User login
August 2008 Instant Poll Results
MARCH 2008
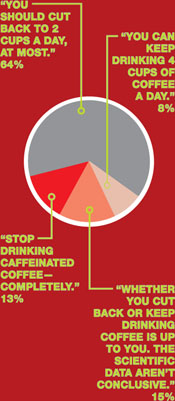
Coffee and conception—what’s your counsel?
A woman drinks 4 cups of caffeinated coffee daily but reports no other source of caffeine, which means that she consumes about 500 mg of caffeine a day. She tells you that she’s concerned about the impact of caffeine on a future pregnancy.
What would you say to this patient about her consumption of caffeine when she begins to try to conceive and, later, while she is pregnant?
APRIL 2008
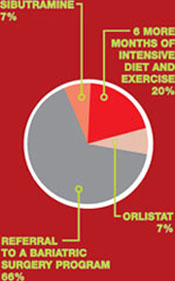
Failed weight loss: Take the next step
Your patient is a 27-year-old woman who has a body mass index of 41 and polycystic ovary syndrome. Her medications are an estrogen–progestin oral contraceptive and metformin, 1,500 mg/day.
She has tried to lose weight many times, without lasting success. She has consulted with nutritionists, personal trainers, and endocrinologists. The next step is yours:
MARCH 2008
Coffee and conception—what’s your counsel?
A woman drinks 4 cups of caffeinated coffee daily but reports no other source of caffeine, which means that she consumes about 500 mg of caffeine a day. She tells you that she’s concerned about the impact of caffeine on a future pregnancy.
What would you say to this patient about her consumption of caffeine when she begins to try to conceive and, later, while she is pregnant?

APRIL 2008
Failed weight loss: Take the next step
Your patient is a 27-year-old woman who has a body mass index of 41 and polycystic ovary syndrome. Her medications are an estrogen–progestin oral contraceptive and metformin, 1,500 mg/day.
She has tried to lose weight many times, without lasting success. She has consulted with nutritionists, personal trainers, and endocrinologists. The next step is yours:

MARCH 2008
Coffee and conception—what’s your counsel?
A woman drinks 4 cups of caffeinated coffee daily but reports no other source of caffeine, which means that she consumes about 500 mg of caffeine a day. She tells you that she’s concerned about the impact of caffeine on a future pregnancy.
What would you say to this patient about her consumption of caffeine when she begins to try to conceive and, later, while she is pregnant?

APRIL 2008
Failed weight loss: Take the next step
Your patient is a 27-year-old woman who has a body mass index of 41 and polycystic ovary syndrome. Her medications are an estrogen–progestin oral contraceptive and metformin, 1,500 mg/day.
She has tried to lose weight many times, without lasting success. She has consulted with nutritionists, personal trainers, and endocrinologists. The next step is yours:

Malpractice minute: June POLL RESULTS
Could a patient’s violent act have been prevented?
A man under outpatient care of the state’s regional behavioral health authority was diagnosed with schizophrenia, paranoid type. He killed his developmentally disabled niece, age 26. The niece’s family claimed the death could have been prevented if the man was civilly committed or heavily medicated. Was the behavioral health authority liable?
⋥ LIABLE: 11% ⋥ NOT LIABLE: 89%
What did the court decide?
The mother was found to be 39% at fault, the patient 11% at fault, and the behavioral health authority 50% at fault for the woman’s death and paid half of the verdict amount to the parents. A $101,740 verdict was returned for the niece’s mother and a $100,625 verdict was returned for the father.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Could a patient’s violent act have been prevented?
A man under outpatient care of the state’s regional behavioral health authority was diagnosed with schizophrenia, paranoid type. He killed his developmentally disabled niece, age 26. The niece’s family claimed the death could have been prevented if the man was civilly committed or heavily medicated. Was the behavioral health authority liable?
⋥ LIABLE: 11% ⋥ NOT LIABLE: 89%
What did the court decide?
The mother was found to be 39% at fault, the patient 11% at fault, and the behavioral health authority 50% at fault for the woman’s death and paid half of the verdict amount to the parents. A $101,740 verdict was returned for the niece’s mother and a $100,625 verdict was returned for the father.
Could a patient’s violent act have been prevented?
A man under outpatient care of the state’s regional behavioral health authority was diagnosed with schizophrenia, paranoid type. He killed his developmentally disabled niece, age 26. The niece’s family claimed the death could have been prevented if the man was civilly committed or heavily medicated. Was the behavioral health authority liable?
⋥ LIABLE: 11% ⋥ NOT LIABLE: 89%
What did the court decide?
The mother was found to be 39% at fault, the patient 11% at fault, and the behavioral health authority 50% at fault for the woman’s death and paid half of the verdict amount to the parents. A $101,740 verdict was returned for the niece’s mother and a $100,625 verdict was returned for the father.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Cases are selected by Current Psychiatry from Medical Malpractice Verdicts, Settlements & Experts, with permission of its editor, Lewis Laska of Nashville, TN (www.verdictslaska.com). Information may be incomplete in some instances, but these cases represent clinical situations that typically result in litigation.
Divorce, custody, and parental consent for psychiatric treatment
Dear Dr. Mossman:
I treat children and adolescents in an acute inpatient setting. Sometimes a child of divorced parents—call him “Johnny”—is admitted to the hospital by one parent—for example the mother—but she doesn’t inform the father. Although the parents have joint custody, Mom doesn’t want me to contact Dad.
I tell Mom that I’d like to get clinical information and consent from Dad, but she refuses, saying, “This will make me look bad, and my ex-husband will try to take emergency custody of Johnny.” My hospital’s legal department says consent from both parents isn’t needed.
These scenarios always leave me feeling upset and confused. I’d appreciate clarification on how to handle these matters.—Submitted by “Dr. K”
Knowing the correct legal answer to a question often doesn’t supply the best clinical solution for your patient. Dr. K received a legally sound response from hospital administrators: a parent who has legal custody may authorize medical treatment for a minor child without first asking or informing the other parent. But Dr. K feels unsatisfied because the hospital didn’t provide what Dr. K sought: a clinically sound answer.
This article reviews custody arrangements and the legal rights they give divorced parents. Also, we will discuss the mother’s concerns and explain why—despite her fears—notifying and involving Johnny’s father can be important, even when it’s not legally required.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online market-place of risk management publications and resources (www.prms.com).
Custody and urgent treatment
A minor—defined in most states as a person younger than age 18—legally cannot give consent for medical care except in limited circumstances, such as contraceptive care.1,2 When a minor undergoes psychiatric hospitalization, physicians usually must obtain consent from the minor’s legal custodian.
Married parents both have legal custody of their children. They also have equal rights to spend time with their children and make major decisions about their welfare, such as authorizing medical care. When parents divorce, these rights must be reassigned in a court-approved divorce decree. Table 1 explains some key terms used to describe custody arrangements after divorce.2,3
Several decades ago, children—especially those younger age 10—usually remained with their mothers, who received sole legal custody; fathers typically had visitation privileges.4 Now, however, most states’ statutes presume that divorced mothers and fathers will have joint legal custody.3
Joint legal custody lets both parents retain their individual legal authority to make decisions on behalf of minor children, although the children may spend most of their time in the physical custody of 1 parent. This means that when urgent medical care is needed—such as a psychiatric hospitalization—1 parent’s consent is sufficient legal authorization for treatment.1,2
What if a child’s parent claims to have legal custody, but the doctor isn’t sure? A doctor who in good faith relies on a parent’s statement can properly provide urgent treatment without delving into custody arrangements.2 In many states, noncustodial parents may authorize treatment in urgent situations—and even some nonurgent ones—if they happen to have physical control of the child when care is needed, such as during a visit.1
Table 1
Child custody: Key legal terms
| Term | Refers to |
|---|---|
| Custody arrangement | The specified times each parent will spend with a minor child and which parent(s) can make major decisions about a child’s welfare |
| Legal custody | A parent’s right to make major decisions about a child’s welfare, including medical care |
| Visitation | The child’s means of maintaining contact with a noncustodial parent |
| Physical custody | Who has physical possession of the child at a particular time, such as during visitation |
| Sole legal custody | A custody arrangement in which only one parent retains the right to make major decisions for the child |
| Joint legal custody | A custody arrangement in which both parents retain the right to make major decisions affecting the child |
| Modification of custody | A legal process in which a court changes a previous custody order |
| Source: Adapted from references 2,3 | |
Nonurgent treatment
After receiving urgent treatment, psychiatric patients typically need continuing, nonurgent care. Dr. K’s inquiry may be anticipating this scenario. In general, parents with joint custody have an equal right to authorize nonurgent care for their children, and Johnny’s treatment could proceed with only Mom’s consent.1 However, if Dr. K knows or has reason to think that Johnny’s father would refuse to give consent for ongoing, nonurgent psychiatric care, providing treatment over the father’s objection may be legally questionable. Under some joint legal custody agreements, both parents need to give consent for medical care and receive clinical information about their children.2
Moreover, trying to treat Johnny in the face of Dad’s explicit objection may be clinically unwise. Unfortunately, many couples’ conflicts are not resolved by divorce, and children can become pawns in ongoing postmarital battles. Such situations can exacerbate children’s emotional problems, which is the opposite of what Dr. K hopes to do for Johnny.
What can Dr. K do?
Address a parent’s fears. Few parents are at their levelheaded best when their children need psychiatric hospitalization. To help Mom and Johnny, Dr. K can point out these things:
- Many states, such as Ohio,5 give Dad the right to learn about Johnny’s treatment and access to treatment records.
- Sooner or later, Dad will find out about the hospitalization. The next time Johnny visits his father, he’ll probably tell Dad what happened. In a few weeks, Dad may receive insurance paperwork or a bill from the hospital.
- Dad may be far more upset and prone to retaliate if he finds out later and is excluded from Johnny’s treatment than if he is notified immediately and gets to participate in his son’s care.
- Realistically, Dad cannot take Johnny away because Mom has arranged for appropriate medical care. If hospitalization is indicated, Mom’s failure to get treatment for Johnny could be grounds for Dad to claim she’s an unfit parent.
Why both parents are needed
Johnny’s hospital care probably will benefit from Dad’s involvement for several reasons (Table 2).
More information. Child and adolescent psychiatrists agree that in most clinical situations it helps to obtain information from as many sources as possible.6-9 Johnny’s father might have crucial information relevant to diagnosis or treatment, such as family history details that Mom doesn’t know.
Debiasing. If Johnny spends time living with both parents, Dr. K should know how often symptoms appear in both environments. Dad’s perspective may be vital, but when postdivorce relationships are strained, what parents convey about each other can be biased. Getting information directly from both parents will give Dr. K a more realistic picture of the child’s environment and psychosocial stressors.7
Treatment planning. After a psychiatric hospitalization, both parents should be aware of Johnny’s diagnosis and treatment. Johnny may need careful supervision for recurrence of symptoms, such as suicidal or homicidal ideation, that can have life-threatening implications.
Medication management. If Johnny is taking medication, he’ll need to receive it regularly. Missing medication when Johnny is with Dad would reduce effectiveness and in some cases could be dangerous. Both parents also should know about possible side effects so they can provide good monitoring.
Psychotherapy. Often, family therapy is an important element of a child’s recovery and will achieve optimum results only if all family members participate. Also, children need consistency. If a behavioral plan is part of Johnny’s treatment, Mom and Dad will need to agree on the rules and implement them consistently at both homes.
Table 2
Why both parents’ input is valuable
| More information from different perspectives concerning behavior in a variety of contexts and settings |
| Less biased information |
| Better treatment planning |
| Better medication management |
| More effective therapy |
Work with parents
When one divorced parent is reluctant to inform the other about their child’s hospitalization, you can respond empathically to fears and concerns. Despite mental health professionals’ best efforts, psychiatric illness still generates feelings of stigma and shame. Divorced parents often feel guilty about the stress the divorce has brought to their children, and they may consciously or unconsciously blame themselves for their child’s illness. In the midst of an ongoing custody dispute, the parent initiating a psychiatric hospitalization may feel especially vulnerable and reluctant to inform the other parent about what’s happening.
Being attuned to these issues will help you address and normalize a parent’s fears. Parents should know that a court could support their seeking treatment for their children’s illness, and they could be contributing to medical neglect if they do not seek this treatment.
In rare instances, not informing the other parent may be the best clinical decision. In situations involving child abuse or extreme domestic violence, a parent’s learning about the hospitalization could create safety issues. In most instances, however, both Mom and Dad will see their child soon after hospitalization, so one parent cannot hope to conceal a hospitalization for very long. Involving both parents from the outset usually will give the child and his family the best shot at a positive outcome.
1. Berger JE. Consent by proxy for nonurgent pediatric care. Pediatrics 2003;112:1186-95.
2. Quinn KM, Weiner BA. Legal rights of children. In: Weiner BA, Wettstein RM, eds. Legal issues in mental health care. New York, NY: Plenum Press; 1993:309-47.
3. Kelly JB. The determination of child custody. Future Child 1994;4:121-242.
4. Melton GB, Petrila J, Poythress NG, Slobogin C. Psychological evaluations for the courts: a handbook for mental health professionals and lawyers. 3rd ed. New York, NY: Guilford Press; 2007.
5. Ohio Rev Code § 3109. 051(H).
6. American Academy of Child and Adolescent Psychiatry. Practice parameters for the psychiatric assessment of children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(10 suppl):4S-20S.
7. American Academy of Child and Adolescent Psychiatry. Practice parameter for the assessment of the family. J Am Acad Child Adolesc Psychiatry 2007;46:922-37.
8. Bostic JQ, King RA. Clinical assessment of children and adolescents: content and structure. In: Martin A, Volkmar FR, eds. Lewis’s child and adolescent psychiatry: a comprehensive textbook. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:323-44.
9. Weston CG, Klykylo WM. The initial psychiatric evaluation of children and adolescents. In: Tasman A, Kay J, Lieberman J, eds. Psychiatry. 3rd ed. London, UK: John Wiley & Sons; 2008:546-54.
Dear Dr. Mossman:
I treat children and adolescents in an acute inpatient setting. Sometimes a child of divorced parents—call him “Johnny”—is admitted to the hospital by one parent—for example the mother—but she doesn’t inform the father. Although the parents have joint custody, Mom doesn’t want me to contact Dad.
I tell Mom that I’d like to get clinical information and consent from Dad, but she refuses, saying, “This will make me look bad, and my ex-husband will try to take emergency custody of Johnny.” My hospital’s legal department says consent from both parents isn’t needed.
These scenarios always leave me feeling upset and confused. I’d appreciate clarification on how to handle these matters.—Submitted by “Dr. K”
Knowing the correct legal answer to a question often doesn’t supply the best clinical solution for your patient. Dr. K received a legally sound response from hospital administrators: a parent who has legal custody may authorize medical treatment for a minor child without first asking or informing the other parent. But Dr. K feels unsatisfied because the hospital didn’t provide what Dr. K sought: a clinically sound answer.
This article reviews custody arrangements and the legal rights they give divorced parents. Also, we will discuss the mother’s concerns and explain why—despite her fears—notifying and involving Johnny’s father can be important, even when it’s not legally required.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online market-place of risk management publications and resources (www.prms.com).
Custody and urgent treatment
A minor—defined in most states as a person younger than age 18—legally cannot give consent for medical care except in limited circumstances, such as contraceptive care.1,2 When a minor undergoes psychiatric hospitalization, physicians usually must obtain consent from the minor’s legal custodian.
Married parents both have legal custody of their children. They also have equal rights to spend time with their children and make major decisions about their welfare, such as authorizing medical care. When parents divorce, these rights must be reassigned in a court-approved divorce decree. Table 1 explains some key terms used to describe custody arrangements after divorce.2,3
Several decades ago, children—especially those younger age 10—usually remained with their mothers, who received sole legal custody; fathers typically had visitation privileges.4 Now, however, most states’ statutes presume that divorced mothers and fathers will have joint legal custody.3
Joint legal custody lets both parents retain their individual legal authority to make decisions on behalf of minor children, although the children may spend most of their time in the physical custody of 1 parent. This means that when urgent medical care is needed—such as a psychiatric hospitalization—1 parent’s consent is sufficient legal authorization for treatment.1,2
What if a child’s parent claims to have legal custody, but the doctor isn’t sure? A doctor who in good faith relies on a parent’s statement can properly provide urgent treatment without delving into custody arrangements.2 In many states, noncustodial parents may authorize treatment in urgent situations—and even some nonurgent ones—if they happen to have physical control of the child when care is needed, such as during a visit.1
Table 1
Child custody: Key legal terms
| Term | Refers to |
|---|---|
| Custody arrangement | The specified times each parent will spend with a minor child and which parent(s) can make major decisions about a child’s welfare |
| Legal custody | A parent’s right to make major decisions about a child’s welfare, including medical care |
| Visitation | The child’s means of maintaining contact with a noncustodial parent |
| Physical custody | Who has physical possession of the child at a particular time, such as during visitation |
| Sole legal custody | A custody arrangement in which only one parent retains the right to make major decisions for the child |
| Joint legal custody | A custody arrangement in which both parents retain the right to make major decisions affecting the child |
| Modification of custody | A legal process in which a court changes a previous custody order |
| Source: Adapted from references 2,3 | |
Nonurgent treatment
After receiving urgent treatment, psychiatric patients typically need continuing, nonurgent care. Dr. K’s inquiry may be anticipating this scenario. In general, parents with joint custody have an equal right to authorize nonurgent care for their children, and Johnny’s treatment could proceed with only Mom’s consent.1 However, if Dr. K knows or has reason to think that Johnny’s father would refuse to give consent for ongoing, nonurgent psychiatric care, providing treatment over the father’s objection may be legally questionable. Under some joint legal custody agreements, both parents need to give consent for medical care and receive clinical information about their children.2
Moreover, trying to treat Johnny in the face of Dad’s explicit objection may be clinically unwise. Unfortunately, many couples’ conflicts are not resolved by divorce, and children can become pawns in ongoing postmarital battles. Such situations can exacerbate children’s emotional problems, which is the opposite of what Dr. K hopes to do for Johnny.
What can Dr. K do?
Address a parent’s fears. Few parents are at their levelheaded best when their children need psychiatric hospitalization. To help Mom and Johnny, Dr. K can point out these things:
- Many states, such as Ohio,5 give Dad the right to learn about Johnny’s treatment and access to treatment records.
- Sooner or later, Dad will find out about the hospitalization. The next time Johnny visits his father, he’ll probably tell Dad what happened. In a few weeks, Dad may receive insurance paperwork or a bill from the hospital.
- Dad may be far more upset and prone to retaliate if he finds out later and is excluded from Johnny’s treatment than if he is notified immediately and gets to participate in his son’s care.
- Realistically, Dad cannot take Johnny away because Mom has arranged for appropriate medical care. If hospitalization is indicated, Mom’s failure to get treatment for Johnny could be grounds for Dad to claim she’s an unfit parent.
Why both parents are needed
Johnny’s hospital care probably will benefit from Dad’s involvement for several reasons (Table 2).
More information. Child and adolescent psychiatrists agree that in most clinical situations it helps to obtain information from as many sources as possible.6-9 Johnny’s father might have crucial information relevant to diagnosis or treatment, such as family history details that Mom doesn’t know.
Debiasing. If Johnny spends time living with both parents, Dr. K should know how often symptoms appear in both environments. Dad’s perspective may be vital, but when postdivorce relationships are strained, what parents convey about each other can be biased. Getting information directly from both parents will give Dr. K a more realistic picture of the child’s environment and psychosocial stressors.7
Treatment planning. After a psychiatric hospitalization, both parents should be aware of Johnny’s diagnosis and treatment. Johnny may need careful supervision for recurrence of symptoms, such as suicidal or homicidal ideation, that can have life-threatening implications.
Medication management. If Johnny is taking medication, he’ll need to receive it regularly. Missing medication when Johnny is with Dad would reduce effectiveness and in some cases could be dangerous. Both parents also should know about possible side effects so they can provide good monitoring.
Psychotherapy. Often, family therapy is an important element of a child’s recovery and will achieve optimum results only if all family members participate. Also, children need consistency. If a behavioral plan is part of Johnny’s treatment, Mom and Dad will need to agree on the rules and implement them consistently at both homes.
Table 2
Why both parents’ input is valuable
| More information from different perspectives concerning behavior in a variety of contexts and settings |
| Less biased information |
| Better treatment planning |
| Better medication management |
| More effective therapy |
Work with parents
When one divorced parent is reluctant to inform the other about their child’s hospitalization, you can respond empathically to fears and concerns. Despite mental health professionals’ best efforts, psychiatric illness still generates feelings of stigma and shame. Divorced parents often feel guilty about the stress the divorce has brought to their children, and they may consciously or unconsciously blame themselves for their child’s illness. In the midst of an ongoing custody dispute, the parent initiating a psychiatric hospitalization may feel especially vulnerable and reluctant to inform the other parent about what’s happening.
Being attuned to these issues will help you address and normalize a parent’s fears. Parents should know that a court could support their seeking treatment for their children’s illness, and they could be contributing to medical neglect if they do not seek this treatment.
In rare instances, not informing the other parent may be the best clinical decision. In situations involving child abuse or extreme domestic violence, a parent’s learning about the hospitalization could create safety issues. In most instances, however, both Mom and Dad will see their child soon after hospitalization, so one parent cannot hope to conceal a hospitalization for very long. Involving both parents from the outset usually will give the child and his family the best shot at a positive outcome.
Dear Dr. Mossman:
I treat children and adolescents in an acute inpatient setting. Sometimes a child of divorced parents—call him “Johnny”—is admitted to the hospital by one parent—for example the mother—but she doesn’t inform the father. Although the parents have joint custody, Mom doesn’t want me to contact Dad.
I tell Mom that I’d like to get clinical information and consent from Dad, but she refuses, saying, “This will make me look bad, and my ex-husband will try to take emergency custody of Johnny.” My hospital’s legal department says consent from both parents isn’t needed.
These scenarios always leave me feeling upset and confused. I’d appreciate clarification on how to handle these matters.—Submitted by “Dr. K”
Knowing the correct legal answer to a question often doesn’t supply the best clinical solution for your patient. Dr. K received a legally sound response from hospital administrators: a parent who has legal custody may authorize medical treatment for a minor child without first asking or informing the other parent. But Dr. K feels unsatisfied because the hospital didn’t provide what Dr. K sought: a clinically sound answer.
This article reviews custody arrangements and the legal rights they give divorced parents. Also, we will discuss the mother’s concerns and explain why—despite her fears—notifying and involving Johnny’s father can be important, even when it’s not legally required.
- Submit your malpractice-related questions to Dr. Mossman at [email protected].
- Include your name, address, and practice location. If your question is chosen for publication, your name can be withheld by request.
- All readers who submit questions will be included in quarterly drawings for a $50 gift certificate for Professional Risk Management Services, Inc’s online market-place of risk management publications and resources (www.prms.com).
Custody and urgent treatment
A minor—defined in most states as a person younger than age 18—legally cannot give consent for medical care except in limited circumstances, such as contraceptive care.1,2 When a minor undergoes psychiatric hospitalization, physicians usually must obtain consent from the minor’s legal custodian.
Married parents both have legal custody of their children. They also have equal rights to spend time with their children and make major decisions about their welfare, such as authorizing medical care. When parents divorce, these rights must be reassigned in a court-approved divorce decree. Table 1 explains some key terms used to describe custody arrangements after divorce.2,3
Several decades ago, children—especially those younger age 10—usually remained with their mothers, who received sole legal custody; fathers typically had visitation privileges.4 Now, however, most states’ statutes presume that divorced mothers and fathers will have joint legal custody.3
Joint legal custody lets both parents retain their individual legal authority to make decisions on behalf of minor children, although the children may spend most of their time in the physical custody of 1 parent. This means that when urgent medical care is needed—such as a psychiatric hospitalization—1 parent’s consent is sufficient legal authorization for treatment.1,2
What if a child’s parent claims to have legal custody, but the doctor isn’t sure? A doctor who in good faith relies on a parent’s statement can properly provide urgent treatment without delving into custody arrangements.2 In many states, noncustodial parents may authorize treatment in urgent situations—and even some nonurgent ones—if they happen to have physical control of the child when care is needed, such as during a visit.1
Table 1
Child custody: Key legal terms
| Term | Refers to |
|---|---|
| Custody arrangement | The specified times each parent will spend with a minor child and which parent(s) can make major decisions about a child’s welfare |
| Legal custody | A parent’s right to make major decisions about a child’s welfare, including medical care |
| Visitation | The child’s means of maintaining contact with a noncustodial parent |
| Physical custody | Who has physical possession of the child at a particular time, such as during visitation |
| Sole legal custody | A custody arrangement in which only one parent retains the right to make major decisions for the child |
| Joint legal custody | A custody arrangement in which both parents retain the right to make major decisions affecting the child |
| Modification of custody | A legal process in which a court changes a previous custody order |
| Source: Adapted from references 2,3 | |
Nonurgent treatment
After receiving urgent treatment, psychiatric patients typically need continuing, nonurgent care. Dr. K’s inquiry may be anticipating this scenario. In general, parents with joint custody have an equal right to authorize nonurgent care for their children, and Johnny’s treatment could proceed with only Mom’s consent.1 However, if Dr. K knows or has reason to think that Johnny’s father would refuse to give consent for ongoing, nonurgent psychiatric care, providing treatment over the father’s objection may be legally questionable. Under some joint legal custody agreements, both parents need to give consent for medical care and receive clinical information about their children.2
Moreover, trying to treat Johnny in the face of Dad’s explicit objection may be clinically unwise. Unfortunately, many couples’ conflicts are not resolved by divorce, and children can become pawns in ongoing postmarital battles. Such situations can exacerbate children’s emotional problems, which is the opposite of what Dr. K hopes to do for Johnny.
What can Dr. K do?
Address a parent’s fears. Few parents are at their levelheaded best when their children need psychiatric hospitalization. To help Mom and Johnny, Dr. K can point out these things:
- Many states, such as Ohio,5 give Dad the right to learn about Johnny’s treatment and access to treatment records.
- Sooner or later, Dad will find out about the hospitalization. The next time Johnny visits his father, he’ll probably tell Dad what happened. In a few weeks, Dad may receive insurance paperwork or a bill from the hospital.
- Dad may be far more upset and prone to retaliate if he finds out later and is excluded from Johnny’s treatment than if he is notified immediately and gets to participate in his son’s care.
- Realistically, Dad cannot take Johnny away because Mom has arranged for appropriate medical care. If hospitalization is indicated, Mom’s failure to get treatment for Johnny could be grounds for Dad to claim she’s an unfit parent.
Why both parents are needed
Johnny’s hospital care probably will benefit from Dad’s involvement for several reasons (Table 2).
More information. Child and adolescent psychiatrists agree that in most clinical situations it helps to obtain information from as many sources as possible.6-9 Johnny’s father might have crucial information relevant to diagnosis or treatment, such as family history details that Mom doesn’t know.
Debiasing. If Johnny spends time living with both parents, Dr. K should know how often symptoms appear in both environments. Dad’s perspective may be vital, but when postdivorce relationships are strained, what parents convey about each other can be biased. Getting information directly from both parents will give Dr. K a more realistic picture of the child’s environment and psychosocial stressors.7
Treatment planning. After a psychiatric hospitalization, both parents should be aware of Johnny’s diagnosis and treatment. Johnny may need careful supervision for recurrence of symptoms, such as suicidal or homicidal ideation, that can have life-threatening implications.
Medication management. If Johnny is taking medication, he’ll need to receive it regularly. Missing medication when Johnny is with Dad would reduce effectiveness and in some cases could be dangerous. Both parents also should know about possible side effects so they can provide good monitoring.
Psychotherapy. Often, family therapy is an important element of a child’s recovery and will achieve optimum results only if all family members participate. Also, children need consistency. If a behavioral plan is part of Johnny’s treatment, Mom and Dad will need to agree on the rules and implement them consistently at both homes.
Table 2
Why both parents’ input is valuable
| More information from different perspectives concerning behavior in a variety of contexts and settings |
| Less biased information |
| Better treatment planning |
| Better medication management |
| More effective therapy |
Work with parents
When one divorced parent is reluctant to inform the other about their child’s hospitalization, you can respond empathically to fears and concerns. Despite mental health professionals’ best efforts, psychiatric illness still generates feelings of stigma and shame. Divorced parents often feel guilty about the stress the divorce has brought to their children, and they may consciously or unconsciously blame themselves for their child’s illness. In the midst of an ongoing custody dispute, the parent initiating a psychiatric hospitalization may feel especially vulnerable and reluctant to inform the other parent about what’s happening.
Being attuned to these issues will help you address and normalize a parent’s fears. Parents should know that a court could support their seeking treatment for their children’s illness, and they could be contributing to medical neglect if they do not seek this treatment.
In rare instances, not informing the other parent may be the best clinical decision. In situations involving child abuse or extreme domestic violence, a parent’s learning about the hospitalization could create safety issues. In most instances, however, both Mom and Dad will see their child soon after hospitalization, so one parent cannot hope to conceal a hospitalization for very long. Involving both parents from the outset usually will give the child and his family the best shot at a positive outcome.
1. Berger JE. Consent by proxy for nonurgent pediatric care. Pediatrics 2003;112:1186-95.
2. Quinn KM, Weiner BA. Legal rights of children. In: Weiner BA, Wettstein RM, eds. Legal issues in mental health care. New York, NY: Plenum Press; 1993:309-47.
3. Kelly JB. The determination of child custody. Future Child 1994;4:121-242.
4. Melton GB, Petrila J, Poythress NG, Slobogin C. Psychological evaluations for the courts: a handbook for mental health professionals and lawyers. 3rd ed. New York, NY: Guilford Press; 2007.
5. Ohio Rev Code § 3109. 051(H).
6. American Academy of Child and Adolescent Psychiatry. Practice parameters for the psychiatric assessment of children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(10 suppl):4S-20S.
7. American Academy of Child and Adolescent Psychiatry. Practice parameter for the assessment of the family. J Am Acad Child Adolesc Psychiatry 2007;46:922-37.
8. Bostic JQ, King RA. Clinical assessment of children and adolescents: content and structure. In: Martin A, Volkmar FR, eds. Lewis’s child and adolescent psychiatry: a comprehensive textbook. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:323-44.
9. Weston CG, Klykylo WM. The initial psychiatric evaluation of children and adolescents. In: Tasman A, Kay J, Lieberman J, eds. Psychiatry. 3rd ed. London, UK: John Wiley & Sons; 2008:546-54.
1. Berger JE. Consent by proxy for nonurgent pediatric care. Pediatrics 2003;112:1186-95.
2. Quinn KM, Weiner BA. Legal rights of children. In: Weiner BA, Wettstein RM, eds. Legal issues in mental health care. New York, NY: Plenum Press; 1993:309-47.
3. Kelly JB. The determination of child custody. Future Child 1994;4:121-242.
4. Melton GB, Petrila J, Poythress NG, Slobogin C. Psychological evaluations for the courts: a handbook for mental health professionals and lawyers. 3rd ed. New York, NY: Guilford Press; 2007.
5. Ohio Rev Code § 3109. 051(H).
6. American Academy of Child and Adolescent Psychiatry. Practice parameters for the psychiatric assessment of children and adolescents. J Am Acad Child Adolesc Psychiatry 1997;36(10 suppl):4S-20S.
7. American Academy of Child and Adolescent Psychiatry. Practice parameter for the assessment of the family. J Am Acad Child Adolesc Psychiatry 2007;46:922-37.
8. Bostic JQ, King RA. Clinical assessment of children and adolescents: content and structure. In: Martin A, Volkmar FR, eds. Lewis’s child and adolescent psychiatry: a comprehensive textbook. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:323-44.
9. Weston CG, Klykylo WM. The initial psychiatric evaluation of children and adolescents. In: Tasman A, Kay J, Lieberman J, eds. Psychiatry. 3rd ed. London, UK: John Wiley & Sons; 2008:546-54.
Listen to the Patient
As the healthcare system struggles with the definition of quality and the implementation of patient-centered care, renewed attention is being given to patient satisfaction.
Now, this performance measure has moved from the hospital’s marketing department into the C-suite, where senior administrators at some hospitals have patient satisfaction scores tied to their compensation.
Pressure is being applied to nudge key hospital care providers, including hospitalists, to keep their patients happy while giving them the care they deserve.
With the recent publishing of the Hospital Consumer Assessment of Healthcare providers and Systems (HCAHPS) scorecards for each hospital on the Hospital Compare Web site (www.hospitalcompare.hhs.gov), patients can see and compare local hospitals.
Because hospitalists are managing an ever-increasing portion of the hospital census, we can count on being right in the middle of all this. Coupled with the fact that 40% of hospitalists are directly employed by their hospital and a significant portion of other hospitalist groups have contracts with hospitals tied to quality improvement, we can expect a lot of pressure to not only improve patient satisfaction, but to make the “numbers” look better.
What Survey Measures
An important starting point for hospitalists and especially their leaders, who will be engaged in conversations with the C-suite about patient satisfaction data, is to better understand what the data indicate.
First, you need to know that the patient questionnaires were designed by several large vendors, the largest being Press Ganey.
While it is possible to segment the patients by those treated by a hospitalist and those not, the questions were not meant to describe, define, or compare the performance of different physicians. Remember, non-hospitalists for this purpose includes not only internists, but also surgeons, obstetricians, and other specialists.
Some questions on the survey about physicians include:
- During this hospital stay, how often did doctors treat you with respect? (never, sometimes, usually, always);
- During this hospital stay, how often did doctors explain things in a way you could understand? and
- During this hospital stay, how often did doctors listen carefully to you?
Other questions that might pertain to care directed by hospitalists but also relate to the entire care team include:
- How often was your pain controlled?
- Before giving you a new medicine, how often did staff tell you what it was for? and
- Before giving you a new medicine, how often did staff describe possible side effects in a way you could understand?
While you might aggregate all the replies specifically about the doctors’ performance and grade all the doctors separately, the all-important questions to the C-suite are the last two sections:
- How do patients rate the hospital? and
- Would patients recommend the hospital to friends and family?
Patients Are Different
It is important to understand the unique characteristics of the patients admitted and managed by hospitalists and to understand how these patients may respond differently to the standard patient satisfaction surveys than others in the patient population.
More often than not, hospitalists admit patients who are acutely ill, presenting through the emergency department (ED) with medical problems. Some studies have estimated that more than 70% of hospitalists’ patients come through the ED, while for the rest of the staff it is closer to 30% to 40%.
It is well known that patients admitted electively are more satisfied than those with an acute illness who come through the ED. In addition, patients admitted for medical problems have lower satisfaction ratings than those admitted for general surgery, subspecialty surgery, or obstetrics.
Therefore, if your hospital administration has pulled together statistics that purport to compare patient satisfaction for your hospitalist group versus all other admissions, you need to make sure that comparisons are made to a similar population, i.e., acutely ill patients admitted through the ED with medical diagnoses. The survey companies should be able to produce just such a comparison.
It is equally as important to make sure you focus on the total experience at the hospital and not just the questions specifically concerning only the doctors. Since hospitalists not only do front-line, face-to-face patient care, but also work with the team and attempt to improve the system to provide better overall quality, make sure to focus on questions like “How do patients rate the hospital?” and “Would patients recommend the hospital to friends and family?”
The other consideration is to understand how close the top quartile is to the bottom quartile, when comparisons are made with this data. In many of these surveys the patients are giving ratings on a scale of one to four, with many of the responses at three or four. Therefore, the top score might be a 3.6 and the bottom score average 3.2. It is important to understand if you are just minor adjustments away from being in a good range or if you are either so far above or below the standard of care that a real situation exists.
HM’s Role
Does the hospitalist model lead to better patient satisfaction? Like most things in hospital medicine, the answer is yes, no, and maybe. There are certain aspects of hospital medicine that should lead to happier patients:
- Present and easily available;
- Expert in hospital care;
- Improved coordination of care by specialists;
- Availability for multiple visits if patient condition changes;
- Availability to visit with loved-ones at their convenience; and
- Rapid response to nurse’s concerns.
There are aspects of getting your care from a hospitalist that may initially make the patient more concerned:
- They may be unfamiliar with the hospitalist and the hospitalist model;
- The hospitalist may demonstrate little or no knowledge of the patient’s history;
- The referring physician may not introduce the patient to the hospitalist; and
- The hospitalist may not explain the relationship with the referring physician.
How to Be Proactive
With all we have to do every day (and the list seems to get longer by the minute), it is easy to get perplexed by having to be responsible for the patients’ satisfaction with their hospital experience. That being said, hospitalists perform well when we step up to the plate and take action in these ways:
- Proactively meet with the person in the C-suite who oversees the patient satisfaction survey process or relates to the hospitalist group (e.g., vice president of medical affairs or chief medical officer) to better understand the survey results;
- Make sure if the data are being used to compare hospitalist care with non-hospitalist care that the comparison group of patients is equivalent (i.e., acutely ill medical patients admitted through the ED, not surgical or obstetrical patients);
- Make sure to focus not only on the “doctor-related” questions, but on patients’ overall satisfaction with the hospital; and
- Offer to help the C-suite improve patient satisfaction, but don’t attempt to “own” this performance measure for the entire hospital. Hospitalists can be helpful, but this is broader than any one group of physicians.
Further, make improving patient satisfaction a core goal for your group. Some strategies that may work include:
- Have a script for each patient encounter (“Hi, I’m Dr. Smith, I take care of Dr. Jones’ patients in the hospital. The way we communicate about your care is … The advantages to our partnership are …”);
- Hand out a brochure with your group’s hospitalists’ pictures, answers to frequently asked questions, and how to contact the hospitalist; and
- Sit down and shut up (i.e., patients will perceive you are taking time with them and listening if you are seated and let them speak without interruption).
Hospitals have been doing patient surveys for some time now. The Centers for Medicare and Medicaid Services and other payers are placing more emphasis on this quality measure. Now that the results easily are available to the public, major newspapers and broadcast media are calling attention to patient perspectives on their hospital care.
Once hospitalist groups understand the data, there is an opportunity to partner with their hospitals to better understand how our patients see their hospital care and allow for hospitalists to have an appropriate role in working with the other health professionals to improve patients’ experience with their care. TH
Dr. Wellikson is the CEO of SHM.
Note to readers: I would like to acknowledge SHM co-founder Win Whitcomb, MD, and SHM Senior Vice President Joe Miller for their assistance with this column.
As the healthcare system struggles with the definition of quality and the implementation of patient-centered care, renewed attention is being given to patient satisfaction.
Now, this performance measure has moved from the hospital’s marketing department into the C-suite, where senior administrators at some hospitals have patient satisfaction scores tied to their compensation.
Pressure is being applied to nudge key hospital care providers, including hospitalists, to keep their patients happy while giving them the care they deserve.
With the recent publishing of the Hospital Consumer Assessment of Healthcare providers and Systems (HCAHPS) scorecards for each hospital on the Hospital Compare Web site (www.hospitalcompare.hhs.gov), patients can see and compare local hospitals.
Because hospitalists are managing an ever-increasing portion of the hospital census, we can count on being right in the middle of all this. Coupled with the fact that 40% of hospitalists are directly employed by their hospital and a significant portion of other hospitalist groups have contracts with hospitals tied to quality improvement, we can expect a lot of pressure to not only improve patient satisfaction, but to make the “numbers” look better.
What Survey Measures
An important starting point for hospitalists and especially their leaders, who will be engaged in conversations with the C-suite about patient satisfaction data, is to better understand what the data indicate.
First, you need to know that the patient questionnaires were designed by several large vendors, the largest being Press Ganey.
While it is possible to segment the patients by those treated by a hospitalist and those not, the questions were not meant to describe, define, or compare the performance of different physicians. Remember, non-hospitalists for this purpose includes not only internists, but also surgeons, obstetricians, and other specialists.
Some questions on the survey about physicians include:
- During this hospital stay, how often did doctors treat you with respect? (never, sometimes, usually, always);
- During this hospital stay, how often did doctors explain things in a way you could understand? and
- During this hospital stay, how often did doctors listen carefully to you?
Other questions that might pertain to care directed by hospitalists but also relate to the entire care team include:
- How often was your pain controlled?
- Before giving you a new medicine, how often did staff tell you what it was for? and
- Before giving you a new medicine, how often did staff describe possible side effects in a way you could understand?
While you might aggregate all the replies specifically about the doctors’ performance and grade all the doctors separately, the all-important questions to the C-suite are the last two sections:
- How do patients rate the hospital? and
- Would patients recommend the hospital to friends and family?
Patients Are Different
It is important to understand the unique characteristics of the patients admitted and managed by hospitalists and to understand how these patients may respond differently to the standard patient satisfaction surveys than others in the patient population.
More often than not, hospitalists admit patients who are acutely ill, presenting through the emergency department (ED) with medical problems. Some studies have estimated that more than 70% of hospitalists’ patients come through the ED, while for the rest of the staff it is closer to 30% to 40%.
It is well known that patients admitted electively are more satisfied than those with an acute illness who come through the ED. In addition, patients admitted for medical problems have lower satisfaction ratings than those admitted for general surgery, subspecialty surgery, or obstetrics.
Therefore, if your hospital administration has pulled together statistics that purport to compare patient satisfaction for your hospitalist group versus all other admissions, you need to make sure that comparisons are made to a similar population, i.e., acutely ill patients admitted through the ED with medical diagnoses. The survey companies should be able to produce just such a comparison.
It is equally as important to make sure you focus on the total experience at the hospital and not just the questions specifically concerning only the doctors. Since hospitalists not only do front-line, face-to-face patient care, but also work with the team and attempt to improve the system to provide better overall quality, make sure to focus on questions like “How do patients rate the hospital?” and “Would patients recommend the hospital to friends and family?”
The other consideration is to understand how close the top quartile is to the bottom quartile, when comparisons are made with this data. In many of these surveys the patients are giving ratings on a scale of one to four, with many of the responses at three or four. Therefore, the top score might be a 3.6 and the bottom score average 3.2. It is important to understand if you are just minor adjustments away from being in a good range or if you are either so far above or below the standard of care that a real situation exists.
HM’s Role
Does the hospitalist model lead to better patient satisfaction? Like most things in hospital medicine, the answer is yes, no, and maybe. There are certain aspects of hospital medicine that should lead to happier patients:
- Present and easily available;
- Expert in hospital care;
- Improved coordination of care by specialists;
- Availability for multiple visits if patient condition changes;
- Availability to visit with loved-ones at their convenience; and
- Rapid response to nurse’s concerns.
There are aspects of getting your care from a hospitalist that may initially make the patient more concerned:
- They may be unfamiliar with the hospitalist and the hospitalist model;
- The hospitalist may demonstrate little or no knowledge of the patient’s history;
- The referring physician may not introduce the patient to the hospitalist; and
- The hospitalist may not explain the relationship with the referring physician.
How to Be Proactive
With all we have to do every day (and the list seems to get longer by the minute), it is easy to get perplexed by having to be responsible for the patients’ satisfaction with their hospital experience. That being said, hospitalists perform well when we step up to the plate and take action in these ways:
- Proactively meet with the person in the C-suite who oversees the patient satisfaction survey process or relates to the hospitalist group (e.g., vice president of medical affairs or chief medical officer) to better understand the survey results;
- Make sure if the data are being used to compare hospitalist care with non-hospitalist care that the comparison group of patients is equivalent (i.e., acutely ill medical patients admitted through the ED, not surgical or obstetrical patients);
- Make sure to focus not only on the “doctor-related” questions, but on patients’ overall satisfaction with the hospital; and
- Offer to help the C-suite improve patient satisfaction, but don’t attempt to “own” this performance measure for the entire hospital. Hospitalists can be helpful, but this is broader than any one group of physicians.
Further, make improving patient satisfaction a core goal for your group. Some strategies that may work include:
- Have a script for each patient encounter (“Hi, I’m Dr. Smith, I take care of Dr. Jones’ patients in the hospital. The way we communicate about your care is … The advantages to our partnership are …”);
- Hand out a brochure with your group’s hospitalists’ pictures, answers to frequently asked questions, and how to contact the hospitalist; and
- Sit down and shut up (i.e., patients will perceive you are taking time with them and listening if you are seated and let them speak without interruption).
Hospitals have been doing patient surveys for some time now. The Centers for Medicare and Medicaid Services and other payers are placing more emphasis on this quality measure. Now that the results easily are available to the public, major newspapers and broadcast media are calling attention to patient perspectives on their hospital care.
Once hospitalist groups understand the data, there is an opportunity to partner with their hospitals to better understand how our patients see their hospital care and allow for hospitalists to have an appropriate role in working with the other health professionals to improve patients’ experience with their care. TH
Dr. Wellikson is the CEO of SHM.
Note to readers: I would like to acknowledge SHM co-founder Win Whitcomb, MD, and SHM Senior Vice President Joe Miller for their assistance with this column.
As the healthcare system struggles with the definition of quality and the implementation of patient-centered care, renewed attention is being given to patient satisfaction.
Now, this performance measure has moved from the hospital’s marketing department into the C-suite, where senior administrators at some hospitals have patient satisfaction scores tied to their compensation.
Pressure is being applied to nudge key hospital care providers, including hospitalists, to keep their patients happy while giving them the care they deserve.
With the recent publishing of the Hospital Consumer Assessment of Healthcare providers and Systems (HCAHPS) scorecards for each hospital on the Hospital Compare Web site (www.hospitalcompare.hhs.gov), patients can see and compare local hospitals.
Because hospitalists are managing an ever-increasing portion of the hospital census, we can count on being right in the middle of all this. Coupled with the fact that 40% of hospitalists are directly employed by their hospital and a significant portion of other hospitalist groups have contracts with hospitals tied to quality improvement, we can expect a lot of pressure to not only improve patient satisfaction, but to make the “numbers” look better.
What Survey Measures
An important starting point for hospitalists and especially their leaders, who will be engaged in conversations with the C-suite about patient satisfaction data, is to better understand what the data indicate.
First, you need to know that the patient questionnaires were designed by several large vendors, the largest being Press Ganey.
While it is possible to segment the patients by those treated by a hospitalist and those not, the questions were not meant to describe, define, or compare the performance of different physicians. Remember, non-hospitalists for this purpose includes not only internists, but also surgeons, obstetricians, and other specialists.
Some questions on the survey about physicians include:
- During this hospital stay, how often did doctors treat you with respect? (never, sometimes, usually, always);
- During this hospital stay, how often did doctors explain things in a way you could understand? and
- During this hospital stay, how often did doctors listen carefully to you?
Other questions that might pertain to care directed by hospitalists but also relate to the entire care team include:
- How often was your pain controlled?
- Before giving you a new medicine, how often did staff tell you what it was for? and
- Before giving you a new medicine, how often did staff describe possible side effects in a way you could understand?
While you might aggregate all the replies specifically about the doctors’ performance and grade all the doctors separately, the all-important questions to the C-suite are the last two sections:
- How do patients rate the hospital? and
- Would patients recommend the hospital to friends and family?
Patients Are Different
It is important to understand the unique characteristics of the patients admitted and managed by hospitalists and to understand how these patients may respond differently to the standard patient satisfaction surveys than others in the patient population.
More often than not, hospitalists admit patients who are acutely ill, presenting through the emergency department (ED) with medical problems. Some studies have estimated that more than 70% of hospitalists’ patients come through the ED, while for the rest of the staff it is closer to 30% to 40%.
It is well known that patients admitted electively are more satisfied than those with an acute illness who come through the ED. In addition, patients admitted for medical problems have lower satisfaction ratings than those admitted for general surgery, subspecialty surgery, or obstetrics.
Therefore, if your hospital administration has pulled together statistics that purport to compare patient satisfaction for your hospitalist group versus all other admissions, you need to make sure that comparisons are made to a similar population, i.e., acutely ill patients admitted through the ED with medical diagnoses. The survey companies should be able to produce just such a comparison.
It is equally as important to make sure you focus on the total experience at the hospital and not just the questions specifically concerning only the doctors. Since hospitalists not only do front-line, face-to-face patient care, but also work with the team and attempt to improve the system to provide better overall quality, make sure to focus on questions like “How do patients rate the hospital?” and “Would patients recommend the hospital to friends and family?”
The other consideration is to understand how close the top quartile is to the bottom quartile, when comparisons are made with this data. In many of these surveys the patients are giving ratings on a scale of one to four, with many of the responses at three or four. Therefore, the top score might be a 3.6 and the bottom score average 3.2. It is important to understand if you are just minor adjustments away from being in a good range or if you are either so far above or below the standard of care that a real situation exists.
HM’s Role
Does the hospitalist model lead to better patient satisfaction? Like most things in hospital medicine, the answer is yes, no, and maybe. There are certain aspects of hospital medicine that should lead to happier patients:
- Present and easily available;
- Expert in hospital care;
- Improved coordination of care by specialists;
- Availability for multiple visits if patient condition changes;
- Availability to visit with loved-ones at their convenience; and
- Rapid response to nurse’s concerns.
There are aspects of getting your care from a hospitalist that may initially make the patient more concerned:
- They may be unfamiliar with the hospitalist and the hospitalist model;
- The hospitalist may demonstrate little or no knowledge of the patient’s history;
- The referring physician may not introduce the patient to the hospitalist; and
- The hospitalist may not explain the relationship with the referring physician.
How to Be Proactive
With all we have to do every day (and the list seems to get longer by the minute), it is easy to get perplexed by having to be responsible for the patients’ satisfaction with their hospital experience. That being said, hospitalists perform well when we step up to the plate and take action in these ways:
- Proactively meet with the person in the C-suite who oversees the patient satisfaction survey process or relates to the hospitalist group (e.g., vice president of medical affairs or chief medical officer) to better understand the survey results;
- Make sure if the data are being used to compare hospitalist care with non-hospitalist care that the comparison group of patients is equivalent (i.e., acutely ill medical patients admitted through the ED, not surgical or obstetrical patients);
- Make sure to focus not only on the “doctor-related” questions, but on patients’ overall satisfaction with the hospital; and
- Offer to help the C-suite improve patient satisfaction, but don’t attempt to “own” this performance measure for the entire hospital. Hospitalists can be helpful, but this is broader than any one group of physicians.
Further, make improving patient satisfaction a core goal for your group. Some strategies that may work include:
- Have a script for each patient encounter (“Hi, I’m Dr. Smith, I take care of Dr. Jones’ patients in the hospital. The way we communicate about your care is … The advantages to our partnership are …”);
- Hand out a brochure with your group’s hospitalists’ pictures, answers to frequently asked questions, and how to contact the hospitalist; and
- Sit down and shut up (i.e., patients will perceive you are taking time with them and listening if you are seated and let them speak without interruption).
Hospitals have been doing patient surveys for some time now. The Centers for Medicare and Medicaid Services and other payers are placing more emphasis on this quality measure. Now that the results easily are available to the public, major newspapers and broadcast media are calling attention to patient perspectives on their hospital care.
Once hospitalist groups understand the data, there is an opportunity to partner with their hospitals to better understand how our patients see their hospital care and allow for hospitalists to have an appropriate role in working with the other health professionals to improve patients’ experience with their care. TH
Dr. Wellikson is the CEO of SHM.
Note to readers: I would like to acknowledge SHM co-founder Win Whitcomb, MD, and SHM Senior Vice President Joe Miller for their assistance with this column.
Maternity Maneuvers
Maternity Maneuvers
How do most hospitalist groups manage maternity leave? I recently took six weeks for maternity leave. My colleagues worked my shifts, and I have virtually paid them all back. To do so I often would end up working 18 to 20 days consecutively and numerous weekends. This was not ideal on many levels. I most likely will not [receive a] bonus this year as well. Is there a better way?
New Mom in Midwest
Dr. Hospitalist responds: Congratulations on the birth of your child. As you recognize, becoming a parent is a wonderful experience but also can be stressful. It is not easy to balance the competing demands of family and work.
Medical leave is not unique to hospitalists—but with the average age of hospitalists being 37, it is commonplace to have hospitalist staff start families at this stage of their lives. In fact, as a hospitalist director, it would be foolish for me not to expect and plan for maternity and paternity leaves.
Medical leaves often are stressful for hospitalist programs because of the need to find replacement staff to fill the work schedule. There is no “best” way to cover the schedule during medical leaves. One thing is certain: Not offering medical leave is not only unrealistic, it may be against the law.
Hospitalist directors and those contemplating medical leave from work should familiarize themselves with the federal government’s Family Medical Leave Act (FMLA). Of course, I’m not an attorney; anyone who is looking for accurate advice concerning FMLA and other legal matters should consult a lawyer.
Briefly stated, the FMLA requires that “covered employers must grant an eligible employee up to a total of 12 work weeks of unpaid leave during any 12-month period for one or more of the following reasons:
- Birth and care of the newborn child of the employee;
- Placement with the employee of a son or daughter for adoption or foster care;
- To care for an immediate family member (spouse, child, or parent) with a serious health condition; or
- To take medical leave when the employee is unable to work because of a serious health condition.
It is important to know that the FMLA strictly defines eligibility criteria. For example, a covered employer is one who “employs 50 or more employees for each working day during each of 20 or more calendar work weeks in the current or preceding calendar year.” There also are strict criteria that define whether one is an eligible employee. It is important to note that FMLA does not guarantee paid time off—it only requires unpaid leave. You can find additional information about the FMLA online at the government’s Web site: www.dol.gov/esa/whd/fmla.
Peer Pressure
I am an attorney who often represents physicians in hospital peer-review matters. I represent a hospitalist whom the medical staff has recommended be terminated. Two internists have been appointed to the peer-review committee; one has an office-based practice, and the other is a cardiologist. Neither is a hospitalist.
I am trying to convince the medical staff that there should be a hospitalist on the peer-review committee because I believe what a hospitalist does each day is fundamentally different in scope and patient mix than the other two internists. My argument will be much stronger if it is the case that a hospitalist’s practice is now its own medical specialty.
Can you point me to any information or articles that support my belief that hospital practice is now a separate specialty?
Anxious Attorney
Dr. Hospitalist responds: Is a hospitalist practice sufficiently different than that of an office-based internist or cardiologist, so much so that peer-review activities would necessitate at a minimum some involvement of other hospitalists? To answer this question, I think we need to understand the definition of a hospitalist.
I recently heard a doctor describe himself as a hospitalist despite working clinically in the hospital only one month annually. Is he correct in defining himself as a hospitalist? If so, how would we distinguish him from primary care doctors who spend one-twelfth of their work life caring for hospitalized patients?
SHM defines hospitalists as “physicians whose primary professional focus is the general medical care of hospitalized patients. Their activities include patient care, teaching, research, and leadership related to hospital medicine.” Based on this definition, the doctor who spends one month annually caring for inpatients could be a hospitalist if the remainder of his work involved teaching, research, and leadership related to hospital medicine.
In your example, you cite two physicians on the peer-review committee: an office based internist and a cardiologist. Is it reasonable to consider their work similar to or different from that of a hospitalist? In the case of the internist, I think the key point is the fact you described him as office-based. That suggests to me his primary professional focus does not involve hospitalized patients.
One could argue that since both the hospitalist and the office-based internist were trained in internal medicine and both have American Board of Internal Medicine certification, they should be considered peers. I would point out that one’s specialty training has nothing to do with the definition of a hospitalist.
Although the majority of hospitalists in this country are internists, many others are family physicians and pediatricians. Some have subspecialty training, some don’t. Even obstetricians and surgeons are defining themselves as hospitalists.
With all that in mind, would we consider the cardiologist a hospitalist? Again, I think it would depend on the nature of the cardiologist practice. If this cardiologist has a primarily outpatient practice, that would be quite different from a hospitalist practice.
What if this cardiologist’s practice primarily is inpatient? I think it is reasonable to think about the scope of these physicians’ practices. Assuming the cardiologist practice is limited to the care of patients with primary cardiac issues, this would be a much narrower scope than that of most hospitalists.
It also is important to consider the training of the hospitalist. Take geriatrics hospitalists, for instance. The scope of their practice may be quite similar to that of a geriatrician who spends the majority of time caring for hospitalized patients.
Does the hospitalist have additional cardiology training? Does the focus of discussion at peer-review committee involve care of patients with primarily cardiac needs? The issue of which physicians should serve on peer-review committees when evaluating hospitalists is a complicated one that demands further scrutiny. TH
Maternity Maneuvers
How do most hospitalist groups manage maternity leave? I recently took six weeks for maternity leave. My colleagues worked my shifts, and I have virtually paid them all back. To do so I often would end up working 18 to 20 days consecutively and numerous weekends. This was not ideal on many levels. I most likely will not [receive a] bonus this year as well. Is there a better way?
New Mom in Midwest
Dr. Hospitalist responds: Congratulations on the birth of your child. As you recognize, becoming a parent is a wonderful experience but also can be stressful. It is not easy to balance the competing demands of family and work.
Medical leave is not unique to hospitalists—but with the average age of hospitalists being 37, it is commonplace to have hospitalist staff start families at this stage of their lives. In fact, as a hospitalist director, it would be foolish for me not to expect and plan for maternity and paternity leaves.
Medical leaves often are stressful for hospitalist programs because of the need to find replacement staff to fill the work schedule. There is no “best” way to cover the schedule during medical leaves. One thing is certain: Not offering medical leave is not only unrealistic, it may be against the law.
Hospitalist directors and those contemplating medical leave from work should familiarize themselves with the federal government’s Family Medical Leave Act (FMLA). Of course, I’m not an attorney; anyone who is looking for accurate advice concerning FMLA and other legal matters should consult a lawyer.
Briefly stated, the FMLA requires that “covered employers must grant an eligible employee up to a total of 12 work weeks of unpaid leave during any 12-month period for one or more of the following reasons:
- Birth and care of the newborn child of the employee;
- Placement with the employee of a son or daughter for adoption or foster care;
- To care for an immediate family member (spouse, child, or parent) with a serious health condition; or
- To take medical leave when the employee is unable to work because of a serious health condition.
It is important to know that the FMLA strictly defines eligibility criteria. For example, a covered employer is one who “employs 50 or more employees for each working day during each of 20 or more calendar work weeks in the current or preceding calendar year.” There also are strict criteria that define whether one is an eligible employee. It is important to note that FMLA does not guarantee paid time off—it only requires unpaid leave. You can find additional information about the FMLA online at the government’s Web site: www.dol.gov/esa/whd/fmla.
Peer Pressure
I am an attorney who often represents physicians in hospital peer-review matters. I represent a hospitalist whom the medical staff has recommended be terminated. Two internists have been appointed to the peer-review committee; one has an office-based practice, and the other is a cardiologist. Neither is a hospitalist.
I am trying to convince the medical staff that there should be a hospitalist on the peer-review committee because I believe what a hospitalist does each day is fundamentally different in scope and patient mix than the other two internists. My argument will be much stronger if it is the case that a hospitalist’s practice is now its own medical specialty.
Can you point me to any information or articles that support my belief that hospital practice is now a separate specialty?
Anxious Attorney
Dr. Hospitalist responds: Is a hospitalist practice sufficiently different than that of an office-based internist or cardiologist, so much so that peer-review activities would necessitate at a minimum some involvement of other hospitalists? To answer this question, I think we need to understand the definition of a hospitalist.
I recently heard a doctor describe himself as a hospitalist despite working clinically in the hospital only one month annually. Is he correct in defining himself as a hospitalist? If so, how would we distinguish him from primary care doctors who spend one-twelfth of their work life caring for hospitalized patients?
SHM defines hospitalists as “physicians whose primary professional focus is the general medical care of hospitalized patients. Their activities include patient care, teaching, research, and leadership related to hospital medicine.” Based on this definition, the doctor who spends one month annually caring for inpatients could be a hospitalist if the remainder of his work involved teaching, research, and leadership related to hospital medicine.
In your example, you cite two physicians on the peer-review committee: an office based internist and a cardiologist. Is it reasonable to consider their work similar to or different from that of a hospitalist? In the case of the internist, I think the key point is the fact you described him as office-based. That suggests to me his primary professional focus does not involve hospitalized patients.
One could argue that since both the hospitalist and the office-based internist were trained in internal medicine and both have American Board of Internal Medicine certification, they should be considered peers. I would point out that one’s specialty training has nothing to do with the definition of a hospitalist.
Although the majority of hospitalists in this country are internists, many others are family physicians and pediatricians. Some have subspecialty training, some don’t. Even obstetricians and surgeons are defining themselves as hospitalists.
With all that in mind, would we consider the cardiologist a hospitalist? Again, I think it would depend on the nature of the cardiologist practice. If this cardiologist has a primarily outpatient practice, that would be quite different from a hospitalist practice.
What if this cardiologist’s practice primarily is inpatient? I think it is reasonable to think about the scope of these physicians’ practices. Assuming the cardiologist practice is limited to the care of patients with primary cardiac issues, this would be a much narrower scope than that of most hospitalists.
It also is important to consider the training of the hospitalist. Take geriatrics hospitalists, for instance. The scope of their practice may be quite similar to that of a geriatrician who spends the majority of time caring for hospitalized patients.
Does the hospitalist have additional cardiology training? Does the focus of discussion at peer-review committee involve care of patients with primarily cardiac needs? The issue of which physicians should serve on peer-review committees when evaluating hospitalists is a complicated one that demands further scrutiny. TH
Maternity Maneuvers
How do most hospitalist groups manage maternity leave? I recently took six weeks for maternity leave. My colleagues worked my shifts, and I have virtually paid them all back. To do so I often would end up working 18 to 20 days consecutively and numerous weekends. This was not ideal on many levels. I most likely will not [receive a] bonus this year as well. Is there a better way?
New Mom in Midwest
Dr. Hospitalist responds: Congratulations on the birth of your child. As you recognize, becoming a parent is a wonderful experience but also can be stressful. It is not easy to balance the competing demands of family and work.
Medical leave is not unique to hospitalists—but with the average age of hospitalists being 37, it is commonplace to have hospitalist staff start families at this stage of their lives. In fact, as a hospitalist director, it would be foolish for me not to expect and plan for maternity and paternity leaves.
Medical leaves often are stressful for hospitalist programs because of the need to find replacement staff to fill the work schedule. There is no “best” way to cover the schedule during medical leaves. One thing is certain: Not offering medical leave is not only unrealistic, it may be against the law.
Hospitalist directors and those contemplating medical leave from work should familiarize themselves with the federal government’s Family Medical Leave Act (FMLA). Of course, I’m not an attorney; anyone who is looking for accurate advice concerning FMLA and other legal matters should consult a lawyer.
Briefly stated, the FMLA requires that “covered employers must grant an eligible employee up to a total of 12 work weeks of unpaid leave during any 12-month period for one or more of the following reasons:
- Birth and care of the newborn child of the employee;
- Placement with the employee of a son or daughter for adoption or foster care;
- To care for an immediate family member (spouse, child, or parent) with a serious health condition; or
- To take medical leave when the employee is unable to work because of a serious health condition.
It is important to know that the FMLA strictly defines eligibility criteria. For example, a covered employer is one who “employs 50 or more employees for each working day during each of 20 or more calendar work weeks in the current or preceding calendar year.” There also are strict criteria that define whether one is an eligible employee. It is important to note that FMLA does not guarantee paid time off—it only requires unpaid leave. You can find additional information about the FMLA online at the government’s Web site: www.dol.gov/esa/whd/fmla.
Peer Pressure
I am an attorney who often represents physicians in hospital peer-review matters. I represent a hospitalist whom the medical staff has recommended be terminated. Two internists have been appointed to the peer-review committee; one has an office-based practice, and the other is a cardiologist. Neither is a hospitalist.
I am trying to convince the medical staff that there should be a hospitalist on the peer-review committee because I believe what a hospitalist does each day is fundamentally different in scope and patient mix than the other two internists. My argument will be much stronger if it is the case that a hospitalist’s practice is now its own medical specialty.
Can you point me to any information or articles that support my belief that hospital practice is now a separate specialty?
Anxious Attorney
Dr. Hospitalist responds: Is a hospitalist practice sufficiently different than that of an office-based internist or cardiologist, so much so that peer-review activities would necessitate at a minimum some involvement of other hospitalists? To answer this question, I think we need to understand the definition of a hospitalist.
I recently heard a doctor describe himself as a hospitalist despite working clinically in the hospital only one month annually. Is he correct in defining himself as a hospitalist? If so, how would we distinguish him from primary care doctors who spend one-twelfth of their work life caring for hospitalized patients?
SHM defines hospitalists as “physicians whose primary professional focus is the general medical care of hospitalized patients. Their activities include patient care, teaching, research, and leadership related to hospital medicine.” Based on this definition, the doctor who spends one month annually caring for inpatients could be a hospitalist if the remainder of his work involved teaching, research, and leadership related to hospital medicine.
In your example, you cite two physicians on the peer-review committee: an office based internist and a cardiologist. Is it reasonable to consider their work similar to or different from that of a hospitalist? In the case of the internist, I think the key point is the fact you described him as office-based. That suggests to me his primary professional focus does not involve hospitalized patients.
One could argue that since both the hospitalist and the office-based internist were trained in internal medicine and both have American Board of Internal Medicine certification, they should be considered peers. I would point out that one’s specialty training has nothing to do with the definition of a hospitalist.
Although the majority of hospitalists in this country are internists, many others are family physicians and pediatricians. Some have subspecialty training, some don’t. Even obstetricians and surgeons are defining themselves as hospitalists.
With all that in mind, would we consider the cardiologist a hospitalist? Again, I think it would depend on the nature of the cardiologist practice. If this cardiologist has a primarily outpatient practice, that would be quite different from a hospitalist practice.
What if this cardiologist’s practice primarily is inpatient? I think it is reasonable to think about the scope of these physicians’ practices. Assuming the cardiologist practice is limited to the care of patients with primary cardiac issues, this would be a much narrower scope than that of most hospitalists.
It also is important to consider the training of the hospitalist. Take geriatrics hospitalists, for instance. The scope of their practice may be quite similar to that of a geriatrician who spends the majority of time caring for hospitalized patients.
Does the hospitalist have additional cardiology training? Does the focus of discussion at peer-review committee involve care of patients with primarily cardiac needs? The issue of which physicians should serve on peer-review committees when evaluating hospitalists is a complicated one that demands further scrutiny. TH
We’re Hiring
At the 2008 SHM Annual Meeting in San Diego, I had the pleasure of serving as moderator for a panel commenting on the opportunities and challenges faced by hospitalists. I’m not sure how well our predictions will withstand the test of time, but two things came up that I’ll discuss here:
1) Nearly every group is recruiting, and many seem to think the hospitalist shortage will last throughout the careers of those in practice today.
2) Nearly all hospitalist groups are looking for more doctors. I asked the approximately 1,600 in attendance how many are recruiting for more hospitalists. Nearly every hand in the room shot up. It was impressive; one friend (Bob Reynolds) told me he was sitting in the back and could feel a breeze in the room from all the hands being raised. Only about three hands went up when I asked how many thought their staffing was adequate.
Bear in mind that based on the show of hands nearly every group in the country is recruiting. Many groups are looking to add three to six hospitalists this year alone. This is on top of the average group growing about 20% to 25% the past two years, based on my study of data from the “Society of Hospital Medicine 2007-08 Survey: The Authoritative Source on the State of the Hospitalist Movement.” The survey showed the number of FTE doctors in the average hospitalist group grew from a median six to eight hospitalists (the average went from eight to 9.7).
Hospital medicine is the fastest-growing field in the history of American medicine, and it looks like the demand for hospitalists may be increasing even faster than the supply.
I was tempted to ask for a show of hands from doctors at the meeting who were looking for a hospitalist position, but feared it could disrupt the whole conference as those seeking new doctors pounced on the potential candidates in a piranha-like feeding frenzy. So there is good news for anyone interested in joining a hospitalist group: You should have a lot of choices. If you’re recruiting, you’d better get to work to make sure you have really good plan. Let me offer a few ideas.
Never stop recruiting. Dr. Greg Mappin, VPMA at Self Regional Hospital in Greenwood, S.C., told me his philosophy is to “recruit forever, and hire when necessary.” I agree.
You should build and maintain a robust candidate pipeline by ensuring your practice maintains a high level of visibility before your best source of new doctors. The best source for most groups is the closest residency training program, though other nearby hospitalist or outpatient practices might be a secondary source of new manpower.
I suggest you engage residents by hosting a dinner near their hospital once or twice a year and inviting all second- and third-year residents to attend regardless of their interest in becoming hospitalists. You might do this even in years you may not need to add hospitalists to ensure your dinner becomes a regular event for them and to ensure they’re very familiar with your program. Some hospitals develop night and weekend moonlighting programs that employ nearby residents, which increases the chance some will join the practice upon completion of their training.
Ensure all hospitalists—especially the group leader—actively participate in recruiting. Your hospital or medical group’s physician recruiter can be a terrific asset. He/she can provide advice regarding how to find candidates, arranging interviews, etc. Yet, it is critical for the hospitalist group leader to actively communicate with every candidate, including responding to every inquiry within a day or so.
Too many group leaders make a big mistake by waiting many days to respond to new inquiries, or letting the recruiter handle all communication in advance of an interview. During the interview, be sure the candidate spends time with many of the current group members and provides contact information for every group member in case the candidate would like to call any who weren’t available on the interview day. Consider providing the candidate with a copy of the group schedule, any orientation documents you have, and other such printed materials to review after the visit.
Recruit specifically for short-term members of your practice. Despite concerns about turnover, I think it is reasonable to actively pursue candidates who may have as little as two years to work in your practice. For example, they may plan to move to another town (e.g., when their spouse finishes training) or start fellowship training. In my experience, at least half of new doctors who plan to be a hospitalist for only a year or two will choose to stay on long term.
If you want your classified ad to stand out, think about writing one that specifically targets short-term hospitalists. It could say something like: “Do you have only two years to work as a hospitalist? Then this is the place for you.” You even could add benefits, such as tuition to attend conferences that would be of value for the doctor regardless of their future specialty or practice setting. If you desperately need additional doctors, get creative in recruiting those who plan to stay with you for only a couple years. I’m confident some will end up staying long term.
Continue “recruiting” the doctors in your practice. For a number of reasons, hospitalist turnover may be higher than most other specialties. So it is particularly important to take steps to minimize it. SHM’s white paper on hospitalist career satisfaction (“A Challenge for a New Specialty: A White Paper on Hospitalist Career Satisfaction”) offers observations and valuable suggestions for any practice. Find it under the “Publications” link on SHM’s Web site, www. hospitalmedicine.org.
No End to Shortage
Now back to that panel discussion at SHM’s Annual Meeting in April. I asked the panelists what things would be like if in 10 years the demand for hospitalists decreased, and the supply finally caught up with and ultimately exceeded demand.
I thought this could be a provocative question that would lead to a discussion about how much of our current situation, such as recent increases in hospital financial support provided per hospitalist, are due to the current hospitalist shortage. Will hospitals decrease their support if there is ever an excess of hospitalists?
No one was buying it. Everyone was convinced that despite the incredible growth in numbers of doctors practicing as hospitalists, the demand for hospitalists will continue to grow even faster than the supply. Panelist Ron Greeno, MD, FCCP, chief medical officer of Cogent Healthcare in Irvine, Calif., thought this hospitalist shortage would continue throughout our lifetime. I’m not sure how long Ron thinks he (or I) will live, but that’s a pretty bold prediction.
It looks like the current intense recruiting environment is here to stay for a long time. Every practice should be thinking about how best to manage it. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
At the 2008 SHM Annual Meeting in San Diego, I had the pleasure of serving as moderator for a panel commenting on the opportunities and challenges faced by hospitalists. I’m not sure how well our predictions will withstand the test of time, but two things came up that I’ll discuss here:
1) Nearly every group is recruiting, and many seem to think the hospitalist shortage will last throughout the careers of those in practice today.
2) Nearly all hospitalist groups are looking for more doctors. I asked the approximately 1,600 in attendance how many are recruiting for more hospitalists. Nearly every hand in the room shot up. It was impressive; one friend (Bob Reynolds) told me he was sitting in the back and could feel a breeze in the room from all the hands being raised. Only about three hands went up when I asked how many thought their staffing was adequate.
Bear in mind that based on the show of hands nearly every group in the country is recruiting. Many groups are looking to add three to six hospitalists this year alone. This is on top of the average group growing about 20% to 25% the past two years, based on my study of data from the “Society of Hospital Medicine 2007-08 Survey: The Authoritative Source on the State of the Hospitalist Movement.” The survey showed the number of FTE doctors in the average hospitalist group grew from a median six to eight hospitalists (the average went from eight to 9.7).
Hospital medicine is the fastest-growing field in the history of American medicine, and it looks like the demand for hospitalists may be increasing even faster than the supply.
I was tempted to ask for a show of hands from doctors at the meeting who were looking for a hospitalist position, but feared it could disrupt the whole conference as those seeking new doctors pounced on the potential candidates in a piranha-like feeding frenzy. So there is good news for anyone interested in joining a hospitalist group: You should have a lot of choices. If you’re recruiting, you’d better get to work to make sure you have really good plan. Let me offer a few ideas.
Never stop recruiting. Dr. Greg Mappin, VPMA at Self Regional Hospital in Greenwood, S.C., told me his philosophy is to “recruit forever, and hire when necessary.” I agree.
You should build and maintain a robust candidate pipeline by ensuring your practice maintains a high level of visibility before your best source of new doctors. The best source for most groups is the closest residency training program, though other nearby hospitalist or outpatient practices might be a secondary source of new manpower.
I suggest you engage residents by hosting a dinner near their hospital once or twice a year and inviting all second- and third-year residents to attend regardless of their interest in becoming hospitalists. You might do this even in years you may not need to add hospitalists to ensure your dinner becomes a regular event for them and to ensure they’re very familiar with your program. Some hospitals develop night and weekend moonlighting programs that employ nearby residents, which increases the chance some will join the practice upon completion of their training.
Ensure all hospitalists—especially the group leader—actively participate in recruiting. Your hospital or medical group’s physician recruiter can be a terrific asset. He/she can provide advice regarding how to find candidates, arranging interviews, etc. Yet, it is critical for the hospitalist group leader to actively communicate with every candidate, including responding to every inquiry within a day or so.
Too many group leaders make a big mistake by waiting many days to respond to new inquiries, or letting the recruiter handle all communication in advance of an interview. During the interview, be sure the candidate spends time with many of the current group members and provides contact information for every group member in case the candidate would like to call any who weren’t available on the interview day. Consider providing the candidate with a copy of the group schedule, any orientation documents you have, and other such printed materials to review after the visit.
Recruit specifically for short-term members of your practice. Despite concerns about turnover, I think it is reasonable to actively pursue candidates who may have as little as two years to work in your practice. For example, they may plan to move to another town (e.g., when their spouse finishes training) or start fellowship training. In my experience, at least half of new doctors who plan to be a hospitalist for only a year or two will choose to stay on long term.
If you want your classified ad to stand out, think about writing one that specifically targets short-term hospitalists. It could say something like: “Do you have only two years to work as a hospitalist? Then this is the place for you.” You even could add benefits, such as tuition to attend conferences that would be of value for the doctor regardless of their future specialty or practice setting. If you desperately need additional doctors, get creative in recruiting those who plan to stay with you for only a couple years. I’m confident some will end up staying long term.
Continue “recruiting” the doctors in your practice. For a number of reasons, hospitalist turnover may be higher than most other specialties. So it is particularly important to take steps to minimize it. SHM’s white paper on hospitalist career satisfaction (“A Challenge for a New Specialty: A White Paper on Hospitalist Career Satisfaction”) offers observations and valuable suggestions for any practice. Find it under the “Publications” link on SHM’s Web site, www. hospitalmedicine.org.
No End to Shortage
Now back to that panel discussion at SHM’s Annual Meeting in April. I asked the panelists what things would be like if in 10 years the demand for hospitalists decreased, and the supply finally caught up with and ultimately exceeded demand.
I thought this could be a provocative question that would lead to a discussion about how much of our current situation, such as recent increases in hospital financial support provided per hospitalist, are due to the current hospitalist shortage. Will hospitals decrease their support if there is ever an excess of hospitalists?
No one was buying it. Everyone was convinced that despite the incredible growth in numbers of doctors practicing as hospitalists, the demand for hospitalists will continue to grow even faster than the supply. Panelist Ron Greeno, MD, FCCP, chief medical officer of Cogent Healthcare in Irvine, Calif., thought this hospitalist shortage would continue throughout our lifetime. I’m not sure how long Ron thinks he (or I) will live, but that’s a pretty bold prediction.
It looks like the current intense recruiting environment is here to stay for a long time. Every practice should be thinking about how best to manage it. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
At the 2008 SHM Annual Meeting in San Diego, I had the pleasure of serving as moderator for a panel commenting on the opportunities and challenges faced by hospitalists. I’m not sure how well our predictions will withstand the test of time, but two things came up that I’ll discuss here:
1) Nearly every group is recruiting, and many seem to think the hospitalist shortage will last throughout the careers of those in practice today.
2) Nearly all hospitalist groups are looking for more doctors. I asked the approximately 1,600 in attendance how many are recruiting for more hospitalists. Nearly every hand in the room shot up. It was impressive; one friend (Bob Reynolds) told me he was sitting in the back and could feel a breeze in the room from all the hands being raised. Only about three hands went up when I asked how many thought their staffing was adequate.
Bear in mind that based on the show of hands nearly every group in the country is recruiting. Many groups are looking to add three to six hospitalists this year alone. This is on top of the average group growing about 20% to 25% the past two years, based on my study of data from the “Society of Hospital Medicine 2007-08 Survey: The Authoritative Source on the State of the Hospitalist Movement.” The survey showed the number of FTE doctors in the average hospitalist group grew from a median six to eight hospitalists (the average went from eight to 9.7).
Hospital medicine is the fastest-growing field in the history of American medicine, and it looks like the demand for hospitalists may be increasing even faster than the supply.
I was tempted to ask for a show of hands from doctors at the meeting who were looking for a hospitalist position, but feared it could disrupt the whole conference as those seeking new doctors pounced on the potential candidates in a piranha-like feeding frenzy. So there is good news for anyone interested in joining a hospitalist group: You should have a lot of choices. If you’re recruiting, you’d better get to work to make sure you have really good plan. Let me offer a few ideas.
Never stop recruiting. Dr. Greg Mappin, VPMA at Self Regional Hospital in Greenwood, S.C., told me his philosophy is to “recruit forever, and hire when necessary.” I agree.
You should build and maintain a robust candidate pipeline by ensuring your practice maintains a high level of visibility before your best source of new doctors. The best source for most groups is the closest residency training program, though other nearby hospitalist or outpatient practices might be a secondary source of new manpower.
I suggest you engage residents by hosting a dinner near their hospital once or twice a year and inviting all second- and third-year residents to attend regardless of their interest in becoming hospitalists. You might do this even in years you may not need to add hospitalists to ensure your dinner becomes a regular event for them and to ensure they’re very familiar with your program. Some hospitals develop night and weekend moonlighting programs that employ nearby residents, which increases the chance some will join the practice upon completion of their training.
Ensure all hospitalists—especially the group leader—actively participate in recruiting. Your hospital or medical group’s physician recruiter can be a terrific asset. He/she can provide advice regarding how to find candidates, arranging interviews, etc. Yet, it is critical for the hospitalist group leader to actively communicate with every candidate, including responding to every inquiry within a day or so.
Too many group leaders make a big mistake by waiting many days to respond to new inquiries, or letting the recruiter handle all communication in advance of an interview. During the interview, be sure the candidate spends time with many of the current group members and provides contact information for every group member in case the candidate would like to call any who weren’t available on the interview day. Consider providing the candidate with a copy of the group schedule, any orientation documents you have, and other such printed materials to review after the visit.
Recruit specifically for short-term members of your practice. Despite concerns about turnover, I think it is reasonable to actively pursue candidates who may have as little as two years to work in your practice. For example, they may plan to move to another town (e.g., when their spouse finishes training) or start fellowship training. In my experience, at least half of new doctors who plan to be a hospitalist for only a year or two will choose to stay on long term.
If you want your classified ad to stand out, think about writing one that specifically targets short-term hospitalists. It could say something like: “Do you have only two years to work as a hospitalist? Then this is the place for you.” You even could add benefits, such as tuition to attend conferences that would be of value for the doctor regardless of their future specialty or practice setting. If you desperately need additional doctors, get creative in recruiting those who plan to stay with you for only a couple years. I’m confident some will end up staying long term.
Continue “recruiting” the doctors in your practice. For a number of reasons, hospitalist turnover may be higher than most other specialties. So it is particularly important to take steps to minimize it. SHM’s white paper on hospitalist career satisfaction (“A Challenge for a New Specialty: A White Paper on Hospitalist Career Satisfaction”) offers observations and valuable suggestions for any practice. Find it under the “Publications” link on SHM’s Web site, www. hospitalmedicine.org.
No End to Shortage
Now back to that panel discussion at SHM’s Annual Meeting in April. I asked the panelists what things would be like if in 10 years the demand for hospitalists decreased, and the supply finally caught up with and ultimately exceeded demand.
I thought this could be a provocative question that would lead to a discussion about how much of our current situation, such as recent increases in hospital financial support provided per hospitalist, are due to the current hospitalist shortage. Will hospitals decrease their support if there is ever an excess of hospitalists?
No one was buying it. Everyone was convinced that despite the incredible growth in numbers of doctors practicing as hospitalists, the demand for hospitalists will continue to grow even faster than the supply. Panelist Ron Greeno, MD, FCCP, chief medical officer of Cogent Healthcare in Irvine, Calif., thought this hospitalist shortage would continue throughout our lifetime. I’m not sure how long Ron thinks he (or I) will live, but that’s a pretty bold prediction.
It looks like the current intense recruiting environment is here to stay for a long time. Every practice should be thinking about how best to manage it. TH
Dr. Nelson has been a practicing hospitalist since 1988 and is co-founder and past president of SHM. He is a principal in Nelson/Flores Associates, a national hospitalist practice management consulting firm. He is also part of the faculty for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. This column represents his views and is not intended to reflect an official position of SHM.
Bueller ... Bueller?
A sea of pimples and drooling yawns. That’s what was staring, glassy-eyed back at me.
More realistically they were staring through me—through space, actually—to 50 minutes into the future. The look inhabited the hinterlands between boredom and loathing. A less-trained eye would mistake their tranquil countenances as anticipatory rapture. However, years of educating medical students and residents taught me that this was the look of those residing serenely in their own world, eons away from the classroom.
Then the ring of the first-period bell jolted me from my thoughts.
It was 7:45 a.m., and I found myself back at my hometown high school—jettisoned 20 years back in time. Months earlier I had agreed to teach a health and biology class as a visiting teacher as part of a career-planning program. I was instructed to teach them about what I do in my professional life. At the time I foolishly imagined a cohort of eagerly engrossed students hanging on my every word. What I found was more in line with a pack of sedated sloths sleepily hanging from the tedium tree.
My folly became even more obvious when I introduced my title slide: “The Epidemic of Medical Error.”
What was I thinking trying to teach 14-year-old kids about patient safety and medical error? Months before the talk I had agreed to this topic only after the principal assured me this would be of great interest to high school freshman and sophomores. It wasn’t until the week before the talk that the unease set in.
Could I really interest hormone-raged pubescent teenagers in the intricacies of hospital patient safety? My wife, ever helpful, was instrumental in triggering this epiphany. She noted that 14-year-olds are really only interested in … well, nothing. The prospect of engaging them in the complexities of hospital healthcare seemed about as likely as getting a trout excited over a fish fry.
Engaged they were not; in fact, some narcoleptic kid in the back had already engaged REM sleep. I was only four minutes into this and I was already foundering miserably. I had become that teacher. You know the one. He is so exceptionally mind-numbing that you wonder if he was brought in as some sort of social experiment testing the currently known limits of boredom.
Then someone had an, er, gastric accident.
The ensuing hilarity made it difficult to pinpoint the exact source or even if this was a true gastroenterological event or its ever-comical cousin, the armpit version. Now, I’m young enough to remember the comic genius of well-timed and executed classroom flatus. As such I understood that this was clearly intended as a territorial marking. The natives had spoken; there was an enemy among them.
As the clock metamorphosed into one of those melting Dali timepieces, we commenced discussion of three cases. The first involved a man who had the wrong leg amputated. The second reviewed the case of young women who suffered devastating consequences after an ICU medication was dropped off her medication list upon transfer to a medical ward team. The final case involved a patient with a myocardial infarction who did not receive aspirin upon admission to the hospital.
An interesting thing happened after I presented the first case. They were interested. It seems cutting off the wrong leg resonates with high school students. Moreover they were aghast that these types of medical errors were occurring. They were shocked that such smart people could make such dumb mistakes. Mr. Narcolepsy slid out of stage 1 sleep long enough to sarcastically note that even he knew that heart attack patients should get aspirin.
I asked them how they thought we could avoid these mistakes. A girl in the front wondered if we couldn’t just ask the patient which leg they wanted cut off. I noted that patients are anesthetized when we meet them for the surgery. She then proffered that perhaps we could ask them while they were awake and then mark the correct leg with a marker prior to going into the operating room.
Regarding the ICU transfer patient who had a medication drop off her med list, a quiet kid in the front asked, “Why don’t you just compare the list of medications used in the ICU to those outside of the ICU?” Another suggested that the two different teams of doctors could sit down and discuss the patient’s medications to be sure nothing was left off.
They wondered if we could avoid forgetting important medications—such as was done with the aspirin for the MI patient—by making a list of the things every patient with a heart attack would need. For example, didn’t they need an EKG, some lab tests, and some medications? Wouldn’t it be best to just have this list so that we didn’t have to remember all these things?
Unwittingly, these teen-agers—none old enough to shave—had just in their own words recited some of the key tenets of the patient safety movement:
- Active communication with the patient prior to surgery;
- Time out prior to surgery to ensure correct patient and surgery;
- Marking the site of surgery;
- Improved communication around patient handoffs;
- Medication reconciliation at every transfer of care; and
- Use of protocols to ensure best practices.
I was floored. In 30 minutes, a group of teenagers had developed a list of hospital safety measures that it has taken modern medicine generations to grasp.
The amount of medical errors has risen in step with the complexity of the medical care we provide. However, this does not mean that the causes of these medical errors are complex. Rather, most errors result from simple mistakes and systems issues. In fact, as I was taught on that fateful spring morning, I learned everything I need to know about patient safety in high school. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado, Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
A sea of pimples and drooling yawns. That’s what was staring, glassy-eyed back at me.
More realistically they were staring through me—through space, actually—to 50 minutes into the future. The look inhabited the hinterlands between boredom and loathing. A less-trained eye would mistake their tranquil countenances as anticipatory rapture. However, years of educating medical students and residents taught me that this was the look of those residing serenely in their own world, eons away from the classroom.
Then the ring of the first-period bell jolted me from my thoughts.
It was 7:45 a.m., and I found myself back at my hometown high school—jettisoned 20 years back in time. Months earlier I had agreed to teach a health and biology class as a visiting teacher as part of a career-planning program. I was instructed to teach them about what I do in my professional life. At the time I foolishly imagined a cohort of eagerly engrossed students hanging on my every word. What I found was more in line with a pack of sedated sloths sleepily hanging from the tedium tree.
My folly became even more obvious when I introduced my title slide: “The Epidemic of Medical Error.”
What was I thinking trying to teach 14-year-old kids about patient safety and medical error? Months before the talk I had agreed to this topic only after the principal assured me this would be of great interest to high school freshman and sophomores. It wasn’t until the week before the talk that the unease set in.
Could I really interest hormone-raged pubescent teenagers in the intricacies of hospital patient safety? My wife, ever helpful, was instrumental in triggering this epiphany. She noted that 14-year-olds are really only interested in … well, nothing. The prospect of engaging them in the complexities of hospital healthcare seemed about as likely as getting a trout excited over a fish fry.
Engaged they were not; in fact, some narcoleptic kid in the back had already engaged REM sleep. I was only four minutes into this and I was already foundering miserably. I had become that teacher. You know the one. He is so exceptionally mind-numbing that you wonder if he was brought in as some sort of social experiment testing the currently known limits of boredom.
Then someone had an, er, gastric accident.
The ensuing hilarity made it difficult to pinpoint the exact source or even if this was a true gastroenterological event or its ever-comical cousin, the armpit version. Now, I’m young enough to remember the comic genius of well-timed and executed classroom flatus. As such I understood that this was clearly intended as a territorial marking. The natives had spoken; there was an enemy among them.
As the clock metamorphosed into one of those melting Dali timepieces, we commenced discussion of three cases. The first involved a man who had the wrong leg amputated. The second reviewed the case of young women who suffered devastating consequences after an ICU medication was dropped off her medication list upon transfer to a medical ward team. The final case involved a patient with a myocardial infarction who did not receive aspirin upon admission to the hospital.
An interesting thing happened after I presented the first case. They were interested. It seems cutting off the wrong leg resonates with high school students. Moreover they were aghast that these types of medical errors were occurring. They were shocked that such smart people could make such dumb mistakes. Mr. Narcolepsy slid out of stage 1 sleep long enough to sarcastically note that even he knew that heart attack patients should get aspirin.
I asked them how they thought we could avoid these mistakes. A girl in the front wondered if we couldn’t just ask the patient which leg they wanted cut off. I noted that patients are anesthetized when we meet them for the surgery. She then proffered that perhaps we could ask them while they were awake and then mark the correct leg with a marker prior to going into the operating room.
Regarding the ICU transfer patient who had a medication drop off her med list, a quiet kid in the front asked, “Why don’t you just compare the list of medications used in the ICU to those outside of the ICU?” Another suggested that the two different teams of doctors could sit down and discuss the patient’s medications to be sure nothing was left off.
They wondered if we could avoid forgetting important medications—such as was done with the aspirin for the MI patient—by making a list of the things every patient with a heart attack would need. For example, didn’t they need an EKG, some lab tests, and some medications? Wouldn’t it be best to just have this list so that we didn’t have to remember all these things?
Unwittingly, these teen-agers—none old enough to shave—had just in their own words recited some of the key tenets of the patient safety movement:
- Active communication with the patient prior to surgery;
- Time out prior to surgery to ensure correct patient and surgery;
- Marking the site of surgery;
- Improved communication around patient handoffs;
- Medication reconciliation at every transfer of care; and
- Use of protocols to ensure best practices.
I was floored. In 30 minutes, a group of teenagers had developed a list of hospital safety measures that it has taken modern medicine generations to grasp.
The amount of medical errors has risen in step with the complexity of the medical care we provide. However, this does not mean that the causes of these medical errors are complex. Rather, most errors result from simple mistakes and systems issues. In fact, as I was taught on that fateful spring morning, I learned everything I need to know about patient safety in high school. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado, Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
A sea of pimples and drooling yawns. That’s what was staring, glassy-eyed back at me.
More realistically they were staring through me—through space, actually—to 50 minutes into the future. The look inhabited the hinterlands between boredom and loathing. A less-trained eye would mistake their tranquil countenances as anticipatory rapture. However, years of educating medical students and residents taught me that this was the look of those residing serenely in their own world, eons away from the classroom.
Then the ring of the first-period bell jolted me from my thoughts.
It was 7:45 a.m., and I found myself back at my hometown high school—jettisoned 20 years back in time. Months earlier I had agreed to teach a health and biology class as a visiting teacher as part of a career-planning program. I was instructed to teach them about what I do in my professional life. At the time I foolishly imagined a cohort of eagerly engrossed students hanging on my every word. What I found was more in line with a pack of sedated sloths sleepily hanging from the tedium tree.
My folly became even more obvious when I introduced my title slide: “The Epidemic of Medical Error.”
What was I thinking trying to teach 14-year-old kids about patient safety and medical error? Months before the talk I had agreed to this topic only after the principal assured me this would be of great interest to high school freshman and sophomores. It wasn’t until the week before the talk that the unease set in.
Could I really interest hormone-raged pubescent teenagers in the intricacies of hospital patient safety? My wife, ever helpful, was instrumental in triggering this epiphany. She noted that 14-year-olds are really only interested in … well, nothing. The prospect of engaging them in the complexities of hospital healthcare seemed about as likely as getting a trout excited over a fish fry.
Engaged they were not; in fact, some narcoleptic kid in the back had already engaged REM sleep. I was only four minutes into this and I was already foundering miserably. I had become that teacher. You know the one. He is so exceptionally mind-numbing that you wonder if he was brought in as some sort of social experiment testing the currently known limits of boredom.
Then someone had an, er, gastric accident.
The ensuing hilarity made it difficult to pinpoint the exact source or even if this was a true gastroenterological event or its ever-comical cousin, the armpit version. Now, I’m young enough to remember the comic genius of well-timed and executed classroom flatus. As such I understood that this was clearly intended as a territorial marking. The natives had spoken; there was an enemy among them.
As the clock metamorphosed into one of those melting Dali timepieces, we commenced discussion of three cases. The first involved a man who had the wrong leg amputated. The second reviewed the case of young women who suffered devastating consequences after an ICU medication was dropped off her medication list upon transfer to a medical ward team. The final case involved a patient with a myocardial infarction who did not receive aspirin upon admission to the hospital.
An interesting thing happened after I presented the first case. They were interested. It seems cutting off the wrong leg resonates with high school students. Moreover they were aghast that these types of medical errors were occurring. They were shocked that such smart people could make such dumb mistakes. Mr. Narcolepsy slid out of stage 1 sleep long enough to sarcastically note that even he knew that heart attack patients should get aspirin.
I asked them how they thought we could avoid these mistakes. A girl in the front wondered if we couldn’t just ask the patient which leg they wanted cut off. I noted that patients are anesthetized when we meet them for the surgery. She then proffered that perhaps we could ask them while they were awake and then mark the correct leg with a marker prior to going into the operating room.
Regarding the ICU transfer patient who had a medication drop off her med list, a quiet kid in the front asked, “Why don’t you just compare the list of medications used in the ICU to those outside of the ICU?” Another suggested that the two different teams of doctors could sit down and discuss the patient’s medications to be sure nothing was left off.
They wondered if we could avoid forgetting important medications—such as was done with the aspirin for the MI patient—by making a list of the things every patient with a heart attack would need. For example, didn’t they need an EKG, some lab tests, and some medications? Wouldn’t it be best to just have this list so that we didn’t have to remember all these things?
Unwittingly, these teen-agers—none old enough to shave—had just in their own words recited some of the key tenets of the patient safety movement:
- Active communication with the patient prior to surgery;
- Time out prior to surgery to ensure correct patient and surgery;
- Marking the site of surgery;
- Improved communication around patient handoffs;
- Medication reconciliation at every transfer of care; and
- Use of protocols to ensure best practices.
I was floored. In 30 minutes, a group of teenagers had developed a list of hospital safety measures that it has taken modern medicine generations to grasp.
The amount of medical errors has risen in step with the complexity of the medical care we provide. However, this does not mean that the causes of these medical errors are complex. Rather, most errors result from simple mistakes and systems issues. In fact, as I was taught on that fateful spring morning, I learned everything I need to know about patient safety in high school. TH
Dr. Glasheen is associate professor of medicine at the University of Colorado, Denver, where he serves as director of the Hospital Medicine Program and the Hospitalist Training Program, and as associate program director of the Internal Medicine Residency Program.
How can we Reduce Indwelling Urinary Catheter Use and Complications?
Case
A 68-year-old male with a history of Alzheimer’s dementia and incontinence presents with failure to thrive. A Foley catheter is placed due to the patient’s incontinence and fall risk. Three days after admission while awaiting placement in a skilled nursing facility (SNF), he develops a urinary tract infection (UTI) complicated by delirium delaying his transfer to the SNF. What could have been done to prevent this complication?
Overview
It has been 50 years since Beeson, et al., recognized the potential harms stemming from urethral catheterization and penned an editorial to the American Journal of Medicine titled “The case against the catheter.”1
Since then, there has been considerable exploration of ways to limit urethral catheterization and ultimately decrease catheter-associated urinary tract infections (CAUTIs). Unfortunately, little progress has been made; indwelling urinary catheters remain ubiquitous in hospitals and CAUTIs remain the most common hospital-acquired infection in the United States.2 Given the emphasis on the quality and costs of healthcare, it is an opportune time to revisit catheter management and use as a way to combat the clinical and economic consequences of CAUTIs.
Clinicians may be lulled into thinking the clinical impact of CAUTI is less than that of other nosocomial infections. However, beyond the obvious patient harm from UTIs, associated bacteremia, and even death, the public health implications of CAUTI cannot be denied. Urinary tract infections constitute 40% of all nosocomial infections; accounting for an estimated 1 million cases annually.3 Further, 80% of all UTIs are associated with indwelling catheter use.
On average, nosocomial UTI necessitates one extra hospital day per patient, or approximately one million excess hospital days per year.4 Pooled cost analysis shows that UTIs consume an additional $400-$1,700 per event, or an estimated $425 million per year in the United States.5,6 Clearly, we cannot wait another 50 years to address this problem.
Review of the Data
Catheter duration as a risk factor for CAUTI: The indwelling catheter creates a portal of entry into a usually sterile body cavity and provides a surface on which microorganisms can colonize. At a finite rate of colonization—the incidence of bacteriuria is 3% to 10% per catheter day—the duration of urinary catheterization becomes the strongest predictor of catheter-associated bacteriuria.7 Even in relatively short-term catheter use of two to 10 days, the pooled cumulative incidence of developing bacteriuria is 26%.
Given the magnitude of these numbers, it should be no surprise that after one month of catheterization, bacteriuria develops in almost all patients. Twenty-four percent of patients with bacteriuria develop symptomatic UTIs with close to 5% suffering bacteremia. Consequently, nosocomial UTIs cause 15% of all hospital-acquired bacteremia.
Optimal catheter management: The easiest and most effective means to prevent CAUTI is to limit the use of urinary catheters to clearly identified medical indications (see Table 1, above). However, as simple as this prevention practice may sound, studies have demonstrated that as many as 20% of patients have indwelling catheters initially placed for unjustified or even unknown medical indications.8 Additionally, continued catheter use is inappropriate in one-third to one-half of all catheter days.9 These data confirm misuse and overuse of indwelling urinary catheters in the hospital setting is common.
In 1981, the Centers for Disease Control and Prevention (CDC) recognized the importance of addressing this situation and published a guideline to aid prevention of CAUTIs.10 The CDC urged the limitation of catheter use to a carefully selected patient population. Furthermore, the report strongly stressed the importance of catheter removal as soon as possible and advised against the use of catheters solely for the convenience of healthcare workers.
Evidence-based techniques for insertion and catheter care also were outlined in the guideline (see Table 2, p. 31). However, these recommendations have been poorly implemented, likely due to the competing priorities of providers and the difficulty operationalizing the guidelines. Additionally, evidence from the intervening 25 years has not yet been incorporated into the guideline, although a revision currently is underway.
Until that revision is complete, the Joanna Briggs Institute guideline published in 2001 addresses some of the same management techniques and incorporates newer evidence.11 Of note, practices that have been discredited due to contradictory evidence include aggressive meatal cleaning, bladder irrigation, and the application of antimicrobial agents in the drainage bag.12
Strategies to reduce unnecessary catheter days: One of the remediable reasons for catheter misuse lies in the fact physicians often are unaware of the presence of an indwelling catheter in their hospitalized patients.
Saint, et al., showed physicians were unaware of catheterization in 28% of their patients and that attending physicians were less conscious of a patient’s catheter status than residents, interns, or medical students.13 Further, the “forgotten” catheters were more likely to be unnecessary than those remembered by the healthcare team.
This information has prompted the use of various computer-based and multidisciplinary feedback protocols to readdress and re-evaluate the need for continued catheterization in a patient. For example, a study at the VAMC Puget Sound demonstrated that having a computerized order protocol for urinary catheters significantly increased the rate of documentation as well as decreased the duration of catheterization by an average of three days.14
Similar interventions to encourage early catheter removal have included daily reminders from nursing staff, allowing a nurse to discontinue catheter use independent of a physician’s order, and feedback in which nursing staff is educated about the incidence of UTI.15-17 All these relatively simple interventions showed significant improvement in the catheter removal rate and incidence of CAUTIs as well as documented cost savings.
Alternatives to indwelling catheters: In addition to efforts to decrease catheter days, alternatives to the indwelling catheters also should be explored. One such alternative method is intermittent catheterization.
Several studies in postoperative patients with hip fractures have demonstrated that the development of UTI is lower with intermittent catheterization when compared with indwelling catheterization.18 Nevertheless, since the risk of bacteriuria is 1% to 3% per episode of catheterization, after a few weeks the majority of patients will have bacteriuria. However, as the bulk of this bacteriuria often is asymptomatic, intermittent catheterization may still be an improvement. This is particularly true in postoperative patients undergoing rehabilitation and those patients only requiring catheterization for a limited number of days.
More recent studies have evaluated the use of bedside bladder ultrasound in an attempt to determine when intermittent catheterization is needed and thereby limit its use compared with standard timed catheterization. Frederickson, et al., demonstrated that this intervention resulted in significantly fewer catheterizations in surgical patients, thus delaying or avoiding the need for catheterization in 81% of the cases.19 Given this drastic improvement, it is no surprise bladder ultrasound use reduced the rates of UTI.20
External condom catheters present another alternative to indwelling catheter use but the outcomes data is conflicting. While the risk of bacteriuria is approximately 12% per month, this rate becomes increasingly higher with frequent manipulation of the condom catheter. 21,22
Two parallel cohort studies in a VA nursing home showed the incidence of symptomatic UTI to be 2.5 times greater in men with an indwelling catheter than those with a condom catheter.23 On the other hand, a cross-sectional Danish study reported higher rates of UTI with external condom catheters than urethral catheters in hospitalized patients.24 Complications from condom catheters include phimosis and local skin maceration, necessitating meticulous care with the use of these devices. Although the data surrounding external catheterization is somewhat contradictory, this device warrants consideration in incontinent males without urinary tract obstruction.
There are several other alternatives to urethral catheterization (see Table 3, p. 31), many of which have excellent face validity even in the absence of rigorous evidence.
Antimicrobial catheters: The development of antimicrobial urinary catheters, including silver-alloy and nitrofurazone-coated catheters, has been greeted with much excitement, however, the jury is still out about their best use. A 2006 systematic literature review reported that in comparison to standard catheters, antimicrobial catheters can delay or even prevent the development of bacteriuria with short-term usage.25
However, not all antimicrobial catheters are equally effective; assorted studies lack data about clinically relevant endpoints such as prevention of symptomatic UTI, bloodstream infection or death.26, 27 In addition, there are no good trials comparing nitrofurazone to silver-alloy catheters. Therefore, the level of excitement surrounding antimicrobial catheters—particularly silver-alloy catheters—must be tempered by the additional costs incurred by their use.
To date, the cost-effectiveness of antimicrobial catheters has not been demonstrated. Although additional research in this topic is still needed, some experts currently recommend the consideration of silver-alloy catheters in patients at the highest risk for developing serious consequences from UTIs.
Efforts to reduce CAUTI: In response to significant public interest in hospital-acquired infections including CAUTI, the federal government and many state governments are beginning to demand change. In August 2007, the Centers for Medicare and Medicaid Services instituted a mandate making hospitals financially responsible for selected preventable hospital-acquired harms, including CAUTIs.28 In addition, beginning with Pennsylvania in 2006, several states have mandated public reporting of hospital-acquired infections.29
Given the available information about CAUTI prevalence, risks, and preventive techniques, it is surprising the majority of hospitals in the United States have not taken appropriate measures to limit indwelling catheter use. A recent study by Saint, et al., demonstrated the startling fact that only a minority of hospitals monitor the use of urethral catheters in their patients.30
Among study hospitals, there was no widely used technique to prevent CAUTI including evidence-based practices such as daily catheter reminders. The results of this investigation illustrate the urgent need for a national strategy to reduce CAUTI. Until that time, however, hospital-based physicians must take the lead to champion collaborative efforts, to promote evidence-based catheter use.
Back to the Case
As incontinence and fall risk are not medically appropriate indications for a urethral catheter, a Foley catheter should not have been utilized. Alternatives to indwelling catheterization in this patient would include a bedside commode with nursing assistance, a timed voiding program, intermittent catheterization with or without bladder ultrasound, incontinence pads, or a condom catheter.
Attentiveness to the appropriate medical indications for catheter use, familiarity with catheter alternatives, and recognition of the clinical and economic impact of CAUTI may have prevented this patient’s UTI-induced delirium and facilitated his early transfer to SNF. TH
Dr. Wald is a getriatric hospitalist and assistant professor of medicine at the University of Colorado, Denver. Dr. Furfari is a hospital medicine fellow at the University of Colorado Denver.
References
- Beeson PB. The case against the catheter. Am J Med. 1958;24:1-3.
- Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28:68-75.
- Sedor J, Mulholland SG. Hospital-acquired UTIs associated with the indwelling catheter. Urol Clin North Am. 1999;26:821-828.
- Foxman B. Epidemiology of UTI: Incidence, morbidity and economic costs. Am J Med. 2002;113(1A):5S-13S.
- Tambyah PA, Knasinski V, Maki D. The direct costs of nosocomial catheter-associated UTI in the era of managed care. Infect Control Hosp Epidemiol. 2002;23:27-31.
- Jarvis, WR. Selected aspects of socioeconomic impact of nosocomial infections. Infect Control Hosp Epidemiol. 1996;17:552-557.
- Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11:609-622.
- Jain P, Parada JP, David A, Smith L. Overuse of the indwelling urinary catheter in hospitalized medical patients. Arch Internal Med. 1995;155:1425-1429.
- Hartstein AI, Garber SB, Ward TT, Jones SR, Morthland VH. Nosocomial urinary tract infection: a prospective evaluation of 108 catheterized patients. Infect Control. 1981;2:380-386.
- Wong E. Guideline for prevention of catheter-associated urinary tract infections. Center for Disease Control and Prevention 1981. Available at: www.cdc.gov/ncidod/dhqp/gl_catheter_assoc.html . Accessed May 8, 2008.
- Joanna Briggs Institute. Management of short term indwelling urethral catheters to prevent urinary tract infections. 2000;4(1):ISSN 1329-1874.
- Burke JP, Garibaldi RA, Britt MR, Jacobson JA, Conti M, Alling DW. Prevention of catheter-associated urinary tract infections. Am J Med. 1981;70:655-658.
- Saint S, Wiese J, Amory JK, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109:476-480.
- Cornia PB, Amory JK, Fraser S, Saint S, Lipsky BA. Computer-based order entry decreases duration of indwelling urinary catheterization in hospitalized patients. Am J Med. 2003;114:404-406.
- Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978.
- Topal J, Conklin S, Camp K, Morris TB, Herbert P. Prevention of nosocomial catheter-associated urinary tract infections through computerized feedback to physicians and a nurse-directed protocol. Am J Med Qual. 2005;20(3):121-126.
- Goetz AM, Kedzuf S, Wagener M, Muder R. Feedback to nursing staff as an intervention to reduce catheter-associated urinary tract infections. Am J Infect Control. 1999;27(5):402-404.
- Johansson I, Athlin E, Frykholm L, Bolinder H, Larsson G. Intermittent versus indwelling catheters for older patients with hip fractures. J Clin Nurs. 2002;11:651-656.
- Frederickson M, Neitzel JJ, Miller EH, Reuter S, Graner T, Heller J. The implementation of bedside bladder ultrasound technology: Effects of patient and cost postoperative outcomes in tertiary care. Orthop Nurs. 2000;19(3):79-87.
- Slappendel R, Weber EWG. Non-invasive measurement of bladder volume as an indication for bladder catheterization after orthopedic surgery and its effect on urinary tract infections. Eur J Anesthesiol. 1999;16:503-506.
- Hirsh D, Fainstein V, Musher DM. Do condom catheter collecting systems cause urinary tract infections? JAMA. 1979;242:340-341.
- Wong ES. Guideline for prevention of catheter-associated urinary tract infections. Am J Infect Control. 1983;11:28-36.
- Saint S, Lipsky BA. Preventing catheter-related bacteriuria. Should We? Can We? How? Arch Internal Med. 1999;159:800-808.
- Zimakoff J, Stickler DJ, Pontoppidan B, Larsen SO. Bladder management and urinary tract infection in Danish hospitals, nursing homes and home care: A national prevalence study. Infect Control Hosp Epidemiol. 1996;17(4):215-221.
- Johnson JR, Kuskowski MA, Wilt TJ. Systematic Review: Antimicrobial urinary catheters to prevent catheter-associated urinary tract infections in hospitalized patients. Ann Internal Med. 2006;144(2):116-126.
- Saint S, Elmore JG, Sullivan SD, Emerson SS, Koepsell TD. The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infections; a meta-analysis. Am J Med. 1998;105(3):236-241.
- Bronahan J, Jull A, Tracy C. Cochrane incontinence group. Types of urethral catheters for management of short-term voiding problems in hospitalized adults. Cochrane Database Syst Rev. 2004;1:CD004013.
- Wald HL, Kramer AM. Nonpayment for harms resulting from medical care. JAMA. 2007;298(23):2782-2784.
- Goldstein J. Hospital infections’ cost tallied. The Philadelphia Inquirer. Nov. 15, 2006.
- Saint S, Kowalski CP, Kaufman SR, et al. Preventing hospital-acquired urinary tract infection in the United States: A national study. Clin Infect Dis. 2008;46(2):243-250.
Case
A 68-year-old male with a history of Alzheimer’s dementia and incontinence presents with failure to thrive. A Foley catheter is placed due to the patient’s incontinence and fall risk. Three days after admission while awaiting placement in a skilled nursing facility (SNF), he develops a urinary tract infection (UTI) complicated by delirium delaying his transfer to the SNF. What could have been done to prevent this complication?
Overview
It has been 50 years since Beeson, et al., recognized the potential harms stemming from urethral catheterization and penned an editorial to the American Journal of Medicine titled “The case against the catheter.”1
Since then, there has been considerable exploration of ways to limit urethral catheterization and ultimately decrease catheter-associated urinary tract infections (CAUTIs). Unfortunately, little progress has been made; indwelling urinary catheters remain ubiquitous in hospitals and CAUTIs remain the most common hospital-acquired infection in the United States.2 Given the emphasis on the quality and costs of healthcare, it is an opportune time to revisit catheter management and use as a way to combat the clinical and economic consequences of CAUTIs.
Clinicians may be lulled into thinking the clinical impact of CAUTI is less than that of other nosocomial infections. However, beyond the obvious patient harm from UTIs, associated bacteremia, and even death, the public health implications of CAUTI cannot be denied. Urinary tract infections constitute 40% of all nosocomial infections; accounting for an estimated 1 million cases annually.3 Further, 80% of all UTIs are associated with indwelling catheter use.
On average, nosocomial UTI necessitates one extra hospital day per patient, or approximately one million excess hospital days per year.4 Pooled cost analysis shows that UTIs consume an additional $400-$1,700 per event, or an estimated $425 million per year in the United States.5,6 Clearly, we cannot wait another 50 years to address this problem.
Review of the Data
Catheter duration as a risk factor for CAUTI: The indwelling catheter creates a portal of entry into a usually sterile body cavity and provides a surface on which microorganisms can colonize. At a finite rate of colonization—the incidence of bacteriuria is 3% to 10% per catheter day—the duration of urinary catheterization becomes the strongest predictor of catheter-associated bacteriuria.7 Even in relatively short-term catheter use of two to 10 days, the pooled cumulative incidence of developing bacteriuria is 26%.
Given the magnitude of these numbers, it should be no surprise that after one month of catheterization, bacteriuria develops in almost all patients. Twenty-four percent of patients with bacteriuria develop symptomatic UTIs with close to 5% suffering bacteremia. Consequently, nosocomial UTIs cause 15% of all hospital-acquired bacteremia.
Optimal catheter management: The easiest and most effective means to prevent CAUTI is to limit the use of urinary catheters to clearly identified medical indications (see Table 1, above). However, as simple as this prevention practice may sound, studies have demonstrated that as many as 20% of patients have indwelling catheters initially placed for unjustified or even unknown medical indications.8 Additionally, continued catheter use is inappropriate in one-third to one-half of all catheter days.9 These data confirm misuse and overuse of indwelling urinary catheters in the hospital setting is common.
In 1981, the Centers for Disease Control and Prevention (CDC) recognized the importance of addressing this situation and published a guideline to aid prevention of CAUTIs.10 The CDC urged the limitation of catheter use to a carefully selected patient population. Furthermore, the report strongly stressed the importance of catheter removal as soon as possible and advised against the use of catheters solely for the convenience of healthcare workers.
Evidence-based techniques for insertion and catheter care also were outlined in the guideline (see Table 2, p. 31). However, these recommendations have been poorly implemented, likely due to the competing priorities of providers and the difficulty operationalizing the guidelines. Additionally, evidence from the intervening 25 years has not yet been incorporated into the guideline, although a revision currently is underway.
Until that revision is complete, the Joanna Briggs Institute guideline published in 2001 addresses some of the same management techniques and incorporates newer evidence.11 Of note, practices that have been discredited due to contradictory evidence include aggressive meatal cleaning, bladder irrigation, and the application of antimicrobial agents in the drainage bag.12
Strategies to reduce unnecessary catheter days: One of the remediable reasons for catheter misuse lies in the fact physicians often are unaware of the presence of an indwelling catheter in their hospitalized patients.
Saint, et al., showed physicians were unaware of catheterization in 28% of their patients and that attending physicians were less conscious of a patient’s catheter status than residents, interns, or medical students.13 Further, the “forgotten” catheters were more likely to be unnecessary than those remembered by the healthcare team.
This information has prompted the use of various computer-based and multidisciplinary feedback protocols to readdress and re-evaluate the need for continued catheterization in a patient. For example, a study at the VAMC Puget Sound demonstrated that having a computerized order protocol for urinary catheters significantly increased the rate of documentation as well as decreased the duration of catheterization by an average of three days.14
Similar interventions to encourage early catheter removal have included daily reminders from nursing staff, allowing a nurse to discontinue catheter use independent of a physician’s order, and feedback in which nursing staff is educated about the incidence of UTI.15-17 All these relatively simple interventions showed significant improvement in the catheter removal rate and incidence of CAUTIs as well as documented cost savings.
Alternatives to indwelling catheters: In addition to efforts to decrease catheter days, alternatives to the indwelling catheters also should be explored. One such alternative method is intermittent catheterization.
Several studies in postoperative patients with hip fractures have demonstrated that the development of UTI is lower with intermittent catheterization when compared with indwelling catheterization.18 Nevertheless, since the risk of bacteriuria is 1% to 3% per episode of catheterization, after a few weeks the majority of patients will have bacteriuria. However, as the bulk of this bacteriuria often is asymptomatic, intermittent catheterization may still be an improvement. This is particularly true in postoperative patients undergoing rehabilitation and those patients only requiring catheterization for a limited number of days.
More recent studies have evaluated the use of bedside bladder ultrasound in an attempt to determine when intermittent catheterization is needed and thereby limit its use compared with standard timed catheterization. Frederickson, et al., demonstrated that this intervention resulted in significantly fewer catheterizations in surgical patients, thus delaying or avoiding the need for catheterization in 81% of the cases.19 Given this drastic improvement, it is no surprise bladder ultrasound use reduced the rates of UTI.20
External condom catheters present another alternative to indwelling catheter use but the outcomes data is conflicting. While the risk of bacteriuria is approximately 12% per month, this rate becomes increasingly higher with frequent manipulation of the condom catheter. 21,22
Two parallel cohort studies in a VA nursing home showed the incidence of symptomatic UTI to be 2.5 times greater in men with an indwelling catheter than those with a condom catheter.23 On the other hand, a cross-sectional Danish study reported higher rates of UTI with external condom catheters than urethral catheters in hospitalized patients.24 Complications from condom catheters include phimosis and local skin maceration, necessitating meticulous care with the use of these devices. Although the data surrounding external catheterization is somewhat contradictory, this device warrants consideration in incontinent males without urinary tract obstruction.
There are several other alternatives to urethral catheterization (see Table 3, p. 31), many of which have excellent face validity even in the absence of rigorous evidence.
Antimicrobial catheters: The development of antimicrobial urinary catheters, including silver-alloy and nitrofurazone-coated catheters, has been greeted with much excitement, however, the jury is still out about their best use. A 2006 systematic literature review reported that in comparison to standard catheters, antimicrobial catheters can delay or even prevent the development of bacteriuria with short-term usage.25
However, not all antimicrobial catheters are equally effective; assorted studies lack data about clinically relevant endpoints such as prevention of symptomatic UTI, bloodstream infection or death.26, 27 In addition, there are no good trials comparing nitrofurazone to silver-alloy catheters. Therefore, the level of excitement surrounding antimicrobial catheters—particularly silver-alloy catheters—must be tempered by the additional costs incurred by their use.
To date, the cost-effectiveness of antimicrobial catheters has not been demonstrated. Although additional research in this topic is still needed, some experts currently recommend the consideration of silver-alloy catheters in patients at the highest risk for developing serious consequences from UTIs.
Efforts to reduce CAUTI: In response to significant public interest in hospital-acquired infections including CAUTI, the federal government and many state governments are beginning to demand change. In August 2007, the Centers for Medicare and Medicaid Services instituted a mandate making hospitals financially responsible for selected preventable hospital-acquired harms, including CAUTIs.28 In addition, beginning with Pennsylvania in 2006, several states have mandated public reporting of hospital-acquired infections.29
Given the available information about CAUTI prevalence, risks, and preventive techniques, it is surprising the majority of hospitals in the United States have not taken appropriate measures to limit indwelling catheter use. A recent study by Saint, et al., demonstrated the startling fact that only a minority of hospitals monitor the use of urethral catheters in their patients.30
Among study hospitals, there was no widely used technique to prevent CAUTI including evidence-based practices such as daily catheter reminders. The results of this investigation illustrate the urgent need for a national strategy to reduce CAUTI. Until that time, however, hospital-based physicians must take the lead to champion collaborative efforts, to promote evidence-based catheter use.
Back to the Case
As incontinence and fall risk are not medically appropriate indications for a urethral catheter, a Foley catheter should not have been utilized. Alternatives to indwelling catheterization in this patient would include a bedside commode with nursing assistance, a timed voiding program, intermittent catheterization with or without bladder ultrasound, incontinence pads, or a condom catheter.
Attentiveness to the appropriate medical indications for catheter use, familiarity with catheter alternatives, and recognition of the clinical and economic impact of CAUTI may have prevented this patient’s UTI-induced delirium and facilitated his early transfer to SNF. TH
Dr. Wald is a getriatric hospitalist and assistant professor of medicine at the University of Colorado, Denver. Dr. Furfari is a hospital medicine fellow at the University of Colorado Denver.
References
- Beeson PB. The case against the catheter. Am J Med. 1958;24:1-3.
- Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28:68-75.
- Sedor J, Mulholland SG. Hospital-acquired UTIs associated with the indwelling catheter. Urol Clin North Am. 1999;26:821-828.
- Foxman B. Epidemiology of UTI: Incidence, morbidity and economic costs. Am J Med. 2002;113(1A):5S-13S.
- Tambyah PA, Knasinski V, Maki D. The direct costs of nosocomial catheter-associated UTI in the era of managed care. Infect Control Hosp Epidemiol. 2002;23:27-31.
- Jarvis, WR. Selected aspects of socioeconomic impact of nosocomial infections. Infect Control Hosp Epidemiol. 1996;17:552-557.
- Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11:609-622.
- Jain P, Parada JP, David A, Smith L. Overuse of the indwelling urinary catheter in hospitalized medical patients. Arch Internal Med. 1995;155:1425-1429.
- Hartstein AI, Garber SB, Ward TT, Jones SR, Morthland VH. Nosocomial urinary tract infection: a prospective evaluation of 108 catheterized patients. Infect Control. 1981;2:380-386.
- Wong E. Guideline for prevention of catheter-associated urinary tract infections. Center for Disease Control and Prevention 1981. Available at: www.cdc.gov/ncidod/dhqp/gl_catheter_assoc.html . Accessed May 8, 2008.
- Joanna Briggs Institute. Management of short term indwelling urethral catheters to prevent urinary tract infections. 2000;4(1):ISSN 1329-1874.
- Burke JP, Garibaldi RA, Britt MR, Jacobson JA, Conti M, Alling DW. Prevention of catheter-associated urinary tract infections. Am J Med. 1981;70:655-658.
- Saint S, Wiese J, Amory JK, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109:476-480.
- Cornia PB, Amory JK, Fraser S, Saint S, Lipsky BA. Computer-based order entry decreases duration of indwelling urinary catheterization in hospitalized patients. Am J Med. 2003;114:404-406.
- Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978.
- Topal J, Conklin S, Camp K, Morris TB, Herbert P. Prevention of nosocomial catheter-associated urinary tract infections through computerized feedback to physicians and a nurse-directed protocol. Am J Med Qual. 2005;20(3):121-126.
- Goetz AM, Kedzuf S, Wagener M, Muder R. Feedback to nursing staff as an intervention to reduce catheter-associated urinary tract infections. Am J Infect Control. 1999;27(5):402-404.
- Johansson I, Athlin E, Frykholm L, Bolinder H, Larsson G. Intermittent versus indwelling catheters for older patients with hip fractures. J Clin Nurs. 2002;11:651-656.
- Frederickson M, Neitzel JJ, Miller EH, Reuter S, Graner T, Heller J. The implementation of bedside bladder ultrasound technology: Effects of patient and cost postoperative outcomes in tertiary care. Orthop Nurs. 2000;19(3):79-87.
- Slappendel R, Weber EWG. Non-invasive measurement of bladder volume as an indication for bladder catheterization after orthopedic surgery and its effect on urinary tract infections. Eur J Anesthesiol. 1999;16:503-506.
- Hirsh D, Fainstein V, Musher DM. Do condom catheter collecting systems cause urinary tract infections? JAMA. 1979;242:340-341.
- Wong ES. Guideline for prevention of catheter-associated urinary tract infections. Am J Infect Control. 1983;11:28-36.
- Saint S, Lipsky BA. Preventing catheter-related bacteriuria. Should We? Can We? How? Arch Internal Med. 1999;159:800-808.
- Zimakoff J, Stickler DJ, Pontoppidan B, Larsen SO. Bladder management and urinary tract infection in Danish hospitals, nursing homes and home care: A national prevalence study. Infect Control Hosp Epidemiol. 1996;17(4):215-221.
- Johnson JR, Kuskowski MA, Wilt TJ. Systematic Review: Antimicrobial urinary catheters to prevent catheter-associated urinary tract infections in hospitalized patients. Ann Internal Med. 2006;144(2):116-126.
- Saint S, Elmore JG, Sullivan SD, Emerson SS, Koepsell TD. The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infections; a meta-analysis. Am J Med. 1998;105(3):236-241.
- Bronahan J, Jull A, Tracy C. Cochrane incontinence group. Types of urethral catheters for management of short-term voiding problems in hospitalized adults. Cochrane Database Syst Rev. 2004;1:CD004013.
- Wald HL, Kramer AM. Nonpayment for harms resulting from medical care. JAMA. 2007;298(23):2782-2784.
- Goldstein J. Hospital infections’ cost tallied. The Philadelphia Inquirer. Nov. 15, 2006.
- Saint S, Kowalski CP, Kaufman SR, et al. Preventing hospital-acquired urinary tract infection in the United States: A national study. Clin Infect Dis. 2008;46(2):243-250.
Case
A 68-year-old male with a history of Alzheimer’s dementia and incontinence presents with failure to thrive. A Foley catheter is placed due to the patient’s incontinence and fall risk. Three days after admission while awaiting placement in a skilled nursing facility (SNF), he develops a urinary tract infection (UTI) complicated by delirium delaying his transfer to the SNF. What could have been done to prevent this complication?
Overview
It has been 50 years since Beeson, et al., recognized the potential harms stemming from urethral catheterization and penned an editorial to the American Journal of Medicine titled “The case against the catheter.”1
Since then, there has been considerable exploration of ways to limit urethral catheterization and ultimately decrease catheter-associated urinary tract infections (CAUTIs). Unfortunately, little progress has been made; indwelling urinary catheters remain ubiquitous in hospitals and CAUTIs remain the most common hospital-acquired infection in the United States.2 Given the emphasis on the quality and costs of healthcare, it is an opportune time to revisit catheter management and use as a way to combat the clinical and economic consequences of CAUTIs.
Clinicians may be lulled into thinking the clinical impact of CAUTI is less than that of other nosocomial infections. However, beyond the obvious patient harm from UTIs, associated bacteremia, and even death, the public health implications of CAUTI cannot be denied. Urinary tract infections constitute 40% of all nosocomial infections; accounting for an estimated 1 million cases annually.3 Further, 80% of all UTIs are associated with indwelling catheter use.
On average, nosocomial UTI necessitates one extra hospital day per patient, or approximately one million excess hospital days per year.4 Pooled cost analysis shows that UTIs consume an additional $400-$1,700 per event, or an estimated $425 million per year in the United States.5,6 Clearly, we cannot wait another 50 years to address this problem.
Review of the Data
Catheter duration as a risk factor for CAUTI: The indwelling catheter creates a portal of entry into a usually sterile body cavity and provides a surface on which microorganisms can colonize. At a finite rate of colonization—the incidence of bacteriuria is 3% to 10% per catheter day—the duration of urinary catheterization becomes the strongest predictor of catheter-associated bacteriuria.7 Even in relatively short-term catheter use of two to 10 days, the pooled cumulative incidence of developing bacteriuria is 26%.
Given the magnitude of these numbers, it should be no surprise that after one month of catheterization, bacteriuria develops in almost all patients. Twenty-four percent of patients with bacteriuria develop symptomatic UTIs with close to 5% suffering bacteremia. Consequently, nosocomial UTIs cause 15% of all hospital-acquired bacteremia.
Optimal catheter management: The easiest and most effective means to prevent CAUTI is to limit the use of urinary catheters to clearly identified medical indications (see Table 1, above). However, as simple as this prevention practice may sound, studies have demonstrated that as many as 20% of patients have indwelling catheters initially placed for unjustified or even unknown medical indications.8 Additionally, continued catheter use is inappropriate in one-third to one-half of all catheter days.9 These data confirm misuse and overuse of indwelling urinary catheters in the hospital setting is common.
In 1981, the Centers for Disease Control and Prevention (CDC) recognized the importance of addressing this situation and published a guideline to aid prevention of CAUTIs.10 The CDC urged the limitation of catheter use to a carefully selected patient population. Furthermore, the report strongly stressed the importance of catheter removal as soon as possible and advised against the use of catheters solely for the convenience of healthcare workers.
Evidence-based techniques for insertion and catheter care also were outlined in the guideline (see Table 2, p. 31). However, these recommendations have been poorly implemented, likely due to the competing priorities of providers and the difficulty operationalizing the guidelines. Additionally, evidence from the intervening 25 years has not yet been incorporated into the guideline, although a revision currently is underway.
Until that revision is complete, the Joanna Briggs Institute guideline published in 2001 addresses some of the same management techniques and incorporates newer evidence.11 Of note, practices that have been discredited due to contradictory evidence include aggressive meatal cleaning, bladder irrigation, and the application of antimicrobial agents in the drainage bag.12
Strategies to reduce unnecessary catheter days: One of the remediable reasons for catheter misuse lies in the fact physicians often are unaware of the presence of an indwelling catheter in their hospitalized patients.
Saint, et al., showed physicians were unaware of catheterization in 28% of their patients and that attending physicians were less conscious of a patient’s catheter status than residents, interns, or medical students.13 Further, the “forgotten” catheters were more likely to be unnecessary than those remembered by the healthcare team.
This information has prompted the use of various computer-based and multidisciplinary feedback protocols to readdress and re-evaluate the need for continued catheterization in a patient. For example, a study at the VAMC Puget Sound demonstrated that having a computerized order protocol for urinary catheters significantly increased the rate of documentation as well as decreased the duration of catheterization by an average of three days.14
Similar interventions to encourage early catheter removal have included daily reminders from nursing staff, allowing a nurse to discontinue catheter use independent of a physician’s order, and feedback in which nursing staff is educated about the incidence of UTI.15-17 All these relatively simple interventions showed significant improvement in the catheter removal rate and incidence of CAUTIs as well as documented cost savings.
Alternatives to indwelling catheters: In addition to efforts to decrease catheter days, alternatives to the indwelling catheters also should be explored. One such alternative method is intermittent catheterization.
Several studies in postoperative patients with hip fractures have demonstrated that the development of UTI is lower with intermittent catheterization when compared with indwelling catheterization.18 Nevertheless, since the risk of bacteriuria is 1% to 3% per episode of catheterization, after a few weeks the majority of patients will have bacteriuria. However, as the bulk of this bacteriuria often is asymptomatic, intermittent catheterization may still be an improvement. This is particularly true in postoperative patients undergoing rehabilitation and those patients only requiring catheterization for a limited number of days.
More recent studies have evaluated the use of bedside bladder ultrasound in an attempt to determine when intermittent catheterization is needed and thereby limit its use compared with standard timed catheterization. Frederickson, et al., demonstrated that this intervention resulted in significantly fewer catheterizations in surgical patients, thus delaying or avoiding the need for catheterization in 81% of the cases.19 Given this drastic improvement, it is no surprise bladder ultrasound use reduced the rates of UTI.20
External condom catheters present another alternative to indwelling catheter use but the outcomes data is conflicting. While the risk of bacteriuria is approximately 12% per month, this rate becomes increasingly higher with frequent manipulation of the condom catheter. 21,22
Two parallel cohort studies in a VA nursing home showed the incidence of symptomatic UTI to be 2.5 times greater in men with an indwelling catheter than those with a condom catheter.23 On the other hand, a cross-sectional Danish study reported higher rates of UTI with external condom catheters than urethral catheters in hospitalized patients.24 Complications from condom catheters include phimosis and local skin maceration, necessitating meticulous care with the use of these devices. Although the data surrounding external catheterization is somewhat contradictory, this device warrants consideration in incontinent males without urinary tract obstruction.
There are several other alternatives to urethral catheterization (see Table 3, p. 31), many of which have excellent face validity even in the absence of rigorous evidence.
Antimicrobial catheters: The development of antimicrobial urinary catheters, including silver-alloy and nitrofurazone-coated catheters, has been greeted with much excitement, however, the jury is still out about their best use. A 2006 systematic literature review reported that in comparison to standard catheters, antimicrobial catheters can delay or even prevent the development of bacteriuria with short-term usage.25
However, not all antimicrobial catheters are equally effective; assorted studies lack data about clinically relevant endpoints such as prevention of symptomatic UTI, bloodstream infection or death.26, 27 In addition, there are no good trials comparing nitrofurazone to silver-alloy catheters. Therefore, the level of excitement surrounding antimicrobial catheters—particularly silver-alloy catheters—must be tempered by the additional costs incurred by their use.
To date, the cost-effectiveness of antimicrobial catheters has not been demonstrated. Although additional research in this topic is still needed, some experts currently recommend the consideration of silver-alloy catheters in patients at the highest risk for developing serious consequences from UTIs.
Efforts to reduce CAUTI: In response to significant public interest in hospital-acquired infections including CAUTI, the federal government and many state governments are beginning to demand change. In August 2007, the Centers for Medicare and Medicaid Services instituted a mandate making hospitals financially responsible for selected preventable hospital-acquired harms, including CAUTIs.28 In addition, beginning with Pennsylvania in 2006, several states have mandated public reporting of hospital-acquired infections.29
Given the available information about CAUTI prevalence, risks, and preventive techniques, it is surprising the majority of hospitals in the United States have not taken appropriate measures to limit indwelling catheter use. A recent study by Saint, et al., demonstrated the startling fact that only a minority of hospitals monitor the use of urethral catheters in their patients.30
Among study hospitals, there was no widely used technique to prevent CAUTI including evidence-based practices such as daily catheter reminders. The results of this investigation illustrate the urgent need for a national strategy to reduce CAUTI. Until that time, however, hospital-based physicians must take the lead to champion collaborative efforts, to promote evidence-based catheter use.
Back to the Case
As incontinence and fall risk are not medically appropriate indications for a urethral catheter, a Foley catheter should not have been utilized. Alternatives to indwelling catheterization in this patient would include a bedside commode with nursing assistance, a timed voiding program, intermittent catheterization with or without bladder ultrasound, incontinence pads, or a condom catheter.
Attentiveness to the appropriate medical indications for catheter use, familiarity with catheter alternatives, and recognition of the clinical and economic impact of CAUTI may have prevented this patient’s UTI-induced delirium and facilitated his early transfer to SNF. TH
Dr. Wald is a getriatric hospitalist and assistant professor of medicine at the University of Colorado, Denver. Dr. Furfari is a hospital medicine fellow at the University of Colorado Denver.
References
- Beeson PB. The case against the catheter. Am J Med. 1958;24:1-3.
- Saint S. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am J Infect Control. 2000;28:68-75.
- Sedor J, Mulholland SG. Hospital-acquired UTIs associated with the indwelling catheter. Urol Clin North Am. 1999;26:821-828.
- Foxman B. Epidemiology of UTI: Incidence, morbidity and economic costs. Am J Med. 2002;113(1A):5S-13S.
- Tambyah PA, Knasinski V, Maki D. The direct costs of nosocomial catheter-associated UTI in the era of managed care. Infect Control Hosp Epidemiol. 2002;23:27-31.
- Jarvis, WR. Selected aspects of socioeconomic impact of nosocomial infections. Infect Control Hosp Epidemiol. 1996;17:552-557.
- Warren JW. Catheter-associated urinary tract infections. Infect Dis Clin North Am. 1997;11:609-622.
- Jain P, Parada JP, David A, Smith L. Overuse of the indwelling urinary catheter in hospitalized medical patients. Arch Internal Med. 1995;155:1425-1429.
- Hartstein AI, Garber SB, Ward TT, Jones SR, Morthland VH. Nosocomial urinary tract infection: a prospective evaluation of 108 catheterized patients. Infect Control. 1981;2:380-386.
- Wong E. Guideline for prevention of catheter-associated urinary tract infections. Center for Disease Control and Prevention 1981. Available at: www.cdc.gov/ncidod/dhqp/gl_catheter_assoc.html . Accessed May 8, 2008.
- Joanna Briggs Institute. Management of short term indwelling urethral catheters to prevent urinary tract infections. 2000;4(1):ISSN 1329-1874.
- Burke JP, Garibaldi RA, Britt MR, Jacobson JA, Conti M, Alling DW. Prevention of catheter-associated urinary tract infections. Am J Med. 1981;70:655-658.
- Saint S, Wiese J, Amory JK, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med. 2000;109:476-480.
- Cornia PB, Amory JK, Fraser S, Saint S, Lipsky BA. Computer-based order entry decreases duration of indwelling urinary catheterization in hospitalized patients. Am J Med. 2003;114:404-406.
- Huang WC, Wann SR, Lin SL, et al. Catheter-associated urinary tract infections in intensive care units can be reduced by prompting physicians to remove unnecessary catheters. Infect Control Hosp Epidemiol. 2004;25(11):974-978.
- Topal J, Conklin S, Camp K, Morris TB, Herbert P. Prevention of nosocomial catheter-associated urinary tract infections through computerized feedback to physicians and a nurse-directed protocol. Am J Med Qual. 2005;20(3):121-126.
- Goetz AM, Kedzuf S, Wagener M, Muder R. Feedback to nursing staff as an intervention to reduce catheter-associated urinary tract infections. Am J Infect Control. 1999;27(5):402-404.
- Johansson I, Athlin E, Frykholm L, Bolinder H, Larsson G. Intermittent versus indwelling catheters for older patients with hip fractures. J Clin Nurs. 2002;11:651-656.
- Frederickson M, Neitzel JJ, Miller EH, Reuter S, Graner T, Heller J. The implementation of bedside bladder ultrasound technology: Effects of patient and cost postoperative outcomes in tertiary care. Orthop Nurs. 2000;19(3):79-87.
- Slappendel R, Weber EWG. Non-invasive measurement of bladder volume as an indication for bladder catheterization after orthopedic surgery and its effect on urinary tract infections. Eur J Anesthesiol. 1999;16:503-506.
- Hirsh D, Fainstein V, Musher DM. Do condom catheter collecting systems cause urinary tract infections? JAMA. 1979;242:340-341.
- Wong ES. Guideline for prevention of catheter-associated urinary tract infections. Am J Infect Control. 1983;11:28-36.
- Saint S, Lipsky BA. Preventing catheter-related bacteriuria. Should We? Can We? How? Arch Internal Med. 1999;159:800-808.
- Zimakoff J, Stickler DJ, Pontoppidan B, Larsen SO. Bladder management and urinary tract infection in Danish hospitals, nursing homes and home care: A national prevalence study. Infect Control Hosp Epidemiol. 1996;17(4):215-221.
- Johnson JR, Kuskowski MA, Wilt TJ. Systematic Review: Antimicrobial urinary catheters to prevent catheter-associated urinary tract infections in hospitalized patients. Ann Internal Med. 2006;144(2):116-126.
- Saint S, Elmore JG, Sullivan SD, Emerson SS, Koepsell TD. The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infections; a meta-analysis. Am J Med. 1998;105(3):236-241.
- Bronahan J, Jull A, Tracy C. Cochrane incontinence group. Types of urethral catheters for management of short-term voiding problems in hospitalized adults. Cochrane Database Syst Rev. 2004;1:CD004013.
- Wald HL, Kramer AM. Nonpayment for harms resulting from medical care. JAMA. 2007;298(23):2782-2784.
- Goldstein J. Hospital infections’ cost tallied. The Philadelphia Inquirer. Nov. 15, 2006.
- Saint S, Kowalski CP, Kaufman SR, et al. Preventing hospital-acquired urinary tract infection in the United States: A national study. Clin Infect Dis. 2008;46(2):243-250.
OR Opportunity
In this era of increasing synergy between the surgical and hospital medicine services, Minnesota hospitalist David Frenz, MD, has taken perioperative management of surgical patients a step further.
One or two days a week, Dr. Frenz can be found in the operating room (OR) of St. Joseph’s Hospital in St. Paul, assisting on multilevel spine surgery cases.
Although Dr. Frenz may be a one-of-a-kind hospitalist acting as first assistant in the OR, the approach offers many advantages to his hospital and hospital medicine service, says Robert C. Moravec, MD.
“It seems more efficient having one assistant surgeon [rather than several scrub technicians] who knows exactly what’s going to happen next,” says Dr. Moravec, medical director for both the hospital service and St. Joseph’s Hospital. “More importantly, it’s a way to develop some expertise in the perioperative arena and to develop collaborative relationships with the surgeons.” In addition, the hospital service is able to bill for an assistant surgeon’s fee, which covers much of Dr. Frenz’ salary. And when he’s not on the medical floors seeing patients, Dr. Frenz is engaged in a monthslong quality improvement (QI) project to improve perioperative care and reduce same-day surgery cancellations at his institution.
The effectiveness of this QI project, which Dr. Moravec believes will go to HealthEast’s other two acute care hospitals in nine months, would not be possible without Dr. Frenz’ conversance with problems in the OR.
“When you are involved in this type of process improvement project, you don’t want, as a do-gooder, to create more cancellations and delays,” says Dr. Frenz. “And you don’t want to screw up their referral relationships. You’ve got to be super-sensitive to those issues as you’re trying to slowly bring about change. The fact that I’m known to the surgeons and that I’m in the OR getting dirty lends credibility to our efforts to bring change.”
Value in Surgical Assisting?
In medical school, Dr. Frenz had considered becoming a general surgeon before switching to family-practice medicine, so he is comfortable in the OR and finds assisting to be a stimulating change of pace. Although this long-standing pilot project is unique, it raises provocative possibilities for other hospitalists.
“Having a hospitalist go into the OR to assist with cases creates an interesting situation,” says Richard Rohr, MD, vice president for medical affairs at Cortland Regional Medical Center in Cortland, N.Y. “The hospitalist is then able to engage with more surgical aspects of the case, as well as the medical management.” Adding surgical assisting to the hospitalist’s role—although it could complicate scheduling and malpractice coverage—might dovetail with some hospitals’ difficulties retaining general surgeons, he says.
Combining the two functions could add to the hospital medicine group’s bottom line if relevant malpractice costs could be worked out, says hospitalist Kenneth Patrick, MD, the ICU director at Chestnut Hill Hospital in Philadelphia. Dr. Frenz’ malpractice is provided by his hospital, and pre-certification for his assistance on cases is handled by the neurosurgeon’s office staff.
In Dr. Patrick’s experience, there could be benefits to the patient if the hospitalist has direct involvement in the OR. For instance, the hospitalist would be better able to anticipate and deal with pre- and post-operative problems.
—David Frenz, MD, hospitalist, St. Joseph’s Hospital, St. Paul, Minn.
Surgery and ‘Outer Space’
Whether or not surgical assisting could become a new frontier for hospitalists, it illustrates the multiple collaborative roles the specialty increasingly offers.
David A. Hoffmann, MD, is medical director of a hospitalist group in Chambersburg, Pa. The group is made up of half family practice and half internal medicine hospitalists. Like so many other hospitalists, he’s seen tremendous growth in the number of surgical co-management cases his group handles at Chambersburg Hospital (see “The Surgical Surge,” December 2007, p. 1). His group tries “to make inroads with the surgeons,” he says. “We send a member of our group to their meetings, and we work with them on management protocols [such as DVT prophylaxis]. I can see the benefits of getting to know what’s going on down there [in the OR]. The truth of matter is, [despite co-management], sending the patient to the OR is like sending someone into outer space for the hospitalist. The rocket goes off, and you don’t see the patient until they come back in for a landing.”
Depending on the location of hospitalist groups, involvement as first assistants could represent additional opportunities for family medicine physicians, Dr. Hoffmann believes.
Air Force Maj. Heather Cereste, MD, agrees that the degree of symbiosis between surgeons and hospitalists likely will continue to be a location - and hospital-specific phenomenon. While serving in Iraq, she had significant experience with surgical procedures, and felt that from an internist’s perspective, she was more valuable to the surgical team. During her third-year residency in Maine, she observed many who planned to go into family practice assisting with gastrointestinal procedures and the like. “Certainly, in a smaller setting, with fewer available resources, the more autonomous a hospitalist can be, the better,” says Dr. Cereste, co-director of the geriatric medicine service at Wilford Hall Medical Center, Lackland Air Force Base, Texas, and chair of the bioethics committee.
Cautionary Tales
Though surgical assisting is an intriguing idea, such a set up “could have its own set of unintended consequences,” especially for a private model hospitalist group, says Brian Bossard, MD, medical director of Inpatient Physician Associates in Lincoln, Neb.
Dr. Bossard has personal experience with this configuration. When an internist in his hospitalist group began to do surgical assisting, the privately owned group (which contracts with Bryan LGH Hospital in Lincoln to provide hospitalist services) did not find this advantageous. The physician’s surgical participation was at times disruptive for the group, since he was unable to be immediately available and on call or to run codes while in the OR.
“It’s not clear to me that there would be an advantage to have a hospitalist [assisting in the OR], as opposed to another physician extender such as a physician assistant or a nurse practitioner,” says Jack M. Percelay, MD, a pediatric hospitalist at Saint Barnabas Medical Center in Livingston, N.J. Co-management of surgical patients is another matter, however, and Dr. Percelay does see value in having hospitalists help with maintenance of lines, wound care, and other post-surgical management duties.
“There is a certain set of procedures we’re supposed to master, such as vascular access and airway support,” Dr. Percelay continues. “But our value as hospitalists is in our cognitive skill set. I don’t know any hospitalists who consider a scalpel as one of their routine tools.”
Bryan Fine, MD, a pediatric hospital at Children’s National Medical Center in Washington, D.C., recently joined a general hospitalist group after spending three and a half years as the hospitalist in charge of medical management for the gastroenterology service. His opinion of hospitalists assisting in surgery? “I think it’s definitely valuable if it’s done in the context of a larger goal and to gain credibility from a hospital administrative level,” he suggests. However, he said, professional satisfaction for a hospitalist might be limited since he or she essentially would be serving as a physician extender.
Barriers
Family-practice physicians often are differentiated from their internal medicine colleagues by their skill sets in procedures.
“To the extent that a family-medicine physician may want to demonstrate that they can have a skill set that adds value in order to be hired or accepted, I think surgical assisting could have very specific application in specific places,” says A. Neal Axon, MD, assistant professor in the departments of internal medicine and pediatrics at the Medical University of South Carolina in Charleston. “I’ve certainly tried to market myself as a med-peds person, and as somebody who’s good at more than one thing.”
Dr. Axon concedes hospitalists as surgical assistants would not work at his institution. “In academic medical centers, the dividing lines between divisions and disciplines are very concrete,” he explains. “I think many people carry those cultural barriers or dividers—even if they are somewhat artificial outside the academic environment—when they leave and go into community practice.”
Those divisions are not felt as keenly in the Midwest, according to Dr. Frenz, where “family medicine has a long tradition.” St. Joseph’s Hospital has a family - medicine residency program, and more than half the credentialed physicians there are family- medicine trained.
“We think that family-medicine physicians have a skill set that is valuable in certain clinical settings,” he says. “For example, we do a lot of work on the behavioral health floors and are the principal medical providers on a 28-bed chemical dependency unit.” Dr. Frenz had a patient who was pregnant and alcohol dependent. Because of his expertise in addiction medicine (another of his self-described “insurgencies”) and residency training in obstetrics, Dr. Frenz is managing the patient without incurring an ob/gyn consultation.
How to Prepare
Every hospitalist’s path and skill set is unique, but for those medical students or residents who might be interested in combining some surgical work with hospitalist skills, Dr. Frenz advises adopting a calculated approach to electives. Besides taking as many surgical electives as possible, trainees should try to pick small community hospitals where they will not have to compete with surgical residents for time in the OR.
Although she thinks expanding into surgical assisting could improve recruitment (offering a varied hospital experience), Dr. Cereste also emphasizes that many questions regarding training standards, care standards, and expense hurdles would have to be addressed.
The bottom line, says Dr. Hoffmann, is that hospitalists “need to be able to play a lot of different roles. I think we’re like a utility infielder. If [surgical assisting] improves patient care, is a valuable service to the health system, and is viewed by consultants, specialists, and family doctors as an additional skill, it’s clearly going to benefit your program and your hospital. The key is to see what works in everyone’s little pond and try to be a team builder.” TH
Gretchen Henkel is a medical writer based in California
In this era of increasing synergy between the surgical and hospital medicine services, Minnesota hospitalist David Frenz, MD, has taken perioperative management of surgical patients a step further.
One or two days a week, Dr. Frenz can be found in the operating room (OR) of St. Joseph’s Hospital in St. Paul, assisting on multilevel spine surgery cases.
Although Dr. Frenz may be a one-of-a-kind hospitalist acting as first assistant in the OR, the approach offers many advantages to his hospital and hospital medicine service, says Robert C. Moravec, MD.
“It seems more efficient having one assistant surgeon [rather than several scrub technicians] who knows exactly what’s going to happen next,” says Dr. Moravec, medical director for both the hospital service and St. Joseph’s Hospital. “More importantly, it’s a way to develop some expertise in the perioperative arena and to develop collaborative relationships with the surgeons.” In addition, the hospital service is able to bill for an assistant surgeon’s fee, which covers much of Dr. Frenz’ salary. And when he’s not on the medical floors seeing patients, Dr. Frenz is engaged in a monthslong quality improvement (QI) project to improve perioperative care and reduce same-day surgery cancellations at his institution.
The effectiveness of this QI project, which Dr. Moravec believes will go to HealthEast’s other two acute care hospitals in nine months, would not be possible without Dr. Frenz’ conversance with problems in the OR.
“When you are involved in this type of process improvement project, you don’t want, as a do-gooder, to create more cancellations and delays,” says Dr. Frenz. “And you don’t want to screw up their referral relationships. You’ve got to be super-sensitive to those issues as you’re trying to slowly bring about change. The fact that I’m known to the surgeons and that I’m in the OR getting dirty lends credibility to our efforts to bring change.”
Value in Surgical Assisting?
In medical school, Dr. Frenz had considered becoming a general surgeon before switching to family-practice medicine, so he is comfortable in the OR and finds assisting to be a stimulating change of pace. Although this long-standing pilot project is unique, it raises provocative possibilities for other hospitalists.
“Having a hospitalist go into the OR to assist with cases creates an interesting situation,” says Richard Rohr, MD, vice president for medical affairs at Cortland Regional Medical Center in Cortland, N.Y. “The hospitalist is then able to engage with more surgical aspects of the case, as well as the medical management.” Adding surgical assisting to the hospitalist’s role—although it could complicate scheduling and malpractice coverage—might dovetail with some hospitals’ difficulties retaining general surgeons, he says.
Combining the two functions could add to the hospital medicine group’s bottom line if relevant malpractice costs could be worked out, says hospitalist Kenneth Patrick, MD, the ICU director at Chestnut Hill Hospital in Philadelphia. Dr. Frenz’ malpractice is provided by his hospital, and pre-certification for his assistance on cases is handled by the neurosurgeon’s office staff.
In Dr. Patrick’s experience, there could be benefits to the patient if the hospitalist has direct involvement in the OR. For instance, the hospitalist would be better able to anticipate and deal with pre- and post-operative problems.
—David Frenz, MD, hospitalist, St. Joseph’s Hospital, St. Paul, Minn.
Surgery and ‘Outer Space’
Whether or not surgical assisting could become a new frontier for hospitalists, it illustrates the multiple collaborative roles the specialty increasingly offers.
David A. Hoffmann, MD, is medical director of a hospitalist group in Chambersburg, Pa. The group is made up of half family practice and half internal medicine hospitalists. Like so many other hospitalists, he’s seen tremendous growth in the number of surgical co-management cases his group handles at Chambersburg Hospital (see “The Surgical Surge,” December 2007, p. 1). His group tries “to make inroads with the surgeons,” he says. “We send a member of our group to their meetings, and we work with them on management protocols [such as DVT prophylaxis]. I can see the benefits of getting to know what’s going on down there [in the OR]. The truth of matter is, [despite co-management], sending the patient to the OR is like sending someone into outer space for the hospitalist. The rocket goes off, and you don’t see the patient until they come back in for a landing.”
Depending on the location of hospitalist groups, involvement as first assistants could represent additional opportunities for family medicine physicians, Dr. Hoffmann believes.
Air Force Maj. Heather Cereste, MD, agrees that the degree of symbiosis between surgeons and hospitalists likely will continue to be a location - and hospital-specific phenomenon. While serving in Iraq, she had significant experience with surgical procedures, and felt that from an internist’s perspective, she was more valuable to the surgical team. During her third-year residency in Maine, she observed many who planned to go into family practice assisting with gastrointestinal procedures and the like. “Certainly, in a smaller setting, with fewer available resources, the more autonomous a hospitalist can be, the better,” says Dr. Cereste, co-director of the geriatric medicine service at Wilford Hall Medical Center, Lackland Air Force Base, Texas, and chair of the bioethics committee.
Cautionary Tales
Though surgical assisting is an intriguing idea, such a set up “could have its own set of unintended consequences,” especially for a private model hospitalist group, says Brian Bossard, MD, medical director of Inpatient Physician Associates in Lincoln, Neb.
Dr. Bossard has personal experience with this configuration. When an internist in his hospitalist group began to do surgical assisting, the privately owned group (which contracts with Bryan LGH Hospital in Lincoln to provide hospitalist services) did not find this advantageous. The physician’s surgical participation was at times disruptive for the group, since he was unable to be immediately available and on call or to run codes while in the OR.
“It’s not clear to me that there would be an advantage to have a hospitalist [assisting in the OR], as opposed to another physician extender such as a physician assistant or a nurse practitioner,” says Jack M. Percelay, MD, a pediatric hospitalist at Saint Barnabas Medical Center in Livingston, N.J. Co-management of surgical patients is another matter, however, and Dr. Percelay does see value in having hospitalists help with maintenance of lines, wound care, and other post-surgical management duties.
“There is a certain set of procedures we’re supposed to master, such as vascular access and airway support,” Dr. Percelay continues. “But our value as hospitalists is in our cognitive skill set. I don’t know any hospitalists who consider a scalpel as one of their routine tools.”
Bryan Fine, MD, a pediatric hospital at Children’s National Medical Center in Washington, D.C., recently joined a general hospitalist group after spending three and a half years as the hospitalist in charge of medical management for the gastroenterology service. His opinion of hospitalists assisting in surgery? “I think it’s definitely valuable if it’s done in the context of a larger goal and to gain credibility from a hospital administrative level,” he suggests. However, he said, professional satisfaction for a hospitalist might be limited since he or she essentially would be serving as a physician extender.
Barriers
Family-practice physicians often are differentiated from their internal medicine colleagues by their skill sets in procedures.
“To the extent that a family-medicine physician may want to demonstrate that they can have a skill set that adds value in order to be hired or accepted, I think surgical assisting could have very specific application in specific places,” says A. Neal Axon, MD, assistant professor in the departments of internal medicine and pediatrics at the Medical University of South Carolina in Charleston. “I’ve certainly tried to market myself as a med-peds person, and as somebody who’s good at more than one thing.”
Dr. Axon concedes hospitalists as surgical assistants would not work at his institution. “In academic medical centers, the dividing lines between divisions and disciplines are very concrete,” he explains. “I think many people carry those cultural barriers or dividers—even if they are somewhat artificial outside the academic environment—when they leave and go into community practice.”
Those divisions are not felt as keenly in the Midwest, according to Dr. Frenz, where “family medicine has a long tradition.” St. Joseph’s Hospital has a family - medicine residency program, and more than half the credentialed physicians there are family- medicine trained.
“We think that family-medicine physicians have a skill set that is valuable in certain clinical settings,” he says. “For example, we do a lot of work on the behavioral health floors and are the principal medical providers on a 28-bed chemical dependency unit.” Dr. Frenz had a patient who was pregnant and alcohol dependent. Because of his expertise in addiction medicine (another of his self-described “insurgencies”) and residency training in obstetrics, Dr. Frenz is managing the patient without incurring an ob/gyn consultation.
How to Prepare
Every hospitalist’s path and skill set is unique, but for those medical students or residents who might be interested in combining some surgical work with hospitalist skills, Dr. Frenz advises adopting a calculated approach to electives. Besides taking as many surgical electives as possible, trainees should try to pick small community hospitals where they will not have to compete with surgical residents for time in the OR.
Although she thinks expanding into surgical assisting could improve recruitment (offering a varied hospital experience), Dr. Cereste also emphasizes that many questions regarding training standards, care standards, and expense hurdles would have to be addressed.
The bottom line, says Dr. Hoffmann, is that hospitalists “need to be able to play a lot of different roles. I think we’re like a utility infielder. If [surgical assisting] improves patient care, is a valuable service to the health system, and is viewed by consultants, specialists, and family doctors as an additional skill, it’s clearly going to benefit your program and your hospital. The key is to see what works in everyone’s little pond and try to be a team builder.” TH
Gretchen Henkel is a medical writer based in California
In this era of increasing synergy between the surgical and hospital medicine services, Minnesota hospitalist David Frenz, MD, has taken perioperative management of surgical patients a step further.
One or two days a week, Dr. Frenz can be found in the operating room (OR) of St. Joseph’s Hospital in St. Paul, assisting on multilevel spine surgery cases.
Although Dr. Frenz may be a one-of-a-kind hospitalist acting as first assistant in the OR, the approach offers many advantages to his hospital and hospital medicine service, says Robert C. Moravec, MD.
“It seems more efficient having one assistant surgeon [rather than several scrub technicians] who knows exactly what’s going to happen next,” says Dr. Moravec, medical director for both the hospital service and St. Joseph’s Hospital. “More importantly, it’s a way to develop some expertise in the perioperative arena and to develop collaborative relationships with the surgeons.” In addition, the hospital service is able to bill for an assistant surgeon’s fee, which covers much of Dr. Frenz’ salary. And when he’s not on the medical floors seeing patients, Dr. Frenz is engaged in a monthslong quality improvement (QI) project to improve perioperative care and reduce same-day surgery cancellations at his institution.
The effectiveness of this QI project, which Dr. Moravec believes will go to HealthEast’s other two acute care hospitals in nine months, would not be possible without Dr. Frenz’ conversance with problems in the OR.
“When you are involved in this type of process improvement project, you don’t want, as a do-gooder, to create more cancellations and delays,” says Dr. Frenz. “And you don’t want to screw up their referral relationships. You’ve got to be super-sensitive to those issues as you’re trying to slowly bring about change. The fact that I’m known to the surgeons and that I’m in the OR getting dirty lends credibility to our efforts to bring change.”
Value in Surgical Assisting?
In medical school, Dr. Frenz had considered becoming a general surgeon before switching to family-practice medicine, so he is comfortable in the OR and finds assisting to be a stimulating change of pace. Although this long-standing pilot project is unique, it raises provocative possibilities for other hospitalists.
“Having a hospitalist go into the OR to assist with cases creates an interesting situation,” says Richard Rohr, MD, vice president for medical affairs at Cortland Regional Medical Center in Cortland, N.Y. “The hospitalist is then able to engage with more surgical aspects of the case, as well as the medical management.” Adding surgical assisting to the hospitalist’s role—although it could complicate scheduling and malpractice coverage—might dovetail with some hospitals’ difficulties retaining general surgeons, he says.
Combining the two functions could add to the hospital medicine group’s bottom line if relevant malpractice costs could be worked out, says hospitalist Kenneth Patrick, MD, the ICU director at Chestnut Hill Hospital in Philadelphia. Dr. Frenz’ malpractice is provided by his hospital, and pre-certification for his assistance on cases is handled by the neurosurgeon’s office staff.
In Dr. Patrick’s experience, there could be benefits to the patient if the hospitalist has direct involvement in the OR. For instance, the hospitalist would be better able to anticipate and deal with pre- and post-operative problems.
—David Frenz, MD, hospitalist, St. Joseph’s Hospital, St. Paul, Minn.
Surgery and ‘Outer Space’
Whether or not surgical assisting could become a new frontier for hospitalists, it illustrates the multiple collaborative roles the specialty increasingly offers.
David A. Hoffmann, MD, is medical director of a hospitalist group in Chambersburg, Pa. The group is made up of half family practice and half internal medicine hospitalists. Like so many other hospitalists, he’s seen tremendous growth in the number of surgical co-management cases his group handles at Chambersburg Hospital (see “The Surgical Surge,” December 2007, p. 1). His group tries “to make inroads with the surgeons,” he says. “We send a member of our group to their meetings, and we work with them on management protocols [such as DVT prophylaxis]. I can see the benefits of getting to know what’s going on down there [in the OR]. The truth of matter is, [despite co-management], sending the patient to the OR is like sending someone into outer space for the hospitalist. The rocket goes off, and you don’t see the patient until they come back in for a landing.”
Depending on the location of hospitalist groups, involvement as first assistants could represent additional opportunities for family medicine physicians, Dr. Hoffmann believes.
Air Force Maj. Heather Cereste, MD, agrees that the degree of symbiosis between surgeons and hospitalists likely will continue to be a location - and hospital-specific phenomenon. While serving in Iraq, she had significant experience with surgical procedures, and felt that from an internist’s perspective, she was more valuable to the surgical team. During her third-year residency in Maine, she observed many who planned to go into family practice assisting with gastrointestinal procedures and the like. “Certainly, in a smaller setting, with fewer available resources, the more autonomous a hospitalist can be, the better,” says Dr. Cereste, co-director of the geriatric medicine service at Wilford Hall Medical Center, Lackland Air Force Base, Texas, and chair of the bioethics committee.
Cautionary Tales
Though surgical assisting is an intriguing idea, such a set up “could have its own set of unintended consequences,” especially for a private model hospitalist group, says Brian Bossard, MD, medical director of Inpatient Physician Associates in Lincoln, Neb.
Dr. Bossard has personal experience with this configuration. When an internist in his hospitalist group began to do surgical assisting, the privately owned group (which contracts with Bryan LGH Hospital in Lincoln to provide hospitalist services) did not find this advantageous. The physician’s surgical participation was at times disruptive for the group, since he was unable to be immediately available and on call or to run codes while in the OR.
“It’s not clear to me that there would be an advantage to have a hospitalist [assisting in the OR], as opposed to another physician extender such as a physician assistant or a nurse practitioner,” says Jack M. Percelay, MD, a pediatric hospitalist at Saint Barnabas Medical Center in Livingston, N.J. Co-management of surgical patients is another matter, however, and Dr. Percelay does see value in having hospitalists help with maintenance of lines, wound care, and other post-surgical management duties.
“There is a certain set of procedures we’re supposed to master, such as vascular access and airway support,” Dr. Percelay continues. “But our value as hospitalists is in our cognitive skill set. I don’t know any hospitalists who consider a scalpel as one of their routine tools.”
Bryan Fine, MD, a pediatric hospital at Children’s National Medical Center in Washington, D.C., recently joined a general hospitalist group after spending three and a half years as the hospitalist in charge of medical management for the gastroenterology service. His opinion of hospitalists assisting in surgery? “I think it’s definitely valuable if it’s done in the context of a larger goal and to gain credibility from a hospital administrative level,” he suggests. However, he said, professional satisfaction for a hospitalist might be limited since he or she essentially would be serving as a physician extender.
Barriers
Family-practice physicians often are differentiated from their internal medicine colleagues by their skill sets in procedures.
“To the extent that a family-medicine physician may want to demonstrate that they can have a skill set that adds value in order to be hired or accepted, I think surgical assisting could have very specific application in specific places,” says A. Neal Axon, MD, assistant professor in the departments of internal medicine and pediatrics at the Medical University of South Carolina in Charleston. “I’ve certainly tried to market myself as a med-peds person, and as somebody who’s good at more than one thing.”
Dr. Axon concedes hospitalists as surgical assistants would not work at his institution. “In academic medical centers, the dividing lines between divisions and disciplines are very concrete,” he explains. “I think many people carry those cultural barriers or dividers—even if they are somewhat artificial outside the academic environment—when they leave and go into community practice.”
Those divisions are not felt as keenly in the Midwest, according to Dr. Frenz, where “family medicine has a long tradition.” St. Joseph’s Hospital has a family - medicine residency program, and more than half the credentialed physicians there are family- medicine trained.
“We think that family-medicine physicians have a skill set that is valuable in certain clinical settings,” he says. “For example, we do a lot of work on the behavioral health floors and are the principal medical providers on a 28-bed chemical dependency unit.” Dr. Frenz had a patient who was pregnant and alcohol dependent. Because of his expertise in addiction medicine (another of his self-described “insurgencies”) and residency training in obstetrics, Dr. Frenz is managing the patient without incurring an ob/gyn consultation.
How to Prepare
Every hospitalist’s path and skill set is unique, but for those medical students or residents who might be interested in combining some surgical work with hospitalist skills, Dr. Frenz advises adopting a calculated approach to electives. Besides taking as many surgical electives as possible, trainees should try to pick small community hospitals where they will not have to compete with surgical residents for time in the OR.
Although she thinks expanding into surgical assisting could improve recruitment (offering a varied hospital experience), Dr. Cereste also emphasizes that many questions regarding training standards, care standards, and expense hurdles would have to be addressed.
The bottom line, says Dr. Hoffmann, is that hospitalists “need to be able to play a lot of different roles. I think we’re like a utility infielder. If [surgical assisting] improves patient care, is a valuable service to the health system, and is viewed by consultants, specialists, and family doctors as an additional skill, it’s clearly going to benefit your program and your hospital. The key is to see what works in everyone’s little pond and try to be a team builder.” TH
Gretchen Henkel is a medical writer based in California
HM Goes Public
You know your industry is on the map when a trailblazer takes his company public. That signals the capital markets that top executives in a leading company are confident in their business model’s ability to grow the company enough to satisfy Wall Street’s voracious hunger for profits.
It’s a tall order, and Adam Singer, MD, founder and CEO of IPC-The Hospitalist Company, based in North Hollywood, Calif., accomplished it in January.
During 2007, Dr. Singer set the wheels in motion for taking IPC public the following year. “Our company is built right, has a solid revenue stream, and the nirvana of a real healthcare company—a battle-tested, proven business model,” he says.
Dr. Singer and his management team had the usual reasons for going public: raising capital to pay for operations and to allow for growth by acquisition, reducing debt, and creating liquidity for shareholders. He also had other fish to fry with a public offering. “I wanted to be the first in our industry to make it out of the box,’’ he says. “I also wanted IPC to be a model for the many hospital medicine companies that would like to mature beyond having a bunch of doctors running around a hospital and calling themselves a hospitalist medicine company.”
For Dr. Singer, going public went beyond the desire to produce quarterly financial results that would warm investors’ hearts. It spoke to his core belief in what the business of hospital medicine should be and, from his vantage point, isn’t.
“By leading a publicly traded hospital medicine company I am debunking the myth that hospitalist groups need hospital subsidies to survive,” he says. “This is a powerful myth, one that is mired in a work force’s idea that its members should get full-time pay for less than full-time work. IPC has raised the bar for our industry. We think that hospitals should demand the overall level of sophistication, physician commitment and productivity that IPC has.”
With few publicly traded physician companies for templates for a public offering, Dr. Singer looked to a colleague, Roger Medel, MD, for direction. Dr. Medel took his company public in 1979 and has grown Sunrise, Fla.-based Pediatrix Medical Group, a provider of neonatal, maternal-fetal, and pediatric intensivist/hospitalist services, from a company with $100 million in market capitalization to $3.32 billion and a recent stock price of $67 per share. The company has a “buy” consensus rating from analysts and respectable price-to-earning and earnings-per-share values.
Economic Evolution
Medical staffing firm IPOs, such as IPC’s, are relatively rare. Most venture capital chases the next hot thing in medical information technology, biotechnology, and medical testing. The quirks of the medical staffing industry, such as hospitalist hiring—where salary increases can consume sizable chunks of a firm’s revenues—may deter potential investors.
Why are publicly traded medical staffing companies like IPC and PDX the exception rather than the rule? Because they rely so heavily on human capital—primarily physicians—for bottom-line results, they must contend with recruiting and retaining from a highly sought after talent pool that have their choice of job opportunities.
Also, many physicians fear the corporatization of medicine, an anxiety that working for a publicly traded company tends to arouse. Physician idealism—wanting to make the world a better place—may clash with a public corporation’s raison d’etre: making money. In a young field like hospital medicine, where performance metrics are evolving, balancing shareholder demands for ROI versus quality patient care requires a delicate touch.
Larry Wellikson, MD, SHM’s chief executive officer, says IPC’s entry into the public markets has been received well, indicating the maturation and strong growth of hospital medicine.
IPC’s public offering took place Jan. 30. Lead underwriters Credit Suisse Securities and Jeffries & Co. offered 5.9 million shares of IPC stock under the ticker symbol IPCM, a four-letter symbol conforming with NASDAQ’s listing requirements. The stock traded mostly at $19 per share, above the original per share estimate of between $15 to $17, raising approximately $38.3 million in net proceeds.
Since then, IPCM shares have ranged from $16.25 to $23.09 per share, trading at a thin average daily volume of 162,000. As of March 31, IPC’s market capitalization was $296 million. Six months from the IPO, average daily volume should increase, as regulations on shareholder sales are eased according to stock exchange rules.
IPC’s latest financial results were upbeat. The firm reported record operating results for the fourth quarter and full year 2007. Total patient encounters rose 29%, compared with 460,000 over the same period in 2006. Fourth-quarter net revenues were $52.6 million, a 31% increase from $40.2 million in fourth-quarter 2006. Physician practice salaries and other expenses for the period were $36.9 million vs. $29.5 million for fourth-quarter 2006. As a percentage of net revenue, physician salaries declined to 70% from 73% in the fourth quarter of 2007 vs. 2006. Dr. Singer attributed the change to higher physician productivity and increased revenue per encounter.
With the stock market retrenching since its all-time high in October, IPC’s timing on going public might seem a bit off. Yet, venture capitalists are bullish on healthcare. They sank a record $9.97 billion into the sector in 2007, topping the previous high of $9.47 billion in 2000, during the dot.com craze. Three large venture capital firms specializing in healthcare have $1.25 billion looking for good homes. The worry on Wall Street is that there won’t be enough healthcare IPOs to satisfy demand; there were only 31 in 2007 vs. 60 in 2000.
Alternatives
Going public is not the only way hospital medicine companies and other physician-intensive enterprises can raise needed capital. Venture capital plays a critical role. Brentwood, Tenn.-based Cogent Healthcare received its first round of such funding in 1997, a second infusion in 2000, and $15 million in 2002. IPC is no stranger to venture capital either: It raised $47 million in venture capital since 1998. Such capital infusions helped IPC and Cogent in their early days by providing the money needed to start hospital medicine groups, recruit top managers, expand into new markets, and improve IT and communication infrastructures.
Some entrepreneurs favor keeping their companies private. For example, John Erickson, founder and CEO of Baltimore-based Erickson Retirement Communities (ERC), is committed to growing his business without going public. There are 19 Erickson campuses in 11 states, with 21,000 residents. ERC’s business plan involves adding sites, anticipating growth to 55,000 residents in five years. Such steady expansion gobbles up capital, but Erickson is adamant about staying private. On going public he says: “I consider it whenever I need capital, but it’s hard to keep public markets happy. If your business slows down for any reason, your stock tanks and the market will punish you harshly. I will not have stock analysts pressuring me about how to run and grow my business.”
Erickson admits the competing demands of raising capital and keeping the company private aren’t easy to reconcile. “We go to midsized and large banks and [real estate investment trusts], get letters of credit, tax bonds, debt financing, mezzanine financing, etc.,” he says. He considers that others in his industry, Sunrise Senior Living (SRZ) and Brookdale Senior Living (BKD) that have gone public show the industry’s strength. “Multiple sources of capitalization in an industry provide greater options for all,” he adds.
That said, Erickson intends to resist any temptation to go public because “I must have the flexibility to implement our five-year plan correctly. If I want to invest $30 million in a medical group or hire seven doctors at $150,000 a pop, I don’t have to answer to some 30-year-old stock analyst who doesn’t like that.”
As for IPC’s public offering, Erickson says the first company to do so in an industry opens new avenues for raising capital in the public arena. “Dr. Singer’s pushing the envelope for hospital medicine, and if he can tolerate the pressure of the market—even when the strings are very tight—that’s great,” he emphasizes.
Commenting on the legal and governance issues of a public offering, Peter Olberg, corporate and finance partner at Manhattan-based law firm of Manatt, Philips and Philips says IPC’s being the first publicly traded hospitalist medicine company is a sound way to raise capital and isn’t risky in terms of disclosure. However, a specialty care provider like IPC can “become a victim of its own success. Public payers can say reimbursement is too high and cut it based on the leader’s financial performance.”
Peer Perspective
SHM President Patrick Cawley, MD, MBA, calls IPC’s public offering a major milestone because it demonstrates the maturity of the hospitalist movement. He expects IPC to use the infusion of capital to step up physician hiring, acquire more groups, and improve its proprietary IT infrastructure by refining its tools to further link outcomes and performance.
“IPC’s emphasis on quality outcomes is clearly where medicine is going,” Dr. Cawley says. And, “Putting pressure on hospitalists to be more productive has a huge potential in helping hospital medicine get more efficient by seeing more patients.”
To put the IPC public offering in perspective Dr. Cawley captures Dr. Singer’s vision. “To run a public company, you focus beyond daily stock prices and on the intermediate and short-term. What Wall Street thinks about your business matters. Our product [hospital medicine] has a great future, and I applaud Adam Singer for taking this step.”
Giving Dr. Singer the last word, he says “I’m a big believer in not worrying about things I can’t control like stock market fluctuations. We can handle good news and bad. We have a good business model and we’ll stick to the knitting.” TH
Marlene Piturro is a medical writer based in New York.
You know your industry is on the map when a trailblazer takes his company public. That signals the capital markets that top executives in a leading company are confident in their business model’s ability to grow the company enough to satisfy Wall Street’s voracious hunger for profits.
It’s a tall order, and Adam Singer, MD, founder and CEO of IPC-The Hospitalist Company, based in North Hollywood, Calif., accomplished it in January.
During 2007, Dr. Singer set the wheels in motion for taking IPC public the following year. “Our company is built right, has a solid revenue stream, and the nirvana of a real healthcare company—a battle-tested, proven business model,” he says.
Dr. Singer and his management team had the usual reasons for going public: raising capital to pay for operations and to allow for growth by acquisition, reducing debt, and creating liquidity for shareholders. He also had other fish to fry with a public offering. “I wanted to be the first in our industry to make it out of the box,’’ he says. “I also wanted IPC to be a model for the many hospital medicine companies that would like to mature beyond having a bunch of doctors running around a hospital and calling themselves a hospitalist medicine company.”
For Dr. Singer, going public went beyond the desire to produce quarterly financial results that would warm investors’ hearts. It spoke to his core belief in what the business of hospital medicine should be and, from his vantage point, isn’t.
“By leading a publicly traded hospital medicine company I am debunking the myth that hospitalist groups need hospital subsidies to survive,” he says. “This is a powerful myth, one that is mired in a work force’s idea that its members should get full-time pay for less than full-time work. IPC has raised the bar for our industry. We think that hospitals should demand the overall level of sophistication, physician commitment and productivity that IPC has.”
With few publicly traded physician companies for templates for a public offering, Dr. Singer looked to a colleague, Roger Medel, MD, for direction. Dr. Medel took his company public in 1979 and has grown Sunrise, Fla.-based Pediatrix Medical Group, a provider of neonatal, maternal-fetal, and pediatric intensivist/hospitalist services, from a company with $100 million in market capitalization to $3.32 billion and a recent stock price of $67 per share. The company has a “buy” consensus rating from analysts and respectable price-to-earning and earnings-per-share values.
Economic Evolution
Medical staffing firm IPOs, such as IPC’s, are relatively rare. Most venture capital chases the next hot thing in medical information technology, biotechnology, and medical testing. The quirks of the medical staffing industry, such as hospitalist hiring—where salary increases can consume sizable chunks of a firm’s revenues—may deter potential investors.
Why are publicly traded medical staffing companies like IPC and PDX the exception rather than the rule? Because they rely so heavily on human capital—primarily physicians—for bottom-line results, they must contend with recruiting and retaining from a highly sought after talent pool that have their choice of job opportunities.
Also, many physicians fear the corporatization of medicine, an anxiety that working for a publicly traded company tends to arouse. Physician idealism—wanting to make the world a better place—may clash with a public corporation’s raison d’etre: making money. In a young field like hospital medicine, where performance metrics are evolving, balancing shareholder demands for ROI versus quality patient care requires a delicate touch.
Larry Wellikson, MD, SHM’s chief executive officer, says IPC’s entry into the public markets has been received well, indicating the maturation and strong growth of hospital medicine.
IPC’s public offering took place Jan. 30. Lead underwriters Credit Suisse Securities and Jeffries & Co. offered 5.9 million shares of IPC stock under the ticker symbol IPCM, a four-letter symbol conforming with NASDAQ’s listing requirements. The stock traded mostly at $19 per share, above the original per share estimate of between $15 to $17, raising approximately $38.3 million in net proceeds.
Since then, IPCM shares have ranged from $16.25 to $23.09 per share, trading at a thin average daily volume of 162,000. As of March 31, IPC’s market capitalization was $296 million. Six months from the IPO, average daily volume should increase, as regulations on shareholder sales are eased according to stock exchange rules.
IPC’s latest financial results were upbeat. The firm reported record operating results for the fourth quarter and full year 2007. Total patient encounters rose 29%, compared with 460,000 over the same period in 2006. Fourth-quarter net revenues were $52.6 million, a 31% increase from $40.2 million in fourth-quarter 2006. Physician practice salaries and other expenses for the period were $36.9 million vs. $29.5 million for fourth-quarter 2006. As a percentage of net revenue, physician salaries declined to 70% from 73% in the fourth quarter of 2007 vs. 2006. Dr. Singer attributed the change to higher physician productivity and increased revenue per encounter.
With the stock market retrenching since its all-time high in October, IPC’s timing on going public might seem a bit off. Yet, venture capitalists are bullish on healthcare. They sank a record $9.97 billion into the sector in 2007, topping the previous high of $9.47 billion in 2000, during the dot.com craze. Three large venture capital firms specializing in healthcare have $1.25 billion looking for good homes. The worry on Wall Street is that there won’t be enough healthcare IPOs to satisfy demand; there were only 31 in 2007 vs. 60 in 2000.
Alternatives
Going public is not the only way hospital medicine companies and other physician-intensive enterprises can raise needed capital. Venture capital plays a critical role. Brentwood, Tenn.-based Cogent Healthcare received its first round of such funding in 1997, a second infusion in 2000, and $15 million in 2002. IPC is no stranger to venture capital either: It raised $47 million in venture capital since 1998. Such capital infusions helped IPC and Cogent in their early days by providing the money needed to start hospital medicine groups, recruit top managers, expand into new markets, and improve IT and communication infrastructures.
Some entrepreneurs favor keeping their companies private. For example, John Erickson, founder and CEO of Baltimore-based Erickson Retirement Communities (ERC), is committed to growing his business without going public. There are 19 Erickson campuses in 11 states, with 21,000 residents. ERC’s business plan involves adding sites, anticipating growth to 55,000 residents in five years. Such steady expansion gobbles up capital, but Erickson is adamant about staying private. On going public he says: “I consider it whenever I need capital, but it’s hard to keep public markets happy. If your business slows down for any reason, your stock tanks and the market will punish you harshly. I will not have stock analysts pressuring me about how to run and grow my business.”
Erickson admits the competing demands of raising capital and keeping the company private aren’t easy to reconcile. “We go to midsized and large banks and [real estate investment trusts], get letters of credit, tax bonds, debt financing, mezzanine financing, etc.,” he says. He considers that others in his industry, Sunrise Senior Living (SRZ) and Brookdale Senior Living (BKD) that have gone public show the industry’s strength. “Multiple sources of capitalization in an industry provide greater options for all,” he adds.
That said, Erickson intends to resist any temptation to go public because “I must have the flexibility to implement our five-year plan correctly. If I want to invest $30 million in a medical group or hire seven doctors at $150,000 a pop, I don’t have to answer to some 30-year-old stock analyst who doesn’t like that.”
As for IPC’s public offering, Erickson says the first company to do so in an industry opens new avenues for raising capital in the public arena. “Dr. Singer’s pushing the envelope for hospital medicine, and if he can tolerate the pressure of the market—even when the strings are very tight—that’s great,” he emphasizes.
Commenting on the legal and governance issues of a public offering, Peter Olberg, corporate and finance partner at Manhattan-based law firm of Manatt, Philips and Philips says IPC’s being the first publicly traded hospitalist medicine company is a sound way to raise capital and isn’t risky in terms of disclosure. However, a specialty care provider like IPC can “become a victim of its own success. Public payers can say reimbursement is too high and cut it based on the leader’s financial performance.”
Peer Perspective
SHM President Patrick Cawley, MD, MBA, calls IPC’s public offering a major milestone because it demonstrates the maturity of the hospitalist movement. He expects IPC to use the infusion of capital to step up physician hiring, acquire more groups, and improve its proprietary IT infrastructure by refining its tools to further link outcomes and performance.
“IPC’s emphasis on quality outcomes is clearly where medicine is going,” Dr. Cawley says. And, “Putting pressure on hospitalists to be more productive has a huge potential in helping hospital medicine get more efficient by seeing more patients.”
To put the IPC public offering in perspective Dr. Cawley captures Dr. Singer’s vision. “To run a public company, you focus beyond daily stock prices and on the intermediate and short-term. What Wall Street thinks about your business matters. Our product [hospital medicine] has a great future, and I applaud Adam Singer for taking this step.”
Giving Dr. Singer the last word, he says “I’m a big believer in not worrying about things I can’t control like stock market fluctuations. We can handle good news and bad. We have a good business model and we’ll stick to the knitting.” TH
Marlene Piturro is a medical writer based in New York.
You know your industry is on the map when a trailblazer takes his company public. That signals the capital markets that top executives in a leading company are confident in their business model’s ability to grow the company enough to satisfy Wall Street’s voracious hunger for profits.
It’s a tall order, and Adam Singer, MD, founder and CEO of IPC-The Hospitalist Company, based in North Hollywood, Calif., accomplished it in January.
During 2007, Dr. Singer set the wheels in motion for taking IPC public the following year. “Our company is built right, has a solid revenue stream, and the nirvana of a real healthcare company—a battle-tested, proven business model,” he says.
Dr. Singer and his management team had the usual reasons for going public: raising capital to pay for operations and to allow for growth by acquisition, reducing debt, and creating liquidity for shareholders. He also had other fish to fry with a public offering. “I wanted to be the first in our industry to make it out of the box,’’ he says. “I also wanted IPC to be a model for the many hospital medicine companies that would like to mature beyond having a bunch of doctors running around a hospital and calling themselves a hospitalist medicine company.”
For Dr. Singer, going public went beyond the desire to produce quarterly financial results that would warm investors’ hearts. It spoke to his core belief in what the business of hospital medicine should be and, from his vantage point, isn’t.
“By leading a publicly traded hospital medicine company I am debunking the myth that hospitalist groups need hospital subsidies to survive,” he says. “This is a powerful myth, one that is mired in a work force’s idea that its members should get full-time pay for less than full-time work. IPC has raised the bar for our industry. We think that hospitals should demand the overall level of sophistication, physician commitment and productivity that IPC has.”
With few publicly traded physician companies for templates for a public offering, Dr. Singer looked to a colleague, Roger Medel, MD, for direction. Dr. Medel took his company public in 1979 and has grown Sunrise, Fla.-based Pediatrix Medical Group, a provider of neonatal, maternal-fetal, and pediatric intensivist/hospitalist services, from a company with $100 million in market capitalization to $3.32 billion and a recent stock price of $67 per share. The company has a “buy” consensus rating from analysts and respectable price-to-earning and earnings-per-share values.
Economic Evolution
Medical staffing firm IPOs, such as IPC’s, are relatively rare. Most venture capital chases the next hot thing in medical information technology, biotechnology, and medical testing. The quirks of the medical staffing industry, such as hospitalist hiring—where salary increases can consume sizable chunks of a firm’s revenues—may deter potential investors.
Why are publicly traded medical staffing companies like IPC and PDX the exception rather than the rule? Because they rely so heavily on human capital—primarily physicians—for bottom-line results, they must contend with recruiting and retaining from a highly sought after talent pool that have their choice of job opportunities.
Also, many physicians fear the corporatization of medicine, an anxiety that working for a publicly traded company tends to arouse. Physician idealism—wanting to make the world a better place—may clash with a public corporation’s raison d’etre: making money. In a young field like hospital medicine, where performance metrics are evolving, balancing shareholder demands for ROI versus quality patient care requires a delicate touch.
Larry Wellikson, MD, SHM’s chief executive officer, says IPC’s entry into the public markets has been received well, indicating the maturation and strong growth of hospital medicine.
IPC’s public offering took place Jan. 30. Lead underwriters Credit Suisse Securities and Jeffries & Co. offered 5.9 million shares of IPC stock under the ticker symbol IPCM, a four-letter symbol conforming with NASDAQ’s listing requirements. The stock traded mostly at $19 per share, above the original per share estimate of between $15 to $17, raising approximately $38.3 million in net proceeds.
Since then, IPCM shares have ranged from $16.25 to $23.09 per share, trading at a thin average daily volume of 162,000. As of March 31, IPC’s market capitalization was $296 million. Six months from the IPO, average daily volume should increase, as regulations on shareholder sales are eased according to stock exchange rules.
IPC’s latest financial results were upbeat. The firm reported record operating results for the fourth quarter and full year 2007. Total patient encounters rose 29%, compared with 460,000 over the same period in 2006. Fourth-quarter net revenues were $52.6 million, a 31% increase from $40.2 million in fourth-quarter 2006. Physician practice salaries and other expenses for the period were $36.9 million vs. $29.5 million for fourth-quarter 2006. As a percentage of net revenue, physician salaries declined to 70% from 73% in the fourth quarter of 2007 vs. 2006. Dr. Singer attributed the change to higher physician productivity and increased revenue per encounter.
With the stock market retrenching since its all-time high in October, IPC’s timing on going public might seem a bit off. Yet, venture capitalists are bullish on healthcare. They sank a record $9.97 billion into the sector in 2007, topping the previous high of $9.47 billion in 2000, during the dot.com craze. Three large venture capital firms specializing in healthcare have $1.25 billion looking for good homes. The worry on Wall Street is that there won’t be enough healthcare IPOs to satisfy demand; there were only 31 in 2007 vs. 60 in 2000.
Alternatives
Going public is not the only way hospital medicine companies and other physician-intensive enterprises can raise needed capital. Venture capital plays a critical role. Brentwood, Tenn.-based Cogent Healthcare received its first round of such funding in 1997, a second infusion in 2000, and $15 million in 2002. IPC is no stranger to venture capital either: It raised $47 million in venture capital since 1998. Such capital infusions helped IPC and Cogent in their early days by providing the money needed to start hospital medicine groups, recruit top managers, expand into new markets, and improve IT and communication infrastructures.
Some entrepreneurs favor keeping their companies private. For example, John Erickson, founder and CEO of Baltimore-based Erickson Retirement Communities (ERC), is committed to growing his business without going public. There are 19 Erickson campuses in 11 states, with 21,000 residents. ERC’s business plan involves adding sites, anticipating growth to 55,000 residents in five years. Such steady expansion gobbles up capital, but Erickson is adamant about staying private. On going public he says: “I consider it whenever I need capital, but it’s hard to keep public markets happy. If your business slows down for any reason, your stock tanks and the market will punish you harshly. I will not have stock analysts pressuring me about how to run and grow my business.”
Erickson admits the competing demands of raising capital and keeping the company private aren’t easy to reconcile. “We go to midsized and large banks and [real estate investment trusts], get letters of credit, tax bonds, debt financing, mezzanine financing, etc.,” he says. He considers that others in his industry, Sunrise Senior Living (SRZ) and Brookdale Senior Living (BKD) that have gone public show the industry’s strength. “Multiple sources of capitalization in an industry provide greater options for all,” he adds.
That said, Erickson intends to resist any temptation to go public because “I must have the flexibility to implement our five-year plan correctly. If I want to invest $30 million in a medical group or hire seven doctors at $150,000 a pop, I don’t have to answer to some 30-year-old stock analyst who doesn’t like that.”
As for IPC’s public offering, Erickson says the first company to do so in an industry opens new avenues for raising capital in the public arena. “Dr. Singer’s pushing the envelope for hospital medicine, and if he can tolerate the pressure of the market—even when the strings are very tight—that’s great,” he emphasizes.
Commenting on the legal and governance issues of a public offering, Peter Olberg, corporate and finance partner at Manhattan-based law firm of Manatt, Philips and Philips says IPC’s being the first publicly traded hospitalist medicine company is a sound way to raise capital and isn’t risky in terms of disclosure. However, a specialty care provider like IPC can “become a victim of its own success. Public payers can say reimbursement is too high and cut it based on the leader’s financial performance.”
Peer Perspective
SHM President Patrick Cawley, MD, MBA, calls IPC’s public offering a major milestone because it demonstrates the maturity of the hospitalist movement. He expects IPC to use the infusion of capital to step up physician hiring, acquire more groups, and improve its proprietary IT infrastructure by refining its tools to further link outcomes and performance.
“IPC’s emphasis on quality outcomes is clearly where medicine is going,” Dr. Cawley says. And, “Putting pressure on hospitalists to be more productive has a huge potential in helping hospital medicine get more efficient by seeing more patients.”
To put the IPC public offering in perspective Dr. Cawley captures Dr. Singer’s vision. “To run a public company, you focus beyond daily stock prices and on the intermediate and short-term. What Wall Street thinks about your business matters. Our product [hospital medicine] has a great future, and I applaud Adam Singer for taking this step.”
Giving Dr. Singer the last word, he says “I’m a big believer in not worrying about things I can’t control like stock market fluctuations. We can handle good news and bad. We have a good business model and we’ll stick to the knitting.” TH
Marlene Piturro is a medical writer based in New York.











