User login
Seek Work Wisely
Hospital medicine has come a long way since the term hospitalist was coined slightly more than a decade ago. SHM estimates the need for 30,000 practicing hospitalists within the next decade.
Filling an available hospitalist position is a two-way process that involves considerations and negotiations at various levels. When looking for the suitable hospitalist job, it is critical that you think both about what your potential employer needs and what you expect from the role you seek. The following insights provide a gauge of what an employer is looking for in a hospitalist applicant.
1) Clinical and procedural skills. Good clinical acumen is fundamental to being a successful hospitalist. As you complete residency training, your professional references are a reliable means for others to judge clinical skills. It’s important that your references comment on your clinical proficiency in their letters. Procedural skills always are welcome but by no means mandatory.
In larger facilities, where residents in training or specialists do many procedures, the program may not insist on procedural skills. On the other hand, some hospital medicine programs may require a proficiency in ICU procedures, which include intubations, central line placement, and A-line placements to mention a few. The SHM publication The Core Competencies in Hospital Medicine: A Framework for Curriculum Development is a great resource for understanding the knowledge and skills expected of a hospitalist physician.
2) Professionalism and teamwork. There are an extraordinary number of healthcare providers a hospitalist needs to work with. In addition to establishing a courteous rapport with patients and their families, good communication with primary care physicians, specialists, nursing staff, case managers, midlevel providers, and administrative and secretarial staff is essential. With this diversity of interactions, professionalism and teamwork are highly regarded and go a long way in establishing you as proficient hospitalist. An applicant’s professionalism is not only judged during the interview period but also confirmed by references. An unwavering positive attitude and commitment to a healthy work environment also are attributes that are recognized by a potential employer.
3) Quality improvement focus. Quality improvement activities and participation in such programs have rightly received unprecedented attention. SHM data indicate that 86% of hospitalist groups are active in quality improvement initiatives. Many hospital medicine programs participate in some form of Medicare pay-for-performance initiatives in order to ensure evidence-based patient care, better health outcomes, and reduce preventable complications.
A commitment to and active interest in quality improvement is highly desirable. Prior participation in and/or research for programs such as venous thromboembolism (VTE) prophylaxis, inpatient glycemic control, fall preventions, CHF optimization, medicine reconciliation pathways, and other evidence-based measures are a definite plus. In addition, specific training in areas such as perioperative care, improving safety of transitions of care, and stroke management are beneficial. Elaborating on any systems enhancement projects undertaken especially during hospital medicine clinical rotations/electives and/or fellowships will be invaluable.
4) Leadership skills. Nonclinical and administrative responsibilities are an important element of many hospitalist programs. Interest in various committees and an ability to assume leadership roles reflect favorably on your application. A good hospital medicine program will often encourage your interest in fostering the program and invite your involvement in initiatives to promote good patient care and facilitate fiscal strength.
An applicant should inquire about opportunities to participate in organizational committees and develop leadership skills, as this will be important for your professional growth. Take the time to point out any previous committee involvement in national healthcare organizations such as SHM.
5) Workflow efficiency. The ability to multitask and be organized are great skills to have as a hospitalist. Hospitalist work often involves managing several things during a short time span (i.e., rounding, admitting, teaching, holding family conferences, answering pages, and running codes). Successfully completing these responsibilities involves patience, structure, and resourcefulness during the course of any given day.
6) Teaching and research skills. In academic hospital medicine programs, good teaching and research skills can be very desirable. Chief residency or assistant chief residency experience is a good sign of teaching experience. Participation in research projects will boost your chances when looking for an academic hospitalist job. In non-academic practices, the employer may not focus much on these skills. Nevertheless, it is of significant value when the practice also hires midlevel practitioners like nurse practitioners or physician’s assistants or is thinking about how to evaluate the effects of a new program or intervention.
7) Local ties and durability. In view of the significant demand for hospitalists, recruiting can be challenging for any program. Another important aspect an employer looks at is whether you have any local ties or other compelling reasons to stay in the area for a long time. If you do have some geographic attachments or other reasons to be in the area for an extended duration, it will make the program more receptive toward you. Also, obtaining or applying for state licensure will save significant time and put you ahead of the curve.
8) Board certification. Most programs require you to be board certified or eligible when hired. Many programs expect you to obtain board certification within one to two years of starting your job. The sooner this is accomplished the more beneficial for the applicant.
Other Considerations
The diversity of hospital medicine programs provides an array of opportunities to choose from. Broadly speaking, the practice type could be academic or community based. The choice would depend upon your interest and proficiency in teaching.
In terms of schedules offered, several models exist. Many hospitalist programs are increasingly becoming 24/7, and it may be expected that you work different shifts. Also look into the licensure requirements of the state where you want to practice and be prepared with the required documentation, as some states may take longer to issue the license.
Above all, always remember: As much as it is important for you to find a befitting job, it is similarly essential for hospital medicine programs to hire worthy and valuable physicians. TH
Dr. Asudani is assistant clinical professor of medicine and a hospitalist at Baystate Medical Center, Tufts School of Medicine. Dr. Gandla is program medical director, Cogent Healthcare, High Point Regional Health System.
Hospital medicine has come a long way since the term hospitalist was coined slightly more than a decade ago. SHM estimates the need for 30,000 practicing hospitalists within the next decade.
Filling an available hospitalist position is a two-way process that involves considerations and negotiations at various levels. When looking for the suitable hospitalist job, it is critical that you think both about what your potential employer needs and what you expect from the role you seek. The following insights provide a gauge of what an employer is looking for in a hospitalist applicant.
1) Clinical and procedural skills. Good clinical acumen is fundamental to being a successful hospitalist. As you complete residency training, your professional references are a reliable means for others to judge clinical skills. It’s important that your references comment on your clinical proficiency in their letters. Procedural skills always are welcome but by no means mandatory.
In larger facilities, where residents in training or specialists do many procedures, the program may not insist on procedural skills. On the other hand, some hospital medicine programs may require a proficiency in ICU procedures, which include intubations, central line placement, and A-line placements to mention a few. The SHM publication The Core Competencies in Hospital Medicine: A Framework for Curriculum Development is a great resource for understanding the knowledge and skills expected of a hospitalist physician.
2) Professionalism and teamwork. There are an extraordinary number of healthcare providers a hospitalist needs to work with. In addition to establishing a courteous rapport with patients and their families, good communication with primary care physicians, specialists, nursing staff, case managers, midlevel providers, and administrative and secretarial staff is essential. With this diversity of interactions, professionalism and teamwork are highly regarded and go a long way in establishing you as proficient hospitalist. An applicant’s professionalism is not only judged during the interview period but also confirmed by references. An unwavering positive attitude and commitment to a healthy work environment also are attributes that are recognized by a potential employer.
3) Quality improvement focus. Quality improvement activities and participation in such programs have rightly received unprecedented attention. SHM data indicate that 86% of hospitalist groups are active in quality improvement initiatives. Many hospital medicine programs participate in some form of Medicare pay-for-performance initiatives in order to ensure evidence-based patient care, better health outcomes, and reduce preventable complications.
A commitment to and active interest in quality improvement is highly desirable. Prior participation in and/or research for programs such as venous thromboembolism (VTE) prophylaxis, inpatient glycemic control, fall preventions, CHF optimization, medicine reconciliation pathways, and other evidence-based measures are a definite plus. In addition, specific training in areas such as perioperative care, improving safety of transitions of care, and stroke management are beneficial. Elaborating on any systems enhancement projects undertaken especially during hospital medicine clinical rotations/electives and/or fellowships will be invaluable.
4) Leadership skills. Nonclinical and administrative responsibilities are an important element of many hospitalist programs. Interest in various committees and an ability to assume leadership roles reflect favorably on your application. A good hospital medicine program will often encourage your interest in fostering the program and invite your involvement in initiatives to promote good patient care and facilitate fiscal strength.
An applicant should inquire about opportunities to participate in organizational committees and develop leadership skills, as this will be important for your professional growth. Take the time to point out any previous committee involvement in national healthcare organizations such as SHM.
5) Workflow efficiency. The ability to multitask and be organized are great skills to have as a hospitalist. Hospitalist work often involves managing several things during a short time span (i.e., rounding, admitting, teaching, holding family conferences, answering pages, and running codes). Successfully completing these responsibilities involves patience, structure, and resourcefulness during the course of any given day.
6) Teaching and research skills. In academic hospital medicine programs, good teaching and research skills can be very desirable. Chief residency or assistant chief residency experience is a good sign of teaching experience. Participation in research projects will boost your chances when looking for an academic hospitalist job. In non-academic practices, the employer may not focus much on these skills. Nevertheless, it is of significant value when the practice also hires midlevel practitioners like nurse practitioners or physician’s assistants or is thinking about how to evaluate the effects of a new program or intervention.
7) Local ties and durability. In view of the significant demand for hospitalists, recruiting can be challenging for any program. Another important aspect an employer looks at is whether you have any local ties or other compelling reasons to stay in the area for a long time. If you do have some geographic attachments or other reasons to be in the area for an extended duration, it will make the program more receptive toward you. Also, obtaining or applying for state licensure will save significant time and put you ahead of the curve.
8) Board certification. Most programs require you to be board certified or eligible when hired. Many programs expect you to obtain board certification within one to two years of starting your job. The sooner this is accomplished the more beneficial for the applicant.
Other Considerations
The diversity of hospital medicine programs provides an array of opportunities to choose from. Broadly speaking, the practice type could be academic or community based. The choice would depend upon your interest and proficiency in teaching.
In terms of schedules offered, several models exist. Many hospitalist programs are increasingly becoming 24/7, and it may be expected that you work different shifts. Also look into the licensure requirements of the state where you want to practice and be prepared with the required documentation, as some states may take longer to issue the license.
Above all, always remember: As much as it is important for you to find a befitting job, it is similarly essential for hospital medicine programs to hire worthy and valuable physicians. TH
Dr. Asudani is assistant clinical professor of medicine and a hospitalist at Baystate Medical Center, Tufts School of Medicine. Dr. Gandla is program medical director, Cogent Healthcare, High Point Regional Health System.
Hospital medicine has come a long way since the term hospitalist was coined slightly more than a decade ago. SHM estimates the need for 30,000 practicing hospitalists within the next decade.
Filling an available hospitalist position is a two-way process that involves considerations and negotiations at various levels. When looking for the suitable hospitalist job, it is critical that you think both about what your potential employer needs and what you expect from the role you seek. The following insights provide a gauge of what an employer is looking for in a hospitalist applicant.
1) Clinical and procedural skills. Good clinical acumen is fundamental to being a successful hospitalist. As you complete residency training, your professional references are a reliable means for others to judge clinical skills. It’s important that your references comment on your clinical proficiency in their letters. Procedural skills always are welcome but by no means mandatory.
In larger facilities, where residents in training or specialists do many procedures, the program may not insist on procedural skills. On the other hand, some hospital medicine programs may require a proficiency in ICU procedures, which include intubations, central line placement, and A-line placements to mention a few. The SHM publication The Core Competencies in Hospital Medicine: A Framework for Curriculum Development is a great resource for understanding the knowledge and skills expected of a hospitalist physician.
2) Professionalism and teamwork. There are an extraordinary number of healthcare providers a hospitalist needs to work with. In addition to establishing a courteous rapport with patients and their families, good communication with primary care physicians, specialists, nursing staff, case managers, midlevel providers, and administrative and secretarial staff is essential. With this diversity of interactions, professionalism and teamwork are highly regarded and go a long way in establishing you as proficient hospitalist. An applicant’s professionalism is not only judged during the interview period but also confirmed by references. An unwavering positive attitude and commitment to a healthy work environment also are attributes that are recognized by a potential employer.
3) Quality improvement focus. Quality improvement activities and participation in such programs have rightly received unprecedented attention. SHM data indicate that 86% of hospitalist groups are active in quality improvement initiatives. Many hospital medicine programs participate in some form of Medicare pay-for-performance initiatives in order to ensure evidence-based patient care, better health outcomes, and reduce preventable complications.
A commitment to and active interest in quality improvement is highly desirable. Prior participation in and/or research for programs such as venous thromboembolism (VTE) prophylaxis, inpatient glycemic control, fall preventions, CHF optimization, medicine reconciliation pathways, and other evidence-based measures are a definite plus. In addition, specific training in areas such as perioperative care, improving safety of transitions of care, and stroke management are beneficial. Elaborating on any systems enhancement projects undertaken especially during hospital medicine clinical rotations/electives and/or fellowships will be invaluable.
4) Leadership skills. Nonclinical and administrative responsibilities are an important element of many hospitalist programs. Interest in various committees and an ability to assume leadership roles reflect favorably on your application. A good hospital medicine program will often encourage your interest in fostering the program and invite your involvement in initiatives to promote good patient care and facilitate fiscal strength.
An applicant should inquire about opportunities to participate in organizational committees and develop leadership skills, as this will be important for your professional growth. Take the time to point out any previous committee involvement in national healthcare organizations such as SHM.
5) Workflow efficiency. The ability to multitask and be organized are great skills to have as a hospitalist. Hospitalist work often involves managing several things during a short time span (i.e., rounding, admitting, teaching, holding family conferences, answering pages, and running codes). Successfully completing these responsibilities involves patience, structure, and resourcefulness during the course of any given day.
6) Teaching and research skills. In academic hospital medicine programs, good teaching and research skills can be very desirable. Chief residency or assistant chief residency experience is a good sign of teaching experience. Participation in research projects will boost your chances when looking for an academic hospitalist job. In non-academic practices, the employer may not focus much on these skills. Nevertheless, it is of significant value when the practice also hires midlevel practitioners like nurse practitioners or physician’s assistants or is thinking about how to evaluate the effects of a new program or intervention.
7) Local ties and durability. In view of the significant demand for hospitalists, recruiting can be challenging for any program. Another important aspect an employer looks at is whether you have any local ties or other compelling reasons to stay in the area for a long time. If you do have some geographic attachments or other reasons to be in the area for an extended duration, it will make the program more receptive toward you. Also, obtaining or applying for state licensure will save significant time and put you ahead of the curve.
8) Board certification. Most programs require you to be board certified or eligible when hired. Many programs expect you to obtain board certification within one to two years of starting your job. The sooner this is accomplished the more beneficial for the applicant.
Other Considerations
The diversity of hospital medicine programs provides an array of opportunities to choose from. Broadly speaking, the practice type could be academic or community based. The choice would depend upon your interest and proficiency in teaching.
In terms of schedules offered, several models exist. Many hospitalist programs are increasingly becoming 24/7, and it may be expected that you work different shifts. Also look into the licensure requirements of the state where you want to practice and be prepared with the required documentation, as some states may take longer to issue the license.
Above all, always remember: As much as it is important for you to find a befitting job, it is similarly essential for hospital medicine programs to hire worthy and valuable physicians. TH
Dr. Asudani is assistant clinical professor of medicine and a hospitalist at Baystate Medical Center, Tufts School of Medicine. Dr. Gandla is program medical director, Cogent Healthcare, High Point Regional Health System.
Medical Board Maneuvers
There are a few pieces of mail that bring an instant feeling of dread—an audit letter from the IRS, a credit card bill after a Las Vegas vacation, and a letter from the medical board. We have no good solutions for the first two pieces of correspondence, but we have a few suggestions when communicating with the medical board.
1) Understand the medical board’s purpose. Every state regulates the practice of medicine for the same reason: Medicine requires highly specialized knowledge, and the average patient does not have the knowledge or experience to determine which physicians are qualified to practice.
Think of the harm that could result if incompetent physicians could practice medicine without oversight. Even worse, think of the harm that could result if non-physicians could provide medical services without proper education and training. That’s why, in every state, the legislatures have passed laws to regulate and control the practice of medicine so people can be properly protected against the unauthorized, unqualified, and improper practice of medicine. Almost everyone agrees regulation of this nature serves a legitimate public purpose.
Consequently, whenever a physician deals with a medical board, they are best served by remembering that the medical board exists to protect the public from the unauthorized, unqualified and improper practice of medicine. The physician’s ultimate goal is to reassure that medical board that their practice is authorized, well-grounded in medicine, and within the standards of professional care. Even if the patient has complained because of a questionable motive, such as attempting to gain an advantage in a billing dispute, a physician cannot use the patient’s motive as grounds for defending poor medical care. Medical boards often distrust physicians who try to shift the focus from the adequacy of their medical care to a patient’s shortcomings.
2) Do I need a lawyer? In most states, the medical board will ask a physician to respond to every patient complaint—even if the complaint is outlandish. Rather than judging the complaint when it arrives, the medical board is more interested in assessing the physician’s response to the complaint. An unhappy patient may lack the acumen to explain the course of treatment and the specifics of their condition, so the medical board relies upon the physician to describe their conduct and the course of care.
Unless the patient’s complaint is in the category of “the doctor placed transmitters in my brain and now the aliens won’t leave me alone,” we always recommend a physician review the complaint and the proposed response with an attorney. In every state, there are attorneys who specialize in representing physicians before medical boards.
Because they’ve dealt with the medical board in many cases throughout a number of years, these attorneys have a good idea of what the medical board expects to see in a response, and, more importantly, what the medical board does not want to see in a response. Investing in an attorney’s services at the outset is money well spent.
Far too often, we see physicians who tried to save a couple of hundred dollars by responding to the medical board, but their response was ineffective. The physician is then faced with spending several thousand dollars defending a disciplinary proceeding. Even worse, if the physician has made a sufficiently serious mistake in the initial response, the physician is going to be stuck with that mistake, severely limiting the attorney’s ability to defend the disciplinary proceeding. Some medical malpractice insurers reimburse physicians for attorney’s fees incurred in responding to a medical board complaint, so check your policy.
3) Candor is your friend. Undoubtedly, there are occasions when a patient complains about medical care without justification. Patients have unrealistic expectations and often fail to understand that each patient’s condition presents a unique challenge. Conversely, some complaints absolutely are legitimate. Every physician makes mistakes, and the medical board will react negatively to a physician who defends an unreasonable course of care. In fact, the medical board will view the physician’s defense of unreasonable care as evidence the mistake is not an aberration in the physician’s practice.
When confronted with one of those instances where the patient’s complaint is legitimate, we doubly recommend you confer with an attorney about your response. At a minimum, however, a physician must be able to explain:
- Why a mistake occurred;
- What steps the physician took to minimize the consequences of the mistake for the patient;
- Why the mistake represents an aberration, not a reason for continued concern; and
- What changes the physician has implemented to ensure the mistake will not reoccur.
In preparing a response to the medical board, we’ve recommended physicians take continuing education in the areas of the patients’ complaints. By taking this remedial measure voluntarily, a physician reduces the likelihood the medical board will impose it as a remedial sanction.
When we first began defending healthcare professionals before their licensing agencies, we thought we’d be spending lots of time dealing with complicated medical issues. We were wrong.
By an overwhelming proportion, the majority of disciplinary actions against physicians arise from three sources:
- Allegations of improper sexual conduct;
- Allegations of substance abuse; or
- Allegations of financial impropriety.
Physicians face the same problems that affect non-physicians—but a physician’s breach of the obligations owed to patients allows a medical board to take disciplinary action. The physician-patient relationship has an inherent disparity of power that makes patients vulnerable to a physician’s abuse of trust. For this reason, medical boards view allegations of this nature quite seriously.
The first question a physician has to ask when accused of these form of misconduct is, “Is it true?” If you are tempted, to answer, “no,” even if the real answer is “yes,” think twice. If you lie to the medical board about one of these issues, you almost certainly will lose your medical license. You will have demonstrated to the board that you not only lack judgment, but that you can’t be trusted. If it even crossed your mind to alter the medical or billing records, don’t. The medical board will probably obtain copies of those records from another source.
If the answer to the question, “Is it true?” is “yes,” the physician faces the prospect that the medical board will revoke or suspend their license. In these situations, we regularly recommend physicians embark on a course of action designed to save the medical license—even if the physician will be subject to arduous probationary terms.
We will recommend the physicians engage practice monitors, seek substance abuse counseling, and repay any wrongfully obtained money. In many states, there are specialized programs that provide mental health and addiction counseling for physicians, and these programs represent potential lifelines for physicians in crisis. Your goal is demonstrate to the medical board that you’ve seen the error of your ways and have committed to a program that will return you to good standing.
Responding to the medical board is a scary proposition. The majority of complaints are dismissed without any disciplinary action against a physician—but no physician should take a complaint lightly. Be thoughtful and candid in your response to maximize the likelihood that the medical board will dismiss the complaint. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
There are a few pieces of mail that bring an instant feeling of dread—an audit letter from the IRS, a credit card bill after a Las Vegas vacation, and a letter from the medical board. We have no good solutions for the first two pieces of correspondence, but we have a few suggestions when communicating with the medical board.
1) Understand the medical board’s purpose. Every state regulates the practice of medicine for the same reason: Medicine requires highly specialized knowledge, and the average patient does not have the knowledge or experience to determine which physicians are qualified to practice.
Think of the harm that could result if incompetent physicians could practice medicine without oversight. Even worse, think of the harm that could result if non-physicians could provide medical services without proper education and training. That’s why, in every state, the legislatures have passed laws to regulate and control the practice of medicine so people can be properly protected against the unauthorized, unqualified, and improper practice of medicine. Almost everyone agrees regulation of this nature serves a legitimate public purpose.
Consequently, whenever a physician deals with a medical board, they are best served by remembering that the medical board exists to protect the public from the unauthorized, unqualified and improper practice of medicine. The physician’s ultimate goal is to reassure that medical board that their practice is authorized, well-grounded in medicine, and within the standards of professional care. Even if the patient has complained because of a questionable motive, such as attempting to gain an advantage in a billing dispute, a physician cannot use the patient’s motive as grounds for defending poor medical care. Medical boards often distrust physicians who try to shift the focus from the adequacy of their medical care to a patient’s shortcomings.
2) Do I need a lawyer? In most states, the medical board will ask a physician to respond to every patient complaint—even if the complaint is outlandish. Rather than judging the complaint when it arrives, the medical board is more interested in assessing the physician’s response to the complaint. An unhappy patient may lack the acumen to explain the course of treatment and the specifics of their condition, so the medical board relies upon the physician to describe their conduct and the course of care.
Unless the patient’s complaint is in the category of “the doctor placed transmitters in my brain and now the aliens won’t leave me alone,” we always recommend a physician review the complaint and the proposed response with an attorney. In every state, there are attorneys who specialize in representing physicians before medical boards.
Because they’ve dealt with the medical board in many cases throughout a number of years, these attorneys have a good idea of what the medical board expects to see in a response, and, more importantly, what the medical board does not want to see in a response. Investing in an attorney’s services at the outset is money well spent.
Far too often, we see physicians who tried to save a couple of hundred dollars by responding to the medical board, but their response was ineffective. The physician is then faced with spending several thousand dollars defending a disciplinary proceeding. Even worse, if the physician has made a sufficiently serious mistake in the initial response, the physician is going to be stuck with that mistake, severely limiting the attorney’s ability to defend the disciplinary proceeding. Some medical malpractice insurers reimburse physicians for attorney’s fees incurred in responding to a medical board complaint, so check your policy.
3) Candor is your friend. Undoubtedly, there are occasions when a patient complains about medical care without justification. Patients have unrealistic expectations and often fail to understand that each patient’s condition presents a unique challenge. Conversely, some complaints absolutely are legitimate. Every physician makes mistakes, and the medical board will react negatively to a physician who defends an unreasonable course of care. In fact, the medical board will view the physician’s defense of unreasonable care as evidence the mistake is not an aberration in the physician’s practice.
When confronted with one of those instances where the patient’s complaint is legitimate, we doubly recommend you confer with an attorney about your response. At a minimum, however, a physician must be able to explain:
- Why a mistake occurred;
- What steps the physician took to minimize the consequences of the mistake for the patient;
- Why the mistake represents an aberration, not a reason for continued concern; and
- What changes the physician has implemented to ensure the mistake will not reoccur.
In preparing a response to the medical board, we’ve recommended physicians take continuing education in the areas of the patients’ complaints. By taking this remedial measure voluntarily, a physician reduces the likelihood the medical board will impose it as a remedial sanction.
When we first began defending healthcare professionals before their licensing agencies, we thought we’d be spending lots of time dealing with complicated medical issues. We were wrong.
By an overwhelming proportion, the majority of disciplinary actions against physicians arise from three sources:
- Allegations of improper sexual conduct;
- Allegations of substance abuse; or
- Allegations of financial impropriety.
Physicians face the same problems that affect non-physicians—but a physician’s breach of the obligations owed to patients allows a medical board to take disciplinary action. The physician-patient relationship has an inherent disparity of power that makes patients vulnerable to a physician’s abuse of trust. For this reason, medical boards view allegations of this nature quite seriously.
The first question a physician has to ask when accused of these form of misconduct is, “Is it true?” If you are tempted, to answer, “no,” even if the real answer is “yes,” think twice. If you lie to the medical board about one of these issues, you almost certainly will lose your medical license. You will have demonstrated to the board that you not only lack judgment, but that you can’t be trusted. If it even crossed your mind to alter the medical or billing records, don’t. The medical board will probably obtain copies of those records from another source.
If the answer to the question, “Is it true?” is “yes,” the physician faces the prospect that the medical board will revoke or suspend their license. In these situations, we regularly recommend physicians embark on a course of action designed to save the medical license—even if the physician will be subject to arduous probationary terms.
We will recommend the physicians engage practice monitors, seek substance abuse counseling, and repay any wrongfully obtained money. In many states, there are specialized programs that provide mental health and addiction counseling for physicians, and these programs represent potential lifelines for physicians in crisis. Your goal is demonstrate to the medical board that you’ve seen the error of your ways and have committed to a program that will return you to good standing.
Responding to the medical board is a scary proposition. The majority of complaints are dismissed without any disciplinary action against a physician—but no physician should take a complaint lightly. Be thoughtful and candid in your response to maximize the likelihood that the medical board will dismiss the complaint. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
There are a few pieces of mail that bring an instant feeling of dread—an audit letter from the IRS, a credit card bill after a Las Vegas vacation, and a letter from the medical board. We have no good solutions for the first two pieces of correspondence, but we have a few suggestions when communicating with the medical board.
1) Understand the medical board’s purpose. Every state regulates the practice of medicine for the same reason: Medicine requires highly specialized knowledge, and the average patient does not have the knowledge or experience to determine which physicians are qualified to practice.
Think of the harm that could result if incompetent physicians could practice medicine without oversight. Even worse, think of the harm that could result if non-physicians could provide medical services without proper education and training. That’s why, in every state, the legislatures have passed laws to regulate and control the practice of medicine so people can be properly protected against the unauthorized, unqualified, and improper practice of medicine. Almost everyone agrees regulation of this nature serves a legitimate public purpose.
Consequently, whenever a physician deals with a medical board, they are best served by remembering that the medical board exists to protect the public from the unauthorized, unqualified and improper practice of medicine. The physician’s ultimate goal is to reassure that medical board that their practice is authorized, well-grounded in medicine, and within the standards of professional care. Even if the patient has complained because of a questionable motive, such as attempting to gain an advantage in a billing dispute, a physician cannot use the patient’s motive as grounds for defending poor medical care. Medical boards often distrust physicians who try to shift the focus from the adequacy of their medical care to a patient’s shortcomings.
2) Do I need a lawyer? In most states, the medical board will ask a physician to respond to every patient complaint—even if the complaint is outlandish. Rather than judging the complaint when it arrives, the medical board is more interested in assessing the physician’s response to the complaint. An unhappy patient may lack the acumen to explain the course of treatment and the specifics of their condition, so the medical board relies upon the physician to describe their conduct and the course of care.
Unless the patient’s complaint is in the category of “the doctor placed transmitters in my brain and now the aliens won’t leave me alone,” we always recommend a physician review the complaint and the proposed response with an attorney. In every state, there are attorneys who specialize in representing physicians before medical boards.
Because they’ve dealt with the medical board in many cases throughout a number of years, these attorneys have a good idea of what the medical board expects to see in a response, and, more importantly, what the medical board does not want to see in a response. Investing in an attorney’s services at the outset is money well spent.
Far too often, we see physicians who tried to save a couple of hundred dollars by responding to the medical board, but their response was ineffective. The physician is then faced with spending several thousand dollars defending a disciplinary proceeding. Even worse, if the physician has made a sufficiently serious mistake in the initial response, the physician is going to be stuck with that mistake, severely limiting the attorney’s ability to defend the disciplinary proceeding. Some medical malpractice insurers reimburse physicians for attorney’s fees incurred in responding to a medical board complaint, so check your policy.
3) Candor is your friend. Undoubtedly, there are occasions when a patient complains about medical care without justification. Patients have unrealistic expectations and often fail to understand that each patient’s condition presents a unique challenge. Conversely, some complaints absolutely are legitimate. Every physician makes mistakes, and the medical board will react negatively to a physician who defends an unreasonable course of care. In fact, the medical board will view the physician’s defense of unreasonable care as evidence the mistake is not an aberration in the physician’s practice.
When confronted with one of those instances where the patient’s complaint is legitimate, we doubly recommend you confer with an attorney about your response. At a minimum, however, a physician must be able to explain:
- Why a mistake occurred;
- What steps the physician took to minimize the consequences of the mistake for the patient;
- Why the mistake represents an aberration, not a reason for continued concern; and
- What changes the physician has implemented to ensure the mistake will not reoccur.
In preparing a response to the medical board, we’ve recommended physicians take continuing education in the areas of the patients’ complaints. By taking this remedial measure voluntarily, a physician reduces the likelihood the medical board will impose it as a remedial sanction.
When we first began defending healthcare professionals before their licensing agencies, we thought we’d be spending lots of time dealing with complicated medical issues. We were wrong.
By an overwhelming proportion, the majority of disciplinary actions against physicians arise from three sources:
- Allegations of improper sexual conduct;
- Allegations of substance abuse; or
- Allegations of financial impropriety.
Physicians face the same problems that affect non-physicians—but a physician’s breach of the obligations owed to patients allows a medical board to take disciplinary action. The physician-patient relationship has an inherent disparity of power that makes patients vulnerable to a physician’s abuse of trust. For this reason, medical boards view allegations of this nature quite seriously.
The first question a physician has to ask when accused of these form of misconduct is, “Is it true?” If you are tempted, to answer, “no,” even if the real answer is “yes,” think twice. If you lie to the medical board about one of these issues, you almost certainly will lose your medical license. You will have demonstrated to the board that you not only lack judgment, but that you can’t be trusted. If it even crossed your mind to alter the medical or billing records, don’t. The medical board will probably obtain copies of those records from another source.
If the answer to the question, “Is it true?” is “yes,” the physician faces the prospect that the medical board will revoke or suspend their license. In these situations, we regularly recommend physicians embark on a course of action designed to save the medical license—even if the physician will be subject to arduous probationary terms.
We will recommend the physicians engage practice monitors, seek substance abuse counseling, and repay any wrongfully obtained money. In many states, there are specialized programs that provide mental health and addiction counseling for physicians, and these programs represent potential lifelines for physicians in crisis. Your goal is demonstrate to the medical board that you’ve seen the error of your ways and have committed to a program that will return you to good standing.
Responding to the medical board is a scary proposition. The majority of complaints are dismissed without any disciplinary action against a physician—but no physician should take a complaint lightly. Be thoughtful and candid in your response to maximize the likelihood that the medical board will dismiss the complaint. TH
Patrick O’Rourke works in the Office of University Counsel, Department of Litigation, University of Colorado, Denver.
Sort Out Surgical Cases
Hospitalists often are involved in the care of a surgical patient. Reimbursement for surgical procedures includes payment for pre-, intra-, and post-operative care.
Knowing the billing and coding responsibilities apart from those of the surgeon is imperative for the hospitalist’s accurate charge capture. There are several critical misconceptions in this regard:
- Hospitalists cannot bill for services when involved in a surgical case;
- Surgeons are not responsible for inpatient care if the patient is stable and does not require additional inpatient post-op visits; and
- Modifiers are not required for hospitalist claims unless the hospitalist reports under the same tax identification number as the surgeon.
Determine Global Period
Procedures are categorized as major or minor surgery. A global period is assigned to each procedure code, designating post-operative periods of zero, 10, or 90 days. Physician services during this global period are considered part of the packaged payment and not separately reimbursed.
The global period for any given CPT code can be identified in the Medicare Physician Fee Schedule and accessed at www.cms.hhs.gov/PfsLookup. In addition to zero, 10, and 90 days, services can be noted with:
- XXX, indicating the global period concept does not apply; or
- ZZZ, indicating an “add-on” procedure that must always be reported with the relevant primary procedure code; “add-on” procedures assume the global period of the primary procedure.
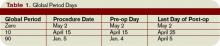
Major surgery routinely is allotted 90-day global periods. Therefore, the surgeon is responsible for the patient and must provide all related care one day prior to the surgery forward thru 90 postoperative days at no additional charge. Minor surgery, including endoscopy, has zero or 10-day postoperative periods, bundling all services on the surgical day only, or the surgical day and the subsequent 10 days, respectively (see Table 1, p. above).
The Surgeon Defined
Any qualified physician able to perform “surgical” services within his scope of practice is considered a “surgeon” for billing purposes. For example, a pulmonologist, or primary care physician, must meet the surgical billing and documentation requirements when performing bronchoscopies or uncomplicated incision-and-drainage services, respectively.
Surgical services easily are identified as any code included in range 20000-69999. This code series includes major, minor, and endoscopic procedures. The “surgeon” and all physicians in the same group practice (i.e., reporting services under the same tax identification number) with the same specialty designation must adhere to the global period billing rules.
Alternately, physicians with different specialty designations in the same group practice (e.g., multispecialty group that reports services under the same tax identification number) or different group practices can perform and separately report medically necessary services during the surgeon’s global period, as long as a formal (mutually agreed upon) transfer of care did not occur. Information on physician specialty designations is available at www.highmarkmedicareservices.com/partb/refman/appendix-d.html.
Package Components
The following services are included in the surgeon’s packaged payment:
- Preoperative visits after the decision for surgery is made beginning one day prior to surgery;
- All additional post-operative medical or surgical services provided by the surgeon related to complications, but not require additional trips to the operating room;
- Post-operative visits by the surgeon related to recovery from surgery, including but not limited to dressing changes; local incisional care; removal of cutaneous sutures and staples; line removals; changes and removal of tracheostomy tubes; and discharge services; and
- Post-operative pain management provided by the surgeon.
Services not included are:
- The initial consultation or evaluation of the problem by the surgeon to determine the need for surgery. Append modifier 57 to this visit if provided the day before or day of major surgery to alert the payer that the service resulted in the decision for surgery. Append modifier 25 to this visit if provided the day of minor surgery;
- Services of other physicians except where the other physicians are providing coverage for the surgeon or agree on a transfer of care. This agreement may be in the form of a letter or an annotation in the discharge summary, hospital record, or ASC record;
- Post-operative visits by the surgeon unrelated to the diagnosis for which the surgical procedure is performed, unless the visits occur due to complications of the surgery. These services only are payable after the patient has been discharged from the hospitalization in which the surgery occurred. Append modifier 24 to these unrelated post-op visits;
- Diagnostic tests and procedures, including diagnostic radiological procedures;
- Clearly distinct surgical procedures during the post-operative period that do not result in repeat operations or treatment for complications;
- Treatment for post-operative complications that require a return trip to the operating room, catheterization lab, or endoscopy suite;
- Immunosuppressive therapy for organ transplants; and
- Critical care services (CPT codes 99291 and 99292) unrelated to the surgery in which a seriously injured or burned patient is critically ill and requires constant attendance of the surgeon. Append modifier 24 to these unrelated critical care services (see Table 2, above).
Payer Variations
While Medicare does not require modifier usage by hospitalists providing medically necessary services on surgical cases, some private payers do. Their electronic claim systems may not differentiate services by non-surgical specialists, requiring all physicians to append the appropriate modifier depending on the reason and timing of the service (see “Key Modifiers” below). TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is on the faculty of SHM’s inpatient coding course.
Hospitalists often are involved in the care of a surgical patient. Reimbursement for surgical procedures includes payment for pre-, intra-, and post-operative care.
Knowing the billing and coding responsibilities apart from those of the surgeon is imperative for the hospitalist’s accurate charge capture. There are several critical misconceptions in this regard:
- Hospitalists cannot bill for services when involved in a surgical case;
- Surgeons are not responsible for inpatient care if the patient is stable and does not require additional inpatient post-op visits; and
- Modifiers are not required for hospitalist claims unless the hospitalist reports under the same tax identification number as the surgeon.
Determine Global Period
Procedures are categorized as major or minor surgery. A global period is assigned to each procedure code, designating post-operative periods of zero, 10, or 90 days. Physician services during this global period are considered part of the packaged payment and not separately reimbursed.
The global period for any given CPT code can be identified in the Medicare Physician Fee Schedule and accessed at www.cms.hhs.gov/PfsLookup. In addition to zero, 10, and 90 days, services can be noted with:
- XXX, indicating the global period concept does not apply; or
- ZZZ, indicating an “add-on” procedure that must always be reported with the relevant primary procedure code; “add-on” procedures assume the global period of the primary procedure.
Major surgery routinely is allotted 90-day global periods. Therefore, the surgeon is responsible for the patient and must provide all related care one day prior to the surgery forward thru 90 postoperative days at no additional charge. Minor surgery, including endoscopy, has zero or 10-day postoperative periods, bundling all services on the surgical day only, or the surgical day and the subsequent 10 days, respectively (see Table 1, p. above).
The Surgeon Defined
Any qualified physician able to perform “surgical” services within his scope of practice is considered a “surgeon” for billing purposes. For example, a pulmonologist, or primary care physician, must meet the surgical billing and documentation requirements when performing bronchoscopies or uncomplicated incision-and-drainage services, respectively.
Surgical services easily are identified as any code included in range 20000-69999. This code series includes major, minor, and endoscopic procedures. The “surgeon” and all physicians in the same group practice (i.e., reporting services under the same tax identification number) with the same specialty designation must adhere to the global period billing rules.
Alternately, physicians with different specialty designations in the same group practice (e.g., multispecialty group that reports services under the same tax identification number) or different group practices can perform and separately report medically necessary services during the surgeon’s global period, as long as a formal (mutually agreed upon) transfer of care did not occur. Information on physician specialty designations is available at www.highmarkmedicareservices.com/partb/refman/appendix-d.html.
Package Components
The following services are included in the surgeon’s packaged payment:
- Preoperative visits after the decision for surgery is made beginning one day prior to surgery;
- All additional post-operative medical or surgical services provided by the surgeon related to complications, but not require additional trips to the operating room;
- Post-operative visits by the surgeon related to recovery from surgery, including but not limited to dressing changes; local incisional care; removal of cutaneous sutures and staples; line removals; changes and removal of tracheostomy tubes; and discharge services; and
- Post-operative pain management provided by the surgeon.
Services not included are:
- The initial consultation or evaluation of the problem by the surgeon to determine the need for surgery. Append modifier 57 to this visit if provided the day before or day of major surgery to alert the payer that the service resulted in the decision for surgery. Append modifier 25 to this visit if provided the day of minor surgery;
- Services of other physicians except where the other physicians are providing coverage for the surgeon or agree on a transfer of care. This agreement may be in the form of a letter or an annotation in the discharge summary, hospital record, or ASC record;
- Post-operative visits by the surgeon unrelated to the diagnosis for which the surgical procedure is performed, unless the visits occur due to complications of the surgery. These services only are payable after the patient has been discharged from the hospitalization in which the surgery occurred. Append modifier 24 to these unrelated post-op visits;
- Diagnostic tests and procedures, including diagnostic radiological procedures;
- Clearly distinct surgical procedures during the post-operative period that do not result in repeat operations or treatment for complications;
- Treatment for post-operative complications that require a return trip to the operating room, catheterization lab, or endoscopy suite;
- Immunosuppressive therapy for organ transplants; and
- Critical care services (CPT codes 99291 and 99292) unrelated to the surgery in which a seriously injured or burned patient is critically ill and requires constant attendance of the surgeon. Append modifier 24 to these unrelated critical care services (see Table 2, above).
Payer Variations
While Medicare does not require modifier usage by hospitalists providing medically necessary services on surgical cases, some private payers do. Their electronic claim systems may not differentiate services by non-surgical specialists, requiring all physicians to append the appropriate modifier depending on the reason and timing of the service (see “Key Modifiers” below). TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is on the faculty of SHM’s inpatient coding course.
Hospitalists often are involved in the care of a surgical patient. Reimbursement for surgical procedures includes payment for pre-, intra-, and post-operative care.
Knowing the billing and coding responsibilities apart from those of the surgeon is imperative for the hospitalist’s accurate charge capture. There are several critical misconceptions in this regard:
- Hospitalists cannot bill for services when involved in a surgical case;
- Surgeons are not responsible for inpatient care if the patient is stable and does not require additional inpatient post-op visits; and
- Modifiers are not required for hospitalist claims unless the hospitalist reports under the same tax identification number as the surgeon.
Determine Global Period
Procedures are categorized as major or minor surgery. A global period is assigned to each procedure code, designating post-operative periods of zero, 10, or 90 days. Physician services during this global period are considered part of the packaged payment and not separately reimbursed.
The global period for any given CPT code can be identified in the Medicare Physician Fee Schedule and accessed at www.cms.hhs.gov/PfsLookup. In addition to zero, 10, and 90 days, services can be noted with:
- XXX, indicating the global period concept does not apply; or
- ZZZ, indicating an “add-on” procedure that must always be reported with the relevant primary procedure code; “add-on” procedures assume the global period of the primary procedure.
Major surgery routinely is allotted 90-day global periods. Therefore, the surgeon is responsible for the patient and must provide all related care one day prior to the surgery forward thru 90 postoperative days at no additional charge. Minor surgery, including endoscopy, has zero or 10-day postoperative periods, bundling all services on the surgical day only, or the surgical day and the subsequent 10 days, respectively (see Table 1, p. above).
The Surgeon Defined
Any qualified physician able to perform “surgical” services within his scope of practice is considered a “surgeon” for billing purposes. For example, a pulmonologist, or primary care physician, must meet the surgical billing and documentation requirements when performing bronchoscopies or uncomplicated incision-and-drainage services, respectively.
Surgical services easily are identified as any code included in range 20000-69999. This code series includes major, minor, and endoscopic procedures. The “surgeon” and all physicians in the same group practice (i.e., reporting services under the same tax identification number) with the same specialty designation must adhere to the global period billing rules.
Alternately, physicians with different specialty designations in the same group practice (e.g., multispecialty group that reports services under the same tax identification number) or different group practices can perform and separately report medically necessary services during the surgeon’s global period, as long as a formal (mutually agreed upon) transfer of care did not occur. Information on physician specialty designations is available at www.highmarkmedicareservices.com/partb/refman/appendix-d.html.
Package Components
The following services are included in the surgeon’s packaged payment:
- Preoperative visits after the decision for surgery is made beginning one day prior to surgery;
- All additional post-operative medical or surgical services provided by the surgeon related to complications, but not require additional trips to the operating room;
- Post-operative visits by the surgeon related to recovery from surgery, including but not limited to dressing changes; local incisional care; removal of cutaneous sutures and staples; line removals; changes and removal of tracheostomy tubes; and discharge services; and
- Post-operative pain management provided by the surgeon.
Services not included are:
- The initial consultation or evaluation of the problem by the surgeon to determine the need for surgery. Append modifier 57 to this visit if provided the day before or day of major surgery to alert the payer that the service resulted in the decision for surgery. Append modifier 25 to this visit if provided the day of minor surgery;
- Services of other physicians except where the other physicians are providing coverage for the surgeon or agree on a transfer of care. This agreement may be in the form of a letter or an annotation in the discharge summary, hospital record, or ASC record;
- Post-operative visits by the surgeon unrelated to the diagnosis for which the surgical procedure is performed, unless the visits occur due to complications of the surgery. These services only are payable after the patient has been discharged from the hospitalization in which the surgery occurred. Append modifier 24 to these unrelated post-op visits;
- Diagnostic tests and procedures, including diagnostic radiological procedures;
- Clearly distinct surgical procedures during the post-operative period that do not result in repeat operations or treatment for complications;
- Treatment for post-operative complications that require a return trip to the operating room, catheterization lab, or endoscopy suite;
- Immunosuppressive therapy for organ transplants; and
- Critical care services (CPT codes 99291 and 99292) unrelated to the surgery in which a seriously injured or burned patient is critically ill and requires constant attendance of the surgeon. Append modifier 24 to these unrelated critical care services (see Table 2, above).
Payer Variations
While Medicare does not require modifier usage by hospitalists providing medically necessary services on surgical cases, some private payers do. Their electronic claim systems may not differentiate services by non-surgical specialists, requiring all physicians to append the appropriate modifier depending on the reason and timing of the service (see “Key Modifiers” below). TH
Carol Pohlig is a billing and coding expert with the University of Pennsylvania Medical Center, Philadelphia. She also is on the faculty of SHM’s inpatient coding course.
Presidential Opportunity
Next year, a new president will take the White House and likely will be the one to lead the United States toward much-needed healthcare reform. What does the near future hold? What should hospitalists know about each candidate’s healthcare policies and proposals? Here, a hospitalist and a government advocate for hospitalists each weigh in.
Are the Times a-Changin’?
Laura Allendorf, SHM’s senior adviser for advocacy and government affairs, keeps a close eye on healthcare legislation, values, and trends in Washington, D.C. She predicts that regardless of which candidate takes office in 2009, change is coming fast.
“Healthcare will definitely be a top priority for the new administration … regardless of who wins the White House,” she says. “There’s been an unprecedented level of discussion already. Congressional committees have already held hearings to prepare for changes next year. They’re laying the groundwork now.”
However, not everyone agrees that we’ll see healthcare reform so soon: Bradley Flansbaum, DO, MPH, chief of hospitalist section at Lenox Hill Hospital in New York City, believes other major issues, such as the slow economy and the war in Iraq, may take precedence.
—Laura Allendorf, SHM senior adviser for advocacy and government affairs
“There are a lot of things on the agenda in Congress right now,” he points out. “I’m not sure how fast [healthcare reform] will really happen.” He says regardless of which man (Barack Obama or John McCain) wins, “he will have two or three top priorities as soon as he takes office—if healthcare reform is one of those priorities, some changes will happen.”
However, the business of Washington still can get in the way of a new administration. Dr. Flansbaum points to a House bill (HR 6331) that requests a delay in implementation of the Medicare competitive bidding program for durable medical equipment. “Lobbyists have sway over what legislators do in Washington, D.C.,” he notes. “Just because Obama or McCain come into office doesn’t mean those lobbyists will go away.”
Despite the forces against change, each candidate is touting major changes to healthcare access.
McCain and Tax Credits
Republican candidate McCain has released a healthcare plan based on instituting a federal tax credit to be used by individuals to purchase their own health insurance—regardless of whether they are covered (or can be covered) through an employer or through the non-group market.
His plan would replace a tax break for those who receive health insurance from their employers with a refundable tax credit of as much as $2,500 per individual and $5,000 per family, to be used for buying private coverage of their choice.
McCain’s plan proposes compensating physicians and hospitals based on performance, including tying Medicaid and Medicare reimbursements to results. His plan also includes ideas for containing healthcare spending by better treating chronic diseases, such as diabetes and heart disease.
“I believe that the best way to help small businesses and employers afford health care is not to increase government control of health care but to bring the rising cost of care under control and give people the option of having personal, portable health insurance,” McCain has said. He added that his proposal would allow individuals to retain their health insurance “even when they move or change jobs.”
Obama’s Funding Plan
Meanwhile, Democratic candidate Obama approaches the issue with a different solution. He proposes universal coverage through the following:
- The proposal would mandate all children have healthcare coverage, and would expand eligibility for Medicaid and SCHIP (State Children’s Health Insurance Program);
- A new public insurance program that would bridge the gap of the uninsured, covering Americans who don’t quality for Medicaid or SCHIP and have no access to coverage through their employer. The coverage would be similar to that offered to members of Congress; and
- A National Health Insurance Exchange to aid individuals and businesses that want to purchase private health insurance directly. Obama’s plan would require all employers to contribute toward health coverage for their employees or toward the cost of the public plan—all, that is, except small businesses who meet certain exemptions.
Congress Is the Decider
“McCain’s plan is, far and away, the more daring, and will present a greater shock to the system,” Dr. Flansbaum maintains. “It would probably lead to gridlock in Congress, because it would need bipartisan agreement to pass and I don’t think the Democrats would agree to it.” If, on the other hand, Obama wins the election, he would almost certainly have a sympathetic Democratic Congress to work with. “He’d have a greater chance of leading change along his lines,” predicts Dr. Flansbaum. “In this case, we might see a Massachusetts-esque plan.”
In either case, the candidate’s proposal may not become reality. “Folks have to remember, it’s Congress that has to come up with the plan” for reform, Allendorf cautions.
A strong president may carry some weight in this regard. “Like all presidents able to effect change, once [the 2009 electee] has the bully pulpit and can sway opinion, Congress should fall into line,” says Dr. Flansbaum. “There will be pressure to change things.”
That change, whatever shape it takes, is almost certain to include some belt-tightening for hospitals, he says. “You have to look at the facts: One-third of healthcare dollars are spent in hospitals … and the numbers given for waste in care in the system are upwards of 30%. You have to assume that hospitals are the logical place to cut.” Regardless of the election outcome, he cautions: “Hospitals will probably have to make painful cuts and changes. It’s going to happen at some point, though I’m not sure that Congress has the political will to push through any changes soon.”
Luckily, hospitalists are accustomed to continuous change and shifting policies, roles, and responsibilities. Their skills at adapting to changing conditions should serve them well in the post-election months. TH
Jane Jerrard is a medical writer based in Chicago.
Next year, a new president will take the White House and likely will be the one to lead the United States toward much-needed healthcare reform. What does the near future hold? What should hospitalists know about each candidate’s healthcare policies and proposals? Here, a hospitalist and a government advocate for hospitalists each weigh in.
Are the Times a-Changin’?
Laura Allendorf, SHM’s senior adviser for advocacy and government affairs, keeps a close eye on healthcare legislation, values, and trends in Washington, D.C. She predicts that regardless of which candidate takes office in 2009, change is coming fast.
“Healthcare will definitely be a top priority for the new administration … regardless of who wins the White House,” she says. “There’s been an unprecedented level of discussion already. Congressional committees have already held hearings to prepare for changes next year. They’re laying the groundwork now.”
However, not everyone agrees that we’ll see healthcare reform so soon: Bradley Flansbaum, DO, MPH, chief of hospitalist section at Lenox Hill Hospital in New York City, believes other major issues, such as the slow economy and the war in Iraq, may take precedence.
—Laura Allendorf, SHM senior adviser for advocacy and government affairs
“There are a lot of things on the agenda in Congress right now,” he points out. “I’m not sure how fast [healthcare reform] will really happen.” He says regardless of which man (Barack Obama or John McCain) wins, “he will have two or three top priorities as soon as he takes office—if healthcare reform is one of those priorities, some changes will happen.”
However, the business of Washington still can get in the way of a new administration. Dr. Flansbaum points to a House bill (HR 6331) that requests a delay in implementation of the Medicare competitive bidding program for durable medical equipment. “Lobbyists have sway over what legislators do in Washington, D.C.,” he notes. “Just because Obama or McCain come into office doesn’t mean those lobbyists will go away.”
Despite the forces against change, each candidate is touting major changes to healthcare access.
McCain and Tax Credits
Republican candidate McCain has released a healthcare plan based on instituting a federal tax credit to be used by individuals to purchase their own health insurance—regardless of whether they are covered (or can be covered) through an employer or through the non-group market.
His plan would replace a tax break for those who receive health insurance from their employers with a refundable tax credit of as much as $2,500 per individual and $5,000 per family, to be used for buying private coverage of their choice.
McCain’s plan proposes compensating physicians and hospitals based on performance, including tying Medicaid and Medicare reimbursements to results. His plan also includes ideas for containing healthcare spending by better treating chronic diseases, such as diabetes and heart disease.
“I believe that the best way to help small businesses and employers afford health care is not to increase government control of health care but to bring the rising cost of care under control and give people the option of having personal, portable health insurance,” McCain has said. He added that his proposal would allow individuals to retain their health insurance “even when they move or change jobs.”
Obama’s Funding Plan
Meanwhile, Democratic candidate Obama approaches the issue with a different solution. He proposes universal coverage through the following:
- The proposal would mandate all children have healthcare coverage, and would expand eligibility for Medicaid and SCHIP (State Children’s Health Insurance Program);
- A new public insurance program that would bridge the gap of the uninsured, covering Americans who don’t quality for Medicaid or SCHIP and have no access to coverage through their employer. The coverage would be similar to that offered to members of Congress; and
- A National Health Insurance Exchange to aid individuals and businesses that want to purchase private health insurance directly. Obama’s plan would require all employers to contribute toward health coverage for their employees or toward the cost of the public plan—all, that is, except small businesses who meet certain exemptions.
Congress Is the Decider
“McCain’s plan is, far and away, the more daring, and will present a greater shock to the system,” Dr. Flansbaum maintains. “It would probably lead to gridlock in Congress, because it would need bipartisan agreement to pass and I don’t think the Democrats would agree to it.” If, on the other hand, Obama wins the election, he would almost certainly have a sympathetic Democratic Congress to work with. “He’d have a greater chance of leading change along his lines,” predicts Dr. Flansbaum. “In this case, we might see a Massachusetts-esque plan.”
In either case, the candidate’s proposal may not become reality. “Folks have to remember, it’s Congress that has to come up with the plan” for reform, Allendorf cautions.
A strong president may carry some weight in this regard. “Like all presidents able to effect change, once [the 2009 electee] has the bully pulpit and can sway opinion, Congress should fall into line,” says Dr. Flansbaum. “There will be pressure to change things.”
That change, whatever shape it takes, is almost certain to include some belt-tightening for hospitals, he says. “You have to look at the facts: One-third of healthcare dollars are spent in hospitals … and the numbers given for waste in care in the system are upwards of 30%. You have to assume that hospitals are the logical place to cut.” Regardless of the election outcome, he cautions: “Hospitals will probably have to make painful cuts and changes. It’s going to happen at some point, though I’m not sure that Congress has the political will to push through any changes soon.”
Luckily, hospitalists are accustomed to continuous change and shifting policies, roles, and responsibilities. Their skills at adapting to changing conditions should serve them well in the post-election months. TH
Jane Jerrard is a medical writer based in Chicago.
Next year, a new president will take the White House and likely will be the one to lead the United States toward much-needed healthcare reform. What does the near future hold? What should hospitalists know about each candidate’s healthcare policies and proposals? Here, a hospitalist and a government advocate for hospitalists each weigh in.
Are the Times a-Changin’?
Laura Allendorf, SHM’s senior adviser for advocacy and government affairs, keeps a close eye on healthcare legislation, values, and trends in Washington, D.C. She predicts that regardless of which candidate takes office in 2009, change is coming fast.
“Healthcare will definitely be a top priority for the new administration … regardless of who wins the White House,” she says. “There’s been an unprecedented level of discussion already. Congressional committees have already held hearings to prepare for changes next year. They’re laying the groundwork now.”
However, not everyone agrees that we’ll see healthcare reform so soon: Bradley Flansbaum, DO, MPH, chief of hospitalist section at Lenox Hill Hospital in New York City, believes other major issues, such as the slow economy and the war in Iraq, may take precedence.
—Laura Allendorf, SHM senior adviser for advocacy and government affairs
“There are a lot of things on the agenda in Congress right now,” he points out. “I’m not sure how fast [healthcare reform] will really happen.” He says regardless of which man (Barack Obama or John McCain) wins, “he will have two or three top priorities as soon as he takes office—if healthcare reform is one of those priorities, some changes will happen.”
However, the business of Washington still can get in the way of a new administration. Dr. Flansbaum points to a House bill (HR 6331) that requests a delay in implementation of the Medicare competitive bidding program for durable medical equipment. “Lobbyists have sway over what legislators do in Washington, D.C.,” he notes. “Just because Obama or McCain come into office doesn’t mean those lobbyists will go away.”
Despite the forces against change, each candidate is touting major changes to healthcare access.
McCain and Tax Credits
Republican candidate McCain has released a healthcare plan based on instituting a federal tax credit to be used by individuals to purchase their own health insurance—regardless of whether they are covered (or can be covered) through an employer or through the non-group market.
His plan would replace a tax break for those who receive health insurance from their employers with a refundable tax credit of as much as $2,500 per individual and $5,000 per family, to be used for buying private coverage of their choice.
McCain’s plan proposes compensating physicians and hospitals based on performance, including tying Medicaid and Medicare reimbursements to results. His plan also includes ideas for containing healthcare spending by better treating chronic diseases, such as diabetes and heart disease.
“I believe that the best way to help small businesses and employers afford health care is not to increase government control of health care but to bring the rising cost of care under control and give people the option of having personal, portable health insurance,” McCain has said. He added that his proposal would allow individuals to retain their health insurance “even when they move or change jobs.”
Obama’s Funding Plan
Meanwhile, Democratic candidate Obama approaches the issue with a different solution. He proposes universal coverage through the following:
- The proposal would mandate all children have healthcare coverage, and would expand eligibility for Medicaid and SCHIP (State Children’s Health Insurance Program);
- A new public insurance program that would bridge the gap of the uninsured, covering Americans who don’t quality for Medicaid or SCHIP and have no access to coverage through their employer. The coverage would be similar to that offered to members of Congress; and
- A National Health Insurance Exchange to aid individuals and businesses that want to purchase private health insurance directly. Obama’s plan would require all employers to contribute toward health coverage for their employees or toward the cost of the public plan—all, that is, except small businesses who meet certain exemptions.
Congress Is the Decider
“McCain’s plan is, far and away, the more daring, and will present a greater shock to the system,” Dr. Flansbaum maintains. “It would probably lead to gridlock in Congress, because it would need bipartisan agreement to pass and I don’t think the Democrats would agree to it.” If, on the other hand, Obama wins the election, he would almost certainly have a sympathetic Democratic Congress to work with. “He’d have a greater chance of leading change along his lines,” predicts Dr. Flansbaum. “In this case, we might see a Massachusetts-esque plan.”
In either case, the candidate’s proposal may not become reality. “Folks have to remember, it’s Congress that has to come up with the plan” for reform, Allendorf cautions.
A strong president may carry some weight in this regard. “Like all presidents able to effect change, once [the 2009 electee] has the bully pulpit and can sway opinion, Congress should fall into line,” says Dr. Flansbaum. “There will be pressure to change things.”
That change, whatever shape it takes, is almost certain to include some belt-tightening for hospitals, he says. “You have to look at the facts: One-third of healthcare dollars are spent in hospitals … and the numbers given for waste in care in the system are upwards of 30%. You have to assume that hospitals are the logical place to cut.” Regardless of the election outcome, he cautions: “Hospitals will probably have to make painful cuts and changes. It’s going to happen at some point, though I’m not sure that Congress has the political will to push through any changes soon.”
Luckily, hospitalists are accustomed to continuous change and shifting policies, roles, and responsibilities. Their skills at adapting to changing conditions should serve them well in the post-election months. TH
Jane Jerrard is a medical writer based in Chicago.
Beat the Boss Blues
A sour relationship with your immediate superior can ruin an otherwise fulfilling job. When you report to someone you continually disagree with or simply don’t understand, just showing up for work can become a misery. If you’re in a situation like this, don’t despair; there is a possible solution.
Power Struggle
Whether the conflict you feel with your boss is over care decisions, personal style, or scope of work, it really boils down to who gets control over your time and your patients.
“For physicians especially, autonomy is very important,” says Tosha B. Wetterneck, MD, associate professor of medicine at University of Wisconsin Hospital/Clinics in Madison. “Physicians are people who work hard, are very smart, and like to control what they do. There is obviously a lot of complexity and variation to the job, which adds to the workload. Plus, decision-making processes need to be happening all the time. This creates stress—and the way to control that stress is to have control over what they do.”
—Russell L. Holman, MD, chief operating officer, Cogent Healthcare, and immediate past president of SHM
A hospitalist who continually butts heads with a superior over issues—or one who subjugates his or her opinion and decisions to the boss’s—is not likely to be satisfied with their job.
“Certainly, an individual’s autonomy is influenced by what they want to have control over and they’re allowed to have control over,” says Dr. Wetterneck. “If there’s a discrepancy between the two, that’s definitely going to have a negative effect on that hospitalist. If there’s a mismatch between what they want control over and what their boss wants, that’s going to be a problem.”
Manage Up
Russell L. Holman, MD, chief operating officer for Brentwood, Tenn.-based Cogent Healthcare and immediate past president of SHM, has worked his way through problems like this—both as the reportee and the boss. He worked out some particularly valuable lessons in a past job where, as medical director, he had trouble connecting with his boss.
“There seemed to be a tremendous communication gap, and there was a mismatch between what I felt was important and what my superior felt was important,” he recalls. “It seemed really hard to get on the same page.”
So he set out to solve the problem: “What I learned was that it’s not sufficient in a leadership role to just focus on who is reporting to you and manage in that direction,” says Dr. Holman. “No one ever tells you this, but you need to spend time managing up.”
Managing up primarily means initiating conversations to get information you need to better work with your boss.
“You need a clear understanding about the priorities and hot buttons of the person you’re reporting to, what they’re personally invested in, how they’re being managed, and what their incentives are,” advises Dr. Holman. “In my situation, I felt that I needed to understand my superior’s background—his career progression, areas of interest, things he felt were important in the organization.”
How do you uncover these facts? It’s simple: Request a one-on-one meeting with your superior and have a direct conversation where you ask those questions.
Next, continues Dr. Holman: “Have what I would call a translational conversation … ‘How do your priorities translate to me and my daily work?’ Again, ask this directly.”
But be warned. “This can be a very productive conversation, but it’s not an easy one to have,” he says. “The reason it’s hard is because whether you’re a frontline hospitalist or a group leader of some kind, you’re a highly educated, highly paid professional. Why would you want to redirect yourself to someone else’s priorities?”
That is the crux of the problem in working for a boss you don’t agree with—you need to relinquish some control to make the situation work.
“This may be difficult for some people but by giving up a little bit, you’ll get a much more productive relationship,” says Dr. Holman. “It also helps you understand how your daily work fits into the broader organizational vision, and you build political capital. You’ll build trust, respect, and equity. If there’s a project you want to engage in and you want support for it, you can trade on that equity.”
Learn their Style
Even as you’re practicing the art of managing up, you may face barriers in dealing with the boss. Consider whether it is a matter of understanding their personal and professional style.
“Maybe you’re just having trouble connecting,” Dr. Holman suggests. “Learn their style, how they communicate. Invest a little time to get a better understanding of their personality style. One way is to ask about their preferences—do they prefer e-mail, phone, or in-person conversations?—and to observe.”
You may discover that the boss is brusque with everyone, not just you, or that they don’t reply to your e-mails because they never check their in-box. The better you understand them, the less stress you’ll suffer from interactions.
The Last Resort
If you’re not getting along with your boss, or don’t like the answers you’re getting, should you consider going over their head to the next level up?
“The temptation may be to use workarounds or back channels—what I call leapfrogging—until you get the answer you want,” Dr. Holman says. “But there’s a lot of damage you can do in leapfrogging. I typically do not recommend that someone going over or around their supervisor unless the circumstances are egregious.”
Ultimately, if you’re still at odds with your boss and the conflict makes you unhappy with your job, you may need to consider finding a better environment.
“If your superior’s personal priorities are in conflict with yours, you owe it to both the boss and yourself to try to converse and reconcile those priorities,” says Dr. Holman. “You should still use the steps, but you may end up leaving anyway. [Managing up] doesn’t guarantee success, but it stacks the deck in your favor.”
He recalls an example where he was the superior to a dissatisfied hospitalist: “There was a hospitalist working for me who had a priority of working in an environment where he could use subjective judgment to make patient decisions. My priority was to standardize care as much as possible. The individual viewed [guidelines, checklists] as an encroachment on his autonomy. This came down to a very fundamental issue. I knew he’d be unhappy in this environment, and we agreed that he would be better off working for another group.”
Perhaps the best advice for coping with a difficult hospitalist-boss relationship is to avoid it in the first place. By recognizing what’s most important to you—what areas you need autonomy in—you can ask questions and perhaps negotiate during the interview or promotion stages. Dr. Wetterneck suggests that hospitalists take the control/autonomy survey included in the SHM white paper “A Challenge for a New Specialty: A White Paper on Hospitalist Career Satisfaction,” which she co-wrote. (The white paper is available under “Publications” on www.hospitalmedicine.org). TH
Jane Jerrard also writes “Public Policy” for The Hospitalist.
A sour relationship with your immediate superior can ruin an otherwise fulfilling job. When you report to someone you continually disagree with or simply don’t understand, just showing up for work can become a misery. If you’re in a situation like this, don’t despair; there is a possible solution.
Power Struggle
Whether the conflict you feel with your boss is over care decisions, personal style, or scope of work, it really boils down to who gets control over your time and your patients.
“For physicians especially, autonomy is very important,” says Tosha B. Wetterneck, MD, associate professor of medicine at University of Wisconsin Hospital/Clinics in Madison. “Physicians are people who work hard, are very smart, and like to control what they do. There is obviously a lot of complexity and variation to the job, which adds to the workload. Plus, decision-making processes need to be happening all the time. This creates stress—and the way to control that stress is to have control over what they do.”
—Russell L. Holman, MD, chief operating officer, Cogent Healthcare, and immediate past president of SHM
A hospitalist who continually butts heads with a superior over issues—or one who subjugates his or her opinion and decisions to the boss’s—is not likely to be satisfied with their job.
“Certainly, an individual’s autonomy is influenced by what they want to have control over and they’re allowed to have control over,” says Dr. Wetterneck. “If there’s a discrepancy between the two, that’s definitely going to have a negative effect on that hospitalist. If there’s a mismatch between what they want control over and what their boss wants, that’s going to be a problem.”
Manage Up
Russell L. Holman, MD, chief operating officer for Brentwood, Tenn.-based Cogent Healthcare and immediate past president of SHM, has worked his way through problems like this—both as the reportee and the boss. He worked out some particularly valuable lessons in a past job where, as medical director, he had trouble connecting with his boss.
“There seemed to be a tremendous communication gap, and there was a mismatch between what I felt was important and what my superior felt was important,” he recalls. “It seemed really hard to get on the same page.”
So he set out to solve the problem: “What I learned was that it’s not sufficient in a leadership role to just focus on who is reporting to you and manage in that direction,” says Dr. Holman. “No one ever tells you this, but you need to spend time managing up.”
Managing up primarily means initiating conversations to get information you need to better work with your boss.
“You need a clear understanding about the priorities and hot buttons of the person you’re reporting to, what they’re personally invested in, how they’re being managed, and what their incentives are,” advises Dr. Holman. “In my situation, I felt that I needed to understand my superior’s background—his career progression, areas of interest, things he felt were important in the organization.”
How do you uncover these facts? It’s simple: Request a one-on-one meeting with your superior and have a direct conversation where you ask those questions.
Next, continues Dr. Holman: “Have what I would call a translational conversation … ‘How do your priorities translate to me and my daily work?’ Again, ask this directly.”
But be warned. “This can be a very productive conversation, but it’s not an easy one to have,” he says. “The reason it’s hard is because whether you’re a frontline hospitalist or a group leader of some kind, you’re a highly educated, highly paid professional. Why would you want to redirect yourself to someone else’s priorities?”
That is the crux of the problem in working for a boss you don’t agree with—you need to relinquish some control to make the situation work.
“This may be difficult for some people but by giving up a little bit, you’ll get a much more productive relationship,” says Dr. Holman. “It also helps you understand how your daily work fits into the broader organizational vision, and you build political capital. You’ll build trust, respect, and equity. If there’s a project you want to engage in and you want support for it, you can trade on that equity.”
Learn their Style
Even as you’re practicing the art of managing up, you may face barriers in dealing with the boss. Consider whether it is a matter of understanding their personal and professional style.
“Maybe you’re just having trouble connecting,” Dr. Holman suggests. “Learn their style, how they communicate. Invest a little time to get a better understanding of their personality style. One way is to ask about their preferences—do they prefer e-mail, phone, or in-person conversations?—and to observe.”
You may discover that the boss is brusque with everyone, not just you, or that they don’t reply to your e-mails because they never check their in-box. The better you understand them, the less stress you’ll suffer from interactions.
The Last Resort
If you’re not getting along with your boss, or don’t like the answers you’re getting, should you consider going over their head to the next level up?
“The temptation may be to use workarounds or back channels—what I call leapfrogging—until you get the answer you want,” Dr. Holman says. “But there’s a lot of damage you can do in leapfrogging. I typically do not recommend that someone going over or around their supervisor unless the circumstances are egregious.”
Ultimately, if you’re still at odds with your boss and the conflict makes you unhappy with your job, you may need to consider finding a better environment.
“If your superior’s personal priorities are in conflict with yours, you owe it to both the boss and yourself to try to converse and reconcile those priorities,” says Dr. Holman. “You should still use the steps, but you may end up leaving anyway. [Managing up] doesn’t guarantee success, but it stacks the deck in your favor.”
He recalls an example where he was the superior to a dissatisfied hospitalist: “There was a hospitalist working for me who had a priority of working in an environment where he could use subjective judgment to make patient decisions. My priority was to standardize care as much as possible. The individual viewed [guidelines, checklists] as an encroachment on his autonomy. This came down to a very fundamental issue. I knew he’d be unhappy in this environment, and we agreed that he would be better off working for another group.”
Perhaps the best advice for coping with a difficult hospitalist-boss relationship is to avoid it in the first place. By recognizing what’s most important to you—what areas you need autonomy in—you can ask questions and perhaps negotiate during the interview or promotion stages. Dr. Wetterneck suggests that hospitalists take the control/autonomy survey included in the SHM white paper “A Challenge for a New Specialty: A White Paper on Hospitalist Career Satisfaction,” which she co-wrote. (The white paper is available under “Publications” on www.hospitalmedicine.org). TH
Jane Jerrard also writes “Public Policy” for The Hospitalist.
A sour relationship with your immediate superior can ruin an otherwise fulfilling job. When you report to someone you continually disagree with or simply don’t understand, just showing up for work can become a misery. If you’re in a situation like this, don’t despair; there is a possible solution.
Power Struggle
Whether the conflict you feel with your boss is over care decisions, personal style, or scope of work, it really boils down to who gets control over your time and your patients.
“For physicians especially, autonomy is very important,” says Tosha B. Wetterneck, MD, associate professor of medicine at University of Wisconsin Hospital/Clinics in Madison. “Physicians are people who work hard, are very smart, and like to control what they do. There is obviously a lot of complexity and variation to the job, which adds to the workload. Plus, decision-making processes need to be happening all the time. This creates stress—and the way to control that stress is to have control over what they do.”
—Russell L. Holman, MD, chief operating officer, Cogent Healthcare, and immediate past president of SHM
A hospitalist who continually butts heads with a superior over issues—or one who subjugates his or her opinion and decisions to the boss’s—is not likely to be satisfied with their job.
“Certainly, an individual’s autonomy is influenced by what they want to have control over and they’re allowed to have control over,” says Dr. Wetterneck. “If there’s a discrepancy between the two, that’s definitely going to have a negative effect on that hospitalist. If there’s a mismatch between what they want control over and what their boss wants, that’s going to be a problem.”
Manage Up
Russell L. Holman, MD, chief operating officer for Brentwood, Tenn.-based Cogent Healthcare and immediate past president of SHM, has worked his way through problems like this—both as the reportee and the boss. He worked out some particularly valuable lessons in a past job where, as medical director, he had trouble connecting with his boss.
“There seemed to be a tremendous communication gap, and there was a mismatch between what I felt was important and what my superior felt was important,” he recalls. “It seemed really hard to get on the same page.”
So he set out to solve the problem: “What I learned was that it’s not sufficient in a leadership role to just focus on who is reporting to you and manage in that direction,” says Dr. Holman. “No one ever tells you this, but you need to spend time managing up.”
Managing up primarily means initiating conversations to get information you need to better work with your boss.
“You need a clear understanding about the priorities and hot buttons of the person you’re reporting to, what they’re personally invested in, how they’re being managed, and what their incentives are,” advises Dr. Holman. “In my situation, I felt that I needed to understand my superior’s background—his career progression, areas of interest, things he felt were important in the organization.”
How do you uncover these facts? It’s simple: Request a one-on-one meeting with your superior and have a direct conversation where you ask those questions.
Next, continues Dr. Holman: “Have what I would call a translational conversation … ‘How do your priorities translate to me and my daily work?’ Again, ask this directly.”
But be warned. “This can be a very productive conversation, but it’s not an easy one to have,” he says. “The reason it’s hard is because whether you’re a frontline hospitalist or a group leader of some kind, you’re a highly educated, highly paid professional. Why would you want to redirect yourself to someone else’s priorities?”
That is the crux of the problem in working for a boss you don’t agree with—you need to relinquish some control to make the situation work.
“This may be difficult for some people but by giving up a little bit, you’ll get a much more productive relationship,” says Dr. Holman. “It also helps you understand how your daily work fits into the broader organizational vision, and you build political capital. You’ll build trust, respect, and equity. If there’s a project you want to engage in and you want support for it, you can trade on that equity.”
Learn their Style
Even as you’re practicing the art of managing up, you may face barriers in dealing with the boss. Consider whether it is a matter of understanding their personal and professional style.
“Maybe you’re just having trouble connecting,” Dr. Holman suggests. “Learn their style, how they communicate. Invest a little time to get a better understanding of their personality style. One way is to ask about their preferences—do they prefer e-mail, phone, or in-person conversations?—and to observe.”
You may discover that the boss is brusque with everyone, not just you, or that they don’t reply to your e-mails because they never check their in-box. The better you understand them, the less stress you’ll suffer from interactions.
The Last Resort
If you’re not getting along with your boss, or don’t like the answers you’re getting, should you consider going over their head to the next level up?
“The temptation may be to use workarounds or back channels—what I call leapfrogging—until you get the answer you want,” Dr. Holman says. “But there’s a lot of damage you can do in leapfrogging. I typically do not recommend that someone going over or around their supervisor unless the circumstances are egregious.”
Ultimately, if you’re still at odds with your boss and the conflict makes you unhappy with your job, you may need to consider finding a better environment.
“If your superior’s personal priorities are in conflict with yours, you owe it to both the boss and yourself to try to converse and reconcile those priorities,” says Dr. Holman. “You should still use the steps, but you may end up leaving anyway. [Managing up] doesn’t guarantee success, but it stacks the deck in your favor.”
He recalls an example where he was the superior to a dissatisfied hospitalist: “There was a hospitalist working for me who had a priority of working in an environment where he could use subjective judgment to make patient decisions. My priority was to standardize care as much as possible. The individual viewed [guidelines, checklists] as an encroachment on his autonomy. This came down to a very fundamental issue. I knew he’d be unhappy in this environment, and we agreed that he would be better off working for another group.”
Perhaps the best advice for coping with a difficult hospitalist-boss relationship is to avoid it in the first place. By recognizing what’s most important to you—what areas you need autonomy in—you can ask questions and perhaps negotiate during the interview or promotion stages. Dr. Wetterneck suggests that hospitalists take the control/autonomy survey included in the SHM white paper “A Challenge for a New Specialty: A White Paper on Hospitalist Career Satisfaction,” which she co-wrote. (The white paper is available under “Publications” on www.hospitalmedicine.org). TH
Jane Jerrard also writes “Public Policy” for The Hospitalist.
Use Outside Help
Addressing the clinical and political issues raised by the use of non-housestaff services is one of the biggest challenges facing hospitalists at academic medical centers, according to a paper in this month’s issue of the Journal of Hospital Medicine.
Lead author Niraj Sehgal, MD, assistant clinical professor of medicine at the University of California, San Francisco (UCSF), and colleagues studied the non-housestaff services at five academic medical centers around the United States to identify what it takes to make the best use of non-housestaff services.
Reliance on these services will grow largely because of restrictions established in 2003 by the Accreditation Council of Graduate Medical Education (ACGME), which limit residents to an 80-hour workweek.
What’s more, it is possible that the ACGME may cut hours even more, given that many other countries have lower restrictions.
In other words, “most academic medical centers now realize residents no longer will be providing as much patient care as they used to,” Dr. Sehgal says.
For example, at UCSF, residents’ hours have been reduced by one-third since the restrictions were established. One way to handle the situation was to reduce the number of patient-hours per resident.
However, at the same time that ruling went into effect, UCSF also increased the number of beds in its hospital. In an effort to determine who is best suited to care for these patients, UCSF and other academic centers turned to non-housestaff services to pick up the slack. “Every residency program has struggled with different models,” he explains.
In their paper, Dr. Sehgal and his colleagues identify nine questions to consider in developing non-housestaff medicine services. The questions reflect key challenges facing medical centers that are building these services, such as:
- System equities: Avoid creating a two-tiered system in which non-housestaff hospitalists who mostly provide clinical care are viewed as second-class citizens compared with academic hospitalists, who also teach and conduct research. This also raises the question of how to define an academic hospitalist;
- Define the patient mix: Should non-housestaff physicians handle less acute patients, specific patient populations, or all patients above the limit for residents?;
- Recruitment and retention: Academic centers may have to offer non-housestaff hospitalists incentives, such as teaching or research opportunities, or financial rewards such as student loan forgiveness, to attract talented clinicians; and
- Compensation and incentives: This should reflect all aspects of the physician’s job, including quality improvement efforts, research activities, and excellence in teaching, as well as clinical productivity.
“How these questions are answered is often driven by local factors, such as the vision of local leadership and the availability of important resources,” Dr. Sehgal and his coauthors write. “Nevertheless, it is important for hospitals to share their experiences since best practices remain unclear.”
To explore how different centers and services address these issues, they compared the non-housestaff medicine services at Brigham and Women’s Hospital in Boston, Emory University in Atlanta, the University of Michigan in Ann Arbor, Northwestern University Medical Center in Chicago, and UCSF. The information was obtained from representatives at each center and was current as of July 2007.
The services ranged in age from two to five years old and covered 168 to 212 clinical days per year, or 15 to 20 shifts per month. Depending on the number of hospitalists in the service, they saw anywhere from 12 to 95 patients per day.
Further, they all provided coverage for 50% of weekends. For night coverage, one service used dedicated nocturnists, two relied exclusively on moonlighters, and the remainder split coverage between the two.
All the services were located within the university hospital, except for the one at UCSF, which was at an outlying affiliated hospital.
Compensation for non-housestaff hospitalists matched that of staff physicians at two hospitals; the other three hospitals offered non-housestaff physicians some type of financial incentive, either in the form of higher salary, student loan forgiveness, or a combination of the two.
This is an interesting era for hospitalists, who are striving to carve an academic niche for themselves while still performing their clinical duties, Dr. Sehgal notes.
Among the ways they can achieve that is by becoming more involved in other areas of medical center operations, such as information technology, quality and system improvements, and committee work.
Handled properly, these opportunities to collaborate can increase hospitalist prestige and visibility, as they become more involved in hospital leadership and research and share management responsibilities with their medical and surgical colleagues.
Smoothly integrating non-housestaff services into day-to-day function is another opportunity for hospitalists to demonstrate their leadership skills, because the use of these services will increase, Dr. Sehgal adds. This makes it necessary to keep studying the outside services and identifying the ways in which they differ from their housestaff counterparts in order to maximize their contributions.
This study shows that creating non-housestaff services involves the consideration of several important elements, including the patients to be seen by those services, and staffing issues, such as whether the service should be composed exclusively of hospitalists, or if other specialties also should be included.
Hospitalists will have to monitor quality control issues and staff retention, and make sure a two-tiered system does not develop between housestaff and non-housestaff physicians. “So far, there has been very little written about this,” Dr. Sehgal concludes. TH
Norra MacReady is a medical writer based in California.
Addressing the clinical and political issues raised by the use of non-housestaff services is one of the biggest challenges facing hospitalists at academic medical centers, according to a paper in this month’s issue of the Journal of Hospital Medicine.
Lead author Niraj Sehgal, MD, assistant clinical professor of medicine at the University of California, San Francisco (UCSF), and colleagues studied the non-housestaff services at five academic medical centers around the United States to identify what it takes to make the best use of non-housestaff services.
Reliance on these services will grow largely because of restrictions established in 2003 by the Accreditation Council of Graduate Medical Education (ACGME), which limit residents to an 80-hour workweek.
What’s more, it is possible that the ACGME may cut hours even more, given that many other countries have lower restrictions.
In other words, “most academic medical centers now realize residents no longer will be providing as much patient care as they used to,” Dr. Sehgal says.
For example, at UCSF, residents’ hours have been reduced by one-third since the restrictions were established. One way to handle the situation was to reduce the number of patient-hours per resident.
However, at the same time that ruling went into effect, UCSF also increased the number of beds in its hospital. In an effort to determine who is best suited to care for these patients, UCSF and other academic centers turned to non-housestaff services to pick up the slack. “Every residency program has struggled with different models,” he explains.
In their paper, Dr. Sehgal and his colleagues identify nine questions to consider in developing non-housestaff medicine services. The questions reflect key challenges facing medical centers that are building these services, such as:
- System equities: Avoid creating a two-tiered system in which non-housestaff hospitalists who mostly provide clinical care are viewed as second-class citizens compared with academic hospitalists, who also teach and conduct research. This also raises the question of how to define an academic hospitalist;
- Define the patient mix: Should non-housestaff physicians handle less acute patients, specific patient populations, or all patients above the limit for residents?;
- Recruitment and retention: Academic centers may have to offer non-housestaff hospitalists incentives, such as teaching or research opportunities, or financial rewards such as student loan forgiveness, to attract talented clinicians; and
- Compensation and incentives: This should reflect all aspects of the physician’s job, including quality improvement efforts, research activities, and excellence in teaching, as well as clinical productivity.
“How these questions are answered is often driven by local factors, such as the vision of local leadership and the availability of important resources,” Dr. Sehgal and his coauthors write. “Nevertheless, it is important for hospitals to share their experiences since best practices remain unclear.”
To explore how different centers and services address these issues, they compared the non-housestaff medicine services at Brigham and Women’s Hospital in Boston, Emory University in Atlanta, the University of Michigan in Ann Arbor, Northwestern University Medical Center in Chicago, and UCSF. The information was obtained from representatives at each center and was current as of July 2007.
The services ranged in age from two to five years old and covered 168 to 212 clinical days per year, or 15 to 20 shifts per month. Depending on the number of hospitalists in the service, they saw anywhere from 12 to 95 patients per day.
Further, they all provided coverage for 50% of weekends. For night coverage, one service used dedicated nocturnists, two relied exclusively on moonlighters, and the remainder split coverage between the two.
All the services were located within the university hospital, except for the one at UCSF, which was at an outlying affiliated hospital.
Compensation for non-housestaff hospitalists matched that of staff physicians at two hospitals; the other three hospitals offered non-housestaff physicians some type of financial incentive, either in the form of higher salary, student loan forgiveness, or a combination of the two.
This is an interesting era for hospitalists, who are striving to carve an academic niche for themselves while still performing their clinical duties, Dr. Sehgal notes.
Among the ways they can achieve that is by becoming more involved in other areas of medical center operations, such as information technology, quality and system improvements, and committee work.
Handled properly, these opportunities to collaborate can increase hospitalist prestige and visibility, as they become more involved in hospital leadership and research and share management responsibilities with their medical and surgical colleagues.
Smoothly integrating non-housestaff services into day-to-day function is another opportunity for hospitalists to demonstrate their leadership skills, because the use of these services will increase, Dr. Sehgal adds. This makes it necessary to keep studying the outside services and identifying the ways in which they differ from their housestaff counterparts in order to maximize their contributions.
This study shows that creating non-housestaff services involves the consideration of several important elements, including the patients to be seen by those services, and staffing issues, such as whether the service should be composed exclusively of hospitalists, or if other specialties also should be included.
Hospitalists will have to monitor quality control issues and staff retention, and make sure a two-tiered system does not develop between housestaff and non-housestaff physicians. “So far, there has been very little written about this,” Dr. Sehgal concludes. TH
Norra MacReady is a medical writer based in California.
Addressing the clinical and political issues raised by the use of non-housestaff services is one of the biggest challenges facing hospitalists at academic medical centers, according to a paper in this month’s issue of the Journal of Hospital Medicine.
Lead author Niraj Sehgal, MD, assistant clinical professor of medicine at the University of California, San Francisco (UCSF), and colleagues studied the non-housestaff services at five academic medical centers around the United States to identify what it takes to make the best use of non-housestaff services.
Reliance on these services will grow largely because of restrictions established in 2003 by the Accreditation Council of Graduate Medical Education (ACGME), which limit residents to an 80-hour workweek.
What’s more, it is possible that the ACGME may cut hours even more, given that many other countries have lower restrictions.
In other words, “most academic medical centers now realize residents no longer will be providing as much patient care as they used to,” Dr. Sehgal says.
For example, at UCSF, residents’ hours have been reduced by one-third since the restrictions were established. One way to handle the situation was to reduce the number of patient-hours per resident.
However, at the same time that ruling went into effect, UCSF also increased the number of beds in its hospital. In an effort to determine who is best suited to care for these patients, UCSF and other academic centers turned to non-housestaff services to pick up the slack. “Every residency program has struggled with different models,” he explains.
In their paper, Dr. Sehgal and his colleagues identify nine questions to consider in developing non-housestaff medicine services. The questions reflect key challenges facing medical centers that are building these services, such as:
- System equities: Avoid creating a two-tiered system in which non-housestaff hospitalists who mostly provide clinical care are viewed as second-class citizens compared with academic hospitalists, who also teach and conduct research. This also raises the question of how to define an academic hospitalist;
- Define the patient mix: Should non-housestaff physicians handle less acute patients, specific patient populations, or all patients above the limit for residents?;
- Recruitment and retention: Academic centers may have to offer non-housestaff hospitalists incentives, such as teaching or research opportunities, or financial rewards such as student loan forgiveness, to attract talented clinicians; and
- Compensation and incentives: This should reflect all aspects of the physician’s job, including quality improvement efforts, research activities, and excellence in teaching, as well as clinical productivity.
“How these questions are answered is often driven by local factors, such as the vision of local leadership and the availability of important resources,” Dr. Sehgal and his coauthors write. “Nevertheless, it is important for hospitals to share their experiences since best practices remain unclear.”
To explore how different centers and services address these issues, they compared the non-housestaff medicine services at Brigham and Women’s Hospital in Boston, Emory University in Atlanta, the University of Michigan in Ann Arbor, Northwestern University Medical Center in Chicago, and UCSF. The information was obtained from representatives at each center and was current as of July 2007.
The services ranged in age from two to five years old and covered 168 to 212 clinical days per year, or 15 to 20 shifts per month. Depending on the number of hospitalists in the service, they saw anywhere from 12 to 95 patients per day.
Further, they all provided coverage for 50% of weekends. For night coverage, one service used dedicated nocturnists, two relied exclusively on moonlighters, and the remainder split coverage between the two.
All the services were located within the university hospital, except for the one at UCSF, which was at an outlying affiliated hospital.
Compensation for non-housestaff hospitalists matched that of staff physicians at two hospitals; the other three hospitals offered non-housestaff physicians some type of financial incentive, either in the form of higher salary, student loan forgiveness, or a combination of the two.
This is an interesting era for hospitalists, who are striving to carve an academic niche for themselves while still performing their clinical duties, Dr. Sehgal notes.
Among the ways they can achieve that is by becoming more involved in other areas of medical center operations, such as information technology, quality and system improvements, and committee work.
Handled properly, these opportunities to collaborate can increase hospitalist prestige and visibility, as they become more involved in hospital leadership and research and share management responsibilities with their medical and surgical colleagues.
Smoothly integrating non-housestaff services into day-to-day function is another opportunity for hospitalists to demonstrate their leadership skills, because the use of these services will increase, Dr. Sehgal adds. This makes it necessary to keep studying the outside services and identifying the ways in which they differ from their housestaff counterparts in order to maximize their contributions.
This study shows that creating non-housestaff services involves the consideration of several important elements, including the patients to be seen by those services, and staffing issues, such as whether the service should be composed exclusively of hospitalists, or if other specialties also should be included.
Hospitalists will have to monitor quality control issues and staff retention, and make sure a two-tiered system does not develop between housestaff and non-housestaff physicians. “So far, there has been very little written about this,” Dr. Sehgal concludes. TH
Norra MacReady is a medical writer based in California.
Busy Season in Pharma
It’s been an active summer for pharmaceutical firms, who’ve been particularly busy adding and removing products from the marketplace and providing fresh information to professional users. Here’s a roundup of vital information that has emerged.
Market Withdrawals
Because of an increased risk of death associated with aprotinin injection (Trasylol) compared with either aminocaproic acid or tranexamic acid, Bayer Pharmaceuticals has removed all remaining stocks of the agent from the U.S. market. Subsequent access to aprotinin injection will be limited to investigational use based on a special treatment protocol. For more information on this, call (888) 842-2937.
Meanwhile, nedocromil sodium inhalation aerosol (Tilade) has been discontinued. Once current supplies are depleted from pharmacies, it no longer will be available. A number of factors led to the decision, including the inability to find a qualified manufacturer of the chlorofluorocarbon propellant.
New Approvals
Certolizumab pegol (Cimzia) injection has been approved by the Food and Drug Administration (FDA) to treat adults with moderate to severe Crohn’s disease who have not responded to conventional therapies. It is a pegylated tumor necrosis factor antagonist. The most common side effects are headache, upper respiratory infections, abdominal pain, injection site reactions, and nausea. It is dosed as an initial 400 mg SC injection followed by 400 mg SC injections at weeks two and our.
A maintenance regimen of 400 mg subcutaneous every four weeks is recommended for patients who obtain a clinical response after the initial three injections. The drug is available as a package that includes everything required to reconstitute and inject the drug (also two vials of drug, each with 200 mg Cimzia). Patients need to be evaluated for increased infection risk and opportunistic infections. Patients should be screened for tuberculosis prior to commencing therapy.
Desvenlafaxine 50 mg tablets (Pristiq), a serotonin-norepinephrine reuptake inhibitor (SNRI), have been FDA approved for the treatment of adults with major depressive disorder. It is dosed once daily. To reach the therapeutic dose, titration is unnecessary. Dose adjustments are necessary for severe renal impairment or end-stage renal disease patients, where the dose should be adjusted to 50 mg every other day. Nausea, dizziness, hyperhidrosis, constipation, and decreased appetite are the most common side effects.
Lubiprostone capsules (Amitiza) have been FDA approved for the treatment of irritable bowel syndrome with constipation (IBS-C) in women 18 or older. Common side effects are nausea, diarrhea, and abdominal pain. It is dosed as 8 mcg twice a day with food and water. Patients should be periodically assessed for therapy continuation need.
Methylnaltrexone bromide (Relistor) has been FDA approved to assist in restoring bowel function in patients who are continuously receiving opioids for pain management and have late-stage, advanced illness. It works by blocking opioid entrance into smooth muscle. It is administered by injection as often as needed, but not to exceed more than one dose in a 24-hour period.
New Indications
Aripiprazole (Abilify) has received a number of new indications from the FDA, mostly in adolescents and children. In adults, it has received approval as an adjunctive treatment to either lithium or valproate for patients age 10 or older with manic and mixed episodes associated with bipolar I disorder with or without psychotic features. When used as monotherapy for bipolar I disorder in adults, the recommended starting dose for these indications in adults is 15 mg/day with a target dose of 30 mg/day.
Other new indications include:
- Lisdexamfetamine dimesylate (Vyvanse) once-daily prodrug of dexamphetamine has been FDA approved for the treatment of attention deficit/hyperactivity disorder (ADHD) in adults;
- Olopatadine hydrochloride (available as the ocular product Patanol) is now available as a nasal spray (Patanase). It was FDA approved for treatment of the symptoms of seasonal allergic rhinitis in patients age 12 or older;
- Quetiapine (Seroquel) has been FDA approved for maintenance treatment in patients with bipolar I disorder. Quetiapine was already approved for the treatment of schizophrenia and depressive or manic episodes; and
- Risedronate sodium 150 mg tablets (Actonel) have been FDA approved as a once-monthly dose to treat postmenopausal osteoporosis.
New Information
Varicella zoster vaccine, live, attenuated (Zostavax): The Centers for Disease Control and Prevention recommends that all adults age 60 or older be vaccinated against herpes zoster with this new vaccine. The recommendation includes patients with a prior shingles episode and those with chronic medical conditions.
Zoster vaccination is not indicated to treat acute zoster, to prevent people with acute zoster from developing post-herpetic neuralgia (PHN), or to treat ongoing PHN. Before administering zoster vaccine, patients do not need to be asked about their history of varicella (chickenpox) or to have varicella immunity testing. It is administered as a single subcutaneous 0.65 mL dose in the deltoid region of the arm. A booster dose is not licensed for the vaccine.
Medication Error Warning
The Institute for Safe Medication Practices (ISMP) has described increased reports of mixups between U-100 and U-500 insulin. These errors can result in dangerous hyperglycemia or hypoglycemia. Mistakes have occurred when prescribers accidentally selected U-500 regular insulin (R) from computer order entry screens instead of U-100.
Potential reasons for this error:
- The two dosage forms appear one line apart on the screen, making it easy to select the wrong one;
- Depending on the screen size, you may only see the first few words of the product listing, so the drug concentration may not be visible;
- Since use of U-500 insulin is uncommon, you may assume the only listed R insulin is U-100 and not look for the drug’s concentration.
ISMP suggests that use of U-500 insulin has increased due to the obesity epidemic, use in insulin pumps, and tight glucose control protocols in the hospital. ISMP says the major suppliers of these computer systems have agreed to add the word “concentrated” on their selection screens, after “insulin” and before U-500, which should help solve the problem.
New Warnings
The acquired immunodeficiency syndrome (AIDS) drugs abacavir (Ziagen) and didanosine (Videx) are being evaluated by the FDA for a possible link to increased risk of myocardial infarction (MI). This is related to the analyses of data collected from “The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study,” which is a large, international observational study of 33,347 HIV-1 infected patients evaluating short- and long-term adverse effects of anti-HIV treatments. The excess risk of MI in patients taking these agents appeared to be greater in patients with other heart disease risk factors. This is an ongoing review.
Meanwhile, the anemia drugs darbepoetin alfa (Aranesp) and epoetin alfa (Epogen/Procrit) have received a boxed warning regarding increased mortality and/or more rapid tumor progression in patients with cancer that are receiving these agents. The warnings section of the package labeling also was updated with additional study information.
Becaplermin gel (Regranex) is a recombinant form of human platelet-derived growth factor FDA approved for treating lower-extremity diabetic neuropathic ulcers. The FDA is evaluating the possibility of an increased cancer risk in diabetic patients who apply becaplermin gel directly to foot/leg ulcers. A recent study involving patients with no previous history of cancer had a greater risk of dying from cancer if they were prescribed becaplermin three or more times. The FDA believes there may be evidence of an increased cancer death risk in patients who had repeated becaplermin treatments.
Montelukast (Singulair) is undergoing a safety review regarding a possible association between it and behavior/mood changes, suicidality, and suicide. However, it may take up to nine months to complete the review. Other leukotriene receptor antagonists also are being evaluated (e.g., zafirlukast, zileuton).
Mycophenolate mofetil (MMF) and the ester of the active metabolite mycophenolic acid (MPA), known as Cellcept and Myfortic, have received an FDA alert regarding reports of infants born with serious congenital anomalies. These anomalies have included microtia, and cleft lip and palate. These women were taking these drugs to prevent organ rejection following transplant, however, some women were receiving the drugs to manage systemic lupus erythematosus (SLE), and erythema multiforme. These women were receiving the agents before their pregnancies and continued into the first trimester or until the pregnancy was detected. Both MMF and MPA increase the risk of spontaneous abortion in the first trimester and can cause congenital malformations in the children that received the drugs in utero.
The FDA and the manufacturer of natalizumab injection (Tysabri) have informed healthcare professionals of reports of clinically significant liver injury (e.g., markedly elevated serum hepatic enzymes, elevated total bilirubin) within six days of starting natalizumab. The agent is FDA approved to treat multiple sclerosis and Crohn’s Disease. Natalizumab should be discontinued in patients with jaundice or other evidence of significant liver injury. Physicians need to inform patients that natalizumab may cause liver injury. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a registered pharmacist based in New York City.
It’s been an active summer for pharmaceutical firms, who’ve been particularly busy adding and removing products from the marketplace and providing fresh information to professional users. Here’s a roundup of vital information that has emerged.
Market Withdrawals
Because of an increased risk of death associated with aprotinin injection (Trasylol) compared with either aminocaproic acid or tranexamic acid, Bayer Pharmaceuticals has removed all remaining stocks of the agent from the U.S. market. Subsequent access to aprotinin injection will be limited to investigational use based on a special treatment protocol. For more information on this, call (888) 842-2937.
Meanwhile, nedocromil sodium inhalation aerosol (Tilade) has been discontinued. Once current supplies are depleted from pharmacies, it no longer will be available. A number of factors led to the decision, including the inability to find a qualified manufacturer of the chlorofluorocarbon propellant.
New Approvals
Certolizumab pegol (Cimzia) injection has been approved by the Food and Drug Administration (FDA) to treat adults with moderate to severe Crohn’s disease who have not responded to conventional therapies. It is a pegylated tumor necrosis factor antagonist. The most common side effects are headache, upper respiratory infections, abdominal pain, injection site reactions, and nausea. It is dosed as an initial 400 mg SC injection followed by 400 mg SC injections at weeks two and our.
A maintenance regimen of 400 mg subcutaneous every four weeks is recommended for patients who obtain a clinical response after the initial three injections. The drug is available as a package that includes everything required to reconstitute and inject the drug (also two vials of drug, each with 200 mg Cimzia). Patients need to be evaluated for increased infection risk and opportunistic infections. Patients should be screened for tuberculosis prior to commencing therapy.
Desvenlafaxine 50 mg tablets (Pristiq), a serotonin-norepinephrine reuptake inhibitor (SNRI), have been FDA approved for the treatment of adults with major depressive disorder. It is dosed once daily. To reach the therapeutic dose, titration is unnecessary. Dose adjustments are necessary for severe renal impairment or end-stage renal disease patients, where the dose should be adjusted to 50 mg every other day. Nausea, dizziness, hyperhidrosis, constipation, and decreased appetite are the most common side effects.
Lubiprostone capsules (Amitiza) have been FDA approved for the treatment of irritable bowel syndrome with constipation (IBS-C) in women 18 or older. Common side effects are nausea, diarrhea, and abdominal pain. It is dosed as 8 mcg twice a day with food and water. Patients should be periodically assessed for therapy continuation need.
Methylnaltrexone bromide (Relistor) has been FDA approved to assist in restoring bowel function in patients who are continuously receiving opioids for pain management and have late-stage, advanced illness. It works by blocking opioid entrance into smooth muscle. It is administered by injection as often as needed, but not to exceed more than one dose in a 24-hour period.
New Indications
Aripiprazole (Abilify) has received a number of new indications from the FDA, mostly in adolescents and children. In adults, it has received approval as an adjunctive treatment to either lithium or valproate for patients age 10 or older with manic and mixed episodes associated with bipolar I disorder with or without psychotic features. When used as monotherapy for bipolar I disorder in adults, the recommended starting dose for these indications in adults is 15 mg/day with a target dose of 30 mg/day.
Other new indications include:
- Lisdexamfetamine dimesylate (Vyvanse) once-daily prodrug of dexamphetamine has been FDA approved for the treatment of attention deficit/hyperactivity disorder (ADHD) in adults;
- Olopatadine hydrochloride (available as the ocular product Patanol) is now available as a nasal spray (Patanase). It was FDA approved for treatment of the symptoms of seasonal allergic rhinitis in patients age 12 or older;
- Quetiapine (Seroquel) has been FDA approved for maintenance treatment in patients with bipolar I disorder. Quetiapine was already approved for the treatment of schizophrenia and depressive or manic episodes; and
- Risedronate sodium 150 mg tablets (Actonel) have been FDA approved as a once-monthly dose to treat postmenopausal osteoporosis.
New Information
Varicella zoster vaccine, live, attenuated (Zostavax): The Centers for Disease Control and Prevention recommends that all adults age 60 or older be vaccinated against herpes zoster with this new vaccine. The recommendation includes patients with a prior shingles episode and those with chronic medical conditions.
Zoster vaccination is not indicated to treat acute zoster, to prevent people with acute zoster from developing post-herpetic neuralgia (PHN), or to treat ongoing PHN. Before administering zoster vaccine, patients do not need to be asked about their history of varicella (chickenpox) or to have varicella immunity testing. It is administered as a single subcutaneous 0.65 mL dose in the deltoid region of the arm. A booster dose is not licensed for the vaccine.
Medication Error Warning
The Institute for Safe Medication Practices (ISMP) has described increased reports of mixups between U-100 and U-500 insulin. These errors can result in dangerous hyperglycemia or hypoglycemia. Mistakes have occurred when prescribers accidentally selected U-500 regular insulin (R) from computer order entry screens instead of U-100.
Potential reasons for this error:
- The two dosage forms appear one line apart on the screen, making it easy to select the wrong one;
- Depending on the screen size, you may only see the first few words of the product listing, so the drug concentration may not be visible;
- Since use of U-500 insulin is uncommon, you may assume the only listed R insulin is U-100 and not look for the drug’s concentration.
ISMP suggests that use of U-500 insulin has increased due to the obesity epidemic, use in insulin pumps, and tight glucose control protocols in the hospital. ISMP says the major suppliers of these computer systems have agreed to add the word “concentrated” on their selection screens, after “insulin” and before U-500, which should help solve the problem.
New Warnings
The acquired immunodeficiency syndrome (AIDS) drugs abacavir (Ziagen) and didanosine (Videx) are being evaluated by the FDA for a possible link to increased risk of myocardial infarction (MI). This is related to the analyses of data collected from “The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study,” which is a large, international observational study of 33,347 HIV-1 infected patients evaluating short- and long-term adverse effects of anti-HIV treatments. The excess risk of MI in patients taking these agents appeared to be greater in patients with other heart disease risk factors. This is an ongoing review.
Meanwhile, the anemia drugs darbepoetin alfa (Aranesp) and epoetin alfa (Epogen/Procrit) have received a boxed warning regarding increased mortality and/or more rapid tumor progression in patients with cancer that are receiving these agents. The warnings section of the package labeling also was updated with additional study information.
Becaplermin gel (Regranex) is a recombinant form of human platelet-derived growth factor FDA approved for treating lower-extremity diabetic neuropathic ulcers. The FDA is evaluating the possibility of an increased cancer risk in diabetic patients who apply becaplermin gel directly to foot/leg ulcers. A recent study involving patients with no previous history of cancer had a greater risk of dying from cancer if they were prescribed becaplermin three or more times. The FDA believes there may be evidence of an increased cancer death risk in patients who had repeated becaplermin treatments.
Montelukast (Singulair) is undergoing a safety review regarding a possible association between it and behavior/mood changes, suicidality, and suicide. However, it may take up to nine months to complete the review. Other leukotriene receptor antagonists also are being evaluated (e.g., zafirlukast, zileuton).
Mycophenolate mofetil (MMF) and the ester of the active metabolite mycophenolic acid (MPA), known as Cellcept and Myfortic, have received an FDA alert regarding reports of infants born with serious congenital anomalies. These anomalies have included microtia, and cleft lip and palate. These women were taking these drugs to prevent organ rejection following transplant, however, some women were receiving the drugs to manage systemic lupus erythematosus (SLE), and erythema multiforme. These women were receiving the agents before their pregnancies and continued into the first trimester or until the pregnancy was detected. Both MMF and MPA increase the risk of spontaneous abortion in the first trimester and can cause congenital malformations in the children that received the drugs in utero.
The FDA and the manufacturer of natalizumab injection (Tysabri) have informed healthcare professionals of reports of clinically significant liver injury (e.g., markedly elevated serum hepatic enzymes, elevated total bilirubin) within six days of starting natalizumab. The agent is FDA approved to treat multiple sclerosis and Crohn’s Disease. Natalizumab should be discontinued in patients with jaundice or other evidence of significant liver injury. Physicians need to inform patients that natalizumab may cause liver injury. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a registered pharmacist based in New York City.
It’s been an active summer for pharmaceutical firms, who’ve been particularly busy adding and removing products from the marketplace and providing fresh information to professional users. Here’s a roundup of vital information that has emerged.
Market Withdrawals
Because of an increased risk of death associated with aprotinin injection (Trasylol) compared with either aminocaproic acid or tranexamic acid, Bayer Pharmaceuticals has removed all remaining stocks of the agent from the U.S. market. Subsequent access to aprotinin injection will be limited to investigational use based on a special treatment protocol. For more information on this, call (888) 842-2937.
Meanwhile, nedocromil sodium inhalation aerosol (Tilade) has been discontinued. Once current supplies are depleted from pharmacies, it no longer will be available. A number of factors led to the decision, including the inability to find a qualified manufacturer of the chlorofluorocarbon propellant.
New Approvals
Certolizumab pegol (Cimzia) injection has been approved by the Food and Drug Administration (FDA) to treat adults with moderate to severe Crohn’s disease who have not responded to conventional therapies. It is a pegylated tumor necrosis factor antagonist. The most common side effects are headache, upper respiratory infections, abdominal pain, injection site reactions, and nausea. It is dosed as an initial 400 mg SC injection followed by 400 mg SC injections at weeks two and our.
A maintenance regimen of 400 mg subcutaneous every four weeks is recommended for patients who obtain a clinical response after the initial three injections. The drug is available as a package that includes everything required to reconstitute and inject the drug (also two vials of drug, each with 200 mg Cimzia). Patients need to be evaluated for increased infection risk and opportunistic infections. Patients should be screened for tuberculosis prior to commencing therapy.
Desvenlafaxine 50 mg tablets (Pristiq), a serotonin-norepinephrine reuptake inhibitor (SNRI), have been FDA approved for the treatment of adults with major depressive disorder. It is dosed once daily. To reach the therapeutic dose, titration is unnecessary. Dose adjustments are necessary for severe renal impairment or end-stage renal disease patients, where the dose should be adjusted to 50 mg every other day. Nausea, dizziness, hyperhidrosis, constipation, and decreased appetite are the most common side effects.
Lubiprostone capsules (Amitiza) have been FDA approved for the treatment of irritable bowel syndrome with constipation (IBS-C) in women 18 or older. Common side effects are nausea, diarrhea, and abdominal pain. It is dosed as 8 mcg twice a day with food and water. Patients should be periodically assessed for therapy continuation need.
Methylnaltrexone bromide (Relistor) has been FDA approved to assist in restoring bowel function in patients who are continuously receiving opioids for pain management and have late-stage, advanced illness. It works by blocking opioid entrance into smooth muscle. It is administered by injection as often as needed, but not to exceed more than one dose in a 24-hour period.
New Indications
Aripiprazole (Abilify) has received a number of new indications from the FDA, mostly in adolescents and children. In adults, it has received approval as an adjunctive treatment to either lithium or valproate for patients age 10 or older with manic and mixed episodes associated with bipolar I disorder with or without psychotic features. When used as monotherapy for bipolar I disorder in adults, the recommended starting dose for these indications in adults is 15 mg/day with a target dose of 30 mg/day.
Other new indications include:
- Lisdexamfetamine dimesylate (Vyvanse) once-daily prodrug of dexamphetamine has been FDA approved for the treatment of attention deficit/hyperactivity disorder (ADHD) in adults;
- Olopatadine hydrochloride (available as the ocular product Patanol) is now available as a nasal spray (Patanase). It was FDA approved for treatment of the symptoms of seasonal allergic rhinitis in patients age 12 or older;
- Quetiapine (Seroquel) has been FDA approved for maintenance treatment in patients with bipolar I disorder. Quetiapine was already approved for the treatment of schizophrenia and depressive or manic episodes; and
- Risedronate sodium 150 mg tablets (Actonel) have been FDA approved as a once-monthly dose to treat postmenopausal osteoporosis.
New Information
Varicella zoster vaccine, live, attenuated (Zostavax): The Centers for Disease Control and Prevention recommends that all adults age 60 or older be vaccinated against herpes zoster with this new vaccine. The recommendation includes patients with a prior shingles episode and those with chronic medical conditions.
Zoster vaccination is not indicated to treat acute zoster, to prevent people with acute zoster from developing post-herpetic neuralgia (PHN), or to treat ongoing PHN. Before administering zoster vaccine, patients do not need to be asked about their history of varicella (chickenpox) or to have varicella immunity testing. It is administered as a single subcutaneous 0.65 mL dose in the deltoid region of the arm. A booster dose is not licensed for the vaccine.
Medication Error Warning
The Institute for Safe Medication Practices (ISMP) has described increased reports of mixups between U-100 and U-500 insulin. These errors can result in dangerous hyperglycemia or hypoglycemia. Mistakes have occurred when prescribers accidentally selected U-500 regular insulin (R) from computer order entry screens instead of U-100.
Potential reasons for this error:
- The two dosage forms appear one line apart on the screen, making it easy to select the wrong one;
- Depending on the screen size, you may only see the first few words of the product listing, so the drug concentration may not be visible;
- Since use of U-500 insulin is uncommon, you may assume the only listed R insulin is U-100 and not look for the drug’s concentration.
ISMP suggests that use of U-500 insulin has increased due to the obesity epidemic, use in insulin pumps, and tight glucose control protocols in the hospital. ISMP says the major suppliers of these computer systems have agreed to add the word “concentrated” on their selection screens, after “insulin” and before U-500, which should help solve the problem.
New Warnings
The acquired immunodeficiency syndrome (AIDS) drugs abacavir (Ziagen) and didanosine (Videx) are being evaluated by the FDA for a possible link to increased risk of myocardial infarction (MI). This is related to the analyses of data collected from “The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study,” which is a large, international observational study of 33,347 HIV-1 infected patients evaluating short- and long-term adverse effects of anti-HIV treatments. The excess risk of MI in patients taking these agents appeared to be greater in patients with other heart disease risk factors. This is an ongoing review.
Meanwhile, the anemia drugs darbepoetin alfa (Aranesp) and epoetin alfa (Epogen/Procrit) have received a boxed warning regarding increased mortality and/or more rapid tumor progression in patients with cancer that are receiving these agents. The warnings section of the package labeling also was updated with additional study information.
Becaplermin gel (Regranex) is a recombinant form of human platelet-derived growth factor FDA approved for treating lower-extremity diabetic neuropathic ulcers. The FDA is evaluating the possibility of an increased cancer risk in diabetic patients who apply becaplermin gel directly to foot/leg ulcers. A recent study involving patients with no previous history of cancer had a greater risk of dying from cancer if they were prescribed becaplermin three or more times. The FDA believes there may be evidence of an increased cancer death risk in patients who had repeated becaplermin treatments.
Montelukast (Singulair) is undergoing a safety review regarding a possible association between it and behavior/mood changes, suicidality, and suicide. However, it may take up to nine months to complete the review. Other leukotriene receptor antagonists also are being evaluated (e.g., zafirlukast, zileuton).
Mycophenolate mofetil (MMF) and the ester of the active metabolite mycophenolic acid (MPA), known as Cellcept and Myfortic, have received an FDA alert regarding reports of infants born with serious congenital anomalies. These anomalies have included microtia, and cleft lip and palate. These women were taking these drugs to prevent organ rejection following transplant, however, some women were receiving the drugs to manage systemic lupus erythematosus (SLE), and erythema multiforme. These women were receiving the agents before their pregnancies and continued into the first trimester or until the pregnancy was detected. Both MMF and MPA increase the risk of spontaneous abortion in the first trimester and can cause congenital malformations in the children that received the drugs in utero.
The FDA and the manufacturer of natalizumab injection (Tysabri) have informed healthcare professionals of reports of clinically significant liver injury (e.g., markedly elevated serum hepatic enzymes, elevated total bilirubin) within six days of starting natalizumab. The agent is FDA approved to treat multiple sclerosis and Crohn’s Disease. Natalizumab should be discontinued in patients with jaundice or other evidence of significant liver injury. Physicians need to inform patients that natalizumab may cause liver injury. TH
Michele B. Kaufman, PharmD, BSc, RPh, is a registered pharmacist based in New York City.
In the Literature
Literature at a Glance
A guide to this month’s studies.
- Carotid stenting is equivalent to endarterectomy in carotid artery stenosis.
- Idiopathic ventricular fibrillation is associated with early repolarization.
- Aggressive LDL and blood pressure management may benefit diabetic patients.
- Treatment of hypertension in patients older than age 80 improves outcomes.
- Length of stay predicts mortality in pulmonary embolism patients.
- Poor symptom control and lack of family support during end-of-life hospital stays.
- Potentially inappropriate medication use in hospitalized elders is common.
- Steroids may not help in pediatric meningitis.
- PCI and CABG may be equivalent in the treatment of left main disease.
Is Stenting or Endarterectomy Best for Carotid Artery Stenosis?
Background: Patients with moderate to severe symptomatic carotid artery stenosis and those with severe asymptomatic carotid stenosis benefit from carotid endarterectomy. Carotid stenting may provide an alternative therapy, but the long-term protection against stroke compared with endarterectomy is unclear.
Study Design: Prospective randomized trial.
Setting: 29 centers in the United States.
Synopsis: This article reports the long-term (three years) follow-up of the SAPPHIRE trial, published in 2004, which compared carotid stenting to endarterectomy in patients at high surgical risk. In that trial, 334 patients randomized to either stenting or endarterectomy had similar outcomes at one year. Patients were followed for three years with death and major cardiovascular events as endpoints.
Rates of stroke at three years were approximately 10% with an overall death rate of approximately 20%. There was no difference between carotid stenting and endarterectomy with regards to death, stroke, or other cardiovascular outcome.
Notably, follow-up was not complete (78%), a specific type of stenting procedure was used, and the patient population was at high risk for surgical complications. Therefore, results may not be applicable in other centers or in other patient populations. Yet, this trial provides follow-up, long-term evidence that carotid stenting may be a viable alternative to endarterectomy in patients with carotid artery stenosis.
Bottom line: Carotid stenting and endarterectomy had similar outcomes at three years in high-risk patients with carotid artery stenosis.
Citation: Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572-1579.
Is Early Repolarization on EKG Associated with Sudden Cardiac Arrest?
Background: Electrocardiographic early repolarization, defined as elevation of the QRS-ST junction of at least 0.1mV from baseline in the inferior or lateral leads (manifested as slurring or notching), occurs in 1% to 5% of patients. It is considered benign, but experimental studies have suggested it may be arrhythmogenic.
Study Design: Prospective case-control.
Setting: 22 international tertiary care centers.
Synopsis: Case subjects were less than 60 years of age and were resuscitated after ventricular fibrillation (VF) arrest ultimately deemed idiopathic. All had normal echocardiograms, no evidence of coronary artery disease, and no repolarization abnormalities (including Brugada and long-QT). Of 206 patients, 31% had early repolarization on EKG, versus only 5% in controls without heart disease. In case subjects with prior EKGs, early repolarization was proven to be pre-existing.
The mean magnitude of J-point elevation was 2 mm in cases versus 1.2 mm in controls, and in cases this magnitude increased during later episodes of arrhythmia. Electrophysiologic mapping showed that ectopy originated at sites concordant with the location of abnormal repolarization. During five years of follow-up, arrhythmic recurrence was twice as common in cases with early repolarization.
Although long-term observational studies of persons with early repolarization have shown a benign natural course, this study may change our approach to those with syncope or a family history of sudden death.
Bottom line: Early repolarization on EKG is associated with idiopathic ventricular fibrillation.
Citation: Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358(19):2016-2023.
Does Aggressive Blood Pressure and LDL Treatment in Diabetics Affect Development of Subclinical Atherosclerosis?
Background: There is evidence to suggest more aggressive treatment of LDL cholesterol in patients with known coronary artery disease is beneficial and more aggressive blood pressure control can improve outcomes in some patient populations. However, it is unclear if patients with diabetes without cardiovascular disease would benefit from more aggressive LDL and systolic blood pressure (SBP) treatment.
Study Design: Randomized, open-label, blinded-to-end point trial.
Setting: Four centers in Oklahoma, Arizona, and South Dakota.
Synopsis: Investigators studied 499 type 2 diabetic American Indian men with no history of cardiovascular disease. Patients were randomized to receive treatment to achieve aggressive (70 mg/dL and 115 mmHg) or standard (100 mg/dL and 130 mmHg) targets for their LDL cholesterol and SBP, respectively.
At three years, the aggressive group showed decreased carotid intima-media thickness (IMT) and decreased left ventricular mass, whereas both IMT and left ventricular volume increased in the standard group. There were no differences in clinical cardiovascular events between the aggressive and standard group and both groups had lower-than-expected clinical events.
This study included no women and was limited to an American Indian population. Of note, there was an increase in adverse events related to blood pressure medications in the aggressive group. It also is unclear how the surrogates of cardiovascular disease or subclinical atherosclerosis relate to significant clinical outcomes.
Bottom line: More aggressive LDL and SBP treatment in diabetics without coronary disease decreased subclinical atherosclerosis but did not impact clinical outcomes.
Citation: Howard B, Roman M, Devereux R, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes. JAMA. 2008;299(14):1678-1689.
Should We Treat Hypertension in Patients Older Than 80?
Background: There is debate about whether treatment of hypertension in the elderly is beneficial. Numerous studies suggest blood pressure control does less to prevent strokes in patients older than 80 years than for younger patients. Moreover, other evidence shows controlling blood pressure in elderly patients may result in an increase in mortality even if there was a decreased risk of stroke.
Study Design: Randomized, double-blind, placebo-controlled trial.
Setting: 195 centers in 13 countries in Europe, China, Australasia, and North Africa.
Synopsis: This study evaluated 3,845 patients, age 80 or older, with a sustained systolic blood pressure (SBP) of 160 mmHg and randomized them to receive indapamide (sustained release) or placebo. Perindopril, or placebo, was added if necessary to achieve a target blood pressure of 150/80 mmHg. Patients who received the indapamide with or without the perindopril had lower blood pressure, lower rate of stroke, lower rate of heart failure, lower rate of death from a cardiovascular cause, and a 21% reduction in all-cause mortality (all statistically significant). There were very few adverse drug events and fewer adverse events overall in the treatment group.
Of note, exclusion criteria included a history of heart failure requiring anti-hypertensive medication, dementia, need for nursing care, an inability to stand or walk, and a creatinine more than 1.7 mg/dL. As well, the “target” SBP of 150 mmHg (which only half of the treatment group achieved) is still considered hypertensive according to the JNC 7 guidelines.
Bottom line: In some patients older than 80, treatment of hypertension may reduce the incidence of stroke, death from stroke, heart failure, and all-cause mortality.
Citation: Beckett N, Peters R, Fletcher A, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
What Is the Optimal Hospital LOS for Patients with PE?
Background: Though there are clear trends toward shorter hospital stays after pulmonary embolism (PE), especially with the introduction of low molecular weight heparin, the optimal timing of discharge and the effect of decreased length of stay (LOS) on post-discharge mortality are unknown. Furthermore, there is no risk stratification strategy used to identify low-risk patients with PE who can safely be discharged early or treated in the outpatient setting.
Study Design: Retrospective cohort study.
Setting: 186 acute care hospitals in Pennsylvania from January 2000 to November 2002.
Synopsis: Using a statewide database of 15,531 patients discharged with pulmonary embolism (PE), the authors sought to identify patient and hospital factors associated with LOS and assess whether LOS was associated with post-discharge mortality.
Findings indicate there is considerable variation in LOS for PE between and within hospitals in Pennsylvania. The median LOS for patients with PE was six days; patients with a LOS of four or fewer days had significantly higher post-discharge mortality than patients hospitalized five to eight days. More than half the patients discharged at four or fewer days were classified as high-risk, with Pulmonary Embolism Severity Index (PESI) scores of III-V (3.1% to 24.5% risk of mortality at 30 days).
Although we cannot infer causation (i.e., early discharge=death), clinicians should be aware of the results and consider severity of illness (using PESI or other criteria) in the discharge decision in patients with PE. Future prognostic models and evidence-based criteria would be helpful to identify patients with PE who can be safely discharged early.
Bottom line: Physicians may inappropriately select patients with PE for early discharge who are at increased risk of complications.
Citation: Aujesky D, Stone RA, Kim S, et al. Length of hospital stay and post-discharge mortality in patients with pulmonary embolism. Arch Intern Med. 2008;168(7):706-712.
Do Patients Have a “Good Death” in the Hospital?
Background: Despite an increasing focus on providing appropriate end-of-life care, the majority of patients in developed countries die in the hospital. The circumstances and quality of care provided at the time of death are poorly described.
Study Design: Cross-sectional survey.
Setting: 613 departments in 200 French hospitals.
Synopsis: For 3,793 in-hospital deaths, the investigators surveyed the bedside nurses about the circumstances and details of the death. Twenty-three percent of the patients were admitted for end-of-life care, 29% had a malignancy, and 50% of patients were identified as terminally ill for three days prior to their death.
A family member or relative was present in only 25% of all deaths; 20% of patients were alone at the time of death. In the last few hours of life, up to 70% of patients had symptoms of respiratory distress, while only 44% received opiate analgesia. Only 35% of nurses were satisfied with the quality of death. Satisfaction increased with presence of family members and having written protocols for care at the end of life.
This large, multicenter study has limitations but provides a concerning snapshot of death in the hospital. Hospitalists should be aggressive about symptom control at the end of life as well as attempt to ensure patients are not alone at the time of death.
Bottom line: Many patients die in the hospital in some degree of respiratory distress and without family or friends at the bedside.
Citation: Ferrand E, Jabre P, Vincent-Genod C, et al. Circumstances of death in hospitalized patients and nurses’ perceptions. Arch Intern Med. 2008;168(8):867-875.
How Common Is Potentially Inappropriate Medication Use in the Hospital?
Background: Use of potentially inappropriate medications (PIM) in the elderly based on the Beers’ List is common in nursing homes, the emergency department (ED), and outpatient settings and is associated with adverse outcomes and hospitalization. Frequency of PIM use the inpatient setting has not been well studied.
Study Design: A retrospective cohort study.
Setting: 384 U.S. hospitals.
Synopsis: In this retrospective cohort study of 493,971 inpatients (older than 65) admitted with medical diagnoses to non-surgeons, PIM prescription was evaluated. Forty nine percent of all patients were prescribed at least one PIM, while 6% were prescribed three or more. In a multivariable model, physician specialty was associated with variation in high severity PIM (HSPIM) prescription. In comparison with internal medicine physicians, cardiologists (odds ratio [OR] 1.32) and pulmonologists (OR 1.10) were more likely to prescribe HSPIMs, while hospitalists (OR 0.90) and geriatricians (OR 0.60) were less likely. In addition, patient age older than 85 was associated with decreased HSPIM prescription (OR 0.59) compared with those younger than 85.
Compared with patients in the Midwest, patients in the South (OR 1.63) and West (OR 1.43) were more likely to prescribe HSPIMs, while those in the Northeast (OR 0.85) were less likely. Hospitals with geriatric services had less PIM use. The study couldn’t account for continuation of chronic medications and did not evaluate adverse outcomes from PIM prescribing.
Bottom line: PIM prescription to hospitalized geriatric patients is common and associated with provider and hospital characteristics.
Citation: Rothberg MB, Pekow PS, Liu F, et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3:91-102:91-102.
Is There a Benefit to Corticosteroids When Treating Bacterial Meningitis in Children?
Background: The benefit of adjuvant corticosteroids in the treatment of bacterial meningitis in children in the developed world remains unclear; recent expert guidelines reflect this uncertainty.
Study Design: Retrospective cohort study.
Setting: Twenty-seven tertiary care hospitals in the United States.
Synopsis: Researchers examined 2,780 children with a primary diagnosis of bacterial meningitis discharged from 27 tertiary care centers in the U.S. from 2001-2006. Using a propensity analysis (to control for severity of illness), the study compared those who had received adjunctive corticosteroids with those who had not, with mortality and length of study (LOS) as primary outcomes.
The median age was nine months, 8.9% of children received corticosteroids, and the overall mortality rate was 4.2%. Adjuvent corticosteroids did not reduce mortality or LOS. The outcomes were unchanged in subgroup analyses.
Although limited by its retrospective design and lack of other outcome measures (e.g., hearing loss, neurological deficits), this study provides reasonable evidence that corticosteroid use in bacterial meningitis in children may not save lives or shorten LOS. Pediatric hospitalists may not want to routinely give steroids in this setting pending large randomized-controlled trials.
Bottom line: Adjunctive corticosteroids therapy in children with bacterial meningitis may not save lives or reduce LOS.
Citation: Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055.
Should Unprotected Left Main Disease Be Treated With PCI or CABG?
Background: The current standard of care for the treatment of left main coronary artery disease is coronary-artery bypass grafting (CABG). With the advent of drug-eluting stents, there is growing interest in the use of percutaneous coronary intervention (PCI) to treat left main disease.
Study Design: Prospective observational study.
Setting: Twelve Korean cardiac centers.
Synopsis: From 2000 to 2006, patients with left main disease were treated with PCI or CABG at the discretion of the physician. Nearly 1,100 patients in each cohort were compared and evaluated for death and a composite outcome of death, myocardial infarction, or stroke. Propensity-matching was employed to control for confounders.
In the overall cohort matched by propensity score, there was no significant difference in death or the composite outcome between the PCI and CABG groups after three years. Type of stent (bare metal vs. drug-eluting) did not affect the outcome. Rates of target-vessel revascularization were significantly higher in the group that received stents.
The results are limited by the observational nature and the need for propensity analysis and yet provide an intriguing result. The standard of care for treatment of left main disease remains CABG, but clinicians may be more comfortable treating with stents while we await randomized-controlled trials.
Bottom line: In this observational study, PCI and CABG had similar outcomes in patients with left main disease.
Citation: Seung KB, Park D, Kim Y, Lee S. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781-1792.
Literature at a Glance
A guide to this month’s studies.
- Carotid stenting is equivalent to endarterectomy in carotid artery stenosis.
- Idiopathic ventricular fibrillation is associated with early repolarization.
- Aggressive LDL and blood pressure management may benefit diabetic patients.
- Treatment of hypertension in patients older than age 80 improves outcomes.
- Length of stay predicts mortality in pulmonary embolism patients.
- Poor symptom control and lack of family support during end-of-life hospital stays.
- Potentially inappropriate medication use in hospitalized elders is common.
- Steroids may not help in pediatric meningitis.
- PCI and CABG may be equivalent in the treatment of left main disease.
Is Stenting or Endarterectomy Best for Carotid Artery Stenosis?
Background: Patients with moderate to severe symptomatic carotid artery stenosis and those with severe asymptomatic carotid stenosis benefit from carotid endarterectomy. Carotid stenting may provide an alternative therapy, but the long-term protection against stroke compared with endarterectomy is unclear.
Study Design: Prospective randomized trial.
Setting: 29 centers in the United States.
Synopsis: This article reports the long-term (three years) follow-up of the SAPPHIRE trial, published in 2004, which compared carotid stenting to endarterectomy in patients at high surgical risk. In that trial, 334 patients randomized to either stenting or endarterectomy had similar outcomes at one year. Patients were followed for three years with death and major cardiovascular events as endpoints.
Rates of stroke at three years were approximately 10% with an overall death rate of approximately 20%. There was no difference between carotid stenting and endarterectomy with regards to death, stroke, or other cardiovascular outcome.
Notably, follow-up was not complete (78%), a specific type of stenting procedure was used, and the patient population was at high risk for surgical complications. Therefore, results may not be applicable in other centers or in other patient populations. Yet, this trial provides follow-up, long-term evidence that carotid stenting may be a viable alternative to endarterectomy in patients with carotid artery stenosis.
Bottom line: Carotid stenting and endarterectomy had similar outcomes at three years in high-risk patients with carotid artery stenosis.
Citation: Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572-1579.
Is Early Repolarization on EKG Associated with Sudden Cardiac Arrest?
Background: Electrocardiographic early repolarization, defined as elevation of the QRS-ST junction of at least 0.1mV from baseline in the inferior or lateral leads (manifested as slurring or notching), occurs in 1% to 5% of patients. It is considered benign, but experimental studies have suggested it may be arrhythmogenic.
Study Design: Prospective case-control.
Setting: 22 international tertiary care centers.
Synopsis: Case subjects were less than 60 years of age and were resuscitated after ventricular fibrillation (VF) arrest ultimately deemed idiopathic. All had normal echocardiograms, no evidence of coronary artery disease, and no repolarization abnormalities (including Brugada and long-QT). Of 206 patients, 31% had early repolarization on EKG, versus only 5% in controls without heart disease. In case subjects with prior EKGs, early repolarization was proven to be pre-existing.
The mean magnitude of J-point elevation was 2 mm in cases versus 1.2 mm in controls, and in cases this magnitude increased during later episodes of arrhythmia. Electrophysiologic mapping showed that ectopy originated at sites concordant with the location of abnormal repolarization. During five years of follow-up, arrhythmic recurrence was twice as common in cases with early repolarization.
Although long-term observational studies of persons with early repolarization have shown a benign natural course, this study may change our approach to those with syncope or a family history of sudden death.
Bottom line: Early repolarization on EKG is associated with idiopathic ventricular fibrillation.
Citation: Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358(19):2016-2023.
Does Aggressive Blood Pressure and LDL Treatment in Diabetics Affect Development of Subclinical Atherosclerosis?
Background: There is evidence to suggest more aggressive treatment of LDL cholesterol in patients with known coronary artery disease is beneficial and more aggressive blood pressure control can improve outcomes in some patient populations. However, it is unclear if patients with diabetes without cardiovascular disease would benefit from more aggressive LDL and systolic blood pressure (SBP) treatment.
Study Design: Randomized, open-label, blinded-to-end point trial.
Setting: Four centers in Oklahoma, Arizona, and South Dakota.
Synopsis: Investigators studied 499 type 2 diabetic American Indian men with no history of cardiovascular disease. Patients were randomized to receive treatment to achieve aggressive (70 mg/dL and 115 mmHg) or standard (100 mg/dL and 130 mmHg) targets for their LDL cholesterol and SBP, respectively.
At three years, the aggressive group showed decreased carotid intima-media thickness (IMT) and decreased left ventricular mass, whereas both IMT and left ventricular volume increased in the standard group. There were no differences in clinical cardiovascular events between the aggressive and standard group and both groups had lower-than-expected clinical events.
This study included no women and was limited to an American Indian population. Of note, there was an increase in adverse events related to blood pressure medications in the aggressive group. It also is unclear how the surrogates of cardiovascular disease or subclinical atherosclerosis relate to significant clinical outcomes.
Bottom line: More aggressive LDL and SBP treatment in diabetics without coronary disease decreased subclinical atherosclerosis but did not impact clinical outcomes.
Citation: Howard B, Roman M, Devereux R, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes. JAMA. 2008;299(14):1678-1689.
Should We Treat Hypertension in Patients Older Than 80?
Background: There is debate about whether treatment of hypertension in the elderly is beneficial. Numerous studies suggest blood pressure control does less to prevent strokes in patients older than 80 years than for younger patients. Moreover, other evidence shows controlling blood pressure in elderly patients may result in an increase in mortality even if there was a decreased risk of stroke.
Study Design: Randomized, double-blind, placebo-controlled trial.
Setting: 195 centers in 13 countries in Europe, China, Australasia, and North Africa.
Synopsis: This study evaluated 3,845 patients, age 80 or older, with a sustained systolic blood pressure (SBP) of 160 mmHg and randomized them to receive indapamide (sustained release) or placebo. Perindopril, or placebo, was added if necessary to achieve a target blood pressure of 150/80 mmHg. Patients who received the indapamide with or without the perindopril had lower blood pressure, lower rate of stroke, lower rate of heart failure, lower rate of death from a cardiovascular cause, and a 21% reduction in all-cause mortality (all statistically significant). There were very few adverse drug events and fewer adverse events overall in the treatment group.
Of note, exclusion criteria included a history of heart failure requiring anti-hypertensive medication, dementia, need for nursing care, an inability to stand or walk, and a creatinine more than 1.7 mg/dL. As well, the “target” SBP of 150 mmHg (which only half of the treatment group achieved) is still considered hypertensive according to the JNC 7 guidelines.
Bottom line: In some patients older than 80, treatment of hypertension may reduce the incidence of stroke, death from stroke, heart failure, and all-cause mortality.
Citation: Beckett N, Peters R, Fletcher A, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
What Is the Optimal Hospital LOS for Patients with PE?
Background: Though there are clear trends toward shorter hospital stays after pulmonary embolism (PE), especially with the introduction of low molecular weight heparin, the optimal timing of discharge and the effect of decreased length of stay (LOS) on post-discharge mortality are unknown. Furthermore, there is no risk stratification strategy used to identify low-risk patients with PE who can safely be discharged early or treated in the outpatient setting.
Study Design: Retrospective cohort study.
Setting: 186 acute care hospitals in Pennsylvania from January 2000 to November 2002.
Synopsis: Using a statewide database of 15,531 patients discharged with pulmonary embolism (PE), the authors sought to identify patient and hospital factors associated with LOS and assess whether LOS was associated with post-discharge mortality.
Findings indicate there is considerable variation in LOS for PE between and within hospitals in Pennsylvania. The median LOS for patients with PE was six days; patients with a LOS of four or fewer days had significantly higher post-discharge mortality than patients hospitalized five to eight days. More than half the patients discharged at four or fewer days were classified as high-risk, with Pulmonary Embolism Severity Index (PESI) scores of III-V (3.1% to 24.5% risk of mortality at 30 days).
Although we cannot infer causation (i.e., early discharge=death), clinicians should be aware of the results and consider severity of illness (using PESI or other criteria) in the discharge decision in patients with PE. Future prognostic models and evidence-based criteria would be helpful to identify patients with PE who can be safely discharged early.
Bottom line: Physicians may inappropriately select patients with PE for early discharge who are at increased risk of complications.
Citation: Aujesky D, Stone RA, Kim S, et al. Length of hospital stay and post-discharge mortality in patients with pulmonary embolism. Arch Intern Med. 2008;168(7):706-712.
Do Patients Have a “Good Death” in the Hospital?
Background: Despite an increasing focus on providing appropriate end-of-life care, the majority of patients in developed countries die in the hospital. The circumstances and quality of care provided at the time of death are poorly described.
Study Design: Cross-sectional survey.
Setting: 613 departments in 200 French hospitals.
Synopsis: For 3,793 in-hospital deaths, the investigators surveyed the bedside nurses about the circumstances and details of the death. Twenty-three percent of the patients were admitted for end-of-life care, 29% had a malignancy, and 50% of patients were identified as terminally ill for three days prior to their death.
A family member or relative was present in only 25% of all deaths; 20% of patients were alone at the time of death. In the last few hours of life, up to 70% of patients had symptoms of respiratory distress, while only 44% received opiate analgesia. Only 35% of nurses were satisfied with the quality of death. Satisfaction increased with presence of family members and having written protocols for care at the end of life.
This large, multicenter study has limitations but provides a concerning snapshot of death in the hospital. Hospitalists should be aggressive about symptom control at the end of life as well as attempt to ensure patients are not alone at the time of death.
Bottom line: Many patients die in the hospital in some degree of respiratory distress and without family or friends at the bedside.
Citation: Ferrand E, Jabre P, Vincent-Genod C, et al. Circumstances of death in hospitalized patients and nurses’ perceptions. Arch Intern Med. 2008;168(8):867-875.
How Common Is Potentially Inappropriate Medication Use in the Hospital?
Background: Use of potentially inappropriate medications (PIM) in the elderly based on the Beers’ List is common in nursing homes, the emergency department (ED), and outpatient settings and is associated with adverse outcomes and hospitalization. Frequency of PIM use the inpatient setting has not been well studied.
Study Design: A retrospective cohort study.
Setting: 384 U.S. hospitals.
Synopsis: In this retrospective cohort study of 493,971 inpatients (older than 65) admitted with medical diagnoses to non-surgeons, PIM prescription was evaluated. Forty nine percent of all patients were prescribed at least one PIM, while 6% were prescribed three or more. In a multivariable model, physician specialty was associated with variation in high severity PIM (HSPIM) prescription. In comparison with internal medicine physicians, cardiologists (odds ratio [OR] 1.32) and pulmonologists (OR 1.10) were more likely to prescribe HSPIMs, while hospitalists (OR 0.90) and geriatricians (OR 0.60) were less likely. In addition, patient age older than 85 was associated with decreased HSPIM prescription (OR 0.59) compared with those younger than 85.
Compared with patients in the Midwest, patients in the South (OR 1.63) and West (OR 1.43) were more likely to prescribe HSPIMs, while those in the Northeast (OR 0.85) were less likely. Hospitals with geriatric services had less PIM use. The study couldn’t account for continuation of chronic medications and did not evaluate adverse outcomes from PIM prescribing.
Bottom line: PIM prescription to hospitalized geriatric patients is common and associated with provider and hospital characteristics.
Citation: Rothberg MB, Pekow PS, Liu F, et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3:91-102:91-102.
Is There a Benefit to Corticosteroids When Treating Bacterial Meningitis in Children?
Background: The benefit of adjuvant corticosteroids in the treatment of bacterial meningitis in children in the developed world remains unclear; recent expert guidelines reflect this uncertainty.
Study Design: Retrospective cohort study.
Setting: Twenty-seven tertiary care hospitals in the United States.
Synopsis: Researchers examined 2,780 children with a primary diagnosis of bacterial meningitis discharged from 27 tertiary care centers in the U.S. from 2001-2006. Using a propensity analysis (to control for severity of illness), the study compared those who had received adjunctive corticosteroids with those who had not, with mortality and length of study (LOS) as primary outcomes.
The median age was nine months, 8.9% of children received corticosteroids, and the overall mortality rate was 4.2%. Adjuvent corticosteroids did not reduce mortality or LOS. The outcomes were unchanged in subgroup analyses.
Although limited by its retrospective design and lack of other outcome measures (e.g., hearing loss, neurological deficits), this study provides reasonable evidence that corticosteroid use in bacterial meningitis in children may not save lives or shorten LOS. Pediatric hospitalists may not want to routinely give steroids in this setting pending large randomized-controlled trials.
Bottom line: Adjunctive corticosteroids therapy in children with bacterial meningitis may not save lives or reduce LOS.
Citation: Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055.
Should Unprotected Left Main Disease Be Treated With PCI or CABG?
Background: The current standard of care for the treatment of left main coronary artery disease is coronary-artery bypass grafting (CABG). With the advent of drug-eluting stents, there is growing interest in the use of percutaneous coronary intervention (PCI) to treat left main disease.
Study Design: Prospective observational study.
Setting: Twelve Korean cardiac centers.
Synopsis: From 2000 to 2006, patients with left main disease were treated with PCI or CABG at the discretion of the physician. Nearly 1,100 patients in each cohort were compared and evaluated for death and a composite outcome of death, myocardial infarction, or stroke. Propensity-matching was employed to control for confounders.
In the overall cohort matched by propensity score, there was no significant difference in death or the composite outcome between the PCI and CABG groups after three years. Type of stent (bare metal vs. drug-eluting) did not affect the outcome. Rates of target-vessel revascularization were significantly higher in the group that received stents.
The results are limited by the observational nature and the need for propensity analysis and yet provide an intriguing result. The standard of care for treatment of left main disease remains CABG, but clinicians may be more comfortable treating with stents while we await randomized-controlled trials.
Bottom line: In this observational study, PCI and CABG had similar outcomes in patients with left main disease.
Citation: Seung KB, Park D, Kim Y, Lee S. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781-1792.
Literature at a Glance
A guide to this month’s studies.
- Carotid stenting is equivalent to endarterectomy in carotid artery stenosis.
- Idiopathic ventricular fibrillation is associated with early repolarization.
- Aggressive LDL and blood pressure management may benefit diabetic patients.
- Treatment of hypertension in patients older than age 80 improves outcomes.
- Length of stay predicts mortality in pulmonary embolism patients.
- Poor symptom control and lack of family support during end-of-life hospital stays.
- Potentially inappropriate medication use in hospitalized elders is common.
- Steroids may not help in pediatric meningitis.
- PCI and CABG may be equivalent in the treatment of left main disease.
Is Stenting or Endarterectomy Best for Carotid Artery Stenosis?
Background: Patients with moderate to severe symptomatic carotid artery stenosis and those with severe asymptomatic carotid stenosis benefit from carotid endarterectomy. Carotid stenting may provide an alternative therapy, but the long-term protection against stroke compared with endarterectomy is unclear.
Study Design: Prospective randomized trial.
Setting: 29 centers in the United States.
Synopsis: This article reports the long-term (three years) follow-up of the SAPPHIRE trial, published in 2004, which compared carotid stenting to endarterectomy in patients at high surgical risk. In that trial, 334 patients randomized to either stenting or endarterectomy had similar outcomes at one year. Patients were followed for three years with death and major cardiovascular events as endpoints.
Rates of stroke at three years were approximately 10% with an overall death rate of approximately 20%. There was no difference between carotid stenting and endarterectomy with regards to death, stroke, or other cardiovascular outcome.
Notably, follow-up was not complete (78%), a specific type of stenting procedure was used, and the patient population was at high risk for surgical complications. Therefore, results may not be applicable in other centers or in other patient populations. Yet, this trial provides follow-up, long-term evidence that carotid stenting may be a viable alternative to endarterectomy in patients with carotid artery stenosis.
Bottom line: Carotid stenting and endarterectomy had similar outcomes at three years in high-risk patients with carotid artery stenosis.
Citation: Gurm HS, Yadav JS, Fayad P, et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572-1579.
Is Early Repolarization on EKG Associated with Sudden Cardiac Arrest?
Background: Electrocardiographic early repolarization, defined as elevation of the QRS-ST junction of at least 0.1mV from baseline in the inferior or lateral leads (manifested as slurring or notching), occurs in 1% to 5% of patients. It is considered benign, but experimental studies have suggested it may be arrhythmogenic.
Study Design: Prospective case-control.
Setting: 22 international tertiary care centers.
Synopsis: Case subjects were less than 60 years of age and were resuscitated after ventricular fibrillation (VF) arrest ultimately deemed idiopathic. All had normal echocardiograms, no evidence of coronary artery disease, and no repolarization abnormalities (including Brugada and long-QT). Of 206 patients, 31% had early repolarization on EKG, versus only 5% in controls without heart disease. In case subjects with prior EKGs, early repolarization was proven to be pre-existing.
The mean magnitude of J-point elevation was 2 mm in cases versus 1.2 mm in controls, and in cases this magnitude increased during later episodes of arrhythmia. Electrophysiologic mapping showed that ectopy originated at sites concordant with the location of abnormal repolarization. During five years of follow-up, arrhythmic recurrence was twice as common in cases with early repolarization.
Although long-term observational studies of persons with early repolarization have shown a benign natural course, this study may change our approach to those with syncope or a family history of sudden death.
Bottom line: Early repolarization on EKG is associated with idiopathic ventricular fibrillation.
Citation: Haissaguerre M, Derval N, Sacher F, et al. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358(19):2016-2023.
Does Aggressive Blood Pressure and LDL Treatment in Diabetics Affect Development of Subclinical Atherosclerosis?
Background: There is evidence to suggest more aggressive treatment of LDL cholesterol in patients with known coronary artery disease is beneficial and more aggressive blood pressure control can improve outcomes in some patient populations. However, it is unclear if patients with diabetes without cardiovascular disease would benefit from more aggressive LDL and systolic blood pressure (SBP) treatment.
Study Design: Randomized, open-label, blinded-to-end point trial.
Setting: Four centers in Oklahoma, Arizona, and South Dakota.
Synopsis: Investigators studied 499 type 2 diabetic American Indian men with no history of cardiovascular disease. Patients were randomized to receive treatment to achieve aggressive (70 mg/dL and 115 mmHg) or standard (100 mg/dL and 130 mmHg) targets for their LDL cholesterol and SBP, respectively.
At three years, the aggressive group showed decreased carotid intima-media thickness (IMT) and decreased left ventricular mass, whereas both IMT and left ventricular volume increased in the standard group. There were no differences in clinical cardiovascular events between the aggressive and standard group and both groups had lower-than-expected clinical events.
This study included no women and was limited to an American Indian population. Of note, there was an increase in adverse events related to blood pressure medications in the aggressive group. It also is unclear how the surrogates of cardiovascular disease or subclinical atherosclerosis relate to significant clinical outcomes.
Bottom line: More aggressive LDL and SBP treatment in diabetics without coronary disease decreased subclinical atherosclerosis but did not impact clinical outcomes.
Citation: Howard B, Roman M, Devereux R, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes. JAMA. 2008;299(14):1678-1689.
Should We Treat Hypertension in Patients Older Than 80?
Background: There is debate about whether treatment of hypertension in the elderly is beneficial. Numerous studies suggest blood pressure control does less to prevent strokes in patients older than 80 years than for younger patients. Moreover, other evidence shows controlling blood pressure in elderly patients may result in an increase in mortality even if there was a decreased risk of stroke.
Study Design: Randomized, double-blind, placebo-controlled trial.
Setting: 195 centers in 13 countries in Europe, China, Australasia, and North Africa.
Synopsis: This study evaluated 3,845 patients, age 80 or older, with a sustained systolic blood pressure (SBP) of 160 mmHg and randomized them to receive indapamide (sustained release) or placebo. Perindopril, or placebo, was added if necessary to achieve a target blood pressure of 150/80 mmHg. Patients who received the indapamide with or without the perindopril had lower blood pressure, lower rate of stroke, lower rate of heart failure, lower rate of death from a cardiovascular cause, and a 21% reduction in all-cause mortality (all statistically significant). There were very few adverse drug events and fewer adverse events overall in the treatment group.
Of note, exclusion criteria included a history of heart failure requiring anti-hypertensive medication, dementia, need for nursing care, an inability to stand or walk, and a creatinine more than 1.7 mg/dL. As well, the “target” SBP of 150 mmHg (which only half of the treatment group achieved) is still considered hypertensive according to the JNC 7 guidelines.
Bottom line: In some patients older than 80, treatment of hypertension may reduce the incidence of stroke, death from stroke, heart failure, and all-cause mortality.
Citation: Beckett N, Peters R, Fletcher A, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887-1898.
What Is the Optimal Hospital LOS for Patients with PE?
Background: Though there are clear trends toward shorter hospital stays after pulmonary embolism (PE), especially with the introduction of low molecular weight heparin, the optimal timing of discharge and the effect of decreased length of stay (LOS) on post-discharge mortality are unknown. Furthermore, there is no risk stratification strategy used to identify low-risk patients with PE who can safely be discharged early or treated in the outpatient setting.
Study Design: Retrospective cohort study.
Setting: 186 acute care hospitals in Pennsylvania from January 2000 to November 2002.
Synopsis: Using a statewide database of 15,531 patients discharged with pulmonary embolism (PE), the authors sought to identify patient and hospital factors associated with LOS and assess whether LOS was associated with post-discharge mortality.
Findings indicate there is considerable variation in LOS for PE between and within hospitals in Pennsylvania. The median LOS for patients with PE was six days; patients with a LOS of four or fewer days had significantly higher post-discharge mortality than patients hospitalized five to eight days. More than half the patients discharged at four or fewer days were classified as high-risk, with Pulmonary Embolism Severity Index (PESI) scores of III-V (3.1% to 24.5% risk of mortality at 30 days).
Although we cannot infer causation (i.e., early discharge=death), clinicians should be aware of the results and consider severity of illness (using PESI or other criteria) in the discharge decision in patients with PE. Future prognostic models and evidence-based criteria would be helpful to identify patients with PE who can be safely discharged early.
Bottom line: Physicians may inappropriately select patients with PE for early discharge who are at increased risk of complications.
Citation: Aujesky D, Stone RA, Kim S, et al. Length of hospital stay and post-discharge mortality in patients with pulmonary embolism. Arch Intern Med. 2008;168(7):706-712.
Do Patients Have a “Good Death” in the Hospital?
Background: Despite an increasing focus on providing appropriate end-of-life care, the majority of patients in developed countries die in the hospital. The circumstances and quality of care provided at the time of death are poorly described.
Study Design: Cross-sectional survey.
Setting: 613 departments in 200 French hospitals.
Synopsis: For 3,793 in-hospital deaths, the investigators surveyed the bedside nurses about the circumstances and details of the death. Twenty-three percent of the patients were admitted for end-of-life care, 29% had a malignancy, and 50% of patients were identified as terminally ill for three days prior to their death.
A family member or relative was present in only 25% of all deaths; 20% of patients were alone at the time of death. In the last few hours of life, up to 70% of patients had symptoms of respiratory distress, while only 44% received opiate analgesia. Only 35% of nurses were satisfied with the quality of death. Satisfaction increased with presence of family members and having written protocols for care at the end of life.
This large, multicenter study has limitations but provides a concerning snapshot of death in the hospital. Hospitalists should be aggressive about symptom control at the end of life as well as attempt to ensure patients are not alone at the time of death.
Bottom line: Many patients die in the hospital in some degree of respiratory distress and without family or friends at the bedside.
Citation: Ferrand E, Jabre P, Vincent-Genod C, et al. Circumstances of death in hospitalized patients and nurses’ perceptions. Arch Intern Med. 2008;168(8):867-875.
How Common Is Potentially Inappropriate Medication Use in the Hospital?
Background: Use of potentially inappropriate medications (PIM) in the elderly based on the Beers’ List is common in nursing homes, the emergency department (ED), and outpatient settings and is associated with adverse outcomes and hospitalization. Frequency of PIM use the inpatient setting has not been well studied.
Study Design: A retrospective cohort study.
Setting: 384 U.S. hospitals.
Synopsis: In this retrospective cohort study of 493,971 inpatients (older than 65) admitted with medical diagnoses to non-surgeons, PIM prescription was evaluated. Forty nine percent of all patients were prescribed at least one PIM, while 6% were prescribed three or more. In a multivariable model, physician specialty was associated with variation in high severity PIM (HSPIM) prescription. In comparison with internal medicine physicians, cardiologists (odds ratio [OR] 1.32) and pulmonologists (OR 1.10) were more likely to prescribe HSPIMs, while hospitalists (OR 0.90) and geriatricians (OR 0.60) were less likely. In addition, patient age older than 85 was associated with decreased HSPIM prescription (OR 0.59) compared with those younger than 85.
Compared with patients in the Midwest, patients in the South (OR 1.63) and West (OR 1.43) were more likely to prescribe HSPIMs, while those in the Northeast (OR 0.85) were less likely. Hospitals with geriatric services had less PIM use. The study couldn’t account for continuation of chronic medications and did not evaluate adverse outcomes from PIM prescribing.
Bottom line: PIM prescription to hospitalized geriatric patients is common and associated with provider and hospital characteristics.
Citation: Rothberg MB, Pekow PS, Liu F, et al. Potentially inappropriate medication use in hospitalized elders. J Hosp Med. 2008;3:91-102:91-102.
Is There a Benefit to Corticosteroids When Treating Bacterial Meningitis in Children?
Background: The benefit of adjuvant corticosteroids in the treatment of bacterial meningitis in children in the developed world remains unclear; recent expert guidelines reflect this uncertainty.
Study Design: Retrospective cohort study.
Setting: Twenty-seven tertiary care hospitals in the United States.
Synopsis: Researchers examined 2,780 children with a primary diagnosis of bacterial meningitis discharged from 27 tertiary care centers in the U.S. from 2001-2006. Using a propensity analysis (to control for severity of illness), the study compared those who had received adjunctive corticosteroids with those who had not, with mortality and length of study (LOS) as primary outcomes.
The median age was nine months, 8.9% of children received corticosteroids, and the overall mortality rate was 4.2%. Adjuvent corticosteroids did not reduce mortality or LOS. The outcomes were unchanged in subgroup analyses.
Although limited by its retrospective design and lack of other outcome measures (e.g., hearing loss, neurological deficits), this study provides reasonable evidence that corticosteroid use in bacterial meningitis in children may not save lives or shorten LOS. Pediatric hospitalists may not want to routinely give steroids in this setting pending large randomized-controlled trials.
Bottom line: Adjunctive corticosteroids therapy in children with bacterial meningitis may not save lives or reduce LOS.
Citation: Mongelluzzo J, Mohamad Z, Ten Have TR, Shah SS. Corticosteroids and mortality in children with bacterial meningitis. JAMA. 2008;299(17):2048-2055.
Should Unprotected Left Main Disease Be Treated With PCI or CABG?
Background: The current standard of care for the treatment of left main coronary artery disease is coronary-artery bypass grafting (CABG). With the advent of drug-eluting stents, there is growing interest in the use of percutaneous coronary intervention (PCI) to treat left main disease.
Study Design: Prospective observational study.
Setting: Twelve Korean cardiac centers.
Synopsis: From 2000 to 2006, patients with left main disease were treated with PCI or CABG at the discretion of the physician. Nearly 1,100 patients in each cohort were compared and evaluated for death and a composite outcome of death, myocardial infarction, or stroke. Propensity-matching was employed to control for confounders.
In the overall cohort matched by propensity score, there was no significant difference in death or the composite outcome between the PCI and CABG groups after three years. Type of stent (bare metal vs. drug-eluting) did not affect the outcome. Rates of target-vessel revascularization were significantly higher in the group that received stents.
The results are limited by the observational nature and the need for propensity analysis and yet provide an intriguing result. The standard of care for treatment of left main disease remains CABG, but clinicians may be more comfortable treating with stents while we await randomized-controlled trials.
Bottom line: In this observational study, PCI and CABG had similar outcomes in patients with left main disease.
Citation: Seung KB, Park D, Kim Y, Lee S. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781-1792.
New Voice for HM Administrators
Non-physician administrators are an integral part of most hospital medicine practices. SHM has recognized that the rapidly increasing size and complexity of hospital medicine practices demands increasingly sophisticated administrative capabilities, and that practice administrators bring a unique perspective to the field.
In an effort to better support and integrate this population of its membership, SHM has launched the Administrators’ Task Force.
The task force met at the SHM Annual Meeting in San Diego and was introduced at the Administrators’ Open Forum there.
The task force seeks to identify the needs and interests of hospital medicine practice administrators, as well as the ways this demographic can enhance and assist hospital medicine growth.
Important goals of the task force include identifying educational opportunities and programs for administrators, encouraging other administrators to join SHM, strengthening relationships between administrators and hospitalists, and defining ways to enhance and assist hospital medicine growth. Throughout the coming year, the task force will be striving to carry out its charge. It welcomes the feedback and participation of SHM’s full membership.
Members of this year’s Administrators’ Task Force are as follows:
- Daniel Owens, MBA, chairman of the task force. He is administrative director for the Department of Internal Medicine at Emory Healthcare in Atlanta;
- Jodi Braun, hospitalist program manager at Theda Clark Medical Center in Neenah, Wis.;
- Kim Dickinson, vice president of operations with Cogent Healthcare Inc.;
- Sandy Folkenson, RN, BSN, director of hospital medicine service at St. Joseph’s Hospital, HealthEast Care System, St. Paul, Minn.;
- Mary Germann, RN, MN, FACHE, executive director for physician services in Mt. Laurel, N.J.;
- Ellen Hearne, associate vice president of medical services at McLeod Regional Medical Center in Florence, S.C.;
- Ajay Kharbanda, MBA, CMPE, practice administrator at Harris Methodist Medical Foundation in Fort Worth, Texas;
- JoAnn Weissberger, PHR, hospital medicine practice manager at Duke University Medical Center in Durham, N.C.;
- Jeannine Ramsey, RN, MBA, director of operations for hospitalist services in Nashville, Tenn., and Bowling Green, Ky., with HCA Physician Services;
- Leslie Flores, director of SHM’s Practice Management Institute, is the staff liaison for the task force.
The panel is listed on the SHM Web site (www.hospitalmedicine.org) in the committees section, along with contact information.
We invite you to contact us with your thoughts about how the task force can best serve the interests of SHM and its practice administrator members. TH
Non-physician administrators are an integral part of most hospital medicine practices. SHM has recognized that the rapidly increasing size and complexity of hospital medicine practices demands increasingly sophisticated administrative capabilities, and that practice administrators bring a unique perspective to the field.
In an effort to better support and integrate this population of its membership, SHM has launched the Administrators’ Task Force.
The task force met at the SHM Annual Meeting in San Diego and was introduced at the Administrators’ Open Forum there.
The task force seeks to identify the needs and interests of hospital medicine practice administrators, as well as the ways this demographic can enhance and assist hospital medicine growth.
Important goals of the task force include identifying educational opportunities and programs for administrators, encouraging other administrators to join SHM, strengthening relationships between administrators and hospitalists, and defining ways to enhance and assist hospital medicine growth. Throughout the coming year, the task force will be striving to carry out its charge. It welcomes the feedback and participation of SHM’s full membership.
Members of this year’s Administrators’ Task Force are as follows:
- Daniel Owens, MBA, chairman of the task force. He is administrative director for the Department of Internal Medicine at Emory Healthcare in Atlanta;
- Jodi Braun, hospitalist program manager at Theda Clark Medical Center in Neenah, Wis.;
- Kim Dickinson, vice president of operations with Cogent Healthcare Inc.;
- Sandy Folkenson, RN, BSN, director of hospital medicine service at St. Joseph’s Hospital, HealthEast Care System, St. Paul, Minn.;
- Mary Germann, RN, MN, FACHE, executive director for physician services in Mt. Laurel, N.J.;
- Ellen Hearne, associate vice president of medical services at McLeod Regional Medical Center in Florence, S.C.;
- Ajay Kharbanda, MBA, CMPE, practice administrator at Harris Methodist Medical Foundation in Fort Worth, Texas;
- JoAnn Weissberger, PHR, hospital medicine practice manager at Duke University Medical Center in Durham, N.C.;
- Jeannine Ramsey, RN, MBA, director of operations for hospitalist services in Nashville, Tenn., and Bowling Green, Ky., with HCA Physician Services;
- Leslie Flores, director of SHM’s Practice Management Institute, is the staff liaison for the task force.
The panel is listed on the SHM Web site (www.hospitalmedicine.org) in the committees section, along with contact information.
We invite you to contact us with your thoughts about how the task force can best serve the interests of SHM and its practice administrator members. TH
Non-physician administrators are an integral part of most hospital medicine practices. SHM has recognized that the rapidly increasing size and complexity of hospital medicine practices demands increasingly sophisticated administrative capabilities, and that practice administrators bring a unique perspective to the field.
In an effort to better support and integrate this population of its membership, SHM has launched the Administrators’ Task Force.
The task force met at the SHM Annual Meeting in San Diego and was introduced at the Administrators’ Open Forum there.
The task force seeks to identify the needs and interests of hospital medicine practice administrators, as well as the ways this demographic can enhance and assist hospital medicine growth.
Important goals of the task force include identifying educational opportunities and programs for administrators, encouraging other administrators to join SHM, strengthening relationships between administrators and hospitalists, and defining ways to enhance and assist hospital medicine growth. Throughout the coming year, the task force will be striving to carry out its charge. It welcomes the feedback and participation of SHM’s full membership.
Members of this year’s Administrators’ Task Force are as follows:
- Daniel Owens, MBA, chairman of the task force. He is administrative director for the Department of Internal Medicine at Emory Healthcare in Atlanta;
- Jodi Braun, hospitalist program manager at Theda Clark Medical Center in Neenah, Wis.;
- Kim Dickinson, vice president of operations with Cogent Healthcare Inc.;
- Sandy Folkenson, RN, BSN, director of hospital medicine service at St. Joseph’s Hospital, HealthEast Care System, St. Paul, Minn.;
- Mary Germann, RN, MN, FACHE, executive director for physician services in Mt. Laurel, N.J.;
- Ellen Hearne, associate vice president of medical services at McLeod Regional Medical Center in Florence, S.C.;
- Ajay Kharbanda, MBA, CMPE, practice administrator at Harris Methodist Medical Foundation in Fort Worth, Texas;
- JoAnn Weissberger, PHR, hospital medicine practice manager at Duke University Medical Center in Durham, N.C.;
- Jeannine Ramsey, RN, MBA, director of operations for hospitalist services in Nashville, Tenn., and Bowling Green, Ky., with HCA Physician Services;
- Leslie Flores, director of SHM’s Practice Management Institute, is the staff liaison for the task force.
The panel is listed on the SHM Web site (www.hospitalmedicine.org) in the committees section, along with contact information.
We invite you to contact us with your thoughts about how the task force can best serve the interests of SHM and its practice administrator members. TH
SHM to Debut Fellow in HM
This April, at Hospital Medicine 2008, SHM announced plans to introduce the Fellow in Hospital Medicine (FHM) designation. SHM’s first class of “fellows” will be inducted at Hospital Medicine 2009 in Chicago.
As the fall rollout of the program approaches, and as the electronic and print promotions begin, we wanted to continue to share more of the behind-the-scenes details as preparations enter the home stretch. A good deal of research, deliberation, and hard work has gone into the crafting of this program. It’s important to us to publicly thank those involved, and offer you our perspective on why we’re convinced the FHM program will be positively received throughout the hospital medicine community.
Getting to April’s public announcement was the result of hard work by many, especially SHM’s Membership Committee. This group of volunteer members worked diligently the past year to create a structure the society’s Board of Directors unanimously approved.
Prior to significant deliberations, the committee spent a good deal of time scrutinizing similar programs in comparable medical organizations. This included analyzing applications and other collateral materials. Discussions were held regarding which elements easily could transfer to hospital medicine and which didn’t fit our organizational DNA.
From there, the committee began bimonthly discussions on the best form for a fellows program.
Early in this process, it was decided that criteria should closely dovetail with SHM’s Core Competencies in Hospital Medicine as a method of ensuring the program’s credibility throughout all corners of the hospital medicine community.
With the core competencies as the foundation, the next important decision was reached: that the fellows program should be inclusive within our specialty. This concept led to a core goal publicly identifying those who decided to make hospital medicine their career and to note their continued growth in the specialty through higher levels of recognition. As a result, the “Senior Fellow” and “Masters” designations were added to the mix.
Once a draft set of criteria was created, the committee conducted a pilot program tested on a cross section of members. This, along with feedback from SHM’s Board of Directors, gave the committee additional information with which to fine-tune the program and led to its approval at the January board meeting.
With approval in hand, work has transitioned to creating an application and candidate review process. Again, the committee is hard at work reviewing how others approach this piece of their programs and is deep in deliberation on the best approach for SHM. Even though it’s the summer, this process will be as thorough as all the work to date.
When you’re considering whether or not to complete that first FHM application, and apply to join our inaugural class, please know that the FHM designation:
- Is firmly rooted in the core competencies of our specialty;
- Represents a way to tell your colleagues, your employers, and your patients that you are a hospitalist; and
- Came to be because of the hard work and dedication of a group of your peers.
In the coming months, we will continue to share details of the program and answer common questions.
If you have any questions about the upcoming fellowship program, please do not hesitate to contact our office by calling (800) 843-3360.
This April, at Hospital Medicine 2008, SHM announced plans to introduce the Fellow in Hospital Medicine (FHM) designation. SHM’s first class of “fellows” will be inducted at Hospital Medicine 2009 in Chicago.
As the fall rollout of the program approaches, and as the electronic and print promotions begin, we wanted to continue to share more of the behind-the-scenes details as preparations enter the home stretch. A good deal of research, deliberation, and hard work has gone into the crafting of this program. It’s important to us to publicly thank those involved, and offer you our perspective on why we’re convinced the FHM program will be positively received throughout the hospital medicine community.
Getting to April’s public announcement was the result of hard work by many, especially SHM’s Membership Committee. This group of volunteer members worked diligently the past year to create a structure the society’s Board of Directors unanimously approved.
Prior to significant deliberations, the committee spent a good deal of time scrutinizing similar programs in comparable medical organizations. This included analyzing applications and other collateral materials. Discussions were held regarding which elements easily could transfer to hospital medicine and which didn’t fit our organizational DNA.
From there, the committee began bimonthly discussions on the best form for a fellows program.
Early in this process, it was decided that criteria should closely dovetail with SHM’s Core Competencies in Hospital Medicine as a method of ensuring the program’s credibility throughout all corners of the hospital medicine community.
With the core competencies as the foundation, the next important decision was reached: that the fellows program should be inclusive within our specialty. This concept led to a core goal publicly identifying those who decided to make hospital medicine their career and to note their continued growth in the specialty through higher levels of recognition. As a result, the “Senior Fellow” and “Masters” designations were added to the mix.
Once a draft set of criteria was created, the committee conducted a pilot program tested on a cross section of members. This, along with feedback from SHM’s Board of Directors, gave the committee additional information with which to fine-tune the program and led to its approval at the January board meeting.
With approval in hand, work has transitioned to creating an application and candidate review process. Again, the committee is hard at work reviewing how others approach this piece of their programs and is deep in deliberation on the best approach for SHM. Even though it’s the summer, this process will be as thorough as all the work to date.
When you’re considering whether or not to complete that first FHM application, and apply to join our inaugural class, please know that the FHM designation:
- Is firmly rooted in the core competencies of our specialty;
- Represents a way to tell your colleagues, your employers, and your patients that you are a hospitalist; and
- Came to be because of the hard work and dedication of a group of your peers.
In the coming months, we will continue to share details of the program and answer common questions.
If you have any questions about the upcoming fellowship program, please do not hesitate to contact our office by calling (800) 843-3360.
This April, at Hospital Medicine 2008, SHM announced plans to introduce the Fellow in Hospital Medicine (FHM) designation. SHM’s first class of “fellows” will be inducted at Hospital Medicine 2009 in Chicago.
As the fall rollout of the program approaches, and as the electronic and print promotions begin, we wanted to continue to share more of the behind-the-scenes details as preparations enter the home stretch. A good deal of research, deliberation, and hard work has gone into the crafting of this program. It’s important to us to publicly thank those involved, and offer you our perspective on why we’re convinced the FHM program will be positively received throughout the hospital medicine community.
Getting to April’s public announcement was the result of hard work by many, especially SHM’s Membership Committee. This group of volunteer members worked diligently the past year to create a structure the society’s Board of Directors unanimously approved.
Prior to significant deliberations, the committee spent a good deal of time scrutinizing similar programs in comparable medical organizations. This included analyzing applications and other collateral materials. Discussions were held regarding which elements easily could transfer to hospital medicine and which didn’t fit our organizational DNA.
From there, the committee began bimonthly discussions on the best form for a fellows program.
Early in this process, it was decided that criteria should closely dovetail with SHM’s Core Competencies in Hospital Medicine as a method of ensuring the program’s credibility throughout all corners of the hospital medicine community.
With the core competencies as the foundation, the next important decision was reached: that the fellows program should be inclusive within our specialty. This concept led to a core goal publicly identifying those who decided to make hospital medicine their career and to note their continued growth in the specialty through higher levels of recognition. As a result, the “Senior Fellow” and “Masters” designations were added to the mix.
Once a draft set of criteria was created, the committee conducted a pilot program tested on a cross section of members. This, along with feedback from SHM’s Board of Directors, gave the committee additional information with which to fine-tune the program and led to its approval at the January board meeting.
With approval in hand, work has transitioned to creating an application and candidate review process. Again, the committee is hard at work reviewing how others approach this piece of their programs and is deep in deliberation on the best approach for SHM. Even though it’s the summer, this process will be as thorough as all the work to date.
When you’re considering whether or not to complete that first FHM application, and apply to join our inaugural class, please know that the FHM designation:
- Is firmly rooted in the core competencies of our specialty;
- Represents a way to tell your colleagues, your employers, and your patients that you are a hospitalist; and
- Came to be because of the hard work and dedication of a group of your peers.
In the coming months, we will continue to share details of the program and answer common questions.
If you have any questions about the upcoming fellowship program, please do not hesitate to contact our office by calling (800) 843-3360.