User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Federal judge pauses strict Texas abortion law
Robert Pitman, a federal district court judge in Austin, sided with the Biden administration and granted the Justice Department’s request to halt enforcement of the new law. The Biden administration sued to stop the law, and Mr. Pitman’s decision pauses the law while it moves through federal courts, The New York Times reported.
In a 113-page ruling, Mr. Pitman criticized the law, also known as Senate Bill 8, for delegating enforcement to private individuals, who can sue anyone who performs abortions or “aids and abets” them. Plaintiffs are encouraged to file lawsuits because they recover legal fees and $10,000 if they win.
“From the moment S.B. 8 went into effect, women have been unlawfully prevented from exercising control over their own lives in ways that are protected by the Constitution,” Mr. Pitman wrote in the ruling.
“This court will not sanction one more day of this offensive deprivation of such an important right,” he said.
The Texas law bans abortions once fetal cardiac activity can be detected, which is usually around 6 weeks of pregnancy. Women may not know they’re pregnant yet during that time frame.
It’s not yet clear what effect Mr. Pitman’s ruling will have on women in Texas, the Times reported. Since the law went into effect about a month ago, women have sought abortion providers in other states. Mr. Pitman’s ruling pauses the enforcement of the law, but clinics may still face retroactive lawsuits for abortions provided while the law was temporarily suspended.
Whole Woman’s Health, which operates four clinics in Texas, said Wednesday that it was “making plans to resume abortion care up to 18 weeks as soon as possible,” the newspaper reported. The Center for Reproductive Rights also said that clinics “hope to resume full abortion services as soon as they are able.”
Late Oct. 6, Texas said it would appeal the ruling. The U.S. Court of Appeals for the Fifth Circuit is one of the most conservative in the country, according to the Times, and another decision could come soon.
A version of this article first appeared on WebMD.com.
Robert Pitman, a federal district court judge in Austin, sided with the Biden administration and granted the Justice Department’s request to halt enforcement of the new law. The Biden administration sued to stop the law, and Mr. Pitman’s decision pauses the law while it moves through federal courts, The New York Times reported.
In a 113-page ruling, Mr. Pitman criticized the law, also known as Senate Bill 8, for delegating enforcement to private individuals, who can sue anyone who performs abortions or “aids and abets” them. Plaintiffs are encouraged to file lawsuits because they recover legal fees and $10,000 if they win.
“From the moment S.B. 8 went into effect, women have been unlawfully prevented from exercising control over their own lives in ways that are protected by the Constitution,” Mr. Pitman wrote in the ruling.
“This court will not sanction one more day of this offensive deprivation of such an important right,” he said.
The Texas law bans abortions once fetal cardiac activity can be detected, which is usually around 6 weeks of pregnancy. Women may not know they’re pregnant yet during that time frame.
It’s not yet clear what effect Mr. Pitman’s ruling will have on women in Texas, the Times reported. Since the law went into effect about a month ago, women have sought abortion providers in other states. Mr. Pitman’s ruling pauses the enforcement of the law, but clinics may still face retroactive lawsuits for abortions provided while the law was temporarily suspended.
Whole Woman’s Health, which operates four clinics in Texas, said Wednesday that it was “making plans to resume abortion care up to 18 weeks as soon as possible,” the newspaper reported. The Center for Reproductive Rights also said that clinics “hope to resume full abortion services as soon as they are able.”
Late Oct. 6, Texas said it would appeal the ruling. The U.S. Court of Appeals for the Fifth Circuit is one of the most conservative in the country, according to the Times, and another decision could come soon.
A version of this article first appeared on WebMD.com.
Robert Pitman, a federal district court judge in Austin, sided with the Biden administration and granted the Justice Department’s request to halt enforcement of the new law. The Biden administration sued to stop the law, and Mr. Pitman’s decision pauses the law while it moves through federal courts, The New York Times reported.
In a 113-page ruling, Mr. Pitman criticized the law, also known as Senate Bill 8, for delegating enforcement to private individuals, who can sue anyone who performs abortions or “aids and abets” them. Plaintiffs are encouraged to file lawsuits because they recover legal fees and $10,000 if they win.
“From the moment S.B. 8 went into effect, women have been unlawfully prevented from exercising control over their own lives in ways that are protected by the Constitution,” Mr. Pitman wrote in the ruling.
“This court will not sanction one more day of this offensive deprivation of such an important right,” he said.
The Texas law bans abortions once fetal cardiac activity can be detected, which is usually around 6 weeks of pregnancy. Women may not know they’re pregnant yet during that time frame.
It’s not yet clear what effect Mr. Pitman’s ruling will have on women in Texas, the Times reported. Since the law went into effect about a month ago, women have sought abortion providers in other states. Mr. Pitman’s ruling pauses the enforcement of the law, but clinics may still face retroactive lawsuits for abortions provided while the law was temporarily suspended.
Whole Woman’s Health, which operates four clinics in Texas, said Wednesday that it was “making plans to resume abortion care up to 18 weeks as soon as possible,” the newspaper reported. The Center for Reproductive Rights also said that clinics “hope to resume full abortion services as soon as they are able.”
Late Oct. 6, Texas said it would appeal the ruling. The U.S. Court of Appeals for the Fifth Circuit is one of the most conservative in the country, according to the Times, and another decision could come soon.
A version of this article first appeared on WebMD.com.
Maternal SSRI use linked to more encephalopathy in newborns, risk still small
, although the overall risk remains extremely low, a new study finds.
The findings were presented in a poster at the 50th annual meeting of the Child Neurology Society.
“Our work showed that neonates exposed to SSRI in utero had higher risks of neonatal encephalopathy even when adjusting for confounders such as maternal mental health disorders and age. SSRIs could cause side effects such as encephalopathy in neonates, and these risks need to be balanced carefully with the potential benefits of treatment to the mother,” study lead author Marie Cornet, MD, a neonatology fellow with Benioff Children’s Hospital at the University of California, San Francisco, said in an interview.
According to Dr. Cornet, “we know that SSRI exposure in utero is associated with increased risks of respiratory distress at birth, need for positive-pressure ventilation, and an abnormal neurologic exam.” The researchers launched the new study to determine if the estimated 4%-8% of pregnant women who take SSRIs may be putting their newborns at greater risk of NE.
The researchers retrospectively tracked 305,426 infants who were born in the Kaiser Permanente Northern California health system (≥35 weeks) from 2011 to 2019. The mothers had an average age of 31 years, and approximately 34.7% were White, 34.7% of unknown race, 23.3% Asian, and 6.2% Black.
The researchers defined NE as a “5-minute APGAR score <7 and abnormal level of consciousness, activity, tone, or reflexes.”
A total of 8,024 infants (2.6%) had mothers who used SSRIs in the third trimester, and 510 (0.17%) were determined to have had NE.
After adjustment for maternal depression or anxiety, maternal age, race, and hospital, exposed neonates had 2.7 times higher odds of NE (odds ratio, 2.7).
Each 25 mg per day increase in the dose of SSRIs, as equalized to doses of sertraline (Zoloft), was linked to a significant 31% increase in the odds of developing NE (OR, 1.31).
The study doesn’t examine the benefits of SSRI treatment in pregnancy. Those taking SSRIs were much more likely to have depression during pregnancy (76.5% vs. 13.5%) and anxiety (56.7% vs. 6.9%), compared with those who did not take the drug.
The possible connection between SSRIs and NE is unclear. “SSRIs may contribute to NE by a withdrawal mechanism or by a toxicity mechanism. It is also possible that SSRIs themselves are not responsible for encephalopathy, or that the severity of maternal mental health is itself responsible for increased neonatal encephalopathy,” Dr. Cornet said. “However, we believe we adjusted our results thoroughly for that. Furthermore, in this cohort, neonates born from mothers with untreated depression were not at higher risk of encephalopathy than neonates born to mothers without depression.”
She added: “When infants have acidosis or require prolonged resuscitation after birth, they get treated with therapeutic hypothermia. This invasive treatment was shown to decrease mortality and morbidity in neonates with hypoxic-ischemic encephalopathy. However, therapeutic hypothermia may not be helpful in infants with encephalopathy due to other causes than acute hypoxia-ischemia, such as infection, inflammation, genetic conditions, or exposure to toxins. In the case of SSRIs, our results show that, while neonates often have encephalopathy, this encephalopathy is often mild and self-resolved. We did not see a statistically significant increase in acidosis or treatment with therapeutic hypothermia.”
In the future neurologists should consider SSRI use as a potential cause in cases of NE, Dr. Cornet said. “If there are no signs of hypoxic-ischemic encephalopathy – no evidence of acidosis, acute perinatal event – treatment with therapeutic hypothermia may not be indicated.”
Dr. Cornet said more research is in the works. “Studying the long-term side effect of SSRIs on neonatal brain development and injury is essential,” she said. “We plan to compare brain injury in neonates exposed and unexposed to SSRIs and examine long-term outcomes to assess if the effect of SSRI exposure is transient or has a lasting impact.”
This study was funded by the Thrasher Early Career Research Grant and by the Newborn Brain Research Innovation Award at UCSF. The authors have no relevant disclosures.
, although the overall risk remains extremely low, a new study finds.
The findings were presented in a poster at the 50th annual meeting of the Child Neurology Society.
“Our work showed that neonates exposed to SSRI in utero had higher risks of neonatal encephalopathy even when adjusting for confounders such as maternal mental health disorders and age. SSRIs could cause side effects such as encephalopathy in neonates, and these risks need to be balanced carefully with the potential benefits of treatment to the mother,” study lead author Marie Cornet, MD, a neonatology fellow with Benioff Children’s Hospital at the University of California, San Francisco, said in an interview.
According to Dr. Cornet, “we know that SSRI exposure in utero is associated with increased risks of respiratory distress at birth, need for positive-pressure ventilation, and an abnormal neurologic exam.” The researchers launched the new study to determine if the estimated 4%-8% of pregnant women who take SSRIs may be putting their newborns at greater risk of NE.
The researchers retrospectively tracked 305,426 infants who were born in the Kaiser Permanente Northern California health system (≥35 weeks) from 2011 to 2019. The mothers had an average age of 31 years, and approximately 34.7% were White, 34.7% of unknown race, 23.3% Asian, and 6.2% Black.
The researchers defined NE as a “5-minute APGAR score <7 and abnormal level of consciousness, activity, tone, or reflexes.”
A total of 8,024 infants (2.6%) had mothers who used SSRIs in the third trimester, and 510 (0.17%) were determined to have had NE.
After adjustment for maternal depression or anxiety, maternal age, race, and hospital, exposed neonates had 2.7 times higher odds of NE (odds ratio, 2.7).
Each 25 mg per day increase in the dose of SSRIs, as equalized to doses of sertraline (Zoloft), was linked to a significant 31% increase in the odds of developing NE (OR, 1.31).
The study doesn’t examine the benefits of SSRI treatment in pregnancy. Those taking SSRIs were much more likely to have depression during pregnancy (76.5% vs. 13.5%) and anxiety (56.7% vs. 6.9%), compared with those who did not take the drug.
The possible connection between SSRIs and NE is unclear. “SSRIs may contribute to NE by a withdrawal mechanism or by a toxicity mechanism. It is also possible that SSRIs themselves are not responsible for encephalopathy, or that the severity of maternal mental health is itself responsible for increased neonatal encephalopathy,” Dr. Cornet said. “However, we believe we adjusted our results thoroughly for that. Furthermore, in this cohort, neonates born from mothers with untreated depression were not at higher risk of encephalopathy than neonates born to mothers without depression.”
She added: “When infants have acidosis or require prolonged resuscitation after birth, they get treated with therapeutic hypothermia. This invasive treatment was shown to decrease mortality and morbidity in neonates with hypoxic-ischemic encephalopathy. However, therapeutic hypothermia may not be helpful in infants with encephalopathy due to other causes than acute hypoxia-ischemia, such as infection, inflammation, genetic conditions, or exposure to toxins. In the case of SSRIs, our results show that, while neonates often have encephalopathy, this encephalopathy is often mild and self-resolved. We did not see a statistically significant increase in acidosis or treatment with therapeutic hypothermia.”
In the future neurologists should consider SSRI use as a potential cause in cases of NE, Dr. Cornet said. “If there are no signs of hypoxic-ischemic encephalopathy – no evidence of acidosis, acute perinatal event – treatment with therapeutic hypothermia may not be indicated.”
Dr. Cornet said more research is in the works. “Studying the long-term side effect of SSRIs on neonatal brain development and injury is essential,” she said. “We plan to compare brain injury in neonates exposed and unexposed to SSRIs and examine long-term outcomes to assess if the effect of SSRI exposure is transient or has a lasting impact.”
This study was funded by the Thrasher Early Career Research Grant and by the Newborn Brain Research Innovation Award at UCSF. The authors have no relevant disclosures.
, although the overall risk remains extremely low, a new study finds.
The findings were presented in a poster at the 50th annual meeting of the Child Neurology Society.
“Our work showed that neonates exposed to SSRI in utero had higher risks of neonatal encephalopathy even when adjusting for confounders such as maternal mental health disorders and age. SSRIs could cause side effects such as encephalopathy in neonates, and these risks need to be balanced carefully with the potential benefits of treatment to the mother,” study lead author Marie Cornet, MD, a neonatology fellow with Benioff Children’s Hospital at the University of California, San Francisco, said in an interview.
According to Dr. Cornet, “we know that SSRI exposure in utero is associated with increased risks of respiratory distress at birth, need for positive-pressure ventilation, and an abnormal neurologic exam.” The researchers launched the new study to determine if the estimated 4%-8% of pregnant women who take SSRIs may be putting their newborns at greater risk of NE.
The researchers retrospectively tracked 305,426 infants who were born in the Kaiser Permanente Northern California health system (≥35 weeks) from 2011 to 2019. The mothers had an average age of 31 years, and approximately 34.7% were White, 34.7% of unknown race, 23.3% Asian, and 6.2% Black.
The researchers defined NE as a “5-minute APGAR score <7 and abnormal level of consciousness, activity, tone, or reflexes.”
A total of 8,024 infants (2.6%) had mothers who used SSRIs in the third trimester, and 510 (0.17%) were determined to have had NE.
After adjustment for maternal depression or anxiety, maternal age, race, and hospital, exposed neonates had 2.7 times higher odds of NE (odds ratio, 2.7).
Each 25 mg per day increase in the dose of SSRIs, as equalized to doses of sertraline (Zoloft), was linked to a significant 31% increase in the odds of developing NE (OR, 1.31).
The study doesn’t examine the benefits of SSRI treatment in pregnancy. Those taking SSRIs were much more likely to have depression during pregnancy (76.5% vs. 13.5%) and anxiety (56.7% vs. 6.9%), compared with those who did not take the drug.
The possible connection between SSRIs and NE is unclear. “SSRIs may contribute to NE by a withdrawal mechanism or by a toxicity mechanism. It is also possible that SSRIs themselves are not responsible for encephalopathy, or that the severity of maternal mental health is itself responsible for increased neonatal encephalopathy,” Dr. Cornet said. “However, we believe we adjusted our results thoroughly for that. Furthermore, in this cohort, neonates born from mothers with untreated depression were not at higher risk of encephalopathy than neonates born to mothers without depression.”
She added: “When infants have acidosis or require prolonged resuscitation after birth, they get treated with therapeutic hypothermia. This invasive treatment was shown to decrease mortality and morbidity in neonates with hypoxic-ischemic encephalopathy. However, therapeutic hypothermia may not be helpful in infants with encephalopathy due to other causes than acute hypoxia-ischemia, such as infection, inflammation, genetic conditions, or exposure to toxins. In the case of SSRIs, our results show that, while neonates often have encephalopathy, this encephalopathy is often mild and self-resolved. We did not see a statistically significant increase in acidosis or treatment with therapeutic hypothermia.”
In the future neurologists should consider SSRI use as a potential cause in cases of NE, Dr. Cornet said. “If there are no signs of hypoxic-ischemic encephalopathy – no evidence of acidosis, acute perinatal event – treatment with therapeutic hypothermia may not be indicated.”
Dr. Cornet said more research is in the works. “Studying the long-term side effect of SSRIs on neonatal brain development and injury is essential,” she said. “We plan to compare brain injury in neonates exposed and unexposed to SSRIs and examine long-term outcomes to assess if the effect of SSRI exposure is transient or has a lasting impact.”
This study was funded by the Thrasher Early Career Research Grant and by the Newborn Brain Research Innovation Award at UCSF. The authors have no relevant disclosures.
FROM CNS 2021
Merck’s new COVID-19 pill: ‘Game changer’ or just one more tool?
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Soon after Merck announced on Oct. 1 that it would ask federal regulators for emergency use authorization (EUA) for its auspicious new COVID-19 pill, the accolades began.
Former Food and Drug Administration chief Scott Gottlieb, MD, told CNBC the drug was “a profound game changer.” Top infectious disease expert Anthony S. Fauci, MD, called the early data “impressive.” The World Health Organization termed it “certainly good news,” while saying it awaits more data.
Merck, partnering with Ridgeback Biotherapeutics on the investigational oral antiviral medicine molnupiravir, plans to submit applications to regulatory agencies worldwide, hoping to deliver the first oral antiviral medication for COVID-19.
Interim clinical trial results show that the drug may slash the risk for hospitalization or death by 50% in those with mild to moderate COVID-19.
When the results were found to be so favorable, the study was halted at the recommendation of an independent data-monitoring committee and in consultation with the FDA.
“This anticipated drug has gotten a little more hype than it deserves,” said William Schaffner, MD, professor of preventive medicine and infectious disease specialist at Vanderbilt University Medical Center in Nashville, Tenn. He and others suggest a reality check.
“It’s not exactly a home run, like penicillin for strep throat,” agreed Carl Fichtenbaum, MD, professor of infectious diseases at the University of Cincinnati, who is investigating a similar pill for a rival company, Atea, partnering with Roche.
“But it is encouraging,” he said. “It will probably be an incremental improvement on what we have.” The fact that it can be taken at home is a plus: “Anything we can do to keep people from getting sicker is a good thing.”
“The data show in this higher risk group [those who were studied had at least one risk factor for severe COVID-19, such as age or a medical condition], it reduces the risk of advancing to severe disease by 50%,” Dr. Schaffner said. While that’s a clear benefit for half, it of course leaves the other half without benefit, he said.
Others critiqued the predicted cost of the drug. The U.S. government has already agreed to pay about $700 per patient, according to a new report from Harvard T. H. Chan School of Public Health, Boston, and King’s College Hospital, London. That analysis concluded that the actual cost of production for the 5-day course is $17.74.
“We fully expect that having an oral treatment that reduces the risk of hospitalizations will be significantly cost effective for society,” Melissa Moody, a Merck spokesperson, told this news organization. “We are optimistic that molnupiravir can become an important medicine as part of the global effort to fight the pandemic.”
Merck expects to produce 10 million courses of treatment by the end of the year, with additional doses expected to be produced in 2022, according to a company press release. Earlier in 2021, Merck finalized its agreement with the U.S. government to supply about 1.7 million courses of the drug at the $700 price, once an EUA or FDA approval is given.
Merck also has supply and purchase agreements with other governments worldwide, pending regulatory approval.
Study details
Details about the study findings came from a Merck press release. In the planned interim analysis, Merck and Ridgeback evaluated data from 775 patients initially enrolled in the phase 3 MOVe-OUT trial.
All adults had lab-confirmed mild to moderate COVID-19, and reported onset of symptoms within 5 days of being randomly assigned to the drug or placebo. All had at least one risk factor linked with poor disease outcome (such as older age or obesity).
The drug is a ribonucleoside and works by creating mutations in the virus’s genome, halting the ability of the virus to replicate.
Through day 29 of the study, the drug reduced the risk or hospitalization or death by about 50%. While 7.3% of those who received the drug either died or were hospitalized by day 29, 14.1% of those on placebo did, a statistically significant difference (P = .0012).
Side effects were similar in both groups, with 35% of the drug-treated and 40% of the placebo group reporting some side effect, Merck reported. Adverse drug-related events were 12% in the drug group and 11% in the placebo group. While 1.3% of the drug-treated group quit the study because of an adverse event, 3.4% of the placebo group quit.
Pros, cons, and unknowns
The ability to take the drug orally, and at home, is a definite plus, Dr. Schaffner said, compared with the monoclonal antibody treatment currently approved that must be given intravenously or subcutaneously and in certain locations.
More people could be reached and helped with the option of an at-home, oral medicine, he and others agreed.
The regimen for molnupiravir is four pills, two times daily, for 5 days, even if symptoms are mild. As with other prescription drugs, “there will always be folks who don’t comply completely” with the prescribed regimen, Dr. Schaffner said. With this pill, that might be especially true if the symptoms are very mild.
The 50% reduction is not as effective as the benefit often quoted for monoclonal antibody treatment. In clinical trials of Regeneron’s monoclonal antibody treatment, the regimen reduced COVID-19–related hospitalization or death in high-risk patients by 70%.
Even so, the new pill could change the pandemic’s course, others say. “I think molnupiravir has the potential to change how we take care of people who have COVID and risk factors for developing severe disease,” Rajesh Tim Gandhi, MD, an infectious disease physician at Massachusetts General Hospital and Harvard Medical School in Boston, told this news organization.
“What we’ll need to do, however, is make sure that people get tested quickly after they develop symptoms and, if they’re confirmed to have COVID, start on the pills within 5 days of developing symptoms,” he said, while warning that more data are needed about the drug and the trial results.
Another concern is that the promise of a pill will stall vaccination rates, with some people figuring why get vaccinated when they can obtain the pill if they do get sick.
Relying on treatment alone won’t work, Dr. Schaffner said. “Let’s [also] focus on prevention, which is the vaccine. We have to keep working both sides of the street.”
Dr. Gandhi added: “It’s important to remember that even though molnupiravir reduced the likelihood of hospitalization and death, a number of people who received the drug still got sick enough to end up in the hospital.”
Also unknown, he said, is how severe their disease was and whether they will develop long COVID.
The Merck study included only unvaccinated people. Might it work for those vaccinated people who get a breakthrough infection? “From a purely scientific perspective, there is no reason to believe molnupiravir would not work in people who are vaccinated, but the overall efficacy on top of the vaccine is likely dependent on how well they were able to mount a protective immune response to the vaccine,” Ms. Moody said. Still, Merck believes the pill could be of benefit for these infections too, she added.
As for the expected cost, Ms. Moody said that the company takes into account a number of factors in setting pricing, “but fundamentally we look at the impact of the disease, the benefits that the drug delivers to patients and to society, and at supporting ongoing drug development.”
On Merck’s heels: Pfizer, Roche, Atea
Pfizer is studying an antiviral pill, PF-07321332, a protease inhibitor that blocks the protease enzymes and halts replication of the virus.
In addition to studying the drug in infected patients at high risk of severe illness and in those at typical risk, Pfizer launched a phase 2-3 study in late September that will enroll people who live in the same household as a person with a confirmed, symptomatic COVID-19 infection to see if the drug can prevent disease in those who have been exposed.
Atea and Roche’s COVID pill, AT527, is in phase 3 trials as well. AT527 is an inhibitor of polymerase, an enzyme many viruses have, to stop replications. Atea is evaluating the drug to reduce disease “burden” and for both pre- and postexposure prevention.
Big picture: Role of COVID-19 pills
It may be necessary to target the coronavirus with more than one antiviral agent, said Dr. Fichtenbaum, a principal investigator for the AT527 trials.
“Sometimes viruses require two or three active agents to control their replication,” he said, citing information gleaned from other viral research, such as HIV. For control of HIV infection, a cocktail or combination of antivirals is often recommended.
That may well be the case for COVID-19, Dr. Fichtenbaum said. The goal would be to attack the virus at more than one pathway.
A version of this article first appeared on Medscape.com.
Web of antimicrobials doesn’t hold water
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.
Music plus mushrooms equals therapy
Magic mushrooms have been used recreationally and medicinally for thousands of years, but researchers have found adding music could be a game changer in antidepressant treatment.
The ingredient that makes these mushrooms so magical is psilocybin. It works well for the clinical treatment of mental health conditions and some forms of depression because the “trip” can be contained to one work day, making it easy to administer under supervision. With the accompaniment of music, scientists have found that psilocybin evokes emotion.
This recent study, presented at the European College of Neuropsychopharmacology Congress in Lisbon, tested participants’ emotional response to music before and after the psilocybin. Ketanserin, an antihypertensive drug, was used to test against the effects of psilocybin. The scientist played Mozart and Elgar and found that participants on psilocybin had an emotional response increase of 60%. That response was even greater, compared with ketanserin, which actually lessened the emotional response to music.
“This shows that combination of psilocybin and music has a strong emotional effect, and we believe that this will be important for the therapeutic application of psychedelics if they are approved for clinical use,” said lead researcher Dea Siggaard Stenbæk of the University of Copenhagen.
Professor David J. Nutt of Imperial College in London, who was not involved in the study, said that it supports the use of music for treatment efficacy with psychedelics and suggested that the next step is to “optimise this approach probably through individualising and personalising music tracks in therapy.”
Cue the 1960s LSD music montage.
Chicken ‘white striping is not a disease’
Have you ever sliced open a new pack of chicken breasts to start dinner and noticed white fatty lines running through the chicken? Maybe you thought it was just some extra fat to trim off, but the Humane League calls it “white striping disease.”
Chicken is the No. 1 meat consumed by Americans, so it’s not surprising that chickens are factory farmed and raised to be ready for slaughter quickly, according to CBSNews.com, which reported that the Humane League claims white striping is found in 70% of the chicken in popular grocery stores. The league expressed concern for the chickens’ welfare as they are bred to grow bigger quickly, which is causing the white striping and increasing the fat content of the meat by as much as 224%.
The National Chicken Council told CBS that the league’s findings were unscientific. A spokesperson said, “White striping is not a disease. It is a quality factor in chicken breast meat caused by deposits of fat in the muscle during the bird’s growth and development.” He went on to say that severe white striping happens in 3%-6% of birds, which are mostly used in further processed products, not in chicken breast packages.
Somehow, that’s not making us feel any better.
The itsy bitsy spider lets us all down
Most people do not like spiders. That’s too bad, because spiders are generally nothing but helpful little creatures that prey upon annoying flies and other pests. Then there’s the silk they produce. The ancient Romans used it to treat conditions such as warts and skin lesions. Spiders wrap their eggs in silk to protect them from harmful bacteria.
Of course, we can hardly trust the medical opinions of people from 2,000 years ago, but modern-day studies have not definitively proved whether or not spider silk has any antimicrobial properties.
To settle the matter once and for all, researchers from Denmark built a silk-harvesting machine using the most famous of Danish inventions: Legos. The contraption, sort of a paddle wheel, pulled the silk from several different species of spider pinned down by the researchers. The silk was then tested against three different bacteria species, including good old Escherichia coli.
Unfortunately for our spider friends, their silk has no antimicrobial activity. The researchers suspected that any such activity seen in previous studies was actually caused by improper control for the solvents used to extract the silk; those solvents can have antimicrobial properties on their own. As for protecting their eggs, rather than killing bacteria, the silk likely provides a physical barrier alone.
It is bad news for spiders on the benefit-to-humanity front, but look at the bright side: If their silk had antimicrobial activity, we’d have to start farming them to acquire more silk. And that’s no good. Spiders deserve to roam free, hunt as they please, and drop down on your head from the ceiling.
Anxiety and allergies: Cause, effect, confusion
We’re big fans of science, but as longtime, totally impartial (Science rules!) observers of science’s medical realm, we can see that the day-to-day process of practicing the scientific method occasionally gets a bit messy. And no, we’re not talking about COVID-19.
We’re talking allergies. We’re talking mental health. We’re talking allergic disease and mental health.
We’re talking about a pair of press releases we came across during our never-ending search for material to educate, entertain, and astound our fabulously wonderful and loyal readers. (We say that, of course, in the most impartial way possible.)
The first release was titled, “Allergies including asthma and hay fever not linked to mental health traits” and covered research from the University of Bristol (England). The investigators were trying to determine if “allergic diseases actually causes mental health traits including anxiety, depression, bipolar disorder, and schizophrenia, or vice versa,” according to the release.
What they found, however, was “little evidence of a causal relationship between the onset of allergic disease and mental health.” Again, this is the press release talking.
The second release seemed to suggest the exact opposite: “Study uncovers link between allergies and mental health conditions.” That got our attention. A little more reading revealed that “people with asthma, atopic dermatitis, and hay fever also had a higher likelihood of having depression, anxiety, bipolar disorder, or neuroticism.”
One of the investigators was quoted as saying, “Establishing whether allergic disease causes mental health problems, or vice versa, is important to ensure that resources and treatment strategies are targeted appropriately.”
Did you notice the “vice versa”? Did you notice that it appeared in quotes from both releases? We did, so we took a closer look at the source. The second release covered a group of investigators from the University of Bristol – the same group, and the same study, in fact, as the first one.
So there you have it. One study, two press releases, and one confused journalist. Thank you, science.
Cut risedronate drug holiday to under 2 years in older patients
Any pause in taking the osteoporosis drug risedronate (Actonel) should last no longer than 2 years rather than the 2-3 years currently recommended for bisphosphonates, new research suggests.
In a cohort of patients aged 66 and older in Ontario, Canada, those who had been taking risedronate had a 34% greater risk of a hip fracture during year 2 to year 3 of a pause in taking the drug – a drug holiday – compared with those who had been taking alendronate (Fosamax).
The study showed that “risedronate, which has a shorter half-life, confers relatively less hip fracture protection than alendronate during drug holidays longer than 2 years and careful monitoring and follow-up after 2 years is likely warranted,” Kaley (Kaleen) N. Hayes, Pharm D, PhD, summarized in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research. Dr. Hayes is an assistant professor in the department of health services, policy, and practice at Brown University School of Public Health, Providence, R.I.
“Although alendronate and risedronate have similar effectiveness for preventing fractures on treatment, our findings suggest that older patients on a risedronate drug holiday may benefit from assessment to consider resuming therapy after 2 years to prevent hip fractures,” she elaborated in an email.
Juliet Compston, MD, identified this study as one of the meeting’s clinical science highlights.
“This is the first study to directly compare fracture incidence during a drug holiday after treatment with the two most commonly prescribed oral bisphosphonates, alendronate and risedronate,” she told this news organization in an email.
The difference in fracture incidence during the 3-year drug holiday is “consistent with the known difference in pharmacokinetic properties of the two drugs,” noted Dr. Compston, professor of bone medicine and honorary consultant physician at the University of Cambridge (England) School of Clinical Medicine.
Since the increased risk of fracture after stopping risedronate vs. alendronate was seen by 2 years, “reevaluation of risk in risedronate-treated patients should therefore be considered earlier than the recommended period of 2-3 years after discontinuation,” she said.
“The study does not provide information about the optimal duration of drug holiday for either risedronate or alendronate, but it supports a shorter duration for the former of up to 2 years,” according to Dr. Compston.
Study rationale and findings
“The question of whether people treated for osteoporosis with oral bisphosphonates should have drug holidays is controversial,” Dr. Compston noted, “but many guidelines recommend that in lower-risk individuals who have received bisphosphonates for 5 years, a break from treatment of 2-3 years should be considered.”
Five or more years of bisphosphonate treatment for osteoporosis has been associated with rare adverse effects such as atypical femoral fractures, and these drugs appear to have fracture protection effects that linger for a while, so a drug holiday is recommended for most patients, Dr. Hayes added.
Guidelines such as the 2016 ASBMR task force report on long-term bisphosphonates for osteoporosis, she continued, “acknowledge that evidence for this recommendation comes primarily from the extension trial for alendronate, and patients undergoing a risedronate drug holiday may need to be reassessed earlier because of risedronate’s shorter half-life.”
Compared with alendronate, risedronate accumulates less in the bone and is eliminated more quickly from the body, so its fracture protection during drug holidays may be shorter.
The researchers aimed to estimate the 3-year fracture risk after discontinuing long-term (3 or more years) risedronate vs. alendronate therapy among older adults in Ontario.
From health care administrative data, they identified 120,368 patients aged 66 years and older who had started taking risedronate or alendronate as initial therapy for osteoporosis during the period 2000-2016. They had taken the therapy for 3 or more years (with at least 80% adherence) before stopping it for 120 days or longer.
The researchers found that 45% of patients were taking risedronate and 55% were taking alendronate, which are the main bisphosphonates used in Ontario, Dr. Hayes noted. Etidronate (Didronel) is recommended as second-line therapy and accounts for less than 2% of patients starting oral bisphosphonate therapy.
In an earlier study, the researchers identified a shift toward greater use of risedronate than alendronate since 2008, likely related to newer formulations (for example, monthly and weekly delayed-release formulations of risedronate vs. only weekly alendronate formulations).
The researchers matched 25,077 patients taking alendronate with 25,077 patients taking risedronate, based on fracture risk–related characteristics, including demographics, diagnoses, medication use, and health care use.
The patients had a mean age of 74 when they started taking an oral bisphosphonate; 82% were women and most were White.
Most patients (78%) had received a prescription from a general practitioner and, on average, they took the bisphosphonate therapy for 5.9 years before the drug holiday.
The primary outcome of incident hip fracture during a 3-year drug holiday occurred in 915 patients. There were 12.4 events per 1,000 patients in the risedronate group vs. 10.6 events per 1,000 patients in the alendronate group (hazard ratio, 1.18; 95% confidence interval, 1.04-1.34).
The risks were not significantly higher during year 1 or year 2 of the drug holiday, but the curves began to diverge after 2 years, coauthor Suzanne Cadarette, PhD, of the Leslie Dan Faculty of Pharmacy at the University of Toronto, explained when replying to a question after the presentation. Dr. Cadarette supervised this PhD dissertation research by Dr. Hayes.
The researchers acknowledged that the limitations of their study include a lack of information about race or bone mineral density, and the findings may not apply to a younger, more racially diverse population.
The research was supported by the University of Toronto Dalla Lana School of Public Health and the Leslie Dan Faculty of Pharmacy, a Canadian Institutes of Health Research grant, and a doctoral research award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Any pause in taking the osteoporosis drug risedronate (Actonel) should last no longer than 2 years rather than the 2-3 years currently recommended for bisphosphonates, new research suggests.
In a cohort of patients aged 66 and older in Ontario, Canada, those who had been taking risedronate had a 34% greater risk of a hip fracture during year 2 to year 3 of a pause in taking the drug – a drug holiday – compared with those who had been taking alendronate (Fosamax).
The study showed that “risedronate, which has a shorter half-life, confers relatively less hip fracture protection than alendronate during drug holidays longer than 2 years and careful monitoring and follow-up after 2 years is likely warranted,” Kaley (Kaleen) N. Hayes, Pharm D, PhD, summarized in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research. Dr. Hayes is an assistant professor in the department of health services, policy, and practice at Brown University School of Public Health, Providence, R.I.
“Although alendronate and risedronate have similar effectiveness for preventing fractures on treatment, our findings suggest that older patients on a risedronate drug holiday may benefit from assessment to consider resuming therapy after 2 years to prevent hip fractures,” she elaborated in an email.
Juliet Compston, MD, identified this study as one of the meeting’s clinical science highlights.
“This is the first study to directly compare fracture incidence during a drug holiday after treatment with the two most commonly prescribed oral bisphosphonates, alendronate and risedronate,” she told this news organization in an email.
The difference in fracture incidence during the 3-year drug holiday is “consistent with the known difference in pharmacokinetic properties of the two drugs,” noted Dr. Compston, professor of bone medicine and honorary consultant physician at the University of Cambridge (England) School of Clinical Medicine.
Since the increased risk of fracture after stopping risedronate vs. alendronate was seen by 2 years, “reevaluation of risk in risedronate-treated patients should therefore be considered earlier than the recommended period of 2-3 years after discontinuation,” she said.
“The study does not provide information about the optimal duration of drug holiday for either risedronate or alendronate, but it supports a shorter duration for the former of up to 2 years,” according to Dr. Compston.
Study rationale and findings
“The question of whether people treated for osteoporosis with oral bisphosphonates should have drug holidays is controversial,” Dr. Compston noted, “but many guidelines recommend that in lower-risk individuals who have received bisphosphonates for 5 years, a break from treatment of 2-3 years should be considered.”
Five or more years of bisphosphonate treatment for osteoporosis has been associated with rare adverse effects such as atypical femoral fractures, and these drugs appear to have fracture protection effects that linger for a while, so a drug holiday is recommended for most patients, Dr. Hayes added.
Guidelines such as the 2016 ASBMR task force report on long-term bisphosphonates for osteoporosis, she continued, “acknowledge that evidence for this recommendation comes primarily from the extension trial for alendronate, and patients undergoing a risedronate drug holiday may need to be reassessed earlier because of risedronate’s shorter half-life.”
Compared with alendronate, risedronate accumulates less in the bone and is eliminated more quickly from the body, so its fracture protection during drug holidays may be shorter.
The researchers aimed to estimate the 3-year fracture risk after discontinuing long-term (3 or more years) risedronate vs. alendronate therapy among older adults in Ontario.
From health care administrative data, they identified 120,368 patients aged 66 years and older who had started taking risedronate or alendronate as initial therapy for osteoporosis during the period 2000-2016. They had taken the therapy for 3 or more years (with at least 80% adherence) before stopping it for 120 days or longer.
The researchers found that 45% of patients were taking risedronate and 55% were taking alendronate, which are the main bisphosphonates used in Ontario, Dr. Hayes noted. Etidronate (Didronel) is recommended as second-line therapy and accounts for less than 2% of patients starting oral bisphosphonate therapy.
In an earlier study, the researchers identified a shift toward greater use of risedronate than alendronate since 2008, likely related to newer formulations (for example, monthly and weekly delayed-release formulations of risedronate vs. only weekly alendronate formulations).
The researchers matched 25,077 patients taking alendronate with 25,077 patients taking risedronate, based on fracture risk–related characteristics, including demographics, diagnoses, medication use, and health care use.
The patients had a mean age of 74 when they started taking an oral bisphosphonate; 82% were women and most were White.
Most patients (78%) had received a prescription from a general practitioner and, on average, they took the bisphosphonate therapy for 5.9 years before the drug holiday.
The primary outcome of incident hip fracture during a 3-year drug holiday occurred in 915 patients. There were 12.4 events per 1,000 patients in the risedronate group vs. 10.6 events per 1,000 patients in the alendronate group (hazard ratio, 1.18; 95% confidence interval, 1.04-1.34).
The risks were not significantly higher during year 1 or year 2 of the drug holiday, but the curves began to diverge after 2 years, coauthor Suzanne Cadarette, PhD, of the Leslie Dan Faculty of Pharmacy at the University of Toronto, explained when replying to a question after the presentation. Dr. Cadarette supervised this PhD dissertation research by Dr. Hayes.
The researchers acknowledged that the limitations of their study include a lack of information about race or bone mineral density, and the findings may not apply to a younger, more racially diverse population.
The research was supported by the University of Toronto Dalla Lana School of Public Health and the Leslie Dan Faculty of Pharmacy, a Canadian Institutes of Health Research grant, and a doctoral research award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Any pause in taking the osteoporosis drug risedronate (Actonel) should last no longer than 2 years rather than the 2-3 years currently recommended for bisphosphonates, new research suggests.
In a cohort of patients aged 66 and older in Ontario, Canada, those who had been taking risedronate had a 34% greater risk of a hip fracture during year 2 to year 3 of a pause in taking the drug – a drug holiday – compared with those who had been taking alendronate (Fosamax).
The study showed that “risedronate, which has a shorter half-life, confers relatively less hip fracture protection than alendronate during drug holidays longer than 2 years and careful monitoring and follow-up after 2 years is likely warranted,” Kaley (Kaleen) N. Hayes, Pharm D, PhD, summarized in an oral presentation at the annual meeting of the American Society for Bone and Mineral Research. Dr. Hayes is an assistant professor in the department of health services, policy, and practice at Brown University School of Public Health, Providence, R.I.
“Although alendronate and risedronate have similar effectiveness for preventing fractures on treatment, our findings suggest that older patients on a risedronate drug holiday may benefit from assessment to consider resuming therapy after 2 years to prevent hip fractures,” she elaborated in an email.
Juliet Compston, MD, identified this study as one of the meeting’s clinical science highlights.
“This is the first study to directly compare fracture incidence during a drug holiday after treatment with the two most commonly prescribed oral bisphosphonates, alendronate and risedronate,” she told this news organization in an email.
The difference in fracture incidence during the 3-year drug holiday is “consistent with the known difference in pharmacokinetic properties of the two drugs,” noted Dr. Compston, professor of bone medicine and honorary consultant physician at the University of Cambridge (England) School of Clinical Medicine.
Since the increased risk of fracture after stopping risedronate vs. alendronate was seen by 2 years, “reevaluation of risk in risedronate-treated patients should therefore be considered earlier than the recommended period of 2-3 years after discontinuation,” she said.
“The study does not provide information about the optimal duration of drug holiday for either risedronate or alendronate, but it supports a shorter duration for the former of up to 2 years,” according to Dr. Compston.
Study rationale and findings
“The question of whether people treated for osteoporosis with oral bisphosphonates should have drug holidays is controversial,” Dr. Compston noted, “but many guidelines recommend that in lower-risk individuals who have received bisphosphonates for 5 years, a break from treatment of 2-3 years should be considered.”
Five or more years of bisphosphonate treatment for osteoporosis has been associated with rare adverse effects such as atypical femoral fractures, and these drugs appear to have fracture protection effects that linger for a while, so a drug holiday is recommended for most patients, Dr. Hayes added.
Guidelines such as the 2016 ASBMR task force report on long-term bisphosphonates for osteoporosis, she continued, “acknowledge that evidence for this recommendation comes primarily from the extension trial for alendronate, and patients undergoing a risedronate drug holiday may need to be reassessed earlier because of risedronate’s shorter half-life.”
Compared with alendronate, risedronate accumulates less in the bone and is eliminated more quickly from the body, so its fracture protection during drug holidays may be shorter.
The researchers aimed to estimate the 3-year fracture risk after discontinuing long-term (3 or more years) risedronate vs. alendronate therapy among older adults in Ontario.
From health care administrative data, they identified 120,368 patients aged 66 years and older who had started taking risedronate or alendronate as initial therapy for osteoporosis during the period 2000-2016. They had taken the therapy for 3 or more years (with at least 80% adherence) before stopping it for 120 days or longer.
The researchers found that 45% of patients were taking risedronate and 55% were taking alendronate, which are the main bisphosphonates used in Ontario, Dr. Hayes noted. Etidronate (Didronel) is recommended as second-line therapy and accounts for less than 2% of patients starting oral bisphosphonate therapy.
In an earlier study, the researchers identified a shift toward greater use of risedronate than alendronate since 2008, likely related to newer formulations (for example, monthly and weekly delayed-release formulations of risedronate vs. only weekly alendronate formulations).
The researchers matched 25,077 patients taking alendronate with 25,077 patients taking risedronate, based on fracture risk–related characteristics, including demographics, diagnoses, medication use, and health care use.
The patients had a mean age of 74 when they started taking an oral bisphosphonate; 82% were women and most were White.
Most patients (78%) had received a prescription from a general practitioner and, on average, they took the bisphosphonate therapy for 5.9 years before the drug holiday.
The primary outcome of incident hip fracture during a 3-year drug holiday occurred in 915 patients. There were 12.4 events per 1,000 patients in the risedronate group vs. 10.6 events per 1,000 patients in the alendronate group (hazard ratio, 1.18; 95% confidence interval, 1.04-1.34).
The risks were not significantly higher during year 1 or year 2 of the drug holiday, but the curves began to diverge after 2 years, coauthor Suzanne Cadarette, PhD, of the Leslie Dan Faculty of Pharmacy at the University of Toronto, explained when replying to a question after the presentation. Dr. Cadarette supervised this PhD dissertation research by Dr. Hayes.
The researchers acknowledged that the limitations of their study include a lack of information about race or bone mineral density, and the findings may not apply to a younger, more racially diverse population.
The research was supported by the University of Toronto Dalla Lana School of Public Health and the Leslie Dan Faculty of Pharmacy, a Canadian Institutes of Health Research grant, and a doctoral research award. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2021
Exercise appears to improve bone structure, not density
“Postmenopausal women with low bone mass should obtain adequate calcium and vitamin D and participate in bone-loading exercises,” researchers noted in a recent study published in Osteoporosis International.
“Additional use of bisphosphonates will increase bone mineral density (BMD), especially at the spine,” wrote Nancy Waltman, PhD, College of Nursing, University of Nebraska Medical Center, Omaha, and colleagues.
The findings are partial results from the Heartland Osteoporosis Prevention Study (HOPS), which randomized women who had entered menopause within the previous 6 months and had osteopenia (low bone mass, T score –1.0 to –2.49) to receive one of three treatments for 12 months:
- Bone-loading and resistance exercise plus calcium and vitamin D supplements.
- Risedronate plus calcium and vitamin D supplements.
- Calcium and vitamin D supplements alone (control).
At 1 year, “risedronate significantly increased BMD at the spine, compared to exercise and control, and serum biomarkers of bone turnover also significantly reduced in the risedronate group,” Laura Bilek, PT, PhD, said during an oral presentation of the research at the annual meeting of the American Society for Bone and Mineral Research.
However, the results also showed that, importantly, “in postmenopausal women, exercise appears to improve strength at the hip through changes in structure, not BMD,” stressed Dr. Bilek, of the College of Allied Health Professionals, University of Nebraska Medical Center.
Bone health is about more than just bone mineral density
“The key takeaway for clinicians is that bone health is about more than just density!” she noted in an email.
Current guidelines don’t recommend prescribing risedronate until a woman has overt osteoporosis, she said.
On the other hand, many studies have shown that, to be most effective, bone-loading exercises should be a lifelong habit and women should begin to do them at least during menopause and should not wait until bone loss occurs.
Other studies have shown that exercise changes bone structure (size or geometry), which improves bone strength. The current study supports both prior observations.
And exercise also improves muscle strength and decreases the risk of falls and fractures, Dr. Bilek noted.
Invited to comment, Pauline M. Camacho, MD, cochair of the task force for the American Association of Clinical Endocrinologists (AACE) guidelines for osteoporosis, noted that all three measures – pharmacotherapy, exercise, and calcium/vitamin D – are important in the successful management of osteoporosis.
This study showed that risedronate is superior to calcium/vitamin D supplementation as well as exercise for BMD and for bone turnover in these women with osteopenia, said Dr. Camacho, professor of medicine and director of the Osteoporosis and Metabolic Bone Disease Center, Loyola University Medical Center, Chicago.
“Most women with osteopenia do not receive pharmacologic therapy,” she noted, and receive it only “if there is a history of fractures or they have other features that change that diagnosis to osteoporosis.
“There is no downside to exercise, and this needs to be advised to all patients,” she said. “The other aspect of exercise that was not assessed in this study is its effect on balance. Patients who exercise will have improved balance, which should translate into fewer falls, and thus fewer fractures.”
How can women with osteopenia maintain bone health?
In their article, Dr. Waltman and colleagues say the Lifting Intervention for Training Muscle and Osteoporosis Rehabilitation (LIFTMOR) clinical trial is one of the first to address clinician concerns about the safety and effectiveness of exercise to improve bone health.
In that trial of 101 postmenopausal women with low bone mass, 8 months of 30-minute, twice-weekly, supervised high-intensity resistance and impact training was safe and BMD increased by 2.9% at the lumbar spine and 0.3% at the femoral neck.
“Our [HOPS] study,” Dr. Waltman and colleagues explained, “builds on the LIFTMOR clinical trial and adds further data to inform whether postmenopausal women with low bone mass can effectively maintain or even improve BMD with bone-loading exercises prior to prescriptions for medication.
“Our long-term goal is to contribute to the development of clinical practice guidelines for the prevention of fractures in postmenopausal women with low bone mass,” they said.
They randomized 276 postmenopausal women who were a mean age of 54 (range, 44-63); most were White (78%) or Hispanic (6%).
Women were excluded from the study if they had a diagnosis of osteoporosis (T-score < −2.5); had an increased risk of a major fracture or hip fracture; had been on bisphosphonates within the last 6 months; were currently on estrogen, tamoxifen, or aromatase inhibitors; had a serum vitamin D level < 10 mg/mL or > 100 mg/mL; had any conditions that prohibited prescriptions for calcium and vitamin D supplements, risedronate, or exercise; or weighed more than 300 pounds.
All women received 1,200 mg/day of calcium (from supplements or diet) and 1,000-3,000 IU/day of vitamin D supplements, based on their serum 25(OH) vitamin D levels.
The exercise program consisted of visiting a gym three times a week for 45 minutes of bone-loading exercise – jogging with a weighted vest – and resistance exercises, which were supervised by a trainer for the first 2 weeks.
Women in the risedronate group received a 150-mg tablet of risedronate every 4 weeks.
At baseline, 6 months, and 12 months, the women had DXA scans to determine BMD and hip structure, and had blood tests to determine levels of serum markers for bone formation (bone specific alkaline phosphatase [Alkphase B]) and bone resorption (N-terminal telopeptide [NTx]).
Compared with baseline, at 12 months, the women had the following changes in BMD at the following sites:
- Spine: +1.9%, +0.9%, and –0.4%, in the risedronate, exercise, and control groups.
- Total hip: +0.9%, +0.5%, and +0.5%, in the risedronate, exercise, and control groups.
- Femoral neck: +0.09%, –0.4%, and –0.5%, in the risedronate, exercise, and control groups.
These improvements in BMD were significantly greater in the risedronate group than in the exercise or control groups (P < .01 for both).
The decreases in serum levels of NtX and Alkphase B were also greater with risedronate than in the exercise or control groups (P < .01 for all).
The most frequent adverse effect with the calcium supplement was constipation (n = 4). Some women taking risedronate had gastrointestinal disturbances (n = 4), muscle or joint pain (n = 11), or chest pain and dizziness (n = 2). None of the women had adverse effects from vitamin D. A few women had muscle soreness from exercise that went away after the exercises were adapted. None of the women had a serious injury or fracture from exercise.
More women in the exercise group withdrew from the study (n = 20), with most citing lack of time as the reason; 13 women withdrew from the risedronate group, and 16 withdrew from the control group.
Of the 276 participants who completed the 12-month study, treatment adherence was 92% for calcium, 94% for vitamin D, 75% for risedronate, and 59% for exercise.
Exercise was associated with positive changes in intertrochanter hip structural analysis measures, which will be described in an upcoming study, Dr. Bilek said.
The study was funded by the National Institute of Nursing Research of the National Institutes of Health. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Postmenopausal women with low bone mass should obtain adequate calcium and vitamin D and participate in bone-loading exercises,” researchers noted in a recent study published in Osteoporosis International.
“Additional use of bisphosphonates will increase bone mineral density (BMD), especially at the spine,” wrote Nancy Waltman, PhD, College of Nursing, University of Nebraska Medical Center, Omaha, and colleagues.
The findings are partial results from the Heartland Osteoporosis Prevention Study (HOPS), which randomized women who had entered menopause within the previous 6 months and had osteopenia (low bone mass, T score –1.0 to –2.49) to receive one of three treatments for 12 months:
- Bone-loading and resistance exercise plus calcium and vitamin D supplements.
- Risedronate plus calcium and vitamin D supplements.
- Calcium and vitamin D supplements alone (control).
At 1 year, “risedronate significantly increased BMD at the spine, compared to exercise and control, and serum biomarkers of bone turnover also significantly reduced in the risedronate group,” Laura Bilek, PT, PhD, said during an oral presentation of the research at the annual meeting of the American Society for Bone and Mineral Research.
However, the results also showed that, importantly, “in postmenopausal women, exercise appears to improve strength at the hip through changes in structure, not BMD,” stressed Dr. Bilek, of the College of Allied Health Professionals, University of Nebraska Medical Center.
Bone health is about more than just bone mineral density
“The key takeaway for clinicians is that bone health is about more than just density!” she noted in an email.
Current guidelines don’t recommend prescribing risedronate until a woman has overt osteoporosis, she said.
On the other hand, many studies have shown that, to be most effective, bone-loading exercises should be a lifelong habit and women should begin to do them at least during menopause and should not wait until bone loss occurs.
Other studies have shown that exercise changes bone structure (size or geometry), which improves bone strength. The current study supports both prior observations.
And exercise also improves muscle strength and decreases the risk of falls and fractures, Dr. Bilek noted.
Invited to comment, Pauline M. Camacho, MD, cochair of the task force for the American Association of Clinical Endocrinologists (AACE) guidelines for osteoporosis, noted that all three measures – pharmacotherapy, exercise, and calcium/vitamin D – are important in the successful management of osteoporosis.
This study showed that risedronate is superior to calcium/vitamin D supplementation as well as exercise for BMD and for bone turnover in these women with osteopenia, said Dr. Camacho, professor of medicine and director of the Osteoporosis and Metabolic Bone Disease Center, Loyola University Medical Center, Chicago.
“Most women with osteopenia do not receive pharmacologic therapy,” she noted, and receive it only “if there is a history of fractures or they have other features that change that diagnosis to osteoporosis.
“There is no downside to exercise, and this needs to be advised to all patients,” she said. “The other aspect of exercise that was not assessed in this study is its effect on balance. Patients who exercise will have improved balance, which should translate into fewer falls, and thus fewer fractures.”
How can women with osteopenia maintain bone health?
In their article, Dr. Waltman and colleagues say the Lifting Intervention for Training Muscle and Osteoporosis Rehabilitation (LIFTMOR) clinical trial is one of the first to address clinician concerns about the safety and effectiveness of exercise to improve bone health.
In that trial of 101 postmenopausal women with low bone mass, 8 months of 30-minute, twice-weekly, supervised high-intensity resistance and impact training was safe and BMD increased by 2.9% at the lumbar spine and 0.3% at the femoral neck.
“Our [HOPS] study,” Dr. Waltman and colleagues explained, “builds on the LIFTMOR clinical trial and adds further data to inform whether postmenopausal women with low bone mass can effectively maintain or even improve BMD with bone-loading exercises prior to prescriptions for medication.
“Our long-term goal is to contribute to the development of clinical practice guidelines for the prevention of fractures in postmenopausal women with low bone mass,” they said.
They randomized 276 postmenopausal women who were a mean age of 54 (range, 44-63); most were White (78%) or Hispanic (6%).
Women were excluded from the study if they had a diagnosis of osteoporosis (T-score < −2.5); had an increased risk of a major fracture or hip fracture; had been on bisphosphonates within the last 6 months; were currently on estrogen, tamoxifen, or aromatase inhibitors; had a serum vitamin D level < 10 mg/mL or > 100 mg/mL; had any conditions that prohibited prescriptions for calcium and vitamin D supplements, risedronate, or exercise; or weighed more than 300 pounds.
All women received 1,200 mg/day of calcium (from supplements or diet) and 1,000-3,000 IU/day of vitamin D supplements, based on their serum 25(OH) vitamin D levels.
The exercise program consisted of visiting a gym three times a week for 45 minutes of bone-loading exercise – jogging with a weighted vest – and resistance exercises, which were supervised by a trainer for the first 2 weeks.
Women in the risedronate group received a 150-mg tablet of risedronate every 4 weeks.
At baseline, 6 months, and 12 months, the women had DXA scans to determine BMD and hip structure, and had blood tests to determine levels of serum markers for bone formation (bone specific alkaline phosphatase [Alkphase B]) and bone resorption (N-terminal telopeptide [NTx]).
Compared with baseline, at 12 months, the women had the following changes in BMD at the following sites:
- Spine: +1.9%, +0.9%, and –0.4%, in the risedronate, exercise, and control groups.
- Total hip: +0.9%, +0.5%, and +0.5%, in the risedronate, exercise, and control groups.
- Femoral neck: +0.09%, –0.4%, and –0.5%, in the risedronate, exercise, and control groups.
These improvements in BMD were significantly greater in the risedronate group than in the exercise or control groups (P < .01 for both).
The decreases in serum levels of NtX and Alkphase B were also greater with risedronate than in the exercise or control groups (P < .01 for all).
The most frequent adverse effect with the calcium supplement was constipation (n = 4). Some women taking risedronate had gastrointestinal disturbances (n = 4), muscle or joint pain (n = 11), or chest pain and dizziness (n = 2). None of the women had adverse effects from vitamin D. A few women had muscle soreness from exercise that went away after the exercises were adapted. None of the women had a serious injury or fracture from exercise.
More women in the exercise group withdrew from the study (n = 20), with most citing lack of time as the reason; 13 women withdrew from the risedronate group, and 16 withdrew from the control group.
Of the 276 participants who completed the 12-month study, treatment adherence was 92% for calcium, 94% for vitamin D, 75% for risedronate, and 59% for exercise.
Exercise was associated with positive changes in intertrochanter hip structural analysis measures, which will be described in an upcoming study, Dr. Bilek said.
The study was funded by the National Institute of Nursing Research of the National Institutes of Health. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
“Postmenopausal women with low bone mass should obtain adequate calcium and vitamin D and participate in bone-loading exercises,” researchers noted in a recent study published in Osteoporosis International.
“Additional use of bisphosphonates will increase bone mineral density (BMD), especially at the spine,” wrote Nancy Waltman, PhD, College of Nursing, University of Nebraska Medical Center, Omaha, and colleagues.
The findings are partial results from the Heartland Osteoporosis Prevention Study (HOPS), which randomized women who had entered menopause within the previous 6 months and had osteopenia (low bone mass, T score –1.0 to –2.49) to receive one of three treatments for 12 months:
- Bone-loading and resistance exercise plus calcium and vitamin D supplements.
- Risedronate plus calcium and vitamin D supplements.
- Calcium and vitamin D supplements alone (control).
At 1 year, “risedronate significantly increased BMD at the spine, compared to exercise and control, and serum biomarkers of bone turnover also significantly reduced in the risedronate group,” Laura Bilek, PT, PhD, said during an oral presentation of the research at the annual meeting of the American Society for Bone and Mineral Research.
However, the results also showed that, importantly, “in postmenopausal women, exercise appears to improve strength at the hip through changes in structure, not BMD,” stressed Dr. Bilek, of the College of Allied Health Professionals, University of Nebraska Medical Center.
Bone health is about more than just bone mineral density
“The key takeaway for clinicians is that bone health is about more than just density!” she noted in an email.
Current guidelines don’t recommend prescribing risedronate until a woman has overt osteoporosis, she said.
On the other hand, many studies have shown that, to be most effective, bone-loading exercises should be a lifelong habit and women should begin to do them at least during menopause and should not wait until bone loss occurs.
Other studies have shown that exercise changes bone structure (size or geometry), which improves bone strength. The current study supports both prior observations.
And exercise also improves muscle strength and decreases the risk of falls and fractures, Dr. Bilek noted.
Invited to comment, Pauline M. Camacho, MD, cochair of the task force for the American Association of Clinical Endocrinologists (AACE) guidelines for osteoporosis, noted that all three measures – pharmacotherapy, exercise, and calcium/vitamin D – are important in the successful management of osteoporosis.
This study showed that risedronate is superior to calcium/vitamin D supplementation as well as exercise for BMD and for bone turnover in these women with osteopenia, said Dr. Camacho, professor of medicine and director of the Osteoporosis and Metabolic Bone Disease Center, Loyola University Medical Center, Chicago.
“Most women with osteopenia do not receive pharmacologic therapy,” she noted, and receive it only “if there is a history of fractures or they have other features that change that diagnosis to osteoporosis.
“There is no downside to exercise, and this needs to be advised to all patients,” she said. “The other aspect of exercise that was not assessed in this study is its effect on balance. Patients who exercise will have improved balance, which should translate into fewer falls, and thus fewer fractures.”
How can women with osteopenia maintain bone health?
In their article, Dr. Waltman and colleagues say the Lifting Intervention for Training Muscle and Osteoporosis Rehabilitation (LIFTMOR) clinical trial is one of the first to address clinician concerns about the safety and effectiveness of exercise to improve bone health.
In that trial of 101 postmenopausal women with low bone mass, 8 months of 30-minute, twice-weekly, supervised high-intensity resistance and impact training was safe and BMD increased by 2.9% at the lumbar spine and 0.3% at the femoral neck.
“Our [HOPS] study,” Dr. Waltman and colleagues explained, “builds on the LIFTMOR clinical trial and adds further data to inform whether postmenopausal women with low bone mass can effectively maintain or even improve BMD with bone-loading exercises prior to prescriptions for medication.
“Our long-term goal is to contribute to the development of clinical practice guidelines for the prevention of fractures in postmenopausal women with low bone mass,” they said.
They randomized 276 postmenopausal women who were a mean age of 54 (range, 44-63); most were White (78%) or Hispanic (6%).
Women were excluded from the study if they had a diagnosis of osteoporosis (T-score < −2.5); had an increased risk of a major fracture or hip fracture; had been on bisphosphonates within the last 6 months; were currently on estrogen, tamoxifen, or aromatase inhibitors; had a serum vitamin D level < 10 mg/mL or > 100 mg/mL; had any conditions that prohibited prescriptions for calcium and vitamin D supplements, risedronate, or exercise; or weighed more than 300 pounds.
All women received 1,200 mg/day of calcium (from supplements or diet) and 1,000-3,000 IU/day of vitamin D supplements, based on their serum 25(OH) vitamin D levels.
The exercise program consisted of visiting a gym three times a week for 45 minutes of bone-loading exercise – jogging with a weighted vest – and resistance exercises, which were supervised by a trainer for the first 2 weeks.
Women in the risedronate group received a 150-mg tablet of risedronate every 4 weeks.
At baseline, 6 months, and 12 months, the women had DXA scans to determine BMD and hip structure, and had blood tests to determine levels of serum markers for bone formation (bone specific alkaline phosphatase [Alkphase B]) and bone resorption (N-terminal telopeptide [NTx]).
Compared with baseline, at 12 months, the women had the following changes in BMD at the following sites:
- Spine: +1.9%, +0.9%, and –0.4%, in the risedronate, exercise, and control groups.
- Total hip: +0.9%, +0.5%, and +0.5%, in the risedronate, exercise, and control groups.
- Femoral neck: +0.09%, –0.4%, and –0.5%, in the risedronate, exercise, and control groups.
These improvements in BMD were significantly greater in the risedronate group than in the exercise or control groups (P < .01 for both).
The decreases in serum levels of NtX and Alkphase B were also greater with risedronate than in the exercise or control groups (P < .01 for all).
The most frequent adverse effect with the calcium supplement was constipation (n = 4). Some women taking risedronate had gastrointestinal disturbances (n = 4), muscle or joint pain (n = 11), or chest pain and dizziness (n = 2). None of the women had adverse effects from vitamin D. A few women had muscle soreness from exercise that went away after the exercises were adapted. None of the women had a serious injury or fracture from exercise.
More women in the exercise group withdrew from the study (n = 20), with most citing lack of time as the reason; 13 women withdrew from the risedronate group, and 16 withdrew from the control group.
Of the 276 participants who completed the 12-month study, treatment adherence was 92% for calcium, 94% for vitamin D, 75% for risedronate, and 59% for exercise.
Exercise was associated with positive changes in intertrochanter hip structural analysis measures, which will be described in an upcoming study, Dr. Bilek said.
The study was funded by the National Institute of Nursing Research of the National Institutes of Health. The researchers have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASBMR 2021
2021 update on contraception
A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.
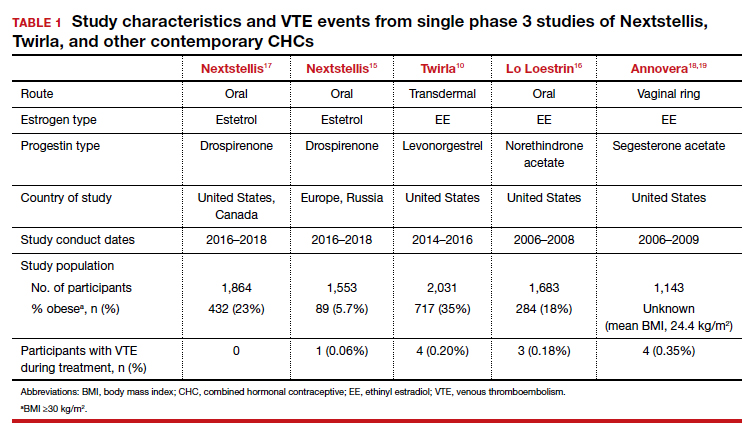
Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%
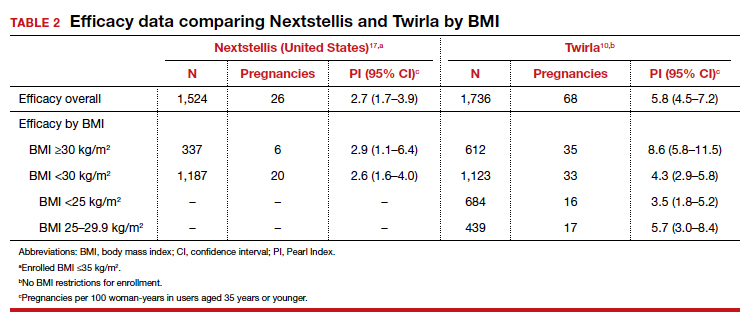
Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.
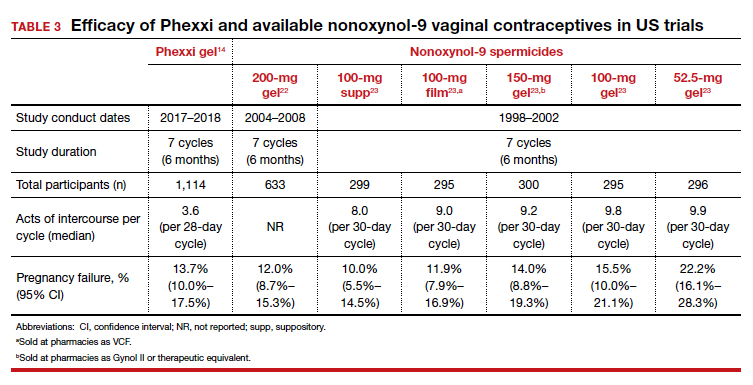
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.

Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%

Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.

A new contraceptive method should ideally provide improved access or a higher quality and safety option. Although unintended pregnancy rates in the United States are decreasing, significant disparities across race and socioeconomic status remain,1 and these disparities actually doubled from 1994 to 2011 even though the overall unintended pregnancy rate decreased.1-3 Specifically, people of color, those with lower income, and people with lower education levels had higher rates of unintended pregnancies than did White people with higher education and income, suggesting disparate access to contraception services.1 Thus, as new contraceptive methods are introduced, we must assess if they have the potential to address this disparity as well as continue to provide higher quality and safer options.
In this Update, we critically review the phase 3 data on efficacy and safety for 3 new methods that were introduced to the US market over the past year to evaluate their impact on the current contraceptive landscape.
The first method, newly approved by the US Food and Drug Administration (FDA), is a combined oral contraceptive (OC) that contains a novel endogenous estrogen, estetrol, or E4 (Nextstellis). E4 is a natural estrogen produced in the fetal liver that has lower potency and a longer half-life than estradiol. Nextstellis is a monophasic 24/4 OC pill that contains E4 14.2 mg and drospirenone 3 mg in each of the 24 hormone-containing pills. Most combined hormonal contraceptives (CHCs) in the United States today contain synthetically made ethinyl estradiol (EE) due to its high potency and oral bioavailability. Outside of the reproductive system, EE upregulates the production of hepatic proteins and alters procoagulant and anticoagulant factors, which results in an overall increase in venous thromboembolic (VTE) risk among CHC users.2
After widespread use of combined oral contraceptives (COCs) started in the 1960s, data emerged regarding increased VTE risk.3 Subsequent research discovered that the type of estrogen used in CHCs directly correlates with the thrombosis risk due to the hepatic upregulation with both first- and second-pass metabolism. Although this risk was reduced as the EE dose decreased below 50 µg and concurrent VTE risk factors were contraindicated, CHC users still faced a 2-fold increase in VTE risk compared with nonusers.4,5 EE in contraceptive formulations increases VTE risk, likely related to upregulation of procoagulant factors and decreasing anticoagulant proteins.2 By contrast, a phase 2 trial of Nextstellis demonstrated more neutral effects of E4/drospirenone on hemostatic parameters compared with EE/levonorgestrel or EE/drospirenone.6 Furthermore, E4/drospirenone exhibited lower increases in hepatic proteins, such as angiotensinogen, triglycerides, and sex-hormone binding globulin.7 These findings together suggest that this novel CHC pill has a more favorable cardiovascular adverse effect profile compared with currently available CHCs.
The second contraceptive method is a new transdermal patch that contains EE and levonorgestrel (Twirla); this is in contrast to the available EE/norelgestromin contraceptive patch (Xulane). Transdermal contraceptive patches can offer some users easier adherence as compared with a daily OC.8 Until this past year, the only transdermal contraceptive available in the United States was Xulane, which contains a daily dose of EE 35 µg and norelgestromin 150 µg. Norelgestromin is eventually metabolized to levonorgestrel derivatives.9 Twirla is administered in the same manner as Xulane and contains a daily hormone exposure equivalent to a COC containing EE 30 µg and levonorgestrel 120 µg. Similar to EE/norelgestromin, the EE/levonorgestrel patch also is contraindicated in obese patients (body mass index [BMI] ≥30 kg/m2) due to decreased efficacy and increased risk for VTE. Additionally, phase 3 data demonstrated decreasing efficacy of Twirla in overweight users (BMI ≥25–30 kg/m2), perhaps further limiting the population that may benefit from this contraceptive method.10 These issues with efficacy and weight likely are related to the fact that levonorgestrel distribution is weight dependent, with evidence of lower plasma levels in obese individuals.11-13
The third new method is a prescription vaginal contraceptive gel with lactic acid, citric acid, and potassium bitartrate (Phexxi) designed to prevent pregnancy by maintaining an acidic vaginal environment that is inhospitable to sperm. For many decades, vaginal contraceptives, including vaginal spermicidal gels, provided easy access to a nonhormonal and flexible method of moderately effective contraception for many users. Phexxi is a prescription vaginal pH regulator administered as a gel and active for 1 hour after application. All previous vaginal gels sold in the United States are applied similarly, are available over the counter, and include nonoxynol-9, which is a surfactant that damages sperm cell membranes. Recent data from a phase 3 trial demonstrated similar contraceptive effectiveness of Phexxi when compared with available nonoxynol-9 alternatives.14
Continue to: New OC with the novel estrogen E4 demonstrates good safety profile for VTE...
New OC with the novel estrogen E4 demonstrates good safety profile for VTE
Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
The COC E4/drospirenone was evaluated in 2 parallel multinational studies. Here, we review the North American data that are more relevant for the US population; the European-Russian data also are published.15
Study examined 1 year’s use of E4/drospirenone
The US–Canadian trial conducted by Creinin and colleagues enrolled 1,864 participants aged 16 to 50 years to evaluate contraceptive efficacy, bleeding patterns, and adverse events with 1-year use (13 cycles) of E4/drospirenone. The primary efficacy group included 1,524 women aged 16 to 35. This study enrolled healthy, heterosexually active participants with a BMI ≤35 kg/m2 and regular menses from 70 sites in the United States and 7 sites in Canada. The dropout rate was 45%, comparable to that in other contraceptive studies. Participants used E4/drospirenone cyclically, taking 1 hormone-containing pill daily for 24 days followed by 4 days of placebo pills.
Contraceptive efficacious, no VTE observed
The researchers reported efficacy as a Pearl Index (PI) of 2.65 pregnancies per 100 woman-years in participants aged 16 to 35 and an overall 13-cycle life-table pregnancy rate of 2.06%. The PI did not differ among nonobese and obese participants in multivariable analysis. Most users experienced scheduled withdrawal bleeding; only 13% to 18% reported absence of scheduled bleeding. Unscheduled bleeding was typically spotting (55.2%), and this decreased with treatment duration from 30% in cycle 1 to 15% to 20% in cycle 5 and on.
Overall, 28.9% of participants reported treatment-related adverse events (AEs), which most commonly were headache (5.0%), metrorrhagia (4.6%), and nausea (3.8%). Investigators reported a minimal change in mean (SD) BMI of 0.4 (1.7) kg/m2 from baseline after 1 year of E4/drospirenone use, and only 0.5% of participants discontinued use due to weight gain. The most common reasons for AE-related treatment discontinuation included metrorrhagia (0.9%), menorrhagia (0.8%), and vaginal hemorrhage (0.5%). Importantly, no cases of VTE occurred in this study of estetrol despite 23% of participants being obese, a known risk factor for VTE.
Nextstellis provides safe, effective contraception with a PI comparable to that of other available CHCs as well as a favorable bleeding profile in healthy users who are adherent to treatment. Importantly, contraceptive efficacy was maintained in obese users with a BMI up to 35 kg/m2. In contrast to EE or estradiol, E4 demonstrates a lower impact on the hepatic system, and preliminary findings suggest a lower VTE risk compared with other CHCs on the market. The European phase 3 trial of 1,553 participants also demonstrated a low rate of VTE, with only 1 case diagnosed.15 By contrast, similar phase 3 trials of available CHCs demonstrated more frequent VTE events despite low-dose EE formulations (TABLE 1).10,15-18 In general, most US phase 3 trials have 3 to 4 VTE events in the studied population, and the Nextstellis North American trial, of which 92% of participants were from the United States, had 0. However, confirmation of any potential lower VTE risk requires further analysis from large, population-based postmarketing studies.

Continue to: Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women...
Efficacy of a new EE/levonorgestrel transdermal patch may be lower in overweight, obese women
Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
To assess the contraceptive efficacy, tolerability, and safety of the transdermal patch Twirla (EE/levonorgestrel) over 1 year of treatment (13 cycles), Nelson and colleagues conducted an open-label, multicenter, US-based phase 3 trial of participants aged 18 years and older with regular cycles. There were no restrictions based on BMI. On average, the study population was overweight, with a mean BMI of 28.3 kg/m2 , and 35% of the population was considered obese (BMI ≥30 kg/m2).
Study design
A total of 2,032 participants enrolled in the study, with separate populations defined for specific analysis on safety, contraceptive efficacy, and cycle control. The primary efficacy group included 1,736 participants. Fifty-one percent discontinued the study, most commonly due to “women’s decision” (15%) and lost to follow-up (11%). Users received bleeding diaries and returned periodically throughout the study for evaluation for efficacy, adherence, and adverse events.
Efficacy associated with BMI
The study results demonstrated an overall PI of 5.8 pregnancies per 100 woman-years for users aged younger than 35. TABLE 2 demonstrates the overall trend of efficacy in relation to BMI.10,15-19 Participants with a higher BMI were found to have a higher PI, revealing lower contraceptive efficacy in more overweight and obese patients. The overall cumulative pregnancy rate over 13 cycles was 5.3%

Participants reported decreasing frequency of bleeding/spotting days over the treatment duration of 13 cycles, from a mean (SD) of 6.2 (4.5) days in cycle 1 to 4.9 (3.5) days in cycle 13. Unscheduled bleeding episodes remained high throughout the study period. Initially, 60% of users reported 1 or more days of unscheduled bleeding in cycle 1, and 42% still reported unscheduled bleeding in cycle 13. In light of this, only 45 participants (2.2%) discontinued the study due to bleeding issues, suggesting perhaps that the bleeding was light. Overall, users experienced acceptable wearability of the patch, and the rate of detachment decreased over the study period from 9.9% in cycle 1 to 2.4% in cycle 13. There were also low rates (0.5%) of moderate to severe irritation. Itching at the adhesion site decreased slightly from 13.1% in cycle 2 to 9.6% in cycle 13.
In general, 27.2% of patch users experienced a study-related AE, most reported as mild to moderate. Nausea (4.1%) and headaches (3.6%) were the most common hormone-related AE. Importantly, 4 obese users experienced 5 VTEs (deep vein thrombosis, n = 2; pulmonary embolism, n = 3) between cycle 5 and 13. Three of these users had additional VTE risk factors, such as air travel and a family history of clots. No users who were of normal weight or overweight experienced VTE.
Available data demonstrate that the EE/norelgestromin patch exposes users to higher serum levels compared with the pill or the ring.20 The higher estrogen exposure with the patch may explain higher estrogen-related adverse effects and may result in increased VTE risk. Initial pharmacokinetic data of the EE/levonorgestrel patch showed lower EE concentrations, similar to marketed COCs and lower than EE/norelgestromin.21 Despite this lower estrogen exposure, the phase 3 trial by Nelson and colleagues did not demonstrate a safer profile with respect to thromboembolic events.
Further, the high PI of 5.8 pregnancies per 100 woman-years calls into question the efficacy of this patch compared with already available CHC options. Indeed, the efficacy appears reasonable in normal-weight individuals, with a PI of 3.5 pregnancies per 100 woman-years; however, this is still higher than its contemporary counterpart, Nextstellis, which has a PI of 2.65 pregnancies per 100 woman-years and included users with a BMI of up to 35 kg/m2 (Table 2). Given the evidence of decreased efficacy, clinicians may consider reserving this option for only normal-weight women who cannot use or prefer not to use another CHC method. Obese individuals (BMI ≥30 kg/m2 ) should not use this patch due to decreased efficacy and increased VTE risk. Lastly, although use in overweight individuals (BMI ≥25 kg/m2) is not absolutely contraindicated, clinicians should counsel the overweight patient on the possibility of decreased contraceptive efficacy due to weight, and they may choose to reserve use of this patch in overweight individuals only when no other comparable or more effective method is an option.
Continue to: Novel vaginal pH buffering spermicide is a new Rx-only option...
Novel vaginal pH buffering spermicide is a new Rx-only option
Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
In an open-label phase 3 study, Thomas and colleagues enrolled 1,384 participants aged 18 to 35 with regular cycles at 112 sites in the United States to assess the contraceptive efficacy, safety, and acceptability of Phexxi vaginal gel (lactic acid, citric acid, and potassium bitartrate) over 7 cycles (6 months). Participants were required to have at least 3 episodes of heterosexual vaginal intercourse per cycle and return throughout the treatment duration for study visits. Fifty-three percent of participants did not complete the study, most frequently due to loss to follow-up (18.1%) and participant withdrawal (12.3%). Most participants were White (69%) and had an average (SD) age of 27.7 (4.5) years.
Efficacy and AE rates
The investigators reported a cumulative pregnancy rate of 13.7% over 7 cycles (6 months). In this study, 45.2% of women experienced 1 AE, and most were noted to be mild (23.9%) to moderate (18.7%). The most reported AE was vulvovaginal burning (20.0%), followed by vulvovaginal pruritus (11.2%), urinary tract infection (5.7%), and vulvovaginal pain (3.8%). Less than 2% of participants discontinued the study due to an AE. Burning and itching decreased with time and with decreased frequency of use. When used twice per day compared with once per day, burning rates decreased from 4.6% to 2.1%, and itching rates decreased from 1.0% to 0.7%. Serious AEs were uncommon, occurring in 1.3% of users; only 1, cystitis, was noted to be “probably” related to the treatment. ●
Prior to the approval of Phexxi, all currently available vaginal contraceptive gels in the United States contained nonoxynol-9 as the active ingredient, which is a surfactant that is spermicidal by damaging cell membranes. Although Phexxi provides a novel mechanism of action as a spermicide, the contraceptive efficacy is about the same as available spermicides on the market (see TABLE 3).14,22,23 The FDA calculated a 13-cycle PI to include in the label (27.5 pregnancies per 100 woman-years) based on the results of this study; however, no reliable statistical method exists to calculate a true PI from a 7-cycle study. Thus, we recommend that clinicians counsel patients appropriately based on the 6-month rate noted in the study, and that this rate is similar to that with currently available over-the-counter products. This point is important, as Phexxi is available only by prescription, which may impact patient cost and access.
Equally important is Phexxi’s potential for sexually transmitted infection (STI) prevention. In a US-based randomized controlled trial, Phexxi use demonstrated significant risk reduction in gonorrhea and chlamydia infections among participants aged 18 to 45 years.24 That study showed a relative risk reduction of 50% and 78% for chlamydia and gonorrhea, respectively.24 Future research is planned to evaluate this spermicide as a novel STI prevention method. Ultimately, Phexxi may provide an alternative spermicide for users interested in moderately effective contraception and unable to tolerate available nonoxynol-9 formulations. Interested users will have to rely on a prescription, possibly limiting access to this novel spermicide. Further data are required to determine its potential as an STI prevention agent.

- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
- Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008–2011. N Engl J Med. 2016;374:843-852.
- Meade TW. Oral contraceptives, clotting factors, and thrombosis. Am J Obstet Gynecol. 1982;142(6 pt 2):758-761.
- Royal College of General Practitioners’ Oral Contraception Study. Oral contraceptives, venous thrombosis, and varicose veins. J R Coll Gen Pract. 1978;28:393-399.
- Dinger JC, Heinemann LA, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance Study on oral contraceptives based on 142,475 women-years of observation. Contraception. 2007;75:344-354.
- Heinemann LA, Dinger JC. Range of published estimates of venous thromboembolism incidence in young women. Contraception. 2007;75:328-336.
- Douxfils J, Klipping C, Duijkers I, et al. Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters. Contraception. 2020;102:396-402.
- Klipping C, Duijkers I, Mawet M, et al. Endocrine and metabolic effects of an oral contraceptive containing estetrol and drospirenone. Contraception. 2021;103:213-221.
- Archer DF, Cullins V, Creasy GW, et al. The impact of improved compliance with a weekly contraceptive transdermal system (Ortho Evra) on contraceptive efficacy. Contraception. 2004;69:189-195.
- Stanczyk FZ, Roy S. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception. 1990;42:67-96.
- Nelson AL, Kaunitz AM, Kroll R; SECURE Investigators. Efficacy, safety, and tolerability of a levonorgestrel/ethinyl estradiol transdermal delivery system: phase 3 clinical trial results. Contraception. 2021;103:137-143.
- Natavio M, Stanczyk FZ, Molins EAG, et al. Pharmacokinetics of the 1.5 mg levonorgestrel emergency contraceptive in women with normal, obese and extremely obese body mass index. Contraception. 2019;99:306-311.
- Praditpan P, Hamouie A, Basaraba CN, et al. Pharmacokinetics of levonorgestrel and ulipristal acetate emergency contraception in women with normal and obese body mass index. Contraception. 2017;95:464-469.
- Westhoff CL, Torgal AH, Mayeda ER, et al. Pharmacokinetics of a combined oral contraceptive in obese and normal-weight women. Contraception. 2010;81:474-480.
- Thomas MA, Chappell BT, Maximos B, et al. A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contracept X. 2020;2:100031.
- Gemzell-Danielsson K, Apter D, Zatik J, et al. Estetrol-drospirenone combination oral contraceptive: a clinical study of contraceptive efficacy, bleeding pattern, and safety in Europe and Russia. BJOG. 2021. doi: 10.1111/1471-0528.16840.
- Archer DF, Nakajima ST, Sawyer AT, et al. Norethindrone acetate 1.0 milligram and ethinyl estradiol 10 micrograms as an ultra low-dose oral contraceptive. Obstet Gynecol. 2013;122:601-607.
- Creinin MD, Westhoff CL, Bouchard C, et al. Estetrol-drospirenone combination oral contraceptive: North American phase 3 efficacy and safety results. Contraception. 2021;104:222-228.
- Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD, et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323-328.
- Safety and efficacy of a contraceptive vaginal ring delivering Nestorone and ethinyl estradiol. Clinicaltrials.gov identifier: NCT00263341. https://clinicaltrials.gov/ct2/show /NCT00263341. Accessed August 23, 2021.
- van den Heuvel MW, van Bragt AJ, Alnabawy AK, et al. Comparison of ethinylestradiol pharmacokinetics in three hormonal contraceptive formulations: the vaginal ring, the transdermal patch and an oral contraceptive. Contraception. 2005;72:168-174.
- Stanczyk FZ, Rubin A, Flood L, et al. Pharmacokinetics, tolerability and cycle control of three transdermal contraceptive delivery systems containing different doses of ethinyl-estradiol and levonorgestrel. Horm Mol Biol Clin Investig. 2011;6:231-240.
- Burke AE, Barnhart K, Jensen JT, et al. Contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels: a randomized controlled trial. Obstet Gynecol. 2010;116:1265-1273.
- Raymond EG, Chen PL, Luoto J; Spermicidal Trial Group. Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstet Gynecol. 2004;103:430-439.
- Chappell BT, Mena LA, Maximos B, et al. EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. Am J Obstet Gynecol. 2021;225:162.e1-162.e14.
Evolving management strategies for patient service excellence: Is your practice up to speed?
Over the past decade, the use of technology with the focus on optimizing the consumer experience has exploded throughout numerous industries, including education, retail, and entertainment. Within health care, we would be naïve to ignore patient expectations for an optimized consumer experience within our offices. Thus, clinicians across all health care disciplines must remain cognizant of and work to optimize the patient experience in the ever-expanding world of health care.
Reengineering one’s practice will continue to be a work in progress. As medicine and technology continuously advance, clinicians must be able to adapt and implement changes. An excellent example of such adaptation is the use of telemedicine during the COVID-19 pandemic.1 We hope that the use of telemedicine remains an integral part of our armamentarium as we move forward.
In this article, we offer perspectives on using telemedicine, improving the patient experience, and implementing the use of social media in your practice. We look for a common denominator when provision of clinical care is the topic of discussion. Knowing the details of your medical practice and addressing its highlights as well as its concerns will benefit patients, staff, and health care providers. We hope that you glean some insights that you can apply in your practice.
Telemedicine: Part of the new normal
The American College of Obstetricians and Gynecologists defines telehealth as a “technology-enhanced health care framework that includes services, such as virtual visits, remote patient monitoring and mobile health care.”2 The American Telemedicine Association and the World Health Organization use the terms telemedicine and telehealth interchangeably.3 We live in a relatively new era since the COVID-19 pandemic necessitated that traditional face-to-face meeting(s) with patients be conducted virtually. The good news is that the outcomes with telehealth visits appear to be on par with those of traditional office visits.4
Telehealth allows clinicians to deliver medical evaluation and management plans right in a patient’s home and to receive appropriate reimbursement for doing so. This is a result of actions by Congress and the Department of Health and Human Services that removed restrictions related to telemedicine.5 The telemedicine approach provides a different perspective on provision of care (FIGURE 1).

For telemedicine practice, prerequisites include having the appropriate hardware, software, and a secure internet connection to maintain quality and patient safety.4 It is wise to check with regulatory laws at the local, state, and federal levels, as some states have separate licensure requirements for delivering this type of health care. Review insurance carrier guidelines as well as medical malpractice coverage for telehealth care provision. Ideally, obtain proof in writing from third-party payers and malpractice insurance carriers. TABLE 1 lists ObGyn-related activities and services that can be provided via telemedicine.3
While in many circumstances the indications for telemedicine are obvious, some remain less apparent. For example, patients may be more receptive to the use of telemedicine for counseling and education for family planning services and termination of pregnancy.6 Psychological counseling lends itself to a telemedicine approach to address levels of anxiety and depression, especially in the postpartum setting.
An initial telemedicine consultation often is complemented by subsequent patient examination when deemed necessary. Pelvic imaging often is ordered to address concerns expressed during the telemedicine visit. Teleradiology is an interesting aspect of telemedicine that is expanding. Telesonography, the use of ultrasonography, is extremely relevant to obstetrics and gynecology. Specifically, the development of self-operated endovaginal telemonitors and 3D as well as 4D imaging incorporates self-operated endovaginal telemonitoring. This technology remains a work in progress.7
Another aspect to telemedicine is telesurgery. Although an operative procedure cannot be performed virtually, pre- and postoperative counseling can be provided via telemedicine, offering tremendous convenience to patients.
Understanding the infrastructure of telemedicine and assuring security, adherence with HIPAA (Health Insurance Portability and Accountability Act), state licensure, reimbursement, and medical malpractice aspects is well worth the effort.
Continue to: Reengineer your office to enhance the patient experience...
Reengineer your office to enhance the patient experience
Create a hospitable environment. One way to do this is by having your front desk staffer standing up to greet patients. The medical management literature has reported an interesting analogy.8 Picture going to a retailer whose job is to sell you the product you are interested in. Where is that person positioned? Standing at the counter, at eye level with you, doing his or her best to convince you to buy a particular product. Having your office front desk personnel standing is analogous to the “atmosphere (when approaching the front desk) that conveys clear energy and a clear tone or readiness,” all of which contribute to a more positive patient experience.8
A hospitable environment at the check-in desk sets the stage for the office visit. When a staffer is sitting at the front desk office entrance point, the concept conveyed to the patient is, “You can wait for us because you need us more than we need you.” Changing the staffer’s posture to a standing position conveys, “Welcome, we are glad to see you and address why you are here.”8
Conduct a flow analysis of your office procedures. It is clear that the front desk serves as an advertisement of what your practice has to offer. A friendly smile from the receptionist goes a long way. In addition, the total time from patient check-in to checkout should be monitored. Having this type of data aids staff evaluation and patient satisfaction.9
Examine your office’s aspects of what the business world calls throughput. In essence, problems related to throughput include that the clinician is chronically late or slow with patients or that inadequate time was allocated per patient visit or per procedure.
It is valuable to allocate staff resources ahead of time, including patient registration and insurance verification details. Staff records review and preparation for the clinician streamlines time with the patient. Having lab tests, other consultations, and so on readily available for the clinician is time well spent by the medical assistants. For procedures, preparation of equipment that is in good working order and having supplies appropriately stocked can help facilitate success and efficiency. Creation of an “electronic on-time board” displays if the clinician is running on time or not.9 These practical tips can result in better patient and staff satisfaction. In addition, periodic surveys help engage patients in the process. TABLE 2 provides sample survey questions to ask patients.10
Taking a careful look at your current office practices and reengineering them as needed is an investment that provides an excellent return.
Continue to: Develop a presence on social media...
Develop a presence on social media
Having a social media presence is becoming one of the most effective strategies for reaching an intended audience. In the United States, more than 70% of the public uses at least one social media platform.11 It can be an effective and efficient tool for clinicians to grow their practice; distribute information about unique areas of the practice; and reach potential patients, referring physicians, and prospective faculty/trainees. Social media also is increasingly being used by clinicians to connect with other health care providers in their own specialty or other specialties. Digital communities have been created where ideas are shared and topics of interest are discussed. Clinicians can listen in on expert opinions, disseminate their research, and discuss practice management challenges or health advocacy. FIGURE 2 provides a snapshot of the social media landscape.
There is a wealth of options when it comes to social media platforms, including but not limited to Facebook, Twitter, LinkedIn, Instagram, YouTube, and blogs (TABLE 3). Facebook has the largest user base of all social media platforms, with approximately 1.7 billion active monthly users; thus, its use creates an opportunity to reach a massive audience.12,13 People use Facebook for both personal and professional reasons. The platform allows for sharing of photos, live videos, posted text, and comments. It can be used as a helpful resource to engage patients and disseminate accurate medical information. Importantly, remember that content posted should comply with the HIPAA Privacy Rule and that information shared should come from a credible source.
The Mayo Clinic is an impressive example of the use of social media for consumer education, research, and expansion of the reach of its brand. They incorporated social media into their strategic marketing plan, and between 2015 and 2016, social media referrals led to a 139% increase in patient appointment requests.13 Of the 20 different social media sites used, Facebook was the top social media referrer, accounting for 81% of social media referrals in 2015 and 88% in 2016. They have expanded their reach through different social media platforms and have more than 1.5 million followers on Twitter. Their videos on YouTube were viewed more than 4.9 million times in 2016 alone. This example illustrates social media’s effectiveness and the potential role it can play in connecting with patients.
Final thoughts
The practice of medicine has undeniably changed over the years and will continue to evolve. Understanding how to implement change to ensure that high-quality, efficient patient care is being delivered is paramount.
We have highlighted various aspects of practice management that you can use to overcome current obstacles and changing standards. The advent of telemedicine has provided easy access to clinicians. Consultation occurs in the comfort of the patient’s home, and the ability to provide local examination telecast to a clinician allows physicians and advanced practice practitioners to reach a wider range of patients. Social media has established an infrastructure for educating patients and providers while at the same time conveying educational tools to patients. This level of communication will continue to expand as time progresses.
Practitioners have a whole new cadre to add to their toolbox to provide patients with state-of-the-art communication and care. ●
- Anifandis G, Tempest H, Oliva R, et al. COVID-19 and human reproduction: a pandemic that packs a serious punch. Systems Biol Reprod Med. 2021;67:3-23.
- American College of Obstetricians and Gynecologists Presidential Task Force on Telehealth. Implementing telehealth in practice: ACOG Committee Opinion No. 798. Obstet Gynecol. 2020;135:e73-e79.
- Lee S, Hitt WC. Clinical applications of telemedicine in gynecology and women’s health. Obstet Gynecol Clin North Am. 2020;47:259-270.
- DeNicola N, Grossman D, Marko K, et al. Telehealth interventions to improve obstetrics and gynecologic health outcomes: a systematic review. Obstet Gynecol. 2020;135:371-382.
- Keesara S, Jonas A, Schulman K. Covid-19 and health care’s digital revolution. N Engl J Med. 2020;382:e82.
- Grossman D, Grindlay K. Safety of medical abortion provided through telemedicine compared with in person. Obstet Gynecol. 2017;130:778-782.
- Pereira I, von Horn K, Depebusch M, et al. Self-operated endovaginal telemonitoring: a prospective clinical validation study. Fertil Steril. 2016;106:306-310e1.
- Massey GG, Hunter DG. Enhancing the patient experience with stand-up check-in. MGMA Connex. 2016;34-36.
- The patient experience, from check-in to check out. MGMA Connex. 2017;17:45-46.
- Swankoski KE, Peikes DN, Morrison N, et al. Patient experience during a large primary care practice transformation initiative. Am J Manag Care. 2018;24:607-613.
- Pew Research Center. Social media fact sheet. https://www .pewinternet.org/fact-sheet/social-media/. April 7. 2021. Accessed September 21, 2021.
- Small Business Trends website. Mansfield M. Social media statistics 2016. https://smallbiztrends.com/2016/11/social -media-statistics-2016.html. Updated June 4, 2021. Accessed September 21, 2021.
- Kotsenas AL, Arce M, Aase L, et al. The strategic imperative for the use of social media in health care. J Am Coll Radiol. 2018;15(1 pt B):155-161.
Over the past decade, the use of technology with the focus on optimizing the consumer experience has exploded throughout numerous industries, including education, retail, and entertainment. Within health care, we would be naïve to ignore patient expectations for an optimized consumer experience within our offices. Thus, clinicians across all health care disciplines must remain cognizant of and work to optimize the patient experience in the ever-expanding world of health care.
Reengineering one’s practice will continue to be a work in progress. As medicine and technology continuously advance, clinicians must be able to adapt and implement changes. An excellent example of such adaptation is the use of telemedicine during the COVID-19 pandemic.1 We hope that the use of telemedicine remains an integral part of our armamentarium as we move forward.
In this article, we offer perspectives on using telemedicine, improving the patient experience, and implementing the use of social media in your practice. We look for a common denominator when provision of clinical care is the topic of discussion. Knowing the details of your medical practice and addressing its highlights as well as its concerns will benefit patients, staff, and health care providers. We hope that you glean some insights that you can apply in your practice.
Telemedicine: Part of the new normal
The American College of Obstetricians and Gynecologists defines telehealth as a “technology-enhanced health care framework that includes services, such as virtual visits, remote patient monitoring and mobile health care.”2 The American Telemedicine Association and the World Health Organization use the terms telemedicine and telehealth interchangeably.3 We live in a relatively new era since the COVID-19 pandemic necessitated that traditional face-to-face meeting(s) with patients be conducted virtually. The good news is that the outcomes with telehealth visits appear to be on par with those of traditional office visits.4
Telehealth allows clinicians to deliver medical evaluation and management plans right in a patient’s home and to receive appropriate reimbursement for doing so. This is a result of actions by Congress and the Department of Health and Human Services that removed restrictions related to telemedicine.5 The telemedicine approach provides a different perspective on provision of care (FIGURE 1).

For telemedicine practice, prerequisites include having the appropriate hardware, software, and a secure internet connection to maintain quality and patient safety.4 It is wise to check with regulatory laws at the local, state, and federal levels, as some states have separate licensure requirements for delivering this type of health care. Review insurance carrier guidelines as well as medical malpractice coverage for telehealth care provision. Ideally, obtain proof in writing from third-party payers and malpractice insurance carriers. TABLE 1 lists ObGyn-related activities and services that can be provided via telemedicine.3
While in many circumstances the indications for telemedicine are obvious, some remain less apparent. For example, patients may be more receptive to the use of telemedicine for counseling and education for family planning services and termination of pregnancy.6 Psychological counseling lends itself to a telemedicine approach to address levels of anxiety and depression, especially in the postpartum setting.
An initial telemedicine consultation often is complemented by subsequent patient examination when deemed necessary. Pelvic imaging often is ordered to address concerns expressed during the telemedicine visit. Teleradiology is an interesting aspect of telemedicine that is expanding. Telesonography, the use of ultrasonography, is extremely relevant to obstetrics and gynecology. Specifically, the development of self-operated endovaginal telemonitors and 3D as well as 4D imaging incorporates self-operated endovaginal telemonitoring. This technology remains a work in progress.7
Another aspect to telemedicine is telesurgery. Although an operative procedure cannot be performed virtually, pre- and postoperative counseling can be provided via telemedicine, offering tremendous convenience to patients.
Understanding the infrastructure of telemedicine and assuring security, adherence with HIPAA (Health Insurance Portability and Accountability Act), state licensure, reimbursement, and medical malpractice aspects is well worth the effort.
Continue to: Reengineer your office to enhance the patient experience...
Reengineer your office to enhance the patient experience
Create a hospitable environment. One way to do this is by having your front desk staffer standing up to greet patients. The medical management literature has reported an interesting analogy.8 Picture going to a retailer whose job is to sell you the product you are interested in. Where is that person positioned? Standing at the counter, at eye level with you, doing his or her best to convince you to buy a particular product. Having your office front desk personnel standing is analogous to the “atmosphere (when approaching the front desk) that conveys clear energy and a clear tone or readiness,” all of which contribute to a more positive patient experience.8
A hospitable environment at the check-in desk sets the stage for the office visit. When a staffer is sitting at the front desk office entrance point, the concept conveyed to the patient is, “You can wait for us because you need us more than we need you.” Changing the staffer’s posture to a standing position conveys, “Welcome, we are glad to see you and address why you are here.”8
Conduct a flow analysis of your office procedures. It is clear that the front desk serves as an advertisement of what your practice has to offer. A friendly smile from the receptionist goes a long way. In addition, the total time from patient check-in to checkout should be monitored. Having this type of data aids staff evaluation and patient satisfaction.9
Examine your office’s aspects of what the business world calls throughput. In essence, problems related to throughput include that the clinician is chronically late or slow with patients or that inadequate time was allocated per patient visit or per procedure.
It is valuable to allocate staff resources ahead of time, including patient registration and insurance verification details. Staff records review and preparation for the clinician streamlines time with the patient. Having lab tests, other consultations, and so on readily available for the clinician is time well spent by the medical assistants. For procedures, preparation of equipment that is in good working order and having supplies appropriately stocked can help facilitate success and efficiency. Creation of an “electronic on-time board” displays if the clinician is running on time or not.9 These practical tips can result in better patient and staff satisfaction. In addition, periodic surveys help engage patients in the process. TABLE 2 provides sample survey questions to ask patients.10
Taking a careful look at your current office practices and reengineering them as needed is an investment that provides an excellent return.
Continue to: Develop a presence on social media...
Develop a presence on social media
Having a social media presence is becoming one of the most effective strategies for reaching an intended audience. In the United States, more than 70% of the public uses at least one social media platform.11 It can be an effective and efficient tool for clinicians to grow their practice; distribute information about unique areas of the practice; and reach potential patients, referring physicians, and prospective faculty/trainees. Social media also is increasingly being used by clinicians to connect with other health care providers in their own specialty or other specialties. Digital communities have been created where ideas are shared and topics of interest are discussed. Clinicians can listen in on expert opinions, disseminate their research, and discuss practice management challenges or health advocacy. FIGURE 2 provides a snapshot of the social media landscape.

There is a wealth of options when it comes to social media platforms, including but not limited to Facebook, Twitter, LinkedIn, Instagram, YouTube, and blogs (TABLE 3). Facebook has the largest user base of all social media platforms, with approximately 1.7 billion active monthly users; thus, its use creates an opportunity to reach a massive audience.12,13 People use Facebook for both personal and professional reasons. The platform allows for sharing of photos, live videos, posted text, and comments. It can be used as a helpful resource to engage patients and disseminate accurate medical information. Importantly, remember that content posted should comply with the HIPAA Privacy Rule and that information shared should come from a credible source.
The Mayo Clinic is an impressive example of the use of social media for consumer education, research, and expansion of the reach of its brand. They incorporated social media into their strategic marketing plan, and between 2015 and 2016, social media referrals led to a 139% increase in patient appointment requests.13 Of the 20 different social media sites used, Facebook was the top social media referrer, accounting for 81% of social media referrals in 2015 and 88% in 2016. They have expanded their reach through different social media platforms and have more than 1.5 million followers on Twitter. Their videos on YouTube were viewed more than 4.9 million times in 2016 alone. This example illustrates social media’s effectiveness and the potential role it can play in connecting with patients.
Final thoughts
The practice of medicine has undeniably changed over the years and will continue to evolve. Understanding how to implement change to ensure that high-quality, efficient patient care is being delivered is paramount.
We have highlighted various aspects of practice management that you can use to overcome current obstacles and changing standards. The advent of telemedicine has provided easy access to clinicians. Consultation occurs in the comfort of the patient’s home, and the ability to provide local examination telecast to a clinician allows physicians and advanced practice practitioners to reach a wider range of patients. Social media has established an infrastructure for educating patients and providers while at the same time conveying educational tools to patients. This level of communication will continue to expand as time progresses.
Practitioners have a whole new cadre to add to their toolbox to provide patients with state-of-the-art communication and care. ●
Over the past decade, the use of technology with the focus on optimizing the consumer experience has exploded throughout numerous industries, including education, retail, and entertainment. Within health care, we would be naïve to ignore patient expectations for an optimized consumer experience within our offices. Thus, clinicians across all health care disciplines must remain cognizant of and work to optimize the patient experience in the ever-expanding world of health care.
Reengineering one’s practice will continue to be a work in progress. As medicine and technology continuously advance, clinicians must be able to adapt and implement changes. An excellent example of such adaptation is the use of telemedicine during the COVID-19 pandemic.1 We hope that the use of telemedicine remains an integral part of our armamentarium as we move forward.
In this article, we offer perspectives on using telemedicine, improving the patient experience, and implementing the use of social media in your practice. We look for a common denominator when provision of clinical care is the topic of discussion. Knowing the details of your medical practice and addressing its highlights as well as its concerns will benefit patients, staff, and health care providers. We hope that you glean some insights that you can apply in your practice.
Telemedicine: Part of the new normal
The American College of Obstetricians and Gynecologists defines telehealth as a “technology-enhanced health care framework that includes services, such as virtual visits, remote patient monitoring and mobile health care.”2 The American Telemedicine Association and the World Health Organization use the terms telemedicine and telehealth interchangeably.3 We live in a relatively new era since the COVID-19 pandemic necessitated that traditional face-to-face meeting(s) with patients be conducted virtually. The good news is that the outcomes with telehealth visits appear to be on par with those of traditional office visits.4
Telehealth allows clinicians to deliver medical evaluation and management plans right in a patient’s home and to receive appropriate reimbursement for doing so. This is a result of actions by Congress and the Department of Health and Human Services that removed restrictions related to telemedicine.5 The telemedicine approach provides a different perspective on provision of care (FIGURE 1).

For telemedicine practice, prerequisites include having the appropriate hardware, software, and a secure internet connection to maintain quality and patient safety.4 It is wise to check with regulatory laws at the local, state, and federal levels, as some states have separate licensure requirements for delivering this type of health care. Review insurance carrier guidelines as well as medical malpractice coverage for telehealth care provision. Ideally, obtain proof in writing from third-party payers and malpractice insurance carriers. TABLE 1 lists ObGyn-related activities and services that can be provided via telemedicine.3
While in many circumstances the indications for telemedicine are obvious, some remain less apparent. For example, patients may be more receptive to the use of telemedicine for counseling and education for family planning services and termination of pregnancy.6 Psychological counseling lends itself to a telemedicine approach to address levels of anxiety and depression, especially in the postpartum setting.
An initial telemedicine consultation often is complemented by subsequent patient examination when deemed necessary. Pelvic imaging often is ordered to address concerns expressed during the telemedicine visit. Teleradiology is an interesting aspect of telemedicine that is expanding. Telesonography, the use of ultrasonography, is extremely relevant to obstetrics and gynecology. Specifically, the development of self-operated endovaginal telemonitors and 3D as well as 4D imaging incorporates self-operated endovaginal telemonitoring. This technology remains a work in progress.7
Another aspect to telemedicine is telesurgery. Although an operative procedure cannot be performed virtually, pre- and postoperative counseling can be provided via telemedicine, offering tremendous convenience to patients.
Understanding the infrastructure of telemedicine and assuring security, adherence with HIPAA (Health Insurance Portability and Accountability Act), state licensure, reimbursement, and medical malpractice aspects is well worth the effort.
Continue to: Reengineer your office to enhance the patient experience...
Reengineer your office to enhance the patient experience
Create a hospitable environment. One way to do this is by having your front desk staffer standing up to greet patients. The medical management literature has reported an interesting analogy.8 Picture going to a retailer whose job is to sell you the product you are interested in. Where is that person positioned? Standing at the counter, at eye level with you, doing his or her best to convince you to buy a particular product. Having your office front desk personnel standing is analogous to the “atmosphere (when approaching the front desk) that conveys clear energy and a clear tone or readiness,” all of which contribute to a more positive patient experience.8
A hospitable environment at the check-in desk sets the stage for the office visit. When a staffer is sitting at the front desk office entrance point, the concept conveyed to the patient is, “You can wait for us because you need us more than we need you.” Changing the staffer’s posture to a standing position conveys, “Welcome, we are glad to see you and address why you are here.”8
Conduct a flow analysis of your office procedures. It is clear that the front desk serves as an advertisement of what your practice has to offer. A friendly smile from the receptionist goes a long way. In addition, the total time from patient check-in to checkout should be monitored. Having this type of data aids staff evaluation and patient satisfaction.9
Examine your office’s aspects of what the business world calls throughput. In essence, problems related to throughput include that the clinician is chronically late or slow with patients or that inadequate time was allocated per patient visit or per procedure.
It is valuable to allocate staff resources ahead of time, including patient registration and insurance verification details. Staff records review and preparation for the clinician streamlines time with the patient. Having lab tests, other consultations, and so on readily available for the clinician is time well spent by the medical assistants. For procedures, preparation of equipment that is in good working order and having supplies appropriately stocked can help facilitate success and efficiency. Creation of an “electronic on-time board” displays if the clinician is running on time or not.9 These practical tips can result in better patient and staff satisfaction. In addition, periodic surveys help engage patients in the process. TABLE 2 provides sample survey questions to ask patients.10
Taking a careful look at your current office practices and reengineering them as needed is an investment that provides an excellent return.
Continue to: Develop a presence on social media...
Develop a presence on social media
Having a social media presence is becoming one of the most effective strategies for reaching an intended audience. In the United States, more than 70% of the public uses at least one social media platform.11 It can be an effective and efficient tool for clinicians to grow their practice; distribute information about unique areas of the practice; and reach potential patients, referring physicians, and prospective faculty/trainees. Social media also is increasingly being used by clinicians to connect with other health care providers in their own specialty or other specialties. Digital communities have been created where ideas are shared and topics of interest are discussed. Clinicians can listen in on expert opinions, disseminate their research, and discuss practice management challenges or health advocacy. FIGURE 2 provides a snapshot of the social media landscape.

There is a wealth of options when it comes to social media platforms, including but not limited to Facebook, Twitter, LinkedIn, Instagram, YouTube, and blogs (TABLE 3). Facebook has the largest user base of all social media platforms, with approximately 1.7 billion active monthly users; thus, its use creates an opportunity to reach a massive audience.12,13 People use Facebook for both personal and professional reasons. The platform allows for sharing of photos, live videos, posted text, and comments. It can be used as a helpful resource to engage patients and disseminate accurate medical information. Importantly, remember that content posted should comply with the HIPAA Privacy Rule and that information shared should come from a credible source.
The Mayo Clinic is an impressive example of the use of social media for consumer education, research, and expansion of the reach of its brand. They incorporated social media into their strategic marketing plan, and between 2015 and 2016, social media referrals led to a 139% increase in patient appointment requests.13 Of the 20 different social media sites used, Facebook was the top social media referrer, accounting for 81% of social media referrals in 2015 and 88% in 2016. They have expanded their reach through different social media platforms and have more than 1.5 million followers on Twitter. Their videos on YouTube were viewed more than 4.9 million times in 2016 alone. This example illustrates social media’s effectiveness and the potential role it can play in connecting with patients.
Final thoughts
The practice of medicine has undeniably changed over the years and will continue to evolve. Understanding how to implement change to ensure that high-quality, efficient patient care is being delivered is paramount.
We have highlighted various aspects of practice management that you can use to overcome current obstacles and changing standards. The advent of telemedicine has provided easy access to clinicians. Consultation occurs in the comfort of the patient’s home, and the ability to provide local examination telecast to a clinician allows physicians and advanced practice practitioners to reach a wider range of patients. Social media has established an infrastructure for educating patients and providers while at the same time conveying educational tools to patients. This level of communication will continue to expand as time progresses.
Practitioners have a whole new cadre to add to their toolbox to provide patients with state-of-the-art communication and care. ●
- Anifandis G, Tempest H, Oliva R, et al. COVID-19 and human reproduction: a pandemic that packs a serious punch. Systems Biol Reprod Med. 2021;67:3-23.
- American College of Obstetricians and Gynecologists Presidential Task Force on Telehealth. Implementing telehealth in practice: ACOG Committee Opinion No. 798. Obstet Gynecol. 2020;135:e73-e79.
- Lee S, Hitt WC. Clinical applications of telemedicine in gynecology and women’s health. Obstet Gynecol Clin North Am. 2020;47:259-270.
- DeNicola N, Grossman D, Marko K, et al. Telehealth interventions to improve obstetrics and gynecologic health outcomes: a systematic review. Obstet Gynecol. 2020;135:371-382.
- Keesara S, Jonas A, Schulman K. Covid-19 and health care’s digital revolution. N Engl J Med. 2020;382:e82.
- Grossman D, Grindlay K. Safety of medical abortion provided through telemedicine compared with in person. Obstet Gynecol. 2017;130:778-782.
- Pereira I, von Horn K, Depebusch M, et al. Self-operated endovaginal telemonitoring: a prospective clinical validation study. Fertil Steril. 2016;106:306-310e1.
- Massey GG, Hunter DG. Enhancing the patient experience with stand-up check-in. MGMA Connex. 2016;34-36.
- The patient experience, from check-in to check out. MGMA Connex. 2017;17:45-46.
- Swankoski KE, Peikes DN, Morrison N, et al. Patient experience during a large primary care practice transformation initiative. Am J Manag Care. 2018;24:607-613.
- Pew Research Center. Social media fact sheet. https://www .pewinternet.org/fact-sheet/social-media/. April 7. 2021. Accessed September 21, 2021.
- Small Business Trends website. Mansfield M. Social media statistics 2016. https://smallbiztrends.com/2016/11/social -media-statistics-2016.html. Updated June 4, 2021. Accessed September 21, 2021.
- Kotsenas AL, Arce M, Aase L, et al. The strategic imperative for the use of social media in health care. J Am Coll Radiol. 2018;15(1 pt B):155-161.
- Anifandis G, Tempest H, Oliva R, et al. COVID-19 and human reproduction: a pandemic that packs a serious punch. Systems Biol Reprod Med. 2021;67:3-23.
- American College of Obstetricians and Gynecologists Presidential Task Force on Telehealth. Implementing telehealth in practice: ACOG Committee Opinion No. 798. Obstet Gynecol. 2020;135:e73-e79.
- Lee S, Hitt WC. Clinical applications of telemedicine in gynecology and women’s health. Obstet Gynecol Clin North Am. 2020;47:259-270.
- DeNicola N, Grossman D, Marko K, et al. Telehealth interventions to improve obstetrics and gynecologic health outcomes: a systematic review. Obstet Gynecol. 2020;135:371-382.
- Keesara S, Jonas A, Schulman K. Covid-19 and health care’s digital revolution. N Engl J Med. 2020;382:e82.
- Grossman D, Grindlay K. Safety of medical abortion provided through telemedicine compared with in person. Obstet Gynecol. 2017;130:778-782.
- Pereira I, von Horn K, Depebusch M, et al. Self-operated endovaginal telemonitoring: a prospective clinical validation study. Fertil Steril. 2016;106:306-310e1.
- Massey GG, Hunter DG. Enhancing the patient experience with stand-up check-in. MGMA Connex. 2016;34-36.
- The patient experience, from check-in to check out. MGMA Connex. 2017;17:45-46.
- Swankoski KE, Peikes DN, Morrison N, et al. Patient experience during a large primary care practice transformation initiative. Am J Manag Care. 2018;24:607-613.
- Pew Research Center. Social media fact sheet. https://www .pewinternet.org/fact-sheet/social-media/. April 7. 2021. Accessed September 21, 2021.
- Small Business Trends website. Mansfield M. Social media statistics 2016. https://smallbiztrends.com/2016/11/social -media-statistics-2016.html. Updated June 4, 2021. Accessed September 21, 2021.
- Kotsenas AL, Arce M, Aase L, et al. The strategic imperative for the use of social media in health care. J Am Coll Radiol. 2018;15(1 pt B):155-161.
Is active (vs expectant) management of a persistent PUL more effective?
Barnhart K, Hansen KR, Stephenson MD, et al; Reproductive Medicine Network. Effect of an active vs expectant management strategy on successful resolution of pregnancy among patients with a persisting pregnancy of unknown location: the ACT or NOT randomized clinical trial. JAMA. 2021;326:390-400.
EXPERT COMMENTARY
Among patients with persistent PUL, it can be difficult to distinguish between ectopic pregnancy and an early nonviable intrauterine pregnancy.1 If untreated, ectopic pregnancy can lead to serious morbidity and mortality.2 Management options for persistent PUL include expectant management, empirical methotrexate, or diagnostic uterine evacuation with methotrexate as needed. Data on the potential for these options to achieve pregnancy resolution is valuable for patients and clinicians choosing a treatment plan.
Details of the study
Barnhart and colleagues conducted a multicenter, randomized controlled trial that enrolled 225 women with persistent PUL (defined by transvaginal ultrasound imaging without a definitive intrauterine or extrauterine gestation and at least 2 consecutive human chorionic gonadotropin [hCG] values with less than a 15% rise per day). Participants were randomly assigned to 1 of 3 treatment groups: expectant management, empirical methotrexate, or uterine evacuation followed by methotrexate if needed.
The primary outcome was pregnancy resolution without a change in management strategy. A secondary outcome was noninferiority of empirical methotrexate compared with uterine evacuation with methotrexate as needed in achieving pregnancy resolution.
Results. The active management groups were significantly more likely to achieve pregnancy resolution without changing strategies than the expectant management group (51.5% vs 36.0%; difference, 15.4%). However, 39% of enrolled participants declined their randomized allocation and crossed over into a different management strategy.
Empirical methotrexate was found to be noninferior to uterine evacuation followed by methotrexate as needed in achieving pregnancy resolution (54.9% vs 48.3%; difference, 6.6%).
Study strengths and limitations
Prior studies of hemodynamically stable patients with persistent PUL or stable tubal ectopic pregnancy and low initial hCG values (<2,000 IU/L) failed to demonstrate that active management with methotrexate or uterine evacuation leads to more successful or faster pregnancy resolution.3-5 Barnhart and colleagues’ study results, however, found that active management with 2-dose empirical methotrexate or uterine evacuation was more likely to lead to pregnancy resolution without requiring a change in management plan than was expectant management. The authors performed both an intention-to-treat and an as-treated analysis to confirm results.
The 39% crossover rate between the treatment groups likely reflected both patient preference and clinical presentation, potentially biasing the results. The low overall rate of adverse events confirms the safety and acceptability of a patient-centered approach to persistent PUL management. ●
Patients with a persistent PUL who undergo active management with either empirical methotrexate or uterine evacuation followed by methotrexate are more likely to experience pregnancy resolution without a change in management strategy than those who undergo expectant management. Given the safety of all 3 options and demonstrated patient preferences, shared decision making should be used when determining a management plan.
SARAH GUTMAN, MD, MSPH, AND
COURTNEY A. SCHRIEBER, MD, MPH
- van Mello NM, Mol F, Opmeer BC, et al. Diagnostic value of serum hCG on the outcome of pregnancy of unknown location: a systematic review and meta-analysis. Hum Reprod Update. 2012:18:603-617.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG practice bulletin no. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- van Mello NM, Mol F, Verhoeve HR, et al. Methotrexate or expectant management in women with an ectopic pregnancy or pregnancy of unknown location and low serum hCG concentrations? A randomized comparison. Hum Reprod. 2013;28:60-67.
- Jurkovic D, Memtsa M, Sawyer E, et al. Single-dose systemic methotrexate vs expectant management for treatment of tubal ectopic pregnancy: a placebo-controlled randomized trial. Ultrasound Obstet Gynecol. 2017;49:171-176.
- Silva PM, Araujo Junior E, Ceccino GN, et al. Effectiveness of expectant management versus methotrexate in tubal ectopic pregnancy: a double-blind randomized trial. Arch Gynecol Obstet. 2015;291:939-943.
Barnhart K, Hansen KR, Stephenson MD, et al; Reproductive Medicine Network. Effect of an active vs expectant management strategy on successful resolution of pregnancy among patients with a persisting pregnancy of unknown location: the ACT or NOT randomized clinical trial. JAMA. 2021;326:390-400.
EXPERT COMMENTARY
Among patients with persistent PUL, it can be difficult to distinguish between ectopic pregnancy and an early nonviable intrauterine pregnancy.1 If untreated, ectopic pregnancy can lead to serious morbidity and mortality.2 Management options for persistent PUL include expectant management, empirical methotrexate, or diagnostic uterine evacuation with methotrexate as needed. Data on the potential for these options to achieve pregnancy resolution is valuable for patients and clinicians choosing a treatment plan.
Details of the study
Barnhart and colleagues conducted a multicenter, randomized controlled trial that enrolled 225 women with persistent PUL (defined by transvaginal ultrasound imaging without a definitive intrauterine or extrauterine gestation and at least 2 consecutive human chorionic gonadotropin [hCG] values with less than a 15% rise per day). Participants were randomly assigned to 1 of 3 treatment groups: expectant management, empirical methotrexate, or uterine evacuation followed by methotrexate if needed.
The primary outcome was pregnancy resolution without a change in management strategy. A secondary outcome was noninferiority of empirical methotrexate compared with uterine evacuation with methotrexate as needed in achieving pregnancy resolution.
Results. The active management groups were significantly more likely to achieve pregnancy resolution without changing strategies than the expectant management group (51.5% vs 36.0%; difference, 15.4%). However, 39% of enrolled participants declined their randomized allocation and crossed over into a different management strategy.
Empirical methotrexate was found to be noninferior to uterine evacuation followed by methotrexate as needed in achieving pregnancy resolution (54.9% vs 48.3%; difference, 6.6%).
Study strengths and limitations
Prior studies of hemodynamically stable patients with persistent PUL or stable tubal ectopic pregnancy and low initial hCG values (<2,000 IU/L) failed to demonstrate that active management with methotrexate or uterine evacuation leads to more successful or faster pregnancy resolution.3-5 Barnhart and colleagues’ study results, however, found that active management with 2-dose empirical methotrexate or uterine evacuation was more likely to lead to pregnancy resolution without requiring a change in management plan than was expectant management. The authors performed both an intention-to-treat and an as-treated analysis to confirm results.
The 39% crossover rate between the treatment groups likely reflected both patient preference and clinical presentation, potentially biasing the results. The low overall rate of adverse events confirms the safety and acceptability of a patient-centered approach to persistent PUL management. ●
Patients with a persistent PUL who undergo active management with either empirical methotrexate or uterine evacuation followed by methotrexate are more likely to experience pregnancy resolution without a change in management strategy than those who undergo expectant management. Given the safety of all 3 options and demonstrated patient preferences, shared decision making should be used when determining a management plan.
SARAH GUTMAN, MD, MSPH, AND
COURTNEY A. SCHRIEBER, MD, MPH
Barnhart K, Hansen KR, Stephenson MD, et al; Reproductive Medicine Network. Effect of an active vs expectant management strategy on successful resolution of pregnancy among patients with a persisting pregnancy of unknown location: the ACT or NOT randomized clinical trial. JAMA. 2021;326:390-400.
EXPERT COMMENTARY
Among patients with persistent PUL, it can be difficult to distinguish between ectopic pregnancy and an early nonviable intrauterine pregnancy.1 If untreated, ectopic pregnancy can lead to serious morbidity and mortality.2 Management options for persistent PUL include expectant management, empirical methotrexate, or diagnostic uterine evacuation with methotrexate as needed. Data on the potential for these options to achieve pregnancy resolution is valuable for patients and clinicians choosing a treatment plan.
Details of the study
Barnhart and colleagues conducted a multicenter, randomized controlled trial that enrolled 225 women with persistent PUL (defined by transvaginal ultrasound imaging without a definitive intrauterine or extrauterine gestation and at least 2 consecutive human chorionic gonadotropin [hCG] values with less than a 15% rise per day). Participants were randomly assigned to 1 of 3 treatment groups: expectant management, empirical methotrexate, or uterine evacuation followed by methotrexate if needed.
The primary outcome was pregnancy resolution without a change in management strategy. A secondary outcome was noninferiority of empirical methotrexate compared with uterine evacuation with methotrexate as needed in achieving pregnancy resolution.
Results. The active management groups were significantly more likely to achieve pregnancy resolution without changing strategies than the expectant management group (51.5% vs 36.0%; difference, 15.4%). However, 39% of enrolled participants declined their randomized allocation and crossed over into a different management strategy.
Empirical methotrexate was found to be noninferior to uterine evacuation followed by methotrexate as needed in achieving pregnancy resolution (54.9% vs 48.3%; difference, 6.6%).
Study strengths and limitations
Prior studies of hemodynamically stable patients with persistent PUL or stable tubal ectopic pregnancy and low initial hCG values (<2,000 IU/L) failed to demonstrate that active management with methotrexate or uterine evacuation leads to more successful or faster pregnancy resolution.3-5 Barnhart and colleagues’ study results, however, found that active management with 2-dose empirical methotrexate or uterine evacuation was more likely to lead to pregnancy resolution without requiring a change in management plan than was expectant management. The authors performed both an intention-to-treat and an as-treated analysis to confirm results.
The 39% crossover rate between the treatment groups likely reflected both patient preference and clinical presentation, potentially biasing the results. The low overall rate of adverse events confirms the safety and acceptability of a patient-centered approach to persistent PUL management. ●
Patients with a persistent PUL who undergo active management with either empirical methotrexate or uterine evacuation followed by methotrexate are more likely to experience pregnancy resolution without a change in management strategy than those who undergo expectant management. Given the safety of all 3 options and demonstrated patient preferences, shared decision making should be used when determining a management plan.
SARAH GUTMAN, MD, MSPH, AND
COURTNEY A. SCHRIEBER, MD, MPH
- van Mello NM, Mol F, Opmeer BC, et al. Diagnostic value of serum hCG on the outcome of pregnancy of unknown location: a systematic review and meta-analysis. Hum Reprod Update. 2012:18:603-617.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG practice bulletin no. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- van Mello NM, Mol F, Verhoeve HR, et al. Methotrexate or expectant management in women with an ectopic pregnancy or pregnancy of unknown location and low serum hCG concentrations? A randomized comparison. Hum Reprod. 2013;28:60-67.
- Jurkovic D, Memtsa M, Sawyer E, et al. Single-dose systemic methotrexate vs expectant management for treatment of tubal ectopic pregnancy: a placebo-controlled randomized trial. Ultrasound Obstet Gynecol. 2017;49:171-176.
- Silva PM, Araujo Junior E, Ceccino GN, et al. Effectiveness of expectant management versus methotrexate in tubal ectopic pregnancy: a double-blind randomized trial. Arch Gynecol Obstet. 2015;291:939-943.
- van Mello NM, Mol F, Opmeer BC, et al. Diagnostic value of serum hCG on the outcome of pregnancy of unknown location: a systematic review and meta-analysis. Hum Reprod Update. 2012:18:603-617.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG practice bulletin no. 193: tubal ectopic pregnancy. Obstet Gynecol. 2018;131:e91-e103.
- van Mello NM, Mol F, Verhoeve HR, et al. Methotrexate or expectant management in women with an ectopic pregnancy or pregnancy of unknown location and low serum hCG concentrations? A randomized comparison. Hum Reprod. 2013;28:60-67.
- Jurkovic D, Memtsa M, Sawyer E, et al. Single-dose systemic methotrexate vs expectant management for treatment of tubal ectopic pregnancy: a placebo-controlled randomized trial. Ultrasound Obstet Gynecol. 2017;49:171-176.
- Silva PM, Araujo Junior E, Ceccino GN, et al. Effectiveness of expectant management versus methotrexate in tubal ectopic pregnancy: a double-blind randomized trial. Arch Gynecol Obstet. 2015;291:939-943.
Can we return to the ABCs of crafting a medical record note?
Prior to 1980, medical record notes were generally hand-written, short, and to the point. Senior physicians often wrote their 3-line notes using a fountain pen in an elegant cursive. With the transition to electronic medical records, notes have become bloated with irrelevant information and frequently lack a focus on the critical clinical insights that optimize patient care. The use of smart phrases to pull vast amounts of raw data into the note is a major contributor to note bloat. The unrestrained use of the copy and paste functionality generates a sequence of cloned notes that grow in length as new information is added and little information from prior notes removed. With each subsequent clone the note often becomes less accurate, lengthier, and more difficult for a reader to understand. In one survey of 253 physicians who wrote electronic notes, 90% reported that they used the copy and paste function, with 71% reporting that use of this function caused inconsistencies within and among notes and increased the repetitive presentation of outdated information in the note.1 Although the surveyed clinicians recognized that the copy and paste function caused problems, 80% reported that they planned to continue to use the copy and paste function.1
The SOAP note
The problem-oriented SOAP note is written in the classic structure of subjective and objective information, followed by an assessment and plan.2 The structure of the SOAP note emphasizes the logical and sequential collection of data followed by data analysis, resulting in a focused assessment and plan. When notes were hand-written and short, the entire SOAP note could be viewed on one page. Like a dashboard, the eye could quickly scan each key component of the note, facilitating the simultaneous integration of all 4 components of the note, facilitating understanding of the patient’s clinical situation. When the SOAP note structure is used to create a multipage electronic note, the result is a note that often confuses rather than enlightens the reader. A 5- to 10-page SOAP note is often useless for patient care but demonstrates the ability of computer-savvy clinicians to quickly generate a note thousands of words in length.
The APSO note, a response to note bloat
When a medical record note becomes a multipage document, clinicians should consider switching from the SOAP note structure to the APSO note, where the assessment and plan are at the top of the note, and the subjective and objective information is below the assessment and plan. The APSO format permits the reader to more quickly grasp the critical thinking of the author and facilitates a focus on key points relevant to the patient’s condition. The note can be written in the SOAP format, but then the assessment and plan are brought to the top of the note. In my clinical experience fewer than 10% of clinicians are using an APSO note structure. I believe that, with a multipage note, the APSO structure improves the experience of the reader and should be more widely utilized, especially by clinicians who are prone to crafting a bloated note. In a survey of more than 3,000 clinicians, approximately two-thirds of the respondents reported that, compared with SOAP notes, APSO notes were easier and faster to read, and APSO notes made it easier to follow the clinical reasoning of the author.3
Continue to: New evaluation and management billing guidelines—An opportunity to reduce note bloat...
New evaluation and management billing guidelines—An opportunity to reduce note bloat
Previous evaluation and management federal billing guidelines emphasized documentation of a myriad of clinically irrelevant details contributing to note bloat. The new federal evaluation and management billing guidelines pivot the focus of the note to the quality and complexity of medical decision making as demonstrated in the assessment and plan.4 Prioritizing the assessment and plan as the key feature of the medical record note should help reduce the length of notes. The American College of Physicians recently recommended deleting the complete review of systems and prior histories from most notes unless relevant to medical decision making and the assessment and plan.5
The open note
The open note mandate was contained in federal regulations developed to implement the 21st Century Cures Act, which required patients to have access to the information in their medical record. In order to comply with the regulation, health systems are sending most notes and test results to the patient through the health system’s patient gateway. The open note process entered my practice through a stealthy progression, from an initial step of permitting a clinician to easily share their note with a patient to a top-down edict that all notes, except some notes that have a high risk of causing patient harm, must be sent immediately to the patient. Obviously, an open note supports “transparency,” but I am unaware of high quality evidence that open notes improve the health of a population or reduce morbidity or mortality from health problems.
The federal mandate that clinicians share their notes or risk fiscal penalties is coercive and undermines the independence of health professionals. Open notes may have many benefits, including:
- improving a patient’s comprehension and sense of control over their health issues
- increasing patient trust in their health system
- increasing the number of questions patients ask their clinician.6
Open notes may also cause unintended adverse emotional trauma to patients, especially when the note communicates “bad news.” In one study of 100 oncology patients, approximately 25% of respondents reported that reading clinical notes was emotionally difficult, and they sometimes regretted having read the note.6 One patient reported, “I think MyChart is great but in this whole cancer thing MyChart has not been a good thing.” Another patient reported, “Reading serious stuff like that is just too taxing for me to be honest with you.”6 An additional finding of the study was that patients reported their notes were written with too much medical jargon and repetition of information.
Open laboratory, pathology, and imaging data—Helpful or harmful?
A component of the open note mandate is that laboratory, pathology, and imaging data must be shared timely with patients. Some health systems incorporate a 3-day pause prior to sharing such data, in order to provide the clinical team with time to communicate with the patient before the test results are shared. Some health systems, including my health system, have engineered the open note data-sharing system to immediately share the results of most completed laboratory, pathology, and imaging studies with the patient. Immediate sharing of data may result in the patient first learning that they have a serious, life-threatening health problem, such as cancer, from their patient portal rather than from a clinician. As an example, a patient may first learn that they have metastatic cancer from a CT scan that was ordered for a benign indication.
Another example is that a patient may first learn that they have an HIV infection from their patient portal. This can be a shocking and emotionally damaging experience for the patient. For many test results, it would be best if a clinician were able to communicate the result to the patient, providing support and context to the meaning of the result, rather than sending sensitive, life-altering information directly from the laboratory or imaging department to the patient. Leaders in medical education have spent decades teaching clinicians how to communicate “bad news” in a sensitive, supportive, and effective manner. The open sharing of laboratory, pathology, and imaging data short-circuits the superior process of relying on a highly capable clinician to communicate bad news.
Continue to: Crafting the open medical record note...
Crafting the open medical record note
Building on the advice that “when life gives you lemons, make lemonade,” I have begun to pivot the purpose of my medical notes from a product useful to myself and other clinicians to a product whose primary purpose is to be helpful for the patient. The open note can facilitate building a trusting relationship with the patient. My notes are becoming a series of written conversations with the patient, emphasizing compassion and empathy. I am increasing significantly the amount of educational information in the note to help the patient understand their situation. In addition, I am replacing traditional medical terms with verbiage more appropriate in the context of a conversation with the patient, reducing the use of medical jargon. For example, I have stopped using “chief complaint” and replaced it with “health issues.” I am diligently avoiding the use of medical terms that have negative connotations, including “obese,” “psychosomatic,” “alcoholic,” and “drug addiction.” I include encouragement and positive comments in many of my notes. For example, “Ms. X is successfully managing her health issues and experiencing improved health. It is a pleasure collaborating with her on achieving optimal health.”
Can we bring sanity back to medical note writing?
The primary role of a clinician is to spend as much time as possible listening to patients, understanding their needs, and helping them achieve optimal health. There are many benefits to an electronic medical record, including legibility, accessibility, interoperability, and efficiency. However, in current practice “note bloat” undermines the potential of the electronic medical record and makes many notes ineffective to the process of advancing the patient’s health. We are competent and highly trained clinicians. We can craft notes that are simple, specific, story-driven, compassionate, and empathetic. If we return to the ABCs of note writing, focusing on accuracy, brevity, and clarity, we will make note writing and reading more rewarding and improve patient care. ●
- O’Donnell HC, Kaushal R, Barron Y, et al. Physicians’ attitudes towards copy and pasting in the electronic note writing. J Gen Intern Med. 2009;24:63-68.
- Weed LL. Medical records, patient care and medical education. Ir J Med Sci. 1964;462:271-282.
- Sieja A, Pell J, Markley K, et al. Successful implementation of APSO notes across a major health system. Am J Account Care. 2017;5:29-34.
- Barbieri RL, Levy B. Major changes in Medicare billing are planned for January 2021: some specialists fare better that others. OBG Manag. 2020;32:9, 10, 12, 14.
- State of the note summit, 2021. Medical specialty dos and don’ts. https://www.acponline.org/system/files/documents/practice-resources/business-resources/coding/state-of-the-note-summit-2021/sotn21-specialtycare.pdf. Accessed September 21, 2021.
- Kayashtha N, Pollak KI, LeBLanc TW. Open oncology notes: a qualitative study of oncology patients’ experiences reading their cancer care notes. Am Soc Clin Oncol. 2018;14:e251-e257.
Prior to 1980, medical record notes were generally hand-written, short, and to the point. Senior physicians often wrote their 3-line notes using a fountain pen in an elegant cursive. With the transition to electronic medical records, notes have become bloated with irrelevant information and frequently lack a focus on the critical clinical insights that optimize patient care. The use of smart phrases to pull vast amounts of raw data into the note is a major contributor to note bloat. The unrestrained use of the copy and paste functionality generates a sequence of cloned notes that grow in length as new information is added and little information from prior notes removed. With each subsequent clone the note often becomes less accurate, lengthier, and more difficult for a reader to understand. In one survey of 253 physicians who wrote electronic notes, 90% reported that they used the copy and paste function, with 71% reporting that use of this function caused inconsistencies within and among notes and increased the repetitive presentation of outdated information in the note.1 Although the surveyed clinicians recognized that the copy and paste function caused problems, 80% reported that they planned to continue to use the copy and paste function.1
The SOAP note
The problem-oriented SOAP note is written in the classic structure of subjective and objective information, followed by an assessment and plan.2 The structure of the SOAP note emphasizes the logical and sequential collection of data followed by data analysis, resulting in a focused assessment and plan. When notes were hand-written and short, the entire SOAP note could be viewed on one page. Like a dashboard, the eye could quickly scan each key component of the note, facilitating the simultaneous integration of all 4 components of the note, facilitating understanding of the patient’s clinical situation. When the SOAP note structure is used to create a multipage electronic note, the result is a note that often confuses rather than enlightens the reader. A 5- to 10-page SOAP note is often useless for patient care but demonstrates the ability of computer-savvy clinicians to quickly generate a note thousands of words in length.
The APSO note, a response to note bloat
When a medical record note becomes a multipage document, clinicians should consider switching from the SOAP note structure to the APSO note, where the assessment and plan are at the top of the note, and the subjective and objective information is below the assessment and plan. The APSO format permits the reader to more quickly grasp the critical thinking of the author and facilitates a focus on key points relevant to the patient’s condition. The note can be written in the SOAP format, but then the assessment and plan are brought to the top of the note. In my clinical experience fewer than 10% of clinicians are using an APSO note structure. I believe that, with a multipage note, the APSO structure improves the experience of the reader and should be more widely utilized, especially by clinicians who are prone to crafting a bloated note. In a survey of more than 3,000 clinicians, approximately two-thirds of the respondents reported that, compared with SOAP notes, APSO notes were easier and faster to read, and APSO notes made it easier to follow the clinical reasoning of the author.3
Continue to: New evaluation and management billing guidelines—An opportunity to reduce note bloat...
New evaluation and management billing guidelines—An opportunity to reduce note bloat
Previous evaluation and management federal billing guidelines emphasized documentation of a myriad of clinically irrelevant details contributing to note bloat. The new federal evaluation and management billing guidelines pivot the focus of the note to the quality and complexity of medical decision making as demonstrated in the assessment and plan.4 Prioritizing the assessment and plan as the key feature of the medical record note should help reduce the length of notes. The American College of Physicians recently recommended deleting the complete review of systems and prior histories from most notes unless relevant to medical decision making and the assessment and plan.5
The open note
The open note mandate was contained in federal regulations developed to implement the 21st Century Cures Act, which required patients to have access to the information in their medical record. In order to comply with the regulation, health systems are sending most notes and test results to the patient through the health system’s patient gateway. The open note process entered my practice through a stealthy progression, from an initial step of permitting a clinician to easily share their note with a patient to a top-down edict that all notes, except some notes that have a high risk of causing patient harm, must be sent immediately to the patient. Obviously, an open note supports “transparency,” but I am unaware of high quality evidence that open notes improve the health of a population or reduce morbidity or mortality from health problems.
The federal mandate that clinicians share their notes or risk fiscal penalties is coercive and undermines the independence of health professionals. Open notes may have many benefits, including:
- improving a patient’s comprehension and sense of control over their health issues
- increasing patient trust in their health system
- increasing the number of questions patients ask their clinician.6
Open notes may also cause unintended adverse emotional trauma to patients, especially when the note communicates “bad news.” In one study of 100 oncology patients, approximately 25% of respondents reported that reading clinical notes was emotionally difficult, and they sometimes regretted having read the note.6 One patient reported, “I think MyChart is great but in this whole cancer thing MyChart has not been a good thing.” Another patient reported, “Reading serious stuff like that is just too taxing for me to be honest with you.”6 An additional finding of the study was that patients reported their notes were written with too much medical jargon and repetition of information.
Open laboratory, pathology, and imaging data—Helpful or harmful?
A component of the open note mandate is that laboratory, pathology, and imaging data must be shared timely with patients. Some health systems incorporate a 3-day pause prior to sharing such data, in order to provide the clinical team with time to communicate with the patient before the test results are shared. Some health systems, including my health system, have engineered the open note data-sharing system to immediately share the results of most completed laboratory, pathology, and imaging studies with the patient. Immediate sharing of data may result in the patient first learning that they have a serious, life-threatening health problem, such as cancer, from their patient portal rather than from a clinician. As an example, a patient may first learn that they have metastatic cancer from a CT scan that was ordered for a benign indication.
Another example is that a patient may first learn that they have an HIV infection from their patient portal. This can be a shocking and emotionally damaging experience for the patient. For many test results, it would be best if a clinician were able to communicate the result to the patient, providing support and context to the meaning of the result, rather than sending sensitive, life-altering information directly from the laboratory or imaging department to the patient. Leaders in medical education have spent decades teaching clinicians how to communicate “bad news” in a sensitive, supportive, and effective manner. The open sharing of laboratory, pathology, and imaging data short-circuits the superior process of relying on a highly capable clinician to communicate bad news.
Continue to: Crafting the open medical record note...
Crafting the open medical record note
Building on the advice that “when life gives you lemons, make lemonade,” I have begun to pivot the purpose of my medical notes from a product useful to myself and other clinicians to a product whose primary purpose is to be helpful for the patient. The open note can facilitate building a trusting relationship with the patient. My notes are becoming a series of written conversations with the patient, emphasizing compassion and empathy. I am increasing significantly the amount of educational information in the note to help the patient understand their situation. In addition, I am replacing traditional medical terms with verbiage more appropriate in the context of a conversation with the patient, reducing the use of medical jargon. For example, I have stopped using “chief complaint” and replaced it with “health issues.” I am diligently avoiding the use of medical terms that have negative connotations, including “obese,” “psychosomatic,” “alcoholic,” and “drug addiction.” I include encouragement and positive comments in many of my notes. For example, “Ms. X is successfully managing her health issues and experiencing improved health. It is a pleasure collaborating with her on achieving optimal health.”
Can we bring sanity back to medical note writing?
The primary role of a clinician is to spend as much time as possible listening to patients, understanding their needs, and helping them achieve optimal health. There are many benefits to an electronic medical record, including legibility, accessibility, interoperability, and efficiency. However, in current practice “note bloat” undermines the potential of the electronic medical record and makes many notes ineffective to the process of advancing the patient’s health. We are competent and highly trained clinicians. We can craft notes that are simple, specific, story-driven, compassionate, and empathetic. If we return to the ABCs of note writing, focusing on accuracy, brevity, and clarity, we will make note writing and reading more rewarding and improve patient care. ●
Prior to 1980, medical record notes were generally hand-written, short, and to the point. Senior physicians often wrote their 3-line notes using a fountain pen in an elegant cursive. With the transition to electronic medical records, notes have become bloated with irrelevant information and frequently lack a focus on the critical clinical insights that optimize patient care. The use of smart phrases to pull vast amounts of raw data into the note is a major contributor to note bloat. The unrestrained use of the copy and paste functionality generates a sequence of cloned notes that grow in length as new information is added and little information from prior notes removed. With each subsequent clone the note often becomes less accurate, lengthier, and more difficult for a reader to understand. In one survey of 253 physicians who wrote electronic notes, 90% reported that they used the copy and paste function, with 71% reporting that use of this function caused inconsistencies within and among notes and increased the repetitive presentation of outdated information in the note.1 Although the surveyed clinicians recognized that the copy and paste function caused problems, 80% reported that they planned to continue to use the copy and paste function.1
The SOAP note
The problem-oriented SOAP note is written in the classic structure of subjective and objective information, followed by an assessment and plan.2 The structure of the SOAP note emphasizes the logical and sequential collection of data followed by data analysis, resulting in a focused assessment and plan. When notes were hand-written and short, the entire SOAP note could be viewed on one page. Like a dashboard, the eye could quickly scan each key component of the note, facilitating the simultaneous integration of all 4 components of the note, facilitating understanding of the patient’s clinical situation. When the SOAP note structure is used to create a multipage electronic note, the result is a note that often confuses rather than enlightens the reader. A 5- to 10-page SOAP note is often useless for patient care but demonstrates the ability of computer-savvy clinicians to quickly generate a note thousands of words in length.
The APSO note, a response to note bloat
When a medical record note becomes a multipage document, clinicians should consider switching from the SOAP note structure to the APSO note, where the assessment and plan are at the top of the note, and the subjective and objective information is below the assessment and plan. The APSO format permits the reader to more quickly grasp the critical thinking of the author and facilitates a focus on key points relevant to the patient’s condition. The note can be written in the SOAP format, but then the assessment and plan are brought to the top of the note. In my clinical experience fewer than 10% of clinicians are using an APSO note structure. I believe that, with a multipage note, the APSO structure improves the experience of the reader and should be more widely utilized, especially by clinicians who are prone to crafting a bloated note. In a survey of more than 3,000 clinicians, approximately two-thirds of the respondents reported that, compared with SOAP notes, APSO notes were easier and faster to read, and APSO notes made it easier to follow the clinical reasoning of the author.3
Continue to: New evaluation and management billing guidelines—An opportunity to reduce note bloat...
New evaluation and management billing guidelines—An opportunity to reduce note bloat
Previous evaluation and management federal billing guidelines emphasized documentation of a myriad of clinically irrelevant details contributing to note bloat. The new federal evaluation and management billing guidelines pivot the focus of the note to the quality and complexity of medical decision making as demonstrated in the assessment and plan.4 Prioritizing the assessment and plan as the key feature of the medical record note should help reduce the length of notes. The American College of Physicians recently recommended deleting the complete review of systems and prior histories from most notes unless relevant to medical decision making and the assessment and plan.5
The open note
The open note mandate was contained in federal regulations developed to implement the 21st Century Cures Act, which required patients to have access to the information in their medical record. In order to comply with the regulation, health systems are sending most notes and test results to the patient through the health system’s patient gateway. The open note process entered my practice through a stealthy progression, from an initial step of permitting a clinician to easily share their note with a patient to a top-down edict that all notes, except some notes that have a high risk of causing patient harm, must be sent immediately to the patient. Obviously, an open note supports “transparency,” but I am unaware of high quality evidence that open notes improve the health of a population or reduce morbidity or mortality from health problems.
The federal mandate that clinicians share their notes or risk fiscal penalties is coercive and undermines the independence of health professionals. Open notes may have many benefits, including:
- improving a patient’s comprehension and sense of control over their health issues
- increasing patient trust in their health system
- increasing the number of questions patients ask their clinician.6
Open notes may also cause unintended adverse emotional trauma to patients, especially when the note communicates “bad news.” In one study of 100 oncology patients, approximately 25% of respondents reported that reading clinical notes was emotionally difficult, and they sometimes regretted having read the note.6 One patient reported, “I think MyChart is great but in this whole cancer thing MyChart has not been a good thing.” Another patient reported, “Reading serious stuff like that is just too taxing for me to be honest with you.”6 An additional finding of the study was that patients reported their notes were written with too much medical jargon and repetition of information.
Open laboratory, pathology, and imaging data—Helpful or harmful?
A component of the open note mandate is that laboratory, pathology, and imaging data must be shared timely with patients. Some health systems incorporate a 3-day pause prior to sharing such data, in order to provide the clinical team with time to communicate with the patient before the test results are shared. Some health systems, including my health system, have engineered the open note data-sharing system to immediately share the results of most completed laboratory, pathology, and imaging studies with the patient. Immediate sharing of data may result in the patient first learning that they have a serious, life-threatening health problem, such as cancer, from their patient portal rather than from a clinician. As an example, a patient may first learn that they have metastatic cancer from a CT scan that was ordered for a benign indication.
Another example is that a patient may first learn that they have an HIV infection from their patient portal. This can be a shocking and emotionally damaging experience for the patient. For many test results, it would be best if a clinician were able to communicate the result to the patient, providing support and context to the meaning of the result, rather than sending sensitive, life-altering information directly from the laboratory or imaging department to the patient. Leaders in medical education have spent decades teaching clinicians how to communicate “bad news” in a sensitive, supportive, and effective manner. The open sharing of laboratory, pathology, and imaging data short-circuits the superior process of relying on a highly capable clinician to communicate bad news.
Continue to: Crafting the open medical record note...
Crafting the open medical record note
Building on the advice that “when life gives you lemons, make lemonade,” I have begun to pivot the purpose of my medical notes from a product useful to myself and other clinicians to a product whose primary purpose is to be helpful for the patient. The open note can facilitate building a trusting relationship with the patient. My notes are becoming a series of written conversations with the patient, emphasizing compassion and empathy. I am increasing significantly the amount of educational information in the note to help the patient understand their situation. In addition, I am replacing traditional medical terms with verbiage more appropriate in the context of a conversation with the patient, reducing the use of medical jargon. For example, I have stopped using “chief complaint” and replaced it with “health issues.” I am diligently avoiding the use of medical terms that have negative connotations, including “obese,” “psychosomatic,” “alcoholic,” and “drug addiction.” I include encouragement and positive comments in many of my notes. For example, “Ms. X is successfully managing her health issues and experiencing improved health. It is a pleasure collaborating with her on achieving optimal health.”
Can we bring sanity back to medical note writing?
The primary role of a clinician is to spend as much time as possible listening to patients, understanding their needs, and helping them achieve optimal health. There are many benefits to an electronic medical record, including legibility, accessibility, interoperability, and efficiency. However, in current practice “note bloat” undermines the potential of the electronic medical record and makes many notes ineffective to the process of advancing the patient’s health. We are competent and highly trained clinicians. We can craft notes that are simple, specific, story-driven, compassionate, and empathetic. If we return to the ABCs of note writing, focusing on accuracy, brevity, and clarity, we will make note writing and reading more rewarding and improve patient care. ●
- O’Donnell HC, Kaushal R, Barron Y, et al. Physicians’ attitudes towards copy and pasting in the electronic note writing. J Gen Intern Med. 2009;24:63-68.
- Weed LL. Medical records, patient care and medical education. Ir J Med Sci. 1964;462:271-282.
- Sieja A, Pell J, Markley K, et al. Successful implementation of APSO notes across a major health system. Am J Account Care. 2017;5:29-34.
- Barbieri RL, Levy B. Major changes in Medicare billing are planned for January 2021: some specialists fare better that others. OBG Manag. 2020;32:9, 10, 12, 14.
- State of the note summit, 2021. Medical specialty dos and don’ts. https://www.acponline.org/system/files/documents/practice-resources/business-resources/coding/state-of-the-note-summit-2021/sotn21-specialtycare.pdf. Accessed September 21, 2021.
- Kayashtha N, Pollak KI, LeBLanc TW. Open oncology notes: a qualitative study of oncology patients’ experiences reading their cancer care notes. Am Soc Clin Oncol. 2018;14:e251-e257.
- O’Donnell HC, Kaushal R, Barron Y, et al. Physicians’ attitudes towards copy and pasting in the electronic note writing. J Gen Intern Med. 2009;24:63-68.
- Weed LL. Medical records, patient care and medical education. Ir J Med Sci. 1964;462:271-282.
- Sieja A, Pell J, Markley K, et al. Successful implementation of APSO notes across a major health system. Am J Account Care. 2017;5:29-34.
- Barbieri RL, Levy B. Major changes in Medicare billing are planned for January 2021: some specialists fare better that others. OBG Manag. 2020;32:9, 10, 12, 14.
- State of the note summit, 2021. Medical specialty dos and don’ts. https://www.acponline.org/system/files/documents/practice-resources/business-resources/coding/state-of-the-note-summit-2021/sotn21-specialtycare.pdf. Accessed September 21, 2021.
- Kayashtha N, Pollak KI, LeBLanc TW. Open oncology notes: a qualitative study of oncology patients’ experiences reading their cancer care notes. Am Soc Clin Oncol. 2018;14:e251-e257.