User login
In reply: The negative U wave in the setting of demand ischemia
In Reply: We appreciate the comments from Drs. Suksaranjit, Cheungpasitporn, Bischof, and Marx on our recent article on the negative U wave in a patient with chronic aortic regurgitation.1 The clinical data including electrocardiography, echocardiography, and coronary angiography were presented to emphasize the importance of identifying the negative U wave in the setting of valvular heart disease. We outlined the common differential diagnosis for a negative U wave (page 506). We believe that in the appropriate clinical setting the presence of a negative U wave provides diagnostic utility.
Several published reports to date have described the occurrence of the negative U wave in the setting of obstructive coronary artery disease2–5 or coronary artery vasospasm.6 We were unable to find similar data in the setting of demand ischemia in the presence of normal coronary arteries (functional ischemia), but we fully recognize its likely occurrence, and we value the helpful insight.
- Venkatachalam S, Rimmerman CM. Electrocardiography in aortic regurgitation: it’s in the details. Cleve Clin J Med 2011; 78:505–506.
- Gerson MC, Phillips JF, Morris SN, McHenry PL. Exercise-induced U-wave inversion as a marker of stenosis of the left anterior descending coronary artery. Circulation 1979; 60:1014–1020.
- Galli M, Temporelli P. Images in clinical medicine. Negative U waves as an indicator of stress-induced myocardial ischemia. N Engl J Med 1994; 330:1791.
- Miwa K, Nakagawa K, Hirai T, Inoue H. Exercise-induced U-wave alterations as a marker of well-developed and well-functioning collateral vessels in patients with effort angina. J Am Coll Cardiol 2000; 35:757–763.
- Rimmerman CM. A 62-year-old man with an abnormal electrocardiogram. Cleve Clin J Med 2001; 68:975–976.
- Kodama-Takahashi K, Ohshima K, Yamamoto K, et al. Occurrence of transient U-wave inversion during vasospastic anginal attack is not related to the direction of concurrent ST-segment shift. Chest 2002; 122:535–541.
In Reply: We appreciate the comments from Drs. Suksaranjit, Cheungpasitporn, Bischof, and Marx on our recent article on the negative U wave in a patient with chronic aortic regurgitation.1 The clinical data including electrocardiography, echocardiography, and coronary angiography were presented to emphasize the importance of identifying the negative U wave in the setting of valvular heart disease. We outlined the common differential diagnosis for a negative U wave (page 506). We believe that in the appropriate clinical setting the presence of a negative U wave provides diagnostic utility.
Several published reports to date have described the occurrence of the negative U wave in the setting of obstructive coronary artery disease2–5 or coronary artery vasospasm.6 We were unable to find similar data in the setting of demand ischemia in the presence of normal coronary arteries (functional ischemia), but we fully recognize its likely occurrence, and we value the helpful insight.
In Reply: We appreciate the comments from Drs. Suksaranjit, Cheungpasitporn, Bischof, and Marx on our recent article on the negative U wave in a patient with chronic aortic regurgitation.1 The clinical data including electrocardiography, echocardiography, and coronary angiography were presented to emphasize the importance of identifying the negative U wave in the setting of valvular heart disease. We outlined the common differential diagnosis for a negative U wave (page 506). We believe that in the appropriate clinical setting the presence of a negative U wave provides diagnostic utility.
Several published reports to date have described the occurrence of the negative U wave in the setting of obstructive coronary artery disease2–5 or coronary artery vasospasm.6 We were unable to find similar data in the setting of demand ischemia in the presence of normal coronary arteries (functional ischemia), but we fully recognize its likely occurrence, and we value the helpful insight.
- Venkatachalam S, Rimmerman CM. Electrocardiography in aortic regurgitation: it’s in the details. Cleve Clin J Med 2011; 78:505–506.
- Gerson MC, Phillips JF, Morris SN, McHenry PL. Exercise-induced U-wave inversion as a marker of stenosis of the left anterior descending coronary artery. Circulation 1979; 60:1014–1020.
- Galli M, Temporelli P. Images in clinical medicine. Negative U waves as an indicator of stress-induced myocardial ischemia. N Engl J Med 1994; 330:1791.
- Miwa K, Nakagawa K, Hirai T, Inoue H. Exercise-induced U-wave alterations as a marker of well-developed and well-functioning collateral vessels in patients with effort angina. J Am Coll Cardiol 2000; 35:757–763.
- Rimmerman CM. A 62-year-old man with an abnormal electrocardiogram. Cleve Clin J Med 2001; 68:975–976.
- Kodama-Takahashi K, Ohshima K, Yamamoto K, et al. Occurrence of transient U-wave inversion during vasospastic anginal attack is not related to the direction of concurrent ST-segment shift. Chest 2002; 122:535–541.
- Venkatachalam S, Rimmerman CM. Electrocardiography in aortic regurgitation: it’s in the details. Cleve Clin J Med 2011; 78:505–506.
- Gerson MC, Phillips JF, Morris SN, McHenry PL. Exercise-induced U-wave inversion as a marker of stenosis of the left anterior descending coronary artery. Circulation 1979; 60:1014–1020.
- Galli M, Temporelli P. Images in clinical medicine. Negative U waves as an indicator of stress-induced myocardial ischemia. N Engl J Med 1994; 330:1791.
- Miwa K, Nakagawa K, Hirai T, Inoue H. Exercise-induced U-wave alterations as a marker of well-developed and well-functioning collateral vessels in patients with effort angina. J Am Coll Cardiol 2000; 35:757–763.
- Rimmerman CM. A 62-year-old man with an abnormal electrocardiogram. Cleve Clin J Med 2001; 68:975–976.
- Kodama-Takahashi K, Ohshima K, Yamamoto K, et al. Occurrence of transient U-wave inversion during vasospastic anginal attack is not related to the direction of concurrent ST-segment shift. Chest 2002; 122:535–541.
Quality, frailty, and common sense
Congestive heart failure, as noted in the review by Samala et al in this issue of the Journal, is more prevalent in the elderly. Particularly in the frail elderly, managing severe congestive heart failure poses ethical, socioeconomic, and medical challenges. The presence of even subtle cognitive impairment requires detailed dialogue with family and caregivers about medications and about symptoms that warrant a trip to the emergency room. Patients on a fixed income may not be able to afford their medications and thus may use them sporadically. And the preprepared foods they often eat are laden with sodium.
The symptoms of congestive heart failure may easily go unrecognized or be attributed to other common problems. Sorting out the reasons for exertional fatigue, especially a generalized sense of fatigue, can be particularly vexing. Anemia and sarcopenia can directly cause exertional fatigue or “weakness” but may also exacerbate heart failure and cause similar symptoms. Pharmacologic and dietary causes for volume overload must be sought. Even intermittent use of over-the-counter nonsteroidal anti-inflammatory drugs can be problematic.
Severe congestive heart failure is a lethal disease. Current quality guidelines for its treatment emphasize the use of multiple drugs and devices. Yet vasoactive drugs may not be well tolerated in frail patients, who are particularly vulnerable to orthostatic hypotension and cerebral hypoperfusion. Digoxin, of marginal benefit in younger patients without tachyarrhythmias, has an even more tenuous risk-benefit ratio in the frail elderly. Beta-blockers may cause fatigue and depression, and even low-dose diuretics can exacerbate symptoms of bladder dysfunction. Previously implanted defibrillators may be inconsistent with the patient’s current end-of-life desires.
Ideal management of the genuinely frail elderly patient with severe congestive heart failure is not always a matter of ventricular assist devices, biventricular pacers, or angiotensin-converting enzyme inhibitors. At some point, referral to palliative care resources, guided by informed input from the patient, family members, and caregivers, may be the most appropriate high-quality care that we can (and should) offer.
Congestive heart failure, as noted in the review by Samala et al in this issue of the Journal, is more prevalent in the elderly. Particularly in the frail elderly, managing severe congestive heart failure poses ethical, socioeconomic, and medical challenges. The presence of even subtle cognitive impairment requires detailed dialogue with family and caregivers about medications and about symptoms that warrant a trip to the emergency room. Patients on a fixed income may not be able to afford their medications and thus may use them sporadically. And the preprepared foods they often eat are laden with sodium.
The symptoms of congestive heart failure may easily go unrecognized or be attributed to other common problems. Sorting out the reasons for exertional fatigue, especially a generalized sense of fatigue, can be particularly vexing. Anemia and sarcopenia can directly cause exertional fatigue or “weakness” but may also exacerbate heart failure and cause similar symptoms. Pharmacologic and dietary causes for volume overload must be sought. Even intermittent use of over-the-counter nonsteroidal anti-inflammatory drugs can be problematic.
Severe congestive heart failure is a lethal disease. Current quality guidelines for its treatment emphasize the use of multiple drugs and devices. Yet vasoactive drugs may not be well tolerated in frail patients, who are particularly vulnerable to orthostatic hypotension and cerebral hypoperfusion. Digoxin, of marginal benefit in younger patients without tachyarrhythmias, has an even more tenuous risk-benefit ratio in the frail elderly. Beta-blockers may cause fatigue and depression, and even low-dose diuretics can exacerbate symptoms of bladder dysfunction. Previously implanted defibrillators may be inconsistent with the patient’s current end-of-life desires.
Ideal management of the genuinely frail elderly patient with severe congestive heart failure is not always a matter of ventricular assist devices, biventricular pacers, or angiotensin-converting enzyme inhibitors. At some point, referral to palliative care resources, guided by informed input from the patient, family members, and caregivers, may be the most appropriate high-quality care that we can (and should) offer.
Congestive heart failure, as noted in the review by Samala et al in this issue of the Journal, is more prevalent in the elderly. Particularly in the frail elderly, managing severe congestive heart failure poses ethical, socioeconomic, and medical challenges. The presence of even subtle cognitive impairment requires detailed dialogue with family and caregivers about medications and about symptoms that warrant a trip to the emergency room. Patients on a fixed income may not be able to afford their medications and thus may use them sporadically. And the preprepared foods they often eat are laden with sodium.
The symptoms of congestive heart failure may easily go unrecognized or be attributed to other common problems. Sorting out the reasons for exertional fatigue, especially a generalized sense of fatigue, can be particularly vexing. Anemia and sarcopenia can directly cause exertional fatigue or “weakness” but may also exacerbate heart failure and cause similar symptoms. Pharmacologic and dietary causes for volume overload must be sought. Even intermittent use of over-the-counter nonsteroidal anti-inflammatory drugs can be problematic.
Severe congestive heart failure is a lethal disease. Current quality guidelines for its treatment emphasize the use of multiple drugs and devices. Yet vasoactive drugs may not be well tolerated in frail patients, who are particularly vulnerable to orthostatic hypotension and cerebral hypoperfusion. Digoxin, of marginal benefit in younger patients without tachyarrhythmias, has an even more tenuous risk-benefit ratio in the frail elderly. Beta-blockers may cause fatigue and depression, and even low-dose diuretics can exacerbate symptoms of bladder dysfunction. Previously implanted defibrillators may be inconsistent with the patient’s current end-of-life desires.
Ideal management of the genuinely frail elderly patient with severe congestive heart failure is not always a matter of ventricular assist devices, biventricular pacers, or angiotensin-converting enzyme inhibitors. At some point, referral to palliative care resources, guided by informed input from the patient, family members, and caregivers, may be the most appropriate high-quality care that we can (and should) offer.
Heart failure in frail, older patients: We can do ‘MORE’
Mr. R. is an 85-year-old with congestive heart failure; the last time his ejection fraction was measured it was 30%. He also has hypertension, coronary artery disease (for which he underwent triple-vessel coronary artery bypass grafting), osteoarthritis, hyperlipidemia, and chronic obstructive pulmonary disease. He currently takes lisinopril (Zestril), carvedilol (Coreg), aspirin, clopidogrel (Plavix), digoxin, simvastatin (Zocor), furosemide (Lasix), an albuterol inhaler (Proventil), and over-the-counter naproxen (Naprosyn), the last two taken as needed.
Accompanied by his daughter, Mr. R. comes to see his primary care physician for a routine follow-up visit. He says he feels fine and has no shortness of breath or chest pain, but he feels light-headed at times, especially when he gets out of bed. He also mentions that he is bothered with having to get up three to four times at night to urinate.
On further questioning, he relates that he uses a cane to walk around the house and gets short of breath when walking from his bed to the bathroom and from one room to the next. He can feed himself, but he needs assistance with bathing and getting dressed.
Mr. R. admits that he has been feeling lonely since his wife died about a year ago. He now lives with his daughter and her family, and they all get along well. His daughter mentions that over the last 6 months he has not been eating well, that he appears to have lost interest in doing some of the things that he used to enjoy, and that he has lost weight. She adds that he has fallen twice in the last month.
On physical examination, Mr. R. is without distress but appears weak. He answers all questions appropriately, although his affect is flat and his daughter fills in some of the details.
Supine, his blood pressure is 160/90 mm Hg and his heart rate is 75; immediately after standing up he feels dizzy and his blood pressure drops to 120/60 mm Hg with a heart rate of 110. Three months ago he weighed 155 pounds (70.3 kg); today he weighs 145 pounds (65.9 kg).
His neck veins are not distended. On chest auscultation, bibasilar coarse crackles are heard, as well as a systolic murmur (grade 2 on a scale of 6), loudest in the second intercostal space at the right parasternal border. No peripheral edema is detected. His Mini-Mental State Exam score is 22 out of 30.
What changes, if any, should be made in Mr. R.’s management? What advice should the primary care physician give Mr. R. and his daughter about the course of his heart failure?
THE IMPORTANCE OF COMPLETE CARE
Mr. R. has multiple convoluted medical issues that plague many elderly patients with heart failure. To provide optimal care to patients like him, physicians need to draw on knowledge from the fields of internal medicine, geriatrics, and cardiology.
In this paper, we discuss how diagnosing and managing heart failure is different in elderly patients. We emphasize the importance of complete care of frail elderly patients, highlighting the pharmacologic and nonpharmacologic interventions that are available. Finally, we will return to Mr. R. and discuss a comprehensive plan for him.
HEART FAILURE, FRAILTY, DISABILITY ARE ALL CONNECTED
The ability to bounce back from physical insults, chiefly medical illnesses, sharply declines in old age. As various stressors accumulate, physical deterioration becomes inevitable. While some older adults can avoid going down this path of morbidity, in an increasing number of frail elderly patients, congestive heart failure inescapably assumes a complicated course.
Frailty is a state of increased vulnerability to stressors due to age-related declines in physiologic reserve.1 Two elements intimately related to frailty are comorbidity and disability.
Fried et al2 analyzed data from more than 5,000 older men and women in the Cardiovascular Health Study and concluded that comorbidity (ie, having two or more chronic diseases) is a risk factor for frailty, which in turn results in disability, falls, hospitalizations, and death.
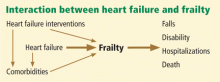
The relationship between congestive heart failure and frailty is complex. Not only does heart failure itself result in frailty, but its multiple therapies can put additional stress on a frail patient. In addition, the heart failure and its treatments can negatively affect coexisting disorders (Figure 1).
BY THE NUMBERS
Heart failure is largely a disorder of the elderly, and as the US population ages, heart failure is rising in prevalence to epidemic numbers.3 The median age of patients admitted to the hospital because of heart failure is 75,4 and patients age 65 and older account for more than 75% of heart failure hospitalizations.5 Every year, in every 1,000 people over age 65, nearly 10 new cases of heart failure are diagnosed.6
Before age 70, men are affected more than women, but the opposite is true at age 70 and beyond. The reason for this reversal is that women live longer and have a better prognosis, as the cause of heart failure in most women is diastolic dysfunction secondary to hypertension rather than systolic dysfunction due to coronary artery disease, as in most men.7
Heart failure is costly and generally has a poor prognosis. The total cost of treating it reached a staggering $37.2 billion in 2009, and it was the leading cause of Medicare hospital admissions.6 Heart failure is the primary cause or a contributory cause of death in about 290,000 patients each year, and the rate of death at 1 year is an astonishing 1 in 5.6 The median survival time after diagnosis is 2.3 to 3.6 years in patients ages 67 to 74, and it is considerably shorter—1.1 to 1.6 years—in patients age 85 and older.8
THE BROKEN HEART
In 2005, the American College of Cardiology and the American Heart Association defined congestive heart failure as “a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood.”9 This characterization captures the intricate nature of the disease: its spectrum of symptoms, its many causes (eg, coronary artery disease, hypertension, nonischemic or idiopathic cardiomyopathy, and valvular heart disease), and the dual pathophysiologic features of systolic and diastolic impairment.
Systolic vs diastolic failure
Of the various ways of classifying heart failure, the most important is systolic vs diastolic.
The hallmark of systolic heart failure is a decreased left ventricular ejection fraction, and it is characterized by a large thin-walled ventricle that is weak and unable to eject enough blood to generate a normal cardiac output.
In contrast, the ejection fraction is normal or nearly normal in diastolic heart failure, but the end-diastolic volume is decreased because the ventricle is hypertrophied and thick-walled. The resultant chamber has become small and stiff and does not have enough volume for sufficient cardiac output.
QUIRKS IN THE HISTORY AND PHYSICAL EXAMINATION
The combination of inactivity and coexisting illnesses in a frail older adult may obscure some of the usual clinical manifestations of heart failure. While shortness of breath on mild exertion, easy fatigability, and leg swelling are common in younger heart failure patients, these symptoms may be due to normal aging in a much older patient. Let us consider some important aspects of the common signs and symptoms associated with heart failure.
Dyspnea on exertion is one of the earliest and most prominent symptoms. The usual question asked of patients to elicit whether this key manifestation is present is, “Do you get short of breath after walking a block?” However, this question may not be appropriate for a frail elderly person whose activity is restricted by comorbidities such as severe arthritis, coronary artery disease, or peripheral arterial disease. For a patient like this, ask instead if he or she gets short of breath after milder forms of exertion, such as making the bed, walking to the bathroom, or changing clothes.10 Also, keep in mind that dyspnea on exertion may be due to other conditions, such as renal failure, lung disease, depression, anemia, or deconditioning.
Orthopnea and paroxysmal nocturnal dyspnea may not be volunteered or elicited if a patient is sleeping in a chair or a recliner.
Leg swelling is less specific in older adults than in younger patients because chronic venous insufficiency is common in older people.
Weight gain almost always accompanies symptomatic heart failure but may also be due to increased appetite secondary to depression.
A change in mental status is common in elderly people with heart failure, especially those with vascular dementia with extensive cerebrovascular atherosclerosis or those who have latent Alzheimer disease.10
Cough, a symptom of a multitude of disorders, may be an early or the only manifestation of heart failure.
Pulmonary crackles are typically detected in most heart failure patients, but they may not be as characteristic in older adults, as they may also be noted in bronchitis, pneumonia, and other chronic lung diseases.
Additional symptoms to watch for include fatigue, syncope, angina, nocturia, and oliguria.
The bottom line is to integrate individual findings with other elements of the history and physical examination in diagnosing heart failure and tracking its progression.
CLINCHING THE DIAGNOSIS
Congestive heart failure is essentially a clinical diagnosis best established even before ordering tests, especially during times and situations in which these tests are not always readily available, such as outside office hours and in a long-term care setting.
A reliable and thorough history and physical examination is the most important component of the diagnostic process.
An echocardiogram is obtained next to measure the ejection fraction, which has both prognostic and therapeutic significance. Echocardiography can also uncover potential contributory cardiac structural abnormalities.
A chest radiograph is also typically obtained to look for pulmonary congestion, but in older adults its interpretation may be skewed by chronic lung disease or spinal deformities such as scoliosis and kyphosis.
The B-type natriuretic peptide (BNP) level is a popular blood test. BNP is commonly elevated in patients with heart failure. However, an elevated level in older adults should always be evaluated within the context of other clinical findings, as it can also result from advancing age and diseases other than heart failure, such as coronary artery disease, chronic pulmonary disease, pulmonary embolism, and renal insufficiency.11,12
PHARMACOTHERAPY PEARLS
Drug treatment for heart failure has evolved rapidly. Robust and sophisticated clinical trials have led to guidelines that call for specific medications. Unfortunately, older patients, particularly the very old and frail, have been poorly represented in these studies.9 Nonetheless, the type and choice of drugs for the young and old are similar.
Take into account age-associated changes in pharmacokinetics
Age-associated changes in pharmacokinetics must be taken into account when prescribing drugs for heart failure.13
Oral absorption of cardiovascular drugs is not significantly affected by the various changes that occur in older adults (eg, reduced gastric acid production, gastric emptying rate, gastrointestinal blood flow, and mobility). However, reductions in both lean body mass and total body water that come with aging result in lower volumes of distribution and higher plasma concentrations of hydrophilic drugs, most notably angiotensin-converting enzyme (ACE) inhibitors and digoxin. In contrast, the plasma concentrations of lipophilic drugs such as beta-blockers and central alpha-agonists tend to decrease as the proportion of body fat increases in older adults.
As the plasma albumin level diminishes with age, the free-drug concentration of salicylates and warfarin (Coumadin), which are extensively albumin-bound, may increase.
The serum concentrations of cardiovascular drugs metabolized in the liver—eg, propranolol (Inderal), lidocaine, labetalol (Trandate), verapamil (Calan), diltiazem (Cardizem), nitrates, and warfarin—may be elevated due to reduced hepatic blood flow, mass, volume, and overall metabolic capacity.
Declines in renal blood flow, glomerular filtration, and tubular function may cause accumulation of drugs that are excreted through the kidneys.
Beware of toxicities
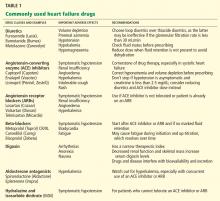
Table 1 lists some of the drugs used in treating heart failure, common adverse affects to watch for, and recommendations for their use.
WIELDING THE SCALPEL
A tenet of heart failure management is to correct the underlying cardiac structural abnormality. This often calls for invasive intervention along with optimization of drug therapy.
For example:
- Diseased coronary arteries may be amenable to revascularization, either by percutaneous coronary intervention or by the much more involved coronary artery bypass grafting, with the aim of enhancing cardiac function.
- Valves can be repaired or replaced in patients with valvular heart disease.
- A pacemaker can be implanted to remedy sick sinus syndrome, especially with concurrent use of heart-rate-lowering agents such as beta-blockers.
- Placement of an implantable cardioverter-defibrillator has been found to be effective in preventing death due to ventricular tachyarrhythmias in patients with an ejection fraction of less than 30%.9
- Cardiac resynchronization with a biventricular pacemaker may increase the ejection fraction and cardiac output by eliminating dyssynchronous contraction of the left and right ventricles.14
In frail older adults, consideration of these invasive therapies must be individualized. While procedures such as percutaneous coronary intervention and pacemaker placement may not be as physically taxing as bypass grafting or valve replacement, the potential for surgical complications must be seriously considered, particularly if the patient has diminished physiologic reserve. Case-to-case consideration is also crucial in cardioverter-defibrillator insertion, as the survival benefit may be diminished in older adults, who likely have coexisting illnesses that predispose them to die of a noncardiac cause.15,16
The bottom line is to contemplate multiple factors—severity of the heart failure, comorbidities, baseline functional status, and social support—when assessing the appropriateness of an invasive intervention.
BEYOND DRUGS AND DEVICES: WE CAN DO ‘MORE’
Much of the spotlight has been on the various drugs and devices used to treat heart failure, but of equal importance for frail elderly patients are complementary approaches that can be used to ease disease progression and boost the quality of life. The acronym MORE highlights these strategies.
M: Multidisciplinary management programs
Heart failure disease-management programs are designed to provide comprehensive multidisciplinary care across different settings (ie, home, outpatient, and inpatient) to high-risk patients who often have multiple medical, social, and behavioral issues.9 Interventions usually include intensive patient education, encouraging patients to be more aggressive participants in their care, closely monitoring patients through telephone follow-up or home nursing, carefully reviewing medications to improve adherence to evidence-based guidelines, and multidisciplinary care with nurse case management directed by a physician.
Studies have shown that management programs, which were largely nurse-directed and targeted at older adults and patients with advanced disease, can improve quality of life and functional status, decrease hospitalizations for both heart failure and other causes, and decrease medical costs.17–19
O: Other diseases
R: Restrictions
Specific limitations in the intake of certain dietary elements are a valuable adjunct in heart failure management.
Sodium intake should be restricted to less than 3 g/day by not adding salt to meals and by avoiding salt-rich foods (eg, canned and processed foods).24 During times of distressing volume overload, a tighter sodium limit of 2 g/day is necessary, and diuretics may be less effective if this restriction is not implemented.
Fluid restriction depends on the patient’s clinical status.25 While it is not necessary to limit fluid intake in the absence of retention, a limit of 2 L/day is recommended if edema is detected. If volume overload is severe, the limit should be 1 L/day.
Alcohol is a myocardial depressant that reduces the left ventricular ejection fraction.26 Abstinence is a must for patients with alcohol-induced heart failure; otherwise, a limit of 1 drink (8 oz of beer, 4 oz of wine, or 1 oz of hard liquor) per day is suggested.24
Calories and fat intake are both important to watch, particularly in patients with obesity, hyperlipidemia, hypertension, or coronary artery disease.
E: End-of-life issues
Usual causes of death in patients with heart failure include sudden cardiac death, arrhythmias, hypotension, end-organ hypoperfusion, and metabolic derangement.27,28
Given the life-limiting nature of the disease in frail older adults, it is very important for clinicians to discuss end-of-life matters with patients and their families as early as possible. Needed are effective communication skills that foster respect, empathy, and mutual understanding.
Advance directives. The primary task is to encourage patients to develop advance health directives. These are legal documents that represent patients’ preferences about interventions available toward the end of life such as do-not-resuscitate orders, appointment of surrogate decision-makers, and use of life-sustaining interventions (eg, a feeding tube, dialysis, blood transfusions). Establishing these directives early on will help ease the transition from one mode of care to another (eg, from acute care to hospice care), prevent pointless use of resources (eg, emergency room visits, hospital admissions), and ensure that the patient’s wishes are carried out.
Palliative measures that aim to alleviate suffering and promote quality of life and dignity are available for patients with severe symptoms. For varying degrees of dyspnea, diuretics, nitrates, morphine, and positive inotropic agents such as dobutamine (Dobutrex) and milrinone (Primacor) can be tried. Thoracentesis is done in patients with extensive pleural effusion. Fatigue and anorexia are due to a combination of factors, namely, decreased cardiac output, increased neurohormone levels, deconditioning, depression, decreased sleep, and anxiety.29 Opioids, caffeine, exercise, oxygen, fluid and salt restriction, and correction of anemia and depression may help ease these symptoms.
For patients with an implantable cardioverter-defibrillator, deactivation is an important matter that needs to be addressed. Deactivation can be carried out with certainty once the goal of care has shifted away from curative efforts and either the patient or a surrogate decision-maker has made the informed decision to turn the device off. Berger30 raised three points that the clinician and decision-maker can discuss in trying to achieve a resolution during times of doubt and indecision:
- The patient may no longer value continued survival
- The device may no longer offer the prospect of increased survival
- The device may impede active dying.
The idea of hospice care should be gradually and gently explored to ensure a prompt and seamless transition when the time comes. The patient and family need to know that the goal of hospice care is to ensure comfort and that they can benefit the most by enrolling early during the course of the terminal illness.
The Medicare hospice benefit is granted to patients who have been certified by two physicians to have a life expectancy of 6 months or less if their terminal illness runs its natural course. The criteria for determining that heart failure is terminal are:
- New York Heart Association class III (symptomatic with less than ordinary activities) or IV (symptomatic at rest)
- Left ventricular ejection fraction less than or equal to 20%
- Persistent symptoms despite optimal medical management
- Inability to tolerate optional management due to hypotension with or without renal failure.31
WHAT CAN WE DO FOR MR. R.?
Mr. R. has systolic heart failure stemming from coronary artery disease, and his symptoms put him in New York Heart Association class III. He is well managed with drugs of different appropriate classes: an ACE inhibitor, a beta-blocker, digoxin, an aldosterone antagonist, and a diuretic. His other drugs all have well-defined indications.
Since he does not have fluid overload, his furosemide can be stopped, and this change will likely relieve his orthostatic hypotension and nocturia. His systolic blood pressure target can be liberalized to 150 mm Hg or less, as tighter control might exacerbate orthostatic hypotension. This change, along with having him start using a walker instead of a cane, will hopefully prevent future falls. Furthermore, his naproxen should be discontinued, as it can worsen heart failure.
Mr. R. has symptoms of depression and thus needs to be started on an antidepressant and encouraged to engage in social activities as much as he can tolerate. These interventions may also help with his mild dementia, which is evidenced by a Mini-Mental State Exam score of 22. He will not benefit from sodium and fat restriction, as he has actually been losing weight.
To keep Mr. R.’s cognitive impairment and overall decline in function from compromising his compliance with his treatment, he will need a substantial amount of assistance, which his daughter alone may not be able to provide. To tackle this concern, a discussion about participating in a heart failure management program can be started with Mr. R. and his family.
More importantly, his advanced directives, including delegating a surrogate decision-maker and deciding on do-not-resuscitate status, have to be clarified. Finally, it would be prudent to introduce the concept of hospice care to the patient and his daughter while he is still coherent and able to state his preferences.
- Walston J, Hadley EC, Ferruci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006; 54:991–1001.
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol 1992; 20:301–306.
- Popovic JR, 1999 National Hospital Discharge Survey: annual summary with detailed diagnosis and procedure data. National Center for Health Statistics. Vital Health Stat 2001; 13:1–206.
- DeFrances CJ, Hall MJ, Podgornik MN. 2003 National Hospital Discharge Survey. Advance data from vital and health statistics; no. 359. Hyattsville (MD): National Center for Health Statistics, 2005.
- American Heart Association. Heart disease and stroke statistics—2009 update: a report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:e21–e181.
- Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA 1996; 275:1557–1562.
- Croft JB, Giles WH, Pollard RA, et al. Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Arch Intern Med 1999; 159:505–510.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). Circulation 2005; 112:e154–e235.
- Ahmed A. Clinical manifestations, diagnostic assessment, and etiology of heart failure in older adults. Clin Geriatr Med 2007; 23:11–30.
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002; 40:976–982.
- Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002; 90:254–258.
- Aronow WS, Frishman WH, Cheng-Lai A. Cardiovascular drug therapy in the elderly. Cardiol Rev 2007; 15:195–215.
- Bakker P, Meijburg H, de Bries J, et al. Biventricular pacing in end-stage heart failure improves functional capacity and left ventricular function. J Interv Card Electrophysiol 2000; 4:395–404.
- Healey JS, Hallstrom AP, Kuck KH, et al. Role of the implantable defibrillator among elderly patients with a history of life-threatening ventricular arrhythmias. Eur Heart J 2007; 28:1746–1749.
- Lee DS, Tu JV, Austin PC, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol 2007; 49:2408–2415.
- Rich MW, Beckham V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995; 333:1190–1195.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- McAlister F, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol 2004; 44:810–819.
- Horwich TB, Fonarow GC, Hamilton MA, et al. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 2002; 39:1780–1786.
- Al-Ahmad A, Rand WM, Manjunath G, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 2001; 38:955–962.
- Singh SN, Fisher SG, Deedwania PC, et al. Pulmonary effect of amiodarone in patients with heart failure: the Congestive Heart Failure-Survival Trial of Antiarrhythmic Therapy (CHF-STAT) Investigators (Veterans Affairs Cooperative Study No. 320). J Am Coll Cardiol 1997; 30:514–517.
- Cohen MB, Mather PJ. A review of the association between congestive heart failure and cognitive impairment. Am J Geriatr Cardiol 2007; 16:171–174.
- Dracup K, Baker DW, Dunbar SB, et al. Management of heart failure. II. Counseling, education, and lifestyle modifications. JAMA 1994; 272:1442–1446.
- Lenihan DJ, Uretsky BF. Non-pharmacologic treatment of heart failure in the elderly. Clin Geriatr Med 2000; 16:477–488.
- Regan TJ. Alcohol and the cardiovascular system. JAMA 1990; 264:377–381.
- Teuteberg JJ, Lewis EF, Nohria A, et al. Characteristics of patients who die with heart failure and a low ejection fraction in the new millennium. J Card Fail 2006; 12:47–53.
- Derfler MC, Jacob M, Wolf RE, et al. Mode of death from congestive heart failure: implications for clinical management. Am J Geriatr Cardiol 2004; 13:299–304.
- Evangelista LS, Moser DK, Westlake C, et al. Correlates of fatigue in patients with heart failure. Prog Cardiovasc Nurs 2008; 23:12–17.
- Berger JT. The ethics of deactivating implanted cardioverter defibrillators. Ann Intern Med 2005; 142:631–634.
- Stuart B, Connor S, Kinzbrunner BM, et al. Medical guidelines for determining prognosis in selected non-cancer diseases, 2nd ed. Arlington VA, National Hospice Organization; 1996.
Mr. R. is an 85-year-old with congestive heart failure; the last time his ejection fraction was measured it was 30%. He also has hypertension, coronary artery disease (for which he underwent triple-vessel coronary artery bypass grafting), osteoarthritis, hyperlipidemia, and chronic obstructive pulmonary disease. He currently takes lisinopril (Zestril), carvedilol (Coreg), aspirin, clopidogrel (Plavix), digoxin, simvastatin (Zocor), furosemide (Lasix), an albuterol inhaler (Proventil), and over-the-counter naproxen (Naprosyn), the last two taken as needed.
Accompanied by his daughter, Mr. R. comes to see his primary care physician for a routine follow-up visit. He says he feels fine and has no shortness of breath or chest pain, but he feels light-headed at times, especially when he gets out of bed. He also mentions that he is bothered with having to get up three to four times at night to urinate.
On further questioning, he relates that he uses a cane to walk around the house and gets short of breath when walking from his bed to the bathroom and from one room to the next. He can feed himself, but he needs assistance with bathing and getting dressed.
Mr. R. admits that he has been feeling lonely since his wife died about a year ago. He now lives with his daughter and her family, and they all get along well. His daughter mentions that over the last 6 months he has not been eating well, that he appears to have lost interest in doing some of the things that he used to enjoy, and that he has lost weight. She adds that he has fallen twice in the last month.
On physical examination, Mr. R. is without distress but appears weak. He answers all questions appropriately, although his affect is flat and his daughter fills in some of the details.
Supine, his blood pressure is 160/90 mm Hg and his heart rate is 75; immediately after standing up he feels dizzy and his blood pressure drops to 120/60 mm Hg with a heart rate of 110. Three months ago he weighed 155 pounds (70.3 kg); today he weighs 145 pounds (65.9 kg).
His neck veins are not distended. On chest auscultation, bibasilar coarse crackles are heard, as well as a systolic murmur (grade 2 on a scale of 6), loudest in the second intercostal space at the right parasternal border. No peripheral edema is detected. His Mini-Mental State Exam score is 22 out of 30.
What changes, if any, should be made in Mr. R.’s management? What advice should the primary care physician give Mr. R. and his daughter about the course of his heart failure?
THE IMPORTANCE OF COMPLETE CARE
Mr. R. has multiple convoluted medical issues that plague many elderly patients with heart failure. To provide optimal care to patients like him, physicians need to draw on knowledge from the fields of internal medicine, geriatrics, and cardiology.
In this paper, we discuss how diagnosing and managing heart failure is different in elderly patients. We emphasize the importance of complete care of frail elderly patients, highlighting the pharmacologic and nonpharmacologic interventions that are available. Finally, we will return to Mr. R. and discuss a comprehensive plan for him.
HEART FAILURE, FRAILTY, DISABILITY ARE ALL CONNECTED
The ability to bounce back from physical insults, chiefly medical illnesses, sharply declines in old age. As various stressors accumulate, physical deterioration becomes inevitable. While some older adults can avoid going down this path of morbidity, in an increasing number of frail elderly patients, congestive heart failure inescapably assumes a complicated course.
Frailty is a state of increased vulnerability to stressors due to age-related declines in physiologic reserve.1 Two elements intimately related to frailty are comorbidity and disability.
Fried et al2 analyzed data from more than 5,000 older men and women in the Cardiovascular Health Study and concluded that comorbidity (ie, having two or more chronic diseases) is a risk factor for frailty, which in turn results in disability, falls, hospitalizations, and death.
The relationship between congestive heart failure and frailty is complex. Not only does heart failure itself result in frailty, but its multiple therapies can put additional stress on a frail patient. In addition, the heart failure and its treatments can negatively affect coexisting disorders (Figure 1).
BY THE NUMBERS
Heart failure is largely a disorder of the elderly, and as the US population ages, heart failure is rising in prevalence to epidemic numbers.3 The median age of patients admitted to the hospital because of heart failure is 75,4 and patients age 65 and older account for more than 75% of heart failure hospitalizations.5 Every year, in every 1,000 people over age 65, nearly 10 new cases of heart failure are diagnosed.6
Before age 70, men are affected more than women, but the opposite is true at age 70 and beyond. The reason for this reversal is that women live longer and have a better prognosis, as the cause of heart failure in most women is diastolic dysfunction secondary to hypertension rather than systolic dysfunction due to coronary artery disease, as in most men.7
Heart failure is costly and generally has a poor prognosis. The total cost of treating it reached a staggering $37.2 billion in 2009, and it was the leading cause of Medicare hospital admissions.6 Heart failure is the primary cause or a contributory cause of death in about 290,000 patients each year, and the rate of death at 1 year is an astonishing 1 in 5.6 The median survival time after diagnosis is 2.3 to 3.6 years in patients ages 67 to 74, and it is considerably shorter—1.1 to 1.6 years—in patients age 85 and older.8
THE BROKEN HEART
In 2005, the American College of Cardiology and the American Heart Association defined congestive heart failure as “a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood.”9 This characterization captures the intricate nature of the disease: its spectrum of symptoms, its many causes (eg, coronary artery disease, hypertension, nonischemic or idiopathic cardiomyopathy, and valvular heart disease), and the dual pathophysiologic features of systolic and diastolic impairment.
Systolic vs diastolic failure
Of the various ways of classifying heart failure, the most important is systolic vs diastolic.
The hallmark of systolic heart failure is a decreased left ventricular ejection fraction, and it is characterized by a large thin-walled ventricle that is weak and unable to eject enough blood to generate a normal cardiac output.
In contrast, the ejection fraction is normal or nearly normal in diastolic heart failure, but the end-diastolic volume is decreased because the ventricle is hypertrophied and thick-walled. The resultant chamber has become small and stiff and does not have enough volume for sufficient cardiac output.
QUIRKS IN THE HISTORY AND PHYSICAL EXAMINATION
The combination of inactivity and coexisting illnesses in a frail older adult may obscure some of the usual clinical manifestations of heart failure. While shortness of breath on mild exertion, easy fatigability, and leg swelling are common in younger heart failure patients, these symptoms may be due to normal aging in a much older patient. Let us consider some important aspects of the common signs and symptoms associated with heart failure.
Dyspnea on exertion is one of the earliest and most prominent symptoms. The usual question asked of patients to elicit whether this key manifestation is present is, “Do you get short of breath after walking a block?” However, this question may not be appropriate for a frail elderly person whose activity is restricted by comorbidities such as severe arthritis, coronary artery disease, or peripheral arterial disease. For a patient like this, ask instead if he or she gets short of breath after milder forms of exertion, such as making the bed, walking to the bathroom, or changing clothes.10 Also, keep in mind that dyspnea on exertion may be due to other conditions, such as renal failure, lung disease, depression, anemia, or deconditioning.
Orthopnea and paroxysmal nocturnal dyspnea may not be volunteered or elicited if a patient is sleeping in a chair or a recliner.
Leg swelling is less specific in older adults than in younger patients because chronic venous insufficiency is common in older people.
Weight gain almost always accompanies symptomatic heart failure but may also be due to increased appetite secondary to depression.
A change in mental status is common in elderly people with heart failure, especially those with vascular dementia with extensive cerebrovascular atherosclerosis or those who have latent Alzheimer disease.10
Cough, a symptom of a multitude of disorders, may be an early or the only manifestation of heart failure.
Pulmonary crackles are typically detected in most heart failure patients, but they may not be as characteristic in older adults, as they may also be noted in bronchitis, pneumonia, and other chronic lung diseases.
Additional symptoms to watch for include fatigue, syncope, angina, nocturia, and oliguria.
The bottom line is to integrate individual findings with other elements of the history and physical examination in diagnosing heart failure and tracking its progression.
CLINCHING THE DIAGNOSIS
Congestive heart failure is essentially a clinical diagnosis best established even before ordering tests, especially during times and situations in which these tests are not always readily available, such as outside office hours and in a long-term care setting.
A reliable and thorough history and physical examination is the most important component of the diagnostic process.
An echocardiogram is obtained next to measure the ejection fraction, which has both prognostic and therapeutic significance. Echocardiography can also uncover potential contributory cardiac structural abnormalities.
A chest radiograph is also typically obtained to look for pulmonary congestion, but in older adults its interpretation may be skewed by chronic lung disease or spinal deformities such as scoliosis and kyphosis.
The B-type natriuretic peptide (BNP) level is a popular blood test. BNP is commonly elevated in patients with heart failure. However, an elevated level in older adults should always be evaluated within the context of other clinical findings, as it can also result from advancing age and diseases other than heart failure, such as coronary artery disease, chronic pulmonary disease, pulmonary embolism, and renal insufficiency.11,12
PHARMACOTHERAPY PEARLS
Drug treatment for heart failure has evolved rapidly. Robust and sophisticated clinical trials have led to guidelines that call for specific medications. Unfortunately, older patients, particularly the very old and frail, have been poorly represented in these studies.9 Nonetheless, the type and choice of drugs for the young and old are similar.
Take into account age-associated changes in pharmacokinetics
Age-associated changes in pharmacokinetics must be taken into account when prescribing drugs for heart failure.13
Oral absorption of cardiovascular drugs is not significantly affected by the various changes that occur in older adults (eg, reduced gastric acid production, gastric emptying rate, gastrointestinal blood flow, and mobility). However, reductions in both lean body mass and total body water that come with aging result in lower volumes of distribution and higher plasma concentrations of hydrophilic drugs, most notably angiotensin-converting enzyme (ACE) inhibitors and digoxin. In contrast, the plasma concentrations of lipophilic drugs such as beta-blockers and central alpha-agonists tend to decrease as the proportion of body fat increases in older adults.
As the plasma albumin level diminishes with age, the free-drug concentration of salicylates and warfarin (Coumadin), which are extensively albumin-bound, may increase.
The serum concentrations of cardiovascular drugs metabolized in the liver—eg, propranolol (Inderal), lidocaine, labetalol (Trandate), verapamil (Calan), diltiazem (Cardizem), nitrates, and warfarin—may be elevated due to reduced hepatic blood flow, mass, volume, and overall metabolic capacity.
Declines in renal blood flow, glomerular filtration, and tubular function may cause accumulation of drugs that are excreted through the kidneys.
Beware of toxicities
Table 1 lists some of the drugs used in treating heart failure, common adverse affects to watch for, and recommendations for their use.
WIELDING THE SCALPEL
A tenet of heart failure management is to correct the underlying cardiac structural abnormality. This often calls for invasive intervention along with optimization of drug therapy.
For example:
- Diseased coronary arteries may be amenable to revascularization, either by percutaneous coronary intervention or by the much more involved coronary artery bypass grafting, with the aim of enhancing cardiac function.
- Valves can be repaired or replaced in patients with valvular heart disease.
- A pacemaker can be implanted to remedy sick sinus syndrome, especially with concurrent use of heart-rate-lowering agents such as beta-blockers.
- Placement of an implantable cardioverter-defibrillator has been found to be effective in preventing death due to ventricular tachyarrhythmias in patients with an ejection fraction of less than 30%.9
- Cardiac resynchronization with a biventricular pacemaker may increase the ejection fraction and cardiac output by eliminating dyssynchronous contraction of the left and right ventricles.14
In frail older adults, consideration of these invasive therapies must be individualized. While procedures such as percutaneous coronary intervention and pacemaker placement may not be as physically taxing as bypass grafting or valve replacement, the potential for surgical complications must be seriously considered, particularly if the patient has diminished physiologic reserve. Case-to-case consideration is also crucial in cardioverter-defibrillator insertion, as the survival benefit may be diminished in older adults, who likely have coexisting illnesses that predispose them to die of a noncardiac cause.15,16
The bottom line is to contemplate multiple factors—severity of the heart failure, comorbidities, baseline functional status, and social support—when assessing the appropriateness of an invasive intervention.
BEYOND DRUGS AND DEVICES: WE CAN DO ‘MORE’
Much of the spotlight has been on the various drugs and devices used to treat heart failure, but of equal importance for frail elderly patients are complementary approaches that can be used to ease disease progression and boost the quality of life. The acronym MORE highlights these strategies.
M: Multidisciplinary management programs
Heart failure disease-management programs are designed to provide comprehensive multidisciplinary care across different settings (ie, home, outpatient, and inpatient) to high-risk patients who often have multiple medical, social, and behavioral issues.9 Interventions usually include intensive patient education, encouraging patients to be more aggressive participants in their care, closely monitoring patients through telephone follow-up or home nursing, carefully reviewing medications to improve adherence to evidence-based guidelines, and multidisciplinary care with nurse case management directed by a physician.
Studies have shown that management programs, which were largely nurse-directed and targeted at older adults and patients with advanced disease, can improve quality of life and functional status, decrease hospitalizations for both heart failure and other causes, and decrease medical costs.17–19
O: Other diseases
R: Restrictions
Specific limitations in the intake of certain dietary elements are a valuable adjunct in heart failure management.
Sodium intake should be restricted to less than 3 g/day by not adding salt to meals and by avoiding salt-rich foods (eg, canned and processed foods).24 During times of distressing volume overload, a tighter sodium limit of 2 g/day is necessary, and diuretics may be less effective if this restriction is not implemented.
Fluid restriction depends on the patient’s clinical status.25 While it is not necessary to limit fluid intake in the absence of retention, a limit of 2 L/day is recommended if edema is detected. If volume overload is severe, the limit should be 1 L/day.
Alcohol is a myocardial depressant that reduces the left ventricular ejection fraction.26 Abstinence is a must for patients with alcohol-induced heart failure; otherwise, a limit of 1 drink (8 oz of beer, 4 oz of wine, or 1 oz of hard liquor) per day is suggested.24
Calories and fat intake are both important to watch, particularly in patients with obesity, hyperlipidemia, hypertension, or coronary artery disease.
E: End-of-life issues
Usual causes of death in patients with heart failure include sudden cardiac death, arrhythmias, hypotension, end-organ hypoperfusion, and metabolic derangement.27,28
Given the life-limiting nature of the disease in frail older adults, it is very important for clinicians to discuss end-of-life matters with patients and their families as early as possible. Needed are effective communication skills that foster respect, empathy, and mutual understanding.
Advance directives. The primary task is to encourage patients to develop advance health directives. These are legal documents that represent patients’ preferences about interventions available toward the end of life such as do-not-resuscitate orders, appointment of surrogate decision-makers, and use of life-sustaining interventions (eg, a feeding tube, dialysis, blood transfusions). Establishing these directives early on will help ease the transition from one mode of care to another (eg, from acute care to hospice care), prevent pointless use of resources (eg, emergency room visits, hospital admissions), and ensure that the patient’s wishes are carried out.
Palliative measures that aim to alleviate suffering and promote quality of life and dignity are available for patients with severe symptoms. For varying degrees of dyspnea, diuretics, nitrates, morphine, and positive inotropic agents such as dobutamine (Dobutrex) and milrinone (Primacor) can be tried. Thoracentesis is done in patients with extensive pleural effusion. Fatigue and anorexia are due to a combination of factors, namely, decreased cardiac output, increased neurohormone levels, deconditioning, depression, decreased sleep, and anxiety.29 Opioids, caffeine, exercise, oxygen, fluid and salt restriction, and correction of anemia and depression may help ease these symptoms.
For patients with an implantable cardioverter-defibrillator, deactivation is an important matter that needs to be addressed. Deactivation can be carried out with certainty once the goal of care has shifted away from curative efforts and either the patient or a surrogate decision-maker has made the informed decision to turn the device off. Berger30 raised three points that the clinician and decision-maker can discuss in trying to achieve a resolution during times of doubt and indecision:
- The patient may no longer value continued survival
- The device may no longer offer the prospect of increased survival
- The device may impede active dying.
The idea of hospice care should be gradually and gently explored to ensure a prompt and seamless transition when the time comes. The patient and family need to know that the goal of hospice care is to ensure comfort and that they can benefit the most by enrolling early during the course of the terminal illness.
The Medicare hospice benefit is granted to patients who have been certified by two physicians to have a life expectancy of 6 months or less if their terminal illness runs its natural course. The criteria for determining that heart failure is terminal are:
- New York Heart Association class III (symptomatic with less than ordinary activities) or IV (symptomatic at rest)
- Left ventricular ejection fraction less than or equal to 20%
- Persistent symptoms despite optimal medical management
- Inability to tolerate optional management due to hypotension with or without renal failure.31
WHAT CAN WE DO FOR MR. R.?
Mr. R. has systolic heart failure stemming from coronary artery disease, and his symptoms put him in New York Heart Association class III. He is well managed with drugs of different appropriate classes: an ACE inhibitor, a beta-blocker, digoxin, an aldosterone antagonist, and a diuretic. His other drugs all have well-defined indications.
Since he does not have fluid overload, his furosemide can be stopped, and this change will likely relieve his orthostatic hypotension and nocturia. His systolic blood pressure target can be liberalized to 150 mm Hg or less, as tighter control might exacerbate orthostatic hypotension. This change, along with having him start using a walker instead of a cane, will hopefully prevent future falls. Furthermore, his naproxen should be discontinued, as it can worsen heart failure.
Mr. R. has symptoms of depression and thus needs to be started on an antidepressant and encouraged to engage in social activities as much as he can tolerate. These interventions may also help with his mild dementia, which is evidenced by a Mini-Mental State Exam score of 22. He will not benefit from sodium and fat restriction, as he has actually been losing weight.
To keep Mr. R.’s cognitive impairment and overall decline in function from compromising his compliance with his treatment, he will need a substantial amount of assistance, which his daughter alone may not be able to provide. To tackle this concern, a discussion about participating in a heart failure management program can be started with Mr. R. and his family.
More importantly, his advanced directives, including delegating a surrogate decision-maker and deciding on do-not-resuscitate status, have to be clarified. Finally, it would be prudent to introduce the concept of hospice care to the patient and his daughter while he is still coherent and able to state his preferences.
Mr. R. is an 85-year-old with congestive heart failure; the last time his ejection fraction was measured it was 30%. He also has hypertension, coronary artery disease (for which he underwent triple-vessel coronary artery bypass grafting), osteoarthritis, hyperlipidemia, and chronic obstructive pulmonary disease. He currently takes lisinopril (Zestril), carvedilol (Coreg), aspirin, clopidogrel (Plavix), digoxin, simvastatin (Zocor), furosemide (Lasix), an albuterol inhaler (Proventil), and over-the-counter naproxen (Naprosyn), the last two taken as needed.
Accompanied by his daughter, Mr. R. comes to see his primary care physician for a routine follow-up visit. He says he feels fine and has no shortness of breath or chest pain, but he feels light-headed at times, especially when he gets out of bed. He also mentions that he is bothered with having to get up three to four times at night to urinate.
On further questioning, he relates that he uses a cane to walk around the house and gets short of breath when walking from his bed to the bathroom and from one room to the next. He can feed himself, but he needs assistance with bathing and getting dressed.
Mr. R. admits that he has been feeling lonely since his wife died about a year ago. He now lives with his daughter and her family, and they all get along well. His daughter mentions that over the last 6 months he has not been eating well, that he appears to have lost interest in doing some of the things that he used to enjoy, and that he has lost weight. She adds that he has fallen twice in the last month.
On physical examination, Mr. R. is without distress but appears weak. He answers all questions appropriately, although his affect is flat and his daughter fills in some of the details.
Supine, his blood pressure is 160/90 mm Hg and his heart rate is 75; immediately after standing up he feels dizzy and his blood pressure drops to 120/60 mm Hg with a heart rate of 110. Three months ago he weighed 155 pounds (70.3 kg); today he weighs 145 pounds (65.9 kg).
His neck veins are not distended. On chest auscultation, bibasilar coarse crackles are heard, as well as a systolic murmur (grade 2 on a scale of 6), loudest in the second intercostal space at the right parasternal border. No peripheral edema is detected. His Mini-Mental State Exam score is 22 out of 30.
What changes, if any, should be made in Mr. R.’s management? What advice should the primary care physician give Mr. R. and his daughter about the course of his heart failure?
THE IMPORTANCE OF COMPLETE CARE
Mr. R. has multiple convoluted medical issues that plague many elderly patients with heart failure. To provide optimal care to patients like him, physicians need to draw on knowledge from the fields of internal medicine, geriatrics, and cardiology.
In this paper, we discuss how diagnosing and managing heart failure is different in elderly patients. We emphasize the importance of complete care of frail elderly patients, highlighting the pharmacologic and nonpharmacologic interventions that are available. Finally, we will return to Mr. R. and discuss a comprehensive plan for him.
HEART FAILURE, FRAILTY, DISABILITY ARE ALL CONNECTED
The ability to bounce back from physical insults, chiefly medical illnesses, sharply declines in old age. As various stressors accumulate, physical deterioration becomes inevitable. While some older adults can avoid going down this path of morbidity, in an increasing number of frail elderly patients, congestive heart failure inescapably assumes a complicated course.
Frailty is a state of increased vulnerability to stressors due to age-related declines in physiologic reserve.1 Two elements intimately related to frailty are comorbidity and disability.
Fried et al2 analyzed data from more than 5,000 older men and women in the Cardiovascular Health Study and concluded that comorbidity (ie, having two or more chronic diseases) is a risk factor for frailty, which in turn results in disability, falls, hospitalizations, and death.
The relationship between congestive heart failure and frailty is complex. Not only does heart failure itself result in frailty, but its multiple therapies can put additional stress on a frail patient. In addition, the heart failure and its treatments can negatively affect coexisting disorders (Figure 1).
BY THE NUMBERS
Heart failure is largely a disorder of the elderly, and as the US population ages, heart failure is rising in prevalence to epidemic numbers.3 The median age of patients admitted to the hospital because of heart failure is 75,4 and patients age 65 and older account for more than 75% of heart failure hospitalizations.5 Every year, in every 1,000 people over age 65, nearly 10 new cases of heart failure are diagnosed.6
Before age 70, men are affected more than women, but the opposite is true at age 70 and beyond. The reason for this reversal is that women live longer and have a better prognosis, as the cause of heart failure in most women is diastolic dysfunction secondary to hypertension rather than systolic dysfunction due to coronary artery disease, as in most men.7
Heart failure is costly and generally has a poor prognosis. The total cost of treating it reached a staggering $37.2 billion in 2009, and it was the leading cause of Medicare hospital admissions.6 Heart failure is the primary cause or a contributory cause of death in about 290,000 patients each year, and the rate of death at 1 year is an astonishing 1 in 5.6 The median survival time after diagnosis is 2.3 to 3.6 years in patients ages 67 to 74, and it is considerably shorter—1.1 to 1.6 years—in patients age 85 and older.8
THE BROKEN HEART
In 2005, the American College of Cardiology and the American Heart Association defined congestive heart failure as “a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood.”9 This characterization captures the intricate nature of the disease: its spectrum of symptoms, its many causes (eg, coronary artery disease, hypertension, nonischemic or idiopathic cardiomyopathy, and valvular heart disease), and the dual pathophysiologic features of systolic and diastolic impairment.
Systolic vs diastolic failure
Of the various ways of classifying heart failure, the most important is systolic vs diastolic.
The hallmark of systolic heart failure is a decreased left ventricular ejection fraction, and it is characterized by a large thin-walled ventricle that is weak and unable to eject enough blood to generate a normal cardiac output.
In contrast, the ejection fraction is normal or nearly normal in diastolic heart failure, but the end-diastolic volume is decreased because the ventricle is hypertrophied and thick-walled. The resultant chamber has become small and stiff and does not have enough volume for sufficient cardiac output.
QUIRKS IN THE HISTORY AND PHYSICAL EXAMINATION
The combination of inactivity and coexisting illnesses in a frail older adult may obscure some of the usual clinical manifestations of heart failure. While shortness of breath on mild exertion, easy fatigability, and leg swelling are common in younger heart failure patients, these symptoms may be due to normal aging in a much older patient. Let us consider some important aspects of the common signs and symptoms associated with heart failure.
Dyspnea on exertion is one of the earliest and most prominent symptoms. The usual question asked of patients to elicit whether this key manifestation is present is, “Do you get short of breath after walking a block?” However, this question may not be appropriate for a frail elderly person whose activity is restricted by comorbidities such as severe arthritis, coronary artery disease, or peripheral arterial disease. For a patient like this, ask instead if he or she gets short of breath after milder forms of exertion, such as making the bed, walking to the bathroom, or changing clothes.10 Also, keep in mind that dyspnea on exertion may be due to other conditions, such as renal failure, lung disease, depression, anemia, or deconditioning.
Orthopnea and paroxysmal nocturnal dyspnea may not be volunteered or elicited if a patient is sleeping in a chair or a recliner.
Leg swelling is less specific in older adults than in younger patients because chronic venous insufficiency is common in older people.
Weight gain almost always accompanies symptomatic heart failure but may also be due to increased appetite secondary to depression.
A change in mental status is common in elderly people with heart failure, especially those with vascular dementia with extensive cerebrovascular atherosclerosis or those who have latent Alzheimer disease.10
Cough, a symptom of a multitude of disorders, may be an early or the only manifestation of heart failure.
Pulmonary crackles are typically detected in most heart failure patients, but they may not be as characteristic in older adults, as they may also be noted in bronchitis, pneumonia, and other chronic lung diseases.
Additional symptoms to watch for include fatigue, syncope, angina, nocturia, and oliguria.
The bottom line is to integrate individual findings with other elements of the history and physical examination in diagnosing heart failure and tracking its progression.
CLINCHING THE DIAGNOSIS
Congestive heart failure is essentially a clinical diagnosis best established even before ordering tests, especially during times and situations in which these tests are not always readily available, such as outside office hours and in a long-term care setting.
A reliable and thorough history and physical examination is the most important component of the diagnostic process.
An echocardiogram is obtained next to measure the ejection fraction, which has both prognostic and therapeutic significance. Echocardiography can also uncover potential contributory cardiac structural abnormalities.
A chest radiograph is also typically obtained to look for pulmonary congestion, but in older adults its interpretation may be skewed by chronic lung disease or spinal deformities such as scoliosis and kyphosis.
The B-type natriuretic peptide (BNP) level is a popular blood test. BNP is commonly elevated in patients with heart failure. However, an elevated level in older adults should always be evaluated within the context of other clinical findings, as it can also result from advancing age and diseases other than heart failure, such as coronary artery disease, chronic pulmonary disease, pulmonary embolism, and renal insufficiency.11,12
PHARMACOTHERAPY PEARLS
Drug treatment for heart failure has evolved rapidly. Robust and sophisticated clinical trials have led to guidelines that call for specific medications. Unfortunately, older patients, particularly the very old and frail, have been poorly represented in these studies.9 Nonetheless, the type and choice of drugs for the young and old are similar.
Take into account age-associated changes in pharmacokinetics
Age-associated changes in pharmacokinetics must be taken into account when prescribing drugs for heart failure.13
Oral absorption of cardiovascular drugs is not significantly affected by the various changes that occur in older adults (eg, reduced gastric acid production, gastric emptying rate, gastrointestinal blood flow, and mobility). However, reductions in both lean body mass and total body water that come with aging result in lower volumes of distribution and higher plasma concentrations of hydrophilic drugs, most notably angiotensin-converting enzyme (ACE) inhibitors and digoxin. In contrast, the plasma concentrations of lipophilic drugs such as beta-blockers and central alpha-agonists tend to decrease as the proportion of body fat increases in older adults.
As the plasma albumin level diminishes with age, the free-drug concentration of salicylates and warfarin (Coumadin), which are extensively albumin-bound, may increase.
The serum concentrations of cardiovascular drugs metabolized in the liver—eg, propranolol (Inderal), lidocaine, labetalol (Trandate), verapamil (Calan), diltiazem (Cardizem), nitrates, and warfarin—may be elevated due to reduced hepatic blood flow, mass, volume, and overall metabolic capacity.
Declines in renal blood flow, glomerular filtration, and tubular function may cause accumulation of drugs that are excreted through the kidneys.
Beware of toxicities
Table 1 lists some of the drugs used in treating heart failure, common adverse affects to watch for, and recommendations for their use.
WIELDING THE SCALPEL
A tenet of heart failure management is to correct the underlying cardiac structural abnormality. This often calls for invasive intervention along with optimization of drug therapy.
For example:
- Diseased coronary arteries may be amenable to revascularization, either by percutaneous coronary intervention or by the much more involved coronary artery bypass grafting, with the aim of enhancing cardiac function.
- Valves can be repaired or replaced in patients with valvular heart disease.
- A pacemaker can be implanted to remedy sick sinus syndrome, especially with concurrent use of heart-rate-lowering agents such as beta-blockers.
- Placement of an implantable cardioverter-defibrillator has been found to be effective in preventing death due to ventricular tachyarrhythmias in patients with an ejection fraction of less than 30%.9
- Cardiac resynchronization with a biventricular pacemaker may increase the ejection fraction and cardiac output by eliminating dyssynchronous contraction of the left and right ventricles.14
In frail older adults, consideration of these invasive therapies must be individualized. While procedures such as percutaneous coronary intervention and pacemaker placement may not be as physically taxing as bypass grafting or valve replacement, the potential for surgical complications must be seriously considered, particularly if the patient has diminished physiologic reserve. Case-to-case consideration is also crucial in cardioverter-defibrillator insertion, as the survival benefit may be diminished in older adults, who likely have coexisting illnesses that predispose them to die of a noncardiac cause.15,16
The bottom line is to contemplate multiple factors—severity of the heart failure, comorbidities, baseline functional status, and social support—when assessing the appropriateness of an invasive intervention.
BEYOND DRUGS AND DEVICES: WE CAN DO ‘MORE’
Much of the spotlight has been on the various drugs and devices used to treat heart failure, but of equal importance for frail elderly patients are complementary approaches that can be used to ease disease progression and boost the quality of life. The acronym MORE highlights these strategies.
M: Multidisciplinary management programs
Heart failure disease-management programs are designed to provide comprehensive multidisciplinary care across different settings (ie, home, outpatient, and inpatient) to high-risk patients who often have multiple medical, social, and behavioral issues.9 Interventions usually include intensive patient education, encouraging patients to be more aggressive participants in their care, closely monitoring patients through telephone follow-up or home nursing, carefully reviewing medications to improve adherence to evidence-based guidelines, and multidisciplinary care with nurse case management directed by a physician.
Studies have shown that management programs, which were largely nurse-directed and targeted at older adults and patients with advanced disease, can improve quality of life and functional status, decrease hospitalizations for both heart failure and other causes, and decrease medical costs.17–19
O: Other diseases
R: Restrictions
Specific limitations in the intake of certain dietary elements are a valuable adjunct in heart failure management.
Sodium intake should be restricted to less than 3 g/day by not adding salt to meals and by avoiding salt-rich foods (eg, canned and processed foods).24 During times of distressing volume overload, a tighter sodium limit of 2 g/day is necessary, and diuretics may be less effective if this restriction is not implemented.
Fluid restriction depends on the patient’s clinical status.25 While it is not necessary to limit fluid intake in the absence of retention, a limit of 2 L/day is recommended if edema is detected. If volume overload is severe, the limit should be 1 L/day.
Alcohol is a myocardial depressant that reduces the left ventricular ejection fraction.26 Abstinence is a must for patients with alcohol-induced heart failure; otherwise, a limit of 1 drink (8 oz of beer, 4 oz of wine, or 1 oz of hard liquor) per day is suggested.24
Calories and fat intake are both important to watch, particularly in patients with obesity, hyperlipidemia, hypertension, or coronary artery disease.
E: End-of-life issues
Usual causes of death in patients with heart failure include sudden cardiac death, arrhythmias, hypotension, end-organ hypoperfusion, and metabolic derangement.27,28
Given the life-limiting nature of the disease in frail older adults, it is very important for clinicians to discuss end-of-life matters with patients and their families as early as possible. Needed are effective communication skills that foster respect, empathy, and mutual understanding.
Advance directives. The primary task is to encourage patients to develop advance health directives. These are legal documents that represent patients’ preferences about interventions available toward the end of life such as do-not-resuscitate orders, appointment of surrogate decision-makers, and use of life-sustaining interventions (eg, a feeding tube, dialysis, blood transfusions). Establishing these directives early on will help ease the transition from one mode of care to another (eg, from acute care to hospice care), prevent pointless use of resources (eg, emergency room visits, hospital admissions), and ensure that the patient’s wishes are carried out.
Palliative measures that aim to alleviate suffering and promote quality of life and dignity are available for patients with severe symptoms. For varying degrees of dyspnea, diuretics, nitrates, morphine, and positive inotropic agents such as dobutamine (Dobutrex) and milrinone (Primacor) can be tried. Thoracentesis is done in patients with extensive pleural effusion. Fatigue and anorexia are due to a combination of factors, namely, decreased cardiac output, increased neurohormone levels, deconditioning, depression, decreased sleep, and anxiety.29 Opioids, caffeine, exercise, oxygen, fluid and salt restriction, and correction of anemia and depression may help ease these symptoms.
For patients with an implantable cardioverter-defibrillator, deactivation is an important matter that needs to be addressed. Deactivation can be carried out with certainty once the goal of care has shifted away from curative efforts and either the patient or a surrogate decision-maker has made the informed decision to turn the device off. Berger30 raised three points that the clinician and decision-maker can discuss in trying to achieve a resolution during times of doubt and indecision:
- The patient may no longer value continued survival
- The device may no longer offer the prospect of increased survival
- The device may impede active dying.
The idea of hospice care should be gradually and gently explored to ensure a prompt and seamless transition when the time comes. The patient and family need to know that the goal of hospice care is to ensure comfort and that they can benefit the most by enrolling early during the course of the terminal illness.
The Medicare hospice benefit is granted to patients who have been certified by two physicians to have a life expectancy of 6 months or less if their terminal illness runs its natural course. The criteria for determining that heart failure is terminal are:
- New York Heart Association class III (symptomatic with less than ordinary activities) or IV (symptomatic at rest)
- Left ventricular ejection fraction less than or equal to 20%
- Persistent symptoms despite optimal medical management
- Inability to tolerate optional management due to hypotension with or without renal failure.31
WHAT CAN WE DO FOR MR. R.?
Mr. R. has systolic heart failure stemming from coronary artery disease, and his symptoms put him in New York Heart Association class III. He is well managed with drugs of different appropriate classes: an ACE inhibitor, a beta-blocker, digoxin, an aldosterone antagonist, and a diuretic. His other drugs all have well-defined indications.
Since he does not have fluid overload, his furosemide can be stopped, and this change will likely relieve his orthostatic hypotension and nocturia. His systolic blood pressure target can be liberalized to 150 mm Hg or less, as tighter control might exacerbate orthostatic hypotension. This change, along with having him start using a walker instead of a cane, will hopefully prevent future falls. Furthermore, his naproxen should be discontinued, as it can worsen heart failure.
Mr. R. has symptoms of depression and thus needs to be started on an antidepressant and encouraged to engage in social activities as much as he can tolerate. These interventions may also help with his mild dementia, which is evidenced by a Mini-Mental State Exam score of 22. He will not benefit from sodium and fat restriction, as he has actually been losing weight.
To keep Mr. R.’s cognitive impairment and overall decline in function from compromising his compliance with his treatment, he will need a substantial amount of assistance, which his daughter alone may not be able to provide. To tackle this concern, a discussion about participating in a heart failure management program can be started with Mr. R. and his family.
More importantly, his advanced directives, including delegating a surrogate decision-maker and deciding on do-not-resuscitate status, have to be clarified. Finally, it would be prudent to introduce the concept of hospice care to the patient and his daughter while he is still coherent and able to state his preferences.
- Walston J, Hadley EC, Ferruci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006; 54:991–1001.
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol 1992; 20:301–306.
- Popovic JR, 1999 National Hospital Discharge Survey: annual summary with detailed diagnosis and procedure data. National Center for Health Statistics. Vital Health Stat 2001; 13:1–206.
- DeFrances CJ, Hall MJ, Podgornik MN. 2003 National Hospital Discharge Survey. Advance data from vital and health statistics; no. 359. Hyattsville (MD): National Center for Health Statistics, 2005.
- American Heart Association. Heart disease and stroke statistics—2009 update: a report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:e21–e181.
- Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA 1996; 275:1557–1562.
- Croft JB, Giles WH, Pollard RA, et al. Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Arch Intern Med 1999; 159:505–510.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). Circulation 2005; 112:e154–e235.
- Ahmed A. Clinical manifestations, diagnostic assessment, and etiology of heart failure in older adults. Clin Geriatr Med 2007; 23:11–30.
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002; 40:976–982.
- Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002; 90:254–258.
- Aronow WS, Frishman WH, Cheng-Lai A. Cardiovascular drug therapy in the elderly. Cardiol Rev 2007; 15:195–215.
- Bakker P, Meijburg H, de Bries J, et al. Biventricular pacing in end-stage heart failure improves functional capacity and left ventricular function. J Interv Card Electrophysiol 2000; 4:395–404.
- Healey JS, Hallstrom AP, Kuck KH, et al. Role of the implantable defibrillator among elderly patients with a history of life-threatening ventricular arrhythmias. Eur Heart J 2007; 28:1746–1749.
- Lee DS, Tu JV, Austin PC, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol 2007; 49:2408–2415.
- Rich MW, Beckham V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995; 333:1190–1195.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- McAlister F, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol 2004; 44:810–819.
- Horwich TB, Fonarow GC, Hamilton MA, et al. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 2002; 39:1780–1786.
- Al-Ahmad A, Rand WM, Manjunath G, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 2001; 38:955–962.
- Singh SN, Fisher SG, Deedwania PC, et al. Pulmonary effect of amiodarone in patients with heart failure: the Congestive Heart Failure-Survival Trial of Antiarrhythmic Therapy (CHF-STAT) Investigators (Veterans Affairs Cooperative Study No. 320). J Am Coll Cardiol 1997; 30:514–517.
- Cohen MB, Mather PJ. A review of the association between congestive heart failure and cognitive impairment. Am J Geriatr Cardiol 2007; 16:171–174.
- Dracup K, Baker DW, Dunbar SB, et al. Management of heart failure. II. Counseling, education, and lifestyle modifications. JAMA 1994; 272:1442–1446.
- Lenihan DJ, Uretsky BF. Non-pharmacologic treatment of heart failure in the elderly. Clin Geriatr Med 2000; 16:477–488.
- Regan TJ. Alcohol and the cardiovascular system. JAMA 1990; 264:377–381.
- Teuteberg JJ, Lewis EF, Nohria A, et al. Characteristics of patients who die with heart failure and a low ejection fraction in the new millennium. J Card Fail 2006; 12:47–53.
- Derfler MC, Jacob M, Wolf RE, et al. Mode of death from congestive heart failure: implications for clinical management. Am J Geriatr Cardiol 2004; 13:299–304.
- Evangelista LS, Moser DK, Westlake C, et al. Correlates of fatigue in patients with heart failure. Prog Cardiovasc Nurs 2008; 23:12–17.
- Berger JT. The ethics of deactivating implanted cardioverter defibrillators. Ann Intern Med 2005; 142:631–634.
- Stuart B, Connor S, Kinzbrunner BM, et al. Medical guidelines for determining prognosis in selected non-cancer diseases, 2nd ed. Arlington VA, National Hospice Organization; 1996.
- Walston J, Hadley EC, Ferruci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006; 54:991–1001.
- Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–M156.
- Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol 1992; 20:301–306.
- Popovic JR, 1999 National Hospital Discharge Survey: annual summary with detailed diagnosis and procedure data. National Center for Health Statistics. Vital Health Stat 2001; 13:1–206.
- DeFrances CJ, Hall MJ, Podgornik MN. 2003 National Hospital Discharge Survey. Advance data from vital and health statistics; no. 359. Hyattsville (MD): National Center for Health Statistics, 2005.
- American Heart Association. Heart disease and stroke statistics—2009 update: a report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2009; 119:e21–e181.
- Levy D, Larson MG, Vasan RS, et al. The progression from hypertension to congestive heart failure. JAMA 1996; 275:1557–1562.
- Croft JB, Giles WH, Pollard RA, et al. Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Arch Intern Med 1999; 159:505–510.
- Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). Circulation 2005; 112:e154–e235.
- Ahmed A. Clinical manifestations, diagnostic assessment, and etiology of heart failure in older adults. Clin Geriatr Med 2007; 23:11–30.
- Redfield MM, Rodeheffer RJ, Jacobsen SJ, et al. Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol 2002; 40:976–982.
- Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. Am J Cardiol 2002; 90:254–258.
- Aronow WS, Frishman WH, Cheng-Lai A. Cardiovascular drug therapy in the elderly. Cardiol Rev 2007; 15:195–215.
- Bakker P, Meijburg H, de Bries J, et al. Biventricular pacing in end-stage heart failure improves functional capacity and left ventricular function. J Interv Card Electrophysiol 2000; 4:395–404.
- Healey JS, Hallstrom AP, Kuck KH, et al. Role of the implantable defibrillator among elderly patients with a history of life-threatening ventricular arrhythmias. Eur Heart J 2007; 28:1746–1749.
- Lee DS, Tu JV, Austin PC, et al. Effect of cardiac and noncardiac conditions on survival after defibrillator implantation. J Am Coll Cardiol 2007; 49:2408–2415.
- Rich MW, Beckham V, Wittenberg C, et al. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995; 333:1190–1195.
- Fonarow GC, Stevenson LW, Walden JA, et al. Impact of a comprehensive heart failure management program on hospital readmission and functional status of patients with advanced heart failure. J Am Coll Cardiol 1997; 30:725–732.
- McAlister F, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol 2004; 44:810–819.
- Horwich TB, Fonarow GC, Hamilton MA, et al. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 2002; 39:1780–1786.
- Al-Ahmad A, Rand WM, Manjunath G, et al. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 2001; 38:955–962.
- Singh SN, Fisher SG, Deedwania PC, et al. Pulmonary effect of amiodarone in patients with heart failure: the Congestive Heart Failure-Survival Trial of Antiarrhythmic Therapy (CHF-STAT) Investigators (Veterans Affairs Cooperative Study No. 320). J Am Coll Cardiol 1997; 30:514–517.
- Cohen MB, Mather PJ. A review of the association between congestive heart failure and cognitive impairment. Am J Geriatr Cardiol 2007; 16:171–174.
- Dracup K, Baker DW, Dunbar SB, et al. Management of heart failure. II. Counseling, education, and lifestyle modifications. JAMA 1994; 272:1442–1446.
- Lenihan DJ, Uretsky BF. Non-pharmacologic treatment of heart failure in the elderly. Clin Geriatr Med 2000; 16:477–488.
- Regan TJ. Alcohol and the cardiovascular system. JAMA 1990; 264:377–381.
- Teuteberg JJ, Lewis EF, Nohria A, et al. Characteristics of patients who die with heart failure and a low ejection fraction in the new millennium. J Card Fail 2006; 12:47–53.
- Derfler MC, Jacob M, Wolf RE, et al. Mode of death from congestive heart failure: implications for clinical management. Am J Geriatr Cardiol 2004; 13:299–304.
- Evangelista LS, Moser DK, Westlake C, et al. Correlates of fatigue in patients with heart failure. Prog Cardiovasc Nurs 2008; 23:12–17.
- Berger JT. The ethics of deactivating implanted cardioverter defibrillators. Ann Intern Med 2005; 142:631–634.
- Stuart B, Connor S, Kinzbrunner BM, et al. Medical guidelines for determining prognosis in selected non-cancer diseases, 2nd ed. Arlington VA, National Hospice Organization; 1996.
KEY POINTS
- Not only does heart failure itself result in frailty, but its treatment can also put additional stress on an already frail patient. In addition, the illness and its treatments can negatively affect coexisting disorders.
- Common signs and symptoms of heart failure are less specific in older adults, and atypical symptoms may predominate.
- Age-associated changes in pharmacokinetics must be taken into account when prescribing drugs for heart failure.
- Effective communication among health professionals, patients, and families is necessary.
- Given the life-limiting nature of heart failure in frail older adults, it is critical for clinicians to discuss end-of-life issues with patients and their families as soon as possible.
PTSD Smartphone Application Wins FCC Award
Eruptive Collagenomas, Suprasellar Meningioma, and Elevated Prolactin in a Patient With a History of Papillary Thyroid Carcinoma
News Roundup: New and Noteworthy Information
Researchers have found that having a transient ischemic attack (TIA) can reduce a person’s life expectancy up to 20%, according to a study published online November 10 in Stroke. “There is a lack of modern-day data quantifying the effect of TIA on survival, and recent data do not take into account expected survival,” the researchers commented. To investigate the impact of a TIA on survival, the investigators analyzed data from 22,157 patients hospitalized with a TIA, then estimated survival relative to the age- and sex-matched general population up to nine years after hospitalization. At one-year follow-up, 91.5% of TIA patients survived, compared with 95.0% expected survival in the general population; by nine-years follow-up, observed survival was 20% lower than expected. Older age, prior hospitalization for stroke (but not TIA), atrial fibrillation, and congestive heart failure significantly increased the risk of excess death in these patients.
Higher levels of urinary sodium excretion were associated with an increased risk of cardiovascular events, while lower levels were associated with cardiovascular death and hospitalization for congestive heart failure, according to a study published in the November 23 JAMA. “[Our objective was] to determine the association between estimated urinary sodium and potassium excretion (surrogates for intake) and cardiovascular events in patients with established cardiovascular disease or diabetes mellitus,” stated the researchers. The results of their observational analyses revealed that “compared with baseline sodium excretion of 4 to 5.99 g per day, sodium excretion of greater than 7 g per day was associated with an increased risk of all cardiovascular events.” In addition, a sodium excretion of less than 3 g per day was associated with increased risk of cardiovascular mortality and hospitalization for congestive heart failure, and a higher estimated potassium excretion was associated with a reduced risk of stroke.
Serum vitamin D levels are significantly lower in patients with recurrent inflammatory spinal cord disease, according to the results of a study published online November 14 in Archives of Neurology. The study authors performed a retrospective analysis evaluating vitamin D levels of 77 patients with monophasic and recurrent inflammatory spinal cord diseases. “Vitamin D levels are significantly lower in patients who developed recurrent spinal cord disease, adjusting for season, age, sex, and race,” the investigators concluded. “This study provides a basis for a prospective trial of measuring 25-hydroxyvitamin D levels in these patient populations and assessing the influence of vitamin D supplementation on the frequency of relapses in those with recurrent inflammatory spinal cord disease.”
The FDA has approved AdaptiveStim with RestoreSensor neurostimulation system for the management of chronic pain. Unlike other implantable neurostimulation devices that require frequent manual adjustments with changes in body positions, the AdaptiveStim system (Medtronic, Inc; Minneapolis) automatically adapts stimulation levels to the needs of people with chronic back and/or leg pain by recognizing and remembering the correlation between a change in body position and the level of stimulation needed. The FDA’s approval was based on data from a clinical trial in which 86.5% of study participants with chronic pain experienced better pain relief and convenience when the system was turned on, compared with a control period when the participants manually adjusted neurostimulation settings; 80.3% of study participants also reported functional improvements, including improved comfort during position changes. The AdaptiveStim system was also approved for use in MRI head scans if recommended by a physician.
Brain overgrowth in boys with autism involves an abnormal excess number of neurons, according to the results of a small preliminary study published in the November 9 JAMA. “Autism often involves early brain overgrowth, including the prefrontal cortex,” the investigators stated. “Although prefrontal abnormality has been theorized to underlie some autistic symptoms, the cellular defects that cause abnormal overgrowth remain unknown.” To investigate whether this overgrowth in children with autism involves excess neuron numbers, the researchers compared postmortem tissue from the prefrontal cortex of seven boys (age range, 2 to 16) who had autism with postmortem tissue of six typically developing boys. They found that children with autism had 67% more neurons in the prefrontal cortex. “Brain weight in the autistic case differed from normative mean weight for age by a mean of 17.6%, while brains in controls differed by a mean of 0.2%,” the researchers reported.
A person’s stroke risk profile may also be helpful in predicting whether a person will develop memory problems and cognitive impairment later in life, according to a study published in the November 8 Neurology. “Participants included subjects without stroke at baseline … with at least two cognitive function assessments during the follow-up,” the researchers explained. “During a mean follow-up of 4.1 years, 1,907 participants met criteria for incident cognitive impairment.” The researchers determined that male sex, black race, less education, older age, and presence of left ventricular hypertrophy were related to the development of cognitive impairment. “Total Framingham Stroke Risk Profile score, elevated blood pressure, and left ventricular hypertrophy predict development of clinically significant cognitive dysfunction,” the investigators concluded. “Prevention and treatment of high blood pressure may be effective in preserving cognitive health.”
The FDA has approved Xarelto (rivaroxaban) to reduce the risk of stroke in people who have nonvalvular atrial fibrillation. Xarelto (Janssen Pharmaceuticals Inc; Titusville, New Jersey) is an oral anti-clotting drug that has also been approved to reduce the risk of blood clots, deep vein thrombosis, and pulmonary embolism following knee or hip replacement surgery. The FDA’s approval was based on the results of a clinical trial with more than 14,000 patients that compared Xarelto with the anti-clotting drug warfarin; Xarelto proved similar to warfarin in its ability to prevent stroke. Bleeding was the most common adverse event reported by patients treated with Xarelto in this clinical trial, and the risk of bleeding was similar to the risk of bleeding associated with warfarin.
Patients with dementia who have a stroke have a higher likelihood of becoming disabled and being institutionalized, compared with patients who did not have dementia at the time of their stroke, according to a report in the November 1 Neurology. Investigators conducted a retrospective cohort study that included 9,304 patients with an acute ischemic stroke, 702 of whom had a history of dementia. “Patients with dementia were older (mean age, 81 vs 70), had more severe strokes, and were more likely to have atrial fibrillation than those without dementia,” the investigators determined. They also found that patients with dementia were slightly less likely to be admitted to a stroke unit, had a higher disability at discharge, and were less likely to be discharged to their prestroke place of residence. “In patients with stroke, preexisting dementia is associated with high rates of disability and institutionalization, representing an increasing challenge for the health care system,” the researchers concluded.
—Ariel Jones
Researchers have found that having a transient ischemic attack (TIA) can reduce a person’s life expectancy up to 20%, according to a study published online November 10 in Stroke. “There is a lack of modern-day data quantifying the effect of TIA on survival, and recent data do not take into account expected survival,” the researchers commented. To investigate the impact of a TIA on survival, the investigators analyzed data from 22,157 patients hospitalized with a TIA, then estimated survival relative to the age- and sex-matched general population up to nine years after hospitalization. At one-year follow-up, 91.5% of TIA patients survived, compared with 95.0% expected survival in the general population; by nine-years follow-up, observed survival was 20% lower than expected. Older age, prior hospitalization for stroke (but not TIA), atrial fibrillation, and congestive heart failure significantly increased the risk of excess death in these patients.
Higher levels of urinary sodium excretion were associated with an increased risk of cardiovascular events, while lower levels were associated with cardiovascular death and hospitalization for congestive heart failure, according to a study published in the November 23 JAMA. “[Our objective was] to determine the association between estimated urinary sodium and potassium excretion (surrogates for intake) and cardiovascular events in patients with established cardiovascular disease or diabetes mellitus,” stated the researchers. The results of their observational analyses revealed that “compared with baseline sodium excretion of 4 to 5.99 g per day, sodium excretion of greater than 7 g per day was associated with an increased risk of all cardiovascular events.” In addition, a sodium excretion of less than 3 g per day was associated with increased risk of cardiovascular mortality and hospitalization for congestive heart failure, and a higher estimated potassium excretion was associated with a reduced risk of stroke.
Serum vitamin D levels are significantly lower in patients with recurrent inflammatory spinal cord disease, according to the results of a study published online November 14 in Archives of Neurology. The study authors performed a retrospective analysis evaluating vitamin D levels of 77 patients with monophasic and recurrent inflammatory spinal cord diseases. “Vitamin D levels are significantly lower in patients who developed recurrent spinal cord disease, adjusting for season, age, sex, and race,” the investigators concluded. “This study provides a basis for a prospective trial of measuring 25-hydroxyvitamin D levels in these patient populations and assessing the influence of vitamin D supplementation on the frequency of relapses in those with recurrent inflammatory spinal cord disease.”
The FDA has approved AdaptiveStim with RestoreSensor neurostimulation system for the management of chronic pain. Unlike other implantable neurostimulation devices that require frequent manual adjustments with changes in body positions, the AdaptiveStim system (Medtronic, Inc; Minneapolis) automatically adapts stimulation levels to the needs of people with chronic back and/or leg pain by recognizing and remembering the correlation between a change in body position and the level of stimulation needed. The FDA’s approval was based on data from a clinical trial in which 86.5% of study participants with chronic pain experienced better pain relief and convenience when the system was turned on, compared with a control period when the participants manually adjusted neurostimulation settings; 80.3% of study participants also reported functional improvements, including improved comfort during position changes. The AdaptiveStim system was also approved for use in MRI head scans if recommended by a physician.
Brain overgrowth in boys with autism involves an abnormal excess number of neurons, according to the results of a small preliminary study published in the November 9 JAMA. “Autism often involves early brain overgrowth, including the prefrontal cortex,” the investigators stated. “Although prefrontal abnormality has been theorized to underlie some autistic symptoms, the cellular defects that cause abnormal overgrowth remain unknown.” To investigate whether this overgrowth in children with autism involves excess neuron numbers, the researchers compared postmortem tissue from the prefrontal cortex of seven boys (age range, 2 to 16) who had autism with postmortem tissue of six typically developing boys. They found that children with autism had 67% more neurons in the prefrontal cortex. “Brain weight in the autistic case differed from normative mean weight for age by a mean of 17.6%, while brains in controls differed by a mean of 0.2%,” the researchers reported.
A person’s stroke risk profile may also be helpful in predicting whether a person will develop memory problems and cognitive impairment later in life, according to a study published in the November 8 Neurology. “Participants included subjects without stroke at baseline … with at least two cognitive function assessments during the follow-up,” the researchers explained. “During a mean follow-up of 4.1 years, 1,907 participants met criteria for incident cognitive impairment.” The researchers determined that male sex, black race, less education, older age, and presence of left ventricular hypertrophy were related to the development of cognitive impairment. “Total Framingham Stroke Risk Profile score, elevated blood pressure, and left ventricular hypertrophy predict development of clinically significant cognitive dysfunction,” the investigators concluded. “Prevention and treatment of high blood pressure may be effective in preserving cognitive health.”
The FDA has approved Xarelto (rivaroxaban) to reduce the risk of stroke in people who have nonvalvular atrial fibrillation. Xarelto (Janssen Pharmaceuticals Inc; Titusville, New Jersey) is an oral anti-clotting drug that has also been approved to reduce the risk of blood clots, deep vein thrombosis, and pulmonary embolism following knee or hip replacement surgery. The FDA’s approval was based on the results of a clinical trial with more than 14,000 patients that compared Xarelto with the anti-clotting drug warfarin; Xarelto proved similar to warfarin in its ability to prevent stroke. Bleeding was the most common adverse event reported by patients treated with Xarelto in this clinical trial, and the risk of bleeding was similar to the risk of bleeding associated with warfarin.
Patients with dementia who have a stroke have a higher likelihood of becoming disabled and being institutionalized, compared with patients who did not have dementia at the time of their stroke, according to a report in the November 1 Neurology. Investigators conducted a retrospective cohort study that included 9,304 patients with an acute ischemic stroke, 702 of whom had a history of dementia. “Patients with dementia were older (mean age, 81 vs 70), had more severe strokes, and were more likely to have atrial fibrillation than those without dementia,” the investigators determined. They also found that patients with dementia were slightly less likely to be admitted to a stroke unit, had a higher disability at discharge, and were less likely to be discharged to their prestroke place of residence. “In patients with stroke, preexisting dementia is associated with high rates of disability and institutionalization, representing an increasing challenge for the health care system,” the researchers concluded.
—Ariel Jones
Researchers have found that having a transient ischemic attack (TIA) can reduce a person’s life expectancy up to 20%, according to a study published online November 10 in Stroke. “There is a lack of modern-day data quantifying the effect of TIA on survival, and recent data do not take into account expected survival,” the researchers commented. To investigate the impact of a TIA on survival, the investigators analyzed data from 22,157 patients hospitalized with a TIA, then estimated survival relative to the age- and sex-matched general population up to nine years after hospitalization. At one-year follow-up, 91.5% of TIA patients survived, compared with 95.0% expected survival in the general population; by nine-years follow-up, observed survival was 20% lower than expected. Older age, prior hospitalization for stroke (but not TIA), atrial fibrillation, and congestive heart failure significantly increased the risk of excess death in these patients.
Higher levels of urinary sodium excretion were associated with an increased risk of cardiovascular events, while lower levels were associated with cardiovascular death and hospitalization for congestive heart failure, according to a study published in the November 23 JAMA. “[Our objective was] to determine the association between estimated urinary sodium and potassium excretion (surrogates for intake) and cardiovascular events in patients with established cardiovascular disease or diabetes mellitus,” stated the researchers. The results of their observational analyses revealed that “compared with baseline sodium excretion of 4 to 5.99 g per day, sodium excretion of greater than 7 g per day was associated with an increased risk of all cardiovascular events.” In addition, a sodium excretion of less than 3 g per day was associated with increased risk of cardiovascular mortality and hospitalization for congestive heart failure, and a higher estimated potassium excretion was associated with a reduced risk of stroke.
Serum vitamin D levels are significantly lower in patients with recurrent inflammatory spinal cord disease, according to the results of a study published online November 14 in Archives of Neurology. The study authors performed a retrospective analysis evaluating vitamin D levels of 77 patients with monophasic and recurrent inflammatory spinal cord diseases. “Vitamin D levels are significantly lower in patients who developed recurrent spinal cord disease, adjusting for season, age, sex, and race,” the investigators concluded. “This study provides a basis for a prospective trial of measuring 25-hydroxyvitamin D levels in these patient populations and assessing the influence of vitamin D supplementation on the frequency of relapses in those with recurrent inflammatory spinal cord disease.”
The FDA has approved AdaptiveStim with RestoreSensor neurostimulation system for the management of chronic pain. Unlike other implantable neurostimulation devices that require frequent manual adjustments with changes in body positions, the AdaptiveStim system (Medtronic, Inc; Minneapolis) automatically adapts stimulation levels to the needs of people with chronic back and/or leg pain by recognizing and remembering the correlation between a change in body position and the level of stimulation needed. The FDA’s approval was based on data from a clinical trial in which 86.5% of study participants with chronic pain experienced better pain relief and convenience when the system was turned on, compared with a control period when the participants manually adjusted neurostimulation settings; 80.3% of study participants also reported functional improvements, including improved comfort during position changes. The AdaptiveStim system was also approved for use in MRI head scans if recommended by a physician.
Brain overgrowth in boys with autism involves an abnormal excess number of neurons, according to the results of a small preliminary study published in the November 9 JAMA. “Autism often involves early brain overgrowth, including the prefrontal cortex,” the investigators stated. “Although prefrontal abnormality has been theorized to underlie some autistic symptoms, the cellular defects that cause abnormal overgrowth remain unknown.” To investigate whether this overgrowth in children with autism involves excess neuron numbers, the researchers compared postmortem tissue from the prefrontal cortex of seven boys (age range, 2 to 16) who had autism with postmortem tissue of six typically developing boys. They found that children with autism had 67% more neurons in the prefrontal cortex. “Brain weight in the autistic case differed from normative mean weight for age by a mean of 17.6%, while brains in controls differed by a mean of 0.2%,” the researchers reported.
A person’s stroke risk profile may also be helpful in predicting whether a person will develop memory problems and cognitive impairment later in life, according to a study published in the November 8 Neurology. “Participants included subjects without stroke at baseline … with at least two cognitive function assessments during the follow-up,” the researchers explained. “During a mean follow-up of 4.1 years, 1,907 participants met criteria for incident cognitive impairment.” The researchers determined that male sex, black race, less education, older age, and presence of left ventricular hypertrophy were related to the development of cognitive impairment. “Total Framingham Stroke Risk Profile score, elevated blood pressure, and left ventricular hypertrophy predict development of clinically significant cognitive dysfunction,” the investigators concluded. “Prevention and treatment of high blood pressure may be effective in preserving cognitive health.”
The FDA has approved Xarelto (rivaroxaban) to reduce the risk of stroke in people who have nonvalvular atrial fibrillation. Xarelto (Janssen Pharmaceuticals Inc; Titusville, New Jersey) is an oral anti-clotting drug that has also been approved to reduce the risk of blood clots, deep vein thrombosis, and pulmonary embolism following knee or hip replacement surgery. The FDA’s approval was based on the results of a clinical trial with more than 14,000 patients that compared Xarelto with the anti-clotting drug warfarin; Xarelto proved similar to warfarin in its ability to prevent stroke. Bleeding was the most common adverse event reported by patients treated with Xarelto in this clinical trial, and the risk of bleeding was similar to the risk of bleeding associated with warfarin.
Patients with dementia who have a stroke have a higher likelihood of becoming disabled and being institutionalized, compared with patients who did not have dementia at the time of their stroke, according to a report in the November 1 Neurology. Investigators conducted a retrospective cohort study that included 9,304 patients with an acute ischemic stroke, 702 of whom had a history of dementia. “Patients with dementia were older (mean age, 81 vs 70), had more severe strokes, and were more likely to have atrial fibrillation than those without dementia,” the investigators determined. They also found that patients with dementia were slightly less likely to be admitted to a stroke unit, had a higher disability at discharge, and were less likely to be discharged to their prestroke place of residence. “In patients with stroke, preexisting dementia is associated with high rates of disability and institutionalization, representing an increasing challenge for the health care system,” the researchers concluded.
—Ariel Jones
Grand Rounds: Pregnant Woman, 33, With Leg Pain and Numbness
A 33-year-old woman in her 32nd week of pregnancy (gravida 3, para 2) presented to the emergency department (ED) with a five-day history of weakness and ascending numbness below the right knee. She related a two-week history of right-sided low back pain that radiated to the right buttock and was associated with severe right lower extremity (RLE) pain, most prominent in the posterolateral aspect of the right calf. She denied perianal numbness, incontinence, or other changes in bowel or bladder function. She also denied left lower extremity involvement or trauma.
The patient had had one uneventful pregnancy to date. Her medical history included hypothyroidism, treated with levothyroxine; and anxiety, for which she was taking sertraline. She denied any history of allergies, alcohol consumption, smoking, or illicit drug use. She had been evaluated twice and received reassurance in the two weeks before her presentation to the ED. She was admitted to the obstetric service secondary to pain, and a stat MRI rather than x-ray was ordered by obstetrics. An orthopedic consult was ordered. A spine surgeon happened to be on call.
Examination revealed that the patient walked plantigrade, with her right foot slightly externally rotated. She was unable to dorsiflex or plantarflex her right foot. She was unable to heel- or toe-walk on the right side, possessed 0 out of 5 strength at the right extensor hallucis longus and 2 to 3 out of 5 at the right tibialis anterior and gastroc soleus complex. She complained of pain with right leg elevation exceeding 30° and had very limited sensation to light touch in the right L5 and S1 dermatomes. Deep tendon reflex was absent at the right ankle. The patient refused a rectal exam or post-void evaluation.
The initial diagnosis considered by the ED clinician was sciatica, with a differential diagnosis that included pelvic pain of pregnancy, lumbar sprain strain, sciatica, lumbar disk, herniated nucleus pulposus with radiculopathy, and cauda equina syndrome. Trauma was considered and ruled out, as were malignancies; inflammatory, infectious, or degenerative conditions; or other compressive processes.1
Lumbar MRI demonstrated a very large, right-sided disk herniation at L5-S1 with an extruded fragment that was severely compressing the thecal sac and the right S1 nerve root, causing severe right foraminal stenosis at the level of L5-S1. Degenerative changes were noted at L4-5 with disk dessication and no lesions seen.
The patient was diagnosed with cauda equina syndrome, which was felt to be causing severe RLE weakness and ascending numbness. The options of observation, analgesia, physical therapy, and epidural injections were discussed with the patient; however, surgery was strongly recommended due to her profound weakness and the severity of pain she was experiencing, in addition to the size of the disk herniation. She opted for surgery.
The patient was given epidural anesthesia at the L3-4 level, with a catheter left in place during the procedure. A test dose of lidocaine (1.5 cc) with epinephrine was injected to ensure proper placement, and bupivacaine 0.5% was given in increments of 5.0 cc three times during the case. Propofol was administered for sedation, and a 2.0-mg dose of a long-acting morphine was given to the patient before removal of the epidural catheter. Fetal monitoring was performed by obstetrics throughout the procedure.
A laminotomy, partial facetectomy, and diskectomy were performed at L5-S1 with excision of a free fragment. Surgical pathology described the disk as fibrocartilaginous tissue measuring 3.5 cm x 1.4 cm x 0.6 cm.
DISCUSSION
Although nearly half of pregnant women experience low back pain, cauda equina syndrome (CES), a complication of lumbar disk herniation, is extremely rare in the gravid patient.2 In a decade-long review of 48,760 consecutive deliveries, LaBan et al3 identified symptomatic lumbar herniated nucleus pulposus in only five patients (approximately one in 10,000 pregnancies). In pregnant women who do experience CES, symptoms most commonly develop between the fifth and seventh month of pregnancy.4 According to Small et al,5 “The major pitfall in diagnosis is not including CES in the back pain differential.”
True CES presents as a triad of symptoms: lower extremity weakness, altered sensation in the skin of the buttocks and upper posterior thighs (saddle anesthesia), and dysfunction or paralysis of the bowel and bladder. However, few patients present with all of the classic symptoms,6 and patients with CES are often dismissed by several clinicians in their search for relief before presenting to a subspecialist. Kostuik et al7 consider “unilateral sciatica with motor and sensory disturbance” a more common presentation of CES; also indicative of this condition, they report, is “urinary dysfunction combined with motor and sensory loss in the presence of a disc lesion.”
The polypeptide relaxin, which is secreted by the corpus luteum to promote joint laxity in late pregnancy, has been associated with low back pain and pelvic pain of pregnancy; it has also been suggested as a possible contributing cause of CES during pregnancy.8,9 Additionally, increased lumbar lordosis with positional and postural stress may cause direct pressure by the gravid uterus on nerve roots. The great vessels may also be compressed by the uterus, resulting in ischemia of the neural element and back pain that radiates to the legs.10 Many cases of lumbar disk prolapse occur during the first and second trimesters. The most clinically incapacitated patients have been found to have the highest levels of relaxin.9
The Diagnosis
Early diagnosis of CES, through proper physical examination and radiologic studies, is paramount. A rectal examination should be performed to assess for sphincter tone (which may be diminished in 80% of patients) and to assess for perineal sensation.5 Catheterization yielding a postvoid residual urine greater than 100/200 cc is reported to have a specificity and sensitivity of 90% or greater for CES. Small et al5 recommend a straight leg raise maneuver to assess for radiculopathy.
Various studies in the literature support the use of MRI in the gravid patient to confirm the diagnosis of CES and to identify the degree and level of disk protrusion.2-4,11
Treatment
CES requires urgent surgical decompression.11 Early recognition of CES attributable to lumbar disk prolapse, report O’Laoire et al,12 is essential to prevent irreversible sphincter paralysis. They liken the condition’s urgency to that of extradural hematoma in a head injury.
Disk surgery during pregnancy—preferably a team effort, with obstetrics performing perioperative fetal monitoring—has been deemed a safe management method.2,4 Spinal or general anesthesia during nonobstetric surgery is generally considered safe for both mother and fetus.13,14 Adequate oxygenation without risk for hyperventilation is considered essential.15
PATIENT OUTCOME
In the immediate postoperative period, the patient continued to complain of RLE pain, which abated significantly by the time she was discharged. When she was seen in follow-up four days later, she was able to heel- and toe-walk on the right side, and her strength had improved to 3 or 4 out of 5 at the RLE. She continued to experience diminished sensation to the plantar aspect of the right foot, which persisted at the one-month follow up. At that visit, the patient also reported occasional pain in the right buttock. Physical therapy was started to strengthen the RLE.
By three months postsurgery, the patient had undergone uneventful vaginal delivery. She had an entirely benign exam with 5 out of 5 strength at the RLE and no neurologic deficits. She was cleared to return to light weightlifting with good technique and lumbar support but was told to refrain from running until the sixth month postsurgery.
CONCLUSION
Although the case patient did not have a “true” (ie, typical) presentation of CES, her symptoms warranted a full workup and treatment to prevent possible long-term sequelae. Medical practitioners should be familiar with the triad presentation of CES. They must differentiate lower back pain of muscular origin from lumbar disk herniation and be able to appreciate the degree of symptom severity reported by the gravid patient. A thorough history and physical assessment must be performed in every such case. When in doubt, the clinician must err on the side of caution, referring the patient for MRI and consulting with a specialist.
REFERENCES
1. Johnston RA. The management of acute spinal cord compression. J Neurol Neurosurg Psychiatr. 1993;56(10):1046-1054.
2. Brown MD, Levi AD. Surgery for lumbar disc herniation during pregnancy. Spine (Phila PA 1976). 2001;26(5):440-443.
3. LaBan MM, Perrin JCS, Latimer FR. Pregnancy and the herniated lumbar disc. Arch Phys Med Rehabil. 1983;64(7):319-321.
4. LaBan MM, Rapp NS, Van Oeyen P, Meerschaert JR. The lumbar herniated disk of pregnancy: a report of six cases identified by magnetic resonance imaging. Arch Phys Med Rehabil. 1995;76(5):476-479.
5. Small SA, Perron AD, Brady WJ. Orthopedic pitfalls: cauda equina syndrome. Am J Emerg Med. 2005;23(2):159-163.
6. Tay EC, Chacha PB. Midline prolapse of a lumbar intervertebral disc with compression of the cauda equina. J Bone Joint Surg. 1979;61(1):43-46.
7. Kostuik JP, Harrington I, Alexander D, et al. Cauda equina syndrome and lumbar disc herniation. J Bone Joint Surg Am. 1986;68(3):386-391.
8. Russell R, Reynolds F. Back pain, pregnancy, and childbirth. BMJ. 1997;314(7087):1062-1063.
9. MacLennan AH, Nicholson R, Green RC, Bath M. Serum relaxin and pelvic pain of pregnancy. Lancet. 1986;2(8501):243-245.
10. Ashkan K, Casey AT, Powell M, Crockard HA. Back pain during pregnancy and after childbirth: an unusual cause not to miss. J R Soc Med. 1998;91(2):88-90.
11. Busse JW, Bhandari M, Schnittker JB, et al. Delayed presentation of cauda equina syndrome secondary to lumbar disc herniation: functional outcomes and health-related quality of life. CJEM. 2001;3(4):285-291.
12. O’Laoire SA, Crockard HA, Thomas DG. Prognosis for sphincter recovery after operation for cauda equina compression owing to lumbar disc prolapse. Br Med J (Clin Res Ed). 1981;282(6279):1852-1854.
13. Kuczkowski KM. The safety of anaesthetics in pregnant women. Expert Opin Drug Saf. 2006; 5(2):251-264.
14. Kuczkowski KM. Nonobstetric surgery during pregnancy: what are the risks of anesthesia? Obstet Gynecol Surv. 2004;59(1):52-56.
15. Birnbach DJ, Browne IM. Anesthesia for obstetrics. In: Miller RD, Eriksson LI, Fleisher LA, et al. Miller’s Anesthesia. Philadelphia, PA: Churchill Livingston, Elsevier Health Science; 2010: 2203-2240.
A 33-year-old woman in her 32nd week of pregnancy (gravida 3, para 2) presented to the emergency department (ED) with a five-day history of weakness and ascending numbness below the right knee. She related a two-week history of right-sided low back pain that radiated to the right buttock and was associated with severe right lower extremity (RLE) pain, most prominent in the posterolateral aspect of the right calf. She denied perianal numbness, incontinence, or other changes in bowel or bladder function. She also denied left lower extremity involvement or trauma.
The patient had had one uneventful pregnancy to date. Her medical history included hypothyroidism, treated with levothyroxine; and anxiety, for which she was taking sertraline. She denied any history of allergies, alcohol consumption, smoking, or illicit drug use. She had been evaluated twice and received reassurance in the two weeks before her presentation to the ED. She was admitted to the obstetric service secondary to pain, and a stat MRI rather than x-ray was ordered by obstetrics. An orthopedic consult was ordered. A spine surgeon happened to be on call.
Examination revealed that the patient walked plantigrade, with her right foot slightly externally rotated. She was unable to dorsiflex or plantarflex her right foot. She was unable to heel- or toe-walk on the right side, possessed 0 out of 5 strength at the right extensor hallucis longus and 2 to 3 out of 5 at the right tibialis anterior and gastroc soleus complex. She complained of pain with right leg elevation exceeding 30° and had very limited sensation to light touch in the right L5 and S1 dermatomes. Deep tendon reflex was absent at the right ankle. The patient refused a rectal exam or post-void evaluation.
The initial diagnosis considered by the ED clinician was sciatica, with a differential diagnosis that included pelvic pain of pregnancy, lumbar sprain strain, sciatica, lumbar disk, herniated nucleus pulposus with radiculopathy, and cauda equina syndrome. Trauma was considered and ruled out, as were malignancies; inflammatory, infectious, or degenerative conditions; or other compressive processes.1
Lumbar MRI demonstrated a very large, right-sided disk herniation at L5-S1 with an extruded fragment that was severely compressing the thecal sac and the right S1 nerve root, causing severe right foraminal stenosis at the level of L5-S1. Degenerative changes were noted at L4-5 with disk dessication and no lesions seen.
The patient was diagnosed with cauda equina syndrome, which was felt to be causing severe RLE weakness and ascending numbness. The options of observation, analgesia, physical therapy, and epidural injections were discussed with the patient; however, surgery was strongly recommended due to her profound weakness and the severity of pain she was experiencing, in addition to the size of the disk herniation. She opted for surgery.
The patient was given epidural anesthesia at the L3-4 level, with a catheter left in place during the procedure. A test dose of lidocaine (1.5 cc) with epinephrine was injected to ensure proper placement, and bupivacaine 0.5% was given in increments of 5.0 cc three times during the case. Propofol was administered for sedation, and a 2.0-mg dose of a long-acting morphine was given to the patient before removal of the epidural catheter. Fetal monitoring was performed by obstetrics throughout the procedure.
A laminotomy, partial facetectomy, and diskectomy were performed at L5-S1 with excision of a free fragment. Surgical pathology described the disk as fibrocartilaginous tissue measuring 3.5 cm x 1.4 cm x 0.6 cm.
DISCUSSION
Although nearly half of pregnant women experience low back pain, cauda equina syndrome (CES), a complication of lumbar disk herniation, is extremely rare in the gravid patient.2 In a decade-long review of 48,760 consecutive deliveries, LaBan et al3 identified symptomatic lumbar herniated nucleus pulposus in only five patients (approximately one in 10,000 pregnancies). In pregnant women who do experience CES, symptoms most commonly develop between the fifth and seventh month of pregnancy.4 According to Small et al,5 “The major pitfall in diagnosis is not including CES in the back pain differential.”
True CES presents as a triad of symptoms: lower extremity weakness, altered sensation in the skin of the buttocks and upper posterior thighs (saddle anesthesia), and dysfunction or paralysis of the bowel and bladder. However, few patients present with all of the classic symptoms,6 and patients with CES are often dismissed by several clinicians in their search for relief before presenting to a subspecialist. Kostuik et al7 consider “unilateral sciatica with motor and sensory disturbance” a more common presentation of CES; also indicative of this condition, they report, is “urinary dysfunction combined with motor and sensory loss in the presence of a disc lesion.”
The polypeptide relaxin, which is secreted by the corpus luteum to promote joint laxity in late pregnancy, has been associated with low back pain and pelvic pain of pregnancy; it has also been suggested as a possible contributing cause of CES during pregnancy.8,9 Additionally, increased lumbar lordosis with positional and postural stress may cause direct pressure by the gravid uterus on nerve roots. The great vessels may also be compressed by the uterus, resulting in ischemia of the neural element and back pain that radiates to the legs.10 Many cases of lumbar disk prolapse occur during the first and second trimesters. The most clinically incapacitated patients have been found to have the highest levels of relaxin.9
The Diagnosis
Early diagnosis of CES, through proper physical examination and radiologic studies, is paramount. A rectal examination should be performed to assess for sphincter tone (which may be diminished in 80% of patients) and to assess for perineal sensation.5 Catheterization yielding a postvoid residual urine greater than 100/200 cc is reported to have a specificity and sensitivity of 90% or greater for CES. Small et al5 recommend a straight leg raise maneuver to assess for radiculopathy.
Various studies in the literature support the use of MRI in the gravid patient to confirm the diagnosis of CES and to identify the degree and level of disk protrusion.2-4,11
Treatment
CES requires urgent surgical decompression.11 Early recognition of CES attributable to lumbar disk prolapse, report O’Laoire et al,12 is essential to prevent irreversible sphincter paralysis. They liken the condition’s urgency to that of extradural hematoma in a head injury.
Disk surgery during pregnancy—preferably a team effort, with obstetrics performing perioperative fetal monitoring—has been deemed a safe management method.2,4 Spinal or general anesthesia during nonobstetric surgery is generally considered safe for both mother and fetus.13,14 Adequate oxygenation without risk for hyperventilation is considered essential.15
PATIENT OUTCOME
In the immediate postoperative period, the patient continued to complain of RLE pain, which abated significantly by the time she was discharged. When she was seen in follow-up four days later, she was able to heel- and toe-walk on the right side, and her strength had improved to 3 or 4 out of 5 at the RLE. She continued to experience diminished sensation to the plantar aspect of the right foot, which persisted at the one-month follow up. At that visit, the patient also reported occasional pain in the right buttock. Physical therapy was started to strengthen the RLE.
By three months postsurgery, the patient had undergone uneventful vaginal delivery. She had an entirely benign exam with 5 out of 5 strength at the RLE and no neurologic deficits. She was cleared to return to light weightlifting with good technique and lumbar support but was told to refrain from running until the sixth month postsurgery.
CONCLUSION
Although the case patient did not have a “true” (ie, typical) presentation of CES, her symptoms warranted a full workup and treatment to prevent possible long-term sequelae. Medical practitioners should be familiar with the triad presentation of CES. They must differentiate lower back pain of muscular origin from lumbar disk herniation and be able to appreciate the degree of symptom severity reported by the gravid patient. A thorough history and physical assessment must be performed in every such case. When in doubt, the clinician must err on the side of caution, referring the patient for MRI and consulting with a specialist.
REFERENCES
1. Johnston RA. The management of acute spinal cord compression. J Neurol Neurosurg Psychiatr. 1993;56(10):1046-1054.
2. Brown MD, Levi AD. Surgery for lumbar disc herniation during pregnancy. Spine (Phila PA 1976). 2001;26(5):440-443.
3. LaBan MM, Perrin JCS, Latimer FR. Pregnancy and the herniated lumbar disc. Arch Phys Med Rehabil. 1983;64(7):319-321.
4. LaBan MM, Rapp NS, Van Oeyen P, Meerschaert JR. The lumbar herniated disk of pregnancy: a report of six cases identified by magnetic resonance imaging. Arch Phys Med Rehabil. 1995;76(5):476-479.
5. Small SA, Perron AD, Brady WJ. Orthopedic pitfalls: cauda equina syndrome. Am J Emerg Med. 2005;23(2):159-163.
6. Tay EC, Chacha PB. Midline prolapse of a lumbar intervertebral disc with compression of the cauda equina. J Bone Joint Surg. 1979;61(1):43-46.
7. Kostuik JP, Harrington I, Alexander D, et al. Cauda equina syndrome and lumbar disc herniation. J Bone Joint Surg Am. 1986;68(3):386-391.
8. Russell R, Reynolds F. Back pain, pregnancy, and childbirth. BMJ. 1997;314(7087):1062-1063.
9. MacLennan AH, Nicholson R, Green RC, Bath M. Serum relaxin and pelvic pain of pregnancy. Lancet. 1986;2(8501):243-245.
10. Ashkan K, Casey AT, Powell M, Crockard HA. Back pain during pregnancy and after childbirth: an unusual cause not to miss. J R Soc Med. 1998;91(2):88-90.
11. Busse JW, Bhandari M, Schnittker JB, et al. Delayed presentation of cauda equina syndrome secondary to lumbar disc herniation: functional outcomes and health-related quality of life. CJEM. 2001;3(4):285-291.
12. O’Laoire SA, Crockard HA, Thomas DG. Prognosis for sphincter recovery after operation for cauda equina compression owing to lumbar disc prolapse. Br Med J (Clin Res Ed). 1981;282(6279):1852-1854.
13. Kuczkowski KM. The safety of anaesthetics in pregnant women. Expert Opin Drug Saf. 2006; 5(2):251-264.
14. Kuczkowski KM. Nonobstetric surgery during pregnancy: what are the risks of anesthesia? Obstet Gynecol Surv. 2004;59(1):52-56.
15. Birnbach DJ, Browne IM. Anesthesia for obstetrics. In: Miller RD, Eriksson LI, Fleisher LA, et al. Miller’s Anesthesia. Philadelphia, PA: Churchill Livingston, Elsevier Health Science; 2010: 2203-2240.
A 33-year-old woman in her 32nd week of pregnancy (gravida 3, para 2) presented to the emergency department (ED) with a five-day history of weakness and ascending numbness below the right knee. She related a two-week history of right-sided low back pain that radiated to the right buttock and was associated with severe right lower extremity (RLE) pain, most prominent in the posterolateral aspect of the right calf. She denied perianal numbness, incontinence, or other changes in bowel or bladder function. She also denied left lower extremity involvement or trauma.
The patient had had one uneventful pregnancy to date. Her medical history included hypothyroidism, treated with levothyroxine; and anxiety, for which she was taking sertraline. She denied any history of allergies, alcohol consumption, smoking, or illicit drug use. She had been evaluated twice and received reassurance in the two weeks before her presentation to the ED. She was admitted to the obstetric service secondary to pain, and a stat MRI rather than x-ray was ordered by obstetrics. An orthopedic consult was ordered. A spine surgeon happened to be on call.
Examination revealed that the patient walked plantigrade, with her right foot slightly externally rotated. She was unable to dorsiflex or plantarflex her right foot. She was unable to heel- or toe-walk on the right side, possessed 0 out of 5 strength at the right extensor hallucis longus and 2 to 3 out of 5 at the right tibialis anterior and gastroc soleus complex. She complained of pain with right leg elevation exceeding 30° and had very limited sensation to light touch in the right L5 and S1 dermatomes. Deep tendon reflex was absent at the right ankle. The patient refused a rectal exam or post-void evaluation.
The initial diagnosis considered by the ED clinician was sciatica, with a differential diagnosis that included pelvic pain of pregnancy, lumbar sprain strain, sciatica, lumbar disk, herniated nucleus pulposus with radiculopathy, and cauda equina syndrome. Trauma was considered and ruled out, as were malignancies; inflammatory, infectious, or degenerative conditions; or other compressive processes.1
Lumbar MRI demonstrated a very large, right-sided disk herniation at L5-S1 with an extruded fragment that was severely compressing the thecal sac and the right S1 nerve root, causing severe right foraminal stenosis at the level of L5-S1. Degenerative changes were noted at L4-5 with disk dessication and no lesions seen.
The patient was diagnosed with cauda equina syndrome, which was felt to be causing severe RLE weakness and ascending numbness. The options of observation, analgesia, physical therapy, and epidural injections were discussed with the patient; however, surgery was strongly recommended due to her profound weakness and the severity of pain she was experiencing, in addition to the size of the disk herniation. She opted for surgery.
The patient was given epidural anesthesia at the L3-4 level, with a catheter left in place during the procedure. A test dose of lidocaine (1.5 cc) with epinephrine was injected to ensure proper placement, and bupivacaine 0.5% was given in increments of 5.0 cc three times during the case. Propofol was administered for sedation, and a 2.0-mg dose of a long-acting morphine was given to the patient before removal of the epidural catheter. Fetal monitoring was performed by obstetrics throughout the procedure.
A laminotomy, partial facetectomy, and diskectomy were performed at L5-S1 with excision of a free fragment. Surgical pathology described the disk as fibrocartilaginous tissue measuring 3.5 cm x 1.4 cm x 0.6 cm.
DISCUSSION
Although nearly half of pregnant women experience low back pain, cauda equina syndrome (CES), a complication of lumbar disk herniation, is extremely rare in the gravid patient.2 In a decade-long review of 48,760 consecutive deliveries, LaBan et al3 identified symptomatic lumbar herniated nucleus pulposus in only five patients (approximately one in 10,000 pregnancies). In pregnant women who do experience CES, symptoms most commonly develop between the fifth and seventh month of pregnancy.4 According to Small et al,5 “The major pitfall in diagnosis is not including CES in the back pain differential.”
True CES presents as a triad of symptoms: lower extremity weakness, altered sensation in the skin of the buttocks and upper posterior thighs (saddle anesthesia), and dysfunction or paralysis of the bowel and bladder. However, few patients present with all of the classic symptoms,6 and patients with CES are often dismissed by several clinicians in their search for relief before presenting to a subspecialist. Kostuik et al7 consider “unilateral sciatica with motor and sensory disturbance” a more common presentation of CES; also indicative of this condition, they report, is “urinary dysfunction combined with motor and sensory loss in the presence of a disc lesion.”
The polypeptide relaxin, which is secreted by the corpus luteum to promote joint laxity in late pregnancy, has been associated with low back pain and pelvic pain of pregnancy; it has also been suggested as a possible contributing cause of CES during pregnancy.8,9 Additionally, increased lumbar lordosis with positional and postural stress may cause direct pressure by the gravid uterus on nerve roots. The great vessels may also be compressed by the uterus, resulting in ischemia of the neural element and back pain that radiates to the legs.10 Many cases of lumbar disk prolapse occur during the first and second trimesters. The most clinically incapacitated patients have been found to have the highest levels of relaxin.9
The Diagnosis
Early diagnosis of CES, through proper physical examination and radiologic studies, is paramount. A rectal examination should be performed to assess for sphincter tone (which may be diminished in 80% of patients) and to assess for perineal sensation.5 Catheterization yielding a postvoid residual urine greater than 100/200 cc is reported to have a specificity and sensitivity of 90% or greater for CES. Small et al5 recommend a straight leg raise maneuver to assess for radiculopathy.
Various studies in the literature support the use of MRI in the gravid patient to confirm the diagnosis of CES and to identify the degree and level of disk protrusion.2-4,11
Treatment
CES requires urgent surgical decompression.11 Early recognition of CES attributable to lumbar disk prolapse, report O’Laoire et al,12 is essential to prevent irreversible sphincter paralysis. They liken the condition’s urgency to that of extradural hematoma in a head injury.
Disk surgery during pregnancy—preferably a team effort, with obstetrics performing perioperative fetal monitoring—has been deemed a safe management method.2,4 Spinal or general anesthesia during nonobstetric surgery is generally considered safe for both mother and fetus.13,14 Adequate oxygenation without risk for hyperventilation is considered essential.15
PATIENT OUTCOME
In the immediate postoperative period, the patient continued to complain of RLE pain, which abated significantly by the time she was discharged. When she was seen in follow-up four days later, she was able to heel- and toe-walk on the right side, and her strength had improved to 3 or 4 out of 5 at the RLE. She continued to experience diminished sensation to the plantar aspect of the right foot, which persisted at the one-month follow up. At that visit, the patient also reported occasional pain in the right buttock. Physical therapy was started to strengthen the RLE.
By three months postsurgery, the patient had undergone uneventful vaginal delivery. She had an entirely benign exam with 5 out of 5 strength at the RLE and no neurologic deficits. She was cleared to return to light weightlifting with good technique and lumbar support but was told to refrain from running until the sixth month postsurgery.
CONCLUSION
Although the case patient did not have a “true” (ie, typical) presentation of CES, her symptoms warranted a full workup and treatment to prevent possible long-term sequelae. Medical practitioners should be familiar with the triad presentation of CES. They must differentiate lower back pain of muscular origin from lumbar disk herniation and be able to appreciate the degree of symptom severity reported by the gravid patient. A thorough history and physical assessment must be performed in every such case. When in doubt, the clinician must err on the side of caution, referring the patient for MRI and consulting with a specialist.
REFERENCES
1. Johnston RA. The management of acute spinal cord compression. J Neurol Neurosurg Psychiatr. 1993;56(10):1046-1054.
2. Brown MD, Levi AD. Surgery for lumbar disc herniation during pregnancy. Spine (Phila PA 1976). 2001;26(5):440-443.
3. LaBan MM, Perrin JCS, Latimer FR. Pregnancy and the herniated lumbar disc. Arch Phys Med Rehabil. 1983;64(7):319-321.
4. LaBan MM, Rapp NS, Van Oeyen P, Meerschaert JR. The lumbar herniated disk of pregnancy: a report of six cases identified by magnetic resonance imaging. Arch Phys Med Rehabil. 1995;76(5):476-479.
5. Small SA, Perron AD, Brady WJ. Orthopedic pitfalls: cauda equina syndrome. Am J Emerg Med. 2005;23(2):159-163.
6. Tay EC, Chacha PB. Midline prolapse of a lumbar intervertebral disc with compression of the cauda equina. J Bone Joint Surg. 1979;61(1):43-46.
7. Kostuik JP, Harrington I, Alexander D, et al. Cauda equina syndrome and lumbar disc herniation. J Bone Joint Surg Am. 1986;68(3):386-391.
8. Russell R, Reynolds F. Back pain, pregnancy, and childbirth. BMJ. 1997;314(7087):1062-1063.
9. MacLennan AH, Nicholson R, Green RC, Bath M. Serum relaxin and pelvic pain of pregnancy. Lancet. 1986;2(8501):243-245.
10. Ashkan K, Casey AT, Powell M, Crockard HA. Back pain during pregnancy and after childbirth: an unusual cause not to miss. J R Soc Med. 1998;91(2):88-90.
11. Busse JW, Bhandari M, Schnittker JB, et al. Delayed presentation of cauda equina syndrome secondary to lumbar disc herniation: functional outcomes and health-related quality of life. CJEM. 2001;3(4):285-291.
12. O’Laoire SA, Crockard HA, Thomas DG. Prognosis for sphincter recovery after operation for cauda equina compression owing to lumbar disc prolapse. Br Med J (Clin Res Ed). 1981;282(6279):1852-1854.
13. Kuczkowski KM. The safety of anaesthetics in pregnant women. Expert Opin Drug Saf. 2006; 5(2):251-264.
14. Kuczkowski KM. Nonobstetric surgery during pregnancy: what are the risks of anesthesia? Obstet Gynecol Surv. 2004;59(1):52-56.
15. Birnbach DJ, Browne IM. Anesthesia for obstetrics. In: Miller RD, Eriksson LI, Fleisher LA, et al. Miller’s Anesthesia. Philadelphia, PA: Churchill Livingston, Elsevier Health Science; 2010: 2203-2240.
Assessment of Ipsilateral Versus Contralateral Proximal Fibula for Use in Distal Radius Osteoarticular Reconstruction
Verruciform Xanthoma: A Special Epidermal Nevus
Disoriented and forgetful
CASE: Disoriented and delusional
Ms. P, a 53-year-old registered nurse, is admitted to the inpatient psychiatric unit with confusion, markedly disorganized thought processes, delayed verbal responsiveness, mood lability, and persecutory delusions. Shortly before hospitalization, Ms. P traveled approximately 360 miles from her daughter’s home with a male companion. Noting changes in her mental status, the man brought Ms. P to the local hospital. She was then transferred to our facility.
At admission, Ms. P is not oriented to time. She denies auditory or visual hallucinations and does not display psychomotor agitation or retardation. She reports her mood as sad and her affect is mildly labile. Insight and judgment are considered poor.
Five years ago, Ms. P and her mother were diagnosed with Fabry’s disease (FD) based on genetic analysis. Both women are carriers for the mutations and Ms. P’s mother was found to have almost absent galactosidase activity.
The authors’ observations
FD is an X-linked recessive glycolipid storage disease caused by deficient activity of the lysosomal storage enzyme α-galactosidase A. The disorder affects both men and women and leads to progressive intracellular accumulation of globotriaosylceramide and other related glycosphingolipids.1,2 The earliest FD symptoms—burning pain and acroparesthesias—typically appear in childhood (Table 1).2 FD often is misdiagnosed in women because women tend to display neurologic symptoms later than men, with typical symptom onset in the teenage years.3,4 Often, these symptoms are confused with psychiatric disorders or vague neurologic or pain syndromes.5 In patients with no family history of FD, accurate diagnosis may not be made until adulthood.
Laboratory, dermatologic, and genetic tests can accurately determine the presence of FD.1 However, because multiple organ systems are involved, initially attributing symptoms to FD is challenging, particularly in women.1,3,5 For men, diagnosis can be established by measuring plasma or urinary globotriaosylceramide or plasma α-galactosidase A in addition to genetic analysis. In women, genetic analysis is a better diagnosis strategy because elevations in globotriaosylceramide or α-galactosidase A may not be prominent. An algorithm for diagnosing and assessing patients with FD has been proposed.2
Table 1
Typical signs and symptoms of Fabry’s disease
| Typical time at onset | Signs/symptoms |
|---|---|
| Childhood and adolescence (age ≤16) | Neuropathic pain Ophthalmologic abnormalities (cornea verticillata and tortuous retinal blood vessels) Hearing impairment Dyshidrosis (hypohidrosis and hyperhidrosis) Hypersensitivity to heat and cold Gastrointestinal disturbances and abdominal pain Lethargy and tiredness Angiokeratomas Onset of renal and cardiac signs (eg, microalbuminuria, proteinuria, abnormal heart rate variability) |
| Early adulthood (age 17 to 30) | Extension of any of the above Proteinuria and progressive renal failure Cardiomyopathy Transient ischemic attacks, strokes Facial dysmorphism |
| Later adulthood (age >30) | Worsening of any of the above Heart disease (eg, left ventricular hypertrophy, angina, arrhythmia, and dyspnea) Transient ischemic attacks, strokes Osteopenia and osteoporosis |
| Source: Mehta A, Beck M, Eyskens F, et al. Fabry disease: a review of current management strategies. QJM. 2010;103(9):641-659, by permission of Oxford University Press | |
HISTORY: Cognitive deterioration
Ms. P has had psychiatric symptoms such as depression and anxiety since childhood. However, 3 years ago she started to experience psychological and cognitive deterioration. Medical records indicate that Ms. P described memory and concentration problems over the previous few years. She also reported pain, weakness, and numbness in her left leg after surgery for a work-related back injury, for which she received a financial settlement through workers’ compensation. Shortly thereafter, Ms. P separated from her third husband, moved in with her parents, and found work as a psychiatric nurse. She was dismissed after 6 weeks because she could not learn the electronic medical record system and had difficulty with memory and attention. Her performance on the Mini-Mental State Exam6 at that time was 28 out of 30, which was within normal limits.
After her parents died 3 years ago, Ms. P lived with her daughter, who became her primary caregiver and legal guardian. Ms. P’s daughter notes that her mother’s impulsive and risky behaviors grew more pronounced. Ms. P went on shopping sprees and became sexually promiscuous.
Ms. P’s psychiatric history includes childhood sexual abuse, hospitalization for a suicide attempt at age 19, and courses of psychotherapy and pharmacotherapy. In addition to FD, Ms. P’s medical history consists of coronary artery disease, type 2 diabetes mellitus, hypercholesterolemia, obesity, arthritis, back pain, fibromyalgia, and gastroesophageal reflux disease. Her family history is notable for alcohol abuse (both parents and a brother), lung cancer (mother), myocardial infarction (father), and Alzheimer’s disease (father).
The authors’ observations
Because α-galactosidase A is ubiquitous throughout the body, in addition to neurologic symptoms, FD involves multiple organ systems, with possible dermatologic, renal, gastrointestinal, cardiac, and cerebrovascular dysfunction. Despite growth in FD research, including the Fabry Outcomes Survey,3 the psychosocial and neuropsychiatric implications of the disease remain unclear.7 Behavioral presentations are idiosyncratic and unstable over time, depending on the structures impacted by progressive glycosphingolipid accumulation. Premature cardiovascular events (onset between age 30 and 40 for women), greater incidence of ischemic stroke or transient ischemic attack (7% to 30%), and frequent evidence of white matter lesions put FD patients at greater risk for developing presenile vascular dementia.1,3 Nearly all male FD patients with dementia show some evidence of stroke or transient ischemic attack; cognitive functioning has not been well explored in female patients.4 In a heterogeneous sample of 15 FD patients age 7 to 61, Segal et al8 noted deficits in attention, processing speed, and executive function .75 to 1.95 standard deviations below normative means. No patients in this study had a history of stroke or transient ischemic attack; neuroimaging studies were not reported. Kolodny and Pastores9 suggested multiple mechanisms for cognitive disruption, suggesting that mild dementia late in the disease course could be secondary to diffuse leukomalacia, multiple strokes, or possibly to lipid storage in hippocampal and frontal lobe neurons.
Psychiatric comorbidity
Psychiatric illness, such as depression or a personality disorder, may be comorbid with FD, although pathologic mechanisms remain unclear.7,10,11 Hypothesized mechanisms include:
- psychosocial stress from chronic disease
- white matter changes
- disruption of impaired L-arginine-nitric oxide pathways.7,12
Crosbie et al13 noted that FD patients presented with greater psychological distress as measured by the Minnesota Multiphasic Personality Inventory-2 than patients with Gaucher disease or chronic heart disease. However, no significant differences were found between patients with FD and those diagnosed with a pain disorder. In the Segal et al study, out of 11 adult FD patients, 4 were diagnosed with major depressive disorder, 1 with schizophrenia, 2 with schizotypal personality disorder, and 1 with borderline personality disorder.8
EVALUATION: Brain abnormalities
Head CT scans (conducted 2 years ago and 6 months ago) revealed prominent cortical sulci likely caused by underlying volume loss, especially in bifrontal areas. A brain MRI performed 2 months ago indicated a moderate degree of subcortical atrophy in bilateral frontal and parietal regions. These radiology findings suggest mild to moderate frontal atrophy, mild degree of white matter changes, and slightly enlarged ventricles. An EEG showed background slowing and lack of an alpha rhythm, indicative of cerebral cortical dysfunction.
Ms. P’s α-galactosidase A level was within normal limits; however, normal enzyme levels frequently are reported in symptomatic and asymptomatic female FD patients.14 A dermatology consult confirmed the presence of skin findings characteristic of FD (ie, multiple cherry red papules extensively distributed throughout Ms. P’s chest, abdomen, and back, as well as upper and lower extremities).
Ms. P completed 2 neuropsychological assessments separated by 5 months. For a summary of the results of these tests, see the table titled “Ms. P’s neuropsychological assessment results”. Both assessments revealed grossly impaired intellectual capacity, memory, processing speed, and motor functioning. During the assessment, Ms. P could understand all directions with minimal changes from standardized protocols. Ms. P became insistent that she would not be able to complete memory tasks successfully. She gave up prematurely on tasks, saying they were too difficult. She admitted to guessing on several items because she did not want to continue the task.
Ms. P’s performance on tasks measuring effort and validity of a person’s neuropsychological presentation was consistent with someone exaggerating neurologic symptoms. A person with true dementia may perform as poorly as Ms. P did. However, Ms. P’s scores likely underestimated her level of functioning, even if she was experiencing dementia. Ms. P could not complete tasks individuals with severe dementia complete successfully, such as simple addition and subtraction and digit repetition. Ms. P recalled several recent and remote events, such as her breakfast menu and location of her first assessment, but could not recall words practiced multiple times. Although Ms. P’s scores on a complex card-sorting task were in the impaired range, a detailed review of her pattern indicated that although Ms. P could not generate any correct sorting categories, she made few repetitive responses and errors. This pattern is consistent with someone who understands task requirements, but deliberately avoids answering correctly. This suggests that she retained some ability for hypotheses generation and problem solving; however, because she exaggerated her symptoms, specific deficits could not be determined.
The authors’ observations
Ms. P presented with an interesting manifestation of neuropsychiatric symptoms in the context of FD; however, common cardiac and cerebrovascular features of the disease were not fully developed. Ms. P experienced progressive cognitive and behavioral changes for 2 years before her admission (Table 2), which may represent a prodromal period leading up to what appeared to be a frontally mediated dementia syndrome. Müller et al15 described a patient with FD who displayed a behavioral profile similar to Ms. P’s that included increasingly unstable mood for at least 3 years, borderline personality disorder features, and rapidly fluctuating mood. A case study reported that risperidone, 1 mg/d, used to treat psychosis in a male FD patient caused extrapyramidal symptoms.16
Ms. P presented with no evidence of stroke or transient ischemic attacks, which is atypical for FD patients with cognitive impairment. However, neuroimaging did reveal frontal atrophy that may be associated with her impulse control deficits, risk-taking behavior, emotional instability, and poor judgment. Her cognitive testing was notable for impairment and exaggeration of symptoms consistent with personality disorder symptoms. Possible reasons for exaggeration include a desire to maintain the sick role or secondary gain related to obtaining disability income.
Ms. P’s behavior pattern could be caused by dementia with frontal features, possibly secondary to FD, in combination with personality and psychiatric pathology.
The mainstay of FD treatment is enzyme replacement therapy (ERT), which addresses the underlying enzyme deficiency. Available research indicates that ERT may reduce symptom severity and slow disease progression; however, further studies are needed to determine if it will reduce outcomes such as stroke, ischemic heart disease, or renal disease.2
Table 2
Symptoms that preceded Ms. P’s admission
| Time frame | Symptoms |
|---|---|
| 24 months before admission | Depressed mood Decreased ability to manage independent activities of daily living (eg, finances, cooking) Minimal objective cognitive impairment |
| 12 months before admission | Increased depression Mild to moderate decline in cognitive functioning Visual and auditory hallucinations Impulsivity/poor impulse control Irrational decision-making Increased risky behavior |
| 6 months before admission | Severe cognitive decline with cognitive symptom exaggeration Psychiatric symptom exaggeration Disorganized thinking Continued risky behavior and poor decision-making |
TREATMENT: Persistent deficits
Ms. P is started on risperidone rapidly titrated to 4 mg/d for delusional thinking and behavioral disturbance. After initially improving, she develops delirium when risperidone is increased to 4 mg/d. She has visual hallucinations, marked confusion with disorientation, worsened short-term memory, and an unsteady, shuffling gait. Risperidone is tapered and discontinued and Ms. P’s motor symptoms resolve within 2 days; however, she remains confused and delusional. We start her on quetiapine, 25 mg/d titrated to 50 mg/d, and her agitation and delusional thinking progressively decline. Memantine, titrated to 20 mg/d, and rivastigmine, started at 3 mg/d titrated to 9 mg/d, are added to address her cognitive symptoms.
Over several weeks, Ms. P’s mental status slowly improves and her drug-induced delirium completely resolves. However, she has persistent cognitive impairment characterized by compromised short-term memory and poor insight into her medical and psychological condition. She maintains unrealistic expectations about her ability to live independently and return to the workforce. The treatment team recommends that Ms. P’s daughter pursue guardianship and that she receive around-the-clock supervision after discharge from the hospital.
Table
Ms. P’s neuropsychological assessment results
| June | November | |
|---|---|---|
| Intellectual functioning | ||
| Wechsler Adult Intelligence Scale-III | ||
| FSIQ | 60 | |
| VIQ | 68 | |
| PIQ | 56 | |
| Ravens Colored Progressive Matrices | 70 | |
| Premorbid intellectual functioning estimates | ||
| Peabody Picture Vocabulary Test-2 | 89 | |
| Barona Demographic Estimate | 104 | 104 |
| North American Adult Reading Test | 99 | |
| Memory functioning | ||
| Wechsler Memory Scale-III | ||
| Immediate memory | 45 | |
| General delay memory | 47 | |
| Auditory recognition delay | 55 | |
| California Verbal Learning Test-II | ||
| Trial 1 (immediate recall) | <60 (raw = 3) | |
| Trial 5 | <60 (raw = 3) | |
| Total Words Learned | <60 (raw = 15) | |
| Short Delay Free Recall | <60 (raw = 2) | |
| Long Delay Free Recall | <60 (raw = 4) | |
| Executive functioning | ||
| Trail Making Test A | 88 | 88 |
| Trail Making Test B | failed to understand | failed to understand |
| Wisconsin Card Sort-64 | ||
| Number of categories | <60 (raw = 0) | |
| Errors | 81 | |
| Percent conceptual level responses | 74 | |
| Perseverative responses | 107 | |
| Perseverative errors | 108 | |
| COWAT FAS | 65 | 69 |
| Category exemplar | 69 | 80 |
| Motor functioning | ||
| Finger Tapping Dominant Hand | 68 | |
| Finger Tapping Non-Dominant Hand | 62 | |
| Invalidity/effort | ||
| TOMM | ||
| Trial 1 | raw = 34 | raw = 37 |
| Trial 2 | raw = 42 | raw = 45 |
| Recognition | raw = 44 | |
| MSVT verbal | fail | |
| MSVT nonverbal | fail | |
| Scores provided are standardized (mean = 100; SD = 15). Raw scores are also provided when indicated. COWAT: Controlled oral word association test; FSIQ: Full Scale IQ; MSVT: Medical Symptom Validity Test; PIQ: Performance IQ; TOMM: Test of Memory Malingering; VIQ: Verbal IQ | ||
Related Resources
- National Institute of Neurological Disorders and Stroke. Fabry disease information page. www.ninds.nih.gov/disorders/fabrys/fabrys.htm.
- National Fabry Disease Foundation. www.thenfdf.org.
- Rozenfeld P, Neumann PM. Treatment of Fabry disease: current and emerging strategies. Curr Pharm Biotechnol. 2011;12(6):916-922.
Drug Brand Names
- Donepezil • Aricept
- Memantine • Namenda
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Rivastigmine • Exelon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Eng CM, Germain DP, Banikazemi M, et al. Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med. 2006;8(9):539-548.
2. Mehta A, Beck M, Eyskens F, et al. Fabry disease: a review of current management strategies. QJM. 2010;103(9):641-659.
3. Deegan PB, Baehner AF, Barba Romero MA, et al. Natural history of Fabry disease in females in the Fabry Outcome Survey. J Med Genet. 2006;43(4):347-352.
4. Fellgiebel A, Müller MJ, Ginsberg L. CNS manifestations of Fabry’s disease. Lancet Neurol. 2006;5(9):791-795.
5. Møller AT, Jensen TS. Neurological manifestations in Fabry’s disease. Nat Clin Pract Neurol. 2007;3(2):95-106.
6. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
7. Müller MJ. Neuropsychiatric and psychosocial aspects of Fabry disease. In: Mehta A Beck M, Sunder-Plassman G, eds. Fabry disease: perspectives from 5 years of FOS. Oxford, United Kingdom: Oxford PharmaGenesis Ltd; 2006. http://www.ncbi.nlm.nih.gov/books/nbk11618. Accessed October 31, 2011.
8. Segal P, Kohn Y, Pollak Y, et al. Psychiatric and cognitive profile in Anderson-Fabry patients: a preliminary study. J Inherit Metab Dis. 2010;33(4):429-436.
9. Kolodny EH, Pastores GM. Anderson-Fabry disease: Extrarenal neurologic manifestations. J Am Soc Nephrol. 2002;13(suppl 2):S150-153.
10. Grewal RP. Psychiatric disorders in patients with Fabry disease. Int J Psychiatry Med. 1993;23(3):307-312.
11. Müller MJ, Müller KM, Dascalescu A, et al. Psychiatric and neuropsychological signs and symptoms in patients with Fabry disease: literature review [in German]. Fortschr Neurol Psychiatr. 2005;73(11):687-693.
12. Segal P, Raas-Rothschild A. Neuropsychiatric manifestations of AFD. In: Elstein D Altarescu G, Beck M, eds. Fabry disease. New York, NY: Springer; 2010:321–324.
13. Crosbie TW, Packman W, Packman S. Psychological aspects of patients with Fabry disease. J Inherit Metab Dis. 2009;32(6):745-753.
14. Linthorst GE, Poorthuis BJ, Hollak CE. Enzyme activity for determination of presence of Fabry disease in women results in 40% false-negative results. J Am Coll Cardiol. 2008;51(21):2082.-
15. Müller MJ, Fellgiebel A, Scheurich A, et al. Recurrent brief depression in female patient with Fabry disease. Bipolar Disord. 2006;8(4):418-419.
16. Shen YC, Haw-Ming L, Lin CC, et al. Psychosis in a patient with Fabry’s disease and treatment with aripiprazole. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):779-780.
CASE: Disoriented and delusional
Ms. P, a 53-year-old registered nurse, is admitted to the inpatient psychiatric unit with confusion, markedly disorganized thought processes, delayed verbal responsiveness, mood lability, and persecutory delusions. Shortly before hospitalization, Ms. P traveled approximately 360 miles from her daughter’s home with a male companion. Noting changes in her mental status, the man brought Ms. P to the local hospital. She was then transferred to our facility.
At admission, Ms. P is not oriented to time. She denies auditory or visual hallucinations and does not display psychomotor agitation or retardation. She reports her mood as sad and her affect is mildly labile. Insight and judgment are considered poor.
Five years ago, Ms. P and her mother were diagnosed with Fabry’s disease (FD) based on genetic analysis. Both women are carriers for the mutations and Ms. P’s mother was found to have almost absent galactosidase activity.
The authors’ observations
FD is an X-linked recessive glycolipid storage disease caused by deficient activity of the lysosomal storage enzyme α-galactosidase A. The disorder affects both men and women and leads to progressive intracellular accumulation of globotriaosylceramide and other related glycosphingolipids.1,2 The earliest FD symptoms—burning pain and acroparesthesias—typically appear in childhood (Table 1).2 FD often is misdiagnosed in women because women tend to display neurologic symptoms later than men, with typical symptom onset in the teenage years.3,4 Often, these symptoms are confused with psychiatric disorders or vague neurologic or pain syndromes.5 In patients with no family history of FD, accurate diagnosis may not be made until adulthood.
Laboratory, dermatologic, and genetic tests can accurately determine the presence of FD.1 However, because multiple organ systems are involved, initially attributing symptoms to FD is challenging, particularly in women.1,3,5 For men, diagnosis can be established by measuring plasma or urinary globotriaosylceramide or plasma α-galactosidase A in addition to genetic analysis. In women, genetic analysis is a better diagnosis strategy because elevations in globotriaosylceramide or α-galactosidase A may not be prominent. An algorithm for diagnosing and assessing patients with FD has been proposed.2
Table 1
Typical signs and symptoms of Fabry’s disease
| Typical time at onset | Signs/symptoms |
|---|---|
| Childhood and adolescence (age ≤16) | Neuropathic pain Ophthalmologic abnormalities (cornea verticillata and tortuous retinal blood vessels) Hearing impairment Dyshidrosis (hypohidrosis and hyperhidrosis) Hypersensitivity to heat and cold Gastrointestinal disturbances and abdominal pain Lethargy and tiredness Angiokeratomas Onset of renal and cardiac signs (eg, microalbuminuria, proteinuria, abnormal heart rate variability) |
| Early adulthood (age 17 to 30) | Extension of any of the above Proteinuria and progressive renal failure Cardiomyopathy Transient ischemic attacks, strokes Facial dysmorphism |
| Later adulthood (age >30) | Worsening of any of the above Heart disease (eg, left ventricular hypertrophy, angina, arrhythmia, and dyspnea) Transient ischemic attacks, strokes Osteopenia and osteoporosis |
| Source: Mehta A, Beck M, Eyskens F, et al. Fabry disease: a review of current management strategies. QJM. 2010;103(9):641-659, by permission of Oxford University Press | |
HISTORY: Cognitive deterioration
Ms. P has had psychiatric symptoms such as depression and anxiety since childhood. However, 3 years ago she started to experience psychological and cognitive deterioration. Medical records indicate that Ms. P described memory and concentration problems over the previous few years. She also reported pain, weakness, and numbness in her left leg after surgery for a work-related back injury, for which she received a financial settlement through workers’ compensation. Shortly thereafter, Ms. P separated from her third husband, moved in with her parents, and found work as a psychiatric nurse. She was dismissed after 6 weeks because she could not learn the electronic medical record system and had difficulty with memory and attention. Her performance on the Mini-Mental State Exam6 at that time was 28 out of 30, which was within normal limits.
After her parents died 3 years ago, Ms. P lived with her daughter, who became her primary caregiver and legal guardian. Ms. P’s daughter notes that her mother’s impulsive and risky behaviors grew more pronounced. Ms. P went on shopping sprees and became sexually promiscuous.
Ms. P’s psychiatric history includes childhood sexual abuse, hospitalization for a suicide attempt at age 19, and courses of psychotherapy and pharmacotherapy. In addition to FD, Ms. P’s medical history consists of coronary artery disease, type 2 diabetes mellitus, hypercholesterolemia, obesity, arthritis, back pain, fibromyalgia, and gastroesophageal reflux disease. Her family history is notable for alcohol abuse (both parents and a brother), lung cancer (mother), myocardial infarction (father), and Alzheimer’s disease (father).
The authors’ observations
Because α-galactosidase A is ubiquitous throughout the body, in addition to neurologic symptoms, FD involves multiple organ systems, with possible dermatologic, renal, gastrointestinal, cardiac, and cerebrovascular dysfunction. Despite growth in FD research, including the Fabry Outcomes Survey,3 the psychosocial and neuropsychiatric implications of the disease remain unclear.7 Behavioral presentations are idiosyncratic and unstable over time, depending on the structures impacted by progressive glycosphingolipid accumulation. Premature cardiovascular events (onset between age 30 and 40 for women), greater incidence of ischemic stroke or transient ischemic attack (7% to 30%), and frequent evidence of white matter lesions put FD patients at greater risk for developing presenile vascular dementia.1,3 Nearly all male FD patients with dementia show some evidence of stroke or transient ischemic attack; cognitive functioning has not been well explored in female patients.4 In a heterogeneous sample of 15 FD patients age 7 to 61, Segal et al8 noted deficits in attention, processing speed, and executive function .75 to 1.95 standard deviations below normative means. No patients in this study had a history of stroke or transient ischemic attack; neuroimaging studies were not reported. Kolodny and Pastores9 suggested multiple mechanisms for cognitive disruption, suggesting that mild dementia late in the disease course could be secondary to diffuse leukomalacia, multiple strokes, or possibly to lipid storage in hippocampal and frontal lobe neurons.
Psychiatric comorbidity
Psychiatric illness, such as depression or a personality disorder, may be comorbid with FD, although pathologic mechanisms remain unclear.7,10,11 Hypothesized mechanisms include:
- psychosocial stress from chronic disease
- white matter changes
- disruption of impaired L-arginine-nitric oxide pathways.7,12
Crosbie et al13 noted that FD patients presented with greater psychological distress as measured by the Minnesota Multiphasic Personality Inventory-2 than patients with Gaucher disease or chronic heart disease. However, no significant differences were found between patients with FD and those diagnosed with a pain disorder. In the Segal et al study, out of 11 adult FD patients, 4 were diagnosed with major depressive disorder, 1 with schizophrenia, 2 with schizotypal personality disorder, and 1 with borderline personality disorder.8
EVALUATION: Brain abnormalities
Head CT scans (conducted 2 years ago and 6 months ago) revealed prominent cortical sulci likely caused by underlying volume loss, especially in bifrontal areas. A brain MRI performed 2 months ago indicated a moderate degree of subcortical atrophy in bilateral frontal and parietal regions. These radiology findings suggest mild to moderate frontal atrophy, mild degree of white matter changes, and slightly enlarged ventricles. An EEG showed background slowing and lack of an alpha rhythm, indicative of cerebral cortical dysfunction.
Ms. P’s α-galactosidase A level was within normal limits; however, normal enzyme levels frequently are reported in symptomatic and asymptomatic female FD patients.14 A dermatology consult confirmed the presence of skin findings characteristic of FD (ie, multiple cherry red papules extensively distributed throughout Ms. P’s chest, abdomen, and back, as well as upper and lower extremities).
Ms. P completed 2 neuropsychological assessments separated by 5 months. For a summary of the results of these tests, see the table titled “Ms. P’s neuropsychological assessment results”. Both assessments revealed grossly impaired intellectual capacity, memory, processing speed, and motor functioning. During the assessment, Ms. P could understand all directions with minimal changes from standardized protocols. Ms. P became insistent that she would not be able to complete memory tasks successfully. She gave up prematurely on tasks, saying they were too difficult. She admitted to guessing on several items because she did not want to continue the task.
Ms. P’s performance on tasks measuring effort and validity of a person’s neuropsychological presentation was consistent with someone exaggerating neurologic symptoms. A person with true dementia may perform as poorly as Ms. P did. However, Ms. P’s scores likely underestimated her level of functioning, even if she was experiencing dementia. Ms. P could not complete tasks individuals with severe dementia complete successfully, such as simple addition and subtraction and digit repetition. Ms. P recalled several recent and remote events, such as her breakfast menu and location of her first assessment, but could not recall words practiced multiple times. Although Ms. P’s scores on a complex card-sorting task were in the impaired range, a detailed review of her pattern indicated that although Ms. P could not generate any correct sorting categories, she made few repetitive responses and errors. This pattern is consistent with someone who understands task requirements, but deliberately avoids answering correctly. This suggests that she retained some ability for hypotheses generation and problem solving; however, because she exaggerated her symptoms, specific deficits could not be determined.
The authors’ observations
Ms. P presented with an interesting manifestation of neuropsychiatric symptoms in the context of FD; however, common cardiac and cerebrovascular features of the disease were not fully developed. Ms. P experienced progressive cognitive and behavioral changes for 2 years before her admission (Table 2), which may represent a prodromal period leading up to what appeared to be a frontally mediated dementia syndrome. Müller et al15 described a patient with FD who displayed a behavioral profile similar to Ms. P’s that included increasingly unstable mood for at least 3 years, borderline personality disorder features, and rapidly fluctuating mood. A case study reported that risperidone, 1 mg/d, used to treat psychosis in a male FD patient caused extrapyramidal symptoms.16
Ms. P presented with no evidence of stroke or transient ischemic attacks, which is atypical for FD patients with cognitive impairment. However, neuroimaging did reveal frontal atrophy that may be associated with her impulse control deficits, risk-taking behavior, emotional instability, and poor judgment. Her cognitive testing was notable for impairment and exaggeration of symptoms consistent with personality disorder symptoms. Possible reasons for exaggeration include a desire to maintain the sick role or secondary gain related to obtaining disability income.
Ms. P’s behavior pattern could be caused by dementia with frontal features, possibly secondary to FD, in combination with personality and psychiatric pathology.
The mainstay of FD treatment is enzyme replacement therapy (ERT), which addresses the underlying enzyme deficiency. Available research indicates that ERT may reduce symptom severity and slow disease progression; however, further studies are needed to determine if it will reduce outcomes such as stroke, ischemic heart disease, or renal disease.2
Table 2
Symptoms that preceded Ms. P’s admission
| Time frame | Symptoms |
|---|---|
| 24 months before admission | Depressed mood Decreased ability to manage independent activities of daily living (eg, finances, cooking) Minimal objective cognitive impairment |
| 12 months before admission | Increased depression Mild to moderate decline in cognitive functioning Visual and auditory hallucinations Impulsivity/poor impulse control Irrational decision-making Increased risky behavior |
| 6 months before admission | Severe cognitive decline with cognitive symptom exaggeration Psychiatric symptom exaggeration Disorganized thinking Continued risky behavior and poor decision-making |
TREATMENT: Persistent deficits
Ms. P is started on risperidone rapidly titrated to 4 mg/d for delusional thinking and behavioral disturbance. After initially improving, she develops delirium when risperidone is increased to 4 mg/d. She has visual hallucinations, marked confusion with disorientation, worsened short-term memory, and an unsteady, shuffling gait. Risperidone is tapered and discontinued and Ms. P’s motor symptoms resolve within 2 days; however, she remains confused and delusional. We start her on quetiapine, 25 mg/d titrated to 50 mg/d, and her agitation and delusional thinking progressively decline. Memantine, titrated to 20 mg/d, and rivastigmine, started at 3 mg/d titrated to 9 mg/d, are added to address her cognitive symptoms.
Over several weeks, Ms. P’s mental status slowly improves and her drug-induced delirium completely resolves. However, she has persistent cognitive impairment characterized by compromised short-term memory and poor insight into her medical and psychological condition. She maintains unrealistic expectations about her ability to live independently and return to the workforce. The treatment team recommends that Ms. P’s daughter pursue guardianship and that she receive around-the-clock supervision after discharge from the hospital.
Table
Ms. P’s neuropsychological assessment results
| June | November | |
|---|---|---|
| Intellectual functioning | ||
| Wechsler Adult Intelligence Scale-III | ||
| FSIQ | 60 | |
| VIQ | 68 | |
| PIQ | 56 | |
| Ravens Colored Progressive Matrices | 70 | |
| Premorbid intellectual functioning estimates | ||
| Peabody Picture Vocabulary Test-2 | 89 | |
| Barona Demographic Estimate | 104 | 104 |
| North American Adult Reading Test | 99 | |
| Memory functioning | ||
| Wechsler Memory Scale-III | ||
| Immediate memory | 45 | |
| General delay memory | 47 | |
| Auditory recognition delay | 55 | |
| California Verbal Learning Test-II | ||
| Trial 1 (immediate recall) | <60 (raw = 3) | |
| Trial 5 | <60 (raw = 3) | |
| Total Words Learned | <60 (raw = 15) | |
| Short Delay Free Recall | <60 (raw = 2) | |
| Long Delay Free Recall | <60 (raw = 4) | |
| Executive functioning | ||
| Trail Making Test A | 88 | 88 |
| Trail Making Test B | failed to understand | failed to understand |
| Wisconsin Card Sort-64 | ||
| Number of categories | <60 (raw = 0) | |
| Errors | 81 | |
| Percent conceptual level responses | 74 | |
| Perseverative responses | 107 | |
| Perseverative errors | 108 | |
| COWAT FAS | 65 | 69 |
| Category exemplar | 69 | 80 |
| Motor functioning | ||
| Finger Tapping Dominant Hand | 68 | |
| Finger Tapping Non-Dominant Hand | 62 | |
| Invalidity/effort | ||
| TOMM | ||
| Trial 1 | raw = 34 | raw = 37 |
| Trial 2 | raw = 42 | raw = 45 |
| Recognition | raw = 44 | |
| MSVT verbal | fail | |
| MSVT nonverbal | fail | |
| Scores provided are standardized (mean = 100; SD = 15). Raw scores are also provided when indicated. COWAT: Controlled oral word association test; FSIQ: Full Scale IQ; MSVT: Medical Symptom Validity Test; PIQ: Performance IQ; TOMM: Test of Memory Malingering; VIQ: Verbal IQ | ||
Related Resources
- National Institute of Neurological Disorders and Stroke. Fabry disease information page. www.ninds.nih.gov/disorders/fabrys/fabrys.htm.
- National Fabry Disease Foundation. www.thenfdf.org.
- Rozenfeld P, Neumann PM. Treatment of Fabry disease: current and emerging strategies. Curr Pharm Biotechnol. 2011;12(6):916-922.
Drug Brand Names
- Donepezil • Aricept
- Memantine • Namenda
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Rivastigmine • Exelon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
CASE: Disoriented and delusional
Ms. P, a 53-year-old registered nurse, is admitted to the inpatient psychiatric unit with confusion, markedly disorganized thought processes, delayed verbal responsiveness, mood lability, and persecutory delusions. Shortly before hospitalization, Ms. P traveled approximately 360 miles from her daughter’s home with a male companion. Noting changes in her mental status, the man brought Ms. P to the local hospital. She was then transferred to our facility.
At admission, Ms. P is not oriented to time. She denies auditory or visual hallucinations and does not display psychomotor agitation or retardation. She reports her mood as sad and her affect is mildly labile. Insight and judgment are considered poor.
Five years ago, Ms. P and her mother were diagnosed with Fabry’s disease (FD) based on genetic analysis. Both women are carriers for the mutations and Ms. P’s mother was found to have almost absent galactosidase activity.
The authors’ observations
FD is an X-linked recessive glycolipid storage disease caused by deficient activity of the lysosomal storage enzyme α-galactosidase A. The disorder affects both men and women and leads to progressive intracellular accumulation of globotriaosylceramide and other related glycosphingolipids.1,2 The earliest FD symptoms—burning pain and acroparesthesias—typically appear in childhood (Table 1).2 FD often is misdiagnosed in women because women tend to display neurologic symptoms later than men, with typical symptom onset in the teenage years.3,4 Often, these symptoms are confused with psychiatric disorders or vague neurologic or pain syndromes.5 In patients with no family history of FD, accurate diagnosis may not be made until adulthood.
Laboratory, dermatologic, and genetic tests can accurately determine the presence of FD.1 However, because multiple organ systems are involved, initially attributing symptoms to FD is challenging, particularly in women.1,3,5 For men, diagnosis can be established by measuring plasma or urinary globotriaosylceramide or plasma α-galactosidase A in addition to genetic analysis. In women, genetic analysis is a better diagnosis strategy because elevations in globotriaosylceramide or α-galactosidase A may not be prominent. An algorithm for diagnosing and assessing patients with FD has been proposed.2
Table 1
Typical signs and symptoms of Fabry’s disease
| Typical time at onset | Signs/symptoms |
|---|---|
| Childhood and adolescence (age ≤16) | Neuropathic pain Ophthalmologic abnormalities (cornea verticillata and tortuous retinal blood vessels) Hearing impairment Dyshidrosis (hypohidrosis and hyperhidrosis) Hypersensitivity to heat and cold Gastrointestinal disturbances and abdominal pain Lethargy and tiredness Angiokeratomas Onset of renal and cardiac signs (eg, microalbuminuria, proteinuria, abnormal heart rate variability) |
| Early adulthood (age 17 to 30) | Extension of any of the above Proteinuria and progressive renal failure Cardiomyopathy Transient ischemic attacks, strokes Facial dysmorphism |
| Later adulthood (age >30) | Worsening of any of the above Heart disease (eg, left ventricular hypertrophy, angina, arrhythmia, and dyspnea) Transient ischemic attacks, strokes Osteopenia and osteoporosis |
| Source: Mehta A, Beck M, Eyskens F, et al. Fabry disease: a review of current management strategies. QJM. 2010;103(9):641-659, by permission of Oxford University Press | |
HISTORY: Cognitive deterioration
Ms. P has had psychiatric symptoms such as depression and anxiety since childhood. However, 3 years ago she started to experience psychological and cognitive deterioration. Medical records indicate that Ms. P described memory and concentration problems over the previous few years. She also reported pain, weakness, and numbness in her left leg after surgery for a work-related back injury, for which she received a financial settlement through workers’ compensation. Shortly thereafter, Ms. P separated from her third husband, moved in with her parents, and found work as a psychiatric nurse. She was dismissed after 6 weeks because she could not learn the electronic medical record system and had difficulty with memory and attention. Her performance on the Mini-Mental State Exam6 at that time was 28 out of 30, which was within normal limits.
After her parents died 3 years ago, Ms. P lived with her daughter, who became her primary caregiver and legal guardian. Ms. P’s daughter notes that her mother’s impulsive and risky behaviors grew more pronounced. Ms. P went on shopping sprees and became sexually promiscuous.
Ms. P’s psychiatric history includes childhood sexual abuse, hospitalization for a suicide attempt at age 19, and courses of psychotherapy and pharmacotherapy. In addition to FD, Ms. P’s medical history consists of coronary artery disease, type 2 diabetes mellitus, hypercholesterolemia, obesity, arthritis, back pain, fibromyalgia, and gastroesophageal reflux disease. Her family history is notable for alcohol abuse (both parents and a brother), lung cancer (mother), myocardial infarction (father), and Alzheimer’s disease (father).
The authors’ observations
Because α-galactosidase A is ubiquitous throughout the body, in addition to neurologic symptoms, FD involves multiple organ systems, with possible dermatologic, renal, gastrointestinal, cardiac, and cerebrovascular dysfunction. Despite growth in FD research, including the Fabry Outcomes Survey,3 the psychosocial and neuropsychiatric implications of the disease remain unclear.7 Behavioral presentations are idiosyncratic and unstable over time, depending on the structures impacted by progressive glycosphingolipid accumulation. Premature cardiovascular events (onset between age 30 and 40 for women), greater incidence of ischemic stroke or transient ischemic attack (7% to 30%), and frequent evidence of white matter lesions put FD patients at greater risk for developing presenile vascular dementia.1,3 Nearly all male FD patients with dementia show some evidence of stroke or transient ischemic attack; cognitive functioning has not been well explored in female patients.4 In a heterogeneous sample of 15 FD patients age 7 to 61, Segal et al8 noted deficits in attention, processing speed, and executive function .75 to 1.95 standard deviations below normative means. No patients in this study had a history of stroke or transient ischemic attack; neuroimaging studies were not reported. Kolodny and Pastores9 suggested multiple mechanisms for cognitive disruption, suggesting that mild dementia late in the disease course could be secondary to diffuse leukomalacia, multiple strokes, or possibly to lipid storage in hippocampal and frontal lobe neurons.
Psychiatric comorbidity
Psychiatric illness, such as depression or a personality disorder, may be comorbid with FD, although pathologic mechanisms remain unclear.7,10,11 Hypothesized mechanisms include:
- psychosocial stress from chronic disease
- white matter changes
- disruption of impaired L-arginine-nitric oxide pathways.7,12
Crosbie et al13 noted that FD patients presented with greater psychological distress as measured by the Minnesota Multiphasic Personality Inventory-2 than patients with Gaucher disease or chronic heart disease. However, no significant differences were found between patients with FD and those diagnosed with a pain disorder. In the Segal et al study, out of 11 adult FD patients, 4 were diagnosed with major depressive disorder, 1 with schizophrenia, 2 with schizotypal personality disorder, and 1 with borderline personality disorder.8
EVALUATION: Brain abnormalities
Head CT scans (conducted 2 years ago and 6 months ago) revealed prominent cortical sulci likely caused by underlying volume loss, especially in bifrontal areas. A brain MRI performed 2 months ago indicated a moderate degree of subcortical atrophy in bilateral frontal and parietal regions. These radiology findings suggest mild to moderate frontal atrophy, mild degree of white matter changes, and slightly enlarged ventricles. An EEG showed background slowing and lack of an alpha rhythm, indicative of cerebral cortical dysfunction.
Ms. P’s α-galactosidase A level was within normal limits; however, normal enzyme levels frequently are reported in symptomatic and asymptomatic female FD patients.14 A dermatology consult confirmed the presence of skin findings characteristic of FD (ie, multiple cherry red papules extensively distributed throughout Ms. P’s chest, abdomen, and back, as well as upper and lower extremities).
Ms. P completed 2 neuropsychological assessments separated by 5 months. For a summary of the results of these tests, see the table titled “Ms. P’s neuropsychological assessment results”. Both assessments revealed grossly impaired intellectual capacity, memory, processing speed, and motor functioning. During the assessment, Ms. P could understand all directions with minimal changes from standardized protocols. Ms. P became insistent that she would not be able to complete memory tasks successfully. She gave up prematurely on tasks, saying they were too difficult. She admitted to guessing on several items because she did not want to continue the task.
Ms. P’s performance on tasks measuring effort and validity of a person’s neuropsychological presentation was consistent with someone exaggerating neurologic symptoms. A person with true dementia may perform as poorly as Ms. P did. However, Ms. P’s scores likely underestimated her level of functioning, even if she was experiencing dementia. Ms. P could not complete tasks individuals with severe dementia complete successfully, such as simple addition and subtraction and digit repetition. Ms. P recalled several recent and remote events, such as her breakfast menu and location of her first assessment, but could not recall words practiced multiple times. Although Ms. P’s scores on a complex card-sorting task were in the impaired range, a detailed review of her pattern indicated that although Ms. P could not generate any correct sorting categories, she made few repetitive responses and errors. This pattern is consistent with someone who understands task requirements, but deliberately avoids answering correctly. This suggests that she retained some ability for hypotheses generation and problem solving; however, because she exaggerated her symptoms, specific deficits could not be determined.
The authors’ observations
Ms. P presented with an interesting manifestation of neuropsychiatric symptoms in the context of FD; however, common cardiac and cerebrovascular features of the disease were not fully developed. Ms. P experienced progressive cognitive and behavioral changes for 2 years before her admission (Table 2), which may represent a prodromal period leading up to what appeared to be a frontally mediated dementia syndrome. Müller et al15 described a patient with FD who displayed a behavioral profile similar to Ms. P’s that included increasingly unstable mood for at least 3 years, borderline personality disorder features, and rapidly fluctuating mood. A case study reported that risperidone, 1 mg/d, used to treat psychosis in a male FD patient caused extrapyramidal symptoms.16
Ms. P presented with no evidence of stroke or transient ischemic attacks, which is atypical for FD patients with cognitive impairment. However, neuroimaging did reveal frontal atrophy that may be associated with her impulse control deficits, risk-taking behavior, emotional instability, and poor judgment. Her cognitive testing was notable for impairment and exaggeration of symptoms consistent with personality disorder symptoms. Possible reasons for exaggeration include a desire to maintain the sick role or secondary gain related to obtaining disability income.
Ms. P’s behavior pattern could be caused by dementia with frontal features, possibly secondary to FD, in combination with personality and psychiatric pathology.
The mainstay of FD treatment is enzyme replacement therapy (ERT), which addresses the underlying enzyme deficiency. Available research indicates that ERT may reduce symptom severity and slow disease progression; however, further studies are needed to determine if it will reduce outcomes such as stroke, ischemic heart disease, or renal disease.2
Table 2
Symptoms that preceded Ms. P’s admission
| Time frame | Symptoms |
|---|---|
| 24 months before admission | Depressed mood Decreased ability to manage independent activities of daily living (eg, finances, cooking) Minimal objective cognitive impairment |
| 12 months before admission | Increased depression Mild to moderate decline in cognitive functioning Visual and auditory hallucinations Impulsivity/poor impulse control Irrational decision-making Increased risky behavior |
| 6 months before admission | Severe cognitive decline with cognitive symptom exaggeration Psychiatric symptom exaggeration Disorganized thinking Continued risky behavior and poor decision-making |
TREATMENT: Persistent deficits
Ms. P is started on risperidone rapidly titrated to 4 mg/d for delusional thinking and behavioral disturbance. After initially improving, she develops delirium when risperidone is increased to 4 mg/d. She has visual hallucinations, marked confusion with disorientation, worsened short-term memory, and an unsteady, shuffling gait. Risperidone is tapered and discontinued and Ms. P’s motor symptoms resolve within 2 days; however, she remains confused and delusional. We start her on quetiapine, 25 mg/d titrated to 50 mg/d, and her agitation and delusional thinking progressively decline. Memantine, titrated to 20 mg/d, and rivastigmine, started at 3 mg/d titrated to 9 mg/d, are added to address her cognitive symptoms.
Over several weeks, Ms. P’s mental status slowly improves and her drug-induced delirium completely resolves. However, she has persistent cognitive impairment characterized by compromised short-term memory and poor insight into her medical and psychological condition. She maintains unrealistic expectations about her ability to live independently and return to the workforce. The treatment team recommends that Ms. P’s daughter pursue guardianship and that she receive around-the-clock supervision after discharge from the hospital.
Table
Ms. P’s neuropsychological assessment results
| June | November | |
|---|---|---|
| Intellectual functioning | ||
| Wechsler Adult Intelligence Scale-III | ||
| FSIQ | 60 | |
| VIQ | 68 | |
| PIQ | 56 | |
| Ravens Colored Progressive Matrices | 70 | |
| Premorbid intellectual functioning estimates | ||
| Peabody Picture Vocabulary Test-2 | 89 | |
| Barona Demographic Estimate | 104 | 104 |
| North American Adult Reading Test | 99 | |
| Memory functioning | ||
| Wechsler Memory Scale-III | ||
| Immediate memory | 45 | |
| General delay memory | 47 | |
| Auditory recognition delay | 55 | |
| California Verbal Learning Test-II | ||
| Trial 1 (immediate recall) | <60 (raw = 3) | |
| Trial 5 | <60 (raw = 3) | |
| Total Words Learned | <60 (raw = 15) | |
| Short Delay Free Recall | <60 (raw = 2) | |
| Long Delay Free Recall | <60 (raw = 4) | |
| Executive functioning | ||
| Trail Making Test A | 88 | 88 |
| Trail Making Test B | failed to understand | failed to understand |
| Wisconsin Card Sort-64 | ||
| Number of categories | <60 (raw = 0) | |
| Errors | 81 | |
| Percent conceptual level responses | 74 | |
| Perseverative responses | 107 | |
| Perseverative errors | 108 | |
| COWAT FAS | 65 | 69 |
| Category exemplar | 69 | 80 |
| Motor functioning | ||
| Finger Tapping Dominant Hand | 68 | |
| Finger Tapping Non-Dominant Hand | 62 | |
| Invalidity/effort | ||
| TOMM | ||
| Trial 1 | raw = 34 | raw = 37 |
| Trial 2 | raw = 42 | raw = 45 |
| Recognition | raw = 44 | |
| MSVT verbal | fail | |
| MSVT nonverbal | fail | |
| Scores provided are standardized (mean = 100; SD = 15). Raw scores are also provided when indicated. COWAT: Controlled oral word association test; FSIQ: Full Scale IQ; MSVT: Medical Symptom Validity Test; PIQ: Performance IQ; TOMM: Test of Memory Malingering; VIQ: Verbal IQ | ||
Related Resources
- National Institute of Neurological Disorders and Stroke. Fabry disease information page. www.ninds.nih.gov/disorders/fabrys/fabrys.htm.
- National Fabry Disease Foundation. www.thenfdf.org.
- Rozenfeld P, Neumann PM. Treatment of Fabry disease: current and emerging strategies. Curr Pharm Biotechnol. 2011;12(6):916-922.
Drug Brand Names
- Donepezil • Aricept
- Memantine • Namenda
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Rivastigmine • Exelon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Eng CM, Germain DP, Banikazemi M, et al. Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med. 2006;8(9):539-548.
2. Mehta A, Beck M, Eyskens F, et al. Fabry disease: a review of current management strategies. QJM. 2010;103(9):641-659.
3. Deegan PB, Baehner AF, Barba Romero MA, et al. Natural history of Fabry disease in females in the Fabry Outcome Survey. J Med Genet. 2006;43(4):347-352.
4. Fellgiebel A, Müller MJ, Ginsberg L. CNS manifestations of Fabry’s disease. Lancet Neurol. 2006;5(9):791-795.
5. Møller AT, Jensen TS. Neurological manifestations in Fabry’s disease. Nat Clin Pract Neurol. 2007;3(2):95-106.
6. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
7. Müller MJ. Neuropsychiatric and psychosocial aspects of Fabry disease. In: Mehta A Beck M, Sunder-Plassman G, eds. Fabry disease: perspectives from 5 years of FOS. Oxford, United Kingdom: Oxford PharmaGenesis Ltd; 2006. http://www.ncbi.nlm.nih.gov/books/nbk11618. Accessed October 31, 2011.
8. Segal P, Kohn Y, Pollak Y, et al. Psychiatric and cognitive profile in Anderson-Fabry patients: a preliminary study. J Inherit Metab Dis. 2010;33(4):429-436.
9. Kolodny EH, Pastores GM. Anderson-Fabry disease: Extrarenal neurologic manifestations. J Am Soc Nephrol. 2002;13(suppl 2):S150-153.
10. Grewal RP. Psychiatric disorders in patients with Fabry disease. Int J Psychiatry Med. 1993;23(3):307-312.
11. Müller MJ, Müller KM, Dascalescu A, et al. Psychiatric and neuropsychological signs and symptoms in patients with Fabry disease: literature review [in German]. Fortschr Neurol Psychiatr. 2005;73(11):687-693.
12. Segal P, Raas-Rothschild A. Neuropsychiatric manifestations of AFD. In: Elstein D Altarescu G, Beck M, eds. Fabry disease. New York, NY: Springer; 2010:321–324.
13. Crosbie TW, Packman W, Packman S. Psychological aspects of patients with Fabry disease. J Inherit Metab Dis. 2009;32(6):745-753.
14. Linthorst GE, Poorthuis BJ, Hollak CE. Enzyme activity for determination of presence of Fabry disease in women results in 40% false-negative results. J Am Coll Cardiol. 2008;51(21):2082.-
15. Müller MJ, Fellgiebel A, Scheurich A, et al. Recurrent brief depression in female patient with Fabry disease. Bipolar Disord. 2006;8(4):418-419.
16. Shen YC, Haw-Ming L, Lin CC, et al. Psychosis in a patient with Fabry’s disease and treatment with aripiprazole. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):779-780.
1. Eng CM, Germain DP, Banikazemi M, et al. Fabry disease: guidelines for the evaluation and management of multi-organ system involvement. Genet Med. 2006;8(9):539-548.
2. Mehta A, Beck M, Eyskens F, et al. Fabry disease: a review of current management strategies. QJM. 2010;103(9):641-659.
3. Deegan PB, Baehner AF, Barba Romero MA, et al. Natural history of Fabry disease in females in the Fabry Outcome Survey. J Med Genet. 2006;43(4):347-352.
4. Fellgiebel A, Müller MJ, Ginsberg L. CNS manifestations of Fabry’s disease. Lancet Neurol. 2006;5(9):791-795.
5. Møller AT, Jensen TS. Neurological manifestations in Fabry’s disease. Nat Clin Pract Neurol. 2007;3(2):95-106.
6. Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198.
7. Müller MJ. Neuropsychiatric and psychosocial aspects of Fabry disease. In: Mehta A Beck M, Sunder-Plassman G, eds. Fabry disease: perspectives from 5 years of FOS. Oxford, United Kingdom: Oxford PharmaGenesis Ltd; 2006. http://www.ncbi.nlm.nih.gov/books/nbk11618. Accessed October 31, 2011.
8. Segal P, Kohn Y, Pollak Y, et al. Psychiatric and cognitive profile in Anderson-Fabry patients: a preliminary study. J Inherit Metab Dis. 2010;33(4):429-436.
9. Kolodny EH, Pastores GM. Anderson-Fabry disease: Extrarenal neurologic manifestations. J Am Soc Nephrol. 2002;13(suppl 2):S150-153.
10. Grewal RP. Psychiatric disorders in patients with Fabry disease. Int J Psychiatry Med. 1993;23(3):307-312.
11. Müller MJ, Müller KM, Dascalescu A, et al. Psychiatric and neuropsychological signs and symptoms in patients with Fabry disease: literature review [in German]. Fortschr Neurol Psychiatr. 2005;73(11):687-693.
12. Segal P, Raas-Rothschild A. Neuropsychiatric manifestations of AFD. In: Elstein D Altarescu G, Beck M, eds. Fabry disease. New York, NY: Springer; 2010:321–324.
13. Crosbie TW, Packman W, Packman S. Psychological aspects of patients with Fabry disease. J Inherit Metab Dis. 2009;32(6):745-753.
14. Linthorst GE, Poorthuis BJ, Hollak CE. Enzyme activity for determination of presence of Fabry disease in women results in 40% false-negative results. J Am Coll Cardiol. 2008;51(21):2082.-
15. Müller MJ, Fellgiebel A, Scheurich A, et al. Recurrent brief depression in female patient with Fabry disease. Bipolar Disord. 2006;8(4):418-419.
16. Shen YC, Haw-Ming L, Lin CC, et al. Psychosis in a patient with Fabry’s disease and treatment with aripiprazole. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):779-780.