User login
Outcomes in Patients With Curative Malignancies Receiving Filgrastim as Primary Prophylaxis
Febrile neutropenia (FN) frequently occurs in patients receiving chemotherapy, with the greatest risk of complications occurring in those who experience profound and prolonged neutropenia. Although granulocyte colony-stimulating factor (G-CSF) prophylaxis has been shown to reduce the risk and duration of chemotherapy-induced neutropenia and FN, there is no well-established optimal regimen.1 The 2022 National Comprehensive Cancer Network guidelines for hematopoietic growth factors recommend prophylaxis with G-CSF in at-risk patients receiving chemotherapy, specifically in chemotherapy regimens considered high risk for FN (incidence > 20%) or intermediate risk for FN (incidence 10%-20%) with additional patient risk factors.2 The incidence of developing FN with at least 1 chemotherapy cycle is estimated at 10% to 50% of patients with solid tumors and > 80% of patients with hematologic malignancies.3 The rate of major complications (eg, hypotension, acute renal, respiratory, or heart failure) in the context of FN is 25% to 30%, and mortality is reported up to 11% in this population.4
Because of the significant consequences of neutropenia and FN, prevention is imperative due to the increase in morbidity and mortality, including chemotherapy delays, increased hospitalizations, chemotherapy dose reductions, and discontinuations that cause delays in care.5 In patients with curative malignancies, these consequences can negatively impact treatment efficacy and overall survival. Additionally, infections occur in 20% to 30% of patients with febrile episodes. Although fever is often the only clinical sign or symptom of infection, patients who are profoundly neutropenic may present with suspected infection and be afebrile or hypothermic.3
For filgrastim, the National Comprehensive Cancer Network guidelines do not specify the total days of required injections but state that a daily dose should be given until the postnadir absolute neutrophil count (ANC) recovers to normal or near normal levels by laboratory standards.2 It is uncommon in clinical practice to track postnadir ANCs due to frequent laboratory monitoring. Clinical trial data suggest an average duration of 11 days of daily filgrastim injections for ANC recovery; however, real-world data exist supporting a range from 4 to 10 days with a median of 7 injections per cycle for prevention of neutropenia or FN.6,7
At the South Texas Veterans Health Care System in San Antonio, daily filgrastim injections are preferred due to cost; patients typically receive a 7-day course for primary prophylaxis for FN.
Electronic health record reviews at the South Texas Veterans Health Care System were performed to identify patients who received filgrastim primary prophylaxis (defined as filgrastim, tbo-filgrastim, or filgrastim-sndz) for a curative cancer diagnosis. Primary prophylaxis refers to the administration of G-CSF in the first cycle of chemotherapy before the onset of neutropenia. Patients received filgrastim prophylaxis if they were undergoing treatment with a chemotherapy regimen with either high risk for FN or a chemotherapy regimen with an intermediate risk for FN and additional patient risk factors. Risk factors for patients included prior chemotherapy or radiation therapy; persistent neutropenia; bone marrow involvement by tumor; recent surgery and/or open wounds; liver dysfunction (defined as total bilirubin > 2 mg/dL); renal dysfunction (defined as creatinine clearance < 50 mL/min); and those aged > 65 years receiving full chemotherapy dose intensity. Neutropenia is defined as a decrease in ANC < 1000 neutrophils/μL, whereas FN is defined as a single temperature of > 38.3 °C or > 38.0 °C for longer than 1 hour with < 500 neutrophils/μL or < 1000 neutrophils/μL predicted to decline to < 500 neutrophils/μL over the next 48 hours. All patients had their filgrastim dispensed for home administration during their chemotherapy appointment.
Descriptive statistics were used to summarize the study population and their health outcomes. Fisher exact test was used to compare FN incidence for high- and intermediate-risk FN groups.
RESULTS
Between September 1, 2015, and September 24, 2020, 381 patients received filgrastim. Of these patients, 59 met the inclusion criteria. Patients receiving filgrastim were excluded due to stem cell transplant mobilization/engraftment (n = 145), a noncurative cancer diagnosis (n = 134), use as a secondary prophylaxis (n = 33), and nononcologic neutropenia (n = 8). Additionally, 2 patients initially received pegfilgrastim and were not included in this data set.
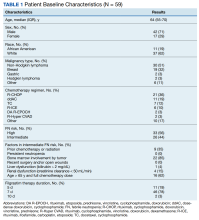
The median (IQR) age was 64 (55-70) years and 42 patients (71%) were male (Table 1).
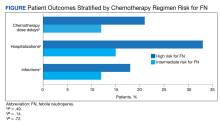
Ten patients (17%) experienced dose delays despite filgrastim use (Table 2).
Nine patients (15%) had the number of filgrastim injections per chemotherapy cycle extended due to various reasons. Five patients required extended days after hospitalization for FN, 3 patients for dose delays due to neutropenia with the previous cycle, and 1 patient with an undocumented reason outside of the prespecified outcomes. Two of these patients experienced continued neutropenia and dose delays after extending filgrastim from 5 to 7 days or 7 to 10 days. One patient who experienced continued neutropenia after extending filgrastim to 10 days was subsequently transitioned to pegfilgrastim without further episodes of neutropenia. The other patient who still experienced neutropenia after extending filgrastim to 7 days was receiving the last chemotherapy cycle and did not require subsequent doses of filgrastim.
Two additional patients were not included in the hospitalizations. The first was a patient on a chemotherapy regimen with a high risk for FN who presented to the emergency department with documented FN but was never admitted since the patient elected to not be hospitalized. This patient developed oral, anal, and vaginal candidiasis, and it was noted by the oncologist at the next clinic visit that this was likely secondary to grade 4 neutropenia (ANC < 500 neutrophils/μL). The second was a patient on a chemotherapy regimen with an intermediate risk for FN who was already hospitalized but had developed FN and sepsis despite filgrastim use.
Finally, out of the hospitalized patients, 9 (15%) had infections. This included 6 patients (18%) in the high risk for FN group and 3 patients (12%) in the intermediate risk for FN group (P = .72). Six patients transitioned to pegfilgrastim for hospitalization, 2 for neutropenia, and 1 for an unspecified reason. Nine patients (15%) who received filgrastim ended up transitioning to pegfilgrastim; 6 (67%) of these patients were transitioned due to hospitalization for FN. Of all the patients who transitioned to pegfilgrastim, 1 patient on a high risk for FN regimen developed sepsis due to herpes zoster in the setting of neutropenia after the previous cycle of chemotherapy.
Real-world data are limited regarding G-CSF practice patterns; however, available data demonstrate patients may receive suboptimal treatment courses of filgrastim leading to increased complications associated with neutropenia and FN, such as dose delays and hospitalizations.8,9 At the South Texas Veterans Health Care System, 48 patients (81%) received a filgrastim course of ≥ 7 days as an initial course for primary prophylaxis. Multivariate analyses performed by Weycker and colleagues described a decreased risk of hospitalization for neutropenia or FN with each additional day of filgrastim prophylaxis; however, such analysis could not be performed in our data set due to the small sample size.8 In this review, 10 patients (17%) experienced treatment delays due to neutropenia or FN, mirroring previously published data. The hospitalization rate of 25% is higher than the published incidence of 5.2% of cancer-related hospitalizations among adults.7,10 This difference may be explained by a difference in health care access for the veteran population.
As an alternative to daily filgrastim injections, the National Comprehensive Cancer Network also recommends a single dose of pegfilgrastim for primary prevention of FN. Efficacy benefits of pegfilgrastim use include increased patient adherence due to a single injection, a reduction in FN incidence and FN-related hospitalizations, and improved time to ANC recovery compared with filgrastim.11 There are reports suggesting pegfilgrastim significantly reduces neutropenia and FN incidence to a greater extent compared with daily filgrastim injections.6 In patients with breast cancer receiving dose-dense adjuvant chemotherapy, there are data demonstrating that patients who received filgrastim were more likely to experience severe neutropenia, dose reductions, and treatment delays leading to lower dose density compared with pegfilgrastim.12 Of the 19 patients with breast cancer included in our population, 26% experienced one of the previously described outcomes leading to either extensions of daily filgrastim injections or transitions to pegfilgrastim to successfully maintain dose density. In patients with acute myeloid leukemia receiving consolidation chemotherapy, filgrastim was found to be associated with a statistically significant increased risk of hospitalizations compared with pegfilgrastim.13 The one patient with acute myeloid leukemia included in our study did not require additional hospitalizations for neutropenia or FN after transitioning to pegfilgrastim.
Given the cost advantage, the South Texas Veterans Health Care System continues to prefer daily filgrastim injections. A recent survey demonstrated that 73% of patients at 23 sites in the Veterans Health Administration used filgrastim rather than pegfilgrastim for cost savings, although it is recognized that daily filgrastim injections are less convenient for patients.14 This analysis did not review costs associated with hospitalization for FN or the appropriateness of G-CSF use. Cancer-related neutropenia accounts for 8.3% of all cancer-related hospitalization costs among adults; the average hospitalization costs nearly $25,000 per stay and about $2.3 billion among adult patients with cancer annually.10,15
Limitations
This study has limitations that affected the applicability and interpretation of the results. This included the study design since it was a retrospective, single-center, descriptive cohort study. Patient adherence to daily filgrastim injections could not be assessed due to the retrospective nature of the study. The small sample size of 59 patients was prohibitive for utilization of additional analytical tools. Additionally, the predominately male veteran population may make applicability to non-VA populations restrictive.
CONCLUSIONS
Based on the incidence of primary and secondary outcomes associated with using daily filgrastim injections as primary prophylaxis in this study, additional measures such as tracking postnadir ANCs should be performed to ensure patients receive an appropriate number of filgrastim doses to prevent complications associated with neutropenia.
Acknowledgments
We thank Eric Dougherty, PharmD, for assistance in producing granulocyte colony-stimulating factor data.
1. Hanna KS, Mancini R, Wilson D, Zuckerman D. Comparing granulocyte colony-stimulating factors prescribing practices versus guideline recommendations in a large community cancer center. J Hematol Oncol Pharm. 2019;9(3):121-126.
2. Griffiths EA, Roy V, Alwan L, et al. NCCN Guidelines insights: hematopoietic growth factors, version 1.2022. J Natl Compr Canc Netw. 2022;20(5):436-442. doi:10.6004/jnccn.2022.0026
3. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93. doi:10.1093/cid/cir073
4. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-1453. doi:10.1200/JCO.2017.77.6211
5. Clemons M, Fergusson D, Simos D, et al. A multicentre, randomized trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy induced febrile neutropenia in early stage breast cancer. Ann Oncol. 2020;31(7):951-957. doi:10.1016/j.annonc.2020.04.005
6. Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404. Published 2011 Sep 23. doi:10.1186/1471-2407-11-404
7. Altwairgi A, Hopman W, Mates M. Real-world impact of granulocyte-colony stimulating factor on febrile neutropenia. Curr Oncol. 2013;20(3):e171-e179. doi:10.3747/co.20.1306
8. Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402-407. doi:10.1345/aph.1G516
9. Link H, Nietsch J, Kerkmann M, Ortner P; Supportive Care Group (ASORS) of the German Cancer Society (DKG). Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy—a representative sample survey in Germany. Support Care Cancer. 2016;24(1):367-376. doi:10.1007/s00520-015-2779-5
10. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266. doi:10.1002/cncr.21847
11. Aapro M, Boccia R, Leonard R, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. 2017;25(11):3295-3304. doi :10.1007/s00520-017-3842-1
12. Kourlaba G, Dimopoulos MA, Pectasides D, et al. Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer. 2015;23(7):2045-2051. doi:10.1007/s00520-014-2555-y
13. Field E, Caimi PF, Cooper B, et al. Comparison of pegfilgrastim and filgrastim to prevent neutropenic fever during consolidation with high dose cytarabine for acute myeloid leukemia. Blood. 2018;132(suppl 1):1404. doi:10.1182/blood-2018-99-118336
14. Knopf K, Hrureshky W, Love BL, Norris L, Bennett CL. Cost-effective use of white blood cell growth factors in the Veterans Administration. Blood. 2018;132(suppl 1):4761. doi:10.1182/blood-2018-99-119724
15. Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552-e561. doi:10.1200/JOP.2016.019588
Febrile neutropenia (FN) frequently occurs in patients receiving chemotherapy, with the greatest risk of complications occurring in those who experience profound and prolonged neutropenia. Although granulocyte colony-stimulating factor (G-CSF) prophylaxis has been shown to reduce the risk and duration of chemotherapy-induced neutropenia and FN, there is no well-established optimal regimen.1 The 2022 National Comprehensive Cancer Network guidelines for hematopoietic growth factors recommend prophylaxis with G-CSF in at-risk patients receiving chemotherapy, specifically in chemotherapy regimens considered high risk for FN (incidence > 20%) or intermediate risk for FN (incidence 10%-20%) with additional patient risk factors.2 The incidence of developing FN with at least 1 chemotherapy cycle is estimated at 10% to 50% of patients with solid tumors and > 80% of patients with hematologic malignancies.3 The rate of major complications (eg, hypotension, acute renal, respiratory, or heart failure) in the context of FN is 25% to 30%, and mortality is reported up to 11% in this population.4
Because of the significant consequences of neutropenia and FN, prevention is imperative due to the increase in morbidity and mortality, including chemotherapy delays, increased hospitalizations, chemotherapy dose reductions, and discontinuations that cause delays in care.5 In patients with curative malignancies, these consequences can negatively impact treatment efficacy and overall survival. Additionally, infections occur in 20% to 30% of patients with febrile episodes. Although fever is often the only clinical sign or symptom of infection, patients who are profoundly neutropenic may present with suspected infection and be afebrile or hypothermic.3
For filgrastim, the National Comprehensive Cancer Network guidelines do not specify the total days of required injections but state that a daily dose should be given until the postnadir absolute neutrophil count (ANC) recovers to normal or near normal levels by laboratory standards.2 It is uncommon in clinical practice to track postnadir ANCs due to frequent laboratory monitoring. Clinical trial data suggest an average duration of 11 days of daily filgrastim injections for ANC recovery; however, real-world data exist supporting a range from 4 to 10 days with a median of 7 injections per cycle for prevention of neutropenia or FN.6,7
At the South Texas Veterans Health Care System in San Antonio, daily filgrastim injections are preferred due to cost; patients typically receive a 7-day course for primary prophylaxis for FN.
Electronic health record reviews at the South Texas Veterans Health Care System were performed to identify patients who received filgrastim primary prophylaxis (defined as filgrastim, tbo-filgrastim, or filgrastim-sndz) for a curative cancer diagnosis. Primary prophylaxis refers to the administration of G-CSF in the first cycle of chemotherapy before the onset of neutropenia. Patients received filgrastim prophylaxis if they were undergoing treatment with a chemotherapy regimen with either high risk for FN or a chemotherapy regimen with an intermediate risk for FN and additional patient risk factors. Risk factors for patients included prior chemotherapy or radiation therapy; persistent neutropenia; bone marrow involvement by tumor; recent surgery and/or open wounds; liver dysfunction (defined as total bilirubin > 2 mg/dL); renal dysfunction (defined as creatinine clearance < 50 mL/min); and those aged > 65 years receiving full chemotherapy dose intensity. Neutropenia is defined as a decrease in ANC < 1000 neutrophils/μL, whereas FN is defined as a single temperature of > 38.3 °C or > 38.0 °C for longer than 1 hour with < 500 neutrophils/μL or < 1000 neutrophils/μL predicted to decline to < 500 neutrophils/μL over the next 48 hours. All patients had their filgrastim dispensed for home administration during their chemotherapy appointment.
Descriptive statistics were used to summarize the study population and their health outcomes. Fisher exact test was used to compare FN incidence for high- and intermediate-risk FN groups.
RESULTS
Between September 1, 2015, and September 24, 2020, 381 patients received filgrastim. Of these patients, 59 met the inclusion criteria. Patients receiving filgrastim were excluded due to stem cell transplant mobilization/engraftment (n = 145), a noncurative cancer diagnosis (n = 134), use as a secondary prophylaxis (n = 33), and nononcologic neutropenia (n = 8). Additionally, 2 patients initially received pegfilgrastim and were not included in this data set.
The median (IQR) age was 64 (55-70) years and 42 patients (71%) were male (Table 1).
Ten patients (17%) experienced dose delays despite filgrastim use (Table 2).
Nine patients (15%) had the number of filgrastim injections per chemotherapy cycle extended due to various reasons. Five patients required extended days after hospitalization for FN, 3 patients for dose delays due to neutropenia with the previous cycle, and 1 patient with an undocumented reason outside of the prespecified outcomes. Two of these patients experienced continued neutropenia and dose delays after extending filgrastim from 5 to 7 days or 7 to 10 days. One patient who experienced continued neutropenia after extending filgrastim to 10 days was subsequently transitioned to pegfilgrastim without further episodes of neutropenia. The other patient who still experienced neutropenia after extending filgrastim to 7 days was receiving the last chemotherapy cycle and did not require subsequent doses of filgrastim.
Two additional patients were not included in the hospitalizations. The first was a patient on a chemotherapy regimen with a high risk for FN who presented to the emergency department with documented FN but was never admitted since the patient elected to not be hospitalized. This patient developed oral, anal, and vaginal candidiasis, and it was noted by the oncologist at the next clinic visit that this was likely secondary to grade 4 neutropenia (ANC < 500 neutrophils/μL). The second was a patient on a chemotherapy regimen with an intermediate risk for FN who was already hospitalized but had developed FN and sepsis despite filgrastim use.
Finally, out of the hospitalized patients, 9 (15%) had infections. This included 6 patients (18%) in the high risk for FN group and 3 patients (12%) in the intermediate risk for FN group (P = .72). Six patients transitioned to pegfilgrastim for hospitalization, 2 for neutropenia, and 1 for an unspecified reason. Nine patients (15%) who received filgrastim ended up transitioning to pegfilgrastim; 6 (67%) of these patients were transitioned due to hospitalization for FN. Of all the patients who transitioned to pegfilgrastim, 1 patient on a high risk for FN regimen developed sepsis due to herpes zoster in the setting of neutropenia after the previous cycle of chemotherapy.
Real-world data are limited regarding G-CSF practice patterns; however, available data demonstrate patients may receive suboptimal treatment courses of filgrastim leading to increased complications associated with neutropenia and FN, such as dose delays and hospitalizations.8,9 At the South Texas Veterans Health Care System, 48 patients (81%) received a filgrastim course of ≥ 7 days as an initial course for primary prophylaxis. Multivariate analyses performed by Weycker and colleagues described a decreased risk of hospitalization for neutropenia or FN with each additional day of filgrastim prophylaxis; however, such analysis could not be performed in our data set due to the small sample size.8 In this review, 10 patients (17%) experienced treatment delays due to neutropenia or FN, mirroring previously published data. The hospitalization rate of 25% is higher than the published incidence of 5.2% of cancer-related hospitalizations among adults.7,10 This difference may be explained by a difference in health care access for the veteran population.
As an alternative to daily filgrastim injections, the National Comprehensive Cancer Network also recommends a single dose of pegfilgrastim for primary prevention of FN. Efficacy benefits of pegfilgrastim use include increased patient adherence due to a single injection, a reduction in FN incidence and FN-related hospitalizations, and improved time to ANC recovery compared with filgrastim.11 There are reports suggesting pegfilgrastim significantly reduces neutropenia and FN incidence to a greater extent compared with daily filgrastim injections.6 In patients with breast cancer receiving dose-dense adjuvant chemotherapy, there are data demonstrating that patients who received filgrastim were more likely to experience severe neutropenia, dose reductions, and treatment delays leading to lower dose density compared with pegfilgrastim.12 Of the 19 patients with breast cancer included in our population, 26% experienced one of the previously described outcomes leading to either extensions of daily filgrastim injections or transitions to pegfilgrastim to successfully maintain dose density. In patients with acute myeloid leukemia receiving consolidation chemotherapy, filgrastim was found to be associated with a statistically significant increased risk of hospitalizations compared with pegfilgrastim.13 The one patient with acute myeloid leukemia included in our study did not require additional hospitalizations for neutropenia or FN after transitioning to pegfilgrastim.
Given the cost advantage, the South Texas Veterans Health Care System continues to prefer daily filgrastim injections. A recent survey demonstrated that 73% of patients at 23 sites in the Veterans Health Administration used filgrastim rather than pegfilgrastim for cost savings, although it is recognized that daily filgrastim injections are less convenient for patients.14 This analysis did not review costs associated with hospitalization for FN or the appropriateness of G-CSF use. Cancer-related neutropenia accounts for 8.3% of all cancer-related hospitalization costs among adults; the average hospitalization costs nearly $25,000 per stay and about $2.3 billion among adult patients with cancer annually.10,15
Limitations
This study has limitations that affected the applicability and interpretation of the results. This included the study design since it was a retrospective, single-center, descriptive cohort study. Patient adherence to daily filgrastim injections could not be assessed due to the retrospective nature of the study. The small sample size of 59 patients was prohibitive for utilization of additional analytical tools. Additionally, the predominately male veteran population may make applicability to non-VA populations restrictive.
CONCLUSIONS
Based on the incidence of primary and secondary outcomes associated with using daily filgrastim injections as primary prophylaxis in this study, additional measures such as tracking postnadir ANCs should be performed to ensure patients receive an appropriate number of filgrastim doses to prevent complications associated with neutropenia.
Acknowledgments
We thank Eric Dougherty, PharmD, for assistance in producing granulocyte colony-stimulating factor data.
Febrile neutropenia (FN) frequently occurs in patients receiving chemotherapy, with the greatest risk of complications occurring in those who experience profound and prolonged neutropenia. Although granulocyte colony-stimulating factor (G-CSF) prophylaxis has been shown to reduce the risk and duration of chemotherapy-induced neutropenia and FN, there is no well-established optimal regimen.1 The 2022 National Comprehensive Cancer Network guidelines for hematopoietic growth factors recommend prophylaxis with G-CSF in at-risk patients receiving chemotherapy, specifically in chemotherapy regimens considered high risk for FN (incidence > 20%) or intermediate risk for FN (incidence 10%-20%) with additional patient risk factors.2 The incidence of developing FN with at least 1 chemotherapy cycle is estimated at 10% to 50% of patients with solid tumors and > 80% of patients with hematologic malignancies.3 The rate of major complications (eg, hypotension, acute renal, respiratory, or heart failure) in the context of FN is 25% to 30%, and mortality is reported up to 11% in this population.4
Because of the significant consequences of neutropenia and FN, prevention is imperative due to the increase in morbidity and mortality, including chemotherapy delays, increased hospitalizations, chemotherapy dose reductions, and discontinuations that cause delays in care.5 In patients with curative malignancies, these consequences can negatively impact treatment efficacy and overall survival. Additionally, infections occur in 20% to 30% of patients with febrile episodes. Although fever is often the only clinical sign or symptom of infection, patients who are profoundly neutropenic may present with suspected infection and be afebrile or hypothermic.3
For filgrastim, the National Comprehensive Cancer Network guidelines do not specify the total days of required injections but state that a daily dose should be given until the postnadir absolute neutrophil count (ANC) recovers to normal or near normal levels by laboratory standards.2 It is uncommon in clinical practice to track postnadir ANCs due to frequent laboratory monitoring. Clinical trial data suggest an average duration of 11 days of daily filgrastim injections for ANC recovery; however, real-world data exist supporting a range from 4 to 10 days with a median of 7 injections per cycle for prevention of neutropenia or FN.6,7
At the South Texas Veterans Health Care System in San Antonio, daily filgrastim injections are preferred due to cost; patients typically receive a 7-day course for primary prophylaxis for FN.
Electronic health record reviews at the South Texas Veterans Health Care System were performed to identify patients who received filgrastim primary prophylaxis (defined as filgrastim, tbo-filgrastim, or filgrastim-sndz) for a curative cancer diagnosis. Primary prophylaxis refers to the administration of G-CSF in the first cycle of chemotherapy before the onset of neutropenia. Patients received filgrastim prophylaxis if they were undergoing treatment with a chemotherapy regimen with either high risk for FN or a chemotherapy regimen with an intermediate risk for FN and additional patient risk factors. Risk factors for patients included prior chemotherapy or radiation therapy; persistent neutropenia; bone marrow involvement by tumor; recent surgery and/or open wounds; liver dysfunction (defined as total bilirubin > 2 mg/dL); renal dysfunction (defined as creatinine clearance < 50 mL/min); and those aged > 65 years receiving full chemotherapy dose intensity. Neutropenia is defined as a decrease in ANC < 1000 neutrophils/μL, whereas FN is defined as a single temperature of > 38.3 °C or > 38.0 °C for longer than 1 hour with < 500 neutrophils/μL or < 1000 neutrophils/μL predicted to decline to < 500 neutrophils/μL over the next 48 hours. All patients had their filgrastim dispensed for home administration during their chemotherapy appointment.
Descriptive statistics were used to summarize the study population and their health outcomes. Fisher exact test was used to compare FN incidence for high- and intermediate-risk FN groups.
RESULTS
Between September 1, 2015, and September 24, 2020, 381 patients received filgrastim. Of these patients, 59 met the inclusion criteria. Patients receiving filgrastim were excluded due to stem cell transplant mobilization/engraftment (n = 145), a noncurative cancer diagnosis (n = 134), use as a secondary prophylaxis (n = 33), and nononcologic neutropenia (n = 8). Additionally, 2 patients initially received pegfilgrastim and were not included in this data set.
The median (IQR) age was 64 (55-70) years and 42 patients (71%) were male (Table 1).
Ten patients (17%) experienced dose delays despite filgrastim use (Table 2).
Nine patients (15%) had the number of filgrastim injections per chemotherapy cycle extended due to various reasons. Five patients required extended days after hospitalization for FN, 3 patients for dose delays due to neutropenia with the previous cycle, and 1 patient with an undocumented reason outside of the prespecified outcomes. Two of these patients experienced continued neutropenia and dose delays after extending filgrastim from 5 to 7 days or 7 to 10 days. One patient who experienced continued neutropenia after extending filgrastim to 10 days was subsequently transitioned to pegfilgrastim without further episodes of neutropenia. The other patient who still experienced neutropenia after extending filgrastim to 7 days was receiving the last chemotherapy cycle and did not require subsequent doses of filgrastim.
Two additional patients were not included in the hospitalizations. The first was a patient on a chemotherapy regimen with a high risk for FN who presented to the emergency department with documented FN but was never admitted since the patient elected to not be hospitalized. This patient developed oral, anal, and vaginal candidiasis, and it was noted by the oncologist at the next clinic visit that this was likely secondary to grade 4 neutropenia (ANC < 500 neutrophils/μL). The second was a patient on a chemotherapy regimen with an intermediate risk for FN who was already hospitalized but had developed FN and sepsis despite filgrastim use.
Finally, out of the hospitalized patients, 9 (15%) had infections. This included 6 patients (18%) in the high risk for FN group and 3 patients (12%) in the intermediate risk for FN group (P = .72). Six patients transitioned to pegfilgrastim for hospitalization, 2 for neutropenia, and 1 for an unspecified reason. Nine patients (15%) who received filgrastim ended up transitioning to pegfilgrastim; 6 (67%) of these patients were transitioned due to hospitalization for FN. Of all the patients who transitioned to pegfilgrastim, 1 patient on a high risk for FN regimen developed sepsis due to herpes zoster in the setting of neutropenia after the previous cycle of chemotherapy.
Real-world data are limited regarding G-CSF practice patterns; however, available data demonstrate patients may receive suboptimal treatment courses of filgrastim leading to increased complications associated with neutropenia and FN, such as dose delays and hospitalizations.8,9 At the South Texas Veterans Health Care System, 48 patients (81%) received a filgrastim course of ≥ 7 days as an initial course for primary prophylaxis. Multivariate analyses performed by Weycker and colleagues described a decreased risk of hospitalization for neutropenia or FN with each additional day of filgrastim prophylaxis; however, such analysis could not be performed in our data set due to the small sample size.8 In this review, 10 patients (17%) experienced treatment delays due to neutropenia or FN, mirroring previously published data. The hospitalization rate of 25% is higher than the published incidence of 5.2% of cancer-related hospitalizations among adults.7,10 This difference may be explained by a difference in health care access for the veteran population.
As an alternative to daily filgrastim injections, the National Comprehensive Cancer Network also recommends a single dose of pegfilgrastim for primary prevention of FN. Efficacy benefits of pegfilgrastim use include increased patient adherence due to a single injection, a reduction in FN incidence and FN-related hospitalizations, and improved time to ANC recovery compared with filgrastim.11 There are reports suggesting pegfilgrastim significantly reduces neutropenia and FN incidence to a greater extent compared with daily filgrastim injections.6 In patients with breast cancer receiving dose-dense adjuvant chemotherapy, there are data demonstrating that patients who received filgrastim were more likely to experience severe neutropenia, dose reductions, and treatment delays leading to lower dose density compared with pegfilgrastim.12 Of the 19 patients with breast cancer included in our population, 26% experienced one of the previously described outcomes leading to either extensions of daily filgrastim injections or transitions to pegfilgrastim to successfully maintain dose density. In patients with acute myeloid leukemia receiving consolidation chemotherapy, filgrastim was found to be associated with a statistically significant increased risk of hospitalizations compared with pegfilgrastim.13 The one patient with acute myeloid leukemia included in our study did not require additional hospitalizations for neutropenia or FN after transitioning to pegfilgrastim.
Given the cost advantage, the South Texas Veterans Health Care System continues to prefer daily filgrastim injections. A recent survey demonstrated that 73% of patients at 23 sites in the Veterans Health Administration used filgrastim rather than pegfilgrastim for cost savings, although it is recognized that daily filgrastim injections are less convenient for patients.14 This analysis did not review costs associated with hospitalization for FN or the appropriateness of G-CSF use. Cancer-related neutropenia accounts for 8.3% of all cancer-related hospitalization costs among adults; the average hospitalization costs nearly $25,000 per stay and about $2.3 billion among adult patients with cancer annually.10,15
Limitations
This study has limitations that affected the applicability and interpretation of the results. This included the study design since it was a retrospective, single-center, descriptive cohort study. Patient adherence to daily filgrastim injections could not be assessed due to the retrospective nature of the study. The small sample size of 59 patients was prohibitive for utilization of additional analytical tools. Additionally, the predominately male veteran population may make applicability to non-VA populations restrictive.
CONCLUSIONS
Based on the incidence of primary and secondary outcomes associated with using daily filgrastim injections as primary prophylaxis in this study, additional measures such as tracking postnadir ANCs should be performed to ensure patients receive an appropriate number of filgrastim doses to prevent complications associated with neutropenia.
Acknowledgments
We thank Eric Dougherty, PharmD, for assistance in producing granulocyte colony-stimulating factor data.
1. Hanna KS, Mancini R, Wilson D, Zuckerman D. Comparing granulocyte colony-stimulating factors prescribing practices versus guideline recommendations in a large community cancer center. J Hematol Oncol Pharm. 2019;9(3):121-126.
2. Griffiths EA, Roy V, Alwan L, et al. NCCN Guidelines insights: hematopoietic growth factors, version 1.2022. J Natl Compr Canc Netw. 2022;20(5):436-442. doi:10.6004/jnccn.2022.0026
3. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93. doi:10.1093/cid/cir073
4. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-1453. doi:10.1200/JCO.2017.77.6211
5. Clemons M, Fergusson D, Simos D, et al. A multicentre, randomized trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy induced febrile neutropenia in early stage breast cancer. Ann Oncol. 2020;31(7):951-957. doi:10.1016/j.annonc.2020.04.005
6. Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404. Published 2011 Sep 23. doi:10.1186/1471-2407-11-404
7. Altwairgi A, Hopman W, Mates M. Real-world impact of granulocyte-colony stimulating factor on febrile neutropenia. Curr Oncol. 2013;20(3):e171-e179. doi:10.3747/co.20.1306
8. Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402-407. doi:10.1345/aph.1G516
9. Link H, Nietsch J, Kerkmann M, Ortner P; Supportive Care Group (ASORS) of the German Cancer Society (DKG). Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy—a representative sample survey in Germany. Support Care Cancer. 2016;24(1):367-376. doi:10.1007/s00520-015-2779-5
10. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266. doi:10.1002/cncr.21847
11. Aapro M, Boccia R, Leonard R, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. 2017;25(11):3295-3304. doi :10.1007/s00520-017-3842-1
12. Kourlaba G, Dimopoulos MA, Pectasides D, et al. Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer. 2015;23(7):2045-2051. doi:10.1007/s00520-014-2555-y
13. Field E, Caimi PF, Cooper B, et al. Comparison of pegfilgrastim and filgrastim to prevent neutropenic fever during consolidation with high dose cytarabine for acute myeloid leukemia. Blood. 2018;132(suppl 1):1404. doi:10.1182/blood-2018-99-118336
14. Knopf K, Hrureshky W, Love BL, Norris L, Bennett CL. Cost-effective use of white blood cell growth factors in the Veterans Administration. Blood. 2018;132(suppl 1):4761. doi:10.1182/blood-2018-99-119724
15. Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552-e561. doi:10.1200/JOP.2016.019588
1. Hanna KS, Mancini R, Wilson D, Zuckerman D. Comparing granulocyte colony-stimulating factors prescribing practices versus guideline recommendations in a large community cancer center. J Hematol Oncol Pharm. 2019;9(3):121-126.
2. Griffiths EA, Roy V, Alwan L, et al. NCCN Guidelines insights: hematopoietic growth factors, version 1.2022. J Natl Compr Canc Netw. 2022;20(5):436-442. doi:10.6004/jnccn.2022.0026
3. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):e56-e93. doi:10.1093/cid/cir073
4. Taplitz RA, Kennedy EB, Bow EJ, et al. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical practice guideline update. J Clin Oncol. 2018;36(14):1443-1453. doi:10.1200/JCO.2017.77.6211
5. Clemons M, Fergusson D, Simos D, et al. A multicentre, randomized trial comparing schedules of G-CSF (filgrastim) administration for primary prophylaxis of chemotherapy induced febrile neutropenia in early stage breast cancer. Ann Oncol. 2020;31(7):951-957. doi:10.1016/j.annonc.2020.04.005
6. Cooper KL, Madan J, Whyte S, Stevenson MD, Akehurst RL. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11:404. Published 2011 Sep 23. doi:10.1186/1471-2407-11-404
7. Altwairgi A, Hopman W, Mates M. Real-world impact of granulocyte-colony stimulating factor on febrile neutropenia. Curr Oncol. 2013;20(3):e171-e179. doi:10.3747/co.20.1306
8. Weycker D, Hackett J, Edelsberg JS, Oster G, Glass AG. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402-407. doi:10.1345/aph.1G516
9. Link H, Nietsch J, Kerkmann M, Ortner P; Supportive Care Group (ASORS) of the German Cancer Society (DKG). Adherence to granulocyte-colony stimulating factor (G-CSF) guidelines to reduce the incidence of febrile neutropenia after chemotherapy—a representative sample survey in Germany. Support Care Cancer. 2016;24(1):367-376. doi:10.1007/s00520-015-2779-5
10. Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258-2266. doi:10.1002/cncr.21847
11. Aapro M, Boccia R, Leonard R, et al. Refining the role of pegfilgrastim (a long-acting G-CSF) for prevention of chemotherapy-induced febrile neutropenia: consensus guidance recommendations. Support Care Cancer. 2017;25(11):3295-3304. doi :10.1007/s00520-017-3842-1
12. Kourlaba G, Dimopoulos MA, Pectasides D, et al. Comparison of filgrastim and pegfilgrastim to prevent neutropenia and maintain dose intensity of adjuvant chemotherapy in patients with breast cancer. Support Care Cancer. 2015;23(7):2045-2051. doi:10.1007/s00520-014-2555-y
13. Field E, Caimi PF, Cooper B, et al. Comparison of pegfilgrastim and filgrastim to prevent neutropenic fever during consolidation with high dose cytarabine for acute myeloid leukemia. Blood. 2018;132(suppl 1):1404. doi:10.1182/blood-2018-99-118336
14. Knopf K, Hrureshky W, Love BL, Norris L, Bennett CL. Cost-effective use of white blood cell growth factors in the Veterans Administration. Blood. 2018;132(suppl 1):4761. doi:10.1182/blood-2018-99-119724
15. Tai E, Guy GP, Dunbar A, Richardson LC. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552-e561. doi:10.1200/JOP.2016.019588