User login
Treating vasculitis with conventional immunosuppressive agents
In 1958, shortly after the first descriptions of granulomatosis with polyangiitis, or GPA (Wegener’s granulomatosis), the 1-year mortality was 18%,1 mainly due to renal failure. Physicians tried to combat the disease using various immunosuppressive drugs (nitrogen mustard and, in later years, azathioprine and methotrexate), but measurable success came only after investigators introduced cyclophosphamide (CYC) in combination with the glucocorticoid prednisone.2
A key 1992 study showed that the CYC/prednisone combination markedly improved the disease status in 91% of patients,3 with 75% achieving complete remission. The treatment came at a price, however, with almost all patients suffering serious morbidity or side effects. The results also highlighted concerns about potential malignancies caused by prolonged use of CYC and glucocorticoids. Those concerns motivated the European Vasculitis Study Group in the late 1980s and early 1990s to design and validate testing for antineutrophil cytoplasmic antibody (ANCA)–associated vasculitides (AAV) and pursue consensus regarding treatment.4
ALTERNATIVES TO STANDARD THERAPY
The accepted therapeutic strategy for GPA is to first induce remission using high doses of CYC and then prevent relapse with longer-term, less toxic therapeutic alternatives. These less toxic therapies include newer agents as well as new methods of delivery, particularly for patients with nonsevere forms of disease.
Methotrexate—effective for early treatment
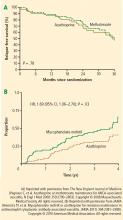
Methotrexate showed early promise in several nonrandomized trials of patients with nonsevere disease. In one such study, de Groot et al subclassified 100 patients at diagnosis according to the extent and severity of the disease.5 Patients were then randomized to receive either standard oral CYC or methotrexate, each combined with prednisolone. Remission rates (90% to 94%) were comparable regardless of whether patients received CYC or methotrexate, although patients with more severe disease who were taking methotrexate took longer to achieve remission. At the same time, relapse rates were higher for methotrexate-taking patients (70%) compared with the CYC group (47%). Thus, while methotrexate could replace CYC for initial treatment of early AAV, CYC had a greater influence on subsequent relapse rates, particularly in patients with more severe forms of disease.
Pulse cyclophosphamide—a new method
Investigators tested pulse delivery of CYC compared with oral daily administration as a means of reducing the CYC dose. An analysis of 14 relatively small studies showed that pulse CYC had the same survival and renal failure rates as continuous therapy.6
One such trial, the CYC Daily Oral Versus Pulsed (CYCLOPS) trial, involved 149 patients with generalized disease (nephritis, GPA, and microscopic polyangiitis [MPA]) who were administered either an intravenous (IV) pulse or a daily oral CYC regimen.7 The pulse CYC neither shortened patients’ time to remission nor increased the proportion of patients who achieved it. Patients receiving pulse CYC suffered one-third the rate of leukopenia experienced by patients who received the oral regimen. Since infection is a source of mortality in vasculitis, this finding is an important consideration when balancing the benefits of day-to-day control offered by oral administration against the safety of at-risk patients such as the elderly.
This treatment strategy may be relevant for patients with renal impairment. It was once thought that patients with renal failure after receiving CYC had more aggressive disease and therefore needed higher dosages. Investigators who studied the impact of renal insufficiency and hemodialysis on the pharmacokinetics of CYC found that clearance of CYC is impaired in patients with reduced renal function.8 Thus, when renal function is suppressed, the CYC dosage should be reduced rather than increased.
Mycophenolate mofetil—efficacy not yet confirmed
Another alternative to CYC, mycophenolate mofetil (MMF), has gained much attention, although its effectiveness is not yet certain. Pilot data show that 13 of 17 patients with MPA achieved remission after 6 months of treatment with MMF.9 Meanwhile, the so-called MYCYC trial, in which patients with newly diagnosed AAV receive either the CYCLOPS regimen or MMF, is under way.10
Deoxyspergualin—remission not sustained
A nonstandard drug that warrants attention is deoxyspergualin (now called gusperimus), licensed in Japan for 15 years. In a prospective, open-label trial of 45 patients with relapsing or refractory GPA, investigators showed that 95% achieved partial remission and 45% full remission, although remission was not sustained when therapy was stopped.11 Because the drug must be administered daily for 21 days by subcutaneous injection, deoxyspergualin is not easy to use. It may represent an alternative, however, because it permitted prednisolone dosage reduction.
EVALUATING RISK AND CHOOSING THERAPIES
CONSIDERATIONS IN CHOOSING REMISSION THERAPY
Overall, when planning remission therapy and its duration, clinicians must balance the efficacy of CYC and glucocorticoids against their toxicity. Close monitoring and the patient’s capacity to adhere to instructions are two critical issues. Other important considerations include the risk and consequences of relapse, which vary in different circumstances, and the association of cancer with CYC therapy.
Relapse risk is variable
Certain patients are at higher risk of relapse than others. Patients with GPA or proteinase-3-ANCA–positive disease are at higher relapse risk than those who have MPA. ANCA-positive disease in remission or rising ANCA markers both increase the risk of relapse. Ear, nose, throat, and lung diseases increase the likelihood of relapse. Patients with GPA who are Staphylococcus aureus carriers have increased risk. Serum creatinine levels of 2.0 to 3.0 mg/dL at the end of induction therapy should arouse concern about renal relapse.
Most relapses affect the ear, nose, and throat system and do not threaten vital organs. Relapse does not increase the risk of end-stage renal disease or death.
Consider mortality and cancer data
Although the strongest predictor of early death is infection, advanced age and renal impairment also predict death. Chronic kidney disease stage at entry and glomerular filtration rate significantly predict mortality.21 More than 36 g CYC (equivalent to 9 to 12 months of standard oral therapy) increases the risk of bladder cancer 10-fold and myeloid leukemia 60-fold, but the cancer risk is time-dependent; malignancy requires 12 years on average to emerge.22
CONCLUSION
Cyclophosphamide in combination with glucocorticoids remains the standard therapy for GPA and related vasculitides, despite the risk of significant treatment-related comorbidities. Several strategies can be employed to reduce exposure, such as sequential withdrawal of CYC and IV administration. The optimization of glucocorticoid dosing will be a major research focus in the next decade. Newer agents may improve the maintenance of remission; for example, azathioprine and methotrexate show equal efficacy and safety, while MMF is less effective. When planning remission maintenance therapy, the relapse risk should be considered carefully because it varies among clinical scenarios. Other factors in the decision include the consequences for the patient, monitoring requirements, and the patient’s ability to understand and adhere to instructions.
- Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J 1958; 2:265–270.
- Novack SN, Pearson CM. Cyclophosphamide therapy in Wegener’s granulomatosis. N Engl J Med 1971; 284:938–942.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Rasmussen N, Jayne DRW, Abramowicz D, et al. European therapeutic trials in ANCA-associated systemic vasculitis: disease scoring, consensus regimens and proposed clinical trials. Clin Exp Immunol 1995; 101 (suppl 1):29–34.
- de Groot K, Rasmussen N, Bacon PA, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005; 52:2461–2469.
- de Groot K, Adu D, Savage COS; for EUVAS (European Vasculitis Study Group). The value of pulse cyclophosphamide in ANCA-associated vasculitis: meta-analysis and critical review. Nephrol Dial Transplant 2001; 16:2018–2027.
- de Groot K, Harper L, Jayne DRW, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2009; 150:670–680.
- Haubitz M, Bohnenstengel F, Brunkhorst R, Schwab M, Hofmann U, Busse D. Cyclophosphamide pharmacokinetics and dose requirements in patients with renal insufficiency. Kidney Int 2002; 61:1495–1501.
- Silva F, Specks U, Kalra S, et al. Mycophenolate mofetil for induction and maintenance of remission in microscopic polyangiitis with mild to moderate renal involvement: a prospective, open-label pilot trial [published online ahead of print January 21, 2010]. Clin J Am Soc Nephrol 2010; 5:445–453. doi: 10.2215/CJN.06010809
- MYCYC clinical trial protocol. The European Vasculitis Society Web site. http://www.vasculitis.org. Updated April 12, 2011. Accessed June 13, 2012.
- Flossmann O, Baslund B, Bruchfeld A, et al. Deoxyspergualin in relapsing and refractory Wegener’s granulomatosis [published online ahead of print August 19, 2008]. Ann Rheum Dis 2009; 68:1125–1130. doi: 10.1136/ard.2008.092429
- Mukhtyar C, Guillevin L, Cid MC, et al; for the European Vasculitis Study Group. EULAR recommendations for the management of primary small and medium vessel vasculitis [published online ahead of print April 15, 2008]. Ann Rheum Dis 2009; 68:310–317. doi: 10.1136/ard.2008.088096
- Mukhtyar C, Guillevin L, Cid MC, et al; for the European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis [published online ahead of print April 15, 2008]. Ann Rheum Dis 2009; 68:318–323. doi: 10.1136/ard.2008.088351
- Hellmich B, Flossmann O, Gross WL, et al; on behalf of the European Vasculitis Study Group. EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti-neutrophil cytoplasm antibody-associated vasculitis [published online ahead of print December 14, 2006]. Ann Rheum Dis 2007; 66:605–617. doi: 10.1136/ard.2006.062711
- Jayne D, Rasmussen N, Andrassy K, et al; for the European Vasculitis Study Group. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Pagnoux C, Mahr A, Hamidou MA, et al; for the French Vasculitis Study Group. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med 2008; 359:2790–2803.
- Hiemstra TF, Walsh M, Mahr A, et al; for the European Vasculitis Study Group (EUVAS). Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibodyassociated vasculitis: a randomized controlled trial [published online ahead of print November 8, 2010]. JAMA 2010; 304:2381–2388. doi: 10.1001/jama.2010.1658
- Metzler C, Miehle N, Manger K, et al; for the German Network of Rheumatic Diseases. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosis [published online ahead of print May 22, 2007]. Rheumatology 2007; 46:1087–1091. doi: 10.1093/rheumatology/kem029
- Walsh M, Merkel PA, Mahr A, Jayne D. Effects of duration of glucocorticoid therapy on relapse rate in antineutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis. Arthritis Care Res (Hoboken) 2010; 62:1166–1173.
- Vanková Z, Ríhová Z, Jancová E, Rysavá R, Merta M, Tesar V. Optimizing the therapeutic strategies in ANCA-associated vasculitis— single centre experience with international randomized trials. Prague Med Rep 2006; 107:199–212.
- Flossmann O, Berden A, de Groot K, et al; for the European Vasculitis Study Group. Long-term patient survival in ANCA-associated vasculitis [published online ahead of print November 24, 2010]. Ann Rheum Dis 2011; 70:488–494. doi: 10.1136/ard.2010.137778
- Faurschou M, Sorensen IJ, Mellemkjaer L, et al. Malignancies in Wegener’s granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients [published online ahead of print October 15, 2007]. J Rheumatol 2008; 35:100–105.
In 1958, shortly after the first descriptions of granulomatosis with polyangiitis, or GPA (Wegener’s granulomatosis), the 1-year mortality was 18%,1 mainly due to renal failure. Physicians tried to combat the disease using various immunosuppressive drugs (nitrogen mustard and, in later years, azathioprine and methotrexate), but measurable success came only after investigators introduced cyclophosphamide (CYC) in combination with the glucocorticoid prednisone.2
A key 1992 study showed that the CYC/prednisone combination markedly improved the disease status in 91% of patients,3 with 75% achieving complete remission. The treatment came at a price, however, with almost all patients suffering serious morbidity or side effects. The results also highlighted concerns about potential malignancies caused by prolonged use of CYC and glucocorticoids. Those concerns motivated the European Vasculitis Study Group in the late 1980s and early 1990s to design and validate testing for antineutrophil cytoplasmic antibody (ANCA)–associated vasculitides (AAV) and pursue consensus regarding treatment.4
ALTERNATIVES TO STANDARD THERAPY
The accepted therapeutic strategy for GPA is to first induce remission using high doses of CYC and then prevent relapse with longer-term, less toxic therapeutic alternatives. These less toxic therapies include newer agents as well as new methods of delivery, particularly for patients with nonsevere forms of disease.
Methotrexate—effective for early treatment
Methotrexate showed early promise in several nonrandomized trials of patients with nonsevere disease. In one such study, de Groot et al subclassified 100 patients at diagnosis according to the extent and severity of the disease.5 Patients were then randomized to receive either standard oral CYC or methotrexate, each combined with prednisolone. Remission rates (90% to 94%) were comparable regardless of whether patients received CYC or methotrexate, although patients with more severe disease who were taking methotrexate took longer to achieve remission. At the same time, relapse rates were higher for methotrexate-taking patients (70%) compared with the CYC group (47%). Thus, while methotrexate could replace CYC for initial treatment of early AAV, CYC had a greater influence on subsequent relapse rates, particularly in patients with more severe forms of disease.
Pulse cyclophosphamide—a new method
Investigators tested pulse delivery of CYC compared with oral daily administration as a means of reducing the CYC dose. An analysis of 14 relatively small studies showed that pulse CYC had the same survival and renal failure rates as continuous therapy.6
One such trial, the CYC Daily Oral Versus Pulsed (CYCLOPS) trial, involved 149 patients with generalized disease (nephritis, GPA, and microscopic polyangiitis [MPA]) who were administered either an intravenous (IV) pulse or a daily oral CYC regimen.7 The pulse CYC neither shortened patients’ time to remission nor increased the proportion of patients who achieved it. Patients receiving pulse CYC suffered one-third the rate of leukopenia experienced by patients who received the oral regimen. Since infection is a source of mortality in vasculitis, this finding is an important consideration when balancing the benefits of day-to-day control offered by oral administration against the safety of at-risk patients such as the elderly.
This treatment strategy may be relevant for patients with renal impairment. It was once thought that patients with renal failure after receiving CYC had more aggressive disease and therefore needed higher dosages. Investigators who studied the impact of renal insufficiency and hemodialysis on the pharmacokinetics of CYC found that clearance of CYC is impaired in patients with reduced renal function.8 Thus, when renal function is suppressed, the CYC dosage should be reduced rather than increased.

Mycophenolate mofetil—efficacy not yet confirmed
Another alternative to CYC, mycophenolate mofetil (MMF), has gained much attention, although its effectiveness is not yet certain. Pilot data show that 13 of 17 patients with MPA achieved remission after 6 months of treatment with MMF.9 Meanwhile, the so-called MYCYC trial, in which patients with newly diagnosed AAV receive either the CYCLOPS regimen or MMF, is under way.10
Deoxyspergualin—remission not sustained
A nonstandard drug that warrants attention is deoxyspergualin (now called gusperimus), licensed in Japan for 15 years. In a prospective, open-label trial of 45 patients with relapsing or refractory GPA, investigators showed that 95% achieved partial remission and 45% full remission, although remission was not sustained when therapy was stopped.11 Because the drug must be administered daily for 21 days by subcutaneous injection, deoxyspergualin is not easy to use. It may represent an alternative, however, because it permitted prednisolone dosage reduction.
EVALUATING RISK AND CHOOSING THERAPIES
CONSIDERATIONS IN CHOOSING REMISSION THERAPY
Overall, when planning remission therapy and its duration, clinicians must balance the efficacy of CYC and glucocorticoids against their toxicity. Close monitoring and the patient’s capacity to adhere to instructions are two critical issues. Other important considerations include the risk and consequences of relapse, which vary in different circumstances, and the association of cancer with CYC therapy.
Relapse risk is variable
Certain patients are at higher risk of relapse than others. Patients with GPA or proteinase-3-ANCA–positive disease are at higher relapse risk than those who have MPA. ANCA-positive disease in remission or rising ANCA markers both increase the risk of relapse. Ear, nose, throat, and lung diseases increase the likelihood of relapse. Patients with GPA who are Staphylococcus aureus carriers have increased risk. Serum creatinine levels of 2.0 to 3.0 mg/dL at the end of induction therapy should arouse concern about renal relapse.
Most relapses affect the ear, nose, and throat system and do not threaten vital organs. Relapse does not increase the risk of end-stage renal disease or death.
Consider mortality and cancer data
Although the strongest predictor of early death is infection, advanced age and renal impairment also predict death. Chronic kidney disease stage at entry and glomerular filtration rate significantly predict mortality.21 More than 36 g CYC (equivalent to 9 to 12 months of standard oral therapy) increases the risk of bladder cancer 10-fold and myeloid leukemia 60-fold, but the cancer risk is time-dependent; malignancy requires 12 years on average to emerge.22
CONCLUSION
Cyclophosphamide in combination with glucocorticoids remains the standard therapy for GPA and related vasculitides, despite the risk of significant treatment-related comorbidities. Several strategies can be employed to reduce exposure, such as sequential withdrawal of CYC and IV administration. The optimization of glucocorticoid dosing will be a major research focus in the next decade. Newer agents may improve the maintenance of remission; for example, azathioprine and methotrexate show equal efficacy and safety, while MMF is less effective. When planning remission maintenance therapy, the relapse risk should be considered carefully because it varies among clinical scenarios. Other factors in the decision include the consequences for the patient, monitoring requirements, and the patient’s ability to understand and adhere to instructions.
In 1958, shortly after the first descriptions of granulomatosis with polyangiitis, or GPA (Wegener’s granulomatosis), the 1-year mortality was 18%,1 mainly due to renal failure. Physicians tried to combat the disease using various immunosuppressive drugs (nitrogen mustard and, in later years, azathioprine and methotrexate), but measurable success came only after investigators introduced cyclophosphamide (CYC) in combination with the glucocorticoid prednisone.2
A key 1992 study showed that the CYC/prednisone combination markedly improved the disease status in 91% of patients,3 with 75% achieving complete remission. The treatment came at a price, however, with almost all patients suffering serious morbidity or side effects. The results also highlighted concerns about potential malignancies caused by prolonged use of CYC and glucocorticoids. Those concerns motivated the European Vasculitis Study Group in the late 1980s and early 1990s to design and validate testing for antineutrophil cytoplasmic antibody (ANCA)–associated vasculitides (AAV) and pursue consensus regarding treatment.4
ALTERNATIVES TO STANDARD THERAPY
The accepted therapeutic strategy for GPA is to first induce remission using high doses of CYC and then prevent relapse with longer-term, less toxic therapeutic alternatives. These less toxic therapies include newer agents as well as new methods of delivery, particularly for patients with nonsevere forms of disease.
Methotrexate—effective for early treatment
Methotrexate showed early promise in several nonrandomized trials of patients with nonsevere disease. In one such study, de Groot et al subclassified 100 patients at diagnosis according to the extent and severity of the disease.5 Patients were then randomized to receive either standard oral CYC or methotrexate, each combined with prednisolone. Remission rates (90% to 94%) were comparable regardless of whether patients received CYC or methotrexate, although patients with more severe disease who were taking methotrexate took longer to achieve remission. At the same time, relapse rates were higher for methotrexate-taking patients (70%) compared with the CYC group (47%). Thus, while methotrexate could replace CYC for initial treatment of early AAV, CYC had a greater influence on subsequent relapse rates, particularly in patients with more severe forms of disease.
Pulse cyclophosphamide—a new method
Investigators tested pulse delivery of CYC compared with oral daily administration as a means of reducing the CYC dose. An analysis of 14 relatively small studies showed that pulse CYC had the same survival and renal failure rates as continuous therapy.6
One such trial, the CYC Daily Oral Versus Pulsed (CYCLOPS) trial, involved 149 patients with generalized disease (nephritis, GPA, and microscopic polyangiitis [MPA]) who were administered either an intravenous (IV) pulse or a daily oral CYC regimen.7 The pulse CYC neither shortened patients’ time to remission nor increased the proportion of patients who achieved it. Patients receiving pulse CYC suffered one-third the rate of leukopenia experienced by patients who received the oral regimen. Since infection is a source of mortality in vasculitis, this finding is an important consideration when balancing the benefits of day-to-day control offered by oral administration against the safety of at-risk patients such as the elderly.
This treatment strategy may be relevant for patients with renal impairment. It was once thought that patients with renal failure after receiving CYC had more aggressive disease and therefore needed higher dosages. Investigators who studied the impact of renal insufficiency and hemodialysis on the pharmacokinetics of CYC found that clearance of CYC is impaired in patients with reduced renal function.8 Thus, when renal function is suppressed, the CYC dosage should be reduced rather than increased.

Mycophenolate mofetil—efficacy not yet confirmed
Another alternative to CYC, mycophenolate mofetil (MMF), has gained much attention, although its effectiveness is not yet certain. Pilot data show that 13 of 17 patients with MPA achieved remission after 6 months of treatment with MMF.9 Meanwhile, the so-called MYCYC trial, in which patients with newly diagnosed AAV receive either the CYCLOPS regimen or MMF, is under way.10
Deoxyspergualin—remission not sustained
A nonstandard drug that warrants attention is deoxyspergualin (now called gusperimus), licensed in Japan for 15 years. In a prospective, open-label trial of 45 patients with relapsing or refractory GPA, investigators showed that 95% achieved partial remission and 45% full remission, although remission was not sustained when therapy was stopped.11 Because the drug must be administered daily for 21 days by subcutaneous injection, deoxyspergualin is not easy to use. It may represent an alternative, however, because it permitted prednisolone dosage reduction.
EVALUATING RISK AND CHOOSING THERAPIES
CONSIDERATIONS IN CHOOSING REMISSION THERAPY
Overall, when planning remission therapy and its duration, clinicians must balance the efficacy of CYC and glucocorticoids against their toxicity. Close monitoring and the patient’s capacity to adhere to instructions are two critical issues. Other important considerations include the risk and consequences of relapse, which vary in different circumstances, and the association of cancer with CYC therapy.
Relapse risk is variable
Certain patients are at higher risk of relapse than others. Patients with GPA or proteinase-3-ANCA–positive disease are at higher relapse risk than those who have MPA. ANCA-positive disease in remission or rising ANCA markers both increase the risk of relapse. Ear, nose, throat, and lung diseases increase the likelihood of relapse. Patients with GPA who are Staphylococcus aureus carriers have increased risk. Serum creatinine levels of 2.0 to 3.0 mg/dL at the end of induction therapy should arouse concern about renal relapse.
Most relapses affect the ear, nose, and throat system and do not threaten vital organs. Relapse does not increase the risk of end-stage renal disease or death.
Consider mortality and cancer data
Although the strongest predictor of early death is infection, advanced age and renal impairment also predict death. Chronic kidney disease stage at entry and glomerular filtration rate significantly predict mortality.21 More than 36 g CYC (equivalent to 9 to 12 months of standard oral therapy) increases the risk of bladder cancer 10-fold and myeloid leukemia 60-fold, but the cancer risk is time-dependent; malignancy requires 12 years on average to emerge.22
CONCLUSION
Cyclophosphamide in combination with glucocorticoids remains the standard therapy for GPA and related vasculitides, despite the risk of significant treatment-related comorbidities. Several strategies can be employed to reduce exposure, such as sequential withdrawal of CYC and IV administration. The optimization of glucocorticoid dosing will be a major research focus in the next decade. Newer agents may improve the maintenance of remission; for example, azathioprine and methotrexate show equal efficacy and safety, while MMF is less effective. When planning remission maintenance therapy, the relapse risk should be considered carefully because it varies among clinical scenarios. Other factors in the decision include the consequences for the patient, monitoring requirements, and the patient’s ability to understand and adhere to instructions.
- Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J 1958; 2:265–270.
- Novack SN, Pearson CM. Cyclophosphamide therapy in Wegener’s granulomatosis. N Engl J Med 1971; 284:938–942.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Rasmussen N, Jayne DRW, Abramowicz D, et al. European therapeutic trials in ANCA-associated systemic vasculitis: disease scoring, consensus regimens and proposed clinical trials. Clin Exp Immunol 1995; 101 (suppl 1):29–34.
- de Groot K, Rasmussen N, Bacon PA, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005; 52:2461–2469.
- de Groot K, Adu D, Savage COS; for EUVAS (European Vasculitis Study Group). The value of pulse cyclophosphamide in ANCA-associated vasculitis: meta-analysis and critical review. Nephrol Dial Transplant 2001; 16:2018–2027.
- de Groot K, Harper L, Jayne DRW, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2009; 150:670–680.
- Haubitz M, Bohnenstengel F, Brunkhorst R, Schwab M, Hofmann U, Busse D. Cyclophosphamide pharmacokinetics and dose requirements in patients with renal insufficiency. Kidney Int 2002; 61:1495–1501.
- Silva F, Specks U, Kalra S, et al. Mycophenolate mofetil for induction and maintenance of remission in microscopic polyangiitis with mild to moderate renal involvement: a prospective, open-label pilot trial [published online ahead of print January 21, 2010]. Clin J Am Soc Nephrol 2010; 5:445–453. doi: 10.2215/CJN.06010809
- MYCYC clinical trial protocol. The European Vasculitis Society Web site. http://www.vasculitis.org. Updated April 12, 2011. Accessed June 13, 2012.
- Flossmann O, Baslund B, Bruchfeld A, et al. Deoxyspergualin in relapsing and refractory Wegener’s granulomatosis [published online ahead of print August 19, 2008]. Ann Rheum Dis 2009; 68:1125–1130. doi: 10.1136/ard.2008.092429
- Mukhtyar C, Guillevin L, Cid MC, et al; for the European Vasculitis Study Group. EULAR recommendations for the management of primary small and medium vessel vasculitis [published online ahead of print April 15, 2008]. Ann Rheum Dis 2009; 68:310–317. doi: 10.1136/ard.2008.088096
- Mukhtyar C, Guillevin L, Cid MC, et al; for the European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis [published online ahead of print April 15, 2008]. Ann Rheum Dis 2009; 68:318–323. doi: 10.1136/ard.2008.088351
- Hellmich B, Flossmann O, Gross WL, et al; on behalf of the European Vasculitis Study Group. EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti-neutrophil cytoplasm antibody-associated vasculitis [published online ahead of print December 14, 2006]. Ann Rheum Dis 2007; 66:605–617. doi: 10.1136/ard.2006.062711
- Jayne D, Rasmussen N, Andrassy K, et al; for the European Vasculitis Study Group. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Pagnoux C, Mahr A, Hamidou MA, et al; for the French Vasculitis Study Group. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med 2008; 359:2790–2803.
- Hiemstra TF, Walsh M, Mahr A, et al; for the European Vasculitis Study Group (EUVAS). Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibodyassociated vasculitis: a randomized controlled trial [published online ahead of print November 8, 2010]. JAMA 2010; 304:2381–2388. doi: 10.1001/jama.2010.1658
- Metzler C, Miehle N, Manger K, et al; for the German Network of Rheumatic Diseases. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosis [published online ahead of print May 22, 2007]. Rheumatology 2007; 46:1087–1091. doi: 10.1093/rheumatology/kem029
- Walsh M, Merkel PA, Mahr A, Jayne D. Effects of duration of glucocorticoid therapy on relapse rate in antineutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis. Arthritis Care Res (Hoboken) 2010; 62:1166–1173.
- Vanková Z, Ríhová Z, Jancová E, Rysavá R, Merta M, Tesar V. Optimizing the therapeutic strategies in ANCA-associated vasculitis— single centre experience with international randomized trials. Prague Med Rep 2006; 107:199–212.
- Flossmann O, Berden A, de Groot K, et al; for the European Vasculitis Study Group. Long-term patient survival in ANCA-associated vasculitis [published online ahead of print November 24, 2010]. Ann Rheum Dis 2011; 70:488–494. doi: 10.1136/ard.2010.137778
- Faurschou M, Sorensen IJ, Mellemkjaer L, et al. Malignancies in Wegener’s granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients [published online ahead of print October 15, 2007]. J Rheumatol 2008; 35:100–105.
- Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J 1958; 2:265–270.
- Novack SN, Pearson CM. Cyclophosphamide therapy in Wegener’s granulomatosis. N Engl J Med 1971; 284:938–942.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Rasmussen N, Jayne DRW, Abramowicz D, et al. European therapeutic trials in ANCA-associated systemic vasculitis: disease scoring, consensus regimens and proposed clinical trials. Clin Exp Immunol 1995; 101 (suppl 1):29–34.
- de Groot K, Rasmussen N, Bacon PA, et al. Randomized trial of cyclophosphamide versus methotrexate for induction of remission in early systemic antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005; 52:2461–2469.
- de Groot K, Adu D, Savage COS; for EUVAS (European Vasculitis Study Group). The value of pulse cyclophosphamide in ANCA-associated vasculitis: meta-analysis and critical review. Nephrol Dial Transplant 2001; 16:2018–2027.
- de Groot K, Harper L, Jayne DRW, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med 2009; 150:670–680.
- Haubitz M, Bohnenstengel F, Brunkhorst R, Schwab M, Hofmann U, Busse D. Cyclophosphamide pharmacokinetics and dose requirements in patients with renal insufficiency. Kidney Int 2002; 61:1495–1501.
- Silva F, Specks U, Kalra S, et al. Mycophenolate mofetil for induction and maintenance of remission in microscopic polyangiitis with mild to moderate renal involvement: a prospective, open-label pilot trial [published online ahead of print January 21, 2010]. Clin J Am Soc Nephrol 2010; 5:445–453. doi: 10.2215/CJN.06010809
- MYCYC clinical trial protocol. The European Vasculitis Society Web site. http://www.vasculitis.org. Updated April 12, 2011. Accessed June 13, 2012.
- Flossmann O, Baslund B, Bruchfeld A, et al. Deoxyspergualin in relapsing and refractory Wegener’s granulomatosis [published online ahead of print August 19, 2008]. Ann Rheum Dis 2009; 68:1125–1130. doi: 10.1136/ard.2008.092429
- Mukhtyar C, Guillevin L, Cid MC, et al; for the European Vasculitis Study Group. EULAR recommendations for the management of primary small and medium vessel vasculitis [published online ahead of print April 15, 2008]. Ann Rheum Dis 2009; 68:310–317. doi: 10.1136/ard.2008.088096
- Mukhtyar C, Guillevin L, Cid MC, et al; for the European Vasculitis Study Group. EULAR recommendations for the management of large vessel vasculitis [published online ahead of print April 15, 2008]. Ann Rheum Dis 2009; 68:318–323. doi: 10.1136/ard.2008.088351
- Hellmich B, Flossmann O, Gross WL, et al; on behalf of the European Vasculitis Study Group. EULAR recommendations for conducting clinical studies and/or clinical trials in systemic vasculitis: focus on anti-neutrophil cytoplasm antibody-associated vasculitis [published online ahead of print December 14, 2006]. Ann Rheum Dis 2007; 66:605–617. doi: 10.1136/ard.2006.062711
- Jayne D, Rasmussen N, Andrassy K, et al; for the European Vasculitis Study Group. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Pagnoux C, Mahr A, Hamidou MA, et al; for the French Vasculitis Study Group. Azathioprine or methotrexate maintenance for ANCA-associated vasculitis. N Engl J Med 2008; 359:2790–2803.
- Hiemstra TF, Walsh M, Mahr A, et al; for the European Vasculitis Study Group (EUVAS). Mycophenolate mofetil vs azathioprine for remission maintenance in antineutrophil cytoplasmic antibodyassociated vasculitis: a randomized controlled trial [published online ahead of print November 8, 2010]. JAMA 2010; 304:2381–2388. doi: 10.1001/jama.2010.1658
- Metzler C, Miehle N, Manger K, et al; for the German Network of Rheumatic Diseases. Elevated relapse rate under oral methotrexate versus leflunomide for maintenance of remission in Wegener’s granulomatosis [published online ahead of print May 22, 2007]. Rheumatology 2007; 46:1087–1091. doi: 10.1093/rheumatology/kem029
- Walsh M, Merkel PA, Mahr A, Jayne D. Effects of duration of glucocorticoid therapy on relapse rate in antineutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis. Arthritis Care Res (Hoboken) 2010; 62:1166–1173.
- Vanková Z, Ríhová Z, Jancová E, Rysavá R, Merta M, Tesar V. Optimizing the therapeutic strategies in ANCA-associated vasculitis— single centre experience with international randomized trials. Prague Med Rep 2006; 107:199–212.
- Flossmann O, Berden A, de Groot K, et al; for the European Vasculitis Study Group. Long-term patient survival in ANCA-associated vasculitis [published online ahead of print November 24, 2010]. Ann Rheum Dis 2011; 70:488–494. doi: 10.1136/ard.2010.137778
- Faurschou M, Sorensen IJ, Mellemkjaer L, et al. Malignancies in Wegener’s granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients [published online ahead of print October 15, 2007]. J Rheumatol 2008; 35:100–105.
Biologic agents in the treatment of granulomatosis with polyangiitis
Granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is a vasculitis that affects the renal and respiratory systems. Remission can be induced in most patients with the combination of glucocorticoids and cyclophosphamide. Unfortunately, patients often suffer disease relapses requiring re-treatment and exposure to the cumulative toxicities of repeated cyclophosphamide use. In recent years, improved understanding of the mechanisms of action of cyclophosphamide has led to investigation of treatment strategies that target the role of B cells more specifically in the pathogenesis of the disease.
This article reviews the results of recent studies involving the use of biologic therapy in the treatment of GPA, with a brief examination of historic events that influenced the design of recent trials.
HISTORICAL PERSPECTIVE
The natural history of GPA was characterized in 19581 in a retrospective study showing that 50% of those afflicted died within 6 months, and 80% died by 18 months. Prednisone and cyclophosphamide changed this dismal outcome. The combination markedly improved the status of 91% to 93% of patients,2,3 with most achieving complete remission. Treatment came with a price, however. Almost all patients suffered serious morbidity or side effects, including chronic renal insufficiency (11% requiring dialysis), recurrent infections, hearing loss, infertility, and diabetes. In addition, most patients (99 of 155 in one study) suffered relapse and a significant number (19 of 155) died because of the disease or its treatment.
Investigators’ pursuit of treatment alternatives included foregoing cyclophosphamide in patients who had limited or early systemic GPA and reducing the duration of treatment for patients with severe disease.4 Studies conducted in the late 1990s defined what eventually became standard therapy for GPA: remission induction with glucocorticoids and methotrexate for limited GPA and with glucocorticoids and cyclophosphamide for severe disease. Following remission induction, after 3 to 6 months cyclophosphamide is replaced by azathioprine or methotrexate for remission maintenance. While helpful, these alternatives still fell short of achieving safe, long-term remission.
THERAPY WITH BIOLOGICS
Targeting tumor necrosis factor
The first randomized placebo-controlled trial of a biologic agent in GPA, the Wegener’s Granulomatosis Etanercept Trial (WGET),4 evaluated whether etanercept, a soluble inhibitor of tumor necrosis factor (TNF), would be an effective adjunct to standard therapy. The results showed that etanercept did not confer any beneficial effect and, in fact, if combined with exposure to cyclophosphamide, etanercept increased the risk for solid tumors. Thus, anti-TNF therapy has a limited or no role in the management of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV).
Targeting B cells
The mechanisms of cyclophosphamide effects on disease activity were not clearly understood. In the late 1970s, however, National Institutes of Health investigators found that cyclophosphamide, at the doses administered for GPA, had a profound effect on B-cell function.5 Later investigations showed that disease activity of GPA was clearly related to the frequency of activated B cells detectable in the peripheral blood, while abnormally activated T cells were also detectable in patients in remission.6 These findings suggested that activated B cells might be responsible for disease activity, whereas persistently activated T cells might explain the chronically relapsing nature of the disease.6
B cells are the precursors of short-lived plasma cells, which are thought to be the primary source of autoantibodies, including ANCA. Based on clinical observations as well as in vitro and some animal model experiments, investigators have ascribed pathogenic roles to ANCA. Consequently, targeting the cells that produce these autoantibodies (short-lived plasma cells of B-cell origin) might form the basis of a novel treatment. Why not target cells of the B-cell lineage, thereby eliminating the short-lived plasma cells that would otherwise produce autoantibodies? This might be achieved with rituximab, a monoclonal antibody directed against the CD20 molecule found on pre-B and mature B cells.7 Our group first successfully deployed this strategy in the early 2000s, followed by an open-label pilot study.8–10
The RAVE trial
The Rituximab in ANCA-Associated Vasculitis (RAVE) trial was a multicenter, randomized, placebo-controlled trial that compared rituximab for remission induction and maintenance with standard therapy consisting of cyclophosphamide followed by azathioprine in patients with severe AAV.11 The results of a pilot trial in 200610 set the stage for the RAVE trial, which hypothesized that treatment with rituximab plus glucocorticoids would not be inferior to daily cyclophosphamide plus glucocorticoids. Both would induce remission and permit discontinuation of prednisone after 6 months.
Nine centers enrolled a total of 197 patients with severe GPA or microscopic polyangiitis (MPA), all positive for ANCA, with active disease severe enough to warrant treatment with prednisone and cyclophosphamide. All participants received 1 to 3 g of methyl-prednisolone intravenously followed by prednisone (1 mg/kg per day). The treatment group received rituximab (375 mg/m2 once weekly for 4 weeks) and the control group received standard therapy with cyclophosphamide (2 mg/kg per day) followed by azathioprine (2 mg/kg per day) after 3 to 6 months when remission was achieved.
The primary end point was complete remission, defined as a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of 0 and successful tapering of prednisone by 6 months. Secondary end points included rates of disease flares, cumulative glucocorticoid doses, rates of adverse events, and Medical Outcomes Study 36-item short-form health survey (SF-36, a measure of quality of life) scores. Among patients receiving rituximab, 64% reached the primary end point compared with 53% of patients in the control group. Rituximab was judged not inferior to standard therapy.
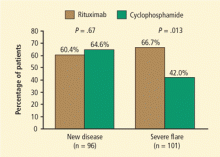
Results were similar for the secondary end point of disease remission while taking less than 10 mg/d of prednisone, with 71% of rituximab patients and 62% of control-group patients achieving remission. Rituximab was also as effective as cyclophosphamide in the treatment of patients with major renal disease or alveolar hemorrhage. Most strikingly, rituximab proved superior to the cyclophosphamide-based regimen for inducing remission in patients who entered the trial with relapsing disease (67% rituximab versus 42% cyclophosphamide) (Figure 1). Those who entered the trial with a new diagnosis did not show the same difference in efficacy.
Rituximab also proved significantly more effective than cyclophosphamide for patients who had proteinase-3 (PR3) ANCA, whereas the efficacy of both agents was equivalent among patients who had myeloperoxidase ANCA. Patients in the cyclophosphamide arm experienced more leukopenia compared with the rituximab arm, but this did not lead to more infections.
In summary, the RAVE trial showed that rituximab matched the efficacy of cyclophosphamide (standard therapy) in inducing remission in patients with severe AAV. The results held true for subsets of patients with major renal disease and those with alveolar hemorrhage. Most strikingly, among patients who entered the trial with a severe relapse, those who received rituximab responded better than those treated with cyclophosphamide. There were no significant differences in flare rates by 6 months and no difference in the rate of severe adverse events. However, participants receiving cyclophosphamide experienced more selected adverse events, particularly leukopenias.
Clinically speaking, rituximab represents the first proven alternative to cyclophosphamide for remission induction in this patient population. The treatment presents the preferred option for patients interested in preserving fertility or who need to be re-treated for a severe disease flare. Based on these data, the US Food and Drug Administration recently extended the labeling of rituximab for treatment of GPA and MPA.
The RITUXVAS trial
The European Vasculitis Study Group (EUVAS) launched another trial comparing the efficacy of rituximab with cyclophosphamide for remission induction.12 The trial design differed from that of the RAVE trial in that investigators did not discontinue prednisone in all patients, followed patients for 12 months, and assessed sustained remission as the primary end point. In this trial, patients in the rituximab arm also received two single intravenous cyclophosphamide infusions, and cyclophosphamide in the control arm was given intravenously. All 44 patients enrolled in the trial and randomized 3:1 to the rituximab versus the cyclophosphamide control arm were ANCA-positive and had active renal disease. The patient population overall was older and had more severe renal disease than the patients enrolled in the RAVE trial. Overall, one course of rituximab achieved the same results as 6 months of intravenous pulse cyclophosphamide followed by oral azathioprine in terms of rate of sustained remission at 12 months, time to relapse, improvement of renal function, and rate of adverse events.
Mayo Clinic cohort study
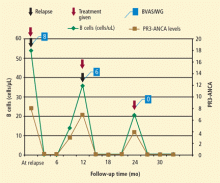
Our group at Mayo Clinic evaluated the safety and effectiveness of rituximab when used repeatedly in order to maintain long-term remission.13 The study involved 53 patients who had a long-term (10 years, on average) diagnosis of refractory AAV. The patients received, on average, four courses of rituximab. All of these patients had GPA and all but one were PR3-ANCA–positive.
In this cohort, rituximab was effective and safe for induction and maintenance of remission in patients with relapsing GPA. The study showed that B-cell depletion effectively maintains remission in these patients, supporting the notion that B cells play an important role in GPA. Because rituximab works by depleting B cells and ANCA, timing of re-treatment can be individualized based on B-cell counts and ANCA levels. Thus, rituximab represents a promising alternative to standard therapy and a means for long-term patient management, particularly for those in whom other agents have failed to achieve or maintain remission in the past.
On a cautionary note, rituximab is an immunosuppressive agent. Risk of infection during treatment seems similar to that associated with carefully monitored cyclophosphamide followed by azathioprine. To avoid complications, physicians should also maintain Pneumocystis prophylaxis for at least the duration of B-cell depletion.
CONCLUSION
Enhanced understanding of the mechanism of action of cyclophosphamide has led to investigation of the role of B cells in the development of AAV and, from there, to the potential for treatment with biologics such as rituximab. Rituximab is equivalent in efficacy to cyclophosphamide for remission induction in AAV. It effectively restores remission and prevents relapse, and it is a better option than cyclophosphamide for PR3-ANCA–associated relapsing vasculitis. Future investigations should further address how to best prevent relapses after B-cell reconstitution.
- Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J 1958; 2:265–270.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Cupps TR, Edgar LC, Fauci AS. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol 1982; 128:2453–2457.
- Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Differential B- and T-cell activation in Wegener’s granulomatosis. J Allergy Clin Immunol 1999; 103:885–894.
- Salama AD, Pusey CD. Drug insight: rituximab in renal disease and transplantation. Nat Clin Pract Nephrol 2006; 2:221–230.
- Specks U, Fervenza FC, McDonald TJ, Hogan MC. Response of Wegener’s granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis Rheum 2001; 44:2836–2840.
- Keogh KA, Wylam ME, Stone JH, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005; 52:262–268.
- Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial [published online ahead of print October 13, 2005]. Am J Respir Crit Care Med 2006; 173:180–187. doi: 10.1164/rccm.200507-1144OC
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
- Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363:211–220.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
Granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is a vasculitis that affects the renal and respiratory systems. Remission can be induced in most patients with the combination of glucocorticoids and cyclophosphamide. Unfortunately, patients often suffer disease relapses requiring re-treatment and exposure to the cumulative toxicities of repeated cyclophosphamide use. In recent years, improved understanding of the mechanisms of action of cyclophosphamide has led to investigation of treatment strategies that target the role of B cells more specifically in the pathogenesis of the disease.
This article reviews the results of recent studies involving the use of biologic therapy in the treatment of GPA, with a brief examination of historic events that influenced the design of recent trials.
HISTORICAL PERSPECTIVE
The natural history of GPA was characterized in 19581 in a retrospective study showing that 50% of those afflicted died within 6 months, and 80% died by 18 months. Prednisone and cyclophosphamide changed this dismal outcome. The combination markedly improved the status of 91% to 93% of patients,2,3 with most achieving complete remission. Treatment came with a price, however. Almost all patients suffered serious morbidity or side effects, including chronic renal insufficiency (11% requiring dialysis), recurrent infections, hearing loss, infertility, and diabetes. In addition, most patients (99 of 155 in one study) suffered relapse and a significant number (19 of 155) died because of the disease or its treatment.
Investigators’ pursuit of treatment alternatives included foregoing cyclophosphamide in patients who had limited or early systemic GPA and reducing the duration of treatment for patients with severe disease.4 Studies conducted in the late 1990s defined what eventually became standard therapy for GPA: remission induction with glucocorticoids and methotrexate for limited GPA and with glucocorticoids and cyclophosphamide for severe disease. Following remission induction, after 3 to 6 months cyclophosphamide is replaced by azathioprine or methotrexate for remission maintenance. While helpful, these alternatives still fell short of achieving safe, long-term remission.
THERAPY WITH BIOLOGICS
Targeting tumor necrosis factor
The first randomized placebo-controlled trial of a biologic agent in GPA, the Wegener’s Granulomatosis Etanercept Trial (WGET),4 evaluated whether etanercept, a soluble inhibitor of tumor necrosis factor (TNF), would be an effective adjunct to standard therapy. The results showed that etanercept did not confer any beneficial effect and, in fact, if combined with exposure to cyclophosphamide, etanercept increased the risk for solid tumors. Thus, anti-TNF therapy has a limited or no role in the management of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV).
Targeting B cells
The mechanisms of cyclophosphamide effects on disease activity were not clearly understood. In the late 1970s, however, National Institutes of Health investigators found that cyclophosphamide, at the doses administered for GPA, had a profound effect on B-cell function.5 Later investigations showed that disease activity of GPA was clearly related to the frequency of activated B cells detectable in the peripheral blood, while abnormally activated T cells were also detectable in patients in remission.6 These findings suggested that activated B cells might be responsible for disease activity, whereas persistently activated T cells might explain the chronically relapsing nature of the disease.6
B cells are the precursors of short-lived plasma cells, which are thought to be the primary source of autoantibodies, including ANCA. Based on clinical observations as well as in vitro and some animal model experiments, investigators have ascribed pathogenic roles to ANCA. Consequently, targeting the cells that produce these autoantibodies (short-lived plasma cells of B-cell origin) might form the basis of a novel treatment. Why not target cells of the B-cell lineage, thereby eliminating the short-lived plasma cells that would otherwise produce autoantibodies? This might be achieved with rituximab, a monoclonal antibody directed against the CD20 molecule found on pre-B and mature B cells.7 Our group first successfully deployed this strategy in the early 2000s, followed by an open-label pilot study.8–10
The RAVE trial
The Rituximab in ANCA-Associated Vasculitis (RAVE) trial was a multicenter, randomized, placebo-controlled trial that compared rituximab for remission induction and maintenance with standard therapy consisting of cyclophosphamide followed by azathioprine in patients with severe AAV.11 The results of a pilot trial in 200610 set the stage for the RAVE trial, which hypothesized that treatment with rituximab plus glucocorticoids would not be inferior to daily cyclophosphamide plus glucocorticoids. Both would induce remission and permit discontinuation of prednisone after 6 months.
Nine centers enrolled a total of 197 patients with severe GPA or microscopic polyangiitis (MPA), all positive for ANCA, with active disease severe enough to warrant treatment with prednisone and cyclophosphamide. All participants received 1 to 3 g of methyl-prednisolone intravenously followed by prednisone (1 mg/kg per day). The treatment group received rituximab (375 mg/m2 once weekly for 4 weeks) and the control group received standard therapy with cyclophosphamide (2 mg/kg per day) followed by azathioprine (2 mg/kg per day) after 3 to 6 months when remission was achieved.
The primary end point was complete remission, defined as a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of 0 and successful tapering of prednisone by 6 months. Secondary end points included rates of disease flares, cumulative glucocorticoid doses, rates of adverse events, and Medical Outcomes Study 36-item short-form health survey (SF-36, a measure of quality of life) scores. Among patients receiving rituximab, 64% reached the primary end point compared with 53% of patients in the control group. Rituximab was judged not inferior to standard therapy.
Results were similar for the secondary end point of disease remission while taking less than 10 mg/d of prednisone, with 71% of rituximab patients and 62% of control-group patients achieving remission. Rituximab was also as effective as cyclophosphamide in the treatment of patients with major renal disease or alveolar hemorrhage. Most strikingly, rituximab proved superior to the cyclophosphamide-based regimen for inducing remission in patients who entered the trial with relapsing disease (67% rituximab versus 42% cyclophosphamide) (Figure 1). Those who entered the trial with a new diagnosis did not show the same difference in efficacy.
Rituximab also proved significantly more effective than cyclophosphamide for patients who had proteinase-3 (PR3) ANCA, whereas the efficacy of both agents was equivalent among patients who had myeloperoxidase ANCA. Patients in the cyclophosphamide arm experienced more leukopenia compared with the rituximab arm, but this did not lead to more infections.
In summary, the RAVE trial showed that rituximab matched the efficacy of cyclophosphamide (standard therapy) in inducing remission in patients with severe AAV. The results held true for subsets of patients with major renal disease and those with alveolar hemorrhage. Most strikingly, among patients who entered the trial with a severe relapse, those who received rituximab responded better than those treated with cyclophosphamide. There were no significant differences in flare rates by 6 months and no difference in the rate of severe adverse events. However, participants receiving cyclophosphamide experienced more selected adverse events, particularly leukopenias.
Clinically speaking, rituximab represents the first proven alternative to cyclophosphamide for remission induction in this patient population. The treatment presents the preferred option for patients interested in preserving fertility or who need to be re-treated for a severe disease flare. Based on these data, the US Food and Drug Administration recently extended the labeling of rituximab for treatment of GPA and MPA.
The RITUXVAS trial
The European Vasculitis Study Group (EUVAS) launched another trial comparing the efficacy of rituximab with cyclophosphamide for remission induction.12 The trial design differed from that of the RAVE trial in that investigators did not discontinue prednisone in all patients, followed patients for 12 months, and assessed sustained remission as the primary end point. In this trial, patients in the rituximab arm also received two single intravenous cyclophosphamide infusions, and cyclophosphamide in the control arm was given intravenously. All 44 patients enrolled in the trial and randomized 3:1 to the rituximab versus the cyclophosphamide control arm were ANCA-positive and had active renal disease. The patient population overall was older and had more severe renal disease than the patients enrolled in the RAVE trial. Overall, one course of rituximab achieved the same results as 6 months of intravenous pulse cyclophosphamide followed by oral azathioprine in terms of rate of sustained remission at 12 months, time to relapse, improvement of renal function, and rate of adverse events.
Mayo Clinic cohort study
Our group at Mayo Clinic evaluated the safety and effectiveness of rituximab when used repeatedly in order to maintain long-term remission.13 The study involved 53 patients who had a long-term (10 years, on average) diagnosis of refractory AAV. The patients received, on average, four courses of rituximab. All of these patients had GPA and all but one were PR3-ANCA–positive.
In this cohort, rituximab was effective and safe for induction and maintenance of remission in patients with relapsing GPA. The study showed that B-cell depletion effectively maintains remission in these patients, supporting the notion that B cells play an important role in GPA. Because rituximab works by depleting B cells and ANCA, timing of re-treatment can be individualized based on B-cell counts and ANCA levels. Thus, rituximab represents a promising alternative to standard therapy and a means for long-term patient management, particularly for those in whom other agents have failed to achieve or maintain remission in the past.
On a cautionary note, rituximab is an immunosuppressive agent. Risk of infection during treatment seems similar to that associated with carefully monitored cyclophosphamide followed by azathioprine. To avoid complications, physicians should also maintain Pneumocystis prophylaxis for at least the duration of B-cell depletion.
CONCLUSION
Enhanced understanding of the mechanism of action of cyclophosphamide has led to investigation of the role of B cells in the development of AAV and, from there, to the potential for treatment with biologics such as rituximab. Rituximab is equivalent in efficacy to cyclophosphamide for remission induction in AAV. It effectively restores remission and prevents relapse, and it is a better option than cyclophosphamide for PR3-ANCA–associated relapsing vasculitis. Future investigations should further address how to best prevent relapses after B-cell reconstitution.
Granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is a vasculitis that affects the renal and respiratory systems. Remission can be induced in most patients with the combination of glucocorticoids and cyclophosphamide. Unfortunately, patients often suffer disease relapses requiring re-treatment and exposure to the cumulative toxicities of repeated cyclophosphamide use. In recent years, improved understanding of the mechanisms of action of cyclophosphamide has led to investigation of treatment strategies that target the role of B cells more specifically in the pathogenesis of the disease.
This article reviews the results of recent studies involving the use of biologic therapy in the treatment of GPA, with a brief examination of historic events that influenced the design of recent trials.
HISTORICAL PERSPECTIVE
The natural history of GPA was characterized in 19581 in a retrospective study showing that 50% of those afflicted died within 6 months, and 80% died by 18 months. Prednisone and cyclophosphamide changed this dismal outcome. The combination markedly improved the status of 91% to 93% of patients,2,3 with most achieving complete remission. Treatment came with a price, however. Almost all patients suffered serious morbidity or side effects, including chronic renal insufficiency (11% requiring dialysis), recurrent infections, hearing loss, infertility, and diabetes. In addition, most patients (99 of 155 in one study) suffered relapse and a significant number (19 of 155) died because of the disease or its treatment.
Investigators’ pursuit of treatment alternatives included foregoing cyclophosphamide in patients who had limited or early systemic GPA and reducing the duration of treatment for patients with severe disease.4 Studies conducted in the late 1990s defined what eventually became standard therapy for GPA: remission induction with glucocorticoids and methotrexate for limited GPA and with glucocorticoids and cyclophosphamide for severe disease. Following remission induction, after 3 to 6 months cyclophosphamide is replaced by azathioprine or methotrexate for remission maintenance. While helpful, these alternatives still fell short of achieving safe, long-term remission.
THERAPY WITH BIOLOGICS
Targeting tumor necrosis factor
The first randomized placebo-controlled trial of a biologic agent in GPA, the Wegener’s Granulomatosis Etanercept Trial (WGET),4 evaluated whether etanercept, a soluble inhibitor of tumor necrosis factor (TNF), would be an effective adjunct to standard therapy. The results showed that etanercept did not confer any beneficial effect and, in fact, if combined with exposure to cyclophosphamide, etanercept increased the risk for solid tumors. Thus, anti-TNF therapy has a limited or no role in the management of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV).
Targeting B cells
The mechanisms of cyclophosphamide effects on disease activity were not clearly understood. In the late 1970s, however, National Institutes of Health investigators found that cyclophosphamide, at the doses administered for GPA, had a profound effect on B-cell function.5 Later investigations showed that disease activity of GPA was clearly related to the frequency of activated B cells detectable in the peripheral blood, while abnormally activated T cells were also detectable in patients in remission.6 These findings suggested that activated B cells might be responsible for disease activity, whereas persistently activated T cells might explain the chronically relapsing nature of the disease.6
B cells are the precursors of short-lived plasma cells, which are thought to be the primary source of autoantibodies, including ANCA. Based on clinical observations as well as in vitro and some animal model experiments, investigators have ascribed pathogenic roles to ANCA. Consequently, targeting the cells that produce these autoantibodies (short-lived plasma cells of B-cell origin) might form the basis of a novel treatment. Why not target cells of the B-cell lineage, thereby eliminating the short-lived plasma cells that would otherwise produce autoantibodies? This might be achieved with rituximab, a monoclonal antibody directed against the CD20 molecule found on pre-B and mature B cells.7 Our group first successfully deployed this strategy in the early 2000s, followed by an open-label pilot study.8–10
The RAVE trial
The Rituximab in ANCA-Associated Vasculitis (RAVE) trial was a multicenter, randomized, placebo-controlled trial that compared rituximab for remission induction and maintenance with standard therapy consisting of cyclophosphamide followed by azathioprine in patients with severe AAV.11 The results of a pilot trial in 200610 set the stage for the RAVE trial, which hypothesized that treatment with rituximab plus glucocorticoids would not be inferior to daily cyclophosphamide plus glucocorticoids. Both would induce remission and permit discontinuation of prednisone after 6 months.
Nine centers enrolled a total of 197 patients with severe GPA or microscopic polyangiitis (MPA), all positive for ANCA, with active disease severe enough to warrant treatment with prednisone and cyclophosphamide. All participants received 1 to 3 g of methyl-prednisolone intravenously followed by prednisone (1 mg/kg per day). The treatment group received rituximab (375 mg/m2 once weekly for 4 weeks) and the control group received standard therapy with cyclophosphamide (2 mg/kg per day) followed by azathioprine (2 mg/kg per day) after 3 to 6 months when remission was achieved.
The primary end point was complete remission, defined as a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of 0 and successful tapering of prednisone by 6 months. Secondary end points included rates of disease flares, cumulative glucocorticoid doses, rates of adverse events, and Medical Outcomes Study 36-item short-form health survey (SF-36, a measure of quality of life) scores. Among patients receiving rituximab, 64% reached the primary end point compared with 53% of patients in the control group. Rituximab was judged not inferior to standard therapy.
Results were similar for the secondary end point of disease remission while taking less than 10 mg/d of prednisone, with 71% of rituximab patients and 62% of control-group patients achieving remission. Rituximab was also as effective as cyclophosphamide in the treatment of patients with major renal disease or alveolar hemorrhage. Most strikingly, rituximab proved superior to the cyclophosphamide-based regimen for inducing remission in patients who entered the trial with relapsing disease (67% rituximab versus 42% cyclophosphamide) (Figure 1). Those who entered the trial with a new diagnosis did not show the same difference in efficacy.
Rituximab also proved significantly more effective than cyclophosphamide for patients who had proteinase-3 (PR3) ANCA, whereas the efficacy of both agents was equivalent among patients who had myeloperoxidase ANCA. Patients in the cyclophosphamide arm experienced more leukopenia compared with the rituximab arm, but this did not lead to more infections.
In summary, the RAVE trial showed that rituximab matched the efficacy of cyclophosphamide (standard therapy) in inducing remission in patients with severe AAV. The results held true for subsets of patients with major renal disease and those with alveolar hemorrhage. Most strikingly, among patients who entered the trial with a severe relapse, those who received rituximab responded better than those treated with cyclophosphamide. There were no significant differences in flare rates by 6 months and no difference in the rate of severe adverse events. However, participants receiving cyclophosphamide experienced more selected adverse events, particularly leukopenias.
Clinically speaking, rituximab represents the first proven alternative to cyclophosphamide for remission induction in this patient population. The treatment presents the preferred option for patients interested in preserving fertility or who need to be re-treated for a severe disease flare. Based on these data, the US Food and Drug Administration recently extended the labeling of rituximab for treatment of GPA and MPA.
The RITUXVAS trial
The European Vasculitis Study Group (EUVAS) launched another trial comparing the efficacy of rituximab with cyclophosphamide for remission induction.12 The trial design differed from that of the RAVE trial in that investigators did not discontinue prednisone in all patients, followed patients for 12 months, and assessed sustained remission as the primary end point. In this trial, patients in the rituximab arm also received two single intravenous cyclophosphamide infusions, and cyclophosphamide in the control arm was given intravenously. All 44 patients enrolled in the trial and randomized 3:1 to the rituximab versus the cyclophosphamide control arm were ANCA-positive and had active renal disease. The patient population overall was older and had more severe renal disease than the patients enrolled in the RAVE trial. Overall, one course of rituximab achieved the same results as 6 months of intravenous pulse cyclophosphamide followed by oral azathioprine in terms of rate of sustained remission at 12 months, time to relapse, improvement of renal function, and rate of adverse events.
Mayo Clinic cohort study
Our group at Mayo Clinic evaluated the safety and effectiveness of rituximab when used repeatedly in order to maintain long-term remission.13 The study involved 53 patients who had a long-term (10 years, on average) diagnosis of refractory AAV. The patients received, on average, four courses of rituximab. All of these patients had GPA and all but one were PR3-ANCA–positive.
In this cohort, rituximab was effective and safe for induction and maintenance of remission in patients with relapsing GPA. The study showed that B-cell depletion effectively maintains remission in these patients, supporting the notion that B cells play an important role in GPA. Because rituximab works by depleting B cells and ANCA, timing of re-treatment can be individualized based on B-cell counts and ANCA levels. Thus, rituximab represents a promising alternative to standard therapy and a means for long-term patient management, particularly for those in whom other agents have failed to achieve or maintain remission in the past.
On a cautionary note, rituximab is an immunosuppressive agent. Risk of infection during treatment seems similar to that associated with carefully monitored cyclophosphamide followed by azathioprine. To avoid complications, physicians should also maintain Pneumocystis prophylaxis for at least the duration of B-cell depletion.
CONCLUSION
Enhanced understanding of the mechanism of action of cyclophosphamide has led to investigation of the role of B cells in the development of AAV and, from there, to the potential for treatment with biologics such as rituximab. Rituximab is equivalent in efficacy to cyclophosphamide for remission induction in AAV. It effectively restores remission and prevents relapse, and it is a better option than cyclophosphamide for PR3-ANCA–associated relapsing vasculitis. Future investigations should further address how to best prevent relapses after B-cell reconstitution.
- Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J 1958; 2:265–270.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Cupps TR, Edgar LC, Fauci AS. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol 1982; 128:2453–2457.
- Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Differential B- and T-cell activation in Wegener’s granulomatosis. J Allergy Clin Immunol 1999; 103:885–894.
- Salama AD, Pusey CD. Drug insight: rituximab in renal disease and transplantation. Nat Clin Pract Nephrol 2006; 2:221–230.
- Specks U, Fervenza FC, McDonald TJ, Hogan MC. Response of Wegener’s granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis Rheum 2001; 44:2836–2840.
- Keogh KA, Wylam ME, Stone JH, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005; 52:262–268.
- Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial [published online ahead of print October 13, 2005]. Am J Respir Crit Care Med 2006; 173:180–187. doi: 10.1164/rccm.200507-1144OC
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
- Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363:211–220.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
- Walton EW. Giant-cell granuloma of the respiratory tract (Wegener’s granulomatosis). Br Med J 1958; 2:265–270.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Cupps TR, Edgar LC, Fauci AS. Suppression of human B lymphocyte function by cyclophosphamide. J Immunol 1982; 128:2453–2457.
- Popa ER, Stegeman CA, Bos NA, Kallenberg CG, Tervaert JW. Differential B- and T-cell activation in Wegener’s granulomatosis. J Allergy Clin Immunol 1999; 103:885–894.
- Salama AD, Pusey CD. Drug insight: rituximab in renal disease and transplantation. Nat Clin Pract Nephrol 2006; 2:221–230.
- Specks U, Fervenza FC, McDonald TJ, Hogan MC. Response of Wegener’s granulomatosis to anti-CD20 chimeric monoclonal antibody therapy. Arthritis Rheum 2001; 44:2836–2840.
- Keogh KA, Wylam ME, Stone JH, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum 2005; 52:262–268.
- Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener’s granulomatosis: report of a prospective, open-label pilot trial [published online ahead of print October 13, 2005]. Am J Respir Crit Care Med 2006; 173:180–187. doi: 10.1164/rccm.200507-1144OC
- Stone JH, Merkel PA, Spiera R, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
- Jones RB, Tervaert JW, Hauser T, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363:211–220.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
History of vasculitis: The life and work of Adolf Kussmaul
Adolf Kussmaul, who lived and practiced medicine in the 19th century, is known for his clinical skills, his scientific acumen, his gift for teaching, and his mastery of diverse areas of knowledge. He was a contemporary of such luminaries as pathologist Rudolf Virchow. In the rheumatology community, he is best known for describing the first case of polyarteritis nodosa (PAN).
FIRST CASE
In the first volume of the first edition of German Archive for Clinical Medicine, Kussmaul, along with his pathology associate Rudolf Maier, reported the case of Carl Seufarth, a 27-year-old tailor’s journeyman. Seufarth arrived at the University of Freiburg internal medicine clinic on May 4, 1865, at 10 am. Kussmaul was at that time head of medicine at Freiburg. Seufarth’s journeyman’s log recorded that he had been healthy when he left his hometown of Gernsbach in southwest Germany on January 30, 1865. His entry indicated that he was 5 feet 2 inches tall, was of strong build, and had healthy facial color.
Kussmaul’s 1866 description of Seufarth upon his arrival at the clinic is among the most memorable passages in medical literature:
Despite his frail appearance, Seufarth was able to walk into the hospital and climb the two flights of stairs to the internal medicine clinic without assistance. He had had a cold followed by a productive cough in the autumn of 1864, but felt well afterward. In the 8 days prior to admission to the University of Freiburg, he developed diarrhea and frequent chills with fevers and sweats. He had felt unwell for the preceding 2 to 3 weeks, during which he was hospitalized briefly for scabies, wandered from one place to another, and eventually arrived in Freiburg. Freiburg police imprisoned him on May 2 for begging and brought him to the internal medicine department on May 4 because of weakness.
Over the next several days, Seufarth experienced rapidly developing weakness, numbness in the left hand and eventually other extremities, and paralysis of the arm and hand muscles. He was closely monitored at the clinic with his temperature recorded every morning and evening. On the 28th day of hospitalization, pea-sized nodules were discovered in the subcutaneous skin of the abdomen and chest. By June 2, the patient was in a state of extreme weakness. He died on June 3, 1865, at 2 am.
This description is what we understand today as typical of vascular involvement in PAN. Maier also examined the tissue microscopically. In his report, he described the aneurysmal dilatations, narrowings, and inflammation occurring at the branches of the arteries. His sketch of involved organs depicted neutrophilic infiltration into the walls of the vessels.
When consulted by Kussmaul for a second opinion, pathologist Rudolf Virchow said he had not observed patients with disease similar to that of Seufarth. In his archives, however, he later found a specimen of an aneurysm in a branch of the superior mesenteric artery.
Kussmaul and Maier published the case under the title “On a previously undescribed peculiar arterial disease (periarteritis nodosa) accompanied by Bright’s disease and rapidly progressive general muscle weakness.” “Periarteritis nodosa” was later termed “polyarteritis nodosa” to better describe the inflammation of multiple medium-and small-vessel arteries rather than inflammation around the arteries as Maier had initially envisioned it.
BIOGRAPHICAL NOTES
The son of a German army surgeon, Kussmaul was born in 1822 in Graben near Karlsruhe, a small town in the Black Forest of southwestern Germany. Kussmaul began his medical studies at the University of Heidelberg in 1840. That same year, he constructed the first ophthalmoscope. The device did not function as intended because he had not discovered the light orientation needed to prevent the iris from contracting. But, as he later said, “It was the best ophthalmoscope of the time. Its only drawback was that it did not work.”
After graduating from the University of Heidelberg, Kussmaul went into private practice in Wiesloch. He returned to the University a year later, after having developed pericarditis, where he served as an assistant in 1846 and 1847 and engaged not only in medicine and medical discovery, but also poetry, publishing, and social movements. He founded a magazine that published short stories, poetry, and spoofs on the government; and he coined the term “Biedermeier,” which refers to a furniture style as well as a German social movement.
With plans to further his medical education, Kussmaul and his friend, Edward Bronner, traveled to Vienna and Prague in 1847 and 1848. In Vienna, they met the anatomic pathologist Karl Rokitansky. Although the young men hoped to study with the renowned scientist, they were soon dissuaded by Rokitansky’s clear dislike of working with students. He also had little use for patients, holding that the best patient was a dead patient because of all that one could learn by doing an autopsy.
Kussmaul and Bronner returned to Germany, Kussmaul having been called to serve as a physician in the Baden battalion during the German-Danish war. There, he contributed significantly to the health of the army by insisting that wounded soldiers not be bled—a common treatment at that time that actually accelerated the deaths of many soldiers in the field.
ACADEMICIAN, SCIENTIST, AND CLINICIAN
Shortly after his 1850 marriage to Luise Amanda Wolf, the daughter of a famous surgeon, Kussmaul developed an ascending polyradiculopathy, which at one time was called Landry-Kussmaul paralysis and later Guillain-Barré syndrome. This condition, along with his previous history of pericarditis, stimulated Kussmaul’s pursuit of medical knowledge for better understanding of his own afflictions as well as medicine in general.
He completed his doctoral dissertation at the University of Würzburg in 1853. There, he worked with pathology professor Virchow, who is known as the father of the theory of coagulation and the cellular theory of disease. It is perhaps less well known that in a treatise on histopathology in 1847, Virchow proposed that vasculitis actually might occur in blood vessels and originate in the adventitia. This profound insight was lost at the time because of inadequate understanding of vasculitic disorders.
Returning to the University of Heidelberg in 1854, Kussmaul earned the rank of assistant professor of medicine and, by 1857, professor of medicine. Two years later, he relocated to the University of Erlangen as a professor of medicine. His inaugural lecture at the University of Erlangen was the presentation of two cases of Landry-Kussmaul paralysis. Kussmaul’s research at Erlangen focused on differentiating the symptoms of mercurialism from syphilis (mercury was used for the treatment of syphilis).
Kussmaul was then called to the University of Freiburg in 1863 as head of the department of medicine. Among Kussmaul’s achievements at the University of Freiburg in the 1860s were the description of paradoxical pulse in obstructive pericarditis that we know as the Kussmaul pulse, and the description of the breathing characteristic of diabetic acidotic coma that we know as Kussmaul respiration. There he also performed the first gastroscopy on a sword-swallowing circus performer using a derivation of a laryngoscope; unfortunately, again his invention was thwarted by lack of an adequate light source. He also studied peptic ulcer disease and described a technique for dilating a stenosed peptic ulcer lesion with a balloon device. He later worked with Czerny and Billroth to develop the surgical procedure used routinely for nearly 100 years to relieve peptic ulcer disease prior to the introduction of drugs such as ranitidine.
RHEUMATOLOGY “WORMS”
Kussmaul and Maier initially published the Seufarth case in abstract form and called it “human worm aneurysm,” because they thought that the vascular pea-shaped or pea-sized structures represented worm and nematode infiltration. When they examined the specimens microscopically, however, they realized that they were viewing an inflammatory disease process.
Ironically, vessel disease of the PAN type was described in 1852 by Rokitansky. Rokitansky reported finding mesenteric aneurysms in the branch points of the arteries; however, because he eschewed technology, he did not examine the specimen microscopically and failed to recognize the inflammatory process. His student, Hans Eppinger, revisited the specimen some 30 years later and, under microscopic examination, clearly defined the aneurysmal dilatations and inflammatory infiltrates (Figure 2).
A final rheumatology worm episode occurred late in Kussmaul’s career in Strasburg, where he had become head of the department of medicine in 1878. Kussmaul asked his assistant and biographer, Albert Kahn, to administer naphthalene to a patient to eradicate intestinal worms. Strangely, the worms survived, but the fever resolved. Due to a pharmacy error, acetanilide, an anti-inflammatory marketed by Bayer, had been dispensed rather than naphthalene. Bayer subsequently marketed the product as Antifebrin.
REMEMBERED AND COMMEMORATED
Kussmaul was a much-loved teacher and a well-respected physician. After he retired in 1888, he returned to Heidelberg as emeritus professor of medicine. He died in 1902 at age 80. His desire to understand disease, his clinical observations, his teaching abilities, and his ability to apply medical technology to the bedside all played roles in his contributions to clinical medicine. One of several Kussmaul commemoration sites is a lunette in Lenox Hill Hospital, New York, New York, where his portrait plaque is displayed alongside those of Ismar Boas and Carl Anton Ewald, the founders of modern gastroenterology.
Adolf Kussmaul, who lived and practiced medicine in the 19th century, is known for his clinical skills, his scientific acumen, his gift for teaching, and his mastery of diverse areas of knowledge. He was a contemporary of such luminaries as pathologist Rudolf Virchow. In the rheumatology community, he is best known for describing the first case of polyarteritis nodosa (PAN).
FIRST CASE
In the first volume of the first edition of German Archive for Clinical Medicine, Kussmaul, along with his pathology associate Rudolf Maier, reported the case of Carl Seufarth, a 27-year-old tailor’s journeyman. Seufarth arrived at the University of Freiburg internal medicine clinic on May 4, 1865, at 10 am. Kussmaul was at that time head of medicine at Freiburg. Seufarth’s journeyman’s log recorded that he had been healthy when he left his hometown of Gernsbach in southwest Germany on January 30, 1865. His entry indicated that he was 5 feet 2 inches tall, was of strong build, and had healthy facial color.
Kussmaul’s 1866 description of Seufarth upon his arrival at the clinic is among the most memorable passages in medical literature:
Despite his frail appearance, Seufarth was able to walk into the hospital and climb the two flights of stairs to the internal medicine clinic without assistance. He had had a cold followed by a productive cough in the autumn of 1864, but felt well afterward. In the 8 days prior to admission to the University of Freiburg, he developed diarrhea and frequent chills with fevers and sweats. He had felt unwell for the preceding 2 to 3 weeks, during which he was hospitalized briefly for scabies, wandered from one place to another, and eventually arrived in Freiburg. Freiburg police imprisoned him on May 2 for begging and brought him to the internal medicine department on May 4 because of weakness.
Over the next several days, Seufarth experienced rapidly developing weakness, numbness in the left hand and eventually other extremities, and paralysis of the arm and hand muscles. He was closely monitored at the clinic with his temperature recorded every morning and evening. On the 28th day of hospitalization, pea-sized nodules were discovered in the subcutaneous skin of the abdomen and chest. By June 2, the patient was in a state of extreme weakness. He died on June 3, 1865, at 2 am.
This description is what we understand today as typical of vascular involvement in PAN. Maier also examined the tissue microscopically. In his report, he described the aneurysmal dilatations, narrowings, and inflammation occurring at the branches of the arteries. His sketch of involved organs depicted neutrophilic infiltration into the walls of the vessels.
When consulted by Kussmaul for a second opinion, pathologist Rudolf Virchow said he had not observed patients with disease similar to that of Seufarth. In his archives, however, he later found a specimen of an aneurysm in a branch of the superior mesenteric artery.
Kussmaul and Maier published the case under the title “On a previously undescribed peculiar arterial disease (periarteritis nodosa) accompanied by Bright’s disease and rapidly progressive general muscle weakness.” “Periarteritis nodosa” was later termed “polyarteritis nodosa” to better describe the inflammation of multiple medium-and small-vessel arteries rather than inflammation around the arteries as Maier had initially envisioned it.
BIOGRAPHICAL NOTES
The son of a German army surgeon, Kussmaul was born in 1822 in Graben near Karlsruhe, a small town in the Black Forest of southwestern Germany. Kussmaul began his medical studies at the University of Heidelberg in 1840. That same year, he constructed the first ophthalmoscope. The device did not function as intended because he had not discovered the light orientation needed to prevent the iris from contracting. But, as he later said, “It was the best ophthalmoscope of the time. Its only drawback was that it did not work.”
After graduating from the University of Heidelberg, Kussmaul went into private practice in Wiesloch. He returned to the University a year later, after having developed pericarditis, where he served as an assistant in 1846 and 1847 and engaged not only in medicine and medical discovery, but also poetry, publishing, and social movements. He founded a magazine that published short stories, poetry, and spoofs on the government; and he coined the term “Biedermeier,” which refers to a furniture style as well as a German social movement.
With plans to further his medical education, Kussmaul and his friend, Edward Bronner, traveled to Vienna and Prague in 1847 and 1848. In Vienna, they met the anatomic pathologist Karl Rokitansky. Although the young men hoped to study with the renowned scientist, they were soon dissuaded by Rokitansky’s clear dislike of working with students. He also had little use for patients, holding that the best patient was a dead patient because of all that one could learn by doing an autopsy.
Kussmaul and Bronner returned to Germany, Kussmaul having been called to serve as a physician in the Baden battalion during the German-Danish war. There, he contributed significantly to the health of the army by insisting that wounded soldiers not be bled—a common treatment at that time that actually accelerated the deaths of many soldiers in the field.
ACADEMICIAN, SCIENTIST, AND CLINICIAN
Shortly after his 1850 marriage to Luise Amanda Wolf, the daughter of a famous surgeon, Kussmaul developed an ascending polyradiculopathy, which at one time was called Landry-Kussmaul paralysis and later Guillain-Barré syndrome. This condition, along with his previous history of pericarditis, stimulated Kussmaul’s pursuit of medical knowledge for better understanding of his own afflictions as well as medicine in general.
He completed his doctoral dissertation at the University of Würzburg in 1853. There, he worked with pathology professor Virchow, who is known as the father of the theory of coagulation and the cellular theory of disease. It is perhaps less well known that in a treatise on histopathology in 1847, Virchow proposed that vasculitis actually might occur in blood vessels and originate in the adventitia. This profound insight was lost at the time because of inadequate understanding of vasculitic disorders.
Returning to the University of Heidelberg in 1854, Kussmaul earned the rank of assistant professor of medicine and, by 1857, professor of medicine. Two years later, he relocated to the University of Erlangen as a professor of medicine. His inaugural lecture at the University of Erlangen was the presentation of two cases of Landry-Kussmaul paralysis. Kussmaul’s research at Erlangen focused on differentiating the symptoms of mercurialism from syphilis (mercury was used for the treatment of syphilis).
Kussmaul was then called to the University of Freiburg in 1863 as head of the department of medicine. Among Kussmaul’s achievements at the University of Freiburg in the 1860s were the description of paradoxical pulse in obstructive pericarditis that we know as the Kussmaul pulse, and the description of the breathing characteristic of diabetic acidotic coma that we know as Kussmaul respiration. There he also performed the first gastroscopy on a sword-swallowing circus performer using a derivation of a laryngoscope; unfortunately, again his invention was thwarted by lack of an adequate light source. He also studied peptic ulcer disease and described a technique for dilating a stenosed peptic ulcer lesion with a balloon device. He later worked with Czerny and Billroth to develop the surgical procedure used routinely for nearly 100 years to relieve peptic ulcer disease prior to the introduction of drugs such as ranitidine.
RHEUMATOLOGY “WORMS”
Kussmaul and Maier initially published the Seufarth case in abstract form and called it “human worm aneurysm,” because they thought that the vascular pea-shaped or pea-sized structures represented worm and nematode infiltration. When they examined the specimens microscopically, however, they realized that they were viewing an inflammatory disease process.
Ironically, vessel disease of the PAN type was described in 1852 by Rokitansky. Rokitansky reported finding mesenteric aneurysms in the branch points of the arteries; however, because he eschewed technology, he did not examine the specimen microscopically and failed to recognize the inflammatory process. His student, Hans Eppinger, revisited the specimen some 30 years later and, under microscopic examination, clearly defined the aneurysmal dilatations and inflammatory infiltrates (Figure 2).
A final rheumatology worm episode occurred late in Kussmaul’s career in Strasburg, where he had become head of the department of medicine in 1878. Kussmaul asked his assistant and biographer, Albert Kahn, to administer naphthalene to a patient to eradicate intestinal worms. Strangely, the worms survived, but the fever resolved. Due to a pharmacy error, acetanilide, an anti-inflammatory marketed by Bayer, had been dispensed rather than naphthalene. Bayer subsequently marketed the product as Antifebrin.
REMEMBERED AND COMMEMORATED
Kussmaul was a much-loved teacher and a well-respected physician. After he retired in 1888, he returned to Heidelberg as emeritus professor of medicine. He died in 1902 at age 80. His desire to understand disease, his clinical observations, his teaching abilities, and his ability to apply medical technology to the bedside all played roles in his contributions to clinical medicine. One of several Kussmaul commemoration sites is a lunette in Lenox Hill Hospital, New York, New York, where his portrait plaque is displayed alongside those of Ismar Boas and Carl Anton Ewald, the founders of modern gastroenterology.
Adolf Kussmaul, who lived and practiced medicine in the 19th century, is known for his clinical skills, his scientific acumen, his gift for teaching, and his mastery of diverse areas of knowledge. He was a contemporary of such luminaries as pathologist Rudolf Virchow. In the rheumatology community, he is best known for describing the first case of polyarteritis nodosa (PAN).
FIRST CASE
In the first volume of the first edition of German Archive for Clinical Medicine, Kussmaul, along with his pathology associate Rudolf Maier, reported the case of Carl Seufarth, a 27-year-old tailor’s journeyman. Seufarth arrived at the University of Freiburg internal medicine clinic on May 4, 1865, at 10 am. Kussmaul was at that time head of medicine at Freiburg. Seufarth’s journeyman’s log recorded that he had been healthy when he left his hometown of Gernsbach in southwest Germany on January 30, 1865. His entry indicated that he was 5 feet 2 inches tall, was of strong build, and had healthy facial color.
Kussmaul’s 1866 description of Seufarth upon his arrival at the clinic is among the most memorable passages in medical literature:
Despite his frail appearance, Seufarth was able to walk into the hospital and climb the two flights of stairs to the internal medicine clinic without assistance. He had had a cold followed by a productive cough in the autumn of 1864, but felt well afterward. In the 8 days prior to admission to the University of Freiburg, he developed diarrhea and frequent chills with fevers and sweats. He had felt unwell for the preceding 2 to 3 weeks, during which he was hospitalized briefly for scabies, wandered from one place to another, and eventually arrived in Freiburg. Freiburg police imprisoned him on May 2 for begging and brought him to the internal medicine department on May 4 because of weakness.
Over the next several days, Seufarth experienced rapidly developing weakness, numbness in the left hand and eventually other extremities, and paralysis of the arm and hand muscles. He was closely monitored at the clinic with his temperature recorded every morning and evening. On the 28th day of hospitalization, pea-sized nodules were discovered in the subcutaneous skin of the abdomen and chest. By June 2, the patient was in a state of extreme weakness. He died on June 3, 1865, at 2 am.
This description is what we understand today as typical of vascular involvement in PAN. Maier also examined the tissue microscopically. In his report, he described the aneurysmal dilatations, narrowings, and inflammation occurring at the branches of the arteries. His sketch of involved organs depicted neutrophilic infiltration into the walls of the vessels.
When consulted by Kussmaul for a second opinion, pathologist Rudolf Virchow said he had not observed patients with disease similar to that of Seufarth. In his archives, however, he later found a specimen of an aneurysm in a branch of the superior mesenteric artery.
Kussmaul and Maier published the case under the title “On a previously undescribed peculiar arterial disease (periarteritis nodosa) accompanied by Bright’s disease and rapidly progressive general muscle weakness.” “Periarteritis nodosa” was later termed “polyarteritis nodosa” to better describe the inflammation of multiple medium-and small-vessel arteries rather than inflammation around the arteries as Maier had initially envisioned it.
BIOGRAPHICAL NOTES
The son of a German army surgeon, Kussmaul was born in 1822 in Graben near Karlsruhe, a small town in the Black Forest of southwestern Germany. Kussmaul began his medical studies at the University of Heidelberg in 1840. That same year, he constructed the first ophthalmoscope. The device did not function as intended because he had not discovered the light orientation needed to prevent the iris from contracting. But, as he later said, “It was the best ophthalmoscope of the time. Its only drawback was that it did not work.”
After graduating from the University of Heidelberg, Kussmaul went into private practice in Wiesloch. He returned to the University a year later, after having developed pericarditis, where he served as an assistant in 1846 and 1847 and engaged not only in medicine and medical discovery, but also poetry, publishing, and social movements. He founded a magazine that published short stories, poetry, and spoofs on the government; and he coined the term “Biedermeier,” which refers to a furniture style as well as a German social movement.
With plans to further his medical education, Kussmaul and his friend, Edward Bronner, traveled to Vienna and Prague in 1847 and 1848. In Vienna, they met the anatomic pathologist Karl Rokitansky. Although the young men hoped to study with the renowned scientist, they were soon dissuaded by Rokitansky’s clear dislike of working with students. He also had little use for patients, holding that the best patient was a dead patient because of all that one could learn by doing an autopsy.
Kussmaul and Bronner returned to Germany, Kussmaul having been called to serve as a physician in the Baden battalion during the German-Danish war. There, he contributed significantly to the health of the army by insisting that wounded soldiers not be bled—a common treatment at that time that actually accelerated the deaths of many soldiers in the field.
ACADEMICIAN, SCIENTIST, AND CLINICIAN
Shortly after his 1850 marriage to Luise Amanda Wolf, the daughter of a famous surgeon, Kussmaul developed an ascending polyradiculopathy, which at one time was called Landry-Kussmaul paralysis and later Guillain-Barré syndrome. This condition, along with his previous history of pericarditis, stimulated Kussmaul’s pursuit of medical knowledge for better understanding of his own afflictions as well as medicine in general.
He completed his doctoral dissertation at the University of Würzburg in 1853. There, he worked with pathology professor Virchow, who is known as the father of the theory of coagulation and the cellular theory of disease. It is perhaps less well known that in a treatise on histopathology in 1847, Virchow proposed that vasculitis actually might occur in blood vessels and originate in the adventitia. This profound insight was lost at the time because of inadequate understanding of vasculitic disorders.
Returning to the University of Heidelberg in 1854, Kussmaul earned the rank of assistant professor of medicine and, by 1857, professor of medicine. Two years later, he relocated to the University of Erlangen as a professor of medicine. His inaugural lecture at the University of Erlangen was the presentation of two cases of Landry-Kussmaul paralysis. Kussmaul’s research at Erlangen focused on differentiating the symptoms of mercurialism from syphilis (mercury was used for the treatment of syphilis).
Kussmaul was then called to the University of Freiburg in 1863 as head of the department of medicine. Among Kussmaul’s achievements at the University of Freiburg in the 1860s were the description of paradoxical pulse in obstructive pericarditis that we know as the Kussmaul pulse, and the description of the breathing characteristic of diabetic acidotic coma that we know as Kussmaul respiration. There he also performed the first gastroscopy on a sword-swallowing circus performer using a derivation of a laryngoscope; unfortunately, again his invention was thwarted by lack of an adequate light source. He also studied peptic ulcer disease and described a technique for dilating a stenosed peptic ulcer lesion with a balloon device. He later worked with Czerny and Billroth to develop the surgical procedure used routinely for nearly 100 years to relieve peptic ulcer disease prior to the introduction of drugs such as ranitidine.
RHEUMATOLOGY “WORMS”
Kussmaul and Maier initially published the Seufarth case in abstract form and called it “human worm aneurysm,” because they thought that the vascular pea-shaped or pea-sized structures represented worm and nematode infiltration. When they examined the specimens microscopically, however, they realized that they were viewing an inflammatory disease process.
Ironically, vessel disease of the PAN type was described in 1852 by Rokitansky. Rokitansky reported finding mesenteric aneurysms in the branch points of the arteries; however, because he eschewed technology, he did not examine the specimen microscopically and failed to recognize the inflammatory process. His student, Hans Eppinger, revisited the specimen some 30 years later and, under microscopic examination, clearly defined the aneurysmal dilatations and inflammatory infiltrates (Figure 2).
A final rheumatology worm episode occurred late in Kussmaul’s career in Strasburg, where he had become head of the department of medicine in 1878. Kussmaul asked his assistant and biographer, Albert Kahn, to administer naphthalene to a patient to eradicate intestinal worms. Strangely, the worms survived, but the fever resolved. Due to a pharmacy error, acetanilide, an anti-inflammatory marketed by Bayer, had been dispensed rather than naphthalene. Bayer subsequently marketed the product as Antifebrin.
REMEMBERED AND COMMEMORATED
Kussmaul was a much-loved teacher and a well-respected physician. After he retired in 1888, he returned to Heidelberg as emeritus professor of medicine. He died in 1902 at age 80. His desire to understand disease, his clinical observations, his teaching abilities, and his ability to apply medical technology to the bedside all played roles in his contributions to clinical medicine. One of several Kussmaul commemoration sites is a lunette in Lenox Hill Hospital, New York, New York, where his portrait plaque is displayed alongside those of Ismar Boas and Carl Anton Ewald, the founders of modern gastroenterology.
Analysis Details the GI Disease Burden in U.S.
Clostridium difficile contributes mightily to the overall burden of gastrointestinal disease in the United States and was associated with a 237% increase in hospitalizations in the last decade.
Researchers who examined the latest data on the nationwide toll of GI and liver disease also found a 314% rise in hospitalizations related to morbid obesity and a continuing national health burden exacted by reflux symptoms, Barrett’s esophagus, and colorectal cancer.
Video from the American Gastroenterological Association (http://www.youtube.com/amergastroassn)
"We compiled the most recently available statistics on GI symptoms, quality of life, outpatient diagnoses, hospitalizations, costs, mortality, and endoscopic utilization from a variety of publicly and privately held databases," Dr. Anne F. Peery of the University of North Carolina, Chapel Hill, and her colleagues reported in the November issue of Gastroenterology (doi:10.1053/j.gastro.2012.08.002).
"Payers, policy makers, clinicians, and others interested in resource utilization may use these statistics to better understand evolving disease trends, and the best way to meet the challenge of these diseases."
The findings are based on data for 2009, the most recent year for which complete information was available, from the National Ambulatory Medical Care Survey, sponsored by the U.S. Centers for Disease Control and Prevention; the United States National Health and Wellness Survey, sponsored by the private company Kantar Health; the Nationwide Inpatient Sample, sponsored by the Agency for Healthcare Research and Quality; the Surveillance, Epidemiology, and End Results database of the National Cancer Institute; the National Vital Statistics System, sponsored by the National Center for Health Statistics and the CDC; and the Thomson Reuters MarketScan’s databases of commercial, Medicare, and Medicaid records.
Among the findings:
• C. difficile hospitalizations have increased 237% since 2000 and were associated with 4% in-hospital mortality. Now the ninth leading GI cause of mortality, with an absolute increase of 230% in the number of C. difficile–related deaths since 2002, the infection also markedly impairs quality of life and the capacity for work and other activities.
• Hospitalizations related to obesity remained relatively stable since 2000, but those associated with morbid obesity rose by 314%, and many were likely caused by the marked increase in bariatric surgery.
• Gastroesophageal reflux remains the most common GI-associated diagnosis in primary care, accounting for 9 million outpatient visits in 2009, and the most common GI-associated discharge diagnosis, with 4.4 million such diagnoses in 2009. Obesity was associated with 1.7 million discharge diagnoses and constipation with 1 million.
• Barrett’s esophagus accounted for almost half a million outpatient visits in 2009, when an estimated 3.3 million Americans had this diagnosis. Given that endoscopic surveillance is recommended every 3-5 years, Barrett’s contributes substantially to resource utilization.
• Colorectal cancer, with an estimated 147,000 patients diagnosed in 2008, accounts for more than half of all GI cancer diagnoses and continues to be the primary cause of GI-associated mortality. Pancreatic and hepatobiliary cancers are the next most frequently diagnosed GI cancers.
• Of the approximately 2.5 million deaths in the United States in 2009, 10% were attributed to an underlying GI cause. Chronic liver disease and cirrhosis are the 12th leading causes of death in the country.
• The total outpatient cost for GI endoscopy in 2009 was estimated to be $32.4 billion, which is higher than previously published estimates. An estimated 6.9 million upper endoscopies, 11.5 million lower endoscopies, and 228,000 biliary endoscopies were performed in the United States in 2009.
• Chronic liver disease and viral hepatitis were associated with 6% mortality and cost an estimated $1.8 billion per year in inpatient cost.
• Hospitalizations for nonalcoholic fatty liver disease increased 97% since 2000.
This study was supported in part by the National Institutes of Health. No financial conflicts of interest were reported.
Digestive (GI and liver) diseases constitute a substantial and growing burden in the United States. As detailed in the report by Dr. Peery and colleagues, there were over 46 million outpatient encounters associated with the top 20 digestive disease diagnoses in 2009, with approximately 10% of deaths nationwide with an underlying digestive disease cause. The observed increased prevalence of hospitalizations for many diagnoses (e.g., a 14% increase with principal discharge diagnosis of chronic liver disease with viral hepatitis) and procedures (e.g., a 17% increase in lower GI endoscopies among commercially insured patients) between 2000 and 2009 is expected given population growth and aging. In addition, dramatic increases in hospitalizations associated with C. difficile and morbid obesity were also noted.
The report provides crucial information for diverse constituencies, including healthcare planners, clinicians and researchers. However, as acknowledged by the authors, there are some important caveats with respect to coverage or quality for some data sources to bear in mind when interpreting these results. This is particularly relevant for non-alcoholic fatty liver disease, which is likely underestimated because of well-known problems in diagnostic code specificity and use.
Several factors suggest that the prevalence and costs of digestive diseases will increase substantially during the next decade. These include: an aging population with the number of people aged 65 years or older projected to be greater than 54 million by 2020;the estimated tens of millions of individuals with newly available healthcare coverage as of 2014 as part of the Affordable Care Act; continued increases in obesity rates; and the recent CDC recommendation that all baby-boomers be screened for hepatitis C. This report will facilitate timely planning and also serve as benchmark to help measure the impact of these forces on the scope and burden of digestive diseases and their clinical management.
DONNA L. WHITE, PH.D., MPH, is an investigator in the Clinical Epidemiology and Outcomes Program in the Houston VA Health Services Research and Development Center of Excellence at the Michael E. DeBakey VA Medical Center, Houston. She also is an assistant professor in the department of medicine at Baylor College of Medicine, Houston.
Digestive (GI and liver) diseases constitute a substantial and growing burden in the United States. As detailed in the report by Dr. Peery and colleagues, there were over 46 million outpatient encounters associated with the top 20 digestive disease diagnoses in 2009, with approximately 10% of deaths nationwide with an underlying digestive disease cause. The observed increased prevalence of hospitalizations for many diagnoses (e.g., a 14% increase with principal discharge diagnosis of chronic liver disease with viral hepatitis) and procedures (e.g., a 17% increase in lower GI endoscopies among commercially insured patients) between 2000 and 2009 is expected given population growth and aging. In addition, dramatic increases in hospitalizations associated with C. difficile and morbid obesity were also noted.
The report provides crucial information for diverse constituencies, including healthcare planners, clinicians and researchers. However, as acknowledged by the authors, there are some important caveats with respect to coverage or quality for some data sources to bear in mind when interpreting these results. This is particularly relevant for non-alcoholic fatty liver disease, which is likely underestimated because of well-known problems in diagnostic code specificity and use.
Several factors suggest that the prevalence and costs of digestive diseases will increase substantially during the next decade. These include: an aging population with the number of people aged 65 years or older projected to be greater than 54 million by 2020;the estimated tens of millions of individuals with newly available healthcare coverage as of 2014 as part of the Affordable Care Act; continued increases in obesity rates; and the recent CDC recommendation that all baby-boomers be screened for hepatitis C. This report will facilitate timely planning and also serve as benchmark to help measure the impact of these forces on the scope and burden of digestive diseases and their clinical management.
DONNA L. WHITE, PH.D., MPH, is an investigator in the Clinical Epidemiology and Outcomes Program in the Houston VA Health Services Research and Development Center of Excellence at the Michael E. DeBakey VA Medical Center, Houston. She also is an assistant professor in the department of medicine at Baylor College of Medicine, Houston.
Digestive (GI and liver) diseases constitute a substantial and growing burden in the United States. As detailed in the report by Dr. Peery and colleagues, there were over 46 million outpatient encounters associated with the top 20 digestive disease diagnoses in 2009, with approximately 10% of deaths nationwide with an underlying digestive disease cause. The observed increased prevalence of hospitalizations for many diagnoses (e.g., a 14% increase with principal discharge diagnosis of chronic liver disease with viral hepatitis) and procedures (e.g., a 17% increase in lower GI endoscopies among commercially insured patients) between 2000 and 2009 is expected given population growth and aging. In addition, dramatic increases in hospitalizations associated with C. difficile and morbid obesity were also noted.
The report provides crucial information for diverse constituencies, including healthcare planners, clinicians and researchers. However, as acknowledged by the authors, there are some important caveats with respect to coverage or quality for some data sources to bear in mind when interpreting these results. This is particularly relevant for non-alcoholic fatty liver disease, which is likely underestimated because of well-known problems in diagnostic code specificity and use.
Several factors suggest that the prevalence and costs of digestive diseases will increase substantially during the next decade. These include: an aging population with the number of people aged 65 years or older projected to be greater than 54 million by 2020;the estimated tens of millions of individuals with newly available healthcare coverage as of 2014 as part of the Affordable Care Act; continued increases in obesity rates; and the recent CDC recommendation that all baby-boomers be screened for hepatitis C. This report will facilitate timely planning and also serve as benchmark to help measure the impact of these forces on the scope and burden of digestive diseases and their clinical management.
DONNA L. WHITE, PH.D., MPH, is an investigator in the Clinical Epidemiology and Outcomes Program in the Houston VA Health Services Research and Development Center of Excellence at the Michael E. DeBakey VA Medical Center, Houston. She also is an assistant professor in the department of medicine at Baylor College of Medicine, Houston.
Clostridium difficile contributes mightily to the overall burden of gastrointestinal disease in the United States and was associated with a 237% increase in hospitalizations in the last decade.
Researchers who examined the latest data on the nationwide toll of GI and liver disease also found a 314% rise in hospitalizations related to morbid obesity and a continuing national health burden exacted by reflux symptoms, Barrett’s esophagus, and colorectal cancer.
Video from the American Gastroenterological Association (http://www.youtube.com/amergastroassn)
"We compiled the most recently available statistics on GI symptoms, quality of life, outpatient diagnoses, hospitalizations, costs, mortality, and endoscopic utilization from a variety of publicly and privately held databases," Dr. Anne F. Peery of the University of North Carolina, Chapel Hill, and her colleagues reported in the November issue of Gastroenterology (doi:10.1053/j.gastro.2012.08.002).
"Payers, policy makers, clinicians, and others interested in resource utilization may use these statistics to better understand evolving disease trends, and the best way to meet the challenge of these diseases."
The findings are based on data for 2009, the most recent year for which complete information was available, from the National Ambulatory Medical Care Survey, sponsored by the U.S. Centers for Disease Control and Prevention; the United States National Health and Wellness Survey, sponsored by the private company Kantar Health; the Nationwide Inpatient Sample, sponsored by the Agency for Healthcare Research and Quality; the Surveillance, Epidemiology, and End Results database of the National Cancer Institute; the National Vital Statistics System, sponsored by the National Center for Health Statistics and the CDC; and the Thomson Reuters MarketScan’s databases of commercial, Medicare, and Medicaid records.
Among the findings:
• C. difficile hospitalizations have increased 237% since 2000 and were associated with 4% in-hospital mortality. Now the ninth leading GI cause of mortality, with an absolute increase of 230% in the number of C. difficile–related deaths since 2002, the infection also markedly impairs quality of life and the capacity for work and other activities.
• Hospitalizations related to obesity remained relatively stable since 2000, but those associated with morbid obesity rose by 314%, and many were likely caused by the marked increase in bariatric surgery.
• Gastroesophageal reflux remains the most common GI-associated diagnosis in primary care, accounting for 9 million outpatient visits in 2009, and the most common GI-associated discharge diagnosis, with 4.4 million such diagnoses in 2009. Obesity was associated with 1.7 million discharge diagnoses and constipation with 1 million.
• Barrett’s esophagus accounted for almost half a million outpatient visits in 2009, when an estimated 3.3 million Americans had this diagnosis. Given that endoscopic surveillance is recommended every 3-5 years, Barrett’s contributes substantially to resource utilization.
• Colorectal cancer, with an estimated 147,000 patients diagnosed in 2008, accounts for more than half of all GI cancer diagnoses and continues to be the primary cause of GI-associated mortality. Pancreatic and hepatobiliary cancers are the next most frequently diagnosed GI cancers.
• Of the approximately 2.5 million deaths in the United States in 2009, 10% were attributed to an underlying GI cause. Chronic liver disease and cirrhosis are the 12th leading causes of death in the country.
• The total outpatient cost for GI endoscopy in 2009 was estimated to be $32.4 billion, which is higher than previously published estimates. An estimated 6.9 million upper endoscopies, 11.5 million lower endoscopies, and 228,000 biliary endoscopies were performed in the United States in 2009.
• Chronic liver disease and viral hepatitis were associated with 6% mortality and cost an estimated $1.8 billion per year in inpatient cost.
• Hospitalizations for nonalcoholic fatty liver disease increased 97% since 2000.
This study was supported in part by the National Institutes of Health. No financial conflicts of interest were reported.
Clostridium difficile contributes mightily to the overall burden of gastrointestinal disease in the United States and was associated with a 237% increase in hospitalizations in the last decade.
Researchers who examined the latest data on the nationwide toll of GI and liver disease also found a 314% rise in hospitalizations related to morbid obesity and a continuing national health burden exacted by reflux symptoms, Barrett’s esophagus, and colorectal cancer.
Video from the American Gastroenterological Association (http://www.youtube.com/amergastroassn)
"We compiled the most recently available statistics on GI symptoms, quality of life, outpatient diagnoses, hospitalizations, costs, mortality, and endoscopic utilization from a variety of publicly and privately held databases," Dr. Anne F. Peery of the University of North Carolina, Chapel Hill, and her colleagues reported in the November issue of Gastroenterology (doi:10.1053/j.gastro.2012.08.002).
"Payers, policy makers, clinicians, and others interested in resource utilization may use these statistics to better understand evolving disease trends, and the best way to meet the challenge of these diseases."
The findings are based on data for 2009, the most recent year for which complete information was available, from the National Ambulatory Medical Care Survey, sponsored by the U.S. Centers for Disease Control and Prevention; the United States National Health and Wellness Survey, sponsored by the private company Kantar Health; the Nationwide Inpatient Sample, sponsored by the Agency for Healthcare Research and Quality; the Surveillance, Epidemiology, and End Results database of the National Cancer Institute; the National Vital Statistics System, sponsored by the National Center for Health Statistics and the CDC; and the Thomson Reuters MarketScan’s databases of commercial, Medicare, and Medicaid records.
Among the findings:
• C. difficile hospitalizations have increased 237% since 2000 and were associated with 4% in-hospital mortality. Now the ninth leading GI cause of mortality, with an absolute increase of 230% in the number of C. difficile–related deaths since 2002, the infection also markedly impairs quality of life and the capacity for work and other activities.
• Hospitalizations related to obesity remained relatively stable since 2000, but those associated with morbid obesity rose by 314%, and many were likely caused by the marked increase in bariatric surgery.
• Gastroesophageal reflux remains the most common GI-associated diagnosis in primary care, accounting for 9 million outpatient visits in 2009, and the most common GI-associated discharge diagnosis, with 4.4 million such diagnoses in 2009. Obesity was associated with 1.7 million discharge diagnoses and constipation with 1 million.
• Barrett’s esophagus accounted for almost half a million outpatient visits in 2009, when an estimated 3.3 million Americans had this diagnosis. Given that endoscopic surveillance is recommended every 3-5 years, Barrett’s contributes substantially to resource utilization.
• Colorectal cancer, with an estimated 147,000 patients diagnosed in 2008, accounts for more than half of all GI cancer diagnoses and continues to be the primary cause of GI-associated mortality. Pancreatic and hepatobiliary cancers are the next most frequently diagnosed GI cancers.
• Of the approximately 2.5 million deaths in the United States in 2009, 10% were attributed to an underlying GI cause. Chronic liver disease and cirrhosis are the 12th leading causes of death in the country.
• The total outpatient cost for GI endoscopy in 2009 was estimated to be $32.4 billion, which is higher than previously published estimates. An estimated 6.9 million upper endoscopies, 11.5 million lower endoscopies, and 228,000 biliary endoscopies were performed in the United States in 2009.
• Chronic liver disease and viral hepatitis were associated with 6% mortality and cost an estimated $1.8 billion per year in inpatient cost.
• Hospitalizations for nonalcoholic fatty liver disease increased 97% since 2000.
This study was supported in part by the National Institutes of Health. No financial conflicts of interest were reported.
FROM GASTROENTEROLOGY
Liver Candidates Decline Many Organ Offers
Eighty-four percent of candidates on the wait-list for liver transplant who either died or were removed from the list before they were able to undergo transplantation declined at least one offer of a donor liver, Dr. Jennifer Cindy Lai of the University of California, San Francisco, and her colleagues reported in the November issue of Gastroenterology.
Even more surprising, most of these candidates declined "not just one or two but a median of six liver offers during their time on the wait-list."
The "declined" donor organs were then successfully transplanted into lower-priority recipients.
These findings suggest that mortality among wait-listed patients "is not simply a result of not having the opportunity for transplantation, as many of us assume. Rather, wait-list mortality appears to result from opportunities for transplantation that were declined," Dr. Lai and her associates wrote.
The reasons that so many viable donor livers were initially declined are not yet clear. General, somewhat vague reasons were listed but not fully explained in the records the researchers analyzed for this study, which they obtained from the United Network for Organ Sharing/Organ Procurement Transplantation Network database.
The investigators assessed organ offers to 33,389 liver transplant candidates aged 18 years and older who were wait-listed across the United States between 2005 and 2010.
The reasons that proffered organs were declined, as listed in the medical records, fit into six broad categories: unfavorable donor age or quality of organ; unfavorable donor organ size/weight; other unfavorable donor factors, such as ABO blood transfusion incompatibility, "social history," "positive serologic tests," or "organ anatomical damage or defect"; unreadiness of the recipient, usually because he or she was ill, unavailable, refused the organ, or required multiple organ transplants at the same time; problems with the transplant program itself, such as a "heavy workload" or unavailability of a surgeon or operating room at the recipient’s medical center, failure to respond to the offer in a timely way, or excessive distance to ship the organ.
A total of 20% of the study population (6,737 patients) died or were removed from the wait-list because they became too sick before they could undergo transplantation. A total of 5,680 (84%) of those patients had been offered one or more donor livers before they died or were taken off the list.
Offers of donor livers were declined most often (68%) because of "unfavorable donor age or quality of organ," whereas 9% were declined because of unfavorable organ size, 15% because of "other donor factors," 4% because the recipient wasn’t ready, and 4% because of transplant program or miscellaneous other factors.
However, the dominant use of the "donor quality or age" refusal code in the database almost certainly "does not accurately or fully capture the true refusal reason," Dr. Lai and her associates said.
Even livers judged to be of high quality according to standard criteria were declined because of supposed "unfavorable donor age or quality of organ." But the investigators found no difference in the risk of graft failure between such high-quality livers that were declined and other high-quality livers that were accepted on the first offer.
Other reasons must be playing an important role in this high rate refusal, but "the nuances of these refusals cannot be determined" without more individualized data, they said.
Dr. Lai and her colleagues suggested that to cut down on refusals of apparently viable organs, the transplant community should "reduce the stigma associated with non–ideal livers, and set realistic expectations for wait-listed candidates" so that they’re less likely to pass up a suitable donation while assuming that a better offer will come along.
Patients also should be educated about the unpredictability of death or of sudden worsening of liver disease while on the wait-list. They should be advised that there is a survival benefit associated with the transplantation of any graft, compared with continuing on the wait-list.
In addition, the current regulatory environment focuses on transplant centers’ outcomes, which may influence some centers to discourage the acceptance of less than optimal donor organs. "This may be especially relevant for low-volume transplant centers, for whom even a small number of poor outcomes ... may make a relatively large difference in the centers’ perceived performance," the researchers wrote.
Finally, wait-list candidates should be encouraged to complete their transplant work-ups as expeditiously as possible to avoid having to refuse a donor offer simply because they have not yet undergone the necessary cardiac testing or cancer screening.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of California, San Francisco. No financial conflicts of interest were reported.
Eighty-four percent of candidates on the wait-list for liver transplant who either died or were removed from the list before they were able to undergo transplantation declined at least one offer of a donor liver, Dr. Jennifer Cindy Lai of the University of California, San Francisco, and her colleagues reported in the November issue of Gastroenterology.
Even more surprising, most of these candidates declined "not just one or two but a median of six liver offers during their time on the wait-list."
The "declined" donor organs were then successfully transplanted into lower-priority recipients.
These findings suggest that mortality among wait-listed patients "is not simply a result of not having the opportunity for transplantation, as many of us assume. Rather, wait-list mortality appears to result from opportunities for transplantation that were declined," Dr. Lai and her associates wrote.
The reasons that so many viable donor livers were initially declined are not yet clear. General, somewhat vague reasons were listed but not fully explained in the records the researchers analyzed for this study, which they obtained from the United Network for Organ Sharing/Organ Procurement Transplantation Network database.
The investigators assessed organ offers to 33,389 liver transplant candidates aged 18 years and older who were wait-listed across the United States between 2005 and 2010.
The reasons that proffered organs were declined, as listed in the medical records, fit into six broad categories: unfavorable donor age or quality of organ; unfavorable donor organ size/weight; other unfavorable donor factors, such as ABO blood transfusion incompatibility, "social history," "positive serologic tests," or "organ anatomical damage or defect"; unreadiness of the recipient, usually because he or she was ill, unavailable, refused the organ, or required multiple organ transplants at the same time; problems with the transplant program itself, such as a "heavy workload" or unavailability of a surgeon or operating room at the recipient’s medical center, failure to respond to the offer in a timely way, or excessive distance to ship the organ.
A total of 20% of the study population (6,737 patients) died or were removed from the wait-list because they became too sick before they could undergo transplantation. A total of 5,680 (84%) of those patients had been offered one or more donor livers before they died or were taken off the list.
Offers of donor livers were declined most often (68%) because of "unfavorable donor age or quality of organ," whereas 9% were declined because of unfavorable organ size, 15% because of "other donor factors," 4% because the recipient wasn’t ready, and 4% because of transplant program or miscellaneous other factors.
However, the dominant use of the "donor quality or age" refusal code in the database almost certainly "does not accurately or fully capture the true refusal reason," Dr. Lai and her associates said.
Even livers judged to be of high quality according to standard criteria were declined because of supposed "unfavorable donor age or quality of organ." But the investigators found no difference in the risk of graft failure between such high-quality livers that were declined and other high-quality livers that were accepted on the first offer.
Other reasons must be playing an important role in this high rate refusal, but "the nuances of these refusals cannot be determined" without more individualized data, they said.
Dr. Lai and her colleagues suggested that to cut down on refusals of apparently viable organs, the transplant community should "reduce the stigma associated with non–ideal livers, and set realistic expectations for wait-listed candidates" so that they’re less likely to pass up a suitable donation while assuming that a better offer will come along.
Patients also should be educated about the unpredictability of death or of sudden worsening of liver disease while on the wait-list. They should be advised that there is a survival benefit associated with the transplantation of any graft, compared with continuing on the wait-list.
In addition, the current regulatory environment focuses on transplant centers’ outcomes, which may influence some centers to discourage the acceptance of less than optimal donor organs. "This may be especially relevant for low-volume transplant centers, for whom even a small number of poor outcomes ... may make a relatively large difference in the centers’ perceived performance," the researchers wrote.
Finally, wait-list candidates should be encouraged to complete their transplant work-ups as expeditiously as possible to avoid having to refuse a donor offer simply because they have not yet undergone the necessary cardiac testing or cancer screening.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of California, San Francisco. No financial conflicts of interest were reported.
Eighty-four percent of candidates on the wait-list for liver transplant who either died or were removed from the list before they were able to undergo transplantation declined at least one offer of a donor liver, Dr. Jennifer Cindy Lai of the University of California, San Francisco, and her colleagues reported in the November issue of Gastroenterology.
Even more surprising, most of these candidates declined "not just one or two but a median of six liver offers during their time on the wait-list."
The "declined" donor organs were then successfully transplanted into lower-priority recipients.
These findings suggest that mortality among wait-listed patients "is not simply a result of not having the opportunity for transplantation, as many of us assume. Rather, wait-list mortality appears to result from opportunities for transplantation that were declined," Dr. Lai and her associates wrote.
The reasons that so many viable donor livers were initially declined are not yet clear. General, somewhat vague reasons were listed but not fully explained in the records the researchers analyzed for this study, which they obtained from the United Network for Organ Sharing/Organ Procurement Transplantation Network database.
The investigators assessed organ offers to 33,389 liver transplant candidates aged 18 years and older who were wait-listed across the United States between 2005 and 2010.
The reasons that proffered organs were declined, as listed in the medical records, fit into six broad categories: unfavorable donor age or quality of organ; unfavorable donor organ size/weight; other unfavorable donor factors, such as ABO blood transfusion incompatibility, "social history," "positive serologic tests," or "organ anatomical damage or defect"; unreadiness of the recipient, usually because he or she was ill, unavailable, refused the organ, or required multiple organ transplants at the same time; problems with the transplant program itself, such as a "heavy workload" or unavailability of a surgeon or operating room at the recipient’s medical center, failure to respond to the offer in a timely way, or excessive distance to ship the organ.
A total of 20% of the study population (6,737 patients) died or were removed from the wait-list because they became too sick before they could undergo transplantation. A total of 5,680 (84%) of those patients had been offered one or more donor livers before they died or were taken off the list.
Offers of donor livers were declined most often (68%) because of "unfavorable donor age or quality of organ," whereas 9% were declined because of unfavorable organ size, 15% because of "other donor factors," 4% because the recipient wasn’t ready, and 4% because of transplant program or miscellaneous other factors.
However, the dominant use of the "donor quality or age" refusal code in the database almost certainly "does not accurately or fully capture the true refusal reason," Dr. Lai and her associates said.
Even livers judged to be of high quality according to standard criteria were declined because of supposed "unfavorable donor age or quality of organ." But the investigators found no difference in the risk of graft failure between such high-quality livers that were declined and other high-quality livers that were accepted on the first offer.
Other reasons must be playing an important role in this high rate refusal, but "the nuances of these refusals cannot be determined" without more individualized data, they said.
Dr. Lai and her colleagues suggested that to cut down on refusals of apparently viable organs, the transplant community should "reduce the stigma associated with non–ideal livers, and set realistic expectations for wait-listed candidates" so that they’re less likely to pass up a suitable donation while assuming that a better offer will come along.
Patients also should be educated about the unpredictability of death or of sudden worsening of liver disease while on the wait-list. They should be advised that there is a survival benefit associated with the transplantation of any graft, compared with continuing on the wait-list.
In addition, the current regulatory environment focuses on transplant centers’ outcomes, which may influence some centers to discourage the acceptance of less than optimal donor organs. "This may be especially relevant for low-volume transplant centers, for whom even a small number of poor outcomes ... may make a relatively large difference in the centers’ perceived performance," the researchers wrote.
Finally, wait-list candidates should be encouraged to complete their transplant work-ups as expeditiously as possible to avoid having to refuse a donor offer simply because they have not yet undergone the necessary cardiac testing or cancer screening.
This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases and the University of California, San Francisco. No financial conflicts of interest were reported.
FROM GASTROENTEROLOGY
Pretreatment Care Predicts HCV Outcomes
Patients with hepatitis C infections are more likely to initiate appropriate antiviral therapy and achieve a sustained virologic response if they receive high quality health care, Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center, Houston, and her colleagues reported in the November issue of Clinical Gastroenterology and Hepatology.
In their study of nearly 35,000 adults with HCV, the odds of initiating antiviral therapy were threefold higher in patients who received optimal care from the moment HCV infection was diagnosed than in those who did not. And among patients who initiated antiviral therapy, the quality of care they received before that therapy even began strongly predicted whether they would complete antiviral therapy and achieve a sustained virologic response, the investigators said.
The study showed, however, that only 11% of patients received all of the appropriate initial care.
Process-of-care measures are frequently used to assess the quality of care for HCV, but until now no study has assessed whether these measures actually correlate with better outcomes. To address this issue, Dr. Kanwal and her associates "evaluated the relationship between adherence to a broad set of process-based measures in HCV and 3 subsequent HCV-specific endpoints: receipt of antiviral treatment, completion of antiviral treatment, and the clinical outcome associated with improved survival: sustained virologic response."
The investigators used data from the VA registry on HCV clinical care, which covers patient demographics, lab tests, pharmacy information, and data on inpatient and outpatient care for approximately 300,000 patients across the country. For this study, they included data on 34,749 adults.
The mean subject age was 53 years, and 97% were men. Approximately half the study population was white and 26% was black; ethnicity was not reported for the others.
The researchers assessed seven process-of-care measures: confirmation of HCV viremia, evaluation by HCV specialists, HCV genotype testing, liver biopsy for those found to have genotype 1 HCV, and the ruling out of liver diseases related to hepatitis B, autoimmunity, or iron overload.
They also assessed seven process-of-care measures related to the prevention and management of comorbid conditions: HIV testing; hepatitis A and B serology testing, hepatitis A and B vaccination if serology results proved negative; treatment of depression; and treatment of substance abuse disorder.
Finally, they assessed six process-of-care measures related to monitoring of antiviral therapy’s effects: testing of viral load before antivirals were initiated and again at weeks 12, 24, and 48; reduction of ribavirin dose if anemia developed during treatment; and avoidance of prescribing growth-stimulating factors for leukopenia during antiviral therapy.
Overall, only 11% of the study subjects received all of the appropriate initial care, and 8% received all the appropriate care related to prevention and management of comorbid conditions. Moreover, of the study subjects who received antiviral therapy, just 37% received all the appropriate monitoring of treatment effects Dr. Kanwal and her associates said.
In patients who received optimal care before a definitive diagnosis was made, the odds of receiving antiviral therapy were 3.2 times higher than in patients who did not receive optimal care before diagnosis, they reported.
Similarly, patients who received optimal preventive and comorbid-condition care showed rates of antiviral therapy that were 36% higher than those of patients who received suboptimal preventive and comorbid-condition care.
The strong association between fulfillment of these process-of-care measures and appropriate antiviral therapy remained robust in a series of sensitivity analyses, which means it’s likely that meeting process-of-care goals directly leads to better HCV outcomes, Dr. Kanwal and her colleagues said.
The investigators could not, however, rule out the possibility that meeting these goals is simply a marker of more compliant patients, which in turn produces better outcomes.
The study findings imply that the effectiveness of the two new direct-acting antiviral agents that recently became available for HCV may hinge on the quality of care patients are receiving before they even start taking these drugs, rather than simply on the effectiveness of the drugs alone, Dr. Kanwal and her associates said.
This study was supported by the U.S. Department of Veterans Affairs Health Services Research and Development Service. No financial conflicts of interest were reported.
Financial reimbursement in medicine has long been driven by volume rather than quality. This incentive structure is changing, and in the near future practitioners will experience increased scrutiny of the quality of care we provide.

|
|
Quality can be divided into structure (having the proper equipment to clean endoscopes), process (testing for latent tuberculosis before beginning anti–tumor necrosis factor therapy), and outcomes (perforation rate during colonoscopy). The latter is, of course, the most important, but it is also the most difficult to measure because of low event rates and inadequate risk adjustment. Therefore, most quality measures are based on processes of care. For quality measurement to yield any true value to the patient, however, it is important that these processes are clearly linked to better patient outcomes.
Hepatitis C, which affects 1.3% of Americans, has recently been a target disease for measuring and improving quality of care. Dr. Kanwal and her colleagues found that patients receiving optimum process-related quality care were more likely to undergo antiviral therapy. Such patients also were more likely to complete treatment once started and to achieve sustained virologic response if treatment was completed. Since these findings persisted despite adjustment for numerous potential confounders such as comorbidities, it appears that higher quality of care (as measured by processes) may truly lead to better patient outcomes.
What does this mean for practitioners? Particularly in the era of triple therapy, hepatitis C virus (HCV) infection cannot be managed like any other disease. Protocols and tracking systems need to be developed to ensure that quality measures are met. Many practices find it helpful to funnel HCV patients to a single person for case management, such as a nurse or midlevel provider. Hopefully, these efforts, in conjunction with newer antivirals, will soon eradicate hepatitis C altogether.
Michael Volk, M.D., is assistant professor of hepatology at the University of Michigan, Ann Arbor.
Financial reimbursement in medicine has long been driven by volume rather than quality. This incentive structure is changing, and in the near future practitioners will experience increased scrutiny of the quality of care we provide.

|
|
Quality can be divided into structure (having the proper equipment to clean endoscopes), process (testing for latent tuberculosis before beginning anti–tumor necrosis factor therapy), and outcomes (perforation rate during colonoscopy). The latter is, of course, the most important, but it is also the most difficult to measure because of low event rates and inadequate risk adjustment. Therefore, most quality measures are based on processes of care. For quality measurement to yield any true value to the patient, however, it is important that these processes are clearly linked to better patient outcomes.
Hepatitis C, which affects 1.3% of Americans, has recently been a target disease for measuring and improving quality of care. Dr. Kanwal and her colleagues found that patients receiving optimum process-related quality care were more likely to undergo antiviral therapy. Such patients also were more likely to complete treatment once started and to achieve sustained virologic response if treatment was completed. Since these findings persisted despite adjustment for numerous potential confounders such as comorbidities, it appears that higher quality of care (as measured by processes) may truly lead to better patient outcomes.
What does this mean for practitioners? Particularly in the era of triple therapy, hepatitis C virus (HCV) infection cannot be managed like any other disease. Protocols and tracking systems need to be developed to ensure that quality measures are met. Many practices find it helpful to funnel HCV patients to a single person for case management, such as a nurse or midlevel provider. Hopefully, these efforts, in conjunction with newer antivirals, will soon eradicate hepatitis C altogether.
Michael Volk, M.D., is assistant professor of hepatology at the University of Michigan, Ann Arbor.
Financial reimbursement in medicine has long been driven by volume rather than quality. This incentive structure is changing, and in the near future practitioners will experience increased scrutiny of the quality of care we provide.

|
|
Quality can be divided into structure (having the proper equipment to clean endoscopes), process (testing for latent tuberculosis before beginning anti–tumor necrosis factor therapy), and outcomes (perforation rate during colonoscopy). The latter is, of course, the most important, but it is also the most difficult to measure because of low event rates and inadequate risk adjustment. Therefore, most quality measures are based on processes of care. For quality measurement to yield any true value to the patient, however, it is important that these processes are clearly linked to better patient outcomes.
Hepatitis C, which affects 1.3% of Americans, has recently been a target disease for measuring and improving quality of care. Dr. Kanwal and her colleagues found that patients receiving optimum process-related quality care were more likely to undergo antiviral therapy. Such patients also were more likely to complete treatment once started and to achieve sustained virologic response if treatment was completed. Since these findings persisted despite adjustment for numerous potential confounders such as comorbidities, it appears that higher quality of care (as measured by processes) may truly lead to better patient outcomes.
What does this mean for practitioners? Particularly in the era of triple therapy, hepatitis C virus (HCV) infection cannot be managed like any other disease. Protocols and tracking systems need to be developed to ensure that quality measures are met. Many practices find it helpful to funnel HCV patients to a single person for case management, such as a nurse or midlevel provider. Hopefully, these efforts, in conjunction with newer antivirals, will soon eradicate hepatitis C altogether.
Michael Volk, M.D., is assistant professor of hepatology at the University of Michigan, Ann Arbor.
Patients with hepatitis C infections are more likely to initiate appropriate antiviral therapy and achieve a sustained virologic response if they receive high quality health care, Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center, Houston, and her colleagues reported in the November issue of Clinical Gastroenterology and Hepatology.
In their study of nearly 35,000 adults with HCV, the odds of initiating antiviral therapy were threefold higher in patients who received optimal care from the moment HCV infection was diagnosed than in those who did not. And among patients who initiated antiviral therapy, the quality of care they received before that therapy even began strongly predicted whether they would complete antiviral therapy and achieve a sustained virologic response, the investigators said.
The study showed, however, that only 11% of patients received all of the appropriate initial care.
Process-of-care measures are frequently used to assess the quality of care for HCV, but until now no study has assessed whether these measures actually correlate with better outcomes. To address this issue, Dr. Kanwal and her associates "evaluated the relationship between adherence to a broad set of process-based measures in HCV and 3 subsequent HCV-specific endpoints: receipt of antiviral treatment, completion of antiviral treatment, and the clinical outcome associated with improved survival: sustained virologic response."
The investigators used data from the VA registry on HCV clinical care, which covers patient demographics, lab tests, pharmacy information, and data on inpatient and outpatient care for approximately 300,000 patients across the country. For this study, they included data on 34,749 adults.
The mean subject age was 53 years, and 97% were men. Approximately half the study population was white and 26% was black; ethnicity was not reported for the others.
The researchers assessed seven process-of-care measures: confirmation of HCV viremia, evaluation by HCV specialists, HCV genotype testing, liver biopsy for those found to have genotype 1 HCV, and the ruling out of liver diseases related to hepatitis B, autoimmunity, or iron overload.
They also assessed seven process-of-care measures related to the prevention and management of comorbid conditions: HIV testing; hepatitis A and B serology testing, hepatitis A and B vaccination if serology results proved negative; treatment of depression; and treatment of substance abuse disorder.
Finally, they assessed six process-of-care measures related to monitoring of antiviral therapy’s effects: testing of viral load before antivirals were initiated and again at weeks 12, 24, and 48; reduction of ribavirin dose if anemia developed during treatment; and avoidance of prescribing growth-stimulating factors for leukopenia during antiviral therapy.
Overall, only 11% of the study subjects received all of the appropriate initial care, and 8% received all the appropriate care related to prevention and management of comorbid conditions. Moreover, of the study subjects who received antiviral therapy, just 37% received all the appropriate monitoring of treatment effects Dr. Kanwal and her associates said.
In patients who received optimal care before a definitive diagnosis was made, the odds of receiving antiviral therapy were 3.2 times higher than in patients who did not receive optimal care before diagnosis, they reported.
Similarly, patients who received optimal preventive and comorbid-condition care showed rates of antiviral therapy that were 36% higher than those of patients who received suboptimal preventive and comorbid-condition care.
The strong association between fulfillment of these process-of-care measures and appropriate antiviral therapy remained robust in a series of sensitivity analyses, which means it’s likely that meeting process-of-care goals directly leads to better HCV outcomes, Dr. Kanwal and her colleagues said.
The investigators could not, however, rule out the possibility that meeting these goals is simply a marker of more compliant patients, which in turn produces better outcomes.
The study findings imply that the effectiveness of the two new direct-acting antiviral agents that recently became available for HCV may hinge on the quality of care patients are receiving before they even start taking these drugs, rather than simply on the effectiveness of the drugs alone, Dr. Kanwal and her associates said.
This study was supported by the U.S. Department of Veterans Affairs Health Services Research and Development Service. No financial conflicts of interest were reported.
Patients with hepatitis C infections are more likely to initiate appropriate antiviral therapy and achieve a sustained virologic response if they receive high quality health care, Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center, Houston, and her colleagues reported in the November issue of Clinical Gastroenterology and Hepatology.
In their study of nearly 35,000 adults with HCV, the odds of initiating antiviral therapy were threefold higher in patients who received optimal care from the moment HCV infection was diagnosed than in those who did not. And among patients who initiated antiviral therapy, the quality of care they received before that therapy even began strongly predicted whether they would complete antiviral therapy and achieve a sustained virologic response, the investigators said.
The study showed, however, that only 11% of patients received all of the appropriate initial care.
Process-of-care measures are frequently used to assess the quality of care for HCV, but until now no study has assessed whether these measures actually correlate with better outcomes. To address this issue, Dr. Kanwal and her associates "evaluated the relationship between adherence to a broad set of process-based measures in HCV and 3 subsequent HCV-specific endpoints: receipt of antiviral treatment, completion of antiviral treatment, and the clinical outcome associated with improved survival: sustained virologic response."
The investigators used data from the VA registry on HCV clinical care, which covers patient demographics, lab tests, pharmacy information, and data on inpatient and outpatient care for approximately 300,000 patients across the country. For this study, they included data on 34,749 adults.
The mean subject age was 53 years, and 97% were men. Approximately half the study population was white and 26% was black; ethnicity was not reported for the others.
The researchers assessed seven process-of-care measures: confirmation of HCV viremia, evaluation by HCV specialists, HCV genotype testing, liver biopsy for those found to have genotype 1 HCV, and the ruling out of liver diseases related to hepatitis B, autoimmunity, or iron overload.
They also assessed seven process-of-care measures related to the prevention and management of comorbid conditions: HIV testing; hepatitis A and B serology testing, hepatitis A and B vaccination if serology results proved negative; treatment of depression; and treatment of substance abuse disorder.
Finally, they assessed six process-of-care measures related to monitoring of antiviral therapy’s effects: testing of viral load before antivirals were initiated and again at weeks 12, 24, and 48; reduction of ribavirin dose if anemia developed during treatment; and avoidance of prescribing growth-stimulating factors for leukopenia during antiviral therapy.
Overall, only 11% of the study subjects received all of the appropriate initial care, and 8% received all the appropriate care related to prevention and management of comorbid conditions. Moreover, of the study subjects who received antiviral therapy, just 37% received all the appropriate monitoring of treatment effects Dr. Kanwal and her associates said.
In patients who received optimal care before a definitive diagnosis was made, the odds of receiving antiviral therapy were 3.2 times higher than in patients who did not receive optimal care before diagnosis, they reported.
Similarly, patients who received optimal preventive and comorbid-condition care showed rates of antiviral therapy that were 36% higher than those of patients who received suboptimal preventive and comorbid-condition care.
The strong association between fulfillment of these process-of-care measures and appropriate antiviral therapy remained robust in a series of sensitivity analyses, which means it’s likely that meeting process-of-care goals directly leads to better HCV outcomes, Dr. Kanwal and her colleagues said.
The investigators could not, however, rule out the possibility that meeting these goals is simply a marker of more compliant patients, which in turn produces better outcomes.
The study findings imply that the effectiveness of the two new direct-acting antiviral agents that recently became available for HCV may hinge on the quality of care patients are receiving before they even start taking these drugs, rather than simply on the effectiveness of the drugs alone, Dr. Kanwal and her associates said.
This study was supported by the U.S. Department of Veterans Affairs Health Services Research and Development Service. No financial conflicts of interest were reported.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Androgen deficiency in older men: Indications, advantages, and pitfalls of testosterone replacement therapy
Editor’s note: This is the second of two articles on hypogonadism in men and focuses on the appropriate use of testosterone therapy. The first article, published last month, focused in more detail on the differential diagnosis of hypogonadism.
As men age, testosterone production gradually decreases. In our increasingly aged population, clinicians will continue to see an increase in the number of men with seemingly nonspecific symptoms of aging that are possibly due to low serum testosterone (eg, low energy level, depressive symptoms, erectile dysfunction, decreased libido). These clinical symptoms, coupled with low serum testosterone, may adversely affect quality of life and life expectancy. Testosterone replacement therapy (TRT) may improve symptoms and quality of life. Given the nonspecific nature of these symptoms, accurate diagnosis and treatment of clinically significant low testosterone with a goal of symptom and quality of life improvement can prove challenging.
These challenges in diagnosis and treatment result in a lack of standardized nomenclature. The terms male menopause and andropause, although popular, are the least helpful, as they have few correlates with the better-defined female menopause. Late-onset hypogonadism implies a well-defined, later age of decline, which is inaccurate since the decline in serum testosterone in men begins in middle age and is gradual. Testosterone deficiency syndrome implies a set of specific and well-defined symptoms. Androgen deficiency in the aging male (ADAM) and Androgen deficiency in the older male are common terms specifying an age cohort (> 40 years old) and an abnormal laboratory value without mention of symptoms. While all these terms have their limitations, we will primarily use ADAM in this discussion.
PREVALENCE OF LOW TESTOSTERONE
Serum testosterone levels begin to decline in men in their mid-40s, with an approximately 1% to 2% decline annually and a marked decline after age 60.1
Araujo and colleagues2 studied the prevalence of androgen-deficient men, with androgen deficiency defined as at least three signs or symptoms and either a total testosterone less than 200 ng/dL or a total testosterone 200 ng/dL to 400 ng/dL with a free testosterone less than 8.91 ng/dL. The overall prevalence of low testosterone on initial measurement was 6%, which doubled to 12% with repeat measurement.
Serial measures are important: one study that followed untreated men over 15 years found normal testosterone on serial measures in 50%.3 In a multicenter cross-sectional study, 11.8% of men had low testosterone and low or normal luteinizing hormone (LH) levels (secondary hypogonadism/hypothalamic-pituitary failure), with 2% of patients with low testosterone and elevated LH (primary hypogonadism/testicular failure).4
CLINICAL PRESENTATION AND DIAGNOSIS
A biochemical diagnosis of low testosterone is dependent on accurate measurement. Testosterone release is diurnal, with the highest levels in the early morning, and often has week-to-week variability. Thus, it is important to collect blood in the early morning and to confirm a diagnosis of low testosterone with at least one repeat measurement several days later, including LH assessment. LH levels will help differentiate primary hypogonadism from secondary hypogonadism, which may alter diagnosis and treatment in certain patients, with secondary hypogonadism associated with pituitary dysfunction, and primary hypogonadism associated with aging.4
Testosterone binds in the bloodstream to sex hormone-binding globulin (SHBG), and this bound form is generally considered biologically inactive, although there are in vitro and animal studies suggesting SHBG-bound androgen may indeed have biological activity. 5,6 “Bioavailable” testosterone is active and includes both free testosterone and testosterone bound to albumin.
There is no general agreement on the acceptable normal range of testosterone, with variability within the literature and between laboratories. “Normal” total testosterone levels have ranged from more than 280 ng/dL to more than 350 ng/dL (12 nmol/L).7,8 Similarly, there is no generally accepted lower limit of normal, although some studies report a threshold level of testosterone less than 230 ng/dL (8 nmol/L) as “abnormal.” Values between these two upper and lower limits are considered “borderline.”7,8 These intermediate or borderline values coupled with clinical symptoms of testosterone deficiency syndrome or ADAM should be considered abnormal.
When total testosterone is borderline, measurement of free or bioavailable testosterone (free plus albumin-bound) should be considered. Total testosterone is typically measured using automated immunoassay platforms, with method-related differences leading to significant variability in measurement accuracy and precision. This variability is seen most dramatically in those with low total testosterone.9 However, the variability of total testosterone measurements is substantially smaller among mass spectrometry assays than among immunoassays. 10
The gold standards for free testosterone measurement are centrifugal ultrafiltration and equilibrium dialysis.9 However, these techniques are laborious and usually unavailable in local laboratories. Calculated free testosterone values using total testosterone and SHBG are most commonly used and are sufficiently accurate for clinical practice.11
Free testosterone levels can be diagnostic when total testosterone levels do not correspond with clinical presentation. However, the clinical utility of free testosterone is difficult to assess due to the variability among laboratory assays and a lack of consensus on threshold parameters. A threshold free testosterone level of more than 225 pmol/L (65 pg/mL) is generally considered normal.7,8 Before starting a patient on TRT, measurement of hemoglobin and prostate-specific antigen (PSA) and digital rectal examination of the prostate (if age is > 39) are essential.
Prolactin levels are recommended when low testosterone is confirmed, especially in patients at high clinical risk for hyperprolactinemia. Once hyperprolactinemia is identified, Endocrine Society guidelines recommend excluding medication use, renal failure, hypothyroidism, and parasellar tumors as possible causes of elevated prolactin levels.12
Low testosterone values should be treated only in patients with clinically significant symptoms that are likely to be caused by the low testosterone itself. Symptoms associated with age-related decline in testosterone that may improve with TRT include low libido,13,14 low energy,14 depressed mood,15–17 low muscle mass, osteoporosis, and hot flashes. Men with erectile dysfunction have also shown a significant improvement with TRT compared with placebo, but with a variable overall response independent of normalization of testosterone. 18,19 This is likely due to the multifactorial nature of erectile dysfunction, including vascular, neurologic, psychogenic, and endocrinologic causes.
Screening questionnaires have been developed for symptoms of low testosterone, but their clinical utility is unclear. The ADAM questionnaire is used as a screening tool for low testosterone but not to monitor response to TRT, and it is highly nonspecific.20 The Aging Male Symptom Scale questionnaire includes psychological, somatovegetative, and sexual components and is used both to screen for low testosterone and to measure outcomes.21 However, a recent observational study comparing the ability of these questionnaires to assess clinical symptoms revealed a low sensitivity and a low specificity to detect androgen deficiency in men with a total testosterone level less than 300 ng/dL.22 Overall, the current data do not conclusively support the use of hypogonadism questionnaires for screening.
The patient history when evaluating for ADAM should include evaluation of sexual and constitutional symptoms as described above and in Table 1. In addition, a history of traumatic, medical, or surgical events that could affect testosterone production should be obtained, including cryptorchidism, scrotal, inguinal, or abdominal surgery, pituitary surgery or radiation, prior issues with infertility, timing of puberty, history of renal or hepatic failure, chemotherapy (for cancer or autoimmune diseases), and prior use of anabolic steroids or opiates.
A complete physical examination should include assessment of virilization, gynecomastia, and the genitalia, including the size, position, and volume of the testes. The size and consistency of the prostate should be assessed on digital rectal examination.
LOW TESTOSTERONE AND ASSOCIATED COMORBIDITIES
Low testosterone is associated with many comorbidities, including metabolic syndrome, depression, type 2 diabetes mellitus, and cardiovascular disease, as discussed later in this section. Low testosterone has also shown associations with osteoporosis, cognitive impairment, hypertension, hyperlipidemia, decreased physical performance, end-stage renal disease, and treatment with steroids or opiates.23–26 However, the studies that found these associations included men younger than 40 years and may not be fully applicable to the ADAM population.
The association of metabolic syndrome and type 2 diabetes mellitus with low testosterone is well established in multiple studies. Grossman and colleagues27 investigated the association of type 2 diabetes mellitus and low testosterone, with low total testosterone defined as below 10 nmol/L and low calculated free testosterone less than 0.23 nmol/L. The prevalence of low total testosterone was 43%, and the prevalence of low free testosterone was 57%. In addition, a recent meta-analysis comparing total testosterone of men with and without metabolic syndrome revealed an association between a baseline decrease in mean total and free testosterone levels in men with metabolic syndrome compared with controls. This study found a total testosterone mean difference of –2.64 nmol/L (95% confidence interval [CI] –2.95 to –2.32) and a free testosterone mean difference of –0.26 pmol/L (95% CI –0.39 to –0.13), respectively, when comparing men with metabolic syndrome against those without.28
Testosterone has also been suggested to be protective against type 2 diabetes mellitus, with 42% lower risk of type 2 diabetes mellitus in men with testosterone levels ranging from 450 ng/dL to 605 ng/dL.29
Obesity has been specifically linked with secondary hypogonadism.4,23,24 A prospective cohort of 58 men with an average age of 46 years and a body mass index ranging from 30 to 45 kg/m2 were monitored on a low-calorie diet for 9 weeks. Afterward, biochemical analysis revealed an increase in free testosterone from 185 pmol/L ± 66 to 208 ± 70 pmol/L (P = .002) with a mean weight loss of 16.3 kg ± 4.5 kg.30 This emphasizes the importance of lifestyle changes in the management of hypogonadal men.
LOW TESTOSTERONE AND THE OVERALL MORTALITY RATE
Low testosterone is associated unfavorably with the rate of all-cause mortality. A retrospective study in male veterans over age 40 with repeated testosterone levels over a 5-year period found that the risk of death from all causes in men with normal testosterone (> 250 ng/dL or free testosterone > 0.75 ng/dL) was 20% (95% CI 16.2%–241%) vs 35% (95% CI 28.5%–41.4%) in men with low testosterone (< 250 ng/dL or free testosterone < 0.75 ng/dL). In multivariate analysis, men with testosterone less than 250 ng/dL (< 8.7 nmol/L) or free testosterone less than 0.75 ng/dL (< 0.03 nmol/L) had up to an 88% higher death rate than men with normal testosterone levels.31
Low testosterone has also been associated with other end-organ, disease-specific mortality. In men with end-stage renal disease, low testosterone was an independent predictor of death from all causes and from cardiovascular disease.32 A prospective European health study revealed an association between low testosterone and increased risk of death from cardiovascular disease and cancer.33 A recent meta-analysis of population-based studies confirmed this association, despite significant interstudy heterogeneity. 34 Although multiple studies show an independent association of low testosterone and increased mortality rate, causality remains unconfirmed. This may be difficult to prove, given the available study designs and the nonspecific nature of symptoms related to low testosterone and potentially associated comorbidities.
TRT: INDICATIONS AND CONTRAINDICATIONS
The indications, benefits, and risks of TRT are controversial, with current data lacking long-term follow-up and consistent biochemical target values. Treatment of low testosterone is not indicated at the present time in the absence of clinical symptoms.
According to recently published guidelines, TRT is recommended for symptomatic men with low or borderline total testosterone or free testosterone (< 350 ng/dL or < 65 pg/mL).7,8 Patients with borderline biochemical values (total testosterone 200–350 ng/dL, free testosterone 40–65 pg/mL) and possible related symptoms should be treated with TRT for at least 3 months and then reevaluated to verify improved testosterone levels and to assess for symptom amelioration or resolution.35 Dose escalation is recommended in patients with subtherapeutic testosterone levels and limited clinical improvement after 3 months of treatment.
Target maintenance testosterone levels have not been defined, with mid to lower young adult male serum testosterone levels recommended at this time.8 Given that the current literature does not specify a target testosterone replacement range, we recommend monitoring the clinical response along with total testosterone to decide adjustments in TRT. Ultimately, treatment goals of TRT should be the resolution of signs and symptoms, including improvement of sexual function, libido, and preservation of bone mineral density.7,8
Contraindications
TRT is not recommended in men with the following:
- Breast cancer
- Polycythemia (hematocrit > 50%)
- Untreated obstructive sleep apnea
- Lower urinary tract symptoms caused by an enlarged prostate; International Prostate Symptom Score > 19
- Poorly controlled heart failure
- Desire for fertility.
The role of TRT in prostate cancer remains controversial (see below) and remains contraindicated in recent Endocrine Society clinical practice guidelines.7 Guidelines recommend urologic consultation prior to initiation of TRT in patients at increased risk of prostate cancer,7 based on age, race, family history, PSA, PSA velocity, and history of prostate biopsy.
One prominent historic concern about androgen replacement therapy regards the potential for de novo development of prostate cancer. Numerous studies have failed to find elevated risk of new diagnosis, progression, or recurrence of prostate cancer in patients on TRT.36,37 Nevertheless, patients who develop elevated PSA, increased PSA velocity, or an abnormal digital rectal examination while on TRT should undergo prostate biopsy.
TRT FORMULATIONS AND TREATMENT OPTIONS
A number of effective formulations of TRT are available (Table 2). Transdermal and parenteral formulations are most commonly used. Enteric testosterone formulations are not available in the United States and are associated with hepatotoxicity. While buccal testosterone therapy is available, it often leads to local gingival irritation and has not gained widespread popularity.
Parenteral TRT can be administered intramuscularly (IM) or subcutaneously (SQ). Testosterone cypionate (Depo-Testosterone) is the only IM form available in the United States and is given every 2 to 3 weeks. It is the least expensive form of TRT, but it requires frequent administration (by either the clinical practitioner or the patient himself). Testosterone cypionate injections lead to markedly wide swings of testosterone levels, ranging from supraphysiologic levels for a few days after administration to hypogonadal levels before the next injection. This may be mitigated by more-frequent injections. The longer-acting form testosterone undecanoate is available outside the United States and is given every 12 weeks when stable levels are reached.
The other parenteral option is SQ slow-release pellets (Testopel). These pellets have 75 mg of testosterone. Typically 8 to 14 pellets are placed subcutaneously in the buttock area, which will provide coverage for 3 to 6 months.38 The insertion procedure is simple with a short learning curve, limited compliance issues, and elimination of risk of transdermal transmission of drug to others. Disadvantages include wound infection and pellet extrusion, seen in 0.3% to 12% of patients in various studies.38
Another route of TRT is transdermal, including patches, liquids, and gels. Patches are applied daily and are rotated to different sites with minimal risk for skin transmission to others, although use may be limited by site dermatitis. Three hydro-alcoholic gel formulations are currently available in the United States: Androgel (1% or 1.62%), which is applied to the chest or the shoulders; Testim 1%, which is applied to the shoulders; and Fortesta (2%), which is applied to the thighs. A liquid preparation, Axiron, is applied to the axillae. Because secondary transfer to women and children is possible, it is important to thoroughly wash hands after application and to cover the treated skin with clothing. In 3 to 4 hours, all the medication is absorbed, and the area should then be washed before direct skin contact with others (Table 2).
MONITORING PATIENTS ON TRT
Patients starting TRT will require clinical and biochemical monitoring to evaluate response to therapy as well as possible side effects. The first set of laboratory values should be obtained 6 to 12 weeks after initiation of therapy and then typically quarterly for 1 year, every 6 months for the second year, and annually thereafter. Laboratory values monitored should include total testosterone, PSA, and hematocrit.
Men on daily therapy (patch, gel, liquid) should have testosterone drawn approximately 2 hours after application. Current TRT regimen data lack an appropriate target testosterone value, and guidelines suggest a mid to lower young adult male testosterone level.8 Since this is not clearly delineated in the current literature, the authors recommend monitoring clinical symptoms along with testosterone levels when adjusting TRT. It is important to document that serum testosterone was actually increased to the normal range in treated men without clinical improvement.
A rise in PSA of up to 24% would be an acceptable response in a benign prostate gland, but a higher increase or increase above 4.0 ng/dL should prompt consideration of prostate biopsy. 39 Similarly, hemoglobin and hematocrit typically increase, but a hematocrit greater than 55% should prompt dose reduction or cessation.7 Transaminases do not need routine monitoring during parenteral or transdermal therapy. Bone mineral density should be monitored every 1 to 2 years.7,8
CLINICAL BENEFITS OF TRT
There are promising data regarding the clinical benefits of TRT in patients with metabolic syndrome and type 2 diabetes mellitus. A recent meta-analysis investigating the effect of TRT on metabolic syndrome revealed an improvement in fasting plasma glucose, homeostatic model assessment index, triglycerides, treadmill duration, high-density lipoprotein cholesterol, and waist circumference.40,41 TRT also decreased insulin resistance and improved glycemic control in type 2 diabetic hypogonadal men.42 Results from a randomized controlled trial comparing 12 weeks of intramuscular testosterone treatment vs placebo in men with metabolic syndrome revealed an improvement in mean waist circumference from 108 cm ± 8 cm to 105.5 cm ± 7.7 cm. Sixty percent of men initially diagnosed with metabolic syndrome and treated with testosterone no longer met diagnostic criteria for metabolic syndrome according to the National Cholesterol Education Program–Third Adult Treatment Panel (NCEP-ATP III) and the International Diabetes Federation (IDF) guidelines.43
Depression has also been associated with low testosterone, with free testosterone levels below 170 pmol/L associated with frank depressive symptoms and levels below 220 pmol/L predictive of future onset of depressive symptoms.15 Testosterone replacement therapy has been shown to improve depressive symptoms in hypogonadal men.16,17 Shores et al16 conducted a randomized placebo-controlled study of testosterone replacement in men older than 50 years with dysthymia or minor depression. Men treated with testosterone gel for 12 weeks showed an improvement of baseline total testosterone levels from 291 ng/dL to 449 ng/dL. Men treated with testosterone also had a 53% rate of depression remission compared with 19% in the placebo group.16
The evidence supporting improved sexual function with TRT is variable. Some studies indicate limited or transient improvement of sexual function after TRT in men with erectile dysfunction,18,19 while others report an improvement in sexual function after 3 months of TRT.44 Because of the multifactorial nature of erectile dysfunction, men with erectile dysfunction and ADAM may require TRT and a phosphodiesterase type 5 (PDE5) inhibitor, as TRT alone may be insufficient. In a prospective observational study of men with erectile dysfunction and an initial testosterone lower than 300 ng/dL, testosterone gel was administered for at least 1 year, and improvement in sexual function was seen. Results revealed a correlation between improvement in sexual function and concurrent therapy with a PDE5 inhibitor.45 In a recent multicenter placebo-controlled study of PDE5 inhibitor nonresponders, the addition of a testosterone gel to tadalafil (Cialis) improved sexual function, again suggesting a synergistic effect when treating erectile dysfunction with both TRT and a PDE5 inhibitor.46
ADVERSE EVENTS RELATED TO TRT
Despite the aforementioned benefits, it must be emphasized that TRT should be used for specific target symptoms related to hypogonadism in older men and that the general health benefits and safety of TRT in an asymptomatic man with a low measured testosterone alone remains unproven.
Cardiovascular events. In a recent study of 209 elderly men with low testosterone and limited mobility associated with other chronic illnesses, 6 months of TRT resulted in the development of cardiovascular-related adverse events in 23 patients compared with 5 men in the placebo group.47 This may have been related to how adverse events were reported, with cumulative adverse events reviewed every 6 months, ranging from peripheral edema, hypertension, arrhythmias, and electrocardiographic changes. Serious adverse events were reviewed as they occurred, including stroke and acute myocardial events.
Other studies41,43 have revealed a favorable effect of TRT on cardiovascular disease and its surrogate markers but have lacked detailed reports and close monitoring of adverse events. Thus, variation of outcome measurement and reporting may obfuscate the detection of adverse cardiovascular events. Outcomes may also depend on the testosterone formulation and the target serum concentration.43
Larger, long-term placebo-controlled trials are needed to elucidate cardiovascular risk as a primary outcome in older androgen-deficient men undergoing TRT.
Other adverse effects related to TRT include erythrocytosis, seen in 3% to 18% of patients with transdermal administration,48,49 and up to 44% of patients undergoing IM therapy.48 Gynecomastia can occur and is more likely to resolve after treatment cessation of transdermal testosterone treatment than IM injections.48 Other potential clinical side effects that should prompt dose-reduction or discontinuation are irritability, bothersome acne, fluid retention, testicular atrophy, worsening of lower urinary tract symptoms from an enlarged prostate, and new or worsening heart failure. Infrequently, obstructive sleep apnea may be worsened by TRT, although currently the data linking sleep apnea and TRT are limited.50
TRT AND PROSTATE CANCER
The relationship between prostate cancer growth and testosterone is well established, with androgen ablation remaining the cornerstone of treatment for metastatic disease. Since androgen deprivation leads to the regression of prostate cancer, there has been concern that TRT may lead to growth or de novo development of prostate cancer. TRT has thus been strongly prohibited in patients with prostate cancer.7 However, recent data challenge this paradigm.
In a retrospective study of 81 men (mean age 56.8 years) treated with TRT, only 4 men (4.9%) developed prostate cancer over a 5-year period.51 This is less than the estimated 16.7% probability of developing prostate cancer in the general US population.52
Recent accumulating data support the concept of testosterone reaching a saturation level when binding androgen receptors within the prostate at extremely low levels. Increases above this level with TRT as with ADAM do not increase the risk of development or progression of prostate cancer.53 In addition, large doses of dihydrotestosterone do not seem to alter PSA, prostate volume, or International Prostate Symptom Score.54 These findings may have implications in future androgen therapies in hypogonadal older men.
Pathologic studies suggest low testosterone is associated with a higher Gleason grade of prostate cancer,55 although this association remains unconfirmed.56
In men with erectile dysfunction after prostate cancer treatment, TRT appears safe after brachytherapy57 or radical prostatectomy.58 A small study of 15 hypogonadal men with castrate-resistant prostate cancer and minimal or no metastatic disease showed only 1 patient had symptomatic progression.59 Moreover, a recent small study of 13 men with known prostate cancer on active surveillance showed that TRT did not lead to local progression or metastatic disease in any of the patients.60
While these data are provocative, it should still be emphasized that the standard of care for prostate cancer screening should be followed in age-appropriate men with ADAM. In addition, hypogonadal men with prostate cancer should only be treated with testosterone in conjunction with careful counseling and ongoing monitoring.
TRT SHOULD NOT REPLACE HEALTHY LIFESTYLE CHANGES
There has been a dramatic increase in TRT initiation for nonspecific symptoms of low testosterone in older androgen-deficient men. With this increase in initiation of TRT, there is a significant risk of overtreating. While there are many encouraging associations between treatment of androgen deficiency and improvement in rates of of morbidity and mortality, much remains unknown about the overall long-term risks and benefits of TRT. It is important to emphasize that TRT should not replace healthy lifestyle changes including regular exercise, weight loss, and diet modifications, which may provide the patient symptom resolution. Thoughtful dialogue with the patient is critical prior to TRT initiation, including thorough disclosure of the risks and benefits of treatment, and the limitations of the data as it evolves.
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002; 87:589–598.
- Araujo AB, O’Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004; 89:5920–5926.
- Travison TG, Shackelton R, Araujo AB, et al. The natural history of symptomatic androgen deficiency in men: onset, progression, and spontaneous remission. J Am Geriatr Soc 2008; 56:831–839.
- Tajar A, Forti G, O’Neill TW, et al; EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010; 95:1810–1818.
- Hammes A, Andreassen TK, Spoelgen R, et al. Role of endocytosis in cellular uptake of sex steroids. Cell 2005; 122:751–762.
- Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol 2010; 316:79–85.
- Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95:2536–2559.
- Wang C, Nieschlag E, Swerdloff R, et al; International Society of Andrology (ISA). Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl 2009; 30:1–9.
- Morley JE, Patrick P, Perry HM. Evaluation of assays available to measure free testosterone. Metabolism 2002; 51:554–559.
- Vesper HW, Bhasin S, Wang C, et al. Interlaboratory comparison study of serum total testosterone [corrected] measurements performed by mass spectrometry methods. Steroids 2009; 74:498–503.
- Ly LP, Sartorius G, Hull L, et al. Accuracy of calculated free testosterone formulae in men. Clin Endocrinol (Oxf) 2010; 73:382–388.
- Melmed S, Casanueva FF, Hoffman AR, et al; Endocrine Society. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:273–288.
- Wu FC, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363:123–135.
- Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab 2004; 89:3813–3817.
- Joshi D, van Schoor NM, de Ronde W, et al. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin Endocrinol (Oxf) 2010; 72:232–240.
- Shores MM, Kivlahan DR, Sadak TI, Li EJ, Matsumoto AM. A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression). J Clin Psychiatry 2009; 70:1009–1016.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the Testim Registry in the US (TRiUS). Aging Male 2012; 15:14–21.
- Jain P, Rademaker AW, McVary KT. Testosterone supplementation for erectile dysfunction: results of a meta-analysis. J Urol 2000; 164:371–375.
- Mulhall JP, Valenzuela R, Aviv N, Parker M. Effect of testosterone supplementation on sexual function in hypogonadal men with erectile dysfunction. Urology 2004; 63:348–352.
- Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000; 49:1239–1242.
- Moore C, Huebler D, Zimmermann T, Heinemann LA, Saad F, Thai DM. The Aging Males’ Symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol 2004; 46:80–87.
- Chueh KS, Huang SP, Lee YC, et al. The Comparison of the Aging Male Symptoms (AMS) Scale and Androgen Deficiency in the Aging Male (ADAM) Questionnaire to Detect Androgen Deficiency in Middle-Aged Men. J Androl 2012[Epub ahead of print]
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006; 60:762–769.
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33:1186–1192.
- Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab 2010; 95:2790–2799.
- Carrero JJ, Qureshi AR, Nakashima A, et al. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant 2011; 26:184–190.
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008; 93:1834–1840.
- Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol 2011; 40:189–207.
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006; 295:1288–1299.
- Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 2004; 6:208–215.
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med 2006; 166:1660–1665.
- Carrero JJ, Qureshi AR, Parini P, et al. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol 2009; 20:613–620.
- Haring R, Völzke H, Steveling A, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J 2010; 31:1494–1501.
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96:3007–3019.
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 2004; 350:482–492.
- Isbarn H, Pinthus JH, Marks LS, et al. Testosterone and prostate cancer: revisiting old paradigms. Eur Urol 2009; 56:48–56.
- Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med 2011; 124:578–587.
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med 2009; 6:3177–3192.
- Gerstenbluth RE, Maniam PN, Corty EW, Seftel AD. Prostate-specific antigen changes in hypogonadal men treated with testosterone replacement. J Androl 2002; 23:922–926.
- Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med 2011; 8:272–283.
- Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol 2011; 165:687–701.
- Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006; 154:899–906.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010; 7:3495–3503.
- Rhoden EL, Morgentaler A. Symptomatic response rates to testosterone therapy and the likelihood of completing 12 months of therapy in clinical practice. J Sex Med 2010; 7:277–283.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. Improved sexual function with testosterone replacement therapy in hypogonadal men: real-world data from the Testim Registry in the United States (TRiUS). J Sex Med 2011; 8:3204–3213.
- Buvat J, Montorsi F, Maggi M, et al. Hypogonadal men nonresponders to the PDE5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (TADTEST study). J Sex Med 2011; 8:284–293.
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med 2010; 363:109–122.
- Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 1999; 84:3469–3478.
- Wang C, Swerdloff RS, Iranmanesh A, et al; Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000; 85:2839–2853.
- Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? J Sex Med 2007; 4:1241–1246.
- Coward RM, Simhan J, Carson CC. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int 2009; 103:1179–1183.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 2009; 55:310–320.
- Page ST, Lin DW, Mostaghel EA, et al. Dihydrotestosterone administration does not increase intraprostatic androgen concentrations or alter prostate androgen action in healthy men: a randomized-controlled trial. J Clin Endocrinol Metab 2011; 96:430–437.
- Botto H, Neuzillet Y, Lebret T, Camparo P, Molinie V, Raynaud JP. High incidence of predominant Gleason pattern 4 localized prostate cancer is associated with low serum testosterone. J Urol 2011; 186:1400–1405.
- Salonia A, Gallina A, Briganti A, et al. Preoperative hypogonadism is not an independent predictor of high-risk disease in patients undergoing radical prostatectomy. Cancer 2011; 117:3953–3962.
- Sarosdy MF. Testosterone replacement for hypogonadism after treatment of early prostate cancer with brachytherapy. Cancer 2007; 109:536–541.
- Khera M. Androgens and erectile function: a case for early androgen use in postprostatectomy hypogonadal men. J Sex Med 2009; 6:(suppl 3):234–238.
- Szmulewitz R, Mohile S, Posadas E, et al. A randomized phase 1 study of testosterone replacement for patients with low-risk castration-resistant prostate cancer. Eur Urol 2009; 56:97–103.
- Morgentaler A, Lipshultz LI, Bennett R, Sweeney M, Avila D, Khera M. Testosterone therapy in men with untreated prostate cancer. J Urol 2011; 185:1256–1260.
Editor’s note: This is the second of two articles on hypogonadism in men and focuses on the appropriate use of testosterone therapy. The first article, published last month, focused in more detail on the differential diagnosis of hypogonadism.
As men age, testosterone production gradually decreases. In our increasingly aged population, clinicians will continue to see an increase in the number of men with seemingly nonspecific symptoms of aging that are possibly due to low serum testosterone (eg, low energy level, depressive symptoms, erectile dysfunction, decreased libido). These clinical symptoms, coupled with low serum testosterone, may adversely affect quality of life and life expectancy. Testosterone replacement therapy (TRT) may improve symptoms and quality of life. Given the nonspecific nature of these symptoms, accurate diagnosis and treatment of clinically significant low testosterone with a goal of symptom and quality of life improvement can prove challenging.
These challenges in diagnosis and treatment result in a lack of standardized nomenclature. The terms male menopause and andropause, although popular, are the least helpful, as they have few correlates with the better-defined female menopause. Late-onset hypogonadism implies a well-defined, later age of decline, which is inaccurate since the decline in serum testosterone in men begins in middle age and is gradual. Testosterone deficiency syndrome implies a set of specific and well-defined symptoms. Androgen deficiency in the aging male (ADAM) and Androgen deficiency in the older male are common terms specifying an age cohort (> 40 years old) and an abnormal laboratory value without mention of symptoms. While all these terms have their limitations, we will primarily use ADAM in this discussion.
PREVALENCE OF LOW TESTOSTERONE
Serum testosterone levels begin to decline in men in their mid-40s, with an approximately 1% to 2% decline annually and a marked decline after age 60.1
Araujo and colleagues2 studied the prevalence of androgen-deficient men, with androgen deficiency defined as at least three signs or symptoms and either a total testosterone less than 200 ng/dL or a total testosterone 200 ng/dL to 400 ng/dL with a free testosterone less than 8.91 ng/dL. The overall prevalence of low testosterone on initial measurement was 6%, which doubled to 12% with repeat measurement.
Serial measures are important: one study that followed untreated men over 15 years found normal testosterone on serial measures in 50%.3 In a multicenter cross-sectional study, 11.8% of men had low testosterone and low or normal luteinizing hormone (LH) levels (secondary hypogonadism/hypothalamic-pituitary failure), with 2% of patients with low testosterone and elevated LH (primary hypogonadism/testicular failure).4
CLINICAL PRESENTATION AND DIAGNOSIS
A biochemical diagnosis of low testosterone is dependent on accurate measurement. Testosterone release is diurnal, with the highest levels in the early morning, and often has week-to-week variability. Thus, it is important to collect blood in the early morning and to confirm a diagnosis of low testosterone with at least one repeat measurement several days later, including LH assessment. LH levels will help differentiate primary hypogonadism from secondary hypogonadism, which may alter diagnosis and treatment in certain patients, with secondary hypogonadism associated with pituitary dysfunction, and primary hypogonadism associated with aging.4
Testosterone binds in the bloodstream to sex hormone-binding globulin (SHBG), and this bound form is generally considered biologically inactive, although there are in vitro and animal studies suggesting SHBG-bound androgen may indeed have biological activity. 5,6 “Bioavailable” testosterone is active and includes both free testosterone and testosterone bound to albumin.
There is no general agreement on the acceptable normal range of testosterone, with variability within the literature and between laboratories. “Normal” total testosterone levels have ranged from more than 280 ng/dL to more than 350 ng/dL (12 nmol/L).7,8 Similarly, there is no generally accepted lower limit of normal, although some studies report a threshold level of testosterone less than 230 ng/dL (8 nmol/L) as “abnormal.” Values between these two upper and lower limits are considered “borderline.”7,8 These intermediate or borderline values coupled with clinical symptoms of testosterone deficiency syndrome or ADAM should be considered abnormal.
When total testosterone is borderline, measurement of free or bioavailable testosterone (free plus albumin-bound) should be considered. Total testosterone is typically measured using automated immunoassay platforms, with method-related differences leading to significant variability in measurement accuracy and precision. This variability is seen most dramatically in those with low total testosterone.9 However, the variability of total testosterone measurements is substantially smaller among mass spectrometry assays than among immunoassays. 10
The gold standards for free testosterone measurement are centrifugal ultrafiltration and equilibrium dialysis.9 However, these techniques are laborious and usually unavailable in local laboratories. Calculated free testosterone values using total testosterone and SHBG are most commonly used and are sufficiently accurate for clinical practice.11
Free testosterone levels can be diagnostic when total testosterone levels do not correspond with clinical presentation. However, the clinical utility of free testosterone is difficult to assess due to the variability among laboratory assays and a lack of consensus on threshold parameters. A threshold free testosterone level of more than 225 pmol/L (65 pg/mL) is generally considered normal.7,8 Before starting a patient on TRT, measurement of hemoglobin and prostate-specific antigen (PSA) and digital rectal examination of the prostate (if age is > 39) are essential.
Prolactin levels are recommended when low testosterone is confirmed, especially in patients at high clinical risk for hyperprolactinemia. Once hyperprolactinemia is identified, Endocrine Society guidelines recommend excluding medication use, renal failure, hypothyroidism, and parasellar tumors as possible causes of elevated prolactin levels.12
Low testosterone values should be treated only in patients with clinically significant symptoms that are likely to be caused by the low testosterone itself. Symptoms associated with age-related decline in testosterone that may improve with TRT include low libido,13,14 low energy,14 depressed mood,15–17 low muscle mass, osteoporosis, and hot flashes. Men with erectile dysfunction have also shown a significant improvement with TRT compared with placebo, but with a variable overall response independent of normalization of testosterone. 18,19 This is likely due to the multifactorial nature of erectile dysfunction, including vascular, neurologic, psychogenic, and endocrinologic causes.
Screening questionnaires have been developed for symptoms of low testosterone, but their clinical utility is unclear. The ADAM questionnaire is used as a screening tool for low testosterone but not to monitor response to TRT, and it is highly nonspecific.20 The Aging Male Symptom Scale questionnaire includes psychological, somatovegetative, and sexual components and is used both to screen for low testosterone and to measure outcomes.21 However, a recent observational study comparing the ability of these questionnaires to assess clinical symptoms revealed a low sensitivity and a low specificity to detect androgen deficiency in men with a total testosterone level less than 300 ng/dL.22 Overall, the current data do not conclusively support the use of hypogonadism questionnaires for screening.
The patient history when evaluating for ADAM should include evaluation of sexual and constitutional symptoms as described above and in Table 1. In addition, a history of traumatic, medical, or surgical events that could affect testosterone production should be obtained, including cryptorchidism, scrotal, inguinal, or abdominal surgery, pituitary surgery or radiation, prior issues with infertility, timing of puberty, history of renal or hepatic failure, chemotherapy (for cancer or autoimmune diseases), and prior use of anabolic steroids or opiates.
A complete physical examination should include assessment of virilization, gynecomastia, and the genitalia, including the size, position, and volume of the testes. The size and consistency of the prostate should be assessed on digital rectal examination.
LOW TESTOSTERONE AND ASSOCIATED COMORBIDITIES
Low testosterone is associated with many comorbidities, including metabolic syndrome, depression, type 2 diabetes mellitus, and cardiovascular disease, as discussed later in this section. Low testosterone has also shown associations with osteoporosis, cognitive impairment, hypertension, hyperlipidemia, decreased physical performance, end-stage renal disease, and treatment with steroids or opiates.23–26 However, the studies that found these associations included men younger than 40 years and may not be fully applicable to the ADAM population.
The association of metabolic syndrome and type 2 diabetes mellitus with low testosterone is well established in multiple studies. Grossman and colleagues27 investigated the association of type 2 diabetes mellitus and low testosterone, with low total testosterone defined as below 10 nmol/L and low calculated free testosterone less than 0.23 nmol/L. The prevalence of low total testosterone was 43%, and the prevalence of low free testosterone was 57%. In addition, a recent meta-analysis comparing total testosterone of men with and without metabolic syndrome revealed an association between a baseline decrease in mean total and free testosterone levels in men with metabolic syndrome compared with controls. This study found a total testosterone mean difference of –2.64 nmol/L (95% confidence interval [CI] –2.95 to –2.32) and a free testosterone mean difference of –0.26 pmol/L (95% CI –0.39 to –0.13), respectively, when comparing men with metabolic syndrome against those without.28
Testosterone has also been suggested to be protective against type 2 diabetes mellitus, with 42% lower risk of type 2 diabetes mellitus in men with testosterone levels ranging from 450 ng/dL to 605 ng/dL.29
Obesity has been specifically linked with secondary hypogonadism.4,23,24 A prospective cohort of 58 men with an average age of 46 years and a body mass index ranging from 30 to 45 kg/m2 were monitored on a low-calorie diet for 9 weeks. Afterward, biochemical analysis revealed an increase in free testosterone from 185 pmol/L ± 66 to 208 ± 70 pmol/L (P = .002) with a mean weight loss of 16.3 kg ± 4.5 kg.30 This emphasizes the importance of lifestyle changes in the management of hypogonadal men.
LOW TESTOSTERONE AND THE OVERALL MORTALITY RATE
Low testosterone is associated unfavorably with the rate of all-cause mortality. A retrospective study in male veterans over age 40 with repeated testosterone levels over a 5-year period found that the risk of death from all causes in men with normal testosterone (> 250 ng/dL or free testosterone > 0.75 ng/dL) was 20% (95% CI 16.2%–241%) vs 35% (95% CI 28.5%–41.4%) in men with low testosterone (< 250 ng/dL or free testosterone < 0.75 ng/dL). In multivariate analysis, men with testosterone less than 250 ng/dL (< 8.7 nmol/L) or free testosterone less than 0.75 ng/dL (< 0.03 nmol/L) had up to an 88% higher death rate than men with normal testosterone levels.31
Low testosterone has also been associated with other end-organ, disease-specific mortality. In men with end-stage renal disease, low testosterone was an independent predictor of death from all causes and from cardiovascular disease.32 A prospective European health study revealed an association between low testosterone and increased risk of death from cardiovascular disease and cancer.33 A recent meta-analysis of population-based studies confirmed this association, despite significant interstudy heterogeneity. 34 Although multiple studies show an independent association of low testosterone and increased mortality rate, causality remains unconfirmed. This may be difficult to prove, given the available study designs and the nonspecific nature of symptoms related to low testosterone and potentially associated comorbidities.
TRT: INDICATIONS AND CONTRAINDICATIONS
The indications, benefits, and risks of TRT are controversial, with current data lacking long-term follow-up and consistent biochemical target values. Treatment of low testosterone is not indicated at the present time in the absence of clinical symptoms.
According to recently published guidelines, TRT is recommended for symptomatic men with low or borderline total testosterone or free testosterone (< 350 ng/dL or < 65 pg/mL).7,8 Patients with borderline biochemical values (total testosterone 200–350 ng/dL, free testosterone 40–65 pg/mL) and possible related symptoms should be treated with TRT for at least 3 months and then reevaluated to verify improved testosterone levels and to assess for symptom amelioration or resolution.35 Dose escalation is recommended in patients with subtherapeutic testosterone levels and limited clinical improvement after 3 months of treatment.
Target maintenance testosterone levels have not been defined, with mid to lower young adult male serum testosterone levels recommended at this time.8 Given that the current literature does not specify a target testosterone replacement range, we recommend monitoring the clinical response along with total testosterone to decide adjustments in TRT. Ultimately, treatment goals of TRT should be the resolution of signs and symptoms, including improvement of sexual function, libido, and preservation of bone mineral density.7,8
Contraindications
TRT is not recommended in men with the following:
- Breast cancer
- Polycythemia (hematocrit > 50%)
- Untreated obstructive sleep apnea
- Lower urinary tract symptoms caused by an enlarged prostate; International Prostate Symptom Score > 19
- Poorly controlled heart failure
- Desire for fertility.
The role of TRT in prostate cancer remains controversial (see below) and remains contraindicated in recent Endocrine Society clinical practice guidelines.7 Guidelines recommend urologic consultation prior to initiation of TRT in patients at increased risk of prostate cancer,7 based on age, race, family history, PSA, PSA velocity, and history of prostate biopsy.
One prominent historic concern about androgen replacement therapy regards the potential for de novo development of prostate cancer. Numerous studies have failed to find elevated risk of new diagnosis, progression, or recurrence of prostate cancer in patients on TRT.36,37 Nevertheless, patients who develop elevated PSA, increased PSA velocity, or an abnormal digital rectal examination while on TRT should undergo prostate biopsy.
TRT FORMULATIONS AND TREATMENT OPTIONS
A number of effective formulations of TRT are available (Table 2). Transdermal and parenteral formulations are most commonly used. Enteric testosterone formulations are not available in the United States and are associated with hepatotoxicity. While buccal testosterone therapy is available, it often leads to local gingival irritation and has not gained widespread popularity.
Parenteral TRT can be administered intramuscularly (IM) or subcutaneously (SQ). Testosterone cypionate (Depo-Testosterone) is the only IM form available in the United States and is given every 2 to 3 weeks. It is the least expensive form of TRT, but it requires frequent administration (by either the clinical practitioner or the patient himself). Testosterone cypionate injections lead to markedly wide swings of testosterone levels, ranging from supraphysiologic levels for a few days after administration to hypogonadal levels before the next injection. This may be mitigated by more-frequent injections. The longer-acting form testosterone undecanoate is available outside the United States and is given every 12 weeks when stable levels are reached.
The other parenteral option is SQ slow-release pellets (Testopel). These pellets have 75 mg of testosterone. Typically 8 to 14 pellets are placed subcutaneously in the buttock area, which will provide coverage for 3 to 6 months.38 The insertion procedure is simple with a short learning curve, limited compliance issues, and elimination of risk of transdermal transmission of drug to others. Disadvantages include wound infection and pellet extrusion, seen in 0.3% to 12% of patients in various studies.38
Another route of TRT is transdermal, including patches, liquids, and gels. Patches are applied daily and are rotated to different sites with minimal risk for skin transmission to others, although use may be limited by site dermatitis. Three hydro-alcoholic gel formulations are currently available in the United States: Androgel (1% or 1.62%), which is applied to the chest or the shoulders; Testim 1%, which is applied to the shoulders; and Fortesta (2%), which is applied to the thighs. A liquid preparation, Axiron, is applied to the axillae. Because secondary transfer to women and children is possible, it is important to thoroughly wash hands after application and to cover the treated skin with clothing. In 3 to 4 hours, all the medication is absorbed, and the area should then be washed before direct skin contact with others (Table 2).
MONITORING PATIENTS ON TRT
Patients starting TRT will require clinical and biochemical monitoring to evaluate response to therapy as well as possible side effects. The first set of laboratory values should be obtained 6 to 12 weeks after initiation of therapy and then typically quarterly for 1 year, every 6 months for the second year, and annually thereafter. Laboratory values monitored should include total testosterone, PSA, and hematocrit.
Men on daily therapy (patch, gel, liquid) should have testosterone drawn approximately 2 hours after application. Current TRT regimen data lack an appropriate target testosterone value, and guidelines suggest a mid to lower young adult male testosterone level.8 Since this is not clearly delineated in the current literature, the authors recommend monitoring clinical symptoms along with testosterone levels when adjusting TRT. It is important to document that serum testosterone was actually increased to the normal range in treated men without clinical improvement.
A rise in PSA of up to 24% would be an acceptable response in a benign prostate gland, but a higher increase or increase above 4.0 ng/dL should prompt consideration of prostate biopsy. 39 Similarly, hemoglobin and hematocrit typically increase, but a hematocrit greater than 55% should prompt dose reduction or cessation.7 Transaminases do not need routine monitoring during parenteral or transdermal therapy. Bone mineral density should be monitored every 1 to 2 years.7,8
CLINICAL BENEFITS OF TRT
There are promising data regarding the clinical benefits of TRT in patients with metabolic syndrome and type 2 diabetes mellitus. A recent meta-analysis investigating the effect of TRT on metabolic syndrome revealed an improvement in fasting plasma glucose, homeostatic model assessment index, triglycerides, treadmill duration, high-density lipoprotein cholesterol, and waist circumference.40,41 TRT also decreased insulin resistance and improved glycemic control in type 2 diabetic hypogonadal men.42 Results from a randomized controlled trial comparing 12 weeks of intramuscular testosterone treatment vs placebo in men with metabolic syndrome revealed an improvement in mean waist circumference from 108 cm ± 8 cm to 105.5 cm ± 7.7 cm. Sixty percent of men initially diagnosed with metabolic syndrome and treated with testosterone no longer met diagnostic criteria for metabolic syndrome according to the National Cholesterol Education Program–Third Adult Treatment Panel (NCEP-ATP III) and the International Diabetes Federation (IDF) guidelines.43
Depression has also been associated with low testosterone, with free testosterone levels below 170 pmol/L associated with frank depressive symptoms and levels below 220 pmol/L predictive of future onset of depressive symptoms.15 Testosterone replacement therapy has been shown to improve depressive symptoms in hypogonadal men.16,17 Shores et al16 conducted a randomized placebo-controlled study of testosterone replacement in men older than 50 years with dysthymia or minor depression. Men treated with testosterone gel for 12 weeks showed an improvement of baseline total testosterone levels from 291 ng/dL to 449 ng/dL. Men treated with testosterone also had a 53% rate of depression remission compared with 19% in the placebo group.16
The evidence supporting improved sexual function with TRT is variable. Some studies indicate limited or transient improvement of sexual function after TRT in men with erectile dysfunction,18,19 while others report an improvement in sexual function after 3 months of TRT.44 Because of the multifactorial nature of erectile dysfunction, men with erectile dysfunction and ADAM may require TRT and a phosphodiesterase type 5 (PDE5) inhibitor, as TRT alone may be insufficient. In a prospective observational study of men with erectile dysfunction and an initial testosterone lower than 300 ng/dL, testosterone gel was administered for at least 1 year, and improvement in sexual function was seen. Results revealed a correlation between improvement in sexual function and concurrent therapy with a PDE5 inhibitor.45 In a recent multicenter placebo-controlled study of PDE5 inhibitor nonresponders, the addition of a testosterone gel to tadalafil (Cialis) improved sexual function, again suggesting a synergistic effect when treating erectile dysfunction with both TRT and a PDE5 inhibitor.46
ADVERSE EVENTS RELATED TO TRT
Despite the aforementioned benefits, it must be emphasized that TRT should be used for specific target symptoms related to hypogonadism in older men and that the general health benefits and safety of TRT in an asymptomatic man with a low measured testosterone alone remains unproven.
Cardiovascular events. In a recent study of 209 elderly men with low testosterone and limited mobility associated with other chronic illnesses, 6 months of TRT resulted in the development of cardiovascular-related adverse events in 23 patients compared with 5 men in the placebo group.47 This may have been related to how adverse events were reported, with cumulative adverse events reviewed every 6 months, ranging from peripheral edema, hypertension, arrhythmias, and electrocardiographic changes. Serious adverse events were reviewed as they occurred, including stroke and acute myocardial events.
Other studies41,43 have revealed a favorable effect of TRT on cardiovascular disease and its surrogate markers but have lacked detailed reports and close monitoring of adverse events. Thus, variation of outcome measurement and reporting may obfuscate the detection of adverse cardiovascular events. Outcomes may also depend on the testosterone formulation and the target serum concentration.43
Larger, long-term placebo-controlled trials are needed to elucidate cardiovascular risk as a primary outcome in older androgen-deficient men undergoing TRT.
Other adverse effects related to TRT include erythrocytosis, seen in 3% to 18% of patients with transdermal administration,48,49 and up to 44% of patients undergoing IM therapy.48 Gynecomastia can occur and is more likely to resolve after treatment cessation of transdermal testosterone treatment than IM injections.48 Other potential clinical side effects that should prompt dose-reduction or discontinuation are irritability, bothersome acne, fluid retention, testicular atrophy, worsening of lower urinary tract symptoms from an enlarged prostate, and new or worsening heart failure. Infrequently, obstructive sleep apnea may be worsened by TRT, although currently the data linking sleep apnea and TRT are limited.50
TRT AND PROSTATE CANCER
The relationship between prostate cancer growth and testosterone is well established, with androgen ablation remaining the cornerstone of treatment for metastatic disease. Since androgen deprivation leads to the regression of prostate cancer, there has been concern that TRT may lead to growth or de novo development of prostate cancer. TRT has thus been strongly prohibited in patients with prostate cancer.7 However, recent data challenge this paradigm.
In a retrospective study of 81 men (mean age 56.8 years) treated with TRT, only 4 men (4.9%) developed prostate cancer over a 5-year period.51 This is less than the estimated 16.7% probability of developing prostate cancer in the general US population.52
Recent accumulating data support the concept of testosterone reaching a saturation level when binding androgen receptors within the prostate at extremely low levels. Increases above this level with TRT as with ADAM do not increase the risk of development or progression of prostate cancer.53 In addition, large doses of dihydrotestosterone do not seem to alter PSA, prostate volume, or International Prostate Symptom Score.54 These findings may have implications in future androgen therapies in hypogonadal older men.
Pathologic studies suggest low testosterone is associated with a higher Gleason grade of prostate cancer,55 although this association remains unconfirmed.56
In men with erectile dysfunction after prostate cancer treatment, TRT appears safe after brachytherapy57 or radical prostatectomy.58 A small study of 15 hypogonadal men with castrate-resistant prostate cancer and minimal or no metastatic disease showed only 1 patient had symptomatic progression.59 Moreover, a recent small study of 13 men with known prostate cancer on active surveillance showed that TRT did not lead to local progression or metastatic disease in any of the patients.60
While these data are provocative, it should still be emphasized that the standard of care for prostate cancer screening should be followed in age-appropriate men with ADAM. In addition, hypogonadal men with prostate cancer should only be treated with testosterone in conjunction with careful counseling and ongoing monitoring.
TRT SHOULD NOT REPLACE HEALTHY LIFESTYLE CHANGES
There has been a dramatic increase in TRT initiation for nonspecific symptoms of low testosterone in older androgen-deficient men. With this increase in initiation of TRT, there is a significant risk of overtreating. While there are many encouraging associations between treatment of androgen deficiency and improvement in rates of of morbidity and mortality, much remains unknown about the overall long-term risks and benefits of TRT. It is important to emphasize that TRT should not replace healthy lifestyle changes including regular exercise, weight loss, and diet modifications, which may provide the patient symptom resolution. Thoughtful dialogue with the patient is critical prior to TRT initiation, including thorough disclosure of the risks and benefits of treatment, and the limitations of the data as it evolves.
Editor’s note: This is the second of two articles on hypogonadism in men and focuses on the appropriate use of testosterone therapy. The first article, published last month, focused in more detail on the differential diagnosis of hypogonadism.
As men age, testosterone production gradually decreases. In our increasingly aged population, clinicians will continue to see an increase in the number of men with seemingly nonspecific symptoms of aging that are possibly due to low serum testosterone (eg, low energy level, depressive symptoms, erectile dysfunction, decreased libido). These clinical symptoms, coupled with low serum testosterone, may adversely affect quality of life and life expectancy. Testosterone replacement therapy (TRT) may improve symptoms and quality of life. Given the nonspecific nature of these symptoms, accurate diagnosis and treatment of clinically significant low testosterone with a goal of symptom and quality of life improvement can prove challenging.
These challenges in diagnosis and treatment result in a lack of standardized nomenclature. The terms male menopause and andropause, although popular, are the least helpful, as they have few correlates with the better-defined female menopause. Late-onset hypogonadism implies a well-defined, later age of decline, which is inaccurate since the decline in serum testosterone in men begins in middle age and is gradual. Testosterone deficiency syndrome implies a set of specific and well-defined symptoms. Androgen deficiency in the aging male (ADAM) and Androgen deficiency in the older male are common terms specifying an age cohort (> 40 years old) and an abnormal laboratory value without mention of symptoms. While all these terms have their limitations, we will primarily use ADAM in this discussion.
PREVALENCE OF LOW TESTOSTERONE
Serum testosterone levels begin to decline in men in their mid-40s, with an approximately 1% to 2% decline annually and a marked decline after age 60.1
Araujo and colleagues2 studied the prevalence of androgen-deficient men, with androgen deficiency defined as at least three signs or symptoms and either a total testosterone less than 200 ng/dL or a total testosterone 200 ng/dL to 400 ng/dL with a free testosterone less than 8.91 ng/dL. The overall prevalence of low testosterone on initial measurement was 6%, which doubled to 12% with repeat measurement.
Serial measures are important: one study that followed untreated men over 15 years found normal testosterone on serial measures in 50%.3 In a multicenter cross-sectional study, 11.8% of men had low testosterone and low or normal luteinizing hormone (LH) levels (secondary hypogonadism/hypothalamic-pituitary failure), with 2% of patients with low testosterone and elevated LH (primary hypogonadism/testicular failure).4
CLINICAL PRESENTATION AND DIAGNOSIS
A biochemical diagnosis of low testosterone is dependent on accurate measurement. Testosterone release is diurnal, with the highest levels in the early morning, and often has week-to-week variability. Thus, it is important to collect blood in the early morning and to confirm a diagnosis of low testosterone with at least one repeat measurement several days later, including LH assessment. LH levels will help differentiate primary hypogonadism from secondary hypogonadism, which may alter diagnosis and treatment in certain patients, with secondary hypogonadism associated with pituitary dysfunction, and primary hypogonadism associated with aging.4
Testosterone binds in the bloodstream to sex hormone-binding globulin (SHBG), and this bound form is generally considered biologically inactive, although there are in vitro and animal studies suggesting SHBG-bound androgen may indeed have biological activity. 5,6 “Bioavailable” testosterone is active and includes both free testosterone and testosterone bound to albumin.
There is no general agreement on the acceptable normal range of testosterone, with variability within the literature and between laboratories. “Normal” total testosterone levels have ranged from more than 280 ng/dL to more than 350 ng/dL (12 nmol/L).7,8 Similarly, there is no generally accepted lower limit of normal, although some studies report a threshold level of testosterone less than 230 ng/dL (8 nmol/L) as “abnormal.” Values between these two upper and lower limits are considered “borderline.”7,8 These intermediate or borderline values coupled with clinical symptoms of testosterone deficiency syndrome or ADAM should be considered abnormal.
When total testosterone is borderline, measurement of free or bioavailable testosterone (free plus albumin-bound) should be considered. Total testosterone is typically measured using automated immunoassay platforms, with method-related differences leading to significant variability in measurement accuracy and precision. This variability is seen most dramatically in those with low total testosterone.9 However, the variability of total testosterone measurements is substantially smaller among mass spectrometry assays than among immunoassays. 10
The gold standards for free testosterone measurement are centrifugal ultrafiltration and equilibrium dialysis.9 However, these techniques are laborious and usually unavailable in local laboratories. Calculated free testosterone values using total testosterone and SHBG are most commonly used and are sufficiently accurate for clinical practice.11
Free testosterone levels can be diagnostic when total testosterone levels do not correspond with clinical presentation. However, the clinical utility of free testosterone is difficult to assess due to the variability among laboratory assays and a lack of consensus on threshold parameters. A threshold free testosterone level of more than 225 pmol/L (65 pg/mL) is generally considered normal.7,8 Before starting a patient on TRT, measurement of hemoglobin and prostate-specific antigen (PSA) and digital rectal examination of the prostate (if age is > 39) are essential.
Prolactin levels are recommended when low testosterone is confirmed, especially in patients at high clinical risk for hyperprolactinemia. Once hyperprolactinemia is identified, Endocrine Society guidelines recommend excluding medication use, renal failure, hypothyroidism, and parasellar tumors as possible causes of elevated prolactin levels.12
Low testosterone values should be treated only in patients with clinically significant symptoms that are likely to be caused by the low testosterone itself. Symptoms associated with age-related decline in testosterone that may improve with TRT include low libido,13,14 low energy,14 depressed mood,15–17 low muscle mass, osteoporosis, and hot flashes. Men with erectile dysfunction have also shown a significant improvement with TRT compared with placebo, but with a variable overall response independent of normalization of testosterone. 18,19 This is likely due to the multifactorial nature of erectile dysfunction, including vascular, neurologic, psychogenic, and endocrinologic causes.
Screening questionnaires have been developed for symptoms of low testosterone, but their clinical utility is unclear. The ADAM questionnaire is used as a screening tool for low testosterone but not to monitor response to TRT, and it is highly nonspecific.20 The Aging Male Symptom Scale questionnaire includes psychological, somatovegetative, and sexual components and is used both to screen for low testosterone and to measure outcomes.21 However, a recent observational study comparing the ability of these questionnaires to assess clinical symptoms revealed a low sensitivity and a low specificity to detect androgen deficiency in men with a total testosterone level less than 300 ng/dL.22 Overall, the current data do not conclusively support the use of hypogonadism questionnaires for screening.
The patient history when evaluating for ADAM should include evaluation of sexual and constitutional symptoms as described above and in Table 1. In addition, a history of traumatic, medical, or surgical events that could affect testosterone production should be obtained, including cryptorchidism, scrotal, inguinal, or abdominal surgery, pituitary surgery or radiation, prior issues with infertility, timing of puberty, history of renal or hepatic failure, chemotherapy (for cancer or autoimmune diseases), and prior use of anabolic steroids or opiates.
A complete physical examination should include assessment of virilization, gynecomastia, and the genitalia, including the size, position, and volume of the testes. The size and consistency of the prostate should be assessed on digital rectal examination.
LOW TESTOSTERONE AND ASSOCIATED COMORBIDITIES
Low testosterone is associated with many comorbidities, including metabolic syndrome, depression, type 2 diabetes mellitus, and cardiovascular disease, as discussed later in this section. Low testosterone has also shown associations with osteoporosis, cognitive impairment, hypertension, hyperlipidemia, decreased physical performance, end-stage renal disease, and treatment with steroids or opiates.23–26 However, the studies that found these associations included men younger than 40 years and may not be fully applicable to the ADAM population.
The association of metabolic syndrome and type 2 diabetes mellitus with low testosterone is well established in multiple studies. Grossman and colleagues27 investigated the association of type 2 diabetes mellitus and low testosterone, with low total testosterone defined as below 10 nmol/L and low calculated free testosterone less than 0.23 nmol/L. The prevalence of low total testosterone was 43%, and the prevalence of low free testosterone was 57%. In addition, a recent meta-analysis comparing total testosterone of men with and without metabolic syndrome revealed an association between a baseline decrease in mean total and free testosterone levels in men with metabolic syndrome compared with controls. This study found a total testosterone mean difference of –2.64 nmol/L (95% confidence interval [CI] –2.95 to –2.32) and a free testosterone mean difference of –0.26 pmol/L (95% CI –0.39 to –0.13), respectively, when comparing men with metabolic syndrome against those without.28
Testosterone has also been suggested to be protective against type 2 diabetes mellitus, with 42% lower risk of type 2 diabetes mellitus in men with testosterone levels ranging from 450 ng/dL to 605 ng/dL.29
Obesity has been specifically linked with secondary hypogonadism.4,23,24 A prospective cohort of 58 men with an average age of 46 years and a body mass index ranging from 30 to 45 kg/m2 were monitored on a low-calorie diet for 9 weeks. Afterward, biochemical analysis revealed an increase in free testosterone from 185 pmol/L ± 66 to 208 ± 70 pmol/L (P = .002) with a mean weight loss of 16.3 kg ± 4.5 kg.30 This emphasizes the importance of lifestyle changes in the management of hypogonadal men.
LOW TESTOSTERONE AND THE OVERALL MORTALITY RATE
Low testosterone is associated unfavorably with the rate of all-cause mortality. A retrospective study in male veterans over age 40 with repeated testosterone levels over a 5-year period found that the risk of death from all causes in men with normal testosterone (> 250 ng/dL or free testosterone > 0.75 ng/dL) was 20% (95% CI 16.2%–241%) vs 35% (95% CI 28.5%–41.4%) in men with low testosterone (< 250 ng/dL or free testosterone < 0.75 ng/dL). In multivariate analysis, men with testosterone less than 250 ng/dL (< 8.7 nmol/L) or free testosterone less than 0.75 ng/dL (< 0.03 nmol/L) had up to an 88% higher death rate than men with normal testosterone levels.31
Low testosterone has also been associated with other end-organ, disease-specific mortality. In men with end-stage renal disease, low testosterone was an independent predictor of death from all causes and from cardiovascular disease.32 A prospective European health study revealed an association between low testosterone and increased risk of death from cardiovascular disease and cancer.33 A recent meta-analysis of population-based studies confirmed this association, despite significant interstudy heterogeneity. 34 Although multiple studies show an independent association of low testosterone and increased mortality rate, causality remains unconfirmed. This may be difficult to prove, given the available study designs and the nonspecific nature of symptoms related to low testosterone and potentially associated comorbidities.
TRT: INDICATIONS AND CONTRAINDICATIONS
The indications, benefits, and risks of TRT are controversial, with current data lacking long-term follow-up and consistent biochemical target values. Treatment of low testosterone is not indicated at the present time in the absence of clinical symptoms.
According to recently published guidelines, TRT is recommended for symptomatic men with low or borderline total testosterone or free testosterone (< 350 ng/dL or < 65 pg/mL).7,8 Patients with borderline biochemical values (total testosterone 200–350 ng/dL, free testosterone 40–65 pg/mL) and possible related symptoms should be treated with TRT for at least 3 months and then reevaluated to verify improved testosterone levels and to assess for symptom amelioration or resolution.35 Dose escalation is recommended in patients with subtherapeutic testosterone levels and limited clinical improvement after 3 months of treatment.
Target maintenance testosterone levels have not been defined, with mid to lower young adult male serum testosterone levels recommended at this time.8 Given that the current literature does not specify a target testosterone replacement range, we recommend monitoring the clinical response along with total testosterone to decide adjustments in TRT. Ultimately, treatment goals of TRT should be the resolution of signs and symptoms, including improvement of sexual function, libido, and preservation of bone mineral density.7,8
Contraindications
TRT is not recommended in men with the following:
- Breast cancer
- Polycythemia (hematocrit > 50%)
- Untreated obstructive sleep apnea
- Lower urinary tract symptoms caused by an enlarged prostate; International Prostate Symptom Score > 19
- Poorly controlled heart failure
- Desire for fertility.
The role of TRT in prostate cancer remains controversial (see below) and remains contraindicated in recent Endocrine Society clinical practice guidelines.7 Guidelines recommend urologic consultation prior to initiation of TRT in patients at increased risk of prostate cancer,7 based on age, race, family history, PSA, PSA velocity, and history of prostate biopsy.
One prominent historic concern about androgen replacement therapy regards the potential for de novo development of prostate cancer. Numerous studies have failed to find elevated risk of new diagnosis, progression, or recurrence of prostate cancer in patients on TRT.36,37 Nevertheless, patients who develop elevated PSA, increased PSA velocity, or an abnormal digital rectal examination while on TRT should undergo prostate biopsy.
TRT FORMULATIONS AND TREATMENT OPTIONS
A number of effective formulations of TRT are available (Table 2). Transdermal and parenteral formulations are most commonly used. Enteric testosterone formulations are not available in the United States and are associated with hepatotoxicity. While buccal testosterone therapy is available, it often leads to local gingival irritation and has not gained widespread popularity.
Parenteral TRT can be administered intramuscularly (IM) or subcutaneously (SQ). Testosterone cypionate (Depo-Testosterone) is the only IM form available in the United States and is given every 2 to 3 weeks. It is the least expensive form of TRT, but it requires frequent administration (by either the clinical practitioner or the patient himself). Testosterone cypionate injections lead to markedly wide swings of testosterone levels, ranging from supraphysiologic levels for a few days after administration to hypogonadal levels before the next injection. This may be mitigated by more-frequent injections. The longer-acting form testosterone undecanoate is available outside the United States and is given every 12 weeks when stable levels are reached.
The other parenteral option is SQ slow-release pellets (Testopel). These pellets have 75 mg of testosterone. Typically 8 to 14 pellets are placed subcutaneously in the buttock area, which will provide coverage for 3 to 6 months.38 The insertion procedure is simple with a short learning curve, limited compliance issues, and elimination of risk of transdermal transmission of drug to others. Disadvantages include wound infection and pellet extrusion, seen in 0.3% to 12% of patients in various studies.38
Another route of TRT is transdermal, including patches, liquids, and gels. Patches are applied daily and are rotated to different sites with minimal risk for skin transmission to others, although use may be limited by site dermatitis. Three hydro-alcoholic gel formulations are currently available in the United States: Androgel (1% or 1.62%), which is applied to the chest or the shoulders; Testim 1%, which is applied to the shoulders; and Fortesta (2%), which is applied to the thighs. A liquid preparation, Axiron, is applied to the axillae. Because secondary transfer to women and children is possible, it is important to thoroughly wash hands after application and to cover the treated skin with clothing. In 3 to 4 hours, all the medication is absorbed, and the area should then be washed before direct skin contact with others (Table 2).
MONITORING PATIENTS ON TRT
Patients starting TRT will require clinical and biochemical monitoring to evaluate response to therapy as well as possible side effects. The first set of laboratory values should be obtained 6 to 12 weeks after initiation of therapy and then typically quarterly for 1 year, every 6 months for the second year, and annually thereafter. Laboratory values monitored should include total testosterone, PSA, and hematocrit.
Men on daily therapy (patch, gel, liquid) should have testosterone drawn approximately 2 hours after application. Current TRT regimen data lack an appropriate target testosterone value, and guidelines suggest a mid to lower young adult male testosterone level.8 Since this is not clearly delineated in the current literature, the authors recommend monitoring clinical symptoms along with testosterone levels when adjusting TRT. It is important to document that serum testosterone was actually increased to the normal range in treated men without clinical improvement.
A rise in PSA of up to 24% would be an acceptable response in a benign prostate gland, but a higher increase or increase above 4.0 ng/dL should prompt consideration of prostate biopsy. 39 Similarly, hemoglobin and hematocrit typically increase, but a hematocrit greater than 55% should prompt dose reduction or cessation.7 Transaminases do not need routine monitoring during parenteral or transdermal therapy. Bone mineral density should be monitored every 1 to 2 years.7,8
CLINICAL BENEFITS OF TRT
There are promising data regarding the clinical benefits of TRT in patients with metabolic syndrome and type 2 diabetes mellitus. A recent meta-analysis investigating the effect of TRT on metabolic syndrome revealed an improvement in fasting plasma glucose, homeostatic model assessment index, triglycerides, treadmill duration, high-density lipoprotein cholesterol, and waist circumference.40,41 TRT also decreased insulin resistance and improved glycemic control in type 2 diabetic hypogonadal men.42 Results from a randomized controlled trial comparing 12 weeks of intramuscular testosterone treatment vs placebo in men with metabolic syndrome revealed an improvement in mean waist circumference from 108 cm ± 8 cm to 105.5 cm ± 7.7 cm. Sixty percent of men initially diagnosed with metabolic syndrome and treated with testosterone no longer met diagnostic criteria for metabolic syndrome according to the National Cholesterol Education Program–Third Adult Treatment Panel (NCEP-ATP III) and the International Diabetes Federation (IDF) guidelines.43
Depression has also been associated with low testosterone, with free testosterone levels below 170 pmol/L associated with frank depressive symptoms and levels below 220 pmol/L predictive of future onset of depressive symptoms.15 Testosterone replacement therapy has been shown to improve depressive symptoms in hypogonadal men.16,17 Shores et al16 conducted a randomized placebo-controlled study of testosterone replacement in men older than 50 years with dysthymia or minor depression. Men treated with testosterone gel for 12 weeks showed an improvement of baseline total testosterone levels from 291 ng/dL to 449 ng/dL. Men treated with testosterone also had a 53% rate of depression remission compared with 19% in the placebo group.16
The evidence supporting improved sexual function with TRT is variable. Some studies indicate limited or transient improvement of sexual function after TRT in men with erectile dysfunction,18,19 while others report an improvement in sexual function after 3 months of TRT.44 Because of the multifactorial nature of erectile dysfunction, men with erectile dysfunction and ADAM may require TRT and a phosphodiesterase type 5 (PDE5) inhibitor, as TRT alone may be insufficient. In a prospective observational study of men with erectile dysfunction and an initial testosterone lower than 300 ng/dL, testosterone gel was administered for at least 1 year, and improvement in sexual function was seen. Results revealed a correlation between improvement in sexual function and concurrent therapy with a PDE5 inhibitor.45 In a recent multicenter placebo-controlled study of PDE5 inhibitor nonresponders, the addition of a testosterone gel to tadalafil (Cialis) improved sexual function, again suggesting a synergistic effect when treating erectile dysfunction with both TRT and a PDE5 inhibitor.46
ADVERSE EVENTS RELATED TO TRT
Despite the aforementioned benefits, it must be emphasized that TRT should be used for specific target symptoms related to hypogonadism in older men and that the general health benefits and safety of TRT in an asymptomatic man with a low measured testosterone alone remains unproven.
Cardiovascular events. In a recent study of 209 elderly men with low testosterone and limited mobility associated with other chronic illnesses, 6 months of TRT resulted in the development of cardiovascular-related adverse events in 23 patients compared with 5 men in the placebo group.47 This may have been related to how adverse events were reported, with cumulative adverse events reviewed every 6 months, ranging from peripheral edema, hypertension, arrhythmias, and electrocardiographic changes. Serious adverse events were reviewed as they occurred, including stroke and acute myocardial events.
Other studies41,43 have revealed a favorable effect of TRT on cardiovascular disease and its surrogate markers but have lacked detailed reports and close monitoring of adverse events. Thus, variation of outcome measurement and reporting may obfuscate the detection of adverse cardiovascular events. Outcomes may also depend on the testosterone formulation and the target serum concentration.43
Larger, long-term placebo-controlled trials are needed to elucidate cardiovascular risk as a primary outcome in older androgen-deficient men undergoing TRT.
Other adverse effects related to TRT include erythrocytosis, seen in 3% to 18% of patients with transdermal administration,48,49 and up to 44% of patients undergoing IM therapy.48 Gynecomastia can occur and is more likely to resolve after treatment cessation of transdermal testosterone treatment than IM injections.48 Other potential clinical side effects that should prompt dose-reduction or discontinuation are irritability, bothersome acne, fluid retention, testicular atrophy, worsening of lower urinary tract symptoms from an enlarged prostate, and new or worsening heart failure. Infrequently, obstructive sleep apnea may be worsened by TRT, although currently the data linking sleep apnea and TRT are limited.50
TRT AND PROSTATE CANCER
The relationship between prostate cancer growth and testosterone is well established, with androgen ablation remaining the cornerstone of treatment for metastatic disease. Since androgen deprivation leads to the regression of prostate cancer, there has been concern that TRT may lead to growth or de novo development of prostate cancer. TRT has thus been strongly prohibited in patients with prostate cancer.7 However, recent data challenge this paradigm.
In a retrospective study of 81 men (mean age 56.8 years) treated with TRT, only 4 men (4.9%) developed prostate cancer over a 5-year period.51 This is less than the estimated 16.7% probability of developing prostate cancer in the general US population.52
Recent accumulating data support the concept of testosterone reaching a saturation level when binding androgen receptors within the prostate at extremely low levels. Increases above this level with TRT as with ADAM do not increase the risk of development or progression of prostate cancer.53 In addition, large doses of dihydrotestosterone do not seem to alter PSA, prostate volume, or International Prostate Symptom Score.54 These findings may have implications in future androgen therapies in hypogonadal older men.
Pathologic studies suggest low testosterone is associated with a higher Gleason grade of prostate cancer,55 although this association remains unconfirmed.56
In men with erectile dysfunction after prostate cancer treatment, TRT appears safe after brachytherapy57 or radical prostatectomy.58 A small study of 15 hypogonadal men with castrate-resistant prostate cancer and minimal or no metastatic disease showed only 1 patient had symptomatic progression.59 Moreover, a recent small study of 13 men with known prostate cancer on active surveillance showed that TRT did not lead to local progression or metastatic disease in any of the patients.60
While these data are provocative, it should still be emphasized that the standard of care for prostate cancer screening should be followed in age-appropriate men with ADAM. In addition, hypogonadal men with prostate cancer should only be treated with testosterone in conjunction with careful counseling and ongoing monitoring.
TRT SHOULD NOT REPLACE HEALTHY LIFESTYLE CHANGES
There has been a dramatic increase in TRT initiation for nonspecific symptoms of low testosterone in older androgen-deficient men. With this increase in initiation of TRT, there is a significant risk of overtreating. While there are many encouraging associations between treatment of androgen deficiency and improvement in rates of of morbidity and mortality, much remains unknown about the overall long-term risks and benefits of TRT. It is important to emphasize that TRT should not replace healthy lifestyle changes including regular exercise, weight loss, and diet modifications, which may provide the patient symptom resolution. Thoughtful dialogue with the patient is critical prior to TRT initiation, including thorough disclosure of the risks and benefits of treatment, and the limitations of the data as it evolves.
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002; 87:589–598.
- Araujo AB, O’Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004; 89:5920–5926.
- Travison TG, Shackelton R, Araujo AB, et al. The natural history of symptomatic androgen deficiency in men: onset, progression, and spontaneous remission. J Am Geriatr Soc 2008; 56:831–839.
- Tajar A, Forti G, O’Neill TW, et al; EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010; 95:1810–1818.
- Hammes A, Andreassen TK, Spoelgen R, et al. Role of endocytosis in cellular uptake of sex steroids. Cell 2005; 122:751–762.
- Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol 2010; 316:79–85.
- Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95:2536–2559.
- Wang C, Nieschlag E, Swerdloff R, et al; International Society of Andrology (ISA). Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl 2009; 30:1–9.
- Morley JE, Patrick P, Perry HM. Evaluation of assays available to measure free testosterone. Metabolism 2002; 51:554–559.
- Vesper HW, Bhasin S, Wang C, et al. Interlaboratory comparison study of serum total testosterone [corrected] measurements performed by mass spectrometry methods. Steroids 2009; 74:498–503.
- Ly LP, Sartorius G, Hull L, et al. Accuracy of calculated free testosterone formulae in men. Clin Endocrinol (Oxf) 2010; 73:382–388.
- Melmed S, Casanueva FF, Hoffman AR, et al; Endocrine Society. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:273–288.
- Wu FC, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363:123–135.
- Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab 2004; 89:3813–3817.
- Joshi D, van Schoor NM, de Ronde W, et al. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin Endocrinol (Oxf) 2010; 72:232–240.
- Shores MM, Kivlahan DR, Sadak TI, Li EJ, Matsumoto AM. A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression). J Clin Psychiatry 2009; 70:1009–1016.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the Testim Registry in the US (TRiUS). Aging Male 2012; 15:14–21.
- Jain P, Rademaker AW, McVary KT. Testosterone supplementation for erectile dysfunction: results of a meta-analysis. J Urol 2000; 164:371–375.
- Mulhall JP, Valenzuela R, Aviv N, Parker M. Effect of testosterone supplementation on sexual function in hypogonadal men with erectile dysfunction. Urology 2004; 63:348–352.
- Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000; 49:1239–1242.
- Moore C, Huebler D, Zimmermann T, Heinemann LA, Saad F, Thai DM. The Aging Males’ Symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol 2004; 46:80–87.
- Chueh KS, Huang SP, Lee YC, et al. The Comparison of the Aging Male Symptoms (AMS) Scale and Androgen Deficiency in the Aging Male (ADAM) Questionnaire to Detect Androgen Deficiency in Middle-Aged Men. J Androl 2012[Epub ahead of print]
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006; 60:762–769.
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33:1186–1192.
- Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab 2010; 95:2790–2799.
- Carrero JJ, Qureshi AR, Nakashima A, et al. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant 2011; 26:184–190.
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008; 93:1834–1840.
- Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol 2011; 40:189–207.
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006; 295:1288–1299.
- Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 2004; 6:208–215.
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med 2006; 166:1660–1665.
- Carrero JJ, Qureshi AR, Parini P, et al. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol 2009; 20:613–620.
- Haring R, Völzke H, Steveling A, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J 2010; 31:1494–1501.
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96:3007–3019.
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 2004; 350:482–492.
- Isbarn H, Pinthus JH, Marks LS, et al. Testosterone and prostate cancer: revisiting old paradigms. Eur Urol 2009; 56:48–56.
- Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med 2011; 124:578–587.
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med 2009; 6:3177–3192.
- Gerstenbluth RE, Maniam PN, Corty EW, Seftel AD. Prostate-specific antigen changes in hypogonadal men treated with testosterone replacement. J Androl 2002; 23:922–926.
- Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med 2011; 8:272–283.
- Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol 2011; 165:687–701.
- Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006; 154:899–906.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010; 7:3495–3503.
- Rhoden EL, Morgentaler A. Symptomatic response rates to testosterone therapy and the likelihood of completing 12 months of therapy in clinical practice. J Sex Med 2010; 7:277–283.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. Improved sexual function with testosterone replacement therapy in hypogonadal men: real-world data from the Testim Registry in the United States (TRiUS). J Sex Med 2011; 8:3204–3213.
- Buvat J, Montorsi F, Maggi M, et al. Hypogonadal men nonresponders to the PDE5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (TADTEST study). J Sex Med 2011; 8:284–293.
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med 2010; 363:109–122.
- Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 1999; 84:3469–3478.
- Wang C, Swerdloff RS, Iranmanesh A, et al; Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000; 85:2839–2853.
- Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? J Sex Med 2007; 4:1241–1246.
- Coward RM, Simhan J, Carson CC. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int 2009; 103:1179–1183.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 2009; 55:310–320.
- Page ST, Lin DW, Mostaghel EA, et al. Dihydrotestosterone administration does not increase intraprostatic androgen concentrations or alter prostate androgen action in healthy men: a randomized-controlled trial. J Clin Endocrinol Metab 2011; 96:430–437.
- Botto H, Neuzillet Y, Lebret T, Camparo P, Molinie V, Raynaud JP. High incidence of predominant Gleason pattern 4 localized prostate cancer is associated with low serum testosterone. J Urol 2011; 186:1400–1405.
- Salonia A, Gallina A, Briganti A, et al. Preoperative hypogonadism is not an independent predictor of high-risk disease in patients undergoing radical prostatectomy. Cancer 2011; 117:3953–3962.
- Sarosdy MF. Testosterone replacement for hypogonadism after treatment of early prostate cancer with brachytherapy. Cancer 2007; 109:536–541.
- Khera M. Androgens and erectile function: a case for early androgen use in postprostatectomy hypogonadal men. J Sex Med 2009; 6:(suppl 3):234–238.
- Szmulewitz R, Mohile S, Posadas E, et al. A randomized phase 1 study of testosterone replacement for patients with low-risk castration-resistant prostate cancer. Eur Urol 2009; 56:97–103.
- Morgentaler A, Lipshultz LI, Bennett R, Sweeney M, Avila D, Khera M. Testosterone therapy in men with untreated prostate cancer. J Urol 2011; 185:1256–1260.
- Feldman HA, Longcope C, Derby CA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 2002; 87:589–598.
- Araujo AB, O’Donnell AB, Brambilla DJ, et al. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab 2004; 89:5920–5926.
- Travison TG, Shackelton R, Araujo AB, et al. The natural history of symptomatic androgen deficiency in men: onset, progression, and spontaneous remission. J Am Geriatr Soc 2008; 56:831–839.
- Tajar A, Forti G, O’Neill TW, et al; EMAS Group. Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab 2010; 95:1810–1818.
- Hammes A, Andreassen TK, Spoelgen R, et al. Role of endocytosis in cellular uptake of sex steroids. Cell 2005; 122:751–762.
- Rosner W, Hryb DJ, Kahn SM, Nakhla AM, Romas NA. Interactions of sex hormone-binding globulin with target cells. Mol Cell Endocrinol 2010; 316:79–85.
- Bhasin S, Cunningham GR, Hayes FJ, et al; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010; 95:2536–2559.
- Wang C, Nieschlag E, Swerdloff R, et al; International Society of Andrology (ISA). Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl 2009; 30:1–9.
- Morley JE, Patrick P, Perry HM. Evaluation of assays available to measure free testosterone. Metabolism 2002; 51:554–559.
- Vesper HW, Bhasin S, Wang C, et al. Interlaboratory comparison study of serum total testosterone [corrected] measurements performed by mass spectrometry methods. Steroids 2009; 74:498–503.
- Ly LP, Sartorius G, Hull L, et al. Accuracy of calculated free testosterone formulae in men. Clin Endocrinol (Oxf) 2010; 73:382–388.
- Melmed S, Casanueva FF, Hoffman AR, et al; Endocrine Society. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2011; 96:273–288.
- Wu FC, Tajar A, Beynon JM, et al; EMAS Group. Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 2010; 363:123–135.
- Kelleher S, Conway AJ, Handelsman DJ. Blood testosterone threshold for androgen deficiency symptoms. J Clin Endocrinol Metab 2004; 89:3813–3817.
- Joshi D, van Schoor NM, de Ronde W, et al. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin Endocrinol (Oxf) 2010; 72:232–240.
- Shores MM, Kivlahan DR, Sadak TI, Li EJ, Matsumoto AM. A randomized, double-blind, placebo-controlled study of testosterone treatment in hypogonadal older men with subthreshold depression (dysthymia or minor depression). J Clin Psychiatry 2009; 70:1009–1016.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. The effect of testosterone supplementation on depression symptoms in hypogonadal men from the Testim Registry in the US (TRiUS). Aging Male 2012; 15:14–21.
- Jain P, Rademaker AW, McVary KT. Testosterone supplementation for erectile dysfunction: results of a meta-analysis. J Urol 2000; 164:371–375.
- Mulhall JP, Valenzuela R, Aviv N, Parker M. Effect of testosterone supplementation on sexual function in hypogonadal men with erectile dysfunction. Urology 2004; 63:348–352.
- Morley JE, Charlton E, Patrick P, et al. Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 2000; 49:1239–1242.
- Moore C, Huebler D, Zimmermann T, Heinemann LA, Saad F, Thai DM. The Aging Males’ Symptoms scale (AMS) as outcome measure for treatment of androgen deficiency. Eur Urol 2004; 46:80–87.
- Chueh KS, Huang SP, Lee YC, et al. The Comparison of the Aging Male Symptoms (AMS) Scale and Androgen Deficiency in the Aging Male (ADAM) Questionnaire to Detect Androgen Deficiency in Middle-Aged Men. J Androl 2012[Epub ahead of print]
- Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006; 60:762–769.
- Dhindsa S, Miller MG, McWhirter CL, et al. Testosterone concentrations in diabetic and nondiabetic obese men. Diabetes Care 2010; 33:1186–1192.
- Krasnoff JB, Basaria S, Pencina MJ, et al. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab 2010; 95:2790–2799.
- Carrero JJ, Qureshi AR, Nakashima A, et al. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant 2011; 26:184–190.
- Grossmann M, Thomas MC, Panagiotopoulos S, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab 2008; 93:1834–1840.
- Brand JS, van der Tweel I, Grobbee DE, Emmelot-Vonk MH, van der Schouw YT. Testosterone, sex hormone-binding globulin and the metabolic syndrome: a systematic review and meta-analysis of observational studies. Int J Epidemiol 2011; 40:189–207.
- Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2006; 295:1288–1299.
- Niskanen L, Laaksonen DE, Punnonen K, Mustajoki P, Kaukua J, Rissanen A. Changes in sex hormone-binding globulin and testosterone during weight loss and weight maintenance in abdominally obese men with the metabolic syndrome. Diabetes Obes Metab 2004; 6:208–215.
- Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med 2006; 166:1660–1665.
- Carrero JJ, Qureshi AR, Parini P, et al. Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol 2009; 20:613–620.
- Haring R, Völzke H, Steveling A, et al. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20–79. Eur Heart J 2010; 31:1494–1501.
- Araujo AB, Dixon JM, Suarez EA, Murad MH, Guey LT, Wittert GA. Clinical review: Endogenous testosterone and mortality in men: a systematic review and meta-analysis. J Clin Endocrinol Metab 2011; 96:3007–3019.
- Rhoden EL, Morgentaler A. Risks of testosterone-replacement therapy and recommendations for monitoring. N Engl J Med 2004; 350:482–492.
- Isbarn H, Pinthus JH, Marks LS, et al. Testosterone and prostate cancer: revisiting old paradigms. Eur Urol 2009; 56:48–56.
- Traish AM, Miner MM, Morgentaler A, Zitzmann M. Testosterone deficiency. Am J Med 2011; 124:578–587.
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med 2009; 6:3177–3192.
- Gerstenbluth RE, Maniam PN, Corty EW, Seftel AD. Prostate-specific antigen changes in hypogonadal men treated with testosterone replacement. J Androl 2002; 23:922–926.
- Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med 2011; 8:272–283.
- Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol 2011; 165:687–701.
- Kapoor D, Goodwin E, Channer KS, Jones TH. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 2006; 154:899–906.
- Aversa A, Bruzziches R, Francomano D, et al. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: results from a 24-month, randomized, double-blind, placebo-controlled study. J Sex Med 2010; 7:3495–3503.
- Rhoden EL, Morgentaler A. Symptomatic response rates to testosterone therapy and the likelihood of completing 12 months of therapy in clinical practice. J Sex Med 2010; 7:277–283.
- Khera M, Bhattacharya RK, Blick G, Kushner H, Nguyen D, Miner MM. Improved sexual function with testosterone replacement therapy in hypogonadal men: real-world data from the Testim Registry in the United States (TRiUS). J Sex Med 2011; 8:3204–3213.
- Buvat J, Montorsi F, Maggi M, et al. Hypogonadal men nonresponders to the PDE5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (TADTEST study). J Sex Med 2011; 8:284–293.
- Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med 2010; 363:109–122.
- Dobs AS, Meikle AW, Arver S, Sanders SW, Caramelli KE, Mazer NA. Pharmacokinetics, efficacy, and safety of a permeation-enhanced testosterone transdermal system in comparison with bi-weekly injections of testosterone enanthate for the treatment of hypogonadal men. J Clin Endocrinol Metab 1999; 84:3469–3478.
- Wang C, Swerdloff RS, Iranmanesh A, et al; Testosterone Gel Study Group. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. J Clin Endocrinol Metab 2000; 85:2839–2853.
- Hanafy HM. Testosterone therapy and obstructive sleep apnea: is there a real connection? J Sex Med 2007; 4:1241–1246.
- Coward RM, Simhan J, Carson CC. Prostate-specific antigen changes and prostate cancer in hypogonadal men treated with testosterone replacement therapy. BJU Int 2009; 103:1179–1183.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 2009; 55:310–320.
- Page ST, Lin DW, Mostaghel EA, et al. Dihydrotestosterone administration does not increase intraprostatic androgen concentrations or alter prostate androgen action in healthy men: a randomized-controlled trial. J Clin Endocrinol Metab 2011; 96:430–437.
- Botto H, Neuzillet Y, Lebret T, Camparo P, Molinie V, Raynaud JP. High incidence of predominant Gleason pattern 4 localized prostate cancer is associated with low serum testosterone. J Urol 2011; 186:1400–1405.
- Salonia A, Gallina A, Briganti A, et al. Preoperative hypogonadism is not an independent predictor of high-risk disease in patients undergoing radical prostatectomy. Cancer 2011; 117:3953–3962.
- Sarosdy MF. Testosterone replacement for hypogonadism after treatment of early prostate cancer with brachytherapy. Cancer 2007; 109:536–541.
- Khera M. Androgens and erectile function: a case for early androgen use in postprostatectomy hypogonadal men. J Sex Med 2009; 6:(suppl 3):234–238.
- Szmulewitz R, Mohile S, Posadas E, et al. A randomized phase 1 study of testosterone replacement for patients with low-risk castration-resistant prostate cancer. Eur Urol 2009; 56:97–103.
- Morgentaler A, Lipshultz LI, Bennett R, Sweeney M, Avila D, Khera M. Testosterone therapy in men with untreated prostate cancer. J Urol 2011; 185:1256–1260.
KEY POINTS
- General health benefits and safety of TRT in asymptomatic patients are not clearly defined by current data.
- Treatment of low testosterone is discouraged in the absence of clinical symptoms.
- A morning serum testosterone should be obtained after ruling out other causes of symptoms. It should also be repeated to confirm androgen deficiency in older men.
- Androgen deficiency in older men is associated with metabolic syndrome, type 2 diabetes mellitus, obesity, osteoporosis, renal failure, anemia, and previous treatment with steroids or opiates.
- TRT in men with a history of prostate cancer remains controversial. The existing limited data suggest that TRT is safe after curative therapy for prostate cancer. Patients treated should be monitored closely and informed of the risks of cancer progression and recurrence while they are on TRT.
Detecting and managing hereditary colorectal cancer syndromes in your practice
Hereditary colorectal cancer syndromes account for 5% to 10% of cases of colorectal cancer.
Identifying these patients in clinical practice begins by assessing a patient’s personal and family health history. An accurate and comprehensive family history should cover three generations and include ethnic background, ages and causes of death of relatives, and any diagnosis of cancer, including age at onset and history of polyps.
Red flags for a hereditary colorectal cancer syndrome in the personal or family history are:
- Early age of onset of cancer (eg, colorectal cancer before age 50)
- More than 10 colorectal adenomas
- Synchronous (ie, occurring at the same time) or metachronous (occurring at different times) primary cancers
- Multiple relatives in successive generations with the same or related cancers (eg, colon or endometrial cancer)
- A family member with a known hereditary colorectal cancer syndrome (Table 1).
Any of these red flags should prompt a referral for genetic counseling.
SYNDROMES ARE CLASSIFIED AS WITH OR WITHOUT POLYPOSIS
Many hereditary syndromes are associated with a higher risk of colorectal cancer. Generally, they can be divided into two categories (Table 2): polyposis syndromes (in which patients have numerous colorectal polyps) and nonpolyposis syndromes (with few or no polyps).
These two main types are subclassified on the basis of the histology of most of the polyps detected: adenomatous, hamartomatous, serrated, or mixed types.
In this review, we will address the three most common of these syndromes: Lynch syndrome (hereditary nonpolyposis colorectal cancer), familial adenomatous polyposis, and MYH-associated polyposis. However, as noted in Table 2, other hereditary colorectal cancer syndromes exist, and suspicion of these conditions should prompt a referral for further evaluation.
LYNCH SYNDROME (HEREDITARY NONPOLYPOSIS COLORECTAL CANCER)
Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer, predisposes people to a variety of cancers.
Colorectal cancer is the most common type of cancer associated with Lynch syndrome. Recent research suggests that the cumulative risk of developing colorectal cancer by age 80 is 42% for all patients with Lynch syndrome.1 The median age at onset is 45 years.1 For patients who undergo segmental resection of their initial cancer, the cumulative risk of metachronous colorectal cancer (ie, a new tumor arising later) is 16% at 10 years, 41% at 20 years, and up to 62% after 30 years.2
Endometrial cancer occurs in 17% to 57% of women with Lynch syndrome by age 70, with a median age at onset of 49 years.1
Other extracolonic cancers in Lynch syndrome include cancers of the:
- Stomach (1%–10% risk by age 70 years)
- Ovaries (1%–20% risk)
- Hepatobiliary tract (1%–2% risk)
- Urinary tract (1%–12% risk)
- Small bowel (1%–2% risk)
- Brain (1%–8% risk)
- Skin (sebaceous adenomas, adenocarcinomas, and keratoacanthomas).1,3,4
Earlier studies reported higher rates of associated cancer than those shown here. However, their data were largely derived from registries and may be overestimates. The numbers shown above are from population-based studies.
Genetics of Lynch syndrome
Lynch syndrome is caused by a germline mutation in the MLH1, MSH2, MSH6, PMS2, or EPCAM genes.5 These genes code for proteins that are responsible DNA mismatch repair—one of the cell’s proofreading mechanisms during DNA replication.
These mutations are inherited in an autosomal dominant manner. Though de novo mutations in these genes have been reported, they are rare and the exact frequency with which they occur is unknown.6
In whom should Lynch syndrome be suspected?
Lynch syndrome can be suspected on the basis of family history and clinical criteria.
In 1991, the same group of experts who coined the term “hereditary nonpolyposis colorectal cancer” developed family history criteria for it1:
- At least three relatives with histologically confirmed colorectal cancer, one of whom is a first-degree relative of the other two
- At least two successive generations involved
- At least one of the cancers diagnosed before age 50
- Familial adenomatous polyposis is excluded.
Known as the Amsterdam criteria, these were to be used in collaborative studies of families with hereditary colorectal cancer.7 In 1999, these criteria were broadened to include extracolonic cancers and became known as the Amsterdam II criteria (Table 3).8
Patients whose families meet the Amsterdam II criteria or who have molecular pathologic evidence of Lynch syndrome (see below) are appropriate candidates for genetic counseling and testing.
Diagnosis of Lynch syndrome
The diagnosis of Lynch syndrome is based on molecular pathologic analysis (performed on tumor samples) and confirmed by genetic testing.
Molecular pathologic evidence of Lynch syndrome includes microsatellite instability and loss of expression of one or more of the DNA mismatch repair proteins (detected using immunohistochemistry) (more on these below). The revised Bethesda guidelines (TABLE 3) were intended to identify individuals whose tumors should be tested for one or both of these phenomena.9
In 2009, the Evaluation of Genomic Applications in Practice and Prevention working group recommended that all patients with newly diagnosed colorectal cancer undergo microsatellite instability analysis, immunohistochemistry testing, or both, regardless of whether they meet the Amsterdam II or the Bethesda guideline criteria.10
Microsatellite instability analysis. Microsatellites are short sequences of repeated DNA. The tumor cells of patients who carry defective mismatch repair genes have microsatellites that are longer or shorter than in normal cells, a condition called microsatellite instability (ie, “MSI-high”).
Microsatellite instability testing, using a standardized panel of five DNA markers, is performed on normal and tumor tissue. If more than two of the five microsatellite markers in the tumor show instability, the lesion is considered to have a high level of microsatellite instability. About 15% of colorectal cancers have this high level, although most are not associated with Lynch syndrome and lose MLH1 expression by promoter methylation.11,12
While only 2% of patients with colorectal cancer have Lynch syndrome, from 90% to 95% of colorectal cancers from patients with Lynch syndrome have high levels of microsatellite instability.10 The presence of MLH1 promoter hypermethylation, the BRAF mutation V600E, or both within the tumor suggests that the cancer is not associated with Lynch syndrome.
Some families that meet the Amsterdam I criteria have microsatellite-stable tumors: their condition has been called familial colorectal cancer type X.13 This condition is associated with a higher risk of colorectal cancer but not the other malignancies observed in Lynch syndrome.
Immunohistochemistry is performed to assess for expression of the mismatch repair proteins MSH2, MSH6, MLH1, and PMS2. Absence of expression of the specific protein within tumor cells compared with normal cells within the specimen suggests dysfunction of the specific gene and guides germline mutation testing (Figure 1). For example, a patient who lacks expression of the MSH2 protein in his or her colon cancer most likely has a mutation in the MSH2 gene. Therefore, germ-line genetic testing should initially target the MSH2 gene. Approximately 88% of Lynch syndrome-associated colorectal cancers have abnormal immunohistochemical staining.10
Testing for microsatellite instability and mismatch repair gene expression ideally precedes germline genetic testing and helps to guide which gene or genes should be tested.9,14
Genetic testing for Lynch syndrome is routinely performed on a blood or saliva sample, using DNA from white blood cells and sequencing the gene or genes involved to look for mutations. Positive results from a germline genetic test confirm the diagnosis of Lynch syndrome and allow for predictive testing for relatives at risk. The term Lynch syndrome is used exclusively to describe individuals with evidence of a mutation in one of the mismatch repair genes.15
If a patient’s results are positive, genetic counseling and genetic testing should be offered to at-risk relatives age 18 and over.
Management of Lynch syndrome
Aggressive cancer surveillance is essential for people with Lynch syndrome and for those who are considered at risk but have not pursued genetic testing, such as a sibling of a person with Lynch syndrome.
Colorectal cancer. Colonoscopy is recommended every 1 to 2 years beginning at the age of 20 to 25 years, or 2 to 5 years earlier than the age of the youngest relative affected with colorectal cancer if the initial diagnosis was before age 25. When patients turn 40 years old, colonoscopy is done annually.16–18 A significant reduction in cancer incidence and in the mortality rate has been shown with colonoscopic surveillance.19–21
Chemoprevention may also have a role. Patients with Lynch syndrome who took aspirin 600 mg per day for an average of 25 months had a significantly lower incidence of colorectal cancer during a 55-month follow-up period compared with patients randomized to placebo.22
For patients with Lynch syndrome who are diagnosed with colorectal cancer, the high risk of metachronous cancers after standard segmental colectomy calls for a more extended resection. Retrospective analysis of 382 Lynch syndrome patients found that none of the 50 who underwent total or subtotal colectomy were diagnosed with metachronous colorectal cancer, whereas a metachronous cancer developed in 74 (22%) of the 332 patients who had had segmented resection.2 Annual surveillance of the remaining colon, rectum, or both is indicated postoperatively.
Gynecologic cancers. Women with Lynch syndrome should also consider gynecologic surveillance and risk-reducing surgery. This includes annual gynecologic examination, transvaginal ultrasonography, and endometrial aspiration, beginning at age 30 to 35 years. Although this surveillance does detect premalignant lesions and early symptomatic cancers, its effect on the mortality rate is unknown. Hysterectomy with bilateral salpingo-oophorectomy has been shown to significantly reduce endometrial and ovarian cancers in women with Lynch syndrome.23,24
Urothelial cancers. Carriers of MSH2 mutations have a significantly higher risk of urothelial cancers.4 Therefore, MSH2 carriers should consider ultrasonography of the urinary tract, urinary cytology, and urinalysis every 1 to 2 years beginning at age 40.4
Other extracolonic cancers. Poor evidence exists for systematic screening for the other extracolonic tumors associated with Lynch syndrome. However, the National Comprehensive Cancer Network advises considering esophagogastroduodenoscopy with extended duodenoscopy as well as capsule endoscopy every 2 to 3 years beginning at age 30 to 35.14
ADENOMATOUS POLYPOSIS SYNDROMES
Familial adenomatous polyposis and MYH-associated polyposis are the next most common hereditary colorectal cancer syndromes. Each of these accounts for about 1% of cases of colorectal cancer. Clinically, these two syndromes can be challenging to distinguish because they overlap phenotypically to a significant degree.
FAMILIAL ADENOMATOUS POLYPOSIS
Familial adenomatous polyposis is caused by mutations in the APC gene. Its prevalence is 2.29 to 3.2 per 100,000 individuals.25,26
Genetics of familial adenomatous polyposis
APC is the only gene known to cause familial adenomatous polyposis. Mutations in APC are inherited in an autosomal dominant manner. Approximately 25% of cases of familial adenomatous polyposis are due to a de novo mutation in APC.27
Clinical presentation of familial adenomatous polyposis
Familial adenomatous polyposis is classified by the burden of colorectal adenomas.
Patients who have fewer than 100 adenomas have an attenuated form of the disease. In this group, polyps usually begin to form in the late teenage years or early 20s and tend to develop in the proximal colon. The attenuated form is associated with an approximately 70% lifetime risk of colorectal cancer.28
Patients who have more than 100 polyps are considered to have the classic form of the disease, and those with more than 1,000 polyps have profuse familial adenomatous polyposis (Figure 2). In these groups, polyps typically begin to develop in the preteenage to mid-teenage years. Without surgery, there is nearly a 100% risk of colorectal cancer. The average age at diagnosis of colorectal cancer is 39 years for patients with classic disease.
Upper gastrointestinal polyps are common in familial adenomatous polyposis. Nearly 90% of patients develop duodenal adenomas by a mean age of 44, with a cumulative lifetime risk of nearly 100%.29 Fundic gland polyposis occurs in nearly 90% of patients,30 while gastric adenomas are reported in fewer than 15% of patients.
Duodenal and periampullary cancer is the second most common malignancy in familial adenomatous polyposis. The lifetime risk ranges from 2% to 36%, depending on the Spigelman stage. People with Spigelman stage I, II, or III have a 2.5% risk of duodenal cancer, while those with stage IV disease have up to a 36% lifetime risk.
Gastric cancer, arising from fundic gland polyps, has been reported but is rare in Western populations.
In familial adenomatous polyposis, the incidence of jejunal adenomas and cancer is less than 10%, and the risk of ileal adenomas and cancer is less than 1%.31
Familial adenomatous polyposis is also associated with a higher risk of other malignancies, including:
- Pancreatic cancer (2% lifetime risk)
- Thyroid cancer (2% to 3% lifetime risk, typically papillary carcinoma)32
- Hepatoblastoma (1% to 2% lifetime risk)
- Brain tumors (< 1% lifetime risk)
- Biliary cancer (higher risk than in the general population).33
Benign extracolonic manifestations that have been observed include osteomas, dental abnormalities (supernumerary teeth, unerupted or absent teeth, odontomas), congenital hypertrophy of the retinal pigment epithelium, benign cutaneous lesions (epidermoid cysts and fibromas), and desmoid tumors.33 The term “Gardner syndrome” has been used to describe patients who have familial adenomatous polyposis but also have osteomas and soft-tissue tumors.34 These patients carry the same risk of colorectal cancer as other patients with familial adenomatous polyposis.
Diagnosing familial adenomatous polyposis
The diagnosis of familial adenomatous polyposis is suspected when a patient has more than 10 adenomatous polyps.
Seventy-five percent of patients with familial adenomatous polyposis have a family history of the condition. Therefore, most cases are identified at a young age on screening sigmoidoscopy or colonoscopy or by predictive gene testing. Patients rarely have cancer at the time of diagnosis.
The other 25% of patients typically are diagnosed when symptoms develop from the polyps or cancer. Over 50% of these symptomatic patients have cancer at the time of diagnosis.
It is recommended that people who have more than 10 adenomas detected on a single colonoscopy or who are first-degree relatives of patients with familial adenomatous polyposis undergo a genetic evaluation and testing for mutations in the APC gene.14 Once an APC mutation is identified in the family, at-risk relatives should be offered testing around age 10 years for families with classic familial adenomatous polyposis or in the mid to late teenage years for those with the attenuated form. It also appropriate to refer patients with desmoid tumors, duodenal adenomas, and bilateral or multifocal congenital hypertrophy of the retinal pigment epithelium for a genetic evaluation.
Management of familial adenomatous polyposis
Flexible sigmoidoscopy every 1 to 2 years beginning at age 10 to 12 years is recommended for individuals and families who have been phenotypically or genetically diagnosed with familial adenomatous polyposis.35–37 If colorectal adenomas are found, surgical options should be discussed and annual colonoscopic surveillance should commence.
For people with the attenuated form, because of the later age of disease onset and the tendency for right-sided disease, colonoscopy every 1 to 2 years should commence at about age 18.35–37 If polyps are found, colonoscopy should be performed every year.
The decision of when to offer colectomy is based on polyp burden (taking into account the number, pathologic appearance, and size of the polyps) and psychosocial factors such as patient maturity. Surgical options include total colectomy and ileorectal anastomosis or total proctocolectomy and ileal pouch anal anastomosis.38 Colonic and extracolonic phenotype as well as genotype should factor into the type of operation recommended. After colectomy, annual endoscopic surveillance of the rectum or ileal pouch is indicated to screen for recurrent polyposis and cancer.
Chemoprevention with sulindac (Clinoril) 150 mg or celecoxib (Celebrex) 400 mg twice a day causes regression of colorectal adenomas in familial adenomatous polyposis and may be useful as an adjunct to endoscopy in managing the colorectal polyp burden.39,40
Forward and side-viewing upper endoscopy should commence at age 20. This should include visualization and biopsy of the papilla and periampulllary region.29 The frequency of endoscopic surveillance depends on the Spigelman stage, which reflects the duodenal polyp burden. It is recommended that patients with Spigelman stage IV duodenal polyposis be seen in consultation with an experienced gastrointestinal surgeon for consideration of a prophylactic, pylorus-preserving, pancreas-sparing duodenectomy. This procedure has been shown to be more effective in polyp control and cancer prevention than endoscopic polyp ablation and local surgical resection.41
Some evidence for the utility of celecoxib 400 mg twice daily for the regression of duodenal polyposis was noted in a 6-month placebo-controlled trial.42 Some experts recommend removal of large duodenal adenomas, with adjunctive celecoxib therapy to control polyposis burden.30
People with familial adenomatous polyposis have been shown to have a 2.6% risk of thyroid cancer, and ultrasonography of the neck with attention to the thyroid is recommended for them.32
MYH-ASSOCIATED POLYPOSIS
Biallelic mutations in the MYH gene result in an adenomatous polyposis syndrome that may be indistinguishable from the attenuated or classic forms of familial adenomatous polyposis. A characteristic autosomal recessive pattern of inheritance in the family can be useful for identifying these patients in the clinic.
Genetics of MYH-associated polyposis
MYH-associated polyposis is the only known autosomal recessive hereditary colorectal cancer syndrome. In white populations, the most commonly reported mutations in MYH are Y179C (previously called Y165C) and G396D (previously called G382D), which account for up to 80% of cases.43 These two mutations are estimated to occur in 1% to 2% of the general population.44
Clinical presentation of MYH-associated polyposis
MYH-associated polyposis typically presents as multiple adenomatous polyps and is diagnosed at a mean age of 47 years. Eleven percent to 42% of affected individuals are reported to have fewer than 100 adenomas, while a minority (7.5% to 29%) of patients present with classic polyposis.45–47 In one study, an estimated 19% of patients presented with colorectal cancer and reported no history of colorectal polyps.48 Synchronous colorectal cancer is seen in more than 60% of patients with biallelic MYH mutations.49 Patients with monoallelic (heterozygous) MYH mutations appear to have the same risk of developing colorectal adenomas and cancer as the general population.49
Upper-gastrointestinal polyps have been reported in MYH-associated polyposis; as many as 17% to 25% of patients have duodenal adenomas.50,51
Diagnosis of MYH-associated polyposis
Genetic testing for biallelic MYH mutations should be performed in patients who test negative for an APC mutation but who have clinical features of familial adenomatous polyposis, a personal history of more than 10 colorectal adenomas, or a recessive family history of polyposis. 14 It has been shown that up to 29% of patients with familial adenomatous polyposis who are APC-negative will have biallelic mutations in the MYH gene.52 The siblings of a patient with biallelic MYH mutations should be offered genetic counseling and testing in their late teens or early 20s. All children of an individual with MYH-associated polyposis will carry one MYH mutation and are only at risk of having the syndrome if the other parent is also a MYH carrier and passed on his or her mutation.
Management of MYH-associated polyposis
The management of patients with MYH-associated polyposis is similar to that recommended for attenuated and classic familial adenomatous polyposis.14 Genetic counseling and testing and colonic and extracolonic surveillance are warranted. There are no data on the use of chemoprevention in MYH-associated polyposis. Surgery should be considered early because of the high risk of colorectal cancer, even in individuals with very few adenomas. Patients with monoallelic MYH mutations should follow the general population screening guidelines for colorectal cancer.49
GENETIC COUNSELING AND GENETIC TESTING
The American College of Gastroenterology advises that patients suspected of having hereditary colorectal cancer syndromes be advised to pursue genetic counseling and, if appropriate, genetic testing.16 They further recommend genetic counseling and informed consent before genetic testing.16
Genetic counseling is a process of working with patients and families whereby:
- A detailed medical and family history is obtained
- A formal risk assessment is performed
- Education about the disease in question and about genetic testing is provided
- Psychosocial concerns are assessed
- Informed consent is obtained when genetic testing is recommended.53
This process is important for helping patients better understand their cancer risks, the benefits and limitations of genetic testing, and the protections that are in place for people who undergo genetic testing, including the Genetic Information Non-Discrimination Act.
In 1996 the American Society of Clinical Oncology issued a policy statement highlighting the essential elements of informed consent for genetic testing for cancer susceptibility, and this was updated in 2003.54 In particular, it notes that patients should be informed of the implications of positive and negative results and of the possibility that the test may be uninformative.
When a hereditary colorectal cancer syndrome is suspected, a positive genetic test result confirms the diagnosis and allows for predictive testing of the patient’s relatives. However, no genetic test for a hereditary colorectal cancer syndrome is 100% sensitive. Therefore, a negative result does not rule out the syndrome in question.
Further, all cancer susceptibility genes have variants of uncertain significance, which are genetic alterations for which there are insufficient data to determine if the mutation is disease-causing or polymorphic (benign). Both negative and uninformative results can be confusing for patients and providers and can lead to false reassurance or undue worry when patients are not properly educated about these potential outcomes of testing.
Genetic testing is an evolving field, and with additional research and improved testing technologies, appropriate diagnoses can be made over time. That is why it is important for the genetic counseling relationship to continue over time.
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011; 305:2304–2310.
- Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 2011; 60:950–957.
- Barrow E, Robinson L, Alduaij W, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet 2009; 75:141–149.
- van der Post RS, Kiemeney LA, Ligtenberg MJ, et al. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J Med Genet 2010; 47:464–470.
- Wijnen JT, Vasen HF, Khan PM, et al. Clinical findings with implications for genetic testing in families with clustering of colorectal cancer. N Engl J Med 1998; 339:511–518.
- Bisgaard ML, Bernstein I. HNPCC mutation rate. Familial Cancer 2003; 2.
- Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum 1991; 34:424–425.
- Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999; 116:1453–1456.
- Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004; 96:261–268.
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: can UGT1A1 genotyping reduce morbidity and mortality in patients with metastatic colorectal cancer treated with irinotecan? Genet Med 2009; 11:15–20.
- Aaltonen LA, Peltomäki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993; 260:812–816.
- Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994; 145:148–156.
- Lindor NM, Rabe K, Petersen GM, et al. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA 2005; 293:1979–1985.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines) colorectal cancer screening version 2.2011. www.nccn.org. Accessed October 2, 2012.
- Jass JR. Hereditary non-polyposis colorectal cancer: the rise and fall of a confusing term. World J Gastroenterol 2006; 12:4943–4950.
- Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM; American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol 2009; 104:739–750.
- Winawer S, Fletcher R, Rex D, et al; Gastrointestinal Consortium Panel. Colorectal cancer screening and surveillance: clinical guidelines and rationale-update based on new evidence. Gastroenterology 2003; 124:544–560.
- Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 2006; 296:1507–1517.
- de Jong AE, Hendriks YM, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 2006; 130:665–671.
- Mecklin JP, Aarnio M, Läärä E, et al. Development of colorectal tumors in colonoscopic surveillance in Lynch syndrome. Gastroenterology 2007; 133:1093–1098.
- Engel C, Rahner N, Schulmann K, et al; German HNPCC Consortium. Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol 2010; 8:174–182.
- Burn J, Gerdes AM, Macrae F, et al; CAPP2 Investigators. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011; 378:2081–2087.
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer). J Med Genet 2007; 44:353–362.
- Manchanda R, Menon U, Michaelson-Cohen R, Beller U, Jacobs I. Hereditary non-polyposis colorectal cancer or Lynch syndrome: the gynaecological perspective. Curr Opin Obstet Gynecol 2009; 21:31–38.
- Burn J, Chapman P, Delhanty J, et al. The UK Northern region genetic register for familial adenomatous polyposis coli: use of age of onset, congenital hypertrophy of the retinal pigment epithelium, and DNA markers in risk calculations. J Med Genet 1991; 28:289–296.
- Järvinen HJ. Epidemiology of familial adenomatous polyposis in Finland: impact of family screening on the colorectal cancer rate and survival. Gut 1992; 33:357–360.
- Bisgaard ML, Fenger K, Bülow S, Niebuhr E, Mohr J. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum Mutat 1994; 3:121–125.
- Neklason DW, Stevens J, Boucher KM, et al. American founder mutation for attenuated familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008; 6:46–52.
- Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc 1999; 49:358–364.
- Bianchi LK, Burke CA, Bennett AE, Lopez R, Hasson H, Church JM. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008; 6:180–185.
- Kadmon M, Tandara A, Herfarth C. Duodenal adenomatosis in familial adenomatous polyposis coli. A review of the literature and results from the Heidelberg Polyposis Register. Int J Colorectal Dis 2001; 16:63–75.
- Jarrar AM, Milas M, Mitchell J, et al. Screening for thyroid cancer in patients with familial adenomatous polyposis. Ann Surg 2011; 253:515–521.
- Jasperson KW, Burt RW. APC-associated polyposis conditions. In:Pagon RA, Bird TD, Dolan CR, et al, eds. GeneReviews (Internet). Seattle, WA: University of Washington; 2011.
- Gardner EJ, Richards RC. Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. Am J Hum Genet 1953; 5:139–147.
- Dunlop MG; British Society for Gastroenterology. Guidance on gastrointestinal surveillance for hereditary non-polyposis colorectal cancer, familial adenomatous polyposis, juvenile polyposis, and Peutz-Jeghers syndrome. Gut 2002; 51(suppl 5):V21–V27.
- Burke W, Petersen G, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. Cancer Genetics Studies Consortium. JAMA 1997; 277:915–919.
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008; 57:704–713.
- Church J. Familial adenomatous polyposis. Surg Oncol Clin N Am 2009; 18:585–598.
- Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993; 328:1313–1316.
- Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000; 342:1946–1952.
- Johnson MD, Mackey R, Brown N, Church J, Burke C, Walsh RM. Outcome based on management for duodenal adenomas: sporadic versus familial disease. J Gastrointest Surg 2010; 14:229–235.
- Phillips RK, Wallace MH, Lynch PM, et al; FAP Study Group. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut 2002; 50:857–860.
- Tenesa A, Campbell H, Barnetson R, Porteous M, Dunlop M, Farrington SM. Association of MUTYH and colorectal cancer. Br J Cancer 2006; 95:239–242.
- Croitoru ME, Cleary SP, Di Nicola N, et al. Association between biallelic and monoallelic germline MYH gene mutations and colorectal cancer risk. J Natl Cancer Inst 2004; 96:1631–1634.
- Croitoru ME, Cleary SP, Berk T, et al. Germline MYH mutations in a clinic-based series of Canadian multiple colorectal adenoma patients. J Surg Oncol 2007; 95:499–506.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet 2003; 362:39–41.
- Nielsen M, Franken PF, Reinards TH, et al. Multiplicity in polyp count and extracolonic manifestations in 40 Dutch patients with MYH associated polyposis coli (MAP). J Med Genet 2005; 42:e54.
- Cleary SP, Cotterchio M, Jenkins MA, et al. Germline MutY human homologue mutations and colorectal cancer: a multisite case-control study. Gastroenterology 2009; 136:1251–1260.
- Lubbe SJ, Di Bernardo MC, Chandler IP, Houlston RS. Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J Clin Oncol 2009; 27:3975–3980.
- Aretz S, Uhlhaas S, Goergens H, et al. MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int J Cancer 2006; 119:807–814.
- Vogt S, Jones N, Christian D, et al. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology 2009; 137:1976–1985.e1–e10.
- Gismondi V, Meta M, Bonelli L, et al. Prevalence of the Y165C, G382D and 1395delGGA germline mutations of the MYH gene in Italian patients with adenomatous polyposis coli and colorectal adenomas. Int J Cancer 2004; 109:680–684.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the national society of genetic counselors. J Genet Couns 2004; 13:83–114.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
Hereditary colorectal cancer syndromes account for 5% to 10% of cases of colorectal cancer.
Identifying these patients in clinical practice begins by assessing a patient’s personal and family health history. An accurate and comprehensive family history should cover three generations and include ethnic background, ages and causes of death of relatives, and any diagnosis of cancer, including age at onset and history of polyps.
Red flags for a hereditary colorectal cancer syndrome in the personal or family history are:
- Early age of onset of cancer (eg, colorectal cancer before age 50)
- More than 10 colorectal adenomas
- Synchronous (ie, occurring at the same time) or metachronous (occurring at different times) primary cancers
- Multiple relatives in successive generations with the same or related cancers (eg, colon or endometrial cancer)
- A family member with a known hereditary colorectal cancer syndrome (Table 1).
Any of these red flags should prompt a referral for genetic counseling.
SYNDROMES ARE CLASSIFIED AS WITH OR WITHOUT POLYPOSIS
Many hereditary syndromes are associated with a higher risk of colorectal cancer. Generally, they can be divided into two categories (Table 2): polyposis syndromes (in which patients have numerous colorectal polyps) and nonpolyposis syndromes (with few or no polyps).
These two main types are subclassified on the basis of the histology of most of the polyps detected: adenomatous, hamartomatous, serrated, or mixed types.
In this review, we will address the three most common of these syndromes: Lynch syndrome (hereditary nonpolyposis colorectal cancer), familial adenomatous polyposis, and MYH-associated polyposis. However, as noted in Table 2, other hereditary colorectal cancer syndromes exist, and suspicion of these conditions should prompt a referral for further evaluation.
LYNCH SYNDROME (HEREDITARY NONPOLYPOSIS COLORECTAL CANCER)
Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer, predisposes people to a variety of cancers.
Colorectal cancer is the most common type of cancer associated with Lynch syndrome. Recent research suggests that the cumulative risk of developing colorectal cancer by age 80 is 42% for all patients with Lynch syndrome.1 The median age at onset is 45 years.1 For patients who undergo segmental resection of their initial cancer, the cumulative risk of metachronous colorectal cancer (ie, a new tumor arising later) is 16% at 10 years, 41% at 20 years, and up to 62% after 30 years.2
Endometrial cancer occurs in 17% to 57% of women with Lynch syndrome by age 70, with a median age at onset of 49 years.1
Other extracolonic cancers in Lynch syndrome include cancers of the:
- Stomach (1%–10% risk by age 70 years)
- Ovaries (1%–20% risk)
- Hepatobiliary tract (1%–2% risk)
- Urinary tract (1%–12% risk)
- Small bowel (1%–2% risk)
- Brain (1%–8% risk)
- Skin (sebaceous adenomas, adenocarcinomas, and keratoacanthomas).1,3,4
Earlier studies reported higher rates of associated cancer than those shown here. However, their data were largely derived from registries and may be overestimates. The numbers shown above are from population-based studies.
Genetics of Lynch syndrome
Lynch syndrome is caused by a germline mutation in the MLH1, MSH2, MSH6, PMS2, or EPCAM genes.5 These genes code for proteins that are responsible DNA mismatch repair—one of the cell’s proofreading mechanisms during DNA replication.
These mutations are inherited in an autosomal dominant manner. Though de novo mutations in these genes have been reported, they are rare and the exact frequency with which they occur is unknown.6
In whom should Lynch syndrome be suspected?
Lynch syndrome can be suspected on the basis of family history and clinical criteria.
In 1991, the same group of experts who coined the term “hereditary nonpolyposis colorectal cancer” developed family history criteria for it1:
- At least three relatives with histologically confirmed colorectal cancer, one of whom is a first-degree relative of the other two
- At least two successive generations involved
- At least one of the cancers diagnosed before age 50
- Familial adenomatous polyposis is excluded.
Known as the Amsterdam criteria, these were to be used in collaborative studies of families with hereditary colorectal cancer.7 In 1999, these criteria were broadened to include extracolonic cancers and became known as the Amsterdam II criteria (Table 3).8
Patients whose families meet the Amsterdam II criteria or who have molecular pathologic evidence of Lynch syndrome (see below) are appropriate candidates for genetic counseling and testing.
Diagnosis of Lynch syndrome
The diagnosis of Lynch syndrome is based on molecular pathologic analysis (performed on tumor samples) and confirmed by genetic testing.
Molecular pathologic evidence of Lynch syndrome includes microsatellite instability and loss of expression of one or more of the DNA mismatch repair proteins (detected using immunohistochemistry) (more on these below). The revised Bethesda guidelines (TABLE 3) were intended to identify individuals whose tumors should be tested for one or both of these phenomena.9
In 2009, the Evaluation of Genomic Applications in Practice and Prevention working group recommended that all patients with newly diagnosed colorectal cancer undergo microsatellite instability analysis, immunohistochemistry testing, or both, regardless of whether they meet the Amsterdam II or the Bethesda guideline criteria.10
Microsatellite instability analysis. Microsatellites are short sequences of repeated DNA. The tumor cells of patients who carry defective mismatch repair genes have microsatellites that are longer or shorter than in normal cells, a condition called microsatellite instability (ie, “MSI-high”).
Microsatellite instability testing, using a standardized panel of five DNA markers, is performed on normal and tumor tissue. If more than two of the five microsatellite markers in the tumor show instability, the lesion is considered to have a high level of microsatellite instability. About 15% of colorectal cancers have this high level, although most are not associated with Lynch syndrome and lose MLH1 expression by promoter methylation.11,12
While only 2% of patients with colorectal cancer have Lynch syndrome, from 90% to 95% of colorectal cancers from patients with Lynch syndrome have high levels of microsatellite instability.10 The presence of MLH1 promoter hypermethylation, the BRAF mutation V600E, or both within the tumor suggests that the cancer is not associated with Lynch syndrome.
Some families that meet the Amsterdam I criteria have microsatellite-stable tumors: their condition has been called familial colorectal cancer type X.13 This condition is associated with a higher risk of colorectal cancer but not the other malignancies observed in Lynch syndrome.
Immunohistochemistry is performed to assess for expression of the mismatch repair proteins MSH2, MSH6, MLH1, and PMS2. Absence of expression of the specific protein within tumor cells compared with normal cells within the specimen suggests dysfunction of the specific gene and guides germline mutation testing (Figure 1). For example, a patient who lacks expression of the MSH2 protein in his or her colon cancer most likely has a mutation in the MSH2 gene. Therefore, germ-line genetic testing should initially target the MSH2 gene. Approximately 88% of Lynch syndrome-associated colorectal cancers have abnormal immunohistochemical staining.10
Testing for microsatellite instability and mismatch repair gene expression ideally precedes germline genetic testing and helps to guide which gene or genes should be tested.9,14
Genetic testing for Lynch syndrome is routinely performed on a blood or saliva sample, using DNA from white blood cells and sequencing the gene or genes involved to look for mutations. Positive results from a germline genetic test confirm the diagnosis of Lynch syndrome and allow for predictive testing for relatives at risk. The term Lynch syndrome is used exclusively to describe individuals with evidence of a mutation in one of the mismatch repair genes.15
If a patient’s results are positive, genetic counseling and genetic testing should be offered to at-risk relatives age 18 and over.
Management of Lynch syndrome
Aggressive cancer surveillance is essential for people with Lynch syndrome and for those who are considered at risk but have not pursued genetic testing, such as a sibling of a person with Lynch syndrome.
Colorectal cancer. Colonoscopy is recommended every 1 to 2 years beginning at the age of 20 to 25 years, or 2 to 5 years earlier than the age of the youngest relative affected with colorectal cancer if the initial diagnosis was before age 25. When patients turn 40 years old, colonoscopy is done annually.16–18 A significant reduction in cancer incidence and in the mortality rate has been shown with colonoscopic surveillance.19–21
Chemoprevention may also have a role. Patients with Lynch syndrome who took aspirin 600 mg per day for an average of 25 months had a significantly lower incidence of colorectal cancer during a 55-month follow-up period compared with patients randomized to placebo.22
For patients with Lynch syndrome who are diagnosed with colorectal cancer, the high risk of metachronous cancers after standard segmental colectomy calls for a more extended resection. Retrospective analysis of 382 Lynch syndrome patients found that none of the 50 who underwent total or subtotal colectomy were diagnosed with metachronous colorectal cancer, whereas a metachronous cancer developed in 74 (22%) of the 332 patients who had had segmented resection.2 Annual surveillance of the remaining colon, rectum, or both is indicated postoperatively.
Gynecologic cancers. Women with Lynch syndrome should also consider gynecologic surveillance and risk-reducing surgery. This includes annual gynecologic examination, transvaginal ultrasonography, and endometrial aspiration, beginning at age 30 to 35 years. Although this surveillance does detect premalignant lesions and early symptomatic cancers, its effect on the mortality rate is unknown. Hysterectomy with bilateral salpingo-oophorectomy has been shown to significantly reduce endometrial and ovarian cancers in women with Lynch syndrome.23,24
Urothelial cancers. Carriers of MSH2 mutations have a significantly higher risk of urothelial cancers.4 Therefore, MSH2 carriers should consider ultrasonography of the urinary tract, urinary cytology, and urinalysis every 1 to 2 years beginning at age 40.4
Other extracolonic cancers. Poor evidence exists for systematic screening for the other extracolonic tumors associated with Lynch syndrome. However, the National Comprehensive Cancer Network advises considering esophagogastroduodenoscopy with extended duodenoscopy as well as capsule endoscopy every 2 to 3 years beginning at age 30 to 35.14
ADENOMATOUS POLYPOSIS SYNDROMES
Familial adenomatous polyposis and MYH-associated polyposis are the next most common hereditary colorectal cancer syndromes. Each of these accounts for about 1% of cases of colorectal cancer. Clinically, these two syndromes can be challenging to distinguish because they overlap phenotypically to a significant degree.
FAMILIAL ADENOMATOUS POLYPOSIS
Familial adenomatous polyposis is caused by mutations in the APC gene. Its prevalence is 2.29 to 3.2 per 100,000 individuals.25,26
Genetics of familial adenomatous polyposis
APC is the only gene known to cause familial adenomatous polyposis. Mutations in APC are inherited in an autosomal dominant manner. Approximately 25% of cases of familial adenomatous polyposis are due to a de novo mutation in APC.27
Clinical presentation of familial adenomatous polyposis
Familial adenomatous polyposis is classified by the burden of colorectal adenomas.
Patients who have fewer than 100 adenomas have an attenuated form of the disease. In this group, polyps usually begin to form in the late teenage years or early 20s and tend to develop in the proximal colon. The attenuated form is associated with an approximately 70% lifetime risk of colorectal cancer.28
Patients who have more than 100 polyps are considered to have the classic form of the disease, and those with more than 1,000 polyps have profuse familial adenomatous polyposis (Figure 2). In these groups, polyps typically begin to develop in the preteenage to mid-teenage years. Without surgery, there is nearly a 100% risk of colorectal cancer. The average age at diagnosis of colorectal cancer is 39 years for patients with classic disease.
Upper gastrointestinal polyps are common in familial adenomatous polyposis. Nearly 90% of patients develop duodenal adenomas by a mean age of 44, with a cumulative lifetime risk of nearly 100%.29 Fundic gland polyposis occurs in nearly 90% of patients,30 while gastric adenomas are reported in fewer than 15% of patients.
Duodenal and periampullary cancer is the second most common malignancy in familial adenomatous polyposis. The lifetime risk ranges from 2% to 36%, depending on the Spigelman stage. People with Spigelman stage I, II, or III have a 2.5% risk of duodenal cancer, while those with stage IV disease have up to a 36% lifetime risk.
Gastric cancer, arising from fundic gland polyps, has been reported but is rare in Western populations.
In familial adenomatous polyposis, the incidence of jejunal adenomas and cancer is less than 10%, and the risk of ileal adenomas and cancer is less than 1%.31
Familial adenomatous polyposis is also associated with a higher risk of other malignancies, including:
- Pancreatic cancer (2% lifetime risk)
- Thyroid cancer (2% to 3% lifetime risk, typically papillary carcinoma)32
- Hepatoblastoma (1% to 2% lifetime risk)
- Brain tumors (< 1% lifetime risk)
- Biliary cancer (higher risk than in the general population).33
Benign extracolonic manifestations that have been observed include osteomas, dental abnormalities (supernumerary teeth, unerupted or absent teeth, odontomas), congenital hypertrophy of the retinal pigment epithelium, benign cutaneous lesions (epidermoid cysts and fibromas), and desmoid tumors.33 The term “Gardner syndrome” has been used to describe patients who have familial adenomatous polyposis but also have osteomas and soft-tissue tumors.34 These patients carry the same risk of colorectal cancer as other patients with familial adenomatous polyposis.
Diagnosing familial adenomatous polyposis
The diagnosis of familial adenomatous polyposis is suspected when a patient has more than 10 adenomatous polyps.
Seventy-five percent of patients with familial adenomatous polyposis have a family history of the condition. Therefore, most cases are identified at a young age on screening sigmoidoscopy or colonoscopy or by predictive gene testing. Patients rarely have cancer at the time of diagnosis.
The other 25% of patients typically are diagnosed when symptoms develop from the polyps or cancer. Over 50% of these symptomatic patients have cancer at the time of diagnosis.
It is recommended that people who have more than 10 adenomas detected on a single colonoscopy or who are first-degree relatives of patients with familial adenomatous polyposis undergo a genetic evaluation and testing for mutations in the APC gene.14 Once an APC mutation is identified in the family, at-risk relatives should be offered testing around age 10 years for families with classic familial adenomatous polyposis or in the mid to late teenage years for those with the attenuated form. It also appropriate to refer patients with desmoid tumors, duodenal adenomas, and bilateral or multifocal congenital hypertrophy of the retinal pigment epithelium for a genetic evaluation.
Management of familial adenomatous polyposis
Flexible sigmoidoscopy every 1 to 2 years beginning at age 10 to 12 years is recommended for individuals and families who have been phenotypically or genetically diagnosed with familial adenomatous polyposis.35–37 If colorectal adenomas are found, surgical options should be discussed and annual colonoscopic surveillance should commence.
For people with the attenuated form, because of the later age of disease onset and the tendency for right-sided disease, colonoscopy every 1 to 2 years should commence at about age 18.35–37 If polyps are found, colonoscopy should be performed every year.
The decision of when to offer colectomy is based on polyp burden (taking into account the number, pathologic appearance, and size of the polyps) and psychosocial factors such as patient maturity. Surgical options include total colectomy and ileorectal anastomosis or total proctocolectomy and ileal pouch anal anastomosis.38 Colonic and extracolonic phenotype as well as genotype should factor into the type of operation recommended. After colectomy, annual endoscopic surveillance of the rectum or ileal pouch is indicated to screen for recurrent polyposis and cancer.
Chemoprevention with sulindac (Clinoril) 150 mg or celecoxib (Celebrex) 400 mg twice a day causes regression of colorectal adenomas in familial adenomatous polyposis and may be useful as an adjunct to endoscopy in managing the colorectal polyp burden.39,40
Forward and side-viewing upper endoscopy should commence at age 20. This should include visualization and biopsy of the papilla and periampulllary region.29 The frequency of endoscopic surveillance depends on the Spigelman stage, which reflects the duodenal polyp burden. It is recommended that patients with Spigelman stage IV duodenal polyposis be seen in consultation with an experienced gastrointestinal surgeon for consideration of a prophylactic, pylorus-preserving, pancreas-sparing duodenectomy. This procedure has been shown to be more effective in polyp control and cancer prevention than endoscopic polyp ablation and local surgical resection.41
Some evidence for the utility of celecoxib 400 mg twice daily for the regression of duodenal polyposis was noted in a 6-month placebo-controlled trial.42 Some experts recommend removal of large duodenal adenomas, with adjunctive celecoxib therapy to control polyposis burden.30
People with familial adenomatous polyposis have been shown to have a 2.6% risk of thyroid cancer, and ultrasonography of the neck with attention to the thyroid is recommended for them.32
MYH-ASSOCIATED POLYPOSIS
Biallelic mutations in the MYH gene result in an adenomatous polyposis syndrome that may be indistinguishable from the attenuated or classic forms of familial adenomatous polyposis. A characteristic autosomal recessive pattern of inheritance in the family can be useful for identifying these patients in the clinic.
Genetics of MYH-associated polyposis
MYH-associated polyposis is the only known autosomal recessive hereditary colorectal cancer syndrome. In white populations, the most commonly reported mutations in MYH are Y179C (previously called Y165C) and G396D (previously called G382D), which account for up to 80% of cases.43 These two mutations are estimated to occur in 1% to 2% of the general population.44
Clinical presentation of MYH-associated polyposis
MYH-associated polyposis typically presents as multiple adenomatous polyps and is diagnosed at a mean age of 47 years. Eleven percent to 42% of affected individuals are reported to have fewer than 100 adenomas, while a minority (7.5% to 29%) of patients present with classic polyposis.45–47 In one study, an estimated 19% of patients presented with colorectal cancer and reported no history of colorectal polyps.48 Synchronous colorectal cancer is seen in more than 60% of patients with biallelic MYH mutations.49 Patients with monoallelic (heterozygous) MYH mutations appear to have the same risk of developing colorectal adenomas and cancer as the general population.49
Upper-gastrointestinal polyps have been reported in MYH-associated polyposis; as many as 17% to 25% of patients have duodenal adenomas.50,51
Diagnosis of MYH-associated polyposis
Genetic testing for biallelic MYH mutations should be performed in patients who test negative for an APC mutation but who have clinical features of familial adenomatous polyposis, a personal history of more than 10 colorectal adenomas, or a recessive family history of polyposis. 14 It has been shown that up to 29% of patients with familial adenomatous polyposis who are APC-negative will have biallelic mutations in the MYH gene.52 The siblings of a patient with biallelic MYH mutations should be offered genetic counseling and testing in their late teens or early 20s. All children of an individual with MYH-associated polyposis will carry one MYH mutation and are only at risk of having the syndrome if the other parent is also a MYH carrier and passed on his or her mutation.
Management of MYH-associated polyposis
The management of patients with MYH-associated polyposis is similar to that recommended for attenuated and classic familial adenomatous polyposis.14 Genetic counseling and testing and colonic and extracolonic surveillance are warranted. There are no data on the use of chemoprevention in MYH-associated polyposis. Surgery should be considered early because of the high risk of colorectal cancer, even in individuals with very few adenomas. Patients with monoallelic MYH mutations should follow the general population screening guidelines for colorectal cancer.49
GENETIC COUNSELING AND GENETIC TESTING
The American College of Gastroenterology advises that patients suspected of having hereditary colorectal cancer syndromes be advised to pursue genetic counseling and, if appropriate, genetic testing.16 They further recommend genetic counseling and informed consent before genetic testing.16
Genetic counseling is a process of working with patients and families whereby:
- A detailed medical and family history is obtained
- A formal risk assessment is performed
- Education about the disease in question and about genetic testing is provided
- Psychosocial concerns are assessed
- Informed consent is obtained when genetic testing is recommended.53
This process is important for helping patients better understand their cancer risks, the benefits and limitations of genetic testing, and the protections that are in place for people who undergo genetic testing, including the Genetic Information Non-Discrimination Act.
In 1996 the American Society of Clinical Oncology issued a policy statement highlighting the essential elements of informed consent for genetic testing for cancer susceptibility, and this was updated in 2003.54 In particular, it notes that patients should be informed of the implications of positive and negative results and of the possibility that the test may be uninformative.
When a hereditary colorectal cancer syndrome is suspected, a positive genetic test result confirms the diagnosis and allows for predictive testing of the patient’s relatives. However, no genetic test for a hereditary colorectal cancer syndrome is 100% sensitive. Therefore, a negative result does not rule out the syndrome in question.
Further, all cancer susceptibility genes have variants of uncertain significance, which are genetic alterations for which there are insufficient data to determine if the mutation is disease-causing or polymorphic (benign). Both negative and uninformative results can be confusing for patients and providers and can lead to false reassurance or undue worry when patients are not properly educated about these potential outcomes of testing.
Genetic testing is an evolving field, and with additional research and improved testing technologies, appropriate diagnoses can be made over time. That is why it is important for the genetic counseling relationship to continue over time.
Hereditary colorectal cancer syndromes account for 5% to 10% of cases of colorectal cancer.
Identifying these patients in clinical practice begins by assessing a patient’s personal and family health history. An accurate and comprehensive family history should cover three generations and include ethnic background, ages and causes of death of relatives, and any diagnosis of cancer, including age at onset and history of polyps.
Red flags for a hereditary colorectal cancer syndrome in the personal or family history are:
- Early age of onset of cancer (eg, colorectal cancer before age 50)
- More than 10 colorectal adenomas
- Synchronous (ie, occurring at the same time) or metachronous (occurring at different times) primary cancers
- Multiple relatives in successive generations with the same or related cancers (eg, colon or endometrial cancer)
- A family member with a known hereditary colorectal cancer syndrome (Table 1).
Any of these red flags should prompt a referral for genetic counseling.
SYNDROMES ARE CLASSIFIED AS WITH OR WITHOUT POLYPOSIS
Many hereditary syndromes are associated with a higher risk of colorectal cancer. Generally, they can be divided into two categories (Table 2): polyposis syndromes (in which patients have numerous colorectal polyps) and nonpolyposis syndromes (with few or no polyps).
These two main types are subclassified on the basis of the histology of most of the polyps detected: adenomatous, hamartomatous, serrated, or mixed types.
In this review, we will address the three most common of these syndromes: Lynch syndrome (hereditary nonpolyposis colorectal cancer), familial adenomatous polyposis, and MYH-associated polyposis. However, as noted in Table 2, other hereditary colorectal cancer syndromes exist, and suspicion of these conditions should prompt a referral for further evaluation.
LYNCH SYNDROME (HEREDITARY NONPOLYPOSIS COLORECTAL CANCER)
Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer, predisposes people to a variety of cancers.
Colorectal cancer is the most common type of cancer associated with Lynch syndrome. Recent research suggests that the cumulative risk of developing colorectal cancer by age 80 is 42% for all patients with Lynch syndrome.1 The median age at onset is 45 years.1 For patients who undergo segmental resection of their initial cancer, the cumulative risk of metachronous colorectal cancer (ie, a new tumor arising later) is 16% at 10 years, 41% at 20 years, and up to 62% after 30 years.2
Endometrial cancer occurs in 17% to 57% of women with Lynch syndrome by age 70, with a median age at onset of 49 years.1
Other extracolonic cancers in Lynch syndrome include cancers of the:
- Stomach (1%–10% risk by age 70 years)
- Ovaries (1%–20% risk)
- Hepatobiliary tract (1%–2% risk)
- Urinary tract (1%–12% risk)
- Small bowel (1%–2% risk)
- Brain (1%–8% risk)
- Skin (sebaceous adenomas, adenocarcinomas, and keratoacanthomas).1,3,4
Earlier studies reported higher rates of associated cancer than those shown here. However, their data were largely derived from registries and may be overestimates. The numbers shown above are from population-based studies.
Genetics of Lynch syndrome
Lynch syndrome is caused by a germline mutation in the MLH1, MSH2, MSH6, PMS2, or EPCAM genes.5 These genes code for proteins that are responsible DNA mismatch repair—one of the cell’s proofreading mechanisms during DNA replication.
These mutations are inherited in an autosomal dominant manner. Though de novo mutations in these genes have been reported, they are rare and the exact frequency with which they occur is unknown.6
In whom should Lynch syndrome be suspected?
Lynch syndrome can be suspected on the basis of family history and clinical criteria.
In 1991, the same group of experts who coined the term “hereditary nonpolyposis colorectal cancer” developed family history criteria for it1:
- At least three relatives with histologically confirmed colorectal cancer, one of whom is a first-degree relative of the other two
- At least two successive generations involved
- At least one of the cancers diagnosed before age 50
- Familial adenomatous polyposis is excluded.
Known as the Amsterdam criteria, these were to be used in collaborative studies of families with hereditary colorectal cancer.7 In 1999, these criteria were broadened to include extracolonic cancers and became known as the Amsterdam II criteria (Table 3).8
Patients whose families meet the Amsterdam II criteria or who have molecular pathologic evidence of Lynch syndrome (see below) are appropriate candidates for genetic counseling and testing.
Diagnosis of Lynch syndrome
The diagnosis of Lynch syndrome is based on molecular pathologic analysis (performed on tumor samples) and confirmed by genetic testing.
Molecular pathologic evidence of Lynch syndrome includes microsatellite instability and loss of expression of one or more of the DNA mismatch repair proteins (detected using immunohistochemistry) (more on these below). The revised Bethesda guidelines (TABLE 3) were intended to identify individuals whose tumors should be tested for one or both of these phenomena.9
In 2009, the Evaluation of Genomic Applications in Practice and Prevention working group recommended that all patients with newly diagnosed colorectal cancer undergo microsatellite instability analysis, immunohistochemistry testing, or both, regardless of whether they meet the Amsterdam II or the Bethesda guideline criteria.10
Microsatellite instability analysis. Microsatellites are short sequences of repeated DNA. The tumor cells of patients who carry defective mismatch repair genes have microsatellites that are longer or shorter than in normal cells, a condition called microsatellite instability (ie, “MSI-high”).
Microsatellite instability testing, using a standardized panel of five DNA markers, is performed on normal and tumor tissue. If more than two of the five microsatellite markers in the tumor show instability, the lesion is considered to have a high level of microsatellite instability. About 15% of colorectal cancers have this high level, although most are not associated with Lynch syndrome and lose MLH1 expression by promoter methylation.11,12
While only 2% of patients with colorectal cancer have Lynch syndrome, from 90% to 95% of colorectal cancers from patients with Lynch syndrome have high levels of microsatellite instability.10 The presence of MLH1 promoter hypermethylation, the BRAF mutation V600E, or both within the tumor suggests that the cancer is not associated with Lynch syndrome.
Some families that meet the Amsterdam I criteria have microsatellite-stable tumors: their condition has been called familial colorectal cancer type X.13 This condition is associated with a higher risk of colorectal cancer but not the other malignancies observed in Lynch syndrome.
Immunohistochemistry is performed to assess for expression of the mismatch repair proteins MSH2, MSH6, MLH1, and PMS2. Absence of expression of the specific protein within tumor cells compared with normal cells within the specimen suggests dysfunction of the specific gene and guides germline mutation testing (Figure 1). For example, a patient who lacks expression of the MSH2 protein in his or her colon cancer most likely has a mutation in the MSH2 gene. Therefore, germ-line genetic testing should initially target the MSH2 gene. Approximately 88% of Lynch syndrome-associated colorectal cancers have abnormal immunohistochemical staining.10
Testing for microsatellite instability and mismatch repair gene expression ideally precedes germline genetic testing and helps to guide which gene or genes should be tested.9,14
Genetic testing for Lynch syndrome is routinely performed on a blood or saliva sample, using DNA from white blood cells and sequencing the gene or genes involved to look for mutations. Positive results from a germline genetic test confirm the diagnosis of Lynch syndrome and allow for predictive testing for relatives at risk. The term Lynch syndrome is used exclusively to describe individuals with evidence of a mutation in one of the mismatch repair genes.15
If a patient’s results are positive, genetic counseling and genetic testing should be offered to at-risk relatives age 18 and over.
Management of Lynch syndrome
Aggressive cancer surveillance is essential for people with Lynch syndrome and for those who are considered at risk but have not pursued genetic testing, such as a sibling of a person with Lynch syndrome.
Colorectal cancer. Colonoscopy is recommended every 1 to 2 years beginning at the age of 20 to 25 years, or 2 to 5 years earlier than the age of the youngest relative affected with colorectal cancer if the initial diagnosis was before age 25. When patients turn 40 years old, colonoscopy is done annually.16–18 A significant reduction in cancer incidence and in the mortality rate has been shown with colonoscopic surveillance.19–21
Chemoprevention may also have a role. Patients with Lynch syndrome who took aspirin 600 mg per day for an average of 25 months had a significantly lower incidence of colorectal cancer during a 55-month follow-up period compared with patients randomized to placebo.22
For patients with Lynch syndrome who are diagnosed with colorectal cancer, the high risk of metachronous cancers after standard segmental colectomy calls for a more extended resection. Retrospective analysis of 382 Lynch syndrome patients found that none of the 50 who underwent total or subtotal colectomy were diagnosed with metachronous colorectal cancer, whereas a metachronous cancer developed in 74 (22%) of the 332 patients who had had segmented resection.2 Annual surveillance of the remaining colon, rectum, or both is indicated postoperatively.
Gynecologic cancers. Women with Lynch syndrome should also consider gynecologic surveillance and risk-reducing surgery. This includes annual gynecologic examination, transvaginal ultrasonography, and endometrial aspiration, beginning at age 30 to 35 years. Although this surveillance does detect premalignant lesions and early symptomatic cancers, its effect on the mortality rate is unknown. Hysterectomy with bilateral salpingo-oophorectomy has been shown to significantly reduce endometrial and ovarian cancers in women with Lynch syndrome.23,24
Urothelial cancers. Carriers of MSH2 mutations have a significantly higher risk of urothelial cancers.4 Therefore, MSH2 carriers should consider ultrasonography of the urinary tract, urinary cytology, and urinalysis every 1 to 2 years beginning at age 40.4
Other extracolonic cancers. Poor evidence exists for systematic screening for the other extracolonic tumors associated with Lynch syndrome. However, the National Comprehensive Cancer Network advises considering esophagogastroduodenoscopy with extended duodenoscopy as well as capsule endoscopy every 2 to 3 years beginning at age 30 to 35.14
ADENOMATOUS POLYPOSIS SYNDROMES
Familial adenomatous polyposis and MYH-associated polyposis are the next most common hereditary colorectal cancer syndromes. Each of these accounts for about 1% of cases of colorectal cancer. Clinically, these two syndromes can be challenging to distinguish because they overlap phenotypically to a significant degree.
FAMILIAL ADENOMATOUS POLYPOSIS
Familial adenomatous polyposis is caused by mutations in the APC gene. Its prevalence is 2.29 to 3.2 per 100,000 individuals.25,26
Genetics of familial adenomatous polyposis
APC is the only gene known to cause familial adenomatous polyposis. Mutations in APC are inherited in an autosomal dominant manner. Approximately 25% of cases of familial adenomatous polyposis are due to a de novo mutation in APC.27
Clinical presentation of familial adenomatous polyposis
Familial adenomatous polyposis is classified by the burden of colorectal adenomas.
Patients who have fewer than 100 adenomas have an attenuated form of the disease. In this group, polyps usually begin to form in the late teenage years or early 20s and tend to develop in the proximal colon. The attenuated form is associated with an approximately 70% lifetime risk of colorectal cancer.28
Patients who have more than 100 polyps are considered to have the classic form of the disease, and those with more than 1,000 polyps have profuse familial adenomatous polyposis (Figure 2). In these groups, polyps typically begin to develop in the preteenage to mid-teenage years. Without surgery, there is nearly a 100% risk of colorectal cancer. The average age at diagnosis of colorectal cancer is 39 years for patients with classic disease.
Upper gastrointestinal polyps are common in familial adenomatous polyposis. Nearly 90% of patients develop duodenal adenomas by a mean age of 44, with a cumulative lifetime risk of nearly 100%.29 Fundic gland polyposis occurs in nearly 90% of patients,30 while gastric adenomas are reported in fewer than 15% of patients.
Duodenal and periampullary cancer is the second most common malignancy in familial adenomatous polyposis. The lifetime risk ranges from 2% to 36%, depending on the Spigelman stage. People with Spigelman stage I, II, or III have a 2.5% risk of duodenal cancer, while those with stage IV disease have up to a 36% lifetime risk.
Gastric cancer, arising from fundic gland polyps, has been reported but is rare in Western populations.
In familial adenomatous polyposis, the incidence of jejunal adenomas and cancer is less than 10%, and the risk of ileal adenomas and cancer is less than 1%.31
Familial adenomatous polyposis is also associated with a higher risk of other malignancies, including:
- Pancreatic cancer (2% lifetime risk)
- Thyroid cancer (2% to 3% lifetime risk, typically papillary carcinoma)32
- Hepatoblastoma (1% to 2% lifetime risk)
- Brain tumors (< 1% lifetime risk)
- Biliary cancer (higher risk than in the general population).33
Benign extracolonic manifestations that have been observed include osteomas, dental abnormalities (supernumerary teeth, unerupted or absent teeth, odontomas), congenital hypertrophy of the retinal pigment epithelium, benign cutaneous lesions (epidermoid cysts and fibromas), and desmoid tumors.33 The term “Gardner syndrome” has been used to describe patients who have familial adenomatous polyposis but also have osteomas and soft-tissue tumors.34 These patients carry the same risk of colorectal cancer as other patients with familial adenomatous polyposis.
Diagnosing familial adenomatous polyposis
The diagnosis of familial adenomatous polyposis is suspected when a patient has more than 10 adenomatous polyps.
Seventy-five percent of patients with familial adenomatous polyposis have a family history of the condition. Therefore, most cases are identified at a young age on screening sigmoidoscopy or colonoscopy or by predictive gene testing. Patients rarely have cancer at the time of diagnosis.
The other 25% of patients typically are diagnosed when symptoms develop from the polyps or cancer. Over 50% of these symptomatic patients have cancer at the time of diagnosis.
It is recommended that people who have more than 10 adenomas detected on a single colonoscopy or who are first-degree relatives of patients with familial adenomatous polyposis undergo a genetic evaluation and testing for mutations in the APC gene.14 Once an APC mutation is identified in the family, at-risk relatives should be offered testing around age 10 years for families with classic familial adenomatous polyposis or in the mid to late teenage years for those with the attenuated form. It also appropriate to refer patients with desmoid tumors, duodenal adenomas, and bilateral or multifocal congenital hypertrophy of the retinal pigment epithelium for a genetic evaluation.
Management of familial adenomatous polyposis
Flexible sigmoidoscopy every 1 to 2 years beginning at age 10 to 12 years is recommended for individuals and families who have been phenotypically or genetically diagnosed with familial adenomatous polyposis.35–37 If colorectal adenomas are found, surgical options should be discussed and annual colonoscopic surveillance should commence.
For people with the attenuated form, because of the later age of disease onset and the tendency for right-sided disease, colonoscopy every 1 to 2 years should commence at about age 18.35–37 If polyps are found, colonoscopy should be performed every year.
The decision of when to offer colectomy is based on polyp burden (taking into account the number, pathologic appearance, and size of the polyps) and psychosocial factors such as patient maturity. Surgical options include total colectomy and ileorectal anastomosis or total proctocolectomy and ileal pouch anal anastomosis.38 Colonic and extracolonic phenotype as well as genotype should factor into the type of operation recommended. After colectomy, annual endoscopic surveillance of the rectum or ileal pouch is indicated to screen for recurrent polyposis and cancer.
Chemoprevention with sulindac (Clinoril) 150 mg or celecoxib (Celebrex) 400 mg twice a day causes regression of colorectal adenomas in familial adenomatous polyposis and may be useful as an adjunct to endoscopy in managing the colorectal polyp burden.39,40
Forward and side-viewing upper endoscopy should commence at age 20. This should include visualization and biopsy of the papilla and periampulllary region.29 The frequency of endoscopic surveillance depends on the Spigelman stage, which reflects the duodenal polyp burden. It is recommended that patients with Spigelman stage IV duodenal polyposis be seen in consultation with an experienced gastrointestinal surgeon for consideration of a prophylactic, pylorus-preserving, pancreas-sparing duodenectomy. This procedure has been shown to be more effective in polyp control and cancer prevention than endoscopic polyp ablation and local surgical resection.41
Some evidence for the utility of celecoxib 400 mg twice daily for the regression of duodenal polyposis was noted in a 6-month placebo-controlled trial.42 Some experts recommend removal of large duodenal adenomas, with adjunctive celecoxib therapy to control polyposis burden.30
People with familial adenomatous polyposis have been shown to have a 2.6% risk of thyroid cancer, and ultrasonography of the neck with attention to the thyroid is recommended for them.32
MYH-ASSOCIATED POLYPOSIS
Biallelic mutations in the MYH gene result in an adenomatous polyposis syndrome that may be indistinguishable from the attenuated or classic forms of familial adenomatous polyposis. A characteristic autosomal recessive pattern of inheritance in the family can be useful for identifying these patients in the clinic.
Genetics of MYH-associated polyposis
MYH-associated polyposis is the only known autosomal recessive hereditary colorectal cancer syndrome. In white populations, the most commonly reported mutations in MYH are Y179C (previously called Y165C) and G396D (previously called G382D), which account for up to 80% of cases.43 These two mutations are estimated to occur in 1% to 2% of the general population.44
Clinical presentation of MYH-associated polyposis
MYH-associated polyposis typically presents as multiple adenomatous polyps and is diagnosed at a mean age of 47 years. Eleven percent to 42% of affected individuals are reported to have fewer than 100 adenomas, while a minority (7.5% to 29%) of patients present with classic polyposis.45–47 In one study, an estimated 19% of patients presented with colorectal cancer and reported no history of colorectal polyps.48 Synchronous colorectal cancer is seen in more than 60% of patients with biallelic MYH mutations.49 Patients with monoallelic (heterozygous) MYH mutations appear to have the same risk of developing colorectal adenomas and cancer as the general population.49
Upper-gastrointestinal polyps have been reported in MYH-associated polyposis; as many as 17% to 25% of patients have duodenal adenomas.50,51
Diagnosis of MYH-associated polyposis
Genetic testing for biallelic MYH mutations should be performed in patients who test negative for an APC mutation but who have clinical features of familial adenomatous polyposis, a personal history of more than 10 colorectal adenomas, or a recessive family history of polyposis. 14 It has been shown that up to 29% of patients with familial adenomatous polyposis who are APC-negative will have biallelic mutations in the MYH gene.52 The siblings of a patient with biallelic MYH mutations should be offered genetic counseling and testing in their late teens or early 20s. All children of an individual with MYH-associated polyposis will carry one MYH mutation and are only at risk of having the syndrome if the other parent is also a MYH carrier and passed on his or her mutation.
Management of MYH-associated polyposis
The management of patients with MYH-associated polyposis is similar to that recommended for attenuated and classic familial adenomatous polyposis.14 Genetic counseling and testing and colonic and extracolonic surveillance are warranted. There are no data on the use of chemoprevention in MYH-associated polyposis. Surgery should be considered early because of the high risk of colorectal cancer, even in individuals with very few adenomas. Patients with monoallelic MYH mutations should follow the general population screening guidelines for colorectal cancer.49
GENETIC COUNSELING AND GENETIC TESTING
The American College of Gastroenterology advises that patients suspected of having hereditary colorectal cancer syndromes be advised to pursue genetic counseling and, if appropriate, genetic testing.16 They further recommend genetic counseling and informed consent before genetic testing.16
Genetic counseling is a process of working with patients and families whereby:
- A detailed medical and family history is obtained
- A formal risk assessment is performed
- Education about the disease in question and about genetic testing is provided
- Psychosocial concerns are assessed
- Informed consent is obtained when genetic testing is recommended.53
This process is important for helping patients better understand their cancer risks, the benefits and limitations of genetic testing, and the protections that are in place for people who undergo genetic testing, including the Genetic Information Non-Discrimination Act.
In 1996 the American Society of Clinical Oncology issued a policy statement highlighting the essential elements of informed consent for genetic testing for cancer susceptibility, and this was updated in 2003.54 In particular, it notes that patients should be informed of the implications of positive and negative results and of the possibility that the test may be uninformative.
When a hereditary colorectal cancer syndrome is suspected, a positive genetic test result confirms the diagnosis and allows for predictive testing of the patient’s relatives. However, no genetic test for a hereditary colorectal cancer syndrome is 100% sensitive. Therefore, a negative result does not rule out the syndrome in question.
Further, all cancer susceptibility genes have variants of uncertain significance, which are genetic alterations for which there are insufficient data to determine if the mutation is disease-causing or polymorphic (benign). Both negative and uninformative results can be confusing for patients and providers and can lead to false reassurance or undue worry when patients are not properly educated about these potential outcomes of testing.
Genetic testing is an evolving field, and with additional research and improved testing technologies, appropriate diagnoses can be made over time. That is why it is important for the genetic counseling relationship to continue over time.
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011; 305:2304–2310.
- Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 2011; 60:950–957.
- Barrow E, Robinson L, Alduaij W, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet 2009; 75:141–149.
- van der Post RS, Kiemeney LA, Ligtenberg MJ, et al. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J Med Genet 2010; 47:464–470.
- Wijnen JT, Vasen HF, Khan PM, et al. Clinical findings with implications for genetic testing in families with clustering of colorectal cancer. N Engl J Med 1998; 339:511–518.
- Bisgaard ML, Bernstein I. HNPCC mutation rate. Familial Cancer 2003; 2.
- Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum 1991; 34:424–425.
- Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999; 116:1453–1456.
- Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004; 96:261–268.
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: can UGT1A1 genotyping reduce morbidity and mortality in patients with metastatic colorectal cancer treated with irinotecan? Genet Med 2009; 11:15–20.
- Aaltonen LA, Peltomäki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993; 260:812–816.
- Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994; 145:148–156.
- Lindor NM, Rabe K, Petersen GM, et al. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA 2005; 293:1979–1985.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines) colorectal cancer screening version 2.2011. www.nccn.org. Accessed October 2, 2012.
- Jass JR. Hereditary non-polyposis colorectal cancer: the rise and fall of a confusing term. World J Gastroenterol 2006; 12:4943–4950.
- Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM; American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol 2009; 104:739–750.
- Winawer S, Fletcher R, Rex D, et al; Gastrointestinal Consortium Panel. Colorectal cancer screening and surveillance: clinical guidelines and rationale-update based on new evidence. Gastroenterology 2003; 124:544–560.
- Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 2006; 296:1507–1517.
- de Jong AE, Hendriks YM, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 2006; 130:665–671.
- Mecklin JP, Aarnio M, Läärä E, et al. Development of colorectal tumors in colonoscopic surveillance in Lynch syndrome. Gastroenterology 2007; 133:1093–1098.
- Engel C, Rahner N, Schulmann K, et al; German HNPCC Consortium. Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol 2010; 8:174–182.
- Burn J, Gerdes AM, Macrae F, et al; CAPP2 Investigators. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011; 378:2081–2087.
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer). J Med Genet 2007; 44:353–362.
- Manchanda R, Menon U, Michaelson-Cohen R, Beller U, Jacobs I. Hereditary non-polyposis colorectal cancer or Lynch syndrome: the gynaecological perspective. Curr Opin Obstet Gynecol 2009; 21:31–38.
- Burn J, Chapman P, Delhanty J, et al. The UK Northern region genetic register for familial adenomatous polyposis coli: use of age of onset, congenital hypertrophy of the retinal pigment epithelium, and DNA markers in risk calculations. J Med Genet 1991; 28:289–296.
- Järvinen HJ. Epidemiology of familial adenomatous polyposis in Finland: impact of family screening on the colorectal cancer rate and survival. Gut 1992; 33:357–360.
- Bisgaard ML, Fenger K, Bülow S, Niebuhr E, Mohr J. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum Mutat 1994; 3:121–125.
- Neklason DW, Stevens J, Boucher KM, et al. American founder mutation for attenuated familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008; 6:46–52.
- Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc 1999; 49:358–364.
- Bianchi LK, Burke CA, Bennett AE, Lopez R, Hasson H, Church JM. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008; 6:180–185.
- Kadmon M, Tandara A, Herfarth C. Duodenal adenomatosis in familial adenomatous polyposis coli. A review of the literature and results from the Heidelberg Polyposis Register. Int J Colorectal Dis 2001; 16:63–75.
- Jarrar AM, Milas M, Mitchell J, et al. Screening for thyroid cancer in patients with familial adenomatous polyposis. Ann Surg 2011; 253:515–521.
- Jasperson KW, Burt RW. APC-associated polyposis conditions. In:Pagon RA, Bird TD, Dolan CR, et al, eds. GeneReviews (Internet). Seattle, WA: University of Washington; 2011.
- Gardner EJ, Richards RC. Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. Am J Hum Genet 1953; 5:139–147.
- Dunlop MG; British Society for Gastroenterology. Guidance on gastrointestinal surveillance for hereditary non-polyposis colorectal cancer, familial adenomatous polyposis, juvenile polyposis, and Peutz-Jeghers syndrome. Gut 2002; 51(suppl 5):V21–V27.
- Burke W, Petersen G, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. Cancer Genetics Studies Consortium. JAMA 1997; 277:915–919.
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008; 57:704–713.
- Church J. Familial adenomatous polyposis. Surg Oncol Clin N Am 2009; 18:585–598.
- Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993; 328:1313–1316.
- Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000; 342:1946–1952.
- Johnson MD, Mackey R, Brown N, Church J, Burke C, Walsh RM. Outcome based on management for duodenal adenomas: sporadic versus familial disease. J Gastrointest Surg 2010; 14:229–235.
- Phillips RK, Wallace MH, Lynch PM, et al; FAP Study Group. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut 2002; 50:857–860.
- Tenesa A, Campbell H, Barnetson R, Porteous M, Dunlop M, Farrington SM. Association of MUTYH and colorectal cancer. Br J Cancer 2006; 95:239–242.
- Croitoru ME, Cleary SP, Di Nicola N, et al. Association between biallelic and monoallelic germline MYH gene mutations and colorectal cancer risk. J Natl Cancer Inst 2004; 96:1631–1634.
- Croitoru ME, Cleary SP, Berk T, et al. Germline MYH mutations in a clinic-based series of Canadian multiple colorectal adenoma patients. J Surg Oncol 2007; 95:499–506.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet 2003; 362:39–41.
- Nielsen M, Franken PF, Reinards TH, et al. Multiplicity in polyp count and extracolonic manifestations in 40 Dutch patients with MYH associated polyposis coli (MAP). J Med Genet 2005; 42:e54.
- Cleary SP, Cotterchio M, Jenkins MA, et al. Germline MutY human homologue mutations and colorectal cancer: a multisite case-control study. Gastroenterology 2009; 136:1251–1260.
- Lubbe SJ, Di Bernardo MC, Chandler IP, Houlston RS. Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J Clin Oncol 2009; 27:3975–3980.
- Aretz S, Uhlhaas S, Goergens H, et al. MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int J Cancer 2006; 119:807–814.
- Vogt S, Jones N, Christian D, et al. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology 2009; 137:1976–1985.e1–e10.
- Gismondi V, Meta M, Bonelli L, et al. Prevalence of the Y165C, G382D and 1395delGGA germline mutations of the MYH gene in Italian patients with adenomatous polyposis coli and colorectal adenomas. Int J Cancer 2004; 109:680–684.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the national society of genetic counselors. J Genet Couns 2004; 13:83–114.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
- Bonadona V, Bonaïti B, Olschwang S, et al. Cancer risks associated with germline mutations in MLH1, MSH2, and MSH6 genes in Lynch syndrome. JAMA 2011; 305:2304–2310.
- Parry S, Win AK, Parry B, et al. Metachronous colorectal cancer risk for mismatch repair gene mutation carriers: the advantage of more extensive colon surgery. Gut 2011; 60:950–957.
- Barrow E, Robinson L, Alduaij W, et al. Cumulative lifetime incidence of extracolonic cancers in Lynch syndrome: a report of 121 families with proven mutations. Clin Genet 2009; 75:141–149.
- van der Post RS, Kiemeney LA, Ligtenberg MJ, et al. Risk of urothelial bladder cancer in Lynch syndrome is increased, in particular among MSH2 mutation carriers. J Med Genet 2010; 47:464–470.
- Wijnen JT, Vasen HF, Khan PM, et al. Clinical findings with implications for genetic testing in families with clustering of colorectal cancer. N Engl J Med 1998; 339:511–518.
- Bisgaard ML, Bernstein I. HNPCC mutation rate. Familial Cancer 2003; 2.
- Vasen HF, Mecklin JP, Khan PM, Lynch HT. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum 1991; 34:424–425.
- Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology 1999; 116:1453–1456.
- Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. J Natl Cancer Inst 2004; 96:261–268.
- Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: can UGT1A1 genotyping reduce morbidity and mortality in patients with metastatic colorectal cancer treated with irinotecan? Genet Med 2009; 11:15–20.
- Aaltonen LA, Peltomäki P, Leach FS, et al. Clues to the pathogenesis of familial colorectal cancer. Science 1993; 260:812–816.
- Kim H, Jen J, Vogelstein B, Hamilton SR. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994; 145:148–156.
- Lindor NM, Rabe K, Petersen GM, et al. Lower cancer incidence in Amsterdam-I criteria families without mismatch repair deficiency: familial colorectal cancer type X. JAMA 2005; 293:1979–1985.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines) colorectal cancer screening version 2.2011. www.nccn.org. Accessed October 2, 2012.
- Jass JR. Hereditary non-polyposis colorectal cancer: the rise and fall of a confusing term. World J Gastroenterol 2006; 12:4943–4950.
- Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM; American College of Gastroenterology. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol 2009; 104:739–750.
- Winawer S, Fletcher R, Rex D, et al; Gastrointestinal Consortium Panel. Colorectal cancer screening and surveillance: clinical guidelines and rationale-update based on new evidence. Gastroenterology 2003; 124:544–560.
- Lindor NM, Petersen GM, Hadley DW, et al. Recommendations for the care of individuals with an inherited predisposition to Lynch syndrome: a systematic review. JAMA 2006; 296:1507–1517.
- de Jong AE, Hendriks YM, Kleibeuker JH, et al. Decrease in mortality in Lynch syndrome families because of surveillance. Gastroenterology 2006; 130:665–671.
- Mecklin JP, Aarnio M, Läärä E, et al. Development of colorectal tumors in colonoscopic surveillance in Lynch syndrome. Gastroenterology 2007; 133:1093–1098.
- Engel C, Rahner N, Schulmann K, et al; German HNPCC Consortium. Efficacy of annual colonoscopic surveillance in individuals with hereditary nonpolyposis colorectal cancer. Clin Gastroenterol Hepatol 2010; 8:174–182.
- Burn J, Gerdes AM, Macrae F, et al; CAPP2 Investigators. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011; 378:2081–2087.
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of Lynch syndrome (hereditary non-polyposis cancer). J Med Genet 2007; 44:353–362.
- Manchanda R, Menon U, Michaelson-Cohen R, Beller U, Jacobs I. Hereditary non-polyposis colorectal cancer or Lynch syndrome: the gynaecological perspective. Curr Opin Obstet Gynecol 2009; 21:31–38.
- Burn J, Chapman P, Delhanty J, et al. The UK Northern region genetic register for familial adenomatous polyposis coli: use of age of onset, congenital hypertrophy of the retinal pigment epithelium, and DNA markers in risk calculations. J Med Genet 1991; 28:289–296.
- Järvinen HJ. Epidemiology of familial adenomatous polyposis in Finland: impact of family screening on the colorectal cancer rate and survival. Gut 1992; 33:357–360.
- Bisgaard ML, Fenger K, Bülow S, Niebuhr E, Mohr J. Familial adenomatous polyposis (FAP): frequency, penetrance, and mutation rate. Hum Mutat 1994; 3:121–125.
- Neklason DW, Stevens J, Boucher KM, et al. American founder mutation for attenuated familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008; 6:46–52.
- Burke CA, Beck GJ, Church JM, van Stolk RU. The natural history of untreated duodenal and ampullary adenomas in patients with familial adenomatous polyposis followed in an endoscopic surveillance program. Gastrointest Endosc 1999; 49:358–364.
- Bianchi LK, Burke CA, Bennett AE, Lopez R, Hasson H, Church JM. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol 2008; 6:180–185.
- Kadmon M, Tandara A, Herfarth C. Duodenal adenomatosis in familial adenomatous polyposis coli. A review of the literature and results from the Heidelberg Polyposis Register. Int J Colorectal Dis 2001; 16:63–75.
- Jarrar AM, Milas M, Mitchell J, et al. Screening for thyroid cancer in patients with familial adenomatous polyposis. Ann Surg 2011; 253:515–521.
- Jasperson KW, Burt RW. APC-associated polyposis conditions. In:Pagon RA, Bird TD, Dolan CR, et al, eds. GeneReviews (Internet). Seattle, WA: University of Washington; 2011.
- Gardner EJ, Richards RC. Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. Am J Hum Genet 1953; 5:139–147.
- Dunlop MG; British Society for Gastroenterology. Guidance on gastrointestinal surveillance for hereditary non-polyposis colorectal cancer, familial adenomatous polyposis, juvenile polyposis, and Peutz-Jeghers syndrome. Gut 2002; 51(suppl 5):V21–V27.
- Burke W, Petersen G, Lynch P, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. I. Hereditary nonpolyposis colon cancer. Cancer Genetics Studies Consortium. JAMA 1997; 277:915–919.
- Vasen HF, Möslein G, Alonso A, et al. Guidelines for the clinical management of familial adenomatous polyposis (FAP). Gut 2008; 57:704–713.
- Church J. Familial adenomatous polyposis. Surg Oncol Clin N Am 2009; 18:585–598.
- Giardiello FM, Hamilton SR, Krush AJ, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med 1993; 328:1313–1316.
- Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000; 342:1946–1952.
- Johnson MD, Mackey R, Brown N, Church J, Burke C, Walsh RM. Outcome based on management for duodenal adenomas: sporadic versus familial disease. J Gastrointest Surg 2010; 14:229–235.
- Phillips RK, Wallace MH, Lynch PM, et al; FAP Study Group. A randomised, double blind, placebo controlled study of celecoxib, a selective cyclooxygenase 2 inhibitor, on duodenal polyposis in familial adenomatous polyposis. Gut 2002; 50:857–860.
- Tenesa A, Campbell H, Barnetson R, Porteous M, Dunlop M, Farrington SM. Association of MUTYH and colorectal cancer. Br J Cancer 2006; 95:239–242.
- Croitoru ME, Cleary SP, Di Nicola N, et al. Association between biallelic and monoallelic germline MYH gene mutations and colorectal cancer risk. J Natl Cancer Inst 2004; 96:1631–1634.
- Croitoru ME, Cleary SP, Berk T, et al. Germline MYH mutations in a clinic-based series of Canadian multiple colorectal adenoma patients. J Surg Oncol 2007; 95:499–506.
- Sampson JR, Dolwani S, Jones S, et al. Autosomal recessive colorectal adenomatous polyposis due to inherited mutations of MYH. Lancet 2003; 362:39–41.
- Nielsen M, Franken PF, Reinards TH, et al. Multiplicity in polyp count and extracolonic manifestations in 40 Dutch patients with MYH associated polyposis coli (MAP). J Med Genet 2005; 42:e54.
- Cleary SP, Cotterchio M, Jenkins MA, et al. Germline MutY human homologue mutations and colorectal cancer: a multisite case-control study. Gastroenterology 2009; 136:1251–1260.
- Lubbe SJ, Di Bernardo MC, Chandler IP, Houlston RS. Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J Clin Oncol 2009; 27:3975–3980.
- Aretz S, Uhlhaas S, Goergens H, et al. MUTYH-associated polyposis: 70 of 71 patients with biallelic mutations present with an attenuated or atypical phenotype. Int J Cancer 2006; 119:807–814.
- Vogt S, Jones N, Christian D, et al. Expanded extracolonic tumor spectrum in MUTYH-associated polyposis. Gastroenterology 2009; 137:1976–1985.e1–e10.
- Gismondi V, Meta M, Bonelli L, et al. Prevalence of the Y165C, G382D and 1395delGGA germline mutations of the MYH gene in Italian patients with adenomatous polyposis coli and colorectal adenomas. Int J Cancer 2004; 109:680–684.
- Trepanier A, Ahrens M, McKinnon W, et al; National Society of Genetic Counselors. Genetic cancer risk assessment and counseling: recommendations of the national society of genetic counselors. J Genet Couns 2004; 13:83–114.
- American Society of Clinical Oncology. American Society of Clinical Oncology policy statement update: genetic testing for cancer susceptibility. J Clin Oncol 2003; 21:2397–2406.
KEY POINTS
- Hereditary colorectal cancer syndromes carry a substantial risk of intestinal and extraintestinal tumors.
- Affected patients need increased cancer surveillance and may benefit from prophylactic surgery.
- Identifying these patients in clinical practice begins by assessing a patient’s personal and family health history.
- Patients suspected of having hereditary colorectal cancer syndromes should be referred for genetic counseling and, if appropriate, for genetic testing.
Nail pigmentation and fatigue
A 49-year-old man presented with pigmentation on the nails of both hands during the past 6 months. He had no history of significant medical conditions, but he complained of generalized asthenia, with no fever, during the previous 6 weeks. He said that he was a volcanologist and had recently returned from an expedition in the mountains of Peru that involved intense physical activity at high altitudes. He was a non-smoker and did not drink alcohol; he was not taking any medications or nutritional supplements. A biochemical profile including a white blood cell count 3 months ago was normal.
On physical examination, he had splenomegaly (about 2 cm below the costal margin) and low systolic blood pressure (90 mm Hg, with the diastolic pressure at 60 mm Hg). Heart and lung examinations, including chest radiography, were normal.
Q: What is the diagnosis?
- Addison disease
- Onychotillomania
- Longitudinal melanonychia in human immunodeficiency virus (HIV) infection
- Systemic lupus erythematosus
A: The patient was diagnosed with longitudinal melanonychia with underlying HIV infection.
In addition to the clinical findings noted above, laboratory testing now revealed a low white blood cell count (2.9 × 109/L, reference range 4.5–11.0) and a low CD4+ T-cell count (182 cells/mL). An enzyme-linked immunosorbent assay and a Western blot assay were positive for HIV, and his viral load was 120,000 copies/mL as determined by reverse transcription-polymerase chain reaction testing. On more detailed questioning, the patient admitted to sexual practices that put him at high risk for HIV infection.
Longitudinal melanonychia is characterized by a tan, brown, or black longitudinal streak, originating in the matrix and resulting from increased synthesis of melanin (by either normal or abnormal melanocytes) and its deposition within the nail plate.1
If the cause is not apparent (eg, trauma, application of chemical agents, concomitant onychomycosis or treatments), this pigmentation usually reflects the presence of a benign lesion within the matrix caused by a melanocytic nevus, simple lentigo, or increased activation of benign melanocytes. This finding has been reported in a US study2 to be more common in people with dark skin, occurring in 77% of blacks over age 20 and in almost 100% of those over age 50.
Melanoma and other nonmelanocytic tumors may present as longitudinal melanonychia, but these rarely involve more than one digit simultaneously, and they are usually detected with specific clinical clues, such as the Hutchinson sign, ie, periungual spread of pigmentation into the proximal and lateral nail folds.
A SIGN OF BENIGN AND MALIGNANT CONDITIONS
Longitudinal melanonychia can have benign causes, and these need to be investigated. It can occur with repeated trauma, friction, or pressure on the nails. The physician should question the patient about habits such as picking or chewing of the finger tips or pushing back the cuticles, as well as about athletic activities or manual work that could affect the nails. In addition, blood thinners can cause nail discoloration via subungual hemorrhage.
A number of pathologic conditions can cause longitudinal discoloration of the nails. Infection with certain dermatophytes can cause discoloration, but this pigmentation is usually wider and more distal at the hyponychium rather than proximal, with a black streak with several pointed extensions proximally.
Adrenocortical insufficiency (Addison disease), nutritional disorders (deficiency of folic acid or vitamin B12), and, more rarely, systemic lupus erythematosus and scleroderma should be ruled out, as they are typically associated with cutaneous and mucosal hyperpigmentation.
Chemotherapeutic agents such as doxorubicin (Adriamycin), hydroxyurea (Droxea), and cyclophosphamide and drugs such as antimalarials and tetracyclines can also cause longitudinal melanonychia.1,3
Nail lesions with a similar pattern are often seen in patients (especially black patients) taking zidovudine (Retrovir), and these lesions seem not to be related to drug dosage, HIV risk group, or severity of the infection. Nail discoloration in HIV patients not taking antiretroviral therapy has not been well documented.
The significance of melanonychia in the course of HIV remains unclear. A key role of overexpression of alpha-melanocyte-stimulating-hormone has been proposed.4 Although rare in fair-skinned people, longitudinal melanonychia represents an important clinical sign of disease progression. In reported cases, however, no significant improvement has been noted with treatment despite favorable virologic and immunologic responses.4,5
- Smith DF, Morgan MB, Bettencourt MS. Longitudinal melanonychia. Arch Dermatol 2003; 139:1209–1214.
- Baran R, Kechijian P. Longitudinal melanonychia (melanonychia striata): diagnosis and management. J Am Acad Dermatol 1989; 21:1165–1175.
- Haneke E, Baran R. Longitudinal melanonychia. Dermatol Surg 2001; 27:580–584.
- Ward HA, Russo GG, Shrum J. Cutaneous manifestations of antiretroviral therapy. J Am Acad Dermatol 2002; 46:284–293.
- Ehnzadeh-Cheemeh P, Grimes RM, Rowan P, Huang YJ, Essien EJ, Lewis ST. Melanonychia in patients infected with human immunodeficiency virus original communication. Adv Infect Dis 2011; 1:15–19.
A 49-year-old man presented with pigmentation on the nails of both hands during the past 6 months. He had no history of significant medical conditions, but he complained of generalized asthenia, with no fever, during the previous 6 weeks. He said that he was a volcanologist and had recently returned from an expedition in the mountains of Peru that involved intense physical activity at high altitudes. He was a non-smoker and did not drink alcohol; he was not taking any medications or nutritional supplements. A biochemical profile including a white blood cell count 3 months ago was normal.
On physical examination, he had splenomegaly (about 2 cm below the costal margin) and low systolic blood pressure (90 mm Hg, with the diastolic pressure at 60 mm Hg). Heart and lung examinations, including chest radiography, were normal.
Q: What is the diagnosis?
- Addison disease
- Onychotillomania
- Longitudinal melanonychia in human immunodeficiency virus (HIV) infection
- Systemic lupus erythematosus
A: The patient was diagnosed with longitudinal melanonychia with underlying HIV infection.
In addition to the clinical findings noted above, laboratory testing now revealed a low white blood cell count (2.9 × 109/L, reference range 4.5–11.0) and a low CD4+ T-cell count (182 cells/mL). An enzyme-linked immunosorbent assay and a Western blot assay were positive for HIV, and his viral load was 120,000 copies/mL as determined by reverse transcription-polymerase chain reaction testing. On more detailed questioning, the patient admitted to sexual practices that put him at high risk for HIV infection.
Longitudinal melanonychia is characterized by a tan, brown, or black longitudinal streak, originating in the matrix and resulting from increased synthesis of melanin (by either normal or abnormal melanocytes) and its deposition within the nail plate.1
If the cause is not apparent (eg, trauma, application of chemical agents, concomitant onychomycosis or treatments), this pigmentation usually reflects the presence of a benign lesion within the matrix caused by a melanocytic nevus, simple lentigo, or increased activation of benign melanocytes. This finding has been reported in a US study2 to be more common in people with dark skin, occurring in 77% of blacks over age 20 and in almost 100% of those over age 50.
Melanoma and other nonmelanocytic tumors may present as longitudinal melanonychia, but these rarely involve more than one digit simultaneously, and they are usually detected with specific clinical clues, such as the Hutchinson sign, ie, periungual spread of pigmentation into the proximal and lateral nail folds.
A SIGN OF BENIGN AND MALIGNANT CONDITIONS
Longitudinal melanonychia can have benign causes, and these need to be investigated. It can occur with repeated trauma, friction, or pressure on the nails. The physician should question the patient about habits such as picking or chewing of the finger tips or pushing back the cuticles, as well as about athletic activities or manual work that could affect the nails. In addition, blood thinners can cause nail discoloration via subungual hemorrhage.
A number of pathologic conditions can cause longitudinal discoloration of the nails. Infection with certain dermatophytes can cause discoloration, but this pigmentation is usually wider and more distal at the hyponychium rather than proximal, with a black streak with several pointed extensions proximally.
Adrenocortical insufficiency (Addison disease), nutritional disorders (deficiency of folic acid or vitamin B12), and, more rarely, systemic lupus erythematosus and scleroderma should be ruled out, as they are typically associated with cutaneous and mucosal hyperpigmentation.
Chemotherapeutic agents such as doxorubicin (Adriamycin), hydroxyurea (Droxea), and cyclophosphamide and drugs such as antimalarials and tetracyclines can also cause longitudinal melanonychia.1,3
Nail lesions with a similar pattern are often seen in patients (especially black patients) taking zidovudine (Retrovir), and these lesions seem not to be related to drug dosage, HIV risk group, or severity of the infection. Nail discoloration in HIV patients not taking antiretroviral therapy has not been well documented.
The significance of melanonychia in the course of HIV remains unclear. A key role of overexpression of alpha-melanocyte-stimulating-hormone has been proposed.4 Although rare in fair-skinned people, longitudinal melanonychia represents an important clinical sign of disease progression. In reported cases, however, no significant improvement has been noted with treatment despite favorable virologic and immunologic responses.4,5
A 49-year-old man presented with pigmentation on the nails of both hands during the past 6 months. He had no history of significant medical conditions, but he complained of generalized asthenia, with no fever, during the previous 6 weeks. He said that he was a volcanologist and had recently returned from an expedition in the mountains of Peru that involved intense physical activity at high altitudes. He was a non-smoker and did not drink alcohol; he was not taking any medications or nutritional supplements. A biochemical profile including a white blood cell count 3 months ago was normal.
On physical examination, he had splenomegaly (about 2 cm below the costal margin) and low systolic blood pressure (90 mm Hg, with the diastolic pressure at 60 mm Hg). Heart and lung examinations, including chest radiography, were normal.
Q: What is the diagnosis?
- Addison disease
- Onychotillomania
- Longitudinal melanonychia in human immunodeficiency virus (HIV) infection
- Systemic lupus erythematosus
A: The patient was diagnosed with longitudinal melanonychia with underlying HIV infection.
In addition to the clinical findings noted above, laboratory testing now revealed a low white blood cell count (2.9 × 109/L, reference range 4.5–11.0) and a low CD4+ T-cell count (182 cells/mL). An enzyme-linked immunosorbent assay and a Western blot assay were positive for HIV, and his viral load was 120,000 copies/mL as determined by reverse transcription-polymerase chain reaction testing. On more detailed questioning, the patient admitted to sexual practices that put him at high risk for HIV infection.
Longitudinal melanonychia is characterized by a tan, brown, or black longitudinal streak, originating in the matrix and resulting from increased synthesis of melanin (by either normal or abnormal melanocytes) and its deposition within the nail plate.1
If the cause is not apparent (eg, trauma, application of chemical agents, concomitant onychomycosis or treatments), this pigmentation usually reflects the presence of a benign lesion within the matrix caused by a melanocytic nevus, simple lentigo, or increased activation of benign melanocytes. This finding has been reported in a US study2 to be more common in people with dark skin, occurring in 77% of blacks over age 20 and in almost 100% of those over age 50.
Melanoma and other nonmelanocytic tumors may present as longitudinal melanonychia, but these rarely involve more than one digit simultaneously, and they are usually detected with specific clinical clues, such as the Hutchinson sign, ie, periungual spread of pigmentation into the proximal and lateral nail folds.
A SIGN OF BENIGN AND MALIGNANT CONDITIONS
Longitudinal melanonychia can have benign causes, and these need to be investigated. It can occur with repeated trauma, friction, or pressure on the nails. The physician should question the patient about habits such as picking or chewing of the finger tips or pushing back the cuticles, as well as about athletic activities or manual work that could affect the nails. In addition, blood thinners can cause nail discoloration via subungual hemorrhage.
A number of pathologic conditions can cause longitudinal discoloration of the nails. Infection with certain dermatophytes can cause discoloration, but this pigmentation is usually wider and more distal at the hyponychium rather than proximal, with a black streak with several pointed extensions proximally.
Adrenocortical insufficiency (Addison disease), nutritional disorders (deficiency of folic acid or vitamin B12), and, more rarely, systemic lupus erythematosus and scleroderma should be ruled out, as they are typically associated with cutaneous and mucosal hyperpigmentation.
Chemotherapeutic agents such as doxorubicin (Adriamycin), hydroxyurea (Droxea), and cyclophosphamide and drugs such as antimalarials and tetracyclines can also cause longitudinal melanonychia.1,3
Nail lesions with a similar pattern are often seen in patients (especially black patients) taking zidovudine (Retrovir), and these lesions seem not to be related to drug dosage, HIV risk group, or severity of the infection. Nail discoloration in HIV patients not taking antiretroviral therapy has not been well documented.
The significance of melanonychia in the course of HIV remains unclear. A key role of overexpression of alpha-melanocyte-stimulating-hormone has been proposed.4 Although rare in fair-skinned people, longitudinal melanonychia represents an important clinical sign of disease progression. In reported cases, however, no significant improvement has been noted with treatment despite favorable virologic and immunologic responses.4,5
- Smith DF, Morgan MB, Bettencourt MS. Longitudinal melanonychia. Arch Dermatol 2003; 139:1209–1214.
- Baran R, Kechijian P. Longitudinal melanonychia (melanonychia striata): diagnosis and management. J Am Acad Dermatol 1989; 21:1165–1175.
- Haneke E, Baran R. Longitudinal melanonychia. Dermatol Surg 2001; 27:580–584.
- Ward HA, Russo GG, Shrum J. Cutaneous manifestations of antiretroviral therapy. J Am Acad Dermatol 2002; 46:284–293.
- Ehnzadeh-Cheemeh P, Grimes RM, Rowan P, Huang YJ, Essien EJ, Lewis ST. Melanonychia in patients infected with human immunodeficiency virus original communication. Adv Infect Dis 2011; 1:15–19.
- Smith DF, Morgan MB, Bettencourt MS. Longitudinal melanonychia. Arch Dermatol 2003; 139:1209–1214.
- Baran R, Kechijian P. Longitudinal melanonychia (melanonychia striata): diagnosis and management. J Am Acad Dermatol 1989; 21:1165–1175.
- Haneke E, Baran R. Longitudinal melanonychia. Dermatol Surg 2001; 27:580–584.
- Ward HA, Russo GG, Shrum J. Cutaneous manifestations of antiretroviral therapy. J Am Acad Dermatol 2002; 46:284–293.
- Ehnzadeh-Cheemeh P, Grimes RM, Rowan P, Huang YJ, Essien EJ, Lewis ST. Melanonychia in patients infected with human immunodeficiency virus original communication. Adv Infect Dis 2011; 1:15–19.
Emergency contraception: Separating fact from fiction
In the United States, nearly 50 million legal abortions were performed between 1973 and 2008.1 About half of pregnancies in American women are unintended, and 4 out of 10 unintended pregnancies are terminated by abortion.2 Of the women who had abortions, 54% had used a contraceptive method during the month they became pregnant.3
It is hoped that the expanded use of emergency contraception will translate into fewer abortions. However, in a 2006–2008 survey conducted by the US Centers for Disease Control and Prevention, only 9.7% of women ages 15 to 44 reported ever having used emergency contraception.4 (To put this figure in perspective, a similar number—about 10%—of women in this age group become pregnant in any given year, half of them unintentionally.4) Clearly, patients need to be better educated in the methods of contraception and emergency contraception.
Hospitals are not meeting the need. Pretending to be in need of emergency contraception, Harrison5 called the emergency departments of all 597 Catholic hospitals in the United States and 615 (17%) of the non-Catholic hospitals. About half of the staff she spoke to said they do not dispense emergency contraception, even in cases of sexual assault. This was the case for both Catholic and non-Catholic hospitals. Of the people she talked to who said they did not provide emergency contraception under any circumstance, only about half gave her a phone number for another facility to try, and most of these phone numbers were wrong, were for facilities that were not open on weekends, or were for facilities that did not offer emergency contraception either. This is in spite of legal precedent, which indicates that failure to provide complete post-rape counseling, including emergency contraception, constitutes inadequate care and gives a woman the standing to sue the hospital.6
Clearly, better provider education is also needed in the area of emergency contraception. The Association of Reproductive Health Professionals has a helpful Web site for providers and for patients. In addition to up-to-date information about contraceptive and emergency contraceptive choices, it provides advice on how to discuss emergency contraception with patients (www.arhp.org). We can test our own knowledge of this topic by reviewing the following questions.
WHICH PRODUCT IS MOST EFFECTIVE?
Q: True or false? Levonorgestrel monotherapy (Plan B One-Step, Next Choice) is the most effective oral emergency contraceptive.
A: False, although this statement was true before the US approval of ulipristal acetate (ella) in August 2010.
For many years levonorgestrel monotherapy has been the mainstay of emergency contraception, having replaced the combination estrogen-progestin (Yuzpe) regimen because of better tolerability and improved efficacy.7 Its main mechanism of action involves delaying ovulation. Levonorgestrel is given in two doses of 0.75 mg 12 hours apart, or as a single 1.5-mg dose (Table 1). Both formulations of levonorgestrel are available over the counter to women age 17 and older, or by prescription if they are under age 17.
However, a randomized controlled trial showed that women treated with ulipristal had about half the number of pregnancies than in those treated with levonorgestrel, with pregnancy rates of 0.9% vs 1.7%.8
HOW WIDE IS THE WINDOW OF OPPORTUNITY?
Q: True or false? Both ulipristal and levonorgestrel can be taken up to 120 hours (5 days) after unprotected intercourse. However, ulipristal maintains its effectiveness throughout this time, whereas levonorgestrel becomes less effective the longer a patient waits to take it.
A: True. Ulipristal is a second-generation selective progesterone receptor modulator. These drugs can function as agonists, antagonists, or mixed agonist-antagonists at the progesterone receptor, depending on the tissue affected. Ulipristal is given as a one-time, 30-mg dose within 120 hours of intercourse.
In a study of 1,696 women, 844 of whom received ulipristal acetate and 852 of whom received levonorgestrel, ulipristal was at least as effective as levonorgestrel when used within 72 hours of intercourse for emergency contraception, with 15 pregnancies in the ulipristal group and 22 pregnancies in the levonorgestrel group (odds ratio [OR] 0.68, 95% confidence interval [CI] 0.35–1.31]). However, ulipristal prevented significantly more pregnancies than levonorgestrel at 72 to 120 hours, with no pregnancies in the ulipristal group and three pregnancies in the levonorgestrel group.9
Because ulipristal has a long half-life (32 hours), it can delay ovulation beyond the life span of sperm, thereby extending the window of opportunity for emergency contraception. However, patients should be advised to avoid further unprotected intercourse after the use of emergency contraception. Because emergency contraception works mainly by delaying ovulation, it may increase the likelihood of pregnancy if the patient has unprotected intercourse again several days later.
IS MIFEPRISTONE AN EMERGENCY CONTRACEPTIVE?
Q: True or false? In the United States, mifepristone (Mifeprex), also known as RU-486, is available for use as an emergency contraceptive in addition to its use in abortion.
A: False, even though mifepristone, another selective progesterone receptor modulator, is highly effective when used up to 120 hours after intercourse. In fact, it might be effective up to 17 days after unprotected intercourse.10
Although mifepristone is one of the most effective forms of emergency contraception, social and political controversy has prevented its approval in the United States. However, it is approved for use as an abortifacient, at a higher dose than would be used for emergency contraception.
Unlike levonorgestrel, mifepristone exerts its effect via two potential mechanisms: delaying ovulation and preventing implantation.11
IUDs AS EMERGENCY CONTRACEPTION
Q: True or false? Insertion of a 5-year intrauterine device (IUD), ie, the levonorgestrel-releasing intrauterine system (Mirena), is 99.8% effective at preventing pregnancy when used within 5 days of unprotected intercourse.
A: False. The Mirena IUD has not been studied as a form of emergency contraception. However, this statement would be true for the 10-year copper IUD ParaGard. Copper-releasing IUDs are considered a very effective method of emergency contraception, with associated pregnancy rates of 0.0% to 0.2% when inserted up until implantation (within 5 days after ovulation).12,13 If desired, the IUD can then be kept in place for up to 10 years as a method of birth control.
However, this method requires the ready availability of a health professional trained to do the insertion. It is also important to make sure that the patient will not be at increased risk of sexually transmitted infections from further unprotected intercourse. The American Congress of Obstetricians and Gynecologists (ACOG) recommends that an IUD be placed within 5 days of unprotected intercourse for use as emergency contraception.
A recent review looked at 42 published studies of copper IUDs used for emergency contraception around the world. It found copper IUDs to be a safe and highly effective method of emergency contraception, with the additional advantage of simultaneously offering one of the most reliable and cost-effective contraceptive options.14
EMERGENCY CONTRACEPTION AT MID-CYCLE
Q: True or false? When choosing a method of emergency contraception, it is important to consider whether a woman is near ovulation during the time of intercourse.
A: True. Emergency contraception can prevent pregnancy after unprotected intercourse, but it does not always work. The most widely used method, levonorgestrel 1.5 mg orally within 72 hours of intercourse, prevents at least 50% of pregnancies that would have occurred in the absence of its use.15 Glasier et al16 showed that emergency contraception was more likely to fail if a woman had unprotected intercourse around the time of ovulation.16
Though it can be difficult for women to tell if they are in the fertile times of their cycle, it might be helpful to try to identify women who have intercourse at mid-cycle, when the risk of pregnancy is greatest. Because insertion of an IUD and use of ulipristal acetate probably prevent more pregnancies, these methods might be preferred over levonorgestrel-based regimens during these higher-risk situations.
OBESE PATIENTS
Q: True or false? Hormonal emergency contraception is more likely to fail in obese patients.
A: True. Most recent evidence shows that whichever oral emergency contraceptive drug is taken, the risk of pregnancy is more than 3 times greater for obese women (OR 3.60, 95% CI 1.96–6.53) and 1.5 times greater for overweight women (OR 1.53, 95% CI 0.75–2.95).16 Of all covariates tested, those that were shown to increase the odds of failure of the emergency contraception were higher body mass index, further unprotected intercourse, and conception probability (based on time of fertility cycle). In fact, among obese women treated with levonorgestrel, the observed pregnancy rate was 5.8%, which is slightly above the overall pregnancy rate expected in the absence of emergency contraception, suggesting that for obese women levonorgestrel-based emergency contraception may even be ineffective.
This is in line with recent reports suggesting that oral contraceptives are less effective in obese women. More effective regimens such as an IUD or ulipristal might be preferred in these women. However, obesity should not be used as a reason not to offer emergency contraception, as this is the last chance these women have to prevent pregnancy.
IS IT ABORTION?
Q: True or false? Emergency contraception does not cause abortion.
A: True, but patients may ask for more details about this. Hormonal emergency contraception works primarily by delaying or inhibiting ovulation and inhibiting fertilization.
Levonorgestrel or combined estrogen-progestin-based methods would be unlikely to have any adverse effects on the endometrium after fertilization, since they would only serve to enhance the progesterone effect. Therefore, they are unlikely to affect the ability of the embryo to attach to the endometrium.
Ulipristal, on the other hand, can have just the opposite effect on the postovulatory endometrium because of its inhibitory action on progesterone. Ulipristal is structurally similar to mifepristone, and its mechanism of action varies depending on the time of administration during the menstrual cycle. When unprotected intercourse occurs during a time when fertility is not possible, ulipristal behaves like a placebo. When intercourse occurs just before ovulation, ulipristal acts by delaying ovulation and thereby preventing fertilization (similar to levonorgestrel). Ulipristal may have an additional action of affecting the ability of the embryo to either attach to the endometrium or maintain its attachment, by a variety of mechanisms of action.17,18 Because of this, some in the popular press and on the Internet have spoken out against the use of ulipristal.
The ACOG considers pregnancy to begin not with fertilization of the egg but with implantation, as demonstrated by a positive pregnancy test.
Of note, the copper IUD also prevents implantation after fertilization, which likely explains its high efficacy.
Women who have detailed questions about this can be counseled that levonorgestrel works mostly by preventing ovulation, and that ulipristal and the copper IUD might also work via postfertilization mechanisms. However, they are not considered to be abortive, based on standard definitions of pregnancy.
If a woman is pregnant and she takes levonorgestrel-based emergency contraception, this has not been shown to have any adverse effects on the fetus (similar to oral contraceptives).
Ulipristal is classified as pregnancy category X, and therefore its use during pregnancy is contraindicated. Based on information provided by the manufacturer, there are no adequate, well-controlled studies of ulipristal use in pregnant women. Although fetal loss was observed in animal studies after ulipristal administration (during the period of organogenesis), no malformations or adverse events were present in the surviving fetuses. Ulipristal is not indicated for termination of an existing pregnancy.
DO THE USUAL CONTRAINDICATIONS TO HORMONAL CONTRACEPTIVES APPLY?
Q: True or false? Because emergency contraception has such a short duration of exposure, the usual medical contraindications to hormonal therapies do not apply to it.
A: True. The usual contraindications to the use of hormonal contraceptives (eg, migraine with aura, hypertension, history of venous thromboembolism) do not apply to emergency contraception because of the short time of exposure.19 Furthermore, the risks associated with pregnancy in these women would likely outweigh any risks associated with emergency contraception.
However, one must be cognizant of potential drug interactions. According to the manufacturer, the use of ulipristal did not inhibit or induce cytochrome P 450 enzymes in vitro; therefore, in vivo studies were not performed. But because ulipristal is metabolized primarily via CYP3A4, an interaction between agents that induce or inhibit CYP3A4 could occur.20 Thus, concomitant use of drugs such as barbiturates, rifampin (Rifadin), St. John’s wort, or antiseizure drugs such as topiramate (Topamax) may lower ulipristal concentrations. These medications may also affect levonorgestrel levels, similar to their effects on combined hormonal contraception. However, it is not known whether this translates to decreased efficacy.
When a woman is taking medications that can potentially decrease the effectiveness of hormonal emergency contraception, a more effective method such as a copper IUD might be more strongly considered. If a woman is not interested in an IUD, oral emergency contraception should still be offered, given that this is one of the last chances to prevent pregnancy, especially if she is on a potential teratogen.
Oral contraceptive pills have not been studied in combination with ulipristal. However, because ulipristal binds with high affinity to progesterone receptors (thus competing with the contraceptive), use of additional barrier contraceptives is recommended for the remainder of the menstrual cycle.
EMERGENCY CONTRACEPTION AND BREASTFEEDING
Q: True of false? Emergency contraceptives can be used if a woman is breastfeeding.
A: That depends on which method is used. Both the ACOG and the World Health Organization state that it is safe for breastfeeding women to use emergency contraception, but these are older guidelines addressing progestin-only regimens (ie, levonorgestrel).19,21 It is unknown whether ulipristal is secreted into human breast milk, although excretion was seen in animal studies. Therefore, ulipristal is not recommended for use by women who are breastfeeding.20,22 To minimize the infant’s exposure to levonorgestrel, mothers should consider not nursing for at least 8 hours after ingestion, but no more than 24 hours is needed.23
- Jones RK, Kooistra K. Abortion incidence and access to services in the United States, 2008. Perspect Sex Reprod Health 2011; 43:41–50.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Jones RK, Darroch JE, Henshaw SK. Contraceptive use among US women having abortions in 2000–2001. Perspect Sex Reprod Health 2002; 34:294–303.
- Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. National Center for Health Statistics. Vital Health Stat 2010; 23. http://www.cdc.gov/NCHS/data/series/sr_23/sr23_029.pdf. Accessed October 1, 2012.
- Harrison T. Availability of emergency contraception: a survey of hospital emergency department staff. Ann Emerg Med 2005; 46:105–110.
- Goldenring JM, Allred G. Post-rape care in hospital emergency rooms. Am J Public Health 2001; 91:1169–1170.
- Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Task Force on Postovulatory Methods of Fertility Regulation. Lancet 1998; 352:428–433.
- Creinin MD, Schlaff W, Archer DF, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol 2006; 108:1089–1097.
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet 2010; 375:555–562.
- Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Mifepristone (RU 486) compared with high-dose estrogen and progestogen for emergency postcoital contraception. N Engl J Med 1992; 327:1041–1044.
- Glasier A. Emergency postcoital contraception. N Engl J Med 1997; 337:1058–1064.
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol 2002; 187:1699–1708.
- Zhou L, Xiao B. Emergency contraception with Multiload Cu-375 SL IUD: a multicenter clinical trial. Contraception 2001; 64:107–112.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod 2012; 27:1994–2000.
- Trussell J, Ellertson C, von Hertzen H, et al. Estimating the effectiveness of emergency contraceptive pills. Contraception 2003; 67:259–265.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception 2011; 84:363–367.
- Miech RP. Immunopharmacology of ulipristal as an emergency contraceptive. Int J Womens Health 2011; 3:391–397.
- Keenan JA. Ulipristal acetate: contraceptive or contragestive? Ann Pharmacother 2011; 45:813–815.
- Medical eligibility criteria for contraceptive use. 3rd ed. Geneva: Reproductive Health and Research, World Health Organization; 2004.
- Ella package insert. Morristown, NJ: Watson Pharmaceuticals; August 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022474s000lbl.pdf. Accessed July 6, 2012.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 112: emergency contraception. Obstet Gynecol 2010; 115:1100–1109.
- Orleans RJ. Clinical review. NDA22-474. Ella (ulipristal acetate 30 mg). US Food and Drug Administration, July 27, 2010. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM295393.pdf. Accessed October 1, 2012.
- Gainer E, Massai R, Lillo S, et al. Levonorgestrel pharmacokinetics in plasma and milk of lactating women who take 1.5 mg for emergency contraception. Hum Reprod 2007; 22:1578–1584.
In the United States, nearly 50 million legal abortions were performed between 1973 and 2008.1 About half of pregnancies in American women are unintended, and 4 out of 10 unintended pregnancies are terminated by abortion.2 Of the women who had abortions, 54% had used a contraceptive method during the month they became pregnant.3
It is hoped that the expanded use of emergency contraception will translate into fewer abortions. However, in a 2006–2008 survey conducted by the US Centers for Disease Control and Prevention, only 9.7% of women ages 15 to 44 reported ever having used emergency contraception.4 (To put this figure in perspective, a similar number—about 10%—of women in this age group become pregnant in any given year, half of them unintentionally.4) Clearly, patients need to be better educated in the methods of contraception and emergency contraception.
Hospitals are not meeting the need. Pretending to be in need of emergency contraception, Harrison5 called the emergency departments of all 597 Catholic hospitals in the United States and 615 (17%) of the non-Catholic hospitals. About half of the staff she spoke to said they do not dispense emergency contraception, even in cases of sexual assault. This was the case for both Catholic and non-Catholic hospitals. Of the people she talked to who said they did not provide emergency contraception under any circumstance, only about half gave her a phone number for another facility to try, and most of these phone numbers were wrong, were for facilities that were not open on weekends, or were for facilities that did not offer emergency contraception either. This is in spite of legal precedent, which indicates that failure to provide complete post-rape counseling, including emergency contraception, constitutes inadequate care and gives a woman the standing to sue the hospital.6
Clearly, better provider education is also needed in the area of emergency contraception. The Association of Reproductive Health Professionals has a helpful Web site for providers and for patients. In addition to up-to-date information about contraceptive and emergency contraceptive choices, it provides advice on how to discuss emergency contraception with patients (www.arhp.org). We can test our own knowledge of this topic by reviewing the following questions.
WHICH PRODUCT IS MOST EFFECTIVE?
Q: True or false? Levonorgestrel monotherapy (Plan B One-Step, Next Choice) is the most effective oral emergency contraceptive.
A: False, although this statement was true before the US approval of ulipristal acetate (ella) in August 2010.
For many years levonorgestrel monotherapy has been the mainstay of emergency contraception, having replaced the combination estrogen-progestin (Yuzpe) regimen because of better tolerability and improved efficacy.7 Its main mechanism of action involves delaying ovulation. Levonorgestrel is given in two doses of 0.75 mg 12 hours apart, or as a single 1.5-mg dose (Table 1). Both formulations of levonorgestrel are available over the counter to women age 17 and older, or by prescription if they are under age 17.
However, a randomized controlled trial showed that women treated with ulipristal had about half the number of pregnancies than in those treated with levonorgestrel, with pregnancy rates of 0.9% vs 1.7%.8
HOW WIDE IS THE WINDOW OF OPPORTUNITY?
Q: True or false? Both ulipristal and levonorgestrel can be taken up to 120 hours (5 days) after unprotected intercourse. However, ulipristal maintains its effectiveness throughout this time, whereas levonorgestrel becomes less effective the longer a patient waits to take it.
A: True. Ulipristal is a second-generation selective progesterone receptor modulator. These drugs can function as agonists, antagonists, or mixed agonist-antagonists at the progesterone receptor, depending on the tissue affected. Ulipristal is given as a one-time, 30-mg dose within 120 hours of intercourse.
In a study of 1,696 women, 844 of whom received ulipristal acetate and 852 of whom received levonorgestrel, ulipristal was at least as effective as levonorgestrel when used within 72 hours of intercourse for emergency contraception, with 15 pregnancies in the ulipristal group and 22 pregnancies in the levonorgestrel group (odds ratio [OR] 0.68, 95% confidence interval [CI] 0.35–1.31]). However, ulipristal prevented significantly more pregnancies than levonorgestrel at 72 to 120 hours, with no pregnancies in the ulipristal group and three pregnancies in the levonorgestrel group.9
Because ulipristal has a long half-life (32 hours), it can delay ovulation beyond the life span of sperm, thereby extending the window of opportunity for emergency contraception. However, patients should be advised to avoid further unprotected intercourse after the use of emergency contraception. Because emergency contraception works mainly by delaying ovulation, it may increase the likelihood of pregnancy if the patient has unprotected intercourse again several days later.
IS MIFEPRISTONE AN EMERGENCY CONTRACEPTIVE?
Q: True or false? In the United States, mifepristone (Mifeprex), also known as RU-486, is available for use as an emergency contraceptive in addition to its use in abortion.
A: False, even though mifepristone, another selective progesterone receptor modulator, is highly effective when used up to 120 hours after intercourse. In fact, it might be effective up to 17 days after unprotected intercourse.10
Although mifepristone is one of the most effective forms of emergency contraception, social and political controversy has prevented its approval in the United States. However, it is approved for use as an abortifacient, at a higher dose than would be used for emergency contraception.
Unlike levonorgestrel, mifepristone exerts its effect via two potential mechanisms: delaying ovulation and preventing implantation.11
IUDs AS EMERGENCY CONTRACEPTION
Q: True or false? Insertion of a 5-year intrauterine device (IUD), ie, the levonorgestrel-releasing intrauterine system (Mirena), is 99.8% effective at preventing pregnancy when used within 5 days of unprotected intercourse.
A: False. The Mirena IUD has not been studied as a form of emergency contraception. However, this statement would be true for the 10-year copper IUD ParaGard. Copper-releasing IUDs are considered a very effective method of emergency contraception, with associated pregnancy rates of 0.0% to 0.2% when inserted up until implantation (within 5 days after ovulation).12,13 If desired, the IUD can then be kept in place for up to 10 years as a method of birth control.
However, this method requires the ready availability of a health professional trained to do the insertion. It is also important to make sure that the patient will not be at increased risk of sexually transmitted infections from further unprotected intercourse. The American Congress of Obstetricians and Gynecologists (ACOG) recommends that an IUD be placed within 5 days of unprotected intercourse for use as emergency contraception.
A recent review looked at 42 published studies of copper IUDs used for emergency contraception around the world. It found copper IUDs to be a safe and highly effective method of emergency contraception, with the additional advantage of simultaneously offering one of the most reliable and cost-effective contraceptive options.14
EMERGENCY CONTRACEPTION AT MID-CYCLE
Q: True or false? When choosing a method of emergency contraception, it is important to consider whether a woman is near ovulation during the time of intercourse.
A: True. Emergency contraception can prevent pregnancy after unprotected intercourse, but it does not always work. The most widely used method, levonorgestrel 1.5 mg orally within 72 hours of intercourse, prevents at least 50% of pregnancies that would have occurred in the absence of its use.15 Glasier et al16 showed that emergency contraception was more likely to fail if a woman had unprotected intercourse around the time of ovulation.16
Though it can be difficult for women to tell if they are in the fertile times of their cycle, it might be helpful to try to identify women who have intercourse at mid-cycle, when the risk of pregnancy is greatest. Because insertion of an IUD and use of ulipristal acetate probably prevent more pregnancies, these methods might be preferred over levonorgestrel-based regimens during these higher-risk situations.
OBESE PATIENTS
Q: True or false? Hormonal emergency contraception is more likely to fail in obese patients.
A: True. Most recent evidence shows that whichever oral emergency contraceptive drug is taken, the risk of pregnancy is more than 3 times greater for obese women (OR 3.60, 95% CI 1.96–6.53) and 1.5 times greater for overweight women (OR 1.53, 95% CI 0.75–2.95).16 Of all covariates tested, those that were shown to increase the odds of failure of the emergency contraception were higher body mass index, further unprotected intercourse, and conception probability (based on time of fertility cycle). In fact, among obese women treated with levonorgestrel, the observed pregnancy rate was 5.8%, which is slightly above the overall pregnancy rate expected in the absence of emergency contraception, suggesting that for obese women levonorgestrel-based emergency contraception may even be ineffective.
This is in line with recent reports suggesting that oral contraceptives are less effective in obese women. More effective regimens such as an IUD or ulipristal might be preferred in these women. However, obesity should not be used as a reason not to offer emergency contraception, as this is the last chance these women have to prevent pregnancy.
IS IT ABORTION?
Q: True or false? Emergency contraception does not cause abortion.
A: True, but patients may ask for more details about this. Hormonal emergency contraception works primarily by delaying or inhibiting ovulation and inhibiting fertilization.
Levonorgestrel or combined estrogen-progestin-based methods would be unlikely to have any adverse effects on the endometrium after fertilization, since they would only serve to enhance the progesterone effect. Therefore, they are unlikely to affect the ability of the embryo to attach to the endometrium.
Ulipristal, on the other hand, can have just the opposite effect on the postovulatory endometrium because of its inhibitory action on progesterone. Ulipristal is structurally similar to mifepristone, and its mechanism of action varies depending on the time of administration during the menstrual cycle. When unprotected intercourse occurs during a time when fertility is not possible, ulipristal behaves like a placebo. When intercourse occurs just before ovulation, ulipristal acts by delaying ovulation and thereby preventing fertilization (similar to levonorgestrel). Ulipristal may have an additional action of affecting the ability of the embryo to either attach to the endometrium or maintain its attachment, by a variety of mechanisms of action.17,18 Because of this, some in the popular press and on the Internet have spoken out against the use of ulipristal.
The ACOG considers pregnancy to begin not with fertilization of the egg but with implantation, as demonstrated by a positive pregnancy test.
Of note, the copper IUD also prevents implantation after fertilization, which likely explains its high efficacy.
Women who have detailed questions about this can be counseled that levonorgestrel works mostly by preventing ovulation, and that ulipristal and the copper IUD might also work via postfertilization mechanisms. However, they are not considered to be abortive, based on standard definitions of pregnancy.
If a woman is pregnant and she takes levonorgestrel-based emergency contraception, this has not been shown to have any adverse effects on the fetus (similar to oral contraceptives).
Ulipristal is classified as pregnancy category X, and therefore its use during pregnancy is contraindicated. Based on information provided by the manufacturer, there are no adequate, well-controlled studies of ulipristal use in pregnant women. Although fetal loss was observed in animal studies after ulipristal administration (during the period of organogenesis), no malformations or adverse events were present in the surviving fetuses. Ulipristal is not indicated for termination of an existing pregnancy.
DO THE USUAL CONTRAINDICATIONS TO HORMONAL CONTRACEPTIVES APPLY?
Q: True or false? Because emergency contraception has such a short duration of exposure, the usual medical contraindications to hormonal therapies do not apply to it.
A: True. The usual contraindications to the use of hormonal contraceptives (eg, migraine with aura, hypertension, history of venous thromboembolism) do not apply to emergency contraception because of the short time of exposure.19 Furthermore, the risks associated with pregnancy in these women would likely outweigh any risks associated with emergency contraception.
However, one must be cognizant of potential drug interactions. According to the manufacturer, the use of ulipristal did not inhibit or induce cytochrome P 450 enzymes in vitro; therefore, in vivo studies were not performed. But because ulipristal is metabolized primarily via CYP3A4, an interaction between agents that induce or inhibit CYP3A4 could occur.20 Thus, concomitant use of drugs such as barbiturates, rifampin (Rifadin), St. John’s wort, or antiseizure drugs such as topiramate (Topamax) may lower ulipristal concentrations. These medications may also affect levonorgestrel levels, similar to their effects on combined hormonal contraception. However, it is not known whether this translates to decreased efficacy.
When a woman is taking medications that can potentially decrease the effectiveness of hormonal emergency contraception, a more effective method such as a copper IUD might be more strongly considered. If a woman is not interested in an IUD, oral emergency contraception should still be offered, given that this is one of the last chances to prevent pregnancy, especially if she is on a potential teratogen.
Oral contraceptive pills have not been studied in combination with ulipristal. However, because ulipristal binds with high affinity to progesterone receptors (thus competing with the contraceptive), use of additional barrier contraceptives is recommended for the remainder of the menstrual cycle.
EMERGENCY CONTRACEPTION AND BREASTFEEDING
Q: True of false? Emergency contraceptives can be used if a woman is breastfeeding.
A: That depends on which method is used. Both the ACOG and the World Health Organization state that it is safe for breastfeeding women to use emergency contraception, but these are older guidelines addressing progestin-only regimens (ie, levonorgestrel).19,21 It is unknown whether ulipristal is secreted into human breast milk, although excretion was seen in animal studies. Therefore, ulipristal is not recommended for use by women who are breastfeeding.20,22 To minimize the infant’s exposure to levonorgestrel, mothers should consider not nursing for at least 8 hours after ingestion, but no more than 24 hours is needed.23
In the United States, nearly 50 million legal abortions were performed between 1973 and 2008.1 About half of pregnancies in American women are unintended, and 4 out of 10 unintended pregnancies are terminated by abortion.2 Of the women who had abortions, 54% had used a contraceptive method during the month they became pregnant.3
It is hoped that the expanded use of emergency contraception will translate into fewer abortions. However, in a 2006–2008 survey conducted by the US Centers for Disease Control and Prevention, only 9.7% of women ages 15 to 44 reported ever having used emergency contraception.4 (To put this figure in perspective, a similar number—about 10%—of women in this age group become pregnant in any given year, half of them unintentionally.4) Clearly, patients need to be better educated in the methods of contraception and emergency contraception.
Hospitals are not meeting the need. Pretending to be in need of emergency contraception, Harrison5 called the emergency departments of all 597 Catholic hospitals in the United States and 615 (17%) of the non-Catholic hospitals. About half of the staff she spoke to said they do not dispense emergency contraception, even in cases of sexual assault. This was the case for both Catholic and non-Catholic hospitals. Of the people she talked to who said they did not provide emergency contraception under any circumstance, only about half gave her a phone number for another facility to try, and most of these phone numbers were wrong, were for facilities that were not open on weekends, or were for facilities that did not offer emergency contraception either. This is in spite of legal precedent, which indicates that failure to provide complete post-rape counseling, including emergency contraception, constitutes inadequate care and gives a woman the standing to sue the hospital.6
Clearly, better provider education is also needed in the area of emergency contraception. The Association of Reproductive Health Professionals has a helpful Web site for providers and for patients. In addition to up-to-date information about contraceptive and emergency contraceptive choices, it provides advice on how to discuss emergency contraception with patients (www.arhp.org). We can test our own knowledge of this topic by reviewing the following questions.
WHICH PRODUCT IS MOST EFFECTIVE?
Q: True or false? Levonorgestrel monotherapy (Plan B One-Step, Next Choice) is the most effective oral emergency contraceptive.
A: False, although this statement was true before the US approval of ulipristal acetate (ella) in August 2010.
For many years levonorgestrel monotherapy has been the mainstay of emergency contraception, having replaced the combination estrogen-progestin (Yuzpe) regimen because of better tolerability and improved efficacy.7 Its main mechanism of action involves delaying ovulation. Levonorgestrel is given in two doses of 0.75 mg 12 hours apart, or as a single 1.5-mg dose (Table 1). Both formulations of levonorgestrel are available over the counter to women age 17 and older, or by prescription if they are under age 17.
However, a randomized controlled trial showed that women treated with ulipristal had about half the number of pregnancies than in those treated with levonorgestrel, with pregnancy rates of 0.9% vs 1.7%.8
HOW WIDE IS THE WINDOW OF OPPORTUNITY?
Q: True or false? Both ulipristal and levonorgestrel can be taken up to 120 hours (5 days) after unprotected intercourse. However, ulipristal maintains its effectiveness throughout this time, whereas levonorgestrel becomes less effective the longer a patient waits to take it.
A: True. Ulipristal is a second-generation selective progesterone receptor modulator. These drugs can function as agonists, antagonists, or mixed agonist-antagonists at the progesterone receptor, depending on the tissue affected. Ulipristal is given as a one-time, 30-mg dose within 120 hours of intercourse.
In a study of 1,696 women, 844 of whom received ulipristal acetate and 852 of whom received levonorgestrel, ulipristal was at least as effective as levonorgestrel when used within 72 hours of intercourse for emergency contraception, with 15 pregnancies in the ulipristal group and 22 pregnancies in the levonorgestrel group (odds ratio [OR] 0.68, 95% confidence interval [CI] 0.35–1.31]). However, ulipristal prevented significantly more pregnancies than levonorgestrel at 72 to 120 hours, with no pregnancies in the ulipristal group and three pregnancies in the levonorgestrel group.9
Because ulipristal has a long half-life (32 hours), it can delay ovulation beyond the life span of sperm, thereby extending the window of opportunity for emergency contraception. However, patients should be advised to avoid further unprotected intercourse after the use of emergency contraception. Because emergency contraception works mainly by delaying ovulation, it may increase the likelihood of pregnancy if the patient has unprotected intercourse again several days later.
IS MIFEPRISTONE AN EMERGENCY CONTRACEPTIVE?
Q: True or false? In the United States, mifepristone (Mifeprex), also known as RU-486, is available for use as an emergency contraceptive in addition to its use in abortion.
A: False, even though mifepristone, another selective progesterone receptor modulator, is highly effective when used up to 120 hours after intercourse. In fact, it might be effective up to 17 days after unprotected intercourse.10
Although mifepristone is one of the most effective forms of emergency contraception, social and political controversy has prevented its approval in the United States. However, it is approved for use as an abortifacient, at a higher dose than would be used for emergency contraception.
Unlike levonorgestrel, mifepristone exerts its effect via two potential mechanisms: delaying ovulation and preventing implantation.11
IUDs AS EMERGENCY CONTRACEPTION
Q: True or false? Insertion of a 5-year intrauterine device (IUD), ie, the levonorgestrel-releasing intrauterine system (Mirena), is 99.8% effective at preventing pregnancy when used within 5 days of unprotected intercourse.
A: False. The Mirena IUD has not been studied as a form of emergency contraception. However, this statement would be true for the 10-year copper IUD ParaGard. Copper-releasing IUDs are considered a very effective method of emergency contraception, with associated pregnancy rates of 0.0% to 0.2% when inserted up until implantation (within 5 days after ovulation).12,13 If desired, the IUD can then be kept in place for up to 10 years as a method of birth control.
However, this method requires the ready availability of a health professional trained to do the insertion. It is also important to make sure that the patient will not be at increased risk of sexually transmitted infections from further unprotected intercourse. The American Congress of Obstetricians and Gynecologists (ACOG) recommends that an IUD be placed within 5 days of unprotected intercourse for use as emergency contraception.
A recent review looked at 42 published studies of copper IUDs used for emergency contraception around the world. It found copper IUDs to be a safe and highly effective method of emergency contraception, with the additional advantage of simultaneously offering one of the most reliable and cost-effective contraceptive options.14
EMERGENCY CONTRACEPTION AT MID-CYCLE
Q: True or false? When choosing a method of emergency contraception, it is important to consider whether a woman is near ovulation during the time of intercourse.
A: True. Emergency contraception can prevent pregnancy after unprotected intercourse, but it does not always work. The most widely used method, levonorgestrel 1.5 mg orally within 72 hours of intercourse, prevents at least 50% of pregnancies that would have occurred in the absence of its use.15 Glasier et al16 showed that emergency contraception was more likely to fail if a woman had unprotected intercourse around the time of ovulation.16
Though it can be difficult for women to tell if they are in the fertile times of their cycle, it might be helpful to try to identify women who have intercourse at mid-cycle, when the risk of pregnancy is greatest. Because insertion of an IUD and use of ulipristal acetate probably prevent more pregnancies, these methods might be preferred over levonorgestrel-based regimens during these higher-risk situations.
OBESE PATIENTS
Q: True or false? Hormonal emergency contraception is more likely to fail in obese patients.
A: True. Most recent evidence shows that whichever oral emergency contraceptive drug is taken, the risk of pregnancy is more than 3 times greater for obese women (OR 3.60, 95% CI 1.96–6.53) and 1.5 times greater for overweight women (OR 1.53, 95% CI 0.75–2.95).16 Of all covariates tested, those that were shown to increase the odds of failure of the emergency contraception were higher body mass index, further unprotected intercourse, and conception probability (based on time of fertility cycle). In fact, among obese women treated with levonorgestrel, the observed pregnancy rate was 5.8%, which is slightly above the overall pregnancy rate expected in the absence of emergency contraception, suggesting that for obese women levonorgestrel-based emergency contraception may even be ineffective.
This is in line with recent reports suggesting that oral contraceptives are less effective in obese women. More effective regimens such as an IUD or ulipristal might be preferred in these women. However, obesity should not be used as a reason not to offer emergency contraception, as this is the last chance these women have to prevent pregnancy.
IS IT ABORTION?
Q: True or false? Emergency contraception does not cause abortion.
A: True, but patients may ask for more details about this. Hormonal emergency contraception works primarily by delaying or inhibiting ovulation and inhibiting fertilization.
Levonorgestrel or combined estrogen-progestin-based methods would be unlikely to have any adverse effects on the endometrium after fertilization, since they would only serve to enhance the progesterone effect. Therefore, they are unlikely to affect the ability of the embryo to attach to the endometrium.
Ulipristal, on the other hand, can have just the opposite effect on the postovulatory endometrium because of its inhibitory action on progesterone. Ulipristal is structurally similar to mifepristone, and its mechanism of action varies depending on the time of administration during the menstrual cycle. When unprotected intercourse occurs during a time when fertility is not possible, ulipristal behaves like a placebo. When intercourse occurs just before ovulation, ulipristal acts by delaying ovulation and thereby preventing fertilization (similar to levonorgestrel). Ulipristal may have an additional action of affecting the ability of the embryo to either attach to the endometrium or maintain its attachment, by a variety of mechanisms of action.17,18 Because of this, some in the popular press and on the Internet have spoken out against the use of ulipristal.
The ACOG considers pregnancy to begin not with fertilization of the egg but with implantation, as demonstrated by a positive pregnancy test.
Of note, the copper IUD also prevents implantation after fertilization, which likely explains its high efficacy.
Women who have detailed questions about this can be counseled that levonorgestrel works mostly by preventing ovulation, and that ulipristal and the copper IUD might also work via postfertilization mechanisms. However, they are not considered to be abortive, based on standard definitions of pregnancy.
If a woman is pregnant and she takes levonorgestrel-based emergency contraception, this has not been shown to have any adverse effects on the fetus (similar to oral contraceptives).
Ulipristal is classified as pregnancy category X, and therefore its use during pregnancy is contraindicated. Based on information provided by the manufacturer, there are no adequate, well-controlled studies of ulipristal use in pregnant women. Although fetal loss was observed in animal studies after ulipristal administration (during the period of organogenesis), no malformations or adverse events were present in the surviving fetuses. Ulipristal is not indicated for termination of an existing pregnancy.
DO THE USUAL CONTRAINDICATIONS TO HORMONAL CONTRACEPTIVES APPLY?
Q: True or false? Because emergency contraception has such a short duration of exposure, the usual medical contraindications to hormonal therapies do not apply to it.
A: True. The usual contraindications to the use of hormonal contraceptives (eg, migraine with aura, hypertension, history of venous thromboembolism) do not apply to emergency contraception because of the short time of exposure.19 Furthermore, the risks associated with pregnancy in these women would likely outweigh any risks associated with emergency contraception.
However, one must be cognizant of potential drug interactions. According to the manufacturer, the use of ulipristal did not inhibit or induce cytochrome P 450 enzymes in vitro; therefore, in vivo studies were not performed. But because ulipristal is metabolized primarily via CYP3A4, an interaction between agents that induce or inhibit CYP3A4 could occur.20 Thus, concomitant use of drugs such as barbiturates, rifampin (Rifadin), St. John’s wort, or antiseizure drugs such as topiramate (Topamax) may lower ulipristal concentrations. These medications may also affect levonorgestrel levels, similar to their effects on combined hormonal contraception. However, it is not known whether this translates to decreased efficacy.
When a woman is taking medications that can potentially decrease the effectiveness of hormonal emergency contraception, a more effective method such as a copper IUD might be more strongly considered. If a woman is not interested in an IUD, oral emergency contraception should still be offered, given that this is one of the last chances to prevent pregnancy, especially if she is on a potential teratogen.
Oral contraceptive pills have not been studied in combination with ulipristal. However, because ulipristal binds with high affinity to progesterone receptors (thus competing with the contraceptive), use of additional barrier contraceptives is recommended for the remainder of the menstrual cycle.
EMERGENCY CONTRACEPTION AND BREASTFEEDING
Q: True of false? Emergency contraceptives can be used if a woman is breastfeeding.
A: That depends on which method is used. Both the ACOG and the World Health Organization state that it is safe for breastfeeding women to use emergency contraception, but these are older guidelines addressing progestin-only regimens (ie, levonorgestrel).19,21 It is unknown whether ulipristal is secreted into human breast milk, although excretion was seen in animal studies. Therefore, ulipristal is not recommended for use by women who are breastfeeding.20,22 To minimize the infant’s exposure to levonorgestrel, mothers should consider not nursing for at least 8 hours after ingestion, but no more than 24 hours is needed.23
- Jones RK, Kooistra K. Abortion incidence and access to services in the United States, 2008. Perspect Sex Reprod Health 2011; 43:41–50.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Jones RK, Darroch JE, Henshaw SK. Contraceptive use among US women having abortions in 2000–2001. Perspect Sex Reprod Health 2002; 34:294–303.
- Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. National Center for Health Statistics. Vital Health Stat 2010; 23. http://www.cdc.gov/NCHS/data/series/sr_23/sr23_029.pdf. Accessed October 1, 2012.
- Harrison T. Availability of emergency contraception: a survey of hospital emergency department staff. Ann Emerg Med 2005; 46:105–110.
- Goldenring JM, Allred G. Post-rape care in hospital emergency rooms. Am J Public Health 2001; 91:1169–1170.
- Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Task Force on Postovulatory Methods of Fertility Regulation. Lancet 1998; 352:428–433.
- Creinin MD, Schlaff W, Archer DF, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol 2006; 108:1089–1097.
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet 2010; 375:555–562.
- Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Mifepristone (RU 486) compared with high-dose estrogen and progestogen for emergency postcoital contraception. N Engl J Med 1992; 327:1041–1044.
- Glasier A. Emergency postcoital contraception. N Engl J Med 1997; 337:1058–1064.
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol 2002; 187:1699–1708.
- Zhou L, Xiao B. Emergency contraception with Multiload Cu-375 SL IUD: a multicenter clinical trial. Contraception 2001; 64:107–112.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod 2012; 27:1994–2000.
- Trussell J, Ellertson C, von Hertzen H, et al. Estimating the effectiveness of emergency contraceptive pills. Contraception 2003; 67:259–265.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception 2011; 84:363–367.
- Miech RP. Immunopharmacology of ulipristal as an emergency contraceptive. Int J Womens Health 2011; 3:391–397.
- Keenan JA. Ulipristal acetate: contraceptive or contragestive? Ann Pharmacother 2011; 45:813–815.
- Medical eligibility criteria for contraceptive use. 3rd ed. Geneva: Reproductive Health and Research, World Health Organization; 2004.
- Ella package insert. Morristown, NJ: Watson Pharmaceuticals; August 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022474s000lbl.pdf. Accessed July 6, 2012.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 112: emergency contraception. Obstet Gynecol 2010; 115:1100–1109.
- Orleans RJ. Clinical review. NDA22-474. Ella (ulipristal acetate 30 mg). US Food and Drug Administration, July 27, 2010. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM295393.pdf. Accessed October 1, 2012.
- Gainer E, Massai R, Lillo S, et al. Levonorgestrel pharmacokinetics in plasma and milk of lactating women who take 1.5 mg for emergency contraception. Hum Reprod 2007; 22:1578–1584.
- Jones RK, Kooistra K. Abortion incidence and access to services in the United States, 2008. Perspect Sex Reprod Health 2011; 43:41–50.
- Finer LB, Zolna MR. Unintended pregnancy in the United States: incidence and disparities, 2006. Contraception 2011; 84:478–485.
- Jones RK, Darroch JE, Henshaw SK. Contraceptive use among US women having abortions in 2000–2001. Perspect Sex Reprod Health 2002; 34:294–303.
- Mosher WD, Jones J. Use of contraception in the United States: 1982–2008. National Center for Health Statistics. Vital Health Stat 2010; 23. http://www.cdc.gov/NCHS/data/series/sr_23/sr23_029.pdf. Accessed October 1, 2012.
- Harrison T. Availability of emergency contraception: a survey of hospital emergency department staff. Ann Emerg Med 2005; 46:105–110.
- Goldenring JM, Allred G. Post-rape care in hospital emergency rooms. Am J Public Health 2001; 91:1169–1170.
- Randomised controlled trial of levonorgestrel versus the Yuzpe regimen of combined oral contraceptives for emergency contraception. Task Force on Postovulatory Methods of Fertility Regulation. Lancet 1998; 352:428–433.
- Creinin MD, Schlaff W, Archer DF, et al. Progesterone receptor modulator for emergency contraception: a randomized controlled trial. Obstet Gynecol 2006; 108:1089–1097.
- Glasier AF, Cameron ST, Fine PM, et al. Ulipristal acetate versus levonorgestrel for emergency contraception: a randomised non-inferiority trial and meta-analysis. Lancet 2010; 375:555–562.
- Glasier A, Thong KJ, Dewar M, Mackie M, Baird DT. Mifepristone (RU 486) compared with high-dose estrogen and progestogen for emergency postcoital contraception. N Engl J Med 1992; 327:1041–1044.
- Glasier A. Emergency postcoital contraception. N Engl J Med 1997; 337:1058–1064.
- Stanford JB, Mikolajczyk RT. Mechanisms of action of intrauterine devices: update and estimation of postfertilization effects. Am J Obstet Gynecol 2002; 187:1699–1708.
- Zhou L, Xiao B. Emergency contraception with Multiload Cu-375 SL IUD: a multicenter clinical trial. Contraception 2001; 64:107–112.
- Cleland K, Zhu H, Goldstuck N, Cheng L, Trussell J. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod 2012; 27:1994–2000.
- Trussell J, Ellertson C, von Hertzen H, et al. Estimating the effectiveness of emergency contraceptive pills. Contraception 2003; 67:259–265.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception 2011; 84:363–367.
- Miech RP. Immunopharmacology of ulipristal as an emergency contraceptive. Int J Womens Health 2011; 3:391–397.
- Keenan JA. Ulipristal acetate: contraceptive or contragestive? Ann Pharmacother 2011; 45:813–815.
- Medical eligibility criteria for contraceptive use. 3rd ed. Geneva: Reproductive Health and Research, World Health Organization; 2004.
- Ella package insert. Morristown, NJ: Watson Pharmaceuticals; August 2010. http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022474s000lbl.pdf. Accessed July 6, 2012.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 112: emergency contraception. Obstet Gynecol 2010; 115:1100–1109.
- Orleans RJ. Clinical review. NDA22-474. Ella (ulipristal acetate 30 mg). US Food and Drug Administration, July 27, 2010. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM295393.pdf. Accessed October 1, 2012.
- Gainer E, Massai R, Lillo S, et al. Levonorgestrel pharmacokinetics in plasma and milk of lactating women who take 1.5 mg for emergency contraception. Hum Reprod 2007; 22:1578–1584.
KEY POINTS
- Levonorgestrel-based emergency contraceptives such as Plan B One-Step, Next Choice, and generics are now available over the counter, which has the advantage of avoiding the delays and hassles of calling the doctor’s office and waiting for prescriptions. But patients still need our guidance on how and when to use emergency contraception.
- Even if patients now have easy access to over-the-counter emergency contraceptives, we physicians should take every opportunity to discuss effective contraceptive options with our patients.
- Ulipristal and copper intrauterine devices (ParaGard) are likely to be more effective than levonorgestrel and should be considered in women at highest risk of pregnancy, such as those who are obese.
- Prescribers should feel comfortable addressing tough questions about mechanisms of action, as controversies and myths about emergency contraception are regularly discussed in the media and on the Internet.