User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Stay tuned for CSI: Olive oil
Cracking down on food fraud
How do you know the olive oil in your pantry is from Greece? Or that the avocados on your toast are from Mexico? The label, right? Well, maybe not. False claims of origin are a huge problem in the food industry, costing over $30 billion in economic damage annually.
Fear not, citizens, because botanists are on the job, and they’ve found a cheaper and more efficient way to expose that non-Greek olive oil.
How? Florian Cueni, PhD, of the University of Basel, Switzerland, and associates developed a new model to simulate oxygen isotope ratios in plants from a specific region, based on the temperature, precipitation, growing season information, and humidity data. Previously, botanists had to collect reference data from the claimed origin country and from other regions to validate where the product actually came from.
“With minor adjustments to the parameters, our model can be used to determine all plant products,” said senior investigator Ansgar Kahmen. This can open up the door for even more plant forensics, including drug confiscations and illegal timber logging, with information that will hold up in court.
Why pay Greek-olive prices for olives from California?
Fear leads to anger, anger leads to unhelpful online reviews
And reading angry online reviews leads to hate and suffering. We may have co-opted Master Yoda’s wise words ever so slightly, but anyone who’s done any shopping online (so everyone) knows that the review section of any product can be downright villainous. Do these reviews affect what we buy?
The angry online product review was the subject of a recent study published in MIS Quarterly. In a series of experiments, participants were shown a series of realistic online reviews with varying amounts of anger but with similar amounts of information. After reading the reviews, participants rated helpfulness, their personal opinion of the product/retailer, and whether or not they would buy the product.
Participants overwhelmingly rated calmly written reviews as more helpful than angrily written ones. One would expect, then, that those unhelpful angry reviews would have little effect on the participant’s view or willingness to buy a product, but the study investigators found the opposite. Reading angry reviews made the participants more likely to reject the product, even though they didn’t think the angry review was useful. And when you think about it, it does make sense. Anger means drama, and we can’t resist a juicy bit of drama.
So while we should all aspire to be Yoda and rise above anger and hatred, in reality we seem to be channeling Emperor Palpatine. We let the hate flow through us, and in our anger, we ignore perfectly good products. On the plus side, now we can shoot lightning out of our hands, so that’s pretty cool.
Health care is heading to the hall of fame
We couldn’t be happier here at LOTME because it’s that time of year again.
No, we’re not talking about Healthcare Security and Safety Week or National Metric Week, although those are both kind of important. Hmm, maybe we should talk about health care security or the metric system. After all, in this country, medicine is one of the metric system’s biggest customers. And who doesn’t love picograms? They’re the unit-of-measurement equivalent of a koala.
So we’re doing the metric system, then? Nah.
We’re excited because the 2022 inductees to the National Inventors Hall of Fame were just announced, and, as usual, the world of health care is well represented.
First up is the surprisingly relevant (thanks to the party guest that won’t leave, SARS-CoV-2) pair of Katalin Karikó, PhD, and Drew Weissman, MD, who worked together in the early 2000s to modify mRNA “so it could avoid immediate immune detection, remain active longer and efficiently instruct cells to create antigens to protect against severe disease.” Their discoveries eventually led to the use of modified mRNA in the COVID-19 vaccines.
The second, albeit posthumous, physician-inductee is Patricia Bath, MD, who was the first Black female physician to receive a U.S. patent for a medical invention. The laserphaco device and technique to remove cataracts “performed all steps of cataract removal: making the incision, destroying the lens, and vacuuming out the fractured pieces.”
Two other inductees have somewhat tenuous connections to medical care. Lonnie Johnson invented the Super Soaker, a powerful squirt gun that has been criticized by psychologists for encouraging violence, and Carl Benz invented the automobile, which sort of means he invented the ambulance, so there you go.
The induction ceremony takes place on May 5, 2022, in Washington, DC. If you’re attending the black-tie dinner at The Anthem, let us know and we’ll split an Uber. It’s our only night to be fancy.
Cracking down on food fraud
How do you know the olive oil in your pantry is from Greece? Or that the avocados on your toast are from Mexico? The label, right? Well, maybe not. False claims of origin are a huge problem in the food industry, costing over $30 billion in economic damage annually.
Fear not, citizens, because botanists are on the job, and they’ve found a cheaper and more efficient way to expose that non-Greek olive oil.
How? Florian Cueni, PhD, of the University of Basel, Switzerland, and associates developed a new model to simulate oxygen isotope ratios in plants from a specific region, based on the temperature, precipitation, growing season information, and humidity data. Previously, botanists had to collect reference data from the claimed origin country and from other regions to validate where the product actually came from.
“With minor adjustments to the parameters, our model can be used to determine all plant products,” said senior investigator Ansgar Kahmen. This can open up the door for even more plant forensics, including drug confiscations and illegal timber logging, with information that will hold up in court.
Why pay Greek-olive prices for olives from California?
Fear leads to anger, anger leads to unhelpful online reviews
And reading angry online reviews leads to hate and suffering. We may have co-opted Master Yoda’s wise words ever so slightly, but anyone who’s done any shopping online (so everyone) knows that the review section of any product can be downright villainous. Do these reviews affect what we buy?
The angry online product review was the subject of a recent study published in MIS Quarterly. In a series of experiments, participants were shown a series of realistic online reviews with varying amounts of anger but with similar amounts of information. After reading the reviews, participants rated helpfulness, their personal opinion of the product/retailer, and whether or not they would buy the product.
Participants overwhelmingly rated calmly written reviews as more helpful than angrily written ones. One would expect, then, that those unhelpful angry reviews would have little effect on the participant’s view or willingness to buy a product, but the study investigators found the opposite. Reading angry reviews made the participants more likely to reject the product, even though they didn’t think the angry review was useful. And when you think about it, it does make sense. Anger means drama, and we can’t resist a juicy bit of drama.
So while we should all aspire to be Yoda and rise above anger and hatred, in reality we seem to be channeling Emperor Palpatine. We let the hate flow through us, and in our anger, we ignore perfectly good products. On the plus side, now we can shoot lightning out of our hands, so that’s pretty cool.
Health care is heading to the hall of fame
We couldn’t be happier here at LOTME because it’s that time of year again.
No, we’re not talking about Healthcare Security and Safety Week or National Metric Week, although those are both kind of important. Hmm, maybe we should talk about health care security or the metric system. After all, in this country, medicine is one of the metric system’s biggest customers. And who doesn’t love picograms? They’re the unit-of-measurement equivalent of a koala.
So we’re doing the metric system, then? Nah.
We’re excited because the 2022 inductees to the National Inventors Hall of Fame were just announced, and, as usual, the world of health care is well represented.
First up is the surprisingly relevant (thanks to the party guest that won’t leave, SARS-CoV-2) pair of Katalin Karikó, PhD, and Drew Weissman, MD, who worked together in the early 2000s to modify mRNA “so it could avoid immediate immune detection, remain active longer and efficiently instruct cells to create antigens to protect against severe disease.” Their discoveries eventually led to the use of modified mRNA in the COVID-19 vaccines.
The second, albeit posthumous, physician-inductee is Patricia Bath, MD, who was the first Black female physician to receive a U.S. patent for a medical invention. The laserphaco device and technique to remove cataracts “performed all steps of cataract removal: making the incision, destroying the lens, and vacuuming out the fractured pieces.”
Two other inductees have somewhat tenuous connections to medical care. Lonnie Johnson invented the Super Soaker, a powerful squirt gun that has been criticized by psychologists for encouraging violence, and Carl Benz invented the automobile, which sort of means he invented the ambulance, so there you go.
The induction ceremony takes place on May 5, 2022, in Washington, DC. If you’re attending the black-tie dinner at The Anthem, let us know and we’ll split an Uber. It’s our only night to be fancy.
Cracking down on food fraud
How do you know the olive oil in your pantry is from Greece? Or that the avocados on your toast are from Mexico? The label, right? Well, maybe not. False claims of origin are a huge problem in the food industry, costing over $30 billion in economic damage annually.
Fear not, citizens, because botanists are on the job, and they’ve found a cheaper and more efficient way to expose that non-Greek olive oil.
How? Florian Cueni, PhD, of the University of Basel, Switzerland, and associates developed a new model to simulate oxygen isotope ratios in plants from a specific region, based on the temperature, precipitation, growing season information, and humidity data. Previously, botanists had to collect reference data from the claimed origin country and from other regions to validate where the product actually came from.
“With minor adjustments to the parameters, our model can be used to determine all plant products,” said senior investigator Ansgar Kahmen. This can open up the door for even more plant forensics, including drug confiscations and illegal timber logging, with information that will hold up in court.
Why pay Greek-olive prices for olives from California?
Fear leads to anger, anger leads to unhelpful online reviews
And reading angry online reviews leads to hate and suffering. We may have co-opted Master Yoda’s wise words ever so slightly, but anyone who’s done any shopping online (so everyone) knows that the review section of any product can be downright villainous. Do these reviews affect what we buy?
The angry online product review was the subject of a recent study published in MIS Quarterly. In a series of experiments, participants were shown a series of realistic online reviews with varying amounts of anger but with similar amounts of information. After reading the reviews, participants rated helpfulness, their personal opinion of the product/retailer, and whether or not they would buy the product.
Participants overwhelmingly rated calmly written reviews as more helpful than angrily written ones. One would expect, then, that those unhelpful angry reviews would have little effect on the participant’s view or willingness to buy a product, but the study investigators found the opposite. Reading angry reviews made the participants more likely to reject the product, even though they didn’t think the angry review was useful. And when you think about it, it does make sense. Anger means drama, and we can’t resist a juicy bit of drama.
So while we should all aspire to be Yoda and rise above anger and hatred, in reality we seem to be channeling Emperor Palpatine. We let the hate flow through us, and in our anger, we ignore perfectly good products. On the plus side, now we can shoot lightning out of our hands, so that’s pretty cool.
Health care is heading to the hall of fame
We couldn’t be happier here at LOTME because it’s that time of year again.
No, we’re not talking about Healthcare Security and Safety Week or National Metric Week, although those are both kind of important. Hmm, maybe we should talk about health care security or the metric system. After all, in this country, medicine is one of the metric system’s biggest customers. And who doesn’t love picograms? They’re the unit-of-measurement equivalent of a koala.
So we’re doing the metric system, then? Nah.
We’re excited because the 2022 inductees to the National Inventors Hall of Fame were just announced, and, as usual, the world of health care is well represented.
First up is the surprisingly relevant (thanks to the party guest that won’t leave, SARS-CoV-2) pair of Katalin Karikó, PhD, and Drew Weissman, MD, who worked together in the early 2000s to modify mRNA “so it could avoid immediate immune detection, remain active longer and efficiently instruct cells to create antigens to protect against severe disease.” Their discoveries eventually led to the use of modified mRNA in the COVID-19 vaccines.
The second, albeit posthumous, physician-inductee is Patricia Bath, MD, who was the first Black female physician to receive a U.S. patent for a medical invention. The laserphaco device and technique to remove cataracts “performed all steps of cataract removal: making the incision, destroying the lens, and vacuuming out the fractured pieces.”
Two other inductees have somewhat tenuous connections to medical care. Lonnie Johnson invented the Super Soaker, a powerful squirt gun that has been criticized by psychologists for encouraging violence, and Carl Benz invented the automobile, which sort of means he invented the ambulance, so there you go.
The induction ceremony takes place on May 5, 2022, in Washington, DC. If you’re attending the black-tie dinner at The Anthem, let us know and we’ll split an Uber. It’s our only night to be fancy.
New approval in early breast cancer: First advance in 20 years
Abemaciclib had already been approved for use in the treatment of HR+, HER2– advanced or metastatic breast cancer.
Now it is also approved for use in HR+, HER2– early breast cancer for patients who have high-risk, node-positive disease and whose tumors have a Ki-67 score of 20% or higher, as determined by a U.S. Food and Drug Administration–approved test.
The FDA also approved the Ki-67 IHC MIB-1 pharmDx (Dako Omnis) assay for use as a companion diagnostic test.
This is the first CDK4/6 inhibitor to be approved for use in this patient population.
Approximately 70% of all breast cancers are of the HR+, HER2– subtype.
The approval is based on some of the results from the monarchE study, which was presented last year at the annual meeting of the European Society of Medical Oncology and was simultaneously published in the Journal of Clinical Oncology.
The results showed that the addition of abemaciclib to endocrine therapy (tamoxifen or aromatase inhibitors) significantly improved invasive disease-free survival (IDFS), which was defined on the basis of the length of time before breast cancer comes back, any new cancer develops, or death.
The 2-year IDFS rates were 92.2% with the combination vs. 88.7% for endocrine therapy alone for the overall patient population.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust, London, said at the meeting, as reported at the time by this news organization.
Reacting to the findings, Giuseppe Curigliano, MD, PhD, head of the division of early drug development at the European Institute of Oncology, Milan, said, “This is a very important trial and the findings will change practice.”
He predicted that once the drug is approved for use in high-risk HR+, HER2– early breast cancer, “the new standard of care for these patients will be to add 2 years of abemaciclib to endocrine therapy.”
In a press release about the new approval from the manufacturer (Lilly), another investigator on the monarchE study, Sara M. Tolaney, MD, MPH, Harvard Medical School and the Dana-Farber Cancer Institute, Boston, agreed that the results are practice changing. She said that the combination of abemaciclib and endocrine therapy is a potential new standard of care for this patient population. “We are encouraged by the marked reduction in the risk of recurrence even beyond the 2-year treatment period in these patients, and I’m grateful to be able to offer this as a treatment option to my patients,” she said.
On Twitter, she commented that restricting the indication to patients who show Ki67 ≥20% is “interesting,” inasmuch as benefits were seen in patients with both low and high Ki67.
Hal Burstein, MD, from Dana-Farber, also found this detail “interesting, as Ki67 testing remains a very controversial topic and difficult to standardize.”
Replying, Pedro Exman, MD, from the Hospital Alemão Oswaldo Cruz, in São Paulo, said: “Does it make sense to approve only in a subset of patients based in a positive subgroup analysis of a positive ITT study that was not even described in the JCO publication?”
Other experts said they were eagerly awaiting further results, particularly on overall survival, from the monarchE trial. New data are due to be presented on Oct. 14 at an ESMO virtual plenary session.
Commenting late last year about these results, George W. Sledge Jr, MD, professor of medicine at Stanford University Medical Center, Palo Alto, Calif., said that the median follow-up time “is still quite short for a study of ER+ adjuvant therapy, where the majority of recurrences and deaths occur after 5 years in many studies.”
Consequently, “we still have a long way to go to understand the ultimate effects of CDK4/6 inhibition on early-stage ER+ breast cancer, particularly on late recurrences,” he told this news organization at the time.
Agreed, said C. Kent Osborne, MD, codirector of the San Antonio Breast Cancer Symposium and founding director of the Duncan Cancer Center at Baylor College of Medicine, Houston, Tex. The results are “very encouraging, especially in the subgroup of tumors with high proliferation” (identified by the K1-67 score).
However, Dr. Osborne also urged caution in the interpretation of the results, “given the still rather short follow-up, given that that ER+ disease is known for its persistent recurrence rate, even past 10 years.”
He also noted that “this class of inhibitors is likely cytostatic, rather than cytocidal, meaning that it blocks cell proliferation rather than killing the cells.” Questions therefore remain over whether the survival curves for combination therapy will come together with those for endocrine therapy alone once patients stop taking the drug.
Study details
The monarchE trial involved patients with HR+, HER2–, high-risk early breast cancer who had undergone surgery and, as indicated, radiotherapy and/or adjuvant/neoadjuvant chemotherapy. Patients with four or more positive nodes or one to three nodes and either tumors of size ≥5 cm, histologic grade 3, or central Ki-67 ≥20% were eligible; 5,637 patients were randomly assigned in a 1:1 ratio to receive standard-of-care adjuvant endocrine therapy (ET) with or without abemaciclib (150 mg twice daily for 2 years).
A preplanned interim analysis was carried out after 323 IDFS events were observed in the intent-to-treat population. The results, as published last year in the Journal of Clinical Oncology, show that abemaciclib plus ET yielded superior IDFS in comparison with ET alone (P = .01; hazard ratio, 0.75; 95% confidence interval, 0.60-0.93), with 2-year IDFS rates of 92.2% vs. 88.7%.
In the press release announcing the approval of the new indication, the manufacturer notes that the approval was based on the results from a subgroup of 2,003 patients whose tumors had a Ki-67 score of ≥20% and who were also at high risk for recurrence (≥four positive axillary lymph nodes [ALN], or one-three positive ALN with grade 3 disease and/or tumor size ≥5 cm).
There was a statistically significant improvement in IDFS for this prespecified subgroup of patients (HR, 0.643; 95% CI, 0.475-0.872; P = .0042).
With additional follow-up, conducted post hoc, the results showed a 37% decrease in the risk for breast cancer recurrence or death, compared with ET alone (HR, 0.626; 95% CI, 0.49-0.80) and an absolute benefit in IDFS event rate of 7.1% at 3 years. IDFS was 86.1% for abemaciclib plus ET vs. 79.0% for ET alone.
Adverse reactions from monarchE were consistent with the known safety profile for abemaciclib, the company noted. Safety and tolerability were evaluated in 5,591 patients. The most common adverse reactions reported (≥10%) with abemaciclib plus ET vs. ET alone were diarrhea (84% vs. 9%), infections (51% vs. 39%), neutropenia (46% vs. 6%), fatigue (41% vs. 18%), leukopenia (38% vs. 7%), nausea (30% vs. 9%), anemia (24% vs. 4%), headache (20% vs. 15%), vomiting (18% vs. 4.6%), stomatitis (14% vs. 5%), lymphopenia (14% vs. 3%), thrombocytopenia (13% vs. 2%), decreased appetite (12% vs. 2.4%), increased ALT (12% vs. 6%), increased AST (12% vs. 5%), dizziness (11% vs. 7%), rash (11% vs. 4.5%), and alopecia (11% vs. 2.7 %).
A version of this article first appeared on Medscape.com.
Abemaciclib had already been approved for use in the treatment of HR+, HER2– advanced or metastatic breast cancer.
Now it is also approved for use in HR+, HER2– early breast cancer for patients who have high-risk, node-positive disease and whose tumors have a Ki-67 score of 20% or higher, as determined by a U.S. Food and Drug Administration–approved test.
The FDA also approved the Ki-67 IHC MIB-1 pharmDx (Dako Omnis) assay for use as a companion diagnostic test.
This is the first CDK4/6 inhibitor to be approved for use in this patient population.
Approximately 70% of all breast cancers are of the HR+, HER2– subtype.
The approval is based on some of the results from the monarchE study, which was presented last year at the annual meeting of the European Society of Medical Oncology and was simultaneously published in the Journal of Clinical Oncology.
The results showed that the addition of abemaciclib to endocrine therapy (tamoxifen or aromatase inhibitors) significantly improved invasive disease-free survival (IDFS), which was defined on the basis of the length of time before breast cancer comes back, any new cancer develops, or death.
The 2-year IDFS rates were 92.2% with the combination vs. 88.7% for endocrine therapy alone for the overall patient population.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust, London, said at the meeting, as reported at the time by this news organization.
Reacting to the findings, Giuseppe Curigliano, MD, PhD, head of the division of early drug development at the European Institute of Oncology, Milan, said, “This is a very important trial and the findings will change practice.”
He predicted that once the drug is approved for use in high-risk HR+, HER2– early breast cancer, “the new standard of care for these patients will be to add 2 years of abemaciclib to endocrine therapy.”
In a press release about the new approval from the manufacturer (Lilly), another investigator on the monarchE study, Sara M. Tolaney, MD, MPH, Harvard Medical School and the Dana-Farber Cancer Institute, Boston, agreed that the results are practice changing. She said that the combination of abemaciclib and endocrine therapy is a potential new standard of care for this patient population. “We are encouraged by the marked reduction in the risk of recurrence even beyond the 2-year treatment period in these patients, and I’m grateful to be able to offer this as a treatment option to my patients,” she said.
On Twitter, she commented that restricting the indication to patients who show Ki67 ≥20% is “interesting,” inasmuch as benefits were seen in patients with both low and high Ki67.
Hal Burstein, MD, from Dana-Farber, also found this detail “interesting, as Ki67 testing remains a very controversial topic and difficult to standardize.”
Replying, Pedro Exman, MD, from the Hospital Alemão Oswaldo Cruz, in São Paulo, said: “Does it make sense to approve only in a subset of patients based in a positive subgroup analysis of a positive ITT study that was not even described in the JCO publication?”
Other experts said they were eagerly awaiting further results, particularly on overall survival, from the monarchE trial. New data are due to be presented on Oct. 14 at an ESMO virtual plenary session.
Commenting late last year about these results, George W. Sledge Jr, MD, professor of medicine at Stanford University Medical Center, Palo Alto, Calif., said that the median follow-up time “is still quite short for a study of ER+ adjuvant therapy, where the majority of recurrences and deaths occur after 5 years in many studies.”
Consequently, “we still have a long way to go to understand the ultimate effects of CDK4/6 inhibition on early-stage ER+ breast cancer, particularly on late recurrences,” he told this news organization at the time.
Agreed, said C. Kent Osborne, MD, codirector of the San Antonio Breast Cancer Symposium and founding director of the Duncan Cancer Center at Baylor College of Medicine, Houston, Tex. The results are “very encouraging, especially in the subgroup of tumors with high proliferation” (identified by the K1-67 score).
However, Dr. Osborne also urged caution in the interpretation of the results, “given the still rather short follow-up, given that that ER+ disease is known for its persistent recurrence rate, even past 10 years.”
He also noted that “this class of inhibitors is likely cytostatic, rather than cytocidal, meaning that it blocks cell proliferation rather than killing the cells.” Questions therefore remain over whether the survival curves for combination therapy will come together with those for endocrine therapy alone once patients stop taking the drug.
Study details
The monarchE trial involved patients with HR+, HER2–, high-risk early breast cancer who had undergone surgery and, as indicated, radiotherapy and/or adjuvant/neoadjuvant chemotherapy. Patients with four or more positive nodes or one to three nodes and either tumors of size ≥5 cm, histologic grade 3, or central Ki-67 ≥20% were eligible; 5,637 patients were randomly assigned in a 1:1 ratio to receive standard-of-care adjuvant endocrine therapy (ET) with or without abemaciclib (150 mg twice daily for 2 years).
A preplanned interim analysis was carried out after 323 IDFS events were observed in the intent-to-treat population. The results, as published last year in the Journal of Clinical Oncology, show that abemaciclib plus ET yielded superior IDFS in comparison with ET alone (P = .01; hazard ratio, 0.75; 95% confidence interval, 0.60-0.93), with 2-year IDFS rates of 92.2% vs. 88.7%.
In the press release announcing the approval of the new indication, the manufacturer notes that the approval was based on the results from a subgroup of 2,003 patients whose tumors had a Ki-67 score of ≥20% and who were also at high risk for recurrence (≥four positive axillary lymph nodes [ALN], or one-three positive ALN with grade 3 disease and/or tumor size ≥5 cm).
There was a statistically significant improvement in IDFS for this prespecified subgroup of patients (HR, 0.643; 95% CI, 0.475-0.872; P = .0042).
With additional follow-up, conducted post hoc, the results showed a 37% decrease in the risk for breast cancer recurrence or death, compared with ET alone (HR, 0.626; 95% CI, 0.49-0.80) and an absolute benefit in IDFS event rate of 7.1% at 3 years. IDFS was 86.1% for abemaciclib plus ET vs. 79.0% for ET alone.
Adverse reactions from monarchE were consistent with the known safety profile for abemaciclib, the company noted. Safety and tolerability were evaluated in 5,591 patients. The most common adverse reactions reported (≥10%) with abemaciclib plus ET vs. ET alone were diarrhea (84% vs. 9%), infections (51% vs. 39%), neutropenia (46% vs. 6%), fatigue (41% vs. 18%), leukopenia (38% vs. 7%), nausea (30% vs. 9%), anemia (24% vs. 4%), headache (20% vs. 15%), vomiting (18% vs. 4.6%), stomatitis (14% vs. 5%), lymphopenia (14% vs. 3%), thrombocytopenia (13% vs. 2%), decreased appetite (12% vs. 2.4%), increased ALT (12% vs. 6%), increased AST (12% vs. 5%), dizziness (11% vs. 7%), rash (11% vs. 4.5%), and alopecia (11% vs. 2.7 %).
A version of this article first appeared on Medscape.com.
Abemaciclib had already been approved for use in the treatment of HR+, HER2– advanced or metastatic breast cancer.
Now it is also approved for use in HR+, HER2– early breast cancer for patients who have high-risk, node-positive disease and whose tumors have a Ki-67 score of 20% or higher, as determined by a U.S. Food and Drug Administration–approved test.
The FDA also approved the Ki-67 IHC MIB-1 pharmDx (Dako Omnis) assay for use as a companion diagnostic test.
This is the first CDK4/6 inhibitor to be approved for use in this patient population.
Approximately 70% of all breast cancers are of the HR+, HER2– subtype.
The approval is based on some of the results from the monarchE study, which was presented last year at the annual meeting of the European Society of Medical Oncology and was simultaneously published in the Journal of Clinical Oncology.
The results showed that the addition of abemaciclib to endocrine therapy (tamoxifen or aromatase inhibitors) significantly improved invasive disease-free survival (IDFS), which was defined on the basis of the length of time before breast cancer comes back, any new cancer develops, or death.
The 2-year IDFS rates were 92.2% with the combination vs. 88.7% for endocrine therapy alone for the overall patient population.
“This is the first time in more than 20 years that we have seen an advance in the adjuvant treatment of this form of breast cancer,” lead investigator Stephen Johnston, MD, PhD, from the Royal Marsden Hospital NHS Foundation Trust, London, said at the meeting, as reported at the time by this news organization.
Reacting to the findings, Giuseppe Curigliano, MD, PhD, head of the division of early drug development at the European Institute of Oncology, Milan, said, “This is a very important trial and the findings will change practice.”
He predicted that once the drug is approved for use in high-risk HR+, HER2– early breast cancer, “the new standard of care for these patients will be to add 2 years of abemaciclib to endocrine therapy.”
In a press release about the new approval from the manufacturer (Lilly), another investigator on the monarchE study, Sara M. Tolaney, MD, MPH, Harvard Medical School and the Dana-Farber Cancer Institute, Boston, agreed that the results are practice changing. She said that the combination of abemaciclib and endocrine therapy is a potential new standard of care for this patient population. “We are encouraged by the marked reduction in the risk of recurrence even beyond the 2-year treatment period in these patients, and I’m grateful to be able to offer this as a treatment option to my patients,” she said.
On Twitter, she commented that restricting the indication to patients who show Ki67 ≥20% is “interesting,” inasmuch as benefits were seen in patients with both low and high Ki67.
Hal Burstein, MD, from Dana-Farber, also found this detail “interesting, as Ki67 testing remains a very controversial topic and difficult to standardize.”
Replying, Pedro Exman, MD, from the Hospital Alemão Oswaldo Cruz, in São Paulo, said: “Does it make sense to approve only in a subset of patients based in a positive subgroup analysis of a positive ITT study that was not even described in the JCO publication?”
Other experts said they were eagerly awaiting further results, particularly on overall survival, from the monarchE trial. New data are due to be presented on Oct. 14 at an ESMO virtual plenary session.
Commenting late last year about these results, George W. Sledge Jr, MD, professor of medicine at Stanford University Medical Center, Palo Alto, Calif., said that the median follow-up time “is still quite short for a study of ER+ adjuvant therapy, where the majority of recurrences and deaths occur after 5 years in many studies.”
Consequently, “we still have a long way to go to understand the ultimate effects of CDK4/6 inhibition on early-stage ER+ breast cancer, particularly on late recurrences,” he told this news organization at the time.
Agreed, said C. Kent Osborne, MD, codirector of the San Antonio Breast Cancer Symposium and founding director of the Duncan Cancer Center at Baylor College of Medicine, Houston, Tex. The results are “very encouraging, especially in the subgroup of tumors with high proliferation” (identified by the K1-67 score).
However, Dr. Osborne also urged caution in the interpretation of the results, “given the still rather short follow-up, given that that ER+ disease is known for its persistent recurrence rate, even past 10 years.”
He also noted that “this class of inhibitors is likely cytostatic, rather than cytocidal, meaning that it blocks cell proliferation rather than killing the cells.” Questions therefore remain over whether the survival curves for combination therapy will come together with those for endocrine therapy alone once patients stop taking the drug.
Study details
The monarchE trial involved patients with HR+, HER2–, high-risk early breast cancer who had undergone surgery and, as indicated, radiotherapy and/or adjuvant/neoadjuvant chemotherapy. Patients with four or more positive nodes or one to three nodes and either tumors of size ≥5 cm, histologic grade 3, or central Ki-67 ≥20% were eligible; 5,637 patients were randomly assigned in a 1:1 ratio to receive standard-of-care adjuvant endocrine therapy (ET) with or without abemaciclib (150 mg twice daily for 2 years).
A preplanned interim analysis was carried out after 323 IDFS events were observed in the intent-to-treat population. The results, as published last year in the Journal of Clinical Oncology, show that abemaciclib plus ET yielded superior IDFS in comparison with ET alone (P = .01; hazard ratio, 0.75; 95% confidence interval, 0.60-0.93), with 2-year IDFS rates of 92.2% vs. 88.7%.
In the press release announcing the approval of the new indication, the manufacturer notes that the approval was based on the results from a subgroup of 2,003 patients whose tumors had a Ki-67 score of ≥20% and who were also at high risk for recurrence (≥four positive axillary lymph nodes [ALN], or one-three positive ALN with grade 3 disease and/or tumor size ≥5 cm).
There was a statistically significant improvement in IDFS for this prespecified subgroup of patients (HR, 0.643; 95% CI, 0.475-0.872; P = .0042).
With additional follow-up, conducted post hoc, the results showed a 37% decrease in the risk for breast cancer recurrence or death, compared with ET alone (HR, 0.626; 95% CI, 0.49-0.80) and an absolute benefit in IDFS event rate of 7.1% at 3 years. IDFS was 86.1% for abemaciclib plus ET vs. 79.0% for ET alone.
Adverse reactions from monarchE were consistent with the known safety profile for abemaciclib, the company noted. Safety and tolerability were evaluated in 5,591 patients. The most common adverse reactions reported (≥10%) with abemaciclib plus ET vs. ET alone were diarrhea (84% vs. 9%), infections (51% vs. 39%), neutropenia (46% vs. 6%), fatigue (41% vs. 18%), leukopenia (38% vs. 7%), nausea (30% vs. 9%), anemia (24% vs. 4%), headache (20% vs. 15%), vomiting (18% vs. 4.6%), stomatitis (14% vs. 5%), lymphopenia (14% vs. 3%), thrombocytopenia (13% vs. 2%), decreased appetite (12% vs. 2.4%), increased ALT (12% vs. 6%), increased AST (12% vs. 5%), dizziness (11% vs. 7%), rash (11% vs. 4.5%), and alopecia (11% vs. 2.7 %).
A version of this article first appeared on Medscape.com.
Oral PTH shows promise for osteoporosis in early phase 2 study
An investigational oral form of parathyroid hormone (PTH 1-34), EB 613 (Entera Bio) met its primary efficacy outcome in a phase 2 dosing study involving postmenopausal women with low bone mineral density (BMD).
The adverse effect profile of the drug was similar to that of the injectable PTH 1-34 teriparatide (Forteo), which is approved for osteoporosis.
Arthur C. Santora, MD, chief medical officer, Entera Bio, presented 6-month findings from the study during an oral session at the annual meeting of the American Society of Bone and Mineral Research. The 3-month findings from the study were reported as a poster.
If the drug demonstrates efficacy and safety in larger phase 3 trials, it could be the first oral bone-building (anabolic) therapy for osteoporosis.
Clifford J. Rosen, MD, PhD, who was not involved with the research, told this news organization: “I think this is an intriguing study.” The most likely patients for oral PTH, he added, “are those that have osteoporosis, previous fracture, or very low BMD, particularly those unlikely or unwilling to take bisphosphonates.”
However, “this is very early in the process before this drug could come to market,” cautioned Dr. Rosen, who is director of the Center for Clinical and Translational Research, Maine Medical Research Institute, Scarborough.
“Much more data on efficacy are required at 12 and 24 months for phase 2, and then a full phase 3 [clinical trial] with high-risk fracture patients,” he said.
The company is seeking input from the Food and Drug Administration to develop the protocol for a phase 3 trial. They expect to start this trial in 2022 at sites in the United States, Europe, and Israel, Dr. Santora said.
Primary outcome met
The study randomly assigned 161 postmenopausal women with osteoporosis or low BMD to receive placebo or the investigational oral PTH for 6 months.
Compared with women who received placebo, those who received the study drug experienced a significantly greater increase in the bone formation marker procollagen type I N-terminal propeptide (P1NP) from baseline to 3 months, thereby meeting the study’s primary outcome.
In secondary outcomes, women who received the 2.5-mg/d dose experienced a similar 6-month increase in BMD at the spine and greater increases in BMD at the total hip and femoral neck than those who received injectable teriparatide, Dr. Santora reported.
“The study’s key takeaway is that a once-daily oral PTH [tablet] has the potential to produce the same BMD effects as subcutaneous injections of PTH,” he said in an interview.
Additionally, “the drug was well tolerated when the dose was titrated by adding additional tablets, which suggests that the dose can be tailored to each patient,” he said.
Other study findings
Injectable teriparatide reduces the risk for vertebral fractures by up to 80%, Dr. Santora noted, but the fact that the drug must be administered by injection may deter some older patients from using it.
The company developed an oral form of biosynthetic human PTH with a proprietary drug delivery.
The researchers conducted the phase 2 study at four sites in Israel between June 2019 and May 2021. They enrolled women aged 50 years and older who had entered menopause at least 3 years earlier and who had osteoporosis or low BMD.
Forty-three women received placebo, and the others received oral PTH at doses of 0.5 mg/d (n = 25), 1.0 mg/d (n = 29), 1.5 mg/d (n = 28), 2.5 mg/d (n = 19), or at a dose that was titrated up to 2.5 mg/d starting at 1.5 mg/d for month 1, then 2 mg/d for month 2, and then 2.5 mg/d for months 3 to 6 (n = 17).
The mean age of the patients was 61 years, the mean body mass index was 25-27 kg/m2, and the mean T score at the spine of –2.2 to –2.45.
Among the women who received 2.5 mg/d of oral PTH for the full 6 months, serum levels of the bone resorption marker C-terminal telopeptide of type I collagen (CTX) decreased 21% from baseline to 6 months, and serum levels of P1NP increased at month 1 and then decreased to baseline by month 6.
The women who received 2.5 mg/d of oral PTH for the full 6 months also demonstrated significantly greater increases in BMD at the lumbar spine (3.8%), total hip (1.4%), and femoral neck (2.4%), compared with women who received placebo.
The safety profile of oral PTH was consistent with that of subcutaneous PTH. Patients experienced headache, nausea, presyncope, and dizziness; there were no treatment-emergent hypercalcemia adverse events.
A few ‘unexpected findings’
Suzanne M. Jan De Beur, MD, outgoing ASBMR president, said, “Oral PTH appeared to increase BMD by [dual-energy x-ray absorptiometry] at the lumbar spine effectively and to a similar degree as teriparatide in previous studies.”
She identified two unexpected findings.
“There were increases in BMD by DXA at the femoral neck and total hip at 6 months that were [greater than those] seen in previous trials of teriparatide. Second, markers of bone resorption (CTX) decreased at 6 months, and this is in stark contrast to the increases observed with teriparatide treatment,” she noted in an interview.
Dr. Rosen also noted that “the decrease in CTX is very unusual for PTH and difficult to explain.” He added: “P1NP, a marker of bone formation, was not increased.”
Dr. Jan de Beur continued: “Teriparatide (PTH1-34) and abaloparatide are effective anabolic agents that we use to treat patients with high risk of osteoporotic fracture. Although effective, the burden of daily subcutaneous injection can be a barrier for older individuals, those with poor dexterity, and those that are averse to self-injection.
“Taken together, these results appear promising, that oral PTH may prove to be an effective anabolic agent for osteoporosis treatment,” she summarized.
She stressed that a larger phase 3 study is needed to demonstrate safety and efficacy.
The study was funded by Entera Bio. Dr. Santora is chief medical officer of Entera Bio.
A version of this article first appeared on Medscape.com .
An investigational oral form of parathyroid hormone (PTH 1-34), EB 613 (Entera Bio) met its primary efficacy outcome in a phase 2 dosing study involving postmenopausal women with low bone mineral density (BMD).
The adverse effect profile of the drug was similar to that of the injectable PTH 1-34 teriparatide (Forteo), which is approved for osteoporosis.
Arthur C. Santora, MD, chief medical officer, Entera Bio, presented 6-month findings from the study during an oral session at the annual meeting of the American Society of Bone and Mineral Research. The 3-month findings from the study were reported as a poster.
If the drug demonstrates efficacy and safety in larger phase 3 trials, it could be the first oral bone-building (anabolic) therapy for osteoporosis.
Clifford J. Rosen, MD, PhD, who was not involved with the research, told this news organization: “I think this is an intriguing study.” The most likely patients for oral PTH, he added, “are those that have osteoporosis, previous fracture, or very low BMD, particularly those unlikely or unwilling to take bisphosphonates.”
However, “this is very early in the process before this drug could come to market,” cautioned Dr. Rosen, who is director of the Center for Clinical and Translational Research, Maine Medical Research Institute, Scarborough.
“Much more data on efficacy are required at 12 and 24 months for phase 2, and then a full phase 3 [clinical trial] with high-risk fracture patients,” he said.
The company is seeking input from the Food and Drug Administration to develop the protocol for a phase 3 trial. They expect to start this trial in 2022 at sites in the United States, Europe, and Israel, Dr. Santora said.
Primary outcome met
The study randomly assigned 161 postmenopausal women with osteoporosis or low BMD to receive placebo or the investigational oral PTH for 6 months.
Compared with women who received placebo, those who received the study drug experienced a significantly greater increase in the bone formation marker procollagen type I N-terminal propeptide (P1NP) from baseline to 3 months, thereby meeting the study’s primary outcome.
In secondary outcomes, women who received the 2.5-mg/d dose experienced a similar 6-month increase in BMD at the spine and greater increases in BMD at the total hip and femoral neck than those who received injectable teriparatide, Dr. Santora reported.
“The study’s key takeaway is that a once-daily oral PTH [tablet] has the potential to produce the same BMD effects as subcutaneous injections of PTH,” he said in an interview.
Additionally, “the drug was well tolerated when the dose was titrated by adding additional tablets, which suggests that the dose can be tailored to each patient,” he said.
Other study findings
Injectable teriparatide reduces the risk for vertebral fractures by up to 80%, Dr. Santora noted, but the fact that the drug must be administered by injection may deter some older patients from using it.
The company developed an oral form of biosynthetic human PTH with a proprietary drug delivery.
The researchers conducted the phase 2 study at four sites in Israel between June 2019 and May 2021. They enrolled women aged 50 years and older who had entered menopause at least 3 years earlier and who had osteoporosis or low BMD.
Forty-three women received placebo, and the others received oral PTH at doses of 0.5 mg/d (n = 25), 1.0 mg/d (n = 29), 1.5 mg/d (n = 28), 2.5 mg/d (n = 19), or at a dose that was titrated up to 2.5 mg/d starting at 1.5 mg/d for month 1, then 2 mg/d for month 2, and then 2.5 mg/d for months 3 to 6 (n = 17).
The mean age of the patients was 61 years, the mean body mass index was 25-27 kg/m2, and the mean T score at the spine of –2.2 to –2.45.
Among the women who received 2.5 mg/d of oral PTH for the full 6 months, serum levels of the bone resorption marker C-terminal telopeptide of type I collagen (CTX) decreased 21% from baseline to 6 months, and serum levels of P1NP increased at month 1 and then decreased to baseline by month 6.
The women who received 2.5 mg/d of oral PTH for the full 6 months also demonstrated significantly greater increases in BMD at the lumbar spine (3.8%), total hip (1.4%), and femoral neck (2.4%), compared with women who received placebo.
The safety profile of oral PTH was consistent with that of subcutaneous PTH. Patients experienced headache, nausea, presyncope, and dizziness; there were no treatment-emergent hypercalcemia adverse events.
A few ‘unexpected findings’
Suzanne M. Jan De Beur, MD, outgoing ASBMR president, said, “Oral PTH appeared to increase BMD by [dual-energy x-ray absorptiometry] at the lumbar spine effectively and to a similar degree as teriparatide in previous studies.”
She identified two unexpected findings.
“There were increases in BMD by DXA at the femoral neck and total hip at 6 months that were [greater than those] seen in previous trials of teriparatide. Second, markers of bone resorption (CTX) decreased at 6 months, and this is in stark contrast to the increases observed with teriparatide treatment,” she noted in an interview.
Dr. Rosen also noted that “the decrease in CTX is very unusual for PTH and difficult to explain.” He added: “P1NP, a marker of bone formation, was not increased.”
Dr. Jan de Beur continued: “Teriparatide (PTH1-34) and abaloparatide are effective anabolic agents that we use to treat patients with high risk of osteoporotic fracture. Although effective, the burden of daily subcutaneous injection can be a barrier for older individuals, those with poor dexterity, and those that are averse to self-injection.
“Taken together, these results appear promising, that oral PTH may prove to be an effective anabolic agent for osteoporosis treatment,” she summarized.
She stressed that a larger phase 3 study is needed to demonstrate safety and efficacy.
The study was funded by Entera Bio. Dr. Santora is chief medical officer of Entera Bio.
A version of this article first appeared on Medscape.com .
An investigational oral form of parathyroid hormone (PTH 1-34), EB 613 (Entera Bio) met its primary efficacy outcome in a phase 2 dosing study involving postmenopausal women with low bone mineral density (BMD).
The adverse effect profile of the drug was similar to that of the injectable PTH 1-34 teriparatide (Forteo), which is approved for osteoporosis.
Arthur C. Santora, MD, chief medical officer, Entera Bio, presented 6-month findings from the study during an oral session at the annual meeting of the American Society of Bone and Mineral Research. The 3-month findings from the study were reported as a poster.
If the drug demonstrates efficacy and safety in larger phase 3 trials, it could be the first oral bone-building (anabolic) therapy for osteoporosis.
Clifford J. Rosen, MD, PhD, who was not involved with the research, told this news organization: “I think this is an intriguing study.” The most likely patients for oral PTH, he added, “are those that have osteoporosis, previous fracture, or very low BMD, particularly those unlikely or unwilling to take bisphosphonates.”
However, “this is very early in the process before this drug could come to market,” cautioned Dr. Rosen, who is director of the Center for Clinical and Translational Research, Maine Medical Research Institute, Scarborough.
“Much more data on efficacy are required at 12 and 24 months for phase 2, and then a full phase 3 [clinical trial] with high-risk fracture patients,” he said.
The company is seeking input from the Food and Drug Administration to develop the protocol for a phase 3 trial. They expect to start this trial in 2022 at sites in the United States, Europe, and Israel, Dr. Santora said.
Primary outcome met
The study randomly assigned 161 postmenopausal women with osteoporosis or low BMD to receive placebo or the investigational oral PTH for 6 months.
Compared with women who received placebo, those who received the study drug experienced a significantly greater increase in the bone formation marker procollagen type I N-terminal propeptide (P1NP) from baseline to 3 months, thereby meeting the study’s primary outcome.
In secondary outcomes, women who received the 2.5-mg/d dose experienced a similar 6-month increase in BMD at the spine and greater increases in BMD at the total hip and femoral neck than those who received injectable teriparatide, Dr. Santora reported.
“The study’s key takeaway is that a once-daily oral PTH [tablet] has the potential to produce the same BMD effects as subcutaneous injections of PTH,” he said in an interview.
Additionally, “the drug was well tolerated when the dose was titrated by adding additional tablets, which suggests that the dose can be tailored to each patient,” he said.
Other study findings
Injectable teriparatide reduces the risk for vertebral fractures by up to 80%, Dr. Santora noted, but the fact that the drug must be administered by injection may deter some older patients from using it.
The company developed an oral form of biosynthetic human PTH with a proprietary drug delivery.
The researchers conducted the phase 2 study at four sites in Israel between June 2019 and May 2021. They enrolled women aged 50 years and older who had entered menopause at least 3 years earlier and who had osteoporosis or low BMD.
Forty-three women received placebo, and the others received oral PTH at doses of 0.5 mg/d (n = 25), 1.0 mg/d (n = 29), 1.5 mg/d (n = 28), 2.5 mg/d (n = 19), or at a dose that was titrated up to 2.5 mg/d starting at 1.5 mg/d for month 1, then 2 mg/d for month 2, and then 2.5 mg/d for months 3 to 6 (n = 17).
The mean age of the patients was 61 years, the mean body mass index was 25-27 kg/m2, and the mean T score at the spine of –2.2 to –2.45.
Among the women who received 2.5 mg/d of oral PTH for the full 6 months, serum levels of the bone resorption marker C-terminal telopeptide of type I collagen (CTX) decreased 21% from baseline to 6 months, and serum levels of P1NP increased at month 1 and then decreased to baseline by month 6.
The women who received 2.5 mg/d of oral PTH for the full 6 months also demonstrated significantly greater increases in BMD at the lumbar spine (3.8%), total hip (1.4%), and femoral neck (2.4%), compared with women who received placebo.
The safety profile of oral PTH was consistent with that of subcutaneous PTH. Patients experienced headache, nausea, presyncope, and dizziness; there were no treatment-emergent hypercalcemia adverse events.
A few ‘unexpected findings’
Suzanne M. Jan De Beur, MD, outgoing ASBMR president, said, “Oral PTH appeared to increase BMD by [dual-energy x-ray absorptiometry] at the lumbar spine effectively and to a similar degree as teriparatide in previous studies.”
She identified two unexpected findings.
“There were increases in BMD by DXA at the femoral neck and total hip at 6 months that were [greater than those] seen in previous trials of teriparatide. Second, markers of bone resorption (CTX) decreased at 6 months, and this is in stark contrast to the increases observed with teriparatide treatment,” she noted in an interview.
Dr. Rosen also noted that “the decrease in CTX is very unusual for PTH and difficult to explain.” He added: “P1NP, a marker of bone formation, was not increased.”
Dr. Jan de Beur continued: “Teriparatide (PTH1-34) and abaloparatide are effective anabolic agents that we use to treat patients with high risk of osteoporotic fracture. Although effective, the burden of daily subcutaneous injection can be a barrier for older individuals, those with poor dexterity, and those that are averse to self-injection.
“Taken together, these results appear promising, that oral PTH may prove to be an effective anabolic agent for osteoporosis treatment,” she summarized.
She stressed that a larger phase 3 study is needed to demonstrate safety and efficacy.
The study was funded by Entera Bio. Dr. Santora is chief medical officer of Entera Bio.
A version of this article first appeared on Medscape.com .
FDA OKs iPLEDGE change for gender-neutral language
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
The Food and Drug Administration has approved a modification to the isotretinoin risk-mitigation program to make it more inclusive for transgender patients.
Beginning on Dec. 13, 2021, Previously, there were three risk categories: females of reproductive potential, females not of reproductive potential, and males.
In recent years, dermatologists and others have advocated for the change, hoping to make the process more inclusive and less intrusive for their transgender patients.
Isotretinoin (Accutane, Absorica, Amnesteem, Claravis, others) has a high risk of severe birth defects, and has been linked with other health issues, making it crucial for those with the ability to become pregnant to take contraceptive precautions while on the medication. Under the iPLEDGE program, physicians, patients, and pharmacies prescribing, using, or dispensing the drug must all be registered, with requirements that include the use of two forms of an effective contraceptive and regular pregnancy testing for patients who can become pregnant.
The FDA had given notification in June 2018 that the REMS modification and labeling change would be required, replacing the gender-specific language with gender-neutral language, according to an FDA spokesperson. The change was based on feedback that the gender-specific language can be a barrier to access for some patients. The FDA approved the modification on Oct. 8.
Expert reactions
“This is an exciting and welcome change from the FDA on iPLEDGE that many dermatologists, myself included, have advocated for quite a few years,” Howa Yeung, MD, MSc, assistant professor of dermatology at Emory University, Atlanta, said in an interview.
In a report on the dermatologic care for lesbian, gay, bisexual, and transgender persons published in the Journal of the American Academy of Dermatology, Dr. Yeung and his colleagues noted that more than 10 million lesbian, gay, bisexual and transgender people live in the United States and that improving their health is a public health priority.
“For cisgender patients, nothing has changed – patients will continue to receive appropriate educational material related to isotretinoin based on their pregnancy potential,” Dr. Yeung said. “For transgender and gender diverse patients, this is a huge step forward.”
Under the previous system, doctors were asked to register patients using gender binary categories, “which were confusing when they did not reflect reality” for these patients, Dr. Yeung said. The new system, Dr. Yeung added, “will make my job easier. I no longer have to struggle between respecting the patient’s gender identity and providing medically necessary care for patients with severe acne.”
“The new terminology is not just respectful, it also is simpler and makes more sense,” agreed Joshua D. Safer, MD, executive director of the Center for Transgender Medicine and Surgery at Mount Sinai Health System and professor of medicine at the Icahn School of Medicine at Mount Sinai, New York. “As it stood, a transgender man with his uterus and ovaries in place might be missed in the pregnancy surveillance system because he could simply be labeled a man and not followed further. At the same time, both transgender women and cisgender women who were at no risk of pregnancy could be subject to more medical scrutiny that might have been consider intrusive.”
The change “validates the important point that pregnancy potential is not exclusively defined by sociocultural constructs of gender and allow dermatologists to focus purely on what matters when prescribing isotretinoin – whether an individual is able to become pregnant or not, regardless of their gender identity,” Klint Peebles, MD, a dermatologist at Kaiser Permanente in Washington, D.C., and suburban Maryland, who has also advocated for the change, said in an interview.
FDA elaborates
The modification includes important changes for doctors, pharmacists, and patients alike, according to the FDA.
Health care providers must assign and confirm their currently enrolled patient’s risk category when they first log in to the IPLEDGE REMS website on or after Dec. 13, the effective date. They should be sure any patient whose prescription RMA (iPLEDGE authorization) expires on Dec. 11-12 is told to obtain their prescription before midnight, Eastern time, Dec. 10.
Pharmacists will be affected, too, since the iPLEDGE REMS changed to a new platform vendor and the current “switch” pharmacy management system will be removed as a method to verify authorization to dispense isotretinoin. With these changes, as of Dec. 13, pharmacists can’t use the switch system to obtain a predispense authorization, or RMA (risk management authorization). They will need to obtain an RMA online by accessing the iPLEDGE REMS website or via telephone to the PLEDGE REMS center, 866-495-0654, before dispensing the prescription.
Patients, beginning Dec. 13, will have the option of presenting a unique QR code at the pharmacy on their smartphone rather than providing the iPLEDGE identification number. The code can be accessed by logging into their account on the iPLEDGE REMS website.
Patients with an isotretinoin prescription RMA that expires Dec. 11-12, must obtain the prescription before 11:59 p.m. Eastern time on Dec. 10. If the RMA expires before the prescription is picked up, the patient must begin the authorization process all over again.
Dr. Safer, Dr. Yeung, and Dr. Peebles have no relevant disclosures.
More information on the update and the isotretinoin REMS program is available on the FDA website.
Omega-3s tame inflammation in elderly COVID-19 patients
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
results of a small randomized controlled trial suggest.
Results of the study, which included 22 patients with multiple comorbidities, were presented at the European Geriatric Medicine Society annual congress, a hybrid live and online meeting.
The patients, who had a median age of 81 years, were randomized to receive an intravenous infusion of an omega-3 polyunsaturated fatty acid (PUFA) emulsion containing 10 g of fish oil per 100 mL or a saline placebo.
Those who received the intravenous infusion had significant decreases from baseline to end of treatment in the neutrophil-to-lymphocyte ratio (NLR), indicating marked reductions in systemic inflammation.
In contrast, patients randomized to a saline placebo had no significant improvements in NLR, Magnus Bäck, MD, PhD, from the Karolinska Institute in Stockholm reported at the meeting.
“Our lipidomic analysis also showed that omega-3 treatment skewed the lipid response, with reduced levels of proinflammatory lipid mediators, and increased levels of proresolving mediators,” according to a late-breaking abstract, which Dr. Bäck presented during the session.
Omega-3 treatment was not significantly associated with reduction in either C-reactive protein (CRP) or the proinflammatory cytokine interleukin-6, however.
‘Eicosanoid storm’
In a review article published in January 2021 in the open-access journal Frontiers in Physiology, Dr. Bäck and colleagues outlined the rationale for their randomized trial.
“Excessive inflammation has been reported in severe cases with respiratory failure and cardiovascular complications,” they wrote. “In addition to the release of cytokines, referred to as cytokine release syndrome or ‘cytokine storm,’ increased proinflammatory lipid mediators derived from the omega-6 polyunsaturated fatty acid (PUFA) arachidonic acid may cause an ‘eicosanoid storm,’ which contributes to the uncontrolled systemic inflammation.”
Omega-3 PUFA contains proresolving mediators that can limit inflammatory reactions, suggesting the possibility of an inflammation-resolving benefit in patients with COVID-19 without concerns about immunosuppression, the authors hypothesized.
Trial details
In the trial, COVID-Omega-F, they enrolled patients with a COVID-19 diagnosis requiring hospitalization. Patients with an allergy to fish oil or who had contraindications to intravenous PUFA administration (for example, risk for bleeding, shock, or emboli) were excluded.
Ten patients were randomly assigned to receive infusions of the omega-3 PUFA and 12 were assigned to receive infusions of the placebo, once daily for 5 days. The primary outcome measure was change in inflammatory biomarkers, including white blood cell counts, CRP, cytokines, and lipid mediators.
Baseline demographic and clinical characteristics were similar between the two study arms, with a median of about 7 days since the onset of symptoms, and 3.5 days since a diagnosis of COVID-19.
All patients had low lymphocyte responses reflected by a high NLR, a prognostic measure for worse outcomes in patients with COVID-19 infections, Dr. Bäck said.
Inflammation was moderate, with a CRP of 65 mg/L in the placebo group and 62 mg/L in the omega-3 group.
Seven patients in each study arm received concomitant corticoid treatment. Two patients in each arm died in hospital, but there were no serious treatment-related adverse events.
Inflammatory markers improve
As noted before, there was a significant decline in NLR from baseline among patients randomized to omega-3 (P = .02) but no corresponding decrease in patients assigned to placebo infusions.
“The significant decrease was largely driven by an increase in the lymphocyte count in the omega-3 treated group (P = .004), whereas lymphocytes did not significantly change,” Dr. Bäck said.
As expected, patients in the omega-3 group had pronounced increases in omega-3 fatty acids, including eicosapentaenoic acid and docosahexaenoic acid.
The metabolism of fatty acids also differed markedly between the groups, with a significant decrease in the omega-3 group but not the placebo group in proinflammatory mediators, and an increase in precursors to proresolving mediators, Dr. Bäck noted.
AFib concerns
In a question-and-answer part of the session, a physician who identified herself as “Senya from Russia” questioned the safety of omega-3 treatment in this population, “because recently there was a meta-analysis which showed that omega-3 fatty acids will increase the risk of atrial fibrillation in older adults especially.”
The systematic review and meta-analysis she referred to, published in Circulation and reported on by this news organization, showed that, among 81,210 patients with a mean age of 65 enrolled in seven randomized controlled trials, omega-3 fatty acid supplementation was associated with a 25% increase in risk for atrial fibrillation. This risk appeared to be higher in trials testing doses greater than 1 g/day, according to the paper.
“This was not monitored in this study,” Dr. Bäck replied. “It is true that the meta-analysis showed an increased incidence of atrial fibrillation, so it would be something to monitor in case this trial would be expanded to a larger population.”
The study was supported by the Karolinska Institute. Dr. Bäck disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EUGMS
True or false: Breast density increases breast cancer risk
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).
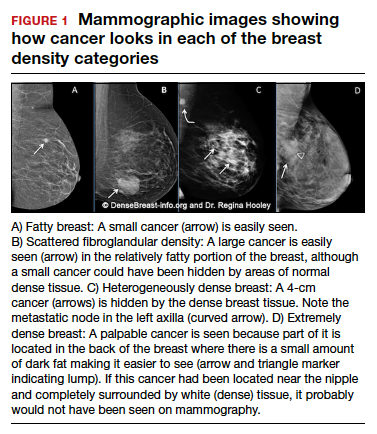
Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8
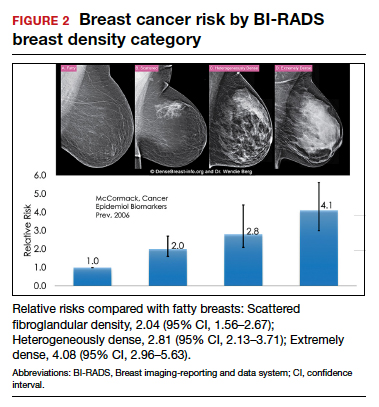
Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).

Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8

Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
Which of the following statements about breast density is TRUE?
Text copyright DenseBreast-info.org.
Answer
D. The risks associated with dense breast tissue are 2-fold: Dense tissue can mask cancer on a mammogram, and having dense breasts also increases the risk of developing breast cancer. As breast density increases, the sensitivity of mammography decreases, and the risk of developing breast cancer increases.
A woman’s breast density is usually determined by a radiologist’s visual evaluation of the mammogram. Breast density also can be measured quantitatively by computer software or estimated on computed tomography scan or magnetic resonance imaging. Breast density cannot be determined by the way a breast looks or feels.
Breast density and mammographic sensitivity
Cancers can be hidden or “masked” by dense tissue. On a mammogram, cancer is white. Normal dense tissue also appears white. If a cancer develops in an area of normal dense tissue, it can be harder or sometimes impossible to see it on the mammogram, like trying to see a snowman in a blizzard. As breast density increases, the ability to see cancer on mammography decreases (FIGURE 1).

Standard 2D mammography has been shown to miss about 40% of cancers present in women with extremely dense breasts and 25% of cancers present in women with heterogeneously dense breasts.1-6 A cancer still can be masked on tomosynthesis (3D mammography) if it occurs in an area of dense tissue (where breast cancers more commonly occur), and tomosynthesis does not improve cancer detection appreciably in women with extremely dense breasts. To find cancer in a woman with dense breasts, additional screening beyond mammography should be considered.
Breast density and breast cancer risk
Dense breast tissue not only reduces mammography effectiveness, it also is a risk factor for the development of breast cancer: the denser the breast, the higher the risk.7 A meta-analysis across many studies concluded that magnitude of risk increases with each increase in density category, and women with extremely dense breasts (category D) have a 4-fold greater risk of developing breast cancer than do women with fatty breasts (category A), with upper limit of nearly 6-fold greater risk (FIGURE 2).8

Most women do not have fatty breasts, however. More women have breasts with scattered fibroglandular density.9 Women with heterogeneously dense breasts (category C) have about a 1.5-fold greater risk of developing breast cancer than those with scattered fibroglandular density (category B), while women with extremely dense breasts (category D) have about a 2-fold greater risk.
There are probably several reasons that dense tissue increases breast cancer risk. One is that cancers arise microscopically in the glandular tissue. The more glandular tissue, the more susceptible tissue where cancer can develop. Glandular cells divide with hormonal stimulation throughout a woman’s lifetime, and each time a cell divides, “mistakes” can be made. An accumulation of mistakes can result in cancer. The more glandular the tissue, the greater the breast cancer risk. Women who have had breast reduction experience a reduced risk for breast cancer: thus, even a reduced absolute amount of glandular tissue reduces the risk for breast cancer. The second is that the local environment around the glands may produce certain growth hormones that stimulate cells to divide, and this is observed with fibrous breast tissue more than fatty breast tissue. ●
For more information, visit medically sourced DenseBreast-info.org. Comprehensive resources include a free CME opportunity, Dense Breasts and Supplemental Screening.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
- Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394-1404. doi: 10.1001 /jama.2012.388.
- Destounis S, Johnston L, Highnam R, et al. Using volumetric breast density to quantify the potential masking risk of mammographic density. AJR Am J Roentgenol. 2017;208:222-227. doi: 10.2214/AJR.16.16489.
- Kerlikowske K, Scott CG, Mahmoudzadeh AP, et al. Automated and clinical breast imaging reporting and data system density measures predict risk for screen-detected and interval cancers: a case-control study. Ann Intern Med. 2018;168:757-765. doi: 10.7326/M17-3008.
- Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165-175. doi: 10.1148/radiol.2251011667.
- Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000;92:1081-1087. doi: 10.1093/jnci/92.13.1081.
- Wanders JOP, Holland K, Karssemeijer N, et al. The effect of volumetric breast density on the risk of screen-detected and interval breast cancers: a cohort study. Breast Cancer Res. 2017;19:67. doi: 10.1186/s13058-017-0859-9.
- Society AC. Breast Cancer Facts & Figures 2019-2020. American Cancer Society, Inc. https://www.cancer.org/content/dam/cancer-org/research/cancer -facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts -and-figures-2019-2020.pdf. Published 2019. Accessed September 23, 2021.
- McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1159-1169. doi: 10.1158/1055-9965.EPI-06-0034.
- Kerlikowske K, Cook AJ, Buist DS, et al. Breast cancer risk by breast density, menopause, and postmenopausal hormone therapy use. J Clin Oncol. 2010;28:3830-3837. doi: 10.1200/JCO.2009.26.4770.
Quiz developed in collaboration with 
USPSTF rules out aspirin for over 60s in primary CVD prevention
New draft recommendations from the U.S. Preventive Services Task Force (USPSTF) on the use of aspirin for the primary prevention of cardiovascular disease (CVD) have been released and appear to limit the population in which it should be considered.
“The USPSTF concludes with moderate certainty that aspirin use for the primary prevention of CVD events in adults ages 40 to 59 years who have a 10% or greater 10-year CVD risk has a small net benefit,” the recommendation notes. They conclude that for these patients, the decision to use aspirin “should be an individual one.”
“Persons who are not at increased risk for bleeding and are willing to take low-dose aspirin daily are more likely to benefit,” they note.
For older individuals, however, the task force concludes.
The new recommendations were posted online Oct. 12 and will be available for public comment until November 8. Once it is finalized, the recommendation will replace the 2016 USPSTF recommendation on aspirin use to prevent CVD and colorectal cancer (CRC), they note.
In that document, the task force recommended initiating low-dose aspirin for the primary prevention of both CVD and CRC in adults 50-59 years of age who had a 10% or greater 10-year CVD risk, were not at increased risk for bleeding, had a life expectancy of at least 10 years, and were willing to take daily low-dose aspirin for at least 10 years, with the decision to start being an individual one.
For older and younger patients, they found at that time that the evidence was “insufficient to assess the balance of benefits and harms of initiating aspirin use for the primary prevention of CVD and CRC in adults younger than age 50 years or adults aged 70 years or older.”
In the new draft document, “the USPSTF has changed the age ranges and grades of its recommendation on aspirin use.” Besides the recommendations for CVD prevention, they have also changed the previous recommendation of aspirin for the prevention of CRC given evidence generated from large primary CVD prevention trials.
“Based on new analyses of the evidence from primary CVD prevention populations, longer-term follow-up data from the Women’s Health Study (WHS) (JE Buring, personal communication, November 23, 2020), and new trial evidence, the USPSTF concluded that the evidence is inadequate that low-dose aspirin use reduces CRC incidence or mortality,” it states.
Optimum dose
On the optimum dose for primary CVD prevention, the task force says the benefit appears similar for a low dose (≤100 mg/d) and all doses that have been studied in CVD prevention trials (50 to 500 mg/d). “A pragmatic approach would be to use 81 mg/d, which is the most commonly prescribed dose in the United States,” it states.
The USPSTF recommends using the ACC/AHA Pooled Cohort Equations to estimate cardiovascular risk but it points out that these equations are imperfect for risk prediction at the individual level, and suggests using these risk estimates as a starting point to discuss with appropriate candidates their desire for daily aspirin use. The benefits of initiating aspirin use are greater for individuals at higher risk for CVD events (eg, those with >15% or >20% 10-year CVD risk), they note.
“Decisions about initiating aspirin use should be based on shared decision-making between clinicians and patients about the potential benefits and harms. Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin use. Persons who place a higher value on the potential harms or on the burden of taking a daily preventive medication than the potential benefits may choose not to initiate low-dose aspirin use,” the task force says.
It also points out that the risk for bleeding increases modestly with advancing age. “For persons who have initiated aspirin use, the net benefits continue to accrue over time in the absence of a bleeding event. The net benefits, however, become smaller with advancing age because of an increased risk for bleeding, so modeling data suggest that it may be reasonable to consider stopping aspirin use around age 75 years,” it states.
Systematic review
The updated draft recommendations are based on a new systematic review commissioned by the USPSTF on the effectiveness of aspirin to reduce the risk of CVD events (myocardial infarction and stroke), cardiovascular mortality, and all-cause mortality in persons without a history of CVD.
The systematic review also investigated the effect of aspirin use on CRC incidence and mortality in primary CVD prevention populations, as well as the harms, particularly bleeding harms, associated with aspirin use.
In addition to the systematic evidence review, the USPSTF commissioned a microsimulation modeling study to assess the net balance of benefits and harms from aspirin use for primary prevention of CVD and CRC, stratified by age, sex, and CVD risk level. Modeling study parameter inputs were informed by the results of the systematic review, and the primary outcomes were net benefits expressed as quality-adjusted life-years and life-years.
The USPSTF found 13 randomized clinical trials (RCTs) that reported on the benefits of aspirin use for the primary prevention of cardiovascular morbidity and mortality. The total number of participants was 161,680, and most trials used low-dose aspirin of 100 mg/d or less or aspirin every other day. The 13 primary prevention trials included a balanced number of male and female participants and included a broad distribution of ages, with mean age ranging from 53 years in the Physicians’ Health Study to 74 years in the ASPREE trial.
This body of evidence shows that aspirin use for primary prevention of CVD is associated with a decreased risk of myocardial infarction and stroke but not cardiovascular mortality or all-cause mortality. Results are quite similar when including studies using all doses of aspirin compared with studies using low-dose aspirin.
The USPSTF reviewed 14 RCTs in CVD primary prevention populations that reported on the bleeding harms of aspirin.
When looking at studies reporting on the harms of low-dose aspirin use (≤100 mg/d), which is most relevant to current practice, a pooled analysis of 10 trials showed that aspirin use was associated with a 58% increase in major gastrointestinal bleeding, and a pooled analysis of 11 trials showed a 31% increase in intracranial bleeds in the aspirin group compared with the control group. Low-dose aspirin use was not associated with a statistically significant increase in risk of fatal hemorrhagic stroke.
Data suggested that the increased risk of bleeding associated with aspirin use occurs relatively quickly after initiating aspirin, and data do not suggest that aspirin has a differential relative bleeding risk based on age, sex, presence of diabetes, level of CVD risk, or race or ethnicity. Although the increase in relative risk does not appear to differ based on age, the absolute risk of bleeding, and thus the magnitude of bleeding harm, does increase with age, and more so in adults age 60 years or older, they note.
The microsimulation model to estimate the magnitude of net benefit of low-dose aspirin use incorporated findings from the systematic review.
Modeling data demonstrated that aspirin use in both men and women ages 40-59 years with 10% or greater 10-year CVD risk generally provides a modest net benefit in both quality-adjusted life-years and life-years gained. Initiation of aspirin use in persons aged 60-69 years results in quality-adjusted life-years gained that range from slightly negative to slightly positive depending on CVD risk level, and life-years gained are generally negative.
In persons aged 70-79 years, initiation of aspirin use results in a loss of both quality-adjusted life-years and life-years at essentially all CVD risk levels modeled (ie, up to 20% 10-year CVD risk).
The USPSTF thus determined that aspirin use has a small net benefit in persons aged 40-59 years with 10% or greater 10-year CVD risk, and initiation of aspirin use has no net benefit in persons age 60 years or older.
When looking at net lifetime benefit of continuous aspirin use until stopping at age 65, 70, 75, 80, or 85 years, modeling data suggest that there is generally little incremental lifetime net benefit in continuing aspirin use beyond the age of 75-80 years.
The task force points out that the net benefit of continuing aspirin use by a person in their 60s or 70s is not the same as the net benefit of initiating aspirin use by a person in their 60s or 70s. This is because, in part, of the fact that CVD risk is heavily influenced by age. Persons who meet the eligibility criteria for aspirin use at a younger age (ie, ≥10% 10-year CVD risk in their 40s or 50s) typically have even higher CVD risk by their 60s or 70s compared with persons who first reach a 10% or greater 10-year CVD risk in their 60s or 70s, and may gain more benefit by continuing aspirin use than a person at lower risk might gain by initiating aspirin use, the USPSTF explains.
A version of this article first appeared on Medscape.com.
New draft recommendations from the U.S. Preventive Services Task Force (USPSTF) on the use of aspirin for the primary prevention of cardiovascular disease (CVD) have been released and appear to limit the population in which it should be considered.
“The USPSTF concludes with moderate certainty that aspirin use for the primary prevention of CVD events in adults ages 40 to 59 years who have a 10% or greater 10-year CVD risk has a small net benefit,” the recommendation notes. They conclude that for these patients, the decision to use aspirin “should be an individual one.”
“Persons who are not at increased risk for bleeding and are willing to take low-dose aspirin daily are more likely to benefit,” they note.
For older individuals, however, the task force concludes.
The new recommendations were posted online Oct. 12 and will be available for public comment until November 8. Once it is finalized, the recommendation will replace the 2016 USPSTF recommendation on aspirin use to prevent CVD and colorectal cancer (CRC), they note.
In that document, the task force recommended initiating low-dose aspirin for the primary prevention of both CVD and CRC in adults 50-59 years of age who had a 10% or greater 10-year CVD risk, were not at increased risk for bleeding, had a life expectancy of at least 10 years, and were willing to take daily low-dose aspirin for at least 10 years, with the decision to start being an individual one.
For older and younger patients, they found at that time that the evidence was “insufficient to assess the balance of benefits and harms of initiating aspirin use for the primary prevention of CVD and CRC in adults younger than age 50 years or adults aged 70 years or older.”
In the new draft document, “the USPSTF has changed the age ranges and grades of its recommendation on aspirin use.” Besides the recommendations for CVD prevention, they have also changed the previous recommendation of aspirin for the prevention of CRC given evidence generated from large primary CVD prevention trials.
“Based on new analyses of the evidence from primary CVD prevention populations, longer-term follow-up data from the Women’s Health Study (WHS) (JE Buring, personal communication, November 23, 2020), and new trial evidence, the USPSTF concluded that the evidence is inadequate that low-dose aspirin use reduces CRC incidence or mortality,” it states.
Optimum dose
On the optimum dose for primary CVD prevention, the task force says the benefit appears similar for a low dose (≤100 mg/d) and all doses that have been studied in CVD prevention trials (50 to 500 mg/d). “A pragmatic approach would be to use 81 mg/d, which is the most commonly prescribed dose in the United States,” it states.
The USPSTF recommends using the ACC/AHA Pooled Cohort Equations to estimate cardiovascular risk but it points out that these equations are imperfect for risk prediction at the individual level, and suggests using these risk estimates as a starting point to discuss with appropriate candidates their desire for daily aspirin use. The benefits of initiating aspirin use are greater for individuals at higher risk for CVD events (eg, those with >15% or >20% 10-year CVD risk), they note.
“Decisions about initiating aspirin use should be based on shared decision-making between clinicians and patients about the potential benefits and harms. Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin use. Persons who place a higher value on the potential harms or on the burden of taking a daily preventive medication than the potential benefits may choose not to initiate low-dose aspirin use,” the task force says.
It also points out that the risk for bleeding increases modestly with advancing age. “For persons who have initiated aspirin use, the net benefits continue to accrue over time in the absence of a bleeding event. The net benefits, however, become smaller with advancing age because of an increased risk for bleeding, so modeling data suggest that it may be reasonable to consider stopping aspirin use around age 75 years,” it states.
Systematic review
The updated draft recommendations are based on a new systematic review commissioned by the USPSTF on the effectiveness of aspirin to reduce the risk of CVD events (myocardial infarction and stroke), cardiovascular mortality, and all-cause mortality in persons without a history of CVD.
The systematic review also investigated the effect of aspirin use on CRC incidence and mortality in primary CVD prevention populations, as well as the harms, particularly bleeding harms, associated with aspirin use.
In addition to the systematic evidence review, the USPSTF commissioned a microsimulation modeling study to assess the net balance of benefits and harms from aspirin use for primary prevention of CVD and CRC, stratified by age, sex, and CVD risk level. Modeling study parameter inputs were informed by the results of the systematic review, and the primary outcomes were net benefits expressed as quality-adjusted life-years and life-years.
The USPSTF found 13 randomized clinical trials (RCTs) that reported on the benefits of aspirin use for the primary prevention of cardiovascular morbidity and mortality. The total number of participants was 161,680, and most trials used low-dose aspirin of 100 mg/d or less or aspirin every other day. The 13 primary prevention trials included a balanced number of male and female participants and included a broad distribution of ages, with mean age ranging from 53 years in the Physicians’ Health Study to 74 years in the ASPREE trial.
This body of evidence shows that aspirin use for primary prevention of CVD is associated with a decreased risk of myocardial infarction and stroke but not cardiovascular mortality or all-cause mortality. Results are quite similar when including studies using all doses of aspirin compared with studies using low-dose aspirin.
The USPSTF reviewed 14 RCTs in CVD primary prevention populations that reported on the bleeding harms of aspirin.
When looking at studies reporting on the harms of low-dose aspirin use (≤100 mg/d), which is most relevant to current practice, a pooled analysis of 10 trials showed that aspirin use was associated with a 58% increase in major gastrointestinal bleeding, and a pooled analysis of 11 trials showed a 31% increase in intracranial bleeds in the aspirin group compared with the control group. Low-dose aspirin use was not associated with a statistically significant increase in risk of fatal hemorrhagic stroke.
Data suggested that the increased risk of bleeding associated with aspirin use occurs relatively quickly after initiating aspirin, and data do not suggest that aspirin has a differential relative bleeding risk based on age, sex, presence of diabetes, level of CVD risk, or race or ethnicity. Although the increase in relative risk does not appear to differ based on age, the absolute risk of bleeding, and thus the magnitude of bleeding harm, does increase with age, and more so in adults age 60 years or older, they note.
The microsimulation model to estimate the magnitude of net benefit of low-dose aspirin use incorporated findings from the systematic review.
Modeling data demonstrated that aspirin use in both men and women ages 40-59 years with 10% or greater 10-year CVD risk generally provides a modest net benefit in both quality-adjusted life-years and life-years gained. Initiation of aspirin use in persons aged 60-69 years results in quality-adjusted life-years gained that range from slightly negative to slightly positive depending on CVD risk level, and life-years gained are generally negative.
In persons aged 70-79 years, initiation of aspirin use results in a loss of both quality-adjusted life-years and life-years at essentially all CVD risk levels modeled (ie, up to 20% 10-year CVD risk).
The USPSTF thus determined that aspirin use has a small net benefit in persons aged 40-59 years with 10% or greater 10-year CVD risk, and initiation of aspirin use has no net benefit in persons age 60 years or older.
When looking at net lifetime benefit of continuous aspirin use until stopping at age 65, 70, 75, 80, or 85 years, modeling data suggest that there is generally little incremental lifetime net benefit in continuing aspirin use beyond the age of 75-80 years.
The task force points out that the net benefit of continuing aspirin use by a person in their 60s or 70s is not the same as the net benefit of initiating aspirin use by a person in their 60s or 70s. This is because, in part, of the fact that CVD risk is heavily influenced by age. Persons who meet the eligibility criteria for aspirin use at a younger age (ie, ≥10% 10-year CVD risk in their 40s or 50s) typically have even higher CVD risk by their 60s or 70s compared with persons who first reach a 10% or greater 10-year CVD risk in their 60s or 70s, and may gain more benefit by continuing aspirin use than a person at lower risk might gain by initiating aspirin use, the USPSTF explains.
A version of this article first appeared on Medscape.com.
New draft recommendations from the U.S. Preventive Services Task Force (USPSTF) on the use of aspirin for the primary prevention of cardiovascular disease (CVD) have been released and appear to limit the population in which it should be considered.
“The USPSTF concludes with moderate certainty that aspirin use for the primary prevention of CVD events in adults ages 40 to 59 years who have a 10% or greater 10-year CVD risk has a small net benefit,” the recommendation notes. They conclude that for these patients, the decision to use aspirin “should be an individual one.”
“Persons who are not at increased risk for bleeding and are willing to take low-dose aspirin daily are more likely to benefit,” they note.
For older individuals, however, the task force concludes.
The new recommendations were posted online Oct. 12 and will be available for public comment until November 8. Once it is finalized, the recommendation will replace the 2016 USPSTF recommendation on aspirin use to prevent CVD and colorectal cancer (CRC), they note.
In that document, the task force recommended initiating low-dose aspirin for the primary prevention of both CVD and CRC in adults 50-59 years of age who had a 10% or greater 10-year CVD risk, were not at increased risk for bleeding, had a life expectancy of at least 10 years, and were willing to take daily low-dose aspirin for at least 10 years, with the decision to start being an individual one.
For older and younger patients, they found at that time that the evidence was “insufficient to assess the balance of benefits and harms of initiating aspirin use for the primary prevention of CVD and CRC in adults younger than age 50 years or adults aged 70 years or older.”
In the new draft document, “the USPSTF has changed the age ranges and grades of its recommendation on aspirin use.” Besides the recommendations for CVD prevention, they have also changed the previous recommendation of aspirin for the prevention of CRC given evidence generated from large primary CVD prevention trials.
“Based on new analyses of the evidence from primary CVD prevention populations, longer-term follow-up data from the Women’s Health Study (WHS) (JE Buring, personal communication, November 23, 2020), and new trial evidence, the USPSTF concluded that the evidence is inadequate that low-dose aspirin use reduces CRC incidence or mortality,” it states.
Optimum dose
On the optimum dose for primary CVD prevention, the task force says the benefit appears similar for a low dose (≤100 mg/d) and all doses that have been studied in CVD prevention trials (50 to 500 mg/d). “A pragmatic approach would be to use 81 mg/d, which is the most commonly prescribed dose in the United States,” it states.
The USPSTF recommends using the ACC/AHA Pooled Cohort Equations to estimate cardiovascular risk but it points out that these equations are imperfect for risk prediction at the individual level, and suggests using these risk estimates as a starting point to discuss with appropriate candidates their desire for daily aspirin use. The benefits of initiating aspirin use are greater for individuals at higher risk for CVD events (eg, those with >15% or >20% 10-year CVD risk), they note.
“Decisions about initiating aspirin use should be based on shared decision-making between clinicians and patients about the potential benefits and harms. Persons who place a higher value on the potential benefits than the potential harms may choose to initiate low-dose aspirin use. Persons who place a higher value on the potential harms or on the burden of taking a daily preventive medication than the potential benefits may choose not to initiate low-dose aspirin use,” the task force says.
It also points out that the risk for bleeding increases modestly with advancing age. “For persons who have initiated aspirin use, the net benefits continue to accrue over time in the absence of a bleeding event. The net benefits, however, become smaller with advancing age because of an increased risk for bleeding, so modeling data suggest that it may be reasonable to consider stopping aspirin use around age 75 years,” it states.
Systematic review
The updated draft recommendations are based on a new systematic review commissioned by the USPSTF on the effectiveness of aspirin to reduce the risk of CVD events (myocardial infarction and stroke), cardiovascular mortality, and all-cause mortality in persons without a history of CVD.
The systematic review also investigated the effect of aspirin use on CRC incidence and mortality in primary CVD prevention populations, as well as the harms, particularly bleeding harms, associated with aspirin use.
In addition to the systematic evidence review, the USPSTF commissioned a microsimulation modeling study to assess the net balance of benefits and harms from aspirin use for primary prevention of CVD and CRC, stratified by age, sex, and CVD risk level. Modeling study parameter inputs were informed by the results of the systematic review, and the primary outcomes were net benefits expressed as quality-adjusted life-years and life-years.
The USPSTF found 13 randomized clinical trials (RCTs) that reported on the benefits of aspirin use for the primary prevention of cardiovascular morbidity and mortality. The total number of participants was 161,680, and most trials used low-dose aspirin of 100 mg/d or less or aspirin every other day. The 13 primary prevention trials included a balanced number of male and female participants and included a broad distribution of ages, with mean age ranging from 53 years in the Physicians’ Health Study to 74 years in the ASPREE trial.
This body of evidence shows that aspirin use for primary prevention of CVD is associated with a decreased risk of myocardial infarction and stroke but not cardiovascular mortality or all-cause mortality. Results are quite similar when including studies using all doses of aspirin compared with studies using low-dose aspirin.
The USPSTF reviewed 14 RCTs in CVD primary prevention populations that reported on the bleeding harms of aspirin.
When looking at studies reporting on the harms of low-dose aspirin use (≤100 mg/d), which is most relevant to current practice, a pooled analysis of 10 trials showed that aspirin use was associated with a 58% increase in major gastrointestinal bleeding, and a pooled analysis of 11 trials showed a 31% increase in intracranial bleeds in the aspirin group compared with the control group. Low-dose aspirin use was not associated with a statistically significant increase in risk of fatal hemorrhagic stroke.
Data suggested that the increased risk of bleeding associated with aspirin use occurs relatively quickly after initiating aspirin, and data do not suggest that aspirin has a differential relative bleeding risk based on age, sex, presence of diabetes, level of CVD risk, or race or ethnicity. Although the increase in relative risk does not appear to differ based on age, the absolute risk of bleeding, and thus the magnitude of bleeding harm, does increase with age, and more so in adults age 60 years or older, they note.
The microsimulation model to estimate the magnitude of net benefit of low-dose aspirin use incorporated findings from the systematic review.
Modeling data demonstrated that aspirin use in both men and women ages 40-59 years with 10% or greater 10-year CVD risk generally provides a modest net benefit in both quality-adjusted life-years and life-years gained. Initiation of aspirin use in persons aged 60-69 years results in quality-adjusted life-years gained that range from slightly negative to slightly positive depending on CVD risk level, and life-years gained are generally negative.
In persons aged 70-79 years, initiation of aspirin use results in a loss of both quality-adjusted life-years and life-years at essentially all CVD risk levels modeled (ie, up to 20% 10-year CVD risk).
The USPSTF thus determined that aspirin use has a small net benefit in persons aged 40-59 years with 10% or greater 10-year CVD risk, and initiation of aspirin use has no net benefit in persons age 60 years or older.
When looking at net lifetime benefit of continuous aspirin use until stopping at age 65, 70, 75, 80, or 85 years, modeling data suggest that there is generally little incremental lifetime net benefit in continuing aspirin use beyond the age of 75-80 years.
The task force points out that the net benefit of continuing aspirin use by a person in their 60s or 70s is not the same as the net benefit of initiating aspirin use by a person in their 60s or 70s. This is because, in part, of the fact that CVD risk is heavily influenced by age. Persons who meet the eligibility criteria for aspirin use at a younger age (ie, ≥10% 10-year CVD risk in their 40s or 50s) typically have even higher CVD risk by their 60s or 70s compared with persons who first reach a 10% or greater 10-year CVD risk in their 60s or 70s, and may gain more benefit by continuing aspirin use than a person at lower risk might gain by initiating aspirin use, the USPSTF explains.
A version of this article first appeared on Medscape.com.
Homicide remains a top cause of maternal mortality
The prevalence of homicide was 16% higher in pregnant women or postpartum women than nonpregnant or nonpostpartum women in the United States, according to 2018 and 2019 mortality data from the National Center for Health Statistics.
Homicide has long been identified as a leading cause of death during pregnancy, but homicide is not counted in estimates of maternal mortality, nor is it emphasized as a target for prevention and intervention, wrote Maeve Wallace, PhD, of Tulane University, New Orleans, and colleagues.
Data on maternal mortality (defined as “death while pregnant or within 42 days of the end of pregnancy from causes related to or aggravated by pregnancy”) were limited until the addition of pregnancy to the U.S. Standard Certificate of Death in 2003; all 50 states had adopted it by 2018, the researchers noted.
In a study published in Obstetrics & Gynecology, the researchers analyzed the first 2 years of nationally available data to identify pregnancy-associated mortality and characterize other risk factors such as age and race.
The researchers identified 4,705 female homicides in 2018 and 2019. Of these, 273 (5.8%) occurred in women who were pregnant or within a year of the end of pregnancy. Approximately half (50.2%) of the pregnant or postpartum victims were non-Hispanic Black, 30% were non-Hispanic white, 9.5% were Hispanic, and 10.3% were other races; approximately one-third (35.5%) were in the 20- to 24-year age group.
Overall, the ratio was 3.62 homicides per 100,000 live births among females who were either pregnant or within 1 year post partum, compared to 3.12 homicides per 100,000 live births in nonpregnant, nonpostpartum females aged 10-44 years (P = .05).
“Patterns were similar in further stratification by both race and age such that pregnancy was associated with more than a doubled risk of homicide among girls and women aged 10–24 in both the non-Hispanic White and non-Hispanic Black populations,” the researchers wrote.
The findings are consistent with previous studies, which “implicates health and social system failures. Although we are unable to directly evaluate the involvement of intimate partner violence (IPV) in this report, we did find that a majority of pregnancy-associated homicides occurred in the home, implicating the likelihood of involvement by persons known to the victim,” they noted. In addition, the data showed that approximately 70% of the incidents of homicide in pregnant and postpartum women involved a firearm, an increase over previous estimates.
The study findings were limited by several factors including the lack of circumstantial information and incomplete data on victim characteristics, the researchers noted. Other key limitations included the potential for false-positives and false-negatives when recording pregnancy status, which could lead to underestimates of pregnancy-associated homicides, and the lack of data on pregnancy outcomes for women who experienced live birth, abortion, or miscarriage within a year of death.
However, the results highlight the need for increased awareness and training of physicians in completing the pregnancy checkbox on death certificates, and the need for action on recommendations and interventions to prevent maternal deaths from homicide, they emphasized.
“Although encouraging, a commitment to the actual implementation of policies and investments known to be effective at protecting and the promoting the health and safety of girls and women must follow,” they concluded.
Data highlight disparities
“This study could not be done effectively prior to now, as the adoption of the pregnancy checkbox on the U.S. Standard Certificate of Death was only available in all 50 states as of 2018,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview.
“This study also demonstrates what was already known, which is that pregnancy is a high-risk time period for intimate partner violence, including homicide. The differences in homicide rates based on race and ethnicity also highlight the clear disparities in maternal mortality in the U.S. that are attributable to racism. There is more attention being paid to maternal mortality and the differential experience based on race, and this demonstrates that simply addressing medical management during pregnancy is not enough – we need to address root causes of racism if we truly want to reduce maternal mortality,” Dr. Prager said.
“The primary take-home message for clinicians is to ascertain safety from every patient, and to try to reduce the impacts of racism on health care for patients, especially during pregnancy,” she said.
Although more detailed records would help with elucidating causes versus associations, “more research is not the answer,” Dr. Prager stated. “The real solution here is to have better gun safety laws, and to put significant resources toward reducing the impacts of racism on health care and our society.”
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose, but serves on the editorial advisory board of Ob.Gyn News.
The prevalence of homicide was 16% higher in pregnant women or postpartum women than nonpregnant or nonpostpartum women in the United States, according to 2018 and 2019 mortality data from the National Center for Health Statistics.
Homicide has long been identified as a leading cause of death during pregnancy, but homicide is not counted in estimates of maternal mortality, nor is it emphasized as a target for prevention and intervention, wrote Maeve Wallace, PhD, of Tulane University, New Orleans, and colleagues.
Data on maternal mortality (defined as “death while pregnant or within 42 days of the end of pregnancy from causes related to or aggravated by pregnancy”) were limited until the addition of pregnancy to the U.S. Standard Certificate of Death in 2003; all 50 states had adopted it by 2018, the researchers noted.
In a study published in Obstetrics & Gynecology, the researchers analyzed the first 2 years of nationally available data to identify pregnancy-associated mortality and characterize other risk factors such as age and race.
The researchers identified 4,705 female homicides in 2018 and 2019. Of these, 273 (5.8%) occurred in women who were pregnant or within a year of the end of pregnancy. Approximately half (50.2%) of the pregnant or postpartum victims were non-Hispanic Black, 30% were non-Hispanic white, 9.5% were Hispanic, and 10.3% were other races; approximately one-third (35.5%) were in the 20- to 24-year age group.
Overall, the ratio was 3.62 homicides per 100,000 live births among females who were either pregnant or within 1 year post partum, compared to 3.12 homicides per 100,000 live births in nonpregnant, nonpostpartum females aged 10-44 years (P = .05).
“Patterns were similar in further stratification by both race and age such that pregnancy was associated with more than a doubled risk of homicide among girls and women aged 10–24 in both the non-Hispanic White and non-Hispanic Black populations,” the researchers wrote.
The findings are consistent with previous studies, which “implicates health and social system failures. Although we are unable to directly evaluate the involvement of intimate partner violence (IPV) in this report, we did find that a majority of pregnancy-associated homicides occurred in the home, implicating the likelihood of involvement by persons known to the victim,” they noted. In addition, the data showed that approximately 70% of the incidents of homicide in pregnant and postpartum women involved a firearm, an increase over previous estimates.
The study findings were limited by several factors including the lack of circumstantial information and incomplete data on victim characteristics, the researchers noted. Other key limitations included the potential for false-positives and false-negatives when recording pregnancy status, which could lead to underestimates of pregnancy-associated homicides, and the lack of data on pregnancy outcomes for women who experienced live birth, abortion, or miscarriage within a year of death.
However, the results highlight the need for increased awareness and training of physicians in completing the pregnancy checkbox on death certificates, and the need for action on recommendations and interventions to prevent maternal deaths from homicide, they emphasized.
“Although encouraging, a commitment to the actual implementation of policies and investments known to be effective at protecting and the promoting the health and safety of girls and women must follow,” they concluded.
Data highlight disparities
“This study could not be done effectively prior to now, as the adoption of the pregnancy checkbox on the U.S. Standard Certificate of Death was only available in all 50 states as of 2018,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview.
“This study also demonstrates what was already known, which is that pregnancy is a high-risk time period for intimate partner violence, including homicide. The differences in homicide rates based on race and ethnicity also highlight the clear disparities in maternal mortality in the U.S. that are attributable to racism. There is more attention being paid to maternal mortality and the differential experience based on race, and this demonstrates that simply addressing medical management during pregnancy is not enough – we need to address root causes of racism if we truly want to reduce maternal mortality,” Dr. Prager said.
“The primary take-home message for clinicians is to ascertain safety from every patient, and to try to reduce the impacts of racism on health care for patients, especially during pregnancy,” she said.
Although more detailed records would help with elucidating causes versus associations, “more research is not the answer,” Dr. Prager stated. “The real solution here is to have better gun safety laws, and to put significant resources toward reducing the impacts of racism on health care and our society.”
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose, but serves on the editorial advisory board of Ob.Gyn News.
The prevalence of homicide was 16% higher in pregnant women or postpartum women than nonpregnant or nonpostpartum women in the United States, according to 2018 and 2019 mortality data from the National Center for Health Statistics.
Homicide has long been identified as a leading cause of death during pregnancy, but homicide is not counted in estimates of maternal mortality, nor is it emphasized as a target for prevention and intervention, wrote Maeve Wallace, PhD, of Tulane University, New Orleans, and colleagues.
Data on maternal mortality (defined as “death while pregnant or within 42 days of the end of pregnancy from causes related to or aggravated by pregnancy”) were limited until the addition of pregnancy to the U.S. Standard Certificate of Death in 2003; all 50 states had adopted it by 2018, the researchers noted.
In a study published in Obstetrics & Gynecology, the researchers analyzed the first 2 years of nationally available data to identify pregnancy-associated mortality and characterize other risk factors such as age and race.
The researchers identified 4,705 female homicides in 2018 and 2019. Of these, 273 (5.8%) occurred in women who were pregnant or within a year of the end of pregnancy. Approximately half (50.2%) of the pregnant or postpartum victims were non-Hispanic Black, 30% were non-Hispanic white, 9.5% were Hispanic, and 10.3% were other races; approximately one-third (35.5%) were in the 20- to 24-year age group.
Overall, the ratio was 3.62 homicides per 100,000 live births among females who were either pregnant or within 1 year post partum, compared to 3.12 homicides per 100,000 live births in nonpregnant, nonpostpartum females aged 10-44 years (P = .05).
“Patterns were similar in further stratification by both race and age such that pregnancy was associated with more than a doubled risk of homicide among girls and women aged 10–24 in both the non-Hispanic White and non-Hispanic Black populations,” the researchers wrote.
The findings are consistent with previous studies, which “implicates health and social system failures. Although we are unable to directly evaluate the involvement of intimate partner violence (IPV) in this report, we did find that a majority of pregnancy-associated homicides occurred in the home, implicating the likelihood of involvement by persons known to the victim,” they noted. In addition, the data showed that approximately 70% of the incidents of homicide in pregnant and postpartum women involved a firearm, an increase over previous estimates.
The study findings were limited by several factors including the lack of circumstantial information and incomplete data on victim characteristics, the researchers noted. Other key limitations included the potential for false-positives and false-negatives when recording pregnancy status, which could lead to underestimates of pregnancy-associated homicides, and the lack of data on pregnancy outcomes for women who experienced live birth, abortion, or miscarriage within a year of death.
However, the results highlight the need for increased awareness and training of physicians in completing the pregnancy checkbox on death certificates, and the need for action on recommendations and interventions to prevent maternal deaths from homicide, they emphasized.
“Although encouraging, a commitment to the actual implementation of policies and investments known to be effective at protecting and the promoting the health and safety of girls and women must follow,” they concluded.
Data highlight disparities
“This study could not be done effectively prior to now, as the adoption of the pregnancy checkbox on the U.S. Standard Certificate of Death was only available in all 50 states as of 2018,” Sarah W. Prager, MD, of the University of Washington, Seattle, said in an interview.
“This study also demonstrates what was already known, which is that pregnancy is a high-risk time period for intimate partner violence, including homicide. The differences in homicide rates based on race and ethnicity also highlight the clear disparities in maternal mortality in the U.S. that are attributable to racism. There is more attention being paid to maternal mortality and the differential experience based on race, and this demonstrates that simply addressing medical management during pregnancy is not enough – we need to address root causes of racism if we truly want to reduce maternal mortality,” Dr. Prager said.
“The primary take-home message for clinicians is to ascertain safety from every patient, and to try to reduce the impacts of racism on health care for patients, especially during pregnancy,” she said.
Although more detailed records would help with elucidating causes versus associations, “more research is not the answer,” Dr. Prager stated. “The real solution here is to have better gun safety laws, and to put significant resources toward reducing the impacts of racism on health care and our society.”
The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The researchers had no financial conflicts to disclose. Dr. Prager had no financial conflicts to disclose, but serves on the editorial advisory board of Ob.Gyn News.
FROM OBSTETRICS & GYNECOLOGY
Synthetic chemical in consumer products linked to early death, study says
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Daily exposure to phthalates, which are synthetic chemicals founds in many consumer products, may lead to hundreds of thousands of early deaths each year among older adults in the United States, according to a new study published Oct. 12, 2021, in the peer-reviewed journal Environmental Pollution.
The chemicals are found in hundreds of types of products, including children’s toys, food storage containers, makeup, perfume, and shampoo. In the study, those with the highest levels of phthalates had a greater risk of death from any cause, especially heart disease.
“This study adds to the growing database on the impact of plastics on the human body and bolsters public health and business cases for reducing or eliminating the use of plastics,” Leonardo Trasande, MD, the lead author and a professor of environmental medicine and population health at New York University Langone Health, told CNN.
Dr. Trasande and colleagues measured the urine concentration of phthalates in more than 5,000 adults aged 55-64 and compared the levels with the risk of early death over an average of 10 years. The research team controlled for preexisting heart diseases, diabetes, cancer, poor eating habits, physical activity, body mass, and other known hormone disruptors such as bisphenol A, or BPA, an industrial chemical that’s been used since the 1950s to make certain plastics and resins, according to the Mayo Clinic
The research team found that phthalates could contribute to 91,000-107,000 premature deaths per year in the United States. These early deaths could cost the nation $40 billion to $47 billion each year in lost economic productivity.
Phthalates interrupt the body’s endocrine system and hormone production. Previous studies have found that the chemicals are linked with developmental, reproductive, and immune system problems, according to NYU Langone Health. They’ve also been linked with asthma, childhood obesity, heart issues, and cancer.
“These chemicals have a rap sheet,” Dr. Trasande told CNN. “And the fact of the matter is that when you look at the entire body of evidence, it provides a haunting pattern of concern.”
Phthalates are often called “everywhere chemicals” because they are so common, CNN reported. Also called “plasticizers,” they are added to products to make them more durable, including PVC plumbing, vinyl flooring, medical tubing, garden hoses, food packaging, detergents, clothing, furniture, and automotive materials.
People are often exposed when they breathe contaminated air or consume food that comes into contact with the chemical, according to the Centers for Disease Control and Prevention. Children may be exposed by touching plastic items and putting their hands in their mouth.
Dr. Trasande told CNN that it’s possible to lessen exposure to phthalates and other endocrine disruptors such as BPA by using unscented lotions, laundry detergents, and cleaning supplies, as well as substituting glass, stainless steel, ceramic, and wood for plastic food storage.
“First, avoid plastics as much as you can. Never put plastic containers in the microwave or dishwasher, where the heat can break down the linings so they might be absorbed more readily,” he said. “In addition, cooking at home and reducing your use of processed foods can reduce the levels of the chemical exposures you come in contact with.”
A version of this article first appeared on WebMD.com.
Women with recurrent UTIs express fear, frustration
Fear of antibiotic overuse and frustration with physicians who prescribe them too freely are key sentiments expressed by women with recurrent urinary tract infections (rUTIs), according to findings from a study involving six focus groups.
“Here in our female pelvic medicine reconstructive urology clinic at Cedars-Sinai and at UCLA, we see many women who are referred for evaluation of rUTIs who are very frustrated with their care,” Victoria Scott, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“So with these focus groups, we saw an opportunity to explore why women are so frustrated and to try and improve the care delivered,” she added.
Findings from the study were published online Sept. 1 in The Journal of Urology.
“There is a need for physicians to modify management strategies ... and to devote more research efforts to improving nonantibiotic options for the prevention and treatment of recurrent urinary tract infections, as well as management strategies that better empower patients,” the authors wrote.
Six focus groups
Four or five participants were included in each of the six focus groups – a total of 29 women. All participants reported a history of symptomatic, culture-proven UTI episodes. They had experienced two or more infections in 6 months or three or more infections within 1 year. Women were predominantly White. Most were employed part- or full-time and held a college degree.
From a qualitative analysis of all focus group transcripts, two main themes emerged:
- The negative impact of taking antibiotics for the prevention and treatment of rUTIs.
- Resentment of the medical profession for the way it managed rUTIs.
The researchers found that participants had a good understanding of the deleterious effects from inappropriate antibiotic use, largely gleaned from media sources and the Internet. “Numerous women stated that they had reached such a level of concern about antibiotics that they would resist taking them for prevention or treatment of infections,” Dr. Scott and colleagues pointed out.
These concerns centered around the risk of developing resistance to antibiotics and the ill effects that antibiotics can have on the gastrointestinal and genitourinary microbiomes. Several women reported that they had developed Clostridium difficile infections after taking antibiotics; one of the patients required hospitalization for the infection.
Women also reported concerns that they had been given an antibiotic needlessly for symptoms that might have been caused by a genitourinary condition other than a UTI. They also reported feeling resentful toward practitioners, particularly if they felt the practitioner was overprescribing antibiotics. Some had resorted to consultations with alternative practitioners, such as herbalists. “A second concern discussed by participants was the feeling of being ignored by physicians,” the authors observed.
In this regard, the women felt that their physicians underestimated the burden that rUTIs had on their lives and the detrimental effect that repeated infections had on their relationships, work, and overall quality of life. “These perceptions led to a prevalent mistrust of physicians,” the investigators wrote. This prompted many women to insist that the medical community devote more effort to the development of nonantibiotic options for the prevention and treatment of UTIs.
Improved management strategies
Asked how physicians might improve their management of rUTIs, Dr. Scott shared a number of suggestions. Cardinal rule No. 1: Have the patient undergo a urinalysis to make sure she does have a UTI. “There is a subset of patients among women with rUTIs who come in with a diagnosis of an rUTI but who really have not had documentation of more than one positive urine culture,” Dr. Scott noted. Such a history suggests that they do not have an rUTI.
It’s imperative that physicians rule out commonly misdiagnosed disorders, such as overactive bladder, as a cause of the patient’s symptoms. Symptoms of overactive bladder and rUTIs often overlap. While waiting for results from the urinalysis to confirm or rule out a UTI, young and healthy women may be prescribed a nonsteroidal anti-inflammatory drug (NSAID), such as naproxen, which can help ameliorate symptoms.
Because UTIs are frequently self-limiting, Dr. Scott and others have found that for young, otherwise healthy women, NSAIDs alone can often resolve symptoms of the UTI without use of an antibiotic. For relatively severe symptoms, a urinary analgesic, such as phenazopyridine (Pyridium), may soothe the lining of the urinary tract and relieve pain. Cystex is an over-the-counter urinary analgesic that women can procure themselves, Dr. Scott added.
If an antibiotic is indicated, those most commonly prescribed for a single episode of acute cystitis are nitrofurantoin and sulfamethoxazole plus trimethoprim (Bactrim). For recurrent UTIs, “patients are a bit more complicated,” Dr. Scott admitted. “I think the best practice is to look back at a woman’s prior urine culture and select an antibiotic that showed good sensitivity in the last positive urine test,” she said.
Prevention starts with behavioral strategies, such as voiding after sexual intercourse and wiping from front to back following urination to avoid introducing fecal bacteria into the urethra. Evidence suggests that premenopausal women who drink at least 1.5 L of water a day have significantly fewer UTI episodes, Dr. Scott noted. There is also “pretty good” evidence that cranberry supplements (not juice) can prevent rUTIs. Use of cranberry supplements is supported by the American Urological Association (conditional recommendation; evidence level of grade C).
For peri- and postmenopausal women, vaginal estrogen may be effective. It’s use for UTI prevention is well supported by the literature. Although not as well supported by evidence, some women find that a supplement such as D-mannose may prevent or treat UTIs by causing bacteria to bind to it rather than to the bladder wall. Probiotics are another possibility, she noted. Empathy can’t hurt, she added.
“A common theme among satisfied women was the sentiment that their physicians understood their problems and had a system in place to allow rapid diagnosis and treatment for UTI episodes,” the authors emphasized.
“[Such attitudes] highlight the need to investigate each patient’s experience and perceptions to allow for shared decision making regarding the management of rUTIs,” they wrote.
Further commentary
Asked to comment on the findings, editorialist Michelle Van Kuiken, MD, assistant professor of urology, University of California, San Francisco, acknowledged that there is not a lot of good evidence to support many of the strategies recommended by the American Urological Association to prevent and treat rUTIs, but she often follows these recommendations anyway. “The one statement in the guidelines that is the most supported by evidence is the use of cranberry supplements, and I do routinely recommended daily use of some form of concentrated cranberry supplements for all of my patients with rUTIs,” she said in an interview.
Dr. Van Kuiken said that vaginal estrogen is a very good option for all postmenopausal women who suffer from rUTIs and that there is growing acceptance of its use for this and other indications. There is some evidence to support D-mannose as well, although it’s not that robust, she acknowledged.
She said the evidence supporting the use of probiotics for this indication is very thin. She does not routinely recommend them for rUTIs, although they are not inherently harmful. “I think for a lot of women who have rUTIs, it can be pretty debilitating and upsetting for them – it can impact travel plans, work, and social events,” Dr. Van Kuiken said.
“Until we develop better diagnostic and therapeutic strategies, validating women’s experiences and concerns with rUTI while limiting unnecessary antibiotics remains our best option,” she wrote.
Dr. Scott and Dr. Van Kuiken have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fear of antibiotic overuse and frustration with physicians who prescribe them too freely are key sentiments expressed by women with recurrent urinary tract infections (rUTIs), according to findings from a study involving six focus groups.
“Here in our female pelvic medicine reconstructive urology clinic at Cedars-Sinai and at UCLA, we see many women who are referred for evaluation of rUTIs who are very frustrated with their care,” Victoria Scott, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“So with these focus groups, we saw an opportunity to explore why women are so frustrated and to try and improve the care delivered,” she added.
Findings from the study were published online Sept. 1 in The Journal of Urology.
“There is a need for physicians to modify management strategies ... and to devote more research efforts to improving nonantibiotic options for the prevention and treatment of recurrent urinary tract infections, as well as management strategies that better empower patients,” the authors wrote.
Six focus groups
Four or five participants were included in each of the six focus groups – a total of 29 women. All participants reported a history of symptomatic, culture-proven UTI episodes. They had experienced two or more infections in 6 months or three or more infections within 1 year. Women were predominantly White. Most were employed part- or full-time and held a college degree.
From a qualitative analysis of all focus group transcripts, two main themes emerged:
- The negative impact of taking antibiotics for the prevention and treatment of rUTIs.
- Resentment of the medical profession for the way it managed rUTIs.
The researchers found that participants had a good understanding of the deleterious effects from inappropriate antibiotic use, largely gleaned from media sources and the Internet. “Numerous women stated that they had reached such a level of concern about antibiotics that they would resist taking them for prevention or treatment of infections,” Dr. Scott and colleagues pointed out.
These concerns centered around the risk of developing resistance to antibiotics and the ill effects that antibiotics can have on the gastrointestinal and genitourinary microbiomes. Several women reported that they had developed Clostridium difficile infections after taking antibiotics; one of the patients required hospitalization for the infection.
Women also reported concerns that they had been given an antibiotic needlessly for symptoms that might have been caused by a genitourinary condition other than a UTI. They also reported feeling resentful toward practitioners, particularly if they felt the practitioner was overprescribing antibiotics. Some had resorted to consultations with alternative practitioners, such as herbalists. “A second concern discussed by participants was the feeling of being ignored by physicians,” the authors observed.
In this regard, the women felt that their physicians underestimated the burden that rUTIs had on their lives and the detrimental effect that repeated infections had on their relationships, work, and overall quality of life. “These perceptions led to a prevalent mistrust of physicians,” the investigators wrote. This prompted many women to insist that the medical community devote more effort to the development of nonantibiotic options for the prevention and treatment of UTIs.
Improved management strategies
Asked how physicians might improve their management of rUTIs, Dr. Scott shared a number of suggestions. Cardinal rule No. 1: Have the patient undergo a urinalysis to make sure she does have a UTI. “There is a subset of patients among women with rUTIs who come in with a diagnosis of an rUTI but who really have not had documentation of more than one positive urine culture,” Dr. Scott noted. Such a history suggests that they do not have an rUTI.
It’s imperative that physicians rule out commonly misdiagnosed disorders, such as overactive bladder, as a cause of the patient’s symptoms. Symptoms of overactive bladder and rUTIs often overlap. While waiting for results from the urinalysis to confirm or rule out a UTI, young and healthy women may be prescribed a nonsteroidal anti-inflammatory drug (NSAID), such as naproxen, which can help ameliorate symptoms.
Because UTIs are frequently self-limiting, Dr. Scott and others have found that for young, otherwise healthy women, NSAIDs alone can often resolve symptoms of the UTI without use of an antibiotic. For relatively severe symptoms, a urinary analgesic, such as phenazopyridine (Pyridium), may soothe the lining of the urinary tract and relieve pain. Cystex is an over-the-counter urinary analgesic that women can procure themselves, Dr. Scott added.
If an antibiotic is indicated, those most commonly prescribed for a single episode of acute cystitis are nitrofurantoin and sulfamethoxazole plus trimethoprim (Bactrim). For recurrent UTIs, “patients are a bit more complicated,” Dr. Scott admitted. “I think the best practice is to look back at a woman’s prior urine culture and select an antibiotic that showed good sensitivity in the last positive urine test,” she said.
Prevention starts with behavioral strategies, such as voiding after sexual intercourse and wiping from front to back following urination to avoid introducing fecal bacteria into the urethra. Evidence suggests that premenopausal women who drink at least 1.5 L of water a day have significantly fewer UTI episodes, Dr. Scott noted. There is also “pretty good” evidence that cranberry supplements (not juice) can prevent rUTIs. Use of cranberry supplements is supported by the American Urological Association (conditional recommendation; evidence level of grade C).
For peri- and postmenopausal women, vaginal estrogen may be effective. It’s use for UTI prevention is well supported by the literature. Although not as well supported by evidence, some women find that a supplement such as D-mannose may prevent or treat UTIs by causing bacteria to bind to it rather than to the bladder wall. Probiotics are another possibility, she noted. Empathy can’t hurt, she added.
“A common theme among satisfied women was the sentiment that their physicians understood their problems and had a system in place to allow rapid diagnosis and treatment for UTI episodes,” the authors emphasized.
“[Such attitudes] highlight the need to investigate each patient’s experience and perceptions to allow for shared decision making regarding the management of rUTIs,” they wrote.
Further commentary
Asked to comment on the findings, editorialist Michelle Van Kuiken, MD, assistant professor of urology, University of California, San Francisco, acknowledged that there is not a lot of good evidence to support many of the strategies recommended by the American Urological Association to prevent and treat rUTIs, but she often follows these recommendations anyway. “The one statement in the guidelines that is the most supported by evidence is the use of cranberry supplements, and I do routinely recommended daily use of some form of concentrated cranberry supplements for all of my patients with rUTIs,” she said in an interview.
Dr. Van Kuiken said that vaginal estrogen is a very good option for all postmenopausal women who suffer from rUTIs and that there is growing acceptance of its use for this and other indications. There is some evidence to support D-mannose as well, although it’s not that robust, she acknowledged.
She said the evidence supporting the use of probiotics for this indication is very thin. She does not routinely recommend them for rUTIs, although they are not inherently harmful. “I think for a lot of women who have rUTIs, it can be pretty debilitating and upsetting for them – it can impact travel plans, work, and social events,” Dr. Van Kuiken said.
“Until we develop better diagnostic and therapeutic strategies, validating women’s experiences and concerns with rUTI while limiting unnecessary antibiotics remains our best option,” she wrote.
Dr. Scott and Dr. Van Kuiken have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fear of antibiotic overuse and frustration with physicians who prescribe them too freely are key sentiments expressed by women with recurrent urinary tract infections (rUTIs), according to findings from a study involving six focus groups.
“Here in our female pelvic medicine reconstructive urology clinic at Cedars-Sinai and at UCLA, we see many women who are referred for evaluation of rUTIs who are very frustrated with their care,” Victoria Scott, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“So with these focus groups, we saw an opportunity to explore why women are so frustrated and to try and improve the care delivered,” she added.
Findings from the study were published online Sept. 1 in The Journal of Urology.
“There is a need for physicians to modify management strategies ... and to devote more research efforts to improving nonantibiotic options for the prevention and treatment of recurrent urinary tract infections, as well as management strategies that better empower patients,” the authors wrote.
Six focus groups
Four or five participants were included in each of the six focus groups – a total of 29 women. All participants reported a history of symptomatic, culture-proven UTI episodes. They had experienced two or more infections in 6 months or three or more infections within 1 year. Women were predominantly White. Most were employed part- or full-time and held a college degree.
From a qualitative analysis of all focus group transcripts, two main themes emerged:
- The negative impact of taking antibiotics for the prevention and treatment of rUTIs.
- Resentment of the medical profession for the way it managed rUTIs.
The researchers found that participants had a good understanding of the deleterious effects from inappropriate antibiotic use, largely gleaned from media sources and the Internet. “Numerous women stated that they had reached such a level of concern about antibiotics that they would resist taking them for prevention or treatment of infections,” Dr. Scott and colleagues pointed out.
These concerns centered around the risk of developing resistance to antibiotics and the ill effects that antibiotics can have on the gastrointestinal and genitourinary microbiomes. Several women reported that they had developed Clostridium difficile infections after taking antibiotics; one of the patients required hospitalization for the infection.
Women also reported concerns that they had been given an antibiotic needlessly for symptoms that might have been caused by a genitourinary condition other than a UTI. They also reported feeling resentful toward practitioners, particularly if they felt the practitioner was overprescribing antibiotics. Some had resorted to consultations with alternative practitioners, such as herbalists. “A second concern discussed by participants was the feeling of being ignored by physicians,” the authors observed.
In this regard, the women felt that their physicians underestimated the burden that rUTIs had on their lives and the detrimental effect that repeated infections had on their relationships, work, and overall quality of life. “These perceptions led to a prevalent mistrust of physicians,” the investigators wrote. This prompted many women to insist that the medical community devote more effort to the development of nonantibiotic options for the prevention and treatment of UTIs.
Improved management strategies
Asked how physicians might improve their management of rUTIs, Dr. Scott shared a number of suggestions. Cardinal rule No. 1: Have the patient undergo a urinalysis to make sure she does have a UTI. “There is a subset of patients among women with rUTIs who come in with a diagnosis of an rUTI but who really have not had documentation of more than one positive urine culture,” Dr. Scott noted. Such a history suggests that they do not have an rUTI.
It’s imperative that physicians rule out commonly misdiagnosed disorders, such as overactive bladder, as a cause of the patient’s symptoms. Symptoms of overactive bladder and rUTIs often overlap. While waiting for results from the urinalysis to confirm or rule out a UTI, young and healthy women may be prescribed a nonsteroidal anti-inflammatory drug (NSAID), such as naproxen, which can help ameliorate symptoms.
Because UTIs are frequently self-limiting, Dr. Scott and others have found that for young, otherwise healthy women, NSAIDs alone can often resolve symptoms of the UTI without use of an antibiotic. For relatively severe symptoms, a urinary analgesic, such as phenazopyridine (Pyridium), may soothe the lining of the urinary tract and relieve pain. Cystex is an over-the-counter urinary analgesic that women can procure themselves, Dr. Scott added.
If an antibiotic is indicated, those most commonly prescribed for a single episode of acute cystitis are nitrofurantoin and sulfamethoxazole plus trimethoprim (Bactrim). For recurrent UTIs, “patients are a bit more complicated,” Dr. Scott admitted. “I think the best practice is to look back at a woman’s prior urine culture and select an antibiotic that showed good sensitivity in the last positive urine test,” she said.
Prevention starts with behavioral strategies, such as voiding after sexual intercourse and wiping from front to back following urination to avoid introducing fecal bacteria into the urethra. Evidence suggests that premenopausal women who drink at least 1.5 L of water a day have significantly fewer UTI episodes, Dr. Scott noted. There is also “pretty good” evidence that cranberry supplements (not juice) can prevent rUTIs. Use of cranberry supplements is supported by the American Urological Association (conditional recommendation; evidence level of grade C).
For peri- and postmenopausal women, vaginal estrogen may be effective. It’s use for UTI prevention is well supported by the literature. Although not as well supported by evidence, some women find that a supplement such as D-mannose may prevent or treat UTIs by causing bacteria to bind to it rather than to the bladder wall. Probiotics are another possibility, she noted. Empathy can’t hurt, she added.
“A common theme among satisfied women was the sentiment that their physicians understood their problems and had a system in place to allow rapid diagnosis and treatment for UTI episodes,” the authors emphasized.
“[Such attitudes] highlight the need to investigate each patient’s experience and perceptions to allow for shared decision making regarding the management of rUTIs,” they wrote.
Further commentary
Asked to comment on the findings, editorialist Michelle Van Kuiken, MD, assistant professor of urology, University of California, San Francisco, acknowledged that there is not a lot of good evidence to support many of the strategies recommended by the American Urological Association to prevent and treat rUTIs, but she often follows these recommendations anyway. “The one statement in the guidelines that is the most supported by evidence is the use of cranberry supplements, and I do routinely recommended daily use of some form of concentrated cranberry supplements for all of my patients with rUTIs,” she said in an interview.
Dr. Van Kuiken said that vaginal estrogen is a very good option for all postmenopausal women who suffer from rUTIs and that there is growing acceptance of its use for this and other indications. There is some evidence to support D-mannose as well, although it’s not that robust, she acknowledged.
She said the evidence supporting the use of probiotics for this indication is very thin. She does not routinely recommend them for rUTIs, although they are not inherently harmful. “I think for a lot of women who have rUTIs, it can be pretty debilitating and upsetting for them – it can impact travel plans, work, and social events,” Dr. Van Kuiken said.
“Until we develop better diagnostic and therapeutic strategies, validating women’s experiences and concerns with rUTI while limiting unnecessary antibiotics remains our best option,” she wrote.
Dr. Scott and Dr. Van Kuiken have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.