User login
Nebulized amphotericin B does not affect aspergillosis exacerbation-free status at 1 year
Topline
Nebulized amphotericin B does not improve exacerbation-free status at 1 year for patients with bronchopulmonary aspergillosis, though it may delay onset and incidence.
Methodology
Investigators searched PubMed and Embase databases for studies that included at least five patients with allergic bronchopulmonary aspergillosis who were managed with nebulized amphotericin B.
They included five studies, two of which were randomized controlled trials (RCTs), and three were observational studies; there was a total of 188 patients.
The primary objective of this systematic review and meta-analysis was to determine the frequency of patients remaining exacerbation free 1 year after initiating treatment with nebulized amphotericin B.
Takeaway
From the studies (one observational, two RCTs; n = 84) with exacerbation data at 1 or 2 years, the pooled proportion of patients who remained exacerbation free with nebulized amphotericin B at 1 year was 76% (I2 = 64.6%).
The pooled difference in risk with the two RCTs that assessed exacerbation-free status at 1 year was 0.33 and was not significantly different between the nebulized amphotericin B and control arms, which received nebulized saline.
Two RCTs provided the time to first exacerbation, which was significantly longer with nebulized amphotericin B than with nebulized saline (337 vs. 177 days; P = .004; I2 = 82%).
The proportion of patients who experienced two or more exacerbations was significantly lower with nebulized amphotericin B than with nebulized saline (9/33 [27.3%] vs 20/38 [52.6%]; P = .03).
In practice
Also, the proportion of subjects experiencing ≥ 2 exacerbations was also lesser with NAB than in the control,” concluded Valliappan Muthu, MD, and colleagues. However, “the ideal duration and optimal dose of LAMB for nebulization are unclear.”
Study details
“Nebulized amphotericin B for preventing exacerbations in allergic bronchopulmonary aspergillosis: A systematic review and meta-analysis” was published online in Pulmonary Pharmacology and Therapeutics.
Limitations
The current review is limited by the small number of included trials and may have a high risk of bias. Therefore, more evidence is required for the use of nebulized amphotericin B in routine care. The authors have disclosed no conflicts of interest.
A version of this article originally appeared on Medscape.com.
Topline
Nebulized amphotericin B does not improve exacerbation-free status at 1 year for patients with bronchopulmonary aspergillosis, though it may delay onset and incidence.
Methodology
Investigators searched PubMed and Embase databases for studies that included at least five patients with allergic bronchopulmonary aspergillosis who were managed with nebulized amphotericin B.
They included five studies, two of which were randomized controlled trials (RCTs), and three were observational studies; there was a total of 188 patients.
The primary objective of this systematic review and meta-analysis was to determine the frequency of patients remaining exacerbation free 1 year after initiating treatment with nebulized amphotericin B.
Takeaway
From the studies (one observational, two RCTs; n = 84) with exacerbation data at 1 or 2 years, the pooled proportion of patients who remained exacerbation free with nebulized amphotericin B at 1 year was 76% (I2 = 64.6%).
The pooled difference in risk with the two RCTs that assessed exacerbation-free status at 1 year was 0.33 and was not significantly different between the nebulized amphotericin B and control arms, which received nebulized saline.
Two RCTs provided the time to first exacerbation, which was significantly longer with nebulized amphotericin B than with nebulized saline (337 vs. 177 days; P = .004; I2 = 82%).
The proportion of patients who experienced two or more exacerbations was significantly lower with nebulized amphotericin B than with nebulized saline (9/33 [27.3%] vs 20/38 [52.6%]; P = .03).
In practice
Also, the proportion of subjects experiencing ≥ 2 exacerbations was also lesser with NAB than in the control,” concluded Valliappan Muthu, MD, and colleagues. However, “the ideal duration and optimal dose of LAMB for nebulization are unclear.”
Study details
“Nebulized amphotericin B for preventing exacerbations in allergic bronchopulmonary aspergillosis: A systematic review and meta-analysis” was published online in Pulmonary Pharmacology and Therapeutics.
Limitations
The current review is limited by the small number of included trials and may have a high risk of bias. Therefore, more evidence is required for the use of nebulized amphotericin B in routine care. The authors have disclosed no conflicts of interest.
A version of this article originally appeared on Medscape.com.
Topline
Nebulized amphotericin B does not improve exacerbation-free status at 1 year for patients with bronchopulmonary aspergillosis, though it may delay onset and incidence.
Methodology
Investigators searched PubMed and Embase databases for studies that included at least five patients with allergic bronchopulmonary aspergillosis who were managed with nebulized amphotericin B.
They included five studies, two of which were randomized controlled trials (RCTs), and three were observational studies; there was a total of 188 patients.
The primary objective of this systematic review and meta-analysis was to determine the frequency of patients remaining exacerbation free 1 year after initiating treatment with nebulized amphotericin B.
Takeaway
From the studies (one observational, two RCTs; n = 84) with exacerbation data at 1 or 2 years, the pooled proportion of patients who remained exacerbation free with nebulized amphotericin B at 1 year was 76% (I2 = 64.6%).
The pooled difference in risk with the two RCTs that assessed exacerbation-free status at 1 year was 0.33 and was not significantly different between the nebulized amphotericin B and control arms, which received nebulized saline.
Two RCTs provided the time to first exacerbation, which was significantly longer with nebulized amphotericin B than with nebulized saline (337 vs. 177 days; P = .004; I2 = 82%).
The proportion of patients who experienced two or more exacerbations was significantly lower with nebulized amphotericin B than with nebulized saline (9/33 [27.3%] vs 20/38 [52.6%]; P = .03).
In practice
Also, the proportion of subjects experiencing ≥ 2 exacerbations was also lesser with NAB than in the control,” concluded Valliappan Muthu, MD, and colleagues. However, “the ideal duration and optimal dose of LAMB for nebulization are unclear.”
Study details
“Nebulized amphotericin B for preventing exacerbations in allergic bronchopulmonary aspergillosis: A systematic review and meta-analysis” was published online in Pulmonary Pharmacology and Therapeutics.
Limitations
The current review is limited by the small number of included trials and may have a high risk of bias. Therefore, more evidence is required for the use of nebulized amphotericin B in routine care. The authors have disclosed no conflicts of interest.
A version of this article originally appeared on Medscape.com.
Palliative Care: Utilization Patterns in Inpatient Dermatology
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2

Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2

Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2

Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
Practice Points
- Although severe dermatologic disease negatively impacts patients’ quality of life, palliative care may be underutilized in this population.
- Palliative care should be an integral part of caring for patients who are admitted to the hospital with serious dermatologic illnesses.
Dangerous grandparents
Many decades ago I wrote a book I brazenly titled: “The Good Grandmother Handbook.” I had been a parent for a scant 7 or 8 years but based on my experiences in the office I felt I had accumulated enough wisdom to suggest to women in their fifth to seventh decades how they might conduct themselves around their grandchildren. Luckily, the book never got further than several hundred pages of crudely typed manuscript. This was before word processing programs had settled into the home computer industry, which was still in its infancy.
But I continue find the subject of grandparents interesting. Now, with grandchildren of my own (the oldest has just graduated from high school) and scores of peers knee deep in their own grandparenting adventures, I hope that my perspective now has a bit less of a holier-than-thou aroma.
My most recent muse-prodding event came when I stumbled across an article about the epidemiology of unintentional pediatric firearm fatalities. Looking at 10 years of data from the National Violent Death Reporting System, the investigators found that in 80% of the cases the firearm owner was a relative of the victim; in slightly more than 60% of the cases the event occurred in the victim’s home.
The data set was not granular enough to define the exact relationship between the child and relative who owned the gun. I suspect that most often the relative was a parent or an uncle or aunt. However, viewed through my septuagenarian prism, this paper prompted me to wonder in how many of these fatalities the firearm owner was a grandparent.
I have only anecdotal observations, but I can easily recall situations here in Maine in which a child has been injured by his or her grandfather’s gun. The data from the study show that pediatric fatalities are bimodal, with the majority occurring in the 1- to 5-year age group and a second peak in adolescence. The grandparent-involved cases I can recall were in the younger demographic.
Unfortunately, firearms aren’t the only threat that other grandparents and I pose to the health and safety of our grandchildren. I can remember before the development of, and the widespread use of, tamper-proof pill bottles, “grandma’s purse” overdoses were an unfortunately common occurrence.
More recently, at least here in Maine, we have been hearing more about motorized vehicle–related injuries and fatalities – grandparents backing over their grandchildren in the driveway or, more often, grandfathers (usually) taking their young grandchildren for rides on their snowmobiles, ATVs, lawn tractors, (fill in the blank). Whenever one of these events occurs, my mind quickly jumps beyond the tragic loss of life to imagining what terrible and long-lasting emotional chaos these incidents have spawned in those families.
During the pandemic, many parents and grandparents became aware of the threat that viral-spewing young children pose to the older and more vulnerable generation. On the other hand, many parents have been told that having a grandparent around can present a risk to the health and safety of their grandchildren. It can be a touchy subject in families, and grandparents may bristle at “being treated like a child” when they are reminded that children aren’t small adults and that their own behavior may be setting a bad example or putting their grandchildren at risk.
My generation had to learn how to buckle infants and toddlers into car seats because it was something that wasn’t done for our children. Fortunately, most new grandparents now already have those buckle-and-click skills and mindset. But,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Many decades ago I wrote a book I brazenly titled: “The Good Grandmother Handbook.” I had been a parent for a scant 7 or 8 years but based on my experiences in the office I felt I had accumulated enough wisdom to suggest to women in their fifth to seventh decades how they might conduct themselves around their grandchildren. Luckily, the book never got further than several hundred pages of crudely typed manuscript. This was before word processing programs had settled into the home computer industry, which was still in its infancy.
But I continue find the subject of grandparents interesting. Now, with grandchildren of my own (the oldest has just graduated from high school) and scores of peers knee deep in their own grandparenting adventures, I hope that my perspective now has a bit less of a holier-than-thou aroma.
My most recent muse-prodding event came when I stumbled across an article about the epidemiology of unintentional pediatric firearm fatalities. Looking at 10 years of data from the National Violent Death Reporting System, the investigators found that in 80% of the cases the firearm owner was a relative of the victim; in slightly more than 60% of the cases the event occurred in the victim’s home.
The data set was not granular enough to define the exact relationship between the child and relative who owned the gun. I suspect that most often the relative was a parent or an uncle or aunt. However, viewed through my septuagenarian prism, this paper prompted me to wonder in how many of these fatalities the firearm owner was a grandparent.
I have only anecdotal observations, but I can easily recall situations here in Maine in which a child has been injured by his or her grandfather’s gun. The data from the study show that pediatric fatalities are bimodal, with the majority occurring in the 1- to 5-year age group and a second peak in adolescence. The grandparent-involved cases I can recall were in the younger demographic.
Unfortunately, firearms aren’t the only threat that other grandparents and I pose to the health and safety of our grandchildren. I can remember before the development of, and the widespread use of, tamper-proof pill bottles, “grandma’s purse” overdoses were an unfortunately common occurrence.
More recently, at least here in Maine, we have been hearing more about motorized vehicle–related injuries and fatalities – grandparents backing over their grandchildren in the driveway or, more often, grandfathers (usually) taking their young grandchildren for rides on their snowmobiles, ATVs, lawn tractors, (fill in the blank). Whenever one of these events occurs, my mind quickly jumps beyond the tragic loss of life to imagining what terrible and long-lasting emotional chaos these incidents have spawned in those families.
During the pandemic, many parents and grandparents became aware of the threat that viral-spewing young children pose to the older and more vulnerable generation. On the other hand, many parents have been told that having a grandparent around can present a risk to the health and safety of their grandchildren. It can be a touchy subject in families, and grandparents may bristle at “being treated like a child” when they are reminded that children aren’t small adults and that their own behavior may be setting a bad example or putting their grandchildren at risk.
My generation had to learn how to buckle infants and toddlers into car seats because it was something that wasn’t done for our children. Fortunately, most new grandparents now already have those buckle-and-click skills and mindset. But,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Many decades ago I wrote a book I brazenly titled: “The Good Grandmother Handbook.” I had been a parent for a scant 7 or 8 years but based on my experiences in the office I felt I had accumulated enough wisdom to suggest to women in their fifth to seventh decades how they might conduct themselves around their grandchildren. Luckily, the book never got further than several hundred pages of crudely typed manuscript. This was before word processing programs had settled into the home computer industry, which was still in its infancy.
But I continue find the subject of grandparents interesting. Now, with grandchildren of my own (the oldest has just graduated from high school) and scores of peers knee deep in their own grandparenting adventures, I hope that my perspective now has a bit less of a holier-than-thou aroma.
My most recent muse-prodding event came when I stumbled across an article about the epidemiology of unintentional pediatric firearm fatalities. Looking at 10 years of data from the National Violent Death Reporting System, the investigators found that in 80% of the cases the firearm owner was a relative of the victim; in slightly more than 60% of the cases the event occurred in the victim’s home.
The data set was not granular enough to define the exact relationship between the child and relative who owned the gun. I suspect that most often the relative was a parent or an uncle or aunt. However, viewed through my septuagenarian prism, this paper prompted me to wonder in how many of these fatalities the firearm owner was a grandparent.
I have only anecdotal observations, but I can easily recall situations here in Maine in which a child has been injured by his or her grandfather’s gun. The data from the study show that pediatric fatalities are bimodal, with the majority occurring in the 1- to 5-year age group and a second peak in adolescence. The grandparent-involved cases I can recall were in the younger demographic.
Unfortunately, firearms aren’t the only threat that other grandparents and I pose to the health and safety of our grandchildren. I can remember before the development of, and the widespread use of, tamper-proof pill bottles, “grandma’s purse” overdoses were an unfortunately common occurrence.
More recently, at least here in Maine, we have been hearing more about motorized vehicle–related injuries and fatalities – grandparents backing over their grandchildren in the driveway or, more often, grandfathers (usually) taking their young grandchildren for rides on their snowmobiles, ATVs, lawn tractors, (fill in the blank). Whenever one of these events occurs, my mind quickly jumps beyond the tragic loss of life to imagining what terrible and long-lasting emotional chaos these incidents have spawned in those families.
During the pandemic, many parents and grandparents became aware of the threat that viral-spewing young children pose to the older and more vulnerable generation. On the other hand, many parents have been told that having a grandparent around can present a risk to the health and safety of their grandchildren. It can be a touchy subject in families, and grandparents may bristle at “being treated like a child” when they are reminded that children aren’t small adults and that their own behavior may be setting a bad example or putting their grandchildren at risk.
My generation had to learn how to buckle infants and toddlers into car seats because it was something that wasn’t done for our children. Fortunately, most new grandparents now already have those buckle-and-click skills and mindset. But,
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
What is the proper treatment for posttraumatic headache? Expert debate
AUSTIN, TEX – There are no guidelines available, nor is there much quality evidence to support one decision or another, according to two experts who debated the question at the annual meeting of the American Headache Society.
Early treatment
Frank Conidi, DO, spoke first, and pointed out the need to define both early treatment and the condition being treated. Is it early-treatment abortive, is it preventative, and if the patient has a concussion, is it a mild traumatic brain injury (TBI), or severe TBI?
The majority of patients with posttraumatic headache will meet criteria for migraine or probable migraine. “It can be anywhere from 58% to upwards of 90%. And if you see these patients, it makes sense, because posttraumatic headache patients are disabled by their headaches,” said Dr. Conidi, director of the Florida Center for Headache and Sports Neurology.
He argued for early treatment to reduce chronification. “We know that if headaches are left untreated, they’re going to start to spiral up and become daily. This leads to the development of peripheral and central sensitization and lowers the threshold for further migraine attacks,” said Dr. Conidi.
He noted that patients with posttraumatic headache often have comorbidities such as sleep issues, neck pain, or posttraumatic stress disorder, all of which are risk factors for chronification. Treatment does not necessarily mean medication, however. “The mainstay of posttraumatic headache treatment is actually physical and cognitive activity to tolerance. And what I call the 20/5 rule: 20 minutes of physical activity with 5-minute chill breaks. In addition, we use light sub-aerobic exercise 3 to 5 days out in concussion, [which] has been shown to improve concussion recovery time,” he said.
Dr. Conidi suggested treatment of triggers, such as neck issues and whiplash symptoms. “Probably the best treatment I’ve ever seen, and I published on this, are pericranial nerve blocks. Pericranial nerve blocks work wonderfully. If you’re going to block the pericranial nerves, block them all, not just the occipital. Block the trigeminal branches. I’ve actually been able to locate a little two-and-a-half-inch plastic Luer-lock catheter that I can hook on a 1-cc syringe with viscous lidocaine, and I can do sphenopalatine ganglion blocks on all my patients now for under 25 cents. So we’ve been combining the nerve blocks, and we’ve been using them early. Oftentimes the patients won’t have any further headaches, especially if it’s [after] a concussion,” he said.
With respect to concussion-related posttraumatic headache, he summed up: “We’re aggressive early. We’re using intervention. We’re layering our treatment. We’re using medications: prednisone, NSAIDS, and now we have gepants. We’ve been having good success with using gepants,” he said.
Treatment of TBI patients is broadly similar, with the main difference being that neurologists typically won’t see such patients early on as they may be in rehab facilities or hospitals for extended periods. “You may not be getting [to see] them for 1 or 2 months. In that case, you want to educate your neurosurgery and your [physical medicine and rehabilitation] colleagues on the treatment.
Finally, he described work that his group has done in using stimulants for posttraumatic headache. “Stimulants not only treat the cognitive symptoms, but they give the patient cognitive reserve and we find that it gets the patient through the day so they actually have less headaches. It’s a form of prevention. I know there are shortages nationally of both Adderall and Ritalin, but we have had excellent results in our posttraumatic patients using these types of medications,” said Dr. Conidi.
Delayed treatment
Amaal J. Starling, MD, offered a counterargument, but she narrowed the question down to whether preventive treatment should be used within one and a half months of the injury, which she defined as early treatment. Her argument against early preventive treatment centered around the core value of beneficence – to act for the benefit of the patient, and avoid harm.
She discussed the natural history of posttraumatic headache, which is largely self-limited. For example, an NCAA study that found 88% of concussions had symptom resolution within 1 week, and 86% of posttraumatic headache resolved within 1 week. “If individuals routinely are having a self-limited course, there is no need for early treatment with a preventive treatment option because the majority of posttraumatic headache is resolving within that one-and-a-half-month postinjury threshold. The better recommendation, as provided in evidence from Dr. Conidi’s presentation, is to provide supportive care, including acute medications or acute treatment options like nerve blocks for acute pain relief and symptom relief,” said Dr. Starling, associate professor of neurology at Mayo Clinic in Scottsdale, Ariz.
Dr. Starling expressed concern that preventive medications could lead to worsening of comorbidities. For example, posttraumatic headache is often associated with autonomic dysfunction and visual vestibular dysfunction. The former commonly occurs with concussion and is similar to postural orthostatic tachycardia syndrome (POTS), and the second most common symptom of POTS is headache, according to Dr. Starling. Posttraumatic POTS is treated similarly to idiopathic POTS, with a nonpharmacologic approach. One element of POTS management is to withdraw exacerbating medications such as beta-blockers, tricyclic antidepressants, and SNRIs. “These look strikingly similar to some of the headache preventive medications that we might consider for somebody, and so the concern is early preventive treatment with these medications to treat the posttraumatic headache may actually worsen some of these comorbidities that are present in our posttraumatic headache patients. We have to be careful about potentially exacerbating comorbidities with early preventive treatment,” she said.
Prevention medications for headache can also worsen visual vestibular dysfunction, such as dizziness. There are some data suggesting that vestibular rehabilitation and vision therapy can improve dizziness, but also headache. “We all know that many of our preventive medications for headache could potentially exacerbate visual vestibular symptoms, so we have to be careful about that. So again, first do no harm. Posttraumatic POTS is common and causes headache. Posttraumatic vestibular dysfunction is common and causes headache. Instead of initiating a headache preventive medication early, we recommend to identify these comorbidities and provide targeted treatment. Treatment of these comorbidities may, in and of itself, improve the headache. We also we have to be careful because some preventive medications may worsen the comorbidities,” said Dr. Starling.
Areas of agreement
Dr. Conidi agreed that preventative treatment is less likely to be needed for concussion patients, but said that TBI patients are more likely to require it to prevent chronification. Dr. Starling agreed that chronification is an important concern, but she noted that many posttraumatic headache patients are athletes, and preventative medications can also lead to issues that might interfere with return to play, such as decreased sweating, or weight gain or loss. This is complicated by the fact that titration and weaning periods can be long. “We have to be very careful about these medications’ side effects, especially when we don’t have the evidence to demonstrate that it is worth the potential risk of being put on these medications,” she said.
The debate led Catherin Chong, PhD, to ask about the state of the field. “There’s a posttraumatic headache special interest section here [at AHS 2023], and the question that really is coming up at every meeting is, is there some coherence in the field? Is it too early or is it time for a position statement?” asked Dr. Chong, a career scientist at Mayo Clinic (Phoenix). Dr. Chong comoderated the debate and ensuing discussion.
Dr. Starling felt it’s too early for a position statement, but a scoping review could identify research questions that could lead to a position statement. “I’m really excited about the work that’s being done to identify the cohort of individuals with acute posttraumatic headache that may chronify to persistent posttraumatic headache so that we can minimize the risk of exposing the large cohort that’s going to be likely self-limited to a treatment option. Then we can identify those individuals where that risk is worth it because they’re the ones that could lead to chronification. Figuring out if that’s looking at levels of allodynia or other factors that can [help identify those at most risk] would be important,” she said.
Dr. Conidi agreed with the need for more information on the parameters to be studied, but he expressed the belief that any position statement would be a consensus statement. “It’s not going to have any hard evidence behind it, but I do think we need [a position statement]. Even in the general neurology world, there’s a huge lack of understanding of how to treat these patients,” he said.
Dr. Conidi did not make any disclosures. Dr. Starling has consulted for AbbVie, Allergan, Amgen, Axsome Therapeutics, Everyday Health, Lundbeck, Med-IQ, Medscape, Neurolief, Satsuma, and WebMD. Dr. Chong has no relevant financial disclosures.
AUSTIN, TEX – There are no guidelines available, nor is there much quality evidence to support one decision or another, according to two experts who debated the question at the annual meeting of the American Headache Society.
Early treatment
Frank Conidi, DO, spoke first, and pointed out the need to define both early treatment and the condition being treated. Is it early-treatment abortive, is it preventative, and if the patient has a concussion, is it a mild traumatic brain injury (TBI), or severe TBI?
The majority of patients with posttraumatic headache will meet criteria for migraine or probable migraine. “It can be anywhere from 58% to upwards of 90%. And if you see these patients, it makes sense, because posttraumatic headache patients are disabled by their headaches,” said Dr. Conidi, director of the Florida Center for Headache and Sports Neurology.
He argued for early treatment to reduce chronification. “We know that if headaches are left untreated, they’re going to start to spiral up and become daily. This leads to the development of peripheral and central sensitization and lowers the threshold for further migraine attacks,” said Dr. Conidi.
He noted that patients with posttraumatic headache often have comorbidities such as sleep issues, neck pain, or posttraumatic stress disorder, all of which are risk factors for chronification. Treatment does not necessarily mean medication, however. “The mainstay of posttraumatic headache treatment is actually physical and cognitive activity to tolerance. And what I call the 20/5 rule: 20 minutes of physical activity with 5-minute chill breaks. In addition, we use light sub-aerobic exercise 3 to 5 days out in concussion, [which] has been shown to improve concussion recovery time,” he said.
Dr. Conidi suggested treatment of triggers, such as neck issues and whiplash symptoms. “Probably the best treatment I’ve ever seen, and I published on this, are pericranial nerve blocks. Pericranial nerve blocks work wonderfully. If you’re going to block the pericranial nerves, block them all, not just the occipital. Block the trigeminal branches. I’ve actually been able to locate a little two-and-a-half-inch plastic Luer-lock catheter that I can hook on a 1-cc syringe with viscous lidocaine, and I can do sphenopalatine ganglion blocks on all my patients now for under 25 cents. So we’ve been combining the nerve blocks, and we’ve been using them early. Oftentimes the patients won’t have any further headaches, especially if it’s [after] a concussion,” he said.
With respect to concussion-related posttraumatic headache, he summed up: “We’re aggressive early. We’re using intervention. We’re layering our treatment. We’re using medications: prednisone, NSAIDS, and now we have gepants. We’ve been having good success with using gepants,” he said.
Treatment of TBI patients is broadly similar, with the main difference being that neurologists typically won’t see such patients early on as they may be in rehab facilities or hospitals for extended periods. “You may not be getting [to see] them for 1 or 2 months. In that case, you want to educate your neurosurgery and your [physical medicine and rehabilitation] colleagues on the treatment.
Finally, he described work that his group has done in using stimulants for posttraumatic headache. “Stimulants not only treat the cognitive symptoms, but they give the patient cognitive reserve and we find that it gets the patient through the day so they actually have less headaches. It’s a form of prevention. I know there are shortages nationally of both Adderall and Ritalin, but we have had excellent results in our posttraumatic patients using these types of medications,” said Dr. Conidi.
Delayed treatment
Amaal J. Starling, MD, offered a counterargument, but she narrowed the question down to whether preventive treatment should be used within one and a half months of the injury, which she defined as early treatment. Her argument against early preventive treatment centered around the core value of beneficence – to act for the benefit of the patient, and avoid harm.
She discussed the natural history of posttraumatic headache, which is largely self-limited. For example, an NCAA study that found 88% of concussions had symptom resolution within 1 week, and 86% of posttraumatic headache resolved within 1 week. “If individuals routinely are having a self-limited course, there is no need for early treatment with a preventive treatment option because the majority of posttraumatic headache is resolving within that one-and-a-half-month postinjury threshold. The better recommendation, as provided in evidence from Dr. Conidi’s presentation, is to provide supportive care, including acute medications or acute treatment options like nerve blocks for acute pain relief and symptom relief,” said Dr. Starling, associate professor of neurology at Mayo Clinic in Scottsdale, Ariz.
Dr. Starling expressed concern that preventive medications could lead to worsening of comorbidities. For example, posttraumatic headache is often associated with autonomic dysfunction and visual vestibular dysfunction. The former commonly occurs with concussion and is similar to postural orthostatic tachycardia syndrome (POTS), and the second most common symptom of POTS is headache, according to Dr. Starling. Posttraumatic POTS is treated similarly to idiopathic POTS, with a nonpharmacologic approach. One element of POTS management is to withdraw exacerbating medications such as beta-blockers, tricyclic antidepressants, and SNRIs. “These look strikingly similar to some of the headache preventive medications that we might consider for somebody, and so the concern is early preventive treatment with these medications to treat the posttraumatic headache may actually worsen some of these comorbidities that are present in our posttraumatic headache patients. We have to be careful about potentially exacerbating comorbidities with early preventive treatment,” she said.
Prevention medications for headache can also worsen visual vestibular dysfunction, such as dizziness. There are some data suggesting that vestibular rehabilitation and vision therapy can improve dizziness, but also headache. “We all know that many of our preventive medications for headache could potentially exacerbate visual vestibular symptoms, so we have to be careful about that. So again, first do no harm. Posttraumatic POTS is common and causes headache. Posttraumatic vestibular dysfunction is common and causes headache. Instead of initiating a headache preventive medication early, we recommend to identify these comorbidities and provide targeted treatment. Treatment of these comorbidities may, in and of itself, improve the headache. We also we have to be careful because some preventive medications may worsen the comorbidities,” said Dr. Starling.
Areas of agreement
Dr. Conidi agreed that preventative treatment is less likely to be needed for concussion patients, but said that TBI patients are more likely to require it to prevent chronification. Dr. Starling agreed that chronification is an important concern, but she noted that many posttraumatic headache patients are athletes, and preventative medications can also lead to issues that might interfere with return to play, such as decreased sweating, or weight gain or loss. This is complicated by the fact that titration and weaning periods can be long. “We have to be very careful about these medications’ side effects, especially when we don’t have the evidence to demonstrate that it is worth the potential risk of being put on these medications,” she said.
The debate led Catherin Chong, PhD, to ask about the state of the field. “There’s a posttraumatic headache special interest section here [at AHS 2023], and the question that really is coming up at every meeting is, is there some coherence in the field? Is it too early or is it time for a position statement?” asked Dr. Chong, a career scientist at Mayo Clinic (Phoenix). Dr. Chong comoderated the debate and ensuing discussion.
Dr. Starling felt it’s too early for a position statement, but a scoping review could identify research questions that could lead to a position statement. “I’m really excited about the work that’s being done to identify the cohort of individuals with acute posttraumatic headache that may chronify to persistent posttraumatic headache so that we can minimize the risk of exposing the large cohort that’s going to be likely self-limited to a treatment option. Then we can identify those individuals where that risk is worth it because they’re the ones that could lead to chronification. Figuring out if that’s looking at levels of allodynia or other factors that can [help identify those at most risk] would be important,” she said.
Dr. Conidi agreed with the need for more information on the parameters to be studied, but he expressed the belief that any position statement would be a consensus statement. “It’s not going to have any hard evidence behind it, but I do think we need [a position statement]. Even in the general neurology world, there’s a huge lack of understanding of how to treat these patients,” he said.
Dr. Conidi did not make any disclosures. Dr. Starling has consulted for AbbVie, Allergan, Amgen, Axsome Therapeutics, Everyday Health, Lundbeck, Med-IQ, Medscape, Neurolief, Satsuma, and WebMD. Dr. Chong has no relevant financial disclosures.
AUSTIN, TEX – There are no guidelines available, nor is there much quality evidence to support one decision or another, according to two experts who debated the question at the annual meeting of the American Headache Society.
Early treatment
Frank Conidi, DO, spoke first, and pointed out the need to define both early treatment and the condition being treated. Is it early-treatment abortive, is it preventative, and if the patient has a concussion, is it a mild traumatic brain injury (TBI), or severe TBI?
The majority of patients with posttraumatic headache will meet criteria for migraine or probable migraine. “It can be anywhere from 58% to upwards of 90%. And if you see these patients, it makes sense, because posttraumatic headache patients are disabled by their headaches,” said Dr. Conidi, director of the Florida Center for Headache and Sports Neurology.
He argued for early treatment to reduce chronification. “We know that if headaches are left untreated, they’re going to start to spiral up and become daily. This leads to the development of peripheral and central sensitization and lowers the threshold for further migraine attacks,” said Dr. Conidi.
He noted that patients with posttraumatic headache often have comorbidities such as sleep issues, neck pain, or posttraumatic stress disorder, all of which are risk factors for chronification. Treatment does not necessarily mean medication, however. “The mainstay of posttraumatic headache treatment is actually physical and cognitive activity to tolerance. And what I call the 20/5 rule: 20 minutes of physical activity with 5-minute chill breaks. In addition, we use light sub-aerobic exercise 3 to 5 days out in concussion, [which] has been shown to improve concussion recovery time,” he said.
Dr. Conidi suggested treatment of triggers, such as neck issues and whiplash symptoms. “Probably the best treatment I’ve ever seen, and I published on this, are pericranial nerve blocks. Pericranial nerve blocks work wonderfully. If you’re going to block the pericranial nerves, block them all, not just the occipital. Block the trigeminal branches. I’ve actually been able to locate a little two-and-a-half-inch plastic Luer-lock catheter that I can hook on a 1-cc syringe with viscous lidocaine, and I can do sphenopalatine ganglion blocks on all my patients now for under 25 cents. So we’ve been combining the nerve blocks, and we’ve been using them early. Oftentimes the patients won’t have any further headaches, especially if it’s [after] a concussion,” he said.
With respect to concussion-related posttraumatic headache, he summed up: “We’re aggressive early. We’re using intervention. We’re layering our treatment. We’re using medications: prednisone, NSAIDS, and now we have gepants. We’ve been having good success with using gepants,” he said.
Treatment of TBI patients is broadly similar, with the main difference being that neurologists typically won’t see such patients early on as they may be in rehab facilities or hospitals for extended periods. “You may not be getting [to see] them for 1 or 2 months. In that case, you want to educate your neurosurgery and your [physical medicine and rehabilitation] colleagues on the treatment.
Finally, he described work that his group has done in using stimulants for posttraumatic headache. “Stimulants not only treat the cognitive symptoms, but they give the patient cognitive reserve and we find that it gets the patient through the day so they actually have less headaches. It’s a form of prevention. I know there are shortages nationally of both Adderall and Ritalin, but we have had excellent results in our posttraumatic patients using these types of medications,” said Dr. Conidi.
Delayed treatment
Amaal J. Starling, MD, offered a counterargument, but she narrowed the question down to whether preventive treatment should be used within one and a half months of the injury, which she defined as early treatment. Her argument against early preventive treatment centered around the core value of beneficence – to act for the benefit of the patient, and avoid harm.
She discussed the natural history of posttraumatic headache, which is largely self-limited. For example, an NCAA study that found 88% of concussions had symptom resolution within 1 week, and 86% of posttraumatic headache resolved within 1 week. “If individuals routinely are having a self-limited course, there is no need for early treatment with a preventive treatment option because the majority of posttraumatic headache is resolving within that one-and-a-half-month postinjury threshold. The better recommendation, as provided in evidence from Dr. Conidi’s presentation, is to provide supportive care, including acute medications or acute treatment options like nerve blocks for acute pain relief and symptom relief,” said Dr. Starling, associate professor of neurology at Mayo Clinic in Scottsdale, Ariz.
Dr. Starling expressed concern that preventive medications could lead to worsening of comorbidities. For example, posttraumatic headache is often associated with autonomic dysfunction and visual vestibular dysfunction. The former commonly occurs with concussion and is similar to postural orthostatic tachycardia syndrome (POTS), and the second most common symptom of POTS is headache, according to Dr. Starling. Posttraumatic POTS is treated similarly to idiopathic POTS, with a nonpharmacologic approach. One element of POTS management is to withdraw exacerbating medications such as beta-blockers, tricyclic antidepressants, and SNRIs. “These look strikingly similar to some of the headache preventive medications that we might consider for somebody, and so the concern is early preventive treatment with these medications to treat the posttraumatic headache may actually worsen some of these comorbidities that are present in our posttraumatic headache patients. We have to be careful about potentially exacerbating comorbidities with early preventive treatment,” she said.
Prevention medications for headache can also worsen visual vestibular dysfunction, such as dizziness. There are some data suggesting that vestibular rehabilitation and vision therapy can improve dizziness, but also headache. “We all know that many of our preventive medications for headache could potentially exacerbate visual vestibular symptoms, so we have to be careful about that. So again, first do no harm. Posttraumatic POTS is common and causes headache. Posttraumatic vestibular dysfunction is common and causes headache. Instead of initiating a headache preventive medication early, we recommend to identify these comorbidities and provide targeted treatment. Treatment of these comorbidities may, in and of itself, improve the headache. We also we have to be careful because some preventive medications may worsen the comorbidities,” said Dr. Starling.
Areas of agreement
Dr. Conidi agreed that preventative treatment is less likely to be needed for concussion patients, but said that TBI patients are more likely to require it to prevent chronification. Dr. Starling agreed that chronification is an important concern, but she noted that many posttraumatic headache patients are athletes, and preventative medications can also lead to issues that might interfere with return to play, such as decreased sweating, or weight gain or loss. This is complicated by the fact that titration and weaning periods can be long. “We have to be very careful about these medications’ side effects, especially when we don’t have the evidence to demonstrate that it is worth the potential risk of being put on these medications,” she said.
The debate led Catherin Chong, PhD, to ask about the state of the field. “There’s a posttraumatic headache special interest section here [at AHS 2023], and the question that really is coming up at every meeting is, is there some coherence in the field? Is it too early or is it time for a position statement?” asked Dr. Chong, a career scientist at Mayo Clinic (Phoenix). Dr. Chong comoderated the debate and ensuing discussion.
Dr. Starling felt it’s too early for a position statement, but a scoping review could identify research questions that could lead to a position statement. “I’m really excited about the work that’s being done to identify the cohort of individuals with acute posttraumatic headache that may chronify to persistent posttraumatic headache so that we can minimize the risk of exposing the large cohort that’s going to be likely self-limited to a treatment option. Then we can identify those individuals where that risk is worth it because they’re the ones that could lead to chronification. Figuring out if that’s looking at levels of allodynia or other factors that can [help identify those at most risk] would be important,” she said.
Dr. Conidi agreed with the need for more information on the parameters to be studied, but he expressed the belief that any position statement would be a consensus statement. “It’s not going to have any hard evidence behind it, but I do think we need [a position statement]. Even in the general neurology world, there’s a huge lack of understanding of how to treat these patients,” he said.
Dr. Conidi did not make any disclosures. Dr. Starling has consulted for AbbVie, Allergan, Amgen, Axsome Therapeutics, Everyday Health, Lundbeck, Med-IQ, Medscape, Neurolief, Satsuma, and WebMD. Dr. Chong has no relevant financial disclosures.
AT AHS 2023
Painful, Nonhealing, Violaceus Plaque on the Right Breast
The Diagnosis: Diffuse Dermal Angiomatosis
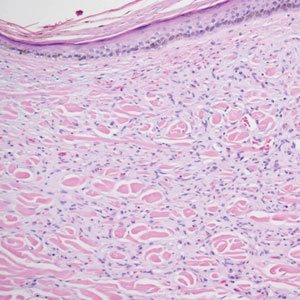
Diffuse dermal angiomatosis (DDA) is an acquired reactive vascular proliferation in the spectrum of cutaneous reactive angioendotheliomatoses. Clinically, DDA presents as violaceous reticulated plaques, often with secondary ulceration and sometimes necrosis.1-3 Diffuse dermal angiomatosis more commonly presents in patients with a history of severe peripheral vascular disease, coagulopathies, or infection, and it frequently arises on the extremities. Diffuse dermal angiomatosis also has been shown to develop on the breasts, particularly in patients with pendulous breast tissue. Vascular proliferation in DDA is hypothesized to be from ischemia and hypoxia, leading to angiogenesis.1-3 Diffuse dermal angiomatosis is characterized histologically by the presence of a diffuse proliferation of spindled endothelial cells distributed between the collagen bundles throughout the dermis (quiz image and Figure 1). Spindle-shaped endothelial cells exhibit a vacuolated cytoplasm. On immunohistochemistry, these dermal spindle cells classically stain positive for CD31, CD34, and erythroblast transformation specific–related gene (Erg) and stain negative for both human herpesvirus 8 (HHV-8) and factor XIIIa.
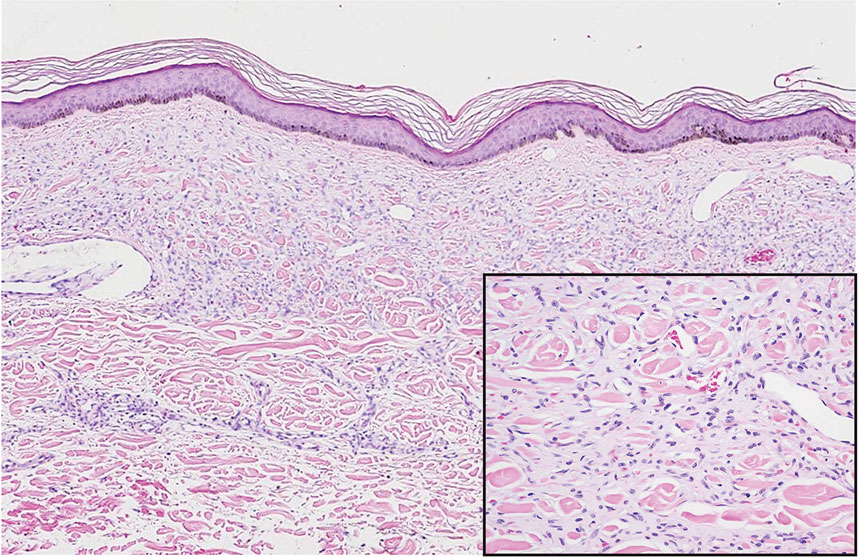
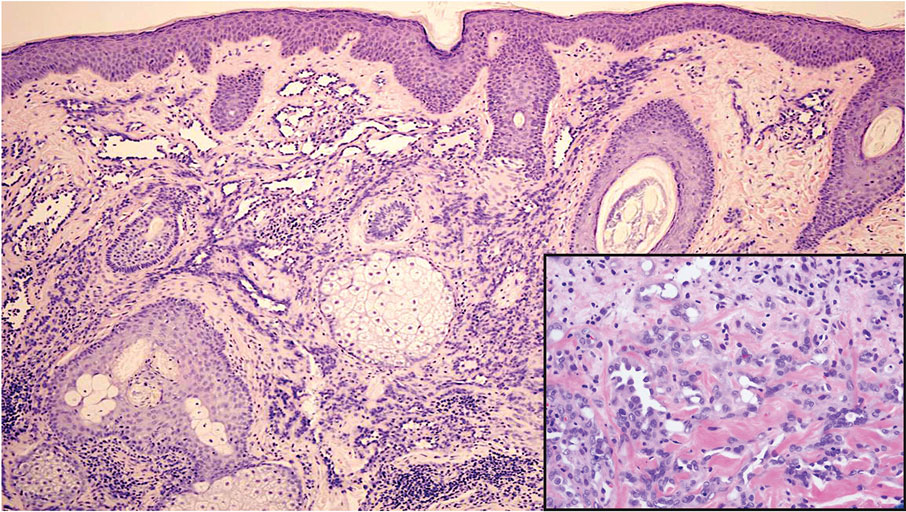
Cutaneous fibrous histiocytoma, more commonly referred to as dermatofibroma, is a common benign lesion that presents clinically as a solitary firm nodule most commonly on the extremities in areas of repetitive trauma or pressure. It classically exhibits dimpling of the overlaying skin with lateral pressure on the lesion, known as the dimple sign.4 Histologically, dermatofibromas share similar features to DDA and demonstrate the presence of bland-appearing spindle cells within the dermis between the collagen bundles, resulting in collagen trapping. However, a distinguishing histologic feature of a dermatofibroma in comparison to DDA is the presence of epidermal hyperplasia overlying the dermatofibroma, leading to tabled rete ridges (Figure 2). Spindle cells in dermatofibromas are fibroblasts and have a distinct immunophenotype that includes factor XIIIa positivity and negative staining for CD31, CD34, and Erg.4,5
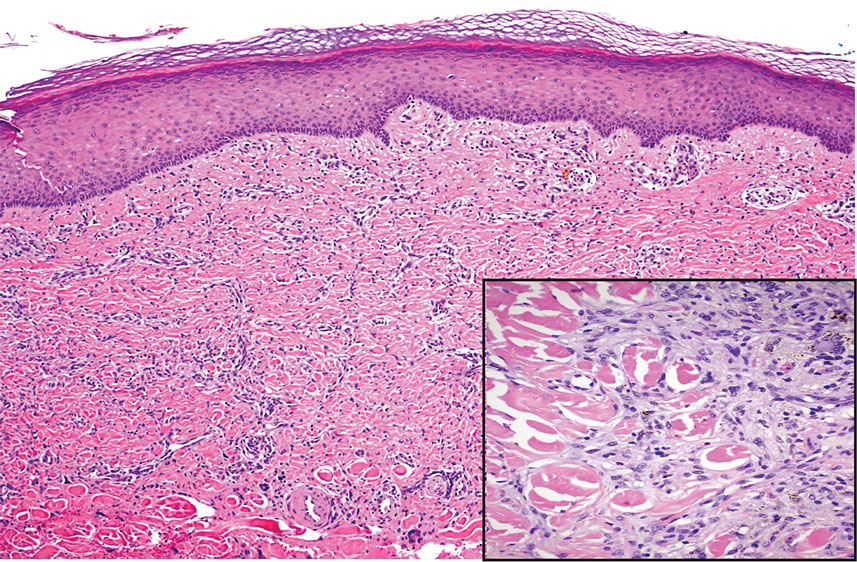
Dermatofibrosarcoma protuberans (DFSP) is a rare malignant soft-tissue sarcoma that clinically presents as a firm, flesh-colored, dermal plaque on the trunk, proximal extremities, head, or neck.5 Histologically, DFSP can be distinguished from DDA by the high density of spindle cells that are arranged in a storiform pattern, extending and infiltrating the underlying subcutaneous fat in a honeycomblike pattern (Figure 3). Spindle cells in DFSP typically show expression of CD34 but are negative for CD31, Erg, and factor XIIIa.5

Kaposi sarcoma (KS) is an endothelial cell–driven angioproliferative neoplasm that is associated with HHV-8 infection.6 The clinical presentation of KS can range from isolated pink or purple papules and patches to more extensive ulcerated plaques or nodules. Histopathology exhibits proliferation of monomorphic spindled endothelial cells within the dermis staining positive for HHV-8, Erg, CD31, and CD34, in conjunction with extravasated erythrocytes arranged within slitlike vascular spaces (Figure 4). Additionally, KS classically exhibits aberrant endothelial cell proliferation and vessel formation around preexisting vessels, which is referred to as the promontory sign (Figure 4).
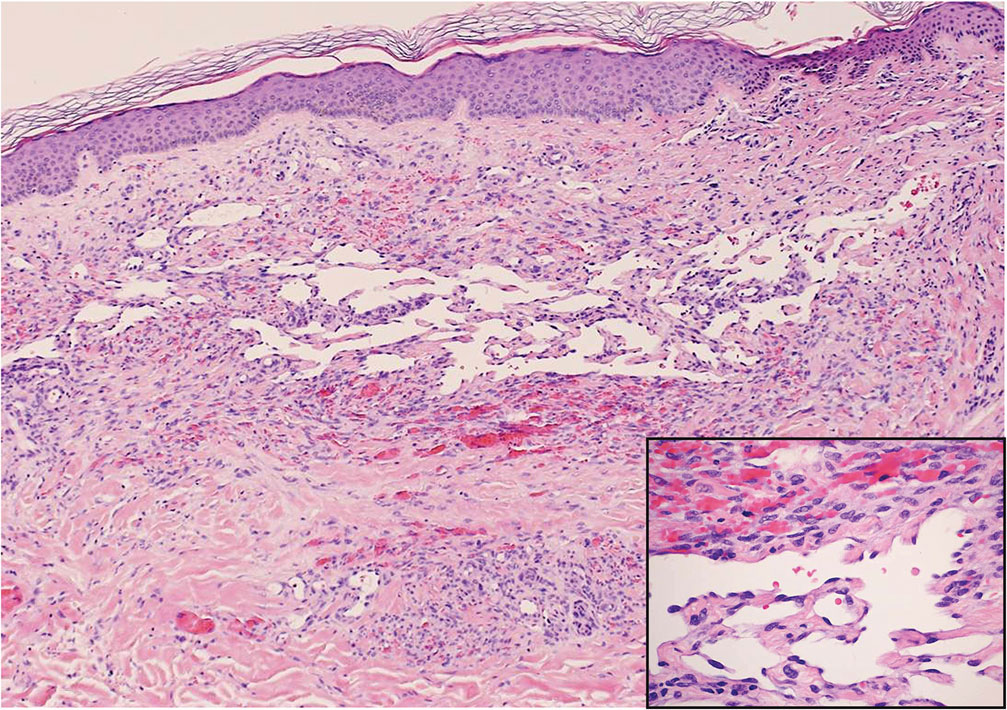
Angiosarcoma is a rare and highly aggressive vascular tumor arising from endothelial cells lining the blood vessels and lymphatics.7,8 Clinically, angiosarcoma presents as ulcerated violaceous nodules or plaques on the head, neck, or trunk. Histologic evaluation of angiosarcoma reveals a complex and poorly demarcated vascular network dissecting between collagen bundles in the dermis (Figure 5). Multilayering of endothelial cells, papillary projections extending into the vessel lumina, and mitoses frequently are seen. On immunohistochemistry, endothelial cells demonstrate prominent cellular atypia and stain positive with CD31, CD34, and Erg.
- Touloei K, Tongdee E, Smirnov B, et al. Diffuse dermal angiomatosis. Cutis. 2019;103:181-184.
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Baylor Univ Med Cent Proc. 2020;33:273-275.
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Etemad SA, Dewan AK. Kaposi sarcoma updates. Dermatol Clin. 2019;37:505-517.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
The Diagnosis: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is an acquired reactive vascular proliferation in the spectrum of cutaneous reactive angioendotheliomatoses. Clinically, DDA presents as violaceous reticulated plaques, often with secondary ulceration and sometimes necrosis.1-3 Diffuse dermal angiomatosis more commonly presents in patients with a history of severe peripheral vascular disease, coagulopathies, or infection, and it frequently arises on the extremities. Diffuse dermal angiomatosis also has been shown to develop on the breasts, particularly in patients with pendulous breast tissue. Vascular proliferation in DDA is hypothesized to be from ischemia and hypoxia, leading to angiogenesis.1-3 Diffuse dermal angiomatosis is characterized histologically by the presence of a diffuse proliferation of spindled endothelial cells distributed between the collagen bundles throughout the dermis (quiz image and Figure 1). Spindle-shaped endothelial cells exhibit a vacuolated cytoplasm. On immunohistochemistry, these dermal spindle cells classically stain positive for CD31, CD34, and erythroblast transformation specific–related gene (Erg) and stain negative for both human herpesvirus 8 (HHV-8) and factor XIIIa.

Cutaneous fibrous histiocytoma, more commonly referred to as dermatofibroma, is a common benign lesion that presents clinically as a solitary firm nodule most commonly on the extremities in areas of repetitive trauma or pressure. It classically exhibits dimpling of the overlaying skin with lateral pressure on the lesion, known as the dimple sign.4 Histologically, dermatofibromas share similar features to DDA and demonstrate the presence of bland-appearing spindle cells within the dermis between the collagen bundles, resulting in collagen trapping. However, a distinguishing histologic feature of a dermatofibroma in comparison to DDA is the presence of epidermal hyperplasia overlying the dermatofibroma, leading to tabled rete ridges (Figure 2). Spindle cells in dermatofibromas are fibroblasts and have a distinct immunophenotype that includes factor XIIIa positivity and negative staining for CD31, CD34, and Erg.4,5

Dermatofibrosarcoma protuberans (DFSP) is a rare malignant soft-tissue sarcoma that clinically presents as a firm, flesh-colored, dermal plaque on the trunk, proximal extremities, head, or neck.5 Histologically, DFSP can be distinguished from DDA by the high density of spindle cells that are arranged in a storiform pattern, extending and infiltrating the underlying subcutaneous fat in a honeycomblike pattern (Figure 3). Spindle cells in DFSP typically show expression of CD34 but are negative for CD31, Erg, and factor XIIIa.5

Kaposi sarcoma (KS) is an endothelial cell–driven angioproliferative neoplasm that is associated with HHV-8 infection.6 The clinical presentation of KS can range from isolated pink or purple papules and patches to more extensive ulcerated plaques or nodules. Histopathology exhibits proliferation of monomorphic spindled endothelial cells within the dermis staining positive for HHV-8, Erg, CD31, and CD34, in conjunction with extravasated erythrocytes arranged within slitlike vascular spaces (Figure 4). Additionally, KS classically exhibits aberrant endothelial cell proliferation and vessel formation around preexisting vessels, which is referred to as the promontory sign (Figure 4).

Angiosarcoma is a rare and highly aggressive vascular tumor arising from endothelial cells lining the blood vessels and lymphatics.7,8 Clinically, angiosarcoma presents as ulcerated violaceous nodules or plaques on the head, neck, or trunk. Histologic evaluation of angiosarcoma reveals a complex and poorly demarcated vascular network dissecting between collagen bundles in the dermis (Figure 5). Multilayering of endothelial cells, papillary projections extending into the vessel lumina, and mitoses frequently are seen. On immunohistochemistry, endothelial cells demonstrate prominent cellular atypia and stain positive with CD31, CD34, and Erg.

The Diagnosis: Diffuse Dermal Angiomatosis
Diffuse dermal angiomatosis (DDA) is an acquired reactive vascular proliferation in the spectrum of cutaneous reactive angioendotheliomatoses. Clinically, DDA presents as violaceous reticulated plaques, often with secondary ulceration and sometimes necrosis.1-3 Diffuse dermal angiomatosis more commonly presents in patients with a history of severe peripheral vascular disease, coagulopathies, or infection, and it frequently arises on the extremities. Diffuse dermal angiomatosis also has been shown to develop on the breasts, particularly in patients with pendulous breast tissue. Vascular proliferation in DDA is hypothesized to be from ischemia and hypoxia, leading to angiogenesis.1-3 Diffuse dermal angiomatosis is characterized histologically by the presence of a diffuse proliferation of spindled endothelial cells distributed between the collagen bundles throughout the dermis (quiz image and Figure 1). Spindle-shaped endothelial cells exhibit a vacuolated cytoplasm. On immunohistochemistry, these dermal spindle cells classically stain positive for CD31, CD34, and erythroblast transformation specific–related gene (Erg) and stain negative for both human herpesvirus 8 (HHV-8) and factor XIIIa.

Cutaneous fibrous histiocytoma, more commonly referred to as dermatofibroma, is a common benign lesion that presents clinically as a solitary firm nodule most commonly on the extremities in areas of repetitive trauma or pressure. It classically exhibits dimpling of the overlaying skin with lateral pressure on the lesion, known as the dimple sign.4 Histologically, dermatofibromas share similar features to DDA and demonstrate the presence of bland-appearing spindle cells within the dermis between the collagen bundles, resulting in collagen trapping. However, a distinguishing histologic feature of a dermatofibroma in comparison to DDA is the presence of epidermal hyperplasia overlying the dermatofibroma, leading to tabled rete ridges (Figure 2). Spindle cells in dermatofibromas are fibroblasts and have a distinct immunophenotype that includes factor XIIIa positivity and negative staining for CD31, CD34, and Erg.4,5

Dermatofibrosarcoma protuberans (DFSP) is a rare malignant soft-tissue sarcoma that clinically presents as a firm, flesh-colored, dermal plaque on the trunk, proximal extremities, head, or neck.5 Histologically, DFSP can be distinguished from DDA by the high density of spindle cells that are arranged in a storiform pattern, extending and infiltrating the underlying subcutaneous fat in a honeycomblike pattern (Figure 3). Spindle cells in DFSP typically show expression of CD34 but are negative for CD31, Erg, and factor XIIIa.5

Kaposi sarcoma (KS) is an endothelial cell–driven angioproliferative neoplasm that is associated with HHV-8 infection.6 The clinical presentation of KS can range from isolated pink or purple papules and patches to more extensive ulcerated plaques or nodules. Histopathology exhibits proliferation of monomorphic spindled endothelial cells within the dermis staining positive for HHV-8, Erg, CD31, and CD34, in conjunction with extravasated erythrocytes arranged within slitlike vascular spaces (Figure 4). Additionally, KS classically exhibits aberrant endothelial cell proliferation and vessel formation around preexisting vessels, which is referred to as the promontory sign (Figure 4).

Angiosarcoma is a rare and highly aggressive vascular tumor arising from endothelial cells lining the blood vessels and lymphatics.7,8 Clinically, angiosarcoma presents as ulcerated violaceous nodules or plaques on the head, neck, or trunk. Histologic evaluation of angiosarcoma reveals a complex and poorly demarcated vascular network dissecting between collagen bundles in the dermis (Figure 5). Multilayering of endothelial cells, papillary projections extending into the vessel lumina, and mitoses frequently are seen. On immunohistochemistry, endothelial cells demonstrate prominent cellular atypia and stain positive with CD31, CD34, and Erg.

- Touloei K, Tongdee E, Smirnov B, et al. Diffuse dermal angiomatosis. Cutis. 2019;103:181-184.
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Baylor Univ Med Cent Proc. 2020;33:273-275.
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Etemad SA, Dewan AK. Kaposi sarcoma updates. Dermatol Clin. 2019;37:505-517.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
- Touloei K, Tongdee E, Smirnov B, et al. Diffuse dermal angiomatosis. Cutis. 2019;103:181-184.
- Nguyen N, Silfvast-Kaiser AS, Frieder J, et al. Diffuse dermal angiomatosis of the breast. Baylor Univ Med Cent Proc. 2020;33:273-275.
- Frikha F, Boudaya S, Abid N, et al. Diffuse dermal angiomatosis of the breast with adjacent fat necrosis: a case report and review of the literature. Dermatol Online J. 2018;24:13030/qt1vq114n7.
- Luzar B, Calonje E. Cutaneous fibrohistiocytic tumours—an update. Histopathology. 2010;56:148-165.
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Etemad SA, Dewan AK. Kaposi sarcoma updates. Dermatol Clin. 2019;37:505-517.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
A 42-year-old woman with a medical history of hypertension and smoking tobacco (5 pack years) presented with a painful, nonhealing, violaceous, reticulated plaque with ulceration on the right breast of 3 months’ duration. Histopathology revealed diffuse, interstitial, bland-appearing spindle cells throughout the papillary and reticular dermis that were distributed between the collagen bundles. Dermal interstitial spindle cells were positive for CD31, CD34, and erythroblast transformation specific–related gene immunostains. Factor XIIIa and human herpesvirus 8 immunostaining was negative.
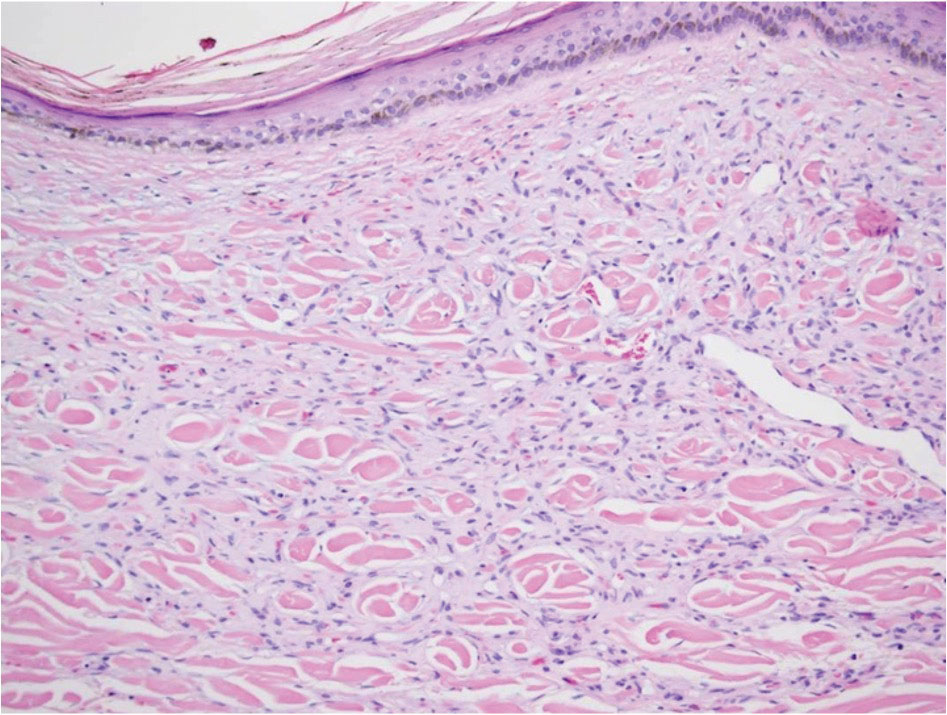
Pulmonary embolism confers higher mortality long term
Topline
Long-term mortality rates among individuals who have had a pulmonary embolism are significantly higher than rates in the general population.
Methodology
Researchers investigated long-term outcomes of patients with pulmonary embolism in a single-center registry.
They followed 896 patients for up to 14 years.
Data were from consecutive cases treated between May 2005 and December 2017.
Takeaway
The total follow-up time was 3,908 patient-years (median, 3.1 years).
One-year and five-year mortality rates were 19.7% (95% confidence interval, 17.2%-22.4%) and 37.1% (95% CI, 33.6%-40.5%), respectively, for patients with pulmonary embolism.
The most frequent causes of death were cancer (28.5%), pulmonary embolism (19.4%), infections (13.9%), and cardiovascular events (11.6%).
Late mortality (>30 days) was more frequent than in the general population for patients with cancer (5-year standardized mortality ratio, 2.77; 95% CI, 2.41-3.16) and for patients without cancer (1.80; 95% CI, 1.50-2.14), compared with expected rates.
In practice
stated Johannes Eckelt, Clinic of Cardiology and Pneumology, University Medical Center Göttingen (Germany).
Source
“Long-term Mortality in Pulmonary Embolism: Results in a Single-Center Registry,” by Mr. Eckelt and colleagues was published in Research and Practice in Thrombosis and Haemostasis.
Limitations
Owing to the single-center study design, selection bias cannot be excluded, limiting the generalizability of the study findings, the authors stated.
Disclosures
The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Topline
Long-term mortality rates among individuals who have had a pulmonary embolism are significantly higher than rates in the general population.
Methodology
Researchers investigated long-term outcomes of patients with pulmonary embolism in a single-center registry.
They followed 896 patients for up to 14 years.
Data were from consecutive cases treated between May 2005 and December 2017.
Takeaway
The total follow-up time was 3,908 patient-years (median, 3.1 years).
One-year and five-year mortality rates were 19.7% (95% confidence interval, 17.2%-22.4%) and 37.1% (95% CI, 33.6%-40.5%), respectively, for patients with pulmonary embolism.
The most frequent causes of death were cancer (28.5%), pulmonary embolism (19.4%), infections (13.9%), and cardiovascular events (11.6%).
Late mortality (>30 days) was more frequent than in the general population for patients with cancer (5-year standardized mortality ratio, 2.77; 95% CI, 2.41-3.16) and for patients without cancer (1.80; 95% CI, 1.50-2.14), compared with expected rates.
In practice
stated Johannes Eckelt, Clinic of Cardiology and Pneumology, University Medical Center Göttingen (Germany).
Source
“Long-term Mortality in Pulmonary Embolism: Results in a Single-Center Registry,” by Mr. Eckelt and colleagues was published in Research and Practice in Thrombosis and Haemostasis.
Limitations
Owing to the single-center study design, selection bias cannot be excluded, limiting the generalizability of the study findings, the authors stated.
Disclosures
The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Topline
Long-term mortality rates among individuals who have had a pulmonary embolism are significantly higher than rates in the general population.
Methodology
Researchers investigated long-term outcomes of patients with pulmonary embolism in a single-center registry.
They followed 896 patients for up to 14 years.
Data were from consecutive cases treated between May 2005 and December 2017.
Takeaway
The total follow-up time was 3,908 patient-years (median, 3.1 years).
One-year and five-year mortality rates were 19.7% (95% confidence interval, 17.2%-22.4%) and 37.1% (95% CI, 33.6%-40.5%), respectively, for patients with pulmonary embolism.
The most frequent causes of death were cancer (28.5%), pulmonary embolism (19.4%), infections (13.9%), and cardiovascular events (11.6%).
Late mortality (>30 days) was more frequent than in the general population for patients with cancer (5-year standardized mortality ratio, 2.77; 95% CI, 2.41-3.16) and for patients without cancer (1.80; 95% CI, 1.50-2.14), compared with expected rates.
In practice
stated Johannes Eckelt, Clinic of Cardiology and Pneumology, University Medical Center Göttingen (Germany).
Source
“Long-term Mortality in Pulmonary Embolism: Results in a Single-Center Registry,” by Mr. Eckelt and colleagues was published in Research and Practice in Thrombosis and Haemostasis.
Limitations
Owing to the single-center study design, selection bias cannot be excluded, limiting the generalizability of the study findings, the authors stated.
Disclosures
The authors have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
How small practices are surviving and thriving, part 1
Key takeaways
- Small-town physicians mostly love their practices; they are close to their patients and community, have the opportunity to practice very varied medicine, and feel like they make a difference. But they also struggle with many issues.
- Small practices are at a disadvantage when it comes to negotiating reimbursements.
- Resources such as access to specialists, equipment, and specialty meds put small-town docs in more precarious situations.
The challenges are mounting for physicians in small-town practices and rural areas, with private equity buying up many practices, the cost of overhead rising, and increased stress in attracting top talent. In the first of a two-part series, this news organization spoke to physicians in small towns around the country to identify some of the pain points squeezing small-town practices’ profits and making patient care more difficult.
Here are how physicians are working to offset the challenges and to make their small-town practices more rewarding.
Low reimbursements remain challenging
Jennifer Bacani McKenney, MD, owner of Fredonia Family Care, a private family medicine practice in Fredonia, Kan. (population 2,132), loves having close relationships with her patients and being an integral part of the community. However, she said that owning the only clinic in her town, which is 90 miles from Wichita, limits her power when negotiating for reimbursements.
“We don’t have bargaining power, so we often will end up getting terribly low reimbursements, especially for Medicaid,” she said. “We pay the price for not being part of a big health system.”
To bolster her ability to get reimbursement price concessions, her practice – which was initially started by her father and now includes four physicians – joined an accountable care organization in 2016.
“By joining other private practices around the state, we made some gains,” said Dr. McKenney, who was born in the hospital where she now works. “It enabled us to sit at the table with Blue Cross/Blue Shield of Kansas, for example, and have conversations that they listen to.”
Talent recruitment is an ongoing issue
For Ann Lima, MD, a family physician who came to Orofino, Idaho (population 3,000), 8 years ago after her residency in Ventura, Calif., practicing small-town medicine and seeing patients with a myriad of medical issues is a fulfilling challenge, but finding trained providers to join her practice remains problematic.
That’s because the physicians in her practice need to be nimble and to be able to routinely pivot from primary care to obstetrics to emergency medicine, owing to the nature of small-town practicing.
“It’s challenging in terms of finding people who are able to stay on top of all facets of hospital and acute care emergency care as well as OB and primary care,” she said. She noted that, for patients who require additional care, the nearest cities are Spokane, Wash., and Coeur D’Alene, Idaho, both approximately 3 hours away.
“It’s a challenge to find well-trained family physicians who want to do this diverse type of medicine.”
When it comes to staffing at her clinic, Dr. McKenney said it’s been more efficient to train employees from the ground up than try to find health care workers who already have significant experience.
“Right now, I have two 19-year-olds, a 21-year-old, and a 24-year-old working for me,” Dr. McKenney said of her clinic staff, which currently includes four doctors, a nurse practitioner, and 14 employees. “I hired the 19-year-old at age 17 and taught her to be a medical assistant.”
In addition to difficulty in recruiting physicians, nurses, and staff to a small-town practice, trying to find affordable housing makes it difficult to attract staffing in certain locations, said Frank Batcha, MD, a family physician in Hailey, Idaho (population 9,463), and chief of staff at St. Luke’s Wood River Regional Hospital in Ketchum, Idaho, where he has worked since the 1990s.
“We’re a resort community, so housing is unaffordable for somebody with an entry-level job,” he said. The region, a valley that includes Sun Valley, a popular ski resort with about 22,000 residents, is home to a handful of celebrities. It’s a popular destination spot and makes for a beautiful back country to call home.
“But it’s difficult to recruit physicians out of residency for this reason,” said Dr. Batcha. “We call it the scenery tax. It comes with a price.” Idaho is 49th out of 50th in physicians per capita for the entire United States.
Resources can be scarce
Another stressor for rural and small-town physicians is access to specialists, resources, and, in some cases, vital equipment.
“We have a general surgeon but no other specialty care,” Dr. Lima said. “This means that we can do acute appendicitis, we can take out gall bladders and do hernia repairs locally, but for significant trauma care and for patients who are very sick with ICU needs, we have to transfer them.”
Weather is also a huge factor that can affect ground ambulance or helicopter travel to a larger hospital.
“If there’s a storm, instead of a 45-minute transfer via helicopter, it’s a 3½ hour drive along mountain and river roads,” said Dr. Lima.
Ultimately, Dr. McKenney wished colleagues better understood the challenges facing rural physicians.
“When I transfer a patient from my hospital to a bigger facility, it’s because I don’t have certain medications on hand or an MRI ready to go,” she said. “It’s not that I don’t know what I’m doing.”
In addition, when she calls for a consult or sends a patient to a larger facility, it’s always because of a lack of resources.
“As rural physicians, we are really well educated and well trained,” she said “Our issue is that we’re practicing in a place with fewer things. But, when we call upon you, just know that we’ve tried everything we can first.”
Dr. McKenney lives and works happily in the town she grew up in and said no place could have given her a warmer welcome. In fact, while she was still finishing school, the townspeople campaigned to get her to come back and practice there – hard to come by that in a big city.
Small-town physicians offered five tactics for making a small-town practice work successfully:
- Develop relationships with specialists in your nearest large facility for referrals.
- Consider joining an ACO to improve work flow, diversify revenue streams, and maintain independence.
- Create a culture that’s welcoming to all incoming young professionals.
- Host medical students and residents as part of their education. “If they learn about your community, your practice, and rural healthcare early on, they will be more likely to be interested in coming back to serve that same community,” said Dr. McKenney.
- Recruit more than one physician if possible. “It’s really scary for new physicians to go out and practice on their own right out of training. Most rural communities need more than one more doctor anyway, and this gives them a built-in support system from the beginning,” said Dr. McKenney.
A version of this article first appeared on Medscape.com.
Key takeaways
- Small-town physicians mostly love their practices; they are close to their patients and community, have the opportunity to practice very varied medicine, and feel like they make a difference. But they also struggle with many issues.
- Small practices are at a disadvantage when it comes to negotiating reimbursements.
- Resources such as access to specialists, equipment, and specialty meds put small-town docs in more precarious situations.
The challenges are mounting for physicians in small-town practices and rural areas, with private equity buying up many practices, the cost of overhead rising, and increased stress in attracting top talent. In the first of a two-part series, this news organization spoke to physicians in small towns around the country to identify some of the pain points squeezing small-town practices’ profits and making patient care more difficult.
Here are how physicians are working to offset the challenges and to make their small-town practices more rewarding.
Low reimbursements remain challenging
Jennifer Bacani McKenney, MD, owner of Fredonia Family Care, a private family medicine practice in Fredonia, Kan. (population 2,132), loves having close relationships with her patients and being an integral part of the community. However, she said that owning the only clinic in her town, which is 90 miles from Wichita, limits her power when negotiating for reimbursements.
“We don’t have bargaining power, so we often will end up getting terribly low reimbursements, especially for Medicaid,” she said. “We pay the price for not being part of a big health system.”
To bolster her ability to get reimbursement price concessions, her practice – which was initially started by her father and now includes four physicians – joined an accountable care organization in 2016.
“By joining other private practices around the state, we made some gains,” said Dr. McKenney, who was born in the hospital where she now works. “It enabled us to sit at the table with Blue Cross/Blue Shield of Kansas, for example, and have conversations that they listen to.”
Talent recruitment is an ongoing issue
For Ann Lima, MD, a family physician who came to Orofino, Idaho (population 3,000), 8 years ago after her residency in Ventura, Calif., practicing small-town medicine and seeing patients with a myriad of medical issues is a fulfilling challenge, but finding trained providers to join her practice remains problematic.
That’s because the physicians in her practice need to be nimble and to be able to routinely pivot from primary care to obstetrics to emergency medicine, owing to the nature of small-town practicing.
“It’s challenging in terms of finding people who are able to stay on top of all facets of hospital and acute care emergency care as well as OB and primary care,” she said. She noted that, for patients who require additional care, the nearest cities are Spokane, Wash., and Coeur D’Alene, Idaho, both approximately 3 hours away.
“It’s a challenge to find well-trained family physicians who want to do this diverse type of medicine.”
When it comes to staffing at her clinic, Dr. McKenney said it’s been more efficient to train employees from the ground up than try to find health care workers who already have significant experience.
“Right now, I have two 19-year-olds, a 21-year-old, and a 24-year-old working for me,” Dr. McKenney said of her clinic staff, which currently includes four doctors, a nurse practitioner, and 14 employees. “I hired the 19-year-old at age 17 and taught her to be a medical assistant.”
In addition to difficulty in recruiting physicians, nurses, and staff to a small-town practice, trying to find affordable housing makes it difficult to attract staffing in certain locations, said Frank Batcha, MD, a family physician in Hailey, Idaho (population 9,463), and chief of staff at St. Luke’s Wood River Regional Hospital in Ketchum, Idaho, where he has worked since the 1990s.
“We’re a resort community, so housing is unaffordable for somebody with an entry-level job,” he said. The region, a valley that includes Sun Valley, a popular ski resort with about 22,000 residents, is home to a handful of celebrities. It’s a popular destination spot and makes for a beautiful back country to call home.
“But it’s difficult to recruit physicians out of residency for this reason,” said Dr. Batcha. “We call it the scenery tax. It comes with a price.” Idaho is 49th out of 50th in physicians per capita for the entire United States.
Resources can be scarce
Another stressor for rural and small-town physicians is access to specialists, resources, and, in some cases, vital equipment.
“We have a general surgeon but no other specialty care,” Dr. Lima said. “This means that we can do acute appendicitis, we can take out gall bladders and do hernia repairs locally, but for significant trauma care and for patients who are very sick with ICU needs, we have to transfer them.”
Weather is also a huge factor that can affect ground ambulance or helicopter travel to a larger hospital.
“If there’s a storm, instead of a 45-minute transfer via helicopter, it’s a 3½ hour drive along mountain and river roads,” said Dr. Lima.
Ultimately, Dr. McKenney wished colleagues better understood the challenges facing rural physicians.
“When I transfer a patient from my hospital to a bigger facility, it’s because I don’t have certain medications on hand or an MRI ready to go,” she said. “It’s not that I don’t know what I’m doing.”
In addition, when she calls for a consult or sends a patient to a larger facility, it’s always because of a lack of resources.
“As rural physicians, we are really well educated and well trained,” she said “Our issue is that we’re practicing in a place with fewer things. But, when we call upon you, just know that we’ve tried everything we can first.”
Dr. McKenney lives and works happily in the town she grew up in and said no place could have given her a warmer welcome. In fact, while she was still finishing school, the townspeople campaigned to get her to come back and practice there – hard to come by that in a big city.
Small-town physicians offered five tactics for making a small-town practice work successfully:
- Develop relationships with specialists in your nearest large facility for referrals.
- Consider joining an ACO to improve work flow, diversify revenue streams, and maintain independence.
- Create a culture that’s welcoming to all incoming young professionals.
- Host medical students and residents as part of their education. “If they learn about your community, your practice, and rural healthcare early on, they will be more likely to be interested in coming back to serve that same community,” said Dr. McKenney.
- Recruit more than one physician if possible. “It’s really scary for new physicians to go out and practice on their own right out of training. Most rural communities need more than one more doctor anyway, and this gives them a built-in support system from the beginning,” said Dr. McKenney.
A version of this article first appeared on Medscape.com.
Key takeaways
- Small-town physicians mostly love their practices; they are close to their patients and community, have the opportunity to practice very varied medicine, and feel like they make a difference. But they also struggle with many issues.
- Small practices are at a disadvantage when it comes to negotiating reimbursements.
- Resources such as access to specialists, equipment, and specialty meds put small-town docs in more precarious situations.
The challenges are mounting for physicians in small-town practices and rural areas, with private equity buying up many practices, the cost of overhead rising, and increased stress in attracting top talent. In the first of a two-part series, this news organization spoke to physicians in small towns around the country to identify some of the pain points squeezing small-town practices’ profits and making patient care more difficult.
Here are how physicians are working to offset the challenges and to make their small-town practices more rewarding.
Low reimbursements remain challenging
Jennifer Bacani McKenney, MD, owner of Fredonia Family Care, a private family medicine practice in Fredonia, Kan. (population 2,132), loves having close relationships with her patients and being an integral part of the community. However, she said that owning the only clinic in her town, which is 90 miles from Wichita, limits her power when negotiating for reimbursements.
“We don’t have bargaining power, so we often will end up getting terribly low reimbursements, especially for Medicaid,” she said. “We pay the price for not being part of a big health system.”
To bolster her ability to get reimbursement price concessions, her practice – which was initially started by her father and now includes four physicians – joined an accountable care organization in 2016.
“By joining other private practices around the state, we made some gains,” said Dr. McKenney, who was born in the hospital where she now works. “It enabled us to sit at the table with Blue Cross/Blue Shield of Kansas, for example, and have conversations that they listen to.”
Talent recruitment is an ongoing issue
For Ann Lima, MD, a family physician who came to Orofino, Idaho (population 3,000), 8 years ago after her residency in Ventura, Calif., practicing small-town medicine and seeing patients with a myriad of medical issues is a fulfilling challenge, but finding trained providers to join her practice remains problematic.
That’s because the physicians in her practice need to be nimble and to be able to routinely pivot from primary care to obstetrics to emergency medicine, owing to the nature of small-town practicing.
“It’s challenging in terms of finding people who are able to stay on top of all facets of hospital and acute care emergency care as well as OB and primary care,” she said. She noted that, for patients who require additional care, the nearest cities are Spokane, Wash., and Coeur D’Alene, Idaho, both approximately 3 hours away.
“It’s a challenge to find well-trained family physicians who want to do this diverse type of medicine.”
When it comes to staffing at her clinic, Dr. McKenney said it’s been more efficient to train employees from the ground up than try to find health care workers who already have significant experience.
“Right now, I have two 19-year-olds, a 21-year-old, and a 24-year-old working for me,” Dr. McKenney said of her clinic staff, which currently includes four doctors, a nurse practitioner, and 14 employees. “I hired the 19-year-old at age 17 and taught her to be a medical assistant.”
In addition to difficulty in recruiting physicians, nurses, and staff to a small-town practice, trying to find affordable housing makes it difficult to attract staffing in certain locations, said Frank Batcha, MD, a family physician in Hailey, Idaho (population 9,463), and chief of staff at St. Luke’s Wood River Regional Hospital in Ketchum, Idaho, where he has worked since the 1990s.
“We’re a resort community, so housing is unaffordable for somebody with an entry-level job,” he said. The region, a valley that includes Sun Valley, a popular ski resort with about 22,000 residents, is home to a handful of celebrities. It’s a popular destination spot and makes for a beautiful back country to call home.
“But it’s difficult to recruit physicians out of residency for this reason,” said Dr. Batcha. “We call it the scenery tax. It comes with a price.” Idaho is 49th out of 50th in physicians per capita for the entire United States.
Resources can be scarce
Another stressor for rural and small-town physicians is access to specialists, resources, and, in some cases, vital equipment.
“We have a general surgeon but no other specialty care,” Dr. Lima said. “This means that we can do acute appendicitis, we can take out gall bladders and do hernia repairs locally, but for significant trauma care and for patients who are very sick with ICU needs, we have to transfer them.”
Weather is also a huge factor that can affect ground ambulance or helicopter travel to a larger hospital.
“If there’s a storm, instead of a 45-minute transfer via helicopter, it’s a 3½ hour drive along mountain and river roads,” said Dr. Lima.
Ultimately, Dr. McKenney wished colleagues better understood the challenges facing rural physicians.
“When I transfer a patient from my hospital to a bigger facility, it’s because I don’t have certain medications on hand or an MRI ready to go,” she said. “It’s not that I don’t know what I’m doing.”
In addition, when she calls for a consult or sends a patient to a larger facility, it’s always because of a lack of resources.
“As rural physicians, we are really well educated and well trained,” she said “Our issue is that we’re practicing in a place with fewer things. But, when we call upon you, just know that we’ve tried everything we can first.”
Dr. McKenney lives and works happily in the town she grew up in and said no place could have given her a warmer welcome. In fact, while she was still finishing school, the townspeople campaigned to get her to come back and practice there – hard to come by that in a big city.
Small-town physicians offered five tactics for making a small-town practice work successfully:
- Develop relationships with specialists in your nearest large facility for referrals.
- Consider joining an ACO to improve work flow, diversify revenue streams, and maintain independence.
- Create a culture that’s welcoming to all incoming young professionals.
- Host medical students and residents as part of their education. “If they learn about your community, your practice, and rural healthcare early on, they will be more likely to be interested in coming back to serve that same community,” said Dr. McKenney.
- Recruit more than one physician if possible. “It’s really scary for new physicians to go out and practice on their own right out of training. Most rural communities need more than one more doctor anyway, and this gives them a built-in support system from the beginning,” said Dr. McKenney.
A version of this article first appeared on Medscape.com.
Nails falling off in a 3-year-old
When the nails peel off from the proximal nail folds, the clinical term is onychomadesis and it is important to ask about recent infections or severe metabolic stressors. In children and adults, onychomadesis on multiple fingers may occur after infections and has been associated with hand-foot-mouth disease caused by common viral infections—especially strains of coxsackievirus.1
Because shed nails show evidence of viral infection, one hypothesis for their peeling off is that the tissue of the nail matrix is infected, leading to metabolic changes. As the nail matrix returns to normal function, a new nail is made and ultimately will replace the nail that has come off. In healthy US adults, fingernails grow 3.47 mm per month on average while toenails grow 1.62 mm per month on average.2
Sometimes it’s hard to elicit a history of a very mild viral illness weeks or months after it has resolved. Asking specifically about mouth ulcers may help. If there is a history of a viral illness, no specific work-up or treatment is necessary. Patients may be reassured that nails will improve over several months without lasting effects.
In this case, the patient and her family were given reassurance and the nails returned to normal within a few months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Kim EJ, Park HS, Yoon HS, et al. Four cases of onychomadesis after hand-foot-mouth disease. Ann Dermatol. 2014;26:777-778. doi: 10.5021/ad.2014.26.6.777
2. Yaemsiri S, Hou N, Slining MM, et al. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24:420-423. doi: 10.1111/j.1468-3083.2009.03426.x

When the nails peel off from the proximal nail folds, the clinical term is onychomadesis and it is important to ask about recent infections or severe metabolic stressors. In children and adults, onychomadesis on multiple fingers may occur after infections and has been associated with hand-foot-mouth disease caused by common viral infections—especially strains of coxsackievirus.1
Because shed nails show evidence of viral infection, one hypothesis for their peeling off is that the tissue of the nail matrix is infected, leading to metabolic changes. As the nail matrix returns to normal function, a new nail is made and ultimately will replace the nail that has come off. In healthy US adults, fingernails grow 3.47 mm per month on average while toenails grow 1.62 mm per month on average.2
Sometimes it’s hard to elicit a history of a very mild viral illness weeks or months after it has resolved. Asking specifically about mouth ulcers may help. If there is a history of a viral illness, no specific work-up or treatment is necessary. Patients may be reassured that nails will improve over several months without lasting effects.
In this case, the patient and her family were given reassurance and the nails returned to normal within a few months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

When the nails peel off from the proximal nail folds, the clinical term is onychomadesis and it is important to ask about recent infections or severe metabolic stressors. In children and adults, onychomadesis on multiple fingers may occur after infections and has been associated with hand-foot-mouth disease caused by common viral infections—especially strains of coxsackievirus.1
Because shed nails show evidence of viral infection, one hypothesis for their peeling off is that the tissue of the nail matrix is infected, leading to metabolic changes. As the nail matrix returns to normal function, a new nail is made and ultimately will replace the nail that has come off. In healthy US adults, fingernails grow 3.47 mm per month on average while toenails grow 1.62 mm per month on average.2
Sometimes it’s hard to elicit a history of a very mild viral illness weeks or months after it has resolved. Asking specifically about mouth ulcers may help. If there is a history of a viral illness, no specific work-up or treatment is necessary. Patients may be reassured that nails will improve over several months without lasting effects.
In this case, the patient and her family were given reassurance and the nails returned to normal within a few months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Kim EJ, Park HS, Yoon HS, et al. Four cases of onychomadesis after hand-foot-mouth disease. Ann Dermatol. 2014;26:777-778. doi: 10.5021/ad.2014.26.6.777
2. Yaemsiri S, Hou N, Slining MM, et al. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24:420-423. doi: 10.1111/j.1468-3083.2009.03426.x
1. Kim EJ, Park HS, Yoon HS, et al. Four cases of onychomadesis after hand-foot-mouth disease. Ann Dermatol. 2014;26:777-778. doi: 10.5021/ad.2014.26.6.777
2. Yaemsiri S, Hou N, Slining MM, et al. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24:420-423. doi: 10.1111/j.1468-3083.2009.03426.x
‘Artificial pancreas’ for all type 1 diabetes pregnancies?
In the largest randomized controlled trial of an automated insulin delivery (AID) system (hybrid closed-loop) versus standard insulin delivery in pregnant women with type 1 diabetes, the automated CamAPS FX system prevailed.
Helen R. Murphy, MD, presented these topline findings from the Automated Insulin Delivery Amongst Pregnant Women With Type 1 Diabetes (AiDAPT) trial during an e-poster session at the annual scientific sessions of the American Diabetes Association.
The “hybrid closed-loop significantly improved maternal glucose and should be offered to all pregnant women with type 1 diabetes,” concluded Dr. Murphy, professor of medicine at the University of East Anglia and a clinician at Norfolk and Norwich University Hospital in the United Kingdom.
CamAPS FX is the only AID system approved in Europe and the United Kingdom for type 1 diabetes from age 1 and during pregnancy. The hybrid closed-loop system is not available in the United States but other systems are available and sometimes used off label in pregnancy. Such systems are sometimes known colloquially as an “artificial pancreas.”
The researchers said their findings provide evidence for the UK National Institute of Clinical Excellence (NICE) to recommend that all pregnant women with type 1 diabetes should be offered the CamAPS FX system.
Asked by an audience member about type 2 diabetes in pregnancy, Dr. Murphy said: “I don’t think we can necessarily extend these data to women with type 2 diabetes. We just don’t have enough data on glucose profiles in type 2 to train an algorithm yet.”
However, the data provide support for earlier use of closed-loop therapy in type 1 diabetes, she said. “The ideal time to start closed-loop is not necessarily between 8 and 12 weeks. Half of all pregnancies are unplanned,” she noted, “so start [AID] as early as possible [in patients with type 1 diabetes].”
Two experts weigh in
Whether pregnant women with type 1 diabetes should be offered hybrid closed-loop therapy “depends,” said Anne L. Peters, MD, who was not involved with the research.
“It is all about being able to set [blood glucose] targets,” according to Dr. Peters, director of the University of Southern California Westside Center for Diabetes in Los Angeles.
“If a woman is on an AID system – except for DIY loop – I have them stop the automation and adjust manually,” she said in an email. “My [patients] do amazingly well in pregnancy – most can get their A1cs below 6%,” she noted. “But if someone can’t do that and their A1cs are higher, automation can help.
“It is always about individualizing care,” said Dr. Peters. “The one thing that helps the most is continuous glucose monitoring (CGM). And I do have patients who remain on [insulin] injections throughout pregnancy.”
And Sarit Polsky, MD, MPH, who was also not involved with the current study, agrees that “AID with CamAPS, which has an option to customize the glucose target in the pregnancy-specific range, appears to be safe and effective in pregnancy and should be offered” to patients in Europe and the United Kingdom.
“Whether other AID systems should be recommended in pregnancy is still unclear, said Dr. Polsky, associate professor of medicine and pediatrics at Barbara Davis Center for Childhood Diabetes, University of Colorado Anschutz Medical Campus.
“Around 48% of [global] pregnancies are unplanned,” Dr. Polsky said in an interview. “Many women do indeed become pregnant while using AID systems and many opt to continue use of these systems.
“Off-label use of these products can be beneficial in pregnancy in select cases, but the systems generally need the use of assistive techniques, which we previously published, to help get glucose levels to pregnancy-specific targets,” she noted in an email.
Study rationale, method, and findings
Pregnant women with diabetes are advised to aim for very tight glucose targets throughout pregnancy and avoid hyperglycemia, to reduce risk of preterm delivery, neonatal weight > 90th percentile, and neonatal morbidity, according to Dr. Murphy and colleagues.
“However, despite increased use of [CGM], continuous subcutaneous insulin infusion (CSII), and improved insulin analogs, achieving and maintaining the recommended glucose targets remains challenging for most pregnant women with type 1 diabetes,” they wrote in their abstract.
Researchers randomized 124 women who had type 1 diabetes for at least 12 months, were at < 13 weeks’ to 6 days’ gestation, and had an A1c of 6.5% to < 10% who were taking intensive standard insulin therapy at nine antenatal clinics in the United Kingdom. Half of the women were using CSII and half were receiving multiple daily injections of insulin.
As explained in the published study protocol, the women were randomized to continue their standard insulin delivery or switch to a closed-loop system consisting of the study insulin pump (Dana Diabecare RS), a CGM transmitter, and an app (CamAPS FX) on an Android smartphone that communicates wirelessly with the insulin pump and CGM transmitter.
Participants in both groups used the same CGM system and received support for insulin dose adjustment from their antenatal clinical care team.
They were a mean age of 31 years, had a mean A1c of 7.7%, and had had type 1 diabetes for 17 years on average. Their body mass index varied; 37% had normal weight, 27% had overweight, and 26% had obesity.
A significantly higher percentage of women in the AID group than in the control group had blood glucose in target range more than 70% of the time (46% vs. 10%; P < .001).
Compared with women in the control group, those in the AID group had larger reductions in hyperglycemia (–11%; P < .001), higher overnight time-in-range (13%; P < .001), and lower A1c (–0.34%; P < .001), without additional insulin, weight gain, or hypoglycemia.
The effect was consistent across clinical sites and maternal age and A1c categories.
Ongoing studies, off-label use
Hybrid closed-loop systems “including Tandem Control IQ, the Omnipod 5, and the Medtronic 780G give insulin continuously on the basis of values obtained from a sensor,” Dr. Peters explained in a recent commentary. “These aren’t fully closed-loop systems because the individual still has to interact with the system and give doses for meals, and then adjust doses for exercise.”
There are currently three studies using commercially available AID systems without pregnancy-specific glucose targets, in type 1 diabetes pregnancies, Dr. Polsky noted.
The Pregnancy Intervention With a Closed-Loop System (PICLS) trial used the Medtronic 670G system in pregnancy and was conducted in the United States. The Closed-Loop Insulin Delivery in Pregnant Women With Type 1 Diabetes (CRISTAL) study is using the Medtronic 780G system in pregnancy and is being conducted in Belgium and the Netherlands. And the Closed-Loop Insulin Delivery in Type 1 Diabetes Pregnancies (CIRCUIT) study is using the Tandem Control IQ system in pregnancy and is being conducted in Canada, she explained.
“The decision to continue to use or to initiate (off-label) use of any of these systems in pregnancy should be individualized, and pregnant individuals should make these decisions by working with an experienced endocrine/diabetes team,” Dr. Polsky stressed.
“The hope is that the results of these exciting trials will show safe and effective use of these systems throughout gestation with improvements in glucose control and quality of life,” she concluded.
The study was funded by the UK National Institute for Health Research, JDRF, and Diabetes Research and Wellness Foundation. Dr. Murphy has reported being on the advisory panel for Medtronic and receiving research support from Dexcom. Dr. Peters disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care, received a research grant from Abbott Diabetes Care, and received stock options from Teladoc and Omada Health. Dr. Polsky has disclosed that she is a contributing writer for diaTribe, was on a medical advisory board for Medtronic MiniMed, has received research funding from DexCom, Eli Lilly, JDRF, Leona & Harry Helmsley Charitable Trust, NIDDK, and Sanofi, and has received research support from Diasome Pharmaceuticals, LabStyle Innovation, Lexicon, Medtronic MiniMed, and Sanofi.
A version of this article first appeared on Medscape.com.
In the largest randomized controlled trial of an automated insulin delivery (AID) system (hybrid closed-loop) versus standard insulin delivery in pregnant women with type 1 diabetes, the automated CamAPS FX system prevailed.
Helen R. Murphy, MD, presented these topline findings from the Automated Insulin Delivery Amongst Pregnant Women With Type 1 Diabetes (AiDAPT) trial during an e-poster session at the annual scientific sessions of the American Diabetes Association.
The “hybrid closed-loop significantly improved maternal glucose and should be offered to all pregnant women with type 1 diabetes,” concluded Dr. Murphy, professor of medicine at the University of East Anglia and a clinician at Norfolk and Norwich University Hospital in the United Kingdom.
CamAPS FX is the only AID system approved in Europe and the United Kingdom for type 1 diabetes from age 1 and during pregnancy. The hybrid closed-loop system is not available in the United States but other systems are available and sometimes used off label in pregnancy. Such systems are sometimes known colloquially as an “artificial pancreas.”
The researchers said their findings provide evidence for the UK National Institute of Clinical Excellence (NICE) to recommend that all pregnant women with type 1 diabetes should be offered the CamAPS FX system.
Asked by an audience member about type 2 diabetes in pregnancy, Dr. Murphy said: “I don’t think we can necessarily extend these data to women with type 2 diabetes. We just don’t have enough data on glucose profiles in type 2 to train an algorithm yet.”
However, the data provide support for earlier use of closed-loop therapy in type 1 diabetes, she said. “The ideal time to start closed-loop is not necessarily between 8 and 12 weeks. Half of all pregnancies are unplanned,” she noted, “so start [AID] as early as possible [in patients with type 1 diabetes].”
Two experts weigh in
Whether pregnant women with type 1 diabetes should be offered hybrid closed-loop therapy “depends,” said Anne L. Peters, MD, who was not involved with the research.
“It is all about being able to set [blood glucose] targets,” according to Dr. Peters, director of the University of Southern California Westside Center for Diabetes in Los Angeles.
“If a woman is on an AID system – except for DIY loop – I have them stop the automation and adjust manually,” she said in an email. “My [patients] do amazingly well in pregnancy – most can get their A1cs below 6%,” she noted. “But if someone can’t do that and their A1cs are higher, automation can help.
“It is always about individualizing care,” said Dr. Peters. “The one thing that helps the most is continuous glucose monitoring (CGM). And I do have patients who remain on [insulin] injections throughout pregnancy.”
And Sarit Polsky, MD, MPH, who was also not involved with the current study, agrees that “AID with CamAPS, which has an option to customize the glucose target in the pregnancy-specific range, appears to be safe and effective in pregnancy and should be offered” to patients in Europe and the United Kingdom.
“Whether other AID systems should be recommended in pregnancy is still unclear, said Dr. Polsky, associate professor of medicine and pediatrics at Barbara Davis Center for Childhood Diabetes, University of Colorado Anschutz Medical Campus.
“Around 48% of [global] pregnancies are unplanned,” Dr. Polsky said in an interview. “Many women do indeed become pregnant while using AID systems and many opt to continue use of these systems.
“Off-label use of these products can be beneficial in pregnancy in select cases, but the systems generally need the use of assistive techniques, which we previously published, to help get glucose levels to pregnancy-specific targets,” she noted in an email.
Study rationale, method, and findings
Pregnant women with diabetes are advised to aim for very tight glucose targets throughout pregnancy and avoid hyperglycemia, to reduce risk of preterm delivery, neonatal weight > 90th percentile, and neonatal morbidity, according to Dr. Murphy and colleagues.
“However, despite increased use of [CGM], continuous subcutaneous insulin infusion (CSII), and improved insulin analogs, achieving and maintaining the recommended glucose targets remains challenging for most pregnant women with type 1 diabetes,” they wrote in their abstract.
Researchers randomized 124 women who had type 1 diabetes for at least 12 months, were at < 13 weeks’ to 6 days’ gestation, and had an A1c of 6.5% to < 10% who were taking intensive standard insulin therapy at nine antenatal clinics in the United Kingdom. Half of the women were using CSII and half were receiving multiple daily injections of insulin.
As explained in the published study protocol, the women were randomized to continue their standard insulin delivery or switch to a closed-loop system consisting of the study insulin pump (Dana Diabecare RS), a CGM transmitter, and an app (CamAPS FX) on an Android smartphone that communicates wirelessly with the insulin pump and CGM transmitter.
Participants in both groups used the same CGM system and received support for insulin dose adjustment from their antenatal clinical care team.
They were a mean age of 31 years, had a mean A1c of 7.7%, and had had type 1 diabetes for 17 years on average. Their body mass index varied; 37% had normal weight, 27% had overweight, and 26% had obesity.
A significantly higher percentage of women in the AID group than in the control group had blood glucose in target range more than 70% of the time (46% vs. 10%; P < .001).
Compared with women in the control group, those in the AID group had larger reductions in hyperglycemia (–11%; P < .001), higher overnight time-in-range (13%; P < .001), and lower A1c (–0.34%; P < .001), without additional insulin, weight gain, or hypoglycemia.
The effect was consistent across clinical sites and maternal age and A1c categories.
Ongoing studies, off-label use
Hybrid closed-loop systems “including Tandem Control IQ, the Omnipod 5, and the Medtronic 780G give insulin continuously on the basis of values obtained from a sensor,” Dr. Peters explained in a recent commentary. “These aren’t fully closed-loop systems because the individual still has to interact with the system and give doses for meals, and then adjust doses for exercise.”
There are currently three studies using commercially available AID systems without pregnancy-specific glucose targets, in type 1 diabetes pregnancies, Dr. Polsky noted.
The Pregnancy Intervention With a Closed-Loop System (PICLS) trial used the Medtronic 670G system in pregnancy and was conducted in the United States. The Closed-Loop Insulin Delivery in Pregnant Women With Type 1 Diabetes (CRISTAL) study is using the Medtronic 780G system in pregnancy and is being conducted in Belgium and the Netherlands. And the Closed-Loop Insulin Delivery in Type 1 Diabetes Pregnancies (CIRCUIT) study is using the Tandem Control IQ system in pregnancy and is being conducted in Canada, she explained.
“The decision to continue to use or to initiate (off-label) use of any of these systems in pregnancy should be individualized, and pregnant individuals should make these decisions by working with an experienced endocrine/diabetes team,” Dr. Polsky stressed.
“The hope is that the results of these exciting trials will show safe and effective use of these systems throughout gestation with improvements in glucose control and quality of life,” she concluded.
The study was funded by the UK National Institute for Health Research, JDRF, and Diabetes Research and Wellness Foundation. Dr. Murphy has reported being on the advisory panel for Medtronic and receiving research support from Dexcom. Dr. Peters disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care, received a research grant from Abbott Diabetes Care, and received stock options from Teladoc and Omada Health. Dr. Polsky has disclosed that she is a contributing writer for diaTribe, was on a medical advisory board for Medtronic MiniMed, has received research funding from DexCom, Eli Lilly, JDRF, Leona & Harry Helmsley Charitable Trust, NIDDK, and Sanofi, and has received research support from Diasome Pharmaceuticals, LabStyle Innovation, Lexicon, Medtronic MiniMed, and Sanofi.
A version of this article first appeared on Medscape.com.
In the largest randomized controlled trial of an automated insulin delivery (AID) system (hybrid closed-loop) versus standard insulin delivery in pregnant women with type 1 diabetes, the automated CamAPS FX system prevailed.
Helen R. Murphy, MD, presented these topline findings from the Automated Insulin Delivery Amongst Pregnant Women With Type 1 Diabetes (AiDAPT) trial during an e-poster session at the annual scientific sessions of the American Diabetes Association.
The “hybrid closed-loop significantly improved maternal glucose and should be offered to all pregnant women with type 1 diabetes,” concluded Dr. Murphy, professor of medicine at the University of East Anglia and a clinician at Norfolk and Norwich University Hospital in the United Kingdom.
CamAPS FX is the only AID system approved in Europe and the United Kingdom for type 1 diabetes from age 1 and during pregnancy. The hybrid closed-loop system is not available in the United States but other systems are available and sometimes used off label in pregnancy. Such systems are sometimes known colloquially as an “artificial pancreas.”
The researchers said their findings provide evidence for the UK National Institute of Clinical Excellence (NICE) to recommend that all pregnant women with type 1 diabetes should be offered the CamAPS FX system.
Asked by an audience member about type 2 diabetes in pregnancy, Dr. Murphy said: “I don’t think we can necessarily extend these data to women with type 2 diabetes. We just don’t have enough data on glucose profiles in type 2 to train an algorithm yet.”
However, the data provide support for earlier use of closed-loop therapy in type 1 diabetes, she said. “The ideal time to start closed-loop is not necessarily between 8 and 12 weeks. Half of all pregnancies are unplanned,” she noted, “so start [AID] as early as possible [in patients with type 1 diabetes].”
Two experts weigh in
Whether pregnant women with type 1 diabetes should be offered hybrid closed-loop therapy “depends,” said Anne L. Peters, MD, who was not involved with the research.
“It is all about being able to set [blood glucose] targets,” according to Dr. Peters, director of the University of Southern California Westside Center for Diabetes in Los Angeles.
“If a woman is on an AID system – except for DIY loop – I have them stop the automation and adjust manually,” she said in an email. “My [patients] do amazingly well in pregnancy – most can get their A1cs below 6%,” she noted. “But if someone can’t do that and their A1cs are higher, automation can help.
“It is always about individualizing care,” said Dr. Peters. “The one thing that helps the most is continuous glucose monitoring (CGM). And I do have patients who remain on [insulin] injections throughout pregnancy.”
And Sarit Polsky, MD, MPH, who was also not involved with the current study, agrees that “AID with CamAPS, which has an option to customize the glucose target in the pregnancy-specific range, appears to be safe and effective in pregnancy and should be offered” to patients in Europe and the United Kingdom.
“Whether other AID systems should be recommended in pregnancy is still unclear, said Dr. Polsky, associate professor of medicine and pediatrics at Barbara Davis Center for Childhood Diabetes, University of Colorado Anschutz Medical Campus.
“Around 48% of [global] pregnancies are unplanned,” Dr. Polsky said in an interview. “Many women do indeed become pregnant while using AID systems and many opt to continue use of these systems.
“Off-label use of these products can be beneficial in pregnancy in select cases, but the systems generally need the use of assistive techniques, which we previously published, to help get glucose levels to pregnancy-specific targets,” she noted in an email.
Study rationale, method, and findings
Pregnant women with diabetes are advised to aim for very tight glucose targets throughout pregnancy and avoid hyperglycemia, to reduce risk of preterm delivery, neonatal weight > 90th percentile, and neonatal morbidity, according to Dr. Murphy and colleagues.
“However, despite increased use of [CGM], continuous subcutaneous insulin infusion (CSII), and improved insulin analogs, achieving and maintaining the recommended glucose targets remains challenging for most pregnant women with type 1 diabetes,” they wrote in their abstract.
Researchers randomized 124 women who had type 1 diabetes for at least 12 months, were at < 13 weeks’ to 6 days’ gestation, and had an A1c of 6.5% to < 10% who were taking intensive standard insulin therapy at nine antenatal clinics in the United Kingdom. Half of the women were using CSII and half were receiving multiple daily injections of insulin.
As explained in the published study protocol, the women were randomized to continue their standard insulin delivery or switch to a closed-loop system consisting of the study insulin pump (Dana Diabecare RS), a CGM transmitter, and an app (CamAPS FX) on an Android smartphone that communicates wirelessly with the insulin pump and CGM transmitter.
Participants in both groups used the same CGM system and received support for insulin dose adjustment from their antenatal clinical care team.
They were a mean age of 31 years, had a mean A1c of 7.7%, and had had type 1 diabetes for 17 years on average. Their body mass index varied; 37% had normal weight, 27% had overweight, and 26% had obesity.
A significantly higher percentage of women in the AID group than in the control group had blood glucose in target range more than 70% of the time (46% vs. 10%; P < .001).
Compared with women in the control group, those in the AID group had larger reductions in hyperglycemia (–11%; P < .001), higher overnight time-in-range (13%; P < .001), and lower A1c (–0.34%; P < .001), without additional insulin, weight gain, or hypoglycemia.
The effect was consistent across clinical sites and maternal age and A1c categories.
Ongoing studies, off-label use
Hybrid closed-loop systems “including Tandem Control IQ, the Omnipod 5, and the Medtronic 780G give insulin continuously on the basis of values obtained from a sensor,” Dr. Peters explained in a recent commentary. “These aren’t fully closed-loop systems because the individual still has to interact with the system and give doses for meals, and then adjust doses for exercise.”
There are currently three studies using commercially available AID systems without pregnancy-specific glucose targets, in type 1 diabetes pregnancies, Dr. Polsky noted.
The Pregnancy Intervention With a Closed-Loop System (PICLS) trial used the Medtronic 670G system in pregnancy and was conducted in the United States. The Closed-Loop Insulin Delivery in Pregnant Women With Type 1 Diabetes (CRISTAL) study is using the Medtronic 780G system in pregnancy and is being conducted in Belgium and the Netherlands. And the Closed-Loop Insulin Delivery in Type 1 Diabetes Pregnancies (CIRCUIT) study is using the Tandem Control IQ system in pregnancy and is being conducted in Canada, she explained.
“The decision to continue to use or to initiate (off-label) use of any of these systems in pregnancy should be individualized, and pregnant individuals should make these decisions by working with an experienced endocrine/diabetes team,” Dr. Polsky stressed.
“The hope is that the results of these exciting trials will show safe and effective use of these systems throughout gestation with improvements in glucose control and quality of life,” she concluded.
The study was funded by the UK National Institute for Health Research, JDRF, and Diabetes Research and Wellness Foundation. Dr. Murphy has reported being on the advisory panel for Medtronic and receiving research support from Dexcom. Dr. Peters disclosed that she served as a consultant for Blue Circle Health, Vertex, and Abbott Diabetes Care, received a research grant from Abbott Diabetes Care, and received stock options from Teladoc and Omada Health. Dr. Polsky has disclosed that she is a contributing writer for diaTribe, was on a medical advisory board for Medtronic MiniMed, has received research funding from DexCom, Eli Lilly, JDRF, Leona & Harry Helmsley Charitable Trust, NIDDK, and Sanofi, and has received research support from Diasome Pharmaceuticals, LabStyle Innovation, Lexicon, Medtronic MiniMed, and Sanofi.
A version of this article first appeared on Medscape.com.
FROM ADA 2023
Thoughts on primary care in 2023
As we all face remarkable challenges in giving great care to our patients and maintaining great care for ourselves, I wanted to share a few thoughts I have had regarding difficult things I have seen in the past few months.
- Call centers: Yikes! I think this is an overlooked stress on the primary care system. In a cost-cutting effort, organizations have gone to call centers to handle incoming calls, and hold times can be enormous. My own organization often has wait times longer than 30 minutes. I recently called another organization and had a wait time more than 30 minutes. Patients become frustrated and will message their primary care team to intervene for scheduling issues then will arrive at their appointments frustrated by all the hassles.
- Difficult encounters: We all have visits that we know will be challenging. I think it is even more difficult when we enter the visit stressed and tired. I have always found that, when I am in a calm place, even the most difficult visits go much better. Our patients arrive at clinic visits more stressed and tired too, as they face the challenge of a stretched and overwhelmed primary care system.
- Limited availability of specialists: My organization has had a sharp increase in wait times for specialty care over the past few years. Waits for some specialties can be almost a year. A study by Reddy and colleagues found a wait time of 3 months for patients referred to gastroenterologists.1 The lack of timely access to specialists adds to the stress and burden of primary care professionals. Managing problems deemed in need of subspecialty care as patients wait for appointments is difficult.
- Patient portals: Some practices are starting to figure this out this problem, others aren’t. Budd reviewed all the factors with the EHR that contribute to physician burnout.2 Portals have added another source of patient care outside face-to-face visits that adds to physician work load; for many practices is not appropriately accounted for in effort or productivity measures. Some practices are now starting to charge for patient messaging, but this may require even more physician time in documentation and billing. Unless this directly helps the physician reduce work hours or improve compensation, then it may make the problem worse.
There is little mystery why it seems so hard ... it is! Many things have been added to the plate of primary care professionals (increased messaging, calming patients frustrated with the medical system, and increased need for bridging care while patients wait for specialty appointments). Our patients need us now more than ever to give excellent, compassionate care in a poorly functioning system. We need to be emotionally and physically healthy enough to be there for our patients. Prioritize your own needs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Reddy K et al. Health Equity. 2018 Jun 1;2(1):103-8.
2. Budd J. J Prim Care Community Health. 2023 Apr 19.
As we all face remarkable challenges in giving great care to our patients and maintaining great care for ourselves, I wanted to share a few thoughts I have had regarding difficult things I have seen in the past few months.
- Call centers: Yikes! I think this is an overlooked stress on the primary care system. In a cost-cutting effort, organizations have gone to call centers to handle incoming calls, and hold times can be enormous. My own organization often has wait times longer than 30 minutes. I recently called another organization and had a wait time more than 30 minutes. Patients become frustrated and will message their primary care team to intervene for scheduling issues then will arrive at their appointments frustrated by all the hassles.
- Difficult encounters: We all have visits that we know will be challenging. I think it is even more difficult when we enter the visit stressed and tired. I have always found that, when I am in a calm place, even the most difficult visits go much better. Our patients arrive at clinic visits more stressed and tired too, as they face the challenge of a stretched and overwhelmed primary care system.
- Limited availability of specialists: My organization has had a sharp increase in wait times for specialty care over the past few years. Waits for some specialties can be almost a year. A study by Reddy and colleagues found a wait time of 3 months for patients referred to gastroenterologists.1 The lack of timely access to specialists adds to the stress and burden of primary care professionals. Managing problems deemed in need of subspecialty care as patients wait for appointments is difficult.
- Patient portals: Some practices are starting to figure this out this problem, others aren’t. Budd reviewed all the factors with the EHR that contribute to physician burnout.2 Portals have added another source of patient care outside face-to-face visits that adds to physician work load; for many practices is not appropriately accounted for in effort or productivity measures. Some practices are now starting to charge for patient messaging, but this may require even more physician time in documentation and billing. Unless this directly helps the physician reduce work hours or improve compensation, then it may make the problem worse.
There is little mystery why it seems so hard ... it is! Many things have been added to the plate of primary care professionals (increased messaging, calming patients frustrated with the medical system, and increased need for bridging care while patients wait for specialty appointments). Our patients need us now more than ever to give excellent, compassionate care in a poorly functioning system. We need to be emotionally and physically healthy enough to be there for our patients. Prioritize your own needs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Reddy K et al. Health Equity. 2018 Jun 1;2(1):103-8.
2. Budd J. J Prim Care Community Health. 2023 Apr 19.
As we all face remarkable challenges in giving great care to our patients and maintaining great care for ourselves, I wanted to share a few thoughts I have had regarding difficult things I have seen in the past few months.
- Call centers: Yikes! I think this is an overlooked stress on the primary care system. In a cost-cutting effort, organizations have gone to call centers to handle incoming calls, and hold times can be enormous. My own organization often has wait times longer than 30 minutes. I recently called another organization and had a wait time more than 30 minutes. Patients become frustrated and will message their primary care team to intervene for scheduling issues then will arrive at their appointments frustrated by all the hassles.
- Difficult encounters: We all have visits that we know will be challenging. I think it is even more difficult when we enter the visit stressed and tired. I have always found that, when I am in a calm place, even the most difficult visits go much better. Our patients arrive at clinic visits more stressed and tired too, as they face the challenge of a stretched and overwhelmed primary care system.
- Limited availability of specialists: My organization has had a sharp increase in wait times for specialty care over the past few years. Waits for some specialties can be almost a year. A study by Reddy and colleagues found a wait time of 3 months for patients referred to gastroenterologists.1 The lack of timely access to specialists adds to the stress and burden of primary care professionals. Managing problems deemed in need of subspecialty care as patients wait for appointments is difficult.
- Patient portals: Some practices are starting to figure this out this problem, others aren’t. Budd reviewed all the factors with the EHR that contribute to physician burnout.2 Portals have added another source of patient care outside face-to-face visits that adds to physician work load; for many practices is not appropriately accounted for in effort or productivity measures. Some practices are now starting to charge for patient messaging, but this may require even more physician time in documentation and billing. Unless this directly helps the physician reduce work hours or improve compensation, then it may make the problem worse.
There is little mystery why it seems so hard ... it is! Many things have been added to the plate of primary care professionals (increased messaging, calming patients frustrated with the medical system, and increased need for bridging care while patients wait for specialty appointments). Our patients need us now more than ever to give excellent, compassionate care in a poorly functioning system. We need to be emotionally and physically healthy enough to be there for our patients. Prioritize your own needs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Reddy K et al. Health Equity. 2018 Jun 1;2(1):103-8.
2. Budd J. J Prim Care Community Health. 2023 Apr 19.