User login
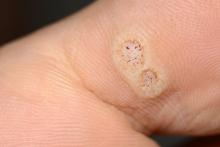
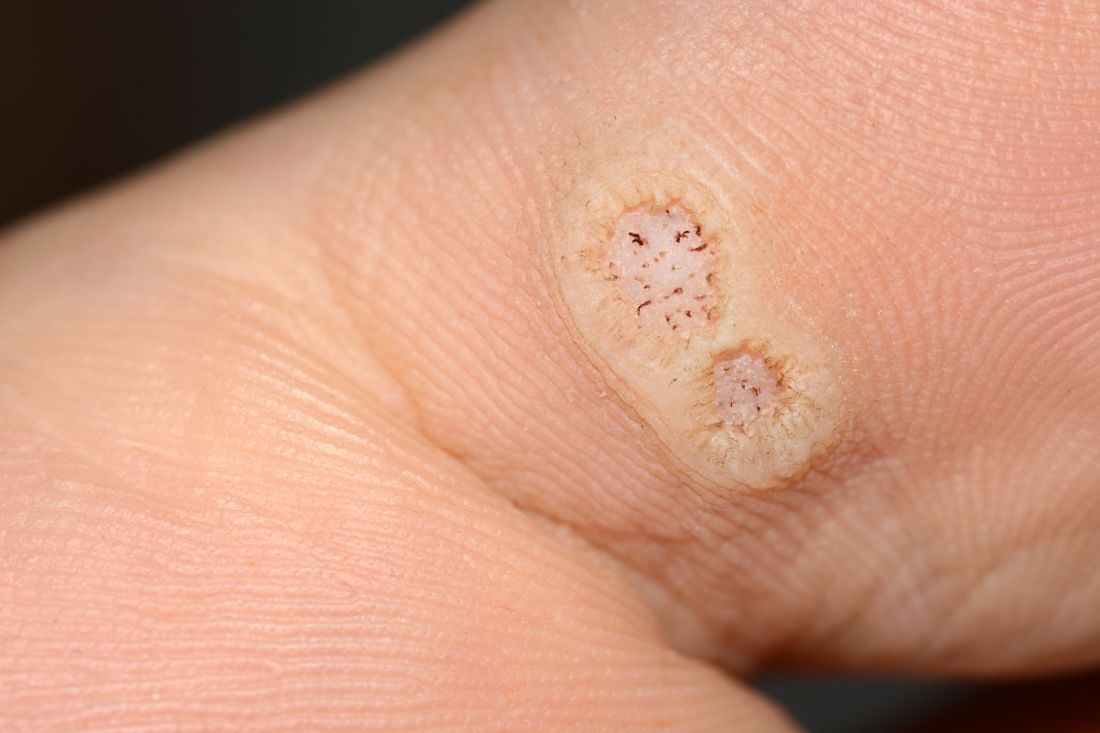
Warts difficult to eradicate in immunocompromised children
.
Only a quarter of patients (24%) who were undergoing active cancer treatment experienced complete resolution of their warts, compared with 63.3% of patients who were not on active treatment.
In addition, warts persisted or worsened in 56.0% of patients receiving active treatment compared with 13.4% of those who were not receiving it.
“These data enable providers treating warts in children with cancer to have an educated discussion regarding the expected clinical progression of warts and the likelihood of response to wart therapy while on and off anti-cancer treatment,” the authors wrote in the study, published in Pediatric Dermatology.
In immunocompromised children, warts are more common than in the general pediatric population, and more resistant to treatment. But as the authors noted, data on the course and prognosis of warts in pediatric patients who are actively receiving anti-cancer therapy compared with patients who have completed treatment are limited.
Tina Ho, MD, PhD, of the department of dermatology, and colleagues from Boston Children’s Hospital, sought to analyze the clinical course of warts treated in this patient population at their institution over a 10-year period. They conducted a retrospective study of 72 children who were treated for cancer between 2011 and 2021, and who had also been treated for warts.
The median age of the cohort was 12 years, and they were followed for a median of 2 years following their diagnosis of warts. Within this group, more than half (55%) had hematologic malignancies, while 27% had a history of bone marrow transplantation.
Of note, the authors pointed out, 54% of the patients had plantar warts, and 60% of patients (38 of 63) with a documented number of warts had more than five at the time of presentation.
The treatment regimens that the children had received varied, with 81% of patients receiving cytotoxic chemotherapy and 23% of patients on targeted therapies that included immunotherapy.
The warts were most commonly treated with cryotherapy and topical salicylic acid; this was the case for those actively receiving oncology treatment or those who had completed their treatment regimens.
Outcomes of wart treatments were available in 25 of the patients undergoing active cancer treatment and in 30 of those who had completed treatment. For children on active oncology treatment, 5 (20%) achieved partial resolution, 6 (24%) achieved complete resolution, and 14 (56%) experienced persistence or worsening of their warts following therapy. Those who had completed treatment had better outcomes: Seven (23.3%) had a partial response, 19 (63.3%) had complete resolution, and 4 (13.4%) had persistence or worsening of warts after treatment of warts.
The authors also pointed out the treatment of warts can be painful, expensive, and time-consuming. “It is thus imperative that the risks and benefits of these treatments are carefully considered before proceeding with treatment,” wrote Dr. Ho and colleagues. “This is especially true in medically complex children with cancer who may be fearful of procedures and spend significant portions of their young lives within the medical system.”
Limitations to the study include its retrospective design and small sample size. Clinical data were not uniformly complete, and follow-up intervals varied among the participants. Also, it was conducted at a single-institution and at a large tertiary center, so the results may not be fully generalizable.
The authors declared no conflict of interest. No outside funding source was listed.
.
Only a quarter of patients (24%) who were undergoing active cancer treatment experienced complete resolution of their warts, compared with 63.3% of patients who were not on active treatment.
In addition, warts persisted or worsened in 56.0% of patients receiving active treatment compared with 13.4% of those who were not receiving it.
“These data enable providers treating warts in children with cancer to have an educated discussion regarding the expected clinical progression of warts and the likelihood of response to wart therapy while on and off anti-cancer treatment,” the authors wrote in the study, published in Pediatric Dermatology.
In immunocompromised children, warts are more common than in the general pediatric population, and more resistant to treatment. But as the authors noted, data on the course and prognosis of warts in pediatric patients who are actively receiving anti-cancer therapy compared with patients who have completed treatment are limited.
Tina Ho, MD, PhD, of the department of dermatology, and colleagues from Boston Children’s Hospital, sought to analyze the clinical course of warts treated in this patient population at their institution over a 10-year period. They conducted a retrospective study of 72 children who were treated for cancer between 2011 and 2021, and who had also been treated for warts.
The median age of the cohort was 12 years, and they were followed for a median of 2 years following their diagnosis of warts. Within this group, more than half (55%) had hematologic malignancies, while 27% had a history of bone marrow transplantation.
Of note, the authors pointed out, 54% of the patients had plantar warts, and 60% of patients (38 of 63) with a documented number of warts had more than five at the time of presentation.
The treatment regimens that the children had received varied, with 81% of patients receiving cytotoxic chemotherapy and 23% of patients on targeted therapies that included immunotherapy.
The warts were most commonly treated with cryotherapy and topical salicylic acid; this was the case for those actively receiving oncology treatment or those who had completed their treatment regimens.
Outcomes of wart treatments were available in 25 of the patients undergoing active cancer treatment and in 30 of those who had completed treatment. For children on active oncology treatment, 5 (20%) achieved partial resolution, 6 (24%) achieved complete resolution, and 14 (56%) experienced persistence or worsening of their warts following therapy. Those who had completed treatment had better outcomes: Seven (23.3%) had a partial response, 19 (63.3%) had complete resolution, and 4 (13.4%) had persistence or worsening of warts after treatment of warts.
The authors also pointed out the treatment of warts can be painful, expensive, and time-consuming. “It is thus imperative that the risks and benefits of these treatments are carefully considered before proceeding with treatment,” wrote Dr. Ho and colleagues. “This is especially true in medically complex children with cancer who may be fearful of procedures and spend significant portions of their young lives within the medical system.”
Limitations to the study include its retrospective design and small sample size. Clinical data were not uniformly complete, and follow-up intervals varied among the participants. Also, it was conducted at a single-institution and at a large tertiary center, so the results may not be fully generalizable.
The authors declared no conflict of interest. No outside funding source was listed.
.
Only a quarter of patients (24%) who were undergoing active cancer treatment experienced complete resolution of their warts, compared with 63.3% of patients who were not on active treatment.
In addition, warts persisted or worsened in 56.0% of patients receiving active treatment compared with 13.4% of those who were not receiving it.
“These data enable providers treating warts in children with cancer to have an educated discussion regarding the expected clinical progression of warts and the likelihood of response to wart therapy while on and off anti-cancer treatment,” the authors wrote in the study, published in Pediatric Dermatology.
In immunocompromised children, warts are more common than in the general pediatric population, and more resistant to treatment. But as the authors noted, data on the course and prognosis of warts in pediatric patients who are actively receiving anti-cancer therapy compared with patients who have completed treatment are limited.
Tina Ho, MD, PhD, of the department of dermatology, and colleagues from Boston Children’s Hospital, sought to analyze the clinical course of warts treated in this patient population at their institution over a 10-year period. They conducted a retrospective study of 72 children who were treated for cancer between 2011 and 2021, and who had also been treated for warts.
The median age of the cohort was 12 years, and they were followed for a median of 2 years following their diagnosis of warts. Within this group, more than half (55%) had hematologic malignancies, while 27% had a history of bone marrow transplantation.
Of note, the authors pointed out, 54% of the patients had plantar warts, and 60% of patients (38 of 63) with a documented number of warts had more than five at the time of presentation.
The treatment regimens that the children had received varied, with 81% of patients receiving cytotoxic chemotherapy and 23% of patients on targeted therapies that included immunotherapy.
The warts were most commonly treated with cryotherapy and topical salicylic acid; this was the case for those actively receiving oncology treatment or those who had completed their treatment regimens.
Outcomes of wart treatments were available in 25 of the patients undergoing active cancer treatment and in 30 of those who had completed treatment. For children on active oncology treatment, 5 (20%) achieved partial resolution, 6 (24%) achieved complete resolution, and 14 (56%) experienced persistence or worsening of their warts following therapy. Those who had completed treatment had better outcomes: Seven (23.3%) had a partial response, 19 (63.3%) had complete resolution, and 4 (13.4%) had persistence or worsening of warts after treatment of warts.
The authors also pointed out the treatment of warts can be painful, expensive, and time-consuming. “It is thus imperative that the risks and benefits of these treatments are carefully considered before proceeding with treatment,” wrote Dr. Ho and colleagues. “This is especially true in medically complex children with cancer who may be fearful of procedures and spend significant portions of their young lives within the medical system.”
Limitations to the study include its retrospective design and small sample size. Clinical data were not uniformly complete, and follow-up intervals varied among the participants. Also, it was conducted at a single-institution and at a large tertiary center, so the results may not be fully generalizable.
The authors declared no conflict of interest. No outside funding source was listed.
FROM PEDIATRIC DERMATOLOGY
Prognostic factors of SCCs in organ transplant recipients worse compared with general population
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
, results from a dual cohort study demonstrated.
The findings build on previous research and underscore the need for early diagnosis and aggressive surveillance in this patient population, corresponding author Adele C. Green, MBBS, PhD, professor and senior scientist at the QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia, and colleagues wrote in the study, which was published online in JAMA Dermatology. “Squamous cell carcinomas (SCCs) of the skin develop up to 77 times more frequently in immunosuppressed organ transplant recipients (OTRs) than the general population,” they wrote. “Because SCCs cause substantially more morbidity and death in the former, they are postulated to be innately more aggressive than in immunocompetent patients, but OTRs’ higher SCC mortality may simply reflect greater SCC tumor burdens per patient.”
In what is believed to be the first study of its kind, Dr. Green and colleagues drew data from two cohort studies to evaluate five key clinicopathologic indicators of poor SCC outcomes in organ transplant recipients, and in those from the general population in Queensland, Australia: cephalic location, perineural invasion, invasion to/beyond subcutaneous fat, poor differentiation, and tumor size greater than 20 mm. The study population included organ transplant recipients at high risk of skin cancer, who were enrolled in the Skin Tumours in Allograft Recipients (STAR) study, and those from a population-based cohort, the QSkin Sun and Health Study. STAR consisted of lung transplant recipients and kidney and liver transplant recipients at high risk of skin cancer who were recruited from tertiary centers and diagnosed with histopathologically confirmed SCC from 2012 to 2015. QSkin consisted of individuals from Queensland’s general adult population diagnosed with SCCs from 2012 to 2015.
SCC cases in QSkin were ascertained through Australia’s universal health insurance agency and linked with histopathology records. Next, the researchers performed data analysis from both cohort studies to determine the prevalence ratio (PR) of head/neck location, perineural invasion, tumor invasion to/beyond subcutaneous fat, poor cellular differentiation, and tumor diameter greater than 20 mm among SCCs among organ transplant recipients compared with the general population.
After combining the two studies, the researchers compared 741 SCCs excised from 191 organ transplant recipients and 2,558 SCCs excised from 1,507 individuals in the general population. Their median ages were similar (62.7 and 63.7 years, respectively) and most were male (78% and 63.4%, respectively).
As for site of involvement, SCCs developed most often on the head and neck in the transplant recipients (38.6%) and on the arms and hands in the general population (35.2%). After adjustment for age and sex, perineural invasion of SCCs was more than twice as common in transplant recipients than among cases in the general population, as was invasion to/beyond subcutaneous fat (PR of 2.37 for both associations).
In other findings, compared with SCCs in the general population, poorly vs. well-differentiated SCCs were more than threefold more common in transplant recipients (PR, 3.45), while the prevalence of tumors greater than 20 mm vs. 20 mm or smaller was moderately higher in transplant recipients (PR, 1.52).
“These findings are considered generalizable, confirming that OTRs’ poorer SCC outcomes are associated with not only their sheer numbers of SCC tumors, but also with a strong shift toward more invasive, less differentiated, and larger SCC tumors, in agreement with previous findings,” the researchers wrote. “This shift is likely associated with decreased immunosurveillance resulting from immunosuppressive therapy (since carcinogenesis decelerates with therapy cessation) interacting with effects of high UV radiation exposure.”
They acknowledged certain limitations of their analysis, chiefly the lack of central review of SCCs to ensure standard assessment of histopathologic features “including caliber of nerves with perineural invasion and cell differentiation; such a review would not have been feasible logistically.”
The study was supported by grants from the National Health and Medical Research Council of Australia. The researchers reported having no disclosures related to the submitted work.
FROM JAMA DERMATOLOGY
Galcanezumab safe and effective for chronic migraine and medication overuse headache
Key clinical point: Galcanezumab was safe and effective in a patient population severely impaired by chronic migraine (CM) and medication overuse headache (MOH).
Major finding: Galcanezumab led to a significant reduction in migraine days per month, painkillers per month, number of days on medication, numeric rating scale scores, 6-item Headache Impact Test scores, and Migraine Disability Assessment questionnaire scores (all P < .001), with improvements being the greatest during the first 3 months of treatment. Adverse events were mostly mild, with only one case of treatment discontinuation because of severe low back pain.
Study details: The data come from a single-center, prospective study including 78 patients with CM and MOH who received galcanezumab.
Disclosures: This study did not report the funding source. S Guerzoni and C Baraldi declared receiving honoraria from various sources. L Pani declared serving as the Chief Scientific Officer of EDRA-LSWR Publishing Company and Inpeco SA Total Lab Automation Company and had ties to other sources. Other authors declared no conflicts of interest.
Source: Guerzoni S et al. Galcanezumab for the treatment of chronic migraine and medication overuse headache: Real-world clinical evidence in a severely impaired patient population. Brain Behav. 2023;13:e2799 (May 19). doi: 10.1002/brb3.2799
Key clinical point: Galcanezumab was safe and effective in a patient population severely impaired by chronic migraine (CM) and medication overuse headache (MOH).
Major finding: Galcanezumab led to a significant reduction in migraine days per month, painkillers per month, number of days on medication, numeric rating scale scores, 6-item Headache Impact Test scores, and Migraine Disability Assessment questionnaire scores (all P < .001), with improvements being the greatest during the first 3 months of treatment. Adverse events were mostly mild, with only one case of treatment discontinuation because of severe low back pain.
Study details: The data come from a single-center, prospective study including 78 patients with CM and MOH who received galcanezumab.
Disclosures: This study did not report the funding source. S Guerzoni and C Baraldi declared receiving honoraria from various sources. L Pani declared serving as the Chief Scientific Officer of EDRA-LSWR Publishing Company and Inpeco SA Total Lab Automation Company and had ties to other sources. Other authors declared no conflicts of interest.
Source: Guerzoni S et al. Galcanezumab for the treatment of chronic migraine and medication overuse headache: Real-world clinical evidence in a severely impaired patient population. Brain Behav. 2023;13:e2799 (May 19). doi: 10.1002/brb3.2799
Key clinical point: Galcanezumab was safe and effective in a patient population severely impaired by chronic migraine (CM) and medication overuse headache (MOH).
Major finding: Galcanezumab led to a significant reduction in migraine days per month, painkillers per month, number of days on medication, numeric rating scale scores, 6-item Headache Impact Test scores, and Migraine Disability Assessment questionnaire scores (all P < .001), with improvements being the greatest during the first 3 months of treatment. Adverse events were mostly mild, with only one case of treatment discontinuation because of severe low back pain.
Study details: The data come from a single-center, prospective study including 78 patients with CM and MOH who received galcanezumab.
Disclosures: This study did not report the funding source. S Guerzoni and C Baraldi declared receiving honoraria from various sources. L Pani declared serving as the Chief Scientific Officer of EDRA-LSWR Publishing Company and Inpeco SA Total Lab Automation Company and had ties to other sources. Other authors declared no conflicts of interest.
Source: Guerzoni S et al. Galcanezumab for the treatment of chronic migraine and medication overuse headache: Real-world clinical evidence in a severely impaired patient population. Brain Behav. 2023;13:e2799 (May 19). doi: 10.1002/brb3.2799
Ketogenic diet may improve sleep complaints in patients with migraine
Key clinical point: Ketogenic diet (KD) significantly improved sleep complaints in patients with migraine, irrespective of migraine improvements and anthropometric modifications.
Major finding: Migraine intensity, headache frequency, and the severity of headache-related disability improved significantly after 3 months of KD therapy (all P < .001) along with a significant decrease in the rate of insomnia (before vs after treatment: 60% vs 40%; P < .001) and number of patients experiencing poor sleep (reduced to half at follow-up; P < .001). The modifications in sleep features showed no correlation with migraine improvements and anthropometric changes.
Study details: This study included 70 patients with migraine who received KD as a preventive therapy for migraine.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Merlino G et al. Sleep of migraine patients is ameliorated by ketogenic diet, independently of pain control. Sleep Med. 2023;107:196-201 (May 9). doi: 10.1016/j.sleep.2023.05.006
Key clinical point: Ketogenic diet (KD) significantly improved sleep complaints in patients with migraine, irrespective of migraine improvements and anthropometric modifications.
Major finding: Migraine intensity, headache frequency, and the severity of headache-related disability improved significantly after 3 months of KD therapy (all P < .001) along with a significant decrease in the rate of insomnia (before vs after treatment: 60% vs 40%; P < .001) and number of patients experiencing poor sleep (reduced to half at follow-up; P < .001). The modifications in sleep features showed no correlation with migraine improvements and anthropometric changes.
Study details: This study included 70 patients with migraine who received KD as a preventive therapy for migraine.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Merlino G et al. Sleep of migraine patients is ameliorated by ketogenic diet, independently of pain control. Sleep Med. 2023;107:196-201 (May 9). doi: 10.1016/j.sleep.2023.05.006
Key clinical point: Ketogenic diet (KD) significantly improved sleep complaints in patients with migraine, irrespective of migraine improvements and anthropometric modifications.
Major finding: Migraine intensity, headache frequency, and the severity of headache-related disability improved significantly after 3 months of KD therapy (all P < .001) along with a significant decrease in the rate of insomnia (before vs after treatment: 60% vs 40%; P < .001) and number of patients experiencing poor sleep (reduced to half at follow-up; P < .001). The modifications in sleep features showed no correlation with migraine improvements and anthropometric changes.
Study details: This study included 70 patients with migraine who received KD as a preventive therapy for migraine.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Merlino G et al. Sleep of migraine patients is ameliorated by ketogenic diet, independently of pain control. Sleep Med. 2023;107:196-201 (May 9). doi: 10.1016/j.sleep.2023.05.006
Migraine history raises susceptibility to Alzheimer’s disease
Key clinical point: The risk of developing Alzheimer’s disease (AD) dementia is significantly higher in individuals with vs without migraine, with younger age, obesity, and chronic migraine being significant risk factors for AD dementia among individuals with migraine.
Major finding: A prior history of migraine is a significant risk factor for AD dementia (hazard ratio [HR] 1.32; 95% CI 1.30-1.35). The risk was prominently higher among individuals with vs without migraine who were younger (HR 1.58; 95% CI 1.52-1.64) and had obesity (HR 1.39; 95% CI 1.35-1.43) and among those with chronic vs episodic migraine (CM HR 1.48, 95% CI 1.44-1.52; vs EM HR 1.26, 95% CI 1.27-1.29).
Study details: This retrospective, nationwide cohort study included individuals without (n = 5,863,348) and with (n = 212,836) migraine.
Disclosures: This study was supported by grants from the National Research Foundation funded by the Ministry of Education, the Technology Development Program funded by the Ministry of SMEs and Startups (Korea), and others. The authors declared no conflicts of interest.
Source: Kim J et al. Association between migraine and Alzheimer’s disease: A nationwide cohort study. Front Aging Neurosci. 2023;15:1196185 (May 25). doi: 10.3389/fnagi.2023.1196185
Key clinical point: The risk of developing Alzheimer’s disease (AD) dementia is significantly higher in individuals with vs without migraine, with younger age, obesity, and chronic migraine being significant risk factors for AD dementia among individuals with migraine.
Major finding: A prior history of migraine is a significant risk factor for AD dementia (hazard ratio [HR] 1.32; 95% CI 1.30-1.35). The risk was prominently higher among individuals with vs without migraine who were younger (HR 1.58; 95% CI 1.52-1.64) and had obesity (HR 1.39; 95% CI 1.35-1.43) and among those with chronic vs episodic migraine (CM HR 1.48, 95% CI 1.44-1.52; vs EM HR 1.26, 95% CI 1.27-1.29).
Study details: This retrospective, nationwide cohort study included individuals without (n = 5,863,348) and with (n = 212,836) migraine.
Disclosures: This study was supported by grants from the National Research Foundation funded by the Ministry of Education, the Technology Development Program funded by the Ministry of SMEs and Startups (Korea), and others. The authors declared no conflicts of interest.
Source: Kim J et al. Association between migraine and Alzheimer’s disease: A nationwide cohort study. Front Aging Neurosci. 2023;15:1196185 (May 25). doi: 10.3389/fnagi.2023.1196185
Key clinical point: The risk of developing Alzheimer’s disease (AD) dementia is significantly higher in individuals with vs without migraine, with younger age, obesity, and chronic migraine being significant risk factors for AD dementia among individuals with migraine.
Major finding: A prior history of migraine is a significant risk factor for AD dementia (hazard ratio [HR] 1.32; 95% CI 1.30-1.35). The risk was prominently higher among individuals with vs without migraine who were younger (HR 1.58; 95% CI 1.52-1.64) and had obesity (HR 1.39; 95% CI 1.35-1.43) and among those with chronic vs episodic migraine (CM HR 1.48, 95% CI 1.44-1.52; vs EM HR 1.26, 95% CI 1.27-1.29).
Study details: This retrospective, nationwide cohort study included individuals without (n = 5,863,348) and with (n = 212,836) migraine.
Disclosures: This study was supported by grants from the National Research Foundation funded by the Ministry of Education, the Technology Development Program funded by the Ministry of SMEs and Startups (Korea), and others. The authors declared no conflicts of interest.
Source: Kim J et al. Association between migraine and Alzheimer’s disease: A nationwide cohort study. Front Aging Neurosci. 2023;15:1196185 (May 25). doi: 10.3389/fnagi.2023.1196185
Opioid use more frequent in patients with chronic migraine
Key clinical point: Use of opioids is still prevalent among patients with migraine, thus indicating non-adherence to evidence-based international guidelines; moreover, the opioid use is more frequent and prolonged among patients with chronic migraine (CM) than among those with episodic migraine (EM).
Major finding: Overall, 13.4% of patients reported ever using an opioid for headache, with 46.3% using opioids occasionally, whereas 27.0% and 11.3% reported using them for >1 month and >1 year, respectively. Additionally, 2.4% of participants used opioids without a prescription. Patients with CM vs EM reported more frequent (21.6% vs 11.7%; P < .001) and prolonged (>1 month: 33.6% vs 24.4%; P < .003; >1 year: 17.7% vs 8.7%; P < .001) opioid use.
Study details: Findings are from a cross-sectional questionnaire-based study including 3712 patients with migraine (CM n = 629; EM n = 3,083).
Disclosures: This study did not receive any funding. GM Terwindt declared receiving consultancy support and independent support from various sources. No other conflicts of interest were declared.
Source: van Welie RF, van Welie FC, et al. Characterizing opioid use in a Dutch cohort with migraine. Cephalalgia. 2023;43(5) (May 11). doi: 10.1177/03331024231174160
Key clinical point: Use of opioids is still prevalent among patients with migraine, thus indicating non-adherence to evidence-based international guidelines; moreover, the opioid use is more frequent and prolonged among patients with chronic migraine (CM) than among those with episodic migraine (EM).
Major finding: Overall, 13.4% of patients reported ever using an opioid for headache, with 46.3% using opioids occasionally, whereas 27.0% and 11.3% reported using them for >1 month and >1 year, respectively. Additionally, 2.4% of participants used opioids without a prescription. Patients with CM vs EM reported more frequent (21.6% vs 11.7%; P < .001) and prolonged (>1 month: 33.6% vs 24.4%; P < .003; >1 year: 17.7% vs 8.7%; P < .001) opioid use.
Study details: Findings are from a cross-sectional questionnaire-based study including 3712 patients with migraine (CM n = 629; EM n = 3,083).
Disclosures: This study did not receive any funding. GM Terwindt declared receiving consultancy support and independent support from various sources. No other conflicts of interest were declared.
Source: van Welie RF, van Welie FC, et al. Characterizing opioid use in a Dutch cohort with migraine. Cephalalgia. 2023;43(5) (May 11). doi: 10.1177/03331024231174160
Key clinical point: Use of opioids is still prevalent among patients with migraine, thus indicating non-adherence to evidence-based international guidelines; moreover, the opioid use is more frequent and prolonged among patients with chronic migraine (CM) than among those with episodic migraine (EM).
Major finding: Overall, 13.4% of patients reported ever using an opioid for headache, with 46.3% using opioids occasionally, whereas 27.0% and 11.3% reported using them for >1 month and >1 year, respectively. Additionally, 2.4% of participants used opioids without a prescription. Patients with CM vs EM reported more frequent (21.6% vs 11.7%; P < .001) and prolonged (>1 month: 33.6% vs 24.4%; P < .003; >1 year: 17.7% vs 8.7%; P < .001) opioid use.
Study details: Findings are from a cross-sectional questionnaire-based study including 3712 patients with migraine (CM n = 629; EM n = 3,083).
Disclosures: This study did not receive any funding. GM Terwindt declared receiving consultancy support and independent support from various sources. No other conflicts of interest were declared.
Source: van Welie RF, van Welie FC, et al. Characterizing opioid use in a Dutch cohort with migraine. Cephalalgia. 2023;43(5) (May 11). doi: 10.1177/03331024231174160
Fremanezumab switch may benefit migraine patients who are not responding to anti-CGRP mAb
Key clinical point: Switching to fremanezumab may provide clinical benefits in patients with difficult-to-treat episodic or chronic migraine who have not responded to prior monoclonal antibody (mAb) therapy targeting the calcitonin gene-related peptide (anti-CGRP) pathway.
Major finding: Overall, 42.8% of patients achieved a 50% reduction in the monthly migraine days (MMD) after switching to fremanezumab. The MMD decreased from 13.6 to 7.2 (P < .0001), Migraine Disability Assessment scores were reduced from 73.3 to 50.3 (P = .0014), and acute migraine medication use decreased from 9.7 to 4.9 days/month (P < .0001) after 3 months of fremanezumab therapy.
Study details: This subgroup analysis of the real-world, non-interventional Finesse study included 153 patients with episodic or chronic migraine who switched to fremanezumab from other anti-CGRP mAb treatments.
Disclosures: This study was funded by TEVA GmbH. Two authors declared being employees of TEVA GmbH. Several authors, including the lead author, declared serving as consultants or on advisory or speaker boards or receiving research grants from various sources, including TEVA GmbH.
Source: Straube A et al. Real-world effectiveness of fremanezumab in patients with migraine switching from another mAb targeting the CGRP pathway: A subgroup analysis of the Finesse Study. J Headache Pain. 2023;24:59 (May 23). doi: 10.1186/s10194-023-01593-2
Key clinical point: Switching to fremanezumab may provide clinical benefits in patients with difficult-to-treat episodic or chronic migraine who have not responded to prior monoclonal antibody (mAb) therapy targeting the calcitonin gene-related peptide (anti-CGRP) pathway.
Major finding: Overall, 42.8% of patients achieved a 50% reduction in the monthly migraine days (MMD) after switching to fremanezumab. The MMD decreased from 13.6 to 7.2 (P < .0001), Migraine Disability Assessment scores were reduced from 73.3 to 50.3 (P = .0014), and acute migraine medication use decreased from 9.7 to 4.9 days/month (P < .0001) after 3 months of fremanezumab therapy.
Study details: This subgroup analysis of the real-world, non-interventional Finesse study included 153 patients with episodic or chronic migraine who switched to fremanezumab from other anti-CGRP mAb treatments.
Disclosures: This study was funded by TEVA GmbH. Two authors declared being employees of TEVA GmbH. Several authors, including the lead author, declared serving as consultants or on advisory or speaker boards or receiving research grants from various sources, including TEVA GmbH.
Source: Straube A et al. Real-world effectiveness of fremanezumab in patients with migraine switching from another mAb targeting the CGRP pathway: A subgroup analysis of the Finesse Study. J Headache Pain. 2023;24:59 (May 23). doi: 10.1186/s10194-023-01593-2
Key clinical point: Switching to fremanezumab may provide clinical benefits in patients with difficult-to-treat episodic or chronic migraine who have not responded to prior monoclonal antibody (mAb) therapy targeting the calcitonin gene-related peptide (anti-CGRP) pathway.
Major finding: Overall, 42.8% of patients achieved a 50% reduction in the monthly migraine days (MMD) after switching to fremanezumab. The MMD decreased from 13.6 to 7.2 (P < .0001), Migraine Disability Assessment scores were reduced from 73.3 to 50.3 (P = .0014), and acute migraine medication use decreased from 9.7 to 4.9 days/month (P < .0001) after 3 months of fremanezumab therapy.
Study details: This subgroup analysis of the real-world, non-interventional Finesse study included 153 patients with episodic or chronic migraine who switched to fremanezumab from other anti-CGRP mAb treatments.
Disclosures: This study was funded by TEVA GmbH. Two authors declared being employees of TEVA GmbH. Several authors, including the lead author, declared serving as consultants or on advisory or speaker boards or receiving research grants from various sources, including TEVA GmbH.
Source: Straube A et al. Real-world effectiveness of fremanezumab in patients with migraine switching from another mAb targeting the CGRP pathway: A subgroup analysis of the Finesse Study. J Headache Pain. 2023;24:59 (May 23). doi: 10.1186/s10194-023-01593-2
Real-world data show benefits of anti-CGRP mAb in migraine patients age ≥ 65 years
Key clinical point: Anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAb) are a safe and effective treatment option for patients age > 65 years with migraine and who did not respond to ≥3 prior migraine preventive medications.
Major finding: At 6 months, monthly migraine days, monthly headache days, and monthly acute medication intake days reduced by 10.1 days (P = .0001), 10.5 days (P < .001), and 9.4 days (P < .001), respectively. Nearly 25.3% of the patients experienced adverse effects at some point during follow-up, which were mostly mild in severity.
Study details: The data come from an observational retrospective study including 162 patients age > 65 years with migraine who did not respond to ≥3 migraine preventive medications and were treated with any one of the three anti-CGRP mAb (erenumab, galcanezumab, or fremanezumab).
Disclosures: This study did not receive any specific grant. Several authors, including the lead author, reported receiving honoraria for consulting, speaking, or advisory board participation; research funding; or travel funding from various sources.
Source: Muñoz-Vendrell A et al. Effectiveness and safety of anti-CGRP monoclonal antibodies in patients over 65 years: A real-life multicentre analysis of 162 patients. J Headache Pain. 2023;24:63 (Jun 2). doi: 10.1186/s10194-023-01585-2
Key clinical point: Anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAb) are a safe and effective treatment option for patients age > 65 years with migraine and who did not respond to ≥3 prior migraine preventive medications.
Major finding: At 6 months, monthly migraine days, monthly headache days, and monthly acute medication intake days reduced by 10.1 days (P = .0001), 10.5 days (P < .001), and 9.4 days (P < .001), respectively. Nearly 25.3% of the patients experienced adverse effects at some point during follow-up, which were mostly mild in severity.
Study details: The data come from an observational retrospective study including 162 patients age > 65 years with migraine who did not respond to ≥3 migraine preventive medications and were treated with any one of the three anti-CGRP mAb (erenumab, galcanezumab, or fremanezumab).
Disclosures: This study did not receive any specific grant. Several authors, including the lead author, reported receiving honoraria for consulting, speaking, or advisory board participation; research funding; or travel funding from various sources.
Source: Muñoz-Vendrell A et al. Effectiveness and safety of anti-CGRP monoclonal antibodies in patients over 65 years: A real-life multicentre analysis of 162 patients. J Headache Pain. 2023;24:63 (Jun 2). doi: 10.1186/s10194-023-01585-2
Key clinical point: Anti-calcitonin gene-related peptide (CGRP) monoclonal antibodies (mAb) are a safe and effective treatment option for patients age > 65 years with migraine and who did not respond to ≥3 prior migraine preventive medications.
Major finding: At 6 months, monthly migraine days, monthly headache days, and monthly acute medication intake days reduced by 10.1 days (P = .0001), 10.5 days (P < .001), and 9.4 days (P < .001), respectively. Nearly 25.3% of the patients experienced adverse effects at some point during follow-up, which were mostly mild in severity.
Study details: The data come from an observational retrospective study including 162 patients age > 65 years with migraine who did not respond to ≥3 migraine preventive medications and were treated with any one of the three anti-CGRP mAb (erenumab, galcanezumab, or fremanezumab).
Disclosures: This study did not receive any specific grant. Several authors, including the lead author, reported receiving honoraria for consulting, speaking, or advisory board participation; research funding; or travel funding from various sources.
Source: Muñoz-Vendrell A et al. Effectiveness and safety of anti-CGRP monoclonal antibodies in patients over 65 years: A real-life multicentre analysis of 162 patients. J Headache Pain. 2023;24:63 (Jun 2). doi: 10.1186/s10194-023-01585-2
Perimenstrual migraine attacks are exclusively migraine attacks without aura, recommends study
Key clinical point: The perimenstrual period was associated with an increased susceptibility to migraine without aura exclusively in both women who experienced migraine with and without aura; hence, the study recommended that only attacks without aura should be considered for a perimenstrual migraine diagnosis.
Major finding: A significant interaction was observed between the perimenstrual window and migraine subtype for migraine attack occurrence (P = .022), with the effect of the perimenstrual window being greater among women with migraine without aura (odds ratio [OR] 1.57; 95% CI 1.45-1.69) vs with aura (OR 1.36; 95% CI 1.24-1.49). Women with migraine with vs without aura showed similar increase in migraine attacks without aura during the perimenstrual window (P = .224).
Study details: This longitudinal electronic diary study included 526 premenopausal women diagnosed with migraine with or without aura.
Disclosures: This study was supported by ZonMw and the Dutch Brain Foundation. Five authors, including the lead author, declared receiving independent support from the study funders. Some authors declared receiving consultancy and independent support from various sources.
Source: Verhagen IE et al. Migraine with and without aura in relation to the menstrual cycle and other hormonal milestones: A prospective cohort study. Cephalalgia. 2023;43(6) (May 31). doi: 10.1177/03331024231164322
Key clinical point: The perimenstrual period was associated with an increased susceptibility to migraine without aura exclusively in both women who experienced migraine with and without aura; hence, the study recommended that only attacks without aura should be considered for a perimenstrual migraine diagnosis.
Major finding: A significant interaction was observed between the perimenstrual window and migraine subtype for migraine attack occurrence (P = .022), with the effect of the perimenstrual window being greater among women with migraine without aura (odds ratio [OR] 1.57; 95% CI 1.45-1.69) vs with aura (OR 1.36; 95% CI 1.24-1.49). Women with migraine with vs without aura showed similar increase in migraine attacks without aura during the perimenstrual window (P = .224).
Study details: This longitudinal electronic diary study included 526 premenopausal women diagnosed with migraine with or without aura.
Disclosures: This study was supported by ZonMw and the Dutch Brain Foundation. Five authors, including the lead author, declared receiving independent support from the study funders. Some authors declared receiving consultancy and independent support from various sources.
Source: Verhagen IE et al. Migraine with and without aura in relation to the menstrual cycle and other hormonal milestones: A prospective cohort study. Cephalalgia. 2023;43(6) (May 31). doi: 10.1177/03331024231164322
Key clinical point: The perimenstrual period was associated with an increased susceptibility to migraine without aura exclusively in both women who experienced migraine with and without aura; hence, the study recommended that only attacks without aura should be considered for a perimenstrual migraine diagnosis.
Major finding: A significant interaction was observed between the perimenstrual window and migraine subtype for migraine attack occurrence (P = .022), with the effect of the perimenstrual window being greater among women with migraine without aura (odds ratio [OR] 1.57; 95% CI 1.45-1.69) vs with aura (OR 1.36; 95% CI 1.24-1.49). Women with migraine with vs without aura showed similar increase in migraine attacks without aura during the perimenstrual window (P = .224).
Study details: This longitudinal electronic diary study included 526 premenopausal women diagnosed with migraine with or without aura.
Disclosures: This study was supported by ZonMw and the Dutch Brain Foundation. Five authors, including the lead author, declared receiving independent support from the study funders. Some authors declared receiving consultancy and independent support from various sources.
Source: Verhagen IE et al. Migraine with and without aura in relation to the menstrual cycle and other hormonal milestones: A prospective cohort study. Cephalalgia. 2023;43(6) (May 31). doi: 10.1177/03331024231164322
Efficacy of galcanezumab after 1 week of treatment for migraine predicts responders at 3 months
Key clinical point: Galcanezumab showed significant efficacy after the first week of treatment, and the treatment efficacy after the first week was a significant predictor of the response rate at 3 months.
Major finding: The mean changes in weekly response rates (RR) at 1, 2, 3, and 4 weeks after galcanezumab initiation were 44.6%, 31.4%, 26.0%, and 32.6%, respectively, with the improvement being greatest at 1 week (P < .001) and the RR at 1 week being the only predictive factor for ≥50% RR at 3 months (adjusted odds ratio 1.029; P = .002). Adverse events were mostly mild.
Study details: This retrospective, observational study included 55 patients with high-frequency episodic migraine or chronic migraine who received galcanezumab treatment (an initial loading dose of 240 mg followed by a dose of 120 mg monthly for at least 2 months).
Disclosures: This study did not receive any funding. Four authors declared receiving lecture fees from various sources. No other conflicts of interest were declared.
Source: Suzuki K et al. Could efficacy at 1 week after galcanezumab administration for patients with migraine predict responders at 3 months? A real world study. J Neurol. 2023 (May 23). doi: 10.1007/s00415-023-11788-x
Key clinical point: Galcanezumab showed significant efficacy after the first week of treatment, and the treatment efficacy after the first week was a significant predictor of the response rate at 3 months.
Major finding: The mean changes in weekly response rates (RR) at 1, 2, 3, and 4 weeks after galcanezumab initiation were 44.6%, 31.4%, 26.0%, and 32.6%, respectively, with the improvement being greatest at 1 week (P < .001) and the RR at 1 week being the only predictive factor for ≥50% RR at 3 months (adjusted odds ratio 1.029; P = .002). Adverse events were mostly mild.
Study details: This retrospective, observational study included 55 patients with high-frequency episodic migraine or chronic migraine who received galcanezumab treatment (an initial loading dose of 240 mg followed by a dose of 120 mg monthly for at least 2 months).
Disclosures: This study did not receive any funding. Four authors declared receiving lecture fees from various sources. No other conflicts of interest were declared.
Source: Suzuki K et al. Could efficacy at 1 week after galcanezumab administration for patients with migraine predict responders at 3 months? A real world study. J Neurol. 2023 (May 23). doi: 10.1007/s00415-023-11788-x
Key clinical point: Galcanezumab showed significant efficacy after the first week of treatment, and the treatment efficacy after the first week was a significant predictor of the response rate at 3 months.
Major finding: The mean changes in weekly response rates (RR) at 1, 2, 3, and 4 weeks after galcanezumab initiation were 44.6%, 31.4%, 26.0%, and 32.6%, respectively, with the improvement being greatest at 1 week (P < .001) and the RR at 1 week being the only predictive factor for ≥50% RR at 3 months (adjusted odds ratio 1.029; P = .002). Adverse events were mostly mild.
Study details: This retrospective, observational study included 55 patients with high-frequency episodic migraine or chronic migraine who received galcanezumab treatment (an initial loading dose of 240 mg followed by a dose of 120 mg monthly for at least 2 months).
Disclosures: This study did not receive any funding. Four authors declared receiving lecture fees from various sources. No other conflicts of interest were declared.
Source: Suzuki K et al. Could efficacy at 1 week after galcanezumab administration for patients with migraine predict responders at 3 months? A real world study. J Neurol. 2023 (May 23). doi: 10.1007/s00415-023-11788-x