand.”
Over the course of the study period, the rate of depression and affective disorders diagnoses slowed while anxiety and stress-related disorders were increasingly diagnosed.
“Particularly given the common co-occurrence of anxiety and depression, the trends we identified may represent physicians’ greater comfort over time with accurately diagnosing anxiety in the primary care setting, potentially for diagnoses that previously would have been classified as depression,” the investigators wrote, noting these findings align with a 2014 study by Olfson and colleagues.
Multiple factors associated with primary care mental health visits
Several variables were associated with significantly greater likelihood that a mental health concern would be addressed at a given visit, including female sex, younger age, payment via Medicare or Medicaid, and the physician being the patient’s regular physician.
“Our study demonstrated that mental health concerns were significantly more likely to be addressed in a visit with one’s usual primary care physician,” Dr. Rotenstein said. “This finding emphasizes the value of the longitudinal, supportive relationship developed in primary care for raising and addressing the full continuum of a patient’s needs, including mental health concerns.”
The investigators also observed significant associations between race/ethnicity and likelihood of addressing a mental health concern.
Compared with White patients, Black patients were 40% less likely to have a primary care visit with a mental health concern (odds ratio, 0.6; P less than .001). Similarly, Hispanic patients were 40% less likely than non-Hispanic patients to have a visit with a mental health concern (OR, 0.6; P less than .001).
“Unfortunately, our data don’t give us insight into why Black and Hispanic patients were less likely to have a mental health concern addressed in the context of a primary care visit,” Dr. Rotenstein said. “However, the data do suggest an urgent need to better understand and subsequently address the underlying causes of these disparities.”
She suggested several possible explanations, including differences in rates of screening, issues with access to care, insurance coverage disparities, and communication or cultural barriers.
Stuck in the reimbursement trap
Michael Klinkman, MD , professor of family medicine and learning health sciences at the University of Michigan Medical School, Ann Arbor, said the data align with his own clinical experience.
“The proportion of visits where depression was addresed went down, but the baseline is going up, so I don’t think we’re dealing with any less depression,” Dr. Klinkman said in an interview. “It’s just that there’s a lot more anxiety and stress that we’re finding and dealing with in primary care.”
While most family doctors are comfortable with best practices in managing these conditions, they may feel increasingly overburdened by the sheer number of patients with mental illness under their care alone, according to Dr. Klinkman.
“Primary care docs are increasingly feeling like they’re on their own in dealing with mental health problems,” he said.
While he agreed in theory with the interventions proposed by Dr. Rotenstein and colleagues, some solutions, like billing code changes, may ultimately worsen the burden on primary care providers.
“My fear in all of this, frankly, is that we’re going to create a better sense of the need for primary care practice in general to address mental health and social care issues, and we’re just going to create a lot more work and more widget-counting around doing that,” said Dr. Klinkman.
Value-based care appears to be a better solution, he said, since “we’re trying to take care of a human being, not the 1,050 pieces of that human being’s care that we’re trying to bundle up with different codes.”
A flat-fee, per-patient model, however, is unlikely to gain traction in the United States.
Dr. Klinkman has been involved in health care system reform up to the federal level, where he has encountered politicians who understood the issues but were incapable of helping because of partisan gridlock, he said. “It’s just politically near impossible to make changes in this basic health care business model.”
Policymakers advised Dr. Klinkman and his colleagues to strive for incremental changes, leaving them to grapple with increasingly complex reimbursement rules.
“We’re kind of stuck in this trap of trying to create new codes for services that we think ought to be better reimbursed,” Dr. Klinkman said. “We’re missing the person in all of this – the human being we’re trying to serve.”
The investigators, Dr. Cain, and Dr. Klinkman disclosed no conflicts of interest.
*This article was updated on 2/27/2023.
FROM HEALTH AFFAIRS
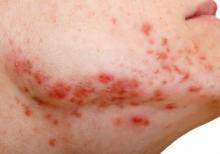
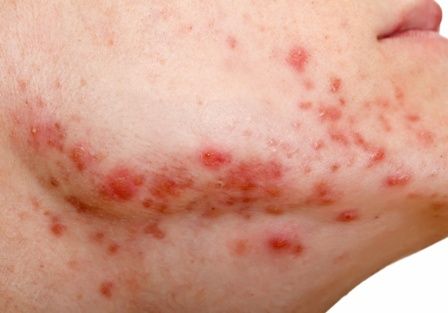
Large cohort study finds isotretinoin not associated with IBD
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.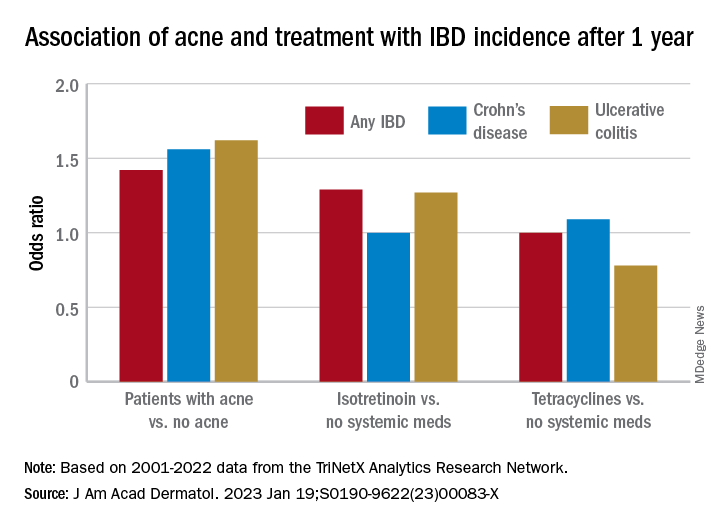
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
that also found no significant association of oral tetracycline-class antibiotics with IBD – and a small but statistically significant association of acne itself with the inflammatory disorders that make up IBD.
For the study, senior author John S. Barbieri, MD, MBA, of the department of dermatology, at Brigham and Women’s Hospital, Boston, and his colleagues used data from the TriNetX global research platform, which mines patient-level electronic medical record data from dozens of health care organizations, mainly in the United States. The network includes over 106 million patients. They looked at four cohorts: Patients without acne; those with acne but no current or prior use of systemic medications; those with acne managed with isotretinoin (and no prior use of oral tetracycline-class antibiotics); and those with acne managed with oral tetracycline-class antibiotics (and no exposure to isotretinoin).
For the acne cohorts, the investigators captured first encounters with a diagnosis of acne and first prescriptions of interest. And studywide, they used propensity score matching to balance cohorts for age, sex, race, ethnicity, and combined oral contraceptive use.
“These data should provide more reassurance to patients and prescribers that isotretinoin does not appear to result in a meaningfully increased risk of inflammatory bowel disease,” they wrote in the study, published online in the Journal of the American Academy of Dermatology.
“These are important findings as isotretinoin is a valuable treatment for acne that can result in a durable remission of disease activity, prevent acne scarring, and reduce our overreliance on oral antibiotics for acne,” they added.
Indeed, dermatologist Jonathan S. Weiss, MD, who was not involved in the research and was asked to comment on the study, said that the findings “are reassuring given the large numbers of patients evaluated and treated.” The smallest cohort – the isotretinoin group – had over 11,000 patients, and the other cohorts had over 100,000 patients each, he said in an interview.
“At this point, I’m not sure we need any other immediate information to feel comfortable using isotretinoin with respect to a potential to cause IBD, but it would be nice to see some longitudinal follow-up data for longer-term reassurance,” added Dr. Weiss, who practices in Snellville, Georgia, and is on the board of the directors of the American Acne and Rosacea Society.
The findings: Risk with acne
To assess the potential association between acne and IBD, the researchers identified more than 350,000 patients with acne managed without systemic medications, and propensity score matched them with patients who did not have acne. Altogether, their mean age was 22; 32.1% were male, and 59.6% were White.
Compared with the controls who did not have acne, they found a statistically significant association between acne and risk of incident IBD (odds ratio, 1.42; 95% confidence interval, 1.23-1.65) and an absolute risk difference of .04%. Separated into Crohn’s disease (CD) and ulcerative colitis (UC), ORs were 1.56 and 1.62, respectively.
Tetracyclines
To assess the association of oral tetracycline use and IBD, they compared more than 144,000 patients whose acne was managed with antibiotics with patients whose acne was managed without systemic medications. The patients had a mean age of 24.4; 34.7% were male, and 68.2% were White.
Compared with the patients who were not on systemic medications, there were no significant associations among those on oral tetracyclines, with an OR for incident IBD of 1 (95% CI, 0.82-1.22), an OR for incident CD of 1.09 (95% CI, 0.86-1.38), and an OR for UC of 0.78 (95% CI, 0.61-1.00).
Isotretinoin
To evaluate the association of isotretinoin and IBD, the researchers compared more than 11,000 patients treated with isotretinoin with two matched groups: patients with acne managed without systemic medications, and patients with acne managed with oral tetracyclines. The latter comparison was made to minimize potential confounding by acne severity. These patients had a mean age of 21.1; 49.5% were male, and 75.3% were White.
In the first comparison, compared with patients not treated with systemic medications, the OR for 1-year incidence of IBD among patients treated with isotretinoin was 1.29 (95% CI, 0.64-2.59), with an absolute risk difference of .036%. The ORs for CD and UC were 1.00 (95% CI, 0.45-2.23) and 1.27 (95% CI, .58-2.80), respectively.
And compared with the antibiotic-managed group, the OR for incident IBD among those on isotretinoin was 1.13 (95% CI, 0.57-2.21), with an absolute risk difference of .018%. The OR for CD was 1.00 (95% CI, 0.45-2.23). The OR for UC could not be accurately estimated because of an insufficient number of events in the tetracycline-treated group.
‘Challenging’ area of research
Researching acne treatments and the potential risk of IBD has been a methodologically “challenging topic to study” because of possible confounding and surveillance bias depending on study designs, Dr. Barbieri, director of the Brigham and Women’s Advanced Acne Therapeutics Clinic, said in an interview.
Studies that have identified a potential association between isotretinoin and IBD often have not adequately controlled for prior antibiotic exposure, for instance. And other studies, including a retrospective cohort study also published recently in JAAD using the same TriNetX database, have found 6-month isotretinoin-related risks of IBD but no increased risk at 1 year or more of follow-up – a finding that suggests a role of surveillance bias, Dr. Barbieri said.
The follow-up period of 1 year in their new study was chosen to minimize the risk of such bias. “Since patients on isotretinoin are seen more often, and since there are historical concerns about isotretinoin and IBD, patients on isotretinoin may be more likely to be screened earlier and thus could be diagnosed sooner than those not on [the medication],” he said.
He and his coauthors considered similar potential bias in designing the no-acne cohort, choosing patients who had routine primary care visits without abnormal findings in order to “reduce potential for bias due to frequency of interaction with the health care system,” they noted in their paper. (Patients had no prior encounters for acne and no history of acne treatments.)
Antibiotics, acne itself
Research on antibiotic use for acne and risk of IBD is scant, and the few studies that have been published show conflicting findings, Dr. Barbieri noted. In the meantime, studies and meta-analyses in the general medical literature – not involving acne – have identified an association between lifetime oral antibiotic exposure and IBD, he said.
While the results of the new study “are reassuring that oral tetracycline-class exposure for acne may not be associated with a significant absolute risk of inflammatory bowel disease, given the potential for antibiotic resistance and other antibiotic-associated complications, it remains important to be judicious” with their use in acne management, he and his coauthors wrote in the study.
The potential association between antibiotics for acne and IBD needs further study, preferably with longer follow-up duration, Dr. Barbieri said in the interview, but researchers are challenged by the lack of datasets with high-quality longitudinal data “beyond a few years of follow-up.”
The extent to which acne itself is associated with IBD is another area ripe for more research. Thus far, it seems that IBD and acne – and other chronic inflammatory skin diseases such as psoriasis – involve similar pathogenic pathways. “We know that in IBD Th17 and TNF immunologic pathways are important, so it’s not surprising that there may be associations,” he said.
In their paper, Dr. Barbieri and his coauthors emphasize, however, that the absolute risk difference between acne and IBD is small. It’s “unlikely that population level screening is warranted among patients with acne,” they wrote.
A second new study
The other study, also published recently in JAAD, used the same TriNetX research platform to identify approximately 77,000 patients with acne starting isotretinoin and matched them with patients starting oral antibiotics.
The investigators, Khalaf Kridin MD, PhD, and Ralf J. Ludwig, MD, of the Lübeck Institute of Experimental Dermatology, University of Lübeck (Germany), found that the lifetime risks (greater than 6 months) for patients on isotretinoin were not significantly elevated, compared with those on oral antibiotics for either CD (hazard ratio 1.05; 95% CI, 0.89-1.24, P = .583) or UC (HR, 1.13; 95% CI, 0.95-1.34; P = .162) They also looked at the risk of irritable bowel syndrome (IBS) and found a lower lifetime risk in the isotretinoin group.
In the short term, during the first 6 months after drug initiation, there was a significant, but slight increase in UC in the isotretinoin group. But this risk decreased to the level of the antibiotic group with longer follow up. “The absolute incidence rates [of IBD] and the risk difference of UC within the first 6 months are of limited clinical significance,” they wrote.
It may be, Dr. Weiss said in commenting on this study, “that isotretinoin unmasks an already-existing genetic tendency to UC early on in the course of treatment, but that it does not truly cause an increased incidence of any type of IBD.”
Both studies, said Dr. Barbieri, “add to an extensive body of literature that supports that isotretinoin is not associated with IBD.”
Dr. Barbieri had no disclosures for the study, for which Matthew T. Taylor served as first author. Coauthor Shawn Kwatra, MD, disclosed that he is an advisory board member/consultant for numerous pharmaceutical companies and has served as an investigator for several. Both are supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The other authors had no disclosures. Dr. Kridin and Dr. Ludwig had no disclosures for their study. Dr. Weiss had no disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Advice on antibiotics for kids during shortages
Pharmacies are running out of the antibiotics used to treat serious infections in children. This leaves parents and doctors frustrated and scared.
After weeks of overcrowded waiting rooms, extended office hours, and countless telephone calls during the viral respiratory surge, pediatricians are now facing a new challenge: an ever-growing list of medication shortages, including many of the most commonly used antibiotics.
These shortages primarily affect liquid formulations, so children – and the pediatricians’ offices and pharmacies serving them – are disproportionately impacted. Though there are multiple factors contributing, antibiotic overuse for viral infections during the surge has undoubtedly catalyzed the current crisis. It can be scary for parents to watch a child miserable with fever, which is why parents and pediatricians look for a quick fix in antibiotics, but unnecessary prescriptions that contribute to ongoing shortages should be avoided. We, as practicing pediatricians, think that this is a moment for reflection on when and why we use antibiotics during viral season. Though antibiotic overuse may have led us into this shortage, better antibiotic stewardship may just lead us out of it.
Since amoxicillin was approved for medical use in 1974, it has been one of the most commonly prescribed antibiotics in children. It is particularly well-suited for use in children because it treats common pediatric infections such as ear infections, strep throat, and pneumonia. These factors, along with its low cost and bubblegum flavor, make it no surprise that amoxicillin was consistently one of the top 25 medications prescribed in the United States between 2013 and 2019, with over 25 million prescriptions annually.
Amoxicillin remains the best first-line treatment option for the most common bacterial respiratory tract infections in children. With liquid formulations scarce, pediatricians, parents, and pharmacists are getting creative with crushed tablets or sprinkling capsules when possible.
However, without liquid amoxicillin readily available in our pediatric arsenal, we have recently had to turn to antibiotics with higher costs and more side effects. These broad-spectrum antibiotics target a more extensive range of bacteria and are rarely necessary for common pediatric infections. Further, their use risks increasing the already dire problem of antibiotic resistance, which causes more than 35,000 deaths in the United States each year. And perhaps most importantly, broader spectrum antibiotics aren’t better than amoxicillin for the treatment of respiratory tract infections; they are sometimes worse.
The urge to turn to antibiotics as a potential cure for childhood illnesses is an understandable one for parents and clinicians alike. A common refrain in pediatrician offices is, “Isn’t there anything we can give them?” as parents look for respite in a long viral season. As viruses continue to surge, it is helpful to remember that children will get 8 to 10 viral infections per year, with most of those occurring in the fall and winter. When parents report that their child is always sick, they aren’t far off.
Most of these infections will be cured by a child’s own immune system rather than our medications. For example, in children older than 2 years, studies have demonstrated that waiting about 2 days to start antibiotics after an ear infection is diagnosed is just as effective as starting the antibiotics right away. As tempting as it is to ask for antibiotics early, that prescription may only worsen the situation if it is a virus. Instead, pediatricians can offer parents support in treating their children at home with humidifiers, pain/fever relievers when appropriate, honey in children over 12 months, and hydration.
This drug shortage is a pivotal moment for parents and clinicians to reconsider how and when we use antibiotics during viral season. Though antibiotics may be one of the greatest inventions of the 20th century, it is how we use them now that will determine our health in the century to come.
Dr. Lockwood is Associate Professor, department of pediatrics, University of Pennsylvania, Philadelphia. Dr. Same is Assistant Professor, department of clinical pediatrics, at the University of Pennsylvania. Neither reported any conflicts of interest.
A version of this article first appeared on Medscape.com.
Pharmacies are running out of the antibiotics used to treat serious infections in children. This leaves parents and doctors frustrated and scared.
After weeks of overcrowded waiting rooms, extended office hours, and countless telephone calls during the viral respiratory surge, pediatricians are now facing a new challenge: an ever-growing list of medication shortages, including many of the most commonly used antibiotics.
These shortages primarily affect liquid formulations, so children – and the pediatricians’ offices and pharmacies serving them – are disproportionately impacted. Though there are multiple factors contributing, antibiotic overuse for viral infections during the surge has undoubtedly catalyzed the current crisis. It can be scary for parents to watch a child miserable with fever, which is why parents and pediatricians look for a quick fix in antibiotics, but unnecessary prescriptions that contribute to ongoing shortages should be avoided. We, as practicing pediatricians, think that this is a moment for reflection on when and why we use antibiotics during viral season. Though antibiotic overuse may have led us into this shortage, better antibiotic stewardship may just lead us out of it.
Since amoxicillin was approved for medical use in 1974, it has been one of the most commonly prescribed antibiotics in children. It is particularly well-suited for use in children because it treats common pediatric infections such as ear infections, strep throat, and pneumonia. These factors, along with its low cost and bubblegum flavor, make it no surprise that amoxicillin was consistently one of the top 25 medications prescribed in the United States between 2013 and 2019, with over 25 million prescriptions annually.
Amoxicillin remains the best first-line treatment option for the most common bacterial respiratory tract infections in children. With liquid formulations scarce, pediatricians, parents, and pharmacists are getting creative with crushed tablets or sprinkling capsules when possible.
However, without liquid amoxicillin readily available in our pediatric arsenal, we have recently had to turn to antibiotics with higher costs and more side effects. These broad-spectrum antibiotics target a more extensive range of bacteria and are rarely necessary for common pediatric infections. Further, their use risks increasing the already dire problem of antibiotic resistance, which causes more than 35,000 deaths in the United States each year. And perhaps most importantly, broader spectrum antibiotics aren’t better than amoxicillin for the treatment of respiratory tract infections; they are sometimes worse.
The urge to turn to antibiotics as a potential cure for childhood illnesses is an understandable one for parents and clinicians alike. A common refrain in pediatrician offices is, “Isn’t there anything we can give them?” as parents look for respite in a long viral season. As viruses continue to surge, it is helpful to remember that children will get 8 to 10 viral infections per year, with most of those occurring in the fall and winter. When parents report that their child is always sick, they aren’t far off.
Most of these infections will be cured by a child’s own immune system rather than our medications. For example, in children older than 2 years, studies have demonstrated that waiting about 2 days to start antibiotics after an ear infection is diagnosed is just as effective as starting the antibiotics right away. As tempting as it is to ask for antibiotics early, that prescription may only worsen the situation if it is a virus. Instead, pediatricians can offer parents support in treating their children at home with humidifiers, pain/fever relievers when appropriate, honey in children over 12 months, and hydration.
This drug shortage is a pivotal moment for parents and clinicians to reconsider how and when we use antibiotics during viral season. Though antibiotics may be one of the greatest inventions of the 20th century, it is how we use them now that will determine our health in the century to come.
Dr. Lockwood is Associate Professor, department of pediatrics, University of Pennsylvania, Philadelphia. Dr. Same is Assistant Professor, department of clinical pediatrics, at the University of Pennsylvania. Neither reported any conflicts of interest.
A version of this article first appeared on Medscape.com.
Pharmacies are running out of the antibiotics used to treat serious infections in children. This leaves parents and doctors frustrated and scared.
After weeks of overcrowded waiting rooms, extended office hours, and countless telephone calls during the viral respiratory surge, pediatricians are now facing a new challenge: an ever-growing list of medication shortages, including many of the most commonly used antibiotics.
These shortages primarily affect liquid formulations, so children – and the pediatricians’ offices and pharmacies serving them – are disproportionately impacted. Though there are multiple factors contributing, antibiotic overuse for viral infections during the surge has undoubtedly catalyzed the current crisis. It can be scary for parents to watch a child miserable with fever, which is why parents and pediatricians look for a quick fix in antibiotics, but unnecessary prescriptions that contribute to ongoing shortages should be avoided. We, as practicing pediatricians, think that this is a moment for reflection on when and why we use antibiotics during viral season. Though antibiotic overuse may have led us into this shortage, better antibiotic stewardship may just lead us out of it.
Since amoxicillin was approved for medical use in 1974, it has been one of the most commonly prescribed antibiotics in children. It is particularly well-suited for use in children because it treats common pediatric infections such as ear infections, strep throat, and pneumonia. These factors, along with its low cost and bubblegum flavor, make it no surprise that amoxicillin was consistently one of the top 25 medications prescribed in the United States between 2013 and 2019, with over 25 million prescriptions annually.
Amoxicillin remains the best first-line treatment option for the most common bacterial respiratory tract infections in children. With liquid formulations scarce, pediatricians, parents, and pharmacists are getting creative with crushed tablets or sprinkling capsules when possible.
However, without liquid amoxicillin readily available in our pediatric arsenal, we have recently had to turn to antibiotics with higher costs and more side effects. These broad-spectrum antibiotics target a more extensive range of bacteria and are rarely necessary for common pediatric infections. Further, their use risks increasing the already dire problem of antibiotic resistance, which causes more than 35,000 deaths in the United States each year. And perhaps most importantly, broader spectrum antibiotics aren’t better than amoxicillin for the treatment of respiratory tract infections; they are sometimes worse.
The urge to turn to antibiotics as a potential cure for childhood illnesses is an understandable one for parents and clinicians alike. A common refrain in pediatrician offices is, “Isn’t there anything we can give them?” as parents look for respite in a long viral season. As viruses continue to surge, it is helpful to remember that children will get 8 to 10 viral infections per year, with most of those occurring in the fall and winter. When parents report that their child is always sick, they aren’t far off.
Most of these infections will be cured by a child’s own immune system rather than our medications. For example, in children older than 2 years, studies have demonstrated that waiting about 2 days to start antibiotics after an ear infection is diagnosed is just as effective as starting the antibiotics right away. As tempting as it is to ask for antibiotics early, that prescription may only worsen the situation if it is a virus. Instead, pediatricians can offer parents support in treating their children at home with humidifiers, pain/fever relievers when appropriate, honey in children over 12 months, and hydration.
This drug shortage is a pivotal moment for parents and clinicians to reconsider how and when we use antibiotics during viral season. Though antibiotics may be one of the greatest inventions of the 20th century, it is how we use them now that will determine our health in the century to come.
Dr. Lockwood is Associate Professor, department of pediatrics, University of Pennsylvania, Philadelphia. Dr. Same is Assistant Professor, department of clinical pediatrics, at the University of Pennsylvania. Neither reported any conflicts of interest.
A version of this article first appeared on Medscape.com.
The new blood pressure target in primary care
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. There are very few things that we treat more often than hypertension, so you’d think the guidelines would have been clear a long time ago. Less than 10 years ago, in 2014, JNC 8 (Eighth Joint National Committee) recommended target blood pressure for individuals under 60 to be less than 140/90, and for those older than 60, less than 150/90.
Then, based primarily on the SPRINT trial (which included only people with or at significantly elevated risk for atherosclerotic cardiovascular disease), in 2017 the American Heart Association’s hypertension guidelines lowered the target BP to less than 130/80 for most individuals. It’s a little more nuanced than that, but most of us don’t remember the nuance. I’ve written about my reservations with that statement in the AHA’s journal, Circulation.
Now the American Academy of Family Physicians has updated its recommendations, and they recommend a BP less than 140/90. This is not a small change, as it often takes additional medication to achieve lower BP targets, and additional medicines lead to additional adverse effects. I’m going share with you some details from the new guideline, and then I’m going share my opinion about it.
The AAFP guideline applies to adults with hypertension, with or without cardiovascular disease. In the comprehensive literature review, the trials ran for an average of 3.7 years, and about 75% of the patients in the trials did not have preexisting cardiovascular disease.
The key to their recommendations is that target BPs lower than 140/90 did not show a statistically significant decrease in total mortality. In regard to serious adverse events, though, lower targets led to a nominal increase that didn’t reach statistical significance. Serious adverse events were defined as death or events that required hospitalization or resulted in significant disability. In regard to all other adverse events, including syncope and hypotension, there was a significant increase, with a relative risk of 1.44 (a 44% increase in adverse events). This reflected an absolute risk increase of 3%, compared with the standard target group (specifically 9.8% vs. 6.8%), with a number needed to harm of 33 over 3.7 years.
Another potential harm of low BP targets was the need for an average of one additional medicine to reach lower BP targets. One systematic review cited an eightfold higher withdrawal rate because of adverse events in the lower-target BP groups.
The AAFP guidelines said that, in the comprehensive review of the literature, while there was no difference in mortality or stroke with lower BP targets, a small additional benefit was observed in myocardial infarction – a 16% lower incidence, with a number needed to treat of 137 over 3.7 years.
So that’s the background. Let me now go over the specifics of the AAFP recommendations.
AAFP gives a strong recommendation for a standard BP target of less than 140/90. They go on to say – and grade this next statement as a weak recommendation – that, while treating to a lower BP target does not provide additional mortality benefit, a target BP of less than 135/85 can be considered to lower the risk for MI, noting that lower BP may increase harms. They state that the lower BP target could be considered based on patient preferences and values.
The AAFP guideline is incredibly helpful. The difference in the recommendations of two large societies – American Heart Association and AAFP — stems from two things. I believe that AHA focused on the composite endpoints in trials such as SPRINT, which included only high-risk patients, and the AAFP uses mortality as the driving endpoint in a broader group of patients that included both high- and lower-risk patients.
In addition, it appears that the two organizations weigh adverse events differently in coming to their conclusions. Clearly, we see more adverse events when aiming for a lower BP level, and in my experience, patients care a lot about adverse events.
Interestingly, the International Society of Hypertension recommends an “essential” BP target of less than 140/90 for most individuals, and for those under 65, they provide the option of an “optimal” BP of less than 130/80. Remember that for certain comorbidities there are also other guidelines out there. The American Diabetes Association this year revised its target BP to less than 130/80 for people with diabetes; for prevention of recurrent stroke, guidelines from the AHA/American Stroke Association in 2021 recommend BP less than 130/80, and the International Society for Hypertension as well as the AHA recommends a BP of less than 130/80 for those with established atherosclerotic cardiovascular disease.
To repeat, though, the main topic for today is that as a general target, the AAFP guidelines recommend a BP less than 140/90.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He disclosed conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
*This article was updated on 2/7/2023.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. There are very few things that we treat more often than hypertension, so you’d think the guidelines would have been clear a long time ago. Less than 10 years ago, in 2014, JNC 8 (Eighth Joint National Committee) recommended target blood pressure for individuals under 60 to be less than 140/90, and for those older than 60, less than 150/90.
Then, based primarily on the SPRINT trial (which included only people with or at significantly elevated risk for atherosclerotic cardiovascular disease), in 2017 the American Heart Association’s hypertension guidelines lowered the target BP to less than 130/80 for most individuals. It’s a little more nuanced than that, but most of us don’t remember the nuance. I’ve written about my reservations with that statement in the AHA’s journal, Circulation.
Now the American Academy of Family Physicians has updated its recommendations, and they recommend a BP less than 140/90. This is not a small change, as it often takes additional medication to achieve lower BP targets, and additional medicines lead to additional adverse effects. I’m going share with you some details from the new guideline, and then I’m going share my opinion about it.
The AAFP guideline applies to adults with hypertension, with or without cardiovascular disease. In the comprehensive literature review, the trials ran for an average of 3.7 years, and about 75% of the patients in the trials did not have preexisting cardiovascular disease.
The key to their recommendations is that target BPs lower than 140/90 did not show a statistically significant decrease in total mortality. In regard to serious adverse events, though, lower targets led to a nominal increase that didn’t reach statistical significance. Serious adverse events were defined as death or events that required hospitalization or resulted in significant disability. In regard to all other adverse events, including syncope and hypotension, there was a significant increase, with a relative risk of 1.44 (a 44% increase in adverse events). This reflected an absolute risk increase of 3%, compared with the standard target group (specifically 9.8% vs. 6.8%), with a number needed to harm of 33 over 3.7 years.
Another potential harm of low BP targets was the need for an average of one additional medicine to reach lower BP targets. One systematic review cited an eightfold higher withdrawal rate because of adverse events in the lower-target BP groups.
The AAFP guidelines said that, in the comprehensive review of the literature, while there was no difference in mortality or stroke with lower BP targets, a small additional benefit was observed in myocardial infarction – a 16% lower incidence, with a number needed to treat of 137 over 3.7 years.
So that’s the background. Let me now go over the specifics of the AAFP recommendations.
AAFP gives a strong recommendation for a standard BP target of less than 140/90. They go on to say – and grade this next statement as a weak recommendation – that, while treating to a lower BP target does not provide additional mortality benefit, a target BP of less than 135/85 can be considered to lower the risk for MI, noting that lower BP may increase harms. They state that the lower BP target could be considered based on patient preferences and values.
The AAFP guideline is incredibly helpful. The difference in the recommendations of two large societies – American Heart Association and AAFP — stems from two things. I believe that AHA focused on the composite endpoints in trials such as SPRINT, which included only high-risk patients, and the AAFP uses mortality as the driving endpoint in a broader group of patients that included both high- and lower-risk patients.
In addition, it appears that the two organizations weigh adverse events differently in coming to their conclusions. Clearly, we see more adverse events when aiming for a lower BP level, and in my experience, patients care a lot about adverse events.
Interestingly, the International Society of Hypertension recommends an “essential” BP target of less than 140/90 for most individuals, and for those under 65, they provide the option of an “optimal” BP of less than 130/80. Remember that for certain comorbidities there are also other guidelines out there. The American Diabetes Association this year revised its target BP to less than 130/80 for people with diabetes; for prevention of recurrent stroke, guidelines from the AHA/American Stroke Association in 2021 recommend BP less than 130/80, and the International Society for Hypertension as well as the AHA recommends a BP of less than 130/80 for those with established atherosclerotic cardiovascular disease.
To repeat, though, the main topic for today is that as a general target, the AAFP guidelines recommend a BP less than 140/90.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He disclosed conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
*This article was updated on 2/7/2023.
This transcript has been edited for clarity.
I’m Dr. Neil Skolnik. There are very few things that we treat more often than hypertension, so you’d think the guidelines would have been clear a long time ago. Less than 10 years ago, in 2014, JNC 8 (Eighth Joint National Committee) recommended target blood pressure for individuals under 60 to be less than 140/90, and for those older than 60, less than 150/90.
Then, based primarily on the SPRINT trial (which included only people with or at significantly elevated risk for atherosclerotic cardiovascular disease), in 2017 the American Heart Association’s hypertension guidelines lowered the target BP to less than 130/80 for most individuals. It’s a little more nuanced than that, but most of us don’t remember the nuance. I’ve written about my reservations with that statement in the AHA’s journal, Circulation.
Now the American Academy of Family Physicians has updated its recommendations, and they recommend a BP less than 140/90. This is not a small change, as it often takes additional medication to achieve lower BP targets, and additional medicines lead to additional adverse effects. I’m going share with you some details from the new guideline, and then I’m going share my opinion about it.
The AAFP guideline applies to adults with hypertension, with or without cardiovascular disease. In the comprehensive literature review, the trials ran for an average of 3.7 years, and about 75% of the patients in the trials did not have preexisting cardiovascular disease.
The key to their recommendations is that target BPs lower than 140/90 did not show a statistically significant decrease in total mortality. In regard to serious adverse events, though, lower targets led to a nominal increase that didn’t reach statistical significance. Serious adverse events were defined as death or events that required hospitalization or resulted in significant disability. In regard to all other adverse events, including syncope and hypotension, there was a significant increase, with a relative risk of 1.44 (a 44% increase in adverse events). This reflected an absolute risk increase of 3%, compared with the standard target group (specifically 9.8% vs. 6.8%), with a number needed to harm of 33 over 3.7 years.
Another potential harm of low BP targets was the need for an average of one additional medicine to reach lower BP targets. One systematic review cited an eightfold higher withdrawal rate because of adverse events in the lower-target BP groups.
The AAFP guidelines said that, in the comprehensive review of the literature, while there was no difference in mortality or stroke with lower BP targets, a small additional benefit was observed in myocardial infarction – a 16% lower incidence, with a number needed to treat of 137 over 3.7 years.
So that’s the background. Let me now go over the specifics of the AAFP recommendations.
AAFP gives a strong recommendation for a standard BP target of less than 140/90. They go on to say – and grade this next statement as a weak recommendation – that, while treating to a lower BP target does not provide additional mortality benefit, a target BP of less than 135/85 can be considered to lower the risk for MI, noting that lower BP may increase harms. They state that the lower BP target could be considered based on patient preferences and values.
The AAFP guideline is incredibly helpful. The difference in the recommendations of two large societies – American Heart Association and AAFP — stems from two things. I believe that AHA focused on the composite endpoints in trials such as SPRINT, which included only high-risk patients, and the AAFP uses mortality as the driving endpoint in a broader group of patients that included both high- and lower-risk patients.
In addition, it appears that the two organizations weigh adverse events differently in coming to their conclusions. Clearly, we see more adverse events when aiming for a lower BP level, and in my experience, patients care a lot about adverse events.
Interestingly, the International Society of Hypertension recommends an “essential” BP target of less than 140/90 for most individuals, and for those under 65, they provide the option of an “optimal” BP of less than 130/80. Remember that for certain comorbidities there are also other guidelines out there. The American Diabetes Association this year revised its target BP to less than 130/80 for people with diabetes; for prevention of recurrent stroke, guidelines from the AHA/American Stroke Association in 2021 recommend BP less than 130/80, and the International Society for Hypertension as well as the AHA recommends a BP of less than 130/80 for those with established atherosclerotic cardiovascular disease.
To repeat, though, the main topic for today is that as a general target, the AAFP guidelines recommend a BP less than 140/90.
Dr. Skolnik is professor, department of family medicine, Sidney Kimmel Medical College, Philadelphia, and associate director, department of family medicine, Abington (Pa.) Jefferson Health. He disclosed conflicts of interest with AstraZeneca, Teva, Eli Lilly, Boehringer Ingelheim, Sanofi, Sanofi Pasteur, GlaxoSmithKline, Merck, and Bayer.
A version of this article first appeared on Medscape.com.
*This article was updated on 2/7/2023.
More data back Guillain-Barré risk with Janssen COVID shot
Over 14 months, GBS reporting rates within 21 and 42 days of administration of Janssen’s replication-incompetent adenoviral vector vaccine were approximately 9 to 12 times higher than after administration of the Pfizer-BioNTech (BNT162b2) or the Moderna (mRNA-1273) mRNA COVID vaccines.
Additionally, observed GBS cases after the Janssen shot were 2 to 3 times greater than expected, based on background rates within 21 and 42 days of vaccination.
Conversely, and confirming prior data, there was no increased risk for GBS with the Pfizer or Moderna vaccines and no significant difference between observed and expected numbers of GBS cases after either mRNA COVID-19 vaccine.
The findings were published online in JAMA Network Open.
More precise risk estimates
Winston Abara, MD, with the U.S. Centers for Disease Control and Prevention, and colleagues analyzed GBS reports submitted to the VAERS between December 2020 and January 2022.
Among 487.6 million COVID-19 vaccine doses administered, 3.7% were Janssen’s Ad26.COV2.S vaccine, 54.7% were Pfizer’s BNT162b2 vaccine, and 41.6% were Moderna’s mRNA-1273 vaccine.
There were 295 verified reports of GBS identified after COVID-19 vaccination. Of these, 209 occurred within 21 days of vaccination and 253 within 42 days.
Within 21 days of vaccination, GBS reporting rates per 1 million doses were 3.29 for the Janssen vaccine versus 0.29 and 0.35 for the Pfizer and Moderna vaccines, respectively. Within 42 days of vaccination, reporting rates per 1 million doses were 4.07, 0.34, and 0.44, respectively.
Also within 21 days of vaccination, GBS reporting rates were significantly higher with the Janssen vaccine than the Pfizer vaccine (reporting rate ratio, 11.40) and the Moderna vaccine (RRR, 9.26). Similar findings were observed within 42 days after vaccination.
The observed-to-expected ratios were 3.79 for 21-day and 2.34 for 42-day intervals after receipt of the Janssen vaccine, and less than 1 (not significant) after the Pfizer or Moderna vaccine within both post-vaccination periods.
“Unlike prior studies, our analysis included all U.S. reports of verified GBS cases that met the Brighton Collaboration GBS case definition criteria (Brighton Levels 1, 2, and 3) submitted over a 14-month surveillance period to the to the Vaccine Adverse Event Reporting System,” Dr. Abara said in an interview. “Because we used all U.S. reports, the sample of verified GBS cases in this analysis is larger than other studies. Therefore, it may provide a more precise estimate of the GBS risk within 21 and 42 days after mRNA and Ad26.COV2.S vaccination,” he said.
‘Remarkably low’ use
Nicola Klein, MD, PhD, Kaiser Permanente Vaccine Study Center, Oakland, Calif., noted that this is a “nice confirmatory analysis that supports and further expands what’s been observed before.”
Last year, as reported by this news organization, Dr. Klein and colleagues reported data from the Vaccine Safety Datalink confirming a small but statistically significant increased risk for GBS in the 3 weeks after receipt of the Janssen COVID-19 vaccine but not the Pfizer or Moderna vaccines.
Unlike VAERS, the Vaccine Safety Datalink is not a reporting system. It’s an active surveillance of medical records in the Kaiser Permanente system. The VAERS is a passive system, so it requires individuals to report GBS cases to the VAERS team, Dr. Klein explained.
So although the two studies are slightly different, overall, the VAERS data is “consistent with what we found,” she said.
Also weighing in, C. Buddy Creech, MD, MPH, director of the Vanderbilt Vaccine Research Program and professor of pediatrics at the Vanderbilt University School of Medicine, Nashville, Tenn., said it is “important to realize that GBS had been observed after adenovirus-vectored vaccines earlier in the pandemic, both for the AstraZeneca vaccine and the Janssen vaccine.”
The Advisory Committee on Immunization Practices (ACIP) preferentially recommends that people age 18 years and older receive an mRNA COVID-19 vaccine rather than the Janssen adenoviral vector vaccine when both types of COVID-19 vaccine are available.
“Thus, the use of the Janssen vaccine is remarkably low in the U.S. right now,” Dr. Creech said.
“Nevertheless, we have a firm commitment, both scientifically and ethically, to track potential side effects after vaccination and to make sure that the vaccines in use for COVID, and other important infectious diseases, are safe and effective,” he added.
The study had no commercial funding. Dr. Abara and Dr. Creech have reported no relevant financial relationships. Dr. Klein reported having received grants from Pfizer research support for a COVID vaccine clinical trial, as well as grants from Merck, GlaxoSmithKline, Sanofi Pasteur, and Protein Science (now Sanofi Pasteur).
A version of this article first appeared on Medscape.com.
Over 14 months, GBS reporting rates within 21 and 42 days of administration of Janssen’s replication-incompetent adenoviral vector vaccine were approximately 9 to 12 times higher than after administration of the Pfizer-BioNTech (BNT162b2) or the Moderna (mRNA-1273) mRNA COVID vaccines.
Additionally, observed GBS cases after the Janssen shot were 2 to 3 times greater than expected, based on background rates within 21 and 42 days of vaccination.
Conversely, and confirming prior data, there was no increased risk for GBS with the Pfizer or Moderna vaccines and no significant difference between observed and expected numbers of GBS cases after either mRNA COVID-19 vaccine.
The findings were published online in JAMA Network Open.
More precise risk estimates
Winston Abara, MD, with the U.S. Centers for Disease Control and Prevention, and colleagues analyzed GBS reports submitted to the VAERS between December 2020 and January 2022.
Among 487.6 million COVID-19 vaccine doses administered, 3.7% were Janssen’s Ad26.COV2.S vaccine, 54.7% were Pfizer’s BNT162b2 vaccine, and 41.6% were Moderna’s mRNA-1273 vaccine.
There were 295 verified reports of GBS identified after COVID-19 vaccination. Of these, 209 occurred within 21 days of vaccination and 253 within 42 days.
Within 21 days of vaccination, GBS reporting rates per 1 million doses were 3.29 for the Janssen vaccine versus 0.29 and 0.35 for the Pfizer and Moderna vaccines, respectively. Within 42 days of vaccination, reporting rates per 1 million doses were 4.07, 0.34, and 0.44, respectively.
Also within 21 days of vaccination, GBS reporting rates were significantly higher with the Janssen vaccine than the Pfizer vaccine (reporting rate ratio, 11.40) and the Moderna vaccine (RRR, 9.26). Similar findings were observed within 42 days after vaccination.
The observed-to-expected ratios were 3.79 for 21-day and 2.34 for 42-day intervals after receipt of the Janssen vaccine, and less than 1 (not significant) after the Pfizer or Moderna vaccine within both post-vaccination periods.
“Unlike prior studies, our analysis included all U.S. reports of verified GBS cases that met the Brighton Collaboration GBS case definition criteria (Brighton Levels 1, 2, and 3) submitted over a 14-month surveillance period to the to the Vaccine Adverse Event Reporting System,” Dr. Abara said in an interview. “Because we used all U.S. reports, the sample of verified GBS cases in this analysis is larger than other studies. Therefore, it may provide a more precise estimate of the GBS risk within 21 and 42 days after mRNA and Ad26.COV2.S vaccination,” he said.
‘Remarkably low’ use
Nicola Klein, MD, PhD, Kaiser Permanente Vaccine Study Center, Oakland, Calif., noted that this is a “nice confirmatory analysis that supports and further expands what’s been observed before.”
Last year, as reported by this news organization, Dr. Klein and colleagues reported data from the Vaccine Safety Datalink confirming a small but statistically significant increased risk for GBS in the 3 weeks after receipt of the Janssen COVID-19 vaccine but not the Pfizer or Moderna vaccines.
Unlike VAERS, the Vaccine Safety Datalink is not a reporting system. It’s an active surveillance of medical records in the Kaiser Permanente system. The VAERS is a passive system, so it requires individuals to report GBS cases to the VAERS team, Dr. Klein explained.
So although the two studies are slightly different, overall, the VAERS data is “consistent with what we found,” she said.
Also weighing in, C. Buddy Creech, MD, MPH, director of the Vanderbilt Vaccine Research Program and professor of pediatrics at the Vanderbilt University School of Medicine, Nashville, Tenn., said it is “important to realize that GBS had been observed after adenovirus-vectored vaccines earlier in the pandemic, both for the AstraZeneca vaccine and the Janssen vaccine.”
The Advisory Committee on Immunization Practices (ACIP) preferentially recommends that people age 18 years and older receive an mRNA COVID-19 vaccine rather than the Janssen adenoviral vector vaccine when both types of COVID-19 vaccine are available.
“Thus, the use of the Janssen vaccine is remarkably low in the U.S. right now,” Dr. Creech said.
“Nevertheless, we have a firm commitment, both scientifically and ethically, to track potential side effects after vaccination and to make sure that the vaccines in use for COVID, and other important infectious diseases, are safe and effective,” he added.
The study had no commercial funding. Dr. Abara and Dr. Creech have reported no relevant financial relationships. Dr. Klein reported having received grants from Pfizer research support for a COVID vaccine clinical trial, as well as grants from Merck, GlaxoSmithKline, Sanofi Pasteur, and Protein Science (now Sanofi Pasteur).
A version of this article first appeared on Medscape.com.
Over 14 months, GBS reporting rates within 21 and 42 days of administration of Janssen’s replication-incompetent adenoviral vector vaccine were approximately 9 to 12 times higher than after administration of the Pfizer-BioNTech (BNT162b2) or the Moderna (mRNA-1273) mRNA COVID vaccines.
Additionally, observed GBS cases after the Janssen shot were 2 to 3 times greater than expected, based on background rates within 21 and 42 days of vaccination.
Conversely, and confirming prior data, there was no increased risk for GBS with the Pfizer or Moderna vaccines and no significant difference between observed and expected numbers of GBS cases after either mRNA COVID-19 vaccine.
The findings were published online in JAMA Network Open.
More precise risk estimates
Winston Abara, MD, with the U.S. Centers for Disease Control and Prevention, and colleagues analyzed GBS reports submitted to the VAERS between December 2020 and January 2022.
Among 487.6 million COVID-19 vaccine doses administered, 3.7% were Janssen’s Ad26.COV2.S vaccine, 54.7% were Pfizer’s BNT162b2 vaccine, and 41.6% were Moderna’s mRNA-1273 vaccine.
There were 295 verified reports of GBS identified after COVID-19 vaccination. Of these, 209 occurred within 21 days of vaccination and 253 within 42 days.
Within 21 days of vaccination, GBS reporting rates per 1 million doses were 3.29 for the Janssen vaccine versus 0.29 and 0.35 for the Pfizer and Moderna vaccines, respectively. Within 42 days of vaccination, reporting rates per 1 million doses were 4.07, 0.34, and 0.44, respectively.
Also within 21 days of vaccination, GBS reporting rates were significantly higher with the Janssen vaccine than the Pfizer vaccine (reporting rate ratio, 11.40) and the Moderna vaccine (RRR, 9.26). Similar findings were observed within 42 days after vaccination.
The observed-to-expected ratios were 3.79 for 21-day and 2.34 for 42-day intervals after receipt of the Janssen vaccine, and less than 1 (not significant) after the Pfizer or Moderna vaccine within both post-vaccination periods.
“Unlike prior studies, our analysis included all U.S. reports of verified GBS cases that met the Brighton Collaboration GBS case definition criteria (Brighton Levels 1, 2, and 3) submitted over a 14-month surveillance period to the to the Vaccine Adverse Event Reporting System,” Dr. Abara said in an interview. “Because we used all U.S. reports, the sample of verified GBS cases in this analysis is larger than other studies. Therefore, it may provide a more precise estimate of the GBS risk within 21 and 42 days after mRNA and Ad26.COV2.S vaccination,” he said.
‘Remarkably low’ use
Nicola Klein, MD, PhD, Kaiser Permanente Vaccine Study Center, Oakland, Calif., noted that this is a “nice confirmatory analysis that supports and further expands what’s been observed before.”
Last year, as reported by this news organization, Dr. Klein and colleagues reported data from the Vaccine Safety Datalink confirming a small but statistically significant increased risk for GBS in the 3 weeks after receipt of the Janssen COVID-19 vaccine but not the Pfizer or Moderna vaccines.
Unlike VAERS, the Vaccine Safety Datalink is not a reporting system. It’s an active surveillance of medical records in the Kaiser Permanente system. The VAERS is a passive system, so it requires individuals to report GBS cases to the VAERS team, Dr. Klein explained.
So although the two studies are slightly different, overall, the VAERS data is “consistent with what we found,” she said.
Also weighing in, C. Buddy Creech, MD, MPH, director of the Vanderbilt Vaccine Research Program and professor of pediatrics at the Vanderbilt University School of Medicine, Nashville, Tenn., said it is “important to realize that GBS had been observed after adenovirus-vectored vaccines earlier in the pandemic, both for the AstraZeneca vaccine and the Janssen vaccine.”
The Advisory Committee on Immunization Practices (ACIP) preferentially recommends that people age 18 years and older receive an mRNA COVID-19 vaccine rather than the Janssen adenoviral vector vaccine when both types of COVID-19 vaccine are available.
“Thus, the use of the Janssen vaccine is remarkably low in the U.S. right now,” Dr. Creech said.
“Nevertheless, we have a firm commitment, both scientifically and ethically, to track potential side effects after vaccination and to make sure that the vaccines in use for COVID, and other important infectious diseases, are safe and effective,” he added.
The study had no commercial funding. Dr. Abara and Dr. Creech have reported no relevant financial relationships. Dr. Klein reported having received grants from Pfizer research support for a COVID vaccine clinical trial, as well as grants from Merck, GlaxoSmithKline, Sanofi Pasteur, and Protein Science (now Sanofi Pasteur).
A version of this article first appeared on Medscape.com.
Genetic testing in the PICU prompts meaningful changes in care
according to a new study presented at the Society of Critical Care Medicine’s 2023 Critical Care Congress.
“We have had a lot of success using genome sequencing to help not only with diagnosis, but also changes in management,” lead author Katherine Rodriguez, MD, a pediatric critical care fellow physician at Rady Children’s Hospital, San Diego, told this news organization.
However, data on the use of rapid whole genome sequencing (rWGS) in the pediatric intensive care unit (PICU) are limited, and data from multiple institutions are lacking, Dr. Rodriguez said. In the current study, data from multiple hospitals allowed the researchers to examine differences in management across institutions, she said.
Dr. Rodriguez, with principal investigator Nicole Coufal, MD, also of Rady Children’s, and colleagues conducted the study at three children’s hospitals from March 2019 to July 2022. The study population included 80 children whose origin of illness was uncertain. The patients underwent rWGS testing in the PICU or cardiac ICU setting. The patients ranged in age from 0 to 17 years; 64% were younger than 1 year, (mean age, 2.8 years); 56% were male, and 59% were White.
After rWGS testing, 65% of the children were positive for a genetic variant. The data prompted changes to care for 42% of these patients; 38% of the changes occurred during the patient’s PICU stay, including medication changes and procedures that were either avoided or completed.
The remaining 62% of the changes were subacute and affected management for the remainder of the child’s hospitalization and after discharge, Dr. Rodriguez explained in her presentation.
The average turnaround time for the testing was 10 days, which is important to an intensivist, who may have been hesitant to order tests because of the time involved, Dr. Rodriguez said. The current study shows that “we can get test results in a reasonable time to make meaningful changes in care,” she told this news organization.
Choosing which patients to test can be a challenge for clinicians, Dr. Rodriguez acknowledged. “We have gotten a sense of which patients are likely to have diagnostic or not diagnostic genomes, but it is also a gut feeling,” she said.
“If this child is your patient and you are concerned, if they seem sicker than expected, or have a concerning family history, then send the test,” she said. “It is becoming more affordable, and can come back quickly enough to guide treatment while the patient is still in the ICU.”
In the current study, the greatest diagnostic utility appeared in patients with cardiac symptoms, such as congenital heart disease, sudden cardiac arrest, or suspected channelopathy, Dr. Rodriguez said in her presentation.
Patients with suspected neurological disease had a 50% rate of molecular diagnosis. “Interestingly, 74% of patients with respiratory disease where an underlying genetic etiology was suspected received a molecular diagnosis,” although rWGS was not applied to general populations with RSV or other respiratory illnesses, she said.
In her presentation, Dr. Rodriguez shared examples of how genetic testing had a dramatic impact on patient survival. In one case, a 14-year-old girl presented in cardiac arrest and was found to have new-onset dilated cardiomyopathy. Whether the etiology was acquired or infectious and possibly reversible or genetic was unclear, she said.
“A diagnostic genome result within 48 hours indicated a genetic etiology,” she said. The patient was listed for heart transplant despite the incomplete infectious workup, and received a successful heart transplant 1 week after admission, Dr. Rodriguez said.
Guidelines for which PICU patients should undergo genetic testing do not yet exist, Dr. Rodriguez told this news organization. “We are trying to find some more meaningful parameters where we can say that a patient has a high pretest possibility of a genetic condition,” she said.
“Increasing availability of rWGS can significantly impact patient care and assist families in making difficult decisions during times of critical illness,” she said.
Insurance coverage and testing access are improving, said Dr. Rodriguez. Medicaid policies exist for neonates/infants in the ICU in several states, including Oregon, California, Michigan, Maryland, and Louisiana, she said. In some areas, hospitals may pay for testing for these children if insurance will not, she added.
Dr. Rodriguez and colleagues are continuing to enroll patients in a prospective study of the impact of rWGS, with the addition of a fourth study site and inclusion of family surveys. “We also will be looking at a secondary analysis of cost savings and benefits,” she said.
Ultimately, the current study should be empowering to physicians, “especially if they don’t have good access to geneticists,” Dr. Rodriguez said in an interview.
The study received no outside funding. Dr. Rodriguez reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study presented at the Society of Critical Care Medicine’s 2023 Critical Care Congress.
“We have had a lot of success using genome sequencing to help not only with diagnosis, but also changes in management,” lead author Katherine Rodriguez, MD, a pediatric critical care fellow physician at Rady Children’s Hospital, San Diego, told this news organization.
However, data on the use of rapid whole genome sequencing (rWGS) in the pediatric intensive care unit (PICU) are limited, and data from multiple institutions are lacking, Dr. Rodriguez said. In the current study, data from multiple hospitals allowed the researchers to examine differences in management across institutions, she said.
Dr. Rodriguez, with principal investigator Nicole Coufal, MD, also of Rady Children’s, and colleagues conducted the study at three children’s hospitals from March 2019 to July 2022. The study population included 80 children whose origin of illness was uncertain. The patients underwent rWGS testing in the PICU or cardiac ICU setting. The patients ranged in age from 0 to 17 years; 64% were younger than 1 year, (mean age, 2.8 years); 56% were male, and 59% were White.
After rWGS testing, 65% of the children were positive for a genetic variant. The data prompted changes to care for 42% of these patients; 38% of the changes occurred during the patient’s PICU stay, including medication changes and procedures that were either avoided or completed.
The remaining 62% of the changes were subacute and affected management for the remainder of the child’s hospitalization and after discharge, Dr. Rodriguez explained in her presentation.
The average turnaround time for the testing was 10 days, which is important to an intensivist, who may have been hesitant to order tests because of the time involved, Dr. Rodriguez said. The current study shows that “we can get test results in a reasonable time to make meaningful changes in care,” she told this news organization.
Choosing which patients to test can be a challenge for clinicians, Dr. Rodriguez acknowledged. “We have gotten a sense of which patients are likely to have diagnostic or not diagnostic genomes, but it is also a gut feeling,” she said.
“If this child is your patient and you are concerned, if they seem sicker than expected, or have a concerning family history, then send the test,” she said. “It is becoming more affordable, and can come back quickly enough to guide treatment while the patient is still in the ICU.”
In the current study, the greatest diagnostic utility appeared in patients with cardiac symptoms, such as congenital heart disease, sudden cardiac arrest, or suspected channelopathy, Dr. Rodriguez said in her presentation.
Patients with suspected neurological disease had a 50% rate of molecular diagnosis. “Interestingly, 74% of patients with respiratory disease where an underlying genetic etiology was suspected received a molecular diagnosis,” although rWGS was not applied to general populations with RSV or other respiratory illnesses, she said.
In her presentation, Dr. Rodriguez shared examples of how genetic testing had a dramatic impact on patient survival. In one case, a 14-year-old girl presented in cardiac arrest and was found to have new-onset dilated cardiomyopathy. Whether the etiology was acquired or infectious and possibly reversible or genetic was unclear, she said.
“A diagnostic genome result within 48 hours indicated a genetic etiology,” she said. The patient was listed for heart transplant despite the incomplete infectious workup, and received a successful heart transplant 1 week after admission, Dr. Rodriguez said.
Guidelines for which PICU patients should undergo genetic testing do not yet exist, Dr. Rodriguez told this news organization. “We are trying to find some more meaningful parameters where we can say that a patient has a high pretest possibility of a genetic condition,” she said.
“Increasing availability of rWGS can significantly impact patient care and assist families in making difficult decisions during times of critical illness,” she said.
Insurance coverage and testing access are improving, said Dr. Rodriguez. Medicaid policies exist for neonates/infants in the ICU in several states, including Oregon, California, Michigan, Maryland, and Louisiana, she said. In some areas, hospitals may pay for testing for these children if insurance will not, she added.
Dr. Rodriguez and colleagues are continuing to enroll patients in a prospective study of the impact of rWGS, with the addition of a fourth study site and inclusion of family surveys. “We also will be looking at a secondary analysis of cost savings and benefits,” she said.
Ultimately, the current study should be empowering to physicians, “especially if they don’t have good access to geneticists,” Dr. Rodriguez said in an interview.
The study received no outside funding. Dr. Rodriguez reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study presented at the Society of Critical Care Medicine’s 2023 Critical Care Congress.
“We have had a lot of success using genome sequencing to help not only with diagnosis, but also changes in management,” lead author Katherine Rodriguez, MD, a pediatric critical care fellow physician at Rady Children’s Hospital, San Diego, told this news organization.
However, data on the use of rapid whole genome sequencing (rWGS) in the pediatric intensive care unit (PICU) are limited, and data from multiple institutions are lacking, Dr. Rodriguez said. In the current study, data from multiple hospitals allowed the researchers to examine differences in management across institutions, she said.
Dr. Rodriguez, with principal investigator Nicole Coufal, MD, also of Rady Children’s, and colleagues conducted the study at three children’s hospitals from March 2019 to July 2022. The study population included 80 children whose origin of illness was uncertain. The patients underwent rWGS testing in the PICU or cardiac ICU setting. The patients ranged in age from 0 to 17 years; 64% were younger than 1 year, (mean age, 2.8 years); 56% were male, and 59% were White.
After rWGS testing, 65% of the children were positive for a genetic variant. The data prompted changes to care for 42% of these patients; 38% of the changes occurred during the patient’s PICU stay, including medication changes and procedures that were either avoided or completed.
The remaining 62% of the changes were subacute and affected management for the remainder of the child’s hospitalization and after discharge, Dr. Rodriguez explained in her presentation.
The average turnaround time for the testing was 10 days, which is important to an intensivist, who may have been hesitant to order tests because of the time involved, Dr. Rodriguez said. The current study shows that “we can get test results in a reasonable time to make meaningful changes in care,” she told this news organization.
Choosing which patients to test can be a challenge for clinicians, Dr. Rodriguez acknowledged. “We have gotten a sense of which patients are likely to have diagnostic or not diagnostic genomes, but it is also a gut feeling,” she said.
“If this child is your patient and you are concerned, if they seem sicker than expected, or have a concerning family history, then send the test,” she said. “It is becoming more affordable, and can come back quickly enough to guide treatment while the patient is still in the ICU.”
In the current study, the greatest diagnostic utility appeared in patients with cardiac symptoms, such as congenital heart disease, sudden cardiac arrest, or suspected channelopathy, Dr. Rodriguez said in her presentation.
Patients with suspected neurological disease had a 50% rate of molecular diagnosis. “Interestingly, 74% of patients with respiratory disease where an underlying genetic etiology was suspected received a molecular diagnosis,” although rWGS was not applied to general populations with RSV or other respiratory illnesses, she said.
In her presentation, Dr. Rodriguez shared examples of how genetic testing had a dramatic impact on patient survival. In one case, a 14-year-old girl presented in cardiac arrest and was found to have new-onset dilated cardiomyopathy. Whether the etiology was acquired or infectious and possibly reversible or genetic was unclear, she said.
“A diagnostic genome result within 48 hours indicated a genetic etiology,” she said. The patient was listed for heart transplant despite the incomplete infectious workup, and received a successful heart transplant 1 week after admission, Dr. Rodriguez said.
Guidelines for which PICU patients should undergo genetic testing do not yet exist, Dr. Rodriguez told this news organization. “We are trying to find some more meaningful parameters where we can say that a patient has a high pretest possibility of a genetic condition,” she said.
“Increasing availability of rWGS can significantly impact patient care and assist families in making difficult decisions during times of critical illness,” she said.
Insurance coverage and testing access are improving, said Dr. Rodriguez. Medicaid policies exist for neonates/infants in the ICU in several states, including Oregon, California, Michigan, Maryland, and Louisiana, she said. In some areas, hospitals may pay for testing for these children if insurance will not, she added.
Dr. Rodriguez and colleagues are continuing to enroll patients in a prospective study of the impact of rWGS, with the addition of a fourth study site and inclusion of family surveys. “We also will be looking at a secondary analysis of cost savings and benefits,” she said.
Ultimately, the current study should be empowering to physicians, “especially if they don’t have good access to geneticists,” Dr. Rodriguez said in an interview.
The study received no outside funding. Dr. Rodriguez reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM SCCM 2023
Black patients less likely to receive opioids for advanced cancer
Opioids are widely regarded as a linchpin in the treatment of moderate to severe cancer-related pain and end-of-life symptoms; however, a new study suggests.
Black patients were more likely to undergo urine drug screening (UDS) despite being less likely to receive any opioids for pain management and receiving lower daily doses of opioids in comparison with White patients, the study found.
The inequities were particularly stark for Black men. “We found that Black men were far less likely to be prescribed reasonable doses than White men were,” said the study’s senior author, Alexi Wright, MD, MPH, a gynecologic oncologist and a researcher in the division of population sciences at Dana-Farber Cancer Institute, Boston. “And Black men were less likely to receive long-acting opioids, which are essential for many patients dying of cancer. Our findings are startling because everyone should agree that cancer patients should have equal access to pain relief at the end of life.”
The study was published on in the Journal of Clinical Oncology.
The researchers gathered data on 318,549 Medicare beneficiaries older than 65 years with poor-prognosis cancers who died between 2007 and 2019. During this time frame, for all groups, access to opioids declined and urine drug testing expanded, owing to the overall opioid epidemic in the United States. Overall, the proportion of patients near end of life (EOL) who received any opioid or long-acting opioids decreased from 42.2% to 32.7% and from 17.9% to 9.4%, respectively.
The investigators used National Drug Codes to identify all Medicare Part D claims for outpatient opioid prescriptions, excluding addiction treatments, cough suppressants, and parenteral opioids. They focused on prescriptions that were filled at least 30 days before death or hospice enrollment.
Among the study participants, the majority (85.5%) of patients were White, 29,555 patients (9.3%) were Black, and 16,636 patients (5.2%) were Hispanic.
Black and Hispanic patients were statistically less likely than White patients to receive opioid prescriptions near EOL (Black, –4.3 percentage points; Hispanic, –3.6 percentage points). They were also less likely to receive long-acting opioid prescriptions (Black, –3.1 percentage points; Hispanic, –2.2 percentage points).
“It’s not just that patients of color are less likely to get opioids, but when they do get them, they get lower doses, and they also are less likely to get long-acting opioids, which a lot of people view as sort of more potential for addiction, which isn’t necessarily true but kind of viewed with heightened concern or suspicion,” the study’s lead author, Andrea Enzinger, MD, a gastrointestinal oncologist and a researcher in Dana-Farber’s division of population sciences, said in an interview.
Dr. Enzinger added that she believes systemic racism and preconceived biases toward minorities and drug addiction may be contributing to these trends.
When Black patients did receive at least one opioid prescription, they received daily doses that were 10.5 morphine milligram equivalents (MMEs) lower than doses given to White patients. Compared with the total opioid dose filled per White decedent near EOL, the total dose filled per Black decedent was 210 MMEs lower.
“We all need to be worried about the potential for misuse or addiction, but this is the one setting that is very low on my priority list when somebody is dying. I mean, we’re looking at the last month of life, so nobody has the potential to become addicted,” Dr. Enzinger commented.
The team also evaluated rates or urine drug screening (UDS), but as these rates were relatively low, they expanded the time frame to 180 days before death or hospice. They found that disparities in UDS disproportionately affected Black men.
From 2007 to 2019, the proportion of patients who underwent UDS increased from 0.6% to 6.7% in the 180 days before death or hospice; however, Black decedents were tested more often than White or Hispanic decedents.
Black decedents were 0.5 percentage points more likely than White decedents to undergo UDS near EOL.
“The disparities in urine drug screening are modest but important, because they hint at underlying systematic racism in recommending patients for screening,” Dr. Wright said. “Screening needs to either be applied uniformly or not at all for patients in this situation.”
The researchers acknowledged that their findings likely do not represent the full spectrum of prescribing disparities and believe that the work should be expanded among younger populations. Nevertheless, the investigators believe the work highlights the persistent racial and ethnic disparities in opioid access.
The study was supported by a grant from the Agency for Healthcare Research and Policy.
A version of this article first appeared on Medscape.com.
Opioids are widely regarded as a linchpin in the treatment of moderate to severe cancer-related pain and end-of-life symptoms; however, a new study suggests.
Black patients were more likely to undergo urine drug screening (UDS) despite being less likely to receive any opioids for pain management and receiving lower daily doses of opioids in comparison with White patients, the study found.
The inequities were particularly stark for Black men. “We found that Black men were far less likely to be prescribed reasonable doses than White men were,” said the study’s senior author, Alexi Wright, MD, MPH, a gynecologic oncologist and a researcher in the division of population sciences at Dana-Farber Cancer Institute, Boston. “And Black men were less likely to receive long-acting opioids, which are essential for many patients dying of cancer. Our findings are startling because everyone should agree that cancer patients should have equal access to pain relief at the end of life.”
The study was published on in the Journal of Clinical Oncology.
The researchers gathered data on 318,549 Medicare beneficiaries older than 65 years with poor-prognosis cancers who died between 2007 and 2019. During this time frame, for all groups, access to opioids declined and urine drug testing expanded, owing to the overall opioid epidemic in the United States. Overall, the proportion of patients near end of life (EOL) who received any opioid or long-acting opioids decreased from 42.2% to 32.7% and from 17.9% to 9.4%, respectively.
The investigators used National Drug Codes to identify all Medicare Part D claims for outpatient opioid prescriptions, excluding addiction treatments, cough suppressants, and parenteral opioids. They focused on prescriptions that were filled at least 30 days before death or hospice enrollment.
Among the study participants, the majority (85.5%) of patients were White, 29,555 patients (9.3%) were Black, and 16,636 patients (5.2%) were Hispanic.
Black and Hispanic patients were statistically less likely than White patients to receive opioid prescriptions near EOL (Black, –4.3 percentage points; Hispanic, –3.6 percentage points). They were also less likely to receive long-acting opioid prescriptions (Black, –3.1 percentage points; Hispanic, –2.2 percentage points).
“It’s not just that patients of color are less likely to get opioids, but when they do get them, they get lower doses, and they also are less likely to get long-acting opioids, which a lot of people view as sort of more potential for addiction, which isn’t necessarily true but kind of viewed with heightened concern or suspicion,” the study’s lead author, Andrea Enzinger, MD, a gastrointestinal oncologist and a researcher in Dana-Farber’s division of population sciences, said in an interview.
Dr. Enzinger added that she believes systemic racism and preconceived biases toward minorities and drug addiction may be contributing to these trends.
When Black patients did receive at least one opioid prescription, they received daily doses that were 10.5 morphine milligram equivalents (MMEs) lower than doses given to White patients. Compared with the total opioid dose filled per White decedent near EOL, the total dose filled per Black decedent was 210 MMEs lower.
“We all need to be worried about the potential for misuse or addiction, but this is the one setting that is very low on my priority list when somebody is dying. I mean, we’re looking at the last month of life, so nobody has the potential to become addicted,” Dr. Enzinger commented.
The team also evaluated rates or urine drug screening (UDS), but as these rates were relatively low, they expanded the time frame to 180 days before death or hospice. They found that disparities in UDS disproportionately affected Black men.
From 2007 to 2019, the proportion of patients who underwent UDS increased from 0.6% to 6.7% in the 180 days before death or hospice; however, Black decedents were tested more often than White or Hispanic decedents.
Black decedents were 0.5 percentage points more likely than White decedents to undergo UDS near EOL.
“The disparities in urine drug screening are modest but important, because they hint at underlying systematic racism in recommending patients for screening,” Dr. Wright said. “Screening needs to either be applied uniformly or not at all for patients in this situation.”
The researchers acknowledged that their findings likely do not represent the full spectrum of prescribing disparities and believe that the work should be expanded among younger populations. Nevertheless, the investigators believe the work highlights the persistent racial and ethnic disparities in opioid access.
The study was supported by a grant from the Agency for Healthcare Research and Policy.
A version of this article first appeared on Medscape.com.
Opioids are widely regarded as a linchpin in the treatment of moderate to severe cancer-related pain and end-of-life symptoms; however, a new study suggests.
Black patients were more likely to undergo urine drug screening (UDS) despite being less likely to receive any opioids for pain management and receiving lower daily doses of opioids in comparison with White patients, the study found.
The inequities were particularly stark for Black men. “We found that Black men were far less likely to be prescribed reasonable doses than White men were,” said the study’s senior author, Alexi Wright, MD, MPH, a gynecologic oncologist and a researcher in the division of population sciences at Dana-Farber Cancer Institute, Boston. “And Black men were less likely to receive long-acting opioids, which are essential for many patients dying of cancer. Our findings are startling because everyone should agree that cancer patients should have equal access to pain relief at the end of life.”
The study was published on in the Journal of Clinical Oncology.
The researchers gathered data on 318,549 Medicare beneficiaries older than 65 years with poor-prognosis cancers who died between 2007 and 2019. During this time frame, for all groups, access to opioids declined and urine drug testing expanded, owing to the overall opioid epidemic in the United States. Overall, the proportion of patients near end of life (EOL) who received any opioid or long-acting opioids decreased from 42.2% to 32.7% and from 17.9% to 9.4%, respectively.
The investigators used National Drug Codes to identify all Medicare Part D claims for outpatient opioid prescriptions, excluding addiction treatments, cough suppressants, and parenteral opioids. They focused on prescriptions that were filled at least 30 days before death or hospice enrollment.
Among the study participants, the majority (85.5%) of patients were White, 29,555 patients (9.3%) were Black, and 16,636 patients (5.2%) were Hispanic.
Black and Hispanic patients were statistically less likely than White patients to receive opioid prescriptions near EOL (Black, –4.3 percentage points; Hispanic, –3.6 percentage points). They were also less likely to receive long-acting opioid prescriptions (Black, –3.1 percentage points; Hispanic, –2.2 percentage points).
“It’s not just that patients of color are less likely to get opioids, but when they do get them, they get lower doses, and they also are less likely to get long-acting opioids, which a lot of people view as sort of more potential for addiction, which isn’t necessarily true but kind of viewed with heightened concern or suspicion,” the study’s lead author, Andrea Enzinger, MD, a gastrointestinal oncologist and a researcher in Dana-Farber’s division of population sciences, said in an interview.
Dr. Enzinger added that she believes systemic racism and preconceived biases toward minorities and drug addiction may be contributing to these trends.
When Black patients did receive at least one opioid prescription, they received daily doses that were 10.5 morphine milligram equivalents (MMEs) lower than doses given to White patients. Compared with the total opioid dose filled per White decedent near EOL, the total dose filled per Black decedent was 210 MMEs lower.
“We all need to be worried about the potential for misuse or addiction, but this is the one setting that is very low on my priority list when somebody is dying. I mean, we’re looking at the last month of life, so nobody has the potential to become addicted,” Dr. Enzinger commented.
The team also evaluated rates or urine drug screening (UDS), but as these rates were relatively low, they expanded the time frame to 180 days before death or hospice. They found that disparities in UDS disproportionately affected Black men.
From 2007 to 2019, the proportion of patients who underwent UDS increased from 0.6% to 6.7% in the 180 days before death or hospice; however, Black decedents were tested more often than White or Hispanic decedents.
Black decedents were 0.5 percentage points more likely than White decedents to undergo UDS near EOL.
“The disparities in urine drug screening are modest but important, because they hint at underlying systematic racism in recommending patients for screening,” Dr. Wright said. “Screening needs to either be applied uniformly or not at all for patients in this situation.”
The researchers acknowledged that their findings likely do not represent the full spectrum of prescribing disparities and believe that the work should be expanded among younger populations. Nevertheless, the investigators believe the work highlights the persistent racial and ethnic disparities in opioid access.
The study was supported by a grant from the Agency for Healthcare Research and Policy.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Loneliness risk elevated among young cancer survivors
findings from a large retrospective study suggest.
Young cancer survivors were more than twice as likely to report loneliness at study baseline and follow-up. Loneliness at these times was associated with an almost 10-fold increased risk for anxiety and a nearly 18-fold increased risk for depression.
“We observed an elevated prevalence of loneliness in survivors, compared to sibling controls, and found that loneliness was associated with emotional, behavioral, and physical health morbidities,” lead study author Chiara Papini, PhD, of St. Jude Children’s Research Hospital, Memphis, and her colleagues write. “Our results highlight the importance of identifying and screening young adult survivors of childhood cancer for loneliness and the need for targeted interventions to reduce loneliness.”
The article was published online in the journal Cancer.
Most young cancer survivors in the United States reach adulthood and need to play catch-up: make up for missed school and work, become reacquainted with old friends, and develop new friendships, social networks, and intimate relationships. Meeting these needs may be hindered by adverse physical and psychosocial problems that linger or develop after treatment, which may leave cancer survivors feeling isolated.
“Young adult survivors of childhood cancer are navigating a developmental period marked by increased social expectations, during which loneliness may have significant impact on physical and mental health,” Dr. Papini and colleagues say.
To better understand the risks for loneliness among young cancer survivors, Dr. Papini and her colleagues analyzed data from the retrospective Childhood Cancer Survivor Study, which followed young survivors who had been diagnosed with a range of cancers before age 21 years. Study participants had been treated at one of 31 study sites in North America and had survived 5 years or longer after diagnosis.
The 9,664 survivors and 2,221 randomly sampled siblings ranged in age from 19 to 39 years at the time they completed a survey that assessed emotional distress at baseline and at follow‐up a median of 6.6 years. At baseline, the median age of the survivors was 27 years, and a median of 17.5 years had passed from the time of their diagnosis.
The most common diagnoses were leukemia (35%), Hodgkin lymphoma (15%), central nervous system (CNS) tumors (14%), and bone tumors (10%). More than half (56%) had received radiation therapy.
Using multivariable models, the researchers found that survivors were more likely than siblings to report moderate to extreme loneliness at either baseline or follow‐up (prevalence ratio, 1.04) and were more than two times more likely to report loneliness at both baseline and follow‐up (PR, 2.21).
Loneliness at baseline and follow‐up was associated with a much greater risk for anxiety (relative risk, 9.75) and depression (RR, 17.86). Loneliness at follow‐up was linked with increased risks for suicidal ideation (RR, 1.52), heavy or risky alcohol consumption (RR, 1.27), and any grade 2-4 new‐onset chronic health condition (RR, 1.29), especially those that were neurologic (RR, 4.37).
Survivors of CNS tumors (odds ratio, 2.59) and leukemia (OR, 2.52) were most likely to report loneliness at both baseline and follow‐up, though survivors of four other cancer types also faced an elevated risk for loneliness: neuroblastoma (OR, 2.32), bone tumor (OR, 2.12), soft tissue sarcoma (OR, 1.78), and Hodgkin lymphoma (OR, 1.69).
Treatment type appeared to matter as well. Survivors who underwent amputation (OR, 1.82) or were treated with cranial radiation greater than or equal to 20 Gy (OR, 1.56) or corticosteroids (OR, 1.31) were more likely to report loneliness at baseline and follow‐up, compared with those who reported no loneliness at both time points.
The authors acknowledge limitations to the study, including the fact that roughly 90% of survivors and siblings were White, which limits the applicability of their results to diverse groups. In addition, the responses were self-reported without external validation.
Overall, though, the findings provide a framework for clinicians to understand and identify loneliness among young cancer survivors and help them cope with their emotions.
“The Childhood Cancer Survivor Study provides the largest and the most comprehensive dataset on childhood cancer survivors and healthy-sibling comparisons, giving us powerful data on survivorship, late effects, and psychosocial and health outcomes,” Rachel M. Moore, PhD, child psychologist at Children’s Mercy Kansas City, Mo., said in an interview.
Asking a simple question – “Are you feeling lonely?” – can identify at-risk survivors and enable health care teams to provide timely interventions that address young patients’ physical and psychological needs, said Dr. Moore, who was not involved in the study.
Dr. Moore noted that within her clinical practice, “adolescent and young adult survivors frequently discuss loneliness in their daily lives. They feel different from their peers and misunderstood. Having a conversation early in survivorship care about the experience of loneliness as a product of cancer treatment can open the door to regular screening and destigmatizing mental health services.”
Supporting young people throughout their survivorship journey is important, said Rusha Bhandari, MD, medical director of the Childhood, Adolescent, and Young Adult Cancer Survivorship Program at City of Hope, Duarte, Calif. This study can help ensure that clinicians “provide comprehensive care, including psychosocial screening and support, to meet the unique needs of our young adult survivors,” said Dr. Bhandari, who also was not involved in the research.
The National Cancer Institute and the American Lebanese Syrian Associated Charities supported the study. One co-author reported receiving corporate consulting fees. Dr. Papini, the remaining co-authors, Dr. Moore, and Dr. Bhandari report no relevant financial involvements.
A version of this article first appeared on Medscape.com.
findings from a large retrospective study suggest.
Young cancer survivors were more than twice as likely to report loneliness at study baseline and follow-up. Loneliness at these times was associated with an almost 10-fold increased risk for anxiety and a nearly 18-fold increased risk for depression.
“We observed an elevated prevalence of loneliness in survivors, compared to sibling controls, and found that loneliness was associated with emotional, behavioral, and physical health morbidities,” lead study author Chiara Papini, PhD, of St. Jude Children’s Research Hospital, Memphis, and her colleagues write. “Our results highlight the importance of identifying and screening young adult survivors of childhood cancer for loneliness and the need for targeted interventions to reduce loneliness.”
The article was published online in the journal Cancer.
Most young cancer survivors in the United States reach adulthood and need to play catch-up: make up for missed school and work, become reacquainted with old friends, and develop new friendships, social networks, and intimate relationships. Meeting these needs may be hindered by adverse physical and psychosocial problems that linger or develop after treatment, which may leave cancer survivors feeling isolated.
“Young adult survivors of childhood cancer are navigating a developmental period marked by increased social expectations, during which loneliness may have significant impact on physical and mental health,” Dr. Papini and colleagues say.
To better understand the risks for loneliness among young cancer survivors, Dr. Papini and her colleagues analyzed data from the retrospective Childhood Cancer Survivor Study, which followed young survivors who had been diagnosed with a range of cancers before age 21 years. Study participants had been treated at one of 31 study sites in North America and had survived 5 years or longer after diagnosis.
The 9,664 survivors and 2,221 randomly sampled siblings ranged in age from 19 to 39 years at the time they completed a survey that assessed emotional distress at baseline and at follow‐up a median of 6.6 years. At baseline, the median age of the survivors was 27 years, and a median of 17.5 years had passed from the time of their diagnosis.
The most common diagnoses were leukemia (35%), Hodgkin lymphoma (15%), central nervous system (CNS) tumors (14%), and bone tumors (10%). More than half (56%) had received radiation therapy.
Using multivariable models, the researchers found that survivors were more likely than siblings to report moderate to extreme loneliness at either baseline or follow‐up (prevalence ratio, 1.04) and were more than two times more likely to report loneliness at both baseline and follow‐up (PR, 2.21).
Loneliness at baseline and follow‐up was associated with a much greater risk for anxiety (relative risk, 9.75) and depression (RR, 17.86). Loneliness at follow‐up was linked with increased risks for suicidal ideation (RR, 1.52), heavy or risky alcohol consumption (RR, 1.27), and any grade 2-4 new‐onset chronic health condition (RR, 1.29), especially those that were neurologic (RR, 4.37).
Survivors of CNS tumors (odds ratio, 2.59) and leukemia (OR, 2.52) were most likely to report loneliness at both baseline and follow‐up, though survivors of four other cancer types also faced an elevated risk for loneliness: neuroblastoma (OR, 2.32), bone tumor (OR, 2.12), soft tissue sarcoma (OR, 1.78), and Hodgkin lymphoma (OR, 1.69).
Treatment type appeared to matter as well. Survivors who underwent amputation (OR, 1.82) or were treated with cranial radiation greater than or equal to 20 Gy (OR, 1.56) or corticosteroids (OR, 1.31) were more likely to report loneliness at baseline and follow‐up, compared with those who reported no loneliness at both time points.
The authors acknowledge limitations to the study, including the fact that roughly 90% of survivors and siblings were White, which limits the applicability of their results to diverse groups. In addition, the responses were self-reported without external validation.
Overall, though, the findings provide a framework for clinicians to understand and identify loneliness among young cancer survivors and help them cope with their emotions.
“The Childhood Cancer Survivor Study provides the largest and the most comprehensive dataset on childhood cancer survivors and healthy-sibling comparisons, giving us powerful data on survivorship, late effects, and psychosocial and health outcomes,” Rachel M. Moore, PhD, child psychologist at Children’s Mercy Kansas City, Mo., said in an interview.
Asking a simple question – “Are you feeling lonely?” – can identify at-risk survivors and enable health care teams to provide timely interventions that address young patients’ physical and psychological needs, said Dr. Moore, who was not involved in the study.
Dr. Moore noted that within her clinical practice, “adolescent and young adult survivors frequently discuss loneliness in their daily lives. They feel different from their peers and misunderstood. Having a conversation early in survivorship care about the experience of loneliness as a product of cancer treatment can open the door to regular screening and destigmatizing mental health services.”
Supporting young people throughout their survivorship journey is important, said Rusha Bhandari, MD, medical director of the Childhood, Adolescent, and Young Adult Cancer Survivorship Program at City of Hope, Duarte, Calif. This study can help ensure that clinicians “provide comprehensive care, including psychosocial screening and support, to meet the unique needs of our young adult survivors,” said Dr. Bhandari, who also was not involved in the research.
The National Cancer Institute and the American Lebanese Syrian Associated Charities supported the study. One co-author reported receiving corporate consulting fees. Dr. Papini, the remaining co-authors, Dr. Moore, and Dr. Bhandari report no relevant financial involvements.
A version of this article first appeared on Medscape.com.
findings from a large retrospective study suggest.
Young cancer survivors were more than twice as likely to report loneliness at study baseline and follow-up. Loneliness at these times was associated with an almost 10-fold increased risk for anxiety and a nearly 18-fold increased risk for depression.
“We observed an elevated prevalence of loneliness in survivors, compared to sibling controls, and found that loneliness was associated with emotional, behavioral, and physical health morbidities,” lead study author Chiara Papini, PhD, of St. Jude Children’s Research Hospital, Memphis, and her colleagues write. “Our results highlight the importance of identifying and screening young adult survivors of childhood cancer for loneliness and the need for targeted interventions to reduce loneliness.”
The article was published online in the journal Cancer.
Most young cancer survivors in the United States reach adulthood and need to play catch-up: make up for missed school and work, become reacquainted with old friends, and develop new friendships, social networks, and intimate relationships. Meeting these needs may be hindered by adverse physical and psychosocial problems that linger or develop after treatment, which may leave cancer survivors feeling isolated.
“Young adult survivors of childhood cancer are navigating a developmental period marked by increased social expectations, during which loneliness may have significant impact on physical and mental health,” Dr. Papini and colleagues say.
To better understand the risks for loneliness among young cancer survivors, Dr. Papini and her colleagues analyzed data from the retrospective Childhood Cancer Survivor Study, which followed young survivors who had been diagnosed with a range of cancers before age 21 years. Study participants had been treated at one of 31 study sites in North America and had survived 5 years or longer after diagnosis.
The 9,664 survivors and 2,221 randomly sampled siblings ranged in age from 19 to 39 years at the time they completed a survey that assessed emotional distress at baseline and at follow‐up a median of 6.6 years. At baseline, the median age of the survivors was 27 years, and a median of 17.5 years had passed from the time of their diagnosis.
The most common diagnoses were leukemia (35%), Hodgkin lymphoma (15%), central nervous system (CNS) tumors (14%), and bone tumors (10%). More than half (56%) had received radiation therapy.
Using multivariable models, the researchers found that survivors were more likely than siblings to report moderate to extreme loneliness at either baseline or follow‐up (prevalence ratio, 1.04) and were more than two times more likely to report loneliness at both baseline and follow‐up (PR, 2.21).
Loneliness at baseline and follow‐up was associated with a much greater risk for anxiety (relative risk, 9.75) and depression (RR, 17.86). Loneliness at follow‐up was linked with increased risks for suicidal ideation (RR, 1.52), heavy or risky alcohol consumption (RR, 1.27), and any grade 2-4 new‐onset chronic health condition (RR, 1.29), especially those that were neurologic (RR, 4.37).
Survivors of CNS tumors (odds ratio, 2.59) and leukemia (OR, 2.52) were most likely to report loneliness at both baseline and follow‐up, though survivors of four other cancer types also faced an elevated risk for loneliness: neuroblastoma (OR, 2.32), bone tumor (OR, 2.12), soft tissue sarcoma (OR, 1.78), and Hodgkin lymphoma (OR, 1.69).
Treatment type appeared to matter as well. Survivors who underwent amputation (OR, 1.82) or were treated with cranial radiation greater than or equal to 20 Gy (OR, 1.56) or corticosteroids (OR, 1.31) were more likely to report loneliness at baseline and follow‐up, compared with those who reported no loneliness at both time points.
The authors acknowledge limitations to the study, including the fact that roughly 90% of survivors and siblings were White, which limits the applicability of their results to diverse groups. In addition, the responses were self-reported without external validation.
Overall, though, the findings provide a framework for clinicians to understand and identify loneliness among young cancer survivors and help them cope with their emotions.
“The Childhood Cancer Survivor Study provides the largest and the most comprehensive dataset on childhood cancer survivors and healthy-sibling comparisons, giving us powerful data on survivorship, late effects, and psychosocial and health outcomes,” Rachel M. Moore, PhD, child psychologist at Children’s Mercy Kansas City, Mo., said in an interview.
Asking a simple question – “Are you feeling lonely?” – can identify at-risk survivors and enable health care teams to provide timely interventions that address young patients’ physical and psychological needs, said Dr. Moore, who was not involved in the study.
Dr. Moore noted that within her clinical practice, “adolescent and young adult survivors frequently discuss loneliness in their daily lives. They feel different from their peers and misunderstood. Having a conversation early in survivorship care about the experience of loneliness as a product of cancer treatment can open the door to regular screening and destigmatizing mental health services.”
Supporting young people throughout their survivorship journey is important, said Rusha Bhandari, MD, medical director of the Childhood, Adolescent, and Young Adult Cancer Survivorship Program at City of Hope, Duarte, Calif. This study can help ensure that clinicians “provide comprehensive care, including psychosocial screening and support, to meet the unique needs of our young adult survivors,” said Dr. Bhandari, who also was not involved in the research.
The National Cancer Institute and the American Lebanese Syrian Associated Charities supported the study. One co-author reported receiving corporate consulting fees. Dr. Papini, the remaining co-authors, Dr. Moore, and Dr. Bhandari report no relevant financial involvements.
A version of this article first appeared on Medscape.com.
FROM CANCER