User login
Vulvar syringoma
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
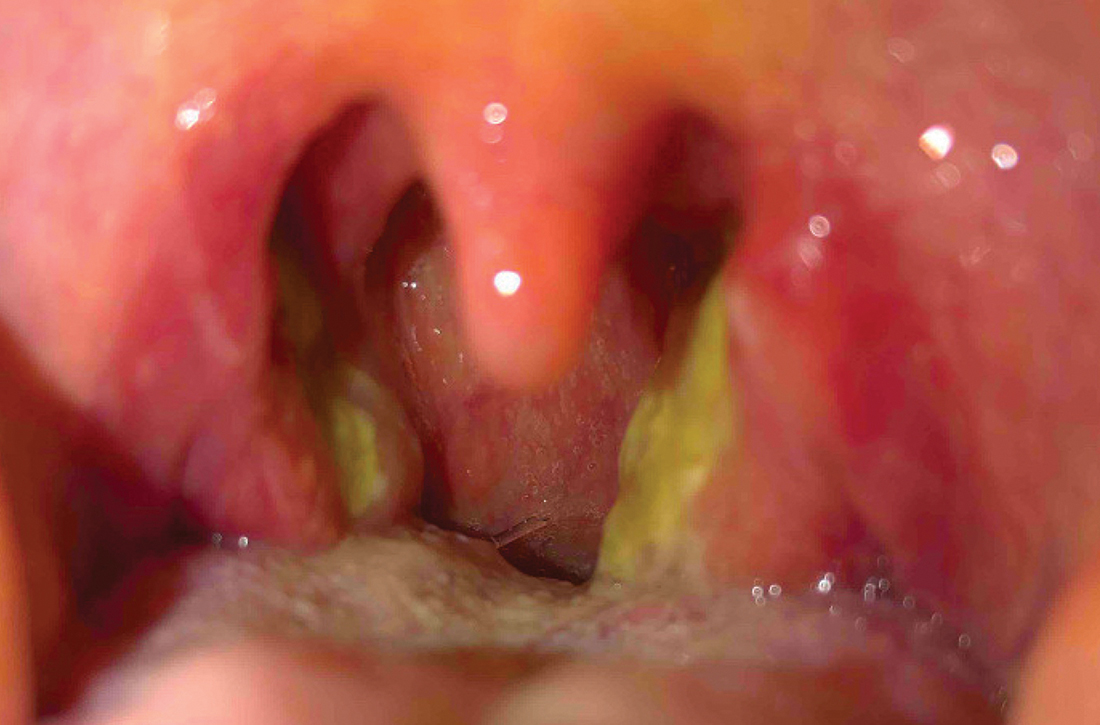
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
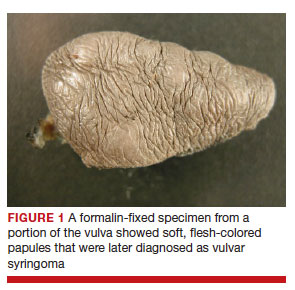
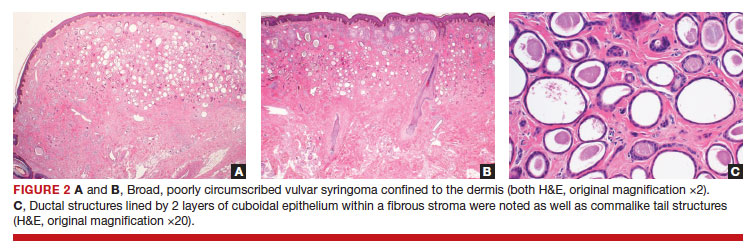
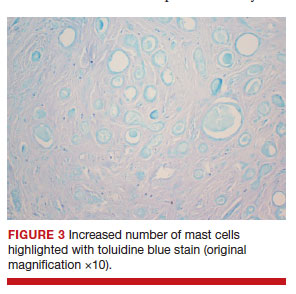
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (FIGURE 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (FIGURES 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (FIGURE 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (FIGURE 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.
Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulvar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (FIGURES 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (FIGURE 3).Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems. ●
- Ensure adequate depth of biopsy to assist in the histologic diagnosis of syringoma vs microcystic adnexal carcinoma.
- Vulvar syringomas also may contribute to notable pruritus and ultimately be the underlying etiology for secondary skin changes leading to a lichen simplex chronicus–like phenotype
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas DermoSifiliográficas. 2008; 99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995; 22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinomaaggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369372.
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (FIGURE 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (FIGURES 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (FIGURE 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (FIGURE 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.



Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulvar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (FIGURES 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (FIGURE 3).Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems. ●
- Ensure adequate depth of biopsy to assist in the histologic diagnosis of syringoma vs microcystic adnexal carcinoma.
- Vulvar syringomas also may contribute to notable pruritus and ultimately be the underlying etiology for secondary skin changes leading to a lichen simplex chronicus–like phenotype
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (FIGURE 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (FIGURES 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (FIGURE 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (FIGURE 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.



Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulvar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (FIGURES 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (FIGURE 3).Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems. ●
- Ensure adequate depth of biopsy to assist in the histologic diagnosis of syringoma vs microcystic adnexal carcinoma.
- Vulvar syringomas also may contribute to notable pruritus and ultimately be the underlying etiology for secondary skin changes leading to a lichen simplex chronicus–like phenotype
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas DermoSifiliográficas. 2008; 99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995; 22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinomaaggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369372.
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas DermoSifiliográficas. 2008; 99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995; 22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinomaaggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369372.
Risk for breast cancer reduced after bariatric surgery
In a matched cohort study of more than 69,000 Canadian women, risk for incident breast cancer at 1 year was 40% higher among women who had not undergone bariatric surgery, compared with those who had. The risk remained elevated through 5 years of follow-up.
The findings were “definitely a bit surprising,” study author Aristithes G. Doumouras, MD, MPH, assistant professor of surgery at McMaster University, Hamilton, Ont., said in an interview. “The patients that underwent bariatric surgery had better cancer outcomes than patients who weighed less than they did, so it showed that there was more at play than just weight loss. This effect was durable [and] shows how powerful the surgery is, [as well as] the fact that we haven’t even explored all of its effects.”
The study was published online in JAMA Surgery.
Protective association
To determine whether there is a residual risk for breast cancer following bariatric surgery for obesity, the investigators analyzed clinical and administrative data collected between 2010 and 2016 in Ontario. They retrospectively matched women with obesity who underwent bariatric surgery with women without a history of bariatric surgery. Participants were matched by age and breast cancer screening status. Covariates included diabetes status, neighborhood income quintile, and measures of health care use. The population included 69,260 women (mean age, 45 years).
Among participants who underwent bariatric surgery for obesity, baseline body mass index was greater than 35 for those with related comorbid conditions, and BMI was greater than 40 for those without comorbid conditions. The investigators categorized nonsurgical control patients in accordance with the following four BMI categories: less than 25, 25-29, 30-34, and greater than or equal to 35. Each control group, as well as the surgical group, included 13,852 women.
Participants in the surgical group were followed for 5 years after bariatric surgery. Those in the nonsurgical group were followed for 5 years after the index date (that is, the date of BMI measurement).
In the overall population, 659 cases of breast cancer were diagnosed in the overall population (0.95%) during the study period. This total included 103 (0.74%) cancers in the surgical cohort; 128 (0.92%) in the group with BMI less than 25; 143 (1.03%) among those with BMI 25-29; 150 (1.08%) in the group with BMI 30-34; and 135 (0.97%) among those with BMI greater than or equal to 35.
Most cancers were stage I. There were 65 cases among those with BMI less than 25; 76 for those with BMI of 25-29; 65 for BMI of 30-34; 67 for BMI greater than or equal to 35, and 60 for the surgery group.
Most tumors were of medium grade and were estrogen receptor positive, progesterone receptor positive, and ERBB2 negative. No significant differences were observed across the groups for stage, grade, or hormone status.
There was an increased hazard for incident breast cancer in the nonsurgical group, compared with the postsurgical group after washout periods of 1 year (hazard ratio, 1.40), 2 years (HR, 1.31), and 5 years (HR, 1.38).
In a comparison of the postsurgical cohort with the nonsurgical cohort with BMI less than 25, the hazard of incident breast cancer was not significantly different for any of the washout periods, but there was a reduced hazard for incident breast cancer among postsurgical patients than among nonsurgical patients in all high BMI categories (BMI ≥ 25).
“Taken together, these results demonstrate that the protective association between substantial weight loss via bariatric surgery and breast cancer risk is sustained after 5 years following surgery and that it is associated with a baseline risk similar to that of women with BMI less than 25,” the investigators write.
Nevertheless, Dr. Doumouras said “the interaction between the surgery and individuals is poorly studied, and this level of personalized medicine is simply not there yet. We are working on developing a prospective cohort that has genetic, protein, and microbiome [data] to help answer these questions.”
There are not enough women in subpopulations such as BRCA carriers to study at this point, he added. “This is where more patients and time will really help the research process.”
A universal benefit?
“Although these findings are important overall for the general population at risk for breast cancer, we raise an important caveat: The benefit of surgical weight loss may not be universal,” write Justin B. Dimick, MD, MPH, surgical innovation editor for JAMA Surgery, and Melissa L. Pilewskie, MD, both of the University of Michigan, Ann Arbor, in an accompanying commentary.
“In addition to lifestyle factors, several nonmodifiable risk factors, such as a genetic predisposition, strong family history, personal history of a high-risk breast lesion, or history of chest wall radiation, impart significant elevation in risk, and the data remain mixed on the impact of weight loss for individuals in these high-risk cohorts,” they add.
“Further study to elucidate the underlying mechanism associated with obesity, weight loss, and breast cancer risk should help guide strategies for risk reduction that are specific to unique high-risk cohorts, because modifiable risk factors may not portend the same benefit among all groups.”
Commenting on the findings, Stephen Edge, MD, breast surgeon and vice president for system quality and outcomes at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., said, “The importance of this study is that it shows that weight loss in midlife can reduce breast cancer risk back to or even below the risk of similar people who were not obese. This has major implications for counseling women.”
The investigators did not have information on the extent of weight loss with surgery or on which participants maintained the lower weight, Dr. Edge noted; “However, overall, most people who have weight reduction surgery have major weight loss.”
At this point, he said, “we can now tell women with obesity that in addition to the many other advantages of weight loss, their risk of getting breast cancer will also be reduced.”
The study was supported by the Ontario Bariatric Registry and ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ontario Ministry of Long-Term Care. Dr. Doumouras, Dr. Dimick, Dr. Pilewskie, and Dr. Edge reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a matched cohort study of more than 69,000 Canadian women, risk for incident breast cancer at 1 year was 40% higher among women who had not undergone bariatric surgery, compared with those who had. The risk remained elevated through 5 years of follow-up.
The findings were “definitely a bit surprising,” study author Aristithes G. Doumouras, MD, MPH, assistant professor of surgery at McMaster University, Hamilton, Ont., said in an interview. “The patients that underwent bariatric surgery had better cancer outcomes than patients who weighed less than they did, so it showed that there was more at play than just weight loss. This effect was durable [and] shows how powerful the surgery is, [as well as] the fact that we haven’t even explored all of its effects.”
The study was published online in JAMA Surgery.
Protective association
To determine whether there is a residual risk for breast cancer following bariatric surgery for obesity, the investigators analyzed clinical and administrative data collected between 2010 and 2016 in Ontario. They retrospectively matched women with obesity who underwent bariatric surgery with women without a history of bariatric surgery. Participants were matched by age and breast cancer screening status. Covariates included diabetes status, neighborhood income quintile, and measures of health care use. The population included 69,260 women (mean age, 45 years).
Among participants who underwent bariatric surgery for obesity, baseline body mass index was greater than 35 for those with related comorbid conditions, and BMI was greater than 40 for those without comorbid conditions. The investigators categorized nonsurgical control patients in accordance with the following four BMI categories: less than 25, 25-29, 30-34, and greater than or equal to 35. Each control group, as well as the surgical group, included 13,852 women.
Participants in the surgical group were followed for 5 years after bariatric surgery. Those in the nonsurgical group were followed for 5 years after the index date (that is, the date of BMI measurement).
In the overall population, 659 cases of breast cancer were diagnosed in the overall population (0.95%) during the study period. This total included 103 (0.74%) cancers in the surgical cohort; 128 (0.92%) in the group with BMI less than 25; 143 (1.03%) among those with BMI 25-29; 150 (1.08%) in the group with BMI 30-34; and 135 (0.97%) among those with BMI greater than or equal to 35.
Most cancers were stage I. There were 65 cases among those with BMI less than 25; 76 for those with BMI of 25-29; 65 for BMI of 30-34; 67 for BMI greater than or equal to 35, and 60 for the surgery group.
Most tumors were of medium grade and were estrogen receptor positive, progesterone receptor positive, and ERBB2 negative. No significant differences were observed across the groups for stage, grade, or hormone status.
There was an increased hazard for incident breast cancer in the nonsurgical group, compared with the postsurgical group after washout periods of 1 year (hazard ratio, 1.40), 2 years (HR, 1.31), and 5 years (HR, 1.38).
In a comparison of the postsurgical cohort with the nonsurgical cohort with BMI less than 25, the hazard of incident breast cancer was not significantly different for any of the washout periods, but there was a reduced hazard for incident breast cancer among postsurgical patients than among nonsurgical patients in all high BMI categories (BMI ≥ 25).
“Taken together, these results demonstrate that the protective association between substantial weight loss via bariatric surgery and breast cancer risk is sustained after 5 years following surgery and that it is associated with a baseline risk similar to that of women with BMI less than 25,” the investigators write.
Nevertheless, Dr. Doumouras said “the interaction between the surgery and individuals is poorly studied, and this level of personalized medicine is simply not there yet. We are working on developing a prospective cohort that has genetic, protein, and microbiome [data] to help answer these questions.”
There are not enough women in subpopulations such as BRCA carriers to study at this point, he added. “This is where more patients and time will really help the research process.”
A universal benefit?
“Although these findings are important overall for the general population at risk for breast cancer, we raise an important caveat: The benefit of surgical weight loss may not be universal,” write Justin B. Dimick, MD, MPH, surgical innovation editor for JAMA Surgery, and Melissa L. Pilewskie, MD, both of the University of Michigan, Ann Arbor, in an accompanying commentary.
“In addition to lifestyle factors, several nonmodifiable risk factors, such as a genetic predisposition, strong family history, personal history of a high-risk breast lesion, or history of chest wall radiation, impart significant elevation in risk, and the data remain mixed on the impact of weight loss for individuals in these high-risk cohorts,” they add.
“Further study to elucidate the underlying mechanism associated with obesity, weight loss, and breast cancer risk should help guide strategies for risk reduction that are specific to unique high-risk cohorts, because modifiable risk factors may not portend the same benefit among all groups.”
Commenting on the findings, Stephen Edge, MD, breast surgeon and vice president for system quality and outcomes at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., said, “The importance of this study is that it shows that weight loss in midlife can reduce breast cancer risk back to or even below the risk of similar people who were not obese. This has major implications for counseling women.”
The investigators did not have information on the extent of weight loss with surgery or on which participants maintained the lower weight, Dr. Edge noted; “However, overall, most people who have weight reduction surgery have major weight loss.”
At this point, he said, “we can now tell women with obesity that in addition to the many other advantages of weight loss, their risk of getting breast cancer will also be reduced.”
The study was supported by the Ontario Bariatric Registry and ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ontario Ministry of Long-Term Care. Dr. Doumouras, Dr. Dimick, Dr. Pilewskie, and Dr. Edge reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a matched cohort study of more than 69,000 Canadian women, risk for incident breast cancer at 1 year was 40% higher among women who had not undergone bariatric surgery, compared with those who had. The risk remained elevated through 5 years of follow-up.
The findings were “definitely a bit surprising,” study author Aristithes G. Doumouras, MD, MPH, assistant professor of surgery at McMaster University, Hamilton, Ont., said in an interview. “The patients that underwent bariatric surgery had better cancer outcomes than patients who weighed less than they did, so it showed that there was more at play than just weight loss. This effect was durable [and] shows how powerful the surgery is, [as well as] the fact that we haven’t even explored all of its effects.”
The study was published online in JAMA Surgery.
Protective association
To determine whether there is a residual risk for breast cancer following bariatric surgery for obesity, the investigators analyzed clinical and administrative data collected between 2010 and 2016 in Ontario. They retrospectively matched women with obesity who underwent bariatric surgery with women without a history of bariatric surgery. Participants were matched by age and breast cancer screening status. Covariates included diabetes status, neighborhood income quintile, and measures of health care use. The population included 69,260 women (mean age, 45 years).
Among participants who underwent bariatric surgery for obesity, baseline body mass index was greater than 35 for those with related comorbid conditions, and BMI was greater than 40 for those without comorbid conditions. The investigators categorized nonsurgical control patients in accordance with the following four BMI categories: less than 25, 25-29, 30-34, and greater than or equal to 35. Each control group, as well as the surgical group, included 13,852 women.
Participants in the surgical group were followed for 5 years after bariatric surgery. Those in the nonsurgical group were followed for 5 years after the index date (that is, the date of BMI measurement).
In the overall population, 659 cases of breast cancer were diagnosed in the overall population (0.95%) during the study period. This total included 103 (0.74%) cancers in the surgical cohort; 128 (0.92%) in the group with BMI less than 25; 143 (1.03%) among those with BMI 25-29; 150 (1.08%) in the group with BMI 30-34; and 135 (0.97%) among those with BMI greater than or equal to 35.
Most cancers were stage I. There were 65 cases among those with BMI less than 25; 76 for those with BMI of 25-29; 65 for BMI of 30-34; 67 for BMI greater than or equal to 35, and 60 for the surgery group.
Most tumors were of medium grade and were estrogen receptor positive, progesterone receptor positive, and ERBB2 negative. No significant differences were observed across the groups for stage, grade, or hormone status.
There was an increased hazard for incident breast cancer in the nonsurgical group, compared with the postsurgical group after washout periods of 1 year (hazard ratio, 1.40), 2 years (HR, 1.31), and 5 years (HR, 1.38).
In a comparison of the postsurgical cohort with the nonsurgical cohort with BMI less than 25, the hazard of incident breast cancer was not significantly different for any of the washout periods, but there was a reduced hazard for incident breast cancer among postsurgical patients than among nonsurgical patients in all high BMI categories (BMI ≥ 25).
“Taken together, these results demonstrate that the protective association between substantial weight loss via bariatric surgery and breast cancer risk is sustained after 5 years following surgery and that it is associated with a baseline risk similar to that of women with BMI less than 25,” the investigators write.
Nevertheless, Dr. Doumouras said “the interaction between the surgery and individuals is poorly studied, and this level of personalized medicine is simply not there yet. We are working on developing a prospective cohort that has genetic, protein, and microbiome [data] to help answer these questions.”
There are not enough women in subpopulations such as BRCA carriers to study at this point, he added. “This is where more patients and time will really help the research process.”
A universal benefit?
“Although these findings are important overall for the general population at risk for breast cancer, we raise an important caveat: The benefit of surgical weight loss may not be universal,” write Justin B. Dimick, MD, MPH, surgical innovation editor for JAMA Surgery, and Melissa L. Pilewskie, MD, both of the University of Michigan, Ann Arbor, in an accompanying commentary.
“In addition to lifestyle factors, several nonmodifiable risk factors, such as a genetic predisposition, strong family history, personal history of a high-risk breast lesion, or history of chest wall radiation, impart significant elevation in risk, and the data remain mixed on the impact of weight loss for individuals in these high-risk cohorts,” they add.
“Further study to elucidate the underlying mechanism associated with obesity, weight loss, and breast cancer risk should help guide strategies for risk reduction that are specific to unique high-risk cohorts, because modifiable risk factors may not portend the same benefit among all groups.”
Commenting on the findings, Stephen Edge, MD, breast surgeon and vice president for system quality and outcomes at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., said, “The importance of this study is that it shows that weight loss in midlife can reduce breast cancer risk back to or even below the risk of similar people who were not obese. This has major implications for counseling women.”
The investigators did not have information on the extent of weight loss with surgery or on which participants maintained the lower weight, Dr. Edge noted; “However, overall, most people who have weight reduction surgery have major weight loss.”
At this point, he said, “we can now tell women with obesity that in addition to the many other advantages of weight loss, their risk of getting breast cancer will also be reduced.”
The study was supported by the Ontario Bariatric Registry and ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ontario Ministry of Long-Term Care. Dr. Doumouras, Dr. Dimick, Dr. Pilewskie, and Dr. Edge reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
Patritumab deruxtecan shows promise for breast cancer patients
according to data presented from Abstract 1240 at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
Patritumab deruxtecan (HER3-DXd) has previously demonstrated an acceptable safety profile and antitumor activity in phase I studies involving heavily pretreated patients with metastatic breast cancer and various levels of HER3 protein expression, said Mafalda Oliveira, MD, of the Vall d’Hebron University Hospital and Vall d’Hebron Institute of Oncology in Barcelona.
Antibody-drug conjugates (ADCs) are a combination of a monoclonal antibody chemically linked to a drug, as defined by the National Cancer Institute. ADCs work by binding to receptors or proteins and selectively delivering cytotoxic drugs to the site of a tumor.
Dr. Oliveira presented results from part B the of SOLTI TOT-HER3 trial, a window-of-opportunity trial that evaluated the effect of a single dose of HER3-Dxd in patients with treatment-naive HR+/HER2– early breast cancer.
In such trials, patients receive one or more new compounds between the time of cancer diagnosis and standard treatment. Biological and clinical activity from part A of the SOLTI TOT-HER3 trial were presented at last year’s ESMO Breast Cancer Congress, Dr. Oliveira said.
In the current study, Dr. Oliveira and colleagues recruited 37 women with HR+/HER2– early breast cancer, including 20 who were hormone receptor–positive and 17 who had triple negative breast cancer (TNBC). The age of the participants ranged from 30 to 81 years, with a median age of 51 years; 54% were premenopausal. The mean tumor size was 21 mm, with a range of 10-81 mm.
Distinct from part A of the SOLTI TOT-HER3 trial, part B included a subset of patients with TNBC to assess preliminary efficacy in this subtype, Dr. Oliveira noted.
All patients in part B received a single dose of 5.6 mg/kg of HER3-DXd. The primary outcome was the variation in the tumor cellularity and tumor-infiltrating lymphocyte (CelTIL) score at baseline and after 21 days via breast ultrasound.
At day 21, the total CelTIL score increased by a significant mean difference of 9.4 points after a single dose; the mean differences for TNBC and HR+/HER2– patients, were 17.9 points and 2.2 points, respectively, Dr. Oliveira said. The overall response rate was 32% (35% in TNBC patients and 30% in HR+/HER2– patients) and was significantly associated with the absolute change in CelTIL (area under the curve = 0.693; P = .049).
In a subtype analysis, a statistically significant change in CelTIL was observed between paired samples overall (P = .046) and in TNBC (P = .016), but not in HR+ (P = .793).
Baseline levels of ERBB3 (also known as human epidermal growth factor receptor type 3, or HER3) were not associated with changes in CelTIL or in overall response rate.
HER3-DXd induced high expression of immune-related genes (such as PD1, CD8, and CD19), and suppressed proliferation-related genes, she said.
A total of 31 patients (84%) reported any adverse events. Of these, the most common were nausea, fatigue, alopecia, diarrhea, constipation, and vomiting, and one patient experienced grade 3 treatment-related nausea. No interstitial lung disease events were reported during the study, and the incidence of hematological and hepatic toxicity was lower with the lower dose in part B, compared with the 6.5 mg/kg dose used in part A, Dr. Oliveira noted.
To further validate the findings of the current study and assess the activity of HER3-DXd in early breast cancer, Dr. Oliveira and colleagues are conducting a neoadjuvant phase II trial known as SOLTI-2103 VALENTINE. In this study, they are testing six cycles of HER3-DXd at a 5.6 mg/kg dose in HR+/HER2– breast cancer patients, she said.
During a question-and-answer session, Dr. Oliveira was asked whether CelTIL is the best endpoint for assessing HER3-DXd. Finding the best endpoint is always a challenge when conducting window-of-opportunity trials, she said. The CelTIL score has been correlated with pathologic complete response (pCR), as well as with disease-free survival and overall survival, she added.
ICARUS-BREAST01
In a presentation of Abstract 1890 during the same session, Barbari Pistilli, MD, of Gustave Roussy Cancer Center, Villejuif, France, shared data from a phase II study known as ICARUS-BREAST.
The study population included women with unresectable locally advanced breast cancer (ABC) who had undergone a median of two previous systemic therapies. In the current study, the patients underwent a median of eight cycles of HER3-DXd. The dosage was 5.6 mg/kg every 3 weeks until disease progression or unacceptable toxicity.
The primary outcome was overall response and disease progression after 3 months. Dr. Pistilli, who is also a coauthor of the research, provided data from 56 evaluable patients.
After 3 months, 16 patients (28.6%) showed a partial response, 30 patients showed stable disease (54%), and 10 (18%) showed disease progression. “No patients had a complete response,” Dr. Pistilli noted.
As for the safety profile, all patients reported at least one treatment-emergent event of any grade, but less than half (48.2%) were grade 3 or higher, and 12.5% led to treatment discontinuation. Fatigue and nausea were the most frequently reported adverse events overall, and occurred in 89.3% and 76.8% of patients, respectively. All grade and grade 3 or higher neutropenia occurred in four patients and six patients, respectively; all grade and grade 3 or higher thrombocytopenia occurred in four patients and two patients, respectively, Dr. Pistilli said.
Data on circulating tumor cells (CTCs) were available for 31 patients, and the researchers reviewed CTC counts after the first HER3-DXd cycle.
“We found that the median number of CTCs decreased by one to two cell cycles of HER3-DXd,” said Dr. Pistilli. She and her coauthors found no substantial impact of the treatment on HER3 negative CTC counts, and “more importantly, no increase of HER3 negative CTC counts at disease progression,” Dr. Pistilli continued.
In addition, patients with higher HER+ CTC counts at baseline or a greater decrease in HER3+ CTC counts after one cycle of HER3-DXd were more likely to have an early treatment response, but this association was not statistically significant.
Looking ahead, further analysis will be performed to evaluate the association between HER3+ CTC counts and dynamics and the main outcomes of overall response rate and progression-free survival to determine the potential of HER3+ CTC counts to identify patients who can benefit from HER3-DXd, said Dr. Pistilli. The ICARUS-BREAST01 study is ongoing, and further efficacy and biomarker analysis will be presented, she added.
In the question-and-answer session, Dr. Pistilli was asked why she chose CTC as a measure.
Dr. Pistilli responded that she and her coauthors wanted to understand whether CTC could serve as a biomarker to help in patient selection.
Also, when asked about which genes might be upregulated and downregulated in responders vs. nonresponders, she noted that some genes related to DNA repair were involved in patients who were responders, but more research is needed.
Early results merit further exploration
Although patritumab deruxtecan is early in development, “there is a clear signal to expand,” based on preliminary research, said Rebecca A. Dent, MD, who served as discussant for the two studies.
“There is no clear role for a specific subtype in both protein and gene expression,” noted Dr. Dent, who is a professor at Duke NUS Medical School, a collaboration between Duke University, Durham, N.C., and the National University of Singapore.
In the SOLTI TOT-HER3 trial, the small numbers make teasing out correlations a challenge, said Dr. Dent. However, changes were observed after just one cycle of the drug, and the upregulation of immune signature genes was reassuring, she said.
“A single dose of HER3-DXd induced an overall response of approximately 30% independently of hormone receptor status,” she emphasized, and the lower incidence of hematological and hepatic toxicity with the lower dose is good news as well. The findings were limited by the small sample size, but the results support moving forward with clinical development of HER3-DXd, she said.
The ICARUS-BREAST01 study researchers tried to show whether they could identify potential markers of early treatment response, and they examined CTCs and gene alterations, said Dr. Dent. “I think it is reassuring that despite these patients being heavily pretreated, HER-DXd seems to be active regardless of most frequent breast cancer genomic alterations,” she noted. Remaining questions include the need for more data on primary resistance.
“We are able to get these patients to respond, but what makes patients resistant to ADCs is just as important,” she said. “We see exciting data across all these subtypes,”
In Dr. Dent’s opinion, future research should focus on triple negative breast cancer, an opinion supported by the stronger response in this subset of patients in the SOLTI TOT-HER3 trial. “I think you need to bring triple negative to the table,” she said.
The SOLTI TOT-HER3 study was funded by Daiichi Sankyo. Dr. Oliveira disclosed relationships with companies including AstraZeneca, Ayala Pharmaceuticals, Boehringer-Ingelheim, Genentech, Gilead, GSK, Novartis, Roche, Seagen, Zenith Epigenetics, Daiichi Sankyo, iTEOS, MSD, Pierre-Fabre, Relay Therapeutics, and Eisai. ICARUS-BREAST01 was sponsored by the Gustave Roussy Cancer Center and supported by Daiichi Sankyo. Dr. Pistilli disclosed relationships with multiple companies including Daiichi-Sankyo, AstraZeneca, Gilead, Seagen, MSD, Novartis, Lilly, and Pierre Fabre. Dr. Dent disclosed financial relationships with companies including AstraZeneca, Roche, Eisai, Lilly, MSD, Novartis, and Pfizer.
according to data presented from Abstract 1240 at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
Patritumab deruxtecan (HER3-DXd) has previously demonstrated an acceptable safety profile and antitumor activity in phase I studies involving heavily pretreated patients with metastatic breast cancer and various levels of HER3 protein expression, said Mafalda Oliveira, MD, of the Vall d’Hebron University Hospital and Vall d’Hebron Institute of Oncology in Barcelona.
Antibody-drug conjugates (ADCs) are a combination of a monoclonal antibody chemically linked to a drug, as defined by the National Cancer Institute. ADCs work by binding to receptors or proteins and selectively delivering cytotoxic drugs to the site of a tumor.
Dr. Oliveira presented results from part B the of SOLTI TOT-HER3 trial, a window-of-opportunity trial that evaluated the effect of a single dose of HER3-Dxd in patients with treatment-naive HR+/HER2– early breast cancer.
In such trials, patients receive one or more new compounds between the time of cancer diagnosis and standard treatment. Biological and clinical activity from part A of the SOLTI TOT-HER3 trial were presented at last year’s ESMO Breast Cancer Congress, Dr. Oliveira said.
In the current study, Dr. Oliveira and colleagues recruited 37 women with HR+/HER2– early breast cancer, including 20 who were hormone receptor–positive and 17 who had triple negative breast cancer (TNBC). The age of the participants ranged from 30 to 81 years, with a median age of 51 years; 54% were premenopausal. The mean tumor size was 21 mm, with a range of 10-81 mm.
Distinct from part A of the SOLTI TOT-HER3 trial, part B included a subset of patients with TNBC to assess preliminary efficacy in this subtype, Dr. Oliveira noted.
All patients in part B received a single dose of 5.6 mg/kg of HER3-DXd. The primary outcome was the variation in the tumor cellularity and tumor-infiltrating lymphocyte (CelTIL) score at baseline and after 21 days via breast ultrasound.
At day 21, the total CelTIL score increased by a significant mean difference of 9.4 points after a single dose; the mean differences for TNBC and HR+/HER2– patients, were 17.9 points and 2.2 points, respectively, Dr. Oliveira said. The overall response rate was 32% (35% in TNBC patients and 30% in HR+/HER2– patients) and was significantly associated with the absolute change in CelTIL (area under the curve = 0.693; P = .049).
In a subtype analysis, a statistically significant change in CelTIL was observed between paired samples overall (P = .046) and in TNBC (P = .016), but not in HR+ (P = .793).
Baseline levels of ERBB3 (also known as human epidermal growth factor receptor type 3, or HER3) were not associated with changes in CelTIL or in overall response rate.
HER3-DXd induced high expression of immune-related genes (such as PD1, CD8, and CD19), and suppressed proliferation-related genes, she said.
A total of 31 patients (84%) reported any adverse events. Of these, the most common were nausea, fatigue, alopecia, diarrhea, constipation, and vomiting, and one patient experienced grade 3 treatment-related nausea. No interstitial lung disease events were reported during the study, and the incidence of hematological and hepatic toxicity was lower with the lower dose in part B, compared with the 6.5 mg/kg dose used in part A, Dr. Oliveira noted.
To further validate the findings of the current study and assess the activity of HER3-DXd in early breast cancer, Dr. Oliveira and colleagues are conducting a neoadjuvant phase II trial known as SOLTI-2103 VALENTINE. In this study, they are testing six cycles of HER3-DXd at a 5.6 mg/kg dose in HR+/HER2– breast cancer patients, she said.
During a question-and-answer session, Dr. Oliveira was asked whether CelTIL is the best endpoint for assessing HER3-DXd. Finding the best endpoint is always a challenge when conducting window-of-opportunity trials, she said. The CelTIL score has been correlated with pathologic complete response (pCR), as well as with disease-free survival and overall survival, she added.
ICARUS-BREAST01
In a presentation of Abstract 1890 during the same session, Barbari Pistilli, MD, of Gustave Roussy Cancer Center, Villejuif, France, shared data from a phase II study known as ICARUS-BREAST.
The study population included women with unresectable locally advanced breast cancer (ABC) who had undergone a median of two previous systemic therapies. In the current study, the patients underwent a median of eight cycles of HER3-DXd. The dosage was 5.6 mg/kg every 3 weeks until disease progression or unacceptable toxicity.
The primary outcome was overall response and disease progression after 3 months. Dr. Pistilli, who is also a coauthor of the research, provided data from 56 evaluable patients.
After 3 months, 16 patients (28.6%) showed a partial response, 30 patients showed stable disease (54%), and 10 (18%) showed disease progression. “No patients had a complete response,” Dr. Pistilli noted.
As for the safety profile, all patients reported at least one treatment-emergent event of any grade, but less than half (48.2%) were grade 3 or higher, and 12.5% led to treatment discontinuation. Fatigue and nausea were the most frequently reported adverse events overall, and occurred in 89.3% and 76.8% of patients, respectively. All grade and grade 3 or higher neutropenia occurred in four patients and six patients, respectively; all grade and grade 3 or higher thrombocytopenia occurred in four patients and two patients, respectively, Dr. Pistilli said.
Data on circulating tumor cells (CTCs) were available for 31 patients, and the researchers reviewed CTC counts after the first HER3-DXd cycle.
“We found that the median number of CTCs decreased by one to two cell cycles of HER3-DXd,” said Dr. Pistilli. She and her coauthors found no substantial impact of the treatment on HER3 negative CTC counts, and “more importantly, no increase of HER3 negative CTC counts at disease progression,” Dr. Pistilli continued.
In addition, patients with higher HER+ CTC counts at baseline or a greater decrease in HER3+ CTC counts after one cycle of HER3-DXd were more likely to have an early treatment response, but this association was not statistically significant.
Looking ahead, further analysis will be performed to evaluate the association between HER3+ CTC counts and dynamics and the main outcomes of overall response rate and progression-free survival to determine the potential of HER3+ CTC counts to identify patients who can benefit from HER3-DXd, said Dr. Pistilli. The ICARUS-BREAST01 study is ongoing, and further efficacy and biomarker analysis will be presented, she added.
In the question-and-answer session, Dr. Pistilli was asked why she chose CTC as a measure.
Dr. Pistilli responded that she and her coauthors wanted to understand whether CTC could serve as a biomarker to help in patient selection.
Also, when asked about which genes might be upregulated and downregulated in responders vs. nonresponders, she noted that some genes related to DNA repair were involved in patients who were responders, but more research is needed.
Early results merit further exploration
Although patritumab deruxtecan is early in development, “there is a clear signal to expand,” based on preliminary research, said Rebecca A. Dent, MD, who served as discussant for the two studies.
“There is no clear role for a specific subtype in both protein and gene expression,” noted Dr. Dent, who is a professor at Duke NUS Medical School, a collaboration between Duke University, Durham, N.C., and the National University of Singapore.
In the SOLTI TOT-HER3 trial, the small numbers make teasing out correlations a challenge, said Dr. Dent. However, changes were observed after just one cycle of the drug, and the upregulation of immune signature genes was reassuring, she said.
“A single dose of HER3-DXd induced an overall response of approximately 30% independently of hormone receptor status,” she emphasized, and the lower incidence of hematological and hepatic toxicity with the lower dose is good news as well. The findings were limited by the small sample size, but the results support moving forward with clinical development of HER3-DXd, she said.
The ICARUS-BREAST01 study researchers tried to show whether they could identify potential markers of early treatment response, and they examined CTCs and gene alterations, said Dr. Dent. “I think it is reassuring that despite these patients being heavily pretreated, HER-DXd seems to be active regardless of most frequent breast cancer genomic alterations,” she noted. Remaining questions include the need for more data on primary resistance.
“We are able to get these patients to respond, but what makes patients resistant to ADCs is just as important,” she said. “We see exciting data across all these subtypes,”
In Dr. Dent’s opinion, future research should focus on triple negative breast cancer, an opinion supported by the stronger response in this subset of patients in the SOLTI TOT-HER3 trial. “I think you need to bring triple negative to the table,” she said.
The SOLTI TOT-HER3 study was funded by Daiichi Sankyo. Dr. Oliveira disclosed relationships with companies including AstraZeneca, Ayala Pharmaceuticals, Boehringer-Ingelheim, Genentech, Gilead, GSK, Novartis, Roche, Seagen, Zenith Epigenetics, Daiichi Sankyo, iTEOS, MSD, Pierre-Fabre, Relay Therapeutics, and Eisai. ICARUS-BREAST01 was sponsored by the Gustave Roussy Cancer Center and supported by Daiichi Sankyo. Dr. Pistilli disclosed relationships with multiple companies including Daiichi-Sankyo, AstraZeneca, Gilead, Seagen, MSD, Novartis, Lilly, and Pierre Fabre. Dr. Dent disclosed financial relationships with companies including AstraZeneca, Roche, Eisai, Lilly, MSD, Novartis, and Pfizer.
according to data presented from Abstract 1240 at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress.
Patritumab deruxtecan (HER3-DXd) has previously demonstrated an acceptable safety profile and antitumor activity in phase I studies involving heavily pretreated patients with metastatic breast cancer and various levels of HER3 protein expression, said Mafalda Oliveira, MD, of the Vall d’Hebron University Hospital and Vall d’Hebron Institute of Oncology in Barcelona.
Antibody-drug conjugates (ADCs) are a combination of a monoclonal antibody chemically linked to a drug, as defined by the National Cancer Institute. ADCs work by binding to receptors or proteins and selectively delivering cytotoxic drugs to the site of a tumor.
Dr. Oliveira presented results from part B the of SOLTI TOT-HER3 trial, a window-of-opportunity trial that evaluated the effect of a single dose of HER3-Dxd in patients with treatment-naive HR+/HER2– early breast cancer.
In such trials, patients receive one or more new compounds between the time of cancer diagnosis and standard treatment. Biological and clinical activity from part A of the SOLTI TOT-HER3 trial were presented at last year’s ESMO Breast Cancer Congress, Dr. Oliveira said.
In the current study, Dr. Oliveira and colleagues recruited 37 women with HR+/HER2– early breast cancer, including 20 who were hormone receptor–positive and 17 who had triple negative breast cancer (TNBC). The age of the participants ranged from 30 to 81 years, with a median age of 51 years; 54% were premenopausal. The mean tumor size was 21 mm, with a range of 10-81 mm.
Distinct from part A of the SOLTI TOT-HER3 trial, part B included a subset of patients with TNBC to assess preliminary efficacy in this subtype, Dr. Oliveira noted.
All patients in part B received a single dose of 5.6 mg/kg of HER3-DXd. The primary outcome was the variation in the tumor cellularity and tumor-infiltrating lymphocyte (CelTIL) score at baseline and after 21 days via breast ultrasound.
At day 21, the total CelTIL score increased by a significant mean difference of 9.4 points after a single dose; the mean differences for TNBC and HR+/HER2– patients, were 17.9 points and 2.2 points, respectively, Dr. Oliveira said. The overall response rate was 32% (35% in TNBC patients and 30% in HR+/HER2– patients) and was significantly associated with the absolute change in CelTIL (area under the curve = 0.693; P = .049).
In a subtype analysis, a statistically significant change in CelTIL was observed between paired samples overall (P = .046) and in TNBC (P = .016), but not in HR+ (P = .793).
Baseline levels of ERBB3 (also known as human epidermal growth factor receptor type 3, or HER3) were not associated with changes in CelTIL or in overall response rate.
HER3-DXd induced high expression of immune-related genes (such as PD1, CD8, and CD19), and suppressed proliferation-related genes, she said.
A total of 31 patients (84%) reported any adverse events. Of these, the most common were nausea, fatigue, alopecia, diarrhea, constipation, and vomiting, and one patient experienced grade 3 treatment-related nausea. No interstitial lung disease events were reported during the study, and the incidence of hematological and hepatic toxicity was lower with the lower dose in part B, compared with the 6.5 mg/kg dose used in part A, Dr. Oliveira noted.
To further validate the findings of the current study and assess the activity of HER3-DXd in early breast cancer, Dr. Oliveira and colleagues are conducting a neoadjuvant phase II trial known as SOLTI-2103 VALENTINE. In this study, they are testing six cycles of HER3-DXd at a 5.6 mg/kg dose in HR+/HER2– breast cancer patients, she said.
During a question-and-answer session, Dr. Oliveira was asked whether CelTIL is the best endpoint for assessing HER3-DXd. Finding the best endpoint is always a challenge when conducting window-of-opportunity trials, she said. The CelTIL score has been correlated with pathologic complete response (pCR), as well as with disease-free survival and overall survival, she added.
ICARUS-BREAST01
In a presentation of Abstract 1890 during the same session, Barbari Pistilli, MD, of Gustave Roussy Cancer Center, Villejuif, France, shared data from a phase II study known as ICARUS-BREAST.
The study population included women with unresectable locally advanced breast cancer (ABC) who had undergone a median of two previous systemic therapies. In the current study, the patients underwent a median of eight cycles of HER3-DXd. The dosage was 5.6 mg/kg every 3 weeks until disease progression or unacceptable toxicity.
The primary outcome was overall response and disease progression after 3 months. Dr. Pistilli, who is also a coauthor of the research, provided data from 56 evaluable patients.
After 3 months, 16 patients (28.6%) showed a partial response, 30 patients showed stable disease (54%), and 10 (18%) showed disease progression. “No patients had a complete response,” Dr. Pistilli noted.
As for the safety profile, all patients reported at least one treatment-emergent event of any grade, but less than half (48.2%) were grade 3 or higher, and 12.5% led to treatment discontinuation. Fatigue and nausea were the most frequently reported adverse events overall, and occurred in 89.3% and 76.8% of patients, respectively. All grade and grade 3 or higher neutropenia occurred in four patients and six patients, respectively; all grade and grade 3 or higher thrombocytopenia occurred in four patients and two patients, respectively, Dr. Pistilli said.
Data on circulating tumor cells (CTCs) were available for 31 patients, and the researchers reviewed CTC counts after the first HER3-DXd cycle.
“We found that the median number of CTCs decreased by one to two cell cycles of HER3-DXd,” said Dr. Pistilli. She and her coauthors found no substantial impact of the treatment on HER3 negative CTC counts, and “more importantly, no increase of HER3 negative CTC counts at disease progression,” Dr. Pistilli continued.
In addition, patients with higher HER+ CTC counts at baseline or a greater decrease in HER3+ CTC counts after one cycle of HER3-DXd were more likely to have an early treatment response, but this association was not statistically significant.
Looking ahead, further analysis will be performed to evaluate the association between HER3+ CTC counts and dynamics and the main outcomes of overall response rate and progression-free survival to determine the potential of HER3+ CTC counts to identify patients who can benefit from HER3-DXd, said Dr. Pistilli. The ICARUS-BREAST01 study is ongoing, and further efficacy and biomarker analysis will be presented, she added.
In the question-and-answer session, Dr. Pistilli was asked why she chose CTC as a measure.
Dr. Pistilli responded that she and her coauthors wanted to understand whether CTC could serve as a biomarker to help in patient selection.
Also, when asked about which genes might be upregulated and downregulated in responders vs. nonresponders, she noted that some genes related to DNA repair were involved in patients who were responders, but more research is needed.
Early results merit further exploration
Although patritumab deruxtecan is early in development, “there is a clear signal to expand,” based on preliminary research, said Rebecca A. Dent, MD, who served as discussant for the two studies.
“There is no clear role for a specific subtype in both protein and gene expression,” noted Dr. Dent, who is a professor at Duke NUS Medical School, a collaboration between Duke University, Durham, N.C., and the National University of Singapore.
In the SOLTI TOT-HER3 trial, the small numbers make teasing out correlations a challenge, said Dr. Dent. However, changes were observed after just one cycle of the drug, and the upregulation of immune signature genes was reassuring, she said.
“A single dose of HER3-DXd induced an overall response of approximately 30% independently of hormone receptor status,” she emphasized, and the lower incidence of hematological and hepatic toxicity with the lower dose is good news as well. The findings were limited by the small sample size, but the results support moving forward with clinical development of HER3-DXd, she said.
The ICARUS-BREAST01 study researchers tried to show whether they could identify potential markers of early treatment response, and they examined CTCs and gene alterations, said Dr. Dent. “I think it is reassuring that despite these patients being heavily pretreated, HER-DXd seems to be active regardless of most frequent breast cancer genomic alterations,” she noted. Remaining questions include the need for more data on primary resistance.
“We are able to get these patients to respond, but what makes patients resistant to ADCs is just as important,” she said. “We see exciting data across all these subtypes,”
In Dr. Dent’s opinion, future research should focus on triple negative breast cancer, an opinion supported by the stronger response in this subset of patients in the SOLTI TOT-HER3 trial. “I think you need to bring triple negative to the table,” she said.
The SOLTI TOT-HER3 study was funded by Daiichi Sankyo. Dr. Oliveira disclosed relationships with companies including AstraZeneca, Ayala Pharmaceuticals, Boehringer-Ingelheim, Genentech, Gilead, GSK, Novartis, Roche, Seagen, Zenith Epigenetics, Daiichi Sankyo, iTEOS, MSD, Pierre-Fabre, Relay Therapeutics, and Eisai. ICARUS-BREAST01 was sponsored by the Gustave Roussy Cancer Center and supported by Daiichi Sankyo. Dr. Pistilli disclosed relationships with multiple companies including Daiichi-Sankyo, AstraZeneca, Gilead, Seagen, MSD, Novartis, Lilly, and Pierre Fabre. Dr. Dent disclosed financial relationships with companies including AstraZeneca, Roche, Eisai, Lilly, MSD, Novartis, and Pfizer.
FROM ESMO BREAST CANCER 2023
sFlt-1:PlGF ratio normal at 24 to 28 weeks: Discontinue aspirin for preterm preeclampsia prevention?

Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.
EXPERT COMMENTARY
Aspirin is, to date, the only proven preventative treatment to reduce the risk of preeclampsia in pregnancy. While aspirin initiation, optimally prior to 16 weeks, generally is accepted, the best timing for discontinuation remains uncertain due to conflicting data on risk of bleeding and different doses used. The American College of Obstetricians and Gynecologists recommends a broad range of patients eligible for low-dose aspirin with continuation through delivery, citing data that support no increase in either maternal or fetal/neonatal complications, including bleeding complications.1 Other guidelines recommend reduction in pregnancy exposure to aspirin with strict guidelines for which patients are considered “high risk” as well as discontinuation at 36 weeks prior to labor onset to reduce the risk of potential bleeding complications.
Recently, Mendoza and colleagues tested the hypothesis that, in patients at high risk for preterm preeclampsia (based on high-risk first-trimester screening followed by a low risk of preeclampsia at 24 to 28 weeks based on a normal sFlt-1:PlGF [soluble fms-like tyrosine kinase-1 to placental growth factor] ratio), discontinuing aspirin is noninferior in preventing preterm preeclampsia compared with continuing aspirin until 36 weeks.2
Details of the study
Mendoza and colleagues conducted a multicenter, open label, randomized, phase 3, noninferiority trial that randomly assigned 968 participants prior to stopping recruitment based on the findings from a planned interim analysis.2
The patient population included women with singleton pregnancies between 24 and 28 weeks who had initiated aspirin 150 mg daily by 16 6/7 weeks due to high-risk first- trimester screening for preterm preeclampsia. Additionally, these patients also had an sFlt-1:PlGR ratio of 38 or less between 24 and 28 weeks’ gestation, which prior studies have demonstrated to exclude the diagnosis of preeclampsia.
Patients were randomly assigned to either discontinue aspirin at 24 to 28 weeks’ gestation (intervention group) or continue aspirin until 36 weeks’ gestation (control group). The primary outcome was delivery due to preeclampsia at less than 37 weeks, with secondary outcomes of preeclampsia at less than 34 weeks, preeclampsia at 37 or more weeks, or other adverse pregnancy outcomes.
Results. For the primary outcome (936 participants’ data analyzed), the incidence of preeclampsia at less than 37 weeks was 1.48% in the intervention group and 1.73% in the control group (absolute difference, -0.25%, which meets study criteria for noninferiority).
No difference occurred in the secondary outcomes of adverse outcomes at less than 34 weeks or at less than 37 weeks. While there was no difference in the incidence of the individual adverse outcomes at 37 or more weeks, the intervention group had a decrease in the incidence of having “any” adverse outcome (-5.04%) as well as a decrease in minor antepartum hemorrhage (nose and/or gum bleeding) (-4.7%).
The authors therefore concluded that aspirin discontinuation at 24 to 28 weeks’ gestation in pregnant patients at high risk for preterm preeclampsia and a normal sFlt-1:PlGF ratio is noninferior to aspirin continuation for prevention of preterm preeclampsia. They also suggested that this discontinuation may reduce the risk of adverse pregnancy outcomes at 37 or more weeks as well as minor bleeding complications.
Study strengths and limitations
The authors cited the novelty of this study at considering using aspirin for the prevention of preterm preeclampsia in a specific patient group for the shortest amount of time needed to achieve this goal. Potential benefits could be decreased bleeding complications, cost, anxiety, and visits.
They also noted the following study limitations: open-label design, a predominantly White patient population, early termination due to the interim analysis, inadequate power for more rare complications, and a query as to the appropriate choice for the threshold for noninferiority. Noninferiority trials have inherent weaknesses as a group that should be considered before major practice changes occur as a result of their findings.
Several other factors in the study limit the generalizability of the authors’ recommendations, especially to patient populations in the United States. For example, the study used an aspirin dose of 150 mg daily, which is almost double the dose recommended in the United States (81 mg). The reasoning for this was that doses higher than 100 mg have been shown to be the most effective for preeclampsia prevention but also may have higher rates of bleeding complications, including placental abruption. The demonstrated increase in complications may not hold at a lower dose.
Additionally, patients in this study were selected for aspirin by a first-trimester algorithm that may not be in general use everywhere (and differs from the US Preventive Services Task Force recommendations for low-dose aspirin use in pregnancy). Finally, although extremely interesting, the use of the sFlt-1:PlFG ratio at 24 to 28 weeks is not in widespread use in the United States and may incur an additional cost not equivalent to the low cost of a daily aspirin.
Essentially, this is an extremely limited study for a very specific population. Before globally discontinuing low-dose aspirin in high-risk patients, the different doses and eligibility criteria should be studied for effect of early discontinuation. ●
Low-dose aspirin should continue to be used for prevention of preeclampsia in high-risk pregnant patients, optimally starting at 12 to 16 weeks’ gestation and continuing either through 36 weeks or delivery. Further study is needed to determine the optimal timing for earlier discontinuation of aspirin based on dose, risk factors, and other measures of preeclampsia risk as the pregnancy progresses.
JAIMEY M. PAULI, MD
- ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52. doi:10.1097/AOG.0000000000002708.
- Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.

Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.
EXPERT COMMENTARY
Aspirin is, to date, the only proven preventative treatment to reduce the risk of preeclampsia in pregnancy. While aspirin initiation, optimally prior to 16 weeks, generally is accepted, the best timing for discontinuation remains uncertain due to conflicting data on risk of bleeding and different doses used. The American College of Obstetricians and Gynecologists recommends a broad range of patients eligible for low-dose aspirin with continuation through delivery, citing data that support no increase in either maternal or fetal/neonatal complications, including bleeding complications.1 Other guidelines recommend reduction in pregnancy exposure to aspirin with strict guidelines for which patients are considered “high risk” as well as discontinuation at 36 weeks prior to labor onset to reduce the risk of potential bleeding complications.
Recently, Mendoza and colleagues tested the hypothesis that, in patients at high risk for preterm preeclampsia (based on high-risk first-trimester screening followed by a low risk of preeclampsia at 24 to 28 weeks based on a normal sFlt-1:PlGF [soluble fms-like tyrosine kinase-1 to placental growth factor] ratio), discontinuing aspirin is noninferior in preventing preterm preeclampsia compared with continuing aspirin until 36 weeks.2
Details of the study
Mendoza and colleagues conducted a multicenter, open label, randomized, phase 3, noninferiority trial that randomly assigned 968 participants prior to stopping recruitment based on the findings from a planned interim analysis.2
The patient population included women with singleton pregnancies between 24 and 28 weeks who had initiated aspirin 150 mg daily by 16 6/7 weeks due to high-risk first- trimester screening for preterm preeclampsia. Additionally, these patients also had an sFlt-1:PlGR ratio of 38 or less between 24 and 28 weeks’ gestation, which prior studies have demonstrated to exclude the diagnosis of preeclampsia.
Patients were randomly assigned to either discontinue aspirin at 24 to 28 weeks’ gestation (intervention group) or continue aspirin until 36 weeks’ gestation (control group). The primary outcome was delivery due to preeclampsia at less than 37 weeks, with secondary outcomes of preeclampsia at less than 34 weeks, preeclampsia at 37 or more weeks, or other adverse pregnancy outcomes.
Results. For the primary outcome (936 participants’ data analyzed), the incidence of preeclampsia at less than 37 weeks was 1.48% in the intervention group and 1.73% in the control group (absolute difference, -0.25%, which meets study criteria for noninferiority).
No difference occurred in the secondary outcomes of adverse outcomes at less than 34 weeks or at less than 37 weeks. While there was no difference in the incidence of the individual adverse outcomes at 37 or more weeks, the intervention group had a decrease in the incidence of having “any” adverse outcome (-5.04%) as well as a decrease in minor antepartum hemorrhage (nose and/or gum bleeding) (-4.7%).
The authors therefore concluded that aspirin discontinuation at 24 to 28 weeks’ gestation in pregnant patients at high risk for preterm preeclampsia and a normal sFlt-1:PlGF ratio is noninferior to aspirin continuation for prevention of preterm preeclampsia. They also suggested that this discontinuation may reduce the risk of adverse pregnancy outcomes at 37 or more weeks as well as minor bleeding complications.
Study strengths and limitations
The authors cited the novelty of this study at considering using aspirin for the prevention of preterm preeclampsia in a specific patient group for the shortest amount of time needed to achieve this goal. Potential benefits could be decreased bleeding complications, cost, anxiety, and visits.
They also noted the following study limitations: open-label design, a predominantly White patient population, early termination due to the interim analysis, inadequate power for more rare complications, and a query as to the appropriate choice for the threshold for noninferiority. Noninferiority trials have inherent weaknesses as a group that should be considered before major practice changes occur as a result of their findings.
Several other factors in the study limit the generalizability of the authors’ recommendations, especially to patient populations in the United States. For example, the study used an aspirin dose of 150 mg daily, which is almost double the dose recommended in the United States (81 mg). The reasoning for this was that doses higher than 100 mg have been shown to be the most effective for preeclampsia prevention but also may have higher rates of bleeding complications, including placental abruption. The demonstrated increase in complications may not hold at a lower dose.
Additionally, patients in this study were selected for aspirin by a first-trimester algorithm that may not be in general use everywhere (and differs from the US Preventive Services Task Force recommendations for low-dose aspirin use in pregnancy). Finally, although extremely interesting, the use of the sFlt-1:PlFG ratio at 24 to 28 weeks is not in widespread use in the United States and may incur an additional cost not equivalent to the low cost of a daily aspirin.
Essentially, this is an extremely limited study for a very specific population. Before globally discontinuing low-dose aspirin in high-risk patients, the different doses and eligibility criteria should be studied for effect of early discontinuation. ●
Low-dose aspirin should continue to be used for prevention of preeclampsia in high-risk pregnant patients, optimally starting at 12 to 16 weeks’ gestation and continuing either through 36 weeks or delivery. Further study is needed to determine the optimal timing for earlier discontinuation of aspirin based on dose, risk factors, and other measures of preeclampsia risk as the pregnancy progresses.
JAIMEY M. PAULI, MD

Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.
EXPERT COMMENTARY
Aspirin is, to date, the only proven preventative treatment to reduce the risk of preeclampsia in pregnancy. While aspirin initiation, optimally prior to 16 weeks, generally is accepted, the best timing for discontinuation remains uncertain due to conflicting data on risk of bleeding and different doses used. The American College of Obstetricians and Gynecologists recommends a broad range of patients eligible for low-dose aspirin with continuation through delivery, citing data that support no increase in either maternal or fetal/neonatal complications, including bleeding complications.1 Other guidelines recommend reduction in pregnancy exposure to aspirin with strict guidelines for which patients are considered “high risk” as well as discontinuation at 36 weeks prior to labor onset to reduce the risk of potential bleeding complications.
Recently, Mendoza and colleagues tested the hypothesis that, in patients at high risk for preterm preeclampsia (based on high-risk first-trimester screening followed by a low risk of preeclampsia at 24 to 28 weeks based on a normal sFlt-1:PlGF [soluble fms-like tyrosine kinase-1 to placental growth factor] ratio), discontinuing aspirin is noninferior in preventing preterm preeclampsia compared with continuing aspirin until 36 weeks.2
Details of the study
Mendoza and colleagues conducted a multicenter, open label, randomized, phase 3, noninferiority trial that randomly assigned 968 participants prior to stopping recruitment based on the findings from a planned interim analysis.2
The patient population included women with singleton pregnancies between 24 and 28 weeks who had initiated aspirin 150 mg daily by 16 6/7 weeks due to high-risk first- trimester screening for preterm preeclampsia. Additionally, these patients also had an sFlt-1:PlGR ratio of 38 or less between 24 and 28 weeks’ gestation, which prior studies have demonstrated to exclude the diagnosis of preeclampsia.
Patients were randomly assigned to either discontinue aspirin at 24 to 28 weeks’ gestation (intervention group) or continue aspirin until 36 weeks’ gestation (control group). The primary outcome was delivery due to preeclampsia at less than 37 weeks, with secondary outcomes of preeclampsia at less than 34 weeks, preeclampsia at 37 or more weeks, or other adverse pregnancy outcomes.
Results. For the primary outcome (936 participants’ data analyzed), the incidence of preeclampsia at less than 37 weeks was 1.48% in the intervention group and 1.73% in the control group (absolute difference, -0.25%, which meets study criteria for noninferiority).
No difference occurred in the secondary outcomes of adverse outcomes at less than 34 weeks or at less than 37 weeks. While there was no difference in the incidence of the individual adverse outcomes at 37 or more weeks, the intervention group had a decrease in the incidence of having “any” adverse outcome (-5.04%) as well as a decrease in minor antepartum hemorrhage (nose and/or gum bleeding) (-4.7%).
The authors therefore concluded that aspirin discontinuation at 24 to 28 weeks’ gestation in pregnant patients at high risk for preterm preeclampsia and a normal sFlt-1:PlGF ratio is noninferior to aspirin continuation for prevention of preterm preeclampsia. They also suggested that this discontinuation may reduce the risk of adverse pregnancy outcomes at 37 or more weeks as well as minor bleeding complications.
Study strengths and limitations
The authors cited the novelty of this study at considering using aspirin for the prevention of preterm preeclampsia in a specific patient group for the shortest amount of time needed to achieve this goal. Potential benefits could be decreased bleeding complications, cost, anxiety, and visits.
They also noted the following study limitations: open-label design, a predominantly White patient population, early termination due to the interim analysis, inadequate power for more rare complications, and a query as to the appropriate choice for the threshold for noninferiority. Noninferiority trials have inherent weaknesses as a group that should be considered before major practice changes occur as a result of their findings.
Several other factors in the study limit the generalizability of the authors’ recommendations, especially to patient populations in the United States. For example, the study used an aspirin dose of 150 mg daily, which is almost double the dose recommended in the United States (81 mg). The reasoning for this was that doses higher than 100 mg have been shown to be the most effective for preeclampsia prevention but also may have higher rates of bleeding complications, including placental abruption. The demonstrated increase in complications may not hold at a lower dose.
Additionally, patients in this study were selected for aspirin by a first-trimester algorithm that may not be in general use everywhere (and differs from the US Preventive Services Task Force recommendations for low-dose aspirin use in pregnancy). Finally, although extremely interesting, the use of the sFlt-1:PlFG ratio at 24 to 28 weeks is not in widespread use in the United States and may incur an additional cost not equivalent to the low cost of a daily aspirin.
Essentially, this is an extremely limited study for a very specific population. Before globally discontinuing low-dose aspirin in high-risk patients, the different doses and eligibility criteria should be studied for effect of early discontinuation. ●
Low-dose aspirin should continue to be used for prevention of preeclampsia in high-risk pregnant patients, optimally starting at 12 to 16 weeks’ gestation and continuing either through 36 weeks or delivery. Further study is needed to determine the optimal timing for earlier discontinuation of aspirin based on dose, risk factors, and other measures of preeclampsia risk as the pregnancy progresses.
JAIMEY M. PAULI, MD
- ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52. doi:10.1097/AOG.0000000000002708.
- Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.
- ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52. doi:10.1097/AOG.0000000000002708.
- Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.
Does the current age cutoff for screening miss too many cases of cervical cancer in older women?
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
EXPERT COMMENTARY
Cervical cancer screening guidelines recommend screening cessation at age 65 once specific exit criteria are met. (According to the American Cancer Society, individuals aged >65 years who have no history of cervical intraepithelial neoplasia [CIN] grade 2 or more severe disease within the past 25 years, and who have documented adequate negative prior screening in the prior 10 years, discontinue all cervical cancer screening.)1 We know, however, that about one-fifth of all cervical cancer cases are diagnosed among individuals aged 65 or older, and for Black women that proportion is even higher when data are appropriately adjusted to account for the increased rate of hysterectomy among Black versus White women.2-4
Early-stage cervical cancer is largely a curable disease with very high 5-year overall survival rates. Unfortunately, more than half of all cervical cancer is diagnosed at a more advanced stage, and survival rates are much lower for this population.5
Cervical cancer incidence rates plummeted in the United States after the introduction of the Pap test for cervical cancer screening. However, the percentage of women who are not up to date with cervical cancer screening may now be increasing, from 14% in 2005 to 23% in 2019 according to one study from the US Preventive Services Task Force.6 When looking at cervical cancer screening rates by age, researchers from the Centers for Disease Control and Prevention estimate that the proportion of patients who have not been recently screened goes up as patients get older, with approximately 845,000 American women aged 61 to 65 not adequately screened in 2015 alone.7
Details of the study
Cooley and colleagues sought to better characterize the cohort of women diagnosed with cervical cancer at a later age, specifically the stage at diagnosis and survival.8 They used data from the California Cancer Registry (CCR), a large state-mandated, population-based data repository that is affiliated with the Surveillance, Epidemiology, and End Results (SEER) program.
The researchers identified 12,442 womenin the CCR who were newly diagnosed with cervical cancer from 2009 to 2018, 17.4% of whom were age 65 or older. They looked at cancer stage at diagnosis as it relates to relative survival rate (“the ratio of the observed survival rate among those who have cancer divided by the expected survival rate for people of the same sex, race/ethnicity, and age who do not have cancer”), Charlson comorbidity score, socioeconomic status, health insurance status, urbanicity, and race/ethnicity.
Results. In this study, 71% of women aged 65 or older presented with advanced-stage disease (FIGO [International Federation of Gynecology and Obstetrics] stage II–IV) as compared with only 48% in those aged 21 to 64. Five-year relative survival rates also were lower in the older cohort—23% to 37%, compared with 42% to 52% in the younger patients. In a sensitivity analysis, late-stage disease was associated with older age, increasing medical comorbidities, and nonadenocarcinoma histology.
Interestingly, older women of Hispanic ethnicity were less likely to be diagnosed with late-stage disease when compared with non-Hispanic White women.
Study strengths and limitations
Although this study’s conclusions—that patients with advanced-stage cancer are more likely to do poorly than those with early-stage cancer—may seem obvious to some even without the proven data, it is still important to highlight what a clinician may intuit with data to support that intuition. It is particularly important to emphasize this risk in older women in light of the aging population in the United States, with adults older than age 65 expected to account for more than 20% of the nation’s population by 2030.9
The study by Cooley and colleagues adds value to the existing literature due to its large study population, which included more than 12,000 patients diagnosed with cervical cancer.8 And although its results may not be completely generalizable as the data were gathered from only a California-specific population, the sample was diverse with significant portions of Hispanic and Black patients. This study supports previous data that showed high rates of advanced cervical cancer in women older than age 65, with resultant worse 5-year relative survival in this population of older women specifically.4 ●
Cervical cancer is both common and deadly in older women. Although current cervical cancer screening guidelines recommend screening cessation after age 65, remember that this is based on strict exit criteria. Consider screening older women (especially with human papillomavirus [HPV] testing) for cervical cancer if they have risk factors (such as smoking, multiple sexual partners, inconsistent or infrequent screening, history of abnormal Pap or HPV tests), and keep cervical cancer on your differential diagnosis in women who present with postmenopausal bleeding, vaginal discharge, pelvic pain, recurrent urinary tract infections, or other concerning symptoms.
SARAH DILLEY, MD, MPH, AND WARNER HUH, MD
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
- Fontham ETH, Wolf AMD, Church TR, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J Clin. 2020;70:321-346. doi:10.3322/caac.21628.
- Dilley S, Huh W, Blechter B, et al. It’s time to re-evaluate cervical cancer screening after age 65. Gynecol Oncol. 2021;162:200-202. doi:10.1016/j.ygyno.2021.04.027.
- Rositch AF, Nowak RG, Gravitt PE. Increased age and racespecific incidence of cervical cancer after correction for hysterectomy prevalence in the United States from 2000 to 2009. Cancer. 2014;120:2032-2038. doi:10.1002/cncr.28548.
- Beavis AL, Gravitt PE, Rositch AF. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer. 2017;123:1044-1050. doi:10.1002 /cncr.30507.
- Cancer Stat Facts. National Cancer Institute Surveillance, Epidemiology, and End Results Program. https://seer.cancer .gov/statfacts/html/cervix.html
- Suk R, Hong YR, Rajan SS, et al. Assessment of US Preventive Services Task Force guideline-concordant cervical cancer screening rates and reasons for underscreening by age, race and ethnicity, sexual orientation, rurality, and insurance, 2005 to 2019. JAMA Netw Open. 2022;5:e2143582. doi:10.1001 /jamanetworkopen.2021.43582.
- White MC, Shoemaker ML, Benard VB. Cervical cancer screening and incidence by age: unmet needs near and after the stopping age for screening. Am J Prev Med. 2017;53:392395. doi:10.1016/j.amepre.2017.02.024.
- Cooley JJ, Maguire FB, Morris CR, et al. Cervical cancer stage at diagnosis and survival among women ≥65 years in California. Cancer Epidemiol Biomarkers Prev. 2023;32:91-97. doi:10.1158/1055-9965.EPI-22-0793.
- Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States. May 2014. United States Census Bureau. Accessed April 12, 2023. https://www.census .gov/library/publications/2014/demo/p25-1140.html
23-year-old woman • fever, fatigue, and sore throat • scleral icterus and hepatosplenomegaly • Dx?
THE CASE
A 23-year-old woman sought care from her primary care physician (PCP) after being sick for 7 days. The illness started with a headache and fatigue, and by Day 6, she also had fever, chills, sore throat, nausea, a poor appetite, and intractable vomiting. The patient had no significant medical history and was socially isolating due to the COVID-19 pandemic. She had no known sick contacts or recent sexual activity and did not use any illicit drugs.
On examination, her vital signs were normal although she appeared ill and diaphoretic. A shallow tonsil ulcer and tonsillar adenopathy were present. Laboratory tests included a complete blood count (CBC), comprehensive metabolic panel, Monospot test, and Epstein-Barr virus (EBV) antibody test. Results were notable for leukocytosis with atypical lymphocytes on her CBC. Her Monospot test and EBV immunoglobulin (Ig) M antibody were positive, and her EBV IgG antibody was negative. She was given a diagnosis of infectious mononucleosis (IM) and told to get adequate rest, drink a lot of fluids, and take ibuprofen or acetaminophen for pain control.
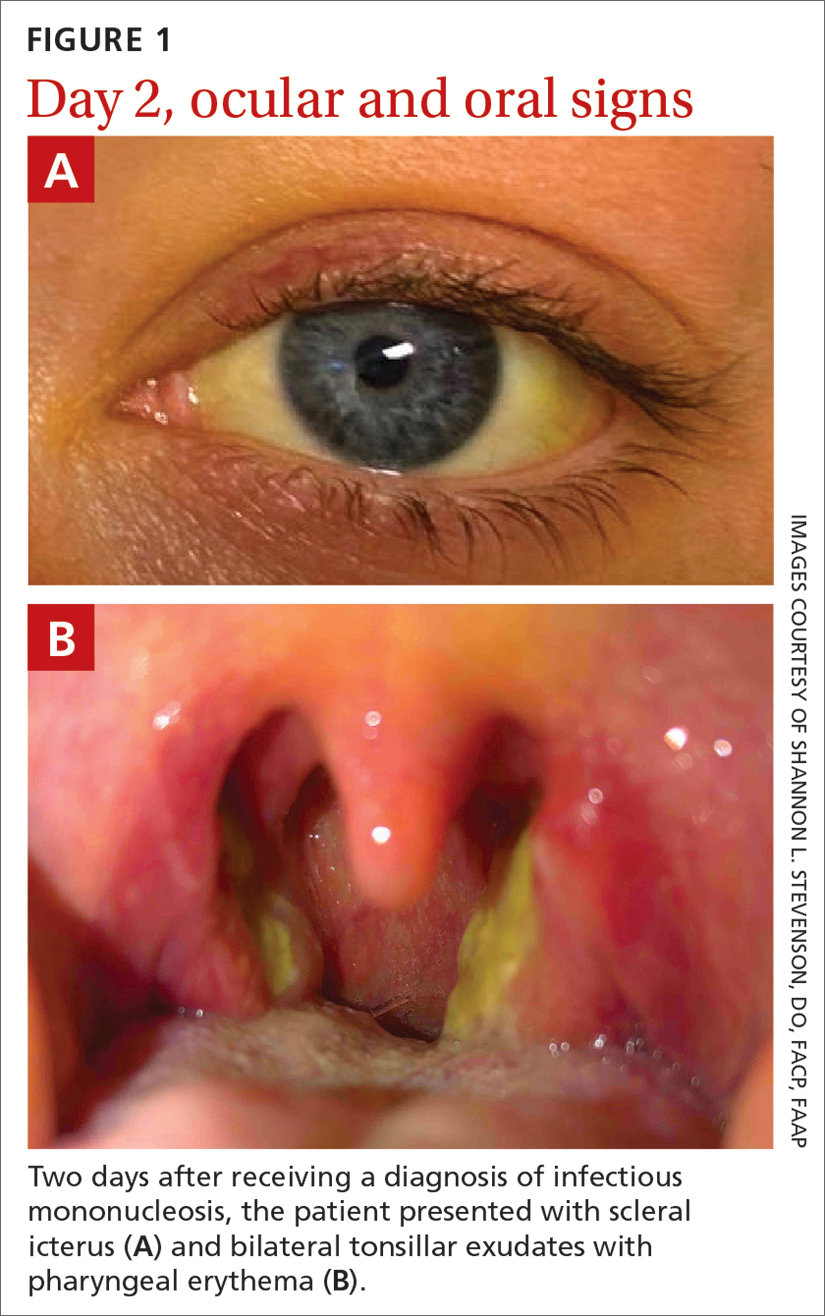
Two days later, she returned to her PCP with scleral icterus (FIGURE 1A), increasingly tender cervical lymphadenopathy, and left-side abdominal pain. Her liver function tests (LFTs) had worsened (TABLE). An abdominal ultrasound revealed mild diffuse decreased hepatic echogenicity and prominent periportal echogenicity, likely related to diffuse hepatic parenchymal disease, as well as splenomegaly and a mildly thickened gallbladder with no gallstones. She also had severe throat discomfort, with bilateral tonsillar exudates and pharyngeal erythema (FIGURE 1B).
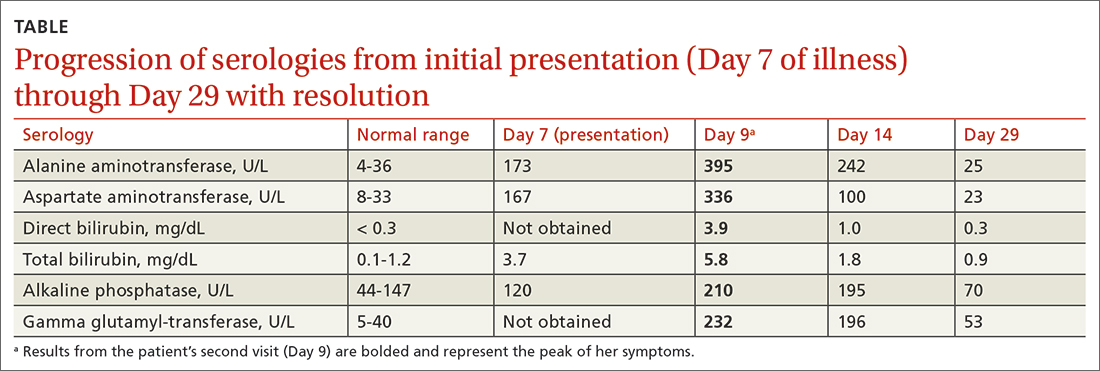
THE DIAGNOSIS
Based on her symptoms and the results of her physical examination, LFTs, EBV serologic assays, and abdominal ultrasound, this patient was given a diagnosis of acute EBV hepatitis.
DISCUSSION
EBV infection, which is the most common cause of IM, causes asymptomatic liver enzyme abnormalities in 80% to 90% of patients.1-3 Although not common, patients can develop acute EBV hepatitis and require hospitalization.4
Be aware of potential complications. Prompt assessment of elevated liver enzymes and accurate diagnosis are key.5 Although acute EBV hepatitis is usually self-limiting, there can be serious gastrointestinal complications such as splenic rupture, liver failure due to acute and/or chronic EBV infection, autoimmune hepatitis, and hepatocellular carcinoma.2 It’s rare for EBV hepatitis to lead to acute liver failure, but when that occurs, it can be fatal.6-9 One case series revealed that while primary EBV infection accounts for less than 1% of adult acute liver failure cases, it has a high case fatality rate of 50%.9
Treatment for patients with EBV hepatitis is usually supportive and includes rest, analgesia, and avoidance of vigorous activity for 1 month to reduce the risk for splenic rupture.1 In patients with nausea and vomiting, intravenous fluids may be necessary and can be administered at an outpatient infusion center. For individuals with severe tonsillar hypertrophy, prednisone (40-60 mg/d for 2-3 days, with subsequent tapering over 1-2 weeks) is indicated to prevent airway obstruction.1 Acyclovir may be used to reduce EBV viral shedding; however, it has no significant clinical impact.1
Continue to: Patients who are hemodynamially stable...
Patients who are hemodynamically stable and have appropriate access to follow-up care can be managed at home.2 If follow-up cannot occur remotely within 1 week or the patient’s clinical status begins to worsen (ie, the patient’s liver enzymes or bilirubin levels dramatically increase), hospitalization is necessary.10
Through shared decision-making, our patient was treated as an outpatient based on her hemodynamic stability and her ability to closely follow up in the clinic and by phone and to access an outpatient infusion center. She was reexamined within 2 days and given ondansetron 8 mg IV with 2 L of normal saline at our outpatient infusion center. We also prescribed ibuprofen (400 mg every 6 hours as needed) for analgesia and issued the standard recommendations that she avoid contact sports (for at least 6 weeks) and excessive alcohol consumption.
On Day 11, the patient followed up with her PCP by telephone. The patient was started on oral prednisone (40 mg/d for 3 days with taper over the next week as symptoms improved) for her severe throat discomfort, exudates, difficulty swallowing, and muffled voice. By Day 14, her aminotransferase levels began to decrease (TABLE), and her symptoms steadily improved thereafter.
THE TAKEAWAY
When a patient presents with unexplained elevated liver enzymes or cholestasis, it is important to assess for signs and symptoms of EBV hepatitis. Although EBV hepatitis is typically self-limiting, it can have serious complications or be fatal. Prompt initiation of outpatient management may avoid these complications and hospitalization.
CORRESPONDENCE
Lydia J. Schneider, MD, 225 East Chicago Avenue, Chicago, IL 60611; [email protected]
1. Cohen JI. Chapter 189: Epstein-Barr virus infections, including infectious mononucleosis. In: Jameson JL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. McGraw Hill; 2020. Accessed March 21, 2023. accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192024765
2. Crum NF. Epstein Barr virus hepatitis: case series and review. South Med J. 2006;99:544-547. doi: 10.1097/01.smj.0000216469.04854.2a
3. Bunchorntavakul C, Reddy KR. Epstein-Barr virus and cytomegalovirus infections of the liver. Gastroenterol Clin North Am. 2020;49:331-346. doi: 10.1016/j.gtc.2020.01.008
4. Leonardsson H, Hreinsson JP, Löve A, et al. Hepatitis due to Epstein-Barr virus and cytomegalovirus: clinical features and outcomes. Scand J Gastroenterol. 2017;52:893-897. doi: 10.1080/ 00365521.2017.1319972
5. Banker L, Bowman PE. Epstein-Barr virus: forgotten etiology of hepatic injury. Clinical Advisor. September 23, 2021. Accessed April 18, 2023. www.clinicaladvisor.com/home/topics/infectious-diseases-information-center/epstein-barr-virus-etiology-hepatic-injury/
6. Fugl A, Lykkegaard Andersen C. Epstein-Barr virus and its association with disease: a review of relevance to general practice. BMC Fam Pract. 2019;20:62. doi: 10.1186/s12875-019-0954-3
7. Markin RS, Linder J, Zuerlein K, et al. Hepatitis in fatal infectious mononucleosis. Gastroenterology. 1987;93:1210-1217. doi: 10.1016/0016-5085(87)90246-0
8. Zhang W, Chen B, Chen Y, et al. Epstein-Barr virus-associated acute liver failure present in a 67-year-old immunocompetent female. Gastroenterology Res. 2016;9:74-78.
9. Mellinğer J, Rossaro L, Naugler W, et al. Epstein-Barr virus (EBV) related acute liver failure: a case series from the US Acute Liver Failure Study Group. Dig Dis Sci. 2014;59:1630-1637. doi: 10.1007/s10620-014-3029-2
10. Uluğ M, Kemal Celen M, Ayaz C, et al. Acute hepatitis: a rare complication of Epstein-Barr virus (EBV) infection. J Infect Dev Ctries. 2010;4:668-673. doi: 10.3855/jidc.871
THE CASE
A 23-year-old woman sought care from her primary care physician (PCP) after being sick for 7 days. The illness started with a headache and fatigue, and by Day 6, she also had fever, chills, sore throat, nausea, a poor appetite, and intractable vomiting. The patient had no significant medical history and was socially isolating due to the COVID-19 pandemic. She had no known sick contacts or recent sexual activity and did not use any illicit drugs.
On examination, her vital signs were normal although she appeared ill and diaphoretic. A shallow tonsil ulcer and tonsillar adenopathy were present. Laboratory tests included a complete blood count (CBC), comprehensive metabolic panel, Monospot test, and Epstein-Barr virus (EBV) antibody test. Results were notable for leukocytosis with atypical lymphocytes on her CBC. Her Monospot test and EBV immunoglobulin (Ig) M antibody were positive, and her EBV IgG antibody was negative. She was given a diagnosis of infectious mononucleosis (IM) and told to get adequate rest, drink a lot of fluids, and take ibuprofen or acetaminophen for pain control.

Two days later, she returned to her PCP with scleral icterus (FIGURE 1A), increasingly tender cervical lymphadenopathy, and left-side abdominal pain. Her liver function tests (LFTs) had worsened (TABLE). An abdominal ultrasound revealed mild diffuse decreased hepatic echogenicity and prominent periportal echogenicity, likely related to diffuse hepatic parenchymal disease, as well as splenomegaly and a mildly thickened gallbladder with no gallstones. She also had severe throat discomfort, with bilateral tonsillar exudates and pharyngeal erythema (FIGURE 1B).

THE DIAGNOSIS
Based on her symptoms and the results of her physical examination, LFTs, EBV serologic assays, and abdominal ultrasound, this patient was given a diagnosis of acute EBV hepatitis.
DISCUSSION
EBV infection, which is the most common cause of IM, causes asymptomatic liver enzyme abnormalities in 80% to 90% of patients.1-3 Although not common, patients can develop acute EBV hepatitis and require hospitalization.4
Be aware of potential complications. Prompt assessment of elevated liver enzymes and accurate diagnosis are key.5 Although acute EBV hepatitis is usually self-limiting, there can be serious gastrointestinal complications such as splenic rupture, liver failure due to acute and/or chronic EBV infection, autoimmune hepatitis, and hepatocellular carcinoma.2 It’s rare for EBV hepatitis to lead to acute liver failure, but when that occurs, it can be fatal.6-9 One case series revealed that while primary EBV infection accounts for less than 1% of adult acute liver failure cases, it has a high case fatality rate of 50%.9
Treatment for patients with EBV hepatitis is usually supportive and includes rest, analgesia, and avoidance of vigorous activity for 1 month to reduce the risk for splenic rupture.1 In patients with nausea and vomiting, intravenous fluids may be necessary and can be administered at an outpatient infusion center. For individuals with severe tonsillar hypertrophy, prednisone (40-60 mg/d for 2-3 days, with subsequent tapering over 1-2 weeks) is indicated to prevent airway obstruction.1 Acyclovir may be used to reduce EBV viral shedding; however, it has no significant clinical impact.1
Continue to: Patients who are hemodynamially stable...
Patients who are hemodynamically stable and have appropriate access to follow-up care can be managed at home.2 If follow-up cannot occur remotely within 1 week or the patient’s clinical status begins to worsen (ie, the patient’s liver enzymes or bilirubin levels dramatically increase), hospitalization is necessary.10
Through shared decision-making, our patient was treated as an outpatient based on her hemodynamic stability and her ability to closely follow up in the clinic and by phone and to access an outpatient infusion center. She was reexamined within 2 days and given ondansetron 8 mg IV with 2 L of normal saline at our outpatient infusion center. We also prescribed ibuprofen (400 mg every 6 hours as needed) for analgesia and issued the standard recommendations that she avoid contact sports (for at least 6 weeks) and excessive alcohol consumption.
On Day 11, the patient followed up with her PCP by telephone. The patient was started on oral prednisone (40 mg/d for 3 days with taper over the next week as symptoms improved) for her severe throat discomfort, exudates, difficulty swallowing, and muffled voice. By Day 14, her aminotransferase levels began to decrease (TABLE), and her symptoms steadily improved thereafter.
THE TAKEAWAY
When a patient presents with unexplained elevated liver enzymes or cholestasis, it is important to assess for signs and symptoms of EBV hepatitis. Although EBV hepatitis is typically self-limiting, it can have serious complications or be fatal. Prompt initiation of outpatient management may avoid these complications and hospitalization.
CORRESPONDENCE
Lydia J. Schneider, MD, 225 East Chicago Avenue, Chicago, IL 60611; [email protected]
THE CASE
A 23-year-old woman sought care from her primary care physician (PCP) after being sick for 7 days. The illness started with a headache and fatigue, and by Day 6, she also had fever, chills, sore throat, nausea, a poor appetite, and intractable vomiting. The patient had no significant medical history and was socially isolating due to the COVID-19 pandemic. She had no known sick contacts or recent sexual activity and did not use any illicit drugs.
On examination, her vital signs were normal although she appeared ill and diaphoretic. A shallow tonsil ulcer and tonsillar adenopathy were present. Laboratory tests included a complete blood count (CBC), comprehensive metabolic panel, Monospot test, and Epstein-Barr virus (EBV) antibody test. Results were notable for leukocytosis with atypical lymphocytes on her CBC. Her Monospot test and EBV immunoglobulin (Ig) M antibody were positive, and her EBV IgG antibody was negative. She was given a diagnosis of infectious mononucleosis (IM) and told to get adequate rest, drink a lot of fluids, and take ibuprofen or acetaminophen for pain control.

Two days later, she returned to her PCP with scleral icterus (FIGURE 1A), increasingly tender cervical lymphadenopathy, and left-side abdominal pain. Her liver function tests (LFTs) had worsened (TABLE). An abdominal ultrasound revealed mild diffuse decreased hepatic echogenicity and prominent periportal echogenicity, likely related to diffuse hepatic parenchymal disease, as well as splenomegaly and a mildly thickened gallbladder with no gallstones. She also had severe throat discomfort, with bilateral tonsillar exudates and pharyngeal erythema (FIGURE 1B).

THE DIAGNOSIS
Based on her symptoms and the results of her physical examination, LFTs, EBV serologic assays, and abdominal ultrasound, this patient was given a diagnosis of acute EBV hepatitis.
DISCUSSION
EBV infection, which is the most common cause of IM, causes asymptomatic liver enzyme abnormalities in 80% to 90% of patients.1-3 Although not common, patients can develop acute EBV hepatitis and require hospitalization.4
Be aware of potential complications. Prompt assessment of elevated liver enzymes and accurate diagnosis are key.5 Although acute EBV hepatitis is usually self-limiting, there can be serious gastrointestinal complications such as splenic rupture, liver failure due to acute and/or chronic EBV infection, autoimmune hepatitis, and hepatocellular carcinoma.2 It’s rare for EBV hepatitis to lead to acute liver failure, but when that occurs, it can be fatal.6-9 One case series revealed that while primary EBV infection accounts for less than 1% of adult acute liver failure cases, it has a high case fatality rate of 50%.9
Treatment for patients with EBV hepatitis is usually supportive and includes rest, analgesia, and avoidance of vigorous activity for 1 month to reduce the risk for splenic rupture.1 In patients with nausea and vomiting, intravenous fluids may be necessary and can be administered at an outpatient infusion center. For individuals with severe tonsillar hypertrophy, prednisone (40-60 mg/d for 2-3 days, with subsequent tapering over 1-2 weeks) is indicated to prevent airway obstruction.1 Acyclovir may be used to reduce EBV viral shedding; however, it has no significant clinical impact.1
Continue to: Patients who are hemodynamially stable...
Patients who are hemodynamically stable and have appropriate access to follow-up care can be managed at home.2 If follow-up cannot occur remotely within 1 week or the patient’s clinical status begins to worsen (ie, the patient’s liver enzymes or bilirubin levels dramatically increase), hospitalization is necessary.10
Through shared decision-making, our patient was treated as an outpatient based on her hemodynamic stability and her ability to closely follow up in the clinic and by phone and to access an outpatient infusion center. She was reexamined within 2 days and given ondansetron 8 mg IV with 2 L of normal saline at our outpatient infusion center. We also prescribed ibuprofen (400 mg every 6 hours as needed) for analgesia and issued the standard recommendations that she avoid contact sports (for at least 6 weeks) and excessive alcohol consumption.
On Day 11, the patient followed up with her PCP by telephone. The patient was started on oral prednisone (40 mg/d for 3 days with taper over the next week as symptoms improved) for her severe throat discomfort, exudates, difficulty swallowing, and muffled voice. By Day 14, her aminotransferase levels began to decrease (TABLE), and her symptoms steadily improved thereafter.
THE TAKEAWAY
When a patient presents with unexplained elevated liver enzymes or cholestasis, it is important to assess for signs and symptoms of EBV hepatitis. Although EBV hepatitis is typically self-limiting, it can have serious complications or be fatal. Prompt initiation of outpatient management may avoid these complications and hospitalization.
CORRESPONDENCE
Lydia J. Schneider, MD, 225 East Chicago Avenue, Chicago, IL 60611; [email protected]
1. Cohen JI. Chapter 189: Epstein-Barr virus infections, including infectious mononucleosis. In: Jameson JL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. McGraw Hill; 2020. Accessed March 21, 2023. accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192024765
2. Crum NF. Epstein Barr virus hepatitis: case series and review. South Med J. 2006;99:544-547. doi: 10.1097/01.smj.0000216469.04854.2a
3. Bunchorntavakul C, Reddy KR. Epstein-Barr virus and cytomegalovirus infections of the liver. Gastroenterol Clin North Am. 2020;49:331-346. doi: 10.1016/j.gtc.2020.01.008
4. Leonardsson H, Hreinsson JP, Löve A, et al. Hepatitis due to Epstein-Barr virus and cytomegalovirus: clinical features and outcomes. Scand J Gastroenterol. 2017;52:893-897. doi: 10.1080/ 00365521.2017.1319972
5. Banker L, Bowman PE. Epstein-Barr virus: forgotten etiology of hepatic injury. Clinical Advisor. September 23, 2021. Accessed April 18, 2023. www.clinicaladvisor.com/home/topics/infectious-diseases-information-center/epstein-barr-virus-etiology-hepatic-injury/
6. Fugl A, Lykkegaard Andersen C. Epstein-Barr virus and its association with disease: a review of relevance to general practice. BMC Fam Pract. 2019;20:62. doi: 10.1186/s12875-019-0954-3
7. Markin RS, Linder J, Zuerlein K, et al. Hepatitis in fatal infectious mononucleosis. Gastroenterology. 1987;93:1210-1217. doi: 10.1016/0016-5085(87)90246-0
8. Zhang W, Chen B, Chen Y, et al. Epstein-Barr virus-associated acute liver failure present in a 67-year-old immunocompetent female. Gastroenterology Res. 2016;9:74-78.
9. Mellinğer J, Rossaro L, Naugler W, et al. Epstein-Barr virus (EBV) related acute liver failure: a case series from the US Acute Liver Failure Study Group. Dig Dis Sci. 2014;59:1630-1637. doi: 10.1007/s10620-014-3029-2
10. Uluğ M, Kemal Celen M, Ayaz C, et al. Acute hepatitis: a rare complication of Epstein-Barr virus (EBV) infection. J Infect Dev Ctries. 2010;4:668-673. doi: 10.3855/jidc.871
1. Cohen JI. Chapter 189: Epstein-Barr virus infections, including infectious mononucleosis. In: Jameson JL, Fauci AS, Kasper DL, et al, eds. Harrison’s Principles of Internal Medicine. 20th ed. McGraw Hill; 2020. Accessed March 21, 2023. accessmedicine.mhmedical.com/content.aspx?bookid=2129§ionid=192024765
2. Crum NF. Epstein Barr virus hepatitis: case series and review. South Med J. 2006;99:544-547. doi: 10.1097/01.smj.0000216469.04854.2a
3. Bunchorntavakul C, Reddy KR. Epstein-Barr virus and cytomegalovirus infections of the liver. Gastroenterol Clin North Am. 2020;49:331-346. doi: 10.1016/j.gtc.2020.01.008
4. Leonardsson H, Hreinsson JP, Löve A, et al. Hepatitis due to Epstein-Barr virus and cytomegalovirus: clinical features and outcomes. Scand J Gastroenterol. 2017;52:893-897. doi: 10.1080/ 00365521.2017.1319972
5. Banker L, Bowman PE. Epstein-Barr virus: forgotten etiology of hepatic injury. Clinical Advisor. September 23, 2021. Accessed April 18, 2023. www.clinicaladvisor.com/home/topics/infectious-diseases-information-center/epstein-barr-virus-etiology-hepatic-injury/
6. Fugl A, Lykkegaard Andersen C. Epstein-Barr virus and its association with disease: a review of relevance to general practice. BMC Fam Pract. 2019;20:62. doi: 10.1186/s12875-019-0954-3
7. Markin RS, Linder J, Zuerlein K, et al. Hepatitis in fatal infectious mononucleosis. Gastroenterology. 1987;93:1210-1217. doi: 10.1016/0016-5085(87)90246-0
8. Zhang W, Chen B, Chen Y, et al. Epstein-Barr virus-associated acute liver failure present in a 67-year-old immunocompetent female. Gastroenterology Res. 2016;9:74-78.
9. Mellinğer J, Rossaro L, Naugler W, et al. Epstein-Barr virus (EBV) related acute liver failure: a case series from the US Acute Liver Failure Study Group. Dig Dis Sci. 2014;59:1630-1637. doi: 10.1007/s10620-014-3029-2
10. Uluğ M, Kemal Celen M, Ayaz C, et al. Acute hepatitis: a rare complication of Epstein-Barr virus (EBV) infection. J Infect Dev Ctries. 2010;4:668-673. doi: 10.3855/jidc.871
► Fever, fatigue, and sore throat
► Scleral icterus and hepatosplenomegaly
Does hormone replacement therapy prevent cognitive decline in postmenopausal women?
Evidence summary
Multiple analyses suggest HRT worsens rather than improves cognition
A 2017 Cochrane review of 22 randomized, double-blind studies compared use of HRT (estrogen only or combination estrogen + progesterone therapies) with placebo in postmenopausal women (N = 43,637). Age ranges varied, but the average age in most studies was > 60 years. Treatment duration was at least 1 year. Various outcomes were assessed across these 22 studies, including cardiovascular disease, bone health, and cognition.1
Cognitive outcomes were assessed with the Mini-Mental Status Exam in 5 of the trials (N = 12,789). Data were not combined due to heterogeneity. The authors found no significant difference in cognitive scores between the treatment and control groups in any of these 5 studies.1
In the largest included study, the Women’s Health Initiative (WHI) Memory Study (N = 10,739), participants were older than 65 years. Among those receiving estrogen-only HRT, there were no statistically significant differences compared to those receiving placebo. However, healthy postmenopausal women taking combination HRT had an increased risk for “probable dementia” compared to those taking placebo (relative risk [RR] = 1.97; 95% CI, 1.16-3.33). When researchers looked exclusively at women taking HRT, the risk for dementia increased from 9 in 1000 to 18 in 1000 (95% CI, 11-30) after 4 years of HRT use. This results in a number needed to harm of 4 to 50 patients.1
Two notable limitations of this evidence are that the average age of this population was > 60 years and 80% of the participants were White.1
A 2021 meta-analysis of 23 RCTs (N = 13,683) studied the effect of HRT on global cognitive function as well as specific cognitive domains including memory, executive function, attention, and language. Mean patient age in the studies varied from 48 to 81 years. Nine of these studies were also included in the previously discussed Cochrane review.2
There was a statistically significant but small decrease in overall global cognition (10 trials; N = 12,115; standardized mean difference [SMD] = –0.04; 95% CI, –0.08 to –0.01) in those receiving HRT compared to placebo. This effect was slightly more pronounced among those who initiated HRT at age > 60 years (8 trials; N = 11,914; SMD = –0.05; 95% CI, –0.08 to –0.01) and among patients with HRT duration > 6 months (7 trials; N = 11,828; SMD = –0.05; 95% CI, –0.08 to –0.01). There were no significant differences in specific cognitive domains.2
In a 2017 follow-up to the WHI trial, researchers analyzed data on long-term cognitive effects in women previously treated with HRT. There were 2 cohorts: participants who initiated HRT at a younger age (50-54; N = 1376) and those who initiated HRT later in life (age 65-79; N = 2880). Cognitive outcomes were assessed using the Telephone Interview for Cognitive Status-modified, with interviews conducted annually beginning 6 to 7 years after HRT was stopped.3
The investigators found no significant change in composite cognitive function in the younger HRT-treated group compared to placebo (estrogen alone: mean deviation [MD] = 0.014; 95% CI, –0.097 to 0.126; estrogen + progesterone: MD = –0.047; 95% CI, –0.134 to 0.04), or in the group who initiated HRT at an older age (estrogen alone: MD = –0.099; 95% CI, –0.202 to 0.004; estrogen + progesterone: MD = –0.022; 95% CI, –0.099 to 0.055). The authors state that although the data did not reach significance, this study also found a trend toward decreases in global cognitive function in the older age group.3
Editor’s takeaway
Abundant, consistent evidence with long-term follow-up shows postmenopausal HRT does not reduce cognitive decline. In fact, it appears to increase cognitive decline slightly. Renewed interest in postmenopausal HRT to alleviate menopausal symptoms should balance the risks and benefits to the individual patient.
1. Marjoribanks J, Farquhar C, Roberts H, et al. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143. doi: 10.1002/14651858.CD004143.pub5
2. Zhou HH, Yu Z, Luo L, et al. The effect of hormone replacement therapy on cognitive function in healthy postmenopausal women: a meta-analysis of 23 randomized controlled trials. Psychogeriatrics. 2021;21:926-938. doi: 10.1111/psyg.12768
3. Espeland MA, Rapp SR, Manson JE, et al. Long-term effects on cognitive trajectories of postmenopausal hormone therapy in two age groups. J Gerontol A Biol Sci Med Sci. 2017;72:838-845. doi: 10.1093/gerona/glw156
Evidence summary
Multiple analyses suggest HRT worsens rather than improves cognition
A 2017 Cochrane review of 22 randomized, double-blind studies compared use of HRT (estrogen only or combination estrogen + progesterone therapies) with placebo in postmenopausal women (N = 43,637). Age ranges varied, but the average age in most studies was > 60 years. Treatment duration was at least 1 year. Various outcomes were assessed across these 22 studies, including cardiovascular disease, bone health, and cognition.1
Cognitive outcomes were assessed with the Mini-Mental Status Exam in 5 of the trials (N = 12,789). Data were not combined due to heterogeneity. The authors found no significant difference in cognitive scores between the treatment and control groups in any of these 5 studies.1
In the largest included study, the Women’s Health Initiative (WHI) Memory Study (N = 10,739), participants were older than 65 years. Among those receiving estrogen-only HRT, there were no statistically significant differences compared to those receiving placebo. However, healthy postmenopausal women taking combination HRT had an increased risk for “probable dementia” compared to those taking placebo (relative risk [RR] = 1.97; 95% CI, 1.16-3.33). When researchers looked exclusively at women taking HRT, the risk for dementia increased from 9 in 1000 to 18 in 1000 (95% CI, 11-30) after 4 years of HRT use. This results in a number needed to harm of 4 to 50 patients.1
Two notable limitations of this evidence are that the average age of this population was > 60 years and 80% of the participants were White.1
A 2021 meta-analysis of 23 RCTs (N = 13,683) studied the effect of HRT on global cognitive function as well as specific cognitive domains including memory, executive function, attention, and language. Mean patient age in the studies varied from 48 to 81 years. Nine of these studies were also included in the previously discussed Cochrane review.2
There was a statistically significant but small decrease in overall global cognition (10 trials; N = 12,115; standardized mean difference [SMD] = –0.04; 95% CI, –0.08 to –0.01) in those receiving HRT compared to placebo. This effect was slightly more pronounced among those who initiated HRT at age > 60 years (8 trials; N = 11,914; SMD = –0.05; 95% CI, –0.08 to –0.01) and among patients with HRT duration > 6 months (7 trials; N = 11,828; SMD = –0.05; 95% CI, –0.08 to –0.01). There were no significant differences in specific cognitive domains.2
In a 2017 follow-up to the WHI trial, researchers analyzed data on long-term cognitive effects in women previously treated with HRT. There were 2 cohorts: participants who initiated HRT at a younger age (50-54; N = 1376) and those who initiated HRT later in life (age 65-79; N = 2880). Cognitive outcomes were assessed using the Telephone Interview for Cognitive Status-modified, with interviews conducted annually beginning 6 to 7 years after HRT was stopped.3
The investigators found no significant change in composite cognitive function in the younger HRT-treated group compared to placebo (estrogen alone: mean deviation [MD] = 0.014; 95% CI, –0.097 to 0.126; estrogen + progesterone: MD = –0.047; 95% CI, –0.134 to 0.04), or in the group who initiated HRT at an older age (estrogen alone: MD = –0.099; 95% CI, –0.202 to 0.004; estrogen + progesterone: MD = –0.022; 95% CI, –0.099 to 0.055). The authors state that although the data did not reach significance, this study also found a trend toward decreases in global cognitive function in the older age group.3
Editor’s takeaway
Abundant, consistent evidence with long-term follow-up shows postmenopausal HRT does not reduce cognitive decline. In fact, it appears to increase cognitive decline slightly. Renewed interest in postmenopausal HRT to alleviate menopausal symptoms should balance the risks and benefits to the individual patient.
Evidence summary
Multiple analyses suggest HRT worsens rather than improves cognition
A 2017 Cochrane review of 22 randomized, double-blind studies compared use of HRT (estrogen only or combination estrogen + progesterone therapies) with placebo in postmenopausal women (N = 43,637). Age ranges varied, but the average age in most studies was > 60 years. Treatment duration was at least 1 year. Various outcomes were assessed across these 22 studies, including cardiovascular disease, bone health, and cognition.1
Cognitive outcomes were assessed with the Mini-Mental Status Exam in 5 of the trials (N = 12,789). Data were not combined due to heterogeneity. The authors found no significant difference in cognitive scores between the treatment and control groups in any of these 5 studies.1
In the largest included study, the Women’s Health Initiative (WHI) Memory Study (N = 10,739), participants were older than 65 years. Among those receiving estrogen-only HRT, there were no statistically significant differences compared to those receiving placebo. However, healthy postmenopausal women taking combination HRT had an increased risk for “probable dementia” compared to those taking placebo (relative risk [RR] = 1.97; 95% CI, 1.16-3.33). When researchers looked exclusively at women taking HRT, the risk for dementia increased from 9 in 1000 to 18 in 1000 (95% CI, 11-30) after 4 years of HRT use. This results in a number needed to harm of 4 to 50 patients.1
Two notable limitations of this evidence are that the average age of this population was > 60 years and 80% of the participants were White.1
A 2021 meta-analysis of 23 RCTs (N = 13,683) studied the effect of HRT on global cognitive function as well as specific cognitive domains including memory, executive function, attention, and language. Mean patient age in the studies varied from 48 to 81 years. Nine of these studies were also included in the previously discussed Cochrane review.2
There was a statistically significant but small decrease in overall global cognition (10 trials; N = 12,115; standardized mean difference [SMD] = –0.04; 95% CI, –0.08 to –0.01) in those receiving HRT compared to placebo. This effect was slightly more pronounced among those who initiated HRT at age > 60 years (8 trials; N = 11,914; SMD = –0.05; 95% CI, –0.08 to –0.01) and among patients with HRT duration > 6 months (7 trials; N = 11,828; SMD = –0.05; 95% CI, –0.08 to –0.01). There were no significant differences in specific cognitive domains.2
In a 2017 follow-up to the WHI trial, researchers analyzed data on long-term cognitive effects in women previously treated with HRT. There were 2 cohorts: participants who initiated HRT at a younger age (50-54; N = 1376) and those who initiated HRT later in life (age 65-79; N = 2880). Cognitive outcomes were assessed using the Telephone Interview for Cognitive Status-modified, with interviews conducted annually beginning 6 to 7 years after HRT was stopped.3
The investigators found no significant change in composite cognitive function in the younger HRT-treated group compared to placebo (estrogen alone: mean deviation [MD] = 0.014; 95% CI, –0.097 to 0.126; estrogen + progesterone: MD = –0.047; 95% CI, –0.134 to 0.04), or in the group who initiated HRT at an older age (estrogen alone: MD = –0.099; 95% CI, –0.202 to 0.004; estrogen + progesterone: MD = –0.022; 95% CI, –0.099 to 0.055). The authors state that although the data did not reach significance, this study also found a trend toward decreases in global cognitive function in the older age group.3
Editor’s takeaway
Abundant, consistent evidence with long-term follow-up shows postmenopausal HRT does not reduce cognitive decline. In fact, it appears to increase cognitive decline slightly. Renewed interest in postmenopausal HRT to alleviate menopausal symptoms should balance the risks and benefits to the individual patient.
1. Marjoribanks J, Farquhar C, Roberts H, et al. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143. doi: 10.1002/14651858.CD004143.pub5
2. Zhou HH, Yu Z, Luo L, et al. The effect of hormone replacement therapy on cognitive function in healthy postmenopausal women: a meta-analysis of 23 randomized controlled trials. Psychogeriatrics. 2021;21:926-938. doi: 10.1111/psyg.12768
3. Espeland MA, Rapp SR, Manson JE, et al. Long-term effects on cognitive trajectories of postmenopausal hormone therapy in two age groups. J Gerontol A Biol Sci Med Sci. 2017;72:838-845. doi: 10.1093/gerona/glw156
1. Marjoribanks J, Farquhar C, Roberts H, et al. Long-term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database Syst Rev. 2017;1:CD004143. doi: 10.1002/14651858.CD004143.pub5
2. Zhou HH, Yu Z, Luo L, et al. The effect of hormone replacement therapy on cognitive function in healthy postmenopausal women: a meta-analysis of 23 randomized controlled trials. Psychogeriatrics. 2021;21:926-938. doi: 10.1111/psyg.12768
3. Espeland MA, Rapp SR, Manson JE, et al. Long-term effects on cognitive trajectories of postmenopausal hormone therapy in two age groups. J Gerontol A Biol Sci Med Sci. 2017;72:838-845. doi: 10.1093/gerona/glw156
EVIDENCE-BASED REVIEW:
NO. Hormone replacement therapy (HRT) does not prevent cognitive decline in postmenopausal women—and in fact, it may slightly increase risk (strength of recommendation, A; systematic review, meta-analysis of randomized controlled trials [RCTs], and individual RCT).
Doctor spots a gunshot victim staggering down his street
It was a quiet day. I got up around 3 o’clock in the afternoon for my shift at 6 p.m. I was shaking off the cobwebs and making coffee at our front window that overlooked Brown Street in North Philadelphia. There was nobody else around so I went outside to see what was going on.
He was in his 50s or 60s, bleeding and obviously in distress. I had him sit down. Then I ran back inside and grabbed a dish towel and some exam gloves that I had in the house.
I ran back out and assessed him. A bullet had gone through one of his hands, but he had other wounds. I had to expose him, so I trauma stripped him on the sidewalk. I got his pants and his shirt off and saw a gunshot going through his lower pelvis. He was bleeding out from there.
I got the towel and started applying deep pressure down into the iliac vein in case they hit something, which I found out later, they had. I held it there. The man was just lying there begging not to die.
I’m someone who is very calm, maybe abnormally calm, as people tell me. I try to use that during my resuscitations and traumas. Just keeping everybody calm makes the situation easier. Afterwards, people asked me, “Weren’t you worried that you were going to get shot?” That does happen in North Philadelphia. But it didn’t even cross my mind.
I didn’t have to think at all about what I was doing. We saw so many gunshots, especially at Einstein Medical Center. We saw them daily. I’d sometimes get more than half a dozen gunshots in one shift.
So, I was holding pressure and some people started to come over. I got somebody to call 911 and asked the man about his medical history. I found out he had diabetes. Five or 10 minutes later, EMS showed up. They looked pretty stunned when I was able to give the handoff presentation to them. I told them what happened and his back-story. I wanted to make sure they would check his sugar and take extra precautions.
They got him on the stretcher, and he eventually made it to the hospital where he had surgery. They had to have a vascular surgeon work on him. I called later, and they told me, “Yeah, he’s alive.” But that’s about the extent of the update I got.
After the ambulance left, it was kind of chaos. All the neighbors poured out of their houses. People were panicked, talking and getting excited about it. I didn’t know, but everyone else had actually been home the whole time. They didn’t come out until then.
I went back inside and tried to get ready for work. I wasn’t planning on talking to the media, but my next door neighbor just walked the news camera crew over to my house and knocked on my door. I wasn’t exactly dressed to be on TV, but they talked to me on camera, and it was on the news later that night.
I went to work and didn’t say anything about it. To be honest, I was trying to avoid telling anyone. Our team had a close-knit bond, and we would often tease each other when we received any type of recognition.
Naturally one of my attendings saw it on the local news and told everybody. So, I got a lot of happy harassment for quite some time. Someone baked me a cake that said, “Hero of Fairmount” (the Philly neighborhood in which I live). Someone else printed out a photo of me that said, “Stop the Bleed Hero of Fairmount,” and put it on every single computer screen.
The man came to see me about 2 weeks later (a neighbor told him where I lived). The man was very tearful and gave me a big hug. We just embraced for a while, and he said how thankful he was. He brought me a bottle of wine, which I thought was really nice.
He told me what happened to him: There was a lot of construction on our street and he was the contractor overseeing a couple of home remodels and demolitions. Sometimes he paid workers in cash and carried it with him. Somebody had tipped off somebody else that he was going to be there that day. The contractor walked into one of the houses and a guy in a ski mask waited there with a gun. The guy shot him and took the cash. The bullet went through his hand into his pelvis.
I had never had to deal with something that intense before outside of work. Most of it really comes down to the basics – the ABCs and bleeding control. You do whatever you can with what you have. In this case, it was just a dish towel, gloves, and my hands to put as much pressure as possible.
It really was strange that I happened to be looking out the window at that moment. I don’t know if it was just a coincidence. The man told me he believed God had put somebody there at the right place at the right time to save his life. I just felt very fortunate to have been able to help him. I never saw him again.
I think something like this gives you a little confidence that you can actually do something and make a meaningful impact anywhere when it’s needed. It lets you know that you’re capable of doing it. You always think about it, but you don’t know until it happens.
A version of this article first appeared on Medscape.com.
It was a quiet day. I got up around 3 o’clock in the afternoon for my shift at 6 p.m. I was shaking off the cobwebs and making coffee at our front window that overlooked Brown Street in North Philadelphia. There was nobody else around so I went outside to see what was going on.
He was in his 50s or 60s, bleeding and obviously in distress. I had him sit down. Then I ran back inside and grabbed a dish towel and some exam gloves that I had in the house.
I ran back out and assessed him. A bullet had gone through one of his hands, but he had other wounds. I had to expose him, so I trauma stripped him on the sidewalk. I got his pants and his shirt off and saw a gunshot going through his lower pelvis. He was bleeding out from there.
I got the towel and started applying deep pressure down into the iliac vein in case they hit something, which I found out later, they had. I held it there. The man was just lying there begging not to die.
I’m someone who is very calm, maybe abnormally calm, as people tell me. I try to use that during my resuscitations and traumas. Just keeping everybody calm makes the situation easier. Afterwards, people asked me, “Weren’t you worried that you were going to get shot?” That does happen in North Philadelphia. But it didn’t even cross my mind.
I didn’t have to think at all about what I was doing. We saw so many gunshots, especially at Einstein Medical Center. We saw them daily. I’d sometimes get more than half a dozen gunshots in one shift.
So, I was holding pressure and some people started to come over. I got somebody to call 911 and asked the man about his medical history. I found out he had diabetes. Five or 10 minutes later, EMS showed up. They looked pretty stunned when I was able to give the handoff presentation to them. I told them what happened and his back-story. I wanted to make sure they would check his sugar and take extra precautions.
They got him on the stretcher, and he eventually made it to the hospital where he had surgery. They had to have a vascular surgeon work on him. I called later, and they told me, “Yeah, he’s alive.” But that’s about the extent of the update I got.
After the ambulance left, it was kind of chaos. All the neighbors poured out of their houses. People were panicked, talking and getting excited about it. I didn’t know, but everyone else had actually been home the whole time. They didn’t come out until then.
I went back inside and tried to get ready for work. I wasn’t planning on talking to the media, but my next door neighbor just walked the news camera crew over to my house and knocked on my door. I wasn’t exactly dressed to be on TV, but they talked to me on camera, and it was on the news later that night.
I went to work and didn’t say anything about it. To be honest, I was trying to avoid telling anyone. Our team had a close-knit bond, and we would often tease each other when we received any type of recognition.
Naturally one of my attendings saw it on the local news and told everybody. So, I got a lot of happy harassment for quite some time. Someone baked me a cake that said, “Hero of Fairmount” (the Philly neighborhood in which I live). Someone else printed out a photo of me that said, “Stop the Bleed Hero of Fairmount,” and put it on every single computer screen.
The man came to see me about 2 weeks later (a neighbor told him where I lived). The man was very tearful and gave me a big hug. We just embraced for a while, and he said how thankful he was. He brought me a bottle of wine, which I thought was really nice.
He told me what happened to him: There was a lot of construction on our street and he was the contractor overseeing a couple of home remodels and demolitions. Sometimes he paid workers in cash and carried it with him. Somebody had tipped off somebody else that he was going to be there that day. The contractor walked into one of the houses and a guy in a ski mask waited there with a gun. The guy shot him and took the cash. The bullet went through his hand into his pelvis.
I had never had to deal with something that intense before outside of work. Most of it really comes down to the basics – the ABCs and bleeding control. You do whatever you can with what you have. In this case, it was just a dish towel, gloves, and my hands to put as much pressure as possible.
It really was strange that I happened to be looking out the window at that moment. I don’t know if it was just a coincidence. The man told me he believed God had put somebody there at the right place at the right time to save his life. I just felt very fortunate to have been able to help him. I never saw him again.
I think something like this gives you a little confidence that you can actually do something and make a meaningful impact anywhere when it’s needed. It lets you know that you’re capable of doing it. You always think about it, but you don’t know until it happens.
A version of this article first appeared on Medscape.com.
It was a quiet day. I got up around 3 o’clock in the afternoon for my shift at 6 p.m. I was shaking off the cobwebs and making coffee at our front window that overlooked Brown Street in North Philadelphia. There was nobody else around so I went outside to see what was going on.
He was in his 50s or 60s, bleeding and obviously in distress. I had him sit down. Then I ran back inside and grabbed a dish towel and some exam gloves that I had in the house.
I ran back out and assessed him. A bullet had gone through one of his hands, but he had other wounds. I had to expose him, so I trauma stripped him on the sidewalk. I got his pants and his shirt off and saw a gunshot going through his lower pelvis. He was bleeding out from there.
I got the towel and started applying deep pressure down into the iliac vein in case they hit something, which I found out later, they had. I held it there. The man was just lying there begging not to die.
I’m someone who is very calm, maybe abnormally calm, as people tell me. I try to use that during my resuscitations and traumas. Just keeping everybody calm makes the situation easier. Afterwards, people asked me, “Weren’t you worried that you were going to get shot?” That does happen in North Philadelphia. But it didn’t even cross my mind.
I didn’t have to think at all about what I was doing. We saw so many gunshots, especially at Einstein Medical Center. We saw them daily. I’d sometimes get more than half a dozen gunshots in one shift.
So, I was holding pressure and some people started to come over. I got somebody to call 911 and asked the man about his medical history. I found out he had diabetes. Five or 10 minutes later, EMS showed up. They looked pretty stunned when I was able to give the handoff presentation to them. I told them what happened and his back-story. I wanted to make sure they would check his sugar and take extra precautions.
They got him on the stretcher, and he eventually made it to the hospital where he had surgery. They had to have a vascular surgeon work on him. I called later, and they told me, “Yeah, he’s alive.” But that’s about the extent of the update I got.
After the ambulance left, it was kind of chaos. All the neighbors poured out of their houses. People were panicked, talking and getting excited about it. I didn’t know, but everyone else had actually been home the whole time. They didn’t come out until then.
I went back inside and tried to get ready for work. I wasn’t planning on talking to the media, but my next door neighbor just walked the news camera crew over to my house and knocked on my door. I wasn’t exactly dressed to be on TV, but they talked to me on camera, and it was on the news later that night.
I went to work and didn’t say anything about it. To be honest, I was trying to avoid telling anyone. Our team had a close-knit bond, and we would often tease each other when we received any type of recognition.
Naturally one of my attendings saw it on the local news and told everybody. So, I got a lot of happy harassment for quite some time. Someone baked me a cake that said, “Hero of Fairmount” (the Philly neighborhood in which I live). Someone else printed out a photo of me that said, “Stop the Bleed Hero of Fairmount,” and put it on every single computer screen.
The man came to see me about 2 weeks later (a neighbor told him where I lived). The man was very tearful and gave me a big hug. We just embraced for a while, and he said how thankful he was. He brought me a bottle of wine, which I thought was really nice.
He told me what happened to him: There was a lot of construction on our street and he was the contractor overseeing a couple of home remodels and demolitions. Sometimes he paid workers in cash and carried it with him. Somebody had tipped off somebody else that he was going to be there that day. The contractor walked into one of the houses and a guy in a ski mask waited there with a gun. The guy shot him and took the cash. The bullet went through his hand into his pelvis.
I had never had to deal with something that intense before outside of work. Most of it really comes down to the basics – the ABCs and bleeding control. You do whatever you can with what you have. In this case, it was just a dish towel, gloves, and my hands to put as much pressure as possible.
It really was strange that I happened to be looking out the window at that moment. I don’t know if it was just a coincidence. The man told me he believed God had put somebody there at the right place at the right time to save his life. I just felt very fortunate to have been able to help him. I never saw him again.
I think something like this gives you a little confidence that you can actually do something and make a meaningful impact anywhere when it’s needed. It lets you know that you’re capable of doing it. You always think about it, but you don’t know until it happens.
A version of this article first appeared on Medscape.com.
Balancing needs and risks as the opioid pendulum swings
Recently, my family had a conversation about the volume of news reports on overdose deaths from the illicit use of opioid drugs—a phenomenon that is complex and stems from many factors. We decided, as a family, that we could have a small impact on the problem. How? By carrying naloxone with us and administering it if we encounter a person with potential opioid overdose. Our decision was made possible by the recent US Food and Drug Administration (FDA) approval of naloxone nasal spray for over-the-counter use.1 At a cost of about $50 for 2 nasal sprays, we decided it would be a reasonable price to pay to potentially save a life.
Prescribing opioids in clinical practice is a different side of the problem. The Centers for Disease Control and Prevention (CDC) reports that prescription opioids account for about one-quarter of opioid overdose deaths.2 This is not trivial, and much effort has gone into addressing how clinicians can do better by their patients. There are training programs and risk-mitigation strategies for opioid prescribing. States have developed prescribing registries to identify patients who receive controlled substances from multiple prescribers, at higher-than-recommended doses, and too early in the pain management process. These efforts have reduced the number of opioid prescriptions and rates of high-dose prescribing (> 90 morphine milligram equivalents). However, that hasn’t translated into a reduction in the number of deaths.2
The article by Posen et al3 in this issue further reminded me how trends in health care, including opioid prescribing, are like a pendulum—swinging from one extreme to the other before eventually centering. I recall conversations with colleagues about how often we undertreated pain—and then later, how relieved we were when new approaches to pain management, using newer opiates, emerged and were reported to be much safer, even for long-term use. We now know the rest of that story: more prescriptions, higher doses, longer duration, addiction, death, and deception by manufacturers.
In our efforts to prevent addiction and decrease opioid deaths, we tried to get patients off opioids completely, thereby increasing demand for addiction therapy, including medication-assisted recovery. This also drove many of our patients to seek opioids from nefarious suppliers, resulting in even more deaths from fentanyl-laced drugs.
At least one positive has arisen from the “no more opioids” movement: We have re-evaluated their true effect on managing pain. Initially, we were told opioids were safe and highly effective—and, having few tools to help our patients, we were Pollyanna-ish in accepting this. But many recent studies have demonstrated that using opioids for pain is no more effective than using other analgesics.4-9 In addition to overdose deaths and addiction, these studies show significantly higher rates of opioid discontinuation due to adverse effects.
We certainly can manage most patients’ pain effectively with other approaches. For some, though—patients whose pain is not adequately controlled and/or interferes with their ability to function, and those who are terminally ill—opioid nihilism has had unintended consequences. Recognizing these issues, the CDC updated its guideline for prescribing opioids in 2022.10 Four areas were addressed: whether to initiate opioids; opioid selection and dosing; duration of therapy and need for follow-up; and assessing risk and addressing potential harms of opioid use. The CDC encourages clinicians to find a balance of the potential benefits and harms and to avoid inflexibility. Finally, the CDC encourages clinicians to identify and treat patients with opioid use disorders.
Clearly, opioid overuse and overdose result from complex medical, economic, and societal factors. Individual clinicians are well equipped to manage things “in their own backyards.” However, what we do can be perceived as a bandage for a much larger problem. Our public health system has the potential for greater impact, but the “cure” will require multimodal solutions addressing many facets of society and government.11 At the very least, we should keep some naloxone close by and vote for political candidates who see broader solutions for addressing this life-and-death crisis.
1. FDA. FDA approves first over-the-counter naloxone nasal spray. Updated March 29, 2023. Accessed April 16, 2023. www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray
2. CDC. Prescription opioid overdose death maps. Updated June 6, 2022. Accessed April 16, 2023. www.cdc.gov/drugoverdose/deaths/prescription/maps.html
3. Posen A, Keller E, Elmes At, et al. Medication-assisted recovery for opioid use disorder: a guide. J Fam Pract. 2023;72:164-171.
4. Fiore JF Jr, El-Kefraoui C, Chay MA, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet. 2022;399:2280-2293. doi: 10.1016/S0140-6736(22)00582-7
5. Moutzouros V, Jildeh TR, Tramer JS, et al. Can we eliminate opioids after anterior cruciate ligament reconstruction? A prospective, randomized controlled trial. Am J Sports Med. 2021;49:3794-3801. doi: 10.1177/03635465211045394
6. Falk J, Thomas B, Kirkwood J, et al. PEER systematic review of randomized controlled trials: management of chronic neuropathic pain in primary care. Can Fam Physician. 2021;67:e130-e140. doi: 10.46747/cfp.6705e130
7. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191. doi: 10.7326/m17-0598
8. Kolber MR, Ton J, Thomas B, et al. PEER systematic review of randomized controlled trials: management of chronic low back pain in primary care. Can Fam Physician. 2021;67:e20-e30. doi: 10.46747/cfp.6701e20
9. O’Brien MDC, Wand APF. A systematic review of the evidence for the efficacy of opioids for chronic non-cancer pain in community-dwelling older adults. Age Ageing. 2020;49:175-183. doi: 10.1093/ageing/afz175
10. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
11. American Academy of Family Physicians. Chronic pain management and opioid misuse: a public health concern (position paper). Accessed April 16, 2023. www.aafp.org/about/policies/all/chronic-pain-management-opiod-misuse.html
Recently, my family had a conversation about the volume of news reports on overdose deaths from the illicit use of opioid drugs—a phenomenon that is complex and stems from many factors. We decided, as a family, that we could have a small impact on the problem. How? By carrying naloxone with us and administering it if we encounter a person with potential opioid overdose. Our decision was made possible by the recent US Food and Drug Administration (FDA) approval of naloxone nasal spray for over-the-counter use.1 At a cost of about $50 for 2 nasal sprays, we decided it would be a reasonable price to pay to potentially save a life.
Prescribing opioids in clinical practice is a different side of the problem. The Centers for Disease Control and Prevention (CDC) reports that prescription opioids account for about one-quarter of opioid overdose deaths.2 This is not trivial, and much effort has gone into addressing how clinicians can do better by their patients. There are training programs and risk-mitigation strategies for opioid prescribing. States have developed prescribing registries to identify patients who receive controlled substances from multiple prescribers, at higher-than-recommended doses, and too early in the pain management process. These efforts have reduced the number of opioid prescriptions and rates of high-dose prescribing (> 90 morphine milligram equivalents). However, that hasn’t translated into a reduction in the number of deaths.2
The article by Posen et al3 in this issue further reminded me how trends in health care, including opioid prescribing, are like a pendulum—swinging from one extreme to the other before eventually centering. I recall conversations with colleagues about how often we undertreated pain—and then later, how relieved we were when new approaches to pain management, using newer opiates, emerged and were reported to be much safer, even for long-term use. We now know the rest of that story: more prescriptions, higher doses, longer duration, addiction, death, and deception by manufacturers.
In our efforts to prevent addiction and decrease opioid deaths, we tried to get patients off opioids completely, thereby increasing demand for addiction therapy, including medication-assisted recovery. This also drove many of our patients to seek opioids from nefarious suppliers, resulting in even more deaths from fentanyl-laced drugs.
At least one positive has arisen from the “no more opioids” movement: We have re-evaluated their true effect on managing pain. Initially, we were told opioids were safe and highly effective—and, having few tools to help our patients, we were Pollyanna-ish in accepting this. But many recent studies have demonstrated that using opioids for pain is no more effective than using other analgesics.4-9 In addition to overdose deaths and addiction, these studies show significantly higher rates of opioid discontinuation due to adverse effects.
We certainly can manage most patients’ pain effectively with other approaches. For some, though—patients whose pain is not adequately controlled and/or interferes with their ability to function, and those who are terminally ill—opioid nihilism has had unintended consequences. Recognizing these issues, the CDC updated its guideline for prescribing opioids in 2022.10 Four areas were addressed: whether to initiate opioids; opioid selection and dosing; duration of therapy and need for follow-up; and assessing risk and addressing potential harms of opioid use. The CDC encourages clinicians to find a balance of the potential benefits and harms and to avoid inflexibility. Finally, the CDC encourages clinicians to identify and treat patients with opioid use disorders.
Clearly, opioid overuse and overdose result from complex medical, economic, and societal factors. Individual clinicians are well equipped to manage things “in their own backyards.” However, what we do can be perceived as a bandage for a much larger problem. Our public health system has the potential for greater impact, but the “cure” will require multimodal solutions addressing many facets of society and government.11 At the very least, we should keep some naloxone close by and vote for political candidates who see broader solutions for addressing this life-and-death crisis.
Recently, my family had a conversation about the volume of news reports on overdose deaths from the illicit use of opioid drugs—a phenomenon that is complex and stems from many factors. We decided, as a family, that we could have a small impact on the problem. How? By carrying naloxone with us and administering it if we encounter a person with potential opioid overdose. Our decision was made possible by the recent US Food and Drug Administration (FDA) approval of naloxone nasal spray for over-the-counter use.1 At a cost of about $50 for 2 nasal sprays, we decided it would be a reasonable price to pay to potentially save a life.
Prescribing opioids in clinical practice is a different side of the problem. The Centers for Disease Control and Prevention (CDC) reports that prescription opioids account for about one-quarter of opioid overdose deaths.2 This is not trivial, and much effort has gone into addressing how clinicians can do better by their patients. There are training programs and risk-mitigation strategies for opioid prescribing. States have developed prescribing registries to identify patients who receive controlled substances from multiple prescribers, at higher-than-recommended doses, and too early in the pain management process. These efforts have reduced the number of opioid prescriptions and rates of high-dose prescribing (> 90 morphine milligram equivalents). However, that hasn’t translated into a reduction in the number of deaths.2
The article by Posen et al3 in this issue further reminded me how trends in health care, including opioid prescribing, are like a pendulum—swinging from one extreme to the other before eventually centering. I recall conversations with colleagues about how often we undertreated pain—and then later, how relieved we were when new approaches to pain management, using newer opiates, emerged and were reported to be much safer, even for long-term use. We now know the rest of that story: more prescriptions, higher doses, longer duration, addiction, death, and deception by manufacturers.
In our efforts to prevent addiction and decrease opioid deaths, we tried to get patients off opioids completely, thereby increasing demand for addiction therapy, including medication-assisted recovery. This also drove many of our patients to seek opioids from nefarious suppliers, resulting in even more deaths from fentanyl-laced drugs.
At least one positive has arisen from the “no more opioids” movement: We have re-evaluated their true effect on managing pain. Initially, we were told opioids were safe and highly effective—and, having few tools to help our patients, we were Pollyanna-ish in accepting this. But many recent studies have demonstrated that using opioids for pain is no more effective than using other analgesics.4-9 In addition to overdose deaths and addiction, these studies show significantly higher rates of opioid discontinuation due to adverse effects.
We certainly can manage most patients’ pain effectively with other approaches. For some, though—patients whose pain is not adequately controlled and/or interferes with their ability to function, and those who are terminally ill—opioid nihilism has had unintended consequences. Recognizing these issues, the CDC updated its guideline for prescribing opioids in 2022.10 Four areas were addressed: whether to initiate opioids; opioid selection and dosing; duration of therapy and need for follow-up; and assessing risk and addressing potential harms of opioid use. The CDC encourages clinicians to find a balance of the potential benefits and harms and to avoid inflexibility. Finally, the CDC encourages clinicians to identify and treat patients with opioid use disorders.
Clearly, opioid overuse and overdose result from complex medical, economic, and societal factors. Individual clinicians are well equipped to manage things “in their own backyards.” However, what we do can be perceived as a bandage for a much larger problem. Our public health system has the potential for greater impact, but the “cure” will require multimodal solutions addressing many facets of society and government.11 At the very least, we should keep some naloxone close by and vote for political candidates who see broader solutions for addressing this life-and-death crisis.
1. FDA. FDA approves first over-the-counter naloxone nasal spray. Updated March 29, 2023. Accessed April 16, 2023. www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray
2. CDC. Prescription opioid overdose death maps. Updated June 6, 2022. Accessed April 16, 2023. www.cdc.gov/drugoverdose/deaths/prescription/maps.html
3. Posen A, Keller E, Elmes At, et al. Medication-assisted recovery for opioid use disorder: a guide. J Fam Pract. 2023;72:164-171.
4. Fiore JF Jr, El-Kefraoui C, Chay MA, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet. 2022;399:2280-2293. doi: 10.1016/S0140-6736(22)00582-7
5. Moutzouros V, Jildeh TR, Tramer JS, et al. Can we eliminate opioids after anterior cruciate ligament reconstruction? A prospective, randomized controlled trial. Am J Sports Med. 2021;49:3794-3801. doi: 10.1177/03635465211045394
6. Falk J, Thomas B, Kirkwood J, et al. PEER systematic review of randomized controlled trials: management of chronic neuropathic pain in primary care. Can Fam Physician. 2021;67:e130-e140. doi: 10.46747/cfp.6705e130
7. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191. doi: 10.7326/m17-0598
8. Kolber MR, Ton J, Thomas B, et al. PEER systematic review of randomized controlled trials: management of chronic low back pain in primary care. Can Fam Physician. 2021;67:e20-e30. doi: 10.46747/cfp.6701e20
9. O’Brien MDC, Wand APF. A systematic review of the evidence for the efficacy of opioids for chronic non-cancer pain in community-dwelling older adults. Age Ageing. 2020;49:175-183. doi: 10.1093/ageing/afz175
10. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
11. American Academy of Family Physicians. Chronic pain management and opioid misuse: a public health concern (position paper). Accessed April 16, 2023. www.aafp.org/about/policies/all/chronic-pain-management-opiod-misuse.html
1. FDA. FDA approves first over-the-counter naloxone nasal spray. Updated March 29, 2023. Accessed April 16, 2023. www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray
2. CDC. Prescription opioid overdose death maps. Updated June 6, 2022. Accessed April 16, 2023. www.cdc.gov/drugoverdose/deaths/prescription/maps.html
3. Posen A, Keller E, Elmes At, et al. Medication-assisted recovery for opioid use disorder: a guide. J Fam Pract. 2023;72:164-171.
4. Fiore JF Jr, El-Kefraoui C, Chay MA, et al. Opioid versus opioid-free analgesia after surgical discharge: a systematic review and meta-analysis of randomised trials. Lancet. 2022;399:2280-2293. doi: 10.1016/S0140-6736(22)00582-7
5. Moutzouros V, Jildeh TR, Tramer JS, et al. Can we eliminate opioids after anterior cruciate ligament reconstruction? A prospective, randomized controlled trial. Am J Sports Med. 2021;49:3794-3801. doi: 10.1177/03635465211045394
6. Falk J, Thomas B, Kirkwood J, et al. PEER systematic review of randomized controlled trials: management of chronic neuropathic pain in primary care. Can Fam Physician. 2021;67:e130-e140. doi: 10.46747/cfp.6705e130
7. Frank JW, Lovejoy TI, Becker WC, et al. Patient outcomes in dose reduction or discontinuation of long-term opioid therapy: a systematic review. Ann Intern Med. 2017;167:181-191. doi: 10.7326/m17-0598
8. Kolber MR, Ton J, Thomas B, et al. PEER systematic review of randomized controlled trials: management of chronic low back pain in primary care. Can Fam Physician. 2021;67:e20-e30. doi: 10.46747/cfp.6701e20
9. O’Brien MDC, Wand APF. A systematic review of the evidence for the efficacy of opioids for chronic non-cancer pain in community-dwelling older adults. Age Ageing. 2020;49:175-183. doi: 10.1093/ageing/afz175
10. Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71:1-95. doi: 10.15585/mmwr.rr7103a1
11. American Academy of Family Physicians. Chronic pain management and opioid misuse: a public health concern (position paper). Accessed April 16, 2023. www.aafp.org/about/policies/all/chronic-pain-management-opiod-misuse.html
Evolve your website
The past few years have seen major transformations in the way health care websites operate and interact with patients. .
In mid-2018, a major Google algorithm change, known to the IT community as the “Medic Update,” significantly changed search criteria for most health and wellness websites. Another big update went live in late 2021. Websites that have not evolved with these changes have dropped in search rankings and provide a poorer user experience all around.
Many potential patients are searching for your services online, so your website cannot be an afterthought. Not only does it need to be designed with your target audience in mind, but it is also important to consider the metrics Google and other search engines now use when assessing the quality of your website so that patients will find it in the first place.
Here are some features that you (or your website company) need to prioritize to keep your site current and atop search results in 2023 and beyond.
Begin with an understandable URL. Search engines use URLs to determine how well your site, or a portion of it, matches search criteria. URLs also need to make sense to searchers, especially when they link specific areas of expertise (more on that in a minute). For example, a URL like “jonesdermatology.com/?p=89021” is meaningless to anyone except programmers; but “jonesdermatology.com/psoriasistreatments” obviously leads to a page about psoriasis treatments. Search engines look for not only the most relevant, but also the most helpful and user-friendly answers to a user’s query.
Incidentally, if the URL for your site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
Continue with a good meta description. That’s the grayish text that follows the title and URL in search results. Searchers will read it to confirm that your site is what they seek, so make sure it describes exactly what you do, including any areas of special expertise.
Make your practice approachable with photos. New patients are more comfortable when they know what you look like, so real photos of you and your staff are always more effective than stock photos of models. Photos or a video tour of your office will reassure prospective patients that they will be visiting a clean, modern, professional facility.
Describe your principal services in detail. You never know which specific service a prospective patient is searching for, so describe everything you offer. Don’t try to summarize everything on a single page; relevance is determined by how deeply a topic is covered, so each principal service should have a detailed description on its own page. Not only will your skills become more visible to search engines, but you can also use the space to enumerate your qualifications and expertise in each area. Whenever possible, write your descriptions in question-and-answer form. Searchers tend to ask questions (“what is the best ... ?”), particularly in voice searches. Search engines increasingly value sites that ask and answer common questions.
Make your site interactive. “Interactivity” is a major buzzword in modern search engine parlance. Once searchers make an appointment, they stop searching. If they have to wait until the next day to call your office, they may keep looking – and might find a competitor with online scheduling. HIPAA-compliant chatbots, secure messaging, and online patient portals to access medical records, lab results, and other important information will also set your site apart.
Testimonials are essential. Amazon.com taught us that candid reviews from customers go a long way toward building the trust necessary to buy products and services, and nowhere is that truer than for medical services. According to one study, when it comes to finding a doctor, 88% of people trust online reviews as much as a personal recommendation. Loyal patients will be happy to write you glowing reviews; feature them prominently.
How does your site look on small screens? More than half of all searches are now made on smartphones, so the more mobile-friendly your site is, the higher it will be ranked. Prospective patients who are forced to scroll forever, or zoom in to tap a link, are likely to become frustrated and move on. Mobile searchers prefer sites that provide the best experience for the least amount of effort, and rankings tend to reflect that preference. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test..
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
The past few years have seen major transformations in the way health care websites operate and interact with patients. .
In mid-2018, a major Google algorithm change, known to the IT community as the “Medic Update,” significantly changed search criteria for most health and wellness websites. Another big update went live in late 2021. Websites that have not evolved with these changes have dropped in search rankings and provide a poorer user experience all around.
Many potential patients are searching for your services online, so your website cannot be an afterthought. Not only does it need to be designed with your target audience in mind, but it is also important to consider the metrics Google and other search engines now use when assessing the quality of your website so that patients will find it in the first place.
Here are some features that you (or your website company) need to prioritize to keep your site current and atop search results in 2023 and beyond.
Begin with an understandable URL. Search engines use URLs to determine how well your site, or a portion of it, matches search criteria. URLs also need to make sense to searchers, especially when they link specific areas of expertise (more on that in a minute). For example, a URL like “jonesdermatology.com/?p=89021” is meaningless to anyone except programmers; but “jonesdermatology.com/psoriasistreatments” obviously leads to a page about psoriasis treatments. Search engines look for not only the most relevant, but also the most helpful and user-friendly answers to a user’s query.
Incidentally, if the URL for your site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
Continue with a good meta description. That’s the grayish text that follows the title and URL in search results. Searchers will read it to confirm that your site is what they seek, so make sure it describes exactly what you do, including any areas of special expertise.
Make your practice approachable with photos. New patients are more comfortable when they know what you look like, so real photos of you and your staff are always more effective than stock photos of models. Photos or a video tour of your office will reassure prospective patients that they will be visiting a clean, modern, professional facility.
Describe your principal services in detail. You never know which specific service a prospective patient is searching for, so describe everything you offer. Don’t try to summarize everything on a single page; relevance is determined by how deeply a topic is covered, so each principal service should have a detailed description on its own page. Not only will your skills become more visible to search engines, but you can also use the space to enumerate your qualifications and expertise in each area. Whenever possible, write your descriptions in question-and-answer form. Searchers tend to ask questions (“what is the best ... ?”), particularly in voice searches. Search engines increasingly value sites that ask and answer common questions.
Make your site interactive. “Interactivity” is a major buzzword in modern search engine parlance. Once searchers make an appointment, they stop searching. If they have to wait until the next day to call your office, they may keep looking – and might find a competitor with online scheduling. HIPAA-compliant chatbots, secure messaging, and online patient portals to access medical records, lab results, and other important information will also set your site apart.
Testimonials are essential. Amazon.com taught us that candid reviews from customers go a long way toward building the trust necessary to buy products and services, and nowhere is that truer than for medical services. According to one study, when it comes to finding a doctor, 88% of people trust online reviews as much as a personal recommendation. Loyal patients will be happy to write you glowing reviews; feature them prominently.
How does your site look on small screens? More than half of all searches are now made on smartphones, so the more mobile-friendly your site is, the higher it will be ranked. Prospective patients who are forced to scroll forever, or zoom in to tap a link, are likely to become frustrated and move on. Mobile searchers prefer sites that provide the best experience for the least amount of effort, and rankings tend to reflect that preference. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test..
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
The past few years have seen major transformations in the way health care websites operate and interact with patients. .
In mid-2018, a major Google algorithm change, known to the IT community as the “Medic Update,” significantly changed search criteria for most health and wellness websites. Another big update went live in late 2021. Websites that have not evolved with these changes have dropped in search rankings and provide a poorer user experience all around.
Many potential patients are searching for your services online, so your website cannot be an afterthought. Not only does it need to be designed with your target audience in mind, but it is also important to consider the metrics Google and other search engines now use when assessing the quality of your website so that patients will find it in the first place.
Here are some features that you (or your website company) need to prioritize to keep your site current and atop search results in 2023 and beyond.
Begin with an understandable URL. Search engines use URLs to determine how well your site, or a portion of it, matches search criteria. URLs also need to make sense to searchers, especially when they link specific areas of expertise (more on that in a minute). For example, a URL like “jonesdermatology.com/?p=89021” is meaningless to anyone except programmers; but “jonesdermatology.com/psoriasistreatments” obviously leads to a page about psoriasis treatments. Search engines look for not only the most relevant, but also the most helpful and user-friendly answers to a user’s query.
Incidentally, if the URL for your site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
Continue with a good meta description. That’s the grayish text that follows the title and URL in search results. Searchers will read it to confirm that your site is what they seek, so make sure it describes exactly what you do, including any areas of special expertise.
Make your practice approachable with photos. New patients are more comfortable when they know what you look like, so real photos of you and your staff are always more effective than stock photos of models. Photos or a video tour of your office will reassure prospective patients that they will be visiting a clean, modern, professional facility.
Describe your principal services in detail. You never know which specific service a prospective patient is searching for, so describe everything you offer. Don’t try to summarize everything on a single page; relevance is determined by how deeply a topic is covered, so each principal service should have a detailed description on its own page. Not only will your skills become more visible to search engines, but you can also use the space to enumerate your qualifications and expertise in each area. Whenever possible, write your descriptions in question-and-answer form. Searchers tend to ask questions (“what is the best ... ?”), particularly in voice searches. Search engines increasingly value sites that ask and answer common questions.
Make your site interactive. “Interactivity” is a major buzzword in modern search engine parlance. Once searchers make an appointment, they stop searching. If they have to wait until the next day to call your office, they may keep looking – and might find a competitor with online scheduling. HIPAA-compliant chatbots, secure messaging, and online patient portals to access medical records, lab results, and other important information will also set your site apart.
Testimonials are essential. Amazon.com taught us that candid reviews from customers go a long way toward building the trust necessary to buy products and services, and nowhere is that truer than for medical services. According to one study, when it comes to finding a doctor, 88% of people trust online reviews as much as a personal recommendation. Loyal patients will be happy to write you glowing reviews; feature them prominently.
How does your site look on small screens? More than half of all searches are now made on smartphones, so the more mobile-friendly your site is, the higher it will be ranked. Prospective patients who are forced to scroll forever, or zoom in to tap a link, are likely to become frustrated and move on. Mobile searchers prefer sites that provide the best experience for the least amount of effort, and rankings tend to reflect that preference. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test..
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].