User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Vaccinations for the ObGyn’s toolbox
CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
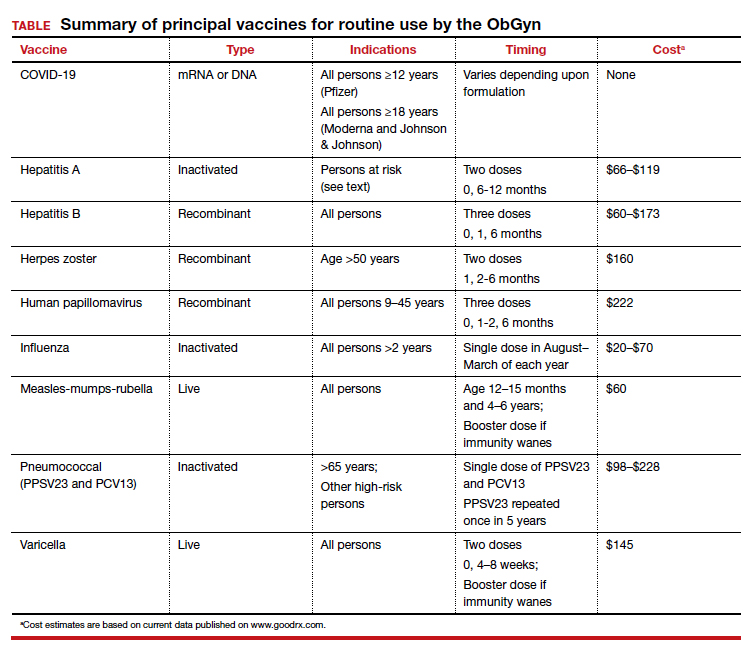
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.
COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.

CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.

COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●

CASE 1st prenatal appointment for young, pregnant migrant
A 21-year-old primigravid woman at 12 weeks’ gestation recently immigrated to the United States from an impoverished rural area of Southeast Asia. On the first prenatal appointment, she is noted to have no evidence of immunity to rubella, measles, or varicella. Her hepatitis B surface antigen and hepatitis C antibody tests are negative. She also has negative test results for gonorrhea, chlamydia, syphilis, and HIV infection. Her pap test is negative.
- What vaccinations should this patient receive during her pregnancy?
- What additional vaccinations are indicated postpartum?
Preventive vaccinations: What to know
As ObGyns, we serve as the primary care physician for many women throughout their early and middle decades of life. Accordingly, we have an obligation to be well informed about preventive health services such as vaccinations. The purpose of this article is to review the principal vaccines with which ObGyns should be familiar. I will discuss the vaccines in alphabetical order and then focus on the indications and timing for each vaccine and the relative cost of each immunization. Key points are summarized in the TABLE.

COVID-19 vaccine
In the latter part of 2020 and early part of 2021, three COVID-19 vaccines received emergency use authorization (EUA) from the US Food and Drug Administration (FDA) for individuals 16 years of age and older (Pfizer-BioNTech) and 18 years of age and older (Moderna and Johnson & Johnson).1 The cost of their administration is borne by the federal government. Two of the vaccines are mRNA agents—Moderna and Pfizer-BioNTech. Both are administered in a 2-dose series, separated by 4 and 3 weeks, respectively. The efficacy of these vaccines in preventing serious or critical illness approaches 95%. The Pfizer-BioNTech vaccine has now been fully FDA approved for administration to individuals older than age 16, with EUA for those down to age 12. Full approval of the Moderna vaccine will not be far behind. Because of some evidence suggesting waning immunity over time and because of growing concerns about the increased transmissibility of the delta variant of the virus, the FDA has been strongly considering a recommendation for a third (booster) dose of each of these vaccines, administered 8 months after the second dose for all eligible Americans. On September 17, 2021, the FDA advisory committee recommended a booster for the Pfizer-BioNTech vaccine for people older than age 65 and for those over the age of 16 at high risk for severe COVID-19. Several days later, full FDA approval was granted for this recommendation. Subsequently, the Director of the Centers for Disease Control and Prevention (CDC) included health care workers and pregnant women in the group for whom the booster is recommended.
The third vaccine formulation is the Johnson & Johnson DNA vaccine, which is prepared with a human adenovirus vector. This vaccine is administered in a single intramuscular dose and has a reported efficacy of 66% to 85%, though it may approach 95% in preventing critical illness. The FDA is expected to announce decisions about booster doses for the Johnson & Johnson and Moderna vaccines in the coming weeks.
Although initial trials of the COVID-19vaccines excluded pregnant and lactating women, the vaccines are safe in pregnancy or postpartum. In fact the vaccines do not contain either a killed or attenuated viral particle that is capable of transmitting infection. Therefore, both the American College of Obstetricians and Gynecologists (ACOG) and the Society for Maternal-Fetal Medicine now support routine immunization during pregnancy.
A recent report by Shimabukuro and colleagues2 demonstrated that the risk of vaccine-related complications in pregnant women receiving the Pfizer-BioNTech or Moderna vaccines was no different than in nonpregnant patients and that there was no evidence of teratogenic effects. The trial included more than 35,000 pregnant women; 2.3% were vaccinated in the periconception period, 28.6% in the first trimester, 43.3% in the second trimester, and 25.7% in the third trimester. Given this, and in light of isolated reports of unusual thromboembolic complications associated with the Johnson & Johnson vaccine, I strongly recommend use of either the Moderna or Pfizer-BioNTech vaccine in our prenatal and postpartum patients.
Continue to: Hepatitis A vaccine...
Hepatitis A vaccine
The hepatitis A vaccine is an inactivated vaccine and is safe for use in pregnancy. It is available in two monovalent preparations—Havrix (GlaxoSmithKline) and Vaqta (Merck & Co.) and is administered in a 2-dose intramuscular injection at time zero and 6 to 12 months later.3 The vaccine is also available in a bivalent form with recombinant hepatitis B vaccine—Twinrix (GlaxoSmithKline). When administered in this form, the vaccine should be given at time zero, 1 month, and 6 months. The wholesale cost of the monovalent vaccine is $66 to $119, depending upon whether the provider uses a multi-dose or a single-dose vial. The cost of Twinrix is $149.
The hepatitis A vaccine is indicated for select pregnant and nonpregnant patients:
- international travelers
- intravenous drug users
- those with occupational exposure (eg, individuals who work in a primate laboratory)
- residents and staff in chronic care facilities
- individuals with chronic liver disease
- individuals with clotting factor disorders
- residents in endemic areas.
Hepatitis B vaccine
The hepatitis B vaccine is a recombinant vaccine that contains an inactivated portion of the hepatitis B surface antigen. It was originally produced in two monovalent formulations: Engerix B (GlaxoSmithKline) and Recombivax-HB (Merck & Co.). These original formulations are given in a 3-dose series at time zero, 1 month, and 6 months. Recently, a new and more potent formulation was introduced into clinical practice. Heplisav-B (Dynavax Technologies Co.) is also a recombinant vaccine that contains a boosting adjuvant. It is programed to be administered in a 2-dose series at time zero and 1 month.4-6
The wholesale cost of the monovalent vaccines varies from $60 to $173, depending upon use of a multi-dose vial versus a single-use vial. The cost of Heplisav-B varies from $146 to $173, depending upon use of a prefilled syringe versus a single-dose vial.
Although the hepatitis B vaccine should be part of the childhood immunization series, it also should be administered to any pregnant woman who has not been vaccinated previously or who does not already have evidence of immunity as a result of natural infection.
Continue to: Herpes zoster vaccine...
Herpes zoster vaccine
Herpes zoster infection (shingles) can be a particularly disabling condition in older patients and results from reactivation of a latent varicella-zoster infection. Shingles can cause extremely painful skin lesions, threaten the patient’s vision, and result in long-lasting postherpetic neuralgia. Both cellular and hormonal immunity are essential to protect against recurrent infection.
The original herpes zoster vaccine (Zoster Vaccine Live; ZVL, Zostavax) is no longer produced in the United States because it is not as effective as the newer vaccine—Recombinant Zoster Vaccine (Shingrix, GlaxoSmithKline).7,8 The antigen in the new vaccine is a component of the surface glycoprotein E, and it is combined with an adjuvant to enhance immunoreactivity. The vaccine is given intramuscularly in two doses at time zero and again at 2 to 6 months and is indicated for all individuals >50 years, including those who may have had an episode of shingles. This newer vaccine is 97% effective in patients >50 years and 90% effective in patients >70. The wholesale cost of each injection is about $160.
Human papillomavirus vaccine
The HPV vaccine (Gardasil-9, Merck & Co.) is a recombinant 9-valent vaccine directed against the human papillomavirus. It induces immunity to serotypes 6 and 11 (which cause 90% of genital warts), 16 and 18 (which cause 80% of genital cancers), and 31, 33, 45, 52, and 58 (viral strains that are responsible for both genital and oropharyngeal cancers). The vaccine is administered intramuscularly in a 3-dose series at time zero, 1-2 months, and 6 months. The principal target groups for the vaccine are males and females, ages 9 to 45 years. Ideally, children of both sexes should receive this vaccine prior to the onset of sexual activity. The wholesale cost of each vaccine injection is approximately $222.9
Influenza vaccine
The inactivated, intramuscular flu vaccine is recommended for anyone over age 2, including pregnant women. Although pregnant women are not more likely to acquire flu compared with those who are not pregnant, if they do become infected, they are likely to become more seriously ill, with higher mortality. Accordingly, all pregnant women should receive, in any trimester, the inactivated flu vaccine beginning in the late summer and early fall of each year and extending through March of the next year.10,11
Multiple formulations of the inactivated vaccine are marketed, all targeting two strains of influenza A and two strains of influenza B. The components of the vaccine vary each year as scientists try to match the new vaccine with the most highly prevalent strains in the previous flu season. The vaccine should be administered in a single intramuscular dose. The cost varies from approximately $20 to $70.
The intranasal influenza vaccine is a live virus vaccine that is intended primarily for children and should not be administered in pregnancy. In addition, there is a higher dose of the inactivated quadrivalent vaccine that is available for administration to patients over age 65. This higher dose is more likely to cause adverse effects and is not indicated in pregnancy.
Continue to: Measles, mumps, rubella vaccine (MMR)...
Measles, mumps, rubella vaccine (MMR)
The MMR is a standard component of the childhood vaccination series. The trivalent preparation is a live, attenuated vaccine that is typically given subcutaneously in a 2-dose series. The first dose is administered at age 12-15 months, and the second dose at age 4-6 years. The vaccine is highly immunogenic, with vaccine-induced immunity usually life-long. In some patients, however, immunity wanes over time. Accordingly, all pregnant women should be screened for immunity to rubella since, of the 3, this infection poses the greatest risk to the fetus. Women who do not have evidence of immunity should be advised to avoid contact with children who may have a viral exanthem. They should then receive a booster dose of the vaccine immediately postpartum and should practice secure contraception for 1 month. The vaccine cost is approximately $60.
Pneumococcal vaccine
The inactivated pneumococcal vaccine is produced in two forms, both of which are safe for administration in pregnancy.12 The original vaccine, introduced in 1983, was PPSV23 (Pneumovax 23, Merck & Co), a 23-serovalent vaccine that was intended primarily for adults. This vaccine is administered in a single subcutaneous or intramuscular dose. The newest vaccine, introduced in 2010, is PCV13 (Prevnar 13, Pfizer Inc), a 13-serovalent vaccine. It was intended primarily for children, in whom it is administered in a 4-dose series beginning at 6 to 8 weeks of age. The cost of the former is approximately $98 to $120; the cost of the latter is $228.
Vaccination against pneumococcal infection is routinely indicated for those older than the age of 65 and for the following at-risk patients, including those who are pregnant11:
- individuals who have had a splenectomy or who have a medical illness that produces functional asplenia (eg, sickle cell anemia)
- individuals with chronic cardiac, pulmonic, hepatic, or renal disease
- individuals with immunosuppressive conditions such as HIV infection or a disseminated malignancy
- individuals who have a cochlear implant
- individuals who have a chronic leak of cerebrospinal fluid.
The recommendations for timing of these 2 vaccines in adults can initially appear confusing. Put most simply, if a high-risk patient first receives the PCV13 vaccine, she should receive the PPSV23 vaccine in about 8 weeks. The PPSV23 vaccine should be repeated in 5 years. If an at-risk patient initially receives the PPSV23 vaccine, the PCV13 vaccine should be given 1 year later.12
Tdap vaccine
The Tdap vaccine contains tetanus toxoid, reduced diptheria toxoid, and an acellular component of the pertussis bacterium. Although it has long been part of the childhood vaccinations series, immunity to each component, particularly pertussis, tends to wane over time.
Pertussis poses a serious risk to the health of the pregnant woman and the newborn infant. Accordingly, the Advisory Committee on Immunization Practices (ACIP), CDC, and the ACOG now advise administration of a booster dose of this vaccine in the early third trimester of each pregnancy.13-15 The vaccine should be administered as a single intramuscular injection. The approximate cost of the vaccine is $64 to $71, depending upon whether the provider uses a single-dose vial or a single-dose prefilled syringe. In nonpregnant patients, the ACIP currently recommends administration of a booster dose of the vaccine every 10 years, primarily to provide durable protection against tetanus.
Continue to: Varicella vaccine...
Varicella vaccine
The varicella vaccine is also one of the main components of the childhood immunization series. This live virus vaccine can be administered subcutaneously as a monovalent agent or as a quadrivalent agent in association with the MMR vaccine.
Pregnant women who do not have a well-documented history of natural infection should be tested for IgG antibody to the varicella-zoster virus at the time of their first prenatal appointment. Interestingly, approximately 70% of patients with an uncertain history actually have immunity when tested. If the patient lacks immunity, she should be vaccinated immediately postpartum.16,17 The vaccine should be administered in a 2-dose series at time zero and then 4 to 8 weeks later. Patients should adhere to secure contraception from the time of the first dose until 1 month after the second dose. The cost of each dose of the vaccine is approximately $145.
Adverse effects of vaccination
All vaccines have many of the same side effects. The most common is simply a reaction at the site of injection, characterized by pain, increased warmth, erythema, swelling, and tenderness. Other common side effects include systemic manifestations, such as low-grade fever, nausea and vomiting, malaise, fatigue, headache, lymphadenopathy, myalgias, and arthralgias. Some vaccines, notably varicella, herpes zoster, measles, and rubella may cause a disseminated rash. Most of these minor side effects are easily managed by rest, hydration, and administration of an analgesic such as acetaminophen or ibuprofen. More serious side effects include rare complications such as anaphylaxis, Bell palsy, Guillain-Barre syndrome, and venous thromboembolism (Johnson & Johnson COVID-19 vaccine). Any of the vaccines discussed above should not be given, or given only with extreme caution, to an individual who has experienced any of these reactions with a previous vaccine.
Barriers to vaccination
Although the vaccines reviewed above are highly effective in preventing serious illness in recipients, the medical profession’s “report card” in ensuring adherence with vaccine protocols is not optimal. In fact, it probably merits a grade no higher than C+, with vaccination rates in the range of 50% to 70%.
One of the major barriers to vaccination is lack of detailed information about vaccine efficacy and safety on the part of both provider and patient. Another is the problem of misinformation (eg, the persistent belief on the part of some individuals that vaccines may cause a serious problem, such as autism).18,19 Another important barrier to widespread vaccination is the logistical problem associated with proper scheduling of multidose regimens (such as those for hepatitis A and B, varicella, and COVID-19). A final barrier, and in my own university-based practice, the most important obstacle is the expense of vaccination. Most, but not all, private insurance companies provide coverage for vaccines approved by the Centers for Disease Control and Prevention and the US Preventive Services Task Force. However, public insurance agencies often provide disappointingly inconsistent coverage for essential vaccines.
By keeping well informed about the most recent public health recommendations for vaccinations for adults and by leading important initiatives within our own practices, we should be able to overcome the first 3 barriers listed above. For example, Morgan and colleagues20 recently achieved a 97% success rate with Tdap administration in pregnancy by placing a best-practice alert in the patients’ electronic medical records. Surmounting the final barrier will require intense effort on the part of individual practitioners and professional organizations to advocate for coverage for essential vaccinations for our patients.
CASE Resolved
This patient was raised in an area of the world where her family did not have easy access to medical care. Accordingly, she did not receive the usual childhood vaccines, such as measles, mumps, rubella, varicella, hepatitis B, and almost certainly, tetanus, diphtheria, and pertussis (Tdap), and the HPV vaccine. The MMR vaccine and the varicella vaccine are live virus vaccines and should not be given during pregnancy. However, these vaccines should be administered postpartum, and the patient should be instructed to practice secure contraception for a minimum of 1 month following vaccination. She also should be offered the HPV vaccine postpartum. During pregnancy, she definitely should receive the COVID-19 vaccine, the 3-dose hepatitis B vaccine series, the influenza vaccine, and Tdap. If her present living conditions place her at risk for hepatitis A, she also should be vaccinated against this illness. ●
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.
- Rasmussen SA, Kelley CF, Horton JP, et al. Coronavirus disease 2019 (COVID-19) vaccines and pregnancy. What obstetricians need to know. Obstet Gynecol. 2021;137:408-414. doi: 10.1097/AOG.0000000000004290.
- Shimabukuro TT, Kim SY, Myers RT, et al. Preliminary findings of mRNA Covid-19 vaccine safety in pregnant persons. N Engl J Med. 2021;384:2273-2282. doi: 10.1056/NEJMoa2104983.
- Duff B, Duff P. Hepatitis A vaccine: ready for prime time. Obstet Gynecol. 1998;91:468-471. doi: 10.1016/s0029-7844(97)00669-8.
- Omer SB. Maternal immunization. N Engl J Med. 2017;376:1256-1267. doi: 10.1056/NEJMra1509044.
- Dionne-Odom J, Tita AT, Silverman NS. Society for Maternal-Fetal Medicine Consult Series: #38: hepatitis B in pregnancy screening, treatment, and prevention of vertical transmission. Am J Obstet Gynecol. 2016;214:6-14. doi: http://dx.doi.org/10.1016/j.ajog.2015.09.100.
- Yawetz S. Immunizations during pregnancy. UpToDate, January 15, 2021.
- Cunningham Al, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016:375:1019-1032. doi: 10.1056/NEJMoa1603800.
- Albrecht MA, Levin MJ. Vaccination for the prevention of shingles (herpes zoster). UpToDate, July 6, 2020.
- ACOG Committee Opinion. Human papillomavirus vaccination. Obstet Gynecol. 2006;108:699-705. doi: 10.1097/00006250-200609000-00047.
- Callaghan WM, Creanga AA, Jamieson DJ. Pregnancy-related mortality resulting from influenza in the United States during the 2009-2010 pandemic. Obstet Gynecol. 2015;126:486-490. doi: 10.1097/AOG.0000000000000996.
- ACOG Committee Opinion. Influenza vaccination during pregnancy. Obstet Gynecol. 2014;124:648-651. doi: 10.1097/01.AOG.0000453599.11566.11.
- Scheller NM, Pasternak B, Molgaard-Nielsen D, et al. Quadrivalent HPV vaccination and the risk of adverse pregnancy outcomes. N Engl J Med. 2017;376:1223-1233. doi: 10.1056/NEJMoa1612296.
- Moumne O, Duff P. Treatment and prevention of pneumococcal infection. Clin Obstet Gynecol. 2019;62:781-789. doi: 10.1097/GRF.0000000000000451.
- ACOG Committee Opinion. Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol. 2017;130:668-669. doi: 10.1097/AOG.0000000000002293.
- Sukumaran L, McCarthy NL, Kharbanda EO, et al. Safety of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis and influenza vaccinations in pregnancy. Obstet Gynecol. 2015;126:1069-1074. doi: 10.1097/AOG.0000000000001066.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165. doi: 10.1155/S1064744994000013.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65. doi: 10.1155/S1064744996000142.
- Desmond A, Offit PA. On the shoulders of giants--from Jenner's cowpox to mRNA COVID vaccines. N Engl. J Med. 2021;384:1081-1083. doi: 10.1056/NEJMp2034334.
- Poland GA, Jacobson RM. The age-old struggle against the antivaccinationists. N Engl J Med. 2011;364:97-99. doi: 10.1056/NEJMp1010594.
- Morgan JL, Baggari SR, Chung W, et al. Association of a best-practice alert and prenatal administration with tetanus toxoid, reduced diptheria toxoid, and acellular pertussis vaccination rates. Obstet Gynecol. 2015;126:333-337. doi: 10.1097/AOG.0000000000000975.
New York’s largest health care provider fires 1,400 unvaccinated employees
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
The employees represented less than 2% of Northwell’s 76,000 employees, who are now all fully vaccinated against COVID-19, Joe Kemp, the assistant vice president of public relations for the company, told The Hill.
“Northwell Health is proud to announce that our workforce -- the largest in New York State -- is 100% vaccinated,” the company said in a statement to several news outlets.
“This allows us to continue to provide exceptional care at all of our facilities, without interruption and remain open and fully operational,” Northwell Health said.
Having a fully vaccinated workforce is part of the health system’s duty to protect others, the company said. Northwell Health includes 23 hospitals and more than 830 outpatient facilities, according to ABC News.
“Northwell regrets losing any employee under such circumstances,” the company said. “We owe it to our staff, our patients, and the communities we serve to be 100% vaccinated against COVID-19.”
Former New York Gov. Andrew Cuomo announced in August that the state would require health care workers to receive at least one COVID-19 vaccine shot by Sept. 27. Employees didn’t have the option for weekly testing or religious exemptions, which is being challenged in several lawsuits, according to The New York Times.
The order went into effect last week, prompting tens of thousands of employees to get vaccinated. As of last week, 87% of hospital staff were fully vaccinated, and 92% of hospital and retirement home workers had received at least one dose, according to state health data.
Northwell announced its own vaccine mandate in August as well, which sparked protests among some workers. The order applied to both clinical and non-clinical staff.
A few thousand Northwell employees got vaccinated as the deadline approached, Mr. Kemp told The New York Times. Some who lost their jobs at first were able to return to work, and those who have been terminated can interview for reinstatement for 30 days. The hospital system is also “openly recruiting” for the vacant positions.
“The goal was to get people vaccinated, not to get people terminated,” Mr. Kemp said.
Hospitalized COVID-19 patients in New York hit a low of 350 in mid-July, according to state hospitalization data. Now, about 2,200 people are hospitalized throughout the state, most of whom are unvaccinated.
As of Oct. 3, nearly 72% of New York residents had received at least one vaccine dose, according to the latest state data. About 64% are fully vaccinated.
A version of this article first appeared on WebMD.com.
Unexpected thrombocytosis could flag occult cancer
A routine blood test may pack a bigger punch than previously suspected, suggests a recent analysis of over 3 million Canadian patient records.
A finding of thrombocytosis (platelet count >450 x 109/L) was associated with a greatly increased risk for some cancers up to 5 years later.
Overall, a high platelet count increased by 2.7 times the odds of receiving a solid-tumor cancer diagnosis within 2 years (95% confidence interval, 2.6-2.8).
The cancers most likely to be associated with unexpected thrombocytosis were those notorious for late-stage diagnosis due to a lack of early symptoms.
The risk was highest (23.3 times) for ovarian cancer. The risk was 3.8 times higher for pancreatic cancer and 3.5 times higher for cervical cancer.
Lung cancer was 4.4 times more likely within 2 years among patients with thrombocytosis compared to patients with normal platelet counts.
Conversely, breast, prostate, and thyroid cancers were not linked to the finding of thrombocytosis.
The study results were published online in JAMA Network Open on Aug. 12).
One of the authors of the article, Stephen A. Narod, MD, director of the Familial Breast Cancer Research Unit at the Women’s College Research Institute, Toronto, said the results were not unexpected but “very striking.”
“I had a hunch we were going to see this because I’ve seen this in other databases,” said Dr. Narod. “I think what struck me about it was how ubiquitous it was.”
Dr. Narod urged physicians, especially those in primary care, to take note: “If the platelets are high, I would certainly have a concern about lung cancer, colon cancer, and ovarian cancer.”
Dr. Narod and coauthor Vasily Giannakeas, a PhD candidate, pointed out that in their analysis that they were unable to single out cases in which a blood test was performed because the patient complained of symptoms that are associated with cancer. In those cases, thrombocytosis may have been diagnostic, rather than a lifesaving serendipitous finding.
Similar findings were reported recently from the United Kingdom.
A study by Sarah Bailey, PhD, MPH, and colleagues that was published last year in the British Journal of General Practice also found a connection between cancer incidence and platelet count. Dr. Bailey is a senior research fellow at the University of Exeter, England.
However, unlike in the Canadian study, the team led by Dr. Bailey was able to distinguish those patients for whom there were alarm symptoms for cancer. Dr. Bailey and colleagues found that two-thirds of men older than 65 had “no recorded alarm features of cancer in the 21 days before their index platelet count.”
Although this suggests that a routine finding of thrombocytosis could uncover unsuspected cancers, Dr. Bailey is cautious about hailing platelet counts as a new cancer-screening tool.
In emailed comments, Dr. Bailey said, “The crucial part of our study is that it was conducted with patients who were ill enough to see their GP [general practitioner]. Opportunistic measurement in patients who are asymptomatic would be quite a different thing. We would have to study the platelet count and subsequent cancers in asymptomatic patients to know if that was worth doing.”
Perhaps most helpfully, the U.K. study showed that cancer risk was increased even among some patients with normal platelet counts. For example, for men aged 60 and older, lung cancer was 4.7 times more likely among those with high-normal counts (≥326 x 109/L).
Because of this somewhat alarming finding, Dr. Bailey suggested moving away from a focus on absolute values. Rising platelet counts might be more clinically useful, she said.
“Physicians should be on the lookout for any unexplained increase in an individual’s platelet count, irrespective of whether the increased value is over or under the local threshold that is applied to define thrombocytosis,” concluded Dr. Bailey.
Dr. Narod has disclosed no relevant financial relationships. Dr. Bailey is a research fellow of the CanTest Collaborative.
A version of this article first appeared on Medscape.com.
A routine blood test may pack a bigger punch than previously suspected, suggests a recent analysis of over 3 million Canadian patient records.
A finding of thrombocytosis (platelet count >450 x 109/L) was associated with a greatly increased risk for some cancers up to 5 years later.
Overall, a high platelet count increased by 2.7 times the odds of receiving a solid-tumor cancer diagnosis within 2 years (95% confidence interval, 2.6-2.8).
The cancers most likely to be associated with unexpected thrombocytosis were those notorious for late-stage diagnosis due to a lack of early symptoms.
The risk was highest (23.3 times) for ovarian cancer. The risk was 3.8 times higher for pancreatic cancer and 3.5 times higher for cervical cancer.
Lung cancer was 4.4 times more likely within 2 years among patients with thrombocytosis compared to patients with normal platelet counts.
Conversely, breast, prostate, and thyroid cancers were not linked to the finding of thrombocytosis.
The study results were published online in JAMA Network Open on Aug. 12).
One of the authors of the article, Stephen A. Narod, MD, director of the Familial Breast Cancer Research Unit at the Women’s College Research Institute, Toronto, said the results were not unexpected but “very striking.”
“I had a hunch we were going to see this because I’ve seen this in other databases,” said Dr. Narod. “I think what struck me about it was how ubiquitous it was.”
Dr. Narod urged physicians, especially those in primary care, to take note: “If the platelets are high, I would certainly have a concern about lung cancer, colon cancer, and ovarian cancer.”
Dr. Narod and coauthor Vasily Giannakeas, a PhD candidate, pointed out that in their analysis that they were unable to single out cases in which a blood test was performed because the patient complained of symptoms that are associated with cancer. In those cases, thrombocytosis may have been diagnostic, rather than a lifesaving serendipitous finding.
Similar findings were reported recently from the United Kingdom.
A study by Sarah Bailey, PhD, MPH, and colleagues that was published last year in the British Journal of General Practice also found a connection between cancer incidence and platelet count. Dr. Bailey is a senior research fellow at the University of Exeter, England.
However, unlike in the Canadian study, the team led by Dr. Bailey was able to distinguish those patients for whom there were alarm symptoms for cancer. Dr. Bailey and colleagues found that two-thirds of men older than 65 had “no recorded alarm features of cancer in the 21 days before their index platelet count.”
Although this suggests that a routine finding of thrombocytosis could uncover unsuspected cancers, Dr. Bailey is cautious about hailing platelet counts as a new cancer-screening tool.
In emailed comments, Dr. Bailey said, “The crucial part of our study is that it was conducted with patients who were ill enough to see their GP [general practitioner]. Opportunistic measurement in patients who are asymptomatic would be quite a different thing. We would have to study the platelet count and subsequent cancers in asymptomatic patients to know if that was worth doing.”
Perhaps most helpfully, the U.K. study showed that cancer risk was increased even among some patients with normal platelet counts. For example, for men aged 60 and older, lung cancer was 4.7 times more likely among those with high-normal counts (≥326 x 109/L).
Because of this somewhat alarming finding, Dr. Bailey suggested moving away from a focus on absolute values. Rising platelet counts might be more clinically useful, she said.
“Physicians should be on the lookout for any unexplained increase in an individual’s platelet count, irrespective of whether the increased value is over or under the local threshold that is applied to define thrombocytosis,” concluded Dr. Bailey.
Dr. Narod has disclosed no relevant financial relationships. Dr. Bailey is a research fellow of the CanTest Collaborative.
A version of this article first appeared on Medscape.com.
A routine blood test may pack a bigger punch than previously suspected, suggests a recent analysis of over 3 million Canadian patient records.
A finding of thrombocytosis (platelet count >450 x 109/L) was associated with a greatly increased risk for some cancers up to 5 years later.
Overall, a high platelet count increased by 2.7 times the odds of receiving a solid-tumor cancer diagnosis within 2 years (95% confidence interval, 2.6-2.8).
The cancers most likely to be associated with unexpected thrombocytosis were those notorious for late-stage diagnosis due to a lack of early symptoms.
The risk was highest (23.3 times) for ovarian cancer. The risk was 3.8 times higher for pancreatic cancer and 3.5 times higher for cervical cancer.
Lung cancer was 4.4 times more likely within 2 years among patients with thrombocytosis compared to patients with normal platelet counts.
Conversely, breast, prostate, and thyroid cancers were not linked to the finding of thrombocytosis.
The study results were published online in JAMA Network Open on Aug. 12).
One of the authors of the article, Stephen A. Narod, MD, director of the Familial Breast Cancer Research Unit at the Women’s College Research Institute, Toronto, said the results were not unexpected but “very striking.”
“I had a hunch we were going to see this because I’ve seen this in other databases,” said Dr. Narod. “I think what struck me about it was how ubiquitous it was.”
Dr. Narod urged physicians, especially those in primary care, to take note: “If the platelets are high, I would certainly have a concern about lung cancer, colon cancer, and ovarian cancer.”
Dr. Narod and coauthor Vasily Giannakeas, a PhD candidate, pointed out that in their analysis that they were unable to single out cases in which a blood test was performed because the patient complained of symptoms that are associated with cancer. In those cases, thrombocytosis may have been diagnostic, rather than a lifesaving serendipitous finding.
Similar findings were reported recently from the United Kingdom.
A study by Sarah Bailey, PhD, MPH, and colleagues that was published last year in the British Journal of General Practice also found a connection between cancer incidence and platelet count. Dr. Bailey is a senior research fellow at the University of Exeter, England.
However, unlike in the Canadian study, the team led by Dr. Bailey was able to distinguish those patients for whom there were alarm symptoms for cancer. Dr. Bailey and colleagues found that two-thirds of men older than 65 had “no recorded alarm features of cancer in the 21 days before their index platelet count.”
Although this suggests that a routine finding of thrombocytosis could uncover unsuspected cancers, Dr. Bailey is cautious about hailing platelet counts as a new cancer-screening tool.
In emailed comments, Dr. Bailey said, “The crucial part of our study is that it was conducted with patients who were ill enough to see their GP [general practitioner]. Opportunistic measurement in patients who are asymptomatic would be quite a different thing. We would have to study the platelet count and subsequent cancers in asymptomatic patients to know if that was worth doing.”
Perhaps most helpfully, the U.K. study showed that cancer risk was increased even among some patients with normal platelet counts. For example, for men aged 60 and older, lung cancer was 4.7 times more likely among those with high-normal counts (≥326 x 109/L).
Because of this somewhat alarming finding, Dr. Bailey suggested moving away from a focus on absolute values. Rising platelet counts might be more clinically useful, she said.
“Physicians should be on the lookout for any unexplained increase in an individual’s platelet count, irrespective of whether the increased value is over or under the local threshold that is applied to define thrombocytosis,” concluded Dr. Bailey.
Dr. Narod has disclosed no relevant financial relationships. Dr. Bailey is a research fellow of the CanTest Collaborative.
A version of this article first appeared on Medscape.com.
Racism a strong factor in Black women’s high rate of premature births, study finds
Dr. Paula Braveman, director of the Center on Social Disparities in Health at the University of California, San Francisco, says her latest research revealed an “astounding” level of evidence that racism is a decisive “upstream” cause of higher rates of preterm birth among Black women.
The tipping point for Dr. Paula Braveman came when a longtime patient of hers at a community clinic in San Francisco’s Mission District slipped past the front desk and knocked on her office door to say goodbye. He wouldn’t be coming to the clinic anymore, he told her, because he could no longer afford it.
It was a decisive moment for Dr. Braveman, who decided she wanted not only to heal ailing patients but also to advocate for policies that would help them be healthier when they arrived at her clinic. In the nearly four decades since, Dr. Braveman has dedicated herself to studying the “social determinants of health” – how the spaces where we live, work, play and learn, and the relationships we have in those places influence how healthy we are.
As director of the Center on Social Disparities in Health at the University of California, San Francisco, Dr. Braveman has studied the link between neighborhood wealth and children’s health, and how access to insurance influences prenatal care. A longtime advocate of translating research into policy, she has collaborated on major health initiatives with the health department in San Francisco, the federal Centers for Disease Control and Prevention, and the World Health Organization.
Dr. Braveman has a particular interest in maternal and infant health. Her latest research reviews what’s known about the persistent gap in preterm birth rates between Black and White women in the United States. Black women are about 1.6 times as likely as White women to give birth more than three weeks before the due date. That statistic bears alarming and costly health consequences, as infants born prematurely are at higher risk for breathing, heart, and brain abnormalities, among other complications.
Dr. Braveman coauthored the review with a group of experts convened by the March of Dimes that included geneticists, clinicians, epidemiologists, biomedical experts, and neurologists. They examined more than two dozen suspected causes of preterm births – including quality of prenatal care, environmental toxics, chronic stress, poverty and obesity – and determined that racism, directly or indirectly, best explained the racial disparities in preterm birth rates.
(Note: In the review, the authors make extensive use of the terms “upstream” and “downstream” to describe what determines people’s health. A downstream risk is the condition or factor most directly responsible for a health outcome, while an upstream factor is what causes or fuels the downstream risk – and often what needs to change to prevent someone from becoming sick. For example, a person living near drinking water polluted with toxic chemicals might get sick from drinking the water. The downstream fix would be telling individuals to use filters. The upstream solution would be to stop the dumping of toxic chemicals.)
KHN spoke with Dr. Braveman about the study and its findings. The excerpts have been edited for length and style.
Q: You have been studying the issue of preterm birth and racial disparities for so long. Were there any findings from this review that surprised you?
The process of systematically going through all of the risk factors that are written about in the literature and then seeing how the story of racism was an upstream determinant for virtually all of them. That was kind of astounding.
The other thing that was very impressive: When we looked at the idea that genetic factors could be the cause of the Black-White disparity in preterm birth. The genetics experts in the group, and there were three or four of them, concluded from the evidence that genetic factors might influence the disparity in preterm birth, but at most the effect would be very small, very small indeed. This could not account for the greater rate of preterm birth among Black women compared to White women.
Q: You were looking to identify not just what causes preterm birth but also to explain racial differences in rates of preterm birth. Are there examples of factors that can influence preterm birth that don’t explain racial disparities?
It does look like there are genetic components to preterm birth, but they don’t explain the Black-White disparity in preterm birth. Another example is having an early elective C-section. That’s one of the problems contributing to avoidable preterm birth, but it doesn’t look like that’s really contributing to the Black-White disparity in preterm birth.
Q: You and your colleagues listed exactly one upstream cause of preterm birth: racism. How would you characterize the certainty that racism is a decisive upstream cause of higher rates of preterm birth among Black women?
It makes me think of this saying: A randomized clinical trial wouldn’t be necessary to give certainty about the importance of having a parachute on if you jump from a plane. To me, at this point, it is close to that.
Going through that paper – and we worked on that paper over a three- or four-year period, so there was a lot of time to think about it – I don’t see how the evidence that we have could be explained otherwise.
Q: What did you learn about how a mother’s broader lifetime experience of racism might affect birth outcomes versus what she experienced within the medical establishment during pregnancy?
There were many ways that experiencing racial discrimination would affect a woman’s pregnancy, but one major way would be through pathways and biological mechanisms involved in stress and stress physiology. In neuroscience, what’s been clear is that a chronic stressor seems to be more damaging to health than an acute stressor.
So it doesn’t make much sense to be looking only during pregnancy. But that’s where most of that research has been done: stress during pregnancy and racial discrimination, and its role in birth outcomes. Very few studies have looked at experiences of racial discrimination across the life course.
My colleagues and I have published a paper where we asked African American women about their experiences of racism, and we didn’t even define what we meant. Women did not talk a lot about the experiences of racism during pregnancy from their medical providers; they talked about the lifetime experience and particularly experiences going back to childhood. And they talked about having to worry, and constant vigilance, so that even if they’re not experiencing an incident, their antennae have to be out to be prepared in case an incident does occur.
Putting all of it together with what we know about stress physiology, I would put my money on the lifetime experiences being so much more important than experiences during pregnancy. There isn’t enough known about preterm birth, but from what is known, inflammation is involved, immune dysfunction, and that’s what stress leads to. The neuroscientists have shown us that chronic stress produces inflammation and immune system dysfunction.
Q: What policies do you think are most important at this stage for reducing preterm birth for Black women?
I wish I could just say one policy or two policies, but I think it does get back to the need to dismantle racism in our society. In all of its manifestations. That’s unfortunate, not to be able to say, “Oh, here, I have this magic bullet, and if you just go with that, that will solve the problem.”
If you take the conclusions of this study seriously, you say, well, policies to just go after these downstream factors are not going to work. It’s up to the upstream investment in trying to achieve a more equitable and less racist society. Ultimately, I think that’s the take-home, and it’s a tall, tall order.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Dr. Paula Braveman, director of the Center on Social Disparities in Health at the University of California, San Francisco, says her latest research revealed an “astounding” level of evidence that racism is a decisive “upstream” cause of higher rates of preterm birth among Black women.
The tipping point for Dr. Paula Braveman came when a longtime patient of hers at a community clinic in San Francisco’s Mission District slipped past the front desk and knocked on her office door to say goodbye. He wouldn’t be coming to the clinic anymore, he told her, because he could no longer afford it.
It was a decisive moment for Dr. Braveman, who decided she wanted not only to heal ailing patients but also to advocate for policies that would help them be healthier when they arrived at her clinic. In the nearly four decades since, Dr. Braveman has dedicated herself to studying the “social determinants of health” – how the spaces where we live, work, play and learn, and the relationships we have in those places influence how healthy we are.
As director of the Center on Social Disparities in Health at the University of California, San Francisco, Dr. Braveman has studied the link between neighborhood wealth and children’s health, and how access to insurance influences prenatal care. A longtime advocate of translating research into policy, she has collaborated on major health initiatives with the health department in San Francisco, the federal Centers for Disease Control and Prevention, and the World Health Organization.
Dr. Braveman has a particular interest in maternal and infant health. Her latest research reviews what’s known about the persistent gap in preterm birth rates between Black and White women in the United States. Black women are about 1.6 times as likely as White women to give birth more than three weeks before the due date. That statistic bears alarming and costly health consequences, as infants born prematurely are at higher risk for breathing, heart, and brain abnormalities, among other complications.
Dr. Braveman coauthored the review with a group of experts convened by the March of Dimes that included geneticists, clinicians, epidemiologists, biomedical experts, and neurologists. They examined more than two dozen suspected causes of preterm births – including quality of prenatal care, environmental toxics, chronic stress, poverty and obesity – and determined that racism, directly or indirectly, best explained the racial disparities in preterm birth rates.
(Note: In the review, the authors make extensive use of the terms “upstream” and “downstream” to describe what determines people’s health. A downstream risk is the condition or factor most directly responsible for a health outcome, while an upstream factor is what causes or fuels the downstream risk – and often what needs to change to prevent someone from becoming sick. For example, a person living near drinking water polluted with toxic chemicals might get sick from drinking the water. The downstream fix would be telling individuals to use filters. The upstream solution would be to stop the dumping of toxic chemicals.)
KHN spoke with Dr. Braveman about the study and its findings. The excerpts have been edited for length and style.
Q: You have been studying the issue of preterm birth and racial disparities for so long. Were there any findings from this review that surprised you?
The process of systematically going through all of the risk factors that are written about in the literature and then seeing how the story of racism was an upstream determinant for virtually all of them. That was kind of astounding.
The other thing that was very impressive: When we looked at the idea that genetic factors could be the cause of the Black-White disparity in preterm birth. The genetics experts in the group, and there were three or four of them, concluded from the evidence that genetic factors might influence the disparity in preterm birth, but at most the effect would be very small, very small indeed. This could not account for the greater rate of preterm birth among Black women compared to White women.
Q: You were looking to identify not just what causes preterm birth but also to explain racial differences in rates of preterm birth. Are there examples of factors that can influence preterm birth that don’t explain racial disparities?
It does look like there are genetic components to preterm birth, but they don’t explain the Black-White disparity in preterm birth. Another example is having an early elective C-section. That’s one of the problems contributing to avoidable preterm birth, but it doesn’t look like that’s really contributing to the Black-White disparity in preterm birth.
Q: You and your colleagues listed exactly one upstream cause of preterm birth: racism. How would you characterize the certainty that racism is a decisive upstream cause of higher rates of preterm birth among Black women?
It makes me think of this saying: A randomized clinical trial wouldn’t be necessary to give certainty about the importance of having a parachute on if you jump from a plane. To me, at this point, it is close to that.
Going through that paper – and we worked on that paper over a three- or four-year period, so there was a lot of time to think about it – I don’t see how the evidence that we have could be explained otherwise.
Q: What did you learn about how a mother’s broader lifetime experience of racism might affect birth outcomes versus what she experienced within the medical establishment during pregnancy?
There were many ways that experiencing racial discrimination would affect a woman’s pregnancy, but one major way would be through pathways and biological mechanisms involved in stress and stress physiology. In neuroscience, what’s been clear is that a chronic stressor seems to be more damaging to health than an acute stressor.
So it doesn’t make much sense to be looking only during pregnancy. But that’s where most of that research has been done: stress during pregnancy and racial discrimination, and its role in birth outcomes. Very few studies have looked at experiences of racial discrimination across the life course.
My colleagues and I have published a paper where we asked African American women about their experiences of racism, and we didn’t even define what we meant. Women did not talk a lot about the experiences of racism during pregnancy from their medical providers; they talked about the lifetime experience and particularly experiences going back to childhood. And they talked about having to worry, and constant vigilance, so that even if they’re not experiencing an incident, their antennae have to be out to be prepared in case an incident does occur.
Putting all of it together with what we know about stress physiology, I would put my money on the lifetime experiences being so much more important than experiences during pregnancy. There isn’t enough known about preterm birth, but from what is known, inflammation is involved, immune dysfunction, and that’s what stress leads to. The neuroscientists have shown us that chronic stress produces inflammation and immune system dysfunction.
Q: What policies do you think are most important at this stage for reducing preterm birth for Black women?
I wish I could just say one policy or two policies, but I think it does get back to the need to dismantle racism in our society. In all of its manifestations. That’s unfortunate, not to be able to say, “Oh, here, I have this magic bullet, and if you just go with that, that will solve the problem.”
If you take the conclusions of this study seriously, you say, well, policies to just go after these downstream factors are not going to work. It’s up to the upstream investment in trying to achieve a more equitable and less racist society. Ultimately, I think that’s the take-home, and it’s a tall, tall order.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Dr. Paula Braveman, director of the Center on Social Disparities in Health at the University of California, San Francisco, says her latest research revealed an “astounding” level of evidence that racism is a decisive “upstream” cause of higher rates of preterm birth among Black women.
The tipping point for Dr. Paula Braveman came when a longtime patient of hers at a community clinic in San Francisco’s Mission District slipped past the front desk and knocked on her office door to say goodbye. He wouldn’t be coming to the clinic anymore, he told her, because he could no longer afford it.
It was a decisive moment for Dr. Braveman, who decided she wanted not only to heal ailing patients but also to advocate for policies that would help them be healthier when they arrived at her clinic. In the nearly four decades since, Dr. Braveman has dedicated herself to studying the “social determinants of health” – how the spaces where we live, work, play and learn, and the relationships we have in those places influence how healthy we are.
As director of the Center on Social Disparities in Health at the University of California, San Francisco, Dr. Braveman has studied the link between neighborhood wealth and children’s health, and how access to insurance influences prenatal care. A longtime advocate of translating research into policy, she has collaborated on major health initiatives with the health department in San Francisco, the federal Centers for Disease Control and Prevention, and the World Health Organization.
Dr. Braveman has a particular interest in maternal and infant health. Her latest research reviews what’s known about the persistent gap in preterm birth rates between Black and White women in the United States. Black women are about 1.6 times as likely as White women to give birth more than three weeks before the due date. That statistic bears alarming and costly health consequences, as infants born prematurely are at higher risk for breathing, heart, and brain abnormalities, among other complications.
Dr. Braveman coauthored the review with a group of experts convened by the March of Dimes that included geneticists, clinicians, epidemiologists, biomedical experts, and neurologists. They examined more than two dozen suspected causes of preterm births – including quality of prenatal care, environmental toxics, chronic stress, poverty and obesity – and determined that racism, directly or indirectly, best explained the racial disparities in preterm birth rates.
(Note: In the review, the authors make extensive use of the terms “upstream” and “downstream” to describe what determines people’s health. A downstream risk is the condition or factor most directly responsible for a health outcome, while an upstream factor is what causes or fuels the downstream risk – and often what needs to change to prevent someone from becoming sick. For example, a person living near drinking water polluted with toxic chemicals might get sick from drinking the water. The downstream fix would be telling individuals to use filters. The upstream solution would be to stop the dumping of toxic chemicals.)
KHN spoke with Dr. Braveman about the study and its findings. The excerpts have been edited for length and style.
Q: You have been studying the issue of preterm birth and racial disparities for so long. Were there any findings from this review that surprised you?
The process of systematically going through all of the risk factors that are written about in the literature and then seeing how the story of racism was an upstream determinant for virtually all of them. That was kind of astounding.
The other thing that was very impressive: When we looked at the idea that genetic factors could be the cause of the Black-White disparity in preterm birth. The genetics experts in the group, and there were three or four of them, concluded from the evidence that genetic factors might influence the disparity in preterm birth, but at most the effect would be very small, very small indeed. This could not account for the greater rate of preterm birth among Black women compared to White women.
Q: You were looking to identify not just what causes preterm birth but also to explain racial differences in rates of preterm birth. Are there examples of factors that can influence preterm birth that don’t explain racial disparities?
It does look like there are genetic components to preterm birth, but they don’t explain the Black-White disparity in preterm birth. Another example is having an early elective C-section. That’s one of the problems contributing to avoidable preterm birth, but it doesn’t look like that’s really contributing to the Black-White disparity in preterm birth.
Q: You and your colleagues listed exactly one upstream cause of preterm birth: racism. How would you characterize the certainty that racism is a decisive upstream cause of higher rates of preterm birth among Black women?
It makes me think of this saying: A randomized clinical trial wouldn’t be necessary to give certainty about the importance of having a parachute on if you jump from a plane. To me, at this point, it is close to that.
Going through that paper – and we worked on that paper over a three- or four-year period, so there was a lot of time to think about it – I don’t see how the evidence that we have could be explained otherwise.
Q: What did you learn about how a mother’s broader lifetime experience of racism might affect birth outcomes versus what she experienced within the medical establishment during pregnancy?
There were many ways that experiencing racial discrimination would affect a woman’s pregnancy, but one major way would be through pathways and biological mechanisms involved in stress and stress physiology. In neuroscience, what’s been clear is that a chronic stressor seems to be more damaging to health than an acute stressor.
So it doesn’t make much sense to be looking only during pregnancy. But that’s where most of that research has been done: stress during pregnancy and racial discrimination, and its role in birth outcomes. Very few studies have looked at experiences of racial discrimination across the life course.
My colleagues and I have published a paper where we asked African American women about their experiences of racism, and we didn’t even define what we meant. Women did not talk a lot about the experiences of racism during pregnancy from their medical providers; they talked about the lifetime experience and particularly experiences going back to childhood. And they talked about having to worry, and constant vigilance, so that even if they’re not experiencing an incident, their antennae have to be out to be prepared in case an incident does occur.
Putting all of it together with what we know about stress physiology, I would put my money on the lifetime experiences being so much more important than experiences during pregnancy. There isn’t enough known about preterm birth, but from what is known, inflammation is involved, immune dysfunction, and that’s what stress leads to. The neuroscientists have shown us that chronic stress produces inflammation and immune system dysfunction.
Q: What policies do you think are most important at this stage for reducing preterm birth for Black women?
I wish I could just say one policy or two policies, but I think it does get back to the need to dismantle racism in our society. In all of its manifestations. That’s unfortunate, not to be able to say, “Oh, here, I have this magic bullet, and if you just go with that, that will solve the problem.”
If you take the conclusions of this study seriously, you say, well, policies to just go after these downstream factors are not going to work. It’s up to the upstream investment in trying to achieve a more equitable and less racist society. Ultimately, I think that’s the take-home, and it’s a tall, tall order.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Clinical Edge Journal Scan Commentary: Uterine Fibroids October 2021
A second study also evaluated the growth uterine fibroids during a different time during a woman’s lifespan - pregnancy. While it’s commonly thought that fibroids grow during pregnancy, a prospective cross-sectional study in the International Journal of Gynecology & Obstetrics (Tian et al) evaluated 394 women with uterine fibroids and found that growth depended on gestational age. In this group, ultrasound examinations were conducted to measure the size of uterine fibroids during weeks 6–7, 11–14, 22–24 and 28–34 of pregnancy and before delivery. The study team found that uterine fibroid size commonly increased before 22–24 weeks of pregnancy with the fastest growth occurring before 11–14 weeks gestation. Later in pregnancy, from 22–24 weeks to the date of delivery, uterine fibroid size remained unchanged.
Lin et al published a large-scale nationwide cohort study in PLoS One that evaluated whether women with uterine fibroids were at increased risk of developing endometriosis. Overall, 31,239 women with uterine fibroids were matched to 124, 956 control participants and followed for 14 years. Compared to controls, patients with uterine fibroids were at a higher risk of developing endometriosis (adjusted hazard ratio [aHR] 6.44, P < 0.05). Other conditions associated with a higher risk of endometriosis included history of tubo-ovarian infection (aHR 2.86, P = 0.01), endometritis (aHR 1.14, P < 0.001), infertility (aHR 1.26, P < 0.001) and allergic diseases (aHR, 1.11, P < .001). Similarly, having both uterine fibroids and infertility significantly increased the risk of endometriosis (aHR 6.95; P < 0.001).
A second study also evaluated the growth uterine fibroids during a different time during a woman’s lifespan - pregnancy. While it’s commonly thought that fibroids grow during pregnancy, a prospective cross-sectional study in the International Journal of Gynecology & Obstetrics (Tian et al) evaluated 394 women with uterine fibroids and found that growth depended on gestational age. In this group, ultrasound examinations were conducted to measure the size of uterine fibroids during weeks 6–7, 11–14, 22–24 and 28–34 of pregnancy and before delivery. The study team found that uterine fibroid size commonly increased before 22–24 weeks of pregnancy with the fastest growth occurring before 11–14 weeks gestation. Later in pregnancy, from 22–24 weeks to the date of delivery, uterine fibroid size remained unchanged.
Lin et al published a large-scale nationwide cohort study in PLoS One that evaluated whether women with uterine fibroids were at increased risk of developing endometriosis. Overall, 31,239 women with uterine fibroids were matched to 124, 956 control participants and followed for 14 years. Compared to controls, patients with uterine fibroids were at a higher risk of developing endometriosis (adjusted hazard ratio [aHR] 6.44, P < 0.05). Other conditions associated with a higher risk of endometriosis included history of tubo-ovarian infection (aHR 2.86, P = 0.01), endometritis (aHR 1.14, P < 0.001), infertility (aHR 1.26, P < 0.001) and allergic diseases (aHR, 1.11, P < .001). Similarly, having both uterine fibroids and infertility significantly increased the risk of endometriosis (aHR 6.95; P < 0.001).
A second study also evaluated the growth uterine fibroids during a different time during a woman’s lifespan - pregnancy. While it’s commonly thought that fibroids grow during pregnancy, a prospective cross-sectional study in the International Journal of Gynecology & Obstetrics (Tian et al) evaluated 394 women with uterine fibroids and found that growth depended on gestational age. In this group, ultrasound examinations were conducted to measure the size of uterine fibroids during weeks 6–7, 11–14, 22–24 and 28–34 of pregnancy and before delivery. The study team found that uterine fibroid size commonly increased before 22–24 weeks of pregnancy with the fastest growth occurring before 11–14 weeks gestation. Later in pregnancy, from 22–24 weeks to the date of delivery, uterine fibroid size remained unchanged.
Lin et al published a large-scale nationwide cohort study in PLoS One that evaluated whether women with uterine fibroids were at increased risk of developing endometriosis. Overall, 31,239 women with uterine fibroids were matched to 124, 956 control participants and followed for 14 years. Compared to controls, patients with uterine fibroids were at a higher risk of developing endometriosis (adjusted hazard ratio [aHR] 6.44, P < 0.05). Other conditions associated with a higher risk of endometriosis included history of tubo-ovarian infection (aHR 2.86, P = 0.01), endometritis (aHR 1.14, P < 0.001), infertility (aHR 1.26, P < 0.001) and allergic diseases (aHR, 1.11, P < .001). Similarly, having both uterine fibroids and infertility significantly increased the risk of endometriosis (aHR 6.95; P < 0.001).
Johnson & Johnson requests FDA approval for vaccine booster doses
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
The company said it filed a request for people ages 18 and older who have received the one-shot vaccine. Johnson & Johnson submitted data for several different booster intervals -- ranging from 2 months to 6 months -- but didn’t formally recommend one to the FDA, The Associated Press reported.
“We’re describing the data to them,” Mathai Mammen, MD, head of global research and development for Janssen, the company’s vaccine division, told CNN.
“The process is not that we asked for a very specific interval -- we’re providing them data and we’re going to be presenting to the committee,” he said. “They’ll take all that into consideration when they ultimately decide on an appropriate interval.”
The FDA’s independent vaccine advisory committee meets next week to review data on booster shots from both Johnson & Johnson and Moderna. It’s the first step in the review process, which then requires approval from leaders at the FDA and Centers for Disease Control and Prevention. If both agencies authorize the extra shots, Americans could receive boosters from Johnson & Johnson and Moderna later this month, the AP reported.
Johnson & Johnson previously released data that showed the vaccine remains highly effective against COVID-19 at least 5 months after vaccination, with 81% efficacy against hospitalizations in the United States.
Two weeks ago, the company reported that a booster dose at 2 months or 6 months further lifted immunity, with a booster at 2 months providing 94% protection against moderate and severe COVID-19. The company said the 6-month booster raised antibodies by 12 times but didn’t release additional data at that time.
In September, the FDA authorized booster shots of the Pfizer vaccine for ages 65 and older, those who live in long-term care facilities, and those with higher risks for contracting COVID-19. The Biden administration is supporting a booster campaign to address potential waning vaccine immunity and remaining surges of the more contagious Delta variant, the AP reported.
A version of this article first appeared on WebMD.com.
MRI screening cost effective for women with dense breasts
Alternatively, if a woman worries that the 4-year screening interval is too long, screening mammography may be offered every 2 years, with MRI screening offered for the second 2-year interval, according to the findings. This strategy would still require the patient to undergo MRI breast cancer screening every 4 years.
“MRI is more effective not only for selected patients. It is actually more effective than mammography for all women,” editorialist Christiane Kuhl, MD, PhD, University of Aachen (Germany), said in an interview.
“But the superior diagnostic accuracy of MRI is more often needed for women who are at higher risk for breast cancer, and therefore the cost-effectiveness is easier to achieve in women who are at higher risk,” she added.
The study was published online Sept. 29 in the Journal of the National Cancer Institute.
DENSE trial
The simulation model used for the study was based on results from the Dense Tissue and Early Breast Neoplasm Screening (DENSE) trial, which showed that additional MRI screening for women with extremely dense breast tissue led to significantly fewer interval cancers in comparison with mammography alone (P < .001). In the DENSE trial, MRI participants underwent mammography plus MRI at 2-year intervals; the control group underwent mammography alone at 2-year intervals.
In the current study, “screening strategies varied in the number of MRIs and mammograms offered to women aged 50-75 years,” explains Amarens Geuzinge, MSc, University Medical Center, Rotterdam, the Netherlands, and colleagues, “and incremental cost-effectiveness ratios (ICERs) were calculated ... with a willingness-to-pay threshold of 22,000 euros (>$25,000 U.S.),” the investigators add.
Analyses indicated that screening every 2 years with mammography alone cost the least of all strategies that were evaluated, but it also resulted in the lowest number of quality-adjusted life years (QALYs) – in other words, it delivered the least amount of benefit for patients, coauthor Eveline Heijnsdijk, PhD, University Medical Center, Rotterdam, the Netherlands, explained to this news organization.
Offering an additional MRI every 2 years resulted in the highest costs but not the highest number of QALYs and was inferior to the other screening strategies analyzed, she added. Alternating mammography with MRI breast cancer screening, each conducted every 2 years, came close to providing the same benefits to patients as the every-4-year MRI screening strategy, Dr. Heijnsdijk noted.
However, when the authors applied the National Institute for Health and Care Excellence (NICE) threshold, MRI screening every 4 years yielded the highest acceptable incremental cost-effectiveness ratio (ICER), at 15,620 euros per QALYs, whereas screening every 3 years with MRI alone yielded an ICER of 37,181 euros per QALY.
If decision-makers are willing to pay more than 22,000 euros per QALY gained, “MRI every 2 or 3 years can also become cost effective,” the authors add.
Asked how acceptable MRI screening might be if performed only once every 4 years, Dr. Heijnsdijk noted that, in another of their studies, most of the women who had undergone MRI screening for breast cancer said that they would do so again. “MRI is not a pleasant test, but mammography is also not a pleasant test,” she said.
“So many women prefer MRI above mammography, especially because the detection rate with MRI is better than mammography,” she noted. Dr. Heijnsdijk also said that the percentage of women with extremely dense breasts who would be candidates for MRI screening is small – no more than 10% of women.
At a unit cost of slightly under 300 euros for MRI screening – compared with about 100 euros for screening mammography in the Netherlands – the cost of offering 10% of women MRI instead of mammography might increase, but any additional screening costs could be offset by reductions in the need to treat late-stage breast cancer more aggressively.
‘Interval’ cancers
Commenting further on the study, Dr. Kuhl pointed out that from 25% to 45% of cancers that occur in women who have undergone screening mammography are diagnosed as “interval” cancers, even among women who participate in the best mammography programs. “For a long time, people argued that these interval cancers developed only after the last respective mammogram, but that’s not true at all, because we know that with MRI screening, we can reduce the interval cancer rate down to zero,” Dr. Kuhl emphasized.
This is partially explained by the fact that mammography is “particularly blind” when it comes to detecting rapidly growing tumors. “The fact is that mammography has a modality-inherent tendency to preferentially detect slow-growing cancers, whereas rapidly growing tumors are indistinguishable from ubiquitous benign changes like cysts. [This] is why women who undergo screening mammography are frequently not diagnosed with the cancers that we really need to find,” she said.
Although there is ample talk about overdiagnosis when it comes to screening mammography, the overwhelmingly important problem is underdiagnosis. Even in exemplary mammography screening programs, at least 20% of tumors that are diagnosed on mammography have already advanced to a stage that is too late, Dr. Kuhl noted.
This means that at least half of women do not benefit from screening mammography nearly to the extent that they – and their health care practitioners – believe they should, she added. Dr. Kuhl underscored that this does not mean that clinicians should abandon screening mammography.
What it does mean is that physicians need to abandon the one-size-fits-all approach to screening mammography and start stratifying women on the basis of their individual risk of developing breast cancer by taking a family or personal history. Most women do undergo screening mammography at least once, Dr. Kuhl pointed out. From that mammogram, physicians can use information on breast density and breast architecture to better determine individual risk.
“We have good ideas about how to achieve risk stratification, but we’re not using them, because as long as mammography is the answer for everybody, there isn’t much motivation to dig deeper into the issue of how to determine risk,” Dr. Kuhl said.
“But we have to ensure the early diagnosis of aggressive cancers, and it’s exactly MRI that can do this, and we should start with women with very dense breasts because they are doubly underserved by mammography,” she said.
The study was supported by the University Medical Center Utrecht, Bayer HealthCare Medical Care, Matakina, and others. Ms. Geuzinge, Dr. Heijnsdijk, and Dr. Kuhl have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alternatively, if a woman worries that the 4-year screening interval is too long, screening mammography may be offered every 2 years, with MRI screening offered for the second 2-year interval, according to the findings. This strategy would still require the patient to undergo MRI breast cancer screening every 4 years.
“MRI is more effective not only for selected patients. It is actually more effective than mammography for all women,” editorialist Christiane Kuhl, MD, PhD, University of Aachen (Germany), said in an interview.
“But the superior diagnostic accuracy of MRI is more often needed for women who are at higher risk for breast cancer, and therefore the cost-effectiveness is easier to achieve in women who are at higher risk,” she added.
The study was published online Sept. 29 in the Journal of the National Cancer Institute.
DENSE trial
The simulation model used for the study was based on results from the Dense Tissue and Early Breast Neoplasm Screening (DENSE) trial, which showed that additional MRI screening for women with extremely dense breast tissue led to significantly fewer interval cancers in comparison with mammography alone (P < .001). In the DENSE trial, MRI participants underwent mammography plus MRI at 2-year intervals; the control group underwent mammography alone at 2-year intervals.
In the current study, “screening strategies varied in the number of MRIs and mammograms offered to women aged 50-75 years,” explains Amarens Geuzinge, MSc, University Medical Center, Rotterdam, the Netherlands, and colleagues, “and incremental cost-effectiveness ratios (ICERs) were calculated ... with a willingness-to-pay threshold of 22,000 euros (>$25,000 U.S.),” the investigators add.
Analyses indicated that screening every 2 years with mammography alone cost the least of all strategies that were evaluated, but it also resulted in the lowest number of quality-adjusted life years (QALYs) – in other words, it delivered the least amount of benefit for patients, coauthor Eveline Heijnsdijk, PhD, University Medical Center, Rotterdam, the Netherlands, explained to this news organization.
Offering an additional MRI every 2 years resulted in the highest costs but not the highest number of QALYs and was inferior to the other screening strategies analyzed, she added. Alternating mammography with MRI breast cancer screening, each conducted every 2 years, came close to providing the same benefits to patients as the every-4-year MRI screening strategy, Dr. Heijnsdijk noted.
However, when the authors applied the National Institute for Health and Care Excellence (NICE) threshold, MRI screening every 4 years yielded the highest acceptable incremental cost-effectiveness ratio (ICER), at 15,620 euros per QALYs, whereas screening every 3 years with MRI alone yielded an ICER of 37,181 euros per QALY.
If decision-makers are willing to pay more than 22,000 euros per QALY gained, “MRI every 2 or 3 years can also become cost effective,” the authors add.
Asked how acceptable MRI screening might be if performed only once every 4 years, Dr. Heijnsdijk noted that, in another of their studies, most of the women who had undergone MRI screening for breast cancer said that they would do so again. “MRI is not a pleasant test, but mammography is also not a pleasant test,” she said.
“So many women prefer MRI above mammography, especially because the detection rate with MRI is better than mammography,” she noted. Dr. Heijnsdijk also said that the percentage of women with extremely dense breasts who would be candidates for MRI screening is small – no more than 10% of women.
At a unit cost of slightly under 300 euros for MRI screening – compared with about 100 euros for screening mammography in the Netherlands – the cost of offering 10% of women MRI instead of mammography might increase, but any additional screening costs could be offset by reductions in the need to treat late-stage breast cancer more aggressively.
‘Interval’ cancers
Commenting further on the study, Dr. Kuhl pointed out that from 25% to 45% of cancers that occur in women who have undergone screening mammography are diagnosed as “interval” cancers, even among women who participate in the best mammography programs. “For a long time, people argued that these interval cancers developed only after the last respective mammogram, but that’s not true at all, because we know that with MRI screening, we can reduce the interval cancer rate down to zero,” Dr. Kuhl emphasized.
This is partially explained by the fact that mammography is “particularly blind” when it comes to detecting rapidly growing tumors. “The fact is that mammography has a modality-inherent tendency to preferentially detect slow-growing cancers, whereas rapidly growing tumors are indistinguishable from ubiquitous benign changes like cysts. [This] is why women who undergo screening mammography are frequently not diagnosed with the cancers that we really need to find,” she said.
Although there is ample talk about overdiagnosis when it comes to screening mammography, the overwhelmingly important problem is underdiagnosis. Even in exemplary mammography screening programs, at least 20% of tumors that are diagnosed on mammography have already advanced to a stage that is too late, Dr. Kuhl noted.
This means that at least half of women do not benefit from screening mammography nearly to the extent that they – and their health care practitioners – believe they should, she added. Dr. Kuhl underscored that this does not mean that clinicians should abandon screening mammography.
What it does mean is that physicians need to abandon the one-size-fits-all approach to screening mammography and start stratifying women on the basis of their individual risk of developing breast cancer by taking a family or personal history. Most women do undergo screening mammography at least once, Dr. Kuhl pointed out. From that mammogram, physicians can use information on breast density and breast architecture to better determine individual risk.
“We have good ideas about how to achieve risk stratification, but we’re not using them, because as long as mammography is the answer for everybody, there isn’t much motivation to dig deeper into the issue of how to determine risk,” Dr. Kuhl said.
“But we have to ensure the early diagnosis of aggressive cancers, and it’s exactly MRI that can do this, and we should start with women with very dense breasts because they are doubly underserved by mammography,” she said.
The study was supported by the University Medical Center Utrecht, Bayer HealthCare Medical Care, Matakina, and others. Ms. Geuzinge, Dr. Heijnsdijk, and Dr. Kuhl have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alternatively, if a woman worries that the 4-year screening interval is too long, screening mammography may be offered every 2 years, with MRI screening offered for the second 2-year interval, according to the findings. This strategy would still require the patient to undergo MRI breast cancer screening every 4 years.
“MRI is more effective not only for selected patients. It is actually more effective than mammography for all women,” editorialist Christiane Kuhl, MD, PhD, University of Aachen (Germany), said in an interview.
“But the superior diagnostic accuracy of MRI is more often needed for women who are at higher risk for breast cancer, and therefore the cost-effectiveness is easier to achieve in women who are at higher risk,” she added.
The study was published online Sept. 29 in the Journal of the National Cancer Institute.
DENSE trial
The simulation model used for the study was based on results from the Dense Tissue and Early Breast Neoplasm Screening (DENSE) trial, which showed that additional MRI screening for women with extremely dense breast tissue led to significantly fewer interval cancers in comparison with mammography alone (P < .001). In the DENSE trial, MRI participants underwent mammography plus MRI at 2-year intervals; the control group underwent mammography alone at 2-year intervals.
In the current study, “screening strategies varied in the number of MRIs and mammograms offered to women aged 50-75 years,” explains Amarens Geuzinge, MSc, University Medical Center, Rotterdam, the Netherlands, and colleagues, “and incremental cost-effectiveness ratios (ICERs) were calculated ... with a willingness-to-pay threshold of 22,000 euros (>$25,000 U.S.),” the investigators add.
Analyses indicated that screening every 2 years with mammography alone cost the least of all strategies that were evaluated, but it also resulted in the lowest number of quality-adjusted life years (QALYs) – in other words, it delivered the least amount of benefit for patients, coauthor Eveline Heijnsdijk, PhD, University Medical Center, Rotterdam, the Netherlands, explained to this news organization.
Offering an additional MRI every 2 years resulted in the highest costs but not the highest number of QALYs and was inferior to the other screening strategies analyzed, she added. Alternating mammography with MRI breast cancer screening, each conducted every 2 years, came close to providing the same benefits to patients as the every-4-year MRI screening strategy, Dr. Heijnsdijk noted.
However, when the authors applied the National Institute for Health and Care Excellence (NICE) threshold, MRI screening every 4 years yielded the highest acceptable incremental cost-effectiveness ratio (ICER), at 15,620 euros per QALYs, whereas screening every 3 years with MRI alone yielded an ICER of 37,181 euros per QALY.
If decision-makers are willing to pay more than 22,000 euros per QALY gained, “MRI every 2 or 3 years can also become cost effective,” the authors add.
Asked how acceptable MRI screening might be if performed only once every 4 years, Dr. Heijnsdijk noted that, in another of their studies, most of the women who had undergone MRI screening for breast cancer said that they would do so again. “MRI is not a pleasant test, but mammography is also not a pleasant test,” she said.
“So many women prefer MRI above mammography, especially because the detection rate with MRI is better than mammography,” she noted. Dr. Heijnsdijk also said that the percentage of women with extremely dense breasts who would be candidates for MRI screening is small – no more than 10% of women.
At a unit cost of slightly under 300 euros for MRI screening – compared with about 100 euros for screening mammography in the Netherlands – the cost of offering 10% of women MRI instead of mammography might increase, but any additional screening costs could be offset by reductions in the need to treat late-stage breast cancer more aggressively.
‘Interval’ cancers
Commenting further on the study, Dr. Kuhl pointed out that from 25% to 45% of cancers that occur in women who have undergone screening mammography are diagnosed as “interval” cancers, even among women who participate in the best mammography programs. “For a long time, people argued that these interval cancers developed only after the last respective mammogram, but that’s not true at all, because we know that with MRI screening, we can reduce the interval cancer rate down to zero,” Dr. Kuhl emphasized.
This is partially explained by the fact that mammography is “particularly blind” when it comes to detecting rapidly growing tumors. “The fact is that mammography has a modality-inherent tendency to preferentially detect slow-growing cancers, whereas rapidly growing tumors are indistinguishable from ubiquitous benign changes like cysts. [This] is why women who undergo screening mammography are frequently not diagnosed with the cancers that we really need to find,” she said.
Although there is ample talk about overdiagnosis when it comes to screening mammography, the overwhelmingly important problem is underdiagnosis. Even in exemplary mammography screening programs, at least 20% of tumors that are diagnosed on mammography have already advanced to a stage that is too late, Dr. Kuhl noted.
This means that at least half of women do not benefit from screening mammography nearly to the extent that they – and their health care practitioners – believe they should, she added. Dr. Kuhl underscored that this does not mean that clinicians should abandon screening mammography.
What it does mean is that physicians need to abandon the one-size-fits-all approach to screening mammography and start stratifying women on the basis of their individual risk of developing breast cancer by taking a family or personal history. Most women do undergo screening mammography at least once, Dr. Kuhl pointed out. From that mammogram, physicians can use information on breast density and breast architecture to better determine individual risk.
“We have good ideas about how to achieve risk stratification, but we’re not using them, because as long as mammography is the answer for everybody, there isn’t much motivation to dig deeper into the issue of how to determine risk,” Dr. Kuhl said.
“But we have to ensure the early diagnosis of aggressive cancers, and it’s exactly MRI that can do this, and we should start with women with very dense breasts because they are doubly underserved by mammography,” she said.
The study was supported by the University Medical Center Utrecht, Bayer HealthCare Medical Care, Matakina, and others. Ms. Geuzinge, Dr. Heijnsdijk, and Dr. Kuhl have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pfizer COVID vaccine antibodies may disappear in 7 months, study says
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
, according to a new study published on the bioRxiv preprint server.
In the study, which hasn’t yet been peer-reviewed or formally published in a medical journal, researchers analyzed blood samples from 46 healthy young or middle-aged adults after receiving two doses, and then 6 months after the second dose.
“Our study shows vaccination with the Pfizer-BioNTech vaccine induces high levels of neutralizing antibodies against the original vaccine strain, but these levels drop by nearly 10-fold by 7 months,” the researchers told Reuters.
In about half of the adults, neutralizing antibodies were undetectable at 6 months after the second dose, particularly against coronavirus variants such as Delta, Beta, and Mu.
Neutralizing antibodies only make up part of the body’s immune defense against the virus, Reuters noted, but they are still “critically important” in protecting against coronavirus infections.
“These findings suggest that administering a booster dose at around 6 to 7 months following the initial immunization will likely enhance protection,” the study authors wrote.
BioNTech said a new vaccine formula will likely be needed by mid-2022 to protect against future mutations of the virus, according to the Financial Times.
“This year, [a different vaccine] is completely unneeded, but by mid-next year, it could be a different situation,” Ugur Sahin, MD, cofounder and CEO of BioNTech, told the news outlet.
Current variants, namely the Delta variant, are more contagious than the original coronavirus strain but not different enough to evade current vaccines, he said. But new strains may be able to evade boosters.
“This virus will stay, and the virus will further adapt,” Dr. Sahin said. “This is a continuous evolution, and that evolution has just started.”
A version of this article first appeared on WebMD.com.
Antibody cocktail reduces chance of developing COVID
A one-time dose of two long-acting monoclonal antibodies reduced the risk of developing symptomatic COVID by 77% in comparison with placebo (P < .001) in a randomized, double-blind, placebo-controlled, phase 3 trial in adults, according to researchers who presented results at IDWeek 2021, an annual scientific meeting on infectious diseases.
The mix of tixagevimab and cilgavimab (AZD7442, Astra Zeneca) in a 300-mg dose is delivered in two intramuscular injections.
“This is the first long-acting combination of monoclonal antibodies that represents a potential new option to augment COVID-19 prevention,” said lead author Myron J. Levin, MD, a professor and pediatric infectious disease specialist at the University of Colorado at Denver, Aurora, who presented the findings of the PROVENT trial.
Both antibodies were taken from B cells donated by patients who had been infected with SARS-CoV-2, and they work synergistically, Dr. Levin said.
“The combination of them is better than adding results of each individually,” he said. “In vitro experiments have already shown that variants of interest and concern, including the Delta variant, are successfully neutralized by this cocktail.”
The trial was conducted in 87 sites in the United States, the United Kingdom, Spain, France, and Belgium. Participants included 5,197 unvaccinated adults who had never been infected with SARS-CoV-2 and either were at higher risk for inadequate response to COVID-19 vaccines because they were immunocompromised or were at high risk for exposure.
“Efficacy was observed through at least 3 months,” Dr. Levin said. “Preliminary pharmacokinetic modeling predicts potential protection for up to 12 months.”
Raymund Razonable, MD, an infectious disease expert with the Mayo Clinic in Rochester, Minn., who was not involved with the trial, told this news organization he was particularly interested in this combination because the developers made use of novel technology that extends the half-life of the antibodies and because of the large number of participants in the study.
Modeling that shows protection could last up to a year is novel and important, he said.
“People won’t need frequent injections,” Dr. Razonable said. With postexposure prophylaxis monoclonal cocktails, people may be given a dose a month, he noted.
Dr. Razonable said, “This is something intended to prevent COVID in people who are unvaccinated. The downside to that is we want people to get vaccinated. The best strategy so far is really vaccination.”
He said AZD7442 could potentially help fill the void for patients who are not able to respond to the COVID vaccines, including some who are immunocompromised or are undergoing chemotherapy.
Dr. Razonable said that, although the 77% reduction for developing symptomatic COVID-19 (95% confidence interval vs. placebo, 46.0-90.0; P < .001) is impressive, it is a reduction in relative risk. Still unknown is how much an individual’s absolute risk is reduced.
He also said it would be helpful to know how many people in the study population were immunocompromised, “because I think that’s where this product will be useful for prevention.”
The primary study endpoints were the first case of SARS-CoV-2 RT-PCR-positive symptomatic illness post dose and prior to day 183 (efficacy) as well as the safety of the product.
The cocktail appeared to be well tolerated. Adverse events occurred in 35% of participants administered AZD7442 and in 34% of the placebo group. Injection-site reactions occurred in 2.4% of the AZD7442 group and in 2.1% of the placebo group. There was one case of severe or critical COVID-19; two COVID-19–related deaths occurred in the placebo group.
AZD7442 is being developed with the help of funding from the U.S. government. Dr. Levin has received support from GlaxoSmithKline companies. Many of the coauthors are employed by AstraZeneca and hold stock in the company. Dr. Razonable has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A one-time dose of two long-acting monoclonal antibodies reduced the risk of developing symptomatic COVID by 77% in comparison with placebo (P < .001) in a randomized, double-blind, placebo-controlled, phase 3 trial in adults, according to researchers who presented results at IDWeek 2021, an annual scientific meeting on infectious diseases.
The mix of tixagevimab and cilgavimab (AZD7442, Astra Zeneca) in a 300-mg dose is delivered in two intramuscular injections.
“This is the first long-acting combination of monoclonal antibodies that represents a potential new option to augment COVID-19 prevention,” said lead author Myron J. Levin, MD, a professor and pediatric infectious disease specialist at the University of Colorado at Denver, Aurora, who presented the findings of the PROVENT trial.
Both antibodies were taken from B cells donated by patients who had been infected with SARS-CoV-2, and they work synergistically, Dr. Levin said.
“The combination of them is better than adding results of each individually,” he said. “In vitro experiments have already shown that variants of interest and concern, including the Delta variant, are successfully neutralized by this cocktail.”
The trial was conducted in 87 sites in the United States, the United Kingdom, Spain, France, and Belgium. Participants included 5,197 unvaccinated adults who had never been infected with SARS-CoV-2 and either were at higher risk for inadequate response to COVID-19 vaccines because they were immunocompromised or were at high risk for exposure.
“Efficacy was observed through at least 3 months,” Dr. Levin said. “Preliminary pharmacokinetic modeling predicts potential protection for up to 12 months.”
Raymund Razonable, MD, an infectious disease expert with the Mayo Clinic in Rochester, Minn., who was not involved with the trial, told this news organization he was particularly interested in this combination because the developers made use of novel technology that extends the half-life of the antibodies and because of the large number of participants in the study.
Modeling that shows protection could last up to a year is novel and important, he said.
“People won’t need frequent injections,” Dr. Razonable said. With postexposure prophylaxis monoclonal cocktails, people may be given a dose a month, he noted.
Dr. Razonable said, “This is something intended to prevent COVID in people who are unvaccinated. The downside to that is we want people to get vaccinated. The best strategy so far is really vaccination.”
He said AZD7442 could potentially help fill the void for patients who are not able to respond to the COVID vaccines, including some who are immunocompromised or are undergoing chemotherapy.
Dr. Razonable said that, although the 77% reduction for developing symptomatic COVID-19 (95% confidence interval vs. placebo, 46.0-90.0; P < .001) is impressive, it is a reduction in relative risk. Still unknown is how much an individual’s absolute risk is reduced.
He also said it would be helpful to know how many people in the study population were immunocompromised, “because I think that’s where this product will be useful for prevention.”
The primary study endpoints were the first case of SARS-CoV-2 RT-PCR-positive symptomatic illness post dose and prior to day 183 (efficacy) as well as the safety of the product.
The cocktail appeared to be well tolerated. Adverse events occurred in 35% of participants administered AZD7442 and in 34% of the placebo group. Injection-site reactions occurred in 2.4% of the AZD7442 group and in 2.1% of the placebo group. There was one case of severe or critical COVID-19; two COVID-19–related deaths occurred in the placebo group.
AZD7442 is being developed with the help of funding from the U.S. government. Dr. Levin has received support from GlaxoSmithKline companies. Many of the coauthors are employed by AstraZeneca and hold stock in the company. Dr. Razonable has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A one-time dose of two long-acting monoclonal antibodies reduced the risk of developing symptomatic COVID by 77% in comparison with placebo (P < .001) in a randomized, double-blind, placebo-controlled, phase 3 trial in adults, according to researchers who presented results at IDWeek 2021, an annual scientific meeting on infectious diseases.
The mix of tixagevimab and cilgavimab (AZD7442, Astra Zeneca) in a 300-mg dose is delivered in two intramuscular injections.
“This is the first long-acting combination of monoclonal antibodies that represents a potential new option to augment COVID-19 prevention,” said lead author Myron J. Levin, MD, a professor and pediatric infectious disease specialist at the University of Colorado at Denver, Aurora, who presented the findings of the PROVENT trial.
Both antibodies were taken from B cells donated by patients who had been infected with SARS-CoV-2, and they work synergistically, Dr. Levin said.
“The combination of them is better than adding results of each individually,” he said. “In vitro experiments have already shown that variants of interest and concern, including the Delta variant, are successfully neutralized by this cocktail.”
The trial was conducted in 87 sites in the United States, the United Kingdom, Spain, France, and Belgium. Participants included 5,197 unvaccinated adults who had never been infected with SARS-CoV-2 and either were at higher risk for inadequate response to COVID-19 vaccines because they were immunocompromised or were at high risk for exposure.
“Efficacy was observed through at least 3 months,” Dr. Levin said. “Preliminary pharmacokinetic modeling predicts potential protection for up to 12 months.”
Raymund Razonable, MD, an infectious disease expert with the Mayo Clinic in Rochester, Minn., who was not involved with the trial, told this news organization he was particularly interested in this combination because the developers made use of novel technology that extends the half-life of the antibodies and because of the large number of participants in the study.
Modeling that shows protection could last up to a year is novel and important, he said.
“People won’t need frequent injections,” Dr. Razonable said. With postexposure prophylaxis monoclonal cocktails, people may be given a dose a month, he noted.
Dr. Razonable said, “This is something intended to prevent COVID in people who are unvaccinated. The downside to that is we want people to get vaccinated. The best strategy so far is really vaccination.”
He said AZD7442 could potentially help fill the void for patients who are not able to respond to the COVID vaccines, including some who are immunocompromised or are undergoing chemotherapy.
Dr. Razonable said that, although the 77% reduction for developing symptomatic COVID-19 (95% confidence interval vs. placebo, 46.0-90.0; P < .001) is impressive, it is a reduction in relative risk. Still unknown is how much an individual’s absolute risk is reduced.
He also said it would be helpful to know how many people in the study population were immunocompromised, “because I think that’s where this product will be useful for prevention.”
The primary study endpoints were the first case of SARS-CoV-2 RT-PCR-positive symptomatic illness post dose and prior to day 183 (efficacy) as well as the safety of the product.
The cocktail appeared to be well tolerated. Adverse events occurred in 35% of participants administered AZD7442 and in 34% of the placebo group. Injection-site reactions occurred in 2.4% of the AZD7442 group and in 2.1% of the placebo group. There was one case of severe or critical COVID-19; two COVID-19–related deaths occurred in the placebo group.
AZD7442 is being developed with the help of funding from the U.S. government. Dr. Levin has received support from GlaxoSmithKline companies. Many of the coauthors are employed by AstraZeneca and hold stock in the company. Dr. Razonable has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Oteseconazole promising for recurrent yeast infections
A phase 3, randomized, double-blind, controlled trial has shown that oteseconazole (Mycovia Pharmaceuticals), an oral antifungal agent, is safe and effective in treating acute and recurrent yeast infections (vulvovaginal candidiasis [VVC]) and in preventing recurrence of acute VVC episodes.
Findings of the ultraVIOLET trial, which compared oteseconazole with the standard fluconazole, were presented at IDWeek 2021, an annual scientific meeting on infectious diseases, by lead author Mark G. Martens, MD, a professor in the department of obstetrics and gynecology at Drexel University College of Medicine in Philadelphia.
About 75% of all women will have a yeast infection in their lifetime, Dr. Martens noted. About 138 million women worldwide have recurring episodes (at least three acute episodes in the last year) of the debilitating condition.
“Recurrent vulvovaginal candidiasis typically requires treatment of the acute episode followed by long-term suppressive therapy with either weekly or biweekly fluconazole,” Dr. Martens said. However, when therapy stops, more than 50% of patients with recurrent VVC experience an infection within the next 6 months, which takes a significant toll on daily life.
Additionally, fluconazole has been linked with safety issues concerning chronic dosing, he said, citing liver toxicity, drug-drug interactions and “increased risk of miscarriage and birth defects when used during pregnancy.”
Topical treatments have been associated with messy application and burning, he noted.
For this study, researchers enrolled 219 women with a history of recurrent VVC at 51 U.S. sites. Participants were randomized either to 600 mg oteseconazole on day 1, 450 mg oteseconazole on day 2 or placebo capsules; or three sequential 150 mg doses (every 72 hours) of fluconazole together with matching placebo capsules.
In the maintenance phase, 185 women with resolved acute VVC (clinical signs and symptoms were scored below 3) on day 14 received 150 mg oteseconazole or placebo weekly for 11 weeks.
Oteseconazole was superior to fluconazole/placebo in the proportion of subjects with at least one culture-verified acute VVC episode through week 50 in the intent-to-treat population (P < .001) which included subjects who failed to clear their infection in the induction phase.
The average percentage of participants with at least one culture-verified acute VVC episode through week 50 was lower in the oteseconazole group (5.1%), compared with the fluconazole/placebo group (42.2%).
Oteseconazole was noninferior to fluconazole in the proportion of subjects with resolved acute VVC infections at day 14 – 93.2% for the oteseconazole group vs. 95.8% for the fluconazole/placebo group.
The percentages of women who had at least one treatment-emergent adverse event (TEAE) were similar – 54% in the oteseconazole group and 64% in the fluconazole/placebo group. Most TEAEs were mild or moderate and there were no drug-related SAEs or adverse effects on liver function.
“There was no difference in the two groups in he baseline characteristics of age, race, and history of diabetes,” he said.
Oluwatosin Goje, MD, an ob.gyn. with the Cleveland Clinic told this news organization that the drug may offer another option for women who don’t respond to azoles.
“The CDC guidelines say, and I agree, that most episodes of recurrent VVC that are caused by Candida albicans will respond to topical azoles, to oral azoles, to the known drugs that are available. You just may have to use them for a prolonged period of time,” Dr. Goje said. But some patients won’t respond to azoles, the currently available drugs, and topical treatments – so new options are welcome for them, she noted.
She pointed out that the U.S. Food and Drug Administration in June approved ibrexafungerp (Brexafemme), the first oral nonazole treatment for vaginal yeast infections. It was the first approved medicine in a novel antifungal class in more than 2 decades.
Dr. Goje, who runs a large clinic with substantial numbers of women with recurrent yeast infections, said the psychosocial problems women with recurrent yeast infections face – and the time off work and money spent trying to get temporary relief from over-the-counter medications – is underestimated.
“Women have long suffered vaginitis. It can be a lot of social and economic burden. So anything in the toolbox to help women is welcome,” Dr. Goje said.
The study was sponsored by Mycovia Pharmaceuticals. Dr. Martens reports no relevant financial relationships. Several coauthors are either employees of Mycovia or receive support from the company. Dr. Goje has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A phase 3, randomized, double-blind, controlled trial has shown that oteseconazole (Mycovia Pharmaceuticals), an oral antifungal agent, is safe and effective in treating acute and recurrent yeast infections (vulvovaginal candidiasis [VVC]) and in preventing recurrence of acute VVC episodes.
Findings of the ultraVIOLET trial, which compared oteseconazole with the standard fluconazole, were presented at IDWeek 2021, an annual scientific meeting on infectious diseases, by lead author Mark G. Martens, MD, a professor in the department of obstetrics and gynecology at Drexel University College of Medicine in Philadelphia.
About 75% of all women will have a yeast infection in their lifetime, Dr. Martens noted. About 138 million women worldwide have recurring episodes (at least three acute episodes in the last year) of the debilitating condition.
“Recurrent vulvovaginal candidiasis typically requires treatment of the acute episode followed by long-term suppressive therapy with either weekly or biweekly fluconazole,” Dr. Martens said. However, when therapy stops, more than 50% of patients with recurrent VVC experience an infection within the next 6 months, which takes a significant toll on daily life.
Additionally, fluconazole has been linked with safety issues concerning chronic dosing, he said, citing liver toxicity, drug-drug interactions and “increased risk of miscarriage and birth defects when used during pregnancy.”
Topical treatments have been associated with messy application and burning, he noted.
For this study, researchers enrolled 219 women with a history of recurrent VVC at 51 U.S. sites. Participants were randomized either to 600 mg oteseconazole on day 1, 450 mg oteseconazole on day 2 or placebo capsules; or three sequential 150 mg doses (every 72 hours) of fluconazole together with matching placebo capsules.
In the maintenance phase, 185 women with resolved acute VVC (clinical signs and symptoms were scored below 3) on day 14 received 150 mg oteseconazole or placebo weekly for 11 weeks.
Oteseconazole was superior to fluconazole/placebo in the proportion of subjects with at least one culture-verified acute VVC episode through week 50 in the intent-to-treat population (P < .001) which included subjects who failed to clear their infection in the induction phase.
The average percentage of participants with at least one culture-verified acute VVC episode through week 50 was lower in the oteseconazole group (5.1%), compared with the fluconazole/placebo group (42.2%).
Oteseconazole was noninferior to fluconazole in the proportion of subjects with resolved acute VVC infections at day 14 – 93.2% for the oteseconazole group vs. 95.8% for the fluconazole/placebo group.
The percentages of women who had at least one treatment-emergent adverse event (TEAE) were similar – 54% in the oteseconazole group and 64% in the fluconazole/placebo group. Most TEAEs were mild or moderate and there were no drug-related SAEs or adverse effects on liver function.
“There was no difference in the two groups in he baseline characteristics of age, race, and history of diabetes,” he said.
Oluwatosin Goje, MD, an ob.gyn. with the Cleveland Clinic told this news organization that the drug may offer another option for women who don’t respond to azoles.
“The CDC guidelines say, and I agree, that most episodes of recurrent VVC that are caused by Candida albicans will respond to topical azoles, to oral azoles, to the known drugs that are available. You just may have to use them for a prolonged period of time,” Dr. Goje said. But some patients won’t respond to azoles, the currently available drugs, and topical treatments – so new options are welcome for them, she noted.
She pointed out that the U.S. Food and Drug Administration in June approved ibrexafungerp (Brexafemme), the first oral nonazole treatment for vaginal yeast infections. It was the first approved medicine in a novel antifungal class in more than 2 decades.
Dr. Goje, who runs a large clinic with substantial numbers of women with recurrent yeast infections, said the psychosocial problems women with recurrent yeast infections face – and the time off work and money spent trying to get temporary relief from over-the-counter medications – is underestimated.
“Women have long suffered vaginitis. It can be a lot of social and economic burden. So anything in the toolbox to help women is welcome,” Dr. Goje said.
The study was sponsored by Mycovia Pharmaceuticals. Dr. Martens reports no relevant financial relationships. Several coauthors are either employees of Mycovia or receive support from the company. Dr. Goje has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A phase 3, randomized, double-blind, controlled trial has shown that oteseconazole (Mycovia Pharmaceuticals), an oral antifungal agent, is safe and effective in treating acute and recurrent yeast infections (vulvovaginal candidiasis [VVC]) and in preventing recurrence of acute VVC episodes.
Findings of the ultraVIOLET trial, which compared oteseconazole with the standard fluconazole, were presented at IDWeek 2021, an annual scientific meeting on infectious diseases, by lead author Mark G. Martens, MD, a professor in the department of obstetrics and gynecology at Drexel University College of Medicine in Philadelphia.
About 75% of all women will have a yeast infection in their lifetime, Dr. Martens noted. About 138 million women worldwide have recurring episodes (at least three acute episodes in the last year) of the debilitating condition.
“Recurrent vulvovaginal candidiasis typically requires treatment of the acute episode followed by long-term suppressive therapy with either weekly or biweekly fluconazole,” Dr. Martens said. However, when therapy stops, more than 50% of patients with recurrent VVC experience an infection within the next 6 months, which takes a significant toll on daily life.
Additionally, fluconazole has been linked with safety issues concerning chronic dosing, he said, citing liver toxicity, drug-drug interactions and “increased risk of miscarriage and birth defects when used during pregnancy.”
Topical treatments have been associated with messy application and burning, he noted.
For this study, researchers enrolled 219 women with a history of recurrent VVC at 51 U.S. sites. Participants were randomized either to 600 mg oteseconazole on day 1, 450 mg oteseconazole on day 2 or placebo capsules; or three sequential 150 mg doses (every 72 hours) of fluconazole together with matching placebo capsules.
In the maintenance phase, 185 women with resolved acute VVC (clinical signs and symptoms were scored below 3) on day 14 received 150 mg oteseconazole or placebo weekly for 11 weeks.
Oteseconazole was superior to fluconazole/placebo in the proportion of subjects with at least one culture-verified acute VVC episode through week 50 in the intent-to-treat population (P < .001) which included subjects who failed to clear their infection in the induction phase.
The average percentage of participants with at least one culture-verified acute VVC episode through week 50 was lower in the oteseconazole group (5.1%), compared with the fluconazole/placebo group (42.2%).
Oteseconazole was noninferior to fluconazole in the proportion of subjects with resolved acute VVC infections at day 14 – 93.2% for the oteseconazole group vs. 95.8% for the fluconazole/placebo group.
The percentages of women who had at least one treatment-emergent adverse event (TEAE) were similar – 54% in the oteseconazole group and 64% in the fluconazole/placebo group. Most TEAEs were mild or moderate and there were no drug-related SAEs or adverse effects on liver function.
“There was no difference in the two groups in he baseline characteristics of age, race, and history of diabetes,” he said.
Oluwatosin Goje, MD, an ob.gyn. with the Cleveland Clinic told this news organization that the drug may offer another option for women who don’t respond to azoles.
“The CDC guidelines say, and I agree, that most episodes of recurrent VVC that are caused by Candida albicans will respond to topical azoles, to oral azoles, to the known drugs that are available. You just may have to use them for a prolonged period of time,” Dr. Goje said. But some patients won’t respond to azoles, the currently available drugs, and topical treatments – so new options are welcome for them, she noted.
She pointed out that the U.S. Food and Drug Administration in June approved ibrexafungerp (Brexafemme), the first oral nonazole treatment for vaginal yeast infections. It was the first approved medicine in a novel antifungal class in more than 2 decades.
Dr. Goje, who runs a large clinic with substantial numbers of women with recurrent yeast infections, said the psychosocial problems women with recurrent yeast infections face – and the time off work and money spent trying to get temporary relief from over-the-counter medications – is underestimated.
“Women have long suffered vaginitis. It can be a lot of social and economic burden. So anything in the toolbox to help women is welcome,” Dr. Goje said.
The study was sponsored by Mycovia Pharmaceuticals. Dr. Martens reports no relevant financial relationships. Several coauthors are either employees of Mycovia or receive support from the company. Dr. Goje has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.