User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Low preconception complement levels linked to adverse pregnancy outcomes in antiphospholipid syndrome
Low serum levels of two complement proteins are linked to worse pregnancy outcomes in women with antiphospholipid syndrome (APS), the results of a multicenter study appear to confirm.
The study evaluated preconception complement levels in 260 pregnancies in 197 women who had APS or carried antiphospholipid antibodies (aPL), and found that low levels of C3 and C4 in the 6 months prior to pregnancy were associated with several gestational complications and resulted in pregnancy losses.
“This study has validated, on large scale, the possible utility of preconception measurement of C3 and C4 levels to predict pregnancy loss in patients with aPL, even at a high-risk profile,” said study investigator Daniele Lini, MD, of ASST Spedali Civili and the University of Brescia (Italy).
“The tests are easy and cheap to be routinely performed, and they could therefore represent a valid aid to identify women that need particular monitoring and management,” he said at the 14th International Congress on Systemic Lupus Erythematosus held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
aPL and adverse obstetric outcomes
aPL, which include lupus anticoagulant, anti–beta2-glycoprotein 1, and anticardiolipin antibodies, have been shown to induce fetal loss in animal models. Their influence on the outcome of human pregnancies, however, has been less clear, with several studies failing to prove a link between their presence and obstetric complications.
Dr. Lini and coinvestigators conducted a multicenter study involving 11 Italian centers and one Russian center, retrospectively looking for women with primary APS or women who had persistently high levels of aPL but no symptoms who had become pregnant. Of 503 pregnancies, information on complement levels before conception was available for 260, of which 184 had occurred in women with APS and 76 in women with persistently high aPL.
The pregnancies were grouped according to whether there were low (n = 93) or normal (n = 167) levels of C3 and C4 in the last 6 months.
“Women with adverse pregnancy outcomes showed significantly lower preconception complement levels than those with successful pregnancies, without any difference between APS and aPL carriers,” Dr. Lini reported.
Comparing those with low to those with high complement levels, the preterm live birth rate (before 37 weeks’ gestation) was 37% versus 18% (P < .0001).
The full-term live birth rates were a respective 42% and 72% (P < .0001).
The rate of pregnancy loss, which included both abortion and miscarriage, was a respective 21% and 10% (P = .008).
A subgroup analysis focusing on where there was triple aPL positivity found that preconception low C3 and/or C4 levels was associated with an increased rate of pregnancy loss (P = .05). This association disappeared if there was just one or two aPL present.
The researchers found no correlation between complement levels and rates of venous thromboembolism or thrombocytopenia.
Study highlights ‘impact and importance’ of complement in APS
The study indicates “the impact and the importance of complement” in APS, said Yehuda Shoenfeld, MD, the founder and head of the Zabludowicz Center for Autoimmune Diseases at the Sheba Medical Center in Tel Hashomer, Israel.
In the early days of understanding APS, said Dr. Shoenfeld, it was thought that complement was not as important as it was in systemic lupus erythematosus (SLE). The importance of raised complement seen in studies of APS would often be discounted or neglected in comparison to SLE.
However, “slowly, slowly” it has been found that “complement [in APS] is activated very similarly to SLE,” Dr. Shoenfeld noted.
“I think that it’s important to assess the component levels,” Dr. Lini said in discussion. “This is needed to be done in the preconception counseling for APS and aPL carrier patients.”
Determining whether there is single, double, or even triple aPL positivity could be useful in guiding clinical decisions.
“If we have triple positivity, that could mean that there may be a more immunologic activation of the system and that it could be useful to administrate hydroxychloroquine [to] those patients who would like to have a pregnancy,” Dr. Lini suggested.
Plus, in those with decreased complement levels, “this could be a very useful tool” to identify where something could go wrong during their pregnancy.
The study had no outside funding. Dr. Lini and Dr. Shoenfeld disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low serum levels of two complement proteins are linked to worse pregnancy outcomes in women with antiphospholipid syndrome (APS), the results of a multicenter study appear to confirm.
The study evaluated preconception complement levels in 260 pregnancies in 197 women who had APS or carried antiphospholipid antibodies (aPL), and found that low levels of C3 and C4 in the 6 months prior to pregnancy were associated with several gestational complications and resulted in pregnancy losses.
“This study has validated, on large scale, the possible utility of preconception measurement of C3 and C4 levels to predict pregnancy loss in patients with aPL, even at a high-risk profile,” said study investigator Daniele Lini, MD, of ASST Spedali Civili and the University of Brescia (Italy).
“The tests are easy and cheap to be routinely performed, and they could therefore represent a valid aid to identify women that need particular monitoring and management,” he said at the 14th International Congress on Systemic Lupus Erythematosus held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
aPL and adverse obstetric outcomes
aPL, which include lupus anticoagulant, anti–beta2-glycoprotein 1, and anticardiolipin antibodies, have been shown to induce fetal loss in animal models. Their influence on the outcome of human pregnancies, however, has been less clear, with several studies failing to prove a link between their presence and obstetric complications.
Dr. Lini and coinvestigators conducted a multicenter study involving 11 Italian centers and one Russian center, retrospectively looking for women with primary APS or women who had persistently high levels of aPL but no symptoms who had become pregnant. Of 503 pregnancies, information on complement levels before conception was available for 260, of which 184 had occurred in women with APS and 76 in women with persistently high aPL.
The pregnancies were grouped according to whether there were low (n = 93) or normal (n = 167) levels of C3 and C4 in the last 6 months.
“Women with adverse pregnancy outcomes showed significantly lower preconception complement levels than those with successful pregnancies, without any difference between APS and aPL carriers,” Dr. Lini reported.
Comparing those with low to those with high complement levels, the preterm live birth rate (before 37 weeks’ gestation) was 37% versus 18% (P < .0001).
The full-term live birth rates were a respective 42% and 72% (P < .0001).
The rate of pregnancy loss, which included both abortion and miscarriage, was a respective 21% and 10% (P = .008).
A subgroup analysis focusing on where there was triple aPL positivity found that preconception low C3 and/or C4 levels was associated with an increased rate of pregnancy loss (P = .05). This association disappeared if there was just one or two aPL present.
The researchers found no correlation between complement levels and rates of venous thromboembolism or thrombocytopenia.
Study highlights ‘impact and importance’ of complement in APS
The study indicates “the impact and the importance of complement” in APS, said Yehuda Shoenfeld, MD, the founder and head of the Zabludowicz Center for Autoimmune Diseases at the Sheba Medical Center in Tel Hashomer, Israel.
In the early days of understanding APS, said Dr. Shoenfeld, it was thought that complement was not as important as it was in systemic lupus erythematosus (SLE). The importance of raised complement seen in studies of APS would often be discounted or neglected in comparison to SLE.
However, “slowly, slowly” it has been found that “complement [in APS] is activated very similarly to SLE,” Dr. Shoenfeld noted.
“I think that it’s important to assess the component levels,” Dr. Lini said in discussion. “This is needed to be done in the preconception counseling for APS and aPL carrier patients.”
Determining whether there is single, double, or even triple aPL positivity could be useful in guiding clinical decisions.
“If we have triple positivity, that could mean that there may be a more immunologic activation of the system and that it could be useful to administrate hydroxychloroquine [to] those patients who would like to have a pregnancy,” Dr. Lini suggested.
Plus, in those with decreased complement levels, “this could be a very useful tool” to identify where something could go wrong during their pregnancy.
The study had no outside funding. Dr. Lini and Dr. Shoenfeld disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Low serum levels of two complement proteins are linked to worse pregnancy outcomes in women with antiphospholipid syndrome (APS), the results of a multicenter study appear to confirm.
The study evaluated preconception complement levels in 260 pregnancies in 197 women who had APS or carried antiphospholipid antibodies (aPL), and found that low levels of C3 and C4 in the 6 months prior to pregnancy were associated with several gestational complications and resulted in pregnancy losses.
“This study has validated, on large scale, the possible utility of preconception measurement of C3 and C4 levels to predict pregnancy loss in patients with aPL, even at a high-risk profile,” said study investigator Daniele Lini, MD, of ASST Spedali Civili and the University of Brescia (Italy).
“The tests are easy and cheap to be routinely performed, and they could therefore represent a valid aid to identify women that need particular monitoring and management,” he said at the 14th International Congress on Systemic Lupus Erythematosus held together with the 6th International Congress on Controversies in Rheumatology and Autoimmunity.
aPL and adverse obstetric outcomes
aPL, which include lupus anticoagulant, anti–beta2-glycoprotein 1, and anticardiolipin antibodies, have been shown to induce fetal loss in animal models. Their influence on the outcome of human pregnancies, however, has been less clear, with several studies failing to prove a link between their presence and obstetric complications.
Dr. Lini and coinvestigators conducted a multicenter study involving 11 Italian centers and one Russian center, retrospectively looking for women with primary APS or women who had persistently high levels of aPL but no symptoms who had become pregnant. Of 503 pregnancies, information on complement levels before conception was available for 260, of which 184 had occurred in women with APS and 76 in women with persistently high aPL.
The pregnancies were grouped according to whether there were low (n = 93) or normal (n = 167) levels of C3 and C4 in the last 6 months.
“Women with adverse pregnancy outcomes showed significantly lower preconception complement levels than those with successful pregnancies, without any difference between APS and aPL carriers,” Dr. Lini reported.
Comparing those with low to those with high complement levels, the preterm live birth rate (before 37 weeks’ gestation) was 37% versus 18% (P < .0001).
The full-term live birth rates were a respective 42% and 72% (P < .0001).
The rate of pregnancy loss, which included both abortion and miscarriage, was a respective 21% and 10% (P = .008).
A subgroup analysis focusing on where there was triple aPL positivity found that preconception low C3 and/or C4 levels was associated with an increased rate of pregnancy loss (P = .05). This association disappeared if there was just one or two aPL present.
The researchers found no correlation between complement levels and rates of venous thromboembolism or thrombocytopenia.
Study highlights ‘impact and importance’ of complement in APS
The study indicates “the impact and the importance of complement” in APS, said Yehuda Shoenfeld, MD, the founder and head of the Zabludowicz Center for Autoimmune Diseases at the Sheba Medical Center in Tel Hashomer, Israel.
In the early days of understanding APS, said Dr. Shoenfeld, it was thought that complement was not as important as it was in systemic lupus erythematosus (SLE). The importance of raised complement seen in studies of APS would often be discounted or neglected in comparison to SLE.
However, “slowly, slowly” it has been found that “complement [in APS] is activated very similarly to SLE,” Dr. Shoenfeld noted.
“I think that it’s important to assess the component levels,” Dr. Lini said in discussion. “This is needed to be done in the preconception counseling for APS and aPL carrier patients.”
Determining whether there is single, double, or even triple aPL positivity could be useful in guiding clinical decisions.
“If we have triple positivity, that could mean that there may be a more immunologic activation of the system and that it could be useful to administrate hydroxychloroquine [to] those patients who would like to have a pregnancy,” Dr. Lini suggested.
Plus, in those with decreased complement levels, “this could be a very useful tool” to identify where something could go wrong during their pregnancy.
The study had no outside funding. Dr. Lini and Dr. Shoenfeld disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Merck seeks FDA authorization for antiviral COVID-19 pill
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
HEPA filters may clean SARS-CoV-2 from the air: Study
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
Abaloparatide significantly reduced fractures, increased BMD in women at high fracture risk
Postmenopausal women at high or very high risk of fracture gained significantly more bone mineral density and were significantly less likely to experience a fracture when taking abaloparatide for 18 months, according to new research presented at the hybrid annual meeting of the North American Menopause Society.
“The findings showed that abaloparatide was better than teriparatide in a number of parameters important in osteoporosis treatment, and similar in others, in high-risk and very-high-risk postmenopausal women with osteoporosis,” Bart Clarke, MD, a professor of medicine at Mayo Clinic in Rochester, Minn., said in an interview. “Abaloparatide is safe and effective for use in high-risk or very-high-risk postmenopausal women,” as defined by the new American Association of Clinical Endocrinology/American College of Endocrinology osteoporosis guidelines.
Ricardo R. Correa, MD, of the department of endocrinology and director of diversity for graduate medical education at the University of Arizona, Phoenix, said that the study demonstrates that abaloparatide and teriparatide have a very similar effect with abaloparatide providing a slightly better absolute risk reduction in fracture. Dr. Correa was not involved in the research.
“What will drive my decision in what to prescribe will be the cost and insurance coverage,” Dr. Correa said. “At the Veterans Administration hospital, the option that we have is abaloparatide, so this is the option that we use.”
Among women at least 65 years old who have already had one fracture, 1 in 10 will experience another fracture within the next year, and 30% will have another fracture within the next 5 years, the authors noted in their background material. Since phase 3 ACTIVE study data in 2016 showed that abaloparatide reduces fracture risk while increasing bone mineral density, compared with placebo, the researchers reanalyzed that data to assess the drug’s efficacy in patients at high or very high risk for fracture.
The study involved 2,463 postmenopausal women with osteoporosis who received one of three interventions: 80 mcg abaloparatide daily, placebo, or 20 mcg subcutaneous teriparatide daily. Only the abaloparatide and placebo groups were double blinded.
“Teriparatide was used as the comparator drug because teriparatide was previously approved as the first anabolic drug for osteoporosis,” Dr. Clarke said in an interview. “The hope was to show that abaloparatide was a better anabolic drug.”
Women were considered at high or very high risk of fracture if they met at least one of the following four criteria from the 2020 American Association of Clinical Endocrinology guidelines:
- Fracture within the past 12 months or prevalent vertebral fracture.
- Very low T-score (less than –3.0) at baseline at any site.
- Multiple fractures at baseline since age 45.
- Very high fracture risk based on the Fracture Risk Assessment Tool (FRAX) (at least 30% for major osteoporotic fracture or at least 4.5% for hip fracture).
Among the 2,026 patients who met at least one of these criteria, 664 received abaloparatide, 685 received teriparatide, and 677 received placebo. Both the abaloparatide and teriparatide significantly reduced new vertebral fracture risk, compared with placebo. In the abaloparatide group, 0.72% of women had a new vertebral fracture, compared with 0.99% in the teriparatide group and 4.77% in the placebo group (P < .0001).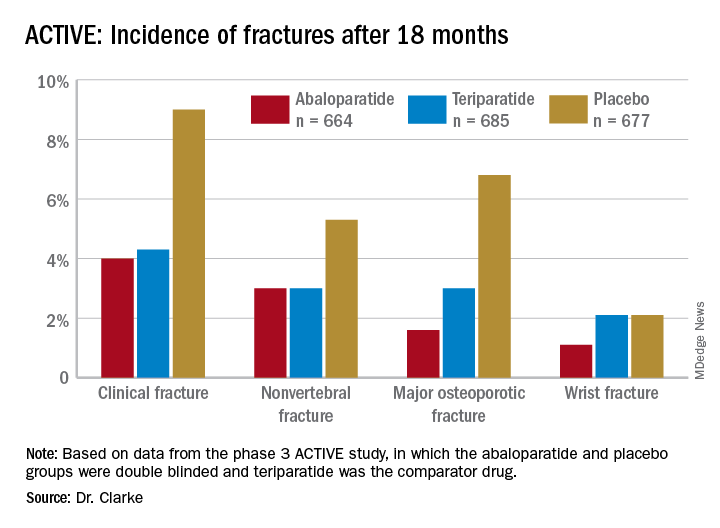
Abaloparatide and teriparatide also led to significant increases in lumbar spine, total hip, and femoral neck bone mineral density, compared with placebo (P < .0001).
The study was limited by its duration of 18 months and the Food and Drug Administration’s restriction on using abaloparatide for more than 2 years because of the theoretical risk of increasing osteosarcoma, although that risk has never been demonstrated in humans, Dr. Correa said. ”We need more data with abaloparitide in more than 2 years,” he added.
In determining which medication clinicians should first prescribe to manage osteoporosis, Dr. Correa said practitioners should consider the type of osteoporosis women have, their preferences, and their labs on kidney function.
With mild to moderate osteoporosis, bisphosphonates will be the first option while denosumab will be preferred for moderate to severe osteoporosis. Teriparatide and abaloparitide are the first-line options for severe osteoporosis, he said.
“If the glomerular filtration rate is low, we cannot use bisphosphonate and we will have to limit our use to denosumab,” he said. Route and frequency of delivery plays a role in patient preferences.
“If the patient prefers an infusion once a year or a pill, then bisphosphonate,” he said, but “if the patient is fine with an injection every 6 months, then denosumab.” Patients who need and can do an injection every day can take abaloparitide or teriparatide.
Failure of previous treatments also guide clinical decisions, he added. ”If the patient has been on one medication and has a fracture or the bone mineral density decreases, then we need to switch to another medication, usually teriparatide or abaloparitide, to build new bone.”
Contraindications for abaloparatide include a high serum calcium before therapy or prior allergic reactions to components in abaloparatide, Dr. Clarke said. No new safety signals showed up in the data analysis.
The research was funded by Radius Health. Dr. Clarke is an advisory board member of Amgen, and another author consults and speaks for Amgen and is a Radius Health Advisory Board member. Two other authors are Radius Health employees who own stock in the company. Dr Correa has no disclosures.
Postmenopausal women at high or very high risk of fracture gained significantly more bone mineral density and were significantly less likely to experience a fracture when taking abaloparatide for 18 months, according to new research presented at the hybrid annual meeting of the North American Menopause Society.
“The findings showed that abaloparatide was better than teriparatide in a number of parameters important in osteoporosis treatment, and similar in others, in high-risk and very-high-risk postmenopausal women with osteoporosis,” Bart Clarke, MD, a professor of medicine at Mayo Clinic in Rochester, Minn., said in an interview. “Abaloparatide is safe and effective for use in high-risk or very-high-risk postmenopausal women,” as defined by the new American Association of Clinical Endocrinology/American College of Endocrinology osteoporosis guidelines.
Ricardo R. Correa, MD, of the department of endocrinology and director of diversity for graduate medical education at the University of Arizona, Phoenix, said that the study demonstrates that abaloparatide and teriparatide have a very similar effect with abaloparatide providing a slightly better absolute risk reduction in fracture. Dr. Correa was not involved in the research.
“What will drive my decision in what to prescribe will be the cost and insurance coverage,” Dr. Correa said. “At the Veterans Administration hospital, the option that we have is abaloparatide, so this is the option that we use.”
Among women at least 65 years old who have already had one fracture, 1 in 10 will experience another fracture within the next year, and 30% will have another fracture within the next 5 years, the authors noted in their background material. Since phase 3 ACTIVE study data in 2016 showed that abaloparatide reduces fracture risk while increasing bone mineral density, compared with placebo, the researchers reanalyzed that data to assess the drug’s efficacy in patients at high or very high risk for fracture.
The study involved 2,463 postmenopausal women with osteoporosis who received one of three interventions: 80 mcg abaloparatide daily, placebo, or 20 mcg subcutaneous teriparatide daily. Only the abaloparatide and placebo groups were double blinded.
“Teriparatide was used as the comparator drug because teriparatide was previously approved as the first anabolic drug for osteoporosis,” Dr. Clarke said in an interview. “The hope was to show that abaloparatide was a better anabolic drug.”
Women were considered at high or very high risk of fracture if they met at least one of the following four criteria from the 2020 American Association of Clinical Endocrinology guidelines:
- Fracture within the past 12 months or prevalent vertebral fracture.
- Very low T-score (less than –3.0) at baseline at any site.
- Multiple fractures at baseline since age 45.
- Very high fracture risk based on the Fracture Risk Assessment Tool (FRAX) (at least 30% for major osteoporotic fracture or at least 4.5% for hip fracture).
Among the 2,026 patients who met at least one of these criteria, 664 received abaloparatide, 685 received teriparatide, and 677 received placebo. Both the abaloparatide and teriparatide significantly reduced new vertebral fracture risk, compared with placebo. In the abaloparatide group, 0.72% of women had a new vertebral fracture, compared with 0.99% in the teriparatide group and 4.77% in the placebo group (P < .0001).
Abaloparatide and teriparatide also led to significant increases in lumbar spine, total hip, and femoral neck bone mineral density, compared with placebo (P < .0001).
The study was limited by its duration of 18 months and the Food and Drug Administration’s restriction on using abaloparatide for more than 2 years because of the theoretical risk of increasing osteosarcoma, although that risk has never been demonstrated in humans, Dr. Correa said. ”We need more data with abaloparitide in more than 2 years,” he added.
In determining which medication clinicians should first prescribe to manage osteoporosis, Dr. Correa said practitioners should consider the type of osteoporosis women have, their preferences, and their labs on kidney function.
With mild to moderate osteoporosis, bisphosphonates will be the first option while denosumab will be preferred for moderate to severe osteoporosis. Teriparatide and abaloparitide are the first-line options for severe osteoporosis, he said.
“If the glomerular filtration rate is low, we cannot use bisphosphonate and we will have to limit our use to denosumab,” he said. Route and frequency of delivery plays a role in patient preferences.
“If the patient prefers an infusion once a year or a pill, then bisphosphonate,” he said, but “if the patient is fine with an injection every 6 months, then denosumab.” Patients who need and can do an injection every day can take abaloparitide or teriparatide.
Failure of previous treatments also guide clinical decisions, he added. ”If the patient has been on one medication and has a fracture or the bone mineral density decreases, then we need to switch to another medication, usually teriparatide or abaloparitide, to build new bone.”
Contraindications for abaloparatide include a high serum calcium before therapy or prior allergic reactions to components in abaloparatide, Dr. Clarke said. No new safety signals showed up in the data analysis.
The research was funded by Radius Health. Dr. Clarke is an advisory board member of Amgen, and another author consults and speaks for Amgen and is a Radius Health Advisory Board member. Two other authors are Radius Health employees who own stock in the company. Dr Correa has no disclosures.
Postmenopausal women at high or very high risk of fracture gained significantly more bone mineral density and were significantly less likely to experience a fracture when taking abaloparatide for 18 months, according to new research presented at the hybrid annual meeting of the North American Menopause Society.
“The findings showed that abaloparatide was better than teriparatide in a number of parameters important in osteoporosis treatment, and similar in others, in high-risk and very-high-risk postmenopausal women with osteoporosis,” Bart Clarke, MD, a professor of medicine at Mayo Clinic in Rochester, Minn., said in an interview. “Abaloparatide is safe and effective for use in high-risk or very-high-risk postmenopausal women,” as defined by the new American Association of Clinical Endocrinology/American College of Endocrinology osteoporosis guidelines.
Ricardo R. Correa, MD, of the department of endocrinology and director of diversity for graduate medical education at the University of Arizona, Phoenix, said that the study demonstrates that abaloparatide and teriparatide have a very similar effect with abaloparatide providing a slightly better absolute risk reduction in fracture. Dr. Correa was not involved in the research.
“What will drive my decision in what to prescribe will be the cost and insurance coverage,” Dr. Correa said. “At the Veterans Administration hospital, the option that we have is abaloparatide, so this is the option that we use.”
Among women at least 65 years old who have already had one fracture, 1 in 10 will experience another fracture within the next year, and 30% will have another fracture within the next 5 years, the authors noted in their background material. Since phase 3 ACTIVE study data in 2016 showed that abaloparatide reduces fracture risk while increasing bone mineral density, compared with placebo, the researchers reanalyzed that data to assess the drug’s efficacy in patients at high or very high risk for fracture.
The study involved 2,463 postmenopausal women with osteoporosis who received one of three interventions: 80 mcg abaloparatide daily, placebo, or 20 mcg subcutaneous teriparatide daily. Only the abaloparatide and placebo groups were double blinded.
“Teriparatide was used as the comparator drug because teriparatide was previously approved as the first anabolic drug for osteoporosis,” Dr. Clarke said in an interview. “The hope was to show that abaloparatide was a better anabolic drug.”
Women were considered at high or very high risk of fracture if they met at least one of the following four criteria from the 2020 American Association of Clinical Endocrinology guidelines:
- Fracture within the past 12 months or prevalent vertebral fracture.
- Very low T-score (less than –3.0) at baseline at any site.
- Multiple fractures at baseline since age 45.
- Very high fracture risk based on the Fracture Risk Assessment Tool (FRAX) (at least 30% for major osteoporotic fracture or at least 4.5% for hip fracture).
Among the 2,026 patients who met at least one of these criteria, 664 received abaloparatide, 685 received teriparatide, and 677 received placebo. Both the abaloparatide and teriparatide significantly reduced new vertebral fracture risk, compared with placebo. In the abaloparatide group, 0.72% of women had a new vertebral fracture, compared with 0.99% in the teriparatide group and 4.77% in the placebo group (P < .0001).
Abaloparatide and teriparatide also led to significant increases in lumbar spine, total hip, and femoral neck bone mineral density, compared with placebo (P < .0001).
The study was limited by its duration of 18 months and the Food and Drug Administration’s restriction on using abaloparatide for more than 2 years because of the theoretical risk of increasing osteosarcoma, although that risk has never been demonstrated in humans, Dr. Correa said. ”We need more data with abaloparitide in more than 2 years,” he added.
In determining which medication clinicians should first prescribe to manage osteoporosis, Dr. Correa said practitioners should consider the type of osteoporosis women have, their preferences, and their labs on kidney function.
With mild to moderate osteoporosis, bisphosphonates will be the first option while denosumab will be preferred for moderate to severe osteoporosis. Teriparatide and abaloparitide are the first-line options for severe osteoporosis, he said.
“If the glomerular filtration rate is low, we cannot use bisphosphonate and we will have to limit our use to denosumab,” he said. Route and frequency of delivery plays a role in patient preferences.
“If the patient prefers an infusion once a year or a pill, then bisphosphonate,” he said, but “if the patient is fine with an injection every 6 months, then denosumab.” Patients who need and can do an injection every day can take abaloparitide or teriparatide.
Failure of previous treatments also guide clinical decisions, he added. ”If the patient has been on one medication and has a fracture or the bone mineral density decreases, then we need to switch to another medication, usually teriparatide or abaloparitide, to build new bone.”
Contraindications for abaloparatide include a high serum calcium before therapy or prior allergic reactions to components in abaloparatide, Dr. Clarke said. No new safety signals showed up in the data analysis.
The research was funded by Radius Health. Dr. Clarke is an advisory board member of Amgen, and another author consults and speaks for Amgen and is a Radius Health Advisory Board member. Two other authors are Radius Health employees who own stock in the company. Dr Correa has no disclosures.
FROM NAMS 2021
Steroid a promising short-term treatment option for major depression?
Study results of an experimental agent that improves symptoms of major depression and boosts quality of life in as little as 3 days suggest it may be an effective short-term treatment option.
Phase 3 results of a randomized, placebo-controlled trial compared zuranolone, an neuroactive steroid that binds to both synaptic and extra-synaptic GABA-A receptors, to placebo in patients with major depressive disorder (MDD). Overall, 30% of participants were already taking antidepressants.
Investigators found the drug was associated with a significant improvement in depression scores versus placebo, with benefit observed as early as day 3. This was accompanied by improved function and well-being.
, said study presenter Colville Brown, MD, Sage Therapeutics, Cambridge, Mass.
“These data continue to support the development of zuranolone as a potential 14-day short course treatment for major depressive disorder episodes.”
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
High placebo response
However, despite being significant, the drug’s benefit was only slightly higher than that of placebo, raising questions about the study design and the true performance of the drug.
Dr. Brown explained that patients with MDD were randomized to oral zuranolone 50 mg or placebo once daily for 14 days, with dose reductions to 40 mg or matching placebo permitted in case of perceived intolerance.
Patients were assessed at baseline and day 15 via the 17-item Hamilton Rating Scale for Depression (HAMD-17) before entering a 28-day follow-up period off the study drug.
Among the 268 participants who received zuranolone, 90.3% completed the study, compared with 87.4% of 269 patients in the placebo group.
The mean age of participants was 40 years. Women made up 69.4% of those who received zuranolone and 61.7% assigned to placebo.
The mean HAMD-17 score at baseline was 26.8 and 26.9 in the zuranolone and placebo groups, respectively. Dr. Brown noted that 29.5% of patients in the zuranolone group and 30.1% of those assigned to placebo were taking antidepressants at baseline.
The study’s primary endpoint was met, with patients taking the study drug experiencing a significantly greater reduction in HAMD-17 scores from baseline to day 15 versus those given placebo, at 14.1 versus 12.3 points (P = .0141).
Dr. Brown highlighted that the difference in reduction in HAMD-17 scores between the zuranolone and placebo groups was already significant at day 3 (P < .0001), and again at day 8 (P < .0001) and day 12 (P < .001).
At day 3, response rates on the HAMD-17 were significantly higher among zuranolone-treated patients than among those given placebo, at 29.3% versus 16.3% (P < .001). However, the differences on day 15 and on day 42 were no longer significant.
A similar effect was seen for HAMD-17 remissions, which were seen in 7.6% of zuranolone-treated patients and 2.3% of those given placebo at day 3 (P < .01), rising to 29.8% versus 27.1% at day 15, and 30.8% versus 29.6% at day 42, and neither difference was significant.
Dr. Brown also showed that, at all time points during the treatment and follow-up periods, improvements in response rates in Global Improvement on the Clinical Global Impression scale favored zuranolone.
On the SF-36v2 quality of life questionnaire, improvements again favored zuranolone on all domains, although the difference between active treatment and placebo was significant only for vitality on day 15, at 12.8 versus 9.7 points (P < .05).
Treatment-emergent adverse events were more common with zuranolone, with 60.1% of patients experiencing at least one event of any grade versus 44.6% with placebo. However, severe events were seen in only 3.0% versus 1.1% of patients, and serious adverse events were recorded in only two patients (0.7%) in both groups.
The most common adverse events were somnolence, dizziness, headache, sedation, and diarrhea, with no increase in suicidal ideation or withdrawal. Dr. Brown noted that there was “no change in the safety signal” between patients with or without prior antidepressant therapy.
From the audience, Marie-Josée Filteau, MD, department of psychiatry, Laval University, Quebec, drew attention to the similarity in the improvement in HAMD-17 scores between the zuranolone and placebo groups, asking: “How is that compelling?”
Dr. Brown replied that “what they are excited about is that change from baseline with zuranolone,” adding: “You do see it in the placebo group as well, and ... this isn’t new to psychiatry.
“This is a heterogeneous disease, and remember this [study] was conducted during COVID, so patients were being seen with clinic visits during COVID.
“What impact did that have? The placebo is not really placebo” in this case.
More effective than results suggest?
Approached for comment by this news organization, Maurizio Fava, MD, executive vice chair, department of psychiatry, and executive director, Clinical Trials Network and Institute, Massachusetts General Hospital, Boston, noted there are several issues with the trial.
Because of those, the drug “is likely to be much more efficacious than it looks because it achieved statistical significance despite an extremely high placebo response,” he said
“Whenever your change on placebo is greater than 10 points on the HAMD, you have an excessive response ... and a very, very low chance of detecting a signal,” he said.
Dr. Fava said that another issue was including patients who were either on or off antidepressants, which meant the population was not sufficiently homogenous.
Another “flaw” was to assume that the placebo effect would be “transient” and deteriorate over time, whereas the results showed the opposite.
Nevertheless, “it’s a positive study because of the sample size ... that provides further evidence for the antidepressant activity of zuranolone” and the drug was “well tolerated.”
Dr. Fava expects zuranolone “will make it to the market,” as an indication from the Food and Drug Administration is likely, “but if you’re asking me: Is the drug as effective as shown in their studies? It’s probably much more effective.”
The study was funded by Sage Therapeutics and Biogen. Dr. Brown is an employee of Sage Therapeutics. Lead investigator Anita Clayton, MD, University of Virginia, Charlottesville, has reported relationships with Dario Bioscience, Janssen, Praxis Precision Medicines, Relmada Therapeutics, Sage Therapeutics, Fabre-Kramer, MindCure, Ovoca Bio, PureTech Health, S1 Biopharma, Vella Bioscience, WCG MedAvante-ProPhase, Ballantine Books/Random House, Guilford Publications, Euthymics, and Mediflix.
A version of this article first appeared on Medscape.com.
Study results of an experimental agent that improves symptoms of major depression and boosts quality of life in as little as 3 days suggest it may be an effective short-term treatment option.
Phase 3 results of a randomized, placebo-controlled trial compared zuranolone, an neuroactive steroid that binds to both synaptic and extra-synaptic GABA-A receptors, to placebo in patients with major depressive disorder (MDD). Overall, 30% of participants were already taking antidepressants.
Investigators found the drug was associated with a significant improvement in depression scores versus placebo, with benefit observed as early as day 3. This was accompanied by improved function and well-being.
, said study presenter Colville Brown, MD, Sage Therapeutics, Cambridge, Mass.
“These data continue to support the development of zuranolone as a potential 14-day short course treatment for major depressive disorder episodes.”
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
High placebo response
However, despite being significant, the drug’s benefit was only slightly higher than that of placebo, raising questions about the study design and the true performance of the drug.
Dr. Brown explained that patients with MDD were randomized to oral zuranolone 50 mg or placebo once daily for 14 days, with dose reductions to 40 mg or matching placebo permitted in case of perceived intolerance.
Patients were assessed at baseline and day 15 via the 17-item Hamilton Rating Scale for Depression (HAMD-17) before entering a 28-day follow-up period off the study drug.
Among the 268 participants who received zuranolone, 90.3% completed the study, compared with 87.4% of 269 patients in the placebo group.
The mean age of participants was 40 years. Women made up 69.4% of those who received zuranolone and 61.7% assigned to placebo.
The mean HAMD-17 score at baseline was 26.8 and 26.9 in the zuranolone and placebo groups, respectively. Dr. Brown noted that 29.5% of patients in the zuranolone group and 30.1% of those assigned to placebo were taking antidepressants at baseline.
The study’s primary endpoint was met, with patients taking the study drug experiencing a significantly greater reduction in HAMD-17 scores from baseline to day 15 versus those given placebo, at 14.1 versus 12.3 points (P = .0141).
Dr. Brown highlighted that the difference in reduction in HAMD-17 scores between the zuranolone and placebo groups was already significant at day 3 (P < .0001), and again at day 8 (P < .0001) and day 12 (P < .001).
At day 3, response rates on the HAMD-17 were significantly higher among zuranolone-treated patients than among those given placebo, at 29.3% versus 16.3% (P < .001). However, the differences on day 15 and on day 42 were no longer significant.
A similar effect was seen for HAMD-17 remissions, which were seen in 7.6% of zuranolone-treated patients and 2.3% of those given placebo at day 3 (P < .01), rising to 29.8% versus 27.1% at day 15, and 30.8% versus 29.6% at day 42, and neither difference was significant.
Dr. Brown also showed that, at all time points during the treatment and follow-up periods, improvements in response rates in Global Improvement on the Clinical Global Impression scale favored zuranolone.
On the SF-36v2 quality of life questionnaire, improvements again favored zuranolone on all domains, although the difference between active treatment and placebo was significant only for vitality on day 15, at 12.8 versus 9.7 points (P < .05).
Treatment-emergent adverse events were more common with zuranolone, with 60.1% of patients experiencing at least one event of any grade versus 44.6% with placebo. However, severe events were seen in only 3.0% versus 1.1% of patients, and serious adverse events were recorded in only two patients (0.7%) in both groups.
The most common adverse events were somnolence, dizziness, headache, sedation, and diarrhea, with no increase in suicidal ideation or withdrawal. Dr. Brown noted that there was “no change in the safety signal” between patients with or without prior antidepressant therapy.
From the audience, Marie-Josée Filteau, MD, department of psychiatry, Laval University, Quebec, drew attention to the similarity in the improvement in HAMD-17 scores between the zuranolone and placebo groups, asking: “How is that compelling?”
Dr. Brown replied that “what they are excited about is that change from baseline with zuranolone,” adding: “You do see it in the placebo group as well, and ... this isn’t new to psychiatry.
“This is a heterogeneous disease, and remember this [study] was conducted during COVID, so patients were being seen with clinic visits during COVID.
“What impact did that have? The placebo is not really placebo” in this case.
More effective than results suggest?
Approached for comment by this news organization, Maurizio Fava, MD, executive vice chair, department of psychiatry, and executive director, Clinical Trials Network and Institute, Massachusetts General Hospital, Boston, noted there are several issues with the trial.
Because of those, the drug “is likely to be much more efficacious than it looks because it achieved statistical significance despite an extremely high placebo response,” he said
“Whenever your change on placebo is greater than 10 points on the HAMD, you have an excessive response ... and a very, very low chance of detecting a signal,” he said.
Dr. Fava said that another issue was including patients who were either on or off antidepressants, which meant the population was not sufficiently homogenous.
Another “flaw” was to assume that the placebo effect would be “transient” and deteriorate over time, whereas the results showed the opposite.
Nevertheless, “it’s a positive study because of the sample size ... that provides further evidence for the antidepressant activity of zuranolone” and the drug was “well tolerated.”
Dr. Fava expects zuranolone “will make it to the market,” as an indication from the Food and Drug Administration is likely, “but if you’re asking me: Is the drug as effective as shown in their studies? It’s probably much more effective.”
The study was funded by Sage Therapeutics and Biogen. Dr. Brown is an employee of Sage Therapeutics. Lead investigator Anita Clayton, MD, University of Virginia, Charlottesville, has reported relationships with Dario Bioscience, Janssen, Praxis Precision Medicines, Relmada Therapeutics, Sage Therapeutics, Fabre-Kramer, MindCure, Ovoca Bio, PureTech Health, S1 Biopharma, Vella Bioscience, WCG MedAvante-ProPhase, Ballantine Books/Random House, Guilford Publications, Euthymics, and Mediflix.
A version of this article first appeared on Medscape.com.
Study results of an experimental agent that improves symptoms of major depression and boosts quality of life in as little as 3 days suggest it may be an effective short-term treatment option.
Phase 3 results of a randomized, placebo-controlled trial compared zuranolone, an neuroactive steroid that binds to both synaptic and extra-synaptic GABA-A receptors, to placebo in patients with major depressive disorder (MDD). Overall, 30% of participants were already taking antidepressants.
Investigators found the drug was associated with a significant improvement in depression scores versus placebo, with benefit observed as early as day 3. This was accompanied by improved function and well-being.
, said study presenter Colville Brown, MD, Sage Therapeutics, Cambridge, Mass.
“These data continue to support the development of zuranolone as a potential 14-day short course treatment for major depressive disorder episodes.”
The findings were presented at the virtual congress of the European College of Neuropsychopharmacology.
High placebo response
However, despite being significant, the drug’s benefit was only slightly higher than that of placebo, raising questions about the study design and the true performance of the drug.
Dr. Brown explained that patients with MDD were randomized to oral zuranolone 50 mg or placebo once daily for 14 days, with dose reductions to 40 mg or matching placebo permitted in case of perceived intolerance.
Patients were assessed at baseline and day 15 via the 17-item Hamilton Rating Scale for Depression (HAMD-17) before entering a 28-day follow-up period off the study drug.
Among the 268 participants who received zuranolone, 90.3% completed the study, compared with 87.4% of 269 patients in the placebo group.
The mean age of participants was 40 years. Women made up 69.4% of those who received zuranolone and 61.7% assigned to placebo.
The mean HAMD-17 score at baseline was 26.8 and 26.9 in the zuranolone and placebo groups, respectively. Dr. Brown noted that 29.5% of patients in the zuranolone group and 30.1% of those assigned to placebo were taking antidepressants at baseline.
The study’s primary endpoint was met, with patients taking the study drug experiencing a significantly greater reduction in HAMD-17 scores from baseline to day 15 versus those given placebo, at 14.1 versus 12.3 points (P = .0141).
Dr. Brown highlighted that the difference in reduction in HAMD-17 scores between the zuranolone and placebo groups was already significant at day 3 (P < .0001), and again at day 8 (P < .0001) and day 12 (P < .001).
At day 3, response rates on the HAMD-17 were significantly higher among zuranolone-treated patients than among those given placebo, at 29.3% versus 16.3% (P < .001). However, the differences on day 15 and on day 42 were no longer significant.
A similar effect was seen for HAMD-17 remissions, which were seen in 7.6% of zuranolone-treated patients and 2.3% of those given placebo at day 3 (P < .01), rising to 29.8% versus 27.1% at day 15, and 30.8% versus 29.6% at day 42, and neither difference was significant.
Dr. Brown also showed that, at all time points during the treatment and follow-up periods, improvements in response rates in Global Improvement on the Clinical Global Impression scale favored zuranolone.
On the SF-36v2 quality of life questionnaire, improvements again favored zuranolone on all domains, although the difference between active treatment and placebo was significant only for vitality on day 15, at 12.8 versus 9.7 points (P < .05).
Treatment-emergent adverse events were more common with zuranolone, with 60.1% of patients experiencing at least one event of any grade versus 44.6% with placebo. However, severe events were seen in only 3.0% versus 1.1% of patients, and serious adverse events were recorded in only two patients (0.7%) in both groups.
The most common adverse events were somnolence, dizziness, headache, sedation, and diarrhea, with no increase in suicidal ideation or withdrawal. Dr. Brown noted that there was “no change in the safety signal” between patients with or without prior antidepressant therapy.
From the audience, Marie-Josée Filteau, MD, department of psychiatry, Laval University, Quebec, drew attention to the similarity in the improvement in HAMD-17 scores between the zuranolone and placebo groups, asking: “How is that compelling?”
Dr. Brown replied that “what they are excited about is that change from baseline with zuranolone,” adding: “You do see it in the placebo group as well, and ... this isn’t new to psychiatry.
“This is a heterogeneous disease, and remember this [study] was conducted during COVID, so patients were being seen with clinic visits during COVID.
“What impact did that have? The placebo is not really placebo” in this case.
More effective than results suggest?
Approached for comment by this news organization, Maurizio Fava, MD, executive vice chair, department of psychiatry, and executive director, Clinical Trials Network and Institute, Massachusetts General Hospital, Boston, noted there are several issues with the trial.
Because of those, the drug “is likely to be much more efficacious than it looks because it achieved statistical significance despite an extremely high placebo response,” he said
“Whenever your change on placebo is greater than 10 points on the HAMD, you have an excessive response ... and a very, very low chance of detecting a signal,” he said.
Dr. Fava said that another issue was including patients who were either on or off antidepressants, which meant the population was not sufficiently homogenous.
Another “flaw” was to assume that the placebo effect would be “transient” and deteriorate over time, whereas the results showed the opposite.
Nevertheless, “it’s a positive study because of the sample size ... that provides further evidence for the antidepressant activity of zuranolone” and the drug was “well tolerated.”
Dr. Fava expects zuranolone “will make it to the market,” as an indication from the Food and Drug Administration is likely, “but if you’re asking me: Is the drug as effective as shown in their studies? It’s probably much more effective.”
The study was funded by Sage Therapeutics and Biogen. Dr. Brown is an employee of Sage Therapeutics. Lead investigator Anita Clayton, MD, University of Virginia, Charlottesville, has reported relationships with Dario Bioscience, Janssen, Praxis Precision Medicines, Relmada Therapeutics, Sage Therapeutics, Fabre-Kramer, MindCure, Ovoca Bio, PureTech Health, S1 Biopharma, Vella Bioscience, WCG MedAvante-ProPhase, Ballantine Books/Random House, Guilford Publications, Euthymics, and Mediflix.
A version of this article first appeared on Medscape.com.
FROM ECNP 2021
Psychiatrists shift stance on gender dysphoria, recommend therapy
A new position statement from the Royal Australian and New Zealand College of Psychiatrists (RANZCP) stresses the importance of a mental health evaluation for people with gender dysphoria – in particular for children and adolescents – before any firm decisions are made on whether to prescribe hormonal treatments to transition, or perform surgeries, often referred to as “gender-affirming care.”
“There is a paucity of quality evidence on the outcomes of those presenting with gender dysphoria. In particular, there is a need for better evidence in relation to outcomes for children and young people,” the guidance states.
Because gender dysphoria “is associated with significant distress ... each case should be assessed by a mental health professional, which will frequently be a psychiatrist, with the person at the center of care. It is important the psychological state and context in which gender dysphoria has arisen is explored to assess the most appropriate treatment,” it adds.
The move by the psychiatry body represents a big shift in the landscape regarding recommendations for the treatment of gender dysphoria in Australia and New Zealand.
Asked to explain the new RANZCP position, Philip Morris, MBBS, FRANZCP, said: “The College acknowledged the complexity of the issues and the legitimacy of different approaches.”
Exploration of a patient’s reasons for identifying as transgender is essential, he said in an interview, especially when it comes to young people.
“There may be other reasons for doing it, and we need to look for those, identify them and treat them. This needs to be done before initiating hormones and changing the whole physical nature of the child,” he said.
“A cautious psychotherapy-first approach makes sense. If we can do that with adolescents, then we will take a big step in the right direction,” stressed Dr. Morris, who is president of the National Association of Practising Psychiatrists in Australia.
Keira Bell case and Scandinavian stance lead to more open discussion
The rapid rise in gender dysphoria among adolescents in the Western world, referred to as “rapid-onset” or “late-onset” gender dysphoria, has seen a huge increase in the number of natal girls presenting and created frenzied debate that has intensified worldwide in the last 12 months about how to best treat youth with gender dysphoria.
Concerns have arisen that some transgender identification is due to social contagion, and there is a growing number of “detransitioners” – people who identified as transgender, transitioned to the opposite gender, but then regretted their decision, changed their minds, and “detransitioned” back to their birth sex. If they have had hormone therapy, and in some cases surgery, they are left with irreversible changes to their bodies.
As a result, Scandinavian countries, most notably Finland, once eager advocates of the gender-affirmative approach, have pulled back and issued new treatment guidelines in 2020 stating that psychotherapy, rather than gender reassignment, should be the first line of treatment for gender-dysphoric youth.
This, along with a landmark High Court decision in the U.K. regarding the use of puberty-blocking drugs for children with gender dysphoria, brought by detransitioner Keira Bell, which was recently overturned by the Appeal Court, but which Ms. Bell now says she will take to the Supreme Court, has led to a considerable shift in the conversation around treating transgender adolescents with hormonal therapy, says Dr. Morris.
“This [has moved from] ... a topic that could previously not be talked about freely to one that we can discuss more openly now. This is a big improvement. Previously, everyone thought it was all settled, but it’s not, certainly not from a medical angle,” he states.
At odds with prior Australian recommendations
The RANZCP had previously endorsed the standard guidelines of the Royal Children’s Hospital (RCH) Melbourne, followed by most gender-identity services in Australia and similar guidance from New Zealand, which both recommend gender-affirming care.
“Increasing evidence demonstrates that with supportive, gender-affirming care during childhood and adolescence, harms can be ameliorated and mental health and well-being outcomes can be significantly improved,” state the RCH guidelines.
But in 2019, RANZCP removed its endorsement of the RCH guidelines and started a consultation, which resulted in the new position statement.
However, Ken Pang, MD, of the Murdoch Children’s Research Institute in Melbourne and an author of the RCH guidelines, says the key recommendations of the new RANZCP position statement are consistent with their own guidelines.
The former note “the need for a skilled mental health clinician in providing comprehensive exploration of a child or adolescent’s biopsychosocial context,” Dr. Pang says.
However, it’s difficult not to see the contrast in stance when the new RANZCP statement maintains: “Research on gender dysphoria is still emerging. There are polarized views and mixed evidence regarding treatment options for people presenting with gender identity concerns, especially children and young people.”
Dr. Pang says the RCH guidelines do, however, recognize the need for further research in the field.
“I look forward to being able to incorporate such research, including from our own Trans20 study, into future revisions of our guidelines,” he told this news organization.
Watch your backs with affirmative therapy: Will there be a compromise?
Dr. Morris says there will obviously be cases where “the child might transition with a medical intervention, but that wouldn’t be the first step.”
And yet, he adds, “There are those who push the pro-trans view that everyone should be allowed to transition, and the doctors are only technicians that provide hormones with no questions asked.”
But from a doctor’s perspective, clinicians will still be held responsible in medical and legal terms for the treatments given, he stressed.
“I don’t think they will ever not be accountable for that. They will always need to determine in their own mind whether their actions have positive value that outweigh any disadvantages,” Dr. Morris continues.
The RANZCP statement does, in fact, stress just this.
All health care professionals need to “be aware of ethical and medicolegal dilemmas” pertaining to affirmative therapy, it indicates. “Psychiatrists should practice within the relevant laws and accepted professional standards in relation to assessing capacity and obtaining consent...”
Dr. Morris hopes there will ultimately be many more checks and balances in place and that courts and clinicians will need to step back and not assume every child who seeks to transition is doing it as a result of pure gender dysphoria.
He predicts that things will end in a compromise.
“In my view, this compromise will treat children with respect and approach them like any other patient that presents with a condition that requires proper assessment and treatment.”
“In the end, some cases will be transitioned, but there will be fewer than [are] transitioned at the moment,” he predicts.
Dr. Morris has reported no relevant financial relationships. Dr. Pang is a member of the Australian Professional Association for Trans Health and its research committee.
A version of this article first appeared on Medscape.com.
A new position statement from the Royal Australian and New Zealand College of Psychiatrists (RANZCP) stresses the importance of a mental health evaluation for people with gender dysphoria – in particular for children and adolescents – before any firm decisions are made on whether to prescribe hormonal treatments to transition, or perform surgeries, often referred to as “gender-affirming care.”
“There is a paucity of quality evidence on the outcomes of those presenting with gender dysphoria. In particular, there is a need for better evidence in relation to outcomes for children and young people,” the guidance states.
Because gender dysphoria “is associated with significant distress ... each case should be assessed by a mental health professional, which will frequently be a psychiatrist, with the person at the center of care. It is important the psychological state and context in which gender dysphoria has arisen is explored to assess the most appropriate treatment,” it adds.
The move by the psychiatry body represents a big shift in the landscape regarding recommendations for the treatment of gender dysphoria in Australia and New Zealand.
Asked to explain the new RANZCP position, Philip Morris, MBBS, FRANZCP, said: “The College acknowledged the complexity of the issues and the legitimacy of different approaches.”
Exploration of a patient’s reasons for identifying as transgender is essential, he said in an interview, especially when it comes to young people.
“There may be other reasons for doing it, and we need to look for those, identify them and treat them. This needs to be done before initiating hormones and changing the whole physical nature of the child,” he said.
“A cautious psychotherapy-first approach makes sense. If we can do that with adolescents, then we will take a big step in the right direction,” stressed Dr. Morris, who is president of the National Association of Practising Psychiatrists in Australia.
Keira Bell case and Scandinavian stance lead to more open discussion
The rapid rise in gender dysphoria among adolescents in the Western world, referred to as “rapid-onset” or “late-onset” gender dysphoria, has seen a huge increase in the number of natal girls presenting and created frenzied debate that has intensified worldwide in the last 12 months about how to best treat youth with gender dysphoria.
Concerns have arisen that some transgender identification is due to social contagion, and there is a growing number of “detransitioners” – people who identified as transgender, transitioned to the opposite gender, but then regretted their decision, changed their minds, and “detransitioned” back to their birth sex. If they have had hormone therapy, and in some cases surgery, they are left with irreversible changes to their bodies.
As a result, Scandinavian countries, most notably Finland, once eager advocates of the gender-affirmative approach, have pulled back and issued new treatment guidelines in 2020 stating that psychotherapy, rather than gender reassignment, should be the first line of treatment for gender-dysphoric youth.
This, along with a landmark High Court decision in the U.K. regarding the use of puberty-blocking drugs for children with gender dysphoria, brought by detransitioner Keira Bell, which was recently overturned by the Appeal Court, but which Ms. Bell now says she will take to the Supreme Court, has led to a considerable shift in the conversation around treating transgender adolescents with hormonal therapy, says Dr. Morris.
“This [has moved from] ... a topic that could previously not be talked about freely to one that we can discuss more openly now. This is a big improvement. Previously, everyone thought it was all settled, but it’s not, certainly not from a medical angle,” he states.
At odds with prior Australian recommendations
The RANZCP had previously endorsed the standard guidelines of the Royal Children’s Hospital (RCH) Melbourne, followed by most gender-identity services in Australia and similar guidance from New Zealand, which both recommend gender-affirming care.
“Increasing evidence demonstrates that with supportive, gender-affirming care during childhood and adolescence, harms can be ameliorated and mental health and well-being outcomes can be significantly improved,” state the RCH guidelines.
But in 2019, RANZCP removed its endorsement of the RCH guidelines and started a consultation, which resulted in the new position statement.
However, Ken Pang, MD, of the Murdoch Children’s Research Institute in Melbourne and an author of the RCH guidelines, says the key recommendations of the new RANZCP position statement are consistent with their own guidelines.
The former note “the need for a skilled mental health clinician in providing comprehensive exploration of a child or adolescent’s biopsychosocial context,” Dr. Pang says.
However, it’s difficult not to see the contrast in stance when the new RANZCP statement maintains: “Research on gender dysphoria is still emerging. There are polarized views and mixed evidence regarding treatment options for people presenting with gender identity concerns, especially children and young people.”
Dr. Pang says the RCH guidelines do, however, recognize the need for further research in the field.
“I look forward to being able to incorporate such research, including from our own Trans20 study, into future revisions of our guidelines,” he told this news organization.
Watch your backs with affirmative therapy: Will there be a compromise?
Dr. Morris says there will obviously be cases where “the child might transition with a medical intervention, but that wouldn’t be the first step.”
And yet, he adds, “There are those who push the pro-trans view that everyone should be allowed to transition, and the doctors are only technicians that provide hormones with no questions asked.”
But from a doctor’s perspective, clinicians will still be held responsible in medical and legal terms for the treatments given, he stressed.
“I don’t think they will ever not be accountable for that. They will always need to determine in their own mind whether their actions have positive value that outweigh any disadvantages,” Dr. Morris continues.
The RANZCP statement does, in fact, stress just this.
All health care professionals need to “be aware of ethical and medicolegal dilemmas” pertaining to affirmative therapy, it indicates. “Psychiatrists should practice within the relevant laws and accepted professional standards in relation to assessing capacity and obtaining consent...”
Dr. Morris hopes there will ultimately be many more checks and balances in place and that courts and clinicians will need to step back and not assume every child who seeks to transition is doing it as a result of pure gender dysphoria.
He predicts that things will end in a compromise.
“In my view, this compromise will treat children with respect and approach them like any other patient that presents with a condition that requires proper assessment and treatment.”
“In the end, some cases will be transitioned, but there will be fewer than [are] transitioned at the moment,” he predicts.
Dr. Morris has reported no relevant financial relationships. Dr. Pang is a member of the Australian Professional Association for Trans Health and its research committee.
A version of this article first appeared on Medscape.com.
A new position statement from the Royal Australian and New Zealand College of Psychiatrists (RANZCP) stresses the importance of a mental health evaluation for people with gender dysphoria – in particular for children and adolescents – before any firm decisions are made on whether to prescribe hormonal treatments to transition, or perform surgeries, often referred to as “gender-affirming care.”
“There is a paucity of quality evidence on the outcomes of those presenting with gender dysphoria. In particular, there is a need for better evidence in relation to outcomes for children and young people,” the guidance states.
Because gender dysphoria “is associated with significant distress ... each case should be assessed by a mental health professional, which will frequently be a psychiatrist, with the person at the center of care. It is important the psychological state and context in which gender dysphoria has arisen is explored to assess the most appropriate treatment,” it adds.
The move by the psychiatry body represents a big shift in the landscape regarding recommendations for the treatment of gender dysphoria in Australia and New Zealand.
Asked to explain the new RANZCP position, Philip Morris, MBBS, FRANZCP, said: “The College acknowledged the complexity of the issues and the legitimacy of different approaches.”
Exploration of a patient’s reasons for identifying as transgender is essential, he said in an interview, especially when it comes to young people.
“There may be other reasons for doing it, and we need to look for those, identify them and treat them. This needs to be done before initiating hormones and changing the whole physical nature of the child,” he said.
“A cautious psychotherapy-first approach makes sense. If we can do that with adolescents, then we will take a big step in the right direction,” stressed Dr. Morris, who is president of the National Association of Practising Psychiatrists in Australia.
Keira Bell case and Scandinavian stance lead to more open discussion
The rapid rise in gender dysphoria among adolescents in the Western world, referred to as “rapid-onset” or “late-onset” gender dysphoria, has seen a huge increase in the number of natal girls presenting and created frenzied debate that has intensified worldwide in the last 12 months about how to best treat youth with gender dysphoria.
Concerns have arisen that some transgender identification is due to social contagion, and there is a growing number of “detransitioners” – people who identified as transgender, transitioned to the opposite gender, but then regretted their decision, changed their minds, and “detransitioned” back to their birth sex. If they have had hormone therapy, and in some cases surgery, they are left with irreversible changes to their bodies.
As a result, Scandinavian countries, most notably Finland, once eager advocates of the gender-affirmative approach, have pulled back and issued new treatment guidelines in 2020 stating that psychotherapy, rather than gender reassignment, should be the first line of treatment for gender-dysphoric youth.
This, along with a landmark High Court decision in the U.K. regarding the use of puberty-blocking drugs for children with gender dysphoria, brought by detransitioner Keira Bell, which was recently overturned by the Appeal Court, but which Ms. Bell now says she will take to the Supreme Court, has led to a considerable shift in the conversation around treating transgender adolescents with hormonal therapy, says Dr. Morris.
“This [has moved from] ... a topic that could previously not be talked about freely to one that we can discuss more openly now. This is a big improvement. Previously, everyone thought it was all settled, but it’s not, certainly not from a medical angle,” he states.
At odds with prior Australian recommendations
The RANZCP had previously endorsed the standard guidelines of the Royal Children’s Hospital (RCH) Melbourne, followed by most gender-identity services in Australia and similar guidance from New Zealand, which both recommend gender-affirming care.
“Increasing evidence demonstrates that with supportive, gender-affirming care during childhood and adolescence, harms can be ameliorated and mental health and well-being outcomes can be significantly improved,” state the RCH guidelines.
But in 2019, RANZCP removed its endorsement of the RCH guidelines and started a consultation, which resulted in the new position statement.
However, Ken Pang, MD, of the Murdoch Children’s Research Institute in Melbourne and an author of the RCH guidelines, says the key recommendations of the new RANZCP position statement are consistent with their own guidelines.
The former note “the need for a skilled mental health clinician in providing comprehensive exploration of a child or adolescent’s biopsychosocial context,” Dr. Pang says.
However, it’s difficult not to see the contrast in stance when the new RANZCP statement maintains: “Research on gender dysphoria is still emerging. There are polarized views and mixed evidence regarding treatment options for people presenting with gender identity concerns, especially children and young people.”
Dr. Pang says the RCH guidelines do, however, recognize the need for further research in the field.
“I look forward to being able to incorporate such research, including from our own Trans20 study, into future revisions of our guidelines,” he told this news organization.
Watch your backs with affirmative therapy: Will there be a compromise?
Dr. Morris says there will obviously be cases where “the child might transition with a medical intervention, but that wouldn’t be the first step.”
And yet, he adds, “There are those who push the pro-trans view that everyone should be allowed to transition, and the doctors are only technicians that provide hormones with no questions asked.”
But from a doctor’s perspective, clinicians will still be held responsible in medical and legal terms for the treatments given, he stressed.
“I don’t think they will ever not be accountable for that. They will always need to determine in their own mind whether their actions have positive value that outweigh any disadvantages,” Dr. Morris continues.
The RANZCP statement does, in fact, stress just this.
All health care professionals need to “be aware of ethical and medicolegal dilemmas” pertaining to affirmative therapy, it indicates. “Psychiatrists should practice within the relevant laws and accepted professional standards in relation to assessing capacity and obtaining consent...”
Dr. Morris hopes there will ultimately be many more checks and balances in place and that courts and clinicians will need to step back and not assume every child who seeks to transition is doing it as a result of pure gender dysphoria.
He predicts that things will end in a compromise.
“In my view, this compromise will treat children with respect and approach them like any other patient that presents with a condition that requires proper assessment and treatment.”
“In the end, some cases will be transitioned, but there will be fewer than [are] transitioned at the moment,” he predicts.
Dr. Morris has reported no relevant financial relationships. Dr. Pang is a member of the Australian Professional Association for Trans Health and its research committee.
A version of this article first appeared on Medscape.com.
Do you use intrapartum warm compresses to the perineum or perineal massage in your practice?
[polldaddy:10937454]
[polldaddy:10937454]
[polldaddy:10937454]
New nonhormonal therapies for hot flashes on the horizon
Hot flashes affect three out of four women and can last 7-10 years, but the current standard of care treatment isn’t necessarily appropriate for all women who experience vasomotor symptoms, according to Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health Clinic in Jacksonville, Fla.
For the majority of women under age 60 who are within 10 years of menopause, hormone therapy currently remains the most effective management option for hot flashes where the benefits outweigh the risks, Dr. Faubion told attendees Sept. 25 during a plenary at the annual meeting of the North American Menopause Society. “But really, individualizing treatment is the goal, and there are some women who are going to need some other options.”
Contraindications for hormone therapy include having a history of breast cancer, coronary heart disease, active liver disease, unexplained vaginal bleeding, high-risk endometrial cancer, transient ischemic attack, and a previous venous thromboembolic event or stroke.
“Fortunately, we have things in development,” Dr. Faubion said. She reviewed a wide range of therapies that are not currently Food and Drug Administration approved for vasomotor symptoms but are either available off label or are in clinical trials.
One of these is oxybutynin, an antimuscarinic, anticholinergic agent currently used to treat overactive bladder and overactive sweating. In a 2016 trial, 73% of women taking 15 mg extended-release oxybutynin once daily rated their symptoms as “much better,” compared with 26% who received placebo. The women experienced reduced frequency and severity of hot flashes and better sleep.
Subsequent research found a 60% reduction in hot flash frequency with 2.5 mg twice a day and a 77% reduction with 5 mg twice a day, compared with a 27% reduction with placebo. The only reported side effect that occurred more often with oxybutynin was dry mouth, but there were no significant differences in reasons for discontinuation between the treatment and placebo groups.
There are, however, some potential long-term cognitive effects from oxybutynin, Dr. Faubion said. Some research has shown an increased risk of dementia from oxybutynin and from an overall higher cumulative use of anticholinergics.
“There’s some concern about that for long-term use,” she said, but it’s effective, it’s “probably not harmful [when] used short term in women with significant, bothersome hot flashes who are unwilling or unable to use hormone therapy, and the adverse effects are tolerable for most women.” Women with bladder symptoms would be especially ideal candidates since the drug already treats those.
Dr. Faubion then discussed a new estrogen called estetrol (E4), a naturally occurring estrogen with selection action in tissues that is produced by the fetal liver and crosses the placenta. It has a long half-life of 28-32 hours, and its potential mechanism may give it a different safety profile than estradiol (E2). “There may be a lower risk of drug-drug interactions; lower breast stimulation, pain or carcinogenic impact; lower impact on triglycerides; and a neutral impact on markers of coagulation,” she said.
Though estetrol was recently approved as an oral contraceptive under the name Estelle, it’s also under investigation as a postmenopausal regimen. Preliminary findings suggest it reduces vasomotor symptom severity by 44%, compared with 30% with placebo, at 15 mg, the apparent minimum effective dose. The safety profile showed no endometrial hyperplasia and no unexpected adverse events. In those taking 15 mg of estetrol, mean endometrial thickness increased from 2 to 6 mm but returned to baseline after progestin therapy.
“The 15-mg dose also positively influenced markers of bone turnover, increased HDL [cholesterol], improved glucose tolerance,” and had no effects on coagulation parameters or triglycerides, Dr. Faubion added.
Another group of potential agents being studied for hot flashes are NK3 antagonists, which aim to exploit the recent discovery that kisspeptin, neurokinin B, and dynorphin (KNDy) neurons may play an important role in the etiology of vasomotor symptoms. Development of one of these, MLE 4901, was halted despite a 45% reduction in hot flashes because 3 of 28 women developed transiently elevated liver function tests, about four to six times the upper limit of normal.
Two others, fezolinetant and NT-814, are in phase 2 trials and have shown a significant reduction in symptoms, compared with placebo. The most commonly reported adverse effect in the phase 2a trial was gastrointestinal effects, but none of the participants stopped the drug because of these, and no elevated liver tests occurred. In the larger phase 2b trial, the most commonly reported treatment-emergent adverse events included nausea, diarrhea, fatigue, urinary tract infection, sinusitis, upper respiratory infection, headache, and cough. Five women discontinued the drug because of elevated liver enzymes.
“Overall, NK3 inhibitors appear to be generally well tolerated,” Dr. Faubion said. “There does seem to be mild transaminase elevation,” though it’s not yet known if this is an effect from this class of drugs as a whole. She noted that follicle-stimulating hormone does not significantly increase, which is important because elevated FSH is associated with poor bone health, nor does estradiol significantly increase, which is clinically relevant for women at high risk of breast cancer.
“We don’t know the effects on the heart, the brain, the bone, mood, weight, or sexual health, so there’s a lot that is still not known,” Dr. Faubion said. “We still don’t know about long-term safety and efficacy with these chemical compounds,” but clinical trials of them are ongoing.
They “would be a welcome alternative to hormone therapy for those who can’t or prefer not to use a hormonal option,” Dr. Faubion said. “However, we may need broad education of clinicians to caution against widespread abandonment of hormone therapy, particularly in women with premature or early menopause.”
Donna Klassen, LCSW, the cofounder of Let’s Talk Menopause, asked whether any of these new therapies were being tested in women with breast cancer and whether anything was known about taking oxybutynin at the same time as letrozole.
“I suspect that most women with chronic diseases would have been excluded from these initial studies, but I can’t speak to that,” Dr. Faubion said, and she wasn’t aware of any data related to taking oxybutynin and letrozole concurrently.
James Simon, MD, medical director and founder of IntimMedicine and one of those who led the research on oxybutynin, responded that his trials excluded breast cancer survivors and anyone taking aromatase inhibitors.
“It will be unlikely that, in the very near future, that data will be available because all the clinical developments on these NK3s or KNDy neuron-modulating drugs exclude cancer patients,” Dr. Simon said.
However, another attendee, Lisa Larkin, MD, of Cincinnati, introduced herself as a breast cancer survivor who takes tamoxifen and said she feels “completely comfortable” prescribing oxybutynin to breast cancer survivors.
“In terms of side effects and effectiveness in patients on tamoxifen and aromatase inhibitors, I’ve had incredibly good luck with it, and I think it’s underutilized,” Dr. Larkin said. “The clinical pearl I would tell you is you can start really low, and the dry mouth really seems to improve with time.” She added that patients should be informed that it takes 2 weeks before it begins working, but the side effects eventually go away. “It becomes very tolerable, so I just encourage all of you to consider it as another great option.”
Dr. Faubion had no disclosures. Disclosure information was unavailable for Dr. Simon, Dr. Larkin, and Ms. Klassen.
Hot flashes affect three out of four women and can last 7-10 years, but the current standard of care treatment isn’t necessarily appropriate for all women who experience vasomotor symptoms, according to Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health Clinic in Jacksonville, Fla.
For the majority of women under age 60 who are within 10 years of menopause, hormone therapy currently remains the most effective management option for hot flashes where the benefits outweigh the risks, Dr. Faubion told attendees Sept. 25 during a plenary at the annual meeting of the North American Menopause Society. “But really, individualizing treatment is the goal, and there are some women who are going to need some other options.”
Contraindications for hormone therapy include having a history of breast cancer, coronary heart disease, active liver disease, unexplained vaginal bleeding, high-risk endometrial cancer, transient ischemic attack, and a previous venous thromboembolic event or stroke.
“Fortunately, we have things in development,” Dr. Faubion said. She reviewed a wide range of therapies that are not currently Food and Drug Administration approved for vasomotor symptoms but are either available off label or are in clinical trials.
One of these is oxybutynin, an antimuscarinic, anticholinergic agent currently used to treat overactive bladder and overactive sweating. In a 2016 trial, 73% of women taking 15 mg extended-release oxybutynin once daily rated their symptoms as “much better,” compared with 26% who received placebo. The women experienced reduced frequency and severity of hot flashes and better sleep.
Subsequent research found a 60% reduction in hot flash frequency with 2.5 mg twice a day and a 77% reduction with 5 mg twice a day, compared with a 27% reduction with placebo. The only reported side effect that occurred more often with oxybutynin was dry mouth, but there were no significant differences in reasons for discontinuation between the treatment and placebo groups.
There are, however, some potential long-term cognitive effects from oxybutynin, Dr. Faubion said. Some research has shown an increased risk of dementia from oxybutynin and from an overall higher cumulative use of anticholinergics.
“There’s some concern about that for long-term use,” she said, but it’s effective, it’s “probably not harmful [when] used short term in women with significant, bothersome hot flashes who are unwilling or unable to use hormone therapy, and the adverse effects are tolerable for most women.” Women with bladder symptoms would be especially ideal candidates since the drug already treats those.
Dr. Faubion then discussed a new estrogen called estetrol (E4), a naturally occurring estrogen with selection action in tissues that is produced by the fetal liver and crosses the placenta. It has a long half-life of 28-32 hours, and its potential mechanism may give it a different safety profile than estradiol (E2). “There may be a lower risk of drug-drug interactions; lower breast stimulation, pain or carcinogenic impact; lower impact on triglycerides; and a neutral impact on markers of coagulation,” she said.
Though estetrol was recently approved as an oral contraceptive under the name Estelle, it’s also under investigation as a postmenopausal regimen. Preliminary findings suggest it reduces vasomotor symptom severity by 44%, compared with 30% with placebo, at 15 mg, the apparent minimum effective dose. The safety profile showed no endometrial hyperplasia and no unexpected adverse events. In those taking 15 mg of estetrol, mean endometrial thickness increased from 2 to 6 mm but returned to baseline after progestin therapy.
“The 15-mg dose also positively influenced markers of bone turnover, increased HDL [cholesterol], improved glucose tolerance,” and had no effects on coagulation parameters or triglycerides, Dr. Faubion added.
Another group of potential agents being studied for hot flashes are NK3 antagonists, which aim to exploit the recent discovery that kisspeptin, neurokinin B, and dynorphin (KNDy) neurons may play an important role in the etiology of vasomotor symptoms. Development of one of these, MLE 4901, was halted despite a 45% reduction in hot flashes because 3 of 28 women developed transiently elevated liver function tests, about four to six times the upper limit of normal.
Two others, fezolinetant and NT-814, are in phase 2 trials and have shown a significant reduction in symptoms, compared with placebo. The most commonly reported adverse effect in the phase 2a trial was gastrointestinal effects, but none of the participants stopped the drug because of these, and no elevated liver tests occurred. In the larger phase 2b trial, the most commonly reported treatment-emergent adverse events included nausea, diarrhea, fatigue, urinary tract infection, sinusitis, upper respiratory infection, headache, and cough. Five women discontinued the drug because of elevated liver enzymes.
“Overall, NK3 inhibitors appear to be generally well tolerated,” Dr. Faubion said. “There does seem to be mild transaminase elevation,” though it’s not yet known if this is an effect from this class of drugs as a whole. She noted that follicle-stimulating hormone does not significantly increase, which is important because elevated FSH is associated with poor bone health, nor does estradiol significantly increase, which is clinically relevant for women at high risk of breast cancer.
“We don’t know the effects on the heart, the brain, the bone, mood, weight, or sexual health, so there’s a lot that is still not known,” Dr. Faubion said. “We still don’t know about long-term safety and efficacy with these chemical compounds,” but clinical trials of them are ongoing.
They “would be a welcome alternative to hormone therapy for those who can’t or prefer not to use a hormonal option,” Dr. Faubion said. “However, we may need broad education of clinicians to caution against widespread abandonment of hormone therapy, particularly in women with premature or early menopause.”
Donna Klassen, LCSW, the cofounder of Let’s Talk Menopause, asked whether any of these new therapies were being tested in women with breast cancer and whether anything was known about taking oxybutynin at the same time as letrozole.
“I suspect that most women with chronic diseases would have been excluded from these initial studies, but I can’t speak to that,” Dr. Faubion said, and she wasn’t aware of any data related to taking oxybutynin and letrozole concurrently.
James Simon, MD, medical director and founder of IntimMedicine and one of those who led the research on oxybutynin, responded that his trials excluded breast cancer survivors and anyone taking aromatase inhibitors.
“It will be unlikely that, in the very near future, that data will be available because all the clinical developments on these NK3s or KNDy neuron-modulating drugs exclude cancer patients,” Dr. Simon said.
However, another attendee, Lisa Larkin, MD, of Cincinnati, introduced herself as a breast cancer survivor who takes tamoxifen and said she feels “completely comfortable” prescribing oxybutynin to breast cancer survivors.
“In terms of side effects and effectiveness in patients on tamoxifen and aromatase inhibitors, I’ve had incredibly good luck with it, and I think it’s underutilized,” Dr. Larkin said. “The clinical pearl I would tell you is you can start really low, and the dry mouth really seems to improve with time.” She added that patients should be informed that it takes 2 weeks before it begins working, but the side effects eventually go away. “It becomes very tolerable, so I just encourage all of you to consider it as another great option.”
Dr. Faubion had no disclosures. Disclosure information was unavailable for Dr. Simon, Dr. Larkin, and Ms. Klassen.
Hot flashes affect three out of four women and can last 7-10 years, but the current standard of care treatment isn’t necessarily appropriate for all women who experience vasomotor symptoms, according to Stephanie Faubion, MD, MBA, director of the Mayo Clinic Women’s Health Clinic in Jacksonville, Fla.
For the majority of women under age 60 who are within 10 years of menopause, hormone therapy currently remains the most effective management option for hot flashes where the benefits outweigh the risks, Dr. Faubion told attendees Sept. 25 during a plenary at the annual meeting of the North American Menopause Society. “But really, individualizing treatment is the goal, and there are some women who are going to need some other options.”
Contraindications for hormone therapy include having a history of breast cancer, coronary heart disease, active liver disease, unexplained vaginal bleeding, high-risk endometrial cancer, transient ischemic attack, and a previous venous thromboembolic event or stroke.
“Fortunately, we have things in development,” Dr. Faubion said. She reviewed a wide range of therapies that are not currently Food and Drug Administration approved for vasomotor symptoms but are either available off label or are in clinical trials.
One of these is oxybutynin, an antimuscarinic, anticholinergic agent currently used to treat overactive bladder and overactive sweating. In a 2016 trial, 73% of women taking 15 mg extended-release oxybutynin once daily rated their symptoms as “much better,” compared with 26% who received placebo. The women experienced reduced frequency and severity of hot flashes and better sleep.
Subsequent research found a 60% reduction in hot flash frequency with 2.5 mg twice a day and a 77% reduction with 5 mg twice a day, compared with a 27% reduction with placebo. The only reported side effect that occurred more often with oxybutynin was dry mouth, but there were no significant differences in reasons for discontinuation between the treatment and placebo groups.
There are, however, some potential long-term cognitive effects from oxybutynin, Dr. Faubion said. Some research has shown an increased risk of dementia from oxybutynin and from an overall higher cumulative use of anticholinergics.
“There’s some concern about that for long-term use,” she said, but it’s effective, it’s “probably not harmful [when] used short term in women with significant, bothersome hot flashes who are unwilling or unable to use hormone therapy, and the adverse effects are tolerable for most women.” Women with bladder symptoms would be especially ideal candidates since the drug already treats those.
Dr. Faubion then discussed a new estrogen called estetrol (E4), a naturally occurring estrogen with selection action in tissues that is produced by the fetal liver and crosses the placenta. It has a long half-life of 28-32 hours, and its potential mechanism may give it a different safety profile than estradiol (E2). “There may be a lower risk of drug-drug interactions; lower breast stimulation, pain or carcinogenic impact; lower impact on triglycerides; and a neutral impact on markers of coagulation,” she said.
Though estetrol was recently approved as an oral contraceptive under the name Estelle, it’s also under investigation as a postmenopausal regimen. Preliminary findings suggest it reduces vasomotor symptom severity by 44%, compared with 30% with placebo, at 15 mg, the apparent minimum effective dose. The safety profile showed no endometrial hyperplasia and no unexpected adverse events. In those taking 15 mg of estetrol, mean endometrial thickness increased from 2 to 6 mm but returned to baseline after progestin therapy.
“The 15-mg dose also positively influenced markers of bone turnover, increased HDL [cholesterol], improved glucose tolerance,” and had no effects on coagulation parameters or triglycerides, Dr. Faubion added.
Another group of potential agents being studied for hot flashes are NK3 antagonists, which aim to exploit the recent discovery that kisspeptin, neurokinin B, and dynorphin (KNDy) neurons may play an important role in the etiology of vasomotor symptoms. Development of one of these, MLE 4901, was halted despite a 45% reduction in hot flashes because 3 of 28 women developed transiently elevated liver function tests, about four to six times the upper limit of normal.
Two others, fezolinetant and NT-814, are in phase 2 trials and have shown a significant reduction in symptoms, compared with placebo. The most commonly reported adverse effect in the phase 2a trial was gastrointestinal effects, but none of the participants stopped the drug because of these, and no elevated liver tests occurred. In the larger phase 2b trial, the most commonly reported treatment-emergent adverse events included nausea, diarrhea, fatigue, urinary tract infection, sinusitis, upper respiratory infection, headache, and cough. Five women discontinued the drug because of elevated liver enzymes.
“Overall, NK3 inhibitors appear to be generally well tolerated,” Dr. Faubion said. “There does seem to be mild transaminase elevation,” though it’s not yet known if this is an effect from this class of drugs as a whole. She noted that follicle-stimulating hormone does not significantly increase, which is important because elevated FSH is associated with poor bone health, nor does estradiol significantly increase, which is clinically relevant for women at high risk of breast cancer.
“We don’t know the effects on the heart, the brain, the bone, mood, weight, or sexual health, so there’s a lot that is still not known,” Dr. Faubion said. “We still don’t know about long-term safety and efficacy with these chemical compounds,” but clinical trials of them are ongoing.
They “would be a welcome alternative to hormone therapy for those who can’t or prefer not to use a hormonal option,” Dr. Faubion said. “However, we may need broad education of clinicians to caution against widespread abandonment of hormone therapy, particularly in women with premature or early menopause.”
Donna Klassen, LCSW, the cofounder of Let’s Talk Menopause, asked whether any of these new therapies were being tested in women with breast cancer and whether anything was known about taking oxybutynin at the same time as letrozole.
“I suspect that most women with chronic diseases would have been excluded from these initial studies, but I can’t speak to that,” Dr. Faubion said, and she wasn’t aware of any data related to taking oxybutynin and letrozole concurrently.
James Simon, MD, medical director and founder of IntimMedicine and one of those who led the research on oxybutynin, responded that his trials excluded breast cancer survivors and anyone taking aromatase inhibitors.
“It will be unlikely that, in the very near future, that data will be available because all the clinical developments on these NK3s or KNDy neuron-modulating drugs exclude cancer patients,” Dr. Simon said.
However, another attendee, Lisa Larkin, MD, of Cincinnati, introduced herself as a breast cancer survivor who takes tamoxifen and said she feels “completely comfortable” prescribing oxybutynin to breast cancer survivors.
“In terms of side effects and effectiveness in patients on tamoxifen and aromatase inhibitors, I’ve had incredibly good luck with it, and I think it’s underutilized,” Dr. Larkin said. “The clinical pearl I would tell you is you can start really low, and the dry mouth really seems to improve with time.” She added that patients should be informed that it takes 2 weeks before it begins working, but the side effects eventually go away. “It becomes very tolerable, so I just encourage all of you to consider it as another great option.”
Dr. Faubion had no disclosures. Disclosure information was unavailable for Dr. Simon, Dr. Larkin, and Ms. Klassen.
FROM NAMS 2021
Major insurers running billions of dollars behind on payments to hospitals and doctors
Anthem Blue Cross, the country’s second-biggest health insurance company, is behind on billions of dollars in payments owed to hospitals and doctors because of onerous new reimbursement rules, computer problems and mishandled claims, say hospital officials in multiple states.
Anthem, like other big insurers, is using the COVID-19 crisis as cover to institute “egregious” policies that harm patients and pinch hospital finances, said Molly Smith, group vice president at the American Hospital Association. “There’s this sense of ‘Everyone’s distracted. We can get this through.’ ”
Hospitals are also dealing with a spike in retroactive claims denials by UnitedHealthcare, the biggest health insurer, for ED care, the AHA said.
Hospitals say it is hurting their finances as many cope with COVID surges – even after the industry has received tens of billions of dollars in emergency assistance from the federal government.
“We recognize there have been some challenges” to prompt payments caused by claims-processing changes and “a new set of dynamics” amid the pandemic, Anthem spokesperson Colin Manning said in an email. “We apologize for any delays or inconvenience this may have caused.”
Virginia law requires insurers to pay claims within 40 days. In a Sept. 24 letter to state insurance regulators, VCU Health, a system that operates a large teaching hospital in Richmond associated with Virginia Commonwealth University, said Anthem owes it $385 million. More than 40% of the claims are more than 90 days old, VCU said.
For all Virginia hospitals, Anthem’s late, unpaid claims amount to “hundreds of millions of dollars,” the Virginia Hospital and Healthcare Association said in a June 23 letter to state regulators.
Nationwide, the payment delays “are creating an untenable situation,” the American Hospital Association said in a Sept. 9 letter to Anthem CEO Gail Boudreaux. “Patients are facing greater hurdles to accessing care; clinicians are burning out on unnecessary administrative tasks; and the system is straining to finance the personnel and supplies” needed to fight Covid.
Complaints about Anthem extend “from sea to shining sea, from New Hampshire to California,” AHA CEO Rick Pollack told KHN.
Substantial payment delays can be seen on Anthem’s books. On June 30, 2019, before the pandemic, 43% of the insurer’s medical bills for that quarter were unpaid, according to regulatory filings. Two years later that figure had risen to 53% – a difference of $2.5 billion.
Anthem profits were $4.6 billion in 2020 and $3.5 billion in the first half of 2021.
Alexis Thurber, who lives near Seattle, was insured by Anthem when she got an $18,192 hospital bill in May for radiation therapy that doctors said was essential to treat her breast cancer.
The treatments were “experimental” and “not medically necessary,” Anthem said, according to Ms. Thurber. She spent much of the summer trying to get the insurer to pay up – placing two dozen phone calls, spending hours on hold, sending multiple emails and enduring unmeasurable stress and worry. It finally covered the claim months later.
“It’s so egregious. It’s a game they’re playing,” said Ms. Thurber, 51, whose cancer was diagnosed in November. “Trying to get true help was impossible.”
Privacy rules prevent Anthem from commenting on Ms. Thurber’s case, said Anthem spokesperson Colin Manning.
When insurers fail to promptly pay medical bills, patients are left in the lurch. They might first get a notice saying payment is pending or denied. A hospital might bill them for treatment they thought would be covered. Hospitals and doctors often sue patients whose insurance didn’t pay up.
Hospitals point to a variety of Anthem practices contributing to payment delays or denials, including new layers of document requirements, prior-authorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers. “This requires providers to literally leave the patient[’s] bedside to get on the phone with Anthem,” AHA said in its letter.
Anthem often hinders coverage for outpatient surgery, specialty pharmacy and other services in health systems listed as in network, amounting to a “bait and switch” on Anthem members, AHA officials said.
“Demanding that patients be treated outside of the hospital setting, against the advice of the patient’s in-network treating physician, appears to be motivated by a desire to drive up Empire’s profits,” the Greater New York Hospital Association wrote in an April letter to Empire Blue Cross, which is owned by Anthem.
Anthem officials pushed back in a recent letter to the AHA, saying the insurer’s changing rules are intended partly to control excessive prices charged by hospitals for specialty drugs and nonemergency surgery, screening and diagnostic procedures.
Severe problems with Anthem’s new claims management system surfaced months ago and “persist without meaningful improvement,” AHA said in its letter.
Claims have gotten lost in Anthem’s computers, and in some cases VCU Health has had to print medical records and mail them to get paid, VCU said in its letter. The cash slowdown imposes “an unmanageable disruption that threatens to undermine our financial footing,” VCU said.
United denied $31,557 in claims for Emily Long’s care after she was struck in June by a motorcycle in New York City. She needed surgery to repair a fractured cheekbone. United said there was a lack of documentation for “medical necessity” – an “incredibly aggravating” response on top of the distress of the accident, Ms. Long said.
The Brooklyn hospital that treated Ms. Long was “paid appropriately under her plan and within the required time frame,” said United spokesperson Maria Gordon Shydlo. “The facility has the right to appeal the decision.”
United’s unpaid claims came to 54% as of June 30, about the same level as 2 years previously.
When Erin Conlisk initially had trouble gaining approval for a piece of medical equipment for her elderly father this summer, United employees told her the insurer’s entire prior-authorization database had gone down for weeks, said Ms. Conlisk, who lives in California.
“There was a brief issue with our prior-authorization process in mid-July, which was resolved quickly,” Gordon Shydlo said.
When asked by Wall Street analysts about the payment backups, Anthem executives said it partly reflects their decision to increase financial reserves amid the health crisis.
“Really a ton of uncertainty associated with this environment,” John Gallina, the company’s chief financial officer, said on a conference call in July. “We’ve tried to be extremely prudent and conservative in our approach.”
During the pandemic, hospitals have benefited from two extraordinary cash infusions. They and other medical providers have received more than $100 billion through the CARES Act of 2020 and the American Rescue Plan of 2021. Last year United, Anthem and other insurers accelerated billions in hospital reimbursements.
The federal payments enriched many of the biggest, wealthiest systems while poorer hospitals serving low-income patients and rural areas struggled.
Those are the systems most hurt now by insurer payment delays, hospital officials said. Federal relief funds “have been a lifeline, but they don’t make people whole in terms of the losses from increased expenses and lost revenue as a result of the COVID experience,” Mr. Pollack said.
Several health systems declined to comment about claims payment delays or didn’t respond to a reporter’s queries. Among individual hospitals “there is a deep fear of talking on the record about your largest business partner,” AHA’s Ms. Smith said.
Alexis Thurber worried she might have to pay her $18,192 radiation bill herself, and she’s not confident her Anthem policy will do a better job next time of covering the cost of her care.
“It makes me not want to go to the doctor anymore,” she said. “I’m scared to get another mammogram because you can’t rely on it.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Anthem Blue Cross, the country’s second-biggest health insurance company, is behind on billions of dollars in payments owed to hospitals and doctors because of onerous new reimbursement rules, computer problems and mishandled claims, say hospital officials in multiple states.
Anthem, like other big insurers, is using the COVID-19 crisis as cover to institute “egregious” policies that harm patients and pinch hospital finances, said Molly Smith, group vice president at the American Hospital Association. “There’s this sense of ‘Everyone’s distracted. We can get this through.’ ”
Hospitals are also dealing with a spike in retroactive claims denials by UnitedHealthcare, the biggest health insurer, for ED care, the AHA said.
Hospitals say it is hurting their finances as many cope with COVID surges – even after the industry has received tens of billions of dollars in emergency assistance from the federal government.
“We recognize there have been some challenges” to prompt payments caused by claims-processing changes and “a new set of dynamics” amid the pandemic, Anthem spokesperson Colin Manning said in an email. “We apologize for any delays or inconvenience this may have caused.”
Virginia law requires insurers to pay claims within 40 days. In a Sept. 24 letter to state insurance regulators, VCU Health, a system that operates a large teaching hospital in Richmond associated with Virginia Commonwealth University, said Anthem owes it $385 million. More than 40% of the claims are more than 90 days old, VCU said.
For all Virginia hospitals, Anthem’s late, unpaid claims amount to “hundreds of millions of dollars,” the Virginia Hospital and Healthcare Association said in a June 23 letter to state regulators.
Nationwide, the payment delays “are creating an untenable situation,” the American Hospital Association said in a Sept. 9 letter to Anthem CEO Gail Boudreaux. “Patients are facing greater hurdles to accessing care; clinicians are burning out on unnecessary administrative tasks; and the system is straining to finance the personnel and supplies” needed to fight Covid.
Complaints about Anthem extend “from sea to shining sea, from New Hampshire to California,” AHA CEO Rick Pollack told KHN.
Substantial payment delays can be seen on Anthem’s books. On June 30, 2019, before the pandemic, 43% of the insurer’s medical bills for that quarter were unpaid, according to regulatory filings. Two years later that figure had risen to 53% – a difference of $2.5 billion.
Anthem profits were $4.6 billion in 2020 and $3.5 billion in the first half of 2021.
Alexis Thurber, who lives near Seattle, was insured by Anthem when she got an $18,192 hospital bill in May for radiation therapy that doctors said was essential to treat her breast cancer.
The treatments were “experimental” and “not medically necessary,” Anthem said, according to Ms. Thurber. She spent much of the summer trying to get the insurer to pay up – placing two dozen phone calls, spending hours on hold, sending multiple emails and enduring unmeasurable stress and worry. It finally covered the claim months later.
“It’s so egregious. It’s a game they’re playing,” said Ms. Thurber, 51, whose cancer was diagnosed in November. “Trying to get true help was impossible.”
Privacy rules prevent Anthem from commenting on Ms. Thurber’s case, said Anthem spokesperson Colin Manning.
When insurers fail to promptly pay medical bills, patients are left in the lurch. They might first get a notice saying payment is pending or denied. A hospital might bill them for treatment they thought would be covered. Hospitals and doctors often sue patients whose insurance didn’t pay up.
Hospitals point to a variety of Anthem practices contributing to payment delays or denials, including new layers of document requirements, prior-authorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers. “This requires providers to literally leave the patient[’s] bedside to get on the phone with Anthem,” AHA said in its letter.
Anthem often hinders coverage for outpatient surgery, specialty pharmacy and other services in health systems listed as in network, amounting to a “bait and switch” on Anthem members, AHA officials said.
“Demanding that patients be treated outside of the hospital setting, against the advice of the patient’s in-network treating physician, appears to be motivated by a desire to drive up Empire’s profits,” the Greater New York Hospital Association wrote in an April letter to Empire Blue Cross, which is owned by Anthem.
Anthem officials pushed back in a recent letter to the AHA, saying the insurer’s changing rules are intended partly to control excessive prices charged by hospitals for specialty drugs and nonemergency surgery, screening and diagnostic procedures.
Severe problems with Anthem’s new claims management system surfaced months ago and “persist without meaningful improvement,” AHA said in its letter.
Claims have gotten lost in Anthem’s computers, and in some cases VCU Health has had to print medical records and mail them to get paid, VCU said in its letter. The cash slowdown imposes “an unmanageable disruption that threatens to undermine our financial footing,” VCU said.
United denied $31,557 in claims for Emily Long’s care after she was struck in June by a motorcycle in New York City. She needed surgery to repair a fractured cheekbone. United said there was a lack of documentation for “medical necessity” – an “incredibly aggravating” response on top of the distress of the accident, Ms. Long said.
The Brooklyn hospital that treated Ms. Long was “paid appropriately under her plan and within the required time frame,” said United spokesperson Maria Gordon Shydlo. “The facility has the right to appeal the decision.”
United’s unpaid claims came to 54% as of June 30, about the same level as 2 years previously.
When Erin Conlisk initially had trouble gaining approval for a piece of medical equipment for her elderly father this summer, United employees told her the insurer’s entire prior-authorization database had gone down for weeks, said Ms. Conlisk, who lives in California.
“There was a brief issue with our prior-authorization process in mid-July, which was resolved quickly,” Gordon Shydlo said.
When asked by Wall Street analysts about the payment backups, Anthem executives said it partly reflects their decision to increase financial reserves amid the health crisis.
“Really a ton of uncertainty associated with this environment,” John Gallina, the company’s chief financial officer, said on a conference call in July. “We’ve tried to be extremely prudent and conservative in our approach.”
During the pandemic, hospitals have benefited from two extraordinary cash infusions. They and other medical providers have received more than $100 billion through the CARES Act of 2020 and the American Rescue Plan of 2021. Last year United, Anthem and other insurers accelerated billions in hospital reimbursements.
The federal payments enriched many of the biggest, wealthiest systems while poorer hospitals serving low-income patients and rural areas struggled.
Those are the systems most hurt now by insurer payment delays, hospital officials said. Federal relief funds “have been a lifeline, but they don’t make people whole in terms of the losses from increased expenses and lost revenue as a result of the COVID experience,” Mr. Pollack said.
Several health systems declined to comment about claims payment delays or didn’t respond to a reporter’s queries. Among individual hospitals “there is a deep fear of talking on the record about your largest business partner,” AHA’s Ms. Smith said.
Alexis Thurber worried she might have to pay her $18,192 radiation bill herself, and she’s not confident her Anthem policy will do a better job next time of covering the cost of her care.
“It makes me not want to go to the doctor anymore,” she said. “I’m scared to get another mammogram because you can’t rely on it.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Anthem Blue Cross, the country’s second-biggest health insurance company, is behind on billions of dollars in payments owed to hospitals and doctors because of onerous new reimbursement rules, computer problems and mishandled claims, say hospital officials in multiple states.
Anthem, like other big insurers, is using the COVID-19 crisis as cover to institute “egregious” policies that harm patients and pinch hospital finances, said Molly Smith, group vice president at the American Hospital Association. “There’s this sense of ‘Everyone’s distracted. We can get this through.’ ”
Hospitals are also dealing with a spike in retroactive claims denials by UnitedHealthcare, the biggest health insurer, for ED care, the AHA said.
Hospitals say it is hurting their finances as many cope with COVID surges – even after the industry has received tens of billions of dollars in emergency assistance from the federal government.
“We recognize there have been some challenges” to prompt payments caused by claims-processing changes and “a new set of dynamics” amid the pandemic, Anthem spokesperson Colin Manning said in an email. “We apologize for any delays or inconvenience this may have caused.”
Virginia law requires insurers to pay claims within 40 days. In a Sept. 24 letter to state insurance regulators, VCU Health, a system that operates a large teaching hospital in Richmond associated with Virginia Commonwealth University, said Anthem owes it $385 million. More than 40% of the claims are more than 90 days old, VCU said.
For all Virginia hospitals, Anthem’s late, unpaid claims amount to “hundreds of millions of dollars,” the Virginia Hospital and Healthcare Association said in a June 23 letter to state regulators.
Nationwide, the payment delays “are creating an untenable situation,” the American Hospital Association said in a Sept. 9 letter to Anthem CEO Gail Boudreaux. “Patients are facing greater hurdles to accessing care; clinicians are burning out on unnecessary administrative tasks; and the system is straining to finance the personnel and supplies” needed to fight Covid.
Complaints about Anthem extend “from sea to shining sea, from New Hampshire to California,” AHA CEO Rick Pollack told KHN.
Substantial payment delays can be seen on Anthem’s books. On June 30, 2019, before the pandemic, 43% of the insurer’s medical bills for that quarter were unpaid, according to regulatory filings. Two years later that figure had risen to 53% – a difference of $2.5 billion.
Anthem profits were $4.6 billion in 2020 and $3.5 billion in the first half of 2021.
Alexis Thurber, who lives near Seattle, was insured by Anthem when she got an $18,192 hospital bill in May for radiation therapy that doctors said was essential to treat her breast cancer.
The treatments were “experimental” and “not medically necessary,” Anthem said, according to Ms. Thurber. She spent much of the summer trying to get the insurer to pay up – placing two dozen phone calls, spending hours on hold, sending multiple emails and enduring unmeasurable stress and worry. It finally covered the claim months later.
“It’s so egregious. It’s a game they’re playing,” said Ms. Thurber, 51, whose cancer was diagnosed in November. “Trying to get true help was impossible.”
Privacy rules prevent Anthem from commenting on Ms. Thurber’s case, said Anthem spokesperson Colin Manning.
When insurers fail to promptly pay medical bills, patients are left in the lurch. They might first get a notice saying payment is pending or denied. A hospital might bill them for treatment they thought would be covered. Hospitals and doctors often sue patients whose insurance didn’t pay up.
Hospitals point to a variety of Anthem practices contributing to payment delays or denials, including new layers of document requirements, prior-authorization hurdles for routine procedures and requirements that doctors themselves – not support staffers – speak to insurance gatekeepers. “This requires providers to literally leave the patient[’s] bedside to get on the phone with Anthem,” AHA said in its letter.
Anthem often hinders coverage for outpatient surgery, specialty pharmacy and other services in health systems listed as in network, amounting to a “bait and switch” on Anthem members, AHA officials said.
“Demanding that patients be treated outside of the hospital setting, against the advice of the patient’s in-network treating physician, appears to be motivated by a desire to drive up Empire’s profits,” the Greater New York Hospital Association wrote in an April letter to Empire Blue Cross, which is owned by Anthem.
Anthem officials pushed back in a recent letter to the AHA, saying the insurer’s changing rules are intended partly to control excessive prices charged by hospitals for specialty drugs and nonemergency surgery, screening and diagnostic procedures.
Severe problems with Anthem’s new claims management system surfaced months ago and “persist without meaningful improvement,” AHA said in its letter.
Claims have gotten lost in Anthem’s computers, and in some cases VCU Health has had to print medical records and mail them to get paid, VCU said in its letter. The cash slowdown imposes “an unmanageable disruption that threatens to undermine our financial footing,” VCU said.
United denied $31,557 in claims for Emily Long’s care after she was struck in June by a motorcycle in New York City. She needed surgery to repair a fractured cheekbone. United said there was a lack of documentation for “medical necessity” – an “incredibly aggravating” response on top of the distress of the accident, Ms. Long said.
The Brooklyn hospital that treated Ms. Long was “paid appropriately under her plan and within the required time frame,” said United spokesperson Maria Gordon Shydlo. “The facility has the right to appeal the decision.”
United’s unpaid claims came to 54% as of June 30, about the same level as 2 years previously.
When Erin Conlisk initially had trouble gaining approval for a piece of medical equipment for her elderly father this summer, United employees told her the insurer’s entire prior-authorization database had gone down for weeks, said Ms. Conlisk, who lives in California.
“There was a brief issue with our prior-authorization process in mid-July, which was resolved quickly,” Gordon Shydlo said.
When asked by Wall Street analysts about the payment backups, Anthem executives said it partly reflects their decision to increase financial reserves amid the health crisis.
“Really a ton of uncertainty associated with this environment,” John Gallina, the company’s chief financial officer, said on a conference call in July. “We’ve tried to be extremely prudent and conservative in our approach.”
During the pandemic, hospitals have benefited from two extraordinary cash infusions. They and other medical providers have received more than $100 billion through the CARES Act of 2020 and the American Rescue Plan of 2021. Last year United, Anthem and other insurers accelerated billions in hospital reimbursements.
The federal payments enriched many of the biggest, wealthiest systems while poorer hospitals serving low-income patients and rural areas struggled.
Those are the systems most hurt now by insurer payment delays, hospital officials said. Federal relief funds “have been a lifeline, but they don’t make people whole in terms of the losses from increased expenses and lost revenue as a result of the COVID experience,” Mr. Pollack said.
Several health systems declined to comment about claims payment delays or didn’t respond to a reporter’s queries. Among individual hospitals “there is a deep fear of talking on the record about your largest business partner,” AHA’s Ms. Smith said.
Alexis Thurber worried she might have to pay her $18,192 radiation bill herself, and she’s not confident her Anthem policy will do a better job next time of covering the cost of her care.
“It makes me not want to go to the doctor anymore,” she said. “I’m scared to get another mammogram because you can’t rely on it.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Old wives’ tales, traditional medicine, and science
Sixteen-year-old Ana and is sitting on the bench with her science teacher, Ms. Tehrani, waiting for the bus to take them back to their village after school. Ana wants to hear her science teacher’s opinion about her grandmother.
Do you respect your grandmother?
Why yes, of course, why to do you ask?
So you think my grandmother is wise when she tells me old wife tales?
Like what?
Well, she says not to take my medicine because it will have bad effects and that I should take her remedies instead.
What else does she tell you?
Well, she says that people are born how they are and that they belong to either God or the Devil, not to their parents.
What else?
She thinks I am a fay child; she has always said that about me.
What does that mean?
It means that I have my own ways, fairy ways, and that I should go out in the forest and listen.
Do you?
Yes.
What do you hear?
I hear about my destiny.
What do you hear?
I hear that I must wash in witch hazel. My grandmother taught me how to find it and how to prepare it. She said I should sit in the forest and wait for a sign.
What sign?
I don’t know.
Well, what do you think about your grandmother?
I love her but …
But what?
I think she might be wrong about all of this, you know, science and all that.
But you do it, anyway?
Yes.
Why?
Aren’t we supposed to respect our elders, and aren’t they supposed to be wise?
Ms. Tehrani is in a bind. What to say? She has no ready answer, feeling caught between two beliefs: the unscientific basis of ineffective old wives’ treatments and the purported wisdom of our elders. She knows Ana’s family and that there are women in that family going back generations who are identified as medicine women or women with the special powers of the forest.
Ana wants to study science but she is being groomed as the family wise mother. Ana is caught between the ways of the past and the ways of the future. She sees that to go with the future is to devalue her family tradition. If she chooses to study medicine, can she keep the balance between magical ways and the ways of science?
Ms. Tehrani decides to expose her class to Indigenous and preindustrial cultural practices and what science has to say. She describes how knowledge is passed down through the generations, and how some of this knowledge has now been proved correct by science, such as the use of opium for pain management and how some knowledge has been corrected by science. She asks the class: What myths have been passed down in your family that science has shown to be effective or ineffective? What does science have to say about how we live our lives?
After a baby in the village dies, Ms. Tehrani asks the local health center to think about implementing a teaching course on caring for babies, a course that will discuss tradition and science. She is well aware of the fact that Black mothers tend not to follow the advice of the pediatricians who now recommend that parents put babies to sleep on their backs. Black women trust the advice of their paternal and maternal grandmothers more than the advice of health care providers, research by Deborah Stiffler, PhD, RN, CNM, shows (J Spec Pediatr Nurs. 2018 Apr;23[2]:e12213). While new Black mothers feel that they have limited knowledge and are eager to learn about safe sleep practices, their grandmothers were skeptical – and the grandmothers often won that argument. Black mothers believed that their own mothers knew best, based on their experience raising infants.
In Dr. Stiffler’s study, one grandmother commented: “Girls today need a mother to help them take care of their babies. They don’t know how to do anything. When I was growing up, our moms helped us.”
One new mother said: I “listen more to the elderly people because like the social workers and stuff some of them don’t have kids. They just go by the book … so I feel like I listen more to like my grandparents.”
Integrating traditions
When Ana enters medical school she is faced with the task of integration of traditional practice and Western medicine. Ana looks to the National Center for Complementary and Integrative Health (NCCIH), the U.S. government’s lead agency for scientific research on complementary and integrative health approaches for support in her task. The NCCIH was established in 1998 with the mission of determining the usefulness and safety of complementary and integrative health approaches, and their roles in improving health and health care.
The NCCIH notes that more than 30% of adults use health care approaches that are not part of conventional medical care or that have origins outside of usual Western practice, and 17.7% of American adults had used a dietary supplement other than vitamins and minerals in the past year, most commonly fish oil. This agency notes that large rigorous research studies extend to only a few dietary supplements, with results showing that the products didn’t work for the conditions studied. The work of the NCCIH is mirrored worldwide.
The 2008 Beijing Declaration called on World Health Organization member states and other stakeholders to integrate traditional medicine and complementary alternative medicines into national health care systems. The WHO Congress on Traditional Medicine recognizes that traditional medicine (TM) may be more affordable and accessible than Western medicine, and that it plays an important role in meeting the demands of primary health care in many developing countries. From 70% to 80% of the population in India and Ethiopia depend on TM for primary health care, and 70% of the population in Canada and 80% in Germany are reported to have used TM as complementary and/or alternative medical treatment.
After graduation and residency, Ana returns to her village and helps her science teacher consider how best to shape the intergenerational transmission of knowledge, so that it is both honored by the elders and also shaped by the science of medicine.
Every village, regardless of where it is in the world, has to contend with finding the balance between the traditional medical knowledge that is passed down through the family and the discoveries of science. When it comes to practicing medicine and psychiatry, a respect for family tradition must be weighed against the application of science: this is a long conversation that is well worth its time.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). Dr. Heru has no conflicts of interest. Contact Dr. Heru at [email protected].
Sixteen-year-old Ana and is sitting on the bench with her science teacher, Ms. Tehrani, waiting for the bus to take them back to their village after school. Ana wants to hear her science teacher’s opinion about her grandmother.
Do you respect your grandmother?
Why yes, of course, why to do you ask?
So you think my grandmother is wise when she tells me old wife tales?
Like what?
Well, she says not to take my medicine because it will have bad effects and that I should take her remedies instead.
What else does she tell you?
Well, she says that people are born how they are and that they belong to either God or the Devil, not to their parents.
What else?
She thinks I am a fay child; she has always said that about me.
What does that mean?
It means that I have my own ways, fairy ways, and that I should go out in the forest and listen.
Do you?
Yes.
What do you hear?
I hear about my destiny.
What do you hear?
I hear that I must wash in witch hazel. My grandmother taught me how to find it and how to prepare it. She said I should sit in the forest and wait for a sign.
What sign?
I don’t know.
Well, what do you think about your grandmother?
I love her but …
But what?
I think she might be wrong about all of this, you know, science and all that.
But you do it, anyway?
Yes.
Why?
Aren’t we supposed to respect our elders, and aren’t they supposed to be wise?
Ms. Tehrani is in a bind. What to say? She has no ready answer, feeling caught between two beliefs: the unscientific basis of ineffective old wives’ treatments and the purported wisdom of our elders. She knows Ana’s family and that there are women in that family going back generations who are identified as medicine women or women with the special powers of the forest.
Ana wants to study science but she is being groomed as the family wise mother. Ana is caught between the ways of the past and the ways of the future. She sees that to go with the future is to devalue her family tradition. If she chooses to study medicine, can she keep the balance between magical ways and the ways of science?
Ms. Tehrani decides to expose her class to Indigenous and preindustrial cultural practices and what science has to say. She describes how knowledge is passed down through the generations, and how some of this knowledge has now been proved correct by science, such as the use of opium for pain management and how some knowledge has been corrected by science. She asks the class: What myths have been passed down in your family that science has shown to be effective or ineffective? What does science have to say about how we live our lives?
After a baby in the village dies, Ms. Tehrani asks the local health center to think about implementing a teaching course on caring for babies, a course that will discuss tradition and science. She is well aware of the fact that Black mothers tend not to follow the advice of the pediatricians who now recommend that parents put babies to sleep on their backs. Black women trust the advice of their paternal and maternal grandmothers more than the advice of health care providers, research by Deborah Stiffler, PhD, RN, CNM, shows (J Spec Pediatr Nurs. 2018 Apr;23[2]:e12213). While new Black mothers feel that they have limited knowledge and are eager to learn about safe sleep practices, their grandmothers were skeptical – and the grandmothers often won that argument. Black mothers believed that their own mothers knew best, based on their experience raising infants.
In Dr. Stiffler’s study, one grandmother commented: “Girls today need a mother to help them take care of their babies. They don’t know how to do anything. When I was growing up, our moms helped us.”
One new mother said: I “listen more to the elderly people because like the social workers and stuff some of them don’t have kids. They just go by the book … so I feel like I listen more to like my grandparents.”
Integrating traditions
When Ana enters medical school she is faced with the task of integration of traditional practice and Western medicine. Ana looks to the National Center for Complementary and Integrative Health (NCCIH), the U.S. government’s lead agency for scientific research on complementary and integrative health approaches for support in her task. The NCCIH was established in 1998 with the mission of determining the usefulness and safety of complementary and integrative health approaches, and their roles in improving health and health care.
The NCCIH notes that more than 30% of adults use health care approaches that are not part of conventional medical care or that have origins outside of usual Western practice, and 17.7% of American adults had used a dietary supplement other than vitamins and minerals in the past year, most commonly fish oil. This agency notes that large rigorous research studies extend to only a few dietary supplements, with results showing that the products didn’t work for the conditions studied. The work of the NCCIH is mirrored worldwide.
The 2008 Beijing Declaration called on World Health Organization member states and other stakeholders to integrate traditional medicine and complementary alternative medicines into national health care systems. The WHO Congress on Traditional Medicine recognizes that traditional medicine (TM) may be more affordable and accessible than Western medicine, and that it plays an important role in meeting the demands of primary health care in many developing countries. From 70% to 80% of the population in India and Ethiopia depend on TM for primary health care, and 70% of the population in Canada and 80% in Germany are reported to have used TM as complementary and/or alternative medical treatment.
After graduation and residency, Ana returns to her village and helps her science teacher consider how best to shape the intergenerational transmission of knowledge, so that it is both honored by the elders and also shaped by the science of medicine.
Every village, regardless of where it is in the world, has to contend with finding the balance between the traditional medical knowledge that is passed down through the family and the discoveries of science. When it comes to practicing medicine and psychiatry, a respect for family tradition must be weighed against the application of science: this is a long conversation that is well worth its time.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). Dr. Heru has no conflicts of interest. Contact Dr. Heru at [email protected].
Sixteen-year-old Ana and is sitting on the bench with her science teacher, Ms. Tehrani, waiting for the bus to take them back to their village after school. Ana wants to hear her science teacher’s opinion about her grandmother.
Do you respect your grandmother?
Why yes, of course, why to do you ask?
So you think my grandmother is wise when she tells me old wife tales?
Like what?
Well, she says not to take my medicine because it will have bad effects and that I should take her remedies instead.
What else does she tell you?
Well, she says that people are born how they are and that they belong to either God or the Devil, not to their parents.
What else?
She thinks I am a fay child; she has always said that about me.
What does that mean?
It means that I have my own ways, fairy ways, and that I should go out in the forest and listen.
Do you?
Yes.
What do you hear?
I hear about my destiny.
What do you hear?
I hear that I must wash in witch hazel. My grandmother taught me how to find it and how to prepare it. She said I should sit in the forest and wait for a sign.
What sign?
I don’t know.
Well, what do you think about your grandmother?
I love her but …
But what?
I think she might be wrong about all of this, you know, science and all that.
But you do it, anyway?
Yes.
Why?
Aren’t we supposed to respect our elders, and aren’t they supposed to be wise?
Ms. Tehrani is in a bind. What to say? She has no ready answer, feeling caught between two beliefs: the unscientific basis of ineffective old wives’ treatments and the purported wisdom of our elders. She knows Ana’s family and that there are women in that family going back generations who are identified as medicine women or women with the special powers of the forest.
Ana wants to study science but she is being groomed as the family wise mother. Ana is caught between the ways of the past and the ways of the future. She sees that to go with the future is to devalue her family tradition. If she chooses to study medicine, can she keep the balance between magical ways and the ways of science?
Ms. Tehrani decides to expose her class to Indigenous and preindustrial cultural practices and what science has to say. She describes how knowledge is passed down through the generations, and how some of this knowledge has now been proved correct by science, such as the use of opium for pain management and how some knowledge has been corrected by science. She asks the class: What myths have been passed down in your family that science has shown to be effective or ineffective? What does science have to say about how we live our lives?
After a baby in the village dies, Ms. Tehrani asks the local health center to think about implementing a teaching course on caring for babies, a course that will discuss tradition and science. She is well aware of the fact that Black mothers tend not to follow the advice of the pediatricians who now recommend that parents put babies to sleep on their backs. Black women trust the advice of their paternal and maternal grandmothers more than the advice of health care providers, research by Deborah Stiffler, PhD, RN, CNM, shows (J Spec Pediatr Nurs. 2018 Apr;23[2]:e12213). While new Black mothers feel that they have limited knowledge and are eager to learn about safe sleep practices, their grandmothers were skeptical – and the grandmothers often won that argument. Black mothers believed that their own mothers knew best, based on their experience raising infants.
In Dr. Stiffler’s study, one grandmother commented: “Girls today need a mother to help them take care of their babies. They don’t know how to do anything. When I was growing up, our moms helped us.”
One new mother said: I “listen more to the elderly people because like the social workers and stuff some of them don’t have kids. They just go by the book … so I feel like I listen more to like my grandparents.”
Integrating traditions
When Ana enters medical school she is faced with the task of integration of traditional practice and Western medicine. Ana looks to the National Center for Complementary and Integrative Health (NCCIH), the U.S. government’s lead agency for scientific research on complementary and integrative health approaches for support in her task. The NCCIH was established in 1998 with the mission of determining the usefulness and safety of complementary and integrative health approaches, and their roles in improving health and health care.
The NCCIH notes that more than 30% of adults use health care approaches that are not part of conventional medical care or that have origins outside of usual Western practice, and 17.7% of American adults had used a dietary supplement other than vitamins and minerals in the past year, most commonly fish oil. This agency notes that large rigorous research studies extend to only a few dietary supplements, with results showing that the products didn’t work for the conditions studied. The work of the NCCIH is mirrored worldwide.
The 2008 Beijing Declaration called on World Health Organization member states and other stakeholders to integrate traditional medicine and complementary alternative medicines into national health care systems. The WHO Congress on Traditional Medicine recognizes that traditional medicine (TM) may be more affordable and accessible than Western medicine, and that it plays an important role in meeting the demands of primary health care in many developing countries. From 70% to 80% of the population in India and Ethiopia depend on TM for primary health care, and 70% of the population in Canada and 80% in Germany are reported to have used TM as complementary and/or alternative medical treatment.
After graduation and residency, Ana returns to her village and helps her science teacher consider how best to shape the intergenerational transmission of knowledge, so that it is both honored by the elders and also shaped by the science of medicine.
Every village, regardless of where it is in the world, has to contend with finding the balance between the traditional medical knowledge that is passed down through the family and the discoveries of science. When it comes to practicing medicine and psychiatry, a respect for family tradition must be weighed against the application of science: this is a long conversation that is well worth its time.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). Dr. Heru has no conflicts of interest. Contact Dr. Heru at [email protected].



